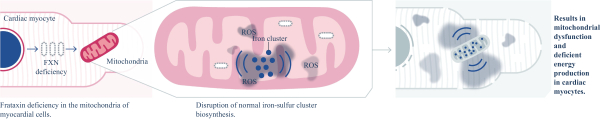
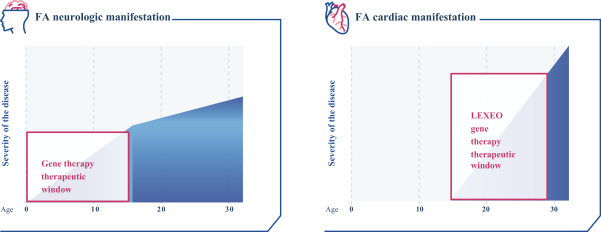
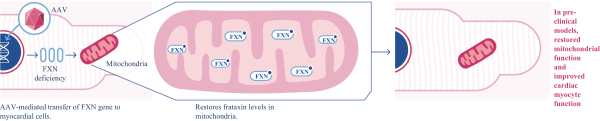
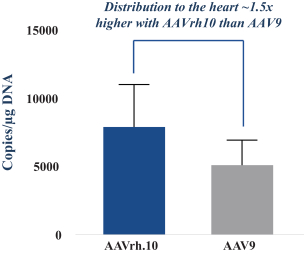
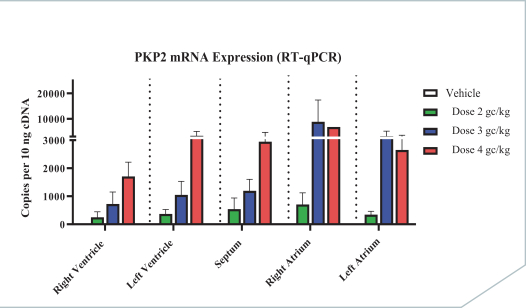
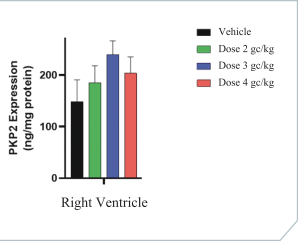
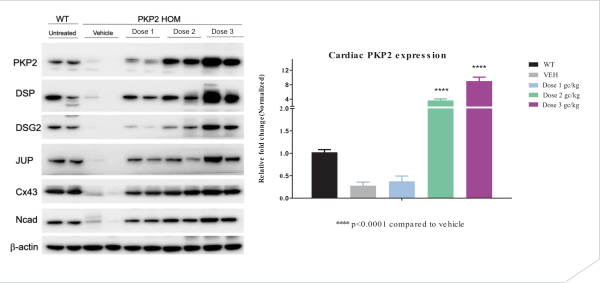
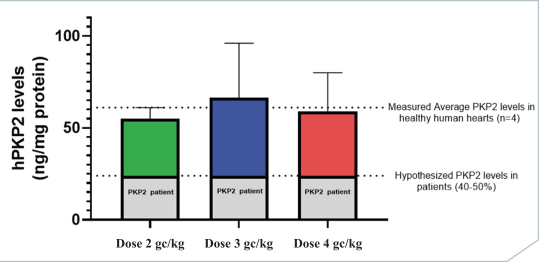
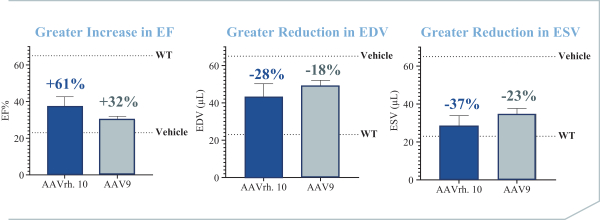
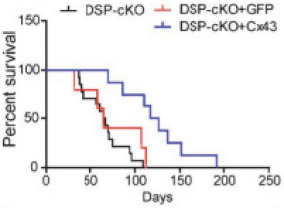
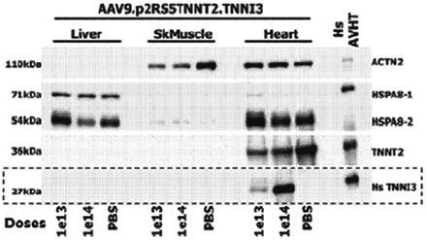
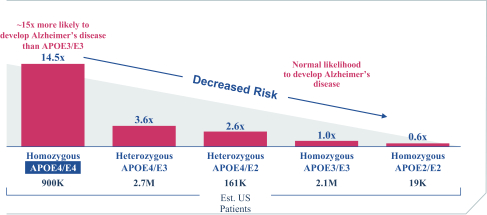
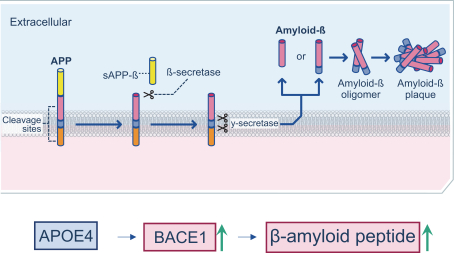
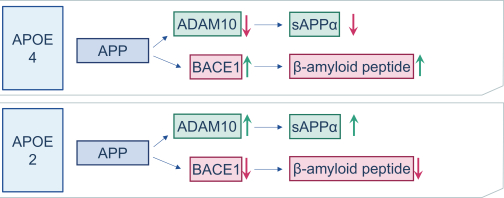
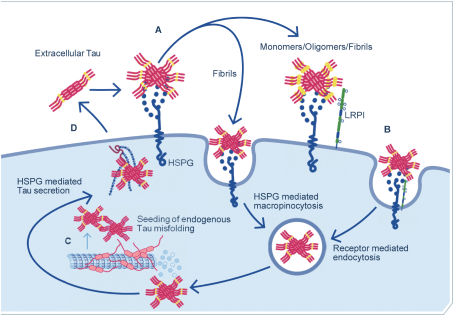
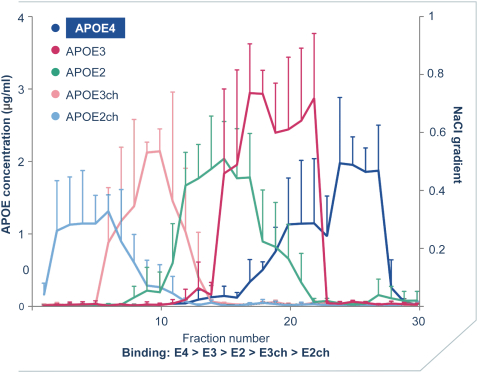
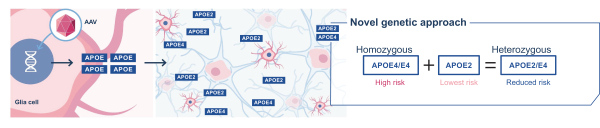
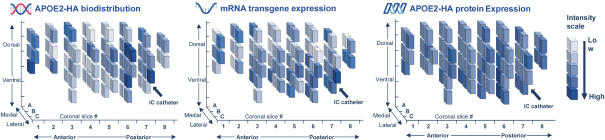
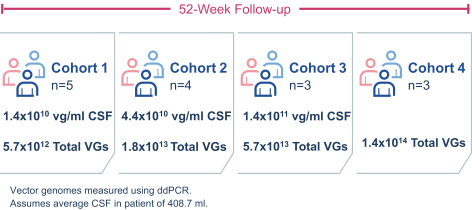
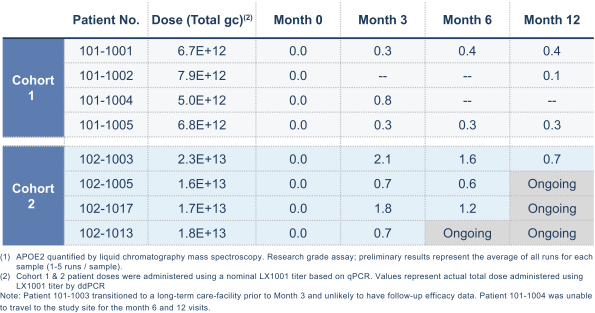
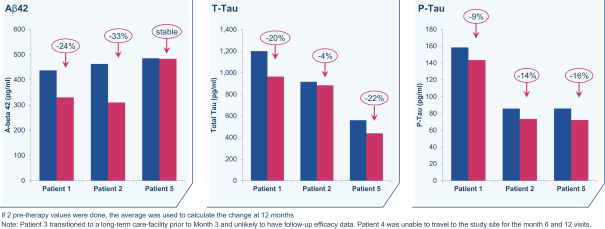
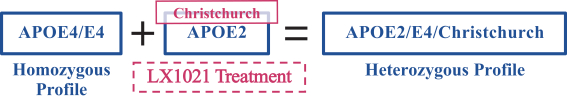
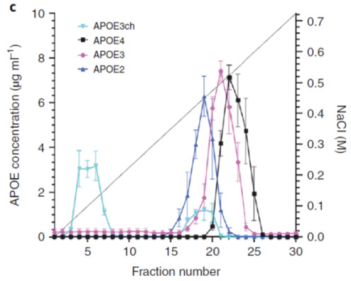
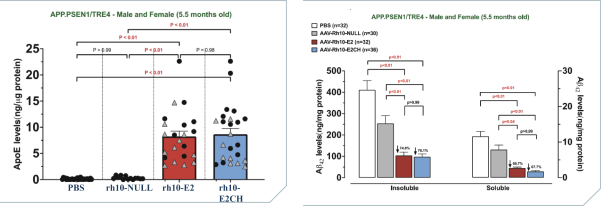
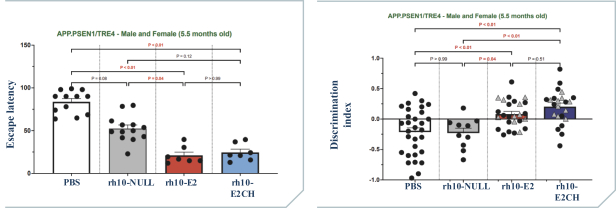
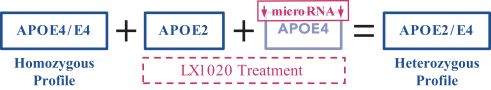
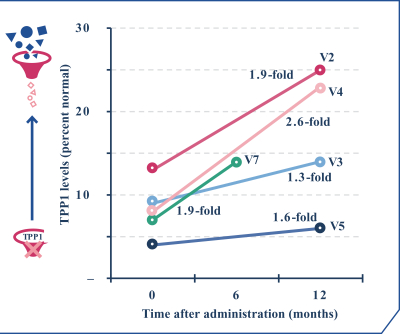
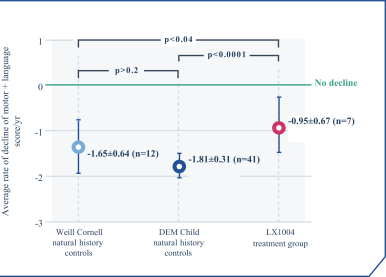
