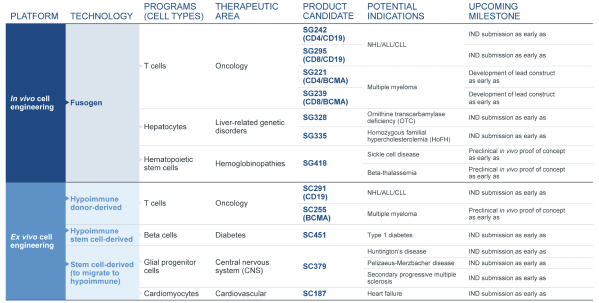
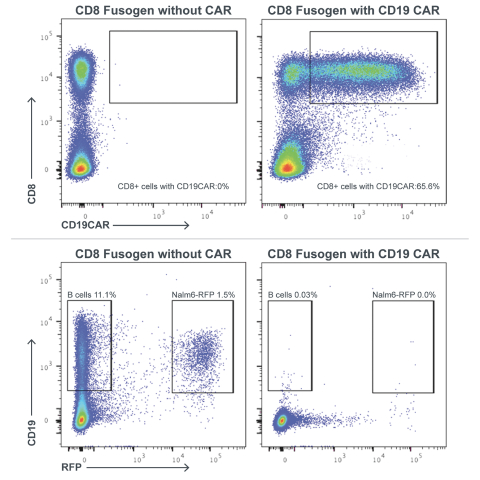
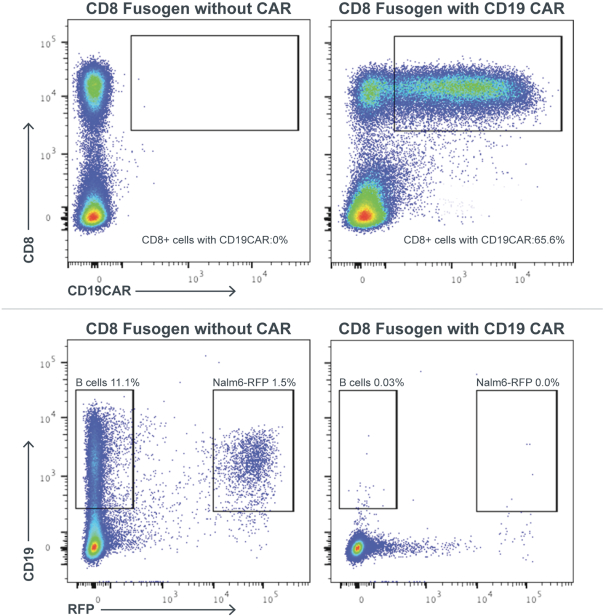
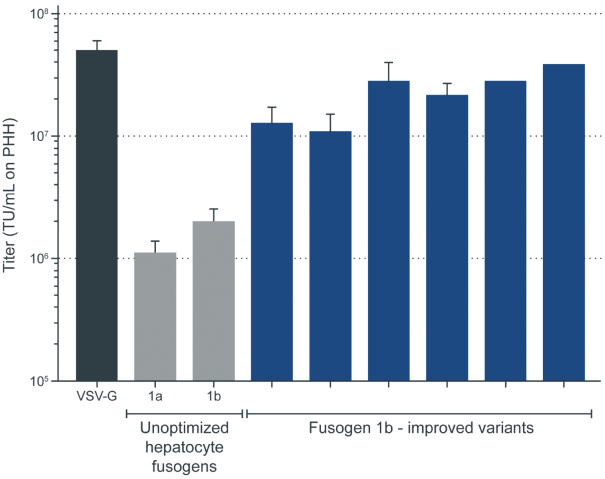
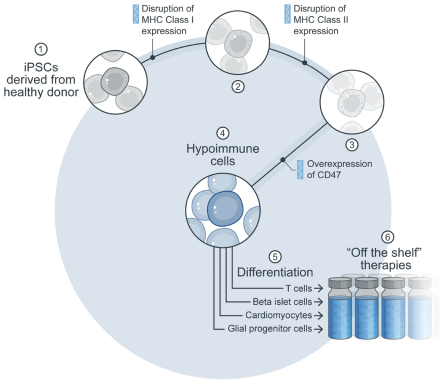
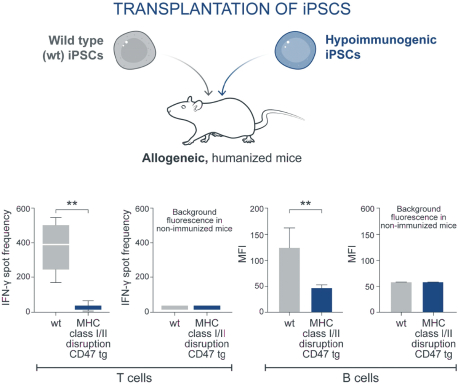
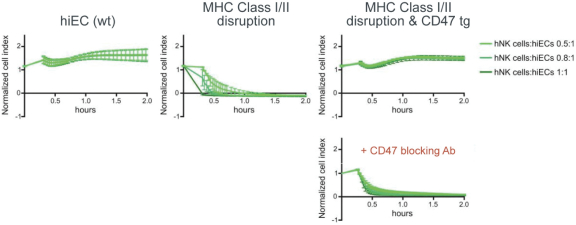
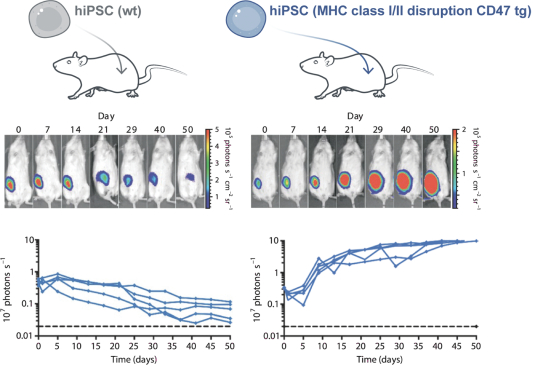
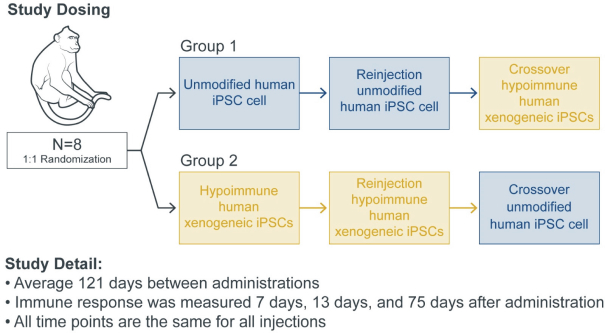
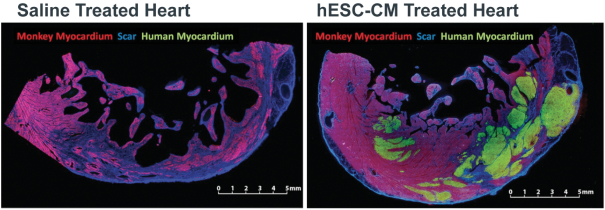
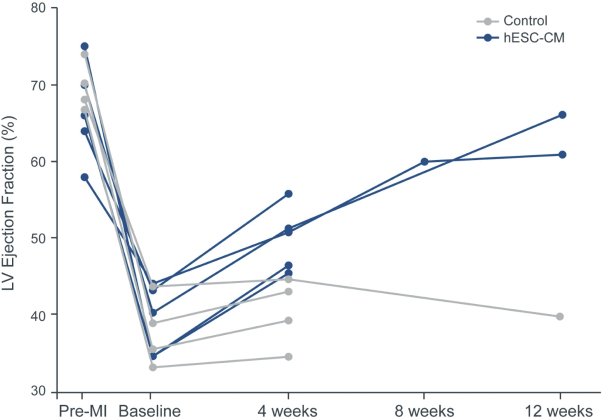
| | • | | establishing, scaling up and scaling out, either alone or with third-party manufacturers, manufacturing capabilities of clinical supply for our clinical trials and commercial manufacturing (including licensure), if any of our product candidates are approved. |
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully develop and commercialize our product candidates, which would materially adversely affect our business, financial condition, and results of operations.
Additionally, clinical or regulatory setbacks to other companies developing similar products or within adjacent fields, including those in gene editing and gene therapy and allogenic cell-based therapies, may impact the clinical development of and regulatory pathway for our current or future product candidates, or may negatively impact the perceptions of value or risk of our technologies.
We expect to continue to expand our development and regulatory capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We have experience rapid growth since our inception in July 2018. As of December 31, 2018, we had 37 full-time employees and, as of September 30, 2020, we grew to 240 full-time employees. We expect continued growth in the number of our employees and the scope of our operations, particularly to continue our IND-enabling studies, establish regulatory, quality, and clinical operations, and begin manufacturing supply chain logistics.
