As filed with the Securities and Exchange Commission on September 14, 2021
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________________________
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
________________________________
RAFAEL HOLDINGS, INC.
(Exact Name of Registrant as Specified in Its Charter)
________________________________
Delaware | | 6719 | | 82-2296593 |
(State or Other Jurisdiction of
Incorporation or Organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
________________________________
520 Broad Street
Newark, New Jersey 07102
Telephone: (212) 658-1450
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Ameet Mallik
Chief Executive Officer
520 Broad Street
Newark, New Jersey 07102
Telephone: (212) 658-1450
(Name, address, including zip code, and telephone number, including area code, of agent for service)
________________________________
With copies to:
Dov T. Schwell, Esq.
Schwell Wimpfheimer & Associates LLP
37 W. 39th Street, Suite 505
New York, NY 10018
Telephone: (646) 328-0795 | | Jeffrey Spindler, Esq.
Mitchell Raab, Esq.
Olshan Frome Wolosky LLP
1325 Avenue of the Americas
New York, New York 10019
Telephone: (212) 451-2300 | | Sanjeev Luther
Rafael Pharmaceuticals, Inc.
1 Duncan Drive Cranbury,
NJ 08512 Telephone: (609) 409-7050 | | David E. Schulman, Esq.
Orrick Herrington &
Sutcliffe LLP
1152 15th Street, N.W.
Washington, DC 20005
Telephone: (202) 339-8654 |
________________________________
Approximate date of commencement of the proposed sale of the securities to the public: As soon as practicable after this Registration Statement becomes effective and upon completion of the mergers and transactions described in the enclosed proxy statement/prospectus.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act of 1933, as amended, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| | Large accelerated filer | | ☐ | | Accelerated filer | | ☐ | | |
| | | Non-accelerated filer | | ☒ | | Smaller reporting company | | ☒ | | |
| | | | | | | Emerging growth company | | ☒ | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13e-4(i) (Cross-Border Issuer Tender Offer) ☐
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) ☐
Table of Contents
CALCULATION OF REGISTRATION FEE
Title of Securities to be Registered | | Amount to be
Registered(1) | | Proposed
Maximum
Aggregate
Offering
Price | | Amount of
Registration
Fee(2) |
Class B Common Stock, par value $.01 per share | | 17,145,038 | | $ | 607,191,521 | | $ | 66,244.59 |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such dates as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary proxy statement/prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary proxy statement/prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY, SUBJECT TO COMPLETION, DATED SEPTEMBER 14, 2021
Dear Rafael Holdings, Inc. Stockholders:
On behalf of the board of directors of Rafael Holdings, Inc. (“Parent” or “Holdings”), I am pleased to enclose the proxy statement/prospectus for the business combination of Holdings and Rafael Pharmaceuticals, Inc. (“Pharma”) and the related facilitating transactions.
As previously announced, on June 17, 2021, Holdings entered into an Agreement and Plan of Merger (the “Merger Agreement”) with RH Merger I, Inc., a Delaware corporation and a wholly-owned subsidiary of Holdings (“Merger Sub I”); RH Merger II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Holdings (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”); and Pharma. The Merger Agreement provides for, among other things, the following shall occur: at the effective time, Merger Sub I will merge with and into Pharma (the “Initial Merger”), and Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger (the “Subsequent Merger” and together with the Initial Merger, the “Mergers”). As a result of the Mergers, Pharma is intended to become a wholly-owned limited liability subsidiary of Holdings. At the Subsequent Merger effective time, the limited liability company agreement of Merger Sub II will be amended and restated to be the certificate of formation and limited liability company agreement of the surviving entity.
As a consequence of the Mergers, at the effective time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Holding’s Class B Common Stock, par value $0.01 per share (the “Parent Class B Common Stock”), per share of Pharma capital stock. This Per Share Merger Consideration ratio for each class of Pharma capital stock is currently anticipated to result in prior holders of Pharma capital stock and contingent interests receiving Parent Class B Common Stock amounting to approximately 44% of the outstanding capitalization of Holdings following the Mergers based on the currently outstanding capitalization, including after giving effect to the issuance of 2,945,986 shares of Parent Class B Common Stock consummated on August 24, 2021 (the “Holdings Financing”). Additional equity in Holdings will be issued pursuant to employment agreements (which will reduce the foregoing percentage proportionately). See “The Merger Agreement — Merger Consideration” beginning on page 66 of the accompanying proxy statement/prospectus.
Pursuant to the Transactions, an aggregate of 17,145,038 shares of Parent Class B Common Stock are expected to be issued to holders of outstanding shares of Pharma capital stock.
The shares of Parent Class B common stock are listed on the New York Stock Exchange (“NYSE”) under the symbol “RFL” and we do not expect that to be impacted by the Mergers. We believe that the Mergers will benefit both Holdings and Pharma stockholders and we ask for your support in voting for the proposals related to the transactions at the special meeting.
In connection with the Mergers, Holdings will grant additional restricted shares of Parent Class B Common Stock to Ameet Mallik, Holdings’ chief executive officer, so that the Mr. Mallik’s equity interest in Holdings represents an indirect, beneficial 5% ownership interest in Pharma after giving effect to the exercise, conversion, or exchange of all outstanding securities that are exercisable or exchangeable for, or convertible into, capital stock of Holdings.
Further, Holdings is in the process of expanding its management team and other personnel and plans to issue incentive awards under the Rafael Holdings, Inc.’s 2018 Equity Incentive Plan, as amended and restated (the “Plan”). Accordingly, we will need to amend the Plan to authorize an additional [•] shares of Parent Class B Common Stock to be issued under the Plan.
Holdings stockholders are cordially invited to attend a special meeting to be held on [•], 2021 at Holdings’ offices at 520 Broad Street, 4th Floor, Newark, New Jersey 07102 at 11:30 a.m., Eastern Time. The matter to be voted on at the special meeting are (i) the approval and adoption of the Merger Agreement, including, but not limited to, the issuance of Parent Class B Common Stock in connection with the Mergers; (ii) the approval of the grant to Ameet Mallik, our chief executive officer, of up to [•] shares of Parent Class B Common Stock as required by his employment agreement upon consummation of the Mergers to be made under the Plan and the necessary amendments to the Plan to provide for such grant; and (iii) to approve an amendment of the Plan to authorize an additional [•] shares of Parent Class B Common Stock to be reserved for issuance thereunder.
Your vote is very important regardless of the number of shares you own. Your proxy is being solicited by the board of directors of Holdings. We cannot consummate the Transactions unless Holdings stockholders adopt the first two proposals listed above. Whether or not you plan to attend the special meeting, please vote as soon as possible by following the instructions in the accompanying proxy statement/prospectus. More information about the Transactions and the proposals are contained in the accompanying proxy statement/prospectus.
Table of Contents
After careful consideration, the board of directors of Holdings and the Special Committee of the board of directors of Holdings have unanimously approved and declared advisable the Merger Agreement and the Transactions, and determined that the terms and provisions of the Merger Agreement are fair to, advisable and in the best interests of Holdings and its stockholders. The board of directors of Holdings unanimously recommends that you vote “FOR” each of the proposals described in the accompanying proxy statement/prospectus, including a proposal to adjourn the special meeting in the event there are not sufficient votes to obtain approval of the three proposals described above. In considering the recommendation of the board of directors of Holdings, you should be aware that certain directors and executive officers of Holdings may have interests in the Transactions that may be different from, or in addition to, the interests of Holdings’ stockholders generally. See “Risk Factors — Risk Factors Relating to the Transaction — Executive officers and directors of Parent may have interests in the Mergers that are different from, or in addition to, the rights of its stockholders.” beginning on page 24 of the accompanying proxy statement/prospectus.
The accompanying proxy statement/prospectus provides important information regarding the special meeting and a detailed description of the Merger Agreement, including, but not limited to, the issuance of shares of Parent Class B Common Stock, and the other proposals related to the Transactions. We urge you to read carefully and in its entirety the accompanying proxy statement/prospectus (including the annexes and any documents incorporated by reference into the accompanying proxy statement/prospectus). Please pay particular attention to the section entitled “Risk Factors” beginning on page 20 of the accompanying proxy statement/prospectus. You can also obtain information about Holdings from documents that Holdings previously has filed with the U.S. Securities and Exchange Commission.
Whether or not you plan to attend the special meeting, please ensure that your shares will be represented and voted at the meeting by submitting a proxy as soon as possible by following the instructions in the accompanying proxy statement/prospectus.
If Holdings stockholders have any questions or require assistance in voting their shares of Parent Class B Common Stock or Class A Common Stock, they should call [•].
We hope to see you at the special meeting and look forward to the successful completion of the Transactions.
On behalf of the board of directors of Holdings, I thank you for your consideration and continued support.
| | Sincerely, |
| | | /s/ Howard S. Jonas |
| | | Howard S. Jonas |
| | | Chairman of the Board of Directors |
Table of Contents
RAFAEL HOLDINGS, INC.
520 Broad Street, Newark, New Jersey 07102
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
To the Stockholders of Rafael Holdings, Inc.:
NOTICE IS HEREBY GIVEN that a special meeting (the “Special Meeting”) of stockholders of Rafael Holdings, Inc., a Delaware corporation (“Holdings” or “Parent”), will be held on [•], 2021 at 11:30 a.m. local time, at 520 Broad Street, 4th floor, Newark, New Jersey 07102. Only stockholders who hold shares of Holdings Class A common stock, par value $0.01 per share (“Parent Class A Common Stock”) and Holdings Class B common stock, par value $0.01 per share (“Parent Class B Common Stock,” together with the Parent Class A Common Stock, the “Common Stock”)), at the close of business on [•], 2021, the record date for the Special Meeting, are entitled to vote at the Special Meeting and any adjournments or postponements of the Special Meeting. You are cordially invited to attend the Special Meeting to conduct the following items of business:
• The Merger Agreement — to consider and vote upon a proposal to adopt the Agreement and Plan of Merger (the “Merger Agreement”) with RH Merger I, Inc., a Delaware corporation and a wholly-owned subsidiary of Holdings (“Merger Sub I”); RH Merger II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Holdings (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”); and Rafael Pharmaceuticals, Inc., a Delaware corporation (“Pharma”), including, but not limited to, the issuances of Parent Class B Common Stock in connection with the Mergers (“Proposal 1”);
• the approval of the grant to Ameet Mallik, our chief executive officer, of up to [•] shares of Parent Class B Common Stock as required by his employment agreement upon consummation of the Mergers to be made under the Plan and the necessary amendments to the Plan to provide for such grant (“Proposal 2”);
• to approve an amendment of the Plan to authorize an additional [•] shares of Parent Class B Common Stock to be reserved for issuance thereunder (“Proposal 3”);
• The Adjournment Proposal — to consider and vote upon a proposal to approve the adjournment of the Special Meeting to a later date or dates, if necessary, to permit further solicitation of proxies in the event there are insufficient votes present in person or by proxy for, or otherwise in connection with, the approval of each of Proposals 1, 2 and 3 (the “Adjournment Proposal”).
Each of Proposals 1, 2, and 3 is conditioned on the approval of each of the other proposals among Proposals 1, 2 and 3 and will be of no force and effect if any one or more of Proposals 1, 2, and 3 is not approved. Further, the consummation of the transactions, contemplated by the Merger Agreement, is conditioned on, among other things, the approval of the Merger by the Pharma stockholders.
No other business will be conducted at the Special Meeting. These proposals are described more fully in the accompanying proxy statement. We urge you to read the proxy statement/prospectus, including the Annexes and the documents incorporated by reference in the accompanying proxy statement/prospectus carefully and in their entirety. In particular, we urge you to read carefully “Risk Factors” beginning on page 20 of the accompanying proxy statement/prospectus. For specific instructions on how to vote your shares, see “The Special Meeting of Holdings Stockholders — Voting Your Shares” beginning on page 46 of the accompanying proxy statement/prospectus.
Your vote is very important, regardless of the number of shares of Holdings Common Stock that you own. The approval of Proposal 1 requires the approval of the holders of a majority of the outstanding shares of Parent Common Stock, not including shares owned directly or indirectly by any affiliate of Holdings, Merger Subs or any of their respective officers or directors on or after the date of the Merger Agreement, including trusts for the benefit of immediate family members of such parties regardless of whether they are affiliates, outstanding and entitled to vote at the Special Meeting. The approval of Proposals 2 and 3 require the approval of the holders of a majority of
Table of Contents
the outstanding shares of Parent Common Stock. The approval of each of Proposals 1, 2, 3 and the Adjournment Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the meeting.
After careful consideration, our board of directors has unanimously determined that the terms and provisions of the Merger Agreement are fair to, advisable and in the best interests of Holdings and its stockholders, and unanimously recommends you vote “FOR” Proposals 1 through 3, as well as the Adjournment Proposal.
Even if you plan to attend the Special Meeting in person, we request that you complete, sign, date and return the enclosed proxy card in the envelope provided, or submit your proxy by telephone or the Internet prior to the Special Meeting, and thus ensure that your shares will be represented and voted at the Special Meeting if you later become unable to attend. If your shares are held in a stock brokerage account or by a bank or other nominee, please follow the instructions that you receive from your broker, bank or other nominee to vote your shares.
| | By order of the board of directors, |
| | | /s/ Joyce Mason |
| | | Corporate Secretary |
| | | [•], 2021 |
Table of Contents
i
Table of Contents
ii
Table of Contents
QUESTIONS AND ANSWERS ABOUT THE TRANSACTION AND THE SPECIAL MEETING
The following questions and answers are intended to address briefly some commonly asked questions regarding the Transactions and the Special Meeting. These questions and answers only highlight some of the information contained in this proxy statement/prospectus. They may not contain all the information that is important to you. You should read carefully this entire proxy statement/prospectus, including the Annexes and the documents incorporated by reference into this proxy statement/prospectus, to understand fully the proposed transactions and the voting procedures for the meeting. See “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus. Unless otherwise indicated or the context requires, all references in this proxy statement/prospectus to:
• “Acquired Shares” refers to the Pharma Preferred Stock and Pharma Common Stock not held by Parent, the Merger Subs or any other wholly owned subsidiary of Parent.
• “Altira Acquisition Agreement” refer to the Altira Acquisition Agreement, a copy of which is included as Annex F to this proxy statement/prospectus.
• the “Business Combination” refer to approval by Holdings’ and Pharma’s stockholders, the satisfaction of the other conditions in the Merger Agreement and the Merger transactions contemplated by the Merger Agreement are consummated, resulting in Pharma becoming a wholly-owned subsidiary of Holdings.
• “dollars” or “$” refer to U.S. dollars.
• the “Exchange Act” refer to the Securities Exchange Act of 1934, as amended.
• the “Holdings Bylaws” refer to the Amended and Restated By-laws of Holdings.
• the “Holdings Charter” refer to the Amended and Restated Certificate of Incorporation of Holdings and in effect immediately prior to the Effective Time (as defined below).
• “Initial Merger” refers to Merger Sub I merging with and into Pharma.
• the “Merger Agreement” refer to the Agreement and Plan of Merger, dated as of June 17, 2021, by and among Parent, Merger Subs and Pharma, a copy of which is included as Annex A to this proxy statement/prospectus.
• “our” or “we” refer to Parent.
• “Lock-Up Agreements” refer to the lock-up agreements between certain stockholders of Pharma and Holdings providing for post-closing restrictions on the ability of such individuals with respect to the transfer of shares of Parent Common Stock to be issued to them pursuant to the Merger Agreement and/or otherwise owned, as applicable, in substantially the form included as Annex B to this proxy statement/prospectus.
• “Mergers” refer to, collectively, the Initial Merger and the Subsequent Merger.
• “Merger Sub I” refer to RH Merger I, Inc., a Delaware corporation and a wholly-owned subsidiary of Parent.
• “Merger Sub II” refer to RH Merger II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Parent.
• “Merger Subs” refer to Merger Sub I, together with Merger Sub II.
• “Parent” or “Holdings” refer to Rafael Holdings, Inc., a Delaware corporation.
• “Parent Class A Common Stock” refer to Parent’s Class A Common Stock, par value $0.01 per share, the holders of which are entitled to three votes per share.
• “Parent Class B Common Stock” refer to Parent’s Class B Common Stock, par value $0.01 per share, the holders of which are entitled to one-tenth of a vote per share.
1
Table of Contents
• “Parent Common Stock” refer to the Parent Class A Common Stock and the Parent Class B Common Stock.
• “Parent Minority Approval” refer to the adoption of the Merger Agreement, and the approval of the transactions contemplated thereby, including the Merger, by the affirmative vote of at least a majority of the issued and outstanding shares of Parent Common Stock, excluding shares owned directly or indirectly by any affiliate of Parent, Merger Subs or any of their respective officers or directors on or after the date of the Merger Agreement, including trusts for the benefit of immediate family members of such parties regardless of whether they are affiliates, outstanding and entitled to vote thereon at the Parent Special Meeting.
• the “Parent Stockholder Approval” refer to the approval of the issuance of the Parent Class B Common Stock issuable pursuant to the Merger and Parent Minority Approval.
• “Pharma” or the “Company” refer to Rafael Pharmaceuticals, Inc., a Delaware corporation.
• “Pharma Charter” refer to the Amended and Restated Certificate of Incorporation and in effect immediately prior to the Effective Time.
• “Pharma Common Stock” refer to Pharma Voting Common Stock and Pharma Non-Voting Common Stock.
• “Pharma Minority Approval” refer to the adoption of the Merger Agreement, and the approval of the transactions contemplated thereby, including the Merger, by the affirmative vote of at least a majority of the issued and outstanding shares of Pharma Capital Stock entitled to vote thereon, excluding shares of Pharma Capital Stock owned directly or indirectly by Parent or any affiliate of Parent.
• “Pharma Non-Voting Common Stock” shall mean Pharma’s Common Stock, par value $0.001 per share, designated as non-voting in the Pharma Charter.
• “Pharma Preferred Stock” refer to collectively the Series A Preferred Stock, Series B-1 Preferred Stock, the Series B-2 Preferred Stock, the Series B-3 Preferred Stock, the Series B-4 Preferred Stock, the Series C Preferred Stock and the Series D Preferred Stock.
• “Pharma Restricted Stock” refer to a share of Pharma Common Stock that is (i) outstanding on the date of the Merger Agreement and (ii) subject to a substantial risk of forfeiture within the meaning of Section 83 of the Code.
• “Pharma Stockholder Approval” refer to the Pharma Minority Approval and approval of the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Pharma as contemplated by the Merger Agreement.
• “Pharma Voting Common Stock” shall mean Pharma’s Common Stock, par value $0.001 per share, designated as voting in the Pharma Charter.
• “Registration Statement” refer to the Registration Statement of which this proxy statement/prospectus forms a part.
• the “SEC” refer to the U.S. Securities and Exchange Commission.
• the “Securities Act” refer to the Securities Act of 1933, as amended.
• “Subsequent Merger” refer to Pharma merging with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger.
• “Subsequent Merger Effective Time” refer the time when the certificate of merger for Subsequent Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as is permissible under the DGCL and the Delaware Limited Liability Company Act.
• “Support Agreements” refer to the support agreements entered into by certain directors and executive officers of Pharma and other Pharma Stockholders with Parent and Pharma, a copy of which in substantially the form annexed hereto as Annex C.
2
Table of Contents
• “Transactions” refer to the Merger Agreement, the Mergers and the other transactions contemplated thereunder, including the Voting Agreements, Support Agreements, Lock-Up Agreements and Altira Acquisition Agreement.
• “Voting Agreements” refer to the Voting Agreement among Howard Jonas and certain of his family members and affiliates, in substantially the form included as Annex D to this proxy statement/prospectus.
If you are in any doubt about the transactions described herein, you should consult your own financial advisor.
Q: What is the Merger Agreement and the Transactions?
A: On June 17, 2021, Parent, Merger Subs and Pharma entered into the Merger Agreement, pursuant to which, among other things, the following shall occur: at the Effective Time, Merger Sub I will merge with and into Pharma, and Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger. As a result of the Mergers, Pharma is intended to become a wholly-owned limited liability subsidiary of Holdings. At the Subsequent Merger Effective Time, the limited liability company agreement of Merger Sub II will be amended and restated to be the certificate of formation and limited liability company agreement of the surviving entity.
Concurrently with the execution of the Merger Agreement, certain key affiliates of both Parent and Pharma entered into binding commitments to support the Mergers. The Support Agreements were executed by affiliates and other related parties of Pharma, and the Voting Agreements were executed by affiliates and other related parties of Holdings. In addition, certain key affiliates of both Parent and Pharma entered into lock-up agreements, which contain certain restrictions on transfer of such Parent Common Stock for a period of six months following closing of the Business Combination.
As a result of the Transactions, at the Effective Time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist, and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Parent Class B Common Stock per share of Pharma capital stock. This Per Share Merger Consideration ratio for each class of Pharma capital stock is currently anticipated to result in prior holders of Pharma capital stock and contingent interests receiving Parent Class B Common Stock amounting to approximately 44% of the outstanding capitalization of Holdings following the Mergers based on the currently outstanding capitalization, including after giving effect to the issuance of the Holdings Financing. Additional equity in Parent will be issued pursuant to employment agreements (which will reduce the foregoing percentage proportionately). See “The Merger Agreement” beginning on page 66 of this proxy statement/prospectus.
Q: Why am I receiving this proxy statement/prospectus?
A: This proxy statement/prospectus serves as the proxy statement through which Parent will solicit proxies to obtain the necessary stockholder approval for the Transactions. Parent is holding a special meeting of stockholders (the “Parent Special Meeting”) in order to obtain the Parent Stockholder Approval necessary to approve the Mergers, and to obtain the necessary Parent stockholder approval of (i) the grant to Ameet Mallik, our chief executive officer, of up to [•] shares of Class B Common Stock as required by his employment agreement upon consummation of the Mergers to be made under the Plan and the necessary amendments to the Plan to provide for such grant and (ii) an amendment of the Plan to authorize an additional [•] shares of Class B Common Stock to be reserved for issuance thereunder. Holdings stockholders will also be asked to approve the adjournment of the Parent Special Meeting (if necessary or appropriate to solicit additional proxies if there are not sufficient votes to obtain the Parent Stockholder Approval).
You are receiving this proxy statement/prospectus because you were a holder of record of Parent Common Stock as of the close of business on the record date for the Parent Special Meeting, and are therefore entitled to vote at the Parent Special Meeting.
This proxy statement/prospectus contains important information about the Transactions. Please also review the Annexes to this proxy statement/prospectus. You should read this information carefully and in its entirety. The enclosed voting materials allow you to vote your shares without attending the Parent Special Meeting. Your vote is very important and we encourage you to submit your proxy as soon as possible.
3
Table of Contents
Q: When were the enclosed solicitation materials first made available to Holdings stockholders?
A: The enclosed materials were first made available to Holdings stockholders on or about [•], 2021.
Q: When and where will the Parent Special Meeting be held?
A: The Parent Special Meeting will be held at 520 Broad Street, 4th Floor, Newark, New Jersey 07102, on [•], 2021 at 11:30 a.m., local time.
Q: What will Holdings stockholders receive as consideration in the Mergers?
A: Holdings stockholders will not receive Merger Consideration and, other than issuance of shares of Parent Class B Common Stock, the Business Combination will have no impact on the outstanding capital stock of Parent, and each share of Parent Class A Common Stock or Parent Class B Common Stock of Parent that is issued and outstanding as of immediately prior to the Effective Time of the Mergers, which will continue to remain outstanding. See “The Merger Agreement — Merger Consideration” beginning on page 66 of this proxy statement/prospectus.
Q: What will Pharma Stockholders receive as consideration in the Mergers?
A: As a consequence of the Mergers, at the Effective Time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist, and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Parent Class B Common Stock per share of Pharma capital stock. The Per Share Merger Consideration ratio for each class of Pharma capital stock provides that the prior holders of Pharma capital stock and contingent interests are intended to receive Parent Class B Common Stock. Additional equity in Parent may be issued in connection with one or more financings and pursuant to employment agreements (which will reduce the foregoing percentage proportionately).
See “The Merger Agreement — Merger Consideration” beginning on page 66 of this proxy statement/prospectus.
Q: What will happen to outstanding Pharma equity awards in the Transactions?
A: At the Effective Time, all compensatory options to purchase Pharma Common Stock shall automatically vest in full, and all holders of such options will receive options to purchase Class B common stock of Holdings using the ratios applicable to determine the Holdings stock in the Business Combination. The parties intend that the Company Convertible Notes will not be assumed or continued by Parent or Pharma in connection with the Merger or the other transactions contemplated by the Merger Agreement. All Company Warrants are intended to terminate at the Effective Time as contemplated by and in accordance with the Merger Agreement and the applicable Support Agreement. See “The Merger Agreement — Treatment of Company Warrants, Company Options, Company Convertible Notes and Contingent Rights” beginning on page 69 of this proxy statement/prospectus.
Q: When are the Transactions expected to be completed?
A: Pursuant to the terms of the Merger Agreement, the Transactions are to be completed on or prior to December 15, 2021 (the “End Date”); provided, however, that, in the event that the SEC has not declared effective under the Securities Act the Registration Statement by the date which is 45 calendar days prior to the End Date, then the End Date shall automatically be extended to February 1, 2022. However, no assurance can be provided as to when or if the Transactions will be completed. The Parent Stockholder Approval and the Pharma Stockholder Approval must be obtained, and other conditions specified in the Merger Agreement must be satisfied or, to the extent applicable, waived prior to the consummation of the Transactions.
Q: What conditions must be satisfied to complete the Transactions?
A: The Merger Agreement. The obligations of each of Parent, Merger Subs and Pharma to complete the Mergers are subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement or applicable law) on or prior to the Effective Time of various conditions, including the following:
• The Registration Statement shall have been declared effective by the SEC under the Securities Act and no stop order suspending the effectiveness of the Registration Statement shall have been issued by the SEC and remain in effect, and no proceedings for that purpose shall be pending or threatened in writing by the SEC and not withdrawn or lapsed.
4
Table of Contents
• No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of any of the Mergers shall have been issued by any court of competent jurisdiction and remain in effect, and no material Law shall have been enacted since the date of the Merger Agreement that makes consummation of any of the Mergers illegal.
• The Merger Agreement shall have been duly adopted by the Required Company Stockholder Vote (as defined in the Merger Agreement) and the Required Parent Stockholder Vote (as defined in the Merger Agreement), and the Required Company Stockholder Vote and the Required Parent Stockholder Vote shall remain in full force and effect and, if the Registration Statement incorporates information by reference as contemplated in General Instruction A.2 of Form S-4 under the Securities Act, at least twenty business days (as defined pursuant to Form S-4) shall have elapsed from the date of the first mailing of the prospectus in the Registration Statement to the Pharma stockholders until the date of the Closing.
• The Merger Agreement shall have been duly adopted by the Parent Minority Approval and the Company Minority Approval, each of the Parent Stockholder Approval and the Company Stockholder Approval have occurred, and all such approvals shall remain in full force and effect.
• The Merger Shares shall have been approved for listing on the NYSE, subject to official notice of issuance.
• The transactions contemplated by the Altira Acquisition Agreement shall have been consummated by all parties thereto in accordance with its terms; provided that such consummation of the Altira Acquisition Agreement shall not be a condition to Parent’s obligations to consummate the Merger if (a) Parent has not funded the payment amount thereunder before the Closing and (b) the Altira Acquisition Agreement has been consummated in all material respects by all parties thereto other than Parent (to the extent that such covenants require performance by such other parties at or before the Closing), including execution and delivery of any and all conveyance documents.
• The Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Pharma in the form of attached to the Merger Agreement, shall have received the required director and stockholder approvals and been filed with the Delaware Secretary of State on a date prior to the Closing Date.
• Parent shall have consummated the Financing (which Financing has since been consummated).
See “The Merger Agreement — Conditions to the Merger” beginning on page 77 of this proxy statement/prospectus.
Q: Who will serve as the management of Parent and the Surviving Company following the Transactions?
A: Following the consummation of the Transactions, the directors and officers of the Initial Surviving Corporation immediately after the Effective Time and the managers and officers of the Surviving Company immediately after the Subsequent Merger Effective Time, shall be the respective individuals listed in a schedule to the Merger Agreement (as may be amended from time to time), each to hold office in accordance with the applicable governing documents and applicable law, until such director’s or officer’s successor is duly elected or appointed and qualified, or until the earlier of their death, resignation or removal. The Surviving Company shall be a wholly owned subsidiary of Parent, to be managed by Parent as its sole member. At the Surviving Company, Patrick Fabbio will serve as Treasurer, Tamara Rhein as Comptroller and Joyce Mason as Secretary. The Merger Agreement does not provide for any changes in the currently existing management of Parent.
Q: Following the Transactions, will Parent Common Stock continue to trade on a stock exchange?
A: Yes. Parent Class B Common Stock is currently listed on the NYSE under the symbol “RFL”. Following the consummation of the Transactions, we expect that Parent Class B Common Stock will continue to be listed on the NYSE.
Q: What vote is required to approve each proposal?
5
Table of Contents
A: Your vote “FOR” each proposal presented at the Special Meeting is very important, and you are encouraged to submit a proxy as soon as possible.
Merger Agreement Proposal. Approval and adoption of the Merger Agreement Proposal requires the affirmative vote of the holders of a majority of the outstanding shares of Holdings Common Stock entitled to vote thereon as of the record date for the special meeting and the approval by Holdings stockholders of at least a majority of the issued and outstanding shares of Holdings Class B Common Stock not owned directly or indirectly by any affiliate of Holdings, the Merger Subs, or any of their respective officers or directors or trusts for the benefit of immediate family members of such parties, outstanding and entitled to vote thereon at the Meeting.
Any abstention by a Holdings stockholder, failure of any Holdings stockholder to submit a vote and broker non-vote will have the same effect as voting against the Merger Agreement Proposal. In connection with the Merger Agreement, it is expected that Holdings’ directors and executive officers who are stockholders of Holdings will vote “FOR” each of the proposals described above, including Holdings’ Chairman and the Board of Directors, Howard Jonas, who along with certain of his family members and affiliates entered into a Voting Agreement, which is attached as Annex D to this proxy statement/prospectus and pursuant to which they have agreed to vote in favor of the consummation of the Mergers and take other actions in furtherance of the consummation of the Merger Agreement.
Ameet Mallik Grant Proposal. Approval and adoption of the Ameet Mallik Grant Proposal requires the affirmative vote of a majority of the issued and outstanding shares of Holdings Common Stock to vote thereon. Abstentions and broker non-votes will not be voted either for or against the Ameet Mallik Proposal and, accordingly, will not affect the outcome of the Ameet Mallik Proposal.
The Plan Amendment Proposal. Approval and adoption of the Plan Amendment Proposal requires the affirmative vote of a majority of the issued and outstanding shares of Holdings Common Stock to vote thereon. Abstentions and broker non-votes will not be voted either for or against the Ameet Mallik Proposal and, accordingly, will not affect the outcome of the Ameet Mallik Proposal.
Q: How does the Holdings board of directors (the “Holdings Board” or “the Board of Directors”) recommend that I vote?
A: The Holdings Board, after considering the recommendation of the Holdings Special Committee and various factors described under “Proposal 1: The Merger Agreement Proposal — Recommendation of the Holdings Board and Reasons for the Transactions,” has unanimously approved the Merger Agreement and determined that the Merger Agreement and the Mergers are fair to, and in the best interests of, Holdings and its stockholders and recommends that Holdings stockholders approve and adopt this Merger Agreement, the Mergers and the other transactions contemplated by this document.
Accordingly, the Holdings Board recommends that Holdings stockholders vote:
• “FOR” the Merger Agreement Proposal,
• “FOR” the Ameet Mallik Grant Proposal and
• “FOR” the Plan Amendment Proposal.
Q: What happens if the Mergers are not completed?
A: If the Merger Agreement is not approved and adopted by Holdings stockholders or the Mergers are not completed for any other reason, Pharma stockholders will not receive any shares of Holdings Class B Common Stock for shares of Pharma’s Common Stock they own. Holdings Class B Common Stock will continue to be listed and traded on the NYSE and registered under the Exchange Act, and Holdings will continue to file periodic reports with the SEC on account of Holdings Class B Common Stock.
Q: Am I entitled to vote at the Special Meeting?
6
Table of Contents
A: Only stockholders of record on [•], 2021, the record date for the Special Meeting, are entitled to receive notice of and to vote at the Special Meeting. As of the close of business on [•], 2021, there were [•] issued and outstanding shares of Holdings Class A Common Stock and [•] issued and outstanding shares of Holdings Class B Common Stock entitled to vote at the Special Meeting, with Stockholders are entitled to three votes for each share of Class A Common Stock held by them and one-tenth of one vote for each share of Class B Common Stock. The holders of Class A Common Stock and Class B Common Stock will vote as a single body on all matters presented to the stockholders.
Q: What constitutes a quorum at the Special Meeting?
A: In order for business to be conducted at the Special Meeting, a quorum must be present. A quorum at the Special Meeting requires the presence, in person or by proxy, of a majority of all the voting power of all shares entitled to vote at the Special Meeting, present in person or by proxy. Abstentions and broker non-votes will be counted towards a quorum. At the close of business on [•], 2021, the record date for the Special Meeting, there were [•] shares of Holdings Class A Common Stock outstanding and [•] shares of Holdings Class B Common Stock outstanding.
Q: What do I need to do now?
A: After you have carefully read and considered the information contained in or incorporated by reference into this proxy statement/prospectus, please submit your proxy via the Internet or by telephone in accordance with the instructions set forth on the enclosed proxy card, or complete, sign, date and return the enclosed proxy card in the postage-prepaid envelope provided as soon as possible so that your shares will be represented and voted at the Special Meeting.
Additional information on voting procedures can be found under the section titled “Special Meeting.”
Q: How will my proxy be voted?
A: If you submit your proxy via the Internet, by telephone or by completing, signing, dating and returning the enclosed proxy card, your proxy will be voted in accordance with your instructions.
If you are a registered stockholder of record and you return your signed proxy card but do not indicate your voting preference, the persons named in the proxy card will vote the shares represented by the proxy as recommended by the Holdings Board. Please note that you may not vote shares held in “street name” by returning a proxy card directly to Holdings, or by voting in person at the Special Meeting, unless you provide a “legal proxy,” which you must obtain from your broker, bank or nominee. If you hold your shares in the name of a broker, bank or other nominee and you do not instruct your broker, bank or nominee how to vote your shares, your broker may not vote your shares of Holdings Common Stock, which will have the same effect as a vote “AGAINST” the Merger Agreement Proposal but will have no effect on the Ameet Mallik Grant and Plan Amendment Proposals.
Additional information on voting procedures can be found under the section titled “Special Meeting.”
Q: Who will count the votes?
A: The votes at the Special Meeting will be counted by an independent inspector of election appointed by the Holdings Board.
Q: May I vote in person?
A: Yes. If you are a stockholder of record of Holdings at the close of business on [•], 2021, you may attend the Special Meeting and vote your shares in person, in lieu of submitting your proxy by Internet or telephone or by completing, signing, dating and returning the enclosed proxy card.
If you are a beneficial holder of Holdings Common Stock, you are also invited to attend the Special Meeting. However, because you are not the stockholder of record, you may not vote your shares in person at the Special Meeting unless you request and obtain a valid “legal proxy” from your broker, bank or nominee.
Q: What must I bring to attend the Special Meeting?
7
Table of Contents
A: Only Holdings stockholders of record, as of the close of business on the record date, beneficial owners of Holdings Common Stock as of the record date, holders of valid proxies for the Special Meeting, and invited guests of Holdings may attend the Special Meeting. All attendees should be prepared to present government-issued photo identification (such as a driver’s license or passport) for admittance. The additional items, if any, that attendees must bring depend on whether they are stockholders of record, beneficial owners or proxy holders.
Additional information on attending the Special Meeting can be found under the section titled “Special Meeting.”
Q: What should I do if I receive more than one set of voting materials for the Special Meeting?
A: You may receive more than one set of voting materials for the Special Meeting, including multiple copies of this proxy statement/prospectus and multiple proxy cards or voting instruction forms. For example, if you hold your Holdings Common Stock in more than one brokerage account, you will receive a separate voting instruction form for each brokerage account in which you hold shares. If you are a holder of record and your shares are registered in more than one name, you will receive more than one proxy card. Please submit each separate proxy or voting instruction form that you receive by following the instructions set forth in each separate proxy or voting instruction form.
Q: What is the difference between holding shares as a “stockholder of record” and holding shares as “beneficial owner” (or in “street name”)?
A: Most stockholders are considered “beneficial owners” of their shares — they hold their shares through a broker, bank or other nominee rather than directly in their own name. As summarized below, there are some distinctions between shares held of record and those owned beneficially or in “street name.”
Stockholder of Record: If your shares are registered directly in your name with Holdings’ transfer agent, you are considered the “stockholder of record” with respect to those shares. As a stockholder of record, you have the right to grant your voting proxy directly to us by submitting your vote by written proxy, telephone or the Internet, or to vote in person at the meeting.
Beneficial Owner: If your shares are held in a stock brokerage account or by a bank or other nominee, you are considered the “beneficial owner” of shares held in “street name,” and the proxy materials are being forwarded to you by your broker, bank or nominee. As a beneficial owner, you have the right to direct your broker, bank or nominee as to how to vote your shares if you follow the instructions you receive from your broker, bank, or nominee. You are also invited to attend the Special Meeting. However, since you are not the stockholder of record, you may not vote these shares in person at the Special Meeting unless you request, complete and deliver the proper documentation provided by your broker, bank or nominee and bring it with you to the Special Meeting.
Q: What is a broker non-vote?
A: If you are a beneficial owner of shares held in “street name” and do not provide your broker, bank or nominee with specific voting instructions, the broker, bank or nominee may generally vote on “routine” matters, but cannot vote on “non-routine” matters. If the broker, bank or nominee does not receive instructions from you on how to vote your shares on a non-routine matter, it will inform the inspector of election that it does not have authority to vote on this matter with respect to your shares. This is referred to as a “broker non-vote.”
You should instruct your broker, bank or other nominee how to vote your shares. Under the rules applicable to broker-dealers, your broker, bank or other nominee does not have discretionary authority to vote your shares on any of the proposals scheduled to be voted on at the Special Meeting. A broker non-vote will have the same effect as a vote “against” the approval and adoption of the Merger Agreement Proposal and will not be voted either for or against the Ameet Mallik Grant Proposal or the Plan Amendment Proposal. Additional information on voting procedures can be found under the section titled “Special Meeting.”
Q: Can I revoke or change my vote?
8
Table of Contents
A: Yes. A stockholder of record may revoke or change a proxy before the proxy is exercised by filing with Holdings’ Secretary a notice of revocation, by delivering to Holdings a new proxy, by attending the meeting and voting in person, or by re-voting by telephone or the Internet. Beneficial owners must follow instructions provided by their broker, bank or other nominee to revoke or change a proxy. A stockholder’s last timely vote, whether via the Internet, by telephone or by mail, is the vote that will be counted.
A beneficial owner of Holdings’ Common Stock may change his, her or its voting instruction by submitting a new voting instruction to the broker, bank or other nominee that holds his, her or its shares of record or by requesting a “legal proxy” from such broker, bank or other nominee and voting in person at the Special Meeting. Additional information can be found under the section titled “Special Meeting.”
Q: What happens if I sell or otherwise transfer my shares of Holdings Common Stock before the Special Meeting?
A: The record date for stockholders entitled to vote at the Special Meeting is [•], 2021, which is earlier than the date of the Special Meeting. If you sell or otherwise transfer your shares after the record date but before the Special Meeting, unless special arrangements (such as provision of a proxy) are made between you and the person to whom you transfer your shares and each of you notifies Holdings in writing of such special arrangements, you will retain your right to vote such shares at the Special Meeting but will otherwise transfer ownership of your shares of Holdings Common Stock.
Q: Where can I find voting results of the Special Meeting?
A: Holdings intends to announce preliminary voting results at the Special Meeting and publish the final results in Current Reports on Form 8-K to be filed with the SEC following the Special Meeting. All reports that Holdings files with the SEC are publicly available when filed. See “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus.
Q: Do stockholders have dissenters’ rights or appraisal rights?
A: Holdings stockholders are not entitled to dissenters’ rights or appraisal rights in connection with the Mergers. For further information relating to appraisal and dissenters’ rights, see “The Merger Agreement — Appraisal Rights.” beginning on page 69 of this proxy statement/prospectus.
Q: How can I find more information about Holdings and Pharma?
A: You can find more information about Holdings and Pharma in this proxy statement/prospectus and more information regarding Holdings from various sources described in the section titled “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus.
Q: Who can answer my questions about the Special Meeting, the Mergers, the other transactions contemplated by the Merger Agreement, or any other related agreement?
A: If you have any questions about the Special Meeting, the Mergers, the other related agreements, or how to submit your proxy, or if you need additional copies of this proxy statement/prospectus or documents incorporated by reference herein, the enclosed proxy card or voting instructions, you should contact:
Rafael Holdings, Inc.
520 Broad Street
Newark, New Jersey 07102
(212) 658-1450
Attention: Joyce Mason, Corporate Secretary
9
Table of Contents
SUMMARY
This summary highlights selected information contained in this proxy statement/prospectus and may not contain all of the information that may be important to you. Accordingly, you should read carefully this entire proxy statement/prospectus, including the Annexes and the documents referred to or incorporated by reference in this proxy statement/prospectus. The page references have been included in this summary to direct you to a more complete description of the topics presented below. See “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus.
Information about the Companies (Page 43)
Rafael Holdings, Inc.
Rafael Holdings, Inc. (“Holdings” or the “Parent”), a Delaware corporation, owns interests in pre-clinical and clinical stage pharmaceutical companies and commercial real estate assets. The assets are operated as two separate lines of business.
The pharmaceutical holdings include preferred and common equity interests and a warrant to purchase additional equity interests in Rafael Pharmaceuticals, Inc., or Rafael Pharmaceuticals, which is a clinical stage, oncology-focused pharmaceutical company committed to the development and commercialization of therapies that exploit the metabolic differences between normal cells and cancer cells; and, a majority equity interest in LipoMedix Pharmaceuticals Ltd., or LipoMedix, a clinical stage oncological pharmaceutical company based in Israel. In addition, in 2019, the Company established the Barer Institute (“Barer”), a wholly-owned early stage venture focused on developing a pipeline of therapeutic compounds, including compounds to regulate cancer metabolism. The venture is pursuing collaborative research agreements with leading scientists from top academic institutions. In addition, the Company has recently initiated efforts to develop other early stage pharmaceutical ventures including Levco Pharmaceuticals Ltd. (“Levco”), an Israeli company, established to partner with Dr. Alberto Gabizon and a top institution in Israel on the development of novel compounds for cancer, and Farber Partners, LLC (“Farber”), formed around an agreement with Princeton University’s Office of Technology Licensing for technology from the laboratory of Professor Joshua Rabinowitz, in the Department of Chemistry, Princeton University, for an exclusive worldwide license to its SHMT (serine hydroxymethyltransferase) inhibitor program. Additionally, in 2021, the Company established Rafael Medical Devices, LLC (“Rafael Medical Devices”), a wholly-owned orthopedic device company developing instruments and implants to advance minimally invasive surgeries in the upper and lower extremities.
The commercial real estate holdings consist of a building at 520 Broad Street in Newark, New Jersey that serves as headquarters for the Company and certain other entities and hosts other tenants and an associated 800-car public garage, and a portion of a building in Israel.
Rafael Pharmaceuticals, Inc.
Pharma is a clinical stage, oncology-focused pharmaceutical company committed to the development and commercialization of therapies that exploit the metabolic differences between normal cells and cancer cells.
Pharma is developing its lead product CPI-613® (devimistat), an investigational new drug for the treatment of solid tumors and hematologic malignancies. This molecule has been developed based on Altered Metabolism Directed (AMD) platform. It is a small molecule and suitable for intravenous (IV) administration.
RH Merger I, Inc.
RH Merger I, Inc., a Delaware corporation, is a wholly-owned subsidiary of Holdings (“Merger Sub I”) formed solely for the purpose of effectuating the Mergers. Merger Sub I has no material assets and does not operate any business. To date, Merger Sub I has not conducted any activities other than those incidental to its formation and the execution of the Merger Agreement. After the consummation of the Mergers, it will cease to exist.
The mailing address of Merger Sub I’s principal executive office is 520 Broad Street, Newark, New Jersey 07102.
10
Table of Contents
RH Merger II, LLC
RH Merger II, LLC, a Delaware limited liability company, is a wholly-owned subsidiary of Holdings (“Merger Sub II”) formed solely for the purpose of effectuating the Mergers. Merger Sub II has no material assets and does not operate any business. To date, Merger Sub II has not conducted any activities other than those incidental to its formation and the execution of the Merger Agreement. After the consummation of the Mergers, Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger and Pharma is intended to become a wholly-owned limited liability subsidiary of Holdings.
The mailing address of Merger Sub II’s principal executive office is 520 Broad Street, Newark, New Jersey 07102.
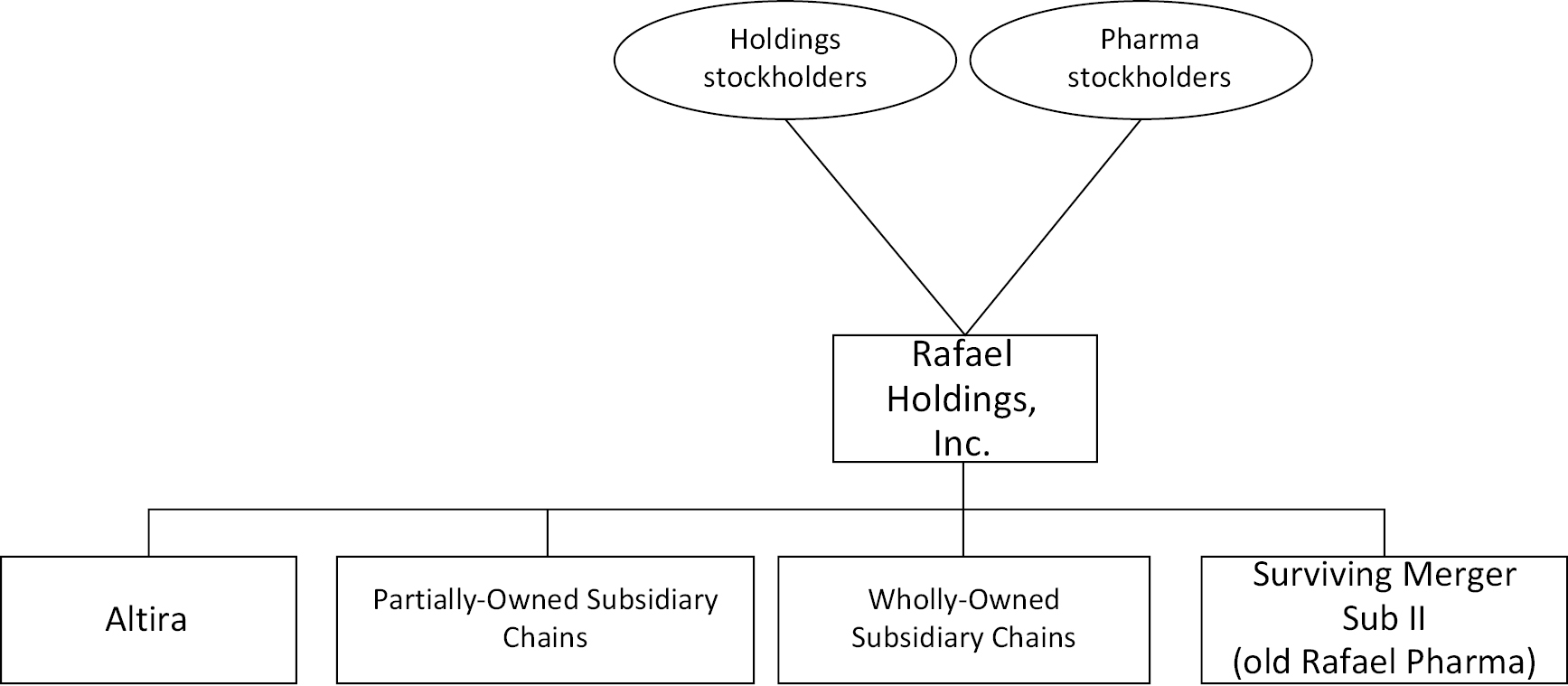
Company Structure — Diagrams (Page 52)
Upon consummation of the Transactions, Pharma is intended to become a direct, wholly-owned subsidiary of Parent. The following diagrams illustrate in simplified terms the current structure of Parent and Pharma and the expected structure of Parent following the consummation of the Transactions.
Current Structure of the Companies

Post-Closing Structure
Holdings Recommendation and Reasons for the Transaction (Page 53)
At a meeting held on June 14, 2021, following receipt of the recommendation of the Holdings Special Committee, the Holdings board of directors met in person and by teleconference to review the Merger. The Holdings board, acting upon the recommendation of the Holdings Special Committee (i) determined that it was fair to and in the best interests of Holdings and its stockholders for Holdings to enter into the Merger Agreement, (ii) declared the Merger Agreement and the transactions contemplated thereby advisable, (iii) adopted the Merger Agreement and approved the
11
Table of Contents
execution, delivery and performance of the Merger Agreement and of certain ancillary agreements thereto agreement, (iv) resolved to recommend adoption of the Merger Agreement and approval of the Mergers and the other transactions contemplated thereby by the holders of shares of Parent Common Stock and (v) directed that the Merger Agreement be submitted to the holders of shares of Parent Common Stock entitled to vote for its adoption.
Pharma Recommendation and Reasons for the Transactions
After consideration, the Pharma board of directors adopted resolutions determining that the Merger Agreement, the Mergers contemplated by the Merger Agreement and the other transactions contemplated by the Merger Agreement were advisable, fair to and in the best interests of Pharma and its stockholders, adopting and approving the Merger Agreement and the transactions contemplated thereby, including the Mergers, and directing that the Merger Agreement be submitted to the Pharma Stockholders for their consideration. Pharma’s board of directors recommends that the Pharma Stockholders adopt and approve the Merger Agreement and the transactions contemplated thereby, including the Mergers.
Opinion of Houlihan Lokey Capital, Inc. (Page 57)
On June 14, 2021, Houlihan Lokey Capital, Inc., which we refer to as Houlihan Lokey, verbally rendered its opinion to the Special Committee (which was subsequently confirmed in writing by delivery of Houlihan Lokey’s written opinion addressed to the Special Committee dated June 14, 2021), as to, as of such date, the fairness, from a financial point of view, to Holdings of the Aggregate Consideration (as defined in the opinion) to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement.
Houlihan Lokey’s opinion was directed to the Special Committee (in its capacity as such) and only addressed the fairness, from a financial point of view, to Holdings of the Aggregate Consideration to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement and did not address any other aspect or implication of the Business Combination or any other agreement, arrangement or understanding. The summary of Houlihan Lokey’s opinion in this prospectus/proxy statement is qualified in its entirety by reference to the full text of its written opinion, which is attached as Annex H to this prospectus and proxy statement and describes the procedures followed, assumptions made, qualifications and limitations on the review undertaken and other matters considered by Houlihan Lokey in connection with the preparation of its opinion. However, neither Houlihan Lokey’s opinion nor the summary of its opinion and the related analyses set forth in this prospectus and proxy statement are intended to be, and do not constitute, advice or a recommendation to the Special Committee, Holdings’s Board of Directors, Holdings, any security holder of Holdings or any other person as to how to act or vote with respect to any matter relating to the Business Combination. See “The Business Combination — Opinion of Houlihan Lokey Capital, Inc.” beginning on page 57 of this proxy statement/prospectus.
Merger Consideration (Page 66)
Merger Agreement
As a consequence of the Mergers, at the Effective Time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist, and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Parent Class B Common Stock per share of Pharma capital stock.
This Per Share Merger Consideration ratio for each class of Pharma capital stock is currently anticipated to result in prior holders of Pharma capital stock and contingent interests receiving Parent Class B Common Stock amounting to approximately 44% of the outstanding capitalization of Holdings following the Mergers based on the currently outstanding capitalization, including after giving effect to the Holdings Financing. Additional equity in Parent may be issued in connection with one or more financings and pursuant to employment agreements (which will reduce the foregoing percentage proportionately).
12
Table of Contents
Treatment of Company Warrants, Company Options, Company Convertible Notes and Contingent Rights (Page 69)
At the Effective Time, all compensatory options to purchase Pharma Common Stock shall automatically vest in full, and all holders of such options will receive options to purchase Class B common stock of Holdings using the ratios applicable to determining the Holdings stock in the Business Combination. The parties intend that the Company Convertible Notes will not be assumed or continued by Parent or Pharma in connection with the Merger or the other transactions contemplated by the Merger Agreement. All Company Warrants are intended to terminate at the Effective Time as contemplated by and in accordance with the Merger Agreement and the applicable Support Agreement.
Conditions to the Merger (Page 77)
Merger Agreement
The obligations of each of Parent, Pharma and Merger Subs to complete the Mergers are subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement or applicable law) on or prior to the Effective Time of various conditions, including the following:
• The Registration Statement shall have been declared effective by the SEC under the Securities Act and no stop order suspending the effectiveness of the Registration Statement shall have been issued by the SEC and remain in effect, and no proceedings for that purpose shall be pending or threatened in writing by the SEC and not withdrawn or lapsed.
• No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of any of the Mergers shall have been issued by any court of competent jurisdiction and remain in effect, and no material Law shall have been enacted since the date of the Merger Agreement that makes consummation of any of the Mergers illegal.
• The Merger Agreement shall have been duly adopted by the Required Company Stockholder Vote and the Required Parent Stockholder Vote, and the Required Company Stockholder Vote and the Required Parent Stockholder Vote shall remain in full force and effect and, if the Registration Statement incorporates information by reference as contemplated in General Instruction A.2 of Form S-4 under the Securities Act, at least twenty business days (as defined pursuant to Form S-4) shall have elapsed from the date of the first mailing of the prospectus in the Registration Statement to Pharma stockholders until the date of the Closing.
• The Merger Agreement shall have been duly adopted by the Parent Minority Approval and the Company Minority Approval, each of the Parent Stockholder Approval and the Company Stockholder Approval have occurred, and all such approvals shall remain in full force and effect.
• The Merger Shares shall have been approved for listing on the NYSE, subject to official notice of issuance.
• The transactions contemplated by the Altira Acquisition Agreement shall have been consummated by all parties thereto in accordance with its terms; provided that such consummation shall not be a condition to Parent’s obligations to consummate the Merger if (a) Parent has not funded the payment amount thereunder before the Closing and (b) the Altira Acquisition Agreement has been consummated in all material respects by all parties thereto other than Parent (to the extent that such covenants require performance by such other parties at or before the Closing), including execution and delivery of any and all conveyance documents.
• The Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Pharma in the form of attached to the Merger Agreement, shall have received the required director and stockholder approvals and been filed with the Delaware Secretary of State on a date prior to the Closing Date.
• Parent shall have consummated the Financing (which Financing has since been consummated).
13
Table of Contents
Conditions to the Obligations of Parent and the Merger Subs to Complete the Merger
In addition, the obligations of Parent and the Merger Sub to complete the Merger are also subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement or applicable law) at or prior to the Closing of each of the following conditions:
• The Specified Representations (as defined in the Merger Agreement) that are qualified by materiality or Company Material Adverse Effect shall be true and correct in all respects as of the date of the Merger Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all respects as of such earlier date). The Specified Representations that are not qualified by materiality or Company Material Adverse Effect shall be true and correct in all material respects as of the date of the Merger Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all material respects as of such earlier date). The other representations and warranties of Pharma shall be true and correct (without giving effect to any limitation as to materiality or Company Material Adverse Effect set forth therein) as of the date of the Merger Agreement and of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct would not, individually or in the aggregate, have a Company Material Adverse Effect.
• Pharma shall have performed and complied with, in all material respects, all of its covenants hereunder at or before the Closing (to the extent that such covenants require performance by Pharma at or before the Closing).
• There shall not be pending any lawsuit or other Legal Proceeding challenging the Merger that (a) has been commenced by a Governmental Body of competent jurisdiction and (b) could reasonably be expected to result in an injunction or judgment in favor of such Governmental Body that would prevent consummation of the Merger; Parent shall have received the following agreements and documents, each of which shall be in full force and effect: (a) the D&O Resignations; (b) the Certificate of Merger, executed by Pharma; (c) a good standing certificate of Pharma from the Secretary of State of the State of Delaware dated within seven days prior to the Closing Date; and (d) the Lock-Up Agreements duly executed by each party thereto with respect to shares of Parent Class B Common Stock issued pursuant to the Merger (other than Parent).
• As of the Closing, the Chief Executive Officer or Chief Financial Officer of Pharma shall have delivered to Parent a certificate to the effect that each of the conditions specified in Sections 7.1 (Accuracy of Representations and Warranties), 7.2 (Performance of Covenants) and 7.8 (No Material Adverse Effect) is satisfied in all respects.
• There shall be no obligation to pay any royalties or similar payments on any sales of products incorporating the Company Intellectual Property (as defined in the Merger Agreement) other than as set forth in Pharma’s disclosure schedule and the third-party consents set forth on the Pharma Disclosure Schedules attached to the Merger Agreement shall have been obtained in form and substance reasonably satisfactory to Holdings.
• No Material Adverse Effect. Since the date of the Merger Agreement, no Company Material Adverse Effect shall have occurred that is continuing.
Conditions to the Obligations of Company to Complete the Merger
The obligation of Pharma to complete the Merger is subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement), at or prior to the Closing, of the following conditions:
• The representations and warranties of Parent and the Merger Subs shall be true and correct in all material respects as of the date of the Merger Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all respects as of such earlier date).
14
Table of Contents
• Parent and the Merger Subs shall have performed and complied with, in all material respects, all of their respective covenants hereunder at or before the Closing (to the extent that such covenants require performance by Parent or Merger Subs at or before the Closing).
• There shall not be pending before any court of competent jurisdiction any lawsuit or other Legal Proceeding challenging the Merger that has been commenced by a Governmental Body.
• Since the date of the Merger Agreement, no Parent Material Adverse Effect shall have occurred that is continuing.
• An authorized officer of Parent and the Merger Subs shall have delivered to Company a certificate to the effect that each of the conditions specified in Sections 8.1 (Accuracy of Representations and Warranties), 8.2 (Performance of Covenants) and 8.4 (No Material Adverse Effect) is satisfied in all respects.
• Parent shall have dissolved all of its direct and indirect subsidiaries (other than Merger Sub I and Merger Sub II) holding shares of Company capital stock or securities exercisable for or convertible into Company capital stock, or distributed or otherwise transferred, the shares of Company capital stock or securities exercisable for or convertible into Company capital stock held by such subsidiaries to Parent and the other members of such subsidiaries.
• The Lock-Up Agreements shall have been duly executed by each party thereto with respect to shares of Parent Class A Common Stock and Parent Class B Common Stock.
See “The Merger Agreement — Conditions to the Merger” beginning on page 77 of this proxy statement/prospectus.
Termination of the Agreements (Page 79)
The Merger Agreement may be terminated prior to the Effective Time (whether before or after the adoption of the Merger Agreement by the Required Company Stockholder Vote, except to the extent otherwise provided below) as follows:
• by mutual written consent of Parent and Pharma;
• by either Parent or Pharma, if:
• the Merger shall not have been consummated by the End Date; provided, however, that the right to terminate the Merger Agreement under Section 9.1(b) (Termination) shall not be available to Pharma, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure of the Merger to occur on or before the End Date and such action or failure to act constitutes a breach of the Merger Agreement;
• a court of competent jurisdiction or other Governmental Body of competent jurisdiction shall have issued a final and non-appealable order, decree or ruling, in each case, having the effect of permanently restraining, enjoining or otherwise prohibiting or making illegal the Merger; provided, however, that the right to terminate the Merger Agreement under the foregoing section shall not be available to Pharma, on the one hand, or to Parent, on the other hand, if such Party did not use commercially reasonable efforts to have such order, decree or ruling vacated prior to its becoming final and non-appealable and such failure to use commercially reasonable efforts constitutes a breach of the Merger Agreement;
• each of the following are satisfied: (i) the Parent Stockholder Solicitation Period shall have run and elapsed and either (A) the Required Parent Stockholder Vote or (B) the Parent Minority Approval shall not have been obtained on or prior to the end of the Parent Stockholder Solicitation Period, or (ii) the Company Stockholder Solicitation Period shall have run and elapsed and either (x) the Required Company Stockholder Vote or (y) the Company Minority Approval shall not have been obtained on or prior to the end of the Company Stockholder Solicitation Period; provided however that the right to terminate the Merger Agreement under Section 9.1(f) (Termination) shall not be available to Pharma, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure to obtain the approvals required pursuant to clauses (i) or (ii) hereof, as applicable, and such action or failure to act constitutes a breach of the Merger
15
Table of Contents
Agreement. Notwithstanding any other term in Section 9.1(f) (Termination), once the Required Company Stockholder Vote, the Company Minority Approval, the Required Parent Stockholder Vote and the Parent Minority Approval have been obtained, neither Parent nor Pharma may terminate the Merger Agreement pursuant to Section 9.1(f) (Termination).
• by Parent, if:
• Pharma shall have breached or failed to perform any of its representations, warranties, covenants, obligations or agreements contained in the Merger Agreement, which breach or failure to perform (i) would give rise to the failure of a condition set forth in Section 7.1 (Accuracy of Representations and Warranties) or Section 7.2 (Performance of Covenants) and (ii) cannot be, or has not been, cured within 30 calendar days following receipt by Pharma of written notice of such material breach or failure to perform; provided that Parent may not terminate the Merger Agreement pursuant to Section 9.1(d) (Termination) if Parent is in breach of the Merger Agreement such that Pharma has the right to terminate the Merger Agreement pursuant to Section 9.1(e) (Termination) but for the proviso thereto;
• by Pharma, if:
• Parent or Merger Subs shall have breached or failed to perform any of their respective representations, warranties, covenants, obligations or agreements contained in the Merger Agreement, which breach or failure to perform (i) would give rise to the failure of a condition set forth in Section 8.1 (Accuracy of Representations and Warranties) or Section 8.2 (Performance of Covenants) and (ii) cannot be, or has not been, cured within 30 calendar days following receipt by Parent of written notice of such material breach or failure to perform; provided that Pharma may not terminate the Merger Agreement pursuant to Section 9.1(e) (Termination) if Pharma is in breach of the Merger Agreement such that Parent has the right to terminate the Merger Agreement pursuant to Section 9.1(d) (Termination) but for the proviso thereto;
The Party desiring to terminate the Merger Agreement pursuant to Section 9.1 (Termination), shall give the other party written notice of such termination, specifying the provisions hereof pursuant to which such termination is made and the basis therefor described in reasonable detail.
See “The Merger Agreement — Termination” beginning on page 79 of this proxy statement/prospectus.
Department of Justice, Federal Trade Commission and Other U.S. Antitrust Authorities (Page 84)
Under Title II of the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and the rules, regulations, and informal interpretations issued thereunder, and any successor to such statute, rules, or regulations (the “HSR Act”), certain transactions may not be completed unless certain waiting period requirements have expired or been terminated. The HSR Act provides that each party must file a premerger notification with the FTC and the DOJ. A transaction notifiable under the HSR Act may not be completed until the expiration of a 30-calendar-day waiting period following the parties’ filings of their respective HSR Act notification forms or the early termination of that waiting period. At any time before the expiration of the initial waiting period, the DOJ or the FTC may issue a request for additional documents and materials. If such a second request is issued, the parties may not complete the transaction until they substantially comply with the second request and observe a second 30-calendar-day waiting period, unless the waiting period is terminated earlier or extended by timing agreement.
The Merger is exempt from the reporting and waiting requirements under the HSR Act, as an acquisition of voting securities not meeting or exceeding a greater notification threshold. See, 16 C.F.R, Section 802.21. The (1) acquiring person in the Merger and all other persons required by the HSR Act to file notification previously filed notification with respect to an earlier acquisition of voting securities of the same issuer; (2) waiting period with respect to the earlier acquisition has expired, or been terminated pursuant to §803.11, (3) Merger is planned to be consummated within 5 years of such expiration or termination; and (4) Merger will not increase the holdings of the acquiring person to meet or exceed a notification threshold (as adjusted) greater than the greatest notification threshold met or exceeded in the earlier acquisition and filing.
However, there can be no assurance that a challenge to the transactions on antitrust grounds will not be made by the FTC, DOJ, or U.S. state attorneys general, or if such a challenge is made, what the result will be.
16
Table of Contents
Timing; Challenges by Governmental and Other Entities (Page 85)
There can be no assurance the ability of the ability to maintain or obtain approvals on satisfactory terms or the absence of any action challenging such approvals. In addition, there can be no assurance that any of the governmental or other entities described above, including the DOJ, FTC, U.S. state attorneys general, state insurance regulators, foreign regulators and private parties, will not challenge the Contemplated Transaction on antitrust, competition, or foreign investment grounds and, if such a challenge is made, there can be no assurance as to its result.
Subject to certain conditions, if the transaction is not completed on or before the End Date (as may be extended in accordance with the Merger Agreement), either Holdings or Pharma may terminate the Merger Agreement. For more information, see the section of this proxy statement/prospectus entitled “The Merger Agreement — Termination” beginning on page 79 of this proxy statement/prospectus.
Voting and Support Agreements, Lock-Up Agreements, Altira Acquisition Agreement and 56% Warrant Extension (Page 82)
Concurrently with the execution of the Merger Agreement on June 17, 2021, certain key affiliates of both Holdings and Pharma entered into binding commitments to support the Merger. The Support Agreement was executed by affiliates and other related parties of Pharma, and the Voting Agreement was executed by affiliates and other related parties of Holdings. In addition, certain key stockholders of Parent and Pharma that will receive Parent Common Stock in the Business Combination entered into Lock-Up Agreements, pursuant to which such holders will be subject to certain restrictions on transfer of such Parent Common Stock for a period of six months following closing of the Business Combination. As a condition to the closing of the Business Combination, Holdings is to acquire for $30 million in cash the remaining membership interest in Altira Capital & Consulting, LLC (“Altira”) that it does not already own.
Pursuant to the Merger Agreement, the expiration date of that certain Warrant to Purchase Shares of Pharma issued on September 16, 2016 to IDT Corporation, a predecessor of Parent, which Warrant to Purchase Shares of Pharma is held by Pharma Holdings LLC, a 90%-owned subsidiary of Holdings of which the remaining 10% is owned by the Instrument (the “56% Warrant”), which was extended by Parent beyond August 15, 2021 and shall remain exercisable by the holder(s) thereof until the earlier of (i) upon the occurrence of the Effective Time, the Effective Time or (ii) if the Effective Time does not occur, the date that is calculated by adding (A) the number of calendar days the Merger Agreement has been in effect prior to its termination in accordance with its terms to (B) August 15, 2021. Prior to the Closing, Holdings shall effect the termination of the 56% Warrant by Pharma Holdings LLC, such termination to be contingent on the consummation of the Merger and effective at the Initial Effective Time. The Support Agreements executed by holders of the 56% Warrant provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable pursuant to the 56% Warrant shall be calculated as of September 30, 2021, as if the Merger were consummated on such date.
See “Description of the Support and Voting Agreements, Lock-Up Agreements, Altira Acquisition Agreement and 56% Warrant Extension” beginning on page 82 of this proxy statement/prospectus.
Holdings Financing (Page 82)
On August 24, 2021, pursuant to securities purchase agreements dated August 19, 2021, in transactions exempt for the registration requirements of the Securities Act, Holdings sold an aggregate of 2,833,425 shares of its Class B Common stock to certain institutional investors at a purchase price equal to $35.00 per share, for aggregate gross proceeds of approximately $99.2 million, and an additional 112,561 shares of its Class B Common Stock to an entity associated with the family of Howard Jonas at a purchase price equal to $44.42 per share, which was equal to the closing price of a share of the Class B Common Stock on the NYSE on August 19, 2021 for gross proceeds of approximately $5.0 million.
In connection with the sales to institutional investors, Holdings entered into a Registration Rights Agreement (the “Registration Rights Agreement”), under which Holdings agreed to prepare and file a registration statement with the SEC within 30 days after the earlier of (i) the date of the closing of the Merger Agreement, and (ii) the date the Merger Agreement is terminated in accordance with its terms (the “Initiation Date”), for purposes of registering the resale of the shares sold to those investors, and to use commercially reasonable efforts to cause this registration statement to be declared effective by the SEC within 90 days after the Initiation Date.
17
Table of Contents
Interests of Certain Persons in the Transactions (page 25)
In considering the recommendation of the Holdings Board, you should be aware that certain directors and executive officers of Holdings may have interests in the Mergers that may be different from, or in addition to, the interests of Holdings Stockholders generally. The Holdings Board and its Special Committee were aware of, and considered, these interests, among other matters, in evaluating and approving the Merger Agreement and the Mergers and in recommending to Holdings Stockholders that the Mergers be approved. Specifically:
• An instrument (the “Instrument”) that is within a trust that is for the benefit of children of Howard Jonas, holds (i) Series C Convertible Notes of Pharma as well as (ii) a 10% interest in Pharma Holdings LLC, which holds the 56% Warrant, and, directly and indirectly, holds Series D Preferred Stock of Pharma. The Instrument will receive Parent Class B Common Stock in the Mergers in respect of those interests in the same manner as other holders of similar interests in Pharma;
• The Instrument holds a contractual right to receive additional shares of Pharma capital stock equal to 10% of the fully diluted Pharma capital stock (the “Bonus Shares”) (subject to the terms of the 56% Warrant and the Line of Credit upon the occurrence of certain events) and the Merger Agreement provides that such events will be deemed satisfied in connection with the Mergers. The Instrument will receive 2,021,802 shares of Parent Class B Common Stock in respect of the Bonus Shares.
• Howard Jonas, Chairman of the Holdings Board, holds 100,000 options to purchase common stock of Pharma and would receive 12,045 shares of Parent Class B Common Stock in the Mergers in respect of that interest.
• An entity controlled by members of Howard Jonas’ family owns a 37.5% interest in RP Finance LLC.
• Under his employment agreement with Holdings, upon consummation of the Mergers, Ameet Mallik is entitled to receive restricted shares of Class B common stock to avoid dilution in his current interest in Holdings, which we expect to be [•] shares.
• Michael Weiss, a member of the Holdings Board, holds a 0.25% interest in CS Pharma LLC and will receive 5,022 shares of Parent Class B Common Stock in respect of his percentage interest in the Series D Preferred Stock of Pharma held by CS Pharma LLC.
These interests are described in further detail in “Risk Factors — Executive officers and directors of Parent may have interests in the Mergers that are different from, or in addition to, the rights of its stockholders.” beginning on page 24 of this proxy statement/prospectus.
Certain Governance Matters Following the Transactions (Page 48)
Following the consummation of the Mergers, the Holdings Board will consist of 11 members, comprised of three representatives from Pharma: Richard Axel, Chi Van Dang and Sanjeev Luther. The executive officers of Holdings will remain the same.
No Appraisal Rights (Page 48)
Appraisal rights are not available to holders of Parent Common Stock or Pharma Common Stock in connection with the Mergers.
NYSE Requirements (Page 86)
Parent Class B Common Stock is currently listed on the NYSE under the symbol “RFL”. On [•], 2021, the last reported sale price for Parent Class B Common Stock was $[•] per share. Following the Mergers, we expect that Parent Class B Common Stock will continue to be listed on the NYSE.
18
Table of Contents
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This proxy statement/prospectus and the documents incorporated herein by reference contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are based on our management’s current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to us. Discussions containing these forward-looking statements may be found, among other places, in the Sections entitled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” incorporated by reference from our most recent Annual Report on Form 10-K and in our Quarterly Reports on Form 10-Q, as well as any amendments thereto, filed with the SEC.
All statements, other than statements of historical fact, included or incorporated herein regarding our strategy, future operations, financial position, future revenues, projected costs, plans, prospects and objectives are forward-looking statements. Words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek,” “estimate,” “think,” “may,” “could,” “will,” “would,” “should,” “continue,” “potential,” “likely,” “opportunity” and similar expressions or variations of such words are intended to identify forward-looking statements, but are not the exclusive means of identifying forward-looking statements. Additionally, statements concerning future matters such as our expectations of business and market conditions, development and commercialization of new products, enhancements of existing products or technologies, and other statements regarding matters that are not historical are forward-looking statements. Such statements are based on currently available operating, financial and competitive information and are subject to various risks, uncertainties and assumptions that could cause actual results to differ materially from those anticipated or implied in our forward-looking statements due to a number of factors including, but not limited to, those set forth above under the section entitled “Risk Factors” in this proxy statement/prospectus and any accompanying prospectus supplement. Given these risks, uncertainties and other factors, many of which are beyond our control, you should not place undue reliance on these forward-looking statements.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements to reflect events or developments occurring after the date of this proxy statement/prospectus, even if new information becomes available in the future.
19
Table of Contents
RISK FACTORS
In addition to the other information contained or incorporated by reference into this proxy statement/prospectus, including the matters addressed in “Cautionary Statement Regarding Forward-Looking Statements” beginning on page 19 of this proxy statement/prospectus, you should carefully consider the following risk factors before deciding how to vote. You also should read and consider the risk factors associated with Parent because these risk factors may affect the operations and financial results of the combined company. These risk factors may be found under Part I, Item 1A, “Risk Factors” in Parent’s Annual Report on Form 10-K for the year ended July 31, 2020, which is on file with the SEC and incorporated by reference into this proxy statement/prospectus. Parent or Pharma may face additional risks and uncertainties that are not presently known to Parent, or that Parent currently deems immaterial, which may also impair Parent’s business or financial condition.
Risk Factors Relating to the Transactions
Changes in the market prices of Parent Class B Common Stock may result from a variety of factors that are beyond the control of Parent, including changes in its business, operations and prospects, regulatory considerations, governmental actions, and legal proceedings and developments.
The market value of Parent Class B Common Stock has fluctuated since the date of the announcement of the Mergers and will continue to fluctuate from the date of this proxy statement/prospectus to the date of the Parent Meeting and the Closing Date, which could occur a considerable amount of time after the date of the Parent Meeting. The market value of Parent Class B Common Stock at the time of the closing of the Mergers may vary significantly from their prices on the date of the Merger Agreement, the date of this proxy statement/prospectus or the date of the Parent Meeting. Changes in the market prices of Parent Class B Common Stock may result from a variety of factors that are beyond the control of Parent, including changes in its business, operations and prospects, regulatory considerations, governmental actions, and legal proceedings and developments. You are urged to obtain up-to-date prices for Parent Class B Common Stock.
The NYSE may not list the Merger Shares on its exchange, which could limit investors’ ability to make transactions in the Merger Shares and subject the Merger Shares to additional trading restrictions.
In connection with the Mergers, in order to continue to maintain the listing of our securities on the NYSE, we will be required to demonstrate compliance with the NYSE’s listing requirements. We will use our reasonable best efforts to cause the shares of Parent Class B Common Stock issuable, and required to be reserved for issuance, in connection with the Mergers (the “Merger Shares”) to be approved for listing on the NYSE, subject to official notice of issuance, prior to the Effective Time. We cannot assure you that we will be able to meet all listing requirements. Even if the Merger Shares are listed on the NYSE, Parent may be unable to maintain the listing of its securities in the future.
If Parent fails to meet the listing requirements and the NYSE does not list its securities on its exchange, Pharma would not be required to consummate the Merger. In the event that Pharma elected to waive this condition, and the Merger was consummated without Parent’s securities being listed on the NYSE or on another national securities exchange, Parent could face significant material adverse consequences, including:
• a limited availability of market quotations for the Merger Shares;
• reduced liquidity for the Merger Shares;
• a determination that the Merger Shares are a “penny stock” which will require brokers trading in the Merger Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for the Merger Shares;
• a limited amount of news and analyst coverage; and
• a decreased ability to issue additional securities or obtain additional financing in the future.
20
Table of Contents
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” If the Mergers Shares are not listed on the NYSE, such securities would not qualify as covered securities and we would be subject to regulation in each state in which we offer our securities because states are not preempted from regulating the sale of securities that are not covered securities.
The parties may not realize the anticipated benefits and cost savings of the Transactions.
While Parent and Pharma will continue to operate independently until the completion of the Transactions, the success of the Transactions will depend, in part, on Parent’s and Pharma’s ability to realize the anticipated benefits and cost savings from combining Parent’s and Pharma’s businesses. The parties’ ability to realize these anticipated benefits and cost savings is subject to certain risks, including, among others:
• the parties’ ability to successfully combine their respective businesses;
• the risk that the combined businesses will not perform as expected;
• the extent to which the parties will be able to realize the expected synergies, which include realizing potential savings from re-assessing priority assets and aligning investments, eliminating duplication and redundancy, adopting an optimized operating model between both companies and leveraging scale, and creating value resulting from the combination of Parent’s and Pharma’s businesses;
• the possibility that the combined company will not achieve the free cash flow that the parties have projected;
• the reduction of cash available for operations and other uses and the incurrence of indebtedness to finance the Mergers;
• the assumption of known and unknown liabilities of Parent, including potential tax and employee-related liabilities; and
• the possibility of costly litigation challenging the Mergers.
If Parent and Pharma are not able to successfully integrate their businesses within the anticipated time frame, or at all, the anticipated cost savings, synergies operational efficiencies and other benefits of the Mergers may not be realized fully or may take longer to realize than expected, and the combined company may not perform as expected.
Integrating Parent’s and Pharma’s businesses may be more difficult, time-consuming or costly than expected.
Parent and Pharma have operated and, until completion of the Mergers will continue to operate, independently, and there can be no assurances that their businesses can be integrated successfully. It is possible that the integration process could result in the loss of key employees, the disruption of either company’s or both companies’ ongoing businesses or unexpected integration issues, such as higher than expected integration costs and an overall post-completion integration process that takes longer than originally anticipated. Specifically, issues that must be addressed in integrating the operations of Parent and Pharma in order to realize the anticipated benefits of the Mergers so the combined business performs as expected include, among others:
• combining the companies’ separate operational, financial, reporting and corporate functions;
• integrating the companies’ technologies, products and services;
• identifying and eliminating redundant and underperforming operations and assets;
• harmonizing the companies’ operating practices, employee development, compensation and benefit programs, internal controls and other policies, procedures and processes;
• addressing possible differences in corporate cultures and management philosophies;
• maintaining employee morale and retaining key management and other employees;
• attracting and recruiting prospective employees;
• consolidating the companies’ corporate, administrative and information technology infrastructure;
21
Table of Contents
• coordinating sales, distribution and marketing efforts;
• managing the movement of certain businesses and positions to different locations;
• maintaining existing agreements with customers and vendors and avoiding delays in entering into new agreements with prospective customers and vendors;
• coordinating geographically dispersed organizations; and
• effecting potential actions that may be required in connection with obtaining regulatory approvals.
In addition, at times, the attention of certain members of each company’s management and each company’s resources may be focused on completion of the Mergers and the integration of the businesses of the two companies and diverted from day-to-day business operations, which may disrupt each company’s ongoing business and, consequently, the business of the combined company.
Failure to complete the Mergers could negatively impact the stock price and the future business and financial results of Parent and Pharma.
The parties’ respective obligations to complete the Mergers are subject to the satisfaction or waiver of a number of conditions set forth in the Merger Agreement. There can be no assurance that the conditions to completion of the Mergers will be satisfied or waived or that the Mergers will be completed. If the Mergers are not completed for any reason, the ongoing businesses of Parent and Pharma may be materially and adversely affected and, without realizing any of the benefits of having completed the Mergers, Parent and Pharma would be subject to a number of risks, including the following:
• Parent may experience negative reactions from the financial markets, including negative impacts on trading prices of Parent Class B Common Stock and from its customers, vendors, regulators and employees;
• Parent may be required to pay certain expenses incurred in connection with the Mergers, whether or not the Mergers are completed;
• the Merger Agreement places certain restrictions on the operation of Parent’s business prior to the closing of the Mergers, and such restrictions, the waiver of which is subject to the consent of the other parties, may prevent Parent from making certain acquisitions, taking certain other specified actions or otherwise pursuing business opportunities during the pendency of the Mergers that Parent would have made, taken or pursued if these restrictions were not in place; and
• matters relating to the Mergers (including integration planning) will require substantial commitments of time and resources by Parent management and the expenditure of significant funds in the form of fees and expenses, which would otherwise have been devoted to day-to-day operations and other opportunities that may have been beneficial to Parent as an independent company.
In addition, each of Parent and Pharma could be subject to litigation related to any failure to complete the Mergers or related to any proceeding to specifically enforce Parent’s or Pharma’s obligations under the Merger Agreement. If any of these risks materialize, they may materially and adversely affect Parent’s business, financial condition, financial results and stock prices.
Parent and Pharma will be subject to business uncertainties and contractual restrictions while the Mergers are pending.
Uncertainty about the effect of the Mergers on employees, vendors and customers may have an adverse effect on Parent and Pharma and consequently on the combined company after the closing of the Mergers. These uncertainties may impair Parent’s or Pharma’s ability to retain and motivate key personnel and could cause customers and others that deal with Parent or Pharma, as applicable, to defer or decline entering into contracts with Parent or Pharma, as applicable, or making other decisions concerning Parent or Pharma, as applicable, or seek to change existing business relationships with Parent or Pharma, as applicable. In addition, if key employees depart because of uncertainty about their future roles and the potential complexities of the Mergers, Parent’s and Pharma’s businesses could be
22
Table of Contents
harmed. Furthermore, the Merger Agreement places certain restrictions on the operation of Parent’s and Pharma’s businesses prior to the closing of the Mergers, which may delay or prevent Parent and Pharma from undertaking certain actions or business opportunities that may arise prior to the consummation of the Mergers. See “The Merger Agreement — Conduct of Business of Company” and “The Merger Agreement — Conduct of Business of Holdings” beginning on pages 71 and 73, respectively, of this proxy statement/prospectus for a description of the restrictive covenants applicable to Parent and Pharma.
Third parties may terminate or alter existing contracts or relationships with Parent or Pharma.
Each of Parent and Pharma has contracts with customers, vendors and other business partners which may require Parent or Pharma, as applicable, to obtain consents from these other parties in connection with the Mergers. If these consents cannot be obtained, the counterparties to these contracts and other third parties with which Parent and/or Pharma currently have relationships may have the ability to terminate, reduce the scope of or otherwise materially adversely alter their relationships with either party in anticipation of the Mergers, or with Parent following the Mergers. The pursuit of such rights may result in Parent or Pharma suffering a loss of potential future revenue, incurring liabilities in connection with a breach of such agreements or losing rights that are material to its business. Any such disruptions could limit Parent’s or Pharma’s ability to achieve the anticipated benefits of the Mergers. The adverse effect of such disruptions could also be exacerbated by a delay in the completion of the Merger or the termination of the Mergers.
The Mergers are subject to a number of closing conditions and, if these conditions are not satisfied, the Merger Agreement may be terminated in accordance with its terms and the Mergers may not be completed. In addition, the parties have the right to terminate the Merger Agreement under other specified circumstances, in which case the Mergers would not be completed.
The Mergers are subject to a number of closing conditions and, if these conditions are not satisfied or waived (to the extent permitted by law), the Mergers will not be completed. These conditions include, among others: (i) the absence of certain legal impediments, (ii) effectiveness of the registration statement on Form S-4 relating to the Mergers, (iii) obtaining the Parent Stockholder Approval, (iv) obtaining the Company Stockholder Approval and (v) the absence of a Parent Material Adverse Effect (as defined in the Merger Agreement) or a Company Material Adverse Effect (as defined in the Merger Agreement) and (vi) the listing of Merger Shares on NYSE, subject to official notice of issuance. In addition, each party’s obligation to complete the Mergers is subject to the accuracy of the other parties’ representations and warranties in the Merger Agreement (subject in most cases to “material adverse effect” qualifications), the other parties’ compliance, in all material respects, with their respective covenants and agreements in the Merger Agreement.
The conditions to the closing may not be fulfilled and, accordingly, the Mergers may not be completed. In addition, if the Mergers are not completed by the End Date, any party may choose not to proceed with the Mergers. Moreover, the parties can mutually decide to terminate the Merger Agreement at any time prior to the consummation of the Mergers, before or after receipt of the Parent Stockholder Approval and the Company Stockholder Approval and each party may elect to terminate the Merger Agreement in certain other circumstances, as described in “The Merger Agreement — Termination”.
Parent or Pharma may waive one or more of the closing conditions to the Mergers without re-soliciting stockholder approval.
Each of Parent and Pharma has the right to waive certain of the closing conditions to the Mergers. Any such waiver may not require re-solicitation of stockholders, in which case stockholders of Parent and Pharma will not have the chance to change their votes as a result of any such waiver and Parent and Pharma will have the ability to complete the Mergers without seeking further stockholder approval. Any determination whether to waive any condition to the Mergers, whether stockholder approval would be re-solicited as a result of any such waiver or whether this proxy statement/prospectus would be amended as a result of any waiver will be made by Parent or Pharma, as applicable, at the time of such waiver based on the facts and circumstances as they exist at that time, and any such waiver could have an adverse effect on Parent.
23
Table of Contents
Parent Stockholders will have a reduced ownership and voting interest after the Mergers and will exercise less influence over management.
After the completion of the Mergers, Parent Stockholders will own a smaller percentage of Parent than they currently own of Parent. Based on the estimated number of shares of Parent that is currently anticipated to be outstanding immediately prior to the consummation of the Mergers on a fully-diluted basis, it is currently anticipated Parent Stockholders will own approximately 56% of Parent immediately after consummation of the Mergers on a fully-diluted basis. Consequently, Parent Stockholders, as a group, will have reduced ownership and voting power in the combined company compared to their current ownership and voting power in Parent.
Executive officers and directors of Parent may have interests in the Mergers that are different from, or in addition to, the rights of its stockholders.
The Holdings Special Committee negotiated the terms of the Mergers and recommended that the Holdings Board approve the Merger Agreement and the Transactions. The Holdings Board approved the Merger Agreement and the Transactions and recommends that you vote in favor of the proposals at the Parent Special Meeting. Certain executive officers and directors of Parent may have interests in the Mergers that may be different from, or in addition to, yours. These interests include:
• The Instrument that is within a trust that is for the benefit of children of Howard Jonas, holds (i) Series C Convertible Notes of Pharma as well as a 10% interest in Pharma Holdings LLC, which holds the 56% Warrant, and, directly and indirectly, holds Series D Preferred Stock of Pharma. The Instrument will receive Parent Class B Common Stock in the Mergers in respect of those interests in the same manner as other holders of similar interests in Pharma;
• The Instrument holds a contractual right to receive the Bonus Shares upon the occurrence of certain events, and the Merger Agreement provides that such events will be deemed satisfied in connection with the Mergers. The Instrument will receive 2,021,802 shares of Parent Class B Common Stock in respect of the Bonus Shares.
• Howard Jonas, Chairman of the Board of Holdings, holds 100,000 options to purchase common stock of Pharma and upon exercising and funding the options, would receive 12,045 shares of Parent Class B Common Stock in the Mergers in respect of that interest.
• An entity controlled by members of Howard Jonas’ family owns a 37.5% interest in RP Finance LLC.
• Under his employment agreement with Holdings, upon consummation of the Mergers, Ameet Mallik is entitled to receive restricted shares of Class B common stock to avoid dilution in his current interest in Holdings, which we expect to be [•] shares.
• Michael Weiss, a member of the Board, holds a 0.25% interest in CS Pharma LLC and will receive 5,022 shares of Parent Class B Common Stock in respect of his percentage interest in the Series D Preferred Stock of Pharma held by CS Pharma LLC.
For a description of the interests of Parent executive officers and directors in the Mergers, see “Risk Factors — Executive officers and directors of Parent may have interests in the Mergers that are different from, or in addition to, the rights of its stockholders” beginning on page 24 of this proxy statement/prospectus.
Parent and Pharma may have difficulty attracting, motivating and retaining executives and other key employees in light of the proposed Mergers.
The combined company’s success after the Mergers will depend in part on each of Parent’s and Pharma’s ability to retain key executives and other employees. The directors and officers of the Initial Surviving Corporation immediately after the Effective Time and the managers and officers of the Surviving Company immediately after the Subsequent Merger Effective Time, shall be the respective individuals listed in a schedule to the Merger Agreement (as may be amended from time to time), each to hold office in accordance with the applicable governing documents and applicable law, until such director’s or officer’s successor is duly elected or appointed and qualified, or until the earlier of their death, resignation or removal. Uncertainty about the effect of the Mergers on Parent’s and Pharma’s employees may have an adverse effect on each company separately and consequently, the combined business. This uncertainty
24
Table of Contents
may impair Parent’s and/or Pharma’s ability to attract, retain and motivate key personnel. Employee retention may be particularly challenging during the pendency of the Mergers, as Parent’s and Pharma’s employees may experience uncertainty about their future roles in the combined business. Additionally, all holders of options to purchase shares of Pharma Common Stock held by employees or independent contractors of Pharma as of immediately prior to the Closing Date shall, to the extent then unvested, vest in full immediately prior to the Closing Date. Because the vesting of these options, it could make their retention more difficult
Furthermore, if any of Parent’s or Pharma’s key employees depart or are at risk of departing, including because of issues relating to the uncertainty and difficulty of integration, financial security or a desire not to become employees of the combined business, Parent or Pharma, as applicable, may have to incur significant costs in retaining such individuals or in identifying, hiring and retaining replacements for departing employees and may lose significant expertise and talent, and the combined company’s ability to realize the anticipated benefits of the Mergers may be materially and adversely affected. No assurance can be given that the combined company will be able to attract or retain key employees to the same extent that Parent or Pharma has been able to attract or retain employees in the past.
Parent will incur significant transaction and merger-related transition costs in connection with the Mergers.
Parent expects that it will incur significant, non-recurring costs in connection with consummating the Mergers and integrating the operations of the two companies post-closing. Parent may incur additional costs to retain key employees. Parent will also incur significant fees and expenses relating to financing arrangements and legal services (including any costs that would be incurred in defending against any potential class action lawsuits and derivative lawsuits in connection with the Mergers if any such proceedings are brought), accounting and other fees and costs, which are currently estimated to be approximately $[•] in the aggregate, associated with consummating the Mergers. Some of these costs are payable regardless of whether the Mergers are completed. Though Parent continues to assess the magnitude of these costs, additional unanticipated costs may be incurred in the Mergers and the integration of the businesses of Parent and Pharma.
Parent and Pharma may be the target of securities class action and derivative lawsuits which could result in substantial costs and may delay or prevent the Mergers from being completed.
Securities class action lawsuits and derivative lawsuits are often brought against public companies that have entered into merger agreements. Even if the lawsuits are without merit, defending against these claims can result in substantial costs and divert management time and resources. An adverse judgment could result in monetary damages, which could have a negative impact on Parent’s or Pharma’s liquidity and financial condition. Additionally, if a plaintiff is successful in obtaining an injunction prohibiting completion of the Mergers, then that injunction may delay or prevent the Mergers from being completed, which may adversely affect Parent’s or Pharma’s or, if the Mergers are completed but delayed, the combined company’s business, financial position and results of operations. As of September 13, 2021, the last practicable day before the date of this proxy statement/prospectus, to our knowledge no such lawsuits have been filed in connection with the Mergers and we cannot predict whether any will be filed.
Additional Risks Relating to Holdings after Completion of the Mergers
The combined company may be subject to the risks that each of Holdings and Pharma faces.
Although Holdings held significant ownership in Pharma prior to the Mergers, following completion of the Mergers, risks faced by Pharma will significantly impact Holdings. Such risks are described in the documents that Holdings has filed with the SEC, including the Annual Report on Form 10-K for the fiscal year ended July 31, 2020 of Holdings filed with the SEC on October 29, 2020, which is incorporated by reference into this proxy statement/prospectus. If any such risks actually occur, the business, financial condition, results of operations or cash flows of the combined company could be materially adversely affected. See “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus.
25
Table of Contents
The unaudited pro forma condensed combined financial statements included in this proxy statement/prospectus are preliminary and the actual financial condition and results of operations of Holdings after the Mergers may differ materially.
This proxy statement/prospectus includes unaudited pro forma condensed combined financial statements for Holdings. The unaudited pro forma condensed combined statements of comprehensive income (loss) for the year ended July 31, 2020 and the nine months ended April 30, 2021, and the unaudited pro forma combined balance sheet as of April 30, 2021. Both the unaudited pro forma condensed combined statements of income and the unaudited pro forma combined balance sheet of Holdings should be read in conjunction with the financial statements and accompanying notes of Holdings, which are incorporated by reference into this proxy statement/prospectus. The unaudited pro forma financial statements are presented for illustrative purposes only, are based on certain assumptions, address a hypothetical situation and reflect limited historical financial data. The unaudited pro forma financial statements do not include, among other things, expenses related to planned growth of the combined companies, adjustments related to restructuring or integration activities, future acquisitions or disposals not yet known or probable or impacts of transaction related change in control provisions that are currently not factually supportable and/or probable of occurring. Therefore, the unaudited pro forma financial statements are presented for informational purposes only and are not necessarily indicative of what Holdings’ actual financial condition or results of operations would have been had the Mergers been completed on the dates indicated. Accordingly, Holdings’ business, assets, results of operations and financial condition may differ significantly from those indicated by the pro forma financial statements included in this proxy statement/prospectus. For more information, see “Unaudited Pro Forma Condensed Combined Financial Information” and “Where You Can Find More Information” beginning on pages 28 and 96, respectively, of this proxy statement/prospectus.
The market price for shares of Parent Class B Common Stock may be affected by factors different from those affecting the value of shares of Pharma Common Stock.
Upon completion of the Mergers, holders of Pharma Common Stock will become holders of Parent Class B Common Stock. Holdings’ and Pharma’s respective business differ, and accordingly the results of operations of the combined company will be affected by factors different from those currently affecting the results of operations of Holdings and Pharma. For a discussion of the business of Holdings and of certain factors to consider in connection with that business, see the documents incorporated by reference into this proxy statement/prospectus, including the Annual Report on Form 10-K for the fiscal year ended July 31, 2020 of Holdings filed with the SEC on October 29, 2020, and referred to in “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus.
The market price for shares of Parent Class B Common Stock may decline as a result of the Mergers, including as a result of some Holdings stockholders adjusting their portfolios.
The market price of Parent Class B Common Stock at the time of consummation of the Mergers may vary significantly from the market price of Parent Class B Common Stock on the date the Merger Agreement was executed, the date of this proxy statement/prospectus and the date of the Special Meeting. Following consummation of the Mergers, the market price of Parent Class B Common Stock may decline if, among other things, the costs related to the Mergers are greater than expected. The market price also may decline if Holdings does not achieve the perceived benefits of the Mergers as rapidly or to the extent anticipated by financial or industry analysts or if the effect of the Mergers on Holdings’ financial position, results of operations or cash flows is not consistent with the expectations of financial or industry analysts.
In addition, sales of Parent Class B Common Stock by Holdings’ stockholders after the completion of the Mergers may cause the market price of Parent Class B Common Stock to decrease. Based on the number of shares of Parent Class B Common Stock and Pharma Common Stock outstanding as of [•], 2021, the latest practicable date before the date of this proxy statement/prospectus, approximately [•] shares of Parent Class B Common Stock are expected to be issued and outstanding immediately after the effective time of the Mergers (including [•] shares of Parent Class B Common Stock to be issued to [•] in connection with the Mergers). Many Holdings stockholders may decide not to hold the shares of Holdings Class B Common Stock that they receive in the Mergers. Such or other sales of Holdings Class B Common Stock could have the effect of depressing the market price for Parent Common Stock and may take place promptly following the Mergers.
26
Table of Contents
Any of these events may make it more difficult for Holdings to sell equity or equity-related securities, dilute your ownership interest in Holdings and have an adverse impact on the price of Parent Class B Common Stock.
The Mergers may not be accretive, and may be dilutive, to the combined company’s earnings per share, which may negatively affect the market price of shares of Parent Class B Common Stock.
Holdings currently believes the Mergers will result in a number of benefits, including cost savings, operating efficiencies, and stronger demand for their respective products and services, and that the Mergers will be accretive to Holdings’ earnings. This belief is based, in part, on preliminary current estimates that may materially change. In addition, future events and conditions, including adverse changes in market conditions, additional transaction and integration-related costs and other factors such as the failure to realize some or all of the anticipated benefits of the Mergers, could decrease or delay the accretion that is currently anticipated or could result in dilution. Any dilution of, or decrease in or delay of any accretion to, the combined company’s earnings per share could cause the price of shares of Parent Class B Common Stock to decline or grow at a reduced rate.
Additional Risks Relating to Holdings and Pharma after the Mergers
Holdings’ and Pharma’s businesses are, and will continue to be, subject to the risks described in Part I, Item 1A in Holdings’ Annual Report on Form 10-K for the year ended July 31, 2020, as filed with the SEC and incorporated by reference into this proxy statement/prospectus. See “Where You Can Find More Information” beginning on page 96 of this proxy statement/prospectus for the location of information incorporated by reference into this proxy statement/prospectus.
27
Table of Contents
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
On June 17, 2021, Rafael Holdings, Inc. (“Holdings” or the “Company”) and Rafael Pharmaceuticals, Inc. (“Pharma”) entered into a merger agreement (the “Merger Agreement”). The Merger Agreement provides for, among other things, the following shall occur: upon the date and time of the filing of such Certificate of Merger with the Secretary of State of the State of Delaware (the “Effective Time”), Merger Sub I will merge with and into Pharma (the “Initial Merger”), and Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger (the “Subsequent Merger” and together with the Initial Merger, the “Mergers”). As a result of the Mergers, Pharma will become a wholly-owned limited liability subsidiary of Holdings. Upon the closing of the Mergers, the stockholders of Pharma will receive consideration in the form of Holdings Class B Common Stock (the “Merger”).
The following unaudited pro forma condensed combined financial statements have been prepared to illustrate the estimated effects of the Merger. The accompanying unaudited pro forma condensed combined balance sheet as of April 30, 2021 combines the historical consolidated balance sheets of Holdings and Pharma, giving effect to the Merger as if it had been completed on April 30, 2021. The unaudited pro forma condensed combined statements of operations for the nine months ended April 30, 2021 and for the year ended July 31, 2020 combine the historical consolidated income statements of Holdings and Pharma, giving effect to the Merger as if it had been completed on August 1, 2019.
These unaudited pro forma condensed combined financial statements are based on, and should be read in conjunction with the accompanying notes as well as the historical audited consolidated financial statements of both Holdings and Pharma as of and for the year ended July 31, 2020, which are included in this proxy statement/prospectus.
As of the date of this proxy statement/prospectus, Holdings has not completed the valuation analysis and calculations in sufficient detail necessary to arrive at the required estimates of the fair value of the Pharma assets to be acquired or liabilities to be assumed, other than a preliminary estimate for In-Process Research & Development and certain financial assets and financial liabilities. Accordingly, apart from the aforementioned, certain Pharma assets and liabilities are presented at their respective carrying amounts and should be treated as preliminary values. A final determination of the fair value of Pharma’s assets and liabilities will be based on Pharma’s actual assets and liabilities as of the closing date and, therefore, cannot be made prior to the consummation of the Merger. In addition, the value of the merger consideration to be paid by Holdings in shares of Holdings Class B Common Stock upon the consummation of the Merger will be determined based on the closing price of Holdings Class B Common Stock on the closing date and the expected number of issued and outstanding shares of Pharma capital stock as of September 30, 2021 pursuant to the Merger Agreement. Pharma capital stock issued and/or outstanding will be cancelled, retired, and cease to exist, and shall be converted into the right to receive the applicable per share merger consideration of 0.12045 shares of Holdings Class B Common Stock for each share of Pharma capital stock previously owned. Actual adjustments may differ from the amounts reflected in the unaudited pro forma condensed combined financial information, and the differences may be material.
The unaudited pro forma condensed combined financial information is provided for illustrative and information purposes only and is not intended to represent or necessarily be indicative of the combined company’s results of operations or financial condition had the Merger been completed on the dates indicated, nor do they purport to project our results of operations or financial condition for any future period or as of any future date. The unaudited pro forma condensed combined financial information does not include any expected cost savings or operating synergies, which may be realized subsequent to the combination, or the impact of any non-recurring activity and one-time transaction-related or integration-related items. Moreover, the pro forma adjustments represent best estimates based upon the information available to date and are preliminary and subject to change after more detailed information is obtained.
28
Table of Contents
Unaudited Pro Forma Condensed Combined Balance Sheet
As of April 30, 2021
(in thousands, except share and per share data)
| | Historical Holdings | | Historical Pharma | | Transaction Accounting Adjustments | | | | Other Transaction Accounting Adjustments | | | | Pro Forma Combined |
ASSETS | | | | | | | | | | | | | | | | | | | | | |
CURRENT ASSETS | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 4,679 | | $ | 2,484 | | $ | 6,386 | | | A | | $ | 72,169 | | | L | | $ | 85,718 |
Trade accounts receivable, net of allowance for doubtful accounts of $183 and $218 at April 30, 2021 and July 31, 2020, respectively | | | 248 | | | — | | | — | | | | | | — | | | | | | 248 |
Marketable securities | | | — | | | 75 | | | — | | | | | | — | | | | | | 75 |
Due from Rafael Pharmaceuticals | | | 480 | | | — | | | — | | | | | | (480 | ) | | M | | | — |
Prepaid expenses and other current assets | | | 625 | | | 5,246 | | | — | | | | | | — | | | | | | 5,871 |
Total current assets | | | 6,032 | | | 7,805 | | | 6,386 | | | | | | 71,689 | | | | | | 91,912 |
| | | | | | | | | | | | | | | | | | | | | | |
Property and equipment, net | | | 43,591 | | | 233 | | | — | | | | | | — | | | | | | 43,824 |
Equity investment – RP Finance LLC | | | 479 | | | — | | | (479 | ) | | B | | | — | | | | | | — |
Due from RP Finance LLC | | | 3,750 | | | — | | | (3,750 | ) | | B | | | 3,750 | | | N | | | 3,750 |
Investments – Rafael Pharmaceuticals | | | 79,141 | | | — | | | (79,141 | ) | | C | | | — | | | | | | — |
Investments – Other Pharmaceuticals | | | 477 | | | — | | | — | | | | | | — | | | | | | 477 |
Investments – Hedge Funds | | | 9,681 | | | — | | | — | | | | | | — | | | | | | 9,681 |
Deferred debt issuance costs | | | — | | | 29,120 | | | (29,120 | ) | | B | | | — | | | | | | — |
In-process research and development and patents | | | 1,575 | | | — | | | 1,132,500 | | | D | | | — | | | | | | 1,134,075 |
Trade names and trademarks | | | — | | | — | | | — | | | | | | — | | | | | | — |
Goodwill | | | — | | | — | | | 433,253 | | | E | | | — | | | | | | 433,253 |
Other assets | | | 1,482 | | | — | | | — | | | | | | — | | | | | | 1,482 |
TOTAL ASSETS | | $ | 146,208 | | $ | 37,158 | | $ | 1,459,649 | | | | | $ | 75,439 | | | | | $ | 1,718,454 |
| | | | | | | | | | | | | | | | | | | | | | |
LIABILITIES AND EQUITY | | | | | | | | | | | | | | | | | | | | | |
CURRENT LIABILITIES | | | | | | | | | | | | | | | | | | | | | |
Trade accounts payable | | $ | 753 | | $ | 6,535 | | $ | — | | | | | $ | — | | | | | $ | 7,288 |
Accrued expenses | | | 861 | | | 6,223 | | | 550 | | | F | | | — | | | | | | 7,634 |
Amount due for purchase of membership interest | | | 3,000 | | | — | | | — | | | | | | — | | | | | | 3,000 |
Other current liabilities | | | 235 | | | 186 | | | — | | | | | | — | | | | | | 421 |
Due to related parties | | | 60 | | | 1,049 | | | — | | | | | | 3,270 | | | M,N | | | 4,379 |
Note payable | | | — | | | 394 | | | — | | | | | | — | | | | | | 394 |
Convertible notes – related parties | | | — | | | 12,239 | | | (12,239 | ) | | G | | | — | | | | | | — |
Total current liabilities | | | 4,909 | | | 26,626 | | | (11,689 | ) | | | | | 3,270 | | | | | | 23,116 |
| | | | | | | | | | | | | | | | | | | | | | |
Deferred income tax liabilities, net | | | 9 | | | — | | | 318,346 | | | Q | | | — | | | | | | 318,355 |
Note payable – long term | | | — | | | 1,020 | | | — | | | | | | — | | | | | | 1,020 |
Derivative liabilities | | | — | | | 54,685 | | | (54,685 | ) | | B | | | — | | | | | | — |
Line of credit | | | — | | | 10,000 | | | (10,000 | ) | | B | | | — | | | | | | — |
Other liabilities | | | 33 | | | — | | | — | | | | | | — | | | | | | 33 |
TOTAL LIABILITIES | | | 4,951 | | | 92,331 | | | 241,972 | | | | | | 3,270 | | | | | | 342,524 |
29
Table of Contents
Unaudited Pro Forma Condensed Combined Balance Sheet
As of April 30, 2021
(in thousands, except share and per share data) — (Continued)
| | Historical Holdings | | Historical Pharma | | Transaction Accounting Adjustments | | | | Other Transaction Accounting Adjustments | | | | Pro Forma Combined |
COMMITMENTS AND CONTINGENCIES | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
EQUITY | | | | | | | | | | | | | | | | | | | | | | | |
Class A common stock, $0.01 par value; 35,000,000 shares authorized, 787,163 shares issued and outstanding as of April 30, 2021 and July 31, 2020, respectively | | | 8 | | | | — | | | | — | | | | | | — | | | | | | 8 |
Class B common stock, $0.01 par value; 200,000,000 shares authorized, 15,969,962 issued and 15,982,349 outstanding as of April 30, 2021, and 15,034,598 issued and 15,028,536 outstanding as of July 31, 2020 | | | 158 | | | | — | | | | 171 | | | H | | | 29 | | | O | | | 358 |
Convertible preferred stock, $0.001 par value; 300,000,000 shares authorized; 95,506,177 shares issued and outstanding as of April 30, 2021, and 82,885,895 as of July 31, 2020 | | | — | | | | 97 | | | | (97 | ) | | I | | | — | | | | | | — |
Common stock, $0.001 par value; 310,000,000 shares authorized; 24,392,839 shares issued and outstanding as of April 30, 2021, and 24,381,591 as of July 31, 2020 | | | — | | | | 24 | | | | (24 | ) | | I | | | — | | | | | | — |
Additional paid-in capital | | | 151,258 | | | | 186,208 | | | | 434,595 | | | J | | | 104,140 | | | O | | | 876,201 |
Accumulated deficit | | | (28,419 | ) | | | (241,502 | ) | | | 783,032 | | | K | | | (32,000 | ) | | P | | | 481,111 |
Accumulated other comprehensive income related to foreign currency translation adjustment | | | 3,766 | | | | — | | | | — | | | | | | — | | | | | | 3,766 |
Total equity attributable to Rafael Holdings, Inc. | | | 126,771 | | | | (55,173 | ) | | | 1,217,677 | | | | | | 72,169 | | | | | | 1,361,444 |
Noncontrolling interests | | | 14,486 | | | | — | | | | — | | | | | | — | | | | | | 14,486 |
TOTAL EQUITY | | | 141,257 | | | | (55,173 | ) | | | 1,217,677 | | | | | | 72,169 | | | | | | 1,375,930 |
TOTAL LIABILITIES AND EQUITY | | $ | 146,208 | | | $ | 37,158 | | | $ | 1,459,649 | | | | | $ | 75,439 | | | | | $ | 1,718,454 |
See accompanying notes to unaudited pro forma condensed combined financial information
30
Table of Contents
Unaudited Pro Forma Condensed Combined Statements of Operations
For the Nine Months Ended April 30, 2021
(in thousands, except share and per share data)
| | Historical
Holdings | | Historical
Pharma | | Transaction
Accounting
Adjustments | | | | Other
Transaction
Adjustments | | | | Pro Forma
Combined |
REVENUE | | | | | | | | | | | | | | | | | | | | | | | | |
Rental – Third Party | | $ | 654 | | | $ | — | | | $ | — | | | | | $ | — | | | | | $ | 654 | |
Rental – Related Party | | | 1,570 | | | | — | | | | — | | | | | | — | | | | | | 1,570 | |
Parking | | | 418 | | | | — | | | | — | | | | | | — | | | | | | 418 | |
Other – Related Party | | | 360 | | | | — | | | | — | | | | | | — | | | | | | 360 | |
Revenue | | | — | | | | 1,930 | | | | — | | | | | | — | | | | | | 1,930 | |
Total revenue | | | 3,002 | | | | 1,930 | | | | — | | | | | | — | | | | | | 4,932 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
COSTS AND EXPENSES | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | 8,365 | | | | 3,782 | | | | — | | | | | | 2,000 | | | HH | | | 14,147 | |
Research and development | | | 3,345 | | | | 28,926 | | | | 21,110 | | | BB | | | — | | | | | | 53,381 | |
Depreciation and amortization | | | 1,079 | | | | — | | | | — | | | | | | — | | | | | | 1,079 | |
Impairment – Altira | | | 7,000 | | | | — | | | | — | | | | | | 30,000 | | | II | | | 37,000 | |
Loss from operations | | | (16,787 | ) | | | (30,778 | ) | | | (21,110 | ) | | | | | (32,000 | ) | | | | | (100,675 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Interest expense, net | | | (2 | ) | | | (6,031 | ) | | | 6,094 | | | CC | | | — | | | | | | 61 | |
Gain on sale of building | | | 749 | | | | — | | | | — | | | | | | — | | | | | | 749 | |
Impairment of investments – Other Pharmaceuticals | | | (724 | ) | | | — | | | | — | | | | | | — | | | | | | (724 | ) |
Unrealized gain on investments – Hedge Funds | | | 4,171 | | | | — | | | | — | | | | | | — | | | | | | 4,171 | |
Unrealized loss on marketable securities | | | — | | | | (8 | ) | | | — | | | | | | — | | | | | | (8 | ) |
Modification of warrants expense | | | — | | | | (11,604 | ) | | | — | | | | | | — | | | | | | (11,604 | ) |
Change in fair value of derivative liabilities | | | — | | | | (17,253 | ) | | | 17,253 | | | DD | | | — | | | | | | — | |
Income (loss) before income taxes | | | (12,593 | ) | | | (65,674 | ) | | | 2,237 | | | | | | (32,000 | ) | | | | | (108,030 | ) |
Provision for income taxes | | | (13 | ) | | | — | | | | — | | | | | | — | | | | | | (13 | ) |
Equity in earnings of RP Finance | | | 288 | | | | — | | | | — | | | | | | — | | | | | | 288 | |
Consolidated net income (loss) | | | (12,318 | ) | | | (65,674 | ) | | | 2,237 | | | | | | (32,000 | ) | | | | | (107,755 | ) |
Net loss attributable to noncontrolling interests | | | (154 | ) | | | — | | | | — | | | | | | — | | | | | | (154 | ) |
Net income (loss) attributable to Rafael Holdings, Inc. | | $ | (12,164 | ) | | $ | (65,674 | ) | | $ | 2,237 | | | | | $ | (32,000 | ) | | | | $ | (107,601 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Loss per share | | | | | | | | | | | | | | | | | | | | | | | | |
Basic and diluted | | $ | (0.75 | ) | | | | | | | | | | | | | | | | | | $ | (2.96 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average number of shares used in calculation of loss per share | | | | | | | | | | | | | | | | | | | | | | | | |
Basic and diluted | | | 16,216,969 | | | | | | | | 20,091,024 | | | EE | | | | | | | | | 36,307,993 | |
See accompanying notes to unaudited pro forma condensed combined financial information
31
Table of Contents
Unaudited Pro Forma Condensed Combined Statements of Operations
For the Year Ended July 31, 2020
(in thousands, except share and per share data)
| | Historical
Holdings | | Historical
Pharma | | Transaction
Accounting
Adjustments | | | | Other
Transaction
Adjustments | | | | Pro Forma
Combined |
REVENUE | | | | | | | | | | | | | | | | | | | | | | | | |
Rental – Third Party | | $ | 1,516 | | | $ | — | | | $ | — | | | | | $ | — | | | | | $ | 1,516 | |
Rental – Related Party | | | 2,082 | | | | — | | | | — | | | | | | — | | | | | | 2,082 | |
Parking | | | 832 | | | | — | | | | — | | | | | | — | | | | | | 832 | |
Other – Related Party | | | 480 | | | | — | | | | — | | | | | | — | | | | | | 480 | |
Total revenue | | | 4,910 | | | | — | | | | — | | | | | | — | | | | | | 4,910 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
COSTS AND EXPENSES | | | | | | | | | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | 9,118 | | | | 3,694 | | | | 550 | | | AA | | | 2,000 | | | HH | | | 15,362 | |
Research and development | | | 2,391 | | | | 36,527 | | | | 21,130 | | | BB | | | — | | | | | | 60,048 | |
Depreciation and amortization | | | 1,866 | | | | — | | | | — | | | | | | — | | | | | | 1,866 | |
Impairment – Altira | | | — | | | | — | | | | — | | | | | | 30,000 | | | II | | | 30,000 | |
Loss from operations | | | (8,465 | ) | | | (40,221 | ) | | | (21,680 | ) | | | | | (32,000 | ) | | | | | (102,366 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Interest expense, net | | | (32 | ) | | | (3,809 | ) | | | 4,219 | | | CC | | | — | | | | | | 378 | |
Net loss resulting from foreign exchange transactions | | | (5 | ) | | | — | | | | — | | | | | | — | | | | | | (5 | ) |
Gain on sale of marketable securities | | | — | | | | 160 | | | | — | | | | | | — | | | | | | 160 | |
Impairment of investments – Other Pharmaceuticals | | | (799 | ) | | | — | | | | — | | | | | | — | | | | | | (799 | ) |
Unrealized gain on investments – Hedge Funds | | | 2,385 | | | | — | | | | — | | | | | | — | | | | | | 2,385 | |
Modification of warrants expense | | | — | | | | (31,530 | ) | | | — | | | | | | — | | | | | | (31,530 | ) |
Change in fair value of derivative liabilities | | | — | | | | (3,851 | ) | | | 3,851 | | | DD | | | — | | | | | | — | |
Unrealized loss on marketable securities | | | — | | | | (49 | ) | | | — | | | | | | — | | | | | | (49 | ) |
Gain on cost investment of investee | | | — | | | | — | | | | 542,559 | | | FF | | | — | | | | | | 542,559 | |
Other income (loss) | | | — | | | | — | | | | (479 | ) | | GG | | | — | | | | | | (479 | ) |
Income (loss) before income taxes | | | (6,916 | ) | | | (79,300 | ) | | | 528,470 | | | | | | (32,000 | ) | | | | | 410,254 | |
Provision for income taxes | | | (29 | ) | | | — | | | | — | | | | | | — | | | | | | (29 | ) |
Impairment of equity method investment of Altira | | | (4,000 | ) | | | — | | | | — | | | | | | — | | | | | | (4,000 | ) |
Equity in earnings of RP Finance | | | 192 | | | | — | | | | — | | | | | | — | | | | | | 192 | |
Consolidated net income (loss) | | | (10,753 | ) | | | (79,300 | ) | | | 528,470 | | | | | | (32,000 | ) | | | | | 406,417 | |
Net loss attributable to noncontrolling interests | | | (338 | ) | | | — | | | | — | | | | | | — | | | | | | (338 | ) |
Net income (loss) attributable to Rafael Holdings, Inc. | | $ | (10,415 | ) | | $ | (79,300 | ) | | $ | 528,470 | | | | | $ | (32,000 | ) | | | | $ | 406,755 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Basic net income (loss) per share | | $ | (0.66 | ) | | | | | | | | | | | | | | | | | | $ | 11.34 | |
Diluted net income (loss) per share | | $ | (0.66 | ) | | | | | | | | | | | | | | | | | | $ | 11.29 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Basic common shares outstanding | | | 15,764,829 | | | | | | | | 20,091,024 | | | EE | | | | | | | | | 35,855,853 | |
Diluted common shares outstanding | | | 15,764,829 | | | | | | | | 20,258,157 | | | EE | | | | | | | | | 36,022,986 | |
See accompanying notes to unaudited pro forma condensed combined financial information
32
Table of Contents
Note 1 — Description of the Merger
On June 17, 2021, Rafael Holdings, Inc. (“Holdings” or the “Company”) and Rafael Pharmaceuticals, Inc. (“Pharma”) entered into a merger agreement (the “Merger Agreement”). The Merger Agreement provides for, among other things, the following shall occur: upon the date and time of the filing of such Certificate of Merger with the Secretary of State of the State of Delaware (the “Effective Time”), Merger Sub I will merge with and into Pharma (the “Initial Merger”), and Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger (the “Subsequent Merger” and together with the Initial Merger, the “Mergers”). As a result of the Mergers, Pharma will become a wholly-owned limited liability subsidiary of Holdings. Upon the closing of the Mergers, the stockholders of Pharma will receive consideration in the form of Holdings Class B Common Stock.
