Filed Pursuant to Rule 433 Issuer Free Writing Prospectus
dated February 3rd, 2021
File No. 333-252234

www.longeveron.com Filed Pursuant to Rule 433 Issuer Free Writing Prospectus dated February 3 rd , 2021 File No. 333 - 252234

Forward Looking Statements 2 This presentation contains forward - looking statements . All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, prospective products and product candidates and their development, the beneficial characteristics, efficacy, and therapeutic effects of our product candidates, the ability of our clinical trials to demonstrate the safety and efficacy of our product candidates and other positive results, regulatory approvals, our ability to commercialize our products and product candidates and attract collaborators, our ability to increase our per donor yield, the size of the market opportunity for our product candidates, including our estimates of the number of patients who suffer from the disease we are targeting, reimbursement for our product candidates, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, our ability to obtain and maintain intellectual property protection for our product candidates and their development and to avoid infringing the intellectual property rights of third parties, competing therapies, and future results of current and anticipated products and product candidates, are forward - looking statements . These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . Because forward - looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward - looking statements as predictions of future events . The events and circumstances reflected in our forward - looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward - looking statements . Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances, or otherwise . Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third - party sources . In addition, no independent source has evaluated the reasonableness or accuracy of Longeveron, LLC internal estimates and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates . We have filed a registration statement (including a preliminary prospectus) with the SEC for the offering to which this communications relates . The registration statement has not yet become effective . Before you invest, you should read the preliminary prospectus in that registration statement (including the risk factors described therein) and other documents that we have filed with the SEC for more complete information about us and this offering . We encourage you to read the registration statement and the prospectus in full for more detailed information on the statistics, reports and clinical trials referenced in this presentation . You may access these documents for free by visiting EDGAR on the SEC Website at http://www.sec.gov . Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectus if you contact James Clavijo, CFO, Longeveron at ( 305 ) 304 - 7700 or jclavijo@longeveron . com .

3 Specialized cells within our body possess unique regenerative qualities to repair and restore damaged tissues and organs . Longeveron is harnessing these special properties to develop safe and effective “off - the - shelf” cellular therapy to treat chronic aging - related disease, improve healthspan and extend longevity.

4 Don Soffer Real Estate entrepreneur and businessman. Joshua Hare, MD Cardiologist, University of Miami Health System Founding Director, University of Miami’s Interdisciplinary Stem Cell Institute $25M investment Manufacturing platform; process; technology; research strategy This was the vision of a wealthy philanthropist and a pioneering research scientist and clinician who formed Longeveron in 2014.

Longeveron At - a - Glance □ Developing safe and effective cellular therapies for chronic aging - related diseases □ Lead product, Lomecel - B, made from bone marrow - derived (allogeneic) Medicinal Signaling Cells □ In - house manufacturing for scalable, “off - the - shelf” cell therapy that can be delivered on - demand in an outpatient setting. □ Well - tolerated in clinical trials to date (> 260 subjects treated) □ Advanced clinical pipeline targeting diverse unmet medical needs and life - threatening conditions □ Multiple near - term value - driving milestones: ▪ Currently 4 ongoing Phase 1 or 2 clinical studies in the US with data in expected in 2021 ▪ Transitioning to Phase 2 Alzheimer’s Disease efficacy trial ▪ 2 research programs with direct relevance to COVID pandemic: ARDS & Vaccine Adjuvant ▪ Research programs in Japan, US and Bahamas □ Encouraging Phase 1 & 2 safety and efficacy data in Alzheimer’s Disease & Aging Frailty subjects 5
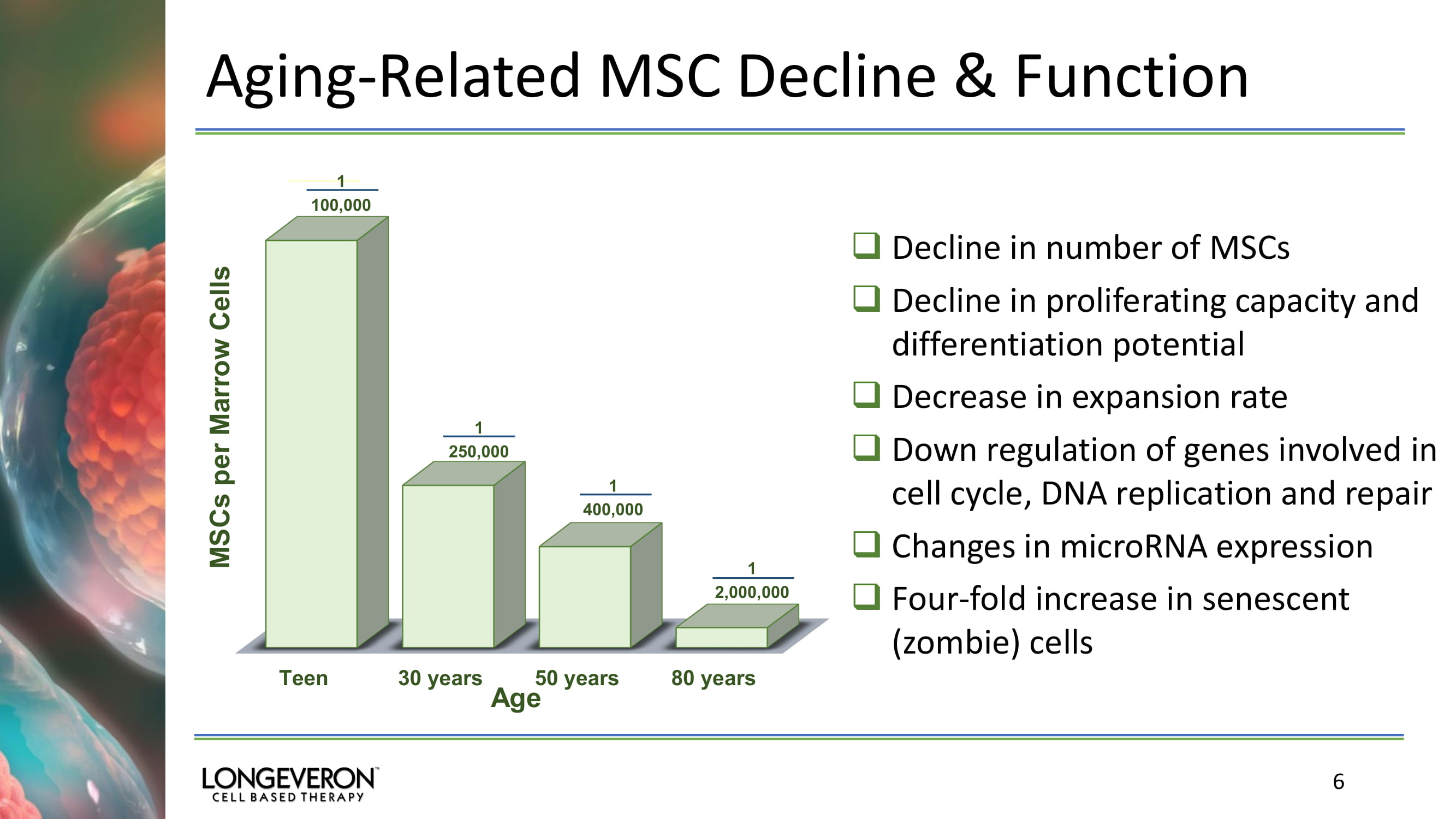
Aging - Related MSC Decline & Function 6 □ Decline in number of MSCs □ Decline in proliferating capacity and differentiation potential □ Decrease in expansion rate □ Down regulation of genes involved in cell cycle, DNA replication and repair □ Changes in microRNA expression □ Four - fold increase in senescent (zombie) cells Teen 30 years 50 years 80 years MSCs per Marrow Cells Age 1 100,000 1 250,000 1 400,000 1 2,000,000
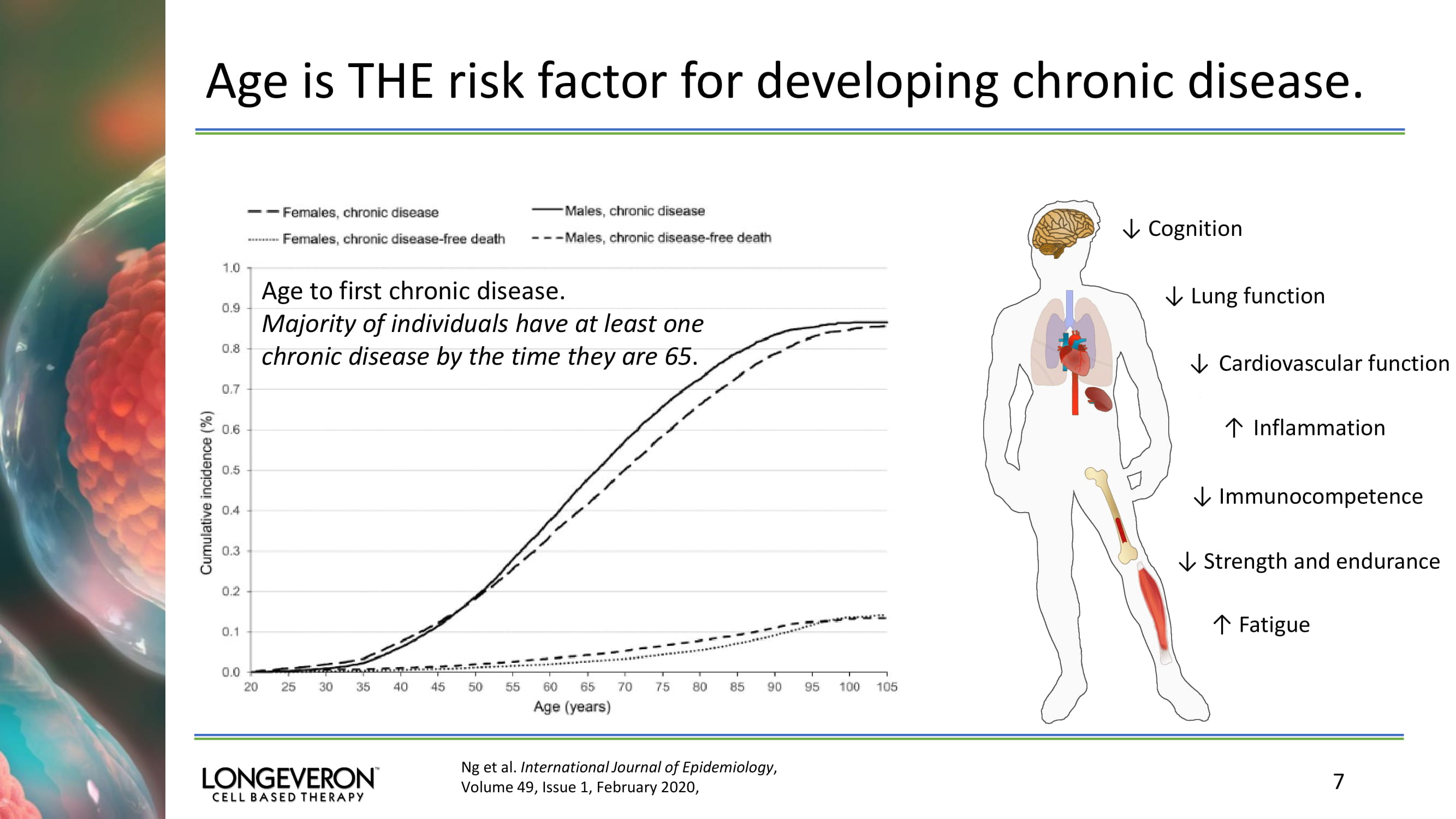
Age is THE risk factor for developing chronic disease. 7 Ng et al. International Journal of Epidemiology , Volume 49, Issue 1, February 2020, Age to first chronic disease. Majority of individuals have at least one chronic disease by the time they are 65 . ↓ Cognition ↓ Lung function ↓ Cardiovascular function ↓ Immunocompetence ↓ Strength and endurance ↑ Fatigue ↑ Inflammation
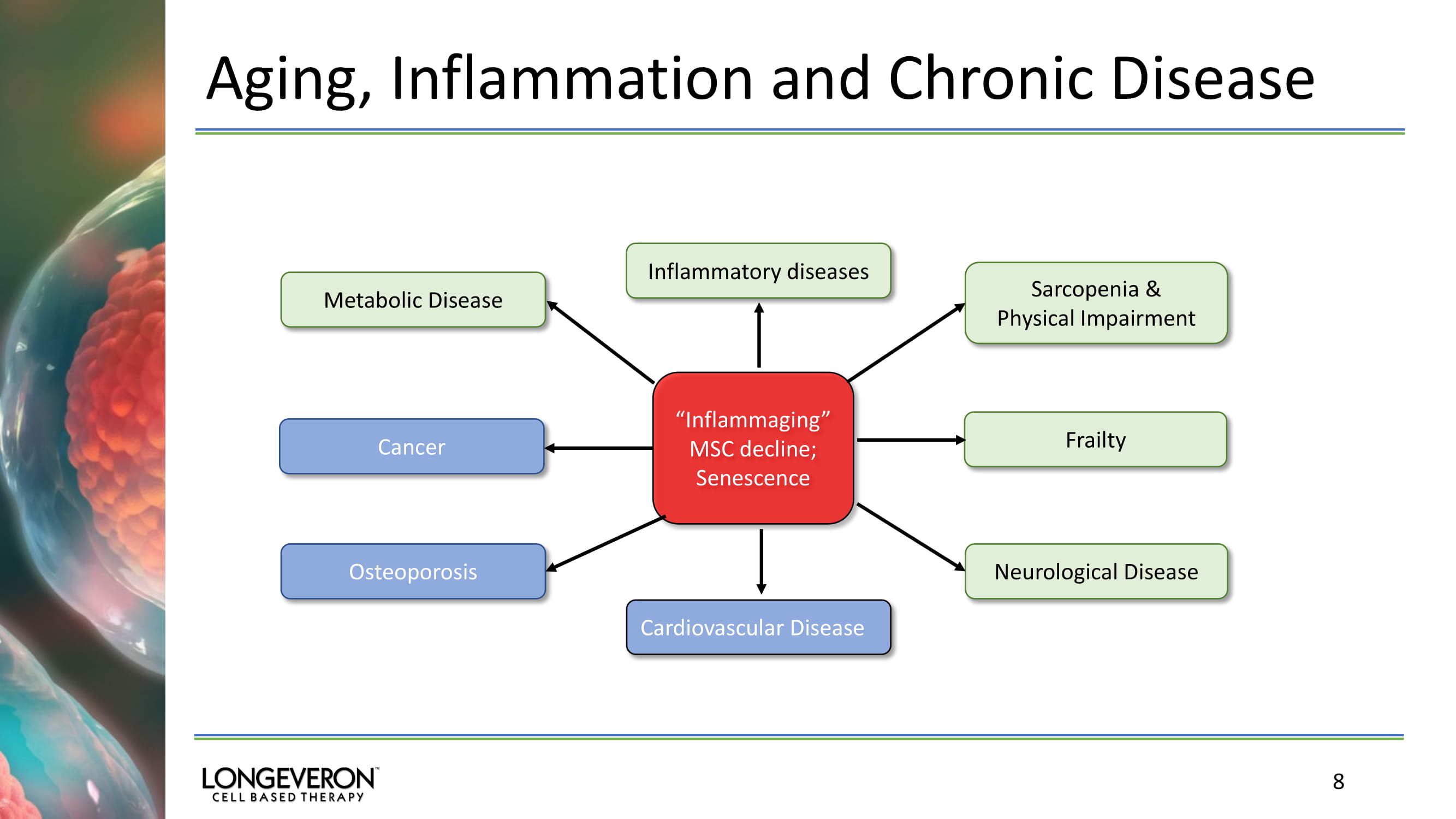
Aging, Inflammation and Chronic Disease 8 “ Inflammaging ” MSC decline; Senescence Sarcopenia & Physical Impairment Frailty Neurological Disease Inflammatory diseases Osteoporosis Cancer Metabolic Disease Cardiovascular Disease
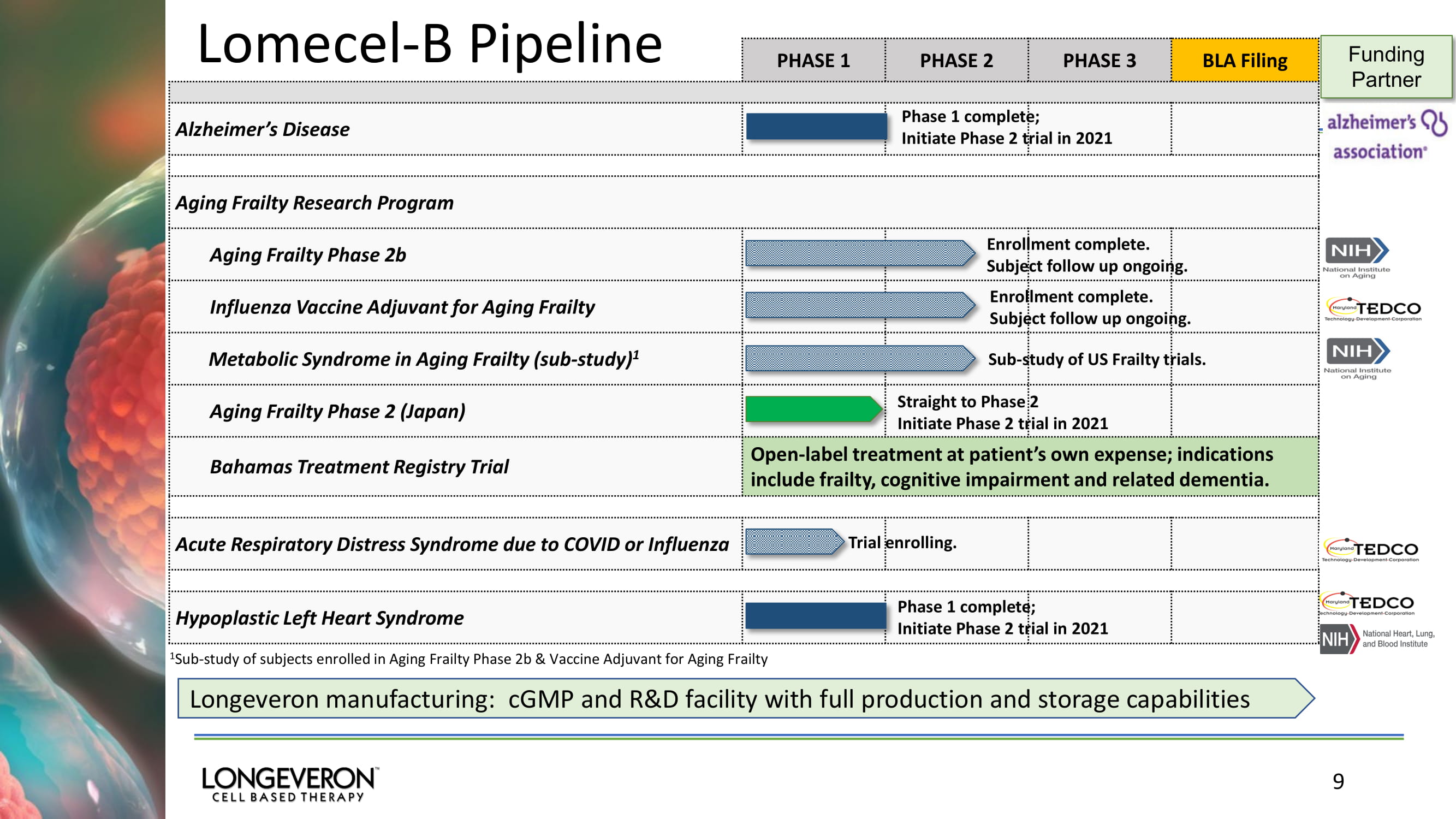
9 PHASE 1 PHASE 2 PHASE 3 BLA Filing Alzheimer’s Disease Aging Frailty Research Program Aging Frailty Phase 2b Influenza Vaccine Adjuvant for Aging Frailty Metabolic Syndrome in Aging Frailty (sub - study) 1 Aging Frailty Phase 2 (Japan) Bahamas Treatment Registry Trial Open - label treatment at patient’s own expense; indications include frailty, cognitive impairment and related dementia. Acute Respiratory Distres s Syndrome due to COVID or Influenza Hypoplastic Left Heart Syndrome Funding Partner 1 Sub - study of subjects enrolled in Aging Frailty Phase 2b & Vaccine Adjuvant for Aging Frailty Phase 1 complete; Initiate Phase 2 trial in 2021 Enrollment complete. Subject follow up ongoing. Straight to Phase 2 Initiate Phase 2 trial in 2021 Phase 1 complete; Initiate Phase 2 trial in 2021 Trial enrolling. Enrollment complete. Subject follow up ongoing. Sub - study of US Frailty trials. Longeveron manufacturing: cGMP and R&D facility with full production and storage capabilities Lomecel - B Pipeline

