| PROSPECTUS | Filed pursuant to Rule 424(b)(3) |
| | Registration No. 333 - 284051 |

5,280,000 Ordinary Shares
Up to 3,520,000 Warrants to Purchase up to 3,520,000 Ordinary Shares
Up to 1,656,271 Pre-Funded Warrants to Purchase up to 1,656,271 Ordinary Shares
This prospectus relates to the resale, from time to time, by the selling shareholder identified in this prospectus under the section “Selling Shareholder,” (the “selling shareholder”) of NeuroSense Therapeutics Ltd. (“NeuroSense,” “we,” “us” or the “Company”) of (i) 103,729 of our ordinary shares, no par value per share (the “Shares”) issued to the selling shareholder under a securities purchase agreement, dated December 2, 2024 (the “Purchase Agreement”), by and between us and the purchasers named therein, (ii) up to 3,520,000 ordinary shares issuable to the selling shareholder upon exercise of warrants to purchase ordinary shares at an exercise price of $1.25 per ordinary share (the “Ordinary Warrants”), (iii) 3,520,000 Ordinary Warrants issued to the selling shareholder, (iv) up to 1,656,271 ordinary shares, issuable to the selling shareholder upon exercise of pre-funded warrants to purchase Ordinary Shares at an exercise price of $0.0001 per ordinary share (the “Pre-Funded Warrants” and together with the Ordinary Warrants, the “Warrants”), (v) 1,656,271 Pre-Funded Warrants. The Shares, the Prefunded Warrants, the ordinary shares underlying Prefunded Warrants (the “PFW Shares”), the Warrants and the ordinary shares underlying the Warrants (the “Ordinary Warrant Shares” and together with the “PFW Shares”, the “Warrant Shares”)) were offered pursuant to the exemptions provided in Section 4(a)(2) under the Securities Act of 1933, as amended (the “Securities Act”), and Regulation D promulgated thereunder.
The selling shareholder will receive all of the proceeds from any sales of the resale of Shares, Warrants and Warrant Shares offered hereby. We will not receive any of the proceeds, but we will incur expenses in connection with such offering. To the extent the Warrants are exercised for cash, if at all, we will receive the exercise price of the Warrants.
The selling shareholder may sell the Shares, the Warrants, and the Warrant Shares covered by this prospectus through public or private transactions at market prices prevailing at the time of sale, at negotiated prices or such other prices as such selling shareholder may determine. The timing and amount of any sale are within the sole discretion of such selling shareholder. Our registration of the Shares, the Warrants, and the Warrant Shares for resale covered by this prospectus does not mean that the selling shareholder will offer or sell any of the Shares, the Warrants, and the Warrant Shares,. For further information regarding the possible methods by which the Shares, the Warrants and the Warrant Shares may be distributed, see “Plan of Distribution.”
We are an “emerging growth company” and a “foreign private issuer”, each as defined under federal securities laws, and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary — Implications of Being an Emerging Growth Company” and “Prospectus Summary — Implications of Being a Foreign Private Issuer” for additional information.
Our ordinary shares and warrants to purchase our ordinary shares are listed on The Nasdaq Capital Market (“Nasdaq”) under the symbols “NRSN” and “NRSNW.” On December 24, 2024, the closing price for our ordinary shares of was $1.28 and the closing price for the warrants to purchase our ordinary shares was $0.46.
Investing in our securities is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 8 to read about factors you should consider before buying the Shares or the Warrants.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of the disclosures in this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is January 16, 2025
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of the registration statement on Form F-3 that we filed with the Securities and Exchange Commission (the “SEC”) for the offering of the Shares, the Warrants and the Warrant Shares by the selling shareholder.
You should not assume that the information contained in, or incorporated by reference into, this prospectus is accurate on any date subsequent to the date set forth on the front cover of this prospectus, even though this prospectus is delivered or the Shares, the Warrants, the Warrant Shares, the Pre-Funded Warrants and PFW Shares covered by this prospectus are sold or otherwise disposed of on a later date. It is important for you to read and consider all information contained in, or incorporated by reference into, this prospectus in making your investment decision. You should also read and consider the information in the documents to which we have referred you under the caption “Where You Can Find Additional Information” in this prospectus.
Neither we nor the selling shareholder have authorized anyone to provide any information or to make any representation other than those contained in, or incorporated by reference into, this prospectus. You must not rely upon any information or representation not contained in, or incorporated by reference into, this prospectus. This prospectus does not constitute an offer to sell or the solicitation of an offer to buy any of our securities other than the securities covered hereby, nor does this prospectus constitute an offer to sell or the solicitation of an offer to buy any of our securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
This prospectus, including the information incorporated by reference herein, contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. See “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.”
TRADEMARKS, SERVICE MARKS AND TRADENAMES
The NeuroSense Therapeutics logo and other trademarks and service marks of NeuroSense Therapeutics Ltd. appearing in this prospectus or the information incorporated by reference herein are the property of the Company. Solely for convenience, some of the trademarks, service marks, logos and trade names referred to in this prospectus are presented without the ® and ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks, service marks and trade names. This prospectus, including the information incorporated by reference herein, contains additional trademarks, service marks and trade names of others. All trademarks, service marks and trade names appearing in this prospectus or the information incorporated by reference herein are, to our knowledge, the property of their respective owners. We do not intend our use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
MARKET AND INDUSTRY DATA
This prospectus, including the information incorporated by reference herein, contains industry, market and competitive position data that are based on industry publications and studies conducted by third parties as well as our own internal estimates and research. These industry publications and third-party studies generally state that the information that they contain has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that each of these publications and third-party studies is reliable, we have not independently verified the market and industry data obtained from these third-party sources. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward-looking statements included in, or incorporated by reference into, this prospectus. While we believe our internal research is reliable and the definition of our market and industry are appropriate, neither such research nor these definitions have been verified by any independent source.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and certain information incorporated by reference herein contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and other securities laws. Many of the forward-looking statements contained in, or incorporated by reference into, this prospectus can be identified by the use of forward-looking words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “should,” “target,” “would” and other similar expressions that are predictions of or indicate future events and future trends, although not all forward-looking statements contain these identifying words.
Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements are subject to substantial risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking statements due to a variety of factors, including, but not limited to, those identified under the section titled “Risk Factors” in this prospectus or the documents incorporated herein. These risks and uncertainties include factors relating to:
| | ● | our ability to maintain the listing of our ordinary shares on Nasdaq; |
| | | |
| | ● | the going concern reference in our financial statements and our need for substantial additional financing to achieve our goals; |
| | | |
| | ● | our limited operating history and history of incurring significant losses and negative cash flows since our inception, which we anticipate will continue for the foreseeable future; |
| | ● | our dependence on the success of our lead product candidate, PrimeC, including our obtaining of regulatory approval to market PrimeC in the United States (“U.S.”); |
| | ● | our limited experience in conducting clinical trials and reliance on clinical research organizations and others to conduct them; |
| | ● | our ability to advance our preclinical product candidates into clinical development and through regulatory approval; |
| | ● | the results of our clinical trials, which may fail to adequately demonstrate the safety and efficacy of our product candidates; |
| | ● | our ability to achieve the broad degree of physician adoption and use and market acceptance necessary for commercial success; |
| | ● | our reliance on third parties in marketing, producing or distributing products and research materials for certain raw materials, compounds and components necessary to produce PrimeC for clinical trials and to support commercial scale production of PrimeC, if approved; |
| | ● | our receipt of regulatory clarity and approvals for our therapeutic candidates and the timing of other regulatory filings and approvals; |
| | ● | estimates of our expenses, revenues, capital requirements and our needs for additional financing; |
| | ● | our efforts to obtain, protect or enforce our patents and other intellectual property rights related to our product candidates and technologies; |
| | ● | the impact of the public health, political and security situation in Israel, the U.S. and other countries in which we may obtain approvals for our products or our business; |
| | ● | the impacts on our ongoing and planned trials and manufacturing as a result of the war in Israel; and |
| | ● | those factors referred to in our most recent Annual Report on Form 20-F incorporated by reference herein in “Item 3. Key Information — D. Risk Factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects,” as well as in our most recent Annual Report on Form 20-F generally, which is incorporated by reference into this prospectus. |
The preceding list is not intended to be an exhaustive list of all of our risks and uncertainties. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus or the documents incorporated herein or therein will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus or any document incorporated herein or therein, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all relevant information.
Unless otherwise required by law, we do not undertake any obligation to update them in light of new information or future developments or to release publicly any revisions to these statements in order to reflect later events or circumstances or to reflect the occurrence of unanticipated events.
You should read this prospectus, the documents incorporated herein and therein, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in or incorporated by reference into this prospectus that we consider important. This summary does not contain all of the information you should consider before investing in our securities. Before you decide to invest in our securities, you should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and related notes incorporated by reference into this prospectus and the other documents incorporated by reference into this prospectus, which are described under “Incorporation by Reference” before making an investment in our securities.
Company Overview
We are a clinical-stage biotechnology company focused on discovering and developing treatments for people living with neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD) and Parkinson’s disease (PD). We believe these diseases represent some of the most significant unmet medical needs of our time, with limited effective therapeutic options available. The burden of these diseases on both patients and society is substantial. For example, the average annual cost of ALS alone is $180,000 per patient, and its estimated annual burden on the U.S. healthcare system is greater than $1 billion. Due to the complexity of neurodegenerative diseases, our strategy is utilizing a combined therapeutic approach to target multiple disease-related pathways.
Our lead therapeutic candidate, PrimeC, is a novel extended-release oral formulation, fixed-dose combination of two FDA-approved drugs, ciprofloxacin and celecoxib. PrimeC is designed to treat ALS by modulating microRNA synthesis, iron accumulation, and neuroinflammation, all of which are hallmarks of ALS pathology. The U.S. Food and Drug Administration, or the FDA and the European Medicines Agency, or the EMA have granted PrimeC orphan drug designation for the treatment of ALS. In addition, the EMA has granted PrimeC the Small and Medium-Sized Enterprise, or SME, status, which offers significant potential benefits leading up to and following drug regulatory approval. We believe PrimeC’s multifunctional mechanism of action has the potential to significantly prolong lifespan and improve ALS patients’ quality of life, thereby reducing the burden of this debilitating disease on both patients and healthcare systems.
