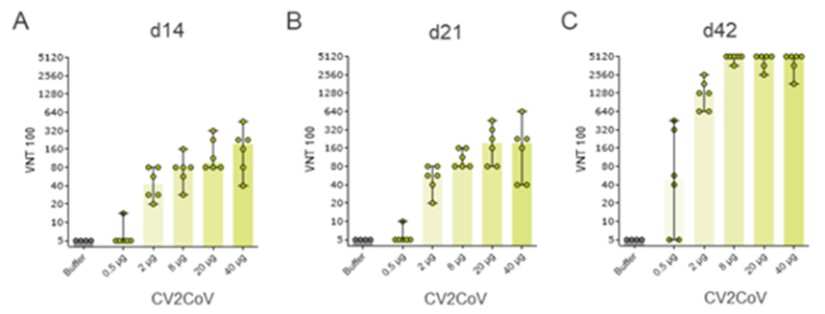
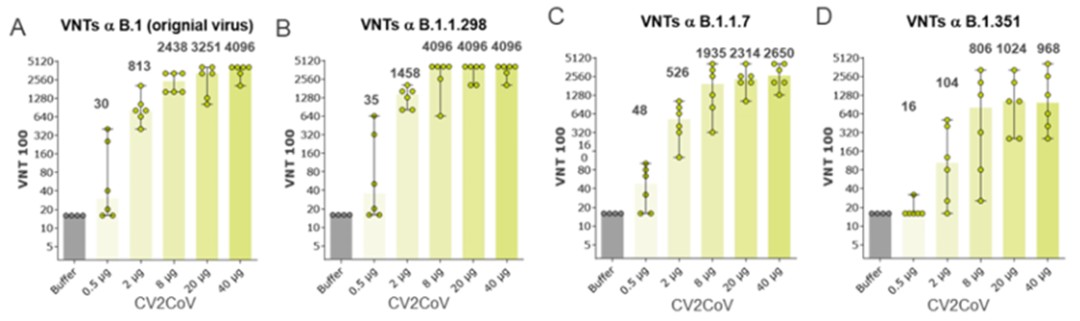
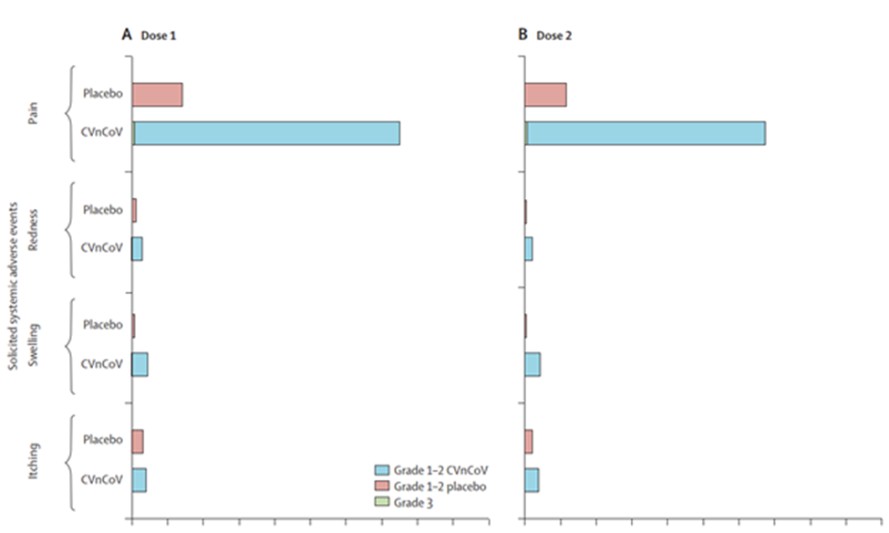
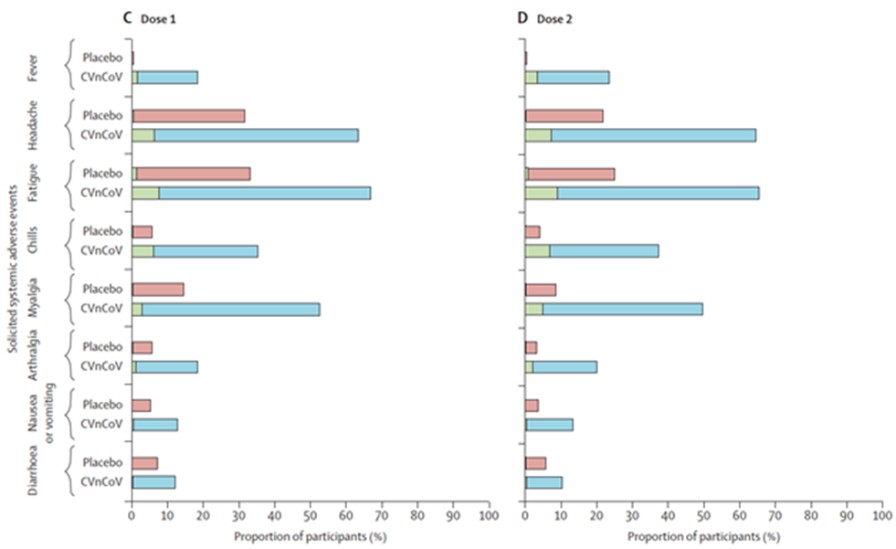
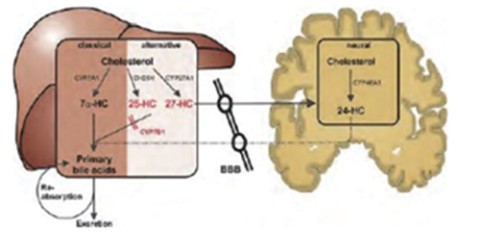
At any time during the stand-by phase, in case there is a public health emergency, the Federal Republic of Germany may exercise its preferred purchase right and/or its preferred manufacturing right. If the preferred purchase right is exercised the CureVac-GSK Consortium will have to deliver up to 80 million doses of the mRNA vaccine of the CureVac-GSK Consortium, and if the preferred manufacturing right is exercised the CureVac-GSK Consortium will have to act as a contract manufacturer and manufacture a third party’s mRNA vaccine in our GMP IV facility. However, there are strict and narrow requirements to be fulfilled before the Federal Republic of Germany may exercise the preferred manufacturing right.
Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary or intellectual property protection for our product candidates, and our core technologies and other know-how, defend and enforce our patents, preserve the confidentiality of our trade secrets, operate our business without infringing, misappropriating or otherwise violating the intellectual property or proprietary rights of third parties and prevent third parties from infringing, misappropriating or otherwise violating our proprietary or intellectual property rights. We seek to protect our proprietary and intellectual property position by, among other methods, seeking and maintaining patents in the United States and other major markets. We also rely on trade secrets and know-how to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, which we generally seek to protect through contractual obligations with third parties.
Patents
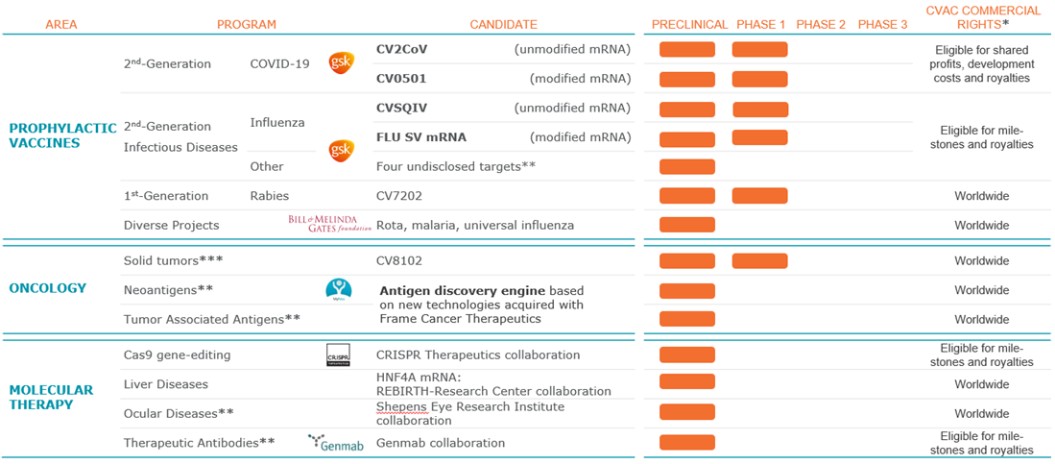
As of April 3, 2023, we own approximately 102 issued U.S. patents, 122 pending U.S. patent applications, 233 issued foreign patents (including 50 European patents, which have been validated in various European countries resulting in a total of approximately 518 national patents in European countries), 347 pending foreign patent applications (including 85 pending European patent applications) and 17 pending Patent Cooperation Treaty, or PCT, patent applications, including several patent