Lomecel - B: “Off - the - Shelf” Therapy 10 Healthy adult donors 18 – 45 years old; Rigorously screened. MSCs isolated from bone marrow aspirate MSCs culture - expanded into the billions of cells 40 minute IV infusion. Key Advantages of Lomecel - B : • Easy to administer, store and freeze (“Off - the - shelf” therapy) • Can be delivered systemically or locally • Can home to sites of tissue damage and inflammation • Not perceived by the host as foreign (Immuno - evasive/Immune - privileged) Longeveron Headquarters Converge Miami 1951 NW 7 th Ave Miami, FL
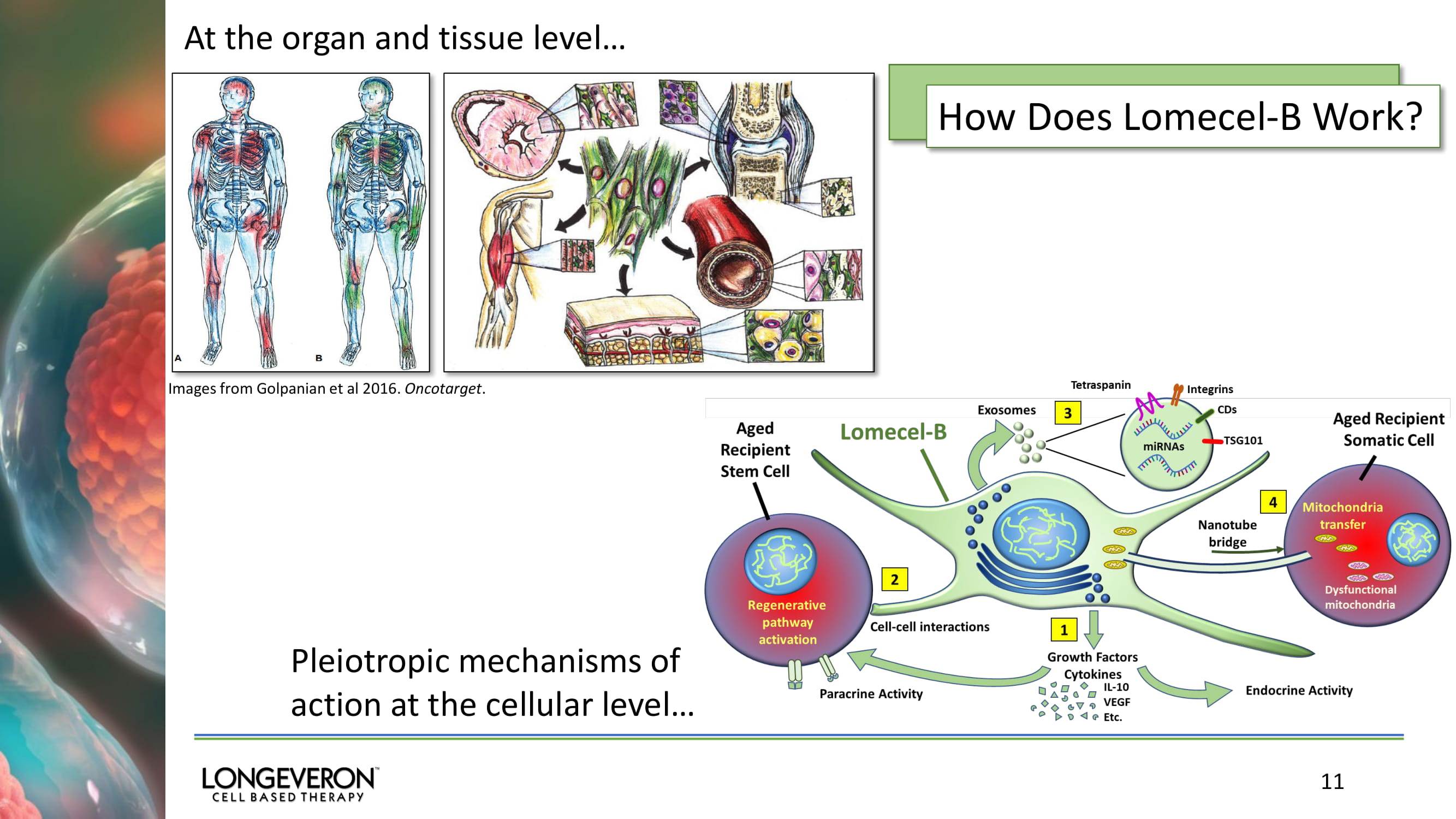
11 Images from Golpanian et al 2016. Oncotarget . At the organ and tissue level… Pleiotropic mechanisms of action at the cellular level… How Does Lomecel - B Work?