PrimeC was evaluated in PARADIGM, a Phase IIb randomized, multi-center, multinational, prospective, double-blind, placebo-controlled study, to evaluate safety, tolerability, and efficacy of PrimeC in 68 people living with ALS. Participants were being administered PrimeC or placebo at a 2:1 ratio, respectively, for the six-month double-blind part. Study participants were allowed to continue standard of care treatment of approved products. The primary endpoints of the study are an evaluation of ALS-biomarkers as well as safety and tolerability assessment. Secondary and exploratory endpoints are the evaluation of clinical efficacy (ALS Functional Rating Scale — Revised, or ALSFRS-R, and slow vital capacity), survival, and improvement in quality of life. All subjects who completed the six-month double-blind, placebo-controlled dosing period had the opportunity to be transferred to the PrimeC active arm for a 12-month open label extension. The study completed enrollment in May 2023, enrolling 69 participants, in which 68 are living with ALS and one participant who was misdiagnosed for ALS and was excluded from the evaluations. Four ALS clinical centers participated in the study in 3 territories: Israel, Italy, and Canada. In December 2023, we reported that we met the primary safety and tolerability endpoints and achieved secondary clinical efficacy endpoints in the top-line results of our 6-month double-blind phase of PARADIGM. In May 2024, we announced new positive data analysis from PARADIGM clinical trial demonstrating statistically significant slowing of disease progression in high-risk ALS patients. In July 2024, we announced results from the 12-month analysis of the PARADIGM clinical trial which showed a significant improvement in the rate of decline of ALS Functional Rating Scale-Revised (ALSFRS-R) scores and survival rates for subjects who received PrimeC from the start of the trial compared to those who started on placebo. In August 2024, we announced positive 12-month biomarker data from the PARADIGM clinical trial, which showed a significant decrease in ferritin levels and a corresponding increase in transferrin levels, both indicating alleviation of the pathology. In October 2024, we completed the full 18-month dosing in PARADIGM and in December 2024 we announced results from the 18-month analysis of the PARADIGM clinical trial which showed statistically significant positive results from the 18-month data analysis of the PARADIGM study, evaluating the efficacy of PrimeC in the treatment of ALS.
Following the FDA’s recommendation for additional non-clinical data to support long term use of Ciprofloxacin (as PrimeC is intended for long-term administration in treating ALS) a long-term tox study was initiated. In September 2024, we announced the successful completion of the in-life phase of the study, as we move towards the initiation of a Phase 3 study in the US. We had a Type C meeting with the FDA in November 2024 and plan to meet with the EMA in the first quarter of 2025 and to subsequently commence a pivotal Phase III clinical trial for PrimeC in ALS treatment in the first half of 2025. Additionally, in November 2023, we concluded a successful Type D meeting with the FDA regarding CMC development plans for the expected Phase 3 pivotal study and subsequent marketing approval. The FDA endorsed our proposed CMC development plan.
In December 2024, we announced the outcome of the Type C meeting held with the FDA in November 2024. The purpose of the meeting was to discuss the design of a proposed Phase 3 clinical study and the plan for submission of an eventual 505(b)(2) marketing application. We had a productive discussion with the FDA regarding the design of the planned Phase 3 pivotal study with PrimeC, including efficacy and safety measurements. In light of the FDA’s positive feedback, we plan to submit a final protocol to the FDA during the first half of 2025 with the aim of commencing enrollment of the pivotal Phase 3 study in mid-2025, which would include approximately 300 patients divided by a ratio of 2:1, PrimeC to placebo. The Phase 3 study is expected to be a randomized, multi-center, multinational, prospective, double-blind, placebo-controlled study, with an open label extension (OLE), to evaluate the efficacy and safety of PrimeC in people living with ALS. Following 12 months of treatment, it is expected that all participants will transition to PrimeC for a 12-month OLE.
PrimeC was previously evaluated in a Phase IIa clinical trial, or NST002, in 15 people living with ALS, conducted at the Tel Aviv Sourasky Medical Center, Israel. The primary endpoint of the NST002 trial, which was safety and tolerability, was met. In this trial, the safety profile observed was consistent with known safety profiles of ciprofloxacin and celecoxib. Side effects were mild and transient in nature. There were no new or unexpected safety signals detected during the trial.
Additionally, we observed positive clinical signals in comparison to virtual controls, and a serum biomarker analysis showed significant changes following treatment, indicating biological activity of the drug in comparison to untreated matched ALS patients. All 12 patients who completed the NST002 trial elected to continue into an extension study with PrimeC, that was conducted as an Investigator Initiated Study. To date, we are still supporting the drug supply for a few of the participants in this study, which is over than 40 months since NST002 was initiated.
We completed three additional studies in 2022 as part of our drug development program to further support our future regulatory submissions. In April 2022, we initiated a pharmacokinetic, or PK, study, or NCT05232461, of PrimeC. The PK open-label, randomized, single-dose, three-treatment, three-period crossover study evaluated the effect of food on the bioavailability of PrimeC as compared to the bioavailability of co-administered ciprofloxacin tablets and celecoxib capsules in adult subjects in the U.S. under an FDA cleared IND protocol.
In August 2022, we completed enrollment and dosing of all subjects in a multi-dose PK study, or NCT05436678. On September 28, 2022, we released the results of the NCT05436678 study. Based on results, we believe the PK profile of PrimeC supports the formulation’s extended-release properties, as the concentrations of the active components have been synchronized, aiming to potentially maximize the synergism between the two compounds. In June 2022, we reported the successful completion of the “in-life” phase of its 90-day GLP toxicology study. In this study, the components of PrimeC, celecoxib and ciprofloxacin, were administered to rodents at doses 4x the maximal clinical dose. All animals appeared normal, with no significant findings observed. We intend to present the data from these studies to the FDA as part of PrimeC’s drug development plan.
We believe we have a strong patent estate, including patents on method of use, combination, and formulation. We have secured U.S. Patent 10,980,780 relating to methods for treatment of ALS using ciprofloxacin and celecoxib, the components of PrimeC, which expires in 2038. Equivalent patents also have been issued in the European Patent Office, Canada, Australia, Israel and Japan. The patent estate also includes US Patent Application 18/047,289, which relates to Prime C formulations. This application was allowed by the United States Patent and Trademark Office in June 2024, and once granted, will expire in October 2042. Equivalent applications are pending in many jurisdictions worldwide. We also expect to take advantage of orphan drug exclusivity for PrimeC, if approved, for seven years in the United States and ten years in the European Union. In addition, U.S. patent application 16/623,467, which relates to methods of treatment of neurodegenerative disease using combinations of ciprofloxacin and celecoxib, is currently pending. This patent application is expected to expire on June 20, 2038.
Our organization is built around a management team with extensive experience in the pharmaceutical industry, with a particular focus on ALS research and clinical trials. We believe that our leadership team is well-positioned to lead us through clinical development, regulatory approval and commercialization of our product candidates. Furthermore, we maintain steadfast and extensive communication and collaboration with patient advocacy groups and associations, underscoring the importance of patient perspectives in advancing therapeutic strategies.
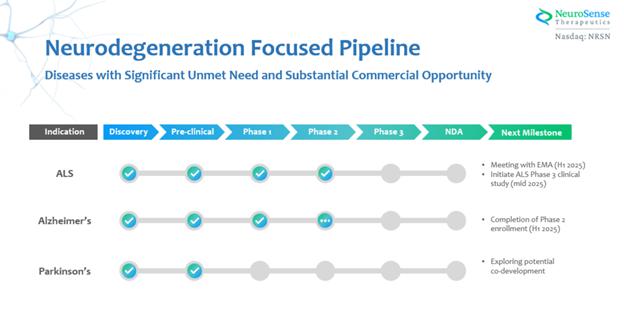
In addition to PrimeC, we extended our pipeline and conducted research and development efforts for AD and PD, with a similar strategy of combined products. The following chart represents our current product development pipeline:
Recent Developments
April 2024 Registered Direct Offering
On April 10, 2024, we entered into a securities purchase agreement pursuant to which we issued and sold to an institutional purchaser in a registered direct offering, (i) an aggregate of 1,732,000 ordinary shares, at an offering price of $1.50 per share; and (ii) an aggregate of 1,248,000 pre-funded warrants, each representing the right to acquire one ordinary share at an offering price of $1.4999 per pre-funded warrant, for gross proceeds of approximately $4.47 million before deducting the placement agent fee and related offering expenses, which included in part the placement agent shares. Each pre-funded warrant represented the right to purchase one ordinary share at an exercise price of $0.0001 per share and were exercised in full. In a concurrent private placement, pursuant to the purchase agreement, we issued and sold to such purchaser warrants to purchase up to an aggregate of 2,980,000 ordinary shares, which are exercisable immediately upon issuance at an exercise price of $1.50 per ordinary share and will expire on the fifth anniversary of the original issuance date.
August 2024 Private Placement
On August 6, 2024, we entered into a securities purchase agreement with certain investors, which include members of our senior management and existing investors, pursuant to which we issued and sold in a private placement offering an aggregate of 800,000 ordinary shares and warrants to purchase an aggregate of 800,000 ordinary shares at a combined purchase price of $0.75 per share and warrant. Each warrant is exercisable immediately upon issuance at an exercise price of $0.75 per ordinary share and will expire on the fifth anniversary of the original issuance date.
August 2024 ATM
On August 16, 2024, we entered into a Capital on Demand™ Sales Agreement (the “Sales Agreement”) with JonesTrading Institutional Services LLC, as sales agent (the “Sales Agent”), pursuant to which we may offer and sell, from time to time, to or through the Sales Agent, ordinary shares having an aggregate offering price of up to gross sale proceeds of up to $2,524,437.
Standby Equity Purchase Agreement with YA
On October 31, 2024, we entered into the Standby Equity Purchase Agreement (“Standby Equity Purchase Agreement”) with YA II PN, LTD., a Cayman Islands exempt limited partnership (“YA”). Pursuant to the Standby Equity Purchase Agreement, the Company has the right, but not the obligation, to sell to YA from time to time (each such occurrence, an “Advance”) up to $30.0 million (the “Commitment Amount”) of our ordinary shares during the 36 months following the execution of the Purchase Agreement, subject to the restrictions and satisfaction of the conditions in the Standby Equity Purchase Agreement. At our option, the ordinary shares would be purchased by YA from time to time at a price equal to 97% of the lowest of the three daily VWAPs during a three consecutive trading day period commencing on the date that we, subject to certain limitations, deliver a notice to YA that the Company is committing Yorkville to purchase such ordinary shares (the “Advance Shares”). We may also specify a certain minimum acceptable price per share in each Advance. “VWAP” means, for any trading day, the daily volume weighted average price of our ordinary shares for such trading day on the Nasdaq Stock Market during regular trading hours as reported by Bloomberg L.P. As consideration for YA’s irrevocable commitment to purchase our ordinary shares up to the Commitment Amount, we issued 224,697 ordinary shares (the “Commitment Shares”) to YA and also paid a $25,000 structuring fee to YA.
Pursuant to the Standby Equity Purchase Agreement, YA shall not be obligated to purchase or acquire any ordinary shares under the Standby Equity Purchase Agreement which, when aggregated with all other ordinary shares beneficially owned by YA and its affiliates, would result in the beneficial ownership of YA and its affiliates (on an aggregated basis) to exceed 4.99% of the then outstanding voting power or number of our ordinary shares.
December 2024 Private Placement
On December 2, 2024, we entered into the Purchase Agreement with the selling shareholder and Alon Ben-Noon, the Company’s Chief Executive Officer pursuant to which we agreed to issue and sell, in a private placement (the “Offering”) by us directly to the purchasers (i) 2,343,729 Shares; (ii) Warrants exercisable for an aggregate of 8,000,000 ordinary shares at an exercise price of $1.25 per share; and (iii) Pre-Funded Warrants exercisable for an aggregate of 1,656,271 ordinary shares at an exercise price of $0.0001 per share. The purchase price of each Share and accompanying two Warrants was $1.25, and the purchase price of each Pre-Funded Warrant and accompanying two Warrants was $1.2499. The gross proceeds to us from the offering were $5.0 million, before deducting offering expenses payable by us.
