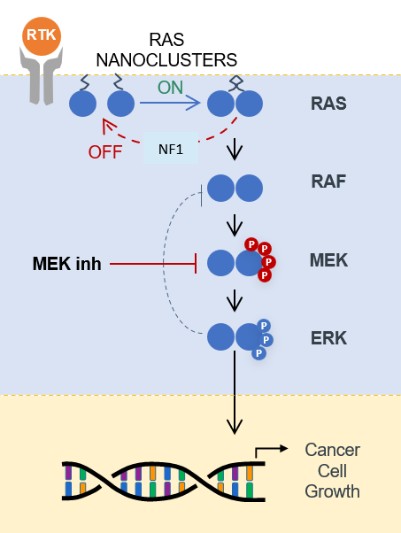
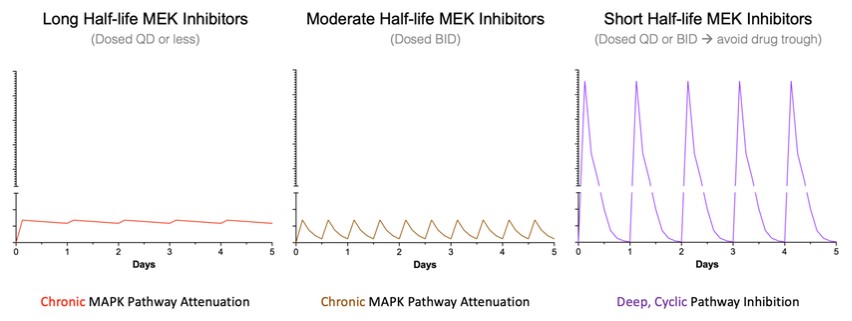
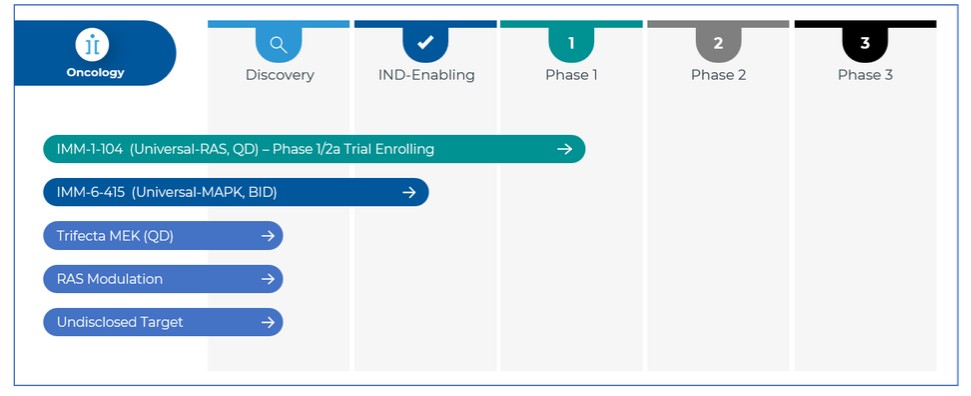
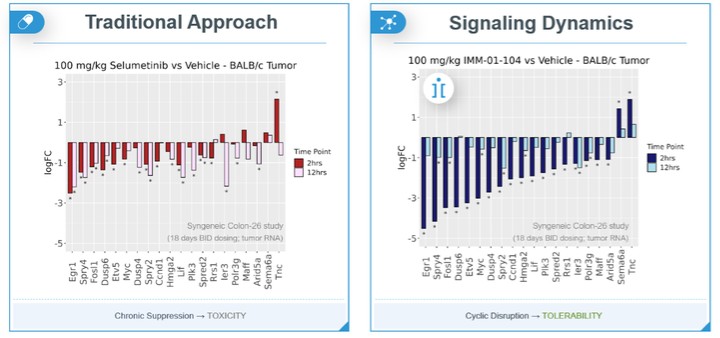
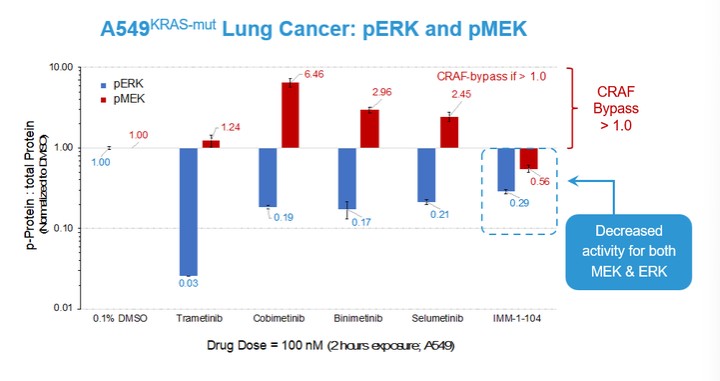
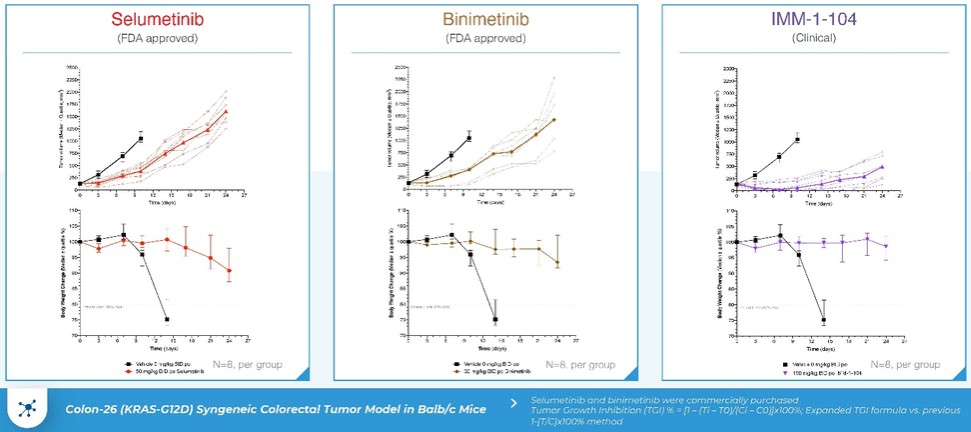
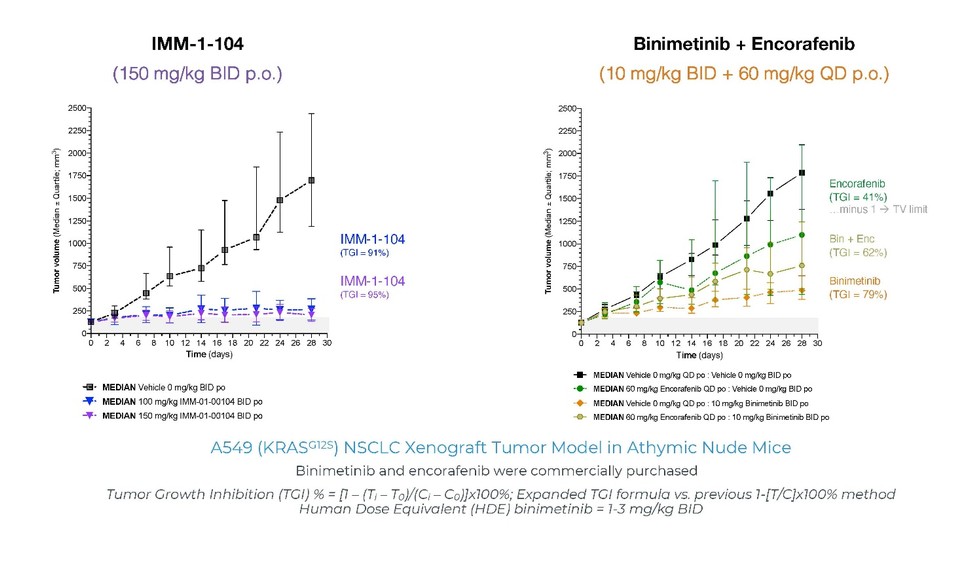
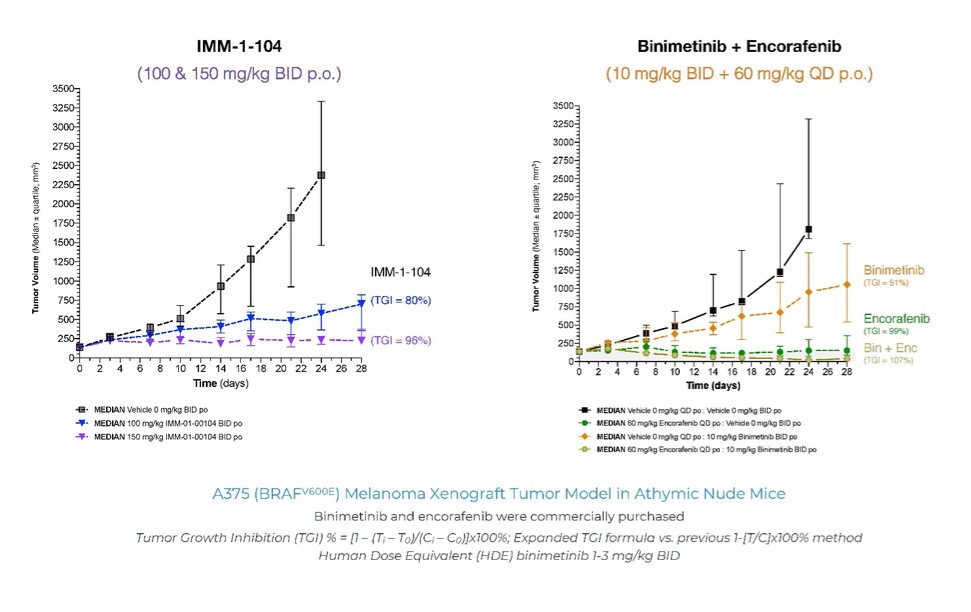
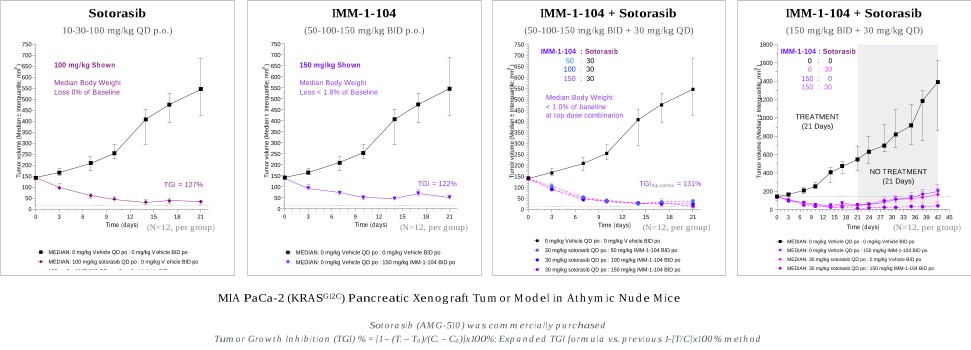
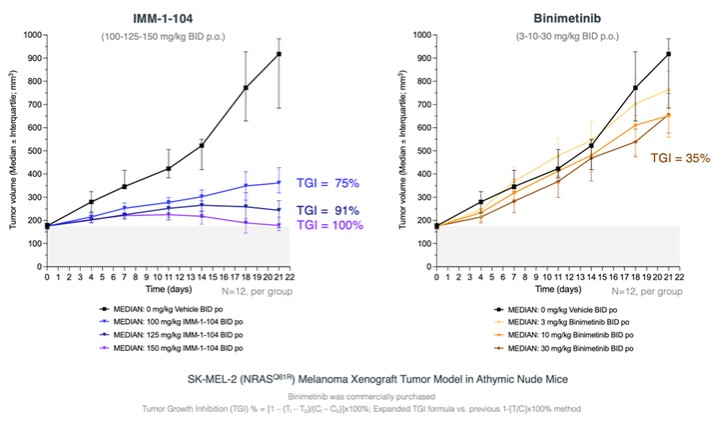
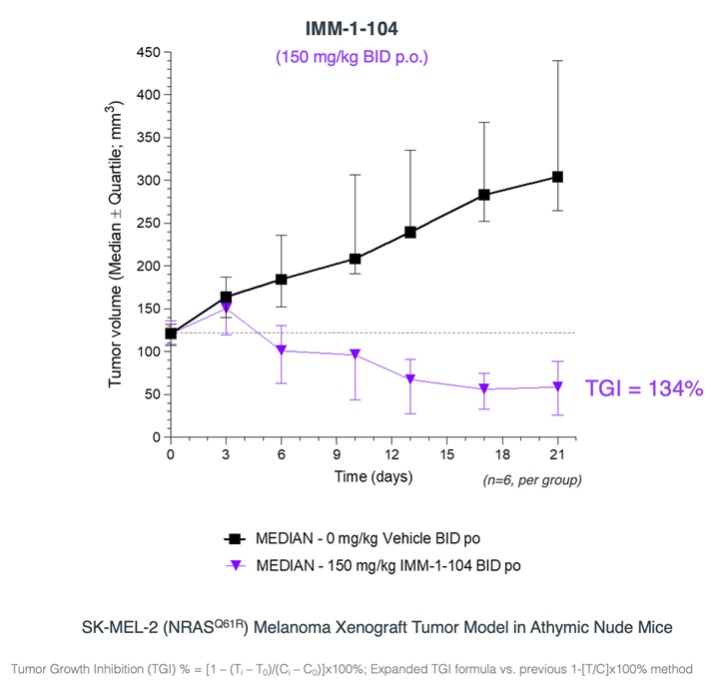
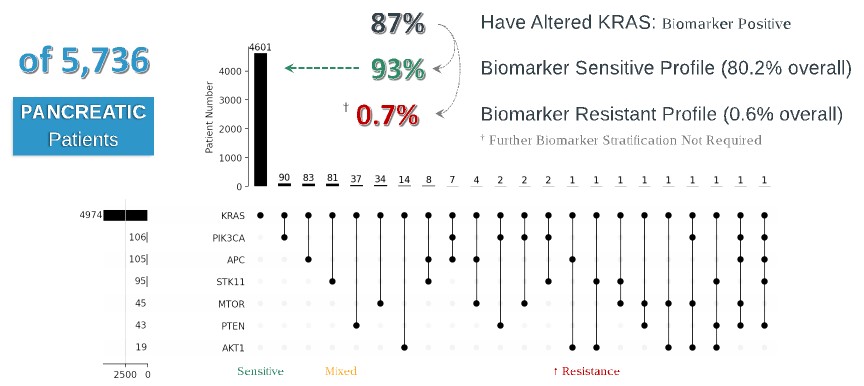
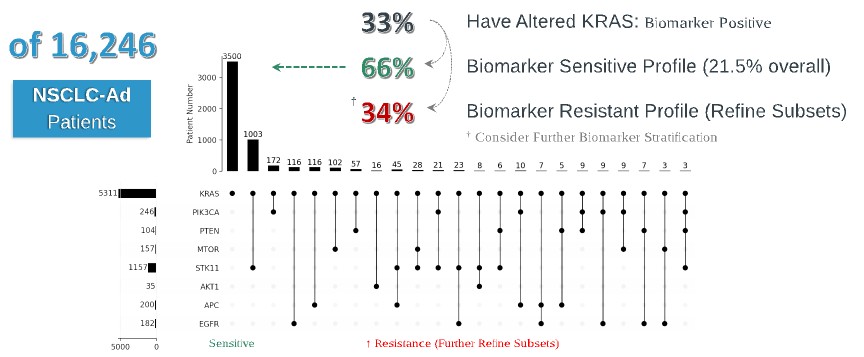
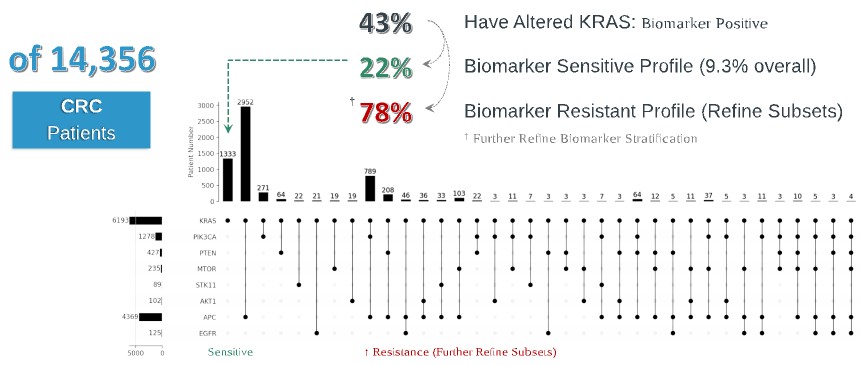
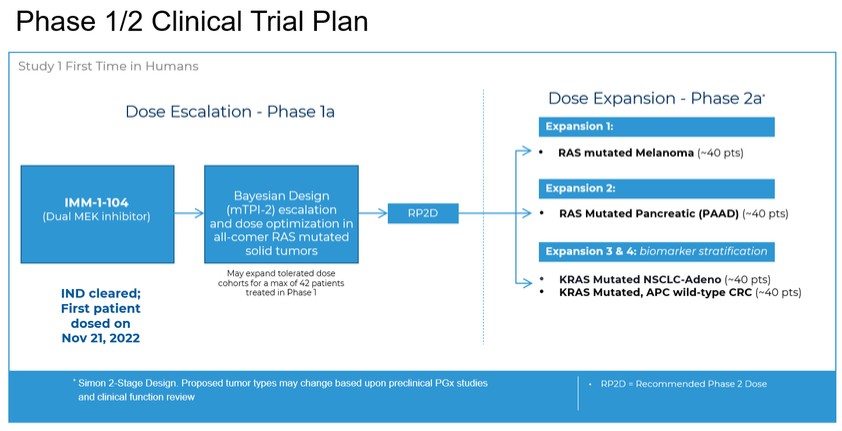
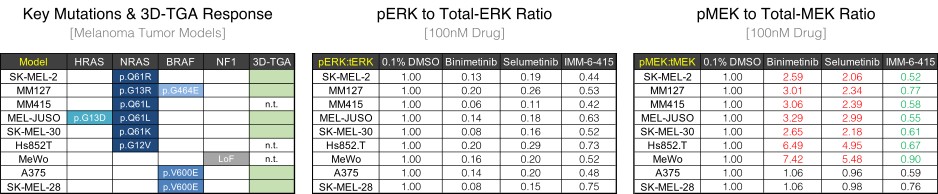
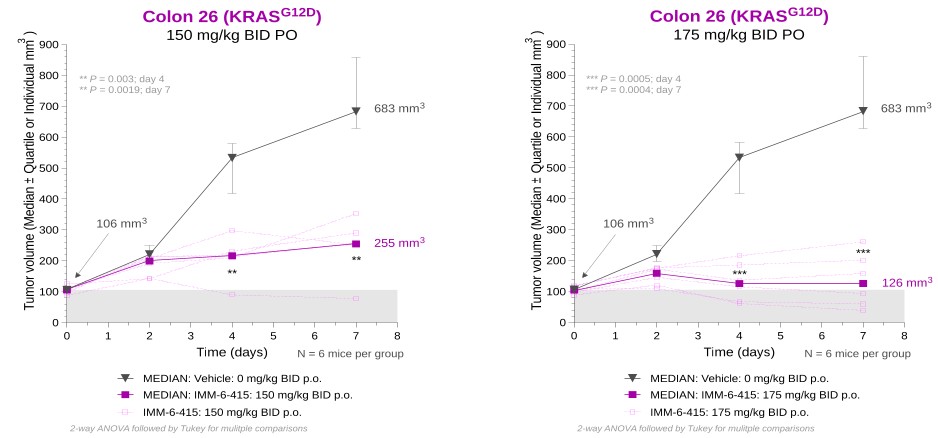