regulations, including those laws requiring the reporting of true, complete and accurate information to the FDA, manufacturing standards, federal and state healthcare laws and regulations, and laws that require the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by these parties could also involve the improper use of individually identifiable information, including, without limitation, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. In connection with this offering, we intend to adopt a code of business conduct and ethics; however, even with such a code of conduct in place, it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, including, without limitation, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations.
Our business and operations would suffer in the event of system failures, cyberattacks or a deficiency in our or our CMOs’, CROs’, manufacturers’ contractors’, consultants’ or collaborators’ cybersecurity.
Despite the implementation of security measures, our internal computer systems, as well as those of third parties on which we rely, are vulnerable to damage from, among other things, computer viruses, malware, unauthorized access, natural disasters, terrorism, war telecommunication and electrical failures, system malfunctions, cyberattacks or cyber-intrusions over the Internet, attachments to emails, phishing attacks, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyberattacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could lead to the loss, destruction, alteration, prevention of access to, disclosure, dissemination of, or damage or unauthorized access to, our data (including trade secrets or other confidential information, intellectual property, proprietary business information and personal data) or data that is processed or maintained on our behalf, and cause interruptions in our operations, which could result in a material disruption of our product candidate development programs. For example, the loss of preclinical study or clinical trial data from completed, ongoing or planned trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, clinical trial data, proprietary business information, personal data and personally identifiable information of our clinical trial subjects and employees, in our data centers and on our networks. The secure processing, maintenance and transmission of this information is critical to our operations. Despite our security measures, we cannot ensure that our information technology and infrastructure will prevent breakdowns or breaches in our or their systems or other cybersecurity incidents that cause loss, destruction, unavailability, alteration, dissemination of, or damage
74