University), BioNTech SE (together with Pfizer Inc.), GlaxoSmithKline plc (GSK) (together with Sanofi S.A.), Heat Biologics, Inc., Inovio Pharmaceuticals, Inc., Johnson & Johnson, Moderna, Inc., Novavax, Inc., Regeneron Pharmaceuticals Inc., and Vaxart, Inc. For example, BioNTech SE (together with Pfizer Inc.), Janssen Pharmaceutical Companies of Johnson & Johnson and Moderna Inc. have developed COVID-19 vaccines that have received authorization for emergency use and are being widely administered. In addition, Pfizer, Inc. recently announced its COVID-19 experimental drug (an orally administered protease inhibitor) which entered the clinic in March 2021, may be available by the end of 2021. Similarly, Merck (together with Ridgeback Bio), is developing the drug Molnupiravir, an antiviral drug currently in a Phase 3 clinical trial which has the potential to become the first oral COVID-19 treatment. The availability of such COVID-19 vaccines and Pfizer’s oral COVID-19 drug may reduce or eliminate the need for our potential COVID therapies to treat the disease and therefore negatively impact the commercial opportunity therefor.
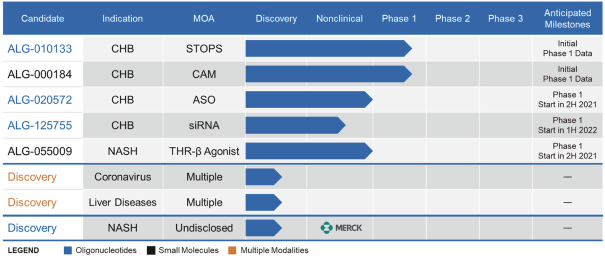
Furthermore, there are companies developing or marketing treatments for NASH, including AbbVie, Inc., AstraZeneca PLC/MedImmune LLC, Bristol-Myers Squibb Company, Eli Lilly and Company, FronThera US Pharmaceuticals LLC, Janssen, Merck, Novartis Pharmaceuticals Corporation (together with Pfizer, Inc.), Novo Nordisk A/S, Pfizer Inc., Roche, Sanofi S.A., Takeda Pharmaceutical Company Limited (together with HemoShear Therapeutics, LLC), 89bio, Inc., Akero Therapeutics, Inc., Blade Therapeutics, Inc., Cirius Therapeutics, Inc., Enanta Pharmaceuticals, Inc., Galectin Therapeutics Inc., Galmed Pharmaceuticals Ltd., Genfit SA, Gilead, Intercept Pharmaceuticals, Inc., Inventiva Pharma SA, Madrigal Pharmaceuticals, Inc., MediciNova, Inc., NGM Biopharmaceuticals, Inc., Pliant Therapeutics, Inc. (together with Novartis), Terns Pharmaceuticals, Inc. and Viking Therapeutics, Inc.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe effects, are more convenient, have a broader label, are marketed more effectively, including gaining exclusivity for their competing products on formularies thereby excluding our products from such formularies, are reimbursed or are less expensive than any products that we may develop. Our competitors also may obtain FDA, EMA or other marketing approval for their products more rapidly than we may obtain approval for ours (if at all), which could result in our competitors establishing a strong market position before we are able to enter the market (if ever). Even if the drug candidates we develop achieve marketing approval, they may be priced at a significant premium over competitive products, resulting in reduced competitiveness of our products.
Smaller and other early stage companies may also prove to be significant competitors. In addition, academic research departments and public and private research institutions may be conducting research on compounds that could prove to be competitive.
These third parties compete with us not only in drug candidate development, but also in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring and/or licensing technologies complementary to, or necessary for, our programs.
In addition, the biopharmaceutical industry is characterized by rapid technological change. If we fail to keep pace with technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our drug candidates obsolete, less competitive or not economical, thereby adversely affecting our business, financial condition and results of operations.
If any of our current or future drug candidates obtain regulatory approval, additional competitors could enter the market with generic versions of such products, which may result in a material decline in sales of our competing products.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments to the Federal Food, Drug, and Cosmetic Act (the FDCA), a pharmaceutical manufacturer may file an abbreviated new drug application (an ANDA) seeking approval of a generic version of an approved innovator
39