Upon consummation of the Subsequent Merger, the limited liability company agreement of Merger Sub II will be amended and restated to be the certificate of formation and limited liability company agreement of the surviving entity. Any shares of Rafael Pharmaceuticals capital stock issued and/or outstanding will be cancelled, retired, and cease to exist, and shall be converted into the right to receive the applicable per share merger consideration (0.12045 shares of Holdings Class B Common Stock for each share of Rafael Pharmaceuticals stock previously owned).
Per Share Merger Consideration ratio for each class of Pharma capital stock will provide that the prior holders of Pharma capital stock and contingent interests will receive Holdings Class B Common Stock. Additional equity in Holdings will be issued in connection with the contemplated financing and pursuant to employment agreements.
Other transactions
Pursuant to the Merger Agreement, one condition precedent (among others) to each party’s obligations to consummate the Merger Transactions is that the Company shall have consummated a financing in which it raised no less than $50 million of aggregate net proceeds (after payment of any fees and expenses associated with such transaction(s)) from one or more of the following sources of financing: (i) a private placement of the Company’s capital stock, (ii) an underwritten public offering of the Company’s capital stock registered with the SEC on Form S-3 (or other applicable form), or (iii) the sale of real property owned by the Company (collectively, the “Financing Condition”).
Pursuant to the Financing Condition, Holdings sold an aggregate of 2,833,425 shares of its Class B common stock, par value $0.01 per share, to investors, at a purchase price equal to $35.00 per share, for aggregate gross proceeds of approximately $99.2 million, before deducting placement agent fees and other offering expenses. Additionally, the Company agreed to sell an aggregate of 112,561 shares of Class B Common Stock at a purchase price equal to $44.42 per share, which was equal to the closing price of a share of the Class B Common Stock on the NYSE on August 19, 2021, which resulted in aggregate gross proceeds to the Company of approximately $5.0 million (collectively, the “PIPE”). The closing of the PIPE occurred on August 24, 2021. In connection with the PIPE, the Company sold an aggregate of 2,945,986 shares of Class B common stock for aggregate gross proceeds of approximately $104 million. The Company believes that, through the closing of the PIPE, the Financing Condition in the Merger Agreement has been satisfied.
Additionally, on May 27, 2021, the Company granted 908,497 restricted shares of Class B common stock of the Company to the Chief Executive Officer. Additionally, in June 2021, the Company paid a $2 million sign-on bonus to the Chief Executive Officer.
As a condition to the closing of the Mergers, Holdings is to acquire for $30 million in cash the remaining membership interest of 33.333% in Altira Capital & Consulting, LLC that it does not already own.
Note 2 — Basis of Presentation
The following unaudited pro forma condensed combined financial information of the combined company is presented to illustrate the proposed effects of the Mergers. The unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, as amended by Securities and Exchange Commission (“SEC”) Final Rule Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.” Release No. 33-10786 replaced the existing pro forma adjustment criteria with simplified requirements to depict the accounting for the transaction (“Transaction Accounting Adjustments”)
33
Table of Contents
and present the reasonably estimable synergies and other transaction effects that have occurred or are reasonably expected to occur (“Management’s Adjustments”). The combined company has elected not to present Management’s Adjustments and will only be presenting Transaction Accounting Adjustments in the unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information and explanatory notes have been prepared to illustrate the effects of the Mergers involving Holdings and Pharma under the acquisition method of accounting in accordance with Accounting Standards Codification (ASC) 805, Business Combinations, with Holdings treated as the accounting acquirer. Under the acquisition method of accounting, the identifiable assets acquired, and liabilities assumed of Pharma are recognized and measured as of the acquisition date at fair value, defined in ASC 820, Fair Value Measurement, and added to those of Holdings which are based on its respective historical financial information.
ASC 820 defines the term “fair value” and sets forth the valuation requirements for any asset or liability measured at fair value, expands related disclosure requirements and specifies a hierarchy of valuation techniques based on the nature of the inputs used to develop the fair value measures. Fair value is defined as “the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.” This is an exit price concept for the valuation of the asset or liability. In addition, market participants are assumed to be buyers and sellers unrelated to the Company in the principal (or the most advantageous) market for the asset or liability. Fair value measurements for an asset assume the highest and best use by these market participants. As a result of these standards, the Company may be required to record assets which are not intended to be used or sold and/or to value assets at fair value measures that do not reflect the intended use of those assets. Many of these fair value measurements can be highly subjective and it is also possible that others, applying reasonable judgment to the same facts and circumstances, could develop and support a range of alternative estimated amounts.
Management has made significant estimates and assumptions in its determination of the Transaction Accounting Adjustments. As the unaudited pro forma condensed combined financial information has been prepared based on these preliminary estimates, the final amounts recorded might differ materially from the information presented.
Upon consummation of the Mergers, a final determination of the fair value of Pharma’s assets acquired and liabilities assumed will be performed. Any changes in the fair values of the net assets or total merger consideration as compared with the information shown in the unaudited pro forma condensed combined financial information may change the amount of the total purchase consideration allocated to goodwill and other assets and liabilities and may impact Holding’s statement of operations following the consummation of the Mergers. The final merger consideration allocation may be materially different than the preliminary allocation presented in the unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information is provided for illustrative and information purposes only and is not intended to represent or necessarily be indicative of the combined company’s results of operations or financial condition had the Mergers been completed on the dates indicated, nor do they purport to project Company’s results of operations or financial condition for any future period or as of any future date. The unaudited pro forma condensed combined financial information does not include any expected cost savings or operating synergies, which may be realized subsequent to the combination or the impact of any non-recurring activity and one-time transaction-related or integration-related items. Moreover, the pro forma adjustments represent best estimates based upon the information available to date and are preliminary and subject to change after more detailed information is obtained.
Note 3 — Accounting Policies and Reclassification Adjustments
Holdings has not identified all adjustments necessary to conform Pharma’s accounting policies to Holdings’ accounting policies. Upon consummation of the Mergers, or as more information becomes available, Holdings will perform a more detailed review of Pharma’s accounting policies. Upon consummation of the Mergers, the Company will perform a comprehensive review of its accounting policies. The Company may, as a result, identify additional differences between the accounting policies of the two companies which, when conformed, could have a material impact on the combined consolidated financial statements.
Under the acquisition method of accounting, the identifiable assets acquired and liabilities assumed of Pharma are recognized and measured as of the acquisition date at fair value and added to those of Holdings. The determination of fair value used in the pro forma adjustments are presented herein.
34
Table of Contents
Note 4 — Estimated Preliminary Merger Consideration
The accompanying unaudited pro forma condensed combined financial statements reflect an estimated preliminary purchase price of approximately $1,243 million (the “Merger Consideration”) comprised of equity consideration of approximately $605 million, the fair value of the Company’s existing ownership interest in Pharma of approximately $622 million, and the fair value of Holdings’ replacement vested stock options for Pharma vested stock options of approximately $16 million.
The Mergers provide for Pharma stockholders to receive 0.12045 shares of Holdings Class B Common Stock for each share of Pharma capital stock they hold immediately prior to the Mergers, inclusive of Pharma’s preferred shares, common shares, conversion of convertible notes, and other instruments.
The consideration payable by Holdings takes into account the value of the Holdings’ assets including its interests in Pharma through shares and warrants. Prior to the closing of the Mergers, Holdings accounted for its existing 50.7% equity interest in Pharma using the cost method, and the net book value was approximately $80 million as of April 30, 2021. The Company accounted for the acquisition of the 49.3% equity interest of Pharma as a step acquisition, which required remeasurement of the Company’s existing 50.7% ownership interest in Pharma to fair value of approximately $622 million prior to completing the acquisition method of accounting.
The table below represents the total estimated preliminary Merger Consideration:
(Dollars in thousands, except per share data) | | Amount |
Holdings Class B Common Stock to be issued to Pharma stockholders(1) | | | 17,145,038 |
Holdings Class B Common Stock closing price per share on September 10, 2021 | | $ | 35.26 |
Total equity consideration for 49.3% interest in Pharma | | $ | 604,534 |
Fair value of Holdings’ existing cost method investment in Pharma(2) | | | 621,700 |
Fair value of Holdings’ replacement vested stock options for Pharma vested stock options(3) | | | 16,440 |
Total | | $ | 1,242,674 |
As described in Note 1 above, the Merger Agreement includes estimated equity portion of the estimated preliminary Merger Consideration of $605 million, the actual value of which will be subject to change based on the final Exchange Ratio determined as of the Closing Date and the underlying market price of the Holdings shares. As a result, changes in Holdings’ stock price will impact the market value of the Holdings shares to be issued in the Mergers. This is also indicated below through the sensitivity analysis performed using the hypothetical change in the closing price of Holdings shares to assess the impact on the number of shares issued to holders of Pharma shares, as part of Merger Consideration on the Effective Date.
The equity portion of the estimated preliminary Merger Consideration is based on Holdings’ closing share price of $35.26 on September 10, 2021. The value of the purchase price consideration will change based on fluctuations in the market price of Holdings’ common shares. The equity portion of the purchase price will vary based on the market price of Holdings’ common shares upon consummation of the Mergers. The Company believes that a 10% fluctuation in market price of common shares is reasonably possible based on historical volatility, and the potential effect on purchase price would be as shown below:
(Dollars in thousands, except per share data) | | Holdings’
Share Price | | Equity portion
of Estimated
Preliminary
Merger
Consideration |
As presented | | $ | 35.26 | | $ | 604,534 |
10% increase | | $ | 38.79 | | $ | 664,986 |
10% decrease | | $ | 31.73 | | $ | 544,081 |
35
Table of Contents
Note 5 — Estimated Preliminary Purchase Price Allocation
Holdings has performed a preliminary allocation of the estimated preliminary Merger Consideration to the identifiable assets acquired and liabilities assumed of Pharma. Using the total estimated preliminary Merger Consideration for the Mergers, the Company has estimated the allocations to such assets and liabilities. The preliminary purchase price allocation is based on financial information of Pharma as of the closing date which represents the best information available to management at the time of this filing.
The following table summarizes the allocation of the estimated preliminary Merger Consideration:
(in thousands) | | Fair Value |
Assets acquired | | | | |
Cash | | $ | 8,870 | |
Marketable securities | | | 75 | |
Prepaid expenses and other current assets | | | 5,246 | |
Property and equipment, net | | | 233 | |
Goodwill(1) | | | 433,253 | |
Acquired In-Process Research & Development | | | 1,132,500 | |
Total assets acquired | | | 1,580,177 | |
| | | | | |
Liabilities assumed | | | | |
Accounts payable | | | (6,535 | ) |
Accrued expenses | | | (6,223 | ) |
Other current liabilities | | | (1,235 | ) |
Note payable | | | (394 | ) |
Note payable long-term | | | (1,020 | ) |
Due to Holdings | | | (3,750 | ) |
Deferred tax liabilities | | | (318,346 | ) |
Total liabilities assumed | | | (337,503 | ) |
Purchase price allocated | | $ | 1,242,674 | |
This preliminary purchase price allocation has been used to prepare the Transaction Accounting Adjustments in the condensed combined pro forma balance sheet and statements of operations. The final purchase price allocation will be determined when Holdings has completed the detailed valuations and necessary calculations as described in more detail in the explanatory notes in Note 6 — Pro Forma Adjustments. The final allocation is expected to be completed when Holdings files its report on Form 10-Q for the quarter ending October 31, 2021, and could differ materially from the preliminary allocation used in the Transaction Accounting Adjustments. The final allocation may include (1) changes in allocations to In-Process Research & Development (“IPR&D”), and (2) other changes to assets and liabilities.
Note 6 — Pro Forma Adjustments
The following is a description of the unaudited pro forma adjustments reflected in the unaudited pro forma condensed combined financial statements:
Adjustments to the Unaudited Pro Forma Condensed Combined Balance Sheet as of April 30, 2021
(A) Represents the net of the amount of (i) cash paid by Pharma due pursuant to the termination of the Line of Credit of approximately $6 million, and (ii) the expected receipt of cash from the proceeds from the exercise of the Warrant to Purchase Shares of Pharma issued on September 16, 2016 to IDT Corporation, a predecessor of Holdings, which Warrant to Purchase Shares of Pharma is held by Pharma Holdings LLC, a 90%-owned subsidiary of Holdings of which the remaining 10% is owned by the Instrument (the “56% Warrant”). The expected proceeds of approximately $13 million are to be received upon the exercise of 10% of the 56% Warrant.
36
Table of Contents
(B) Reflects the elimination of the derivative liabilities, deferred issuance costs, and amounts due Holdings related to the termination of Pharma’s Line of Credit. Upon the consummation of the Mergers, amounts owed of approximately $6 million pursuant to the Line of Credit will be paid by Pharma. Additionally, RP Finance LLP will be dissolved upon consummation of the Mergers, and therefore Holdings’ equity method investment in RP Finance LLC is eliminated (refer also to Item GG). The derivative liabilities and deferred issuance costs related to the Line of Credit will be eliminated.
(C) Reflects the elimination of the cost method investment in Pharma, held by Holdings prior to the Mergers. Upon the consummation of the Mergers, this investment will be eliminated as Pharma is intended to be a wholly owned subsidiary of Holdings and consolidated into the operations of Holdings.
Under ASC 805, a step acquisition in which control of a business is obtained over time is accounted for as a business combination. The accounting guidance also requires that previously held interests be remeasured at fair value and any difference between the fair value and the carrying value of the interest held be recognized as a gain or loss on the statement of income. The resulting gain on the remeasurement to fair value of the previously held cost method investment in Pharma of approximately $543 million has been included as an adjustment to accumulated deficit in the unaudited pro forma condensed combined balance sheet at April 30, 2021 (refer also to Item K) and as a gain on cost investment of investee in the unaudited condensed combined statement of operations for the year ended July 31, 2020 (refer also to Item FF). The estimated related gain is based on a preliminary estimated fair value of Holdings’ Pharma investment and may differ materially once the final fair value is determined.
(D) Reflects the adjustment to record IPR&D of approximately $1,133 million acquired by the Company at its estimated fair value. As part of the preliminary valuation analysis, the Company identified IPR&D. To value the IPR&D, the Company utilized the Multi-Period Excess Earnings Method (“MPEEM”), under the Income Approach. The method reflects the present value of the operating cash flows generated by Pharma’s assets after taking into account the cost to realize the revenue, and an appropriate discount rate to reflect the time value and risk associated with the invested capital. IPR&D acquired represents Pharma’s research and development activities related to oncology-focused pharmaceuticals which exploit the metabolic differences between normal cells and cancer cells.
(E) Reflects preliminary goodwill of approximately $433 million for the estimated preliminary Merger Consideration in excess of the fair value of the net assets acquired in connection with the Mergers (see Note 5).
(F) Represents preliminary estimated transaction costs inclusive of financing, printing, legal, financial advisory and accounting fees, that are expensed as part of the Mergers. The unaudited pro forma condensed combined balance sheet reflects these costs as an increase to “Accrued expenses.” These costs are expensed through “Accumulated deficit” and are included in the unaudited pro forma condensed combined statement of operations for the year ended July 31, 2020 as discussed below. These transaction costs are in addition to the transaction costs that had already been recognized in the Company’s historical financial statements as of April 30, 2021.
(G) Reflects the conversion of all Convertible Notes issued by Pharma, which will be converted to shares of Pharma’s Preferred Stock immediately before the consummation and closing of the Mergers.
(H) Represents the par value of the Class B Common Stock issued by Holdings to Pharma stockholders upon the closing of the Mergers in the amount of $171 thousand.
(I) Reflects elimination of Pharma’s historical convertible preferred stock and common stock par value from the historical Pharma balance sheet.
(J) Reflects elimination of (i) Pharma’s historical additional paid-in capital, and (ii) issuance of the equity portion of the estimated preliminary Merger Consideration of approximately $605 million.
(in thousands) | | Amount |
Elimination of historical additional paid-in capital balance of Pharma(1) | | $ | (186,208 | ) |
Holdings Class B Common Stock to be issued to Pharma stockholders(2) | | | 604,363 | |
Fair value of Holdings’ replacement vested stock options for Pharma vested options | | | 16,440 | |
Total adjustments to Additional paid-in capital | | $ | 434,595 | |
37
Table of Contents
(K) Reflects adjustments to eliminate (i) Pharma’s historical accumulated deficit, (ii) transaction costs incurred in connection with the Mergers, (iii) the gain on the remeasurement of Holdings’ current cost method investment in Pharma, and (iv) the loss on Holdings’ equity method investment in RP Finance.
(in thousands) | | Amount |
Elimination of Pharma historical accumulated deficit(1) | | $ | 241,502 | |
Transaction costs incurred in connection with Mergers(2) | | | (550 | ) |
Gain on remeasurement of Holdings’ existing cost method investment in Pharma(3) | | | 542,559 | |
Loss on Holdings’ equity method investment in RP Finance LLC(4) | | | (479 | ) |
Total adjustments to Accumulated deficit | | $ | 783,032 | |
(L) Represents the net cash receipts related to (i) the PIPE of $104 million, (ii) payment of $30 million to acquire the remaining membership interest of 33.333% in Altira Capital & Consulting, LLC, and (iii) payment of $2 million sign-on bonus to the Chief Executive Officer.
(M) Represents amounts due to/from Holdings and Pharma to the other, which have been considered to be settlements of preexisting relationships between the entities, and are not included in applying the acquisition method of accounting.
(N) Represents amounts due to/from Holdings and Pharma to the other, which have been considered to be settlements of preexisting relationships between the entities, and are not included in applying the acquisition method of accounting.
(O) In connection with the PIPE as described above, Holdings sold an aggregate of 2,945,986 shares of Class B common stock for aggregate proceeds of approximately $104 million on August 24, 2021, with an aggregate par value of approximately $29 thousand, and an increase to additional paid-in capital of approximately $104 million.
(P) Represents the increase to accumulated deficit related to (i) the purchase of the remaining membership interest of 33.333% in Altira Capital & Consulting, LLC (“Altira”) that it does not already own for $30 million as a condition to the Merger Agreement, and (ii) payment of a $2 million sign-on bonus to the Chief Executive Officer which was expensed on May 27, 2021.
The purchase of the additional membership interests in Altira is not considered a business in accordance with the guidance in ASC 805. The membership interests to be acquired do not consist of inputs, processes, and are not generating outputs, as required in ASC 805 to qualify as a business, and is therefore accounted for as an asset acquisition. Although this transaction is considered an asset acquisition, there are no assets or liabilities to be recorded as of the acquisition date as Altira does not have any business operations. The cost of the investment was determined to be $30 million pursuant to the terms of Agreement, and the amount paid will be expensed.
(Q) Reflects adjustment to record deferred tax liabilities established on the preliminary purchase price allocation primarily related to the IPR&D acquired. Because the IPR&D is considered an indefinite-lived asset for accounting purposes, the fair value of the IPR&D on the acquisition date creates a deferred income tax liability (“DTL”) in accordance with ASC 740, Income Taxes. This DTL is computed using the fair value of the IPR&D assets on the acquisition date multiplied by Holdings’ combined federal and state income tax rate of 28.11%. While this DTL would reverse on impairment or sale or commencement of amortization of the related intangible assets, those events are not anticipated under ASC 740 for purposes of predicting reversal of a temporary difference to support the realization of deferred tax assets. As a result, the reversal of the DTL with respect to the acquired IPR&D is not considered a source of future taxable income in accordance with ASC 740. Holdings has a full valuation allowance on its deferred tax assets for all periods presented and this IPR&D DTL is not expected to have any impact on the valuation allowance at the effective date of the Mergers or in Holdings’ historical consolidated financial statements for any period presented.
38
Table of Contents
Adjustments to the Unaudited Pro Forma Condensed Combined Statements of Operations
The pro forma adjustments included in the unaudited pro forma condensed combined statements of operations for the nine months ended April 30, 2021 and for the year ended July 31, 2020 are as follows:
(AA) Reflects the accrual of additional transaction costs incurred by the Company subsequent to April 30, 2021. These costs are in addition to the transaction costs of approximately $226 thousand that had already been recognized in the Company’s historical income statement for the nine months ended April 30, 2021. Additional transaction costs are reflected as if incurred on August 1, 2019, the date the Mergers occurred for the purposes of the unaudited pro forma condensed combined statement of operations.
(BB) Reflects net compensation cost of approximately $21 million and approximately $21 million for the nine months ended April 30, 2021 and the year ended July 31, 2020, respectively, related to the replacement of the Pharma options with options to purchase Holdings Class B Common Stock, and the elimination of the stock based compensation expense for Pharma. No post-Merger services are required for the replacement awards, and Pharma’s employees had rendered all of the required service for the Pharma awards as of the date of the Mergers. The amount attributable to post-Merger service is approximately $22 million, which is the difference between the total value of the replacement awards of approximately $39 million and the portion attributable to pre-Merger service of approximately $16 million. Because no post-Merger service is required for the replacement awards, Holdings will immediately recognize approximately $22 million as compensation cost in its post-Merger financial statements.
(CC) Represents the elimination of interest expense which consists of (i) interest expense related to Pharma’s Convertible Notes of $312 thousand and $428 thousand for the nine months ended April 30, 2021 and the year ended July 31, 2020, respectively and (ii) amortization of deferred issuance costs of $5,782 thousand and $3,791 thousand for the nine months ended April 30, 2021 and the year ended July 31, 2020, respectively, related to the termination of Pharma’s Line of Credit.
(DD) Represents the elimination of the change in the derivative liabilities associated with the termination of Pharma’s Line of Credit.
(EE) Represents the issuance of Class B Common Stock in connection with the Mergers, assuming the shares were outstanding since August 1, 2019. As the Mergers and related equity transactions are being reflected as if they had occurred at the beginning of the periods presented, the calculation of weighted average shares outstanding for basic and diluted loss per share assumes that the shares issuable relating to the Mergers have been outstanding for the entirety of all periods presented.
On May 27, 2021, the Company granted 908,497 restricted shares of Class B common stock of the Company to the Chief Executive Officer. However, the restricted shares of Class B common stock do not begin to vest until one year after service has been rendered, and therefore were not included in the calculation of the denominator for basic and diluted earnings per share as shown below.
In connection with the PIPE, which closed on August 24, 2021, the Company sold an aggregate of 2,945,986 shares of Class B common stock which has been included in the denominator as outstanding for the entire period presented in basic and diluted earnings per share as shown below.
39
Table of Contents
The following table summarizes pro forma adjustments to basic and diluted common shares outstanding:
| | Nine Months
Ended
April 30,
2021(1) | | Year Ended July 31,
2020 |
Denominator for basic and diluted earnings per share calculation: | | | | |
Basic common shares outstanding – Holdings historical | | 16,216,969 | | 15,764,829 |
Class B Common Stock expected to be issued to Pharma in connection with the Mergers | | 17,145,038 | | 17,145,038 |
Class B Common Stock issued pursuant to Securities Purchase Agreements | | 2,945,986 | | 2,945,986 |
Pro forma basic common shares outstanding | | 36,307,993 | | 35,855,853 |
| | | | | |
Diluted common shares outstanding – Holdings historical | | | | 15,764,829 |
Class B Common Stock expected to be issued to Pharma in connection with the Mergers | | | | 17,145,038 |
Holdings stock options | | | | 167,133 |
Class B Common Stock issued pursuant to Securities Purchase Agreements | | | | 2,945,986 |
Pro forma diluted common shares outstanding | | | | 36,022,986 |
(FF) The resulting gain on remeasurement to fair value of Holdings’ previously held cost method investment in Pharma of approximately $543 million has been included as a gain on cost investment of investee transaction in the unaudited condensed combined statement of operations for the year ended July 31, 2020. The estimated related gain is based on a preliminary estimated fair value of its Pharma investment and may differ materially once the final fair value is determined upon closing and consummation of the Mergers.
(GG) The resulting loss on the previously held equity method investment in RP Finance of $479 thousand has been included as other income (loss) in the unaudited condensed combined statement of operations for the year ended July 31, 2020.
(HH) Represents the payment of $2 million sign-on bonus to Ameet Mallik, the Chief Executive Officer.
(II) Represents the payment of $30 million to acquire the remaining membership interest of 33.333% in Altira Capital & Consulting, LLC. The purchase of the remaining 33.333% membership interest in Altira does not include any acquisition of IPR&D, rather it is the purchase of the royalty rights owned by the remaining minority stockholder and considered an asset acquisition as Altira has no assets, liabilities, or operations, and is not a business. Therefore, the investment has been immediately charged as an impairment expense.
40
Table of Contents
COMPARATIVE HISTORICAL AND UNAUDITED PRO FORMA CONSOLIDATED
PER SHARE INFORMATION
The following table sets forth (i) historical per share information of Holdings for the nine months ended April 30, 2021 and the year ended July 31, 2020, and (ii) the unaudited pro forma consolidated per share information of Holdings after giving pro forma effect to the Mergers, including Holdings’ issuance of 0.12045 shares of Holdings Class B Common Stock for each outstanding share of Pharma capital stock not owned by Holdings or its subsidiaries for the nine months ended April 30, 2021 and the year ended July 31, 2020. Historical per share information of Pharma for the nine months ended April 30, 2021 and the year ended July 31, 2020 is not relevant as Pharma common shares are not publicly traded.
This information should be read in conjunction with (i) the historical financial information included elsewhere in this proxy statement/prospectus, (ii) the historical financial statements of Holdings and related notes that are incorporated by reference in this proxy statement/prospectus, (iii) the historical financial statements of Pharma and related notes that are attached to this proxy statement/prospectus and (iv) the “Unaudited Pro Forma Condensed Combined Financial Information” included in this proxy statement/prospectus.
| | Historical
Holdings | | Historical
Pharma | | Pro Forma |
Basic net income (loss) per share of common stock(1) | | | | | | | | | | | | |
Nine months ended April 30, 2021 | | $ | (0.75 | ) | | | N/M | (4) | | $ | (2.96 | ) |
Year Ended July 31, 2020 | | $ | (0.66 | ) | | | N/M | (4) | | $ | 11.34 | |
Diluted net income (loss) per share of common stock | | | | | | | | | | | | |
Nine months ended April 30, 2021 | | $ | (0.75 | ) | | | N/M | (4) | | $ | (2.96 | ) |
Year Ended July 31, 2020 | | $ | (0.66 | ) | | | N/M | (4) | | $ | 11.29 | |
Cash dividends declared per share | | | | | | | | | | | | |
Nine months ended April 30, 2021 | | $ | — | | | $ | — | | | $ | — | |
Year Ended July 31, 2020 | | $ | — | | | $ | — | | | $ | — | |
Book value per share(2) | | | | | | | | | | | | |
As of April 30, 2021 | | $ | 8.42 | | | $ | (2.26 | ) | | $ | 38.14 | |
As of July 31, 2020 | | $ | 8.25 | | | $ | (0.93 | ) | | | N/M | (3) |
41
Table of Contents
COMPARATIVE PER SHARE MARKET PRICE DATA AND DIVIDEND INFORMATION
Shares of Holdings Class B common stock are traded on the NYSE under the symbol “RFL.” The following table sets forth, for the periods indicated, the range of high and low intraday sales prices for Holdings common stock on the NYSE. No class or series of shares of Pharma are traded on a public stock exchange and therefore are not included below. The sales prices are as reported in published financial sources.
| | Holdings Class B
Common Stock |
| | | High | | Low |
Fiscal 2020 | | | | | | |
Quarter ended October 31, 2019 | | $ | 22.33 | | $ | 17.37 |
Quarter ended January 31, 2020 | | | 22.58 | | | 15.46 |
Quarter ended April 30, 2020 | | | 22.13 | | | 10.40 |
Quarter ended July 31, 2020 | | | 17.30 | | | 12.38 |
Fiscal 2021 | | | | | | |
Quarter ended October 31, 2020 | | $ | 19.17 | | $ | 13.95 |
Quarter ended January 31, 2021 | | | 26.29 | | | 17.19 |
Quarter ended April 30, 2021 | | | 47.86 | | | 23.51 |
Quarter ended July 31, 2021 | | | 63.59 | | | 41.68 |
Neither Holdings nor Pharma has ever paid a dividend with respect to the shares of Holdings common stock or Pharma common stock, respectively.
42
Table of Contents
INFORMATION ABOUT THE COMPANIES
Rafael Holdings, Inc.
Rafael Holdings, Inc. (“Holdings” or the “Parent”), a Delaware corporation, owns interests in pre-clinical and clinical stage pharmaceutical companies and commercial real estate assets. The assets are operated as two separate lines of business.
The pharmaceutical holdings include preferred and common equity interests and a warrant to purchase additional equity interests in Rafael Pharmaceuticals, Inc., or Rafael Pharmaceuticals, which is a clinical stage, oncology-focused pharmaceutical company committed to the development and commercialization of therapies that exploit the metabolic differences between normal cells and cancer cells; and, a majority equity interest in LipoMedix Pharmaceuticals Ltd., or LipoMedix, a clinical stage oncological pharmaceutical company based in Israel. In addition, in 2019, the Company established the Barer Institute (“Barer”), a wholly-owned early stage venture focused on developing a pipeline of therapeutic compounds, including compounds to regulate cancer metabolism. The venture is pursuing collaborative research agreements with leading scientists from top academic institutions. In addition, the Company has recently initiated efforts to develop other early stage pharmaceutical ventures including Levco Pharmaceuticals Ltd. (“Levco”), an Israeli company, established to partner with Dr. Alberto Gabizon and a top institution in Israel on the development of novel compounds for cancer, and Farber Partners, LLC (“Farber”), formed around an agreement with Princeton University’s Office of Technology Licensing for technology from the laboratory of Professor Joshua Rabinowitz, in the Department of Chemistry, Princeton University, for an exclusive worldwide license to its SHMT (serine hydroxymethyltransferase) inhibitor program. Additionally, in 2021, the Company established Rafael Medical Devices, LLC (“Rafael Medical Devices”), a wholly-owned orthopedic device company developing instruments and implants to advance minimally invasive surgeries in the upper and lower extremities.
The commercial real estate holdings consist of a building at 520 Broad Street in Newark, New Jersey that serves as headquarters for the Company and certain other entities and hosts other tenants and an associated 800-car public garage, and a portion of a building in Israel.
Our headquarters are located at 520 Broad Street, Newark, New Jersey 07102. The main telephone number at our headquarters is (212) 658-1450 and our corporate web site’s home page is www.rafaelholdings.com.
Rafael Pharmaceuticals, Inc.
Rafael Pharmaceuticals, Inc., a Delaware corporation (“Pharma”), is a clinical stage, oncology-focused pharmaceutical company committed to the development and commercialization of therapies that exploit the metabolic differences between normal cells and cancer cells.
Pharma is developing its lead product CPI-613® (devimistat), an investigational new drug for the treatment of solid tumors and hematologic malignancies. This molecule has been developed based on Altered Metabolism Directed (AMD) platform. It is a small molecule and suitable for intravenous (IV) administration.
RH Merger I, Inc.
RH Merger I, Inc., a Delaware corporation is a wholly-owned subsidiary of Holdings (“Merger Sub I”) formed solely for the purpose of effectuating the Mergers. Merger Sub I has no material assets and does not operate any business. To date, Merger Sub I has not conducted any activities other than those incidental to its formation and the execution of the Merger Agreement. After the consummation of the Mergers, it will cease to exist.
The mailing address of Merger Sub I’s principal executive office is 520 Broad Street, Newark, New Jersey 07102.
RH Merger II, LLC
RH Merger II, LLC, a Delaware limited liability company is a wholly-owned subsidiary of Holdings (“Merger Sub II”) formed solely for the purpose of effectuating the Mergers. Merger Sub II has no material assets and does not operate any business. To date, Merger Sub II has not conducted any activities other than those incidental to its formation and the execution of the Merger Agreement. After the consummation of the Mergers, Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger and Pharma is intended to become a wholly-owned limited liability subsidiary of Holdings.
The mailing address of Merger Sub II’s principal executive office is 520 Broad Street, Newark, New Jersey 07102.
43
Table of Contents
THE SPECIAL MEETING OF HOLDINGS’ STOCKHOLDERS
Overview
This proxy statement/prospectus is being provided to Holdings stockholders as part of a solicitation of proxies by the Board of Directors for use at the Special Meeting and at any adjournments of such meeting. This proxy statement/prospectus is being furnished to Holdings stockholders on or about [•], 2021. In addition, this proxy statement/prospectus is a prospectus for Holdings in connection with the issuance by Holdings of Parent Class B Common Stock to be delivered to Pharma stockholders in connection with the Mergers. This proxy statement/prospectus provides Holdings stockholders with information they need to be able to vote or instruct their vote to be cast at the special meeting.
Date, Time and Place of the Special Meeting
The Special Meeting will be held at [•], on [•], 2021 at [•], local time.
Record Date; Outstanding Shares; Shares Entitled to Vote
The Holdings Board has fixed the close of business in New York, NY on [•], 2021 as the record date (the “Record Date”) for determining the holders of shares of Parent Class A Common Stock and Parent Class B Common Stock entitled to notice of, and to vote at, the Special Meeting. As of the close of business on the Record Date, Holdings had [•] shares issued and outstanding and entitled to vote at the Special Meeting, consisting of 787,163 shares of Parent Class A Common Stock and [•] shares of Parent Class B Common Stock.
Stockholders are entitled to three votes for each share of Parent Class A Common Stock held by them and one-tenth of one vote for each share of Parent Class B Common Stock. The holders of Parent Class A Common Stock and Parent Class B Common Stock will vote as a single body on each proposal and any other matter properly coming before the Special Meeting. There are no dissenters’ rights of appraisal in connection with any proposal.
Attendance
You can vote either in person at the Special Meeting or by proxy without attending the meeting.
Beneficial holders of the Parent Class A Common Stock and Parent Class B Common Stock as of the Record Date whose stock is held of record by another party should receive voting instructions from their bank, broker or other holder of record. If a stockholder’s shares are held through a nominee and the stockholder wants to vote at the meeting, such stockholder must obtain a proxy from the nominee record holder authorizing such stockholder to vote at the Special Meeting.
Stockholders of record should receive a paper copy of our proxy materials and may vote by following the instructions on the proxy card that is included with the proxy materials. As set forth on the proxy card, there are two convenient methods for holders of record to direct their vote by proxy without attending the Special Meeting: on the Internet or by mail. To vote by Internet, visit [www.voteproxy.com]. To vote by mail, mark, date and sign the enclosed proxy card and return it in the postage-paid envelope provided. Holders of record may also vote by attending the Special Meeting and voting by ballot.
All shares for which a proxy has been duly executed and delivered (by Internet or mail) and not revoked will be voted at the Special Meeting. If a stockholder of record signs and returns a proxy card but does not give voting instructions, the shares represented by that proxy will be voted as recommended by the Board of Directors. If any other matters are properly presented at the Special Meeting for consideration and if you have voted your shares by Internet or mail, the persons named as proxies will have the discretion to vote on those matters for you. On the date of filing this proxy statement/prospectus with the SEC, the Board of Directors did not know of any other matter to be raised at the Special Meeting.
44
Table of Contents
Proposals
At the Special Meeting, Holdings stockholders will be asked to vote upon the following proposals:
• The Merger Agreement — to consider and vote upon a proposal to adopt the Merger Agreement with Holdings, Merger Subs and Pharma, including, but not limited to, the issuances of Parent Class B Common Stock in connection with the Mergers (“Proposal 1”);
• the approval of the grant to Ameet Mallik, our chief executive officer of up to [•] shares of Parent Class B Common Stock as required by his employment agreement upon consummation of the Mergers to be made under the Plan and the necessary amendments to the Plan to provide for such grant (“Proposal 2”);
• to approve an amendment of the Plan to authorize an additional [•] shares of Parent Class B Common Stock to be reserved for issuance thereunder (“Proposal 3”);
• The Adjournment Proposal — to consider and vote upon a proposal to approve the adjournment of the Special Meeting to a later date or dates, if necessary, to permit further solicitation of proxies in the event there are insufficient votes present in person or by proxy for, or otherwise in connection with, the approval of each of Proposals 1, 2 and 3 (the “Adjournment Proposal”).
Quorum
The presence at the Special Meeting of a majority of the voting power of the Holdings’ outstanding Parent Class A Common Stock and Parent Class B Common Stock (voting together), either in person or by proxy, will constitute a quorum for the transaction of business at the Special Meeting. Abstentions are counted as present and entitled to vote for purposes of determining whether a quorum is present. A “broker non-vote” on a matter occurs when a broker, bank or your representative may not vote on a particular matter because it does not have discretionary voting authority and has not received instructions from the beneficial owner. Because brokers will not have discretionary authority to vote on any of the proposals, a share held by a broker without any voting instructions will not be deemed present or represented by proxy at the Special Meeting and will not count towards establishing a quorum.
If you are a beneficial owner whose shares are held of record by a broker, you must instruct your broker how to vote your shares. If you do not provide voting instructions, your shares will not be voted on any of the proposals. In the event of a broker non-vote or an abstention with respect to any proposal coming before the Special Meeting, the shares represented by the relevant proxy will not be deemed to be present and entitled to vote on the proposals for the purpose of determining the total number of shares of which a majority is required for adoption, having the practical effect of reducing the number of affirmative votes required to achieve a majority vote for such matters by reducing the total number of shares from which a majority is calculated.
Vote Required; Recommendation of the Holdings Board of Directors
Proposal 1: To Adopt the Merger Agreement, including, but not limited to, the issuances of Parent Class B Common Stock in connection with the Mergers
Holdings stockholders are being asked to consider and vote on a proposal to adopt the Merger Agreement. You should carefully read this proxy statement/prospectus in its entirety for more detailed information concerning the Mergers. In particular, you are directed to the Merger Agreement, which is attached as Annex A to this proxy statement/prospectus.
The approval of Proposal 1 requires (i) the affirmative vote of a majority of the issued and outstanding shares of Parent Common Stock to vote thereon, and (ii) the affirmative vote of at least a majority of the issued and outstanding shares of Parent Common Stock not owned directly or indirectly by any affiliate of Holdings, the Merger Subs, or any of their respective officers or directors on or after the date of the Merger Agreement, including trusts for the benefit of immediate family members of such parties regardless of whether they are affiliates, outstanding and entitled to vote thereon at the Special Meeting. Abstentions and broker non-votes will have the effect of a vote “AGAINST” Proposal 1.
The Board of Directors of Holdings unanimously recommends that you vote “FOR” the approval of Proposal 1.
45
Table of Contents
Proposal 2: To Approve the grant to Ameet Mallik
Holdings stockholders are being asked to consider and vote upon a proposal to approve the grant to Ameet Mallik, our chief executive officer of up to [•] shares of Parent Class B Common Stock as required by his employment agreement upon consummation of the Mergers to be made under the Plan and the necessary amendments to the Plan to provide for such grant.
The approval of Proposal 2 requires the affirmative vote of a majority of the issued and outstanding shares of Parent Common Stock to vote thereon. Abstentions and broker non-votes will have no effect on the outcome of Proposal 2.
Proposal 2 is conditioned on the approval of Proposal 1. If Proposal 1 is not approved, Proposal 2 will be of no force or effect, even if approved by the Holdings stockholders.
The Board of Directors of Holdings unanimously recommends that you vote “FOR” the approval of Proposal 2.
Proposal 3: To Approve an amendment of the Plan
Holdings stockholders are being asked to consider and vote upon a proposal to approve the amendment of the Plan to authorize an additional [•] shares of Parent Class B Common Stock to be reserved for issuance thereunder.
The approval of Proposal 3 requires the affirmative vote of a majority of the issued and outstanding shares of Parent Common Stock to vote thereon. Abstentions and broker non-votes will have no effect on the outcome of Proposal 3.
Proposal 3 is conditioned on the approval of Proposal 1. If Proposal 1 is not approved, Proposal 3 will be of no force or effect, even if approved by the Holdings stockholders.
The Board of Directors of Holdings unanimously recommends that you vote “FOR” the approval of Proposal 3.
Proposal to Adjourn the Special Meeting
Holdings stockholders are being asked to approve a proposal to adjourn the Special Meeting, or any adjournments thereof, to another time or place if necessary or appropriate to solicit additional proxies if there are not sufficient votes to approve Proposals 1, 2 and 3 at the time of the Special Meeting or any adjournment or postponement thereof.
The approval of the Adjournment Proposal requires the affirmative vote of a majority of the votes cast at the Special Meeting (whether or not a quorum, as specified in the Holdings Bylaws, is present). Abstentions and broker non-votes will have no effect on the outcome of the Adjournment Proposal.
The Board of Directors of Holdings recommends that you vote “FOR” the Adjournment Proposal.
Share Ownership and Voting by Holdings’ Officers and Directors
As of the Record Date, Holdings’ directors and executive officers had the right to vote approximately [•] shares of Parent Common Stock, representing approximately [•]% of the shares of Parent Common Stock then outstanding and entitled to vote at the Special Meeting. It is expected that Holdings’ directors and executive officers who are stockholders of Holdings will vote “FOR” each of the proposals described above, including Holdings’ Chairman and the Board of Directors, Howard Jonas, who along with certain of his family members and affiliates entered into a Voting Agreement, which is attached as Annex D to this proxy statement/prospectus and pursuant to which they have agreed to vote in favor of the consummation of the Mergers and take other actions in furtherance of the consummation of the Merger Agreement.
Voting Your Shares
You can vote either in person at the Special Meeting or by proxy without attending the meeting.
Beneficial holders of Parent Class A Common Stock and Parent Class B Common Stock as of the Record Date whose stock is held of record by another party should receive voting instructions from their bank, broker or other holder of record. If a stockholder’s shares are held through a nominee and the stockholder wants to vote at the meeting, such stockholder must obtain a proxy from the nominee record holder authorizing such stockholder to vote at the Special Meeting.
46
Table of Contents
Stockholders of record should receive a paper copy of our proxy materials and may vote by following the instructions on the proxy card that is included with the proxy materials. As set forth on the proxy card, there are two convenient methods for holders of record to direct their vote by proxy without attending the Special Meeting: on the Internet or by mail. To vote by Internet, visit [www.voteproxy.com]. To vote by mail, mark, date and sign the enclosed proxy card and return it in the postage-paid envelope provided. Holders of record may also vote by attending the Special Meeting and voting by ballot.
All shares for which a proxy has been duly executed and delivered (by Internet or mail) and not revoked will be voted at the Special Meeting. If a stockholder of record signs and returns a proxy card but does not give voting instructions, the shares represented by that proxy will be voted as recommended by the Board of Directors. If any other matters are properly presented at the Special Meeting for consideration and if you have voted your shares by Internet or mail, the persons named as proxies will have the discretion to vote on those matters for you. On the date of filing this proxy statement/prospectus with the SEC, the Board of Directors did not know of any other matter to be raised at the Special Meeting.
If you own shares in your own name, you are considered, with respect to those shares, the “stockholder of record”. If you are a beneficial owner of shares registered in the name of your broker, bank or other agent acting as nominee, your shares are held by your broker, bank or other agent as your nominee, or in “street name,” and you will need to obtain a proxy form from the organization that holds your shares and follow the instructions included on that form regarding how to instruct the organization to vote your shares. Your broker, bank or other nominee may have an earlier deadline by which you must provide instructions to it as to how to vote your shares, so you should read carefully the materials provided to you by your broker, bank or other nominee.
Banks, brokers and other agents acting as nominees are permitted to use discretionary voting authority to vote proxies for proposals that are deemed “routine” by the NYSE, but are not permitted to use discretionary voting authority to vote proxies for proposals that are deemed “non-routine” by the NYSE. A “broker non-vote” occurs when a proposal is deemed “non-routine” and a nominee holding shares for a beneficial owner does not have discretionary voting authority with respect to the matter being considered and has not received instructions from the beneficial owner. The determination of which proposals are deemed “routine” versus “non-routine” may not be made by the NYSE until after the date on which this proxy statement/prospectus has been mailed to you. As such, it is important that you provide voting instructions to your bank, broker or other nominee, if you wish to determine the voting of your shares.
If you do not provide voting instructions to your bank, broker or other nominee, your shares will not be voted on any proposal on which your bank, broker or other nominee does not have discretionary authority to vote. In these cases, the bank, broker or other nominee will not be able to vote your shares on those matters for which specific authorization is required. Brokers do not generally have, and we do not expect them to have, discretionary authority to vote on any of the proposals presented at the Special Meeting.
How Can I Change My Vote?
A stockholder of record can revoke his, her or its proxy at any time before it is voted at the Special Meeting by delivering to Holdings (to the attention of Joyce J. Mason, Esq., Corporate Secretary) a written notice of revocation or by executing a later-dated proxy by Internet or mail, or by attending the Special Meeting and voting in person.
If your shares are held in the name of a bank, broker, or other nominee, you must obtain a proxy executed in your favor from the holder of record (that is, your bank, broker, or nominee) to be able to vote at the Special Meeting.
Costs of Solicitation
Holdings will bear all costs and expenses in connection with the solicitation of proxies, including the costs of preparing, printing and mailing this proxy statement/prospectus for the Special Meeting. Holdings has engaged [•] to serve as information agent and assist in the solicitation of proxies for the Special Meeting and will pay an aggregate initial fee of approximately $[•], plus any additional fees to be determined at the conclusion of the solicitation and reimbursement of reasonable out-of-pocket expenses.
47
Table of Contents
Other Business
Holdings is not aware of any other business to be acted upon at the Special Meeting. If, however, other matters are properly brought before the Special Meeting, the proxies will have discretion to vote or act on those matters according to their best judgment and they intend to vote the shares as the Holdings board of directors may recommend.
Assistance
Holdings stockholders who have questions about the Merger Agreement, the Mergers or the other matters to be voted on at the Special Meeting who need assistance submitting their proxy or voting shares or who desire additional copies of this proxy statement/prospectus or additional proxy cards should contact:
[•]
Certain Governance Matters Following the Transactions
Following the consummation of the Mergers, the Holdings Board will consist of 11 members, comprised of the current members of the Holdings Board and three representatives from Pharma: Richard Axel, Chi Van Dang and Sanjeev Luther. The executive officers of Holdings will remain the same.
No Appraisal Rights
Appraisal rights are not available to holders of Parent Common Stock or Pharma Common Stock in connection with the Mergers.
Listing of Holdings Class B Common Stock on NYSE
Parent Class B Common Stock is currently listed on the NYSE under the symbol “RFL”. On [•], 2021, the last reported sale price for Parent Class B Common Stock was $[•] per share. Following the Mergers, we expect that Parent Class B Common Stock will continue to be listed on the NYSE.
Background to the Merger
Pharma (formerly Cornerstone Pharmaceuticals, Inc.) was formed as a result of a merger in 2002 between Cornerstone Ventures, LLC (“Ventures”), a New York limited liability company which was organized on May 14, 1999, and Pharma (which was a New York corporation at the time) which was incorporated on November 1, 2001, with Pharma being the surviving entity. On December 24, 2009, Pharma (New York) was merged with and into a newly formed Delaware corporation by the same name, for the purpose of changing domiciles. This merger had no financial or accounting effects on Pharma. On May 10, 2017, the Pharma board of directors approved the resolution changing the name to “Rafael Pharmaceuticals, Inc.”. IDT Corporation (“IDT”), previously the parent company of Holdings prior to the spinoff of Holdings consummated in March 2018, first invested in Pharma in December 2015, and made subsequent investments in Pharma, including via the Warrant that was issued to IDT Corporation by Pharma in September 2016. Following the consummation of a spinoff transaction on March 26, 2018, Holdings succeeded to own all of the interests in Pharma then held by IDT, including, without limitation, the Warrant. Holdings and its subsidiaries collectively own securities representing 51% of the outstanding capital stock of Pharma and 41% of the capital stock of Pharma on a fully diluted basis (excluding the remainder of the Warrant).
The Holdings board of directors met on March 22, 2019 to discuss interest in exploring a transaction on which the Company would acquire the capital stock of Pharma that it did not already own. The disinterested directors of the Holdings board of directors resolved to pursue such a transaction as a means of unlocking the full value of Holdings’ interests in Pharma and to allow for synergies between the Pharma personnel and the scientific team being built at Holdings, and established the Holdings Special Committee with authorization to evaluate, pursue and negotiate the terms of such a transaction, and to retain such independent advisors as reasonably necessary for the Holdings Special Committee to carry out its responsibilities. The resolutions passed by the Holdings board of directors to establish the Holdings Special Committee authorized the Holdings Special Committee to, among other things, (i) establish, approve, modify, monitor and direct the process and procedures related to the review and evaluation of the possible transaction (or determine not to proceed with the possible transaction), (ii) respond to any communications, inquiries or proposals by regulatory authorities or third parties regarding the possible transaction (to the extent determined appropriate by the Holdings Special Committee), (iii) review, evaluate, investigate, pursue and negotiate the terms and conditions of the
48
Table of Contents
possible transaction (to the extent determined appropriate by the Holdings Special Committee), (iv) determine on behalf of the Holdings board of directors and Holdings whether the possible transaction is fair to, and in the best interests of, Holdings and its stockholders (or any subset thereof to the extent determined appropriate by the Holdings Special Committee), (v) reject or approve the possible transaction or recommend such rejection or approval to the Holdings board of directors, (vi) effect or recommend to the Holdings board of directors the consummation of the possible transaction Stephen Greenberg, Boris Pasche and Michael Weiss were appointed as the members of the Holdings Special Committee.
The Holdings Special Committee convened its first meeting by telephone on March 27, 2019, at which meeting Stephen Greenberg was appointed as Chair and Boris Pasche was appointed as Vice Chair. The Holdings Special Committee resolved to hold a follow up meeting on April 16, 2019 to interview investment banks to provide certain financial advisory services to the Holdings Special Committee, and to consider the appointment of counsel.
On April 18, 2019, Michael Weiss voluntarily withdrew from the Holdings Special Committee and ceased to be a member thereof.
The Holdings Special Committee resolved to appoint Olshan Frome Wolosky LLP (“Olshan”) as independent legal counsel to the Holdings Special Committee at a meeting in New York on April 16, 2019 following completion of a customary conflicts check. The decision to engage Olshan was based on, among other factors, Olshan’s absence of prior or current engagements on behalf of either Holdings, Pharma or any of their affiliates, experience in representing health and life science focused public companies as well as experience in mergers and acquisition transactions. At the same meeting, the Holdings Special Committee interviewed several nationally recognized financial advisory firms to provide certain services to the Holdings Special Committee. As part of these interviews, the Holdings Special Committee requested information from candidates to assess the extent of any relationships with either Holdings, Pharma, or any of their affiliates. Following the completion of the interviews, the Holdings Special Committee resolved to engage Houlihan Lokey based on, among other things, Houlihan Lokey’s qualifications, absence of prior or current engagements on behalf of either Holdings, Pharma, or any of their affiliates, reputation and experience, including in health and life science space. The Holdings Special Committee subsequently negotiated and executed an engagement letter with Houlihan Lokey for certain financial advisory services in connection with the potential business combination with Pharma.
The Holdings Special Committee, working with counsel, assessed its own composition to ensure its independence from Holdings, Pharma, or any of their affiliates, including by completing and reviewing the responses to customary director questionnaires.
On July 20, 2020, the Holdings Special Committee held a telephonic meeting to discuss alternatives to the possible transaction with Pharma after receiving informal feedback from certain members of the Pharma board of directors that Pharma was more interested in pursuing an initial public offering than in undertaking the business combination with Holdings that had been discussed. Accordingly, the Holdings Special Committee discussed in general terms what conditions Holdings would need to set for its support of an initial public offering of Pharma that would be designed to maximize stockholder value for the stockholders of Holdings. In advance of such meeting, Olshan had circulated to the Holdings Special Committee a draft letter setting forth the terms on which the Holdings Special Committee would engage in discussions with the Pharma board of directors on the pursuit of an initial public offering for Pharma. The Holdings Special Committee reviewed and discussed the terms of such proposal, and resolved that following the meeting such draft letter should be transmitted to Thomas Rose of Sichenzia Ross Ference LLP as counsel for Pharma with instructions that he present the letter to the Pharma board of directors and Pharma special committee of the board of directors.
On each of December 31, 2020 and January 18, 2021, the Holdings Special Committee held a telephonic meeting to discuss the value of certain of Holdings’ assets.
Representatives of Olshan and Orrick Herrington & Sutcliffe LLP (Pharma’s counsel) discussed proposals for a revised term sheet to merge Holdings and Pharma with counsel for Holdings and Mr. Greenberg on January 29, 2021. In addition, on such date, the Olshan team reviewed and discussed with Holdings management a draft of the pro forma capitalization table for Pharma in the context of negotiations over percentage ownership of the combined company.
On February 1, 2021, Holdings management and representatives of Schwell Wimpfheimer & Associates LLP (“SWA”) had a call with representatives of Olshan to discuss proposed terms for the merger and potential alternatives if such terms were not acceptable to Pharma.
On February 5, 2021, following further discussions between the Holdings Special Committee and key stakeholders at Pharma, representatives of Olshan sent a draft term sheet for the possible transaction to the Pharma
49
Table of Contents
board of directors. The term sheet proposed an equity value for Pharma of $850 million and set the expectation that each of Pharma and Holdings would be required to obtain a majority of the minority stockholder approval of the merger as a condition precedent. As part of the negotiations following delivery of the revised term sheet, the Holdings Special Committee rejected proposals from Pharma calling for a change to the stockholder structure, additional board seats for certain Pharma Board members and a post-closing ownership split of 50% for each of the pre-closing Pharma and Holdings stockholder groups.
On February 16, 2021, the Holdings Special Committee held a telephonic meeting to discuss the value of Holdings’ assets (excluding Holdings’ interests in Pharma) that was proposed by Pharma as a basis for negotiating the merger of Holdings and Pharma. The Holdings Special Committee discussed reverting to the Pharma board of directors with a compromise position, as Pharma had ascribed a reduced value for the non-Pharma assets of Holdings as compared to the initial proposal made by the Holdings Special Committee. The Holdings Special Committee also discussed the preparation of a draft merger agreement by Holdings’ counsel and Olshan, and determined that counsel should begin preparing such a draft.
Representatives of Olshan and Orrick had a call with Mr. Greenberg to discuss requests for information received from the Pharma board of directors on February 23, 2021.
On March 4, 2021, the Olshan and Orrick teams discussed the Pharma capitalization table with representatives of Holdings management.
On March 10, 2021, representatives of Olshan circulated an initial draft Merger Agreement to the Orrick team.
On March 16, 2021, representatives of Olshan and Orrick convened a call to discuss tax structuring considerations in the transaction.
On March 17, 2021, Holdings’ finance team walked the Orrick and Olshan teams through their understanding of certain elements of the Pharma capitalization table.
On March 23, 2021, the Holdings Special Committee convened a telephonic meeting to discuss the pursuit of an equity financing of Holdings to raise funds necessary to consummate the possible transaction. At this meeting, with members of Holdings management in attendance, the Holdings Special Committee received presentations from several nationally recognized investment banks who could potentially act as underwriters for such transaction. Also on March 23, 2021, representatives of Olshan, Orrick and Raymond James (Pharma’s financial advisor) convened a call to discuss the proposed financing.
On March 25, 2021 counsel for Holdings and representatives of Olshan had discussions with the Orrick team regarding certain points raised by the Pharma board of directors.
On April 1, 2021, representatives of Orrick circulated a revised draft Merger Agreement to the Olshan team, reflecting negotiations over the course of the previous weeks, and including an update to the structure of the Merger to contemplate the merging of Pharma out of existence in order to realize certain tax efficiencies for all parties.
On April 2, 2021, representatives of Orrick, Olshan and Raymond James had a call with Mr. Greenberg in attendance to negotiate certain revised terms in the recent draft Merger Agreement.
On April 15, 2021, representatives of Orrick, Olshan and Holdings management had a call to negotiate certain key points in the Merger Agreement. On April 16, 2021, representatives of Olshan sent a revised draft of the Merger Agreement to Orrick to reflect such discussions. The Olshan and Orrick teams had several conversations to negotiate key terms in the Merger Agreement over the course of the week following the circulation of the revised draft Merger Agreement.
On April 29, 2021, representatives of Olshan circulated an initial draft Lockup Agreement to bind certain key Holdings and Pharma stockholders after the closing of the Merger.
Representatives of Orrick and Olshan convened a call on April 30, 2021 with members of Holdings management to discuss the Pharma capitalization table and negotiate regarding certain deal points, including the treatment of incentive equity at Pharma upon consummation of the business combination.
50
Table of Contents
On May 4, 2021, representatives of Orrick circulated initial drafts of the Support Agreement to be executed by key Pharma stockholders, including Holdings and its affiliates, the Voting Agreement to be executed by certain controlling affiliates of Holdings, and the Note Cancellation Agreement to be executed by certain Pharma noteholders in support of the Merger.
On May 5, 2021, Mr. Greenberg, Mr. Luther, Dr. Scheller, Dr. Dang and representatives of Orrick and Olshan had a call to negotiate certain terms in the Merger Agreement, including the treatment of employee incentive equity subject to vesting.
On May 7, 2021, representatives of Orrick, Olshan and Holdings management had a call to discuss and finalize the tax structure for the transaction.
On May 10, 2021, representatives of Orrick and Olshan discussed with Mr. Luther and Mr. Greenberg regarding the making of a capital call under the Line of Credit and certain other matters related to the transaction. Also on such date, representatives of Orrick sent a revised draft Merger Agreement to representatives of Olshan on May 10, 2021.
Representatives of Olshan circulated revised drafts of the Support Agreement, Voting Agreement and Note Cancellation Agreement to the Orrick team on May 11, 2021. On the same date, representatives of Olshan and Orrick had a call to exchange updates on timing and next steps in order to execute the merger agreement.
On May 13, 2021, representatives of Olshan convened a call with representatives of CohnReznick LLP and SWA, along with members of Holdings management, to discuss required financials for a registration statement following execution of the merger agreement, and to walk through anticipated timing for producing the financials that would be required for inclusion in the registration statement.
On May 16, 2021, representatives of Olshan circulated a revised draft Merger Agreement to representatives of Orrick following further discussions on terms between the parties.
On May 19, 2021, representatives of Olshan circulated a revised version of the prior draft Merger Agreement including for the first time the exact merger consideration ratios to reflect the results of the negotiations between the parties on consideration. Representatives of Olshan and Orrick had a conference call to discuss the changes that same day.
On May 20, 2021, following a conference call with the Olshan team to discuss the Merger Agreement, representatives of Orrick circulated a revised draft Merger Agreement to representatives of Olshan reflecting additional changes in advance of distributing the Merger Agreement for review by Pharma’s board of directors.
Representatives of Orrick circulated revised drafts of the Support Agreement, Note Cancellation Agreement and Lockup Agreement on May 25, 2021 to reflect revised terms after further discussions with the Olshan team on May 24, 2021.
On May 26, 2021, the Holdings Special Committee held a virtual meeting also attended by representatives of Houlihan Lokey. At this meeting, Houlihan Lokey reviewed and discussed with the Holdings Special Committee preliminary financial analyses performed to date with respect to Pharma and Holdings. The Holdings Special Committee also received an update from counsel on the status of negotiations.
The Orrick and Olshan teams discussed the Pharma capitalization table and the Support Agreement on May 27, 2021. Over the course of May 27 and May 28, 2021, Olshan sent revised drafts of all transaction documents, including the Merger Agreement, to Orrick reflecting the results of discussions between parties and seeking to receive any final comments from the Pharma team.
On June 1, 2021, the Orrick and Olshan teams discussed outstanding terms in the Merger Agreement, Support Agreement and other ancillary agreements, and later in the day Orrick circulated revised drafts to the Olshan team.
Representatives of Orrick and Olshan discussed with Mr. Greenberg the timing of Holdings’ funding of the capital call made by Pharma under the Line of Credit on June 2, 2021. Also on such date, representatives of Orrick and Olshan had a call to discuss the most recent version of the capitalization table of Pharma, along with Holdings management.
On June 3, 2021, representatives of Olshan circulated further comments to the Merger Agreement, Support Agreement and Note Cancellation Agreement.
51
Table of Contents
On June 7, 2021, following further discussions, representatives of Olshan circulated a draft of the form of Support Agreement to be executed by Holdings and certain of its affiliates. On June 9 and June 10, 2021, representatives of Olshan circulated proposed final forms of the Merger Agreement reflecting certain incremental changes. Also on June 9, 2021, following further discussion and analysis of the capital structure of Pharma, the parties received an agreed form capitalization table for Pharma embodying the final negotiated Per Share Merger Consideration ratios and including projected allocations of consideration to each Pharma stockholder.
On June 10, 2021, representatives of Olshan circulated a revised draft Merger Agreement to reflect further negotiations between the parties.
The Orrick team circulated revised versions of the Support Agreements, Voting Agreement, and Lockup to the Olshan team on June 11, 2021. Olshan returned further revised drafts of the foregoing agreements and of the Merger Agreement on June 12, 2021. On June 13, 2021, the Orrick and Olshan teams discussed all proposed changes to the foregoing documents, and documents were finalized over the course of the day.
On June 14, 2021, the Holdings Special Committee held a virtual meeting to review the Merger. The members of the Holdings Special Committee discussed key aspects of the Merger Agreement with counsel, and at the request of the Special Committee, Houlihan Lokey then reviewed and discussed its financial analyses. Houlihan Lokey also reviewed with the Holdings Special Committee the conflict checks procedures undertaken by Houlihan Lokey, as well as the results of those procedures, which the Special Committee considered in gaining certain assurance of the satisfactory degree of Houlihan Lokey’s independence in providing financial advisory services in connection with the Merger. Thereafter, Houlihan Lokey verbally rendered its opinion to the Special Committee (which was subsequently confirmed in writing by delivery of Houlihan Lokey’s written opinion addressed to the Special Committee dated June 14, 2021), as to, as of such date, the fairness, from a financial point of view, to the Company of the Aggregate Consideration (defined in the opinion as 17,145,038 shares of Parent Class B Common Stock) to be paid by the Company for the Acquired Shares in the Merger pursuant to the Merger Agreement. After further consideration and discussion, the Holdings Special Committee unanimously resolved (i) that it was in the best interests of Holdings and its stockholders to enter into the Merger Agreement and (ii) to recommend to the Holdings board of directors that it authorize and approve the entry into the Merger Agreement and all such ancillary documents required to be entered into in connection therewith.
On June 14, 2021, following receipt of the recommendation of the Holdings Special Committee, the Holdings board of directors met in person and by teleconference to review the Merger. The Holdings board of directors discussed key aspects of the Merger, and was given an opportunity to review its fiduciary duties with Holdings’ counsel. Counsel for the Holdings Special Committee and representatives of Houlihan Lokey also attended the meeting in order to answer questions regarding the assistance provided to the Holdings Special Committee in its evaluation of the Merger. In connection with describing the recommendation of the Holdings Special Committee, Holdings’ counsel reviewed for the Holdings board of directors the independence criteria which the members of the Holdings Special Committee satisfied in order to serve (and continue to serve) on the Holdings Special Committee. After due consideration and discussion, the Holdings board of directors unanimously resolved (i) that it was advisable and fair to, and in the best interests of, Holdings and Holdings’ stockholders for Holdings to enter into the Merger Agreement, and accordingly that the entry into the Merger Agreement by any duly authorized officer of Holdings was approved;
Over the course of the next three days, the parties worked to collect fully executed Support Agreements and Lockup Agreements from significant stockholders of Pharma as a precondition to execution of the Merger Agreement.
On June 17, 2021, the respective parties executed the Merger Agreement, the Support Agreements, the Voting Agreement, the Lockup Agreements and the Altira Acquisition Agreement. Before the opening of trading on June 21, 2021, Holdings and Pharma issued a joint press release announcing the entry into the Merger Agreement, and Holdings filed a Current Report on Form 8-K with the executed Merger Agreement.
Company Structure — Diagrams
Upon consummation of the Transactions, Pharma is intended to become a direct, wholly-owned subsidiary of Parent. The following diagrams illustrate in simplified terms the current structure of Parent and Pharma and the expected structure of Parent following the consummation of the Transactions.
52
Table of Contents
Current Structure of the Companies

Post-Closing Structure

Holdings Recommendation and Reasons for the Transaction
At a meeting held on June 14, 2021, following receipt of the recommendation of the Holdings Special Committee, the Holdings board of directors met in person and by teleconference to review the Merger. The Holdings board, acting upon the recommendation of the Holdings Special Committee (i) determined that it was fair to and in the best interests of Holdings and its stockholders for Holdings to enter into the Merger Agreement, (ii) declared the Merger Agreement and the transactions contemplated thereby advisable, (iii) adopted the Merger Agreement and approved the execution, delivery and performance of the Merger Agreement and of certain ancillary agreements thereto agreement, (iv) resolved to recommend adoption of the Merger Agreement and approval of the Mergers and the other transactions contemplated thereby by the holders of shares of Parent Common Stock and (v) directed that the Merger Agreement be submitted to the holders of shares of Parent Common Stock entitled to vote for its adoption.
Reasons for Recommendation
In the course of reaching its recommendation, the Holdings board of directors considered the following material factors relating to the Agreements and the Transactions, each of which the Holdings board of directors believes supported its decision:
• The fact that, due to the use of the Per Share Merger Consideration exchange ratio fixed in the Merger Agreement, Holdings stockholders are expected to own a majority of Holdings immediately after consummation of the Mergers, Holdings stockholders would continue to participate in the future performance of the combined company, as well as maintain majority voting power over the combined company;
• The fact that the combined company is expected to have greater financial resources and access to capital markets to realize the full potential of its products;
53
Table of Contents
• The alternatives reasonably available to Holdings, including remaining as a substantial stockholder in Pharma or pursuing other strategic alternatives, which the Holdings board of directors evaluated with the assistance of the Holdings Special Committee;
• The fact that the Mergers will greatly simplify the capital structure of Pharma and the manner of Holdings’ interest therein, allowing the public markets to better understand and value Holdings’ investment in Pharma;
• The fact that the Transactions have the potential to deliver cost savings, synergies and operational efficiencies to the combined company;
• The recommendation of the Holdings Special Committee in favor of the Transactions;
• The fact that the Holdings Special Committee received the opinion of Houlihan Lokey rendered to it on June 14, 2021, as to the fairness, from a financial point of view, as of such date, to Holdings of the Aggregate Consideration (as defined in such opinion) to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement; and
• The Per Share Merger Consideration ratio under the Merger Agreement is fixed (i.e. it will not be adjusted for fluctuations in the market price of Parent Class B Common Stock), creating certainty as to the number of shares of Parent Class B Common Stock to be issued.
The Holdings board of directors considered these advantages and opportunities against a number of other factors identified in its deliberations weighing negatively against the Transactions, including:
• The difficulties of combining the businesses and workforces of Holdings and Pharma based on, among other things, the potential disruption associated with the Transactions and integrating the companies;
• The challenges inherent in the management and operation of Pharma, including the risk that integration costs may be greater than anticipated and may require greater than anticipated management attention and focus post-closing;
• the possibility that the anticipated benefits of the Transactions may not be realized, recognizing the many challenges associated with successfully integrating the businesses of Holdings and Pharma, including the risk of not capturing all of the anticipated cost savings, synergies and operational efficiencies;
• The possibility that the consummation of the Transactions might not occur, or might be delayed, despite the companies’ efforts, including by reason of a failure to obtain either the Parent Stockholder Approval or the Pharma Stockholder Approval;
• The risks and costs to Holdings in connection with the Transactions (including if the Transactions are not completed), either during the pendency of the Transactions or following the closing, including the risks and costs associated with the potential diversion of management and employee attention, potential employee attrition and the potential effect on business and supplier relationships;
• the potential that the fixed Per Share Merger Consideration exchange ratio could result in Holdings delivering greater value to Pharma stockholders than had been anticipated by Holdings should the value of shares of Holdings Common Stock increase disproportionately from the date of the Merger Agreement;
• assuming the consummation of the Transactions, the dilution to Holdings stockholders as stockholders of Holdings due to the issuance of all of the securities to be issued in connection with the Transactions; and
• various other risks associated with the Transactions and the businesses of Holdings and the combined company described in “Risk Factors” beginning on page 20 of this joint proxy statement/prospectus.
The foregoing discussion of the factors considered by the Holdings board of directors is not intended to be exhaustive, but, rather, includes the material factors considered by the Holdings board of directors. In reaching its
54
Table of Contents
decision to adopt and approve, and declare advisable, the Merger Agreement, the Mergers and the other transactions contemplated by the Merger Agreement, the Holdings board of directors did not quantify or assign any relative weights to the factors considered, and individual directors may have given different weights to different factors. The Holdings board of directors considered all these factors as a whole, including through discussions with, and questioning of, Holdings’ management and outside legal counsel, and, overall, considered these factors to be favorable to, and to support, its determination.
The Holdings board of directors concluded that the potentially negative factors associated with the Transactions were outweighed by the potential benefits that it expected Holdings stockholders would receive as a result of the Transactions, including the belief of the Holdings board of directors that the Transactions would maximize the value of Holdings’ existing stake in Pharma and of the Holdings Common Stock.
Pharma Recommendation and Reasons for the Transactions
After consideration, the Pharma board of directors adopted resolutions determining that the Merger Agreement, the Mergers contemplated by the Merger Agreement and the other transactions contemplated by the Merger Agreement were advisable, fair to and in the best interests of Pharma and its stockholders, adopting and approving the Merger Agreement and the transactions contemplated thereby, including the Mergers, and directing that the Merger Agreement be submitted to the Pharma Stockholders for their consideration. Pharma’s board of directors recommends that the Pharma Stockholders adopt and approve the Merger Agreement and the transactions contemplated thereby, including the Mergers.
In reaching its decision to adopt and approve, and declare advisable, the Merger Agreement and resolving to recommend that Pharma Stockholders adopt and approve the Merger Agreement and thereby approve the Mergers and the other transactions contemplated by the Merger Agreement, Pharma’s board of directors consulted with Pharma management, as well as its financial and legal advisors, and considered a number of factors, including its knowledge of Pharma’s business, operations, financial condition, earnings and prospects, and its knowledge of the financial and capital markets. Among the various factors that Pharma’s board of directors considered in favor of its decision are:
• The belief of Pharma’s board of directors, after review of alternative strategic opportunities from time to time, including strategic transactions with other partners and the possibility of, and benefits and risks associated with, continuing to operate Pharma as an independent, stand-alone entity, that the proposed Transactions represent the best potential transaction for Pharma to create greater value for Pharma’s stockholders, while also providing greater liquidity by owning stock in a public company.
• Pharma’s board of directors considered the terms and conditions of the Merger Agreement, including but not limited to the nature and scope of the closing conditions and the likelihood of obtaining any necessary regulatory approvals, in addition to the transactions contemplated thereby, including the Mergers.
• Pharma’s board of directors considered the amount of consideration to be received by the Pharma Stockholders in the proposed Mergers under the terms and conditions of the Merger Agreement.
• Pharma’s board of directors considered the fact that the combined company would provide Pharma’s stockholders with the opportunity to go forward with ownership in a public company with a larger market capitalization.
• Pharma’s board of directors considered the current industry trends and market conditions affecting the Company and the cost of alternative means of raising capital and the belief that the Transactions would be a more time- and cost-effective means to access capital than other options considered.
• Pharma’s board of directors belief that under new public ownership it will have the flexibility and financial resources to pursue and execute a growth strategy to increase stockholder value and will benefit from being publicly traded, and can effectively utilize the broader access to capital and public profile that are associated with being a publicly traded biotechnology company.
• Pharma’s board of directors considered that, pursuant to the Voting Agreements, entered into by Howard Jonas and certain other affiliates of Holdings, such persons agreed during the term of the Support Agreements to, among other things and upon the terms and conditions therein, vote or cause to be voted all of their respective (directly or indirectly held or otherwise beneficially owned) shares: (i) to adopt the
55
Table of Contents
Merger Agreement and approve the Merger and any of the other transactions contemplated by the Merger Agreement, (ii) against any action agreement or proposal (A) made in opposition to or in competition with the consummation of the issuance of the merger consideration or any of the other transactions contemplated by the Merger Agreement or (B) that would reasonably be expected to result in a breach of any covenant, representation or warranty of Holdings, and (iii) against any amendment of the certificate of incorporation or Holdings Bylaws or any other proposal, action or transaction involving Holdings or any of its subsidiaries that would impede, frustrate, prevent or materially delay the Merger and transactions related thereto.
• Pharma’s board of directors also considered that in connection with the execution of the Merger Agreement on June 17, 2021, certain key affiliates of Holdings and Pharma would enter into Lock-Up Agreements, including that the holders generally shall not be able to transfer Parent Common Stock for a period of six months following closing of the Business Combination.
Pharma’s board of directors also considered the following negative factors:
• Pharma’s board of directors considered the risk that the Business Combination might not be consummated in a timely manner or at all, due to a lack of stockholder approval or failure to satisfy various conditions to closing.
• Pharma’s board of directors considered the possibility that the Transactions might not be completed and that there may be an adverse effect on Pharma’s reputation, business and employees upon the public announcement of the agreement to enter into the Merger Agreement or in the event the Mergers are not completed.
• Pharma’s board of directors considered the expenses to be incurred in connection with the Transactions and related administrative challenges associated with combining the companies.
• Pharma’s board of directors considered the additional public company expenses and obligations that Pharma’s business will be subject to following the Closing that it has not previously been subject to as a private company.
• Pharma’s board of directors considered the fact that, although Pharma will continue to exercise, consistent with the terms and conditions of the Merger Agreement, control and supervision over its operations prior to the Closing, the Merger Agreement generally obligates Pharma, subject to the Parent’s prior consent (which consent may not be unreasonably withheld, conditioned or delayed), to conduct its business in the ordinary course of business consistent with past practice and in accordance with specified restrictions, which might delay or prevent Pharma from undertaking certain business opportunities that might arise pending Closing.
• Pharma board of directors considered the fact that certain executive officers and directors of Pharma have interests in the Transactions that may be different from, or in addition to, the interests of Pharma stockholders generally, including the manner in which they would be affected by the Transactions.
• Pharma board of directors considered various other risks associated with the Transactions, including the risks described in the section titled “Risk Factors.”
The foregoing discussion of the factors considered by Pharma’s board of directors is not intended to be exhaustive, but, rather, includes the material factors considered by Pharma’s board of directors. In reaching its decision to adopt and approve, and declare advisable, the Merger Agreement, the Mergers and the other transactions contemplated by the Merger Agreement, Pharma’s board of directors did not quantify or assign any relative weights to the factors considered, and individual directors may have given different weights to different factors. Pharma’s board of directors considered all these factors as a whole, including discussions with, and questioning of, Pharma’s management and financial and legal advisors, and, overall, considered these factors to be favorable to, and to support, its determination.
Pharma’s board of directors concluded that the potentially negative factors associated with the Transactions were outweighed by the potential benefits that it expected Pharma stockholders would receive as a result of the Transactions, including the belief of Pharma’s board of directors that the Transactions would maximize the immediate
56
Table of Contents
value of shares of Pharma Common Stock and Pharma Preferred Stock and eliminate the risk and uncertainty affecting the future prospects of Pharma, including the potential execution risks associated with an initial public offering of Pharma Common Stock and Pharma Preferred Stock and pursuing its business plan as a public company.
Opinion of Houlihan Lokey Capital, Inc.
On June 14, 2021, Houlihan Lokey verbally rendered its opinion to the Special Committee (which was subsequently confirmed in writing by delivery of Houlihan Lokey’s written opinion addressed to the Special Committee dated June 14, 2021), as to, as of such date, the fairness, from a financial point of view, to the Company of the Aggregate Consideration (as defined in the opinion) to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement.
Holdings advised, and directed Houlihan Lokey to assume, that the aggregate consideration issuable by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement would be no more than 17,145,038 shares of Parent Class B Common Stock (which was defined in Houlihan Lokey’s opinion as the “Aggregate Consideration”). Holdings further advised Houlihan Lokey, and Houlihan Lokey assumed, without independent verification, that (a) after giving effect to certain transactions involving the capital structure of Pharma (which was defined in Houlihan Lokey’s opinion as, collectively, the “Pre-Merger Steps”) and as of immediately prior to the consummation of the Initial Merger, the Acquired Shares would represent 49.3% of the total equity value of Pharma (which percentage was defined in Houlihan Lokey’s opinion as the “Acquired Shares Equity Ownership Percentage”) and (b) the Aggregate Consideration would represent 47.0% of the total equity value of Holdings as of immediately after the consummation of the Business Combination (taking into account certain planned equity incentive issuances (including the Mallik Issuance (as defined in the opinion)) but not taking into account any capital raise or other issuances in the interim or the Financing (as defined in the opinion)) (which percentage was defined in Houlihan Lokey’s opinion as the “Pro Forma Equity Ownership Percentage”).
For the purposes of Houlihan Lokey’s analyses and opinion, with the consent and approval of the Special Committee, Houlihan Lokey evaluated the fairness, from a financial point of view, to Holdings of the Aggregate Consideration to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement solely on the basis of a comparison of the Pro Forma Equity Ownership Percentage with implied reference ranges of percentages obtained by dividing (1) implied pre-Business Combination valuation reference ranges for the Acquired Shares (based on the Acquired Shares Equity Ownership Percentage and implied total equity value reference ranges for Pharma) by (2) the sum of implied pre-Business Combination total equity value reference ranges for Pharma plus certain assumed values for assets and liabilities of Holdings (excluding its interests in Pharma), in each case as Houlihan Lokey believed was indicated by its financial analyses of Pharma. No representation was made by Houlihan Lokey in its opinion, either directly or indirectly, as to any legal matter or as to the sufficiency of the basis set forth above for any general or particular purpose other than setting forth the scope of Houlihan Lokey’s opinion.
Houlihan Lokey’s opinion was directed to the Special Committee (in its capacity as such) and only addressed the fairness, from a financial point of view, to Holdings of the Aggregate Consideration to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement and did not address any other aspect or implication of the Business Combination or any other agreement, arrangement or understanding. The summary of Houlihan Lokey’s opinion in this prospectus and proxy statement is qualified in its entirety by reference to the full text of its written opinion, which is attached as Annex H to this prospectus/proxy statement and describes the procedures followed, assumptions made, qualifications and limitations on the review undertaken and other matters considered by Houlihan Lokey in connection with the preparation of its opinion. However, neither Houlihan Lokey’s opinion nor the summary of its opinion and the related analyses set forth in this prospectus and proxy statement are intended to be, and do not constitute, advice or a recommendation to the Special Committee, Holdings’s Board of Directors, Holdings, any security holder of the Company or any other person as to how to act or vote with respect to any matter relating to the Business Combination.
In arriving at its opinion, Houlihan Lokey, among other things:
1. reviewed the draft dated June 13, 2021 of the Merger Agreement;
2. reviewed certain publicly available business and financial information relating to Holdings that Houlihan Lokey deemed to be relevant;
57
Table of Contents
3. reviewed certain information relating to the historical, current and future operations, financial condition and prospects of Pharma and certain information relating to assets and liabilities of Holdings (excluding its interests in Pharma) made available to Houlihan Lokey by Holdings, including (a) probability-adjusted financial projections prepared by the management of Pharma and discussed with Holdings’ management relating to Pharma for the years ending 2021 through 2030 and (b) certain assumed values for assets and liabilities of Holdings (excluding its interests in Pharma) provided by or discussed with Holdings’ management;
4. reviewed certain information regarding the capitalization of Pharma after giving effect to the Pre-Merger Steps and as of immediately prior to the consummation of the Initial Merger and the capitalization of Holdings as of immediately after the consummation of the Business Combination;
5. spoke with certain members of the managements of Holdings and Pharma and certain representatives and advisors of Holdings regarding the businesses, operations, financial condition and prospects of Pharma and Holdings, the Business Combination and related matters;
6. compared certain financial data of Pharma with that of public companies that Houlihan Lokey deemed to be relevant;
7. considered publicly available financial terms of certain transactions that Houlihan Lokey deemed to be relevant;
8. reviewed the current and historical market prices and trading volume for Parent Class B Common Stock; and
9. conducted such other financial studies, analyses and inquiries and considered such other information and factors as Houlihan Lokey deemed appropriate.
Houlihan Lokey relied upon and assumed, without independent verification, the accuracy and completeness of all data, material and other information furnished, or otherwise made available, to it, discussed with or reviewed by it, or publicly available, and did not assume any responsibility with respect to such data, material and other information. In addition, the managements of Holdings and Pharma advised Houlihan Lokey, and Houlihan Lokey assumed, that the probability-adjusted financial projections and other estimates reviewed by Houlihan Lokey were reasonably prepared in good faith on bases reflecting the best currently available estimates and judgments of the managements of Holdings and Pharma, as the case may be, as to the future financial results and condition of Pharma and the other matters covered thereby, and Houlihan Lokey expressed no opinion with respect to the such projections or the assumptions on which they were based. Houlihan Lokey relied upon and assumed, without independent verification, that there had been no change in the businesses, assets, liabilities, financial condition, results of operations, cash flows or prospects of Pharma or Holdings since the respective dates of the most recent financial statements and other information, financial or otherwise, provided to Houlihan Lokey that would have been material to its analyses or opinion and that there was no information or any facts that would have made any of the information reviewed by Houlihan Lokey incomplete or misleading. Houlihan Lokey also relied upon, without independent verification, the assessments of the managements of Holdings and Pharma as to the product candidates and intellectual property of Pharma and Holdings and the validity of, and risks associated with, such product candidates and intellectual property (including, without limitation, the validity and life of patents or other intellectual property, the timing and probability of successful testing, development and commercialization of such product candidates and related indications, approval thereof by appropriate governmental authorities and the potential impact of generic competition), and Houlihan Lokey assumed, at the direction of Holdings, that there would be no developments with respect to any such matters that would affect Houlihan Lokey’s analyses or opinion.
In reaching its conclusions under Houlihan Lokey’s opinion, upon the advice of management of Holdings, Houlihan Lokey relied upon and assumed, without independent verification, that the information regarding the capitalizations of Pharma and Holdings referred to above reflected the material economic aspects of the capitalization of Pharma after giving effect to the Pre-Merger Steps and as of immediately prior to the consummation of the Initial Merger and the capitalization of Holdings as of immediately after the consummation of the Business Combination, that the Acquired Shares Equity Ownership Percentage reflected the correct allocation to the Acquired Shares of the total equity value of Pharma and that the Pro Forma Equity Ownership Percentage reflected the correct allocation to the Aggregate Consideration of the total equity value of Holdings. For purposes of Houlihan Lokey’s analyses and opinion, Houlihan Lokey did not apply any control premium, minority or illiquidity discounts or other premiums or
58
Table of Contents
discounts, or otherwise given effect to any rights, restrictions or limitations, that may be attributable to any security (including, without limitation, any capital stock) of Pharma, Holdings or any other party or blocks of such securities. Houlihan Lokey did not perform or rely upon separate financial analyses of the various classes of capital stock or any other securities of Pharma or Holdings, including, without limitation, any separate financial analyses of preferred stock based on a comparison of the terms thereof with those of debt or preferred equity securities of other companies or a separate discounted cash flow analysis based on potential future preferred dividends or otherwise. In addition, Houlihan Lokey did not consider any differences in the classes of the common stock of Holdings and, with the consent of the Special Committee, Houlihan Lokey relied upon and assumed, without independent verification, that shares of Parent Class B Common Stock are economically equivalent to shares of Class A Common Stock, par value $0.01 per share, of Holdings. With respect to assets and liabilities of Holdings (excluding its interests in Pharma), Houlihan Lokey relied, with the consent and approval of the Special Committee and without independent verification, upon certain assumed values for such assets and liabilities, and, except with respect to the royalty payments from Pharma to Altira, which is majority-owned by Holdings, Houlihan Lokey was not provided with financial projections relating to such assets and liabilities and, accordingly, Houlihan Lokey did not perform or rely upon separate financial analyses of such assets and liabilities.
Houlihan Lokey relied upon and assumed, without independent verification, that (a) the representations and warranties of all parties to the Merger Agreement and all other related documents and instruments that are referred to therein were true and correct, (b) each party to the Merger Agreement and such other related documents and instruments would fully and timely perform all of the covenants and agreements required to be performed by such party, (c) all conditions to the consummation of the Business Combination and related transactions would be satisfied without waiver thereof, and (d) the Business Combination and related transactions (including, among other things, the Pre-Merger Steps and the issuance to Ameet Mallik of restricted shares of Parent Class B Common Stock upon consummation of the Business Combination (the “Mallik Issuance”)) would be consummated in a timely manner in accordance with the terms described in the Merger Agreement and such other related documents and instruments and as otherwise described to Houlihan Lokey by representatives of Holdings, without any amendments or modifications thereto. Houlihan Lokey also assumed, with the consent of the Special Committee, that the Business Combination would qualify for the intended tax treatment described in the Merger Agreement for U.S. federal income tax purposes. Houlihan Lokey relied upon and assumed, without independent verification, that (i) the Business Combination and related transactions would be consummated in a manner that complies in all respects with all applicable federal, state and foreign statutes, rules and regulations, and (ii) all governmental, regulatory, and other consents and approvals necessary for the consummation of the Business Combination and related transactions would be obtained and that no delay, limitations, restrictions or conditions would be imposed or amendments, modifications or waivers made that would result in the disposition of any assets of Holdings or Pharma, or otherwise have an effect on the Business Combination, Holdings or Pharma, that would be material to Houlihan Lokey’s analyses or opinion. Houlihan Lokey also relied upon and assumed, without independent verification, at the direction of Holdings, that the actual number of shares of Parent Class B Common Stock issued as the aggregate consideration to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Agreement will not differ from the Aggregate Consideration that Houlihan Lokey was directed to assume in any respect that would be material to Houlihan Lokey’s analyses or opinion. In addition, Houlihan Lokey relied upon and assumed, without independent verification, that the final form of the Merger Agreement would not differ in any respect from the draft of the Merger Agreement identified above. Houlihan Lokey’s opinion noted that the Merger Agreement provides that the closing of the Initial Merger is conditioned upon financing activities by Holdings by way of a private placement and/or an underwritten public offering of capital stock of Holdings and/or the sale of real property owned by Holdings (which was defined in Houlihan Lokey’s opinion as the “Financing”) and Holdings’s acquisition of the minority interest in Altira not owned by Holdings (which was defined in Houlihan Lokey’s opinion as the “Altira Buyout”). For purposes of Houlihan Lokey’s analyses and opinion, with the consent and approval of the Special Committee, no effect was given to such transactions or any aspect, implication or effect thereof on Pharma, Holdings, the Pro Forma Equity Ownership Percentage or otherwise.
Furthermore, in connection with its opinion, Houlihan Lokey was not requested to make, and did not make, any physical inspection or independent appraisal or evaluation of any of the assets, properties or liabilities (fixed, contingent, derivative, off-balance-sheet or otherwise) of Holdings, Pharma or any other party, nor was Houlihan Lokey provided with any such appraisal or evaluation. With respect to the real estate properties of Holdings or Pharma, Houlihan Lokey expressed no opinion as to the value of any such real estate property, or the price at which any such real estate property may be transferable, at any time. Houlihan Lokey did not estimate, and expressed no opinion regarding, the liquidation value of any entity or business. Houlihan Lokey undertook no independent analysis of any potential
59
Table of Contents
or actual litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which Holdings or Pharma was or may have been a party or was or may have been subject, or of any governmental investigation of any possible unasserted claims or other contingent liabilities to which Holdings or Pharma was or may have been a party or was or may have been subject.
Houlihan Lokey was not requested to, and did not, (a) initiate or participate in any discussions or negotiations with, or solicit any indications of interest from, third parties with respect to the Business Combination or any related transaction, the securities, assets, businesses or operations of Holdings, Pharma or any other party, or any alternatives to the Business Combination or any related transaction, (b) negotiate the terms of the Business Combination or any related transaction, or (c) advise the Special Committee, the Board, Holdings or any other party with respect to alternatives to the Business Combination or any related transaction. Houlihan Lokey expressed no view or opinion as to any such matters, including the terms that could have been obtained if any of the foregoing had been undertaken. Representatives of the Special Committee and Holdings advised Houlihan Lokey, and Houlihan Lokey relied upon and assumed, without independent verification, that the terms of the Business Combination and related transactions were negotiated on an arms-length basis. Houlihan Lokey’s opinion was necessarily based on financial, economic, market and other conditions as in effect on, and the information made available to Houlihan Lokey as of, the date of the opinion. Houlihan Lokey did not undertake, and is under no obligation, to update, revise, reaffirm or withdraw its opinion, or otherwise comment on or consider events occurring or coming to Houlihan Lokey’s attention after the date of the opinion. The credit, financial and stock markets have been experiencing unusual volatility and Houlihan Lokey expressed no opinion or view as to any potential effects of such volatility on the Business Combination, Holdings or Pharma, and Houlihan Lokey’s opinion did not purport to address potential developments in any such markets. Houlihan Lokey was not expressing any opinion as to what the value of Parent Class B Common Stock actually would be when issued pursuant to the Business Combination or any related transaction or the price or range of prices at which Parent Class B Common Stock, any capital stock of Pharma or any other securities of Holdings or Pharma might be purchased or sold, or otherwise be transferable, at any time.
Houlihan Lokey’s opinion was furnished for the use of the Special Committee (in its capacity as such) in connection with its evaluation of the Business Combination and may not be relied upon by any other person or entity (including, without limitation, security holders, creditors or other constituencies of Holdings) or used for any other purpose without Houlihan Lokey’s prior written consent. Houlihan Lokey’s opinion should not be construed as creating any fiduciary duty on Houlihan Lokey’s part to any party. Houlihan Lokey’s opinion is not intended to be, and does not constitute, a recommendation to the Special Committee, the Board, Holdings, any security holder or any other party as to how to act or vote with respect to any matter relating to the Business Combination or any related transaction or otherwise.
Houlihan Lokey was not requested to opine as to, and its opinion did not express an opinion as to or otherwise address, among other things: (i) the underlying business decision of the Special Committee, the Board, Holdings, its security holders or any other party to proceed with or effect the Business Combination or any related transaction, (ii) the terms of any arrangements, understandings, agreements or documents related to, or the form, structure or any other portion or aspect of, the Business Combination (other than the Aggregate Consideration to the extent expressly specified in the opinion) or any related transaction or otherwise, including, without limitation, the form or structure of the Aggregate Consideration, the allocation thereof among the various classes of the Acquired Shares or any terms, aspects or implications of the Pre-Merger Steps, the Mallik Issuance, the Financing, the Altira Buyout or any agreements by Holdings with respect to its shares of the capital stock of Pharma in the event that the Business Combination is not consummated, (iii) the fairness of any portion or aspect of the Business Combination or any related transaction to the holders of any class of securities, creditors or other constituencies of Holdings, or to any other party, except to Holdings if and only to the extent expressly set forth in the last sentence of Houlihan Lokey’s opinion, (iv) the relative merits of the Business Combination or any related transaction as compared to any alternative business strategies or transactions that might have been available for Holdings or any other party, (v) the fairness of any portion or aspect of the Business Combination or any related transaction to any one class or group of Holdings’s, Pharma’s or any other party’s security holders or other constituents vis-à-vis any other class or group of Holdings’s, Pharma’s or such other party’s security holders or other constituents (including, without limitation, the allocation of any consideration amongst or within such classes or groups of security holders or other constituents), (vi) whether or not Holdings, Pharma, their respective security holders or any other party is receiving or paying reasonably equivalent value in the Business Combination or any related transaction, (vii) the solvency, creditworthiness or fair value of Pharma, Holdings or any other participant
60
Table of Contents
in the Business Combination or any related transaction, or any of their respective assets, under any applicable laws relating to bankruptcy, insolvency, fraudulent conveyance or similar matters, (viii) the fairness, financial or otherwise, of the amount, nature or any other aspect of any compensation to or consideration payable to or received by any officers, directors or employees of any party to the Business Combination or any related transaction, any class of such persons or any other party, relative to the Aggregate Consideration or otherwise, or (ix) any dilutive or other effects of any portion or aspect of the Business Combination or any related transaction (including, without limitation, the Mallik Issuance and the Financing) on the existing stockholders of Holdings or the financial or other implications and effects of the Business Combination or any related transaction on Holdings or any other party (including, without limitation, any aspects relating to ongoing operations of Holdings or Pharma following consummation of the Business Combination and related transactions). Furthermore, no opinion, counsel or interpretation was intended in matters that require legal, regulatory, accounting, insurance, tax or other similar professional advice. Houlihan Lokey assumed that such opinions, counsel or interpretations had been or would be obtained from the appropriate professional sources. Furthermore, Houlihan Lokey relied, with the consent of the Special Committee, on the assessments by the Special Committee, Holdings and their respective advisors as to all legal, regulatory, accounting, insurance, tax and other similar matters with respect to Pharma, Holdings and the Business Combination or otherwise.
In performing its analyses, Houlihan Lokey considered general business, economic, industry and market conditions, financial and otherwise, and other matters as they existed on, and could be evaluated as of, the date of its opinion. No company, transaction or business used in Houlihan Lokey’s analyses for comparative purposes is identical to Holdings or the proposed Business Combination and an evaluation of the results of those analyses is not entirely mathematical. The estimates contained in the financial forecasts prepared by the management of Holdings and the implied reference range values indicated by Houlihan Lokey’s analyses are not necessarily indicative of actual values or predictive of future results or values, which may be significantly more or less favorable than those suggested by the analyses. In addition, any analyses relating to the value of assets, businesses or securities do not purport to be appraisals or to reflect the prices at which businesses or securities actually may be sold, which may depend on a variety of factors, many of which are beyond the control of Holdings. Much of the information used in, and accordingly the results of, Houlihan Lokey’s analyses are inherently subject to substantial uncertainty.
Houlihan Lokey’s opinion was only one of many factors considered by the Special Committee in evaluating the proposed Business Combination. Neither Houlihan Lokey’s opinion nor its analyses were determinative of the Aggregate Consideration or of the views of the Special Committee, Holdings’ Board of Directors or Holdings’ management with respect to the Business Combination or the Aggregate Consideration. The type and amount of consideration payable in the Initial Merger were determined through negotiation between the Special Committee and Pharma, and the decision to enter into the Merger Agreement was solely that of the Special Committee and Holdings’ Board of Directors.
Financial Analyses
In preparing its opinion to the Special Committee, Houlihan Lokey performed a variety of analyses, including those described below. The summary of Houlihan Lokey’s analyses is not a complete description of the analyses underlying Houlihan Lokey’s opinion. The preparation of such an opinion is a complex process involving various quantitative and qualitative judgments and determinations with respect to the financial, comparative and other analytical methods employed and the adaptation and application of these methods to the unique facts and circumstances presented. As a consequence, neither Houlihan Lokey’s opinion nor its underlying analyses is readily susceptible to summary description. Houlihan Lokey arrived at its opinion based on the results of all analyses undertaken by it and assessed as a whole and did not draw, in isolation, conclusions from or with regard to any individual analysis, methodology or factor. While the results of each analysis were taken into account in reaching Houlihan Lokey’s overall conclusion with respect to fairness, Houlihan Lokey did not make separate or quantifiable judgments regarding individual analyses. Accordingly, Houlihan Lokey believes that its analyses and the following summary must be considered as a whole and that selecting portions of its analyses, methodologies and factors, without considering all analyses, methodologies and factors, could create a misleading or incomplete view of the processes underlying Houlihan Lokey’s analyses and opinion.
The following is a summary of the material financial analyses performed by Houlihan Lokey in connection with the preparation of its opinion and reviewed with the Special Committee on June 14, 2021. The order of the analyses does not represent relative importance or weight given to those analyses by Houlihan Lokey. The analyses summarized below include information presented in tabular format. The tables alone do not constitute a complete description of the
61
Table of Contents
analyses. Considering the data in the tables below without considering the full narrative description of the analyses, as well as the methodologies underlying, and the assumptions, qualifications and limitations affecting, each analysis, could create a misleading or incomplete view of Houlihan Lokey’s analyses.
Unless the context indicates otherwise, equity values and enterprise values (generally, the value as of a specified date of the relevant company’s outstanding equity securities (taking into account outstanding options and other securities convertible, exercisable or exchangeable into or for equity securities of the company) plus the amount of its net debt (the amount of its outstanding indebtedness, non-convertible preferred stock, capital lease obligations and non-controlling interests less the amount of cash and cash equivalents on its balance sheet)) used in the selected companies analysis described below were calculated using the closing price of the common stock of the selected companies listed below as of June 11, 2021, and implied enterprise values or transaction values for the selected transactions analysis described below were calculated based on the value of the proposed consideration in the selected transactions. The estimates of the future financial performance of Pharma and the royalty payments from Pharma to Altira relied upon for the discounted cash flow financial analyses described below were based on financial projections prepared by the management of Pharma relating to Pharma (including with respect to the royalty payments from Pharma to Altira) for the years 2021 through 2030.
Selected Companies Analysis. Houlihan Lokey reviewed, among other things, enterprise values for selected companies, with publicly traded equity securities, that Houlihan Lokey deemed relevant.
The selected companies and the resulting 25th percentile, mean, median and 75th percentile data for the selected companies were as follows:
• Arcus Biosciences, Inc.
• ImmunoGen, Inc.
• Infinity Pharmaceuticals, Inc.
• Iovance Biotherapeutics, Inc.
• Mersana Therapeutics, Inc.
• SpringWorks Therapeutics, Inc.
• ZIOPHARM Oncology, Inc.
• Zymeworks, Inc.
| | Enterprise
Value
(in millions) |
25% | | $ | 739.0 |
Mean | | | 1,441.0 |
Median | | | 977.9 |
75% | | | 1,747.6 |
Taking into account the results of the selected companies analysis, Houlihan Lokey applied a range of implied enterprise values of $650.0 million to $900.0 million for the clinical assets of Pharma. Adding a range of assumed values of $12.0 million to $27.0 million for the preclinical assets of Pharma described below under the heading “Analysis of Selected Licensing Agreements” and subtracting the net debt of Pharma based on information as provided by the management of Pharma, the selected companies analysis indicated an implied total equity value reference range of Pharma of approximately $653.4 million to $918.4 million.
Houlihan Lokey then compared the Pro Forma Equity Ownership Percentage of 47.0% with the implied reference range of percentages of 48.6% to 48.2% obtained by dividing (1) an implied pre-Business Combination valuation reference range for the Acquired Shares of approximately $401.1 million to $531.7 million (based on the Acquired Shares Equity Ownership Percentage of 49.3% and the sum of the implied total equity value reference range for Pharma of approximately $653.4 million to $918.4 million and the assumed cash proceeds from the exercise of warrants and options of Pharma) by (2) an implied combined valuation reference range of approximately $824.6 million to $1,103.3 million (based on the sum of the implied total equity value reference range for Pharma of approximately
62
Table of Contents
$653.4 million to $918.4 million, certain assumed values for assets and liabilities of Holdings (excluding its interests in Pharma but including, among other values, a range of implied values of approximately $52.2 million to $60.0 million for Holdings’ ownership interest in Altira described below under the heading “Analysis of Altira Interest”) and the assumed cash proceeds from the exercise of warrants and options of Pharma not directly or indirectly held by Holdings and from the exercise of in-the-money warrants and options of Holdings).
Selected Transactions Analysis. Houlihan Lokey considered certain financial terms of certain transactions involving target companies or licenses that Houlihan Lokey deemed relevant, which included, among other things, implied enterprise values based on the upfront consideration paid.
The selected transactions and the resulting 25th percentile, mean, median and 75th percentile data for the selected transactions were as follows:
Date Announced | | Target/Licensee | | Acquiror/Licensor |
3/4/2021 | | Five Prime Therapeutics, Inc.me | | Amgen, Inc. |
12/9/2019 | | ArQule, Inc. | | Merck Sharpe & Dohme Corp. |
5/21/2019 | | Peloton Therapeutics, Inc. | | Merck & Co., Inc. |
10/18/2018 | | Endocyte, Inc. | | Novartis AG |
5/10/2018 | | Armo BioSciences, Inc. | | Eli Lilly and Co. |
1/31/2018 | | Cascadian Therapeutics, Inc. | | Seattle Genetics Inc. |
12/2/2017 | | Ignyta, Inc. | | Roche Holdings, Inc. |
5/31/2016 | | Celator Pharmaceuticals, Inc. | | Jazz Pharmaceuticals plc |
8/24/2015 | | BioMaren Pharmaceuticals, Inc. | | Medivation |
4/24/2015 | | AstraZeneca plc/Medimmune | | Celgene Corp. |
9/28/2014 | | Ambit Biosciences Corp. | | Daiichi Sankyo Co. Ltd. |
| | Enterprise
Value based
on Upfront
Consideration
(in millions) |
25% | | $ | 489.9 |
Mean | | | 1,202.1 |
Median | | | 1,462.6 |
75% | | | 1,704.9 |
Taking into account the results of the selected transactions analysis, Houlihan Lokey applied a range of implied enterprise values of $700.0 million to $1,050.0 million for the clinical assets of Pharma. Adding a range of assumed values of $12.0 million to $27.0 million for the preclinical assets of Pharma described below under the heading “Analysis of Selected Licensing Agreements” and subtracting the net debt of Pharma based on information as provided by the management of Pharma, the selected transactions analysis indicated an implied total equity value reference range of Pharma of approximately $703.4 million to $1,068.4 million.
