Competition
The biotechnology and pharmaceutical industries are characterized by rapid evolution of technologies, fierce competition and strong defense of intellectual property. While we believe that our product candidates, discovery programs, technology, knowledge, experience and scientific resources provide us with competitive advantages, we face competition from major pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions, among others.
Any product candidates that we successfully develop and commercialize could compete with currently approved therapies and new therapies that may become available in the future. Key product features that would affect our ability to effectively compete with other therapeutics include the efficacy, safety and convenience of our products.
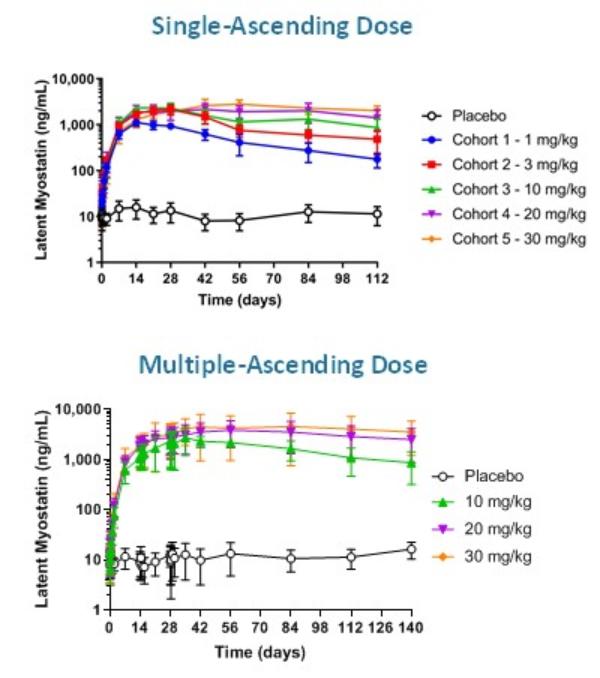
At this time, there are no FDA- or EMA-approved muscle-directed treatments for SMA. We believe apitegromab (SRK-015) may be used in conjunction with SMN upregulators or as a monotherapy in certain settings. Biogen markets SPINRAZA® (nusinersen), the first marketed SMN2 upregulator. Biogen is also developing BIIB110 for SMA and other diseases. BIIB110 is a Phase 1 investigational agent that is intended to work in part through inhibition of the myostatin signaling pathway.
On May 24, 2019, Novartis International AG (“Novartis”) received FDA approval for ZOLGENSMA® (onasemnogene abeparvovec-xioi), the first SMN1 gene replacement therapy in SMA. ZOLGENSMA® (onasemnogene abeparvovec-xioi) is currently available in the U.S. for SMA patients less than 2 years of age. Novartis is also developing an alternate formulation of onasemnogene abeparvovec-xioi for older SMA patients, as well as an oral SMN2 upregulator. Both of these additional investigational agents are in early stage clinical development.
A third SMN upregulator, The Roche Group’s (“Roche’s”)Evrysdi® (risdiplam), received FDA approval on August 7, 2020. Like SPINRAZA® (nusinersen), risdiplam modulates the SMN2 gene but is administered in an oral dosage form.
Cytokinetics, Inc. is developing reldesemtiv, a fast-skeletal muscle troponin activator (“FSTA”), as a potential treatment for amyotrophic lateral sclerosis (“ALS”) and SMA.
Catalyst Pharmaceuticals Inc.is developing an investigational agent with another mechanism of action for the treatment of SMA.
Many companies, such as Regeneron Pharmaceuticals, Inc. and Roche are developing therapies for muscle-wasting diseases, other than SMA, that are intended to work, at least in part, through inhibition of the myostatin signaling pathway.
