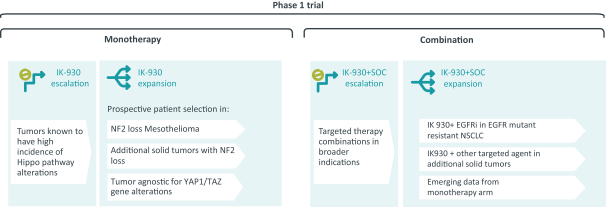
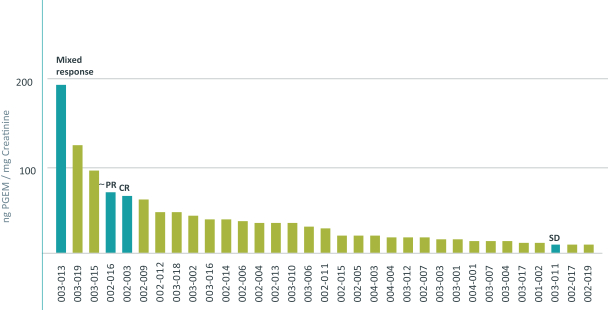
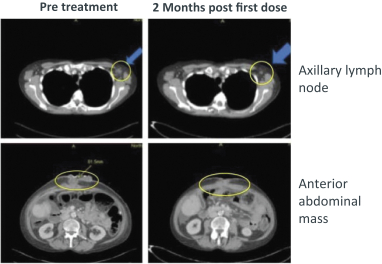
approval is sought can only be extended in connection with one of the approvals. The United States Patent and Trademark Office reviews and approves the application for any patent term extension in consultation with the FDA. In the future, if our product candidates receive approval by the FDA, we expect to apply for patent term extensions on any issued patents covering those products, depending upon the length of the clinical studies for each product and other factors. There can be no assurance that our pending patent applications, and any patent applications that we may in the future file or license from third parties, will issue or that we will benefit from any patent term extension or favorable adjustments to the terms of any patents we may own or in-license in the future. In addition, the actual protection afforded by a patent varies on a product-by-product basis, from country-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent. Patent term may be inadequate to protect our competitive position on our products for an adequate amount of time.
As of December 28, 2020, our overall patent portfolio includes forty-three (43) patent families comprising issued patents, pending U.S. and PCT International patent applications, and pending patent applications in foreign jurisdictions. The patents and patent applications have claims relating to our current product candidates, methods of use and manufacturing processes, as well as claims directed to potential future products and developments.
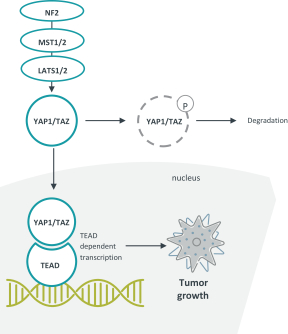
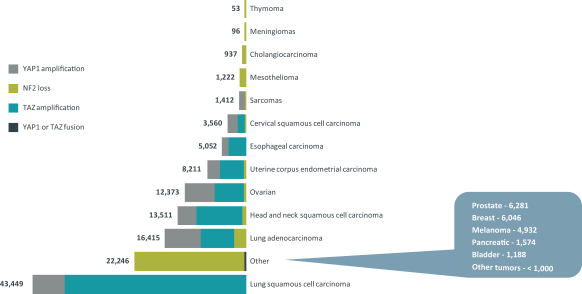
TEAD Inhibitor Patent Families
As of December 20, 2020, we solely own seven patent families related to TEAD inhibitors and degraders, compositions thereof, and methods of their use. Any U.S. or foreign patents that issue from these patent families, if granted and all appropriate maintenance fees paid, are expected to expire from 2040 to 2041, not including any patent term adjustment, patent term extension, or supplementary protection certificate (SPC).