Houlihan Lokey then compared the Pro Forma Equity Ownership Percentage of 47.0% with the implied reference range of percentages of 48.6% to 48.3% obtained by dividing (1) an implied pre-Business Combination valuation reference range for the Acquired Shares of approximately $425.8 million to $605.6 million (based on the Acquired Shares Equity Ownership Percentage of 49.3% and the sum of the implied total equity value reference range for Pharma of approximately $703.4 million to $1,068.4 and the assumed cash proceeds from the exercise of warrants and options of Pharma) by (2) an implied combined valuation reference range of approximately $875.2 million to $1,253.3 million (based on the sum of the implied total equity value reference range for Pharma of approximately $703.4 million to $1,068.4, certain assumed values for assets and liabilities of Holdings (excluding its interests in Pharma but including, among other values, a range of implied values of approximately $52.2 million to $60.0 million for Holdings’s ownership interest in Altira described below under the heading “Analysis of Altira Interest”) and the assumed cash proceeds from the exercise of warrants and options of Pharma not directly or indirectly held by Holdings and from the exercise of in-the-money warrants and options of Holdings).
63
Table of Contents
Discounted Cash Flow Analysis. Houlihan Lokey performed a discounted cash flow analysis of Pharma by calculating the estimated net present value of the projected unlevered free cash flows of Pharma based on the financial projections prepared by the management of Pharma relating to Pharma for the years ending 2021 through 2030. Houlihan Lokey calculated terminal values for Pharma by applying a range of negative perpetuity growth rates of -40.0% to -20.0% to Pharma’s calendar year 2030 estimated unlevered free cash flow. The net present values of Pharma’s projected future cash flows and terminal values were then calculated using discount rates ranging from 13.0% to 18.0%. These calculations resulted in a range of implied enterprise values of approximately $1,106.3 million to $1,605.7 million for the clinical assets of Pharma. Adding a range of assumed values of $12.0 million to $27.0 million for the preclinical assets of Pharma described below under the heading “Analysis of Selected Licensing Agreements” and subtracting the net debt of Pharma based on information as provided by the management of Pharma, the discounted cash flow analysis indicated an implied total equity value reference range of Pharma of approximately $1,109.4 million to $1,624.4 million.
Houlihan Lokey then compared the Pro Forma Equity Ownership Percentage of 47.0% with the implied reference range of percentages of 48.8% to 48.5% obtained by dividing (1) an implied pre-Business Combination valuation reference range for the Acquired Shares of approximately $625.8 million to $879.6 million (based on the Acquired Shares Equity Ownership Percentage of 49.3% and the sum of the implied total equity value reference range for Pharma of approximately $1,109.4 million to $1,624.4 million and the assumed cash proceeds from the exercise of warrants and options of Pharma) by (2) an implied combined valuation reference range of approximately $1,281.2 million to $1,814.1 million (based on the sum of the implied total equity value reference range for Pharma of approximately $703.4 million to $1,068.4, certain assumed values for assets and liabilities of Holdings (excluding its interests in Pharma but including, among other values, a range of implied values of approximately $52.2 million to $60.0 million for Holdings’s ownership interest in Altira described below under the heading “Analysis of Altira Interest”) and the assumed cash proceeds from the exercise of warrants and options of Pharma not directly or indirectly held by Holdings and from the exercise of in-the-money warrants and options of Holdings).
Analysis of Selected Licensing Agreements. In performing the financial analyses described above, Houlihan Lokey included a range of assumed values of $12.0 million to $27.0 million for the preclinical assets of Pharma. Houlihan Lokey considered certain financial terms of 193 licensing transactions involving preclinical assets announced between January 2009 and May 2021, which included, among other things, risk-adjusted transaction values based on the upfront consideration paid and the probability-weighted estimated net present value of contingent consideration assumed to be between 5% and 10%.
The resulting low, 25th percentile, mean, median, 75th percentile and high data for the selected transactions were as follows (with the selected range indicated in bold):
| | | | Risk-Adjusted Transaction Value
(in millions) |
| | | Upfront
Consideration
(in millions) | | Inclusive
of 5%
of Contingent Consideration | | Inclusive of
10% of
Contingent
Consideration |
Low | | $ | 0.1 | | $ | 0.1 | | $ | 0.1 |
25% | | | 4.3 | | | 9.3 | | | 14.4 |
Mean | | | 12.0 | | | 27.0 | | | 42.0 |
Median | | | 32.2 | | | 54.9 | | | 77.5 |
75% | | | 37.0 | | | 62.0 | | | 87.0 |
High | | | 400.0 | | | 539.0 | | | 678.0 |
Analysis of Altira Interest. In performing the financial analyses described above, Houlihan Lokey included a range of implied values of approximately $52.2 million to $60.0 million for Holdings’ ownership interest in Altira. Houlihan Lokey considered the implied value of Holdings’ 66.67% ownership interest in Altira of $60.0 million based on an assumed purchase price for the 33.3% ownership interest not owned by Holdings of $30.0 million provided by Holdings’s management. Houlihan Lokey also performed a discounted cash flow analysis of Altira by calculating the estimated net present value of the projected royalty payments from Pharma to Altira based on the financial projections prepared by the management of Pharma relating to Pharma for the years ending 2021 through 2030. Houlihan Lokey calculated terminal values for the royalty payments by applying a range of negative perpetuity growth rates of -40.0% to -20.0% to the estimated calendar year 2030 royalty payments. The net present values of projected royalty payments
64
Table of Contents
and terminal values were then calculated using discount rates ranging from 13.0% to 18.0%. The discounted cash flow analysis indicated an implied equity value reference range for Altira of approximately $67.0 million to $94.3 million. Based on Holdings’s 66.67% ownership interest in Altira and the midpoints of the foregoing negative perpetuity growth rate and discount rate ranges applied by Houlihan Lokey, the discounted cash flow analysis resulted in an implied equity value of approximately $52.2 million for Holdings’s ownership interest in Altira.
Other Matters
Houlihan Lokey was engaged by the Special Committee to provide certain financial advisory services, including an opinion to the Special Committee as to the fairness, from a financial point of view, to Holdings of the Aggregate Consideration to be paid by Holdings for the Acquired Shares in the Initial Merger pursuant to the Merger Agreement. The Special Committee engaged Houlihan Lokey based on Houlihan Lokey’s experience and reputation. Houlihan Lokey is regularly engaged to render financial opinions in connection with mergers, acquisitions, divestitures, leveraged buyouts, and for other purposes. Pursuant to its engagement by the Special Committee, Houlihan Lokey is entitled to an aggregate fee of $450,000 for its services, a portion of which became payable upon the execution of Houlihan Lokey’s engagement letter and the balance of which became payable upon the delivery of Houlihan Lokey’s opinion. No portion of Houlihan Lokey’s fee is contingent upon the successful completion of the Business Combination. Holdings has also agreed to reimburse Houlihan Lokey for certain expenses and to indemnify Houlihan Lokey, its affiliates and certain related parties against certain liabilities and expenses, including certain liabilities under the federal securities laws, arising out of or related to Houlihan Lokey’s engagement.
In the ordinary course of business, certain of Houlihan Lokey’s employees and affiliates, as well as investment funds in which they may have financial interests or with which they may co-invest, may acquire, hold or sell, long or short positions, or trade, in debt, equity, and other securities and financial instruments (including loans and other obligations) of, or investments in, Holdings, Pharma or any other party that may be involved in the Business Combination or any related transaction and their respective affiliates or security holders or any currency or commodity that may be involved in the Business Combination or any related transaction.
Houlihan Lokey and certain of its affiliates may provide investment banking, financial advisory and/or other financial or consulting services to Holdings, other participants in the Business Combination or any related transaction or certain of their respective affiliates or security holders in the future, for which Houlihan Lokey and its affiliates may receive compensation. Furthermore, in connection with bankruptcies, restructurings, distressed situations and similar matters, Houlihan Lokey and certain of its affiliates may have in the past acted, may currently be acting and may in the future act as financial advisor to debtors, creditors, equity holders, trustees, agents and other interested parties (including, without limitation, formal and informal committees or groups of creditors) that may have included or represented and may include or represent, directly or indirectly, or may be or have been adverse to Holdings, other participants in the Business Combination or any related transaction or certain of their respective affiliates or security holders, for which advice and services Houlihan Lokey and its affiliates have received and may receive compensation.
65
Table of Contents
THE MERGER AGREEMENT
The following is a summary of the material terms and conditions of the Merger Agreement. This summary may not contain all of the information about the Merger Agreement that is important to you. This summary is qualified by reference to the complete text of the Merger Agreement, which is attached as Annex A to, and incorporated by reference into, this proxy statement/prospectus. In the event there is a conflict between the Merger Agreement and the summary in this proxy statement/prospectus, the Merger Agreement shall govern. You are encouraged to read the full text of the Merger Agreement because it is the legal document that governs the Merger.
Explanatory Note Regarding the Merger Agreement and the Summary of the Merger Agreement: Representations, Warranties and Covenants in the Merger Agreement Are Not Intended to Function or Be Relied on as Public Disclosures
The Merger Agreement and the summary of its terms in this proxy statement/prospectus have been included to provide information about the terms and conditions of the Merger Agreement. The representations, warranties, covenants and agreements contained in the Merger Agreement were made by Parent, Company and Merger Subs as of specific dates and were qualified and subject to certain limitations and exceptions agreed to by Parent, Company and Merger Subs in connection with negotiating the terms of the Merger Agreement. In particular, in your review of the representations and warranties contained in the Merger Agreement and described in this summary, it is important to bear in mind that the representations and warranties were negotiated for the purpose of allocating contractual risk among the parties to the Merger Agreement rather than to establish matters as facts. The representations and warranties also may be subject to a contractual standard of materiality or material adverse effect that is different from what may be viewed as material by stockholders or stockholders, as applicable, or other investors and from the materiality standard applicable to reports and documents filed with the SEC and in some cases may be qualified by disclosures made by one party to the other, which are not reflected in the body of the Merger Agreement. Moreover, information concerning the subject matter of the representations and warranties, which do not purport to be accurate as of the date of this proxy statement/prospectus, may have changed since the date of the Merger Agreement.
For the foregoing reasons, the Merger Agreement should not be read alone, but should instead be read in conjunction with the other information regarding the Merger Agreement, the Merger, Parent, Company and Merger Subs and their respective affiliates and businesses, which is contained in or incorporated by reference into this proxy statement/prospectus, as well as in the Forms 10-K, Forms 10-Q and other filings that Parent has made or will make with the SEC.
Structure of the Merger
The Merger Agreement provides for, among other things, the following shall occur: at the Effective Time, Merger Sub I will merge with and into Pharma, and Pharma (as the surviving company of the merger with Merger Sub I) will merge with and into Merger Sub II with Merger Sub II being the surviving entity of the subsequent merger. As a result of the Mergers, it is intended that Pharma is intended to become a wholly-owned limited liability company subsidiary of Parent (the “Business Combination”). At the Subsequent Merger Effective Time, the limited liability company agreement of Merger Sub II will be amended and restated to be the certificate of formation and limited liability company agreement of the surviving entity.
The directors and officers of the Initial Surviving Corporation immediately after the Effective Time and the managers and officers of the Surviving Company immediately after the Subsequent Merger Effective Time, shall be the respective individuals listed in a schedule to the Merger Agreement (as may be amended from time to time), each to hold office in accordance with the applicable governing documents and applicable law, until such director’s or officer’s successor is duly elected or appointed and qualified, or until the earlier of their death, resignation or removal.
Following the Business Combination, Pharma will have a single class of equity authorized and outstanding all of which is intended to be held by Holdings. For U.S. federal income tax purposes, the Mergers are intended to qualify as a “reorganization” within the meaning of Section 368(a)(1) of the Internal Revenue Code of 1986, as amended.
Merger Consideration
As a consequence of the Mergers, at the Effective Time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist, and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Parent Class B Common Stock per share of Pharma capital stock.
66
Table of Contents
This Per Share Merger Consideration ratio for each class of Pharma capital stock is currently anticipated to result in prior holders of Pharma capital stock and contingent interests receiving Parent Class B Common Stock amounting to approximately 44% of the outstanding capitalization of Holdings following the Mergers based on the currently outstanding capitalization, including after giving effect to the issuance of the “Holdings Financing. Additional equity in Parent may be issued in connection with one or more financings and pursuant to employment agreements (which will reduce the foregoing percentage proportionately).
No fractional shares of Parent Common Stock will be issued in connection with the Merger, and holders of Pharma Common Stock who would otherwise be entitled to receive a fraction of a share of Parent Common Stock, shall, in lieu of any such fractional shares to which they would otherwise be entitled, receive a payment in cash determined by multiplying such fraction by the volume weighted average closing sale price of one share of Parent Class B Common Stock as reported on the NYSE for the ten consecutive trading day period ending the fifth trading day prior to the Closing Date.
The Merger Agreement provides that certain key stockholders of Parent and of Pharma that, in the latter case, will receive Parent Common Stock in the Business Combination have agreed to enter into a lock-up agreement, which contains certain restrictions on transfer of such Parent Common Stock for a period of six months following closing of the Business Combination.
The consideration payable by Holdings took into account the value of the Holdings’ assets, including its and its subsidiaries’ interests in Pharma. Other holders of warrants to purchase Pharma stock may elect to exercise their portion of the Warrant prior to the Closing Date. Any other unexercised warrants shall be terminated at the Effective Time in accordance with the terms of the Merger Agreement; provided that it is intended that (i) any Pharma Series C Warrants, to the extent not exercised by its holder prior to the Effective Time, is intended to be exchanged for a commensurate warrant to purchase shares of Parent or otherwise be settled by Parent and (ii) the Common Warrant Option, to the extent not exercised by its holder prior to the Effective Time, is intended to be exchanged for a commensurate warrant to purchase shares of Parent or otherwise be settled by Parent.
In connection with the Mergers, the contractual right, subject to its terms, to receive additional shares of Pharma capital stock equal to 10% of the diluted Pharma capital stock that is held by an instrument that is within a trust for the benefit of the children of Holdings’ Chairman Howard Jonas will also be satisfied by the issuance of Pharma capital stock immediately prior to the effective time of the Mergers, and the holder of the right shall receive the Merger Consideration that would be due to a holder of the shares of Pharma Common Stock that are subject of that right. The Merger Agreement provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable as Bonus Shares shall be calculated as of September 30, 2021, as if the Mergers were consummated on such date.
At the Effective Time, all compensatory options to purchase Pharma Common Stock shall automatically vest in full, and all holders of such options will receive options to purchase Class B common stock of Holdings using the ratios applicable to determining the Holdings stock issuable in the Business Combination.
Closing
The closing of the Merger will occur no later than the second business day following the satisfaction or waiver of the conditions set forth in the Merger Agreement (except for any conditions that by their terms are to satisfied at the Closing, but subject to the satisfaction of such conditions at the Closing or waiver by the party entitled to waive such conditions).
Effective Time
The Mergers will become effective upon the date and time of the filing of certificates of merger relating to the Mergers with the Secretary of State of the State of Delaware, or at such later time as is permissible under the DGCL and as may be mutually agreed in writing by Pharma and Parent and specified in each Certificate of Merger for each Merger. We refer to the Initial Merger Effective Time as the “Effective Time” throughout this proxy statement/prospectus.
67
Table of Contents
Exchange and Payment Procedures
Prior to the Closing Date, Parent will engage a reputable entity to act as exchange agent in the Merger (the “Exchange Agent”). No later than the Closing Date, Parent will deposit with the Exchange Agent (i) certificates or evidence of book-entry shares representing the Parent Class B Common Stock (provided that any evidence of book entry shares to be issued in respect of Pharma Restricted Stock (to the extent consistent with the terms of such shares of Pharma Restricted Stock) will be subject to trading restrictions and a substantial risk of forfeiture under the terms of the applicable restricted stock agreements) and (ii) cash sufficient to make payments in lieu of fractional shares.
Promptly after the Exchange Agent is retained by Parent, the Exchange Agent will mail to the holders of Company Capital Stock immediately prior to the Effective Time (including the former Company Noteholders after giving effect to cancellation and conversion of the Company Convertible Notes immediately prior to the Effective Time): (i) a letter of transmittal (“Letter of Transmittal”), in substantially the form agreed by Company and Parent and attached to the Merger Agreement, which includes a release in favor of Pharma and Parent, confidentiality and other provisions on which Pharma and Parent will rely and (ii) instructions for use in effecting the surrender of Company Stock Certificates (as defined below) or book-entry shares in exchange for the Parent Common Stock and, in respect of fractional amounts, cash amounts payable in accordance with the Merger Agreement. Upon surrender of a Company Stock Certificate and delivery of a completed and duly executed Letter of Transmittal to the Exchange Agent for payment, (A) the holder of such Company Stock Certificate shall be entitled to receive in exchange therefor the applicable Per Share Merger Consideration for each share evidenced by such Company Stock Certificate or book-entry shares determined pursuant to the conversion of shares provision under the Merger Agreement, within 10 Business Days of such surrender and delivery, and (B) the Company Stock Certificate or book-entry shares so surrendered shall be canceled. No holder of any Company Capital Stock or any instruments convertible into Company Capital Stock shall be entitled to receive any of the consideration in accordance with the preceding sentence without returning the completed and duly executed Letter of Transmittal. If any Company Stock Certificate shall have been lost, stolen or destroyed, Parent may, as a condition to the payment of the consideration thereunder, require the owner of such Company Stock Certificate to provide a reasonably appropriate affidavit to Parent (which may include an indemnity or bond in customary form).
Rights of Company Stockholders Following the Effective Time
At the Effective Time all shares of Company Capital Stock outstanding immediately prior to the Effective Time shall automatically be canceled and retired and shall cease to exist, and all holders of certificates representing shares of Company Capital Stock that were outstanding immediately prior to the Effective Time shall cease to have any rights as Company Stockholders, and each certificate representing any such Company Capital Stock (a “Company Stock Certificate”) shall thereafter represent the right to receive the applicable Per Share Merger Consideration, if any.
Transfers Following the Effective Time
In addition, at the Effective Time, the stock transfer books of Pharma shall be closed with respect to all shares of Company Capital Stock outstanding immediately prior to the Effective Time. No further transfer of any such shares of Company Capital Stock shall be made on such stock transfer books after the Effective Time. If, after the Effective Time, a Company Stock Certificate, including any valid certificate representing any shares of Pharma Preferred Stock previously converted into shares of Company Common Stock outstanding immediately prior to the Effective Time, is presented to the Exchange Agent or to the Surviving Company or Parent, such Company Stock Certificate shall be canceled and is intended to be exchanged pursuant to the terms of the Merger Agreement.
Withholding Rights
Each of Parent, Pharma, the Exchange Agent, and their respective agents (each a “Withholding Agent”) will be entitled to deduct and withhold from any amount payable to any person under the Merger Agreement or any other documents associated with the transaction, the amounts such Withholding Agent is required to deduct and withhold under the Internal Revenue Code of 1986, as amended (the “Code”), or any other applicable Law. To the extent that amounts are so withheld and paid over to the applicable Governmental Body, such withheld amounts will be treated as having been paid to the applicable Person in respect of whom such amounts were withheld.
68
Table of Contents
Treatment of Company Warrants, Company Options, Company Convertible Notes and Contingent Rights.
All Company Warrants are intended to terminate at the Effective Time as contemplated by and in accordance with the Merger Agreement and the applicable Support Agreement is intended to be exchanged for a commensurate warrant to purchase shares of Parent or otherwise be settled by Parent and the Common Warrant Option, to the extent not exercised by its holder prior to the Effective Time, is intended to be exchanged for a commensurate warrant to purchase shares of Parent or otherwise be settled by Parent.
All holders of Company Options (“Converted Options”) held by employees or independent contractors of Pharma as of immediately prior to the Closing Date will, to the extent then unvested, vest in full immediately prior to the Closing Date. All holders of Converted Options will receive options to purchase shares of Parent Class B Common Stock as contemplated under the Merger Agreement; provided that the adjustments provided under the Merger Agreement with respect to any Company Options are intended to be effected in a manner that is consistent with Sections 424(a) and 409A of the Code (such replacement options, the “Parent Options”). The number of shares of Parent Class B Common Stock underlying the Parent Options will be determined by multiplying (A) the number of shares of Company Common Stock that were subject to each such Converted Option held by such holder, as in effect immediately prior to the Effective Time, by (B) the Per Common Share Merger Consideration ratio, and rounding the resulting number down to the nearest whole number of shares of Parent Class B Common Stock. The per share exercise price for the Parent Options will be determined by dividing (x) the per share exercise price of such Converted Options held by such holder, as in effect immediately prior to the Effective Time, by (y) the Per Common Share Merger Consideration ratio and rounding the resulting exercise price up to the nearest whole cent. Any other restriction on the exercise of the Converted Options (excluding any vesting restriction) will continue in full force and effect in the corresponding Parent Options. Subject to certain exceptions in the Merger Agreement, the terms of the Converted Options will remain unchanged and the terms of the Converted Options will remain unchanged.
The parties intend that the Company Convertible Notes will not be assumed or continued by Parent or Pharma in connection with the Merger or the other transactions contemplated by the Merger Agreement. Immediately prior to the Effective Time, Parent and Pharma shall use their commercially reasonable efforts to cause each Company Convertible Note to be cancelled and extinguished and converted into the applicable Note Conversion Shares, in accordance with the terms of the Convertible Note Cancellation Agreement, to be executed and delivered by each Company Noteholder prior to the Closing.
Upon satisfaction (or waiver) of certain of the conditions precedent in the Merger Agreement, and as of immediately prior to the Effective Time, shares of Company Capital Stock will be issued to the holders of the Contingent Rights (as reflected in the Closing Payment Schedule (as defined in the Merger Agreement)) in accordance with their respective terms. At the Effective Time, such shares of Company Capital Stock shall be converted in accordance with the conversion of shares provisions in the Merger Agreement into the Per Line of Credit Share Merger Consideration and Per Bonus Share Merger Consideration, as applicable, or, to the extent applicable pursuant to the Merger Agreement, canceled and retired for no consideration.
No Appraisal Rights
No dissenters’ or appraisal rights will be available with respect to the Merger or the other transactions contemplated by the Merger Agreement.
Listing of the Merger Shares and Registration
Approval of listing on NYSE of the Merger Shares is a condition to the obligation to complete the Merger as described in “The Merger Agreement — Conditions to the Merger” beginning on page 77 of this proxy statement/prospectus.
Representations and Warranties
Holdings, Pharma and the Merger Subs each made representations and warranties in the Merger Agreement for the benefit of the other parties. The representations and warranties of the parties are subject, in some cases, to specified exceptions and qualifications contained in confidential disclosure schedules and are also qualified by materiality and material adverse effect and certain documents filed by the parties with the SEC.
69
Table of Contents
For Pharma, the representations and warranties in the Merger Agreement relate to, among other things:
• Due incorporation, etc.;
• certificate of incorporation and bylaws; certificates of designation;
• capitalization;
• financial statements;
• absence of certain changes;
• title to assets;
• real property; leasehold;
• intellectual property;
• material contracts;
• liabilities;
• compliance with laws; permits;
• certain business practices;
• tax matters;
• employee benefit plans;
• employee matters;
• environmental matters;
• insurance;
• legal proceedings; orders;
• authority; binding nature of agreement;
• vote required;
• counterparties;
• non-contravention; consents;
• financial advisor;
• related party transactions;
• regulatory matters;
• disclosure; and
• no additional representations.
For Parent and Merger Subs, the representations and warranties in the Merger Agreement relate to, among other things:
• due incorporation, etc.;
• capitalization, etc.;
• authority; binding nature of agreement;
• absence of certain changes;
70
Table of Contents
• legal proceedings;
• SEC Reports, Financial Statements;
• liabilities;
• compliance with laws;
• subsidiaries; merger subs;
• valid issuance;
• financing;
• takeover statutes;
• ownership of capital stock of Pharma;
• tax matters; and
• no additional representation.
None of the representations and warranties in the Merger Agreement or in any schedule, certificate, instrument or other document pursuant to the Merger Agreement shall survive the Effective Time.
Conduct of Business of Company
The Merger Agreement provides that, during the Pre-Closing Period, subject to limited exceptions, (i) Pharma shall operate in the Ordinary Course of Business and in compliance with applicable Law; (ii) Pharma shall use commercially reasonable efforts to (A) preserve substantially intact its present business organization, (B) preserve its material relationships with suppliers, distributors, licensors, licensees and others to whom Pharma has contractual obligations, (C) prosecute and maintain the Patents licensed by Pharma and other material Company Registered Intellectual Property (including any Patents), and (D) file all Company Tax Returns and pay all Taxes when due (except for Taxes being contested in good faith in appropriate proceedings for which adequate reserves have been have been established in accordance with GAAP, with respect to which Parent has been notified in advance in writing). The Merger Agreement also expressly restricts the ability of Pharma to take the following actions without the consent of Holdings, subject to limited exceptions:
• change or amend Pharma Charter, the Certificates of Designation or Pharma’s bylaws or authorize or propose the same;
• directly or indirectly split, combine or reclassify any of its capital stock (except in connection with the conversion of Pharma Preferred Stock to Company Common Stock, or the exercise of any Company Warrants or Company Options); issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock; declare, set aside, or pay any dividend or make any distribution (whether in cash or in kind) with respect to any of its capital stock, membership interest or other equity interests (as applicable) or redeem, purchase, or otherwise acquire, directly or indirectly, any of its capital stock, membership interests or other equity interests (as applicable);
• issue, deliver, transfer or sell, or authorize to issue, deliver, transfer or sell, any shares of Company Capital Stock or securities convertible into, or subscriptions, rights, calls, conversion rights, warrants or options to acquire, or other agreements or commitments of any character obligating it to issue any such shares or other convertible securities, or authorize or propose any change in its equity capitalization or capital structure; provided, however, that (i) Pharma may issue shares of Company Common Stock in connection with the exercise of Company Options, conversion of Pharma Preferred Stock to Company Common Stock or other rights for Company Common Stock, in each case to the extent outstanding as of the date of the Merger Agreement, (ii) Pharma may issue shares of Company Capital Stock in connection with the exercise of, and in accordance with the terms of, any Company Warrants outstanding as of the date of the Merger Agreement, and (iii) Pharma may issue shares of Company Common Stock in respect of the Contingent Rights in such amounts as set forth in Pharma Disclosure Schedules attached to the Merger Agreement;
71
Table of Contents
• enter into or adopt any plan or agreement of complete or partial liquidation, restructuring, receivership, recapitalization or dissolution, or file a voluntary petition in bankruptcy or commence a voluntary legal procedure for reorganization, arrangement, adjustment, release or composition of Debt in bankruptcy or other similar Laws now or thereafter in effect;
• incur any Debt for borrowed money, or guarantee any such Debt, or issue or sell any debt securities or guarantee any debt securities of other persons;
• make any capital expenditures, capital additions or capital improvements, in excess of $100,000;
• acquire or agree to acquire by merging with, or by purchasing a portion of the stock or assets of, or by any other manner, any business or any Entity;
• (A) initiate any new line of business, (B) make any investment in or advance, loan or capital contribution to any Person (other than business-related advances to its employees in the Ordinary Course of Business), (C) form or acquire any Subsidiary that is not wholly-owned by Pharma or any of its wholly-owned Subsidiaries or (D) otherwise acquire or agree to acquire any securities or assets of a Third Party that are material, individually or in the aggregate, to Pharma;
• terminate, cancel, amend, waive, modify, fail to use commercially reasonable efforts to comply with, maintain or renew any material Permit;
• sell, license, lease, assign or otherwise dispose of or create or incur any Lien on, directly or indirectly, any tangible properties or assets of Pharma or any of its Subsidiaries which are material to Pharma as a whole, other than (A) sales of inventory, of products or services, or obsolete equipment, all in the ordinary course of business, (B) sales, licenses, leases or transfers that are pursuant to Contracts in effect on the date of the Merger Agreement or (C) incurrence of Permitted Liens;
• (i) sell, license, lease, assign or otherwise dispose of or create or incur any Lien on, directly or indirectly, any material Company Owned Intellectual Property, or (ii) acquire, in-license or otherwise obtain any right, title or interest in or to any pending or issued Patents, inventions, patent disclosures or other Intellectual Property material to Pharma from any other Person (other than, with respect to each of clauses (i) and (ii), non-exclusive licenses or other non-exclusive grants of rights entered into in the Ordinary Course of Business, and, as applicable, on terms materially consistent with Pharma’s applicable form agreement(s), provided that any Intellectual Property arising from any such form agreement, for such agreements entered into in the Pre-Closing Period, will be solely owned by Pharma to the extent permitted under applicable Law);
• enter into any Material Contract, amend or modify in any material respect any Material Contract or terminate any Material Contract or waive any material right of Pharma or any of its Subsidiaries under any Material Contract, including without limitation, take any action with respect to the Altira Royalty Agreement;
• enter into any agreement or arrangement that limits or otherwise restricts Pharma or any of its Subsidiaries from engaging or competing in any line of business, in any location or with any Person, or would purport to limit, after the Effective Time, Parent or any of its Subsidiaries;
• make, revoke, or change any material election in respect of Taxes, change an annual Tax accounting period, adopt or change any accounting method in respect of Taxes, file any amended Company Tax Return, enter into any Tax allocation, sharing or indemnity agreement or any agreement with respect to Taxes, settle any claim or assessment in respect of Taxes, surrender or abandon any right to claim a material refund of Taxes, or consent to any extension or waiver of the limitation period applicable to any material claim or assessment in respect of Taxes;
72
Table of Contents
• except in each case as required by the terms of any Company Plan as in effect on the date of the Merger Agreement or as required by applicable Law, (i) adopt, establish, enter into, amend or terminate any Company Equity Plan, Company Plan or any plan, agreement, program, policy, trust, fund or other arrangement that would be a Company Plan if it were in existence as of the date of the Merger Agreement (except for renewals in the Ordinary Course of Business), (ii) increase the compensation or benefits (including severance, termination, retention and change of control compensation or benefits) of, or grant any bonus or other incentive compensation to, any Company Service Provider (except for increases in base salary in the Ordinary Course of Business with respect to Company Service Providers with a total annual base salary under $200,000, (iii) grant any severance or termination pay to any Company Service Provider, (iv) terminate the employment or engagement of any Company Service Provider other than for cause, (v) hire or engage any new Company Service Provider (except in the Ordinary Course of Business with respect to Company Service Providers with a total annual base salary under $200,000), or (vi) take any action to accelerate the vesting, funding or payment of any compensation or benefit under any Company Equity Plan (subject to certain exceptions) or other Company Plan or other Contract.
• terminate or allow to lapse any of the Insurance Policies;
• waive or release in writing, assign, commence, settle or agree to settle any Legal Proceeding, other than waivers, releases, compromises or settlements (i) in the Ordinary Course of Business (ii) that involve only the payment of monetary damages not in excess of $100,000 in the aggregate and (iii) that do not include the imposition of equitable relief on, or the admission of wrongdoing by, Pharma;
• make any loans, advances or capital contributions to, or investments in, any other Person, or form or acquire any Subsidiary that is not wholly-owned by Pharma or any of its wholly-owned Subsidiaries;
• commence, terminate or materially modify any clinical trial;
• terminate any executive employee of Pharma or materially modify the terms and conditions of employment of any executive employee of Pharma; or agree or commit to take certain of the actions described above.
Conduct of Business of Holdings
The Merger Agreement provides that, during the Pre-Closing Period, subject to limited exceptions, Holdings shall:
• not change or amend the Holdings Charter or Holdings Bylaws, or authorize or propose the same;
• not enter into or adopt any plan or agreement of complete or partial liquidation, restructuring, receivership, recapitalization or dissolution, or file a voluntary petition in bankruptcy or commence a voluntary legal procedure for reorganization, arrangement, adjustment, release or composition of Debt in bankruptcy or other similar Laws now or thereafter in effect;
• use its commercially reasonable efforts to conduct its business in the ordinary course of business consistent with past practice in all material respects and, to the extent consistent therewith, Parent shall use its commercially reasonable efforts to preserve the business organizations of Parent substantially intact and maintain existing relations and goodwill with Governmental Bodies, customers, suppliers, production companies, distributors, licensees, licensors, creditors, lessors, employees and business associates and others having material business dealings with Parent, as applicable, and keep available the services of its present employees and agents; and
• not agree or commit to take any of the actions described in clauses above.
In addition, from the execution of the Merger Agreement until the Effective Time, Parent (i) shall not, and shall not cause Pharma to, terminate or materially alter the terms and conditions of employment of any executive employee of Pharma without the prior consent of at least eighty percent (80%) of Pharma Board or (ii) remove any director or vote for (or consent to) any person for appointment or election as a director to Pharma Board without the prior consent of eighty percent (80%) of Pharma Board.
73
Table of Contents
No Solicitation
Pharma has agreed to not, and to cause its Subsidiaries and Representatives not to, directly or indirectly: (i) solicit, initiate or knowingly encourage, knowingly induce or knowingly facilitate the communication, making, submission or announcement of any Takeover Proposal or Takeover Inquiry or take any action that could reasonably be expected to lead to a Takeover Proposal or Takeover Inquiry; (ii) furnish any non-public information regarding Pharma to any Person in connection with or in response to a Takeover Proposal or Takeover Inquiry (other than to inform any Person of the existence of the no solicitation provision in the Merger Agreement; (iii) engage in discussions or negotiations with any Person (other than to inform any Person of the existence of the provisions in no solicitation provision in the Merger Agreement) with respect to any Takeover Proposal or Takeover Inquiry; (iv) approve or recommend any Takeover Proposal; execute or enter into any binding or nonbinding letter of intent, memorandum of understanding or similar understanding or Contract contemplating or otherwise relating to any Takeover; or (v) resolve or agree to do any of the foregoing.
Pharma has agreed to immediately cease and cause to be terminated any discussions, negotiations and communications with any Person that relate to any Takeover Proposal or Takeover Inquiry that were occurring as of the date of the Merger Agreement.
Promptly after the date of the Merger Agreement, Pharma agreed to: (i) request each Person that has received confidential information from Pharma or any of its Representatives on behalf of Pharma or its Representatives at any time during the past 12 months pursuant to a confidentiality or similar agreement in connection with such Person’s consideration of a possible Takeover Proposal to return or destroy all confidential information previously furnished to such Person by or on behalf of any of Pharma to the extent Pharma has the right to make such request pursuant to a confidentiality or similar agreement with such Person; and (ii) except as otherwise permitted by the applicable no solicitation provision in the Merger Agreement, prohibit any Person from having access to any physical or electronic data room relating to any possible Takeover Proposal or Takeover Inquiry.
Parent Special Meeting
Subject to fiduciary obligations under applicable Law, Parent, acting through the Holdings Board (or a committee thereof), has agreed to, as promptly as practicable (and in any event within twenty-five (25) Business Days) after the Registration Statement has been declared effective, take all action necessary, including under the DGCL, to duly call, give notice of, convene and hold a meeting of its stockholders for the approval of the issuance of the Parent Class B Common Stock issuable pursuant to the Parent Stockholder Approval and the other matters contemplated by the Merger Agreement (including any adjournment, recess or postponement thereof) and has further agreed to not postpone, recess or adjourn such Special Meeting, provided, however, for the avoidance of doubt, Holdings may postpone or adjourn the Special Meeting: (A) with the written consent of Pharma; (B) for the absence of a quorum; (C) to the extent necessary to ensure that any required supplement or amendment to the Registration Statement is provided to the holders of Parent Common Stock within a reasonable period of time in advance of the Pharma special meeting; (D) to allow reasonable additional time to solicit additional proxies; or (E) if required by Law. Holdings shall recommend approval and adoption of the Parent Stockholder Approval, including approval of the Merger Agreement and the Contemplated Transactions by Holdings stockholders in the Registration Statement and otherwise comply with all Laws applicable to the Special Meeting. Parent shall use its reasonable best efforts to (i) solicit from its stockholders proxies in favor of (it being understood that a proxy card will be deemed “in favor of” a matter to be acted upon by Holdings stockholders if it provides the stockholder with the ability to either vote for, vote against or abstain from voting on such matter) the adoption of the Merger Agreement and take all other actions reasonably necessary or advisable to secure the approval and adoption of the Merger Agreement and the Contemplated Transactions by Holdings stockholders and (ii) to solicit the Parent Minority Approval. Parent agreed to vote for the adoption of the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Pharma in the form attached to the Merger Agreement prior to the Mergers.
Indemnification and Insurance
All rights to indemnification by Pharma existing in favor of those Persons who are directors and officers of Pharma as of, or prior to, the date of the Merger Agreement (the “D&O Indemnified Persons”) for their acts and omissions occurring prior to the Effective Time as provided in Pharma Charter and Pharma’s bylaws (as in effect as
74
Table of Contents
of the date of the Merger Agreement) and as provided in those indemnification agreements (the “Indemnification Agreements”) between Pharma, shall survive the Merger and shall be complied with and performed by the Surviving Company.
For six years from and after the Effective Time, Parent will cause all rights to indemnification and exculpation by Pharma existing in favor of D&O Indemnified Persons for their acts and omissions occurring or alleged to have occurred prior to the Effective Time, as provided in Pharma Charter and Pharma’s bylaws (as in effect as of the date of the Merger Agreement) and as provided in the Indemnification Agreements, in each case subject to the terms, conditions and limitations thereof, to survive the Merger, including as a result of the amendment of the certificate of incorporation of the Surviving Company pursuant to the Merger Agreement, and for six years from and after the Effective Time, Parent shall cause the Surviving Company to fulfill and honor such obligations to the fullest extent permitted under applicable Law.
Parent will cause the Surviving Company as of the Effective Time, to obtain and fully pay for “tail” insurance policies with a claims period of at least six (6) years from and after the Effective Time from an insurance carrier with a credit rating the same as or better than Pharma’s current insurance carrier with respect to directors’ and officers’ liability insurance and fiduciary liability insurance (collectively, “D&O Insurance”) with respect to matters existing or occurring at or prior to the Effective Time with benefits and levels of coverage at least as favorable as Pharma’s existing policies (including in connection with the Merger Agreement or the transactions or actions contemplated thereby) with respect to those Indemnified Parties who are currently on the date hereof (and any additional Indemnified Parties who prior to the Effective Time become) covered by Pharma’s D&O Insurance; provided, however, that (i) in no event shall the Surviving Company be required to expend for such “tail” insurance policies an annual premium amount in excess of three hundred percent (300%) of the annual premium currently on the date hereof paid by Pharma for such insurance and (ii) if the annual premium of such “tail” insurance policies exceeds three hundred percent (300%) of the annual premium currently paid by Pharma for such insurance, Parent shall cause the Surviving Company to obtain and fully pay for policies covering such Indemnified Parties with the greatest coverage as is then available at a cost up to but not exceeding such amount. Parent shall, and shall cause the Surviving Company to, use its reasonable best efforts to cause such “tail” insurance policies to be maintained in full force and effect, for their full term, and to honor all of its obligations thereunder. After the Effective Time, Parent shall and shall cause the Surviving Company to maintain such policy in full force and effect, and continue to honor the obligations thereunder.
In the event that Parent, Pharma or the Surviving Company or any of their respective successors or assigns (i) consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or Entity of such consolidation or merger or (ii) transfers all or substantially all of its properties and assets to any Person, then, and in each such case, Parent shall ensure that the successors and assigns of Parent, Pharma or the Surviving Company, as the case may be, shall assume the obligations set forth in the applicable sections of the Merger Agreement.
The foregoing provisions of Merger Agreement shall survive the consummation of the Merger and are (i) intended to be for the benefit of, and will be enforceable by, each of the D&O Indemnified Persons and their successors, assigns and heirs and (ii) in addition to, and not in substitution for, any other rights to indemnification or contribution that any such D&O Indemnified Person may have by Contract or otherwise. The Indemnification of Officers and Directors provisions of the Merger Agreement may not be amended, altered or repealed after the Effective Time without the prior written consent of the affected D&O Indemnified Person.
Proxy Statement and Registration Statement Covenant
As promptly as practicable after the date of the Merger Agreement (and in any event within the later of (i) sixty (60) days after the date of the Merger Agreement; provided that as of such date the Pharma financials required to be included in the Registration Statement have been prepared and completed by Pharma or (ii) fifteen (15) days from the date on which the audit of Pharma financials required to be included in the Registration Statement have been completed), the Parties shall prepare, and Parent shall cause to be filed with the SEC, a registration statement on Form S-4 to register under the Securities Act the offer and sale of the Merger Shares pursuant to the Merger, which shall include a prospectus and a proxy statement relating to the Parent Stockholder Approval and the Company Stockholder Approval. Parent covenants and agrees that the Registration Statement will not, at the time that the Registration Statement or any amendments or supplements thereto is filed with the SEC or first mailed to Holdings stockholders contain any untrue statement of a material fact or omit to state any material fact required
75
Table of Contents
to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. Pharma covenants and agrees that the information provided by Pharma to Parent for inclusion in the Registration Statement (including Pharma Financial Statements) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make such information not misleading. Notwithstanding the foregoing, Parent will make no covenant, representation or warranty with respect to statements made in the Registration Statement, if any, based on information provided by Pharma or any of its Representatives specifically for inclusion therein and Pharma makes no covenant, representation or warranty with respect to statements made in the Registration Statement, if any, other than with respect to the information provided by Pharma or any of its Representatives for inclusion therein. Pharma and its legal counsel will be given reasonable opportunity to review and comment on the Registration Statement, including all amendments and supplements thereto, prior to the filing thereof with the SEC, and on the response to any comments of the SEC on the Registration Statement, prior to the filing thereof with the SEC. Parent shall use reasonable efforts to cause the Registration Statement to comply with the applicable rules and regulations promulgated by the SEC, to respond promptly to any comments of the SEC or its staff and to have the Registration Statement declared effective under the Securities Act as promptly as practicable after it is filed with the SEC. Each Party shall promptly furnish to the other Party all information concerning such Party and such Party’s Affiliates and such Party’s stockholders that may be required or reasonably requested in connection with such actions. If Parent or Pharma become aware of any event or information that, pursuant to the Securities Act or the Exchange Act, should be disclosed in an amendment or supplement to the Registration Statement, then such Party, as the case may be, shall promptly inform the other Parties thereof and shall cooperate with such other Parties in filing such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to Holdings stockholders. No filing of, or amendment or supplement to, the Registration Statement will be made by Parent, without the prior written consent of Pharma, which shall not be unreasonably withheld, conditioned or delayed. Pharma and Parent shall each use commercially reasonable efforts to cause the Registration Statement to comply with applicable federal and state securities Laws requirements and the rules and regulations of the NYSE.
The Parties shall reasonably cooperate with each other and provide, and shall use reasonable best efforts to cause their respective Representatives to provide, the other Party and its Representatives, with all true, correct and complete information regarding such Party that is required by Law to be included in the Registration Statement or reasonably requested by the other Party to be included in the Registration Statement. If at any time before the Effective Time the information provided in the Registration Statement has or will become “stale” and new information should, as determined by Parent acting reasonably, be disclosed in an amendment or supplement to the Registration Statement, then Parent shall promptly inform Pharma thereof and each such Party shall cooperate with one another, and shall use reasonable best efforts to cause their accounting and other outside professionals to so cooperate, (x) in providing the financial reporting necessary for such filing and (y) in filing such amendment or supplement with the SEC (and, if related to the proxy statement, mailing such amendment or supplement to Holdings stockholders).
Reasonable Best Efforts and Regulatory Filings
Subject to the terms and conditions of the Merger Agreement, Pharma and Parent shall use reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary, proper or advisable under applicable Law or otherwise to consummate and make effective the Contemplated Transactions as promptly as practicable, including notifying the other of any communication, inquiry, or investigation received from or initiated by a Governmental Body and using reasonable best efforts to cooperate in responding to any such inquiries, investigations, or requests, by using reasonable best efforts to take all action necessary to satisfy all of the conditions to the obligations of the other Parties to the Merger Agreement to effect the Mergers, to obtain all necessary waivers, consents, approvals and other documents required to be delivered hereunder, and to effect all necessary registrations and filings and to remove any injunctions or other impediments or delays, legal or otherwise, in each case in order to consummate and make effective the Merger and the Contemplated Transactions.
Pharma shall use reasonable best efforts to obtain all consents and waivers with respect to (i) the Contracts set forth on Part 2.21 of Pharma Disclosure Schedule attached to the Merger Agreement and (ii) any and all Contracts entered into by Pharma following the date hereof and prior to the Closing, in each case, that are required to be obtained from parties to such Contracts to which Pharma is a party in connection with the Contemplated Transactions.
76
Table of Contents
Financing
Pharma agreed to use its reasonable best efforts to provide, and use its reasonable best efforts to cause its officers, directors, employees, financial advisors, counsel, accountants and other Representatives and Affiliates to provide, all cooperation reasonably requested by Parent or the Merger Subs to assist them in connection with the arrangement of the Financing (which Financing has since been consummated). Such cooperation included but was not limited to the following:
• participating (with Representatives of Pharma of appropriate seniority and expertise) at reasonable times, upon reasonable advance notice, in meetings, presentations, due diligence sessions, and sessions with rating agencies, and otherwise cooperating with the marketing efforts for the Financing;
• assisting Parent with the preparation of all materials reasonably required to be provided or requested by Parent or its Representatives for use in connection with the Financing, including, without limitation, financial statements and financial forecasts; and
• taking corporate actions reasonably requested by Parent to facilitate the consummation of the Financing.
Corporate Governance
Pharma will obtain and deliver to Parent as of the Effective Time (or, at the option of Parent, at a later date) the resignation of each (i) director of Pharma and (ii) officers of Pharma, effective as of the Effective Time (or, at the option of Parent, at a later date).
Additional Covenants
The Merger Agreement contains additional covenants relating to, among other matters, public announcements with respect to the Merger, notice of certain events prior to the Effective Time, preparation of a closing payment schedule and access to certain information.
Conditions to the Merger
Conditions to the Obligations of the Parties to Complete the Merger
The obligations of each of Parent, Pharma and Merger Subs to complete the Merger are subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement or applicable law) on or prior to the Effective Time of various conditions, including the following:
• The Registration Statement shall have been declared effective by the SEC under the Securities Act and no stop order suspending the effectiveness of the Registration Statement shall have been issued by the SEC and remain in effect, and no proceedings for that purpose shall be pending or threatened in writing by the SEC and not withdrawn or lapsed.
• No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of any of the Mergers shall have been issued by any court of competent jurisdiction and remain in effect, and no material Law shall have been enacted since the date of the Merger Agreement that makes consummation of any of the Mergers illegal.
• The Merger Agreement shall have been duly adopted by the Required Company Stockholder Vote and the Required Parent Stockholder Vote, and the Required Company Stockholder Vote and the Required Parent Stockholder Vote shall remain in full force and effect and, if the Registration Statement incorporates information by reference as contemplated in General Instruction A.2 of Form S-4 under the Securities Act, at least twenty business days (as defined pursuant to Form S-4) shall have elapsed from the date of the first mailing of the prospectus in the Registration Statement to Pharma stockholders until the date of the Closing.
• The Merger Agreement shall have been duly adopted by the Parent Minority Approval and the Company Minority Approval, each of the Parent Stockholder Approval and the Company Stockholder Approval have occurred, and all such approvals shall remain in full force and effect.
77
Table of Contents
• The Merger Shares shall have been approved for listing on the NYSE, subject to official notice of issuance.
• The transactions contemplated by the Altira Acquisition Agreement shall have been consummated by all parties thereto in accordance with its terms; provided that such consummation shall not be a condition to Parent’s obligations to consummate the Merger if (a) Parent has not funded the payment amount thereunder before the Closing and (b) the Altira Acquisition Agreement has been consummated in all material respects by all parties thereto other than Parent (to the extent that such covenants require performance by such other parties at or before the Closing), including execution and delivery of any and all conveyance documents.
• The Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Pharma in the form of attached to the Merger Agreement, shall have received the required director and stockholder approvals and been filed with the Delaware Secretary of State on a date prior to the Closing Date.
• Parent shall have consummated the Financing (which Financing has since been consummated).
Conditions to the Obligations of Parent and the Merger Subs to Complete the Merger
In addition, the obligations of Parent and the Merger Sub to complete the Merger are also subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement or applicable law) at or prior to the Closing of each of the following conditions:
• The Specified Representations that are qualified by materiality or Company Material Adverse Effect shall be true and correct in all respects as of the date of the Merger Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all respects as of such earlier date). The Specified Representations that are not qualified by materiality or Company Material Adverse Effect shall be true and correct in all material respects as of the date of the Merger Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all material respects as of such earlier date). The other representations and warranties of Pharma shall be true and correct (without giving effect to any limitation as to materiality or Company Material Adverse Effect set forth therein) as of the date of the Merger Agreement and of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct would not, individually or in the aggregate, have a Company Material Adverse Effect.
• Pharma shall have performed and complied with, in all material respects, all of its covenants hereunder at or before the Closing (to the extent that such covenants require performance by Pharma at or before the Closing).
• There shall not be pending any lawsuit or other Legal Proceeding challenging the Merger that (a) has been commenced by a Governmental Body of competent jurisdiction and (b) could reasonably be expected to result in an injunction or judgment in favor of such Governmental Body that would prevent consummation of the Merger; Parent shall have received the following agreements and documents, each of which shall be in full force and effect: (a) the D&O Resignations; (b) the Certificate of Merger, executed by Pharma; (c) a good standing certificate of Pharma from the Secretary of State of the State of Delaware dated within seven days prior to the Closing Date; and (d) the Lock-Up Agreements duly executed by each party thereto with respect to shares of Parent Class B Common Stock issued pursuant to the Merger (other than Parent).
• As of the Closing, the Chief Executive Officer or Chief Financial Officer of Pharma shall have delivered to Parent a certificate to the effect that each of the conditions specified in Sections 7.1 (Accuracy of Representations and Warranties), 7.2 (Performance of Covenants) and 7.8 (No Material Adverse Effect) is satisfied in all respects.
• There shall be no obligation to pay any royalties or similar payments on any sales of products incorporating Pharma Intellectual Property and the third party consents set forth on Pharma Disclosure Schedules shall have been obtained in form and substance reasonably satisfactory to Holdings.
78
Table of Contents
• No Material Adverse Effect. Since the date of the Merger Agreement, no Company Material Adverse Effect shall have occurred that is continuing.
Conditions to the Obligations of Company to Complete the Merger
The obligation of Pharma to complete the Merger is subject to the satisfaction (or waiver to the extent permissible under the Merger Agreement), at or prior to the Closing, of the following conditions:
• The representations and warranties of Parent and the Merger Subs shall be true and correct in all material respects as of the date of the Merger Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all respects as of such earlier date).
• Parent and the Merger Subs shall have performed and complied with, in all material respects, all of their respective covenants hereunder at or before the Closing (to the extent that such covenants require performance by Parent or Merger Subs at or before the Closing).
• There shall not be pending before any court of competent jurisdiction any lawsuit or other Legal Proceeding challenging the Merger that has been commenced by a Governmental Body.
• Since the date of the Merger Agreement, no Parent Material Adverse Effect shall have occurred that is continuing.
• An authorized officer of Parent and the Merger Subs shall have delivered to Company a certificate to the effect that each of the conditions specified in Sections 8.1 (Accuracy of Representations and Warranties), 8.2 (Performance of Covenants) and 8.4 (No Material Adverse Effect) is satisfied in all respects.
• Parent shall have dissolved all of its direct and indirect subsidiaries (other than Merger Sub I and Merger Sub II) holding shares of Company capital stock or securities exercisable for or convertible into Company capital stock, or distributed or otherwise transferred, the shares of Company capital stock or securities exercisable for or convertible into Company capital stock held by such subsidiaries to Parent and the other members of such subsidiaries.
• The Lock-Up Agreements shall have been duly executed by each party thereto with respect to shares of Parent Class A Common Stock and Parent Class B Common Stock.
Termination
The Merger Agreement may be terminated prior to the Effective Time (whether before or after the adoption of the Merger Agreement by the Required Company Stockholder Vote, except to the extent otherwise provided below) as follows:
• by mutual written consent of Parent and Pharma;
• by either Parent or Pharma, if:
• the Merger shall not have been consummated by the End Date; provided, however, that the right to terminate the Merger Agreement under Section 9.1(b) (Termination) shall not be available to Pharma, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure of the Merger to occur on or before the End Date and such action or failure to act constitutes a breach of the Merger Agreement;
• a court of competent jurisdiction or other Governmental Body of competent jurisdiction shall have issued a final and non-appealable order, decree or ruling, in each case, having the effect of permanently restraining, enjoining or otherwise prohibiting or making illegal the Merger; provided, however, that the right to terminate the Merger Agreement under the foregoing section shall not be available to Pharma, on the one hand, or to Parent, on the other hand, if such Party did not use commercially reasonable efforts to have such order, decree or ruling vacated prior to its becoming final and non-appealable and such failure to use commercially reasonable efforts constitutes a breach of the Merger Agreement;
79
Table of Contents
• each of the following are satisfied: (i) the Parent Stockholder Solicitation Period shall have run and elapsed and either (A) the Required Parent Stockholder Vote or (B) the Parent Minority Approval shall not have been obtained on or prior to the end of the Parent Stockholder Solicitation Period, or (ii) the Company Stockholder Solicitation Period shall have run and elapsed and either (x) the Required Company Stockholder Vote or (y) the Company Minority Approval shall not have been obtained on or prior to the end of the Company Stockholder Solicitation Period; provided however that the right to terminate the Merger Agreement under Section 9.1(f) (Termination) shall not be available to Pharma, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure to obtain the approvals required pursuant to clauses (i) or (ii) hereof, as applicable, and such action or failure to act constitutes a breach of the Merger Agreement. Notwithstanding any other term in Section 9.1(f) (Termination), once the Required Company Stockholder Vote, the Company Minority Approval, the Required Parent Stockholder Vote and the Parent Minority Approval have been obtained, neither Parent nor Pharma may terminate the Merger Agreement pursuant to Section 9.1(f) (Termination).
• by Parent, if:
• Pharma shall have breached or failed to perform any of its representations, warranties, covenants, obligations or agreements contained in the Merger Agreement, which breach or failure to perform (i) would give rise to the failure of a condition set forth in Section 7.1 (Accuracy of Representations and Warranties) or Section 7.2 (Performance of Covenants) and (ii) cannot be, or has not been, cured within 30 calendar days following receipt by Pharma of written notice of such material breach or failure to perform; provided that Parent may not terminate the Merger Agreement pursuant to Section 9.1(d) (Termination) if Parent is in breach of the Merger Agreement such that Pharma has the right to terminate the Merger Agreement pursuant to Section 9.1(e) (Termination) but for the proviso thereto;
• by Pharma, if:
• Parent or Merger Subs shall have breached or failed to perform any of their respective representations, warranties, covenants, obligations or agreements contained in the Merger Agreement, which breach or failure to perform (i) would give rise to the failure of a condition set forth in Section 8.1 (Accuracy of Representations and Warranties) or Section 8.2 (Performance of Covenants) and (ii) cannot be, or has not been, cured within 30 calendar days following receipt by Parent of written notice of such material breach or failure to perform; provided that Pharma may not terminate the Merger Agreement pursuant to Section 9.1(e) (Termination) if Pharma is in breach of the Merger Agreement such that Parent has the right to terminate the Merger Agreement pursuant to Section 9.1(d) (Termination) but for the proviso thereto;
The Party desiring to terminate the Merger Agreement pursuant to Section 9.1 (Termination), shall give the other party written notice of such termination, specifying the provisions hereof pursuant to which such termination is made and the basis therefor described in reasonable detail.
Effect of Termination
If the Merger Agreement is terminated, the Merger Agreement will be of no further force or effect, and there will not be any liability or obligation on the part of any party, except that (i) Section 9.2 (Effect of Termination), Section 5.2(c), (d) and (e), Section 5.7 (Confidentiality), Section 1.11 (Extension of 56% Warrant) and Section 10 (Miscellaneous Provisions) and the definitions of the capitalized terms referenced in such Sections shall survive the termination of the Merger Agreement and shall remain in full force and effect, (ii) nothing therein shall relieve any party from any liability for Fraud or for any knowing and willful material breach of the Merger Agreement by such party prior to the termination of the Merger Agreement, and (iii) no termination of the Merger Agreement shall affect the obligations of the Parties contained in the Confidentiality Agreement (subject to the terms thereof), all of which obligations shall survive termination of the Merger Agreement in accordance with their terms.
80
Table of Contents
Amendments
Subject to applicable Law, the Merger Agreement may be amended, modified and supplemented in any and all respects, whether before or after any vote of the stockholders of Holdings or Pharma contemplated thereby, by written agreement of the Parties to the Merger Agreement, at any time prior to the Closing Date with respect to any of the terms contained herein; provided, however, that after the receipt of the Parent Stockholder Approval, there shall be no amendment or waiver that would require the further approval of the stockholders of Holdings under applicable Law without such approval having first been obtained. A termination of the Merger Agreement pursuant to Section 9.1 (Termination) shall, in order to be effective, require, in the case of Parent and Pharma, action by their respective boards of directors (or a committee thereof) as applicable.
Waivers
No failure on the part of any party to exercise any power, right, privilege or remedy under the Merger Agreement, and no delay on the part of any party in exercising any power, right, privilege or remedy under the Merger Agreement, shall operate as a waiver of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy.
No party shall be deemed to have waived any claim arising out of the Merger Agreement, or any power, right, privilege or remedy under the Merger Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of such party; and any such waiver shall not be applicable or have any effect except in the specific instance in which it is given.
Third Party Beneficiaries
The Merger Agreement is not intended to confer upon any Person (other than the Parties thereto) any right, benefit or remedy of any nature whatsoever under or by reason of the Merger Agreement, except (a) as provided in Section 5.4 (Indemnification of Officers and Directors), which in each case shall be third party beneficiaries of said provisions, provided that no consent of any D&O Indemnified Person shall be required to amend any provision of the Merger Agreement prior to the Effective Time, (b) pursuant to Section 1.11 (Extension of 56% Warrant), (c) pursuant to Section 5.2(e) and (d) from and after the Effective Time, the rights of the Securityholders as of immediately prior to the Effective Time to receive the consideration pursuant to the Merger set forth in the Merger Agreement.
Governing Law; Jurisdiction
The Merger Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of Laws thereof. In any action between any of the Parties arising out of or relating to the Merger Agreement or any of the Contemplated Transactions: (a) each of the Parties irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware (or, only if such court declines to accept jurisdiction over a particular matter, any state or federal court within the State of Delaware); and (b) if any such action is commenced in a state court, then, subject to applicable Law, no party shall object to the removal of such action to any federal court located in Delaware. Each of the Parties waives any defense of inconvenient forum to the maintenance of any action so brought and waives any bond, surety or other security that might be required of any other party with respect thereto. Each Party thereto waives, to the fullest extent permitted by Law, any right to trial by jury of any claim, demand, action, or cause of action (i) arising under the Merger Agreement or (ii) in any way connected with or related or incidental to the dealings of the Parties in respect of the Merger Agreement or any of the transactions related thereto, in each case, whether now existing or thereafter arising, and whether in contract, tort, equity, or otherwise. Each Party thereto further agrees and consents that any such claim, demand, action, or cause of action shall be decided by court trial without a jury and that the Parties may file a copy of the Merger Agreement with any court as written evidence of the consent of the Parties to the waiver of their right to trial by jury.
81
Table of Contents
Specific Performance
Each of the Parties to the Merger Agreement agreed that the Merger Agreement is intended to be legally binding and specifically enforceable pursuant to its terms and that Parent and Pharma would be irreparably harmed if any of the provisions of the Merger Agreement are not performed in accordance with their specific terms and that monetary damages would not provide adequate remedy in such event. Accordingly, in addition to any other remedy to which a Party may be entitled at Law, a Party shall be entitled to injunctive relief to prevent breaches of the Merger Agreement and to specifically enforce the terms and provisions thereof, in each case without posting a bond or undertaking, this being in addition to any other remedy to which they are entitled at Law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of the Merger Agreement on the basis that the other Parties have an adequate remedy at Law or an award of specific performance is not an appropriate remedy for any reason at Law or equity.
Notwithstanding the foregoing or anything else to the contrary in the Merger Agreement, Pharma shall not be entitled to enforce specifically Parent’s and the Merger Subs’ obligations to consummate the Merger unless (i) all of the conditions set forth in Sections 6 and 7 (other than those conditions that by their nature are to be satisfied at the Closing; provided that such conditions would have been satisfied or waived assuming the Closing were to occur) shall have been satisfied (or are capable of being satisfied at the Closing) or (to the extent permissible under applicable Law) waived; (ii) Pharma has irrevocably confirmed in writing to Parent that (A) all of the conditions to Pharma’s obligations have been satisfied or waived other than those conditions that by their nature are to be satisfied at the Closing; provided that such conditions would have been satisfied or waived assuming the Closing were to occur), and (B) if specific performance is granted, then it is ready, willing and able to take the actions within its control to cause the Closing to occur and (iii) Parent and Merger Sub I have failed to complete the Closing by the End Date.
Non-Recourse
Notwithstanding anything in the Merger Agreement to the contrary, no Representative, Affiliate of, or direct or indirect equity owner in, Pharma shall have any personal liability to either Parent or Merger Subs or any other Person as a result of the breach of any representation, warranty, covenant, agreement or obligation of Pharma in the Merger Agreement, and no Representative, Affiliate of, or direct or indirect equity owner in, either Parent or Merger Subs shall have any personal liability to Pharma or any other Person as a result of the breach of any representation, warranty, covenant, agreement or obligation of any of Parent or Merger Subs in, or otherwise in connection with, the Merger Agreement.
Holdings Financing
On August 24, 2021, pursuant to securities purchase agreements dated August 19, 2021, in transactions exempt for the registration requirements of the Securities Act, Holdings sold an aggregate of 2,833,425 shares of its Class B Common stock to certain institutional investors at a purchase price equal to $35.00 per share, for aggregate gross proceeds of approximately $99.2 million, and an additional 112,561 shares of its Class B Common Stock to an entity associated with the family of Howard Jonas at a purchase price equal to $44.42 per share, which was equal to the closing price of a share of the Class B Common Stock on the NYSE on August 19, 2021 for gross proceeds of approximately $5.0 million.
In connection with the sales to institutional investors, Holdings entered into the Registration Rights Agreement, under which Holdings agreed to prepare and file a registration statement with the SEC within 30 days after the earlier of (i) the date of the closing of the Merger Agreement, and (ii) the Initiation Date, for purposes of registering the resale of the shares sold to those investors, and to use commercially reasonable efforts to cause this registration statement to be declared effective by the SEC within 90 days after the Initiation Date.
Description of the Support and Voting Agreements, Lock-Up Agreements, Altira Acquisition Agreement and 56% Warrant Extension
The following is a summary of the material terms and conditions of the Support Agreements, the Voting Agreements, the Lock-Up Agreements, the Altira Acquisition Agreement and 56% Warrant. This summary may not contain all of the information about such agreements that is important to you. This summary is qualified by reference
82
Table of Contents
to the complete text of the Form of Support Agreement, Form of Voting Agreement, Form of Lock-Up Agreement and Form of Altira Acquisition Agreement, which are attached as Annexes C, D, B and F, respectively to, and incorporated by reference into, this proxy statement/prospectus. You are encouraged to read the full text of the agreements.
Concurrently with the execution of the Merger Agreement on June 17, 2021, certain key affiliates of both Holdings and Pharma entered into binding commitments to support the Merger. The Support Agreement was executed by affiliates and other related parties of Pharma, and the Voting Agreement was executed by affiliates and other related parties of Holdings.
Support Agreements
Pursuant to the Support Agreements, certain directors and executive officers of Pharma and other Pharma Stockholders agreed during the term of the Support Agreements to, among other things and upon the terms and conditions therein, vote or cause to be voted all of their respective (directly or indirectly held or otherwise beneficially owned) shares: (i) to adopt the Merger Agreement and approve the Merger and any of the other transactions contemplated by the Merger Agreement, (ii) to adopt the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Pharma in the form attached hereto as Annex G, (iii) against any transaction described or contemplated in Section 4.4 of the Merger Agreement or any other competing or alternative acquisition proposal for Pharma or any of its subsidiaries and (iv) against any other proposal, action or transaction involving Pharma or any of its subsidiaries that would impede, frustrate, prevent or materially delay the Merger and transactions related thereto.
In addition, in the event that Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a Holdings stockholder meeting, each applicable Pharma holder signatory thereto undertakes to take (and cause its subsidiaries and affiliates to take) certain actions in support of an initial public offering of Pharma, including: (i) voting for all amendments to the Certificate of Incorporation of Pharma recommended by the Pharma board in connection with an initial public offering of Company Common Stock, (ii) immediately prior to the applicable IPO and contingent on the conversion of all other Pharma Preferred Stock (except that of Holdings and its affiliates), converting all outstanding Pharma Preferred Stock into Company Common Stock in accordance with applicable terms and conditions and at the ratio applicable for such Pharma Preferred Stock, (iii) refraining from taking certain actions without the prior consent of the managing underwriter for the IPO during a period (not to exceed 180 days) following the date of the final prospectus for the IPO, including the transfer, sale or other disposition of Company Common Stock held prior to the effectiveness of a registration statement for such IPO, and (iv) executing such agreements as may be reasonable requested by the managing underwriter for the IPO. Pharma also agreed that in such case, the Warrant would be exercisable on a cashless basis by Holdings, Mr. Jonas or any affiliate thereof who holds rights under the Warrant at an implied price per share no greater than the price of Company Common Stock to be issued in the IPO.
The Support Agreements also prohibit the Pharma holders signatory thereto from transferring their interests in Pharma subject to the applicable Support Agreement, except under certain limited circumstances described in the Support Agreement. Each Support Agreement contains a covenant requiring the signatory, to the extent applicable, to undertake reasonable best efforts to obtain the signature of any affiliates or family members who also hold interests in Pharma.
The Support Agreements automatically terminate upon the earliest to occur of (i) the termination of the Merger Agreement in accordance with its terms and (ii) in the event of a material modification of any material term or provision of the Merger Agreement that is materially adverse to the applicable Pharma holder, the election of such Pharma holder to terminate the applicable Support Agreement.
Voting Agreements
Pursuant to the Voting Agreements, Howard Jonas and certain of his family members and affiliates agreed during the term of the Support Agreements to, among other things and upon the terms and conditions therein, vote or cause to be voted all of their respective (directly or indirectly held or otherwise beneficially owned) shares: (i) to adopt the Merger Agreement and approve the Merger and any of the other transactions contemplated by the Merger Agreement, (ii) against any action agreement or proposal (A) made in opposition to or in competition with the consummation of the issuance of the merger consideration or any of the other transactions contemplated by the Merger Agreement or (B) that would reasonably be expected to result in a breach of any covenant, representation or warranty of Holdings,
83
Table of Contents
and (iii) against any amendment of the certificate of incorporation or Holdings Bylaws or any other proposal, action or transaction involving Holdings or any of its subsidiaries that would impede, frustrate, prevent or materially delay the Merger and transactions related thereto.
The Voting Agreements also prohibit the holders signatory thereto from transferring their interests in Holdings subject to the applicable Voting Agreement, except under certain limited circumstances described in the Voting Agreement. Each Voting Agreement contains a covenant requiring the signatory, to the extent applicable, to undertake reasonable best efforts to obtain the signature of any affiliates or family members who also hold interests in Holdings.
The Voting Agreements automatically terminate upon the earliest to occur of (i) the closing of the Merger, (ii) the termination of the Merger Agreement in accordance with its terms, (iii) in the event of a material modification of any material term or provision of the Merger Agreement that would materially increase the merger consideration issuable by Holdings pursuant to the Merger, unless the signatory holder consents to such modification and (iv) the mutual written consent of the holder and Pharma.
Lock-Up Agreements
Concurrently with the execution of the Merger Agreement on June 17, 2021, certain key affiliates of both Holdings and Pharma entered into Lock-Up Agreements. The Lock-Up Agreements provide that the Parent Class B Common Stock to be received by the holder pursuant to the Merger Agreement, the Parent Class B Common Stock issued upon the exercise of Parent Options and the Parent Class B Common Stock and the Parent Class A Common Stock held by the holder or its Affiliates as of the date of the Lock-Up Agreement shall become subject to limitations on disposition as set forth therein, including that the holders shall not be able to transfer such Parent Common Stock for a period of six months following closing of the Business Combination.
Altira Acquisition Agreement
As a condition to the closing of the Business Combination, Holdings is to acquire for $30 million in cash the remaining membership interest in Altira Capital & Consulting, LLC that it does not already own.
56% Warrant Extension
Pursuant to the Merger Agreement, the expiration date of the 56% Warrant was extended by Parent beyond August 15, 2021 and shall remain exercisable by the holder(s) thereof until the earlier of (i) upon the occurrence of the Effective Time, the Effective Time or (ii) if the Effective Time does not occur, the date that is calculated by adding (A) the number of calendar days the Merger Agreement has been in effect prior to its termination in accordance with its terms, to (B) August 15, 2021. Prior to the Closing, Holdings shall effect the termination of the 56% Warrant by Pharma Holdings LLC, such termination to be contingent on the consummation of the Merger and effective at the Initial Effective Time. The Support Agreements executed by holders of the 56% Warrant provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable pursuant to the 56% Warrant shall be calculated as of September 30, 2021, as if the Merger were consummated on such date.
Department of Justice, Federal Trade Commission and Other U.S. Antitrust Authorities
Under Title II of the HSR Act, certain transactions may not be completed unless certain waiting period requirements have expired or been terminated. The HSR Act provides that each party must file a premerger notification with the FTC and the DOJ. A transaction notifiable under the HSR Act may not be completed until the expiration of a 30-calendar-day waiting period following the parties’ filings of their respective HSR Act notification forms or the early termination of that waiting period. At any time before the expiration of the initial waiting period, the DOJ or the FTC may issue a request for additional documents and materials. If such a second request is issued, the parties may not complete the transaction until they substantially comply with the second request and observe a second 30-calendar-day waiting period, unless the waiting period is terminated earlier or extended by timing agreement.
The Merger is exempt from the reporting and waiting requirements under the HSR Act, as an acquisition of voting securities not meeting or exceeding a greater notification threshold. See, 16 C.F.R, Section 802.21. The (1) acquiring person in the Merger and all other persons required by the HSR Act to file notification previously filed notification with respect to an earlier acquisition of voting securities of the same issuer; (2) waiting period with respect to the earlier acquisition has expired, or been terminated pursuant to §803.11, (3) Merger is planned to be consummated
84
Table of Contents
within 5 years of such expiration or termination; and (4) Merger will not increase the holdings of the acquiring person to meet or exceed a notification threshold (as adjusted) greater than the greatest notification threshold met or exceeded in the earlier acquisition and filing.
At any time before or after the Contemplated Transactions are completed, the FTC or DOJ could take action under U.S. antitrust laws in opposition to the transaction, including seeking to enjoin completion of the Contemplated Transactions, condition approval of the transaction upon the divestiture of substantial assets of Parent, Pharma, or their respective affiliates, requiring the parties to license, or hold separate, assets or to terminate existing relationships and contractual rights, or impose restrictions on Parent’s post-transaction operations. In addition, U.S. state attorneys general could take such action under the antitrust laws as they deem necessary or desirable in the public interest, including, without limitation, seeking to enjoin completion of the Contemplated Transaction, or permitting completion subject to regulatory concessions or conditions (including seeking divestiture of substantial assets of the parties, or requiring the parties to license, or hold separate, assets or to terminate existing relationships and contractual rights, or imposing restrictions on Parent’s post-closing operations). Private parties also may seek to take legal action under the antitrust laws under some circumstances.
There can be no assurance that a challenge to the transactions on antitrust grounds will not be made, or if such a challenge is made, what the result will be.
Timing; Challenges by Governmental and Other Entities
There can be no assurance the ability of the ability to maintain or obtain approvals on satisfactory terms or the absence of any action challenging such approvals. In addition, there can be no assurance that any of the governmental or other entities described above, including the DOJ, FTC, U.S. state attorneys general, state insurance regulators, foreign regulators and private parties, will not challenge the Contemplated Transaction on antitrust, competition, or foreign investment grounds and, if such a challenge is made, there can be no assurance as to its result.
Subject to certain conditions, if the transaction is not completed on or before the End Date (as may be extended in accordance with the Merger Agreement), either Holdings or Pharma may terminate the Merger Agreement.
85
Table of Contents
NYSE REQUIREMENTS
Overview
Parent Class B Common Stock is currently listed on the NYSE under the symbol “RFL”. On [•], 2021, the last reported sale price for our Class B common stock was $[•] per share. Following the Mergers, we expect that Parent Class B Common Stock will continue to be listed on the NYSE.
Holdings is asking its stockholders to consider and vote upon a proposal to adopt and approve the Merger Agreement for the purposes of complying with the applicable provisions of Section 312.03 of the NYSE’s Listed Company Manual.
Reasons for the Approval for Purposes of NYSE Listing Rule 312.03
Pursuant to Section 312.03(c) of the NYSE’s Listed Company Manual, stockholder approval is required prior to the issuance of common stock, or of securities convertible into or exercisable for common stock, in any transaction or series of related transactions if: (1) the common stock has, or will have upon issuance, voting power equal to or in excess of 20 percent of the voting power outstanding before the issuance of such stock or of securities convertible into or exercisable for common stock or (2) the number of shares of common stock to be issued is, or will be upon issuance, equal to or in excess of 20 percent of the number of shares of common stock outstanding before the issuance of the common stock or of securities convertible into or exercisable for common stock.
Accordingly, the aggregate number of shares of Parent Class B Common Stock that Holdings will issue in connection with the Mergers will exceed 20% of the number of shares of Parent Class B Common Stock outstanding before such issuance, and for this reason, Holdings is seeking the approval of Holdings stockholders for the Merger Agreement, including the issuance of shares of Class B Common Stock to holders of Pharma stockholders. Holdings is required to complete a supplemental listing application to have the shares of Parent Class B Common Stock issued to Pharma stockholders listed on the NYSE.
86
Table of Contents
DESCRIPTION OF HOLDINGS CAPITAL STOCK
Our authorized capital stock consists of (i) 35 million shares of Parent Class A Common Stock, (ii) 200 million shares of Parent Class B Common Stock, and (iii) 10 million shares of Preferred Stock.
The following description of our classes of authorized stock does not purport to be complete and is subject to and qualified in its entirety by reference to our charter and bylaws, copies of which are filed as exhibits to the Annual Report on Form 10-K for the fiscal year ended July 31, 2020 filed with the SEC on October 29, 2020.
Parent Class A Common Stock
Holders of shares of our Parent Class A Common Stock are entitled to three votes for each share on all matters to be voted on by the stockholders. Holders of our Parent Class A Common Stock are entitled to share ratably in dividends, if any, as may be declared from time to time by the Board of Directors in its discretion from funds legally available therefor. Each share of our Parent Class A Common Stock may be converted, at any time and at the option of the holder, and automatically converts upon transfers to unaffiliated parties, into one fully paid and non-assessable share of our Parent Class B Common Stock.
As of [•], 2021, there were [•] of our shares of Parent Class A Common Stock outstanding.
Parent Class B Common Stock
Holders of shares of our Parent Class B Common Stock are entitled to one tenth of one vote for each share on all matters to be voted on by the stockholders. Holders of our Parent Class B Common Stock are entitled to share ratably in dividends, if any, as may be declared from time to time by the Board of Directors in its discretion from funds legally available therefor.
As of [•], 2021, there were [•] shares of Parent Class B common stock outstanding.
Preferred Stock
The Board of Directors has the authority to fix the price, rights, preferences, privileges and restrictions, including voting rights, of those shares without any further vote or action by the stockholders.
As of [•], 2021, there were no shares of our preferred stock were outstanding.
Anti-Takeover Effects of Our Charter and By-Laws
Some provisions of Delaware law and our Certificate of Incorporation and By-Laws could make the following more difficult:
• acquisition of us by means of a tender offer;
• acquisition of us by means of a proxy contest or otherwise; or
• removal of our incumbent officers and directors.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions also are designed to encourage persons seeking to acquire control of us to first negotiate with our Board of Directors. We believe that the benefits of increased protection give us the potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us and outweigh the disadvantages of discouraging those proposals because negotiation of them could result in an improvement of their terms.
Certificate of Incorporation; By-Laws
Our Certificate of Incorporation and By-Laws contain provisions that could make more difficult the acquisition of us by means of a tender offer, a proxy contest or otherwise. These provisions are summarized below.
87
Table of Contents
Undesignated Preferred Stock. The authorization of our undesignated preferred stock makes it possible for our Board of Directors to issue our preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile takeovers or delaying changes of control of our management.
Size of Board and Vacancies. Our Certificate of Incorporation provides that the number of directors on our Board of Directors will be between three and seventeen. Newly created directorships resulting from any increase in our authorized number of directors or any vacancies in our Board of Directors resulting from death, resignation, retirement, disqualification, removal from office or other cause will be filled solely by the vote of our remaining directors in office.
Stockholder Meetings. Under our By-Laws, only our (i) Chief Executive Officer, (ii) President or (iii) Corporate Secretary may call special meetings of our stockholders.
88
Table of Contents
COMPARISON OF THE RIGHTS OF HOLDERS OF hOLDINGS COMMON STOCK
AND PHARMA common Stock
Holdings and Pharma are Delaware corporations and the rights of Holdings and Pharma stockholders are governed by the DGCL. Pharma stockholders’ rights are also governed by Pharma’s Certificate of Incorporation and bylaws. If the transaction is completed, the rights of Pharma stockholders who become Holdings stockholders will be governed by the Holdings Certificate of Incorporation and bylaws.
Holdings and Pharma are both organized under the laws of the State of Delaware; however, differences in rights of holders of Holdings and Pharma capital stock arise from differences between Certificates of Incorporation and bylaws and certificates of designation for series of preferred stock of Pharma. As holders of Holdings Class A Common Stock and Holdings Class B Common Stock, your rights with respect thereto will continue to be governed by Delaware law, including the DGCL, as well as Holdings’ constituent documents. This section summarizes material differences between the rights of Holdings and Pharma stockholders. This summary does not purport to be a complete statement of all the differences, or a complete description of the specific provisions referred to. Further, the identification of specific differences is not intended to indicate that other equally or more significant differences do not exist. Holdings and Pharma stockholders should carefully read the relevant provisions of the Holdings Certificate of Incorporation, the Holdings bylaws, the Pharma Certificate of Incorporation, the Pharma bylaws and the DGCL. Copies of the documents referred to in this summary may be obtained as described under “Where You Can Find More Information.”
Holdings | | Pharma |
Authorized and Outstanding Capital Stock |
Holdings is authorized to issue 245,000,000 shares of stock, consisting of 35,000,000 shares of Holdings Class A common stock, par value $0.001 per share, 200,000,000 shares of Holdings class B common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share. As of the close of business on the Holdings record date, there were [•] shares of Holdings Class B common stock, 787,163 shares of Class A common stock and no shares of serial preferred stock issued and outstanding. | | Pharma is authorized to issue 610,000,000 shares of stock, consisting of (i) 310,000,000 shares of common stock, par value $.001 per share, consisting of (A) 300,000,000 shares of Voting Common Stock, and (B) 10,000,000 shares of Non-Voting Common Stock, and (ii) 300,000,000 shares of preferred stock, par value $.001 per share, of which there are (A) 6,050,000 shares designated as Series A convertible preferred stock, (B) 1,892,072 shares designated as Series B-1 convertible preferred stock, (C) 17,500,000 shares designated as Series B-2 convertible preferred stock, (D) 30,000,000 shares designated as Series B-3 convertible preferred stock, (E) 10,400,000 shares designated as Series B-4 convertible preferred stock, (F) 25,000,000 shares designated as Series C convertible preferred stock, and (G) 131,000,000 shares of Series D convertible preferred stock. As of the close of business on the Pharma record date, there were 24,392,841 shares of Pharma common stock and 96,506,157 shares of preferred stock issued and outstanding. |
Voting Rights |
Holders of Class B Common Stock are entitled to one/tenth of one vote per share on all matters submitted to a vote of the stockholders of the Company. Holders of Class B Common Stock do not have cumulative voting rights. | | Holders of Voting Common Stock are entitled to one vote per share on all matters submitted to a vote of the stockholders of the Company. The holders of Non-Voting Common Stock have no voting rights. Holders of Preferred Stock are entitled to the number of votes for each share of Preferred Stock equal to the number of shares of Voting Common Stock into which such share of Preferred Stock could be converted (pursuant to the Certificate of Designations for the relevant series of preferred stock) (the “Preferred Stock Votes”). Holders of Preferred Stock vote together with the Voting Common Stock as a single class on all matters. With respect to matters required to be submitted to a separate class vote of the holders of a particular series of Preferred Stock or all series of Preferred Stock as a whole, holders of Preferred Stock are entitled to (i) one vote for each share |
89
Table of Contents
Holdings | | Pharma |
| | | of Preferred Stock held by such holder, with respect to a class vote by a particular series, and (ii) the number of applicable Preferred Stock Votes for each share of Preferred Stock held by such holder, with respect to a class vote by all series of Preferred Stock as a whole. Certain major decisions (set forth in the Series D Certificate of Designations) require the approval of holders of a majority of the Series D Preferred Stock. Certain major decisions (set forth in the Series B Certificate of Designations) require the approval of holders of a majority of the Series A Preferred Stock, Series B-1 Preferred Stock, Series B-2 Preferred Stock, Series B-3 Preferred Stock and Series B-4 Preferred Stock voting as a single class. Certain major decisions (set forth in the Series C Certificate of Designations) require the approval of holders of a majority of the Series A Preferred Stock, Series B-1 Preferred Stock, Series B-2 Preferred Stock, Series B-3 Preferred Stock, Series B-4 Preferred Stock and Series C Preferred Stock voting as a single class. |
Distributions and Dividends |
Subject to the rights of the holders of Preferred Stock, and subject to any other provisions of the Amended and Restated Certificate of Incorporation, as it may be amended from time to time, holders of Class B Stock shall be entitled to receive such dividends and other distributions in cash, in property or in shares of Holdings as may be declared thereon by the Board of Directors from time to time out of assets or funds of the Holdings legally available therefor; provided, however, that no cash, property or share dividend or distribution may be declared or paid on the outstanding shares of any Class B Stock unless an identical per share dividend or distribution is simultaneously declared and paid on the outstanding shares of the Class A common stock; provided further, however, that (i) a dividend of shares may be declared and paid in Class A Stock to holders of Class A Stock and in Class B Stock to holders of Class B Stock if the number of shares paid per share to holders of Class A Stock and to holders of Class B Stock shall be the same (ii) a dividend or distribution by the Holdings of the securities of another entity may be declared and paid to holders of Common Shares, if the securities distributed to the holders of differing classes of Common Shares have disparate voting and conversion rights, so long as (A) such shares bear the same relative equity rights in the entity whose securities are being distributed, and (B) the disparate voting and conversion rights of the securities being distributed to the Class A Stock and the Class B Stock are not materially different than the relative voting and conversion rights of the Class A Stock and Class B Stock. If Holdings shall in any manner subdivide, combine or reclassify the outstanding shares of Class A Stock or Class B Stock, the outstanding shares of the other class of common stock shall be subdivided, combined or reclassified proportionately in the same manner and on the same basis as the outstanding shares of Class A Stock or Class B Stock, as the case may be, have been subdivided, combined or reclassified. | | Subject to the rights of the holders of Preferred Stock, and subject to any other provisions of the Amended and Restated Certificate of Incorporation, as it may be amended from time to time, holders of Common Stock shall be entitled to receive such dividends and other distributions in cash, in property or in shares of Pharma as may be declared thereon by Pharma’s Board of Directors from time to time out of assets or funds of Pharma legally available therefor; provided, however, that no cash, property or share dividend or distribution may be declared or paid on the outstanding shares of any (i) Common Stock unless an identical per share dividend or distribution is simultaneously declared and paid on the outstanding shares of Preferred Stock, (ii) Series A Preferred Stock unless an identical per share dividend or distribution is simultaneously declared and paid on the outstanding shares of Series B Preferred Stock, Series C Preferred Stock and Series D Preferred Stock, on an as converted basis, (iii) Series B Preferred Stock unless an identical per share dividend or distribution is simultaneously declared and paid on the outstanding shares of Series C Preferred Stock and Series D Preferred Stock, on an as converted basis, and (iv) Series C Preferred Stock unless an identical per share dividend or distribution is simultaneously declared and paid on the outstanding shares of Series D Preferred Stock, on an as converted basis. |
90
Table of Contents
Holdings | | Pharma |
Quorum |
The Holdings bylaws provide that, unless otherwise provided by law, or the Holdings Certificate of
Incorporation, the holders of a majority in voting power of the stock issued and outstanding and entitled to vote at the meeting, present in person, or represented by proxy, shall constitute a quorum for the transaction of business at all meetings of the stockholders. | | The Pharma bylaws provide that, unless otherwise provided by law, or the Pharma Certificate of Incorporation, the holders of a majority of the shares of capital stock issued and outstanding and entitled to vote at the meeting, present in person, or represented by proxy, shall constitute a quorum for the transaction of business at all meetings of the stockholders. |
Record Date |
The Holdings Board may fix a record date for purposes of, among other things, determining the rights of stockholders to notice of or to vote at any stockholder meeting and determining the identity of stockholders entitled to receive payment of any dividend or other distribution. The record date shall not be more than sixty days nor less than ten days before the date of such meeting, nor more than sixty days prior to any other action. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Holdings Board may fix a new record date for the adjourned meeting. | | The Pharma board may fix a record date for purposes of, among other things, determining the rights of stockholders to notice of or to vote at any stockholder meeting and determining the identity of stockholders entitled to receive payment of any dividend or other distribution. The record date shall not be more than sixty days nor less than ten days before the date of such meeting, nor more than sixty days prior to any other action. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Pharma board may fix a new record date for the adjourned meeting. |
Number of Directors |
The Holdings bylaws provide that the Holdings Board shall consist of not less than three nor more than seventeen members, the exact number of which shall be fixed from time to time by the Holdings Board. There are currently eight Holdings directors. | | The Pharma bylaws provide that the Pharma board shall consist of not less than three nor more than nine members. The number of directors may be increased or decreased from time to time by action of the Board of Directors or of the stockholders. There are currently nine Pharma directors. |
Election of Directors |
Pursuant to the Holdings bylaws, directors shall be elected if the votes cast at the Annual Meeting of Stockholders for each nominee’s election exceed the votes cast against such nominee’s election. Each director so elected shall hold office until the expiration of the term of such director (as set forth in the Holdings Certificate of Incorporation) and until his or her successor is duly elected and qualified, or until his or her earlier death or incapacity, resignation, retirement, disqualification or removal from office. Any director may resign at any time upon notice to Holdings. Directors need not be Stockholders. Subject to the terms of any one or more classes or series of Preferred Stock, newly created directorships resulting from any increase in the number of directors and any vacancies in the board resulting from death or incapacity, resignation, retirement, disqualification or removal from office may be filled only by the affirmative vote of a majority of the directors then in office, though less than a quorum, or by a sole remaining director, and directors so elected shall hold office until the next occurring annual meeting of stockholders following appointment and until their successors are duly elected and qualified, or until their earlier death or incapacity, resignation, retirement, disqualification or removal from office. | | Pursuant to the Pharma bylaws, directors are elected at the annual meeting of stockholders by such stockholders as have the right to vote on such election. Each director so elected shall hold office until the expiration of the term of such director (as set forth in the Pharma bylaws) and until the annual meeting after his or her election) until his or her earlier death, resignation or removal from office. Any director may resign at any time upon notice to Pharma. Directors need not be Stockholders. Any vacancies in the board, however occurring, including from enlargement of the board, may be filled only by the affirmative vote of a majority of the directors then in office, though less than a quorum, or by a sole remaining director, and directors so elected shall hold office until the next election of the class for which such director shall have been chosen, subject to the election and qualification of his successor and to such director’s earlier death, resignation or removal. |
91
Table of Contents
Holdings | | Pharma |
Cumulative Voting |
Holdings stockholders do not have cumulative voting rights. | | Pharma stockholders do not have cumulative voting rights. |
Removal of Directors |
Pursuant to the Holdings bylaws, a director or the entire board may be removed at any time, with or without cause, by the holders of issued and outstanding capital stock of Holdings representing not less than a majority of the voting power of all issued and outstanding capital stock of Holdings entitled to vote at an election of directors. | | Pursuant to the Pharma bylaws, a director may be removed only for cause by the affirmative vote of the holders of at least 75% of the votes which all the stockholders would be entitled to cast in any annual election of directors or class of directors. |
Stockholder Proposals |
Governed by the DGCL and the Rule 14a-8 under the Exchange Act, as amended, and the rules and regulations thereunder. | | Governed by the DGCL. |
Stockholder Action by Written Consent |
Any action required or permitted to be taken at any Annual or Special Meeting of Stockholders of Holdings, may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. An electronic transmission consenting to an action to be taken and transmitted by a stockholder or proxyholder, or by a person or persons authorized to act for a stockholder or proxyholder, shall be deemed to be written, signed, and dated, provided that any such electronic transmission sets forth or is delivered with information from which Holding can determine (1) that the electronic transmission was transmitted by the stockholder or proxyholder or by a person or persons authorized to act for the stockholder or proxyholder and (2) the date on which such stockholder or proxyholder or authorized person or persons transmitted such electronic transmission. Any consent by means of electronic transmission shall be deemed to have been signed on the date on which such electronic transmission was transmitted. No consent given by electronic transmission shall be deemed to have been delivered until such consent is reproduced in paper form and until such paper form shall be delivered to Holdings by delivery to its registered office in the State of Delaware, its principal place of business or an officer or agent of Holdings having custody of the book or books in which proceedings of meetings of stockholders are recorded. | | Whenever the vote of stockholders at a meeting thereof is required or permitted to be taken in connection with any corporate action by any provisions of the DGCL or Pharma’s Certificate of Incorporation or bylaws, the meeting and vote of stockholders may be dispensed with if the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted shall consent in writing to such corporate action being taken. |
92
Table of Contents
Holdings | | Pharma |
Special Stockholder Meetings |
Unless otherwise prescribed by law or by the Holdings Certificate of Incorporation, Special Meetings of Stockholders, for any purpose or purposes, may be called by either (i) the Chairman, (ii) the Chief Executive Officer, (iii) the President, (iv) the Corporate Secretary, or (v) any Assistant Secretary, and shall be called by any such officer at the request in writing of a majority of the Board of Directors or at the request in writing of stockholders owning issued and outstanding capital stock of Holdings representing not less than a majority of the voting power of all issued and outstanding capital stock of Holsings. Such request shall state the purpose or purposes of the proposed meeting. | | Special meetings of stockholders (i) may be called at any time only by Pharma’s Chairman of the Board, President or the Board of Directors, and (ii) must be called at the request in writing of a majority of the directors or stockholders entitled to vote or as otherwise required by the DGCL. Business transacted at any special meeting of stockholders is limited to matters relating to the purpose or purposes stated in the notice of meeting. |
Notice of Stockholder Meetings |
Written notice of stockholders’ meetings, stating the place, date, and hour thereof, and, in the case of a special meeting, the purpose or purposes for which the meeting is called, shall be given to each stockholder entitled to vote thereat by or at whose direction the notice is being issued. A copy of the notice of any meeting shall be delivered in accordance with the provisions of Holdings bylaws not less than ten days but not more than sixty days before the date of such meeting, unless a different period is prescribed by law. | | Written notice of stockholders’ meetings, stating the place, date, and hour thereof, and, in the case of a special meeting, the purpose or purposes for which the meeting is called, shall be given to each stockholder entitled to vote thereat by or at whose direction the notice is being issued. A copy of the notice of any meeting shall be delivered in accordance with the provisions of the Pharma bylaws not less than ten days but not more than sixty days before the date of such meeting, unless a different period is prescribed by law. |
Limitation of Personal Liability of Directors |
The Holdings Certificate of Incorporation provides that no Holdings director will be personally liable to Holdings or any of its stockholders for monetary damages for any breach of his or her fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL as the same exists or hereafter may be amended. | | The Pharma Certificate of Incorporation provides that no Pharma director will be personally liable to Pharma or any of its stockholders for monetary damages for any breach of his or her fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL as the same exists or hereafter may be amended, for any breach of the director’s duty of loyalty to Pharma or its stockholders, for acts or omissions not in good faith or which involve intentional misconduct or knowing violation of law, or for any transaction from which the director derived an improper personal benefit... |
Indemnification of Directors and Officers |
Holdings shall indemnify and hold harmless, to the fullest extent permitted by the DGCL, any current or former director or officer who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative by reason of the fact that the person was a director or officer of Holdings or, while serving as a director or officer of Holdings, is or was serving at the request of Holdings as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, enterprise or non-profit entity, against all liability and loss suffered and expenses (including attorneys’ fees, judgments, fines ERISA excise taxes or penalties and amounts paid in settlement) reasonably incurred by such person in connection with any such proceeding. | | Pharma shall indemnify to the full extent authorized by law any person made or threatened to be made a party to an action or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such person, such person’s testator or intestate, is or was a director, officer or employee or agent of Pharma or any predecessor of Pharma or serves or served any other enterprise as a director, officer or employee or agent at the request of Pharma or any predecessor of Pharma. |
93
Table of Contents
Holdings | | Pharma |
Rights Upon Liquidation |
Holders of Class B Common Stock are not entitled to a liquidation preference. | | In the event of any liquidation, dissolution or winding up of Pharma, the holders of shares of Series D Preferred Stock then outstanding are entitled to be paid out of the assets available for distribution to stockholders, before any payment made to the holders of any other stock, a per share amount equal to the applicable Series D Original Issuance Price per share, plus any dividends declared but unpaid thereon. After the payment of all preferential amounts required to be paid to the holders of Series D Preferred Stock, the holders of shares of Series C Preferred Stock then outstanding are entitled to be paid out of the assets then available for distribution to stockholders, if any, on a pari passu basis with each series of Series B Preferred Stock, and before any payment shall be made to the holders of Series A Preferred Stock and Common Stock or any other class or series of capital stock ranking on liquidation junior to the Series B Preferred Stock, a per share amount equal to the applicable Original Issuance Price per share, plus any dividends declared but unpaid thereon. After the payment of all preferential amounts required to be paid to the holders of Series D Preferred Stock, Series C Preferred Stock and Series B Preferred Stock, the holders of shares of Series A Preferred Stock then outstanding are entitled to be paid out of the assets then available for distribution to its stockholders, if any, and before any payment shall be made to the holders of Common Stock or any other class or series of capital stock ranking on liquidation junior to the Series A Preferred Stock, a per share amount equal to the applicable Original Issuance Price per share, plus any dividends declared but unpaid thereon. After the payment of all preferential amounts required to be paid to the holders of Series D Preferred Stock, Series C Preferred Stock, Series B Preferred Stock and Series A Preferred Stock, the holders of shares of Common Stock then outstanding shall be entitled to receive the remaining assets then available for distribution, if any, to its stockholders as otherwise set forth in the Pharma Certificate of Incorporation. |
94
Table of Contents
Holdings | | Pharma |
| | | Notwithstanding the foregoing clause (a), clause (b), clause (c) and clause (d) above, if, in connection with any voluntary or involuntary liquidation, dissolution or winding up of the Corporation (including a Deemed Liquidation Event), the per share amount that the holders of a particular series of Preferred Stock would, if such holders converted all the shares of such series of Preferred Stock into Common Stock immediately prior to such liquidation, dissolution or winding up of the Corporation (including a Deemed Liquidation Event), be entitled to receive pursuant to clause (d) above with respect to the shares of Common Stock issuable upon such conversion of all of such shares of such series of Preferred Stock is greater than the per share amount that each holder of the applicable series of Preferred Stock would, if such holders did not so convert all of the shares of such series of Preferred Stock into Common Stock immediately prior to such liquidation, dissolution or winding up of the Corporation (including a Deemed Liquidation Event), be entitled to receive pursuant to clause (a), clause (b) and clause (c) above with respect to such shares of Preferred Stock, then such holder shall be entitled to be paid out of the assets available for distribution in accordance such greater amount pursuant to such liquidation, dissolution or winding up of the Corporation (including a Deemed Liquidation Event) in full satisfaction of all amounts to which such holder is (or would otherwise be) entitled pursuant to clause (a), clause (b), clause (c) and clause (d) above, without first having to convert such shares of Preferred Stock into Common Stock. |
Certain Business Combinations |
Section 203 of the DGCL generally prohibits a Delaware corporation from engaging in a business combination with an “interested stockholder” that acquires more than 15% but less than 85% of the corporation’s outstanding voting stock for three years following the time that person becomes an “interested stockholder” (generally defined as a holder who (i) together with its affiliates and associates, owns or (ii) is an affiliate or associate of the corporation and, together with that person’s affiliates and associates, has owned at any time within the previous three years, at least 15% of the corporation’s outstanding shares), unless prior to the date the person becomes an interested stockholder, the corporation’s board of directors approves either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder or the business combination is approved by the corporation’s board of directors and by the affirmative vote of at least two-thirds of the corporation’s outstanding voting stock that is not owned by the interested stockholder at a meeting of stockholders (and not by written consent) or other specified exceptions are met. The DGCL allows a corporation’s certificate of incorporation to contain a provision expressly electing not to be governed by Section 203, and Holdings Certificate of Incorporation provides that Holdings is not governed by Section 203. | | |
95
Table of Contents
LEGAL MATTERS
Schwell Wimpfheimer & Associates LLP, counsel for Holdings, will provide an opinion regarding the validity of the Parent Class B Common Stock to be issued in connection with the Transactions.
EXPERTS
The financial statements of Rafael Holdings, Inc. as of and for the years ended July 31, 2020 and 2019 appearing in Rafael Holdings, Inc.’s Annual Report on Form 10-K for the year ended July 31, 2020 have been audited by CohnReznick LLP, independent registered public accounting firm, as set forth in their report thereon, included therein, and incorporated herein by reference. Such financial statements are incorporated herein by reference in reliance upon such report of CohnReznick LLP given on the authority of such firm as experts in accounting and auditing.
The financial statements of Rafael Pharmaceuticals, Inc. as of and for the years ended July 31, 2020 and 2019 have been audited by CohnReznick LLP, independent registered public accounting firm, as set forth in their report thereon, which includes an explanatory paragraph regarding Rafael Pharmaceuticals, Inc.’s ability to continue as a going concern, included herein. Such financial statements are included herein in reliance upon such report of CohnReznick LLP given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement on Form S-4 under the Securities Act with respect to the securities we are offering under this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, where our SEC filings are also available. The address of the SEC’s web site is http://www.sec.gov. We maintain a website at http://www.rafaelholdings.com. Information contained in or accessible through our website does not constitute a part of this prospectus.
96
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information that we file with it into this prospectus, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the information in this prospectus. We incorporate by reference into this registration statement and prospectus the following documents, and any future filings we will make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement but prior to effectiveness of the registration statement and after the date of this prospectus but prior to the termination of the offering of the securities covered by this prospectus (other than current reports or portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K):
• Our Annual Report on Form 10-K for the year ended July 31, 2020, filed with the SEC on October 29, 2020;
• Our Quarterly Reports on Form 10-Q for the quarters ended October 31, 2020, filed with the SEC on December 15, 2020, January 31, 2021 filed with the SEC on March 17, 2021 and April 30, 2021 filed with the SEC on June 14, 2021;
• Our Current Reports on Form 8-K filed with the SEC on September 1, 2020, October 29, 2020, December 11, 2020, December 15, 2020, January 19, 2021, January 28, 2021, March 11, 2021 and March 17, 2021, June 14, 2021, July 15, 2021, August 24, 2021 and September 14, 2021.
• Our definitive proxy statement on Schedule 14A filed with the SEC on November 23, 2020; and
• Description of our Parent Class B Common Stock set forth under Item 11 in Post-Effective Amendment No. 3 to the Registrant’s Registration Statement on Form 10, filed with the SEC on March 26, 2018, and contained in Exhibit 4.2 to our Annual Report on 10-K filed with SEC on October 29, 2020, including any amendment or report filed for the purpose of updating such information.
We will provide each person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated by reference into this prospectus but not delivered with this prospectus upon written or oral request at no cost to the requester. Requests should be directed to: Rafael Holdings, Inc., 520 Broad Street, Newark, New Jersey 07102, Attn: Investor Relations, or you may call us at (212) 658-1450.
97
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
TABLE OF CONTENTS
F-1
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
BALANCE SHEETS
(in thousands, except share and per share data)
| | April 30,
2021 | | July 31,
2020 |
| | | (Unaudited) | | (Note 2) |
ASSETS | | | | | | | | |
CURRENT ASSETS | | | | | | | | |
Cash | | $ | 2,484 | | | $ | 4,932 | |
Marketable securities | | | 75 | | | | 1,403 | |
Prepaid expenses and other current assets | | | 5,246 | | | | 4,354 | |
Total current assets | | | 7,805 | | | | 10,689 | |
| | | | | | | | | |
NON-CURRENT ASSETS | | | | | | | | |
Property and equipment, net | | | 233 | | | | 236 | |
Deferred debt issuance costs | | | 29,120 | | | | 34,902 | |
TOTAL ASSETS | | $ | 37,158 | | | $ | 45,827 | |
| | | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| | | | | | | | | |
CURRENT LIABILITIES | | | | | | | | |
Accounts payable | | $ | 6,535 | | | $ | 7,739 | |
Accrued expenses | | | 6,223 | | | | 6,538 | |
Deferred officers’ salaries | | | 186 | | | | 248 | |
Advances payable to related parties | | | 1,049 | | | | 1,346 | |
Note payable-in default | | | 394 | | | | 298 | |
Convertible notes – related parties | | | 12,239 | | | | 12,239 | |
Total current liabilities | | | 26,626 | | | | 28,408 | |
| | | | | | | | | |
LONG TERM LIABILITIES | | | | | | | | |
Note payable – long term | | | 1,020 | | | | 301 | |
Line of credit | | | 10,000 | | | | — | |
Derivative liabilities | | | 54,685 | | | | 37,432 | |
TOTAL LIABILITIES | | | 92,331 | | | | 66,141 | |
| | | | | | | | | |
COMMITMENTS AND CONTINGENCIES | | | | | | | | |
| | | | | | | | | |
STOCKHOLDERS’ DEFICIT | | | | | | | | |
Convertible Preferred stock, $0.001 par value; 300,000,000 shares authorized; 95,506,177 and 82,885,915 shares issued and outstanding as of April 30, 2021 and July 31, 2020, respectively, liquidation preference of $31,125 as of April 30, 2021 and July 31, 2020 | | | 97 | | | | 83 | |
Common stock, $0.001 par value; 310,000,000 shares authorized; 24,392,841 and 24,381,591 shares issued and outstanding as of April 30, 2021 and July 31, 2020, respectively | | | 24 | | | | 24 | |
Additional paid-in capital | | | 186,208 | | | | 155,407 | |
Accumulated deficit | | | (241,502 | ) | | | (175,828 | ) |
Total stockholders’ deficit | | | (55,173 | ) | | | (20,314 | ) |
TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 37,158 | | | $ | 45,827 | |
See accompanying notes to the financial statements.
F-2
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
(in thousands, except share data)
(Unaudited)
| | For The Nine Months Ended
April 30, |
| | | 2021 | | 2020 |
Revenue | | $ | 1,930 | | | $ | — | |
| | | | | | | | | |
OPERATING EXPENSES | | | | | | | | |
Research and development | | | 28,926 | | | | 24,916 | |
General and administrative | | | 3,782 | | | | 2,760 | |
TOTAL OPERATING EXPENSES | | | 32,708 | | | | 27,676 | |
LOSS FROM OPERATIONS | | | 30,778 | | | | 27,676 | |
| | | | | | | | | |
OTHER INCOME AND (EXPENSE) | | | | | | | | |
Interest income | | | 36 | | | | 353 | |
Interest expense | | | (6,067 | ) | | | (2,156 | ) |
Modification of warrants expense | | | (11,604 | ) | | | (31,530 | ) |
Gain on sales of marketable securities | | | — | | | | 32 | |
Unrealized (loss) gain on marketable securities | | | (8 | ) | | | 115 | |
Change in fair value of derivative liabilities | | | (17,253 | ) | | | (1,832 | ) |
TOTAL OTHER INCOME AND (EXPENSE) | | | (34,896 | ) | | | (35,018 | ) |
NET LOSS | | $ | 65,674 | | | $ | 62,694 | |
See accompanying notes to the financial statements.
F-3
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except share data)
(Unaudited)
| | Preferred Stock | | Common Stock | | Additional Paid-in Capital | | Accumulated Deficit | | Total Stockholders’ Deficiency |
| | | Series A | | Series B | | Series C | | Series D | | Shares | | Par Value | |
| | | Shares | | Par Value | | Shares | | Par Value | | Shares | | Par Value | | Shares | | Par Value | |
Balance as of July 31, 2019 | | 3,057,500 | | $ | 3 | | 26,454,278 | | $ | 27 | | — | | $ | — | | 53,374,137 | | $ | 53 | | 21,769,278 | | $ | 22 | | $ | 117,421 | | $ | (96,528 | ) | | $ | 20,998 | |
Stock-based compensation resulting from stock options granted to non-employees and employees | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 1,009 | | | — | | | | 1,009 | |
Common stock issued for related party LOC | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 2,612,313 | | | 2 | | | 5,110 | | | — | | | | 5,112 | |
Common stock issued in exchange for notes and interest | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | | — | |
Common stock issued in exchange for warrants | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | | — | | | | — | |
Modification of warrants | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 31,530 | | | — | | | | 31,530 | |
Net loss | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | | (62,694 | ) | | | (62,694 | ) |
Balance as of April 30, 2020 | | 3,057,500 | | | 3 | | 26,454,278 | | | 27 | | — | | | — | | 53,374,137 | | | 53 | | 24,381,591 | | | 24 | | | 155,070 | | | (159,222 | ) | | | (4,045 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of July 31, 2020 | | 3,057,500 | | | 3 | | 26,454,278 | | | 27 | | — | | | — | | 53,374,137 | | | 53 | | 24,381,591 | | | 24 | | | 155,407 | | | (175,828 | ) | | | (20,314 | ) |
Stock-based compensation resulting from stock options granted to non-employees and employees | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 1,367 | | | — | | | | 1,367 | |
Preferred stock issued for exercise of warrants | | — | | | — | | — | | | — | | 6,321,312 | | | 6 | | — | | | — | | — | | | — | | | 8,685 | | | — | | | | 8,691 | |
Preferred stock issued for exercise of warrants | | — | | | — | | — | | | — | | — | | | — | | 7,298,950 | | | 8 | | — | | | — | | | 9,116 | | | — | | | | 9,124 | |
Common stock issued for exercise of warrants | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 11,250 | | | — | | | 28 | | | — | | | | 28 | |
Modification of warrants | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 11,605 | | | — | | | | 11,605 | |
Net loss | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | | (65,674 | ) | | | (65,674 | ) |
Balance as of April 30, 2021 | | 3,057,500 | | $ | 3 | | 26,454,278 | | $ | 27 | | 6,321,312 | | $ | 6 | | 60,673,087 | | $ | 61 | | 24,392,841 | | $ | 24 | | $ | 186,208 | | $ | (241,502 | ) | | $ | (55,173 | ) |
See accompanying notes to the financial statements.
F-4
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
(Unaudited)
| | For The Nine Months Ended
April 30, |
| | | 2021 | | 2020 |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | |
Net loss | | $ | (65,674 | ) | | $ | (62,694 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization | | | 51 | | | | 55 | |
Amortization of debt issuance cost | | | 5,782 | | | | 1,842 | |
Noncash stock-based compensation | | | 1,367 | | | | 1,009 | |
Income from investments in marketable securities | | | (110 | ) | | | (145 | ) |
Change in fair value of derivative liabilities | | | 17,253 | | | | 1,832 | |
Change in fair value of warrants due to modification | | | 11,604 | | | | 31,530 | |
Changes in operating assets and liabilities: | | | | | | | | |
Prepaid expenses and other current assets | | | (892 | ) | | | 438 | |
Accounts payable | | | (1,204 | ) | | | 445 | |
Accrued expenses | | | 585 | | | | 5,444 | |
Payment of deferred officers’ salaries | | | (62 | ) | | | (323 | ) |
Due to related party | | | 360 | | | | (280 | ) |
NET CASH USED IN OPERATING ACTIVITIES | | | (30,940 | ) | | | (20,846 | ) |
| | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
Purchases of property and equipment | | | (48 | ) | | | (77 | ) |
Proceeds from sale and maturity of marketable securities | | | 1,438 | | | | 7,184 | |
NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | | | 1,391 | | | | 7,107 | |
| | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
Proceeds from note payable | | | 815 | | | | — | |
Proceeds from line of credit | | | 10,000 | | | | — | |
Preferred stock issued for exercise of warrants | | | 16,258 | | | | — | |
Common stock issued for exercise of options | | | 28 | | | | — | |
NET CASH PROVIDED BY FINANCING ACTIVITIES | | | 27,101 | | | | — | |
| | | | | | | | | |
NET DECREASE IN CASH | | | (2,448 | ) | | | (13,739 | ) |
CASH AT BEGINNING OF PERIOD | | | 4,932 | | | | 17,426 | |
CASH AT END OF PERIOD | | $ | 2,484 | | | $ | 3,687 | |
| | | | | | | | | |
NONCASH FINANCING ACTIVITY | | | | | | | | |
Conversion of due to related party payble for exercise of warrants | | $ | 657 | | | $ | — | |
Conversion of accrued expenses for exercise of warrants | | $ | 900 | | | $ | — | |
Common stock issued for related party LOC | | $ | — | | | $ | 5,112 | |
Deferred debt issuance costs for related party LOC | | $ | — | | | $ | 40,943 | |
See accompanying notes to the financial statements.
