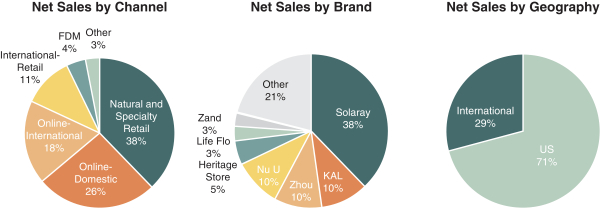
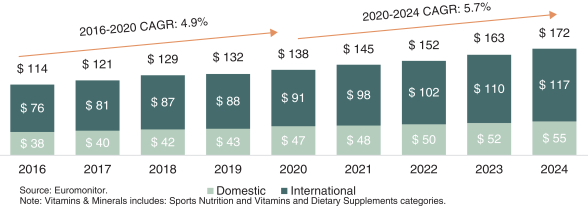
As filed with the Securities and Exchange Commission on July 6, 2021.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Nutrition Topco, LLC
to be converted as described herein to a corporation named
The Better Being Co.
(Exact name of registrant as specified in its charter)
| | | | |
| Delaware | | 2833 | | 82-1823161 |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification No.) |
222 Main Street, Suite 1600
Salt Lake City, Utah 84101
Telephone: (435) 655-6000
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Monty Sharma
Nutrition Topco, LLC
Chief Executive Officer
222 Main Street, Suite 1600
Salt Lake City, Utah 84101
(435) 655-6000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of all communications, including communications sent to agent for service, should be sent to:
| | |
Robert M. Hayward, P.C. Robert E. Goedert, P.C. Alexander M. Schwartz
Kirkland & Ellis LLP
300 North LaSalle
Chicago, Illinois 60654
(312) 862-2000 | | Ian D. Schuman Stelios G. Saffos Scott W. Westhoff Latham & Watkins LLP 1271 Avenue of the Americas New York, New York 10020 (212) 906-1200 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☐
If this Form is filed to registered additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | |
| Large accelerated filer | | ☐ | | Accelerated Filer | | ☐ |
| | | |
| Non-accelerated filer | | ☐ | | Smaller Reporting Company | | ☐ |
| | | |
| | | | Emerging Growth Company | | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
| | | | |
|
Title of Each Class of
Securities to be Registered | | Proposed Maximum Aggregate
Offering Price(1)(2) | | Amount of Registration Fee |
Common Stock, par value $0.001 per share | | $100,000,000 | | $10,910 |
|
|
| (1) | Includes the aggregate offering price of shares of common stock subject to the underwriters’ option to purchase additional shares. |
| (2) | Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.