PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes incorporated by reference in this prospectus. You should also consider, among other things, the matters described under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in our Annual Report on Form 10-K for the year ended December 31, 2020, in each case incorporated by reference in this prospectus. Unless the context otherwise requires, the terms “Relay,” “Relay Therapeutics,” “the Company,” “we,” “us,” and “our” in this prospectus refer to Relay Therapeutics, Inc. and its subsidiary.
Overview
We are a clinical-stage precision medicines company transforming the drug discovery process with the goal of bringing life-changing therapies to patients. We are among the first of a new breed of biotech created at the intersection of disparate disciplines. Our Dynamo™ platform integrates an array of leading-edge computational and experimental approaches to effectively drug protein targets that have previously been intractable. Our initial focus is on enhancing small molecule therapeutic discovery in targeted oncology and genetic disease.
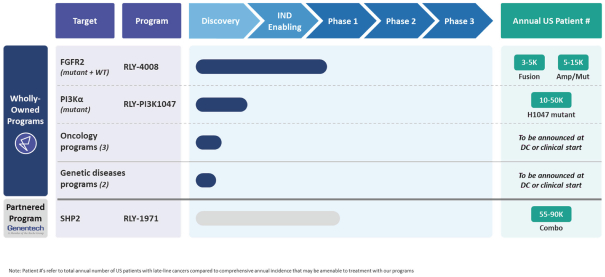
We are advancing a pipeline of medicines to address targets in precision oncology, including our lead product candidates, RLY-1971 and RLY-4008, as well as our PI3Kα mutant selective program, or the RLY-PI3K1047 program. We initiated a Phase 1 clinical trial for RLY-1971, our inhibitor of Src homology region 2 domain-containing phosphatase-2, or SHP2, in patients with advanced solid tumors in the first quarter of 2020. In December 2020, we entered into a global collaboration and license agreement, or the Genentech Agreement, with Genentech, Inc., a member of the Roche Group, or Genentech, for the development and commercialization of RLY-1971. We initiated a first-in-human clinical trial of RLY-4008, our inhibitor of fibroblast growth factor receptor 2, or FGFR2, enriched for patients with advanced solid tumors having oncogenic FGFR2 alterations in the third quarter of 2020. We anticipate the RLY-PI3K1047 program, our program for molecules targeting cancer-associated mutant variants of phosphoinostide 3-kinase alpha, or PI3Kα, to be in Investigational New Drug, or IND, enabling studies in 2021. While our initial focus is on precision oncology, we believe our Dynamo platform may also be broadly applied to other areas of precision medicine, such as genetic disease. In addition to the three product candidates described above, we have five discovery stage programs across precision oncology and genetic disease. We are focused on using the novel insights derived from our approach to transform the lives of patients suffering from debilitating and life-threatening diseases through the discovery, development and commercialization of our therapies.
Precision medicine emerged as an approach for disease treatment as the understanding of the link between genetic alterations, protein dysfunction and diseases evolved. Precision medicine aims to specifically and potently drug genetically validated target proteins (i.e., genetic variants potentially implicated in biology of disease). However, some target proteins thus far have been intractable using conventional drug discovery tools, such as structure-based drug design, or SBDD. While SBDD is well-suited to solving some drug discovery problems such as orthosteric site kinase inhibitors, its reliance on static images of protein fragments limits its ability to gain accurate insights into the dynamic behavior of proteins in their natural state, which in turn limits its ability to discover medicines with exquisite specificity. Our approach pivots the understanding of protein targets from the industry-standard, static view, to a novel paradigm based on fundamental insights into protein motion. We then apply these novel insights into protein motion to drug discovery and design, which we term Motion Based Drug DesignTM, or MBDD.