F-5
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
1. ORGANIZATION, BUSINESS OVERVIEW AND MANAGEMENT’S PLAN
Rafael Pharmaceuticals, Inc. (formerly Cornerstone Pharmaceuticals, Inc.) was formed as a result of a merger in 2002 between Cornerstone Ventures, LLC (“Ventures”), a New York limited liability company which was organized on May 14, 1999, and Rafael Pharmaceuticals, Inc., a New York corporation which was incorporated on November 1, 2001, with Rafael Pharmaceuticals, Inc. (the “Company”) being the surviving entity. On December 24, 2009, the Company (New York) was merged with and into a newly formed Delaware corporation by the same name, for the purpose of changing domiciles. This merger had no financial or accounting effects on the Company. On May 10, 2017, the Board approved the resolution changing the name to Rafael Pharmaceuticals, Inc.
The Company is a privately held, clinical stage, oncology-focused biopharmaceutical company engaged in developing therapeutics that exploit and target cancer cell metabolism. The Company is currently developing a technology platform. The drug candidate is in the development phase and have not received regulatory approval. The Company has yet to generate any revenues from its product candidate in development. The Company has performed certain research related activities under grant and service agreements for which revenue was earned and recognized.
Since inception, the Company has incurred significant losses from operations and has not generated positive cash flows from operations. In addition, at April 30, 2021, the Company does not have any revenue stream to support its cost structure. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts and the classification of liabilities that might be necessary from the outcome of this uncertainty.
Management’s Plan
The development of the Company’s products will require a commitment of substantial funds to conduct the costly and time-consuming research, preclinical and clinical testing necessary to bring such products to market and to establish, acquire or contract for manufacturing and marketing capabilities. Future capital requirements will depend on factors including scientific progress in research and development programs, the Company’s ability to establish collaborative arrangements with others for drug development, progress with preclinical and clinical trials, the time and costs involved in obtaining regulatory approvals and effective commercialization activities. Since inception, the Company has incurred significant losses from operations and has not generated positive cash flows from operations. In addition, at April 30, 2021, the Company does not have any revenue stream to support its cost structure. The Company will need to raise substantial additional capital in order to bring its products to market and reach additional milestones in its licensing agreement in order to recognize revenue. Additional capital is necessary to fund planned activities beyond that date or expand operating activities. The Company has secured a $50 million Line of Credit which the Company will use for planned activities and to expand its activities (see Note 7). As of April 30, 2021, the Company has drawn down $10,000,000 on its Line of Credit and the remaining amount available to draw down is $40,000,000. There can be no assurance that such new offerings will be consummated or that such collaborative ventures will be entered into on favorable terms, if at all. If such new offerings are not consummated or additional financing is not otherwise available, the Company will be required to modify its business development plans or reduce or cease certain or all operations. In the event that any additional funding is obtained, such financings may have a dilutive effect to current stockholders. The Company plans on merging with Rafael Holdings (see Note 16).
As of April 30, 2021, the Company is in default on approximately $12.2 million of convertible notes as they are beyond their payment terms.
F-6
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation — The Company’s financial statements are prepared using accounting principles generally accepted in the United States of America (“U.S. GAAP”) applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
Operating results for the nine months ended April 30, 2021 and 2020 are not necessarily indicative of the results that may be expected for the fiscal year ending July 31, 2021. The balance sheet at July 31, 2020 has been derived from the Company’s audited financial statements at that date but does not include all of the information and footnotes required by U.S. GAAP for complete financial statements. Therefore, these financial statements should be read in conjunction with the Company’s audited financial statements and notes thereto for the years ended July 31, 2020 and 2019.
Use of Estimates — The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. Estimates also affect the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. The useful life of certain acquired property and equipment, fair value of leasehold improvements for the Cranbury, New Jersey facility (see notes 14 and 15), fair value of the of stock-based compensation expense, allocation of certain expenses between general and administrative and research and development expenses, accounting for the issuance of warrants, and accounting for certain income tax items including our conclusion to establish a valuation allowance, require the significant use of estimates and assumptions. Management believes that amounts recorded based on estimates and assumptions are reasonable and any differences between estimates and actual amounts will not have a material impact on the Company’s financial statements.
Cash and Marketable Securities — Cash consists of cash and deposits of all highly liquid debt instruments of three months or less that are readily convertible into cash.
The Company’s investments in marketable securities have been classified and accounted for as trading securities. The Company’s marketable securities are measured at fair value with gains and losses recognized in other income/(expense). The cost of securities sold is determined using the specific identification method.
Property and Equipment — Property and equipment consist of office equipment, laboratory equipment and leasehold improvements. Property and equipment are recorded at cost less accumulated depreciation and amortization. The related depreciation and amortization is computed using the straight-line method over the estimated useful lives of the related assets as follows: office equipment and laboratory equipment — three to five years; and leasehold improvements — 10 years, which represents the lesser of the estimated life of the assets or the lease term. Minor maintenance and repairs, and minor renewals and betterments are charged to expense as incurred.
Advances Payable to Related Parties — Funds were advanced to the Company from time to time for operating purposes from related parties. Advances payable to related parties were $1.0 million and $1.3 million at April 30, 2021 and July 31, 2020, respectively.
Long-Lived Assets — The Company reviews its long-lived assets for possible impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be fully recoverable. An impairment loss is recognized whenever the carrying amount of a long-lived asset exceeds the expected future cash flows, on an undiscounted basis, to be generated from the asset, including eventual disposition. If such review indicates that the carrying amount of long-lived assets is not recoverable, the carrying amount of such assets is reduced to fair value. The Company’s management determined that no events or changes in circumstances have indicated that asset-carrying values were no longer recoverable. No impairment charges were recorded during the nine months ended April 30, 2021 and 2020.
F-7
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Income Taxes — Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. Valuation allowances have been established as it is more likely than not that the deferred tax assets will not be realized.
The Company files income tax returns in the U.S. federal jurisdiction and certain state jurisdictions. The tax years subject to audit are 2016 through 2019. The Company does not believe there will be a material change in its recognized tax positions over the next 12 months.
The Company recognizes the effect of uncertain income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs.
The Company has sustained losses since inception, which has generally resulted in a zero percent effective tax rate.
The Company’s policy is to recognize interest and penalties accrued on tax matters as a component of income tax expense. The Company has not incurred any interest or penalties.
Revenue Recognition — In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606), or ASU 2014-09. The objective of the ASU is to establish a single comprehensive model for entities to use in accounting for revenue arising from contracts with customers, which supersedes most of the existing revenue recognition guidance, including industry-specific guidance. The core principle is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In applying the ASU, companies will perform a five-step analysis of transactions to determine when and how revenue is recognized. The five-step analysis consists of the following: (i) identifying the contract with a customer, (ii) identifying the performance obligations in the contract, (iii) determining the transaction price, (iv) allocating the transaction price to the performance obligations in the contract and (v) recognizing revenue when (or as) the entity satisfies a performance obligation. ASU 2014-09 applies to all contracts with customers except those that are within the scope of other topics in the FASB’s ASC. The Company adopted ASU 2014-09 effective August 1, 2020 using the modified retrospective approach. The Company reviewed all contracts that were not completed as of August 1, 2020 and the adoption did not have a material impact on the Company’s financial statements.
The Company derives its revenue from the License of the Licensed Compounds and products which falls within the scope of accounting standard codification ASC 606, Revenue from Contracts with Customers (“ASC 606”) and is accounted for at the point in time when control of the goods or services transfers to the customer and the Company’s performance obligation is satisfied, consistent with the Company’s previous accounting.
In June 2019, the Company signed a license agreement with a third party whereby the licensor was granted exclusive license and rights to develop, manufacture, and commercialize the licensed compounds and the licensed products in the licensor territory. The licensor territory consists of Japan, South Korea, Taiwan, and all ASEAN Countries which are Indonesia, Thailand, Malaysia, Singapore, Philippines, Vietnam, Myanmar, Cambodia, Laos and Brunei. The Company recognized $1.9 million in revenue for the nine months ended April 30, 2021 due to milestones reached under, the license agreement.
Research and Development Costs and Expenses — Research and development costs and expenses consist primarily of salaries and related personnel expenses, stock-based compensation, fees paid to external service providers, laboratory supplies, costs for facilities and equipment, license costs, and other costs for research and development
F-8
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
activities. Research and development expenses are recorded in operating expenses in the period in which they are incurred. Estimates have been used in determining the liability for certain costs where services have been performed but not yet invoiced. The Company monitors levels of performance under each significant contract for external service providers, including the extent of patient enrollment and other activities through communications with the service providers to reflect the actual amount expended.
Concentrations of Risk — Financial instruments which potentially subject the Company to concentrations of risk principally consist of cash. At times the Company invests excess cash primarily in money market funds backed by U.S. Treasury collateral. At times, such funds may exceed federally insured limits. At times during April 30,2021 and July 31, 2021, the Company had cash that was in excess of the federally insured limits. The Company places its temporary cash investments with high credit quality institutions.
Any products developed by the Company will require approval from the U.S. Food and Drug Administration (“FDA”) or foreign regulatory agencies prior to commercial sales. There can be no assurance that the Company’s products will receive the necessary approvals. If the Company is denied such approvals or such approvals are delayed, it could have a material adverse effect on the Company.
The Company’s drug candidates are in the development phase and none have received regulatory approval. To achieve profitable operations, the Company must successfully develop, test, manufacture and market its products. There can be no assurance that any such products can be developed successfully or manufactured at an acceptable cost and with appropriate performance characteristics, or that such products will be successfully marketed. These factors could have a material adverse effect on the Company’s future financial results.
Stock-Based Compensation — The Company accounts for its stock-based compensation awards in accordance with ASC Topic 718, Compensation — Stock Compensation (“ASC 718”). ASC 718 requires all grants of employee stock options to be recognized in the statement of operations based on their grant date fair values. For stock options granted to employees and to members of the Board of Directors for their services on the Board of Directors, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option- pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock. The Company records compensation cost over the related service periods.
The Company recorded stock-based option compensation as follows (in thousands):
| | For the Nine Months Ended April 30, |
| | | 2021 | | 2020 |
Research and development | | $ | 1,264 | | $ | 928 |
Administrative | | | 103 | | | 81 |
Total | | $ | 1,367 | | $ | 1,009 |
No related tax benefits from stock compensation expense were recognized for the nine months ended April 30, 2021 and 2020.
Warrants — The Company accounts for warrants to purchase stock based on guidelines provided in ASC Topic 815, Derivatives and Hedging — Contracts in Entity’s Own Equity (“ASC 815”) which provides guidance on contracts that are settled in the Company’s own shares as either a liability or as an equity instrument depending on the warrant agreement. The Company uses the Black-Scholes or trinomial pricing models, depending on the applicable terms of the warrant agreement, to value the derivative warrant liabilities in bifurcating such amount from convertible notes payable. (See Notes 8 and 11).
F-9
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its financial position, results of operations or cash flows upon adoption.
In February 2016, the FASB issued Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842). ASU 2016-02 increases the transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. Certain qualitative and quantitative disclosures are required, as well as a retrospective recognition and measurement of impacted leases. The new ASU is effective for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022, with early adoption permitted. We are currently evaluating ASU 2016-02 to determine the impact on the Company’s financial position, results of operations or cash flows.
In August 2017, the FASB issued ASU 2017-12, Derivatives and Hedging (Topic 850), the objective of which is to improve the financial reporting of hedging relationships to better portray the economic results of an entity’s risk management activities in its financial statements. In addition, the amendments in this update make certain targeted improvements to simplify the application and disclosure of the hedge accounting guidance in current generally accepted accounting principles. For public business entities, the amendments in this update are effective for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2019, and interim periods beginning after December 15, 2020. Early adoption is permitted in any period after issuance. For cash flow and net investment hedges existing at the date of adoption, an entity should apply a cumulative-effect adjustment related to eliminating the separate measurement of ineffectiveness to accumulated other comprehensive income with a corresponding adjustment to the opening balance of retained earnings as of the beginning of the fiscal year that an entity adopts the amendments in this update. The amended presentation and disclosure guidance is required only prospectively. The Company adopted this update on August 1, 2020, however the adoption of this standard did not have any impact on the Company’s financial position, results of operations or cash flows.
In June 2018, the FASB issued ASU 2018-07, Improvements to Nonemployee Share-Based Payment Accounting, which expands the scope of Topic 718, Compensation — Stock Compensation, to include all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. The guidance is effective for interim and annual periods beginning after December 15, 2018, our fiscal 2020. Early adoption is permitted. The adoption of this standard did not have any impact on the Company’s financial position, results of operations or cash flows.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement — Disclosure Framework (Topic 820). The updated guidance improves the disclosure requirements on fair value measurements. The updated guidance if effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. Early adoption is permitted for any removed or modified disclosures. The adoption of this standard did not have any impact on the Company’s financial position, results of operations or cash flows.
F-10
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
3. FAIR VALUE MEASUREMENTS
ASC Topic 820, Fair Value Measurement and Disclosures establishes a consistent framework in how to value the fair value of assets and liabilities. This framework is intended to increase consistency in how fair value determinations are made under various existing accounting standards that permit, or in some cases require, estimates of fair value. This standard also expands financial statement disclosure requirements about a company’s use of fair value measurements, including the effect of such measures on earnings.
This standard defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This standard also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Three levels of inputs are used to measure fair value:
| | Level 1 — | | Quoted prices in active markets for identical assets or liabilities. |
| | | Level 2 — | | Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| | | Level 3 — | | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Reported amounts for cash, accounts payable and stockholder advances are generally considered to be representative of their respective fair values because of the short-term nature of those instruments and are considered Level 1.
As required by ASC 820-10, assets and liabilities fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability.
Convertible promissory notes were recorded based on the face values as of their respective issuance dates with the exception of the Convertible Promissory Note — D Series (“D Note”) dated September 16, 2016 with a face value of $10,000,000. The proceeds allocated to Series D Note is adjusted for amortization for the difference between the issuance value and the fair value as of the date of issuance. Due to close proximity to the issuance dates, including the relatively short maturity dates, the carrying amounts of the convertible notes were valued as of their issuance dates except for the D Note which was carried at the initial fair value measurement date as adjusted for discount amortization. The Company concluded that an estimation of the fair value of the convertible promissory notes was not practical due to the short-term maturity obligations of the respective instruments. (See Note 8).
The Company determined that the anti-dilution feature of the line of credit (see Note 7) should be recognized as a free-standing instrument that meets the definition of a derivative under ASC 815. Accordingly, the Company recorded the value of the anti-dilution feature as a derivative liability which will be stated at fair value at each reporting period with changes in fair value being recorded as a marked-to-market adjustment in the Company’s statement of operations. For the significant assumptions the Company used to calculate the derivative liability (see note 9).
F-11
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
3. FAIR VALUE MEASUREMENTS (cont.)
The following table sets forth the Company’s assets and liabilities fair value at April 30, 2021 and July 31 2020 by level within the fair value hierarchy:
| | Carrying
Value | | Fair Value Measurement as of April 30, 2021 |
Level 1 | | Level 2 | | Level 3 |
Assets: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 2,484 | | $ | 2,484 | | $ | — | | $ | — |
Marketable Securities | | | 75 | | | 75 | | | — | | | — |
| | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | |
Convertible Notes | | $ | 12,239 | | | — | | $ | — | | $ | 12,239 |
Derivative liabilities | | | 54,685 | | $ | — | | | — | | | 54,685 |
| | Carrying
Value | | Fair Value Measurement as of July 31, 2020 |
Level 1 | | Level 2 | | Level 3 |
Assets: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 4,932 | | $ | 4,932 | | $ | — | | $ | — |
Marketable Securities | | | 1,403 | | | 1,403 | | | — | | | — |
| | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | |
Convertible Notes | | $ | 12,239 | | | — | | $ | — | | $ | 12,239 |
Derivative liabilities | | | 37,432 | | | — | | | — | | | 37,432 |
The following table sets forth the Company’s change in derivative liability for the nine months ended April 30, 2021:
| | Derivative Liability –
Line of Credit |
Balance as of July 31, 2020 | | $ | 37,432 |
Additions during the period | | | — |
Change in fair value | | | 17,253 |
Balance as of April 30, 2021 | | $ | 54,685 |
4. PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets are comprised of the following:
| | April 30,
2021 | | July 31,
2020 |
Deposits with vendors and service providers | | $ | 5,244 | | $ | 4,352 |
Insurance | | | 2 | | | 2 |
Total | | $ | 5,246 | | $ | 4,354 |
F-12
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
5. PROPERTY AND EQUIPMENT
Property and equipment, net is comprised of the following:
| | April 30,
2021 | | July 31,
2020 |
Laboratory equipment | | $ | 985 | | | $ | 947 | |
Computer and office equipment | | | 150 | | | | 140 | |
Leasehold improvements | | | 2,089 | | | | 2,089 | |
| | | | 3,224 | | | | 3,176 | |
Less: Accumulated depreciation and amortization | | | (2,991 | ) | | | (2,940 | ) |
| | | $ | 233 | | | $ | 236 | |
Depreciation and amortization expense was $50 and $55 for the nine months ended April 30,2021 and 2020, respectively.
6. ACCRUED EXPENSES
| | April 30,
2021 | | July 31,
2020 |
Interest (due to convertible note holders) | | $ | 3,029 | | $ | 2,708 |
Compensation | | | 50 | | | 50 |
Clinical and research costs | | | 3,144 | | | 3,586 |
Other | | | — | | | 110 |
Legal and professional fees | | | — | | | 84 |
Total | | $ | 6,223 | | $ | 6,538 |
Compensation costs include unpaid employee salaries and benefits and severance obligations to former executives.
7. NOTES PAYABLE
Paycheck Protection Program Loan
In May 2020, the Company was granted a loan (the “PPP Loan”) in the amount of $599,242, pursuant to the Paycheck Protection Program (the “PPP”) under Division A, Title I of the CARES Act, which was enacted March 27, 2020. The application for these funds required the Company to, in good faith, certify that the current economic uncertainty made the loan request necessary to support the ongoing operations of the Company.
In April 2021, the Company received an additional $814,642 of PPP loan. The maturity of the note is five years and bear interest at a rate of 1.00% per annum, and monthly payments commencing ten months after the loan forgiveness covered period.
The first PPP loan matures two years, and the second PPP loan matures five years after being granted. Both PPP loan’s bears interest at a rate of 1.00% per annum, payable in monthly payments commencing six months after loan disbursements. The notes may be prepaid at any time prior to maturity with no prepayment penalties. Funds from the PPP Loan may only be used for payroll costs and any payments of certain covered interest, lease and utility payments. Under the terms of the PPP, certain amounts of the Loan may be forgiven if they are used for qualifying expenses as described in the CARES Act. The Company is currently applying for forgiveness, but no assurance can be provided that the Company will obtain forgiveness of the PPP Loan in whole or in part. As of April 30, 2021 the current portion of the loan is $394,315 and the non-current portion is $1,019,569.
F-13
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
7. NOTES PAYABLE (cont.)
The following is a schedule by year of future PPP loan payments at April 30, 2021:
Year Ending July 31, | | Note
payable |
Remainder 2021 | | $ | 294,272 |
2022 | | | 312,724 |
2023 | | | 217,133 |
2024 | | | 219,315 |
2025 | | | 221,518 |
Thereafter | | | 148,922 |
Total | | $ | 1,413,884 |
Line of Credit Agreement
On February 3, 2020, the Company entered into a Line of Credit Loan Agreement (“Line of Credit Agreement”) with RP Finance, LLC (“RP Finance”), which provides a revolving commitment of up to $50,000,000 to fund clinical trials and other capital needs.
Rafael Holdings, Inc. owns 37.5% of the equity interests in RP Finance and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. Howard Jonas owns 37.5% of the equity interests in RP Finance, and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. The remaining 25% equity interests in RP Finance is owned by other shareholders of the Company.
Under the Line of Credit Agreement, all funds borrowed will bear interest at the mid-term Applicable Federal Rate published by the U.S. Internal Revenue Service. The maturity date is the earlier of February 3, 2025, upon a change of control of the Company or a sale of the Company or its assets. The Company can draw on the facility on 60 days’ notice. The funds borrowed under the Line of Credit Agreement must be repaid out of certain proceeds from equity sales by the Company.
In connection with entering into the Line of Credit Agreement, the Company agreed to issue to RP Finance 2,612,313 shares of its common stock, representing 12% of the issued and outstanding shares of common stock, with such interest subject to anti-dilution protection as set forth in the Line of Credit Agreement. The common stock issued was valued at a price of $1.95 a share, or $5,112,297. The anti-dilution clause was valued at $33,580,762, which was recorded as a derivative liability. The total aggregate value of the stock issued and the anti-dilution clause was approximately $38,693,059, which the Company recorded as deferred debt issuance costs, net and will be amortized over the life of the line of credit on a straight line basis. For the nine months ended April 30, 2021 and 2020, the Company recorded approximately $5,782,000 and $1,843,000 as amortization expense respectively. As of April 30, 2021, the remaining amount of the deferred debt issuance cost was $29,120,392.
As of April 30, 2021, the Company has drawn down $10,000,000 on its Line of Credit and the remaining amount available to draw down is $40,000,000.
8. CONVERTIBLE NOTES
The Promissory Notes issuance through April 30, 2021 are as follows:
| | Total | | C Notes(1)(2) | | D Notes(3) |
Balance as of July 31, 2020 | | | 12,239 | | $ | 12,239 | | | — |
Converted | | | — | | | — | | | — |
Balance at April 30, 2021 | | $ | 12,239 | | $ | 12,239 | | $ | — |
F-14
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
8. CONVERTIBLE NOTES (cont.)
The Company accounted for the D Note transaction in accordance ASC 470 and ASC 815. The proceeds were allocated based on the fair value of the note, warrant, equity bonus, and a conversion feature using the Black-Scholes calculation as of the date of the transaction. Thus, the D Note was valued at $3.1 million net of deferred financing charges of approximately $125,000 at issuance. The fair value of the note was calculated using the Black-Scholes method.
F-15
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
8. CONVERTIBLE NOTES (cont.)
The following assumptions were utilized in the Black-Scholes method:
Expected term (years) | | 2.0 |
Risk-free interest rate | | 0.69% |
Volatility | | 96.2% |
Dividend yield | | None |
During the nine months ended April 30, 2021 and 2020, the Company recorded $285,584 and $312,102 of convertible note interest expense, respectively.
9. DERIVATIVE LIABILITIES
The Company determined that the anti-dilution feature of the line of credit (see Note 7) should be recognized as a free-standing instrument that meets the definition of a derivative under ASC 815. Accordingly, the Company recorded the value of the anti-dilution feature as a derivative liability which will be stated at fair value at each reporting period with changes in fair value being recorded as a marked-to-market adjustment in the Company’s statement of operations.
The fair value of the derivative liability — Line of Credit is estimated using a Monte Carlo pricing model with the following assumptions:
Market value of common stock | | $2.72 |
Expected volatility | | 93.0% |
Expected term (in years) | | 3.76 |
Risk-free interest rate | | 0.53% |
10. STOCKHOLDERS’ EQUITY
Common Stock — In September 2016, the Company increased the number of authorized shares for common stock from 175,000,000 shares to 310,000,000 including 10,000,000 shares of non-voting common stock. Dividends on common stock will be paid when and if declared by the Board of Directors, subject to restrictions of any other equity security. Each holder is entitled to vote on all matters except for matters exclusively related to other securities. Each holder is entitled to one vote for each common share held. The Company at all times reserves enough common stock to affect a conversion of the shares of other securities into common stock.
On February 3, 2020, the Company entered into a Line of Credit Loan Agreement with RP Finance, LLC (“RP Finance”), which provides a revolving commitment of up to $50,000,000 to fund clinical trials and other capital needs. In connection with entering into the Line of Credit Agreement, the Company agreed to issue to RP Finance 2,612,313 shares of its common stock representing 12% of the issued and outstanding shares of common stock. The shares were valued at a price of $1.95 a share, or $5,112,297.
During the nine months ended April 30, 2021, 11,250 shares of common stock were issued upon exercise of employee options.
Convertible Preferred Stock — In September 2016, the Company increased the number of authorized shares for preferred stock from 100,000,000 shares to 300,000,000. Dividends will be paid when and if declared by the Board of Directors, subject to restrictions of various equity and debt securities. The Company has not declared any dividends on any class of its preferred or common stock. Each preferred stockholder is entitled to vote based on the number of shares held as if converted to common stock. Certain series of preferred stock require separate approval of a majority of the holders of that series. For changes in articles of incorporation, the majority of preferred stock must approve any actions. The various series of preferred stock have convertible features which provide for mandatory or automatic conversions to common stock and non-mandatory conversions to common stock or other securities. Each issue provides for a conversion formula which is based on the lower of the started conversion rate or a rate based on the new equity offering.
F-16
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
10. STOCKHOLDERS’ EQUITY (cont.)
In the event of a liquidation, certain series of preferred stock would receive its stated liquidated values before other series and preferred stock would receive liquidated values prior to the common stockholders. Neither the Company nor the holder of the preferred stock have the right to redeem or cause the redemption of the preferred stock. Certain series of preferred stockholders have the right to be represented by a Board of Director member.
The following summarizes the Preferred Stock:
| | April 30,
2021 | | July 31,
2020 |
| | |
Series | | Shares
Authorized | | Shares
Issued | | Liquidation
Preference | | Shares
Authorized | | Shares
Issued | | Liquidation
Preference |
A | | 6,050,000 | | 3,057,500 | | $ | 3,057,500 | | 6,050,000 | | 3,057,500 | | $ | 3,057,500 |
B-1 | | 1,892,072 | | 1,513,658 | | | 1,892,072 | | 1,892,072 | | 1,513,658 | | | 1,892,072 |
B-2 | | 17,500,000 | | 10,000,000 | | | 10,000,000 | | 17,500,000 | | 10,000,000 | | | 10,000,000 |
B-3 | | 30,000,000 | | 10,000,000 | | | 10,000,000 | | 30,000,000 | | 10,000,000 | | | 10,000,000 |
B-4 | | 10,400,000 | | 4,940,620 | | | 6,175,775 | | 10,400,000 | | 4,940,620 | | | 6,175,775 |
C | | 25,000,000 | | 6,321,312 | | | — | | 25,000,000 | | — | | | — |
D | | 161,000,000 | | 60,673,087 | | | — | | 161,000,000 | | 53,374,137 | | | — |
| | | 251,842,072 | | 95,506,177 | | $ | 31,125,347 | | 251,842,072 | | 82,885,915 | | $ | 31,125,347 |
The above tables include 251,842,072 in shares authorized under their respective series as April 30, 2021, and July 31, 2020; however, the Company has 300,000,000 shares authorized for all series of Preferred Stock.
The following are the various classes of preferred stock that the Company has issued:
Series A — Contemporaneous with the merger in 2002 as discussed in Note 1, the Company issued 3,057,500 shares of $.001 par value Series A Convertible Preferred Stock (“Series A Preferred”) to a group of investors for $1.00 per share. Each share of Series A Preferred is convertible into common stock at a conversion price of $1.00 per share. Series A Preferred is automatically converted into common stock upon the effectiveness of the Company’s filing of an S-1 registration statement with the Securities and Exchange Commission or from the written notification of a majority of the holders of the Series A Preferred share. The conversion price is adjusted for dilutive issuances subject to certain exclusions.
Series B-1, B-2 and B-3 — Prior to 2009, two investors made investments in units, each unit consisting of common stock with a liquidation preference and non-callable warrants. The issuance of Series B-4 Convertible Preferred Stock in 2009 (Series B-4 Preferred), the investment (prior to 2009) for 17,713,658 units were exchanged for shares of Series B-1, B-2, and B-3 Convertible Preferred Stock and non-callable warrants. In 2011, the Company issued an additional 3.8 million units. Series B-1, B-2 and B-3 were entitled to appoint two members of the Board of Directors. As of July 31, 2019, the warrants have either expired or were exchanged for common stock.
Series B-4 — In 2009, a group of investors invested $6,175,750 for 4,940,620 units of Series B-4 Convertible Preferred (Series B-4 Preferred) shares and non-callable warrants. Each warrant provides for the purchase of Series B-4 Preferred at $1.375 per share. Such warrants expired December 2013. The Preferred Series B-4 Preferred Stock stockholders and the Company entered into an Investor’s Rights Agreement providing certain registration rights, rights of participation in subsequent financings excluding among other items underwritten public offerings. Additionally, the Preferred Series B-4 conversion (to common stock) price is adjusted for certain dilutive issuances until the Company receives proceeds of $10 million in equity. In addition, the Company issued non- callable finder warrants to purchase up to 518,764 shares of Series B-4 Preferred, of which 172,922 expired in December 2013 and 345,842 expired on December 31, 2016.
The Series B Preferred have a liquidated value superior to Series A Preferred. Series B Preferred will automatically convert if there is a firm commitment of a fully underwritten public offering of at least $10 million or if the majority of the Series B Preferred holders (of which the B-1, B-2 and B-3 equaled the majority) agreed to convert.
F-17
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
10. STOCKHOLDERS’ EQUITY (cont.)
Series C — The Series C Preferred Stock will automatically convert into common stock in the event of a qualified public offering providing at least $20,000,000 and the common shares are traded on a nationally recognized exchange. The Series C Preferred Stock will also automatically convert to common stock upon the written consent of the majority of outstanding shares of the Preferred Stock voting as a single class. Holders of C Notes can convert into Series C Preferred and warrants to purchase Series C Preferred Stock.
Series D — The Series D Preferred shares will automatically convert into common stock in the event of a qualified public offering which is underwritten by an underwriter of international standing and traded on an internationally recognized exchange or NASDAQ, and such underwriting has a minimum equity valuation of $200,000,000 and results in aggregate cash proceeds of not less than $50,000,000. The Series D Preferred shares will also automatically convert to common stock upon the written consent of the majority of outstanding shares of the Series D preferred shares. The holder of the Series D Preferred shares has liquidation rights superior to other stockholders equal to the Original Issuance Amount based on $1.25 per share. As of July 31, 2020, 53.4 million shares of Series D Preferred stock were issued upon partial exercise of the Series D Warrant and the conversion of the D Note plus accrued interest.
11. WARRANTS
Preferred Convertible Series C Warrants — The warrants were issued in connection with Series C Convertible Notes. The notes convert into “Series C Units”. Each unit is comprised of one share of the Company’s Series C Convertible Preferred Stock (“C Preferred Stock”) at a conversion price of $1.25 per unit, and one warrant to purchase one share of the Company’s C Preferred Stock at an exercise price of $1.375 per share. The warrants expire four years from the initial date of the note unless amended. The Company has extended the expiration dates on certain warrants (see below). In October 2020, the Company received $3,013,028 as a deposit for the exercising of certain C warrants. In December 2020, 6,321,312 of C warrants were exercised at a price of $1.375 for a total of $8,691,801 and 2,410,922 C warrants were forfeited. The company received $7,134,676 in cash and converted $1,557,128 of accrued expenses and related party payable. The total remaining C warrants as of April 30, 2021 was 3,253,948, these are due to expire in February 2022.
Preferred Convertible Series D Warrant — The warrant was issued in connection with the September 16, 2016 private offering which resulted in the issuance of a $10,000,000 D Note. The warrant is for the purchase of up to 56% of the then issued and outstanding stock of the Company on an as-converted and fully diluted basis. The exercise price of the warrant is the lower of 70% of the price sold in an equity financing, or $1.25 per share, subject to certain adjustments. The minimum initial and subsequent exercises of the warrant shall be for such number of shares that will result in at least $5,000,000 of gross proceeds to the Company, or such lesser amount as represents 5% of the outstanding capital stock, or such lesser amount as may then remain unexercised. The warrant will expire upon the earlier of August 15, 2021 or a qualified initial public offering or liquidation event. On January 28, 2021, Rafael Holdings partially exercised warrants to maintain the 51% ownership percentage and purchased 7,298,950 shares of the Company’s Series D Preferred Stock for $9,123,688.
As of April 30, 2021, the Warrant holder owns 60,673,087 shares of Series D Preferred Stock.
Extension of Warrants
On March 2, 2020, the Company extended the expiration dates of the Series C and D warrants to August 31, 2020 and June 30, 2021, respectively. In connection with the extension of these warrants, the Company recorded $31,530,000 of warrant modification expense.
On August 31, 2020, the Company extended the Series C warrants to allow the holders 30 days to determine whether or not to exercise the warrants. If the holders choose to exercise the warrants and provide a 10% deposit, the Company will provide an additional 60-days extension to fund the full amount of the exercise. Additionally, the Company further extended the expiration dates of the Series D warrants to August 15, 2021. In connection with the extension of these warrants, the Company recorded $11,603,525 of warrant modification expense.
F-18
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
12. OPTIONS
In 2009, the Company established the 2009 Stock Incentive Plan (“2009 Plan”) providing for the additional granting of incentive stock options and non-qualified options to purchase the Company’s common stock. A total of 5,500,000 shares were authorized under the 2009 Plan and 929,100 shares are available for grant. In 2018, the Company established the 2018 Stock Incentive Plan (“2018 Plan”) providing for the additional granting of incentive stock options and non-qualified options to purchase the Company’s common stock. A total of 4,500,000 shares were authorized under the 2018 Plan. In 2019, the 2018 plan was amended and an additional 2,000,000 shares were authorized for a total of 6,500,000 authorized shares and 255,598 shares are available for grant.
The Company follows the provisions of the ASC Topic 718, Compensation — Stock Compensation, which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and non-employee directors, including employee stock options. Stock compensation expense based on the grant date fair value estimated in accordance with the provisions of ASC 718 is generally recognized as an expense over the requisite service period.
During the period from July 31, 2019 to April 30, 2021, the following stock option grants were made:
Option Date | | Options
Granted | | Exercise
Price | | Estimated
Fair Value of
Underlying
Stock | | Intrinsic
Value |
August 1, 2019 | | 1,631,264 | | $ | 2.50 | | $ | 1.95 | | None |
December 1, 2019 | | 1,373,750 | | $ | 2.50 | | $ | 1.95 – $2.07 | | None |
February 1-6, 2020 | | 651,300 | | $ | 2.50 | | $ | 1.95 | | None |
June 1, 2020 | | 535,000 | | $ | 2.50 | | $ | 2.11 | | None |
November 1, 2020 | | 130,000 | | $ | 2.50 | | $ | 2.11 | | None |
April 1, 2021 | | 240,000 | | $ | 3.50 | | $ | 2.72 | | None |
The option prices were determined based on factors such as historical option prices, recent equity transactions and other factors as deemed necessary and relevant in the circumstances. The Company uses an option pricing model to estimate the fair value of its stock options, since market values are generally not available for long-term, non-transferable stock options. In order to estimate the fair value of the options granted, we performed a determination of fair value for accounting purposes of our common stock and underlying stock options using a combination of valuation methods described in American Institute of Certified Public Accountant (“AICPA”) Technical Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation.
The exercise prices for options granted were set by our Board of Directors at a premium over fair value of our common stock at the time the grants were authorized. The Board of Directors considered the status of our product pipeline and the progress since the last valuation date, our platform for identifying novel compounds, our asset value (comprised of intellectual property rights and current cash on hand), our present value of future cash flows, our capital structure, the valuation of comparable companies and our acquisition potential. The Board of Directors also considered additional factors, including our existing licensing and research agreements, intellectual property, funding prospects, the funding prospects and valuations of similar companies, the growth prospects of the pharmaceutical industry in general and oncology companies, expected regulatory and commercial hurdles to commercializing or licensing our clinical candidates, the ability of the management team and our access to financing.
The Company utilizes the Black-Scholes valuation method to value stock options and recognize compensation expense over the vesting period. The expected life represents the period that the Company’s stock-based compensation awards are expected to be outstanding. The Company uses a simplified method provided in Securities and Exchange Commission release, Staff Accounting Bulletin (“SAB”) No. 110 (“SAB No. 110”), which averages an award’s weighted average vesting period and contractual term for “plain vanilla” share options. The expected volatility was estimated by analyzing the historic volatility of similar public biotech companies in the oncology field, considering the state of product completion. The Company used an estimated forfeiture rate of 10%.
F-19
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
12. OPTIONS (cont.)
No dividend payouts were assumed as we have not historically paid and are not anticipated to pay dividends in the foreseeable future. The risk-free rate of return reflects the weighted average interest rate offered for U.S. Treasury rates over the expected term of the options. The fair market value of common stock used in the calculation was based upon future cash flow scenarios.
A summary of significant assumptions used to estimate the fair value of the equity awards granted in during the period is as follows:
| | April 30,
2021 | | July 31,
2020 |
Expected term (years) | | 7 | | 6.5 to 7 |
Risk-free interest rate | | 0.04% to 0.14% | | 0.50% to 1.77% |
Volatility | | 85.40% – 94.00% | | 85.40% |
Dividend yield | | None | | None |
No related tax benefits were recorded for the nine months ended April 31, 2021 and 2020. Generally, options are granted with an exercise price at, or in excess of, the fair value of the common stock at the date of issuance. Options typically vest over a one- to four-year period in equal increments and expire not more than 10 years after the grant date.
The Company recorded noncash stock-based compensation of approximately $1,367,000 and $1,009,000 during the nine months ended April 30, 2021 and 2020, respectively.
A summary of option activity for the combined plans for the nine months ended April 30, 2021 is presented as follows:
| | Number of
Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contract
Terms (Years) | | Aggregate
Intrinsic
Value |
Options outstanding – July 31, 2020 | | 11,533,114 | | | 2.29 | | 7.6 | | | — |
Granted | | 370,000 | | | 2.50 | | | | | |
Forfeited | | (401,562 | ) | | 2.50 | | | | | |
Exercised | | (11,250 | ) | | 2.50 | | | | | |
Options outstanding – April 30, 2021 | | 11,490,302 | | | 2.37 | | 6.5 | | $ | 4,129,670 |
Options vested and expected to vest – April 30, 2021 | | 11,058,612 | | | 2.36 | | 6.5 | | | 4,044,645 |
Options exercisable – April 30, 2021 | | 7,173,395 | | | 2.16 | | 5.9 | | | 3,279,415 |
Other Options — The Company has issued other options to investors and others as finders’ fees (collectively “Other Options”) in connection with prior capital raises.
In connection with the Company’s 2003 common stock offerings, the Company entered into an option agreement with an individual in connection with identifying investors. The option agreement grants the right to purchase an option (a “Purchase Option”) to purchase 472,000 Class A Options (“Class A Options”), which allows the purchase of 2.5 shares of common stock for each Class A Option at $1.10 per share. In order to secure this Class A Option, a Purchase Option must initially be purchased for $.005 per potential share of Class A options. Upon exercise of each Class A Option, a right is granted to one Class B Option (“Class B Options”), which allows the purchase of 2.5 shares of common stock for each Class B Option at $1.25 per share. The expiration date of the Class A Options is the later of October 29, 2005 or six months from the date the Company’s shares become publicly traded. The Class B Options expire 180 days from the exercise of the Class A Options.
F-20
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
12. OPTIONS (cont.)
In 2003, 625,000 options (the “Options”) were granted at a strike price of $1.10 per share to a 2003 Investor. These Options are set to expire 180 days following the closing of an IPO, or from the date the Company’s shares become publicly traded.
13. INCOME TAXES
As of April 30, 2021, the Company has approximately $125,200,000 of NOLs that will begin to expire in 2022 and approximately $63,300,000 of New Jersey NOLs that will begin to expire in 2029. The NOLs generated in the current year will not expire. The Company has undergone ownership changes and has determined that a “change in ownership” as defined by IRC Section 382 of the Internal Revenue Code of 1986, as amended, and the rules and regulations promulgated thereunder, did occur during 2008. Accordingly, about $19,875,000 of the Company’s NOLs carryforwards are limited and the Company can only use $3,601,883 for the first five years from the ownership change and $1,488,550 per year going forward. Therefore, all of the limited NOL’s will be available after seven years (2015). The Company has R&D Credit carryovers for Federal and New Jersey of approximately $4,878,000 and $2,163,000, respectively, these will begin to expire in 2029.
The Company records accrued interest and penalties related to unrecorded tax benefits in the interest expense and operating expense. The Company had not accrued any interest or penalties related to unrecognized benefits. The Company is no longer subject to Federal income tax assessment for years before 2017 and 2016 for New Jersey income tax assessment. However, since the Company has incurred net operating losses in every year since inception, all of its income tax returns are subject to examination and adjustments by the Internal Revenue Service for at least three years following the year in which the tax attributes are utilized. The Company does not believe that there will be a material change in its unrecognized tax positions over the next 12 months.
14. COMMITMENTS AND CONTINGENCIES
COVID-19 Pandemic
In December 2019, a new coronavirus, now known as COVID-19, which has proved to be highly contagious, emerged in Wuhan, China and has since spread around the globe. The Company actively monitors the outbreak and its potential impact on its operations.
The impacts on the operations and specifically the ongoing clinical trials have been actively managed by respective pharmaceutical management teams who have worked closely with the appropriate regulatory agencies to continue clinical trial activities with as minimal impact as possible.
Due to both known and unknown risks, including quarantines, closures and other restrictions resulting from the outbreak, operations may be adversely impacted. Additionally, as there is an evolving nature to the COVID-19 situation, we cannot reasonably assess or predict at this time the full extent of the negative impact that the COVID-19 pandemic may have on our business, financial condition, results of operations and cash flows. The impact will depend on future developments such as the ultimate duration and the severity of the spread of the COVID-19 pandemic in the U.S. and globally, the effectiveness of federal, state, local and foreign government actions on mitigation and spread of COVID-19, the pandemic’s impact on the U.S. and global economies, changes in our customers’ behavior emanating from the pandemic and how quickly we can resume our normal operations, among others. For all these reasons, the Company may incur expenses or delays relating to such events outside of the Company’s control, which could have a material adverse impact on the Company’s business.
License Agreements — The Company is a party to two license agreements in connection with certain technology being used for products under development and is required to make certain annual maintenance payments. In addition, royalty payments, calculated on a low single digit percentage of net sales, as defined in the respective agreements, will be required upon the commercialization of licensed technology. Sublicensing fees are calculated and due based upon a percentage of gross sublicense fees. The Company expenses license obligation payments to research and development.
F-21
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
14. COMMITMENTS AND CONTINGENCIES (cont.)
One worldwide license agreement requires the Company to reimburse the other party for costs associated with filing and defending various patents worldwide. Payment obligations under this license agreement remain in effect until the last underlying patents granted under the license agreement expire in their respective countries, the last patent expired in 2019. License maintenance fees are currently $20,000 per year and continue for the term of the agreement. The license maintenance fees are replaced by minimum royalties of $10,000 during the first year following governmental approval to market products and escalate to $1,000,000 during the term of the agreement. The Company is also responsible for sub licensing fees. The Company may credit each annual license maintenance fee in full against all royalties and sublicensing fees due during the same calendar year. The Company may terminate the license agreement upon 90 days’ notice. Either party may terminate the license agreement if the other party commits any material breach of any covenant or promise and does not cure such breach within 30 days of the receipt of written notice of such material breach. In May 2017, the Company renegotiated the agreement referred to as the “second license”. In exchange for a waiver of certain product development milestones, the Company modified the agreement to pay a low single digit percentage royalty for a duration of five years on Net Sales of product sold after the expiration of the licensed patent and potentially up to eight years. As of April 30, 2021, there are no products being marketed which are covered by the patents under the license agreement.
The remaining minimum payments required under the license agreement, assuming the agreements are not terminated by the Company, excluding any escalation for receiving government marketing approval subsequent to July 31, 2018 are $20,000 per year through fiscal year ended 2020.
The Company’s second license continues until the termination of the later of the last to expire patent or royalty obligation under the agreement on a country by country basis (currently, or as otherwise provided in the license agreement). Fifty percent (50%) of the maintenance fee payments, up to $1.1 million, may be credited against the potential future royalty payments, calculated on a single digit percentage of net sales, as defined, that the Company would have to make to the license holder should royalties be paid. The agreement may be terminated on 15 days’ written notice after default by the other party if said default is not cured within 30 days of receipt of notice by the defaulting party. In addition, the Company may terminate the agreement on 15 days’ written notice to the license holder. Royalties are due based on Gross Sales, as defined, for products sold relating to patented and unpatented technology, and shall terminate on the 15th anniversary of the first commercial sale of the product in the corresponding country or territory. Sublicense payments are due in connection with any sublicense fees received relating to patented and non-patented products related to the patented technology and proprietary know-how, as provided in the agreement. As of April 30, 2021, there were no products being marketed which are covered by the patents under the license agreement. There were no additional annual license maintenance fees required beyond 2010.
In addition, Ventures (as discussed in Note 1) had an operating agreement with Altira Capital and Consulting LLC (“Altira”) that provided for the payment of royalties to Altira in exchange for the assignment of intellectual property to the Company. At the time of the operating agreement, Altira was owned by two officers (the Chief Executive Officer and the President) of the Company. The operating agreement was terminated when Ventures and the Company merged in 2002. At the merger date, the Company agreed to continue the specific section of the operating agreement dealing with the payment of royalties to Altira, including the establishment of a royalty pool for certain inventors. Altira would receive a royalty as part of a newly created inventor’s royalty pool to include specific inventors of Company technology for the products and services developed in part by certain Altira principals and other inventors. The Company is obligated to pay royalties based upon percentage (low single digit) of net sales, as defined in the royalty agreement. The royalty obligations remain in effect, on a country by country basis, until the last to expire patent claims associated with such products and services expire or are no longer in force. No payments have been made in connection with a royalty pool. As of April 30, 2021, the last to expire patent claim is to remain in force until fiscal 2034.
Lease Obligations — The Company entered into a 10-year lease agreement on June 1, 2006 for office and laboratory space in Cranbury, New Jersey (“Cranbury”). Such agreement initial term expired during July 2016 and was amended in September 2016. The amended lease agreement renews the lease for an additional seven years commencing on August 1, 2016 and ending on July 31, 2023. The base rent was amended to $217,588 annually through July 31, 2021 increasing to $250,185 commencing on August 1, 2021 for the last two years.
F-22
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
14. COMMITMENTS AND CONTINGENCIES (cont.)
Rent expense including deferred rent charges amounted to $482,000 and $191,000 for the nine months ended April 30, 2021 and 2020, respectively.
The following is a schedule by year of future rent payments at April 30, 2021.
Year Ending July 31, | | Rental
Payment |
2021 – remainder | | $ | 217,588 |
2022 | | | 250,185 |
2023 | | | 250,185 |
Total | | $ | 717,958 |
15. RELATED PARTY TRANSACTIONS
On January 16, 2016, CS Pharma Holdings (a subsidiary of IDT Corporation) and/or IDT Corporation (the “Investor”) and the Company entered into a subscription agreement for the issuance of up to a $10,000,000 D Note to the Investor which is convertible into Series D Preferred Stock subject to due diligence and the conversion of a prior $500,000 advance to a C Note holder. On March 31, 2016, the Company entered into the First Global Amendment to the Loan Document providing for an additional $1,500,000 of funds to the Company in exchange for the issuance of an additional C Note in the same amount which was issued on April 13, 2016. After completion of due diligence and negotiations, on September 16, 2016, the Investor provided the remaining $8,000,000 less expenses. The Company cancelled both C Notes and issued a Series D Note in the amount of $10,000,000. The D Note, including the unpaid interest, has a maturity date of two years. In addition, the Company issued a warrant to the Investor to purchase up to 56% of the outstanding capital stock of the Company. An equity bonus was granted to be calculated based on 10% of the outstanding capital stock subject to certain milestones. In addition, the Investor was granted voting proxies by holders of a majority of the shares of the Company’s common stock outstanding permitting the Investor to veto certain financing or asset transactions for a period of two years. The Amendment provided that the subscriber may appoint two additional Board Members and the Chairman of the Board, as well as Veto Rights for subsequent financing, sale, a change of control or other arrangement, and Equity Bonus Shares.
In March 2018, IDT Corporation spun off a former subsidiary to an independent public company, Rafael Holdings, Inc., which acquired upon spin off, interests/rights in Rafael Pharmaceuticals Inc. through a 90%-owned non-operating subsidiary, Pharma Holdings, LLC (“Pharma Holdings”). Pharma Holdings holds the Preferred Convertible Series D Warrant to purchase a significant stake in Rafael Pharmaceuticals Inc., as well as other equity and governance rights in Rafael Pharmaceuticals Inc., and owns 50% of CS Pharma Holdings, LLC (“CS Pharma”), a non-operating entity which holds the convertible debt and other rights to purchase equity interests in Rafael Pharmaceuticals Inc.
The Company’s Chairman of the Board of Directors (appointed in April 2016), Mr. Howard Jonas, who is also the current Chairman of the Board of the Investor, held jointly with his spouse $525,000 in C Notes as of July 31, 2018. In addition, an affiliated foundation held an additional $525,000 of C Notes. Mr. Jonas also has a stock option for 100,000 shares of common stock at $1.25 per share which expires in 2023.
On February 3, 2020, the Company entered into a Line of Credit Loan Agreement (“Line of Credit Agreement”) with RP Finance, LLC (“RP Finance”), which provides a revolving commitment of up to $50,000,000 to fund clinical trials and other capital needs. Rafael Holdings, Inc. owns 37.5% of the equity interests in RP Finance and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. Howard Jonas owns 37.5% of the equity interests in RP Finance, and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. The remaining 25% equity interests in RP Finance is owned by other stockholders of the Company. (see note 7)
F-23
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
15. RELATED PARTY TRANSACTIONS (cont.)
Altira (see Note 14), a significant investor, is owned by an officer and director of the Company, a former officer and director of the Company and two other significant investors/directors (herein the “Two Investors”) who became partners in Altira in 2004. In June 2006, a company controlled by the Two Investors, Cedar Brook East Corporate Center, L.P. (“Landlord”), leased a facility to the Company under a 10-year non-cancelable lease (see Note 14) which renewed in 2016. Altira is entitled to receive royalties on net sales as described in Note 14. The Two Investors continued to invest in the preferred stock issuances and make advances to the Company.
As of April 30, 2021 and July 31, 2020, the Company has unpaid lease obligations (to companies controlled by the Two Investors) in accounts payable of approximately $0 and $963,000, respectively. During the nine months ended April 30, 2021, the Company converted $900,000 of the unpaid lease obligation in lieu of payment for the exercise of warrants into Preferred C shares and was forgiven on the remaining $63,000. The Company recorded the forgiveness of the $63,000 as interest income.
The Company pays $40,000 per month in Management Fees to Rafael Holdings, Inc. for accounting and other administrative functions. Rafael Holdings is the parent company of Pharma Holdings. At April 30, 2021 and July 31 2020, the Company owed $480,000 and $120,000 to Rafael Holdings, Inc, respectively.
During the nine months ended April 30, 2021, the Company converted $657,128 of due to related parties in lieu of payment for the exercise of warrants into Preferred C shares. As of April 30, 2021, the remaining balance due to related party was $568,522.
During the nine months ended April 30, 2021 and 2020, there were no related parties advances.
16. SUBSEQUENT EVENTS
The Company evaluated events that have occurred from the date of the financial statements through the date the financial statements were available to be issued.
From May 2021 through the date of the issuance of these financial statements, the Company called for a $8.75 million draw on its Line of Credit.
On June 14, 2021, in accordance with the State of New Jersey’s Technology Business Tax Certificate Program, which allowed certain high technology and biotechnology companies to sell unused net operating loss carryforwards (“NOLs”) to other New Jersey-based corporate taxpayers based in New Jersey, the Company sold all its available New Jersey NOLs and received a payment of $2,348,399.
Plan of Merger
On June 17, 2021, Rafael Holdings, Inc. (“Holdings”), entered into an Agreement and Plan of Merger (the “Merger Agreement”), by and among: Holdings; RH Merger I, Inc., a Delaware corporation and a wholly-owned subsidiary of Holdings (“Merger Sub I”); RH Merger II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Holdings (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”); and Rafael Pharmaceuticals, Inc., a Delaware corporation (“Pharma”). Pharma will become a wholly-owned subsidiary of Holdings.
As a consequence of the Mergers, at the Effective Time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist, and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Parent Class B Common Stock per share of Pharma capital stock. This Per Share Merger Consideration ratio for each class of Pharma capital stock will provide that the prior holders of Pharma capital stock and contingent interests will receive Parent Class B Common Stock amounting to approximately 48% of the currently outstanding capitalization of Parent. Additional equity in Parent will be issued in connection with the contemplated financing and pursuant to employment agreements (which will reduce the foregoing percentage proportionately).
F-24
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE NINE MONTHS ENDED APRIL 30, 2021 AND 2020
16. SUBSEQUENT EVENTS (cont.)
The consideration payable by Holdings takes into account the value of the Holdings’ assets including its interests in Pharma through shares and warrants. Other holders of warrants to purchase Pharma stock may elect to exercise their portion of the Warrant prior to the Closing Date. Any other unexercised warrants shall be terminated at the Effective Time in accordance with the terms of the Merger Agreement; provided that any Pharma Series C Warrants, to the extent not exercised by its holder prior to the Effective Time, shall be exchanged for a commensurate warrant to purchase shares of Holdings or otherwise be settled by Holdings and the Common Warrant Option, to the extent not exercised by its holder prior to the Effective Time, shall be exchanged for a commensurate warrant to purchase shares of Holdings or otherwise be settled by Holdings.
In connection with the Mergers, the contractual right to receive additional shares of Pharma capital stock equal to 10% of the fully diluted Pharma capital stock (the “Bonus Shares”) that is held by an instrument (the “Instrument”) that is within a trust for the benefit of the children of Holdings’ Chairman Howard Jonas will also be satisfied by the issuance of Pharma capital stock immediately prior to the effective time of the Mergers and the holder of the right shall receive the Merger Consideration that would be due to a holder of the shares of Pharma Common Stock as would be issuable in respect of that right. The Merger Agreement provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable as Bonus Shares shall be calculated as of September 30, 2021, as if the Mergers were consummated on such date.
At the Effective Time, all compensatory options to purchase Pharma Common Stock (i) shall automatically vest in full, and all holders of such options will receive options to purchase Class B common stock of Holdings using the ratios applicable to determining the Holdings stock in the Business Combination.
Convertible Note Cancellation Agreement
Under the terms of the Merger Agreement, the parties intend that the Pharma Convertible Notes shall not be assumed or continued by Holdings or Pharma in connection with the Merger or the other transactions contemplated thereby. Immediately prior to the Effective Time, Holdings and Pharma shall use their commercially reasonable efforts to cause each Pharma Convertible Note to be cancelled and extinguished and converted into the applicable Note Conversion Shares, in accordance with the terms of Pharma Convertible Notes and of that certain form of Convertible Note Cancellation Agreement, to be executed and delivered by each Pharma Noteholder prior to the Closing. The Convertible Note Cancellation Agreement provides that, contingent on the consummation of the Merger, the applicable Note Conversion Shares to which a Pharma Noteholder is entitled shall be calculated based on accrued interest as of September 30, 2021, as if the Mergers were consummated on such date.
Warrant held By Holdings
Prior to the Closing, Holdings shall effect the termination of the Series D warrants, such termination to be contingent on the consummation of the Merger and effective at the Initial Effective Time. The Support Agreements executed by holders of the Series D warrants provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable pursuant to the Series D warrants shall be calculated as of September 30, 2021, as if the Merger were consummated on such date.
Line of Credit Amendment
RP Finance and Pharma have amended the Line of Credit to modify the definition of “Change of Control” to provide that the Line of Credit, which has an outstanding principal balance of $18.75 million on the date hereof, and all accrued and unpaid interest thereon will be due and payable on consummation of the Merger and to shorten the call period for draws to thirty days. The Merger Agreement provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable pursuant to the Line of Credit shall be calculated as of September 30, 2021, as if the Mergers were consummated on such date.
F-25
Table of Contents
Independent Auditor’s Report
To the Management of
Rafael Pharmaceuticals, Inc.
We have audited the accompanying financial statements of Rafael Pharmaceuticals, Inc., which comprise the balance sheets as of July 31, 2020 and 2019, and the related statements of operations, stockholders’ equity (deficit) and cash flows for the years then ended, and the related notes to the financial statements.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Rafael Pharmaceuticals Inc. as of July 31, 2020 and 2019, and the results of its operations and its cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and negative net cash flows from operating activities, and has stated that the substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
New York, New York
September 2, 2021
F-26
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
BALANCE SHEETS
(in thousands, except share and per share data)
| | July 31, |
| | | 2020 | | 2019 |
ASSETS | | | | | | | | |
CURRENT ASSETS | | | | | | | | |
Cash | | $ | 4,932 | | | $ | 17,426 | |
Marketable securities | | | 1,403 | | | | 18,917 | |
Prepaid expenses and other current assets | | | 4,354 | | | | 5,764 | |
Total current assets | | | 10,689 | | | | 42,107 | |
NON-CURRENT ASSETS | | | | | | | | |
Property and equipment, net | | | 236 | | | | 226 | |
Deferred debt issuance costs | | | 34,902 | | | | — | |
TOTAL ASSETS | | $ | 45,827 | | | $ | 42,333 | |
| | | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
CURRENT LIABILITIES | | | | | | | | |
Accounts payable | | $ | 7,738 | | | $ | 2,957 | |
Accrued expenses | | | 6,538 | | | | 4,310 | |
Deferred officers’ salaries | | | 248 | | | | 323 | |
Advances payable to related parties | | | 1,346 | | | | 1,506 | |
Note payable | | | 298 | | | | — | |
Convertible notes – related parties | | | 12,239 | | | | 12,239 | |
Total current liabilities | | | 28,407 | | | | 21,335 | |
LONG TERM LIABILITIES | | | | | | | | |
Note payable – long term | | | 301 | | | | — | |
Derivative liabilities | | | 37,432 | | | | — | |
TOTAL LIABILITIES | | | 66,140 | | | | 21,335 | |
COMMITMENTS AND CONTINGENCIES | | | | | | | | |
STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
Convertible Preferred stock, $0.001 par value; 300,000,000 shares authorized; 82,885,915 shares issued and outstanding as of July 31, 2020 and 2019, respectively | | | 83 | | | | 83 | |
Common stock, $0.001 par value; 310,000,000 shares authorized; 24,381,591 and 21,769,278 shares issued and outstanding as of July 31, 2020 and 2019, respectively | | | 24 | | | | 22 | |
Additional paid-in capital | | | 155,408 | | | | 117,421 | |
Accumulated deficit | | | (175,828 | ) | | | (96,528 | ) |
Total stockholders’ equity (deficit) | | | (20,313 | ) | | | 20,998 | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | $ | 45,827 | | | $ | 42,333 | |
See accompanying notes to the financial statements.
F-27
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
(in thousands, except share data)
| | For the Years Ended
July 31, |
| | | 2020 | | 2019 |
REVENUE | | | | | | | | |
Revenue | | $ | — | | | $ | 12,884 | |
TOTAL REVENUE | | | — | | | | 12,884 | |
OPERATING EXPENSES | | | | | | | | |
Research and Development | | | 36,527 | | | | 24,417 | |
General and Administrative | | | 3,694 | | | | 2,478 | |
TOTAL OPERATING EXPENSES | | | 40,221 | | | | 26,895 | |
LOSS FROM OPERATIONS | | | 40,221 | | | | 14,011 | |
OTHER INCOME AND (EXPENSE) | | | | | | | | |
Interest income | | | 401 | | | | 349 | |
Interest expense | | | (4,210 | ) | | | (1,354 | ) |
Modification of warrants expense | | | (31,530 | ) | | | — | |
Gain on sales of marketable securities | | | 160 | | | | 64 | |
Unrealized gain on marketable securities | | | (49 | ) | | | 156 | |
Change in fair value of derivative liabilities | | | (3,851 | ) | | | — | |
TOTAL OTHER INCOME AND (EXPENSE) | | | (39,079 | ) | | | (785 | ) |
NET LOSS | | $ | 79,300 | | | $ | 14,796 | |
See accompanying notes to the financial statements.
F-28
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For the Years Ended July 31, 2020 and 2019
(in thousands, except share data)
| | Preferred Stock | | Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | Total
Stockholders’
Equity
(Deficit) |
| | | Series A | | Series B | | Series D | | | | | |
| | | Shares | | Par Value | | Shares | | Par Value | | Shares | | Par Value | | Shares | | Par Value | |
Balance as of July 31, 2018 | | 3,057,500 | | $ | 3 | | 26,454,278 | | $ | 27 | | $ | — | | | — | | 21,769,278 | | $ | 22 | | $ | 50,539 | | $ | (81,732 | ) | | $ | (31,141 | ) |
Stock-based compensation resulting from stock options granted to non-employees and employees | | — | | | — | | — | | | — | | | — | | | — | | — | | | — | | | 218 | | | — | | | | 218 | |
Common stock issued in exchange for notes and interest | | — | | | — | | — | | | — | | | 8,678,105 | | | 9 | | — | | | — | | | 10,839 | | | — | | | | 10,848 | |
Common stock issued in exchange for warrants | | — | | | — | | — | | | — | | | 44,696,032 | | | 44 | | — | | | — | | | 55,825 | | | — | | | | 55,869 | |
Net loss | | — | | | — | | — | | | — | | | — | | | — | | — | | | — | | | — | | | (14,796 | ) | | | (14,796 | ) |
Balance as of July 31, 2019 | | 3,057,500 | | | 3 | | 26,454,278 | | | 27 | | | 53,374,137 | | | 53 | | 21,769,278 | | | 22 | | | 117,421 | | | (96,528 | ) | | | 20,998 | |
Stock-based compensation resulting from stock options granted to non-employees and employees | | — | | | — | | — | | | — | | | — | | | — | | — | | | — | | | 1,347 | | | — | | | | 1,347 | |
Common stock issued for related party LOC | | — | | | — | | — | | | — | | | — | | | — | | 2,612,313 | | | 2 | | | 5,110 | | | — | | | | 5,112 | |
Modification of warrants | | — | | | — | | — | | | — | | | — | | | — | | — | | | — | | | 31,530 | | | — | | | | 31,530 | |
Net loss | | — | | | — | | — | | | — | | | — | | | — | | — | | | — | | | — | | | (79,300 | ) | | | (79,300 | ) |
Balance as of July 31, 2020 | | 3,057,500 | | $ | 3 | | 26,454,278 | | $ | 27 | | | 53,374,137 | | $ | 53 | | 24,381,591 | | $ | 24 | | $ | 155,408 | | $ | (175,828 | ) | | $ | (20,313 | ) |
See accompanying notes to the financial statements.
F-29
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
| | For the Years Ended
July 31, |
| | | 2020 | | 2019 |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | |
Net loss | | $ | (79,300 | ) | | $ | (14,796 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization | | | 75 | | | | 63 | |
Amortization of debt discount | | | 3,791 | | | | 737 | |
Deferred taxes | | | — | | | | 1,184 | |
Interest expense | | | — | | | | 616 | |
Noncash stock-based compensation | | | 1,347 | | | | 218 | |
Income from investments in marketable securities | | | (110 | ) | | | (407 | ) |
Change in fair value of derivative liabilities | | | 3,851 | | | | — | |
Change in fair value of warrants due to modification Changes in operating assets and liabilities: | | | 31,530 | | | | — | |
Prepaid expenses and other current assets | | | 1,410 | | | | (5,651 | ) |
Accounts payable | | | 4,781 | | | | (37 | ) |
Accrued expenses | | | 2,228 | | | | 1,317 | |
Payment of deferred officers’ salaries | | | (75 | ) | | | (175 | ) |
Due to related party | | | (160 | ) | | | 280 | |
NET CASH USED IN OPERATING ACTIVITIES | | | (30,632 | ) | | | (16,651 | ) |
| | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
Purchases of property and equipment | | | (85 | ) | | | (4 | ) |
Investment in marketable securities | | | — | | | | (31,251 | ) |
Proceeds from sale and maturity of marketable securities | | | 17,624 | | | | 12,741 | |
NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | | | 17,539 | | | | (18,514 | ) |
| | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
Proceeds from note payable | | | 599 | | | | — | |
Advances from related parties | | | — | | | | 135 | |
Repayment of advances from related parties | | | — | | | | (3,470 | ) |
Proceeds from sale of Series D Preferred Stock | | | — | | | | 55,870 | |
NET CASH PROVIDED BY FINANCING ACTIVITIES | | | 599 | | | | 52,535 | |
| | | | | | | | | |
NET INCREASE (DECREASE) IN CASH | | | (12,494 | ) | | | 17,370 | |
CASH AT BEGINNING OF YEAR | | | 17,426 | | | | 56 | |
CASH AT END OF YEAR | | $ | 4,932 | | | $ | 17,426 | |
| | | | | | | | | |
NONCASH FINANCING ACTIVITY | | | | | | | | |
Conversion of Series D Note and accrued interest | | $ | — | | | $ | 10,848 | |
Common stock issued for related party LOC | | $ | 5,112 | | | $ | — | |
Deferred debt issuance costs for related party LOC | | $ | 33,581 | | | $ | — | |
See accompanying notes to the financial statements.
F-30
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
1. ORGANIZATION, BUSINESS OVERVIEW AND MANAGEMENT’S PLAN
Rafael Pharmaceuticals, Inc. (formerly Cornerstone Pharmaceuticals, Inc.) was formed as a result of a merger in 2002 between Cornerstone Ventures, LLC (“Ventures”), a New York limited liability company which was organized on May 14, 1999, and Rafael Pharmaceuticals, Inc., a New York corporation which was incorporated on November 1, 2001, with Rafael Pharmaceuticals, Inc. (the “Company”) being the surviving entity. On December 24, 2009, the Company (New York) was merged with and into a newly formed Delaware corporation by the same name, for the purpose of changing domiciles. This merger had no financial or accounting effects on the Company. On May 10, 2017, the Board approved the resolution changing the name to Rafael Pharmaceuticals, Inc.
The Company is a privately held, clinical stage, oncology-focused biopharmaceutical company engaged in developing therapeutics that exploit and target cancer cell metabolism. The Company is currently developing a technology platform. The drug candidate is in the development phase and have not received regulatory approval. The Company has yet to generate any revenues from its product candidate in development. The Company has performed certain research related activities under grant and service agreements for which revenue was earned and recognized.
Since inception, the Company has incurred significant losses from operations and has not generated positive cash flows from operations. In addition, at July 31, 2020, the Company does not have any revenue stream to support its cost structure. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts and the classification of liabilities that might be necessary from the outcome of this uncertainty.
Management’s Plan
The development of the Company’s products will require a commitment of substantial funds to conduct the costly and time-consuming research, preclinical and clinical testing necessary to bring such products to market and to establish, acquire or contract for manufacturing and marketing capabilities. Future capital requirements will depend on factors including scientific progress in research and development programs, the Company’s ability to establish collaborative arrangements with others for drug development, progress with preclinical and clinical trials, the time and costs involved in obtaining regulatory approvals and effective commercialization activities. Since inception, the Company has incurred significant losses from operations and has not generated positive cash flows from operations. In addition, at July 31, 2020, the Company does not have any revenue stream to support its cost structure. The Company will need to raise substantial additional capital in order to bring its products to market and reach additional milestones in its licensing agreement in order to recognize revenue. Additional capital is necessary to fund planned activities beyond that date or expand operating activities. The Company has secured a $50 million Line of Credit which the Company will use for planned activities and to expand its activities (see note 7). There can be no assurance that such new offerings will be consummated or that such collaborative ventures will be entered into on favorable terms, if at all. If such new offerings are not consummated or additional financing is not otherwise available, the Company will be required to modify its business development plans or reduce or cease certain or all operations. In the event that any additional funding is obtained, such financings may have a dilutive effect to current stockholders. The Company plans on merging with Rafael Holding (see note 16).
As of July 31, 2020, the Company is in default on approximately $12.2 million of convertible notes as they are beyond their payment terms.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation — The Company’s financial statements are prepared using accounting principles generally accepted in the United States of America (“U.S. GAAP”) applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
F-31
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Use of Estimates — The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. Estimates also affect the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. The useful life of certain acquired property and equipment, fair value of leasehold improvements for the Cranbury, New Jersey facility (see notes 14 and 15), fair value of the of stock-based compensation expense, allocation of certain expenses between general and administrative and research and development expenses, accounting for the issuance of warrants, and accounting for certain income tax items including our conclusion to establish a valuation allowance, require the significant use of estimates and assumptions. Management believes that amounts recorded based on estimates and assumptions are reasonable and any differences between estimates and actual amounts will not have a material impact on the Company’s financial statements.
Cash and Marketable Securities — Cash consists of cash and deposits of all highly liquid debt instruments of three months or less that are readily convertible into cash.
The Company’s investments in marketable securities have been classified and accounted for as trading securities. The Company’s marketable securities are measured at fair value with gains and losses recognized in other income/(expense). The cost of securities sold is determined using the specific identification method.
Property and Equipment — Property and equipment consist of office equipment, laboratory equipment and leasehold improvements. Property and equipment are recorded at cost less accumulated depreciation and amortization. The related depreciation and amortization is computed using the straight-line method over the estimated useful lives of the related assets as follows: office equipment and laboratory equipment — three to five years; and leasehold improvements - 10 years, which represents the lesser of the estimated life of the assets or the lease term. Minor maintenance and repairs, and minor renewals and betterments are charged to expense as incurred.
Advances Payable to Related Parties — Funds were advanced to the Company from time to time for operating purposes from related parties. Advances payable to related parties were $1.3 million and $1.5 million at July 31, 2020 and 2019, respectively.
Long-Lived Assets — The Company reviews its long-lived assets for possible impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be fully recoverable. An impairment loss is recognized whenever the carrying amount of a long-lived asset exceeds the expected future cash flows, on an undiscounted basis, to be generated from the asset, including eventual disposition. If such review indicates that the carrying amount of long-lived assets is not recoverable, the carrying amount of such assets is reduced to fair value. The Company’s management determined that no events or changes in circumstances have indicated that asset-carrying values were no longer recoverable. No impairment charges were recorded during the years ended July 31, 2020 and 2019.
Income Taxes — Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. Valuation allowances have been established as it is more likely than not that the deferred tax assets will not be realized.
The Company files income tax returns in the U.S. federal jurisdiction and certain state jurisdictions. The tax years subject to audit are 2016 through 2019. The Company does not believe there will be a material change in its recognized tax positions over the next 12 months.
F-32
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
The Company recognizes the effect of uncertain income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs.
The Company has sustained losses since inception, which has generally resulted in a zero percent effective tax rate.
The Company’s policy is to recognize interest and penalties accrued on tax matters as a component of income tax expense. The Company has not incurred any interest or penalties.
Revenue Recognition — Revenue is recognized by the Company when earned, which is at the time that ownership and assumed risk of loss are transferred, collection of relevant receivables is probable, persuasive evidence of an arrangement exists, and the price of the arrangement is fixed and determinable. The Company has historically earned revenue from the performance of certain research related activities under grant and service agreements which, at the time of the provision of such services, were considered part of the Company’s planned principal operations. The Company has continued to pursue additional grant and service agreements and plans to perform such services in the future.
In June 2019, the Company signed a license agreement with a third party whereby the licensor was granted exclusive license and rights to develop, manufacture, and commercialize the licensed compounds and the licensed products in the licensor Territory. The licensor Territory consists of Japan, South Korea, Taiwan, and all ASEAN Countries which are Indonesia, Thailand, Malaysia, Singapore, Philippines, Vietnam, Myanmar, Cambodia, Laos and Brunei. The Company recognized $12.9 million in revenue in fiscal year 2019 due to milestones reached under a license agreement.
Research and Development Costs and Expenses — Research and development costs and expenses consist primarily of salaries and related personnel expenses, stock-based compensation, fees paid to external service providers, laboratory supplies, costs for facilities and equipment, license costs, and other costs for research and development activities. Research and development expenses are recorded in operating expenses in the period in which they are incurred. Estimates have been used in determining the liability for certain costs where services have been performed but not yet invoiced. The Company monitors levels of performance under each significant contract for external service providers, including the extent of patient enrollment and other activities through communications with the service providers to reflect the actual amount expended.
Concentrations of Risk — Financial instruments which potentially subject the Company to concentrations of risk principally consist of cash. At times the Company invests excess cash primarily in money market funds backed by U.S. Treasury collateral. At times, such funds may exceed federally insured limits. At times during fiscal years 2020 and 2019 and at July 31, 2020, the Company had cash that was in excess of the federally insured limits. The Company places its temporary cash investments with high credit quality institutions.
Any products developed by the Company will require approval from the U.S. Food and Drug Administration (“FDA”) or foreign regulatory agencies prior to commercial sales. There can be no assurance that the Company’s products will receive the necessary approvals. If the Company is denied such approvals or such approvals are delayed, it could have a material adverse effect on the Company.
The Company’s drug candidates are in the development phase and none have received regulatory approval. To achieve profitable operations, the Company must successfully develop, test, manufacture and market its products. There can be no assurance that any such products can be developed successfully or manufactured at an acceptable cost and with appropriate performance characteristics, or that such products will be successfully marketed. These factors could have a material adverse effect on the Company’s future financial results.
F-33
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Stock-Based Compensation — The Company accounts for its stock-based compensation awards in accordance with Accounting Standards Codification (“ASC”) Topic 718, Compensation — Stock Compensation (“ASC 718”). ASC 718 requires all grants of employee stock options to be recognized in the statement of operations based on their grant date fair values. For stock options granted to employees and to members of the Board of Directors for their services on the Board of Directors, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option- pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock. The Company records compensation cost over the related service periods.
The Company recorded stock-based option compensation as follows (in thousands):
| | For the Years Ended
July 31, |
| | | 2020 | | 2019 |
Research and development | | $ | 109 | | $ | 103 |
Administrative | | | 1,238 | | | 115 |
Total | | $ | 1,347 | | $ | 218 |
No related tax benefits from stock compensation expense were recognized for the years ended July 31, 2020 and 2019.
Warrants — The Company accounts for warrants to purchase stock based on guidelines provided in ASC Topic 815, Derivatives and Hedging — Contracts in Entity’s Own Equity (“ASC 815”) which provides guidance on contracts that are settled in the Company’s own shares as either a liability or as an equity instrument depending on the warrant agreement. The Company uses the Black-Scholes or trinomial pricing models, depending on the applicable terms of the warrant agreement, to value the derivative warrant liabilities in bifurcating such amount from convertible notes payable. (See notes 8 and 11).
Deferred Financing Cost — The Company accounted for deferred financing costs in accordance with ASU 2015-03. These costs were incurred before an associated debt liability is recorded in the financial statements, therefore would be presented as an asset on the Company’s balance sheet. These costs represent the common stock issued and the anti-dilution provision associated with obtaining the Line of Credit (see note 7). These fees are amortized over the terms of the respective financing agreements, on a straight-line basis. Unamortized deferred financing fees are expensed in full when the associated debt is refinanced, repaid before maturity, or otherwise extinguished.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its financial position, results of operations or cash flows upon adoption.
In February 2016, the FASB issued Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842). ASU 2016-02 increases the transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. Certain qualitative and quantitative disclosures are required, as well as a retrospective recognition and measurement of impacted leases. The new ASU is effective for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022, with early adoption permitted. We are currently evaluating ASU 2016-02 to determine the impact on the Company’s financial position, results of operations or cash flows.
F-34
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
In August 2017, the FASB issued ASU 2017-12, Derivatives and Hedging (Topic 850), the objective of which is to improve the financial reporting of hedging relationships to better portray the economic results of an entity’s risk management activities in its financial statements. In addition, the amendments in this update make certain targeted improvements to simplify the application and disclosure of the hedge accounting guidance in current generally accepted accounting principles. For public business entities, the amendments in this update are effective for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2019, and interim periods beginning after December 15, 2020. Early adoption is permitted in any period after issuance. For cash flow and net investment hedges existing at the date of adoption, an entity should apply a cumulative-effect adjustment related to eliminating the separate measurement of ineffectiveness to accumulated other comprehensive income with a corresponding adjustment to the opening balance of retained earnings as of the beginning of the fiscal year that an entity adopts the amendments in this update. The amended presentation and disclosure guidance is required only prospectively. The Company is currently evaluating the impact of the adoption of this update, however the adoption of this standard is not expected to have any impact on the Company’s financial position, results of operations or cash flows.
In June 2018, the FASB issued ASU 2018-07, Improvements to Nonemployee Share-Based Payment Accounting, which expands the scope of Topic 718, Compensation — Stock Compensation, to include all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. The guidance is effective for interim and annual periods beginning after December 15, 2018, our fiscal 2019. Early adoption is permitted. The adoption of this standard did not have any impact on the Company’s financial position, results of operations or cash flows.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement — Disclosure Framework (Topic 820). The updated guidance improves the disclosure requirements on fair value measurements. The updated guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. Early adoption is permitted for any removed or modified disclosures. The adoption of this standard did not have any impact on the Company’s financial position, results of operations or cash flows.
3. FAIR VALUE MEASUREMENTS
ASC Topic 820, Fair Value Measurement and Disclosures establishes a consistent framework in how to value the fair value of assets and liabilities. This framework is intended to increase consistency in how fair value determinations are made under various existing accounting standards that permit, or in some cases require, estimates of fair value. This standard also expands financial statement disclosure requirements about a company’s use of fair value measurements, including the effect of such measures on earnings.
This standard defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This standard also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Three levels of inputs are used to measure fair value:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
F-35
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
3. FAIR VALUE MEASUREMENTS (cont.)
Reported amounts for cash, accounts payable and stockholder advances are generally considered to be representative of their respective fair values because of the short-term nature of those instruments and are considered Level 1.
As required by ASC 820-10, assets and liabilities fair values are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability.
Convertible promissory notes were recorded based on the face values as of their respective issuance dates with the exception of the Convertible Promissory Note — D Series (“D Note”) dated September 16, 2016 with a face value of $10,000,000. The proceeds allocated to D Note is adjusted for amortization for the difference between the issuance value and the fair value as of the date of issuance. Due to close proximity to the issuance dates, including the relatively short maturity dates, the carrying amounts of the convertible notes were valued as of their issuance dates except for the D Note which was carried at the initial fair value measurement date as adjusted for discount amortization. The Company concluded that an estimation of the fair value of the convertible promissory notes was not practical due to the short-term maturity obligations of the respective instruments. (See note 8).
The Company determined that the anti-dilution feature of the line of credit (see note 7) should be recognized as a free-standing instrument that meets the definition of a derivative under ASC 815. Accordingly, the Company recorded the value of the anti-dilution feature as a derivative liability which will be stated at fair value at each reporting period with changes in fair value being recorded as a marked-to-market adjustment in the Company’s statement of operations. For the significant assumptions the Company used to calculate the derivative liability see note 9.
The following table sets forth the Company’s assets and liabilities fair value at July 31, 2020 and 2019 by level within the fair value hierarchy:
| | Carrying
Value | | Fair Value Measurement as of July 31, 2020 |
Level 1 | | Level 2 | | Level 3 |
Assets: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 4,932 | | $ | 4,932 | | $ | — | | $ | — |
Marketable Securities | | | 1,403 | | | 1,403 | | | — | | | — |
Liabilities: | | | | | | | | | | | | |
Convertible Notes | | $ | 12,239 | | $ | — | | $ | — | | $ | 12,239 |
Derivative liabilities | | | 37,432 | | | — | | | — | | | 37,432 |
| | Carrying
Value | | Fair Value Measurement as of July 31, 2019 |
Level 1 | | Level 2 | | Level 3 |
Assets: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 17,426 | | $ | 17,426 | | $ | — | | $ | — |
Marketable Securities | | | 18,917 | | | 18,917 | | | — | | | — |
Liabilities: | | | | | | | | | | | | |
Convertible Notes | | $ | 12,239 | | $ | — | | $ | — | | $ | 12,239 |
F-36
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
3. FAIR VALUE MEASUREMENTS (cont.)
The following table sets forth the Company’s change in derivative liability for the year ended July 31, 2020:
| | Derivative
Liability – Line
of Credit |
Balance as of July 31, 2019 | | $ | — |
Additions during the period | | | 33,581 |
Change in fair value | | | 3,851 |
Change due to exercise/redemptions | | | — |
Balance as of July 31, 2020 | | $ | 37,432 |
4. PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets are comprised of the following:
| | July 31, |
| | | 2020 | | 2019 |
Deposits with vendors and service providers | | $ | 4,352 | | $ | 5,762 |
Insurance | | | 2 | | | 2 |
Total | | $ | 4,354 | | $ | 5,764 |
5. PROPERTY AND EQUIPMENT
Property and equipment, net is comprised of the following:
| | July 31, |
| | | 2020 | | 2019 |
Laboratory equipment | | $ | 947 | | | $ | 894 | |
Computer and office equipment | | | 140 | | | | 108 | |
Leasehold improvements | | | 2,089 | | | | 2,089 | |
| | | | 3,176 | | | | 3,091 | |
Less: Accumulated depreciation and amortization | | | (2,940 | ) | | | (2,865 | ) |
| | | $ | 236 | | | $ | 226 | |
Depreciation and amortization expense was $75 and $63 for the years ended July 31, 2020 and 2019, respectively.
6. ACCRUED EXPENSES
| | July 31, |
| | | 2020 | | 2019 |
Interest (due to convertible note holders) | | $ | 2,708 | | $ | 2,289 |
Compensation | | | 50 | | | 50 |
Clinical and research costs | | | 3,586 | | | 1,777 |
Other | | | 110 | | | 110 |
Legal and professional fees | | | 84 | | | 84 |
Total | | $ | 6,538 | | $ | 4,310 |
Compensation costs include unpaid employee salaries and benefits and severance obligations to former executives.
F-37
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
7. NOTES PAYABLE
Paycheck Protection Program Loan
In May 2020, the Company was granted a loan (the “PPP Loan”) in the amount of $599,242, pursuant to the Paycheck Protection Program (the “PPP”) under Division A, Title I of the CARES Act, which was enacted March 27, 2020. The application for these funds required the Company to, in good faith, certify that the current economic uncertainty made the loan request necessary to support the ongoing operations of the Company.
The PPP Loan matures two years after being granted and bears interest at a rate of 1.00% per annum, payable in monthly payments commencing six months after loan disbursements. The notes may be prepaid at any time prior to maturity with no prepayment penalties. Funds from the PPP Loan may only be used for payroll costs and any payments of certain covered interest, lease and utility payments. Under the terms of the PPP, certain amounts of the Loan may be forgiven if they are used for qualifying expenses as described in the CARES Act. The Company is currently applying for forgiveness, but no assurance can be provided that the Company will obtain forgiveness of the PPP Loan in whole or in part. As of July 31, 2020 the current portion of the loan is $298,490 and the non-current portion is $300,752.
The following is a schedule by year of future PPP loan payments at July 31, 2020.
Year Ending July 31, | | Note
payable |
2021 | | $ | 298,490 |
2022 | | | 300,752 |
Total | | $ | 599,242 |
Line of Credit Agreement
On February 3, 2020, the Company entered into a Line of Credit Loan Agreement (“Line of Credit Agreement”) with RP Finance, LLC (“RP Finance”), which provides a revolving commitment of up to $50,000,000 to fund clinical trials and other capital needs.
Rafael Holdings, Inc. owns 37.5% of the equity interests in RP Finance and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. Howard Jonas owns 37.5% of the equity interests in RP Finance, and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. The remaining 25% equity interests in RP Finance is owned by other stockholders of the Company.
Under the Line of Credit Agreement, all funds borrowed will bear interest at the mid-term Applicable Federal Rate published by the U.S. Internal Revenue Service. The maturity date is the earlier of February 3, 2025, upon a change of control of the Company or a sale of the Company or its assets. The Company can draw on the facility on 60 days’ notice. The funds borrowed under the Line of Credit Agreement must be repaid out of certain proceeds from equity sales by the Company.
In connection with entering into the Line of Credit Agreement, the Company agreed to issue to RP Finance 2,612,313 shares of its common stock, representing 12% of the issued and outstanding shares of common stock, with such interest subject to anti-dilution protection as set forth in the Line of Credit Agreement. The common stock issued was valued at a price of $1.95 a share, or $5,112,297. The anti-dilution clause was valued at $33,580,762, which was recorded as a derivative liability. The total aggregate value of the stock issued and the anti-dilution clause was $38,693,059, which the Company recorded as deferred debt issuance costs, net and will be amortized over the life of the line of credit on a straight line basis. For the year ended July 31, 2020, the Company recorded $3,790,946 as amortization expense. As of July 31, 2020, the Company has not drawn down any cash and the remaining amount of the deferred debt issuance cost was $34,902,113.
F-38
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
8. CONVERTIBLE NOTES
The Promissory Notes issuance through July 31, 2020 was as followed:
| | Total | | C Notes(1)(2) | | D Notes(3) |
Balance as of July 31, 2018 | | $ | 22,239 | | | $ | 12,239 | | $ | 10,000 | |
Converted during 2019 | | | (10,000 | ) | | | — | | | (10,000 | ) |
Balance at July 31, 2019 and 2020 | | $ | 12,239 | | | $ | 12,239 | | $ | — | |
F-39
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
8. CONVERTIBLE NOTES (cont.)
The Company accounted for the D Note transaction in accordance ASC 470 and ASC 815. The proceeds were allocated based on the fair value of the note, warrant, equity bonus, and a conversion feature using the Black-Scholes calculation as of the date of the transaction. Thus, the D Note was valued at $3.1 million net of deferred financing charges of approximately $125,000 at issuance. The fair value of the note was calculated using the Black-Scholes method.
The following assumptions were utilized in the Black-Scholes method:
Expected term (years) | | 2.0 |
Risk-free interest rate | | 0.69% |
Volatility | | 96.2% |
Dividend yield | | None |
During the years ended July 31, 2020 and 2019, $0 and $737,000 was recorded in noncash interest expense, respectively.
9. DERIVATIVE LIABILITIES
The Company determined that the anti-dilution feature of the line of credit (see note 7) should be recognized as a free-standing instrument that meets the definition of a derivative under ASC 815. Accordingly, the Company recorded the value of the anti-dilution feature as a derivative liability which will be stated at fair value at each reporting period with changes in fair value being recorded as a marked-to-market adjustment in the Company’s statement of operations.
The fair value of the derivative liability — Line of Credit is estimated using a Monte Carlo pricing model with the following assumptions:
Market value of common stock | | |
Expected volatility | | 63.0% – 94.6% |
Expected term (in years) | | 4.51 – 5.00 |
Risk-free interest rate | | 0.26% – 1.50% |
10. STOCKHOLDERS’ EQUITY
Common Stock — In September 2016, the Company increased the number of authorized shares for common stock from 175,000,000 shares to 310,000,000 including 10,000,000 shares of non-voting common stock. Dividends on common stock will be paid when and if declared by the Board of Directors, subject to restrictions of any other equity security. Each holder is entitled to vote on all matters except for matters exclusively related to other securities. Each holder is entitled to one vote for each common share held. The Company at all times reserves enough common stock to affect a conversion of the shares of other securities into common stock.
F-40
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
10. STOCKHOLDERS’ EQUITY (cont.)
On February 3, 2020, the Company entered into a Line of Credit Loan Agreement with RP Finance, LLC (“RP Finance”), which provides a revolving commitment of up to $50,000,000 to fund clinical trials and other capital needs. In connection with entering into the Line of Credit Agreement, the Company agreed to issue to RP Finance 2,612,313 shares of its common stock representing 12% of the issued and outstanding shares of common stock. The shares were valued at a price of $1.95 a share, or $5,112,297.
Convertible Preferred Stock — In September 2016, the Company increased the number of authorized shares for preferred stock from 100,000,000 shares to 300,000,000. Dividends will be paid when and if declared by the Board of Directors, subject to restrictions of various equity and debt securities. The Company has not declared any dividends on any class of its preferred or common stock. Each preferred stockholder is entitled to vote based on the number of shares held as if converted to common stock. Certain series of preferred stock require separate approval of a majority of the holders of that series. For changes in articles of incorporation, the majority of preferred stock must approve any actions. The various series of preferred stock have convertible features which provide for mandatory or automatic conversions to common stock and non-mandatory conversions to common stock or other securities. Each issue provides for a conversion formula which is based on the lower of the started conversion rate or a rate based on the new equity offering. In the event of a liquidation, certain series of preferred stock would receive its stated liquidated values before other series and preferred stock would receive liquidated values prior to the common stockholders. Neither the Company nor the holder of the preferred stock have the right to redeem or cause the redemption of the preferred stock. Certain series of preferred stockholders have the right to be represented by a Board of Directors member.
The following summarizes the Preferred Stock:
| | July 31, |
| | | 2020 | | 2019 |
Series | | Shares
Authorized | | Shares
Issued | | Liquidation
Preference | | Shares
Authorized | | Shares
Issued | | Liquidation
Preference |
A | | 6,050,000 | | 3,057,500 | | $ | 3,057,500 | | 6,050,000 | | 3,057,500 | | $ | 3,057,500 |
B-1 | | 1,892,072 | | 1,513,658 | | | 1,892,072 | | 1,892,072 | | 1,513,658 | | | 1,892,072 |
B-2 | | 17,500,000 | | 10,000,000 | | | 10,000,000 | | 17,500,000 | | 10,000,000 | | | 10,000,000 |
B-3 | | 30,000,000 | | 10,000,000 | | | 10,000,000 | | 30,000,000 | | 10,000,000 | | | 10,000,000 |
B-4 | | 10,400,000 | | 4,940,620 | | | 6,175,775 | | 10,400,000 | | 4,940,620 | | | 6,175,775 |
C | | 25,000,000 | | — | | | — | | 25,000,000 | | — | | | — |
D | | 161,000,000 | | 53,374,137 | | | — | | 161,000,000 | | 53,374,137 | | | — |
| | | 251,842,072 | | 82,885,915 | | $ | 31,125,347 | | 251,842,072 | | 82,885,915 | | $ | 31,125,347 |
The above tables include 251,842,072 in shares authorized under their respective series as July 31, 2020 and 2019; however, the Company has 300,000,000 shares authorized for all series of Preferred Stock.
The following are the various classes of preferred stock that the Company has issued:
Series A — Contemporaneous with the merger in 2002 as discussed in note 1, the Company issued 3,057,500 shares of $.001 par value Series A Convertible Preferred Stock (“Series A Preferred”) to a group of investors for $1.00 per share. Each share of Series A Preferred is convertible into common stock at a conversion price of $1.00 per share. Series A Preferred is automatically converted into common stock upon the effectiveness of the Company’s filing of an S-1 registration statement with the Securities and Exchange Commission or from the written notification of a majority of the holders of the Series A Preferred share. The conversion price is adjusted for dilutive issuances subject to certain exclusions.
Series B-1, B-2 and B-3 — Prior to 2009, two investors made investments in units, each unit consisting of common stock with a liquidation preference and non-callable warrants. The issuance of Series B-4 Convertible Preferred Stock in 2009 (Series B-4 Preferred), the investment (prior to 2009) for 17,713,658 units were exchanged for shares of Series B-1, B-2, and B-3 Convertible Preferred Stock and non-callable warrants. In 2011, the Company issued an additional 3.8 million units. Series B-1, B-2 and B-3 were entitled to appoint two members of the Board of Directors. As of July 31, 2019, the warrants have either expired or were exchanged for common stock.
F-41
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
10. STOCKHOLDERS’ EQUITY (cont.)
Series B-4 — In 2009, a group of investors invested $6,175,750 for 4,940,620 units of Series B-4 Convertible Preferred (Series B-4 Preferred) shares and non-callable warrants. Each warrant provides for the purchase of Series B-4 Preferred at $1.375 per share. Such warrants expired December 2013. The Preferred Series B-4 Preferred Stock stockholders and the Company entered into an Investor’s Rights Agreement providing certain registration rights, rights of participation in subsequent financings excluding among other items underwritten public offerings. Additionally, the Preferred Series B-4 conversion (to common stock) price is adjusted for certain dilutive issuances until the Company receives proceeds of $10 million in equity. In addition, the Company issued non- callable finder warrants to purchase up to 518,764 shares of Series B-4 Preferred, of which 172,922 expired in December 2013 and 345,842 expired on December 31, 2016.
The Series B Preferred have a liquidated value superior to Series A Preferred and will automatically convert if there is a firm commitment of a fully underwritten public offering of at least $10 million or if the majority of the Series B Preferred holders (of which the B-1, B-2 and B-3 equaled the majority) agreed to convert.
Series C —The Series C Preferred Stock will automatically convert into common stock in the event of a qualified public offering providing at least $20,000,000 and the common shares are traded on a nationally recognized exchange. The Series C Preferred Stock will also automatically convert to common stock upon the written consent of the majority of outstanding shares of the Preferred Stock voting as a single class. Holders of C Notes can convert into Series C Preferred and warrants to purchase Series C Preferred Stock. No C Notes have converted into Series C Preferred Stock.
Series D — The Series D Preferred shares will automatically convert into common stock in the event of a qualified public offering which is underwritten by an underwriter of international standing and traded on an internationally recognized exchange or NASDAQ, and such underwriting has a minimum equity valuation of $200,000,000 and results in aggregate cash proceeds of not less than $50,000,000. The Series D Preferred shares will also automatically convert to common stock upon the written consent of the majority of outstanding shares of the Series D preferred shares. The holder of the Series D Preferred shares has liquidation rights superior to other stockholders equal to the Original Issuance Amount based on $1.25 per share. As of July 31, 2020, 53.4 million shares of Series D Preferred stock were issued upon partial exercise of the Series D Warrant and the conversion of the D Note plus accrued interest.
11. WARRANTS
Preferred Convertible Series C Warrants — The warrants were issued in connection with Series C Convertible Notes. The notes convert into “Series C Units”. Each unit is comprised of one share of the Company’s Series C Convertible Preferred Stock (“C Preferred Stock”) at a conversion price of $1.25 per unit, and one warrant to purchase one share of the Company’s C Preferred Stock at an exercise price of $1.375 per share. The warrants expire four years from the initial date of the note unless amended. The Company has extended the expiration dates on certain warrants (see below).
Preferred Convertible Series D Warrant — The warrant was issued in connection with the September 16, 2016 private offering which resulted in the issuance of a $10,000,000 D Note. The warrant is for the purchase of up to 56% of the then issued and outstanding stock of the Company on an as-converted and fully diluted basis. The exercise price of the warrant is the lower of 70% of the price sold in an equity financing, or $1.25 per share, subject to certain adjustments. The minimum initial and subsequent exercises of the warrant shall be for such number of shares that will result in at least $5,000,000 of gross proceeds to the Company, or such lesser amount as represents 5% of the outstanding capital stock, or such lesser amount as may then remain unexercised. The warrant will expire upon the earlier of December 31, 2020 or a qualified initial public offering or liquidation event.
As of July 31, 2020, the Warrant holder owns 53,400,000 shares of Series D Preferred Stock.
F-42
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
11. WARRANTS (cont.)
Extension of Warrants
On March 2, 2020, the Company extended the expiration dates of the Series C and D warrants to August 31, 2020 and June 30, 2021, respectively. In connection with the extension of these warrants, the Company recorded $32,447,000 of warrant modification expense. In August 2021, the Company further extended the expiration date of the warrants (see note 16).
12. OPTIONS
In 2009, the Company established the 2009 Stock Incentive Plan (“2009 Plan”) providing for the additional granting of incentive stock options and non-qualified options to purchase the Company’s common stock. A total of 5,500,000 shares were authorized under the 2009 Plan and 669,100 shares are available for grant. In 2018, the Company established the 2018 Stock Incentive Plan (“2018 Plan”) providing for the additional granting of incentive stock options and non-qualified options to purchase the Company’s common stock. A total of 4,500,000 shares were authorized under the 2018 Plan. In 2019, the 2018 plan was amended and an additional 2,000,000 shares were authorized for a total of 6,500,000 authorized shares. As of July 31, 2020, 222,786 shares are available for grant.
The Company follows the provisions of the ASC Topic 718, Compensation — Stock Compensation, which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and non-employee directors, including employee stock options. Stock compensation expense based on the grant date fair value estimated in accordance with the provisions of ASC 718 is generally recognized as an expense over the requisite service period.
During the period from August 1, 2018 to July 31, 2020, the following stock option grants were made:
Option Date | | Options
Granted | | Exercise
Price | | Estimated
Fair Value of
Underlying
Stock | | Intrinsic
Value |
January 4, 2019 | | 800,000 | | $ | 2.50 | | $ | 1.14 | | None |
March 16, 2019 | | 150,000 | | $ | 2.50 | | $ | 1.14 | | None |
June 1, 2019 | | 1,375,900 | | $ | 2.50 | | $ | 1.14 | | None |
June 1, 2019 | | 25,000 | | $ | 1.25 | | $ | 1.14 | | None |
August 1, 2019 | | 1,626,264 | | $ | 2.50 | | $ | 1.95 | | None |
December 1, 2019 | | 1,366,750 | | $ | 2.50 | | $ | 1.95 | | None |
February 1-6, 2020 | | 651,300 | | $ | 2.50 | | $ | 1.95 | | None |
June 1, 2020 | | 850,000 | | $ | 2.50 | | $ | 2.11 | | None |
The option prices were determined based on factors such as historical option prices, recent equity transactions and other factors as deemed necessary and relevant in the circumstances. The Company uses an option pricing model to estimate the fair value of its stock options, since market values are generally not available for long-term, non-transferable stock options. In order to estimate the fair value of the options granted, we performed a determination of fair value for accounting purposes of our common stock and underlying stock options using a combination of valuation methods described in American Institute of Certified Public Accountants (“AICPA”) Technical Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation.
F-43
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
12. OPTIONS (cont.)
The exercise prices for options granted were set by our Board of Directors at a premium over fair value of our common stock at the time the grants were authorized. The Board of Directors considered the status of our product pipeline and the progress since the last valuation date, our platform for identifying novel compounds, our asset value (comprised of intellectual property rights and current cash on hand), our present value of future cash flows, our capital structure, the valuation of comparable companies and our acquisition potential. The Board of Directors also considered additional factors, including our existing licensing and research agreements, intellectual property, funding prospects, the funding prospects and valuations of similar companies, the growth prospects of the pharmaceutical industry in general and oncology companies, expected regulatory and commercial hurdles to commercializing or licensing our clinical candidates, the ability of the management team and our access to financing.
The Company utilizes the Black-Scholes valuation method to value stock options and recognize compensation expense over the vesting period. The expected life represents the period that the Company’s stock-based compensation awards are expected to be outstanding. The Company uses a simplified method provided in Securities and Exchange Commission release, Staff Accounting Bulletin (“SAB”) No. 110 (“SAB No. 110”), which averages an award’s weighted average vesting period and contractual term for “plain vanilla” share options. The expected volatility was estimated by analyzing the historic volatility of similar public biotech companies in the oncology field, considering the state of product completion. The Company used an estimated forfeiture rate of 10%.
No dividend payouts were assumed as we have not historically paid and are not anticipated to pay dividends in the foreseeable future. The risk-free rate of return reflects the weighted average interest rate offered for U.S. Treasury rates over the expected term of the options. The fair market value of common stock used in the calculation was based upon future cash flow scenarios.
A summary of significant assumptions used to estimate the fair value of the equity awards granted in during the period is as follows:
| | July 31, |
| | | 2020 | | 2019 |
Expected term (years) | | 6.5 to 7 | | 7 |
Risk-free interest rate | | 0.50% to 1.77% | | 2.03% to 2.56% |
Volatility | | 85.40% | | 74% |
Dividend yield | | None | | None |
No related tax benefits were recorded for the years ended July 31, 2020 and 2019. Generally, options are granted with an exercise price at, or in excess of, the fair value of the common stock at the date of issuance. Options typically vest over a one- to four-year period in equal increments and expire not more than 10 years after the grant date.
The Company recorded noncash stock-based compensation of approximately $1,347,000 and $218,000 during the years ended July 31, 2020 and 2019, respectively. As of July 31, 2020, options to purchase 4,920,447 shares of common stock were fully vested. As of July 31, 2020, there was approximately $5,063,000 of remaining unamortized stock-based compensation expense associated with stock options, which will be expensed over the next three years.
F-44
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
12. OPTIONS (cont.)
A summary of option activity for the combined plans for the years ended July 31, 2020 and 2019, and the changes for the years then ended, is presented as follows:
| | Number of
Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contract
Terms (Years) | | Aggregate
Intrinsic
Value |
Options outstanding – July 31, 2018 | | 6,200,900 | | | $ | 1.91 | | 7.0 | | — |
Granted | | 2,400,900 | | | | 2.49 | | — | | — |
Expired | | (585,000 | ) | | | 1.00 | | — | | — |
Forfeited | | (250,000 | ) | | | 2.50 | | — | | — |
Options outstanding – July 31, 2019 | | 7,766,800 | | | | 2.14 | | 7.4 | | — |
Granted | | 4,494,314 | | | | 2.50 | | — | | — |
Forfeited | | (728,000 | ) | | | 2.07 | | — | | — |
Options outstanding – July 31, 2020 | | 11,533,114 | | | | 2.29 | | 7.6 | | — |
Options vested and expected to vest – July 31, 2020 | | 10,871,848 | | | | 2.28 | | 7.5 | | — |
Options exercisable – July 31, 2020 | | 4,920,447 | | | $ | 2.12 | | 6.0 | | — |
Other Options — The Company has issued other options to investors and others as finders’ fees (collectively “Other Options”) in connection with prior capital raises.
In connection with the Company’s 2003 common stock offerings, the Company entered into an option agreement with an individual in connection with identifying investors. The option agreement grants the right to purchase an option (a “Purchase Option”) to purchase 472,000 Class A Options (“Class A Options”), which allows the purchase of 2.5 shares of common stock for each Class A Option at $1.10 per share. In order to secure this Class A Option, a Purchase Option must initially be purchased for $.005 per potential share of Class A options. Upon exercise of each Class A Option, a right is granted to one Class B Option (“Class B Options”), which allows the purchase of 2.5 shares of common stock for each Class B Option at $1.25 per share. The expiration date of the Class A Options is the later of October 29, 2005 or six months from the date the Company’s shares become publicly traded. The Class B Options expire 180 days from the exercise of the Class A Options.
In 2003, 625,000 options (the “Options”) were granted at a strike price of $1.10 per share to a 2003 Investor. These Options are set to expire 180 days following the closing of an IPO, or from the date the Company’s shares become publicly traded.
13. INCOME TAXES
The benefit for income taxes consists of the following for the years ended July 31, 2020 and 2019:
| | For the Years Ending
July, 31 |
| | | 2020 | | 2019 |
Current state | | $ | — | | | $ | — | |
Deferred – Federal | | | 9,893,185 | | | | 4,163,441 | |
Deferred – State | | | 2,375,732 | | | | 2,664,686 | |
Total | | | 12,268,917 | | | | 6,828,127 | |
Change in valuation allowance | | | (12,268,917 | ) | | | (6,828,127 | ) |
| | | $ | — | | | $ | — | |
F-45
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
13. INCOME TAXES (cont.)
Deferred tax assets (liabilities) as of July 31, 2020 and 2019 consist of the following:
| | For the Years Ending
July, 31 |
| | | 2020 | | 2019 |
Net operating loss carryovers | | $ | 30,493,319 | | | $ | 19,729,474 | |
Stock compensation | | | — | | | | 438,875 | |
Tax credits | | | 6,537,201 | | | | 4,183,454 | |
Depreciation | | | 382,967 | | | | 327,013 | |
Accrued compensation | | | — | | | | 7,257 | |
Other | | | (21,622 | ) | | | 15,556 | |
Charity | | | 70,275 | | | | — | |
Deferred debt discount | | | (881,077 | ) | | | — | |
Accrued interest | | | 761,200 | | | | 636,079 | |
Gross deferred tax assets | | | 37,342,263 | | | | 25,337,708 | |
Valuation allowance | | | (37,342,263 | ) | | | (25,337,708 | ) |
Net deferred taxes | | $ | — | | | $ | — | |
A reconciliation between the Company’s effective tax rate and the federal statutory rate for the years ended July 31, 2020 and 2019 is as follows:
| | For the Years Ended
July 31, |
| | | 2020 | | 2019 |
Federal statutory rate | | 21.0 | % | | 21.0 | % |
Permanent differences | | (9.7 | )% | | — | |
Federal research and development credits | | — | | | 7.6 | % |
State taxes | | 4.0 | % | | 7.1 | % |
State NOL Sale | | — | | | — | |
Deferred Tax Rate Change Valuation allowance | | (15.1 | )% | | (46.2 | )% |
Prior year adjustment | | — | | | — | |
Other | | (0.2 | )% | | 4.7 | % |
Effective tax rate | | — | | | (5.8 | )% |
During 2019, in accordance with the State of New Jersey’s Technology Business Tax Certificate Program, which allowed certain high technology and biotechnology companies to sell unused net operating loss carryforwards (“NOLs”) to other New Jersey-based corporate taxpayers based in New Jersey, the Company plans on selling its New Jersey NOLs. The value of the tax attributes have not yet been determined. In fiscal year 2019, the Company received approximately $1.2 million from the program which was the value of the remaining tax attributes from fiscal 2018 after commission to the selling agent. On June 14, 2021 the company received $2.3 million related to the R&D credit of 2020.
As of July 31, 2020, the Company has approximately $125,200,000 of NOLs that will begin to expire in 2022 and approximately $63,700,000 of New Jersey NOLs that will begin to expire in 2029. The NOLs generated in the current year will not expire. The Company has undergone ownership changes and has determined that a “change in ownership” as defined by IRC Section 382 of the Internal Revenue Code of 1986, as amended, and the rules and regulations promulgated thereunder, did occur during 2008. Accordingly, about $19,875,000 of the Company’s NOLs carryforwards are limited and the Company can only use $3,601,883 for the first five years from the ownership change
F-46
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
13. INCOME TAXES (cont.)
and $1,488,550 per year going forward. Therefore, all of the limited NOL’s will be available after seven years (2015). The Company has R&D Credit carryovers for Federal and New Jersey of approximately $4,878,000 and $2,163,000, respectively, these will begin to expire in 2029.
The Company records accrued interest and penalties related to unrecorded tax benefits in the interest expense and operating expense. The Company had not accrued any interest or penalties related to unrecognized benefits. The Company is no longer subject to Federal income tax assessment for years before 2017 and 2016 for New Jersey income tax assessment. However, since the Company has incurred net operating losses in every year since inception, all of its income tax returns are subject to examination and adjustments by the Internal Revenue Service for at least three years following the year in which the tax attributes are utilized. The Company does not believe that there will be a material change in its unrecognized tax positions over the next 12 months.
14. COMMITMENTS
COVID-19 Pandemic
In December 2019, a new coronavirus, now known as COVID-19, which has proved to be highly contagious, emerged in Wuhan, China and has since spread around the globe. The Company actively monitors the outbreak and its potential impact on its operations.
The impacts on the operations and specifically the ongoing clinical trials have been actively managed by respective pharmaceutical management teams who have worked closely with the appropriate regulatory agencies to continue clinical trial activities with as minimal impact as possible.
Due to both known and unknown risks, including quarantines, closures and other restrictions resulting from the outbreak, operations may be adversely impacted. Additionally, as there is an evolving nature to the COVID-19 situation, we cannot reasonably assess or predict at this time the full extent of the negative impact that the COVID-19 pandemic may have on our business, financial condition, results of operations and cash flows. The impact will depend on future developments such as the ultimate duration and the severity of the spread of the COVID-19 pandemic in the U.S. and globally, the effectiveness of federal, state, local and foreign government actions on mitigation and spread of COVID-19, the pandemic’s impact on the U.S. and global economies, changes in our customers’ behavior emanating from the pandemic and how quickly we can resume our normal operations, among others. For all these reasons, the Company may incur expenses or delays relating to such events outside of the Company’s control, which could have a material adverse impact on the Company’s business.
License Agreements — The Company is a party to two license agreements in connection with certain technology being used for products under development and is required to make certain annual maintenance payments. In addition, royalty payments, calculated on a low single digit percentage of net sales, as defined in the respective agreements, will be required upon the commercialization of licensed technology. Sublicensing fees are calculated and due based upon a percentage of gross sublicense fees. The Company expenses license obligation payments to research and development.
One worldwide license agreement requires the Company to reimburse the other party for costs associated with filing and defending various patents worldwide. Payment obligations under this license agreement remain in effect until the last underlying patents granted under the license agreement expire in their respective countries. Currently, the last patent expired in 2019. License maintenance fees are currently $20,000 per year and continue for the term of the agreement. The license maintenance fees are replaced by minimum royalties of $10,000 during the first year following governmental approval to market products and escalate to $1,000,000 during the term of the agreement. The Company is also responsible for sub licensing fees. The Company may credit each annual license maintenance fee in full against all royalties and sublicensing fees due during the same calendar year. The Company may terminate the license agreement upon 90 days’ notice. Either party may terminate the license agreement if the other party commits any material breach of any covenant or promise and does not cure such breach within 30 days of the receipt of written notice of such material breach. In May 2017, the Company renegotiated the agreement referred to as the “second
F-47
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
14. COMMITMENTS (cont.)
license”. In exchange for a waiver of certain product development milestones, the Company modified the agreement to pay a low single digit percentage royalty for a duration of five years on Net Sales of product sold after the expiration of the licensed patent and potentially up to eight years. As of July 31, 2020, there are no products being marketed which are covered by the patents under the license agreement.
