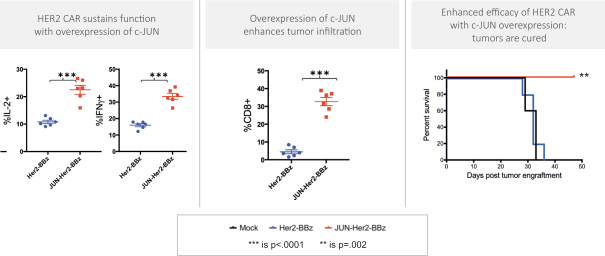
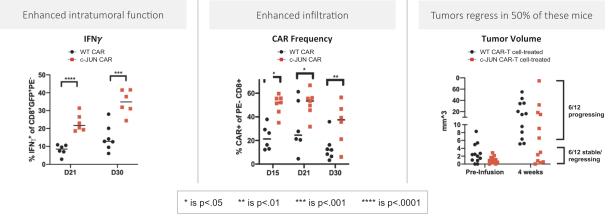
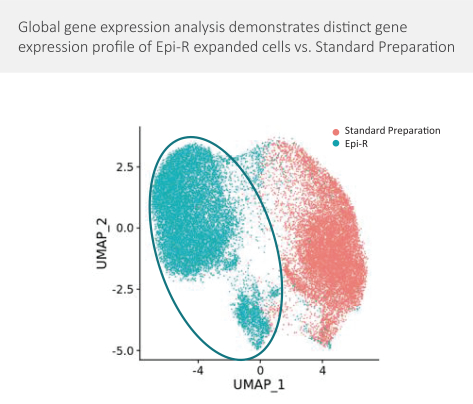
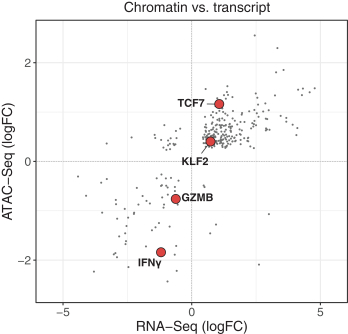
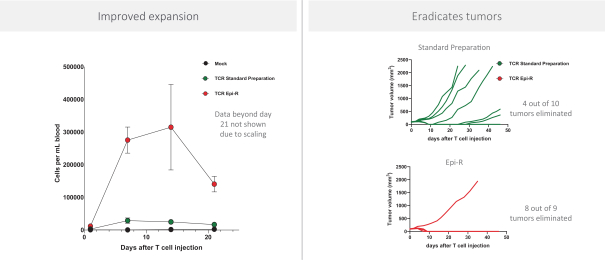
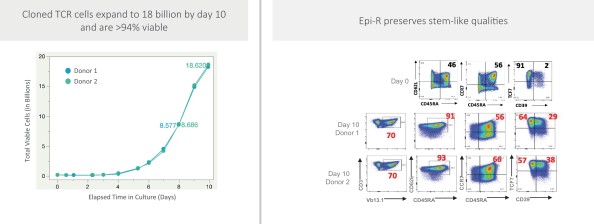
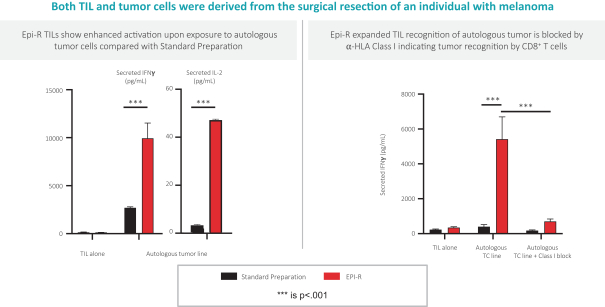
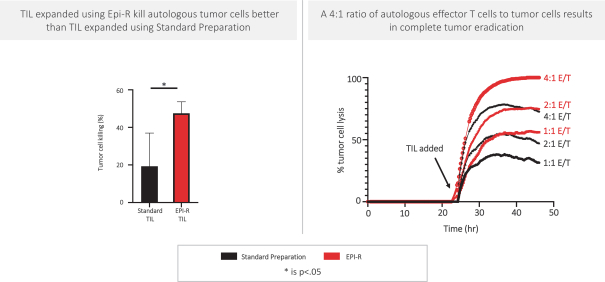
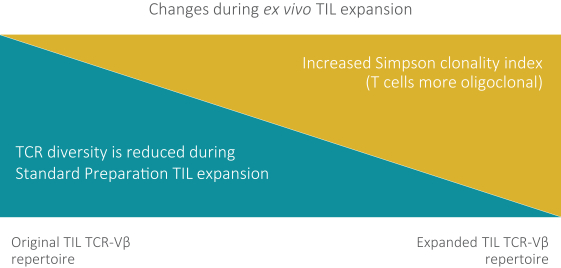
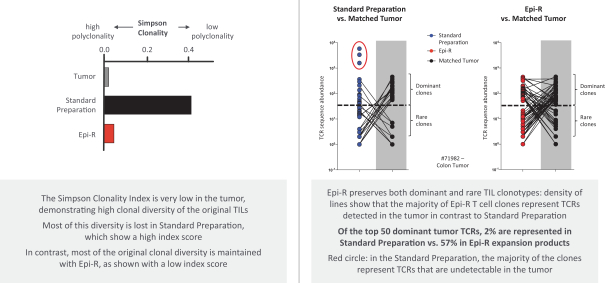
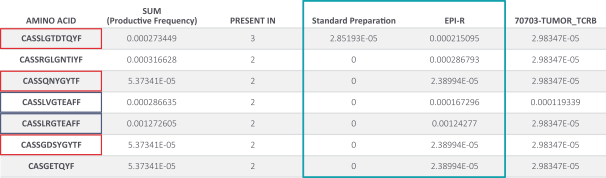
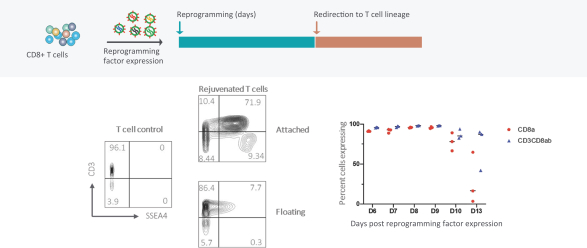
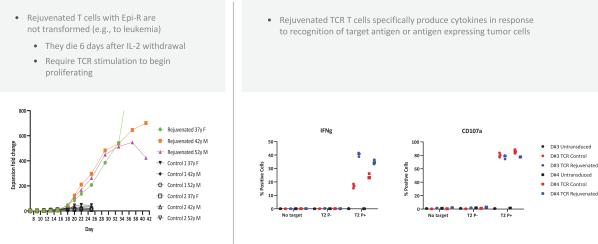
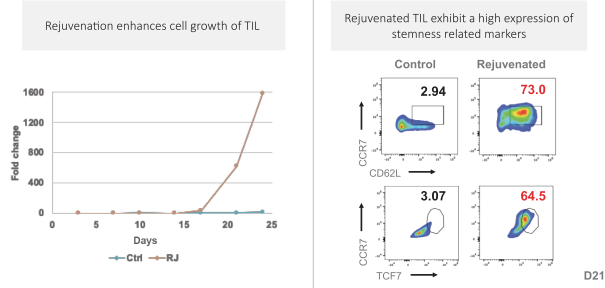
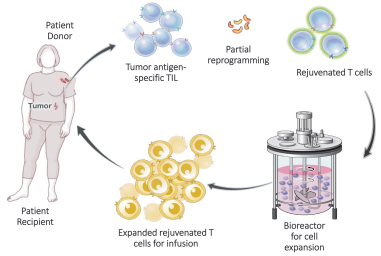
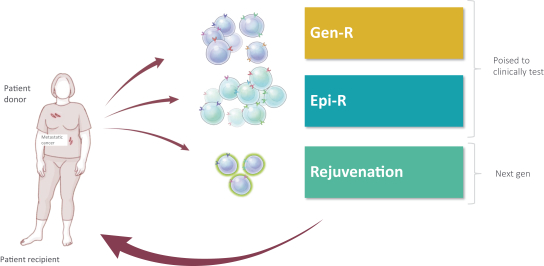
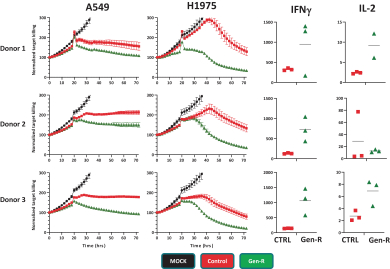
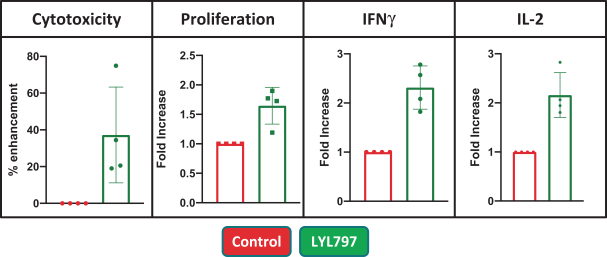
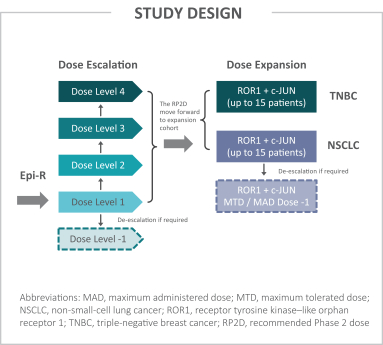
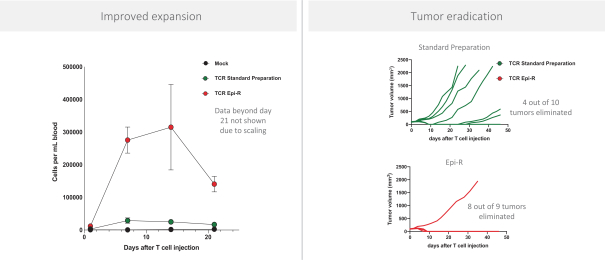
Moreover, because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any particular product candidate can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent. Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. Patent disputes are sometimes interwoven into other business disputes.
