| • | reduced protection for intellectual property rights in some countries; |
| • | export restrictions, trade regulations and foreign tax laws; |
| • | fluctuating foreign currency exchange rates; |
| • | obtaining and maintaining foreign certification and compliance with other regulatory requirements; |
| • | customs clearance and shipping delays; and |
| • | political and economic instability. |
If one or more of these risks were realized, it could require us to dedicate significant resources to remedy the situation, and if we are unsuccessful at finding a solution, our revenue may decline.
The impact of the ongoing Israel-Hamas war could impede our ability to operate and develop, manufacture and deliver products and components and harm our business and financial results.
In October of 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Following the attacks, Israel’s security cabinet declared war against Hamas and commenced a military campaign. Since the commencement of these events, there have been additional active hostilities, including with Hezbollah in Lebanon, the Houthi movement which controls parts of Yemen, and with Iran. During November 2024, a ceasefire in Lebanon was declared. During January 2025, Israel and Hamas have agreed to a Gaza three-phase ceasefire agreement and partial hostage release, the first six-week phase of such ceasefire began on January 19, 2025. It is possible that these hostilities will escalate in the future into a greater regional conflict, and that additional terrorist organizations and countries will actively join the hostilities. The ongoing and revived hostilities in the region, could prevent or delay shipments of our products, harm our operations and product development and cause our sales to decrease. In the event that the hostilities disrupt the ongoing operation of our facilities or the airports and seaports on which we depend to import and export our supplies and products, our operations may be materially adversely affected. The conflict could cause situations where applicable foreign regulatory bodies, such as the FDA, could not be able to visit our third-party manufacturing facilities in Israel in order to review, causing delays. The war could also result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
Further, there have been travel advisories imposed as related to travel to Israel, and restriction on travel, or delays and disruptions as related to imports and exports may be imposed in the future. An inability to receive supplies and materials, shortages of materials or difficulties in procuring our materials, among others, may adversely impact our ability to commercialize and manufacture our product candidates and products in a timely manner. This could cause a number of delays and/or issues for our operations, including delay of the review of our product candidates by regulatory agencies, which in turn would have a material adverse impact on our ability to commercialize our product candidates. Although the resulting sanctions and any related market disruptions are impossible to predict, they could be substantial, particularly if current or new sanctions continue for an extended period of time or if geopolitical tensions result in expanded military operations on a global scale. See “Item 3. Key Information – D. Risk Factors – Risks Related to our Operations in Israel”.
We have limited business in Russia which pose some degree of sanctions risk that cannot be entirely eliminated.
We have a long-standing distribution contract with a Russian distributer which distributes our products in the country and other neighboring countries. Given the nature of our products and our end customers in Russia (typically physicians), our Russia-related business falls within a category of activities with respect to which the sanctions regimes have been historically more permissive and with respect to which there is a relatively lower incidence of sanctions exposure. Furthermore, we are committed, and take measures, to ensure compliance with any applicable Sanctions. However, the sanctions against Russia across various key jurisdictions, including the United States, are extremely dynamic and complex and usually apply on a strict liability basis and sometimes may expose non-U.S. parties to liability or imposition of market restrictions even in the absence of jurisdictional grounds or touchpoints with the country of the governmental authority imposing the sanctions. Accordingly, in the current environment, Russia-related dealings may entail some degree of exposure to sanctions risks that cannot be entirely eliminated.
We outsource almost all of the manufacturing of our products to a small number of manufacturing subcontractors. If our subcontractors’ operations are interrupted or if our orders exceed our subcontractors’ manufacturing capacity, we may not be able to deliver our products on time.
We outsource almost all of the manufacturing of our products to three subcontractors located in Israel, two of which we are substantially dependent on, while we manufacture our laser and intense pulsed light, or IPL, handpieces in-house in Israel. These subcontractors have limited manufacturing capacity that may be inadequate if our customers place orders for unexpectedly large quantities of our products. In addition, because our subcontractors are located in Israel, they on occasion may feel the impact of potential economic or political instability in the region, including the ongoing Israeli-Hamas war. If the operations of one or more of our subcontractors were halted or limited, even temporarily, or if they were unable or unwilling to fulfill large orders, we could experience business interruption, increased costs, damage to our reputation and loss of our customers. In addition, finding new subcontractors that meet our manufacturing requirements, comply with regulatory requirements, and are ISO certified could take several months.
Future changes to U.S. income tax or trade policies impacting multi-national companies could materially affect our financial condition and results of operations.
In recent years, the U.S. government has instituted or proposed changes to international trade policy through the renegotiation, and potential termination, of certain existing bilateral or multilateral trade agreements and treaties with, and the imposition of tariffs on a wide range of products and other goods from China and other countries. Given our contract manufacturing and logistic providers in those countries, policy or regulations changes in the United States or other countries present particular risks for us.
New or increased tariffs could adversely affect many of our products. There also are risks associated with retaliatory tariffs and resulting trade wars. We cannot predict future trade policy and regulations in the United States and other countries, the terms of any renegotiated trade agreements or treaties, or tariffs and their impact on our business. An escalated trade war could have a significant adverse effect on world trade and the world economy. To the extent that trade tariffs and other restrictions imposed by the United States or other countries increase the price of, or limit the amount of, our products or components or materials used in our products imported into the United States or other countries, or create adverse tax consequences, the sales, cost, or gross margin of our products may be adversely affected and the demand from our customers for products and services may be diminished. Uncertainty surrounding international trade policy and regulations as well as disputes and protectionist measures could also have an adverse effect on consumer confidence and spending. If we deem it necessary to alter all or a portion of our activities or operations in response to such policies, agreements, or tariffs, our capital and operating costs may increase.
Components used in our products are complex in design, and defects may not be discovered prior to shipment to customers, which could result in warranty obligations, reducing our revenue and increasing our costs.
In manufacturing our products, we and our subcontractors depend upon third-party suppliers for various components. Many of these components require a significant degree of technical expertise to produce. If our suppliers fail to produce components to specification, or if the suppliers, our subcontractors, or we, use defective materials or workmanship in the manufacturing process, the reliability and performance of our products will be compromised.
If our products contain defects that cannot be repaired easily and inexpensively, we may experience:
| • | loss of customer orders and delay in order fulfillment; |
| • | damage to our brand reputation; |
| • | increased cost of our warranty program due to product repair or replacement; |
| • | inability to attract new customers; |
| • | diversion of resources from our manufacturing and research and development departments into our service department; |
The occurrence of any one or more of the foregoing could materially harm our business, financial condition and results of operations.
We and our manufacturing subcontractors depend upon third-party suppliers, making us vulnerable to supply shortages, price fluctuations or other degradations in performance of these suppliers, which could harm our business and financial condition.
Many of the components that comprise our products are currently manufactured by a limited number of suppliers. Although each of our components can be obtained from more than one supplier, we do not have the ability to manufacture the components we outsource. Additionally, our subcontractors rely on a limited number of suppliers, or in some cases, one supplier, for some of the materials and components used in our products. If our subcontractors were to lose such suppliers, there can be no assurance that they will be able to identify or enter into agreements with alternative suppliers on a timely basis on acceptable terms, if at all, which could cause interruptions in their operations. If any of these third-party suppliers fails to adequately perform, our revenue and profitability could be adversely affected. A supply interruption or an increase in demand beyond current suppliers’ capabilities could harm our ability to manufacture our products until we identify and qualify a new source of supply, which could take several months.
There is a risk that our suppliers will not always act consistent with our best interests, and may not always supply goods that meet our requirements. Any interruption in the supply of components or materials, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers, which would have an adverse effect on our business, financial condition and results of operations.
Transitioning to a new supplier could be time-consuming and expensive, may result in interruptions in our operations and product delivery, could affect the performance specifications of our products or could require that we modify the design of certain product systems. If a change in manufacturer results in a significant change to any product, a new 510(k) clearance from the FDA or similar international regulatory authorization may be necessary before we implement the change, which could cause substantial delays.
Disruptions or interruptions to our suppliers could occur for many reasons, including fire, floods, hurricanes, typhoons, droughts, tsunamis, volcanoes, earthquakes, disease or other similar natural disasters, unplanned maintenance or other manufacturing problems, labor shortages, power outages or shortages, telecommunications failures, strikes, transportation interruption, government regulation, economic or political instability, terrorism or other extraordinary events, including epidemics and related travel restrictions. Such disruptions may continue over a sustained period and could cause direct injury or damage to our supplier’s employees and property with significant indirect consequences to us. Alternative facilities with sufficient capacity or capabilities may not be available, may cost substantially more or may take a significant time to start manufacturing, each of which could negatively affect our ability to fill customer orders and our business and financial performance.
There exists potential for misuse of our products, over which we have very little to no control, which could harm our reputation and our business.
In the United States, federal regulations allow us to sell our products to or on the order of “licensed practitioners”. The definition of “licensed practitioners” varies from state to state. As a result, depending on state law, our products may be purchased or operated by physicians or other licensed practitioners, including nurse practitioners, chiropractors and technicians. Outside the United States, many jurisdictions do not require specific qualifications or training for purchasers or operators of our products. Although we offer training on the use of our products, we do not supervise the treatments performed. Purchase and use of our products by non-physicians may result in product misuse. The potential misuse of our products by physicians and non-physicians may result in adverse treatment outcomes, which could harm our reputation and expose us to costly product liability litigation.
Our products include a limited time warranty which could result in substantial additional costs to us should we fail to monitor product quality effectively.
We generally provide a 12-month warranty on our products. After the warranty period, maintenance and support is provided on a service contract basis. If our products malfunction, warranty claims may become significant, which could cause a significant drain on our resources and materially adversely affect our results of operations.
We forecast sales to determine requirements for our products and if our forecasts are incorrect, we may experience either shipment delays or increased costs.
Our subcontractors keep limited materials and components on hand. To help them manage their manufacturing operations and minimize inventory costs, we forecast anticipated product orders to predict our inventory needs up to six months in advance and enter into purchase orders on the basis of these forecasts. If our business expands, our demand will increase and our suppliers may be unable to meet our demand. If we overestimate our requirements, our subcontractors will have excess inventory, and may transfer to us any increase in costs. If we underestimate our requirements, our subcontractors may have inadequate components and materials inventory, which could interrupt, delay or prevent delivery of our products to our customers. Any of these occurrences would negatively affect our financial performance and the level of satisfaction our customers have with our business.
Under applicable employment laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
We generally enter into non-competition agreements with our professional employees, in most cases within the framework of their employment agreements. These agreements prohibit our employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. Under applicable employment laws, we may be unable to enforce these agreements, in whole or in part, and it may be difficult for us to restrict our competitors from gaining the expertise our former employees gained while working for us. For example, Israeli courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, we may be unable to prevent our competitors from benefiting from the expertise of our former employees, which could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
The expense and potential unavailability of insurance coverage for our customers and the Company could adversely affect our ability to sell our products and our financial condition.
Some of our customers and prospective customers are required to maintain liability insurance to cover their operations and use of our products. Medical malpractice carriers are withdrawing coverage in certain states or substantially increasing premiums. If this trend continues or worsens, our customers may discontinue using our products and, industry-wide, potential customers may opt against purchasing light, laser or radio frequency-based products due to the cost or inability to procure insurance coverage.
Outbreaks of contagious disease or similar public health threats could materially and adversely affect our business, financial condition and results of operations.
Outbreaks of contagious disease or other adverse public health developments worldwide could have a material adverse effect on our business, financial condition and results of operations. Outbreaks of contagious disease or other adverse public health developments could affect our business in a number of ways, including but not limited to:
| • | Disruptions or restrictions on our employees’ ability to work effectively due to illness. |
| • | Temporary closures or disruptions at our facilities or the facilities of our customers or suppliers could reduce demand for our products or affect our ability to timely meet our customer’s orders and negatively impact our supply chain. |
| • | Outbreaks of contagious disease could cause delays or disruptions in our supply chain. |
| • | The failure of third parties on which we rely to meet their respective obligations to us, or significant disruptions in their ability to do so, which may be caused by their own financial or operational difficulties, could have an adverse impact on our business, financial condition or results of operations. |
| • | The impact of contagious disease or other adverse public health developments could also exacerbate other risks discussed elsewhere in this section of this report, any of which could have a material adverse effect on us. |
Global economic and social conditions may adversely affect our business, financial condition and results of operations.
Any negative conditions in the national and global economic environments may adversely affect our business, financial condition and results of operations. During uncertain economic times and in tight credit markets, many of our customers may experience financial difficulties or be unable or unwilling to borrow money to fund their operations, including obtaining credit lines for purchasing our products, and may delay or reduce purchases or reduce the extent of their operations. The market for aesthetic procedures and the market for our premium products can be particularly vulnerable to economic uncertainty, since the end-users of our products may decrease the demand for our products when they have less discretionary income or determine not to spend their discretionary income on aesthetic procedures. In addition, in many instances, the ability of our customers to purchase our products depends in part upon the availability of obtaining financing at acceptable interest rates.
These factors could result in reductions in revenues from sales of our products, longer sales cycles, difficulties in collection of accounts receivable, slower adoption of new technologies and increased price competition. Payment by our customers of our receivables is dependent upon the financial stability of the economies of certain countries. In light of the current economic state of many countries outside of the United States, we continue to monitor the creditworthiness of our customers because weakness in the end-user market could negatively affect the cash flows of our customers who could, in turn, delay paying their obligations to us. This would increase our credit risk exposure and cause delays in our recognition of revenues on current and future sales to these customers.
Exchange rate fluctuations may decrease our earnings if we are not able to hedge our currency exchange risks successfully.
A majority of our revenues and a substantial portion of our expenses are denominated in U.S. dollars. However, a portion of our revenues and a portion of our costs, including personnel and some marketing and facilities expenses, are incurred in NIS, Canadian dollars and Euros. Inflation in Israel or Europe may have the effect of increasing the U.S. dollar cost of our operations in that country. If the U.S. dollar declines in value in relation to one or more of these currencies, it will become more expensive for us to fund our operations in the countries that use those other currencies. To date, we have not found it necessary to hedge the risks associated with fluctuations in currency exchange rates. In the future, if we do not successfully engage in hedging transactions, our results of operations may be subject to losses from fluctuations in foreign currency exchange rates.
Cyber-attacks as well as improper disclosure or control of personal information could result in liability and harm our reputation, which could adversely affect our business and results of operations. We may face liability if we breach our obligations related to the protection, security, nondisclosure of confidential customer information or disclosure of sensitive data or fail or are perceived to fail to comply with applicable data protection laws and regulations, or consumer protection laws, regulations and standards.
Our business is heavily dependent on the security of our IT networks. Internal or external attacks on any of those could disrupt the normal operations of our engagements and impede our ability to provide services to our customers, thereby subjecting us to liability under our contracts. Additionally, our business involves the use, storage and transmission of information about our employees, our customers and clients of our customers. Attacks upon information technology systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. While we take measures to protect the security of, and unauthorized access to, our systems, as well as the privacy of personal and proprietary information, it is possible that our security controls of our systems, as well as other security practices we follow or those systems of our customers which we rely upon, may not prevent the improper access to or disclosure of personally identifiable or proprietary information. Such disclosure could harm our reputation and subject us to liability under our contracts and laws that protect personal data, resulting in increased costs or loss of revenue.
Further, the global data protection landscape is rapidly evolving, and we are or may become subject to numerous state, federal and foreign laws, requirements and regulations governing the collection, use, disclosure, retention, and security of personal data, such as information that we may collect about individuals in the U.S. and abroad. Implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future, and we cannot yet determine the impact future laws, regulations, standards, or perception of their requirements may have on our business. This evolution may create uncertainty in our business, affect our ability to operate in certain jurisdictions or to collect, store, transfer use and share personal information, necessitate the acceptance of more onerous obligations in our contracts, result in liability or impose additional costs on us. The cost of compliance with these laws, regulations and standards is high and is likely to increase in the future. Any failure or perceived failure by us to comply with federal, state or foreign laws or regulation, our internal policies and procedures or our contracts governing our processing of personal information could result in negative publicity, government investigations and enforcement actions, claims by third parties and damage to our reputation, any of which could have a material adverse effect on our operations, financial performance and business.
As our operations and business grow, we may become subject to or affected by new or additional data protection laws and regulations and face increased scrutiny or attention from regulatory authorities. We are subject to U.S. federal and state laws regarding data privacy and security including Section 5 of the Federal Trade Commission Act, or FTC Act, the California Consumer Privacy Act, or the CCPA and the California Privacy Rights Act, or the CPRA. Further, the Health Insurance Portability and Accountability Act of 1996, as amended, and regulations implemented thereunder, or HIPAA, imposes, among other things, certain standards relating to the privacy, security, transmission and breach reporting of individually identifiable health information. Even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Federal and state consumer protection laws are increasingly being applied by the FTC, and states’ attorneys general to regulate the collection, use, storage and disclosure of personal or personally identifiable information, through websites or otherwise, and to regulate the presentation of website content.
Certain states have also adopted comparable privacy and security laws and regulations, some of which may be more stringent than HIPAA. For example, the CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. Both the CCPA and CRPA are generally more stringent than similar U.S. federal regulations. In the event that we are subject to or affected by HIPAA, the CCPA, the CPRA or other domestic privacy and data protection laws, any liability from failure to comply with the requirements of these laws could adversely affect our financial condition.
We are also subject to foreign data privacy and security laws, including the Israeli Protection of Privacy Law of 1981 and the Privacy Protection Regulations (Data Security) 5777-2017, the European Union General Data Protection Regulation, or GDPR, and the United Kingdom GDPR, or UK GDPR. The GDPR went into effect in May 2018 and imposes strict requirements for processing the personal data of individuals within the European Economic Area, or the EEA. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and significant penalties for non-compliance, including potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. The UK GDPR mirrors the fines under the GDPR, e.g., fines up to the greater of €20 million (£17.5 million) or 4% of global turnover.
As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the standard contractual clauses cannot be used, and/or start taking enforcement action, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results.
Our failure to adhere to or successfully implement processes in response to changing regulatory requirements in this area could result in legal liability or impairment to our reputation in the marketplace, which could have a material adverse effect on our business, financial condition and results of operations.
In the course of providing services to our customers, we may have access to confidential customer information, including nonpublic personal data. We are bound by certain agreements to use and disclose this information in a manner consistent with the privacy standards under regulations applicable to our customers and are subject to numerous U.S. and foreign jurisdiction laws and regulations designed to protect this information, such as the GDPR and various U.S. federal and state laws governing the protection of health or other individually identifiable information. If any person, including a team member of ours, misappropriates customer confidential information, or if customer confidential information is inappropriately disclosed due to a security breach of our computer systems, system failures or otherwise, we may have substantial liabilities to our customers or our customers’ clients and may incur substantial liability and penalties in connection with any violation of applicable privacy laws and/or criminal prosecution. In addition, in the event of any breach or alleged breach of our confidentiality agreements with our customers, these customers may terminate their engagements with us or sue us for breach of contract, resulting in the associated loss of revenue and increased costs and damaged reputation. We may also be subject to civil or criminal liability if we are deemed to have violated applicable regulations. We cannot assure you that we will adequately address the risks created by the regulations to which we may be contractually obligated to abide.
Although we work to comply with applicable laws, regulations and standards, our contractual obligations and other legal obligations, these requirements are evolving and may be modified, interpreted and applied in an inconsistent manner from one jurisdiction to another, and may conflict with one another or other legal obligations with which we must comply. Any failure or perceived failure by us or our employees, representatives, contractors, consultants, collaborators, or other third parties to comply with such requirements or adequately address privacy and security concerns, even if unfounded, could result in additional cost and liability to us, damage our reputation, and adversely affect our business and results of operations.
We may become subject to numerous foreign, federal, and state healthcare statutes and regulations and our failure to comply could result in a material adverse effect to our business and operations.
Although none of our products or procedures using our products are currently covered by any state or federal government healthcare programs, or any private commercial payor, we may become subject to foreign, federal, and state laws intended to prevent healthcare fraud and abuse, including those that apply to all payors. These laws could include state anti-kickback and false claims laws, which may extend to services reimbursable by any payor, as well as state consumer protection laws. Although we currently are not subject to transparency laws, we may become subject to such laws in the future. Such laws could include requirements to disclose payments to certain healthcare professionals and healthcare entities or disclosures related to sales and marketing, or that could require healthcare professionals to provide notice to their patients of ownership or financial arrangements with manufacturers.
Efforts to ensure that our internal operations and business arrangements with third parties comply with future applicable healthcare laws and regulations may involve substantial costs. These laws and regulations, among other things, could constrain our business, marketing and other promotional activities by limiting the kinds of financial arrangements, including financing programs, we may have with physicians or other potential purchasers of our products. It is possible that governmental authorities may conclude that our business practices, including our arrangements with physicians, some of whom received stock options as compensation for services provided, as well as fees for marketing to other physicians, are subject to and do not comply with current or future statutes, regulations, agency guidance or case law involving applicable healthcare laws. If our current or future operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties which could adversely affect our ability to operate our business and pursue our strategy.
We are subject to anti-bribery, and corruption and anti-money laundering laws, including the U.S. Foreign Corrupt Practices Act, as well as export control laws, customs laws, sanctions laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, results of operations and financial condition.
As we continue to grow our international presence and global operations, we will be increasingly exposed to trade and economic sanctions and other restrictions imposed by the United States, the European Union, the State of Israel and other governments and organizations. The U.S. Departments of Justice, Commerce, State and Treasury and other federal agencies and authorities have a broad range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of economic sanctions laws, export control laws, the U.S. Foreign Corrupt Practices Act, or the FCPA, and other federal statutes and regulations, including those established by the Office of Foreign Assets Control, or OFAC. In addition, the U.K. Bribery Act of 2010, or the Bribery Act, prohibits both domestic and international bribery, as well as bribery across both private and public sectors. An organization that “fails to prevent bribery” by anyone associated with the organization can be charged under the Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. Under these laws and regulations, as well as other anti-corruption laws, anti-money laundering laws, export control laws, customs laws, sanctions laws and other laws governing our operations, various government agencies may require export licenses, may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries or with sanctioned persons or entities and modifications to compliance programs, which may increase compliance costs, and may subject us to fines, penalties and other sanctions. A violation of these laws or regulations would negatively affect our business, financial condition and results of operations.
We have implemented policies and procedures designed to ensure compliance by us and our directors, officers, employees, representatives, consultants and agents with the FCPA, OFAC restrictions, the Bribery Act and other export control, anti-corruption, anti-money-laundering and anti-terrorism laws and regulations. We cannot assure you, however, that our policies and procedures are or will be sufficient or that directors, officers, employees, representatives, consultants and agents have not engaged and will not engage in conduct for which we may be held responsible, nor can we assure you that our business partners have not engaged and will not engage in conduct that could materially affect their ability to perform their contractual obligations to us or even result in our being held liable for such conduct. Violations of the FCPA, OFAC restrictions, the Bribery Act or other export control, anti-corruption, anti-money laundering and anti-terrorism laws or regulations may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could have a material adverse effect on our business, financial condition and results of operations.
Our operating costs and business operations could be adversely affected by climate-related events and increasing regulatory requirements and security.
The effects of climate change (such as drought, flooding, heat waves, wildfires, increased storm severity, and sea level rise, etc.) could affect our ability to continue our operations and cause delays in our product development, manufacturing and shipment, all of which could cause reputational harm or otherwise have an adverse effect on our business and operating results. In addition, the impacts of climate change on the global economy and our industry are rapidly evolving. Changing market dynamics, global policy developments and the increasing frequency and impact of extreme weather events on critical infrastructure across different countries could have the potential to disrupt our business, the business of our third-party suppliers and the business of our customers, and may cause us to experience higher attrition, losses and additional costs to maintain or resume operations. We also expect to face increasing regulatory requirements and regulatory scrutiny related to climate matters, resulting in higher associated compliance costs. Failure to uphold, meet or make timely forward progress against our public commitments and goals related to climate action could adversely affect our reputation with suppliers and customers, financial performance or the ability to recruit and retain talent.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights, our competitive position could be harmed. Our success and ability to compete depends in large part upon our ability to protect our proprietary technology.
Our success and ability to compete depends in large part upon our ability to protect our proprietary technology. We rely primarily upon a combination of patents and trademarks, as well as nondisclosure, confidentiality and other contractual agreements to protect the intellectual property related to our brands, products and other proprietary technologies.
We generally apply for patents only in those countries where we intend to make, have made, use, offer for sale, or sell products. To date, we have issued patents in the United States, which we consider to be our main target market, and one issued patent in South Korea. Most of our revenues for the years ended December 31, 2024, 2023 and 2022 were derived from the United States where we have patent protection. We do not seek protection in all countries where we sell products and we may not accurately predict all the countries where patent protection would ultimately be desirable. At this time, the countries in which we have not sought patent protection, but intend to offer our products for sale, are not our main target markets. We acknowledge that competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories in which we do not have patent protection. Such activity may prevent us from protecting our proprietary technology, and thus, may harm our competitive position.
Our patent portfolio consists of fifteen issued U.S. patents, one issued Korean patent and eleven pending patent applications in the United States relating to our technology and products Our pending and future patent applications may not issue as patents or, even if issued, may not issue in a form that will be advantageous to us. Any issued patents may be challenged, invalidated or legally circumvented by third parties. We cannot be certain that our patents will be upheld as valid, proven enforceable or prevent the development of competitive products. Other companies may also design around technologies we have patented. Third parties may have blocking patents that could prevent us from marketing our products or practicing our own patented technology. In addition, competitors could purchase one of our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, design around our protected technology, or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property is not adequately protected against competitors’ products and methods, our competitive position could be adversely affected, as could our business and financial results.
The U.S. Supreme Court and the U.S. Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. Similarly, foreign courts have made, and will likely continue to make, changes in how the patent laws in their respective jurisdictions are interpreted. We cannot predict future changes in the interpretation of patent laws or changes to patent laws that might be enacted into law by U.S. and foreign legislative bodies.
We rely on a combination of patent and other intellectual property laws and confidentiality, non-disclosure and assignment of inventions agreements, as appropriate, with our employees and consultants, to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not be adequate to protect our technology from unauthorized disclosure, third-party infringement or misappropriation. Parties may breach these agreements, and we may not have adequate remedies for any breach. Also, the laws of certain countries in which we develop, manufacture or sell our products may not protect our intellectual property rights to the same extent as the laws of the United States or Israel.
The aesthetics industry is highly competitive and marked by frequent litigation. New patent applications may be pending or may be filed in the future by third parties covering technology that we currently use or may ultimately use. Third parties have claimed, and may in the future claim, that our current or future products infringe their patent or other intellectual property rights and may seek to prevent, limit or interfere with our ability to make, use, sell or import our products. Moreover, if such a claim were to be decided adversely to us or if we settled such a claim on adverse terms, we could be forced to pay substantial damages, to license the technology in question at high rates or to redesign or modify our products so as to avoid any infringement. Any of those results could adversely affect our sales, margins and results of operations.
If it appears necessary or desirable, we may try to obtain licenses for those patents or intellectual property rights that we are allegedly infringing, may infringe, or desire to use. Although holders of these types of intellectual property rights commonly offer these licenses, we cannot assure you that licenses will be offered or that the terms of any offered licenses will be acceptable to us. Our failure to obtain a license for key intellectual property rights from a third party for technology used by us could cause us to incur substantial liabilities and to suspend the manufacturing and selling of products utilizing the technology.
Alternatively, we could be required to expend significant resources to develop non-infringing technology. We cannot assure you that we would be successful in developing non-infringing technology.
Third parties have and may in the future commence litigation against us claiming that our products infringe upon their patents or other intellectual property rights.
From time to time, we may be party to, or threatened with, litigation or other proceedings with third parties, including non-practicing entities, who allege that our products, components of our products, services, and/or proprietary technologies infringe, misappropriate or otherwise violate their intellectual property rights. The types of situations in which we may become a party to such litigation or proceedings include:
| • | we or our collaborators may initiate litigation or other proceedings against third parties seeking to invalidate the patents held by those third parties or to obtain a judgment that our products or processes do not infringe those third parties’ patents; |
| • | we or our collaborators may participate at substantial cost in International Trade Commission proceedings to abate importation of products that would compete unfairly with our products; |
| • | if our competitors file patent applications that claim technology also claimed by us, we may be required to participate in interference, derivation or opposition proceedings to determine the priority of invention, which could jeopardize our patent rights and potentially provide a third party with a dominant patent position; |
| • | if third parties initiate litigation claiming that our processes or products infringe their patent or other intellectual property rights, we and our collaborators will need to defend against such proceedings; |
| • | if third parties initiate litigation or other proceedings seeking to invalidate patents owned by or licensed to us or to obtain a declaratory judgment that their product, service, or technology does not infringe our patents or patents licensed to us, we will need to defend against such proceedings; |
| • | we may be subject to ownership disputes relating to intellectual property, including disputes arising from conflicting obligations of consultants or others who are involved in developing our products; and |
| • | if a license to necessary technology is terminated, the licensor may initiate litigation claiming that our processes or products infringe or misappropriate its patent or other intellectual property rights and/or that we breached our obligations under the license agreement, and we and our collaborators would need to defend against such proceedings. |
These lawsuits and proceedings, regardless of merit, are time-consuming and expensive to initiate, maintain, defend or settle, and could divert the time and attention of managerial and technical personnel, which could materially adversely affect our business. Any such claim could also force us to do one or more of the following:
| • | incur substantial monetary liability for infringement or other violations of intellectual property rights, which we may have to pay if a court decides that the product, service, or technology at issue infringes or violates the third party’s rights, and if the court finds that the infringement was willful, we could be ordered to pay treble damages and the third party’s attorneys’ fees; |
| • | pay substantial damages to our customers or end users to discontinue use or replace infringing technology with non-infringing technology; |
| • | stop manufacturing, offering for sale, selling, using, importing, exporting or licensing the product or technology incorporating the allegedly infringing technology or stop incorporating the allegedly infringing technology into such product, service, or technology; |
| • | obtain from the owner of the infringed intellectual property right a license, which may require us to pay substantial upfront fees or royalties to sell or use the relevant technology and which may not be available on commercially reasonable terms, or at all; |
| • | redesign our products, services, and technology so they do not infringe or violate the third party’s intellectual property rights, which may not be possible or may require substantial monetary expenditures and time; |
| • | enter into cross-licenses with our competitors, which could weaken our overall intellectual property position; |
| • | lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property against others; |
| • | find alternative suppliers for non-infringing products and technologies, which could be costly and create significant delay; or |
| • | relinquish rights associated with one or more of our patent claims, if our claims are held invalid or otherwise unenforceable. |
On February 14, 2024, a purported shareholder of the Company filed a putative shareholder class action (the “Securities Class Action”) in the United States District Court for the Central District of California, captioned Cement Masons and Plasterers Local No. 502 Pension Fund v. InMode Ltd. et al., Case No. 2:24-cv-01219, against the Company and certain of its officers and directors. The complaint alleges claims under Sections 10(b) and 20(a) of the Exchange Act based on allegedly false or misleading statements related to the Company’s business, operations, sales practices and financial outlook. The lawsuit seeks unspecified damages and other relief. On April 16, 2024, multiple shareholders moved to be appointed lead plaintiff. On December 4, 2024, the Court entered an order appointing a group of shareholder funds as the lead plaintiffs. On January 31, 2025, the lead plaintiffs filed an amended complaint. The amended complaint purportedly brings claims on behalf of purchasers of the Company’s common stock between February 18, 2020 and December 6, 2023, inclusive. The Company’s deadline to respond to the amended complaint is April 1, 2025. The Securities Class Action is in the preliminary stages, and the Company has not yet responded to the amended complaint. As of the date of this filing, the Company is unable to estimate a range of loss, if any, that could result were there to be an adverse final decision in the Securities Class Action, and an estimated liability has not been recorded in the Company’s financial statements. The defendants intend to deny the allegations of wrongdoing and vigorously defend against the claims in the Securities Class Action.
Although we may try to resolve any potential future claims or actions, we may not be able to do so on reasonable terms, if at all. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate, and could divert management’s attention from our core business. If we lose this kind of litigation, a court could require us to pay substantial damages and could prohibit us from using technologies essential to our products, either of which would have a material adverse effect on our business, results of operations and financial condition.
We may become involved in litigation to protect the trademark rights associated with the Company name or the names of our products. If we have to change the name of the Company or products, we may experience a loss in goodwill associated with our brand name, customer confusion and a reduction in sales.
Some of our competitors may be able to sustain the costs of complex intellectual property litigation more effectively than we can because they have substantially greater resources. In addition, intellectual property litigation, regardless of its outcome, may cause negative publicity, adversely impact prospective customers, cause product shipment delays, or prohibit us from manufacturing, marketing or otherwise commercializing our products, services and technology. Any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operation, financial condition or cash flows.
In addition, we may indemnify our customers and distributors against claims relating to the infringement of intellectual property rights of third parties related to our products. Third parties may assert infringement claims against our customers or distributors. These claims may require us to initiate or defend protracted and costly litigation on behalf of our customers or distributors, regardless of the merits of these claims. If any of these claims succeed, we may be forced to pay damages on behalf of our customers, suppliers or distributors, or may be required to obtain licenses for the products or services they use. If we cannot obtain all necessary licenses on commercially reasonable terms, our customers may be forced to stop using our products or services.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments, which could have a material adverse effect on the price of our ordinary shares. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our ordinary shares.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented, declared generic or determined to be infringing on other marks. We may not be able to protect our rights in these trademarks and trade names, which we need in order to build name recognition with potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of their former employers or other third parties.
We do and may employ individuals who were previously employed at universities or other pharmaceutical or medical device companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, and we are not currently subject to any significant claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties, we may in the future be subject to such claims. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because even granted intellectual property rights have limitations, and may not adequately protect our business, provide a barrier to entry against our competitors or potential competitors or permit us to maintain our competitive advantage. Moreover, if a third party has intellectual property rights that cover the practice of our technology, we may not be able to fully exercise or extract value from our intellectual property rights. The following examples are illustrative:
| • | others may be able to develop and/or practice technology that is similar to our technology or aspects of our technology, but that are not covered by the claims of the patents that we own or control, assuming such patents have issued or do issue; |
| • | we or any future strategic partners might not have been the first to conceive or reduce to practice the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed; |
| • | we or any future strategic partners might not have been the first to file patent applications covering certain of our inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| • | it is possible that our pending patent applications will not lead to issued patents; |
| • | issued patents that we own may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; |
| • | our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| • | third parties performing manufacturing or testing for us using our products or technologies could use the intellectual property of others without obtaining a proper license; |
| • | parties may assert an ownership interest in our intellectual property and, if successful, such disputes may preclude us from exercising exclusive rights over that intellectual property; |
| • | we may not develop or in-license additional proprietary technologies that are patentable; |
| • | we may not be able to obtain and maintain necessary licenses on commercially reasonable terms, or at all; and |
| • | the patents of others may have an adverse effect on our business. |
Should any of these events occur, they could significantly harm our business and results of operations.
Risks Related to Government Regulation
Our business is subject to extensive and continuing regulatory compliance obligations. If we fail to obtain and maintain necessary FDA clearances for our products, if clearances for future products and proposed indications are delayed or not issued, if we or any of our third-party suppliers or manufacturers fail to comply with applicable regulatory requirements, or if there are regulatory changes, our commercial operations could be harmed.
Our products are medical devices subject to extensive regulation by the applicable regulatory authorities where our products are or will be sold prior to their marketing for commercial use. In the United States, our products are subject to extensive regulation by the FDA for developing, testing, manufacturing, labeling, sale, marketing, advertising, promotion, distribution, import, export, shipping, establishment registration and device listing, inspections and audits, record keeping, recalls and field safety corrective actions and post-market surveillance, including reporting of certain events.
Before a new medical device, or a new use of, or claim for, an existing product can be marketed in the United States, it must first receive marketing authorization from the FDA unless it is exempt. The FDA marketing authorizations include a 510(k) clearance or premarket approval. A relatively small number of devices may be exempt from 510(k) clearance or may receive marketing authorization through the de novo classification pathway. These processes can be expensive and lengthy. The FDA’s 510(k) clearance process usually takes from 3 to 12 months, but it can last longer. The process of obtaining premarket approval is much more costly and uncertain than the 510(k)-clearance process and it generally takes from one to three years, or even longer, from the time the application is filed with the FDA. Our future products and enhancements or changes to products may require new 510(k) clearance or premarket approval from the FDA. All products that we currently market in the United States that require an FDA marketing authorization have received 510(k) clearance for the uses for which they are marketed.
Medical devices may be marketed only for the indications for which they are approved or cleared. We have obtained 510(k) clearance for the current treatments for which we offer our products. However, our clearances can be revoked under certain circumstances. If the FDA disagrees with us concerning the scope or applicability of a clearance or exemption with respect to a device, we may be required to change our promotional and/or labeling materials and/or stop marketing that device. Changes or modifications to an FDA-cleared device that could significantly affect its safety or effectiveness or that constitute a major change or modification in its intended use would require a new 510(k) clearance or possibly premarket approval. We may not be able to obtain additional 510(k) clearances or premarket approvals for new products or for modifications to, or additional indications for, our existing products in a timely fashion, or at all. Delays in obtaining future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our revenue and future profitability.
We have made modifications to our devices in the past and may make additional modifications in the future that we believe do not or will not require additional clearances or approvals. If the FDA disagrees, and requires new clearances or approvals for the modifications, we may be required to recall and to stop marketing the modified devices. We also are subject to the FDA’s Medical Device Reporting regulations, which require us to report to the FDA if our products cause or contribute to a death or serious injury, or malfunction in a way that would likely cause or contribute to a death or serious injury.
The FDA or the applicable foreign regulatory bodies can delay, limit or deny clearance or approval of a device for many reasons, including:
| • | our inability to demonstrate to the satisfaction of the FDA or the applicable foreign regulatory bodies that our products are safe or effective for their intended uses; |
| • | the disagreement of the FDA or the applicable foreign regulatory bodies with the design or implementation of our clinical trials or the interpretation of data from pre-clinical studies or clinical trials; |
| • | serious and unexpected adverse device effects experienced by participants in our clinical trials; |
| • | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; |
| • | our inability to demonstrate that the clinical and other benefits of the device outweigh the risks; |
| • | the manufacturing process or facilities we use may not meet applicable requirements; and |
| • | the potential for approval policies or regulations of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval. |
In addition, the FDA or applicable foreign regulatory bodies may change their clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain new clearances, increase the costs of compliance or restrict our ability to maintain our current clearances. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the Food, Drug and Cosmetic Act of 1938, as amended (the “FDCA”). Among other things, the FDA announced that it plans to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals include possible plans to sunset certain older devices that were used as predicates under the 510(k)-clearance pathway, and to publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. The FDA also announced that it intends to finalize guidance to establish a premarket review pathway for “manufacturers of certain well-understood device types” as an alternative to the 510(k) clearance pathway and that such premarket review pathway would allow manufacturers to rely on objective safety and performance criteria recognized by the FDA to demonstrate substantial equivalence, obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. These proposals have not yet been finalized or adopted, and the FDA announced that it would seek public feedback prior to publication of any such proposals, and may work with Congress to implement such proposals through legislation. Accordingly, it is unclear the extent to which any proposals, if adopted, could impose additional regulatory requirements on us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance, or restrict our ability to maintain our current clearances, or otherwise create competition that may negatively affect our business.
