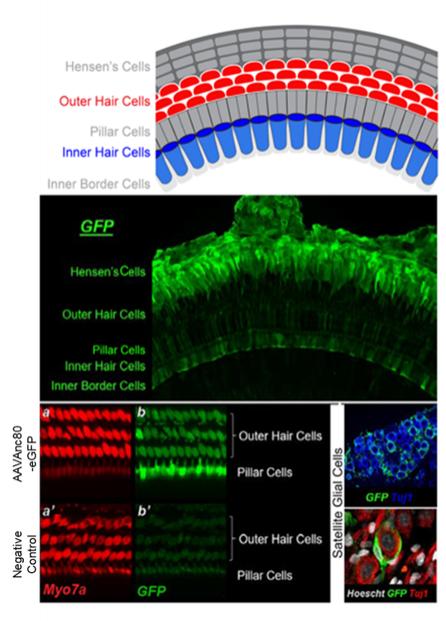
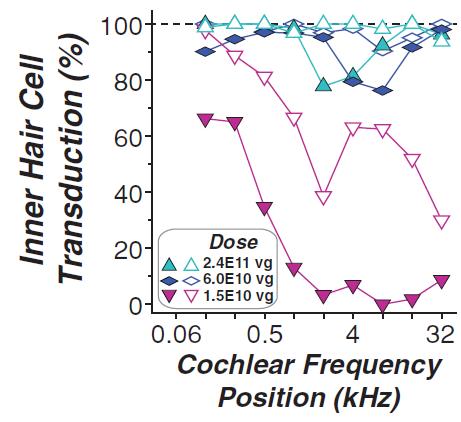
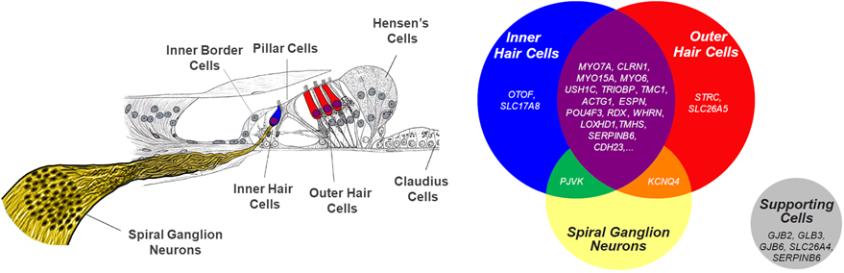
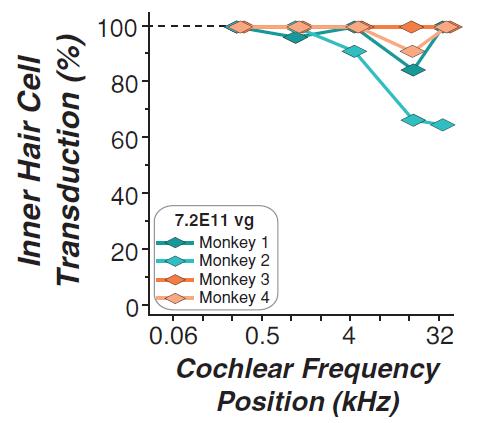
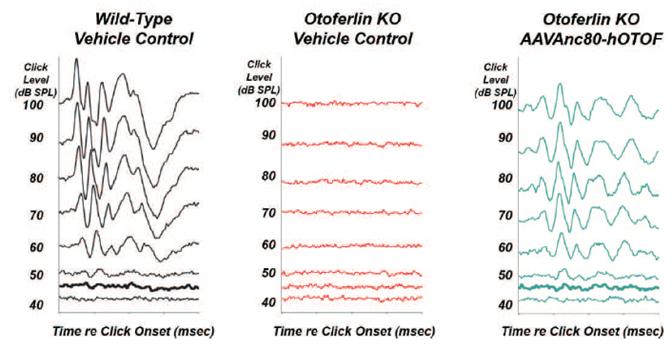
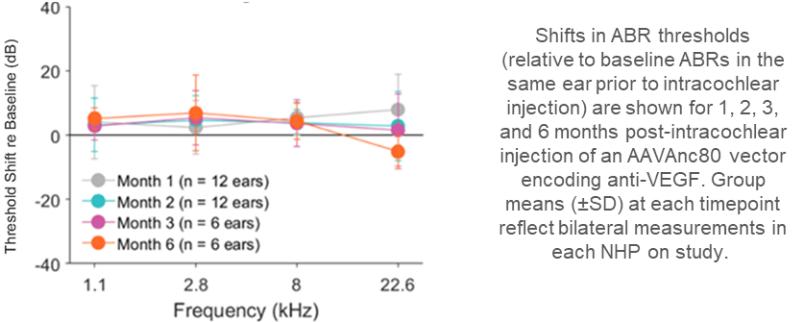
may nevertheless challenge such claims in our patents. If any such claims are invalidated or rendered unenforceable for any reason, we will lose valuable intellectual property rights, and our ability to prevent others from competing with us would be impaired. Any U.S. or ex-U.S. patents that may issue from pending applications that we control, if any, for our hearing program, including our product candidate for vestibular schwannoma, are projected to have a statutory expiration date in between 2034 and 2041, excluding any additional term for patent term adjustments or patent term extensions, if applicable.