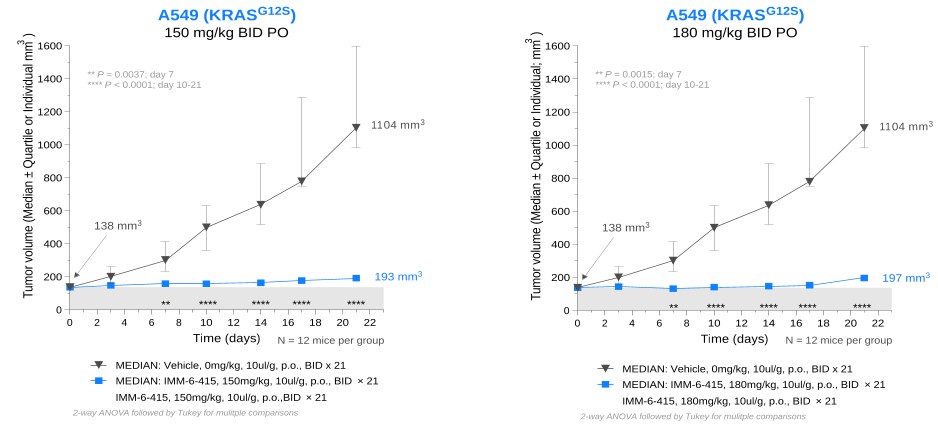
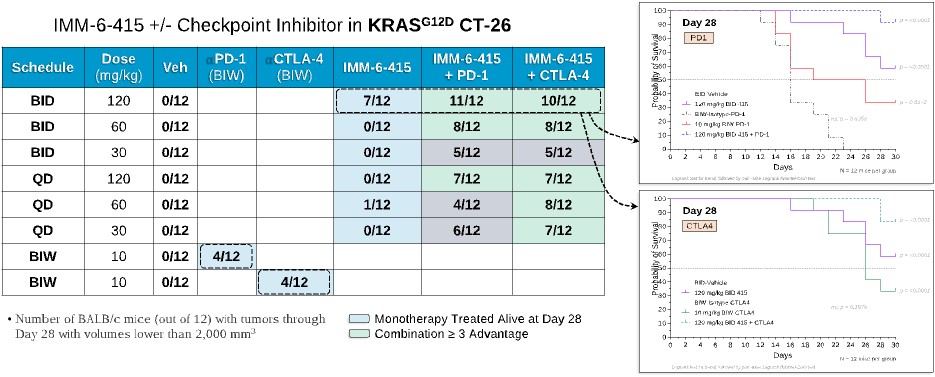
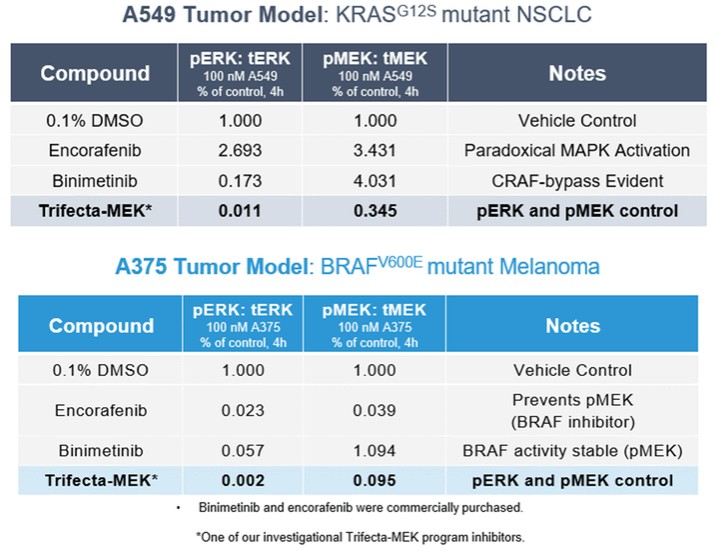
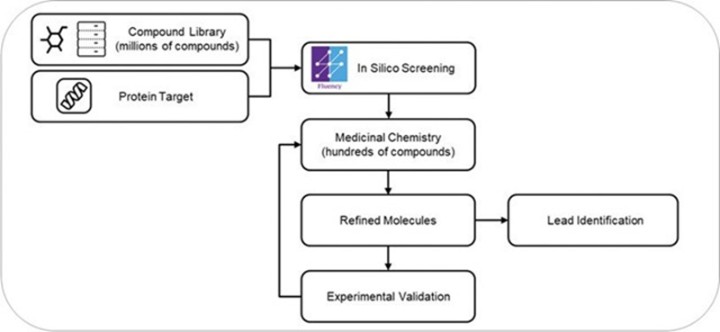
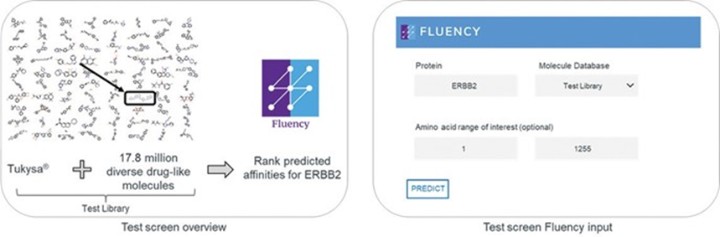
programs targeting the MAPK pathway may compete with current FDA-approved therapies or clinical programs targeting KRAS mutant tumors that are being advanced by certain pharmaceutical and biotechnology companies.
Intellectual Property
Our ability to obtain and maintain intellectual property protection for our products and technology is fundamental to the long-term success of our business. We rely on a combination of intellectual property protection strategies, including