12 Alzheimer’s Disease Research Program □ Completed Phase 1 safety & tolerability study: ▪ Mild Alzheimer’s disease patients (n=33) ▪ Single infusion (Lomecel - B 20 million cells, Lomecel - B 100 million cells or Placebo) ▪ Randomized, placebo - controlled, double - blind ▪ 52 week follow - up ▪ Efficacy endpoints: MRIs, cognitive function, QOL assessments, biomarkers □ Summary Results: ▪ Achieved primary endpoint: well - tolerated; no treatment - related SAEs or brain edema ▪ Several exploratory efficacy endpoints showed encouraging results for Lomecel - B compared to placebo □ Supports immediate transition to efficacy - powered multi - dose Phase 2 trial
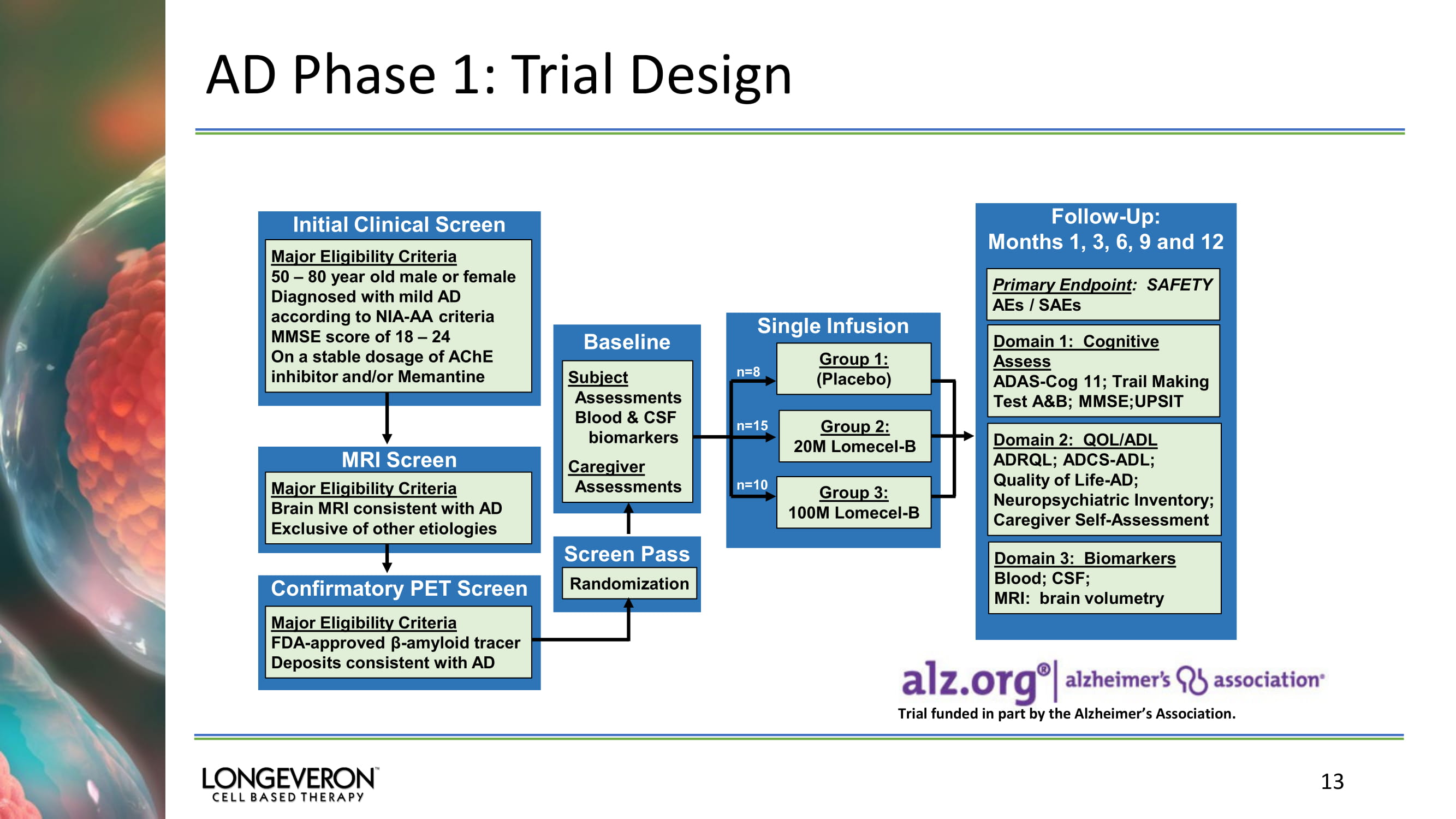
AD Phase 1: Trial Design 13 Initial Clinical Screen MRI Screen Confirmatory PET Screen Major Eligibility Criteria 50 – 80 year old male or female Diagnosed with mild AD according to NIA - AA criteria MMSE score of 18 – 24 On a stable dosage of AChE inhibitor and/or Memantine Major Eligibility Criteria Brain MRI consistent with AD Exclusive of other etiologies Major Eligibility Criteria FDA - approved β - amyloid tracer Deposits consistent with AD Single Infusion n=15 n=10 Group 3: 100M Lomecel - B n=8 Group 1: ( Placebo) Follow - Up: Months 1, 3, 6, 9 and 12 Domain 1: Cognitive Assess ADAS - Cog 11; Trail Making Test A&B; MMSE;UPSIT Domain 3: Biomarkers Blood; CSF; MRI: brain volumetry Primary Endpoint : SAFETY AEs / SAEs Domain 2: QOL/ADL ADRQL; ADCS - ADL; Quality of Life - AD; Neuropsychiatric Inventory; Caregiver Self - Assessment Baseline Screen Pass Randomization Group 2: 20M Lomecel - B ClinicalTrials.gov: NCT02600130 Subject Assessments Blood & CSF biomarkers Caregiver Assessments Trial funded in part by the Alzheimer’s Association.
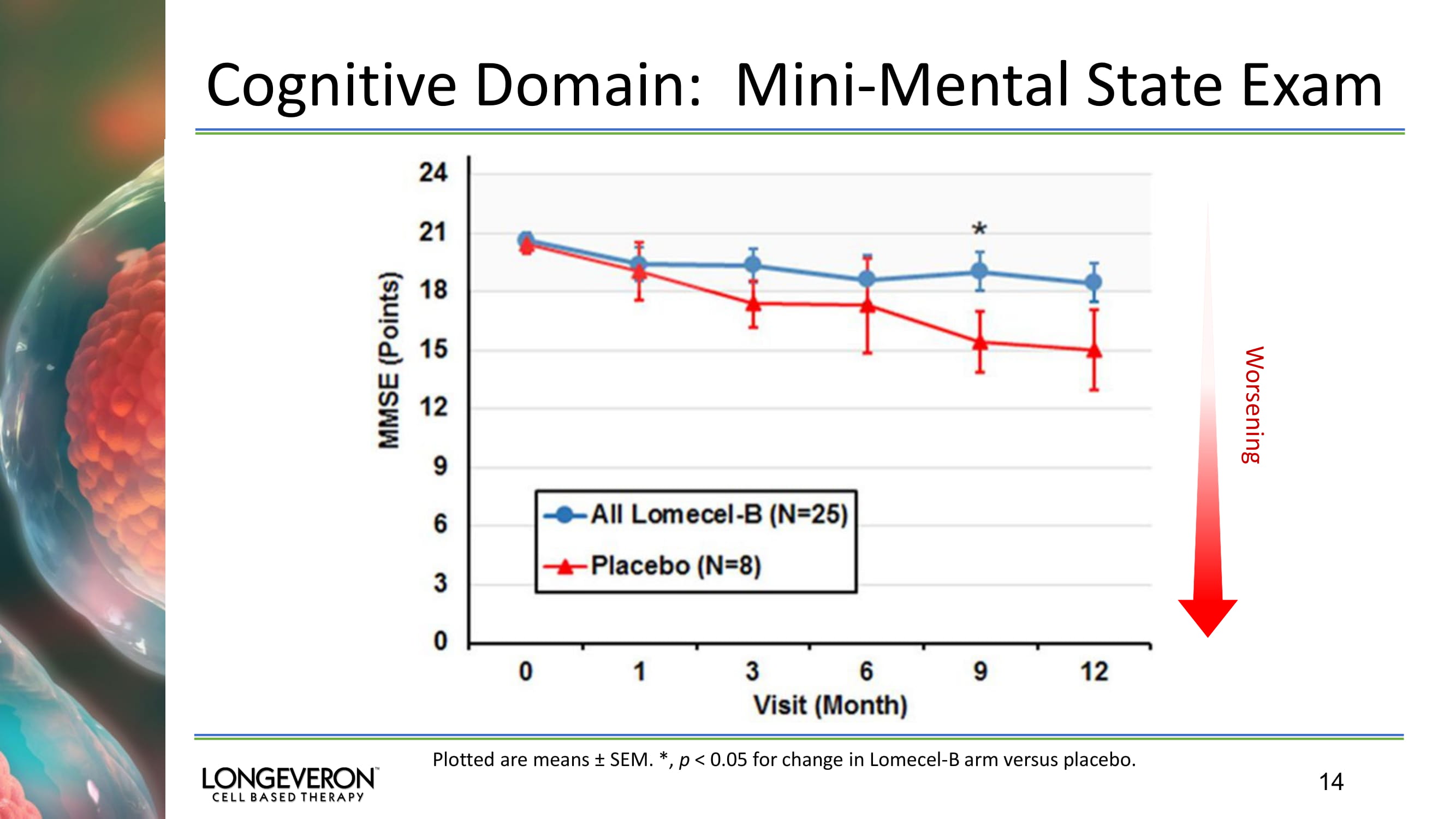
14 Worsening Cognitive Domain: Mini - Mental State Exam Plotted are means ± SEM. *, p < 0.05 for change in Lomecel - B arm versus placebo.
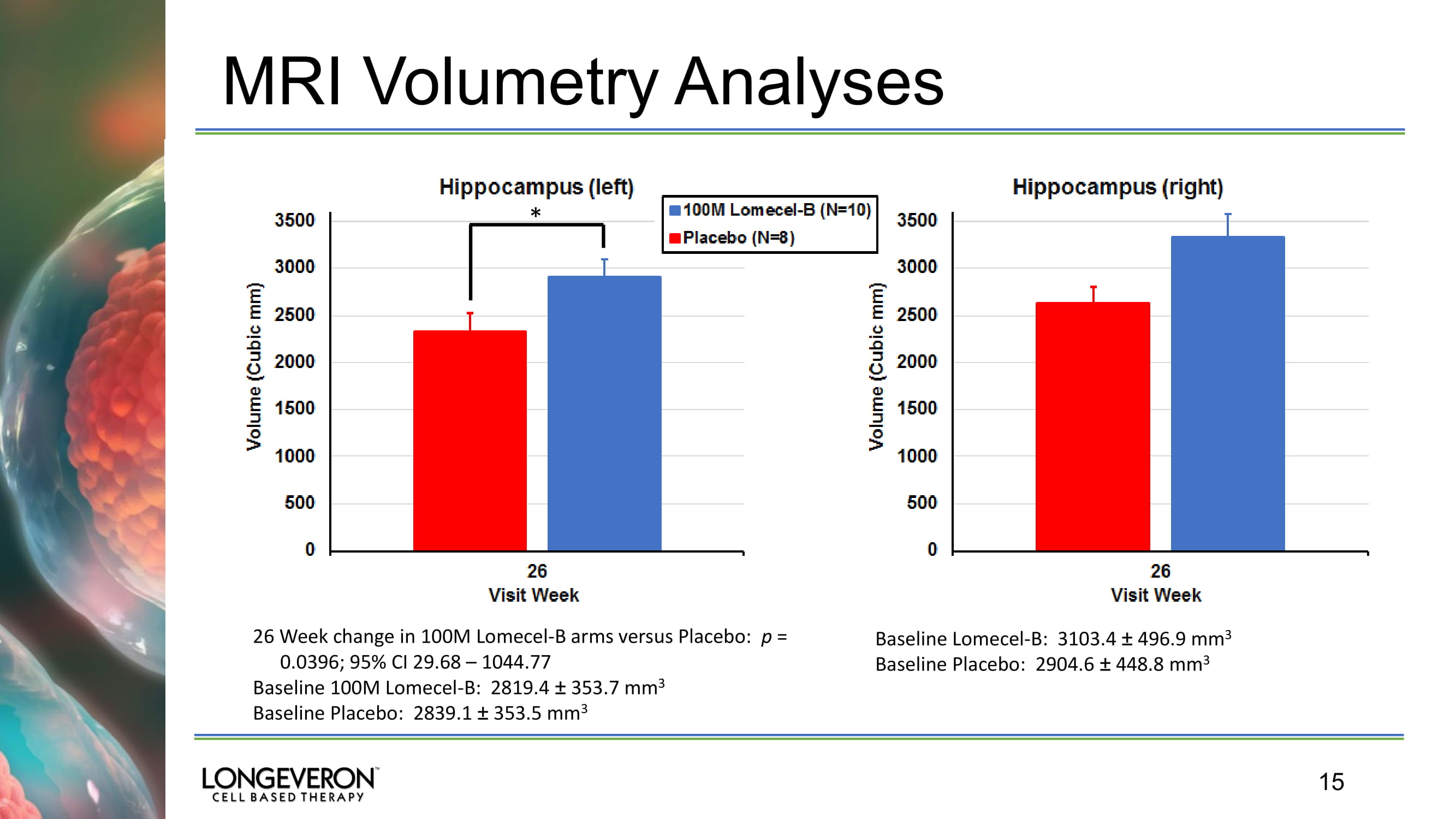
15 MRI Volumetry Analyses * 26 Week change in 100M Lomecel - B arms versus Placebo: p = 0.0396; 95% CI 29.68 – 1044.77 Baseline 100M Lomecel - B : 2819.4 “ 353.7 mm 3 Baseline Placebo : 2839.1 “ 353.5 mm 3 Baseline Lomecel - B: 3103.4 “ 496.9 mm 3 Baseline Placebo : 2904.6 “ 448.8 mm 3

