
C O R P O R A T E P R E S E N T A T I O N | O C T O B E R 2 0 2 1 Saving Lives Through Innovative Molecular Diagnostic Solutions Filed Pursuant to Rule 433 of the Securities Act of 1933 Issuer Free Writing Prospectus dated October 26, 2021 Relating to Preliminary Prospectus dated October 26, 2021 Registration No. 333-260176

A D V I S O R Y Filed pursuant to Rule 433 of the Securities Act of 1933 , as amended . This free writing prospectus related to the proposed initial public offering of ordinary shares of Mainz Biomed N . V . (“Mainz” or the “Company”), which are being registered on a registration statement (including a prospectus) on Form F - 1 /A (the “Registration Statement”) . The Registration Statement has not yet been declared effective . Before you invest, you should read the prospectus in the Registration Statement (including the risk factors described therein) and other documents Mainz has filed with the United States Securities and Exchange Commission (“SEC”) for more complete information about Mainz and the proposed offering . You may get these documents for free by visiting EDGAR on the SEC web site at www . sec . gov . Alternatively, Mainz and any underwriter or dealer participating in the offering will arrange to send you the prospectus if you request it by calling Boustead Securities, LLC at 949 . 502 . 4408 or by email at offerings@boustead 1828 . com or standard mail at Boustead Securities, LLC, Attn : Equity Capital Markets, 6 Venture, Suite 395 , Irvine, CA 92618 , USA . This document contains forward - looking statements . In addition, from time to time, we or our representatives may make forward - looking statements orally or in writing . We base these forward - looking statements on our expectations and projections about future events, which we derive from the information currently available to us . Such forward - looking statements relate to future events or our future performance, including : our financial performance and projections ; our growth in revenue and earnings ; and our business prospects and opportunities . You can identify forward - looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms . In evaluating these forward - looking statements, you should consider various factors, including : our ability to change the direction of the Company ; our ability to keep pace with new technology and changing market needs ; and the competitive environment of our business . These and other factors may cause our actual results to differ materially from any forward - looking statement . Forward - looking statements are only predictions . 2 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. The forward - looking events discussed in this document and other statements made from time to time by us or our representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties and assumptions about us . We are not obligated to publicly update or revise any forward - looking statement, whether as a result of uncertainties and assumptions, the forward - looking events discussed in this document and other statements made from time to time by us or our representatives might not occur . See offering documents for further risks and disclosures . Past performance is not indicative of future results . There is no guarantee that any specific outcome will be achieved . Investments may be speculative, illiquid and there is a total risk of loss . Form CRS/Reg BI Disclaimer : Boustead Securities, LLC is registered with the SEC as a broker - dealer and is a member of the Financial Industry Regulatory Authority (FINRA) and the Securities Investor Protection Corporation (SIPC) . Brokerage and investment advisory services and fees differ, and it is important for you to understand these differences . Free and simple tools are available to research firms and financial professionals at Investor . gov/CRS, which also provides educational materials about broker - dealers, investment advisers, and investing . When we provide you with a recommendation, we have to act in your best interest and not put our interest ahead of yours . At the same time, the way we make money creates a conflict with your interests . Please strive to understand and ask us about these conflicts because they can affect the recommendations we provide you . There are many risks involved with investing . For Boustead Securities, LLC’s customers and clients, please see our ‘Regulation Best Interest Relationship Guide’ on the Form CRS Reg BI page which can be found on our website at https : //www . boustead 1828 . com/form - crs - reg - b i . For FlashFunders’ visitors, you may review the Form CRS of Boustead Securities, LLC under the Form CRS section . Please also carefully review and verify the accuracy of the information you provide us on account applications, subscription documents and others .