The remaining minimum payments required under the license agreement, assuming the agreements are not terminated by the Company, excluding any escalation for receiving government marketing approval subsequent to July 31, 2018 are $20,000 per year through fiscal year ended 2020.
The Company’s second license continues until the termination of the later of the last to expire patent or royalty obligation under the agreement on a country by country basis (currently, or as otherwise provided in the license agreement). Fifty percent (50%) of the maintenance fee payments, up to $1.1 million, may be credited against the potential future royalty payments, calculated on a single digit percentage of net sales, as defined, that the Company would have to make to the license holder should royalties be paid. The agreement may be terminated on 15 days’ written notice after default by the other party if said default is not cured within 30 days of receipt of notice by the defaulting party. In addition, the Company may terminate the agreement on 15 days’ written notice to the license holder. Royalties are due based on Gross Sales, as defined, for products sold relating to patented and unpatented technology, and shall terminate on the 15th anniversary of the first commercial sale of the product in the corresponding country or territory. Sublicense payments are due in connection with any sublicense fees received relating to patented and non-patented products related to the patented technology and proprietary know-how, as provided in the agreement. As of July 31, 2020, there were no products being marketed which are covered by the patents under the license agreement. There were no additional annual license maintenance fees required beyond 2010.
In addition, Ventures (as discussed in Note 1) had an operating agreement with Altira Capital and Consulting LLC (“Altira”) that provided for the payment of royalties to Altira in exchange for the assignment of intellectual property to the Company. At the time of the operating agreement, Altira was owned by two officers (the Chief Executive Officer and the President) of the Company. The operating agreement was terminated when Ventures and the Company merged in 2002. At the merger date, the Company agreed to continue the specific section of the operating agreement dealing with the payment of royalties to Altira, including the establishment of a royalty pool for certain inventors. Altira would receive a royalty as part of a newly created inventor’s royalty pool to include specific inventors of Company technology for the products and services developed in part by certain Altira principals and other inventors. The Company is obligated to pay royalties based upon percentage (low single digit) of net sales, as defined in the royalty agreement. The royalty obligations remain in effect, on a country by country basis, until the last to expire patent claims associated with such products and services expire or are no longer in force. No payments have been made in connection with a royalty pool. As of July 31, 2020, the last to expire patent claim is to remain in force until fiscal 2034.
Lease Obligations — The Company entered into a 10-year lease agreement on June 1, 2006 for office and laboratory space in Cranbury, New Jersey (“Cranbury”). Such agreement’s initial term expired during July 2016 and was amended in September 2016. The amended lease agreement renews the lease for an additional seven years commencing on August 1, 2016 and ending on July 31, 2023. The base rent was amended to $217,588 annually through July 31, 2021 increasing to $250,185 commencing on August 1, 2021 for the last two years.
Rent expense including deferred rent charges amounted to $255,000 and $265,000 for the years ended July 31, 2020 and 2019, respectively.
F-48
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
14. COMMITMENTS (cont.)
The following is a schedule by year of future rent payments at July 31, 2020.
Year Ending July 31, | | Rental
Payment |
2021 | | $ | 217,588 |
2022 | | | 250,185 |
2023 | | | 250,185 |
Total | | $ | 717,958 |
15. RELATED PARTY TRANSACTIONS
On January 16, 2016, CS Pharma Holdings (a subsidiary of IDT Corporation) and/or IDT Corporation (the “Investor”) and the Company entered into a subscription agreement for the issuance of up to a $10,000,000 D Note to the Investor which is convertible into Series D Preferred Stock subject to due diligence and the conversion of a prior $500,000 advance to a C Note holder. On March 31, 2016, the Company entered into the First Global Amendment to the Loan Document providing for an additional $1,500,000 of funds to the Company in exchange for the issuance of an additional C Note in the same amount which was issued on April 13, 2016. After completion of due diligence and negotiations, on September 16, 2016, the Investor provided the remaining $8,000,000 less expenses. The Company cancelled both C Notes and issued a Series D Note in the amount of $10,000,000. The D Note, including the unpaid interest, has a maturity date of two years. In addition, the Company issued a warrant to the Investor to purchase up to 56% of the outstanding capital stock of the Company. An equity bonus was granted to be calculated based on 10% of the outstanding capital stock subject to certain milestones. In addition, the Investor was granted voting proxies by holders of a majority of the shares of the Company’s common stock outstanding permitting the Investor to veto certain financing or asset transactions for a period of two years. The Amendment provided that the subscriber may appoint two additional Board Members and the Chairman of the Board, as well as Veto Rights for subsequent financing, sale, a change of control or other arrangement, and Equity Bonus Shares.
In March 2018, IDT Corporation spun off a former subsidiary to an independent public company, Rafael Holdings, Inc., which acquired upon spin off, interests/rights in Rafael Pharmaceuticals Inc. through a 90%-owned non-operating subsidiary, Pharma Holdings, LLC (“Pharma Holdings”). Pharma Holdings holds the Preferred Convertible Series D Warrant to purchase a significant stake in Rafael Pharmaceuticals Inc., as well as other equity and governance rights in Rafael Pharmaceuticals Inc., and owns 50% of CS Pharma Holdings, LLC (“CS Pharma”), a non-operating entity which holds the convertible debt and other rights to purchase equity interests in Rafael Pharmaceuticals Inc.
The Company’s Chairman of the Board of Directors (appointed in April 2016), Mr. Howard Jonas, who is also the current Chairman of the Board of the Investor, held jointly with his spouse $525,000 in C Notes as of July 31, 2018. In addition, an affiliated foundation held an additional $525,000 of C Notes. Mr. Jonas also has a stock option for 100,000 shares of common stock at $1.25 per share which expires in 2023.
On February 3, 2020, the Company entered into a Line of Credit Loan Agreement (“Line of Credit Agreement”) with RP Finance, LLC (“RP Finance”), which provides a revolving commitment of up to $50,000,000 to fund clinical trials and other capital needs. Rafael Holdings, Inc. owns 37.5% of the equity interests in RP Finance and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. Howard Jonas owns 37.5% of the equity interests in RP Finance, and is required to fund 37.5% of funding requests from the Company under the Line of Credit Agreement. The remaining 25% equity interests in RP Finance is owned by other stockholders of the Company (see note 7).
F-49
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
15. RELATED PARTY TRANSACTIONS (cont.)
Altira (see note 14), a significant investor, is owned by an officer and director of the Company, a former officer and director of the Company and two other significant investors/directors (herein the “Two Investors”) who became partners in Altira in 2004. In June 2006, a company controlled by the Two Investors, Cedar Brook East Corporate Center, L.P. (“Landlord”), leased a facility to the Company under a 10-year non-cancelable lease (see note 14) which renewed in 2016. Altira is entitled to receive royalties on net sales as described in note 14. The Two Investors continued to invest in the preferred stock issuances and make advances to the Company.
As of July 31, 2020 and 2019, the Company has unpaid lease obligations (to companies controlled by the Two Investors) in accounts payable of approximately $963,000.
The Company pays $40,000 per month in Management Fees to Rafael Holdings, Inc. for accounting and other administrative functions. Rafael Holdings is the parent company of Pharma Holdings. At July 31, 2020 and 2019, the Company owed $120,000 and $280,000 to Rafael Holdings, Inc., respectively.
During the years ended July 31, 2020 and 2019, there were no related parties advances.
16. SUBSEQUENT EVENTS
The Company evaluated events that have occurred from the date of the financial statements through the date the financial statements were available to be issued.
From August 2020 through the date of the issuance of these financial statements, the Company called for a $17 million draw on its Line of Credit.
On August 31, 2020, the Company extended the Series C warrants to allow the holders 30 days to determine whether or not to exercise the warrants. If the holders choose to exercise the warrants and provide a 10% deposit, the Company will provide an additional 60-days extension to fund the full amount of the exercise. Additionally, the Company further extended the expiration dates of the Series D warrants to August 15, 2021.
In October 2020, the Company received $3,013,028 as a deposit for the exercising of certain C warrants. In December 2020, 6,321,312 of C warrants were exercised at a price of $1.375 for a total of $8,691,801 and 2,410,922 C warrants were forfeited. The total remaining C warrants as of the issuance of these financial statements was 3,253,948 that expire in February 2022.
On January 28, 2021, Rafael Holdings partially exercised warrants to maintain the 51% ownership percentage and purchased 7.3 million shares of the Company’s Series D Preferred Stock for $9.1 million.
In April 2021, the Company was granted an additional PPP Loan in the amount of $814,642.
On June 14, 2021, in accordance with the State of New Jersey’s Technology Business Tax Certificate Program, which allowed certain high technology and biotechnology companies to sell unused net operating loss carryforwards (“NOLs”) to other New Jersey-based corporate taxpayers based in New Jersey, the Company sold all its available New Jersey NOLs and received a payment of $2,348,399.
Plan of Merger
On June 17, 2021, Rafael Holdings, Inc. (“Holdings”), entered into an Agreement and Plan of Merger (the “Merger Agreement”), by and among: Holdings; RH Merger I, Inc., a Delaware corporation and a wholly-owned subsidiary of Holdings (“Merger Sub I”); RH Merger II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Holdings (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”); and Rafael Pharmaceuticals, Inc., a Delaware corporation (“Pharma”). Pharma will become a wholly-owned subsidiary of Holdings.
F-50
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
16. SUBSEQUENT EVENTS (cont.)
As a consequence of the Mergers, at the Effective Time, each outstanding share of each class of Pharma capital stock will be automatically cancelled and retired and cease to exist, and will entitle a holder of shares of a given class of Pharma capital stock to receive 0.12045 shares of Parent Class B Common Stock per share of Pharma capital stock. This Per Share Merger Consideration ratio for each class of Pharma capital stock will provide that the prior holders of Pharma capital stock and contingent interests will receive Parent Class B Common Stock amounting to approximately 43% of the currently outstanding capitalization of Parent. Additional equity in Parent will be issued in connection with the contemplated financing and pursuant to employment agreements (which will reduce the foregoing percentage proportionately).
The consideration payable by Holdings takes into account the value of the Holdings’ assets including its interests in Pharma through shares and warrants. Other holders of warrants to purchase Pharma stock may elect to exercise their portion of the Warrant prior to the Closing Date. Any other unexercised warrants shall be terminated at the Effective Time in accordance with the terms of the Merger Agreement; provided that any Pharma Series C Warrants, to the extent not exercised by its holder prior to the Effective Time, shall be exchanged for a commensurate warrant to purchase shares of Holdings or otherwise be settled by Holdings and the Common Warrant Option, to the extent not exercised by its holder prior to the Effective Time, shall be exchanged for a commensurate warrant to purchase shares of Holdings or otherwise be settled by Holdings.
In connection with the Mergers, the contractual right to receive additional shares of Pharma capital stock equal to 10% of the fully diluted Pharma capital stock (the “Bonus Shares”) that is held by an instrument (the “Instrument”) that is within a trust for the benefit of the children of Holdings’ Chairman Howard Jonas will also be satisfied by the issuance of Pharma capital stock immediately prior to the effective time of the Mergers and the holder of the right shall receive the Merger Consideration that would be due to a holder of the shares of Pharma Common Stock as would be issuable in respect of that right. The Merger Agreement provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable as Bonus Shares shall be calculated as of September 30, 2021, as if the Mergers were consummated on such date.
At the Effective Time, all compensatory options to purchase Pharma Common Stock (i) shall automatically vest in full, and all holders of such options will receive options to purchase Class B common stock of Holdings using the ratios applicable to determining the Holdings stock in the Business Combination.
Convertible Note Cancellation Agreement
Under the terms of the Merger Agreement, the parties intend that the Pharma Convertible Notes shall not be assumed or continued by Holdings or Pharma in connection with the Merger or the other transactions contemplated thereby. Immediately prior to the Effective Time, Holdings and Pharma shall use their commercially reasonable efforts to cause each Pharma Convertible Note to be cancelled and extinguished and converted into the applicable Note Conversion Shares, in accordance with the terms of Pharma Convertible Notes and of that certain form of Convertible Note Cancellation Agreement, to be executed and delivered by each Pharma Noteholder prior to the Closing. The Convertible Note Cancellation Agreement provides that, contingent on the consummation of the Merger, the applicable Note Conversion Shares to which a Pharma Noteholder is entitled shall be calculated based on accrued interest as of September 30, 2021, as if the Mergers were consummated on such date.
Warrant held By Holdings
Prior to the Closing, Holdings shall effect the termination of the Series D warrants, such termination to be contingent on the consummation of the Merger and effective at the Initial Effective Time. The Support Agreements executed by holders of the Series D warrants provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable pursuant to the Series D warrants shall be calculated as of September 30, 2021, as if the Merger were consummated on such date.
F-51
Table of Contents
RAFAEL PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED JULY 31, 2020 AND 2019
16. SUBSEQUENT EVENTS (cont.)
Line of Credit Amendment
RP Finance and Pharma have amended the Line of Credit to modify the definition of “Change of Control” to provide that the Line of Credit, which has an outstanding principal balance of $17 million on the date hereof, and all accrued and unpaid interest thereon will be due and payable on consummation of the Merger and to shorten the call period for draws to thirty days. The Merger Agreement provides that, contingent on the consummation of the Merger, the anti-dilution accrual of additional shares issuable pursuant to the Line of Credit shall be calculated as of September 30, 2021, as if the Mergers were consummated on such date.
F-52
Table of Contents
Annex A
AGREEMENT AND PLAN OF MERGER
By and among
RAFAEL HOLDINGS, INC.,
RH MERGER I, INC.,
RH MERGER II, LLC,
and
RAFAEL PHARMACEUTICALS, INC.
Dated as of June 17, 2021
Table of Contents
Table of Contents
| | | | | | Annex A
Page |
1. | | TRANSACTION | | A-2 |
| | | 1.1 | | The Mergers | | A-2 |
| | | 1.2 | | Effect of the Mergers | | A-2 |
| | | 1.3 | | Closing; Effective Time | | A-2 |
| | | 1.4 | | Certificate of Incorporation and Bylaws; Directors and Officers. | | A-3 |
| | | 1.5 | | Conversion of Shares | | A-3 |
| | | 1.6 | | Treatment of Company Warrants, Company Options, Company Convertible Notes and Contingent Rights. | | A-4 |
| | | 1.7 | | Further Action. | | A-5 |
| | | 1.8 | | Closing of the Company’s Transfer Books | | A-5 |
| | | 1.9 | | Exchange/Payment | | A-6 |
| | | 1.10 | | No Appraisal or Dissenters’ Rights. | | A-6 |
| | | 1.11 | | Extension of 56% Warrant. | | A-6 |
2. | | REPRESENTATIONS AND WARRANTIES OF THE COMPANY | | A-6 |
| | | 2.1 | | Due Incorporation; Etc. | | A-7 |
| | | 2.2 | | Certificate of Incorporation and Bylaws; Certificates of Designation. | | A-7 |
| | | 2.3 | | Capitalization, Etc. | | A-7 |
| | | 2.4 | | Financial Statements. | | A-8 |
| | | 2.5 | | Absence of Certain Changes. | | A-9 |
| | | 2.6 | | Title to Assets. | | A-9 |
| | | 2.7 | | Real Property; Leasehold | | A-9 |
| | | 2.8 | | Intellectual Property. | | A-10 |
| | | 2.9 | | Material Contracts. | | A-16 |
| | | 2.10 | | Liabilities. | | A-18 |
| | | 2.11 | | Compliance with Laws; Permits. | | A-18 |
| | | 2.12 | | Certain Business Practices | | A-19 |
| | | 2.13 | | Tax Matters. | | A-19 |
| | | 2.14 | | Employee Benefit Plans. | | A-21 |
| | | 2.15 | | Employee Matters. | | A-22 |
| | | 2.16 | | Environmental Matters | | A-24 |
| | | 2.17 | | Insurance | | A-24 |
| | | 2.18 | | Legal Proceedings; Orders | | A-24 |
| | | 2.19 | | Authority; Binding Nature of Agreement | | A-24 |
| | | 2.20 | | Vote Required. | | A-25 |
| | | 2.21 | | Counterparties. | | A-25 |
| | | 2.22 | | Non-Contravention; Consents | | A-25 |
| | | 2.23 | | Financial Advisor | | A-25 |
| | | 2.24 | | Related Party Transactions | | A-25 |
| | | 2.25 | | Regulatory Matters. | | A-26 |
| | | 2.26 | | Disclosure. | | A-28 |
| | | 2.27 | | No Additional Representations. | | A-28 |
3. | | REPRESENTATIONS AND WARRANTIES OF PARENT AND THE MERGER SUBS | | A-29 |
| | | 3.1 | | Due Incorporation, Etc. | | A-29 |
| | | 3.2 | | Capitalization, Etc. | | A-29 |
| | | 3.3 | | Authority; Binding Nature of Agreement | | A-30 |
| | | 3.4 | | Non-Contravention; Consents | | A-30 |
| | | 3.5 | | Absence of Certain Changes | | A-31 |
| | | 3.6 | | Legal Proceedings | | A-31 |
| | | 3.7 | | SEC Reports, Financial Statements. | | A-31 |
Annex A-i
Table of Contents
| | | | | | Annex A
Page |
| | | 3.8 | | Liabilities | | A-32 |
| | | 3.9 | | Compliance with Laws | | A-32 |
| | | 3.10 | | Subsidiaries; Merger Subs | | A-32 |
| | | 3.11 | | Valid Issuance. | | A-32 |
| | | 3.12 | | Financing | | A-32 |
| | | 3.13 | | Takeover Statutes | | A-32 |
| | | 3.14 | | Ownership of Capital Stock of the Company | | A-32 |
| | | 3.15 | | Tax Matters | | A-33 |
| | | 3.16 | | No Additional Representations | | A-33 |
4. | | CERTAIN COVENANTS | | A-33 |
| | | 4.1 | | Access. | | A-33 |
| | | 4.2 | | Conduct of the Business of the Company | | A-33 |
| | | 4.3 | | Conduct of the Business of Parent | | A-35 |
| | | 4.4 | | No Solicitation. | | A-36 |
| | | 4.5 | | Takeover Statutes. | | A-36 |
| | | 4.6 | | Certain Tax Matters | | A-37 |
5. | | ADDITIONAL COVENANTS OF THE PARTIES | | A-37 |
| | | 5.1 | | Registration Statement. | | A-37 |
| | | 5.2 | | Stockholder Consent or Approval; Required Stockholder Votes. | | A-38 |
| | | 5.3 | | Reasonable Best Efforts. | | A-40 |
| | | 5.4 | | Indemnification of Officers and Directors. | | A-40 |
| | | 5.5 | | Disclosure | | A-41 |
| | | 5.6 | | Notification of Certain Events. | | A-41 |
| | | 5.7 | | Confidentiality. | | A-42 |
| | | 5.8 | | Company Options. | | A-42 |
| | | 5.9 | | Resignations | | A-42 |
| | | 5.10 | | Employee Benefits | | A-42 |
| | | 5.11 | | Stock Exchange Listing. | | A-43 |
| | | 5.12 | | Royalty Buyout. | | A-43 |
| | | 5.13 | | The 56% Warrant. | | A-43 |
| | | 5.14 | | Financing. | | A-43 |
| | | 5.15 | | Agency Communications and Filings. | | A-43 |
6. | | CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY | | A-44 |
| | | 6.1 | | Effectiveness of Registration Statement. | | A-44 |
| | | 6.2 | | No Restraints. | | A-45 |
| | | 6.3 | | Stockholder Approval. | | A-45 |
| | | 6.4 | | Majority of the Minority Approval | | A-45 |
| | | 6.5 | | Listing. | | A-45 |
| | | 6.6 | | Royalty Buyout. | | A-45 |
| | | 6.7 | | Governmental Consents. | | A-45 |
| | | 6.8 | | Charter Amendment | | A-45 |
| | | 6.9 | | Closing of Financing. | | A-45 |
7. | | CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND THE MERGER SUBS | | A-45 |
| | | 7.1 | | Accuracy of Representations and Warranties | | A-45 |
| | | 7.2 | | Performance of Covenants. | | A-46 |
| | | 7.3 | | No Governmental Litigation | | A-46 |
| | | 7.4 | | Agreements and Documents | | A-46 |
| | | 7.5 | | Closing Certificate | | A-46 |
| | | 7.6 | | Royalties; Consents. | | A-46 |
| | | 7.7 | | No Material Adverse Effect | | A-46 |
Annex A-ii
Table of Contents
| | | | | | Annex A
Page |
8. | | CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY | | A-46 |
| | | 8.1 | | Accuracy of Representations and Warranties. | | A-46 |
| | | 8.2 | | Performance of Covenants | | A-46 |
| | | 8.3 | | No Governmental Litigation. | | A-46 |
| | | 8.4 | | No Material Adverse Effect | | A-46 |
| | | 8.5 | | Closing Certificate. | | A-47 |
| | | 8.6 | | Reserved | | A-47 |
| | | 8.7 | | Dissolution of Subsidiaries. | | A-47 |
| | | 8.8 | | Lock-Up Agreements | | A-47 |
9. | | TERMINATION | | A-47 |
| | | 9.1 | | Termination | | A-47 |
| | | 9.2 | | Effect of Termination. | | A-48 |
10. | | MISCELLANEOUS PROVISIONS | | A-48 |
| | | 10.1 | | No Survival of Representations and Warranties | | A-48 |
| | | 10.2 | | Amendment. | | A-48 |
| | | 10.3 | | Expenses. | | A-48 |
| | | 10.4 | | Waiver | | A-48 |
| | | 10.5 | | Entire Agreement; Counterparts | | A-49 |
| | | 10.6 | | Applicable Law; Jurisdiction; Waiver of Jury Trial | | A-49 |
| | | 10.7 | | Attorneys’ Fees. | | A-49 |
| | | 10.8 | | Assignability | | A-49 |
| | | 10.9 | | Third Party Beneficiaries | | A-49 |
| | | 10.10 | | Notices | | A-49 |
| | | 10.11 | | Severability. | | A-50 |
| | | 10.12 | | Knowledge. | | A-50 |
| | | 10.13 | | Specific Performance. | | A-51 |
| | | 10.14 | | Construction | | A-51 |
| | | 10.15 | | Disclosure Schedule. | | A-51 |
| | | 10.16 | | Non-Recourse | | A-52 |
Exhibits and Schedules: | | |
| | | |
Exhibit A: Certain Definitions | | |
Exhibit B-1: Support Agreement | | |
Exhibit B-2: Company Stockholder Written Consent | | |
Exhibit B-3: Voting Agreement | | |
Exhibit B-4: Company Note Cancellation Agreement | | |
Exhibit C: Lock-Up Agreement | | |
Exhibit D: Letter of Transmittal | | |
Exhibit E: Altira Acquisition Agreement | | |
Exhibit F: Form of Amendment of Certificate of Incorporation | | |
Schedule I: Lock-Up Parties | | |
Schedule II: Post-Closing Managers and Officers of the Surviving Corporation | | |
Company Disclosure Schedule | | |
Parent Disclosure Schedule | | |
Annex A-iii
Table of Contents
AGREEMENT AND PLAN OF MERGER
THIS AGREEMENT AND PLAN OF MERGER (as may be amended from time to time, this “Agreement”) is made and entered into as of June 17, 2021, by and among: RAFAEL HOLDINGS, INC., a Delaware corporation (“Parent”); RH MERGER I, INC., a Delaware corporation and a wholly-owned subsidiary of Parent (“Merger Sub I”); RH MERGER II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Parent (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”); and RAFAEL PHARMACEUTICALS, INC., a Delaware corporation (the “Company”). Certain capitalized terms used in this Agreement are defined in Exhibit A.
RECITALS
A. Parent, Merger Sub I and the Company intend to effect a merger of Merger Sub I with and into the Company (the “Merger”) in accordance with this Agreement and the Delaware General Corporation Law, as amended (the “DGCL”). Upon consummation of the Merger, Merger Sub I will cease to exist as a separate corporate entity, and the Company will become a wholly-owned subsidiary of Parent.
B. Parent, Merger Sub II and the Company thereafter intend that, on the terms and subject to the conditions set forth in this Agreement and immediately after the Effective Time, the Company shall merge with and into Merger Sub II (the “Subsequent Merger” and, together with the Merger, the “Mergers”), with Merger Sub II surviving the Subsequent Merger, pursuant to the provisions of the DGCL and the Delaware Limited Liability Company Act (the “DLLCA”).
C. The Board of Directors of the Company (the “Company Board”), has (a) determined that this Agreement, the Mergers and the other transactions contemplated by this Agreement, including the Voting Agreements, Support Agreements and Lock-Up Agreements (the “Contemplated Transactions”), are advisable and fair and in the best interests of the Company and its stockholders (other than Parent, its Affiliates and Howard Jonas, and their respective affiliates, as to which fairness the Company Board has not made any determination), (b) authorized and approved the execution, delivery and performance of this Agreement by the Company and approved the Mergers, and (c) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the Company Stockholders vote to adopt this Agreement and approve the consummation of the Mergers;
D. The board of directors of Parent (the “Parent Board”), following a resolution from its special committee (the “Parent Special Committee”) recommending entry into this Agreement and consummation of the Mergers, has (a) determined that this Agreement, the Mergers and the Contemplated Transactions are advisable and fair and in the best interests of the Parent and its stockholders, (b) authorized and approved the execution, delivery and performance of this Agreement by the Parent and approved the Mergers, and (c) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the Parent Stockholders vote to adopt this Agreement and approve the consummation of the Mergers (the “Special Committee Recommendation”);
E. The board of directors of Merger Sub I has (a) determined that this Agreement, the Mergers and the Contemplated Transactions are advisable and fair and in the best interests of Merger Sub I and its stockholder, (b) authorized and approved the execution, delivery and performance of this Agreement by Merger Sub I and approved the Mergers subject to the required stockholder votes to adopt this Agreement and approve the consummation of the Mergers;
F. Merger Sub II has authorized and approved, in accordance with its limited liability company agreement and the DLLCA, the execution, delivery and performance of this Agreement by Merger Sub II and approved the Subsequent Merger subject to the required member vote to adopt this Agreement and approve the consummation of the Subsequent Merger;
G. The Parties intend that, for U.S. federal income tax purposes, the Mergers, taken together, will qualify as a “reorganization” within the meaning of Section 368(a) of the Code (the “Intended Tax Treatment”), and that this Agreement be, and is hereby adopted as, a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3;
Annex A-1
Table of Contents
H. Concurrently with the execution and delivery of this Agreement, as a condition and inducement to the willingness of Parent and Merger Sub I and Merger Sub II to enter into this Agreement, (a) each of the directors and executive officers of the Company and certain other Company Stockholders have entered into a Support Agreement with Parent and the Company in substantially the form attached hereto as Exhibit B-2 (and collectively with the Company stockholder written consent appended thereto (the “Company Stockholder Written Consent”) and in the form attached to this Agreement as Exhibit B-1 (the “Support Agreements”), which support agreements include an agreement from the holders of Company Preferred Stock (including any Company Preferred Stock issuable upon exercise of convertible securities) who are affiliated with Parent or Howard Jonas to convert their Company Preferred Stock to Company Common Stock automatically upon a conversion to Company Common Stock of all other Company Preferred Stock in the limited circumstance specified therein and (b) each of those stockholders of the Company and the Parent listed on Schedule I hereto has entered into a lock-up agreement providing for post-Closing restrictions on the ability of such individuals with respect to the transfer of shares of Parent Common Stock to be issued to them pursuant to this Agreement and/ or otherwise owned, as applicable, in substantially the form attached hereto as Exhibit C (collectively, the “Lock-Up Agreements”); and
I. Concurrently with the execution and delivery of this Agreement, as a condition and inducement to the willingness of the Company to enter into this Agreement, Howard Jonas shall have entered into a Voting Agreement with the Company in substantially the form attached hereto as Exhibit B-3 (the “Voting Agreement”).
AGREEMENT
In consideration of the mutual covenants and agreements contained in this Agreement and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Parties agree as follows:
1. TRANSACTION
1.1 The Mergers.
(a) Upon the terms and subject to the conditions set forth in this Agreement, at the Effective Time, Merger Sub I shall be merged with and into the Company and the separate corporate existence of Merger Sub I shall thereupon cease. The Company shall be the surviving corporation in the Merger (sometimes hereinafter referred to as the “Initial Surviving Corporation”) and, after the Merger, shall be a direct wholly owned Subsidiary of Parent and the separate corporate existence of the Company, with all of its rights, privileges, immunities, powers, franchises and authority, shall continue unaffected by the Merger, except as set forth in Section 1.2 (Effect of the Mergers). The Merger shall have the effects specified in the DGCL.
(b) Upon the terms and subject to the conditions set forth in this Agreement, at the Subsequent Merger Effective Time, the Initial Surviving Corporation shall be merged with and into Merger Sub II and the separate corporate existence of the Initial Surviving Corporation shall thereupon cease. Merger Sub II shall be the surviving entity in the Subsequent Merger (sometimes hereinafter referred to as the “Surviving Company”) and, after the Subsequent Merger, shall continue to be an direct wholly owned Subsidiary of Parent and the separate corporate existence of Merger Sub II, with all of its rights, privileges, immunities, powers, franchises and authority, shall continue unaffected by the Subsequent Merger, except as set forth in Section 1.2 (Effect of the Mergers). The Subsequent Merger shall have the effects specified in the DGCL and the DLLCA.
1.2 Effect of the Mergers. The Mergers shall have the effects set forth in this Agreement and in the applicable provisions of the DGCL and the DLLCA. Without limiting the generality of the foregoing, and subject thereto, (a) at the Effective Time, except as otherwise agreed pursuant to the terms of this Agreement, all of the property, rights, privileges, powers and franchises of the Company and Merger Sub I shall vest in the Initial Surviving Corporation, and all debts, liabilities and duties of the Company and Merger Sub I shall become the debts, liabilities and duties of the Initial Surviving Corporation, and (b) at the Subsequent Merger Effective Time, except as otherwise agreed pursuant to the terms of this Agreement, all of the property, rights, privileges, powers and franchises of the Initial Surviving Corporation and Merger Sub II shall vest in the Surviving Company, and all debts, liabilities and duties of the Initial Surviving Corporation and Merger Sub II shall become the debts, liabilities and duties of the Surviving Company.
1.3 Closing; Effective Time. The Parties shall cause the Merger to be consummated at a closing (the “Closing”) to take place by means of a virtual closing through electronic exchange of signatures at 9:00 a.m., Eastern time, on a date to be mutually designated by the Company and Parent, which shall be no later than the second Business
Annex A-2
Table of Contents
Day after the satisfaction or waiver of the last to be satisfied or waived of the conditions set forth in Sections 6 (Conditions Precedent to the Obligations of Each Party), 7 (Conditions Precedent to Obligations of Parent and Merger Sub) and 8 (Conditions Precedent to Obligations of Company) (other than those conditions that by their terms are to be satisfied at the Closing, but subject to the satisfaction or waiver of such conditions). The date on which the Closing actually occurs in accordance with this Agreement is referred to herein as the “Closing Date.” Subject to the provisions of this Agreement, on the Closing Date, Merger Sub I and the Company shall file with the Secretary of State of the State of Delaware a certificate of merger relating to the Merger (the “Certificate of Merger”) executed in accordance with the relevant provisions of the DGCL, and the Parties shall make all other filings or recordings required under the DGCL in connection with the Merger. The Merger shall become effective upon the date and time of the filing of such Certificate of Merger with the Secretary of State of the State of Delaware, or at such later time as is permissible under the DGCL and as may be mutually agreed in writing by the Company and Parent and specified in the Certificate of Merger for the Merger (the “Effective Time”). Immediately following the Effective Time, the Initial Surviving Corporation and Merger Sub II shall file with the Secretary of State of the State of Delaware a certificate of merger relating to the Subsequent Merger (the “Certificate of Merger for Subsequent Merger”) executed in accordance with the relevant provisions of the DGCL and the DLLCA, and the Parties shall make all other filings or recordings required under the DGCL and the DLLCA in connection with the Subsequent Merger. The Subsequent Merger shall become effective at the time when the Certificate of Merger for Subsequent Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as is permissible under the DGCL and the DLLCA and as may be agreed by the Parties in writing and specified in the Certificate of Merger for the Subsequent Merger (the “Subsequent Merger Effective Time”).
1.4 Certificate of Incorporation and Bylaws; Directors and Officers.
(a) At the Effective Time, the certificate of incorporation of the Initial Surviving Corporation shall be amended and restated in its entirety to be the certificate of incorporation of Merger Sub I as in effect immediately prior to the Effective Time. At the Subsequent Merger Effective Time, the certificate of formation and the limited liability company agreement of Merger Sub II shall be amended and restated in their entirety to be the certificate of formation and limited liability company agreement of the Surviving Company as in effect immediately prior to the Subsequent Merger Effective Time.
(b) The bylaws of the Initial Surviving Corporation shall be amended and restated immediately in their entirety as of the Effective Time to conform to the bylaws of Merger Sub I as in effect immediately prior to the Effective Time. Immediately after the Subsequent Merger Effective Time, the certificate of formation and limited liability company agreement of Merger Sub II shall be the certificate of formation and limited liability company agreement of the Surviving Company as in effect immediately prior to the Subsequent Merger Effective Time.
(c) The directors and officers of the Initial Surviving Corporation immediately after the Effective Time and the managers and officers of the Surviving Company immediately after the Subsequent Merger Effective Time shall be the respective individuals set forth on Schedule II, until his or her successor has been duly elected or appointed and qualified or until his or her earlier death, resignation or removal pursuant to the applicable certificate of incorporation, bylaws, limited liability company agreement and applicable Law. Parent may amend Schedule II to the extent any person named thereon shall become unavailable to serve in the capacity set forth therein.
1.5 Conversion of Shares. At the Effective Time, by virtue of the Merger and without any further action on the part of Parent, Merger Sub I, Merger Sub II, the Company or any stockholder of the Company:
(a) any shares of Company Capital Stock then held by the Company (or held in the Company’s treasury) immediately prior to the Effective Time shall be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor;
(b) any shares of Company Capital Stock then held by Parent, Merger Sub I, Merger Sub II or any other Subsidiary of Parent immediately prior to the Effective Time shall be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor; provided that with respect to Subsidiaries of Parent that are not wholly owned such cancellation shall be with respect to the portion of the Company Capital Stock that Parent would own if the Subsidiary were dissolved and the Company Capital Stock was distributed to the owners of the Subsidiary;
Annex A-3
Table of Contents
(c) except as provided in Section 1.5(a) and Section 1.5(b) of this Section 1.5 (Conversion of Shares) and subject to Section 1.9 (Exchange/Payment), each share of Company Capital Stock issued and outstanding immediately prior to the Effective Time shall cease to exist, and shall be converted, by virtue of the Merger and without any action on the part of the holders thereof, into the right to receive, without interest, the applicable Per Share Merger Consideration; and
(d) each share of the common stock, $0.01 par value per share, of Merger Sub I issued and outstanding immediately prior to the Effective Time shall be converted into one share of validly issued, fully paid and nonassessable common stock of the Initial Surviving Corporation and each stock certificate of Merger Sub I evidencing ownership of any such shares shall thereupon evidence ownership only of such shares of capital stock of the Initial Surviving Corporation; and at the Subsequent Merger Effective Time, by virtue of the Subsequent Merger and without any action on the part of the holder of any capital stock of the Initial Surviving Corporation or on the part of the sole member of Merger Sub II (i) each membership unit of Merger Sub II, issued and outstanding immediately prior to the Subsequent Merger Effective Time, shall remain outstanding immediately following the Subsequent Merger Effective Time, and (ii) each share of common stock, par value $0.01 per share, of the Initial Surviving Corporation, issued and outstanding immediately prior to the Effective Time, shall be cancelled for no consideration.
(e) No fractional shares of Parent Common Stock shall be issued in connection with the Merger, and no certificates or scrip for any such fractional shares shall be issued. Any holder of Company Common Stock who would otherwise be entitled to receive a fraction of a share of Parent Common Stock (after aggregating all fractional shares of Parent Common Stock issuable to such holder) shall, in lieu of such fraction of a share and upon surrender by such holder of a Letter of Transmittal in accordance with Section 1.9 (Exchange/Payment) and any accompanying documents as required therein, be paid in cash the dollar amount (rounded to the nearest whole cent), without interest, determined by multiplying such fraction by the Parent Trading Price.
(f) If, between the date of the Agreement and the Effective Time, the outstanding shares of Parent Common Stock shall have been changed into, or exchanged for, a different number of shares or a different class, by reason of any stock dividend, subdivision, reclassification, recapitalization, split, reverse-split, combination or exchange of shares or other like change (by merger, consolidation or otherwise), the number of Merger Shares and each applicable Per Share Merger Consideration ratio shall, to the extent necessary, be equitably adjusted to reflect such change to the extent necessary to provide the holders of Company Capital Stock, Company Options and Company Warrants with the same economic effect as contemplated by this Agreement prior to such stock dividend, subdivision, reclassification, recapitalization, split, reverse-split, combination or exchange of shares or other like change (by merger, consolidation or otherwise); provided, however, that nothing herein will be construed to permit the Company or Parent to take any action with respect to Company Capital Stock or Parent Common Stock, respectively, that is prohibited by the terms of this Agreement.
(g) No dividends or other distributions declared or made with respect to Parent Common Stock with a record date on or after the Effective Time shall be paid to the holder of any unsurrendered Company Stock Certificate or book-entry share with respect to the shares of Parent Common Stock that such holder has the right to receive in the Merger until such holder surrenders such Company Stock Certificate or book-entry share or provides an affidavit of loss or destruction in lieu thereof in accordance with Section 1.9 (Exchange/Payment) (at which time (or, if later, on the applicable payment date) such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar Laws, to receive all such dividends and distributions, without interest).
1.6 Treatment of Company Warrants, Company Options, Company Convertible Notes and Contingent Rights.
(a) All Company Warrants shall be terminated at the Effective Time as contemplated by and in accordance with Section 5.13 (The 56% Warrant) and the applicable Support Agreement; provided that the Company Series C Warrant, to the extent not exercised by its holder prior to the Effective Time, shall be exchanged for a commensurate warrant to purchase shares of Parent or otherwise be settled by Parent and the Common Warrant Option, to the extent not exercised by its holder prior to the Effective Time, shall be exchanged for a commensurate warrant to purchase shares of Parent or otherwise be settled by Parent.
Annex A-4
Table of Contents
(b) All holders of Company Options (“Converted Options”) held by employees or independent contractors of the Company as of immediately prior to the Closing Date shall, to the extent then unvested, vest in full immediately prior to the Closing Date. All holders of Converted Options (whether or not accelerated pursuant to the prior sentence) shall receive options to purchase shares of Parent Class B Common Stock as contemplated by this Section 1.6(b) (Treatment of Company Warrants, Company Options and the Company Convertible Notes); provided that the adjustments provided in this Section 1.6(b) with respect to any Company Options are intended to be effected in a manner that is consistent with Sections 424(a) and 409A of the Code (such replacement options, the “Parent Options”). The number of shares of Parent Class B Common Stock underlying the Parent Options shall be determined by multiplying (A) the number of shares of Company Common Stock that were subject to each such Converted Option held by such holder, as in effect immediately prior to the Effective Time, by (B) the Per Common Share Merger Consideration ratio, and rounding the resulting number down to the nearest whole number of shares of Parent Class B Common Stock. The per share exercise price for the Parent Options shall be determined by dividing (x) the per share exercise price of such Converted Options held by such holder, as in effect immediately prior to the Effective Time, by (y) the Per Common Share Merger Consideration ratio and rounding the resulting exercise price up to the nearest whole cent. Any other restriction on the exercise of the Converted Options (excluding any vesting restriction) shall continue in full force and effect in the corresponding Parent Options. Except as set forth in this Section 1.6(b) (Treatment of Company Warrants, Company Options and Company Convertible Notes), the terms of the Converted Options shall remain unchanged. Except as set forth in this Section 1.6(b) (Treatment of Company Warrants and Company Options and Company Convertible Notes), the terms of the Converted Options shall remain unchanged
(c) The Parties hereto intend that the Company Convertible Notes shall not be assumed or continued by Parent or the Company in connection with the Merger or the other transactions contemplated hereby. Immediately prior to the Effective Time, Parent and the Company shall use their commercially reasonable efforts to cause each Company Convertible Note to be cancelled and extinguished and converted into the applicable Note Conversion Shares, in accordance with the terms of that certain form of Convertible Note Cancellation Agreement attached as Exhibit B-4 hereto, to be executed and delivered by each Company Noteholder prior to the Closing.
(d) For the avoidance of doubt, the Parties hereto acknowledge and agree that, upon satisfaction (or waiver) of the conditions precedent in Articles VI, VII and VIII, and as of immediately prior to the Effective Time, shares of Company Capital Stock shall be issued to the holders of the Contingent Rights (as reflected in the Closing Payment Schedule) in accordance with their respective terms. At the Effective Time, such shares of Company Capital Stock shall be converted in accordance with the terms of Section 1.5 (Conversion of Shares) into the Per Line of Credit Share Merger Consideration and Per Bonus Share Merger Consideration, as applicable, or, to the extent applicable pursuant to Sections 1.5(a) or 1.5(b), canceled and retired for no consideration.
1.7 Further Action. If, at any time after the Effective Time, any further action is necessary or desirable to carry out the purposes of this Agreement or to vest the Surviving Company with full right, title and possession of and to all rights and property of Merger Sub and the Company, the officers and directors of the Surviving Company and Parent shall take such action, so long as such action is not inconsistent with this Agreement.
1.8 Closing of the Company’s Transfer Books. At the Effective Time: (a) all shares of Company Capital Stock outstanding immediately prior to the Effective Time shall automatically be canceled and retired and shall cease to exist, and all holders of certificates representing shares of Company Capital Stock that were outstanding immediately prior to the Effective Time shall cease to have any rights as Company Stockholders, and each certificate representing any such Company Capital Stock (a “Company Stock Certificate”) shall thereafter represent the right to receive the consideration referred to in Section 1.5 (Conversion of Shares), if any; and (b) the stock transfer books of the Company shall be closed with respect to all shares of Company Capital Stock outstanding immediately prior to the Effective Time. No further transfer of any such shares of Company Capital Stock shall be made on such stock transfer books after the Effective Time. If, after the Effective Time, a Company Stock Certificate, including any valid certificate representing any shares of Company Preferred Stock previously converted into shares of Company Common Stock outstanding immediately prior to the Effective Time, is presented to the Exchange Agent or to the Surviving Company or Parent, such Company Stock Certificate shall be canceled and shall be exchanged as provided in Section 1.9 (Exchange/Payment).
Annex A-5
Table of Contents
1.9 Exchange/Payment.
(a) Prior to the Closing Date, Parent shall select a reputable entity to act as exchange agent in the Merger (the “Exchange Agent”). No later than the Closing Date, Parent shall deposit with the Exchange Agent (i) certificates or evidence of book-entry shares representing the Parent Class B Common Stock issuable pursuant to Section 1.5 (Conversion of Shares) (provided that any evidence of book entry shares to be issued in respect of Company Restricted Stock (to the extent consistent with the terms of such shares of Company Restricted Stock) will be subject to trading restrictions and a substantial risk of forfeiture under the terms of the applicable restricted stock agreements) and (ii) cash sufficient to make payments in lieu of fractional shares in accordance with Section 1.5 (Conversion of Shares).
(b) Promptly after the Exchange Agent is retained by Parent (and in any event no more than three days following the Effective Time), the Exchange Agent shall mail to the holders of Company Capital Stock immediately prior to the Effective Time (including the former Company Noteholders after giving effect to cancellation and conversion of the Company Convertible Notes immediately prior to the Effective Time): (i) a letter of transmittal in substantially the form attached hereto as Exhibit D which includes a release in favor of the Company and Parent, confidentiality and other provisions on which the Company and Parent will rely (the “Letter of Transmittal”) and (ii) instructions for use in effecting the surrender of Company Stock Certificates or book-entry shares in exchange for the Parent Common Stock and cash amounts payable in accordance with Section 1.5 (Conversion of Shares). Upon surrender of a Company Stock Certificate and delivery of a completed and duly executed Letter of Transmittal to the Exchange Agent for payment, (A) the holder of such Company Stock Certificate shall be entitled to receive in exchange therefor the applicable Per Share Merger Consideration for each share evidenced by such Company Stock Certificate or book-entry shares determined pursuant to Section 1.5 (Conversion of Shares), within 10 Business Days of such surrender and delivery, and (B) the Company Stock Certificate or book-entry shares so surrendered shall be canceled. No holder of any Company Capital Stock or any instruments convertible into Company Capital Stock shall be entitled to receive any of the consideration in accordance with the preceding sentence without returning the completed and duly executed Letter of Transmittal. If any Company Stock Certificate shall have been lost, stolen or destroyed, Parent may, as a condition to the payment of the consideration hereunder, require the owner of such Company Stock Certificate to provide a reasonably appropriate affidavit to Parent (which may include an indemnity or bond in customary form).
(c) Neither Parent, the Surviving Company nor any of their respective Affiliates shall be liable to any holder or former holder of Company Capital Stock with respect to any shares properly delivered to any public official pursuant to any applicable abandoned property Law or escheat Law.
(d) Each of Parent, the Company, the Exchange Agent, and their respective agents (each a “Withholding Agent”) will be entitled to deduct and withhold from any amount payable to any Person under this Agreement or any other documents associated with the transaction, the amounts such Withholding Agent is required to deduct and withhold under the Code or any other applicable Law. To the extent that amounts are so withheld and paid over to the applicable Governmental Body, such withheld amounts will be treated as having been paid to the applicable Person in respect of whom such amounts were withheld.
1.10 No Appraisal or Dissenters’ Rights. In accordance with Section 262 of the DGCL, no dissenters’ or appraisal rights shall be available with respect to the Mergers or the other transactions contemplated by this Agreement.
1.11 Extension of 56% Warrant. The expiration date of the 56% Warrant is hereby extended by the Company beyond August 15, 2021 and shall remain exercisable by the holder(s) thereof until the earlier of (i) upon the occurrence of the Effective Time, the Effective Time or (ii) if the Effective Time does not occur, the date that is calculated by adding (A) the number of calendar days this Agreement has been in effect prior to its termination in accordance with its terms, including the date hereof, to (B) August 15, 2021. Pharma Holdings, LLC, as the holder of the 56% Warrant, shall be an express Third Party beneficiary of this Section 1.11.
2. REPRESENTATIONS AND WARRANTIES OF THE COMPANY
The Company represents and warrants to Parent and the Merger Subs as of the date hereof, (a) except to the extent such representations and warranties are specifically made as of a particular date, in which case the Company makes the representations and warranties as of such particular date, and (b) except as set forth in (i) the Company
Annex A-6
Table of Contents
Disclosure Schedule (subject to the qualifications set forth in Section 10.15 (Disclosure Schedule)) or (ii) as disclosed in the Parent SEC Reports filed with the SEC and publicly available on the SEC’s Electronic Data Gathering Analysis and Retrieval system prior to the date of this Agreement (excluding, in each case, any disclosures contained or referenced therein under the captions “Risk Factors,” “Forward-Looking Statements,” “Quantitative and Qualitative Disclosures About Market Risk” and any other disclosures contained or referenced therein of information, factors or risks that are cautionary, predictive or forward-looking in nature), as follows:
2.1 Due Incorporation; Etc.
(a) The Company is a corporation duly organized and validly existing and in good standing under the Laws of Delaware.
(b) The Company has no Subsidiaries and owns no equity interest in any other Person.
(c) The Company is qualified to do business as a foreign corporation, and is in good standing, under the Laws of all states where the nature of its business requires such qualification, except where the failure to be so qualified or in such good standing has not had and would not reasonably be expected, individually or in the aggregate, (i) to have a Company Material Adverse Effect or (ii) to prevent, materially delay or materially impair the ability of the Company to perform its obligations under this Agreement or to consummate the transactions contemplated hereby.
2.2 Certificate of Incorporation and Bylaws; Certificates of Designation. The Company has made available to Parent true and complete copies, in each case including all amendments thereto and as in effect as of the date of this Agreement, of (a) the Company Charter, (b) the Company’s bylaws and (c) each of the Certificates of Designation, and the Company is not in violation of, in conflict with, or in default under, any of the respective terms thereof, and there exists no condition or event which, after notice, lapse of time or both, would result in any such violation, conflict or default.
2.3 Capitalization, Etc.
(a) As of the date of this Agreement: (i) the authorized Company Capital Stock consists solely of the authorized shares of Company Common Stock and Company Preferred Stock as set forth in Part 2.3(a)(i) of the Company Disclosure Schedule, (ii) the issued and outstanding shares of Company Capital Stock consist solely of such shares as are set forth on Part 2.3(a)(ii) of the Company Disclosure Schedule, (iii) all of the shares of Company Capital Stock issuable pursuant to the Company Warrants (including, without limitation, the 56% Warrant) are set forth in Part 2.3(a)(iii) of the Company Disclosure Schedule, and (iv) all of the shares of Company Capital Stock issuable pursuant to the Contingent Rights are set forth in Part 2.3(a)(iv) of the Company Disclosure Schedule. As of the date of this Agreement, the Company has reserved shares of Company Common Stock for issuance to employees, non-employee directors and consultants pursuant to the Company Equity Plan, of which 4,721,365 shares are subject to outstanding and unexercised Company Options.
(b) Except as set forth in Part 2.3(a) of the Company Disclosure Schedule, (A) there are no existing options, warrants, calls, rights (including contingent rights, conversion rights, preemptive rights, co-sale rights, rights of first refusal, convertible securities, subscription rights or other agreements or commitments of any character obligating the Company to issue any such shares or other convertible securities) issued or granted by the Company or Contracts to which the Company, any Company Stockholder or holder of Company Options or Company Warrants, is a party requiring, and there are no securities of the Company outstanding which upon conversion or exchange would require, the issuance, sale or transfer of any additional shares of capital stock or other equity securities of the Company or other securities convertible into, exchangeable for or evidencing the right to subscribe for or purchase shares of Company Capital Stock or other equity securities of the Company, (B) there are no obligations, contingent or otherwise, of the Company to (1) repurchase, redeem or otherwise acquire any shares of Company Capital Stock or (2) to make any material investment in (in the form of a loan, capital contribution or otherwise), or to provide any guarantee (excluding indemnification obligations) with respect to the obligations of, any Person and (C) there are no outstanding restricted shares, stock appreciation rights, phantom stock, contingent value rights, profit participation or similar rights with respect to the Company.
Annex A-7
Table of Contents
(c) There are no bonds, debentures, notes or other Debt of the Company having the right to vote or consent (or, convertible into, or exchangeable for, securities having the right to vote or consent) on any matters on which the Company Stockholders may vote. There are no voting trusts, irrevocable proxies or other Contracts or understandings to which the Company, or any holder of Company Warrants or Company Options is a party or is bound with respect to the voting or consent of any shares of Company Capital Stock.
(d) All of the outstanding shares of Company Capital Stock and other securities (including Company Options and Company Warrants) of the Company have been duly authorized and validly issued, and are fully paid and nonassessable, are not subject to any preemptive rights, purchase options, call options, rights of first refusal or similar rights or any other Liens and have been issued and granted in all material respects in compliance with (i) all applicable securities Laws and other applicable Laws; and (ii) all requirements set forth in applicable Contracts in all material respects. Each share of Company Preferred Stock is convertible into shares of Company Common Stock as set forth in the respective Certificates of Designation.
(e) Part 2.3(e) of the Company Disclosure Schedule contains a true, correct and complete list, as of the date of this Agreement, of all holders of Company Capital Stock, Company Warrants, Company Options and all rights set forth in Part 2.3(a) of the Company Disclosure Schedule. The Company has made available to Parent a true and complete copy of all instruments related to all such rights.
(f) All outstanding Company Options have been granted under the Company Equity Plan. The Company has made available to Parent true and complete copies of the Company Equity Plan and the forms of all stock option agreements and grant notices evidencing such Company Options. Part 2.3(f) of the Company Disclosure Schedule contains a true, correct and complete list, as of the date of this Agreement of (i) the name and country of residence (if outside of the U.S.) of the holder of the Company Options, (ii) the number of shares of the Company Common Stock subject to such Company Options, (iii) the vesting schedule of such Company Option, including the number of vested and unvested shares as of the date of this Agreement and any acceleration provisions, (iv) the grant date of such Company Option, (v) the exercise price of such Company Option, (vi) the expiration date of such Company Option, (vii) whether early exercise is permitted with respect to such Company Option, (viii) whether such Company Option is an “incentive stock option” (as defined in Section 422 of the Code) or a non-qualified stock option, and (ix) whether the holder is a current or former employee or service provider of the Company. Except as set forth in Section 1.6(b) (Treatment of Company Warrants, Company Options and Company Convertible Notes), no vesting of Company Options will accelerate in connection with the consummation of the Contemplated Transactions. No Company Option is or has been a “nonqualified deferred compensation plan” within the meaning of Section 409A(d)(1) of the Code. The treatment of the Company Options that have been granted under the Company Equity Plan as provided in this Agreement is permitted pursuant to the terms of the Company Equity Plan.
2.4 Financial Statements.
(a) The Company has made available to Parent true and complete copies of (i) the Company’s unaudited balance sheet for the year ended July 31, 2020 (“Balance Sheet” and the date thereof, the “Balance Sheet Date”) and the Company’s unaudited statement of operations and statement of cash flows for the years then ended, (ii) the Company’s audited balance sheet for the year ended July 31, 2019 and the Company’s audited statement of operations and statement of cash flows for the year then ended, and (iii) the Company’s unaudited interim financial statements for the quarter ended December 31, 2020 (all of the foregoing financial statements of the Company and any notes thereto are hereinafter collectively referred to as the “Company Financial Statements”). The Company Financial Statements (x) were prepared in accordance with GAAP and fairly present in all material respects the financial condition of the Company at the dates therein indicated and the results of operations of the Company for the periods therein specified in accordance with GAAP, except (A) as may be indicated in the footnotes to the Company Financial Statements and (B) that the unaudited financial statements do not contain footnotes and are subject to normal year-end adjustments and (y) are consistent with, and were prepared from, the books and records of the Company, which books and records are complete in all material respects.
(b) The Company has established and maintains a system of internal accounting controls sufficient to provide reasonable assurances (i) that transactions, receipts and expenditures of the Company are being executed and made only in accordance with appropriate authorizations of management and the Company Board, (ii) that transactions are recorded as necessary (A) to permit preparation of the Company Financial Statements in conformity with GAAP and (B) to maintain accountability for assets, (iii) regarding prevention or timely detection of unauthorized acquisition,
Annex A-8
Table of Contents
use or disposition of the assets of Company and (iv) the assets of the Company have been recorded in conformity with GAAP. None of the Company, the Company’s independent auditors nor, to the Knowledge of the Company, any Company Service Provider, has identified or been made aware of any Fraud, whether or not material, that involves the Company’s management or other Company Service Provider who have a role in the preparation of the Company Financial Statements or the internal accounting controls utilized by the Company, or any claim or allegation regarding any of the foregoing. Neither the Company nor, to the Knowledge of the Company, any Representative of the Company has received or otherwise had or obtained knowledge of any material complaint, allegation, assertion or claim, whether written or oral, in each case, regarding deficient accounting or auditing practices, procedures, methodologies or methods of the Company or its internal accounting controls or any material inaccuracy in the Company Financial Statements. No attorney representing the Company has reported to the Company Board or any committee thereof or to any director or officer of the Company evidence of a material violation of securities Laws, breach of fiduciary duty or similar violation by the Company or its Representatives. There are no significant deficiencies or material weaknesses in the design or operation of the Company’s internal controls that could adversely affect the Company’s ability to record, process, summarize and report financial data. At the Balance Sheet Date, there were no material loss contingencies (as such term is used in Statement of Financial Accounting Standards No. 5 (“Statement No. 5”) issued by the Financial Accounting Standards Board in March 1975 and revised by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification Topic 450-20, Loss Contingencies) that are not adequately provided for in the Balance Sheet as required by Statement No. 5 as revised. There has been no material change in the Company’s accounting policies since the Company’s inception, except as described in the Company Financial Statements.
2.5 Absence of Certain Changes. Except as expressly contemplated by this Agreement, between December 31, 2020 and the date of this Agreement: (a) no event or series of related events that has had or would reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect; (b) no event or action has occurred that, if taken after the date of this Agreement, would require the consent of Parent pursuant to Section 4.2 (Conduct of the Business of the Company) if such event or action occurred during the commencement of the Pre-Closing Period and ending on the Execution Date; or (c) except in connection with the Contemplated Transactions, the Company have used or held its assets and properties for use and has operated and conducted its business in all material respects, in the Ordinary Course of Business.
2.6 Title to Assets. Other than as would not be, individually or in the aggregate, material to the Company, the Company has good and valid title to all tangible assets owned or purported to be owned by it as of the date of this Agreement, other than Intellectual Property assets (which are covered by Section 2.8 (Intellectual Property)), including all such tangible assets (other than capitalized or operating leases) reflected on the Balance Sheet (except for assets sold or otherwise disposed of since the date of the Balance Sheet in the Ordinary Course of Business). Other than as would not be material to the Company, individually or in the aggregate, the Company has good and valid title to, or a valid and binding leasehold or other interest in, all tangible personal property that is currently used by the Company in, and necessary for the conduct of, the Company’s business as currently conducted, free and clear of all Liens (other than Permitted Liens). All of the material tangible assets of the Company (excluding Leased Real Property) have been maintained in a reasonably prudent manner and are in good operating condition (ordinary wear and tear excepted) and are not in need of maintenance, repair or replacement except for ordinary, routine maintenance, repair or replacement not material in nature or cost.
2.7 Real Property; Leasehold. The Company does not own, nor has it ever owned, any real property, or any interest in real property, except for the leaseholds created under the real property leases (including all amendments, extensions, renewals, guarantees, and other agreements with respect thereto) identified in Part 2.7 of the Company Disclosure Schedule (the “Leased Real Property”). The Company has made available to Parent true and complete copies of each lease and document related thereto listed in Part 2.7 of the Company Disclosure Schedule (the “Real Property Leases”). Except as would not reasonably be expected to be, individually or in the aggregate, material to the Company: (a) the Company is in compliance with the Real Property Leases, and has a valid and subsisting leasehold interest in all Leased Real Property, in each case free and clear of all Liens, other than Permitted Liens; and (b) the Company has not granted any other Person the right to occupy or use any Leased Real Property and the Company’s quiet enjoyment of the Leased Real Property under such lease has not been disturbed in any material respect; there are no written or oral subleases, licenses, concessions, occupancy agreements or other Contracts granting to any other Person the right of use or occupancy of any Leased Real Property. The Company has not received written notice of default, or intention to terminate or not renew, any real property lease or any eminent domain, condemnation or similar proceeding pending or threatened, against all or any portion of any Leased Real Property.
Annex A-9
Table of Contents
2.8 Intellectual Property.
(a) As used herein, the following terms have the meanings indicated below:
(i) “Company Data” shall mean all Personal Data or Confidential Information Processed by the Company in the operation of its business.
(ii) “Company Intellectual Property Agreements” shall mean the Outbound Licenses, the Inbound Licenses and the Other IP Contracts.
(iii) “Company Licensed Intellectual Property” shall have the meaning set forth in Section 2.8(m)(i) of this Agreement.
(iv) “Company Owned Intellectual Property” shall mean any and all Intellectual Property owned or purported to be owned by the Company.
(v) “Company Owned Intellectual Property Rights” means any and all Intellectual Property Rights that are owned by or purported to be owned by the Company.
(vi) “Company Privacy Policies” shall mean, collectively, any and all (A) of the Company’s data privacy and security policies published on Company Websites or otherwise made publicly available by the Company and (B) public representations (including representations on Company Websites) of the Company regarding data privacy, integrity, availability or security with regard to the Company’s Processing of Personal Data; in each case since January 1, 2018.
(vii) “Company Products” shall mean all products and services that the Company currently intends to make commercially available following receipt of all approvals under applicable Laws.
(viii) “Company Registered Intellectual Property” shall mean United States, international and foreign: (A) Patents; (B) registered trademarks and service marks, applications to register trademarks and service marks, intent-to-use applications, or other registrations or applications related to trademarks and service marks; (C) registered Internet domain names; (D) registered copyrights and applications for copyright registration; and (E) any other Intellectual Property Rights that are the subject of a filing or registration anywhere in the world, in each case registered or filed in the name of, or owned or purported to be owned by, the Company, excluding any of the foregoing that expired (with no opportunity to renew) prior to the date of this Agreement, or that the Company, in its reasonable business judgment, elected to abandon or allow to lapse (and in fact lapsed or were abandoned) prior to the date of this Agreement.
(ix) “Company Source Code” shall mean, collectively any software source code, human-readable software instructions or programs that embody any Company Owned Intellectual Property Rights that is part of or used in the development or provision of Company Products.
(x) “Company Websites” shall mean all websites owned, operated or hosted by the Company (including those websites operated using the domain names listed in Part 2.8(c) of the Company Disclosure Schedule).
(xi) “Governmental Grant” shall mean any grant, funding, loan, incentive, subsidy or other economic benefit provided or made available by or on behalf of or under the authority of any Governmental Body.
(xii) “Intellectual Property” shall means (A) Intellectual Property Rights; and (B) Technology.
(xiii) “Intellectual Property Rights” shall mean any and all rights (including database rights) in, arising out of, or associated therewith, throughout the world: (A) Patents, utility models, and applications therefor and all reissues, divisions, re-examinations, renewals, extensions, provisionals, continuations and continuations-in-part thereof and equivalent or similar rights in inventions and discoveries anywhere in the world, including invention disclosures; (B) rights under Law associated with Trade Secrets, confidential and proprietary information, know-how, industrial designs and any registrations and applications therefor; (C) trade names, logos, trade dress, trademarks and service marks, trademark and service mark registrations, trademark and service mark applications and any and all goodwill associated with and symbolized by the foregoing items, (D) Internet domain name registrations;
Annex A-10
Table of Contents
(E) copyrights, copyright registrations and applications therefor and all other rights corresponding thereto; (F) database rights under Law; and (G) moral and economic rights of authors and inventors, in each case, however denominated and any similar or equivalent rights to any of the foregoing.
(xiv) “Open Source Materials” shall mean software or other material that is distributed as “free software,” “open source software” or under substantially similar licensing or distribution terms.
(xv) “Personal Data” shall mean information that (A) identifies, directly or indirectly, a natural person; and (B) any other information that is considered “personally identifiable information”, “personal information”, “protected health information”, “individual health information”, or “personal data” under applicable Law (including all applicable Privacy Laws).
(xvi) “Privacy Laws” shall mean each applicable Law concerning privacy, or security of Personal Data, such as the breach, retention, security, protection, disposal, international transfer or other Processing of Personal Data, by the Company, including, as applicable, (A) the EU General Data Protection Regulation 2016/679, the EU e-Privacy Directive 2002/58/EC as amended by Directive 2009/136/EC or further amended or replaced from time to time, and any relevant national implementing legislation, and any substantially similar local legislation, (B) Laws regarding the Processing of Personal Data in direct marketing, e-mails, text messages or telemarketing, (C) Laws regarding the secure disposal of records containing Personal Data, (D) Laws regarding international data transfers and/or on-soil requirements of Personal Data, (E) Laws regarding banking secrecy and outsourcing requirements with respect to the Processing of Personal Data, (F) Laws regarding incident reporting and data breach notification requirements with respect to Personal Data, (G) the California Consumer Privacy Act of 2018, (H) the Health Insurance Portability and Accountability Act of 1996, as amended, (I) Laws regarding unfair or deceptive practices with respect to the Processing of Personal Data; and (J) state consumer protection Laws with respect to the Processing of Personal Data.
(xvii) “Process”, “Processed” or “Processing” shall mean, with respect to Personal Data, the use, access, collection, processing, storage, recording, organization, adaption, modification, alteration, transfer, retrieval, consultation, disclosure, dissemination or combination of such data.
(xviii) “Shrink-Wrap Software” shall mean any Technology (including Technology offered on a SaaS, PaaS, or IaaS or similar basis and software available through retail stores, distribution networks or that is pre-installed as a standard part of hardware purchased by the Company) that (A) is generally commercially available on standard non-exclusive terms, (B) has not been modified or customized by or for the Company, and (C) has an annual license or subscription fee or total replacement cost of less than $105,000.
(xix) “Technology” shall mean any and all of the following to the extent material to the Company’s business: works of authorship, computer programs, source code and executable code, whether embodied in software, firmware or otherwise, assemblers, applets, compilers, user interfaces, application programming interfaces, protocols, architectures, documentation, annotations, comments, designs, files, records, schematics, test methodologies, test vectors, emulation and simulation tools and reports, hardware development tools, models, tooling, prototypes, breadboards and other devices, data structures, databases, data compilations and collections, inventions (whether or not patentable), invention disclosures, discoveries, improvements, technology, proprietary and confidential ideas, data and information (including customer lists and supplier lists, to the extent proprietary or confidential), know-how and information maintained as Trade Secrets, tools, concepts, techniques, methods, processes, formulae, patterns, algorithms and specifications, and any and all instantiations or embodiments of the foregoing in any form and embodied in any media.
(xx) “Trade Secrets” shall mean all of the following to the extent treated by Company as a trade secret under applicable law: inventions (whether or not patentable) and improvements thereto, know-how, research and development information, business plans, specifications, designs, processes, process libraries, technical data, customer data, financial information, pricing and cost information, bills of material, or other confidential information exclusively owned by a Person, including any formula, pattern, compilation, program, device, method, technique, or process, that (i) provides an actual or potential independent economic value from not being generally known to and not being readily ascertainable by, other Persons, and (ii) is the subject of efforts that are reasonable under the circumstances to maintain its secrecy.
Annex A-11
Table of Contents
(b) The Company exclusively owns all rights, title and interest in and to all Company Owned Intellectual Property, free and clear of any Liens (other than Permitted Liens). Other than Permitted Liens, the Company has not transferred (or agreed to transfer) full or partial ownership of, or granted (or agreed to grant) any exclusive license with respect to, any Intellectual Property to any Third Party that is, or at any time in the past was, material Company Owned Intellectual Property.
(c) Part 2.8(c) of the Company Disclosure Schedule lists all Company Registered Intellectual Property, and, for each item of Company Registered Intellectual Property, the jurisdictions in which it has been issued or registered or in which any application for such issuance and registration has been filed, or in which any other filing or recordation has been made; and to Company’s Knowledge all actions that are required to be taken by the Company vis-à-vis the applicable authorities with which such Company Registered Intellectual Property was registered or filed within 60 days of the date of this Agreement. Each item of Company Registered Intellectual Property that has been issued is valid and subsisting. All registration, maintenance and renewal fees currently due in connection with such Company Registered Intellectual Property or any pending applications therefor have been paid and all documents, recordations and certificates in connection with such Company Registered Intellectual Property currently required to be filed have been filed with the relevant patent, copyright, trademark or other authorities in the United States and foreign jurisdictions, as the case may be, for the purposes of prosecuting and maintaining such Company Registered Intellectual Property and recording and perfecting the ownership interests of the Company therein (provided that, for any item of Company Registered Intellectual Property that the Company, in its reasonable business discretion, has elected not to maintain in force, Part 2.8(c) of the Company Disclosure Schedule so indicates). No item of Company Registered Intellectual Property has been registered with “small entity” or “micro entity” status.
(d) There are no inventorship challenges, opposition or nullity proceedings or interferences declared, commenced or provoked, or to the Knowledge of the Company threatened, with respect to any Patents included in the Company Registered Intellectual Property. The Company has complied with its duty of candor and disclosure to the United States Patent and Trademark Office and any relevant foreign patent office with respect to all patent and trademark applications filed by or on behalf of the Company and has made no material misrepresentation in such applications. The Company has no knowledge of any information affecting the validity of any Company Registered Intellectual Property.
(e) Each item of Company Owned Intellectual Property and Company Licensed Intellectual Property is owned by the Company or available for use by the Company. The Company Owned Intellectual Property and Company Licensed Intellectual Property constitutes all Intellectual Property necessary to conduct the Company’s business in all material respects in the manner currently conducted and contemplated to be conducted in the future by Parent. Neither the execution, delivery or performance of this Agreement nor the consummation of the Contemplated Transactions would reasonably be expected to result in or give any other Person the right or option to cause, create, impose or declare: (i) a loss of, or Lien on, any Company Owned Intellectual Property or any Company Licensed Intellectual Property; or (ii) the grant, assignment or transfer to any other Person of any license or other right or interest under, to or in any Company Owned Intellectual Property or Company Licensed Intellectual Property.
(f) No Company Products or material Patents included in the Company Owned Intellectual Property were developed or derived from using, in whole or in part, funding or resources provided by a Governmental Body. No facilities of a university, college, other educational institution or research center was used in the development of the Company Owned Intellectual Property. Part 2.8(f) of the Company Disclosure Schedule lists all Company Products (and the current or most recent version number or identifier (if any) of each such Company Product as of the date of this Agreement). If any Company Products are or were the subject of clinical studies, Part 2.8(e) of the Company Disclosure Schedule lists all such clinical studies for each such Company Product.
(g) The Company has secured from all Company Service Providers who independently or jointly contributed to or participated in the conception, reduction to practice, creation or development of any Company Owned Intellectual Property (each such Person an “Author”), to the extent permitted by applicable Law, unencumbered and unrestricted exclusive ownership of, all of the Authors’ rights, title and interest in such contribution and has obtained the waiver of all non-assignable rights. Without limiting the foregoing, the Company has obtained written and enforceable proprietary information and invention disclosure agreements and Intellectual Property assignments from all current and former Authors and, in the case of Patents, such assignments have been recorded with the relevant authorities in
Annex A-12
Table of Contents
the applicable jurisdiction or jurisdictions. The Company has made available to Parent true and complete copies of all such agreements and assignments covering Company Owned Intellectual Property claiming the Company Products that are material to the research, development or commercialization plans of the Company.
(h) No Company Service Provider currently engaged with or employed by the Company is, to the Knowledge of the Company: (i) bound by or otherwise subject to any Contract with a Third Party restricting such Company Service Provider from performing his/her duties for the Company; or (ii) in breach of any Contract with any former employer or other Person concerning Intellectual Property Rights or confidentiality due to his/her activities as an employee or contractor of the Company.
(i) The Company takes and has taken commercially reasonable steps designed to protect and preserve the confidentiality of: (i) confidential or non-public information of the Company (including all Trade Secrets owned or possessed by the Company), excluding any information that the Company, in its reasonable business judgment, elected to publish or elected to make available to one or more Third Parties without an obligation of confidentiality; as well as (ii) confidential or non-public information provided by any Third Party to the Company under a written obligation of confidentiality, to the extent required pursuant to that written obligation (all such information, collectively, “Confidential Information”). The Company has implemented in the past three years and maintains reasonable security, disaster recovery and business continuity plans and measures consistent with industry practices of companies of similar size offering similar products or services, and conducts its business in material compliance therewith. To the Knowledge of the Company, in the three years prior to the date of this Agreement, the Company has not experienced any material breach of security or other material unauthorized access by a Third Party to any Confidential Information, including Personal Data, in the Company’s’ possession, custody or control, and there has been no material breach by the Company or, to the Knowledge of the Company, material breach of any Third Party’s obligations to the Company under any Contract relating to any Confidential Information.
(j) To the Knowledge of the Company, the operation of the business of the Company as previously conducted in the three years prior to the date of this Agreement and as currently conducted, including (i) the design, development, manufacturing, reproduction, marketing, licensing, sale, offer for sale, importation, distribution, provision and/or use of any Company Product and (ii) the use by the Company of any product, device, process or service used in the business of the Company as previously conducted in the three years prior to the date of this Agreement and as currently conducted, has not, and when operated in the manner currently conducted as of immediately prior to Closing, does not, infringe (directly or indirectly, including via contribution or inducement), misappropriate or violate any Intellectual Property Rights of a Third Party.
(k) The Company has not been sued in any Legal Proceeding, nor has the Company received any written notice alleging that it has infringed, misappropriated, or violated or, by conducting the business of the Company, would infringe, misappropriate, or violate, any Intellectual Property Rights of any other Person, or has conducted any activity constituting unfair competition or unfair trade practices.
(l) There is no, and there has not in the past three years been any infringement or misappropriation of any Company Owned Intellectual Property by any Third Party. The Company has not brought any Legal Proceeding against a Third Party for infringement or misappropriation of any Intellectual Property Rights.
(m) Licenses; Agreements.
(i) Part 2.8(m)(i) of the Company Disclosure Schedule lists all Contracts (other than Contracts that have expired or been terminated prior to the date of this Agreement) pursuant to which Intellectual Property material to the Company is or has been licensed, sold, assigned or otherwise conveyed or provided to the Company by a Third Party (other than those that are Non-Scheduled Inbound Contracts) (“Inbound Licenses”, and the Intellectual Property therein, “Company Licensed Intellectual Property”). The Company is current with all payment obligations under the Inbound Licenses and, in its reasonable judgment, does not anticipate any payments to become due under the Inbound Licenses within twelve (12) months after the Closing Date.
(ii) Part 2.8(m)(ii) of the Company Disclosure Schedule lists all Contracts (other than Contracts that have expired or been terminated prior to the date of this Agreement) pursuant to which any Third Party has been granted any license under, or otherwise has received or acquired any material right (whether or not currently exercisable) or interest in, any Company Owned Intellectual Property material to the Company (in each case, other
Annex A-13
Table of Contents
than those that are Non-Scheduled Outbound Contracts) (“Outbound Licenses”). The Company is not currently bound by, and no Company Owned Intellectual Property Rights are subject to, any Contract containing any covenant or other provision that in any way materially limits or restricts the ability of the Company to use, exploit, assert, or enforce any Company Owned Intellectual Property anywhere in the world in connection with its business. Except as set forth in any Inbound Licenses, the Company has not agreed to indemnify any person or entity against any infringement, violation or misappropriation of any Intellectual Property with respect to any Third-Party Intellectual Property. Company is not a member of or party to any patent pool, industry standards body, trade association or other organization pursuant to the rules of which it is obligated to license any existing or future Intellectual Property to any person.
(iii) Part 2.8(m)(iii) of the Company Disclosure Schedule lists all Contracts (other than those that are Inbound Licenses or Outbound Licenses) in effect as of the date of this Agreement containing any (A) restrictions, in any material respect, on the Company’s rights to patent, register, enforce, use or otherwise exploit any Company Owned Intellectual Property or other Intellectual Property used or held for use in the business of the Company by or on behalf of the Company, including covenants not to sue, (B) right of first refusal, option or any other material right to acquire any right, title or interest, including any exclusive license, in or to any Company Owned Intellectual Property or (C) obligation to make any payment due or payable in connection with any change in control of the Company, or any earn-out, milestone or other contingent payments that have not yet been paid under any Contract pursuant to which any Intellectual Property is or has been licensed, sold, assigned, or otherwise conveyed or provided to the Company (excluding any contingent payments arising pursuant to recruiting agreements for Company Service Providers entered into in the Ordinary Course of Business) (all of the foregoing, the “Other IP Contracts”).
(n) With respect to the Company Intellectual Property Agreements:
(i) The Company is not (nor will it be as a result of the execution and delivery or effectiveness of this Agreement or the performance of the Company’s obligations under this Agreement), in material breach of any Company Intellectual Property Agreement, and the consummation of the Contemplated Transactions will not result in the modification, cancellation, termination, suspension of, or acceleration of any payments, rights, obligations, or remedies with respect to any Company Intellectual Property Agreements, or give any party to any Company Intellectual Property Agreement the right to do any of the foregoing, other than, if a Material Consent is required pursuant to a Company Intellectual Property Agreement from the counterparty thereto, as a result of a failure to obtain such corresponding Material Consent;
(ii) At and immediately after the Closing, the Surviving Company that is party to the applicable Company Intellectual Property Agreement will be permitted to enforce all of the obligations and exercise all of the rights of the Company under the Company Intellectual Property Agreements necessary to conduct its business without the payment of any additional amounts or consideration other than (A) ongoing fees, royalties or payments that the Company would otherwise be required to pay, (B) if a Material Consent is required pursuant to a Company Intellectual Property Agreement from the counterparty thereto, as a result of a failure to obtain such corresponding Material Consent;
(iii) No Company Intellectual Property Agreement requires the Company to include any Third-Party Intellectual Property in any Company Product or to obtain any Person’s approval of a Company Product for the development, manufacture or commercialization of such Company Product; and
(iv) As of the date of this Agreement, there are no pending Legal Proceedings that the Company has been provided written notice of (or otherwise has Knowledge of), and to the Knowledge of the Company, no other disputes or threatened Legal Proceedings, regarding the scope of any Company Intellectual Property Agreements or performance under any such agreements including with respect to any payments to be made or received by the Company thereunder.
(o) None of the execution and performance of this Agreement, the consummation of the Contemplated Transactions and the assignment to Parent by operation of Law or otherwise of any Contracts to which the Company is a party or by which any of its assets is bound, will result in: (i) Parent or any of its Affiliates (other than the Surviving Company) granting to any Third Party any right to or with respect to any material Intellectual Property Rights or Personal Data owned by, or licensed to Parent or any of its Affiliates, (ii) any Party being obligated to pay any royalties or other material amounts to any Third Party in excess of those payable by any of them, respectively, in the absence
Annex A-14
Table of Contents
of this Agreement or the Contemplated Transactions or (iii) any termination of, or other material adverse impact to, any Company Intellectual Property Agreements (other than if a Material Consent is required pursuant to a Company Intellectual Property Agreement from the counterparty thereto, as a result of a failure to obtain such corresponding Material Consent).
(p) The Company has not disclosed, delivered or licensed to any Person or agreed or obligated itself to disclose, deliver or license to any Person, or permitted the disclosure or delivery to any escrow agent or other Person of, any Company Source Code, other than disclosure, delivery, license, or other availability made to (A) Company Service Providers and Company Consultants involved in the development of Company Products, or (B) independent third-party escrow agents pursuant to a valid and enforceable written source code escrow agreement providing for limited release only upon the occurrence of specified release events, no such event has occurred, and no circumstance or condition of which the Company has Knowledge exists that (with or without notice or lapse of time, or both) will (or the Company reasonably expects to) result in the occurrence of such an event. Without limiting the foregoing, neither the execution nor performance of this Agreement nor the consummation of any of the Contemplated Transactions will result in a release from escrow or other delivery to a Third Party of any Company Source Code.
(q) [RESERVED]
(r) Privacy and Personal Data.
(i) The Company, at all times during the past three years, has complied in all material respects with (A) all of the Company Privacy Commitments (as defined below); and (B) Privacy Laws; except in each case of (A) and (B), as would not reasonably be expected to result in liability material to the Company.
(ii) The Company has at all times during the past three years, except as would not reasonably be expected to result in liability material to the Company: (A) obtained or received any consents from data subjects required for the Company to comply with applicable Privacy Laws governing the Processing of Personal Data as conducted by or for the Company, (B) abided by any privacy choices (including opt-out preferences) of data subjects relating to Personal Data exercised pursuant to applicable Privacy Laws, and (C) complied in all material respects with all (1) then-current Company Privacy Policies; (2) obligations of the Company under Contracts relating to the Processing of Company Data; and (3) Third Party access program agreements to which the Company is a party, in each case, as required by applicable Privacy Law or by the terms of any Contract by which the Company is bound, or by the terms of the applicable Company Privacy Policy (collectively, Sections 2.8(q)(ii)(A) through 2.8(q)(ii)(C) (Privacy and Personal Data), the “Company Privacy Commitments”). The execution, delivery and performance of this Agreement, including the consummation of the Contemplated Transactions, will not cause, constitute, or result in a material breach or violation by the Company of any Company Privacy Commitments or Privacy Laws. The Company has made available to Parent complete copies of Company Privacy Policies posted at any time in the past three years. No disclosures made or contained in any Company Privacy Policy in the past three years have been materially inaccurate, misleading or deceptive, including by containing any material omission.
(iii) Except as would not reasonably be expected to result in liability material to the Company, in the past three years, (i) the Company has at all times taken commercially reasonable steps (including implementing and maintaining security systems and technologies in material compliance with all Privacy Laws and in compliance in all material respects with all Company Privacy Commitments) designed to preserve and protect Company Data against (A) loss; (B) theft; and (C) accidental, unauthorized or unlawful Processing in a manner appropriate to the risks represented by the Processing of such data by the Company and for the Company by its data processors or service providers; and (ii) the Company has taken commercially reasonable steps designed to ensure the reliability of the employees and contractors that have access to Company Data and designed to ensure that all employees and contractors with the right to access such data are under written obligations of confidentiality with respect to such data. At all times during the past five years, except as would not reasonably be expected to result in liability material to the Company, taken as a whole, the Company has contractually obligated third parties that service, host, manage, access or otherwise Process Company Data to comply with applicable Privacy Laws and applicable obligations under Company Privacy Commitments. The Company has no Knowledge that any such third parties that service, host, manage, access or otherwise process Company Data, in their provision of services to the Company, have failed to comply in any material respect with applicable Privacy Laws or applicable Company Privacy Commitments.
Annex A-15
Table of Contents
(iv) Except as would not reasonably be expected to result in liability material to the Company, in the three years prior to the date of this Agreement, to the Knowledge of the Company, (i) no unauthorized access to any Company systems used by the Company to maintain Company Data, or any Company Products, or any Company Data in the possession, custody or control of any data processor or service provider of the Company, and (ii) no loss, theft, unauthorized access to, or unauthorized use, acquisition, handling, disclosure, or other Processing of, any Company Data or Personal Data maintained by or otherwise in the possession, custody or control of the Company (each, a “Security Incident”) has occurred. Except as would not reasonably be expected to result in liability material to the Company, taken as a whole, (i) to the Knowledge of the Company, as of the date of this Agreement, no “high” or “critical” security vulnerabilities exist in the Company Product or any of the Company’s on-premises or cloud-based software implemented by the Company, which vulnerabilities have not been remediated or otherwise remediated in a commercially reasonable manner; and (ii) the Company has taken commercially reasonable actions to address, and where applicable, remedy the cause of, all Security Incidents that have occurred in the three years prior to the date of this Agreement. The Company has made all notifications to Persons, Governmental Bodies, media, customers or other third parties required under Privacy Laws or Company Privacy Commitments arising out of or relating to any Security Incident that has occurred in the three years prior to the date of this Agreement.
(v) As of the date of this Agreement, the Company has not received and to the Knowledge of the Company, there is no circumstance, except as would not reasonably be expected to result in liability material to the Company, taken as whole, that has arisen in the three years prior to the date of this Agreement that would reasonably be expected to give rise to any (A) written notice of any Legal Proceeding, order, regulatory opinion, audit result or allegation from a Governmental Body, (B) written notice from any Governmental Body, or (C) any written notice from any other Person (including a data subject): (1) alleging or confirming non-compliance by the Company with an applicable requirement of Privacy Laws or Company Privacy Commitments, (2) requiring or requesting the Company to amend, rectify, cease Processing, de-combine, permanently anonymize, block or delete any Company Data, (3) permitting or mandating relevant Governmental Bodies to investigate, requisition information from, or enter the premises of, the Company relating to the Company’s actual or alleged noncompliance with Privacy Laws or Company Privacy Commitments; or (4) claiming compensation from the Company relating to the Company’s actual or alleged noncompliance with Privacy Laws or Company Privacy Commitments; excluding, in the case of all subparts of item (C), any notice relating to customer service or any requests by individuals that would not reasonably be expected to result in liability material to the Company. The Company has not received written notice of being involved in any Legal Proceedings involving a breach or alleged breach of Privacy Laws or Company Privacy Commitments by the Company.
2.9 Material Contracts.
(a) Part 2.9(a) of the Company Disclosure Schedule lists each Contract (other than any Company Plan set forth on Part 2.14(a) of the Company Disclosure Schedule) that is in effect, and that has not expired or been terminated in accordance with its terms, as of the date of this Agreement to which the Company is a party or by which any of its properties or assets are otherwise bound of the following categories (such Contracts required to be disclosed under Part 2.9(a) of the Company Disclosure Schedule, the “Material Contracts”):
(i) any Contract (or group of related Contracts), other than a Company Plan, that requires future payments by or to the Company in excess of $200,000 in any calendar year, including any such Contract (or group of such Contracts that are related) for the purchase, lease or sale of real property, raw materials, goods, commodities, utilities, equipment, supplies, products or other personal property, or for the provision or receipt of services, in each case to the extent the Contract is not terminable without penalty on 90 days’ or shorter notice;
(ii) (A) any Contract relating to the acquisition or disposition by the Company of any operating business or assets (other than pursuant to non-exclusive licenses or grants of non-exclusive rights); (B) any Contract relating to the acquisition or disposition by the Company of any operating business or assets (other than pursuant to non-exclusive licenses or grants of non-exclusive rights) under which the Company has any executory covenants or indemnification or other obligations or rights (including put or call options); or (C) any Contract under which the Company have any indemnification obligations, other than any such Contracts entered into in the Ordinary Course of Business;
Annex A-16
Table of Contents
(iii) (A) any guaranty, surety or performance bond or letter of credit issued or posted, as applicable, by the Company; (B) any Contract evidencing or relating to Debt of the Company or providing for the creation of or granting any Lien upon any of the property or assets of the Company (excluding Permitted Liens); (C) any Contract (1) relating to any loan or advance to any Person which is outstanding as of the date of this Agreement (other than immaterial advances to employees and consultants in the Ordinary Course of Business) or (2) obligating or committing the Company to make any such loans or advances; and (D) any currency, commodity or other hedging or swap Contract;
(iv) (A) any Contract creating or purporting to create any partnership, alliance or joint venture or any sharing of profits or losses by the Company with any Third Party; or (B) any Contract that provides for “earn-outs” or other contingent payments by or to the Company that have not yet been paid to the Company (excluding any contingent payments arising pursuant to recruiting agreements for Company Service Providers entered in into in the Ordinary Course of Business);
(v) any collective bargaining agreement or similar Contract with any trade union, works council or other labor organization;
(vi) any offer letter, employment agreement, independent contractor agreement or other Contract with any current Company Service Provider pursuant to which the Company is or reasonably could be obligated to pay compensation (excluding variable compensation) in excess of $200,000 annually;
(vii) any Contract that is a settlement, conciliation, or similar agreement with any Governmental Body or that imposes any monetary or other material obligations upon the Company to any Governmental Body after the date of this Agreement;
(viii) all joint venture, partnership (involving sharing of profits) or similar Contracts (and not including any sharing of profits by a Third Party with the Company that are based on sales of goods or services other than Company Products);
(ix) any Contract under which any Governmental Body has any material rights;
(x) (A) any Contract containing covenants restricting or purporting to restrict competition which, in either case, have, would have or purport to have the effect of prohibiting the Company, or, after the Closing, Parent or the Surviving Company from engaging in any business or activity in any geographic area or other jurisdiction, other than any such covenant set forth in this Agreement or the agreements ancillary hereto; (B) any Contract in which the Company has granted “exclusivity” or that requires the Company to deal exclusively with, or grant exclusive rights or rights of first refusal to, any customer, vendor, supplier, distributor, contractor or other Person or that is a requirements contract; (C) any Contract that includes minimum purchase conditions or other requirements, in either case that exceed $100,000 in any calendar year to the extent the Contract is not terminable without penalty on 90 days’ or shorter notice; or (D) any Contract containing a “most-favored-nation,” “best pricing” or other similar term or provision by which another party to such Contract or any other Person is, or could become, entitled to any benefit, right or privilege which, under the terms of such Contract, is required to be at least as favorable to such party as those offered to another Person;
(xi) any Contract involving a sales agent, representative, distributor, reseller, middleman, marketer, broker, franchisor or similar Person who is entitled to receive commissions, fees or markups related to the provision or resale of goods or services of the Company;
(xii) any Contract involving commitments to make capital expenditures or to Contract, purchase or sell assets involving $100,000 or more;
(xiii) any lease, sublease, rental or occupancy agreement, license (not relating to Intellectual Property), installment, and conditional sale agreement or agreement under which the Company is the lessee or lessor of, or own, use or operate any leasehold or other interest in any real or personal property;
(xiv) the Company Intellectual Property Agreements; and
Annex A-17
Table of Contents
(xv) any Contract (excluding any Contract disclosed in Part 2.14(f) of the Company Disclosure Schedule) that contains a change in control clause or similar provision that would be reasonably be expected to be triggered in connection with the consummation of the Contemplated Transactions and would result in payments by the Company or any successor thereto in excess of $100,000, individually or in the aggregate; and
(xvi) any Contract not otherwise listed or required to be listed in Part 2.9(a) of the Company Disclosure Schedule (including Company Intellectual Property Agreements) that, if terminated, or if such Contract expired without being renewed, would have a Company Material Adverse Effect.
(b) With respect to each Material Contract listed in Part 2.9(a) of the Company Disclosure Schedule, such Material Contract is, to the Knowledge of the Company, binding and enforceable against the Company and, to the Knowledge of the Company, against each party thereto other than the Company, in accordance with its terms, subject to (A) Laws of general application relating to bankruptcy, insolvency and the relief of debtors and (B) rules of Law governing specific performance, injunctive relief and other equitable remedies. Except for breaches, violations or defaults which have not had, and would reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect, the Company is not in violation of any provision of, or taken or failed to take any which, with or without notice, lapse of time, or both, would constitute a default under the provisions of, any Material Contract, and, to the Knowledge of the Company, no other party to such Material Contract is in violation of any provision, or taken or failed to take any act which, with or without notice, lapse of time, or both, would constitute a default under the provisions of any Material Contract. Since January 1, 2020, the Company has not received any written notice or, to the Knowledge of the Company, other communication regarding any actual or possible violation or breach of, or default under, any Material Contract by the Company. The Company has made available to Parent true and complete copies of each such Material Contract in all material respects (including all modifications, amendments and supplements thereto and waivers thereunder, but not including purchase orders and similar confirmatory documents not specific to provisions that make such Contract a Material Contract).
2.10 Liabilities. The Company has no liabilities required by GAAP to be reflected in the Balance Sheet or footnotes to the Balance Sheet other than: (a) those set forth on the face of, or reserved in, the Balance Sheet or the footnotes thereto; (b) those incurred in the Ordinary Course of Business since the date of the Balance Sheet (none of which arose out of, in connection with or as a result of any breach of Contract, tort, infringement of Intellectual Property or violation of Law, and none of which are, individually or in the aggregate, material to the business of the Company as presently operated); (c) those incurred pursuant to performance of this Agreement; and (d) those that do not, individually or in the aggregate, constitute a Company Material Adverse Effect.
2.11 Compliance with Laws; Permits.
(a) The Company is in material compliance with, and at all times during the past three years has been in material compliance with, applicable Laws, including those relating to employment, and as of the date of this Agreement, the Company has not received any written notices of any violation with respect to such Laws.
(b) The Company has obtained each material federal, state, county, local or foreign governmental consent, license, permit, grant or other authorization of a Governmental Body (i) pursuant to which the Company currently operates or holds any interest in any of its assets or properties or (ii) that is required for the conduct of the business of the Company or the holding of any such interest (all of the foregoing consents, business licenses, permits, grants and other authorizations, collectively, the “Permits”), and all of the Permits are in full force and effect, except for those the absence of which or the noncompliance with which does not constitute a Company Material Adverse Effect. Part 2.11(b) of the Company Disclosure Schedule lists all Permits as of the date of this Agreement, other than Permits which the Company’s failure to obtain or possess would not reasonably be expected to have, individually or in the aggregate, have a Company Material Adverse Effect. As of the date of this Agreement, the Company has not received any written notice from any Governmental Body regarding (A) any actual or possible violation of any Permit or any failure to comply with any term or requirement of any Permit or (B) any actual or possible revocation, withdrawal, suspension, cancellation, termination or modification of any Permit, and to the Knowledge of the Company, no such notice or other communication is forthcoming. The Company has materially complied with all of the terms of the Permits and none of the Permits will be terminated or impaired, or will become terminable, in whole or in part, as a result of the consummation of the Merger, except for those the absence of which or the noncompliance with which does not constitute a Company Material Adverse Effect.
Annex A-18
Table of Contents
2.12 Certain Business Practices. The Company, and to the Knowledge of the Company, the Company Service Providers while acting on behalf of the Company or other Representatives while acting on behalf of the Company, during the past three years (a) have not used, and are not currently using, any funds for any unlawful contributions, unlawful gifts, unlawful entertainment or other unlawful expenses; (b) have not made any direct or indirect unlawful payments to any foreign or domestic Government Official; (c) have not violated and are not violating any Anti-Corruption Laws; (d) have not established or maintained, and are not maintaining, any unlawful or unrecorded fund of monies or other properties; (e) has not made, and are not making, any false or fictitious entries on its accounting books and records; (f) have not made, and are not making, any bribe, rebate, payoff, influence payment, kickback or other unlawful payment of any nature, and have not paid, and are not paying, any fee, commission or other payment that has not been properly recorded on its accounting books and records as required by the Anti-Corruption Laws; and (g) have not otherwise given or received anything of value to or from a Government Official, an intermediary for unlawful payment to any individual including Government Officials, any political party or customer for the purpose of obtaining or retaining business.
2.13 Tax Matters.
(a) The Company has:
(i) duly and timely filed, or caused to be filed, in accordance with applicable law all income and other material Company Tax Returns, each of which is true, correct and complete in all material respects,
(ii) duly and timely paid in full, or caused to be paid in full, all material Company Taxes due and payable (whether or not shown on any Company Tax Return) on or prior to the Closing Date, and
(iii) properly accrued in accordance with GAAP on their respective books and records a provision for the payment of all material Company Taxes that are due, are claimed to be due, or may or will become due with respect to any Tax period ending on or prior to the Closing Date.
(b) No extension of time to file a Company Tax Return, which such Company Tax Return has not since been filed in accordance with applicable law, has been filed, other than extensions obtained in the Ordinary Course of Business. There is no power of attorney in effect with respect or relating to any Company Tax or Company Tax Return.
(c) No Company Tax Return has ever been filed, and no Company Tax has ever been determined, on a consolidated, combined, unitary or other similar basis (including, but not limited to, a consolidated federal income Tax Return), other than for a group the common parent of which is Parent or the Company. The Company does not have any material liability for the Taxes of any Person (other than the Company) (i) under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local or foreign Law), (ii) as a transferee or successor or (iii) by contract or otherwise (except, in each case, for liabilities pursuant to commercial contracts not primarily relating to Taxes).
(d) The Company has complied in all material respects with all applicable law relating to the deposit, collection, withholding, payment or remittance of any material Tax (including, but not limited to, Sections 1441, 1442, 3121, and 3402 of the Code).
(e) There is no lien for any material Tax upon any asset or property of the Company (except for any statutory lien for any Tax not yet due).
(f) No Proceeding is pending, threatened or proposed in writing with regard to any Company Tax or Company Tax Return.
(g) The statute of limitations applicable or relating to any Company Tax or any Company Tax Return has never been modified, extended or waived, nor has any request been made in writing for any such modification, extension or waiver, with respect to which the statute of limitations has not expired (and in all cases excluding any modification or extension of the statute of limitations resulting from the extension of the due date for filing of a Company Tax Return).
Annex A-19
Table of Contents
(h) No jurisdiction where no Company Tax Return has been filed or no Company Tax has been paid has made or threatened in writing to make a claim for the payment of any Company Tax or the filing of any Company Tax Return.
(i) The Company is not a party to any agreement with any Governmental Body or any Government Official relating to a material amount of Tax (including, but not limited to, any closing agreement within the meaning of Section 7121 of the Code or any analogous provision of applicable law). No private letter or other ruling or determination from any Governmental Body and Government Official relating to any Company Tax or Company Tax Return has ever been requested or received.
(j) The Company will not be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or any portion thereof) ending after the Closing Date as a result of any: (A) change in method of accounting made prior to the Closing for a taxable period ending on or prior to the Closing Date under Section 481 of the Code (or any corresponding provision of state, local or other law), (B) use prior to the Closing of an improper method of accounting for any taxable period ending on or prior to the Closing Date, (C) installment sale or open transaction disposition made prior to the Closing, (D) prepaid amount received on or prior to the Closing Date outside the Ordinary Course of Business (including, without limitation, pursuant to Sections 451(c), 455 or 456 of the Code, Treasury Regulation Section 1.451-5 and Revenue Procedure 2004-34), (E) “closing agreement” as described in Section 7121 of the Code (or any corresponding provision of state, local or other law), (F) intercompany transaction or excess loss account described in the Treasury Regulation under Section 1502 of the Code, (G) the application of Section 951 of the Code or Section 951A of the Code with respect to income earned or recognized or payments received, (H) any election under Section 108(i) or Section 965(h) of the Code, or (J) any provision of local, state or non-U.S. Tax law comparable to any of the foregoing.
(k) The Company has not made an election to defer any Taxes under any provision of the Coronavirus Aid, Relief, and Economic Security Act for 2020 (“CARES Act”) (including, but not limited to, Section 2302 (Delay of Payment of Employer Payroll Taxes)), or any other similar election under state or local Tax law.
(l) The Company has not claimed any credit under Section 2301 of the CARES Act or division G of the Families First Coronavirus Response Act (Tax Credits for Paid Sick and Paid Family and Medical Leave), or any other similar credit under state or local Tax Law.
(m) The Company has never been a beneficiary or otherwise participated in any “reportable transaction” as defined in Section 6707A(c)(1) of the Code and Treasury Regulation Section 1.6011-4(b)(1).
(n) The Company has not distributed stock of another Person nor has their stock been distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code.
(o) The Company is not and has never been, a “United States real property holding corporation” within the meaning of Section 897(c)(2) of the Code at any time during the applicable period specified in Section 897(c)(l)(A)(ii) of the Code.
(p) The Company has not and has never had any (i) place of management, (ii) branch, (iii) office (or any other place of business), (iv) operations of employees, (v) agent with binding authority or (vi) other activities, in each case that gives rise to a permanent establishment in any country other than the country where the Company was organized, and (vii) has never entered into a gain recognition agreement pursuant to Treasury Regulation Section 1.367(a)-8, and (viii) has never transferred an intangible the transfer of which would be subject to the rules of Section 367(d) of the Code.
(q) The Company is not a party to any joint venture, partnership or other agreement, contract or arrangement (whether written or oral) which could be treated as a partnership for Tax purposes, other than a partnership that is wholly-owned, directly or indirectly, by the Company.
Annex A-20
Table of Contents
(r) The Company (i) has filed or caused to be filed all reports and has created and retained all records required under Section 6038A of the Code with respect to its ownership by, and transactions with, related parties, and (ii) has disclosed on the appropriate Company Tax Return all positions that could reasonably be expected to give rise to a substantial understatement of Tax within the meaning of Section 6662 of the Code.
(s) The Company is in material compliance with all applicable transfer pricing laws and regulations (including Section 482 of the Code and its corresponding Treasury Regulations and any corresponding provision of non-U.S. Tax law), including the maintenance of contemporaneous documentation substantiating the transfer pricing practices and methodologies in all material respects.
(t) The Company is and always has been treated as a corporation for all Tax purposes.
(u) The Company has not participated in or is participating in an international boycott within the meaning of Section 999 of the Code.
(v) The Company has, in all material respects, timely collected and properly maintained all resale certificates and other documentation required for any exemption from the collection of any applicable sales or use or similar Taxes.
(w) The Company has not taken any action, nor to the Knowledge of the Company are there any facts or circumstances not set forth in this Agreement, that could reasonably be expected to prevent the Mergers from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code.
2.14 Employee Benefit Plans.
(a) Part 2.14(a) of the Company Disclosure Schedule sets forth a list of all Company Plans and written Company employment agreements providing for severance payments (“Service Provider Agreements”) in effect as of the date of this Agreement (other than (i) Company Service Provider Agreements with employees that are terminable at-will by the Company without a required notice period or severance or change of control pay or benefits, in which case only the form of such Company Service Provider Agreements will be listed, (ii) individual equity award agreements that do not deviate from the Company’s standard forms, in which case only such standard forms of equity award agreement will be listed, and (iii) Company Service Provider Agreements with consultants that are terminable without penalty on not more than thirty (30) days’ notice, in which case only forms of such Company Service Provider Agreements will be listed, unless any such Company Service Provider Agreements provides (x) severance or change of control pay or benefits that are, in each case, greater than required by applicable Law or (y) could reasonably obligate the Company to pay compensation in excess of $100,000 annually).
(b) With respect to each Company Plan and Company Service Provider Agreement (other than those that are materially consistent with a Standard Form IP Agreement), the Company has made available to Parent true and complete copies of (to the extent applicable): (i) each writing constituting a part of any written Company Plan and Company Service Provider Agreement and all amendments thereto, and all trusts or service agreements relating to the administration and recordkeeping of the Company Plan or Company Service Provider Agreement, and written summaries of the material terms of all unwritten Company Plans and Company Service Provider Agreements; (ii) the three most recent annual reports (Form 5500 and all schedules and financial statements attached thereto) as filed with the IRS; (iii) the most recent summary plan description and all summaries of material modifications; (iv) the most recent IRS determination, opinion or advisory letter issued with respect thereto; (v) the most recent summary annual reports, nondiscrimination testing reports and actuarial reports; (vi) all written reports constituting a valuation of the Company Capital Stock for purposes of Sections 409A or 422 of the Code, whether prepared internally by the Company or by an outside, third-party valuation firm; and (vii) all material written correspondence given to such Company Plan or Company Service Provider Agreement or the Company by any Governmental Body (including any foreign Governmental Body responsible for the regulation of such Company Plan or Company Service Provider Agreement) during the three years preceding the date of this Agreement relating to such Company Plan or Company Service Provider Agreement or provided to any such Entity by the Company Plan or Company Service Provider Agreement, the Company during the three years preceding the date of this Agreement with respect to such Company Plan or Company Service Provider Agreement.
Annex A-21
Table of Contents
(c) In all material respects, each Company Plan and Company Service Provider Agreement has been established, funded, operated, administered and maintained in accordance with its terms and in compliance with all applicable Laws, including the Code. There are no Legal Proceedings or claims pending, or, to the Knowledge of the Company, threatened or reasonably anticipated (other than individual claims for benefits payable in the normal operation of the Company Plan or Company Service Provider Agreement) with respect to any Company Plan or Company Service Provider Agreement. There are no audits, inquiries, investigations or proceedings pending or, to the Knowledge of the Company, threatened by any Governmental Body with respect to any Company Plan or Company Service Provider Agreement. Company has timely made all contributions, distributions, reimbursements and payments that are due with respect to each Company Plan and Company Service Provider Agreement, and all contributions, distributions, reimbursements and payments for any period ending on or before the Closing Date that are not yet due have been made or properly accrued with respect to each Company Plan and Company Service Provider Agreement. Each Company Plan and Company Service Provider Agreement can be amended, terminated or otherwise discontinued at any time in accordance with its terms, without liability to Parent or the Company (other than ordinary administration expenses).
(d) The Company Plans which are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and which are intended to meet the qualification requirements of Section 401(a) of the Code have received determination, opinion or advisory letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code and the related trusts are exempt from federal income Taxes under Section 501(a) of the Code, respectively, and to the Knowledge of the Company, nothing has occurred that could reasonably be expected to adversely affect the qualification of such Company Plan or the tax exempt status of the related trust.
(e) Neither the execution or delivery of this Agreement nor the consummation of the Merger will (either alone or in connection with any other event): (i) result in any payment by the Company becoming due to any Company Service Provider, (ii) increase any amount of compensation or benefits otherwise payable under any Company Plan or Company Service Provider Agreement, (iii) result in the acceleration of the time of payment, funding or vesting of any payments or benefits under any Company Plan or Company Service Provider Agreement, (iv) require any contribution or payment to fund any obligation under any Company Plan or Company Service Provider Agreement, or (v) limit the right to amend or terminate any Company Plan or Company Service Provider Agreement.
(f) No Company Plan or Company Service Provider Agreement is, and the Company does not have nor has it ever sponsored, maintained contributed to, been required to contribute to or had or has any obligations or liability (current or contingent) under or with respect to any plan that is or was (i) subject to Section 302 or Title IV of ERISA or Section 412 of the Code (including any “defined benefit plan” within the meaning of Section 3(35) of ERISA), (ii) a “multiemployer plan” within the meaning of Section 3(37) of ERISA, (iii) any “multiple employer plan” within the meaning of Section 413 of the Code, or (iv) any “multiple employer welfare arrangement” within the meaning of Section 3(40) of ERISA. The Company has no current or contingent liability or obligation as a consequence of at any time being considered a single employer under Section 414 of the Code with any other Person.
(g) No Company Plan or Company Service Provider Agreement provides death, medical, dental, vision, life insurance or other welfare benefits beyond termination of service or retirement, other than coverage mandated by Law, and neither the Company nor Company ERISA Affiliates has made a written or oral representation promising the same to any Company Service Provider.
(h) Each Company Plan or Company Service Provider Agreement providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A of the Code) is, and has been, established, administered and maintained in compliance with the requirements of Section 409A of the Code.
2.15 Employee Matters.
(a) The Company has made available to the Parent a true and correct list, as of the date of this Agreement, containing: (i) the names of all current Company Service Providers and identification as either a full-time, part-time or temporary employee or independent contractor; (ii) the annual dollar amount of all compensation (including wages, salary or fees, commissions and bonuses) payable to each such Company Service Provider; (iii) dates of first employment or service; (iv) current job title of each employee of the Company; (v) with respect to employees, whether
Annex A-22
Table of Contents
any such employment is “at-will”; (vi) visa status, if applicable; and (vii) with respect to employees, a designation of whether they are classified as exempt or non-exempt for purposes of the Fair Labor Standards Act, as amended and any similar state law.
(b) The Company: (i) is, and at all times during the prior five years has been, in material compliance with all applicable Laws, and with any order, ruling, decree, judgment or arbitration award of any arbitrator or any court or other Governmental Body, relating to employment and employment practices, including, but not limited to, payment of wages, hours of work, employment taxes and withholdings, labor relations, discrimination, worker classification (including the proper classification of workers as employees or independent contractors and/or consultants), harassment, retaliation, immigration, wrongful discharge or other violation of the rights of any current, former, or prospective Company Service Provider; (ii) has withheld and reported all amounts required by any Law or Contract to be withheld and reported with respect to wages, salaries and other payments to any Company Service Provider; and (iii) has no liability for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body with respect to unemployment compensation benefits, social security or other benefits or obligations for any Company Service Provider (other than routine payments to be made in the normal course of business). To the Knowledge of the Company, each current Company Service Provider is legally authorized to work in his, her or their current position under applicable United States or foreign immigration laws.
(c) The Company is not a party to or otherwise bound by any collective bargaining agreement, Contract or other agreement or understanding with a labor union or labor organization, or works council or similar body, nor is any such Contract or agreement presently being negotiated. To the Knowledge of the Company, there is currently no representation campaign or organizing activity with respect to any Company Service Provider.
(d) There are no currently pending, nor have there within the three (3) years prior to the date hereof been any, actions, suits, claims, charges, complaints, grievances, arbitrations, investigations or other Legal Proceedings against the Company by or with any Governmental Body, arbitrator, or other dispute resolution forum in connection with the application, employment or engagement of any current, former or prospective Company Service Provider including, without limitation, any claim relating to unfair labor practices, employment discrimination, harassment, retaliation, equal pay, wage or hours violations, unpaid wages, unpaid commissions, wrongful termination or any other employment related matter arising under applicable Laws, nor, to the Knowledge of the Company, are any such Legal Proceedings threatened to be brought or filed.
(e) To the Knowledge of the Company, (i) no allegations of sexual harassment or misconduct have within the three (3) years prior to the date hereof been made against (A) any officer, director or executive employee of the Company or (B) any Company Service Provider who, directly or indirectly, supervises other Company Service Providers, and (ii) the Company has not within the three (3) years prior to the date hereof entered into any settlement agreement or conducted any investigation related to allegations of sexual harassment or sexual misconduct by any Company Service Provider or other Person.
(f) As of the date of this Agreement, no current Company officer, director or executive employee of the Company has provided written notice to the Company of his, her or their intent to terminate his, her, or their relationship with the Company as of the date of this Agreement.
(g) (i) All current Company Service Providers who are employees are employed on an “at-will” basis and their employment can be terminated at any time for any reason without notice or payment of severance or other compensation or consideration being owed to such individual other than amounts owed as of the date of termination from employment based on service before that date or as required under applicable Law; (ii) the Company’s relationships with all other current non-employee Company Service Providers can be terminated at any time for any reason without notice or any amounts being owed to such individual other than with respect to compensation or payments accrued before the termination; (iii) no current Company Service Provider is unable to perform services for the Company as a result of a leave of absence; (iv) each Company Service Provider who has rendered services to the Company who is or was classified by the Company as having the status of an independent contractor or other non-employee status for any purpose (including for purposes of taxation and Tax reporting and under any Company Plans or prior employee benefit plans) is properly so characterized, and the Company does not have any material liability as a result of the failure to properly classify any such Company Service Provider as an employee of the Company; (v) the Company has never had any temporary or leased employees that were not treated and accounted for in all respects as employees; and (vi) each
Annex A-23
Table of Contents
Company Service Provider who was or is classified as an employee has been correctly classified as exempt or non-exempt for purposes of all applicable Laws, and overtime has been properly recorded and paid for all such employees classified as non-exempt.