16 Aging Frailty Research Program Phase 1/2 HERA Influenza Vaccine Trial Objective: Can Lomecel - B improve the immune response in Aging Frailty patients receiving influenza vaccine, a group known to mount inadequate immune response to vaccines. □ Phase 1 status : complete (n=22 (3 safety “run - in” subjects)) □ Objectives : ▪ Safety & tolerability ▪ Exploration of timing of Lomecel - B infusion relative to influenza vaccine administration: ▪ Cohort 1: Lomecel - B 100 million cell infusion 4 weeks before flu vaccine (n=10) ▪ Cohort 2: Lomecel - B 100 million cell infusion 1 week before flu vaccine (n=9) SELECTED FOR PHASE 2 □ Efficacy Endpoints : ▪ Antibody production post - vaccination ▪ Surrogate measures of frailty: 6MWT, grip strength, walking speed, patient - reported outcomes of mobility, function and strength; others □ Results : ▪ Safe and well - tolerated ▪ Lomecel - B infusion one week prior to vaccination selected for Phase 2 RCT ▪ Antibody production response suggests Lomecel - B may improve immune response to flu vaccine in Aging Frailty

17 Aging Frailty Research Program Phase 1/2 HERA Influenza Vaccine Trial PHASE 2: ENROLLMENT COMPLETE (N=39); SUBJECT FOLLOW - UP ONGOING □ Phase 2 status : enrollment complete (n=39); subject follow - up ongoing □ Primary Objectives : ▪ Compare post - vaccination antibody production in Lomecel - B vs. placebo through 52 weeks ▪ Surrogate measures of frailty and physical function: 6MWT , grip strength, walking speed, patient - reported outcomes of mobility, function and strength; others □ Cohorts : ▪ Cohort 1: Lomecel - B 100 million cells 1 week prior to flu vaccine (n=19) ▪ Cohort 2: Placebo 1 week prior to flu vaccine (n=20) □ Summary Interim Results : ▪ HAI data not yet available; full analysis at completion of trial ▪ Significant improvement in 6 minute walk distance at 6 months (pooled interim results from Phase 1 & available Phase 2 data)

