(ii)three issued U.S. patents, 18 issued foreign patents in Australia, Austria, Belgium, Canada, Denmark, France, Germany, Ireland, Italy, Japan, Liechtenstein, Netherlands, Spain, Sweden, Switzerland, Turkey, and the UK, one pending U.S. non-provisional patent application, and three pending foreign patent applications in Europe and Canada, which we co-own, and
(iii)three issued U.S. patents, 23 issued foreign patents in Australia, Austria, Belgium, Canada, Denmark, France, Germany, Ireland, Italy, Liechtenstein, Netherlands, Spain, Sweden, Switzerland, Turkey, and the UK, one pending U.S. non-provisional patent application, and seven pending foreign patent applications in Australia, Canada, Europe, Japan, China, and Hong Kong, which we exclusively license.
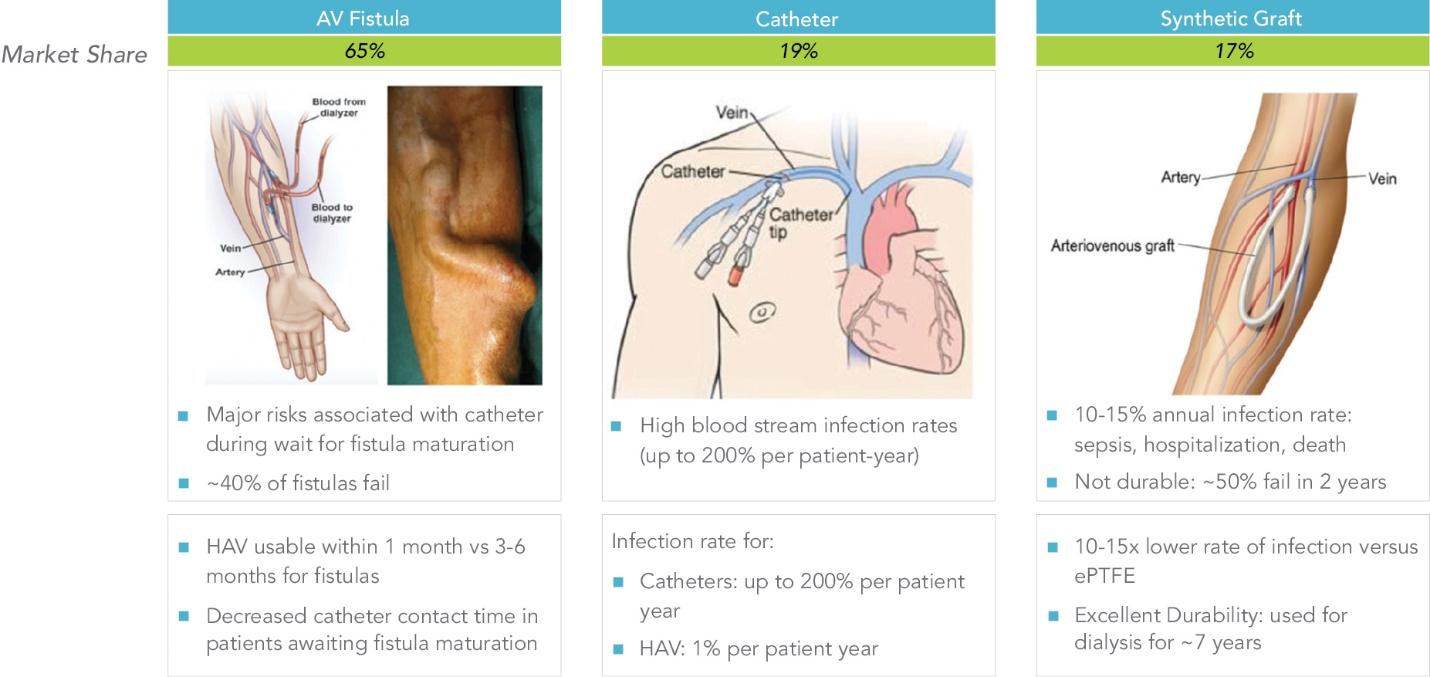
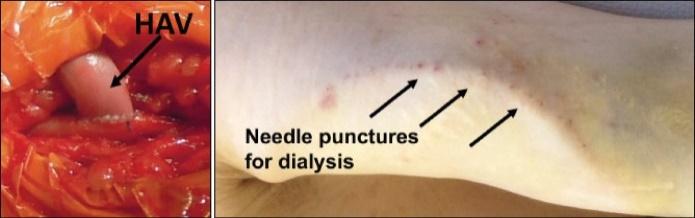
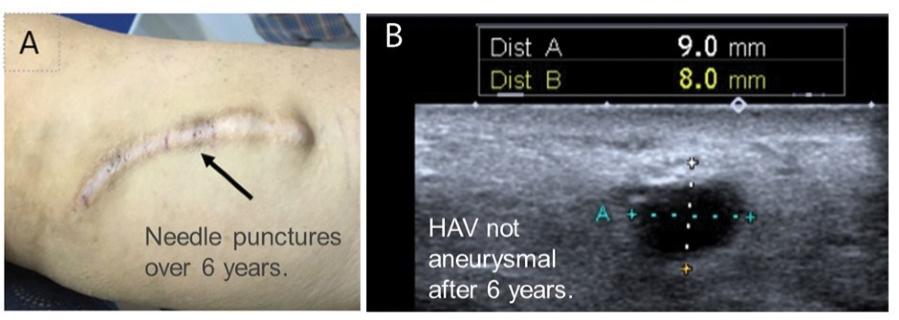
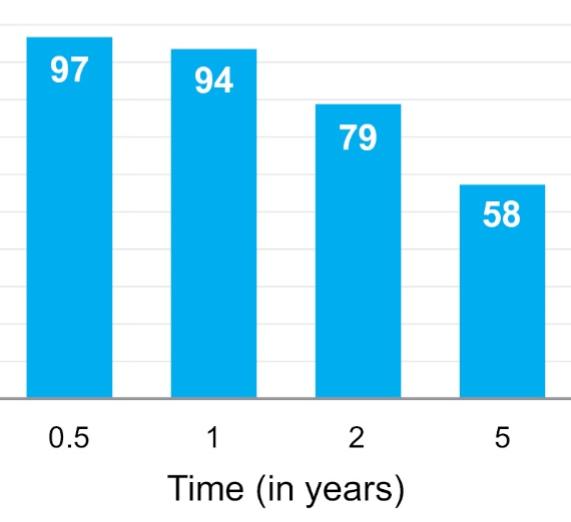
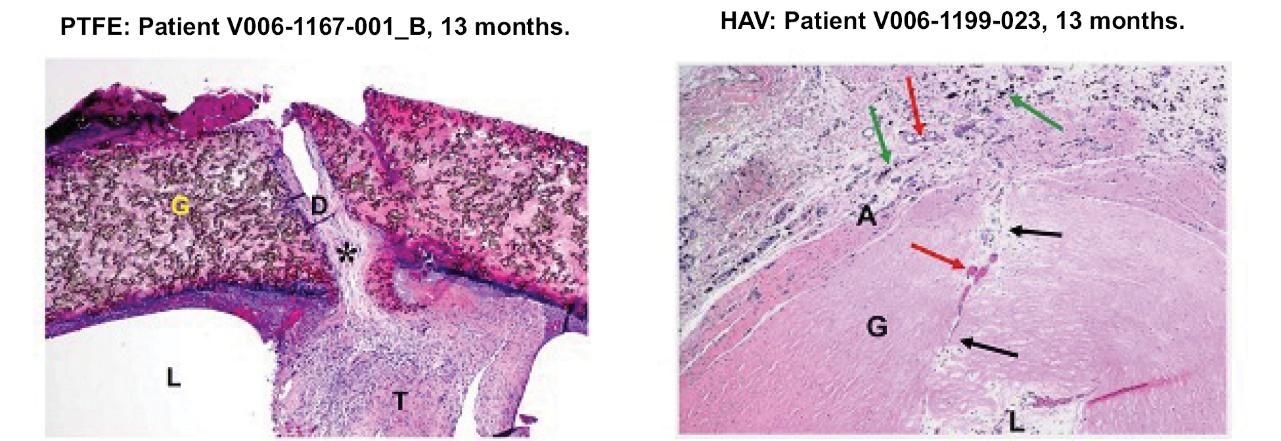
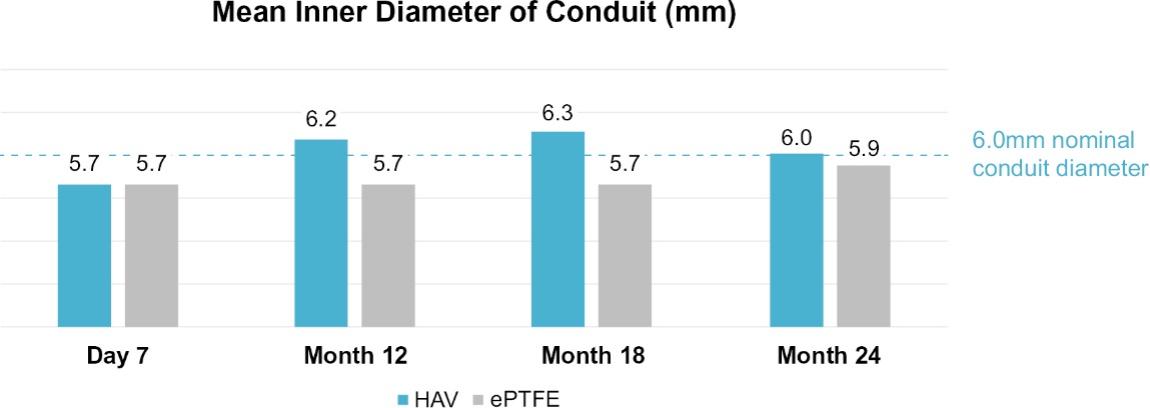
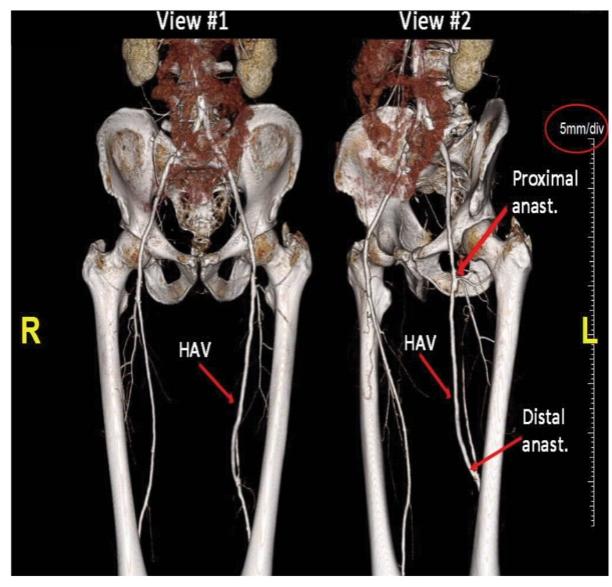
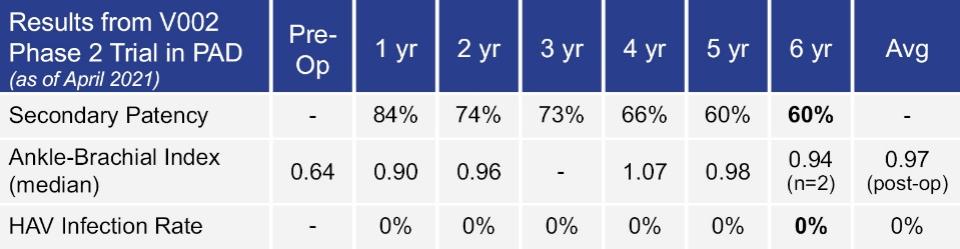
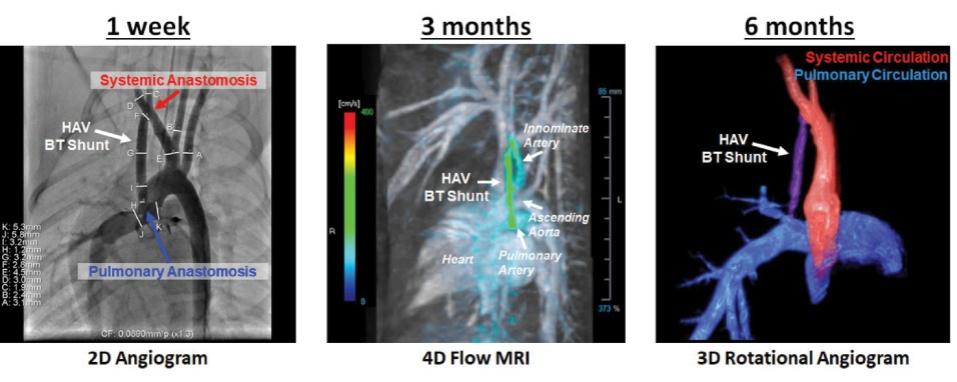
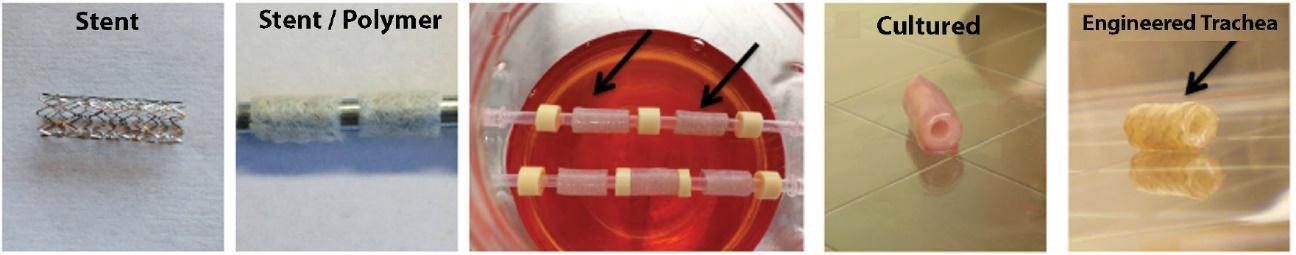
