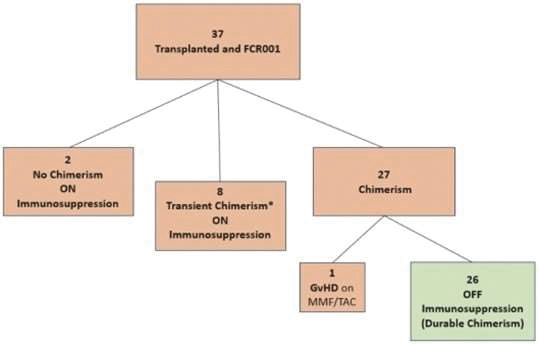
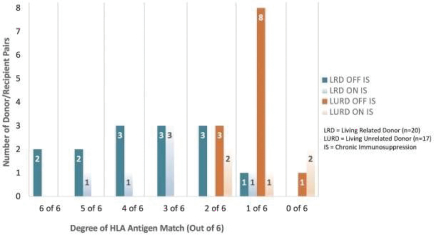
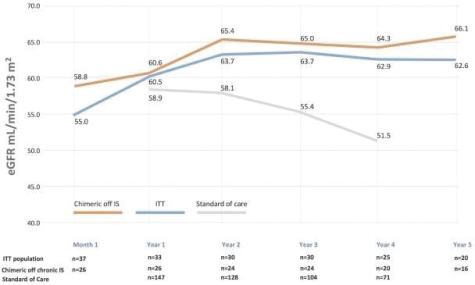
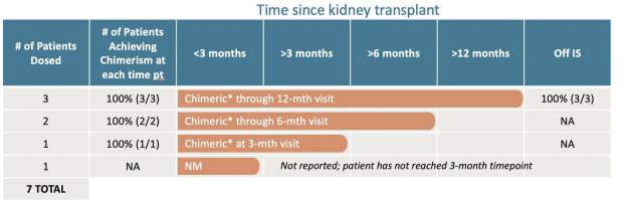
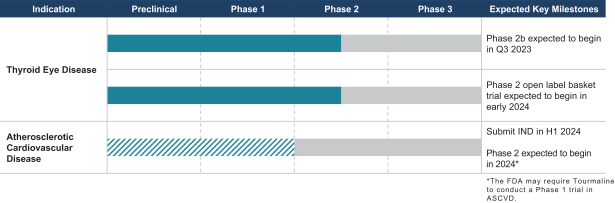
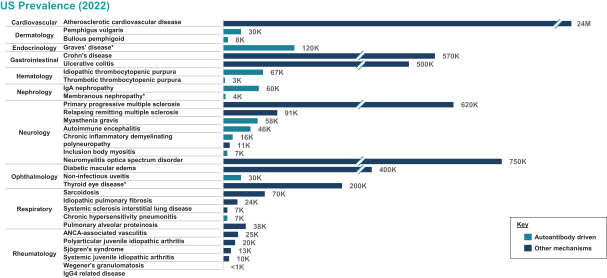
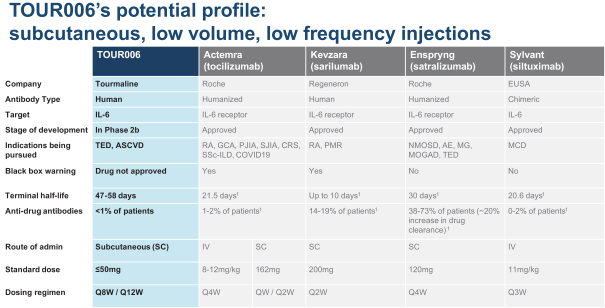
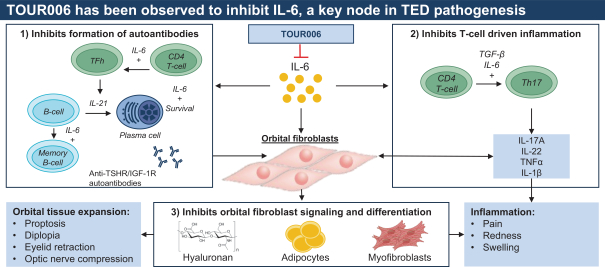
| | • | | “Terrain Valuation” means the sum of (i) $82,500,000 plus (ii) the Terrain Legacy Proceeds (if any), minus (iii) the amount (if any) by which Terrain’s Net Cash is less than $62,437,500 (i.e., 92.5% of Target Net Cash), plus (iv) the amount (if any) by which Terrain’s Net Cash (excluding any Terrain Legacy Proceeds) exceeds $72,562,500 (i.e., 107.5% of Target Net Cash). |
Set forth on Section 1.1(a)(i) of the Terrain Disclosure Schedule is an illustrative example of Exchange Ratio calculations.