Additionally regulatory clearances or approvals to market a product can contain limitations on the indicated uses for such product. Product clearances and approvals can be withdrawn due to failure to comply with regulatory standards or the occurrence of unforeseen problems following initial clearance or approval. FDA regulations depend heavily on administrative interpretation, and there can be no assurance that future interpretations made by the FDA or other regulatory bodies will not adversely affect our operations. We and our manufacturers may be inspected by the FDA from time to time to determine whether we or our manufacturers are in compliance with applicable laws, including the cGMP regulations set forth in the FDA’s Quality System Regulation/Medical Device Current Good Manufacturing Practices, or QSR, including those relating to specifications, development, documentation, validation, testing, quality control and product labeling. A determination that we are in violation of FDA or other applicable foreign regulations or any of our product clearances or approvals could lead to imposition of civil penalties, including fines, product recalls or product seizures and, in certain cases, criminal sanctions.
The use, misuse or off-label use of our products may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
The use, misuse or off-label use of our products may harm our reputation or the image of our products in the marketplace, result in injuries that lead to product liability suits, which could be costly to our business, or result in legal sanctions if we are deemed or alleged to have engaged in off-label promotion.
A medical device may be authorized by the FDA for marketing through several regulatory mechanisms. The FDA classifies medical devices as Class I, Class II, or Class III, in increasing order of risk. Most of our products are Class I or Class II medical devices. As such, they are either exempt from premarketing authorization requirements or are subject to the 510(k)-clearance process, and all are listed with the FDA pursuant to FDA’s medical device listing requirements.
Under FDA regulations, for each of our products we must only use labeling, including advertising and promotional materials, that is consistent with the specific indication(s) for use included in the FDA exemption regulation, clearance, or approval, which is applicable to the specific product. If the FDA or other authorities determine that our promotional or training materials constitute the unlawful promotion of an off-label use, they could request that we modify our training or promotional materials and/or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, civil money penalties, seizure, injunction or criminal fines and penalties. Other federal, state or foreign governmental authorities might also take action if they consider our promotion or training materials to constitute promotion of an uncleared or unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement, or exclusion from participation in federal health programs. In each event, our reputation could be damaged and the use of our products in the marketplace could be impaired, or our business could face significant hardship.
In addition, there may be increased risk of injury if physicians or others attempt to use our products off-label. The FDA does not restrict or regulate a physician’s use of a medical product within the practice of medicine, and we cannot prevent a physician from using our products for an off-label use. The use of our products for indications other than those for which our products have been approved or cleared by the FDA may not effectively treat the conditions not referenced in product indications, which could harm our reputation in the marketplace among physicians and patients. Physicians may also misuse our products or use improper techniques if they are not adequately trained in the particular use, potentially leading to injury and an increased risk of product liability. Product liability claims are expensive to defend and could divert management’s attention from our primary business and result in substantial damage awards against us. Any of these events could harm our business, results of operations and financial condition.
We are subject to ongoing regulatory obligations and a failure to comply with post-marketing regulatory requirements could subject us to enforcement actions, including substantial penalties, and might require us to recall or withdraw a product from the market.
Even after we have obtained the proper regulatory clearance or approval to market a product, we have ongoing responsibilities under FDA regulations and applicable foreign laws and regulations. The FDA, state and foreign regulatory authorities have broad enforcement powers. The regulations to which we are subject are complex and have become more stringent over time. Regulatory changes could result in restrictions on our ability to continue or expand our operations, higher than anticipated costs, or lower than anticipated sales.
Our products and/or their use are also subject to state regulations and additional regulations in other foreign jurisdictions outside of the United States, which may change at any time. We cannot predict the impact or effect of future legislation or regulations and any changes in regulations may impede sales.
Furthermore, the FDA and state authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
| • | warning letters or untitled letters, fines, injunctions, consent decrees and civil penalties; |
| • | repair, replacement, refunds, recalls, termination of distribution, administrative detention or seizure of our products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing our requests for 510(k) clearance or premarket approval of new products, new intended uses, or modifications to existing products; |
| • | withdrawing 510(k) clearances or premarket approvals or foreign regulatory approvals that have already been granted, resulting in prohibitions on sales of our products; and |
The occurrence of any of these events could harm our business, financial condition and results of operations.
If we or our subcontractors fail to comply with federal and state regulation, including the FDA’s Quality System Regulation/Medical Device Good Manufacturing Practices and performance standards, our or our subcontractors’ manufacturing operations could be halted, and our business would suffer.
We and our subcontractors currently are required to demonstrate and maintain compliance with the QSR. The QSR is a complex regulatory scheme that covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. Because our products use optical energy, including lasers, our products also are covered by a performance standard for lasers set forth in FDA regulations. The laser performance standard imposes specific record-keeping, reporting, product testing and product labeling requirements. These requirements include affixing warning labels to laser products, as well as incorporating certain safety features in the design of laser products. The FDA enforces the QSR and laser performance standards through periodic announced or unannounced inspections. We and our subcontractors are subject to such inspections. Although we place our own quality control employee at each of our subcontractor’s facilities, we do not have complete control over our subcontractor’s compliance with these standards.
Any failure by us or our subcontractors to take satisfactory corrective action in response to an adverse QSR inspection or to comply with applicable laser performance standards could result in enforcement actions against us or our subcontractors, including warning letters or untitled letters; fines, injunctions or civil penalties; suspension or withdrawal of approvals or clearances; seizures or recalls of our products; total or partial suspension of production or distribution; administrative or judicially imposed sanctions; the FDA’s refusal to grant pending or future clearances or approvals for our products; clinical holds; refusal to permit the import or export of our products; and criminal prosecution of us or our employees. Any of these actions could significantly and negatively impact the supply of our products, and could cause our sales and business to suffer. In addition, we are subject to standards imposed on our activities outside of the United States, such as obtaining CE mark certification in Europe (by our notified body DEKRA) and the Standards Institution of Israel (imposed on our activities in Israel), and failure to comply with such standards could adversely impact our business.
Our products may cause or contribute to adverse medical events or other undesirable side effects that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
We are subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of the product. If we fail to comply with our reporting obligations, the FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device clearance, seizure of our products or delay in clearance of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. Product defects or other errors may occur in the future. Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals or clearances for the product before we may market or distribute the corrected product. Seeking such approvals or clearances may delay our ability to replace the recalled products in a timely manner. Moreover, if we do not adequately address problems associated with our products, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales.
We may be unable to obtain or maintain international regulatory qualifications or approvals for our current or future products and indications, which could harm our business. Additionally, obtaining and maintaining regulatory approval in one jurisdiction does not mean we will be successful in obtaining regulatory approvals for our products in other jurisdictions.
Sales of our products outside the United States are subject to foreign regulatory requirements that vary widely from country to country, including some regulatory requirements that we may not be fully aware of, or that may change in ways that affect our ability to sell our products in those jurisdictions. Complying with international regulatory requirements can be an expensive and time-consuming process and approval is not certain. The regulatory process in foreign jurisdictions includes all the risks associated with obtaining FDA clearance, as well as additional risks not present in the FDA process. For example, the time required to obtain foreign clearance or approvals may be longer than that required for FDA clearance or approvals, and requirements for such clearances or approvals may significantly differ from FDA requirements, adding costs and variability. Foreign regulatory authorities may not approve our product for the same uses cleared by the FDA. Although we have obtained regulatory clearances to sell our products in the European Union and other countries outside the United States, we may be unable to maintain regulatory qualifications, clearances or approvals in these countries or to obtain approvals in other countries. We also may incur significant costs in attempting to obtain and in maintaining foreign regulatory approvals, clearances or qualifications.
If we experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the United States, or if we fail to receive those qualifications, clearances or approvals, we may be unable to market some of our products or enhancements in certain international markets effectively, or at all. Since the enactment of the Israeli Medical Equipment Law, 2012, or the Medical Equipment Law, the manufacturing and marketing of medical and certain aesthetic devices, including our products, in Israel requires registration with the Israeli Ministry of Health. The Medical Equipment Law offers a fast-track registration process for devices that received approval from certain non-Israeli regulatory agencies, including FDA clearance or CE marks. We have taken advantage of such fast-track registration process in the past. If we are unable to obtain and maintain the necessary registration for any of our products in Israel, we may have to move the manufacturing of such unregistered products to a location outside of Israel and stop selling these products in Israel until the products are registered. We may also suffer harm to our reputation as a result.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new devices to be reviewed and/or cleared by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities.
New regulations may limit our ability to sell to non-physicians in the future.
Currently, we sell our products solely to physicians. However, where permitted under applicable laws, we intend to introduce certain of our products in the developing medical spa market, where aesthetic procedures are being performed at dedicated facilities by non-physicians under physician supervision. U.S., state and international regulations could change at any time, disallowing sales of our products to aestheticians, and/or limiting the ability of aestheticians and non-physicians to operate our products. We cannot predict the impact or effect of changes in U.S., state or international laws or regulations.
Risks Related to Our Ordinary Shares
The price of our ordinary shares may be volatile, and you may lose all or part of your investment.
The price of our ordinary shares has historically fluctuated and has experienced volatility in the past. The market price for our ordinary shares may fluctuate as a result of a number of factors, many of which are beyond our control, including:
| • | fluctuations in our operating results or the operating results of our competitors; |
| • | changes in the estimates of the future size and growth rate of our market opportunities; |
| • | changes in the general economic, industry and market conditions; |
| • | success of competitive technologies and procedures; |
| • | recruitment or departure of key personnel; |
| • | the announcement of new products or enhancements by us or our competitors; |
| • | the commencement or outcome of litigation against us, or involving our general industry or both; |
| • | new laws or regulations or new interpretations of existing laws or regulations applicable to our business; |
| • | changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates; |
| • | developments in our industry, including the announcement of significant new technologies, procedures or acquisitions by us or our competitors; |
| • | actual or expected sales of our ordinary shares by the holders of our ordinary shares; and |
| • | the trading volume of our ordinary shares. |
In addition, the stock prices of companies in the medical device industry have experienced wide fluctuations that often have been unrelated to the operating performance of those companies. These fluctuations may be attributed, among other reasons, to the general global economic environment and the instability in markets. These factors and price fluctuations may materially and adversely affect the market price of our ordinary shares.
Future sales of our ordinary shares could reduce the price of our ordinary shares.
Sales by shareholders of substantial amounts of our ordinary shares, or the perception that these sales may occur in the future, could materially and adversely affect the market price of our ordinary shares and could impair our ability to raise capital through the sale of additional equity securities and our ability to acquire other companies by using our ordinary shares as consideration.
As of December 31, 2024, there are 69,558,670 ordinary shares outstanding (not including a total of 18,025,003 ordinary shares held at that date as treasury shares). Sales of these shares, or the perception that these sales may occur in the future, into the market could cause the market price of our ordinary shares to drop significantly. In addition, we have registered all ordinary shares that we may issue under our equity compensation plans, and, as such, these shares can be freely sold in the public market upon issuance.
We have not paid dividends in the past and may not pay dividends in the future, and any return on investment may be limited to the value of our ordinary shares.
We have never declared or paid cash dividends on our ordinary shares and may not distribute cash or other dividends on our ordinary shares in the foreseeable future. The distribution of dividends on our ordinary shares will depend on our earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. We may only distribute dividends out of “profits,” as defined by the Companies Law and provided that the distribution is not reasonably expected to impair our ability to fulfill our outstanding and anticipated obligations as they become due. If we do not distribute dividends, our ordinary shares may be less valuable because a return on your investment will only occur if the price of our ordinary shares appreciates.
We incur significant costs operating as a public company in the United States, and our management is required to devote substantial time to compliance matters.
As a public company whose ordinary shares are listed in the United States, we are subject to an extensive regulatory regime, requiring us, among other things, to maintain various internal controls and facilities and to prepare and file periodic and current reports and statements. Complying with these requirements is costly and time consuming. In the event that we are unable to demonstrate ongoing compliance with our obligations as a public company, or are unable to produce timely or accurate financial statements, we may be subject to sanctions or investigations by regulatory authorities and investors may lose confidence in our operating results, and the price of our ordinary shares could decline. As of December 31, 2020, we became a “large accelerated filer” beginning from December 31, 2020. We have incurred significant management time and cost complying with the more stringent reporting requirements applicable to “accelerated filers,” including complying with auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002.
We are subject to legal proceedings that may adversely affect our business, financial condition or results of operations.
We are subject to various litigation claims and legal proceedings including securities class actions and potential derivative claims and proceedings based thereon, personal injury, customer contract, environmental and employment claims. Certain of these lawsuits or potential future lawsuits, if decided adversely to us or settled by us, may result in liability and expense material to our consolidated financial condition and consolidated results of operations.
We may be subject to shareholder activism, including proposals or demands by our shareholders to effect changes in our governance, corporate structure or business strategy.
Activist shareholders may seek to influence or control decisions regarding matters such as our operations, distributions, management, compensation or other key aspects of our business or operations. Such activism could result in significant costs and management distraction, diverting resources away from our strategic priorities. Shareholder activism could give rise to perceived uncertainties as to our future prospects and adversely affect our business, operations, financial condition and stock price. Additionally, increased activist activity may lead to volatility in our stock price and potentially adversely impact our relationship with investors, customers, employees, and other stakeholders.
U.S. investors in the Company could suffer adverse tax consequences if we are characterized as a passive foreign investment company.
We believe that we were not a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for our taxable year ended December 31, 2024, and we do not expect to be classified as a PFIC for the current year ending December 31, 2025 or the foreseeable future. However, the determination of whether we are a PFIC is a factual determination made annually based on all the facts and circumstances and thus is subject to change. The relevant rules for determining whether or not we are a PFIC as applied to our business are not entirely clear and certain aspects of the relevant tests will be outside our control. Therefore, no assurance can be given that we will not be a PFIC for any taxable year.
If we are determined to be a PFIC at any time during which a U.S. Holder (as defined in “Item 10E. Additional Information–Taxation–Material U.S. Federal Income Tax Considerations to U.S. Holders”) holds our shares, such U.S. Holder may be subject to materially adverse tax consequences, including additional U.S. federal income tax liability and tax filing obligations. See “Item 10E. Additional Information–Taxation–Material U.S. Federal Income Tax Considerations to U.S. Holders–Passive Foreign Investment Company Considerations.” U.S. Holders are strongly urged to consult their tax advisors as to whether or not we will be a PFIC.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our ordinary shares.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports. As a “large accelerated filer” we are responsible for establishing and maintaining internal controls and procedures that will allow our management to report on, and our independent registered public accounting firm to attest to, our internal controls over financial reporting under Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404. Although our independent registered public accounting firm is required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002 and our management is required to report on our internal controls over financial reporting under Section 404, any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us, as and when required, conducted in connection with Section 404 or any subsequent testing by our independent registered public accounting firm, as and when required, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our ordinary shares.
We are a “foreign private issuer” and have disclosure obligations that are different from those of U.S. domestic reporting companies.
We are a foreign private issuer and are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. For example, we are not required to issue quarterly reports or proxy statements that comply with the requirements applicable to U.S. domestic reporting companies. Furthermore, although under regulations promulgated under the Companies Law, as an Israeli public company listed on the Nasdaq, we are required to disclose the compensation of our five most highly compensated officers on an individual basis, this disclosure will not be as extensive as that required of U.S. domestic reporting companies. We also have four months after the end of each fiscal year to file our annual report with the SEC and are not required to file current reports as frequently or promptly as U.S. domestic reporting companies. Furthermore, our officers, directors and principal shareholders are exempt from the requirements to report transactions and short-swing profit recovery required by Section 16 of the Exchange Act. Also, as a “foreign private issuer,” we are not subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act. Even though we may voluntarily elect to comply with Regulation FD and have done so in the past, these exemptions and leniencies reduce the frequency and scope of information and protections available to you in comparison to those applicable to a U.S. domestic reporting company.
We would lose our foreign private issuer status if, as of June 30 in any calendar year, a majority of our outstanding shares are held of record by U.S. residents and a majority of our directors or executive officers are U.S. citizens or residents or we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we lose our foreign private issuer status, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we would lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
As a “foreign private issuer,” we are permitted to follow certain home country corporate governance practices instead of otherwise applicable SEC and Nasdaq requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.
As a “foreign private issuer,” we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the listing rules of Nasdaq for domestic U.S. issuers. For instance, we follow our home country law instead of the listing rules of Nasdaq that require that we obtain shareholder approval for certain dilutive events, such as the establishment or amendment of certain equity-based compensation plans, an issuance that will result in a change of control of us, certain transactions other than a public offering involving issuances of a 20% or greater interest in the Company, and certain acquisitions of the stock or assets of another company. Under the Companies Law as currently applicable to us, there is no requirement to receive shareholder approval for the issuance of securities for such dilutive events, and under our amended and restated articles of association our board of directors is authorized to issue securities, including ordinary shares, warrants and convertible notes. Additionally, under the Companies Law, unless the articles of association otherwise provide, the quorum required for an ordinary meeting of shareholders must consist of at least two shareholders who hold at least 25% of the voting rights (instead of the 33 and 1/3% required under Nasdaq rules), and we are not required to have a nominating committee consisting solely of independent directors for the nomination of directors. See “Item 16G. Corporate Governance” for details on the differences between Israeli corporate governance practices and comparable U.S. requirements and other home country practices we follow instead of the listing rules of Nasdaq. We may in the future elect to follow home country corporate governance practices in Israel with regard to other matters. Following our home country corporate governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on Nasdaq may provide less protection to you than what is accorded to investors under the listing rules of Nasdaq applicable to domestic U.S. issuers.
Risks Related to Our Operations in Israel
The impact of political, economic and military conditions in Israel, including the ongoing Israel-Hamas war, and the surrounding region could directly affect our business.
Our principal executive offices and research and development facilities as well as our third-party manufacturers are located in Israel. In addition, all of our subcontractors are located in Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region could directly affect our business.
On the military front, since the establishment of the State of Israel in 1948, a number of armed conflicts have occurred between Israel and its Arab neighbors, Hamas (an Islamist militia and political group that controls the Gaza Strip), Hezbollah (an Islamist militia and political group that controls large portions of southern Lebanon), and with Iranian-backed military forces in Syria and the region. Some of these hostilities were accompanied by missiles being fired from the Gaza Strip, Lebanon and Syria against civilian targets in various parts of Israel, including areas in which our employees and third-party manufacturers are located. Any hostilities involving Israel, regional political instability or the interruption or curtailment of trade between Israel and its trading partners could materially and adversely affect our operations and results of operations.
In particular, in October 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on the Israeli population and industrial centers. These attacks resulted in extensive deaths, injuries, and kidnapping of civilians and soldiers, as well as evacuations of tens of thousands of civilians from their homes. Following the attacks, Israel’s security cabinet declared war against Hamas and commenced a military campaign and the Israel Defense Force (the “IDF”), the national military of Israel, began to call-up reservists for active duty. During January 2025, Israel and Hamas have agreed to a Gaza three-phase ceasefire agreement and partial hostage release, the first six-week phase of such ceasefire began on January 19, 2025. There is no certainty that the subsequent phases of this ceasefire will be agreed and consummated and that the ceasefire will last in the long term.
Several of our employees are subject to military reserve service in the IDF and have been and may be called to serve. Since the war with Hamas broke out and as of the date of this Annual Report, 12 of our employees served in active duty. Military reserve service call ups that result in absences of personnel for an extended period of time may materially and adversely affect our business, prospects, financial condition and results of operations.
In addition, since the commencement of these events, there have been additional active hostilities, including with Hezbollah in Lebanon, the Houthi movement which controls parts of Yemen, and with Iran who joined the hostilities against Israel by firing hundreds of drones, ballistic missiles and guided missiles to Israel causing further uncertainty in the region. During November 2024, a ceasefire agreement was reached between Israel and Hezbollah with sporadic breaches. It is possible that these hostilities will escalate in the future into a greater regional conflict, and that additional terrorist organizations, including Hezbollah, the rebels in Syria, Palestinian military organizations in the West Bank, as well as other hostile countries, such as Iran, will actively join or escalate the hostilities.
In addition, in Syria, a country bordering Israel, a civil war is taking place and during December 2024, Syrian Islamist rebel forces seized control of Syria, and the Assad regime collapsed, adding additional instability to the region.
Such hostilities may include terror and missile attacks. In the event that our facilities are damaged as a result of hostile actions, or hostilities otherwise disrupt our ongoing operations, our ability to deliver or provide products and services in a timely manner to meet our contractual obligations towards customers and vendors could be materially and adversely affected.
The scope, intensity and duration of Israel’s current war against Hamas is difficult to predict, as are such war’s economic implications on our business and operations and on Israel’s economy in general. For example, these events may be intertwined with wider macroeconomic indications of a deterioration of Israel’s economic and political standing, which may involve, for instance, a downgrade in Israel’s credit rating by rating agencies (such as the recent downgrade by Moody’s of its credit rating of Israel from A1 to A2, as well as the downgrade of its outlook rating from “stable” to “negative”, followed by additional downgrade from A2 to Baa1 while maintaining “negative” outlook. Any of these implications on Israel’s economy or financial conditions may have a material adverse effect on us and our ability to effectively conduct our business and operations.
Further, as an Israeli company, there is a heightened risk of cyberattacks on our and our supply chain’s IT networks by our adversaries in general, and more so as a result of the ongoing war with Hamas and other conflicts in the region. Although the current war has not materially impacted our business or operations as of the date of this report, any escalation or expansion of the war could have a negative impact on both global and regional conditions and may adversely affect our business, financial condition and results of operations.
Furthermore, following Hamas’ attack on Israel and Israel’s security cabinet declaration of war against Hamas, the Houthi movement, which controls parts of Yemen, launched a number of attacks on marine vessels traversing the Red Sea, which marine vessels were thought to either be in route towards Israel or to be partly owned by Israeli businessmen. The Red Sea is a vital maritime route for international trade traveling to or from Israel. As a result of such disruptions, we may experience in the future delays in supplier deliveries, extended lead times, increased cost of freight, increased insurance costs, and increased materials and manufacturing labor costs. The risk of ongoing supply disruptions may further result in delayed deliveries of our products.
Moreover, political uprisings and conflicts in various countries in the Middle East are affecting the political stability of those countries. This instability has raised concerns regarding security in the region and the potential for armed conflict. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and certain other countries. Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions, could harm our results of operations and could make it more difficult for us to raise capital. Parties with whom we do business may be disinclined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary, in order to meet our business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
In addition, the potential of further escalation of Israel-Hamas conflict and other conflicts in the region could cause disruptions to our operations, and a delay in the development, production and shipment of our products and the heightened tension in our relations with various Middle Eastern countries could adversely affect our customer relationships as well as our sales and performance of existing or future contracts, which in turn could adversely affect our operating results, financial conditions and the expansion of our business. Furthermore, the ongoing Israel-Hamas war and other conflicts in the region and the resulting measures that have been taken, and could be taken in the future, by NATO, the United States, the United Kingdom, the European Union, Israel and its neighboring states and other countries have created global security concerns that could have a lasting impact on regional and global economies. Although the length and impact of this ongoing conflict are highly unpredictable, they could lead to market disruptions, including significant volatility in commodity prices and supply chain disruptions. For more information about the impact of the Israel-Hama war on our operations and financial results, please see “Item 3. Key Information – D. Risk Factors–Risks to Our Business and Industry”.
Finally, on the political front, Israel has held five general elections between 2019 and 2022, and prior to the Hamas attack in October 2023, the Israeli government had been pursuing legislative changes, which, if adopted, will alter the current state of separation of powers among the three branches of government and, as a result, have sparked a considerable political debate. Many individuals, organizations and institutions, within and outside of Israel, voiced concerns over the potential negative impacts of such changes and the controversy surrounding them on the business and financial environment in Israel. Such negative impacts may include, among other things, increased interest rates, currency fluctuations, inflation, civil unrest and volatility in the securities markets, which could adversely affect the conditions in which we operate and potentially deter foreign investors and organizations from investing or transacting business with Israeli-based companies. To date, these initiatives have been substantially put on hold, but if such changes to Israel’s judicial system are again pursued by the government and approved by the parliament, or if any of the foregoing negative impacts were to materialize, it may have an adverse effect on our business, our results of operations and our ability to raise additional funds.
Our commercial insurance may leave us subject to a risk of a loss if a terrorist attack or act of war occurs.
Our insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East or for any resulting disruption in our operations. Although the Israeli government has in the past covered the reinstatement value of direct damages that were caused by terrorist attacks or acts of war, we cannot be assured that this government coverage will be maintained or, if maintained, will be sufficient to compensate us fully for damages incurred and the government may cease providing such coverage or the coverage might not suffice to cover potential damages. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions generally and could harm our results of operations.
Boycotts and various Middle Eastern business restrictions in the region may adversely impact our ability to operate and sell our products.
Several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies whether as a result of the ongoing and revived hostilities in the region, the ongoing Israel-Hamas war or otherwise. In addition, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods based on Israeli government policies. Although Israel had signed bilateral peace agreements with several Middle Eastern countries for forging new economic ties with them, the performance of such bilateral peace agreements could be adversely affected by the going and revived hostilities in Israel region, which has caused a substantial strain on Israel’s regional relations with a number of Middle Eastern countries, such as Egypt and Jordan whose relations with Israel had long been cold but worsened since the outbreak of the war. The restrictive laws and policies and the actions by boycott activists, if become more widespread and successful, and the fast-moving changes to Israel’s geopolitical relations as a result of the war, may seriously limit our ability to sell our products in these countries and may have an adverse impact on our operating results, financial conditions or the expansion of our business.
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
We have entered into assignment of invention agreements with our employees pursuant to which such individuals agree to assign to us all rights to any inventions created during their employment or engagement with us. A significant portion of our intellectual property has been developed by our employees in the course of their employment with us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived by an employee during the scope of his or her employment with a company and as a result thereof are regarded as “service inventions,” which belong to the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patent Law also provides that if there is no agreement between an employer and an employee with respect to the employee’s right to receive compensation for such “service inventions,” and to what extent and under which conditions, the Israeli Compensation and Royalties Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for his or her service inventions and the scope and conditions for such remuneration. The Patent Law further provides criteria for assisting the Committee in making its decisions. Case law clarifies that the right to receive consideration for “service inventions” can be waived by the employee and that in certain circumstances, such waiver does not necessarily have to be explicit. The Committee will examine, on a case-by-case basis, the general contractual framework between the parties, applying interpretation rules of the general Israeli contract laws. Further, the Committee has not yet determined one specific formula for calculating this remuneration, nor the criteria or circumstances under which an employee’s waiver of his right to remuneration will be disregarded. Similarly, it remains unclear whether waivers by employees in their employment agreements of the alleged right to receive consideration for service inventions should be declared as void being a depriving provision in a standard contract. Although our employees have agreed to assign to us service invention rights and have specifically waived their right to receive any special remuneration for such service inventions beyond their regular salary and benefits, as a result of uncertainty under Israeli law with respect to the efficacy of waivers of service invention rights, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could negatively affect our business.
Our operations may be affected by negative economic conditions or labor unrest in Israel.
General strikes or work stoppages, including at Israeli ports, have occurred periodically or have been threatened in the past by Israeli trade unions due to labor disputes. These general strikes or work stoppages may have an adverse effect on the Israeli economy and on our business, including our ability to deliver products to our customers and to receive raw materials from our suppliers in a timely manner and could have a material adverse effect on our results of operations.
You may have difficulties enforcing a U.S. judgment against us, our executive officers and directors and Israeli experts named in this Annual Report on Form 20-F in Israel or the United States, asserting U.S. securities laws claims in Israel or serving process on our officers and directors and these experts in Israel.
We are incorporated in Israel and our corporate headquarters is located in Israel. Many of our executive officers and directors reside outside of the United States, and a significant portion of our assets and the assets of certain of our directors and executive officers are located outside the United States. Therefore, a judgment obtained against us or any of them in the United States, including one based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. Further, if a foreign judgment is enforced by an Israeli court, it will be payable in Israeli currency. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
Moreover, among other reasons, including but not limited to, fraud or absence of due process, or the existence of a judgment which is at variance with another judgment that was given in the same matter if a suit in the same matter between the same parties was pending before a court or tribunal in Israel, an Israeli court will not enforce a foreign judgment if it was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases), or if its enforcement is likely to prejudice the sovereignty or security of the State of Israel.
Provisions of our amended and restated articles of association and Israeli law may delay, prevent or make difficult a merger with, or an acquisition of, the Company, which could prevent a change of control even when the terms of such transaction are favorable to us and our shareholders and, therefore, could depress the price of our ordinary shares.
As a company incorporated under the laws of the State of Israel, we are subject to Israeli corporate law. Israeli corporate law regulates mergers, requires tender offers for acquisitions of ordinary shares above specified thresholds, requires special approvals for transactions involving directors, officers or certain significant shareholders and regulates other matters that may be relevant to these types of transactions. In addition, our amended and restated articles of association contain provisions that may make it more difficult to acquire us, such as classified board provisions. Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to some of our shareholders whose country of residence does not have a tax treaty with Israel granting tax relief to such shareholders from Israeli tax obligations. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of numerous conditions, including, in some cases, a holding period of two years from the date of the transaction during which certain sales and dispositions of shares of the participating companies are subject to certain restrictions. See “Item 10E. Additional Information–Taxation–Israeli Material Tax Considerations” for additional information. These provisions of our amended and restated articles of association and Israeli law may delay, prevent or make difficult an acquisition of us, which could prevent a change of control and therefore depress the price of our ordinary shares.
In addition, as a corporation incorporated under the laws of the State of Israel, we are subject to the Israeli Economic Competition Law, 1988, and the regulations promulgated thereunder (formerly known as the Israeli Antitrust Law, 1988), under which we may be required in certain circumstances to obtain the approval of the Israel Competition Authority (formerly known as the Israel Antitrust Authority) in order to consummate a merger or a sale of all or substantially all of our assets.
Your rights and responsibilities as a holder of our ordinary shares will be governed by Israeli law, which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
Since we are incorporated under Israeli law, the rights and responsibilities of the holders of our ordinary shares are governed by our articles of association and by Israeli law, including the Companies Law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in typical U.S. corporations. In particular, pursuant to the Companies Law, a shareholder of an Israeli company has certain duties, including to act in good faith and fairness and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders and to refrain from abusing its power in the company including, among other things, in voting at the general meeting of shareholders on certain matters, such as an amendment to the company’s articles of association, an increase of the company’s authorized share capital, a merger of the company, and approval of related party transactions that require shareholder approval. A shareholder also has a general duty to refrain from discriminating against other shareholders. In addition, a controlling shareholder or a shareholder who knows that it possesses the power to determine the outcome of a shareholders’ vote or to appoint or prevent the appointment of an officer of the company has a duty to act in fairness towards the company with regard to such vote or appointment. See “Item 6C. Directors, Senior Management and Employees–Board Practices–Approval of Related Party Transactions under Israeli Law” for additional information. There is limited case law available to assist in understanding the nature of these duties or the implications of these provisions. These provisions may be interpreted to impose additional obligations on holders of our ordinary shares that are not typically imposed on shareholders of U.S. corporations.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
We were incorporated as a limited liability company in the State of Israel on January 2, 2008, and operate under its laws. In November 2017, our corporate name was changed from Invasix Ltd. to InMode Ltd. Our registered office is located at Tavor Building, Sha’ar Yokneam, P.O. Box 533, Yokneam 2069206, Israel. The phone number of our registered office is +972-4-9096313. Our wholly‑owned subsidiary, Invasix Inc., a Delaware corporation, acts as our agent for service of process in the United States and is located at 17 Hughes Irvine, California 92618. The phone number for Invasix Inc. is 949-387-5711.
In August 2019, we completed our initial public offering of 10,000,000 of our ordinary shares at an initial public offering price of $7.00 per ordinary share. The effective date of the registration statement on Form F-1 (File No. 333-232615) was August 7, 2019. Also, during August 2019, the underwriters partially exercised their over-allotment option and purchased an additional 1,000,000 ordinary shares at the same price per share. Our ordinary shares commenced trading on Nasdaq on August 8, 2019, under the symbol “INMD”.
Our capital expenditures for the years ended December 31, 2024, 2023 and 2022 amounted to approximately $$0.7 million, $0.8 and $1.6 million, respectively. Our capital expenditures consist principally of the purchase of molds for manufacturing. We anticipate our capital expenditures in 2025 to be up to $1 million and to be financed from our existing cash and cash equivalents.
The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers, including InMode, that file electronically with the SEC. The address of that site is www.sec.gov. We maintain a corporate website at www.inmodemd.com. Our periodic filings are available, free of charge (other than an investor’s own Internet access charges), on our website soon as reasonably practicable after we electronically file or furnish such material with the SEC. The information contained on our website or available through our website is not incorporated by reference into and should not be considered a part of this Annual Report on Form 20-F, and the reference to our website in this Annual Report on Form 20-F is an inactive textual reference only.
B. Business Overview
Overview
We are a leading global provider of innovative, energy-based, minimally invasive surgical medical treatment solutions. Within the global aesthetics market, our products and solutions are primarily designed to address three energy-based treatment categories comprised of: (i) face and body contouring; (ii) medical aesthetics; and (iii) women’s wellness. We have developed and commercialized products utilizing medically accepted RF energy technology, which can penetrate deep into the subdermal fat, allowing adipose tissue remodeling. We believe our RF energy-based proprietary technologies—(i) Radio Frequency Assisted Lipolysis, or RFAL; (ii) Deep Subdermal Fractional RF; (iii) Simultaneous Fat Destruction and Skin Tightening; and (iv) Deep Heating Collagen Remodeling for skin and human natural openings – represent a paradigm shift in the minimally invasive aesthetic solutions market. These technologies are used by physicians to remodel subdermal adipose, or fatty tissue in a variety of procedures including liposuction with simultaneous skin tightening, face and body contouring, ablative skin rejuvenation treatments and treatment of Genitourinary Syndrome of Menopause (GSM). Our products, developed with our proprietary RF energy-based technologies, overcome many of the shortcomings of other surgical options by delivering surgical-grade results under local anesthetics while significantly minimizing risks of scarring, downtime, pain and other complications typically accompanying surgical procedures. In addition to our minimally invasive solutions, we design, develop, manufacture and market differentiated, non-invasive medical aesthetic products that target a wide array of procedures. These include simultaneous fat destruction and skin tightening, permanent hair reduction through the use of our innovative dual wavelength technology and other treatments targeting skin appearance and texture through the use of our high-power IPL technology. Our products, which we market and sell traditionally to plastic and facial surgeons, aesthetic surgeons, dermatologists and OB/GYNs may be used on a variety of body parts including the face, neck, abdomen, upper arms, thighs and intimate feminine regions.
In addition to the existing group of patients who currently undergo full surgical aesthetic procedures, we believe our minimally invasive solutions satisfy an unmet market demand in two incremental groups of patients: (i) those whose skin laxity or other physical attributes have previously precluded them from undergoing surgical aesthetic procedures and (ii) those who would entertain the idea of surgical or minimally invasive aesthetic procedures, but are averse to the associated costs, downtime and potential safety risks. We believe these patient populations will continue to represent a significant opportunity for our differentiated minimally invasive aesthetic solutions.
We believe our products have consistently been at the forefront of technological development in the aesthetic solutions market. Since 2010, we have launched fourteen product platforms: BodyTite, Optimas, Votiva, Contoura, Triton, EmbraceRF, EvolveX, Evoke, Morpheus8, EmpowerRF, Define, Envision, IgniteRF and OptimasMAX. Each product consists of the following components: a platform that incorporates multiple energy sources, one or more handpieces or hands-free applicators, our proprietary software and a simple user interface with touch screen. Our platforms have a small footprint and are lightweight compared to our competitors’ systems, which are typically larger and heavier. Our products can be upgraded easily by the user in order to perform additional treatments by adding handpieces, hands-free applicators and/or installing software in the existing platform. The ease of upgrades enables our customers to meet demand for aesthetic solutions through additional service offerings.
Our focus on innovation has resulted in a strong track record of sustained new and next-generation product development. We believe our ability to bring new products to market and continuously innovate is a distinct competitive advantage.
We expect that the new product platforms will complement our existing portfolio of products, allowing us to increase our offerings to existing customers and attract new customers. We believe that introducing new product platforms is important in order to satisfy consumer demand and respond to evolving technological developments and consumer needs.
In response to customers’ desires to enhance and expand their offering of our aesthetic and wellness office-based procedures, we are developing additional RF energy-based platforms, handpieces and applicators targeted towards several medical specialties.
| • | For ENTs, we are in the initial stage of developing a new platform and handpieces that we believe will provide patients with a medical treatment solution for snoring and rhinitis. The handpiece for treatment of turbinates is based on our Deep Subdermal Fractional RF technology and is expected to contract the turbinate to enable free breathing. This platform and handpiece is in the concept design phase. |
| • | For urologists, we bring to the market a device using RF energy to treat ED (Erectile Dysfunction). We registered a patent application to protect our technological concept, and we believe that our technological concept will work well for this indication but much more research and development is needed. |
We are focused on establishing and using clinical evidence to support and broaden our marketing claims and drive customer awareness and acceptance of our products. Traditionally, the aesthetic solutions market has relied heavily on marketing efforts and “before-and-after” pictures in an attempt to distinguish products. We believe our focus on establishing clinical evidence for the efficacy of our products has been important for adoption by our surgically trained customers, who are accustomed to seeing extensive clinical data in their non-aesthetic practices. To date, 95 third-party clinical studies have been completed and ten third-party clinical studies are in the process of being conducted using our products. We also have a portfolio of over 100 peer-reviewed publications. While we did not have any involvement in the clinical studies mentioned above, such studies provide qualitative results that we believe are meaningful. However, because these were third-party studies, we do not have access to any raw data to conduct any quantitative analyses.
To complement our surgical aesthetic and medical treatment solutions, we offer post-sales training and support services. We provide physicians with training focused on the most beneficial ways to utilize our products, including safety and instructional videos to expand procedural offerings and hands-on, personalized marketing support. We believe that we provide one of the most extensive training and ongoing support programs available to physicians throughout the aesthetic solutions market.
Our revenue decreased to approximately $394.8 million for the year ended December 31, 2024, from approximately $492.0 million for the year ended December 31, 2023 and from approximately $454.3 million for the year ended December 31, 2022. For the years ended December 31, 2024, 2023 and 2022, we recorded a gross margin of approximately 80%, 84% and 84%, respectively, and net income of approximately $181.3 million, $197.9 million and $161.5 million, respectively. Our principal market is in the U.S., where our revenue decreased to approximately $244.8 million for the year ended December 31, 2024, from approximately $307.8 million for the year ended December 31, 2023 and from approximately $298.6 million for the year ended December 31, 2022. Outside of the U.S., our revenue decreased to approximately $150.0 million for the year ended December 31, 2024, from approximately $184.2 million for the year ended December 31, 2023 and from approximately $155.7 million for the year ended December 31, 2022.
