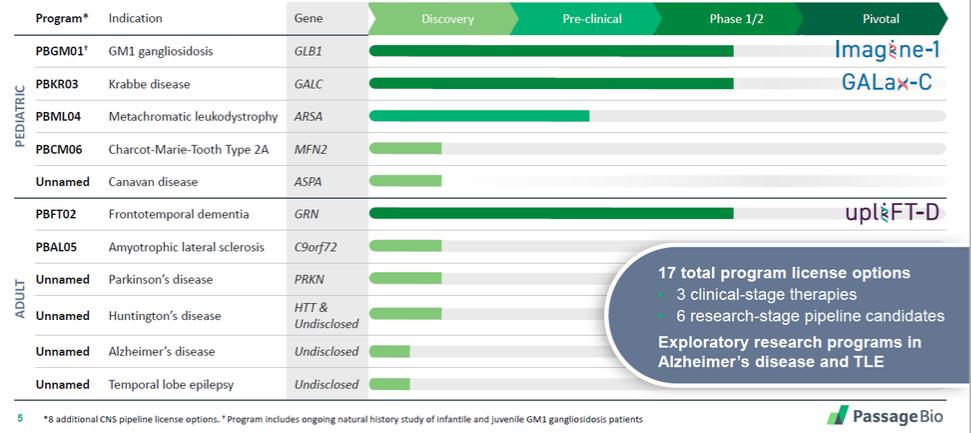
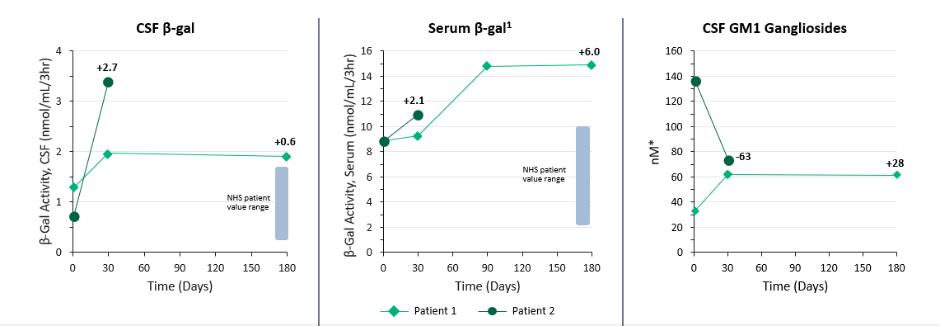
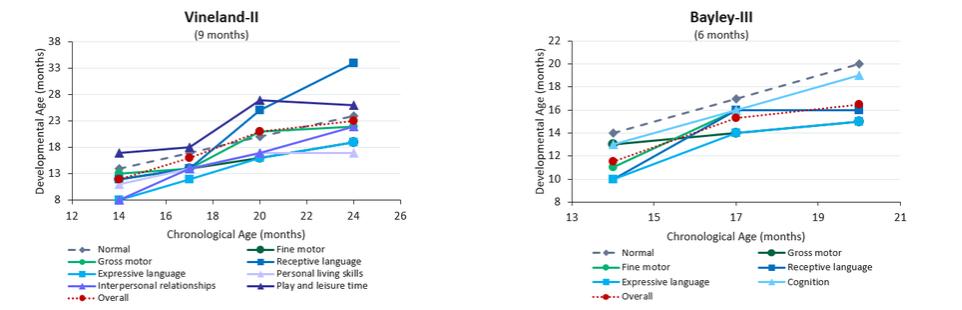
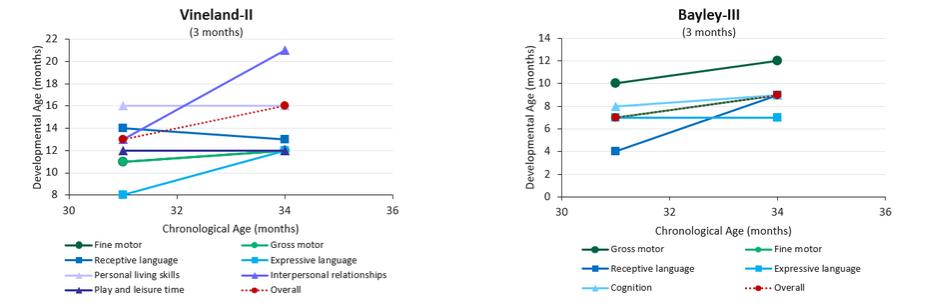
| | adjustments and extensions. The second patent family includes two pending unpublished U.S. provisional patent applications. Any patents that may issue from applications in this family are expected to expire in September 2042. |
The term of individual patents may vary based on the countries in which they are obtained. Generally, patents issued from applications filed in the United States are effective for 20 years from the earliest effective non-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a result of FDA regulatory review period. The restoration period cannot be longer than five years and the total patent term, including the restoration period, must not exceed 14 years following FDA approval. The duration of patents outside of the United States varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective national filing date.
In addition to patents and patent applications that we license, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant aspects of our AAV manufacturing capabilities and gene therapy technology are based upon trade secrets and know-how. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, and obtain and maintain control and/or ownership of certain technologies, in part, through confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners. We also seek to preserve the integrity and confidentiality of our data, trade secrets and know-how, including by implementing measures intended to maintain the physical security of our premises and the physical and electronic security of our information technology systems.
Our ability to stop third parties from making, using, selling, offering to sell or importing our products may depend on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities. With respect to our licensed intellectual property, we cannot be sure that patents will issue with respect to any of the pending patent applications to which we license rights or with respect to any patent applications that we or our licensors may file in the future, nor can we be sure that any of our licensed patents or any patents that may be issued in the future to us or our licensors will be commercially useful in protecting our product candidates and methods of manufacturing the same. Moreover, we may be unable to obtain patent protection for certain of our product candidates generally, as well as with respect to certain indications. See the section entitled “Risk Factors—Risks Related to Our Intellectual Property” for a more comprehensive description of risks related to our intellectual property.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Biological products used for the prevention, treatment, or cure of a disease or condition of a human being are subject to regulation under the FDC Act, except the section of the FDC Act which governs the approval of New Drug Applications, or NDAs. Biological products, such as gene therapy products, are approved for marketing under provisions of the Public Health Service Act, or PHSA, via a Biologics License Application, or BLA. However, the application process and requirements for approval of BLAs are very similar to those for NDAs. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as clinical hold, FDA refusal to approve pending NDAs or BLAs,