To manage our anticipated future growth, we will continue to implement and improve our managerial, operational, and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the complexity in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. In addition, we have limited experience in managing the manufacturing processes necessary for making cell and gene therapies. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
In addition, future growth imposes significant added responsibilities on members of management, including: identifying, recruiting, integrating, maintaining, and motivating additional employees; managing our internal development efforts effectively, including the clinical and FDA review process for our product candidates, while complying with our contractual obligations to contractors and other third parties; and improving our operational, financial and management controls, reporting systems, and procedures.
We currently rely on certain independent organizations, advisors, and consultants to provide certain services, including strategic, financial, business development, and research and development services, as well as certain aspects of regulatory approval and manufacturing. There can be no assurance that the services of independent organizations, advisors, and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements. In addition, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by consultants or contract manufacturing organizations is compromised for any reason, our clinical trials may be extended, delayed, or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business. There can be no assurance that we will be able to manage our existing consultants or find other competent outside contractors and consultants on reasonable terms, or at all.
The outbreak of the novel coronavirus disease, COVID-19, could materially and adversely affect our preclinical studies and development, any clinical trials we subsequently commence, and our business, financial condition, and results of operations.
In December 2019, the coronavirus disease, COVID-19, was identified in Wuhan, China. Since then, COVID-19 has spread globally. In March 2020, the World Health Organization declared COVID-19 a global
25