Regarding U.S. revenues for the years ended December 31, 2024, 2023 and 2022, we derived approximately $223.7 million, or 92%, $264.7 million, or 86% and $253.8 million, or 85%, of our total U.S. revenues from the sale of minimally invasive platforms. We derived approximately $15.2 million, or 6%, $33.9 million, or 11% and $35.8 million, or 12%, respectively, of our total U.S. revenues from the sale of hands-free platforms, and approximately $5.9 million, or 2%, $9.2 million, or 3% and $8.9 million, or 3%, respectively, of our total U.S. revenues from the sale of non-invasive platforms.
We have 35 FDA clearances and, in addition to the United States, where we have an installed base of over 11,860 product platforms, we are permitted to sell our products in most countries. As of December 31, 2024, we sell and market our products in the United States, Canada, United Kingdom, Ireland, Spain, Portugal, France, Belgium, Luxembourg, Italy, Germany, Austria, Japan, Australia and India through a direct sales force (including distributor sales managers) of approximately 267 representatives. We also sell and market our products through over 60 distributors in over 85 countries. As of December 31, 2024, we had a global installed base of approximately 27,090 product platforms capable of running various multi-use applicators and utilizing minimally invasive consumables.
Our Solution
Key benefits of our minimally invasive surgical aesthetic and medical treatment solutions include:
| • | Small to no incisions, which reduces the drawbacks and risks typically associated with surgical procedures such as significant pain, local or widespread scarring, infection, perforation and hemorrhage. |
| • | Outpatient procedures that typically do not require general anesthesia, which can decrease patient downtime, discomfort and other potential complications and typically reduces cost. |
| • | Minimally invasive procedures with similar efficacy to surgical procedures that have the ability to expand the addressable patient population for aesthetics procedures. |
| • | Effective and long-lasting aesthetic solutions, many of which are supported by compelling clinical data, including over 100 peer-reviewed publications. |
| • | Differentiated, RF energy-based technology simultaneously destroys fat and tightens skin, overcoming the many shortcomings of traditional surgical, minimally and non-invasive aesthetic procedures. |
| • | Innovative dual wavelength laser technology that allows for permanent hair reduction on a wider range of skin types and hair textures than other aesthetic solutions currently on the market, reducing the number of treatments required. |
| • | Typically less expensive than other aesthetic solutions on the market that provide comparable results as a result of less required physician time and training required. |
Leader in RF Energy
We believe we are the leader in using RF energy for both minimally invasive and subdermal ablative aesthetic purposes. RF energy is different from optical energy because it is not absorbed by the epidermis and is able to be targeted to penetrate deep into the tissue. The application of RF energy in medicine is a well-established practice. For example, RF energy is the basis of magnetic resonance imaging, or MRI, and surgical diathermy, used to cauterize blood vessels to prevent excessive bleeding, both commonplace applications administered regularly in medical practice. RF energy is also used in cardiology for ablative interventions and in oncology surgery for tumor/metastasis ablation.
RF energy can be delivered to the skin in a variety of ways, the most common being monopolar delivery, whereby RF energy is delivered through a single probe placed on the skin with a grounding pad distant to the probe site. Alternatively, in bipolar delivery, RF energy is delivered from a probe with two electrodes placed over the treatment area. Bipolar delivery has an important advantage over monopolar delivery: depth of penetration of the RF energy is not dependent on the tissue impedance, or electrical resistance, which varies from person to person, or the cross-sectional area of the probe. That is not the case with monopolar delivery. Instead, in bipolar delivery, depth of penetration of the RF energy depends on the distance between the two electrodes on the probe, with increasing distance resulting in increased depth of penetration. We believe we are the leader in the development, design and commercialization of bipolar RF energy devices for minimally invasive and subdermal ablative aesthetic purposes.
Radio-Frequency Assisted Lipolysis
Using our expertise in bipolar RF energy delivery, we developed what we believe is the next generation of lipolysis and adipose tissue remodeling technology, a new category that delivers a thermal response to the adipose tissue, skin and subdermal matrix. Our RFAL products deliver directional RF energy into the subcutaneous fat to coagulate, liquefy and remodel adipose tissue and heat the subcutaneous fibrous septa, or partitions, resulting in substantial collagen contraction of subdermal space. We believe we are the first company to utilize bipolar radio frequency in a minimally invasive manner. Our RFAL products generate a higher power and more efficient energy transfer than laser energy systems and allow the treatment of larger volumes of the subcutaneous tissue with optimal thermal profiles, facilitating the significant tightening of the tissue. The shrinkage of tissue is significant and can reach double-digit percentages of the heated tissue volume. The thermal energy is delivered by an innovative handpiece comprised of two electrodes: the internal electrode is inserted into the fat layer while the other larger electrode is applied externally to the skin surface above the cannula tip. The internal cannula is passed through the subcutaneous fat while the external electrode is moved above and over the skin’s surface. The small, conductive tip of the cannula delivers RF energy into the subcutaneous fat, liquefying it and simultaneously contracting fibrous septa. The liquefied fat can then be removed from the body through a suction cannula. Our RFAL products also apply gentle uniform heating of the dermis, thereby promoting skin tightening. Figure 1 below shows how the RF energy is delivered through the handpiece to simultaneously liquefy fat and tighten the skin.
Our BodyTite, EmbraceRF and IgniteRF platforms, and the BodyTite, FaceTite and QuantumRF handpieces rely on our proprietary RFAL technology. To date, there have been more than 280,000 successful RFAL procedures conducted with positive clinical results using our BodyTite, EmbraceRF and IgniteRF platforms, and the BodyTite, FaceTite and QuantumRF handpieces. We have demonstrated that RFAL has the potential to elicit three-dimensional soft tissue contraction reliably and predictably to both serve otherwise non-traditional liposuction candidates, as well as to improve outcomes in patients for whom liposuction is an option.
Figure 1: RFAL mechanism of action.
Deep Subdermal Fractional RF
Our Deep Subdermal Fractional RF delivers RF energy into the subdermal fat tissue to depths of up to four millimeters, or mm. Deep Subdermal Fractional RF provides skin tightening and adipose tissue remodeling directly under the dermal layer. Our Deep Subdermal Fractional RF products deliver RF energy under the dermis through an array of pins producing localized heat and small micro-lesion dots in the treatment area. The heat generated by the pins in the subdermal tissue promotes collagen restructuring and tissue reshaping. Physicians can offer a versatile fractional treatment creating a three-dimensional matrix of coagulation volumes inside the tissue. Deep Subdermal Fractional RF is used for wrinkle reduction, skin tightening and treatment of cellulite appearance. Our deep subdermal fractional RF can be applied to both face and body. Our Morpheus8, Morpheus8 Body, Morpheus Burst and Morpheus8 V handpieces rely on our proprietary Deep Subdermal Fractional RF technology and are used in conjunction with our BodyTite, Embrace RF, Optimas, IgniteRF, OptimasMAX , EmpowerRF, Envision and Define platforms. Figure 2 below shows how the RF energy is delivered through the coated pins on the handpiece to reshape tissue under the dermal layer.
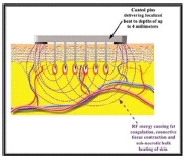
Figure 2: Deep Subdermal Fractional RF mechanism of action.
Simultaneous Fat Destruction and Skin Tightening
Our Simultaneous Fat Destruction and Skin Tightening proprietary technology combines vacuum and bipolar RF energy with high and low amplitudes to both permanently destroy adipose tissue and contract the skin. We believe our Simultaneous Fat Destruction and Skin Tightening technology is the first and only RF-based, non-invasive body contouring technology that permanently destroys adipose tissue. Our BodyFX handpiece and MiniFX handpiece utilize our Simultaneous Fat Destruction and Skin Tightening technology to address problematic fatty tissue in larger body areas such as the abdomen, back and thighs.
Deep Heating Collagen Remodeling for Skin and Human Natural Openings
Our Deep Heating Collagen Remodeling propriety technology delivers heat in a uniform and volumetric form targeting deep into tissue while providing collagen remodeling with real-time control of the device’s temperature. The versatility of this technology allows the operator to provide a customized solution to address a variety of women’s wellness concerns that occur due to aging, hormonal stress or physical damage. Our Forma, FormaV, and Morpheus8V handpieces utilize Deep Heating Collagen Remodeling technology while harnessing continuous bipolar RF energy with real-time temperature measurement. RF energy generated heat is delivered uniformly to vaginal tissue through a consumable applicator to provide vaginal and labia contraction with patients often seeing effects of the procedure immediately.
Pulse/Continuous Bipolar RF
Continuous Bi-polar RF is electrical energy in the RF spectrum (1 MHz) that results from the flow of an electric charge between two electrodes. This conducted energy increases ion movement in the tissue and generates kinetic energy that is transformed to thermal energy (heating). In turn, this thermal energy causes controlled damage to the tissue and triggers a natural healing mechanism and tissue-renewal resulting in tissue tightening and remodeling. The distance between the electrodes allows for control of the depth of penetration of the bi-polar RF energy into the tissue. The distance between the electrodes is chosen based on the particular treatment and according to the tissue to be treated (generally varies between a few millimeters to 3-4 centimeters). Bi-polar RF can be delivered to the tissue in one of two modes: either pulse or continuous. In pulse mode, pulse duration is pre-determined and RF energy automatically stops. In continuous mode, the RF energy is delivered uninterrupted into the tissue for as long as the operator deems appropriate. As part of the design, continuous bi-polar RF energy allows real-time measurement of the patient’s skin temperature. This allows our products to provide real-time feedback to the operator throughout the treatment process and enhances overall safety and efficiency. All of our RF platforms (both existing and expected) and RF handpieces (both minimally and non-invasive) have both pulse bi-polar RF and continuous bi-polar RF capabilities. Our proprietary RFAL-based products (such as our BodyTite and IngiteRF platforms with BodyTite, FaceTite and QuantumRF handpiece) and Deep Heating Collagen Remodeling based products (such as our Forma and Plus handpieces) primarily utilize the continuous bipolar RF feature. Our proprietary Deep Subdermal Fractional RF based products (such as our Morpheus8 and Moprheus8 Burst handpieces) and Simultaneous Fat Destruction and Skin Tightening products (such as our BodyFX and Mini FX handpieces) primarily utilize the pulse feature depending on the procedure and target result. Figure 3 below shows how RF energy is delivered through the handpiece by an electric charge between two electrodes continuously into the tissue.

Figure 3: Pulse/Continuous Bi-polar RF mechanism of action.
Non-invasive Medical Aesthetic Technologies
In addition to our proprietary minimally invasive solutions, we continue to develop innovative non-invasive medical aesthetic solutions, including:
| • | Simultaneous non-invasive fat destruction and skin tightening. We believe our technology is the first and only RF-based, noninvasive body contouring technology that permanently destroys adipose tissue while simultaneously contracting the skin. This technology addresses problematic fatty tissue in large body areas such as the abdomen, back and thighs. Customers use this technology with the Contoura platform and the BodyFX and MiniFX handpieces. |
| • | Dual wavelength for permanent hair reduction. Our single-pulse, dual wavelength product for permanent hair reduction, Triton, combines two wavelengths in one platform, overcoming certain limitations of standard lasers. This optimal mix of wavelengths allows the highest efficiency and safety. We believe Triton is the only FDA-cleared, single-pulse, dual wavelength product for permanent hair reduction. Customers use this technology with the Triton Duo Light and Triton Duo Dark handpieces. |
| • | High-power Intense Pulsed Light. Our high-power IPL is a breakthrough technology that delivers up to three times more energy than typical IPL devices within the 500 to 600 nanometers, or nm, range to improve efficacy for vascular and pigmented lesions. It is optimized to treat a variety of skin types and conditions in a single session. Customers use this technology with the Optimas and the OptimasMAX platforms and the Lumecca handpiece. |
| • | Controlled continuous RF heating. We believe our controlled continuous RF technology is the first auto-adjusting non-invasive thermal skin treating technology for deep and uniform tissue stimulation. This technology uses bipolar RF energy delivery that allows uniformity between the electrodes to provide a comfortable thermal effect with immediate and subsequent contraction. Customers use this technology with the Optimas, OptimasMAX, Votiva, Define, Contoura, Evoke and EvolveX hands-free platforms and EmpowerRF for women’s wellness, as well as the Envision. |
Differentiated and Comprehensive Post-Sales Support
To complement our innovative aesthetic solutions, we offer post-sales training and support services. We provide physicians training focused on the most beneficial ways to utilize our products, including a disciplined focus on safety. Our clinical training and support program consists of three key components:
| i. | A visit by a new physician to one of our many highly qualified plastic surgery facilities for instruction followed by a live patient demonstration; |
| ii. | A visit to the new physician’s office by a trained registered nurse or physician’s assistant to attend the first day of treatments to in-service; and |
| iii. | Open house workshops organized by us, wherein a new physician invites this patient base and we assist them in “kick starting” marketing efforts. These events typically secure significant procedural revenues for the physician. |
In addition, we offer ongoing livestream classes for customers where they can observe and interact in real-time with both our training staff and highly qualified physician instructors on a regular basis. Advanced training is also available for physicians who choose to expand their education on highly skilled procedures, including non-excisional breast lifting or brachioplasty. We are continuing to build a library of on-line instructional videos as both a reference tool and to expand physicians’ procedural offerings. We also provide support to help customers educate and engage patients about the new minimally invasive procedures available to them through our In-Practice program. This program provides hands-on, personalized marketing support for customers’ practices including signage, educational collateral, digital marketing and advertising assets. We believe that we provide one of the most extensive training and ongoing support programs available to physicians throughout the aesthetics industry.
Our Competitive Strengths
We attribute the growing commercial success of our various platforms and products to the following:
| • | Pioneer of the minimally invasive aesthetic solutions market. We believe our proprietary technologies represent a paradigm shift in the minimally invasive and surgical aesthetic solutions market. We believe our technologies and products demonstrate numerous performance advantages over other aesthetic options and enable physicians and patients to obtain results that previously could only be generally achieved with more expensive and invasive surgical procedures. Our RF proprietary energy-based technology simultaneously destroys fat and tightens skin, overcoming many of the limitations of other surgical, minimally and non-invasive procedures, positioning us to address unmet patient needs and expand the addressable patient population for aesthetic solutions. Unlike many of our competitors, our technology is not exclusively laser-based or limited to superficial treatment of the skin. Instead, we have developed and commercialized products utilizing medically-accepted RF energy technology, which can penetrate deep into the subdermal fat, allowing adipose tissue remodeling. |
| • | Strong brand recognition. Our brand is associated with product leadership, significant technological advances and extensive clinical data, which has led to strong customer loyalty. We believe our brand is synonymous throughout the physician and patient communities with having the broadest RF energy-based portfolio in the minimally invasive aesthetics market for fat destruction and remodeling, face and body contouring and skin tightening. |
| • | Provide comprehensive solutions for physicians and patients. We have an extensive product portfolio that includes solutions for a wide range of both minimally and non-invasive procedures across the aesthetic solutions market. Although each of our product platforms has a primary handpiece or applicator that is either minimally or non-invasive, our platforms are designed to be modular, which enables the user to provide complementary treatments using a single platform by attaching different handpieces or applicators. For each of our products, we offer post-sales support services including training, installation, practice growth consulting and repair support that minimizes product downtime and associated lost revenues to physicians. |
| • | Broad regulatory approvals supported by extensive clinical data. We have 35 FDA clearances and in addition to the United States, are permitted to sell in United States, Canada, United Kingdom, Ireland, Spain, Portugal, France, Belgium, Luxembourg, Italy, Germany, Austria, Japan, Australia and India. To date, we also have a portfolio of over 100 peer-reviewed publications and there are over 95 completed and 10 ongoing third-party clinical studies on a number of our products (BodyTite, FaceTite, NeckTite, Optimas, Fractora, Morpheus8, Forma, Lumecca, DiolazeXL, Votiva, Morpheus8V, FormaV, Contoura, BodyFX, MiniFX, Evoke, EvolveX, Morpheus8, AccuTite and QuantumRF). While we did not have any involvement in the clinical studies mentioned above, such studies provide qualitative results that we believe are significant. However, because these were third-party studies, we do not have access to any raw data to conduct any quantitative analyses. We believe our focus on demonstrated clinical data and effectiveness differentiates us from our competition and helps to validate our technology with surgically-trained physicians, who we believe are typically the most difficult segment of the market to penetrate. |
| • | Strong management team with proven track record. Our management team has significant expertise in the medical aesthetics industry with a proven track record of successfully developing and commercializing innovative technologies. Moshe Mizrahy and Dr. Michael Kreindel, our co-founders, previously founded Syneron Medical Ltd. Our senior executive team has an average of over 15 years of medical aesthetics industry experience and has served in various leadership positions at Syneron Medical Ltd. and Cynosure, Inc. |
Our Growth Strategy
Our objective is to expand our technological leadership in the surgical solutions market and to leverage our RF proprietary technologies to expand into additional medical solutions markets. We intend to achieve this goal by implementing the following strategies:
| • | Increase our sales presence to target and expand our addressable market globally. We plan to continue to expand our direct sales organization and our distribution network and seek to recruit and train exceptionally talented sales representatives in existing and new markets to help us broaden the adoption of our products, drive further market penetration and expand beyond our traditional customer base. |
| • | United States: We have a direct sales presence in United States and plan to keep expanding our direct sales team. |
| • | Canada: We have a direct sales presence in Canada and plan to keep expanding our direct sales team. |
| • | Europe: We intend to establish sales and marketing organizations and a network of exclusive European distributors in addition to our subsidiaries network in the United Kingdom, Spain, Italy, France and Germany. |
| • | Latin America: We plan to strengthen our network of exclusive distributors in all countries in Latin America. |
| • | Asia-Pacific: In addition to our direct sales presence in India, Australia and Japan, we may also establish direct sales presence in China through our fully-owned subsidiary in China, Guangzhou InMode Medical Technology Ltd., as well as enhance our network of exclusive distributors in all other countries in Asia. |
| • | Continue to further penetrate our existing customer base and drive recurring revenues. We believe that there are opportunities for us to generate additional revenue from existing customers who are already familiar with our products. Since inception, we have sold over 3.4 million consumables. We expect that as our customer base grows, the percentage of our revenues attributable to consumables will increase. We also expect that certain customers will be candidates for technology upgrades to enhance the capabilities of their existing InMode products. In addition, as we continue to grow our support services program, we expect to seek to increase the number of customers that enter into extended warranties, which would provide us with additional recurring revenues. |
| • | Leverage our existing technology to expand into new minimally and non-invasive applications. We have an active research and development pipeline focused on additional solutions targeted to our traditional customer base. Our near-term product development portfolio consists of new and second generation solutions for various conditions, including wearable, noninvasive face and body reshaping products, cellulite, large area lipolysis, fractional RF treatment of SUI (stress urinary incontinence), vaginal laxity pelvic floor muscle restoration, labiaplasty procedures, post-partum treatments and other GSM symptoms, snoring and rhinitis treatments, dry eye and eyelid treatments, TMJ (Temporomandibular Joint Disorders) and ED (Erectile Dysfunction). |
| • | Expand our customer base beyond traditional customers. We intend to develop products that leverage our minimally and non-invasive technologies to address the unmet market needs of a new customer base, which includes OB/GYNs, ENTs, ophthalmologists, general practitioners and aesthetic clinicians. We intend to adapt our products to the expertise and skill level of these providers, further expanding our addressable market. |
| • | Actively pursue business development opportunities. We may seek to engage in targeted business development activities, including acquisitions of technologies and strategic partnerships, in order to augment our product and technology portfolio in our existing and potentially adjacent markets. We believe we can leverage our global infrastructure and existing relationships to implement a disciplined tuck-in acquisition strategy. |
| • | Expand our intellectual properly and patent portfolio. We intend to expand our existing intellectual property and patent portfolio as we develop additional applications and continue to aggressively defend against potential infringement by our competitors. |
Our Products
We offer a broad portfolio of aesthetic and medical treatment solutions that consist of a variety of platforms providing minimally and non-invasive applications. The following tables provide information concerning our product platforms and their applications.
Minimally Invasive and Ablative Platforms
Product Platform | Energy Source(s) | Year Introduced | Handpiece(s) | Primary (not Exclusive) Applications* |
| BodyTite | Bipolar RF | 2010 | BodyTite | Body Contouring (MI) |
| | | | FaceTite | Face Contouring (MI) |
| | | | NeckTite | Neck Contouring (MI) |
| | | | AccuTite | Face/Body Contouring (MI) |
| Optimas | Laser Bipolar RF IPL | 2016 | Morpheus8 | Skin Rejuvenation (MI) |
| Optimas MAX | | 2024 | Forma Lumecca DiolazeXL Vasculaze | Skin Rejuvenation (NI) |
| | | | Skin Rejuvenation & Pigmentation (NI) |
| | | | Hair Removal (NI) |
| | | | Vascular Lesion (NI) |
| | | | Facial Wrinkles and Texture (MI) |
| | | | | |
| EmbraceRF | Bipolar RF | 2018 | FaceTite | Face Remodeling (MI) |
| | | | Morpheus8 | Facial Wrinkles and Texture (MI) |
| | | | AccuTite | Face/Body Contouring (MI) |
| Votiva | Bipolar RF | 2017 | FormaV | Women’s Wellness (MI) |
| | | | | Women’s Wellness (NI) |
| Morpheus8 | Bipolar RF | 2021 | Morpheus8 | Face and body fractional RF treatment (MI) |
| | | | Morpheus8 Body | |
| Envision | Bipolar RF and IPL | 2023 | Forma i Lumecca I Morpheus8 | Treatment of dry eyes Facial Rejuvenation |
| Morpheus8 Burst | Bipolar RF | 2024 | Morpheus8 Burst Morpheus8 Burst Deep | Face and body fractional RF treatment (MI) |
| EmpowerRF | Bipolar RF and EMS | 2021 | FormaV, Morpheus8V, VTone, Aviva | Women’s Wellness (MI) |
| IgniteRF | Bipolar RF | 2024 | QuantumRF | Body and face contouring (MI) |
Non-Invasive Platforms
| Product Platform | Energy Source(s) | Year Introduced | Handpiece(s) | Primary (not Exclusive) Applications* |
| Contoura | Bipolar RF | 2017 | BodyFX | Body Contouring |
| | | | MiniFX | Face/Neck Contouring |
| | | | Plus | Skin Tightening |
| Triton | Laser | 2018 | Triton Duo Light | Hair Removal |
| | | | Triton Duo Dark | Hair Removal |
Hand-Free Platforms
| Product Platform | Energy Source(s) | Year Introduced | Handpiece(s) | Primary (not Exclusive) Applications* |
| EvolveX | Bipolar RF EMS | 2021 | Tite (HF) | Skin Tightening |
| | | | Transform (HF) | Body Contouring |
| | | | Tone (HF) | EMS |
| Evoke | Bipolar RF | 2020 | Cheek (HF) | Skin Rejuvenation |
| | | | Chin (HF) | Skin Rejuvenation |
| Define | Bipolar RF | 2023 | Cheek (HF) Chin (HF) | Skin Rejuvenation Skin Rejuvenation |
| * | “MI” = Minimally Invasive, “NI” = Non-Invasive, “HF” = Hands-free application |
In addition to the products described above, prior versions of our products continue to be used by customers. Outside of the United States, we also offer some alternative versions of our aesthetic treatment solutions, in some cases under different trademarks, which are tailored to the specific preferences and needs of certain countries and regions.
Components of Our Products
Each of our products consists of the following components:
| • | one or more handpieces or hands-free applicators; |
| • | One-time disposable handpiece or tip; and |
| • | our proprietary software. |
Platforms
Our platforms are mostly electronic boxes, comprised of RF energy generators and modules supporting lasers and IPL, as applicable, a 110/220VAC input power supply, controller and a user interface with touch screen. The user interface allows the physician to select the handpiece and set treatment parameters to meet the requirements of a particular application and patient. Using the touch screen, the physician can independently adjust the energy level, pulse width and other parameters depending on application to optimize the treatment’s safety and effectiveness. The user interface on our multiple energy workstations also allows the user to change energy sources with the press of a button. The control system communicates the operator’s settings from the user interface to the system’s modules and manages system operation and performance.
Handpieces and Hands-Free Applicators
Our handpieces and hands-free applicators are used to apply the energy to the patient treatment area. The handpieces and hands-free applicators are designed for specific targeted body areas, type of energy to maximize treatment safety and efficacy for specific treatment. Certain of our handpieces and hands-free applicators have a contained thermal field that ensures a controlled and safe treatment through our Acquire, Control and Extend, or ACE, technology. Our ACE technology ensures that no areas are under- or over-treated using therapeutic temperatures safely and efficiently. Built-in safeguards, including real time measurements of skin temperature, impedance monitoring, power cut-off and audible feedback, help ensure patient safety throughout the procedure. A number of our handpieces and hands-free applicators are, or contain, one-time use applicators, or consumables, which must be replaced following each treatment.
Minimally Invasive
BodyTite – The minimally invasive, consumable Bodyrite handpiece, introduced in 2010, utilizes directional RF energy for RFAL treatments using needle-size cannula and external electrodes to apply RF energy to the subcutaneous adipose tissue. The tissue is heated to 50°C to 70°C to destroy fat and contract connective tissue, simultaneously remodeling the dermis at external temperatures of up to 42°C. This handpiece allows tissue treatment using a 17cm cannula that provides treatment depth up to 50mm.
Facerite/NeckTite – The minimally invasive, consumable FaceTite and NeckTite handpieces, introduced in 2012, utilize directional RF energy for RFAL treatments using cannula with diameters of 1.8mm and 2.2mm and external electrodes to apply RF energy to the subcutaneous adipose tissue. The tissue is heated to 50°C to 70°C to destroy fat and contract connective tissue, simultaneously remodeling the dermis at external temperatures up to 42°C. This handpiece allows tissue treatment using a 10cm cannula that provides treatment depth up to 25mm.
AccuTite – The minimally invasive AccuTite handpiece, introduced in 2019, utilizes directional RF energy for RFAL treatments using sub-millimeter cannula with diameters of 0.9mm and external electrodes to apply RF energy to subcutaneous adipose tissue. The tissue is heated to 50°C to 70°C to destroy fat and contract connective tissue, simultaneously remodeling the dermis at external temperatures of up to 42°C. This handpiece allows tissue treatment using a 60mm cannula that provides treatment depth up to 25mm. Additionally, in 2019, we began marketing AccuTite for Aviva, a minimally invasive procedure that restores the function and appearance of the vulva by offering a non-excisional alternative to a labiaplasty. Aviva is powered by AccuTite to deliver safe and uniform heat to the entire soft tissue matrix of the labia minora, labia majora, clitoral hood, vaginal introitus and perineal body.
QuantumRF 10 and QuantumRF25 – The minimally invasive disposable handpieces for minimally invasive treatment of face and body for simultaneous treatment of destroying fat and skin tightening. The QuantumRF handpieces are operated on the IgniteRF platform.
Fractora – The minimally invasive Fractora handpiece, introduced in 2011, uses customizable fractional energy and superficial fractional resurfacing for subdermal adipose tissue remodeling. The handpiece offers two treatment depths (skin surface and subdermal) and is safe on all skin types including type IV. The consumable applicator tip contains 24-coated pins with a length of up to 4mm.
Morpheus8/Morpehus8 Body/moprheus8 Burst/Noprheus8 Deep/Morpheus8V – The minimally invasive Morpheus8 and Morpheus8 Body handpieces use RF energy for subdermal adipose tissue remodeling, which is programmable by the user according to treatment area. The handpieces offer treatment depth up to 7mm. The upper part of the needle and external electrodes are coated with a polymer to prevent skin surface thermal damage while delivering RF energy into the subdermal space.
Non-Invasive
BodyFX/MiniFX – The non-invasive BodyFX and MiniFX handpieces, introduced in 2013, combine vacuum and bipolar RF energy with high and low amplitudes to both permanently destroy adipose tissue and contract the skin. The BodyFX handpiece, intended for use on various parts of the body, comprises a vacuum cavity with a size of 1.36in x 1.2in. The MiniFX handpiece, better suited to address problematic fatty tissue in smaller areas, comprises a vacuum cavity with a size of 1.24in x 0.87in.
DiolazeXL (810nm) – The non-invasive DiolazeXL (810nm) handpiece, introduced in 2017, is a high-speed, gold standard 810nm (diode) laser indicated for permanent hair reduction. DiolazeXL’s differentiated triple contact cooling technology (pre, parallel and post), or 3PC technology, provides for a safe and comfortable patient experience. The handpiece covers a spot size of 12mm x 26mm to allow for the removal of a variety of hair colors and thickness. This handpiece offers short and long pulse durations and repetition rates that enable treatment times up to 6cm2/second.
Triton Duo Light (755nm & 810nm) – The non-invasive Triton Duo Light handpiece, introduced in 2017, combines two wavelengths for optimal treatment of light skin patients. The handpiece utilizes a blend of 755nm (Alexandrite) and 810nm (diode) laser wavelengths that have been optimized for hair removal on patients with skin types I to IV. We believe the Triton platform is the only FDA-cleared device capable of firing two wavelengths in one pulse. The handpiece covers a spot size of 12mm x 26mm and provides two pulse durations and high repetition rates.
Triton Duo Dark (810nm & 1064nm) – The non-invasive Triton Duo Dark handpiece, introduced in 2017, combines two wavelengths for optimal treatment of dark skin patients. The handpiece utilizes a blend of 810nm (diode) and 1064nm (Nd:YAG) laser wavelengths that have been optimized for hair removal on patients with skin types Ito IV. We believe the Triton platform is the only FDA-cleared device capable of firing two wavelengths in one pulse. The handpiece covers a spot size of 12mm x 26mm and provides two pulse durations and high repetition rates.
Forma – The non-invasive Forma handpiece, introduced in 2013, uses our ACE technology to deliver auto-adjusting uniform RF energy-generated heat (up to 43°C) for collagen remodeling and skin contraction of the face and neck. We believe Forma is the first thermal face and neck skin tightening device to have both temperature monitoring and automatic, user programmable, RF on/off control. This handpiece has an RF energy output power of up to 65 watts and covers a spot size of 22mm x 20mm.
Forma I – The non-invasive handpiece introduced in 2022 for treatment of peri-orbital and meibomian glands, and for dry eye indication by Health Canada. The energy level is similar to Forma, and the spot size is 10 sq. millimeters.
FormaV – The non-invasive FormaV handpiece, introduced in 2017, uses our ACE technology to deliver auto-adjusting uniform RF energy generated heat (up to 43°C) to vaginal tissue through a consumable applicator.
VTone – Intervaginal EMS device to treat SUI and pelvic floor muscle restorations.
Plus – The non-invasive Plus handpiece, introduced in 2013, uses our ACE technology to deliver auto-adjusting uniform RF generated heat (up to 43°C) for collagen remodeling and skin contraction of the body. We believe Plus is the first thermal body skin tightening device to have both temperature monitoring and automatic, user programmable, RF on/off control. This handpiece has an RF energy output of up to 65 watts and covers a spot size of 45mm x 45mm.
Lumecca Peak – The non-invasive Lumecca and Lumecca Peak handpiece, introduced in 2015 and 2024, are IPL handpieces optimized for both light and dark skin that uses a xenon flash lamp to deliver filtered optical energy in the 515nm to 1200nm range for light skin treatment and 580nm to 1200nm range for darker skin. Lumecca is intended for treatment of superficial vascular and pigmented lesions. The handpiece covers a spot size of 30mm x 10mm with a peak optical power of 10,000 watts.
Vasculaze – The non-invasive Vasculaze handpiece, introduced in 2018, is a 1064nm wavelength diode laser intended for use in the coagulation and hemostasis of benign vascular legions such as, but not limited to, reticular leg veins, spider veins, hemangiomas, port wine stains and venous lakes. Vasculaze is optimized with high peak power, strong contact cooling and an ergonomic head intended to maximize treatment efficiency. The handpiece covers a spot size of 3mm x 4mm and has pulse duration of 20 to 100 milliseconds, or msec.
Hands-Free
Transform – The non-invasive Transform, introduced in 2021, is a set of six hands-free applicators for the EvolveX platform. The mechanism of action is a combination of Bipolar RF and EMS. The Transform is mounted on a belt and works automatically without the assistance of a physician or technician.
Tite – The non-invasive Tite, introduced in 2019, is a set of eight hands-free applicators for the EvolveX platform. The mechanism of action is similar to the Plus handpiece. The Tite is mounted on a belt for treatment of different body parts and areas without the assistance of a physician or technician.
Tone – The non-invasive Tone, introduced in 2019, is a set of four hands-free electro-muscle stimulation applicators. The Tone is designed to be used on the EvolveX platform for muscle stimulation and improving skin tone.
Cheek – The non-invasive Cheek, introduced in 2020, is a hands-free device containing eight applicators that is mounted on the face and designed to be used with the Evoke platform or Define platform. The mechanism of action is similar to the Forma handpiece, but works automatically on both sides of the face without the assistance of a physician or technician.
Chin – The non-invasive Chin, introduced in 2020, is a hands-free device containing two applicators that is mounted on the chin and designed to be used with the Evoke platform or Define platform. The mechanism of action is similar to the Forma handpiece, but works automatically on the chin without the assistance of a physician or technician.
Proprietary Software
Our software permits the user to define treatment parameters to be communicated to the electronic modules in the platform and deliver RF or optical energy through the handpiece or hands-free applicator to the patient. In addition, our software controls and manages proper system performance and automatic temperature control, system self-calibration, system setup and detection of any malfunction of the system. We believe our software’s automotive capabilities allow physicians to dedicate their attention and focus to patient treatment rather than system monitoring. Our users upgrade their products through the purchase of additional treatment applicators and corresponding software plugs. All of our software complies with applicable medical specifications and regulations.
Applications and Procedures
Our products provide our customers with a broad range of applications among both traditional procedures and emerging applications.
Face and Body Contouring
Minimally Invasive
Generally performed by a physician, RFAL delivers directional RF energy into a patient’s subcutaneous fat to coagulate and liquefy adipose tissue and heat the subcutaneous fibrous septa, resulting in substantial collagen contraction of the subdermal space. RF energy is delivered through an innovative handpiece comprised of two electrodes. The internal electrode is inserted into the fat layer, while the other larger electrode is applied externally to the skin surface above the cannula tip. The internal cannula passes through the subcutaneous fat, while the external electrode slides over the skin’s surface. The small conductive tip of the cannula concentrates RF energy in the subcutaneous fat, liquefying it and simultaneously contracting the fiber septa. This liquefied fat can then be removed from the body. Our RFAL technology can be administered on all regions of the body and typical treatments are approximately 30 to 90 minutes each under local anesthesia. We received 510(k) FDA clearance for our RFAL technology in 2016. Users conduct minimally invasive face and body contouring using RFAL technology with the BodyTite and Embrace platforms and BodyTite, FaceTite and AccuTite handpieces. In 2024, we introduced the IgniteRF platform with the QuantumRF handpiece which is also based on RFAL technology and received 510 K FDA clearance for the QuantumRF in 2024.
Generally performed by a physician, Deep Subdermal Fractional RF, delivers RF energy into the subcutaneous adipose tissue to depths of up to 4mm through an array of coated needles producing localized heat and matrix of small lesions in the subcutaneous fat. The heat generated by the pins in the subdermal tissue promotes connective tissue restructuring. As a result, physicians can offer a versatile fractional treatment creating a three-dimensional collagen contraction and subdermal fat coagulation. The deep fractional remodeling is used when fat layer is not thick enough to use RFAL technology or when patient wants only superficial result. Deep Subdermal Fractional RF can be used for wrinkle reduction, skin tightening and treatment of cellulite appearance. In addition to reshaping, Deep Subdermal Fractional RF provides long-term results for inflammatory acne by coagulating enlarged sebaceous glands. The most common areas of treatment are the face and neck. Patients generally receive between one to three treatments for approximately 30 minutes each. Treatments are typically spaced two to three weeks apart. We received two 510(k) FDA clearances for Fractora in 2011 and 2016, and 510(k) FDA clearance for the Morpheus8 in 2019. In addition, we received 510K clearance for Morpheus8soft tissue coagulation/contraction in 2024. Customers use this technology with the BodyTite, Embrace, IgniteRF, Votiva, Empower, Optimas and OptimasMAX platforms.
Non-Invasive
Handpieces: Administered by physicians and other aesthetic practitioners, our differentiated fat reduction solution is based on skin shaping using a vacuum and delivering both low amplitude bipolar RF energy for gentle deep tissue heating and high amplitude RF energy to simultaneously destroy fat and tighten skin. The handpiece is placed over the desired area of the body and vacuum energy shapes the skin into the cavity for safe and effective RF energy delivery allowing greater volumes of fat to be treated (up to 2.5cm in depth). Subsequently, temperature-controlled RF energy is applied to preheat the tissue and fat uniformly to 42°C to 43°C. High amplitude RF energy delivered in ultra-short pulse duration is then administered via electrodes causing apoptosis of adipose tissue and resulting in simultaneous skin contraction. Our technology can be administered on all regions of the body. Patients generally receive six treatments for approximately 10 to 20 minutes each. Treatments are typically spaced one to two weeks apart. We received 510(k) FDA clearance for this technology in 2013. Users conduct non-invasive face and body contouring with the Contoura platform using the BodyFX or MiniFX handpieces.
Hands-Free
Hands-Free Applicators: Applied by physicians and other aesthetic practitioners, our differentiated skin tightening, fat reduction and muscle stimulation solution is based on bipolar RF and EMS technologies, delivered through a set of hands-free applicators mounted or placed over the body or face. The setup of the parameters is determined by a doctor. Subsequently, temperature-controlled RF energy is applied to preheat the tissue and fat uniformly to 42°C to 43°C. High amplitude RF energy delivered in ultra-short pulse duration is then administered via electrodes causing apoptosis of adipose tissue and resulting in simultaneous skin contraction. Our technology can be administered on all regions of the body and face. Patients generally receive three to six treatments for approximately 30 minutes each. Treatments are typically spaced one to two weeks apart. We received 510(k) FDA clearance for the Evoke and Evolve in 2019. In 2021 we received 510(k) FDA clearance for the EvolveX which replace the Evolve platform. Users conduct hands-free face and body contouring with the Evoke and EvolveX platforms using the Transform, Tite and Tone hands-free applicators for the EvolveX, and Cheek and Chin hands-free applicators for the Evoke and Define.
Medical Aesthetics
Skin Rejuvenation/Vascular & Pigmented Lesion Treatment
Generally performed by a physician or other aesthetic professional, different types of energy and treatments are provided depending on the handpiece. The application of JPL energy enables the improvement of photo damage, as well as other pigmented abnormalities and superficial vascular lesions. A 1064nm laser is used for the treatment of larger and deeper veins. Patients often see results after a single treatment but typically two to three treatments of approximately 15 minutes each are recommended. Treatments are typically spaced two to four weeks apart. We received 510(k) FDA clearance for superficial vascular and pigmented lesion treatments in 2013 and for hair removal and permanent hair reduction in 2017 and 2018, respectively. Users rejuvenate the skin with the Optimas and OptimasMAX platforms using the Vasculaze, Lumecca and Lumecca Peak handpieces.