(h) The Company is, and at all times during the past five years has been, in compliance with all obligations due to or in connection with any Company Service Provider. There are no sums owing to any Company Service Provider, other than reimbursements of expenses and fees for the applicable current work period.
2.16 Environmental Matters. The Company is and for the past five years has been in material compliance with all applicable Environmental Laws. During the past five years (or earlier if unresolved), the Company has not received any written notices, demand letters or requests for information from any Governmental Body or any other Person indicating that the Company is or may be in violation of, or may be liable under, any Environmental Law, and the Company is not subject to any pending or, to Knowledge of the Company, threatened action or investigation by any Governmental Body under any Environmental Law. To the Knowledge of the Company, no current or prior owner of any property leased or controlled by the Company has received any written notice from a Governmental Body or any other Person during the past five years (or earlier if unresolved) that alleges that such current or prior owner or the Company are materially violating or have materially violated, or are liable under any Environmental Law. The Company is and for the past five years has been in compliance in all material respects with, and have no material liability under, any provisions of leases relating in any way to any Environmental Laws or to the use, management, handling, disposal or release of Hazardous Substances under such leases. All Environmental Permits, if any, required to be obtained and maintained by the Company under any Environmental Law in connection with its operations as they have been or are currently being conducted, including those relating to the management of Hazardous Substances, have been obtained and maintained by the Company, are in full force and effect, and the Company is and for the past five years has been in material compliance with the terms thereof. The Company has not treated, stored, disposed of, arranged for or permitted the disposal of, handled, released or exposed any Person to any Hazardous Substances on, in or under any real property, or owned or operated any property contaminated by any such Hazardous Substances, in each case that has resulted in or would reasonably be expected to result in material liability to the Company under Environmental Laws. The Company has made available to Parent true and complete copies of any environmental investigation, study, test, audit, review or other analysis in its possession in relation to the current or prior business or real properties of the Company.
2.17 Insurance. As of the date of this Agreement, the Company has the insurance of the types and in the amounts set forth in Part 2.17 of the Company Disclosure Schedule (the “Insurance Policies”). The Insurance Policies are in full force and effect and all premiums due and payable under such Insurance Policies have been paid on a timely basis. As of the date of this Agreement, there is no material claim pending under any of the Insurance Policies as to which coverage has been questioned, denied or disputed by the underwriters of such policies. The Company is in compliance in all material respects with the terms of such policies. The Company has no Knowledge as of the date of this Agreement of any threatened termination of, or material premium increase with respect to, any of such policies. The Company has made available to Parent true and complete copies of the Insurance Policies.
2.18 Legal Proceedings; Orders. There is no pending Legal Proceeding, and, to the Knowledge of the Company, no Person has threatened to commence any Legal Proceeding that involves the Company, or any of the assets owned or used by the Company or any Person whose liability the Company has retained or assumed, either contractually or by operation of Law. There is no Legal Proceeding that challenges, or that may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Merger or any of the Contemplated Transactions, and, to the Knowledge of the Company, no Person has threatened to commence any such Legal Proceeding. There is no order, writ, injunction, judgment or decree to which the Company or any of the assets owned or used by the Company or any of the Company’s officers or directors (in their respective capacities as such), is subject.
2.19 Authority; Binding Nature of Agreement. Subject to receipt of the Required Company Stockholder Vote, the Company has all necessary corporate power and authority to enter into and to perform its obligations under this Agreement. As of the date of this Agreement, the Company Board has (a) determined that this Agreement, the Merger and the Contemplated Transactions, are advisable and fair and in the best interests of the Company and its stockholders (other than Parent, its Affiliates and Howard Jonas, and their respective affiliates, as to which fairness the Company Board has not made any determination), (b) authorized and approved the execution, delivery and performance of this Agreement by the Company and approved the Merger, and (c) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the Company Stockholder vote to approve entry into this
Annex A-24
Table of Contents
Agreement and the consummation of the Merger. This Agreement constitutes the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to (i) Laws of general application relating to bankruptcy, insolvency and the relief of debtors and (ii) rules of Law governing specific performance, injunctive relief and other equitable remedies. The Company Board has (x) (A) determined that it is fair to and in the best interests of the Company and the holders of the Company Capital Stock to enter into this Agreement and declared this Agreement and the transactions contemplated by this Agreement advisable, (B) adopted this Agreement and approved the execution, delivery and performance of this Agreement by the Company and the consummation of the Mergers and the other transactions contemplated by this Agreement and (C) resolved to recommend adoption of this Agreement and approval of the Mergers and the other transactions contemplated by this Agreement by the holders of Company Capital Stock and (y) directed that this Agreement be submitted to the holders of Company Capital Stock entitled to vote for adoption of this Agreement and approval of the Mergers and the other transactions contemplated by this Agreement. The Company Board has (aa) received an opinion of Raymond James & Associates, Inc., the Company Board’s financial advisor, to the effect that, as of the date of such opinion and subject to the limitations, qualifications and assumptions set forth therein, the aggregate consideration to be received by the Company (comprised of the Merger Shares to be issued by Parent) in the Merger is fair to the Company, from a financial point of view, and as of the date this Agreement, such opinion has not been withdrawn, revoked or modified and (bb) determined that it is fair to and in the best interests of the Company and the holders of the Company Capital Stock to enter into this Agreement and declared this Agreement and the transactions contemplated by this Agreement advisable.
2.20 Vote Required. Assuming that the Company Minority Approval has been obtained, the Company has taken all action necessary to permit the execution, delivery and performance of this Agreement and the consummation of the Mergers and the other transactions contemplated by this Agreement under (i) the DGCL and any other “fair price,” “moratorium,” “control share acquisition” or other similar anti-takeover statute or regulation (each, a “Takeover Statute”) that is applicable to the Company or (ii) any anti-takeover provision in the Company’s certificate of incorporation or bylaws that may restrict the execution, delivery and performance of this Agreement and the consummation of the Mergers and the other transactions contemplated by this Agreement.
2.21 Counterparties. The Company has no outstanding material disputes any of its vendors, partners, medical centers suppliers, clinical trial sites or other material service providers involving good or services for which the Company paid an aggregate amount during 2020 exceeding $200,000 (each, a “Counterparty”). Each Counterparty is listed on Part 2.21(a) of the Company Disclosure Schedule. As of the date of this Agreement, the Company has not received any written or other information from any Counterparty that such Counterparty shall not continue in its relationship with the Company (or the Surviving Company or Parent or any of their respective Subsidiaries) after the Closing or that such Counterparty intends to terminate or materially modify existing Contracts with the Company (or the Surviving Company or Parent or any of their respective Subsidiaries). There is no material dissatisfaction on the part of the Company with respect to any Counterparty and there is no material dissatisfaction on the part of any Counterparty with respect to the Company.
2.22 Non-Contravention; Consents. Except for filings, permits, authorizations, consents, approvals and other applicable requirements as may be required under the Securities Act, the Exchange Act, state securities or blue sky laws, the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the “HSR Act”), Council Regulation (EC) No. 139/2004 of 20 January 2004 on the control of concentrations between undertakings (published in the Official Journal of the European Union on January 29, 2004 at L 24/1) (the “EC Merger Regulation”) or other foreign antitrust or competition Laws, and the filing of the Certificate of Merger as required by the DGCL, respectively, no filing with or notice to, and no permit, authorization, consent or approval of, any Governmental Body is necessary for the execution and delivery by the Company of this Agreement or the consummation by the Company of the transactions contemplated by this Agreement.
2.23 Financial Advisor. Except for Raymond James & Associates, Inc., no broker, finder or investment banker is entitled to any brokerage or finder’s fee in connection with the Merger or any of the Contemplated Transactions based upon arrangements made by or on behalf of the Company.
2.24 Related Party Transactions. Except as set forth in Part 2.24(a) of the Company Disclosure Schedule, there are no obligations of the Company to, or Contracts with, current or former officers, directors, stockholders or employees of the Company, or its respective Affiliates or family members other than (a) for payment of ordinary course salaries and bonuses for services rendered, (b) reimbursement of customary and reasonable expenses incurred on behalf of the Company, (c) benefits due under Company Plans and ordinary course fringe benefits not required
Annex A-25
Table of Contents
to be listed on Part 2.14(a) of the Company Disclosure Schedule, (d) Company Plans and (e) agreements relating to Company Options (each Contract required to be set forth on Part 2.24(a) of the Company Disclosure Schedule, an “Affiliated Agreement”). Except as set forth in Part 2.24(a) of the Company Disclosure Schedule, no officer, director or employee of the Company or Company Stockholder is directly interested in any Material Contract. Except as set forth in Part 2.24(b) of the Company Disclosure Schedule, neither the Company nor any of its Affiliates, directors, officers or employees of the Company possess, directly or indirectly, any financial interest in, or is a director, officer or employee of, any Entity that is a material supplier, contractor, lessor, lessee or competitor of the Company.
2.25 Regulatory Matters.
(a) As used herein, “IND” means an investigational new drug application or clinical trial application filed with the FDA or any comparable foreign Governmental Authority, including all documents, data and other information concerning the applicable drug that are necessary for or filed with such application and “FDA” means the U.S. Food and Drug Administration.
(b) The Company has obtained all Permits required by the FDA to conduct the business of the Company as it is currently conducted (the “FDA Permits”). All of the FDA Permits are in full force and effect, and the Company is in material compliance with each such permit held by or issued to it. Except as disclosed in Part 2.25(b) of the Company Disclosure Schedule, the Company has not received any written notice of any threatened or pending complaint, charge, investigation, hearing, warning letter, untitled letter, finding of deficiency or non-compliance, adverse inspection report, FDA Form 483, penalty, fine, sanction, request for recall, or other remedial action, audit, audit report, or any revocation, withdrawal, or suspension of any approval, permit, registration, license or other authorization, or other adverse regulatory action by any Governmental Authority pursuant to any Laws applicable to the Company.
(c) The Company’s product candidates for human use or anticipated to be for human use (the “Product Candidates”) are being, and at all times have been, developed, tested, labeled, manufactured, stored, imported, exported and distributed, as applicable, in compliance in all material respects with the FDCA and applicable implementing regulations issued by the FDA and applicable Laws and applicable implementing regulation issued by the European Medicines Agency (“EMA”) and any other applicable Governmental Authorities, including, as applicable, those Laws relating to the FDA’s current good manufacturing practices, good laboratory practices, good clinical practices, investigational use and applications to market a new pharmaceutical product and, to the knowledge of the Company without investigation, all Laws referred to in EudraLex Volume 10 (Guidelines for Clinical Trials).
(d) The Company has made available to Parent as of the date of this Agreement a complete and correct copy of (w) all material submissions to the FDA or EMA regarding the Product Candidates , including all INDs and all supplements and amendments thereto (“Regulatory Submissions”), (x) all material correspondence to or from the FDA or EMA with respect to the Product Candidates, (y) all material contact reports or similar reports documenting meetings, phone calls or other communications with the FDA or EMA and (z) all study reports (including any interim or preliminary study reports). All Regulatory Submissions (and any supporting documentation thereto) and, any and all requests for authorizations, approvals, certificates, waivers, certifications, clearances, notifications, licenses or permits of the FDA, EMA or any other comparable non-U.S. Governmental Authority relating to the Company, the business currently conducted by the Company and the Company’s products, when submitted to the FDA or any other comparable non-U.S. Governmental Authority, including institutional review boards, independent ethics committees, or similar bodies, were, to the Company’s Knowledge, true and correct in all material respects as of the date of submission or subsequently corrected.
(e) Except as disclosed in Part 2.25(e) of the Company Disclosure Schedule, the Company has not sponsored or conducted any clinical trial (including but not limited to any trial conducted pursuant to an exploratory IND or exploratory CTA).
(f) To the extent required by applicable Laws, all clinical studies, preclinical studies and tests conducted by or on behalf of the Company have been, and if still pending are being, conducted in material compliance with the research protocols submitted to the FDA or other Governmental Authority, good laboratory practices, good clinical practices, and all applicable Laws, including, but not limited to, the FDCA and the Laws of the EMA and, to the Company’s Knowledge, all clinical studies and preclinical studies and tests conducted by or on behalf of the Company have been or, if pending, are being conducted in material compliance, to the extent applicable with such
Annex A-26
Table of Contents
practices and Laws. No clinical study conducted by or on behalf of the Company has been terminated or suspended prior to completion. None of the FDA, EMA or any other applicable Governmental Authority, clinical investigator that has participated or is participating in, or institutional review board that has or has had jurisdiction over, a clinical study conducted by or on behalf of the Company has commenced, or, to the Company’s Knowledge, threatened to initiate, any action to place a clinical hold order on, or otherwise terminate or suspend or refuse to commence, any proposed or ongoing investigation or study conducted or proposed to be conducted by or on behalf of the Company.
(g) To the extent required by applicable Laws, all preclinical tests performed in connection with or as the basis for any regulatory approval that has been sought or obtained for the Product Candidates, and, to the Company’s Knowledge, all other preclinical tests prepared in connection with or as the basis for any such regulatory approval, either (x) have been conducted in material compliance with all applicable Laws and rules, including, where applicable, “Good Laboratory Practice” (“GLP”) as set forth in 21 CFR Part 58, the United States Animal Welfare Act, the International Conference on Harmonization’s (ICH) Guideline on Nonclinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals or the ICH Guideline on Safety Pharmacology Studies for Human Pharmaceuticals or (y) involved experimental research techniques that were performed for informational purposes only, whether or not included in a regulatory filing, or could not be performed by a GLP-compliant testing facility (with appropriate notice being given to the FDA in regulatory filings) and have employed the procedures and controls generally used by qualified experts in animal or preclinical studies of products comparable to those being developed by Company.
(h) The Company is not subject to any investigation that is pending and of which the Company has been notified in writing or, to the Company’s Knowledge, which has been threatened, in each case by (i) the FDA or (ii) the Department of Health and Human Services Office of Inspector General or Department of Justice pursuant to the Federal Healthcare Program Anti-Kickback Statute (42 U.S.C. §1320a-7b(b)) or the Federal False Claims Act (31 U.S.C. §3729).
(i) The Company has not submitted any claim for payment to any government healthcare program in connection with any referrals related to any of the Product Candidates that violated in any material respect any applicable self-referral law, including the Federal Ethics in Patient Referrals Act, 42 U.S.C. §1395nn, or any applicable state self-referral law.
(j) The Company has not submitted any claim for payment to any government healthcare program related to any of the Product Candidates in material violation of any laws relating to false claims or fraud, including the Federal False Claim Act, 31 U.S.C. § 3729, or any applicable state false claim or fraud law.
(k) The Company has complied in all material respects with all applicable security and privacy standards regarding protected health information under (i) the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, including the regulations promulgated thereunder and (ii) any applicable state privacy laws.
(l) All manufacturing operations conducted by or for the benefit of the Company with respect to the Product Candidates have been and are being conducted in material compliance with applicable Laws, including, to the extent applicable, the provisions of the FDA’s current good manufacturing practice regulations, and the respective counterparts thereof promulgated by the EMA and other Governmental Authorities in countries outside the United States. Except as disclosed in Part 2.25(l)(i) of the Company Disclosure Schedule, the Company has not recalled any Product Candidates and no Governmental Authority has suspended or otherwise restricted the manufacture of any of the Product Candidates. Except as disclosed in Part 2.25(l)(ii) of the Company Disclosure Schedule, the Company has not received any notice that the FDA, EMA or any other Governmental Authority, any relevant institutional review board, independent ethics committee or any other similar body has initiated, or threatened to initiate, any action to suspend or otherwise restrict the manufacture of any of the Product Candidates.
(m) Neither the Company nor any Company personnel, Representative or controlled Affiliate has committed any act, made any statement or failed to make any statement that would reasonably be expected to provide a basis for the FDA, the EMA or any other Governmental Authority to invoke its policy with respect to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” or any such similar policies set forth in any applicable Laws. None of the Company or, to the Company’s Knowledge, any of its officers, directors, Representatives or other Company personnel, has been convicted of any crime or engaged in any conduct that has resulted, or would
Annex A-27
Table of Contents
reasonably be expected to result, in debarment or exclusion under applicable Laws, including, without limitation, 21 U.S.C. Section 335a and 42 U.S.C. Section 1320a-7. No claims, actions, proceedings or investigations that would reasonably be expected to result in such a material debarment or exclusion of the Company are pending or, to the Company’s Knowledge, threatened, against the Company or any of its officers, directors, Representatives or other Company personnel.
(n) The Company has not marketed, advertised, distributed, sold, or commercialized any product and is not currently marketing, distributing, selling, or otherwise commercializing any product.
(o) Except as would not, individually or in the aggregate, reasonably be expected to be material to the Company, (x) the Company owns, and has possession of or control over, all of the Company’s personal identifiable information (“PII”) and pre-clinical, clinical and other similar material data and information, including any databases containing any such data and information, and (y) such data and information (i) does not include non-key-coded clinical trial participant information or the means for reversing key coding, (ii) is located at the Company’s premises and in the Company’s systems and is generally available and accessible to the Company and is stored and backed-up on a regular basis, and (iii) will be owned, in the possession and control of, and to the Knowledge of the Company, available for use by, Parent immediately following the Closing Date, free and clear of any restrictions, limitations or obligations. Except as would not, individually or in the aggregate, reasonably be expected to be material to the Company, (A) the Company has obtained all consents and approvals that are necessary to collect, process, use and disclose the PII in its possession, and (B) there is no unauthorized use by the Company or, to the Knowledge of the Company, its Third Party Service Providers, of such PII. The Company maintains commercially reasonable security, disaster recovery and business continuity plans, procedures and facilities, and, if tested, such plans and procedures have been proven effective upon testing in all material respects.
(p) Since January 1, 2018 through the date hereof, there has not occurred any Key Clinical Event. As used in this Section 2.25, “Key Clinical Event” means any action, or any event, fact, circumstance, occurrence or adverse audit or inspection finding that would reasonably be expected to lead to an action, by a Governmental Authority to place a clinical hold order on, or otherwise terminate, suspend or restrict, any ongoing clinical trial conducted, which, in the case of any of the foregoing, would reasonably be expected to materially delay or materially impair submission of the NDA seeking regulatory approval of CPI 613 proposed to be made by the Company (the “Key Product NDA”).
(q) To the Company’s Knowledge, there have been no Serious Adverse Events that have not been described in the final clinical study reports for clinical studies of Product Candidates or otherwise communicated in writing to Parent prior to the Effective Date. For purposes hereof, (i) “Serious Adverse Event” means, with respect to any Product Candidate, any Adverse Experience that results in any of the following outcomes: death, a life threatening Adverse Experience, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant disability or incapacity, a congenital anomaly or birth defect or any other effect that may otherwise jeopardize the patient or clinical study subject or may require intervention to prevent one of the aforementioned outcomes and (ii) “Adverse Experience” means any untoward medical occurrence in a patient or clinical investigation subject administered any Product Candidate, and which does not necessarily have a causal relationship with the treatment for which such Product Candidate is intended to be used, including any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of such Product Candidate, or the worsening in severity of a pre-existing condition after administration of such Product Candidate, whether or not related to such Product Candidate.
2.26 Disclosure. The information supplied by the Company for inclusion in the Registration Statement (including any of the Company Financial Statements) will not, as of the date of the Registration Statement or as of the date such information is prepared or presented, (i) contain any statement that is materially inaccurate or misleading with respect to any material facts, or (ii) omit to state any material fact necessary in order to make such information, in light of the circumstances under which such information will be provided, not false or misleading.
2.27 No Additional Representations. Except for the representations and warranties expressly made by Parent and the Merger Subs in Article 3, the Company acknowledges that none of Parent, the Merger Subs or any other Person makes, and that the Company has not relied upon, any express or implied representation or warranty whatsoever and specifically (but without limiting the foregoing), that none of Parent, the Merger Subs or any of their Representatives makes, nor has the Company relied upon, any representation or warranty with respect to (a) Parent, the Merger Subs
Annex A-28
Table of Contents
or any of their respective Subsidiaries or any of their respective businesses, operations, assets, liabilities, conditions (financial or otherwise), prospects or any other matter relating to Parent, the Merger Subs or any of their respective Subsidiaries or (b) any documentation, forecasts, budgets, projections, estimates or other information (including the accuracy or completeness of, or the reasonableness of the assumptions underlying, such documentation, forecasts, budgets, projections, capitalization information, agreements, estimates or other information) provided by Parent, the Merger Subs or any of their respective Representatives, including in any “data rooms” or management presentations.
3. REPRESENTATIONS AND WARRANTIES OF PARENT AND THE MERGER SUBS
Parent and the Merger Subs represent and warrant to Company as of the date hereof, (a) except to the extent such representations and warranties are specifically made as of a particular date, in which case the Parent and the Merger Subs make the representations and warranties as of such particular date, and except as set forth in (b) the Parent Disclosure Schedule (subject to (i) the qualifications set forth in Section 11.15 (Disclosure Schedule)) or (ii) as disclosed in the Parent SEC Reports filed with the SEC and publicly available on the SEC’s Electronic Data Gathering Analysis and Retrieval system prior to the date of this Agreement (excluding, in each case, any disclosures contained or referenced therein under the captions “Risk Factors,” “Forward-Looking Statements,” “Quantitative and Qualitative Disclosures About Market Risk” and any other disclosures contained or referenced therein of information, factors or risks that are cautionary, predictive or forward-looking in nature), as follows:
3.1 Due Incorporation, Etc. Parent is a corporation duly organized and validly existing under the Laws of Delaware. Merger Sub I is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. Merger Sub II is a limited liability company duly organized, validly existing and in good standing under the Laws of the State of Delaware. Each of the Subsidiaries of Parent is corporation or limited liability company duly organized or formed, validly existing and in good standing under the Laws of the State of Delaware. Parent and each of its Subsidiaries (other than the Company) is qualified to do business as a foreign corporation, and is in good standing, under the Laws of all states where the nature of its business requires such qualification, except where the failure to be so qualified or in such good standing has not had and would not reasonably be expected, individually or in the aggregate, (i) to have a Parent Material Adverse Effect or (ii) to prevent, materially delay or materially impair the ability of Parent to perform its obligations under this Agreement or to consummate the transactions contemplated hereby.
3.2 Capitalization, Etc.
(a) As of May 27, 2021 (the “Parent Capitalization Date”), the authorized Parent Capital Stock consists solely of 35 million shares of Parent Class A Common Stock, 200 million shares of Parent Class B Common Stock. A total of 787,163 shares of Parent Class A Common Stock, 15,984,127 shares of Parent Class B Common Stock and no shares of Parent Preferred Stock are issued and outstanding as of the Parent Capitalization Date. As of the Parent Capitalization Date, Parent has reserved the following shares of Parent Common Stock for issuance to employees, non-employee directors and consultants pursuant to the Parent Equity Plan or otherwise: 689,210 shares are subject to outstanding and unexercised options to purchase Parent Common Stock, 999,547 shares are subject unvested restricted stock awards, and 48,746 shares remain available for issuance thereunder. The Debt of Parent as of the Parent Capitalization Date is listed on Part 3.2(a) of the Parent Disclosure Schedule.
(b) As of the Parent Capitalization Date, except as described in Section 3.2(a) (Capitalization, Etc.), (A) there are no other existing options, warrants, calls, rights (including contingent rights, conversion rights, preemptive rights, co-sale rights, rights of first refusal, convertible securities, subscription rights or other agreements or commitments of any character obligating the Parent to issue any shares or other convertible securities) issued or granted by Parent, and there are no securities of Parent outstanding which upon conversion or exchange would require, the issuance, sale or transfer of any additional shares of capital stock or other equity securities of Parent or other securities convertible into, exchangeable for or evidencing the right to subscribe for or purchase shares of Parent Capital Stock or other equity securities of Parent, (B) there are no obligations, contingent or otherwise, of Parent to (1) repurchase, redeem or otherwise acquire any shares of Parent Capital Stock or (2) to make any material investment in (in the form of a loan, capital contribution or otherwise), or to provide any guarantee (excluding indemnification obligations) with respect to the obligations of, any Person.
(c) As of the Parent Capitalization Date, there are no outstanding restricted shares, stock appreciation rights, phantom stock, contingent value rights, profit participation or similar rights with respect to the Parent.
Annex A-29
Table of Contents
(d) There are no voting trusts, irrevocable proxies or other Contracts or understandings to which Parent, or any holder of the warrants or options to purchase Parent Common Stock is a party or is bound with respect to the voting or consent of any shares of Parent Capital Stock.
(e) All of the outstanding shares of Parent Capital Stock are and have been duly authorized and validly issued, and are fully paid and nonassessable, are not subject to any preemptive rights, purchase options, call options, rights of first refusal or similar rights or any other Liens and have been issued and granted in all material respects in compliance with all applicable securities Laws and other applicable Laws.
(f) The authorized capital stock of Merger Sub I consists solely of 1,000 shares of common stock, par value $0.01 per share, all of which are validly issued and outstanding. The authorized units of Merger Sub II consists solely of 1,000 units, all of which are validly issued and outstanding. All of the issued and outstanding capital stock and units, as applicable, of each the Merger Subs is, and as of the Effective Time shall be, directly owned by Parent.
(g) As of the Parent Capitalization Date, Pharma Holdings LLC is the beneficial owner of the 56% Warrant. Part 3.2(g) of the Parent Disclosure Schedule sets forth each date of exercise of the 56% Warrant, and the number, class and series of shares of Company capital stock issued to Pharma Holdings LLC upon each exercise of the 56% Warrant.
3.3 Authority; Binding Nature of Agreement. Parent and the Merger Subs have all necessary corporate power and authority to enter into and to perform their obligations under this Agreement and any Ancillary Agreements to which they are a party, and the execution, delivery and performance by Parent and the Merger Subs of this Agreement and any Ancillary Agreements to which they are a party have been duly authorized by all necessary action on the part of Parent, the Merger Subs and their respective boards of directors. This Agreement constitutes the legal, valid and binding obligation of Parent and the Merger Subs, enforceable against them in accordance with its terms, subject to (a) Laws of general application relating to bankruptcy, insolvency and the relief of debtors and (b) rules of Law governing specific performance, injunctive relief and other equitable remedies. The Parent Board, acting upon the Special Committee Recommendation, has (x) (A) determined that it is fair to and in the best interests of the Parent and the holders of the Parent Common Stock to enter into this Agreement and declared this Agreement and the transactions contemplated by this Agreement advisable, (B) adopted this Agreement and approved the execution, delivery and performance of this Agreement by the Parent and the consummation of the Mergers and the other transactions contemplated by this Agreement and (C) resolved to recommend adoption of this Agreement and approval of the Mergers and the other transactions contemplated by this Agreement by the holders of Parent Common Stock (the “Parent Recommendation”) and (y) directed that this Agreement be submitted to the holders of Parent Common Stock entitled to vote for adoption of this Agreement and approval of the Mergers and the other transactions contemplated by this Agreement. The Special Committee has (i) been duly established, (ii) received an opinion of Houlihan Lokey Capital, Inc., the Special Committee’s financial advisor, to the effect that, as of the date of such opinion and subject to the limitations, qualifications and assumptions set forth therein, the aggregate consideration to be paid by the Parent (comprised of the Merger Shares to be issued by Parent) in the Merger is fair to Parent, from a financial point of view, and as of the date this Agreement, such opinion has not been withdrawn, revoked or modified, (iii) determined that it is fair to and in the best interests of the Parent and the holders of the Parent Common Stock to enter into this Agreement and declared this Agreement and the transactions contemplated by this Agreement advisable, and (iv) recommended to the Parent Board that the Parent Board make the Parent Recommendation.
3.4 Non-Contravention; Consents. The execution and delivery of this Agreement or any of the Ancillary Agreements by Parent and the Merger Subs and the consummation by Parent and the Merger Subs of the Contemplated Transactions will not cause: (a) a violation of any of the provisions of the certificate of incorporation or bylaws of Parent or Merger Sub I, or the certificate of formation or the limited liability company agreement of Merger Sub II, (b) a violation by Parent or the Merger Subs of any Law applicable to Parent or the Merger Subs, except as would not reasonably be expected to materially and adversely impact Parent’s or the Merger Subs’ ability to consummate the Contemplated Transactions, or (c) a default on the part of Parent or the Merger Subs under any material Contract of Parent or the Merger Subs, except as would not reasonably be expected to materially and adversely impact Parent’s or the Merger Subs’ ability to consummate the Contemplated Transactions. Except as may be required by the (i) the filing with the SEC of the Registration Statement and the declaration of effectiveness of the Registration Statement by the SEC, and other filings required under, and compliance with other applicable requirements of, the Exchange Act as may be required in connection with this Agreement and the Contemplated Transactions; (ii) such filings and approvals as may be required under Securities Act of 1933, as amended, or the rules and regulations of the NYSE, (iii) the DGCL,
Annex A-30
Table of Contents
the DLLCA or governmental regulation, none of Parent, Merger Sub I or Merger Sub II is required to obtain any Consent from any Governmental Body or party to a material Contract of Parent, Merger Sub I or Merger Sub II at any time prior to the Closing in connection with the execution and delivery of this Agreement or the consummation by Parent and the Merger Subs of the Merger.
3.5 Absence of Certain Changes. Except as expressly contemplated by this Agreement, between January 31, 2021 and the date of this Agreement (a) no event or series of related events that has had or would reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect; (b) no event or action has occurred that would require the consent of the Company pursuant to Section 4.3 (Conduct of the Business of Parent) if such event or action occurred during the Pre-Closing Period; or (c) except in connection with the Contemplated Transactions, Parent and its Subsidiaries have used or held its assets and properties for use and has operated and conducted its business in all material respects, in the Ordinary Course of Business.
3.6 Legal Proceedings. As of the date of this Agreement, there is no Legal Proceeding pending (or, to the knowledge of Parent or the Merger Subs, being threatened) against Parent or the Merger Subs (a) challenging the Merger or (b) that would be material to Parent and its Subsidiaries, taken as a whole.
3.7 SEC Reports, Financial Statements.
(a) Parent and its Subsidiaries have timely filed or furnished each form, report, schedule, registration statement, definitive proxy statement and other document (together with all amendments thereof and supplements thereto) required to be filed or furnished by Parent or any of its Subsidiaries pursuant to the Securities Act or the Exchange Act with the SEC since January 1, 2020 (as such documents have since the time of their filing been amended or supplemented, the “Parent SEC Reports”). As of their respective dates, after giving effect to any amendments or supplements thereto, the Parent SEC Reports (A) complied in all material respects with the requirements of the Securities Act and the Exchange Act, as applicable, and, to the extent applicable, Sarbanes-Oxley Act of 2002 (“SOX”), and (B) did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading, provided, however, that no representation is made as to the accuracy of any financial projections or forward-looking statements or the completeness of any information filed or furnished by Parent to the SEC solely for the purposes of complying with Regulation FD promulgated under the Exchange Act. As of the date of this Agreement, there are no material outstanding or unresolved comments in comment letters from the SEC staff with respect to any of the Parent SEC Reports.
(b) Each of the principal executive officer of Parent and the principal financial officer of Parent (or each former principal executive officer of Parent and each former principal financial officer of Parent, as applicable) has made all applicable certifications required by Rule 13a-14 or 15d-14 under the Exchange Act and Sections 302 and 906 of SOX with respect to the Parent SEC Reports. For purposes of the preceding sentence, “chief executive officer” and “chief financial officer” shall have the meanings given to such terms in SOX.
(c) The audited consolidated financial statements and unaudited interim consolidated financial statements (including, in each case, the notes, if any, thereto) included in the Parent SEC Reports (the “Parent Financial Statements”) complied in all material respects with the published rules and regulations of the SEC with respect thereto in effect at the time of filing or furnishing the applicable Parent SEC Report, were prepared in accordance with GAAP, applied on a consistent basis during the periods involved (except as may be indicated therein or in the notes thereto and except with respect to unaudited statements as permitted by the SEC on Form 8-K, Form 10-Q or any successor or like form under the Exchange Act) and fairly present (subject, in the case of the unaudited interim financial statements, to normal, recurring year end audit adjustments that would not be, individually or in the aggregate, materially adverse to Parent) in all material respects the consolidated financial position of Parent and its consolidated subsidiaries, as of the respective dates thereof, and the consolidated results of their operations and cash flows for the respective periods then ended.
(d) Parent maintains a system of internal control over financial reporting (as defined in Rules 13a–15(f) and 15d–15(f) of the Exchange Act) sufficient to provide reasonable assurances regarding the reliability of financial reporting. Parent (A) maintains disclosure controls and procedures (as defined in Rules 13a–15(e) and 15d–15(e) of the Exchange Act) to provide reasonable assurance that all information required to be disclosed by Parent in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods
Annex A-31
Table of Contents
specified in the SEC’s rules and forms and is accumulated and communicated to Parent’s management as appropriate to allow timely decisions regarding required disclosure, and (B) has disclosed, based on its most recent evaluation of internal control over financial reporting, to Parent’s outside auditors and the audit committee of the board of directors of Parent (x) all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting that are reasonably likely to adversely affect Parent’s ability to record, process, summarize and report financial information and(y) any fraud, whether or not material, that involves management or other employees who have a significant role in Parent’s internal control over financial reporting, and the information described in the foregoing clauses (x) and (y) has been disclosed to the Company prior to the date of this Agreement. Neither Parent nor, to the knowledge of Parent, Parent’s independent registered accountant has received or otherwise had or obtained knowledge of any material complaint, allegation, assertion or claim, whether written or oral, in each case, regarding deficient accounting or auditing practices, procedures, methodologies or methods of Parent or its internal accounting controls or any material inaccuracy in the Parent Financial Statements.
3.8 Liabilities. Parent has no liabilities required by GAAP to be reflected in the audited consolidated balance sheet or footnotes thereto as of July 31, 2020 (the “Parent Audited Balance Sheet”) included in the Parent Financial Statements, other than: (a) those set forth on the face of the Parent Audited Balance Sheet; (b) those incurred in the Ordinary Course of Business since the date of the Parent Audited Balance Sheet (none of which arose out of, in connection with or as a result of any breach of Contract, tort, infringement of Intellectual Property or violation of Law, and none of which are, individually or in the aggregate, material to Parent’s business as presently operated); (c) those incurred pursuant to performance of this Agreement; and (d) those that are not, individually or in the aggregate, material to Parent and its Subsidiaries, taken as a whole.
3.9 Compliance with Laws. Parent and the Merger Subs are in material compliance with, and at all times during the past five years have been in material compliance with, applicable Laws, including those relating to employment and the listing and other rules and regulations of NYSE, and as of the date of this Agreement, neither Parent nor the Merger Subs has received any written notices of any violation with respect to such Laws.
3.10 Subsidiaries; Merger Subs. Merger Sub I and Merger Sub II (a) were formed solely for the purpose of engaging in the Contemplated Transactions and are wholly owned subsidiaries of Parent, (b) have engaged in no other business activities and (c) have conducted their operations only as contemplated by this Agreement. Parent has no Subsidiaries other than Merger Sub I, Merger Sub II and the Subsidiaries set forth in Note 1 to the financial statement included in the Parent’s Quarterly Report on Form 10-Q for the fiscal quarter ended January 31, 2021.
3.11 Valid Issuance. The Parent Class B Common Stock to be issued as Merger Shares pursuant to the terms hereof, when issued as provided in and pursuant to the terms of this Agreement, will be duly authorized and validly issued, fully paid and nonassessable, and (other than restrictions under applicable securities Laws, or restrictions created by any Company Stockholder) will be free of restrictions on transfer.
3.12 Financing. Assuming satisfaction of the condition contained in Section 6.9, Parent, Merger Sub I, Merger Sub II will have on the Closing Date, sufficient cash, available lines of credit or other sources of immediately available funds to enable them to make all payments required to be made pursuant to the terms of this Agreement.
3.13 Takeover Statutes. Assuming that the Company Stockholder Approval, including the Company Minority Approval, has been obtained, Parent has taken all action necessary to authorize and approve this Agreement, the Support Agreements, the Voting Agreements and the transactions contemplated hereby and thereby (including the Parent Share Issuance) under Section 203 of the DGCL and, accordingly, none of Section 203 of the DGCL, any other Takeover Statute that is applicable to Parent or any anti-takeover provision in Parent’s certificate of incorporation or bylaws prohibits the execution, delivery and performance of this Agreement, the Support Agreements, the Voting Agreements or the transactions contemplated hereby or thereby (including the Parent Share Issuance).
3.14 Ownership of Capital Stock of the Company. As of the close of business on May 31, 2021, Parent and its Subsidiaries (including for purposes of this provision, CS Pharma LLC and Pharma Holdings LLC) and Howard Jonas collectively (a) beneficially own 1,959,235 shares of Company Common Stock, no shares of Company Series A Preferred Stock, no shares of Company Series B-1 Preferred Stock, no shares of Company Series B-2 Preferred Stock, no shares of Company Series B-3 Preferred Stock, no shares of Company Series B-4 Preferred Stock, 1,081,504 shares of Company Series C Preferred Stock issuable to Howard Jonas, Debbie Jonas and the Jonas Foundation upon conversion of its Convertible Note and 60,673,088 shares of Company Series D Preferred Stock and (b) based upon
Annex A-32
Table of Contents
the terms and conditions of this Agreement, have the right to acquire, (I) through the exercise of the 56% Warrant, 100,874,073 shares of Series D Preferred Stock, and (II) as lenders under the Line of Credit, 14,925,035 shares of Company Common Stock.
3.15 Tax Matters. Parent has not taken any action, nor to the knowledge of Parent are there any facts or circumstances not set forth in this Agreement, that could reasonably be expected to prevent the Mergers from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code. Merger Sub II is, and has been since formation, disregarded as an entity (within the meaning of Treasury Regulations Section 301.7701-3) separate from Parent for U.S. federal income Tax purposes, and no election has been made or will be made, nor has any action been taken or will be taken, to treat Merger Sub II as a corporation for U.S. federal income Tax purposes.
3.16 No Additional Representations. Except for the representations and warranties expressly made by the Company in Article 3, each of Parent and the Merger Subs acknowledges that neither the Company nor any other Person makes, and that none of Parent or Merger Subs have relied upon, any express or implied representation or warranty whatsoever and specifically (but without limiting the foregoing), that neither the Company nor any Representative of the Company makes, nor has Parent or Merger Subs relied upon, any representation or warranty with respect to (a) the Company or its Subsidiaries or any of their respective businesses, operations, assets, liabilities, conditions (financial or otherwise), prospects or any other matter relating to the Company or its Subsidiaries or (b) any documentation, forecasts, budgets, projections, estimates or other information (including the accuracy or completeness of, or the reasonableness of the assumptions underlying, such documentation, forecasts, budgets, projections, estimates or other information) provided by the Company or any Representative of the Company, including in any “data rooms” or management presentations.
4. CERTAIN COVENANTS
4.1 Access. During the period from the date of this Agreement through the earlier of the Effective Time or the termination of this Agreement pursuant to Section 10.1 (Termination) (the “Pre-Closing Period”), and upon reasonable advance notice to the Company, the Company shall (a) provide Parent and Parent’s Representatives with reasonable access during normal business hours to the Company’s personnel, facilities, properties, the existing books, records, Contracts, Company Tax Returns, Company Plans, work papers and other documents and information relating to the Company for the purpose of enabling Parent to verify the accuracy of the Company’s representations and warranties contained in this Agreement or as Parent may otherwise reasonably request and (b) promptly provide Parent and Parent’s Representatives with all reasonably requested information regarding the business of the Company, including copies of the existing books, records, Contracts, Company Tax Returns, Company Plans, work papers and other documents and information relating to the Company, in each of cases (a) and (b), as Parent may reasonably request; provided, however, that any such access shall be conducted at Parent’s expense and in such a manner as to maintain compliance with the confidentiality provisions of this Agreement and of the Contemplated Transactions in accordance with the terms hereof and thereof and not to unduly and materially interfere with the normal operation of the business of the Company. Nothing herein shall require the Company to disclose any information to Parent if such disclosure would, in the Company’s reasonable judgment (based on the advice of outside counsel) (a) waive any attorney-client or other legal privilege (so long as the Company has reasonably cooperated with Parent to permit such inspection of or to disclose such information on a basis that does not result in the loss of such privilege, including disclosing information subject to execution of a joint defense agreement in customary form or limiting disclosure to external counsel for Parent) or (b) contravene any applicable Law or binding agreement entered into prior to the date of this Agreement (including any confidentiality agreement to which the Company is a party, so long as the Company has used commercially reasonable efforts to make appropriate substitute arrangements to permit reasonable disclosure not in violation of such Law, agreement or duty).
4.2 Conduct of the Business of the Company. During the Pre-Closing Period, except (w) as set forth in Schedule 4.2, (x) to the extent necessary to comply with the Company’s obligations under this Agreement, (y) as necessary to ensure that the Company complies with applicable Laws and obligations under any Material Contract, or (z) with Parent’s consent (which shall not be unreasonably withheld, conditioned or delayed): (i) the Company shall operate in the Ordinary Course of Business and in compliance with applicable Law; (ii) the Company shall use commercially reasonable efforts to (A) preserve substantially intact its present business organization, (B) preserve its material relationships with suppliers, distributors, licensors, licensees and others to whom the Company has contractual obligations, (C) prosecute and maintain the Patents licensed by the Company and other material Company Registered Intellectual Property (including any Patents), and (D) file all Company Tax Returns and pay all Taxes when
Annex A-33
Table of Contents
due (except for Taxes being contested in good faith in appropriate proceedings for which adequate reserves have been have been established in accordance with GAAP, with respect to which Parent has been notified in advance in writing); and (iii) except as set forth in Schedule 4.2, the Company shall not:
(a) change or amend the Company Charter, the Certificates of Designation or the Company’s bylaws or authorize or propose the same;
(b) directly or indirectly split, combine or reclassify any of its capital stock (except in connection with the conversion of Company Preferred Stock to Company Common Stock, or the exercise of any Company Warrants or Company Options); issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock; declare, set aside, or pay any dividend or make any distribution (whether in cash or in kind) with respect to any of its capital stock, membership interest or other equity interests (as applicable) or redeem, purchase, or otherwise acquire, directly or indirectly, any of its capital stock, membership interests or other equity interests (as applicable);
(c) issue, deliver, transfer or sell, or authorize to issue, deliver, transfer or sell, any shares of Company Capital Stock or securities convertible into, or subscriptions, rights, calls, conversion rights, warrants or options to acquire, or other agreements or commitments of any character obligating it to issue any such shares or other convertible securities, or authorize or propose any change in its equity capitalization or capital structure; provided, however, that (i) the Company may issue shares of Company Common Stock in connection with the exercise of Company Options, conversion of Company Preferred Stock to Company Common Stock or other rights for Company Common Stock, in each case to the extent outstanding as of the date of this Agreement, (ii) the Company may issue shares of Company Capital Stock in connection with the exercise of, and in accordance with the terms of, any Company Warrants outstanding as of the date of this Agreement, and (iii) the Company may issue shares of Company Common Stock in respect of the Contingent Rights in such amounts as set forth on Part 4.2(c) of the Company Disclosure Schedules;
(d) enter into or adopt any plan or agreement of complete or partial liquidation, restructuring, receivership, recapitalization or dissolution, or file a voluntary petition in bankruptcy or commence a voluntary legal procedure for reorganization, arrangement, adjustment, release or composition of Debt in bankruptcy or other similar Laws now or hereafter in effect;
(e) incur any Debt for borrowed money, or guarantee any such Debt, or issue or sell any debt securities or guarantee any debt securities of other Persons;
(f) make any capital expenditures, capital additions or capital improvements, in excess of $100,000;
(g) acquire or agree to acquire by merging with, or by purchasing a portion of the stock or assets of, or by any other manner, any business or any Entity;
(h) (A) initiate any new line of business, (B) make any investment in or advance, loan or capital contribution to any Person (other than business-related advances to its employees in the Ordinary Course of Business), (C) form or acquire any Subsidiary that is not wholly-owned by the Company or any of its wholly-owned Subsidiaries or (D) otherwise acquire or agree to acquire any securities or assets of a Third Party that are material, individually or in the aggregate, to the Company;
(i) terminate, cancel, amend, waive, modify, fail to use commercially reasonable efforts to comply with, maintain or renew any material Permit;
(j) sell, license, lease, assign or otherwise dispose of or create or incur any Lien on, directly or indirectly, any tangible properties or assets of the Company or any of its Subsidiaries which are material to the Company as a whole, other than (A) sales of inventory, of products or services, or obsolete equipment, all in the ordinary course of business, (B) sales, licenses, leases or transfers that are pursuant to Contracts in effect on the date hereof or (C) incurrence of Permitted Liens;
(k) (i) sell, license, lease, assign or otherwise dispose of or create or incur any Lien on, directly or indirectly, any material Company Owned Intellectual Property, or (ii) acquire, in-license or otherwise obtain any right, title or interest in or to any pending or issued Patents, inventions, patent disclosures or other Intellectual Property material to the Company from any other Person (other than, with respect to each of clauses (i) and (ii), non-exclusive
Annex A-34
Table of Contents
licenses or other non-exclusive grants of rights entered into in the Ordinary Course of Business, and, as applicable, on terms materially consistent with the Company’s applicable form agreement(s), provided that any Intellectual Property arising from any such form agreement, for such agreements entered into in the Pre-Closing Period, will be solely owned by the Company to the extent permitted under applicable Law);
(l) enter into any Material Contract, amend or modify in any material respect any Material Contract or terminate any Material Contract or waive any material right of the Company or any of its Subsidiaries under any Material Contract, including without limitation, take any action with respect to the Altira Royalty Agreement;
(m) enter into any agreement or arrangement that limits or otherwise restricts the Company or any of its Subsidiaries from engaging or competing in any line of business, in any location or with any Person, or would purport to limit, after the Effective Time, Parent or any of its Subsidiaries;
(n) make, revoke, or change any material election in respect of Taxes, change an annual Tax accounting period, adopt or change any accounting method in respect of Taxes, file any amended Company Tax Return, enter into any Tax allocation, sharing or indemnity agreement or any agreement with respect to Taxes, settle any claim or assessment in respect of Taxes, surrender or abandon any right to claim a material refund of Taxes, or consent to any extension or waiver of the limitation period applicable to any material claim or assessment in respect of Taxes;
(o) except in each case as required by the terms of any Company Plan as in effect on the date hereof or as required by applicable Law, (i) adopt, establish, enter into, amend or terminate any Company Equity Plan, Company Plan or any plan, agreement, program, policy, trust, fund or other arrangement that would be a Company Plan if it were in existence as of the date of this Agreement (except for renewals in the Ordinary Course of Business), (ii) increase the compensation or benefits (including severance, termination, retention and change of control compensation or benefits) of, or grant any bonus or other incentive compensation to, any Company Service Provider (except for increases in base salary in the Ordinary Course of Business with respect to Company Service Providers with a total annual base salary under $200,000, (iii) grant any severance or termination pay to any Company Service Provider, (iv) terminate the employment or engagement of any Company Service Provider other than for cause, (v) hire or engage any new Company Service Provider (except in the Ordinary Course of Business with respect to Company Service Providers with a total annual base salary under $200,000), or (vi) take any action to accelerate the vesting, funding or payment of any compensation or benefit under any Company Equity Plan (except as provided in Section 5.8 (Company Options)) or other Company Plan or other Contract.
(p) terminate or allow to lapse any of the Insurance Policies;
(q) waive or release in writing, assign, commence, settle or agree to settle any Legal Proceeding, other than waivers, releases, compromises or settlements (i) in the Ordinary Course of Business (ii) that involve only the payment of monetary damages not in excess of $100,000 in the aggregate and (iii) that do not include the imposition of equitable relief on, or the admission of wrongdoing by, the Company;
(r) make any loans, advances or capital contributions to, or investments in, any other Person, or form or acquire any Subsidiary that is not wholly-owned by the Company or any of its wholly-owned Subsidiaries;
(s) commence, terminate or materially modify any clinical trial;
(t) terminate any executive employee of the Company or materially modify the terms and conditions of employment of any executive employee of the Company; or
(u) agree or commit to take any of the actions described in clauses (a) through (t) of this Section 4.2 (Conduct of the Business of the Company).
4.3 Conduct of the Business of Parent. During the Pre-Closing Period, except (w) as set forth in Schedule 4.3, (x) to the extent necessary to comply with Parent’s obligations under this Agreement, (y) as necessary to ensure that the Parent complies with applicable Laws, or (z) with the Company’s consent (which shall not be unreasonably withheld, conditioned or delayed), Parent shall:
(a) not change or amend the Parent Charter or Parent’s bylaws, or authorize or propose the same;
Annex A-35
Table of Contents
(b) not enter into or adopt any plan or agreement of complete or partial liquidation, restructuring, receivership, recapitalization or dissolution, or file a voluntary petition in bankruptcy or commence a voluntary legal procedure for reorganization, arrangement, adjustment, release or composition of Debt in bankruptcy or other similar Laws now or hereafter in effect;
(c) use its commercially reasonable efforts to conduct its business in the ordinary course of business consistent with past practice in all material respects and, to the extent consistent therewith, Parent shall use its commercially reasonable efforts to preserve the business organizations of Parent substantially intact and maintain existing relations and goodwill with Governmental Bodies, customers, suppliers, production companies, distributors, licensees, licensors, creditors, lessors, employees and business associates and others having material business dealings with Parent, as applicable, and keep available the services of its present employees and agents; and
(d) not agree or commit to take any of the actions described in clauses above in this Section 4.3 (Conduct of the Business of Parent).
Without limiting Section 4.3(c), and in furtherance of, the foregoing, from the execution of this Agreement until the Effective Time, Parent (i) shall not, and shall not cause the Company to, terminate or materially alter the terms and conditions of employment of any executive employee of the Company without the prior consent of at least eighty percent (80%) of the Company Board or (ii) remove any director or vote for (or consent to) any person for appointment or election as a director to the Company Board without the prior consent of eighty percent (80%) of the Company Board.
4.4 No Solicitation.
(a) The Company shall not, and shall cause its Subsidiaries and Representatives not to, directly or indirectly: (i) solicit, initiate or knowingly encourage, knowingly induce or knowingly facilitate the communication, making, submission or announcement of any Takeover Proposal or Takeover Inquiry or take any action that could reasonably be expected to lead to a Takeover Proposal or Takeover Inquiry; (ii) furnish any non-public information regarding the Company to any Person in connection with or in response to a Takeover Proposal or Takeover Inquiry (other than to inform any Person of the existence of the provisions in this Section 4.4 (No Solicitation)); (iii) engage in discussions or negotiations with any Person (other than to inform any Person of the existence of the provisions in this Section 4.4 (No Solicitation)) with respect to any Takeover Proposal or Takeover Inquiry; (iv) approve or recommend any Takeover Proposal; execute or enter into any binding or nonbinding letter of intent, memorandum of understanding or similar understanding or Contract contemplating or otherwise relating to any Takeover; or (v) resolve or agree to do any of the foregoing.
(b) The Company shall immediately cease and cause to be terminated any discussions, negotiations and communications with any Person that relate to any Takeover Proposal or Takeover Inquiry that were occurring as of the date of this Agreement.
(c) Promptly after the date of this Agreement, the Company shall: (i) request each Person that has received confidential information from the Company or any of its Representatives on behalf of the Company or its Representatives at any time during the past 12 months pursuant to a confidentiality or similar agreement in connection with such Person’s consideration of a possible Takeover Proposal to return or destroy all confidential information previously furnished to such Person by or on behalf of any of the Company to the extent the Company has the right to make such request pursuant to a confidentiality or similar agreement with such Person; and (ii) except as otherwise permitted by Section 4.4(a) (No Solicitation), prohibit any Person from having access to any physical or electronic data room relating to any possible Takeover Proposal or Takeover Inquiry.
4.5 Takeover Statutes. If any state takeover statute or similar Law shall become applicable to the Contemplated Transactions, each of the Company and Parent and their respective boards of directors shall grant such approvals and take such actions as are reasonably necessary so that the Contemplated Transactions may be consummated as promptly as practicable on the terms contemplated hereby and otherwise act to eliminate or minimize the effects of such statute or regulation on the Contemplated Transactions.
Annex A-36
Table of Contents
4.6 Certain Tax Matters. None of the Company, Parent, Merger Sub I, Merger Sub II, or the Surviving Company shall take, agree to take or fail to take any action that would reasonably be expected to prevent or impede the Mergers from qualifying for the Intended Tax Treatment. The Parties will prepare and file, and cause their Affiliates and agents to prepare and file, all Tax Returns consistent with the Intended Tax Treatment and will not take, and will cause their Affiliates and agents not to take, any inconsistent position on any Tax Return or during the course of any audit, litigation or other proceeding with respect to Taxes, except as otherwise required by a change in applicable Law after the date hereof or by a Governmental Authority pursuant to a determination within the meaning of Section 1313(a) of the Code. Each Party shall cooperate in good faith with reasonable requests made by the other Parties to document and support the Intended Tax Treatment, including in connection with the preparation and filing of the Registration Statement. Such cooperation shall include, if applicable, providing a certificate executed by an officer of the applicable Party with applicable covenants, representations and warranties reasonably requested by another Party’s tax advisors in connection with the delivery of an opinion regarding the Intended Tax Treatment.
5. ADDITIONAL COVENANTS OF THE PARTIES
5.1 Registration Statement.
(a) As promptly as practicable after the date of this Agreement (and in any event within the later of (i) sixty (60) days after the date of this Agreement; provided that as of such date the Company financials required to be included in the Registration Statement have been completed and approved by the Company’s auditors or (ii) fifteen (15) days from the date on which the Company financials required to be included in the Registration Statement have been completed and approved by the Company’s auditors), the Parties shall prepare, and Parent shall cause to be filed with the SEC, a registration statement on Form S-4 to register under the Securities Act the offer and sale of the Merger Shares pursuant to the Merger, which shall include a prospectus and a proxy statement relating to the Parent Stockholder Approval and the Company Stockholder Approval (as amended from time to time, the “Registration Statement”). Parent covenants and agrees that the Registration Statement will not, at the time that the Registration Statement or any amendments or supplements thereto is filed with the SEC or first mailed to Parent Stockholders contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The Company covenants and agrees that the information provided by the Company to Parent for inclusion in the Registration Statement (including the Company Financial Statements) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make such information not misleading. Notwithstanding the foregoing, Parent makes no covenant, representation or warranty with respect to statements made in the Registration Statement, if any, based on information provided by the Company or any of its Representatives specifically for inclusion therein and the Company makes no covenant, representation or warranty with respect to statements made in the Registration Statement, if any, other than with respect to the information provided by the Company or any of its Representatives for inclusion therein. The Company and its legal counsel shall be given reasonable opportunity to review and comment on the Registration Statement, including all amendments and supplements thereto, prior to the filing thereof with the SEC, and on the response to any comments of the SEC on the Registration Statement, prior to the filing thereof with the SEC. Parent shall use reasonable efforts to cause the Registration Statement to comply with the applicable rules and regulations promulgated by the SEC, to respond promptly to any comments of the SEC or its staff and to have the Registration Statement declared effective under the Securities Act as promptly as practicable after it is filed with the SEC. Each Party shall promptly furnish to the other Party all information concerning such Party and such Party’s Affiliates and such Party’s stockholders that may be required or reasonably requested in connection with any action contemplated by this Section 5.1 (Registration Statement). If Parent or the Company become aware of any event or information that, pursuant to the Securities Act or the Exchange Act, should be disclosed in an amendment or supplement to the Registration Statement, then such Party, as the case may be, shall promptly inform the other Parties thereof and shall cooperate with such other Parties in filing such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to Parent’s stockholders. No filing of, or amendment or supplement to, the Registration Statement will be made by Parent, without the prior written consent of the Company, which shall not be unreasonably withheld, conditioned or delayed. The Company and Parent shall each use commercially reasonable efforts to cause the Registration Statement to comply with applicable federal and state securities Laws requirements and the rules and regulations of the NYSE.
Annex A-37
Table of Contents
(b) The Parties shall reasonably cooperate with each other and provide, and shall use reasonable best efforts to cause their respective Representatives to provide, the other Party and its Representatives, with all true, correct and complete information regarding such Party that is required by Law to be included in the Registration Statement or reasonably requested by the other Party to be included in the Registration Statement. If at any time before the Effective Time the information provided in the Registration Statement has or will become “stale” and new information should, as determined by Parent acting reasonably, be disclosed in an amendment or supplement to the Registration Statement, then Parent shall promptly inform the Company thereof and each such Party shall cooperate with one another, and shall use reasonable best efforts to cause their accounting and other outside professionals to so cooperate, (x) in providing the financial reporting necessary for such filing and (y) in filing such amendment or supplement with the SEC (and, if related to the proxy statement, mailing such amendment or supplement to the Parent stockholders).
5.2 Stockholder Consent or Approval; Required Stockholder Votes.
(a) Subject to fiduciary obligations under applicable Law, Parent, acting through the Parent Board (or a committee thereof), shall, as promptly as practicable (and in any event within twenty-five (25) Business Days) after the Registration Statement has been declared effective, take all action necessary, including under the DGCL, to duly call, give notice of, convene and hold a meeting of its stockholders for the approval of the issuance of the Parent Class B Common Stock issuable pursuant to the Merger and Parent Minority Approval (the “Parent Stockholder Approval”) and the other matters contemplated by this Agreement (including any adjournment, recess or postponement thereof, the “Parent Meeting”) and shall not postpone, recess or adjourn such Parent Meeting, provided, however, for the avoidance of doubt, the Parent may postpone or adjourn the Parent Meeting: (A) with the written consent of the Company; (B) for the absence of a quorum; (C) to the extent necessary to ensure that any required supplement or amendment to the Registration Statement is provided to the holders of Parent Common Stock within a reasonable period of time in advance of the Company Meeting; (D) to allow reasonable additional time to solicit additional proxies; or (E) if required by Law. The Parent shall recommend approval and adoption of the Parent Stockholder Approval, including approval of this Agreement and the Contemplated Transactions by the Parent’s stockholders in the Registration Statement and otherwise comply with all Laws applicable to the Parent Meeting. Parent shall use its reasonable best efforts to (i) solicit from its stockholders proxies in favor of (it being understood that a proxy card will be deemed “in favor of” a matter to be acted upon by the Parent’s stockholders if it provides the stockholder with the ability to either vote for, vote against or abstain from voting on such matter) the adoption of this Agreement and take all other actions reasonably necessary or advisable to secure the approval and adoption of this Agreement and the Contemplated Transactions by the Parent’s stockholders and (ii) to solicit the Parent Minority Approval. Parent hereby agrees to vote for the adoption of the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Company in the form of Exhibit F hereto prior to the Mergers.
(b) Subject to fiduciary obligations under applicable Law, the Company, acting through the Company Board (or a committee thereof), shall, as promptly as practicable (and in any event within twenty-five (25) Business Days) after the Registration Statement has been declared effective, take all action necessary, including under the DGCL, to solicit the Company Stockholder Consent from, or duly call, give notice of, convene and hold a meeting of its stockholders for the Company Minority Approval and approval of the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Company as contemplated by Section 6.8 (Charter Amendment) below (the “Company Stockholder Approval”) and the other matters contemplated by this Agreement (including any adjournment, recess or postponement thereof, the “Company Meeting”) and shall not postpone, recess or adjourn such Company Meeting, provided, however, for the avoidance of doubt, the Company may postpone or adjourn the Company Meeting: (A) with the consent of the Parent; (B) for the absence of a quorum; (C) to the extent necessary to ensure that any required supplement or amendment to the Registration Statement is provided to the holders of Company Capital Stock within a reasonable period of time in advance of the Company Meeting; (D) to allow reasonable additional time to solicit additional proxies; or (E) if required by Law. The Company shall recommend approval and adoption of this Agreement and the Contemplated Transactions by the Company’s stockholders and otherwise comply with all Laws applicable to the Company Meeting. The Company shall use its reasonable best efforts to (i) solicit from its stockholders proxies in favor of (it being understood that a proxy card will be deemed “in favor of” a matter to be acted upon by the Company’s stockholders if it provides the stockholder with the ability to either vote for, vote against or abstain from voting on such matter) the adoption of this Agreement and take all other actions reasonably necessary or advisable to secure the approval and adoption of this Agreement and the Contemplated Transactions by the Company’s stockholders and (ii) to solicit the Company Minority Approval.
Annex A-38
Table of Contents
(c) In the event that the Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a stockholder meeting prior to the termination of this Agreement, Parent hereby covenants and agrees (and covenants and agrees to cause its subsidiaries and Affiliates to):
(i) to vote for all amendments to the Certificate of Incorporation of the Company necessary or desirable and approved by the Company Board to effect a public offering of the Company Common Stock pursuant to the Securities Act of 1933, as amended, (the “IPO”), including without limitation the increase of the authorized shares of Company Common Stock and reverse stock-splits, as applicable;
(ii) immediately prior to the IPO and, subject to the requirements under the Certificate of Incorporation of the Company, contingent upon the conversion of all other outstanding Company Preferred Stock to Company Common Stock (other than Preferred Stock held by Parent or its Affiliates), Parent shall convert all Company Preferred Stock (including Preferred Stock issuable upon exercise of Company Warrants or other rights) into Company Common Stock in accordance with the applicable conversion terms and conditions applicable to the conversion and conversion ratio of the Company Preferred Stock;
(iii) to not, without the prior written consent of the managing underwriter for the IPO and solely to the same extent as all officers, directors, holders of in excess of 3% of the outstanding Company Capital Stock on a fully diluted basis are so bound on terms equivalent to those of this clause (iii), during the period commencing on the date of the final prospectus relating to the Company’s IPO and ending on the date specified by the Company and the managing underwriter (such period not to exceed one hundred eighty (l80) days) (x) lend, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of Company Common Stock (or securities convertible into Company Common Stock) held immediately prior to the effectiveness of the registration statement for the IPO, or (y) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Company Common Stock (or securities convertible into Company Common Stock), whether any such transaction described in subclauses (x) or (y) above is to be settled by delivery of securities, in cash or otherwise.
(d) In the event that the Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a stockholder meeting prior to the termination of this Agreement, Parent agrees (and covenants and agrees to cause its subsidiaries and Affiliates to): (i) to execute such agreements as may be reasonably requested by the underwriters in the IPO that are consistent with Section 5.2(c) above or that are necessary to give further effect thereto; and (ii) that, in order to enforce the foregoing covenants in Section 5.2(c), the Company may impose stop-transfer instructions with respect to the shares of Company Common Stock of Parent (and transferees and assignees thereof) until the end of the lock-up period described in Section 5.2(c)(iii). Parent further hereby constitutes and appoints as the proxies of Parent and hereby grants a power of attorney to the Chief Executive Officer of the Company and designees with full power of substitution, with respect to the matters set forth in Section 5.2(c) above and hereby authorizes each of them to represent and vote in favor of the matters set forth in Section 5.2(c) above. Each of the proxy and power of attorney granted pursuant to this Section 5.2(d) is given in consideration of the agreements and covenants of the Company and the parties in connection with the transactions contemplated by this Agreement and, as such, each is coupled with an interest and shall be irrevocable following any termination of this Agreement. Parent hereby revokes any and all previous proxies or powers of attorney with respect to the Company Common Stock and shall not hereafter purport to grant any other proxy or power of attorney with respect to any of the Company Common Stock, deposit any of the Company Common Stock into a voting trust or enter into any agreement (other than this Agreement), arrangement or understanding with any person, directly or indirectly, to vote, grant any proxy or give instructions with respect to the voting of any of the Company Common Stock, in each case, with respect to any of the matters set forth in Section 5.2(c) above.
(e) In the event that the Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a stockholder meeting prior to the termination of this Agreement, the Company agrees that Parent and Howard Jonas or any Affiliate of any of the foregoing who holds rights, directly or indirectly, under the 56% Warrant shall be permitted (prior to effecting the conversion of its Company Preferred Stock in accordance with Section 5.2(c)(ii)) to exercise on a cashless basis upon customary terms the 56% Warrant at an implied price per share no greater than the price of Company Common Stock to be issued in the IPO. Howard Jonas and any such Affiliate who holds rights under the 56% Warrant shall be an express third party beneficiary of this Section 5.2(e).
Annex A-39
Table of Contents
5.3 Reasonable Best Efforts.
(a) Subject to the terms and conditions hereof, the Company and Parent shall use reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary, proper or advisable under applicable Law or otherwise to consummate and make effective the Contemplated Transactions as promptly as practicable, including notifying the other of any communication, inquiry, or investigation received from or initiated by a Governmental Body and using reasonable best efforts to cooperate in responding to any such inquiries, investigations, or requests, by using reasonable best efforts to take all action necessary to satisfy all of the conditions to the obligations of the other Parties hereto to effect the Merger, to obtain all necessary waivers, consents, approvals and other documents required to be delivered hereunder, and to effect all necessary registrations and filings and to remove any injunctions or other impediments or delays, legal or otherwise, in each case in order to consummate and make effective the Merger and the Contemplated Transactions.
(b) The Company shall use reasonable best efforts to obtain all consents and waivers with respect to (i) the Contracts set forth on Part 2.21 of the Company Disclosure Schedule and (ii) any and all Contracts entered into by the Company following the date hereof and prior to the Closing, in each case, that are required to be obtained from parties to such Contracts to which the Company is a party in connection with the Contemplated Transactions.
(c) The Parties shall reasonably cooperate in the preparation of a spreadsheet (the “Closing Payment Schedule”) setting forth: (i) the name, address and email address (if available) of each holder of Company Capital Stock, the Company Warrants the Company Options immediately prior to the Effective Time, (ii) the number of shares of Company Capital Stock held by each holder thereof immediately prior to the Effective Time (including the number of shares of Company Capital Stock for which Company Options are exercisable), (iii) a calculation of the Merger Shares and, as of the Closing Date, which each holder of Company Capital Stock, Company Warrants and Company Options is eligible to receive, and (iv) for each Securityholder and any other Person to whom payments are due and payable in connection with the Closing, whether any Taxes are required to be withheld (and, if so, what type of withholding applies).
5.4 Indemnification of Officers and Directors.
(a) All rights to indemnification by the Company existing in favor of those Persons who are directors and officers of the Company as of, or prior to, the date of this Agreement (the “D&O Indemnified Persons”) for their acts and omissions occurring prior to the Effective Time as provided in the Company Charter and the Company’s bylaws (as in effect as of the date of this Agreement) and as provided in those indemnification agreements (the “Indemnification Agreements”) between the Company, shall survive the Merger and shall be complied with and performed by the Surviving Company.
(b) For six years from and after the Effective Time, Parent shall cause all rights to indemnification and exculpation by the Company existing in favor of D&O Indemnified Persons for their acts and omissions occurring or alleged to have occurred prior to the Effective Time, as provided in the Company Charter and the Company’s bylaws (as in effect as of the date of this Agreement) and as provided in the Indemnification Agreements, in each case subject to the terms, conditions and limitations thereof, to survive the Merger, including as a result of the amendment of the certificate of incorporation of the Surviving Company pursuant to Section 1.4(a) (Certificate of Incorporation and Bylaws; Directors and Officers), and for six years from and after the Effective Time, Parent shall cause the Surviving Company to fulfill and honor such obligations to the fullest extent permitted under applicable Law.
(c) Parent shall cause the Surviving Company as of the Effective Time, to obtain and fully pay for “tail” insurance policies with a claims period of at least six (6) years from and after the Effective Time from an insurance carrier with a credit rating the same as or better than the Company’s current insurance carrier with respect to directors’ and officers’ liability insurance and fiduciary liability insurance (collectively, “D&O Insurance”) with respect to matters existing or occurring at or prior to the Effective Time with benefits and levels of coverage at least as favorable as the Company’s existing policies (including in connection with this Agreement or the transactions or actions contemplated hereby) with respect to those Indemnified Parties who are currently on the date hereof (and any additional Indemnified Parties who prior to the Effective Time become) covered by the Company’s D&O Insurance; provided, however, that (i) in no event shall the Surviving Company be required to expend for such “tail” insurance policies an annual premium amount in excess of three hundred percent (300%) of the annual premium currently on the date hereof paid by the Company for such insurance and (ii) if the annual premium of such “tail”
Annex A-40
Table of Contents
insurance policies exceeds three hundred percent (300%) of the annual premium currently paid by the Company for such insurance, Parent shall cause the Surviving Company to obtain and fully pay for policies covering such Indemnified Parties with the greatest coverage as is then available at a cost up to but not exceeding such amount. Parent shall, and shall cause the Surviving Company to, use its reasonable best efforts to cause such “tail” insurance policies to be maintained in full force and effect, for their full term, and to honor all of its obligations thereunder. After the Effective Time, Parent shall and shall cause the Surviving Company to maintain such policy in full force and effect, and continue to honor the obligations thereunder.
(d) In the event that Parent, the Company or the Surviving Company or any of their respective successors or assigns (i) consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or Entity of such consolidation or merger or (ii) transfers all or substantially all of its properties and assets to any Person, then, and in each such case, Parent shall ensure that the successors and assigns of Parent, the Company or the Surviving Company, as the case may be, shall assume the obligations set forth in this Section 5.4 (Indemnification of Officers and Directors).
(e) The provisions of this Section 5.4 (Indemnification of Officers and Directors) shall survive the consummation of the Merger and are (i) intended to be for the benefit of, and will be enforceable by, each of the D&O Indemnified Persons and their successors, assigns and heirs and (ii) in addition to, and not in substitution for, any other rights to indemnification or contribution that any such D&O Indemnified Person may have by Contract or otherwise. This Section 5.4 (Indemnification of Officers and Directors) may not be amended, altered or repealed after the Effective Time without the prior written consent of the affected D&O Indemnified Person.
5.5 Disclosure. The initial announcement regarding this Agreement shall be by press release as pre-agreed between the Parent and the Company, and thereafter Parent and the Company shall consult with each other before issuing any further press release(s) or otherwise making any public statement or making any announcement to Company Service Providers (to the extent not previously issued or made in accordance with this Agreement) with respect to the Merger, this Agreement or any of the Contemplated Transactions and shall not issue any such press release, public statement or announcement to Company Service Providers without the other Party’s written consent (which shall not be unreasonably withheld, conditioned or delayed). Notwithstanding the foregoing: (a) each Party may, without such consultation or consent, make any public statement in response to questions from the press, analysts, investors or those attending industry conferences, and make internal announcements to employees, so long as such statements are consistent with previous press releases, public disclosures or public statements made jointly by the Parties (or individually, if approved by the other Party), and (b) a Party may, without the prior consent of the other Party but subject to giving advance notice to the other Party (to the extent practical) and giving due consideration to comments from the other Party, issue any such press release or make any such public announcement or statement as may be required by applicable Law.
5.6 Notification of Certain Events.
(a) During the Pre-Closing Period, the Company shall promptly notify Parent of, and furnish Parent with any information it may reasonably request with respect to, the occurrence of any event or condition or the existence of any fact that may reasonably be expected to cause any of the conditions to the obligations of Parent to consummate the Merger set forth in Article 7 (Conditions Precedent to Obligations of Parent and the Merger Subs) not to be satisfied (including any breaches of inaccuracies of the representations and warranties set forth in Article 2 (Representations and Warranties of the Company)). The Company’s satisfaction of its obligations in the foregoing sentence shall not relieve the Company of any of its other obligations under this Agreement.
(b) During the Pre-Closing Period, Parent shall promptly notify the Company of, and furnish the Company with any information it may reasonably request with respect to, the occurrence of any event or condition or the existence of any fact that may reasonably be expected to cause any of the conditions to the obligations of the Company to consummate the Merger set forth in Article 8 (Conditions Precedent to Obligations of the Company) not to be satisfied (including any breaches of inaccuracies of the representations and warranties set forth in Article 3 (Representations and Warranties of Parent and the Merger Subs)). Parent’s satisfaction of its obligations in the foregoing sentence shall not relieve Parent of any of its other obligations under this Agreement.
Annex A-41
Table of Contents
5.7 Confidentiality. The provisions of the Confidentiality Agreement, to the extent not inconsistent with the express terms of this Agreement, are hereby ratified, confirmed and agreed to as though fully set forth herein, and Parent and the Company agrees that any information obtained pursuant to the provisions hereunder or the negotiation and execution of this Agreement or the effectuation of the Contemplated Transactions, shall be governed by the terms of the Confidentiality Agreement. The Confidentiality Agreement shall remain in effect until the Effective Time, at which point it shall terminate.
5.8 Company Options.
(a) Prior to the Closing, the Company shall take all actions (including providing any required notices, obtaining any necessary determinations and/or obtaining resolutions of the Company Board or a committee thereof) that may be necessary (under the Company Equity Plan and award agreements pursuant to which Company Options are outstanding or otherwise) to cause the treatment (as applicable) of each Company Option then outstanding as set forth in Section 1.6 (Treatment of Company Warrants, Company Options and Company Convertible Notes).
(b) At the Effective Time, Parent shall assume all of the obligations of the Company relating to the Converted Options outstanding immediately prior to the Effective Time. Prior to the delivery of any Parent Common Stock in respect of any Parent Options issued pursuant to Section 1.6(c) (Treatment of Company Warrants, Company Options and Company Convertible Notes), Parent shall file a registration statement on Form S-8 (or other applicable form), promptly but no later than thirty (30) days following the Effective Date, with respect to the Parent Common Stock subject to such Parent Options and shall maintain the effectiveness of such registration statement or registration statements (and maintain the current status of the prospectus or prospectuses contained therein) for so long as such Parent Options remain outstanding. As soon as practicable after the Effective Time, Parent shall deliver to the holders of Converted Options appropriate notices setting forth such holders’ rights pursuant to the respective applicable Parent Options, and the agreements evidencing the grants of such Converted Options shall continue in effect on the same terms and conditions, subject to the adjustments required by Section 1.6(c) (Treatment of Company Warrants, Company Options and Company Convertible Notes) after giving effect to the transactions contemplated by this Agreement.
5.9 Resignations. The Company shall obtain and deliver to Parent as of the Effective Time (or, at the option of Parent, at a later date) the resignation of each (i) director of the Company and (ii) officers of the Company, effective as of the Effective Time (or, at the option of Parent, at a later date) (the “D&O Resignations”).
5.10 Employee Benefits. With respect to those employees of the Company who are employed by the Company as of immediately prior to the Effective Time (the “Continuing Employees”):
(a) Parent shall assume the liability for accrued but unused personal, sick or vacation time to which any Continuing Employee is entitled pursuant to the personal, sick or vacation policies applicable to Continuing Employees immediately prior to the Effective Time.
(b) All Continuing Employees shall be eligible to continue to participate in the Surviving Company’s health and welfare benefit plans (to the same extent such Continuing Employees were eligible to participate under the Company’s health and welfare benefit plans immediately prior to the Effective Time); provided, however, that (i) nothing in this Section 5.10 (Employee Benefits) or elsewhere in this Agreement shall limit the right of Parent or the Surviving Company to amend or terminate any such health or welfare benefit plan or migrate the Continuing Employees to a successor health or welfare benefit plan at any time and (ii) if Parent or the Surviving Company terminates any such health or welfare benefit plan or migrates the Continuing Employees to a successor health or welfare benefit plan, then the Continuing Employees shall be eligible to participate in the Surviving Company’s health and welfare benefit plans for similarly situated employees to the extent that coverage under such plans is replacing comparable coverage under a Company Plan in which such Continuing Employee participated immediately before the Effective Time (each, a “New Plan”). To the extent that service is relevant for eligibility, vesting or allowances (including paid time off) under any such New Plan, then Parent shall use its reasonable best efforts to (i) waive all limitations as to pre-existing conditions, exclusions and waiting periods with respect to participation and coverage requirements applicable to the Continuing Employees and their eligible dependents, to the extent that such conditions, exclusions and waiting periods would not apply under a similar employee benefit plan in which such employees participated immediately prior to the commencement of participation in such New Plan, and (ii) ensure that such health or welfare benefit plan shall, for purposes of eligibility, vesting, deductibles, co-payments and out-of-pocket maximums and allowances (including
Annex A-42
Table of Contents
paid time off), credit Continuing Employees for service and amounts paid immediately prior to the commencement of participation in such New Plan to the same extent that such service and amounts paid were recognized prior to such commencement of participation under the corresponding health or welfare benefit plan of the Company.
(c) Nothing in this Section 5.10 (Employee Benefits) or elsewhere in this Agreement shall be construed to create a right in any employee to employment with Parent, the Surviving Company or any other Affiliate of the Surviving Company and the employment of each Continuing Employee shall be “at will” employment. The provisions of this Section 5.10 (Employee Benefits) are solely for the benefit of the Parties to this Agreement, and no provision of this Section 5.10 (Employee Benefits) is intended to, or shall, constitute the establishment or adoption of or an amendment to any employee benefit plan for purposes of ERISA or otherwise and no current or former employee or any other individual associated therewith shall be regarded for any purpose as a Third Party beneficiary of the Agreement or have the right to enforce the provisions hereof.
5.11 Stock Exchange Listing. Parent shall use reasonable best efforts to cause the shares of Parent Class B Common Stock issuable, and required to be reserved for issuance, in connection with the Merger to be approved for listing on the New York Stock Exchange, subject to official notice of issuance, prior to the Effective Time.
5.12 Royalty Buyout. The Company shall use its reasonable best efforts to effect the consummation of the transactions under the Altira Acquisition Agreement attached hereto as Exhibit E in accordance with its terms (the “Altira Acquisition Agreement”).
5.13 The 56% Warrant. Prior to the Closing, the Parent shall effect the termination of the 56% Warrant by Pharma Holdings, LLC, such termination to be contingent on the consummation of the Merger and effective at the Initial Effective Time.
5.14 Financing. The Company agrees to use its reasonable best efforts to provide, and shall use its reasonable best efforts to cause its officers, directors, employees, financial advisors, counsel, accountants and other Representatives and Affiliates to provide, all cooperation reasonably requested by Parent or the Merger Subs to assist them in connection with the arrangement of the Financing. Such cooperation shall include but not be limited to the following:
(a) participating (with Representatives of the Company of appropriate seniority and expertise) at reasonable times, upon reasonable advance notice, in meetings, presentations, due diligence sessions, and sessions with rating agencies, and otherwise cooperating with the marketing efforts for the Financing;
(b) assisting Parent with the preparation of all materials reasonably required to be provided or requested by Parent or its Representatives for use in connection with the Financing, including, without limitation, financial statements and financial forecasts; and
(c) taking corporate actions reasonably requested by Parent to facilitate the consummation of the Financing.
5.15 Agency Communications and Filings.
(a) As and to the extent applicable, with respect to the Contemplated Transactions, the Parties agree to use their reasonable best efforts to take promptly any and all steps necessary to avoid or eliminate each and every impediment under the Antitrust Laws, that may be asserted by any Governmental Body, so as to enable the Closing to occur as promptly as practicable, but in no case later than the End Date, including providing as promptly as reasonably practicable and advisable all non-legally privileged information reasonably required by another Party or any Governmental Body pursuant to its evaluation of the Contemplated Transactions under the HSR Act. Subject to the terms of this Section 5.15, the Parties shall use their reasonable best efforts to obtain from any Governmental Body all consents, approvals authorizations or orders required to be obtained under the Antitrust Laws or to avoid the entry or enactment of any injunction or other order or decree relating to any Antitrust Law that would delay, restrain, prevent, enjoin or otherwise prohibit consummation of the Transactions. Parent shall file with the FTC and the Antitrust Division of the DOJ Notification and Report Forms as soon as practicable after the date of this Agreement but in no event later than thirty (30) calendar days following the date of this Agreement.
Annex A-43
Table of Contents
(b) As and to the extent applicable, with respect to the Contemplated Transactions and subject to the terms and conditions of this Agreement, each of the Parties shall (and shall cause their respective Affiliates, if applicable, to): (i) as promptly as reasonably practicable cooperate with each other in determining whether, and promptly preparing and making, any filings, notifications or other consents are required to be made with, or obtained from, any other Governmental Bodies in connection with the Contemplated Transactions.
(c) As and to the extent applicable, with respect to the Contemplated Transactions, without limiting the generality of anything contained in this Section 5.15, during the Pre-Closing Period, each Party shall (i) give the other Parties prompt notice of the making or commencement of any request, inquiry, investigation, action or Legal Proceeding brought by a Governmental Body or brought by a third party before any Governmental Body, in each case, with respect to the Contemplated Transactions under the Antitrust Laws, (ii) keep the other Parties reasonably informed as to the status of any such request, inquiry, investigation, action or Legal Proceeding, (iii) promptly inform the other Parties of, and wherever practicable give the other party reasonable advance notice of, and the opportunity to participate in, any communication to or from the FTC, DOJ or any other Governmental Body in connection with any such request, inquiry, investigation, action or Legal Proceeding, (iv) promptly furnish to the other Party, subject to an appropriate confidentiality agreement to limit disclosure to counsel and outside consultants, with copies of documents received from any Governmental Body in connection with any such request, inquiry, investigation, action or Legal, (v) subject to an appropriate confidentiality agreement to limit disclosure to counsel and outside consultants, and to the extent reasonably practicable, consult and cooperate with the other Parties and consider in good faith the views of the other Parties in connection with any written analysis, appearance, presentation, memorandum, brief, argument, opinion or proposal made or submitted in connection with any such request, inquiry, investigation, action or Legal Proceeding, and (vi) except as may be prohibited by any Governmental Body or by any Laws, in connection with any such request, inquiry, investigation, action or Legal Proceeding in respect of the Contemplated Transactions, give the other Party reasonable prior notice and permit authorized Representatives of the other Party to be present at each meeting or conference, including by telephone, relating to such request, inquiry, investigation, action or Legal Proceeding and to have access to and be consulted in advance in connection with any argument, opinion or proposal made or submitted to any Governmental Body in connection with such request, inquiry, investigation, action or Legal Proceeding.
(d) Notwithstanding anything to the contrary contained in this Agreement, but subject to Parent’s obligations set forth in this Section 5.15, Parent shall have the right, following consultation with the Company and after giving due consideration to its views and acting reasonably and in good faith, to direct all matters, strategies, communications, and timing with any Governmental Authority consistent with its obligations hereunder and Parent shall have the responsibility for devising and implementing the strategy for obtaining any necessary or advisable antitrust or competition or foreign investment merger clearances, and the Company shall use its commercially reasonable efforts to provide full, effective, and timely support of Parent. Parent covenants and agrees to pay any Government Body filing fees under any applicable Laws, and any and all fines, penalties, fees and expenses incurred in preparing any HSR filing, or otherwise related to Parent or the Company taking any and all steps necessary to avoid or eliminate each and every impediment under the HSR Act and related regulations.
(e) The Company covenants and agrees that it shall notify Parent at least five (5) days in advance of the filing or submission of, and provide to Parent a copy of, any material application or request with or any material communication with, the FDA, EMA or other Governmental Body performing similar functions.
6. CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY
The obligations of each Party to consummate the Merger are subject to the satisfaction (or the written waiver by each of the Parties) at or prior to the Closing, of each of the following conditions:
6.1 Effectiveness of Registration Statement. The Registration Statement shall have been declared effective by the SEC under the Securities Act and no stop order suspending the effectiveness of the Registration Statement shall have been issued by the SEC and remain in effect, and no proceedings for that purpose shall be pending or threatened in writing by the SEC and not withdrawn or lapsed.
Annex A-44
Table of Contents
6.2 No Restraints. No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of any of the Mergers shall have been issued by any court of competent jurisdiction and remain in effect, and no material Law shall have been enacted since the date of this Agreement that makes consummation of any of the Mergers illegal.
6.3 Stockholder Approval. This Agreement shall have been duly adopted by the Required Company Stockholder Vote and the Required Parent Stockholder Vote, and the Required Company Stockholder Vote and the Required Parent Stockholder Vote shall remain in full force and effect and, if the Registration Statement incorporates information by reference as contemplated in General Instruction A.2 of Form S-4 under the Securities Act, at least twenty business days (as defined pursuant to Form S-4) shall have elapsed from the date of the first mailing of the prospectus in the Registration Statement to the Company Stockholders until the date of the Closing.
6.4 Majority of the Minority Approval. This Agreement shall have been duly adopted by the Parent Minority Approval and the Company Minority Approval, each of the Parent Stockholder Approval and the Company Stockholder Approval have occurred, and all such approvals shall remain in full force and effect.
6.5 Listing. The Merger Shares shall have been approved for listing on the NYSE, subject to official notice of issuance.
6.6 Royalty Buyout. The transactions contemplated by the Altira Acquisition Agreement shall have been consummated by all parties thereto in accordance with its terms; provided that such consummation shall not be a condition to Parent’s obligations to consummate the Merger if (a) Parent has not funded the payment amount thereunder before the Closing and (b) the Altira Acquisition Agreement has been consummated in all material respects by all parties thereto other than Parent (to the extent that such covenants require performance by such other parties at or before the Closing), including execution and delivery of any and all conveyance documents.
6.7 Governmental Consents. Any waiting period (or any extension thereof) applicable to the Contemplated Transactions under the HSR Act shall have expired or been terminated.
6.8 Charter Amendment. The Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Company in the form of Exhibit F hereto, shall have received the required director and stockholder approvals and been filed with the Delaware Secretary of State on a date prior to the Closing Date.
6.9 Closing of Financing. Parent shall have consummated the Financing.
7. CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND THE MERGER SUBS
The obligations of Parent and the Merger Subs to consummate the Merger are subject to the satisfaction (or waiver by Parent), at or prior to the Closing, of each of the following conditions:
7.1 Accuracy of Representations and Warranties. The Specified Representations that are qualified by materiality or Company Material Adverse Effect shall be true and correct in all respects as of the date of this Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all respects as of such earlier date). The Specified Representations that are not qualified by materiality or Company Material Adverse Effect shall be true and correct in all material respects as of the date of this Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all material respects as of such earlier date). The other representations and warranties of the Company set forth in Section 2 (Representations and Warranties of the Company) shall be true and correct (without giving effect to any limitation as to materiality or Company Material Adverse Effect set forth therein) as of the date of this Agreement and of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct would not, individually or in the aggregate, have a Company Material Adverse Effect.
Annex A-45
Table of Contents
7.2 Performance of Covenants. The Company shall have performed and complied with, in all material respects, all of its covenants hereunder at or before the Closing (to the extent that such covenants require performance by the Company at or before the Closing).
7.3 No Governmental Litigation. There shall not be pending any lawsuit or other Legal Proceeding challenging the Merger that (a) has been commenced by a Governmental Body of competent jurisdiction and (b) could reasonably be expected to result in an injunction or judgment in favor of such Governmental Body that would prevent consummation of the Merger.
7.4 Agreements and Documents. Parent shall have received the following agreements and documents, each of which shall be in full force and effect:
(a) the D&O Resignations;
(b) the Certificate of Merger, executed by the Company;
(c) a good standing certificate of the Company from the Secretary of State of the State of Delaware dated within seven days prior to the Closing Date; and
(d) the Lock-Up Agreements duly executed by each party thereto with respect to shares of Parent Class B Common Stock issued pursuant to the Merger (other than Parent).
7.5 Closing Certificate. As of the Closing, the Chief Executive Officer or Chief Financial Officer of the Company shall have delivered to Parent a certificate (the “Company Officers Certificate”) to the effect that each of the conditions specified above in Sections 7.1 (Accuracy of Representations and Warranties), 7.2 (Performance of Covenants) and 7.8 (No Material Adverse Effect) is satisfied in all respects.
7.6 Royalties; Consents.
(a) Except as set forth on Part 7.6(a) of the Company Disclosure Schedules, there shall be no obligation to pay any royalties or similar payments on any sales of products incorporating the Company Intellectual Property.
(b) The third party consents set forth on Part 7.6(b) of the Company Disclosure Schedules shall have been obtained in form and substance reasonably satisfactory to the Parent.
7.7 No Material Adverse Effect. Since the date of this Agreement, no Company Material Adverse Effect shall have occurred that is continuing.
8. CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY
The obligation of the Company to consummate the Merger is subject to the satisfaction (or waiver by the Company), at or prior to the Closing, of the following conditions:
8.1 Accuracy of Representations and Warranties. The representations and warranties of Parent and the Merger Subs set forth in Section 3 (Representations and Warranties of Parent and the Merger Subs) shall be true and correct in all material respects as of the date of this Agreement and as of the Closing Date with the same effect as though made on and as of the Closing (except to the extent expressly made as of an earlier date, in which case such representations and warranties shall be true and correct in all respects as of such earlier date).
8.2 Performance of Covenants. Parent and the Merger Subs shall have performed and complied with, in all material respects, all of their respective covenants hereunder at or before the Closing (to the extent that such covenants require performance by Parent or Merger Subs at or before the Closing).
8.3 No Governmental Litigation. There shall not be pending before any court of competent jurisdiction any lawsuit or other Legal Proceeding challenging the Merger that has been commenced by a Governmental Body.
8.4 No Material Adverse Effect. Since the date of this Agreement, no Parent Material Adverse Effect shall have occurred that is continuing.
Annex A-46
Table of Contents
8.5 Closing Certificate. An authorized officer of Parent and the Merger Subs shall have delivered to Company a certificate (the “Parent Officers Certificate”) to the effect that each of the conditions specified above in Sections 8.1 (Accuracy of Representations and Warranties), 8.2 (Performance of Covenants) and 8.4 (No Material Adverse Effect) is satisfied in all respects.
8.6 Reserved.
8.7 Dissolution of Subsidiaries. Parent shall have dissolved all of its direct and indirect subsidiaries (other than Merger Sub I and Merger Sub II) holding shares of Company capital stock or securities exercisable for or convertible into Company capital stock, or distributed or otherwise transferred, the shares of Company capital stock or securities exercisable for or convertible into Company capital stock held by such subsidiaries to Parent and the other members of such subsidiaries.
8.8 Lock-Up Agreements. The Lock-Up Agreements shall have been duly executed by each party thereto with respect to shares of Parent Class A Common Stock and Parent Class B Common Stock.
9. TERMINATION
9.1 Termination. This Agreement may be terminated prior to the Effective Time (whether before or after the adoption of this Agreement by the Required Company Stockholder Vote, except to the extent otherwise provided below) as follows:
(a) by mutual written consent of Parent and the Company;
(b) by either Parent or the Company if the Merger shall not have been consummated by the End Date; provided, however, that the right to terminate this Agreement under this Section 9.1(b) (Termination) shall not be available to the Company, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure of the Merger to occur on or before the End Date and such action or failure to act constitutes a breach of this Agreement;
(c) by either Parent or the Company if a court of competent jurisdiction or other Governmental Body of competent jurisdiction shall have issued a final and non-appealable order, decree or ruling, in each case, having the effect of permanently restraining, enjoining or otherwise prohibiting or making illegal the Merger; provided, however, that the right to terminate this Agreement under this Section 9.1(c) (Termination) shall not be available to the Company, on the one hand, or to Parent, on the other hand, if such Party did not use commercially reasonable efforts to have such order, decree or ruling vacated prior to its becoming final and non-appealable and such failure to use commercially reasonable efforts constitutes a breach of this Agreement;
(d) by Parent, if the Company shall have breached or failed to perform any of its representations, warranties, covenants, obligations or agreements contained in this Agreement, which breach or failure to perform (i) would give rise to the failure of a condition set forth in Section 7.1 (Accuracy of Representations and Warranties) or Section 7.2 (Performance of Covenants) and (ii) cannot be, or has not been, cured within 30 calendar days following receipt by the Company of written notice of such material breach or failure to perform; provided that Parent may not terminate this Agreement pursuant to this Section 9.1(d) (Termination) if Parent is in breach of this Agreement such that the Company has the right to terminate this Agreement pursuant to Section 9.1(e) (Termination) but for the proviso thereto;
(e) by the Company, if Parent or Merger Subs shall have breached or failed to perform any of their respective representations, warranties, covenants, obligations or agreements contained in this Agreement, which breach or failure to perform (i) would give rise to the failure of a condition set forth in Section 8.1 (Accuracy of Representations and Warranties) or Section 8.2 (Performance of Covenants) and (ii) cannot be, or has not been, cured within 30 calendar days following receipt by Parent of written notice of such material breach or failure to perform; provided that the Company may not terminate this Agreement pursuant to this Section 9.1(e) (Termination) if the Company is in breach of this Agreement such that Parent has the right to terminate this Agreement pursuant to Section 9.1(d) (Termination) but for the proviso thereto; and
Annex A-47
Table of Contents
(f) by Parent or the Company if each of the following are satisfied: (i) the Parent Stockholder Solicitation Period shall have run and elapsed and either (A) the Required Parent Stockholder Vote or (B) the Parent Minority Approval shall not have been obtained on or prior to the end of the Parent Stockholder Solicitation Period, or (ii) the Company Stockholder Solicitation Period shall have run and elapsed and either (x) the Required Company Stockholder Vote or (y) the Company Minority Approval shall not have been obtained on or prior to the end of the Company Stockholder Solicitation Period; provided however that the right to terminate this Agreement under this Section 9.1(f) (Termination) shall not be available to the Company, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure to obtain the approvals required pursuant to clauses (i) or (ii) hereof, as applicable, and such action or failure to act constitutes a breach of this Agreement. Notwithstanding any other term in this Section 9.1(f) (Termination), once the Required Company Stockholder Vote, the Company Minority Approval, the Required Parent Stockholder Vote and the Parent Minority Approval have been obtained, neither Parent nor the Company may terminate this Agreement pursuant to this Section 9.1(f) (Termination).
The Party desiring to terminate this Agreement pursuant to Section 9.1 (Termination), shall give the other party written notice of such termination, specifying the provisions hereof pursuant to which such termination is made and the basis therefor described in reasonable detail.
9.2 Effect of Termination. In the event of the termination of this Agreement as provided in Section 9.1 (Termination), this Agreement shall be of no further force or effect and no party hereto (or any of its Affiliates, directors, trustees, executors, officers, agents or Representatives) shall have any liability or obligation hereunder; provided, however, that (i) this Section 9.2 (Effect of Termination), Section 5.2(c), (d) and (e), Section 5.7 (Confidentiality), Section 1.11 (Extension of 56% Warrant) and Section 10 (Miscellaneous Provisions) and the definitions of the capitalized terms referenced in such Sections shall survive the termination of this Agreement and shall remain in full force and effect, (ii) nothing herein shall relieve any party from any liability for Fraud or for any knowing and willful material breach of this Agreement by such party prior to the termination of this Agreement, and (iii) no termination of this Agreement shall affect the obligations of the Parties contained in the Confidentiality Agreement (subject to the terms thereof), all of which obligations shall survive termination of this Agreement in accordance with their terms.
10. MISCELLANEOUS PROVISIONS
10.1 No Survival of Representations and Warranties. None of the representations and warranties in this Agreement or in any schedule, certificate, instrument or other document pursuant to this Agreement shall survive the Effective Time. This Section 10.1(a) (No Survival of Representations and Warranties) shall not limit any covenant or agreement of the Parties which by its terms contemplates performance after the Effective Time, including without limitation, the covenants set forth in Section 5.4 (Indemnification of Directors and Officers). All representations and warranties set forth in this Agreement are contractual in nature only and subject to the sole and exclusive remedies set forth herein.
10.2 Amendment. Subject to applicable Law, this Agreement may be amended, modified and supplemented in any and all respects, whether before or after any vote of the shareholders of the Parent or the Company contemplated hereby, by written agreement of the Parties hereto, at any time prior to the Closing Date with respect to any of the terms contained herein; provided, however, that after the receipt of the Parent Stockholder Approval, there shall be no amendment or waiver that would require the further approval of the shareholders of the Parent under applicable Law without such approval having first been obtained. A termination of this Agreement pursuant to Section 9.1 (Termination) shall, in order to be effective, require, in the case of Parent and the Company, action by their respective boards of directors (or a committee thereof) as applicable.
10.3 Expenses. All fees and expenses incurred in connection with this Agreement and the Contemplated Transactions shall be paid by the party incurring such expenses, whether or not the Merger is consummated.
10.4 Waiver.
(a) No failure on the part of any party to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of any party in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy.
Annex A-48
Table of Contents
(b) No party shall be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under this Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of such party; and any such waiver shall not be applicable or have any effect except in the specific instance in which it is given.
10.5 Entire Agreement; Counterparts. This Agreement (including the documents and instruments referred to herein), the Support Agreements, the Voting Agreements, Lock-Up Agreements and the Confidentiality Agreement constitute the entire agreement, and supersede all prior agreements and understandings, both written and oral, among the Parties with respect to the subject matter of this Agreement. This Agreement may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same instrument. This Agreement may be executed by electronic transmission, each of which shall be deemed an original.
10.6 Applicable Law; Jurisdiction; Waiver of Jury Trial. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of Laws thereof. In any action between any of the Parties arising out of or relating to this Agreement or any of the Contemplated Transactions: (a) each of the Parties irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware (or, only if such court declines to accept jurisdiction over a particular matter, any state or federal court within the State of Delaware); and (b) if any such action is commenced in a state court, then, subject to applicable Law, no party shall object to the removal of such action to any federal court located in Delaware. Each of the Parties waives any defense of inconvenient forum to the maintenance of any action so brought and waives any bond, surety or other security that might be required of any other party with respect thereto. Each Party hereby waives, to the fullest extent permitted by Law, any right to trial by jury of any claim, demand, action, or cause of action (i) arising under this Agreement or (ii) in any way connected with or related or incidental to the dealings of the Parties in respect of this Agreement or any of the transactions related hereto, in each case, whether now existing or hereafter arising, and whether in contract, tort, equity, or otherwise. Each Party hereby further agrees and consents that any such claim, demand, action, or cause of action shall be decided by court trial without a jury and that the Parties may file a copy of this Agreement with any court as written evidence of the consent of the Parties to the waiver of their right to trial by jury.
10.7 Attorneys’ Fees. In any action at Law or suit in equity to enforce this Agreement or the rights of any of the Parties hereunder, the prevailing party in such action or suit, as determined by a court of competent jurisdiction in a final, non-appealable order, shall be entitled to receive a reasonable sum for its out-of-pocket attorneys’ fees and all other reasonable costs and expenses incurred in such action or suit.
10.8 Assignability. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties hereto and their respective successors and assigns. Neither this Agreement nor any of the rights or obligations hereunder may be assigned (whether by merger, consolidation, sale or otherwise) by the Company without the prior written consent of Parent, or by Parent without the prior written consent of the Company, and any attempted assignment of this Agreement or any of such rights without such consent shall be void and of no effect (except that Parent may assign its rights, and delegate its obligations, under this Agreement to an Affiliate without the prior written consent of the Company so long as Parent remains bound to this Agreement and liable for its obligations hereunder).
10.9 Third Party Beneficiaries. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than the Parties hereto) any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement, except (a) as provided in Section 5.4 (Indemnification of Officers and Directors), which in each case shall be third party beneficiaries of said provisions, provided that no consent of any D&O Indemnified Person shall be required to amend any provision of this Agreement prior to the Effective Time, (b) pursuant to Section 1.11 (Extension of 56% Warrant), (c) pursuant to Section 5.2(e) and (d) from and after the Effective Time, the rights of the Securityholders as of immediately prior to the Effective Time to receive the consideration pursuant to the Merger set forth in this Agreement.
10.10 Notices. Any notice or other communication required or permitted to be delivered to any party under this Agreement shall be in writing and shall be deemed properly delivered, given and received (a) upon receipt when delivered by hand, (b) upon transmission, if sent by electronic transmission, or (c) one Business Day after being sent by
Annex A-49
Table of Contents
courier or express delivery service, provided that in each case the notice or other communication is sent to the address electronic mail address set forth beneath the name of such party below (or to such other address or electronic mail address as such party shall have specified in a written notice given to the other Parties hereto):
if to Parent or Merger Sub I or Merger Sub II:
Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Attention: David Polinsky, Chief Financial Officer
E-mail: david.polinsky@rafaelholdings.com
With a copy (which shall not constitute notice) to:
Olshan Frome Wolosky LLP
1325 Avenue of the Americas
New York, NY 10019
Attention: Mitchell Raab
E-mail: mraab@olshanlaw.com
And:
Schwell Wimpfheimer & Associates
37 West 39th Street, Suite 505
New York, NY 10018
E-mail: dov.schwell@swalegal.com
if to the Company (prior to Closing):
Rafael Pharmaceuticals, Inc.
1 Duncan Drive
Cranbury, NJ 08512
Attention: Sanjeev Luther, Chief Executive Officer
E-mail: sanjeev.luther@rafaelpharma.com
With a copy (which shall not constitute notice) to:
Orrick Herrington & Sutcliffe LLP
1152 15th Street, N.W.
Washington, DC 20005 – 1706
Attention: David E. Schulman
E-mail: dschulman@orrick.com
10.11 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If the final judgment of a court of competent jurisdiction declares that any term or provision hereof is invalid or unenforceable, the Parties hereto agree that the court making such determination shall have the power to limit the term or provision, to delete specific words or phrases, or to replace any invalid or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the Parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term.
10.12 Knowledge. “Knowledge” of the Company shall mean the knowledge of a fact or other matter, after reasonable inquiry, of the Knowledge Individuals. With respect to Intellectual Property, “reasonable inquiry” does not require the Company or any individual to obtain any freedom-to-operate opinions or similar opinions of counsel or any Intellectual Property clearance searches.
Annex A-50
Table of Contents
10.13 Specific Performance.
(a) Each of the Parties hereto agrees that this Agreement is intended to be legally binding and specifically enforceable pursuant to its terms and that Parent and the Company would be irreparably harmed if any of the provisions of this Agreement are not performed in accordance with their specific terms and that monetary damages would not provide adequate remedy in such event. Accordingly, in addition to any other remedy to which a Party may be entitled at Law, a Party shall be entitled to injunctive relief to prevent breaches of this Agreement and to specifically enforce the terms and provisions hereof, in each case without posting a bond or undertaking, this being in addition to any other remedy to which they are entitled at Law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this Agreement on the basis that the other Parties have an adequate remedy at Law or an award of specific performance is not an appropriate remedy for any reason at Law or equity.
(b) Notwithstanding Section 10.13(a) or anything else to the contrary in this Agreement, the Company shall not be entitled to enforce specifically Parent’s and the Merger Subs’ obligations to consummate the Merger unless (i) all of the conditions set forth in Sections 6 and 7 (other than those conditions that by their nature are to be satisfied at the Closing; provided that such conditions would have been satisfied or waived assuming the Closing were to occur) shall have been satisfied (or are capable of being satisfied at the Closing) or (to the extent permissible under applicable Law) waived; (ii) the Company has irrevocably confirmed in writing to Parent that (A) all of the conditions to the Company’s obligations have been satisfied or waived other than those conditions that by their nature are to be satisfied at the Closing; provided that such conditions would have been satisfied or waived assuming the Closing were to occur), and (B) if specific performance is granted, then it is ready, willing and able to take the actions within its control to cause the Closing to occur and (iii) Parent and Merger Sub I have failed to complete the Closing by the End Date.
10.14 Construction.
(a) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine and feminine genders.
(b) The Parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting party shall not be applied in the construction or interpretation of this Agreement.
(c) As used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
(d) Except as otherwise indicated, all references in this Agreement to “Sections,” “Articles,” “Exhibits” and “Schedules” are intended to refer to Sections or Articles of this Agreement and Exhibits or Schedules to this Agreement.
(e) The headings contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
(f) All amounts payable under this Agreement, including the amounts to be set forth in the Estimated Closing Statement and Closing Statement, shall be calculated and paid in U.S. Dollars.
10.15 Disclosure Schedule. The Company Disclosure Schedule and Parent Disclosure Schedule have been arranged, for purposes of convenience only, as separate Parts corresponding to the subsections of Section 2 (Representations and Warranties of the Company) and Section 3 (Representations and Warranties of the Parent and the Merger Subs), respectively, of this Agreement. The representations and warranties contained in Section 2 (Representations and Warranties of the Company) and Section 3 (Representations and Warranties of the Parent and the Merger Subs), of this Agreement are subject to (a) the exceptions and disclosures set forth in the part of the Disclosure Schedule corresponding to the particular subsection of Section 2 (Representations and Warranties of the Company) and Section 3 (Representations and Warranties of the Parent and the Merger Subs) in which such representation and warranty appears; (b) any exceptions or disclosures explicitly cross-referenced in such part of the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable, by reference to another part of the Company Disclosure
Annex A-51
Table of Contents
Schedule or Parent Disclosure Schedule, as applicable; and (c) any exception or disclosure set forth in any other part of the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable, to the extent it is reasonably apparent on the face of such disclosure that such exception or disclosure is intended to qualify such representation and warranty. No reference to or disclosure of any item or other matter in the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable, shall be construed as an admission or indication that such item or other matter is material (nor shall it establish a standard of materiality for any purpose whatsoever) or that such item or other matter is required to be referred to or disclosed in the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable. The information set forth in the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable, is disclosed solely for the purposes of this Agreement, and no information set forth therein shall be deemed to be an admission by any party hereto to any Third Party of any matter whatsoever, including of any violation of Law or breach of any agreement. The Company Disclosure Schedule or Parent Disclosure Schedule, as applicable, and the information and disclosures contained therein are intended only to qualify and limit the representations, warranties and covenants of the Company contained in this Agreement. Matters reflected in the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable, are not necessarily limited to matters required by this Agreement to be reflected in the Company Disclosure Schedule or Parent Disclosure Schedule, as applicable.
10.16 Non-Recourse. Notwithstanding anything herein to the contrary, no Representative, Affiliate of, or direct or indirect equity owner in, the Company shall have any personal liability to either Parent or Merger Subs or any other Person as a result of the breach of any representation, warranty, covenant, agreement or obligation of the Company in this Agreement, and no Representative, Affiliate of, or direct or indirect equity owner in, either Parent or Merger Subs shall have any personal liability to the Company or any other Person as a result of the breach of any representation, warranty, covenant, agreement or obligation of any of Parent or Merger Subs in, or otherwise in connection with, this Agreement.
[SIGNATURE PAGE FOLLOWS]
Annex A-52
Table of Contents
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| | RAFAEL HOLDINGS, INC |
| | | By: | | /s/ Ameet Mallik |
| | | | | Name: | | Ameet Mallik |
| | | | | Title: | | CEO |
| | | RH MERGER I, INC. |
| | | By: | | /s/ David Polinsky |
| | | | | Name: | | David Polinsky |
| | | | | Title: | | President |
| | | RH MERGER II, LLC |
| | | | | /s/ David Polinsky |
| | | | | Name: | | David Polinsky |
| | | | | Title: | | President |
| | | RAFAEL PHARMACEUTICALS, INC. |
| | | By: | | /s/ Sanjeev Luther |
| | | | | Name: | | Sanjeev Luther |
| | | | | Title: | | CEO |
[SIGNATURE PAGE TO AGREEMENT AND PLAN OF MERGER]
Annex A-53
Table of Contents
EXHIBIT A
CERTAIN DEFINITIONS
For purposes of this Agreement (including this Exhibit A):
“56% Warrant” shall mean that certain Warrant to Purchase Shares of the Company issued on September 16, 2016 to IDT Corporation, a predecessor of Parent, which Warrant to Purchase Shares of the Company is, as of the Execution Date, held by Pharma Holdings LLC.
“Adverse Experience” shall have the meaning set forth in Section 2.25(q) of this Agreement.
“Affiliate” shall mean, with respect to any specified Person, any other Person that, directly or indirectly, through one or more intermediaries, controls, is controlled by or is under common control with such specified Person.
“Affiliated Agreement” shall have the meaning set forth in Section 2.24 of this Agreement.
“Affiliated Group” means any affiliated group within the meaning of Code Section 1504(a) or any similar group defined under a similar provision of state, local, or non-U.S. law.
“Agreement” shall have the meaning set forth in the preamble of this Agreement.
“Altira Royalty” shall mean the entitlement to a percentage of the Company’s net sales created pursuant to the Altira Royalty Agreement.
“Altira Royalty Agreement” shall mean that certain Royalty Agreement, dated January 31, 2007 by and among the Company, Altira Capital and Consulting, LLC, Robert Shorr and Robert Rodriguez, including for clarity to the extent assigned or transferred.
“Ancillary Agreements” shall mean, collectively, the Voting Agreements, the Lock-Up Agreements, the Support Agreements and each other agreement, document instrument and/or certificate contemplated by this Agreement to be executed in connection with the Contemplated Transactions, including the Letters of Transmittal.
“Anti-Corruption Laws” shall mean the Foreign Corrupt Practices Act of 1977, as amended, the Anti-Kickback Act of 1986 or any applicable Laws of similar effect, and the related regulations.
“Antitrust Laws” shall mean the Sherman Act, as amended, the Clayton Act, as amended, the HSR Act, the Federal Trade Commission Act, as amended, all applicable foreign anti-trust laws and all other applicable Laws that are designed or intended to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition.
“Author” shall have the meaning set forth in Section 2.8(f) of this Agreement
“Balance Sheet” shall have the meaning set forth in Section 2.4(a) of this Agreement.
“Balance Sheet Date” shall have the meaning set forth in Section 2.4(a) of this Agreement.
“Bonus Shares Agreement” shall mean that certain First Global Amendment to Loan Documents dated April 13, 2016 by and among the Company, IDT Corporation (a predecessor of Parent), Joseph Stern and Aaron Drillick, including for clarity to the extent assigned or transferred.
“Business Day” shall mean any day that is not a Saturday, a Sunday or other day on which banks are required or authorized by Law to be closed in the City of New York, NY.
