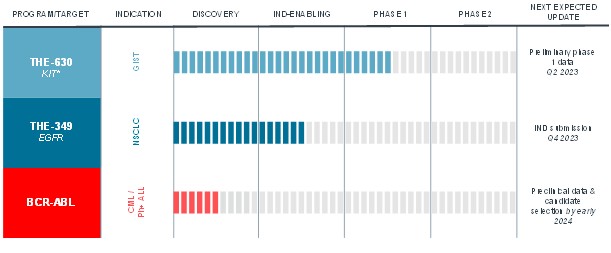
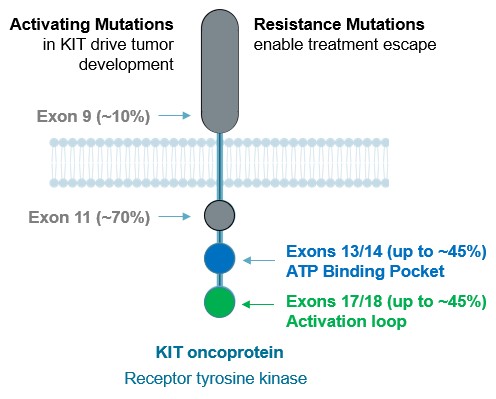
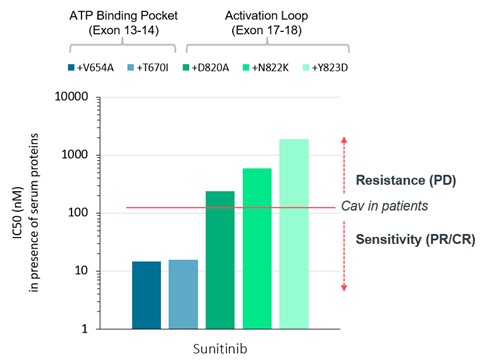
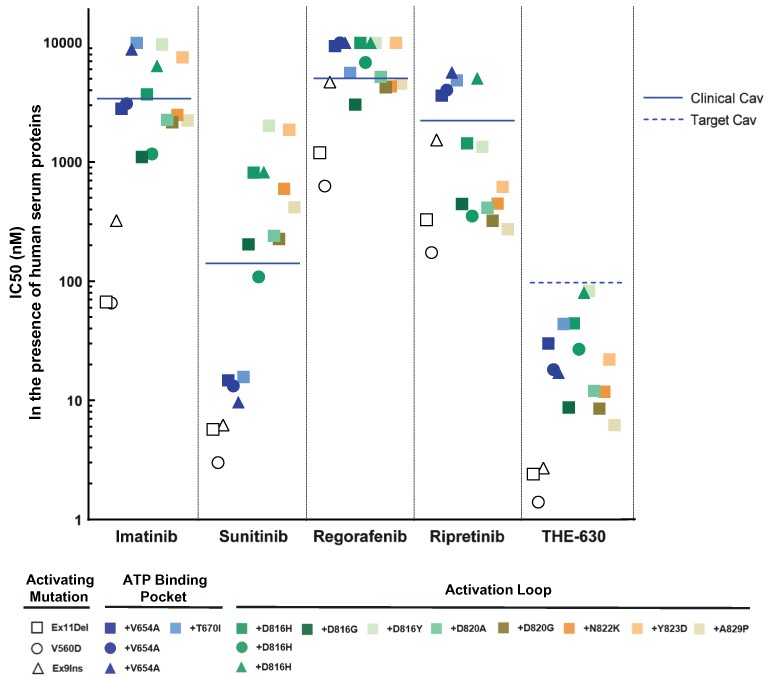
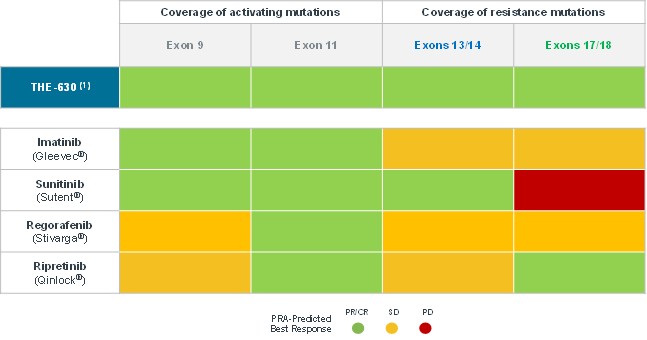
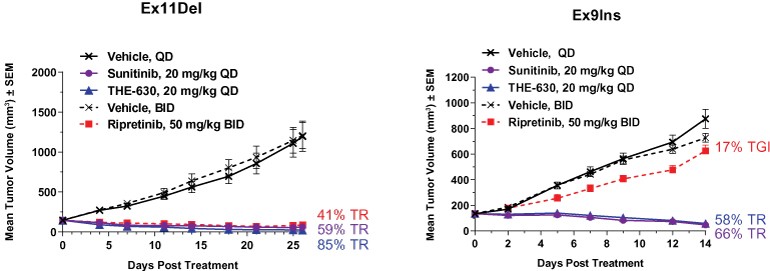
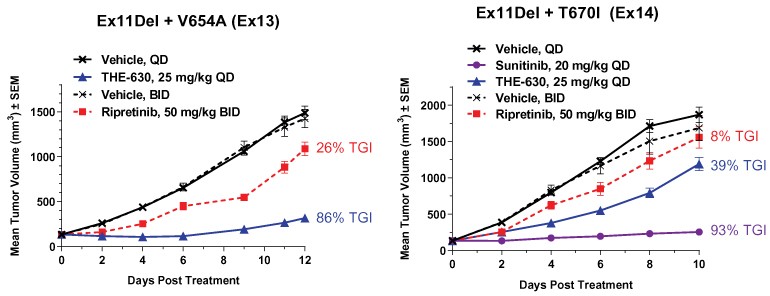
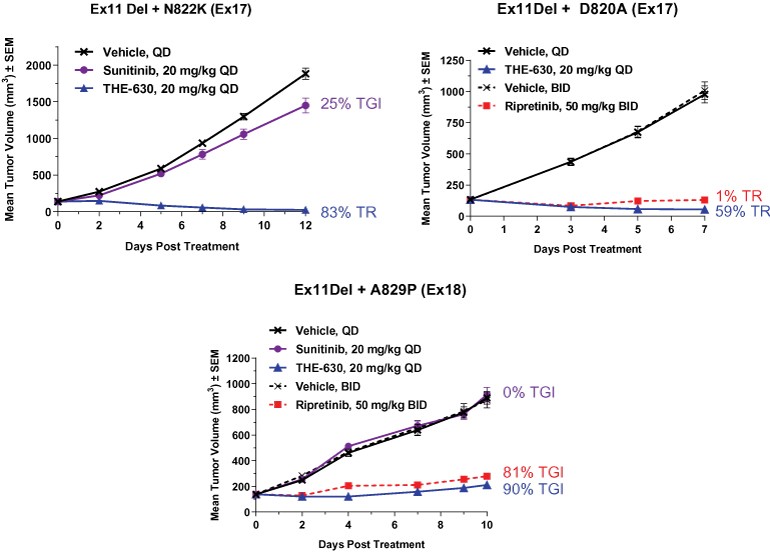
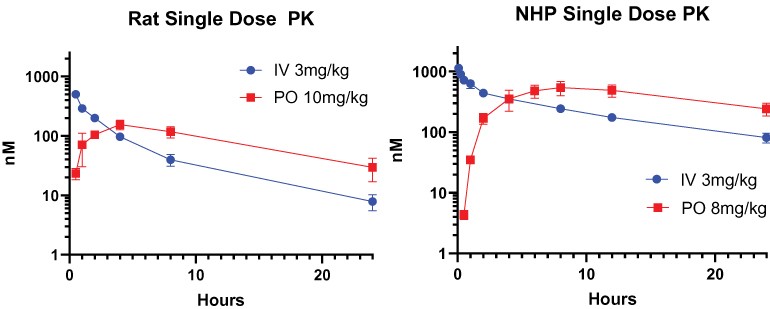
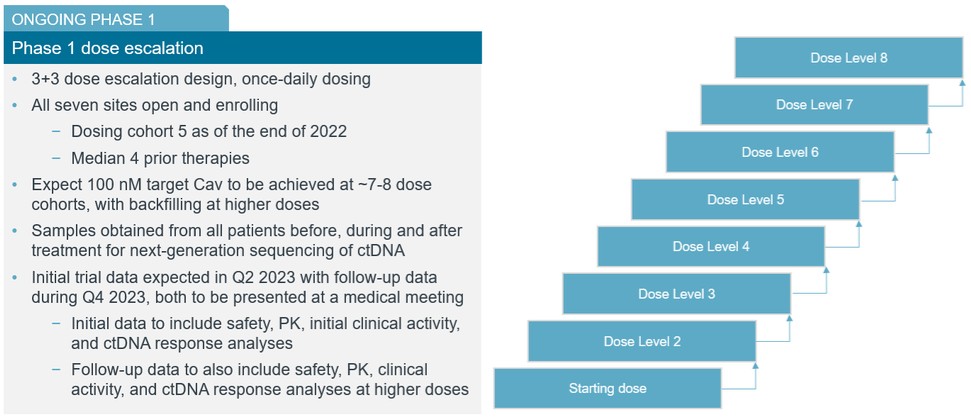
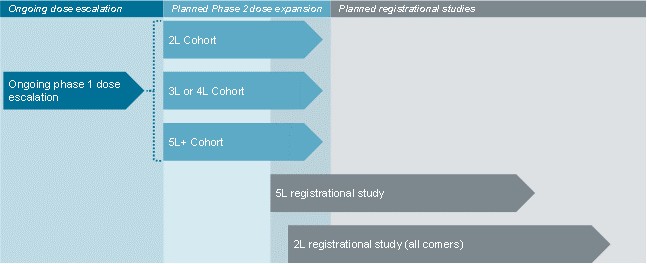
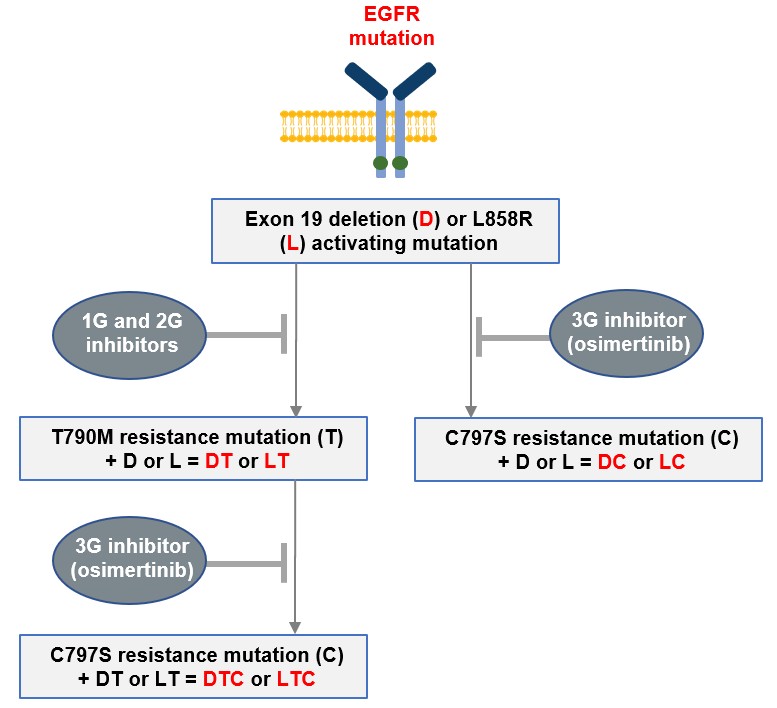
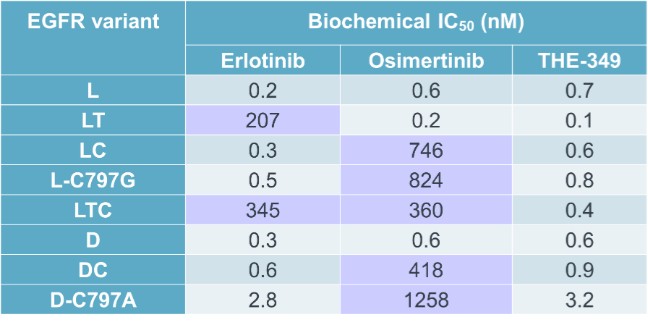
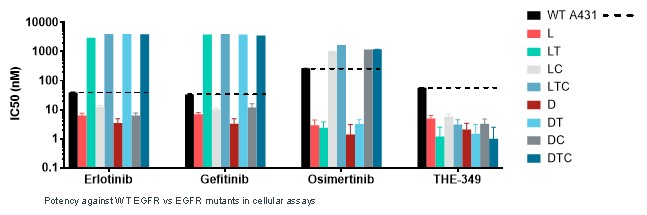
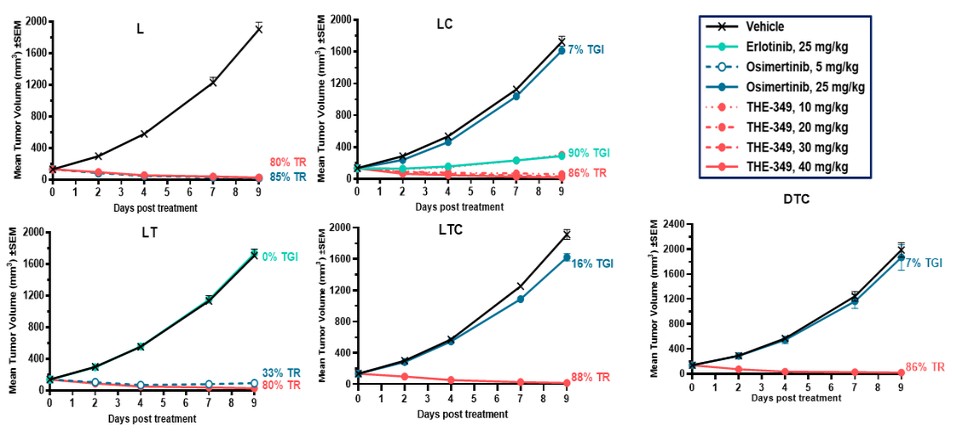
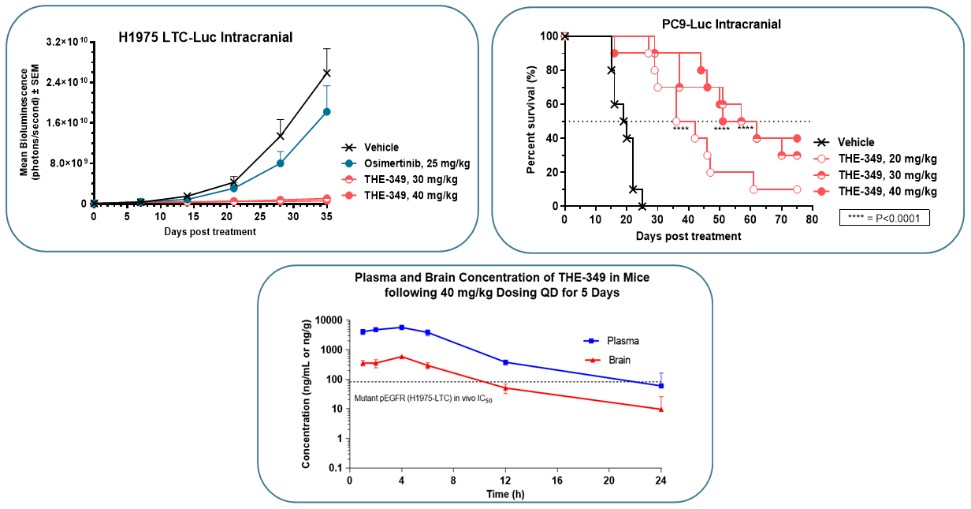
guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment, especially in light of the lack of applicable precedent and regulations. Ensuring that our business arrangements with third parties comply with applicable healthcare and data privacy laws and regulations, as well as responding to investigations by government authorities, can be time- and resource-consuming and can divert management’s attention from the business.
If our operations are found to be in violation of any of the federal and state healthcare laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, private actions brought by individual whistleblowers in the name of the government, debarment or refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, additional reporting requirements and/or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. Defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which could have a material adverse effect on our business, results of operations, financial condition and prospects.
Our employees, independent contractors, consultants, collaborators, principal investigators, CROs, suppliers and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, consultants, collaborators, principal investigators, CROs, suppliers and vendors may engage in misconduct or other improper activities. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (1) the Federal Food, Drug and Cosmetic Act, FDA regulations or those of comparable foreign regulatory authorities, (2) GCP or cGMP, (3) federal and state health and data privacy, security, fraud and abuse, government price reporting, transparency reporting requirements, and other healthcare laws and regulations in the US and abroad, (4) sexual harassment and other workplace misconduct, or (5) laws that require the true, complete and accurate reporting of clinical information or data. In particular, research, sales, marketing and business arrangements in the health care industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by these parties could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
We have adopted a code of conduct and have contracts in place with third parties, but it is not always possible to identify and deter misconduct by these parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant penalties, including civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings and the curtailment or restructuring of our operations.