patents, trademarks, copyrights, trade secrets, license agreements, confidentiality policies and procedures, non-disclosure agreements, invention assignment agreements and technical measures designed to protect the intellectual property and confidential information and data used in our business.
As of February 27, 2023, we have: one issued U.S. patent; five pending U.S. patent applications; 20 pending patent applications outside the U.S.; three U.S. provisional applications; and one Patent Cooperation Treaty, or PCT, application that has not entered national stage. These patents and patent applications relate to subject matter, including: our lead product candidate, IMM-1-104, our Universal-MAPK candidate, IMM-6-415, our DCT, and Fluency. Excluding any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, as applicable; our owned issued U.S. patent and any patents that may issue from our owned pending U.S. patent applications are expected to expire in February, 2039 or January 2041; any patents that may issue from our owned pending foreign patent applications are expected to expire in January, 2041, and any patents that may issue from national phase filings from our PCT application are expected to expire in January, 2043.
With respect to IMM-1-104, as of February 27, 2023, we have one pending U.S. patent application and 20 pending patent applications outside the U.S. This U.S. patent application, and these pending patent applications outside the U.S., include pending claims directed to compounds, pharmaceutical compositions, and methods of use. Any patent that may issue, based upon these pending applications related to IMM-1-104, is expected to expire in January, 2041, excluding any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, as applicable.
With respect to IMM-6-415, as of February 27, 2023, we have one pending provisional U.S. application and one pending PCT application. Any patent that may issue, based upon this provisional U.S. application related to IMM-6-415, is expected to expire in November, 2043 and any patent that may issue based upon the pending PCT application is expected to expire in January, 2043, excluding any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, as applicable.
With respect to our DCT, as of February 27, 2023, we have one issued U.S. patent and one pending U.S. patent application. The issued claims of this U.S. patent and the pending claims of this U.S. patent application are directed to methods (processes) and systems. Our issued U.S. patent related to our DCT and any patent that may issue from our pending patent application related to our DCT are expected to expire in February, 2039, excluding any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, as applicable.
With respect to Fluency, as of February 27, 2023, we have three pending U.S. patent applications. The pending claims of these U.S. patent applications are directed to methods (processes) and systems. Any patent that may issue from our pending patent application related to Fluency is expected to expire in February, 2039, excluding any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees, as applicable.
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. We cannot be sure that our pending patent applications that we have filed or may file in the future will result in issued patents, and we can give no assurance that any patents that have