18 HERA Trial: Antibody Production Against Influenza Phase 1 Subject Data (n=19) □ All subjects, regardless of timing of Lomecel - B infusion, mounted statistically and clinically significant immune response based on antibody production compared to baseline through 4 weeks. □ The CDC found an overall adjusted vaccine effectiveness (VE) of just 18% against flu - associated medically - attended acute respiratory illness in those ≥ 65 years of age (CI = - 25 – 47 %) during 2017 - 2018 flu season. “Index Value” of ≥1.1 considered to be minimum threshold needed for adequate protection. Shown are mean ± SD. p - values are for the change at the respective time - point versus Baseline. ***, p < 0.001
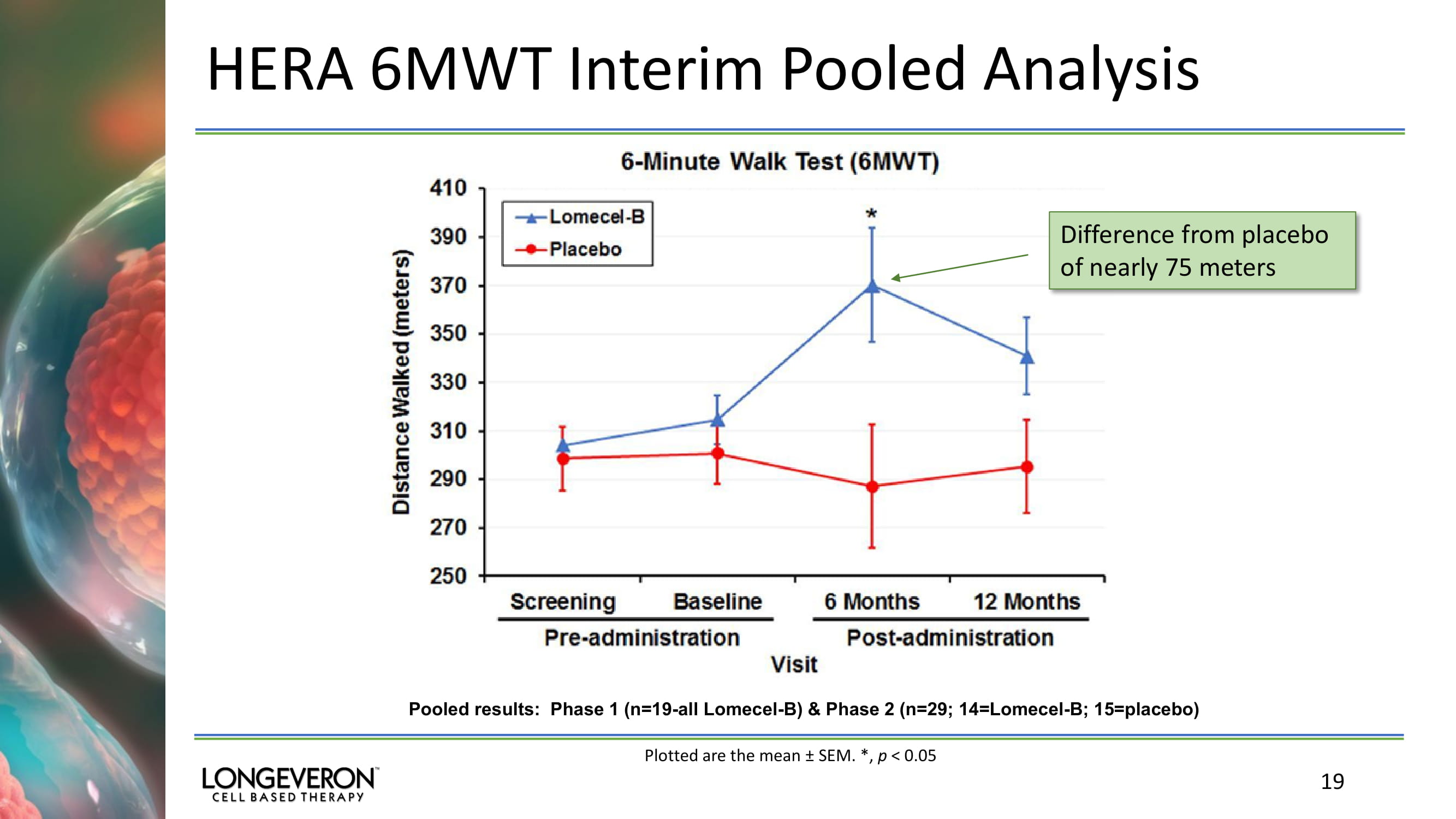
HERA 6MWT Interim Pooled Analysis 19 Pooled results: Phase 1 (n=19 - all Lomecel - B) & Phase 2 (n=29; 14=Lomecel - B; 15=placebo) Difference from placebo of nearly 75 meters Plotted are the mean ± SEM. *, p < 0.05
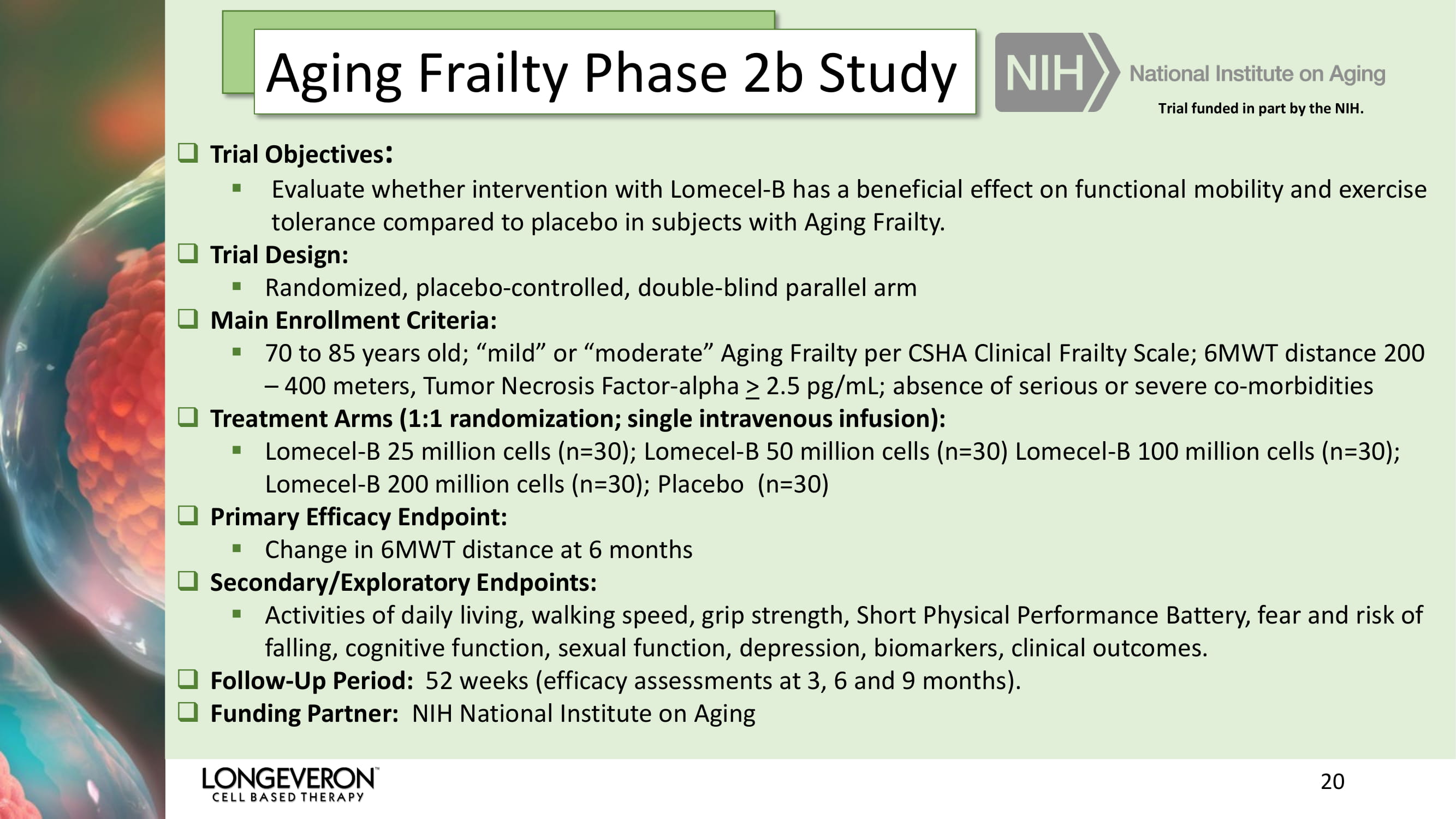
20 Aging Frailty Phase 2b Study □ Trial Objectives : ▪ Evaluate whether intervention with Lomecel - B has a beneficial effect on functional mobility and exercise tolerance compared to placebo in subjects with Aging Frailty . □ Trial Design: ▪ Randomized, placebo - controlled, double - blind parallel arm □ Main Enrollment Criteria: ▪ 70 to 85 years old; “mild” or “moderate” Aging Frailty per CSHA Clinical Frailty Scale; 6MWT distance 200 – 400 meters, Tumor Necrosis Factor - alpha > 2.5 pg /mL; absence of serious or severe co - morbidities □ Treatment Arms (1:1 randomization; single intravenous infusion): ▪ Lomecel - B 25 million cells (n=30); Lomecel - B 50 million cells (n=30) Lomecel - B 100 million cells (n=30); Lomecel - B 200 million cells (n=30); Placebo (n=30) □ Primary Efficacy Endpoint: ▪ Change in 6MWT distance at 6 months □ Secondary/Exploratory Endpoints: ▪ Activities of daily living, walking speed, grip strength, Short Physical Performance Battery, fear and risk of falling, cognitive function, sexual function, depression, biomarkers, clinical outcomes. □ Follow - Up Period: 52 weeks (efficacy assessments at 3, 6 and 9 months). □ Funding Partner: NIH National Institute on Aging Trial funded in part by the NIH.
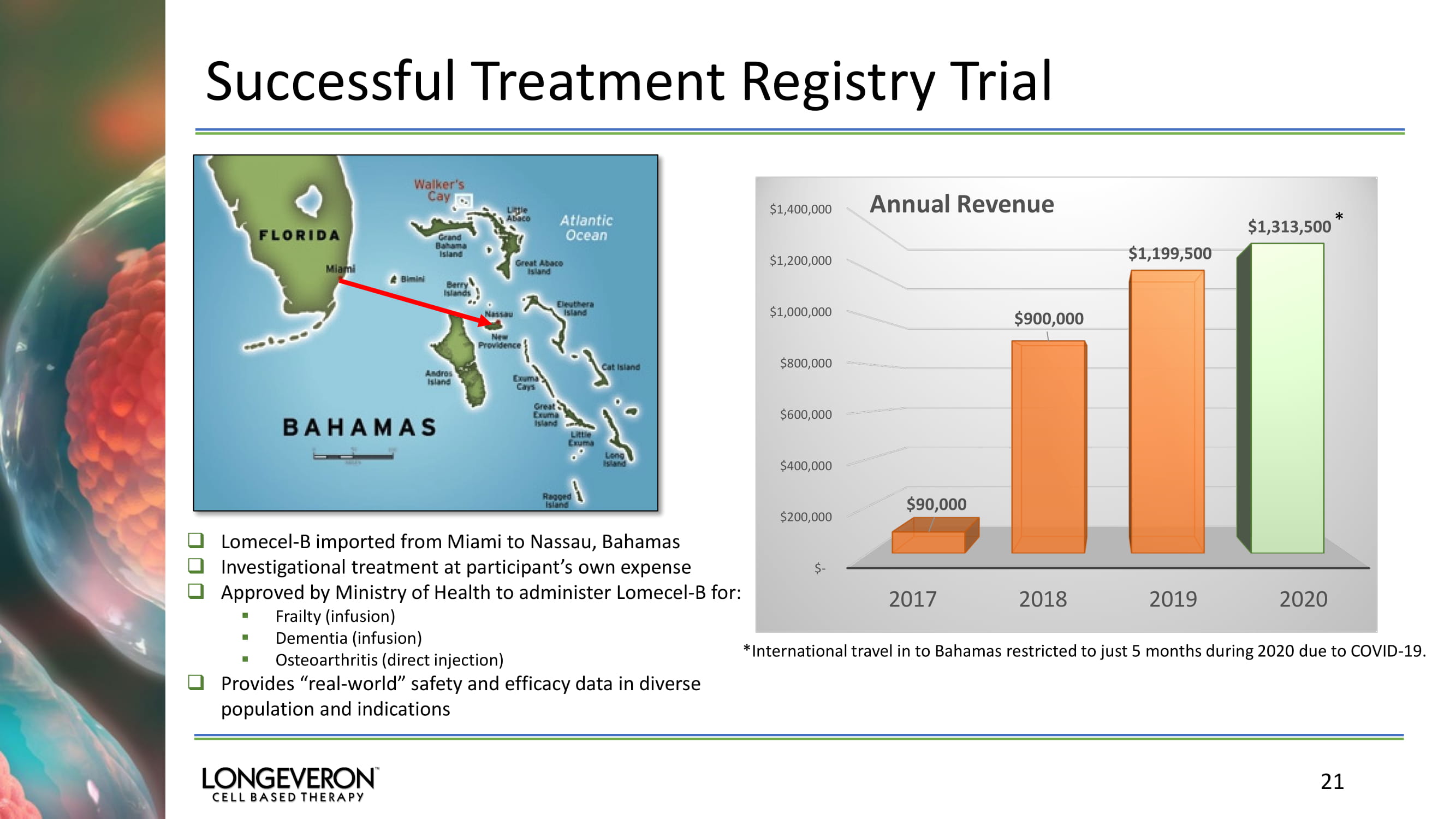
Successful Treatment Registry Trial 21 □ Lomecel - B imported from Miami to Nassau, Bahamas □ Investigational treatment at participant’s own expense □ Approved by Ministry of Health to administer Lomecel - B for: ▪ Frailty (infusion) ▪ Dementia (infusion) ▪ Osteoarthritis (direct injection) □ Provides “real - world” safety and efficacy data in diverse population and indications $- $200,000 $400,000 $600,000 $800,000 $1,000,000 $1,200,000 $1,400,000 2017 2018 2019 2020 $90,000 $900,000 $1,199,500 $1,313,500 Annual Revenue *International travel in to Bahamas restricted to just 5 months during 2020 due to COVID - 19. *