families that are jointly owned with third parties. These patents include claims relating to our RNAoptimizer technology platform, CV8102, CV7202, CVSQIV, our COVID-19 vaccine candidates, and our proprietary LNP technology, as described further below.
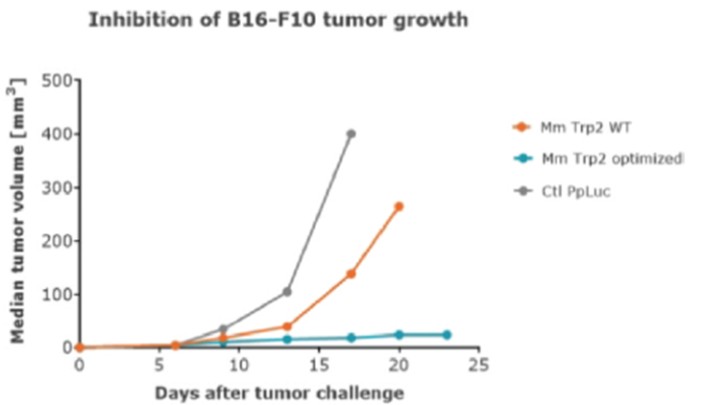
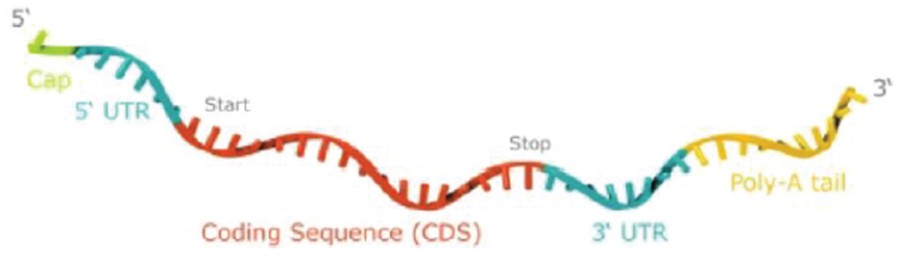
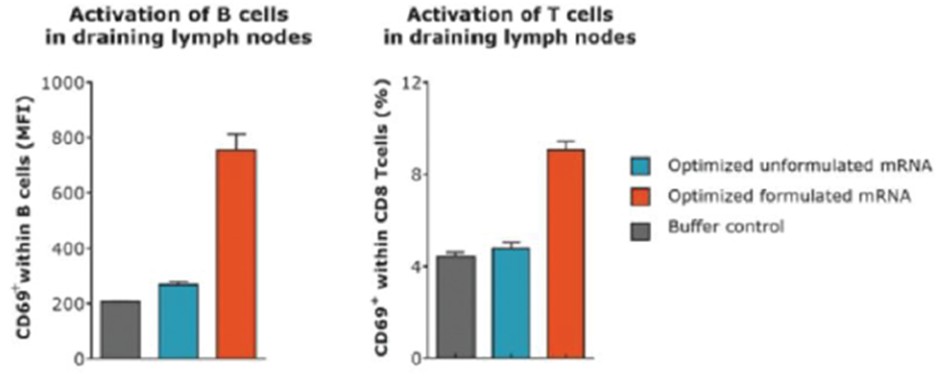
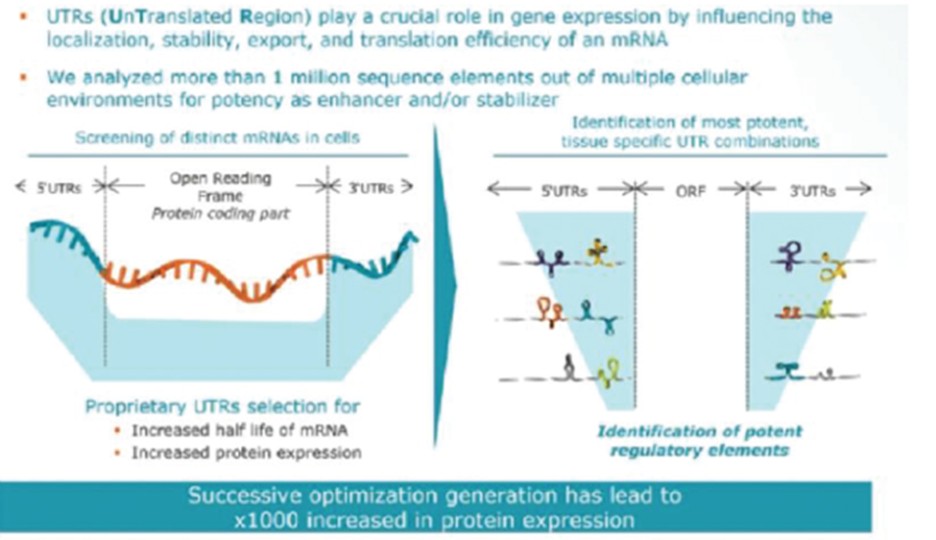
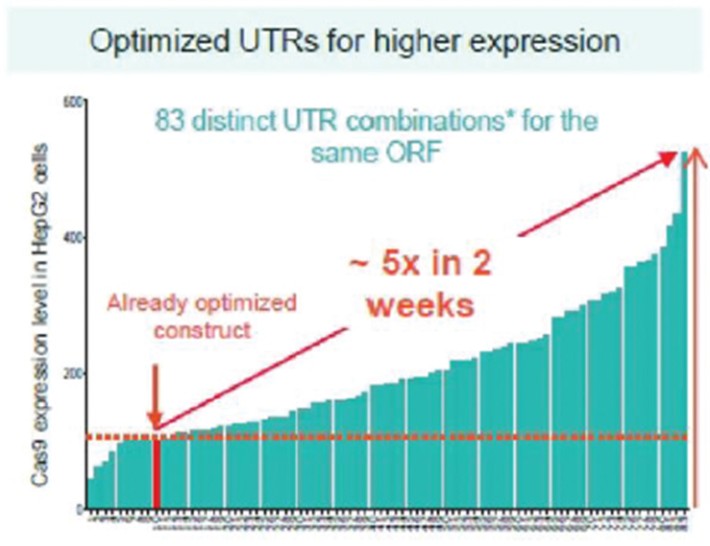
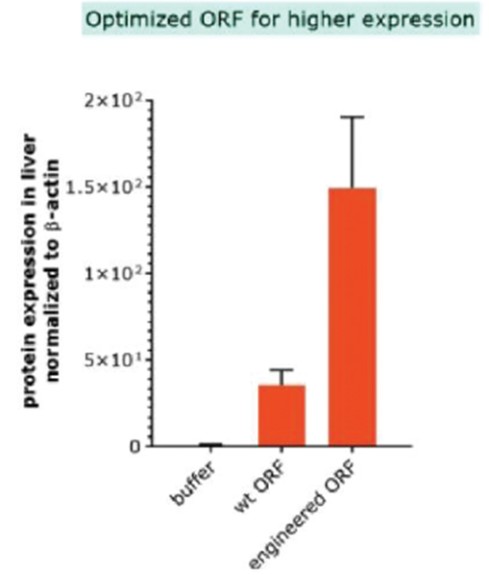
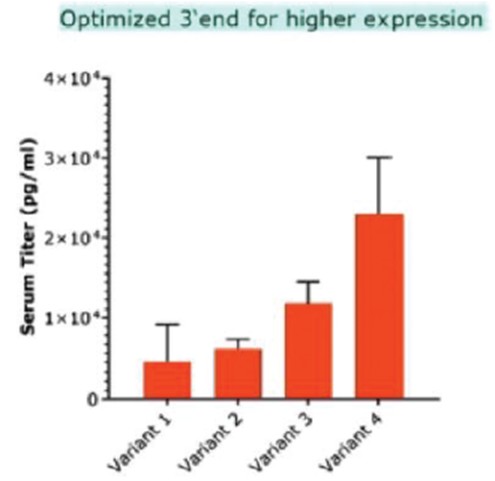
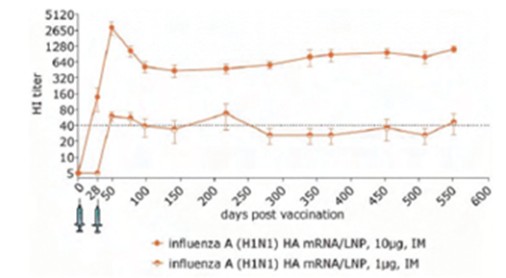
RNAoptimizer
As of April 3, 2023, we own 26 issued U.S. patents, 18 pending U.S. patent applications, 106 issued foreign patents, including in Europe, Canada, China, India, Japan, the Republic of Korea, Singapore, Russia, Mexico and Australia, and 103 pending foreign patent applications and five PCT patent applications relating to our RNAoptimizer technology, including patents and patent applications relating to ORF optimization, UTR optimization, protein optimization and formulation. Our RNAoptimizer technology is used in our CV7202, CVSQIV and SARS-CoV-2 product candidates. The issued patents are expected to expire between 2025 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending patent applications would be expected to expire between 2023 and 2044, excluding any additional term for patent term adjustments or patent term extensions.