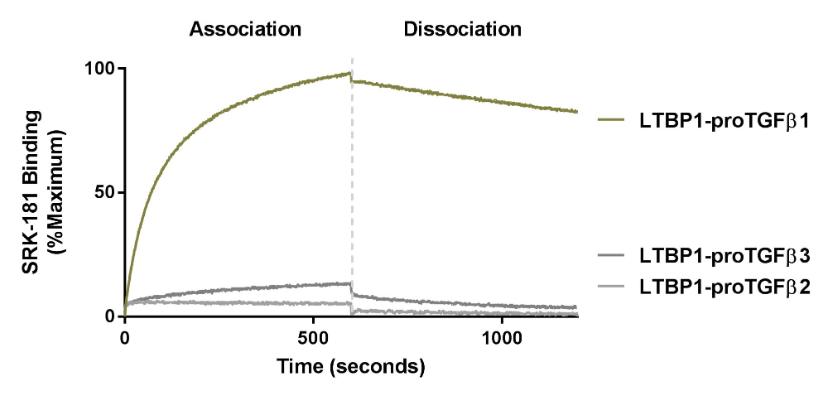
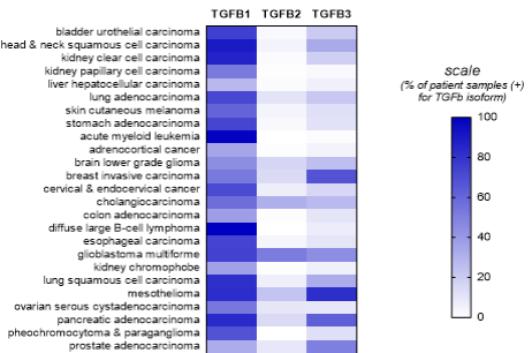
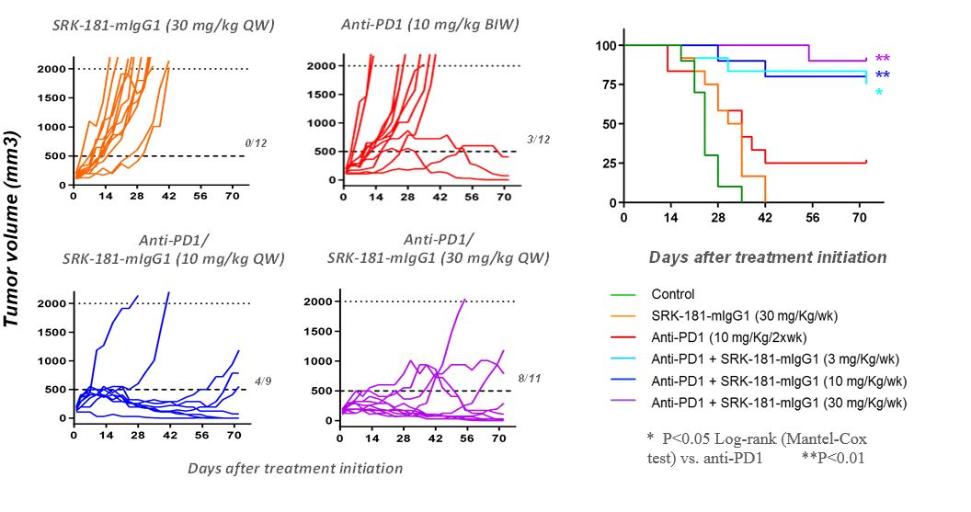
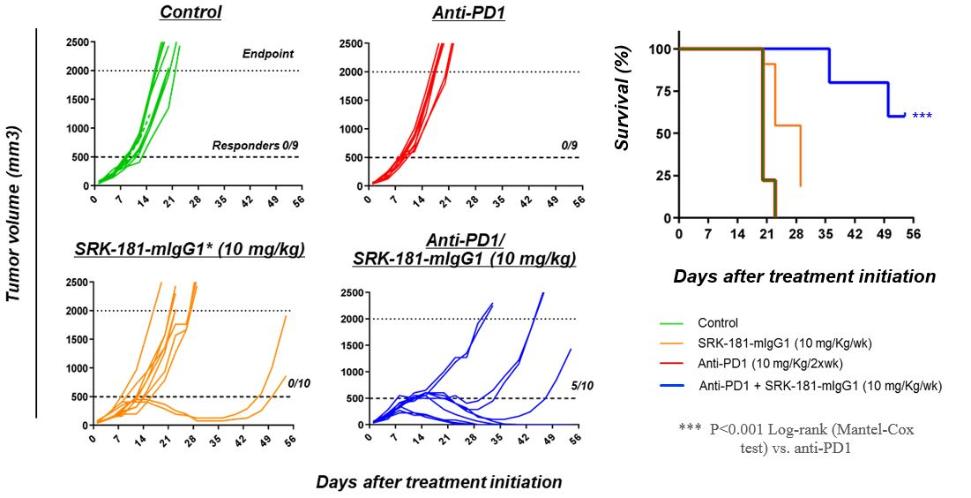
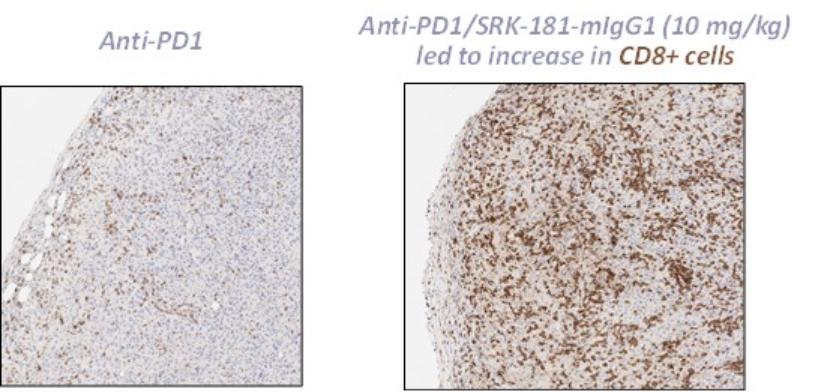
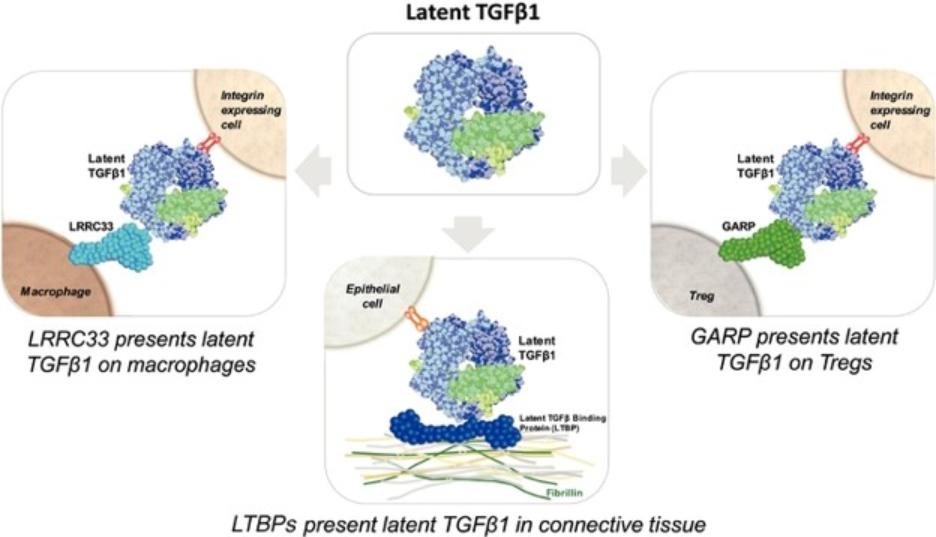
Our competitors for SRK-181 may include other companies developing cancer immunotherapies to be used in combination with CPI therapy. Merck KGaA’s bintrafusp alfa, a bifunctional TGF-β trap/PD-L1 antibody that is partnered with GSK, the leading investigational agent of this emerging class of therapies intended to improve outcomes in patients non-responsive to CPI inhibition. Bintrafusp alfa is currently is in Phase 2 and 3 trials for the treatment of biliary tract cancer (“BTC”), non-small cell lung cancer (“NSCLC”) and cervical cancer, as well as in multiple early-stage clinical studies in a variety of solid tumor types.
Other companies, including Novartis, Merck (acquired Tilos Therapeutics), Sanofi S.A., Bristol Meyers Squibb (acquired Forbius), Gilead and AbbVie Inc. are developing therapies for cancer immunotherapy in combination with CPI therapy, that are intended to work, at least in part, through inhibition of the TGFβ signaling pathway.
Our competitors may also include companies that are or will be developing therapies for the same therapeutic areas that we are targeting within our early pipeline, including other neuromuscular disorders, cancer and fibrosis.
Many of the companies against which we may compete have significantly greater financial resources and expertise than we do in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.