U.S Market Opportunity: Annual Prevalence 22 Aging Frailty Alzheimer’s Disease The Metabolic Syndrome Acute Respiratory Distress Syndrome (ARDS) Hypoplastic Left Heart Syndrome (HLHS) 8.1 million 1 5.8 million 2 87.2 million 3 150,000 annually 4 (unadjusted for COVID - 19 cases) 1,025 annually 5 1 Company estimate based on US Census Bureau Population > 65 years old of 54.06 million (2019 estimate) and community - dwelling Aging Frailty prevalence estimates over the age of 65 (15%) from Bandeen - Roche et al; Gerontol A Biol Sci Med Sci . 2015. Prevalence estimates vary depending on definition criteria used and population studied. 2 Alzheimer’s Association estimate: https://www.alz.org/alzheimers - dementia/facts - figures. 3 Company estimate based US Census Bureau 2019 total population estimate > 18 (255,200,373), and Metabolic Syndrome prevalence estimate of 34.2% from Moore et al ; Prev Chronic Dis 2017 . Prevalence estimates vary depending on definition criteria used and population studied. 4 National Heart and Lung Institute Task Force on Respiratory Disease syndromes. Crit Care . 1998; 2(1): 29 – 34. 5 Centers for Disease Control and Prevention estimate. www.cdc.gov/ncbddd/heartdefects/hlhs.html Large markets — Unmet medical needs — Serious and life - threatening conditions
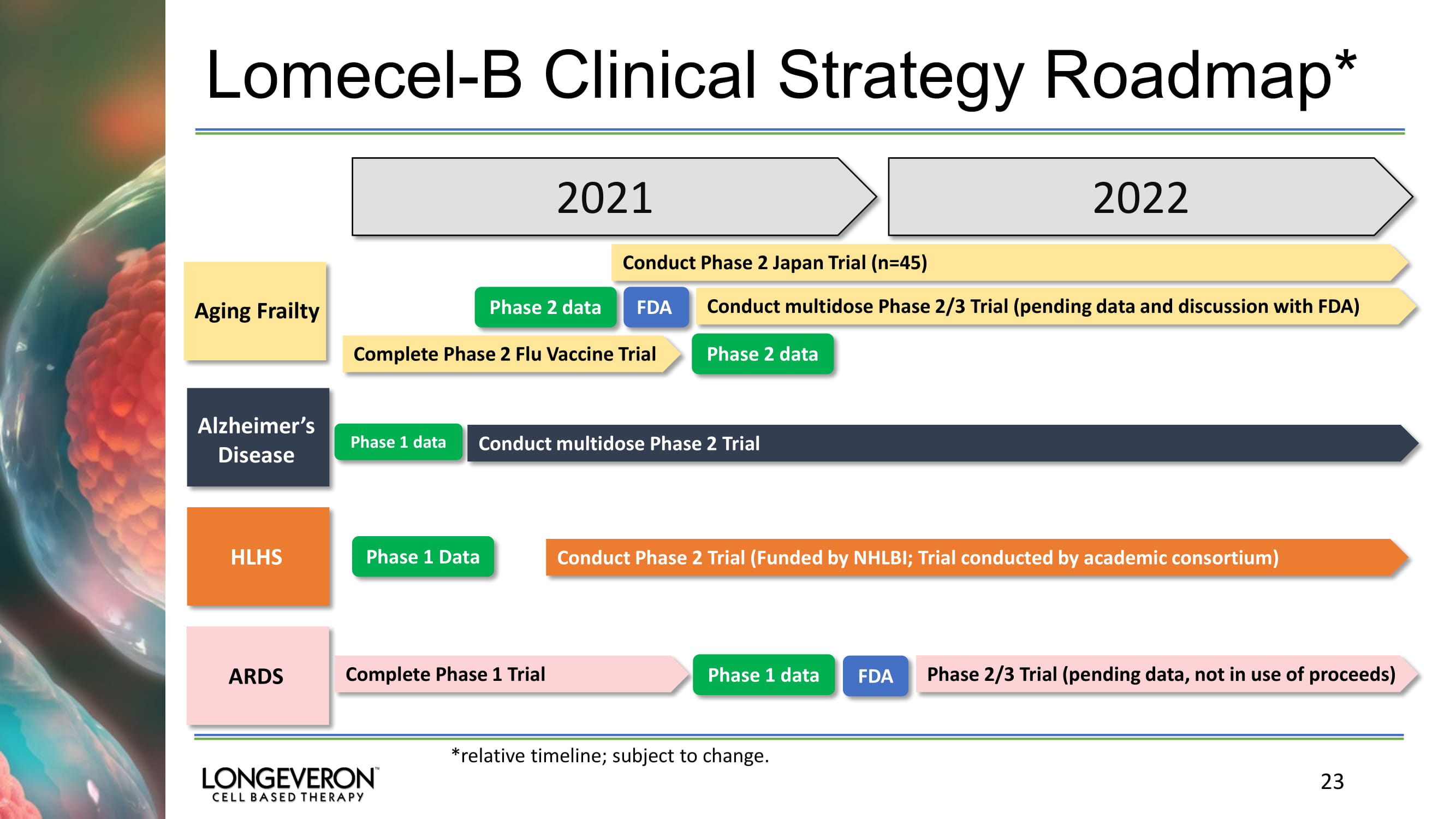
Lomecel - B Clinical Strategy Roadmap* 23 2021 2022 Conduct Phase 2 Japan Trial (n=45) Aging Frailty FDA Conduct multidose Phase 2/3 Trial (pending data and discussion with FDA) Alzheimer’s Disease Conduct multidose Phase 2 Trial HLHS Conduct Phase 2 Trial (Funded by NHLBI; Trial conducted by academic consortium) ARDS Complete Phase 1 Trial FDA Phase 2/3 Trial (pending data, not in use of proceeds ) Phase 1 data Phase 1 Data Phase 2 data Phase 2 data Complete Phase 2 Flu Vaccine Trial Phase 1 data *relative timeline; subject to change.

Estimated Use of Proceeds 2021 - 2022 Research and Development , $13.8 Manufacturing/ Product/Process Development , $2.0 General Corporate Purposes , $7.2 24 • Initiate Phase 2 Alzheimer’s Disease Trial $4.9M • Complete Phase 2b Frailty Trial $1.0M • Initiate Japan Phase 2 Trial $2.2M • Initiate Phase 2/3 US Aging Frailty Trial $3.9M • Provide support for Bahamas Registry $0.5M • Support HLHS – NHLBI trial $0.3M • Complete Phase 1 ARDS Trial $1.0M (general estimates) $13.8M Target Raise: $25.0 million (estimated net $ 23.0 million) Pre - Money Valuation Range: $163 - $175 million Pre - Money Target Share Price Range: $10 to $12/share
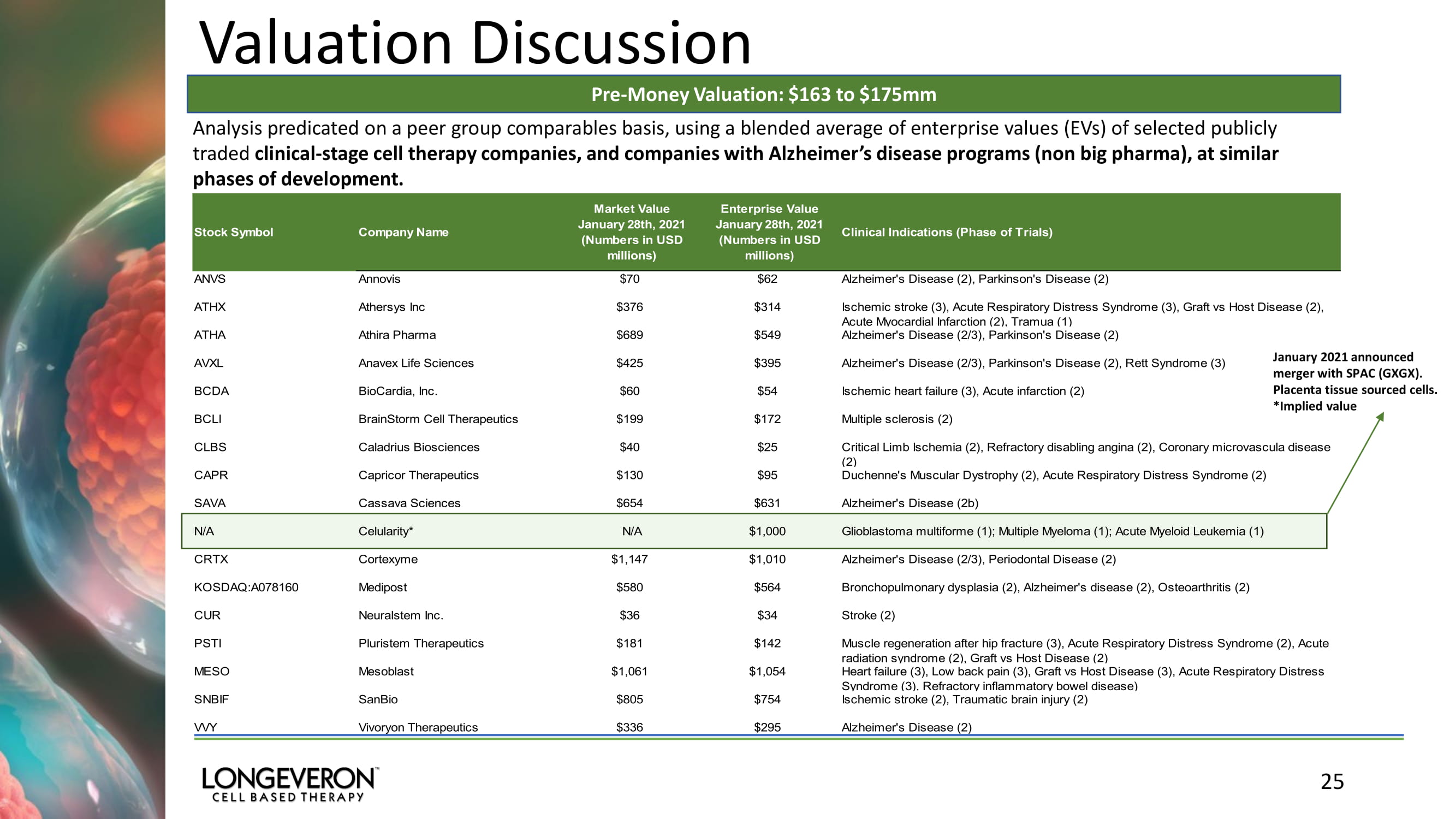
Valuation Discussion 25 Analysis predicated on a peer group comparables basis, using a blended average of enterprise values (EVs) of selected publicly traded clinical - stage cell therapy companies, and companies with Alzheimer’s disease programs (non big pharma), at similar phases of development. Pre - Money Valuation: $163 to $175mm January 2021 announced merger with SPAC (GXGX). Placenta tissue sourced cells. *Implied value Stock Symbol Company Name Market Value January 28th, 2021 (Numbers in USD millions) Enterprise Value January 28th, 2021 (Numbers in USD millions) Clinical Indications (Phase of Trials) ANVS Annovis $70 $62 Alzheimer's Disease (2), Parkinson's Disease (2) ATHX Athersys Inc $376 $314 Ischemic stroke (3), Acute Respiratory Distress Syndrome (3), Graft vs Host Disease (2), Acute Myocardial Infarction (2), Tramua (1) ATHA Athira Pharma $689 $549 Alzheimer's Disease (2/3), Parkinson's Disease (2) AVXL Anavex Life Sciences $425 $395 Alzheimer's Disease (2/3), Parkinson's Disease (2), Rett Syndrome (3) BCDA BioCardia, Inc. $60 $54 Ischemic heart failure (3), Acute infarction (2) BCLI BrainStorm Cell Therapeutics $199 $172 Multiple sclerosis (2) CLBS Caladrius Biosciences $40 $25 Critical Limb Ischemia (2), Refractory disabling angina (2), Coronary microvascula disease (2) CAPR Capricor Therapeutics $130 $95 Duchenne's Muscular Dystrophy (2), Acute Respiratory Distress Syndrome (2) SAVA Cassava Sciences $654 $631 Alzheimer's Disease (2b) N/A Celularity* N/A $1,000 Glioblastoma multiforme (1); Multiple Myeloma (1); Acute Myeloid Leukemia (1) CRTX Cortexyme $1,147 $1,010 Alzheimer's Disease (2/3), Periodontal Disease (2) KOSDAQ:A078160 Medipost $580 $564 Bronchopulmonary dysplasia (2), Alzheimer's disease (2), Osteoarthritis (2) CUR Neuralstem Inc. $36 $34 Stroke (2) PSTI Pluristem Therapeutics $181 $142 Muscle regeneration after hip fracture (3), Acute Respiratory Distress Syndrome (2), Acute radiation syndrome (2), Graft vs Host Disease (2) MESO Mesoblast $1,061 $1,054 Heart failure (3), Low back pain (3), Graft vs Host Disease (3), Acute Respiratory Distress Syndrome (3), Refractory inflammatory bowel disease) SNBIF SanBio $805 $754 Ischemic stroke (2), Traumatic brain injury (2) VVY Vivoryon Therapeutics $336 $295 Alzheimer's Disease (2)

Comparables Stratified: Cell Therapy, Alzheimer’s Disease, and “Anti - Aging” Categories 26 Public Cell Therapy Companies Public AD Companies Public & Private “Anti - Aging” Cos. Name Symbol Market Value ($M) Athersys ATHX $376 Biocardia BCDA $60 Brainstorm Cell Tx BCLA $ 199 Caladirius Bio CLBS $40 Capricor CAPR $130 Medipost (KOSDAQ) 78160 $580 Celularity CELU $1,000* Mesoblast MESO $ 1,061 Neuralstem CUR $36 Pluristem PSTI $ 181 SanBio SNBIF $805 Name Symbol Market Value ($M) Annovis ANVS $70 Athira ATHA $689 Anavex AVXL $425 Cassava SAVA $654 Cortexyme CRTX $1,147 Vivoryon VVY $336 Name Symbol Market Value ($M) Samumed N/A N/A BlueRock Therapeutics (Bayer) N/A N/A Unity Biotechnology UBX $314 Juvenesence N/A N/A Alkahest N/A N/A Elevian N/A N/A Bioquark N/A N/A AgeX AGE $61 BioAge N/A N/A *Implied enterprise value pending closing of merger with SPAC (GXGX; GX Acquisition Corp. 1 - 11 - 2021)

