BXCL701 Lifecycle Management Considerations
We envision employing computational and medicinal chemistry approaches to advance development of a next-generation BXCL701 molecule to introduce enhanced life cycle management capabilities. We anticipate that a next-generation molecule may embrace characteristics such as the inhibition of DPP8/9, while providing an improved orally bioavailable pharmacokinetic profile, including a linear pharmacokinetic, a half-life of between 12 and 48 hours, with multiple mechanisms of elimination that are clearly understood and are not burdened with potential drug-drug interaction liabilities. This next generation molecule would be intended to demonstrate anti-cancer activity as either a monotherapy or in combination with an approved CPI.
Intellectual Property
Our policy is to protect and enhance the proprietary technologies, inventions, and improvements that are commercially important to our business by filing patent applications in the U.S. and other jurisdictions related to our proprietary technology, inventions, improvements, and product candidates. We also rely on trademarks, trade secrets, and know-how relating to our proprietary technologies and product candidates, continuing innovation, and in-licensing technology and products. This reliance is expected to develop, maintain, and strengthen our proprietary position for novel therapeutics and novel formulations of existing therapeutics across multiple therapeutic areas. We also plan to rely on data exclusivity, market exclusivity, and patent term extensions when available.
Patent Portfolio
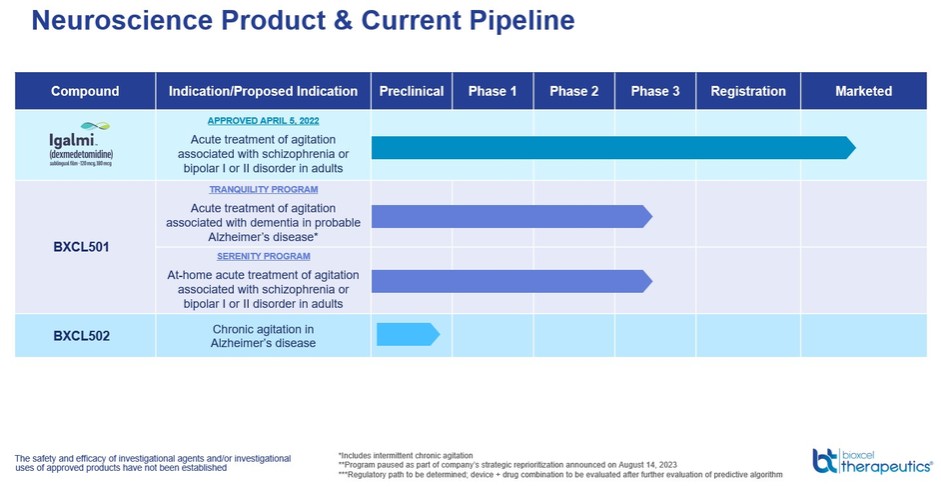
We have multiple patent families filed to protect our Neuroscience program, including BXCL501. On October 4, 2023, the Company announced it had received two Notices of Allowance (“NOAs”) from the U.S. Patent and Trademark Office (“USPTO”) for patent applications related to the method of use of sublingual dexmedetomidine for the treatment of agitation associated with bipolar disorders and schizophrenia. US Patent No. 11,786,508 issued from one of those NOAs on October 17, 2023, and has been listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the “Orange Book”) for IGALMI™ with an expiration date of December 29, 2037. US Patent No. 11,806,334 issued on November 7, 2023, from another NOA and has been listed in the Orange Book with an expiration date of January 12, 2043. As of November 7, 2023, our neuroscience patent portfolio included four Patent Cooperation Treaty (“PCT”) applications, 18 U.S. utility applications, six U.S. provisional applications, one allowed US application, eight issued U.S. utility patents, 112 pending non-U.S. utility applications, 15 allowed or granted non-U.S. patents (including three in Japan), one pending U.S. design patent application, and 34 allowed or registered design patents (including two in Japan). Four U.S. utility patents, directed to our proprietary sublingual film formulation of Dex and set to expire no earlier than 2039, are now listed in the “Orange Book”. In the same family, we also have a granted patent in China, two granted patents in Eurasia, and pending applications in the U.S., China, and other major markets. We expect that patents issuing in this family will expire no earlier than 2039. We have also filed applications in additional patent families that are relevant to BXCL501. We have applications pending in the U.S., Europe and Japan directed to methods of treating insomnia using sublingual Dex. We expect that patents issued from these applications, if any, will expire no earlier than 2035. We also have applications filed in 16 regions/countries, including the U.S., Europe, Japan, and China, directed to methods of treating agitation. We expect that patents issued from these applications, if any, will expire no earlier than 2042. We also have one PCT application directed to treating mania and another to treating depression. If patents issue from those cases, we expect them to expire no earlier than 2041 and 2042, respectively.
We have multiple patent families filed to protect our immuno-oncology program, including our core patent family directed to methods of using BXCL701 with immune checkpoint inhibitors, which is granted in the U.S., Japan, Australia, Canada, Russia, China, South Africa, Mexico, New Zealand, and United Arab Emirates, and with at least one pending application in the U.S., China, Mexico, the Republic of Korea, New Zealand, Russia, Australia, Brazil, Hong Kong, and Europe. Patents issued from this family, if any, are expected to expire no earlier than 2036. We have one additional patent issued in the U.S. directed to a method of selecting patients based on a biomarker, with an expected expiration date no earlier than 2039.
Additional applications are directed to administering BXCL701 in combinations with various other molecules, biomarkers, and dosing regimens. We also have four provisional applications directed to novel formulations of