reserved against in the Company Balance Sheet and (b) for liabilities and obligations incurred in the ordinary course of business consistent with past practice (none of which is a liability for a breach or default under any contract, breach of warranty, tort, infringement, misappropriation or violation of law) since the date of the Company Balance Sheet that are not individually or in the aggregate material to the Company.
Section 4.8Absence of Certain Changes or Events. Except as set forth in Section 4.8 of the Company Disclosure Letter, since the date of the Company Balance Sheet: (i) except in connection with the execution of this Agreement and the consummation of the Contemplated Transactions, the Company and its Subsidiaries have conducted their business only in the ordinary course consistent with past practice; (ii) there has not been any change, event or development or prospective change, event or development that, individually or in the aggregate, has had or would reasonably be expected to have a Material Adverse Effect; and (iii) neither the Company nor any of its Subsidiaries has:
(a) (i) declared, set aside or paid any dividends on, or made any other distributions (whether in cash, stock or property) in respect of, any of its capital stock or other equity interests, (ii) purchased, redeemed or otherwise acquired shares of capital stock or other equity interests of the Company or any of its Subsidiaries or any options, warrants, or rights to acquire any such shares or other equity interests, or (iii) split, combined, reclassified or otherwise amended the terms of any of its capital stock or other equity interests or issued or authorized the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock or other equity interests;
(b)amended or otherwise changed, or authorized or proposed to amend or otherwise change, its certificate of incorporation or by-laws (or similar organizational documents);
(c)adopted or entered into a plan of complete or partial liquidation, dissolution, restructuring, recapitalization or reorganization; or
(d)changed its financial or Tax accounting methods, principles or practices, except insofar as may have been required by a change in GAAP or applicable Law, or revalued any of its material assets.
Section 4.9 Litigation. There is no action, suit, claim, arbitration, investigation, inquiry, grievance or other proceeding (each, an “Action”) (or basis therefor) pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its Subsidiaries, its properties or assets, or any present or former officer, director or employee of the Company or any of its Subsidiaries in such individual’s capacity as such, other than any Action that (a) does not involve an amount in controversy in excess of $100,000 and (b) does not seek injunctive or other non-monetary relief. Neither the Company nor any of its Subsidiaries nor any of their respective properties or assets is subject to any outstanding judgment, order, injunction, rule or decree of any Governmental Entity. There is no Action pending or, to the knowledge of the Company, threatened seeking to prevent, hinder, modify, delay or challenge the Merger or any of the other Contemplated Transactions.
Section 4.10 Compliance with Laws. The Company and each of its Subsidiaries are and have been in compliance in all material respects with all Laws applicable to their businesses, operations, properties or assets. Neither the Company nor any of its Subsidiaries has received, since January 1, 2021, a notice or other written communication alleging or relating to a possible material violation of any Law applicable to their businesses, operations, properties, assets or Company Products (as defined below). The Company and each of its Subsidiaries have in effect all material permits, licenses, variances, exemptions, applications, approvals, clearances, authorizations, registrations, formulary listings, consents, operating certificates, franchises, orders and approvals (collectively, “Permits”) of all Governmental Entities necessary or advisable for them to own, lease or operate its properties and assets and to carry on its businesses and operations as now conducted, and there has occurred no violation of, default (with or without notice or lapse of time or both) under or event giving to others any right of revocation, non-renewal, adverse modification or cancellation of, with or without notice or lapse of time or both, any such Permit, nor would any such revocation, non-renewal, adverse modification or cancellation result from the consummation of the Contemplated Transactions.
Section 4.11 Health Care Regulatory Matters. Except as set forth in Section 4.11 of the Company Disclosure Letter:
(a)The Company, and to the knowledge of the Company, each of its directors, officers, management employees, agents (while acting in such capacity), contract manufacturers, suppliers, and distributors are, and for the past six (6) years have been, in material compliance with all health care laws to the extent applicable to the Company or any of its products or activities, including, but not limited to the following: the Federal Food, Drug & Cosmetic Act (“FDCA”); the Public Health Service Act (42 U.S.C. § 201 et seq.),


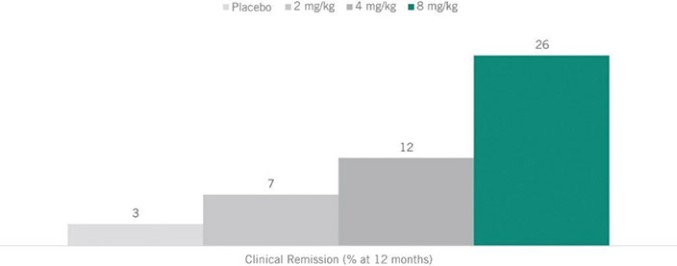
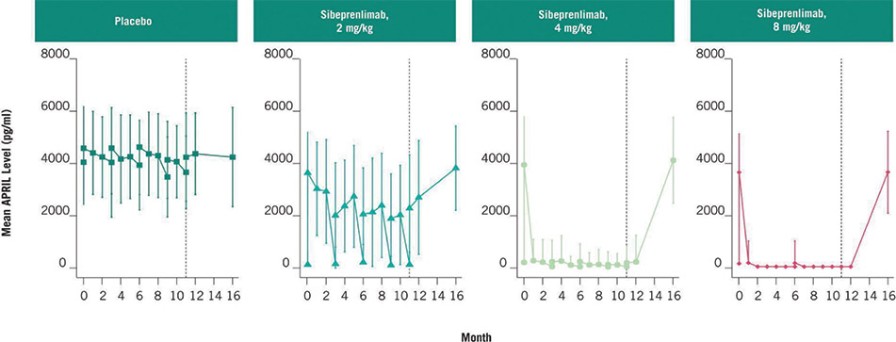


 ) is void.
) is void. ).
).