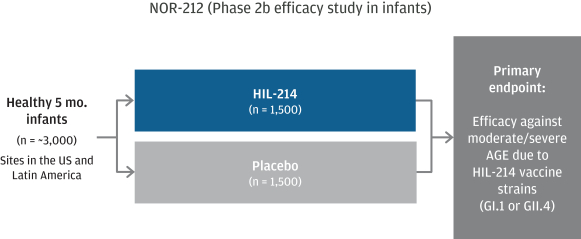
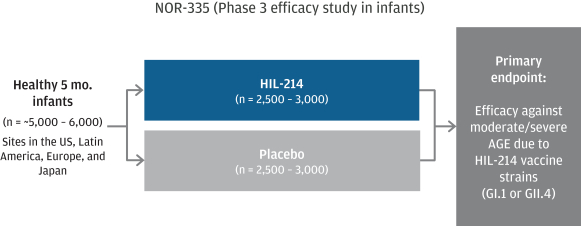
March 31, 2022, our portfolio consists of approximately 22 issued U.S. patents, 5 pending U.S. patent applications, 65 issued foreign patents including 6 issued European patents subsequently validated in individual European countries, and 54 foreign patent applications pending in major international markets. The issued patents and pending applications have nominal expiration dates ranging from 2027 to 2039, without accounting for any available patent term adjustments or extensions.
More specifically, of the 8 distinct patent families, we have in-licensed two patent families relating to manufacturing methods for norovirus VLPs. One of these families contains four U.S. patents projected to expire from 2028 to 2029, as well as a granted patent in each of Canada, Hong Kong, Europe, Republic of Korea and Singapore and two granted patents in Australia, also projected to expire in 2028, in each case without accounting for any available patent term adjustments or extensions. The European patent in this family was validated in Belgium, Bulgaria, Switzerland, Czech Republic, Germany, Denmark, France, the United Kingdom, Hungary, Ireland, Netherlands, Poland and Sweden. There is an additional pending application in Singapore, projected to expire in 2028, without accounting for any available patent term adjustments or extensions.
The other patent family covering manufacturing methods contains two U.S. patents projected to expire from 2033 to 2035, as well as a granted patent in each of Australia, Europe, Hong Kong, Jordan, Lebanon, Republic of Korea, Mexico and Taiwan, all projected to expire in 2033, in each case without accounting for any available patent term adjustments or extensions. The European patent in this family was validated in Belgium, Switzerland, Czech Republic, Germany, France, the United Kingdom, Ireland and Netherlands. There are two additional pending patent applications in China and Argentina and an additional pending application in each of Bangladesh, Canada, Gulf Co-Operation Council, India, Iran, Pakistan, Singapore, Uruguay, Venezuela and the U.S., all projected to expire in 2033, in each case without accounting for any available patent term adjustments or extensions.
We have also in-licensed six patent families covering VLP compositions for HIL-214 and methods of use of HIL-214. One of these families contains seven U.S. patents, all projected to expire in 2027, as well as two granted patents in Europe, two granted patents in Hong Kong and a granted patent in each of Australia, Canada, Republic of Korea, Singapore, also projected to expire in 2027, in each case without accounting for any available patent term adjustments or extensions. One European patent in this family was validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, the United Kingdom, Greece, Ireland, Italy, Lithuania, Netherlands, Sweden and Turkey. A second European patent in this family was validated in Belgium, Bulgaria, Switzerland, Czech Republic, Germany, Denmark, Spain, Finland, France, the United Kingdom, Hungary, Ireland, Italy, Netherlands, Poland and Sweden. There is an additional pending application in Singapore, projected to expire in 2027, without accounting for any available patent term adjustments or extensions.
A second family covering VLP compositions and methods of use contains four U.S. patents projected to expire from 2027 to 2028, as well as two granted patents in each of Australia and China and a granted patent in each of Canada, Macau and Hong Kong, projected to expire from 2027 to 2028, in each case without accounting for any available patent term adjustments or extensions. There is an additional pending application in the U.S. and two in Singapore, projected to expire in 2028, in each case without accounting for any available patent term adjustments or extensions.
A third family covering VLP compositions and methods of use contains two U.S. patents projected to expire in 2029, as well as two granted patents in Republic of Korea and a granted patent in each of Australia, Canada, China, Macau, Europe, Hong Kong and Singapore, projected to expire in 2029, in each case without accounting for any available patent term adjustments or extensions. The European patent in this family was validated in Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, Netherlands, Norway, Poland, Spain, Sweden, Switzerland, Turkey and the United Kingdom. There is an additional pending application in each of
127