Table of Contents
Delaware | 3841 | 92-2182207 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☒ | |||||
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be issued until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and does not constitute the solicitation of an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 31, 2024
PRELIMINARY PROSPECTUS

ALLURION TECHNOLOGIES, INC.
Up to 30,191,900 Shares of Common Stock
This prospectus relates to the potential offer and sale from time to time by the selling securityholder identified in this prospectus of up to an aggregate of 30,191,900 shares of common stock, par value $0.0001 per share (“common stock”), of Allurion Technologies, Inc. (“we,” “us,” the “Company” or “Allurion”) issuable upon the conversion of convertible senior secured notes (the “Notes”) held by the selling securityholder, exclusive of any accrued but unpaid interest on the Notes that may be added to the outstanding principal amount of such Notes at the time of conversion. The Notes were issued and sold to accredited investors in a private placement pursuant to a Note Purchase Agreement, dated as of April 14, 2024, as amended by the First Amendment to Note Purchase Agreement, dated as of April 16, 2024, by and between us, RTW Investments, LP (“RTW”), as agent for the purchasers (the “Purchasers”) party thereto from time to time (RTW in such capacity, the “Principal Purchaser”), and Acquiom Agency Services LLC (“Acquiom”), as collateral agent for the Purchasers and the Principal Purchaser (as amended, the “Amended Note Purchase Agreement”). We are not selling any shares of common stock under this prospectus and will not receive any of the proceeds from the sale or other disposition of the common stock by the selling securityholder. See “Private Placement of Notes” for a description of the Amended Note Purchase Agreement and the Notes and “Selling Securityholder” for additional information regarding the Purchasers.
This prospectus provides you with a general description of such securities and the general manner in which the selling securityholder may offer or sell the securities. More specific terms of any securities that the selling securityholder may offer or sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus.
Our registration of the shares of common stock covered by this prospectus does not mean that the selling securityholder will offer or sell any of the shares. The selling securityholder named in this prospectus, or their donees, pledgees, transferees or other successors-in-interest, may resell the common stock covered by this prospectus through public or private transactions at prevailing market prices, at prices related to prevailing market prices or at privately negotiated prices. For additional information on the possible methods of sale that may be used by the selling securityholder, you should refer to the section of this prospectus entitled “Plan of Distribution.”
Any common stock subject to resale hereunder will have been issued by us and acquired by the selling securityholder prior to any resale of such shares pursuant to this prospectus. No underwriter or other person has been engaged to facilitate the sale of the shares in this offering. The selling securityholder will bear all commissions and discounts, if any, attributable to its sales of the shares of common stock offered hereby. We will incur costs and expenses in connection with the registration of the shares of our common stock offered hereby, including filing, legal and accounting fees. You should read this prospectus and any prospectus supplement or amendment carefully before you invest in our securities.
Our common stock is listed on The New York Stock Exchange (“NYSE”) under the symbol “ALUR.” On May 30, 2024, the last quoted sale price for the shares of our common stock as reported on the NYSE was $1.51 per share.
Sales of a substantial number of shares of common stock in the public market, including the resale of the shares of common stock held by the selling securityholder pursuant to this prospectus, could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock and make it more difficult for you to sell your stockholdings at times and prices that you determine are appropriate. Furthermore, we expect that, because there is a large number of shares being registered pursuant to this registration statement of which this prospectus forms a part, the selling securityholder will continue to offer the securities covered thereby pursuant to this prospectus for a significant period of time, the precise duration of which cannot be predicted. Accordingly, the adverse market and price pressures resulting from an offering pursuant to the registration statement may continue for an extended period of time.
Investing in our securities involves a high degree of risk. Before buying any securities, you should carefully read the discussion of the risks of investing in our securities in “Risk Factors” beginning on page 22 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is May 31, 2024.
Table of Contents
TABLE OF CONTENTS
| Page | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 12 | ||||
| 14 | ||||
| 21 | ||||
| 22 | ||||
| 77 | ||||
| 79 | ||||
| 80 | ||||
| 81 | ||||
| 82 | ||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF ALLURION | 108 | |||
| 130 | ||||
| 141 | ||||
| 146 | ||||
| 148 | ||||
| 150 | ||||
| 153 | ||||
| 158 | ||||
| 169 | ||||
| 170 | ||||
| 172 | ||||
| 172 | ||||
| 172 | ||||
INDEX TO FINANCIAL STATEMENTS ALLURION TECHNOLOGIES, INC. AND SUBSIDIARIES | F-1 | |||
ALLURION TECHNOLOGIES, INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (dollars in thousands) | F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-8 | ||||
i
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-1 that we filed with the SEC whereby the selling securityholder may, from time to time, sell the securities offered by it described in this prospectus. We will not receive any proceeds from the sale by such selling securityholder of the securities offered by it described in this prospectus.
Neither we nor the selling securityholder has authorized anyone to provide you with any information or to make any representations other than those contained in this prospectus or any applicable prospectus supplement or any free writing prospectuses prepared by or on behalf of us or to which we have referred you. Neither we nor the selling securityholder takes responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Neither we nor the selling securityholder will make an offer to sell these securities in any jurisdiction where such offer or sale is not permitted. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus. You should assume that the information appearing in this prospectus or any prospectus supplement is accurate as of the date on the front of those documents only, regardless of the time of delivery of this prospectus or any applicable prospectus supplement, or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
The selling securityholder and its permitted transferees may use this shelf registration statement to sell securities from time to time through any means described in the section titled “Plan of Distribution.” More specific terms of any securities that the selling securityholder and its permitted transferees offer and sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering.
We may also provide a prospectus supplement or post-effective amendment to the registration statement to add information to, or update or change information contained in, this prospectus. Any statement contained in this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in such prospectus supplement or post-effective amendment modifies or supersedes such statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus. You should read both this prospectus and any applicable prospectus supplement or post-effective amendment to the registration statement together with the additional information to which we refer you in the sections of this prospectus titled “Where You Can Find More Information.”
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed or will be filed as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under “Where You Can Find More Information.”
1
Table of Contents
BASIS OF PRESENTATION
The Business Combination (as defined below) between Allurion Technologies, Inc., a Delaware corporation formed on January 25, 2023 for purposes of consummating the transactions contemplated by the Business Combination Agreement (formerly known as Allurion Technologies Holdings, Inc. prior to the consummation of the Business Combination, or “Allurion”) and Compute Health Acquisition Corp., a Delaware corporation formed on October 7, 2020 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or other similar business combination with one or more businesses or entities (“Compute Health”) was effected pursuant to that certain Business Combination Agreement, dated as of February 9, 2023 (as amended, the “Business Combination Agreement”), by and among Allurion, the entity formerly known as Allurion Technologies, Inc. prior to the Business Combination (now known as Allurion Technologies, LLC or “Legacy Allurion”), Compute Health, Compute Health Corp. (“Merger Sub I”) and Compute Health LLC (“Merger Sub II” and, together with Merger Sub I, the “Merger Subs”).
The Business Combination was effected in three steps:
(1) Compute Health merged with and into Allurion (the “CPUH Merger”), with Allurion surviving the CPUH Merger as a publicly listed entity (the time at which the CPUH Merger became effective, the “CPUH Merger Effective Time”) and becoming the sole owner of each Merger Sub;
(2) following the consummation of the CPUH Merger, Merger Sub I merged with and into Legacy Allurion (the “Intermediate Merger”), with Legacy Allurion surviving the Intermediate Merger and becoming a direct, wholly-owned subsidiary of Allurion (the time at which the Intermediate Merger became effective, the “Intermediate Merger Effective Time”); and
(3) thereafter, Legacy Allurion merged with and into Merger Sub II (the “Final Merger” and, collectively with the CPUH Merger and the Intermediate Merger, the “Mergers” and, together with all other transactions contemplated by the Business Combination Agreement, the “Business Combination”), with Merger Sub II surviving the Final Merger and remaining a direct, wholly-owned subsidiary of Allurion (the time at which the Final Merger became effective, the “Final Merger Effective Time”).
References to a year refer to our fiscal year ended on December 31 of the specified year.
Certain monetary amounts, percentages and other figures included herein have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables and charts may not be the arithmetic aggregation of the figures that precede them, and figures expressed as percentages in the text may not total 100% or, as applicable, when aggregated may not be the arithmetic aggregation of the percentages that precede them.
Unless the context otherwise requires, all references herein to “Allurion,” the “Company,” “we,” “us,” or “our” refer to the business and operations of Legacy Allurion (as defined below) and its consolidated subsidiaries prior to consummation of the Business Combination and to New Allurion (as defined below) and its consolidated subsidiaries following the consummation of the Business Combination. “Legacy Allurion” refers to Allurion Technologies, LLC, which was previously known as Allurion Technologies Opco, Inc. (formerly Allurion Technologies, Inc.) prior to the consummation of the Business Combination. “New Allurion” refers to Allurion Technologies, Inc., which was previously known as Allurion Technologies Holdings, Inc. prior to the consummation of the Business Combination.
2
Table of Contents
TRADEMARKS
This document contains references to trademarks and service marks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to those trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
3
Table of Contents
SELECTED DEFINITIONS
Unless the context otherwise requires, all references in this section to “we,” “us,” or “our” refer to the business and operations of Legacy Allurion and its consolidated subsidiaries prior to the Business Combination and to New Allurion and its subsidiaries following the Business Combination. In addition, as used in this prospectus, unless otherwise noted or the context otherwise requires, references to the following capitalized terms have the meanings set forth below:
“2021 Term Loan” refers to that loan and security agreement that we entered into in March 2021 with Runway Growth Credit Fund, Inc.
“2023 Plan” refers to the Allurion 2023 Stock Option and Incentive Plan.
“Aggregate Intermediate Merger Closing Merger Consideration” refers to the shares of our common stock issued or issuable to Historical Rollover Equityholders in connection with the consummation of the Intermediate Merger.
“AI” refers to artificial intelligence.
“Allurion Balloon” refers to the swallowable, ProcedurelessTM intragastric balloon for weight loss developed by Allurion.
“Amended and Restated RTW Side Letter” refers to the Original RTW Side Letter, as amended by the Side Letter Amendment.
“Amended Note Purchase Agreement” refers to the Original RTW Note Purchase Agreement, as amended by that certain First Amendment to the Note Purchase Agreement, dated as of April 16, 2024.
“Backstop Agreement” refers to the Backstop Agreement, dated as of May 2, 2023, by and among RTW, the Fortress Investor, Allurion, Legacy Allurion, and HVL, pursuant to which the Backstop Purchasers purchased an aggregate of $4.0 million of the HVL Bridge Note at the Backstop Closing.
“Backstop Closing” refers to the closing of the Backstop Purchasers’ purchase from HVL of the relevant portion of the HVL Bridge Note, as contemplated by the Backstop Agreement.
“Backstop Purchasers” refers to, collectively, the Fortress Investor and RTW.
“Backstop Shares” refers to, with respect to the Fortress Investor, 700,000 shares of our common stock and, with respect to RTW, 700,000 shares of our common stock, which, in each case, is the number of shares of our common stock we issued to such Backstop Purchaser pursuant to the Backstop Agreement.
“BCA Amendment” refers to the amendment to the Business Combination Agreement, dated as of May 2, 2023, by and among Compute Health, Merger Sub I, Merger Sub II, Allurion and Legacy Allurion,
“Board” refers to our Board of Directors.
“Bridge Notes” refers to $28.7 million aggregate principal amount of convertible unsecured promissory notes sold in a private placement by Legacy Allurion to various investors from February 15, 2023 until August 1, 2023.
“Business Combination” refers to the transactions contemplated by the Business Combination Agreement.
4
Table of Contents
“Business Combination Agreement” refers to the Existing Business Combination Agreement, as amended by the BCA Amendment.
“Bylaws” refers to the Amended and Restated Bylaws of Allurion.
“Chardan” refers to Chardan Capital Markets LLC, a New York limited liability company.
“Chardan Equity Facility” refers to the committed equity facility established pursuant to the Chardan Purchase Agreement, pursuant to which, among other things, Allurion has agreed to issue and sell to Chardan and Chardan has agreed to purchase from Allurion up to $100.0 million of freely tradable shares of common stock.
“Chardan Purchase Agreement” refers to the ChEF Purchase Agreement, dated as of December 18, 2023, by and between Allurion and Chardan.
“Chardan Registration Rights Agreement” refers to the Registration Rights Agreement, dated as of December 18, 2023, by and between Allurion and Chardan.
“Closing Date” refers to August 1, 2023.
“Closings” refers to, collectively, the closing of each of the CPUH Merger, the Intermediate Merger and the Final Merger.
“Code” refers to the Internal Revenue Code of 1986, as amended.
“common stock” refers to the common stock, par value $0.0001 per share, of Allurion.
“Compute Health” refers to Compute Health Acquisition Corp., a Delaware corporation.
“Compute Health Class A Common Stock” refers to the Class A common stock, par value $0.0001 per share, of Compute Health issued in the IPO.
“Compute Health Class B Common Stock” refers to the Class B common stock, par value $0.0001 per share, of Compute Health.
“Compute Health Private Warrants” refers to the warrants of Compute Health issued in a private placement that closed contemporaneously with the IPO, each entitling the holder thereof to purchase one share of Compute Health Class A Common Stock at an exercise price of $11.50, subject to adjustment as set forth in the Warrant Agreement.
“Compute Health Public Warrants” refers to the warrants of Compute Health issued as part of the Compute Health Units in the IPO, each entitling the holder thereof to purchase one share of Compute Health Class A Common Stock at an exercise price of $11.50, subject to adjustment as set forth in the Warrant Agreement.
“Compute Health Units” refers to the units issued in the IPO, each comprised of one share of Compute Health Class A Common Stock and one-quarter of one Compute Health Public Warrant.
“CPUH” refers to Compute Health Acquisition Corp., a Delaware corporation.
“CPUH Exchange Ratio” refers to 1.420455.
“CPUH Merger” refers to the merger, on the Closing Date, of Compute Health with and into Allurion, with Allurion surviving the merger as a publicly listed entity.
5
Table of Contents
“CPUH Merger Closing” refers to the closing of the CPUH Merger.
“CPUH Merger Effective Time” refers to the date and time at which the CPUH Merger became effective.
“CPUH Recapitalization” refers to the recapitalization of the Sponsor’s and the Additional Class B Holders’ shares of Compute Health Class B Common Stock and the Sponsor’s Compute Health Private Warrants into shares of Compute Health Class A Common Stock, pursuant to and subject to the terms and conditions set forth in the Sponsor Support Agreement.
“DGCL” refers to the Delaware General Corporation Law, as may be amended from time to time.
“dollars” or “$” refers to U.S. dollars.
“Exchange Act” refers to the Securities Exchange Act of 1934, as amended.
“Existing Business Combination Agreement” refers to the Business Combination Agreement, dated as of February 9, 2023, by and among Compute Health, Merger Sub I, Merger Sub II, Legacy Allurion and Allurion.
“FDA” refers to the U.S. Food and Drug Administration, or any successor agency thereto.
“Final Merger” refers to the merger, on the Closing Date, of Legacy Allurion with and into Merger Sub II, with Merger Sub II surviving the merger and remaining a direct, wholly-owned subsidiary of Allurion.
“Final Merger Effective Time” refers to the date and time at which the Final Merger became effective.
“Forfeited RSUs” refers to the 79,232 Legacy Allurion RSU Awards previously forfeited by Krishna Gupta.
“Fortress” refers to Fortress Credit Corp. and/or one or more of its affiliates, as applicable.
“Fortress Credit Agreement” refers to the Credit Agreement and Guaranty, dated as of August 1, 2023, by and among Legacy Allurion, as the borrower, Allurion, the guarantors from time to time party thereto, the lenders from time to time parties thereto and Fortress, as administrative agent, as amended by Amendment No. 1 to the Credit Agreement and Guaranty, dated as of December 29, 2023.
“Fortress Investor” refers to CFIP2 ALLE LLC, an affiliate of Fortress Credit Corp. that is party to the Backstop Agreement and the Fortress Letter Agreement.
“Fortress Letter Agreement” refers to the Side Letter Agreement, dated as of May 2, 2023, by and among Legacy Allurion, the Fortress Investor and Fortress Credit Corp.
“Fortress Term Loan” refers to the term loan in an amount of $60 million provided by Fortress to Legacy Allurion pursuant to and subject to the terms and conditions set forth in the Fortress Credit Agreement.
“GAAP” refers to the generally accepted accounting principles in the United States, as applied on a consistent basis.
“Gaur Contribution Agreement” refers to that certain Contribution Agreement by and between Allurion and The Shantanu K. Gaur Revocable Trust of 2021 (the “Gaur Trust”), dated as of May 2, 2023, pursuant to which the Gaur Trust contributed certain shares of common stock to Allurion as a contribution to capital, effective immediately following the Closings and the issuance of common stock to the Gaur Trust pursuant to the terms of the Business Combination Agreement.
“HHS” refers to the U.S. Department of Health & Human Services.
6
Table of Contents
“HIPAA” refers to the Health Insurance Portability and Accountability Act of 1996, as amended.
“Historical Rollover Equityholders” refers to the holders of shares of Legacy Allurion Common Stock, Legacy Allurion Preferred Stock, vested Legacy Allurion Options, Legacy Allurion RSU Awards and Legacy Allurion Warrants.
“HVL” refers to Hunter Ventures Limited, an existing limited partner of a fund managed by Krishna Gupta, a member of our Board.
“HVL Bridge Note” refers to that certain $13 million Bridge Note sold to HVL on February 15, 2023.
“HVL Termination Agreement” refers to the Side Letter Termination Agreement, dated as of May 2, 2023, by and among Legacy Allurion, Allurion, Romulus Growth Allurion L.P. and HVL.
“Incremental Financing” refers to $28.7 million of additional financing obtained by Legacy Allurion prior to the consummation of the Business Combination pursuant to one or more private sales of Legacy Allurion Convertible Notes which converted into shares of Legacy Allurion Common Stock immediately prior to the Intermediate Merger Effective Time.
“Initial Financing” refers to the entry by Legacy Allurion into the Side Letters with the Side Letter Holders, pursuant to which, in the event the Side Letter Holders’ Bridge Notes converted in connection with the consummation of the Business Combination, the conversion rate for such Bridge Notes would be adjusted after the Closing Date to provide each of the Side Letter Holders with additional shares of our common stock, in the event that the trading price of the shares of our common stock was lower than the applicable conversion price of such Bridge Notes, as adjusted for the Intermediate Merger Exchange Ratio.
“Intermediate Merger” refers to the merger, on the Closing Date, of Merger Sub I with and into Legacy Allurion, with Legacy Allurion surviving the merger as a direct, wholly-owned subsidiary of Allurion.
“Intermediate Merger Closing” refers to the closing of the Intermediate Merger.
“Intermediate Merger Effective Time” refers to the date and time at which the Intermediate Merger became effective.
“Intermediate Merger Exchange Ratio” refers to the ratio obtained by dividing (a) the Aggregate Intermediate Merger Closing Merger Consideration by (b) the Fully Diluted Company Capitalization (as defined in the Business Combination Agreement).
“Initial Public Offering” or “IPO” refers to the initial public offering of CPUH, which closed on February 9, 2021.
“Investor” refers to the Sponsor, certain Legacy Allurion Stockholders, and certain other parties, each a party to the Investor Rights Agreement.
“Investor Rights Agreement” refers to the Investor Rights and Lock-up Agreement, dated August 1, 2023, by and among Allurion, the Sponsor, certain Legacy Allurion Stockholders and certain other parties.
“JOBS Act” refers to the Jumpstart Our Business Startups Act of 2012.
“Legacy Allurion” refers to the entity formerly known as Allurion Technologies, Inc., prior to the consummation of the Business Combination, which is now known as Allurion Technologies, LLC.
7
Table of Contents
“Legacy Allurion Common Stock” refers to the common stock, par value $0.0001 per share, of Legacy Allurion.
“Legacy Allurion Convertible Notes” refers to the convertible unsecured promissory notes issued by Legacy Allurion pursuant to that certain (i) Convertible Note Purchase Agreement, dated December 22, 2021, by and among Legacy Allurion and the investors listed on Exhibit A thereto, (ii) Convertible Note Purchase Agreement, dated February 15, 2023, by and among Legacy Allurion and the investors listed on Exhibit A thereto and (iii) Convertible Note Purchase Agreement, dated June 14, 2023, by and among Legacy Allurion and the investors listed on Exhibit A thereto.
“Legacy Allurion Options” refers to, as of any determination time, each option to purchase shares of Legacy Allurion Common Stock granted to any current or former director, manager, officer, employee or other service provider of Legacy Allurion or any of its subsidiaries that was outstanding and unexercised as of immediately prior to the Intermediate Merger Effective Time.
“Legacy Allurion Preferred Stock” refers to, collectively, the Legacy Allurion Series A Preferred Stock, the Legacy Allurion Series A-1 Preferred Stock, the Legacy Allurion Series B Preferred Stock, the Legacy Allurion Series C Preferred Stock, Legacy Allurion Series D-1 Preferred Stock, the Legacy Allurion Series D-2 Preferred Stock and the Legacy Allurion Series D-3 Preferred Stock.
“Legacy Allurion RSU Award” refers to all outstanding restricted stock unit awards denominated in shares of Legacy Allurion Common Stock immediately prior to the Intermediate Merger Effective Time, whether or not vested.
“Legacy Allurion Series A Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series A Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Series A-1 Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series A-1 Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Series B Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series B Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Series C Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series C Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Series D-1 Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series D-1 Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Series D-2 Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series D-2 Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Series D-3 Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Legacy Allurion designated as “Series D-3 Preferred Stock” that were outstanding prior to the Closings.
“Legacy Allurion Stockholders” refers to the holders of Legacy Allurion Common Stock and/or Legacy Allurion Preferred Stock prior to the Business Combination.
“Legacy Allurion Warrants” refers to the warrants exercisable for a number of shares of Legacy Allurion Common Stock or Legacy Allurion Preferred Stock that were outstanding prior to the Closings.
“Medtronic” refers to Medtronic plc.
8
Table of Contents
“Medtronic Collaboration” refers to the collaboration entered into by and between Legacy Allurion and Medtronic pursuant to the Medtronic Sales Agency Agreement.
“Medtronic Sales Agency Agreement” refers to the Sales Agency Agreement, dated May 15, 2023, between Covidien AG, an affiliate of Medtronic, and Legacy Allurion.
“Merger Sub I” refers to Compute Health Corp., a Delaware corporation
“Merger Sub II” refers to Compute Health LLC, a Delaware limited liability company.
“Merger Subs” refers to Merger Sub I and Merger Sub II.
“Mergers” refers to, collectively, the CPUH Merger, the Intermediate Merger, and the Final Merger.
“Notes” refers to the convertible senior secured notes, in the principal amount of $48 million, issued by Allurion pursuant to the Amended Note Purchase Agreement.
“NYSE” refers to The New York Stock Exchange.
“Original RIFA” refers to the Revenue Interest Financing Agreement, dated as of February 9, 2023, by and among Legacy Allurion and RTW, as modified by that certain Assignment and Assumption, dated April 27, 2023, that certain Assignment Agreement, dated as of July 28, 2023, and that certain Company Assumption Agreement, dated as of August 1, 2023.
“Original RTW Note Purchase Agreement” refers to that certain Note Purchase Agreement, dated as of April 14, 2024, by and among Allurion, Allurion Opco, Allurion Australia Pty Ltd, a proprietary limited company organized under the laws of Australia and a wholly-owned subsidiary of Allurion, the Original RIFA Investors (as defined below) and RTW.
“Original RTW Side Letter” refers to that certain letter agreement, dated February 9, 2023, by and among Allurion, Legacy Allurion, Compute Health, Merger Sub II and RTW.
“PCAOB” refers to the Public Company Accounting Oversight Board.
“PIPE Investment” refers to the private placement pursuant to which PIPE Investors made a private investment in the aggregate amount of $37.9 million in public equity in the form of the PIPE Shares on the terms and conditions set forth in the PIPE Subscription Agreements.
“PIPE Investors” refers to the investors that signed PIPE Subscription Agreements.
“PIPE Shares” refers to the 5,386,695 shares of our common stock sold to the PIPE Investors pursuant to the PIPE Subscription Agreements.
“PIPE Subscription Agreements” refers to the subscription agreements, dated as of February 9, 2023, by and among Compute Health, Allurion and the PIPE Investors, pursuant to which Allurion agreed to issue an aggregate of 5,386,695 shares of our common stock to the PIPE Investors following the CPUH Merger Closing and immediately prior to the Intermediate Merger Closing at a purchase price of $7.04 per share.
“Preferred Stock” refers to the shares of preferred stock, par value $0.0001 per share, of Allurion.
“Public Warrants” refers to the public warrants to purchase, in the aggregate, up to 18,759,838 shares of our common stock, at an exercise price of $8.10 per share, that were originally issued in the Initial Public Offering of Compute Health and assumed by us in connection with the Business Combination.
9
Table of Contents
“Registration Statement” refers to the registration statement on Form S-1 of which this prospectus form a part.
“Revenue Interest Financing” refers to the financing, in the initial amount equal to $40 million, provided by RTW to Allurion, pursuant to and subject to the terms and conditions set forth in the Revenue Interest Financing Agreement.
“Revenue Interest Financing Agreement” refers to the Original RIFA, as amended by the RIFA Amendment.
“RIFA Amendment” refers to the Omnibus Amendment to the Revenue Interest Financing Agreement, dated as of April 14, 2024, by and among Allurion, Legacy Allurion, RTW and other parties thereto.
“Rollover Warrants” refers to the privately placed warrants to acquire up to an aggregate of 403,658 shares of our common stock, at exercise prices that range from $0.02 per share to $12.14 per share, which were converted from Legacy Allurion Warrants at the Intermediate Merger Effective Time.
“RSU Forfeiture Agreement” refers to the letter agreement, dated as of May 2, 2023, by and between Krishna Gupta, a member of the Allurion Board, and Legacy Allurion, pursuant to which, among other things, upon the terms and subject to the conditions set forth therein, Mr. Gupta agreed to forfeit to Allurion the Forfeited RSUs.
“RTW” refers to certain entities that have engaged RTW Investments, LP as investment manager.
“SEC” refers to the U.S. Securities and Exchange Commission.
“Securities Act” refers to the Securities Act of 1933, as amended.
“Sponsor” refers to Compute Health Sponsor LLC, a Delaware limited liability company.
“Sponsor Contribution Agreement” refers to that certain Contribution Agreement between Compute Health and the Sponsor, dated as of May 2, 2023, pursuant to which the Sponsor agreed to contribute certain shares of Compute Health Class A Common Stock to Compute Health as a contribution to capital, effective immediately following the CPUH Recapitalization and immediately prior to the CPUH Merger.
“Sponsor Loans” refers to loans borrowed by Compute Health from the Sponsor or any of its affiliates to meet Compute Health’s reasonable funding requirements pursuant to those certain promissory notes for working capital loans, dated April 6, 2021, July 28, 2022 and February 9, 2023.
“Sponsor Loan Equity Issuance” refers to the issuance of 525,568 shares of common stock upon conversion of the amounts of the Sponsor Loans outstanding as of the consummation of the Business Combination that were in excess of $2,500,000 (such excess, the “Sponsor Loan Excess”) up to an amount not to exceed $5,250,000, at a price per share equal to $7.04.
“Sponsor Support Agreement” refers to the Sponsor Support Agreement, by and among Compute Health, the Sponsor, Allurion, Legacy Allurion and the Additional Class B Holders, dated as of February 9, 2023.
“Transfer Agent” refers to Continental Stock Transfer & Trust Company, in its capacity as transfer agent and registrar for the common stock and Public Warrants.
“VWAP” refers to volume weighted average price of a security on the NYSE, or any other national securities exchange on which such securities are then traded, as applicable.
10
Table of Contents
“Warrants” refers to (i) the Public Warrants and (ii) the Rollover Warrants, each exercisable for shares of common stock.
“Warrant Agent” refers to Continental Stock Transfer & Trust Company, in its capacity as agent for the Public Warrants.
“Warrant Agreement” refers to the Warrant Agreement, dated February 4, 2021, by and between Compute Health and the Warrant Agent, as amended by the Warrant Amendment and the Warrant Assumption Agreement.
“Warrant Amendment” refers to the First Amendment to Warrant Agreement, dated as of August 1, 2023, by and between Compute Health and the Warrant Agent.
“Warrant Assumption Agreement” refers to the Warrant Assignment, Assumption and Amendment Agreement, entered into by and among Compute Health, Allurion and the Warrant Agent in connection with the CPUH Merger Closing, pursuant to which the Warrant Agreement was assigned to, and assumed by, Allurion.
11
Table of Contents
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this prospectus may constitute “forward-looking statements” within the meaning of the U.S. federal securities laws, including the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, statements regarding the plans, strategies and prospects, both business and financial, of Allurion. These statements are based on the beliefs and assumptions of our management. Although we believe that our plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, we cannot assure you that we will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events, or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes,” “estimates,” “expects,” “projects,” “target,” “goal,” “forecasts,” “may,” “will,” “potential,” “should,” “would,” “could,” “future,” “seeks,” “plans,” “predicts,” “propose,” “scheduled,” “anticipates,” “intends,” or similar expressions.
Forward-looking statements in this prospectus include, but are not limited to, statements about our ability to:
| • | realize the benefits expected from the Business Combination; |
| • | successfully succeed in or defend litigation or proceedings that may be instituted by or against us; |
| • | manage various conflicts of interest that could arise among us or our affiliates, investors, directors, and officers; |
| • | successfully manage our cash and cash equivalents and any anticipated proceeds from the Chardan Equity Facility (as defined herein); |
| • | maintain the listing of our securities on the NYSE, and the potential liquidity and trading of such securities; |
| • | achieve the benefits of the Medtronic Collaboration; |
| • | acquire sufficient sources of funding if and when needed; |
| • | attract and retain key employees, officers, and directors; |
| • | implement and achieve business plans, forecasts, and other expectations, including any financial projections provided to PIPE Investors (as defined herein) in connection with the Business Combination, and identify and realize additional opportunities; |
| • | manage risks associated with our management having limited experience operating as a public company; |
| • | commercialize current and future products and services and create sufficient demand among health care providers and patients for such products; |
| • | successfully complete current and future preclinical studies and clinical trials of the Allurion Balloon and any other future product candidates; |
| • | obtain market acceptance of the Allurion Balloon as safe and effective; |
| • | cost-effectively sell existing and future products through existing distribution arrangements with distributors and/or successfully adopt a direct sales force as part of a hybrid sales model that includes both distributors and a direct sales effort; |
| • | timely collect accounts receivable from our customers; |
| • | obtain regulatory approval or clearance in the U.S. and certain non-U.S. jurisdictions for current and future products and maintain previously obtained approvals and/or clearances in those jurisdictions where products and services are currently offered; |
12
Table of Contents
| • | accurately forecast customer demand and manufacture sufficient quantities of products that patients and health care providers request; |
| • | successfully compete in the highly competitive and rapidly changing regulated industries in which we operate, and effectively address changes in such industries, including changes in competitors’ products and services and changes in the laws and regulations that affect us; |
| • | successfully manage any future international expansion of our business and navigate business, regulatory, political, operational, financial, and economic risks associated with doing business internationally; |
| • | successfully manage any future growth in our business; |
| • | contract with third-party suppliers and providers and monitor their ability to perform adequately under those arrangements; |
| • | comply with applicable legal and regulatory obligations; |
| • | obtain and maintain intellectual property protection for our products and technologies and acquire or license (on commercially reasonable terms) intellectual property from third parties; |
| • | sell products, and use proprietary technologies, without infringing, misappropriating, or otherwise violating the proprietary rights or intellectual property of third parties; |
| • | manage the impact of any significant acquisitions, dispositions, and other similar or material transactions; |
| • | implement and maintain effective internal controls; |
| • | manage the effects of natural disasters, terrorist attacks, the spread and/or abatement of infectious diseases such as COVID-19, and the occurrence of other events beyond our reasonable control, including with respect to potential operational disruptions, labor disruptions, increased costs, and impacts to demand related thereto, on the ability to implement business plans, forecasts, and other expectations; and |
| • | other factors detailed under the section entitled “Risk Factors.” |
We have based the forward-looking statements contained in this prospectus primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations, prospects, business strategy, and financial needs. The outcome of the events described in these forward-looking statements is subject to risks, uncertainties, assumptions and other factors described in the section entitled “Risk Factors” beginning on page 22 and elsewhere in this prospectus. These risks are not exhaustive. Other sections of this prospectus include additional factors that could adversely impact our business and financial performance. Moreover, we operate in very competitive and rapidly changing environments. New risks and uncertainties emerge from time to time and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this prospectus.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and such statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements. We undertake no obligation to update or revise any forward- looking statements, whether as a result of new information, future events, or otherwise, except as may be required under applicable securities laws.
13
Table of Contents
PROSPECTUS SUMMARY
The following summary highlights information contained elsewhere in this prospectus. It does not contain all the information you should consider before investing in our securities. You should read this entire prospectus carefully, including the sections entitled “Risk Factors,” “Business,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Where You Can Find More Information,” and our consolidated financial statements and related notes included elsewhere in this prospectus, before making an investment decision. In this prospectus, unless the context requires otherwise, all references to “we,” “our,” “us,” “Allurion,” and the “Company” refer to Allurion Technologies, Inc. and its consolidated subsidiaries.