The Warrants are exercisable immediately upon issuance at an exercise price of $1.25 per share and expire five years from the date of issuance. The Pre-Funded Warrants are exercisable immediately upon issuance at an exercise price of $0.0001 per share and may be exercised at any time until the pre-funded warrants are exercised in full. A holder of pre-funded warrants or Warrants (together with its affiliates) may not exercise any portion of such warrants to the extent that the holder would own more than 9.99% of our outstanding ordinary shares immediately after exercise.
Pursuant to the terms of the Purchase Agreement, on December 5, 2024 we issued to the purchasers the subscribed for Shares and Warrants, as applicable against payment for $2.8 million. On December 17, 2024, we received the remaining proceeds of $2.2 million.
Pursuant to the terms of the Purchase Agreement, we registered for resale on the registration statement on Form F-3 filed with the Securities Exchange Commission on December 6, 2024 (File No. 333-283656) (i) 2,200,000 Shares issued to the selling shareholder, (ii) up to 4,400,000 ordinary shares issuable to the selling shareholder upon exercise of the Warrants, and (iii) 4,400,000 Warrants issued to the selling shareholder, which are the securities attributable to the payment made by the selling shareholder to us on December 5, 2024.
Pursuant to the terms of the Purchase Agreement, we are registering for resale on this registration statement on Form F-3 (i) 103,729 Shares issued to the selling shareholder, (ii) up to 3,520,000 ordinary shares issuable to the selling shareholder upon exercise of the Ordinary Warrants (iii) 3,520,000 Ordinary Warrants issued to the selling shareholder, (iv) up to 1,656,271 ordinary shares, issuable to the selling shareholder upon exercise of the Pre-Funded Warrants and (v) 1,656,271 Pre-Funded Warrants issuable to the selling shareholder, which are the securities attributable to the payment made by the selling shareholder to us on December 17, 2024.
Nasdaq Non-Compliance
To continue to be listed on Nasdaq, we need to satisfy a number of conditions, including a minimum closing bid price per share of $1.00 for 30 consecutive business days and shareholders’ equity of at least $2.5 million. On June 21, 2024, we received a notification letter from Nasdaq that we had not regained compliance with Nasdaq’s Listing Rule 5550(b) (the “Minimum Equity Rule”) due to our stockholders’ equity falling below the required minimum of $2,500,000. We promptly requested a hearing before the Nasdaq Hearings Panel (the “Panel”), which was held on August 1, 2024, where we presented a comprehensive plan to regain compliance. On August 25, 2024, we received a written notice from Nasdaq that the Panel granted our request for an exception to continue its listing on Nasdaq until October 31, 2024. On November 11, 2024, we received a written notice from Nasdaq stating that the Panel had granted our request for continued listing on Nasdaq, subject to our filing a public disclosure on or before December 18, 2024, describing the transactions we had undertaken to achieve compliance and demonstrate long-term compliance with Minimum Equity Rule and providing an indication of our equity following those transactions and the provision of income projections to Nasdaq. This extension allowed us additional time to demonstrate compliance with the Minimum Equity Rule and meet the required conditions. On December 17, 2024, we filed a Current Report on Form 6-K where we stated that we as of such date, we believe we have shareholders’ equity of $3 million, which is above the $2.5 million required by the Minimum Equity Rule. We are currently awaiting Nasdaq’s confirmation that we have evidenced compliance with the Minimum Equity Rule and that the matter has been closed.
Separately, on August 26, 2024, we received a notification letter from Nasdaq notifying us that we are not in compliance with the minimum bid price requirement set forth in Nasdaq Listing Rules for continued listing on Nasdaq, since the closing bid price for the Company’s ordinary shares listed on Nasdaq was below $1.00 for 30 consecutive trading days. Nasdaq Listing Rule 5550(a)(2) requires listed securities to maintain a minimum bid price of $1.00 per share for 30 consecutive business days (the “Minimum Bid Price Rule”), and Nasdaq Listing Rule 5810(c)(3)(A) provides that a failure to meet the minimum bid price requirement exists if the deficiency continues for a period of 30 consecutive business days. In accordance with Listing Rule 5810(c)(3)(A), we had a period of 180 calendar days from the date of notification, or until February 24, 2025, to regain compliance with the Minimum Bid Price Rule. On September 23, 2024, Nasdaq notified us that we had regained compliance with the Minimum Bid Price Rule.
No assurance can be given that we will be able to regain compliance with either the Minimum Equity Rule or comply with the other standards that we are required to meet in order to maintain a listing on such exchange, and no assurance can be given that even if we regain compliance with the Minimum Equity Rule we will maintain sufficient shareholders’ equity, or alternatively that the price of the ordinary shares will not again be in violation of Nasdaq’s Minimum Bid Price Rule in the future. Our failure to meet these requirements may result in our securities being delisted from Nasdaq.
Corporate Information
Our legal and commercial name is NeuroSense Therapeutics Ltd. We were incorporated on February 13, 2017 and were registered as a private company limited by shares under the laws of the State of Israel. We completed our initial public offering on the Nasdaq in December 2021. Our ordinary shares and warrants to purchase our ordinary shares are traded on the Nasdaq under the symbol “NRSN” and “NRSNW,” respectively.
Our principal executive offices are located at 11 HaMenofim Street, Building B, Herzliya, 4672562 Israel, and our telephone number is +972-9-7996183. Our website address is www.neurosense-tx.com. The information on our website does not constitute a part of this prospectus. Our agent for service of process in the United States is Cogency Global Inc., 122 East 42nd Street, 18th Floor, New York, NY 10168.
Implications of Being an Emerging Growth Company
As a company with less than $1.235 billion in revenue during our last fiscal year, we are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). As such, we may take advantage of certain exemptions from various reporting requirements that are applicable to other publicly traded entities that are not emerging growth companies. These exemptions include:
| | ● | not being required to have our registered independent public accounting firm attest to management’s assessment of our internal control over financial reporting; |
| | ● | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”), regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); |
| | ● | not being required to submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay,” “say-on-frequency” and “say-on-golden parachutes”; |
| | ● | the audit reports of our independent registered public accounting firm do not require communication of critical audit matters; and |
| | ● | not being required to disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation. |
As a result, the information contained in this prospectus and the documents incorporated by reference herein may be different from the information you receive from other public companies in which you hold shares. We may take advantage of these provisions for up until we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earliest to occur of: (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.235 billion; (ii) the date on which we have issued more than $1 billion in non-convertible debt securities during the previous three years; (iii) the date on which we are deemed to be a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act; or (iv) the last day of the fiscal year following the fifth anniversary of our initial public offering.
Implications of Being a Foreign Private Issuer
We are also a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we continue to qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| | ● | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| | ● | the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| | ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial statements and other specified information, and current reports on Form 8-K upon the occurrence of specified significant events. |
Notwithstanding these exemptions, we will file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an Annual Report on Form 20-F containing financial statements audited by an independent registered public accounting firm.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents; (ii) more than 50% of our assets are located in the U.S.; or (iii) our business is administered principally in the U.S.
Both foreign private issuers and emerging growth companies also are exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
THE OFFERING
| Securities offered by the selling shareholder for resale | | (i) 103,729 Shares, (ii) 3,520,000 ordinary shares issuable upon exercise of Ordinary Warrants, (iii) 3,520,000 Ordinary Warrants, (iv) 1,656,271 ordinary shares, issuable upon exercise of Pre-Funded Warrants, (v) 1,656,271 Pre-Funded Warrants. |
| | | |
| Ordinary shares as of December 25, 2024 | | 23,228,941 ordinary shares. |
| | | |
| Use of proceeds | | All of the Shares, the Warrants, and the Warrant Shares offered by the selling shareholder for resale pursuant to this prospectus will be sold by the selling shareholder. We will not receive any proceeds from such sales. See the section titled “Use of Proceeds” in this prospectus for more information. However, to the extent the Warrants are exercised for cash, if at all, we will receive the exercise price of the Warrants. The exercise price of the Warrants may exceed the trading price of our ordinary shares. If the price of our ordinary shares is below $1.25, we believe that the holder of the Ordinary Warrants will be unlikely to exercise their Ordinary Warrants, resulting in little to no cash proceeds to us. If all the Warrants were exercised, we would receive gross proceeds of approximately $4.4 million. See the section titled “Use of Proceeds” in this prospectus for more information. |
| | | |
| Offering price | | The exercise price of the Ordinary Warrants is $1.25 per ordinary share and the exercise price of the Pre-Funded Warrants is $0.0001 per ordinary share. The Shares, the Warrants and the Warrant Shares offered by the selling shareholder under this prospectus may be offered and sold at prevailing market prices, negotiated prices or such other prices as the selling shareholder may determine. See the section titled “Plan of Distribution” in this prospectus for more information. |
| | | |
| Risk factors | | See the section titled “Risk Factors” beginning on page 8 and the other information included in this prospectus for a discussion of factors you should consider before deciding to invest in the Shares, the Warrants, the Warrant Shares, the Pre-Funded Warrants and PFW Shares. |
| | | |
| Listing | | Our ordinary shares and warrants to purchase ordinary shares are listed on The Nasdaq Capital Market under the symbols “NRSN” and “NRSNW, respectively.” |
Unless otherwise stated, all information in this prospectus, is based on 23,228,941 ordinary shares outstanding as of December 25, 2024, and does not include the following as of that date:
| | ● | 1,069,128 ordinary shares issuable upon the exercise of options outstanding, at a weighted average exercise price of $2.31 per share under our 2018 Employee Share Option Plan; |
| | ● | 200,000 ordinary shares issuable upon the vesting of restricted share units outstanding under our 2018 Share Incentive Plan; |
| | ● | 848,705 ordinary shares reserved for issuance and available for future grant under our 2018 Share Incentive Plan; |
| | ● | 8,535,000 ordinary shares underlying warrants with a weighted average exercise price of $2.37 per share; |
| | ● | 1,656,271 ordinary shares underlying Pre-Funded Warrants issued in the Offering; and |
| | ● | 8,000,000 ordinary shares underlying Warrants issued in the Offering. |
Except as otherwise indicated, all information in this prospectus assumes no exercise of any Pre-Funded Warrants or Warrants.
RISK FACTORS
You should carefully consider the risks described below and the risks described in our 2023 Annual Report, which are incorporated by reference herein, as well as the financial or other information included in this prospectus or incorporated by reference in this prospectus, including our consolidated financial statements and the related notes, before you decide to buy our securities. The risks and uncertainties described below are not the only risks facing us. We may face additional risks and uncertainties not currently known to us or that we currently deem to be immaterial. Any of the risks described below, and any such additional risks, could materially adversely affect our business, financial condition or results of operations. In such case, you may lose all or part of your original investment. The discussion of risks includes or refers to forward-looking statements; you should read the explanation of the qualifications and limitations on such forward-looking statements discussed elsewhere in this prospectus supplement under the caption “Cautionary Statement Regarding Forward-Looking Statements” above.