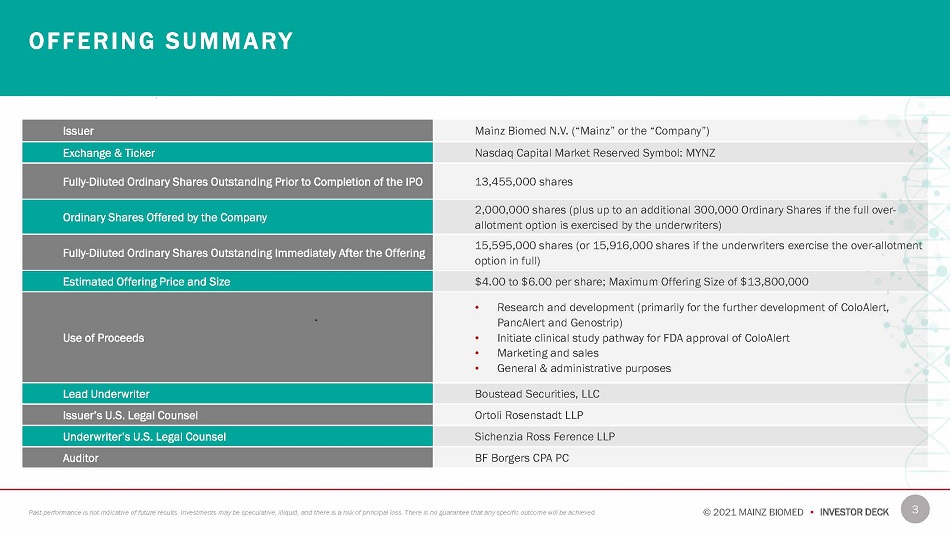
O F F E R I N G S U M M A R Y Issuer Mainz Biomed N.V. (“Mainz” or the “Company”) Exchange & Ticker Nasdaq Capital Market Reserved Symbol: MYNZ Fully - Diluted Ordinary Shares Outstanding Prior to Completion of the IPO 13,455,000 shares Ordinary Shares Offered by the Company 2,000,000 shares (plus up to an additional 300,000 Ordinary Shares if the full over - allotment option is exercised by the underwriters) Fully - Diluted Ordinary Shares Outstanding Immediately After the Offering 15,595,000 shares (or 15,916,000 shares if the underwriters exercise the over - allotment option in full) Estimated Offering Price and Size $4.00 to $6.00 per share; Maximum Offering Size of $13,800,000 Use of Proceeds • Research and development (primarily for the further development of ColoAlert, PancAlert and Genostrip) • Initiate clinical study pathway for FDA approval of ColoAlert • Marketing and sales • General & administrative purposes Lead Underwriter Boustead Securities, LLC Issuer’s U.S. Legal Counsel Ortoli Rosenstadt LLP Underwriter’s U.S. Legal Counsel Sichenzia Ross Ference LLP Auditor BF Borgers CPA PC 3 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved.

4 © 2021 MAINZ BIOMED • INVESTOR DECK A B O U T Mainz BioMed – founded in Germany and aiming to become a leading provider of easy - to - use diagnostic solutions for patients everywhere. Early identification of cancer saves lives. To be effective, sample collection needs to be simple, readily available, and affordable. We develop innovative products and product candidates that quickly and easily identify the early onset of several leading deadly conditions – such as colorectal (“CRC”) and pancreatic cancer. Our goal is to place effective solutions where they need to be… In your hands. Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved.
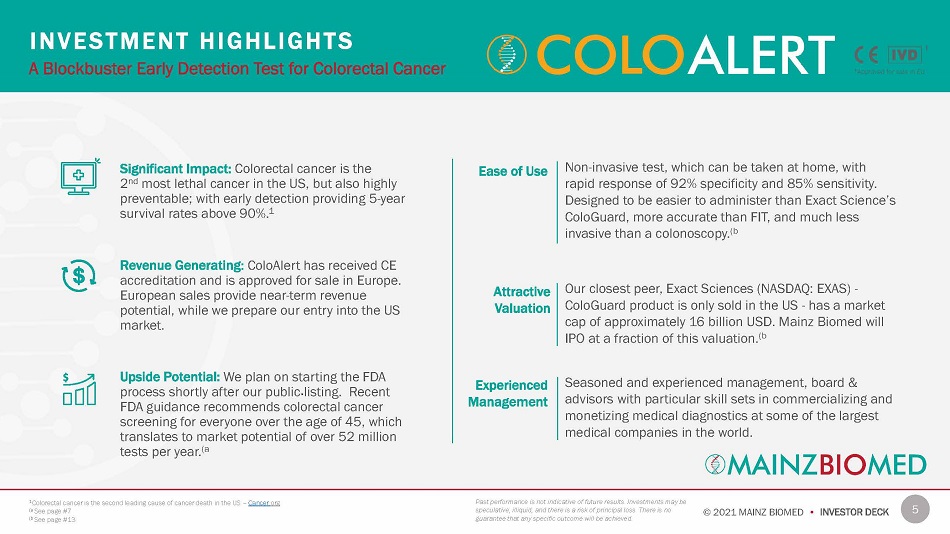
5 © 2021 MAINZ BIOMED • INVESTOR DECK Significant Impact: Colorectal cancer is the 2 nd most lethal cancer in the US, but also highly preventable; with early detection providing 5 - year survival rates above 90%. 1 Revenue Generating: ColoAlert has received CE accreditation and is approved for sale in Europe. European sales provide near - term revenue potential, while we prepare our entry into the US market. Upside Potential: We plan on starting the FDA process shortly after our public listing. Recent FDA guidance recommends colorectal cancer screening for everyone over the age of 45, which translates to market potential of over 52 million tests per year. (a I N V E S T ME N T H I G H L I GH T S A Blockbuster Early Detection Test for Colorectal Cancer Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. 