“CARES Act” shall have the meaning set forth in Section 2.13(k) of this Agreement.
Annex A-54
Table of Contents
“Cash and Cash Equivalents” shall mean, the aggregate amount of unrestricted cash and cash equivalents held by the Company, as determined in accordance with GAAP, consistently applied, less (without duplication for any exclusions provided for under GAAP) (a) the aggregate amount of outstanding checks or drafts of the Company that have not posted, plus (without duplication for any inclusions provided for under GAAP) (b) checks received by the Company that have not been posted.
“Certificates of Designation” shall mean collectively the Company’s certificates of designation for each series of Company Preferred Stock, as amended.
“Certificate of Merger” shall have the meaning set forth in Section 1.3 of this Agreement.
“Certificate of Merger for Subsequent Merger” shall have the meaning set forth in Section 1.3 of this Agreement.
“Closing” shall have the meaning set forth in Section 1.3 of this Agreement.
“Closing Date” shall have the meaning set forth in Section 1.3 of this Agreement.
“Closing Payment Schedule” shall have the meaning set forth in Section 5.3(c) of this Agreement.
“Code” shall mean the Internal Revenue Code of 1986, as amended.
“Common Warrant Option” means the options issued to investors in the Company to purchase an aggregate of 2,985,000 shares of Common Stock of the Company at various exercise prices.
“Company” shall have the meaning set forth in the preamble to this Agreement.
“Company Board” shall have the meaning set forth in the recitals of this Agreement.
“Company Board Recommendation” shall have the meaning set forth in Section 5.2(d) of this Agreement.
“Company Capital Stock” shall mean, collectively, the Company Common Stock and the Company Preferred Stock.
“Company Charter” shall mean the Company’s Amended and Restated Certificate of Incorporation and in effect immediately prior to the Effective Time.
“Company Common Stock” shall mean together the Company Voting Common Stock and Company Non-Voting Common Stock.
“Company Consultant” shall mean any current or former consultant or individual service provider of the Company (other than those that have a Contract with Company in their individual capacity).
“Company Convertible Notes” shall mean those 3.5% Convertible Promissory Notes, issued between February 26, 2013 and September 15, 2016, by the Company to the holders thereof, with an aggregate outstanding total principal amount of $12,189,320.
“Company Data” shall have the meaning set forth in Section 2.8(a)(i) of this Agreement.
“Company Disclosure Schedule” shall mean the disclosure schedule that has been prepared by the Company and delivered to Parent and the Merger Subs on the date of, and prior to the execution of, this Agreement.
“Company Equity Plan” shall mean, collectively, the Company’s 2005 Incentive Stock Plan, 2009 Incentive Stock Plan, and 2018 Equity Incentive Plan.
“Company ERISA Affiliate” shall mean any Person with which the Company is or would be at any relevant time considered a single employer under Section 414(b), (c), (m), or (o) of the Code.
Annex A-55
Table of Contents
“Company Financial Statements” shall have the meaning set forth in Section 2.4(a) of this Agreement.
“Company Intellectual Property Agreements” shall have the meaning set forth in Section 2.8(a)(ii) of this Agreement.
“Company Licensed Intellectual Property” shall have the meaning set forth in Section 2.8(m)(i) of this Agreement.
“Company Material Adverse Effect” shall mean any occurrence, change, event, circumstance or effect that, individually or in the aggregate with any other occurrences, changes, events, circumstances and/or effects, (i) has had or would reasonably be expected to have a material adverse effect on the liabilities, financial condition, existing business or assets of the Company, or (ii) that would prevent the Company from consummating the Merger in accordance with the terms hereof; provided, however, that none of the following (individually or in combination) shall be deemed to constitute, or shall be taken into account in determining whether there exists or has occurred or would reasonably be expected to exist or occur, a Company Material Adverse Effect under clause (i) above: (a) any adverse effect resulting directly or indirectly from general business or economic conditions, except to the extent such general business or economic conditions have a materially disproportionate effect on the Company as compared to companies in the Company’s industry; (b) any adverse effect resulting directly or indirectly from conditions generally affecting any industry or industry sector in which the Company operates or competes, except to the extent such adverse effect has a materially disproportionate effect on the Company as compared to companies in such industry or industry sector; (c) any adverse effect resulting from any change in accounting requirements or principles or any change in applicable Laws or the interpretation thereof, except to the extent such adverse effect has a materially disproportionate effect on the Company as compared to companies in the Company’s industry; (d) any act of terrorism, war or other armed hostilities, any regional, national or international calamity or natural disaster, any pandemic, epidemic or disease outbreak (including the COVID-19 pandemic), except to the extent such events have a materially disproportionate effect on the Company as compared to companies in the Company’s industry; (e) any failures, in and of themselves, by the Company to meet any projections, budgets, forecasts or estimates of financial performance or results of operations (it being understood that the facts or occurrences giving rise or contributing to such failure that are not otherwise excluded from this definition of “Company Material Adverse Effect” may be taken into account); (f) any adverse effect directly resulting from any action taken (or failure to take action) by the Company at the express direction of the Parent, its Affiliates or Representatives or that was expressly approved or ratified by the Parent in its capacity as a stockholder in the Company or (g) the execution, delivery, announcement or pendency of the Agreement or the consummation of the Contemplated Transactions, including departures of employees or changes in commercial or business relationships, including customers, suppliers and distributors arising therefrom.
“Company Meeting” shall have the meaning set forth in Section 5.2(b) of this Agreement.
“Company Minority Approval” means the adoption of this Agreement, and the approval of the transactions contemplated hereby, including the Merger, by the affirmative vote of at least a majority of the issued and outstanding shares of Company Capital Stock entitled to vote thereon, excluding shares of Company Capital Stock owned directly or indirectly by Parent or any Affiliate of Parent.
“Company Non-Voting Common Stock” shall mean the Company’s Common Stock, par value $0.001 per share, designated as non-voting in the Company Charter.
“Company Officers Certificate” shall have the meaning set forth in Section 7.5 of this Agreement.
“Company Option” shall mean any option to purchase shares of Company Common Stock.
“Company Owned Intellectual Property” shall have the meaning set forth in Section 2.8(a)(iv) of this Agreement.
“Company Owned Intellectual Property Rights” shall have the meaning set forth in Section 2.8(a)(iv) of this Agreement.
“Company Noteholders” shall mean each holder of a Company Convertible Note as of immediately prior to the Effective Time.
Annex A-56
Table of Contents
“Company Plans” shall mean “employee benefit plans” (as defined in Section 3(3) of ERISA, whether or not subject to ERISA), all severance, employment, incentive or bonus, retention, change in control, deferred compensation, profit sharing, retirement, welfare, post-employment welfare, fringe benefit, vacation or paid time off, equity or equity-based or other benefit or compensation plan, policy, program, agreement, Contract or arrangement that is sponsored, maintained, contributed to or required to be contributed to by the Company or any Company ERISA Affiliate for the benefit of any Company Service Provider or any spouse, dependent or beneficiary of any such individual, including as a participating employer in any plan, policy, program, agreement, Contract or arrangement sponsored or maintained by a professional employer organization, or under or with respect to which the Company or any Company ERISA Affiliate has or would reasonably be expected to have any liability or obligation (whether current or contingent) with respect to any Company Service Provider or any spouse, dependent or beneficiary of any such individual.
“Company Preferred Stock” shall mean collectively the “Series A Preferred Stock, Series B-1 Preferred Stock, the Series B-2 Preferred Stock, the Series B-3 Preferred Stock, the Series B-4 Preferred Stock, the Series C Preferred Stock and the Series D Preferred Stock.
“Company Privacy Commitments” shall have the meaning set forth in Section 2.8(r)(ii) of this Agreement.
“Company Privacy Policies” shall have the meaning set forth in Section 2.8(a)(vi) of this Agreement.
“Company Products” shall have the meaning set forth in Section 2.8(a)(vii) of this Agreement.
“Company Registered Intellectual Property” shall have the meaning set forth in Section 2.8(a)(viii) of this Agreement.
“Company Restricted Stock” shall mean a share of Company Common Stock that is (i) outstanding on the date of this Agreement and (ii) subject to a substantial risk of forfeiture within the meaning of Section 83 of the Code.
“Company Series C Warrant” shall mean the Warrant to purchase shares of Series C Preferred Stock issued to a Company Noteholder pursuant to a Subscription Agreement dated February 20, 2014 and any subsequent agreements relating thereto.
“Company Service Provider” shall mean any current or former employee, consultant, director or individual service provider of the Company or any other Company ERISA Affiliate.
“Company Service Provider Agreement” shall mean any employment or engagement agreement between the Company on one hand and a Company Service Provider on the other hand.
“Company Source Code” shall have the meaning set forth in Section 2.8(a)(ix) of this Agreement.
“Company Stock Certificate” shall have the meaning set forth in Section 1.8 of this Agreement.
“Company Stockholder Approval” shall have the meaning set forth in Section 5.2(b) of this Agreement.
“Company Stockholder Solicitation Period” means the period following the date hereof, which period, in all cases, shall end on the End Date.
“Company Stockholder Written Consent” shall have the meaning set forth in the recitals of this Agreement.
“Company Stockholders” shall mean the holders of Company Capital Stock.
“Company Tax” means any Tax, if and to the extent that the Company (or any predecessor) is or may be liable under any applicable law, under contract or on any other grounds (including, but not limited to, as a transferee, successor, or member of an Affiliated Group under Section 6901 of the Code or Treasury Regulation Section 1.1502-6, as a result of any Tax sharing or other agreement, or by operation of law) for any such Tax, in each case other than commercial contracts not primarily relating to Taxes.
Annex A-57
Table of Contents
“Company Tax Return” means any Tax Return, election, document, information, opinion, or any amendment to any of the foregoing (including, without limitation, any consolidated, combined or unitary return) filed or required to be filed with any Governmental Body or Government Official, if, in any manner or to any extent, relating to or inclusive of the Company (or any predecessor).
“Company Voting Common Stock” shall mean the Company’s Common Stock, par value $0.001 per share, designated as voting in the Company Charter.
“Company Warrants” shall mean the Common Warrant Option, the Company Series C Warrant and the 56% Warrant.
“Company Websites” shall have the meaning set forth in Section 2.8(a)(x) of this Agreement.
“Confidential Information” shall have the meaning set forth in Section 2.8(i) of this Agreement.
“Confidentiality Agreement” shall mean all confidentiality agreements entered by and between Parent and the Company.
“Consent(s)” shall mean any consent, approval or waiver.
“Contemplated Transactions” shall have the meaning set forth in the recitals of this Agreement.
“Contingent Rights” shall mean collectively the rights to receive Company Capital Stock pursuant to the Line of Credit and the Bonus Shares Agreement.
“Continuing Employee” shall have the meaning set forth in Section 5.10 of this Agreement.
“Contract” shall mean any contract, undertaking, concession, agreement, franchise, permit, instrument, license, lease, sublease, note, bond, indenture, deed of trust, mortgage, loan agreement or other binding commitment (including any binding plan, arrangement or agreement in principle), whether written or oral.
“Converted Options” shall have the meaning set forth in Section 1.6(b) of this Agreement.
“Counterparty” shall have the meaning set forth in Section 2.21 of this Agreement.
“D&O Indemnified Persons” shall have the meaning set forth in Section 5.4(a) of this Agreement.
“D&O Insurance” shall have the meaning set forth in Section 5.4(c) of this Agreement.
“D&O Resignations” shall have the meaning set forth in Section 5.9 of this Agreement.
“Debt” shall mean the outstanding principal amount of, and all interest, fees, prepayment premiums, expenses, breakage costs, indemnities, penalties or other amounts accrued in respect of and all amounts otherwise owing or payable at retirement of, (a) any indebtedness for borrowed money of the Company, (b) any obligation of the Company evidenced by bonds, debentures, notes, mortgages, security arrangements, indentures or other similar instruments, (c) any reimbursement obligation of the Company with respect to letters of credit (including standby letters of credit to the extent drawn upon), bankers’ acceptances or similar facilities issued for the account of the Company, (d) all obligations of the Company as lessee under leases that are required to be recorded as capital leases in accordance with GAAP, (e) all liabilities or obligations of the Company under any interest rate, currency, swap or other hedging agreements, (f) all liabilities or obligations of the Company for the deferred purchase price of property or services and other earn-out, milestone or other contingent payment obligations, (g) any Pre-Closing Taxes (other than Transaction Payroll Taxes), whether due prior to, on, or after the Closing, that have not been paid as of the Closing, and (h) any obligation of the type referred to in clauses (a) through (f) of another Person the payment of which the Company has guaranteed or for which the Company is responsible or liable, directly or indirectly, jointly or severally, as obligor or guarantor.
“DGCL” shall have the meaning set forth in the recitals of this Agreement.
“DLLCA” shall have the meaning set forth in the recitals of this Agreement.
Annex A-58
Table of Contents
“EC Merger Regulation” shall have the meaning set forth in Section 2.22 of this Agreement.
“Effective Time” shall have the meaning set forth in Section 1.3 of this Agreement.
“End Date” shall mean December 15, 2021; provided, however, that, in the event that the SEC has not declared effective under the Securities Act the Registration Statement by the date which is 45 calendar days prior to the End Date, then the End Date shall automatically be extended to February 1, 2022.
“Entity” shall mean any corporation (including any nonprofit corporation), general partnership, limited partnership, limited liability partnership, joint venture, estate, trust, company (including any limited liability company or joint stock company), firm or other enterprise, association, organization or entity.
“Environmental Law” shall mean any Law, any judicial order relating to (a) pollution or the protection, preservation or restoration of the environment (including, air, water vapor, surface water, groundwater, drinking water supply, surface land, subsurface land, plant and animal life or any other natural resource); (b) the exposure to, or the use, storage, recycling, treatment, generation, transportation, processing, handling, labeling, production, release or disposal of, any Hazardous Substances; or (c) or health safety issues (including human and occupational safety and health), in each case as amended and as in effect on or prior to the date hereof.
“Environmental Permit” shall mean any permit, license, review, certification, approval, registration, consent or other authorization issued or required pursuant to any Environmental Laws.
“ERISA” shall mean the Employee Retirement Income Security Act of 1974, as amended.
“Exchange Act” shall mean the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder.
“Exchange Agent” shall have the meaning set forth in Section 1.9(a) of this Agreement.
“FASB” shall have the meaning set forth in Section 2.4(b) of this Agreement.
“Financing” shall mean Parent’s raising of no less than $50 million of aggregate net proceeds (after payment of any fees and expenses associated with such transaction(s)) from one or more of the following sources of financing: (i) a private placement of Parent Capital Stock, (ii) an underwritten public offering of Parent Capital Stock registered with the SEC on Form S-3 (or other applicable form), or (iii) the sale of real property owned by Parent.
“Fraud” shall mean any actual fraud committed with the intent to deceive, as determined under Delaware law.
“GAAP” shall mean United States generally accepted accounting principles.
“Government Official” shall mean (a) any officer or employee of any Governmental Body, (b) any Person acting in an official capacity on behalf of a Governmental Body or quasi-governmental power or authority (including, but not limited to, the National Association of Securities Dealers (NASD), any stock exchange or any securities market, or any Person with any power or authority to administer, assess, audit, calculate, collect, impose, investigate, review or otherwise act with respect to any Tax or any Tax–related matter), (c) any officer or employee of a Person that is majority or wholly owned by a Governmental Body, (d) any officer or employee of a public international organization, such as the World Bank or the United Nations, (e) any officer or employee of a political party or any Person acting in an official capacity on behalf of a political party or (f) any candidate for political office with any Governmental Body.
“Governmental Body” shall mean any national, federal, regional, state, provincial, local, county, municipal or foreign or other governmental authority or quasi-governmental organization or any component part (including, but not limited to, any officer, official, branch, court, arbitration panel, agency, department, regulatory body, authority, tribunal, commission, instrumentality or agency) or instrumentality, legislative body, court, administrative agency, regulatory body, commission or instrumentality, including any multinational authority having governmental or quasi-governmental powers, or any other industry self-regulatory authority or arbitral body.
“Governmental Grant” shall have the meaning set forth in Section 2.8(a)(xi) of this Agreement.
Annex A-59
Table of Contents
“Hazardous Substance” means any substance for which exposure is regulated by any Governmental Body or any Environmental Law, including any toxic waste, pollutant, contaminant, hazardous substance, toxic substance, hazardous waste, special waste, petroleum or any derivative or by-product thereof, radon, radioactive material, asbestos, or asbestos containing material, urea formaldehyde foam insulation, lead, mold, mold spores and mycotoxins or polychlorinated biphenyls or other similar substances.
“HSR Act” shall have the meaning set forth in Section 2.22 of this Agreement.
“Inbound Licenses” shall have the meaning set forth in Section 2.8(m)(i) of this Agreement.
“Indemnification Agreements” shall have the meaning set forth in Section 5.4(a) of this Agreement.
“Initial Surviving Corporation” shall have the meaning set forth in Section 1.1(a) of this Agreement.
“Insurance Policies” shall have the meaning set forth in Section 2.17 of this Agreement.
“Intellectual Property” shall have the meaning set forth in Section 2.8(a)(xii) of this Agreement.
“Intellectual Property Rights” shall have the meaning set forth in Section 2.8(a)(xiii) of this Agreement.
“Intended Tax Treatment” shall have the meaning set forth in the recitals of this Agreement.
“IRS” shall mean the Internal Revenue Service.
“Knowledge” shall have the meaning set forth in Section 10.12 of this Agreement.
“Knowledge Individuals” shall mean the following Persons: Sanjeev Luther, Jenna Vadas and Wendy McDermott.
“Law” shall mean, with respect to any Person, any federal, state, local, municipal, foreign or other, statute, constitution, principle of common law, rule, regulation, executive order, injunction, judgment, order, award, decree, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Body that is binding on such Person.
“Leased Real Property” shall have the meaning set forth in Section 2.7 of this Agreement.
“Legal Proceeding” shall mean any suit, charge, complaint, litigation, arbitration or other proceeding (including any civil, criminal, administrative, investigative or appellate proceeding) asserting claims against the Company, or any hearing, inquiry, audit, examination or investigation of which Company has received written notice commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Body or any arbitrator or arbitration panel.
“Letter of Transmittal” shall have the meaning set forth in Section 1.9(b) of this Agreement.
“Lien” or “Liens” shall mean all mortgages, encumbrances, security interests, claims, charges or pledges.
“Line of Credit” shall mean that certain Line of Credit Loan Agreement by and between the Company and RP Finance LLC, dated as of February 3, 2020.
“Lock-Up Agreements” shall have the meaning set forth in the recitals of this Agreement.
“Material Contract” shall have the meaning set forth in Section 2.9(a) of this Agreement.
“Merger” shall have the meaning set forth in the recitals of this Agreement.
“Merger Shares” shall mean the aggregate number of shares of Parent Class B Common Stock to be issued in the Merger pursuant to this Agreement.
“Merger Sub I” shall have the meaning set forth in the preamble of this Agreement.
Annex A-60
Table of Contents
“Merger Sub II” shall have the meaning set forth in the preamble of this Agreement.
“New Plan” shall have the meaning set forth in Section 5.10(b) of this Agreement.
“NYSE” shall mean the New York Stock Exchange.
“Non-Scheduled Inbound Contracts” means Contracts that are: (i) licenses or other grants of rights to the Company for Shrink-Wrap Software pursuant to the standard terms therefor, (ii) licenses to the Company for Open Source Materials pursuant to the publicly available open source terms therefor, (iii) non-disclosure agreements, (iv) entered into with Company Service Providers or Company Consultants without material modification from a Standard Form IP Agreement, (v) privacy policies, or (vi) Contracts in which the only Intellectual Property licenses or Intellectual Property Rights granted to the Company are with respect to feedback, suggestions, or a trademark for inclusion on customer lists or use in the provision of services.
“Non-Scheduled Outbound Contracts” means (i) Contracts in which the only Intellectual Property licenses or Intellectual Property Rights granted by the Company are non-exclusive rights granted to a Third Party to use Intellectual Property for the sole benefit of the Company, (ii) Contracts governing the use of Company Products entered into with end user customers of the Company Products that are materially consistent with the Company’s form of customer agreement, (iii) privacy policies, (iv) non-disclosure agreements, (v) non-exclusive licenses of Intellectual Property Rights by the Company to a Third Party entered into the Ordinary Course of Business, and (vi) Contracts in which the only Intellectual Property licenses or rights granted by the Company are with respect to feedback, suggestions, or a Company trademark for inclusion on customer lists or use in the provision of services.
“Note Conversion Shares” shall mean the total number of shares of Company Series C Preferred Stock or Company Series D Preferred Stock into which the Company Convertible Notes are converted as of immediately prior to the Effective Time pursuant to the Company Convertible Notes and the Convertible Note Cancellation Agreements.
“Open Source Materials” shall have the meaning set forth in Section 2.8(a)(xiv) of this Agreement.
“Ordinary Course of Business” means, at any given time, the ordinary and usual course of operations of the business of the Company, consistent with past practice, subject to any reasonable changes required to address any then current facts and circumstances (including requirements to comply with applicable Law and to reasonably preserve the health and safety of current Company Service Providers).
“Other IP Contracts” shall have the meaning set forth in Section 2.8(m)(iii) of this Agreement.
“Outbound Licenses” shall have the meaning set forth in Section 2.8(m)(ii) of this Agreement.
“Parent” shall have the meaning set forth in the preamble of this Agreement.
“Parent Audited Balance Sheet” shall have the meaning set forth in Section 3.8 of this Agreement.
“Parent Board” shall have the meaning set forth in the recitals of this Agreement.
“Parent Capital Stock” shall mean the Parent Common Stock, the Class A common stock, par value $0.01 per share of the Parent and the Parent Preferred Stock.
“Parent Capitalization Date” shall have the meaning set forth in Section 3.2(a) of this Agreement.
“Parent Charter” shall mean Parent’s Amended and Restated Certificate of Incorporation and in effect immediately prior to the Effective Time.
“Parent Class A Common Stock” shall mean the Parent’s Class A Common Stock, par value $0.01 per share, the holders of which are entitled to three votes per share.
“Parent Class B Common Stock” shall mean the Parent’s Class B Common Stock, par value $0.01 per share, the holders of which are entitled to one-tenth of a vote per share.
Annex A-61
Table of Contents
“Parent Common Stock” shall mean the together the Parent Class A Common Stock and the Parent Class B Common Stock.
“Parent Disclosure Schedule” shall mean the disclosure schedule that has been prepared by Parent and the Merger Subs and delivered to the Company on the date of, and prior to the execution of, this Agreement.
“Parent Equity Plan” shall mean the Parent 2018 Equity Incentive Plan.
“Parent Financial Statements” shall have the meaning set forth in Section 3.7(c) of this Agreement.
“Parent Material Adverse Effect” shall mean any occurrence, change, event, circumstance or effect that, individually or in the aggregate with any other occurrences, changes, events, circumstances and/or effects, (i) has had or would reasonably be expected to have a material adverse effect on the liabilities, financial condition, existing business or assets of Parent and its Subsidiaries, taken as a whole, or (ii) that would prevent Parent from consummating the Merger in accordance with the terms hereof; provided, however, that none of the following (individually or in combination) shall be deemed to constitute, or shall be taken into account in determining whether there exists or has occurred or would reasonably be expected to exist or occur, a Parent Material Adverse Effect under clause (i) above: (a) any adverse effect resulting directly or indirectly from general business or economic conditions, except to the extent such general business or economic conditions have a materially disproportionate effect on Parent and its Subsidiaries as compared to any companies in Parent’s industry; (b) any adverse effect resulting directly or indirectly from conditions generally affecting any industry or industry sector in which Parent and its Subsidiaries operate or compete, except to the extent such adverse effect has a materially disproportionate effect on Parent and its Subsidiaries as compared to companies in such industry or industry sector; (c) any adverse effect resulting from any change in accounting requirements or principles or any change in applicable Laws or the interpretation thereof, except to the extent such adverse effect has a materially disproportionate effect on Parent as compared to companies in Parent’s industry; (d) any act of terrorism, war or other armed hostilities, any regional, national or international calamity or natural disaster, any pandemic, epidemic or disease outbreak (including the COVID-19 pandemic), except to the extent such events have a material disproportionate effect on Parent and its Subsidiaries as compared to companies in Parent’s industry; (e) any failures, in and of themselves, by Parent to meet any projections, budgets, forecasts or estimates of financial performance or results of operations (it being understood that the facts or occurrences giving rise or contributing to such failure that are not otherwise excluded from this definition of “Parent Material Adverse Effect” may be taken into account); (f) any adverse effect directly resulting from the Company’s failure to take any action (or to delay or not take any action) in accordance with express direction of the Parent, its Affiliates or Representatives exercising Parent’s authority as a controlling stockholder in the Company or (g) the execution, delivery, announcement or pendency of the Agreement or the consummation of the Contemplated Transactions, including departures of employees or changes in commercial or business relationships, including customers, suppliers and distributors arising therefrom.
“Parent Meeting” shall have the meaning set forth in Section 5.2(a) of this Agreement.
“Parent Minority Approval” means the adoption of this Agreement, and the approval of the transactions contemplated hereby, including the Merger, by the affirmative vote of at least a majority of the issued and outstanding Shares of Parent Common Stock, excluding Shares owned directly or indirectly by any Affiliate of Parent, Merger Subs or any of their respective officers or directors on or after the date hereof, including trusts for the benefit of immediate family members of such parties regardless of whether they are Affiliates, outstanding and entitled to vote thereon at the Parent Meeting.
“Parent Officers Certificate” shall have the meaning set forth in Section 8.5 of this Agreement.
“Parent Recommendation” shall have the meaning set forth in Section 3.3 of this Agreement.
“Parent Preferred Stock” shall mean the Preferred Stock, $0.01 par value per share, of Parent.
“Parent SEC Reports” shall have the meaning set forth in Section 3.7(a) of this Agreement.
“Parent Special Committee” shall have the meaning set forth in the recitals of this Agreement.
“Parent Stockholders” shall mean the holders of Parent Capital Stock.
Annex A-62
Table of Contents
“Parent Stockholder Approval” shall have the meaning set forth in Section 5.2(a) of this Agreement.
“Parent Stockholder Solicitation Period” means the period following the date that the Registration Statement is first mailed to all Parent Stockholders of record, which period, in all cases, shall end on the End Date.
“Parent Trading Price” shall mean the volume weighted average closing sale price of one share of Parent Class B Common Stock as reported on the NYSE for the ten consecutive trading day period ending the fifth trading day prior to the Closing Date.
“Parties” mean Parent, Merger Sub I, Merger Sub II and the Company.
“Patents” shall mean all patents and patent applications (including provisional applications) and patent disclosures, and including all divisionals, continuations, substitutions, continuations-in-part, re-examinations, re-issues, additions, renewals, extensions, confirmations, registrations, any confirmation patent or registration patent or patent of addition based on any such patent, patent term extensions, and supplemental protection certificates or requests for continued examinations, foreign counterparts, and the like of any of the foregoing.
“Per Share Merger Consideration” shall mean, subject to Section 1.5(f), the applicable Per Series A Share Merger Consideration, Per Series B-1 Share Merger Consideration, Per Series B-2 Share Merger Consideration, Per Series B-3 Share Merger Consideration, Per Series B-4 Share Merger Consideration, Per Series C Share Merger Consideration, Per Series D Share Merger Consideration, Per Common Share Merger Consideration, Per Bonus Share Merger Consideration or Per Line of Credit Share Merger Consideration, as applicable to a holder of Company Capital Stock or Contingent Rights as of the Effective Time.
“Per Series A Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series A Preferred issued and outstanding as of the Effective Time.
“Per Series B-1 Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series B-1 Preferred issued and outstanding as of the Effective Time.
“Per Series B-2 Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series B-2 Preferred issued and outstanding as of the Effective Time.
“Per Series B-3 Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series B-3 Preferred issued and outstanding as of the Effective Time.
“Per Series B-4 Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series B-4 Preferred issued and outstanding as of the Effective Time.
“Per Series C Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series C Preferred issued and outstanding as of the Effective Time.
“Per Series D Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Series D Preferred issued and outstanding as of the Effective Time.
“Per Common Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Common Stock issued and outstanding as of the Effective Time.
“Per Bonus Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Common Stock issuable pursuant to the Bonus Shares Agreement as of September 30, 2021 (as if the Mergers were consummated on such date).
Annex A-63
Table of Contents
“Per Line of Credit Share Merger Consideration” shall mean the right to receive 0.12045 shares of Parent Class B Common Stock for each share of Company Common Stock issuable pursuant to the Line of Credit as of September 30, 2021 (as if the Mergers were consummated on such date).
“Permits” shall have the meaning set forth in Section 2.11(b) of this Agreement.
“Permitted Liens” shall mean: (a) Liens for current Taxes not yet delinquent or that are being contested in good faith by appropriate proceedings and for which adequate reserves have been established on the face of the Balance Sheet in accordance with GAAP, (b) Liens securing Debt that is reflected on the Balance Sheet; (c) statutory or common Liens to secure obligations to landlords, lessors or renters under leases or rental agreements; (d) deposits or pledges made in connection with, or to secure payment of, workers’ compensation, unemployment insurance or similar programs mandated by applicable Law or governmental regulations; (e) statutory or common Liens in favor of carriers, warehousemen, mechanics and materialmen, to secure claims for labor, materials or supplies, and other like Liens; (f) easements, reservations, rights-of-way, restrictions, minor defects or irregularities in title and other similar Liens affecting real property not interfering in any material respect with the ordinary conduct of the business of the Company; (g) Liens in favor of customs and revenue authorities arising as a matter of Law to secure payment of customs duties in connection with the importation of goods; (h) Liens arising solely by virtue of any statutory or common provision relating to banker’s Liens, rights of setoff or similar rights and remedies as to deposit accounts or other funds maintained with a creditor depository institution; (i) Liens in favor of other financial institutions arising in connection with the Company’s deposit accounts or securities accounts held at such institutions to secure customary fees, charges, and the like; (j) Liens or encumbrances imposed on the underlying fee interest in real property subject to a Real Property Lease; or (k) restrictions associated with (1) Third Party rights and licenses set forth in Intellectual Property Contracts listed on Part 2.8(l) of the Company Disclosure Schedule or in Non-Scheduled Inbound Contracts or (2) non-exclusive rights granted under Intellectual Property by the Company pursuant to Company Intellectual Property Agreements or Non-Scheduled Outbound Contracts.
“Person” shall mean any individual, Entity or Governmental Body.
“Personal Data” shall have the meaning set forth in Section 2.8(a)(xv) of this Agreement.
“Pre-Closing Period” shall have the meaning set forth in Section 4.1 of this Agreement.
“Pre-Closing Tax Period” means any Tax period ending on or prior to the Closing Date and any portion of any Straddle Period ending on or prior to the Closing Date.
“Pre-Closing Taxes” shall mean (i) all Taxes of or with respect to the Company for Pre-Closing Tax Periods, including employer-portion payroll and employment Taxes with respect to compensation accruing in any Pre-Closing Tax Period, regardless of when such Taxes are required to be paid (ii) all Taxes of any member of an affiliated, consolidated, combined or unitary group of which the Company (or any predecessor thereto) is or was a member on or prior to the Closing Date, including pursuant to Treasury Regulation Section 1.1502-6 or any analogous or similar state, local, or non-U.S. Law or regulation, (iii) all Taxes of any Person imposed on the Company as a transferee or successor, by Contract or by operation of Law, or otherwise, which Taxes relate to an event or transaction occurring before the Closing, (iv) any Transaction Payroll Taxes, and (v) any Taxes imposed on income includible by the Subsidiaries of the Company pursuant to Sections 951, 951A or 965 of the Code arising out of, resulting from or attributable to income earned by any “controlled foreign corporation” (as such term is defined in Section 957 of the Code) in any Pre-Closing Tax Period. In the case of any taxable period that includes (but does not end on) the Closing Date, the amount of any Taxes based on or measured by income, receipts or payroll of the Company for the Pre-Closing Tax Period or imposed in connection with any transaction that is allocable to the portion of the period ending on the Closing Date shall be determined based on an interim closing of the books as of the close of business on the Closing Date (and in the case of any Taxes attributable to the ownership of any equity interest in any partnership or other flow-through Entity or “controlled foreign corporation” (within the meaning of Section 957(a) of the Code or any comparable state, local or non-U.S. Law), as if the taxable period of such partnership or other flow-through Entity or “controlled foreign corporation” ended as of the end of the Closing Date), except that exemptions, allowances or deductions that are calculated on an annual basis (including depreciation and amortization deductions), other than with respect to property placed in service after the Closing, will be allocated on a per diem basis, and the amount of other Taxes of the Company that relate to the Pre-Closing Tax Period shall be deemed to be the amount of such Tax for the entire taxable period multiplied by a fraction the numerator of which is number of days in the taxable period ending on
Annex A-64
Table of Contents
the Closing Date and the denominator of which is the number of days in the entire taxable period. For the avoidance of doubt, for purposes of allocating Taxes imposed under Section 951A of the Code, the “qualified business asset investment” (as such term is used in Section 951A(d) of the Code) in respect of the Pre-Closing Tax Period shall equal the product of (i) the applicable Subsidiary’s “qualified business asset investment” (as defined in Section 951A(d)(1) of the Code) for the taxable year of such Subsidiary that includes the Closing Date (determined as though such taxable year ended on the Closing Date), and (ii) a fraction, the numerator of which is the number of days in the portion of such taxable year ending on the Closing Date and the denominator of which is the total number of days in such taxable year.
“Privacy Laws” shall have the meaning set forth in Section 2.8(a)(xvi) of this Agreement.
“Process”, “Processed” or “Processing” shall have the meaning set forth in Section 2.8(a)(xvii) of this Agreement.
“Proceeding” means any audit, administrative action, assessment, case, deposition, examination, executive action, filing, hearing, information request, injunction, inquiry, investigation, judgment, levy, litigation, order, reassessment, review, seizure, subpoena, suit, summons, testimony, or other activity involving or conducted by or on behalf of any Governmental Body and or Government Official.
“Real Property Leases” shall have the meaning set forth in Section 2.7 of this Agreement.
“Registration Statement” shall have the meaning set forth in Section 5.1(a) of this Agreement.
“Representatives” of a Person mean officers, directors, employees, attorneys, accountants, investment bankers, consultants, agents, financial advisors, other advisors and other representatives of such Person.
“Required Company Stockholder Vote” shall mean with respect to the Company, the affirmative vote or consent Company Stockholders holding: (i) a majority of the issued and outstanding shares of Company Common Stock (including shares of Company Preferred Stock voting on an as-converted to Common Stock basis); (ii) a majority of the issued and outstanding shares of Series A Preferred Stock, Series B-1 Preferred Stock, Series B-2 Preferred Stock, Series B-3 Preferred Stock and Series B-4 Preferred Stock (voting as a single class on an as-converted to Common Stock basis); (iii) a majority of the issued and outstanding shares of Series A Preferred Stock, Series B-1 Preferred Stock, Series B-2 Preferred Stock, Series B-3 Preferred Stock, Series B-4 Preferred Stock and Series C Preferred Stock (voting as a single class on an as-converted to Common Stock basis); (iv) a majority of the issued and outstanding shares of Series D Preferred Stock.
“Required Parent Stockholder Vote” shall mean the affirmative vote of the holders of Parent Common Stock who hold as of the applicable record date a majority of the votes entitled to vote on the adoption of this Agreement and the Merger and, if applicable, the issuance of the Parent Class B Common Stock contemplated by this Agreement.
“Altira Acquisition Agreement” shall have the meaning set forth in Section 5.12 of this Agreement.
“Securities Act” shall mean Securities Act of 1933, as amended, and the rules and regulations thereunder.
“Security Incident” shall have the meaning set forth in Section 2.8(r)(iv) of this Agreement
“Securityholders” shall mean the holders of Company Capital Stock, Company Options, the Company Warrants, or other securities convertible into Company Capital Stock.
“Series A Preferred Stock” shall mean the Series A Preferred Stock, $0.001 par value per share, of the Company.
“Series B-1 Preferred Stock” shall mean the Series B-1 Preferred Stock, $0.001 par value per share, of the Company.
“Series B-2 Preferred Stock” shall mean the Series B-2 Preferred Stock, $0.001 par value per share, of the Company.
“Series B-3 Preferred Stock” shall mean the Series B-3 Preferred Stock, $0.001 par value per share, of the Company.
Annex A-65
Table of Contents
“Series B-4 Preferred Stock” shall mean the Series B-4 Preferred Stock, $0.001 par value per share, of the Company.
“Series C Preferred Stock” shall mean the Series C Preferred Stock, $0.001 par value per share, of the Company.
“Series D Preferred Stock” shall mean the Series D Preferred Stock, $0.001 par value per share, of the Company.
“Serious Adverse Event” shall have the meaning set forth in Section 2.25(q) of this Agreement.
“Shrink-Wrap Software” shall have the meaning set forth in Section 2.8(a)(xviii) of this Agreement.
“SOX” shall have the meaning set forth in Section 3.7(a) of this Agreement.
“Special Committee Recommendation” shall have the meaning set forth in the recitals of this Agreement.
“Specified Representations” shall mean the representations and warranties set forth in Sections Section 2.13 (Tax Matters), Section 2.19 (Authority; Binding Nature of Agreement) and Section 2.23 (Financial Advisor).
“Standard Form IP Agreement” means a Company form of a proprietary information and invention disclosure agreements or Intellectual Property assignment currently or historically (during the prior 3 years) used by the Company for Company Service Providers who independently or jointly contributed to or participated in the conception, reduction to practice, creation or development of any Company Owned Intellectual Property.
“Statement No. 5” shall have the meaning set forth in Section 2.4(b) of this Agreement.
“Straddle Period” means any Tax period beginning before the Closing Date and ending after the Closing Date.
“Subsequent Merger” shall have the meaning set forth in the recitals of this Agreement.
“Subsequent Merger Effective Time” shall have the meaning set forth in Section 1.3 of this Agreement.
“Subsidiary” of any Person means (a) a corporation more than 50% of the combined voting power of the outstanding voting securities of which is owned, directly or indirectly, by such Person or by one or more other Subsidiaries of such Person or by such Person and one or more other Subsidiaries thereof, (b) a partnership of which such Person, or one or more other Subsidiaries of such Person or such Person and one or more other Subsidiaries thereof, directly or indirectly, is the general partner and has the power to direct the policies, management and affairs of such partnership, (c) a limited liability company of which such Person or one or more other Subsidiaries of such Person and one or more other Subsidiaries thereof, directly or indirectly, is the managing member and has the power to direct the policies, management and affairs of such company, or (d) any other Person (other than a corporation, partnership or limited liability company) in which such Person, or one or more other Subsidiaries of such Person or such Person and one or more other Subsidiaries thereof, directly or indirectly, has at least a majority ownership and power to direct the policies, management and affairs thereof.
“Support Agreement” shall have the meaning set forth in the recitals.
“Surviving Company” shall have the meaning set forth in Section 1.1(b) of this Agreement.
“Takeover Inquiry” means, with respect to the Company, an inquiry, indication of interest or request for information (other than an inquiry, indication of interest or request for information made or submitted by the Company to Parent) that would reasonably be expected to lead to a Takeover Proposal.
“Takeover Proposal” means, with respect to the Company, an offer, proposal or indication of interest involving the Company and any Person relating to a Takeover Transaction; provided, however, that a “Takeover Proposal” shall not include (or take into account) the transactions contemplated by this Agreement.
Annex A-66
Table of Contents
“Takeover Statute” shall have the meaning set forth in Section 2.20 of this Agreement.
“Takeover Transaction” means, with respect to the Company, any transaction or series of related transactions involving (i) any acquisition or purchase by any Person, directly or indirectly, of any class of outstanding voting securities of the Company (including pursuant to any tender offer (including a self-tender) or exchange offer), in each case, that, if consummated, would result in a Person acquiring beneficial ownership of 15% or more of any class of outstanding voting securities of the Company, (ii) any merger, acquisition, amalgamation, consolidation, business combination, joint venture or other similar transaction in which the Company is a constituent party and which would result in a Person acquiring beneficial ownership of 15% or more of the outstanding voting securities of the Company or (iii) any sale, transfer or disposition of any business or businesses, or assets, of the Company which constitute 15% or more of the fair market value of the Company, or any liquidation or dissolution of the Company.
“Tax” means, to any extent relating to or inclusive of the Company (or any predecessor), any direct or indirect Subsidiary of the Company (or any predecessor): (a) any tax, charge, deficiency, duty, fee, levy, toll or other similar amount in each case in the nature of a tax (including, without limitation, any net income, gross income, profits, gross receipts, escheat, excise, property, sales, ad valorem, withholding, social security, retirement, employment, unemployment, minimum, estimated, severance, stamp, occupation, environmental, premium, capital stock, disability, windfall profits, use, service, net worth, payroll, franchise, license, gains, customs, transfer, recording, registration, value added, surtax, capital gains, goods and services, registration, alternative or add-on or other tax) assessed or otherwise imposed under the Code or any other similar applicable Law and or by the Internal Revenue Service or any other similar agency under any Governmental Body or any Government Official, together with any interest, penalties or any other additions or increases; (b) any liability for any amount described in clause (a) whether as a result of transferee or successor liability, of being a member of an affiliated, consolidated, combined or unitary group for any period or otherwise through operation of law; and (c) any liability for the payment of amounts described in clauses (a) or (b) as a result of any tax sharing, tax indemnity or tax allocation agreement or any other express or implied agreement to indemnify any other Person.
“Tax Returns” shall mean any return, declaration, notice, statement, report, tax filing or form (including estimated Tax returns and reports, withholding Tax returns and reports, any schedule or attachment, and information returns and reports) filed or required to be filed with respect to Taxes (including any amendments thereof), or maintained by any Person, or required to be maintained by any Person, in connection with the determination, assessment or collection of any Tax of any party or the administration of any Laws, regulations or administrative requirements relating to any Tax.
“Technology” shall have the meaning set forth in Section 2.8(a)(xix) of this Agreement.
“Third Party” shall mean, with respect to any party, any Person other than such party or an Affiliate of such party.
“Trade Secrets” shall have the meaning set forth in Section 2.8(a)(xx) of this Agreement.
“Transaction Payroll Taxes” shall mean the employer portion of any payroll or employment Taxes incurred or accrued with respect to any bonuses, option exercises or other compensatory payments in connection with the Contemplated Transactions to the extent the foregoing are paid on or about or prior to the Closing Date.
“Voting Agreements” shall have the meaning set forth in the recitals of this Agreement.
“Withholding Agent” shall have the meaning set forth in Section 1.9(d) of this Agreement.
Annex A-67
Table of Contents
Annex B
FORM OF LOCK-UP AGREEMENT
THIS LOCK-UP AGREEMENT (this “Agreement”) is made and entered into as of June 17, 2021 by and among RAFAEL HOLDINGS, INC., a Delaware corporation (the “Parent”), RAFAEL PHARMACEUTICALS, INC., a Delaware corporation (the “Company”), and the undersigned (the “Holder”). The Parent, the Company and the Holder are sometimes referred to herein individually as a “Party” and, collectively, as the “Parties”. Any capitalized term used but not defined in this Agreement will have the meaning ascribed to such term in the Merger Agreement (as defined below).
WHEREAS, the Parent, RH MERGER I, INC., a Delaware corporation and a wholly-owned subsidiary of Parent (“Merger Sub I”); RH MERGER II, LLC, a Delaware limited liability company and a wholly-owned subsidiary of Parent (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”), and the Company, entered into that certain Agreement and Plan of Merger on June 17, 2021 (as amended from time to time in accordance with the terms thereof, the “Merger Agreement”), pursuant to which, subject to the terms and conditions thereof, the parties thereto will consummate a series of transactions, including (i) the issuance of shares of the Parent’s Class B Common Stock, par value $0.01 per share, the holders of which are entitled to one-tenth of a vote per share (“Parent Class B Common Stock”), and (ii) the exchange of Company Options for options to purchase shares of Parent Class B Common Stock (such replacement options, the “Parent Options”).
WHEREAS, following completion of the transactions contemplated by the Merger Agreement, the Holder will hold Parent Options and/or Parent Class B Common Stock.
WHEREAS, pursuant to the Merger Agreement, and in view of the valuable consideration to be received by the Holder thereunder, the Parent, the Company and the Holder desire to enter into this Agreement, pursuant to which the Parent Class B Common Stock to be received by the Holder pursuant to the Merger Agreement, the Parent Class B Common Stock issued upon the exercise of Parent Options and the Parent Class B Common Stock and the Parent Class A Common Stock held by the Holder or its Affiliates as of the date hereof (together with any securities paid as dividends or distributions with respect to such securities or into which such securities are exchanged or converted, the “Restricted Securities”) shall become subject to limitations on disposition as set forth herein.
NOW, THEREFORE, in consideration of the premises set forth above, which are incorporated in this Agreement as if fully set forth below, and intending to be legally bound hereby, the Parties hereby agree as follows:
1. Lock-Up Provisions.
(a) The Holder hereby agrees not to Transfer any Parent Class B Common Stock (including Parent Class B Common Stock issued or issuable upon the exercise of the Parent Options), during the period commencing from Subsequent Merger Effective Time and ending on the earlier of (a) six (6) months after the Subsequent Merger Effective Time or (b) the date on which the Parent completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of the Parent’s stockholders having the right to exchange their Parent Class B Common Stock for cash, securities or other property (the “Lock-Up Period”). Any discretionary waiver or termination of the restrictions of any or all of such agreements by the Parent shall apply pro rata to all stockholders of the Parent (including the former stockholders of the Company) that are subject to such agreements, based on the number of shares subject to such agreements.
(b) Notwithstanding the provisions set forth in Section 1(a), Transfers of the Restricted Securities that are held by the Holder are permitted (a) to any affiliates of the Holder; (b) in the case of an individual, transfers by gift to a member of the individual’s immediate family, to a trust, the beneficiary of which is a member of the individual’s immediate family or an affiliate of such person, or to a charitable organization; (c) in the case of an individual, transfers by virtue of laws of descent and distribution upon death of the individual; (d) in the case of an individual, transfers pursuant to a qualified domestic relations order; (e) to the Parent or any of its Affiliates or upon exercise of the Parent’s or its Affiliates’ right to repurchase or reacquire any Parent capital stock, including without limitation pursuant to the equity incentive plans, “early exercise” documents or other arrangements of the Parent or its Affiliates; and (f) pursuant to a bona fide third-party tender offer for all outstanding shares of the Parent, merger, consolidation or other similar transaction made to all holders of the Parent’s securities involving a Change of Control of the Parent (including,
Annex B-1
Table of Contents
without limitation, entering into any lock-up, voting or similar agreement pursuant to which the Holder may agree to transfer, sell, tender or otherwise dispose of Restricted Securities or other such securities in connection with such transaction, or vote any Restricted Securities or other such securities in favor of any such transaction) (provided that in the event that such tender offer, merger, consolidation or other such transaction is not completed, the Restricted Securities held by the Holder shall remain subject to the provisions of this Agreement); provided, however, that any of these permitted transferees (other than a permitted transferee under clauses (e) and (f)) must enter into a written agreement agreeing to be bound by the restrictions herein. For the avoidance of doubt the restrictions set forth herein shall not apply to the exercise of any Parent Options. For purposes of this Agreement, “Change of Control” shall mean shall mean the Transfer (whether by tender offer, merger, consolidation or other similar transaction), in one transaction or a series of related transactions, to a person or group of affiliated persons of the Parent’s voting securities if, after such transfer, such person or group of affiliated persons would hold more than 50% of the outstanding voting securities of the Parent (or the surviving entity).
(c) If any Transfer is made or attempted contrary to the provisions of this Agreement, such Transfer shall be null and void ab initio, and the Parent and the Company shall refuse to recognize any such transferee of the Restricted Securities as one of its equity holders for any purpose. In order to enforce this Section 1, the Parent and the Company may impose stop-transfer instructions with respect to the Restricted Securities of the Holder (and any permitted transferees and assigns thereof) until the end of the Lock-Up Period.
(d) During the Lock-Up Period, each certificate evidencing any Restricted Securities shall be stamped or otherwise imprinted with a legend in substantially the following form, in addition to any other applicable legends:
“THE SECURITIES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO RESTRICTIONS ON TRANSFER SET FORTH IN A LOCK-UP AGREEMENT, DATED AS OF JUNE __, 2021, BY AND AMONG THE ISSUER OF SUCH SECURITIES (THE “ISSUER”) AND THE ISSUER’S SECURITY HOLDER NAMED THEREIN, AS AMENDED. A COPY OF SUCH LOCK-UP AGREEMENT WILL BE FURNISHED WITHOUT CHARGE BY THE ISSUER TO THE HOLDER HEREOF UPON WRITTEN REQUEST.”
(e) For the avoidance of any doubt, the Holder shall retain all of its rights as a stockholder of the Parent with respect to the Restricted Securities during the Lock-Up Period, including the right to vote any Restricted Securities.
(f) For the purposes of this Section 1, “Transfer” shall mean the (a) sale of, offer to sell, contract or agreement to sell, hypothecate, pledge, grant of any option to purchase or otherwise dispose of or agreement to dispose of, directly or indirectly, or establishment or increase of a put equivalent position or liquidation with respect to or decrease of a call equivalent position within the meaning of Section 16 of the Securities Exchange Act of 1934, as amended, and the rules and regulations of the US Securities and Exchange Commission promulgated thereunder with respect to, any security, (b) entry into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (c) public announcement of any intention to effect any transaction specified in clause (a) or (b).
2. Miscellaneous.
(a) Effective Date. Section 1 of this Agreement shall become effective upon the Subsequent Merger Effective Time, subject to the consummation of the transactions contemplated by the Merger Agreement on and before the Subsequent Merger Effective Time.
(b) Termination of the Merger Agreement. Notwithstanding anything to the contrary contained herein, in the event that the Merger Agreement is terminated in accordance with its terms prior to the Subsequent Merger Effective Time, this Agreement and all rights and obligations of the Parties hereunder shall automatically terminate and be of no further force or effect.
Annex B-2
Table of Contents
(c) Binding Effect; Assignment. This Agreement and all of the provisions hereof shall be binding upon and inure to the benefit of the Parties hereto and their respective permitted successors and assigns. Except as otherwise provided in this Agreement, this Agreement and all obligations of the Parties are personal to the Parties and may not be transferred or delegated by the Parties at any time.
(d) Third Parties. Nothing contained in this Agreement or in any instrument or document executed by any party in connection with the transactions contemplated hereby shall create any rights in, or be deemed to have been executed for the benefit of, any person or entity that is not a Party hereto or thereto or a successor or permitted assign of such a Party.
(e) Governing Law; Jurisdiction. This Agreement and any dispute or controversy arising out of or relating to this Agreement shall be governed by and construed in accordance with the laws of the State of Delaware, without regard to the conflict of law principles thereof. All actions arising out of or relating to this Agreement shall be heard and determined exclusively in the Court of Chancery of the State of Delaware (or, only if such court declines to accept jurisdiction over a particular matter, any state or federal court within the State of Delaware) (the “Specified Courts”). Each Party hereto hereby (i) submits to the exclusive jurisdiction of any Specified Court for the purpose of any action arising out of or relating to this Agreement brought by any party hereto and (ii) irrevocably waives, and agrees not to assert by way of motion, defense or otherwise, in any such action, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune from attachment or execution, that the action is brought in an inconvenient forum, that the venue of the action is improper, or that this Agreement or the transactions contemplated hereby may not be enforced in or by any Specified Court. Each Party agrees that a final judgment in any action shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by Law. Each Party irrevocably consents to the service of the summons and complaint and any other process in any other action or proceeding relating to the transactions contemplated by this Agreement, on behalf of itself, or its property, by personal delivery of copies of such process to such Party at the applicable address set forth in Section 2(h). Nothing in this Section 2(e) shall affect the right of any party to serve legal process in any other manner permitted by applicable law.
(f) WAIVER OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY WITH RESPECT TO ANY ACTION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY. EACH PARTY HERETO (i) CERTIFIES THAT NO REPRESENTATIVE OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF ANY ACTION, SEEK TO ENFORCE THAT FOREGOING WAIVER AND (ii) ACKNOWLEDGES THAT IT AND THE OTHER PARTIES HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 2(f).
(g) Interpretation. The titles and subtitles used in this Agreement are for convenience only and are not to be considered in construing or interpreting this Agreement. In this Agreement, unless the context otherwise requires: (i) any pronoun used in this Agreement shall include the corresponding masculine, feminine or neuter forms, and the singular form of nouns, pronouns and verbs shall include the plural and vice versa; (ii) “including” (and with correlative meaning “include”) means including without limiting the generality of any description preceding or succeeding such term and shall be deemed in each case to be followed by the words “without limitation”; (iii) the words “herein,” “hereto,” and “hereby” and other words of similar import in this Agreement shall be deemed in each case to refer to this Agreement as a whole and not to any particular section or other subdivision of this Agreement; and (iv) the term “or” means “and/or”. The Parties have participated jointly in the negotiation and drafting of this Agreement. Consequently, in the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties hereto, and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement.
(h) Notices. Any notice or other communication required or permitted to be delivered to any party under this Agreement shall be in writing and shall be deemed properly delivered, given and received (a) upon receipt when delivered by hand, (b) upon transmission, if sent by electronic transmission, or (c) one (1) Business Day after
Annex B-3
Table of Contents
being sent by courier or express delivery service, provided that in each case the notice or other communication is sent to the address electronic mail address set forth beneath the name of such party below (or to such other address or electronic mail address as such party shall have specified in a written notice given to the other Parties hereto):
If to Parent or Merger Sub I or Merger Sub II, to: Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Attention: Chief Financial Officer
E-mail: david.polinsky@rafaelholdings.com | | With copies to (which shall not constitute notice): Olshan Frome Wolosky LLP
1325 Avenue of the Americas
New York, NY 10019
Attention: Mitchell Raab
E-mail: mraab@olshanlaw.com And: Schwell Wimpfheimer & Associates
37 West 39th Street, Suite 505
New York, NY 10018
Attention: Dov Schwell
E-mail: dov.schwell@swalegal.com |
If to the Company (prior to the Closing), to: Rafael Pharmaceuticals, Inc.
1 Duncan Drive
Cranbury, NJ 08512
Attention: Sanjeev Luther, Chief Executive Officer
E-mail: sanjeev.luther@rafaelpharma.com | | With copies to (which shall not constitute notice): Orrick Herrington & Sutcliffe LLP
1152 15th Street, N.W.
Washington, DC 20005 – 1706
Attention: David E. Schulman
E-mail: dschulman@orrick.com |
If to the Holder, to: the address set forth under the Holder’s name on the signature page hereto. |
(i) Amendments and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally or in a particular instance, and either retroactively or prospectively) only with the written consent of the Parent, the Company and the Holder. No failure or delay by a Party in exercising any right hereunder shall operate as a waiver thereof. No waivers of or exceptions to any term, condition, or provision of this Agreement, in any one or more instances, shall be deemed to be or construed as a further or continuing waiver of any such term, condition, or provision.
(j) Severability. In case any provision in this Agreement shall be held invalid, illegal or unenforceable in a jurisdiction, such provision shall be modified or deleted, as to the jurisdiction involved, only to the extent necessary to render the same valid, legal and enforceable, and the validity, legality and enforceability of the remaining provisions hereof shall not in any way be affected or impaired thereby nor shall the validity, legality or enforceability of such provision be affected thereby in any other jurisdiction. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties will substitute for any invalid, illegal or unenforceable provision a suitable and equitable provision that carries out, so far as may be valid, legal and enforceable, the intent and purpose of such invalid, illegal or unenforceable provision.
(k) Specific Performance. The Holder acknowledges that its obligations under this Agreement are unique, recognizes and affirms that in the event of a breach of this Agreement by the Holder, money damages will be inadequate and the Parent and the Company will have no adequate remedy at law, and agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed by the Holder in accordance with their specific terms or were otherwise breached. Accordingly, the Parent and the Company shall be entitled to an injunction or restraining order to prevent breaches of this Agreement by the Holder and to enforce specifically the terms and provisions hereof, without the requirement to post any bond or other security or to prove that money damages would be inadequate, this being in addition to any other right or remedy to which such Party may be entitled under this Agreement, at law or in equity.
(l) Entire Agreement. This Agreement constitutes the full and entire understanding and agreement among the Parties with respect to the subject matter hereof, and any other written or oral agreement relating to the subject matter hereof existing between the Parties is expressly canceled; provided, that, for the avoidance of doubt, the foregoing shall not affect the rights and obligations of the Parties under the Merger Agreement or any of the
Annex B-4
Table of Contents
other Ancillary Documents. Notwithstanding the foregoing, nothing in this Agreement shall limit any of the rights or remedies of the Company or the Parent or any of the obligations of the Holder under any other agreement between the Holder and the Company or the Parent or any certificate or instrument executed by the Holder in favor of the Company or the Parent, and nothing in any other agreement, certificate or instrument shall limit any of the rights or remedies of the Company or the Parent or any of the obligations of the Holder under this Agreement.
(m) Further Assurances. From time to time, at another Party’s request and without further consideration (but at the requesting Party’s reasonable cost and expense), each Party shall execute and deliver such additional documents and take all such further action as may be reasonably necessary to consummate the transactions contemplated by this Agreement.
(n) Counterparts; Facsimile. This Agreement may also be executed and delivered by facsimile signature or by email in portable document format in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
[Remainder of Page Intentionally Left Blank; Signature Pages Follow]
Annex B-5
Table of Contents
CONFIDENTIAL
DRAFT
IN WITNESS WHEREOF, the parties have executed this Lock-Up Agreement as of the date first written above.
| | THE COMPANY: |
| | | RAFAEL PHARMACEUTICALS, INC. |
| | | By: | | |
| | | Name: | | |
| | | Title: | | |
| | | THE PARENT: |
| | | RAFAEL HOLDINGS, INC. |
| | | By: | | |
| | | Name: | | |
| | | Title: | | |
[Signature Page to Lock-Up Agreement]
Annex B-6
Table of Contents
CONFIDENTIAL
IN WITNESS WHEREOF, the parties have executed this Lock-Up Agreement as of the date first written above.
Holder: | | | | |
Name of Holder: | | | | |
Print Name: | | | | |
Signature: | | | | |
Address for Notice: | | |
Address: | | | | |
| | |
| | |
Telephone No.: | | | | |
Email: | | | | |
[Signature Page to Lock-Up Agreement]
Annex B-7
Table of Contents
Annex C
FORM OF SUPPORT AGREEMENT
This SUPPORT AGREEMENT (this “Agreement”), dated as of June 17, 2021, is made and entered into by and among Rafael Holdings, Inc., a Delaware corporation (“Parent”), Rafael Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and the equityholder of the Company party hereto (“Equityholder” and together with other equityholders of the Company, “Equityholders”). Capitalized terms used and not otherwise defined herein shall have the meanings ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
WHEREAS, the Company, Parent, RH Merger I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Parent (“Merger Sub I”), and RH Merger II, LLC, a Delaware limited liability company and a direct, wholly owned subsidiary of Parent (“Merger Sub II” and together with Merger Sub I. “Merger Subs”), are entering into an Agreement and Plan of Merger (as the same may be amended or modified, the “Merger Agreement”), providing for, among other things, the merger of Merger Sub I with and into the Company (the “Merger”), with the Company surviving the Merger and becoming a direct, wholly owned subsidiary of Parent, followed by the merger of the Company with and into Merger Sub II (the “Subsequent Merger”), with Merger Sub II surviving the Subsequent Merger as a direct, wholly owned subsidiary of Parent, in each case on the terms and subject to the conditions set forth in the Merger Agreement.
WHEREAS, (i) Equityholder and Equityholder’s Affiliates and Family Members (each as defined below) own beneficially and of record: (A) the number and class of shares of Company Capital Stock set forth on Exhibit C hereto (such Company Capital Stock, together with any other Company Capital Stock now beneficially owned or acquired (whether by purchase, gift, distribution, exercise of options or warrants, conversion of notes or otherwise) by Equityholder after the date hereof, being collectively referred to herein as the “Equityholder Shares”); (B) to the extent applicable, the number of Options set forth on Exhibit C hereto (the “Equityholder Options”); (C) to the extent applicable, the number of shares of Company Common Stock issuable pursuant to the Bonus Shares Agreement set forth on Exhibit C hereto, (D) to the extent applicable, the number of shares of Company Common Stock issuable pursuant to the Line of Credit set forth on Exhibit C hereto ((C) and (D) together the “Equityholder Contingent Rights”), and (E) to the extent applicable, the number and class of Company Warrants set forth under his, her or its name on the signature page hereto (the “Equityholder Warrants” and, together with the Equityholder Shares, Equityholder Options, Equityholder Contingent Rights and Equityholder Warrants, the “Equityholder Securities”); and (ii) Equityholder is the promisee of the number of promised options set forth under his, her or its name on Exhibit C hereto (the “Equityholder Promised Options”);
WHEREAS, the entitlement to shares of Parent Class B Common Stock in respect of any Equityholder Contingent Rights or Equityholder Warrants is to be calculated based on accrued interest and application of any and all anti-dilution rights through September 30, 2021; and
WHEREAS, in connection with the Merger Agreement and the consummation of the transactions contemplated thereby, the Company, Parent and Merger Subs have requested that Equityholder, and Equityholder has agreed to, enter into this Agreement.
NOW THEREFORE, in consideration of the foregoing and the mutual representations, warranties, covenants, agreements, terms and conditions contained herein, the parties hereto do hereby agree as follows:
ARTICLE I
VOTING AGREEMENT; RELEASE; WAIVER
1.1 Voting Agreement.
(a) Equityholder, who is a holder of Equityholder Securities, hereby agrees, from and after the date hereof and until the earlier of the Effective Time and the Termination Date (as defined below), at any meeting of the stockholders of the Company, however called, at any adjournment or postponement thereof, and in connection with any Company Stockholder Written Consent (as defined below) of the stockholders of the Company, in each case called or provided with respect to any of the matters described in the following clause (ii): (i) to appear at each such
Annex C-1
Table of Contents
meeting in person or by proxy or otherwise cause the Company Capital Stock that Equityholder is entitled to vote to be counted as present thereat for purposes of establishing a quorum; and (ii) to vote in person or by proxy or otherwise cause the Company Capital Stock that Equityholder is entitled to vote to be voted (or deliver a duly executed Company Stockholder Written Consent in lieu thereof) all of the Company Capital Stock that Equityholder is entitled to vote at the time of any vote or Company Stockholder Written Consent (A) to adopt the (I) Merger Agreement and approve the Merger and (II) the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Company in substantially the form of Exhibit F to the Merger Agreement, in each case as and when submitted for the consideration and vote of the stockholders of the Company (not including any amendment or supplement), (B) against any action that would reasonably be expected to result in any of the conditions set forth in Section 6, Section 7 or Section 8 of the Merger Agreement not being satisfied, (C) against any transaction described or contemplated in Section 4.4 of the Merger Agreement or any other competing or alternative acquisition proposal for the Company or any of its subsidiaries or any other proposal, action or transaction involving the Company or any of its subsidiaries, which proposal, action or transaction would impede, frustrate, prevent or materially delay the consummation of the transactions contemplated by the Merger Agreement and the Transaction Documents or this Agreement and (D) against any other action that is intended or reasonably expected to materially impair, prevent or delay the Merger.
(b) Nothing in this Agreement, including Section 1.1(a), shall limit or restrict Equityholder, or any Affiliate or designee of Equityholder, who serves as a member of the Board of Directors of the Company or as an officer of the Company in acting in his or her capacity as a director or officer of the Company and exercising his or her fiduciary duties and responsibilities in such capacity, it being understood that this Agreement shall apply to Equityholder solely in such Equityholder’s capacity as a stockholder of the Company and shall not apply to Equityholder’s, its Affiliate’s or its designee’s actions, judgments or decisions as a director or officer of the Company.
(c) In furtherance of, and without limiting the generality of, the foregoing, within two days following the effectiveness of the Registration Statement, Equityholder shall execute and deliver to the Company (with a copy thereof to Parent) an action by written consent of the Company’s stockholders in substantially the form attached hereto as Exhibit A (the “Company Stockholder Written Consent”), which Company Stockholder Written Consent shall be irrevocable in accordance with its terms. Equityholder shall use Equityholder’s reasonable best efforts to procure that its Affiliates and Family Members who hold Company Capital Stock (including in each case Affiliates and Family Members as listed on Exhibit C) shall execute and deliver to the Company (with a copy thereof to Parent) the Company Stockholder Written Consent. For purposes of this Agreement, “Family Members” shall mean an Equityholder’s spouse, parents, children, grandchildren, siblings, mothers and fathers-in-law, sons and daughters-in-law, brothers and sisters-in-law, anyone (other than domestic employees) who shares Equityholder’s home and any trust established for the benefit of any of the foregoing.
(d) Equityholder hereby covenants and agrees that, except for this Agreement, the Company Stockholder Written Consent, and, if applicable, the agreement to which the undersigned is a party listed on Exhibit B hereto, Equityholder (i) has not entered into, and shall not enter into at any time while this Agreement remains in effect, any voting agreement or voting trust with respect to the Company Capital Stock owned beneficially or of record by Equityholder, (ii) has not granted, and shall not grant at any time while this Agreement remains in effect, a proxy, a consent or power of attorney with respect to the Company Capital Stock owned beneficially or of record by Equityholder, with any such proxy, consent or power of attorney purported to be granted by Equityholder being void from the outset, and (iii) has not entered into any agreement or taken any action (and shall not enter into any agreement or take any action) that would make any representation or warranty of Equityholder contained herein untrue or incorrect in any material respect or have the effect of preventing Equityholder from performing any of its material obligations under this Agreement.
(e) Except as set forth in Section 1.1(a), Equityholder shall not be restricted from voting in favor of, against or abstaining with respect to any matter presented to the Equityholders. Without limiting the generality of the foregoing, nothing in this Agreement shall preclude Equityholder from exercising full power and authority to vote in Equityholder’s sole discretion for or against any proposal submitted to a vote of the Equityholders to approve any payment that would, in the absence of such approval, constitute a parachute payment under Section 280G of the Internal Revenue Code of 1986, as amended.
Annex C-2
Table of Contents
(f) Equityholder shall use reasonable best efforts to cause its Affiliates and Family Members who hold Company Capital Stock (in each case as listed on Exhibit C) at any meeting of the stockholders of the Company, however called, at any adjournment or postponement thereof, and in connection with any Company Stockholder Written Consent (as defined below), in each case called or provided with respect to any of the matters described in the following clause (ii): (i) to appear at each such meeting in person or by proxy or otherwise cause the Company Capital Stock that such person is entitled to vote to be counted as present thereat for purposes of establishing a quorum; and (ii) to vote in person or by proxy or otherwise cause the all Company Capital Stock that such person is entitled to vote to be voted (or deliver a duly executed Company Stockholder Written Consent in lieu thereof) (A) to adopt the (I) Merger Agreement and approve the Merger and the other transactions contemplated by the Merger Agreement, and (II) the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Company in substantially the form of Exhibit F to the Merger Agreement, in each case as and when submitted for the consideration and vote of the stockholders of the Company (not including any amendment or supplement), (B) against any action that would reasonably be expected to result in any of the conditions set forth in Section 6, Section 7 or Section 8 of the Merger Agreement not being satisfied, (C) against any transaction described or contemplated in Section 4.4 of the Merger Agreement or any other competing or alternative acquisition proposal for the Company or any of its subsidiaries or any other proposal, action or transaction involving the Company or any of its subsidiaries, which proposal, action or transaction would impede, frustrate, prevent or materially delay the consummation of the transactions contemplated by the Merger Agreement and the Transaction Documents or this Agreement and (D) against any other action that is intended or reasonably expected to materially impair, prevent or delay the Merger.
1.2 Release.
(a) Equityholder, for himself, herself or itself and on behalf of his, her or its Affiliates, and any of their respective officers, directors, employees, agents, representatives, successors, members, managers, partners and permitted assigns (each a “Waiving Party”), acknowledges and agrees that, as of the Effective Time, to the fullest extent permitted under applicable Law, any and all rights, claims and causes of action he, she or it may have as of the Effective Time against Parent, Merger Subs, or any of their respective Affiliates (including but not limited to the Company), or any of their respective officers, directors, employees, agents, representatives, successors and permitted assigns (the “Parent Group”) relating to the business of the Company, the operation of the Company, or its businesses or relating to the subject matter of the Merger Agreement or any Exhibit or the Schedules thereto, or any ancillary agreement, certificate or other document entered into, made, delivered, or made available in connection therewith, or as a result of any of the transactions contemplated thereby, whether arising under, or based upon, any federal, state, local or foreign statute, Law, ordinance, rule or regulation or otherwise (including any right, whether arising at law or in equity, to seek indemnification, contribution, cost recovery, damages or any other recourse or remedy, including as may arise under common law) are hereby irrevocably waived by the Waiving Parties (the “Waived Claims”); provided, however, this Section 1.2 shall not apply to, and “Waived Claims” shall not include, (i) any rights explicitly set forth in the Merger Agreement (including any equity award or rollover agreement), this Agreement or any ancillary agreement, certificate or other document entered into in connection with the Merger Agreement, or as a result of any of the transactions contemplated thereby, to which the Equityholder is a party, (ii) any rights to indemnification, exculpation or advancement of expenses and insurance as an officer or director of the Company, including in accordance with Section 5.4 of the Merger Agreement or as provided in any indemnification agreement entered into with an officer or director or in the Company Charter and the Company’s bylaws, (iii) in the case of an Equityholder who is also an employee of the Company, any rights provided for under any employment agreement, offer letter or other compensation arrangement or benefit plan with the Company or Parent, (iv) in the case of an Equityholder who is also an employee of, or consultant to, the Company, in respect of claims for accrued and unpaid compensation and/or benefits or other vested rights under any employee benefit programs or policies of the Company at or prior to the Effective Time, (v) any claims first arising (and based solely on facts and circumstances) after the Effective Time and (vi) any claims which may not be waived as a matter of Law, including, but not limited to, any Waiving Party’s right to file a charge with or participate in a charge by any Governmental Body that is authorized to enforce or administer Laws related to employment (in the case of an Equityholder who is also an employee). Moreover, nothing in this Agreement waives any Waiving Party’s right or otherwise prohibits any Waiving Party from reporting violations of law or regulation to any Governmental Body or from otherwise participating, to the extent requested by a Governmental Body, in any investigation or proceeding that may be conducted by an any Governmental Body, including providing documents or other information to the extent requested by a Governmental Body. No Waiving Party needs the prior authorization of the Parent Group to make any such reports or disclosures to any Governmental Body or to participate in any such investigation or proceeding that may be conducted by any Governmental Body, and no Waiving Party is required to notify the Parent Group that such
Annex C-3
Table of Contents
Waiving Party has made such reports or disclosures or has participated or is participating in any such investigation or proceeding. This Agreement also does not limit any Waiving Party’s right to file a charge or complaint with any Governmental Body related to such Equityholder’s employment with the Company or receive an award for information provided to any Governmental Body. Furthermore, without limiting the generality of this Section 1.2, no claim will be brought or maintained by, or on behalf of, Equityholder or any other Waiving Party against any member of the Parent Group, and no recourse will be sought or granted against any of them, by virtue of, or based upon, any alleged misrepresentation or inaccuracy in, or breach of, any of the representations, warranties or covenants of any Person set forth or contained in the Merger Agreement or any Exhibit or Schedules thereto, or any ancillary agreement, certificate or other document entered into, made, delivered, or made available in connection therewith, or as a result of any of the transactions contemplated thereby, in each case except as set forth in clauses (i) through (vi) of the first sentence of this Section 1.2(a). Equityholder acknowledges and agrees that the Waiving Parties may not avoid such limitation on liability by (A) seeking damages for breach of contract, tort (other than claims based on gross negligence, recklessness or fraud) or pursuant to any other theory of liability, all of which are hereby waived or (B) asserting or threatening any claim against any Person that is not a party hereto or to the Merger Agreement (or a successor to a party thereto) for breaches of the representations, warranties, covenants or agreements contained in this Agreement or the Merger Agreement. The parties hereto acknowledge and agree that the limits imposed on the Waiving Parties’ remedies with respect to this Agreement and the Merger Agreement and the transactions contemplated hereby and thereby were bargained for between sophisticated parties and were specifically taken into account in the determination of the amounts to be paid by Parent and Merger Subs under the Merger Agreement.
(b) EQUITYHOLDER, ON BEHALF OF THE WAIVING PARTIES, EXPRESSLY IRREVOCABLY WAIVES ALL RIGHTS AFFORDED BY ANY STATUTE OR COMMON LAW PRINCIPLES WHICH LIMITS THE EFFECT OF A RELEASE WITH RESPECT TO UNKNOWN CLAIMS. EQUITYHOLDER ON BEHALF OF THE WAIVING PARTIES, ACKNOWLEDGES THAT HE, SHE OR IT UNDERSTANDS THE SIGNIFICANCE OF THIS RELEASE OF UNKNOWN CLAIMS AND WAIVER OF ANY STATUTORY PROTECTION AGAINST A RELEASE OF UNKNOWN CLAIMS. EQUITYHOLDER, ON BEHALF OF THE WAIVING PARTIES, ACKNOWLEDGES AND AGREES THAT THIS WAIVER IS AN ESSENTIAL AND MATERIAL TERM OF THIS AGREEMENT.
IN ADDITION, EQUITYHOLDER AND EACH WAIVING PARTY SPECIFICALLY WAIVES THE BENEFIT OF ANY AND ALL PROVISIONS, RIGHTS AND BENEFITS CONFERRED BY ANY LAW OF ANY STATE OR TERRITORY OF THE UNITED STATES, OR PRINCIPLE OF COMMON LAW, WHICH IS SIMILAR, COMPARABLE OR EQUIVALENT TO CALIFORNIA CIVIL CODE SECTION 1542, AS WELL AS THE RIGHTS AND BENEFITS CONFERRED BY CALIFORNIA CIVIL CODE SECTION 1542 ITSELF, WHICH READS AS FOLLOWS: A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS WHICH THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH DEBTOR OR RELEASED PARTY.
(c) This release shall become effective only upon the consummation of the Merger at the Effective Time pursuant to the terms and conditions of the Merger Agreement.
1.3 Waiver of Rights.
(a) Equityholder, on behalf of his, her or itself and its Affiliates, hereby waives any and all preemptive rights, anti-dilution rights (except to the limited extent such rights are reflected in the share numbers on Exhibit C), rights of first refusal, rights of first offer or other similar rights that Equityholder and its Affiliates may have, or ever have had, with respect to any Company Capital Stock and waives any right Equityholder and its Affiliates may have, or ever have had, under the Equityholder Agreements (as defined below) or otherwise to acquire any Company Capital Stock being exchanged pursuant to, or as contemplated by, the Merger Agreement or any transfer that occurred prior to the Effective Time.
(b) EQUITYHOLDER, BY EXECUTING AND DELIVERING THIS AGREEMENT AND BY ACCEPTING THE APPLICABLE MERGER CONSIDERATION PAYABLE TO EQUITYHOLDER UNDER THE MERGER AGREEMENT WAIVES AND AGREES NOT TO ASSERT HIS, HER OR ITS RIGHTS, IF ANY, TO
Annex C-4
Table of Contents
DISSENT OR SEEK STATUTORY APPRAISAL IN RESPECT OF EQUITYHOLDER’S EQUITY INTERESTS IN THE COMPANY PURSUANT TO APPLICABLE LAW (INCLUDING SECTION 262 OF THE DELAWARE GENERAL CORPORATION LAW).
(c) The waivers set forth in this Section 1.3 shall become effective only upon the consummation of the Merger pursuant to the terms and conditions of the Merger Agreement.
1.4 Prior Proxies. Equityholder hereby revokes (or agrees to cause to be revoked) any proxies that Equityholder has heretofore granted with respect to the Company Capital Stock owned by Equityholder.
1.5 No Transfer. Other than pursuant to the terms of this Agreement or the Merger Agreement (including without limitation pursuant to Section 8.6 of the Merger Agreement (Dissolution of Subsidiaries)), without the prior written consent of Parent, during the term of this Agreement, Equityholder agrees not to, directly or indirectly, sell, assign, transfer, pledge, encumber or otherwise dispose of (including, without limitation, by merger, consolidation, sale, liquidation, dissolution, dividend, distribution or otherwise by operation of law), or enter into any contract, option or other arrangement or understanding with respect to the direct or indirect assignment, transfer, encumbrance or other disposition of (including, without limitation, by merger, consolidation or otherwise by operation of law), any share of Company Capital Stock or any right, option or warrant with respect to Company Capital Stock (each, a “Transfer”). Any action taken in violation of the foregoing sentence shall be null and void ab initio. If any involuntary Transfer of any of the Company Capital Stock or any right, option or warrant with respect to Company Capital Stock shall occur (including, but not limited to, a sale by Equityholder’s trustee in any bankruptcy, or a sale to a purchaser at any creditor’s or court sale), the transferee (which term, as used herein, shall include any and all transferees and subsequent transferees of the initial transferee) shall take and hold such Company Capital Stock and/or any right, option or warrant with respect to Company Capital Stock subject to all of the restrictions, liabilities and rights under this Agreement, which shall continue in full force and effect until the termination of this Agreement.
1.6 Merger Consideration Acknowledgement.
(a) General. The Equityholder for himself, herself and itself, , hereby acknowledges and agrees that all Equityholder Securities that it holds, in each case calculated based on accrued interest and application of anti-dilution rights through September 30, 2021 (which aggregate amounts are attached to this Agreement) shall, at the Effective Time, convert into the number of shares of Parent Class B Common Stock calculated by applying the Per Share Merger Consideration ratio applicable to the class (or classes) of Company Capital Stock (or any rights therefor) held by Equityholder, in each case as set forth in the Merger Agreement, a copy of which Equityholder has received and had the opportunity to review. In accordance with Section 3.4 below, the Equityholder hereby undertakes to use reasonable best efforts to ensure that its Affiliates and Family Members accept and assert no claim otherwise that the Company Capital Stock, as calculated based on accrued interest and anti-dilution through September 30, 2021 (which aggregate amounts are set forth in Exhibit C attached to this Agreement) shall, at the Effective Time, convert into the number of shares of Parent Class B Common Stock calculated by applying the Per Share Merger Consideration ratio applicable to the class (or classes) of Company Capital Stock (or any rights therefor) held by such Affiliate or Family Member, in each case as set forth in the Merger Agreement.
(b) Equityholder Contingent Rights and Warrants Cancellation. Equityholder hereby irrevocably acknowledges and agrees that, notwithstanding anything to the contrary set forth in the Bonus Shares Agreement, the Line of Credit or any Equityholder Warrant, and, effective as of immediately prior to the Effective Time, each Equityholder Contingent Right and each Equityholder Warrant is hereby terminated and canceled and is null and void and of no further effect. Each Equityholder further irrevocably acknowledges and agrees that from and after the Closing, such Equityholder’s only right with respect to such Equityholder’s Contingent Rights and Equityholder Warrants is the right to receive the consideration described in the Merger Agreement. Each Equityholder hereby irrevocably acknowledges and agrees that such Equityholder shall be responsible for any federal, state or local income or similar taxes required to be paid by such Holder with respect to such Equityholder’s Contingent Rights, Equityholder Warrants and this Agreement.
Annex C-5
Table of Contents
(c) Tax Indemnification. Each Equityholder shall indemnify and hold the Parent Group (as defined below) harmless from and against any claim, demand, action, cause of action, loss, liability, damage, cost, penalty or expense whatsoever, including reasonable and documented legal fees, suffered or incurred by the Parent Group by reason of such Equityholder’s failure to pay or discharge any federal, state or local income or similar taxes required to be paid by such Equityholder resulting from any amount paid or deemed to be paid to such Equityholder.
(d) Anti-Dilution. Without limiting Section 1.6(a) or (b), Equityholder hereby (i) acknowledges and agrees that the Equityholder Shares listed on Exhibit C hereto reflect all shares issuable as a result anti-dilution rights of such Equityholder through September 30, 2021 and Equityholder hereby waives receipt of anti-dilution rights that would have accrued after September 30, 2021 (such waiver to become effective only upon the consummation of the Merger pursuant to the terms and conditions of the Merger Agreement); (ii) acknowledges and agrees that the maximum number of shares issuable to Equityholder in connection with such Equityholder’s Contingent Rights and Equityholder Warrants is set forth on Exhibit C hereto; and (iii) acknowledges and agrees that effective upon the Effective Time, without any further action required by the Company or such Equityholder, such Equityholder’s Contingent Rights and Equityholder Warrants and all obligations of the Company set forth thereunder shall be immediately deemed satisfied in full and extinguished in their entirety, and any rights of such Equityholder pursuant to such Equityholder’s Contingent Rights and Equityholder Warrants shall be terminated and be of no further force or effect, other than the right to receive the consideration set forth in the Merger Agreement.
(e) Merger Consideration. Equityholder acknowledges and agrees that the amount of consideration to be paid to Equityholder in connection with the Merger pursuant and subject to the terms of the Merger Agreement is the sole amount owed to Equityholder by Parent and Merger Subs as consideration for any and all Equityholder Securities of Equityholder.
ARTICLE II
REPRESENTATIONS AND WARRANTIES OF EQUITYHOLDER
Equityholder hereby represents and warrants to Parent, Merger Subs, the Company, and the Surviving Company that the following representations and warranties are true and accurate on the date hereof and as of the Closing Date as if made as of the Closing Date, and acknowledges that such Persons may rely on such representations and warranties as if each such Person was a party to this Agreement and each shall have the rights, remedies and benefits under this Agreement as if such Person was a party hereto:
2.1 Organization. If Equityholder is not a natural person, Equityholder is validly existing and in good standing under the Laws of the jurisdiction of its organization.
2.2 Authorization; Enforceability. Equityholder has requisite power and authority or, if Equityholder is an individual, legal capacity, to execute and deliver this Agreement and each other agreement contemplated hereby to which Equityholder is a party and to perform Equityholder’s obligations hereunder and thereunder. If Equityholder is not a natural person, the execution and delivery of this Agreement by Equityholder and each other agreement contemplated hereby to which Equityholder is a party and the consummation by Equityholder of the transactions contemplated hereby and thereby have been duly authorized by all necessary action and no other proceedings on the part of Equityholder and no stockholder votes are necessary to authorize this Agreement or the other agreements contemplated hereby to which Equityholder is a party or to consummate the transactions contemplated hereby and thereby. This Agreement has been duly executed and delivered by Equityholder and, assuming the due authorization, execution and delivery by the other parties hereto, constitutes the valid and legally binding obligation of Equityholder, enforceable in accordance with its terms and conditions, subject to Laws of general application relating to public policy, bankruptcy, insolvency and the relief of debtors and rules of Law governing specific performance, injunctive relief and other equitable remedies.
2.3 Non-Contravention.
(a) Neither the execution and delivery of this Agreement or any other agreement contemplated hereby to which Equityholder is a party, nor the consummation of the transactions contemplated hereby or thereby, will (i) violate any Laws to which Equityholder is subject, (ii) if Equityholder is not a natural person, violate any provision of Equityholder’s formation documents and bylaws or other similar document, or (iii) conflict with, result in a breach of, constitute a default under, result in the acceleration of, create in any Person the right to accelerate, terminate,
Annex C-6
Table of Contents
modify or cancel or require any notice or consent under, or result in the imposition of any Lien (other than Permitted Liens) upon any of the assets of Equityholder under, any agreement, contract, lease, license, instrument or other arrangement to which Equityholder is a party or by which Equityholder is bound or to which any of Equityholder’s assets is subject, except in each case of clause (i) or (iii) where the violation, conflict, breach, default, acceleration, termination, modification, cancellation, failure to give notice or obtain consent or Lien would not adversely affect or delay Equityholder’s performance under this Agreement or any other agreement contemplated hereby to which Equityholder is a party or the consummation of the transactions contemplated by this Agreement or such other agreements.
(b) Equityholder does not need to give any notice to, make any filing with or obtain any authorization, consent or approval of any Governmental Body for the parties to consummate the transactions contemplated by this Agreement.
2.4 Litigation. There are no Legal Proceedings pending against, or, to the knowledge of Equityholder, threatened against, Equityholder that would adversely affect or delay Equityholder’s performance under this Agreement or the consummation of the transactions contemplated by this Agreement.
2.5 Ownership of Equityholder Shares. Equityholder is the sole (subject to any community property laws, if applicable) record and beneficial owner of the Equityholder Securities and Equityholder’s Affiliates and Family Members are the sole (subject to any community property laws, if applicable) record and beneficial owners of the Company Capital Stock set forth on Exhibit C. The Equityholder Securities constitute Equityholder’s entire interest in Company Capital Stock. Except for the Equityholder Securities set forth on Exhibit C, Equityholder does not own or hold any other Company Capital Stock or any options, warrants, rights or other securities to purchase or otherwise acquire Company Capital Stock. The Equityholder Securities are not subject to any Liens (other than securities law restrictions of general applicability or pursuant to the Equityholder Agreements). Equityholder’s principal residence or place of business is set forth on the signature page hereto.
ARTICLE III
COVENANTS OF EQUITYHOLDER
3.1 Confidentiality. Except with the prior written consent of Parent, Equityholder agrees to at all times keep confidential and not disclose (other than to his, her or its spouse, family members, officers, directors, employees, members, partners, Affiliates, attorneys and other advisors or representatives; provided, that such Persons are directed to keep confidential and not disclose consistent with the confidentiality restrictions contained herein and Equityholder will be directly responsible for any breach of this Section 3.1 by any such Persons): (a) the existence, terms and conditions of the Merger Agreement and the transactions contemplated thereby; (b) matters regarding the interpretation, performance, breach or termination hereof or thereof; and (c) all confidential, proprietary or non-public information any the Company of any kind or nature, including, without limitation, Intellectual Property developed, used or owned by the Company, obtained by Equityholder or its directors, officers, employees, agents or representatives prior to the Closing, except in each such case to the extent that (i) such information is used in connection with Equityholder’s employment with or engagement by the Company or Parent and only to the extent required to perform Equityholder’s duties as an employee or in connection with such engagement, (ii) such information has otherwise been made public or is publicly available through no fault or action of Equityholder, (iii) Equityholder receives such information from a third party that, to Equityholder’s knowledge, owes no duty of confidentiality to the Company with respect to such information, (iv) such information is independently developed by Equityholder without use of or reference to any confidential, proprietary or non-public information of the Company, (v) Equityholder is required by applicable Law or legal or judicial process to divulge or disclose any such information, in which case Equityholder shall, to the extent legally permissible, promptly notify Parent in writing in advance of disclosing such information and use commercially reasonable efforts to cooperate with Parent (at Parent’s sole expense) to limit such disclosure to the extent so required by applicable Law or legal or judicial process, or (vi) such information as is reasonably necessary for enforcing Equityholder’s rights hereunder or thereunder; provided; however, if Equityholder is not a natural person and is an investment fund, Equityholder may disclose the terms and conditions regarding the transactions contemplated by the Merger Agreement and the nature of Equityholder’s investment in the Company, including, but not limited to the fact that it sold its interest in the Company, the amount of Equityholder’s investment in the Company, co-investors in the Company, the identity of Parent and its proceeds in the sale of its interest in the Company to its stockholders, members, limited partners, investors and prospective stockholders, members, limited partners and investors so long as such Persons are informed by Equityholder of the confidential nature of such information and agree or have agreed to
Annex C-7
Table of Contents
treat such information confidentially. Nothing in this Section 3.1 shall limit or restrict Equityholder who serves as a member of the Board of Directors, an officer, or employee of the Company, the Company or the Surviving Company in acting in his or her capacity as a director, officer, or employee to perform his or her employment duties in the ordinary course, and/or exercising his or her fiduciary duties and responsibilities in such capacity as required by applicable Law.
3.2 Termination of the Equityholder Agreements. Equityholder and the Company consent, effective as of and contingent upon the Effective Time, to the termination of the agreements listed on Exhibit B hereto, in accordance with their respective terms.
3.3 [Included for certain holders] Actions in Connection with Initial Public Offering.
(a) In the event that the Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a stockholder meeting prior to the termination of this Agreement, Equityholder hereby covenants and agrees (and covenants and agrees to cause its subsidiaries and Affiliates to): (i) vote for all amendments to the Certificate of Incorporation of the Company recommended by the Company Board in connection with the public offering of the Company Common Stock pursuant to the Securities Act of 1933, as amended (the “IPO”), including without limitation the increase of the authorized shares of Company Common Stock and reverse stock-splits, as applicable; (ii) immediately prior to the IPO and, subject to the requirements under the Certificate of Incorporation of the Company, contingent upon the conversion of all other outstanding Company Preferred Stock to Company Common Stock (except that of Parent and its Affiliates), convert all of the Company Preferred Stock held directly or indirectly by Equityholder into Company Common Stock in accordance with the applicable conversion terms and conditions applicable to the conversion and conversion ratio of the Preferred Stock; and (iii) not to, without the prior written consent of the managing underwriter for the IPO and solely to the same extent as all officers, directors, holders of in excess of 3% of the outstanding Company Capital Stock on a fully diluted basis are so bound on terms equivalent to those of this clause (iii), during the period commencing on the date of the final prospectus relating to the Company’s IPO and ending on the date specified by the Company and the managing underwriter (such period not to exceed one hundred eighty (l80) days) (x) lend, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of Company Common Stock (or securities convertible into Company Common Stock) held immediately prior to the effectiveness of the registration statement for the IPO, or (y) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Company Common Stock (or securities convertible into Company Common Stock), whether any such transaction described in subclauses (x) or (y) above is to be settled by delivery of securities, in cash or otherwise.
(b) In the event that the Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a stockholder meeting prior to the termination of this Agreement, Equityholder agrees (and covenants and agrees to cause its subsidiaries and Affiliates to): (i) to execute such agreements as may be reasonably requested by the underwriters in the IPO that are consistent with Section 3.3(a) above or that are necessary to give further effect thereto; and (ii) that, in order to enforce the foregoing covenants in Section 3.3(a) above, the Company may impose stop-transfer instructions with respect to the shares of Company Common Stock of Equityholder (and transferees and assignees thereof) until the end of the lock-up period described in Section 3.3(a)(iii). Equityholder further hereby constitutes and appoints as the proxies of Equityholder and hereby grants a power of attorney to the Chief Executive Officer of the Company and designees with full power of substitution, with respect to the matters set forth in Section 3.3(a) above and hereby authorizes each of them to represent and vote in favor of the matters set forth in Section 3.3(a) above. Each of the proxy and power of attorney granted pursuant to this Section 3.3(b) is given in consideration of the agreements and covenants of the Company in connection with the transactions contemplated by the Merger Agreement and, as such, each is coupled with an interest and shall be irrevocable following any termination of the Merger Agreement. Equityholder hereby revokes any and all previous proxies or powers of attorney with respect to the Company Common Stock and shall not hereafter purport to grant any other proxy or power of attorney with respect to any of the Company Common Stock, deposit any of the Company Common Stock into a voting trust or enter into any agreement (other than this Agreement), arrangement or understanding with any person, directly or indirectly, to vote, grant any proxy or give instructions with respect to the voting of any of the Company Common Stock, in each case, with respect to any of the matters set forth in Section 3.3 above.
Annex C-8
Table of Contents
(c) In the event that the Parent Stockholder Approval is sought but not obtained following the setting of a record date and tabulation of votes therefor at a stockholder meeting prior to the termination of this Agreement, the Company agrees that Parent and Howard Jonas or any Affiliate of any of the foregoing who holds rights, directly or indirectly, under the 56% Warrant shall be permitted (prior to effecting the conversion of its Company Preferred Stock in accordance with Section 5.2(c)(ii)) to exercise on a cashless basis upon customary terms the 56% Warrant at an implied price per share no greater than the price of Company Common Stock to be issued in the IPO.
(d) Notwithstanding anything to the contrary in the Merger Agreement, this Agreement or otherwise, the provisions of this Section 3.3 shall survive any termination of the Merger Agreement and/or this Agreement.
3.4 Joinder of Affiliates and Family Members. The Equityholder will use reasonable best efforts to cause each of its Affiliates and Family Members who hold Company Capital Stock (whether directly or indirectly, as listed on Exhibit C) to execute and deliver a joinder to this Agreement within five days of the date hereof affirming such person’s agreement to be bound by the covenants, agreements and acknowledgments of this Agreement as an Equityholder and agreeing to be bound by the terms of that certain Lockup Agreement executed by the Equityholder on the date hereof.
ARTICLE IV
AGREEMENT TO BE BOUND BY CERTAIN
PROVISIONS OF THE MERGER AGREEMENT
4.1 Agreement to be Bound.
(a) Equityholder has received the Merger Agreement and the other agreements, instruments, exhibits, schedules, certificates and other documents referenced in the Merger Agreement or in the foregoing agreements and documents (collectively, the “Transaction Documents”) to which it is a party or is bound, and hereby acknowledges having had sufficient opportunity to review the Merger Agreement and the applicable Transaction Documents and agrees that Equityholder is a Company Stockholder for purposes of the Merger Agreement and, accordingly, agrees to be bound by, solely in Equityholder’s capacity as such Company Stockholder and as fully as though Equityholder were a signatory thereto, the terms and conditions of the Merger Agreement in relation to the Company Stockholders.
(b) Equityholder acknowledges, approves of and agrees to be bound by the provisions set forth in the Merger Agreement related to Tax withholding (as set forth in Section 1.9(d) of the Merger Agreement, to the extent applicable).
(c) Contingent upon the Closing, Parent acknowledges and agrees that Equityholder is entitled to receive the consideration required to be paid to Equityholder pursuant to the terms of the Merger Agreement as though the Equityholder were a signatory thereto, including without limitation, Section 1.6.
ARTICLE V
MISCELLANEOUS
5.1 Governing Law.
(a) This Agreement and any Legal Proceeding of any kind or nature (whether at law or in equity, based upon contract, tort or otherwise) that is in any way related to this Agreement or any of the transactions related hereto (including the interpretation, construction, validity and enforcement of this Agreement, or the negotiation, execution or performance of any of the transactions related hereto (including any Legal Proceeding based upon, arising out of, or related to any representation or warranty expressly made in this Agreement)) shall be governed by and construed in accordance with the domestic Laws of the State of Delaware, including its statutes of limitations, without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the Laws of any jurisdiction other than the State of Delaware and without regard to any borrowing statute that would result in the application of the statute of limitations of any other jurisdictions. In furtherance of the foregoing, the laws of the State of Delaware will control even if under such jurisdiction’s choice of law or conflict of law analysis, the substantive law of some other jurisdiction would ordinarily or necessarily apply.
Annex C-9
Table of Contents
(b) The parties hereto submit to the exclusive jurisdiction of the Chancery Court of the State of Delaware and any state appellate court therefrom within the State of Delaware (or if the Chancery Court of the State of Delaware declines to accept jurisdiction over a particular matter, any federal court sitting in the State of Delaware and any federal appellate court therefrom) in respect of the interpretation and enforcement of the provisions of this Agreement and any related agreement, certificate or other document delivered in connection herewith and by this Agreement waive, and agree not to assert, any defense in any action for the interpretation or enforcement of this Agreement and any related agreement, certificate or other document delivered in connection herewith that they are not subject to such jurisdiction or that such action may not be brought or is not maintainable in such courts or that this Agreement may not be enforced in or by such courts, that the action is brought in an inconvenient forum, or that the venue of the action is improper.
(c) Each party hereto agrees that service in person or by certified or by nationally recognized overnight courier to its address set forth in Section 5.7 shall constitute valid in personam service upon such party and its successors and assigns in any arbitration proceeding commenced pursuant to this Section 5.1. Each party hereto hereby acknowledges that this is a commercial transaction, that the foregoing provisions for consent to arbitration, service of process and waiver of jury trial have been read, understood and voluntarily agreed to by each party hereto and that by agreeing to such provisions each party hereto is waiving important legal rights.
5.2 Succession and Assignment. This Agreement shall be binding upon and inure to the benefit of the parties named herein and their respective successors and permitted assigns. No party hereto may assign either this Agreement or any of its rights, interests or obligations hereunder without the prior written approval of the other parties hereto.
5.3 Severability. Whenever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable Law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable Law by which this Agreement is governed, such invalidity, illegality or unenforceability shall not affect any other provision; provided, that such provision shall be construed to give effect to the parties’ intent regarding such provision to the maximum extent permitted by applicable Law.
5.4 Specific Performance. Equityholder agrees that irreparable damage would occur in the event any of the provisions of this Agreement were not performed by the parties hereto in accordance with their specific terms or were otherwise breached. It is accordingly agreed that Parent, on the one hand, and Equityholder, on the other hand, shall be entitled to seek an injunction or injunctions to prevent breaches of this Agreement by the other (as applicable) and to enforce specifically the terms and provisions of this Agreement and to thereafter cause the transactions contemplated by this Agreement to be consummated on the terms and subject to the conditions thereto set forth in this Agreement. The foregoing rights are in addition to and without limitation of any other remedy to which the parties hereto may be entitled at law or in equity. The parties hereto further agree not to assert that a remedy of specific performance is unenforceable, invalid, contrary to law or inequitable for any reason, nor to assert that a remedy of monetary damages would provide an adequate remedy. Each of the parties hereto hereby waives: (a) any defenses in any action for specific performance, including the defense that a remedy at law would be adequate’ and (b) any requirement under any Law to post a bond or other security as a prerequisite to obtaining equitable relief.
5.5 Amendments and Waivers. No amendment of any provision of this Agreement shall be valid unless the same shall be in writing and signed by Parent and Equityholder. No waiver by Parent or Equityholder of any provision of this Agreement or any default, misrepresentation or breach of warranty or covenant hereunder, whether intentional or not, shall be valid unless the same shall be in writing and signed by the party hereto making such waiver, nor shall such waiver be deemed to extend to any prior or subsequent default, misrepresentation or breach of warranty or covenant hereunder or affect in any way any rights arising by virtue of any prior or subsequent such occurrence.
5.6 Expenses. Each party hereto will bear his, her or its own costs and expenses (including legal fees and expenses) incurred in connection with this Agreement and the transactions contemplated hereby.
Annex C-10
Table of Contents
5.7 Notices. All notices, requests, demands, claims and other communications hereunder will be in writing. Any notice, request, demand, claim or other communication hereunder shall be deemed duly given: (a) when delivered personally to the recipient; (b) one (1) Business Day after being sent to the recipient on a priority basis by reputable overnight courier service (charges prepaid); or (c) if sent by electronic mail, when received if received before 5:00 p.m. local time of the recipient on a Business Day, and otherwise on the next following Business Day, in each case addressed to the intended recipient as set forth below:
(a) If to Parent to:
Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Attention: Chief Financial Officer
E-mail: david.polinsky@rafaelholdings.com
with a copy (which shall not constitute notice) to:
Olshan Frome Wolosky LLP
1325 Avenue of the Americas
New York, NY 10019
Attention: Mitchell Raab
E-mail: mraab@olshanlaw.com
and:
Schwell Wimpfheimer & Associates
37 West 39th Street, Suite 505
New York, NY 10018
E-mail: dov.schwell@swalegal.com
(b) If to the Company (prior to Closing) to:
Rafael Pharmaceuticals, Inc.
1 Duncan Drive
Cranbury, NJ 08512
Attention: Sanjeev Luther, Chief Executive Officer
E-mail: sanjeev.luther@rafaelpharma.com
With a copy (which shall not constitute notice) to
Orrick Herrington & Sutcliffe LLP
1152 15th Street, N.W.
Washington, DC 20005 – 1706
Attention: David E. Schulman
E-mail: dschulman@orrick.com
(c) If to Equityholder, at the address set forth on the signature page hereto, or if no address is set forth therein, at the last address of Equityholder in the Company’s or the Surviving Company’s records.
Any party hereto may change the address to which notices, requests, demands, claims and other communications hereunder are to be delivered by giving the other parties hereto notice in the manner set forth in this Section 5.7.
5.8 Waiver of Jury Trial. EACH PARTY HERETO HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS AGREEMENT, THE OTHER AGREEMENTS CONTEMPLATED HEREBY OR THE TRANSACTIONS CONTEMPLATED BY THIS AGREEMENT. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH
Annex C-11
Table of Contents
OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (II) SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (III) SUCH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (IV) SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 5.8.
5.9 Interpretation; Rules of Construction. Unless otherwise expressly provided or unless the context requires otherwise: (a) all references in this Agreement to Articles, Sections, Schedules and Exhibits shall mean and refer to Articles, Sections, Schedules and Exhibits of this Agreement; (b) all references to statutes and related regulations shall include all amendments of the same and any successor or replacement statutes and regulations; (c) words using the singular or plural number also shall include the plural or singular number, respectively; (d) references to “hereof,” “herein,” “hereby” and similar terms shall refer to this entire Agreement (including the Schedules and Exhibits hereto); (e) references to any Person shall be deemed to mean and include the successors and permitted assigns of such Person (or, in the case of a Governmental Body, Persons succeeding to the relevant functions of such Person); (f) the term “including” shall be deemed to mean “including, without limitation”; (g) words of any gender shall include each other gender; (h) whenever this Agreement refers to a number of days, such number shall refer to calendar days, unless such reference is specifically to “Business Days”; and (i) the term “or” has the inclusive meaning represented by the phrase “and/or.” The Article and Section headings contained in this Agreement are solely for the purpose of reference, are not part of the agreement of the parties and shall not in any way affect the meaning or interpretation of this Agreement. When calculating the period before which, within which or following which, any act is to be done or step taken pursuant to this Agreement, the date that is the reference date in calculating such period shall be excluded. If the last day of such period is a non-Business Day, the period in question shall end on the next succeeding Business Day. This Agreement is the product of negotiations among the parties hereto, each of which is represented by legal counsel, and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement. Rules of construction relating to interpretation against the drafter of an agreement shall not apply to this Agreement and are expressly waived by each party hereto. As used in this Agreement, an Equityholder’s undertaking to use “reasonable best efforts” to cause any trust to take any action or to refrain from taking any action shall specifically mean recommending such course of action to the trustee(s) of such trust as being in the best interest of the trust and its beneficiaries, recognizing that whether or not a trustee determines to adopt such course of action remains a decision to be made by such trustee in the exercise of its fiduciary duties to the applicable trust. Any covenant of the undersigned Equityholder in this Agreement requiring the undersigned Equityholder to use its reasonable best efforts to cause or procure, or to otherwise cause or procure, that any of its Affiliates take any action hereunder in their capacity as Equityholders shall not apply to the undersigned Equityholder with respect to an Affiliate if such Affiliate has entered into, with, inter alia, the Company, a support agreement in substantially the form of this Agreement in its own right as an “Equityholder.”
5.10 Third-Party Beneficiary. The parties hereto hereby acknowledge and agree that (a) the execution and delivery of this Agreement is a condition precedent, and a material inducement, to the consummation by Parent of the transactions under the Merger Agreement and (b) each of the Non-Party Affiliates shall be an express third party beneficiary of Section 5.14 of this Agreement and that each of them shall be entitled to enforce all rights set forth in Section 5.14 of this Agreement.
5.11 Entire Agreement. This Agreement, the Merger Agreement and the other documents referred to herein: (a) constitute the entire agreement among the parties hereto and supersede any prior understandings, agreements or representations by or among the parties hereto, written or oral, to the extent they relate in any way to the subject matter hereof; and (b) are not intended to confer, and shall not be construed as conferring, upon any Person other than the Company and Parent any rights or remedies hereunder, except as set forth in Section 5.10. If the provisions of this Agreement conflict in any way with the provisions of the Merger Agreement, the provisions of the Merger Agreement shall control.
5.12 Counterparts. This Agreement may be executed (a) in one or more partially or fully executed counterparts, each of which will be deemed an original and will bind the signatory, but all of which together will constitute the same instrument, and (b) by electronic means, PDF or facsimile.
Annex C-12
Table of Contents
5.13 Termination. This Agreement shall terminate upon the earliest of (a) the termination of the Merger Agreement in accordance with its terms and (b) the election of Equityholder in its sole discretion to terminate this Agreement following any amendment, supplement, waiver or other modification of any material term or provision of the Merger Agreement which is materially adverse to Equityholder (the earliest such date under clause (a) and (b), being referred to herein as the “Termination Date”); provided, that (i) the provisions set forth in Sections 3.5, 5.1 to 5.3 and Section 5.5 to this Section 5.13 shall survive the termination of this Agreement and (ii) the termination of this Agreement will not relieve any party hereto from liability arising in respect of any breach prior to such termination.
5.14 Non-Recourse. All claims or causes of action (whether in contract or in tort, in law or in equity) that may be based upon, arise out of or relate to this Agreement, or the negotiation, execution or performance of this Agreement (including any representation or warranty made in or in connection with this Agreement or as an inducement to enter into this Agreement), may be made only against the entities that are expressly identified as parties hereto. No Person who is not a named party to this Agreement, including any past, present or future director, officer, employee, incorporator, member, partner, equityholder, Affiliate, agent, attorney or representative of any named party to this Agreement (“Non-Party Affiliates”), shall have any liability (whether in contract or in tort, in law or in equity, or based upon any theory that seeks to impose liability of an entity party against its owners or affiliates) for any obligations or liabilities arising under, in connection with or related to this Agreement or for any claim based on, in respect of, or by reason of this Agreement or its negotiation or execution; and each party hereto waives and releases all such liabilities, claims and obligations against any such Non-Party Affiliate.
*****
Annex C-13
Table of Contents
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the date first above written:
| | PARENT: |
| | | | | |
| | | RAFAEL HOLDINGS, INC. |
| | | | | |
| | | By: | | |
| | | | | Name: |
| | | | | Title: |
| | | | | |
| | | COMPANY: |
| | | | | |
| | | RAFAEL PHARMACEUTICALS, INC. |
| | | | | |
| | | By: | | |
| | | | | Name: |
| | | | | Title: |
[Signature Page to Support Agreement]
Annex C-14
Table of Contents
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the date first above written:
| | EQUITYHOLDER: |
| | | |
| | | (Print Name of Holder) |
| | | |
| | | (Signature) |
| | | |
| | | (Print name if signing on behalf of an entity) |
| | | |
| | | (Print title if signing on behalf of an entity) |
Annex C-15
Table of Contents
EXHIBIT A
Company Stockholder Written Consent
See attached.
Annex C-16
Table of Contents
EXHIBIT B
Terminating Agreements
Annex C-17
Table of Contents
EXHIBIT C
Equityholder Name:
Annex C-18
Table of Contents
Annex D
FORM OF SUPPORT AGREEMENT
THIS SUPPORT AGREEMENT, dated as of June 17, 2021 (this “Agreement”), is between Rafael Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and the undersigned (the “Stockholder”).
WHEREAS, concurrently with the execution of this Agreement, the Company, Rafael Holdings, Inc., a Delaware corporation (“Parent”), RH Merger I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Parent (“Merger Sub I”), and RH Merger II, LLC, a Delaware limited liability company and a direct, wholly owned subsidiary of Parent (“Merger Sub II”), are entering into an Agreement and Plan of Merger (the “Merger Agreement”), providing for, among other things, the merger of Merger Sub I with and into the Company (the “Merger”), with the Company surviving the Merger and becoming a direct, wholly owned subsidiary of Parent, followed by the merger of the Company with and into Merger Sub II (the “Subsequent Merger”), with Merger Sub II surviving the Subsequent Merger as a direct, wholly owned subsidiary of Parent, in each case on the terms and subject to the conditions set forth in the Merger Agreement;
WHEREAS, as of the date hereof, the Stockholder and the Stockholders Family Members are the record and beneficial owner of the number of shares of Parent Class A Common Stock and Parent Class B Common Stock set forth opposite such Stockholder’s and Family Member’s name on Schedule A (such shares of Parent Common Stock, together with any other shares of capital stock of Parent acquired by the Stockholder after the date hereof and during the term of this Agreement, being collectively referred to as the “Subject Shares” of the Stockholder); and
WHEREAS, as a condition to its willingness to enter into the Merger Agreement, the Company has requested that the Stockholder enter into this Agreement, and the Stockholder desires to enter into this Agreement to induce the Company to enter into the Merger Agreement.
NOW, THEREFORE, the parties hereto, intending to be legally bound hereby, agree as follows:
ARTICLE I
Definitions; Interpretation
SECTION 1.01. Definitions. (a) For purposes of this Agreement, the following terms shall have the following meanings:
“Agreement” has the meaning set forth in the preamble.
“Company” has the meaning set forth in the preamble.
“Merger” has the meaning set forth in the recitals.
“Merger Agreement” has the meaning set forth in the recitals.
“Merger Sub I” has the meaning set forth in the recitals.
“Merger Sub II” has the meaning set forth in the recitals.
“Parent” has the meaning set forth in the recitals.
“Stockholder” has the meaning set forth in the preamble.
“Subject Shares” has the meaning set forth in the recitals.
“Subsequent Merger” has the meaning set forth in the recitals.
“Transfer” has the meaning set forth in Section 3.03.
(b) Capitalized terms used but not defined herein shall have the meanings given to such terms in the Merger Agreement.
