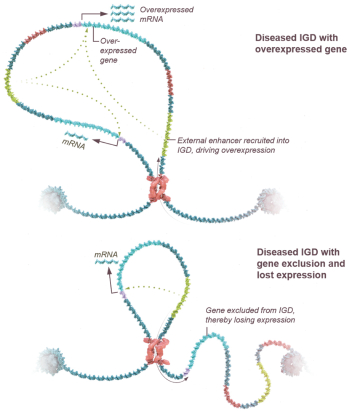
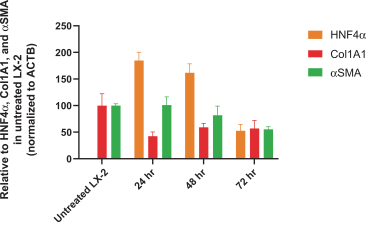
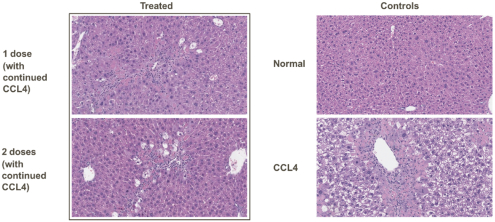
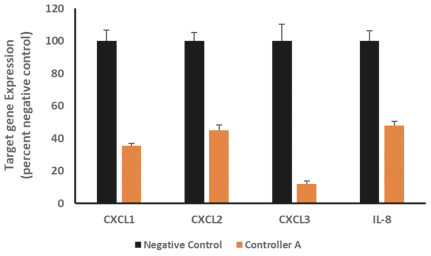
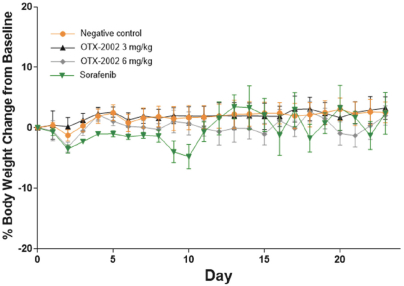
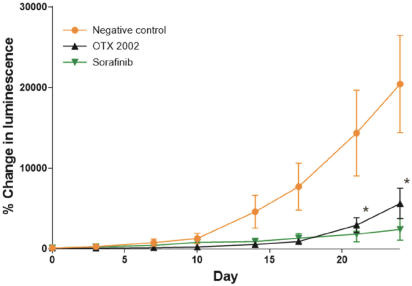
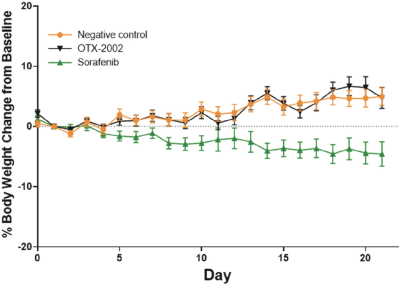
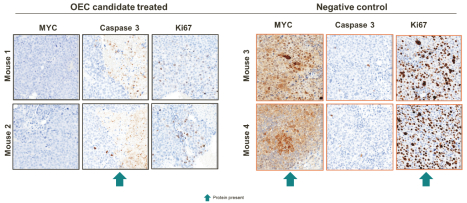
Risks Related to Healthcare Laws and Other Legal Compliance Matters
We will be subject to extensive and costly government regulation.
Our product candidates will be subject to extensive and rigorous domestic government regulation, including regulation by the FDA, the Centers for Medicare & Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice, state and local governments, and their respective equivalents outside of the United States. The FDA regulates the research, development, preclinical and clinical testing, manufacture, safety, effectiveness, record-keeping, reporting, labeling, packaging, storage, approval, advertising, promotion, sale, distribution, import, and export of pharmaceutical products. If our products are marketed abroad, they will also be subject to extensive regulation by foreign governments, whether or not they have obtained FDA approval for a given product and its uses. Such foreign regulation may be equally or more demanding than corresponding United States regulation.
Government regulation substantially increases the cost and risk of researching, developing, manufacturing, and selling our products. The regulatory review and approval process, which includes preclinical testing and clinical trials of each product candidate, is lengthy, expensive, and uncertain. We must obtain and maintain regulatory authorization to conduct preclinical studies and clinical trials. We must obtain regulatory approval for each product we intend to market, and the manufacturing facilities used for the products must be inspected and meet legal requirements. Securing regulatory approval requires the submission of extensive preclinical and clinical data and other supporting information for each proposed therapeutic indication in order to establish the product’s safety and efficacy, potency, and purity, for each intended use. The development and approval process takes many years, requires substantial resources, and may never lead to the approval of a product.
Even if we are able to obtain regulatory approval for a particular product, the approval may limit the indicated medical uses for the product, may otherwise limit our ability to promote, sell, and distribute the product, may require that we conduct costly post-marketing surveillance, and/or may require that we conduct ongoing post-marketing studies. Material changes to an approved product, such as, for example, manufacturing changes or revised labeling, may require further regulatory review and approval. Once obtained, any approvals may be withdrawn, including, for example, if there is a later discovery of previously unknown problems with the product, such as a previously unknown safety issue.
If we, our consultants, CDMOs, CROs, or other vendors, fail to comply with applicable regulatory requirements at any stage during the regulatory process, such noncompliance could result in, among other things, delays in the approval of applications or supplements to approved applications; refusal of a regulatory authority, including the FDA or other regulatory authorities, to review pending market approval applications or supplements to approved applications; warning letters; fines; import and/or export restrictions; product recalls or seizures; injunctions; total or partial suspension of production; civil penalties; withdrawals of previously approved marketing applications or licenses; recommendations by the FDA or other regulatory authorities against governmental contracts; and/or criminal prosecutions.
Enacted and future healthcare legislation and policies may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and could adversely affect our business.
In the United States, the EU and other jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could prevent or delay marketing approval of our products in development, restrict or regulate post-approval activities involving any product candidates for which we obtain marketing approval, impact
37