1 Colorectal cancer is the second leading cause of cancer death in the US – Cancer . org (a See page #7 (b See page #13 † † Approved for sale in EU Non - invasive test, which can be taken at home, with rapid response of 92% specificity and 85% sensitivity. Designed to be easier to administer than Exact Science’s ColoGuard, more accurate than FIT, and much less invasive than a colonoscopy. (b Our closest peer, Exact Sciences (NASDAQ: EXAS) - ColoGuard product is only sold in the US - has a market cap of approximately 16 billion USD. Mainz Biomed will IPO at a fraction of this valuation. (b Seasoned and experienced management, board & advisors with particular skill sets in commercializing and monetizing medical diagnostics at some of the largest medical companies in the world. Ease of Use At t rac t iv e V alu a tion Experienced M ana g e m ent

6 © 2021 MAINZ BIOMED • INVESTOR DECK Clinical laboratory tests save costs and lives by enabling early detection and prevention of disease . Patients with cancers and other conditions are living longer and enjoying better health because of medical revolutions in diagnostic technology . At its center are genetic and genomic tests that identify the unique genetic profile of individual patients or their disease and allow physicians to tailor treatment to those unique characteristics . D I A G N O S T I C O P P O RTU N I T Y Accurate, Non - Invasive, Simple, Safe Diagnostic Solution to Increase Patient Compliance Mainz BioMed develops market - ready molecular genetic diagnostic solutions for life threatening conditions. ColoAlert detects tumors better than the Fecal Immunochemical Test (FIT)* o Higher patient acceptance than FIT test.* o Accurate, non - invasive, simple, safe diagnostic solution to increase patient compliance. o Proposed partnerships with clinical labs aims to speed test collection & results. o Designed to offer significant advantages compared to our major competitor (“ColoGuard”), manufactured by Exact Sciences Corp. Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. Co l or ec t al Ca ncer is the #2nd leading killer (Men & women combined) 1 5 - year survival rate >90% (If detected at stage A). 2 52,980 d e at hs due to CRC in USA (2021) 3 * Dollinger MM et al. (2018), ClinLab 64 (10), 1719 - 1730; internal data comparing ColoAlert to FIT. NOTE: the globally most - used non - invasive test for screening programs. Fecal immunochemical test (FIT) is a screening test for hidden blood in the stool, which can be an early sign of cancer. FIT only detects human blood from the lower intestines. Blood in stool is often a late - stage sign. 1 Colorectal cancer is the second leading cause of cancer death in the US – Cancer.org 2 5 - Year Survival rate 91% at Stage A – 2018 American Cancer Society Report 3 Predicted deaths from CRC in USA – Cancer.org . † † Approved for sale in EU
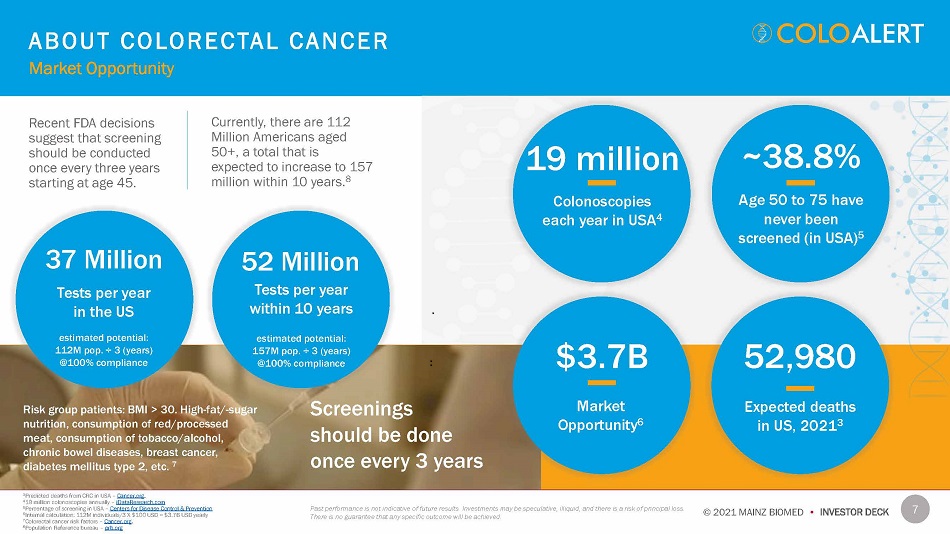