“Exclusivity Agreement” means the letter agreement dated April 24, 2023, between the Company and Terrain, as amended or modified from time to time.
“Federal Health Care Program” means any federal health program as defined in 42 U.S.C. § 1320a-7b(f), including Medicare, Medicaid, TRICARE, CHAMPVA, and state healthcare programs (as defined therein).
“Governmental Authority” means any: (a) nation, state, commonwealth, province, territory, county, municipality, district or other jurisdiction of any nature, (b) federal, state, local, municipal, supra-national, foreign or other government, (c) governmental or quasi-governmental authority of any nature (including any governmental division, department, agency, commission, bureau, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any Tax authority) or (d) self-regulatory organization (including Nasdaq).
“Governmental Authorization” means any: (a) permit, license, certificate, franchise, permission, variance, exception, order, clearance, registration, qualification, approval or authorization issued, granted, given or otherwise made available by or under the authority of any Governmental Authority or pursuant to any Law or (b) right under any Contract with any Governmental Authority.
“Hazardous Materials” means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable chemical, or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation, control or remediation under any Environmental Law, including without limitation, crude oil or any fraction thereof, and petroleum products or by-products.
“Health Care Laws” means all applicable Laws and implementing regulations pertaining to U.S. health care regulatory matters to the extent applicable, including as amended from time to time, any such Law pertaining to: (a) the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.), the Public Health Service Act (42 U.S.C. §262 et seq.) and the regulations promulgated thereunder, (b) any Federal Health Care Program, including those pertaining to providers of goods or services that are paid for by any Federal Health Care Program, including, the False Claims Act, as amended (31 U.S.C. §§ 3729 et seq.), the criminal False Claims Law (42 U.S.C. § 1320a-7b(a)), the Federal Anti-kickback statute (42 U.S.C. § 1320a-7b(b)), the Anti-Kickback Act of 1986 (41 U.S.C. §§ 51-58), the federal Stark Law (42 U.S.C. § 1395nn), the federal False Statements Statute (42 U.S.C. § 1320a-7b(a)), the Exclusion Law (42 U.S.C. § 1320a-7), the Beneficiary Inducement Statute (42 U.S.C. § 1320a-7a(a)(5)), the Federal Program Fraud Civil Remedies Act (31 U.S.C. §§3801-3812), HIPAA (as defined below), the Civil Monetary Penalties Law (42 U.S.C. § 1320a-7a and 1320a-7b), the Physician Payment Sunshine Act (42 U.S.C. § 1320a-7h), (c) Medicare (Title XVIII of the Social Security Act), Medicaid (Title XIX of the Social Security Act), the Medicare and Medicaid Program Integrity Provisions (42 U.S.C. §1320a-7k(d)), TRICARE (d) the Patient Protection and Affordable Care Act (P.L. 111-1468), (e) the Health Care and Education Reconciliation Act of 2010 (P.L. 111-152), (f) accreditation standards and requirements for health care and related services, products, clinicians, and facilities issued by all applicable Governmental Authorities, (g) any and all applicable Laws pertaining to pharmaceutical, biological and medical device products, (h) any and all other applicable comparable state and foreign Laws, and (i) all regulations promulgated pursuant to the foregoing, each of (a) through (i) as amended or modified from time to time.
“HIPAA” means the following, as the same may be amended, modified or supplemented from time to time, and any successor statute thereto, and any and all rules or regulations promulgated from time to time thereunder:
A-12