Overview
We are dedicated to ending obesity by creating a best-in-class weight loss platform to treat the estimated two billion people globally who are overweight. Our platform, the Allurion Program (the “Allurion Program”), features the Allurion Balloon and offers access to AI-powered remote patient monitoring tools, a proprietary behavior change program, secure messaging and video telehealth that are delivered by the Allurion Virtual Care Suite (“VCS”). Over 150,000 patients have already been treated commercially with the Allurion Balloon in over 50 countries globally outside of the United States.
The Allurion Balloon is swallowed as a capsule under the guidance of a health care provider without surgery, endoscopy, or anesthesia. The placement takes approximately 15 minutes during an outpatient visit (though times may vary across different outpatient offices). We believe the proprietary technologies that differentiate the Allurion Balloon enable improved safety and efficacy outcomes. In a prospective, non-randomized, open-label, registry trial, the Allurion Balloon demonstrated low device or procedure related rates of serious adverse events, which we believe compare favorably to that of our competitors.
The VCS is comprised of the following tools to support patients’ weight loss experience, which we believe benefit both patients and health care providers:
| • | For Allurion Program patients: Every current Allurion Program patient receives an Allurion Connected Scale (“Allurion Connected Scale”) and access to our mobile app (“App”), which integrates data from the Allurion Connected Scale, to conveniently monitor weight, body fat, activity, sleep, and several other critical metrics. The App can also enable secure messaging and video telehealth with the patient’s care team and can deliver content from Allurion’s proprietary behavior change program—a library of over 100 weight loss actions related to diet, nutrition, mental health, sleep, goal setting, and a number of other topics—directly to the patient. The App is available in over 15 languages. |
| • | For Allurion Program providers: Every Allurion Program provider receives access to our clinic dashboard (“Allurion Insights”), which provides end-to-end remote patient monitoring powered by the Allurion Iris AI platform, which leverages machine learning to deliver key insights and streamline workflow. Allurion Insights offers real-time access to patient data and AI-powered analytics, 1:1 video telehealth and secure messaging directly to the patient’s App, note functionality to keep track of patient encounters, and clinic-wide metrics that provide a snapshot of the clinic’s overall performance. |
In addition to its use by Allurion Balloon patients, we believe the VCS can potentially be a platform for optimal long-term follow-up after other medical and surgical weight loss interventions in the future.
For example, VCS includes a Treatment Tracking and Clinic-Led Onboarding feature that enables seamless onboarding and management of patients undergoing one or multiple weight loss treatments including gastric
14
Table of Contents
balloons such as the Allurion Balloon, surgery, or medications. In addition, in connection with our collaboration with Medtronic, we expect to develop bundled offerings that incorporate the VCS to onboard and manage Medtronic’s patients.
Recent Developments
RTW Note Purchase Agreement
On April 14, 2024, we entered into the Original Note Purchase Agreement with RTW, as agent for the purchasers (the “Purchasers”) party thereto from time to time (RTW in such capacity, the “Principal Purchaser”), and Acquiom Agency Services LLC (“Acquiom”), as collateral agent for the Purchasers and the Principal Purchaser. Subsequently, on April 16, 2024, we, the Principal Purchaser, the Purchasers and Acquiom entered into the First Amendment to the Original Note Purchase Agreement (as amended, the “Amended Note Purchase Agreement”).
Pursuant to the Amended Note Purchase Agreement, we issued and sold the Notes to the Purchasers in a private placement transaction. We used the proceeds from the issuance of the Notes to refinance our outstanding obligations under the Fortress Credit Agreement in full and to pay fees and expenses in connection therewith and in connection with the transactions contemplated by the Amended Note Purchase Agreement. We have terminated and repaid in full the outstanding borrowings and other obligations under the Fortress Credit Agreement (defined below).
The Notes bear interest at the annual rate of 6.0%, which interest is payable quarterly in cash or, at our option, in kind for the first three years. The maturity date for the Notes is April 16, 2031. The Notes are guaranteed by Allurion Technologies, LLC, a Delaware limited liability company and wholly-owned subsidiary of us (“Allurion Opco”), and certain other of our current and future subsidiaries, and are secured by substantially all the assets of the Company and the guarantors.
The Notes are convertible into shares of our common stock, at a Purchaser’s election at any time after the earliest of (i) the date on which Stockholder Approval (as defined below) is obtained, (ii) December 31, 2025, (iii) the date of a Fundamental Change Company Notice (as defined in the Amended Note Purchase Agreement), and (iv) the Make-Whole Fundamental Change Effective Date (as defined in the Amended Note Purchase Agreement), subject to certain terms and limitations in the Amended Note Purchase Agreement, based on the higher of (x) an initial conversion rate of 307.0797 shares of Common Stock per $1,000 principal amount of Notes (equivalent to an initial conversion price of approximately $3.26 per share, which represents a 35% premium to the trailing 30 day VWAP of our Common Stock on the NYSE as of the close of business on April 12, 2024) and (y) a 35% conversion premium to the lowest price per share in an equity financing for capital raising purposes ending on the date on which we have raised aggregate gross offering proceeds of at least $15,000,000 (the “Next Equity Financing”). If certain corporate events that constitute a “Make-Whole
Fundamental Change” (as defined in the Amended Note Purchase Agreement) occur, then the conversion rate
will, in certain circumstances, be increased for a specified period of time up to a maximum rate of an additional
321.9182 shares of common stock per $1,000 principal amount of Notes. In addition, calling any note for
redemption will also constitute a Make-Whole Fundamental Change with respect to that Note, in which case the
conversion rate applicable to the conversion of that Note will be increased in certain circumstances if it is
converted after it is called for redemption. Although the initial conversion price based on clause (x) above for the Notes is above the NYSE’s “Minimum Price” (as such term is defined in Section 312.03 of the NYSE Listed Company Manual), it is subject to a reset provision at the time of the Next Equity Financing that could result in the conversion price falling below such Minimum Price. Therefore, the Amended Note Purchase Agreement provides that unless and until requisite approval of our stockholders is obtained (“Stockholder Approval”), we
15
Table of Contents
will not deliver Common Stock upon conversion of the Notes in excess of 1% of the number of shares of our Common Stock outstanding as of April 14, 2024. We are required to include a proposal in our definitive proxy statement on Schedule 14A seeking Stockholder Approval no later than December 31, 2025. If we do not obtain Stockholder Approval at such meeting, we will call a special meeting of stockholders each 90-day period thereafter at least two times, and thereafter at each subsequent annual meeting until Stockholder Approval is obtained or the Notes are no longer outstanding; provided, that shares of Common Stock issued upon conversion of the Notes prior to obtaining Stockholder Approval shall not be entitled to vote in favor of Stockholder Approval.
Subject to specified conditions, on or after April 16, 2028, the Notes are redeemable by us at a redemption price equal to 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date. Pursuant to the terms of the Amended Note Purchase Agreement, each Purchaser is subject to a beneficial ownership conversion limitation such that no Purchaser shall be permitted to convert Notes to the extent it would result in such Purchaser and its affiliates beneficially owning more than the beneficial ownership limitation, currently set at 9.99% of our common stock.
Pursuant to the terms of the Amended Note Purchase Agreement, until the Notes are converted or repaid in full, RTW will be entitled to designate one representative who will serve as a non-voting board observer to our Board. We also must include an additional director nominee in our proxy statement for the election of the Class I directors at our 2024 annual meeting of stockholders, with the recommendation of the Board to vote in favor of such additional nominee as well as the “RTW Designated Director” (as defined in the Investor Rights Agreement). Such additional director nominee shall go through our director nomination process led by the nominating and corporate governance committee of the Board. RTW shall have the right to approve such additional nominee, with such approval not to be unreasonably withheld.
The Amended Note Purchase Agreement contains customary terms and covenants, including negative covenants, such as limitations on indebtedness, liens, disposition of assets and mergers, as well as a covenant not to increase the size of our Board of Directors prior to December 31, 2024, without RTW’s prior written consent, which may not be unreasonably withheld.
The Amended Note Purchase Agreement also contains financial maintenance covenants, which require (i) Allurion and Allurion Opco to maintain not less than $12,500,000 in unrestricted cash in controlled accounts in the U.S. at all times, (ii) Allurion to receive minimum trailing twelve-month consolidated Revenue (as defined in the Amended Note Purchase Agreement) at amounts designated in the Amended Note Purchase Agreement, tested quarterly beginning with the twelve-month period ending March 31, 2025, and (iii) Allurion and its subsidiaries’ consolidated business operations outside the United States to be profitable for the trailing three- month period, tested quarterly beginning with the three-month period ending December 31, 2025. The Amended Note Purchase Agreement also contains customary events of default, including defaults related to payment compliance, material inaccuracy of representations and warranties, covenant compliance, bankruptcy and insolvency proceedings, cross-payment defaults and cross-acceleration to certain other material indebtedness agreements, and judgment default.
RIFA Amendment
On February 9, 2023, Allurion Opco entered into the Original RIFA with 4010 Royalty Investments ICAV, an Irish collective asset-management vehicle, for and on behalf of its sub-fund, 4010 Royalty Investments Fund 1, and 4010 Royalty Master Fund LP, a Cayman Islands limited partnership (as transferees of the investors initially party to the Original RIFA) (collectively, together with their successors and permitted assigns, the “Original RIFA Investors”).
16
Table of Contents
On April 14, 2024, the Original RIFA was amended pursuant to the RIFA Amendment to reflect certain modifications agreed between the parties thereto in connection with the Purchasers’ purchase of the Notes and the refinancing of the Fortress Credit Agreement. Among other things, the RIFA Amendment waived the existing event of default under the Original RIFA, increased the rate of revenue interest payments to be paid to RTW on all current and future products and digital solutions developed and to be developed by Allurion (the “Royalty Rate”) for net sales less than or equal to $100 million prior to December 31, 2026 from 6% to 12%, and increased the Royalty Rate on net sales less than or equal to $100 million on or after January 1, 2027 from 10% to 12%, subject to the terms and conditions of the RIFA Amendment.
Amended and Restated RTW Side Letter
On February 9, 2023, Allurion, Allurion Opco, RTW Master Fund, Ltd., RTW Innovation Master Fund, Ltd. and RTW Biotech Opportunities Operating Ltd. (as transferee from RTW Biotech Opportunities Ltd. (formerly known as RTW Venture Fund Limited)) (in each case, together with their successors and permitted assigns, the “Additional RIFA Investors”) entered into the Original RTW Side Letter.
On April 14, 2024, Allurion, Allurion Opco and the Additional RIFA Investors entered into the Side Letter Amendment to reflect certain modifications to the Original RTW Side Letter in connection with the Purchasers’ purchase of the Notes. The Side Letter Amendment provides, among other things, that the Additional RIFA Investors may make a single election in certain circumstances to convert up to $7,500,000 of the purchase price that the Additional RIFA Investors paid for certain equity interests in Allurion into an amount of financing provided by the Additional RIFA Investors to Allurion Opco pursuant to an additional revenue interest financing agreement with Allurion Opco.
Fortress Credit Agreement
On April 16, 2024, we terminated and repaid in full the outstanding borrowings under the Credit Agreement and Guaranty, dated as of August 1, 2023 (as amended by the Fortress Credit Agreement), by and among Allurion, Allurion Opco, the subsidiary guarantors from time to time party thereto, the lenders from time to time party thereto (the “Lenders” and each, a “Lender”) and Fortress, as administrative agent for the Lenders, including the release of all guarantees and liens related thereto.
Summary Risk Factors
Investing in our securities involves risks. If any of these risks actually occur, our business, financial condition and results of operations would likely be materially adversely affected. You should carefully consider all the information contained in this prospectus before making a decision to invest in our securities. In particular, you should consider the risk factors described under “Risk Factors” beginning on page 22. Some of these risks related to our business, operations, financial performance, industry, as well as this offering and our securities, are summarized below.
| • | We expect to incur losses for the foreseeable future, our ability to achieve and maintain profitability depends on the commercial success of the Allurion Balloon, and we expect our revenues to continue to be driven primarily by sales of the Allurion Balloon. |
| • | We have a limited operating history and may face difficulties encountered by companies early in their commercialization in competitive and rapidly evolving markets. |
| • | The failure of the Allurion Balloon to achieve and maintain market acceptance could result in achieving sales or profitability below our expectations, which would cause our business, financial condition, and operating results to be materially and adversely affected. |
17
Table of Contents
| • | There is no guarantee that the FDA or non-U.S. regulatory agencies will grant approval or clearance for our current or future products, and failure to obtain regulatory approvals or clearances in the United States and other international jurisdictions, or revocation of approvals or clearances in those jurisdictions, will prevent us from marketing our products. |
| • | The Allurion Balloon is not currently approved for commercial sale in the United States. Obtaining such approval is costly and time consuming, and we may not obtain the regulatory approval required to sell the Allurion Balloon in the United States. |
| • | The weight loss and obesity-management industries are highly competitive. |
| • | If our competitors are able to develop and market products, whether medical devices or otherwise, that are safer, more effective, easier to use, or more readily adopted by patients and health care providers, our commercial opportunities will be reduced or eliminated. |
| • | Continued international expansion of our business will expose us to business, regulatory, political, operational, financial, and economic risks associated with doing business internationally. |
| • | We depend on a limited number of single source suppliers to manufacture components, sub-assemblies, and materials, which makes us vulnerable to supply shortages and price fluctuations. |
| • | The regulatory approval process is expensive, time consuming, and uncertain, and may prevent us from obtaining approvals for the commercialization of the Allurion Balloon or other products. |
| • | Even if we receive regulatory approval for the Allurion Balloon, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and subject us to penalties if we fail to comply with applicable regulatory requirements. |
| • | If patients using our products experience adverse events or other undesirable side effects, regulatory authorities could withdraw or modify our regulatory approvals, which would adversely affect our reputation and commercial prospects and/or result in other significant negative consequences. |
| • | The medical device industry is characterized by patent litigation and we could become subject to adversarial proceedings or litigation that could be costly, result in the diversion of management’s time and efforts, result in a loss of our intellectual property, require us to pay damages, or prevent us from marketing our existing or future products. |
| • | If we are not able to obtain and maintain intellectual property protection for our products and technologies, if the scope of our patents is not sufficiently broad or our patents are invalidated, or if competitors gain broader patent protection, we may not be able to effectively maintain our market leading technology position. |
| • | We have incurred net operating losses in the past and expect to incur net operating losses for the foreseeable future. |
| • | We have a significant amount of debt, which may affect our ability to operate our business and secure additional financing in the future. |
| • | We may need additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all, which would force us to delay, reduce, or suspend our planned development and commercialization efforts. Raising additional capital may subject us to unfavorable terms, cause dilution to our existing stockholders, restrict our operations, or require us to relinquish rights to our products and technologies. |
| • | We receive the majority of our revenue from sales to health care providers and other third-party distributors, and the failure to collect receivables from them could adversely affect our financial position and results of operations. |
| • | Our share price may be volatile, and purchasers of our securities could incur substantial losses. |
18
Table of Contents
| • | The issuances of our common stock to the selling securityholder upon conversion of the Notes will cause substantial dilution to our existing stockholders, and the sale of the shares of common stock acquired by the selling securityholder, or the perception that such sales may occur, could cause the market price of our common stock to fall. |
| • | Future sales of substantial amounts of the shares of our common stock by the selling securityholder and others could adversely affect the price of our common stock. |
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We are an “emerging growth company” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a registration statement under the Securities Act declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparability of our financial statements with another public company that is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
We will remain an emerging growth company until the earlier of: (1) the last day of the fiscal year (a) following the fifth anniversary of the effectiveness of the registration statement filed in connection with our Business Combination, (b) in which we have total annual gross revenue of at least $1.235 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common equity that is held by non-affiliates exceeds $700 million as of the end of the prior fiscal year’s second fiscal quarter; and (2) the date on which we have issued more than $1.00 billion in non-convertible debt securities during the prior three-year period. References herein to “emerging growth company” have the meaning associated with it in the JOBS Act.
As a result of this status, we have taken advantage of reduced reporting requirements in this prospectus. In particular, in this prospectus, we have not included all of the executive compensation-related information that would be required if we were not an emerging growth company.
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K, which allows us to take advantage of certain exemptions from disclosure requirements, including exemption from compliance with the auditor attestation requirements of Section 404. We will remain a smaller reporting company until the last day of the fiscal year in which (i) the market value of the shares of our common stock held by non-affiliates exceeds $250 million as of the prior June 30, and (ii) our annual revenue exceeded $100 million during such completed fiscal year or the market value of the shares of our common stock held by non-affiliates
19
Table of Contents
exceeds $700 million as of the prior June 30. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
Company Information
We were incorporated under the laws of the State of Delaware on January 25, 2023. The mailing address of our principal executive office is 11 Huron Drive, Natick, MA 01760, and the telephone number is (508) 647-4000.
We file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains an Internet website at www.sec.gov that contains reports, proxy and information statements and other information about issuers, like us, that file electronically with the SEC. We also maintain a website at www.allurion.com. We make available, free of charge, on our investor relations website at investors.allurion.com, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to these reports as soon as reasonably practicable after electronically filing or furnishing those reports to the SEC. Information contained on or accessible through our website is not a part of or incorporated by reference into this prospectus and the inclusion of our website and investor relations website addresses in this prospectus is an inactive textual reference only.
20
Table of Contents
THE OFFERING
| Issuer | Allurion Technologies, Inc., a Delaware corporation. | |
| Shares of Common Stock offered by RTW | Up to 30,191,900 shares of our common stock.
The actual number of shares of our common stock that may be issued upon conversion of the Notes will vary depending on the then-current conversion price of the Notes sold, not to exceed 479,196 shares of common stock, or 1% of the number of shares of the common stock outstanding as of April 14, 2024, unless we obtain Stockholder Approval, in accordance with the applicable stock exchange rules. We are registering the number of shares of common stock issuable assuming the shares are issued at an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of Notes, subject to Stockholder Approval for such issuance and exclusive of any accrued but unpaid interest on the Notes that may be added to the outstanding principal amount of such Notes at the time of conversion. If certain corporate events that constitute a Make-Whole Fundamental Change occur, then the conversion rate will, in certain circumstances, be increased for a specified period of time up to a maximum rate of an additional 321.9182 shares of common stock per $1,000 principal amount of Notes. In addition, calling any Note for redemption will also constitute a Make-Whole Fundamental Change with respect to that Note, in which case the conversion rate applicable to the conversion of that note will be increased in certain circumstances if it is converted after it is called for redemption. In addition, the number of shares of common stock issuable upon conversion of the Notes may increase as interest on the Notes accrues. | |
| Terms of the Offering | RTW will determine when and how it will dispose of any shares of our common stock acquired under the Amended Note Purchase Agreement that are registered under this prospectus for resale. | |
| Use of Proceeds | All shares of common stock offered by this prospectus are being registered for the account of the selling securityholder and we will not receive any proceeds from the resale of shares of our common stock by RTW. | |
| Common stock ticker symbol | “ALUR.” | |
| Risk factors | Any investment in the securities offered hereby is speculative and involves a high degree of risk. You should carefully consider the information set forth under “Risk Factors” and elsewhere in this prospectus. | |
21
Table of Contents
RISK FACTORS
Investing in our securities involves a high degree of risk. Before you decide to invest in our securities, you should consider carefully the risks described below, together with the other information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus. We believe the risks described below are the risks that are material to us as of the date of this prospectus. If any of the following risks actually occur, our business, results of operations and financial condition would likely be materially and adversely affected. In these circumstances, the market price of our securities could decline, and you may lose part or all of your investment.
Risks Related to the Development and Commercialization of Our Products
We expect to incur losses for the foreseeable future, our ability to achieve and maintain profitability depends on the commercial success of the Allurion Balloon, and we expect our revenues to continue to be driven primarily by sales of the Allurion Balloon.
We have incurred losses to date and expect to continue to incur losses for the foreseeable future. Sales of the Allurion Balloon and related accessories, which have occurred outside of the U.S. because we have not yet obtained the regulatory approval required to sell our products within the U.S., accounted for substantially all of our revenues for the years ended December 31, 2023 and 2022 and the three months ended March 31, 2024 and 2023, and we expect our revenues to continue to be driven primarily by sales of the Allurion Balloon. In order to achieve and sustain profitability, our revenues from sales of the Allurion Balloon will need to grow beyond the levels we have achieved in the past. If health care providers and/or patients do not perceive our products to be competitive in features, efficacy and safety when compared to other products in the market, or if demand for the Allurion Balloon or for weight loss procedures and programs in general decreases, we may fail to achieve sales levels that provide for future profitability.
Our ability to successfully market the Allurion Balloon and our other current and future product and service offerings depends on numerous factors, including but not limited to:
| • | outcomes of current and future clinical trials of, and trials involving, the Allurion Balloon; |
| • | acceptance of the Allurion Balloon as safe and effective by patients, caregivers and the medical community; |
| • | an acceptable safety profile of the Allurion Balloon in markets where we have obtained regulatory approvals; |
| • | whether key thought leaders in the medical community accept that such clinical trials are sufficiently meaningful to influence their or their patients’ choices of product; |
| • | maintenance of our existing regulatory approvals and expansion of the geographies in which we have regulatory approvals; |
| • | commercially viable processes at a scale sufficient to meet anticipated demand at an adequate cost of manufacturing, and that are compliant with ISO 13485 Quality Management System requirements and/or good manufacturing practice requirements, as set forth in the FDA’s QSR and other international regulations; |
| • | our success in educating health care providers and patients about the benefits, administration and use of the Allurion Balloon; |
| • | the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments; |
| • | the willingness of patients to pay out-of-pocket for the Allurion Balloon and/or VCS in the absence of coverage and reimbursement for such treatment; |
22
Table of Contents
| • | the success of our internal sales and marketing organization and the sales forces of our distributors; and |
| • | continued demand for weight loss using balloon products, which may be adversely affected by events involving our products or those of our competitors, among other things. |
Some of these factors are beyond our control. If we are unable to continue to commercialize the Allurion Balloon and our other current and future products and services, or are unable to obtain a distributor or partner to commercialize them, we may not be able to produce any incremental revenues related to the Allurion Balloon and our other current and future products and services. This would result in an adverse effect on our business, financial condition, results of operations and growth prospects.
We have a limited operating history and may face difficulties encountered by companies early in their commercialization in competitive and rapidly evolving markets.
The Allurion Balloon has been marketed in countries outside of the United States since 2016, and as such, we have a limited operating history upon which to evaluate our business and forecast our future revenue and operating results. In assessing our business prospects, you should consider the various risks and difficulties frequently encountered by companies early in their commercialization in competitive markets, particularly companies that develop and sell medical devices. These risks include our ability to:
| • | implement and execute our business strategy; |
| • | expand and improve the productivity of our direct sales force, distributors and marketing programs to grow sales of our existing and proposed products and services; |
| • | increase awareness of our brand and build loyalty among health care providers and patients; |
| • | manage growth and expanding operations; |
| • | respond effectively to competitive pressures and developments; |
| • | enhance our existing products and develop new products; |
| • | obtain regulatory approval or clearance to enhance our existing products and commercialize new products, including any label expansions for use of our products in adolescents; |
| • | respond to changing regulations associated with medical devices across all geographies; |
| • | perform clinical trials with respect to our existing products and any new products, including products under development; |
| • | attract, retain and motivate qualified personnel in various areas of our business; and |
| • | obtain and maintain coverage and adequate levels of reimbursement for our products. |
Due to our limited operating history, we may not have the institutional knowledge or experience to be able to effectively address these and other risks that we may face. In addition, we may not be able to develop insights into trends that could emerge and negatively affect our business and may fail to respond effectively to those trends. As a result of these or other risks, we may not be able to execute key components of our business strategy, and our business, financial condition and operating results may suffer.
We do not expect that health care providers or patients will receive third-party reimbursement for treatment with our products. As a result, we expect that our success will depend on the ability and willingness of health care providers to adopt self-pay practice management infrastructure and of patients to pay out-of-pocket for treatment with our products.
Certain elective treatments, such as an intragastric balloon, are typically not covered by insurance. Accordingly, we do not expect that any third-party payors will cover or reimburse health care providers or patients for the Allurion Program. As a result, we expect that our success will depend on the ability and
23
Table of Contents
willingness of health care providers that may not have historically operated a self-pay practice to adopt the policies and procedures needed to successfully operate such a practice. Our sales and marketing efforts have historically targeted bariatric surgeons, gastroenterologists, plastic surgeons and other health care providers.
Although many of these health care providers are accustomed to selling cash-pay services in their practices, some are primarily accustomed to providing services that are reimbursed by third-party payors. As a result, these health care providers may need to augment their administrative staff and billing procedures to address the logistics of a self-pay practice. If health care providers are unable or unwilling to make such changes, adoption of our products may be slower than anticipated.
Our success will also depend on the ability and willingness of patients to pay out-of-pocket for treatment with our products. Adverse changes in the economy, including from heightened inflation, higher interest rates, and geopolitical conflicts such as the Russia-Ukraine war and the Israel-Hamas war, may cause consumers to reassess their spending choices and reduce the demand for elective treatments and could have an adverse effect on consumer spending. This shift could have an adverse effect on our revenues and operating results. In addition, the operations of the medical device distributors upon whom we rely to sell our products may be negatively impacted by any such adverse economic changes. If our distributors are unable to maintain their operations and effectively market and sell our products, our results of operations and business may suffer. Furthermore, consumer preferences and trends may shift due to a variety of factors, including changes in demographic and social trends, public health initiatives and product innovations, which may reduce consumer demand for our products. The decision by a patient to elect to undergo treatment with the Allurion Balloon may be influenced by a number of additional factors, such as:
| • | the success of any sales and marketing programs, including direct-to-consumer marketing efforts, that we, or any third parties we engage, undertake; |
| • | the extent to which health care providers offer the Allurion Balloon to their patients; |
| • | the extent to which the Allurion Balloon satisfies patient expectations; |
| • | the cost, safety, comfort, tolerability, ease of use, and effectiveness of the Allurion Program as compared to other treatments; and |
| • | general consumer confidence, which may be impacted by economic and political conditions. |
Our financial performance will be materially harmed if we cannot generate significant customer demand for the Allurion Balloon.
Changes in coverage and reimbursement for obesity treatments and procedures could affect the adoption of the Allurion Program and our future revenues.
Historically, intragastric balloon products are not reimbursed by third-party payors, although a very limited number of balloon procedures have recently been subject to reimbursement in the U.K. market. We do not currently plan on submitting any requests to any third-party payor for coverage or billing codes specific to our products other than our partnership with the National Health Service in the United Kingdom. However, payors may change their coverage and reimbursement policies for intragastric balloon products as a category and/or for other obesity treatments and procedures, and these changes could negatively impact our business. For example, healthcare reform legislation or regulation that may be proposed or enacted in the future that results in a favorable change in coverage and reimbursement for competitive products and procedures in weight loss and obesity could also negatively impact adoption of our products and our future revenues, and our business could be harmed as we would be at an economic disadvantage when competing for customers. For more information, see section entitled “Business–Government Regulation–Other U.S. Healthcare Law-Coverage, Reimbursement and Healthcare Reform.”
24
Table of Contents
The failure of the Allurion Balloon to achieve and maintain market acceptance could result in us achieving sales below our expectations, which would cause our business, financial condition and operating results to be materially and adversely affected.
Our current business and growth strategy is highly dependent on the Allurion Balloon achieving and maintaining market acceptance. In order for us to sell our products to healthcare providers and, ultimately, weight loss patients, we must convince them that our products are an attractive alternative to competitive treatments for patients who are obese and overweight, including traditional pharmaceutical therapies and more aggressive bariatric surgical treatments, such as gastric bypass and sleeve gastrectomy. Market acceptance and adoption of the Allurion Balloon depends on educating health care providers on its safe and appropriate use, as well as the cost, safety, comfort, tolerability, ease of use, and effectiveness of the Allurion Program compared to other treatments. If we are not successful in convincing existing and potential customers of the benefits of our product, or if we are not able to achieve the support of health care providers for our product, our sales may decline or we may achieve sales below our expectations.
| • | Market acceptance of our products could be negatively impacted by many factors, including: |
| • | the willingness of patients to pay out-of-pocket for the Allurion Program in the absence of coverage and reimbursement for such program; |
| • | the failure of our products to achieve and maintain wide acceptance among patients who are obese and overweight, their health care providers, third-party payors and key opinion leaders in the weight loss treatment community; |
| • | lack of evidence supporting the safety, ease-of-use or other perceived benefits of the Allurion Balloon over competitive products or other currently available weight loss treatment alternatives; |
| • | perceived risks or uncertainties, or actual adverse events or other undesirable side effects, associated with the use of our gastric balloons, or components thereof, or of similar products or technologies of our competitors; |
| • | any adverse legal action, including products liability litigation, against us or our competitors relating to the Allurion Balloon or similar products or technologies; |
| • | the withdrawal or modification of any regulatory approvals for our products; and |
| • | results of clinical studies relating to the Allurion Balloon or similar competitive products. |
In addition, the rapid evolution of technology and treatment options within our industry may cause consumers to delay the purchase of our products in anticipation of advancements or breakthroughs, or the perception that advancements or breakthroughs could occur, in our products or the products offered by our competitors. It is also possible that consumers interested in purchasing any of our future products currently under development may delay the purchase of one of our current products. In addition, customers may delay their purchasing decisions, or health care providers may refrain from providing our products, as a result of a global pandemic or unfavorable changes in general economic conditions.
If the Allurion Balloon, or any other therapy or product that we may develop, does not achieve and maintain widespread market acceptance, we may fail to achieve sales consistent with our projections, in which case our business, financial condition and operating results could be materially and adversely affected.
A substantial proportion of our sales are through third-party distributors, and we do not have direct control over the efforts these distributors may use to sell our products. If our relationships with these distributors deteriorate, or if these distributors fail to sell our products or engage in activities that harm our reputation, or fail to adhere to medical device regulations, our financial results may be negatively affected.
Historically, our sales model has been to sell primarily through distributors rather than through our own sales force, but recently we have begun to transition certain territories to both a direct sales model and a hybrid sales model that includes both distributors and a direct sales effort. We believe that our reliance on distributors
25
Table of Contents
improves the economics of our business, as we do not carry the high fixed costs of a large direct sales force in many of the countries in which the Allurion Balloon is commercially available. If we are unable to maintain or enter into such distribution arrangements on acceptable terms, or at all, we may not be able to successfully commercialize our products in certain countries. Furthermore, distributors can choose the level of effort that they apply to selling our products relative to others in their portfolio. The selection, training, and compensation of distributors’ sales personnel are within the distributor’s control rather than our own and may vary significantly in quality from distributor to distributor.
In addition, although our contract terms require our distributors to comply with all applicable laws regarding the sale of our products, including anti-competition, anti-money laundering, sanctions laws and FDA and other health care regulations, we may not be able to ensure proper compliance. If our distributors fail to effectively market and sell our products in full compliance with applicable laws, our results of operations and business may suffer.
In certain large markets, we engage in direct sales efforts. We may fail to maintain and develop our direct sales force, and our revenues and financial outcomes could suffer as a result. Furthermore, our direct sales personnel may not effectively sell our products.
We engage in direct sales efforts in over 20 countries. We have hired and will need to retain and motivate a significant number of sales and marketing personnel in order to support our anticipated growth in these and other new countries. There is significant competition for quality personnel experienced in such activities, including from companies with greater financial resources than ours. If we are not successful in our efforts to continue recruiting, retaining, and motivating such personnel, we may not be able to increase our revenues, or we may increase our expenses in greater measure than our revenues, negatively impacting our operating results.
We are also working on creating a direct sales structure and strategy in certain markets. We are working to put in place the correct legal and business structure to comply with taxation and operational requirements. These structures may not ultimately be implemented or, if implemented, be successful or effective and may not be able to increase our revenues or improve our gross margins. In addition, our expenses or tax-related costs may increase in greater measure than our revenues, negatively impacting our operating results.
Furthermore, our sales force may operate independently with limited day-to-day oversight from management. They may engage in sales practices that increase certain risks to our business, including the risk of scrutiny from regulatory authorities and the risk that we violate anti-corruption regulations in one or more countries. These and other independent actions may result in unexpected costs, news that might impair our reputation or revenues, litigation in various jurisdictions, and/or sanctions. Any of these could impair the trading price of our common stock and adversely impact our results.
The effectiveness and safety of the Allurion Balloon depends critically on our ability to educate health care providers on its safe and proper use. If we are unable to do so, we may not achieve our expected growth and may be subject to risks and liabilities.
In addition to educating health care providers on the clinical benefits of the Allurion Balloon, we must also train health care providers on the safe and appropriate use of the Allurion Balloon. If we are unable to provide an adequate training program with respect to the Allurion Balloon, product misuse may occur that could lead to serious adverse events. Many health care providers may be unfamiliar with such treatments or find it more complex than competitive products or alternative treatments. As such, there is a learning process involved for health care providers to become proficient in the use of our products and it may take several procedures for a health care provider to be able to use the Allurion Balloon comfortably. In addition, it is also critical for health care providers to be educated and trained on best practices in order to achieve optimal results, including patient selection and eligibility criteria, as well as complementary methods of use such as diet or behavioral modification programs. Convincing health care providers to dedicate the time and energy necessary for adequate training is
26
Table of Contents
challenging, and we cannot assure you that we will be successful in these efforts. This training process may also take longer than we expect. In the event that health care providers are not properly trained in the use of the Allurion Balloon, they may misuse or ineffectively use our products for the treatment of patients. As a result, patients may experience adverse events or not be able to enjoy the benefits of our program or achieve the weight loss outcomes they expect, leading to dissatisfaction and market rejection of our products. In addition, misuse of our products in any stage of the treatment may result in, among other things, patient injury, adverse side effects, negative publicity or lawsuits against us. Any of these events could have an adverse effect on our business and reputation.
The misuse or off-label use of our products may harm our image in the marketplace, result in injuries that lead to product liability suits or result in costly investigations and sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
The Allurion Balloon has been approved or cleared by regulatory authorities in the countries in which we sell it or in which we conduct our operations for specific indications. We do not promote the Allurion Balloon for uses outside of approved or cleared indications for use, known as “off-label uses.” We cannot, however, prevent a health care provider from using our product off-label, when in the health care provider’s independent professional medical judgment he or she deems it appropriate. There may be increased risk of injury to patients if health care providers attempt to use our product off-label. Furthermore, the use of our product for indications other than those approved or cleared by regulatory authorities may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
Health care providers may also misuse our products, use improper techniques, ignore or disregard product warnings, contraindications or other information provided in training materials or product labeling, fail to obtain adequate training, fail to inform patients of the risks associated with procedures that utilize our product or fail to solicit sufficient information from patients regarding their health status or medical histories, any of which may potentially lead to injury and an increased risk of product liability claims. If our product is misused or used with improper techniques or insufficient information, we may become subject to costly litigation by our health care providers or their patients. Moreover, if patients fail to disclose medical conditions or to follow the pre- and post-placement instructions, medication program, and dietary guidelines in connection with their treatment with the Allurion Balloon, there is the risk of injury. Such patients may also fail to achieve their desired results, which could harm our image in the marketplace.
There is no guarantee that the FDA or non-U.S. regulatory agencies will grant approval or clearance for our current or future products, including the Allurion Balloon. Failure to obtain regulatory approvals or clearances in the United States and other international jurisdictions, or revocation of approvals or clearances in those jurisdictions, will prevent us from marketing our products in such jurisdictions.
We intend to seek regulatory approval or clearance of our current and future products in the United States and certain non-U.S. jurisdictions. We have obtained a CE Mark for the Allurion Balloon and are therefore authorized to sell in the EU; however, in order to market in regions such as the United States, the Asia Pacific region and many other jurisdictions, we must obtain separate regulatory approvals or clearances.
The procedures for approval vary among countries and can involve additional clinical testing, and the time required to obtain approval or clearance may differ from that required to obtain the CE Mark or FDA approval. As a result of the United Kingdom leaving the EU, since January 1, 2021, the regulatory framework and regimes for medical devices in the United Kingdom and the EU have diverged. In particular, a new UKCA Mark was introduced for medical devices placed on the Great Britain market (which includes England, Scotland and Wales), and Northern Ireland adopted a hybrid approach as a result of the divergence in accordance with the Northern Ireland Protocol. Of note, on June 30, 2023, the UK Government introduced legislation, confirming that, subject to certain conditions, general medical devices compliant with the MDD with a valid declaration and
27
Table of Contents
CE mark can be placed on the Great Britain market up until the sooner of the expiry of the CE certificate or June 30, 2028, and general medical devices compliant with the Medical Device Regulation (“MDR”) with a valid declaration and CE mark can be placed on the Great Britain market up until June 30, 2030.
Moreover, clinical studies or manufacturing processes conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more international regulatory authorities does not ensure approval by regulatory authorities in other countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. An international regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain regulatory approvals on a timely basis, if at all. We may not be able to submit for regulatory approvals or clearances and even if we submit we may not receive necessary approvals or clearances to commercialize our products in any market.
Before obtaining regulatory approval or clearance for the sale of a product, we may be required to conduct extensive preclinical studies and clinical trials to demonstrate the safety and efficacy of our planned products in human patients. Preclinical studies and clinical trials can be expensive, difficult to design and implement, can take many years to complete, and are uncertain as to outcome. A failure of one or more of our trials could occur at any stage of testing. In connection with the initiation of a clinical trial in the U.S., we filed an investigational device exemption (“IDE”) application, which was approved by the FDA in 2016. After we conducted that trial and submitted a premarket approval (“PMA”) application to the FDA, in 2020, the FDA requested additional data. Therefore, we withdrew the PMA application and in 2021 submitted an IDE application for our AUDACITY trial, which the FDA approved in 2021. We are currently conducting that clinical trial.
Numerous unforeseen events during, or as a result of, preclinical and clinical trials could occur, which would delay or prevent our ability to receive regulatory approval or commercialize the Allurion Balloon or any of our future products, including the following:
| • | preclinical studies and clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional studies or abandon product development programs; |
| • | the number of patients required for clinical trials may be larger than we anticipate, enrollment in these clinical trials may be insufficient or slower than we anticipate, or patients may drop out of these clinical studies at a higher rate than we anticipate; |
| • | the cost of preclinical studies and clinical trials may be greater than we anticipate; |
| • | third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | we might suspend or terminate clinical trials of our products for various reasons, including a finding that our products have unanticipated serious side effects or other unexpected characteristics, or that the trial subjects are being exposed to unacceptable health risks; |
| • | regulators may not approve our proposed clinical development plans; |
| • | regulators or independent institutional review boards (“IRBs”) may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| • | regulators or IRBs may require that we, or our investigators, suspend or terminate clinical studies for various reasons, including non-compliance with regulatory requirements; |
| • | regulators in countries where our products are currently marketed may require that we suspend commercial distribution if there is non-compliance with regulatory requirements or safety concerns; |
| • | the supply or quality of our products or other materials necessary to conduct clinical studies of our products may be insufficient or inadequate; and |
28
Table of Contents
| • | the enactment of new regulatory requirements in the EU under the MDR effective since May 26, 2021 may make approval times longer and standards more difficult to pass. In particular, manufacturers are required to: (i) assign a unique device identification (“UDI”) to a medical device before it is placed on the EU market in order to improve traceability of the medical device; and (ii) register themselves, the medical device and the UDI, among other things, with a new European medical device database. |
If we or any future collaboration or distribution partner are required to conduct additional clinical trials or other testing of the Allurion Balloon or any future products, those clinical studies or other testing may not be successfully completed. If the results of these studies or tests are not positive, or are only modestly positive or if they raise safety concerns, we may:
| • | be delayed in obtaining marketing approvals for the Allurion Balloon or our future products; |
| • | not obtain marketing approval at all; |
| • | obtain approval for indications that are not as broad as desired; |
| • | have a product removed from the market after obtaining marketing approval; or |
| • | be subject to restrictions on how the product is distributed or used. |
Even if we obtain regulatory approvals or clearances in a jurisdiction, our products may be removed from the market due to a variety of factors, including adverse events, recalls, suspension of regulatory clearance to sell, or other factors.
Although we launched the Allurion Balloon commercially in January 2016 and have placed over 150,000 units to date in various countries outside the U.S., we do not have as much post-market surveillance data as our competitors and may not have clearly identified all possible or actual risks of our products. Furthermore, if our clinical trials do not produce patient data that compares favorably with products that are already on the market, health care providers and patients may opt not to use our products, and our business would suffer.
Our product development costs will also increase if we experience delays to our clinical trials or approvals.
Significant clinical trial delays also could allow our competitors to bring products to market before we do, which would impair our ability to commercialize our products and harm our business and results of operations.
The Allurion Balloon is not currently approved for commercial sale in the United States. Obtaining such approval is costly and time consuming, and we may not obtain the regulatory approval required to sell our products in the U.S.
Neither we, nor any future collaboration or distributor partner, can commercialize the Allurion Balloon in the U.S. without first obtaining regulatory approval from the FDA. Extensive preclinical and clinical testing is required to support FDA approval.
The FDA approval process is expensive and will take at least several years to complete, and FDA approval may never be obtained. We must also demonstrate that our manufacturing facilities, processes and controls are adequate to support FDA approval and that our clinical investigators complied with good clinical practices in the conduct of the Allurion Balloon clinical trial.
The FDA has substantial discretion in the approval process. Despite the time and expense exerted, failure may occur at any stage, and we could encounter problems that cause us to abandon or repeat clinical trials. The FDA can delay, limit, or deny approval of a product for many reasons, including, but not limited, to:
| • | a product may not be deemed to be safe and effective; |
| • | the FDA may not find the data from clinical trials and preclinical studies sufficient; |
29
Table of Contents
| • | the opportunity for bias in the clinical trials as a result of the open-label design may not be adequately handled and may cause our trial to fail; |
| • | the FDA may not approve suppliers’ processes or facilities; or |
| • | the FDA may change its approval policies or adopt new regulations. |
If the Allurion Balloon or our future products fail to demonstrate safety and efficacy in further clinical trials that may be required for FDA approval, or do not gain regulatory approval, our business and results of operations will be harmed.
Additionally, we expect that the initial FDA approval of the Allurion Balloon, if obtained, will be subject to a lengthy and expensive follow-up period, during which we must monitor patients enrolled in clinical studies and collect data on their safety outcomes. Even if FDA approval is obtained, the FDA has authority to impose post-market approval conditions, which can include (i) restrictions on the device’s sale, distribution, or use, (ii) continuing evaluation of the device’s safety and efficacy, (iii) additional warning/hazard labeling requirements, (iv) significant record management, (v) periodic reporting requirements, and (vi) any other requirements the FDA determines necessary to provide reasonable assurance of the device’s safety and effectiveness.
Completion of this follow-up trial, in a manner which results in data sufficient to maintain FDA approval, is subject to multiple risks, many of which are outside of our control. These include, but are not limited to, our ability to fund the ongoing trial from our operations or via additional fundraising; trial participants’ willingness and ability to return for follow-up trial visits; and maintenance of a suitable trial database over a long period of time. Even if completed and appropriately evaluated, the trial follow-up may reveal safety or other issues that impact the approved labeling, or may result in withdrawal of the Allurion Balloon from the marketplace in the U.S. or elsewhere.
Even if clinical trials demonstrate acceptable safety and efficacy for the Allurion Balloon in some patient populations, the FDA or similar regulatory authorities outside the U.S. may not approve the marketing of the Allurion Balloon or may approve it with restrictions on the label, which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
It is possible the FDA or similar regulatory authorities may not consider the results of our clinical trials to be sufficient for approval of the Allurion Balloon for our desired indications for use. Moreover, even if the FDA or other regulatory authorities approve the marketing of the Allurion Balloon, the approval may include additional restrictions on the label that could make the Allurion Balloon less attractive to health care providers and patients compared to other products that may be approved for broader indications, which could limit potential sales of the Allurion Balloon.
If we fail to obtain FDA or other regulatory approval of the Allurion Balloon, or if the approval is narrower than what we seek, it could impair our ability to realize value from the Allurion Balloon, and therefore may have a material adverse effect on our business, financial condition, results of operations and growth prospects.
The results of preclinical studies and earlier clinical trials may not be predictive of the results of later preclinical studies and clinical trials, and the results of our current and future clinical trials may not satisfy the requirements of the FDA or other comparable regulatory authorities. If we cannot replicate the positive results from our preclinical studies or earlier clinical trials of the Allurion Balloon in our current or future clinical trials, we may be unable to successfully develop, obtain regulatory approval for and commercialize our current or future product candidates.
We will be required to demonstrate with sufficient valid scientific evidence through well-controlled clinical trials, that our product candidates are safe and effective for their intended uses before we can seek marketing approvals for their commercial sale. Positive results from our preclinical studies of the Allurion Balloon, and any
30
Table of Contents
positive results we may obtain from our early clinical trials of our current or future product candidates, may not necessarily be predictive of the results from subsequent preclinical studies and clinical trials. Similarly, even if we are able to complete our planned preclinical studies or any clinical trials of the Allurion Balloon according to our current development timeline, the positive results from such preclinical studies and clinical trials of the Allurion Balloon may not be replicated in subsequent preclinical studies or clinical trial results.
Additionally, several of our past, planned and ongoing clinical trials utilize an “open-label” trial design. An “open-label” clinical trial is one where both the patient and investigator know whether the patient is receiving the investigational product candidate. Open-label clinical trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label clinical trials are aware when they are receiving treatment. Open-label clinical trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. In addition, open-label clinical trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. The results from an open-label trial may not be predictive of future clinical trial results with any of our product candidates for which we include an open-label clinical trial when studied in a controlled environment with a sham procedure.
Many companies in the pharmaceutical, biotechnology and medical device industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in preclinical studies and clinical trials, including previously unreported adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain approval from the FDA or a comparable foreign regulatory authority. If we fail to produce positive results in our planned preclinical studies or clinical trials of the Allurion Balloon, the development timeline and regulatory approval and commercialization prospects for the Allurion Balloon, and, correspondingly, our business and financial prospects, would be materially adversely affected. Thus, even if the results from our initial research and preclinical activities appear positive, we do not know whether subsequent clinical trials we may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market the Allurion Balloon.
Commercial success of the Allurion Balloon in the United States or elsewhere depends on our ability to accurately forecast customer demand and manufacture sufficient quantities of product that patients and health care providers request, and to manage inventory effectively. The failure to do so could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Manufacturing of the Allurion Balloon requires capital expenditures and a highly-skilled workforce. There is a significant lead time to build and certify a new manufacturing facility. Although we believe our current facilities will give us adequate manufacturing capacity to meet demand for at least the next two years, we have, in the past, been unable to fill all incoming orders to meet growing demand. If we obtain FDA approval, we intend to rely on our existing manufacturing facilities to supply products in the U.S. If demand increases faster than we expect, or if we are unable to produce the quantity of goods that we expect with our current facilities, we may not be able to grow revenue at an optimal rate. There may be other negative effects from supply shortages, including loss of our reputation in the marketplace and a negative impact on our relationships with our distributors, which could have a material adverse effect on our business, financial condition, results of operations, and growth prospects.
On the other hand, if demand for our products declines, or if market supply surpasses demand, we may not be able to reduce manufacturing expenses or overhead costs proportionately. We have invested significantly in our manufacturing capacity. If an increase in supply outpaces the increase in market demand, or if demand decreases, the resulting oversupply could adversely impact our sales and result in the underutilization of our
31
Table of Contents
manufacturing capacity, higher inventory carrying costs and associated working capital, changes in revenue mix, and/or price erosion, any of which would lower our margins and adversely impact our financial results, which could have a material adverse effect on our business, financial condition, results of operations, and growth prospects.
Our business depends on maintaining our brand, reputation, and ongoing demand for our products and services, and a significant reduction in sentiment or demand could affect our results of operations.
Our success depends on awareness and the reputation of our brand, which depends on factors such as the safety and quality of our products, our communication activities, including marketing and education efforts, customer acquisition and retention strategies, and our management of our heath care provider and patient experience. Maintaining, promoting and positioning our brand is important to expanding our customer base. This will depend largely on the success of our education and marketing efforts and our ability to provide a consistent, high-quality experience to health care providers and patients. If we do not successfully continue our education and marketing efforts, particularly to health care systems and large institutions, or if existing users decrease their level of engagement, our revenue, financial results and business may be significantly harmed.
A decrease in customer retention, growth or engagement with our products may have a material and adverse impact on our revenue, business, financial condition and results of operations. Any number of factors could negatively affect customer retention, growth and engagement, including:
| • | customers increasingly engaging with competing products; |
| • | inability to maintain high quality products, including any failure to introduce new and improved products; |
| • | inability to continue to develop or maintain applications for mobile devices that customers find engaging, that work with a variety of mobile operating systems and networks and that achieve a high level of market acceptance; |
| • | changes in customer sentiment about the safety, quality or usefulness of our products, including concerns related to privacy and data sharing, security or other factors; |
| • | inability to manage and prioritize information to ensure customers are presented with content that is engaging, useful and relevant to them; |
| • | adverse changes in our products that are mandated by legislation or regulatory agencies, both in the United States and internationally; or |
| • | technical or other problems preventing us from delivering products in a rapid and reliable manner or otherwise affecting the user experience. |
We may need to make substantial investments in the areas of education and marketing in order to maintain and enhance our brand and awareness of our products. Ineffective marketing, negative publicity, significant discounts by our competitors, product defects, serious adverse events and related liability litigation, failure to obtain regulatory approval or clearance for our products, counterfeit products, unfair labor practices and failure to protect our intellectual property rights are some of the potential threats to the strength of our business. We may need to make substantial expenditures to mitigate the impact of such threats.
We believe that maintaining and enhancing awareness of our products and brand in the countries in which we currently sell our products and in new countries where we have limited awareness or brand recognition is important to expanding our customer base. As such, our growth will depend on the further development and commercialization of our current products, and marketing authorization of our future products. If we are unable to increase awareness of our products or enhance the strength of our brand in the countries in which we currently sell our products and in new countries in a timely manner, then our growth strategy could be adversely affected.
32
Table of Contents
Risks Related to our Business and Industry
The weight loss and obesity management industries are highly competitive. We also compete with companies that make weight loss drugs and other weight loss solutions outside the medical device industry. If our competitors are able to develop and market products that are safer, more effective, easier to use or more readily adopted by patients and health care providers, our commercial opportunities will be reduced or eliminated.
The weight loss and obesity management industries are highly competitive, subject to rapid change and significantly affected by new product introductions, results of clinical research, corporate combinations, actions by regulatory bodies, changes by public and private payers and other factors relating to our industry. We compete both with companies that offer medical devices as a weight loss therapy as well as companies that make weight loss drugs and other weight loss solutions outside the medical device industry. Because of the market opportunity and the high growth potential of the non-surgical device market for weight loss and obesity, in particular recent pharmaceutical therapies known as GLP-1s, competitors and potential competitors have historically dedicated, and will continue to dedicate, significant resources to aggressively develop and commercialize their products. Any one of these factors could reduce the demand for our devices or services or require substantial resources and expenditures for research, design and development to avoid technological or market obsolescence.
Outside the U.S., we compete with a variety of local and regional competitive intragastric balloon manufacturers including SC MedSil, Medicone and Spatz Laboratories. In the U.S., there are three manufacturers with an intragastric balloon approved by the FDA at this time: Boston Scientific Corporation, ReShape Lifesciences, Inc. and Spatz FGIA Inc. All of these balloons require endoscopy and anesthesia for placement and/or removal.
We also compete against the manufacturers of pharmaceuticals that are directed at treating weight loss, such as NovoNordisk A/S, Eli Lilly & Co., Roche Holding AG, GlaxoSmithKline plc, Arena Pharmaceuticals, Inc., VIVUS, Inc. and Orexigen Therapeutics, Inc.
At any time, these or other competitors may introduce new or alternative products that compete directly or indirectly with our products and services. They may also develop and patent products and processes earlier than we can or obtain regulatory clearance or approvals before we are able to obtain required approvals, which could impair our ability to develop and commercialize similar products or services. If clinical outcomes of procedures performed with our competitors’ products are, or are perceived to be, superior to the outcomes of treatments performed with our products, sales of our products could be negatively affected and our business, results of operations and financial condition could suffer.
Our success will depend on our ability to enhance our current products and technologies and develop or acquire and market new products and technologies to keep pace with technological developments and evolving industry standards, while responding to changes in customer needs. A failure to adequately develop or acquire device enhancements or new devices that will address changing technologies and customer requirements adequately, or to introduce such devices on a timely basis, may have a material adverse effect on our business, financial condition and results of operations.
Many of our competitors, or their parent companies, have significantly greater financial and other resources than we do, as well as:
| • | well-established reputations and name recognition with key opinion leaders and health care provider networks; |
| • | an established base of long-time customers with strong brand loyalty; |
| • | products supported by long-term data; |
33
Table of Contents
| • | longer operating histories; |
| • | significantly larger installed bases and distributors and established distribution channels; |
| • | greater existing market share in the obesity and weight management market; |
| • | broader product offerings; |
| • | greater ability to cross-sell products; |
| • | the ability to offer rebates or bundle products to offer higher discounts or incentives; and |
| • | more experience in conducting research and development, manufacturing, performing clinical trials and obtaining regulatory approvals or clearances. |
We might have insufficient financial resources to improve existing devices, advance technologies, develop new devices, and market them at competitive prices. Technological advances by one or more competitors or future entrants into the field may result in our current devices becoming non-competitive or obsolete, which may decrease revenues and profits and adversely affect our business and results of operations. Competition with these companies could result in significant price-cutting, reduced profit margins and loss of market share, any of which would harm our business, financial condition and results of operations. In addition, competitors with greater financial resources than ours could acquire other companies to gain enhanced name recognition and market share, as well as new technologies or products that could effectively compete with our existing and future products, which may cause our revenues to decline and harm our business. In addition, many of our competitors are well-established manufacturers with significant resources and may engage in aggressive marketing tactics. Competitors may also possess the ability to commercialize additional lines of products, bundle products or offer higher discounts and incentives to customers in order to gain a competitive advantage. If the prices of competing products are lowered as a result, we may not be able to compete effectively.
Continued international expansion of our business will expose us to business, regulatory, political, operational, financial and economic risks associated with doing business internationally.
Our products are registered to be sold in over 50 countries, and we operate subsidiaries in Australia, France, the United Arab Emirates, Hong Kong, the United Kingdom, Italy, Spain, Australia and Mexico. Our business strategy contemplates continued international expansion in key markets, including partnering with medical device distributors and introducing the Allurion Balloon and other products outside the U.S. The sale and shipment of our products internationally, as well as the purchase of components from international sources, subjects us to potential trade, export, import and customs, and economic sanctions regulations and laws.
Compliance with these regulations and laws is costly and exposes us to penalties for non-compliance. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export or import privileges, seizure of shipments, restrictions on certain business activities and exclusion or debarment from government contracting. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our shipping and sales activities.
In addition, several of the countries in which we sell our products or conduct our operations are, to some degree, subject to political, economic or social instability. Doing business in other countries outside the U.S. involves a number of other risks, including:
| • | compliance with the free zone regime regulations under which the manufacturing sites operate; |
| • | different regulatory requirements for device approvals in international markets; |
| • | multiple, conflicting and changing laws and regulations such as tariffs and tax laws, export and import restrictions, employment laws, and other governmental approvals, permits and licenses; |
34
Table of Contents
| • | potential failure by us or our distributors to obtain and/or maintain regulatory approvals for the sale or use of our products in various countries; |
| • | difficulties in managing global operations; |
| • | logistics and regulations associated with shipping products, including infrastructure conditions and transportation delays; |
| • | limits on our ability to penetrate international markets if our distributors do not execute successfully; |
| • | governmental price controls, differing reimbursement regimes and other market regulations; |
| • | financial risks, such as longer payment cycles, difficulty enforcing contracts and collecting accounts receivable; |
| • | reduced protection for intellectual property rights, or lack of them in certain jurisdictions, forcing more reliance on our trade secrets, if available; |
| • | economic weakness, political and economic instability, including wars, terrorism and political unrest (such as attacks on commercial ships by Houthi rebels), outbreak of disease, boycotts, curtailment of trade and other business restrictions; |
| • | failure to comply with the Foreign Corrupt Practices Act (the “FCPA”), including its books and records provisions and its anti-bribery provisions, by maintaining accurate information and control over sales activities and distributors’ activities; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | compliance with tax, employment, immigration and labor laws; |
| • | taxes, including withholding of payroll taxes; |
| • | currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in the U.S.; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business and shipping interruptions resulting from natural or other disasters including earthquakes, volcanic activity, hurricanes, floods and fires. |
Any of these risks, if encountered, could harm our future international expansion and operations and, consequently, have an adverse effect on our financial condition, results of operations and cash flows.
We depend on a limited number of single source suppliers to manufacture our components, sub-assemblies and materials, which makes us vulnerable to supply shortages and price fluctuations.
We rely on single source suppliers for some of the components, sub-assemblies and materials for our products. These components, sub-assemblies and materials are critical and, for certain items, there are relatively few alternative sources of supply. These single source suppliers may be unwilling or unable to supply the necessary materials and components reliably and at the levels we anticipate or that are required by the market. We also have two suppliers with which we do not maintain a formal contractual relationship. We typically have at least a six month supply of the materials provided by each of these suppliers but we cannot guarantee that we could find an alternative before our inventory ran out and therefore the loss of these relationships could cause a substantial disruption to our business. We would also have little to no recourse if one of these two suppliers became unwilling or unable to continue to supply materials. While our suppliers have generally met our demand for their products and services on a timely basis in the past, we cannot guarantee that they will in the future be
35
Table of Contents
able to meet our demand for their products, either because of an increase in the level of such demand, acts of nature, the nature of our agreements with those suppliers or our relative importance to them as a customer. Our suppliers may decide in the future to discontinue or reduce the level of business they conduct with us.
We have not qualified or obtained necessary regulatory approvals for additional suppliers for some of these components, sub-assemblies and materials, but we do carry a significant inventory of these items ourselves. While we believe that alternative sources of supply or sterilization may be available, we cannot be certain whether they will be available if and when we need them, or that any alternative suppliers or providers would be able to provide the quantity and quality of components, materials and sterilization that we would need to manufacture and ship our products if our existing suppliers and providers were unable to satisfy our requirements. To utilize other sources, we would need to identify and qualify new providers to our quality standards and obtain any additional regulatory approvals required to change providers, which could result in manufacturing delays and increase our expenses.
Our dependence on third parties subjects us to a number of risks that could impact our ability to manufacture our products and harm our business, including:
| • | interruption of supply or sterilization resulting from modifications to, or discontinuation of, a third party’s operations; |
| • | delays in product shipments resulting from uncorrected defects, reliability issues or a third party’s failure to produce components or complete sterilizations that consistently meet our quality specifications; |
| • | price fluctuations due to a lack of long-term supply arrangements with our third parties for key components or sterilization requirements; |
| • | inability to obtain adequate supply or services in a timely manner or on commercially reasonable terms; |
| • | difficulty identifying and qualifying alternative third parties for the supply of components; |
| • | inability of third parties to comply with applicable provisions of the FDA’s QSR, or other applicable laws or regulations enforced by the FDA, foreign and state regulatory authorities; |
| • | inability to ensure the quality of products manufactured or sterilization conducted by third parties; |
| • | production delays related to the evaluation and testing of products and services from alternative third parties and corresponding regulatory qualifications; and |
| • | delays in delivery by our suppliers and service providers. |
Although we require our third-party suppliers and providers to supply us with components and services that meet our specifications and other applicable legal and regulatory requirements in our agreements and contracts, and we perform incoming inspection, testing or other acceptance activities to ensure the components meet our requirements, there is a risk that these third parties will not always act consistently with our best interests, and may not always supply components or provide services that meet our requirements or in a timely manner.
Negative publicity, product defects and any resulting litigation concerning our products or our competitors’ products could harm our reputation and reduce demand for the Allurion Balloon, either of which could negatively impact our financial results.
The responses of potential patients, health care providers, the media, legislative and regulatory bodies and others to information about complications or alleged complications of our products, or products liability litigation against us or our competitors, could result in negative publicity and could materially reduce market acceptance of our products. These responses or any investigations and potential resulting negative publicity may have a
36
Table of Contents
material adverse effect on our business and reputation and negatively impact our financial condition, results of operations or the market price of our common stock. In addition, significant negative publicity could result in an increased number of product liability claims against us.
We depend on our senior management team and the loss of one or more key employees or an inability to attract and retain highly skilled employees could harm our business.
Our success largely depends upon the continued services of our executive management team and key employees and the loss of one or more of our executive officers or key employees could harm us and directly impact our financial results. Although we have entered into employment agreements with some of our executive officers and key employees, each of them may terminate their employment with us at any time. Changes in our executive management team resulting from the hiring or departure of executives could disrupt our business. We could experience disruptions as each of these individuals begins to integrate into the business and build his or her respective departments. In addition, our Chief Executive Officer, Shantanu Gaur, has been with us since inception and has been instrumental in building operational capabilities, raising capital and guiding product development and regulatory strategy. If Dr. Gaur was no longer working at our company, our industry credibility and operational capabilities would be harmed.
To execute our growth plan, we must attract and retain highly qualified personnel. Competition for skilled personnel is intense, especially for engineers with high levels of experience in designing and developing medical devices and for sales executives. We have, from time to time, experienced, and we expect to continue to experience, difficulty in hiring and retaining employees with appropriate qualifications. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize medical devices.
Many of the companies with which we compete for experienced personnel have greater resources than we have. If we hire employees from competitors or other companies, their former employers may attempt to assert that these employees or we have breached legal obligations, resulting in a diversion of our time and resources and, potentially, damages. In addition, job candidates and existing employees often consider the value of the stock awards they receive in connection with their employment. If the perceived value of our stock awards declines, either because we are a public company or otherwise, it may harm our ability to recruit and retain highly skilled employees. In addition, we invest significant time and expense in training our employees, which increases their value to competitors who may seek to recruit them. If we fail to attract new personnel or fail to retain and motivate our current personnel, our business and future growth prospects would be harmed.
We may acquire other businesses or form joint ventures or make investments in other companies or technologies in the future. If we are not successful in integrating these businesses, as well as identifying and controlling risks associated with the past operations of these businesses, we may incur significant costs, receive penalties or other sanctions from various regulatory agencies, and/or incur significant diversions of management time and attention.
We believe our business growth will be enhanced if we continually seek opportunities to enhance and broaden our product offerings. As part of our business strategy, we may pursue acquisitions or licenses of assets, or acquisitions of businesses. We also may pursue strategic alliances and joint ventures that leverage our core technology and industry experience to expand our product offerings or sales and distribution resources.
However, we may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent
37
Table of Contents
liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have an adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company may also disrupt ongoing operations and require management resources that would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a negative effect on our results of operations.
We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, license, strategic alliance or joint venture. To finance such a transaction, we may choose to issue our common stock as consideration, which would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our common stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings, royalty or debt financings. Additional funds may not be available on terms that are favorable to us, or at all.
We do not know whether we will be able to successfully integrate any acquired business, product or technology. The success of any given acquisition may depend on our ability to retain any key employees related thereto, and we may not be successful at retaining or integrating such key personnel. Integrating any business, product or technology we acquire could be expensive and time-consuming, disrupt our ongoing business, impact our liquidity, and/or distract our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business may suffer. Whether as a result of unsuccessful integration, unanticipated costs, including those associated with assumed liabilities and indemnification obligations, negative accounting impact, or other factors, we may not realize the economic benefits we anticipate from acquisitions. In addition, any amortization or charges resulting from the costs of acquisitions could increase our expenses.
If changes in the economy and/or consumer spending, consumer preference and other trends reduce consumer demand for our products, our sales and profitability would suffer.
We are subject to the risks arising from adverse changes in general economic and market conditions. Certain elective procedures, including those for weight loss, are typically not covered by insurance. Adverse changes in the economy may cause consumers to reassess their spending choices, which could have an adverse effect on consumer spending, reduce the demand for these procedures, and therefore have an adverse effect on our revenues. Furthermore, consumer preferences and trends may shift due to a variety of factors, including changes in demographic and social trends, public health initiatives and product innovations, which may reduce consumer demand for our products.
Our overall performance depends, in part, on worldwide economic conditions. In recent quarters, we have observed increased economic uncertainty in the U.S. and abroad. Impacts of such economic weakness include:
| • | falling overall demand for goods and services, leading to reduced profitability; |
| • | reduced credit availability; |
| • | higher borrowing costs; |
| • | reduced liquidity; |
| • | volatility in credit, equity and foreign exchange markets; and |
| • | bankruptcies. |
These developments could lead to supply chain disruption, inflation, higher interest rates, and uncertainty about business continuity, which may adversely affect our business and our results of operations. As our customers react to global economic conditions and the potential for a global recession, we may see them reduce spending on our products and take additional precautionary measures to limit or delay expenditures and preserve capital and liquidity. Reductions in spending on our products, delays in purchasing decisions, failure to complete
38
Table of Contents
the Allurion Program, and inability to attract new customers, as well as pressure for extended billing terms or pricing discounts, would limit our ability to grow our business and negatively affect our operating results and financial condition.
Changes in our business and operations have placed, and may continue to place, significant demands on our management team and infrastructure. If we fail to manage these demands effectively, we may be unable to execute our business plan, maintain high levels of customer and patient satisfaction, or address competitive challenges adequately.
Our business, headcount, and operations have grown, in the United States and abroad, since our inception, and we anticipate operational changes in the future as we enhance our product development efforts and refine our marketing and distribution strategies. While we expect to continue to grow headcount and operations over the long-term, in January 2024 we announced a restructuring plan designed to more closely align our cost structure with near-term revenue expectations, improve our capital structure, and accelerate the path to profitability (the “Plan”). The Plan anticipates a total reduction of approximately 30% of our global workforce as of December 2023, and we may be unable to manage effectively the changes to the business and potential disruption occasioned by such reductions. The implementation of the Plan, including the impact of a leaner organization, may result in delays in delivering our products and services, declines in customer and patient satisfaction, loss of customers, or difficulties in executing new strategies such as new sales and marketing strategies. We may experience employee attrition, decreased employee morale, and difficulty recruiting and retaining new employees in the future, all of which will require the time and attention of our management team. In addition, our ability to complete the restructuring plan and achieve the anticipated benefits from the Plan within the expected time frame, or at all, are subject to successful execution of management’s estimates and assumptions and may vary materially from our expectations, including as a result of factors that are beyond our control. If we do not realize the expected benefits of the Plan on a timely basis, or at all, our business, results of operations and financial condition could be adversely affected. Furthermore, following completion of the Plan, our business may not be more efficient or flexible than prior to implementation.
In the future, should demand for our products and services significantly increase, including as a result of regulatory approvals in the United States and elsewhere, we may need to increase the number of our employees and the scope of our operations, particularly in the areas of regulatory affairs and sales and marketing. We also intend, now and in the future, to continue to improve our operational, financial and management controls and reporting systems and procedures, which may require additional personnel and capital investments and will increase our costs. Such business growth could place a strain on our existing administrative and operational infrastructure, and we may not be able to make improvements to our personnel infrastructure in an efficient or timely manner, or at all. In addition, we may discover deficiencies in existing systems and controls.
Some new personnel likely would be operating in countries outside the jurisdiction of our corporate headquarters, which adds additional complexity, and may require us to expand our facilities. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Managing personnel across a global enterprise requires expertise and resources and places a strain on our management, administrative and financial infrastructure. Our failure to effectively manage change and accomplish any of these tasks could delay the execution of our business plans or disrupt our operations, and prevent us from growing successfully. We may also be exposed or subject to additional unforeseen or undisclosed liabilities as well as increased levels of indebtedness.
We may be subject to substantial warranty or product liability claims or other litigation in the ordinary course of business that may adversely affect our business, financial condition and operating results.
We face an inherent risk of product liability exposure related to the sale of the Allurion Balloon and any products in clinical trials. The marketing, sale and use, misuse or off-label use of the Allurion Balloon and our other current and future products could lead to the filing of product liability claims against us if someone alleges
39
Table of Contents
that our products failed to perform as designed or caused significant adverse events in patients. We may also be subject to liability for a misunderstanding of, or inappropriate reliance upon, the information we provide. If we cannot successfully defend ourselves against claims that the Allurion Balloon or our other current or future products caused injuries, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any products we may develop; |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of patients from clinical trials or cancellation of trials; |
| • | significant costs to defend the related litigation and distraction to our management team; |
| • | substantial monetary awards to plaintiffs; |
| • | loss of revenue; and |
| • | the inability to commercialize any products that we may develop. |
We currently hold $5.0 million in product liability insurance coverage, which may not be adequate to cover all liabilities we may incur. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Any future collaboration agreements (including with respect to product distribution or commercialization) we may enter into with respect to our current or future products may place the development or commercialization of such products outside our control, or may otherwise be on terms unfavorable to us.
We may enter into additional collaboration agreements with third parties with respect to our current or future products, including for distribution or commercialization in or outside the U.S. Our likely collaborators for any distribution, marketing, licensing or other collaboration arrangements include large and mid-size medical device and diagnostic companies, regional and national medical device and diagnostic companies, and distribution or group purchasing organizations. We will have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our products. Our ability to generate revenue from these arrangements will depend in part on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
We rely on third parties to conduct certain components of our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, which could interfere with or delay our ability to get regulatory approval or commercialize our products.
We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to perform various functions for our clinical trials. Our reliance on third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. We remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the International Council for Harmonization and the FDA require us to comply with standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to ensure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of patients in clinical trials are protected. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our planned products and will not be able to, or may be delayed in our efforts to, successfully commercialize our planned products.
40
Table of Contents
The failure of third parties to meet their contractual, regulatory, and other obligations could adversely affect our business.
We rely on suppliers, vendors, outsourcing partners, consultants, alliance partners and other third parties to research, develop, manufacture and commercialize our products and manage certain parts of our business. Using these third parties poses a number of risks, such as: (i) they may not perform to our standards or legal requirements; (ii) they may not produce reliable results; (iii) they may not perform in a timely manner; (iv) they may not maintain confidentiality of our proprietary information; (v) disputes may arise with respect to ownership of rights to technology developed with our partners; and (vi) disagreements could cause delays in, or termination of, the research, development or commercialization of our products or result in litigation or arbitration.
Moreover, some third parties are located in markets subject to political and social risk, corruption, infrastructure problems and natural disasters, in addition to country-specific privacy and data security risk given current legal and regulatory environments. Failure of third parties to meet their contractual, regulatory, and other obligations may materially affect our business.
We have significant exposure to the economic and political situations in emerging market countries, and developments in these countries could materially impact our financial results, or our business more generally.
Many of the countries in which our products are sold are emerging markets. Our global growth strategy contemplates the expansion of our existing sales activities in Latin America, the Middle East, Africa and the Asia-Pacific region. Our exposure to emerging markets has increased in recent years, as have the number and importance of our distributor arrangements. Economic and political developments in the emerging markets, including economic crises, currency inflation, or political instability, have had in the past, and may have in the future, a material adverse effect on our financial condition and results of operations. Moreover, as these markets continue to grow, competitors may seek to enter these markets and existing market participants will likely try to aggressively protect or increase their market shares. Increased competition may result in price reductions, reduced margins and our inability to gain or hold market share, which could have an adverse effect on our financial condition and results of operations.
Increasing scrutiny and changing expectations from investors with respect to our environmental, social and governance practices may impose additional costs on us or expose us to reputational or other risks.
Investors have increased their emphasis on the environmental, social and governance (“ESG”) practices of companies across all industries, including the environmental impact of operations and human capital management. Certain stockholders use third-party benchmarks or scores to measure a company’s ESG practices and decide whether to invest in its common stock or engage with the company to require changes to its practices.
A failure to comply with investor expectations and standards, which are evolving and vary considerably, or the perception that we have not responded appropriately to the growing concern for ESG issues, could result in reputational harm to our business and could have an adverse effect on us.
Risks Related to Government Regulation
The regulatory approval process is expensive, time consuming and uncertain, and may prevent us from obtaining approvals for the commercialization of the Allurion Balloon or our planned products.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of medical devices are subject to extensive regulation by the FDA and other regulatory authorities in the U.S. and other countries, where regulations differ from country to country. Our products are registered to be sold in over 50 countries, but we are not permitted to market our products in the U.S. until we receive the requisite approval or clearance from the FDA; we have not received such FDA approval to date. In addition, failure to comply with
41
Table of Contents
FDA and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including the following:
| • | warning or untitled letters; |
| • | civil or criminal penalties and fines; |
| • | injunctions; |
| • | suspension or withdrawal of regulatory approval; |
| • | suspension of any ongoing clinical trials; |
| • | voluntary or mandatory product recalls and publicity requirements; |
| • | refusal to accept or approve applications for marketing approval of new devices or supplements to approved applications filed by us; |
| • | restrictions on operations, including costly new manufacturing requirements; or |
| • | seizure or detention of our products or import bans. |
Prior to receiving approval to commercialize any of our products in the U.S. or abroad, we may be required to demonstrate with substantial evidence from preclinical and well-controlled clinical trials, to the satisfaction of the FDA or other regulatory authorities abroad, that such products are safe and effective for their intended uses. Results from preclinical studies and clinical trials can be interpreted in different ways. Even if we believe the preclinical or clinical data for our products are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities. Administering any of our products to humans may produce undesirable side effects, which could interrupt, delay or cause suspension of clinical trials of our planned products and result in the FDA or other regulatory authorities denying approval of our products for any or all targeted indications.
Regulatory approval from the FDA is not guaranteed, and the approval process is expensive and may take several years. The FDA also has substantial discretion in the approval process. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat clinical trials, or perform additional preclinical studies and clinical trials. For example, we previously conducted a clinical trial on the Allurion Balloon and submitted a PMA application based on data from that trial. When the FDA requested additional data, we withdrew the PMA application and sought FDA approval to conduct our AUDACITY trial, which the FDA granted in 2021. We are currently conducting that clinical trial. The number of preclinical studies and clinical trials that will be required for FDA approval varies depending on the product, the indication that the product is designed to address and the regulations applicable to any particular product. The FDA can delay, limit or deny approval of a planned product for many reasons, including, but not limited to, the following:
| • | a planned product or one or more of its features may not be deemed safe or effective; |
| • | the FDA may not find the data from preclinical studies and clinical trials sufficient; |
| • | the FDA might not approve our manufacturing or our third-party supplier’s processes or facilities; or |
| • | the FDA may change its approval policies or adopt new regulations. |
If the Allurion Balloon or any of our other products fail to demonstrate safety and efficacy in preclinical studies and clinical trials or do not gain requisite regulatory approval, our business and results of operations will likely be harmed.
42
Table of Contents
Inadequate funding for the FDA, the SEC and other government agencies, including from government shutdowns, or other disruptions to these agencies’ operations, could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, the ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new product candidates to be reviewed and/or approved by necessary government agencies, which could adversely affect our business. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Upon receipt of regulatory approval to market the Allurion Balloon in a given jurisdiction, we are (or will be) subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and subject us to penalties if we fail to comply with applicable regulatory requirements.
When a regulatory approval is obtained, the approved product and its manufacturer are subject to continual review by regulatory authorities (including, if applicable, the FDA). Our non-U.S. regulatory approvals for the Allurion Balloon, as well as any future regulatory approval that we receive for the Allurion Balloon or for any of our other products, may be subject to limitations on the indicated uses for which the product may be marketed. Future approvals may contain requirements for potentially costly post-marketing follow-up trials to monitor the safety and efficacy of the approved product. In addition, we are subject to extensive and ongoing regulatory requirements by the FDA and other regulatory authorities with regard to the labeling, packaging, adverse event reporting, storage, advertising, promotion and recordkeeping for our products. In addition, we are required to comply with regulations regarding the manufacture of the Allurion Balloon, which include requirements related to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Further, regulatory authorities must inspect these manufacturing facilities and determine they are in compliance with FDA good manufacturing practice requirements as set forth in the QSR before the products can be approved. These facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with the QSR and similar regulations. If we or a third party discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory authority may impose restrictions on that product, the manufacturer or us, including requiring withdrawal of the product from the market or suspension of manufacturing.
If patients using our products experience adverse events or other undesirable side effects, regulatory authorities could withdraw or modify our regulatory approvals, which would adversely affect our reputation and commercial prospects and/or result in other significant negative consequences.
Undesirable side effects caused by the Allurion Balloon could cause us, the FDA or other applicable regulatory authorities to interrupt, delay or halt clinical trials, and could result in more restrictive labeling than originally required, cause the FDA or other regulatory authorities to subsequently withdraw or modify our PMA, if we obtain approval, or other regulatory approvals, or result in the delay or denial of regulatory approval by regulatory authorities. For example, in the 1980s and early 1990s, the FDA required post-market safety and
43
Table of Contents
efficacy data be collected on an earlier version of an intragastric balloon after patients suffered severe side effects and complications with the device, which ultimately resulted in the withdrawal of the PMA approval.
As of March 31, 2024, we had sold over 150,000 units of the Allurion Balloon in international markets. In our commercial experience, the serious adverse event (“SAE”) rate has been less than 0.2% and has been similar to the SAE profile reported in the literature.
If we are unable to demonstrate that any adverse events are not related to our product, the FDA or other regulatory authorities could order us to cease further development of, require more restrictive indications for use and/or additional warnings, precautions and/or contraindications in the labeling than originally required, or delay or deny approval of any of our products. Even if we are able to do so, such event(s) could affect patient recruitment or the ability of enrolled patients to complete the trial. Moreover, if we elect, or are required, to not initiate, delay, suspend or terminate any future clinical trial of any of our products, the commercial prospects of such product may be harmed and our ability to generate product revenues from our product may be delayed or eliminated. Any of these occurrences may harm our ability to develop other products, and may harm our business, financial condition and prospects significantly.
In addition, we or others may later identify undesirable side effects caused by the product (or any other similar product), resulting in potentially significant consequences, including:
| • | regulatory authorities may withdraw or limit their approval of the product; |
| • | regulatory authorities may require the addition of labeling statements, such as a contraindication; |
| • | we may be required to change the way the product is distributed or administered, conduct additional clinical trials or change the labeling of the product; |
| • | we may be required to correct or remove the product from the marketplace or decide to conduct a voluntary recall; |
| • | we may decide to alert physicians through customer notifications; |
| • | regulatory authorities may use publicity such as a press release to alert our customers and the public of the issue; |
| • | health care providers and patients may be dissatisfied, seek refunds and refuse to use our products; |
| • | we could be sued and held liable for injury caused to individuals using our product; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the Allurion Balloon and could substantially increase the costs of commercializing our product and significantly impact our ability to successfully commercialize our product and generate product sales.
Health care reform measures could hinder or prevent our planned products’ commercial success.
In the U.S., there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the health care system in ways that could affect our future revenue and future profitability and the future revenue and future profitability of our potential customers. Federal and state lawmakers regularly propose and, at times, enact legislation, that could result in significant changes to the health care system, some of which are intended to contain or reduce the costs of medical products and services. For example, one of the most significant health care reform measures in decades, ACA, was enacted in 2010. The ACA contains a number of provisions, including those governing enrollment in federal health care programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government health care programs and will result in the development of new programs. For more information, see section entitled “Business–Government Regulation–Other U.S. Healthcare Laws-Coverage, Reimbursement and Healthcare Reform.”
44
Table of Contents
There have been judicial and Congressional challenges to certain aspects of the ACA, as well as executive efforts to repeal or replace certain aspects of the ACA. The Tax Cuts and Jobs Act passed in 2017 included a provision that would repeal one of the primary pillars of the law, the ACA’s individual mandate penalty, which essentially assessed a monetary penalty or fine on certain individuals who fail to maintain qualifying health coverage for all or part of a year. The U.S. Congress may consider other legislation to repeal or replace elements of the ACA on a provision-by-provision basis. We cannot assure you that the ACA, as currently enacted or as amended in the future, will not adversely affect our business and financial results and we cannot predict how future federal or state legislative or administrative changes relating to health care reform will affect our business.
We cannot predict the impact that such actions against the ACA or other health care reform under the Biden administration will have on our business, and there is uncertainty as to what health care programs and regulations may be implemented or changed at the federal and/or state level in the U.S., or the effect of any future legislation or regulation. However, it is possible that such initiatives could have an adverse effect on our ability to obtain approval and/or successfully commercialize products in the U.S. in the future. For example, any changes that reduce, or impede the ability to obtain, reimbursement for the type of products we intend to commercialize in the U.S. (or our products more specifically, if approved) or reduce medical procedure volumes could adversely affect our business plan to introduce our products in the U.S.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, the Budget Control Act of 2011 and subsequent legislation resulted in reductions to Medicare payments to providers of up to 2% per fiscal year to 2031 unless additional Congressional action is taken. In addition, the American Taxpayer Relief Act of 2012, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers, cancer centers and other treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
We cannot predict whether any additional legislative changes will affect our business.
There have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care. The implementation of cost-containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products. Such reforms could have an adverse effect on anticipated revenue from our products and product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop future product candidates. We cannot predict the initiatives that may be adopted in the future or their full impact. The continuing efforts of the government, insurance companies, managed care organizations and other payors of health care services to contain or reduce costs of health care may adversely affect:
| • | the demand for our product(s) and product candidates, if approved; |
| • | our ability to set a price that we believe is fair for our products; |
| • | our ability to generate revenue and achieve or maintain profitability; and |
| • | the availability of capital. |
If we fail to comply with health care regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
Even though we do not and will not control referrals of health care services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state health care laws and regulations pertaining to fraud and abuse and patients’ rights may be applicable to our business. If we are approved by the FDA to market our products in the U.S., we could be subject to health care fraud and abuse, transparency, and patient privacy regulation by both the federal government and the states in which we conduct our business. For more information, see section entitled “Business–Government Regulation-Other U.S. Healthcare Laws.”
45
Table of Contents
Similar regulations would also apply to our business in countries where we have direct sales operations where there are different regulations at European and national levels. There is a high degree of complication in complying with the different levels of regulation and the singular differences in the different countries and markets.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from participation in Medicare, Medicaid and other federal health care programs, additional reporting and government oversight, if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations. Any such penalties or curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal, state or international privacy, security and fraud laws may prove costly.
We have obtained the authorization to distribute our products in regions/countries through the certification of our quality system by the corresponding regulatory entities. Failing to demonstrate that our quality system is in place and consistently and systematically ensures compliance with regulations from such regions/countries might imply losing the certifications and as such, the rights to freely distribute the products which would adversely impact our revenue and reputation.
We have not historically maintained a compliance policy relating to U.S. or foreign economic sanctions, export controls or anti-corruption laws and regulations, and failure to comply with these regimes creates the potential for significant liabilities, penalties and reputational harm.
We have not historically maintained a compliance policy relating to U.S. economic sanctions, export controls or anti-corruption laws and regulations. Failure to comply with such laws and regulations creates the potential for significant liabilities, penalties and reputational harm. We are subject to a number of laws and regulations governing commercial activities with and payments and contributions to third parties, including restrictions imposed by the FCPA, as well as trade sanctions and export control laws administered by the U.S. Department of the Treasury’s Office of Foreign Assets Control (“OFAC”), the U.S. Department of Commerce and the U.S. Department of State.
The FCPA, among other things, prohibits bribery of foreign governments and their officials and political parties and requires U.S. public companies to keep books and records that accurately and fairly reflect those companies’ transactions. OFAC, the U.S. Department of Commerce and the U.S. Department of State administer and enforce various export control laws and regulations and economic sanctions based on U.S. foreign policy and national security goals against targeted foreign states, organizations and individuals.
Similar laws in non-U.S. jurisdictions, such as UK sanctions, EU sanctions or the U.K. Bribery Act, as well as other applicable anti-bribery, anti-corruption, anti-money laundering, sanctions or export control laws, may also impose stricter or more onerous requirements than U.S. economic sanctions, export controls, and anti-corruption laws and regulations, and implementing compliance measures may disrupt our business or cause us to incur significantly more costs. Different laws may also contain conflicting provisions, making compliance more difficult. If we fail to comply with these laws and regulations, we could be exposed to claims for damages, civil or criminal financial penalties, reputational harm, incarceration of our employees, restrictions on our operations and other liabilities, which could materially and adversely affect our business, results of operations and financial condition.
While we have implemented policies and procedures designed to promote compliance by us and our personnel with the FCPA and other anti-corruption laws, they may not be effective in all instances to prevent
46
Table of Contents
violations. Any determination that we have violated the FCPA or other applicable anti-corruption, sanctions or export control laws could subject us to, among other things, civil and criminal penalties, material fines, profit disgorgement, injunctions on future conduct, securities litigation and a general loss of investor confidence, any one of which could adversely affect our business, financial condition and results of operations.
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and financial condition and results of operations.
Actual events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. On March 10, March 12, and May 1, 2023, the Federal Deposit Insurance Corporation took control and was appointed receiver of Silicon Valley Bank (“SVB”), Signature Bank, and First Republic Bank, respectively, after each bank was unable to continue its operations. We are unable to predict the extent or nature of the impacts of the failures of SVB, Signature Bank and First Republic Bank and related circumstances at this time. Similarly, we cannot predict the impact that the high market volatility and instability of the banking sector more broadly could have on economic activity and our business in particular. The failure of other banks and financial institutions and measures taken, or not taken, by governments, businesses and other organizations in response to these events could adversely impact our business, financial condition and results of operations.
Although we assess our banking relationships as we believe necessary or appropriate, our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, the financial institutions with which we have credit agreements or arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships, but could also include factors involving financial markets or the financial services industry generally.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
We may be affected by regulatory responses to climate-related issues.
The Biden Administration has made climate change and the limitation of greenhouse gas (“GHG”) emissions one of its primary objectives. Several states and other geographic regions in the United States have also adopted legislation and regulations to reduce emissions of GHGs.
On March 6, 2024, the SEC finalized new rules for public companies that will require extensive climate-related disclosures and significant analysis of the impact of climate-related issues on our business strategy, results of operations, and financial condition (the “SEC Climate Disclosure Rules”). The new rules will require
47
Table of Contents
us to disclose our material climate-related risks and opportunities, GHG emissions inventory, climate-related targets and goals, and financial impacts of physical and transition risks. As a result of the SEC Climate Disclosure Rules, our legal, accounting, and other compliance expenses may increase significantly, and compliance efforts may divert management time and attention. We may also be exposed to legal or regulatory action or claims as a result of these new regulations. All of these risks could have a material adverse effect on our business, financial position, and/or stock price.
Risks Related to Intellectual Property
The medical device industry is characterized by patent litigation and we could become subject to adversarial proceedings or litigation that could be costly, result in the diversion of management’s time and efforts, result in a loss of our intellectual property, require us to pay damages or prevent us from marketing our existing or future products.
Patent litigation and other adversarial proceedings are prevalent in the medical device and diagnostic sectors. Our commercial success depends in part upon our ability and that of our distributors, contract manufacturers, and suppliers to manufacture, market, and sell our planned products, and to use our proprietary technologies without infringing, misappropriating or otherwise violating the proprietary rights or intellectual property of third parties. We have been, and in the future may become, party to, or be threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology. Third parties may assert infringement claims against us based on existing or future intellectual property rights, or may assert that our patents are invalid or unenforceable. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that we may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Accordingly, third parties may assert infringement claims against us based on intellectual property rights that exist now or arise in the future. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. Medical device and diagnostic industries have produced a significant number of patents and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use or manufacture. The scope of protection afforded by a patent is subject to interpretation by the courts and administrative agencies, and the interpretation is not always uniform. If we were sued for patent infringement, we would need to demonstrate that the relevant product or methods of using the product either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable and we may not be able to do this. Proving invalidity is difficult. For example, in the U.S., proving invalidity in United States District Courts requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could significantly harm our business and operating results. In addition, parties making claims against us may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources, and we may not have sufficient resources to bring these actions to a successful conclusion.
If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third-party to continue developing and marketing our products and technology. We may also elect to enter into such a license in order to settle pending or threatened litigation. However, we may not be able to obtain any required license on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us, and could require us to pay significant royalties and other fees. We could be forced, including by court order, to cease commercializing the infringing technology or product if we are found to infringe a third party’s intellectual property rights. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our planned products in commercially important territories, or force us to cease some of our business operations, which could harm our business. Many of our employees were previously employed at, and many of our current advisors and consultants are employed by, universities or other biotechnology, medical device or pharmaceutical companies, including our competitors or potential competitors.
48
Table of Contents
Although we instruct our employees, advisors and consultants not to, and otherwise endeavor to ensure that they do not, use or disclose the proprietary information or know-how of others in their work for us, we may be subject to claims that we, or these service providers, have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such service providers’ current or former employer or other third party. These and other claims that we have misappropriated the confidential information or trade secrets of third parties can have a similar negative impact on our business to the infringement claims discussed above.
Even if we are successful in defending against intellectual property claims, litigation or other legal proceedings relating to such claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of litigation or other intellectual property related proceedings could have a material adverse effect on our ability to compete in the marketplace.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain future patents, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the U.S. Patent and Trademark Office (“USPTO”), the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents or future patents.
If we fail to comply with our obligations in our intellectual property agreements, we could lose intellectual property rights that are important to our business.
We are a party, and expect to become party in the future, to certain intellectual property agreements that impose various obligations on us. If we fail to comply with these obligations, any licensor may have the right to terminate such agreements, in which event we may not be able to develop and market any product that is covered by such agreements. Termination of such agreements, or reduction or elimination of our rights under such agreements, may result in our having to negotiate new or reinstated arrangements on less favorable terms, or our not having sufficient intellectual property rights to operate our business. The occurrence of such events could harm our business and financial condition.
The risks described elsewhere in this prospectus pertaining to our intellectual property rights also apply to any intellectual property rights that we may license, and any failure by us or any future licensor to obtain, maintain, defend and enforce these rights could have a material adverse effect on our business.
If we are not able to obtain and maintain intellectual property protection for our products and technologies, or if the scope of our patents is not sufficiently broad, or our patents are invalidated, we may not be able to effectively maintain our market leading technology position.
As of March 31, 2024, we owned or had rights to 18 issued and five pending patents in the United States related to various aspects of the Allurion Balloon such as a swallowable, self-deflating and naturally passing
49
Table of Contents
gastric balloon, improvements to the fill and release valves therein, methods for deploying and releasing a gastric balloon within the body, and next generation fill and release valves. In addition, we had 37 issued and five patents pending outside of the United States. Our success depends in large part on our ability to obtain and maintain patent and other intellectual property protection in the U.S. and in other countries with respect to our proprietary technology and products.
The patent position of medical device and diagnostic companies generally is highly uncertain and involves complex legal and factual questions which are dependent upon the current legal and intellectual property context, extant legal precedent and interpretations of the law by individuals, and for which certain legal principles remain unresolved. In recent years, patent rights have been the subject of significant litigation. As a result, the issuance, scope, validity, enforceability and commercial value of the patent rights we rely on are highly uncertain.
Pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Pending and future patent applications may not result in patents being issued at all, may not result in patents being issued in a manner which protect our technology or products, or may not result in patents being issued which effectively prevent others from commercializing competitive technologies and products. Assuming the other requirements for patentability are met, currently, the first to file a patent application is generally entitled to the patent. However, prior to March 16, 2013, in the U.S., the first to invent was entitled to the patent. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. If third parties have filed prior patent applications on inventions claimed in our patents or applications that were filed on or before March 15, 2013, an interference proceeding in the U.S. can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. If third parties have filed such prior applications after March 15, 2013, a derivation proceeding in the U.S. can be initiated by such third parties to determine whether our invention was derived from theirs. The determination that a patent application or patent claim meets all the requirements for patentability is a subjective determination based on the application of law and jurisprudence. The ultimate determination by the USPTO, or by a court or other trier of fact in the U.S., or corresponding foreign national patent offices or courts, on whether a claim meets all requirements of patentability cannot be assured. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our owned (jointly or fully) or licensed-in patents or patent applications.
We cannot provide assurances that any invention that is the subject of our patent applications, whether licensed-in or owned jointly or completely by us, will be found to be patentable, including over our own prior art publications or patent literature, or any such that application will result in an issued patent. We cannot make assurances as to the scope of any claims that may issue from our pending and future patent applications or to the outcome of any proceedings by any potential third parties that could challenge the patentability, validity or enforceability of our patents and patent applications in the U.S. or foreign jurisdictions. Any such challenge, if successful, could limit patent protection for our technology and products and/or materially harm our business.
Even if the patent applications we rely on issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and the patents we rely on may be challenged in the courts or patent offices in the U.S. and abroad. There is no assurance that all the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it may be used to invalidate a patent, or may prevent a patent from issuing from a pending patent application. For example, such patent filings may be subject to a third-party submission of prior art to the USPTO or to other patent offices around the world.
50
Table of Contents
Alternately or additionally, we may become involved in post-grant review procedures, oppositions, derivation proceedings, ex parte re-examinations, inter partes review, supplemental examinations, or interference proceedings or challenges in district court, in the U.S. or in various foreign patent offices, including both national and regional, challenging patents or patent applications in which we have rights, including patents on which we rely to protect our business. Patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable or otherwise may not provide any competitive advantage. An adverse determination in any such challenge may result in loss of the patent or in patent application or patent claims being narrowed, invalidated or held unenforceable, in whole or in part, or in denial of the patent application or loss or reduction of the scope of one or more claims of the patent or patent application, any of which could limit our ability to stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products.
As another example, a European Unified Patent Court (“UPC”) has entered into force on June 1, 2023. The UPC is a common patent court to hear patent infringement and revocation proceedings effective for member states of the European Union (“EU”). This could enable third parties to seek revocation of any of our European patents or licensed-in European patents in a single proceeding at the UPC rather than through multiple proceedings in each of the jurisdictions in which any such European patent is validated. Any such revocation and loss of patent protection could have a material adverse impact on our business and our ability to commercialize or license our technology and products. Moreover, the controlling laws and regulations of the UPC will develop over time, and may adversely affect our ability to enforce our European patents, whether owned or licensed-in, or defend the validity thereof. We, or any future licensor, may decide to opt out our European patents and patent applications from the UPC. If certain formalities and requirements are not met, however, these European patents and patent applications could be challenged for non-compliance and brought under the jurisdiction of the UPC. We cannot be certain that our owned (jointly or fully) or licensed-in European patents or European patent applications will avoid falling under the jurisdiction of the UPC, if we, or any future licensor, decide to opt out of the UPC. Our competitors, who may have greater resources and may have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or eliminate our ability to make, use, and sell our technologies and products. Given the amount of time required for the development, testing and regulatory review of new planned products, patents protecting such products might expire before or shortly after such products are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours or otherwise provide us with a competitive advantage.
Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of the patents we rely on or narrow the scope of our patent protection. The laws of other countries may not protect our rights to the same extent as the laws of the U.S. For example, patent laws in various jurisdictions, including jurisdictions covering significant commercial markets, such as the European Patent Office, China and Japan, restrict the patentability of methods of treatment of the human body more than U.S. law does. Our inability to protect our innovations could have a material adverse effect on our ability to generate revenue. There may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the U.S. for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns. Countries other than the U.S. may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop, and market competing products. Countries other than the U.S. may, under certain circumstances, force us to grant a license under our patents to a competitor, thus allowing the competitor to compete with us in that jurisdiction or forcing us to lower the price of our product in that jurisdiction.
Furthermore, the degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| • | it is possible that one or more of our pending patent applications will not become an issued patent or, if issued, that the patent(s) claims will have sufficient scope to protect all of our planned products, provide us with commercially viable patent protection or provide us with any competitive advantages; |
51
Table of Contents
| • | if our pending applications issue as patents, they may be challenged by third parties as invalid or unenforceable under U.S. or foreign laws; |
| • | we may not successfully commercialize all of our planned products, if approved, before our relevant patents expire; |
| • | we may not be the first to make the inventions covered by each of our patents and pending patent applications; or |
| • | we may not develop additional proprietary technologies or products that are separately patentable. |
In addition, to the extent that we are unable to obtain and maintain patent protection for our technologies or product, or in the event that such patent protection expires, it may no longer be cost-effective to extend our portfolio by pursuing additional development of any future products.
If our trademarks and tradenames are not adequately protected, then we may not be able to build name recognition in our markets and our business may be adversely affected.
We rely on trademarks, service marks, tradenames and brand names to distinguish our products from the products of our competitors, and have registered or applied to register these trademarks. We cannot assure you that our trademark applications will be approved. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in proceedings before the USPTO and comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources towards advertising and marketing new brands. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. Certain of our current or future trademarks may become so well known by the public that their use becomes generic and they lose trademark protection. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business, financial condition and results of operations may be adversely affected.
We may be subject to claims that we or our employees have misappropriated the intellectual property of a third party, including trade secrets or know-how, or are in breach of non-competition or non-solicitation agreements with our competitors.
Many of our employees and consultants were previously employed at or engaged by other medical device, biotechnology or pharmaceutical companies, including our competitors or potential competitors. Some of these employees, consultants and contractors may have executed proprietary rights, non-disclosure and non-competition agreements in connection with such employment. Although we instruct our employees and consultants not to, and otherwise endeavor to ensure that they do not, use or disclose the intellectual property, proprietary information, know-how or trade secrets of others in their work for us, we may be subject to claims that we or these individuals have, inadvertently or otherwise, misappropriated the intellectual property or disclosed the alleged trade secrets or other proprietary information of such employers or competitors.
Additionally, we may be subject to claims from third parties challenging our ownership interest in intellectual property we regard as our own, based on claims that our employees or consultants have breached an obligation to assign inventions to another employer, to a former employer, or to another person or entity. Litigation may be necessary to defend against any other claims, and it may be necessary or we may desire to enter into a license to settle any such claim; however, there can be no assurance that we would be able to obtain a license on commercially reasonable terms, if at all. If our defense to those claims fails, in addition to paying
52
Table of Contents
monetary damages, a court could prohibit us from using technologies or features that are essential to our products, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. An inability to incorporate technologies or features that are important or essential to our products could have a material adverse effect on our business, financial condition and results of operations, and may prevent us from selling our products. In addition, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. Any litigation or the threat thereof may adversely affect our ability to hire employees or contract with independent sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our products, which could have an adverse effect on our business, financial condition and results of operations.
Others may challenge inventorship or claim an ownership interest in our intellectual property, which could expose us to litigation and have a significant adverse effect on our prospects.
Determinations of inventorship can be subjective. While we undertake to accurately identify correct inventorship of inventions made on our behalf by our employees, consultants and contractors, an employee, consultant or contractor may disagree with our determination of inventorship and assert a claim of inventorship. Any disagreement over inventorship could result in our being forced to defend our determination of inventorship in a legal action, which could result in substantial costs and be a distraction to our senior management and scientific personnel.
While we typically require employees, consultants and contractors who may develop intellectual property on our behalf to execute agreements assigning such intellectual property to us, we may be unsuccessful in obtaining execution of assignment agreements with each party who in fact develops intellectual property that we regard as our own. If we are unsuccessful in obtaining assignment agreements from an employee, consultant or contractor who develops intellectual property on our behalf, the employee, consultant or contractor may later claim ownership of the invention. Any disagreement over ownership of intellectual property could result in our losing ownership, or exclusive ownership, of the contested intellectual property, paying monetary damages and/ or being enjoined from clinical testing, manufacturing and marketing of the affected product candidate(s). Even if we are successful in defending against such claims, a dispute could result in substantial costs and be a distraction to our senior management and scientific personnel.
We may become involved in legal proceedings to protect or enforce our intellectual property rights, which could be expensive, time consuming, or unsuccessful.
Competitors may infringe or otherwise violate the patents we rely on, or our other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims that we assert against perceived infringers could also provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property rights, or that our intellectual property is invalid. In an infringement proceeding, a court may decide that a patent we are asserting is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that the patents we are asserting do not cover the technology in question. With respect to a counterclaim of invalidity, we cannot be certain that there is no invalidating prior art of which we and the patent examiner were unaware during prosecution. An adverse result in any litigation proceeding could put one or more patents at risk of being invalidated or interpreted narrowly, or prevent us from stopping the other party from using the invention at issue on the grounds that our patent claims do not cover the invention. If any of our patents are found invalid or unenforceable, or construed narrowly, our ability to stop the other party from launching a competitive product would be materially impaired. Further, such adverse outcomes could limit our ability to assert those patents against future competitors. Loss of patent protection would have a material adverse impact on our business.
Even if we establish infringement of any of our patents by a competitive product, a court may decide not to grant an injunction against further infringing activity, thus allowing the competitive product to continue to be
53
Table of Contents
marketed by the competitor. It is difficult to obtain an injunction in U.S. litigation and a court could decide that the competitor should instead pay us a “reasonable royalty” as determined by the court, and/or other monetary damages. A reasonable royalty or other monetary damages may or may not be an adequate remedy. Loss of exclusivity and/or competition from a related product would have a material adverse impact on our business.
Litigation often involves significant amounts of public disclosures. Such disclosures could have a materially adverse impact on our competitive position or our stock prices. During any litigation, we would be required to produce voluminous records related to our patents and our research and development activities in a process called discovery. The discovery process may result in the disclosure of some of our confidential information. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments.
Litigation is inherently expensive, and the outcome is often uncertain. Any litigation likely would substantially increase our operating losses and reduce our resources available for development activities. Further, we may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. As a result, we may conclude that even if a competitor is infringing any of our patents, the risk-adjusted cost of bringing and enforcing such a claim or action may be too high or not in the best interest of us or our stockholders. In such cases, we may decide that the more prudent course of action is to simply monitor the situation or initiate or seek some other non-litigious action or solution.
Concurrently with an infringement litigation, third parties may also be able to challenge the validity of our patents before administrative bodies in the U.S. or abroad. Such mechanisms include re-examination, post grant review and equivalent proceedings in foreign jurisdictions, e.g., opposition proceedings. Such proceedings could result in revocation or amendment of our patents in such a way that they no longer cover our products, potentially negatively impacting any concurrent litigation.
Interference or derivation proceedings provoked by third parties or brought by the USPTO or any other patent authority may be necessary to determine the priority of inventions or other matters of inventorship with respect to patents and patent applications. In addition to challenges during litigation, third parties can challenge the validity of our patents in the U.S. using post-grant review and inter partes review proceedings, which some third parties have been using to cause the cancellation of selected or all claims of issued patents of competitors. For a patent filed March 16, 2013 or later, a petition for post-grant review can be filed by a third party in a nine-month window from issuance of the patent. A petition for inter partes review can be filed immediately following the issuance of a patent if the patent has an effective filing date prior to March 16, 2013. A petition for inter partes review can be filed after the nine-month period for filing a post-grant review petition has expired for a patent with an effective filing date of March 16, 2013 or later. Post-grant review proceedings can be brought on any ground of invalidity, whereas inter partes review proceedings can only raise an invalidity challenge based on published prior art and patents. These adversarial actions at the USPTO review patent claims without the presumption of validity afforded to U.S. patents in lawsuits in U.S. federal courts and use a lower burden of proof than used in litigation in U.S. federal courts. Therefore, it is generally considered easier for a competitor or third party to have a U.S. patent invalidated in a USPTO post-grant review or inter partes review proceeding than invalidated in a litigation in a U.S. federal court. If any of our patents are challenged by a third party in such a USPTO proceeding, there is no guarantee that we or any future licensors or collaborators will be successful in defending the patent, which may result in a loss of the challenged patent right to us. We may become involved in proceedings, including oppositions, interferences, derivation proceedings inter partes reviews, patent nullification proceedings, or re-examinations, challenging our patent rights or the patent rights of others, and the outcome of any such proceedings are highly uncertain. An adverse determination in any such proceeding could reduce the scope of, or invalidate, important patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Our business also could be harmed if a prevailing party does
54
Table of Contents
not offer us a license on commercially reasonable terms, if any license is offered at all. Litigation or other proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may also become involved in disputes with others regarding the ownership of intellectual property rights. If we are unable to resolve these disputes, we could lose valuable intellectual property rights.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical or management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. Uncertainties resulting from the initiation and continuation of intellectual property litigation or other proceedings could have an adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, harming our business and competitive position.
In addition to our patented technology and products, we rely upon confidential proprietary information, including trade secrets, unpatented know-how, technology and other proprietary information, to develop and maintain our competitive position. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in the market. Although we have taken steps to protect our confidential proprietary information, in part, by confidentiality agreements with our employees and our collaborators, consultants, vendors and advisors, we cannot provide assurances that all such agreements have been duly executed. Third parties may still obtain this information or may come upon this or similar information independently, and we cannot be certain that our trade secrets and other confidential information will not be disclosed or that competitors will not otherwise gain access to our trade secrets, or that technology relevant to our business will not be independently developed by a person that is not a party to such an agreement. Furthermore, if the employees, consultants, collaborators, vendors or advisors that are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could be disclosed, misappropriated or otherwise become known or be independently discovered by our competitors. In addition, intellectual property laws in foreign countries may not protect trade secrets and confidential information to the same extent as the laws of the U.S. If we are unable to prevent disclosure of the intellectual property related to our technologies to third parties, we may not be able to establish or maintain a competitive advantage in our market, which would harm our ability to protect our rights and have an adverse effect on our business.
We may not be able to protect or enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our planned products throughout the world may be prohibitively expensive to us. The requirements for patentability may differ in certain countries, particularly in developing countries; thus, even in countries where we do pursue patent protection, there can be no assurance that any patents will issue with claims that cover our products.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, laws of some countries outside of the U.S. and Europe do not afford intellectual property protection to the same extent as the laws of the U.S. and Europe. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able
55
Table of Contents
to prevent third parties from practicing our inventions in certain countries outside the U.S. and Europe or from selling or importing products made from our inventions in and into the U.S. or other jurisdictions. Consequently, competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as in the U.S. These products may compete with our products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in international jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Further, such proceedings could put our patents (in that or other jurisdictions) at risk of being invalidated, held unenforceable or interpreted narrowly; put our pending patent applications at risk of not issuing; and provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Furthermore, we cannot ensure that we will be able to initiate or maintain the same level or quality of patent protection in all jurisdictions in which we may wish to market our products. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
Changes in the interpretation of patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
In the U.S., the U.S. Congress is responsible for passing laws establishing patentability standards, and, as with any laws, implementation is left to federal agencies and the federal courts based on their interpretations of the laws. In the U.S., interpretation of patent standards can vary significantly within the USPTO, and across the various federal courts, including the U.S. Supreme Court. Recently, the U.S. Supreme Court has ruled on several patent cases, generally limiting the types of inventions that can be patented. Further, there are open questions regarding interpretation of patentability standards that the U.S. Supreme Court has yet to decisively address. Absent clear guidance from the U.S. Supreme Court, the USPTO has become increasingly conservative in its interpretation of patent laws and standards. Similar tensions between government administrations and judicial interpretation of patent laws in other jurisdictions may resulting in changes to the scope or validity of our patents in such jurisdictions.
In addition to increasing uncertainty with regard to our ability to obtain patents in the future, the legal landscape in the U.S. and outside the U.S. has created uncertainty with respect to the value of patents. Depending on any actions by applicable legislating bodies, and future decisions by the entities implementing such laws, the laws and regulations governing patents could change in unpredictable ways and could weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents or applications will be due to be paid by us to the USPTO and various governmental patent agencies outside of the U.S. in several stages over the lifetime of the patents or applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Though we use commercially reasonable efforts to comply with all applicable maintenance requirements, we may fail to do so on occasion. In many cases, such an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules.
56
Table of Contents
However, there are situations in which non-compliance, whether intentional or not, can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to use our technologies and this circumstance would have a material adverse effect on our business.
We may need to acquire or license intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights, that we may determine are important or necessary to the development of our technology and products. In addition, it may be necessary for us to use the patented or proprietary technology of one or more third parties to commercialize our current and future products. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
If we determine to license or acquire third-party intellectual property and we are unable to acquire such intellectual property outright, or obtain licenses to such intellectual property from such third parties when needed or on commercially reasonable terms, our ability to commercialize our products at such time would likely be delayed or we may have to abandon development of that product or program and our business and financial condition could suffer.
If we in-license additional technologies or products in the future, we might become dependent on proprietary rights from third parties with respect to those technologies or products. Any termination of such licenses could result in the loss of significant rights and would cause material adverse harm to our ability to develop and commercialize any product subject to such licenses.
Disputes may also arise between us and any future licensors regarding intellectual property subject to a license agreement. If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product(s).
The risks described elsewhere pertaining to our intellectual property rights also apply to the intellectual property rights that we may determine to in-license, and any failure by us or any future licensors to obtain, maintain, defend and enforce such rights could have an adverse effect on our business. In some cases we may not have control over the prosecution, maintenance or enforcement of the patents that we determine to license, and may not have sufficient ability to provide input into the patent prosecution, maintenance and defense process with respect to such patents, and potential future licensors may fail to take the steps that we believe are necessary or desirable in order to obtain, maintain, defend and enforce the licensed patents.
The Allurion VCS and other products or services contain third-party open source software components. Certain use of such open source components with our proprietary software could adversely affect our ability to charge fees for, or otherwise protect the value of, our offerings.
The Allurion VCS and our other products and services contain software licensed to us by third-party authors under “open source” licenses. Use of such software may entail greater risks than use of non-open source third-party commercial software, as open source licensors generally do not provide support, warranties, indemnification or other contractual protections regarding infringement claims or the quality of the code. Although we seek to monitor our use of open source software to avoid such consequences and to comply with the terms thereof, the terms of many open source licenses have not been interpreted by U.S. or foreign courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to provide or distribute our platform. If we are held to have breached the terms of an
57
Table of Contents
open source software license, we could face liability which may result in an injunction against providing our offering, or be required to seek costly licenses from third parties to continue providing our offerings on terms that are not economically feasible, to re-engineer our platform, to discontinue or delay the provision of our offerings if re-engineering could not be accomplished on a timely basis or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, financial condition and results of operations.
Our internal computer systems, or those used by third parties which we rely on, may fail or suffer security breaches.
Despite the implementation of security measures, our internal computer systems, or those used by third parties which we rely on, are vulnerable to damage from computer viruses and unauthorized access, malware, natural disasters, fire, terrorism, war, telecommunication failures, electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. Although our information security program is in compliance with the global ISO 27001:2013 standards, it does not yet fully comply with all of the additions and changes in the updated ISO 27001:2022 version of the standards, which we anticipate complying with prior to the required transition date of October 31, 2025 to maintain ISO 27001 security certification.
If our security measures are breached, whether due to failure to comply with the ISO 27001:2022 version of the standards or otherwise, or if design flaws in our software or information systems are exposed and exploited, and, as a result, a third party obtains unauthorized access to any of our or our customer’s data, our relationships with our customers and distributors may be damaged, and we could incur significant liability and reputational harm. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. While we have not experienced any such material system failure or security breach to our knowledge to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of data from completed, ongoing or future trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our current and future products could be delayed.
We rely on internet infrastructure, bandwidth providers, third-party computer hardware and software and other third parties for providing services to our customers and patients, and any failure or interruption in the services provided by these third parties could expose us to litigation and negatively impact our relationships with customers and patients, adversely affecting our operating results.
Our ability to deliver our internet-based services depends on the development and maintenance of the infrastructure of the internet by third parties. This includes maintenance of a reliable network backbone with the necessary speed, data capacity, bandwidth capacity and security. Our services are designed to operate without interruption. However, we may experience future interruptions and delays in services and availability from time to time. In the event of a catastrophic event with respect to one or more of our systems, we may experience an extended period of system unavailability, which could negatively impact our relationship with clients and members. To operate without interruption, both we and our service providers must guard against:
| • | damage from fire, power loss, natural disasters and other force of nature events outside our control; |
| • | communications failures; |
| • | software and hardware errors, failures, and crashes; |
58
Table of Contents
| • | security breaches, computer viruses, hacking, denial-of-service attacks, and similar disruptive problems; and |
| • | other potential interruptions. |
We also rely on software licensed from third parties in order to offer our services. These licenses are generally commercially available on varying terms. However, it is possible that this software may not continue to be available on commercially reasonable terms, or at all. Any loss of the right to use any of this software could result in delays in the provisioning of our services until equivalent technology is either developed by us, or, if available, is identified, obtained and integrated. Furthermore, our use of additional or alternative third-party software would require us to enter into license agreements with third parties, and integration of our software with new third-party software may require significant work and require substantial investment of our time and resources. Also, any undetected errors or defects in third-party software could prevent the deployment or impair the functionality of our software, delay new updates or enhancements to our platform, result in a failure of our platform, and injure our reputation.
Our failure to adequately protect personal information in compliance with evolving legal requirements could harm our business.
In the ordinary course of our business, we collect and store sensitive data, including legally protected patient health information and personally identifiable information. We collect this kind of information on our customers for purposes of servicing potential warranty claims and for post-marketing safety vigilance. Data protection and privacy-related laws and regulations are evolving and may result in ever-increasing regulatory and public scrutiny and escalating levels of enforcement and sanctions.
There are a number of state, federal and international laws protecting the privacy and security of health information and personal data. As part of the American Recovery and Reinvestment Act 2009 (“ARRA”), the U.S. Congress amended the privacy and security provisions of HIPAA. HIPAA imposes limitations on the use and disclosure of an individual’s protected health information by certain health care providers, health care clearinghouses, and health insurance plans, collectively referred to as covered entities, that involve the creation, use, maintenance or disclosure of protected health information. The HIPAA amendments also impose compliance obligations and corresponding penalties for non-compliance on individuals and entities that provide services to health care providers and other covered entities, collectively referred to as business associates. Most recently, on December 10, 2020, HHS issued a Notice of Proposed Rulemaking (the public comment period to which was further extended in March 2021) which, if finalized, would make changes to some of HIPAA’s regulatory requirements, which would impact us, to the extent we are a business associate. ARRA also significantly increased the penalties for improper use or disclosure of an individual’s protected health information under HIPAA and extended enforcement authority to state attorneys general. The amendments also create notification requirements for individuals whose protected health information has been inappropriately accessed or disclosed, notification requirements to federal regulators and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by HHS. Most states have laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the protected health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms to ensure ongoing protection of personal information.
In addition, even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the FTCA, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
59
Table of Contents
Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
Many foreign countries and governmental bodies, including the EU, Canada, Australia and other relevant jurisdictions, have laws and regulations concerning the collection and use of personal or sensitive data obtained from their residents or by businesses operating within their jurisdiction. For example, the European Commission adopted the General Data Protection Regulation (“GDPR”), effective on May 25, 2018, that supersedes previous EU data protection legislation, imposes more stringent EU data protection requirements and provides for greater penalties for non-compliance. The GDPR applies to any company established in the EU as well as to those outside the EU if they collect and use personal data in connection with the offering goods or services to individuals in the EU or the monitoring of their behavior.
In addition, following the United Kingdom’s exit from the EU on January 31, 2020, the GDPR ceased to apply in the United Kingdom at the end of the transition period on December 31, 2020. However, as of January 1, 2021, the United Kingdom’s European Union (Withdrawal) Act 2018 incorporated the GDPR (as it existed on December 31, 2020 but subject to certain UK specific amendments) into United Kingdom law (referred to as the UK GDPR). The UK GDPR and the UK Data Protection Act 2018 set out the UK’s data protection regime, which is independent from but aligned to the EU’s data protection regime. In this prospectus, “GDPR” refers to both the EU and the UK GDPR, unless specified otherwise. The GDPR enhances data protection obligations for processors and controllers of personal data, including, for example, expanded disclosures about how personal information is to be used, limitations on retention of information, mandatory data breach notification requirements and onerous new obligations on services providers. Non-compliance with the GDPR can trigger steep fines of up to €20 million (£17.5 million) or 4% of total worldwide annual revenues, whichever is higher. Given the breadth and depth of changes in data protection obligations, meeting the GDPR’s requirements requires time, resources and a review of the technology and systems currently in use against the GDPR’s requirements.
EU Member States have adopted national laws to implement the EU GDPR, which may partially deviate from the EU GDPR, and the competent authorities in the EU Member States may interpret GDPR obligations slightly differently from country to country, such that we do not expect to operate in a uniform legal landscape in the EU with respect to data protection laws. In addition, the UK has announced plans to reform the UK data protection regime.
The GDPR imposes strict rules on the transfer of personal data out of the European Economic Area (“EEA”), or the United Kingdom to third countries, including the U.S. On June 4, 2021, the European Commission issued new forms of standard contractual clauses for data transfers from controllers or processors in the EEA, or otherwise subject to the GDPR, to controllers or processors established outside the EEA, and not subject to the GDPR. The new forms of standard contractual clauses have replaced the standard contractual clauses that were adopted previously under the Data Protection Directive. The UK is not subject to the European Commission’s new standard contractual clauses but has published its own transfer mechanism, the International Data Transfer Agreement, which enables transfers from the UK. We will be required to transition to the new forms of standard contractual clauses and doing so will require significant effort and cost. Although the United Kingdom is regarded as a third country under the EU GDPR, the European Commission has issued a decision recognizing the United Kingdom as providing adequate protection under the EU GDPR and, therefore, transfers of personal data originating in the EEA to the United Kingdom remain unrestricted. Like the EU GDPR, the UK GDPR restricts personal data transfers outside the United Kingdom to countries not regarded by the United Kingdom as providing adequate protection. The United Kingdom government has confirmed that personal data transfers from the United Kingdom to the EEA remain free flowing.
We may be at risk of enforcement actions taken by certain EU or UK data protection authorities until such point in time that we may be able to ensure that all transfers of personal data to us from the EEA or the United Kingdom are conducted in compliance with all applicable regulatory obligations, the guidance of data protection
60
Table of Contents
authorities and evolving best practices. We may find it necessary to establish systems to maintain personal data originating from the EU/UK in the EEA or the United Kingdom (as applicable), which may involve substantial expense and may cause us to need to divert resources from other aspects of our business, all of which may adversely affect our business. Our failure to comply with applicable laws and regulations, or to protect such data, could result in enforcement actions against us, including fines, imprisonment of company officials and public censure, claims for damages by end-customers and other affected individuals, damage to our reputation and loss of goodwill, any of which could harm on our operations, financial performance, and business. Evolving and changing definitions of personal data and personal information, within the European Union, the United Kingdom, the U.S., and elsewhere, may limit or inhibit our ability to operate or expand our business, including limiting strategic partnerships that may involve the sharing of data. Moreover, if the relevant laws and regulations change, or are interpreted and applied in a manner that is inconsistent with our data practices or the operation of our products, we may need to expend resources in order to change our business operations, data practices, or the manner in which our products operate. Even the perception of privacy concerns, whether or not valid, may harm our reputation and inhibit adoption of our products.
There is the risk that the limits we obtained for our cyber liability insurance may not cover the total loss experienced in the event of a data security incident, including the financial loss, legal costs, and business and reputational harm, particularly if there is an interruption to our systems. Additionally, there is the risk of a data privacy or security incident by an employee, which may expose us to liability. If personal information of our customers or employees is misappropriated, our reputation with our customers and employees may be injured resulting in loss of business and/or morale, and we may incur costs to remediate possible injury to our customers and employees or be required to pay fines or take other action with respect to judicial or regulatory actions arising out of such incidents.
If we are not able to satisfy data protection, security, privacy, and other government- and industry-specific requirements, our business could be harmed.
There are a number of data protection, security, privacy and other government- and industry-specific requirements, including those that require companies to notify individuals of data security incidents involving certain types of personal data. Security compromises experienced by other companies, by our customers or by us may lead to public disclosures, which could harm our reputation, erode customer confidence in the effectiveness of our security measures, and negatively impact our other products and our ability to attract new customers. As we expand into new regions, we will need to comply with new requirements. If we cannot comply or if we incur a violation in one or more of these requirements, our growth could be adversely impacted, and we could incur significant liability.
We have incorporated, and plan to incorporate in the future, artificial intelligence, or AI, into some of our products. This technology is new and developing and may present risks that could affect our business.
We have incorporated, and plan to incorporate in the future, AI, including large language models, such as GPT, into our products. AI is a new and emerging technology that is in its early stages of commercial use, particularly within the medical device industry. If any of our products that incorporate AI have perceived or actual negative impacts on the clinicians or patients who use them, we may experience brand or reputational harm, competitive harm or legal liability. The rapid evolution of AI may also require the application of significant resources to develop, test and maintain our products and services that incorporate AI in order to help ensure that it is implemented in a socially responsible manner, to minimize any real or perceived unintended harmful impacts. In addition, AI is subject to a complex and evolving regulatory landscape, including data protection, privacy, and potentially other laws and different jurisdictions have taken and may take in the future varying approaches to regulating AI. Compliance with these laws and regulations can be complex, costly and time-consuming, and there is a risk of regulatory enforcement actions or litigation if we fail to comply with these requirements. As regulations evolve, we may have to alter our business practices or products in order to comply with regulatory requirements.
61
Table of Contents
Risks Related to Our Financial Condition and Capital Requirements
We have incurred net operating losses in the past and expect to incur net operating losses for the foreseeable future.
We have incurred net operating losses since our inception, and we continue to incur significant research and development, general and administrative, and sales and marketing expenses related to our operations. We do not expect to be profitable in 2024, and in future years we expect to incur significant sales and marketing expenses to expand our business and clinical research expenses related to, among other things, the AUDACITY trial for the Allurion Balloon in the U.S. Investment in medical device product development, particularly clinical trials, is highly speculative. It entails substantial upfront capital expenditures and significant risk that any potential planned product will fail to demonstrate adequate safety or effectiveness. We expect to generate significant operating losses for the foreseeable future. As of March 31, 2024 and December 31, 2023, we had an accumulated deficit of $207.2 million and $212.8 million, respectively. Based on our recurring losses and expectations to incur significant expenses and negative cash flow for the foreseeable future, the need to raise additional capital to finance our future operations, we have concluded that there is substantial doubt about our ability to continue as a going concern for a period of one year from the date that the consolidated financial statements for the quarter ended March 31, 2024 are issued.
We expect that our future financial results will depend primarily on our success in launching, selling and supporting the Allurion Balloon and other products that are part of our weight loss platform. This will require us to be successful in a range of activities, including manufacturing, marketing, and selling the Allurion Balloon. We may not succeed in these activities and may never generate revenue that is sufficient to be profitable in the future. Even if we are profitable, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to achieve sustained profitability would depress the value of our company and could impair our ability to raise capital, expand our business, diversify our planned products, market our current and future products, or continue our operations.
We have a significant amount of debt, which may affect our ability to operate our business and secure additional financing in the future.
On April 16, 2024, we issued an aggregate principal amount of $48 million of Notes due April 16, 2031. The interest rate is fixed at 6.00% per annum and is payable quarterly in cash or, at our option, in kind for the first three years. In addition, upon consummation of the Business Combination, we received an investment of $40 million from RTW in exchange for future royalty payments pursuant to the Revenue Interest Financing. We may also incur additional indebtedness to meet future financing needs.
Our ability to make scheduled payments of debt principal and interest on our existing or future indebtedness, or to refinance our indebtedness, and to pay our royalty obligations, depends on our future performance, which is subject to economic, financial, competitive, and other factors beyond our control. Our debt, including the Amended Note Purchase Agreement, contains certain financial covenants relating to minimum liquidity and minimum revenue requirements. We have received waivers of minimum liquidity and minimum revenue requirements under debt arrangements in the past, but there is no guarantee that we will not need to obtain waivers of financial covenants in the future. To the extent that we are unable to continue to comply with such ongoing minimum liquidity and revenue requirements, including as a result of any weakness in our business or macroeconomic trends, and are unable to procure additional waivers from RTW or other lenders in the future, such lenders may pursue a number of actions, including declaring us in breach of our covenants, requiring conditions to cure such breaches and/or exercising foreclosure remedies. Any or all of these actions may materially impact our working capital, and our business may not continue to generate sufficient cash flows from operations to fund operations, service our debt, and satisfy our royalty payment obligations. If we are unable to generate such cash flows, we may be required to adopt one or more alternatives, such as raising additional capital on terms that may be unfavorable or selling assets or portions of our business. Our ability to refinance our existing or any future indebtedness will depend on the capital markets and our financial condition at such time.
62
Table of Contents
We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
In addition, our indebtedness owed to RTW is collateralized by substantially all of our assets and subject to customary financial and operating covenants limiting our ability to, among other things, incur additional indebtedness, change our material line of business, modify our organization documents, create liens, sell assets, or prepay other indebtedness. These covenants may make it difficult to operate our business. We are also subject to standard event of default provisions under the Revenue Interest Financing Agreement and the Amended Note Purchase Agreement, that, if triggered, would allow the debt to be accelerated, which could significantly deplete our cash resources, cause us to raise additional capital at unfavorable terms, require us to sell portions of our business or result in us becoming insolvent. The existing collateral pledged to RTW, and the covenants to which we are bound, may prevent us from being able to secure additional debt or equity financing on favorable terms, or at all, or to pursue business opportunities, including potential acquisitions, heighten our vulnerability to downturns in our business or our industry or the general economy, limit our ability to adjust to changing market conditions and place us at a competitive disadvantage compared to our competitors who have greater capital resources.
We may need additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all, which would force us to delay, reduce or suspend our planned development and commercialization efforts. Raising additional capital may subject us to unfavorable terms, cause dilution to our existing stockholders, restrict our operations, or require us to relinquish rights to our products and technologies.
Our operations have consumed substantial amounts of cash since our inception, and we expect to incur significant expenses in connection with our planned clinical research, development and product commercialization efforts. If our available cash resources and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements, we may seek to sell equity or convertible debt securities, to obtain another form of third-party funding, or to enter into other debt financing. Any failure to raise the funds necessary to support our operations may force us to delay, reduce or suspend our planned clinical trials, research and development programs, or other commercialization efforts.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through strategic collaborations or partnerships, or marketing, distribution, royalty or licensing arrangements with third parties, we may be required to do so at an earlier stage than would otherwise be ideal and/or may have to limit valuable rights to our intellectual property, technologies, products, or future revenue streams, or grant licenses or other rights on terms that are not favorable to us. Furthermore, any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our products.
We receive the majority of our revenue from sales to health care providers and other third-party distributors, and the failure to collect receivables from them could adversely affect our financial position and results of operations.
We receive the majority of our revenue from sales to health care providers and other third-party distributors. We extend credit to our customers for a significant portion of our sales and receivables from our customers are not secured by any type of collateral. We are therefore subject to the risk that our customers may not pay for the products they have purchased, pay at a slower rate than we have historically experienced, or may seek extended
63
Table of Contents
payment terms, which may, in turn, result in delays in our cash collection and increases in our accounts receivable. Our customers may encounter cash flow or operating difficulties, which may reduce their demand for our products or delay their payments to us, thereby increasing our accounts receivable turnover days, or increasing the risk that they may default on their payment obligations. These risks are heightened during periods of global or industry-specific economic downturn or uncertainty and during periods of rising interest rates. Our liquidity and cash flows from operations may be adversely affected if we are unable to settle our accounts receivable on a timely basis, if our accounts receivable cycles or collection periods lengthen or if we encounter a material increase in defaults of payment of our accounts receivable or repayments of amounts we have extended to our customers on credit.
If our estimates or judgments relating to our critical accounting policies prove to be incorrect, our results of operations could be adversely affected.
The preparation of our financial statements requires us to make estimates and assumptions affecting the reported amounts of our assets, liabilities, revenues, expenses and earnings. If these estimates or assumptions are incorrect, it could have a material adverse effect on our results of operations or financial condition. We have identified several accounting policies as being critical to the fair presentation of our financial condition and results of operations because they involve major aspects of our business and require us to make judgments about matters that are inherently uncertain. These policies are described under the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and should be considered in conjunction with our audited consolidated financial statements and notes thereto included elsewhere in this prospectus. The implementation of new accounting requirements or other changes to GAAP, could have a material adverse effect on our reported results of operations and financial condition. Our results of operations may be adversely affected if our assumptions change or if actual circumstances differ from those in our assumptions, which could cause our results of operations to fall below our expectations and the expectations of securities analysts and investors, resulting in a decline in the trading price of our common stock.
Risks Related to this Offering and Ownership of Our Securities
The issuances of our common stock to the selling securityholder upon conversion of the Notes will cause substantial dilution to our existing stockholders, and the sale of the shares of common stock acquired by the selling securityholder, or the perception that such sales may occur, could cause the market price of our common stock to fall.
We are registering up to 30,191,900 shares of our common stock for resale under this prospectus, which reflects the number of shares of common stock issuable upon conversion of the Notes (exclusive of any accrued but unpaid interest on the Notes that may be added to the outstanding principal amount of such Notes at the time of conversion) assuming the shares are issued at an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of Notes, subject to Stockholder Approval for such issuance. If certain corporate events that constitute a “Make-Whole Fundamental Change” (as defined in the Amended Note Purchase Agreement) occur, then the conversion rate will, in certain circumstances, be increased for a specified period of time up to a maximum rate of an additional 321.9182 shares of common stock per $1,000 principal amount of Notes. In addition, calling any note for redemption will also constitute a Make-Whole Fundamental Change with respect to that Note, in which case the conversion rate applicable to the conversion of that Note will be increased in certain circumstances if it is converted after it is called for redemption. Although such conversion price for the Notes is above the NYSE’s “Minimum Price” (as such term is defined in Section 312.03 of the NYSE Listed Company Manual), it is subject to a reset provision at the time of the Next Equity Financing that could result in the conversion price falling below such Minimum Price. As such, the actual number of shares of our common stock that may be issued upon conversion of the Notes will vary depending on the then-current conversion price of the Notes, not to exceed 479,196 shares of common stock, or 1% of the number of shares of the common stock outstanding as of April 14, 2024, unless we obtain Stockholder Approval of such issuance, in accordance with
64
Table of Contents
the applicable stock exchange rules. We are required to include a proposal in our definitive proxy statement on Schedule 14A seeking Stockholder Approval no later than December 31, 2025. In addition, the number of shares of common stock issuable upon conversion of the Notes may increase as interest on the Notes accrues. Depending on market liquidity at the time, issuances and any subsequent sales of our common stock by the selling securityholder may cause the trading price of our common stock to fall.
If and when the selling securityholder converts the Notes, after the selling securityholder has acquired the shares, the selling securityholder may resell all, some, or none of those shares at any time or from time to time in its discretion. Therefore, issuances to the selling securityholder upon conversion of the Notes will result in dilution to the interests of other holders of our common stock. Moreover, depending on the then-current conversion price of the Notes, the selling securityholder may receive shares of common stock at a discounted price to the then-prevailing market price for our common stock. As a result, the selling securityholder may have an incentive to sell its shares because it will still profit, and may sell such shares immediately after receipt of such shares, which could cause the price of our common stock to decrease. Additionally, the issuance of a substantial number of shares of our common stock to the selling securityholder, or the anticipation of such issuances, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales.
Future sales of substantial amounts of the shares of our common stock by the selling securityholder and others could adversely affect the price of our common stock.
The shares of our common stock issued upon conversion of the Notes will be freely tradable without restriction or further registration under the Securities Act. As a result, a substantial number of shares of our common stock may be sold by the selling securityholder in the public market. If there are significantly more shares of our common stock offered for sale than buyers are willing to purchase, then the market price of our common stock will decline to a market price at which buyers are willing to purchase the offered common stock and sellers remain willing to sell our common stock. The sale of the shares by the selling securityholder or any future sales of a substantial number of shares of our common stock in the public market, or the perception that such sales may occur, could also adversely affect the price of our common stock. We cannot predict the effect, if any, that market sales of those shares of common stock or the availability of those shares for sale will have on the market price of our common stock.
If we or our existing stockholders, our directors or their affiliates or certain of our executive officers, sell a substantial number of our common stock in the public market, including the shares issuable upon conversion of the Notes, the market price of our common stock could decrease significantly. The perception in the public market that we or our stockholders might sell our common stock could also depress the market price of our common stock and could impair our future ability to obtain capital, especially through an offering of equity securities.
Our share price may be volatile, and purchasers of our securities could incur substantial losses.
Our share price is likely to be volatile. The securities markets in general, and the market for biotechnology and medical device companies in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. You may not be able to resell your shares at an attractive price due to a number of factors, including the following:
| • | our ability to successfully commercialize, and realize revenues from sales of, the Allurion Balloon; |
| • | the success of competitive products or technologies; |
| • | results of clinical trials of the Allurion Balloon or other current or future products or those of our competitors; |
65
Table of Contents
| • | regulatory or legal developments in the U.S. and other countries, especially changes in laws or regulations applicable to our products; |
| • | introductions and announcements of new products by us, our commercialization partners, or our competitors, and the timing of these introductions or announcements; |
| • | actions taken by regulatory agencies with respect to our products, clinical trials, manufacturing processes or sales and marketing terms; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | the success of our efforts to acquire or in-license additional products or planned products; |
| • | developments concerning our collaborations, including but not limited to those with our sources of manufacturing supply and our commercialization partners; |
| • | developments concerning our ability to bring our manufacturing processes to scale in a cost-effective manner; |
| • | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | developments or disputes concerning patents or other proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our products; |
| • | our ability or inability to raise additional capital and the terms on which we raise it; |
| • | the recruitment or departure of key personnel; |
| • | changes in the structure of health care payment systems; |
| • | market conditions in the medical device, pharmaceutical and biotechnology sectors; |
| • | actual or anticipated changes in earnings estimates or changes in securities analyst recommendations regarding our common stock, other comparable companies or our industry generally; |
| • | trading volume of our common stock; |
| • | guidance or projections, if any, that we provide to the public, any changes in this guidance or projections or our failure to meet this guidance or projections; |
| • | sales of our common stock by us or our stockholders; |
| • | general economic and political conditions such as recessions, interest rates, fuel prices, trade wars, pandemics (such as COVID-19), currency fluctuations, geopolitical conflicts and acts of war or terrorism; |
| • | the effects of natural disasters, terrorist attacks and the spread and/or abatement of infectious diseases, including with respect to potential operational disruptions, labor disruptions, increased costs, and impacts to demand related thereto; and |
| • | the other risks described in this “Risk Factors” section. |
These broad market and industry factors may harm the market price of our securities, regardless of our operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could adversely affect our business, financial condition, results of operations and growth prospects.
We do not intend to pay cash dividends for the foreseeable future.
We currently intend to retain our future earnings, if any, to finance the further development and expansion of our business and do not intend to pay cash dividends in the foreseeable future. Any future determination to pay
66
Table of Contents
dividends will be at the discretion of our Board and will depend on our financial condition, results of operations, capital requirements, restrictions contained in our current or future credit agreements and financing instruments, business prospects and such other factors as our Board deems relevant.
Future sales of our common stock, or the perception that future sales may occur, may cause the market price of our common stock to decline, regardless of our operating performance.
Sales of a substantial number of shares of our common stock in the public market, including the resale of shares held by various holders of our securities registered for resale, could occur at any time (after the expiration of any applicable lock-up period). These sales, or the perception in the market that the holders of a large number of shares of our common stock intend to sell shares, could increase the volatility of the market price of our common stock or result in a significant decline in the public trading price of our common stock.
In addition, on December 18, 2023, we entered into the Purchase Agreement with Chardan related to the Chardan Equity Facility. Pursuant to the Chardan Purchase Agreement, Chardan shall purchase from us up to $100.0 million of shares of our common stock, upon the terms and subject to the conditions and limitations set forth in the Chardan Purchase Agreement. As of March 31, 2024, we have sold $0.4 million of shares of our common stock to Chardan as Purchase Shares (as defined in the Chardan Purchase Agreement) under the Chardan Purchase Agreement, for aggregate net cash proceeds to us of approximately $0.37 million. Additional shares of our common stock under the Chardan Purchase Agreement may be sold by us to Chardan at our discretion from time to time. Sales of shares of our common stock under the Chardan Purchase Agreement in the future may cause the trading price of shares of our common stock to decrease.
The resale, or expected or potential resale, of a substantial number of shares of our common stock in the public market could adversely affect the market price for our common stock and make it more difficult for stockholders to sell their holdings at times and prices that they determine are appropriate. Furthermore, we expect that, because there is a large number of shares of our common stock registered pursuant to various resale registration statements, the selling securityholders thereunder will continue to offer the securities covered thereby for a significant period of time, the precise duration of which cannot be predicted. Accordingly, the adverse market and price pressures resulting from an offering pursuant to the effective resale registration statements may continue for an extended period of time. Sales of a substantial number of such shares in the public market could adversely affect the market price of our common stock.
Additionally, a significant portion of the shares of our common stock registered for resale were purchased by securityholders pursuant to investments in Legacy Allurion that date from 2013 onwards at prices considerably below the current market price of our common stock. The sale of such shares would result in the securityholders realizing a significant gain even if other securityholders experience a negative rate of return. For example, holders of Legacy Allurion Common Stock, many of whom purchased their shares pursuant to investments in Legacy Allurion that date from 2013 through the closing of the Business Combination, paid, on average, an effective purchase price of approximately $0.22 for each share of our common stock they received in connection with the Business Combination. Even if our trading price is significantly below $7.04, the offering price for the units offered in Compute Health’s IPO after giving effect to the CPUH Exchange Ratio, certain of the securityholders, including such holders of Legacy Allurion Common Stock, may still have an incentive to sell shares of our common stock because they purchased the shares at prices lower than the public investors or the current trading price of our common stock. For example, based on the closing price of our common stock of $1.75 as of March 31, 2024, such holders of Legacy Allurion common stock would experience a potential profit, on average, of up to approximately $1.53 per share, or approximately $40.3 million in the aggregate upon the sale of all such shares.
67
Table of Contents
Future sales and issuances of our common stock could result in additional dilution of the percentage ownership of our stockholders.
Significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner as determined from time to time. If we sell common stock, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our Common Stock.
We will incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could adversely affect our business, results of operations, and financial condition.
As a public company, we are subject to the reporting requirements of the Exchange Act, the listing standards of the NYSE, and other applicable securities rules and regulations. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time-consuming and costly, and place significant strain on our personnel, systems and resources. For example, the Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and results of operations. As a result of the complexity involved in complying with the rules and regulations applicable to public companies, our management’s attention may be diverted from other business concerns, which could harm our business, results of operations and financial condition. Although we have already hired additional employees to assist us in complying with these requirements, we may need to hire more employees in the future or engage outside consultants, which will increase our operating expenses.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs, and making some activities more time-consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest substantial resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from business operations to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
We also expect that being a public company and these new rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our Board, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
As a result of disclosure of information in the filings required of a public company, our business and financial condition will become more visible, which may result in an increased risk of threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and results of operations could be harmed, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and harm our business, results of operations, and financial condition.
68
Table of Contents
Certain parties have the right to nominate directors to our Board, and their interests may conflict with ours or yours in the future.
Pursuant to our Investor Rights Agreement, our Board consists of seven directors, a majority of which are required to be “independent” directors for purposes of NYSE rules, and the following persons have the following nomination rights with respect to our Board: (i) one director and one independent director was required to be nominated by Shantanu Gaur; (ii) one director and one independent director was required to be nominated by Remus Group Management, LLC and its affiliates (“Remus Capital”); (iii) one director was required to be nominated by the Sponsor; and (iv) two independent directors are required be nominated by Allurion (one of which shall be designated by RTW until such time as all obligations under the Revenue Interest Financing Agreement or any additional revenue interest financing agreement have been paid by Allurion).
In addition, pursuant to the Amended Note Purchase Agreement, we agreed to include an additional nominee in our proxy statement for the election of Class I directors at the 2024 annual meeting of stockholders, which additional nominee will go through our director nomination process. RTW has the right to approve such nominee, such approval not to be unreasonably withheld.
As a result of the foregoing, Shantanu Gaur, Remus Capital, the Sponsor and RTW or their respective nominees to our Board have the ability to control the appointment of our management, the entering into of mergers, sales of substantially all or all of our assets and other extraordinary transactions and influence amendments to our amended and restated certificate of incorporation (“Charter”) and Bylaws. In any of these matters, the interests of the parties to the Investor Rights Agreement with the right to nominate directors may differ from or conflict with your interests. Moreover, this control over the nomination of directors to our Board may also adversely affect the trading price for our common stock to the extent investors perceive disadvantages in owning stock of a company with these corporate governance provisions.
We are an “emerging growth company” and a “smaller reporting company” within the meaning of the Securities Act, and we intend to take advantage of certain exemptions from disclosure requirements available to emerging growth companies and/or smaller reporting companies, which could make our securities less attractive to investors and may make it more difficult to compare our performance with that of other public companies.
We are “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (“Section 404”), reduced disclosure obligations regarding executive compensation in their periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a registration statement under the Securities Act declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparability of our financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
69
Table of Contents
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K, which allows us to take advantage of certain exemptions from disclosure requirements including exemption from compliance with the auditor attestation requirements of Section 404 and reduced disclosure obligations regarding executive compensation in this prospectus and in our periodic reports and proxy statements. We will remain a smaller reporting company until the last day of the fiscal year in which (i) the market value of the shares of our common stock held by non-affiliates exceeds $250 million as of the prior June 30, and (ii) our annual revenue exceeded $100 million during such completed fiscal year or the market value of the shares of our common stock held by non-affiliates exceeds $700 million as of the prior June 30. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Securities litigation brought against us following volatility in the price of our common stock, regardless of the merit or ultimate results of such litigation, could result in substantial costs, which would hurt our financial condition and results of operations and divert management’s attention and resources from our business.
We have previously identified material weaknesses in our internal control over financial reporting and may identify additional material weaknesses in the future. If we fail to remediate a material weakness or if we otherwise fail to establish and maintain effective control over financial reporting, it may adversely affect our ability to accurately and timely report our financial results, and may adversely affect investor confidence and business operations.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
In connection with the audit of our consolidated financial statements as of and for the years ended December 31, 2023 and 2022, we identified material weaknesses in our internal control over financial reporting that we are currently working to remediate, which relate to: (a) insufficient segregation of duties in the financial statement close process; (b) a lack of sufficient levels of staff with public company and technical accounting experience to maintain proper control activities and perform risk assessment and monitoring activities; and (c) insufficient information systems controls, including access and change management controls. We have concluded that these material weaknesses in our internal control over financial reporting occurred because we do not have the necessary business processes, personnel and related internal controls to operate in a manner to satisfy the accounting and financial reporting timeline requirements of a public company.
We are focused on designing and implementing effective internal controls measures to improve our evaluation of disclosure controls and procedures, including internal control over financial reporting, and remediating the material weaknesses. We have taken steps to remediate including consulting with experts on technical accounting matters and in the preparation of our financial statements. We have also hired additional senior level experienced staff with public company experience and upgraded our enterprise resource planning system to SAP in August of 2022.
However, we cannot assure you that the measures we are taking to remediate the material weaknesses will prevent or avoid potential future material weaknesses. Further, additional weaknesses in our disclosure controls and internal controls over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls or any difficulties encountered in their implementation or improvement could limit our ability to prevent or detect a misstatement of our accounts or disclosures that could result in a material misstatement of our annual or interim financial statements. In such a case, we may be unable to maintain compliance with
70
Table of Contents
securities law requirements regarding timely filing of periodic reports in addition to the listing requirements of the NYSE, investors may lose confidence in our financial reporting and our stock price may decline as a result.
If we are unable to implement and maintain effective internal control over financial reporting in the future, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock may decrease.
We are in the process of designing and implementing our internal controls over financial reporting, which will be time-consuming, costly and complicated. We have identified gaps in our internal control environment in the past and cannot provide assurances that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. If we identify additional material weaknesses in our internal control over financial reporting, if we are unable to comply with the requirements of Section 404 in a timely manner, if we are unable to assert that our internal control over financial reporting is effective or, once required, if our independent registered public accounting firm is unable to attest that our internal control over financial reporting is effective, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could decrease. We could also become subject to stockholder or other third-party litigation as well as investigations by the NYSE, the SEC or other regulatory authorities, which could require additional financial and management resources and could result in fines, trading suspensions or other remedies.
As a public reporting company, we are subject to filing deadlines for reports that we file pursuant to the Exchange Act, and our failure to timely file such reports may have material adverse consequences on our business.
We did not file our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023 (the “Second Quarter 2023 10-Q”) within the timeframe required by the SEC; thus, we have not remained current in our reporting requirements with the SEC since we became an SEC reporting company on August 1, 2023. Although we have since regained status as a current filer given that the Second Quarter 2023 10-Q was filed on October 20, 2023, we will not be eligible to use a registration statement on Form S-3 that would allow us to continuously incorporate by reference our SEC reports into the registration statement, or to use “shelf” registration statements to conduct offerings, until approximately one year from the date we regained (and maintain) status as a current filer. Until such time, if we determine to pursue an offering, we would be required to conduct the offering on an exempt basis, such as in accordance with Rule 144A, or file a registration statement on Form S-1. Using a Form S-1 registration statement for a public offering would likely take significantly longer than using a registration statement on Form S-3 and increase our transaction costs, and could, to the extent we are not able to conduct offerings using alternative methods, adversely impact our liquidity, ability to raise capital or complete acquisitions in a timely manner.
We cannot guarantee that in the future our reporting will always be timely. If we are unable to satisfy SEC filing deadlines or otherwise provide disclosures of material information on a timely basis, stockholders and potential investors in our common stock may have incomplete information about our business and results of operations, which may impact their ability to make an informed investment decision, result in a reduction in the trading price, trading volume or analyst coverage of our common stock or expose us to potential liability.
An active trading market may not develop or be sustained.
The market for our securities may be highly volatile or may decline regardless of our operating performance. An active public market for our securities may not develop or be sustained. We cannot predict the extent to which investor interest in Allurion will lead to the development of an active trading market in our common stock or how liquid that market might become. If an active market does not develop or is not sustained, or if we fail to satisfy the continued listing standards of the NYSE for any reason and our securities are delisted, it may be difficult for you to sell your securities at the time you wish to sell them, at a price that is attractive to
71
Table of Contents
you, or at all. An inactive trading market may also impair our ability to both raise capital by selling shares of capital stock, attract and motivate employees through equity incentive awards and acquire other companies, products or technologies by using shares of capital stock as consideration.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, our common stock share price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. If few or no securities or industry analysts cover us, the trading price for our common stock would likely be negatively impacted. If one or more of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, our share price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our share price would likely decline. If one or more of these analysts cease coverage of Allurion or fail to publish reports on us regularly, demand for our common stock could decrease, which might cause our share price and trading volume to decline.
Provisions in our Charter and Bylaws could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our Charter and Bylaws may discourage, delay, or prevent a merger, acquisition, or other change in control of us that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for our common stock, thereby depressing the market price of our common stock. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board. Because our Board is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. Among others, these provisions include the following:
| • | our Board is divided into three classes with staggered three-year terms which may delay or prevent a change of our management or a change in control; |
| • | our Board has the right to elect directors to fill a vacancy created by the expansion of our Board or the resignation, death, or removal of a director, which will prevent stockholders from being able to fill vacancies on our Board; |
| • | our stockholders are not able to act by written consent, and as a result, a holder, or holders, controlling a majority of our shares are not be able to take certain actions other than at annual stockholders’ meetings or special stockholders’ meetings; |
| • | our Charter does not allow cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| • | amendments of our Charter and Bylaws require the approval of stockholders holding 66 2/3% of our outstanding voting shares (unless amended by our Board); |
| • | our stockholders are required to provide advance notice and additional disclosures in order to nominate individuals for election to our Board or to propose matters that can be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of Allurion; and |
| • | our Board is able to issue, without stockholder approval, preferred shares with voting or other rights or preferences that could impede the success of any attempt to acquire us. |
72
Table of Contents
Sales of shares of our common stock may cause the market price of our common stock to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of our common stock intend to sell shares, could increase the volatility of the market price of our common stock or result in a significant decline in the public trading price of our common stock.
While the Sponsor and certain Legacy Allurion Stockholders have signed the Investor Rights Agreement which contains lock-up restrictions for a period of either 18 months or 12 months following the consummation of the Business Combination, as applicable, and certain other Legacy Allurion Stockholders are subject to similar lock-up restrictions pursuant to our Bylaws, the lock-up restrictions shall not apply to: (a) any shares of our common stock purchased pursuant to the PIPE Subscription Agreements (as defined below), (b) 100 shares of our common stock held by each Investor (as defined in the Investor Rights Agreement), (c) shares issued to the Sponsor in the Sponsor Loan Equity Issuance, (d) certain incremental shares of PIPE Investors who are Legacy Allurion Stockholders or holders of Legacy Allurion Convertible Notes, or shares issued upon conversion of the convertible notes issued between February 2023 and August 2023 (“2023 Convertible Notes”), (e) the Backstop Shares or the shares of our common stock issued to each of HVL, RTW and Fortress, and such shares of our common stock will be freely tradeable subject to federal securities laws and other applicable rules and regulations.
In addition, the effective purchase prices at which certain independent directors of Compute Health, certain Legacy Allurion Stockholders, the PIPE Investors, RTW, a Fortress affiliate and HVL acquired their shares of our common stock are generally substantially less than the IPO price of $7.04 per share, after giving effect to the CPUH Exchange Ratio. Consequently, such stockholders may realize a positive rate of return on the sale of their shares of common stock even if the market price per share of our common stock is below $7.04 per share. While some of our securityholders may experience a positive rate of return based on the current trading price, public securityholders may not experience a similar rate of return on the securities they purchased due to differences in the purchase prices they paid and the trading price at the time of sale and may instead experience a negative rate of return on their investment. On May 30, 2024, the last quoted sale price for our common stock as reported on the NYSE was $1.51 per share.
Consequently, these securityholders may have an incentive to sell their shares of our common stock even if the trading price is below the price paid by investors in Compute Health’s IPO, which could cause the market price of our common stock to decline.
In addition, on December 18, 2023, we entered into the Chardan Purchase Agreement with Chardan related to the Chardan Equity Facility. Pursuant to the Chardan Purchase Agreement, Chardan shall purchase from us up to $100.0 million of shares of our common stock, upon the terms and subject to the conditions and limitations set forth in the Chardan Purchase Agreement.
The shares of our common stock that may be issued under the Chardan Purchase Agreement may be sold by us to Chardan at our discretion from time to time. The purchase price for shares of our common stock that we may sell to Chardan under the Chardan Purchase Agreement will fluctuate based on the trading price of shares of our common stock. As a result, investors who purchase shares from Chardan at different times will likely pay different prices for those shares, and so may experience different levels of dilution and in some cases substantial dilution and different outcomes in their investment results. Investors may experience a decline in the value of the shares they purchase from Chardan as a result of future sales made by us to Chardan at prices lower than the prices such investors paid for their shares.
Depending on market liquidity at the time, sales of shares of our common stock may cause the trading price of shares of our common stock to decrease. We generally have the right to control the timing and amount of any future sales of shares of our common stock to Chardan. Additional sales of shares of our common stock, if any, to
73
Table of Contents
Chardan will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to Chardan all, some or none of the additional shares of our common stock that may be available for us to sell pursuant to the Chardan Purchase Agreement. If and when we do sell shares of our common stock to Chardan, after Chardan has acquired shares of our common stock, Chardan may resell all, some or none of such shares of our common stock at any time or from time to time in its discretion. Therefore, sales to Chardan by us could result in substantial dilution to the interests of other holders of shares of our common stock. In addition, if we sell a substantial number of shares of our common stock to Chardan under the Chardan Purchase Agreement, or if investors expect that we will do so, the actual sales of shares of our common stock or the mere existence of our arrangement with Chardan may make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect such sales.
Under applicable NYSE rules, in no event may we issue to Chardan shares of our common stock representing more than the lower of the 19.99% voting power threshold and the 19.99% share and share equivalent thresholds referenced in Section 312.03(c) of the NYSE Listed Company Manual, unless we obtain prior stockholder approval or if such approval is not required in accordance with the applicable NYSE rules. In addition, Chardan is not obligated to buy any common stock under the Chardan Purchase Agreement if such shares, when aggregated with all other common stock then beneficially owned by Chardan and its affiliates (as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 promulgated thereunder), would result in Chardan beneficially owning common stock in excess of 4.99% of our outstanding voting power or shares of common stock.
Although the initial conversion for the Notes sold to the purchasers and RTW pursuant to the Amended Note Purchase Agreement is above the NYSE’s “Minimum Price” (as such term is defined in Section 312.03 of the NYSE Listed Company Manual), it is subject to a reset provision at the time of the Next Equity Financing that could result in the conversion price falling below such Minimum Price. Therefore, the Amended Note Purchase Agreement provides that unless and until requisite Stockholder Approval is obtained, we will not deliver common stock upon conversion of the Notes in excess of 1% of the number of shares of the common stock outstanding as of April 14, 2024. We are required to include a proposal in our definitive proxy statement on Schedule 14A seeking Stockholder Approval no later than December 31, 2025. If we do not obtain Stockholder Approval at such meeting, we shall call a special meeting of stockholders each 90-day period thereafter at least two times, and thereafter at each subsequent annual meeting until Stockholder Approval is obtained or the Notes are no longer outstanding; provided, that shares of common stock issued upon conversion of the Notes prior to obtaining Stockholder Approval shall not be entitled to vote in favor of Stockholder Approval.
Other than as described above, there are no lock-up, beneficial ownership or stock exchange restrictions that would prevent the foregoing stockholders from selling some or all of their common stock subject to compliance with applicable rules and regulations.
Our existing warrants are exercisable for common stock, the exercise of which would increase the number of shares eligible for future resale in the public market and result in dilution to our stockholders.
As of March 31, 2024, there were 13,206,822 Public Warrants to purchase an aggregate of 18,759,696 shares of common stock at an exercise price of $8.10 per share outstanding and 403,658 Rollover Warrants to purchase an aggregate of 403,658 shares of common stock at exercise prices ranging from $0.02 per share to $12.14 per share outstanding. To the extent such existing warrants are exercised, additional shares of our common stock will be issued, which will result in dilution to the holders of our common stock and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market could adversely affect the market price of our common stock, the impact of which increases as the value of our stock price increases.
74
Table of Contents
Our existing warrants may not be exercised at all and we may not receive any cash proceeds from the exercise of such warrants.
Due to the significant number of redemptions of Compute Health Class A Common Stock in connection with the Business Combination, there was a significantly lower number of shares of Compute Health Class A Common Stock that converted into shares of our common stock in connection with the Business Combination. As a result, the shares of our common stock previously registered for resale (a substantial portion of which may not be resold until the expiration of the applicable lock-up period) are anticipated to constitute a considerable percentage of our public float. Additionally, a significant portion of the shares of our common stock registered for resale were purchased by securityholders pursuant to investments in Legacy Allurion that date from 2013 through the closing of the Business Combination at prices considerably below the current market price of our common stock. This discrepancy in purchase prices may have an impact on the market perception of our common stock’s value and could increase the volatility of the market price of our common stock or result in a significant decline in the public trading price of our common stock. The registration of these shares for resale creates the possibility of a significant increase in the supply of our common stock in the market. The increased supply, coupled with the potential disparity in purchase prices, may lead to heightened selling pressure, which could negatively affect the public trading price of our common stock.
The exercise prices of the existing warrants, in certain circumstances, may be higher than the prevailing market price of our underlying common stock and the cash proceeds to us associated with the exercise of such warrants are contingent upon our stock price. The value of our common stock may fluctuate and may not exceed the exercise price of the existing warrants at any given time. As of the date of this prospectus, all of our Public Warrants, each of which has an exercise price of $8.10 per share, are “out of the money,” meaning the exercise price is higher than the market price of our common stock. Of the 403,658 Rollover Warrants outstanding as of March 31, 2024, 264,801 of such warrants (44,272 of which have an exercise price of $2.43, 130,053 of which have an exercise price of $6.73, and 90,476 of which have an exercise price of $12.14) are “out of the money.” Holders of such “out of the money” warrants are not likely to exercise such warrants. There can be no assurance that such warrants will be in the money prior to their respective expiration dates, and therefore, we may not receive any cash proceeds from the exercise of such warrants.
Certain of our existing Warrants are accounted for as liabilities and the changes in value of such Warrants could have a material effect on, or cause volatility in, our financial results.
In connection with the Business Combination, we assumed our Public Warrants to purchase up to 18,759,696 shares of our common stock (which were originally issued as warrants to purchase shares of Compute Health Class A common stock in connection with Compute Health’s IPO) and Rollover Warrants to purchase up to 403,658 shares of our common stock (which were originally issued as warrants to purchase shares of Legacy Allurion Common Stock and Legacy Allurion Preferred Stock). We evaluated the accounting treatment of such Warrants and determined to classify certain of such Warrants as liabilities measured at fair value. The fair value of such Warrants is remeasured on a quarterly basis with changes in the estimated fair value recorded in Other (expense) income on the consolidated statement of operations and comprehensive loss. Due to the recurring fair value measurement, we expect that we will recognize non-cash gains or losses on such Warrants each reporting period and that the amount of such gains or losses could materially impact or cause volatility in our financial results. For example, upon consummation of the Business Combination, the total value of the liability associated with the Public Warrants was $13.8 million measured at fair value based on the Public Warrant quoted price. However, at March 31, 2024, the fair value of the liability associated with the Public Warrants was determined to be $3.3 million.
Our Earn-Out Shares are accounted for as liabilities and the changes in value of such shares could have a material effect on, or cause volatility in, our financial results.
In connection with the Business Combination, holders of Legacy Allurion common stock and Legacy Allurion preferred stock and holders of vested options, warrants and restricted stock units exercisable or
75
Table of Contents
convertible into Legacy Allurion capital stock received the contingent right to receive additional shares of our common stock (the “Earn-Out Shares”) upon the achievement of certain earn-out targets. We evaluated the accounting treatment of our Earn-Out Shares and determined to classify such shares as liabilities measured at fair value. The fair value of such shares is remeasured on a quarterly basis over the earn-out period with changes in the estimated fair value recorded in other income (expense) on the consolidated statement of operations and comprehensive loss. Due to the recurring fair value measurement, we expect that we will recognize non-cash gains or losses on our Earn-Out Shares each reporting period and that the amount of such gains or losses could materially impact or cause volatility in our financial results. For example, upon consummation of the Business Combination, the fair value of the liability associated with the Earn-Out Shares was initially valued and recorded as $53.0 million. However, at March 31, 2024, the fair value of the liability associated with the Earn-Out Shares was determined to be $9.8 million.
The provisions of our Bylaws requiring exclusive forum in the Court of Chancery of the State of Delaware and the federal district courts of the United States for certain types of lawsuits may have the effect of discouraging lawsuits against our directors and officers.
Our Bylaws provide that, to the fullest extent permitted by law, and unless we consent in writing to the selection of an alternative forum, the Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) and any appellate court thereof will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of, or a claim based on, a breach of a fiduciary duty owed by any of our current or former directors, officers, or other employees or stockholders to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the DGCL, our Charter or our Bylaws (including the interpretation, validity or enforceability thereof) or as to which the DGCL confers jurisdiction on the Chancery Court of the State of Delaware, or (iv) any action asserting a claim governed by the internal affairs doctrine; provided, however, that the preceding clauses (i) through (iv) will not apply to any causes of action arising under the Securities Act or the Exchange Act, or to any claim for which the federal courts have exclusive jurisdiction. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions of our Bylaws as described above.
Additionally, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Accordingly, both state and federal courts have jurisdiction to entertain such Securities Act claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our Bylaws provide that, unless we consent in writing to the selection of an alternative forum, to the fullest extent permitted by law, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act, the Exchange Act, or the respective rules and regulations promulgated thereunder; however, there is uncertainty as to whether a court would enforce such provision, and investors cannot waive compliance with federal securities laws and the rules and regulations thereunder. These provisions may limit or increase the difficultly in a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors and officers, or may increase the cost for such stockholder to bring a claim, both of which may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any applicable action brought against us, a court could find the choice of forum provisions contained in our Bylaws to be inapplicable or unenforceable in such action. If a court were to find the choice of forum provision inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
76
Table of Contents
PRIVATE PLACEMENT OF NOTES
On April 14, 2024, we entered into the Original RTW Note Purchase Agreement with RTW, as agent for the Purchasers party thereto from time to time, and Acquiom, as collateral agent for the Purchasers and RTW. Subsequently, on April 16, 2024, we, RTW, the Purchasers, and Acquiom entered into the First Amendment to the Note Purchase Agreement (the “Amendment” and Amended Note Purchase Agreement.
Pursuant to the Amended Note Purchase Agreement, we issued and sold $48 million aggregate principal amount of Notes to the Purchasers in a private placement transaction. We used the proceeds from the issuance of the Notes to refinance our outstanding obligations under the Fortress Credit Agreement in full and to pay fees and expenses in connection therewith and in connection with the transactions contemplated by the Amended Note Purchase Agreement. We terminated and repaid in full the outstanding borrowings and other obligations under the Fortress Credit Agreement.
The Notes bear interest at the annual rate of 6.0%, which interest is payable quarterly in cash or, at our option, in kind for the first three years. The maturity date for the Notes will be April 16, 2031. The Notes are guaranteed by Allurion Opco, and certain other current and future subsidiaries of Allurion, and are secured by substantially all the assets of Allurion and the guarantors.
The Notes are convertible into shares of our common stock, at a purchaser’s election at any time after the earliest of (i) the date on which Stockholder Approval (as defined below) is obtained, (ii) December 31, 2025, (iii) the date of a Fundamental Change Company Notice (as defined in the Amended Note Purchase Agreement), and (iv) the Make-Whole Fundamental Change Effective Date (as defined in the Amended Note Purchase Agreement), subject to certain terms and limitations in the Amended Note Purchase Agreement, based on the higher of (x) an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of Notes (equivalent to an initial conversion price of approximately $3.26 per share, which represents a 35% premium to the trailing 30 day volume weighted average price of the common stock on the NYSE as of the close of business on April 12, 2024) and (y) a 35% conversion premium to the lowest price per share in the “Next Equity Financing”. If certain corporate events that constitute a “Make-Whole Fundamental Change” (as defined in the Amended Note Purchase Agreement) occur, then the conversion rate will, in certain circumstances, be increased for a specified period of time up to a maximum rate of an additional 321.9182 shares of common stock per $1,000 principal amount of Notes. In addition, calling any note for redemption will also constitute a Make-Whole Fundamental Change with respect to that Note, in which case the conversion rate applicable to the conversion of that Note will be increased in certain circumstances if it is converted after it is called for redemption. Although the initial conversion price based on clause (x) above for the Notes is above the NYSE’s “Minimum Price” (as such term is defined in Section 312.03 of the NYSE Listed Company Manual), it is subject to a reset provision at the time of the Next Equity Financing that could result in the conversion price falling below such Minimum Price. Therefore, the Amended Note Purchase Agreement provides that unless and until the Company obtains Stockholder Approval, we will not deliver common stock upon conversion of the Notes in excess of 479,196 shares of common stock, or 1% of the number of shares of the common stock outstanding as of April 14, 2024. We are required to include a proposal in our definitive proxy statement on Schedule 14A seeking Stockholder Approval no later than December 31, 2025. If we do not obtain Stockholder Approval at such meeting, we shall call a special meeting of stockholders each 90-day period thereafter at least two times, and thereafter at each subsequent annual meeting until Stockholder Approval is obtained or the Notes are no longer outstanding; provided, that shares of common stock issued upon conversion of the Notes prior to obtaining Stockholder Approval shall not be entitled to vote in favor of Stockholder Approval.
Subject to specified conditions, on or after April 16, 2028, the Notes are redeemable by us at a redemption price equal to 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date. Pursuant to the terms of the Amended Note Purchase Agreement, each Purchaser is subject to a beneficial ownership conversion limitation such that no Purchaser shall be permitted to convert Notes to the extent it would result in such Purchaser and its affiliates beneficially owning more than the beneficial ownership limitation, currently set at 9.99% of our common stock.
77
Table of Contents
Pursuant to the terms of the Amended Note Purchase Agreement, until the Notes are converted or repaid in full, RTW will be entitled to designate one representative who will serve as a non-voting board observer to our Board. We also must include an additional director nominee in our proxy statement for the election of the Class I directors at our 2024 annual meeting of stockholders, with the recommendation of the Board to vote in favor of such additional nominee as well as the RTW Designated Director. Such additional director nominee shall go through our director nomination process led by the Nominating and Corporate Governance Committee of the Board. RTW shall have the right to approve such additional nominee, with such approval not to be unreasonably withheld. In addition, the Amended Note Purchase Agreement contains a covenant that we not increase the size of our Board of Directors to more than seven members prior to December 31, 2024 without RTW’s prior written consent, which may not be unreasonably withheld.
The Amended Note Purchase Agreement contains customary terms and covenants, including negative covenants, such as limitations on indebtedness, liens, disposition of assets and mergers. The Amended Note Purchase Agreement also contains financial maintenance covenants, which require (i) Allurion and Allurion Opco to maintain not less than $12,500,000 in unrestricted cash in controlled accounts in the United States at all times, (ii) Allurion to receive minimum trailing twelve-month consolidated Revenue (as defined in the Amended Note Purchase Agreement) at amounts designated in the Amended Note Purchase Agreement, tested quarterly beginning with the twelve-month period ending March 31, 2025, and (iii) Allurion and its subsidiaries’ consolidated business operations outside the United States to be profitable for the trailing three-month period, tested quarterly beginning with the three-month period ending December 31, 2025. The Amended Note Purchase Agreement also contains customary events of default, including defaults related to payment compliance, material inaccuracy of representations and warranties, covenant compliance, bankruptcy and insolvency proceedings, cross-payment defaults and cross-acceleration to certain other material indebtedness agreements, and judgment default.
78
Table of Contents
USE OF PROCEEDS
All of the shares of our common stock offered by the selling securityholder will be solely for its account. We will not receive any of the proceeds from these sales.
The selling securityholder will pay any underwriting fees, discounts, selling commissions, stock transfer taxes and certain legal expenses incurred by such selling securityholder in disposing of its shares of common stock, and we will bear all other costs, fees and expenses incurred in effecting the registration of such securities covered by this prospectus, including, without limitation, all registration, listing and qualifications fees, printers fees, and fees and disbursements of our counsel and our independent registered public accountants.