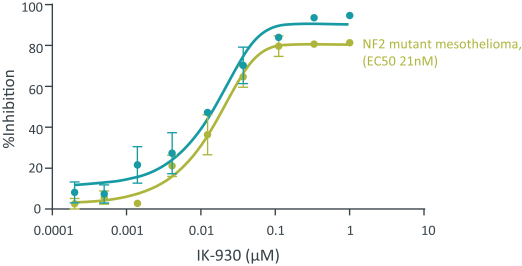
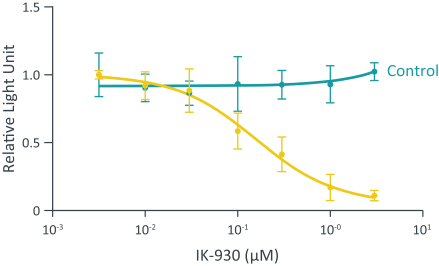
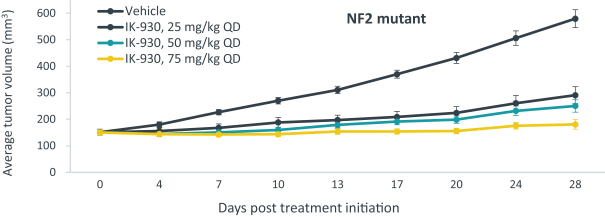
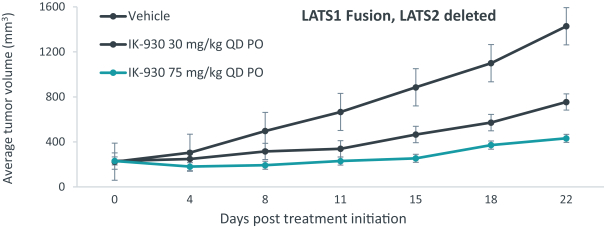
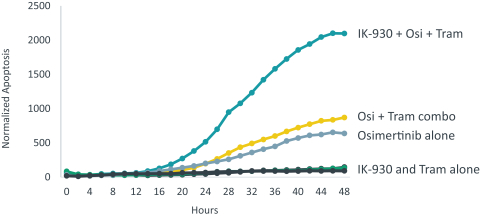
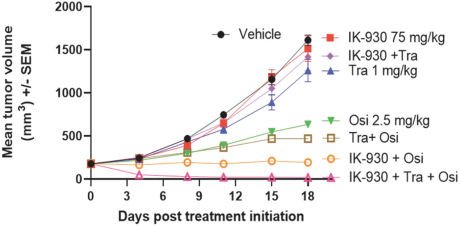
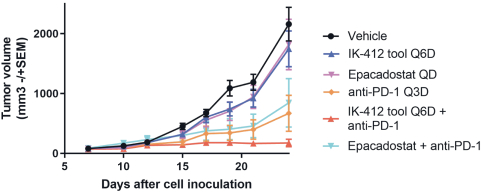
Our current lead TEAD inhibitor, IK-930, compositions thereof, and methods of the use, are covered by pending U.S., PCT international, Taiwanese, and Argentinian patent applications that, if granted and all appropriate maintenance fees paid, are expected to expire in 2040, not including any patent term adjustment, patent term extension, or supplementary protection certificate, or SPC.
EP4 Antagonists Patent Families
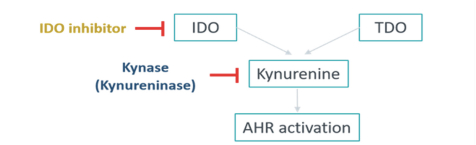
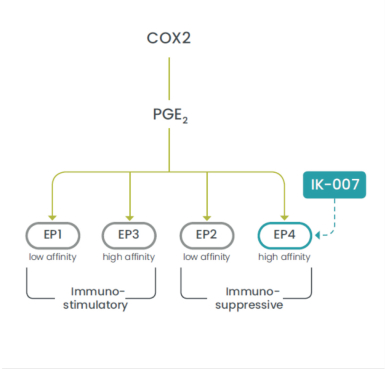
As of December 28, 2020, we have an exclusive license to seven patent families directed to EP4 antagonists, crystal forms thereof, compositions thereof, and methods of their use. Any U.S. or foreign patents that issue from these patent families, if granted and all appropriate maintenance fees paid, are expected to expire from 2021 to 2036, not including any patent term adjustment, patent term extension, or SPC.
As of December 20, 2020, we and AskAt Inc. jointly own three patent families directed to EP4 antagonist compositions, methods of making certain EP4 antagonists and their formulations, and methods of their use. Any U.S. or foreign patents that issue from these patent families, if granted and all appropriate maintenance fees paid, are expected to expire in 2039, not including any patent term adjustment, patent term extension, or SPC.
As of December 28, 2020, we solely own two patent families directed to EP4 antagonist salts and crystal forms, and methods of using EP4 antagonists. Any U.S. or foreign patents that issue from these patent families, if granted and all appropriate maintenance fees paid, are expected to expire from 2039 to 2041, not including any patent term adjustment, patent term extension, or SPC.
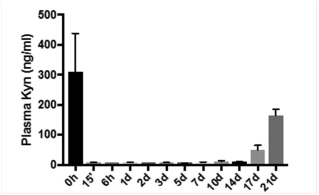
As of December 28, 2020, our current lead EP4 antagonist, IK-007, salts and crystal forms thereof, compositions thereof, and methods of the use, are covered by over ten (10) issued U.S. patents, and by a number of pending U.S. and PCT international patent applications, and issued patents and pending patent applications in foreign jurisdictions that, if granted and all appropriate maintenance fees paid, are expected to expire from 2021 to 2041, not including any patent term adjustment, patent term extension, or SPC.
141