Sub-Necrotic Thermal Tissue Remodeling
Administered by physicians and other aesthetic practitioners, the application is based on uniform and deep heating of the skin and subdermal layer using bipolar RF energy. The handpiece is moved over the desired area of the treatment area, while maintaining the designated temperature for the predetermined time for safe and effective collagen remodeling. Subsequently, temperature-controlled RF energy is applied automatically to heat tissue uniformly to 42°C to 43°C. The Forma handpiece is utilized to target fat and heats the tissue to depths of up to 4.5mm while the Plus handpiece is used for larger body areas and provides effect up to 6mm in depth. Patients generally receive six treatments for approximately 10 to 20 minutes each. Treatments are typically spaced one to two weeks apart. We received three 510(k) FDA clearances for this technology in 2014, 2016 and 2017. Users conduct facial treatment with the Optimas and OptimasMAX platforms using the Forma handpiece and body contouring with the Contoura platform using the Plus handpiece.
Permanent Hair Reduction
Administered by a user, who is not necessarily a physician, our differentiated dual wavelength technology incorporated in the Triton platform fires two wavelengths in a single pulse destroying the hair follicles located in the dermis and subdermal layers. This procedure is continued over the target area and can last from a few minutes to 90 minutes depending on the size of the treatment area. In general, permanent hair reduction requires four to six treatments spaced four to eight weeks apart. More sessions may be required for stubborn hairs. Due to our unique dual wavelength technology, our Triton platform allows practitioners to address all skin types and tones. We received 510(k) FDA clearance for our Optimas and dual wavelength Triton platforms for permanent hair reduction treatments in 2016 and 2018, respectively. Users perform permanent hair reduction procedures with our Optimas and Triton platforms using the DiolazeXL and Triton Duo Light and Triton Duo Dark handpieces, respectively.
Women’s Wellness
We have two platforms for women’s wellness: the Votiva and the EmpowerRF. Procedures with these platforms are performed by a physician. Depending on the handpiece, the administration of bipolar RF energy or subdermal heating is applied to gently warm and massage the internal vaginal tissue (FormaV), the external vaginal tissue (Morpheus8V), or to deliver uniform heat to the entire soft tissue matrix of the labia minora, labia majora, clitoral hood, vaginal introitus and perineal body (Aviva procedure administered by the AccuTite handpiece), and pelvic floor muscle restoration (with VTone). Depending on the treatment type, patients typically receive between two to three treatments of approximately 15 minutes each. Treatments are typically spaced two to three weeks apart. We received 510(k) FDA clearance for FormaV for certain indications in 2017. In July 2018, we received a letter from the FDA seeking information as to the regulatory basis for marketing of our FormaV handpiece based on our promotion and labeling of this device for use in certain women’s wellness conditions and procedures. We timely responded to the FDA by immediately altering the wording of our promotional and labeling materials, and we submitted a response letter in August 2018 answering the FDA’s questions and advising the agency that we had modified our promotional and labeling materials to remove certain statements regarding uses of our products for conditions and procedures that were questioned by the FDA in the agency’s informational request letter. The FDA responded in September 2018 by stating that the agency had reviewed our response letter and verified the changes in terminology made to our website. Moreover, the FDA further responded in November 2018 and confirmed we addressed all items raised by the agency in its letter, and that the FDA continues to expect us to conduct a review of our marketing and promotional materials to make appropriate changes and remove materials containing uncleared claims. We have received no further communications from the agency regarding this matter.
Sales and Marketing
Our primary strategy to increase market penetration relies on selling directly to our traditional customer base of plastic and facial surgeons, aesthetic surgeons, dermatologists and OB/GYNs. We believe we are the only company commercializing minimally invasive aesthetic solutions specifically targeting the surgical community and believe our products represent a significant opportunity for these practitioners to deliver improved patient treatment results and significantly increase their ability to generate additional revenue. We are also targeting newer market opportunities consisting of OB/GYNs, ENTs, ophthalmologists, general practitioners and aesthetic clinicians as an incremental growth opportunity.
We target potential customers through office visits, trade shows, professional journals and various forms of paid and unpaid media. We also conduct clinical workshops featuring recognized expert panelists and key opinion leaders to promote existing and new treatment techniques using our products. We believe that these workshops enhance customer loyalty and provide us with new sales opportunities. We plan to continue to offer a large number of workshops spanning from single-day workshops to three-day workshops. We also use direct mail programs to target specific segments of the market that we seek to access, such as members of medical societies and attendees at meetings sponsored by medical societies or associations. In addition, we maintain an active public relations program that has resulted in treatments based on our products being featured in various televised and printed media outlets including InStyle, Shape, The Doctors and Harper’s Bazaar. In September 2022, international actress and producer, Eva Longoria, agreed to join us as our brand ambassador to share her positive experience with our EvolveX and Morpheus8 technologies, replacing our previous brand ambassador, international pop icon, Paula Abdul.
We currently sell and market our products in the United States, Canada, the United Kingdom, Ireland, Spain, Portugal, France, Belgium, Luxemburg, Italy, Germany, Austria, Japan, Australia and India, through a direct sales force of approximately 260 representatives. We also sell and market our products through over 60 distributors in over 85 countries. Our U.S. sales efforts are headquartered in Irvine, California. To support the continued roll-out of our products and further penetrate the market, we anticipate that our direct salesforce in the United States will continue to increase.
In international markets, to complement our direct sales force in Canada, the United Kingdom, Spain, Portugal, France, Belgium, Luxemburg, Italy, Australia and India, we sell our products through a network of distributors. Our Canadian sales efforts are headquartered in Toronto, Canada. As of December 31, 2024, we had an international sales management team of seven employees supporting over 60 independent distributors. The percentage of our revenues from customers located outside of the United States for each of the years ended December 31, 2024 and 2023 was 38%, and 37%, respectively. We intend to continue to increase penetration of our customer base in international markets and expand into attractive new international markets by identifying and training qualified distributors. In addition, we may opportunistically hire a direct sales force and expand our marketing campaigns in select international markets. We require our distributors to provide customer training, to invest in service and demonstration equipment and marketing, and to attend certain exhibitions and industry meetings.
Service and Support
We support our customers with a range of services, including business and practice development consulting and product service and maintenance.
We service our products in three service centers: (1) the U.S. market is serviced through our facility in Irvine, California, (2) the Canadian market is serviced through our facility in Toronto, Canada, and (3) the rest of the world is serviced through our distributors and our facility in Yokneam, Israel. In the event of a technical malfunction, our customers first contact us (if in the United States or Canada) or our distributors (if outside of the United States or Canada) telephonically. If a product requires service or repair that cannot be addressed telephonically, we or our distributors ship a temporary “loaner” system to the customer as soon as possible, often overnight. This unique “loaner” system reduces any product downtime and associated lost revenues for the physician. We then arrange for shipment of the out-of-order product to one of our service centers in the United States or Canada. Outside the United States or Canada, the product is sent to our distributors or our facility in Israel. Either we or our distributors quickly repair the product and ship it back to the customer. We have designed our products in a lightweight, modular fashion to enable quick and efficient service and support. Specifically, we build our platforms to be less than 35 kilograms in weight to ensure acceptance by traditional, commercial third-party logistics providers for next-day delivery of replacement products without requiring specialized shipping procedures. We believe our depot service and support model provides for more efficient and less costly operations.
Our standard warranty term is 12 months, however, certain products are sold with multi-year warranties. Our standard warranty covers parts, labor, participation in our loaner program and a white glove, door-to-door, shipping service for expedited repair service, and can be extended for an additional charge. We believe that we have a significant opportunity to increase our recurring customer revenue by increasing the number of our customers that enter into service contracts and extended warranties for our systems. All of our distributors have a service department and are required by us to maintain a full inventory of spare parts. All service staff is trained by our service department in Israel.
Manufacturing and Supply
We rely primarily on outsourced manufacturing to produce our devices while maintaining control over the production process. Outsourcing allows us to carry lower inventory levels and maintain fixed unit costs without incurring significant capital expenditures than would be in place if products were self-manufactured. We outsource almost all of the manufacturing of our products to three subcontractors located in Israel, two of which we are substantially dependent on as part of our business. Through our strategic arrangement with Flextronics (Israel) Ltd., or Flex, and (BY) Medimor Ltd., or Medimor, we maintain dedicated manufacturing lines supervised by us in Flex and Medimor’s medical-grade manufacturing facilities in Migdal Haemek and Poriya, Israel. Within the Flex and Medimor facilities, all proprietary manufacturing, testing and assembly equipment has been built and is owned by us. We also use a separate manufacturer in addition to Flex and Medimor to produce our handpieces and disposables.
We believe our outsourced manufacturers’ processes comply with all applicable U.S. and international quality and safety standards, such as ISO 13485:2016, CE and the FDA quality system regulations. We conduct in-house prototype development and present detailed manufacturing documents to our subcontractors, who then purchase most of the necessary components and manufacture the product. These manufacturing subcontractors provide us fully assembled, or “turn-key,” services. We control and monitor the quality of our products by having one of our quality control employees at each of our subcontractor’s facilities.
The contracts we have with our main manufacturing subcontractors (Flex and Medimor) do not have minimum purchase requirements and allow us to purchase end products entered into on a purchase order basis. Under these contracts, our manufacturing subcontractors provide manufacturing services pursuant to our written specifications. These manufacturing services include labor, materials, testing, packaging and delivery, as well as allocating production and storage space within their facilities for our products. Pricing under these contracts is reviewed between us and the manufacturing subcontractors every three months. These contracts typically have one-year terms that automatically renew for successive one-year terms unless either we or the manufacturing subcontractor provide three months written notice prior to the expiration of the term. To date, we have not experienced any significant manufacturing delays. These contracts can be terminated by either party, without cause, with four to six months prior written notice.
We manufacture all laser and IPL handpieces in our facility in Yokneam, Israel and procure other major components of our products on behalf of our third-party manufacturers from a limited number of suppliers. We have flexibility to adjust the number of lasers and other components that we either manufacture or procure as well as the delivery schedules. The forecasts that we use are based on historical demands and expected future plans. Lead times may vary significantly depending on the size of the order, time required to fabricate and test the components, specific supplier requirements and current market demand for the components. We intend to reduce any potential for delays of supply by maintaining relationships with multiple suppliers of major components. To date, we have not experienced significant delays in obtaining our major components.
In November 2019, we signed a Share Purchase and Shareholders Agreement, or the SPA, with Medimor, one of our turnkey manufacturing subcontractors. Pursuant to the SPA, we have invested an aggregate of $600,000, in consideration for 1,369,863 ordinary shares of Medimor (which reflected a 10.34% ownership interest in Medimor at the signing date, on a fully diluted basis), at a price per share of $0.438, of which 414,384 ordinary shares were issued upon consummation of the initial closing on December 31, 2019, and the remaining 955,479 ordinary shares were issued in July 2020 following Medimor achieving certain pre-defined milestone events. In November 2023, we signed an additional Share Purchase and Shareholders Agreement (the “Purchase Agreement”) in which we invested an additional $100,000 in consideration of 117,440 ordinary shares of Medimor.
As of December 31, 2024, we hold 14.78% ownership interest on an as-issued basis and 11.23% ownership interest on a fully diluted basis of Medimor.
Research and Development
Our research and development activities are conducted internally by a team of 43 research and development staff based mainly in Israel. Our research and development efforts are focused on the development of new products, as well as on the extension of our existing products to introduce new applications in the minimally and non-invasive and medical aesthetic markets. We have a number of new projects and products under development. We expect to concentrate our research and development efforts in the coming years on developing procedures and platforms based on our proprietary technologies: (i) RFAL, (ii) Deep Subdermal Fractional RF, (iii) Simultaneous Fat Destruction and Skin Tightening, and (iv) Deep Heating Collagen Remodeling, and developing new technologies.
Our research and development expenditures for the years ended December 31, 2024 and 2023 were approximately $13.1 million and $13.4, respectively.
Seasonality
Our business is not significantly impacted by seasonality; however, our fourth quarter has historically generated slightly stronger operating results. We have historically experienced stronger sales in the fourth quarter in correlation with our customers’ spending patterns and budget cycles. Most physicians operate on an annual budget cycle with a fiscal year that begins on January 1. It is not uncommon to experience a higher level of purchasing activity from physicians in the final months and weeks of their fiscal year. Consequently, our fourth quarter revenues may be greater than other quarters. Our business is also impacted by general economic conditions, which may impact future seasonal variations.
Intellectual Property
We rely on a combination of patent, trademark and copyright laws to protect our intellectual property rights.
Patents and Patent Applications
As of February 4, 2025, we own fifteen issued U.S. patents and one issued Korean patent. As of February 4, 2025, we have filed eleven patent applications that are pending in the United States Patent and Trademark Office. Our issued U.S. patents are projected to expire between 2027 and 2042 (assuming pending U.S. patent applications are approved). These patents and patent applications cover the technologies described herein, and contribute to the protection of our rights to our proprietary technology. Our patents relate to radio frequency (RF) based technology that may be used for minimally invasive aesthetic solutions, such as fat destruction, and fractional skin ablation relating to skin tightening and fat destruction, among others, and cover our existing products. Without these patents, we cannot guarantee that we can prevent others from manufacturing similar products that are covered by our patent rights. We also rely on our issued patents to make, use, sell, and distribute our products. The term of the patents depends on the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. We also rely upon trademarks in various jurisdictions covering the InMode brand and our product lines, as well as upon U.S. copyright law for protection of the software programs associated with our products.
We cannot assure you that patents will issue from any of our pending applications or that, if patents issue, they will be of sufficient scope or strength to provide meaningful protection for our technology. Our policy is to obtain patents and to seek to operate without infringing on the intellectual property rights of third parties. Loss or invalidation of our patents, or a finding of unenforceability or limitation of scope of our patents, could have a material adverse effect on us. The patent position of many inventions in the areas related to our business is highly uncertain, involves many complex legal, factual and technical issues and has recently been the subject of litigation industry-wide. There is no certainty in predicting the breadth of allowable patent claims or the degree of protection afforded under any issued patents.
Notwithstanding the scope of the patent protection available to us, a competitor could develop treatment methods or devices that are not covered by our patents. Furthermore, numerous U.S. and foreign issued patents and patent applications owned by third parties exist in the fields in which we are developing products. Because patent applications can take many years to issue, there may be applications unknown to us, which applications may later result in issued patents that our existing or future products or proprietary technologies may be alleged to infringe. Third parties may also obtain patents that we may need to license from them in order to conduct our business.
It is possible that patents issued to us will be successfully challenged or that patents issued to others may preclude us from commercializing our products under development. Litigation to establish or challenge the validity of patents, to defend against infringement, unenforceability or invalidity claims or to assert infringement, invalidity or unenforceability claims against others, if required, can be lengthy and expensive, and may result in determinations materially adverse to us. We cannot assure you that the products currently marketed or under development by us will not be found to infringe patents issued or licensed to others.
Patent Litigation
We have received, and we may in the future receive, allegations from third parties contending that we are infringing their patents. If such third parties were to commence infringement suits against us, and such third-party patents were found by a court to be valid, enforceable and infringed upon by us, then we could be required to pay damages and/or make royalty payments, and we could also be enjoined from continuing the infringing activity. Depending on the nature of the patent found to be infringed upon by us, a court order requiring us to cease such infringement could have a material adverse effect on us. We might be unable to design around such patents or continue offering the products or services found to be infringing, or we could suffer other adverse consequences.
On October 11, 2023, we filed a complaint of patent infringement against BTL Industries Ltd. (“BTL”) in the United States District Court, Central District of California, alleging that BTL infringed our U.S. patent no. 8961511. In December 2024, the litigation was transferred to the District of Massachusetts, and BTL has filed an answer to our complaint. On April 10, 2024, BTL filed a petition for inter partes review (IPR) before the Patent Trial and Appeal Board of the United States Patent Office, and the Board instituted review of the patent on October 8, 2024. BTL has filed a motion to stay the district court case pending the IPR proceeding, which motion currently is pending.
Although we may try to resolve any potential future claims or actions, we may not be able to do so on reasonable terms, if at all. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate, and could divert management’s attention from our core business. If we lose this kind of litigation, a court could require us to pay substantial damages and could prohibit us from using technologies essential to our products, either of which would have a material adverse effect on our business, results of operations and financial condition. See “Item 3. Key Information – D. Risk Factors-Risks Related to Our Intellectual Property – If we are unable to protect our intellectual property rights, our competitive position could be harmed. Our success and ability to compete depends in large part upon our ability to protect our proprietary technology.”
Copyrights, Trademarks and Trade Secrets
The software programs associated with our products are protected by U.S. copyright law.
We also filed for protection available under trademark law. As of December 31, 2024, we own 16 registered trademarks in the United States and we own at least 38 registered trademarks in various jurisdictions outside the United States, including for the marks “InMode” and “RFAL” and certain key product names, in particular, BodyTite, Contoura by InMode, FaceTite, InMode, Optimas by InMode, Triton by InMode, Votiva by InMode, Triton by InMode, AccuTite, Morpheus, BodyFX, Diolaze, Fractora and Lumecca. We also have at least 6 pending foreign trademark applications. We also have 9 trademark applications in the United States pending for “Define by InMode”, “Fusion Dark”, Fusion Light”, and “QuantumRF”.
We also rely upon know-how and continuing technological innovation, and may pursue licensing opportunities in the future, to develop and maintain our competitive position. We seek to protect our proprietary rights through a variety of methods, including confidentiality agreements and proprietary information agreements with suppliers, employees, consultants and others who may have access to proprietary information, under which they are bound to assign to us inventions made during the term of their employment or term of service.
All professional employees and technical consultants are required to execute confidentiality agreements in connection with their employment and consulting relationships with us. We also require them to agree to disclose and assign to us all inventions conceived in connection with their services to us. However, there can be no assurance that these confidentiality and invention assignment agreements will be enforceable or that they will provide us with adequate protection.
Competition
Our industry is subject to intense competition, subject to rapid change and highly sensitive to the introduction of new products or other market activities of industry participants. We compete against products offered by public companies, including AbbVie, Cutera, Inc., Apyx Medical Corporation, Venus Concept Inc. and Sisram Medical Ltd., as well as by private companies, such as Cynosure LLC/Lutronic, Lumenis Ltd., BTL Aesthetics, Inc. and Candela Medical Inc. In the past few years, several large pharmaceutical and medical device companies have also entered the aesthetic device market, including Valeant Pharmaceuticals International Inc. and Merz Pharma Group. Our products compete against conventional medical products, including Botox®, hyaluronic acid injections and collagen injections, and aesthetic procedures, such as face lifts, liposuction, sclerotherapy, electrolysis, chemical peels and microdermabrasion, which are unrelated to laser, light and RF-based technologies. Our products also compete against laser and other light and radio frequency-based products.
Competition among providers of laser and other light and radio frequency-based products for the aesthetic medical market is characterized by extensive research efforts and rapid technological progress. While we attempt to protect our products through patents and other intellectual property rights, there are few barriers to entry that would prevent new entrants or existing competitors from developing products that would compete directly with ours. There are many companies, both public and private, that are developing innovative devices that use laser and other energy-based and alternative technologies. Many of these competitors have significantly greater financial and human resources than we do and have established reputations, greater brand name recognition, broader product lines, and larger customer bases, as well as worldwide distribution channels that are more effective than ours. Additional competitors may enter the market, and we are likely to compete with new companies in the future. Any business combinations or mergers among our competitors that result in larger competitors with greater resources or distribution networks, or the acquisition of a competitor by a major medical or technology corporation seeking to enter this business, could further result in increased competition.
To compete effectively, we have to demonstrate that our products are attractive alternatives to other devices and treatments by differentiating our products on the basis of performance, brand name, reputation and price. We have encountered and expect to continue to encounter potential customers who, due to existing relationships with our competitors, are committed to, or prefer the products offered by, these competitors. We expect that competitive pressures may over time result in price reductions and reduced margins for our products.
Other medical companies, academic and research institutions, or others, may develop new technologies or therapies, including medical devices, surgical procedures or pharmacological treatments and obtain regulatory approval for products utilizing such techniques that are more effective in treating the conditions that we target or are less expensive than our current or future products. Our technologies and products could be rendered obsolete by such developments.
Government Regulation
Our products and operations are subject to extensive and rigorous regulation by the relevant governmental authorities in the countries where we market and sell or products. These governmental authorities include the FDA, which enforces, the FDCA as well as other similar laws, and regulatory bodies worldwide. In addition, the Federal Trade Commission, or FTC, regulates the advertising of our products in the United States. Further, we are subject to laws directed at preventing fraud and abuse, which subject our sales and marketing, training and other practices to government scrutiny.
In some jurisdictions, such as the United States, Canada, South Korea and Israel, we must complete an application process with the relevant regulator, which includes submitting the results of clinical trials for their review.
In addition to the requirements regarding product clearance, many countries also impose product standards, packaging requirements, environmental requirements, labeling requirements and import and export restrictions on our devices. Each country also has its own tariff regulations, duties and tax requirements. Failure to comply with applicable regulatory requirements may result in fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, criminal prosecution, or other consequences.
In the United States, the FDCA and its implementing regulations govern the following activities that we perform and will continue to perform to help ensure that medical products distributed within the United States are safe and effective for their intended uses:
| • | product design and development; |
| • | premarket clearance or approval; |
| • | advertising and promotion; |
| • | manufacturing and production; |
| • | product sales and distribution; |
| • | import, export and shipping; |
| • | establishment registration and device listing; and |
| • | recalls, field-safety corrective actions and post-market surveillance. |
Each of our currently marketed products has received 510(k) clearance for the uses for which they are being marketed.
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States requires 510(k) clearance or premarket approval. The FDA classifies medical devices into one of three classes – Class I, Class II or Class III – depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to ensure safety and effectiveness. Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be assured by adherence to the FDA’s General Controls for medical devices, which include compliance with the applicable portions of the QSR, facility registration and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising, and promotional materials. Class II devices are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. While most Class I devices are exempt from the 510(k) premarket notification requirement, manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a 510(k) premarket notification is generally known as 510(k) clearance. All of our current products are Class II devices subject to the 510(k) clearance requirements.
Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a premarket approval application, or PMA. Some pre-amendment devices are unclassified, but are subject to the FDA’s premarket notification and clearance process in order to be commercially distributed.
510(k) Clearance Pathway
When a 510(k) clearance is required, we must submit a premarket notification demonstrating that our proposed device is “substantially equivalent,” as defined in the statute, to a previously cleared 510(k) device or a device that was in commercial distribution in the United States before May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted, for which the FDA has not yet called for the submission of premarket approval applications.
After a 510(k) premarket notification is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) notification. If it is accepted for filing, the FDA begins a substantive review. By statute, the FDA is required to complete its review of a 510(k) notification within 90 days of receiving the 510(k) notification. As a practical matter, if the FDA requires additional information, clearance often takes far longer, and clearance is never assured. Although most 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including clinical data, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
If the FDA determines that the device is not “substantially equivalent” to a predicate device, or if the device is automatically classified into Class III, the device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA approval process, or seek reclassification of the device through the de novo process. A manufacturer can also submit a petition for direct de novo review if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application or de novo classification. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. Many minor modifications are accomplished by a letter-to-file in which the manufacture documents the change in an internal letter-to-file. The letter-to-file is in lieu of submitting a new 510(k) to obtain clearance for such change. The FDA can always review these letters to file in an inspection. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA is obtained. In addition, in these circumstances, the FDA can impose significant regulatory fines or penalties for failure to submit the requisite PMA(s).
Over the last several years, the FDA has proposed reforms to its 510(k)-clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k)-clearance process for their products. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the FFDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals included plans to potentially sunset certain older devices that were used as predicates under the 510(k)-clearance pathway and to potentially publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. In May 2019, the FDA solicited public feedback on these proposals. These proposals have not yet been finalized or adopted, and the FDA may work with Congress to implement such proposals through legislation.
In September 2019, the FDA finalized guidance describing an optional “safety and performance based” premarket review pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k) clearance pathway by showing that such device meets objective safety and performance criteria established by the FDA, thereby obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. The FDA maintains a list of device types appropriate for the “safety and performance based” pathway and will continue to develop product-specific guidance documents that identify the performance criteria for each such device type, as well as the testing methods recommended in the guidance documents, where feasible.
Laser devices used for aesthetic procedures, such as hair removal, have generally qualified for clearance under 510(k) procedures.
The table below presents the specific FDA 510(k) clearances, dates and summary of cleared indications for our BodyTite (Inmode RF/IgniteRF), Optimas, Votiva, Contoura, Triton, EmbraceRF, Morpheus8, EvolveX, Define and Evoke platforms:
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| Radiofrequency (RF) | Cheek Chin Forma MiniFX Morpheus8 | K242598 (11/24/2024)
The Define System with the Cheek and Chin Applicators is indicated for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
The Define System with the Forma Applicator is intended for the relief of minor muscle aches and pain, relief of muscle spasm, and for the temporary improvement of local blood circulation. The Define System with the MiniFX Applicator is intended for the relief of minor muscle aches and pain, relief of muscle spasm, for the temporary improvement of local blood circulation and for the temporary reduction in the appearance of cellulite. The Define System with the Morpheus8 Applicator is intended for use in dermatologic procedures where coagulation/contraction of soft tissue or hemostasis is needed. At higher energy levels greater than 62 mJ/pin, the use of the Morpheus8 Applicator is limited to Skin Types I-IV. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| InMode RF / IgniteRF | Radiofrequency (RF) | QuantumRF 25 minimally invasive handpiece
QuantumRF 10 minimally invasive handpiece | K240780 (07/24/2024)
InMode RF/IgniteRF platform with the FaceTite or BodyTite is indicated for use in dermatologic procedures where coagulation/contraction of soft tissue or hemostasis is needed.
|
| InMode system with Morpheus8 90 applicators (coagulation/contraction of software tissue) | Radiofrequency (RF) | • The standard Morpheus 8 90 Applicator is limited to use with the Morpheus8 90 tip head. | K240017 (06/13/2024)
The InMode System with the Morpheus8 90 Applicator is intended for use in dermatological procedures where coagulation/contraction of soft tissue or hemostasis is needed. At higher energy levels greater than 62 mJ/pin, the use of the Morpheus8 Applicator is limited to Skin Types I-IV.
|
| InMode RF / IgniteRF | Radiofrequency (RF) | BodyTite Turbo minimally invasive handpiece
FaceTite Turbo minimally invasive handpiece | K233642 (03/20/2024) InMode RF/IgniteRF platform with the FaceTite or BodyTite is indicated for use in dermatologic procedures where coagulation/contraction of soft tissue or hemostasis is needed. |
| Evolve system with the Transform applicator | Sequential RF/EMS | Transform | K231495 (10/13/2023)
The Evolve System with the Transform Applicator employs RF technology and EMS-TENS technology for the treatment of selected medical conditions.
The Transform Applicator in RF mode is intended for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
The Transform Applicator in EMS mode is intended for:
• Relaxation of muscle spasms • Prevention or retardation of disuse atrophy • Increasing local blood circulation • Muscle re-education • Maintaining or increasing range of motion • Immediate postsurgical stimulation of calf muscles to prevent venous thrombosis
The Transform Applicator in TENS mode is intended for:
• Symptomatic relief and management of chronic, intractable pain • Post-surgical acute pain • Post-trauma acute pain
Additionally, the Transform Applicator in sequential RF/EMS mode is intended for:
• Non-invasive lipolysis (breakdown of fat) of the abdomen. • Reduction in circumference of the abdomen |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| InMode system with Morpheus8 applicators (coagulation/contraction of software tissue) | Radiofrequency (RF) | • The standard Morpheus 8 Applicator is limited to use with the 12, 24 & T tip heads. • The Morpheus 8 Body Applicator is limited to use with the 40-tip head. | K231790 (07/20/2023)
The InMode System with the Morpheus8 Applicators is intended for use in dermatological procedures where coagulation/contraction of soft tissue or hemostasis is needed. At higher energy levels greater than 62 mJ/pin, the use of the Morpheus8 Applicator is limited to Skin Types I-IV. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| InMode Multi System (interface screen was slightly enlarged from 10 inch to 12 inch) | Radiofrequency (RF), Laser, IPL | Laser Applicators:
• Diolaze XL 810nm • Diolaze XL 755/810nm • Diolaze XL 810/1064nm • VLaze (Vasculaze) • IPL Applicator: • SR IPL (Lumecca 580, Lumecca 515)
Non-Invasive RF Applicators:
• Forma (Plus) • Plus (Plus Plus) • Plus90 • i-Forma • BodyFX™ (WMBody) • MiniFX™ • WMFace
Fractional RF Applicators: Fractora
• 24 pins tip (FRF) • 60 pins tip • Morpheus8™ • 12 pins tip (Prime Tip) • 24 pins tip )Fractora 3D) • 40 pins tip (Body Tip) • T tip | K221571 (05/26/2022)
The InMode Multi System with the Diode laser Applicators is indicated for:
• Diolaze XL 810nm Applicator is intended for hair removal and permanent hair reduction defined as the stable, long-term reduction in hair counts at 6, 9, or 12 months following a treatment regime. • Diolaze XL 755/810nm & 810/1064nm Applicators are intended for hair removal. • VLaze Applicator is intended for the treatment of vascular lesions, including angiomas, hemangiomas, telangiectasia, port wine stains, leg veins and other benign vascular lesions.
The InMode Multi System with the IPL Applicator is indicated for:
• IPL Applicator with wavelengths (515-l200nm) is indicated for use for the following treatments: The treatment of benign pigmented epidermal lesions, including dyschromia, hyperpigmentation, melasma, ephelides (freckles); The treatment of benign cutaneous vascular lesions, including port wine stains, facial, truncal and leg telangiectasias, rosacea, erythema of rosacea, angiomas and spider angiomas, poikiloderma of Civatte, superficial leg veins and venous malformations.
The InMode Multi System with the non-invasive RF Applicators is indicated for:
• BodyFX (WMBody) and MiniFX Applicators are intended for the treatment of the following medical conditions: Relief of minor muscle aches and pains, relief of muscle spasm, temporary improvement of local blood circulation; and temporary reduction in the appearance of cellulite. • Plus/ Plus90/Plus-Plus (Forma) and i-Forma Applicators are indicated for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation. • FaceFX (WMFace) Applicator is intended for use in dermatologic procedures, for noninvasive treatment of mild to moderate facial wrinkles and rhytids.
The InMode Multi System with the Fractional RF Applicators is indicated for:
• FRACTORA 60 pin Applicator is intended for use in dermatological procedures requiring ablation and resurfacing of the skin. • FRF 24 pin Applicator is intended for use in dermatologic and general surgical procedures for electrocoagulation and hemostasis.
Fractora3D and Morpheus8 Applicators are intended for use in dermatologic and general surgical procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62 mJ/pin, use is limited to Skin Types I-IV. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| InMode RF Multi Platform – Contoura | Radiofrequency (RF) | Forma(Plus), Plus90, Plus(Plus-Plus) BodyFX,
MiniFX,
Wmface, Fractora 24 pins tip
Fractora 60 pins tip
Morpheus8 24 Pin
Applicator
Morpheus8 40
Pin treatment tip • Morpheus8 12 Pin treatment tip • Morpheus8 T Pin treatment tip | K201150 (07/21/2020)
The InMode RF Multi-System with the Non-invasive RF Applicators employs RF energy for various applications:
• Forma (Plus), Plus (Plus Plus) and Plus90 for relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation. • WMface is intended for use in dermatologic procedures for non-invasive treatment of mild to moderate facial wrinkles and rhytids. • BodyFX™ (WMBody)/MiniFX™ for relief of minor muscle aches and pain, relief of muscle spasm, temporary improvement of local blood circulation and temporary reduction in the appearance of cellulite.
The InMode RF Multi-System with the Fractional Applicators employs RF energy for various applications:
• Fractora Applicator with 60 pins tip is designed for use in dermatological procedures requiring ablation and resurfacing of the skin. • Fractora Applicator with 24 pins tip is intended for use in dermatological and general surgical procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62mJ/pin, use of the applicator is limited to skin types I-IV. • Morpheus8™ for dermatological and general surgical procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62mJ/pin, use of the applicator is limited to skin types I-IV.
|
| Inmode | Powered muscle stimulator | Tone | K192249 (12/17/2019)
The Evolve platform is used in EMS mode for:
• prevention or retardation of disuse atrophy; • maintaining or increasing range of motion; • muscle re-education; • relaxation of muscle spasms; • increasing local blood circulation; and • immediate postsurgical stimulation of calf muscles to prevent venous thrombosis.
And in TENS mode for:
• symptomatic relief and management of chronic, intractable pain; • post-surgical acute pain; and • post-traumatic acute pain.
|
| InMode | Powered muscle stimulator | vTone | K200293 (05/05/2020)
The InMode System with the vTone Applicator is intended to provide electrical stimulation and neuromuscular re-education for the purpose of rehabilitation of weak pelvic floor muscles for the treatment of stress, urge, and mixed urinary incontinence in women. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| EmFace (Evoke) | Radiofrequency (RF) | Cheek Chin | K191855 (10/29/2019)
The EmFace (Evoke) device with the Cheek and Chin applicators is indicated for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
|
| EmBody (Evolve) | Radiofrequency (RF) | EMBodyPlus – Tite EmBodyFX – Trim | K183450 (06/20/2019)
The EmBody (Evolve) platform with its designated applicators is intended for the treatment of the following medical conditions:
The EmBodyPlus (Tite) hands-fee applicator is intended for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
The EmBodyFX(Tite) hands-free applicator is intended for the treatment of the following medical conditions using RF combined with massage:
• relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation; and • temporary reduction in the appearance of cellulite.
|
| InMode RF / Bodite / EmbraceRF | Radiofrequency (RF) | Bodyrite minimally invasive handpiece for thick body areas (>20mm) | K171593 (10/10/2017)
The InMode RFIBodyTitel EmbraceRF platform with the minimally invasive Bodyrite handpiece for thick body areas is indicated for use in dermatological and general surgical procedures for electrocoagulation and hemostasis.
|
| InMode RF / Bodyrite / EmbraceRF | Radiofrequency (RF) | Bodyrite minimally invasive handpiece for thin body areas (<20mm) or for large specialty areas | K163190 (12/12/2016)
The InMode RFIBodyTitel EmbraceRF platform with the minimally invasive Bodyrite handpiece for thin body and large specialty areas is indicated for use in dermatological and general surgical procedures for electrocoagulation and hemostasis.
|
| InMode RF / EmbraceRF | Radiofrequency (RF) | FaceTite | K151793 (02/19/2016)
The InMode RF/EmbraceRF platform with the FaceTite handpiece is indicated for use in dermatological and general surgical procedures for electrocoagulation and hemostasis.
|
| Optimas / InMode RF | Radiofrequency (RF) | Fractora (60 pin tip) | K102461 (06/02/2011)
The Optimas/InMode RF platform with the Fractora 60 pin tip handpiece is indicated for use in dermatological procedures requiring ablation and resurfacing of the skin. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| Optimas / InMode RF / EmbraceRF | Radiofrequency (RF) | Fractora (24 pin tip) FractoraV | K151273 (01/04/2016)
The OptimaslInMode RFI EmbraceRF platform with the Fractora 24 pin tip handpiece is indicated for use in dermatologic and general surgical procedures for electrocoagulation and hemostasis.
|
| Optimas | Radiofrequency (RF) | Morpheus8 | K180189 (06/01/2018)
The Optimas platform with the Morpheus8 handpiece is indicated for use in dermatological and general surgical procedures for electrocoagulation and homeostasis.
|
| InMode RF | Radiofrequency (RF) | Morpheus8 24 Pin Applicator Morpheus8 40 Pin treatment tip (New tip) • Morpheus8 12 Pin treatment tip (New tip) • Morpheus8 T Pin treatment tip (New tip) (maximal treatment depth 4.00 mm) | K192695 (12/27/2019)
The InMode System with the Morpheus8 (Fractora) Applicators is intended for use in dermatological procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62 mJ/pin, use of the Morpheus8 (Fractora) Applicator is limited to Skin Types I-IV.
|
| InMode | Radiofrequency (RF) | Morpheus8 24 Pin Applicator Morpheus8 40 Pin treatment tip (New tip) • Morpheus8 12 Pin treatment tip (New tip) • Morpheus8 T Pin treatment tip (New tip) maximal treatment depth 7.00 mm) | K200947 (06/12/2020)
The InMode System with the Morpheus8 Applicators is intended for use in dermatological procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62 mJ/pin, use of the Morpheus8 (Fractora) Applicator is limited to Skin Types I-IV. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| EmBody (Evolve) | Radiofrequency (RF) | Tone | K201285 (03/05/2021)
The EmBody (Evolve) platform with its designated applicators is intended for the treatment of the following medical conditions:
The EmBody (Evolve) System with Tone Applicator is designed to operate in two modes – EMS and TENS. In EMS mode it is used for:
• Relaxation of muscle spasms • Prevention or retardation of disuse atrophy • Increasing local blood circulation • Muscle re-education • Maintaining or increasing range of motion • Immediate postsurgical stimulation of calf muscles to prevent venous thrombosis
And in TENS mode is intended for:
• Symptomatic relief and management of chronic, intractable pain • Post-surgical acute pain • Post-trauma acute pain
|
| EmBody (Evolve) | Radiofrequency (RF) | EMBodyPlus – Tite T3-Transform | K210877 (07/19/2021)
The EvolveX System with the T3 Applicator employs RF technology or EMS-TENS technology for the treatment of selected medical conditions.
The T3 Applicator in RF mode is intended for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
The T3 Applicator in EMS mode is intended for:
• Relaxation of muscle spasms • Prevention or retardation of disuse atrophy • Increasing local blood circulation • Muscle re-education • Maintaining or increasing range of motion • Immediate postsurgical stimulation of calf muscles to prevent venous thrombosis
The T3 Applicator in TENS mode is intended for:
• Symptomatic relief and management of chronic, intractable pain • Post-surgical acute pain • Post-trauma acute pain
The RF treatment mode and EMS/TENS mode should not be used in combination or sequentially. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| InMode RF Pro Platform – Empower | Radiofrequency (RF) | i-Forma Forma(Plus), Plus90, Plus(Plus-Plus) BodyFX, MiniFX, Wmface, Fractora 24 pins tip Fractora 60 pins tip Morpheus8 24 Pin Applicator Morpheus8 40 Pin treatment tip • Morpheus8 12 Pin treatment tip • Morpheus8 T Pin treatment tip | K201150 (07/21/2021)
The InMode RF Pro System with the Non-invasive RF Applicators employs RF energy for various applications:
• i-Forma, Forma (Plus), Plus (Plus Plus) and Plus90 for relief of minor muscle aches and pain, relief of muscle spasm, and temporary improvement of local blood circulation. • WMface is intended for use in dermatologic procedures for non-invasive treatment of mild to moderate facial wrinkles and rhytids. • BodyFX™ (WMBody)/MiniFX™ for relief of minor muscle aches and pain, relief of muscle spasm, temporary improvement of local blood circulation and temporary reduction in the appearance of cellulite.