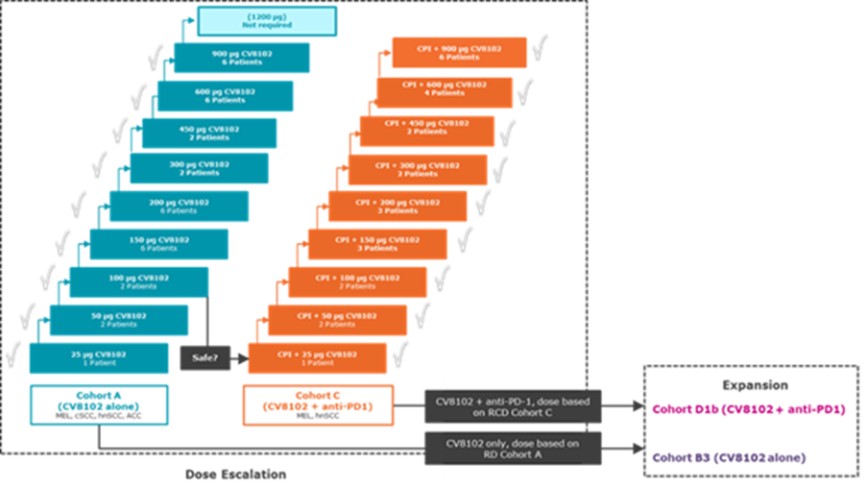
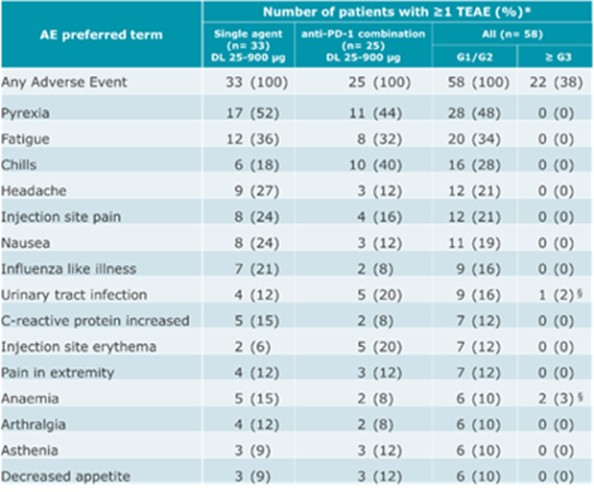
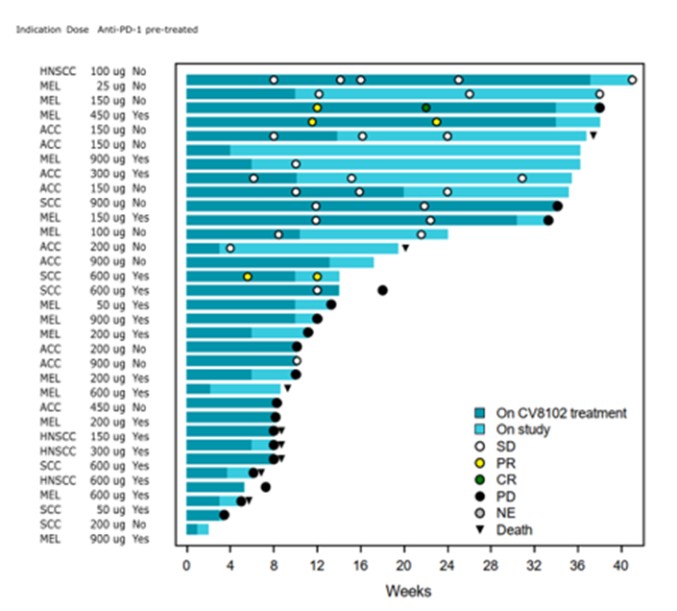
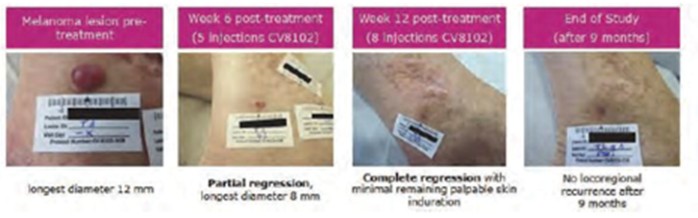
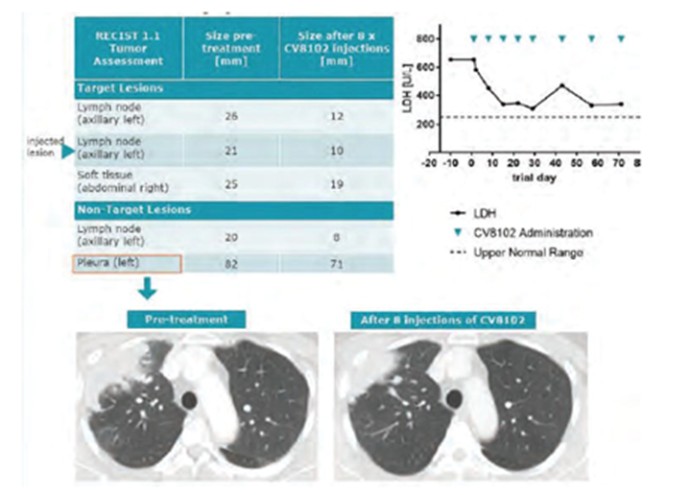
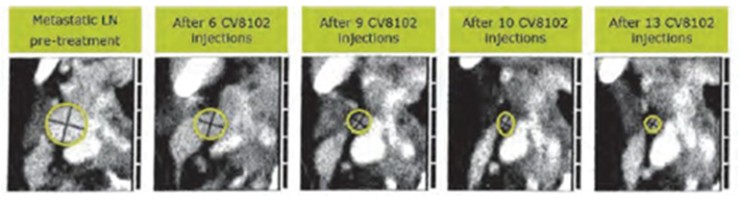
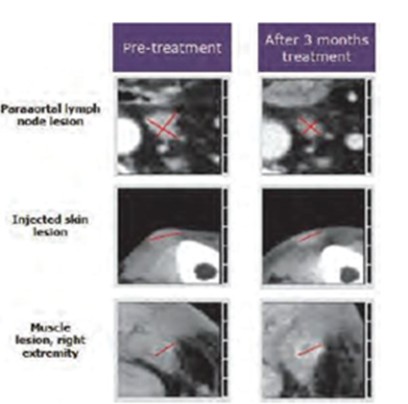
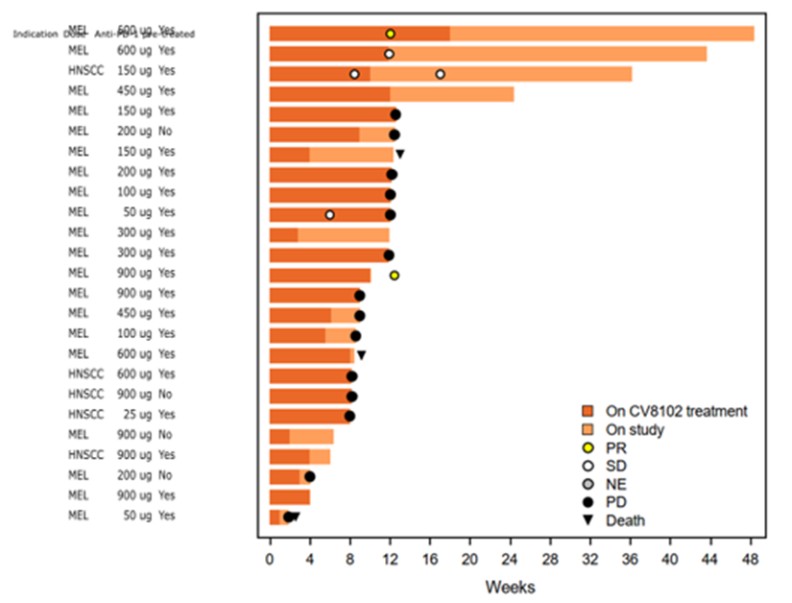
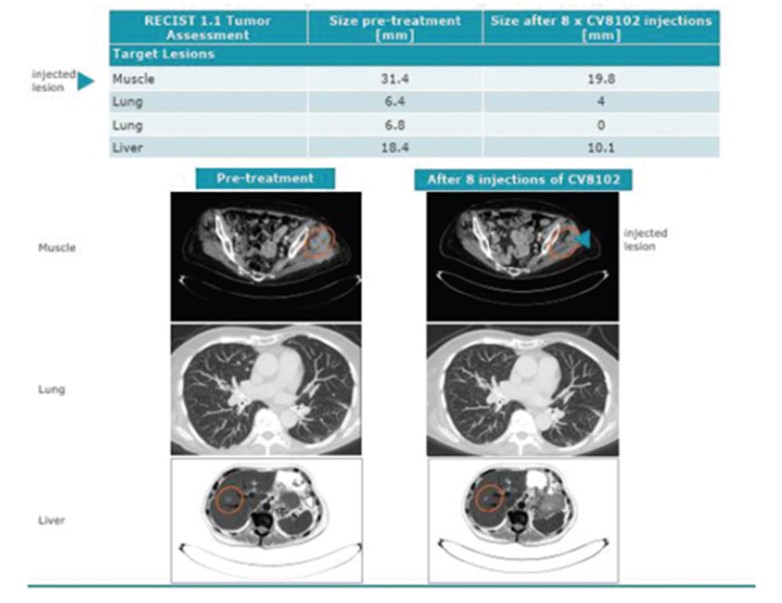
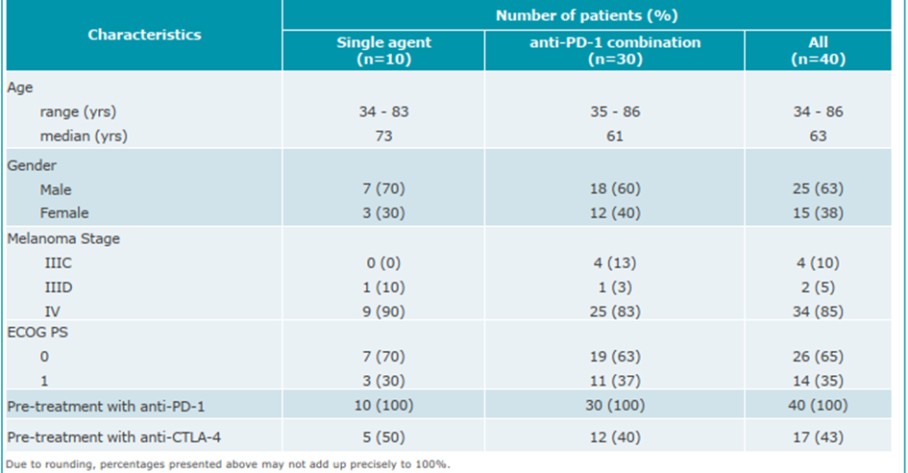