7 © 2021 MAINZ BIOMED • INVESTOR DECK Recent FDA decisions suggest that screening should be conducted once every three years starting at age 45. Currently, there are 112 Million Americans aged 50+, a total that is expected to increase to 157 million within 10 years. 8 19 million Colonoscopies each year in USA 4 ~38.8% Age 50 to 75 have never been screened (in USA) 5 $3.7B Market Oppo r tu ni t y 6 52,980 Expected deaths in US, 2021 3 37 Million Tests per year in the US estimated potential: 112M pop. ¸ 3 (years) @100% compliance 52 Million Tests per year within 10 years estimated potential: 157M pop. ¸ 3 (years) @100% compliance Screenings should be done once every 3 years Risk group patients: BMI > 30. High - fat/ - sugar nutrition, consumption of red/processed meat, consumption of tobacco/alcohol, chronic bowel diseases, breast cancer, diabetes mellitus type 2, etc. 7 A B O U T C O L ORE C T A L C A N CE R Market Opportunity Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. 3 Predicted deaths from CRC in USA – Cancer.org . 4 19 million colonoscopies annually – iDataResearch.com 5 Percentage of screening in USA – Centers for Disease Control & Prevention 6 Internal calculation: 112M individuals/3 X $100 USD = $3.7B USD yearly 7 Colorectal cancer risk factors – Cancer.org . 8 Population Reference bureau – prb.org

8 © 2021 MAINZ BIOMED • INVESTOR DECK ✓ A PCR - based CRC early detection stool test ✓ Up to 60% fewer missed cases compared to fecal immunochemical test (FIT) 9 ✓ Non - invasive, no preparation or sedation, no time off work ✓ 98% patient satisfaction – Easy product to use 10 ✓ Designed to offer affordable CRC screening solutions N E X T G E N C R C P R EVENT I O N Simple, Fast, Accurate and Non - Invasive Patients receive a simple kit that includes instructions, a stool collector and shipping instructions to return the kit through regular mail to their local lab for testing and results. IT’S THAT EASY. visit www.coloalert.com Europe’s answer to Exact Sciences’ ColoGuard Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. 9 Comparing ColoAlert sensitivity with FITs (Gies et al. Gastroenterology 154/2018) 10 98% overall satisfaction with ColoAlert in our internal customer survey. Approved for sale in the European Union 9

C O M M E R C I A L I Z A T I O N Strategic Roll Out Aiming to become the leading global brand for CRC detection through extensive US and European market partnerships 9 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. The competition’s approach… The largest U.S. provider independently markets and distributes its product directly. All nationwide samples must come from, and be returned to, a single corporate laboratory – a time - consuming process. Mainz BioMed intends to develop and leverage scalable dissemination through a collaborative partner program PARTNERSHIPS: Large lab chains incentivized to support sales & marketing efforts to physician clients & consumers. RELATIONSHIPS: Established regional and national labs offer existing client relationships (i.e.: physicians, clinics, hospitals, universities, government institutions, health organizations, etc). PROFITABILITY: ColoAlert is designed for profitability, rapid commercial uptake, and broad consumer acceptance. PROTECTION: Mainz BioMed protects its intellectual property through trade secrets to control all critical reagents, processes and formulations.