The InMode RF Multi-System with the Fractional Applicators employs RF energy for various applications:
• Fractora Applicator with 60 pins tip is designed for use in dermatological procedures requiring ablation and resurfacing of the skin. • Fractora Applicator with 24 pins tip is intended for use in dermatological and general surgical procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62mJ/pin, use of the applicator is limited to skin types I-IV. • Morpheus8™ for dermatological and general surgical procedures for electrocoagulation and hemostasis. At higher energy levels greater than 62mJ/pin, use of the applicator is limited to skin types I-IV.
|
| InMode RF / EmbraceRF | Radiofrequency (RF) | AccuTite | K182325 (08/27/2018)
The InMode RFI EmbraceRF platform with the AccuTite handpiece is indicated for use in dermatological and general surgical procedures for electrocoagulation and hemostasis.
|
| Contoura / Optimas | Radiofrequency (RF) | Plus Plus90 Plus-Plus | K172302 (12/08/2017)
The Contoura/Optimas platform with the Forma Plus, Plus90, Plus-Plus handpieces is indicated for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
|
| Optimas | Intense Pulsed Light (IPL) | Lumecca 515 Lumecca 580 | K123860 (04/02/2013)
The Optimas platform with the Lumecca 515 and Lumecca 580 handpieces is indicated for:
• the treatment of benign pigmented epidermal lesions, including dyschromia, hyperpigmentation, melasma, ephelides (freckles); and • the treatment of benign cutaneous vascular lesions, including port wine stains, facial truncal and leg telangiectasias, rosacea, erythema of rosacea, angiomas and spider angiomas, poikiloderma of civatte, superficial leg veins and venous malformations. |
| Product Platform | Energy Source | Handpiece | FDA 510(k) Clearance and Cleared Indications |
| Triton / Optimas | Laser | DiolazeXL | K170738 (08/07/2017)
The Triton/Optimas platform with the DiolazeXL handpiece is indicated for hair removal and permanent hair reduction defined as stable, long-term reduction in hair counts at six, nine or 12 months following a treatment regime.
|
| Triton / Optimas | Powered Laser | Vasculaze | K173677 (02/23/2018)
The Triton/Optimas platform with the Vasculaze handpiece is indicated for the treatment of vascular lesions, including angiomas, hemangiomas, telangiectasia, port wine stains, leg veins and other benign vascular lesions.
|
| Votiva | Radiofrequency (RF) | FormaV | f (07/12/2016)*
The InMode Plus90 (Votiva) platform with the Forma V handpiece is indicated for the temporary relief of minor muscle aches and pain, temporary relief of muscle spasm, and temporary improvement of local blood circulation.
|
| Contoura / Optimas | Radiofrequency (RF) | BodyFX | K131362 (10/08/2013)
The Contoura/Optimas platform with the BodyFX handpiece is indicated for the treatment of:
• relief of minor muscle aches and pains, muscle spasms and temporary improvement of blood circulation; and • temporary reduction in the appearance of cellulite.
|
| Contoura / Optimas | Radiofrequency (RF) | MiniFX | K160329 (08/19/2016)
The Contoura/Optimas platform with the MiniFX handpiece is indicated for the treatment of:
• relief of minor muscle aches and pain, muscle spasms, and temporary improvement of local blood circulation; and • temporary reduction in the appearance of cellulite.
|
| Triton / Optimas | Laser | Triton Duo Light/ Triton Duo Dark InMode Diolaze XL 755/810nm InMode Diolaze XL 810/1064nm InMode Diolaze XL 810nm | K180719 (06/14/2018)
The Triton/Optimas platform with the Triton Duo Light and Triton Duo Dark handpieces is indicated for hair removal and permanent hair reduction.
|
| InMode | Laser | Diolaze | K142952 (11/24/2014) The InMode Diolaze device is indicated for use for hair removal and for permanent reduction in hair regrowth, defined as the long-term, stable reduction in the number of hairs regrowing when measured at 6, 9, and 12 months after the completion of a treatment regime
|
| InMode | Laser | Diolaze | K123682 (27/02/2013)
The InMode Diolaze device is indicated for use for hair removal
|
| InMode | Radiofrequency (RF) | WMface | k140926 (12/03/2014)
The InMode WMface device is intended for use in dermatologic procedures for noninvasive treatment of mild to moderate facial wrinkles and rhytids. |
| * | In addition to the 510(k) clearance, we also market the FormaV for use with the Votiva platform pursuant to a classification regulation for “genital vibrators for therapeutic use” under 21 C.F.R. 884.5960, which permits “electronically operated devices intended and labeled for therapeutic use in the treatment of sexual dysfunction or as an adjunct to Kegel’s exercise (tightening of the muscles of the pelvic floor to increase muscle tone)” to be marketed without a 510(k) clearance. |
Premarket Approval Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process. A PMA must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling, to demonstrate the safety and effectiveness of the device to the FDA’s satisfaction.
No device that we have developed has required premarket approval, nor do we currently expect that any future device or indication will require premarket approval.
Pervasive and Continuing Regulation
After the FDA permits a device to enter commercial distribution, numerous regulatory requirements apply. These include:
| • | QSR, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| • | clearance or approval of product modifications to 510(k)-cleared or PMA-approved devices that could affect safety or effectiveness; |
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or “off-label” uses; |
| • | advertising and promotion requirements; |
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their devices may have caused or contributed to deaths or serious injuries or malfunctioned in ways that would likely cause or contribute to deaths or serious injuries if the malfunctions were to recur; |
| • | medical device correction and removal reporting regulations, which require the manufacturers to report to the FDA corrections and removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; and |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the devices. |
We may be subject to similar foreign laws that may include applicable post-marketing requirements such as safety surveillance. Our manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. As a manufacturer, our facilities, records and manufacturing processes are subject to periodic scheduled or unscheduled inspections by the FDA. Our failure to maintain compliance with the QSR or other applicable regulatory requirements could result in the shut-down of, or restrictions on, our manufacturing operations and the recall or seizure of our products. The discovery of previously unknown problems with any of our products, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls.
The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections may include the facilities of our manufacturing subcontractors.
We also are regulated under the Radiation Control for Health and Safety Act, which requires laser products to comply with performance standards, including design and operation requirements, and manufacturers to certify in product labeling and in reports to the FDA that their products comply with all such standards. The law also requires laser manufacturers to file new product and annual reports, maintain manufacturing, testing and sales records, and report product defects. Various warning labels must be affixed and certain protective devices installed, depending on the class of the product.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| • | warning or untitled letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures, repair, replacement, refunds, recalls, administrative detention or seizure of products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusing requests for 510(k) clearance or PMAs of new products or new intended uses; |
| • | withdrawing 510(k) clearance or PMAs that have already been granted; and |
The FDA also has the authority to require us to repair, replace or refund the cost of any medical device that we have manufactured or distributed. If any of these events were to occur, they could have a material adverse effect on our business.
We are also subject to a wide range of federal, state and local laws and regulations, including those related to the environment, health and safety, land use, and quality assurance. We believe that we are in compliance with these laws and regulations as currently in effect, and our compliance with such laws will not have a material adverse effect on our capital expenditures, earnings, and competitive and financial position.
Federal Communications Commission and other U.S. governmental agencies governing the use of radio frequency energy
Our products generate and use radio frequency energy and therefore may be subject to technical equipment authorization and other regulatory requirements in the countries and regions where they are marketed or distributed. In the United States, our products are subject to the Federal Communications Commission’s equipment verification procedures, under which the manufacturer is required to determine or verify that the equipment complies with the applicable technical standards and to keep a record of test measurements demonstrating compliance before the equipment can be marketed or sold in the United States. Any modifications to our products may require reverification before we are permitted to market and distribute the modified devices.
We obtain regulatory approvals in countries requiring advance clearance of our products before they are marketed or distributed in those countries. Our failure to comply with the technical, equipment authorization or other regulatory requirements of a specific country or region could impair our ability to commercially market and distribute our products in that country or region.
Other Healthcare Laws
Although none of our products or procedures using our products are currently covered by any state or federal government healthcare programs, or any private commercial payor, we may be subject to a number of foreign, federal, and state laws and regulations that may restrict our business practices, including, without limitation, anti-kickback, false claims, physician payment transparency and data privacy and security laws. The government has interpreted these laws broadly as they apply to the marketing and sales activities of manufacturers and distributors. Companies targeted in such prosecutions and in civil litigation have paid substantial fines, penalties, and settlements in the hundreds of millions of dollars or more, have been forced to implement extensive corrective action plans, can be excluded from federal health care programs, and have often become subject to consent decrees, settlement agreements or corporate integrity agreements severely restricting the manner in which they conduct their business. Many U.S. states and countries outside the United States have similar fraud and abuse statutes or regulations that may be broader in scope than the U.S. federal laws, and may apply regardless of payor, in addition to items and services reimbursed under government programs.
International Regulations
International manufacturing and sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain clearance or approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may be different. For example, the European Union has adopted numerous specific directives regulating the design, manufacture, clinical investigations, conformity assessment, labeling and adverse event reporting for medical devices that do not necessarily have an exact equivalent under U.S. law.
Devices that comply with the essential requirements of the EU Medical Devices Directive (Directive 93/42/EEC) will be entitled to bear CE conformity marking, indicating that the device conforms with the essential requirements of the EU Medical Devices Directive and, accordingly, can be commercially distributed throughout the member states of the European Union, the member states of the European Economic Area, or EEA (which is comprised of the 27 EU countries, plus Norway, Liechtenstein, and Iceland), and countries that have entered into a Mutual Recognition Agreement. The method of assessing conformity varies depending on the type and (risk) class of the product but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a notified body, an independent organization designed by an EU country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. An assessment by a notified body in one member state of the European Union, the EEA or a country that has entered into a Mutual Recognition Agreement is required in order for a manufacturer to commercially distribute the product throughout these countries. European Standardization Committees and the International Organization for Standardization, or ISO, have promulgated voluntary harmonized standards. Compliance with applicable standards establishes the presumption of conformity with the essential requirements for a CE Marking.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. All manufacturers placing medical devices into the market in the EEA must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the Member States of the EEA, and manufacturers are required to take Field Safety Corrective Actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An incident is defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its legal representative to its customers and/or to the end users of the device through Field Safety Notices.
On May 25, 2017, the EU passed the Medical Devices Regulation (Regulation 2017/745) (“MDR”) entered into force, which repeals and replaces the EU Medical Devices Directive. The MDR allows devices lawfully placed on the market prior to May 26, 2021 pursuant to the EU Medical Devices Directive (discussed above), to generally continue to be made available on the market or put into service until May 26, 2025. Once the MDR phase-in period is complete after May 26, 2025, some of our products that entered the market prior to May 26, 2021 may come out of service.
The Medicines and Healthcare Products Regulatory Agency of the United Kingdom (“MHRA”) is responsible for monitoring the UK medical device market. The MHRA requires that medical devices sold in the U.K. be required with the MHRA. Manufacturers based outside the UK need to appoint a UK Responsible Person to register devices with the MHRA in line with the grace periods. From July 1, 2023, all medical devices sold in the UK were required to carry a UKCA (UK Conformity Assessed) mark. However, UKCA marking alone will not be recognized in the EU. The rules for placing medical devices on the Northern Ireland market differs from those in the UK. All of our platforms and applicators require UKCA marking.
Several member states of the EEA have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. Other countries, such as Switzerland, have entered into Mutual Recognition Agreements and allow the marketing of medical devices that meet European Union requirements.
In Israel, the Medical Equipment Law generally governs the regulatory process and authorizations required for the manufacture, marketing and use of medical and certain aesthetic products in Israel. Under the Medical Equipment Law, we are required to register our products with the Israeli Ministry of Health. The Medical Equipment Law offers a fast-track approval process for devices that received approval from certain non-Israeli regulatory agencies, including FDA clearance or CE marks. The registration process under this fast-track process involves submitting an application to the Israeli Ministry of Health which includes, among other things, documentation confirming receipt of the necessary regulatory approvals, such as 510(k) clearance or CE mark certification. In addition, we must provide details regarding the approval, including the period for which the applicable device was authorized for marketing in the applicable country, registration terms, any limitations, and any instructions given with respect to the labeling and packaging of such device. If approved, the registration of the device in Israel will be valid for the same period that such device was authorized to be marketed in the applicable non-Israeli country (but in any event not more than five years from the date of registration in Israel), and the device will be subject to the terms and conditions imposed by the relevant non-Israeli regulatory agency, if any. We have taken advantage of such fast-track approval in the past. Most of our products that we currently sell in Israel are registered with the Israeli Ministry of Health, with registrations that are set to expire on June 10, 2029. Our new products are in the process of being registered with the Israeli Ministry of Health. As necessary, we applied for extensions as required to maintain active registrations, and such registrations expired on January 31, 2024.
Data Protection
Our business involves the use, storage and transmission of information about our employees, our customers and, to a certain extent, clients of our customers. In the course of our operations, we may gain access to confidential customer information, including nonpublic personal data. We are bound by certain agreements to use and disclose this information in a manner consistent with the privacy standards under regulations applicable to our customers and are subject to numerous U.S. and foreign jurisdiction laws and regulations designed to protect this information. With the increase in publicity regarding data breaches resulting in improper dissemination of consumer information, many states have passed laws regulating the actions that a business must take if it experiences a data breach, such as prompt disclosure to affected customers, governmental entities, and the media. In addition to data breach notification laws, some states have enacted statutes and rules requiring businesses to protect certain types of personal information they hold or to otherwise comply with certain specified data security requirements for personal information. We intend to protect all personal information and to comply with all applicable laws regarding the protection of such information.
As an Israeli headquartered company, we are subject to the Israeli Protection of Privacy Law of 1981 and the Privacy Protection Regulations (Data Security) 5777-2017. Further, in the U.S., numerous federal and state laws and regulations, including data breach notification laws, health information privacy laws and consumer protection and advertising laws (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators and third-party providers.
In the European Union and the EEA, the GDPR imposes several stringent requirements for controllers and processors of personal data, including, for example, higher standards for obtaining consent from individuals to process their personal data, more robust disclosures to individuals and a strengthened individual data rights regime, shortened timelines for data breach notifications, limitations on retention and secondary use of information, increased requirements pertaining to health data and pseudonymized (i.e., key-coded) data and additional obligations when we contract third-party processors in connection with the processing of the personal data. The GDPR provides that EU and EEA member states may make their own further laws and regulations limiting the processing of genetic, biometric or health data, which could limit our ability to use and share personal data or could cause our costs to increase, and harm our business and financial condition. Failure to comply with the requirements of GDPR and the applicable national data protection laws of the EU and EEA member states may result in fines of up to €20 million or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, and other administrative penalties. To comply with the new data protection rules imposed by GDPR, we may be required to put in place additional mechanisms ensuring compliance. Further, since January 1, 2021, we are subject to the GDPR and also the UK GDPR, which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, e.g., fines up to the greater of €20 million or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how UK data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the United Kingdom will be regulated in the long term. It is not clear whether (and when) an adequate decision may be granted by the European Commission enabling data transfers from European Union member states to the United Kingdom long-term without additional measures. These changes will lead to additional costs and increase our overall risk exposure.
Corporate and Social Responsibility
Our management continues to participate in Corporate Social Responsibility (“CSR”) Reporting on our website to respond to increased awareness of the importance of environmental and sustainability topics and non-material company activities towards the long-term success of the company. As a manufacturer of medical devices, we already adhere to strict quality and safety requirements dictated by regulatory bodies such as the FDA, CE, Health Canada, TGA (Australia), Brazil, Japan, China’s National Medical Products Administration (“NMPA”), ISO, as well as international GMP standards. We successfully completed all audits conducted by the regulatory bodies in 2024, including a physical audit of our facilities and the facilities of our Tier 1 subcontractors – Medimor, Flex, and Resonetics.
Our mission is to develop and produce innovative, life-changing technologies to enable patients to become their ‘best possible self’, in the safest and most effective manner. Our commitment to CSR topics complements InMode’s core values to provide the ultimate in customer care and support.
Management Discussion of Corporate and Social Responsibility
In 2023, we completed reporting on all six topics defined by The SASB (Sustainability Accounting Standards Board) Sustainability Accounting Standard for Medical Equipment and Supplies. These topics were: Affordability & Pricing, Ethical Marketing, Product Design & Lifecycle Management, Business Ethics, Product Safety and Supply Chain Management.
Our CSR Reporting also highlights our impact on the local economies in government-designated development areas in the periphery where we work with contract suppliers and subcontractors (https://inmodemd.com/investors/corporate-social-responsibility/).
The CSR Reporting specifically focuses on our primary assembly and manufacturing facilities in Israel, the location of the Company’s headquarters. Further information on our corporate social responsibility efforts and SASB data reporting is available on our website. The information contained on our website or available through our website is not incorporated by reference into and should not be considered a part of this Annual Report on Form 20-F, and the reference to our website in this Annual Report on Form 20-F is an inactive textual reference only.
Community Involvement in the Periphery
Robotics Mentoring in Dimona
Since 2017, Moshe Mizrahy and InMode engineers have been involved with the FIRST Robotics Group at Amit Zinman High School in Dimona. Dimona is located in southern Israel and is an area in the lower rungs of the socioeconomic scale.
InMode supports the robotics team of Amit Zinman High School with more than just financial resources. Our engineers provide ongoing mentoring and opportunities to design electromechanical devices. One of the program’s goals is to introduce STEM subjects into the community through projects and our outreach programs and activities for kindergartens and elementary schools. We continue to support the high school’s robotics team with mentoring, resources, and funds and will continue in 2025.
Technology Project at ORT Maale Tiberias High School
InMode, together with Medimor, our Tier 1 supplier, has established a partnership with ORT Maale Tiberias school to create a sustainable technology project. We have earmarked one hundred thousand dollars to this educational project. Tiberias is in an economically depressed area of Israel and there have been few industry or business partnerships with Tiberias. We are proud to sponsor such an exciting and impactful project.
C. Organizational Structure
Our subsidiaries, the countries of their incorporation/residence and proportion of ownership interest are as follows:
| Name | Jurisdiction of Incorporation | | Percentage Ownership |
|
| Invasix Inc. | Delaware, USA | | 100% |
|
| Invasix Corp. | Canada | | |
|
| InMode M.D. Ltd. | Israel | | |
|
| Invasix UK Ltd. | United Kingdom | | 100% |
|
| InMode Japan KK | Japan | | |
|
| Invasix Iberia S.L. | Spain | | |
|
| Guangzhou InMode Medical Technology Ltd. | China | | |
|
| InMode Asia Limited. | Hong Kong | | |
|
| InMode India Private Limited | India | | |
|
| InMode Australia Pty Ltd | Australia | | |
|
| IMD France EURL | France | | |
|
| InMode Innovations Ltd. | Israel | | |
|
| InMode Italy S.r.l | Italy | | |
|
| InMode Germany GmbH | Germany | | |
|
D. Property, Plants and Equipment
We lease our main office, manufacturing and research and development facilities, located in the Industrial Zone in Yokneam, Israel, pursuant to a lease that expires in December 2027. The original agreement was signed in May 2018. In January 2019, February 2020, March 2021, May 2023, June 2024 and July 2024, we signed supplemental lease agreements, further expanding our headquarters in Israel.
We currently lease approximately 44.4 thousand square feet in the Israeli facility. Our current monthly rent payment is approximately $76.7 thousand. In addition, we currently lease a warehouse in Zarzir, Israel with approximately 10.1 square feet and our current monthly rent payment for such warehouse is approximately $8.9 thousand.
In August of 2020, our U.S. subsidiary, signed a new leasing agreement, for an approximately 24-thousand-square-foot facility located in Orange County, California, to support our expanding operations in the U.S. The new lease agreement is for seven years and four months and occupation of the new facility commenced in the middle of April of 2021. The current monthly rent payment is approximately $28 thousand.
Our Canadian subsidiary has signed a new lease agreement in April 2022 for an approximately 12-thousand-square-foot facility in Richmond Hill, Ontario. The new lease agreement is for three years which began in July 2022. The current monthly rent payment is approximately $17 thousand.
From time to time, we also lease small properties, mainly for offices for our subsidiaries around the world, for periods that generally last up to three years.
We believe our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following discussion should be read in conjunction with our consolidated financial statements and related notes for the years ended December 31, 2024, 2023 and 2022, which are included elsewhere in this 2024 Annual Report.
Overview
We design, develop, manufacture and commercialize innovative, energy-based, non-invasive, minimally invasive surgical aesthetic and medical treatment solutions. Since 2010, we have launched 14 product platforms (BodyTite, Optimas, Votiva, Contoura, Triton, EmbraceRF, EvolveX, Evoke, Morpheus8, EmpowerRF, Define, Envision, IgniteRF and OptimasMAX) that we market and sell traditionally to plastic and facial surgeons, aesthetic surgeons, medical spas, dermatologists and OB/GYNs.
As of December 31, 2024, we had a global installed base of approximately 27,090 product platforms capable of running various multi-use applicators and minimally invasive consumables.
We anticipate that our quarterly results of operations may fluctuate from quarter to quarter due to several factors, including seasonality, unexpected delays in the introduction and market acceptance of our products, unexpected delays in our manufacturing operations, introduction of new and improved products by our competitors and the performance of our direct sales organization.
Components of Our Operating Results
Revenues
We generate our revenues primarily from the sale of energy-based medical aesthetic products, which consist of platforms and non-consumable handpieces and hands-free applicators. To a lesser extent, we generate revenue from the sale of consumables and from the sale of extended warranties. For the year ended December 31, 2024, we derived approximately 80% of our revenues from the sale of medical aesthetic products and approximately 20% of our revenues from the sale of consumables and extended warranties. We expect our revenues from the sale of consumables and extended warranties to increase over time as our installed base continues to grow. we expect continued growth in sales of consumables as a result of, increased patient and physician awareness of our medical aesthetic products and additional sales representatives. We have expanded our sales and marketing organization as well as our number of platforms, to help us drive and support revenue growth and intend to continue this expansion.
For the years ended December 31, 2024 and 2023 we derived approximately $344.0 million, or 87% and $406.6 million, or 83%, respectively, of our total revenues from the sale of minimally invasive platforms, and we derived approximately $20.1 million, or 5% and $43.1 million, or 8%, respectively, of our total revenues from the sale of hands-free platforms and approximately $30.7 million, or 8% and $42.4 million, or 9%, respectively, of our total revenues from the sale of non-invasive platforms. This resulted in the year ended December 31, 2024 a decrease of approximately $62.6 million, or 15%, $23.0 million, or 53% and $11.7 million, or 28% in revenues from the sale of minimally invasive platforms, hands-free platforms and non-invasive platforms, respectively. The decrease in the total revenues for the year ended December 31, 2024 was primarily generated by a decrease in the volume of medical aesthetic products sold by us as a result of the economic situation.
In the future, we expect that revenues from the sale of minimally invasive platforms will continue to be a major contributor to our revenues.
We sell our products directly in the United States, Canada, United Kingdom, Ireland, Spain, Portugal, France, Belgium, Luxemburg, Italy, Germany, Austria, Japan, Australia and India, and indirectly through third-party distributors in other countries.
The following table provides information regarding the breakdown of our revenue by geographic region for the years ended December 31, 2024, 2023 and 2022:
| | | | |
| Geographic region | | | | | | | | | |
| United States | | 244,774 | | | 307,818 | | | 298,612 | |
| Europe | | 63,441 | | | 62,532 | | | 49,274 | |
Asia
| | 42,974 | | | 47,744 | | | 36,492 | |
| Other | | | | | | | | | |
| Total | | | | | | | | | |
We believe that there are opportunities for us to generate additional revenue from existing customers who are already familiar with our products. We intend to continue to invest in research and development activities, increase the number of sales representatives in our sales and marketing organization and introduce innovative next-generation pipeline products to our customers. As a result, we expect that certain existing customers will be candidates for technology upgrades to enhance the capabilities of their existing InMode products. In addition, as we continue to grow our support services program, we expect to increase the number of customers that enter into service contracts and extended warranties with us, which would result in additional recurring revenues. We also plan to expand our current product line in order to reach non-traditional customers, such as ENTs, ophthalmologists, general practitioners and aesthetic clinicians, and generate additional revenue.
Cost of Revenues
Our cost of revenues consists primarily of the expenses we incur to have our products manufactured and assembled by third parties and the direct costs we incur in order to obtain the materials, labor and overhead that are needed to manufacture and assemble our products.
Our cost of revenues also includes shipping, handling, service and warranty expenses, as well as salaries and personnel-related expenses, including share-based compensation expenses, for our operations management team, which is comprised of subcontractor supervisors and purchasing and quality control employees. We expect our cost of revenues to increase in absolute dollars primarily as, and to the extent, our revenue grows.
Our cost of revenues as a percentage of revenues has been, and we expect it to continue to be, affected by a variety of factors, including the average selling price of our products, promotional prices being offered to existing customers for our new products, manufacturing costs, the maturity of our existing products, and to a lesser extent the sales mix between the United States and the rest of the world as our average selling price in the United States tends to be higher than in the rest of the world after accounting for differences in currency value. We expect our gross margin to be maintained at current levels over time to the extent we are successful in maintaining our average selling prices, as well as, offsetting increased material and shipping costs with reducing manufacturing costs as our sales volume increases. However, our gross margin may fluctuate from period to period.
Research and Development Expenses
Our research and development expenses consist of salaries and personnel-related expenses, including share-based compensation expenses, for our employees that are primarily engaged in research, development and engineering activities. Our research and development expenses also include regulatory-related costs and expenses, external engineering fees, materials used and other overhead expenses that are incurred in connection with the design and development of our products. For further details with respect to government regulations we are subject to, see “Item 4B. Information on the Company-Business Overview-Government Regulations.” We expense all of our research and development costs as incurred. While we do not track our research and development spending by technology, product or application, we do expect that our overall research and development costs will increase in absolute dollars in the future as we develop more products and technologies. We expect research and development expenses as a percentage of our total revenue to vary over time depending on the level and timing of initiating new product development efforts.
Sales and Marketing Expenses
Our sales and marketing expenses consist primarily of salaries, commissions and personnel-related expenses, including share-based compensation expenses, for our employees that are engaged in sales and marketing activities, which include marketing and public support of our products, participation in trade shows and industry events, promotional and public relations activities, and administrative functions in support of sales and marketing. We expect sales and marketing expenses to continue to increase in absolute dollars as we continue to expand our marketing organization to both drive and support our planned growth in revenue.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries and personnel-related expenses, including share-based compensation expenses, for executive, accounting and administrative personnel, professional fees and other general corporate expenses. We expect general and administrative expenses to grow at a steady state as our operations will continue to expand. However, general and administrative expenses may vary over time, due to increasing legal and insurance premium costs and involvement in IP related litigations.
Income Taxes
We are subject to income taxes in Israel, the United States and numerous foreign jurisdictions.
Our facilities in Israel were previously granted the status of “Benefited Enterprise” which provided us with a ten-year corporate tax exemption for undistributed income from 2012 to 2021. From 2022, our corporate tax rate under the Encouragement of Capital Investments Regulations (Preferred Technology Income and Capital Profits for a Technological Enterprise), 2017 and our Israeli subsidiary is expected to be approximately 7.5%. See “Item 10E. Additional Information-Taxation.”
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of our operations is based upon our audited consolidated financial statements as of and for the years ended December 31, 2024, 2023 and 2022, which have been prepared in accordance with U.S. GAAP. The preparation of our consolidated financial statements requires us to make estimates and judgments that affect the reported amount of assets, liabilities, sales and expenses and related disclosure of contingent assets and liabilities. We base our estimates on historical experience, authoritative pronouncements and various other assumptions that we believe to be reasonable under the circumstances. On a periodic basis, we evaluate our estimates. Actual results could differ from those estimates.
The following are our critical accounting policies and the significant judgments and estimates affecting the application of those policies in our consolidated financial statements.
Revenue Recognition
Product revenue, Net
Revenues from product sales are recognized when the customer obtains control over our product, typically upon shipment to the customer. Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from revenues.
Variable consideration includes price concessions related to installment sales contracts. We estimate variable consideration using the most likely outcome amount. Amounts included in the transaction price are recognized only when it is probable that a significant reversal of cumulative revenues will not occur.
We do not grant any right of return, refund, cancelation or termination. From time to time, we participate in our customers’ marketing activities and deduct costs related to such activities from revenue.
Service Revenue
We also generate revenues from long-term maintenance contracts or extended warranties. Revenue from extended warranties is recognized ratably, on a straight-line basis, over the period of the applicable service contract. Revenue from repairs performed in the absence of extended warranties is recognized when the related services are performed.
More information regarding revenue recognition is discussed in Note 2q in our consolidated financial statements.
Income Taxes
The provision for income tax is calculated based on our assumptions as to our entitlement to various benefits under the applicable tax laws in the jurisdictions in which we operate. The entitlement to such benefits depends upon our compliance with the terms and conditions set out in these laws. We are subject to income taxes in Israel, the U.S. and other foreign jurisdictions. Our effective tax rate is primarily impacted by the geographical mix of taxable income and loss. We record a tax provision for the anticipated tax consequences of our reported operating results. Changes in tax laws, regulations, agreements and treaties, currency exchange restrictions or the level of operations or profitability in each taxing jurisdiction could have an impact upon the amount of current and deferred tax balances and hence our net income.
We estimate the degree to which deferred tax assets will result in a benefit, after consideration of all positive and negative evidence, and provide a valuation allowance for deferred tax assets that we believe more likely than not will not be realized. In situations in which we are able to determine that our deferred tax assets will be realized, that determination generally relies on future reversals of taxable temporary differences and expected future taxable income. Significant judgment required in determining any valuation allowance recorded against deferred tax assets. In assessing the need for a valuation allowance, we considered all available evidence, including past operating results, the most recent projections for taxable income, and prudent and feasible tax planning strategies.
During the year ended December 31, 2024, we determined that the positive evidence overcame any negative evidence, primarily due to cumulative income in recent years, including the effect of permanent adjustments, and the expectation of profitability in future periods, and concluded that it was more likely than not that the United States net deferred tax assets were realizable. As a result, we released the valuation allowance of $60.1 million related to the United States net deferred tax assets during the year ended December 31, 2024, with $5.4 million used during 2024, and $54.7 million recorded as defer tax assets. As of December 31, 2024, we concluded that the deferred tax assets for certain jurisdictions would not be realized and therefore reserved valuation allowance in the amount of $4.2 million with respect to these deferred tax assets.
Significant judgment is required in evaluating our uncertain tax positions. In evaluating the exposure associated with our various tax filing positions, we record reserves for uncertain tax positions in accordance with US GAAP, based on the technical support for the positions and our past audit experience with similar positions. For those tax positions where it is more likely than not that a tax benefit will be sustained, we record the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. We believe our tax positions comply with applicable tax laws and we intend to defend our positions, no assurance can be given that the final tax outcome of these matters will not be different from that which is reflected in our historical income tax reserves and accruals. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences will impact the provision for income taxes in the period in which such determination is made. The provision for income taxes includes the impact of reserve provisions and changes to reserves that are considered appropriate. The liability for these unrecognized tax benefits totaled $3.4 million on December 31, 2024 (see Note 13 to our consolidated financial statements for additional information).
Operating Results
The following table summarizes the results of our operations for the periods presented:
| | | Years Ended December 31, | |
| | | 2024 | | | | |
| | | | | | | | | | | | | |
| | | | |
| Revenues | | | 394,818 | | | | 100 | | | | 492,048 | | | | 100 | |
| Cost of revenues | | | 77,752 | | | | 20 | | | | 80,708 | | | | 16 | |
| Gross profit | | | 317,066 | | | | 80 | | | | 411,340 | | | | 84 | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 13,137 | | | | 3 | | | | 13,410 | | | | 3 | |
| Sales and marketing | | | 181,366 | | | | 46 | | | | 193,042 | | | | 39 | |
| General and administrative | | | 10,032 | | | | 3 | | | | 9,228 | | | | 2 | |
| Total operating expenses | | | 204,535 | | | | 52 | | | | 215,680 | | | | 44 | |
| Operating income | | | 112,531 | | | | 28 | | | | 195,660 | | | | 40 | |
| Finance income, net | | | 30,938 | | | | 8 | | | | 21,607 | | | | 4 | |
| Income before income taxes | | | 143,469 | | | | 36 | | | | 217,267 | | | | 44 | |
| Income taxes benefit (expenses) | | | 37,806 | | | | 10 | | | | (19,348 | ) | | | 4 | |
| Net income | | | 181,275 | | | | 46 | | | | 197,919 | | | | 40 | |
Comparison of the Year Ended December 31, 2024 to the Year Ended December 31, 2023
Revenues
Our revenues decreased by approximately $97.2 million, or 20%, to approximately $394.8 million for the year ended December 31, 2024, compared to approximately $492.0 million for the year ended December 31, 2023. This decrease was attributable to a decrease in sales of our minimal invasive platforms worldwide in the amount of $62.6 million, a decrease in the sales of our hand free platforms worldwide in the amount of $23.0 million, and a decrease of $11.7 decrease from non-invasive. The decrease in the total revenues for the year ended December 31, 2024 as compared to the prior year was primarily generated by an decrease in the volume of medical aesthetic products sold by us during the period as a result of decrease in patient and physician demand due to economic situation. Our revenues in the United States decreased by approximately $63.0 million, or 20%, to approximately $244.8 million for the year ended December 31, 2024, compared to approximately $307.8 million for the year ended December 31, 2023. This decrease was primarily attributable to the overall slowdown in the demand for aesthetic procedures, in addition to challenging financing market in the United States.
Our revenues outside of the United States decreased by approximately $34.2, or 19%, to approximately $150.0 million for the year ended December 31, 2024, compared to approximately $184.2 million for the year ended December 31, 2023. This decrease was primarily due to global slowdown in demand for aesthetic procedures and capital equipment sales.
Our revenues from the sale of consumables and extended warranties for the year ended December 31, 2024, decreased by approximately 1% compared to the year ended December 31, 2023. This decrease was primarily attributable to slowdown in demand for aesthetic procedures.
Cost of revenues
Our cost of revenues decreased by approximately $2.9 million, or 4%, to approximately $77.8 million for the year ended December 31, 2024, compared to approximately $80.7 million for the year ended December 31, 2023. This decrease was primarily due to decrease in sales. Our gross margin decreased to 80% for the year ended December 31, 2024, compared to approximately 84% for the year ended December 31, 2023. This decrease was primarily attributable to an increase of material costs and a different sales mix between the United States and rest of the world..
Research and development expenses
Our research and development expenses decreased to approximately $13.1 million for the year ended December 31, 2024, compared to approximately $13.4 million for the year ended December 31, 2023. This decrease was primarily attributable to a decrease in share-based compensation in the amount of $0.5 million.
Sales and marketing expenses
Our sales and marketing expenses decreased by approximately $11.6 million, or 6%, to approximately $181.4 million for the year ended December 31, 2024, compared to approximately $193.0 million for the year ended December 31, 2023. This decrease was primarily attributable to decrease in commission for sales in the amount of $11.4 million, decrease in share-based compensation in the amount of $5.8 million which was offset with increase in salaries in the amount of $1.8 million, related expenses in the amount of $1.2 million and, trade show and other marketing expenses in amount of $2.2 million.
General and administrative expenses
Our general and administrative expenses increased by approximately $0.8 million, or 9%, to approximately $10.0 million for the year ended December 31, 2024, compared to approximately $9.2 million for the year ended December 31, 2023. This increase was primarily attributable to the increase in professional services in the amount of $0.9 million, increase in product liability provision in the amount of $0.5 million which was offset with decrease in share-based compensation in the amount of $0.5 million.
Finance income, net
Our finance income, net was approximately $30.9 million for the year ended December 31, 2024, compared to approximately $21.6 million for the year ended December 31, 2023. This increase in finance income, net was primarily attributable to increase in interest income from our portfolio of investments in bonds and corporate debt securities and short-term bank deposits in the amount of $12.5 million.
Income taxes benefit (expenses)
Our income taxes decreased by approximately $57.1 million, or -295%, to result in a benefit of approximately $37.8 million for the year ended December 31, 2024, compared to expenses of approximately $19.3 million for the year ended December 31, 2023. This decrease was primarily attributable to recognition of a NOL deferred tax asset in amount of $55.6 million . See “Item 10E. Additional Information–Taxation”.
Liquidity and Capital Resources
Historically, we have funded our operations primarily from cash flows from operations, from private placements of our ordinary shares, from our initial public offering in August 2019 and from exercise of options. Since inception in January 2008, we have not received any debt financing from banks or issued any preferred or debt securities. We have received aggregate net proceeds of approximately $109.8 million from issuances of our ordinary shares, including approximately $69.8 million from our initial public offering.
In May 2024, we approved a share repurchase program of up to 8.37 million ordinary shares, to be purchased out of our cash reserve. In September 2024, we approved a share repurchase program of up to 7.68 million ordinary shares, to be purchased out of our cash reserve. As of December 31, 2024, we purchased 16.05 million ordinary shares in the amount of $285.4 million under these repurchase programs.
As of December 31, 2024, we had working capital of approximately $644.6 million, and our primary source of liquidity was approximately $596.5 million in cash and cash equivalents, marketable securities and short-term bank deposits. Our major cash requirements are obligations to support our ongoing operations which consist primarily of salary and commissions expenses for employees, and contractual obligations for our subcontractors and lease agreements. We expect our working capital to be sufficient for the Company’s present requirements.
If existing cash and cash generated from operations are insufficient to satisfy our liquidity requirements, we may seek to sell equity or debt securities or obtain a credit facility. If we raise funds by issuing equity securities, our shareholders would experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt financing or equity that we raise may contain terms that are not favorable to us or our shareholders. Financing may not be available at all, or in amounts or on terms unacceptable to us. If we are unable to obtain financing, we may be required to delay the development, commercialization and marketing of our aesthetic medical products.
Cash Flows
The following table represents a summary of our cash flow for the periods indicated
| | | Years ended December 31, | |
| | | 2024 | | | | |
| Net cash provided by (used in): | | (in thousands) | |
| Operating activities | | $ | 132,664 | | | $ | 176,826 | |
| Investing activities | | | 162,206 | | | | (136,064 | ) |
| Financing activities | | | (282,771 | ) | | | 5,504 | |
| Effects of exchange rate changes on cash | | | (1,181 | ) | | | 605 | |
| Net increase in cash and cash equivalents | | $ | 10,918 | | | $ | 46,871 | |
Net Cash Provided by Operating Activities
For the year ended December 31, 2024, our net cash provided by operating activities was $132.7 million. The primary reason for net cash provided by operating activities was the net profit of $181.3 million, decrease in accounts receivable of $5.9 million and increase in contract liabilities of $5.4 million. The outflow from deferred tax income in the amount of $55.2 million was primarily due to release of valuation allowance in the U.S. and increase in inventory of $14.5 million and other receivables of $5.7 million. Additionally, our net profit for the year ended December 31, 2024, included $16.6 million in non-cash expenses primarily comprised of share-based compensation expense.