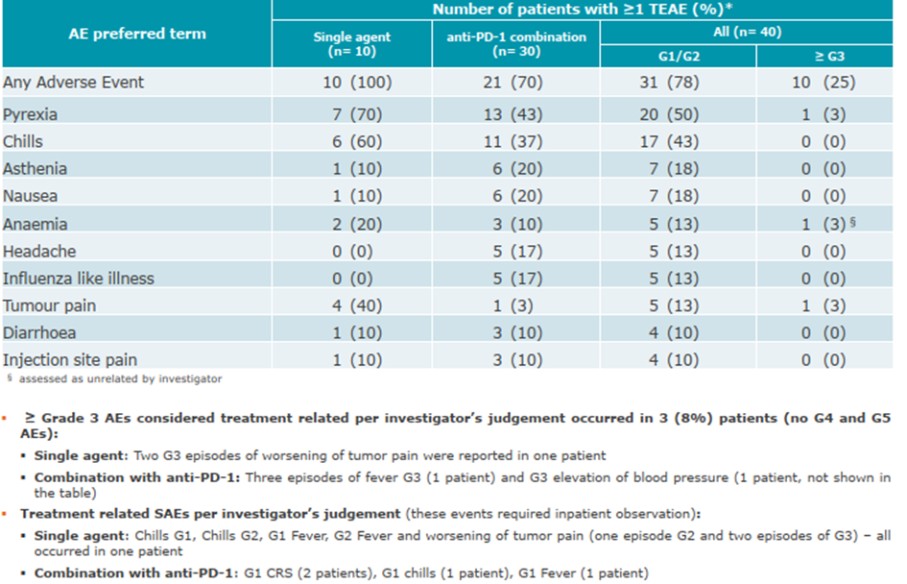
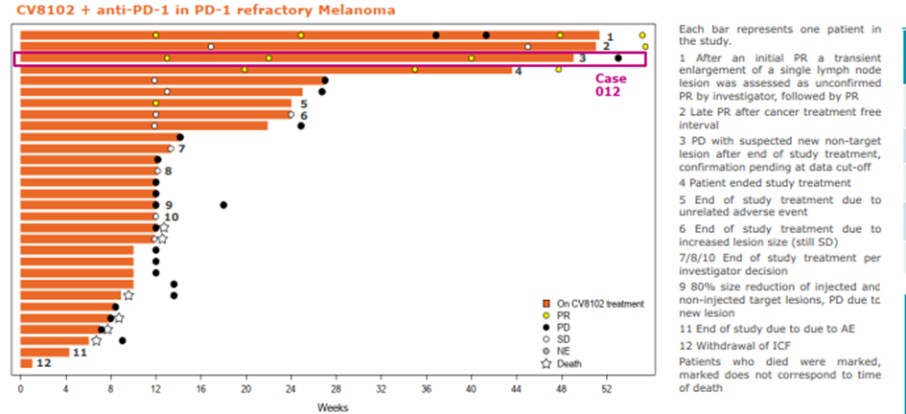
CV8102
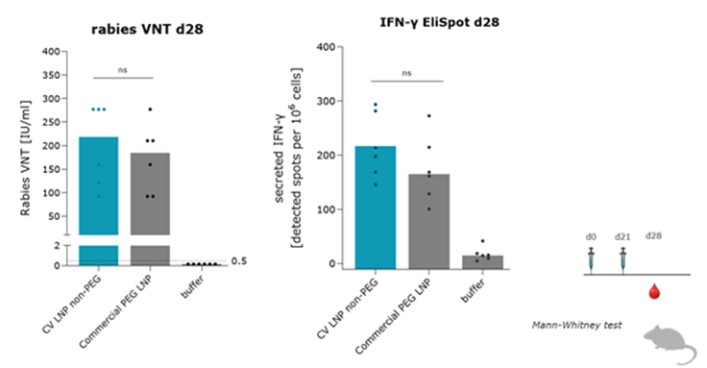
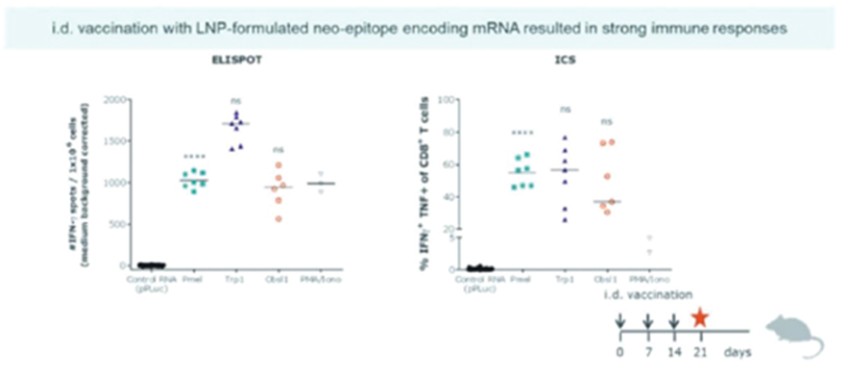
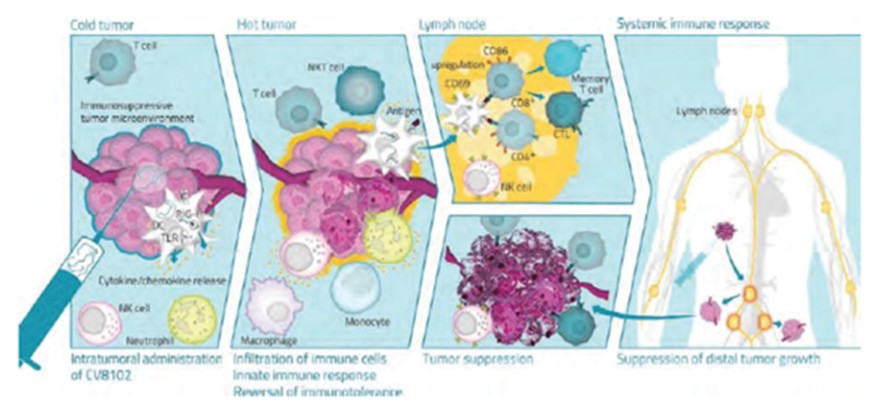
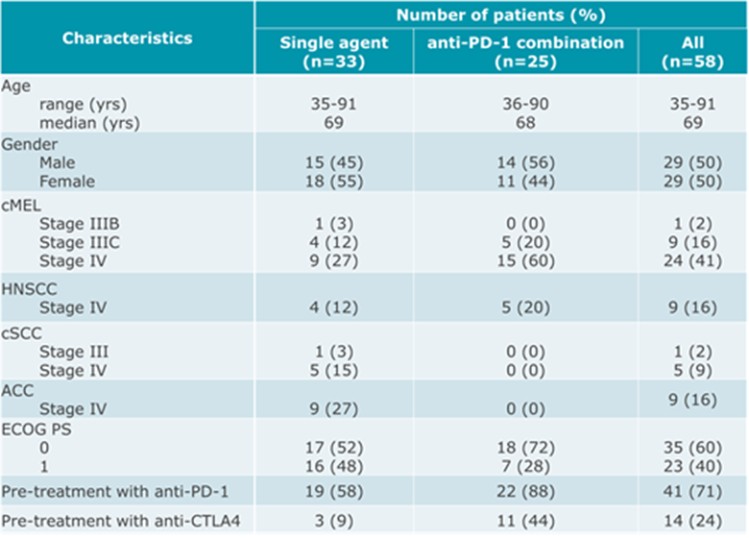
As of April 3, 2023, we own four issued U.S. patents, five pending U.S. patent applications, 26 issued foreign patents, including in Europe, Brazil, Canada, China, India, Japan, the