Trademark Protection
As of December 31, 2021, we held 13 U.S. trademark applications, 104 foreign trademark applications, and 43 foreign trademark registrations for the marks “Akouos,” the Akouos logo, “Resonate,” the Resonate by Akouos logo, “The Sing Registry,” and the Sing logo in connection with our biological products and related services.
Licensed Intellectual Property
Massachusetts Eye and Ear License
In October 2017, we entered into a License Agreement with MEE, or the MEE License, under which we received an exclusive, non-transferable, sublicensable, worldwide, royalty-bearing license to certain patent rights and know-how, including rights related to AAVAnc-, including AAVAnc80, to research, develop, make, have made, manufacture, use, sell, offer to sell, import, export, market, promote, distribute, register and otherwise commercially exploit licensed products in the treatment, diagnosis, prevention, and palliation of any and all balance disorders or diseases pertaining to the inner ear and/or any and all hearing diseases or disorders, in each case, with a total prevalence in the United States of less than 3,000 patients, and an exclusive, non-transferable, sublicensable, worldwide, royalty bearing license under MEE’s rights, title and interest in certain patents co-owned by MEE and Children’s Medical Center Corporation, or BCH, to research, develop, make, have made, manufacture, use, sell, offer to sell, import, export, market, promote, distribute, register and otherwise commercially exploit licensed products in the treatment, diagnosis, prevention, and palliation of any and all balance disorders or diseases pertaining to the inner ear and/or any and all hearing diseases or disorders, including, but not limited to, those with a total prevalence in the United States of less than 3,000 patients. We are obligated to use commercially reasonable efforts to develop and commercialize the MEE licensed products, including filing an IND or Investigational Medicinal Product Dossier in any country in the European Union or an equivalent application in any country within 18 months of completion of GLP toxicology studies for a licensed product. Under certain circumstances, we may be obligated to pursue a program directed to a gene target in order to retain our rights under the agreement to such gene target. MEE is responsible for the prosecution and maintenance of licensed patent rights. If MEE elects not to file, continue to prosecute or maintain such licensed patent rights in our field of use, MEE must notify us within a specified period before any applicable deadline. We would then have the right, but not the obligation, to file, continue to prosecute, or maintain the licensed patent rights. MEE has the first right, but not the duty, to enforce the licensed patents against use by a third party. If MEE does not initiate enforcement of the patents within a specified period after learning of an infringement, then we have the right, but not the duty, to initiate such enforcement efforts within our field of use of the MEE License. If a third party brings an infringement action regarding the patents within our field of use, we have the right, with respect to certain intellectual property rights in the MEE License, to defend at our own cost and expense. MEE has the right to intervene and assume control of such a defense at its own expense.
Upon entering into the MEE License, we issued shares of our common stock to MEE. We are also subject to development, regulatory, and sales milestone payment obligations in amounts corresponding to achievements denoted in the MEE License, totaling approximately $17.7 million. We are additionally obligated to pay certain royalties on a licensed product-by licensed product and country- by- country basis, beginning after the first commercial sale of an MEE licensed product in a given country and lasting until the later of (i) the expiration of the last valid claim in the licensed patents or (ii) ten years after the first commercial sale of such MEE licensed product or products commercialized using BCH’s patent rights, or the MEE Royalty Term. Such royalties shall be based on the portion of annual worldwide net sales within certain royalty tiers at percentages in the mid to high single digits denoted in the MEE License. The mere manufacture of an MEE licensed product or a product commercialized using BCH’s patent rights without a sale or other transfer does not give rise to a royalty claim under the MEE License.