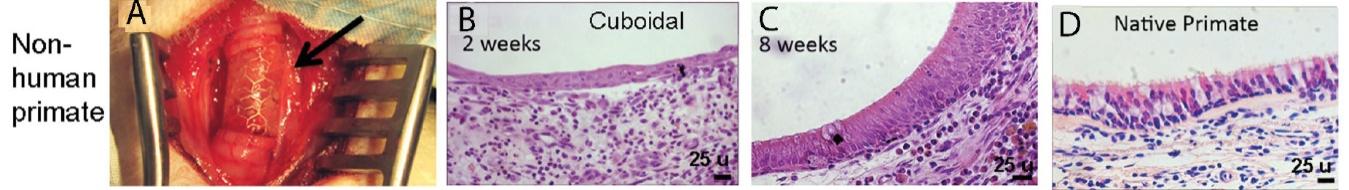
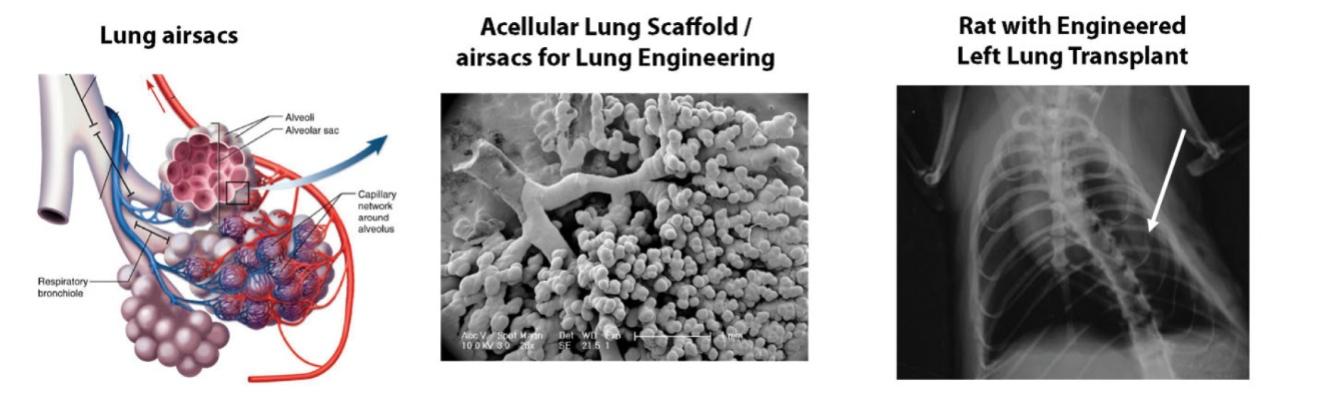
Many of these patents and patent applications generally relate to the scaffolds used to make our vessels, the composition of our vessels, and systems and methods of manufacturing our vessels. Excluding any patent term adjustment or patent term extension, the U.S. patent relating to the scaffold used to make our vessels expires in 2032, the U.S. patents relating to the composition of our vessels expire in 2032 and the U.S. patents relating to the systems and methods of manufacturing our vessels expires in 2032. The U.S. patent relating to the entangler machinery used to make tubular scaffolds expires in 2035. Included in our patent portfolio are three pending, Humacyte-owned non-provisional applications relating to the manufacturing of engineered tissues at commercial scale. If these three non-provisional applications are allowed, such additional patents issuing therefrom would be expected to expire around 2040.