Risks Related to this Offering
If we fail to maintain compliance with NASDAQ’s continued listing requirements, our shares may be delisted from the NASDAQ Capital Market.
To continue to be listed on Nasdaq, we need to satisfy a number of conditions, including the Minimum Bid Price Rule and the Minimum Equity Rule. On June 21, 2024, we received a notification letter from Nasdaq that we had not regained compliance with the Minimum Equity Rule due to our stockholders’ equity falling below the required minimum of $2,500,000. We promptly requested a hearing before the Panel, which was held on August 1, 2024, where we presented a comprehensive plan to regain compliance. On August 25, 2024, we received a written notice from Nasdaq that the Panel granted our request for an exception to continue its listing on Nasdaq until October 31, 2024. On November 11, 2024, we received a written notice from Nasdaq, stating that the Panel had granted our request for continued listing on Nasdaq, subject to our filing a public disclosure on or before December 18, 2024, describing the transactions we had undertaken to achieve compliance and demonstrate long-term compliance with Minimum Equity Rule and providing an indication of our equity following those transactions and the provision of income projections to Nasdaq. This extension allowed us additional time to demonstrate compliance with the Minimum Equity Rule and meet the required conditions. On December 17, 2024, we filed a Current Report on Form 6-K where we stated that we as of such date, we believe we have shareholders’ equity of $3 million, which is above the $2.5 million required by the Minimum Equity Rule. We are currently awaiting Nasdaq’s confirmation that we have evidenced compliance with the Minimum Equity Rule and that the matter has been closed.
No assurance can be given that we will be able to regain compliance with the Minimum Equity Rule or comply with the other standards that we are required to meet in order to maintain a listing on such exchange, such as the Minimum Bid Price Rule, and no assurance can be given that even if we regain compliance with the Minimum Equity Rule we will maintain sufficient shareholders’ equity or the price of the ordinary shares will not again be in violation of Nasdaq’s Minimum Bid Price Rule in the future. Our failure to meet these requirements may result in our securities being delisted from Nasdaq.
If the ordinary shares are delisted from Nasdaq, we may seek to list them on other markets or exchanges or the ordinary shares may trade on the pink sheets. In the event of such delisting, our shareholders’ ability to trade, or obtain quotations of the market value of, our ordinary shares would be severely limited because of lower trading volumes and transaction delays. These factors could contribute to lower prices and larger spreads in the bid and ask prices for our securities. In addition, the substantially decreased trading in the ordinary shares and decreased market liquidity of the ordinary shares as a result of the loss of market efficiencies associated with Nasdaq and the loss of federal preemption of state securities laws, which could materially adversely affect our ability to obtain financing on acceptable terms, if at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities. Additionally, the market price of the ordinary shares may decline further and shareholders may lose some or all of their investment. There can be no assurance that the ordinary shares, if delisted from Nasdaq in the future, would be listed on another national or international securities exchange or on a national quotation service, the Over-The-Counter Markets or the pink sheets.
The sale of a substantial amount of our ordinary shares or ordinary shares, including resale of the shares held by the selling shareholder in the public market could adversely affect the prevailing market price of our ordinary shares.
Sales of substantial amounts of shares of our ordinary shares or ordinary shares in the public market, or the perception that such sales might occur, could adversely affect the market price of our ordinary shares, and the market value of our other securities. We cannot predict if and when the selling shareholder may sell such shares in the public markets. Furthermore, in the future, we may issue additional ordinary shares or ordinary shares or other equity or debt securities convertible into ordinary shares or ordinary shares. Any such issuance could result in substantial dilution to our existing shareholders and could cause our share price to decline.
The price of our ordinary shares may be volatile.
The market price of our ordinary shares has fluctuated in the past. Consequently, the current market price of our ordinary shares may not be indicative of future market prices, and we may be unable to sustain or increase the value of your investment in our ordinary shares.
There is no guarantee that the Ordinary Warrants will be in the money, and they may expire worthless.
The exercise price of the Ordinary Warrants is $1.25 per ordinary share, subject to adjustment. The exercise price of the Ordinary Warrants has at times exceeded the market price of our ordinary shares. To the extent the price of our ordinary shares remains below $1.25, we believe that holders of the Ordinary Warrants will be unlikely to exercise their warrants, resulting in little to no cash proceeds to us. There is no guarantee the exercise price of the Ordinary Warrants will ever exceed the market price of our ordinary shares in the future and, as such, the Ordinary Warrants may expire worthless.
We do not intend to apply for any listing of the Warrants on any exchange or nationally recognized trading system, and we do not expect a market to develop for the Warrants.
We do not intend to apply for any listing of the Warrants on Nasdaq or any other securities exchange or nationally recognized trading system, and we do not expect a market to develop for the Warrants. Without an active market, the liquidity of the Warrants will be limited. Further, the existence of the Warrants may act to reduce both the trading volume and the trading price of our ordinary shares.
Holders of Warrants will have no rights as shareholders of ordinary shares until such holders exercise their Warrants and acquire ordinary shares.
The Warrants do not confer any rights of ordinary share ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire ordinary shares at a fixed price. A holder of (i) a Pre-Funded Warrant may exercise the right to acquire an ordinary share and pay an exercise price of $0.0001 at any time; and (ii) an Ordinary Warrant may exercise the right to acquire an ordinary share and pay an exercise price of $1.25 at any time. Upon exercise of any of the Warrants, the holders thereof will be entitled to exercise the rights of a holder of our ordinary shares only as to matters for which the record date occurs after the exercise date.
We conduct some of our operations in Israel. Conditions in Israel, including the recent attack by Hamas and other terrorist organizations from the Gaza Strip and Israel’s war against them, may affect our operations.
Our corporate headquarters is located in Herzliya, Israel. Because we are incorporated under the laws of the State of Israel, and most of our officers and fourteen out of sixteen of our employees are residents of Israel, our business and operations are directly affected by economic, political, geopolitical and military conditions in Israel. Since the establishment of the State of Israel in 1948, a number of armed conflicts have occurred between Israel and its neighboring countries and terrorist organizations active in the region. These conflicts have involved missile strikes, hostile infiltrations and terrorism against civilian targets in various parts of Israel, which have negatively affected business conditions in Israel.
In October 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on Israeli population and industrial centers located along Israel’s border with the Gaza Strip and in other areas within the State of Israel. These attacks resulted in thousands of deaths and injuries, and Hamas additionally kidnapped many Israeli civilians and soldiers. Following the attack, Israel’s security cabinet declared war against Hamas and a military campaign against these terrorist organizations commenced in parallel to their continued rocket and terror attacks. In parallel, border clashes between Israel and the Hezbollah terrorist group on Israel’s northern border with Lebanon intensified and may escalate into a greater regional conflict.
In addition, since the commencement of these events, there have been continued hostilities along Israel’s northern border with Lebanon (with the Hezbollah terror organization) and on other fronts from various extremist groups in region, such as the Houthis in Yemen and various rebel militia groups in Syria and Iraq. In addition, in April 2024 and October 2024, Iran (in concert with other regional actors) launched direct attacks on Israel involving hundreds of drones and missiles and has threatened to continue to attack Israel and is widely believed to be developing nuclear weapons. Such attacks may continue due to continuing tensions in the region. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza, Hezbollah in Lebanon, the Houthi movement in Yemen and various rebel militia groups in Syria and Iraq. These situations may potentially escalate in the future to more violent events which may affect Israel and us. Additionally, Yemeni rebel group, the Houthis, launched series of attacks on global shipping routes in the Red Sea, causing disruptions of supply chain. These geopolitical developments may adversely affect our ability to continue carrying out various administrative, research, operational and commercial functions and activities both in Israel and globally.
Any hostilities, armed conflicts, terrorist activities involving Israel or the interruption or curtailment of trade between Israel and its trading partners, or any political instability in the region could directly or indirectly adversely affect business conditions and our results of operations and could make it more difficult for us to raise capital and could adversely affect the market price of our ordinary share. An escalation of tensions or violence might result in a significant downturn in the economic or financial condition of Israel, which could have a material adverse effect on our operations in Israel and our business. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary in order to meet our business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
The Israel Defense Force, or IDF, the national military of Israel, is a conscripted military service, subject to certain exceptions. Since October 7, 2023, the IDF has called up several hundred thousand of its reserve forces to serve. Fourteen out of our current 16 employees are resident in Israel. Three of our five executive officers and the 11 other non-management employees of the Company reside in Israel. Currently none of our executive officers nor non-management employees have been called up to perform reserve military service; however, some employees may be called for service in the current or future wars or other armed conflicts with Hamas, Hezbollah or other regional threat actors, and such persons may be absent for an extended period of time. As a result, our operations in Israel may be disrupted by such absences, which disruption may materially and adversely affect our business, prospects, financial condition and results of operations.
Since the war broke out on October 7, 2023, our operations have not been adversely affected by this situation, and we have not experienced disruptions to our clinical studies. As such, our clinical and business development activities remain on track. However, the intensity and duration of Israel’s current war against Hamas and Hezbollah is difficult to predict at this stage, as are such war’s economic implications on our business and operations and on Israel’s economy in general. If the war extends for a long period of time or expands to other fronts, such as Iran, Lebanon, Syria and the West Bank, our operations may be adversely affected.
All of our clinical and pre-clinical research and development is currently being conducted outside of Israel, other than our 12-month open label-extension, or OLE, study of the PARADIGM trial, partially conducted in Tel Aviv, and a planned Phase 2 trial with PrimeC for AD that we plan to conduct in Haifa, Israel. The OLE has not been affected by the war, although the quality of the study may be adversely affected if as a result of the war patients are unable to visit the study center or the study coordinator is not able to conduct home visits and monitor the patients. In addition, in the event of a significant escalation of hostilities in northern Israel, there may be a delay in the planned AD trial. We do not believe the planned AD trial will be materially affected by the war and do not anticipate that any such delay as a result of the war would have a material impact on us. We may also elect to set up a site in Israel for a Phase 3 pivotal ALS trial of PrimeC, but this would be in addition to numerous other sites in Europe and the United States, and as a result we do not expect the timeline or quality of this trial to be adversely affected by the war. Our manufacturing is conducted in India. We do not currently anticipate any disruption to the supply chain relevant to our ongoing clinical trials and believe there are alternative sources of supply from whom we could obtain the necessary finished drug product to conduct our clinical trials. In addition, we believe we have sufficient finished product in inventory to continue our ongoing clinical trials for at least the next few months.
Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, there can be no assurance that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business, financial condition and results of operations.
Finally, political conditions within Israel may affect our operations. Israel has held five general elections between 2019 and 2022, and prior to October 2023, the Israeli government pursued extensive changes to Israel’s judicial system, which sparked extensive political debate and unrest. To date, these initiatives have been substantially put on hold. Actual or perceived political instability in Israel or any negative changes in the political environment, may individually or in the aggregate adversely affect the Israeli economy and, in turn, our business, financial condition, results of operations and growth prospects.