Republic of Korea, Singapore, Taiwan, Russia, Mexico and Australia, and 19 pending foreign patent applications relating to our CV8102 product candidate. The issued patents are expected to expire between 2028 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending applications would be expected to expire between 2029 and 2037, excluding any additional term for patent term adjustments or patent term extensions.
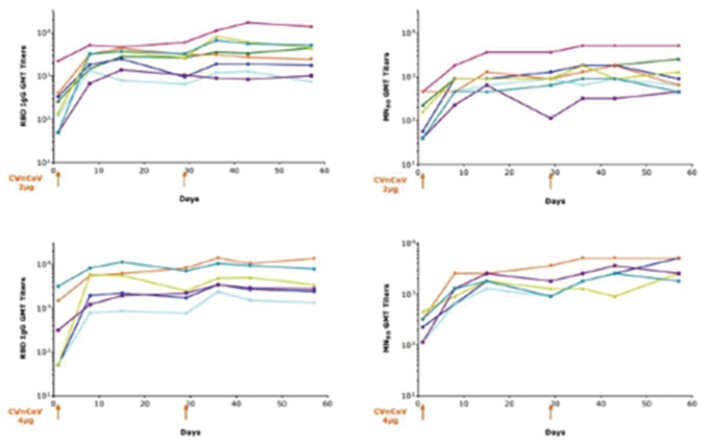
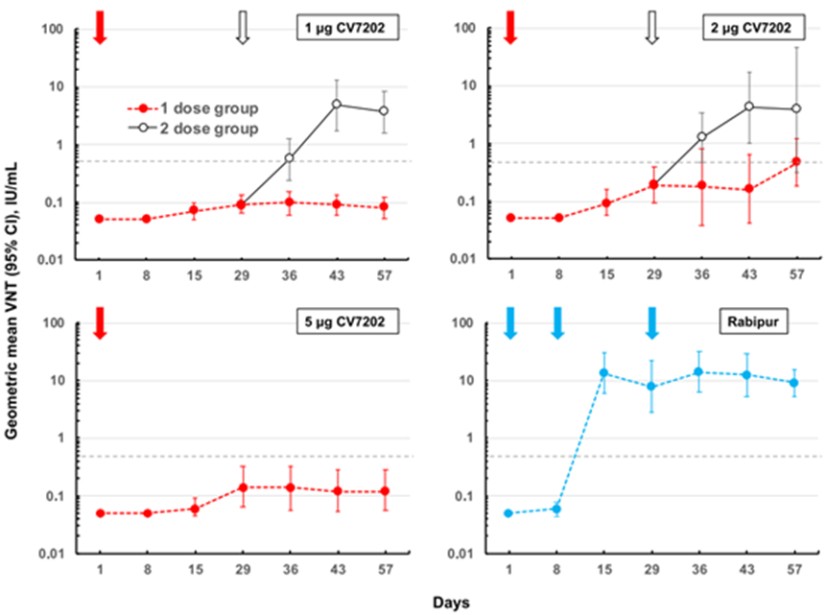
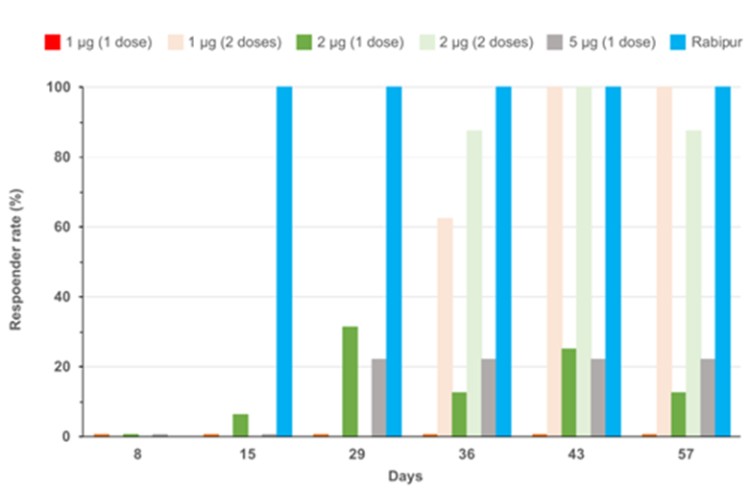
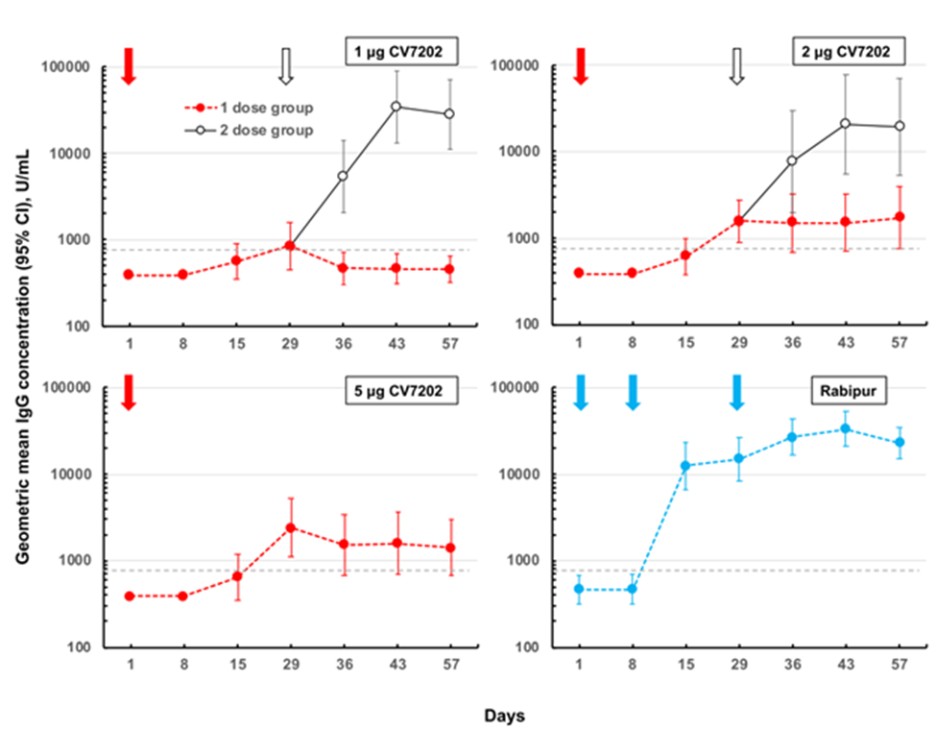
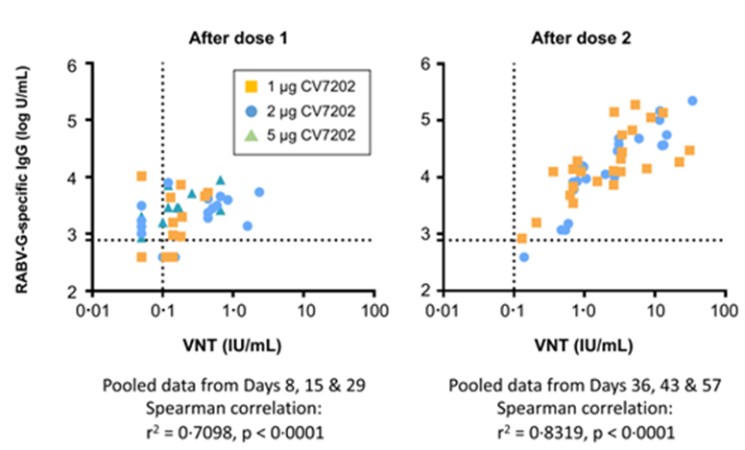
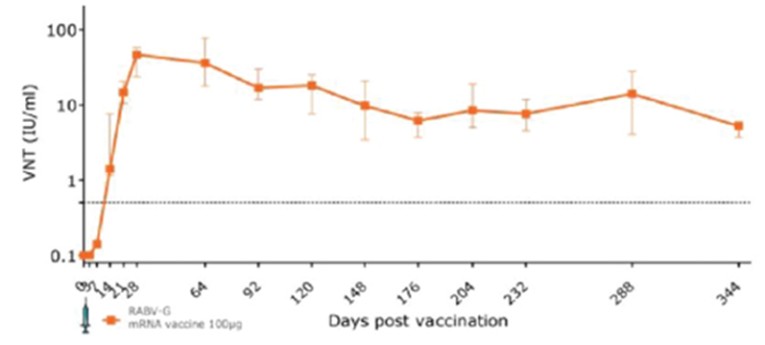
CV7202
As of April 3, 2023, we own eight issued U.S. patents, five pending U.S. patent applications, 25 issued foreign patents, including in Europe, Canada, China, India, Japan, the Republic of Korea, Singapore, Russia, Mexico and Australia, and 23 pending foreign patent applications relating to our CV7202 product candidate. The issued patents are expected to expire between 2025 and 2037, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending patent applications would be expected to expire between 2023 and 2037, excluding any additional term for patent term adjustments or patent term extensions.