For the year ended December 31, 2023, our net cash provided by operating activities was $176.8 million. The primary reason for net cash provided by operating activities was the net profit of $197.9 million. The outflow from operating assets and liabilities in the amount of $41.4 million was primarily due to an increase of $16.1 million in accounts receivable due to an increase in sales through distributors, and an increase in other liabilities of $12.6 million, primarily in income tax due to one-time payments of the Company to the Israeli tax authority discussed above, and an increase of $5.2 million in inventories to meet growth in anticipated sales. As we expect our revenues to continue to grow, we anticipate our accounts receivables, inventory and accounts payable will similarly continue to grow, including our available working capital.
Net Cash Used in Investing Activities
For the year ended December 31, 2024, net cash provided in investing activities was $162.2 million, which primarily related to investment of short-term bank deposits and marketable securities of $504.4 million. These outflows were partially offset by inflows of $667.3 million related to proceeds from short-term bank deposits and marketable securities.
For the year ended December 31, 2023, net cash used in investing activities was $136.1 million, which primarily related to short-term bank deposits and marketable securities of $478.6 million. These outflows were partially offset by inflows of $343.3 million related to proceeds from short-term bank deposits and marketable securities.
Net Cash Used in Financing Activities
For the year ended December 31, 2024, net cash used in financing activities was $282.8 million, which consisted of $285.4 million outflow as part of our share repurchase programs. This outflow was partially offset by inflow of $2.6 million related to proceeds from the exercise of options.
For the year ended December 31, 2023, net cash used in financing activities was $5.5 million, which consisted of proceeds from the exercise of options.
Research and Development, Patents and Licenses, etc.
For a description of the Company’s research and development policies, see “Item 4B. Information on the Company–Business Overview–Intellectual Property”.
Trend Information
Other than as disclosed elsewhere in this Annual Report on Form 20-F, we are not aware of any trends, uncertainties, demands, commitments or events for the year ended December 31, 2024 that are reasonably likely to have a material effect on our net revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future results of operations or financial condition.
Recently Issued Accounting Pronouncements
Certain recently issued accounting pronouncements are discussed in Note 2 in our consolidated financial statements.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth the name, age and position of each of our executive officers and directors as of the date of this Annual Report on Form 20-F.
| Name | | Age | | Position |
| Moshe Mizrahy | | 72 | | Chief Executive Officer |
| Yair Malca | | 47 | | Chief Financial Officer |
| Dr. Michael Kreindel | | 58 | | Chief Technology Officer and Director |
Dr. Michael Anghel(1)(2) | | 86 | | Chairman of the Board of Directors |
Dr. Hadar Ron, M.D.(1)(2) | | 66 | | Director |
Bruce Mann(1)(2)(3) | | 90 | | Director |
Mr. Nadav Kenneth(1)(2)(3) | | 65 | | Director |
(1) Member of Audit & Investment Committee
(2) Member of Compensation, Nominating and Corporate Governance Committee
(3) Mr. Nadav Kenneth replaced Dr. Bruce Mann as a Director in April 2024
A brief biography of each person who serves as an executive officer and/or director of the Company is set forth below:
Moshe Mizrahy. Moshe Mizrahy co-founded the Company in 2008 and has been our Chief Executive Officer since inception and served as the Chairman of our board of directors from 2008 to July 2024. Prior to that, Mr. Mizrahy was co-founder and chief executive officer of Syneron Medical Ltd., a medical aesthetic device company based in Israel. Mr. Mizrahy was also the former chief executive officer of Home Skinovations Ltd., an international medical aesthetic consumer devices company active in the home use market, and is currently the chairman of its board since 2007. In addition to Home Skinovations Ltd., Mr. Mizrahy currently sits on the board of directors of the following companies: SipNose Ltd., Pet Novations Ltd., Peri-Ness Technologies Ltd., Urifer Ltd., Escape Rescue Systems Ltd., New Forest Wood Products (2012) Ltd., Med Smart Hub Ltd., Ivy Next Ltd., M.N. Business Strategy Ltd. and Himalaya Family Office Advising Ltd. Mr. Mizrahy has a B.S. in Engineering from the Tel Aviv University and an MBA from Pace University, New York.
Yair Malca. Yair Malca has served as our Chief Financial Officer since 2017. In his previous role, Mr. Malca was the Director of Finance for Jazz Semiconductor, Inc., a developer of integrated circuits and semiconductors, from 2013 to 2017. Before that, he served as the controller of Syneron from 2008 to 2013, as assistant controller of EZchip Semiconductor, a provider of network processors, from 2007 to 2008, as subsidiary controller of Bermad, a provider of hydraulic control valves, from 2005 to 2007, and began his career in public accounting at Ernst & Young from 2002 to 2005. Mr. Malca holds a B.A. in Accounting and Economics from Haifa University and an MBA from Tel Aviv University, and is a Certified Public Accountant in Israel.
Dr. Michael Anghel. Dr. Michael Anghel became the Chairman of the board of directors effective July 25, 2024. Dr. Anghel has been a director of the Company in August 2019. Dr. Anghel has previously served on the board of directors and on the Investment Monitoring Committee of BiolineRx Ltd. (Nasdaq: BLRX) since 2010 and until 2024. From 1977 to 1999, he led the Discount Investment Corporation Ltd. (of the IDB Group) activities in the fields of technology and communications. In 1999, he founded CAP Ventures, an advanced technology investment company. From 2004 to 2005, Dr. Anghel served as CEO of DCM, the investment banking arm of the Israel Discount Bank (TASE: DSCT). Dr. Anghel has also previously served as the Chairman of the Audit Committee and the Chairman of the Remuneration Committee of Ellomay Capital Ltd. {NYSE, TASE : ELLO) until December 2023. Mr. Anghel also previously served as the chairman of the Center for Educational Technology and as the Chairman of the board of directors of Lahav Ltd (Tel-Aviv University Executive Program). Prior to launching his business career, Dr. Anghel served as a full-time member of the Graduate School of Business Administration of the Tel Aviv University, where he taught finance and corporate strategy. Dr. Anghel holds a B.A. in Economics from the Hebrew University in Jerusalem and an M.B.A. and Ph.D. in Finance from Columbia University, New York.
Dr. Michael Kreindel. Dr. Michael Kreindel co-founded the Company in 2008 and has served as our Chief Technology Officer since inception. Dr. Kreindel became a director for the Company in August 2019. He previously was a co-founder of, and served as CTO of, Syneron Medical Ltd. from 2001 to 2007. Dr. Kreindel has a Ph.D. in physics and mathematics, and also graduated as an engineer and physicist in experimental and theoretical nuclear physics from Ural Politechnical Institute, Russia.
Dr. Hadar Ron, M.D. L.L.B. Dr. Hadar Ron became a director of the Company in August 2019. Since 2000, Dr. Ron has been the founding and managing partner of Israel Healthcare Ventures, an Israeli life science venture capital fund. Dr. Ron is also the chief executive officer of the management company for Israel Healthcare Ventures 2 LP Incorporated, or IHCV2, an Israeli life sciences venture capital fund. Dr. Ron serves as chairperson of G.I. View Ltd., a medical device company specializing in colorectal screenings, and CyTwist Ltd., an information technology start-up company, and as a board member of the following companies: Home Skinovations Ltd., SipNose Ltd., Pet Novations Ltd., Peri-Ness Technologies Ltd., Viroblock Ltd., O.G.D.H. Ltd., OrSense Ltd., and NanoPass Technologies Ltd. In addition, Dr. Ron serves as an external director of Together Pharma Ltd. In addition, Dr. Ron serves as a member of the advisory board of the Momentum Fund Tech Transfer of Tel Aviv University, a board member of BIRAD Ltd., the tech transfer company of Bar Ilan University, and is the chairperson of the scientific advisory board of Social Finance Israel’s Social Impact Bond for the prevention of diabetes and colon cancer. Dr. Ron is a physician and attorney by education. She holds M.D. and L.L.B. degrees from Tel Aviv University and has studied at the School of Business Administration at Tel Aviv University.
Bruce Mann. Mr. Bruce Mann served as a director of the Company from August 2019 to April 2024. Bruce Mann is an independent advisor and consultant on corporate governance, corporate law, and capital markets matters, primarily for emerging technology companies. He was a senior partner, partner, or senior of counsel of Morrison & Foerster LLP for 30 years prior to his retirement in 2017. Mr. Mann has been a Governor-at-Large of the National Association of Securities Dealers (NASD) and a member of the New York Stock Exchange Legal Advisory Committee. He has held numerous positions in the American Bar Association, including chairing the Senior Lawyers Division, the Federal Regulation of Securities Committee and the Venture Capital and Private Equity Committee of the ABA Business Law Section. Mr. Mann holds a BBA from the University of Wisconsin and a JD from the University of Wisconsin Law School.
Mr. Nadav Kenneth. Mr. Nadav Kenneth became a director of the Company in April 2024, replacing Mr. Bruce Mann. Mr. Kenneth served for 22 years in the Israeli Army’s Intelligence corps (Unit 8200) where he led large R&D functions, was in charge of the strategic planning branch (plans, methodology, budgeting and monitoring) and commanded an intelligence unit. Following his service, Mr. Kenneth co-founded, managed and served as a board member of several startup companies, worked for a large cellular operator, and provided consultation to companies and official entities. In 2009, Mr. Kenneth co-founded Qrative Ltd., which developed an AI-based platform for analysis and crystallization of intellectual property and served as its CEO and board member from 2009 to 2022. From 2007 to 2011, Mr. Kenneth provided consultation to the Government of Zanzibar with respect to restructuring of the Zanzibar Revenue Authority and setting up the government’s HR and payroll systems. In 2004, Mr. Kenneth co-founded Trisixty Security, which developed an automated system that monitored compliance with IT security regulations, and served as its country manager and board member from 2004 to 2006. From 2014 to 2020, Mr. Kenneth served as volunteer Chairman of a pre-service education NGO (Mechinat Aderet). Mr. Kenneth Holds a B.A. in Economics and Political Sciences from the Bar-Ilan University and an M.A. in Business Entrepreneurship from the Swinburne University in Australia/Israel.
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as of the date of this annual report.
| Board Diversity Matrix |
| Country of Principal Executive Offices: | Israel |
| Foreign Private Issuer | Yes |
| Disclosure Prohibited under Home Country Law | No |
| Total Number of Directors | 5 |
| | Female | Male | Non- Binary | Did Not
Disclose
Gender |
| Part I: Gender Identity | |
| Directors | 1 | 4 | 0 | 0 |
| Part II: Demographic Background | |
| Underrepresented Individual in Home Country Jurisdiction | – |
| LGBTQ+ | – |
| Did Not Disclose Demographic Background | – |
Family Relationships
There are no family relationships among any of our directors or officers.
Special Arrangements
There are no special arrangements or understandings with major shareholders, customers, suppliers or others, pursuant to which any person referred to above was selected as a director or member of senior management.
B. Compensation
Employment and Consulting Agreements
We have entered into employment or consulting agreements with all of our executive officers and key employees. These agreements contain standard provisions for a company in our industry regarding non-solicitation, confidentiality of information, non-competition and assignment of inventions. Our executive officers will not receive benefits upon the termination of their respective employment with us, other than mandatory severance payments and payment of salary and benefits (and limited accrual of vacation days) during the required notice period for termination of their employment, which varies for each individual. The agreements are terminable by us at will, subject to prior notice, which varies for each individual.
Compensation of Individual Covered Executives
The table and summary below outline the compensation granted to our five most highly compensated officeholders (as defined in the Companies Law) with respect to the year ended December 31, 2024. We refer to the five individuals for whom disclosure is provided herein as our “Covered Executives.” For purposes of the table and the summary below, “compensation” includes amounts accrued or paid in connection with salary costs, consulting fees, bonuses, equity-based compensation, retirement or termination payments, benefits and perquisites such as car, phone and social benefits and any undertaking to provide such compensation. All amounts reported in the table are in terms of cost to the Company, as recognized in our consolidated financial statements for the year ended December 31, 2024, plus compensation paid to such officers following the end of the year in respect of services provided during the year.
Name and Principal Position (USD in thousands) | | Salary (1) | | | Bonus | | | Commission | | | Equity-Based
Compensation (2) | | | Total | |
Shakil Lakhani
Former President, North America (3) | | $ | 1,608 | | | $ | 2,391 | | | | - | | | $ | 129 | | | $ | 4,128 | |
Brandon Nye
VP, Sales of West US (4) | | $ | 270 | | | | – | | | $ | 1,385 | | | $ | 360 | | | $ | 2,015 | |
Michael Dennison
VP, Sales of East US (5) | | $ | 276 | | | | – | | | $ | 1,106 | | | $ | 202 | | | $ | 1,584 | |
Yair Malca
Chief Financial Officer (6) | | $ | 455 | | | $ | 266 | | | | – | | | $ | 979 | | | $ | 1,700 | |
Matt Rodgers
VP, Sales of Canada (7) | | $ | 156 | | | | – | | | $ | 522 | | | $ | 432 | | | $ | 1,110 | |
| (1) | Salary includes Covered Executives’ gross salary plus payment of social benefits made by us on behalf of such Covered Executive. Such benefits may include, to the extent applicable to the Covered Executive, payments, pension, car expenses, medical and other insurances, 401K company contribution, payments for social security and tax gross-up payments, severance pay, vacation and other benefits consistent with our policies. |
| (2) | Represents the share-based compensation expenses recorded in our consolidated financial statements for the year ended December 31, 2024, based on the Share-based Compensation fair value, calculated in accordance with accounting guidance for share-based compensation. For a discussion of the assumptions used in reaching this valuation, see Note 13 to our consolidated financial statements. |
| (3) | On February 12, 2024, Mr. Lakhani was granted with 50,000 RSUs under our 2018 Incentive Plan, all of which were forfeited and are expired as of December 31, 2024. As of October 1, 2024, Mr. Lakhani is no longer serving in the position as President of North America and is no longer employed by the Company. In addition to the salary and bonus amounts indicated in the table above, as part of Mr. Lakhani’s departure from the company, he received a severance payment equal to ninety days of his base salary in the amount of $248,125 and certain payments for the continuation of benefits under COBRA for a period of 18 months from October 1, 2024. |
| (4) | On February 12, 2024, Mr. Nye was granted with 12,500 RSUs under our 2018 Incentive Plan, none of which were vested as of December 31, 2024. |
| (5) | On February 12, 2024, Mr. Dennison was granted with 7,000 RSUs under our 2018 Incentive Plan, none of which were vested as of December 31, 2024. |
| (6) | On February 12, 2024, Mr. Malca was granted with 34,000 RSUs under our 2018 Incentive Plan, none of which were vested as of December 31, 2024. |
| (7) | On February 12, 2024, Mr. Rodgers was granted with 15,000 RSUs under our 2018 Incentive Plan, none of which were vested as of December 31, 2024. |
Compensation of Directors as a Group
The aggregate compensation paid by us to our directors for the year ended December 31, 2024, was approximately $308 thousand, including share-based compensation expenses of approximately $115 thousand. This amount does not include reimbursements or coverage of expenses.
We do not have any written agreement with any director providing for benefits upon the termination of such director’s relationship with our Company, other than our consultancy agreement with our Chief Executive Officer and our employment agreement with our Chief Medical Officer.
Employee Benefit Plans
2008 Plans
On January 30, 2008, our board of directors adopted the 2008 Israeli Option Plan, or 2008 Israeli Plan, pursuant to the Companies Law and Section 102 of the Israeli Income Tax Ordinance, 1961, or the Tax Ordinance, allowing us to grant options to purchase our ordinary shares to our and our current and future affiliates’ Israeli employees, officers, directors, consultants, and service providers. Under Israeli law, no shareholder approval was required to approve the 2008 Israeli Plan.
On January 30, 2008, concurrently with the adoption of the 2008 Israeli Plan, our board of directors also adopted the 2008 Rest of the World Options Plan, or 2008 ROW Plan, allowing us to grant options to purchase our ordinary shares to our and our current and future affiliates’ non-Israeli employees, consultants and service providers. The 2008 ROW Plan was approved by our shareholders on March 16, 2008.
Options granted under the 2008 Israeli Plan and 2008 ROW Plan generally vest over a period of three years, but shorter or longer vesting schedules have been set. Any option that is cancelled or forfeited before expiration of its vesting period was available for future grants until the expiration of these plans in January 2018. Since that date, shares underlying any option that is cancelled or forfeited under these plans return to the authorized and un-issued share capital of the company. Additionally, options under the 2008 Israeli Plan and 2008 ROW Plan generally expire seven years after the initial grant date, unless extended by the board of directors. Our board of directors has previously extended the expiration period of certain options prior to their original expiration date. Under the 2008 Israeli Plan, as of December 31, 2024, we have granted options to purchase a total of 4,182,684 ordinary shares, of which 3,739,012 ordinary shares have been issued upon the exercise of such options and 443,672 options have been expired and forfeited. Under the 2008 ROW Plan, as of December 31, 2024, we have granted options to purchase a total of 19,204,710 ordinary shares, of which 15,142,716 ordinary shares have been issued upon the exercise of such options and 4,061,994 options have been expired and forfeited. As of December 31, 2024, there are no options exercisable under the 2008 Israeli Plan and the 2008 ROW Plan in the aggregate.
For more information, see Note 12 to our consolidated financial statements included elsewhere in this Annual Report on Form 20-F.
Israeli tax law allows us to choose from among three alternative sets of tax treatment for our 2008 Israeli Plan and for future plans. In approving the 2008 Israeli Plan, our board of directors selected the capital gains tax treatment under Section 102 of the Tax Ordinance described below for grants to Israeli employees and other office holders, including directors. In accordance with the capital gains tax treatment under Section 102 of the Tax Ordinance, the 2008 Israeli Plan allowed for beneficial tax treatment for options issued through a trustee to Israeli employees and other office holders, including directors, provided that options granted thereunder or, upon their exercise, the underlying ordinary shares, are held by a trustee for at least two years following the date of the option grant. Under Section 102 of the Tax Ordinance, Israeli employees and other office holders, including directors, are (i) entitled to defer any taxable event with respect to the options until the underlying ordinary shares are sold or withdrawn from the trust, and (ii) subject to capital gains tax of 25% on the sale of the underlying ordinary shares. In addition, we may not recognize expenses pertaining to the options for Israeli tax purposes.
Under the 2008 ROW Plan, we were able to grant our non-Israeli employees, officers, directors, consultants and service providers options to purchase our ordinary shares. The 2008 ROW Plan did not allow favorable tax treatment for our U.S., Canadian and other non-Israeli directors, officers, employees and consultants.
The 2008 Israeli Plan and the 2008 ROW Plan expired in January 2018 and additional grants may not be made thereunder; however, options granted under such plans prior to their expiration remain valid following such expiration.
2018 Incentive Plan
On June 17, 2018, our board of directors adopted the 2018 Incentive Plan allowing us to grant shares, options to purchase our ordinary shares, restricted shares and restricted share units to our and our current and future affiliates’ Israeli and other non-U.S. employees, officers, directors, consultants and service providers. In approving the 2018 Incentive Plan, our board of directors selected the capital gains tax treatment described above for grants to Israeli employees and other office holders, including directors, under the 2018 Incentive Plan. The 2018 Incentive Plan also includes as an appendix a sub-plan, or the U.S. Sub-Plan, allowing us to grant shares, options to purchase our ordinary shares, restricted shares and restricted share units to our and our current and future affiliates’ U.S. employees, officers, directors, consultants, and service providers.
Under the 2018 Incentive Plan, as of December 31, 2024, we have granted restricted share units and options to purchase ordinary shares in a total of 7,753,180, of which 5,527,233 ordinary shares have been issued upon exercise of such options and 772,952 options and restricted share units have been forfeited and returned to the reserved authorized and unissued ordinary shares of the company under the 2018 Incentive Plan. The grant above does not include 2,533,300 options, which we granted during January and February 2020, of which 2,518,300 were cancelled and regranted, in March 2020, following repricing of exercise price, and 15,000 were forfeited prior to the repricing of those options.
As of December 31, 2024, 628,090 options are exercisable.
As of December 31, 2024, up to 8,378,000 of our authorized and unissued ordinary shares may be issued pursuant to awards under the 2018 Incentive Plan. Upon adoption of the 2018 Incentive Plan, our board of directors resolved that the number of reserved authorized and unissued ordinary shares of the company for issuance of awards pursuant to the 2018 Incentive Plan, shall automatically increase on an annual basis in such manner that on the first business day of each calendar year beginning in 2019 such number of reserved ordinary shares equal to the lesser of (i) 800,000 ordinary shares, (ii) three percent (3%) of the number of ordinary shares outstanding as of such date, or (iii) a lesser number of ordinary shares determined by the board of directors, will be added to the reserved authorized and unissued ordinary shares of the company for issuance of awards pursuant to the 2018 Incentive Plan. Accordingly, in January 2023, the number of reserved authorized and unissued ordinary shares of the company for issuance of awards pursuant to the 2018 Incentive Plan was last increased by an additional 800,000 ordinary shares to 8,378,000. Awards are made pursuant to agreements and are subject to vesting and other restrictions as determined by the board of directors or the compensation committee. In January 2024, our board of directors determined that no increase to the number of shares reserved for issuance is required for 2024 calendar year. In January 2025, our board of directors approved the automatic increase for 2025 calendar year in the number of reserved, authorized and unissued ordinary shares available for issuance pursuant to the 2018 Incentive Plan by an additional 800,000 ordinary shares to a total of 9,178,000.
The following paragraphs summarize the terms of the 2018 Incentive Plan:
Plan Administration. Our board of directors or the compensation committee acts as the plan administrator.
Types of Awards. The 2018 Incentive Plan permits grants of options to purchase ordinary shares, ordinary shares, restricted shares, or restricted share units.
Exercise Period. Options granted under the 2018 Incentive Plan are exercisable for a period of seven years following the date of grant, unless otherwise determined by our board of directors or our compensation committee.
Exercise Price. Our board of directors or compensation committee has discretion in determining the exercise price of awards, subject to certain limitations. The 2018 Incentive Plan provides procedures for the cashless exercise of options.
Transactions. The 2018 Incentive Plan provides that in the event of a “Transaction” (which is defined as (i) a merger, acquisition or consolidation of the Company with one or more other entities in which the Company is not the surviving entity; or (ii) a sale or other disposition of all or substantially all, as determined by our board of directors in its discretion, of the outstanding ordinary shares of the Company; or (iii) a sale or other disposition of all or substantially all, as determined by our board of directors in its discretion, of the consolidated assets of the Company and its affiliates), if the unexercised awards then outstanding under the 2018 Incentive Plan are assumed or substituted for securities of the successor company pursuant to the Transaction, then the board of directors or compensation committee, in their sole discretion and subject to applicable laws, may adjust the exercise price and number and type of shares of such unexercised awards to reflect such assumption and/or substitution. All other terms and conditions of the award agreements shall remain unchanged, including but not limited to the vesting period. The 2018 Incentive Plan further provides that our board of directors shall have full power and authority to determine that all outstanding awards shall terminate and cease to be outstanding, except to the extent assumed or substituted as aforesaid. In the event that awards are not assumed or substituted by the successor company, our board of directors may provide the participant the right to exercise the vested awards under such terms and conditions as our board of directors shall determine prior to the Transaction. In addition, our 2018 Incentive Plan further provides that, subject to any applicable law, our board of directors or our compensation committee shall have full power and authority to determine that in certain award agreements there shall be a clause providing different provisions with respect to the vesting period of awards underlying such award agreement or any portion thereof in the event of a Transaction.
Termination. The 2018 Incentive Plan provides that in the event of a participant’s termination of services as an employee, director, consultant or contractor of the Company and/or its subsidiaries, other than by reason of such participant’s death or disability or due to termination for cause, all options which are vested upon the date of termination shall be exercisable during a period of 90 days from the date of termination, unless otherwise determined by our board of directors or our compensation committee. All options that are not vested upon the date of termination shall terminate immediately. The participant shall forfeit any ordinary shares acquired pursuant to an award of restricted stock that remains unvested as of the date of termination.
Plan Term. Unless terminated earlier, the 2018 Incentive Plan will continue in effect for a term of ten years from the date of its adoption.
C. Board Practices
Board of Directors
Under the Companies Law, our board of directors is responsible for setting our general policies and supervising the performance of management. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors, subject to the terms of the consultancy agreement that we have entered into with him. All other executive officers who are not directors are appointed by our Chief Executive Officer, and are subject to the terms of any applicable employment or consultancy agreements that we may enter into with each of them.
Under our amended and restated articles of association, our board of directors must consist of at least three directors and not more than seven directors. Our board of directors consists of five directors. Our directors are elected in three staggered classes by the vote of a majority of the ordinary shares present, in person or by proxy, at a shareholders’ meeting (excluding abstentions). Each class of directors consists, as nearly as possible, of one-third of the total number of directors constituting the entire board of directors (other than the external directors, if applicable). At each annual general meeting of our shareholders, the election or re-election of directors following the expiration of the term of office of the directors of that class of directors will be for a term of office that expires on the third annual general meeting following such election or re-election, such that from 2020 and after, at each annual general meeting the term of office of only one class of directors will expire. Each director holds office until the third annual general meeting of our shareholders, unless the tenure of such director expires earlier pursuant to the Companies Law or unless removed from office as described below.
Our directors are divided among three classes as follows: the Class I director, consisting of Dr. Hadar Ron, will hold office until our annual general meeting of shareholders to be held in 2026; the Class II directors, consisting of Dr. Michael Anghel and Mr. Nadav Kenneth, will hold office until our annual general meeting of shareholders to be held in 2027 and until their successors are duly elected and qualified, or until their earlier resignation or retirement; and the Class III directors, consisting of Mr. Moshe Mizrahy and Dr. Michael Kreindel, will hold office until our upcoming annual general meeting of shareholders to be held in 2025.
The provisions of our amended and restated articles of association relating to the number of directors, staggered board and election and removal of a director from office prior to the lapse of their tenure may be changed only by a resolution adopted by two-thirds of our ordinary shares voting on the proposed change.
Under the Companies Law, our board of directors is required to employ independent judgment and discretion when voting, and is prohibited from entering into any voting arrangements with respect to actions taken at meetings of the board. Further, the Companies Law provides that in the event a director learns about an alleged breach of law or improper conduct of business relating to a company matter, said director must promptly take action to summon a meeting of the board of directors to address any such breach.
In accordance with the exemptions available to foreign private issuers under Nasdaq rules, we do not intend to follow the requirements of Nasdaq rules with regard to the process of nominating directors. Instead, we intend to follow Israeli law and practice, in accordance with which our board of directors (or a committee thereof) is authorized to recommend to our shareholders director nominees for election.
In addition, our amended and restated articles of association allow our board of directors to appoint directors to fill vacancies on our board of directors, including filling empty board seats up to the maximum number of directors permitted under our amended and restated articles of association, for a term of office equal to the remaining period of the term of office of each director whose office has been vacated. Vacancies on our board of directors may be filled by a vote of a simple majority of the directors then in office even if they do not constitute a quorum (subject to the limitation on the number of directors and their qualifications). A director so appointed will hold office until the next applicable annual general meeting of our shareholders in which such director’s class is to be replaced.
Directors may be removed from office by a resolution at a general meeting of shareholders adopted by holders of two-thirds of our ordinary shares voting on the proposed removal, provided that the director being removed from office is given a reasonable opportunity to state his or her case before the general meeting, or under other circumstances set forth in our amended and restated articles of association. If a director is removed from office as set forth above, the general meeting will be entitled, in the same session, to elect another director in his or her place subject to the maximum number of directors permitted as stated above. Should it fail to do so, the board of directors will be entitled to do so. Any director who is appointed in this manner will serve in office for the remainder of the removed director’s term in office, and will be eligible for re-election.
Under the Companies Law, we would be required to include on our board of directors at least two members, each of whom qualifies as an external director, and as to whom special qualifications, voting requirements and other provisions would be applicable. We would also be required to include one such external director on each of our board committees.
However, under regulations promulgated under the Companies Law, Israeli companies whose shares are traded on stock exchanges such as Nasdaq that do not have a controlling shareholder (as defined therein) and which comply with the requirements of the jurisdiction where the company’s shares are traded with respect to the appointment of independent directors and the composition of an audit committee and compensation committee, may elect not to follow the Companies Law requirements with respect to the composition of its audit committee and compensation committee and the appointment of external directors (provided that in the event that upon the appointment of a certain director all members of the board of directors of the company are from one and the same gender only, a director from the opposite gender will be appointed). As we do not have a controlling shareholder, we have elected to comply with the requirements of Nasdaq with respect to the composition of our board and such committees, and therefore we are exempt from the Companies Law requirements with respect thereto, including the appointment of external directors.
Alternate Directors
Our amended and restated articles of association provide, as allowed by the Companies Law, that any director may, by written notice to us, appoint another person to serve as an alternate director at a meeting of the board of directors. The alternate director will be regarded as a director. Under the Companies Law, a person who is not qualified to be appointed as a director, a person who is already serving as a director or a person who is already serving as an alternate director for another director, may not be appointed as an alternate director, unless otherwise permitted by applicable law. Nevertheless, a director who is already serving as a director may be appointed as an alternate director for a member of a committee of the board of directors so long as he or she is not already serving as a member of such committee. The term of appointment of an alternate director may be for one meeting of the board of directors or until notice is given of the cancellation of the appointment.
Leadership Structure of the Board
In accordance with the Companies Law and our amended and restated articles of association, our board of directors is required to appoint one of its members to serve as chairman of the board of directors. Dr. Michael Anghel currently serves as the Chairman of our board of directors.
Under the Companies Law, the chairman of the board of directors of a public company or his relatives cannot be vested with the authority of the chief executive officer of such a public company, without the approval of a special majority of such company’s shareholders. The shareholders’ approval can be provided for a period of five years following an initial public offering, and subsequently, for additional periods of up to three years. The shareholders’ special majority consists of a majority vote of the shares present and voting at a shareholders meeting, provided that either:
| • | such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in approving such resolution that are voted at the meeting, excluding abstentions; or |
| • | the total number of shares voted by non-controlling shareholders and by shareholders who do not have a personal interest against approving such resolution does not exceed 2% of the aggregate voting rights in the company. |
In addition, a person who is subordinated, directly or indirectly, to the chief executive officer may not serve as the chairperson of the board of directors; the chairperson of the board of directors may not be vested with authorities that are granted to persons who are subordinated to the chief executive officer; and the chairperson of the board of directors may not serve in any other position in the company or in a controlled subsidiary, but may serve as a director or chairperson of a controlled subsidiary.
Following the rejection by our shareholders, at the 2024 Annual General Meeting of our shareholders held in April 2024, of the proposed resolution to authorize Mr. Moshe Mizrahy to continue serving as the Company’s chief executive officer and chairman of the board of directors, our board of directors has duly appointed Dr. Michael Anghel as the new Chairman of the Board as of July 2024.
Committees of the Board of Directors
Under the Companies Law and our amended and restated articles of association, our board of directors is permitted to form committees, and to delegate to any such committee powers allotted to the board of directors, subject to certain exceptions. In general, the board of directors may overturn a resolution adopted by a committee it has formed; however, the board’s decision shall not affect the ability of third parties, who were not aware of such decision, to rely on the committee’s resolution prior to the time it is overturned. Only members of the board of directors can be members of a board committee, unless the committee is solely advisory.
Audit and Investment Committee
Our audit and investment committee consists of Dr. Michael Anghel, Dr. Hadar Ron and Mr. Nadav Kenneth. Dr. Anghel serves as chairperson of the audit and investment committee.
Israeli Companies Law Requirements
Under the Companies Law, we are required to maintain an audit committee.
Nasdaq Listing Requirements
Under Nasdaq rules, we are required to maintain an audit committee consisting of at least three independent directors, each of whom is financially literate and one of whom has accounting or related financial management expertise.
All members of our audit and investment committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq. Our board of directors has determined that Dr. Michael Anghel is an audit committee financial expert as such term is defined by the SEC rules and has the requisite financial experience as defined by Nasdaq rules. Each of the members of our audit and investment committee is “independent” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act and satisfies the independent director requirements under Nasdaq rules.
Audit and Investment Committee Role
Our audit and investment committee charter sets forth the responsibilities of the audit and investment committee consistent with the rules and regulations of the SEC and Nasdaq rules as well as the requirements for such committee under the Companies Law, including the following:
| • | oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors in accordance with Israeli law; |
| • | recommending the engagement or termination of the person filling the office of our internal auditor; and |
| • | recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors. |
Our audit and investment committee provides assistance to our board of directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, risk assessment and risk management, internal control and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit and investment committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the auditors are independent of management.
Our audit and investment committee also periodically reviews the Company’s investment policy and guidelines, the investments made by the Company, the Company’s investment strategy and its compliance with the Company’s investment policy, and suggest to the board of directors modifications to be made to the Company’s investment policy.
Under the Companies Law, our audit and investment committee is responsible for:
| • | determining whether there are deficiencies in the business management practices of the Company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices; |
| • | determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and whether such transaction is extraordinary or material under the Companies Law) (see “—Approval of Related Party Transactions under Israeli Law—Office Holders”); |
| • | establishing the approval process (including by conducting competitive proceedings) for certain transactions with a controlling shareholder or in which a controlling shareholder has a personal interest; |
| • | where the board of directors approves the working plan of the internal auditor, examining such working plan before its submission to the board of directors and proposing amendments thereto; |
| • | examining our internal audit controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to fulfill his responsibilities; |
| • | examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment of our auditor; and |
| • | establishing procedures for the handling of employees’ complaints as to deficiencies in the management of our business and the protection to be provided to such employees. |
Compensation, Nominating and Governance Committee
Following our initial public offering, we established a compensation, nominating and corporate governance committee. The members of this committee are Dr. Hadar Ron, Dr. Michael Anghel and Mr. Nadav Kenneth. Dr. Ron serves as chairperson of the committee.
Israeli Companies Law Requirements
Under the Companies Law, the board of directors of a public company must appoint a compensation committee. The duties of the compensation, nominating and corporate governance committee include the recommendation to our board of directors of a policy regarding the terms of engagement of office holders (as defined in the Companies Law), to which we refer as a compensation policy. The term “office holder” is defined under the Companies Law as a chief executive officer (referred to in the Companies Law as the general manager), chief business manager, deputy general manager, vice general manager, any other person assuming the responsibilities of any of these positions regardless of that person’s title, a director and any other manager directly subordinate to the general manager. That policy must be adopted by the board of directors, after considering the recommendations of the compensation, nominating and corporate governance committee, and needs to be approved by the company’s shareholders, which approval requires what we refer to as a Special Majority Approval for Compensation. A Special Majority Approval for Compensation requires shareholder approval by a majority vote of the ordinary shares present and voting at a meeting of shareholders called for such purpose, provided that either: (i) such majority includes at least a majority of the ordinary shares held by all shareholders who are not controlling shareholders and do not have a personal interest in such compensation arrangement, excluding abstentions; or (ii) the total number of ordinary shares of non-controlling shareholders and shareholders who do not have a personal interest in the compensation arrangement and who vote against the arrangement does not exceed 2% of the company’s aggregate voting rights.
Even if the shareholders do not approve the compensation policy, the board of directors may resolve to approve the compensation policy if and to the extent the compensation committee and the board determine, in its judgment following internal discussions and after reconsidering the compensation policy, that approval of the compensation policy is in the best interests of the company. Our compensation committee and board of directors have exercised such authority in May 2024.
Pursuant to regulations promulgated under the Companies Law, if a company adopts a compensation policy in advance of its initial public offering and describes it in its prospectus, then the compensation policy shall be deemed a validly adopted policy and will remain in effect for a term of five years from the date the company becomes a public company. Following the rejection by our shareholders at the 2024 Annual General Meeting of the proposed resolution to approve our updated compensation policy for an additional three year period, our compensation committee and board of directors made the decision to nonetheless approve our updated compensation for an additional three year period until the annual general meeting of the shareholders to be held in 2027 consistent with their authorities under the Companies Law. The compensation policy will be reviewed from time to time by our compensation committee and our board of directors, according to the requirements of the Companies Law.
The compensation policy must serve as the basis for decisions concerning the financial terms of employment or engagement of office holders, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. Under the Companies Law, the compensation policy must relate to certain factors, including advancement of the company’s long-term objectives, business plan and policies, and creation of appropriate incentives for office holders. It must also consider, among other things, the company’s risk management, size and the nature of its operations. The compensation policy must furthermore consider the following additional factors:
| • | the education, skills, expertise and accomplishments of the relevant office holder; |
| • | the office holder’s roles and responsibilities and prior compensation agreements with him or her; |
| • | the ratio between the cost of the terms offered and the cost of the employment of other employees of the company, including those employed through outsourcing firms, in particular the ratio between such cost to the average and median salary of such employees of the company; |
| • | the impact of disparities in salary upon work relationships in the company; |
| • | the possibility of reducing variable compensation at the discretion of the board of directors; |
| • | the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and |
| • | as to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company. |
The compensation policy must also include the following principles:
| • | with the exception of office holders who report directly to the chief executive officer, determining the link between variable compensation and long-term performance and measurable criteria; however, the company may determine that an immaterial part of the variable components of an office holder’s compensation package shall be awarded based on non-measurable criteria, if such amount is not higher than three months’ salary per annum, while taking into account such office holder’s contribution to the company; |
| • | the ratio between variable and fixed compensation, and the ceiling for the value of variable compensation at the time of their payment, or in the case of share-based compensation, at the time of grant; |
| • | the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was restated in the company’s financial statements; |
| • | the minimum holding or vesting period for variable, equity-based compensation while referring to an appropriate long-term perspective based incentives; and |
| • | maximum limits for retirement payments. |
Our compensation policy is designed to promote retention and motivation of directors and executive officers. Additionally, our compensation policy is designed to align the interests of our directors and executive officers with our long-term performance and serves as a risk management tool. Under our compensation policy, a portion of an executive officer’s compensation package is targeted to reflect our short- and long-term goals as well as the executive officer’s individual performance. Our compensation policy also includes measures designed to reduce an executive officer’s incentives to take excessive risks that may harm us in the long term. Such measures include limits on the value of cash bonuses and equity-based compensation for executive officers, limits on the ratio between an executive officer’s variable and total compensation, and minimum vesting periods for equity-based compensation.
Our compensation policy takes into account an executive officer’s individual characteristics, such as his or her respective position, educational background, scope of responsibilities and contributions to the attainment of our goals, as the basis for compensation variation among our executive officers and considers the internal ratios between compensation of our executive officers and directors and other employees.
Pursuant to our compensation policy, compensation that may be granted to an executive officer may include base salary, an annual bonus, other cash bonuses (such as a signing bonus or special bonus for special achievements, such as an outstanding personal achievement, outstanding personal effort or outstanding company performance), equity-based compensation, benefits and retirement compensation and termination of service arrangements. All cash bonuses are limited to a maximum amount linked to the executive officer’s base salary. In addition, the total variable compensation components (cash bonuses and equity-based compensation) may not exceed 90% of each executive officer’s total compensation package with respect to any given calendar year.