THE PRIVATE PLACEMENT
On December 2, 2024, we entered into the Purchase Agreement with the selling shareholder and Alon Ben-Noon, the Company’s Chief Executive Officer pursuant to which we agreed to issue and sell, in a private placement by us directly to the purchasers (i) 2,343,729 Shares; (ii) Warrants exercisable for an aggregate of 8,000,000 ordinary shares at an exercise price of $1.25 per share; and (iii) Pre-Funded Warrants exercisable for an aggregate of 1,656,271 ordinary shares at an exercise price of $0.0001 per share. The purchase price of each Share and accompanying two Warrants was $1.25, and the purchase price of each Pre-Funded Warrant and accompanying two Warrants was $1.2499. The gross proceeds to us from the offering are expected were $5.0 million, before deducting offering expenses payable by us.
The Warrants are exercisable immediately upon issuance at an exercise price of $1.25 per share and expire five years from the date of issuance. The Pre-Funded Warrants are exercisable immediately upon issuance at an exercise price of $0.0001 per share and may be exercised at any time until the pre-funded warrants are exercised in full. A holder of pre-funded warrants or Warrants (together with its affiliates) may not exercise any portion of such warrants to the extent that the holder would own more than 9.99% of our outstanding ordinary shares immediately after exercise.
Pursuant to the terms of the Purchase Agreement, on December 5, 2024 we issued to the purchasers the subscribed for Shares and Warrants, as applicable against payment for $2.8 million. On December 17, 2024, we received the remaining proceeds of $2.2 million.
Pursuant to the terms of the Purchase Agreement, we registered for resale on the registration statement on Form F-3 filed with the Securities Exchange Commission on December 6, 2024 (File No. 333-283656) (i) 2,200,000 Shares issued to the selling shareholder, (ii) up to 4,400,000 ordinary shares issuable to the selling shareholder upon exercise of the Warrants, and (iii) 4,400,000 Warrants issued to the selling shareholder, which are the securities attributable to the payment made by the selling shareholder to us on December 5, 2024.
Pursuant to the terms of the Purchase Agreement, we are registering for resale on this registration statement on Form F-3 (i) 103,729 Shares issued to the selling shareholder, (ii) up to 3,520,000 ordinary shares issuable to the selling shareholder upon exercise of the Warrants (iii) 3,520,000 Warrants issued to the selling shareholder, (iv) up to 1,656,271 ordinary shares, issuable to the selling shareholder upon exercise of the Pre-Funded Warrants and (v) 1,656,271 Pre-Funded Warrants issuable to the selling shareholder, which are the securities attributable to the payment made by the selling shareholder to us on December 17, 2024.
USE OF PROCEEDS
All of the Shares, the Warrants, and the Warrant Shares offered by the selling shareholder for resale pursuant to this prospectus will be sold by the selling shareholder. We will not receive any of the proceeds from such sales. The selling shareholder will receive all of the proceeds from any sales of the Shares, the Warrants, and the Warrant Shares offered hereby. However, we will incur expenses in connection with the registration of the Shares, the Warrants, and the Warrant Shares offered hereby.
We will receive the exercise price upon any exercise of the Warrants, to the extent exercised on a cash basis. If all of the Warrants were exercised, we would receive gross proceeds of approximately $4.4 million. However, the holders of the Warrants are not obligated to exercise the Warrants, and we cannot predict whether or when, if ever, the holders of the Warrants will choose to exercise the Warrants, in whole or in part. The exercise price of the Ordinary Warrants may exceed the trading price of our ordinary shares. If the price of our ordinary shares is below $1.25, we believe that holder of the Ordinary Warrants will be unlikely to exercise its warrants, resulting in little to no cash proceeds to us. Accordingly, we currently intend to use the proceeds received upon such exercise, if any, to advance the clinical development of our lead product candidate, PrimeC for ALS, for the clinical development and preclinical and clinical research and development in support of potential investigational new drug applications for Alzheimer’s disease, for Parkinson’s disease and for other indications, for future research and development of new product candidates and for working capital and general corporate purposes.
MARKET FOR ORDINARY SHARES AND DIVIDEND POLICY
Our ordinary shares are traded on the Nasdaq Capital Market under the symbol “NRSN.” The last reported sale price of our ordinary shares on December 24, 2024 on the Nasdaq Capital Market was $1.28 per share. We do not plan on applying to list the Warrants on any exchange or nationally recognized trading system.
We have never declared or paid any cash dividends on our ordinary shares, and we anticipate that, for the foreseeable future, we will retain any future earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends for at least the next several years.
The distribution of dividends may also be limited by the Israeli Companies Law, 5759-1999 (the “Companies Law”), which permits the distribution of dividends only out of retained earnings or earnings derived over the two most recent fiscal years, whichever is higher, provided that there is no reasonable concern that payment of a dividend will prevent a company from satisfying its existing and foreseeable obligations as they become due. As of December 31, 2023, we did not have distributable earnings pursuant to the Companies Law. Dividend distributions may be determined by our board of directors, as our amended and restated articles of association do not provide that such distributions require shareholder approval.
CAPITALIZATION
The table below sets forth our total capitalization:
| | ● | on an actual basis as of September 30, 2024; and |
| | ● | on a pro forma basis, giving effect to (i) the issuance of 66,000 ordinary shares as a result of vested RSUs; (ii) the issuance of 224,697 ordinary shares as Commitment Shares to YA pursuant to the Standby Equity Purchase Agreement; (iii) the sale of an aggregate of 785,606 ordinary shares at an average price of $1.21 per ordinary share, for aggregate gross proceeds of $949,327 (net proceeds of approximately $921,821 thousand, after deducting sales agent fees); (iv) the issuance and sale of (A) 2,343,729 ordinary shares, (B) 8,000,000 Warrants and (C) 1,656,271 Pre-Funded Warrants at a combined purchase price of $1.25 per ordinary share and accompanying Warrants and $1.2499 per Pre-Funded Warrant and accompanying Warrants for aggregate net proceeds of $4,936,000, after deducting estimated offering expenses payable by us, and (v) the irrevocable waiver of our officers of their right to future payment of certain and potential annual bonuses, as if such events had occurred on September 30, 2024. |
“The amounts shown below are unaudited, represent management’s estimate, and their accounting treatment has not been completed.”
The following table should be read in conjunction with “Use of Proceeds,” our financial statements and related notes that are incorporated by reference into this prospectus and the other financial information included or incorporated by reference into this prospectus. Our historical results do not necessarily indicate our expected results for any future periods.
| As of September 30, 2024 |
| (unaudited) | | Actual
(in thousands) | | | Pro Forma
(in thousands) | |
| Cash | | $ | 344 | | | $ | 6,201 | |
| Long term liabilities | | | 19 | | | | 19 | |
| Shareholders’ equity: | | | | | | | | |
| Ordinary shares, no par value per share | | | — | | | | — | |
| Share premium and capital reserve | | | 31,712 | | | | 37,569 | |
| Accumulated deficit | | | (34,530 | ) | | | (33,232 | ) |
| Total shareholders’ equity | | | (2,818 | ) | | | 4,337 | |
| | | | | | | | | |
| Total capitalization | | $ | (2,799 | ) | | $ | 4,356 | |
The table above is based on 19,808,909 ordinary shares issued and outstanding as of September 30, 2024. This number excludes:
| | ● | 1,069,128 ordinary shares issuable upon the exercise of options outstanding, at a weighted average exercise price of $2.31 per share under our 2018 Employee Share Option Plan; |
| | ● | 266,000 ordinary shares issuable upon the vesting of restricted share units outstanding, some of which are under our 2018 Share Incentive Plan; |
| | ● | 848,705 ordinary shares reserved for issuance and available for future grant under our 2018 Share Incentive Plan; |
| | ● | 8,535,000 ordinary shares underlying warrants with a weighted average exercise price of $2.38 per share; |
| | ● | 1,010,303 ordinary shares issued pursuant to the Sales Agreement and Standby Equity Purchase Agreement; |
| | ● | 2,343,729 ordinary shares issued in the Offering; |
| | ● | 1,656,271 ordinary shares underlying Pre-Funded Warrants issued in the Offering; and |
| | ● | 8,000,000 ordinary shares underlying Warrants issued in the Offering. |
Except as otherwise indicated, all information in this prospectus assumes no exercise of any Pre-Funded Warrants or Warrants.
SELLING SHAREHOLDER
The ordinary shares being offered by the selling shareholder pursuant to this prospectus are those issuable to such selling shareholder, including without limitation upon exercise of the Warrants. For additional information regarding the issuance of the Warrants, see “The Private Placement” above. We are registering the Shares, the Warrants, and the Warrant Shares in order to permit the selling shareholder to offer the Shares, the Warrants, and the Warrant Shares for resale from time to time. To our knowledge, the selling shareholder has not had any material relationship with us or our affiliates within the past three years. Our knowledge is based on information provided by the selling shareholder in connection with the filing of this prospectus.
The table below lists the selling shareholder and other information regarding the beneficial ownership of the Shares, the Warrants, and the Warrant Shares, by the selling shareholder. The second column lists the number of our ordinary shares beneficially owned by the selling shareholder, based on its ownership of our ordinary shares, as of December 25, 2024, and assuming exercise of all of the Warrants held by the selling shareholder on that date, without regard to any limitations on exercises. The third column lists the maximum number of our ordinary shares that may be sold or otherwise disposed of by the selling shareholder pursuant to the registration statement of which this prospectus forms a part. The selling shareholder may sell or otherwise dispose of some, all or none of their Shares, the Warrants, and the Warrant Shares in this offering. Pursuant to the rules of the SEC, beneficial ownership includes any of the ordinary shares as to which a shareholder has sole or shared voting power or investment power, as well as any ordinary shares that a selling shareholder has the right to acquire within 60 days of December 25, 2024. The percentage of beneficial ownership for the selling shareholder is based on 23,228,941 ordinary shares outstanding as of December 25, 2024 and the number of ordinary shares issuable upon exercise or conversion of convertible securities that are currently exercisable or convertible or are exercisable or convertible within 60 days of December 25, 2024 beneficially owned by the applicable selling shareholder. The fourth and eighth columns assumes the sale of all of the Shares, the Warrants, and the Warrant Shares, offered by the selling shareholder pursuant to this prospectus.