Barriers to Competition 27 Company - Owned Patent Applications • Allogeneic MSCs for use as a vaccine adjuvant • Allogeneic MSCs for improving humoral immunity • Allogeneic MSCS to treat sexual dysfunction • MSC protein expression from stimuli (potency assay) • MSCs to treat Alzheimer’s disease Licensed Patents & Applications • CD271+ MSCs for cardiac repair • License is for use of CD721+ cells for aging - related disorders Proprietary Manufacturing Process • Exclusively licensed from University of Miami • Improved and optimized by Longeveron • In - house manufacturing to maintain proprietary trade secrets Statutory Exclusivity • BLA approval for new indication allows for 12 years of potential market exclusivity • Other regulatory exclusivity options will be pursued (e.g. Orphan Drug Exclusivity)

Leadership 28 Geoff Green, MBA, CEO James Clavijo, CFO Paul Lehr, JD, General Counsel International Executive Director Joshua M. Hare, MD, Co - Founder & CSO Lisa McClain - Moss, Sr. Director, Manufacturing Anthony Oliva, PhD, Senior VP, Scientific Affairs Mr . Green has been with LGVN since 2016 , first as SVP of Clinical Operations, and then President and CEO . He has held leadership positions in public and private life sciences companies for the past 20 years, and has experience in clinical drug development, business development, corporate operations and development . Dr . Hare co - founded Longeveron in 2014 utilizing intellectual property and technology exclusively licensed from the University of Miami, where he is also the Founding Director of the university’s Interdisciplinary Stem Cell Institute (ISCI) . Dr . Hare is a graduate of the University of Pennsylvania & Johns Hopkins University School of Medicine . He served as a research fellow at Harvard University & is Board Certified in cardiovascular medicine . Mr . Lehr leads the legal affairs and international programs . He earned his BA from Brown University, and JD from University of Florida . Mr . Lehr has held senior legal & executive positions in corporate and research settings . Mr . Clavijo joined in 2019 . Mr . Clavijo has 25 years of experience as a CFO for pharmaceutical, healthcare and manufacturing companies . He served as Chief Financial Officer for Aeterna Zentaris (NASDAQ : AEZS) & Tri - source Pharma & as Chief Accounting Officer at Soligenix (NASDAQ : SNGX) Dr . Oliva joined Longeveron in 2015 as the company’s senior scientist . Earning his undergraduate degree in Biological Sciences from the University of Chicago and his Ph . D . in Neuroscience from Baylor College of Medicine, Dr . Oliva brings with him extensive experience in the fields of health science and education . Ms . McClain - Moss joined Longeveron in 2017 to lead the Longeveron’s cell therapy manufacturing and product development activity . She has held previous leadership positions at Cognate BioServices and St . Jude’s Children Research Hospital .

Summary x Leader in developing cell - based therapies for aging - related chronic disease x Well - developed allogeneic MSC product x State - of - the - art GMP facility for stringent manufacturing x Preliminary safety profile and supportive early clinical data x Robust clinical pipeline x Revenue - generating Bahamas Registry Trial programs x Significant value inflection milestones 2021 – 2022 x Proven management, scientific, and manufacturing teams 29
Investor Relations Contact 30 Investor Relations Crescendo Communications, LLC David Waldman/Natalya Rudman Tel: (212) 671 - 1021 lgvn@crescendo - ir.com

31 Additional Information
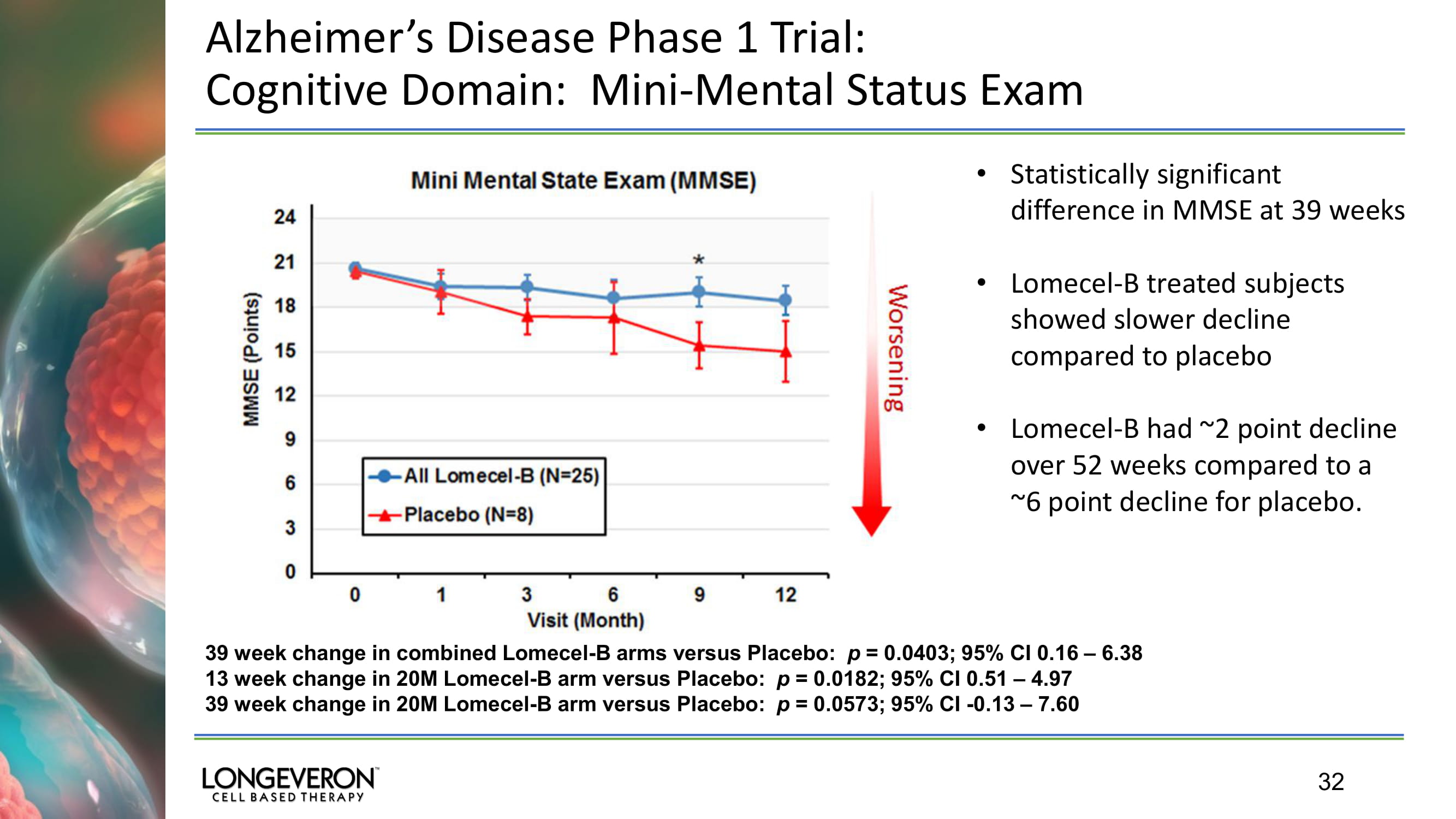
32 Alzheimer’s Disease Phase 1 Trial: Cognitive Domain: Mini - Mental Status Exam 39 week change in combined Lomecel - B arms versus Placebo: p = 0.0403; 95% CI 0.16 – 6.38 13 week change in 20M Lomecel - B arm versus Placebo: p = 0.0182; 95% CI 0.51 – 4.97 39 week change in 20M Lomecel - B arm versus Placebo: p = 0.0573; 95% CI - 0.13 – 7.60 • Statistically significant difference in MMSE at 39 weeks • Lomecel - B treated subjects showed slower decline compared to placebo • Lomecel - B had ~2 point decline over 52 weeks compared to a ~6 point decline for placebo.

33 Alzheimer’s Disease Phase 1: QOL/ADL Domain: ADCS - ADL Improvement 26 Week change in combined Lomecel - B arms versus Placebo: p = 0.0750; 95% CI - 0.93 – 16.86 26 Week change in 20M Lomecel - B arm versus Placebo: p = 0.0118; 95% CI 1.99 – 13.94 • Statistically significant difference in ADCS - ADL at 26 weeks

Frailty Progression 34 Lifelong wear & tear Subclinical disease Chronic sterile inflammation Mobility & strength limitations; Sarcopenia ; W eight loss; Immune system dysfunction; Infections; neurological disease Disability, dependence & death Inflammation Function
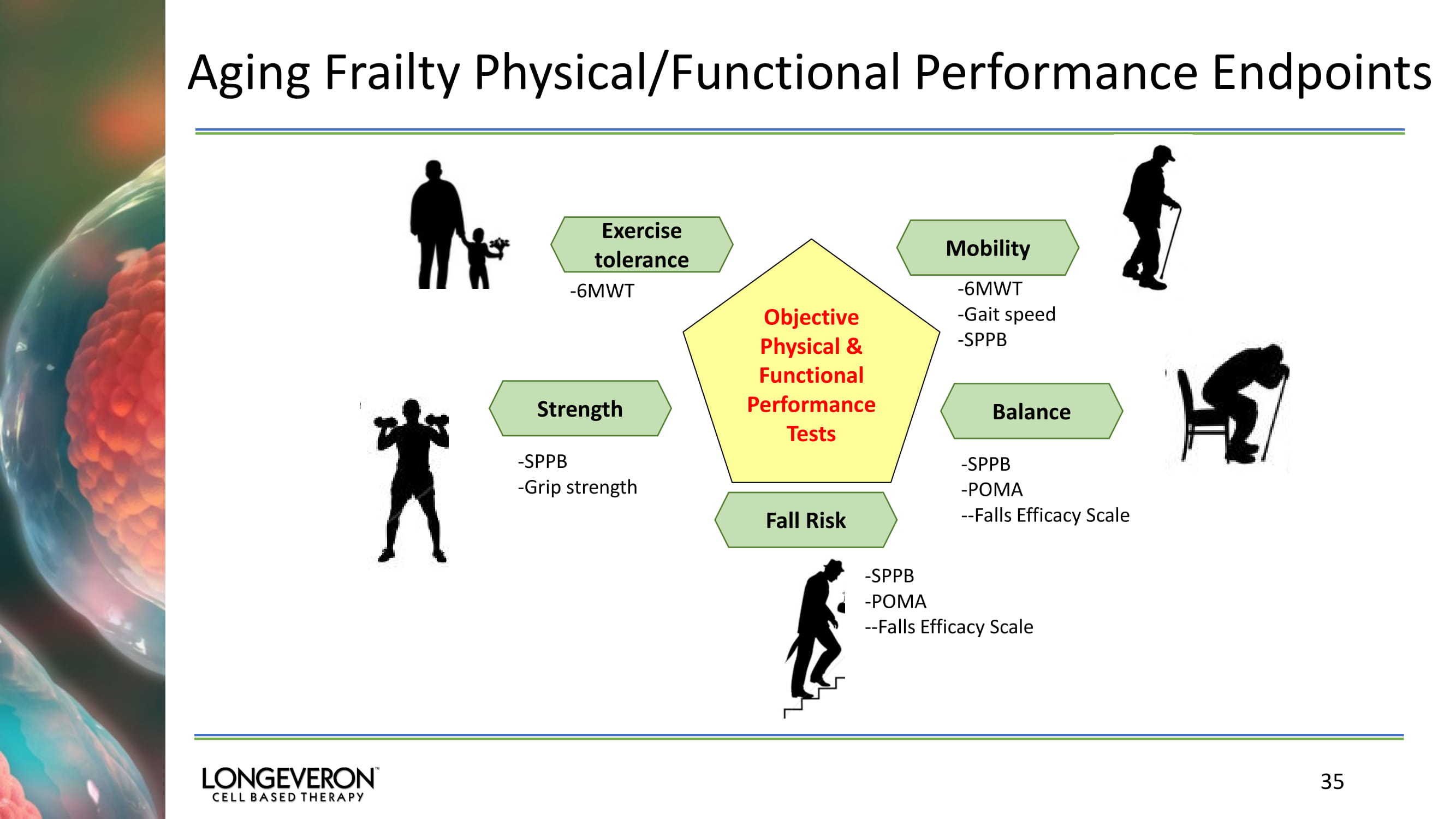
Aging Frailty Physical/Functional Performance Endpoints 35 Exercise tolerance Objective Physical & Functional Performance Tests Balance Mobility Strength Fall Risk - 6MWT - 6MWT - Gait speed - SPPB - SPPB - POMA -- Falls Efficacy Scale - SPPB - POMA -- Falls Efficacy Scale - SPPB - Grip strength