An annual cash bonus may be awarded to executive officers upon the attainment of pre-set periodic objectives and individual targets. The annual cash bonus that may be granted to our executive officers (excluding our chief executive officer) will be based on performance objectives and a discretionary evaluation of the executive officer’s overall performance by our chief executive officer and is subject to minimum thresholds. The annual cash bonus that may be granted to executive officers (excluding our chief executive officer) may be based entirely on a discretionary evaluation. Furthermore, our chief executive officer will be entitled to recommend performance objectives, and such performance objectives will be approved by our compensation committee and, if required by law, by our board of directors.
The performance-measurable objectives of our chief executive officer will be determined annually by our compensation committee and board of directors. Such objectives will include the weight assigned to each achievement in the overall evaluation. A less significant portion of the chief executive officer’s annual cash bonus may be based on a discretionary evaluation of the chief executive officer’s overall performance by the compensation committee and the board of directors based on quantitative and qualitative criteria.
Under our compensation policy, equity-based compensation for executive officers (including members of our board of directors) is designed in a manner consistent with the underlying objectives in determining such person’s annual cash bonus; namely, to enhance the alignment between such person’s interests with the company’s long-term interests and those of our shareholders and to strengthen the retention and motivation of such persons in the medium to long term.
Our compensation policy provides for executive officer’s compensation to be in the form of share options or other equity-based awards, such as restricted shares and restricted share units, in accordance with our share incentive plan then in place. All equity-based incentives granted to executive officers shall be subject to vesting periods in order to promote long-term retention of the awarded executive officers.
Equity-based compensation shall be granted from time to time and will be individually determined and awarded based on the performance, educational background, prior business experience, qualifications, role and the personal responsibilities of the executive officer.
In addition, our compensation policy contains compensation recovery provisions that allow the Company, under certain conditions, to recover bonuses paid in excess. Moreover, the compensation policy enables our chief executive officer to approve immaterial changes to the terms of an executive officer’s employment (provided that the changes of the terms of employment are in accordance our compensation policy) and allows the Company to exculpate, indemnify and insure our executive officers and directors subject to certain limitations.
Our compensation policy also provides for compensation for the members of our board of directors to be determined either (i) in accordance with the amounts set forth in the Companies Regulations (Rules Regarding the Compensation and Expenses of an External Director) of 2000, as amended by the Companies Regulations (Relief for Public Companies Traded in Stock Exchange Outside of Israel) of 2000, as such regulations may be amended from time to time, or (ii) in accordance with the amounts determined in our compensation policy.
Compensation, Nominating and Corporate Governance Committee Roles
The compensation, nominating and corporate governance committee is responsible for (i) recommending the compensation policy to our board of directors for its approval (and subsequent approval by our shareholders) and (ii) undertaking duties related to the compensation policy and to the compensation of our office holders, including:
| • | recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than five years from the Company’s initial public offering, or otherwise three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur five years from the Company’s initial public offering, or otherwise every three years); |
| • | recommending to the board of directors periodic updates to the compensation policy; |
| • | assessing implementation of the compensation policy; |
| • | determining whether to approve the terms of compensation of certain office holders which, according to the Companies Law, require the committee’s approval; |
| • | determining whether the compensation terms of a candidate for the position of the chief executive officer of the Company needs to be brought to approval of the shareholders according to the Companies Law; and |
| • | determining, subject to the approval of the board of directors and under special circumstances, whether to override a determination of the Company’s shareholders regarding certain compensation related issues. |
Our compensation, nominating and corporate governance charter sets forth the responsibilities of the compensation, nominating and corporate governance committee, which include:
| • | the responsibilities set forth in the compensation policy; |
| • | reviewing and approving the granting of options and other incentive awards to the extent such authority is delegated by our board of directors; and |
| • | reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors. |
In addition, our compensation, nominating and corporate governance committee is responsible for:
| • | overseeing our corporate governance functions on behalf of the board; |
| • | making recommendations to the board regarding corporate governance issues; |
| • | identifying and evaluating candidates to serve as our directors consistent with the criteria approved by the board; |
| • | reviewing and evaluating the performance of the board; |
| • | serving as a focal point for communication between director candidates, non-committee directors and our management; |
| • | selecting or recommending to the board for selection candidates to the board; and |
| • | making other recommendations to the board regarding affairs relating to our directors. |
Internal Auditor
Under the Companies Law, the board of directors of an Israeli public company must appoint an internal auditor recommended by the audit committee. An internal auditor may not be:
| • | a person (or a relative of a person) who holds more than 5% of the company’s outstanding shares or voting rights; |
| • | a person (or a relative of a person) who has the power to appoint a director or the general manager of the company (i.e., the chief executive officer); |
| • | an office holder (including a director) of the company (or a relative thereof); or |
| • | a member of the company’s independent accounting firm, or anyone on its behalf. |
The role of the internal auditor is to examine, among other things, our compliance with applicable law and orderly business procedures, and to report to the chief executive officer, the chairman of the board and the chairman of the audit committee. The internal auditor is entitled to receive notice of audit committee meetings and to participate in them. In addition, the internal auditor may request that the chairman of the audit committee convene a meeting within a reasonable time to discuss an issue raised by the internal auditor. The internal auditor is responsible for preparing a proposal for an annual or periodical audit plan and submit such plan to the board of directors or the audit committee for their approval. Somech Chaikin of KPMG currently serves as our internal auditor.
Approval of Related Party Transactions under Israeli Law
Office Holders
The Companies Law codifies the fiduciary duties that office holders owe to a company. Each person listed in the table above under “Item 6A. Directors, Senior Management and Employees-Directors and Senior Management” who is engaged by us is an office holder under the Companies law.
Fiduciary duties. An office holder’s fiduciary duties consist of a duty of loyalty and a duty of care. The duty of loyalty requires the office holder to act in good faith and for the benefit of the company, and includes, among other things, the duty to avoid any conflict of interest between the office holder’s position in the company and personal affairs, and proscribes any competition with the company or the exploitation of any business opportunity of the company in order to receive personal advantage for himself or herself or others. This duty also requires him or her to reveal to the company any information or documents relating to the company’s affairs that the office holder has received due to his or her position as an office holder. The duty of care requires an office holder, among other things, to act with a level of care that a reasonable office holder in the same position would employ under the same circumstances. This includes the duty to use reasonable means to obtain information regarding the advisability of a given action submitted for his or her approval or performed by virtue of his or her position and all other relevant information pertaining to these actions. We may approve an act specified above which would otherwise constitute a breach of an office holder’s duty of loyalty, provided that the office holder acted in good faith, the act or its approval does not harm the Company, and the office holder discloses his or her personal interest, including any related material information or document, in a timely manner before the date for discussion of approval of such act. Any such approval is subject to the terms of the Companies Law, setting forth, among other things, the appropriate parties of the company entitled to provide such approval, and the methods of obtaining such approval.
Disclosure of personal interest. The Companies Law requires that an office holder promptly disclose to the company any personal interest that he or she may have and all related documents and material information known to him or her, in connection with any existing or proposed transaction by the company. “Personal interest,” as defined by the Companies Law, includes a personal interest of any person in an act or transaction of the company, including a personal interest of his or her relative or of a corporate body in which that person or a relative of that person is a 5% or greater shareholder, a holder of 5% or more of the voting rights, a director or chief executive officer, or in which he or she has the right to appoint at least one director or the chief executive officer. “Personal interest” does not apply to a personal interest stemming merely from one’s ownership of shares in the company. A personal interest also includes (1) a personal interest of a person who votes according to a proxy of another person, including in the event that the other person has no personal interest, and (2) a personal interest of a person who gave a proxy to another person to vote on his or her behalf, regardless of whether or not the discretion of how to vote lies with the person voting.
The office holder must make the disclosure of his personal interest promptly and no later than the first meeting of the company’s board of directors that discusses the particular transaction. This duty to disclose such information does not apply if the personal interest of the office holder derives solely from the personal interest of a relative of the office holder in a transaction unless it is an “extraordinary transaction”. The Companies Law defines an extraordinary transaction as a transaction not in the ordinary course of business, not on market terms or that is likely to have a material impact on the company’s profitability, assets or liabilities, and defines a relative as a spouse, sibling, parent, grandparent, descendent, spouse’s descendant, sibling or parent, and the spouse of any of the foregoing.
Approvals of related party transactions. The Companies Law provides that a transaction with an office holder or a transaction in which an office holder has a personal interest may not be approved if it is adverse to the company’s interest. In addition, such a transaction generally requires board approval, unless the transaction is an extraordinary transaction or the articles of association provide otherwise and provided that the transaction is in the company’s interest and is performed by the office holder in good faith. If the transaction is an extraordinary transaction, first the audit committee and then the board of directors, in that order, must approve the transaction. Under certain circumstances, shareholder approval may also be required. Generally, a director (and any person in general) who has a personal interest in an extraordinary transaction that is considered at a meeting of the board of directors or the audit committee may not attend that meeting or vote on that matter, unless (1) the chairman of the audit committee or board of directors (as applicable) determines that he or she should be present to present the transaction that is subject to approval, or (2) a majority of the board of directors or the audit committee, as the case may be, also has a personal interest in the matter then, in such event, all directors may participate in discussions of the audit committee or the board of directors (as applicable) on such transaction and the voting on approval thereof. If a majority of the board of directors or the audit committee has a personal interest in the transaction, shareholder approval also would be required for the approval of such transaction. See “-Approval of Related Party Transactions under Israeli Law-Office Holders-Approval of office holders’ compensation”.
Approval of office holders’ compensation. The compensation of, or an undertaking to indemnify or insure, an office holder who is not a director generally requires approval first by our compensation committee, then by our board of directors. If the compensation arrangement or undertaking to indemnify or insure is inconsistent with our compensation policy, or if the office holder is the chief executive officer (apart from a number of specific exceptions), then the arrangement is further subject to a Special Majority Approval for Compensation. If the shareholders of a company do not approve the compensation terms of office holders at a meeting of the shareholders, other than directors, the compensation committee and board of directors may override the shareholders’ decision, subject to certain conditions. Arrangements regarding the compensation, indemnification or insurance of a director require the approval of the compensation committee, board of directors and shareholders by simple majority, in that order, and under certain circumstances, a Special Majority Approval for Compensation.
Controlling Shareholders
The Companies Law imposes the same disclosure requirements that apply to directors and office holders, as described above, on a controlling shareholder of a public company. The term “controlling shareholder” is defined in the Companies Law as a shareholder with the ability to direct the activities of the company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in a company or has the right to appoint the majority of the directors of the company or its general manager. In the context of a transaction involving a shareholder of the company, a controlling shareholder is deemed to include any shareholder holding 25% or more of the voting rights if no other shareholder owns more than 50% of the voting rights in the company. For this purpose, two or more shareholders with a personal interest in the approval of the same transaction are deemed to be one shareholder.
Approval of the audit committee or the compensation committee (with respect to compensation arrangements) as the case may be, the board of directors and our shareholders, in that order, is required for:
| • | extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest; and |
| • | transactions for the provision of services, whether directly or indirectly, by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, and transactions concerning the terms of engagement of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee. |
The shareholder approval must include the majority of shares voted at the meeting. In addition, either:
| • | at least a majority of the shares held by the shareholders who have no personal interest in the transaction and are present and voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or |
| • | the total shareholdings of those who have no personal interest in the transaction and who vote against the transaction must not represent more than 2% of the aggregate voting rights in the company. |
In addition, any extraordinary transaction with a controlling shareholder or in which a controlling shareholder has a personal interest with a term of more than three years, and under certain conditions, five years from a company’s initial public offering, requires the abovementioned approval at the end of such period; however, such transactions not involving the receipt of services or compensation can be approved for a longer term, provided that the audit committee determines that such longer term is reasonable under the circumstances.
Pursuant to regulations promulgated under the Companies Law, certain transactions with a controlling shareholder or his or her relative, or with directors or other office holders, which would otherwise require approval of a company’s shareholders, may be exempt from shareholder approval upon certain conditions.
Shareholder Duties
Under the Companies Law, a shareholder has a duty to act in good faith and in a customary manner towards the company and other shareholders and to refrain from abusing his or her power in the company including, among other things, when voting in a general meeting of shareholders or in a class meeting on the following matters:
| • | an amendment to the articles of association; |
| • | an increase in the company’s authorized share capital; |
| • | approval of related party transactions and acts of office holders that require shareholder approval. |
A shareholder also has a general duty to refrain from discriminating against other shareholders.
The remedies generally available upon a breach of contract will also apply to a breach of the shareholder duties mentioned above, and in the event of discrimination against other shareholders, additional remedies may be available to the injured shareholder.
In addition, any controlling shareholder, any shareholder who knows that it possesses the power to determine the outcome of a shareholder vote and any shareholder who, under the company’s articles of association, has the power to appoint or prevent the appointment of an office holder in the company, or has any other power with respect to the company, is under a duty to act with fairness towards the company. The Companies Law does not describe the substance of this duty of fairness except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account. There is limited case law available to assist in understanding the nature of these duties or the implications of these provisions.
Approval of Private Placements
Under the Companies Law and the regulations promulgated thereunder, a private placement of securities does not require approval at a general meeting of the shareholders of a company; provided however, that in special circumstances, such as a private placement completed in lieu of a special tender offer (see “Item 10.–Additional Information–B. Articles of Association”) or a private placement which qualifies as a related party transaction (see “Item 6. –Directors, Senior Management and Employees–C. Board Practices–Approval of Related Party Transactions under Israeli Law”), approval at a general meeting of the shareholders of a company is required.
Exculpation, Indemnification and Insurance of Directors and Officers
Our amended and restated articles of association allow us to indemnify, exculpate and insure our office holders, either pursuant to an undertaking made in advance of an event or following an event, to the fullest extent permitted by the Companies Law, the Israeli Securities Law, 5738-1968, or the Securities Law, and the Economic Competition Law, 5748-1988, or the Economic Competition Law, in respect of liabilities, payments and expenses incurred for acts performed and omissions committed as an office holder. Our articles of association also allow us to exculpate, insure or indemnify any person who is not an office holder, including any employee, agent, consultant or contractor who is not an office holder.
Under the Companies Law, the Securities Law and the Economic Competition Law, a company may indemnify an office holder against the following liabilities, payments and expenses incurred in his or her capacity as an office holder, either in advance of the act or following the act, provided its articles of association authorize such indemnification:
| • | a monetary liability incurred by or imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitration award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount, or according to criteria, determined by the board of directors as reasonable under the circumstances. Such undertaking shall detail the foreseen events and amount or criteria mentioned above; |
| • | reasonable litigation expenses, including reasonable attorneys’ fees, incurred by the office holder (i) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (a) no indictment was filed against such office holder as a result of such investigation or proceeding, and (b) no financial liability was imposed upon him or her as a substitute for a criminal proceeding against them as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that did not require proof of criminal intent; and (ii) in connection with a monetary sanction; |
| • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; |
| • | expenses incurred by the office holder with respect to proceedings held pursuant to certain provisions of the Economic Competition Law; |
| • | a monetary liability imposed on the office holder in favor of a payment for a breach offended at an Administrative Procedure (as defined below) as set forth in Section 52(54)(a)(1)(a) of the Securities Law; |
| • | expenses expended by the office holder with respect to an Administrative Procedure under the Securities Law, including reasonable litigation expenses and reasonable attorneys’ fees; and |
| • | any other obligation or expense in respect of which it is permitted or will be permitted under applicable law to indemnify an office holder, including, without limitation, matters referenced in Section 56H(b)(1) of the Securities Law. |
An “Administrative Procedure” is defined as a procedure pursuant to Chapters H3 (Monetary Sanction by the Israeli Securities Authority), H4 (Administrative Enforcement Procedures of the Administrative Enforcement Committee) or Il (Arrangement to Prevent Procedures or Interruption of Procedures Subject to Conditions) of the Securities Law.
Under the Companies Law, the Securities Law and the Economic Competition Law, a company may obtain insurance for an office holder against the following liabilities incurred in his or her capacity as an office holder, to the extent provided in the company’s articles of association:
| • | a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company; |
| • | a breach of the duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder; |
| • | a financial liability imposed on the office holder in favor of a third party; |
| • | expenses incurred by the office holder with respect to proceedings held pursuant to certain provisions of the Economic Competition Law; |
| • | a monetary liability imposed on the office holder in favor of an injured party at an Administrative Procedure pursuant to Section 52(54)(a)(1)(a) of the Securities Law; and |
| • | expenses incurred by an office holder in connection with an Administrative Procedure, including reasonable litigation expenses and reasonable attorneys’ fees. |
Under the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. A company may exculpate an office holder in advance from liability to the company, in whole or part, for damages caused to the company as a result of a breach of the duty of care, but only if a provision authorizing such exculpation is included in its articles of association. A company may not exculpate in advance a director from liability arising out of a breach of a duty of care with respect to a distribution.
Under the Companies Law, however, a company may not indemnify, exculpate or insure an office holder against any of the following:
| • | a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company; |
| • | a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| • | an act or omission committed with intent to derive illegal personal benefit; or |
| • | a fine, civil fine, monetary sanction or forfeit levied against the office holder. |
The Securities Law and the Economic Competition Law also provide certain limitations on the ability of a company to indemnify, exculpate and insure office holders.
We have obtained directors’ and officers’ liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by applicable law. In addition, we have entered into agreements with each of our directors and executive officers, exculpating them from a breach of their duty of care to us to the fullest extent permitted by law, and undertaking to indemnify them to the fullest extent permitted by law. Such indemnification is in addition to any amounts available under our directors’ and office holders’ liability insurance policy. Each office holder who agreed to receive this letter of indemnification also agreed to give his or her approval to terminate all previous letters of indemnification that we have provided to him or her, if any. The maximum and aggregate indemnification amount for all current and future indemnified persons under such agreements is the greater of (i) an amount equal to 25% of our shareholders’ equity on a consolidated basis, based on our most recent financial statements made publicly available before the date on which the indemnity payment is made and (ii) $40 million.
D. Employees
As of December 31, 2024, we had 599 employees worldwide, across 4 departments, including 3 employees on the executive team, 51 employees in finance, IT and administration, 385 employees in sales and marketing, 42 employees in research and development and 118 employees in manufacturing and assembly and supply chain. As of December 31, 2024, 318 of our employees are located in the United States and Canada, 112 are located in Israel and the remainder are located in Europe, Asia and Latin America. We believe our employee relations are good.
Israeli labor laws govern the length of the workday and workweek, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination of employment, equal opportunity and antidiscrimination laws, and other conditions of employment. Subject to certain exceptions, Israeli law generally requires severance pay upon the retirement, death or dismissal of employees and requires us and our employees to make payments to the National Insurance Institute, which is similar to the U.S. Social Security Administration. Our Israeli employees have pension plans that comply with applicable Israeli legal requirements, which also include the mandatory pension payments required by applicable law and allocation of severance pay.
While none of our Israeli employees work under any collective bargaining agreements, certain provisions of the collective bargaining agreements between the Histadrut (General Federation of Labor Law in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Associations) are applicable to our employees in Israel by extension orders issued by the Israeli Ministry of Economy and Industry. These provisions primarily affect such matters as length of working hours and workweek, recuperation pay, travel expenses and pension rights with respect to our Israeli employees.
All of our employment and consulting agreements include employees’ and consultants’ undertakings with respect to confidentiality, noncompetition and assignment to us of intellectual property rights developed in the course of their employment or engagement with us. However, there can be no assurance that these agreements will be enforceable or that they will provide us with adequate protection.
E. Share Ownership
The beneficial ownership of our ordinary shares is determined in accordance with the rules of the SEC. Under these rules, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. For purposes of the table below, we deem ordinary shares issuable pursuant to options, restricted share units or warrants that are currently exercisable or exercisable within 60 days of December 31, 2024, if any, to be outstanding and to be beneficially owned by the person holding the options, restricted share units or warrants for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person.
All arrangements for involving employees in our capital is discussed in “Item 6B. Directors, Senior Management and Employees–Compensation–Employee Benefit Plans” above.
Unless otherwise noted below, each shareholder’s address is do InMode Ltd., Tavor Building, Sha’ar Yokneam, P.O. Box 533, Yokneam 2069200, Israel.
| Name of Beneficial Owner: | | Number of
Ordinary
Shares | | | Percentage(1) | |
| Directors and Named Executive Officers | | | | | | |
Dr. Michael Kreindel(2) | | | 3,114,762 | | | | 4.47 | % |
Moshe Mizrahy(3) | | | 3,499,226 | | | | 5.02 | % |
Dr. Hadar Ron, M.D.(4) | | | 91,270 | | | | * | |
| Bruce Mann | | | - | | | | - | |
Dr. Michael Anghel (5) | | | 18,000 | | | | * | |
Yair Malca(6) | | | 114,242 | | | | * | |
Nadav Kenneth(7) | | | 798 | | | | * | |
| Shakil Lakhani | | | - | | | | - | |
| Total for all directors and executive officers as a group (8 persons) | | | 6,838,298 | | | | 9.82 | % |
| * | Represents less than 1.0%. |
| (1) | Percentage ownership is based on 69,558,670 ordinary shares outstanding (excluding treasury shares) as of December 31, 2024, and (ii) restricted share units and options to purchase ordinary shares in a total of 109,000 exercisable within 60 days of December 31, 2024, of our officers, directors and major shareholders (see “Item 7A. Major Shareholders and Related Party Transactions–Major Shareholders”). |
| (2) | Consists of 3,114,762 ordinary shares. |
| (3) | Consists of 3,499,226 ordinary shares. |
| (4) | Consists of: (i) 59,270 ordinary shares, (ii) options to purchase 30,000 ordinary shares exercisable within 60 days of December 31, 2024, with an exercise price of $7. These options expire on August 13, 2026, and (iii) 2,000 restricted share units vested within 60 days of December 31, 2024. |
| (5) | Consists of: (i) 5,000 ordinary shares, (ii) options to purchase 11,000 ordinary shares exercisable within 60 days of December 31, 2024, with an exercise price of $7. These options expire on August 13, 2026, and (ii) 2,000 restricted share units vested within 60 days of December 31, 2024. |
| (6) | Consists of: (i) 50,242 ordinary shares, (ii) options to purchase 30,000 ordinary shares exercisable within 60 days of December 31, 2024, with an exercise price of $9.845. These options expire on March 14, 2027, and (iii) 34,000 restricted share units vested within 60 days of December 31, 2024. |
| (7) | Consists of 798 ordinary shares. |
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation.
We were not required to prepare an accounting restatement, in accordance with the final clawback rules adopted by the SEC under Section 10D and Rule 10-D1 of the Exchange Act during the period covered by this Annual Report on Form 20-F which would require recoupment of any erroneously awarded incentive compensation.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
The following table sets forth certain information regarding the beneficial ownership of our outstanding ordinary shares, as of the date of this Annual Report on Form 20-F, by each person or entity who we know beneficially owns 5% or more of the outstanding ordinary shares. For purposes of the table below, we deem ordinary shares issuable pursuant to options, restricted share units or warrants that are currently exercisable or exercisable within 60 days of the date of this Annual Report on Form 20-F, if any, to be outstanding and to be beneficially owned by the person holding the options, restricted share units or warrants for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person. Percentages for table below are based on 69,558,670 ordinary shares (excluding treasury shares) outstanding as of December 31, 2024, and options to purchase ordinary shares and restricted share units in a total of 109,000 exercisable within 60 days of December 31, 2024, of our officers, directors and major shareholders (see “Item 6E. Directors, Senior Management and Employees-Share Ownership”).
As of December 23, 2024, we have approximately 52,541 shareholders of record of our ordinary shares, approximately 47,802 of which are U.S. persons. These U.S. persons hold approximately 60% of our outstanding share capital. The actual number of beneficial owners is substantially greater than the number of shareholders of record because a large portion of our ordinary shares are held in street name by brokers and other nominees. This number of shareholders of record also does not include shareholders whose shares may be held in trust by other entities.
None of our shareholders has different voting rights from other shareholders. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of the Company.
| | | Number of
Ordinary
Shares | | | Percentage | |
| | | | | | | |
BlackRock, Inc. (1) | | | 6,587,699 | | | | 9.46 | % |
| (1) | According to a beneficial ownership report on Schedule 13G filed with the SEC by BlackRock Inc. (“Blackrock”), on February 6, 2024, BlackRock and its consolidated subsidiaries beneficially owned an aggregate of 6,587,699 outstanding ordinary shares of the Company. Other than as described in the table above, we have not independently confirmed this beneficial ownership information. |
B. Related Party Transactions
Relationship with Home Skinovations Ltd. (under voluntary liquidation)
Mr. Moshe Mizrahy, our Chief Executive Officer, is a substantial shareholder and board member of Home Skinovations, and Dr. Hadar Ron, one of our directors, serves on the board of directors of Home Skinovations.
Home Skinovations is involved in the development, manufacture and distribution of home-use light-based devices for aesthetic applications, which include hair removal, anti-aging, microdermabrasion, cellulite and acne treatments. Except as detailed below, we have no commitments to, or agreements with, Home Skinovations or any of its subsidiaries, including with respect to any mutual research and development, indebtedness, financing, debt or credit lines, or any jointly-owned intellectual property or like arrangements, and we do not share tangible or intangible assets with Home Skinovations or any of its subsidiaries. Any future agreements with Home Skinovations must be reviewed and approved by our audit committee and board of directors.
Service Agreements
We receive certain services from, and provide certain services to, Home Skinovations. We do not consider these services to be material. The services include an office sublease in Israel, mobile phone services, use of certain computer hardware and switchboard infrastructure, certain software licenses and limited personnel services. In relation to these services, Home Skinovations did not invoice us in the year ended December 31, 2024, nor did the Canadian subsidiary of Home Skinovations invoiced our Canadian subsidiary.
Relationship with Himalaya Family Office Consulting Ltd.
Mr. Moshe Mizrahy, our Chief Executive Officer, is a minor shareholder and board member of Himalaya Family Office Consulting Ltd., a company engaged in providing global investment portfolio management and risk management & analysis services.
We receive certain investment portfolio management services from Himalaya Family Office Consulting Ltd., with respect to part of our investment portfolio, and recorded expenses related to those services in the amount of $333 thousand for the year ended December 31, 2024.
Agreements and Arrangements with Directors and Executive Officers
We have entered into written employment or consulting agreements with each of our executive officers. See “Item 6. Directors, Senior Management and Employees – B. Compensation- Employment and Consulting Agreements”.
Members of our board of directors are entitled to certain compensation for their services. See “Item 6. Directors, Senior Management and Employees – C. Board Practices – Committees of the Board of Directors – Compensation, Nominating and Governance Committee”.
Options
Since our inception, we have granted options to purchase our ordinary shares to our executive officers and certain of our directors. The options are generally subject to the further terms of the respective option plans, which we describe under “Item 6.B. Compensation-Employee Benefit Plans”.
RSUs (restricted share units)
Under the 2018 Incentive Plan, we have granted RSUs to our executive officers and certain of our directors. The RSUs are generally subject to the further terms of the 2018 Incentive Plan, which we describe under “Item 6.B. Compensation-Employee Benefit Plans-2018 Incentive Plan.”
Directors and Officers Insurance Policy and Indemnification and Exculpation Agreements
We have entered into separate indemnification agreements with each of our current directors, office holders and other executives exculpating them from a breach of their duty of care to us to the fullest extent permitted by law and undertaking to indemnify them to the fullest extent permitted by law. We have also obtained directors’ and officers’ liability insurance for each of our executive officers and directors. See “Item 6C. Directors, Senior Management and Employees-Board Practices-Exculpation, Indemnification and Insurance of Directors and Officers” for additional information.
C. Interests of Experts and Counsel
Not applicable.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and other Financial Information
See “Item 18. Financial Statements”.
Legal Proceedings
On February 14, 2024, a purported shareholder of the Company filed a putative shareholder class action in the United States District Court for the Central District of California, captioned Cement Masons and Plasterers Local No. 502 Pension Fund v. InMode Ltd. et al., Case No. 2:24-cv-01219, against the Company and certain of its officers and directors. The complaint alleges claims under Sections 10(b) and 20(a) of the Exchange Act based on allegedly false or misleading statements related to the Company’s business, operations, sales practices and financial outlook. The lawsuit seeks unspecified damages and other relief. On April 16, 2024, multiple shareholders moved to be appointed lead plaintiff. On December 4, 2024, the Court entered an order appointing a group of shareholder funds as the lead plaintiffs. On January 31, 2025, the lead plaintiffs filed an amended complaint. The amended complaint purportedly brings claims on behalf of purchasers of the Company’s common stock between February 18, 2020 and December 6, 2023, inclusive. The Company’s deadline to respond to the amended complaint is April 1, 2025. This proceeding is in the preliminary stages, and the Company has not yet responded to the amended complaint. As of the date of this filing, the Company is unable to estimate a range of loss, if any, that could result were there to be an adverse final decision, and an estimated liability has not been recorded in the Company’s financial statements. The defendants intend to deny the allegations of wrongdoing and vigorously defend against the claims.
We may be party from time to time to various other lawsuits, claims and other legal proceedings that arise in the ordinary course of our business. There can be no assurance that matters that arise in the future, individually or in aggregate, will not have a material adverse effect on our financial condition or results of operations.
B. Significant Changes
Since December 31, 2024, no significant changes have occurred.
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing Details
Our ordinary shares have been trading on the Nasdaq Global Select Market under the symbol “INMD” since August 8, 2019. Prior to that date, there was no public trading market for our ordinary shares.
B. Plan of Distribution
Not applicable.
C. Markets
Our ordinary shares trade on the Nasdaq Global Select Market under the symbol “INMD”.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Articles of Association
The information set forth in our Registration Statement on Form F-1 filed with the SEC on July 11, 2019 (File No.: 333-232615), under the heading “Description of Share Capital” is incorporated herein by reference.
C. Material Contracts
[Not applicable.]
D. Exchange Controls
There are currently no Israeli government laws, decrees or regulations that restrict or that affect our export or import of capital or the remittance of dividends, interest or other payments to non-resident holders of our securities, including the availability of cash and cash equivalents for use by us and our wholly-owned subsidiaries, except under certain circumstances, for shareholders who are subjects of countries that are, or have been, in a state of war with Israel, and except or otherwise as set forth under “Item 10E. Additional Information–Taxation”.
However, under current Israeli laws, currency controls may be imposed by administrative action at any time. Israeli residents also have an obligation to file reports with the Bank of Israel regarding certain transactions.
E. Taxation
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our ordinary shares, both referred to in this Item 10E as the ordinary shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign, including Israeli, or other taxing jurisdiction.
Material Israeli Tax Considerations
The following is a summary of the material Israeli tax laws applicable to us, and some Israeli Government programs benefiting us. This section also contains a discussion of some Israeli tax consequences to persons acquiring ordinary shares. This summary does not discuss all the acts of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of this kind of investor include residents of Israel, traders in securities or persons that own, directly or indirectly, 10% or more of our outstanding voting capital, all of whom are subject to special tax regimes not covered in this discussion. To the extent that the discussion is based on new tax legislation which has not yet been subject to judicial or administrative interpretation, we cannot assure you that Israeli governmental and tax authority or the Israeli courts will accept the views expressed below. The discussion below is subject to amendment under Israeli law or changes to the applicable judicial or administrative interpretations of Israeli law, which could affect the tax consequences described below. The discussion should not be construed as legal or professional tax advice and does not cover all possible tax considerations.
Potential investors are urged to consult their own tax advisors as to the Israeli or other tax consequences of the purchase, ownership and disposition of our ordinary shares, including, in particular, the effect of any foreign, state or local taxes.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to tax on their taxable income at the corporate tax rate of 23% in 2018 and thereafter. Capital gains derived by an Israeli resident company are subject to tax at the regular corporate tax rate.
Tax Benefits under the Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, or the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
Tax Benefits Under the 2005 Amendment
An amendment to the Investment Law, which became effective as of April 1, 2005, or the 2005 Amendment, changed certain provisions of the Investment Law. An eligible investment program under the 2005 Amendment qualifies for benefits as a “Benefited Enterprise”. Prior to the 2005 Amendment, investment programs under the Investment Law were called “Approved Enterprises”. According to the 2005 Amendment, only Approved Enterprises receiving cash grants require the prior approval of the Investment Center. Rather, a company may claim the tax benefits offered by the Investment Law directly in its tax returns, provided that its facilities meet the criteria for tax benefits set forth in the 2005 Amendment. A company that has a Benefited Enterprise may, in its discretion, approach the Israel Tax Authority for a pre-ruling confirming that it is in compliance with the provisions of the Investment Law.
The duration of the tax benefits for a Benefited Enterprise is limited to the earlier of seven or ten years (depending on the geographic location of the Benefited Enterprise within Israel) from the Commencement Year (as described below) or 12 years from the first day of the year of election. Commencement Year is defined as the later of the first tax year in which a company had derived taxable income for tax purposes from the Benefited Enterprise, or the year of election, which is the year in which a company requested to have the tax benefits apply to the Benefited Enterprise. The tax benefits granted to a Benefited Enterprise are determined, depending on the geographic location of the Benefited Enterprise within Israel, according to one of the following:
(i) Exemption from corporate tax may be available on undistributed income for a period of two to ten years, depending on the geographic location of the Benefited Enterprise within Israel, and a reduced corporate tax rate of 10% to 25% for the remainder of the benefits period, depending on the level of foreign investment in each year.
In addition, a company that has a Benefited Enterprise program is eligible for further tax benefits if it qualifies as a Foreign Investors’ Company, or FIC. The level of foreign investment is measured as the percentage of rights in the company (in the terms of shares, rights to profits, voting and appointment of directors) and of combined share capital and shareholder loans that are owned, directly or indirectly, by persons who are not residents of Israel. The determination as to whether a company qualifies as an FIC is made on an annual basis.
The 2011 Amendment as described below has eliminated the definition of a FIC. However, according to the 2011 Amendment’s transitional provisions, the tax benefits of companies with Benefited Enterprise plans that opt to remain under the Benefited Enterprise regime in accordance with the Investment Law prior to the 2011 Amendment will be preserved.
If the company pays a dividend out of income derived from the Benefited Enterprise during the tax exemption period, such income will be subject to deferred corporate tax with respect to the amount distributed (grossed up to reflect such pre-tax income that it would have had to earn in order to distribute the dividend) at the corporate tax rate which would have otherwise been applied. The company is required to withhold tax on such distribution at a rate of 15%, or such lower rate may be provided in an applicable tax treaty (subject to the receipt in advance of a valid certificate from the Israel Tax Authority allowing for a reduced tax rate); or
(ii) Reduced corporate tax rates for companies with facilities in certain geographical locations in Israel.
Our facilities in Israel were granted Benefited Enterprise status and thereunder we enjoyed a ten-year tax exemption from corporate tax on our undistributed income derived from the Benefited Enterprise. The first year in which we were exempted from tax was 2012 and the ten-year eligibility period of tax exemption ended in 2021.
Tax Benefits Under the 2011 Amendment
In December 2010, the Israeli Parliament approved amendment 68 to the Investment Law, or the 2011 Amendment. The 2011 Amendment significantly revised the tax incentive regime in Israel and it commenced on January 1, 2011.
The 2011 Amendment provided for a new and additional status of a “Preferred Enterprise,” which introduced new benefits for income generated by a “Preferred Company” through its Preferred Enterprise. The definition of a Preferred Company, includes, inter alia, a company incorporated in Israel that (1) is not wholly owned by a government entity, (2) owns a Preferred Enterprise and (3) is controlled and managed from Israel and is subject to further conditions set forth in the Investment Law. Moreover, a Preferred Company needs to meet certain condition stipulated in the Investment Law such as being an industrial company (including a minimum threshold of 25% export).
A Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to income attributable to its Preferred Enterprise, unless the Preferred Enterprise is located in a specified development zone, known as Development Zone “A,” in which case the rate for a beneficial activity is currently 7.5%.
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to tax at the rate of 20% or such lower rate as may be provided in an applicable tax treaty. Claim of tax benefits afforded by an applicable tax treaty is subject to the receipt in advance of a valid certificate from the Israel Tax Authority, allowing for a reduced tax rate. However, if such dividends are paid to an Israeli company, no tax is required to be withheld (although, if the funds are subsequently distributed to individuals or non-Israeli residents (individuals and corporations), withholding tax would apply when distributing the dividend to such individuals or non-Israeli residents).
Tax Benefits Under the 2017 Amendment
Additional amendments to the Investment Law became effective in January 2017, or the 2017 Amendment. Under the 2017 Amendment, and provided the conditions stipulated therein are met, income derived by Preferred Companies from “Preferred Technological Enterprises,” or PTE (as defined in the 2017 Amendment), would be subject to reduced corporate tax rates of 7.5% in Development Zone “A” and 12% elsewhere in Israel, or 6% in the case of a “Special Preferred Technological Enterprise,” or SPTE (as defined in the 2017 Amendment) regardless of the company’s geographical location within Israel. A Preferred Company distributing dividends from income derived from its PTE or SPTE, would subject the recipient to a 20% tax (or lower, if so provided under an applicable tax treaty). The 2017 Amendment further provides that, in certain circumstances, a dividend distributed to a corporate shareholder who is not an Israeli resident for tax purposes would be subject to a 4% tax (inter alia, if the amount of foreign investors in the distributing company exceeds 90%). Such taxes would generally be withheld at source by the distributing company.
On June 14, 2017, the Encouragement of Capital Investments Regulations (Preferred Technology Income and Capital Profits for a Technological Enterprise), 2017, or the Regulations, were published, which adopted Action 5 under the base erosion and profit shifting, or BEPS, regulations. The Regulations describe, inter alia, the mechanism used to determine the calculation of the benefits under the PTE and under the SPTE regimes and determine certain requirements relating to documentation of intellectual property for the purpose of a PTE. According to these provisions, a company that complies with the terms under the PTE regime may be entitled to certain tax benefits with respect to income generated during the company’s regular course of business and derived from the preferred intangible asset (as determined in the Investment Law), excluding income derived from intangible assets used for marketing and income attributed to production activity. In the event that intangible assets used for marketing purposes generate over 10% of the PTE’s income, the relevant portion, calculated using a transfer pricing study, would be subject to regular corporate income tax. If such income does not exceed 10%, the PTE will not be required to exclude the marketing income from the PTE’s total income. The Regulations set a presumption of direct production expenses plus 10% with respect to income related to production, which can be countered by the results of a supporting transfer pricing study. Tax rates applicable to such production income expenses will be similar to the tax rates under the Preferred Enterprise regime, to the extent such income would be considered as eligible. In order to calculate the preferred income, the PTE is required to take into account the income and the research and development expenses that are attributed to each single preferred intangible asset. However, the transitional provisions allow companies to take into account the income and research and development expenses attributed to all of the preferred intangible assets they have. Under the Regulations, our corporate tax rate for the Company for a beneficial activity is expected to be approximately 7.5%.
Under the transitional provisions of the 2017 Amendment, a company is allowed to continue to enjoy the tax benefits available under the Investment Law prior to the 2017 Amendment until the end of the period of benefits, as defined in the Investment Law. In each year during the period of benefit under its Benefited Enterprise status, the Company will be able to opt-in for application of the 2017 Amendment, thereby making itself available to the tax rates described above. A company’s decision to opt-in for application of the 2017 Amendment is irrecoverable.