| Name of Selling Shareholder | | Ordinary
Shares
Beneficially
Owned Prior
to the
Offering(1) | | | Percentage of
Outstanding
Ordinary
Shares(1) | | | Maximum Number of Ordinary Shares To Be Sold Pursuant to this Prospectus | | | Number of Ordinary Shares Beneficially Owned
After the
Offering(2) | | | Percentage of
Outstanding
Ordinary
Shares after the
Offering(2) | | | Warrants
Beneficially
Owned
Prior to the
Offering | | | Percentage of
Outstanding
Warrants | | | Maximum
Number of
Warrants To Be Sold
Pursuant
to this Prospectus | | | Number of Warrants Beneficially Owned
After the
Offering | | | Percentage of
Outstanding
Warrants after the
Offering | |
| Rimon Gold Assets, Ltd.(3) | | | 11,880,000 | (4) | | | 36.21 | % | | | 5,280,000 | (5) | | | 6,660,000 | (6) | | | 20.12 | % | | | 9,576,271 | (7) | | | 100 | % | | | 5,176,271 | (8) | | | 4,400,000 | (9) | | | 45.9 | % |
| (1) | Assumes that (i) all of the Warrants covered by the registration statement of which this prospectus is a part are exercised in this offering, and (ii) the selling shareholder does not acquire additional warrants. |
| (2) | Assumes that (i) all of the Shares covered by the registration statement of which this prospectus is a part are sold in this offering and (ii) the selling shareholder does not acquire additional ordinary shares after the date of this prospectus and prior to completion of this offering. The number and percentage of ordinary shares listed do not take into account any limitations on exercise of warrants preventing the selling shareholder from exercising any portion of such warrants if such exercise would result in the selling shareholder owning greater than 9.99% of the outstanding ordinary shares following such exercise. |
| (3) | The securities are directly held by Rimon Gold Assets, Ltd., an Israeli private company (“Rimon Gold”) wholly owned by the Goldfinger Trust (the “Trust”), whose trustee is Abir Raveh (the “Trustee”) and whose beneficiary is Yair Goldfinger. The Trust directs the management of Rimon Gold, its investment and voting decisions and the Trustee directs the management of the Trust, its investment and voting decisions. The address of Rimon Gold is Raveh Ravid & Co. 32A Habarzel Street, Tel Aviv, Israel. The pre-funded warrants and Warrants are subject to a beneficial ownership limitation of 9.99%, which such limitation restricts the selling shareholder from exercising that portion of the pre-funded warrants and warrants that would result in the selling stockholder and its affiliates owning, after exercise, a number of ordinary shares in excess of the beneficial ownership limitation. The number of ordinary shares set forth in the above table does not reflect the application of this limitation. |
| | |
| (4) | Consists of (i) 2,303,729 ordinary shares, (ii) 1,656,271 ordinary shares issuable upon exercise of Pre-Funded warrants, and (iii) 7,920,000 ordinary shares issuable upon exercise of Ordinary Warrants. |
| | |
| (5) | Consists of (i) 103,729 ordinary shares, (ii) 1,656,271 ordinary shares issuable upon exercise of Pre-Funded Warrants, and (iii) 3,520,000 ordinary shares issuable upon exercise of Ordinary Warrants. |
| | |
| (6) | Consists of (i) 2,200,000 ordinary shares, and (ii) 4,400,000 ordinary shares issuable upon exercise of Ordinary Warrants. |
| | |
| (7) | Consists of (i) 1,656,271 ordinary shares issuable upon exercise of Pre-Funded Warrants, and (ii) 7,920,000 ordinary shares issuable upon exercise of Ordinary Warrants. |
| | |
| (8) | Consists of (i) 1,656,271 ordinary shares issuable upon exercise of Pre-Funded Warrants, and (ii) 3,520,000 ordinary shares issuable upon exercise of Ordinary Warrants. |
| | |
| (9) | Consists of 4,400,000 ordinary shares issuable upon exercise of Ordinary Warrants. |
PLAN OF DISTRIBUTION
The selling shareholder and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the Nasdaq Capital Market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A selling shareholder may use any one or more of the following methods when selling securities:
| | ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| | ● | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| | ● | purchases by a broker-dealer as principal and the subsequent resale by the broker-dealer for its account; |
| | ● | an exchange distribution in accordance with the rules of the applicable exchange; |
| | ● | an over-the-counter distribution in accordance with the rules of Nasdaq; |
| | ● | through trading plans entered into by the selling shareholder pursuant to Rule 10b5-1 under the Exchange Act that are in place at the time of an offering pursuant to this prospectus and any applicable prospectus supplement hereto that provide for periodic sales of their securities on the basis of parameters described in such trading plans; |
| | ● | transactions other than on such exchanges or in the over-the-counter market; |
| | ● | directly to purchasers, including through a specific bidding, auction or other process or in privately negotiated transactions; |
| | ● | settlement of short sales; |
| | ● | in transactions through broker-dealers that agree with the selling shareholder to sell a specified number of such securities at a stipulated price per security; |
| | ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| | ● | a combination of any such methods of sale; or |
| | ● | any other method permitted pursuant to applicable law. |
The selling shareholder may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the selling shareholder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling shareholder (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, the selling shareholder may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The selling shareholder may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The selling shareholder may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling shareholder and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. The selling shareholder has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
We are required to pay certain fees and expenses incurred by us incident to the registration of the resale of the Shares, the Warrants, the Warrant Shares, the Pre-Funded Warrants and PFW Shares covered by this prospectus. We have agreed to indemnify the selling shareholder against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
DESCRIPTION OF OUR SECURITIES BEING REGISTERED
The securities to be registered for resale on this registration statement on Form F-3 of which this prospectus forms a part include up to an aggregate amount of (i) 103,729 Shares issued to the selling shareholder, (ii) up to 3,520,000 ordinary shares issuable to the selling shareholder upon exercise of Ordinary Warrants (iii) 3,520,000 Ordinary Warrants issued to the selling shareholder, (iv) up to 1,656,271 ordinary shares, issuable to the selling shareholder upon exercise of Pre-Funded Warrants, and (v) 1,656,271 Pre-Funded Warrants.
Our Ordinary Shares
Our authorized share capital consists of 90,000,000 ordinary shares, no par value per share, of which 23,228,941 ordinary shares were issued and outstanding as of December 25, 2024.
All of the outstanding ordinary shares are validly issued, fully paid and non-assessable. Our ordinary shares are not redeemable and do not have any pre-emptive rights.
Voting Rights and Conversion
All ordinary shares will have identical voting and other rights in all respects. None of our major shareholders have different voting rights than our other shareholders.
Transfer of Shares
Fully paid ordinary shares are issued in registered form and may be freely transferred under our amended and restated articles of association, unless the transfer is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trading. The ownership or voting of ordinary shares by non-residents of Israel is not restricted in any way by the amended and restated articles of association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
Liability to Further Capital Calls
Our board of directors may make, from time to time, such calls as it may deem fit upon shareholders with respect to any sum unpaid with respect to ordinary shares held by such shareholders, which is not payable at a fixed time. Such shareholder shall pay the amount of every call so made upon him. Unless otherwise stipulated by our board of directors, each payment in response to a call shall be deemed to constitute a pro rata payment on account of all shares with respect to which such call was made. A shareholder shall not be entitled to his rights as shareholder, including the right to dividends, unless such shareholder has fully paid all the notices of call delivered to him, or which according to our amended and restated articles of association are deemed to have been delivered to him, together with interest, linkage and expenses, if any, unless otherwise determined by our board of directors.
Business Combinations
Under our amended and restated articles of association, we may not engage in any “business combinations” with any “interested shareholder” for a three-year period following the time that such shareholder became an interested shareholder, unless:
| | ● | prior to the time that such shareholder became an interested shareholder, our board of directors approved either the business combination or the transaction which resulted in the shareholder becoming an interested shareholder; |
| | ● | upon consummation of the transaction which resulted in the shareholder becoming an interested shareholder, the interested shareholder owned at least 85% of our voting shares outstanding at the time the transaction commenced, excluding for purposes of determining our voting shares outstanding (but not the outstanding voting shares owned by the interested shareholder) those shares owned by persons who are directors and also officers; or |
| | ● | at the time that such shareholder became an interested shareholder, or subsequent to such time, the business combination is approved by our board of directors and authorized at a general meeting of shareholders by the affirmative vote of at least 662/3% of our voting shares outstanding that are not owned by the interested shareholder. |
Generally, a “business combination” includes any merger, consolidation, sale or other transaction resulting in a financial benefit to the interested shareholder. Subject to certain exceptions, an “interested shareholder” is any person (other than us and any of our direct or indirect majority-owned subsidiaries) who, together with such person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of our voting shares.
Under certain circumstances, this provision will make it more difficult for a person who would be an “interested shareholder” to effect various business combinations with a corporation for a three-year period. This provision may encourage companies interested in acquiring us to negotiate in advance with our board of directors because the shareholder approval requirement would be avoided if our board of directors approves either the business combination or the transaction which results in the shareholder becoming an interested shareholder. These provisions also may have the effect of preventing changes in our board of directors and may make it more difficult to accomplish transactions which shareholders may otherwise deem to be in their best interests.
Election of Directors
Our ordinary shares do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect our directors, subject to the special approval requirements for external directors under the Companies Law. Under our amended and restated articles of association, the number of directors on our board of directors must be no less than three and no more than nine, including any external directors required to be appointed under the Companies Law. The minimum and maximum number of directors may be changed, at any time and from time to time, by a special vote of the holders of at least 66⅔% of the outstanding shares.
Other than external directors, for whom special election requirements apply under the Companies Law, the vote required to appoint a director is a simple majority vote. In addition, under our amended and restated articles of association, our board of directors may elect new directors to fill vacancies (whether such vacancy is due to a director no longer serving or due to the number of directors serving being less than the maximum required in our amended and restated articles of association), provided that the total number of directors shall not, at any time, exceed nine directors and provided that our board of directors may not elect external directors. Our amended and restated articles of association provide that the term of a director appointed by our board of directors to fill any vacancy will be for the remaining term of office of the director(s) whose office(s) have been vacated. Furthermore, under our amended and restated articles of association, our directors, other than external directors, are divided into three classes with staggered three-year terms. Each class of directors consists, as nearly as possible, of 1/3 of the total number of directors constituting the entire board of directors (other than the external directors).
External directors are elected for an initial term of three years, may be elected for additional three-year terms, and may be removed from office pursuant to the terms of the Companies Law.
Dividend and Liquidation Rights
We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Companies Law, dividend distributions are determined by our board of directors and do not require the approval of the shareholders of a company unless our articles of association provide otherwise. Our amended and restated articles of association do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the Companies Law, subject to certain exceptions with the respect to the buyback by us of our ordinary shares, the distribution amount is limited to the greater of retained earnings or earnings generated over the previous two years, according to our then last reviewed or audited financial statements (less the amount of previously distributed dividends, if not reduced from the earnings), provided that the end of the period to which the financial statements relate is not more than six months prior to the date of the distribution. If we do not meet such criteria, then the we may distribute dividends only with court approval. In each case, we are only permitted to distribute a dividend if our board of directors and, if applicable, the court determines that there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due. In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Exchange Controls
There are currently no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of ordinary shares or interest or other payments to non-residents of Israel, except for shareholders who are subjects of certain countries that are, or have been, in a state of war with Israel at such time.
