marketing-related products or could limit the term of patent protection that otherwise may exist for our product candidates. In addition, the scope of the rights granted under any issued in-licensed patents may not provide us with protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies that are outside the scope of the rights granted under any issued patents. For these reasons, we may face competition with respect to our product candidates. Moreover, because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any particular product candidate can be commercialized, any patent directed to such product may expire or remain in force for only a short period following commercialization, thereby reducing the commercial advantage the patent provides.
In-licensed Patents and Patent Applications
Recurium IP Holdings, LLC or Zeno Management, Inc., are currently the listed owner/assignee, or retained the exclusive license to 48 families of patent applications directed to our technology across our pipeline. As of July 8, 2020, our in-licensed portfolio consists of twelve U.S. patents and eleven foreign patents in six jurisdictions, Europe, Hong Kong, India, Japan, Singapore and Taiwan.
As of July 8, 2020, 20 of the 48 families have a single application pending, and 27 of 48 families have multiple applications pending. The 48 families include 60 U.S. applications (including pending U.S. provisional patent applications and pending U.S. non-provisional patent applications), 9 PCT applications and 188 international applications in approximately 17 countries, including major markets in North America, Europe and Asia, each having a nominal expiration date ranging from 2034 to 2040. The nominal expiration of our patents and patent applications does not account for any applicable patent term adjustments or extensions.
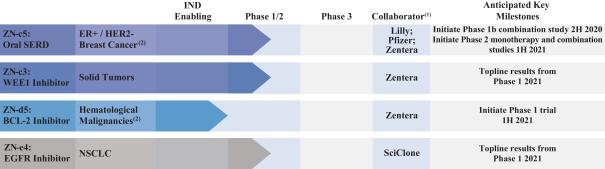
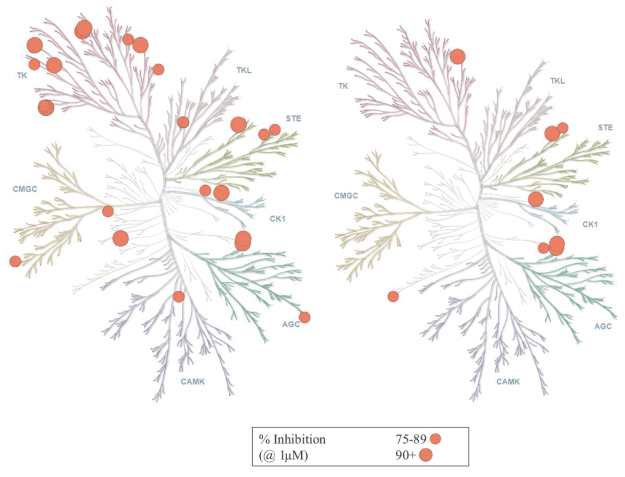
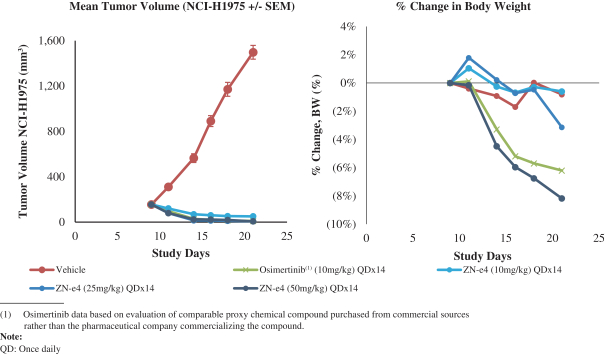
U.S. Patent No. 10,513,509, or the ‘509 Patent, includes claims directed to composition of matter, including ZN-e4, a pharmaceutical composition, a method for inhibiting replication of a malignant growth or a tumor, a method for ameliorating or treating a cancer and a method for inhibiting the activity of EGFR. The ‘509 Patent has an expected expiration date in May 2037. However, we believe the ‘509 Patent may be eligible for a patent term extension under the Hatch-Waxman Act.
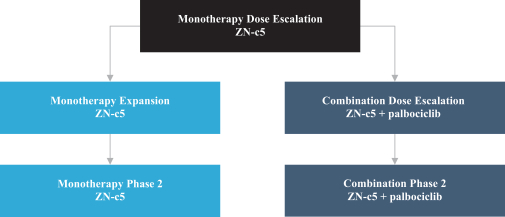
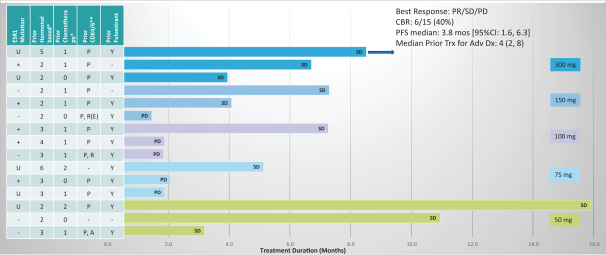
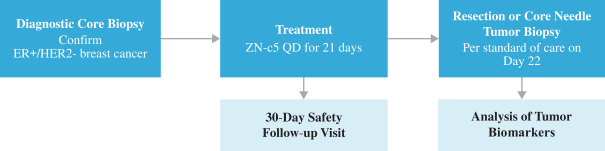
One of the aforementioned pending U.S. and PCT patent applications includes claims directed to ZN-c5, ZN-c3 or ZN-d5, and has an expected expiration in 2037 (ZN-c5) and 2039 (ZN-c3 and ZN-d5). However, there can be no assurance that any of our pending in-licensed patent applications will issue. Furthermore, there can be no assurance that we will benefit from any patent term extension or favorable adjustments to the term of any of our in-licensed issued patents or patents that are issued in the future. The applicable authorities, including the FDA in the United States, may not agree with our assessment of whether such patent term extensions should be granted, and, if granted, they may grant more limited extensions than we request.
Trademarks
As of July 8, 2020, our trademark portfolio contains the following trademarks applications or registrations. U.S. trademark applications are pending for each of the marks ZENTALIS and the stylized “Z” mark. The mark ZENO has a registered U.S. trademark. Applications to register the marks ZENO and ZENTALIS have been filed internationally. The portfolio has an International Madrid Trademark Application designating Australia, Europe, Israel, Japan, Mexico, New Zealand, the Russian Federation, the United Kingdom and Singapore for the mark ZENO. The portfolio also has pending applications for registration and/or a registration has issued for one or more classes in Argentina, Brazil, Canada, Hong Kong and Taiwan for the mark ZENO. The portfolio also has an International Madrid Trademark Application designating Australia, Brazil, Canada, China, Europe, the United Kingdom, Israel, India, Japan, Korea, Mexico, New Zealand, the Russian Federation and Singapore for the mark ZENTALIS. The portfolio also has pending applications for registration in Argentina, Hong Kong, and Taiwan for the mark ZENTALIS.
147