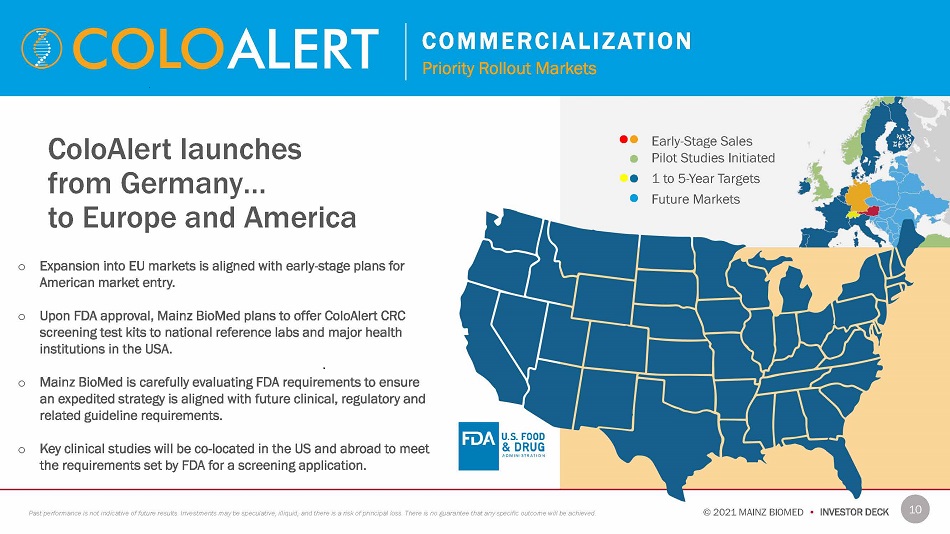
C O M M E R C I A L I Z A T I O N Priority Rollout Markets ColoAlert launches from Germany… to Europe and America o Expansion into EU markets is aligned with early - stage plans for American market entry . o Upon FDA approval, Mainz BioMed plans to offer ColoAlert CRC screening test kits to national reference labs and major health institutions in the USA . o Mainz BioMed is carefully evaluating FDA requirements to ensure an expedited strategy is aligned with future clinical, regulatory and related guideline requirements. o Key clinical studies will be co - located in the US and abroad to meet the requirements set by FDA for a screening application. Early - Stage Sales Pilot Studies Initiated 1 to 5 - Year Targets Future Markets 10 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved.
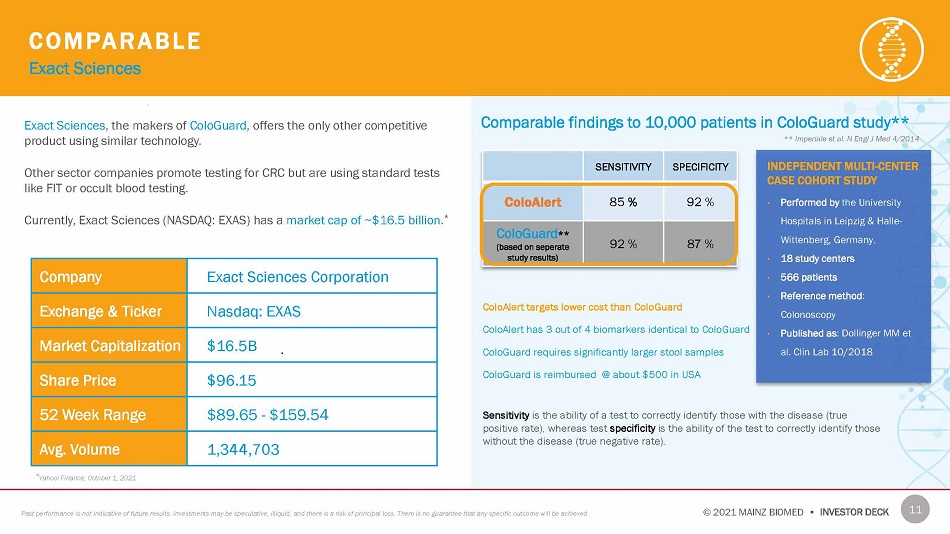
C O M P AR A B L E Exact Sciences * Yahoo! Finance, October 1, 2021 Exact Sciences , the makers of ColoGuard , offers the only other competitive product using similar technology. Other sector companies promote testing for CRC but are using standard tests like FIT or occult blood testing. Currently, Exact Sciences (NASDAQ: EXAS) has a market cap of ~$16.5 billion . * SENSITIVITY SPECIFICITY ColoAlert 85 % 92 % ColoGuard ** (based on seperate study results) 92 % 87 % ColoAlert targets lower cost than ColoGuard ColoAlert has 3 out of 4 biomarkers identical to ColoGuard ColoGuard requires significantly larger stool samples ColoGuard is reimbursed @ about $500 in USA 11 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. INDEPENDENT MULTI - CENTER CASE COHORT STUDY • Performed by the University Hospitals in Leipzig & Halle - Wittenberg, Germany . • 18 study centers • 566 patients • Ref er e n ce me t ho d : Colonoscopy • Published as : Dollinger MM et al. Clin Lab 10/2018 Comparable findings to 10,000 patients in ColoGuard study** ** Imperiale et al. N Engl J Med 4/2014 Sensitivity is the ability of a test to correctly identify those with the disease (true positive rate), whereas test specificity is the ability of the test to correctly identify those without the disease (true negative rate). Company Exact Sciences Corporation Exchange & Ticker Nasdaq: EXAS Market Capitalization $16.5B Share Price $96.15 52 Week Range $89.65 - $159.54 Avg. Volume 1,344,703

THE WORLD'S FIRST EARLY DETECTION PANCREATIC CANCER SCREENING TEST based on real - time PCR - based detection of biomarkers in stool samples. M A R K E T O P P O RTU N I T Y Commercial Pipeline Development – Future Product E A R L Y D E C T E C T I O N o As GenX individuals age into their 40's and 50's they become part of the age group recommended to begin testing for CRC and more. 12 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. o Mainz BioMed is currently developing proprietary genetic testing methods for pancreatic cancer. o Fighting what could soon become the world’s second most deadly cancer.