Shareholder Meetings
Under Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be held no later than 15 months after the date of the previous annual general meeting. All general meetings other than the annual meeting of shareholders are referred to in our amended and restated articles of association as special meetings. Our board of directors may call special meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides that our board of directors is required to convene a special general meeting upon the written request of (i) any two of our directors or one-quarter of the members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of our outstanding issued shares and 1% or more of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Under Israeli law, one or more shareholders holding at least 1% of the voting rights at the general meeting may request that the board of directors include a matter in the agenda of a general meeting to be convened in the future, such as nominating a director candidate, provided that it is appropriate to discuss such a matter at the general meeting. Our amended and restated articles of association contain procedural guidelines and disclosure items with respect to the submission of shareholder proposals for shareholders meetings.
Subject to the provisions of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date of the meeting. Furthermore, the Companies Law requires that resolutions regarding, among other things, the following matters must be passed at a general meeting of our shareholders:
| | ● | amendments to our amended and restated articles of association; |
| | ● | appointment or termination of our auditors; |
| | ● | election of directors, including external directors (unless otherwise determined in our amended and restated articles of association); |
| | ● | approval of certain related party transactions; |
| | ● | increases or reductions of our authorized share capital; |
| | ● | the exercise of our board of directors’ powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management. |
Under our amended and restated articles of association, we are not required to give notice to our registered shareholders pursuant to the Companies Law, unless otherwise required by law. The Companies Law requires that a notice of any annual general meeting or special general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, or an approval of a merger, or as otherwise required under applicable law, notice must be provided at least 35 days prior to the meeting. Under the Companies Law, shareholders of a public company are not permitted to take action by written consent in lieu of a meeting.
Voting Rights
Quorum Requirements
Pursuant to our amended and restated articles of association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting. Under our amended and restated articles of association, the quorum required for general meetings of shareholders must consist of at least two shareholders present in person or by proxy (including by voting deed) holding 25% or more of our voting rights. A meeting adjourned for lack of a quorum will generally be adjourned to the same day of the following week at the same time and place, or to such other day, time or place as indicated by our board of directors if so specified in the notice of the meeting. At the reconvened meeting, any number of shareholders present in person or by proxy shall constitute a lawful quorum.
Vote Requirements
Our amended and restated articles of association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Companies Law or by our amended and restated articles of association. Our amended and restated articles of association provide that all resolutions of our board of directors require a simple majority vote of the directors present and voting at such meeting, unless otherwise required by the Companies Law or by our amended and restated articles of association. Pursuant to our amended and restated articles of association, in the event the vote is tied, the chair of the board of directors will have a casting vote.
Pursuant to our amended and restated articles of association, an amendment to our amended and restated articles of association regarding any change of the composition or election procedures of our directors will require a special majority vote (66⅔%). In addition, any change to the rights and privileges of the holders of any class of our shares requires a simple majority of the class so affected (or otherwise in accordance with the terms of such class), in addition to the simple majority vote of all classes of shares voting together as a single class at a shareholder meeting.
Under the Companies Law, each of (i) the approval of an extraordinary transaction with a controlling shareholder and (ii) the terms of employment or other engagement of the controlling shareholder of the company or such controlling shareholder’s relative (even if not extraordinary) requires the approval of the compensation committee, board of directors, and shareholders, subject to limited exceptions. Certain transactions with respect to remuneration of our office holders and directors require approvals of the compensation committee, board of directors, and in the case of the chief executive officer and directors, shareholders, subject to limited exceptions Another exception to the simple majority vote requirement is a resolution for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350 of the Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting, in person or by proxy and voting on the resolution.
Access to Corporate Records
Under the Companies Law, shareholders are provided access to: minutes of our general meetings; our shareholders register and material shareholders register; our amended and restated articles of association; our financial statements; and any document that we are required by law to file publicly with the Israeli Companies Registrar (the “ISA”). In addition, shareholders may request to be provided with any document related to an action or transaction requiring shareholder approval under the related party transaction provisions of the Companies Law. We may deny this request if we believe it has not been made in good faith or if such denial is necessary to protect our interest or protect a trade secret or patent.
Modification of Class Rights
Under the Companies Law and our amended and restated articles of association, the rights attached to any class of share, such as voting, liquidation and dividend rights, may be amended by adoption of a resolution by the holders of a majority of the shares of that class present at a separate class meeting, or otherwise in accordance with the terms of such class of shares, in addition to the simple majority vote of all classes of shares voting together as a single class at a shareholder meeting, as set forth in our amended and restated articles of association.
Acquisitions Under Israeli Law
Full Tender Offer. A person wishing to acquire shares of an Israeli public company and who would as a result hold over 90% of the target company’s issued and outstanding share capital is required by the Companies Law to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of an Israeli public company and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of shares.
Upon a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli court as described above.
If (a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company or of the applicable class or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
Special Tender Offer. The Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company. Similarly, the Companies Law provides that an acquisition of shares in a public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the voting rights in the company. These requirements do not apply if, in general, the acquisition (1) was made in a private placement that received shareholder approval, (2) was from a 25% or greater shareholder of the company which resulted in the acquirer becoming a 25% or greater shareholder of the company, or (3) was from a 45% or greater shareholder of the company which resulted in the acquirer becoming a 45% or greater shareholder of the company.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer (excluding the purchaser and its controlling shareholders, holders of 25% or more of the voting rights in the company or any person having a personal interest in the acceptance of the tender offer or any other person acting on their behalf, including the relatives and entities under such person’s control). If a special tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger. The Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described under the Companies Law are met, by a majority vote of each party’s shares, and, in the case of the target company, a majority vote of each class of its shares, voted on the proposed merger at a shareholders meeting.
For purposes of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of shares represented at the shareholders meeting that are held by parties other than the other party to the merger, or by any person (or group of persons acting in concert) who holds (or hold, as the case may be) 25% or more of the voting rights or the right to appoint 25% or more of the directors of the other party, vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same special majority approval that governs all extraordinary transactions with controlling shareholders. See “Management — Approval of Related Party Transactions under Israeli Law — Fiduciary Duties of Directors and Officers — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions.”
If the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value to the parties to the merger and the consideration offered to the shareholders of the company.
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each party with the IRC and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
Israeli tax law treats some acquisitions, such as share-for-share exchanges between an Israeli company and a foreign company, less favorably than U.S. tax laws. For example, Israeli tax law may, under certain circumstances, subject a shareholder who exchanges his ordinary shares for shares in another corporation to taxation prior to the sale of the shares received in such share-for-share swap.
Anti-Takeover Measures Under Israeli Law
The Companies Law allows us to create and issue shares having rights different from those attached to ordinary shares, including shares providing certain preferred rights with respect to voting, distributions or other matters and shares having pre-emptive rights. As of the date of this prospectus, no preferred shares were authorized under our amended and restated articles of association. In the future, if we do authorize, create and issue a specific class of preferred shares, such class of shares, depending on the specific rights that may be attached to it, may have the ability to frustrate or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization and designation of a class of preferred shares will require an amendment to our amended and restated articles of association, which requires the prior approval of the holders of a majority of the voting power attaching to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled to participate and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Companies Law as described above in “— Voting Rights.”
Borrowing Powers
Pursuant to the Companies Law and our amended and restated articles of association, our board of directors may exercise all powers and take all actions that are not required under law or under our amended and restated articles of association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
Changes in Capital
Our amended and restated articles of association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Companies Law and must be approved by a resolution duly adopted by our shareholders at a general meeting. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits, require the approval of both our board of directors and an Israeli court.
Establishment
We were incorporated under the laws of the State of Israel on February 13, 2017. We are registered with the IRC.
History of Share Capital
Since January 1, 2021, our issued share capital has changed as provided below.
In February, May, June and July 2021, we entered into Simple Agreements for Future Equity (each, a “SAFE”) with four separate investors for aggregate proceeds of $800 thousand. Pursuant to the terms of each SAFE, upon consummation of an equity financing, we were required to issue to each investor the number of ordinary shares equal to the purchase amount divided by the SAFE price, which was defined as the price per share equal to 80% of the equity financing valuation (to be no less than $25,000 thousand). The SAFE Agreements also provide the investor the right to automatically receive ordinary shares in the case of a liquidity event, defined as a change of control event or an initial public offering. In the case of a liquidity event the investors are entitled to the number of ordinary shares equal to the investment amount divided by the liquidity price. The liquidity price is defined as the price per share equal to our valuation at the time of the liquidity event, multiplied by 80%, and divided by our capitalization (to be no less than $25,000 thousand). As part of our initial public offering, the SAFEs were converted at a price per share of $2.892 into 276,672 ordinary shares.
In May and October 2021, we issued 240,000 and 45,000 ordinary shares, respectively, pursuant to the exercise of options at an exercise price of $0.033 per share.
In August and September 2021, we issued 1,837,500 ordinary shares pursuant to the exercise of warrants at a weighted average price of $0.67 per share.
On November 9, 2021, we implemented a 1-for-3 split of our share capital, resulting in an increase of the issued and outstanding ordinary shares.
On December 13, 2021, we completed our initial public offering and issued 2,000,000 units, each consisting of one ordinary share and a warrant representing the right to purchase one ordinary share with an exercise price of $6.00 per share, at an initial public offering price of $6.00 per unit.
In connection with the closing of our initial public offering, on December 13, 2021, we issued 961,440 ordinary shares pursuant to the exercise of options at a weighted average exercise price of $0.079 per share.
In March 2022, we issued 645,000 ordinary shares upon exercise of warrants issued in our initial public offering at a price per share of $6.00.
In October 2022, we issued 35,980 ordinary shares pursuant to vesting of restricted stock units (“RSUs”) that were granted to one of our officers in October 2021.
In December 2022, we issued 72,000 ordinary shares pursuant to vesting of RSUs that were granted to our officers in November 2021.
In 2022, we issued 85,449 ordinary shares to certain consultants in exchange for their services. One of the consultants received 44,000 shares of restricted stock which were issued in 4 equal installments and subject to 2 year lock-up period. The other consultant’s received ordinary shares subject to a 6 month lock-up.
In March, June, September and December 2023, we issued 72,000 ordinary shares pursuant to vesting of RSUs that were granted to our officers in November 2021.
In May 2023, we issued 3,600 ordinary shares for gross proceeds of $7,200 in connection with our at-the-market offering program. The ordinary shares were sold at market prices at the time of sale.
In May and June 2023 we issued 320,479 ordinary shares pursuant to vesting of RSUs that were granted to our officers in March 2023.
In June, September, December 2023 and in March 2024, we issued a total of 100,000 ordinary shares pursuant to vesting of RSUs that were granted to our employees in March 2023.
In June 2023, we issued 126,000 ordinary shares pursuant to the exercise of options at an exercise price of $0.033 per share.
In June 2023, we issued 1,330,000 ordinary shares pursuant to a registered direct offering at a price per share of $1.50.
In October 2023, we issued 1,670,000 ordinary shares pursuant to the exercise of pre-funded warrants that were granted pursuant to a registered direct offering at a price per share of $0.0001.
In March 2024, we issued 160,000 ordinary shares pursuant to vesting of RSUs that were granted to our non-management directors in March 2023.