Pursuant to the amendment to the Investments Law which became effective on November 15, 2021, a company that elects by November 15, 2022 to pay a reduced corporate tax rate as set forth in that amendment (rather than the regular corporate tax rate applicable to Approved Enterprise income) with respect to undistributed exempt income accumulated by the company until December 31, 2020 will be entitled to distribute a dividend from such income or to be used for any other reason found by the Company, without being required to pay additional corporate tax. A company that has so elected must make certain qualified investments in Israel over the five-year period commencing on the year of which the Company has elected to pay the reduced corporate tax rate. A company that has elected to apply the amendment cannot withdraw from its election. The Company elected to take advantage of the amendment, and has paid NIS 42.5 million (approximately $12 million) as a one-time payment, and as a result NIS 591 million (approximately $165.7 million) of the Company’s undistributed exempt income for years 2012 until 2020 will be entitled to be distributed as dividend or to be used for any other reason found by the Company without being required to pay additional corporate tax. As a result, the Company is required to invest NIS 32 million (approximately $9 million) in its industrial enterprises in Israel over a five year period. Such investment may be in the form of the acquisition of industrial assets (excluding real estate assets), investment in R&D in Israel, or payroll payments to new employees to be hired by the enterprise.
In February 2022, the Company settled the 2017-2020 income tax assessment with the Israeli tax authority, paying $1.3 million. In addition, the Company reached an agreement of tax assessment with the Israeli tax authority under which the Company paid during January 2023 NIS 50.2 million (approximately $14.3 million) on its undistributed exempt income for the year ended December 31, 2021. As a result, NIS 517.8 million (approximately $147.5 million) of the Company’s undistributed exempt income for 2021 may be distributed or used by the Company without being subject to additional corporate tax.
Law for the Encouragement of Industry (Taxes), 5729-1969
We believe that we currently qualify as an “Industrial Company” within the meaning of the Law for the Encouragement of Industry (Taxes), 5729-1969, or the Industry Encouragement Law. The Industry Encouragement Law defines an “Industrial Company” as an Israeli-resident company, incorporated in Israel, of which 90% or more of its income in any tax year, other than of income from defense loans, capital gains, interest and dividends, is derived from an “Industrial Enterprise” owned by it and located in Israel or in the “Area,” in accordance with the definition in section 3a of the Tax Ordinance. An “Industrial Enterprise” is defined as an enterprise which is held by an Industrial Company whose principal activity in a given tax year is industrial production.
The following tax benefits, among others, are available to Industrial Companies:
| • | amortization of the cost of purchased know-how, patents and certain other intangible property rights (other than goodwill), which are used for the development or promotion of the Industrial Enterprise, over an eight-year period for tax purposes, commencing in the year where the Industrial Company began to utilize them; |
| • | accelerated depreciation rates on equipment and buildings; |
| • | under specified conditions, an election to file consolidated tax returns with additional related Israeli Industrial Companies; and |
| • | expenses related to a public offering are deductible in equal amounts over three years, beginning from the year of the offering. |
Eligibility for the benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority. We cannot assure that we will continue to qualify as an “Industrial Company” or that the benefits described above will be available to us in the future.
Capital Gains Taxes Applicable to Israeli Resident and Non-Israeli Resident Shareholders
Capital gains tax is imposed on the sale of capital assets by an Israeli resident and on the sale of such assets by non-Israeli residents if those assets are either: (i) located in Israel, (ii) shares or rights to shares in Israeli resident companies or (iii) represent, directly or indirectly, rights to assets located in Israel, unless a specific exemption is available under Israeli tax law or unless a treaty between Israel and the country of the nonresident provides otherwise. The Tax Ordinance distinguishes between “Real Capital Gain” and the “Inflationary Surplus”. Real Capital Gain is the excess of the total capital gain over Inflationary Surplus computed generally on the basis of the increase in the Israeli Consumer Price Index or, in certain circumstances, a foreign currency exchange rate, between the date of purchase and the date of disposition. You should consult with your own tax advisor as to the method you should use to determine the Inflationary Surplus. Inflationary Surplus is not subject to tax in Israel.
Generally, the tax rate applicable to real capital gains derived by individuals on the sale of our ordinary shares will be taxed at the rate of 25%, unless such shareholder claims a deduction for interest and linkage differences expenses in connection with such shares, in which case the gain will generally be taxed at a rate of 30%. If the individual shareholder is a Controlling Shareholder at the time of the sale or at any time during the 12-month period preceding such sale, such gain will be taxed at the rate of 30%. A “Controlling Shareholder” is defined as a person who holds, directly or indirectly, including together with others, at least 10% of any means of control in the company (including, among other things, the right to receive profits of the company, voting rights, the right to receive the proceeds upon the company’s liquidation and the right to appoint a director).
Real capital gains derived by corporations will be generally subject to the regular corporate tax rate (23% in 2018 and thereafter). Individual and corporate shareholders dealing in securities are taxed at the tax rates applicable to business income: 23% for corporations in 2018 and thereafter and a marginal tax rate of up to 47% in 2018 and thereafter for individuals plus an additional excess tax of 3% as described below.
Non-Israeli Resident Shareholders
Non-Israeli residents (individuals and corporations) are generally exempt from Israeli capital gains tax on any gains derived from the sale, exchange or disposition of shares of Israeli companies publicly traded on a recognized stock exchange outside of Israel, provided, among other things, that such shareholders did not acquire their shares prior to the company’s initial public offering and the gains were not derived from a permanent establishment of such shareholders in Israel. However, shareholders that are non-Israeli entities will not be entitled to such exemption if Israeli residents: (1) have directly or indirectly, alone or together with another, a controlling interest of more than 25% of any of the means of control in such non-Israeli corporation or (2) are the beneficiaries of or are entitled to 25% or more of the revenues or profits of such non-Israeli entity, whether directly or indirectly. This exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares are deemed to be business income.
In addition, a sale of shares may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty and subject to the receipt in advance of a valid certificate from the Israeli tax authority allowing of such exemption.
For example, pursuant to the Convention Between the Governments of the United States and Israel with respect to Taxes of Income, as amended, or the U.S.-Israel Tax Treaty, the sale, exchange or disposition of ordinary shares by a person who qualifies as a resident of the United States within the meaning of the U.S.-Israel Tax Treaty and who holds the shares as a capital asset and is entitled to claim the benefits afforded to such person by the U.S.-Israel Tax Treaty generally will not be subject to the Israeli capital gains tax unless: (i) the capital gain arising from such sale, exchange or disposition is attributed to real estate located in Israel; (ii) the capital gain arising from such sale, exchange or disposition is attributed to royalties; (iii) such person holds, directly or indirectly, shares representing 10% or more of our voting power during any part of the 12-month period preceding such sale, exchange or disposition, subject to certain conditions; (iv) the capital gains arising from such sale, exchange or disposition can be allocated to a permanent establishment of the shareholder in Israel, under certain terms; or (v) such person is an individual and was present in Israel for a period or periods of 183 days or more in the aggregate during the relevant tax year. In any such case, the sale, exchange or disposition of such shares would be subject to Israeli tax, to the extent applicable. Eligibility to benefit from tax treaties is conditioned upon the shareholder presenting a withholding certificate issued by the Israel Tax Authority prior to the applicable payment.
Withholding and Reporting
Either the purchaser, the Israeli stockbrokers or financial institutions through which the shares are held is obliged to withhold tax on the amount of consideration paid upon the sale of our ordinary shares (or on the capital gain realized on the sale, if known), at the Israeli corporate tax rate for Israeli companies (23% in 2018 and thereafter). In case the seller is an individual, the applicable withholding tax rate would be 25% of the amount of consideration paid upon the sale of shares (or on the capital gain realized on the sale, if known).
In some instances where our shareholders may be liable for Israeli tax on the sale of our ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source. Shareholders, including non-Israeli resident shareholders, may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale. In transactions involving a sale of all of the securities of an Israeli resident company, in the form of a merger or otherwise, the Israel Tax Authority may require non-Israeli resident shareholders who are not liable for Israeli tax to sign a declaration in a form specified by the Israel Tax Authority or obtain a specific exemption from the Israel Tax Authority to confirm their status as a non-resident of Israel, and, in the absence of such declarations or exemptions, may require the purchaser of the securities to withhold taxes at source.
The sale of securities traded on a stock exchange, requires that a detailed return, including a computation of the tax due, be filed and an advanced payment must be paid on January 31 and July 31 of every tax year in respect of sales of securities made within the previous six months. However, if all tax due was withheld at source according to applicable provisions of the Tax Ordinance and the regulations promulgated thereunder, then the aforementioned return need not be filed and no advance payment must be made.
Taxation of Dividend Distributions
A distribution of dividends from income, which is not attributed to an Approved Enterprise/Benefited Enterprise/Preferred Enterprise/Technology Enterprises to an Israeli resident individual, will generally be subject to income tax at a rate of 25%. However, a 30% tax rate will apply if the dividend recipient is a “Controlling Shareholder” (as defined above) at the time of the distribution or at any time during the preceding 12-month period, and a non-Israeli resident individual will be generally subject to withholding tax at source at the rate of 15% or such lower rate as may be provided in an applicable tax treaty.
The Tax Ordinance generally provides that a non-Israeli resident (either individual or corporation) is subject to Israeli income tax on the receipt of dividends at the rate of 25% (30% if the dividends recipient is a “Controlling Shareholder” (as defined above), at the time of the distribution or at any time during the preceding 12 months).
Generally, Israeli resident corporations are not subject to Israeli tax on the receipt of dividends paid on shares of Israeli corporations, other than with respect to dividends distributed from income that has accrued during the benefits period and attributed to a Benefited Enterprise as described above.
Payers of dividends on our shares, including the Israeli stockbrokers or the financial institutions through which the shares are held, are generally required, subject to reduced tax rates and the demonstration of a shareholder of his, her or its foreign residency, to withhold taxes upon the distribution of dividends at a rate of 25% (whether or not the recipient is a “Controlling Shareholder”) provided that the shares are registered with a nominee company in Israel (for corporations and individuals), unless a reduced tax rate under the provisions of an applicable double tax treaty (subject to the receipt in advance of a valid certificate from the Israel Tax Authority allowing for a reduced tax rate) is available.
Dividends paid out of income attributed to a Preferred Technology Enterprises are generally subject to withholding tax at source at the rate of 20% or such lower rate as may be provided in an applicable tax treaty.
For example, under the U.S.-Israel Tax Treaty, the following rates will apply in respect of dividends distributed by an Israeli resident company to a U.S. resident (for purposes of the U.S.-Israel Treaty): (i) with regard to a dividend distributed from income which is not attributed to an Approved Enterprise/Benefited Enterprise/Preferred Enterprise/Preferred Technology Enterprise or Special Preferred Technology Enterprise, if the U.S. resident is a corporation which holds during that portion of the taxable year which precedes the date of payment of the dividend and during the whole of its prior taxable year (if any), at least 10% of the outstanding shares of the voting stock of the Israeli resident paying corporation and not more than 25% of the gross income of the Israeli resident paying corporation for such prior taxable year (if any) consists of certain types of interest or dividends – the maximum tax rate of withholding is 12.5% if a certificate for a reduced withholding tax rate would be provided in advance from the Israel Tax Authority, (ii) with regard to a dividend distributed from income derived from an Approved Enterprise/Benefited Enterprise/Preferred Enterprise under the Investment Law, if the U.S. resident is a corporation which holds during that portion of the taxable year which precedes the date of payment of the dividend and during the whole of its prior taxable year (if any), at least 10% of the outstanding shares of the voting stock of the Israeli resident paying corporation and not more than 25% of the gross income of the Israeli resident paying corporation for such prior taxable year (if any) consists of certain types of interest or dividends, the tax rate of withholding 15% will be applicable if a certificate for a reduced withholding tax rate would be provided in advance from the Israel Tax Authority and (iii) in all other cases, the tax rate is 25%, or the domestic rate (if such is lower). The aforementioned rates under the U.S.-Israel Tax Treaty will not apply if the dividend income was derived through a permanent establishment of the U.S. resident in Israel.
If the dividend is attributable partly to income derived from an Approved Enterprise, Benefited Enterprise or Preferred Enterprise, and partly from other sources of income, the income tax rate will be a blended rate reflecting the relative portions of the types of income.
A non-Israeli resident who receives dividend income derived from or accrued from Israel, from which the full amount of tax was withheld at source, is generally exempt from the obligation to file tax returns in Israel with respect to such income, provided that (i) such income was not generated from a business conducted in Israel by the taxpayer, and (ii) the taxpayer has no other taxable sources of income in Israel with respect to which a tax return is required to be filed.
We have never declared or paid cash dividends to our shareholders and currently we do not intend to distribute cash or other dividends in the foreseeable future. We cannot assure you that, in the event we declare a dividend, we will designate the profits that are being distributed in a way that will reduce shareholders’ tax liability.
Foreign Exchange Regulations
Non-residents of Israel who hold our ordinary shares are able to receive any dividends, and any amounts payable upon the dissolution, liquidation and winding up of our affairs, freely repayable in non-Israeli currency at the rate of exchange prevailing at the time of conversion. However, Israeli income tax is generally required to have been paid or withheld on these amounts. In addition, the statutory framework for the potential imposition of currency exchange controls has not been eliminated, and may be restored at any time by administrative action.
Excess Tax
Individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 3% on annual income exceeding a certain threshold (NIS 721,560 for 2024), which amount is linked to the annual change in the Israeli consumer price index, including, but not limited to income derived from dividends, interest and capital gains, subject to the provisions of an applicable tax treaty.
Estate and Gift Tax
Israeli law presently does not impose estate or gift taxes.
Material U.S. Federal Income Tax Considerations to U.S. Holders
The following discussion is a description of the material U.S. federal income tax considerations applicable to an investment in our ordinary shares by U.S. Holders who hold the ordinary shares as capital assets for U.S. federal income tax purposes, (generally, property held for investment). As used in this section, the term “U.S. Holder” means a beneficial owner of an ordinary share who, for U.S. federal income tax purposes, is or is treated as any of the following:
| • | a citizen or resident of the United States; |
| • | a corporation created or organized in or under the laws of the United States or any political subdivision thereof, including the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust if the trust has elected validly to be treated as a United States person for U.S. federal income tax purposes or if a U.S. court is able to exercise primary supervision over the trust’s administration and one or more United States persons have the authority to control all of the trust’s substantial decisions. |
This description is based on provisions of the U.S. Internal Revenue Code of 1986, as amended, referred to in this discussion as the Code, existing U.S. Treasury regulations and administrative and judicial interpretations, each as available and in effect as of the date of this prospectus. These sources may change and are open to differing interpretations, possibly with retroactive effect in a manner that could adversely affect a U.S. Holder. This description does not discuss all aspects of U.S. federal income taxation that may be applicable to investors in light of their particular circumstances or to investors who are subject to special treatment under U.S. federal income tax law, including:
| • | dealers in stocks, securities or currencies; |
| • | financial institutions and financial services entities; |
| • | real estate investment trusts; |
| • | regulated investment companies; |
| • | partnerships and other pass-through entities, and investors in such entities; |
| • | persons that receive ordinary shares as compensation for the performance of services; |
| • | tax-exempt organizations; |
| • | persons that hold ordinary shares as a position in a straddle or as part of a hedging, conversion or other integrated instrument or persons entering into a constructive sale with respect to the ordinary shares; |
| • | persons subject to special tax accounting rules under Section 451(b) of the Code; |
| • | individual retirement and other tax-deferred accounts; |
| • | expatriates of the United States; |
| • | persons having a functional currency other than the U.S. dollar; and |
| • | direct, indirect or constructive owners of 10% or more of our ordinary shares and/or other equity by vote or value. |
This discussion does not address any U.S. state or local or non-U.S. tax consequences or any U.S. federal gift or estate tax or alternative minimum tax considerations.
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds ordinary shares, the U.S. federal income tax consequences relating to an investment in the ordinary shares will depend in part upon the status and activities of such entity or arrangement and the particular partner and certain determinations made at the partner level. Any such entity or arrangement should consult its own tax advisor regarding the U.S. federal income tax consequences applicable to it and its partners of the purchase, ownership and disposition of ordinary shares.
We urge you to consult with your own tax advisor regarding the tax consequences of investing in the ordinary shares, including the effects of federal, state, local, foreign and other tax laws.
Distributions Paid on the Ordinary Shares
We have never paid cash dividends on our ordinary shares and we may not distribute cash or other dividends on our ordinary shares in the foreseeable future. Subject to the discussion below under “- Passive Foreign Investment Company Considerations,” a U.S. Holder generally will be required to include in gross income as ordinary dividend income the amount of any distributions paid on the ordinary shares, including the amount of any Israeli taxes withheld, to the extent that those distributions are paid out of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes. Subject to the discussion below under “-Passive Foreign Investment Company Considerations,” distributions in excess of our earnings and profits will be applied against and will reduce the U.S. Holder’s tax basis in its ordinary shares and, to the extent they exceed that tax basis, will be treated as gain from a sale or exchange of those ordinary shares. Our dividends will not qualify for the dividends-received deduction applicable in some cases to U.S. corporations. Dividends paid in NIS, including the amount of any Israeli taxes withheld, will be includible in the income of a U.S. Holder in a U.S. dollar amount calculated by reference to the exchange rate in effect on the day they are received by the U.S. Holder. Any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend is includible in the income of the U.S. Holder to the date that payment is converted into U.S. dollars generally will be treated as ordinary income or loss. Because we may not account for our earnings and profits in accordance with U.S. federal income tax principles, U.S. Holders should expect all distributions to be reported to them as dividends. Distributions on ordinary shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute passive category income. Subject to certain complex conditions and limitations, Israeli taxes withheld on any distributions on ordinary shares may be eligible for credit against a U.S. Holder’s federal income tax liability. The rules relating to the determination of the U.S. foreign tax credit are complex, and U.S. Holders should consult their tax advisors regarding the availability of a foreign tax credit in their particular circumstances and the possibility of claiming an itemized deduction (in lieu of the foreign tax credit) for any foreign taxes paid or withheld.
Dividends paid by a “qualified foreign corporation” to non-corporate U.S. Holders are eligible for taxation at a reduced capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that certain requirements are met. Each U.S. Holder is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular circumstances. Distributions on ordinary shares that are treated as dividends generally will not be eligible for the “dividends received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations.
A corporation organized outside of the United States, or a non-U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on ordinary shares that are readily tradable on an established securities market in the United States. We believe that we qualify as a resident of Israel for purposes of, and are eligible for the benefits of, the U.S.-Israel Treaty, although there can be no assurance in this regard. Further, the Internal Revenue Service, or IRS, has determined that the U.S.-Israel Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange of information provision. Therefore, subject to the discussion below under “- Passive Foreign Investment Company Considerations,” if the U.S.-Israel Treaty is applicable, such dividends will generally be “qualified dividend income” in the hands of individual U.S. Holders, provided that certain conditions are met, including holding period and the absence of certain risk reduction transaction requirements. Each U.S. Holder is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular circumstances.
Disposition of Ordinary Shares
Subject to the discussion below under “- Passive Foreign Investment Company Considerations,” a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of ordinary shares in an amount equal to the difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received) on the sale, exchange or other disposition and such U.S. Holder’s adjusted tax basis in the ordinary shares. Such capital gain or loss generally will be long-term capital gain taxable at a reduced rate for non-corporate U.S. Holders or long-term capital loss if, on the date of sale, exchange or other disposition, the ordinary shares were held by the U.S. Holder for more than one year. Any capital gain of a non-corporate U.S. Holder that is not long-term capital gain is taxed at ordinary income rates. The deductibility of capital losses is subject to limitations. Any gain or loss recognized from the sale or other disposition of ordinary shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
Passive Foreign Investment Company Considerations
We believe that we were not a PFIC for our taxable year ended December 31, 2024, and we do not expect to be classified as a PFIC for U.S. federal income tax purposes for the current year ending December 31, 2025, or the foreseeable future. However, the determination of whether we are a PFIC is a factual determination made annually based on all the facts and circumstances and thus is subject to change. The relevant rules for determining whether or not we are a PFIC as applied to our business are not entirely clear and certain aspects of the relevant tests will be outside our control. Therefore, no assurance can be given that we will not be a PFIC for any taxable year.
In general, a non-U.S. corporation will be treated as a PFIC, for any taxable year in which either (1) at least 75% of its gross income is “passive income,” referred to as the PFIC income test, or (2) on average at least 50% of its assets, determined on a quarterly basis, are assets that produce passive income or are held for the production of passive income, referred to as the PFIC asset test. Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income generally include cash, even if held as working capital or raised in a public offering, marketable securities, and other assets that may produce passive income. Generally, in determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
A separate determination must be made after the close of each taxable year as to whether we were a PFIC for that year. Because the value of our assets for purposes of the PFIC test will generally be determined by reference to the market price of our ordinary shares, fluctuations in the market price of our ordinary shares may cause us to become a PFIC. In addition, changes in the composition of our income or assets may cause us to become a PFIC.
If we are a PFIC in any taxable year during which a U.S. Holder owns ordinary shares, the U.S. Holder could be liable for additional taxes and interest charges under the “PFIC excess distribution regime” upon (1) a distribution paid during a taxable year that is greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the ordinary shares, and (2) any gain recognized on a sale, exchange or other disposition, including, under certain circumstances, a pledge, of the ordinary shares, whether or not we continue to be a PFIC. Under the PFIC excess distribution regime, the tax on such distribution or gain would be determined by allocating the distribution or gain ratably over the U.S. Holder’s holding period for ordinary shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the current taxable year. The amount allocated to other taxable years will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable, to ordinary income for each such taxable year, and an interest charge, generally applicable to underpayments of tax, will be added to the tax.
If we are a PFIC for any year during which a U.S. Holder holds ordinary shares, we must generally continue to be treated as a PFIC by such U.S. Holder for all succeeding years during which such U.S. Holder holds the ordinary shares, unless we cease to meet the requirements for PFIC status and such U.S. Holder makes a “deemed sale” election with respect to the ordinary shares. If the election is made, the U.S. Holder will be deemed to sell the ordinary shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime. After the deemed sale election, the U.S. Holder’s ordinary shares would not be treated as shares of a PFIC unless we subsequently become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds ordinary shares and one of our non-U.S. corporate subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions. Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries.
If we are a PFIC, a U.S. Holder will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on ordinary shares if such U.S. Holder makes a valid “mark-to-market” election for our ordinary shares. A mark-to-market election is available to a U.S. Holder only for “marketable stock.” Our ordinary shares will be marketable stock as long as they remain listed on Nasdaq and are regularly traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. There can be no assurance that the ordinary shares will be “marketable stock” for purposes of the mark-to-market election. If a mark-to-market election is in effect, a U.S. Holder generally would take into account, as ordinary income for each taxable year of the U.S. Holder, the excess of the fair market value of ordinary shares held at the end of such taxable year over the adjusted tax basis of such ordinary shares. The U.S. Holder would also take into account, as an ordinary loss each year, the excess of the adjusted tax basis of such ordinary shares over their fair market value at the end of the taxable year, but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the mark-to-market election. The U.S. Holder’s tax basis in ordinary shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. Any gain from a sale, exchange or other disposition of ordinary shares in any taxable year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated first as ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as capital loss.
A mark-to-market election will not apply to ordinary shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any non-U.S. subsidiaries that we may organize or acquire in the future. Accordingly, a U.S. Holder may continue to be subject to tax under the PFIC excess distribution regime with respect to any lower-tier PFICs that we may organize or acquire in the future notwithstanding the U.S. Holder’s mark-to-market election for the ordinary shares.
The tax consequences that would apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing fund, or QEF, election. At this time, we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make a QEF election. Prospective investors should assume that a QEF election will not be available.
Each U.S. person that is an investor of a PFIC is generally required to file an annual information return on IRS Form 8621 containing such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
The U.S. federal income tax rules relating to PFICs are very complex. Prospective U.S. investors are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of ordinary shares, the consequences to them of an investment in a PFIC, any elections available with respect to the ordinary shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of ordinary shares of a PFIC.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of ordinary shares. If you are a U.S. person that is an individual, estate or trust, you are encouraged to consult your tax advisors regarding the applicability of this Medicare tax to your income and gains in respect of your investment in ordinary shares.
Information Reporting and Back-up Withholding
U.S. Holders may be required to file certain U.S. information reporting returns with the IRS with respect to an investment in ordinary shares, including, among others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “– Passive Foreign Investment Company Considerations,” each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information. U.S. Holders paying more than $100,000 for ordinary shares may be required to file IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to comply with the required information reporting.
Dividends on and proceeds from the sale or other disposition of ordinary shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if the holder (1) fails to provide an accurate United States taxpayer identification number or otherwise establish a basis for exemption (usually on IRS Form W-9), or (2) is described in certain other categories of persons. However, U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
U.S. Holders should consult their own tax advisors regarding the backup withholding tax and information reporting rules.
EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN ORDINARY SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and under those requirements will file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an Annual Report on Form 20-F containing financial statements that have been examined and reported on, with and opinion expressed by an independent registered public accounting firm, and we intend to submit quarterly interim consolidated financial data to the SEC under cover of the SEC’s Form 6-K.
We maintain a corporate website at www.inmodemd.com. We intend to post our Annual Report on Form 20-F on our website promptly following it being filed with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report on Form 20-F. We have included our website address in this Annual Report on Form 20-F solely as an inactive textual reference.
Our SEC filings are available to you on the SEC’s website at http://www.sec.gov. This site contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. The information on that website is not part of this Annual Report on Form 20-F.
With respect to references made in this Annual Report on Form 20-F to any contract or other document of InMode, such references are not necessarily complete and you should refer to the exhibits attached or incorporated by reference to this Annual Report on Form 20-F for copies of the actual contract or document.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders.
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Foreign Currency Risk
Our consolidated revenues are generated primarily in U.S. dollars. In addition, a substantial portion of our consolidated costs are incurred in dollars. We believe that the U.S. dollar is the primary currency of the economic environment we operate in. Thus, the U.S. dollar is our primary functional and reporting currency.
Our transactions and balances originally denominated in dollars are presented at their original amounts. Balances in non-dollar currencies are translated into U.S. dollars using historical and current exchange rates for non-monetary and monetary balances, respectively. For non-U.S. dollar transactions and other items in the consolidated statements of income (indicated below), the following exchange rates are used: (i) for transactions, exchange rates at transaction dates or average rates; and (ii) for other items (derived from non-monetary balance sheet items such as depreciation and amortization), historical exchange rates. Currency translation gains and losses are presented in finance income or expenses, as appropriate.
A significant portion of our operations is conducted through operations in countries other than the United States and Israel. Revenues from our global operations that were recorded in U.S. dollars represented approximately 72%, 74% and 76% for the years ended December 31, 2024, 2023 and 2022, respectively.
The functional currency of our subsidiaries is the U.S. dollar. For purposes of consolidation, the financial statements of the Foreign Subsidiaries are translated into U.S. dollars in accordance with ASC No. 830, “Foreign Currency Matters” (“ASC 830”).
Interest Rate Risk
The primary objective of our investment activities is to preserve principal while maximizing the interest income we receive from our investments, without increasing risk. Currently, we do not have any outstanding borrowings. We intend to invest our cash balances primarily in bank deposits and fixed-income securities issued by corporations, the United States and non-U.S. governments. We are exposed to market risks resulting from changes in interest rates relating primarily to our financial investments in cash, cash equivalents, deposits and marketable securities. We do not use derivative financial instruments to limit exposure to interest rate risk. Our interest gains may decline in the future as a result of changes in the financial markets; however, we believe any such potential loss would be immaterial to us.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Not applicable.
PART II
ITEM 13. DEFAULTS, DIVIDENDS ARREARAGES AND DELINQUENCIES
Not applicable.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
In May 2024, we approved a share repurchase program of up to 8.37 million ordinary shares, to be purchased out of our cash reserve. As of December 31, 2024, we purchased 8.37 shares in the amount of $150.5 million under this repurchase program.
In September 2024, we approved an additional share repurchase program of up to 7.68 million ordinary shares, to be purchased out of our cash reserve. As of December 31, 2024, we purchased 7.68 shares in the amount of $134.9 million under this repurchase program.
ITEM 15. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We have performed an evaluation, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer (our principal executive officer and principal financial officer, respectively), of the effectiveness of our disclosure controls and procedures that are designed to provide a reasonable level of assurance that the information required to be disclosed in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the specified time periods and accumulated and communicated to our management, including our Chief Executive Officer and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Based on our evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) promulgated under the Exchange Act) as of the end of the period covered by this report are effective in ensuring that the information required to be disclosed in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the specified time periods and accumulated and communicated to our management, including our Chief Executive Officer and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) promulgated under the Exchange Act. Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the reliability of financial reporting and the preparation and fair presentation of published financial statements for external purposes in accordance with generally accepted accounting principles. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate.
Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2024 using criteria described in the Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Based on its assessment, management concluded that the internal control over financial reporting was effective as of December 31, 2024, based on the criteria established in the Internal Control-Integrated Framework (2013).
The effectiveness of our internal control over financial reporting as of December 31, 2024 has been audited by Kesselman & Kesselman, Certified Public Accountants (Isr.), a member of PricewaterhouseCoopers International Limited, independent registered public accounting firm, as stated in their report on pages F-2 to F-3 under “Item 18. Financial Statements” in this Annual Report on Form 20-F.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 20-F that have materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16. [RESERVED]
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that all members of our audit committee are financially literate as determined in accordance with Nasdaq rules and that Dr. Michael Anghel is qualified to serve as an “audit committee financial expert” as defined by SEC rules. Dr. Anghel is independent, as defined by Nasdaq listing standards.
ITEM 16B. CODE OF ETHICS
We have adopted a Code of Business Conduct and Ethics applicable to all of our directors and employees, including our Chief Executive Officer, Chief Financial Officer, controller or principal accounting officer, or other persons performing similar functions. The full text of the Code of Business Conduct and Ethics has been posted on our website at www.inmodemd.com. If we make any amendment to the Code of Business Conduct and Ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the SEC.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Fees Paid to Independent Registered Public Accounting Firm
The following table sets forth, for each of the years indicated, the fees billed by Kesselman & Kesselman, Certified Public Accountants (Isr.), a member of PricewaterhouseCoopers International Limited, our independent registered public accounting firm..
| | | Year ended December 31, | |
| | | | | | | |
| | | USD, in thousands | |
Audit fees(1) | | | 664 | | | | 630 | |
Audit-related fees(2) | | | — | | | | — | |
Tax fees(3) | | | 95 | | | | 116 | |
| All other fees | | | — | | | | — | |
| Total | | | 759 | | | | 746 | |
| (1) | Audit fees consist of fees billed or expected to be billed for the annual audit services engagement and other audit services, which are those services that only the external auditor can reasonably provide, and include the Company audit; statutory audits; comfort letters and consents; attest services; and assistance with and review of documents filed with the SEC. |
| (2) | Audit-related fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of our financial statements or that are traditionally performed by the external auditor, and include consultations concerning financial accounting and reporting standards; internal control reviews of new systems, programs and projects; review of security controls and operational effectiveness of systems; review of plans and control for shared service centers, due diligence related to acquisitions; accounting assistance and audits in connection with proposed or completed acquisitions; and employee benefit plan audits. |
| (3) | Tax fees include fees billed for tax compliance services that were rendered during the most recent fiscal year, including the preparation of original and amended tax returns and claims for refund; tax consultations, such as assistance and representation in connection with tax audits and appeals, tax advice related to mergers and acquisitions, transfer pricing, and requests for rulings or technical advice from taxing authority; tax planning services; and expatriate tax planning and services. |
Our Audit and Investment Committee, in accordance with its charter, reviews and pre-approves all audit services and permitted non-audit services (including the fees and other terms) to be provided by our independent auditors.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
In September 2020, we approved a share repurchase program of up to 2 million ordinary shares, to be purchased out of our cash reserve and to be paid solely from our IPO proceeds. In February 2022, the board approved that the share repurchase program could also be funded from the proceeds of exercised options. In March 2022, we approved an additional repurchase program of up to 1 million ordinary shares, to be purchased out of our cash reserve and to be paid from our remaining IPO proceeds and from the proceeds of exercised options. In May 2024, we approved a share repurchase program of up to 8.37 million ordinary shares, to be purchased out of our cash reserve. In September 2024, we approved an additional share repurchase program of up to 7.68 million ordinary shares, to be purchased out of our cash reserve. As of December 31, 2024, we purchased 18,607,829 shares in the amount of $380.6 million. For the year ended December 31, 2024, we purchased 16,050,000 shares in the amount of $285.4 million. See below for the Company’s purchases of shares under the share repurchase programs in 2024:
Issuer Purchases of Equity Securities in 2024
Period | | (a) Total Number of Shares (or Units Purchased) | | | (b) Average Price Paid per Share (or Unit) | | | (c) Total Number of Shares (or Units) Purchased as Part of Publicly Announced Share Repurchase Plans or Programs | | | (d) Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Publicly Announced Share Repurchase Plans or Programs(1) | |
| May 1, 2024 through July 31, 2024 | | | 8,370,000 | | | $ | 17.974 | | | | 8,370,000 | | | | 0 | |
| | | | | | | | | | | | | | | | | |
| September 11, 2024 through December 31, 2024 | | | 7,680,000 | | | | 17.571 | | | | 7,680,000 | | | | 0 | |
| Total | | | 15,060,000 | | | | 17,781 | | | | 15,060,000 | | | | 0 | |
| (1) | Under a repurchase plan announced in September 2020, with up to 2 million ordinary shares, to be purchased out of our cash reserve and to be paid solely from our IPO proceeds and from proceeds of exercised options. |
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G. CORPORATE GOVERNANCE
Nasdaq Listing Rules and Home Country Practices
Companies incorporated under the laws of the State of Israel, whose shares are publicly traded, including companies with shares listed on Nasdaq, are considered public companies under the Companies Law and are required to comply with various corporate governance requirements under Israeli law relating to such matters as the composition and responsibilities of the audit committee and the compensation committee (subject to certain exceptions that we intend to utilize), and a requirement to have an internal auditor. This is the case even if our ordinary shares are not listed on the Tel Aviv Stock Exchange, which our ordinary shares are not expected to be. These requirements are in addition to the corporate governance requirements imposed by Nasdaq rules and other applicable provisions of U.S. securities laws to which we are subject (as a foreign private issuer). Under Nasdaq rules, a foreign private issuer may generally follow its home country rules of corporate governance in lieu of the comparable requirements of Nasdaq rules, except for certain matters including the composition and responsibilities of the audit committee and the independence of its members within the meaning of the rules and regulations of the SEC.
In accordance with Israeli law and practice and subject to the “home country practice exemption” set forth in Rule 5615 of the Nasdaq rules, we currently follow the provisions of the Companies Law, rather than the Nasdaq requirements, with respect to the following requirements:
| • | Quorum. As permitted under the Companies Law and pursuant to our amended and restated articles of association, the quorum required for an ordinary meeting of shareholders consists of at least two shareholders present in person, by proxy or by other voting instrument in accordance with the Companies Law, who hold at least 25% of the voting rights in the Company (and in an adjourned meeting, with some exceptions, at least one shareholder holding any number of the voting rights in the Company), instead of 33 1/3% of the issued share capital required under Nasdaq rules. A proxy may be deemed to be two (2) or more shareholders pursuant to the number of shareholders represented by the proxy holder. |
| • | Nomination of Directors. Our directors are elected through a staggered board mechanism. With the exception of directors elected by our board of directors due to vacancy, our directors are elected by an annual general meeting of our shareholders to hold office until the next annual meeting following three years from his or her election. The nominations for directors, which are presented to our shareholders by our board of directors, are generally made by the board of directors itself or a duly authorized committee thereof, but nominations may be made by one or more of our shareholders, all in accordance with the provisions of our amended and restated articles of association and the Companies Law. Nominations need not be made by a nominating committee of our board of directors consisting solely of independent directors, as required under Nasdaq rules. |
| • | Compensation Committee. Nasdaq rules require a listed company to have a compensation committee composed entirely of independent directors that operates pursuant to a written charter addressing its purpose, responsibilities and membership qualifications and may receive counselling from independent consultants, after evaluating their independence. The purpose, responsibilities and membership qualifications of our compensation committee are governed by the Companies Law, rather than the Nasdaq rules. In addition, under the Companies Law, there are no specific independence evaluation requirements for outside consultants. |
| • | Compensation of Officers. We comply with the requirements set forth under the Companies Law with respect to the approval of officer compensation. For a discussion regarding the approvals required under the Companies Law and the regulations promulgated thereunder for the approval of compensation of the chief executive officer, all other executive officers and directors, see “Item 6.C – Board Practices –Approval of Related Party Transactions and under Israeli Law”. |
| • | Proxy Statements. We are not required to and, in reliance on home country practice, we do not intend to, comply with certain Nasdaq rules regarding the provision of proxy statements for general meetings of shareholders. Israeli corporate law does not have a regulatory regime for the solicitation of proxies. We intend to provide notice convening an annual general meeting, including an agenda and other relevant documents. |
| • | Shareholder Approval. We are not required to and, in reliance on home country practice, we do not intend to comply with certain Nasdaq rules regarding shareholder approval for certain issuances of securities under Nasdaq Rule 5635. Instead, we will seek our shareholders’ approval for all corporate actions requiring such approval under the requirements of the Companies Law. In accordance with the provisions of our amended and restated articles of association and the Companies Law, our board of directors is authorized to issue securities, including ordinary shares, warrants and convertible notes. |
| • | Executive Sessions. We are not required to and, in reliance on home country practice, we do not intend to comply with certain Nasdaq rules regarding regularly scheduled meetings at which only independent directors are present. |
| • | Approval of Related Party Transactions. All related party transactions are approved in accordance with the requirements and procedures for approval of interested party acts and transactions, set forth in the Companies Law and the regulations promulgated thereunder, which require the approval of the audit committee or the compensation committee, as the case may be, the board of directors and shareholders, as may be applicable, for specified transactions, rather than approval by the audit committee or other independent body of our board of directors as required under the Nasdaq rules. |
| • | Third Party Director Compensation. We follow Israeli law requirements with respect to disclosure of compensation for our directors and executive officers. Israeli law does not require that we disclose information regarding third party compensation of our directors or director nominees. As a result, our practice varies from the third-party compensation disclosure requirements of Nasdaq. |
| • | Annual Shareholders Meeting. As opposed to the Nasdaq Rule 5620(a), which mandates that a listed company hold its annual shareholders meeting within one year of the company’s fiscal year-end, we are required, under the Companies Law, to hold an annual shareholder meeting each calendar year and within 15 months of the last annual shareholders meeting. |
Other than as stated above, we currently intend to take all actions necessary for us to maintain compliance as a foreign private issuer under the applicable corporate governance requirements of the Sarbanes-Oxley Act of 2002, the rules adopted by the SEC and Nasdaq’s listing standards.
We may in the future elect to follow home country corporate governance practices in Israel with regard to other matters. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on Nasdaq, may provide less protection than is accorded to investors under Nasdaq rules applicable to domestic issuers.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.