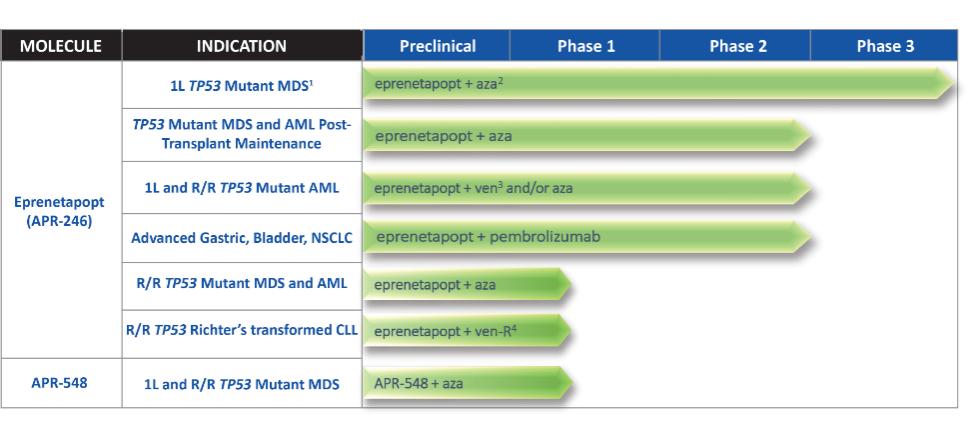
Eprenetapopt Formulation Family
As of December 31, 2021, we exclusively own a patent family directed to formulations of eprenetapopt. This patent family includes one U.S. issued patent and approximately 44 issued patents in Europe (validated in Austria, Belgium, Switzerland-Lichtenstein, Cyprus, Czech Republic, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Italy, Lithuania, Luxembourg, Latvia, Monaco, Malta, Netherlands, Norway, Poland, Portugal, Sweden, Slovakia, San Marino and Turkey), Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan, South Korea, Philippines, Russia, Singapore, and South Africa. This patent family also includes approximately one pending patent application in Thailand. The granted patents and pending applications, if issued, in this family are expected to expire in 2031, not giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Eprenetapopt Dosing Family
As of December 31, 2021, we exclusively own a pending US nonprovisional patent application directed to dosing regimens involving eprenetapopt, which was filed in 2019. Any future U.S. patents that may issue from this US patent application (assuming all the necessary and applicable requirements are satisfied) are expected to expire in 2039, not giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Eprenetapopt Process and Solid Form Family
As of December 31, 2021, we exclusively own a pending U.S. nonprovisional patent application directed to improved processes for large scale preparation of eprenetapopt and to a crystalline solid form comprising eprenetapopt, which was filed in 2020. Any future U.S. patents that may issue from this nonprovisional patent application (assuming all applicable requirements are satisfied) are expected to expire in 2040, not giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Combination Therapy of Eprenetapopt With A Bcl-2 Inhibitor
As of December 31, 2021, we exclusively own a pending PCT application directed to a method of treatment using a combination therapy of eprenetapopt with a Bcl-2 inhibitor which was filed in 2020. This PCT patent application is not eligible to become an issued patent until, among other things, we file a national phase patent application in a PCT contracting state before the expiration of the PCT patent application in that state. If we do not timely file any national phase patent applications, we may lose our priority date with respect to our PCT patent application and any patent protection on the inventions disclosed in our PCT patent application. Any future U.S. patents that may issue from this PCT patent application (assuming all applicable requirements are satisfied) are expected to expire in 2040, not giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Next-Generation Patent Family/APR-548
As of December 31, 2021, we exclusively own a patent family directed to next-generation p53 reactivators, including APR-548. In this family, we own one pending U.S. nonprovisional application and approximately 12 foreign pending applications including a European, Australian, Brazilian, Canadian, Chinese, Israeli, Indian, Japanese, Korean, New Zealand, Singapore and Taiwanese patent application, each with a filing date in 2019. In this patent family claims are directed to compositions of matter and methods-of-use of APR-548. Any future patents that may issue from these patent applications (assuming all applicable requirements are satisfied) are expected to expire in 2039, not giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.