DESCRIPTION OF SHARE CAPITAL
General
We are an exempted company incorporated in June 2014 with limited liability under the laws of the Cayman Islands and our affairs are governed by:
| | • | | Our Seventh Amended and Restated Memorandum and Articles of Association, or our Articles; |
| | • | | the Companies Law (as amended) of the Cayman Islands, or the Companies Law; and |
| | • | | the common law of the Cayman Islands. |
As of the date of this prospectus, our authorized share capital is NT$5,000,000,000 divided into 500,000,000 ordinary shares, with a par value of NT$10.00 per ordinary share. As of the date of this prospectus, there are 160,248,940 ordinary shares issued and outstanding.
For our initial public offering in Taiwan, we conducted a restructuring between one of our subsidiaries, ASLAN Pharmaceuticals Pte. Ltd., a Singapore entity, and us. After the restructuring, we became the parent company of ASLAN Pharmaceuticals Pte. Ltd. and the listing entity in Taiwan. The restructuring was consummated through a share swap according to a reconstruction agreement between ASLAN Pharmaceuticals Pte. Ltd., its then shareholders, and us in September 2014 pursuant to which the shares of ASLAN Pharmaceuticals Pte. Ltd. held by its then shareholders, including ordinary shares, Series A and Series B Preference shares, were swapped into our ordinary shares, Series A and Series B Preference shares at a ratio of 1:1.
Further, we also underwent a share capital restructuring to change the par value of our ordinary shares to NT$10.00.
We raised $41,189,000 by issuing 17,047,095 and 4,861,948 Series C Preference shares at $1.88 per share in November 2015 and January 2016, respectively. In June 2016, we raised $22,224,000 by issuing 19,667,144 ordinary shares at $1.13 per share. In our initial public offering in Taiwan on June 1, 2017, we issued 14,458,000 ordinary shares at a subscription price of NT$68.92 per ordinary share, raising, after deducting underwriting discounts and commissions and offering expenses, an aggregate of NT$996,465,000. Our ordinary shares began trading in the TPEx on June 1, 2017.
In October 2018, we increased our authorized share capital from NT$2,000,000,000 to NT$5,000,000,000.
The following are summaries of material provisions of our Articles and the Companies Law insofar as they relate to the material terms of our share capital.
Seventh Amended and Restated Memorandum and Articles of Association
Subject to other provisions in our Articles, our shareholders may by ordinary resolution increase our authorized share capital or by special resolution reduce the share capital and may also by special resolution amend our Articles.
Ordinary Shares
General. All of our outstanding ordinary shares are fully paid andnon-assessable. No certificates representing the ordinary shares have been issued. The ordinary shares are not entitled to any preemptive conversion or redemption rights at the sole option of the holder of ordinary shares. Our shareholders may freely hold and vote their shares (subject to certain restrictions such as the number of proxies that may be held by a shareholder at a general meeting).
60

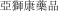 ..” In China, we have a trademark registration for “
..” In China, we have a trademark registration for “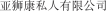 ..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “
..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “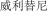 ” (wei li ti ni). This prospectus supplement and accompanying prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus supplement and accompanying prospectus, including logos, artwork and other visual displays, may appear without the™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
” (wei li ti ni). This prospectus supplement and accompanying prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus supplement and accompanying prospectus, including logos, artwork and other visual displays, may appear without the™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.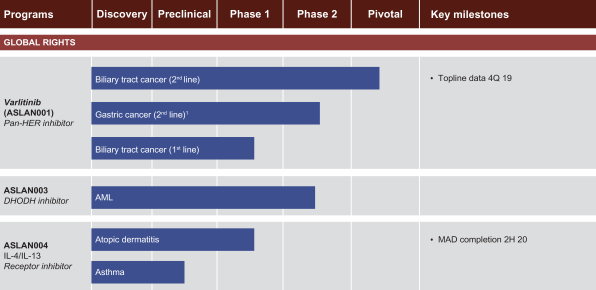
 ” In China, we have a trademark registration for “
” In China, we have a trademark registration for “ ..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “
..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “ ” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos,
” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos,