This is a confidential draft submission to the U.S. Securities and Exchange Commission pursuant to Section106(a) of the Jumpstart Our Business Startups Act of 2012 on [ ], 2023 and is not being filed publicly under the Securities Act of 1933, as amended.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE
SECURITIES ACT OF 1933
KINDLY MD, INC.
(Exact name of registrant as specified in its charter)
| Utah | | 8049 | | 84-3829824 |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification No.) |
230 W 400 South
Suite 201
Salt Lake City, UT 84104
(385) 388-8220
(Address and telephone number of registrant’s principal executive offices)
Timothy Pickett
Chief Executive Officer
230 W 400 South
Suite 201
Salt Lake City, UT 84194
(385)388-8220
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Callie T. Jones, Esq. | Richard A. Friedman, Esq. |
| Brunson Chandler & Jones, PLLC | Stephen Cohen, Esq. |
| 175 South Main Street, Suite 1410 | Sheppard, Mullin, Richter & Hampton LLP |
| Salt Lake City, UT 84111 | 30 Rockefeller Plaza |
| Tel.: (801) 303-5721 | New York, NY 10112-0015 |
| | Tel.: (212) 653-8700 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| | |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| | |
| | Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. The securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION, DATED [●], 2023 |
KINDLY MD, INC.

[ ] Units
Each Unit Consisting of One Share of Common Stock
One Warrant to Purchase One Share of Common Stock and
One Non-tradeable Warrant to Purchase One-half of One Share of Common Stock
This is the initial public offering of KindlyMD, Inc. (the “Company,” “KindlyMD,” “we,” “our,” or “us”). We are offering [ ] units (each a “Unit” and collectively, the “Units”), at an initial public offering price of $[ ] per Unit with each Unit consisting of one share of our common stock, no par value (“Common Stock”), one tradeable warrant (each, a “Tradeable Warrant,” collectively, the “Tradeable Warrants”) to purchase one share of Common Stock at an exercise price of $[ ] per share, and one non-tradeable warrant to purchase one-half of one share of Common Stock (each, a “Non-tradeable Warrant,” collectively, the “Non-tradeable Warrants”; together with the Tradeable Warrants, each a “Warrant,” collectively, the “Warrants”) at an anticipated exercise price of $[ ] per share. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The shares of our Common Stock and the Warrants comprising the Units are immediately separable upon issuance and will be issued separately. The Warrants included in the Units will be exercisable immediately upon issuance, and will expire [●] years from the date of issuance. This offering also includes the shares of Common Stock issuable from time to time upon exercise of the Warrants. The Warrants will be issued in book-entry form pursuant to a warrant agency agreement between us and [●] as warrant agent.
Prior to this offering, there has been no public market for our Common Stock or Warrants. It is currently estimated that the initial public offering price will be between $ and $ per unit.
In connection with this offering, we have applied to have our Common Stock and Warrants listed on the Nasdaq Capital Market under the symbols “[ ]” and “[ ],” respectively. No assurance can be given that our listing application will be approved or, if we receive approval, that a trading market will develop, if developed, that it will be sustained. If our listing application is not approved by The Nasdaq Stock Market LLC (“Nasdaq”), we will not consummate the offering and will terminate this offering.
While we may be a “controlled company” under the rules of Nasdaq, immediately after consummation of this offering, we do not intend to avail ourselves of the corporate governance exemptions afforded to a “controlled company” under the rules of Nasdaq. See “Risk Factors—Risks Related to Our Common Stock and this Offering.”
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus, before purchasing any of the securities offered by this prospectus.
| | | Per Unit | | | Total | |
| Offering price | | $ | [ ] | | | $ | [ ] | |
| Underwriter’s discounts and commissions (1) | | $ | [ ] | | | $ | [ ] | |
| Proceeds to our company before expenses | | $ | [ ] | | | $ | [ ] | |
| (1) | We have agreed to issue WallachBeth Capital, LLC, the representative of the underwriters (“WallachBeth” or the “Representative”), warrants to purchase shares of our Common Stock (the “Representative Warrants”), and to reimburse the underwriters for certain expenses. See “Underwriting” on page 61 for additional information regarding total underwriter compensation. |
We have granted the representative of the underwriters an option to purchase from us, at the public offering price, less the underwriting discounts and commissions, up to an additional shares of Common Stock and/or additional Tradeable Warrants to purchase shares of Common Stock, and/or Non-Tradeable Warrants to purchase shares of Common Stock, in any combination thereof, within 45 days from the date of this prospectus to cover over-allotments, if any.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The underwriters expect to deliver the securities against payment in New York, New York on or about __________________, 2023.
Sole Book-Running Manager

TABLE OF CONTENTS
Through and including __________________, 2023 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriter and with respect to their unsold allotments or subscriptions.
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not rely on any unauthorized information or representations. This prospectus is an offer to sell only the shares of Common Stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
You should rely only on the information contained in this prospectus. Neither we nor the placement agent have authorized anyone to provide any information or to make any representations other than those contained in this prospectus we have prepared. We take no responsibility for and can provide no assurance as to the reliability of any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date. You should also read this prospectus together with the additional information described under “Where You Can Find More Information.”
Unless the context otherwise requires, we use the terms “we,” “us,” “Company,” “KindlyMD,” “Kindly,” and “our” to refer to Kindly MD, Inc. and its consolidated subsidiaries.
Solely for convenience, our trademarks and tradenames referred to in this prospectus, may appear without the ® or ™ symbols, but such references are not intended to indicate in any way that we will not assert, to the fullest extent under applicable law, our rights to these trademarks and tradenames. All other trademarks, service marks and trade names included or incorporated by reference into this prospectus, or the accompanying prospectus are the property of their respective owners.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our Common Stock. You should read the entire prospectus carefully, including the “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our combined financial statements and the related notes thereto that are included elsewhere in this prospectus, before making an investment decision. Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “KindlyMD,” the “Company,” “we,” “us,” “Kindly,” and “our” refer to Kindly MD, Inc., and its subsidiaries.
Overview
Kindly MD, Inc. (“We”, “Us”, “Company”, KindlyMD” or “Kindly”) is a Utah company formed in 2019. KindlyMD is a healthcare data company, focused on holistic pain management and ending the opioid epidemic. KindlyMD offers direct health care to patients integrating prescription medicine and behavioral health services to reduce opioid use in the chronic pain patient population. Kindly believes these methods will help prevent and reduce addiction and dependency on opiates. Our specialty outpatient clinical services are offered on a subscription and fee-for-service basis to augment traditional healthcare The Company offers evaluation and management, including, but not limited to chronic pain, functional medicine, cognitive behavioral therapy, intravenous infusion therapy, trauma and addiction therapy, recovery support services, overdose education efforts, peer support, limited urgent care, dermatology, preventative medicine, travel services, and hormone therapy. Through its focus on an imbedded model of prescriber and therapist teams, KindlyMD is working to develop patient-specific care programs with a specific mission to reduce opioid use in the patient population while successfully treating patients with effective and evidence based non-opioid alternatives in close conjunction with behavioral therapy.
Beyond its treatment of patients, KindlyMD collects data focused on why and how patients turn to alternative treatments to reduce prescription medication use and addiction. The Company captures all relevant datapoints to assist and appropriately treat each individual patient. This also results in valuable data for the Company and the Company’s investors. We strive to become a source for evidence-based guidelines, data, treatment models, and education in the fight against the opioid crisis in America.
Business Revenue Streams
We operate across various revenue streams: (i) medical evaluation and treatment visits for chronic pain and illness, (ii) subscription outpatient medicine (pain clinic medicine and behavioral therapy), (iii) data collection and research, (iv) education partnerships, (vi) online and email campaign marketing revenue, (vii) service affiliate agreements, and (viii) retail sale of dietary supplements, and products.
Further information about our revenue streams can be found in the “Business” section on page 38.
Recent Developments
KindlyMD recently executed a four-year lease for 5,321 square feet of clinic and office space in Murray Utah. This expansion complements our existing clinic location in Millcreek Utah and offers eight more exam and consultation rooms to our local clinical capacity. It also allowed KindlyMD to consolidate a smaller location into a larger location, which will improve the cost-per-patient-visit ratio. We anticipate this expansion to increase revenue opportunity by up to $270,000 per month in the summer of 2023. This additional space coincides with the expansion of services offering to occur in the fall of 2022 to include medication management and imbedded behavioral health services for patients.
Market Opportunity
In the Utah market alone, KindlyMD has a unique opportunity for growth based on service line expansion into pain medicine management, and hormone therapy. Demand for both opioid and non-opioid pain treatment continues to increase due to the growing geriatric population, safe and effective access to non-opioid drugs, and increased prevalence of diagnoses such as osteoarthritis and migraines. Rising demand for surgeries, increasing awareness, availability of treatment options, and the willingness to seek treatment are expected to complement the growth of the population of patients seeking treatment for pain and/or chronic pain medication use. KindlyMD, already a large market share player in the Utah non-opioid treatment space, expects expansion of patients with the inclusion of opioid medication management.
Furthermore, the behavioral therapy industry is slated to grow with the integration of addiction and trauma based cognitive behavioral therapy (CBT) and inclusion of Ketamine and other infusion-based treatment options. Integration of these therapies with traditional pain management will provide a source of revenue as well as behavioral data and clinical research to develop valuable treatment programs, products, and further enhance legislative lobbying efforts toward wider acceptance of safe and effective non-opioid alternative therapies.
Growth Strategy
KindlyMD is leveraging healthcare standards and infrastructure to build a multi-state network of in-person clinics, telemedicine resources, and wholly owned subsidiaries in the pain treatment space. Our expansion approach considers metrics such as prescribing laws and regulations, rates of opioid prescriptions, inclusion of behavioral therapy outcomes, non-opioid alternative medicine access, including medical cannabis, and existing specialty clinic operations in each market. Our goal is to expand into other areas of Utah as well as five additional markets in 2023 through expansion of clinic locations and acquisitions.
KindlyMD collects valuable data from interactions with people online, via telecommunication, in-person patient interactions, and through our products. Clients provide some of this data directly, as do clinicians, and staff by collecting data about interactions, product and medication use, experiences, and behavior. In collecting data from these interactions, we collect and collate data from different contexts and third parties to provide a more seamless, consistent, and uniquely personalized experience, to make informed business decisions, to make clinical decisions, and for other legitimate business purposes. We intend to further use and analyze such data to allow us to become a large and specialized healthcare data company working to reduce opioid use, track product use and sales data, which will be highly valuable to the healthcare industry, the alternative medicine industry, and the pharmaceutical industry.
Competitive Strengths
KindlyMD is one of the largest providers of medical evaluation and management services related to treatment recommendations within the medical cannabis program in Utah. We operate with normative traditional medical standards and practices and set a high standard of care. Kindly MD with year over year profitability to-date, including during the COVID-19 Pandemic. Our leadership team is highly skilled in healthcare technology, customer service, patient care, and high-touch interactions. We value a culture of service to the patient above all.
Our model of healthcare is unique, blending prescribers and licensed behavioral health clinicians into every patient care plan while leveraging non-opioid alternative medicine where indicated. Although there are several large healthcare networks using an integrated model in low-income and high-risk population care, we know of no other large pain clinic in Utah or the US which uses this integration model combined with a willingness to incorporate non-traditional medicine. We are also one of a limited number of specialty providers who allow patients to utilize non-opioid alternative medications, such as medical cannabis, concomitantly with opioids with medical supervision by a licensed integration team.
Our competition, respectively, are traditional medication pain clinics as well as other non-opioid specialty alternative medicine clinics in Utah.
Corporate Information
Our principal executive offices are located at 230 W 400 S, Suite 201 Salt Lake City, UT 84104. Our telephone number is (385)388-8220. Our corporate website address is located at www.kindlymd.com. The information contained in, or accessible from, our website or any other website does not constitute a part of this prospectus.
Impact of COVID-19 Pandemic
The recent outbreak of COVID-19 has spread across the globe and is impacting worldwide economic activity. In response to the COVID-19 pandemic, during 2020 and 2021, the Company established policies and protocols to address safety considerations. The extent to which the COVID-19 pandemic will continue to affect the Company’s business, financial condition, liquidity, and the Company’s operating results will depend on future developments, which are highly uncertain and cannot be predicted. It will depend on various factors including the duration and severity of the outbreak, the severity, or variants of COVID-19, including the omicron variant and its subvariants, and the effectiveness, acceptance, and availability of vaccines in countries throughout the world, and new information which may emerge concerning the appropriate responses if and to the extent that the availability of vaccines reduces restrictions imposed during the pandemic.
Inflation Risk
We do not believe that inflation has had a material effect on our business, results of operations, or financial condition. Nonetheless, if our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs. Our inability or failure to do so could harm our business, results of operations, or financial condition.
Implications of Being a Smaller Reporting Company
As a smaller reporting company, we are eligible for exemptions from various reporting requirements applicable to other public companies that are not smaller reporting companies, including, but not limited to:
| | ● | Reduced disclosure obligations (e.g., matters regarding executive compensation) in our periodic reports, proxy statements and registration statements; and |
| | | |
| | ● | Not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). |
We will remain a smaller reporting company until the end of the fiscal year in which (i) we have a public common equity float of more than $250 million, or (ii) we have annual revenues for the most recently completed fiscal year of more than $100 million plus we have a public common equity float or public float of more than $700 million. We also would not be eligible for status as smaller reporting company if we become an investment company, an asset-backed issuer or a majority-owned subsidiary of a parent company that is not a smaller reporting company.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our shareholders may be different from what you might receive from other public reporting companies in which you hold equity interests.
THE OFFERING
| Issuer: | | Kindly MD, Inc. |
| | | |
| Securities offered(1): | | [ ] Units, at a public offering price of $[ ] per Unit, each consisting of (i) one share of Common Stock, (ii) one Tradeable Warrant to purchase one share of Common Stock and (iii) one Non-tradeable Warrant to purchase one-half of one share of Common Stock. The Units will not be certificated or issued in stand-alone form. The shares of our Common Stock and the Warrants comprising the Units are immediately separable upon issuance and will be issued separately; but will be purchased together in this offering. |
| Description of Warrants included in Units: | | Each Unit consists of one share of Common Stock and two Warrants: one Tradeable Warrant to purchase one share of Common Stock and one Non-tradeable Warrant to purchase one-half of one share of Common Stock. The exercise price of the Tradeable Warrants is $[●] per share ([●]% of the public offering price per Unit), and the exercise price of the Non-tradeable Warrant iswhole $[ ] per share ([ ]% of the public offering price per one Unit). Each Warrant will be exercisable immediately upon issuance and will expire five years after the initial issuance date. The terms of the Warrants will be governed by a warrant agency agreement, dated as of the effective date of this offering, between us and [●], Inc. as the warrant agent (the “Warrant Agent”). This prospectus also relates to the offering of the shares of Common Stock issuable upon exercise of the Warrants. For more information regarding the Warrants, you should carefully read the section titled “Description of Our Securities—Warrants” in this prospectus. |
| | | |
| Over-allotment option | | We have granted the underwriters an option for a period of up to 45 days to purchase, at the public offering price, less underwriting discounts and commissions, up to shares of additional Common Stock, and/or Tradeable Warrants to purchase an additional shares of Common Stock, and/or Non-tradeable Warrants to purchase an additional shares of Common Stock, or any combination of additional shares of Common Stock and Warrants, representing, in the aggregate, up to [ ]% of the number of Units sold in this offering to cover over-allotments, if any. |
| | | |
| Common Stock outstanding before this offering: | | 4,434,596 shares |
| | | |
| Common Stock outstanding after the offering(2): | | [ ] shares (assuming that none of the Warrants are exercised) or shares if the Warrants offered hereby are exercised in full. If the underwriters exercise in full the over-allotment option to purchase an additional shares of Common Stock and/or Warrants to purchase an additional shares of Common Stock, the total number of shares of Common Stock immediately after this offering would be shares (assuming that none of the Warrants are exercised) or shares if the Warrants offered hereby are exercised in full, subject to the assumptions set forth below. |
| | | |
| Use of proceeds: | | We estimate that the net proceeds to us from this offering will be approximately $[ ] million, or approximately $[●] million if the underwriters exercise their over-allotment option in full, assuming an offering price of $[●] per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of this offering primarily for general corporate purposes, including capital expenditures, labor, real estate, marketing and sales, technology development, and other expenses. See “Use of Proceeds” for additional information. |
| | | |
| Underwriters’ compensation: | | In connection with this offering, the underwriters will receive an underwriting discount equal to 9% of the gross proceeds from the sale of Units in the offering. We will also reimburse the underwriters for certain out-of-pocket actual expenses related to the offering and pay them 1.5% of the aggregate sales price sold in the Offering as non-accountable expenses. For additional information regarding our arrangement with the underwriters, please see “Underwriting.” |
| | | |
| Representative Warrants: | | Upon the closing of this offering, we have agreed to issue to WallachBeth warrants that will expire on the fifth anniversary of the commencement date of sales in this offering, entitling the representative to purchase 6% of the number of shares of Common Stock sold in this offering will have an exercise price equal to 115% of the public offering price per Unit set forth on the cover page of this prospectus (or $[ ] per share, which is the midpoint of the price range set forth on the cover page of this prospectus), will provide for a “cashless” exercise, and will contain certain antidilution adjustments (but excluding any price based antidilution). For additional information regarding the representative’s warrants, see “Underwriting—Representative’s Warrants”. |
| Proposed Nasdaq Capital Market trading symbol and listing: | | We have applied to the Nasdaq Capital Market to list our Common Stock under the symbol “[ ]” and our Tradeable Warrants under the symbol “[ ].” No assurance can be given that our listing application will be approved. |
| | | |
| Dividend policy: | | We have not historically paid dividends on our Common Stock and do not anticipate paying dividends on our Common Stock for the foreseeable future. |
| | | |
Transfer agent/Warrant Agent: | | VStock Transfer, LLC |
| | | |
| Risk factors: | | See “Risk Factors” beginning on page 10 and the other information contained in this prospectus for a discussion of factors you should carefully consider before investing in our securities. |
| (1) | The actual number of Units we will offer and the actual price per Unit will be determined based on the actual public offering. |
| (2) | The total number of shares of Common Stock that will be outstanding after this offering is based on 4,434,956 shares of Common Stock outstanding as of April 25; 2023. Unless otherwise indicated, the shares outstanding after this offering excludes the following: |
| | ● | [●] shares of our Common Stock issuable upon the exercise of the Tradeable Warrants, and/or the exercise of Non-tradeable Warrants to be issued as part of the Units; |
| | | |
| | ● | [●] shares of our Common Stock issuable upon exercise of the Representative Warrants. |
Except as otherwise indicated herein, all information in this prospectus assumes, including the number of shares of common stock that will be outstanding after this offering, assumes or gives effect to
| | ● | no exercise by the underwriters of their option to purchase an additional [●] shares of common stock and/or Warrant; |
| | | |
| | ● | no exercise of outstanding options after; |
SUMMARY OF CONSOLIDATED FINANCIAL INFORMATION
The following table summarizes our financial data. The following summary consolidated statements of operations and balance sheet data for the fiscal years ended December 31, 2022 and 2021, have been derived from our unaudited consolidated financial statements included elsewhere in this prospectus. The historical financial data presented below is not necessarily indicative of our financial results in future periods. You should read the summary consolidated financial data in conjunction with those financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our consolidated financial statements are prepared and presented in accordance with United States generally accepted accounting principles, or U.S. GAAP. Our consolidated financial statements have been prepared on a basis consistent with our audited financial statements and include all adjustments, consisting of normal and recurring adjustments that we consider necessary for a fair presentation of the financial position and results of operations as of and for such periods.
Statement of Operations Data:
| | | For the Years Ended
December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| REVENUE | | $ | 3,787,077 | | | $ | 2,504,319 | |
| | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | |
| Costs of goods sold | | | 152,385 | | | | 87,124 | |
| General and administrative | | | 2,151,563 | | | | 853,582 | |
| Personnel | | | 4,176,542 | | | | 1,502,273 | |
| TOTAL OPERATING EXPENSES | | | 6,480,490 | | | | 2,442,979 | |
| | | | | | | | | |
| INCOME (LOSS) FROM OPERATIONS | | | (2,693,413 | ) | | | 61,340 | |
| | | | | | | | | |
| OTHER INCOME (EXPENSE) | | | | | | | | |
| Other income and expense | | | 152,820 | | | | 87,996 | |
| | | | | | | | | |
| TOTAL OTHER INCOME (EXPENSE) | | | 152,820 | | | | 87,996 | |
| | | | | | | | | |
| INCOME (LOSS) BEFORE INCOME TAXES | | | (2,540,593 | ) | | | 149,336 | |
| | | | | | | | | |
| Provision for income taxes | | | - | | | | - | |
| NET INCOME (LOSS) | | $ | (2,540,593 | ) | | $ | 149,336 | |
| | | | | | | | | |
| NET INCOME (LOSS) PER COMON SHARE-BASIC AND DILUTED | | $ | (1.56 | ) | | $ | - | |
| | | | | | | | | |
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING – BASIC AND DILUTED | | | 1,623,386 | | | | - | |
1 See Note to our financial statements for an explanation of the method used to compute basic and diluted net loss per share.
Balance Sheet Data:
| | | December 31, 2022
(unaudited) | |
| | | Actual | | | Pro
Forma | | | Pro Forma,
As Adjusted(1)(2) | |
| Cash and cash equivalents | | $ | 186,918 | | | $ | | | | $ | | |
| Working capital | | $ | (40,506 | ) | | | | | | | | |
| Total assets | | $ | 1,001,269 | | | | | | | | | |
| Total liabilities | | $ | 620,255 | | | | | | | | | |
| Accumulated deficit | | $ | (2,540,593 | ) | | | | | | | | |
| Total stockholders’ equity | | $ | 381,014 | | | | | | | | | |
1 On a pro forma as adjusted basis to give further effect to our issuance and sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
2 Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity (deficit) by approximately $ , assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1.0 million shares in the number of shares offered by us at the assumed initial public offering price per share, the midpoint of the price range listed on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity (deficit) by approximately $ .
RISK FACTORS
An investment in our securities involves a high degree of risk. Before making an investment decision, you should carefully consider the risks that are described in this section You should also read the sections entitled “Cautionary Note Regarding Forward-Looking Statements” on page 24 of this prospectus. Additional risks not presently known or that we currently deem immaterial could also materially and adversely affect us. You should consult your own financial and legal advisors as to the risks entailed by an investment in our securities and the suitability of investing in our securities in light of your particular circumstances. If any of the risks contained in this prospectus develop into actual events, our assets, business, cash flows, condition (financial or otherwise), credit quality, financial performance, liquidity, long-term performance goals, prospects, and/or results of operations could be materially and adversely affected, the trading price of our Common Stock could decline and you may lose all or part of your investment. Some statements in this prospectus, including such statements in the following risk factors, constitute forward-looking statements.
The Company operates in an environment that involves many risks and uncertainties. The risks and uncertainties described in this section are not the only risks and uncertainties that we face. Additional risks and uncertainties that presently are not considered material or are not known to us, and therefore are not mentioned herein, may impair our business operations. If any of the risks described actually occur, our business, operating results, financial position, and value of our securities could be adversely affected.
RISKS RELATED TO OUR BUSINESS
The novel coronavirus (COVID-19) pandemic may have unexpected effects on our business, financial condition and results of operations.
In March 2020, the World Health Organization declared COVID-19 a global pandemic, and governmental authorities around the world have implemented measures to reduce the spread of COVID-19. These measures have adversely affected workforces, customers, supply chains, consumer sentiment, economies, and financial markets, and, along with decreased consumer spending, have led to an economic downturn across many global economies.
The COVID-19 pandemic has rapidly escalated in the United States, creating significant uncertainty and economic disruption, and leading to record levels of unemployment nationally. Numerous state and local jurisdictions have imposed, and others in the future may impose, shelter-in-place orders, quarantines, shut-downs of non-essential businesses, and similar government orders and restrictions on their residents to control the spread of COVID-19. Such orders or restrictions have resulted in temporary facility closures (including certain of our third-party VRCs), work stoppages, slowdowns and travel restrictions, among other effects, thereby adversely impacting our operations. In addition, we expect to be impacted by a downturn in the United States economy, which could have an adverse impact on discretionary consumer spending and may have a significant impact on our business operations and/or our ability to generate revenues and profits.
In response to the COVID-19 disruptions, we have implemented a number of measures designed to protect the health and safety of our staff and contractors. These measures include restrictions on non-essential business travel, the institution of work-from-home policies wherever feasible and the implementation of strategies for workplace safety at our facilities that remain open. We are following the guidance from public health officials and government agencies, including implementation of enhanced cleaning measures, social distancing guidelines and wearing of masks.
The extent to which COVID-19 ultimately impacts our business, financial condition and results of operations will depend on future developments, which are highly uncertain and unpredictable, including new information which may emerge concerning the severity and duration of the COVID-19 outbreak and the effectiveness of actions taken to contain the COVID-19 outbreak or treat its impact, among others. Additionally, while the extent to which COVID-19 ultimately impacts our operations will depend on a number of factors, many of which will be outside of our control. The COVID-19 outbreak is evolving and new information emerges daily; accordingly, the ultimate consequences of the COVID-19 outbreak cannot be predicted with certainty.
In addition to the COVID-19 disruptions possibility adversely impacting our business and financial results, they may also have the effect of heightening many of the other risks described in “Risk Factors,” including risks relating to changes due to our limited operating history; our ability to generate sufficient revenue, to generate positive cash flow; our relationships with third parties, and many other factors. We will endeavor to minimize these impacts, but there can be no assurance relative to the potential impacts that may be incurred.
Uncertainty of profitability
Our business strategy may result in meaningful volatility of revenues, loses and/or earnings. As we will only develop a limited number of business efforts, services and products at a time, our overall success will depend on a limited number of business initiatives, which may cause variability and unsteady profits and losses depending on the products and/or services offered and their market acceptance.
Our revenues and our profitability may be adversely affected by economic conditions and changes in the market for our products and/or services. Our business is also subject to general economic risks that could adversely impact the results of operations and financial condition.
We may not be able to continue our business as a going concern.
Management plans to raise additional capital through the sale of shares of Common Stock to pursue business development activities, but there are no assurances of success relative to the efforts.
Because of the anticipated nature of the services that we offer and attempt to develop, it is difficult to accurately forecast revenues and operating results and these items could fluctuate in the future due to a number of factors. These factors may include, among other things, the following:
| ● | Our ability to raise sufficient capital to take advantage of opportunities and generate sufficient revenues to cover expenses. |
| | | |
| ● | Our ability to source strong opportunities with sufficient risk adjusted returns. |
| | | |
| ● | Our ability to manage our capital and liquidity requirements based on changing market conditions. |
| | | |
| ● | The amount and timing of operating and other costs and expenses. |
| | | |
| ● | The nature and extent of competition from other companies that may reduce market share and create pressure on pricing and investment return expectations. |
Our business may suffer if we are unable to attract or retain talented personnel.
Our success will depend in large measure on the abilities, expertise, judgment, discretion, integrity, and good faith of Management, as well as other personnel. We have a small management team, and the loss of a key individual or our inability to attract suitably qualified replacements or additional staff could adversely affect our business. Our success also depends on the ability of Management to form and maintain key commercial relationships within the marketplace. No assurance can be given that key personnel will continue their association or employment with us or that replacement personnel with comparable skills will be found. If we are unable to attract and retain key personnel and additional employees, our business may be adversely affected. We do not maintain key-man life insurance on any of our executive employees.
The loss of key Management personnel could adversely affect our business.
We depend on the continued services of our executive officer and senior consulting team and are responsible for our day-to-day operations. Our success depends in part on our ability to retain executive officers, to compensate executive officers at attractive levels, and to continue to attract additional qualified individuals to our management team. Although we have entered into an employment agreement with our Chief Executive Officer, and do not believe our Chief Executive Officer is planning to leave or retire in the near term, we cannot assure you that he will remain with us. The loss or limitation of the services of any of our executives or members of our senior management team, or the inability to attract additional qualified management personnel, could have a material adverse effect on our business, financial condition, results of operations, or independent associate relations.
The lack of available and cost-effective directors and officer’s insurance coverage in our industry may cause us to be unable to attract and retain qualified executives, and this may result in our inability to further develop our business.
Our business depends on attracting independent directors, executives, and senior management to advance our business plans. We currently do not have directors and officer’s insurance to protect our directors, officers, and the company against the possible third-party claims. This is due to the significant lack of availability of such policies in the cannabis industry at reasonably competitive prices. As a result, the Company and our executive directors and officers are susceptible to liability claims arising by third parties, and as a result, we may be unable to attract and retain qualified independent directors and executive management causing the development of our business plans to be impeded as a result.
Management of growth will be necessary for us to be competitive.
Successful expansion of our business will depend on our ability to effectively attract and manage staff, strategic business relationships, and shareholders. Specifically, we will need to hire skilled management and technical personnel as well as manage partnerships to navigate shifts in the general economic environment. Expansion has the potential to place significant strains on financial, management, and operational resources, yet failure to expand will inhibit our profitability goals.
The failure to enforce and maintain our intellectual property rights could adversely affect the value of the Company.
The success of our business will partially depend on our ability to protect our intellectual property. As of the date hereof, we do not own any federally registered patents or trademarks. The unauthorized use of our intellectual property could diminish the value of our business, which would have a material adverse effect on our financial condition and results of operation.
Laws and regulations affecting the medical marijuana industry are constantly changing, which could detrimentally affect our operation.
Local, state, and federal medical marijuana laws and regulations are broad in scope and subject to evolving interpretations, which could require us to incur substantial costs associated with compliance or alter certain aspects of our business plan. In addition, violations of these laws, or allegations of such violations, could disrupt certain aspects of our business plan and result in a material adverse effect on certain aspects of our planned operations. In addition, it is possible that regulations may be enacted in the future that will be directly applicable to certain aspects of our businesses. We cannot predict the nature of any future laws, regulations, interpretations, or applications, nor can we determine what effect additional governmental regulations or administrative policies and procedures, when and if promulgated, could have on our business.
If we incur substantial liability from litigation, complaints, or enforcement actions, our financial condition could suffer.
Our participation in the medical marijuana industry may lead to litigation, formal or informal complaints, enforcement actions, and inquiries by various federal, state, or local governmental authorities against us. Litigation, complaints, and enforcement actions could consume considerable amounts of financial and other corporate resources, which could have a negative impact on our sales, revenue, profitability, and growth prospects.
There can be no assurance that our current and future strategic alliances or expansions of scope of existing relationships will have a beneficial impact on our business, financial condition and results of operations.
We may enter into strategic alliances and partnerships with third parties that we believe will complement or augment our existing business. Our ability to complete strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance our business and may involve risks that could adversely affect us, including significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of additional debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that our existing strategic alliances will continue to achieve, the expected benefits to our business or that we will be able to consummate future strategic alliances on satisfactory terms, if at all. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
We may be subject to growth-related risks.
We may be subject to growth-related risks, including capacity constraints and pressure on our internal systems and controls. Our ability to manage growth effectively will require us to continue to implement and improve our operational and financial systems and to expand, train and manage our employee base. Our inability to deal with this growth may have a material adverse effect on our business, prospects, revenue, results of operation and financial condition.
We may be subject to litigation.
We may become party to litigation from time to time in the ordinary course of business, which could adversely affect our business. Should any litigation in which we become involved be determined against us, such a decision could adversely affect our ability to continue operating and could potentially use significant resources. Even if we are involved in litigation and win, litigation can redirect significant resources of Kindly MD Inc. and/or its subsidiaries.
We may face unfavorable publicity or consumer perception.
Management believes the pain management, cannabis, and alternative medicine industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the treatment offered and outcomes produced. Consumer perception of our services may be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding opioids, cannabis, as well as alternative medicine services. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the prescription medicine, behavioral therapy industry, cannabis, or alternative medicine market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that is perceived as less favorable than, or questions earlier research reports, findings or publicity could have a material adverse effect on the demand for our services. Our dependence upon consumer perceptions means that such adverse reports, whether or not accurate or with merit, could ultimately have a material adverse effect on our business, results of operations, financial condition and cash flows. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of treatments in general, or our services specifically, or associating the consumption of prescription or non-prescription medications, cannabis, or any other products with illness or other negative effects or events, could have such a material adverse effect.
We are subject to general economic risks.
Our operations could be affected by the economic context should the unemployment level, interest rates or inflation reach levels that influence consumer trends and spending and, consequently, impact our sales and profitability.
Provisions in our governing documents and Utah law may have an anti-takeover effect, and there are substitutional regulatory limitations on changes of control of bank holding companies.
Our corporate organizational documents and provisions of federal and state law to which we are subject contain certain provisions that could have an anti-takeover effect and may delay, make more difficult or prevent an attempted acquisition that you may favor or an attempted replacement of our board of directors or management.
RISKS OF GOVERNMENT ACTION AND REGULATORY UNCERTAINTY
Our use, disclosure, and other processing of personal information, including health information, is subject to the Health Insurance Portability and Accountability Act (HIPAA), and other federal, state, and foreign data privacy and security laws and regulations, and our failure to comply with those laws and regulations or to appropriately secure the information we hold could result in significant liability or reputational harm and, in turn, a material adverse effect on our client base, customer base and revenue.
In the course of offering personalized health and wellness recommendations, we collect a substantial amount of personalized health information. Numerous state and federal laws and regulations govern the collection, dissemination, use, privacy, confidentiality, security, availability, integrity and other processing of protected health information (PHI), and other types of personal information. For example, HIPAA establishes a set of national privacy and security standards for the protection PHI by health plans, healthcare clearinghouses and certain healthcare providers, referred to as covered entities, and the business associates with whom such covered entities contract for services, as well as their covered subcontractors. When we act in the capacity of a business associate under HIPAA, we execute business associate agreements with our clients.
HIPAA requires covered entities and business associates, such as us, to develop and maintain policies and procedures with respect to PHI that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information.
Violations of HIPAA may result in significant civil and criminal penalties. HIPAA also authorizes state attorneys general to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of duties related to PHI.
In addition, HIPAA mandates that the Secretary of HHS conduct periodic compliance audits of HIPAA covered entities and business associates for compliance with the HIPAA privacy and security rules.
HIPAA further requires that patients be notified of any unauthorized acquisition, access, use or disclosure of their unsecured PHI that compromises the privacy or security of such information, with certain exceptions related to unintentional or inadvertent use or disclosure by employees or authorized individuals. HIPAA requires such notifications to be made “without unreasonable delay and in no case later than 60 calendar days after discovery of the breach.” If a breach affects 500 patients or more, it must be reported to HHS without unreasonable delay, and HHS will post the name of the breaching entity on its public web site. Breaches affecting 500 patients or more in the same state or jurisdiction must also be reported to the local media. If a breach involves fewer than 500 people, the covered entity must record it in a log and notify HHS at least annually.
In addition to HIPAA, numerous other federal, state, and foreign laws and regulations protect the confidentiality, privacy, availability, integrity and security of health-related and other personal information. These laws and regulations in many cases are more restrictive than, and may not be preempted by, HIPAA and its implementing rules. These laws and regulations are often uncertain, contradictory, and subject to changed or differing interpretations, and we expect new laws, rules and regulations regarding privacy, data protection, and to be proposed and enacted in the future. Further, many state attorneys general are interpreting existing federal and state consumer protection laws to impose evolving standards for the online collection, use, dissemination and security of health-related and other personal information. Courts may also adopt the standards for fair information practices promulgated by the FTC, which concern consumer notice, choice, security and access. Consumer protection laws require us to publish statements that describe how we handle personal information and choices individuals may have about the way we handle their personal information. If such information that we publish is considered untrue, we may be subject to government claims of unfair or deceptive trade practices, which could lead to significant liabilities and consequences. Furthermore, according to the FTC, violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of Section 5(a) of the FTC Act.
We may become subject to the Anti-Kickback Statute, Stark Law, False Claims Act, Civil Monetary Penalties Law and may be subject to analogous provisions of applicable state laws and could face substantial penalties if we fail to comply with such laws.
There are several federal laws addressing fraud and abuse that apply to businesses that receive reimbursement from a federal health care program. There are also a number of similar state laws covering fraud and abuse with respect to, for example, private payors, self-pay and insurance. Currently, we receive a substantial percentage of our revenue from private payors and from Medicare. Accordingly, our business is subject to federal fraud and abuse laws, such as the Anti-Kickback Statute, the Stark Law, the False Claims Act, the Civil Monetary Penalties Law and other similar laws. Moreover, we are already subject to similar state laws. We believe we have operated, and intend to continue to operate, our business in compliance with these laws. However, these laws are subject to modification and changes in interpretation and are enforced by authorities vested with broad discretion. Federal and state enforcement entities have significantly increased their scrutiny of healthcare companies and providers which has led to investigations, prosecutions, convictions and large settlements. We continually monitor developments in this area. If these laws are interpreted in a manner contrary to our interpretation or are reinterpreted or amended, or if new legislation is enacted with respect to healthcare fraud and abuse, illegal remuneration, or similar issues, we may be required to restructure our affected operations to maintain compliance with applicable law. There can be no assurances that any such restructuring will be possible or, if possible, would not have a material adverse effect on our results of operations, financial position, or cash flows.
Anti-Kickback Statute
A federal law commonly referred to as the “Anti-Kickback Statute” prohibits the knowing and willful offer, payment, solicitation or receipt of remuneration, directly or indirectly, in return for the referral of patients or arranging for the referral of patients, or in return for the recommendation, arrangement, purchase, lease or order of items or services that are covered, in whole or in part, by a federal healthcare program such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value such as gifts, discounts, rebates, waiver of payments or providing anything at less than its fair market value. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the PPACA, amended the intent requirement of the Anti-Kickback Statute such that a person or entity can be found guilty of violating the statute without actual knowledge of the statute or specific intent to violate the statute. Further, the PPACA now provides that claims submitted in violation of the Anti-Kickback Statute constitute false or fraudulent claims for purposes of the federal False Claims Act, or FCA, including the failure to timely return an overpayment. Many states have adopted similar prohibitions against kickbacks and other practices that are intended to influence the purchase, lease or ordering of healthcare items and services reimbursed by a governmental health program or state Medicaid program. Some of these state prohibitions apply to remuneration for referrals of healthcare items or services reimbursed by any third-party payor, including commercial payors and self-pay patients.
Stark Law
Section 1877 of the Social Security Act, or the Stark Law, prohibits a physician from referring a patient to an entity for certain “designated health services” reimbursable by Medicare if the physician (or close family members) has a financial relationship with that entity, including an ownership or investment interest, a loan or debt relationship or a compensation relationship, unless an exception to the Stark Law is fully satisfied. The designated health services covered by the law include, among others, laboratory and imaging services. Some states have self-referral laws similar to the Stark Law for Medicaid claims and commercial claims.
Violation of the Stark Law may result in prohibition of payment for services rendered, a refund of any Medicare payments for services that resulted from an unlawful referral, $15,000 civil monetary penalties for specified infractions, criminal penalties, and potential exclusion from participation in government healthcare programs, and potential false claims liability. The repayment provisions in the Stark Law are not dependent on the parties having an improper intent; rather, the Stark Law is a strict liability statute and any violation is subject to repayment of all amounts arising out of tainted referrals. If physician self-referral laws are interpreted differently or if other legislative restrictions are issued, we could incur significant sanctions and loss of revenues, or we could have to change our arrangements and operations in a way that could have a material adverse effect on our business, prospects, damage to our reputation, results of operations and financial condition.
False Claims Act
The FCA prohibits providers from, among other things, (1) knowingly presenting or causing to be presented, claims for payments from the Medicare, Medicaid or other federal healthcare programs that are false or fraudulent; (2) knowingly making, using or causing to be made or used, a false record or statement to get a false or fraudulent claim paid or approved by the federal government; or (3) knowingly making, using or causing to be made or used, a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government. The “qui tam” or “whistleblower” provisions of the FCA allow private individuals to bring actions under the FCA on behalf of the government. These private parties are entitled to share in any amounts recovered by the government, and, as a result, the number of “whistleblower” lawsuits that have been filed against providers has increased significantly in recent years. Defendants found to be liable under the FCA may be required to pay three times the actual damages sustained by the government, plus civil penalties ranging between $5,500 and $11,000 for each separate false claim.
There are many potential bases for liability under the FCA. The government has used the FCA to prosecute Medicare and other government healthcare program fraud such as coding errors, billing for services not provided, and providing care that is not medically necessary or that is substandard in quality. The PPACA also provides that claims submitted in connection with patient referrals that result from violations of the Anti-Kickback Statute constitute false claims for the purpose of the FCA, and some courts have held that a violation of the Stark law can result in FCA liability, as well. In addition, a number of states have adopted their own false claims and whistleblower provisions whereby a private party may file a civil lawsuit in state court. We are required to provide information to our employees and certain contractors about state and federal false claims laws and whistleblower provisions and protections.
Civil Monetary Penalties Law
The Civil Monetary Penalties Law prohibits, among other things, the offering or giving of remuneration to a Medicare or Medicaid beneficiary that the person or entity knows or should know is likely to influence the beneficiary’s selection of a particular provider or supplier of items or services reimbursable by a federal or state healthcare program. This broad provision applies to many kinds of inducements or benefits provided to patients, including complimentary items, services or transportation that are of more than a nominal value. This law could affect how we have to structure our operations and activities.
RISK ASSOCIATED WITH OUR INDUSTRY
Our business and financial performance may be adversely affected by downturns in the target markets that we serve or reduced demand for the types of services we offer.
Demand for our services is often affected by general economic conditions as well as trends in our target markets. These changes may result in decreased demand for our services. The occurrence of these conditions is beyond our ability to control and, when they occur, they may have a significant impact on our results of operations. The inability or unwillingness of our customers to pay a premium for our services due to general economic conditions or a downturn in the economy may have a significant adverse impact on results of operations.
Changes within the cannabis industry or the opioid industry may adversely affect our financial performance.
Changes in the identity, ownership structure and strategic goals of our competitors and the emergence of new competitors in our target markets may harm our financial performance.
The Company’s industry is highly competitive, and we have less capital and resources than many of our competitors which may give them an advantage in marketing services similar to ours or make our services obsolete.
We are involved in a highly competitive industry where we may compete with numerous other companies who offer alternative methods or approaches, who may have far greater resources, more experience, and personnel perhaps more qualified than we do. Such resources may give our competitors an advantage in developing and marketing services similar to ours or services that make our services less desirable to consumers or obsolete. There can be no assurance that we will be able to successfully compete against these other entities.
We may be unable to respond to the rapid technological change in the industry and such change may increase costs and competition that may adversely affect our business.
Rapidly changing technologies, frequent new product and service introductions and evolving industry standards characterize our market. Intense competition in our industry exacerbates these market characteristics. Our future success will depend on our ability to adapt to rapidly changing technologies by continually improving the performance features and reliability of our services. We may experience difficulties that could delay or prevent the successful development, introduction or marketing of our services. In addition, any new enhancements must meet the requirements of our current and prospective customers and must achieve significant market acceptance. We could also incur substantial costs if we need to modify our services or infrastructures to adapt to these changes.
RELATED TO OUR COMMON STOCK
We may need additional capital that will dilute the ownership interest of investors.
We may require additional capital to fund our future business operations. If we raise additional funds through the issuance of equity, equity-related or convertible debt securities, these securities may have rights, preferences or privileges senior to those of the rights of holders of our shares of common stock, who may experience dilution of their ownership interest of our shares of Common Stock. We cannot predict whether additional financing will be available to us on favorable terms when required, or at all. The issuance of additional shares of Common Stock by our board of directors may have the effect of further diluting the proportionate equity interest and voting power of holders of our shares of Common Stock.
We will be controlled by existing shareholders.
Our directors and officers currently in place control a significant portion of our shares. Thus, they will continue to oversee the Company’s operations. As a result, our directors and officers will likely have a significant influence on the affairs and management of the Company, as well as on all matters requiring stockholder approval, including electing and removing members of its board of directors, causing the Company to engage in transactions with affiliated entities, causing or restricting the sale or merger of the Company and changing the company’s dividend policy. Such concentration of ownership and control could have the effect of delaying, deferring, or preventing a change in control of the Company, even when such a change of control would be in the best interests of the company’s other stockholders.
Our voting control is concentrated.
Our senior executives exercise a significant majority of the voting power with respect to our outstanding shares. These executives potentially have the ability to control the outcome of matters submitted to our shareholders for approval, including the election and removal of directors and any arrangement or sale of all or substantially all of our assets.
This concentrated control could delay, defer or prevent a change of control, arrangement or merger or sale of all or substantially all of our assets that our other shareholders may support. Conversely, this concentrated control could allow the holders of the majority of the shares to consummate such a transaction that our other shareholders do not support.
If you purchase Common Stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
The offering price of the Common Stock is substantially higher than the net tangible book value per share of our Common Stock (attributing no value to the Warrants). Therefore, if you purchase Common Stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent outstanding options or warrants are exercised, you will incur further dilution. Based on an assumed offering price of $[ ] per share, which is the midpoint of the price range set forth on the cover page of this prospectus, you will experience immediate dilution of $[ ] per share, representing the difference between our pro forma net tangible book value per share of Common Stock after giving effect to this offering and the offering price. See “Dilution” for further information.
The Warrants are speculative in nature.
The Warrants do not confer any rights of Common Stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of Common Stock at a fixed price. Specifically, each Tradeable Warrant will have an exercise price equal to $ , and each Non-tradeable Warrant will have an exercise price equal to $ per whole share. Moreover, following this offering, the market value of the Warrants is uncertain and there can be no assurance that the market value of the Warrants will equal or exceed their exercise price. Furthermore, each Warrant will expire five years from the original issuance date. In the event our Common Stock price does not exceed the exercise price of the Warrants during the period when the Warrants are exercisable, the Warrants may not have any value.
Holders of the Warrants will have no rights as a holder of our Common Stock until they acquire our Common Stock.
Until you acquire our Common Stock upon exercise of your Warrants, you will have no rights with respect to Common Stock issuable upon exercise of your Warrants. Upon exercise of your Warrants, you will be entitled to exercise the rights of a holder of our Common Stock as to the security exercised only as to matters for which the record date occurs after the exercise.
Provisions of the Warrants could discourage an acquisition of us by a third party.
In addition to the provisions of our amended and restated certificate of formation and amended and restated bylaws, certain provisions of the Warrants could make it more difficult or expensive for a third party to acquire us. The Warrants prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the Warrants. These and other provisions of the Warrants could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
We have broad discretion in the use of our cash, cash equivalents, and investments, including the net proceeds from this offering, and may not use them effectively.
Our management will have broad discretion in the application of our cash, cash equivalents, and investments, including the net proceeds from this offering, and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse impact on our business, cause the price of our common stock to decline, and delay the development of additional services or the opening of new locations. Pending their use, we may invest our cash, cash equivalents, and investments, including the net proceeds from this offering, in a manner that does not produce income or that loses value.
We do not expect to pay any dividends on our common stock.
We do not anticipate that we will pay any cash dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment.
RISKS RELATED TO THE OFFERING
Our existing shareholders may experience significant dilution from the sale of our shares of Common Stock.
The perceived risk of dilution may cause our shareholders to sell their shares, which may cause a decline in the price of our shares of Common Stock. By increasing the number of shares offered for sale, material amounts of short selling could further contribute to progressive price declines in our shares of Common Stock.
The issuance of shares of our common stock may have a significant dilutive effect.
Depending on the number of shares we issue, it could have a significant dilutive effect upon our existing shareholders. Although the number of shares that we may issue pursuant to the Purchase Agreement will vary based on our stock price (the higher our stock price, the less shares we have to issue) the information set out below indicates the potential dilutive effect to our shareholders, based on different potential future stock prices, if the full amount of the
Unless the Company becomes public and an active trading market develops for our securities, investors may not be able to sell their shares.
The Company is not a public company and there is not currently an active trading market for our shares of Common Stock and an active trading market may never develop or, if it does develop, may not be maintained. Failure to develop or maintain an active trading market will have a generally negative effect on the price of our shares of Common Stock, and you may be unable to sell your shares of Common Stock or any attempted sale of such shares of Common Stock may have the effect of lowering the market price and therefore your investment could be a partial or complete loss. Investors may have difficulty reselling shares of our Common Stock, either at or above the price they paid for our stock, or even at fair market value.
There could be unidentified risks involved with an investment in our securities.
The foregoing risk factors are not a complete list or explanation of the risks involved with an investment in the securities. Additional risks will likely be experienced that are not presently foreseen by the Company. Prospective investors must not construe this, and the information provided herein as constituting investment, legal, tax or other professional advice. Before making any decision to invest in our securities, you should read this entire prospectus and consult with your own investment, legal, tax and other professional advisors. An investment in our securities is suitable only for investors who can assume the financial risks of an investment in the Company for an indefinite period of time and who can afford to lose their entire investment. The Company makes no representations or warranties of any kind with respect to the likelihood of the success or the business of the Company, the value of our securities, any financial returns that may be generated or any tax benefits or consequences that may result from an investment in the Company.
The market price for the Common Shares may be volatile, which may affect the price at which you could sell the Subordinate Voting Shares.
The market price for securities of cannabis-related companies generally are likely to be volatile. In addition, the market price for the common shares may be subject to wide fluctuations in response to numerous factors beyond our control, including, but not limited to:
| | ● | actual or anticipated fluctuations in our quarterly results of operations; |
| | | |
| | ● | recommendations by securities research analysts; |
| | | |
| | ● | changes in the economic performance or market valuations of companies in the industry in which we operate; |
| | | |
| | ● | addition or departure of our executive officers and other key personnel; |
| | | |
| | ● | release or expiration of transfer restrictions on outstanding Subordinate Voting Shares; |
| | | |
| | ● | sales or perceived sales of additional Subordinate Voting Shares; |
| | | |
| | ● | operating and financial performance that varies from the expectations of management, securities analysts and investors; |
| | | |
| | ● | regulatory changes affecting our industry generally and our business and operations both domestically and abroad; |
| | | |
| | ● | announcements of developments and other material events by us or our competitors; |
| | | |
| | ● | fluctuations in the costs of vital services; |
| | | |
| | ● | changes in global financial markets and global economies and general market conditions; |
| | ● | significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving us or our competitors; |
| | | |
| | ● | operating and share price performance of other companies that investors deem comparable to us or from a lack of market comparable companies; and |
| | | |
| | ● | news reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in our industry or target markets. |
Financial markets have at times historically experienced significant price and volume fluctuations that: (i) have particularly affected the market prices of equity securities of companies and (ii) have often been unrelated to the operating performance, underlying asset values or prospects of such companies. Accordingly, the market price of the Subordinate Voting Shares from time to time may decline even if our operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related factors, may cause decreases in asset values that may result in impairment losses to us. Further fluctuations in price and volume of equity securities may occur in the future. If increased levels of volatility and market turmoil continue, our operations could be adversely impacted, and the trading price of the Subordinate Voting Shares may be materially adversely affected.
We face liquidity risks.
Our Subordinate Voting Shares do not currently trade on any U.S. securities exchange. In the event our Subordinate Voting Shares do trade on any U.S. securities exchange, we cannot predict at what prices the Subordinate Voting Shares will trade and there is no assurance that an active trading market will develop or be sustained. There is a significant liquidity risk associated with an investment in us.
We are eligible to be treated as an “emerging growth company” as defined in the JOBS Act, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make the Subordinate Voting Shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, (2) reduced disclosure obligations regarding executive compensation in this prospectus and periodic reports and proxy statements, and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find the Subordinate Voting Shares less attractive because we may rely on these exemptions. If some investors find the Subordinate Voting Shares less attractive as a result, there may be a less active trading market for the Subordinate Voting Shares, and the stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies.
We have broad discretion in the use of our cash, cash equivalents, and investments, including the net proceeds from this offering, and may not use them effectively.
Our management will have broad discretion in the application of our cash, cash equivalents, and investments, including the net proceeds from this offering, and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse impact on our business, cause the price of our common stock to decline, and delay the development of additional services or the opening of new locations. Pending their use, we may invest our cash, cash equivalents, and investments, including the net proceeds from this offering, in a manner that does not produce income or that loses value. See the section titled “Use of Proceeds” appearing elsewhere in this prospectus.
Costs and expenses of being a reporting company under the 1934 Securities Exchange Act may be burdensome and prevent us from achieving profitability.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and parts of the Sarbanes-Oxley Act. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time-consuming and costly, and place significant strain on our personnel, systems and resources.
Since our shares of Common Stock may be thinly traded it is more susceptible to extreme rises or declines in price, and you may not be able to sell your shares at or above the price paid.
Since our shares of Common Stock may be thinly traded its trading price is likely to be highly volatile and could be subject to extreme fluctuations in response to various factors, many of which are beyond our control, including (but not necessarily limited to): the trading volume of our shares, the number of analysts, market-makers and brokers following our shares of Common Stock, new products or services introduced or announced by us or our competitors, actual or anticipated variations in quarterly operating results, conditions or trends in our business industries, additions or departures of key personnel, sales of our shares of Common Stock and general stock market price and volume fluctuations of publicly traded, and particularly microcap, companies.
Investors may have difficulty reselling shares of our Common Stock, either at or above the price they paid for our stock, or even at fair market value. The stock markets often experience significant price and volume changes that are not related to the operating performance of individual companies, and because our shares of Common Stock are thinly traded it is particularly susceptible to such changes. These broad market changes may cause the market price of our shares of Common Stock to decline regardless of how well we perform as a company. In addition, there is a history of securities class action litigation following periods of volatility in the market price of a company’s securities. Although there is no such litigation currently pending or threatened against us, such a suit against us could result in the incursion of substantial legal fees, potential liabilities and the diversion of management’s attention and resources from our business.
We do not expect to pay any dividends on our common stock.
We do not anticipate that we will pay any cash dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment.
Unless an active trading market develops for our securities, investors may not be able to sell their shares.
There is not currently an active trading market for our shares of Common Stock and an active trading market may never develop or, if it does develop, may not be maintained. Failure to develop or maintain an active trading market will have a generally negative effect on the price of our shares of Common Stock, and you may be unable to sell your shares of Common Stock or any attempted sale of such shares of Common Stock may have the effect of lowering the market price and therefore your investment could be a partial or complete loss.
Since our shares of Common Stock is thinly traded it is more susceptible to extreme rises or declines in price, and you may not be able to sell your shares at or above the price paid.
Since our shares of Common Stock are thinly traded its trading price is likely to be highly volatile and could be subject to extreme fluctuations in response to various factors, many of which are beyond our control, including (but not necessarily limited to): the trading volume of our shares, the number of analysts, market-makers and brokers following our shares of Common Stock, new products or services introduced or announced by us or our competitors, actual or anticipated variations in quarterly operating results, conditions or trends in our business industries, additions or departures of key personnel, sales of our shares of Common Stock and general stock market price and volume fluctuations of publicly traded, and particularly microcap, companies.
Investors may have difficulty reselling shares of our Common Stock, either at or above the price they paid for our stock, or even at fair market value. The stock markets often experience significant price and volume changes that are not related to the operating performance of individual companies, and because our shares of Common Stock are thinly traded it is particularly susceptible to such changes. These broad market changes may cause the market price of our shares of Common Stock to decline regardless of how well we perform as a company. In addition, there is a history of securities class action litigation following periods of volatility in the market price of a company’s securities. Although there is no such litigation currently pending or threatened against us, such a suit against us could result in the incursion of substantial legal fees, potential liabilities and the diversion of management’s attention and resources from our business.
Our existing directors, executive officers and principal stockholders will continue to have substantial control over us after this offering, which could limit your ability to influence the outcome of key transactions, including a change of control.
After this offering our directors, executive officer, principal stockholders and their affiliates will beneficially own or control, directly or indirectly, a significant majority of our shares. As a result, these stockholders, acting together, could have significant influence over the outcome of matters submitted to our stockholders for approval, including the election or removal of directors, any amendments to our certificate of incorporation or bylaws and any merger, consolidation or sale of all or substantially all of our assets, and over the management and affairs of our company. This concentration of ownership may also have the effect of delaying or preventing a change in control of our company or discouraging others from making tender offers for our shares and might affect the market price of our common stock.
Because we do not expect to pay any dividends on our common stock for the foreseeable future, investors in this offering may never receive a return on their investment.
We do not anticipate that we will pay any cash dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment.
There could be unidentified risks involved with an investment in our securities.
The foregoing risk factors are not a complete list or explanation of the risks involved with an investment in the securities. Additional risks will likely be experienced that are not presently foreseen by the Company. Prospective investors must not construe this and the information provided herein as constituting investment, legal, tax or other professional advice. Before making any decision to invest in our securities, you should read this entire prospectus and consult with your own investment, legal, tax and other professional advisors. An investment in our securities is suitable only for investors who can assume the financial risks of an investment in the Company for an indefinite period of time and who can afford to lose their entire investment. The Company makes no representations or warranties of any kind with respect to the likelihood of the success or the business of the Company, the value of our securities, any financial returns that may be generated or any tax benefits or consequences that may result from an investment in the Company.
Because the risk factors referred to above, as well as other risks not mentioned above, could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us, you should not place undue reliance on any such forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made. We undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which such statement is made or reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which ones will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements relate to future events including, without limitation, the terms, timing and closing of our proposed acquisitions or our future financial performance. We have attempted to identify forward-looking statements by using terminology such as “anticipates,” “believes,” “expects,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predict,” “should,” “will,” or the negative of these terms or other comparable terminology. These statements are only predictions; uncertainties and other factors may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels or activity, performance, or achievements expressed or implied by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Our expectations are as of the date this prospectus is filed, and we do not intend to update any of the forward-looking statements after the date this prospectus is filed to confirm these statements to actual results, unless required by law.
You should not place undue reliance on forward looking statements. The cautionary statements set forth in this prospectus identify important factors which you should consider in evaluating our forward-looking statements. These factors include, among other things:
| | ● | Our ability to effectively execute our business plan; |
| | | |
| | ● | Our ability to manage our expansion, growth, and operating expenses; |
| | | |
| | ● | Our ability to protect our brands and reputation; |
| | | |
| | ● | Our ability to repay our debts; |
| | | |
| | ● | Our ability to comply with new regulations that affect our business; |
| | | |
| | ● | Our ability to evaluate and measure our business, prospects, and performance metrics; |
| | | |
| | ● | Our ability to compete and succeed in a highly competitive and evolving industry; |
| | | |
| | ● | Our ability to respond and adapt to changes in technology and customer behavior; |
| | | |
| | ● | Risks in connection with completed or potential acquisitions, dispositions and other strategic growth opportunities and initiatives; |
| | | |
| | ● | Risks related to the anticipated timing of the closing of any potential acquisitions; |
| | ● | Risks related to the integration with regards to potential or completed acquisitions; |
| | | |
| | ● | Various risks related to health epidemics, pandemics and similar outbreaks, such as the coronavirus disease 2019 pandemic, which may have material adverse effects on our business, financial position, results of operations and/or cash flows; |
| | ● | Our ability to obtain, maintain and defend patent protection for our technology and products or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively; and |
| | | |
| | ● | We depend on our proprietary technology which we may not be able to protect. |
The foregoing list of important factors does not include all such factors, nor necessarily present them in order of importance. In addition, you should consult other disclosures made by the Company (such as in our other filings with the SEC or in our press releases) for other factors that may cause actual results to differ materially from those projected by the Company. For additional information regarding risk factors that could affect the Company’s results, see “Risk Factors” beginning on page 10 of this prospectus, and as may be included from time-to-time in our reports filed with the SEC.
The Company intends the forward-looking statements to speak only as of the time of such statements and does not undertake or plan to update or revise such forward-looking statements as more information becomes available or to reflect changes in expectations, assumptions, or results. The Company can give no assurance that such expectations or forward-looking statements will prove to be correct. An occurrence of, or any material adverse change in, one or more of the risk factors or risks and uncertainties referred to in this prospectus, could materially and adversely affect our results of operations, financial condition, and liquidity, and our future performance.
Industry Data and Forecasts
This prospectus also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other industry data. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the statistical and other industry data generated by independent parties and contained in this prospectus. In addition, projections, assumptions, and estimates of our future performance and the future performance of the industries in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors. Our actual results could differ materially from those anticipated in the forward-looking statements for many reasons, including, but not limited to, the possibility that we may fail to preserve our expertise in consumer product development; that existing and potential distribution partners may opt to work with, or favor the products of, competitors if our competitors offer more favorable products or pricing terms; that we may be unable to maintain or grow sources of revenue; that we may be unable maintain profitability; that we may be unable to attract and retain key personnel; or that we may not be able to effectively manage, or to increase, our relationships with customers; and that we may have unexpected increases in costs and expenses. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
USE OF PROCEEDS
We expect to receive net proceeds from this offering of approximately $ million, after deducting underwriting discounts and commissions and estimated offering expenses (assuming a per Unit price of $ , which is the midpoint of the price range set forth on the cover page of this prospectus), or approximately $ million if the underwriters’ representative exercises the over-allotment option, with respect to both shares of Common Stock and Warrants, in full.
A $1.00 increase or decrease in the assumed public offering price of $ (which is the midpoint of the range set forth on the cover page of this prospectus) per Unit would increase or decrease the net proceeds from this offering by approximately $ million, assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions. Similarly, each increase or decrease of Units offered would increase or decrease our net proceeds by approximately $ million, assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. This information is illustrative only and will depend on the actual initial public offering price and other terms of this offering determined at pricing. We do not expect that a change in the initial public offering price or the number of Units by these amounts would have a material effect on our intended uses of the net proceeds from this offering, although a decrease in the initial offering price without a corresponding increase in the number of Units offered may accelerate the time at which we will need to seek additional capital.
We plan to use the net proceeds we receive from this offering for the following purposes:
| | | Use of Net Proceeds Used If % of Offering Raised | |
| Working Capital | | | 100% | | | | 75% | | | | 50% | | | | 25% | |
| Capital Expenditures | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
| Labor | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
| Real Estate | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
| Marketing and Sales | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
| Technology Development | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
| Other | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
| Total Allocated | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | | | $ | [ ] | |
We believe that our existing cash and cash equivalents, along with the net proceeds from this offering and any proceeds from the exercise of Warrants, together with interest on cash balances, will be sufficient to fund our operating expenses and capital expenditure requirements through at least the next 12 months. The foregoing represents our current intentions based upon our present plans and business conditions to use and allocate the net proceeds of this offering. However, the nature, amounts and timing of our actual expenditures may vary significantly depending on numerous factors. As a result, our management has and will retain broad discretion over the allocation of the net proceeds from this offering. We may find it necessary or advisable to use the net proceeds from this offering for other purposes, and we will have broad discretion in the application of net proceeds from this offering. To the extent that the net proceeds we receive from this offering are not immediately used for the above purposes, we intend to invest our net proceeds in short-term, interest-bearing bank deposits or debt instruments.
MARKET FOR OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Market and Other Information
Nasdaq Listing Application
In connection with this offering, we have applied to have our Common Stock and Tradeable Warrants listed on the Nasdaq Capital Market under the symbols “[ ]” and “[ ],” respectively. If approved, we expect to list our Common Stock and the Tradeable Warrants offered in this offering on Nasdaq upon consummation of this offering. No assurance can be given that our listing application will be approved. This offering will occur only if Nasdaq or another securities exchange approves the listing of our Common Stock and Tradeable Warrants. If Nasdaq or another U.S. securities exchange does not approve the listing of our Common Stock and Tradeable Warrants, we will not proceed with this offering. There can be no assurance that our Common Stock and Tradeable Warrants will be listed on the Nasdaq or another securities exchange. For more information see the section “Risk Factors.”
Holders
As of April 25, 2023, there were 4,434,596 shares of Common Stock issued and outstanding and approximately 45 registered holders of record of our Common Stock. The number of shareholders of record does not include certain beneficial owners of our Common Stock whose shares are held in the names of various dealers, clearing agencies, banks, brokers and other fiduciaries.
Transfer Agent
VStock Transfer, LLC with offices located at 18 Lafayette Place, Woodmere, NY 11598, and a telephone number of (212) 828-8436. Our transfer agent will also serve as the Warrant Agent for the Warrants underlying the Units sold in this offering.
Dividend Policy
We have not paid dividends during the three most recently completed fiscal years and have no current plans to pay dividends on our Common Stock. We currently intend to retain all earnings, if any, for use in our business.
CAPITALIZATION
The following table sets forth our capitalization as of December 31, 2022. Such information is set forth on the following basis:
| | ● | an actual basis; and |
| | ● | on a pro-forma basis to give effect to our sale of [ ] Units at $[ ] per Unit, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The as adjusted information below is illustrative only and our capitalization following the closing of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this information together with our financial statements and the related notes thereto included elsewhere in this prospectus and the information set forth under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| | | as of December 31, 2022 | |
| | | Actual | | | Pro Forma(1) | | | Pro Forma as Adjusted | |
| | | (U.S. dollars in thousands) | |
| | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| Cash and cash equivalents | | $ | 186,918 | | | $ | | | | $ | | |
| | | | | | | | | | | | | |
| Shareholders’ equity (deficit): | | | | | | | | | | | | |
| Preferred Stock, $0.001 par value per share, issued and outstanding as of September 30, 2022 | | | — | | | | — | | | | — | |
| Common Stock, par value $.001per share, 4,434,596 issued and outstanding, actual; shares issued and outstanding, pro forma; and shares issued and outstanding pro forma as adjusted | | | 4,434 | | | | | | | | | |
| Additional paid-in capital | | | 2,917,173 | | | | | | | | | |
| Accumulated equity | | | (2,540,593 | ) | | | | | | | | |
| | | | | | | | | | | | | |
| Total shareholders’ (deficit) equity | | | (2,540,593 | ) | | | | | | | | |
| | | | | | | | | | | | | |
| Total capitalization | | $ | 381,014 | | | | | | | | | |
| (1) | If the underwriters’ option to purchase up to an additional [ ] shares of Common Stock is exercised we would receive approximately $[ ] million in additional net proceeds, based on the assumed initial public offering price of $[ ] per Unit, which is the midpoint of the range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us; and (ii) cash and cash equivalents, total shareholders’ equity and total capitalization would each also increase by approximately $[ ] million. |
Each $1.00 increase (decrease) in the assumed public offering price of $[ ] per Unit would increase (decrease) the as adjusted amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $[ ], assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of Units we are offering. Each increase (decrease) of [ ] Units in the number of Units we are offering would increase (decrease) the as adjusted amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $[ ], assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
The number of shares of our Common Stock to be outstanding after this offering is based on 4,434,596 shares of our Common Stock outstanding as of December 31, 2022 and excludes:
| | ● | [●] shares of our Common Stock issuable upon the exercise of the Tradeable Warrants and/or the Non-tradeable Warrants to be issued as part of the Units; |
| | | |
| | ● | [●] shares of our Common Stock issuable upon exercise of the Representative Warrants. |
DILUTION
Each Unit, with an assumed public offering price of $[ ] per Unit, which consists of (i) one share of Common Stock, (ii) one Tradeable Warrant to purchase one share of Common Stock and (iii) one Non-tradeable Warrant to purchase one-half of one share of our Common Stock.
If you invest in our Units, your interest will be diluted immediately to the extent of the difference between the offering price per share of our Common Stock that is part of the Unit and the as adjusted net tangible book value per share of our Common Stock immediately after giving effect to this offering.
As of December 31, 2022 our historical net tangible book value was $[ ] or $[ ] per share of Common Stock. Historical net tangible book value per share represents the amount of our total tangible assets reduced by total liabilities, divided by [ ] the number of shares of Common Stock outstanding on December 31, 2022.
After giving effect to the sale of 1,000,000 Units, at the assumed offering price of $[ ] per Unit, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our net tangible book value as of December 31, 2022 would have been $[ ] or $[ ] per share of Common Stock. This amount represents an immediate increase in net tangible book value of $[ ] per share to our existing stockholders. Investors purchasing our Common Stock in this offering will have paid $[ ] more than the as adjusted net tangible book value per share of Common Stock after this offering.
The following table illustrates this dilution on a per share basis:
| Assumed offering price per share (attributing no value to the Warrants) | | $ | [ ] | |
| Historical net tangible book value per share as of December 31, 2022 | | $ | [ ] | |
| Increase in net tangible book value per share attributable to new investors | | $ | [ ] | |
| Net tangible book value per share after the offering | | $ | [ ] | |
| Dilution per share to new investors | | $ | [ ] | |
Each $1.00 increase (decrease) in the assumed public offering price of $[ ] per Unit would increase (decrease) our net tangible book value after this offering by approximately $[ ] per share, and increase (decrease) the dilution per share to new investors by approximately $[ ] per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us full.
An increase or decrease of 1 million Units in the number of Units offered by us would increase or decrease our pro forma net tangible book value after this offering by approximately $ million, would increase or decrease pro forma net tangible book value per share after this offering by $ per share and would increase or decrease the dilution per share to new investors by $ , after deducting estimated underwriting discounts and estimated offering expenses payable by us.
The following table summarizes, on a pro forma basis as of , the differences between the number of shares of Common Stock acquired from us, the total amount paid and the average price per share paid by the existing holders of our Common Stock and by investors in this offering, based upon an assumed public offering price of $ per Unit, which is the midpoint of the price range set forth on the cover page of this prospectus.
| | | Shares | | | Total Consideration | | | Average
Price Per | |
| | | Number | | | Percent | | | Amount | | | Percent | | | Share | |
| Existing shareholders | | | 45 | | | | | | | | | | | | | | | | | |
| New investors | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | | | | | | | | | | | | | | | | | | | |
The number of shares of our Common Stock to be outstanding after this offering is based on 4,434,596 shares of our Common Stock outstanding as of December 31, 2022, and excludes:
| | ● | [●] shares of our Common Stock issuable upon the exercise of the Tradeable Warrants and/or the Non-tradeable Warrants to be issued as part of the Units; |
| | | |
| | ● | [●] shares of our Common Stock issuable upon exercise of the Representative Warrants. |
If we issue additional shares of Common Stock in the future, there could be further dilution to investors participating in this offering. In addition, we anticipate needing to raise additional capital before generating positive cash flows and we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations for the years ended December 31, 2022 and 2021 should be read in conjunction with the information included under “Business,” “Selected Consolidated Financial Data” and our consolidated financial statements and the accompanying notes included elsewhere in this prospectus. The discussion and analysis below are based on comparisons between our historical financial data for different periods and include certain forward-looking statements about our business, operations, and financial performance. These forward-looking statements are subject to risks, uncertainties, assumptions, and other factors described in “Risk Factors.” Our actual results may differ materially from those expressed in, or implied by, those forward-looking statements. See “Special Note Regarding Forward-Looking Statements.”
We caution that the foregoing list of factors is not exclusive, and new factors may emerge, or changes to the foregoing factors may occur, that could impact our business. We undertake no obligation to publicly update or revise these statements, whether as a result of new information, future events or otherwise, except to the extent required by the federal securities laws.
Certain information contained in this discussion and elsewhere in this prospectus may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 and is subject to the safe harbor created by that act. The safe harbor created by the Private Securities Litigation Reform Act will not apply to certain “forward looking statements” because we issued “penny stock” (as defined in Section 3(a)(51) of the Securities Exchange Act of 1934, as amended, and Rule 3(a)(51-1) under the Exchange Act) during the three year period preceding the date(s) on which those forward looking statements were first made, except to the extent otherwise specifically provided by rule, regulation or order of the Securities and Exchange Commission (the “SEC”). We caution readers that certain important factors may affect our actual results and could cause such results to differ materially from any forward-looking statements which may be deemed to have been made in this prospectus or which are otherwise made by or on our behalf. For this purpose, any statements contained in this prospectus that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as “may,” “will,” “expect,” “believe,” “explore,” “consider,” “anticipate,” “intend,” “could,” “estimate,” “plan,” or “propose” or the negative variations of those words or comparable terminology are intended to identify forward-looking statements. Factors that may affect our results include, but are not limited to, the risks and uncertainties associated with:
| | ● | Our ability to raise capital necessary to sustain our anticipated operations and implement our business plan; |
| | | |
| | ● | Our ability to implement our business plan; |
| | | |
| | ● | Our ability to generate sufficient cash to survive; |
| | | |
| | ● | The degree and nature of our competition; |
| | | |
| | ● | The lack of diversification of our business plan; |
| | | |
| | ● | The general volatility of the capital markets and the establishment of a market for our shares; and |
| | | |
| | ● | Disruption in the economic and financial conditions primarily from the impact of past terrorist attacks in the United States, threats of future attacks, police, and military activities overseas and other disruptive worldwide political and economic events and environmental weather conditions. |
We are also subject to other risks detailed from time to time in our other filings with the SEC and elsewhere in this prospectus. Any one or more of these uncertainties, risks and other influences could materially affect our results of operations and whether forward-looking statements made by us ultimately prove to be accurate. Our actual results, performance and achievements could differ materially from those expressed or implied in these forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether from new information, future events or otherwise.
We are also subject to other risks detailed from time to time in our other filings with SEC and elsewhere in this prospectus. Any one or more of these uncertainties, risks and other influences could materially affect our results of operations and whether forward-looking statements made by us ultimately prove to be accurate. Our actual results, performance and achievements could differ materially from those expressed or implied in these forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether from new information, future events or otherwise.
Overview
KindlyMD is a healthcare data company, focused on holistic pain management and ending the opioid epidemic. KindlyMD offers direct health care to patients integrating prescription medicine and behavioral health services to reduce opioid use in the chronic pain patient population. Kindly believes these methods to be superior in managing the root cause of symptoms and improve outcomes while lowering dependency on opiates. Our specialty outpatient clinical services are offered on a subscription and fee-for-service basis to augment traditional healthcare. The Company offers evaluation and management, including, but not limited to chronic pain, functional medicine, cognitive behavioral therapy, intravenous infusion therapy, trauma and addiction therapy, recovery support services, overdose education efforts, peer support, limited urgent care, dermatology, preventative medicine, travel services, and hormone therapy. Through its focus on an integrated model of prescriber and therapist teams, KindlyMD is working to develop patient-specific care programs with a specific mission to reduce opioid use in the patient population while successfully treating patients with effective and evidence based non-opioid alternatives and in conjunction with behavioral therapy.
Beyond its treatment of patients, KindlyMD collects data focused on why and how patients turn to alternative treatments to reduce prescription medication use and addiction. The Company captures all relevant datapoints to assist and appropriately treat each individual patient. This also results in valuable data for the company and the company’s investors. We strive to become a source for evidence-based guidelines, data, and education in the fight against the opioid crisis in America.
Business Revenue Streams
We operate across various revenue streams: (i) medical evaluation and treatment visits for chronic pain and illness, (ii) subscription outpatient medicine (pain clinic medicine), (iii) data collection and research, (iv) education partnerships, (v) online and email campaign marketing revenue, (vi) service affiliate agreements, and (vii) retail sale of dietary supplements, and products.
KindlyMD is a licensed healthcare facility performing routine evaluation and management for non-emergent conditions in a direct care and subscription model. KindlyMD focuses primarily on three visit types. 1) Patients with pain who are using opioid medications, 2) patients seeking evaluation and management for non-opioid alternative treatments that require the recommendation of a licensed prescriber. 3) Behavioral Therapy and guided therapy visits billed through traditional insurance or out of pocket.
Patients with pain who are using opioid medications
New patients pay an initial evaluation fee of $249 and a monthly subscription fee of $119 per month, plus labs and testing on an individual basis as needed. Visits are required once per month for programs involving management of a controlled substance, such as opioid medication.
Patients seeking evaluation and management for non-opioid alternative treatments that require the recommendation of a licensed prescriber
New patients pay an initial evaluation fee of $249 and $189 for a follow-up appointment. KindlyMD sees between 1600 and 1900 visits per month in total under this program in Utah.
Behavioral Therapy and guided therapy visits billed through traditional insurance or out of pocket
Behavioral health therapy visits are also performed either on a fee for service basis or paid through traditional insurance contracts. These are typically 50 minute therapy sessions with licensed therapists trained in trauma-based therapy.
Data Collection and Research
KindlyMD collects valuable data from interactions with people online, via telecommunication, and patient interactions and through our products. Clients provide some of this data directly, as do clinicians, and staff by collecting data about interactions, product and medication use, experiences, and behavior. The data we collect depends on the context of the interactions with KindlyMD and the choices people make, including their privacy settings and the products and features they use. We also obtain data about customers, patients, and clients from third parties.
Patient data is governed by HIPAA. Patients consent to allowing KindlyMD use of their data upon signing up for our services. Patients, clients, and customers of KindlyMD have choices when it comes to the technology they use and the data they share. If someone chooses not to share their data with KindlyMD, We may not provide services. Where providing data is optional, clients, patients, and customers are offered the choice to provide the data or not to.
In collecting data from these interactions, we combine data we collect from different contexts or obtain from third parties to provide a more seamless, consistent, and personalized experience, to make informed business decisions, to make clinical decisions, and for other legitimate business purposes.
Our processing of personal and healthcare data for these purposes includes both automated and manual (human) methods of processing. Our automated methods often are related to and supported by our manual methods. For example, our automated methods may include artificial intelligence (AI), which is a set of technologies that enable computers to perceive, learn, reason, and assist in decision-making to solve problems in ways that are similar to what people do. To build, train, and improve the accuracy of our automated methods of processing (including AI), we manually review some of the predictions and inferences produced by the automated methods against the underlying data from which the predictions and inferences were made. This manual review may be conducted by KindlyMD employees or vendors who are working on KindlyMD’s behalf. All such vendors enter a BAA with KindlyMD.
We share personal data with consent or to complete any transaction or provide any product an individual has requested or authorized. We also share data with KindlyMD controlled affiliates and subsidiaries; with vendors working on our behalf; when required by law or to respond to legal process; to protect our customers; to protect our customers and patients; to protect lives; to maintain the security of our products; and to protect the rights and property of KindlyMD and its customers and patients.
KindlyMD is required to protect the information it collects and maintains. KindlyMD respects the right to privacy and will protect the information it maintains about clients and patients in accordance with all required laws, regulations and standards. All healthcare research and published data regarding clinical outcomes is anonymized and governed by HIPAA. Personally identifying information is protected and removed from any research, publication, or sale to a third party.
Education Partnerships
We strive to build the largest audience of those seeking honest, evidence-based, holistic and practical information about alternatives to opioids. Our in-house education center creates educational content for all of KindlyMD’s programs. Some of the educational content is subsidized through local and regional partnership agreements, currently representing approximately 3% of overall revenue.
Online and Email Campaign Marketing Revenue
KindlyMD has a combined reach of over 32,000 email contacts that have opted-in to our education and marketing communication. Paid messaging is offered to local and regional businesses for a fee.
Affiliate Agreements
KindlyMD partners with local healthcare clinics and product manufacturers in geographic markets to maximize our ability to increase service and product offerings to more individuals. We partner with local healthcare clinics including those specializing in the evaluation and management of similar conditions as well as organizations specializing in behavioral therapy. We may white-label or manufacture supplements and products suited to our customers. KindlyMD may also sublease space within a primary clinic in order to expand services by an affiliate organization. These service agreements are governed by a healthcare Business Associate Agreement (BAA) as well as financial contracts.
Effects of the COVID-19 Pandemic
The current outbreak of COVID-19 has globally resulted in loss of life, business closures, restrictions on travel, and widespread cancellation of social gatherings. While the disruption is currently expected to be temporary, there is considerable uncertainty around the duration. The extent to which the COVID-19 pandemic impacts our business will depend on future developments, which are highly uncertain and cannot be predicted at this time, including:
| | ● | new information which may emerge concerning the severity of the disease, its relationship to other illnesses, medication interactions and side effects of treatments, protocols surrounding transmission, effects and long-term effects, among other medically related information; |
| | ● | the duration and spread of the outbreak; |
| | | |
| | ● | the severity of travel restrictions imposed by geographic areas in which we operate, mandatory or voluntary business closures; |
| | | |
| | ● | regulatory actions taken in response to the pandemic; |
| | | |
| | ● | other business disruptions that affect our workforce; |
| | | |
| | ● | the impact on capital and financial markets; and |
| | | |
| | ● | actions taken throughout the world, including in markets in which we operate, to contain the COVID-19 outbreak or treat its impact. |
In addition, the current outbreak of COVID-19 has resulted in a widespread global health crisis and adversely affected global economies and financial markets, and similar public health threats could do so in the future. If the disruptions posed by the COVID-19 pandemic or other matters of global concern continue for an extensive period of time, the operations of our business may be materially adversely affected.
To the extent the COVID-19 pandemic or a similar public health threat has an impact on our business, it is likely to also have the effect of heightening many of the other risks described in the “Risk Factors” section.
Critical Accounting Policies, Estimates and Assumptions
Use of Estimates. The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates in the accompanying financial statements include the amortization period for intangible assets, valuation and impairment valuation of intangible assets, valuation of share-based payments.
Changes in Accounting Principles. No significant changes in accounting principles were adopted during fiscal 2021 and 2022.
Impairment of Long-Lived Assets. The Company accounts for long-lived assets in accordance with the provisions of Statement of Financial Accounting Standards ASC 360-10, “Accounting for the Impairment or Disposal of Long-Lived Assets.” This statement requires that long-lived assets and certain identifiable intangibles be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Fair Value of Financial Instruments and Fair Value Measurements. The Company measures their financial assets and liabilities in accordance with generally accepted accounting principles. For certain of our financial instruments, including cash, accounts payable, accrued expenses and short-term loans the carrying amounts approximate fair value due to their short maturities.
We have adopted accounting guidance for financial and non-financial assets and liabilities. The adoption did not have a material impact on our results of operations, financial position, or liquidity. This standard defines fair value, provides guidance for measuring fair value and requires certain disclosures. This standard does not require any new fair value measurements, but rather applies to all other accounting pronouncements that require or permit fair value measurements. This guidance does not apply to measurements related to share-based payments. This guidance discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow), and the cost approach (cost to replace the service capacity of an asset or replacement cost). The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use.
Revenue Recognition. The Company records revenue in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 606. This Accounting Standards Update (“ASU”) is based on the principle that revenue is recognized to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASU also requires additional disclosure about the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer purchase orders, including significant judgments.
The Company recognizes revenue from: (i) medical evaluation and treatment, (ii) subscription outpatient medicine (pain clinic medicine and behavioral therapy), (iii) data collection and research, (iv) education partnerships, (v) online and email campaign marketing revenue, (vi) service affiliate agreements, and (vii) retail sale of dietary supplements, and products.
We recognize revenue when control of the promised goods or services is transferred to our customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. We account for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance, and collectability of consideration is probable.
The Company recognizes revenue after the transaction price has been allocated and when it satisfies the performance obligation. Usually, there is only a single performance obligation in the contract, and therefore the entire transaction price is allocated to the single performance obligation. This typically occurs at a point in time when products and or services are delivered or shipped.
Stock-Based Compensation. The Company accounts for stock-based instruments issued to employees in accordance with ASC Topic 718. ASC Topic 718 requires companies to recognize in the statement of operations the grant-date fair value of stock options and other equity-based compensation issued to employees. The value of the portion of an award that is ultimately expected to vest is recognized as an expense over the requisite service periods using the straight-line attribution method.
Allowance for Doubtful Accounts. The Company establishes an allowance for doubtful accounts to ensure trade receivable are not overstated due to non-collectability. The Company’s allowance is based on a variety of factors, including age of the receivable, significant one-time events, historical experience, and other risk considerations. The Company had an allowance at December 31, 2022 and 2021 of $45,689 and $0, respectively. The Company had bad debt expense of $59,639 and $1,371 for the years ended December 31, 2022, and 2021, respectively.
Plan of Operation
The 2023 operational plan consists of:
| | 1. | Continued establishment and expansion of current and associated service lines within the scope of KindlyMD’s operations. The expansion of pain management services with integrated behavioral health will be our central focus as we continue to expand into new Utah based locations. As funding is secured or made available, it is our intention to establish footprints in new markets (states) with populations in need of a medical model such as ours. We hope to be able to enter four new markets in the twelve calendar months following the closing of the Offering. KindlyMD may also expand upon its existing service lines. |
a. Data Collection and Research
Data collection, research, and analyzation are core tenets within KindlyMD and will continue through the 2023 operational plan and beyond. Key implementation projects may allow us to improve clinical research and development of best practice guidelines within the pain treatment market as well as develop products and services to enhance opioid prescription safety and risk mitigation programs for patients. The main data collection and research projects in 2023 are as follows:
| | ● | Clinical process customization of integrated behavioral health team models and documentation standardization. Highly focused patient evaluations and efficient documentation methods may produce clinical data sets that illustrate and help produce potentially highly effective patient treatment methods. Creating standard workflows based on provider/patient interaction can be both art and science and may yield new processes that could improve patient outcomes in relation to the opioid epidemic in the United States. |
| | | |
| | ● | Interface creation between Electronic Health Record systems and Cloud Data Models (Health Cloud). Data points gathered from staff, providers, patients, and third parties, are entered into multiple systems with discreet data fields and reporting capabilities. We take this data, collate it, and analyze it to identify potentially efficacious treatment methodologies. Large data sets of this type may have many applications that could range from improved patient outcomes to licensed access. |
| | | |
| | ● | Data extraction and analytics may be used to create a Data Lake. A data lake is a centralized repository designed to store, process, and secure large amounts of structured, semi structured, and unstructured data. It can store data in its native format and process any variety of it, ignoring size limits. This level of ability and agility in the healthcare data field can be combined with machine learning and may be extremely productive and lucrative in its application. |
| | | |
| | ● | Continued enhancements to data security policies and protocols. The healthcare and technology industries have constantly changing and increasing requirements. It is imperative that we remain abreast of current and emerging technologies if we wish to be at the forefront of the fight against the opioid epidemic. It is our plan to constantly assess and improve our processes, policies, and procedures to safeguard our data and our patients. |
| | | |
| | ● | Integration with internal and partner resources. Kindly may work with partners, vendors, third parties, as well as internally to enhance the flow of bi-directional information where possible. Once created, integrations of this type can greatly increase the speed of information gathering and data research and may even be licensed for use with other entities. |
b. Subscription Outpatient Medicine (Pain Clinic Medicine)
KindlyMD will expand services of evaluation and management of chronic pain patients as well as patients seeking management of alternative, non-opioid medicine. This effort will initially include expansion of services within the Salt Lake City area Utah market, leasing of a larger facility in Utah County, and expansion into the Southern Utah market. Expansion will increase the KindlyMD labor force in a linear fashion of approximately one employee for every 200 new patients acquired. KindlyMD has been able to hire highly skilled and motivated individuals who advocate for non-opioid drugs and who are motivated to be involved in the deprescribing movement. We anticipate increasing labor costs due to macroeconomic factors. Currently labor is approximately 58% of Kindly’s budgetary allowance.
Clinic operations will continue to require a high number of licensed prescribers and a growing number of licensed clinical behavioral therapists and consultants. We intend to maintain approximately one licensed provider (MD, NP, PA) and one licensed Behaviorist per 3,000 active patients in the pain management program. Growth of the program will also require an increasing number of Licensed Clinical Social Workers (LCSWS) at a rate sufficient to maintain patient care and capacity. We anticipate five LCSW hires in 2023 in the Utah market.
KindlyMD is one of the largest providers of medical evaluation and management services related to treatment recommendations within the medical cannabis program in Utah. We plan to continue this service line as a core revenue driver and awareness builder. Currently we treat approximately 14,800 patients in this program and continue to see increases year over year. Operationally we anticipate slower growth in 2023 due to limiting legislation. Lobbying efforts are underway and appear promising to improve our capacity to increase patient numbers, however this will not be determined until March 2023 and may not materialize.
Our model of healthcare blends prescribers and licensed behavioral health clinicians into every patient care plan at the point of service. Although there are a number of small to large networks using this model in low-income and high-risk population care, we know of no other large pain clinic in Utah, or the US, which uses this integration model. Importantly, we are also one of a limited number of specialty providers who allow patients to utilize non-opioid alternative medications, such as medical cannabis, concomitantly with opioids under guided supervision by a licensed integration team.
Expansion into markets outside of Utah will commence upon secured funding. These will include similarly sized facilities as found in the Salt Lake City area market (approximately 5,000-6000 square feet), requiring budgets of approximately $570,000 and two and a half to three months to open each location.
c. Education Partnerships with Cannabis Companies and Healthcare Organizations
Existing partnerships include education with five medical cannabis production facilities in Utah. Limited advertising and promotion laws restrict these companies from sending marketing material to consumers and potential patients. Additionally, barriers to entry continue to exist in these programs for patients due to cost, continued stigma, and lack of local access. All these variables are targets for KindlyMD to facilitate aid to at-risk communities of patients in order to improve access to non-opioid pain management as well as improve current prescribing and risk mitigation practices within patient groups who are at higher risk of opioid complications.
Training will expand to handle this growth. Training will require developing further behavior integration process manuals and development of internal training protocols. Due to limited psychological integration with prescription medicine practices, these training tools will require custom development internally. Each care team will travel to SLC for immersive two-week training and integration practice work before launching in their own unique locations. Four full-time training and implementation staff will be hired to facilitate these outlined processes and ensure appropriate adherence to the program is attained.
d. Service Affiliate Agreements
KindlyMD partners with local healthcare clinics and product manufacturers in geographic markets to maximize our ability to increase service and product offerings to more individuals. We partner with local healthcare clinics including those specializing in the evaluation and management of similar conditions as well as organizations specializing in behavioral therapy. We may white-label or manufacture supplements and products suited to our customers. KindlyMD may also sublease space within a primary clinic in order to expand services by an affiliate organization. These service agreements are governed by a healthcare Business Associate Agreement (BAA) as well as financial contracts.
In 2023 KindlyMD plans to continue and hopes to increase these affiliate agreements as needed.
e. Online and Email Campaign Marketing
Online marketing and email campaign marketing was a small portion of revenue in 2022/2021 but will continue to improve as the audience grows. Limiting factors have been the lack of paid advertising options, but will improve with the KindlyMD model of pain medicine management and data driven healthcare.
| | 2. | Adopt a comprehensive branding, marketing, digital and social media strategy for its revenue lines. |
| | | Marketing is currently based in-house with supplemental contracted work on an as needed basis. We have an in-house design and content creation team which maintains expertise in promoting the safe and effective use of non-opioid products, including medical cannabis, where legal markets allow for its consumption. KindlyMD also aggressively markets its services through paid channels including but not limited to paid online advertising, social media, emails to a list of over 32,000 contacts and patients, podcasting, YouTube channel content, blogging, press releases, and more. Ongoing spending of approximately 7.4% of total income is slated to continue in order to improve saturation and brand building within the Utah market as well as research and breakout in each new market entry. |
| | 3. | Acquisition growth strategy. KindlyMD will continue to leverage its growth potential as a leader in specialized data collection and healthcare in the opioid and alternative medicine space. We intend to research and negotiate acquisitions as much as we are able. We seek out specialty clinics focused on non-opioid evaluation and management of pain and other chronic illnesses. Clinics with large patient numbers that are involved in state legal medical cannabis programs, where patients are not able to receive behavioral healthcare, addiction services, or prescription management and education. These programs will be evaluated by a small team led by the CEO, COO, and consultant evaluators in order to acquire them as wholly owned subsidiaries or integrated healthcare clinics. We hope to acquire five to ten clinics in 2023/2024. |
| | | |
| 4. | Apply for Grant funding through US and State funded opioid and stimulant misuse programs. In May 2022 President Biden announced grant funding for improved substance use disorder treatment and mental health services. $1.6 Billion was announced awarded in September 2022. We plan to watch closely for funding opportunities in 2023, hiring one full time grant funding analyst to work on increasing KindlyMD’s capacity for government grants related to services of opioid treatment, education, and addiction mitigation. |
Kindly MD’s Commercial operations are in Salt Lake City, Utah.
No assurances can be given that any of these plans will come to fruition or that if implemented they will necessarily yield positive results.
Results of Operations
For the Years Ended December 31, 2022 and 2021
Revenue
The Company had revenue of $3,787,077 for the year ended December 31, 2022, compared to $2,504,319 for the year end December 31, 2021. The increase by $1,282,758, or 51.2%, is primarily attributable to continued growth of customer acquisition, improvement of clinic utilization and standardization of operational processes.
Operating Expenses
The Company had operating expenses of $6,480,490 for the year ended December 31, 2022, compared to $2,442,979 for the comparable period in 2021. The increase in operating expenses is primarily due to the following:
| 1. | Costs of goods sold increased by $65,261, or 74.9%, from $87,124 in 2021 to $152,385 in 2022, primarily due to increased retail sales volume. |
| 2. | General and administrative expenses increased by $1,297,981, or 152.1%, from $853,582 in 2021 to $2,151,563 in 2022, primarily due to our expansion efforts, increased personnel, and additional investments in infrastructure and technology. |
| 3. | Personnel expenses increased by $2,674,269, or 17.8%, from $1,502,273 in 2021 to $4,176,542 in 2022, primarily as a result of our expanded workforce to support our growth initiatives. |
Other Income (Expense)
Our other income increased by $64,824, or 73.7%, from $87,996 for the year ended December 31, 2021, to $152,820 for the year ended December 31, 2022. This increase is primarily attributable to gains from expanded marketing and education income and other non-operating income.
Net Income (Loss)
The Company had a net loss of $2,540,593 for the year ended December 31, 2022, compared to net income of $149,336 for the year ended December 31, 2021. This was due to the expansion of the key personnel and investment into a larger clinic location in Murray, Utah. This was also due to the use of stock as compensation of early key talent.
Liquidity and Capital Resources
As of December 31, 2022, we had total assets of $1,001,269, an increase of $553,626, or 123.7%, compared to $447,643 as of December 31, 2021. The increase in total assets is primarily due to the growth in our business and strategic investments in property, equipment, and operating right-of-use assets.
Our total liabilities increased by $445,698, or 255.3%, from $174,557 as of December 31, 2021, to $620,255 as of December 31, 2022. This increase is primarily due to the increase in accounts payable and accrued expenses, deferred revenue, operating lease liabilities, and amounts due to related parties.
We have sufficient resources to effectuate our business without additional expansion. Without additional funding, we expect to incur expenses offset by revenues during the next twelve months of operations.
Our stockholders’ equity increased by $107,939, or 39.5%, from $273,086 as of December 31, 2021, to $381,014 as of December 31, 2022. This increase is primarily due to the issuance of common stock, additional paid-in capital, and accumulated deficit.
Cash Flow
During the year ended December 31, 2022, our net cash used in operating activities was ($140,383), compared to net cash provided by operating activities of $74,100 during the year ended December 31, 2021. The change in net cash from operating activities is primarily due to the increase in recruiting and expanding the workforce in key personnel and the increase in accrued liabilities.
Net cash used in investing activities was $(317,388) during the year ended December 31, 2022, compared to $(63,602) during the year ended December 31, 2021. The increase in cash used in investing activities is primarily due to investments in property and equipment to support our growth.
Net cash provided by financing activities was $550,000 during the year ended December 31, 2022, compared to $2,000 during the year ended December 31, 2021. The increase in cash provided by financing activities is primarily due to the issuance of common stock and additional paid-in capital.
As a result of these cash flow activities, our net cash increased by $92,229, or 97.4%, from $94,689 as of December 31, 2021, to $186,918 as of December 31, 2022.
Outlook
We expect to continue investing in our growth initiatives, including expanding our client base, enhancing our product and service offerings, and improving our operational efficiency. We believe that these investments will support our long-term growth and profitability.
However, we recognize that there are inherent risks and uncertainties associated with our growth strategy, including the ability to maintain and grow our client base, successfully execute on our strategic initiatives, and manage our operating expenses. We will continue to monitor our financial performance and make adjustments to our growth strategy as necessary to ensure the long-term success of our business.
BUSINESS
Overview
KindlyMD is a holistically focused pain management clinic and healthcare data company formed in 2019. KindlyMD offers direct health care to patients integrating prescription medicine and behavioral health services to reduce opioid use in the chronic pain patient population. Its specialty outpatient clinical services are offered on a subscription and fee-for-service basis to augment traditional healthcare. The Company offers evaluation and management, including, but not limited to chronic pain, functional medicine, cognitive behavioral therapy, intravenous infusion therapy, recovery support services, overdose education efforts, peer support, limited urgent care, dermatology, preventative medicine, travel services, and hormone therapy. Through its focus on an embedded model of prescriber and therapist teams, KindlyMD is working to develop patient-specific care programs with a specific mission to reduce opioid use in the patient population while successfully treating patients with successful and evidence based non-opioid alternatives and behavioral therapy.
Beyond its treatment of patients, KindlyMD collects data focused on why and how patients turn to alternative treatments to reduce prescription medication use and addiction. The Company captures all relevant datapoints to assist and appropriately treat each individual patient. This also results in valuable data for the company and the company’s investors. We strive to become a source for evidence-based guidelines, data, and education in the fight against the opioid crisis in America.
KindlyMD offers direct healthcare focused on what patients want and need, not what the doctor wants or needs. Its prescribers and therapists listen, use data and evidence, then help patients decide on a care plan. Through its focus on the de-stigmatization of alternative therapies and by taking a collaborative approach to counsel patients on every option available to them, KindlyMD is develops patient-specific care programs that imbed behavioral therapy in every visit. KindlyMD providers are experts in de-prescribing and using alternatives to opioids, such as medical cannabis, Ketamine infusion therapy, and other prescription and non-prescription alternatives.
To further reduce inefficacious and opioid use we destigmatize behavioral health by imbedding behavioral health into every program we offer. Sessions are paid either through insurance or out of pocket. Additional work is done for addictions counseling, naloxone education, and risk mitigation strategies as part of our work on the Biden-Harris opioid initiative which places emphasis on decreasing overprescribing while improving access to addiction treatment and mental health initiatives. We offer naloxone to each chronic opioid patient as well as education and monitoring for addiction and recovery.
KindlyMD is most successful when patients report positive health outcomes as a result of our care. KindlyMD imbeds prescribers with behavioral health consultants to develop person-specific care programs for patients. Its medical advisory committee evaluates the efficacy of those programs. Impact goals are set within individual programs and measured against national benchmarks and “Clinical Quality Measures.”
KindlyMD’s Clinical Quality Measure goals for 2023/2024 include:
● Opioid dose reduction at a level pain scale rating
● Pain brought under control within 48 hours
● Preventive Care and Screening: Screening for depression and follow-up plan
● Functional status change for patients with low back pain
● Implementation of improvements causing more timely communication of test results
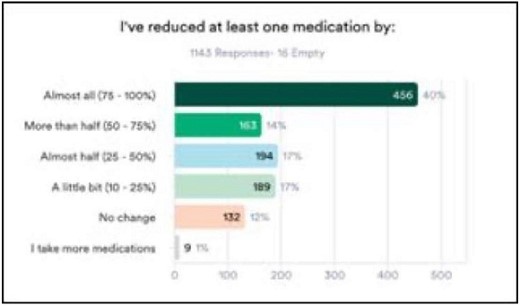
Beyond its treatment of patients, KindlyMD collects data focused on why and how patients may turn to non-opioid alternatives to reduce prescription medication use and addiction. The Company captures all available data from patient visits (including prescriptions, use history, purchase history, and trauma history) and has become the largest existing alternative medicine data source of its kind in Utah. Such data is used to study the effects and habits of patients using alternative treatments versus traditional medications, primarily in the pain and addiction space and medical cannabis space.
KindlyMD’s Role in Fighting the Opioid Epidemic
Sixty nine percent of Americans aged 45-64 use prescription medication, and most of these prescriptions do not help enough to offset the significant risks associated with long term side effects or do not help at all. Over age 65, it gets much worse, with opioids and benzodiazepines the most worrisome. Almost 108,000 Americans died of overdose in the U.S. in 2021 spurring the government to initiate the largest opioid treatment grant funding ever. Yet the opioid market is forecast to grow 3.5% year over year despite heavy regulations and stricter guidelines for providers who prescribe opioids. The aging U.S. population will increase the diagnosis of osteoarthritis beyond 67 million individuals by 2025 and about 100 million Americans suffer from chronic pain each year.
Rising demand for surgeries, increasing awareness, availability of treatment options, and the willingness to seek treatment are expected to complement the growth of the population of patients seeking treatment for pain and/or chronic pain medication use. Furthermore, while non-opioid treatment options, including medical cannabis, are becoming much more widespread, they are not included in clinical guidelines in any helpful fashion. Doctors are not trained in non-opioid alternatives and generally feel unprepared to prescribe or recommend alternatives to opioids. Only 1 in 10 medical doctors are prepared to recommend these non-opioid drugs in their practice.
A growing number of adults are using long term medications that they do not need. Many of these medications cause long term negative side effects and even death. Few providers offer education and guidance around formulary products with simple guidelines targeted to reducing dangerous opioid use. Research on patient behavior and non-opioid alternative use is lacking quality and consistency in large part due to the patchy regulatory framework, inadequate training, and stigma. Patients often seek out alternative medical interventions or turn to dangerous drugs such as fentanyl when traditional healthcare systems fail to meet their needs. Additionally, mental health stressors are known to contribute to chronic medical illness and have skyrocketed in the past three years.
Life stressors and trauma, stress-related physical symptoms, and ineffective patterns of healthcare utilization led KindlyMD to develop a holistically focused solution for patients. KindlyMD offers direct health care focused on prescribing what is needed and getting rid of what is not, blending prescription medicine, alternative medicine, and behavioral health services at an affordable rate. This increases access for the many patients seeking treatment who now have a clear path of integrated care. It also focuses data and evidence collection on the patients and their treatment programs in order to develop clear clinical guidelines, services, and products designed to lower opioid use and improve outcomes using the safest and most effective products and services available.
The four pillars of KindlyMD are:
| 1. | Listen First. KindlyMD is the first healthcare company of its kind to blend prescriptions, alternative medicine, and behavioral health services into every patient care plan. |
| 2. | Integrate. Each “Integration Team” consists of a prescriber, BHC (behavioral health consultant), and Care Coordinator — because it works best when everyone is on the same team under the same roof. Our care is outcome-focused by being patient-focused. |
| | | |
| 3. | Track the data. KindlyMD captures and tracks sought after patient data. Our national database strives to become the first and largest non-opioid alternative medicine data source in the U.S. working to end the opioid epidemic. |
| | | |
| 4. | Understand the need. KindlyMD understands the burden of the opioid epidemic on at-risk populations. This is why KindlyMD connects at-risk veterans, the terminally ill, and low-income individuals with healthcare resources and education through its charitable contributions. |
KindlyMD Headquarters
Our headquarters are located at 230 W 400 S, Suite 201 Salt Lake City, UT 84101.
Recent Acquisitions
KindlyMD recently executed a four-year lease for 5,321 square feet of clinic and office space in Murray Utah. This expansion will complement our existing clinic location in Millcreek Utah and offer up to ten more exam and consultation rooms to our local clinical capacity. It will also allow KindlyMD to consolidate a smaller location into a larger location, which will improve the cost-per-patient-visit ratio. We anticipate this expansion to increase revenue by up to $270,000 per month in the summer of 2023.
Business Revenue Streams
We operate across various revenue streams: (i) medical evaluation and treatment visits for chronic illness, (ii) subscription outpatient medicine (pain clinic medicine), (iii) data collection and research, (iv) education partnerships with various entities, (v) online and email campaign marketing revenue, (vi) product/supplement affiliate agreements, and (vii) retail sale of dietary supplements, and products.
KindlyMD is a licensed healthcare facility performing routine evaluation and management for non-emergent conditions in a direct care and subscription model. KindlyMD focuses primarily on three visit types. 1) Patients with pain who are using opioid medications, 2) patients seeking evaluation and management for non-opioid alternative treatments that require the recommendation of a licensed prescriber. 3) Behavioral Therapy and guided therapy visits billed through traditional insurance or out of pocket.
Patients with pain who are using opioid medications
New patients pay an initial evaluation fee of $249 and a monthly subscription fee of $119 per month, plus labs and testing on an individual basis as needed. Non subscription follow up visits are billed $189. Visits are required at least once per month for programs involving management of a controlled substance, such as opioid medications. Insurance billing is also available in select markets and with select payers, broadening the patient base and improving payment flexibility and access.
Patients seeking evaluation and management for non-opioid alternative treatments that require the recommendation of a licensed prescriber
New patients pay an initial evaluation fee of $249 and $189 for a follow-up appointment. KindlyMD sees between 1600 and 1900 visits per month in total under this program in Utah.
Behavioral Therapy and guided therapy visits billed through traditional insurance or out of pocket
Behavioral health therapy visits are embedded into the Company’s clinical model and also performed either on a fee for service basis or paid through traditional insurance contracts. The clinical model includes behavioral therapy for 15-minute sessions at each visit, plus the Company offers 50 minute therapy sessions with licensed therapists trained in trauma-based therapy.
Data Collection and Research
KindlyMD collects valuable data from interactions with people online, via telecommunication, and patient interactions and through our products. Clients provide some of this data directly, as do clinicians, and staff by collecting data about interactions, product and medication use, experiences, and behavior. The data we collect depends on the context of the interactions with KindlyMD and the choices people make, including their privacy settings and the products and features they use. We also obtain data about customers, patients, and clients from third parties.
Patient data is governed by HIPAA. Patients consent to allowing KindlyMD use of their data upon signing up for our services. Patients, clients, and customers of KindlyMD have choices when it comes to the technology they use and the data they share. If someone chooses not to share their data with KindlyMD, We may not provide services. Where providing data is optional, clients, patients, and customers are offered the choice to provide the data or not to.
In collecting data from these interactions, we combine data we collect from different contexts or obtain from third parties to provide a more seamless, consistent, and personalized experience, to make informed business decisions, to make clinical decisions, and for other legitimate business purposes.
Our processing of personal and healthcare data for these purposes includes both automated and manual (human) methods of processing. Our automated methods often are related to and supported by our manual methods. For example, our automated methods may include artificial intelligence (AI), which is a set of technologies that enable computers to perceive, learn, reason, and assist in decision-making to solve problems in ways that are similar to what people do. To build, train, and improve the accuracy of our automated methods of processing (including AI), we manually review some of the predictions and inferences produced by the automated methods against the underlying data from which the predictions and inferences were made. This manual review may be conducted by KindlyMD employees or vendors who are working on KindlyMD’s behalf.
We share personal data with consent or to complete any transaction or provide any product an individual has requested or authorized. We also share data with KindlyMD controlled affiliates and subsidiaries; with vendors working on our behalf; when required by law or to respond to legal process; to protect our customers; to protect our customers and patients; to protect lives; to maintain the security of our products; and to protect the rights and property of KindlyMD and its customers and patients.
KindlyMD is required to protect the information it collects and maintains. KindlyMD respects the right to privacy and will protect the information it maintains about clients and patients in accordance with all required laws, regulations and standards. All healthcare research and published data regarding clinical outcomes is anonymized and governed by HIPAA. Personally identifying information is protected and removed from any research, publication, or sale to a third party.
Education Partnerships with various entities
We strive to build the largest audience of those seeking honest, evidence-based, holistic and practical information about alternatives to opioids. Our in-house education center creates educational content for all KindlyMD’s programs. Much of the educational content is subsidized through local and regional partnership agreements, currently representing approximately 3% of overall revenue.
Online and Email Campaign Marketing Revenue
KindlyMD has a combined reach of over 32,000 email contacts that have opted-in to our education and marketing communication. Paid messaging is offered to local and regional businesses for a fee.
Product/Supplement Affiliate Agreements
KindlyMD partners with local healthcare clinics and product manufacturers in geographic markets to maximize our ability to increase service and product offerings to more individuals. We partner with local healthcare clinics including those specializing in the evaluation and management of similar conditions as well as organizations specializing in behavioral therapy. We may white-label or manufacture supplements and products suited to our customers. KindlyMD may also sublease space within a primary clinic in order to expand services by an affiliate organization. These service agreements are governed by a healthcare Business Associate Agreement (BAA) as well as financial contracts.
Retail Sales of Dietary Supplements and Products
In early 2023, the company expanded service lines to include full-spectrum hemp-based products, including “gummies”. Our mission is to enhance our patient’s and customers’ overall quality of life and reduce the use of prescription medications, specifically opioids. We launched a line of hemp-derived delta 9 products supported by our direct care of patients. The company’s three initial gummy product offerings contain a variety of cannabinoids and terpenes in addition to CBD while maintaining THC amounts within the limits set in the 2018 Farm Bill.
Our Competitive Strengths
KindlyMD is one of the largest providers of medical evaluation and management services related to treatment recommendations within the medical cannabis program in Utah. Our model of healthcare is unique in Utah, blending prescribers and licensed behavioral health clinicians into every patient care plan. We know of no other large pain clinic in Utah that has this integration model. We are one of a limited number of specialty providers who allow patients to utilize non-opioid alternative medications with their opioids with supervision by a licensed prescriber. Our competition are pain clinics as well as other non-opioid specialty alternative medicine clinics in Utah. KindlyMD providers are experts in de-prescribing and using alternatives to opioids, so much so that in one recent survey, 88% of our patients saw a reduction in other medication in just 6 months.
In additional to its in-person clinics, KindlyMD offers telehealth online to patients in select markets. KindlyMD believes its business model could thrive in other states where a pain clinic can build a profitable base in treating pain patients with prescription medication regardless of the state-legal alternative, non-opioid, options available to patients. With KindlyMD’s data driven model, positive outcomes could contribute to more favorable legislation toward many non-opioid alternative medication options in the US, such as cannabis and psychedelics to augment treatment protocols.
In addition, KindlyMD’s significant database and data collection model sets it apart from its competitors. Each visit is charted by two clinicians, one prescriber and one behaviorist. This data focuses on why and how patients may turn to alternative to reduce prescription medication use and addiction and is a valuable tool in establishing treatment protocols and advocating the industry for more diverse treatment options. We know of no other data pool or data collection model that matches this specificity or level of functionality.
Competition
KindlyMD’s patient services faces competition from existing chronic pain clinics, alternative pain treatment centers, and direct to patient healthcare subscription companies (such as goforward.com or Medalus). Competition also comes from imbedded integration company EvolvedMD who are a behavioral health company imbedding BHC’s into existing clinics in Arizona and Utah through an affiliate model. Further, it also faces competition from other non-opioid focused healthcare data collection companies. However, the Company believes that there is a high barrier for entry in this industry that requires specialized expertise, licensure, and that will require significant capital. The Company believes it can compete with these other companies due to the experience of the team and the unique model of healthcare provided.
Marketing and Customers
KindlyMD uses various marketing techniques to advertise its services and attract potential new clients and patients. Such techniques include releasing weekly podcasts and posting videos on a YouTube channel, aggressive online and social media marketing, billboard campaigns in target markets, publishing research, and participating in speaking engagements. KindlyMD has two specific target markets: patients and businesses. KindlyMD attempts to target patients suffering from chronic pain, adults on opioids or more than one prescription medication, and adults seeking access to medical cannabis in state-legal markets. Further, the Company targets businesses looking for data resources, clinic operations and centers dedicated to wellness, research and analysis, market research, and consumer analyses.
Corporate History
On December 2, 2019, the Company filed their original articles of organization with the state of Utah under the name “Utah Therapeutic Health Center, PLLC.”
On April 15, 2020, the Company filed Articles of conversion with the State of Utah converting the entity from a PLLC to an LLC.
On March 11, 2022, the Company filed Articles of Conversion with the State of Utah converting the entity from a limited liability company to a corporation and formally changing the name of the Company to “Kindly MD, Inc.”
On July 5, 2022, the Company filed Amended and Restated Articles of Incorporation with the State of Utah, adding a preferred class of stock to its authorized shares and increasing the amount of common shares authorized, among other corporate governance matters.
Intellectual Property
The Company has no intellectual Property as of the filing date.
Properties
Currently, the Company operates within a leased property located at 230 W 400 S, Suite 201 Salt Lake City, UT 84104. The lease has a flat monthly rate of $2,498.00 per month and the twelve-month lease term runs through May 2023.
The Company also operates clinics located at the following locations:
In Millcreek, Utah at 740 E 3900 S, Suite 108 Salt Lake City, Utah 84107. The lease is for a 24 month term through March 31, 2022, with an option for a one year extension through April 30, 2023. The monthly rate is $2,334 per month.
In Ogden, Utah at 2485 Grant Ave, Suite 105 Ogden, Utah 84401. The lease expired on August 31, 2022 and is currently on a month-to-month basis. The monthly rate is $978.33 per month.
In Bountiful, Utah at 580 W 100 N, Suite 4 Bountiful, Utah 84010. The lease is for a 48-month term through April 30, 2025. The monthly rate is $1,152 per month.
In Provo, Utah at 222 Draper Lane, Suite 2 Provo, Utah 84601. The lease is for a 15-year term through February 28, 2035. The monthly rate is $510 per month.
In Murray, Utah at 5097 S 900 E, Suite 100, Murray, Utah 84117. The lease is for a 48 month term through January 31, 2027. The monthly rate is $6,278.96 per month.
In Cedar City, Utah at 301 S Main St. Ste B, Cedar City, Utah 84720. The lease is for a 24-month term through August 8, 2023. The monthly rate is $1,200 per month.
Seasonality
Full healthcare clinics and data collection and research businesses typically operate on a full-time basis, twelve months a year, without much seasonal impact or variation.
Employees and Human Capital
We currently have a total of 104 employees, consisting of 40 full-time employees and 62 part-time employees.
Government Regulation
We are subject to local, state, federal and international laws, statutes, rules, policies, and regulations (collectively “Regulations”) that relate directly or indirectly to our operations. These include privacy and data protection regulations. Our business operations involve the collection, transfer, use, disclosure, security, and disposal of personal or sensitive information. As a result, our business is subject to complex and evolving U.S. and international laws and regulations regarding privacy and data protection. We are also subject to common business and tax rules and regulations pertaining to the operation of our business. Below is a discussion of the federal and state-level regulatory regimes in those jurisdictions where we are currently directly involved.
Federal Regulation of Cannabis
In 2005, the U.S. Supreme Court ruled that Congress has the power to regulate cannabis.
The U.S. federal government regulates drugs through the Controlled Substances Act, which places controlled substances, including cannabis, in a schedule. Cannabis is classified as a Schedule I controlled substance. A Schedule I controlled substance is defined as a substance that has no currently accepted medical use in the United States, a lack of safety for use under medical supervision and a high potential for abuse. The Department of Justice defines Schedule I drugs, substances or chemicals as “drugs with no currently accepted medical use and a high potential for abuse.” However, the Food and Drug Administration has approved Epidiolex, which contains a purified form of the drug CBD, a non-psychoactive ingredient in the cannabis plant, for the treatment of seizures associated with two epilepsy conditions. The Food and Drug Administration has not approved cannabis or cannabis compounds as a safe and effective drug for any other condition. Moreover, under the 2018 Farm Bill or Agriculture Improvement Act of 2018, CBD remains a Schedule I controlled substance under the Controlled Substances Act, with a narrow exception for CBD derived from hemp with a tetrahydrocannabinol, which is commonly referred to as THC, concentration of less than 0.3%.
Marijuana is largely regulated at the state level.
State laws that permit and regulate the production, distribution and use of cannabis for adult use or medical purposes are in direct conflict with the Controlled Substances Act, which makes cannabis use and possession federally illegal. Although certain states and territories of the U.S. authorize medical and/or adult use cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts under federal law under any and all circumstances under the Controlled Substances Act. Although our activities are believed to be compliant with applicable state and local laws, strict compliance with state and local laws with respect to cannabis may neither absolve us of liability under U.S. federal law, nor may it provide a defense to any federal proceeding which may be brought
The risk of federal enforcement and other risks associated with our business are described in the section entitled “Risk Factors.”
Regulation of the Cannabis Market at State and Local Level
The Utah Medical Cannabis Act (H.B. 3001 Utah Medical Cannabis Act) directs the Utah Department of Health (UDOH) to issue medical cannabis cards to patients, register medical providers who wish to recommend medical cannabis treatment for their patients, and license medical cannabis pharmacies. Physicians, Advanced Practice Registered Nurses, and physician assistants who are licensed to prescribe a controlled substance are allowed to recommend medical cannabis treatment for their patients. Providers must register as a qualified medical provider through an Electronic Verification System (EVS) established by the UDOH. Registered providers may only recommend medical cannabis treatment to a patient in the course of the physician-patient relationship after completing and documenting in the patient’s record a thorough assessment of the patient’s condition and medical history.
Telemedicine is allowed under the Utah Medical Cannabis Act only after the Provider has evaluated the patient, at least once, face-to-face. All interactions between the treating provider and patient are considered healthcare interactions and therefore are governed by HIPAA privacy laws.
Legal Proceedings
From time to time, the Company may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We know of no existing or pending legal proceedings against us, nor are we involved as a plaintiff in any proceeding or pending litigation. There are no proceedings in which any of our directors, officers or any of their respective affiliates, or any beneficial stockholder, is an adverse party or has a material interest adverse to our interest.
MANAGEMENT
Directors and Executive Officers
The following table sets forth information about our directors and executive officers as of December, 2022. We intend to appoint three independent directors upon the consummation of this offering.
| Name | | Age | | Position | | Date of Appointment |
| Timothy Pickett | | 44 | | Chief Executive Officer, Director | | December 2019 |
Adam Cox Jared Barrera | | 45 41 | | Chief Operating Officer, Director Chief Financial Officer | | April 2022 September 2022 |
Biographies of Directors and Officers
The following is a brief account of the education and business experience during at least the past five years of each director, executive officer, and key employee of our Company, indicating the person’s principal occupation during that period, and the name and principal business of the organization in which such occupation and employment were carried out.
The following noteworthy experience, qualifications, attributes, and skills for each Board member, together with the biographical information for each nominee described below, led to our conclusion that the person should serve as a director in light of our business and structure:
Tim Pickett, Chief Executive Officer, Chairman
Tim Pickett, MPAS-C, age 44, is the founder and CEO since the Company was founded in 2019. He graduated from the University of Utah with a Master’s Degree in Physician Assistant Studies (2014). He previously worked in General Surgery, Trauma, and Emergency Medicine for Steward Medical Group’s Physician Group of Utah from 2014 to October, 2020. His experience in surgery and critical care shaped his view of the opioid crisis. He currently teaches at the University of Utah Physician Assistant program as a guest lecturer on medical cannabis. He lobbies governments for improved legislation for non-opioid medicine access and is an advocate for the practical use of safe, evidence-based alternatives, in medical treatment. He was voted Utah’s best Medical Cannabis Doctor in 2020 and 2021, respectively.
Adam Cox, Chief Operating Officer, Director
Adam Cox, age 45, is an experienced businessperson, and inspiring leader. Adam’s notable business ventures include working in the healthcare sector since 2009 transitioning healthcare from paper charting to electronic records under the CMS mandated Meaningful Use. He pioneered several streamlining initiatives in Healthcare Data Analytics, training, and management in Utah. Mr. Cox guided nationwide IS Acquisitions and Analytics for the largest private hospital operator in the United States. After 13 years in corporate healthcare, he joined KindlyMD to pursue a solution to the opioid epidemic through data analytics and optimum operational management.
Jared Barrera, Chief Financial Officer
Jared Barrera, MBA, age 41 has been in both the banking and healthcare industries for the last 20 years, specifically with an emphasis in finance and accounting. He has a wide array of experience ranging from revenue optimization, business intelligence, GAAP accounting, budgeting and forecasting, financial modeling, lending and credit analysis, revenue cycle management, collections management, auditing and payroll processing. Jared graduated with Master’s degree in Business Administration with an emphasis in accounting from Utah Valley University, 2015. He also received a Bachelor’s of Science degree in accounting from Utah Valley University in 2011.
Indemnification of Directors and Officers
Subject to Title 16, Chapter 10a, Part 9 of the Utah Revised Business Corporation Act (the “Act”) and the laws of the State of Utah, officers shall be indemnified by the Corporation, so long as the officer acted in a manner substantially similar to and consistent with the standard of care for directors. Any officer indemnification shall be limited to proceedings that are directly related to or have arisen out of the officer’s acts on behalf of the Corporation.
Family Relationships
There are no family relationships between our officers and members of our Board of Directors.
Significant Employees
The significant employees are Timothy Pickett and Adam Cox and Jared Barrera.
Director Compensation
There are no formal agreements with our directors for compensation and will be formalized upon consummation of this offering.
Director Independence
The listing rules of Nasdaq require that independent directors must comprise a majority of a listed company’s board of directors. In addition, the rules of Nasdaq require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating and governance committees be independent. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. Under the rules of Nasdaq, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Our Board has undertaken a review of the independence of our directors and considered whether any director has a material relationship with it that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, the Board has determined that no members are “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing standards of Nasdaq. In making these determinations, our Board considered the current and prior relationships that each non-employee director has with the Company and all other facts and circumstances our Board deemed relevant in determining their independence, including the beneficial ownership of the Company’s capital stock by each non-employee director, and any transactions involving them described in the section captioned “Certain Relationships and Related Party Transactions.” The Company intends to appoint three independent board members upon the completion of the Offering.
Board Leadership Structure and Risk Oversight
The Board oversees our business and considers the risks associated with our business strategy and decisions. The Board currently implements its risk oversight function as a whole. As such, it is important for us to have our Chief Executive Officer serve on the Board as he plays key roles in the risk oversight of our Company. Each of the Board committees, when established prior to the effectiveness of the registration statement of which this prospectus is a part, will also provide risk oversight in respect of its areas of concentration and report material risks to the Board for further consideration.
Board Committees
As of the closing of the offering, our Board will have established the following three standing committees: audit committee (the “Audit Committee”); compensation committee (the “Compensation Committee”); and nominating and governance committee (the “Nominating Committee”). Each of our independent directors, [●], will serve on each committee. Our Board will adopt written charters for each of these committees. Upon completion of this offering, copies of the charters will be available on our website at www.kindlymd.com. Our Board may establish other committees as it deems necessary or appropriate from time to time.
The Board may create committees to delegate certain powers to act on behalf of the Board, provided the Board passes a resolution indicating such creation or delegation. The Board may delegate to a committee the power to appoint directors to fill vacancies on the Board. The creation or appointment of a committee does not relieve the Board or its members from their standard of care
Audit Committee
The Audit Committee, among other things, will be responsible for:
| | ● | appointing; approving the compensation of; overseeing the work of; and assessing the independence, qualifications, and performance of the independent auditor; |
| | | |
| | ● | reviewing the internal audit function, including its independence, plans, and budget; |
| | | |
| | ● | approving, in advance, audit and any permissible non-audit services performed by our independent auditor; |
| | | |
| | ● | reviewing our internal controls with the independent auditor, the internal auditor, and management; |
| | | |
| | ● | reviewing the adequacy of our accounting and financial controls as reported by the independent auditor, the internal auditor, and management; |
| | | |
| | ● | overseeing our financial compliance system; and |
| | | |
| | ● | overseeing our major risk exposures regarding the Company’s accounting and financial reporting policies, the activities of our internal audit function, and information technology. |
The Board has affirmatively determined that each member of the Audit Committee meets the additional independence criteria applicable to audit committee members under SEC rules and Nasdaq listing rules. Effective upon the completion of this offering the Board will adopt a written charter setting forth the authority and responsibilities of the Audit Committee. The Board has affirmatively determined that each member of the Audit Committee is financially literate, and that Jared Barrera meets the qualifications of an Audit Committee financial expert under the rules promulgated by the SEC.
The Audit Committee will consist of [●] will chair the Audit Committee. We believe that, after consummation of this offering, the functioning of the Audit Committee will comply with the applicable requirements of the rules and regulations of the Nasdaq listing rules and the SEC.
Compensation Committee
The Compensation Committee will be responsible for:
| | ● | reviewing and making recommendations to the Board with respect to the compensation of our officers and directors, including the CEO; |
| | | |
| | ● | overseeing and administering the Company’s executive compensation plans, including equity-based awards; |
| | | |
| | ● | negotiating and overseeing employment agreements with officers and directors; and |
| | | |
| | ● | overseeing how the Company’s compensation policies and practices may affect the Company’s risk management practices and/or risk-taking incentives. |
Effective upon the completion of this offering, the Board will adopt a written charter setting forth the authority and responsibilities of the Compensation Committee.
The Compensation Committee will consist of [●], [●] will serve as chairman of the Compensation Committee. The Board has affirmatively determined that each member of the Compensation Committee meets the independence criteria applicable to compensation committee members under SEC rules and Nasdaq listing rules. The Company believes that, after the consummation of the offering, the composition of the Compensation Committee will meet the requirements for independence under, and the functioning of such Compensation Committee will comply with, any applicable requirements of the rules and regulations of Nasdaq listing rules and the SEC.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee, among other things, will be responsible for:
| | ● | reviewing and assessing the development of the executive officers and considering and making recommendations to the Board regarding promotion and succession issues; |
| | | |
| | ● | evaluating and reporting to the Board on the performance and effectiveness of the directors, committees and the Board as a whole; |
| | | |
| | ● | working with the Board to determine the appropriate and desirable mix of characteristics, skills, expertise and experience, including diversity considerations, for the full Board and each committee; |
| | | |
| | ● | annually presenting to the Board a list of individuals recommended to be nominated for election to the Board; |
| | | |
| | ● | reviewing, evaluating, and recommending changes to the Company’s corporate governance principles and committee charters; |
| | | |
| | ● | recommending to the Board individuals to be elected to fill vacancies and newly created directorships; |
| | | |
| | ● | overseeing the Company’s compliance program, including the code of business conduct and ethics; and |
| | | |
| | ● | overseeing and evaluating how the Company’s corporate governance and legal and regulatory compliance policies and practices, including leadership, structure, and succession planning, may affect the Company’s major risk exposures. |
Effective upon completion of this offering, the Board will adopt a written charter setting forth the authority and responsibilities of the Nominating and Corporate Governance Committee.
The Nominating and Corporate Governance Committee will consist of [●], will serve as chairperson. The Board has determined that each member of the Nominating and Corporate Governance Committee is independent within the meaning of the independent director guidelines of Nasdaq listing rules.
Compensation Committee Interlocks and Insider Participation
None of the Company’s executive officers serves, or in the past has served, as a member of the Board or the Compensation Committee, or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of the Board or its Compensation Committee. None of the members of the Compensation Committee is, or has ever been, an officer or employee of the company.
Code of Business Conduct and Ethics
Prior to the completion of this offering, the Board will adopt a code of business conduct and ethics applicable to its employees, directors, and officers, in accordance with applicable U.S. federal securities laws and the corporate governance rules of Nasdaq. The code of business conduct and ethics will be publicly available on the Company’s website at www.kindlymd.com. Any substantive amendments or waivers of the code of business conduct and ethics or code of ethics for senior financial officers may be made only by the Board and will be promptly disclosed as required by applicable U.S. federal securities laws and the corporate governance rules of Nasdaq.
Corporate Governance Guidelines
Prior to the completion of this offering, the Board will adopt corporate governance guidelines in accordance with the corporate governance rules of Nasdaq.
Involvement in Certain Material Legal Proceedings During the Last Ten Years
During the past ten years, none of our current directors or executive officers has been:
| ● | the subject of any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| | |
| ● | convicted in a criminal proceeding or is subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| | |
| ● | subject to any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, or banking activities; |
| | |
| ● | found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, that has not been reversed, suspended, or vacated; |
| | |
| ● | subject of, or a party to, any order, judgment, decree or finding, not subsequently reversed, suspended, or vacated, relating to an alleged violation of a federal or state securities or commodities law or regulation, law or regulation respecting financial institutions or insurance companies, law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| | |
| ● | subject of, or a party to, any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity, or organization that has disciplinary authority over its members or persons associated with a member. |
None of our directors, officers or affiliates, or any beneficial owner of 5% or more of our Common Stock, or any associate of such persons, is an adverse party in any material proceeding to, or has a material interest adverse to, us or any of our subsidiaries.
Meetings of the Board of Directors
During its fiscal year ended December 31, 2022, there were multiple meetings of the Board. The Board acted by written consent from its members at that time on numerous occasions.
Directors’ and Officers’ Liability Insurance
The Corporation may purchase and maintain insurance or make other financial arrangements on behalf of any Indemnitee for any liability asserted against him or her and liability and expenses incurred by him or her in his or her capacity as a director, officer, employee, member, managing member or agent, or arising out of his or her status as such, whether or not the Corporation has the authority to indemnify him or her against such liability and expenses.
EXECUTIVE COMPENSATION
Summary Compensation Table
The table below sets forth, for our last fiscal year, the compensation of our officers.
| Name and Principal Position | | Fiscal Year | | | Salary | | | Bonus | | | Stock Awards | | | All Other Compensation | | | Total | |
| | | | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | |
| Timothy Pickett, CEO (a)(b) | | | 2020 | | | | 30,000 | | | | - | | | | - | | | | 25,000 | | | | 55,000 | |
| | | | 2021 | | | | 120,502 | | | | - | | | | - | | | | 160,000 | | | | 280,502 | |
| | (a) | Appointed as CEO, December 2019. |
| | (b) | Includes amounts paid to related entity. |
We have health insurance benefits and a 2022 bonus plan for the officers but do not have pension, annuity, profit sharing or similar benefit plans.
Employment Agreements
The Company entered into a three-year employment agreement with Tim Pickett, CEO, effective May 1, 2022. The agreement also provides that the executive will continue as a director. The agreement provides for an initial term, commencing on the effective date of the agreement and ending on April 30, 2025, and continuing on a year-to-year basis thereafter unless terminated by either party on not less than 60 days’ notice given prior to the expiration of the initial term or any one-year extension. For his services to the Company during the term of the agreement, Mr. Pickett receives and annual salary of $150,000 per annum, commencing on the effective date of the agreement and increasing to $240,000 per annum in the month in which the Company shall have received not less than $2,500,00 from one or more public or private financings of the Company’s equity securities subsequent to the date of the agreement.
On October 10, 2022, Mr. Pickett received issuance of restricted shares of Company stock in the amount of 35,000 shares.
Wade Rivers, LLC
The Company entered into a consulting agreement with Wade Rivers, a WY Limited Liability Company on January 1, 2021. The agreement provides for a continuous term unless terminated by either party on not less than 30 days’ notice given. For services to the Company during the term of the agreement, Wade Rivers received $120,000 per annum, which was modified from U.S. dollars to receiving the same in restricted Company Stock, starting on July 1, 2022. Tim Pickett is the managing member of Wade Rivers, LLC.
The Company entered into a three-year employment agreement with Adam Cox, COO, effective May 1, 2022. The agreement provides for an initial term, commencing on the effective date and ending on April 30, 2023, and continuing a year-to-year basis thereafter unless terminated by either party on not less than 60 day’s notice given prior to the expiration of the initial term or any on-year extension. For his services to the Company during the term of the agreement, Mr. Cox receives and annual salary of $138,000 per annum, commencing on the effective date of the agreement and increasing to $224,000 per annum in the month in which the Company shall have received not less than $5,000,000 from one or more public or private financings of the Company’s equity securities subsequent to the date of the agreement.
On August 17 and October 10, 2022, Mr. Cox received issuance of shares of restricted Company stock, in the amounts of 20,000, and 14,992, respectively.
The Company entered into a Compensation Agreement with Jared Barrera, CFO, effective September 28, 2022. The agreement provides for an ongoing basis unless terminated by either party on not less than 60 days’ notice given. For his services to the Company during the agreement, Mr. Barrera receives and annual salary of $130,000 per annum, commencing on the effective date of the agreement.
On October 10, 2022, Mr. Barrera received issuance of shares of restricted Company stock, in the amount of 10,000 shares.
Outstanding Equity Awards
There were no equity awards made to any named executive officer that were outstanding at December 31, 2022.
Director Compensation
The Company will include director compensation when appointed and prior to listing on the NASDAQ public market.
Change-in-Control Agreements
None.
Incentive Stock Plan
On October 10, 2022, the Company’s shareholders and Board approved the Incentive Stock Plan (the “Plan”).
Indemnification
The Company shall indemnify any and all of its directors, officers, former directors, former officers and any person who may have served at its request as a director or officer of another company in which it owns shares or of which it is a creditor, who were or are made a party or are threatened to be made a party to or are involved in, any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative, arbitrative or investigative (each a “Proceeding”), or any appeal in such a Proceeding or any inquiry or investigation that could lead to such a Proceeding, against any and all liabilities, damages, reasonable and documented expenses (including reasonably incurred and substantiated attorneys’ fees), financial effects of judgments, fines, penalties (including excise and similar taxes and punitive damages) and amounts paid in settlement in connection with such Proceeding by any of them. Such indemnification shall not be deemed exclusive of any other rights to which those indemnified may be entitled otherwise.
To the extent that indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our Company pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. If a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of our Company in the successful defense of any action, suit or proceeding) is asserted by any of our directors, officers or controlling persons in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of that issue.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
SEC rules require us to disclose any transaction since the beginning of our last fiscal year and for the two fiscal years preceding our last fiscal year, or any currently proposed transaction in which we are a participant in which the amount involved exceeded or will exceed $120,000 and in which any related person has or will have a direct or indirect material interest. A related person is any executive officer, director, nominee for director, or holder of 5% or more of our Common Stock, or an immediate family member of any of those persons.
The Company has transactions with related parties, including officers, directors, and their affiliates. The transactions are conducted in the ordinary course of business and are generally on terms no less favorable than those available to unrelated third parties.
As of December 31, 2021, the company had a note receivable from Wade Rivers, LLC, a company owned 50% by the Chief Executive Officer of KindlyMD, in the amount of $66,212. In June 2022 the Company received $151,100 from Wade Rivers, LLC. This paid the balance in full and the remaining proceeds were established as a note payable to Wade Rivers, LLC per a written agreement between parties. As of December 31, 2022, the Company had an outstanding unsecured loan payable to Wade Rivers, LLC, in the amount of $92,545.
The Company reimburses officers and directors for reasonable and necessary expenses incurred in the course of performing their duties for the Company. The Company also provides certain officers and directors with health insurance, retirement benefits, and other fringe benefits.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table lists, as of April 25, 2022, the number of shares of Common Stock of our Company that are beneficially owned by (i) each person or entity known to our Company to be the beneficial owner of more than 10% of the outstanding Common Stock; (ii) each officer and director of our Company; and (iii) all officers and directors as a group. Information relating to beneficial ownership of Common Stock by our principal shareholders and management is based upon information furnished by each person using beneficial ownership’ concepts under the rules of the SEC. Under these rules, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or direct the voting of the security, or investment power, which includes the power to vote or direct the voting of the security. The person is also deemed to be a beneficial owner of any security of which that person has a right to acquire beneficial ownership within 60 days. Under the SEC rules, more than one person may be deemed to be a beneficial owner of the same securities, and a person may be deemed to be a beneficial owner of securities as to which he or she may not have any pecuniary beneficial interest. Except as noted below, each person has sole voting and investment power.
The percentages below are calculated based on 4,434,596 shares of our Common Stock issued and outstanding as of April 25, 2023. Except as disclosed herein, we do not have any outstanding options, or other securities exercisable for or convertible into shares of our Common Stock. Unless otherwise indicated, the address of each person listed is c/o Kindly MD, Inc., 230 W 400 S, Suite 20, Salt Lake City, UT 84104.
To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our Common Stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company.
| Name and Address of Beneficial Owner | | Title | | Title of Class | | Beneficially Owned Before Offering(1) | | | Beneficially Owned After Offering | | | Percent of Class Before Offering(2) | | | Percent of Class After Offering | |
| Officers and Directors | | | | | | | | | | | | | | | | | | | | |
| Tim Pickett (3) | | CEO and Director | | Common | | | 3,215,945 | | | | 3,215,945 | | | | 73 | % | | | | % |
| Adam Cox | | COO and Director | | Common | | | 34,992 | | | | 34,992 | | | | 0.8 | % | | | | % |
| Jared Barrera | | CFO | | Common | | | 10,000 | | | | 10,000 | | | | 0.2 | % | | | | % |
| | | | | | | | | | | | | | | | | | | | | |
| Officers and Directors as a Group (total of 3 persons) | | | | | | | 3,260,937 | | | | | | | | | | | | | |
| | (1) | The number and percentage of shares beneficially owned is determined under the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has sole or shared voting power or investment power and also any shares, which the individual has the right to acquire within 60 days through the exercise of any stock option or other right. The persons named in the table have sole voting and investment power with respect to all shares of Common Stock shown as beneficially owned by them, subject to community property laws where applicable and the information contained in the footnotes to this table. |
| | (2) | SEC Rule 13d-3 generally provides that beneficial owners of securities include any person who, directly or indirectly, has or shares voting power and/or investment power with respect to such securities, and any person who has the right to acquire beneficial ownership of such security within 60 days. Any securities not outstanding which are subject to such options, warrants or conversion privileges exercisable within 60 days are treated as outstanding for the purpose of computing the percentage of outstanding securities owned by that person. Such securities are not treated as outstanding for the purpose of computing the percentage of the class owned by any other person. At the present time there are no outstanding options or warrants. |
| | | |
| | (3) | Mr. Pickett beneficially owns these shares through an entity, Wade Rivers, LLC, that is owned by The Pickett Legacy Trust for which he and his spouse serve as trustees. |
DESCRIPTION OF OUR SECURITIES
General
The following description of our Common Stock and provisions of our Articles of Incorporation and bylaws are summaries and are qualified by reference to such Articles of Incorporation and bylaws that will be in effect upon the closing of this offering. By becoming a shareholder in our Company, you will be deemed to have notice of and consented to these provisions of our Articles of Incorporation and bylaws.
Authorized Stock
Our Articles of Incorporation authorizes us to issue up to 100,000,000 shares of Common Stock (the “Common Stock”) and up to 10,000,000 shares of Preferred Stock (the “Preferred Stock). The authorized but unissued shares of our Common Stock and Preferred Stock are available for future issuance without shareholder approval. These additional shares may be used for a variety of corporate finance transactions, acquisitions, and employee benefit plans. The existence of authorized but unissued and unreserved Common Stock and Preferred Stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Voting Rights
Every shareholder entitled to vote at any meeting shall be entitled to one vote for each share of stock entitled to vote and held by him of record on the date fixed as the record date for said meeting and may so vote in person or by proxy. Any corporate action, other than the election of directors, shall be authorized by a simple majority of the votes cast in favor of or against such action by the holders of shares entitled to vote thereon except as may otherwise be provided by statute or the Articles of Incorporation. An abstention shall not count as a vote cast.
Liquidation or Dissolution
In the event of our liquidation or dissolution, the holders of Common Stock are entitled to receive proportionately all assets available for distribution to shareholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of Common Stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences, and privileges of holders of Common Stock are subject to and may be adversely affected by the rights of the holders of shares of any series of Preferred Stock that we may designate and issue in the future.
Dividends
The dividend rights, if any, of such class or series, the dividend preferences, if any, as between such class or series and any other class or series of stock, whether and the extent to which shares of such class or series shall be entitled to participate in dividends with shares of any other class or series of stock, whether and the extent to which dividends on such class or series shall be cumulative, and any limitations, restrictions or conditions on the payment of such dividends is determined by our Board.
Preemptive Rights
The holders of our Common Stock generally do not have preemptive rights to purchase or subscribe for any of our capital stock or other Common Stock.
Redemption
The terms and conditions, if any, of any purchase, retirement, or sinking fund which may be provided for the shares of such class or series is subject to the authorization of the Board.
Preferred Stock
Our Board is empowered, without stockholder approval, to issue shares of Preferred Stock with dividend, liquidation, redemption, voting or other rights which could adversely affect the voting power or other rights of the holders of Common Stock. In addition, the Preferred Stock could be utilized as a method of discouraging, delaying, or preventing a change in control of us. Although no shares of Preferred Stock are currently issued and outstanding, and we do not currently intend to issue any shares of Preferred Stock, we cannot assure you that we will not do so in the future.
Transfer Agent and Registrar
Our transfer agent for our Common Stock is VStock Transfer, LLC, 18 Lafayette Place, Woodmere, NY, 11598, (212) 828-8436.
Options
We currently have no outstanding options to purchase shares of our Common Stock.
Warrants
We currently have no outstanding warrants to purchase shares of our Common Stock.
Warrants to Be Issued in the Offering
Overview. The following summary of certain terms and provisions of the Warrants, included in the Units offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Warrant Agent Agreement between us and VStock Transfer, LLC, as Warrant Agent, and the forms of Tradeable Warrant and Non-tradeable Warrant, all of which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the Warrant Agent Agreement, including the annexes thereto, and forms of Warrant. The Tradeable Warrant and the Non-tradeable Warrant have identical terms except that (i) unlike the Non-tradeable Warrant, the Tradeable Warrant will be tradeable and will be listed on the Nasdaq Capital Market, and (ii) the exercise price per share of Common Stock is $[ ] per share for the Tradeable Warrant and $[ ] per whole share for the Non-tradeable Warrant.
Duration. The Warrants will be immediately exercisable and will expire on the [●] anniversary of the original issuance date. The exercise price and number of shares of Common Stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our Common Stock and the exercise price. The Warrants will be exercisable immediately upon issuance, will be issued separately from the Common Stock and may be transferred separately immediately thereafter. A Warrant to purchase one share of our Common Stock will be issued for every share of Common Stock purchased in this offering.
Exercise Price. The exercise price of the Tradeable Warrant is $[ ] per share, which is [ ]% of the assumed offering price of the Units, and exercise price of the Non-tradeable Warrant is $[ ] per whole share, which is [ ]% of the assumed offering price of the Units. The exercise price of both the Tradeable Warrants and Non-tradeable Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Exercisability. The Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our Common Stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the Warrant to the extent that the holder would own more than 4.99% of the outstanding Common Stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s Warrants up to 9.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants. No fractional shares of Common Stock will be issued in connection with the exercise of a Warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder exercises its Warrants, a registration statement registering the issuance of the shares of common stock underlying the Warrants under the Securities Act is not then effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Common Stock determined according to a formula set forth in the Warrants.
Transferability. Subject to applicable laws, a Warrant in book entry form may be transferred at the option of the holder through the facilities of The Depository Trust Company (“DTC”) and Warrants in physical form may be transferred upon surrender of the Warrant to the Warrant Agent together with the appropriate instruments of transfer. Pursuant to a warrant agency agreement between us and the Warrant Agent, the Warrants initially will be issued in book-entry form and will be represented by one or more global certificates deposited with DTC and registered in the name of [●], a nominee of DTC, or as otherwise directed by DTC.
Exchange Listing. We have applied for listing our Common Stock and the Tradeable Warrants on The Nasdaq Capital Market under the symbols “[ ]” and “[ ],” respectively. No assurance can be given that our listing application will be approved.
Right as a Stockholder. Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of shares of our Common Stock, the holders of the Warrants do not have the rights or privileges of holders of our Common Stock, including any voting rights, until they exercise their Warrants.
Fundamental Transaction. In the event of any fundamental transaction, as described in the Warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our shares of Common Stock, then upon any subsequent exercise of a Warrant, the holder will have the right to receive as alternative consideration, for each share of Common Stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of Common Stock of the successor or acquiring corporation of our Company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of Common Stock for which the Warrant is exercisable immediately prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the Warrants have the right to require us or a successor entity to redeem the Warrants for cash in the amount of the Black Scholes Value (as defined in each Warrant) of the unexercised portion of the Warrants concurrently with or within [30 days] following the consummation of a fundamental transaction. However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our Board, the holders of the Warrants will only be entitled to receive from us or our successor entity, as of the date of consummation of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the Warrant, that is being offered and paid to the holders of our Common Stock in connection with the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of our Common Stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction.
Governing Law; and Exclusive Forum. The Warrants and the Warrant Agent Agreement are governed by New York law. The warrant certificates governing the Tradeable Warrants and Non-tradeable Warrants provide that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by the warrant certificate (whether brought against a party to the warrant certificate or their respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York, Borough of Manhattan. The warrant certificates further provide that we and the Warrant holders irrevocably submit to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan, for the adjudication of any dispute under the warrant certificate or in connection with it or with any transaction contemplated by it or discussed in it. Furthermore, we and the Warrant holders irrevocably waive, and agree not to assert in any suit, action or proceeding, any claim that we or they are not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. With respect to any complaint asserting a cause of action arising under the Securities Act or the rules and regulations promulgated thereunder, we note, however, that there is uncertainty as to whether a court would enforce this provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the exclusive forum provision in the Warrant certificates expressly does not apply to suits brought to enforce any duty or liability created by the Exchange Act. We irrevocably waive any right we may have to, and agree not to request, a jury trial for the adjudication of any dispute under, in connection with, or arising out of the Warrant or any transaction contemplated by the Warrant.
Representative Warrants
The registration statement of which this prospectus forms a part also registers for sale the Representative Warrants, as a portion of the underwriting compensation in connection with this offering. The Representative Warrants will be exercisable for a five-year period commencing on the date of commencement of sales pursuant to the registration statement of which this prospectus forms a part at an exercise price of $[●] per share (115% of the assumed public offering price per Unit). See “Underwriting” for a description of the Representative Warrants.
Listing
We have applied to have our Common Stock listed on the Nasdaq Capital Market under the symbol “[ ].” We also have applied to have our Tradeable Warrants listed on the Nasdaq Capital Market under the symbol “[ ].” We will not proceed with this offering in the event our listing application is not approved for listing on the Nasdaq Capital Market.
Holders
On April 25, 2023, there were approximately 45 record holders of our Common Stock.
Certain Provisions Potentially Having an Anti-Takeover Effect
Several provisions of our Articles and Bylaws, may have anti-takeover effects. These provisions are intended to avoid costly takeover battles, lessen our vulnerability to a hostile change of control and enhance the ability of our board of directors to maximize shareholder value in connection with any unsolicited offer to acquire us.
Control Shares Acquisition Act
The Corporation elects to opt out of the provisions of the Control Share Acquisitions Act, UTAH CODE ANN. § 61-6-1, et seq., as they may apply to the Corporation or any transaction involving the Corporation. The provisions of the Control Share Acquisitions Act, UTAH CODE ANN. § 61-6-1, et seq., shall not be applicable to control share acquisition of the securities of the Corporation. This election is made in accordance with the provisions of Utah Code Ann. Section 61-6-1 et seq.
Limitation of Liability and Indemnification of Directors and Officers
Under the provisions of our Articles of Incorporation and Bylaws, as of the date of this Registration Statement, each person who is or was a director, officer or employee of registrant shall be indemnified by the registrant to the full extent permitted or authorized by Title 16, Chapter 10a, Part 9 of the Utah Revised Business Corporation Act (“URBCA”), provided that no such indemnification shall be made if a judgment or other final adjudication adverse to such person establishes that his or her acts were committed in bad faith or were the result of active and deliberate dishonesty and were material to the cause of action so adjudicated, or that he or she personally gained in fact a financial profit or other advantage to which he or she was not legally entitled, and provided further that no such indemnification shall be required with respect to any settlement or other non-adjudicated disposition of any threatened or pending action or proceeding unless the Company has given its prior consent to such settlement or other disposition.
The limitation of liability and indemnification provisions in our Articles and Bylaws may discourage shareholders from bringing a lawsuit against directors for breach of their fiduciary duties. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our shareholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our Common Stock in the public market, including shares issued upon the exercise of outstanding options or warrants, or upon debt conversion, or the anticipation of these sales, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of equity securities.
Upon completion of this offering, we estimate that we will have [ ] outstanding shares of our Common Stock, calculated as of [ ], 2023, assuming no exercise of outstanding options or warrants, if any.
Sale of Restricted Securities
The shares of our Common Stock sold pursuant to this offering will be registered under the Securities Act and therefore freely transferable, except for our affiliates. Our affiliates will be deemed to own “control” securities that are not registered for resale under the registration statement covering this prospectus. Individuals who may be considered our affiliates after this offering include individuals who control, are controlled by or are under common control with us, as those terms generally are interpreted for federal securities law purposes. These individuals may include some or all of our directors and executive officers. Individuals who are our affiliates are not permitted to resell their shares of our Common Stock unless such shares are separately registered under an effective registration statement under the Securities Act or an exemption from the registration requirements of the Securities Act is available, such as Rule 144.
Rule 144
In general, under Rule 144 as currently in effect, a person (or persons whose shares are aggregated), including an affiliate, who beneficially owns “restricted securities” (i.e., securities that are not registered by an effective registration statement) of a “reporting company” may not sell these securities until the person has beneficially owned them for at least six months. Thereafter, affiliates may not sell within any three-month period a number of shares in excess of the greater of: (i) 1% of the then outstanding shares of Common Stock as shown by the most recent report or statement published by the issuer; and (ii) the average weekly reported trading volume in such securities during the four preceding calendar weeks.
Sales under Rule 144 by our affiliates will also be subject to restrictions relating to manner of sale, notice and the availability of current public information about us and may be affected only through unsolicited brokers’ transactions.
Persons not deemed to be affiliates who have beneficially owned “restricted securities” for at least six months but for less than one year may sell these securities, provided that current public information about the Company is “available,” which means that, on the date of sale, we have been subject to the reporting requirements of the Exchange Act for at least 90 days and are current in our Exchange Act filings. After beneficially owning “restricted securities” for one year, our non-affiliates may engage in unlimited re-sales of such securities.
Shares received by our affiliates in this offering or upon exercise of stock options or upon vesting of other equity-linked awards may be “control securities” rather than “restricted securities.” “Control securities” are subject to the same volume limitations as “restricted securities” but are not subject to holding period requirements.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of the Company’s Common Stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of the Company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits affiliates of the Company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701 and until expiration of the lock-up period described below.
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of the material U.S. federal income tax considerations relating to the purchase, ownership and disposition of our Common Stock purchased in this offering, which we refer to collectively as our securities, but is for general information purposes only and does not purport to be a complete analysis of all the potential tax considerations. This summary is based upon the provisions of the Code, final, temporary, and proposed Treasury regulations promulgated thereunder, administrative rulings and pronouncements and judicial decisions, all as of the date hereof. These authorities may change, possibly retroactively, resulting in U.S. federal income and estate tax consequences different from those set forth below. There can be no assurance that the Internal Revenue Service (the “IRS”) will not challenge one or more of the tax consequences described herein, and we have not obtained, and do not intend to obtain, an opinion of counsel or ruling from the IRS with respect to the U.S. federal income tax considerations relating to the purchase, ownership, or disposition of our securities.
This summary does not address any alternative minimum tax considerations, any considerations regarding the Medicare tax, any considerations regarding the tax on net investment income, or the tax considerations arising under the laws of any state, local or non-U.S. jurisdiction, or under any non-income tax laws, including U.S. federal gift and estate tax laws, except to the limited extent set forth below. In addition, this summary does not address all of the tax consequences that may be relevant to investors, nor does it address tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
| | ● | banks, insurance companies or other financial institutions; |
| | | |
| | ● | tax-exempt entities or governmental organizations, including agencies or instrumentalities thereof; |
| | | |
| | ● | regulated investment companies and real estate investment trusts; |
| | | |
| | ● | controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; |
| | | |
| | ● | brokers or dealers in securities or currencies; |
| | | |
| | ● | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
| | | |
| | ● | persons that own, or are deemed to own, more than five percent of our capital stock (except to the extent specifically set forth below); |
| | | |
| | ● | tax-qualified retirement plans; |
| | | |
| | ● | certain former citizens or long-term residents of the United States; |
| | | |
| | ● | partnerships or entities or arrangements classified as partnerships for U.S. federal income tax purposes and other pass-through entities including S corporations and trusts (and any investors therein); |
| | | |
| | ● | persons who hold our securities as a position in a hedging transaction, “straddle,” “conversion transaction” or other risk reduction transaction or integrated investment; |
| | | |
| | ● | persons who do not hold our securities as a capital asset within the meaning of Section 1221 of the Code; or |
| | | |
| | ● | persons deemed to sell our securities under the constructive sale provisions of the Code, or persons holding the securities as part of a “straddle,” hedge, conversion transaction, integrated transaction, or other similar transaction. |
In addition, if a partnership (or entity or arrangement classified as a partnership for U.S. federal income tax purposes) holds our securities, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our securities, and partners in such partnerships, should consult their tax advisors.
You are urged to consult your own tax advisors with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our securities arising under the U.S. federal estate or gift tax laws or under the laws of any state, local, non-U.S., or other taxing jurisdiction or under any applicable tax treaty.
Consequences to U.S. Holders
The following is a summary of the U.S. federal income tax consequences that will apply to a U.S. holder of our securities. For purposes of this discussion, you are a U.S. holder if, for U.S. federal income tax purposes, you are a beneficial owner of our securities, other than a partnership, that is:
| | ● | an individual citizen or resident of the United States; |
| | ● | a corporation or other entity taxable as a corporation created or organized in the United States or under the laws of the United States, any State thereof or the District of Columbia; |
| | | |
| | ● | an estate trust whose income is subject to U.S. federal income tax regardless of its source; or |
| | | |
| | ● | a trust (x) whose administration is subject to the primary supervision of a U.S. court, and which has one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) who have the authority to control all substantial decisions of the trust or (y) which has made a valid election to be treated as a “United States person.” |
Distributions
As described in the section titled “Dividend Policy,” we have never declared or paid cash dividends on our Common Stock and do not anticipate paying any dividends on our Common Stock in the foreseeable future. However, if we do make distributions in cash or other property on our Common Stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent our distributions exceed both our current and our accumulated earnings and profits, the excess will constitute a return of capital that will first reduce your basis in our Common Stock, but not below zero, and then will be treated as gain from the sale or other disposition of stock as described below under “—Sale, Exchange or Other Taxable Disposition of Common Stock.”
Dividend income may be taxed to an individual U.S. holder at rates applicable to long-term capital gains, provided that a minimum holding period and other limitations and requirements are satisfied with certain exemptions. Any dividends that we pay to a U.S. holder that is a corporation will qualify for the dividends received deduction if the requisite holding period is satisfied, subject to certain limitations. U.S. holders should consult their own tax advisors regarding the holding period and other requirements that must be satisfied in order to qualify for the reduced tax rate on dividends or the dividends-received deduction.
Sale, Exchange or Other Taxable Disposition of Common Stock
A U.S. holder will generally recognize capital gain or loss on the sale, exchange, or other taxable disposition of our Common Stock. The amount of gain or loss will equal the difference between the amount realized on the sale and such U.S. holder’s adjusted tax basis in such Common Stock. The amount realized will include the amount of any cash and the fair market value of any other property received in exchange for such Common Stock. A U.S. holder’s adjusted tax basis in its Common Stock will generally equal the U.S. holder’s acquisition cost or purchase price, less any prior distributions treated as a return of capital. Gain or loss will be long-term capital gain or loss if the U.S. holder has held the Common Stock for more than one year. Long-term capital gains of non-corporate U.S. holders are generally taxed at preferential rates. The deductibility of capital losses is subject to certain limitations.
Information Reporting and Backup Withholding
In general, information reporting requirements may apply to dividends paid to a U.S. holder and to the proceeds of the sale or other disposition of our Common Stock, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against a U.S. holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS.
Unearned Income Medicare Tax
A 3.8% Medicare contribution tax will generally apply to all or some portion of the net investment income of a U.S. holder that is an individual with adjusted gross income that exceeds a threshold amount ($200,000, or $250,000 if married filing jointly).
Consequences to Non-U.S. Holders
The following is a summary of the U.S. federal income tax consequences that will apply to a non-U.S. holder of our securities. A “non-U.S. holder” is a beneficial owner of our securities (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that, for U.S. federal income tax purposes, is not a U.S. holder. The term “non-U.S. holder” includes:
| | ● | a non-resident alien individual (other than certain former citizens and residents of the U.S. subject to U.S. tax as expatriates); |
| | | |
| | ● | a foreign corporation; |
| | | |
| | ● | an estate or trust that is not a U.S. holder; or |
| | | |
| | ● | any other Person that is not a U.S. holder. |
But generally does not include an individual who is present in the U.S. for 183 days or more or who is otherwise treated as a U.S. resident in the taxable year. If you are such an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the acquisition, ownership or sale or other disposition of our securities.
Distributions
Subject to the discussion below regarding effectively connected income, any distribution paid to a non-U.S. holder, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will constitute a dividend for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the non-U.S. holder’s conduct of a trade or business within the U.S., will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, a non-U.S. holder must provide us with an €RS Form W-8BEN, IRS Form W-8BEN-E or other applicable IRS Form W-8 properly certifying qualification for the reduced rate. These forms must be provided prior to the payment of dividends and must be updated periodically. A non-U.S. holder eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty should consult with its individual tax advisor to determine if you may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. If a non-U.S. holder holds our securities through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, which then may be required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dividends received by a non-U.S. holder that are effectively connected with its conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States) are generally exempt from such withholding tax if the non-U.S. holder satisfies certain certification and disclosure requirements. In order to obtain this exemption, the non-U.S. holder must provide us with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated U.S. federal income tax rates applicable to U.S. holders, net of certain deductions and credits. In addition, dividends received by a corporate non-U.S. holder that are effectively connected with its conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. Non-U.S. holders should consult their own tax advisors regarding any applicable tax treaties that may provide for different rules.
Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. holder’s adjusted tax basis in its Common Stock and, to the extent such distribution exceeds the Non-U.S. holder’s adjusted tax basis, as gain realized from the sale or other disposition of the Common Stock, which will be treated as described under “Non-U.S. Holders — Gain on Sale, Exchange or Other Taxable Disposition of Common Stock” below.
Gain on Sale, Exchange, or Other Taxable Disposition of Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, a non-U.S. holder generally will not be required to pay U.S. federal income tax on any gain realized upon the sale, exchange, or other taxable disposition of our Common Stock unless:
| | ● | the gain is effectively connected with the non-U.S. holder’s conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States); |
| | | |
| | ● | the non-U.S. holder is a non-resident alien individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met; or |
| | | |
| | ● | shares of our Common Stock constitute U.S. real property interests by reason of our status as a “United States real property holding corporation” (a USRPHC) for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the non-U.S. holder’s disposition of, or the non-U.S. holder’s holding period for, our Common Stock (provided that an exception does not apply), and, in the case where shares of our Common Stock are regularly traded on an established securities market, the non-U.S. holder has owned, directly or constructively, more than 5% of our Common Stock at any time within the shorter of the five-year period preceding the disposition or such non-U.S. holder’s holding period for the shares of our Common Stock. |
We believe that we are not currently and will not become a USRPHC for U.S. federal income tax purposes, and the remainder of this discussion so assumes. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our Common Stock is regularly traded on an established securities market, such Common Stock will be treated as U.S. real property interests only if the non-U.S. holder actually or constructively hold more than five percent of such regularly traded Common Stock at any time during the shorter of the five-year period preceding the non-U.S. holder’s disposition of, or the non-U.S. holder’s holding period for, our Common Stock.
If the non-U.S. holder is described in the first bullet above, it will be required to pay tax on the net gain derived from the sale, exchange or other taxable disposition under regular graduated U.S. federal income tax rates, and a corporate non-U.S. holder described in the first bullet above also may be subject to the branch profits tax at a rate of 30%, or (in each case) such lower rate as may be specified by an applicable income tax treaty. An individual non-U.S. holder described in the second bullet above will be required to pay a flat 30% tax (or such lower rate specified by an applicable income tax treaty) on the gain derived from the sale, exchange, or other taxable disposition, which gain may be offset by U.S. source capital losses for the year (provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses). Non-U.S. holders should consult their own tax advisors regarding any applicable income tax or other treaties that may apply.
Federal Estate Tax
Common Stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for U.S. federal estate tax purposes) at the time of their death will generally be includable in the decedent’s gross estate for U.S. federal estate tax purposes. Such shares, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax treaty provides otherwise.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
A non-U.S. holder may have to comply with certification procedures to establish that it is not a United States person in order to avoid information reporting and backup withholding requirements. The certification procedures required to claim a reduced rate of withholding under a treaty generally will satisfy the certification requirements necessary to avoid the backup withholding as well for example, by properly certifying your non-U.S. status on an IRS Form W-8BEN or IRS Form W-8BEN-E or other applicable IRS Form W-8.Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that you are a U.S. person.
Backup withholding is not an additional tax; rather, the U.S. federal income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Foreign Account Tax Compliance
The Foreign Account Tax Compliance Act generally imposes withholding tax at a rate of 30% on dividends on and gross proceeds from the sale or other disposition of our securities paid to a “foreign financial institution” (as specially defined under these rules), unless any such institution (1) enters into, and complies with, an agreement with the IRS to report, on an annual basis, information with respect to interests in, and accounts maintained by, the institution that are owned by certain U.S. persons and by certain non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments, or (2) if required under an intergovernmental agreement between the United States and an applicable foreign country, reports such information to its local tax authority, which will exchange such information with the U.S. authorities. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Accordingly, the entity through which our securities are held will affect the determination of whether such withholding is required. Similarly, dividends in respect of our securities held by an investor that is a non-financial non-U.S. entity that does not qualify under certain exceptions will generally be subject to withholding at a rate of 30%, unless such entity either (1) certifies to us or the applicable withholding agent that such entity does not have any “substantial United States owners” or (2) provides certain information regarding the entity’s “substantial United States owners,” which will in turn be provided to the U.S. Department of Treasury. Non-U.S. holders should consult their own tax advisors regarding the possible implications of this legislation on their investment in our securities.
Each prospective investor should consult its own tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, owning and disposing of our securities, including the consequences of any proposed changes in applicable laws.
UNDERWRITING
We are offering our Units described in this prospectus through the underwriters named below. WallachBeth Capital, LLC is acting as the sole representative (the “Representative”) of the underwriters. We have entered into an underwriting agreement with the Representative and the other underwriters. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase, and we have agreed to sell to the underwriters, the number of shares of Units listed next to its name in the following table.
| Underwriters | | | Number of Units | |
| WallachBeth Capital, LLC | | | [●] | |
| Total | | | [●] | |
The underwriting agreement provides that the underwriters must buy all of the Units offered by this prospectus if they buy any of them. However, the underwriters are not required to take or pay for the Units covered by the underwriters’ option to purchase additional Units as described below. Our Units are offered subject to a number of conditions, including:
| | ● | receipt and acceptance of our Units by the underwriters; and |
| | | |
| | ● | the underwriters’ right to reject orders in whole or in part. |
The underwriters’ obligation to purchase the Units is subject to satisfaction of certain conditions, including, among others, the continued accuracy of representations and warranties made by us in the underwriting agreement, delivery of legal opinions and the absence of any material changes in our assets, business, or prospects after the date of this prospectus.
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
After the initial public offering of the Units, the offering price and other selling terms may be changed by the underwriters. Sales of Units made outside the United States may be made by affiliates of certain of the underwriters.
Underwriting Discount
Units sold by the underwriters to the public will initially be offered at the initial offering price set forth on the cover of this prospectus. Any Units sold by the underwriters to securities dealers may be sold at a discount of up to $[●] per share from the public offering price. The underwriters may offer the Units through one or more of their affiliates or selling agents. If all the Units are not sold at the public offering price, the Representative may change the offering price and the other selling terms. Upon execution of the underwriting agreement, the underwriters will be obligated to purchase the Units at the prices and upon the terms stated therein.
The underwriting discount is equal to the public offering price per Unit, less the amount paid by the underwriters to us per Unit. The underwriting discount was determined through an arms’ length negotiation between us and the underwriters. We have agreed to sell the Units to the underwriters at the offering price of $[ ] per Unit, which represents the public offering price of our Units set forth on the cover page of this prospectus less an 9% underwriting discount.
The following table shows the per share and total underwriting discount we will pay to the underwriters.
| | | Per Unit | | | With Underwriting Discount | |
| Public offering price | | $ | [ ] | | | $ | [ ] | |
| Underwriting discounts and commissions (9%) | | $ | [ ] | | | $ | [ ] | |
| Proceeds to us, before fees and expenses, to us | | $ | [ ] | | | $ | [ ] | |
| Non-accountable expense allowance (1.5%) | | $ | [ ] | | | $ | [ ] | |
| Accountable expense allowance | | $ | [ ] | | | $ | [ ] | |
| Net Total Proceeds | | $ | [ ] | | | $ | [ ] | |
We have agreed to reimburse the Representative for its accountable expenses, including the Representative’s actual and out-of-pocket expenses, including but not limited to reasonable and documented travel, legal fees, and other expenses incurred in connection with the offering up to a maximum amount of $145,000. We have also agreed to pay an additional fee of 1.5% of the aggregate sales price of securities sold in any public offering as non-accountable expenses. We have paid $20,000 to the Representative as an advance to be applied towards reasonable out-of-pocket expenses (the “Advance”). Any portion of the advance shall be returned back to us to the extent not actually incurred in compliance with FINRA Rule 5110(g)(4)(A). We estimate that the total expenses payable by us in connection with this offering, other than the underwriting discounts referred to above and the fee for non-accountable expenses, will be approximately $145,000.
We will be also responsible for and will pay all expenses relating to the offering, including, without limitation, (a) all filing fees and expenses relating to the registration of the securities with the Commission; (b) all fees and expenses relating to the listing of the Common Stock and the Warrants underlying the Units on the Nasdaq Capital Market; (c) all fees, expenses and disbursements relating to the registration or qualification of the securities under the “blue sky” securities laws of such states and other jurisdictions as the Representative may reasonably designate (including, without limitation, all filing and registration fees, and the reasonable fees and disbursements of the Company’s “blue sky” counsel) unless such filings are not required in connection with the Company’s proposed listing on the Nasdaq Capital Market, if applicable; (d) all fees, expenses and disbursements relating to the registration, qualification or exemption of the securities under the securities laws of such foreign jurisdictions as the Representative may reasonably designate; (e) the costs of all mailing and printing of the offering documents; (f) transfer and/or stamp taxes, if any, payable upon the transfer of securities from the Company to the underwriters; (g) the fees and expenses of the Company’s accountants; and (h) fees and expenses including “road show,” diligence, and reasonable legal fees and disbursements for the underwriters’ counsel. The Company shall be responsible for the underwriters’ external counsel legal costs irrespective of whether or not the offering is consummated. Additionally, one percent (1.5%) of the gross proceeds of the offering shall be provided to the underwriters for non-accountable expenses.
We estimate the total expenses payable by us for this offering to be approximately $[ ], which amount includes (i) the underwriting discount of $[ ] (9%), (ii) a non-accountable expense of $[ ] (1.5%), and (iii) reimbursement of the accountable expenses of the representative equal to up to $145,000 including the legal fees of the Representative being paid by us.
Representative Warrants
We have agreed to issue warrants to the Representative to purchase up to a total of [ ] shares of Common Stock equal to (6%) of the shares of Common Stock underlying the Units sold in this offering. We are registering hereby the issuance of the Representative Warrants and the shares of Common Stock issuable upon exercise of such warrants. The Representative Warrants will be exercisable as of the date of the commencement of sales of the offering and will expire on the fifth anniversary of the effective date of the registration statement of which this prospectus forms a part and in compliance with FINRA Rule 5110(f)(2)(G). The Representative Warrants will be exercisable at a price equal to 115% of the public offering price in connection with this offering. The Representative Warrants shall not be redeemable. The Representative Warrants may not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days beginning on the date of commencement of sales of the offering, except as provided for in FINRA Rule 5110(e)(2). Notwithstanding the foregoing, the Representative Warrants may be assigned, in whole or in part, to any officer, manager or member of the Representative (or to officers, managers or members of any such successor or member), and to members of the underwriting syndicate or selling group. The Representative Warrants may be exercised as to all or a lesser number of shares of common stock for a period of five (5) years following the commencement of sales of the offering, will provide for cashless exercise and will contain provisions for one demand registration of the sale of the underlying shares of Common Stock, provided, there is no effective registration statement for such shares, at the Company’s expense, and unlimited “piggyback” registration rights at the Company’s expense. The sole demand registration right provided at the issuer’s expense will not be greater than five (5) years from the commencement of sales of the offering in compliance with FINRA Rule 5110(g)(8)(C). The piggyback registration rights provided will not be greater than seven (7) years from the commencement of sales of the offering in compliance with FINRA Rule 5110(g)(8)(D). The Representative Warrants shall further provide for anti-dilution protection (adjustment in the number and price of such warrants and the shares underlying such warrants) resulting from corporate events (which would include dividends, reorganizations, mergers, etc.) when the public shareholders have been proportionally affected and otherwise in compliance with FINRA Rule 5110(g)(8)(E).
Indemnification
We have agreed to indemnify the several underwriters against certain liabilities, including certain liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters may be required to make in respect of those liabilities.
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
Stock Exchange Listing Application
In connection with this offering, we have applied to have our Common Stock and Warrants listed on the NASDAQ Market under the symbols “[●]” and “[●],” respectively. No assurance can be given that our applications will be approved. We will not proceed with this offering in the event our Common Stock is not approved for listing on the NASDAQ Market.
Price Stabilization, Short Positions
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our shares of Common Stock during and after this offering, including:
| | ● | stabilizing transactions; |
| | | |
| | ● | short sales; |
| | | |
| | ● | purchases to cover positions created by short sales; |
| | | |
| | ● | imposition of penalty bids; and |
| | | |
| | ● | syndicate covering transactions. |
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our shares of Common Stock while this offering is in progress. Stabilization transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. These transactions may also include making short sales of our shares of Common Stock, which involve the sale by the underwriters of a greater number of shares of Common Stock than they are required to purchase in this offering and purchasing shares of Common Stock on the open market to cover short positions created by short sales. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked short sales,” which are short positions in excess of that amount.
The underwriters may close out any covered short position by either exercising their option, in whole or in part, or by purchasing shares in the open market. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of Common Stock in the open market that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because WallachBeth has repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
These stabilizing transactions, short sales, purchases to cover positions created by short sales, the imposition of penalty bids and syndicate covering transactions may have the effect of raising or maintaining the market price of our Common Stock or preventing or retarding a decline in the market price of our Common Stock. As a result of these activities, the price of our Common Stock may be higher than the price that otherwise might exist in the open market. The underwriters may carry out these transactions on the Nasdaq Capital Market, in the over-the-counter market or otherwise. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of the shares. Neither we, nor any of the underwriters make any representation that the underwriters will engage in these stabilization transactions or that any transaction, once commenced, will not be discontinued without notice.
Determination of Offering Price
The public offering price of the Units, including the exercise price of the Warrants, will be negotiated between us and the Representative. The principal factors to be considered in determining the public offering price include:
| | ● | the information set forth in this prospectus and otherwise available to [●]; |
| | | |
| | ● | our history and prospects and the history and prospects for the industry in which we compete; |
| | | |
| | ● | our past and present financial performance; |
| | | |
| | ● | our prospects for future earnings and the present state of our development; |
| | | |
| | ● | the general condition of the securities market at the time of this offering; |
| | | |
| | ● | the recent market prices of, and demand for, publicly traded shares of generally comparable companies; and |
| | | |
| | ● | other factors deemed relevant by the underwriters and us. |
The estimated public offering price range set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors. Neither we nor the underwriters can assure investors that an active trading market will develop for our shares of Common Stock or that the shares of Common Stock will trade in the public market at or above the public offering price.
Affiliations
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing, and brokerage activities. The underwriters and their affiliates may from time to time in the future engage with us and perform services for us or in the ordinary course of their business for which they will receive customary fees and expenses. In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of us. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of these securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in these securities and instruments.
Electronic Distribution
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by one or more of the underwriters participating in this offering, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the particular underwriter, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be relied upon by investors.
Selling Restrictions
Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Regulation, or each, a Relevant Member State, an offer to the public of any shares of our Common Stock may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any shares of our Common Stock may be made at any time under the following exemptions under the Prospectus Regulation, if they have been implemented in that Relevant Member State:
| | (i) | to any legal entity which is a qualified investor as defined in the Prospectus Regulation; |
| | | |
| | (ii) | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or |
| | | |
| | (iii) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, provided that no such offer of shares of our Common Stock shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Regulation. |
For the purposes of this provision, the expression an “offer to the public” in relation to any shares of our Common Stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares of our Common Stock to be offered so as to enable an investor to decide to purchase any shares of our Common Stock, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
United Kingdom
Each underwriter has represented and agreed that:
| | (a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (“FSMA”) received by it in connection with the issue or sale of the shares of our Common Stock in circumstances in which Section 21(1) of the FSMA does not apply to us; and |
| | | |
| | (b) | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of our Common Stock in, from or otherwise involving the United Kingdom. |
Hong Kong
Shares of our Common Stock may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to shares of our Common Stock may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares of our Common Stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder.
Japan
No registration pursuant to Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) (the “FIEL”) has been made or will be made with respect to the solicitation of the application for the acquisition of the shares of Common Stock.
Accordingly, the shares of Common Stock have not been, directly or indirectly, offered or sold and will not be, directly or indirectly, offered or sold in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan) or to others for re-offering or re-sale, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan except pursuant to an exemption from the registration requirements, and otherwise in compliance with, the FIEL and the other applicable laws and regulations of Japan.
For Qualified Institutional Investors (“QII”)
Please note that the solicitation for newly-issued or secondary securities (each as described in Paragraph 2, Article 4 of the FIEL) in relation to the shares of Common Stock constitutes either a “QII only private placement” or a “QII only secondary distribution” (each as described in Paragraph 1, Article 23-13 of the FIEL). Disclosure regarding any such solicitation, as is otherwise prescribed in Paragraph 1, Article 4 of the FIEL, has not been made in relation to the shares of Common Stock. The shares of Common Stock may only be transferred to QIIs.
For Non-QII Investors
Please note that the solicitation for newly-issued or secondary securities (each as described in Paragraph 2, Article 4 of the FIEL) in relation to the shares of Common Stock constitutes either a “small number private placement” or a “small number private secondary distribution” (each as is described in Paragraph 4, Article 23-13 of the FIEL). Disclosure regarding any such solicitation, as is otherwise prescribed in Paragraph 1, Article 4 of the FIEL, has not been made in relation to the shares of Common Stock. The shares of Common Stock may only be transferred en bloc without subdivision to a single investor.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of shares of our Common Stock may not be circulated or distributed, nor may the shares of our Common Stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where shares of our Common Stock are subscribed or purchased under Section 275 by a relevant person which is: (i) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (ii) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired shares of our Common Stock under Section 275 except: (a) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (b) where no consideration is given for the transfer; or (c) by operation of law.
LEGAL MATTERS
The validity of the shares of Common Stock offered hereby and certain other legal matters will be passed upon for us by Brunson Chandler & Jones PLLC, Salt Lake City, UT. Certain legal matters in connection with this offering will be passed upon for the underwriters by Sheppard, Mullin, Richter & Hampton LLP, New York, NY.
EXPERTS
The financial statements of KindlyMD as of December 31, 2022 and 2021 appearing in this prospectus and registration statement of which this prospectus forms a part, are undergoing an audit by Sadler Gibb & Associates, LLC., independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report, given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of Common Stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement on Form S-1 and its exhibits. For further information with respect to KindlyMD and the Common Stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. We also maintain a website at www.kindlymd.com.
We are subject to the information reporting requirements of the Exchange Act, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available on the website of the SEC referred to above. The information contained in, or that can be accessed through, our website is not part of this prospectus, and you should not consider the contents of our website in making an investment decision with respect to our Common Stock.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Kindly MD, Inc.:
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Kindly MD, Inc. (“the Company”) as of December 31, 2022 and 2021, the related statements of operations, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with the auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Sadler, Gibb & Associates, LLC
We have served as the Company’s auditor since 2022
Draper, UT
May 1, 2023
Kindly MD, Inc.
(formerly Utah Therapeutic Health Center LLC)
BALANCE SHEETS
| | | December 31, 2022 | | | December 31, 2021 | |
| | | | | | | |
| ASSETS | | | | | | | | |
| CURRENT ASSETS | | | | | | | | |
| Cash and cash equivalents | | $ | 186,918 | | | $ | 94,689 | |
| Accounts receivable, net | | | 12,123 | | | | 13,474 | |
| Inventory, net | | | 49,568 | | | | 59,770 | |
| Prepaid expenses and other current assets | | | 60,958 | | | | 35,299 | |
| Due from related parties | | | - | | | | 66,212 | |
| TOTAL CURRENT ASSETS | | | 309,567 | | | | 269,444 | |
| Property and equipment, net | | | 326,509 | | | | 62,566 | |
| Operating lease right-of-use asset | | | 350,688 | | | | 109,003 | |
| Security deposits | | | 14,505 | | | | 6,630 | |
| | | | 691,702 | | | | 178,199 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 1,001,269 | | | $ | 447,643 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| CURRENT LIABILITIES | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 166,258 | | | $ | 47,496 | |
| Deferred revenue | | | 8,600 | | | | 17,250 | |
| Current portion of operating lease liability | | | 82,670 | | | | 53,544 | |
| Due to related parties | | | 92,545 | | | | - | |
| TOTAL CURRENT LIABILITIES | | | 350,073 | | | | 118,290 | |
| | | | | | | | | |
| Operating lease liability, net of current portion | | | 270,182 | | | | 56,267 | |
| | | | | | | | | |
| TOTAL LIABILITIES | | | 620,255 | | | | 174,557 | |
| | | | | | | | | |
| Commitments and contingencies | | | - | | | | - | |
| | | | | | | | | |
| STOCKHOLDERS’ EQUITY | | | | | | | | |
| Member’s equity | | $ | - | | | $ | 273,086 | |
| Retained Earnings | | | | | | | | |
| Preferred stock, $0.001 par value, 10,000,000 shares authorized, -0- outstanding | | | - | | | | - | |
| Common stock, $0.001 par value, 100,000,000 shares authorized, 4,434,596 and -0- shares issued and outstanding at December 31, 2022 and 2021 respectively. | | | 4,434 | | | | - | |
| Additional paid-in capital | | | 2,917,173 | | | | - | |
| Accumulated deficit | | | (2,540,593 | ) | | | | |
| TOTAL STOCKHOLDERS’ EQUITY | | $ | 381,014 | | | $ | 273,086 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 1,001,269 | | | $ | 447,643 | |
Kindly MD, Inc.
(Formerly Utah Therapeutic Health Center LLC)
STATEMENTS OF OPERATIONS
| | | For the Years Ended
December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| REVENUE | | $ | 3,787,077 | | | $ | 2,504,319 | |
| | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | |
| Costs of goods sold | | | 152,385 | | | | 87,124 | |
| General and administrative | | | 2,151,563 | | | | 853,582 | |
| Personnel | | | 4,176,542 | | | | 1,502,273 | |
| TOTAL OPERATING EXPENSES | | | 6,480,490 | | | | 2,442,979 | |
| | | | | | | | | |
| INCOME (LOSS) FROM OPERATIONS | | | (2,693,413 | ) | | | 61,340 | |
| | | | | | | | | |
| OTHER INCOME (EXPENSE) | | | | | | | | |
| Other income and expense | | | 152,820 | | | | 87,996 | |
| | | | | | | | | |
| TOTAL OTHER INCOME (EXPENSE) | | | 152,820 | | | | 87,996 | |
| | | | | | | | | |
| INCOME (LOSS) BEFORE INCOME TAXES | | $ | (2,540,593 | ) | | $ | 149,336 | |
| | | | | | | | | |
| Provision for income taxes | | | - | | | | - | |
| NET INCOME (LOSS) | | $ | (2,540,593 | ) | | | 149,336 | |
| | | | | | | | | |
| NET INCOME (LOSS) PER COMON SHARE-BASIC AND DILUTED | | $ | (1.56 | ) | | $ | - | |
| | | | | | | | | |
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING – BASIC AND DILUTED | | | 1,623,386 | | | | - | |
Kindly MD, Inc.
(formerly Utah Therapeutic Health Center LLC)
STATEMENTS OF STOCKHOLDERS’ EQUITY
| | | Members’ | | | Common Stock | | | Additional Paid-in | | | Accumulated | | | | |
| | | Equity | | | Shares | | | Amount | | | Capital | | | Deficit | | | Total | |
| Balance - December 31, 2020 | | $ | 123,550 | | | | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 123,550 | |
| Member Contributions | | | 2,000 | | | | - | | | | - | | | | - | | | | - | | | | 2,000 | |
| Net income for the year ended December 31, 2021 | | | 149,336 | | | | - | | | | - | | | | - | | | | - | | | | 149,336 | |
| Balance - December 31, 2021 | | | 273,086 | | | | - | | | | - | | | | - | | | | - | | | | 273,086 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income prior to effect of reorganization | | | 28,629 | | | | - | | | | - | | | | - | | | | - | | | | 28,629 | |
| Effect of reorganization on members’ capital | | | (301,715 | ) | | | 3,868,287 | | | | 3,868 | | | | 297,847 | | | | - | | | | - | |
| Equity-based compensation subsequent to reorganization | | | - | | | | 352,553 | | | | 352 | | | | 1,628,443 | | | | - | | | | 1,628,795 | |
| Common stock issued for cash | | | - | | | | 86,581 | | | | 86 | | | | 399,918 | | | | - | | | | 400,004 | |
| Common stock issued for compensation | | | - | | | | 88,119 | | | | 88 | | | | 407,022 | | | | - | | | | 407,110 | |
| Common stock issued for services | | | - | | | | 39,056 | | | | 40 | | | | 180,399 | | | | - | | | | 180,439 | |
| Net loss for the year ended December 31, 2022 | | | - | | | | - | | | | - | | | | - | | | | (2,540,593 | ) | | | (2,540,593 | ) |
| Balance – December 31, 2022 | | $ | - | | | | 4,434,596 | | | $ | 4,434 | | | $ | 2,917,173 | | | $ | (2,540,593 | ) | | $ | 381,014 | |
Kindly MD, Inc.
(formerly Utah Therapeutic Health Center LLC)
STATEMENTS OF CASH FLOWS
| | | For the Years Ended December 31, | |
| | | 2022 | | | 2021 | |
| Cash flows from operating activities: | | | | | | | | |
| Net income (loss) | | $ | (2,540,593 | ) | | $ | 149,336 | |
| Adjustments to reconcile net income (loss) to net cash from operating activities: | | | | | | | | |
| Net income prior to effect of reorganization | | | 28,629 | | | | - | |
| Depreciation expense | | | 53,445 | | | | 4,855 | |
| Amortization of operating lease right-of-use assets | | | 52,737 | | | | 58,543 | |
| Equity-based compensation subsequent to reorganization | | | 1,628,795 | | | | - | |
| Common stock issued for services | | | 587,549 | | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable, net | | | (7,123 | ) | | | (401 | ) |
| Inventory | | | 10,202 | | | | (30,643 | ) |
| Prepaid expenses | | | (17,185 | ) | | | (38,847 | ) |
| Other assets | | | (7,875 | ) | | | (3,571 | ) |
| Interest accrued related party payable | | | 8,757 | | | | - | |
| Accounts payable and accrued liabilities | | | 122,311 | | | | 23,189 | |
| Customer deposits | | | (8,650 | ) | | | 17,250 | |
| Operating lease liabilities | | | (51,382 | ) | | | (59,072 | ) |
| Net cash from operating activities | | | (140,383 | ) | | | 121,441 | |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchases of property and equipment | | | (317,388 | ) | | | (63,602 | ) |
| Net cash from investing activities | | | (317,388 | ) | | | (63,602 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Net proceeds from related party notes payable | | | 150,000 | | | | (47,341 | ) |
| Capital contribution from member | | | - | | | | 2,000 | |
| Proceeds from common stock issued for cash | | | 400,000 | | | | - | |
| Net cash from financing activities | | | 550,000 | | | | (45,341 | ) |
| | | | | | | | | |
| Net change in cash | | | 92,229 | | | | 12,498 | |
| Cash at beginning of year | | | 94,689 | | | | 82,191 | |
| Cash at end of year | | $ | 186,918 | | | $ | 94,689 | |
| | | | | | | | | |
| Non Cash Investing and Financing Activities | | | | | | | | |
| Operating lease right-of-use asset and liability | | $ | 294,422 | | | $ | 101,490 | |
| Transfer of related party receivable to payable | | $ | 83,745 | | | $ | - | |
| Effect of reorganization on members’ capital | | $ | 301,715 | | | $ | - | |
Kindly MD, Inc.
(Formerly Utah Therapeutic Health Center LLC)
NOTES TO FINANCIAL STATEMENTS
December 31, 2022 and 2021
NOTE 1 – ORGANIZATION AND NATURE OF BUSINESS
Kindly MD, Inc. (“KindlyMD” or “the Company”) was formed on December 2, 2019. The Company filed their original articles of organization in the State of Utah under the name Utah Therapeutic Health Center, PLLC. The Company is located in Salt Lake City, Utah and is a healthcare and healthcare data company, focused on holistic pain management and ending the opioid epidemic.
In April 2020, the Company converted from a PLLC to an LLC. In March 2022, the Company converted from an LLC to a corporation and changed its name to “Kindly MD, Inc.” In July 2022, the Company amended its articles of incorporation, including the addition of stock and authorized common shares.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The financial statements of the Company are prepared in accordance with generally accepted accounting principles in the Unites States of America (“GAAP”) and are presented in US dollars. In the opinion of management, all adjustments considered necessary for a fair presentation have been included.
Cash and Cash Equivalents
The Company considers all highly liquid investments with the original maturities of three months or less to be cash equivalents. At December 31, 2022 and 2021, the Company had no cash equivalents that exceeded the $250,000 federally insured limits.
Use of Estimates
The preparation of the financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Revenue Recognition
The Company records revenue in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 606. This Accounting Standards Update (“ASU”) is based on the principle that revenue is recognized to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASU also requires additional disclosure about the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer purchase orders, including significant judgments.
The Company recognizes revenue from: (i) medical evaluation and treatment, (ii) education partnerships, (vi) online and email campaign marketing revenue, (iii) service affiliate agreements, and (iv) retail sales.
We recognize revenue when control of the promised goods or services is transferred to our customers, or when services are rendered to the patient, in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. We account for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance, and collectability of consideration is probable.
| | 1. | For medical evaluation and treatment, revenue is recognized upon completion of the service and payment of the patient’s obligation under the contract or agreement to receive treatment. |
| | | |
| | 2. | For education partnerships, online and email campaign marketing revenue, and service affiliate agreements, revenue is recognized when the contract has approval and collectability is probable. |
| | | |
| | 3. | For retail sales, revenue is recognized upon receipt of payment for distinct goods. |
The Company recognizes revenue after the transaction and/or service price has been allocated and when it satisfies the performance obligation. Usually, there is only a single performance obligation in the sale and service, and therefore the entire transaction price is allocated to the single performance obligation. This typically occurs at a point in time when products and or services are rendered, delivered, or shipped.
Performance Obligations
A performance obligation is a promise to transfer a distinct good or service to the customer or patient and is the unit of account in the revenue standard. The contract transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. For the Company’s different revenue service types, the performance obligation is satisfied at different times.
The Company’s performance obligations include providing products and professional services. The Company recognizes product revenue performance obligations in most cases when the product has shipped to the customer. When we perform professional service work, we recognize revenue when we have the right to invoice the customer for the work completed, which typically occurs on an interaction basis for the work performed during any particular billable interaction.
All revenue recognized on the Statement or Operations are considered to be revenue from contracts with customers.
Deferred Revenue
Deferred revenue is typically deposits for services scheduled to be delivered at a future date. Amounts are recognized only when services are delivered as per the Revenue Recognition policy above.
Accounts Receivable
Accounts receivable consist of trade receivables in the normal course of business. The Company historically collects substantially all its trade receivables from customers and bad debt expense at the point of service and bad debt expense has been historically immaterial to the financial statements. Uncollectible balances are expensed in the period they are determined to be uncollectible.
The Company establishes an allowance for doubtful accounts to ensure trade receivable are not overstated due to non-collectability. The Company’s allowance is based on a variety of factors, including age of the receivable, significant one-time events, historical experience, and other risk considerations. The Company had an allowance at December 31, 2022 and 2021 of $45,689 and $0, respectively. The Company had bad debt expense of $59,639 and $1,371 for the years ended December 31, 2022, and 2021, respectively.
Inventory
Inventory is set at the lower of cost or net realizable value. The cost of inventory is determined using the first-in, first-out (“FIFO”) method. An allowance is made for the estimated losses due to obsolescence. In determining excess or obsolescence reserves for its products, the Company considers assumptions such as changes in business and economic conditions, other-than-temporary decreases in demand for its products, and changes in technology or customer requirements. In determining the lower of cost or net realizable value reserves, the Company considers assumptions such as recent historical sales activity and selling prices, as well as estimates of future selling prices. The Company fully reserves for inventories deemed obsolete.
Property and Equipment
Property and equipment are stated at the historical cost and depreciated on a straight-line method over the estimated useful life of the asset. Leasehold improvements are amortized over the estimated life of the leasehold improvements. Expected useful lives of property and equipment vary but generally are 3 years for computer hardware and software, and 5-7 years for medical equipment.
Long-lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When projections indicate that the carrying value of the long-lived asset is not recoverable, the carrying value is reduced by the estimated excess of the carrying value over the fair value. An impairment charge is recognized if the carrying amount is not recoverable and the carrying amount exceeds the fair value of the long-lived assets as determined by projected discounted net future cashflows.
Operating Leases
The Company accounts for leases in accordance with ASC 842. The Company determines whether a contract is a lease at contract inception or for a modified contract at the modification date. At inception or modification, the Company recognizes right-of-use (“ROU”) assets and related lease liabilities on the balance sheet for all leases greater than one year in duration. Lease liabilities and their corresponding ROU assets are initially measured at the present value of the unpaid lease payments as of the lease commencement date. If the lease contains a renewal and/or termination option, the exercise of the option is included in the term of the lease if the Company is reasonably certain that a renewal or termination option will be exercised. As the Company’s leases do not provide an implicit rate, the Company uses an estimated incremental borrowing rate (“IBR”) based on the information available at the commencement date of the respective lease to determine the present value of future payments. The IBR is determined by estimating what it would cost the Company to borrow a collateralized amount equal to the total lease payments over the lease term based on the contractual terms of the lease and the location of the leased asset.
Operating lease payments are recognized as an expense on a straight-line basis over the lease term in equal amounts of rent expense attributed to each period during the term of the lease, regardless of when actual payments are made. This generally results in rent expense in excess of cash payments during the early years of a lease and rent expense less than cash payments in later years. The difference between rent expense recognized and actual rental payments is typically represented as the spread between the ROU asset and lease liability.
When calculating the present value of minimum lease payments, we account for leases as one single lease component if a lease has both lease and non-lease fixed cost components. Variable lease and non-lease cost components are expensed as incurred. We do not recognize ROU assets and lease liabilities for short-term leases that have an initial lease term of 12 months or less. We recognize the lease payments associated with short-term leases as an expense on a straight-line basis over the lease term.
Income Taxes
As of December 31, 2021, the Company was a limited liability entity, and any income or losses were passed directly to the members who assume their own tax liabilities. The company converted from an LLC to a Corporation in March 2022. The Company is subject to income taxes in Utah. We recognize deferred tax assets to the extent that it is more likely than not that the asset will be realized and have evaluated the realizability of the deferred tax assets and determined that they are fully realizable. It is important to note that the tax laws and regulations governing the calculation of income taxes are complex and subject to interpretation. As a result, the ultimate tax liability may differ from the amounts recorded in the financial statements. The Company regularly assesses its tax positions and adjusts the amounts recorded as necessary.
The Company incurred a net loss before income taxes in the current year, resulting in no provision for income taxes being recorded. As of December 31, 2022, the Company concluded that a full valuation allowance was necessary for all of its net deferred tax assets. The Company had no amounts recorded for uncertain tax positions, interest or penalties in the accompanying consolidated financial statements. The Company regularly assesses its tax positions and adjusts amounts recorded as necessary to ensure compliance with applicable tax laws and regulations.
Concentration of Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash. The Company’s cash and cash equivalents are concentrated primarily in banks or credit unions. At times, such deposits could be in excess of insured limits. Management believes that the financial institutions that hold the Company’s financial instruments are financially sound and, accordingly, minimal credit risk is believed to exist with respect to these financial instruments.
Earnings Per Share
The Company calculates earnings per share (EPS) in accordance with the FASB ASC Topic 260. The computation of Basic EPS is based on the weighted average number of shares of common stock outstanding during each period. Basic EPS is calculated by dividing net income available to common shareholders by the weighted average number of common shares outstanding during the period. Diluted EPS accounts for the potential dilution from securities that could be converted into common stock. It is calculated in the same manner as Basic EPS, but includes the impact of potentially dilutive securities such as stock options, convertible shares, and debt. In the event of a net loss, diluted EPS is equivalent to basic EPS as dilutive securities are anti-dilutive. Please note that EPS may not fully represent our cash generation capacity. It should be considered alongside other financial measures provided in our financial statements.
Stock-Based Compensation
The Company accounts for stock-based instruments issued to employees in accordance with ASC Topic 718. ASC Topic 718 requires companies to recognize in the statement of operations the grant-date fair value of stock options and other equity-based compensation issued to employees. The value of the portion of an award that is ultimately expected to vest is recognized as an expense over the requisite service periods using the straight-line attribution method.
Fair Value of Financial Instruments
The fair value of a financial instrument is the amount that could be received upon the sale of an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Financial assets are marked to bid prices and financial liabilities are marked to offer prices. Fair value measurements do not include transaction costs. A fair value hierarchy is used to prioritize the quality and reliability of the information used to determine fair values. Categorization within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. Cash, accounts receivable, inventory, prepaid expenses, accounts payable, and accrued expenses approximate fair value due to the short-term nature. The fair value hierarchy is defined in the following three categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market-based inputs or inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not corroborated by market data.
The carrying value of the Company’s financial instruments including cash and cash equivalents, accounts receivable, prepaid expenses, and accrued expenses approximate their fair value due to the short maturities of these financial instruments. As of December 31, 2022 and 2021, there were no financial assets or liabilities that required disclosure.
Impact of COVID-19
In December 2019, a novel strain of coronavirus (“COVID-19”) emerged in China. On March 11, 2020, the World Health Organization declared the outbreak of COVID-19 a pandemic. The extent of the COVID-19 pandemic’s continued effect on our operational and financial performance and those of third parties on which the Company relies will depend on future developments, including the duration, spread and intensity of the outbreak, the pace at which jurisdictions across the country re-open and restrictions begin to lift. The ultimate impact of the COVID-19 pandemic is highly uncertain and subject to change. The Company does not yet know the full extent of potential impacts on its business and financing. However, these effects could have a material impact on the Company’s liquidity, capital resources, operations and business and those of the third parties on which the Company relies.
Recent Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. This ASU removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. This ASU is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The adoption of this standard did not have a material impact on our combined financial statements.
The Company has reviewed all other FASB-issued ASU accounting pronouncements and interpretations thereof that have effective dates during the period reported and in future periods. The Company has carefully considered the new pronouncements that alter previous GAAP and does not believe that any new or modified principles will have a material impact on the company’s reported financial position or operations in the near term. The applicability of any standard is subject to the formal review of the Company’s financial management and certain standards are under consideration.
NOTE 3 – REVENUE
The Company recognized revenue from the sale of outpatient medical services and retail sales. Revenue recognized amounted to $3,787,077 and $2,504,319 for the years ended December 31, 2022 and 2021, respectively.
The following table presents the Company’s revenues disaggregated by type of customer of such revenue recognized during the years ended December 31, 2022 and 2021.
| | | For the Year Ended
December 31, 2022 | | | For the Year Ended
December 31, 2021 | |
| | | | | | | |
| Net Service Revenue | | $ | 3,594,355 | | | $ | 2,412,847 | |
| Product Revenue | | | 192,722 | | | | 91,472 | |
| Net Sales | | $ | 3,787,077 | | | $ | 2,504,319 | |
NOTE 4 – INVENTORY
| | | For the Year Ended
December 31, 2022 | | | For the Year Ended
December 31, 2021 | |
| Finished goods inventory | | $ | 53,268 | | | $ | 62,770 | |
| Allowance for Inventory Obsolescence | | | (3,700 | ) | | | (3,000 | ) |
| Inventory, net | | $ | 49,568 | | | $ | 59,770 | |
NOTE 5 – PROPERTY AND EQUIPMENT
Property and equipment consist of the following at December 31, 2022 and 2021
| | | December 31, 2022 | | | December 31, 2021 | |
| | | | | | | |
| Leasehold Improvements | | $ | 132,027 | | | $ | 33,404 | |
| Furniture | | | 87,234 | | | | 15,118 | |
| Equipment - Computer | | | 84,756 | | | | 16,057 | |
| Equipment - Other | | | 8,841 | | | | 2,916 | |
| Software | | | 72,025 | | | | - | |
| Less: Accumulated Depreciation | | | (58,374 | ) | | | (4,929 | ) |
| Property and equipment, net | | $ | 326,509 | | | $ | 62,566 | |
Depreciation expense for the years ended December 31, 2022 and 2021 was $58,374 and $4,929, respectively.
NOTE 6 –LEASES
We have leases for office space. The real property leases typically are for two-to-five year terms with many containing options for similar renewal periods.
KindlyMD recently executed a four-year lease for 5,321 square feet of clinic and office space in Murray Utah. This expansion complements our existing clinic location in Millcreek Utah and offers eight more exam and consultation rooms to our local clinical capacity. It also allowed KindlyMD to consolidate a smaller location into a larger location, which will improve the cost-per-patient-visit ratio. We anticipate this expansion to increase revenue opportunity by up to $270,000 per month in the summer of 2023. This additional space coincides with the expansion of services offering to occur in the fall of 2022 to include medication management and imbedded behavioral health services for patients.
The Company also operates clinics located at the following locations:
In Millcreek, Utah at 740 E 3900 S, Suite 108 Salt Lake City, Utah 84107. The lease is for a 24 month term through March 31, 2022, with an option for a one year extension through April 30, 2023. The monthly rate is $2,334 per month.
In Ogden, Utah at 2485 Grant Ave, Suite 105 Ogden, Utah 84401. The lease expired on August 31, 2022 and is currently on a month-to-month basis. The monthly rate is $978.33 per month.
In Bountiful, Utah at 580 W 100 N, Suite 4 Bountiful, Utah 84010. The lease is for a 48-month term through April 30, 2025. The monthly rate is $1,152 per month.
In Provo, Utah at 222 Draper Lane, Suite 2 Provo, Utah 84601. The lease is for a 15-year term through February 28, 2035. The monthly rate is $510 per month.
In Murray, Utah at 5097 S 900 E, Suite 100, Murray, Utah 84117. The lease is for a 48 month term through January 31, 2027. The monthly rate is $6,278.96 per month.
In Cedar City, Utah at 301 S Main St. Ste B, Cedar City, Utah 84720. The lease is for a 24-month term through August 8, 2023. The monthly rate is $1,200 per month.
The following table shows the components of lease expense:
| Lease Cost | | 2022 | | | 2021 | |
| Total lease expense | | $ | 102,150 | | | $ | 70,969 | |
Other information related to operating leases as of December 31 was as follows:
| Weighted average remaining lease term, in years | | | 3.62 | | | | 2.52 | |
| Weighted average discount rate | | | 6.11 | % | | | 6.11 | % |
Future minimum lease payments under operating leases as of December 31, 2021 were as follows:
| Years ending December 31 | | 2022 | | | 2021 | |
| 2022 | | | - | | | $ | 58,635 | |
| 2023 | | $ | 121,617 | | | | 28,852 | |
| 2024 | | | 104,580 | | | | 20,592 | |
| 2025 | | | 96,655 | | | | 10,988 | |
| 2026 | | | 87,381 | | | | | |
| 2027 | | | 7,294 | | | | | |
| Total minimum lease payments | | | 417,526 | | | | 119,067 | |
| Less: Imputed interest | | | (66,838 | ) | | | (9,255 | ) |
| Total present value of minimum lease payments | | $ | 350,688 | | | $ | 109,812 | |
| Current portion operating lease liabilities | | $ | 82,670 | | | $ | 53,544 | |
| Non-Current operating lease liabilities | | | 270,182 | | | $ | 56,268 | |
| Total operating lease liabilities | | $ | 350,688 | | | $ | 109,812 | |
NOTE 7 – INCOME TAXES
As of December 31, 2022 the Company had net operating loss carryforwards of $966,438, giving rise to a deferred tax asset of $250,781 that has been fully allowed for.
Realization of the deferred tax asset is dependent on generating sufficient taxable income to offset the tax items that will be deductible in the future. Although realization is not assured, Management believes it is more likely than not that all of the deferred tax assets will be realized. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income are reduced. The Company has recorded a valuation allowance on December 31, 2022 to allow against all deferred tax assets.
| | | For the Year Ended December 31, 2022 | |
| Deferred Tax Liabilities | | | | |
| Taxable temporary differences, net: | | $ | 326,510 | |
| Expected tax rate | | | 26 | % |
| Deferred tax liabilities | | $ | 84,403 | |
| | | | | |
| Deferred Tax Assets | | | | |
| Deductible temporary differences, net: | | | 3,700 | |
| Loss carryforwards | | | 966,438 | |
| | | | 970,138 | |
| Expected tax rate | | | 26 | % |
| | | | 250,781 | |
| Tax credit carryforwards | | | - | |
| Preliminary deferred tax assets | | | - | |
| Deferred tax asset valuation allowance | | | (250,781 | ) |
| Net deferred tax assets | | $ | - | |
| | | | | |
| Deferred Tax Expense (Benefit) | | | | |
| Beginning net deferred tax asset (liability), net: | | | (166,378 | ) |
| Ending net deferred tax asset (liability), net: | | | 166,378 | |
| Deferred tax benefit | | $ | - | |
The table below outlines the components of income tax expense (benefit):
| | | For the Year Ended December 31, 2022 | |
| Taxable Income | | | | |
| Financial Statement pretax income (loss) | | $ | (2,670,410 | ) |
| Nondeductible expenses | | | 1,953,920 | |
| Tax-exempt income | | | - | |
| | | | (716,490 | ) |
| (Increase) decrease in taxable temporary differences, net: | | | (250,648 | ) |
| (Increase) decrease in tax basis adjustments for taxable temporary differences, net: | | | | |
| Increase (decrease) in deductible temporary differences, net: | | | 700 | |
| Increase (decrease) in tax basis adjustments for deductible temporary differences, net: | | | | |
| Loss (operating, capital, passive, and contribution) carryforwards used this period | | | - | |
| Capital losses, passive losses, and charitable contributions not deductible in the current period | | | - | |
| Deduction for state and local taxes | | | - | |
| Federal taxable income (loss) | | $ | (966,438 | ) |
| Taxes on taxable income (loss) | | | - | |
| Taxes on current period income | | | - | |
| Tax benefit from carryback of current period loss and tax credits to prior periods | | | - | |
| Tax credit carryforwards used this period | | | - | |
| | | | - | |
| Increase (decrease) in unrecognized tax benefit liabilities, net: | | | | |
| Cash payments for settlements of uncertain tax positions | | | - | |
| Adjustments of income taxes receivable (payable) | | | - | |
| Current tax expense | | $ | - | |
The tax provision differs from amounts that would be calculated by applying federal statutory rates to income before income taxes primarily because no tax benefits have been recorded for nondeductible expenses totaling $1,953,920. Deferred tax liabilities recognized for taxable temporary differences total $84,403. Deferred tax assets recognized for deductible temporary differences and loss carryforwards total $250,781. The Company has the following carryforwards available at December 31, 2022: Operating Losses in the amount of $966,438 that expire indefinitely.
The Company has not recognized a liability for uncertain tax positions as of December 31, 2022, and there are no significant tax audits or disputes in progress. The Company has not made any payments for tax liabilities during the year ended December 31, 2022. It is important to note that tax laws and regulations are complex and subject to interpretation. As a result, the ultimate tax liability may differ from the amounts recorded in the financial statements. The Company regularly assesses its tax positions and adjusts the amounts recorded as necessary to ensure compliance with applicable tax laws and regulations.
NOTE 8 – STOCKHOLDER’S EQUITY
Upon conversion from an LLC to a corporate structure in March 2022, the company originally authorized 42,000,000 shares. This was amended July 5, 2022, to authorize a total of 110,000,000 shares, consisting of 10,000,000 shares of preferred stock having a par value of $0.001 per share and 100,000,000 shares of common stock having a par value $0.001 per share.
Common Stock - As of December 31, 2022, 4,434,596 shares were issued and outstanding. The common stockholders are entitled to one vote per share and are entitled to receive dividends when and if declared by the board of directors.
Preferred Stock - As of December 31, 2022, no shares of preferred stock were issued or outstanding.
The Company has granted stock to employees and directors. The fair value of each award is estimated on the date of grant using a Black-Scholes option-pricing model, and the expense is recognized over the vesting period of the award.
On August 17, 2022 the company issued 3,868,287 shares of common stock to Wade Rivers, LLC as an effect of reorganization and 352,553 shares of common stock to founders at a at a price of $4.62 per share, which resulted in a recognized expense of $1,628,395, for a total of 4,220,840 shares.
In September 2022, the company issued 54,113 and 32,468 shares of common stock at a price of $4.62 per share pursuant to the Purchase Agreement for total cash proceeds to the company of $400,000.
On September 30, 2022 the Company issued 88,119 restricted shares of common stock at a fair value of $4.62 per share that immediately vested for compensation of $407,110 to employees.
On December 31, 2022, the Company issued 39,056 restricted shares of common stock at a fair value of $4.62 per share that immediately vested for services valued at $108,439 through December 31, 2022.
NOTE 9 – RELATED PARTY TRANSACTIONS
The Company has transactions with related parties, including officers, directors, and their affiliates. The transactions are conducted in the ordinary course of business and are generally on terms no less favorable than those available to unrelated third parties.
As of December 31, 2021, the company had a note receivable from Wade Rivers, LLC, a company owned 50% by the Chief Executive Officer of KindlyMD, in the amount of $66,212. In June 2022 the Company received $151,100 from Wade Rivers, LLC. This paid the balance in full and the remaining proceeds were established as a note payable to Wade Rivers, LLC per a written agreement between parties. As of December 31, 2022, the Company had an outstanding unsecured loan payable to Wade Rivers, LLC, in the amount of $92,545.
The Company reimburses officers and directors for reasonable and necessary expenses incurred in the course of performing their duties for the Company. The Company also provides certain officers and directors with health insurance, retirement benefits, and other fringe benefits.
[ ] Units
Each Unit Consisting of One Share of Common Stock,
One Warrant to Purchase One Share of Common Stock and
One Non-tradeable Warrant to Purchase One-half of One Share of Common Stock
(and the shares of Common Stock underlying such Warrants)
KINDLY MD, INC.

PROSPECTUS
Sole Book-Running Manager

__________________, 2023
Through and including __________________, 2023 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses payable in connection with the sale and distribution of the securities being registered. All amounts are estimated except the Securities Exchange Commission (“SEC”) registration fee and the Financial Industry Regulatory Authority, Inc. (“FINRA”) filing fee. Except as otherwise noted, all the expenses below will be paid by us.
| Offering Expenses | | | | |
| SEC registration fee | | $ | [●] | |
| FINRA filing fee | | $ | [●] | |
| Legal fees and expenses | | $ | [●] | |
| Total | | $ | [●] | |
Item 14. Indemnification of Directors and Officers
We are a Utah corporation and are governed by the Utah Revised Business Corporation Act (the “URBCA”).
The Utah Revised Business Corporation Act (the “URBCA”) provides, in pertinent part, as follows:
Except as otherwise provided in the URBCA, a corporation may indemnify an individual made a party to a proceeding because the individual is or was a director of the corporation against liability incurred in the proceeding if:
| ● | His conduct was in good faith. |
| ● | He reasonably believed that his conduct was in, or not opposed to, the corporation’s best interests. |
| ● | In the case of any criminal proceeding, he had no reasonable cause to believe his conduct was unlawful. |
However, a corporation may not indemnify a director in connection with either:
| ● | A proceeding by or in the right of the corporation in which the director was determined to be liable to the corporation. |
| ● | Any other proceeding charging that the director derived an improper personal benefit (whether or not the proceeding involved action in the director’s official capacity), in which proceeding the director was determined to be liable on the basis that the director derived an improper personal benefit. |
A corporation may pay for or reimburse reasonable expenses incurred by a director who is a party to a proceeding in advance of a final disposition if:
| ● | The director furnishes the corporation a written affirmation of his good faith belief that he has met the applicable standard of conduct described in Section 16-10a-902 of the Utah Code. |
| ● | The director furnishes to the corporation a written undertaking, executed personally or on his behalf, to repay the advance if it is ultimately determined that he did not meet the standard of conduct. |
| ● | A determination is made that the facts then known to those making the determination would not preclude indemnification. |
A corporation must indemnify a director who was successful in the defense of any proceeding or claim to which the director was a party because of the director’s status as a director of the corporation against reasonable expenses incurred in defending the proceeding or claim for which the director was successful.
Unless a corporation’s articles of incorporation provide otherwise:
| ● | An officer of a corporation is entitled to mandatory indemnification to the same extent as a director of the corporation. |
| ● | A corporation may indemnify and advance expenses to an officer, employee, fiduciary, or agent of the corporation to the same extent as to a director. |
| ● | A corporation may indemnify and advance expenses to an officer, employee, fiduciary, or agent who is not a director to a greater extent than to a director. However, this must be consistent with public policy and provided for in the corporation’s articles of incorporation, bylaws, action of its board of directors, or contract. |
Provided the director complies with the standard of care described in the Bylaws and Section 16-10a-840 of the URBCA, the Corporation shall indemnify any director made a party to a proceeding, brought or threatened, as a consequence of the director acting in their official capacity. In the event a director is entitled to indemnification by the Corporation, the director shall be indemnified pursuant to the process outlined in Title 16, Chapter 10a, Part 9 of the URBCA.
Each director is required, individually and collectively, to act in good faith, with reasonable and prudent care, and in the best interest of the Corporation. If a director acts in accordance with Section 16-10a-840 of the URBCA, then they shall be immune from liability arising from official acts on behalf of the Corporation.
Directors who fail to comply with Section 16-10a-840 of the URBCA shall be personally liable to the Corporation, pursuant to Section 16-10a-842 of the Act, for any improper distributions and as otherwise described in Section 16-10a-841 of the URBCA and KindlyMD’s Bylaws.
Item 15: Recent Sales of Unregistered Securities
We claimed exemption from registration under the Securities Act for the sales and issuances of securities in the following transactions under Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder, in that such sales and issuances did not involve a public offering, or under Rule 701 promulgated under the Securities Act, in that they were offered and sold either pursuant to written compensatory plans or pursuant to a written contract relating to compensation, as provided by Rule 701. All of the purchasers of unregistered securities for which we relied on Section 4(a)(2) and/or Regulation D represented that they were accredited investors as defined under the Securities Act. We claimed such exemption on the basis that (a) the purchasers in each case represented that they intended to acquire the securities for investment only and not with a view to the distribution thereof and that they either received adequate information about the registrant or had access, through employment or other relationships, to such information and (b) appropriate legends were affixed to the stock certificates issued in such transactions.
On September 13, 2022, the Company issued 27,057 shares of its common stock to Michael Krupski in a private placement transaction. These shares were issued pursuant to an exemption from the registration requirements of the Securities Act of 1933, as amended pursuant to Section 4(a)(2) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder since, among other things, the transactions did not involve a public offering.
On September 13, 2022, the Company issued 27,056 shares of its common stock to Laurel Krupski in a private placement transaction. These shares were issued pursuant to an exemption from the registration requirements of the Securities Act of 1933, as amended pursuant to Section 4(a)(2) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder since, among other things, the transactions did not involve a public offering.
On September 19, 2022, the Company issued 36,468 shares of its common stock to Shaun Fuhriman in a private placement transaction. These shares were issued pursuant to an exemption from the registration requirements of the Securities Act of 1933, as amended pursuant to Section 4(a)(2) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder since, among other things, the transactions did not involve a public offering.
Item 16. Exhibits and Financial Statement Schedules
| Exhibit | | |
| Number | | Exhibit Description |
| 1.1* | | Form of Underwriting Agreement |
| 3.1 | | Certificate of Organization of Utah Therapeutic Health Center, PLLC |
| 3.2 | | Certificate of Conversion to Utah Therapeutic Health Center, LLC |
| 3.3 | | Certificate of Conversion to Kindly MD, Inc. |
| 3.4 | | Amended and Restated Articles of Incorporation of Kindly MD, Inc. |
| 3.5 | | Bylaws |
| 4.1* | | Specimen Stock Certificate evidencing the shares of Common Stock |
| 4.2* | | Form of (Tradeable) Common Stock Purchase Warrant |
| 4.3* | | Form of (Non-tradeable) Common Stock Purchase Warrant |
| 4.4* | | Form of Warrant Agent Agreement |
| 4.5* | | Form of Representative Warrant |
| 5.1* | | Legal Opinion of Brunson Chandler & Jones, PLLC |
| 10.1 | | Incentive Stock Plan |
| 10.2+ | | Employment Agreement by and between the Company and Timothy Pickett dated May 1, 2022 |
| 10.3+ | | Consulting Agreement by and between the Company and Wade Rivers, LLC dated January 1, 2021 |
| 10.4+ | | Employment Agreement by and between the Company and Adam Cox dated May 1, 2022 |
| 10.5+ | | Compensation Agreement by and between the Company and Jared Barrera dated September 28, 2022 |
| 21.1 | | List of Subsidiaries |
| 23.1 | | Consent of Sadler Gibb & Associates, LLC, independent registered public accounting firm |
| 23.2 | | Consent of Brunson Chandler & Jones, PLLC (included in Exhibit 5.1) |
| 24.1 | | Power of Attorney (included in signature page) |
| 107 | | Filing Fee Table |
| | * | To be filed by amendment |
| | + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| (b) | Financial statement schedules. |
No financial statement schedules are provided because the information called for is not required or is shown in the consolidated financial statements or related notes.
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of KindlyMD, Inc. pursuant to the foregoing provisions, or otherwise, Kindly MD, Inc. has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by KindlyMD, Inc. of expenses incurred or paid by a director, officer or controlling person of KindlyMD, Inc. in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, KindlyMD, Inc. will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction, the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(c) The undersigned hereby further undertakes that:
(1) For purposes of determining any liability under the Securities Act the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by KindlyMD, Inc. pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Salt Lake City, State of Utah, on [ ] [●], 2023.
| KINDLYMD, INC. | |
| | | |
| By: | /s/ Tim Pickett | |
| | Tim Pickett | |
| | Chief Executive Officer | |
POWER OF ATTORNEY: KNOW ALL PERSONS BY THESE PRESENTS that each individual whose signature appears below constitutes and appoints Tim Pickett, his true and lawful attorneys-in-fact and agents with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement, and to sign any registration statement for the same offering covered by the Registration Statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or his substitute or substitutes, may lawfully do or cause to be done or by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated:
| Name | | Title | | Date |
| | | | | |
| /s/ Tim Pickett | | Chief Executive Officer and Director | | [ ] [●], 2023 |
| Tim Pickett | | (Principal Executive Officer) | | |
| | | | | |
| /s/ Adam Cox | | Chief Operating Officer | | [ ] [●], 2023 |
| Adam Cox | | | | |
| | | | | |
| /s/ Jared Barrera | | Chief Financial Officer and Director | | [ ] [●], 2023 |
| Jared Barrera | | (Principal Financial and Accounting Officer) | | |