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify our proprietary and intellectual property position for our product candidates will depend on our success in obtaining effective patent claims and enforcing those claims if granted. However, our owned and licensed pending patent applications, and any patent applications that we may in the future file or license from third parties, may not result in the issuance of patents. For more information, see the section entitled “Risk Factors—Risks Related to Our Intellectual Property.”
We have also registered trademarks for use in connection with our products. These include registrations for HUMACYL™ in the United States, Europe, Australia, Canada, China, and Israel; HUMAGRAFT™ in Australia, China, Europe, and Israel; HUMAPASS™ in Europe, Australia, and Israel; and HUMACYTE, in the United States, Europe, Australia, Canada, and Israel. We may pursue additional registrations for future products in markets of interest.
In addition to the above, we have established expertise and development capabilities focused in the areas of preclinical research and development, manufacturing process scale-up, cGMP manufacturing, quality control, quality assurance, compliance, regulatory affairs and clinical trial design and execution. We believe that our focus and expertise will help us develop and expand technology-based applications leveraging our proprietary intellectual property.
Finally, we rely, in some circumstances, on trade secrets to protect our technology. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems.
In addition to the intellectual property that we have developed internally, we license rights to certain intellectual property that is material to our business prospects. We have summarized our material license agreements below.
License Agreement with Duke University
In March 2006, we entered into a license agreement with Duke University (“Duke”), which was subsequently amended in 2011, 2014, 2015, 2018 and 2019. We refer to the license agreement as amended, as the Duke License Agreement. Under the Duke License Agreement, Duke granted us a worldwide, exclusive, sublicensable license to certain patents related to decellularized tissue engineering, which we refer to as the patent rights, as well as a non-exclusive license to use and practice certain know-how related to the patent rights. The relevant licensed patent on decellularization of tissue will expire in 2021. We have agreed to use commercially reasonable efforts to develop, register, market and sell products utilizing the patent rights, which we refer to as the licensed products. Any services provided to a third party utilizing licensed products are referred to as licensed services. We have also agreed to meet certain benchmarks in our development efforts, including as to development events, clinical trials, regulatory submissions and marketing approval, within specified timeframes. Under the Duke License Agreement, Duke retains the right to use the patent rights for its own educational and research purposes, and to provide the patent rights to other non-profit, governmental or higher-learning institutions for non-commercial purposes without paying royalties or other fees.
In connection with our entry into the Duke License Agreement, we granted equity consideration to Duke in the form of 200,666 shares of our common stock. Under the Duke License Agreement, we have also agreed to pay Duke: a low single-digit percentage royalty on eligible sales of licensed products and licensed services, plus a low double-digit percentage of any sublicensing revenue; an