79
Table of Contents
DETERMINATION OF OFFERING PRICE
We cannot currently determine the price or prices at which the shares of our common stock may be sold by the selling securityholder under this prospectus. Our common stock is listed on the NYSE under the symbol “ALUR.”
80
Table of Contents
MARKET PRICE AND DIVIDEND INFORMATION
Market Price
Our common stock and Public Warrants are listed on the NYSE under the symbols “ALUR” and “ALUR WS,” respectively.
The closing price of our common stock and our Public Warrants as reported the NYSE on May 30, 2024 was $1.51 per share and $0.19 per Public Warrant.
Holders
As of March 31, 2024, there were 248 holders of record of our common stock and one holder of record of our Public Warrants. The number of holders of record does not include, for example a substantially greater number of “street name” holders or beneficial holders whose shares are held of record by banks, brokers and other financial institutions.
Dividend Policy
We have not paid any cash dividends to date. The payment of cash dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any cash dividends will be within the discretion of our Board at such time. Our ability to pay dividends may also be restricted by the terms of any current or future credit agreement, or any of our or our subsidiaries’ future debt, or preferred equity securities. See the subsection entitled “Risk Factors—Risks Related to Our Financial Condition and Capital Requirements—We have a significant amount of debt, which may affect our ability to operate our business and secure additional financing in the future.”
81
Table of Contents
BUSINESS
Unless the context otherwise requires, all references in this section to the “company,” “we,” “us,” or “our” refer to the business of Legacy Allurion and its subsidiaries prior to the consummation of the Business Combination and to Allurion (formerly known as Allurion Technologies Holdings, Inc.) and its subsidiaries after giving effect to the Business Combination.
Overview
Our company is dedicated to ending obesity by creating a best-in-class weight loss platform to treat the estimated two billion people globally who are overweight. Our platform, the Allurion Program, features the Allurion Balloon and offers access to AI-powered remote patient monitoring tools, a proprietary behavior change program, secure messaging and video telehealth that are delivered by the VCS. Over 150,000 patients have already been treated commercially with the Allurion Balloon in over 50 countries outside of the United States.
The Allurion Balloon is swallowed as a capsule under the guidance of a health care provider without surgery, endoscopy, or anesthesia. The placement takes approximately 15 minutes during an outpatient visit (though times may vary across different outpatient offices). We believe the proprietary technologies that differentiate the Allurion Balloon enable improved safety and efficacy outcomes. In a prospective, non-randomized, open-label, registry trial, the Allurion Balloon demonstrated significant weight loss and low device- or procedure-related rates of serious adverse events, both of which results we believe compare favorably to that of our competitors.
The VCS is comprised of the following tools to support patients’ weight loss experience, which we believe benefit both patients and health care providers:
| • | For Allurion Program patients: Every current Allurion Program patient receives an Allurion Connected Scale and access to our App, which integrates data from the Allurion Connected Scale, to conveniently monitor weight, body fat, activity, sleep, and several other critical metrics. The App can also enable secure messaging and video telehealth with the patient’s care team and can deliver content from Allurion’s proprietary behavior change program – a library of over 100 weight loss actions related to diet, nutrition, mental health, sleep, goal setting, and a number of other topics-directly to the patient. The App also provides access to Coach Iris, a generative AI-powered health coach designed to enhance outcomes with the Allurion Program, offer always-on support, education and motivation, and maximize clinical efficiency. The App is available in over 15 languages. |
| • | For Allurion Program providers: Every Allurion Program provider receives access to Allurion Insights, which provides end-to-end remote patient monitoring powered by the Allurion Iris AI platform, which leverages machine learning to deliver key insights and streamline workflow. Allurion Insights offers real-time access to patient data and AI-powered analytics, 1:1 video telehealth and secure messaging directly to the patient’s App, note functionality to keep track of patient encounters, and clinic-wide metrics that provide a snapshot of the clinic’s overall performance. |
In addition to its use by Allurion Balloon patients, we believe the VCS can potentially be a platform for optimal long-term follow-up after other medical and surgical weight loss interventions in the future.
For example, VCS includes a Treatment Tracking and Clinic-Led Onboarding feature that enables seamless onboarding and management of patients undergoing one or multiple weight loss treatments, including gastric balloons such as the Allurion Balloon, surgery, or medications. In addition, in connection with our collaboration with Medtronic, we expect to develop bundled offerings that incorporate the VCS to onboard and manage Medtronic’s patients.
In November 2021, the FDA approved the IDE for our AUDACITY trial, a 48-week, prospective, randomized, open-label trial, in the United States. Enrollment in the AUDACITY trial is complete, with 550
82
Table of Contents
patients in the trial across 17 sites in the United States, the first of which was treated in July 2022. The results of the trial are expected by year-end 2024, and, if positive, are expected to support a PMA application to the FDA.
We have assembled a broad portfolio of intellectual property related to our medical device, the Allurion Balloon, and our supporting technology platform, the VCS. We believe this intellectual property, combined with proprietary manufacturing processes and the regulatory approvals we have successfully obtained outside of the United States, provides us with a strong market position. As of March 31, 2024, we owned or had rights to 18 issued and five pending patents in the United States related to various aspects of our Allurion Balloon such as a swallowable, self-deflating and naturally passing gastric balloon, improvements to the fill and release valves therein, methods for deploying and releasing a gastric balloon within the body, and next generation fill and release valves. In addition, as of March 31, 2024, we had 37 issued and five patents pending outside of the United States. We intend to continue to expand our intellectual property portfolio and invest in protecting new innovations.
To date, most of our revenues have been generated from sales of the Allurion Balloon. We began selling the Allurion Balloon in Europe in January 2016 and to date have launched in over 50 countries outside of the United States. We currently sell our products either via our direct sales force or, in certain countries, distributors.
Our Market Opportunity
According to the World Health Organization (“WHO”), over two billion people around the world are overweight and by 2030, one billion people globally will have obesity, defined as a body mass index (“BMI”) of 30 or greater. Likewise, according to WHO, the number of obese adults worldwide has nearly tripled since 1975. The global obesity treatment market is expected to be $54 billion by 2030.
Moreover, according to WHO, obesity is the leading cause of chronic diseases worldwide and leads to a higher risk of cardiovascular disease, type 2 diabetes, infertility, liver disease, and certain cancers. According to McKinsey, the annual global economic impact of obesity is estimated to be over $2 trillion.
We expect the rates of obesity to rise globally as access to calorie-rich foods increases and lifestyles become increasingly sedentary, especially among adolescents. According to WHO, the prevalence of obesity in children and adolescents has increased 10-fold in the past four decades and will fuel higher rates of adult obesity in the decades to come.
Despite the significant medical and economic burden that obesity poses, there remains a significant unmet need for safer, more effective, and more consumer-centric treatments.
Based on a market research study we conducted with 9,800 consumers in eight countries, where we assessed each participant’s weight, income level, and interest in various weight loss alternatives, we estimate that 4.3% of the population eligible for the Allurion Balloon and interested in treatment would consider the Allurion Program as a treatment for obesity. Furthermore, based on our market research, we estimate that 3% of the adult U.S. population, or approximately 10 million adults, would consider the Allurion Program as a weight loss treatment. We believe this is an $18 billion total addressable market in the United States.
Current Therapeutic Interventions Used in Weight Loss
Current treatment alternatives for patients who are obese and overweight begin with lifestyle modification, such as diet and exercise. If this course of treatment fails to produce the desired results, as is often the case, physicians may prescribe pharmaceutical therapies, and in patients with more severe obesity, physicians may pursue aggressive bariatric surgical treatments, such as gastric bypass and sleeve gastrectomy. These approaches are associated with concerns around safety, permanence, lifestyle impact, ease of use, cost and compliance issues, as well as the significant weight re-gain associated with such approaches that have limited their adoption.
83
Table of Contents
Lifestyle Modification
Lifestyle modification, which includes diet, exercise and behavior modification delivered either in-person or digitally, is usually prescribed as an initial treatment for a patient who is obese or overweight. However, lifestyle modification alone has generally been ineffective in producing sustainable weight loss in patients with obesity due to poor adherence over an extended period. Many studies have shown that a significant majority of dieters will regain lost weight and many will gain more than they originally lost.
Pharmaceutical Therapy
Pharmaceutical therapy often represents a first option in the treatment of patients with obesity who have failed to achieve weight loss goals through lifestyle modifications alone. Pharmaceutical therapies can have limited effectiveness due to non-adherence and, in most cases, need to be taken for life. In addition, more recent pharmaceutical therapies, commonly known as GLP-1s, require once weekly injections and impose significant financial costs on the patient. Since these drugs are absorbed into the bloodstream, they have been shown to pose significant safety risks and negative systemic side effects, such as adverse gastrointestinal, cardiovascular and central nervous system issues, some of which are serious or life threatening.
Bariatric Surgery
Bariatric surgery is a treatment option generally reserved for cases of severe obesity in patients with a BMI greater than 40. Each year, approximately 580,000 people undergo bariatric surgery worldwide. The most common forms of bariatric surgery, gastric bypass and sleeve gastrectomy, promote weight loss by surgically restricting the stomach’s capacity and outlet size. Gastric bypass also affects weight loss by restricting the body’s ability to absorb nutrients. These procedures are highly invasive, inherently risky in a high BMI population, expensive for the patient, and irreversible. Moreover, patients cite fear of complications as the primary reason to not pursue bariatric surgery. Only 1% of patients who qualify for bariatric surgery actually get it.
Bariatric surgery patients are generally required to make significant postoperative lifestyle changes, including strict dietary changes, vitamin supplementation and long-term medical follow-up programs. Side effects of bariatric surgery include a high rate of re-operation, nausea, vomiting, dumping syndrome, dehydration, and even death. Moreover, up to 25% of patients undergoing bariatric surgery will regain all of the weight previously lost as a result of the surgery.
Recently Developed Treatment Alternatives
Given the shortcomings and limitations of existing treatment alternatives, new medical procedures have recently been introduced. Endoscopic balloon therapy involves an endoscopic procedure with anesthesia to implant a balloon in the stomach that leads to satiety, followed by another endoscopic procedure with anesthesia several months later to remove the balloon.
We believe high costs, procedural complexity, poor consumer experiences, lack of ongoing patient support and follow-up, and the risk of serious side effects have limited the adoption of endoscopic balloon therapy:
| 1. | Rate of SAEs. In the ReShape Lifesciences, Inc. pivotal clinical trial for its ReShape Integrated Duo Balloon System, 31 device- or procedure-related serious adverse events were reported in 20 patients, resulting in a SAE rate of approximately 7.5%. Similarly, in the Apollo Endosurgery, Inc. pivotal clinical trial for its ORBERA Intragastric Balloon System, 17 SAEs were reported in 16 patients, resulting in an SAE rate of approximately 10%. In both trials, there were multiple SAEs related to the endoscopy and anesthesia required for placement and removal of the balloons. |
| 2. | Lack of comfort and tolerability. The ReShape Duo Balloon and ORBERA Balloon are manufactured from thick silicone containing rigid components. We believe that the materials used in these balloons can lead to discomfort, trauma to the stomach lining, and growth of bacteria and fungi |
84
Table of Contents
| on the balloon surface. Intolerance rates in endoscopic balloons requiring the balloons to be removed were approximately 14-17%, compared with approximately 1-3% for the Allurion Balloon. |
| 3. | Limited ability to provide progressive and sustained weight loss. For patients receiving balloon treatment in the ReShape Duo Balloon pivotal trial, the mean weight loss at 24 weeks was just 14.3 pounds. Furthermore, the average treatment subject in ReShape’s pivotal trial with weight loss at 24 weeks regained 40% of the weight loss at 48 weeks, resulting in a mean weight loss of 9.9 pounds at 48 weeks. |
| 4. | Inconvenient placement procedure. The placement procedures for the ReShape Duo Balloon and the ORBERA Balloon require both the device placement and the device removal to be performed in an endoscopic procedure using anesthesia. The patient cannot immediately return to normal activities and must be placed under medical observation for at least a few hours until cleared to go home. |
Our Platform
The Allurion Program
The Allurion Program features the Allurion Balloon and offers access to the VCS, a cutting-edge digital therapeutic that combines AI-powered remote patient monitoring tools with a proprietary behavior change program.
The Allurion Balloon
The Allurion Balloon is a first of its kind, ProcedurelessTM intragastric balloon, that does not require any surgery, endoscopy, or anesthesia for placement. The balloon is swallowed in a capsule during a discrete outpatient office visit that takes approximately 15 minutes (though times may vary across different outpatient offices). Once the capsule is in the stomach, a delivery catheter is used to fill the Allurion Balloon with approximately 550 milliliters of filling fluid. Approximately four months later, a patented time-activated ReleaseValve™ opens and allows the balloon to empty and pass out of the body naturally, although in rare cases, endoscopic or surgical intervention may be required for removal.
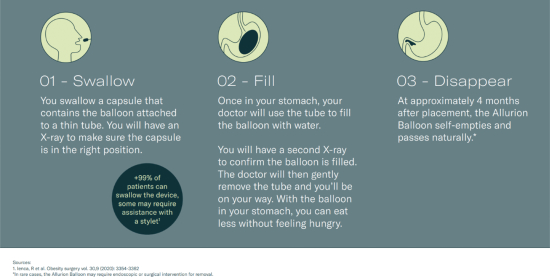
The Allurion Balloon is designed to help reduce a patient’s food intake by taking up space in the stomach and slowing the rate at which the stomach empties. By the time the Allurion Balloon passes, patients develop new food preferences, including smaller portion sizes, which we believe leads to maintainable, long-lasting
85
Table of Contents
results. We believe our clinical studies support that the Allurion Balloon can be swallowed, filled, and passed, and provide short and long-term therapeutic benefit while minimizing risks.
The Allurion Balloon is comprised of several novel and innovative features that differentiate it from previous intragastric balloons and enable it to be swallowed and then naturally passed, including:
| • | Dissolvable capsule. We designed the capsule to be large enough to accommodate the folded balloon, yet small enough to be swallowed. The capsule is titrated to optimize dissolution timing. If the capsule dissolves too quickly, the balloon could be prematurely released before entering the stomach, and if too slowly, the patient and physician are inconvenienced by having to wait longer to fill the balloon. |
| • | Balloon film. Our film is made from a polyether polyurethane that was specifically chosen to be extrudable into a film thin enough to fit into a capsule and pass through the gastrointestinal tract yet impervious to the chemical environment and mechanical forces of the stomach. The film is biocompatible, cost-effective to extrude and manufacture, and puncture resistant, all while being smooth and atraumatic to the gastrointestinal lining. |
| • | Balloon valves. Our Allurion Balloon contains two valves: a fill valve and ReleaseValve™. The valves are constructed from polyurethane film and, unlike valves used in other intragastric balloons, there are no rigid parts. This design ensures that the valves are atraumatic to the stomach lining and can pass through the gastrointestinal tract without obstructing. Both valves are small and flexible so that they can be folded to fit inside the capsule. |
| o | The fill valve is designed to reseal after the delivery catheter is removed. It also contains a radiopaque marker so that the Allurion Balloon can be visualized on x-ray. |
| o | The ReleaseValve™ is constructed from a degradable polymer that faces the inside of the Balloon. Once the degradable polymer is fully degraded, the ReleaseValve™ opens, the Allurion Balloon empties and then passes through the gastrointestinal tract to be excreted. |
| • | Delivery catheter. Our delivery catheter is designed to quickly fill the Allurion Balloon. It is small, flexible, and smooth in order to minimize any potential discomfort to the patient during balloon placement. In addition, the catheter contains length markings to measure transit through the esophagus and into the stomach and is radiopaque to facilitate visualization on x-ray. |
The Allurion Virtual Care Suite
The VCS is a cutting-edge digital therapeutic that combines AI-powered remote patient monitoring tools with a proprietary behavior change program.

The VCS is comprised of tools to support patients’ weight loss experience, which we believe benefit both patients and health care providers:
| • | For patients, the App integrates data from the Allurion Connected Scale, smart watches and other wearables and trackers to conveniently monitor weight, body fat, activity, sleep, and several other |
86
Table of Contents
critical metrics. The App also enables secure messaging and video telehealth with the patient’s care team and delivers content from our proprietary behavior change program-a library of over 100 weight loss actions developed by our team of behavior change experts related to diet, nutrition, mental health, sleep, goal setting, and a number of other topics-directly to the patient. The App also provides access to Coach Iris, a generative AI-powered health coach designed to enhance outcomes with the Allurion Program, offer always-on support, education and motivation, and maximize clinical efficiency. The App is available in over 15 languages. |
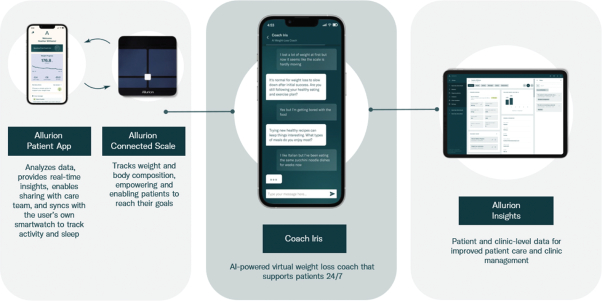
| • | For health care providers, Allurion Insights provides end-to-end remote patient monitoring powered by the Allurion Iris AI platform, which leverages machine learning to deliver key insights and streamline workflow. Allurion Insights offers real-time access to patient data and AI-powered analytics, 1:1 video telehealth and secure messaging directly to the patient’s App, note functionality to keep track of patient encounters, and clinic-wide metrics that provide a snapshot of the clinic’s overall performance. |
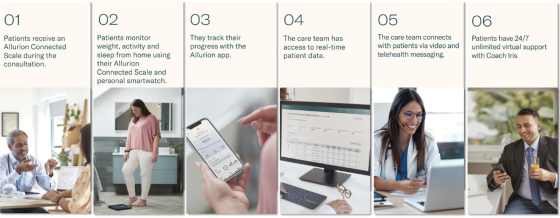
In addition to its use by Allurion Balloon patients, we believe the VCS can potentially be a platform for optimal long-term follow up after other medical and surgical weight loss interventions in the future. In June 2022, we incorporated a Treatment Tracking and Clinic-Led Onboarding feature into the VCS, which enables seamless onboarding and management of patients undergoing one or multiple weight loss treatments including
87
Table of Contents
gastric balloons such as the Allurion Balloon, surgery, or medications. In addition, in connection with our collaboration with Medtronic, we expect to develop bundled offerings that incorporate the VCS to onboard and manage Medtronic’s patients.
Allurion Program Trials
The Allurion Balloon has demonstrated favorable short and long-term results for weight loss and resolution of co-morbidities in multiple trials, with few adverse events.
In a prospective, non-randomized, open-label, registry trial of 1,770 patients, Allurion Balloon patients lost 14% of total body weight or 30 pounds on average after just four months. In another trial of 509 patients, average weight loss was 13.9% of total body weight at four months and 13.3% of total body weight at one year after balloon passage, representing a 96% maintenance of total body weight loss. In a third trial of 42 patients treated with a second balloon, average total body weight loss was 23%. In a study of 5,003 patients treated with the Allurion program, patients lost an average of 14% after 4 months.
In a trial of 232 Allurion Balloon patients who maintained ongoing lifestyle modification after balloon passage, average weight loss was 17% of total body weight at one year; 96% of weight loss was maintained one year after removal of the Allurion Balloon. In a trial of 181 patients that combined Allurion Balloon treatment with a GLP-1 weight loss drug, the first multi-center trial of balloon and drug combination therapy demonstrating significant synergies, average weight loss was 19% of total body weight at eight months.
In a trial of 518 patients (225 with type 2 diabetes and 293 with pre-diabetes) treated with the Allurion Program, those with type 2 diabetes reduced their hemoglobin A1c (HbA1c) on average by 1.6 points, or 23%, and those with pre-diabetes reduced their HbA1c by 0.8 points, or 14%. In a separate trial of 115 patients treated with the Allurion Balloon, co-morbidities were reduced meaningfully – 56% resolution of Type 2 diabetes, 59% resolution of hypertension, and 58% resolution of obstructive sleep apnea. When combined with GLP-1 drugs, patients showed 17.6% weight-loss on average after four months. After two sequential balloons, 26% percent of patients achieved weigh loss of 30% or more.
The FDA approved the IDE for our AUDACITY trial, a 48-week, prospective, randomized, open-label trial comparing the Allurion Program to moderate intensity lifestyle intervention. We received approval of the IDE from the FDA in November 2021 to initiate the AUDACITY clinical trial in the United States. The first patient was treated in July 2022. During the third quarter of 2023, we completed the enrollment of 550 patients in the trial across 17 sites in the United States.
Subjects in the treatment arm will be eligible for up to two Allurion Balloons. Co-primary endpoints of the trial include a 50% responder rate, defined as greater than 5.0% of total body weight loss, at 48 weeks following balloon placement, and a 3.0% total body weight loss superiority margin at 48 weeks. The results of the trial, if positive, are expected to support a PMA application to the FDA.
Prior ENLIGHTEN Trial
AUDACITY’s trial design reflects the FDA’s updated recommendations for weight loss devices and builds upon the ENLIGHTEN trial, our prior IDE trial which was conducted in 2018-2019. The ENLIGHTEN trial featured a sham-controlled design with one balloon cycle. The Allurion Balloon met the co-primary endpoint related to responder rate but failed to meet the co-primary endpoint on superiority margin due to sham overperformance.
In 2019, the FDA issued a White Paper on Weight Loss Devices after safety issues were encountered with other weight loss balloons (ReShape and ORBERA) on the market. The FDA’s updated guidance for clinical
88
Table of Contents
trials for weight loss balloons required increased efficacy and increased the minimum treatment duration to 6 months with a preference for one-year outcomes. We designed the AUDACITY trial in collaboration with the FDA in 2021 to address these new criteria.
We believe that AUDACITY improves upon ENLIGHTEN given the open-label trial design (i.e., no sham), utilization of multiple balloon cycles, and alignment with the FDA’s updated guidance on intragastric balloons for weight loss.
Our Business Model
We believe that our business-to-business-to-consumer business model creates an economic benefit for all key stakeholders. Health care providers may benefit from providing the Allurion Program because it addresses a significant unmet need for their patients and is designed to not require time-consuming surgery, endoscopy, or anesthesia. Moreover, we can provide our product to health care providers who have historically not been able to provide cash pay weight loss procedures, because the Allurion Balloon does not require endoscopy or anesthesia for placement and hence there are fewer restrictions on the type of doctor that can use the Allurion Balloon versus other balloons. Patients may benefit because placement procedures do not require invasive surgery, endoscopy, or anesthesia and hence may reduce the overall cost and inconvenience of getting treated.
We believe our platform addresses the following limitations of current weight loss treatments:
| • | Poor patient and health care provider experience. Many other weight-loss innovations lack remote patient monitoring or behavior modification. By combining a therapeutic medical device with remote patient monitoring and behavior modification, we believe we can improve both the patient and provider experiences. |
| • | More complex safety profile. Weight loss treatments that require surgery, endoscopy, or anesthesia may result in SAEs that limit adoption. These invasive procedures are inherently risky in a high BMI population, especially where patients must undergo anesthesia. The Allurion Balloon has been observed to result in fewer SAEs when compared to other balloons. |
| • | Poor economics. All-in costs for cash-pay bariatric surgery can cost patients approximately $14,000 out-of-pocket on average and up to approximately $33,000 with limited insurance coverage. Weight loss drugs can cost patients as much as $1,000 per month, and weight loss is dependent on patients continuing to use the drugs for life. By removing endoscopy and anesthesia from the placement and removal (except in rare cases) of the Allurion Balloon, we believe that the Allurion Program is significantly more affordable for patients than the alternatives while maintaining attractive health care provider economics. |
| • | Limited channels. We believe most health care providers lack the necessary infrastructure and training to deliver a comprehensive weight-loss platform. Moreover, interventions that require surgery, endoscopy or anesthesia must be performed by gastroenterologists or surgeons, many of whom are not weight-loss specialists. We believe that the Allurion Program can be delivered by a much wider group of health care providers across multiple specialties. |
| • | Flawed go-to-market strategy. We believe other weight-loss companies have deployed strategies which fail to embrace modern-day digital advertising and account training techniques. We believe that the strategies we have developed to acquire leads, increase conversion through the funnel, and educate providers significantly improve the scalability of our business compared to the competition. |
89
Table of Contents

Our Competitive Strengths
We developed the Allurion Program to overcome the limitations of other weight loss treatments, including the other intragastric balloons. Based on our commercial experience in over 150,000 patients, we believe that the Allurion Program provides considerable advantages to patients and providers:
Consumer-centric, ProcedurelessTM technology with favorable safety profile. The Allurion Balloon does not require surgery, endoscopy, or anesthesia for placement; in rare cases, endoscopic or surgical intervention may be required for removal. We believe this results in a safer, easier, faster, and more convenient patient experience at a lower cost and a device that can be administered by a wide array of providers. Though the Allurion Balloon has not been compared in head-to-head trials with other liquid-filled intragastric balloons, Allurion has reported a lower device or procedure-related SAE rate than competing intragastric balloons, with considerably better weight loss results.
More than just a balloon: an end-to-end weight management platform powered by AI and data. The Allurion Program features a uniquely designed medical device and offers access to a proprietary clinically-proven behavior change program and AI-powered remote patient monitoring through the VCS. We believe this holistic approach can improve outcomes for patients, streamline provider workflow, enable end-to-end weight management, and open the door to a life-long relationship with the patient. Further, we believe that the ongoing stream of data we receive on patient outcomes and provider productivity will enable us to enhance the capabilities of our AI platform and further expand our data moat.
90
Table of Contents

Life-changing clinical outcomes that are fast yet durable. On average, Allurion patients lose 14% of their total body weight (approximately 14kg or 30lbs) over just four months and sustain 95% of that weight loss at one year. We have also observed similarly significant effects on obesity-related co-morbidities like type 2 diabetes
Attractive economics for patients and providers. By eliminating endoscopy and anesthesia from balloon placement and removal, we believe that we have made our weight loss product more affordable to the patient and more lucrative for the provider compared to the competition. Health care providers can treat patients with a high- margin device in just a 15-minute office visit, which when compared to devices that require hospital stays, significantly improves profitability per hour.
Broad patent portfolio and proprietary manufacturing capabilities. We have a broad portfolio of intellectual property-including 55 issued patents protecting our products as of March 31, 2024, which we believe, when combined with our proprietary manufacturing processes and know-how, leads to a significant competitive moat. Currently, the Allurion Balloon is manufactured and assembled in-house using components and sub-assemblies at our facilities in Natick, Massachusetts, which further enhances our ability to maintain high levels of quality and protect manufacturing trade secrets that we have developed since inception.
Proven management team with expansive industry experience. Our executive team consists of seasoned medical device and digital health professionals with deep industry experience and expertise, who have led and managed high-growth private and public companies that have introduced and commercialized multiple new products.
Our Growth Strategy
Our primary objective is to become the world’s leading weight loss treatment provider and fulfill our mission to end obesity. The key elements to our strategy are the following:
| • | Broaden our Global Presence. The Allurion Program is currently sold in over 50 countries around the world. In these existing markets, we employ a multi-faceted marketing strategy that includes online |
91
Table of Contents
advertising, co-op marketing campaigns, and professional education programs. This approach enables us to engage with and educate patients and providers, increase the awareness and credibility of our program, provide qualified leads to our accounts, and increase the productivity of our providers. We intend to invest strategically in our existing markets and leverage the commercial strategies we have deployed successfully thus far to drive procedure and revenue growth by increasing the productivity of existing accounts and opening new, high-potential accounts. |
| • | Launch the Allurion Program in New Markets, including the U.S. The addressable market for the Allurion Balloon in the United States is currently over $10 billion. To commercialize in the United States, we are conducting our FDA-approved clinical trial, AUDACITY. We expect readout data for this trial by year-end 2024, which, if positive, will support our PMA submission to the FDA. In addition to the United States, we anticipate expanding in new, key markets in the future. |
| • | Scale and Monetize VCS. The Allurion Program is an end-to-end weight management platform powered by AI and data through the VCS. Currently, providers report that the VCS is driving increased productivity, improved outcomes, and higher patient engagement during the balloon phase. We have also expanded the sale of the VCS in a Software as a Service model, including the launch of the VCS in the United States, to be used in clinics for their patients before and after balloon therapy to drive even better short and long-term outcomes. In addition, VCS can be a stand-alone opportunity that can be used by patients undergoing other weight loss interventions, including the use of GLP-1s. We believe this opportunity to be complimentary to, not just competitive with, other weight loss interventions that can be a key to our growth. 45% of our providers believed that other anti-obesity medications boosted awareness and/or interest in the Allurion Balloon. |
| • | Expand label, advance product pipeline, and strengthen weight loss platform. We plan to leverage our proprietary product technology and research and development expertise to expand our current label, if approved, into adolescents and a lower BMI population. We also plan to develop products for weight loss that improve clinical outcomes, increase ease of use, and reduce costs. |
| • | Strengthen Gross Margin and Improve Profitability. Our gross margin was 78% in the year ended December 31, 2023, and we believe that we can continue to increase our gross margin through increased sales volume and production efficiencies and from scaling the VCS software. In addition, we have implemented a number of operational efficiency initiatives to improve our profitability, reducing expected cash used in operating activities. With this expected continued improvement in profitability, we expect to be able to make key, strategic investments in our initiatives to drive growth. |
Our Competition
We have developed, manufactured, and commercialized the world’s first and only swallowable, ProcedurelessTM gastric balloon for weight loss, which we offer as part of our Allurion Program, a multi-faceted weight loss platform. Weight-loss treatments range from behavioral modification, to drugs and medical devices, and surgery. Outside the U.S., we compete with a variety of local and regional competitive intragastric balloon manufacturers including SC MedSil, Medicone and Spatz Laboratories. In the U.S., there are three manufacturers with an intragastric balloon approved by the FDA at this time: Boston Scientific Corporation, Inc., ReShape Lifesciences, Inc. and Spatz FGIA Inc. All of these balloons require endoscopy and anesthesia for placement and/or removal.
We also compete against the manufacturers of pharmaceuticals that are directed at treating weight loss, such as Novo Nordisk A/S, Eli Lilly & Co., Roche Holding AG, GlaxoSmithKline plc, Arena Pharmaceuticals, Inc., VIVUS, Inc. and Orexigen Therapeutics, Inc. We believe that the principal competitive factors in our market include:
| • | acceptance by health care providers and patients; |
| • | published rates of safety and efficacy; |
92
Table of Contents
| • | reliability and high-quality performance; |
| • | effectiveness at controlling co-morbidities such as diabetes and hypertension; |
| • | invasiveness and the inherent reversibility of the procedure or device; |
| • | cost and average selling price of products and relative rates of reimbursement, if any; |
| • | effective marketing, training, education, sales and distribution; |
| • | regulatory expertise; |
| • | technological leadership and superiority; and |
| • | speed of product innovation and time to market. |
| • | Many of our competitors, or their parent companies, are larger than we are, and they may enjoy several competitive advantages over us, including: |
| • | stronger name recognition; |
| • | existing relationships with health care professionals, customers and third-party payers; |
| • | established distribution networks; |
| • | significant experience in research and development, manufacturing, preclinical testing, clinical trials, obtaining regulatory approvals, obtaining reimbursement and marketing approved products; and |
| • | greater financial, sales and marketing, and manufacturing resources. |
As a result, we cannot assure you that we will be able to compete effectively against these companies or their products.
Intellectual Property
We have assembled a broad portfolio of intellectual property related to our medical device, the Allurion Balloon, and our supporting technology platform, the VCS. We believe this intellectual property, combined with proprietary manufacturing processes and the regulatory approvals we have successfully obtained in over 50 countries outside of the United States, provides us with a strong market position.
As of March 31, 2024, we owned or had rights to 18 issued patents and five pending patent applications in the U.S. related to various aspects of our Allurion Balloon, such as a swallowable, self-deflating and naturally passing gastric balloon, improvements to the fill and release valves therein, methods for deploying and releasing a gastric balloon within the body, and next generation fill and release valves. In addition, outside of the United States we had 37 issued and five patents pending that generally parallel the U.S. portfolio in 17 countries as of March 31, 2024. We are not currently licensing any patents. Allurion owns and possesses all right, title and interest in and to each patent and patent application noted herein free and clear of all liens, other than any liens granted to RTW pursuant to our royalty arrangements with RTW.
Our issued patents are expected to expire at various times between February 21, 2033 and November 27, 2040. The following table sets forth a summary of our patents and patent applications, including where patent applications have been filed, the exemplary subject matter being pursed in the applications, and expected expiration dates.