In March, June and September 2024, we issued 54,000 ordinary shares pursuant to vesting of RSUs that were granted to our officers in November 2021. In April 2024, we issued 20,000 ordinary shares pursuant to vesting of RSUs that were granted to our officers in April 2023.
In April 2024, we issued 1,732,000 ordinary shares pursuant to a registered direct offering at a price per share of $1.50.
In April 2024, we issued 70,964 ordinary shares to our placement agent as partial consideration in exchange for their services, in connection with a registered direct offering.
In May 2024, we issued 632,000 ordinary shares pursuant to the exercise of pre-funded warrants that were granted pursuant to a registered direct offering at a price per share of $0.0001.
In July 2024, we issued 616,000 ordinary shares pursuant to the exercise of pre-funded warrants that were granted pursuant to a registered direct offering at a price per share of $0.0001.
In August 2024, we issued 800,000 ordinary shares pursuant to a private placement offering at a combined purchase price of $0.75 per share and accompanying warrant.
In October and November 2024 we issued 48,000 ordinary shares pursuant to vesting of RSUs that were granted to service providers in May 2024.
In November 2024, we issued 224,697 ordinary shares as the Commitment Shares issued to YA under the Standby Equity Purchase Agreement.
In December 2024, we issued 2,343,729 ordinary shares, 1,656,271 pre-funded warrants and warrants to purchase 8,000,000 ordinary shares in a private placement. The purchase price of each ordinary share and accompanying warrants was $1.25, and the purchase price of each pre-funded warrant and accompanying warrants was $1.2499.
Between August 2024 through to December 25, 2024, we have issued 1,105,509 ordinary shares pursuant to the Sales Agreement.
Ordinary Warrants and Pre-Funded Warrants
The following summary of certain terms and provisions of the Ordinary Warrants and Pre-Funded Warrants that are being offered hereby for resale is not complete and is subject to, and qualified in its entirety by, the provisions of the Ordinary Warrants and Pre-Funded Warrants, the form of which is filed as Exhibit 10.3 and Exhibit 10.2 respectively to our Report on Form 6-K filed on December 2, 2024. Prospective investors should carefully review the terms and provisions of the form of Warrant for a complete description of the terms and conditions of the Ordinary Warrants and Pre-Funded Warrants.
Duration and exercise price
Each Ordinary Warrant offered hereby will have an exercise price per ordinary share equal to $1.25. The Ordinary Warrants became immediately exercisable at any time on or after December 5, 2024 and will expire upon 5:00 p.m., New York City time, on December 5, 2029. The exercise price and number of ordinary shares issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares and the exercise price.
Each Pre-Funded Warrant offered hereby will have an exercise price per Share equal to $0.0001. The Pre-Funded Warrants became immediately exercisable at any time on or after December 5, 2024 and will expire upon 5:00 p.m., New York City time, on December 5, 2029. The exercise price and number of Shares issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our Shares and the exercise price.
Exercisability
The Ordinary Warrants and Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of Shares purchased upon such exercise (except in the case of a cashless exercise as discussed below).
A holder (together with its affiliates) may not exercise any portion of such holder’s Ordinary Warrant or Pre-Funded Warrant to the extent that the holder would beneficially own more than 9.99% of the outstanding shares immediately following the consummation of this offering and assuming the exercise of such warrant or pre-funded warrant. No fractional shares will be issued in connection with the exercise of a Ordinary Warrant or Pre-Funded Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless exercise
A holder of the Ordinary Warrants or Pre-Funded Warrants will have the right to exercise such warrants or pre-funded warrants on a “cashless” basis if there is no effective registration statement registering the issuance of the shares issuable upon the exercise of the Ordinary Warrants or Pre-Funded Warrants or the prospectus included within such registration statement is not available for use.
Fundamental transactions
In the event of any fundamental transaction, as described in the Ordinary Warrants or Pre-Funded Warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our shares, then upon any subsequent exercise of a Ordinary Warrant or Pre-Funded Warrant, the holder will have the right to receive as alternative consideration, for each share that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares for which the Ordinary Warrant or Pre-Funded Warrant is exercisable immediately prior to such event.
Exchange Listing
We do not intend to list the Ordinary Warrants or Pre-Funded Warrant on any securities exchange or nationally recognized trading system.
Transferability
The Ordinary Warrants or Pre-Funded Warrant may be offered for sale, sold, transferred or assigned without our consent.
Transfer Agent and Registrar of Ordinary Shares
The transfer agent and registrar for our ordinary shares is American Stock Transfer & Trust Company, LLC. Its address is 6201 15th Avenue, Brooklyn, New York 11219, and its telephone number is (800) 937-5449.
EXPENSES
The following are the estimated expenses of this offering payable by us with respect to the issuance and distribution of the securities covered by the registration statement of which this prospectus forms a part. With the exception of the SEC registration fee, all amounts are estimates and may change:
| SEC registration fee | | $ | 1,009 | |
| Printer fees and expenses | | | 2,000 | |
| Legal fees and expenses | | | 20,000 | |
| Accountants’ fees and expenses | | | 5,000 | |
| Total | | $ | 28,009 | |
LEGAL MATTERS
Certain legal matters with respect to Israeli law and with respect to the validity of the offered securities under Israeli law will be passed upon for us by Goldfarb Gross Seligman & Co. Certain legal matters with respect to U.S. law will be passed upon for us by Greenberg Traurig, P.A.
EXPERTS
The consolidated financial statements of NeuroSense Therapeutics Ltd. as of December 31, 2023 and 2022 and for each of the years in the three-year period ended December 31, 2023 have been incorporated by reference herein in reliance upon the report of Somekh Chaikin, a member firm of KPMG International, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the December 31, 2023 consolidated financial statements contains an explanatory paragraph that states that the Company’s recurring losses and its expectation to incur significant additional losses raise substantial doubt about the entity’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of that uncertainty.
ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in this prospectus, substantially all of whom reside outside the U.S., may be difficult to obtain within the U.S. Furthermore, because substantially all of our assets and substantially all of our directors and officers are located outside the U.S., any judgment obtained in the U.S. against us or any of our directors and officers may not be collectible within the U.S.
We have irrevocably appointed Cogency Global Inc. as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of this offering or any purchase or sale of securities in connection with this offering. The address of our agent is 122 East 42nd Street, 18th Floor, New York, NY 10168.
We have been informed by our legal counsel in Israel, Goldfarb Gross Seligman & Co., that it may be difficult to initiate an action with respect to U.S. securities law in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum to hear such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact by expert witnesses which can be a time-consuming and costly process. Certain matters of procedure may also be governed by Israeli law.
Subject to certain time limitations and legal procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain exceptions, is non-appealable, including judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that:
| | ● | the judgment was rendered by a court which was, according to the laws of the state of the court, competent to render the judgment; |
| | ● | the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and |
| | ● | the judgment is executory in the state in which it was given. |
| | | Even if these conditions are met, an Israeli court will not declare a foreign civil judgment enforceable if: |
| | ● | the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases); |
| | ● | the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel; |
| | ● | the judgment was obtained by fraud; |
| | ● | the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the Israeli court; |
| | ● | the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in Israel; |
| | ● | the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid; or |
| | ● | at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before a court or tribunal in Israel. |
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
WHERE CAN YOU FIND MORE INFORMATION
This prospectus is part of the registration statement on Form F-3 we have filed with the SEC under the Securities Act, with respect to the securities offered by this prospectus. However, as is permitted by the rules and regulations of the SEC, this prospectus, which constitutes part of the registration statement, omits certain non-material information, exhibits, schedules and undertakings set forth in the registration statement. For further information about us, and the securities offered by this prospectus, please refer to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on the information contained in this prospectus or incorporated by reference. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted.
We are subject to the reporting requirements of the Exchange Act that are applicable to a foreign private issuer. In accordance with the Exchange Act, we file reports, including annual reports on Form 20-F by April 30th of each year. We also furnish to the SEC under cover of Form 6-K material information required to be made public in Israel, filed with and made public by any stock exchange or distributed by us to our shareholders.
The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers, such as us, that file electronically with the SEC (http://www.sec.gov).
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders and our officers, directors and principal shareholders are exempt from the “short-swing profits” reporting and liability provisions contained in Section 16 of the Exchange Act and related Exchange Act rules.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus and information we file later with the SEC will automatically update and supersede this information. The documents we are incorporating by reference as of their respective dates of filing are:
| | ● | our Annual Report on Form 20-F for the fiscal year ended December 31, 2023, filed with the SEC on April 4, 2024; |
| | ● | our Reports on Form 6-K filed with the SEC on April 12, 2024, May 7, 2024, May 23, 2024, May 28, 2024, June 17, 2024, June 21, 2024, June 24, 2024, June 27, 2024, July 1, 2024, July 3, 2024, July 9, 2024, August 1, 2024, August 7, 2024, August 16, 2024, August 26, 2024, August 28, 2024, August 30, 2024, September 9, 2024, September 24, 2024, September 30, 2024 October 9, 2024, October 15, 2024, October 24, 2024, October 28, 2024, October 30, 2024, October 31, 2024, November 12, 2024, December 2, 2024, December 4, 2024, December 11, 2024, December 17, 2024, December 18, 2024 and December 23, 2024, (in each case, to the extent expressly incorporated by reference into our effective registration statements filed by us under the Securities Act); and |
| | ● | the description of our ordinary shares contained under the heading “Item 1. Description of Registrant’s Securities to be Registered” in our registration statement on Form 8-A, as filed with the SEC on November 18, 2021, including any subsequent amendment or any report filed for the purpose of updating such description. |
All subsequent annual reports filed by us pursuant to the Exchange Act on Form 20-F prior to the termination of an offering shall be deemed to be incorporated by reference to this prospectus and to be a part hereof from the date of filing of such documents. We may also incorporate part or all of any Form 6-K subsequently submitted by us to the SEC prior to the termination of an offering by identifying in such Forms 6-K that they, or certain parts of their contents, are being incorporated by reference herein, and any Forms 6-K so identified shall be deemed to be incorporated by reference in this prospectus and to be a part hereof from the date of submission of such documents. Any statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document which also is incorporated or deemed to be incorporated by reference herein modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
The information we incorporate by reference is an important part of this prospectus, and later information that we file with the SEC will automatically update and supersede the information contained in this prospectus.
We will provide each person, including any beneficial owner to whom a prospectus is delivered, without charge, upon a written or oral request, a copy of any of the documents incorporated by reference in this prospectus, other than exhibits to such documents which are not specifically incorporated by reference into such documents. Please direct your written or telephone requests to NeuroSense Therapeutics Ltd., 11 HaMenofim Street, Building B, Herzliya, 4672562, Israel, Attn: Or Eisenberg, telephone number +972587531153. You may also obtain information about us by visiting our website at www.neurosense-tx.com. Information contained in our website is not part of this prospectus.
5,280,000 Ordinary Shares
Up to 3,520,000 Warrants to Purchase up to 3,520,000 Ordinary Shares
Up to 1,656,271 Pre-Funded Warrants to Purchase up to 1,656,271 Ordinary Shares

Prospectus
The date of this prospectus is January 16, 2025.

