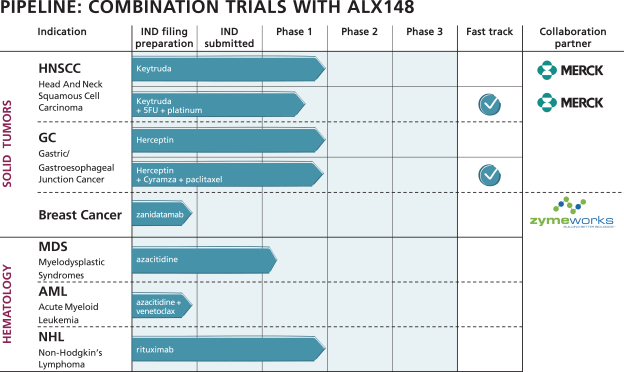
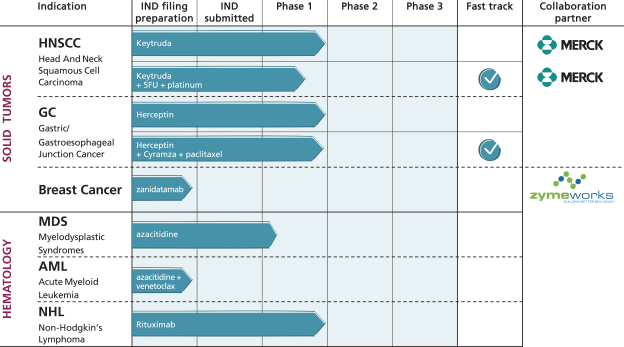
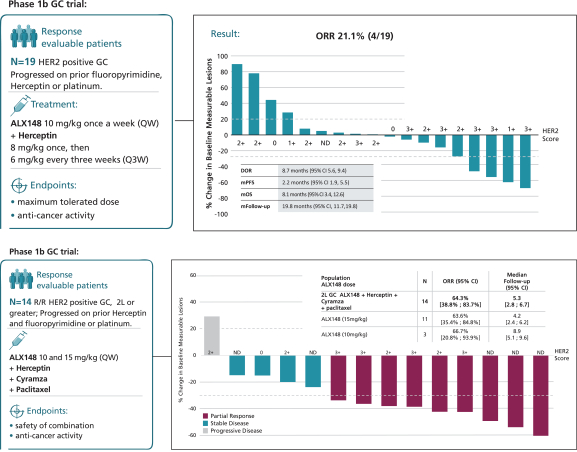
by the second half of 2021. Data from this trial would confirm whether ALX148 improves the response rate to anti-HER2-based therapy in patients with HER2-positive gastric/GEJ cancer. Of note, there are multiple emerging agents, predominantly antibody-based therapies, in development for patients with HER2-positive cancer. Because these agents target HER2 as does trastuzumab, we believe that ALX148 has the potential to maximize the anti-cancer activity of these novel agents should they supplant trastuzumab in the treatment paradigm for these patients.
ALX148 in HER2-expressing breast cancer and other solid tumors
We plan to continue developing ALX148 in a broad range of tumor types and in novel combinations. In November 2020, we announced a clinical trial collaboration with Zymeworks to investigate ALX148 in combination with Zymeworks’ HER2-targeted bispecific antibody, zanidatamab, in patients with advanced HER2-expressing tumors.
Under the terms of the agreement, Zymeworks will conduct an open label, multi-center Phase 1b trial to assess the safety and efficacy of the combination of zanidatamab and ALX148 in a two-part trial. The first part of the trial will evaluate the safety of the combination treatment. The second part of the trial will evaluate the safety, tolerability and anti-tumor activity of the combination in separate cohorts of subjects with HER2-overexpressing breast cancer, HER2-low breast cancer and non-breast HER2-expressing solid tumors.
Research Programs
In addition to ALX148, we are also developing preclinical programs that may offer additional ways to engage the innate and adaptive immune response to cancer.
Licensing and Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our current and future product candidates, novel discoveries, product development technologies and knowhow and to operate without infringing on the proprietary rights of others. We seek to protect our proprietary position by, among other methods, filing or in-licensing U.S. and foreign patents and patent applications related to technology, inventions and improvements that are important to the development and implementation of our business. Our patent portfolio is intended to cover our product candidates and related components, their methods of use and processes for their manufacture and any other inventions that are commercially important to our business. We also rely on trademarks, trade secrets, knowhow, continuing technological innovation and confidential information to develop and maintain our proprietary position.
As of November 19, 2020, we own two issued U.S. patents, seven pending U.S. nonprovisional patent applications, eleven pending U.S. provisional patent applications and a portfolio of national patent application filings in a variety of non-U.S. jurisdictions, including Europe, Hong Kong, Brazil, Mexico, New Zealand, Japan, Australia, Canada, China, India, Israel, Republic of Korea, Singapore and Russia. Of these patents and patent applications, the following relate to ALX148: two issued U.S. patents, four pending U.S. nonprovisional patent applications, eleven pending U.S. provisional patent applications and a portfolio of national patent application filings in a variety of non-U.S. jurisdictions, including Europe, Hong Kong, Brazil, Mexico, New Zealand, Japan, Australia, Canada, China, India, Israel, Republic of Korea, Singapore and Russia.
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. Our two U.S. issued patents and, if issued as U.S. patents, our eleven U.S. nonprovisional patent applications and ten U.S. provisional patent applications are expected to expire between August 2036 and November 2041, excluding any additional term for patent term adjustments or patent term extensions, with an expiration of between August 2036 and November 2041 with respect to our patent and patent applications related to ALX148, excluding any additional term for patent term extensions.
We obtained a worldwide, royalty-bearing, sublicensable license from the Board of Trustees of the Leland Stanford Junior University, or Stanford, under certain patents relating to high-affinity SIRPa variant polypeptides, to develop, manufacture and commercialize products for use in certain licensed fields, the scope of which would include the application of the licensed intellectual property in oncology. Our portfolio of exclusively licensed patents from Stanford includes six issued patents (one of which is in the United States) and applications are pending in six
113