Annex D-1
Table of Contents
SECTION 1.02. Interpretation. When a reference is made in this Agreement to an Article, Section, recital, preamble or Schedule, such reference shall be to an Article, Section, recital, preamble or Schedule of this Agreement unless otherwise indicated. The headings herein are for convenience of reference only, do not constitute part of this Agreement and shall not be deemed to limit or otherwise affect any of the provisions hereof. Unless the express context otherwise requires: (i) whenever the words “include,” “includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without limitation”; (ii) the words “hereto,” “hereof,” “herein” and “hereunder” and words of similar import when used in this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement; (iii) the terms defined in the singular have a comparable meaning when used in the plural and vice versa; (iv) any pronoun used in this Agreement shall include the corresponding masculine, feminine and neutral forms; (v) the term “or” is not exclusive and has the meaning represented by the phrase “and/or”; (vi) the word “extent” in the phrase “to the extent” shall mean the degree to which a subject or other thing extends and such phrase shall not mean simply “if” and (vii) except as otherwise specifically provided herein, all references in this Agreement to any statute include the rules and regulations promulgated thereunder, in each case as amended, re-enacted, consolidated or replaced from time to time and in the case of any such amendment, re-enactment, consolidation or replacement, reference herein to a particular provision shall be read as referring to such amended, re-enacted, consolidated or replaced provision and also include, unless the context otherwise requires, all applicable guidelines, bulletins or policies made in connection therewith. The parties have participated jointly in negotiating and drafting this Agreement. In the event that an ambiguity or a question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the parties, and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement. As used in this Agreement, a Stockholder’s undertaking to use “reasonable best efforts” to cause any trust to take any action or to refrain from taking any action shall specifically mean recommending such course of action to the trustee(s) of such trust as being in the best interest of the trust and its beneficiaries, recognizing that whether or not a trustee determines to adopt such course of action remains a decision to be made by such trustee in the exercise of its fiduciary duties to the applicable trust.
ARTICLE II
Representations and Warranties of Each Stockholder
The Stockholder represents and warrants to the Company that:
SECTION 2.01. Organization. In the event that such Stockholder is an entity, such Stockholder is duly organized, validly existing and in good standing under the laws of its jurisdiction of its organization (in the case of good standing, to the extent such jurisdiction recognizes such concept).
SECTION 2.02. Ownership of Subject Shares. As of the date hereof, Stockholder or its Family Members, as applicable, is the record and beneficial owner of, and has good and valid title to, the Subject Shares that are indicated opposite its name or the names of its Family Members on Schedule A, free and clear of all Liens, except for any Liens created by this Agreement. Stockholder does not own, of record or beneficially, any shares of capital stock of Parent other than the Subject Shares set forth on Schedule A. Stockholder or each of its Family Members set forth on Schedule A, as applicable, has the sole right to vote its Subject Shares.
SECTION 2.03. Authority; Execution and Delivery; Enforceability. In the event that such Stockholder is an individual, such Stockholder has full power and capacity to execute and deliver this Agreement and to perform their obligations hereunder. This Agreement has been duly executed and delivered by such Stockholder, and, in the event such Stockholder is an individual and is married and the Subject Shares constitute community property or otherwise require spousal approval in order for this Agreement to be a legally valid and binding obligation of such Stockholder, this Agreement has been duly executed and delivered by such Stockholder’s spouse. In the event such Stockholder is an entity, such Stockholder has all requisite power and authority and has taken all action necessary to execute and deliver this Agreement and to perform its obligations hereunder. The execution and delivery by such Stockholder of this Agreement and the performance by such Stockholder of its obligations hereunder have been duly authorized by all necessary action, and no other proceedings on the part of such Stockholder (or such Stockholder’s governing body, members, stockholders, partners, trustees or similar Persons, as applicable) are necessary to authorize this Agreement or the performance by such Stockholder of its obligations hereunder. This Agreement has been duly executed and delivered by such Stockholder, and, assuming due authorization, execution and delivery by the Company, this Agreement constitutes the legal, valid and binding agreement of such Stockholder, enforceable against such Stockholder in accordance with its terms.
Annex D-2
Table of Contents
SECTION 2.04. No Conflicts; Governmental Approvals.
(a) The execution, and delivery by such Stockholder of this Agreement do not, and the performance by such Stockholder of its obligations hereunder will not, constitute or result in (i) in the event that such Stockholder is an entity, a conflict with, a breach or violation of, or a default under, the certificate of incorporation and the bylaws, the limited liability company agreement, the partnership or trust agreement or comparable organizational documents of such Stockholder, (ii) with or without notice, lapse of time or both, a breach or violation of, a termination (or right of termination) of or a default under, the loss of any benefit under, the creation, modification or acceleration of any obligations under or the creation of any Lien on any of the properties, rights or assets of such Stockholder pursuant to any Contract binding upon such Stockholder or under any applicable Law to which such Stockholder is subject or (iii) any change in the rights or obligations of any party under any Contract legally binding upon such Stockholder, except in the case of each of clauses (ii) and (iii) directly above, for any such conflict, breach, violation, termination, default, loss, creation, modification, acceleration or change that would not, individually or in the aggregate, reasonably be expected to prevent or materially delay or impair the ability of such Stockholder to perform its obligations hereunder.
(b) No approval by any Governmental Body is required to be obtained or made by or with respect to such Stockholder in connection with the execution, delivery and performance of this Agreement, other than compliance by such Stockholder with and filings under Sections 13(d) and 16 of the Exchange Act.
SECTION 2.05. Litigation. There is no Legal Proceeding pending or, to the knowledge of such Stockholder, any claim that has been asserted against or affecting such Stockholder with respect to a Legal Proceeding (and such Stockholder is not aware of any basis for any such Legal Proceeding or claim), nor is there any order outstanding against such Stockholder or to which any of its properties or assets is subject that would, individually or in the aggregate, reasonably be expected to prevent or materially delay or impair the ability of such Stockholder to perform its obligations hereunder.
ARTICLE III
Covenants of the Stockholders
SECTION 3.01. Agreement to Vote.
(a) From the period commencing with the execution and delivery of this Agreement and continuing until the termination of this Agreement pursuant to Section 4.11, the Stockholder agrees that:
(i) at any meeting of the stockholders of Parent called to seek the Parent Stockholder Approval or in any other circumstances upon which a vote, consent or other approval of such Stockholder with respect to the Merger Agreement and the issuance of the Parent Class B Common Stock pursuant to the Merger (the “Parent Share Issuance”) or any of the other transactions contemplated by the Merger Agreement is sought, such Stockholder shall cause its Subject Shares to be present in person or by proxy for purposes of constituting a quorum and vote, or cause to be voted, including by executing a written consent if requested by the Company, its Subject Shares in favor of granting the Parent Stockholder Approval and the Required Parent Stockholder Vote and any other actions presented to the stockholders of Parent as necessary or desirable in connection with the Parent Stockholder Approval, the Required Parent Stockholder Vote and the Merger Agreement, the Parent Share Issuance or any of the other transactions contemplated by the Merger Agreement; and
(ii) at any meeting of the stockholders of Parent or in any other circumstances upon which a vote, consent or other approval of such Stockholder is sought, such Stockholder shall cause its Subject Shares to be present in person or by proxy for purposes of constituting a quorum and vote, or cause to be voted, including by executing a written consent if requested by the Company, its Subject Shares against (A) any action, agreement or proposal made in opposition to or in competition with the consummation of the Parent Share Issuance or any of the other transactions contemplated by the Merger Agreement, (B) any action, agreement or proposal involving Parent or any of its Subsidiaries that would reasonably be expected to result in a breach of any covenant, representation or warranty of Parent, Merger Sub I or Merger Sub II under the Merger Agreement and (C) any amendment of the certificate of incorporation or bylaws of Parent or any other action, agreement or proposal
Annex D-3
Table of Contents
involving Parent or any of its Subsidiaries that would in any manner impede, frustrate, prevent or nullify any provision of the Merger Agreement, the Parent Share Issuance or any of the other transactions contemplated by the Merger Agreement or change in any manner the voting rights of any class of the capital stock of Parent.
(b) The Stockholder shall not commit or agree to take any action inconsistent with any provision of Section 3.01(a).
(c) The Stockholder will use reasonable best efforts to cause each of its Family Members who hold any Parent Common Stock (whether directly or indirectly, as listed on Schedule A) to comply with the provisions of Section 3.01(a)(i) hereof as though such person were a party hereto.
SECTION 3.02. Irrevocable Proxy. The Stockholder hereby irrevocably grants to, and appoints, each officer of the Company, and any other individual designated in writing by the Company, and each of them individually, as the Stockholder’s proxy and attorney-in-fact (with full power of substitution), for and in the name, place and stead of the Stockholder, to vote its Subject Shares, or grant a consent or approval in respect of its Subject Shares, in a manner consistent with Section 3.01 if such Stockholder has not voted or caused to be voted such Subject Shares in a manner consistent with Section 3.01 at least five (5) Business Days prior to the applicable voting deadline. The Stockholder understands and acknowledges that the Company is entering into the Merger Agreement in reliance upon such Stockholder’s execution and delivery of this Agreement. The Stockholder hereby affirms that the irrevocable proxy set forth in this Section 3.02 is given in connection with the execution of the Merger Agreement, and that such irrevocable proxy is given to secure the performance of the duties of such Stockholder under this Agreement. The Stockholder hereby further affirms that the irrevocable proxy set forth in this Section 3.02 is coupled with an interest and may under no circumstances be revoked. The Stockholder hereby ratifies and confirms all that such irrevocable proxy may lawfully do or cause to be done by virtue hereof. Such irrevocable proxy is executed and intended to be irrevocable in accordance with the provisions of Section 212(e) of the DGCL. Notwithstanding the foregoing, the proxy and appointment granted hereby shall be automatically revoked, without any action by the Stockholder, upon any termination of this Agreement pursuant to Section 4.11.
SECTION 3.03. Transfer and Other Restrictions. Except pursuant to this Agreement, the Stockholder shall not, directly or indirectly, (i) sell, transfer, pledge, assign or otherwise dispose of (including by gift, merger or otherwise by operation of law) (collectively, “Transfer”), or enter into any Contract, option or other arrangement or understanding with respect to the Transfer of, any of its Subject Shares to any Person, (ii) enter into any voting arrangement, whether by proxy, voting agreement or otherwise, with respect to any of its Subject Shares, (iii) take any other action that would make any representation or warranty of such Stockholder contained herein untrue or incorrect or would in any way restrict, limit or interfere with the performance of such Stockholder’s obligations hereunder or (iv) commit or agree to take any of the foregoing actions; provided, however, that, notwithstanding the foregoing, nothing in this Agreement shall be deemed to prohibit Stockholder from selling or disposing of Subject Shares to any Permitted Transferee (as defined below) so long as such Permitted Transferee executes a signature page to this Agreement and delivers the same to the Company, pursuant to which such Permitted Transferee agrees to be a “Stockholder” pursuant to this Agreement with respect to such Subject Shares that are the subject of such Transfer. “Permitted Transferee” means (i) any other Stockholder, (ii) a spouse, lineal descendant or antecedent, brother or sister, adopted child or grandchild or the spouse of any child, adopted child, grandchild or adopted grandchild of such Stockholder, (iii) any trust, the trustees of which include only the persons named in clauses (i) or (ii) and the beneficiaries of which include only the persons named in clauses (i) or (ii), (iv) any corporation, limited liability company or partnership, the stockholders, members or general or limited partners of which include only the persons named in clauses (i) or (ii), or (v) any person by will, for estate or tax planning purposes, for charitable purposes or as charitable gifts or donations. The Stockholder hereby authorizes and will instruct Parent or its counsel to notify Parent’s transfer agent that there is a stop transfer order with respect to all of the Subject Shares of such Stockholder (and that this Agreement places limits on the voting and transfer of such Subject Shares), subject to the provisions hereof. Notwithstanding the foregoing, any such stop transfer order and notice will immediately be withdrawn and terminated upon any termination of this Agreement pursuant to Section 4.11.
Annex D-4
Table of Contents
SECTION 3.04. Directors and Officers. Notwithstanding any provision of this Agreement to the contrary, nothing in this Agreement shall limit or restrict the Stockholder (or an affiliate or designee of any Stockholder) who is a director or officer of Parent or the Company from acting in such capacity or fulfilling the obligations of such office (including, for the avoidance of doubt, exercising their fiduciary duties), including by voting, in their capacity as a director or officer of Parent or the Company, in such Stockholder’s (or its affiliate’s or designee’s) sole discretion on any matter as a director or officer (it being understood that this Agreement shall apply to the Stockholder solely in such Stockholder’s capacity as a stockholder of Parent or the Company). In this regard, the Stockholder shall not be deemed to make any agreement or understanding in this Agreement in the Stockholder’s capacity as a director or officer of Parent or the Company.
SECTION 3.05. Joinder of Family Members. The Stockholder will use reasonable best efforts to cause each of its Family Members who hold any Parent Common Stock (whether directly or indirectly, as listed on Schedule A) to execute and deliver a joinder to this Agreement within five days of the date hereof affirming such person’s agreement to be bound by the covenants, agreements and acknowledgments of this Agreement as a Stockholder and agreeing to be bound by the terms of that certain Lockup Agreement executed by the Stockholder on the date hereof. For purposes of this Agreement, “Family Members” shall mean a Stockholder’s spouse, parents, children, grandchildren, siblings, mothers and fathers-in-law, sons and daughters-in-law, brothers and sisters-in-law, anyone (other than domestic employees) who shares Stockholder’s home and any trust established for the benefit of any of the foregoing.
ARTICLE IV
General Provisions
SECTION 4.01. Notices. All notices, requests, claims, demands, waivers and other communications under this Agreement shall be in writing (including, for the avoidance of doubt, via email) and shall be addressed to a party at the following address for such party:
(a) if to the Company, to:
Rafael Pharmaceuticals, Inc.
1 Duncan Drive
East Windsor, NJ 08512
Attention: Sanjeev Luther
Email: sanjeev.luther@rafaelpharma.com
with a copy to:
Orrick Herrington & Sutcliffe LLP
1152 15th Street, N.W.
Washington, DC 20005 – 1706
Attention: David E. Schulman
E-mail: dschulman@orrick.com
(b) if to the Stockholder, to:
c/o Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Attention: David Polinsky, Chief Financial Officer
E-mail: david.polinsky@rafaelholdings.com
or to such other address(es) as shall be furnished in writing by any such party to the other parties hereto in accordance with the provisions of this Section 4.01.
Annex D-5
Table of Contents
SECTION 4.02. Severability. The provisions of this Agreement shall be deemed severable and the illegality, invalidity or unenforceability of any provision shall not affect the legality, validity or enforceability of any other provision hereof. If any provision of this Agreement, or the application thereof to any Person or any circumstance, is illegal, invalid or unenforceable, (a) a suitable and equitable provision shall be substituted therefor in order to carry out, so far as may be legal, valid and enforceable, the intent and purpose of such illegal, invalid or unenforceable provision, and (b) the remainder of this Agreement and the application of such provision to other Persons or circumstances shall not be affected by such illegality, invalidity or unenforceability, nor shall such illegality, invalidity or unenforceability affect the legality, validity or enforceability of such provision, or the application thereof, in any other jurisdiction.
SECTION 4.03. Counterparts. This Agreement may be executed in one or more counterparts, each of which shall be deemed an original and all of which shall be considered one and the same agreement, and shall become effective when one or more counterparts have been signed by each of the parties hereto and delivered to the other parties. A signed copy of this Agreement delivered by facsimile, email or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Agreement.
SECTION 4.04. Entire Agreement; No Third Party Beneficiaries. This Agreement, together with the Merger Agreement to the extent referenced herein, constitutes the entire agreement, and supersedes all prior agreements and understandings, both written and oral, between the parties hereto with respect to the subject matter hereof and is not intended to confer upon any Person other than the parties hereto any rights or remedies.
SECTION 4.05. Governing Law. This Agreement shall be deemed to be made in and in all respects shall be interpreted, construed and governed by and in accordance with the Law of the State of Delaware without regard to the conflicts of laws, rules or principles thereof (or any other jurisdiction) to the extent that such laws, rules or principles would direct a matter to another jurisdiction.
SECTION 4.06. Venue. The parties hereby irrevocably submit to the exclusive jurisdiction of the Court of Chancery of the State of Delaware, or, only if such court declines to accept jurisdiction over a particular matter, any state or federal court within the State of Delaware, solely in respect of the interpretation and enforcement of the provisions of this Agreement and of the documents referred to in this Agreement, and in respect of the transactions contemplated hereby and thereby, and hereby waive, and agree not to assert, as a defense in any Legal Proceeding for the interpretation or enforcement hereof or of any such document, that it is not subject thereto or that such Legal Proceeding may not be brought or is not maintainable in said courts or that the venue thereof may not be appropriate or that this Agreement or any such document may not be enforced in or by such courts, and the parties irrevocably agree that all claims relating to such Legal Proceeding or transactions shall be heard and determined in such a Delaware state or federal court. The parties hereby consent to and grant any such court jurisdiction over the person of such parties and, to the extent permitted by Law, over the subject matter of such dispute and agree that mailing of process or other papers in connection with any such Legal Proceeding in the manner provided in Section 4.01 or in such other manner as may be permitted by Law shall be valid and sufficient service thereof.
SECTION 4.07. Waiver of Jury Trial. EACH PARTY ACKNOWLEDGES AND AGREES THAT ANY CONTROVERSY WHICH MAY ARISE UNDER THIS AGREEMENT IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES, AND THEREFORE EACH SUCH PARTY HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS AGREEMENT, THE DOCUMENTS REFERRED TO HEREIN OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (i) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (ii) EACH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (iii) EACH PARTY MAKES THIS WAIVER VOLUNTARILY AND (iv) EACH PARTY HAS BEEN INDUCED BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS CONTEMPLATED IN THIS SECTION 4.07, TO ENTER INTO THIS AGREEMENT, THE AGREEMENTS CONTEMPLATED BY THE DOCUMENTS REFERRED TO HEREIN AND THE TRANSACTIONS CONTEMPLATED HEREBY AND THEREBY, AS APPLICABLE.
Annex D-6
Table of Contents
SECTION 4.08. Assignment. Neither this Agreement nor any of the rights, interests or obligations under this Agreement shall be assignable or delegable (as the case may be), in whole or in part, by operation of Law or otherwise, and any attempted or purported assignment or delegation in violation of this Section 4.08 shall be null and void. Subject to the immediately preceding sentence, this Agreement will be binding upon, inure to the benefit of and be enforceable by the parties hereto and their respective successors and assigns.
SECTION 4.09. Enforcement. The parties hereto agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. Accordingly, each party agrees that, in addition to any other available remedies the parties may have in equity or at law, each party shall, unless this Agreement has been terminated in accordance with its terms, be entitled to specific performance and injunctive relief as a remedy for any such breach including an injunction restraining any breach or violation or threatened breach or violation of the provisions of this Agreement and to enforce specifically the terms and provisions of this Agreement exclusively in the courts specified in Section 4.06, in each case without necessity of posting a bond or other form of security. In the event that any Legal Proceeding should be brought in equity to enforce the provisions of this Agreement, no party shall allege, and each party hereby waives the defense, that there is an adequate remedy at law.
SECTION 4.10. Amendment; Waiver. This Agreement may be amended by the parties hereto at any time. Any amendment to this Agreement shall be valid only if set forth in an instrument in writing signed on behalf of each of the parties hereto. The parties hereto may, to the extent permitted under applicable Law, waive compliance with any of the terms or conditions contained in this Agreement. Any agreement on the part of a party to any such waiver shall be valid only if set forth in an instrument in writing signed on behalf of such party. The failure of any party to this Agreement to assert any of its rights hereunder or otherwise shall not constitute a waiver of such rights.
SECTION 4.11. Termination. This Agreement, and all obligations of the parties hereunder shall automatically terminate, without further action by any party hereto, upon the earliest of (i) the Effective Time, (ii) the termination of the Merger Agreement pursuant to Article 9 thereof, (iii) any amendment of the Merger Agreement which would materially increase the number of Merger Shares issuable pursuant to the Merger or the other consideration payable by Parent under the Merger Agreement, unless the Stockholder has consented in writing to such amendment, and (iv) the mutual written agreement of the Stockholder and the Company. In the event of any such termination of this Agreement, this Agreement shall forthwith become void and have no effect, without any liability or obligation on the part of the Company or the Stockholder, other than (A) this Article IV, which provisions shall survive such termination, and (B) liability for any breach of this Agreement prior to such termination.
[Signature Page Follows]
Annex D-7
Table of Contents
IN WITNESS WHEREOF, the parties have duly executed this Agreement, all as of the date first written above.
| | RAFAEL PHARMACEUTICALS, INC. |
| | | | | |
| | | By: | | |
| | | | | Name: |
| | | | | Title: |
[Signature Page to Voting Agreement]
Annex D-8
Table of Contents
IN WITNESS WHEREOF, the parties have duly executed this Agreement, all as of the date first written above.
| | STOCKHOLDER: |
| | | Howard S. Jonas |
| | | |
[Signature Page to Voting Agreement]
Annex D-9
Table of Contents
Annex D-10
Table of Contents
Annex E
FORM OF CONVERTIBLE NOTE CANCELLATION AGREEMENT
This CONVERTIBLE NOTE CANCELLATION AGREEMENT (this “Agreement”), dated as of [•], 2021, is made and entered into by and among Rafael Holdings, Inc., a Delaware corporation (“Parent”), Rafael Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and the holder of an outstanding Company Convertible Note (as defined below) party hereto (“Holder” and together with other holders of note of the Company, “Holders”). Capitalized terms used and not otherwise defined herein shall have the meanings ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
WHEREAS, the Company issued 3.5% Series C Company Convertible Notes (each, as amended, a “Company Convertible Note” and together the “Company Convertible Notes”) for an aggregate amount equal to $15,442,102 to the Holders between February 26, 2013 and September 19, 2016 in the amount set forth opposite the Holder’s name on Annex A hereto pursuant to the form of Subscription Agreement between the Company and the applicable Holder.
WHEREAS, the Company, Parent, RH Merger I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Parent (“Merger Sub I”), and RH Merger II, LLC, a Delaware limited liability company and a direct, wholly owned subsidiary of Parent (“Merger Sub II” and together with Merger Sub I, “Merger Subs”), are entering into an Agreement and Plan of Merger (the “Merger Agreement”), providing for, among other things, the merger of Merger Sub I with and into the Company (the “Merger”), with the Company surviving the Merger and becoming a direct, wholly owned subsidiary of Parent, followed by the merger of the Company with and into Merger Sub II (the “Subsequent Merger”), with Merger Sub II surviving the Subsequent Merger as a direct, wholly owned subsidiary of Parent, in each case on the terms and subject to the conditions set forth in the Merger Agreement.
WHEREAS, in connection with the Merger Agreement and the consummation of the transactions contemplated thereby, the Company and each Holder agrees to terminate and cancel the Company Convertible Notes, effective as of immediately prior to the Effective Time, and the Company, Parent and each Holder have agreed to enter into this Agreement regarding the terms of the termination and cancellation of the Company Convertible Notes.
WHEREAS, notwithstanding anything to the contrary set forth in the Company Convertible Note, pursuant to the terms of the Merger Agreement and this Agreement, each Company Convertible Note, together with interest thereon through September 30, 2021, will be cancelled and extinguished and converted into the applicable Note Conversion Shares (as defined below), subject to the execution and delivery of this Agreement by each Holder, and each Holder shall be entitled to receive in consideration of each Note Conversion Share, at the Closing, subject to Section 1.6(c) of the Merger Agreement, a number of shares of Parent Class B Common Stock calculated in accordance with Section 1.5(c) of the Merger Agreement.
NOW THEREFORE, in consideration of the foregoing and the mutual representations, warranties, covenants, agreements, terms and conditions contained herein, the parties hereto do hereby agree as follows:
ARTICLE I
CONVERSION; RELEASE; WAIVER
1.1 Conversion of Company Convertible Notes.
(a) Note Conversion Shares. Immediately prior to the Effective Time, (a) the outstanding principal and any accrued but unpaid interest on each Company Convertible Note through September 30, 2021 (the “Outstanding Amount”) is set forth next to the Holder’s name on Annex A hereto and (b) the Outstanding Amount under each Company Convertible Note shall convert into (the “Conversion”) a number of shares of Company Series C Preferred Stock equal to the quotient of (i) the Outstanding Amount divided by (ii) $1.25 (such number of shares of Company Series C Preferred Stock, the “Note Conversion Shares”). The number of Note Conversion Shares into which the Outstanding Amount of each Company Convertible Note shall be converted is set forth next to each Holder’s name on Annex A hereto.
Annex E-1
Table of Contents
(b) Company Convertible Note Cancellation. Each Holder hereby irrevocably acknowledges and agrees that, notwithstanding anything to the contrary set forth in any Company Convertible Note, effective as of immediately prior to the Effective Time, each Company Convertible Note is hereby terminated and canceled and is null and void and of no further effect. Each Holder further irrevocably acknowledges and agrees that from and after the Closing, such Holder’s only right with respect to such Holder’s Company Convertible Note is the right to receive the consideration described in Section 3.1 below. Each Holder hereby irrevocably acknowledges and agrees that such Holder shall be responsible for any federal, state or local income or similar taxes required to be paid by such Holder with respect to the Company Convertible Note, the Note Conversion Shares and this Agreement. Each Holder shall indemnify and hold the Released Parties (as defined below) harmless from and against any claim, demand, action, cause of action, loss, liability, damage, cost, penalty or expense whatsoever, including reasonable and documented legal fees, suffered or incurred by the Released Parties by reason of such Holder’s failure to pay or discharge any federal, state or local income or similar taxes required to be paid by such Holder resulting from any amount paid or deemed to be paid to such Holder.
(c) Interest. Without limiting Section 1.1(b), Holder hereby (i) acknowledges and agrees that the principal and accrued interest set forth on Annex A hereto accurately reflect all principal and accrued interest owed through September 30, 2021 and that such Holder waives receipt of interest that would have accrued after September 30, 2021; (ii) acknowledges and agrees that the entire amount owed to such Holder under the Company Convertible Note(s), including all accrued interest thereunder, shall automatically convert at the Effective Time into the number of Note Conversion Shares set forth opposite such Holder’s name on Annex A hereto without the requirement of any further action on the part of Holder; (iii) waives in connection with such conversion any and all notices required by the terms of such Company Convertible Note(s) or any related subscription agreement, note purchase agreement or similar agreement (“Note Purchase Agreement”); (iv) acknowledges and agrees that the number of Note Conversion Shares set forth opposite Holder’s name on Annex A hereto correctly reflects any conversion rate discounts contemplated by such Company Convertible Note(s) or the Note Purchase Agreement (or is as agreed by the Company and such Holder as a result of an agreed amendment of the applicable Company Convertible Note(s)); (v) waives as of the Effective Time any rights to receive payment pursuant to the Company Convertible Notes; and (vi) acknowledges and agrees that effective upon the Effective Time, without any further action required by the Company or such Holder, such Company Convertible Note(s) and all obligations of the Company set forth thereunder shall be immediately deemed satisfied in full and extinguished in their entirety, and any rights of such Holder pursuant to such Company Convertible Note(s) and Note Purchase Agreement shall be terminated and be of no further force or effect, including the right to payment with respect to any residual principal or interest under the Company Convertible Note(s) not converted into whole Note Conversion Shares at the Effective Time. The Company and the Holder acknowledge and agree that the terms of the Company Convertible Notes are amended and restated to provide that the Company Convertible Notes convert into the shares set forth on Annex A hereto and to give effect to the foregoing provisions of this Section 1.1(c).
(d) Approval of Mergers. Holder hereby approves the Merger Agreement and the Mergers for all purposes, including for purposes of any and all voting or approval rights associated with ownership of a Company Convertible Note.
1.2 Release.
(a) Holder, for himself, herself or itself and on behalf of his, her or its Affiliates, and any of their respective officers, directors, employees, agents, representatives, successors, members, managers, partners and permitted assigns (each a “Waiving Party”), acknowledges and agrees that, as of the Effective Time, to the fullest extent permitted under applicable Law, any and all rights, claims and causes of action he, she or it may have as of the Effective Time against Parent, Merger Subs, or any of their respective Affiliates, or any of their respective officers, directors, employees, agents, representatives, successors and permitted assigns (the “Parent Group”) relating to the business of the Company, the operation of the Company, or its businesses or relating to the subject matter of the Merger Agreement or any Exhibit or the Schedules thereto, or any ancillary agreement, certificate or other document entered into, made, delivered, or made available in connection therewith, or as a result of any of the transactions contemplated thereby, whether arising under, or based upon, any federal, state, local or foreign statute, Law, ordinance, rule or regulation or otherwise (including any right, whether arising at law or in equity, to seek indemnification, contribution, cost recovery, damages or any other recourse or remedy, including as may arise under common law) are hereby irrevocably waived by the Waiving Parties (the “Waived Claims”); provided, however, this Section 1.2 shall not apply to, and “Waived Claims” shall not include, (i) any rights explicitly set forth in the Merger Agreement (including any equity award or rollover
Annex E-2
Table of Contents
agreement), this Agreement or any ancillary agreement, certificate or other document entered into in connection with the Merger Agreement, or as a result of any of the transactions contemplated thereby, to which the Holder is a party, (ii) any rights to indemnification, exculpation or advancement of expenses and insurance as an officer or director of the Company, including in accordance with Section 5.4 of the Merger Agreement or as provided in any indemnification agreement entered into with an officer or director or in the Company Charter and the Company’s bylaws, (iii) in the case of an Holder who is also an employee of the Company, any rights provided for under any employment agreement, offer letter or other compensation arrangement or benefit plan with the Company or Parent, (iv) in the case of an Holder who is also an employee of, or consultant to, the Company, in respect of claims for accrued and unpaid compensation and/or benefits or other vested rights under any employee benefit programs or policies of the Company at or prior to the Effective Time, (v) any claims first arising (and based solely on facts and circumstances) after the Effective Time and (vi) any claims which may not be waived as a matter of Law, including, but not limited to, any Waiving Party’s right to file a charge with or participate in a charge by any Governmental Body that is authorized to enforce or administer Laws related to employment (in the case of an Holder who is also an employee). Moreover, nothing in this Agreement waives any Waiving Party’s right or otherwise prohibits any Waiving Party from reporting violations of law or regulation to any Governmental Body or from otherwise participating, to the extent requested by a Governmental Body, in any investigation or proceeding that may be conducted by an any Governmental Body, including providing documents or other information to the extent requested by a Governmental Body. No Waiving Party needs the prior authorization of the Parent Group to make any such reports or disclosures to any Governmental Body or to participate in any such investigation or proceeding that may be conducted by any Governmental Body, and no Waiving Party is required to notify the Parent Group that such Waiving Party has made such reports or disclosures or has participated or is participating in any such investigation or proceeding. This Agreement also does not limit any Waiving Party’s right to file a charge or complaint with any Governmental Body related to such Holder’s employment with the Company or receive an award for information provided to any Governmental Body. Furthermore, without limiting the generality of this Section 1.2, no claim will be brought or maintained by, or on behalf of, Holder or any other Waiving Party against any member of the Parent Group, and no recourse will be sought or granted against any of them, by virtue of, or based upon, any alleged misrepresentation or inaccuracy in, or breach of, any of the representations, warranties or covenants of any Person set forth or contained in the Merger Agreement or any Exhibit or Schedules thereto, or any ancillary agreement, certificate or other document entered into, made, delivered, or made available in connection therewith, or as a result of any of the transactions contemplated thereby, in each case except as set forth in clauses (i) through (vi) of the first sentence of this Section 1.2(a). Holder acknowledges and agrees that the Waiving Parties may not avoid such limitation on liability by (A) seeking damages for breach of contract, tort (other than claims based on gross negligence, recklessness or fraud) or pursuant to any other theory of liability, all of which are hereby waived or (B) asserting or threatening any claim against any Person that is not a party hereto or to the Merger Agreement (or a successor to a party thereto) for breaches of the representations, warranties, covenants or agreements contained in this Agreement or the Merger Agreement. The parties hereto acknowledge and agree that the limits imposed on the Waiving Parties’ remedies with respect to this Agreement and the Merger Agreement and the transactions contemplated hereby and thereby were bargained for between sophisticated parties and were specifically taken into account in the determination of the amounts to be paid by Parent and Merger Subs under the Merger Agreement.
(b) HOLDER, ON BEHALF OF THE WAIVING PARTIES, EXPRESSLY IRREVOCABLY WAIVES ALL RIGHTS AFFORDED BY ANY STATUTE OR COMMON LAW PRINCIPLES WHICH LIMITS THE EFFECT OF A RELEASE WITH RESPECT TO UNKNOWN CLAIMS. HOLDER ON BEHALF OF THE WAIVING PARTIES, ACKNOWLEDGES THAT HE, SHE OR IT UNDERSTANDS THE SIGNIFICANCE OF THIS RELEASE OF UNKNOWN CLAIMS AND WAIVER OF ANY STATUTORY PROTECTION AGAINST A RELEASE OF UNKNOWN CLAIMS. HOLDER, ON BEHALF OF THE WAIVING PARTIES, ACKNOWLEDGES AND AGREES THAT THIS WAIVER IS AN ESSENTIAL AND MATERIAL TERM OF THIS AGREEMENT.
IN ADDITION, HOLDER AND EACH WAIVING PARTY SPECIFICALLY WAIVES THE BENEFIT OF ANY AND ALL PROVISIONS, RIGHTS AND BENEFITS CONFERRED BY ANY LAW OF ANY STATE OR TERRITORY OF THE UNITED STATES, OR PRINCIPLE OF COMMON LAW, WHICH IS SIMILAR, COMPARABLE OR EQUIVALENT TO CALIFORNIA CIVIL CODE SECTION 1542, AS WELL AS THE RIGHTS AND BENEFITS CONFERRED BY CALIFORNIA CIVIL CODE SECTION 1542 ITSELF, WHICH READS AS FOLLOWS: A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS WHICH THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH DEBTOR OR RELEASED PARTY.
Annex E-3
Table of Contents
(c) This release shall become effective only upon the consummation of the Merger at the Effective Time pursuant to the terms and conditions of the Merger Agreement.
1.3 Waiver of Rights.
(a) Holder, on behalf of his, her or itself and its Affiliates, hereby waives any and all preemptive rights, anti-dilution rights, rights of first refusal, rights of first offer or other similar rights that Holder and its Affiliates may have, or ever have had, with respect to any Company Capital Stock and/or the Company Convertible Notes, and waives any right Holder and its Affiliates may have, or ever have had, under the agreements listed on Exhibit A hereto or otherwise to acquire any Company Capital Stock being exchanged pursuant to, or as contemplated by, the Merger Agreement or any transfer that occurred prior to the Effective Time.
(b) The waivers set forth in this Section 1.3 shall become effective only upon the consummation of the Mergers pursuant to the terms and conditions of the Merger Agreement.
1.4 No Transfer. Other than pursuant to the terms of this Agreement or the Merger Agreement, without the prior written consent of Parent, during the term of this Agreement, Holder agrees not to, directly or indirectly, sell, assign, transfer, pledge, encumber or otherwise dispose of (including, without limitation, by merger, consolidation, sale, liquidation, dissolution, dividend, distribution or otherwise by operation of law), or enter into any contract, option or other arrangement or understanding with respect to the direct or indirect assignment, transfer, encumbrance or other disposition of (including, without limitation, by merger, consolidation or otherwise by operation of law), any Company Convertible Note or the Series C Preferred Stock issuable upon conversion thereof (each, a “Transfer”). Any action taken in violation of the foregoing sentence shall be null and void ab initio. If any involuntary Transfer of any of Company Convertible Note or the Series C Preferred Stock issuable upon conversion thereof shall occur (including, but not limited to, a sale by Holder’s trustee in any bankruptcy, or a sale to a purchaser at any creditor’s or court sale), the transferee (which term, as used herein, shall include any and all transferees and subsequent transferees of the initial transferee) shall take and hold such Company Convertible Note or the Series C Preferred Stock issuable upon conversion thereof subject to all of the restrictions, liabilities and rights under this Agreement, which shall continue in full force and effect until the termination of this Agreement.
ARTICLE II
REPRESENTATIONS AND WARRANTIES OF HOLDER
Holder hereby represents and warrants to Parent, Merger Subs, the Company, and the Surviving Company that the following representations and warranties are true and accurate on the date hereof and as of the Closing Date as if made as of the Closing Date, and acknowledges that such Persons may rely on such representations and warranties as if each such Person was a party to this Agreement and each shall have the rights, remedies and benefits under this Agreement as if such Person was a party hereto:
2.1 Organization. If Holder is not a natural person, Holder is validly existing and in good standing under the Laws of the jurisdiction of its organization.
2.2 Authorization; Enforceability. Holder has requisite power and authority or, if Holder is an individual, legal capacity, to execute and deliver this Agreement and each other agreement contemplated hereby to which Holder is a party and to perform Holder’s obligations hereunder and thereunder. If Holder is not a natural person, the execution and delivery of this Agreement by Holder and each other agreement contemplated hereby to which Holder is a party and the consummation by Holder of the transactions contemplated hereby and thereby have been duly authorized by all necessary action and no other proceedings on the part of Holder and no stockholder votes are necessary to authorize this Agreement or the other agreements contemplated hereby to which Holder is a party or to consummate the transactions contemplated hereby and thereby. This Agreement has been duly executed and delivered by Holder and, assuming the due authorization, execution and delivery by the other parties hereto, constitutes the valid and legally binding obligation of Holder, enforceable in accordance with its terms and conditions, subject to Laws of general application relating to public policy, bankruptcy, insolvency and the relief of debtors and rules of Law governing specific performance, injunctive relief and other equitable remedies.
Annex E-4
Table of Contents
2.3 Non-Contravention.
(a) Neither the execution and delivery of this Agreement or any other agreement contemplated hereby to which Holder is a party, nor the consummation of the transactions contemplated hereby or thereby, will (i) violate any Laws to which Holder is subject, (ii) if Holder is not a natural person, violate any provision of Holder’s formation documents and bylaws or other similar document, or (iii) conflict with, result in a breach of, constitute a default under, result in the acceleration of, create in any Person the right to accelerate, terminate, modify or cancel or require any notice or consent under, or result in the imposition of any Lien (other than Permitted Liens) upon any of the assets of Holder under, any agreement, contract, lease, license, instrument or other arrangement to which Holder is a party or by which Holder is bound or to which any of Holder’s assets is subject, except in each case of clause (i) or (iii) where the violation, conflict, breach, default, acceleration, termination, modification, cancellation, failure to give notice or obtain consent or Lien would not adversely affect or delay Holder’s performance under this Agreement or any other agreement contemplated hereby to which Holder is a party or the consummation of the transactions contemplated by this Agreement or such other agreements.
(b) Holder does not need to give any notice to, make any filing with or obtain any authorization, consent or approval of any Governmental Body for the parties to consummate the transactions contemplated by this Agreement.
2.4 Litigation. There are no Legal Proceedings pending against, or, to the knowledge of Holder, threatened against, Holder that would adversely affect or delay Holder’s performance under this Agreement or the consummation of the transactions contemplated by this Agreement.
2.5 Ownership of Company Convertible Note and Note Conversion Shares. Such Holder is the beneficial and record owner of and has good and marketable title to the Company Convertible Note set forth next to such Holder’s name on Annex A hereto and, as of immediately prior to the Effective Time, will be the beneficial and record owners and have good and marketable title to the Note Conversion Shares into which such Company Convertible Note is converted as set forth on Annex A hereto pursuant to this Agreement and such Note Conversion Shares will be free and clear of all Liens other than (a) Liens created by Parent or any of Parent’s Affiliates and (b) Liens imposed by securities Laws. The outstanding principal amount and all accrued interest under such Holder’s Company Convertible Note and the number of Note Conversion Shares into which such Holder’s Company Convertible Note will be converted is set forth next to such Holder’s name on Annex A. Except for this Agreement, there are no agreements or other rights or arrangements existing which provide for the sale, purchase, exchange or other transfer by Holder of the Company Convertible Note or any interests therein. Other than the Company Convertible Note and shares of Company Capital Stock set forth under the Holder’s name on the signature page hereto, neither the Holder or any of his Affiliates owns any Company Capital Stock or rights to or securities convertible into, exercisable for, Company Capital Stock.
2.6 Tax Matters. Such Holder has had an opportunity to review with his own tax advisors the tax consequences of the termination and cancelation of such Holder’s Company Convertible Note, the Conversion, the Merger and the other transactions contemplated by the Merger Agreement and this Agreement. Such Holder understands that he must rely solely on its tax advisors and not on any statements or representations regarding tax matters made by Parent, Merger Sub, the Company or any of their respective Representatives. Such Holder understands that such Holder (and not Parent, Merger Sub, the Company or the Surviving Corporation) shall be responsible for any Tax liability for such Holder that may arise as a result of the termination and cancelation of such Holder’s Company Convertible Note, the Conversion, the Merger or any of the other transactions contemplated by the Merger Agreement or this Agreement.
2.7 Review. Such Holder has had an opportunity to carefully read this Agreement and the Merger Agreement, and such Holder has had reasonable time and opportunity to discuss the requirements of such agreements with such Holder’s financial, legal and other advisors, to the extent such Holder has determined necessary, prior to executing this Agreement. Such Holder acknowledges he is relying solely on his own counsel and not on any statements or representations of Parent, Merger Subs or the Company or their respective Representatives for legal advice with respect to this Agreement, the Merger Agreement and the transactions contemplated hereby and thereby.
Annex E-5
Table of Contents
ARTICLE III
COVENANTS OF HOLDER
3.1 Merger Consideration. Holder acknowledges and agrees that the amount of consideration to be paid to Holder in connection with the Merger pursuant and subject to the terms of the Merger Agreement, including Section 1.5 (c) and Section 1.6 (c) of the Merger Agreement, is the sole amount owed to Holder by Parent and Merger Subs as consideration for any and all Company Convertible Notes and Note Conversion Shares.
3.2 Confidentiality. Except with the prior written consent of Parent, Holder agrees to at all times keep confidential and not disclose (other than to his, her or its spouse, family members, officers, directors, employees, members, partners, Affiliates, attorneys and other advisors or representatives; provided, that such Persons are directed to keep confidential and not disclose consistent with the confidentiality restrictions contained herein and Holder will be directly responsible for any breach of this Section 3.2 by any such Persons): (a) the existence, terms and conditions of the Merger Agreement and the transactions contemplated thereby; (b) matters regarding the interpretation, performance, breach or termination hereof or thereof; and (c) all confidential, proprietary or non-public information any the Company of any kind or nature, including, without limitation, Intellectual Property developed, used or owned by the Company, obtained by Holder or its directors, officers, employees, agents or representatives prior to the Closing, except in each such case to the extent that (i) such information is used in connection with Holder’s employment with or engagement by the Company or Parent and only to the extent required to perform Holder’s duties as an employee or in connection with such engagement, (ii) such information has otherwise been made public or is publicly available through no fault or action of Holder, (iii) Holder receives such information from a third party that, to Holder’s knowledge, owes no duty of confidentiality to the Company with respect to such information, (iv) such information is independently developed by Holder without use of or reference to any confidential, proprietary or non-public information of the Company, (v) Holder is required by applicable Law or legal or judicial process to divulge or disclose any such information, in which case Holder shall, to the extent legally permissible, promptly notify Parent in writing in advance of disclosing such information and use commercially reasonable efforts to cooperate with Parent (at Parent’s sole expense) to limit such disclosure to the extent so required by applicable Law or legal or judicial process, or (vi) such information as is reasonably necessary for enforcing Holder’s rights hereunder or thereunder; provided; however, if Holder is not a natural person and is an investment fund, Holder may disclose the terms and conditions regarding the transactions contemplated by the Merger Agreement and the nature of Holder’s investment in the Company, including, but not limited to the fact that it sold its interest in the Company, the amount of Holder’s investment in the Company, co-investors in the Company, the identity of Parent and its proceeds in the sale of its interest in the Company to its stockholders, members, limited partners, investors and prospective stockholders, members, limited partners and investors so long as such Persons are informed by Holder of the confidential nature of such information and agree or have agreed to treat such information confidentially. Nothing in this Section 3.2 shall limit or restrict Holder who serves as a member of the Board of Directors, an officer, or employee of the Company, the Company or the Surviving Company in acting in his or her capacity as a director, officer, or employee to perform his or her employment duties in the ordinary course, and/or exercising his or her fiduciary duties and responsibilities in such capacity as required by applicable Law.
3.3 Termination of the Holder Agreements. Holder and the Company consent, effective as of and contingent upon the Effective Time, to the termination of the agreements listed on Exhibit A hereto, in accordance with their respective terms.
ARTICLE IV
AGREEMENT TO BE BOUND BY CERTAIN
PROVISIONS OF THE MERGER AGREEMENT
4.1 Agreement to be Bound.
(a) Holder has received the Merger Agreement and the other agreements, instruments, exhibits, schedules, certificates and other documents referenced in the Merger Agreement or in the foregoing agreements and documents (collectively, the “Transaction Documents”) to which it is a party or is bound, and hereby acknowledges having had sufficient opportunity to review the Merger Agreement and the applicable Transaction Documents and agrees that Holder is a Company Stockholder for purposes of the Merger Agreement and, accordingly, agrees to be bound by, solely in Holder’s capacity as such Company Stockholder and as fully as though Holder were a signatory thereto, the terms and conditions of the Merger Agreement in relation to the Company Stockholders.
Annex E-6
Table of Contents
(b) Holder acknowledges, approves of and agrees to be bound by the provisions set forth in the Merger Agreement related to Tax withholding (as set forth in Section 1.9(d) of the Merger Agreement, to the extent applicable).
(c) Contingent upon the Closing, Parent acknowledges and agrees that Holder is entitled to receive the consideration required to be paid to Holder pursuant to the terms of the Merger Agreement as though the Holder were a signatory thereto, including without limitation, Section 1.6.
ARTICLE V
MISCELLANEOUS
5.1 Governing Law.
(a) This Agreement and any Legal Proceeding of any kind or nature (whether at law or in equity, based upon contract, tort or otherwise) that is in any way related to this Agreement or any of the transactions related hereto (including the interpretation, construction, validity and enforcement of this Agreement, or the negotiation, execution or performance of any of the transactions related hereto (including any Legal Proceeding based upon, arising out of, or related to any representation or warranty expressly made in this Agreement)) shall be governed by and construed in accordance with the domestic Laws of the State of Delaware, including its statutes of limitations, without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the Laws of any jurisdiction other than the State of Delaware and without regard to any borrowing statute that would result in the application of the statute of limitations of any other jurisdictions. In furtherance of the foregoing, the laws of the State of Delaware will control even if under such jurisdiction’s choice of law or conflict of law analysis, the substantive law of some other jurisdiction would ordinarily or necessarily apply.
(b) The parties hereto submit to the exclusive jurisdiction of the Chancery Court of the State of Delaware and any state appellate court therefrom within the State of Delaware (or if the Chancery Court of the State of Delaware declines to accept jurisdiction over a particular matter, any federal court sitting in the State of Delaware and any federal appellate court therefrom) in respect of the interpretation and enforcement of the provisions of this Agreement and any related agreement, certificate or other document delivered in connection herewith and by this Agreement waive, and agree not to assert, any defense in any action for the interpretation or enforcement of this Agreement and any related agreement, certificate or other document delivered in connection herewith that they are not subject to such jurisdiction or that such action may not be brought or is not maintainable in such courts or that this Agreement may not be enforced in or by such courts, that the action is brought in an inconvenient forum, or that the venue of the action is improper.
(c) Each party hereto agrees that service in person or by certified or by nationally recognized overnight courier to its address set forth in Section 5.7 shall constitute valid in personam service upon such party and its successors and assigns in any arbitration proceeding commenced pursuant to this Section 5.1. Each party hereto hereby acknowledges that this is a commercial transaction, that the foregoing provisions for consent to arbitration, service of process and waiver of jury trial have been read, understood and voluntarily agreed to by each party hereto and that by agreeing to such provisions each party hereto is waiving important legal rights.
5.2 Succession and Assignment. This Agreement shall be binding upon and inure to the benefit of the parties named herein and their respective successors and permitted assigns. No party hereto may assign either this Agreement or any of its rights, interests or obligations hereunder without the prior written approval of the other parties hereto; provided, however, Parent may assign this Agreement to any Affiliate of Parent without the prior written consent of Holder if any of Parent’s rights under the Merger Agreement are also assigned to such Affiliate; provided, further, no such assignment shall relieve Parent of its obligations hereunder.
5.3 Severability. Whenever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable Law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable Law by which this Agreement is governed, such invalidity, illegality or unenforceability shall not affect any other provision; provided, that such provision shall be construed to give effect to the parties’ intent regarding such provision to the maximum extent permitted by applicable Law.
Annex E-7
Table of Contents
5.4 Specific Performance. Holder agrees that irreparable damage would occur in the event any of the provisions of this Agreement were not performed by the parties hereto in accordance with their specific terms or were otherwise breached. It is accordingly agreed that Parent, on the one hand, and Holder, on the other hand, shall be entitled to seek an injunction or injunctions to prevent breaches of this Agreement by the other (as applicable) and to enforce specifically the terms and provisions of this Agreement and to thereafter cause the transactions contemplated by this Agreement to be consummated on the terms and subject to the conditions thereto set forth in this Agreement. The foregoing rights are in addition to and without limitation of any other remedy to which the parties hereto may be entitled at law or in equity. The parties hereto further agree not to assert that a remedy of specific performance is unenforceable, invalid, contrary to law or inequitable for any reason, nor to assert that a remedy of monetary damages would provide an adequate remedy. Each of the parties hereto hereby waives: (a) any defenses in any action for specific performance, including the defense that a remedy at law would be adequate’ and (b) any requirement under any Law to post a bond or other security as a prerequisite to obtaining equitable relief.
5.5 Amendments and Waivers. No amendment of any provision of this Agreement shall be valid unless the same shall be in writing and signed by Parent and Holder. No waiver by Parent or Holder of any provision of this Agreement or any default, misrepresentation or breach of warranty or covenant hereunder, whether intentional or not, shall be valid unless the same shall be in writing and signed by the party hereto making such waiver, nor shall such waiver be deemed to extend to any prior or subsequent default, misrepresentation or breach of warranty or covenant hereunder or affect in any way any rights arising by virtue of any prior or subsequent such occurrence.
5.6 Expenses. Each party hereto will bear his, her or its own costs and expenses (including legal fees and expenses) incurred in connection with this Agreement and the transactions contemplated hereby.
5.7 Notices. All notices, requests, demands, claims and other communications hereunder will be in writing. Any notice, request, demand, claim or other communication hereunder shall be deemed duly given: (a) when delivered personally to the recipient; (b) one (1) Business Day after being sent to the recipient on a priority basis by reputable overnight courier service (charges prepaid); or (c) if sent by electronic mail, when received if received before 5:00 p.m. local time of the recipient on a Business Day, and otherwise on the next following Business Day, in each case addressed to the intended recipient as set forth below:
(a) If to Parent to:
Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Attention: David Polinsky, Chief Financial Officer
E-mail: david.polinsky@rafaelholdings.com
with a copy (which shall not constitute notice) to:
Olshan Frome Wolosky LLP
1325 Avenue of the Americas
New York, NY 10019
Attention: Mitchell Raab
E-mail: mraab@olshanlaw.com
and:
Schwell Wimpfheimer & Associates
37 West 39th Street, Suite 505
New York, NY 10018
E-mail: dov.schwell@swalegal.com
(b) If to Holder, at the address set forth on the signature page hereto, or if no address is set forth therein, at the last address of Holder in the Company’s or the Surviving Company’s records.
Annex E-8
Table of Contents
Any party hereto may change the address to which notices, requests, demands, claims and other communications hereunder are to be delivered by giving the other parties hereto notice in the manner set forth in this Section 5.7.
5.8 Waiver of Jury Trial. EACH PARTY HERETO HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS AGREEMENT, THE OTHER AGREEMENTS CONTEMPLATED HEREBY OR THE TRANSACTIONS CONTEMPLATED BY THIS AGREEMENT. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (II) SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (III) SUCH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (IV) SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 5.8.
5.9 Interpretation; Rules of Construction. Unless otherwise expressly provided or unless the context requires otherwise: (a) all references in this Agreement to Articles, Sections, Schedules and Exhibits shall mean and refer to Articles, Sections, Schedules and Exhibits of this Agreement; (b) all references to statutes and related regulations shall include all amendments of the same and any successor or replacement statutes and regulations; (c) words using the singular or plural number also shall include the plural or singular number, respectively; (d) references to “hereof,” “herein,” “hereby” and similar terms shall refer to this entire Agreement (including the Schedules and Exhibits hereto); (e) references to any Person shall be deemed to mean and include the successors and permitted assigns of such Person (or, in the case of a Governmental Body, Persons succeeding to the relevant functions of such Person); (f) the term “including” shall be deemed to mean “including, without limitation”; (g) words of any gender shall include each other gender; (h) whenever this Agreement refers to a number of days, such number shall refer to calendar days, unless such reference is specifically to “Business Days”; and (i) the term “or” has the inclusive meaning represented by the phrase “and/or.” The Article and Section headings contained in this Agreement are solely for the purpose of reference, are not part of the agreement of the parties and shall not in any way affect the meaning or interpretation of this Agreement. When calculating the period before which, within which or following which, any act is to be done or step taken pursuant to this Agreement, the date that is the reference date in calculating such period shall be excluded. If the last day of such period is a non-Business Day, the period in question shall end on the next succeeding Business Day. This Agreement is the product of negotiations among the parties hereto, each of which is represented by legal counsel, and no presumption or burden of proof shall arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement. Rules of construction relating to interpretation against the drafter of an agreement shall not apply to this Agreement and are expressly waived by each party hereto.
5.10 Third-Party Beneficiary. The parties hereto hereby acknowledge and agree that (a) the execution and delivery of this Agreement is a condition precedent, and a material inducement, to the consummation by Parent of the transactions under the Merger Agreement and (b) each of the Non-Party Affiliates shall be an express third party beneficiary of Section 5.14 of this Agreement and that each of them shall be entitled to enforce all rights set forth in Section 5.14 of this Agreement.
5.11 Entire Agreement. This Agreement, the Merger Agreement and the other documents referred to herein: (a) constitute the entire agreement among the parties hereto and supersede any prior understandings, agreements or representations by or among the parties hereto, written or oral, to the extent they relate in any way to the subject matter hereof; and (b) are not intended to confer, and shall not be construed as conferring, upon any Person other than the Company and Parent any rights or remedies hereunder, except as set forth in Section 5.10. If the provisions of this Agreement conflict in any way with the provisions of the Merger Agreement, the provisions of the Merger Agreement shall control.
5.12 Counterparts. This Agreement may be executed (a) in one or more partially or fully executed counterparts, each of which will be deemed an original and will bind the signatory, but all of which together will constitute the same instrument, and (b) by electronic means, PDF or facsimile.
Annex E-9
Table of Contents
5.13 Termination. This Agreement shall terminate upon the earliest of (a) the termination of the Merger Agreement in accordance with its terms and (b) the election of Holder in its sole discretion to terminate this Agreement following any amendment, supplement, waiver or other modification of any material term or provision of the Merger Agreement which is materially adverse to Holder (the earliest such date under clause (a) and (b), being referred to herein as the “Termination Date”); provided, that (i) the provisions set forth in Sections 5.1 to 5.3 and Section 5.5 to this Section 5.13 shall survive the termination of this Agreement and (ii) the termination of this Agreement will not relieve any party hereto from liability arising in respect of any breach prior to such termination.
5.14 Non-Recourse. All claims or causes of action (whether in contract or in tort, in law or in equity) that may be based upon, arise out of or relate to this Agreement, or the negotiation, execution or performance of this Agreement (including any representation or warranty made in or in connection with this Agreement or as an inducement to enter into this Agreement), may be made only against the entities that are expressly identified as parties hereto. No Person who is not a named party to this Agreement, including any past, present or future director, officer, employee, incorporator, member, partner, Holder, Affiliate, agent, attorney or representative of any named party to this Agreement (“Non-Party Affiliates”), shall have any liability (whether in contract or in tort, in law or in equity, or based upon any theory that seeks to impose liability of an entity party against its owners or affiliates) for any obligations or liabilities arising under, in connection with or related to this Agreement or for any claim based on, in respect of, or by reason of this Agreement or its negotiation or execution; and each party hereto waives and releases all such liabilities, claims and obligations against any such Non-Party Affiliate.
*****
Annex E-10
Table of Contents
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the date first above written:
| | PARENT: |
| | | RAFAEL HOLDINGS, INC. |
| | | By: | | |
| | | | | Name: |
| | | | | Title: |
| | | COMPANY: |
| | | RAFAEL PHARMACEUTICALS, INC. |
| | | By: | | |
| | | | | Name: |
| | | | | Title: |
[Signature Page to Convertible Note Cancellation Agreement]
Annex E-11
Table of Contents
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the date first above written:
| | HOLDER: |
| | | |
| | | (Print Name of Holder) |
| | | |
| | | (Signature) |
| | | |
| | | (Print name if signing on behalf of an entity) |
| | | |
| | | (Print title if signing on behalf of an entity) |
| | | | | |
| | | Address: | | |
| | | | | |
| | | | | |
| | | Email: | | |
| | | Phone: | | |
[Signature Page to Convertible Note Cancellation Agreement]
Annex E-12
Table of Contents
Annex A
Conversion Shares
Company Noteholder | | Convertible Note
Principal Amount | | Outstanding Principal and
Accrued Interest as of
September 30, 2021 | | Note Conversion Shares |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Annex E-13
Table of Contents
EXHIBIT A
Terminating Agreements
Company Convertible Note in the principal amount of $_________.
Related Subscription Agreement
Annex E-14
Table of Contents
Annex F
FORM OF ACQUISITION AGREEMENT
This Acquisition Agreement (“Agreement”) is made as of June 17, 2021 (the “Effective Date”), by and between Rafael Holdings, Inc., a Delaware corporation (“Buyer”) and each of A. Joseph Stern (“Stern”), an individual residing at [____________] and Aaron Drillick, an individual residing at [____________] (each of Stern and Aaron Drillick (“Drillick”), a “Seller” and, together, the “Sellers”). Capitalized terms used but not defined herein shall have the meaning ascribed to them in that certain Agreement and Plan of Merger, by and among Rafael Holdings, Inc., Pharma, RH Merger I, Inc., a Delaware corporation, and RH Merger II, LLC, a Delaware limited liability company (the “Merger Agreement”).
RECITALS
WHEREAS, the Sellers owns membership interests (the “Interests”) representing one-third of the membership interests in Altira Capital & Consulting, LLC, a New Jersey limited liability company (“Altira”) and thereby an equivalent one-third interest in that certain Royalty Agreement dated January 31, 2007 among Altira, Robert Shorr, Robert Rodriguez and Pharma, (the “Royalty Agreement”);
WHEREAS, Stern owns eighty-five percent (85%) of the Interests (the “Stern Interest”) and Drillick owns fifteen percent (15%) of the Interests (the “Drillick Interest”);
WHEREAS, each Seller desires to sell, and Buyer desires to purchase the Interests at the Closing on the terms and conditions set forth herein (the “Transaction”); and
NOW THEREFORE, in consideration of the mutual promises and covenants contained herein, the Parties, intending to be legally bound, agree as follows:
AGREEMENT
For good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties, intending to be legally bound, agree as follows:
ARTICLE I
DEFINITIONS
Section 1.1 Certain Definitions. For purposes of this Agreement, the following terms have the meanings specified or referred to in this Section 1.1:
“Affiliate” means any Person or entity that directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, a specified Person. For purposes of this definition only, the term “control” means the possession, directly or indirectly, of the then present right to vote more than fifty percent (50%) of the equity or elect more than fifty percent (50%) of the directors (or Persons of similar position), whether through ownership of securities, by contract or otherwise.
“Contract” means any agreement, contract, obligation, promise, or undertaking (whether written or oral and whether express or implied) that is legally binding.
“Damages” shall have the meaning ascribed thereto in Section 6.2.
“Encumbrance” means any charge, claim, community property interest, condition, equitable interest, lien, option, pledge, security interest, right of first refusal, or restriction of any kind, including any restriction on use, voting, transfer, receipt of income, or exercise of any other attribute of ownership.
“Governmental Body” means any:
(a) nation, state, county, city, town, village, district, or other jurisdiction of any nature;
Annex F-1
Table of Contents
(b) federal, state, local, municipal, foreign, or other government;
(c) governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal);
(d) multi-national organization or body; or
(e) body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature.
“Knowledge” or “knowledge” means the actual knowledge of any Party.
“Legal Requirement” means any federal, state, local, municipal, foreign, international, multinational, or other administrative order, constitutional provision, law, ordinance, principle of common law, rule, regulation, statute, treaty, or interpretation of any Governmental Body.
“Order” means any award, decision, injunction, judgment, order, ruling, subpoena, or verdict entered, issued, made, or rendered by any court, administrative agency, or other Governmental Body or by any arbitrator.
“Organizational Documents” means (a) the articles or certificate of incorporation and the bylaws of a corporation; (b) the partnership agreement and any statement of partnership of a general partnership; (c) the limited partnership agreement and the certificate of limited partnership of a limited partnership; (d) the certificate of formation and operating agreement of a limited liability company; (e) any charter or similar document adopted or filed in connection with the creation, formation, or organization of a Person; and (e) any amendment to any of the foregoing.
“Parties” means Buyer, each Seller, Altira and Pharma and “Party” shall refer to any one of Buyer, either Seller, Altira or Pharma, as applicable.
“Person” means any individual, corporation (including any non-profit corporation), general or limited partnership, limited liability company, joint venture, estate, trust, association, organization, labor union, or other entity or Governmental Body.
“Proceeding” means any action, arbitration, audit, hearing, investigation, litigation, or suit (whether civil, criminal, administrative, investigative, or informal) commenced, brought, conducted, or heard by or before, or otherwise involving, any Governmental Body or arbitrator.
ARTICLE II
SALE AND TRANSFER OF INTEREST; CLOSING
Section 2.1 Sale of the Interest. Subject to the terms and conditions of this Agreement, effective as of immediately prior to the consummation of the Mergers under the Merger Agreement (the “Closing”), Sellers will sell and transfer to Buyer free and clear of all Encumbrances, and Buyer will purchase from Seller, the Interests.
Section 2.2 Purchase Price. The purchase price for the Interests shall be THIRTY MILLION DOLLARS ($30,000,000.00) payable in immediately available funds at the Closing as follows: (a) TWENTY-FIVE MILLION FIVE HUNDRED THOUSAND DOLLARS ($25,500,000.00) payable to Stern, and (b) FOUR MILLION FIVE HUNDRED THOUSAND DOLLARS ($4,500,000.00) payable to Drillick. The payments payable by Buyer pursuant to this Section 2.2 shall be paid by wire transfer of immediately available funds in US dollars to the bank accounts previously designated by the Sellers in writing. With respect to each of the payments to be made by Buyer to Seller pursuant to this Section 2.2, Buyer shall deliver to Seller all such documents, certificates, agreements, instruments, third party consents, opinions and certificates in connection with the contemplated transaction herein reasonably required or as Sellers may reasonably request, in form and substance reasonably satisfactory to Sellers, duly executed by Buyer.
Section 2.3 Closing. The consummation of the Transaction will take place at the Closing at the offices of Buyer at 520 Broad Street, Newark, NJ, or at such other place and method as the Buyer and Sellers may agree (including exchange of counterpart signatures via overnight mail, fax or email).
Annex F-2
Table of Contents
Section 2.4 Closing Obligations. At the Closing:
(a) Each Seller will deliver to Buyer a Membership Interest Transfer Power for the transfer of the Interest to Buyer, attached hereto as Exhibit A.
(b) Buyer will deliver to Seller all such other documents, certificates, agreements, instruments, third party consents, opinions and certificates in connection with the contemplated transaction herein reasonably required or as Seller may reasonably request, in form and substance reasonably satisfactory to Sellers, duly executed by Buyer.
(c) Sellers will deliver to Buyer all such other documents, certificates, agreements, instruments, third party consents, opinions and certificates in connection with the contemplated transaction herein reasonably required or as Buyer may reasonably request, in form and substance reasonably satisfactory to Buyer, duly executed by Sellers.
Section 2.5 Conditions to Closing. The obligations of Buyer and Sellers to consummate the Transaction are subject to and conditional on the occurrence of the Closing pursuant to the Merger Agreement.
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF SELLER
Each Seller separately (but not jointly) represents and warrants to Buyer, and acknowledges that Buyer is entering into this Agreement in reliance thereon, as follows:
Section 3.1 Authority. Such Seller has full power and authority to execute and deliver this Agreement and to perform his obligations hereunder. This Agreement constitutes the valid and legally binding obligation of such Seller, enforceable in accordance with its terms and conditions.
Section 3.2 No Conflict. The execution, delivery and performance of this Agreement by such Seller will not directly or indirectly (a) result in the imposition of any Encumbrance against, in the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest, (b) contravene, conflict with or result in the breach of any provision of any contract, agreement, indenture, mortgage, instrument, lease, license, arrangement, or undertaking of any nature, written or oral, of such Seller, or (c) give any Governmental Body or other Person the right to challenge Buyer’s acquisition of, in the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest, or to exercise any remedy or obtain any relief with respect thereto.
Section 3.3 Ownership of Interest. Such Seller is the lawful owner, beneficially and of record, of, in the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest, free and clear of all Encumbrances. In the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest, is fully paid and was issued in compliance with all Legal Requirements. Such Seller owns one hundred percent (100%) of, in the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest. No other person, firm or corporation has any interest whatsoever in any of, in the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest. The sale of, in the case of Stern, the Stern Interest, and, in the case of Drillick, the Drillick Interest, vests title to each such portion of the Interests in Buyer directly, free of any Encumbrances.
Section 3.4 Legal Proceedings. No action, suit or proceeding before any court or Governmental Body or authority, pertaining to the transaction contemplated by this Agreement or to its consummation has been instituted or, to such Seller’s knowledge, threatened.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF BUYER
Buyer represents and warrants to Sellers, and acknowledges that Sellers is entering into this Agreement in reliance thereon, as follows:
Section 4.1 Organization and Good Standing. Buyer is a corporation duly organized, validly existing, and in good standing under the laws of the State of Delaware and has the full power and authority under its Organizational Documents, and applicable Legal Requirements to execute and deliver this Agreement, and other agreements contemplated hereby or which are ancillary hereto.
Annex F-3
Table of Contents
Section 4.2 Authority; No Conflict.
(a) This Agreement constitutes the legal, valid, and binding obligation of Buyer and its successors and permitted assigns, enforceable against such in accordance with its terms.
(b) Neither the execution nor delivery of this Agreement by Buyer will give any Person the right to prevent, delay, or otherwise interfere with any of the contemplated Transactions pursuant to (i) any provision of Buyer’s Organizational Documents; (ii) any resolution adopted by the board of directors or the stockholders of Buyer; (iii) any Legal Requirement or Order to which Buyer is subject prior to Closing; or (iv) any Contract to which Buyer is a party or by which Buyer may be bound prior to Closing.
ARTICLE V
TERMINATION
Section 5.1 This Agreement shall automatically terminate and be of no further force or effect upon the termination of the Merger Agreement in accordance with its terms.
ARTICLE VI
INDEMNIFICATION; REMEDIES
Section 6.1 Survival, Right to Indemnification Not Affected by Knowledge. All representations, warranties, covenants, and obligations in this Agreement, and any other certificate or document delivered pursuant to this Agreement will survive the closing of the Transaction. The right to indemnification, payment of Damages or other remedy based on such representations, warranties, covenants, and obligations will not be affected by (a) any investigation conducted, or (b) any knowledge acquired (or capable of being acquired) at any time, whether before or after the execution and delivery of this Agreement or the closing of the Transaction, in each case with respect to the accuracy or inaccuracy of or compliance with, any such representation, warranty, covenant, or obligation.
Section 6.2 Indemnification of Buyer. Each Seller severally (but not jointly) will indemnify and hold harmless Buyer and its respective officers, directors, stockholders and Affiliates (collectively, the “Buyer Indemnified Persons”) for, and will pay to the Buyer Indemnified Persons the amount of, any loss, liability, claim, damage (including incidental and consequential damages payable to any third party), expense (including costs of investigation and defense and reasonable attorneys’ fees) or diminution of value, whether or not involving a third-party claim (collectively, “Damages”), arising, directly or indirectly, from or in connection with:
(a) any breach of any representation or warranty made by such Seller in this Agreement, or any other certificate or document delivered by such Seller pursuant to this Agreement;
(b) any breach by such Seller of any covenant or obligation of such Seller in this Agreement; and
(c) any claim by any Person for brokerage or finder’s fees or commissions or similar payments based upon any agreement or understanding alleged to have been made by any such Person with such Seller (or any Person acting on its behalf) in connection with this Agreement.
Section 6.3 Indemnification of Sellers. Buyer will indemnify and hold harmless each Seller and its respective officers, directors, stockholders and Affiliates (collectively, the “Seller Indemnified Persons”) for, and will pay to the Seller Indemnified Persons the amount of Damages arising, directly or indirectly, from or in connection with:
(a) any breach of any representation or warranty made by Buyer in this Agreement, or any other certificate or document delivered by Buyer pursuant to this Agreement;
(b) any breach by Buyer of any covenant or obligation of Buyer in this Agreement; and
(c) any claim by any Person for brokerage or finder’s fees or commissions or similar payments based upon any agreement or understanding alleged to have been made by any such Person with Buyer (or any Person acting on its behalf) in connection with this Agreement.
Annex F-4
Table of Contents
Section 6.4 Procedures for Indemnification -Third Party Claims.
(a) Promptly after receipt by a Buyer Indemnified Person or Seller Indemnified Person (each, an “Indemnified Person”) under Section 6.2 or Section 6.3, respectively, of notice of the commencement of any Proceeding against it, such Indemnified Person will, if a claim is to be made against an indemnifying party, give notice to such indemnifying party of the commencement of such claim, but the failure to notify the indemnifying party will not relieve the indemnifying party of any liability that it may have to any Indemnified Person, except to the extent that the indemnifying party demonstrates that the defense of such action is prejudiced by the failure to give such notice.
(b) If any Proceeding referred to in Section 6.2 or Section 6.3 is brought against an Indemnified Person and it gives notice to the indemnifying party of the commencement of such Proceeding, the indemnifying party will be entitled to participate in such Proceeding and, to the extent that it requests in writing to the Indemnified Party (unless (i) it is also a party to such Proceeding and the Indemnified Person determines in good faith that joint representation would be inappropriate, or (ii) the indemnifying party fails to provide reasonable assurance to the Indemnified Person of its financial capacity to defend such Proceeding and provide indemnification with respect to such Proceeding), assume the defense of such Proceeding with counsel satisfactory to the Indemnified Person (not to be unreasonably withheld). If the indemnifying party assumes the defense of a Proceeding, (i) it will be conclusively established for purposes of this Agreement that the claims made in that Proceeding are within the scope of and subject to indemnification; (ii) no compromise or settlement of such claims may be effected by the indemnifying party without the Indemnified Person’s consent unless (A) there is no finding or admission of any violation of Legal Requirements or any violation of the rights of any Person and no effect on any other claims that may be made against the Indemnified Person, and (B) the sole relief provided is monetary damages that are paid in full by the indemnifying party; and (iii) the Indemnified Person will have no liability with respect to any compromise or settlement of such claims effected without its consent. If notice is given to the indemnifying party of the commencement of any Proceeding and it does not, within ten days after the Indemnified Person’s notice is given, give notice to the Indemnified Person of its election to assume the defense of such Proceeding, the indemnifying party will be bound by any determination made in such Proceeding or any compromise or settlement effected by the Indemnified Person.
(c) Notwithstanding the foregoing, if an Indemnified Person determines in good faith that there is a reasonable probability that a Proceeding may adversely affect it or its Affiliates other than as a result of monetary damages for which it would be entitled to indemnification under this Agreement, the Indemnified Person may, by notice to the indemnifying party, assume the exclusive right to defend, compromise, or settle such Proceeding, but the indemnifying party will not be bound by any determination of a Proceeding so defended or any compromise or settlement effected without its consent (which may not be unreasonably withheld).
(d) The indemnifying party hereby consents to the non-exclusive jurisdiction of any court in which a Proceeding is brought against any Indemnified Person for purposes of any claim that an Indemnified Person may have under this Agreement with respect to such Proceeding or the matters alleged therein, and agree that process may be served on Seller with respect to such a claim anywhere in the world.
Section 6.5 Procedure for Indemnification-Other Claims. A claim for indemnification for any matter not involving a third-party claim may be asserted by notice to the indemnifying party.
Section 6.6 Certain Limitations.
Notwithstanding anything to the contrary, the aggregate maximum liability of Buyer and of Seller for all claims arising under this Agreement at law or in equity, including for indemnification under Section 6.2 or Section 6.3, as applicable, or other breach of this Agreement or action between Buyer and Seller, but excluding indirect damages payable by an Indemnified Person to a third party in connection with damages for which an indemnifying party is responsible pursuant to Sections 6.2 or 6.3 above, will not exceed the Purchase Price, together with interest equal to ten percent (10%) per annum, compounded monthly, based upon a 360 day year.
Annex F-5
Table of Contents
ARTICLE VII
GENERAL PROVISIONS
Section 7.1 Expenses. Except as otherwise expressly provided in this Agreement, each Party to this Agreement will bear its respective expenses incurred in connection with the preparation, execution, and performance of this Agreement, including all fees and expenses of agents, representatives, counsel, and accountants. In the event of termination of this Agreement, the obligation of each Party to pay its own expenses will be subject to any rights of such Party arising from a breach of this Agreement by another Party.
Section 7.2 Notices. All notices, consents, waivers, and other communications under this Agreement must be in writing and will be deemed to have been duly given when (a) delivered by hand (with written confirmation of receipt), (b) sent by email (with written confirmation of receipt), provided that a copy is mailed by registered mail, return receipt requested, or (c) when received by the addressee, if sent by a nationally recognized overnight delivery service (receipt requested), in each case to the appropriate addresses and email addresses set forth below (or to such other addresses as the addressee Party may designate by notice to the other Parties):
Sellers:
A. Joseph Stern
[____________]
[____________]
Aaron Drillick
[____________]
[____________]
Buyer:
Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Attention: Menachem Ash, General Counsel
Altira:
Altira Capital & Consulting, LLC
[____________]
[____________]
Pharma:
Rafael Pharmaceuticals, Inc.
1 Duncan Drive
East Windsor, NJ 08512
Attention: Sanjeev Luther
Section 7.3 Arbitration. Any dispute between or among any of the Parties shall be submitted to and settled by commercial arbitration in a forum of the American Arbitration Association (“AAA”) located in Newark, New Jersey. In such arbitration: (a) the arbitrator shall have no authority to amend or modify any of the terms of this Agreement and (b) the arbitrator shall have ten (10) business days from the closing statements or submission of post-hearing briefs by the parties to render his or her decision. Any arbitration award shall be final and binding upon the Parties, and any court (state or federal) having jurisdiction may enter a judgment on the award. Each Party shall bear its/his own costs of participating in any arbitration proceedings. In the event Buyer fails to make the payments (if required) to Sellers in accordance with Article II of this Agreement and Sellers bring and Sellers prevail in any arbitration proceeding relating thereto, Sellers shall be entitled to its legal fees for such proceeding.
Annex F-6
Table of Contents
Section 7.4 Further Assurances. Each Party agrees (a) to furnish upon request to each other such further information, (b) to execute and deliver to each other such other documents, and (c) to do such other acts and things, all as another Party hereto may reasonably request for the purpose of carrying out the intent of this Agreement and the documents referred to in this Agreement.
Section 7.5 Waiver. The rights and remedies of the Parties to this Agreement are cumulative and not alternative. Neither the failure nor any delay by any Party in exercising any right, power, or privilege under this Agreement or the documents referred to in this Agreement will operate as a waiver of such right, power, or privilege, and no single or partial exercise of any such right, power, or privilege will preclude any other or further exercise of such right, power, or privilege or the exercise of any other right, power, or privilege. To the maximum extent permitted by applicable law, (a) no claim or right arising out of this Agreement or the documents referred to in this Agreement can be discharged by one Party, in whole or in part, by a waiver or renunciation of the claim or right unless in writing signed by the other Party as to which such waiver or renunciation is sought; (b) no waiver that may be given by a Party will be applicable except in the specific instance for which it is given; and (c) no notice to or demand on one Party will be deemed to be a waiver of any obligation of such Party or of the right of the Party giving such notice or demand to take further action without notice or demand as provided in this Agreement or the documents referred to in this Agreement.
Section 7.6 Entire Agreement; Modification. This Agreement supersedes all prior agreements between the Parties with respect to its subject matter and constitutes a complete and exclusive statement of the terms of the agreement between the Parties with respect to its subject matter. This Agreement may not be amended except by a written agreement executed by the Parties.
Section 7.7 Assignments, Successors, and no Third-party Rights. Neither Buyer nor any Seller may assign any of its rights or obligations under this Agreement without the prior consent of the Buyer and such Seller (except that Buyer may make such assignment to any of its subsidiaries without the requirement of such consent); it being understood and agreed that each Seller shall be entitled at all times to assign its right to receive any or all amounts that from time to time may be owed to each Seller to one or more Person(s) as such Seller may designate in a writing to the Buyer. This Agreement will apply to, be binding in all respects upon, and inure to the benefit of the successors and permitted assigns of the Parties. Nothing expressed or referred to in this Agreement will be construed to give any Person other than the Parties to this Agreement any legal or equitable right, remedy, or claim under or with respect to this Agreement or any provision of this Agreement. This Agreement and all of its provisions and conditions are for the sole and exclusive benefit of the Parties to this Agreement and their successors and assigns.
Section 7.8 Severability. The invalidity or unenforceability of any provision herein shall not affect the validity or enforceability of any other provision herein. If a court of competent jurisdiction determines that any portion of this Agreement is in violation of any statute or public policy only the portions of this Agreement that violate such statute or public policy shall be stricken, and all other portions of this Agreement that do not violate any statute or public policy shall continue in full force and effect. Further, if any one or more of the provisions contained in this Agreement is determined by a court of competent jurisdiction in any State to be excessively broad as to duration, scope, activity or subject, or is unreasonable or unenforceable under the laws of such State, such provisions will be construed by limiting, reducing, modifying or amending them so as to be enforceable to the maximum extent permitted by the law of that State. If the Agreement is held unenforceable in any jurisdiction, such holding will not impair the enforceability of the Agreement in any other jurisdiction.
Section 7.9 Section Headings, Construction. The headings of Sections in this Agreement are provided for convenience only and will not affect its construction or interpretation. All references to “Section” or “Sections” refer to the corresponding Section or Sections of this Agreement. All words used in this Agreement will be construed to be of such gender or number as the circumstances require. Unless otherwise expressly provided, the word “including” does not limit the preceding words or terms.
Section 7.10 Governing Law. This Agreement will be governed by the laws of the State of New York without regard to conflicts of laws principles.
Section 7.11 Counterparts; Format. This Agreement may be executed in one or more identical counterparts, and delivered via facsimile or e-mail (PDF format) transmission, each of which will be deemed to be an original and, which taken together, shall be deemed to constitute the Agreement.
[SIGNATURE PAGE FOLLOWS]
Annex F-7
Table of Contents
IN WITNESS WHEREOF, the Parties have executed and delivered this Agreement as of the date first written above.
| | Buyer: RAFAEL HOLDINGS, INC. |
| | | |
| | | Name: David Polinsky |
| | | Title: Chief Financial Officer |
| | | |
| | | Sellers: |
| | | |
| | | A. Joseph Stern |
| | | |
| | | Aaron Drillick |
[Signature Page to Acquisition Agreement]
Annex F-8
Table of Contents
EXHIBIT A
Membership Interest Transfer Power
Annex F-9
Table of Contents
Annex G
FORM OF
CERTIFICATE OF AMENDMENT TO
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION OF
RAFAEL PHARMACEUTICALS, INC.
Rafael Pharmaceuticals, Inc., a corporation organized and existing under the General Corporation Law of the State of Delaware (the “Corporation”), hereby certifies that:
1. This Certificate of Amendment to the Certificate of Incorporation (this “Certificate of Amendment”) as set forth below has been duly adopted in accordance with the provisions of Sections 228, 242 and 141(f) of the General Corporation Law of the State of Delaware by the stockholders and directors of the corporation.
2. Article Fourth, Section A of the Corporation’s Amended and Restated Certificate of Incorporation (as amended by all Certificates of Designations and amendments thereto filed with the Delaware Secretary of State, the “Certificate of Incorporation”) is amended and restated to read in its entirety as follows:
“Authorization of Stock. This corporation is authorized to issue two classes of stock to be designated, respectively, common stock and preferred stock. The total number of shares that this corporation is authorized to issue is _______________ (__________). The total number of shares of common stock authorized to be issued is _______________ (__________)., par value $0.001 per share (the “Common Stock”). The total number of shares of preferred stock authorized to be issued is _______________ (__________)., par value $0.001 per share (the “Preferred Stock”), of which _______________ (__________).shares are designated as “Series A Preferred Stock,”. _______________ (__________).shares are designated as “Series B-1 Preferred Stock,” _______________ (__________).shares are designated as “Series B-2 Preferred Stock,” _______________ (__________).shares are designated as “Series B-3 Preferred Stock,” _______________ (__________).shares are designated as “Series B-4 Preferred Stock,” _______________ (__________).shares are designated as “Series C Preferred Stock,” and _______________ (__________).shares are designated as “Series D Preferred Stock,”;”
3. The following paragraph shall be added as new Section at the end of Article Fourth of the Certificate of Incorporation:
“[___]. Merger Consideration In Lieu of Any Other Consideration. Notwithstanding anything to the contrary set forth in the Certificate of Incorporation (including each Certificate of Designations with respect to capital stock of the Corporation) with respect to liquidation rights of the Common Stock and each series of Preferred Stock of the Corporation (collectively, the “Waterfall Provisions”), pursuant to the terms of that certain Agreement and Plan of Merger, dated as of June 16, 2021 by and among the Rafael Holdings, Inc., a Delaware corporation (“Parent”), RH Merger I, Inc., a Delaware corporation and wholly-owned subsidiary of Parent, and RH Merger II, LLC, a Delaware limited liability company and wholly-owned subsidiary of Parent (as such agreement may be amended and/or restated from time to time, the “Merger Agreement”), the holders of shares of Common Stock and Preferred Stock shall not be entitled to receive or be required to accept any amounts or consideration on account of such shares in connection with the Waterfall Provisions, but shall only be entitled to receive or be required to take such amounts as provided pursuant to the Merger Agreement.”
4. All other provisions of the Amended and Restated Certificate of Incorporation remain in full force and effect.
Annex G-1
Table of Contents
IN WITNESS WHEREOF, the corporation has caused this Certificate to be signed by Sanjeev Luther, its Chief Executive Officer, this ___ day of _____________, 2021.
| | RAFAEL PHARMACEUTICALS, INC. |
| | | By: | | |
| | | Name: | | Sanjeev Luther |
| | | Title: | | Chief Executive Officer |
[Signature Page to Charter Amendment]
Annex G-2
Table of Contents
Annex H

June 14, 2021
The Special Committee of the Board of Directors
Rafael Holdings, Inc.
520 Broad Street
Newark, NJ 07102
Dear Special Committee:
We understand that Rafael Holdings, Inc. (the “Company”), Rafael Merger Sub I, Inc. (“Merger Sub I”), which is a wholly-owned subsidiary of the Company, Rafael Merger Sub II, LLC (“Merger Sub II”), which is also a wholly-owned subsidiary of the Company, and Rafael Pharmaceuticals, Inc. (“Rafael Pharmaceuticals”) propose to enter into the Agreement (defined below) pursuant to which, among other things, Merger Sub I will be merged with and into Rafael Pharmaceuticals (the “Merger”) and, thereafter, Rafael Pharmaceuticals will be merged with and into Merger Sub II (such subsequent merger, together with the Merger, the “Transaction”) and that, in connection with the Merger, each of the outstanding shares of the Common Stock, par value $0.001 per share, designated as voting, the Common Stock, par value $0.001 per share, designated as non-voting, and the Series A Convertible Preferred Stock, Series B-1 Convertible Preferred Stock, the Series B-2 Convertible Preferred Stock, the Series B-3 Convertible Preferred Stock, the Series B-4 Convertible Preferred Stock, the Series C Convertible Preferred Stock and the Series D Convertible Preferred Stock of Rafael Pharmaceuticals (collectively, “RP Capital Stock”) not then held by the Company, Merger Sub I, Merger Sub II or any other wholly owned subsidiary of the Company (such RP Capital Stock, collectively, the “Acquired Shares”) will be converted into the right to receive 0.12045 of a share of Class B Common Stock, par value $0.01 per share, of the Company (“Company Class B Common Stock”). The Company has advised us, and has directed us to assume, that the aggregate consideration issuable by the Company for the Acquired Shares in the Merger pursuant to the Agreement will be no more than 17,145,038 shares of Company Class B Common Stock (the “Aggregate Consideration”). The Company further has advised us, and we have assumed, without independent verification, that (a) after giving effect to certain transactions involving the capital structure of Rafael Pharmaceuticals (collectively, the “Pre-Merger Steps”) and as of immediately prior to the consummation of the Merger, the Acquired Shares will represent 49.3% of the total equity value of Rafael Pharmaceuticals (the “Acquired Shares Equity Ownership Percentage”) and (b) the Aggregate Consideration will represent 47.0% of the total equity value of the Company as of immediately after the consummation of the Transaction (taking into account certain planned equity incentive issuances (including the Mallik Issuance (defined below)) but not taking into account any capital raise or other issuances in the interim or the Financing (defined below)) (the “Pro Forma Equity Ownership Percentage”).
The Special Committee (the “Special Committee”) of the Board of Directors of the Company (the “Board”) has requested that Houlihan Lokey Capital, Inc. (“Houlihan Lokey”) provide an opinion (the “Opinion”) to the Special Committee as to whether, as of the date hereof, the Aggregate Consideration to be paid by the Company for the Acquired Shares in the Merger pursuant to the Agreement is fair to the Company from a financial point of view. For the purposes of our analyses and this Opinion, with the consent and approval of the Special Committee, we have evaluated the foregoing solely on the basis of a comparison of the Pro Forma Equity Ownership Percentage with implied reference ranges of percentages obtained by dividing (1) implied pre-Transaction valuation reference ranges for the Acquired Shares (based on the Acquired Shares Equity Ownership Percentage and implied total equity value

Annex H-1
Table of Contents
The Special Committee of the Board of Directors
Rafael Holdings, Inc.
June 14, 2021
reference ranges for Rafael Pharmaceuticals) by (2) the sum of implied pre-Transaction total equity value reference ranges for Rafael Pharmaceuticals plus certain assumed values for assets and liabilities of the Company (excluding its interests in Rafael Pharmaceuticals), in each case as we believe is indicated by our financial analyses of Rafael Pharmaceuticals. No representation is made by Houlihan Lokey herein, either directly or indirectly, as to any legal matter or as to the sufficiency of the basis set forth above for any general or particular purpose other than setting forth the scope of this Opinion.
In connection with this Opinion, we have made such reviews, analyses and inquiries as we have deemed necessary and appropriate under the circumstances. Among other things, we have:
1. reviewed the draft dated June 13, 2021 of the Agreement and Plan of Merger to be entered into by the Company, Merger Sub I, Merger Sub II and Rafael Pharmaceuticals (the “Agreement”);
2. reviewed certain publicly available business and financial information relating to the Company that we deemed to be relevant;
3. reviewed certain information relating to the historical, current and future operations, financial condition and prospects of Rafael Pharmaceuticals and certain information relating to assets and liabilities of the Company (excluding its interests in Rafael Pharmaceuticals) made available to us by the Company, including (a) probability-adjusted financial projections prepared by the management of Rafael Pharmaceuticals and discussed with the management of the Company relating to Rafael Pharmaceuticals for the years 2021 through 2030 and (b) certain assumed values for assets and liabilities of the Company (excluding its interests in Rafael Pharmaceuticals) provided by or discussed with the management of the Company;
4. reviewed certain information regarding the capitalization of Rafael Pharmaceuticals after giving effect to the Pre-Merger Steps and as of immediately prior to the consummation of the Merger and the capitalization of the Company as of immediately after the consummation of the Transaction;
5. spoken with certain members of the managements of the Company and Rafael Pharmaceuticals and certain representatives and advisors of the Company regarding the businesses, operations, financial condition and prospects of Rafael Pharmaceuticals and the Company, the Transaction and related matters;
6. compared certain financial data of Rafael Pharmaceuticals with that of public companies that we deemed to be relevant;
7. considered publicly available financial terms of certain transactions that we deemed to be relevant;
8. reviewed the current and historical market prices and trading volume for Company Class B Common Stock; and
9. conducted such other financial studies, analyses and inquiries and considered such other information and factors as we deemed appropriate.
We have relied upon and assumed, without independent verification, the accuracy and completeness of all data, material and other information furnished, or otherwise made available, to us, discussed with or reviewed by us, or publicly available, and do not assume any responsibility with respect to such data, material and other information. In addition, managements of the Company and Raphael Pharmaceuticals have advised us, and we have assumed, that the probability-adjusted financial projections and other estimates reviewed by us have been reasonably prepared in good faith on bases reflecting the best currently available estimates and judgments of the managements of the Company and Raphael Pharmaceuticals, as the case may be, as to the future financial results and condition of Raphael Pharmaceuticals and the other matters covered thereby, and we express no opinion with respect to such projections or the assumptions on which they are based. We have relied upon and assumed, without independent verification, that there has been no change in the businesses, assets, liabilities, financial condition, results of operations, cash flows or prospects of Raphael Pharmaceuticals or the Company since the respective dates of the most recent financial statements and other information, financial or otherwise, provided to us that would be material to our analyses or this Opinion, and that there is no information or any facts that would make any of the information reviewed by us incomplete or misleading.
Annex H-2
Table of Contents
The Special Committee of the Board of Directors
Rafael Holdings, Inc.
June 14, 2021
We also have relied upon, without independent verification, the assessments of the managements of the Company and Raphael Pharmaceuticals to the product candidates and intellectual property of Raphael Pharmaceuticals and the Company and the validity of, and risks associated with, such product candidates and intellectual property (including, without limitation, the validity and life of patents or other intellectual property, the timing and probability of successful testing, development and commercialization of such product candidates and related indications, approval thereof by appropriate governmental authorities and the potential impact of generic competition), and we have assumed, at the direction of the Company, that there will be no developments with respect to any such matters that would affect our analyses or this Opinion.
In reaching our conclusions hereunder, upon the advice of management of the Company, we have relied upon and assumed, without independent verification, that the information regarding the capitalizations of Rafael Pharmaceuticals and the Company referred to above reflects the material economic aspects of the capitalization of Rafael Pharmaceuticals after giving effect to the Pre-Merger Steps and as of immediately prior to the consummation of the Merger and the capitalization of the Company as of immediately after the consummation of the Transaction, that the Acquired Shares Equity Ownership Percentage reflects the correct allocation to the Acquired Shares of the total equity value of Rafael Pharmaceuticals and that the Pro Forma Equity Ownership Percentage reflects the correct allocation to the Aggregate Consideration of the total equity value of the Company. For purposes of our analyses and this Opinion, we have not applied any control premium, minority or illiquidity discounts or other premiums or discounts, or otherwise given effect to any rights, restrictions or limitations, that may be attributable to any security (including, without limitation, any RP Capital Stock) of Rafael Pharmaceuticals, the Company or any other party or blocks of such securities. We have not performed or relied upon separate financial analyses of the various classes of RP Capital Stock or any other securities of Rafael Pharmaceuticals or the Company, including, without limitation, any separate financial analyses of preferred stock based on a comparison of the terms thereof with those of debt or preferred equity securities of other companies or a separate discounted cash flow analysis based on potential future preferred dividends or otherwise. In addition, we have not considered any differences in the classes of the common stock of the Company and, with the consent of the Special Committee, we have relied upon and assumed, without independent verification, that shares of Company Class B Common Stock are economically equivalent to shares of Class A Common Stock, par value $0.01 per share, of the Company. With respect to assets and liabilities of the Company (excluding its interests in Rafael Pharmaceuticals), we have relied, with the consent and approval of the Special Committee and without independent verification, upon certain assumed values for such assets and liabilities, and, except with respect to the royalty payments from Rafael Pharmaceuticals to Altira Capital & Consulting, LLC (“Altira”), which is majority-owned by the Company, we have not been provided with financial projections relating to such assets and liabilities and, accordingly, have not performed or relied upon separate financial analyses of such assets and liabilities.
We have relied upon and assumed, without independent verification, that (a) the representations and warranties of all parties to the Agreement and all other related documents and instruments that are referred to therein are true and correct, (b) each party to the Agreement and such other related documents and instruments will fully and timely perform all of the covenants and agreements required to be performed by such party, (c) all conditions to the consummation of the Transaction and related transactions will be satisfied without waiver thereof, and (d) the Transaction and related transactions (including, among other things, the Pre-Merger Steps and the issuance to Ameet Mallik of restricted shares of Company Class B Common Stock upon consummation of the Transaction (the “Mallik Issuance”)) will be consummated in a timely manner in accordance with the terms described in the Agreement and such other related documents and instruments and as otherwise described to us by representatives of the Company, without any amendments or modifications thereto. We have also assumed, with the consent of the Special Committee, that the Transaction will qualify for the intended tax treatment described in the Agreement for U.S. federal income tax purposes. We have relied upon and assumed, without independent verification, that (i) the Transaction and related transactions will be consummated in a manner that complies in all respects with all applicable federal, state and foreign statutes, rules and regulations, and (ii) all governmental, regulatory, and other consents and approvals necessary for the consummation of the Transaction and related transactions will be obtained and that no delay, limitations, restrictions or conditions will be imposed or amendments, modifications or waivers made that would result in the disposition of any assets of the Company or Rafael Pharmaceuticals, or otherwise have an effect on the Transaction, the Company or Rafael Pharmaceuticals, that would be material to our analyses or this Opinion. We have also relied upon and assumed,
Annex H-3
Table of Contents
The Special Committee of the Board of Directors
Rafael Holdings, Inc.
June 14, 2021
without independent verification, at the direction of the Company, that the actual number of shares of Company Class B Common Stock issued as the aggregate consideration to be paid by the Company for the Acquired Shares in the Merger pursuant to the Agreement will not differ from the Aggregate Consideration that we have been directed to assume in any respect that would be material to our analyses or this Opinion. In addition, we have relied upon and assumed, without independent verification, that the final form of the Agreement will not differ in any respect from the draft of the Agreement identified above. As you are aware, the Agreement provides that the closing of the Merger is conditioned upon financing activities by the Company by way of a private placement and/or an underwritten public offering of capital stock of the Company and/or the sale of real property owned by the Company (the “Financing”) and the Company’s acquisition of the minority interest in Altira not owned by the Company (the “Altira Buyout”). For purposes of our analyses and this Opinion, with the consent and approval of the Special Committee, no effect has been given to such transactions or any aspect, implication or effect thereof on Rafael Pharmaceuticals, the Company, the Pro Forma Equity Ownership Percentage or otherwise.
Furthermore, in connection with this Opinion, we have not been requested to make, and have not made, any physical inspection or independent appraisal or evaluation of any of the assets, properties or liabilities (fixed, contingent, derivative, off-balance-sheet or otherwise) of the Company, Rafael Pharmaceuticals or any other party, nor were we provided with any such appraisal or evaluation. With respect to the real estate properties of the Company or Rafael Pharmaceuticals, we express no opinion as to the value of any such real estate property, or the price at which any such real estate property may be transferable, at any time. We did not estimate, and express no opinion regarding, the liquidation value of any entity or business. We have undertaken no independent analysis of any potential or actual litigation, regulatory action, possible unasserted claims or other contingent liabilities, to which the Company or Rafael Pharmaceuticals is or may be a party or is or may be subject, or of any governmental investigation of any possible unasserted claims or other contingent liabilities to which the Company or Rafael Pharmaceuticals is or may be a party or is or may be subject.
We have not been requested to, and did not, (a) initiate or participate in any discussions or negotiations with, or solicit any indications of interest from, third parties with respect to the Transaction or any related transaction, the securities, assets, businesses or operations of the Company, Rafael Pharmaceuticals or any other party, or any alternatives to the Transaction or any related transaction, (b) negotiate the terms of the Transaction or any related transaction, or (c) advise the Special Committee, the Board, the Company or any other party with respect to alternatives to the Transaction or any related transaction. We express no view or opinion as to any such matters, including the terms that could have been obtained if any of the foregoing had been undertaken. Representatives of the Special Committee and the Company have advised us, and we have relied upon and assumed, without independent verification, that the terms of the Transaction and related transactions have been negotiated on an arms-length basis. This Opinion is necessarily based on financial, economic, market and other conditions as in effect on, and the information made available to us as of, the date hereof. We have not undertaken, and are under no obligation, to update, revise, reaffirm or withdraw this Opinion, or otherwise comment on or consider events occurring or coming to our attention after the date hereof. As you are aware, the credit, financial and stock markets have been experiencing unusual volatility and we express no opinion or view as to any potential effects of such volatility on the Transaction, the Company or Rafael Pharmaceuticals, and this Opinion does not purport to address potential developments in any such markets. We are not expressing any opinion as to what the value of Company Class B Common Stock actually will be when issued pursuant to the Transaction or any related transaction or the price or range of prices at which Company Class B Common Stock, any RP Capital Stock or any other securities of the Company or Rafael Pharmaceuticals may be purchased or sold, or otherwise be transferable, at any time.
This Opinion is furnished for the use of the Special Committee (in its capacity as such) in connection with its evaluation of the Transaction and may not be used for any other purpose without our prior written consent. This Opinion should not be construed as creating any fiduciary duty on Houlihan Lokey’s part to any party. This Opinion is not intended to be, and does not constitute, a recommendation to the Special Committee, the Board, the Company, any security holder or any other party as to how to act or vote with respect to any matter relating to the Transaction or any related transaction or otherwise. Except as set forth in our engagement letter, this Opinion may not be disclosed, reproduced, disseminated, quoted, summarized or referred to at any time, in any manner or for any purpose, nor shall any references to Houlihan Lokey or any of its affiliates be made, without the prior written consent of Houlihan Lokey.
Annex H-4
Table of Contents
The Special Committee of the Board of Directors
Rafael Holdings, Inc.
June 14, 2021
In the ordinary course of business, certain of our employees and affiliates, as well as investment funds in which they may have financial interests or with which they may co-invest, may acquire, hold or sell, long or short positions, or trade, in debt, equity, and other securities and financial instruments (including loans and other obligations) of, or investments in, the Company, Rafael Pharmaceuticals or any other party that may be involved in the Transaction or any related transaction and their respective affiliates or security holders or any currency or commodity that may be involved in the Transaction or any related transaction.
Houlihan Lokey and certain of its affiliates may provide investment banking, financial advisory and/or other financial or consulting services to the Company, Rafael Pharmaceuticals, other participants in the Transaction or any related transaction or certain of their respective affiliates or security holders in the future, for which Houlihan Lokey and its affiliates may receive compensation. Furthermore, in connection with bankruptcies, restructurings, distressed situations and similar matters, Houlihan Lokey and certain of its affiliates may have in the past acted, may currently be acting and may in the future act as financial advisor to debtors, creditors, equity holders, trustees, agents and other interested parties (including, without limitation, formal and informal committees or groups of creditors) that may have included or represented and may include or represent, directly or indirectly, or may be or have been adverse to, the Company, Rafael Pharmaceuticals, other participants in the Transaction or any related transaction or certain of their respective affiliates or security holders, for which advice and services Houlihan Lokey and its affiliates have received and may receive compensation.
We will receive a fee for rendering this Opinion, which is not contingent upon the successful completion of the Transaction or the conclusion contained in this Opinion. The Company has agreed to reimburse certain of our expenses and to indemnify us and certain related parties for certain potential liabilities arising out of our engagement.
We have not been requested to opine as to, and this Opinion does not express an opinion as to or otherwise address, among other things: (i) the underlying business decision of the Special Committee, the Board, the Company, its security holders or any other party to proceed with or effect the Transaction or any related transaction, (ii) the terms of any arrangements, understandings, agreements or documents related to, or the form, structure or any other portion or aspect of, the Transaction (other than the Aggregate Consideration to the extent expressly specified herein) or any related transaction or otherwise, including, without limitation, the form or structure of the Aggregate Consideration, the allocation thereof among the various classes of the Acquired Shares or any terms, aspects or implications of the Pre-Merger Steps, the Mallik Issuance, the Financing, the Altira Buyout or any agreements by the Company with respect to its shares of the capital stock of Rafael Pharmaceuticals in the event that the Transaction is not consummated, (iii) the fairness of any portion or aspect of the Transaction or any related transaction to the holders of any class of securities, creditors or other constituencies of the Company, or to any other party, except to the Company if and only to the extent expressly set forth in the last sentence of this Opinion, (iv) the relative merits of the Transaction or any related transaction as compared to any alternative business strategies or transactions that might be available for the Company or any other party, (v) the fairness of any portion or aspect of the Transaction or any related transaction to any one class or group of the Company’s, Target’s or any other party’s security holders or other constituents vis-à-vis any other class or group of the Company’s, Target’s or such other party’s security holders or other constituents (including, without limitation, the allocation of any consideration amongst or within such classes or groups of security holders or other constituents), (vi) whether or not the Company, Rafael Pharmaceuticals, their respective security holders or any other party is receiving or paying reasonably equivalent value in the Transaction or any related transaction, (vii) the solvency, creditworthiness or fair value of Rafael Pharmaceuticals, the Company or any other participant in the Transaction or any related transaction, or any of their respective assets, under any applicable laws relating to bankruptcy, insolvency, fraudulent conveyance or similar matters, (viii) the fairness, financial or otherwise, of the amount, nature or any other aspect of any compensation to or consideration payable to or received by any officers, directors or employees of any party to the Transaction or any related transaction, any class of such persons or any other party, relative to the Aggregate Consideration or otherwise, or (ix) any dilutive or other effects of any portion or aspect of the Transaction or any related transaction (including, without limitation, the Mallik Issuance and the Financing) on the existing stockholders of the Company or the financial or other implications and effects of the Transaction or any related transaction on the Company or any other party (including, without limitation, any aspects relating to ongoing operations of the Company or Rafael Pharmaceuticals following consummation of the Transaction and related transactions). Furthermore, no opinion, counsel or interpretation is intended in matters that require legal,
Annex H-5
Table of Contents
The Special Committee of the Board of Directors
Rafael Holdings, Inc.
June 14, 2021
regulatory, accounting, insurance, tax or other similar professional advice. It is assumed that such opinions, counsel or interpretations have been or will be obtained from the appropriate professional sources. Furthermore, we have relied, with the consent of the Special Committee, on the assessments by the Special Committee, the Company and their respective advisors, as to all legal, regulatory, accounting, insurance, tax and other similar matters with respect to Rafael Pharmaceuticals, the Company and the Transaction or otherwise. The issuance of this Opinion was approved by a committee authorized to approve opinions of this nature.
Based upon and subject to the foregoing, and in reliance thereon, it is our opinion that, as of the date hereof, the Aggregate Consideration to be paid by the Company for the Acquired Shares in the Merger pursuant to the Agreement is fair to the Company from a financial point of view.
Very truly yours,
HOULIHAN LOKEY CAPITAL, INC.
Annex H-6
Table of Contents
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 20. Indemnification of Directors and Officers
Delaware law authorizes corporations to eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breach or alleged breach of the directors’ “duty of care”. While the relevant statute does not change directors’ duty of care, it enables corporations to limit available relief to equitable remedies such as injunction or rescission. The statute has no effect on directors’ duty of loyalty, acts or omissions not in good faith or involving intentional misconduct or knowing violations of law, illegal payment of dividends and approval of any transaction from which a director derives an improper personal benefit.
Holdings has adopted provisions in its Amended and Restated Certificate of Incorporation, as amended, which eliminate the personal liability of its directors to Holdings and its stockholders for monetary damages for breach or alleged breach of their duty of care. The bylaws of Holdings provide for indemnification of its directors, officers, employees and agents to the fullest extent permitted by the General Corporation Law of the State of Delaware, Holdings’ state of incorporation, including those circumstances in which indemnification would otherwise be discretionary under Delaware Law. Section 145 of the General Corporation Law of the State of Delaware permits a corporation to indemnify any director or officer of the corporation against expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred in connection with any action, suit, or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reason to believe his or her conduct was unlawful. In a derivative action, or an action brought by or on behalf of the corporation, indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought shall determine that the defendant is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
Holdings may enter into indemnification agreements with certain of its executive officers and directors, indemnifying them against certain potential liabilities that may arise as a result of their service to Holdings, and providing certain other protections. Pharma also maintains insurance policies which insure the officers and directors against certain liabilities.
II-1
Table of Contents
Item 21. Exhibits and Financial Statement Schedules
(a) The exhibits listed below in the “Exhibit Index” are filed as part of, or are incorporated by reference in, this proxy/registration statement.
| | | | Incorporated Herein By Reference To |
Exhibit
Number | | Exhibit Description | | Document | | Filed On | | Exhibit Number |
1.1* | | Form of Underwriting Agreement | | | | | | |
2.1 | | Agreement and Plan of Merger, dated as of June 17, 2021, by and among Holdings, Merger Sub I, Merger Sub II and Pharma. | | 8-K | | 06/21/2021 | | 2.01 |
3.1 | | Amended and Restated Certificate of Incorporation of Rafael Holdings, Inc. | | 10-12G/A | | 03/26/2018 | | 3.1 |
3.2 | | Amended and Restated By-Laws of Rafael Holdings, Inc. | | 8-K | | 09/26/2019 | | 3.1 |
4.1* | | Specimen Parent Class A Common Stock Certificate.* | | | | | | |
4.2* | | Specimen Parent Class B Common Stock Certificate.* | | | | | | |
5.1* | | Opinion of Schwell Wimpfheimer & Associates LLP regarding the legality of the securities being issued. | | | | | | |
10.1 | | Form of Lock-Up Agreement | | 8-K | | 06/21/2021 | | 10.01 |
10.2 | | Form of Voting Agreement | | 8-K | | 06/21/2021 | | 10.02 |
10.3 | | Form of Support Agreement | | 8-K | | 06/21/2021 | | 10.03 |
10.4 | | Form of Convertible Note Cancellation Agreement | | 8-K | | 06/21/2021 | | 10.04 |
10.5 | | Form of Altira Acquisition Agreement | | 8-K | | 06/21/2021 | | 10.05 |
10.6 | | Form of Letter of Transmittal | | 8-K | | 06/21/2021 | | 10.06 |
21.1* | | Subsidiaries of the Registrant | | | | | | |
23.1* | | Consent of Schwell Wimpfheimer & Associates LLP (included as part of its opinion filed as Exhibit 5.1). | | | | | | |
23.2 | | Consent of CohnReznick LLP, Independent Registered Public Accounting Firm of Rafael Holdings, Inc. | | | | | | |
23.3 | | Consent of CohnReznick LLP, Independent Registered Public Accounting Firm of Rafael Pharmaceuticals, Inc. | | | | | | |
25.1* | | Form T-1 Statement of Eligibility of Trustee for Indenture under the Trust Indenture Act of 1939 | | | | | | |
99.1* | | Form of Proxy Card of Rafael Pharmaceuticals, Inc. | | | | | | |
99.2* | | Consent of Richard Axel to be named as a director of Parent. | | | | | | |
99.3* | | Consent of Chi Van Dang to be named as a director of Parent. | | | | | | |
99.4* | | Consent of Sanjeev Luther to be named as a director of Parent. | | | | | | |
Item 22. Undertakings.
(a) The undersigned registrant hereby undertakes:
1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
• To include any prospectus required by section 10(a)(3) of the Securities Act of 1933.
• To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be
II-2
Table of Contents
reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
• To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
• Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
• Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
• The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
• Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) The undersigned registrant hereby undertakes as follows: that prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this registration statement,
II-3
Table of Contents
by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), the issuer undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form.
(d) The registrant undertakes that every prospectus (i) that is filed pursuant to the paragraph immediately preceding, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act of 1933 and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(e) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
(f) The undersigned registrant hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Item 4, 10(b), 11, or 13 of this form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(g) The undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in City of Newark, State of New Jersey, on September 14, 2021.
| | RAFAEL HOLDINGS, INC. |
| | | By: | | /s/ Ameet Mallik |
| | | Name: | | Ameet Mallik |
| | | Title: | | Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities indicated below on September 14, 2021.
Signature | | Title |
| | | |
/s/ Ameet Mallik | | Chief Executive Officer |
Ameet Mallik | | (Principal Executive Officer) |
/s/ Patrick Fabbio | | Chief Financial Officer |
Patrick Fabbio | | (Principal Financial Officer and Principal Accounting Officer) |
/s/ Howard S. Jonas | | Director and Chairman of the Board |
Howard S. Jonas | | |
/s/ Stephen Greenberg | | Director |
Stephen Greenberg | | |
/s/ Rachel Jonas | | Director |
Rachel Jonas | | |
/s/ Shannon Thyme Klinger | | Director |
Shannon Thyme Klinger | | |
/s/ Mark McCamish | | Director |
Mark McCamish | | |
/s/ Boris C. Pasche | | Director |
Boris C. Pasche | | |
/s/ Michael J. Weiss | | Director |
Michael J. Weiss | | |
II-5