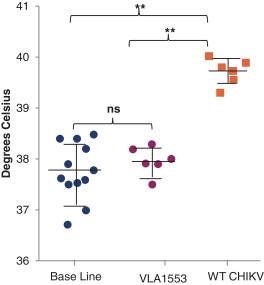
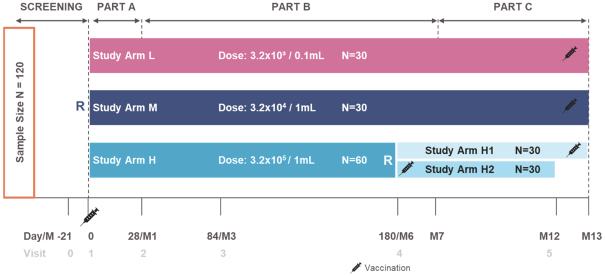
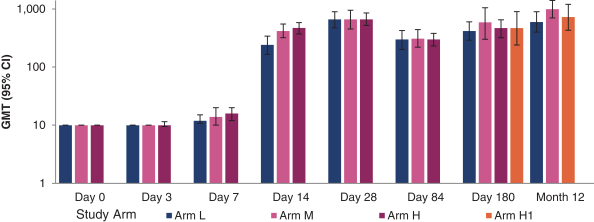
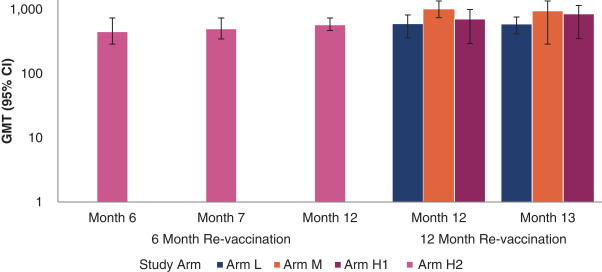
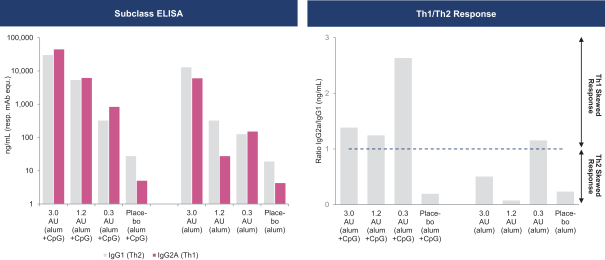
have a co-exclusive right to deliver, distribute, market, sell, promote, and import IXIARO in Germany solely with respect to certain non-profit organizations. Pursuant to the GSK Distribution Agreement, GSK is required to use reasonable commercial efforts to promote, sell and distribute IXIARO in Germany and is required to purchase an agreed upon minimum quantity of IXIARO doses during each year of the agreement. In connection with the GSK Distribution Agreement, we are obligated to supply (or designate a third-party entity to supply) GSK with all of its IXIARO supply requirements, subject to our reserved right to modify or discontinue manufacture and sale of IXIARO at our discretion. The GSK Distribution Agreement further provides that GSK must not manufacture, market, file applications for regulatory approval, distribute, sell or promote, in Germany manufacture, market, file applications for regulatory approval, distribute, sell or promote, in Germany a directly competing product that is a generic substitute for IXIARO.
The GSK Distribution Agreement shall continue until December 31, 2021. Either party may terminate the agreement upon (a) an uncured material breach of the agreement by, insolvency of, or change of control of the other party, or (b) withdrawal of marketing authorization for IXIARO in Germany. GSK may terminate this agreement if we fail to supply IXIARO under a firm purchase order for a specified period of time. In addition, we may terminate the agreement if GSK ceases to carry on business marketing pharmaceutical products in Germany, fails to comply with anti-corruption laws, does not achieve specified minimum purchase quantities, or breaches diligence obligations under that certain distribution agreement between the parties for the distribution of DUKORAL and we terminate such DUKORAL agreement for this same reason.
Bavarian Nordic Distribution Agreements
In November 2020, Valneva Austria GmbH, or Valneva Austria, entered into a distribution agreement, or the IXIARO Distribution Agreement, with Bavarian Nordic A/S, or BN, pursuant to which Valneva Austria granted BN an exclusive right to import, market, promote, distribute and sell IXIARO in Germany. In parallel, Valneva Sweden AB, or Valneva Sweden, entered into a distribution agreement, or the DUKORAL Distribution Agreement, with BN pursuant to which Valneva Sweden granted BN an exclusive right to import, market, promote, distribute and sell DUKORAL in Germany. The IXIARO Distribution Agreement and the DUKORAL Distribution Agreement together are referred to as the BN Distribution Agreements.
The BN Distribution Agreements include sub-distribution rights. Each of Valneva Austria and Valneva Sweden has a co-exclusive right to deliver, distribute, market, sell, promote, and import IXIARO and DUKORAL, as applicable, in Germany solely with respect to certain non-profit organizations. Pursuant to the BN Distribution Agreements, BN is required to use reasonable commercial efforts to promote, sell and distribute IXIARO and DUKORAL in Germany and is required to purchase an agreed upon minimum quantity of IXIARO and DUKORAL doses during each year of the BN Distribution Agreements. The BN Distribution Agreements shall commence on January 1, 2022 and continue until December 31, 2024. Unless terminated earlier this initial term will automatically extend by two years to terminate on December 31, 2026.
VaccGen Sublicense Agreement
In April 2003, we (through our predecessor company Intercell Biomedical Ltd.) entered into a sublicense agreement, or the VaccGen Agreement, with VaccGen International, LLC, or VaccGen. We subsequently amended the VaccGen Agreement in October 2003, June 2004, March 2005, October 2005, April 2006, November 2006, December 2006, August 2007, and February 2010. Pursuant to this agreement, we obtained (a) an exclusive, worldwide (except the Caribbean), sublicensable sublicense under a prophylactic vaccine for Japanese encephalitis, the Vaccine, related patents and other intellectual property related to improvements made during the term of the agreement to develop, gain regulatory approval for, manufacture, have manufactured, distribute, use, offer for sale, import, sell, market, and otherwise commercially exploit the Vaccine and (b) an exclusive, worldwide (except for the Caribbean), royalty-free, transferable, sublicensable right and license under VaccGen’s interest in certain Vaccine information to use, reproduce, distribute, display, prepare derivative works of and otherwise modify, make, sell, offer to sell, import and otherwise use and exploit such information in connection with the foregoing license.
160