to us. We could also be forced, including by court order, to cease commercializing the infringing product or technology. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our products or force us to cease some of our business operations. For more information regarding these risks, please see “Risk Factors—Risks Related to Our Intellectual Property.”
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions.
Some of our potential competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
The key competitive factors that will differentiate AV-101, if approved, are likely to be its efficacy, safety, convenience, price, and the availability of reimbursement from commercial, government and other third-party payors.
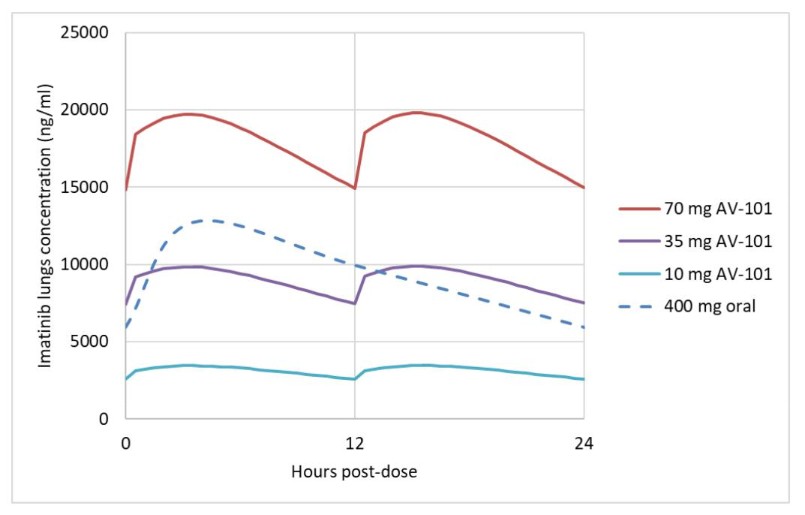
We intend to seek approval for AV-101 initially in patients for the treatment of PAH as an add-on therapy to currently approved standard of care. We recognize that physicians have many treatment options for patients already on existing treatments for PAH, including prostanoids available in oral form as Orenitram (United Therapeutics Corporation, or United Therapeutics) and Uptravi (Janssen Pharmaceuticals, Inc., or Janssen), by inhalation as Tyvaso and Tyvaso DPI (United Therapeutics), Ventavis (Janssen), and by infusion as Remodulin (United Therapeutics), Flolan (GlaxoSmithKline plc) and Veletri (Janssen). We believe that AV-101, if approved, could be used prior to or in combination with prostanoids, and in combination with existing front-line agents such as the oral PDE5 inhibitors, including Revatio (Pfizer) and Adcirca (United Therapeutics); the sGC stimulator Adempas (Bayer AG); and oral ERAs, including Tracleer (Janssen), Letairis (Gilead) and Opsumit (Janssen). PAH is also an active indication for investigational drugs, and we may face competition in the future from sotatercept (Acceleron Pharma, Inc., a wholly-owned subsidiary of Merck & Co., Inc.) under review by the FDA and EMA, and/or seralutinib (Gossamer Bio, Inc.). To our knowledge, Aerami Therapeutics, Inc. is developing other formulations of imatinib for PAH, and have completed a Phase 1 clinical trial and indicated they intend to pursue further clinical development.
Government Regulation
United States—FDA Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The FDCA and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. We, along with third-party contractors, will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory authorities of the countries in which we wish to conduct studies or seek approval of our product candidates. Failure to comply with applicable United States requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, withdrawal of an approval, warning or untitled letters, clinical holds, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, civil penalties, and criminal prosecution.