* o Convenient stool test for at - home use. o Potential for over 50 million tests per year in Europe alone o Supported by federal grant from Germany’s Federal Ministry for Education and Research. o Cost of goods sold (COGS) & reimbursement analogous to ColoAlert program. *https:// www.pancan.org/press - releases/pancreatic - cancer - still - on - path - to - become - second - leading - cause - of - cancer - related - death - in - u - s - by - 2 020/

C O M P A N Y P R O F I L E Management Team Guido Baechler CEO & Director • 30 years of global experience leading public & private life science companies with sustained revenue and EBITDA growth. • Founded Berkeley Life science Advisors, a diagnostic and life science start - up consulting firm. • Former CEO at SummerBio, a leading high throughput COVID testing laboratory. • Former President & CEO at Singulex, Inc . Alameda California – raised over $ 180 M through multiple financings . • Former Roche molecular diagnostics executive, leading global program management. 13 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. William Caragol CFO • 30 years of experience working with growth stage technology companies. • Ex - Board Chair of Thermomedics, Inc., a privately held medical diagnostic equipment company. • Director & Audit Committee Chair at Greenbox POS (NASDAQ: GBOX) a financial tech company. • Former Chairman & CEO of PositiveID, a holding company in the fields of bio detection systems and molecular diagnostics. • Served as Director, Executive VP, COO and CFO of Hawaiian Springs LLC natural spring artesian bottled water company. • Member of the American Institute of Certified Public Accountants.
S U M M A R Y ColoAlert Holds Potential as a Blockbuster Early Detection Test for Colorectal Cancer. 14 © 2021 MAINZ BIOMED • INVESTOR DECK Past performance is not indicative of future results. Investments may be speculative, illiquid, and there is a risk of principal loss. There is no guarantee that any specific outcome will be achieved. Mainz biomed aims to become a leader in the multi - billion - dollar colorectal cancer diagnostics market This is Europe’s answer to Exact Sciences’ (~$16 billion market cap) ColoGuard product . Designed for profitability, rapid commercial uptake, and broad consumer acceptance. Mainz BioMed protects its intellectual property through trade secrets to control all critical reagents, processes and formulations. Mainz BioMed is developing proprietary genetic testing methods for pancreatic cancer.

C O R P O R A T E P R E S E N T A T I O N | O C T O B E R 2 0 2 1 T H AN K Y O U Mainz Biomed B.V. Robert - Koch - Straße 50 55129 Mainz, Germany Tel: +49 (0) 6131 / 55428 - 68 Inquiries: info@mainzbiomed.com