36 Independence & Improved Clinical Outcomes Decreased Inflammation Improved Functional Capacity Better Quality of Life Treatment Goals For Aging Frailty
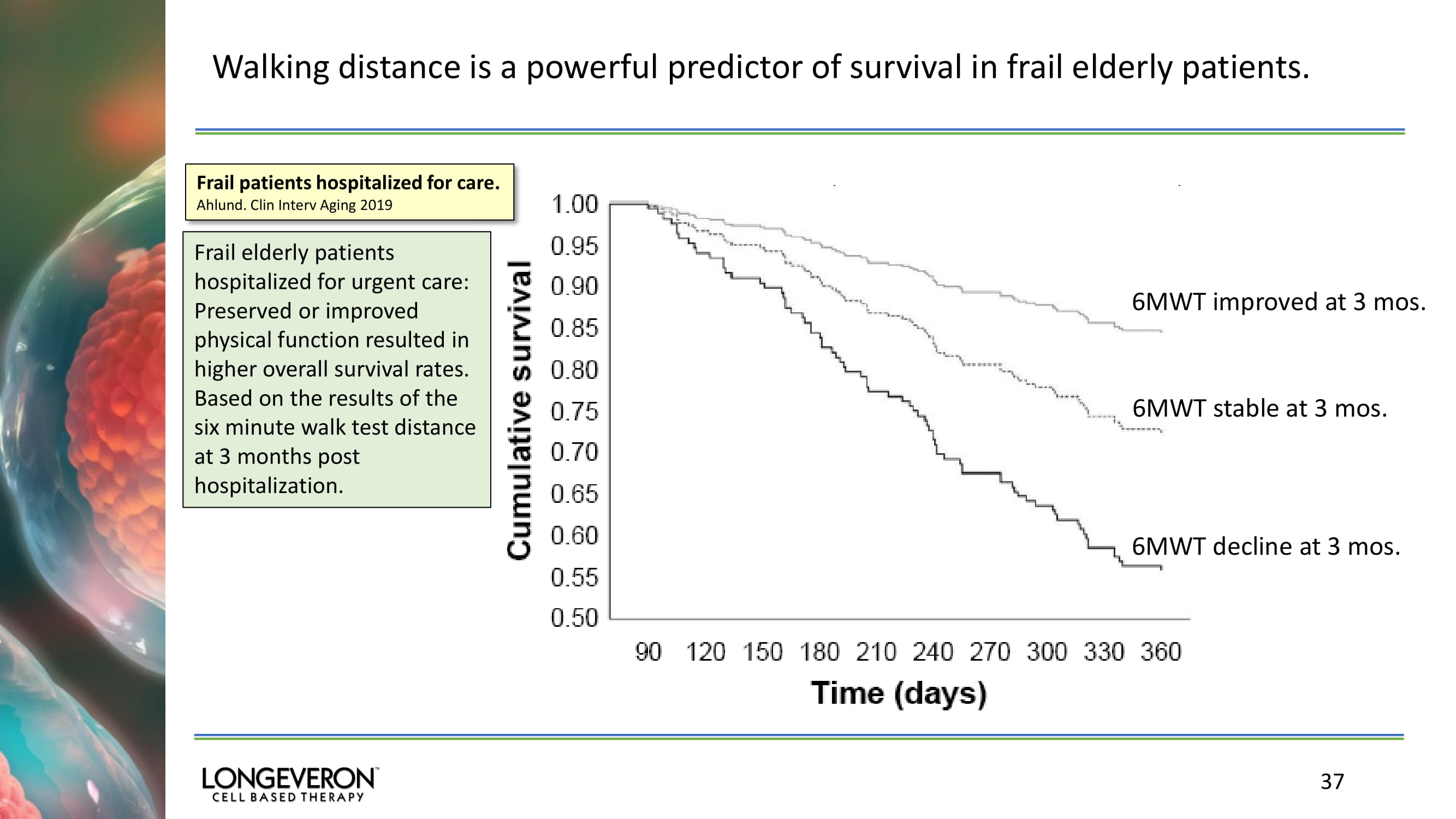
Walking distance is a powerful predictor of survival in frail elderly patients. 37 Frail patients hospitalized for care. Ahlund . Clin Interv Aging 2019 6MWT improved at 3 mos. 6MWT stable at 3 mos. 6MWT decline at 3 mos. Frail elderly patients hospitalized for urgent care: Preserved or improved physical function resulted in higher overall survival rates. Based on the results of the six minute walk test distance at 3 months post hospitalization.
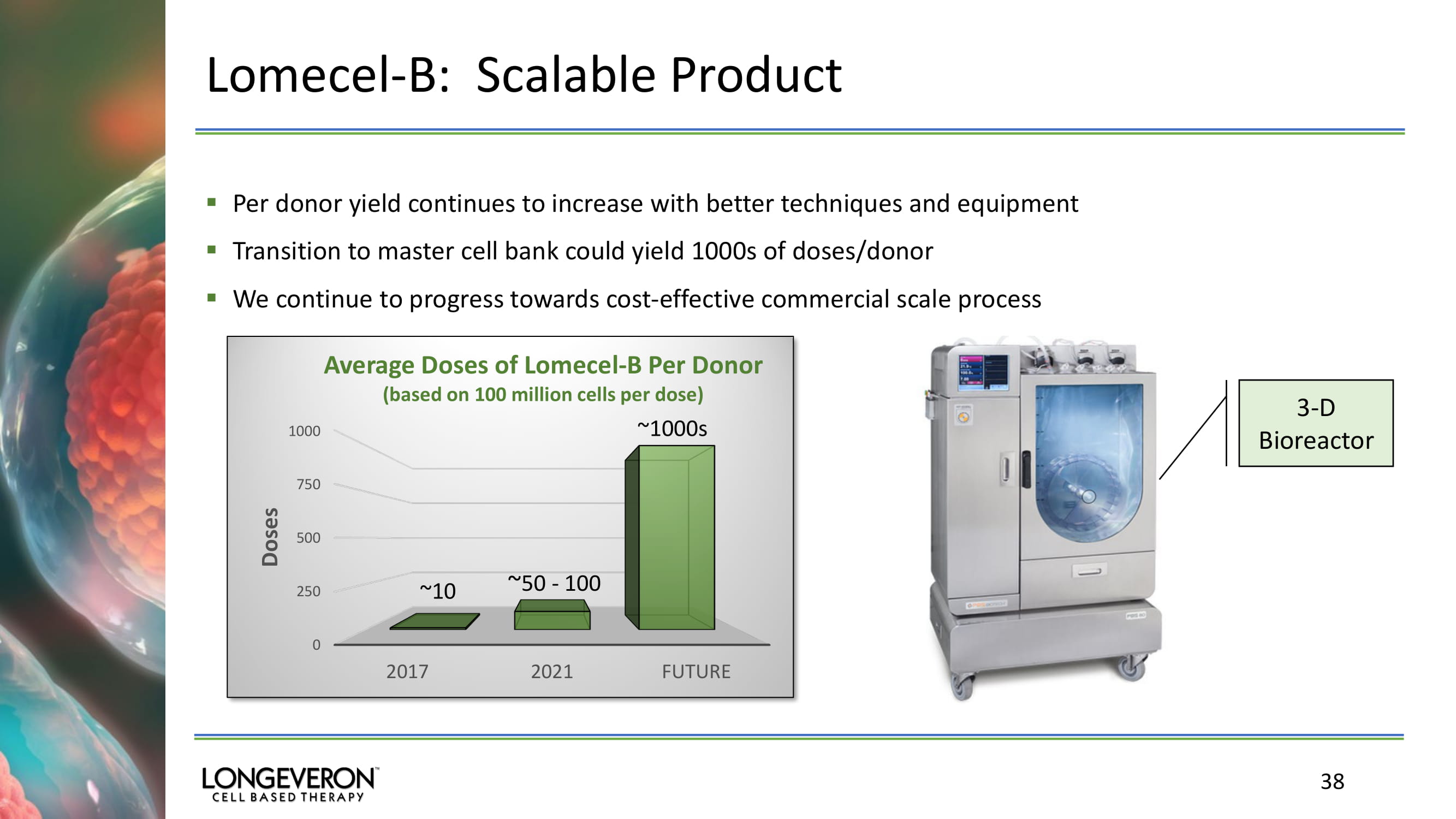
Lomecel - B: Scalable Product ▪ Per donor yield continues to increase with better techniques and equipment ▪ Transition to master cell bank could yield 1000s of doses/donor ▪ We continue to progress towards cost - effective commercial scale process 38 0 250 500 750 1000 2017 2021 FUTURE Doses Average Doses of Lomecel - B Per Donor (based on 100 million cells per dose) 3 - D Bioreactor ~10 ~ 50 - 100 ~1000s

Board of Directors 39 Don Soffer Co - founder Longeveron. Founded Turnberry Associates Brandeis University Joshua Hare, M.D. Co - founded Longeveron Louis Lemberg Professor of Medicine Founding Director of the University of Miami’s Interdisciplinary Stem Cell Institute. Neil Hare, JD President of Global Vision Communications Licensed attorney in the District of Columbia Bar J.D. American University's Washington College of Law B.A. International Relations, Tufts University. Rock Soffer Turnberry Associates O versees leasing, asset acquisitions, zoning and site approvals, and development of specialty projects. Douglas Losordo , M.D. * Caladrius Biosciences Executive Vice President, Global Head of Research and Development, Chief Medical Officer Erin Borger * UBS Northern California Market Head, Wealth Management Advisor Group *Joining Longeveron Board upon Initial Public Offering

Scientific Advisory Board 40 Jeremy Walston, MD, Johns Hopkins Medicine • Co - Director, Biology of Healthy Aging Program, Division of Geriatric Medicine and Gerontology • Co - Director, Biology of Frailty Program • Co - Principal Investigator, Older Americans Independence Center Elena Volpi , MD, PhD, University of Texas Medical Branch - Galveston Daisy Emery Allen Distinguished Chair in Geriatric Medicine; Director, Sealy Center on Aging Director, UTMB Claude D. Pepper Older Americans Independence Center Associate Director, Institute for Translational Sciences Professor, Departments of Internal Medicine - Geriatrics, Neuroscience and Cell Biology, Nutrition and Metabolism Hidenori Arai, MD, PhD, Director, National Center for Geriatrics and Gerontology (Japan) Chair, Japan Frailty Society Professor, Kyoto University Graduate School of Medicine PI, Japanese Study Group on Sarcopenia and Frailty Joe G. N. Garcia, MD, University of Arizona College of Medicine – Tucson Elected member of the Institute of Medicine of the National Academies. Leading authority on prevention and treatment of inflammatory lung disease
Longeveron LLC Financial History 41 Financial History (9/30/20) ▪ Founded: Oct 2014 ▪ Financings: $27.0M (equity) ▪ Revenue: $ 3.3M ▪ Grant Awards: $16.2M ▪ Debt: $ 0.5M








