As of March 31, 2021, our registered trademark portfolio currently contains approximately 27 registered trademarks and pending trademark applications, consisting of approximately four pending trademark applications in the United States, approximately two foreign pending trademark applications in Canada and India, and trademark registrations in the following countries through national filings: Australia, Brazil, China, European Union, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Republic of Korea, Switzerland and the United Kingdom.
We may also rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets are difficult to protect. We seek to protect our technology and product candidates, in part, by entering into confidentiality agreements with those who have access to our confidential information, including our employees, contractors, consultants, collaborators and advisors. We also seek to preserve the integrity and confidentiality of our proprietary technology and processes by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations and systems, agreements or security measures may be breached and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or may be independently discovered by competitors. To the extent that our employees, contractors, consultants, collaborators and advisors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For this and more comprehensive risks related to our proprietary technology, inventions, improvements and product candidates, see the subsection titled “Risk Factors —Risks Relating to Our Intellectual Property.”
Sales and Marketing
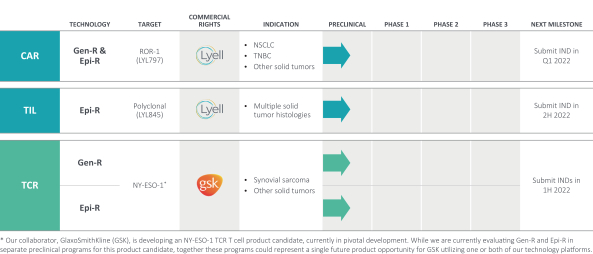
Given our stage of development, we have not yet established a commercial organization or distribution capabilities. We intend to either build a commercial infrastructure to support sales of any approved products, or outsource this function to third parties. We intend to continue evaluating opportunities to work with partners that enhance our capabilities with respect to the development and commercialization of LYL797 or LYL845. In addition, we intend to commercialize our product candidates, if approved, in key markets either alone or with partners in order to maximize the worldwide commercial potential of our programs.
Government Regulation
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of biologics such as those we are developing. We, along with third-party contractors, will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct trials or seek approval or licensure of our product candidates. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
150