93
Table of Contents
Family No. | Jurisdictions | Patent/Application & Status | Exemplary Subject Matter and Scope | Expiration | Type | |||||
| 1 | Australia; Brazil; Canada; China; Europe; Israel; India; Japan; South Korea; Mexico; United States | Granted European patent validated in Germany; Spain; France; United Kingdom; Ireland; Italy; granted Australia; Brazil; Canada; China; Israel; India; Japan; South Korea; Mexico; United States | Medical devices for temporary implantation within the body, such as a gastric space, and methods for temporarily occupying a space in the body, such as a gastric space | 2033 | Utility | |||||
| 2 | United States | Granted | Ingestible Delivery Systems and methods | 2033 | Utility | |||||
| 3 | Australia; Brazil; China; Europe; United States | Granted European patent validated in France; United Kingdom; Ireland; granted Australia; Brazil; China; United States | Medical devices for temporary implantation within the body, such as a gastric space, and methods for temporarily occupying a space in the body, such as a gastric space; improved fill valves for use with the medical devices | 2033, 2036 | Utility | |||||
| 3 | European Patent Convention; United States | Pending patent applications | Medical devices for temporary implantation within the body, such as a gastric space, and methods for temporarily occupying a space in the body, such as a gastric space; improved fill valves for use with the medical devices | 2033, 2036 | Utility | |||||
| 4 | United States | Granted | Automatic-Sealing Balloon-Filling Catheter Systems and methods of use | 2039 | Utility | |||||
| 4 | Europe; United States | Pending patent applications | Automatic-Sealing Balloon-Filling Catheter Systems and methods of use | 2039 | Utility | |||||
| 5 | China; United States | Granted China; United States | Binary fluid control valve systems | 2039 | Utility | |||||
| 5 | Europe; United States | Pending patent applications | Binary fluid control valve systems | 2039 | Utility | |||||
| 6 | United States | Granted | Enhanced Fluid Delivery System | 2040 | Utility | |||||
| 7 | China; United States | Pending patent applications | Fluid Delivery Catheter | 2041 | Utility | |||||
| 8 | United States | Pending patent application | Automatic-Sealing Balloon-Filling Catheter Systems and methods of use | 2043 | Utility | |||||
94
Table of Contents
We are protecting three Allurion-related trademarks in three classes: medical devices (the balloon), downloadable software and digital scale (the mobile app), and medical services (provided by our physicians). As of March 31, 2024, we have 69 registered trademarks and one pending Allurion trademark application among 14 jurisdictions. It is our intention to maintain these registrations indefinitely and to expand the number of jurisdictions in which we have registered trademarks as deemed necessary to protect our freedom to use the marks and/or block competitors in additional markets.
We also hold registrations to the “Elipse” trademark in three classes in four jurisdictions; it is our intention to allow these registrations to lapse at the end of their current terms as we are no longer identified by this trademark.
In addition to pursuing patents on our products, we have taken steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners, and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. In addition, we intend to expand our international operations, and effective patent, copyright, trademark and trade secret protection may not be available or may be limited in foreign countries.
In general, the medical device industry is characterized by the existence of a large number of patents and frequent allegations and related litigation regarding patent and other intellectual property rights. Third parties, including our competitor companies, may assert patent, copyright, trademark and other intellectual property rights against us, our partners or our customers. Our standard license and other agreements may obligate us to indemnify our partners and customers against such claims. We could incur substantial costs and diversion of the attention of our management and technical personnel in defending against any such claims. Successful claims of infringement of a valid patent by a third party could prevent us from selling or distributing our products or performing certain services, require us to expend time and resources to develop non-infringing products, or force us to pay substantial damages (including treble damages if we are found to have willfully infringed patents), royalties or other fees. We cannot assure you that we do not currently infringe, or that we will not in the future infringe, upon any third-party patents or other proprietary rights.
We intend to continue to expand our intellectual property portfolio and invest in protecting new innovations developed in our pipeline programs.
Third-party open source software components
The Allurion VCS and our other products and services contain software licensed to us by third-party authors under “open source” licenses. Use of such software may entail greater risks than use of non-open source third-party commercial software, as open source licensors generally do not provide support, warranties, indemnification or other contractual protections regarding infringement claims or the quality of the code. Although we seek to monitor our use of open source software to avoid such consequences and to comply with the terms thereof, the terms of many open source licenses have not been interpreted by U.S. or foreign courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to provide or distribute our platform. Although we try to mitigate the risk of our use of open source software by managing software development with an information security program that is in compliance with the global standard International Organization for standardization (ISO) 27001:2013, our information security program does not yet comply fully with all of the additions and changes in the updated ISO 27001:2022 version of the standard. We anticipate transitioning to compliance with the ISO 27001:2022 version of the standard prior to the required transition date of October 31, 2025. Using an automated static code analysis
95
Table of Contents
tool, we regularly examine all VCS software code, as well as included open source code, for security vulnerabilities, code quality, as well as open source licensing that is in alignment with our software distribution requirements.
Sales and Marketing
We currently sell our products either through our direct sales force, or in certain countries, through distributors. As of March 31, 2024, our sales and marketing organization consisted of approximately 98 employees and consultants.
Our sales personnel are equipped with a suite of resources including extensive in-depth training, marketing resource tools, and access to a robust schedule of education events. In the regions where we have distributors, we provide clinical training and support to build positive relationships with physicians and clinics and to position our product in the marketplace as a premium product with consequent premium pricing.
We employ a multi-faceted marketing strategy focused on social media engagement with patient success stories, conferences, advertisements and education.
Manufacturing Capabilities
Allurion Balloons are manufactured in-house using components and sub-assemblies at our 10,000 square foot, ISO 13485 certified manufacturing facility in Natick, Massachusetts. We rely on suppliers for the extruded film to manufacture our Allurion Balloon and suppliers for stylets, filler kits, accessories, and scales. All critical component suppliers undergo strict quality system audits and component inspections to ensure they meet our quality standards. All suppliers and materials must be qualified prior to being approved for manufacturing activities. Our suppliers have no contractual obligations to supply us with components, and we are not contractually obligated to purchase such components from any of our suppliers. Order quantities and lead times for components purchased from our suppliers are based on our forecasts derived from anticipated future demand.
Lead times for components may vary significantly depending on the size of the order, time required to fabricate and test the components, specific supplier requirements and current market demand for the components and sub-assemblies. However, some of these components are critical to our products and there are relatively few alternative sources of supply. To date, we have not experienced significant delays in obtaining any of our components or sub-assemblies.
We have registered with the FDA as a medical device manufacturer and have obtained a manufacturing license from the Center for Devices and Radiological Health. We and our component suppliers manufacture our products in compliance with the FDA’s Quality System Regulation (“QSR”) in 21 CFR part 820 of the Federal Food, Drug and Cosmetic Act (“FDCA”).
We are also subject to periodic inspections and audits by various international regulatory and notified bodies, and we believe our past performance in these audits reflects the strength of our quality system and production and process controls. We consider this to be a key element of our risk management and business continuity strategies and a competitive advantage as we have full control of the product life-cycle. Our in-house manufacturing team included 19 employees at March 31, 2024, all of whom undergo well defined training programs throughout their period of employment. We believe our manufacturing experience, know-how, and process-related trade secrets are a competitive advantage.
Additionally, we will need to increase our manufacturing capabilities over time in order to satisfy any increased demand for our balloon system, and we have no experience manufacturing our balloon system in such quantities. If we are unable to keep up with demand for our balloon system, our revenue could be impaired, market acceptance for our balloon system could be harmed and our customers might instead purchase our competitors’ products.
96
Table of Contents
Government Regulation
The healthcare industry, and thus our business as a medical device company, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. In addition, these laws and their interpretations are subject to change.
Regulatory System for Medical Devices in the United States
Unless an exemption applies, each new or significantly modified medical device a company seeks to commercially distribute in the United States will require a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, a de novo classification request, or approval from the FDA of a PMA application. Our Allurion Balloon will require approval from the FDA of a PMA application. The 510(k) clearance, de novo classification request and PMA processes can be resource intensive, expensive and lengthy, and require payment of significant user fees, unless an exemption is available.
Device Classification
Under the FDCA, medical devices are classified into one of three classes-Class I, Class II or Class III-depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurances with respect to safety and effectiveness.
Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be reasonably assured by adherence to a set of FDA regulations, referred to as General Controls, which require compliance with the applicable portions of the QSR facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. Some Class I devices, also called Class I reserved devices, also require premarket clearance by the FDA through the 510(k) premarket notification process described below. Most Class I products are exempt from the premarket notification requirements.
Class II devices are those that are subject to the General Controls, and Special Controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These Special Controls can include performance standards, patient registries, FDA guidance documents and post-market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process.
Class III devices include devices deemed by the FDA to pose the greatest risk such as life-supporting or life-sustaining devices, or implantable devices, in addition to those deemed novel and not substantially equivalent following the 510(k) process. The safety and effectiveness of Class III devices cannot be reasonably assured solely by the General Controls and Special Controls described above. Therefore, these devices are subject to the PMA application process, described below, which is generally more costly and time consuming than the 510(k) process.
The Investigational Device Process
In the United States, absent certain limited exceptions, human clinical trials intended to support 510(k) clearance, de novo classification, or PMA approval require an IDE application. Some types of trials considered to present “non-significant risk” are deemed to have an approved IDE once certain requirements are addressed, and investigational review board (“IRB”) approval is obtained. If the device presents a “significant risk” to human health, as defined by the FDA, the sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound.
97
Table of Contents
The IDE application must be approved in advance by the FDA for a specified number of subjects. The FDA also may issue a conditional approval, in which case the trial may be conducted subject to the FDA’s conditions, which the sponsor must address in order to conduct the trial as originally requested. Generally, clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the trial protocol and informed consent are approved by appropriate IRBs at the clinical trial sites. There can be no assurance that submission of an IDE application will result in the ability to commence clinical trials, and although the FDA’s approval of an IDE application allows clinical testing to go forward for a specified number of subjects, it does not bind the FDA to accept the results of the trial as sufficient to prove the product’s safety and efficacy, even if the trial meets its intended success criteria, unless the sponsor has obtained a binding protocol agreement.
All clinical trials must be conducted in accordance with the FDA’s IDE regulations that govern investigational device labeling, prohibit promotion and specify an array of recordkeeping, reporting and monitoring responsibilities of trial sponsors and trial investigators. Clinical trials must further comply with the FDA’s good clinical practice regulations for IRB approval and for informed consent and other human subject protections. Required records and reports are subject to inspection by the FDA. Clinical trial investigators must disclose certain financial interests to clinical trial sponsors. The commencement or completion of any clinical trial may be delayed or halted, including from the FDA imposing a clinical hold on a trial, or be inadequate to support approval of a PMA application, de novo classification or clearance of a 510(k), for numerous reasons.
The 510(k) Clearance Process
Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification 90 days before it seeks to commercially distribute its device, demonstrating that the device is “substantially equivalent,” as defined in the statute, to a legally marketed predicate device.
A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I, or a device that was previously found substantially equivalent through the 510(k) process.
To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence.
After a 510(k) premarket notification is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) premarket notification. If it is accepted for filing, the FDA begins a substantive review. As a practical matter, clearance often takes longer than 90 days, and clearance is never assured. Although many 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including clinical data, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
If the FDA determines that the device is not “substantially equivalent” to a predicate device, or if the device is automatically classified into Class III, the device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA approval process, or seek reclassification of the device through the de novo process. A manufacturer can also submit a petition for direct de novo review if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk.
After a device receives 510(k) clearance, any modification, including modification to or deviation from design, manufacturing processes, materials, packaging and sterilization that could significantly affect the
98
Table of Contents
device’s safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application or de novo classification. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. Many minor modifications are accomplished by a letter-to-file in which the manufacturer documents the change in an internal letter-to-file. The letter-to-file is in lieu of submitting a new 510(k) to obtain clearance for such change. The FDA can always review these letters to file in an inspection. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA application is obtained. In addition, in these circumstances, the FDA can impose significant regulatory fines or penalties for failure to submit the requisite PMA application(s).
The PMA Approval Process
Through the PMA application process, the applicant must submit data and information demonstrating reasonable assurance of the safety and effectiveness of the device for its intended use to the FDA’s satisfaction. Accordingly, a PMA application typically includes, but is not limited to, extensive technical information regarding device design and development, preclinical study and clinical trial data, manufacturing information, labeling and financial disclosure information for the clinical investigators in device trials. The PMA application must provide valid scientific evidence that demonstrates to the FDA’s satisfaction a reasonable assurance of the safety and effectiveness of the device for its intended use.
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA, by statute and by regulation, has 180 days to review a filed PMA application, although the review of an application more often occurs over a significantly longer period. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA. The FDA considers a PMA or PMA supplement to have been voluntarily withdrawn if an applicant fails to respond to an FDA request for information (e.g., major deficiency letter) within a total of 360 days. Before approving or denying a PMA, an FDA advisory committee may review the PMA at a public meeting and provide the FDA with the committee’s recommendation on whether the FDA should approve the submission, approve it with specific conditions, or not approve it. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Prior to approval of a PMA, the FDA may conduct inspections of the clinical trial data and clinical trial sites, as well as inspections of the manufacturing facility and processes. Overall, the FDA review of a PMA application generally takes between one and three years, but may take significantly longer.
If the FDA evaluation of a PMA is favorable, the FDA will issue either an approval letter, or an approvable letter, the latter of which usually contains several conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval letter authorizing commercial marketing of the device, subject to the conditions of approval and the limitations established in the approval letter. If the FDA’s evaluation of a PMA application or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. The FDA also may determine that additional tests or clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and data is submitted in an amendment to the PMA, or the PMA is withdrawn and resubmitted when the data are available. The PMA process can be expensive, uncertain and lengthy.
99
Table of Contents
New PMA applications or PMA supplements are required for modification to the manufacturing process, equipment or facility, quality control procedures, sterilization, packaging, expiration date, labeling, device specifications, ingredients, materials or design of a device that has been approved through the PMA process. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the approved PMA application and may or may not require as extensive technical or clinical data or the convening of an advisory panel, depending on the nature of the proposed change.
In approving a PMA application, as a condition of approval, the FDA may also require some form of post-approval trial or post-market surveillance, whereby the applicant conducts a follow-up trial or follows certain patient groups for several years and makes periodic reports to the FDA on the clinical status of those patients when necessary to protect the public health or to provide additional or longer-term safety and effectiveness data for the device. The FDA may also require post-market surveillance for certain devices cleared under a 510(k) notification, such as implants or life-supporting or life-sustaining devices used outside a device user facility. The FDA may also approve a PMA application with other post-approval conditions intended to ensure the safety and effectiveness of the device, such as, among other things, restrictions on labeling, promotion, sale, distribution and use.
Pervasive and Continuing FDA Regulation
After the FDA permits a device to enter commercial distribution, numerous regulatory requirements continue to apply. These include:
| • | labeling regulations, unique device identification requirements and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses; |
| • | advertising and promotion requirements; |
| • | restrictions on sale, distribution or use of a device; |
| • | PMA annual reporting requirements; |
| • | PMA approval or clearance of a 510(k) for product modifications; |
| • | MDR regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
| • | medical device correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
| • | recall requirements, including a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death; |
| • | an order of repair, replacement or refund; |
| • | device tracking requirements; and |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
Our manufacturing processes are required to comply with the applicable portions of the FDA’s QSR that covers the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation, and servicing of finished devices intended for human use. In February 2024, the FDA issued the Quality Management System Regulation Final Rule to amend the QSR, incorporating by reference the international standard for medical device quality
100
Table of Contents
management systems set by the ISO, ISO 13485:2016. The rule will become effective on February 2, 2026. Until then, manufacturers are required to comply with the QSR. We actively maintain compliance with the FDA’s QSR, and the European Union’s Quality Management Systems requirements, ISO 13485:2016.
Since February 2017, the FDA has issued three separate letters to health care providers warning of SAEs, including deaths, which are specific to liquid-filled intragastric balloons. We are aware of the filing of additional reports of SAEs, including deaths, associated with liquid-filled balloons since the issuance of the FDA letters to health care providers. While the advisory letters were specific to liquid-filled intragastric balloons and not the Allurion Balloon, these letters could create negative perceptions of the entire gastric balloon category, which may cause negative consequences for us including requiring additional warnings, precautions and/or contraindications in the labeling, delaying or denying approval of our products, or possible review or withdrawal of any approval that we may obtain.
The FDA has broad post-market and regulatory enforcement powers. Medical device manufacturers are subject to unannounced inspections by the FDA and other state, local and foreign regulatory authorities to assess compliance with the QSR and other applicable regulations, and these inspections may include the manufacturing facilities of any suppliers.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| • | warning or untitled letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures, repair, replacement, refunds, recall or seizure of products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | the FDA’s refusal of requests for 510(k) clearance, de novo classification or premarket approval of new products, new intended uses or modifications to existing products; |
| • | the FDA’s refusal to issue certificates to foreign governments needed to export products for sale in other countries; |
| • | withdrawing premarket approvals that have already been granted or reclassifying our devices; and |
| • | criminal prosecution. |
Other U.S. Healthcare Laws
Our business is regulated by laws pertaining to healthcare fraud and abuse, including anti-kickback laws and false claims laws, and other healthcare laws. Violations of these laws are punishable by significant administrative, criminal and civil penalties, including damages, disgorgement, monetary fines, possible exclusion from participation in federal and state healthcare programs, such as Medicare and Medicaid, imprisonment, and integrity oversight and reporting obligations.
Anti-Kickback Statute
The federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or paying remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the furnishing, recommending, purchasing, leasing, ordering, or arranging for, a good or service for which payment may be made under a federal healthcare program such as Medicare and Medicaid. The term “remuneration” has been broadly interpreted to include anything of value, including payments to physicians or other providers, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, waiver of payments and providing anything of value at less than fair market value. There are a number of statutory exceptions and regulatory safe harbors protecting some common
101
Table of Contents
activities from prosecution, but the exceptions and safe harbors are drawn narrowly and require strict compliance in order to offer protection. These exceptions and safe harbors exist for various types of arrangements, including certain investment interests, leases, personal service arrangements, discounts and management contracts. The failure of a particular activity to comply with all requirements of an applicable safe harbor regulation does not mean that the activity violates the federal Anti-Kickback Statute or that prosecution will be pursued. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of the relevant facts and circumstances. Activities and business arrangements that do not fully satisfy each applicable exception or safe harbor may result in increased scrutiny by government enforcement authorities such as the Office of the Inspector General.
Additionally, the intent standard under the federal Anti-Kickback Statute was amended by the Affordable Care Act (“ACA”) to a stricter standard such that a person or entity no longer needs to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. Rather, if “one purpose” of the renumeration is to induce referrals, the federal Anti-Kickback Statute is violated. In addition, the ACA codified case law that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act (“FCA”) (discussed below).
Further, certain states have adopted prohibitions similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare services reimbursed by any source, not only by government healthcare programs such as the Medicare and Medicaid programs, and do not include comparable exceptions and/or safe harbors to those provided by the federal Anti-Kickback Statute.
Federal False Claims Act
The FCA prohibits, among other things, knowingly filing or causing the filing of a false claim or the knowing use of false statements to obtain payment from the federal government. A claim that is filed pursuant to an unlawful kickback may be a false claim under this law and, in a number of cases, manufacturers of medical products have entered into settlements based on FCA allegations that their financial relationships with customers “caused” these customers to submit false claims. When an entity is determined to have violated the FCA, it may be required to pay up to three times the actual damages sustained by the government, plus mandatory civil penalties for each separate false claim. Private individuals can file suits under the FCA on behalf of the government. These lawsuits are known as “qui tam” actions, and the individuals bringing such suits, sometimes known as “relators” or, more commonly, “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. Since complaints related to “qui tam” actions are initially filed under seal, the action may be pending for some time before a defendant is even aware of such action.
HIPAA
HIPAA created new federal crimes, including healthcare fraud and false statements relating to healthcare matters. HIPAA prohibits knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government-sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), also protects the security and privacy of individually identifiable health information maintained or transmitted by certain healthcare providers, health plans and healthcare clearinghouses and their business
102
Table of Contents
associates. HIPAA restricts the use and disclosure of patient health information, including patient records. Although we believe that HIPAA does not apply directly to us, most of our customers have significant obligations under HIPAA, and we intend to cooperate with customers and others to ensure compliance with HIPAA with respect to patient information. HITECH created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. Depending on the facts and circumstances, we could be subject to significant penalties if we violate HIPAA. Failure to comply with HIPAA obligations can result in civil fines and/or criminal penalties. Some states have also enacted rigorous laws or regulations protecting the security and privacy of patient information.
Transparency Reporting
In March 2010, the U.S. Congress enacted the ACA. The U.S. Physician Payments Sunshine Act, which is part of the ACA, requires manufacturers of drugs, biologics, devices and medical supplies that are subject to (or used in procedures subject to) Medicare and Medicaid reimbursement to record payments and transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists, and chiropractors), certain non-physician healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals as well as ownership and investment interests held by physicians and their immediate family members and to report this data to the Centers for Medicare & Medicaid Services, for public disclosure. Failure to report may result in civil or criminal fines and/or penalties. Similar reporting requirements have also been enacted in several states, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with healthcare professionals. In addition to reporting, some states such as Massachusetts and Vermont impose a ban or limit the ability of medical device and pharmaceutical manufacturers providing certain items of value or payments to physicians or other healthcare practitioners licensed in these states. In recent years, the federal government and several states have enacted legislation requiring biotechnology, pharmaceutical and medical device companies to establish marketing compliance programs and file periodic reports on sales, marketing and other activities.
Analogous State and Foreign Laws and Regulations
Additionally, we are subject to state and foreign equivalents of each of the healthcare laws and regulations described above, among others, some of which may be broader in scope and may apply regardless of the payor. Many U.S. states have adopted laws similar to the federal Anti-Kickback Statute and False Claims Act, and may apply to our business practices, including, but not limited to, research, distribution, sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental payors, including private insurers. In addition, some states have passed laws that require pharmaceutical companies to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals. Several states also impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state and require the registration of pharmaceutical sales representatives. State and foreign laws, including for example the European Union General Data Protection Regulation, which became effective May 2018 also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement we could be subject to penalties.
Coverage, Reimbursement and Healthcare Reform
Patients in the U.S. and elsewhere generally rely on third-party payors to reimburse part or all of the costs associated with their healthcare treatment. Accordingly, market acceptance of our products is dependent on the extent to which third-party coverage and reimbursement is available from third-party payors, which can differ
103
Table of Contents
significantly from payor to payor and may change from time to time. Further, from time to time, typically on an annual basis, payment amounts are updated and revised by third-party payors. In cases where the cost of certain of our products are recovered by the healthcare provider as part of the payment for performing a procedure and not separately reimbursed or paid directly by the patient, these updates could directly impact the demand for our products.
Coverage and reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is:
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
All third-party payors, whether governmental or commercial and whether inside the U.S. or outside, are developing increasingly sophisticated methods of controlling healthcare costs. These cost-control methods include prospective payment systems, bundled payment models, capitated arrangements, group purchasing, benefit redesign, pre-authorization processes and requirements for second opinions prior to procedures. These cost-control methods also potentially limit the amount that healthcare providers may be willing to pay for our products. Therefore, coverage or reimbursement for medical devices may decrease in the future. In addition, consolidation in the healthcare industry could lead to demands for price concessions, which may impact our ability to sell our products at prices necessary to support our current business strategies.
Federal and state governments in the U.S. and outside the U.S. may enact legislation to modify the healthcare system, which may result in increased government price controls, additional regulatory mandates and other measures designed to constrain medical costs. These reform measures may limit the amounts that federal and state governments will pay for healthcare products and services, and also indirectly affect the amounts that private payors are willing to pay. The pricing pressure resulting from healthcare cost containment and reimbursement changes could decrease demand for our products, the prices that customers are willing to pay and the frequency of use of our products, which could have an adverse effect on our business.
Foreign Government Regulation
In addition to U.S. regulations, we are subject to a variety of foreign government regulations applicable to medical devices, medicinal products and combination products.
Regulation of Medical Devices in the European Union
The European Union (“EU”), has adopted specific directives and regulations regulating the design, manufacture, clinical investigation, conformity assessment, labeling and adverse event reporting for medical devices.
Until May 25, 2021, medical devices were regulated by the Council Directive 93/42/EEC, or Medical Devices Directive (“MDD”), which has been repealed and replaced by Regulation (EU) No 2017/745, or MDR. There is a transition period during which certificates issued under the MDD remain valid; however, when such certificates expire (or, if earlier, by May 26, 2024), the devices must be certified under the new regime set forth in the Medical Devices Regulation. An extension for devices transitioning to the EU Medical Devices Regulation has been changed from May 26, 2024 to May 26, 2026 for class III implantable custom-made devices and December 31, 2027 for class III and implantable class IIb devices.
104
Table of Contents
Medical Devices Directive
Under the MDD, all medical devices placed on the market in the EU must meet the relevant essential requirements laid down in Annex I to the MDD, including the requirement that a medical device must be designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others. In addition, the device must achieve the performance intended by the manufacturer and be designed, manufactured, and packaged in a suitable manner. The European Commission has adopted various standards applicable to medical devices and there are additionally harmonized standards relating to the design and manufacture of medical devices which are not mandatory but, if complied with, indicate that the device satisfies the applicable element of the essential requirements.
To demonstrate compliance with the essential requirements laid down in Annex I to the MDD, medical device manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification with respect to risk. As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can self-declare the conformity of its products with the essential requirements (except for any parts which relate to sterility or metrology), a conformity assessment procedure requires the intervention of a notified body. Notified bodies are independent organizations designated by EU member states to assess the conformity of devices before being placed on the market. A notified body would typically audit and examine a product’s technical dossiers and the manufacturers’ quality system. If satisfied that the relevant product conforms to the relevant essential requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE Mark to the device, which allows the device to be placed on the market throughout the EU.
Medical Devices Regulation
On April 5, 2017, MDR was adopted with the aim of ensuring better protection of public health and patient safety. Unlike the MDD, the MDR is directly applicable in EU member states without the need for member states to implement into national law. This aims at increasing harmonization across the EU.
The MDR became effective on May 26, 2021. The new Regulation follows the same process of conformity assessment, certificates of conformity and applying a CE mark to devices before they can be placed on the market. However, among other things, the MDR:
| • | strengthens the rules on placing devices on the market (e.g. reclassification of certain devices and wider scope than the MDD) and reinforces surveillance once they are available; |
| • | establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| • | establishes explicit provisions on importers’ and distributors’ obligations and responsibilities; |
| • | imposes an obligation to identify a responsible person who is ultimately responsible for all aspects of compliance with the requirements of the new regulation; |
| • | improves the traceability of medical devices throughout the supply chain to the end-user or patient through the introduction of a UDI, to increase the ability of manufacturers and regulatory authorities to trace specific devices through the supply chain and to facilitate the prompt and efficient recall of medical devices that have been found to present a safety risk; |
105
Table of Contents
| • | sets up a European medical device database (“Eudamed”) to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and |
| • | strengthens rules for the assessment of certain high-risk devices, such as implants, which may have to undergo a clinical evaluation consultation procedure by experts before they are placed on the market. |
Devices lawfully placed on the market pursuant to the MDD prior to May 26, 2021 may generally continue to be made available on the market or put into service until May 26, 2025, provided that the requirements of the transitional provisions are fulfilled. In particular, the certificate in question must still be valid. However, even in this case, manufacturers must comply with a number of new or reinforced requirements set forth in the Medical Devices Regulation, in particular the obligations described below.
The MDR requires that before placing a device, other than a custom-made device, on the market, manufacturers (as well as other economic operators such as authorized representatives and importers) must register by submitting identification information to Eudamed, unless they have already registered. The information to be submitted by manufacturers (and authorized representatives) also includes the name, address and contact details of the person or persons responsible for regulatory compliance.
The MDR also requires that before placing a device, other than a custom-made device, on the market, manufacturers must assign a unique identifier to the device and provide it along with other core data to the UDI database. These new requirements aim at ensuring better identification and traceability of the devices.
Manufacturers are responsible for entering the necessary data on Eudamed, which includes the UDI database, and for keeping it up to date. Eudamed is not yet fully functional, however the European Commission is aiming to have a fully functional version of the Eudamed medical device database available in the fourth quarter of 2027. The Medical Device Coordination Group has published guidance on administrative practices for manufacturers until Eudamed is fully functional.
All manufacturers placing medical devices on the market in the EU must also comply with the EU medical device vigilance system, which has been reinforced by the MDR. Under this system, serious incidents and Field Safety Corrective Actions must be reported to the relevant authorities of the EU member states. These reports will have to be submitted through Eudamed - once functional - and aim to ensure that, in addition to reporting to the relevant authorities of the EU member states, other actors such as the economic operators in the supply chain will also be informed.
Among the new requirements, manufacturers (and authorized representatives) must have available within their organization at least one person responsible for regulatory compliance (“PRRC”), who possesses the requisite expertise in the field of medical devices. The PRRC is responsible for compliance with post-market surveillance and vigilance requirements.
The aforementioned EU rules are generally applicable in the European Economic Area, which consists of the 27 EU Member States plus Norway, Liechtenstein and Iceland.
Brexit
As a result of the United Kingdom leaving the EU, since January 1, 2021, the regulatory framework and regimes for medical devices in the United Kingdom and the EU have diverged. In particular, a new UKCA Mark was introduced for medical devices placed on the Great Britain market (which includes England, Scotland and Wales). Northern Ireland has adopted a hybrid approach as a result of the divergence in accordance with the Northern Ireland Protocol. Manufactures can continue placing CE marked medical devices on the Great Britain market until June 30, 2024. From July 1, 2024, transitional arrangements will apply for CE and UKCA marked medical devices placed on the Great Britain market.
106
Table of Contents
Environmental Laws
The costs and effects of our compliance with applicable environmental laws during fiscal years 2022 and 2023 and the three months ended March 31, 2024 were, and historically have been, immaterial.
Human Capital Resources
As of March 31, 2024, we had 209 employees, 207 of whom were full-time, consisting of clinical, research and development, operations, regulatory and quality, sales, marketing, technology, finance, business analytics and human resources. This included 93 employees located in the United States. From time to time, we also engage contractors, consultants and temporary employees to support our operations. None of the U.S. employees are subject to collective bargaining agreements or represented by a labor union, however, our employees in France are subject to a collective bargaining agreement. We consider our relationship with our employees to be good.
Corporate & Available Information
We were incorporated under the laws of the State of Delaware on January 25, 2023. The mailing address of our principal executive office is 11 Huron Drive, Natick, MA 01760, and the telephone number is (508) 647-4000. Our website address is https://allurion.com/. Further corporate governance information, including our Code of Conduct, Corporate Governance Guidelines and the charters of our Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee are available on our investor relations website under the heading “Governance Documents.”
The SEC maintains an Internet site that contains current and periodic reports, proxy and information statements, and other information regarding issuers, including us, that file electronically with the SEC at www.sec.gov. Additional information about our filings can also be obtained at our website at www.investors.allurion.com under “Financials - SEC Filings.”
We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The contents of our website are not a part of this prospectus and should not be considered to be a part of, or incorporated into, this prospectus or any of our other securities filings unless specifically incorporated herein or therein by reference.
Legal Proceedings
From time to time, we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not currently a party to any material legal proceedings. Regardless of outcome, such proceedings or claims can have an adverse impact on us because of defense and settlement costs, diversion of resources and other factors, and there can be no assurances that favorable outcomes will be obtained.
107
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
The following discussion and analysis includes information that our management believes is relevant to an assessment and understanding of our consolidated results of operations and financial condition. The discussion and analysis should be read together with the consolidated financial statements as of and for the three months ended March 31, 2024 and March 31, 2023, and for the years ended December 31, 2023 and December 31, 2022 that are included in this prospectus. This discussion may contain forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth in the sections titled “Cautionary Statement Regarding Forward-Looking Statements” and “Risk Factors” in this prospectus. For purposes of this section, (i) all references in this discussion and analysis to “Allurion,” the “Company”, “we,” “us,” or “our” refers to the business and operations of Allurion and its consolidated subsidiaries following the consummation of the Business Combination and to Legacy Allurion and its consolidated subsidiaries prior to the consummation of the Business Combination. “Legacy Allurion” refers to Allurion Technologies, LLC, which was previously known as Allurion Technologies Opco, Inc. (formerly Allurion Technologies, Inc.) prior to the consummation of the Business Combination, (ii) all references to Notes are to the notes to the consolidated financial statements included in this prospectus, and (iii) all capitalized terms used herein without definition shall have the meanings assigned to such terms in the notes to the financial statements included in this prospectus.
Overview
Allurion is a leading medical device company that is focused on creating a best-in-class weight loss platform to treat obese and overweight patients. Our platform, the Allurion Program, features the world’s first and only swallowable, ProcedurelessTM intragastric balloon for weight loss and offers AI-powered remote patient monitoring tools, a proprietary behavior change program, secure messaging and video telehealth that are delivered by the VCS.
Our proprietary intragastric balloon, the Allurion Balloon, is swallowed as a capsule under the guidance of a health care provider without surgery, endoscopy, or anesthesia.
The Allurion VCS is comprised of tools to support patients’ weight loss experience, which we believe benefit both patients and health care providers:
| 1. | For Allurion Program patients: Every current Allurion Program patient receives an Allurion Connected Scale and access to our App, which integrates data from the Allurion Connected Scale to conveniently monitor weight, body fat, activity, sleep, and several other critical metrics. The App can also enable secure messaging and video telehealth with the patient’s care team and can deliver content from Allurion’s proprietary behavior change program—a library of 100 weight loss actions related to diet, nutrition, mental health, sleep, goal setting, and a number of other topics—directly to the patient. The App is available in over 15 languages. |
| 2. | For Allurion Program providers: Allurion Insights provides end-to-end remote patient monitoring powered by the Allurion Iris AI platform, which leverages machine learning to deliver key insights related to patient tracking data. Allurion Insights offers real-time access to patient data and AI-powered analytics, note functionality to keep track of patient encounters, and clinic-wide metrics that provide a snapshot of the clinic’s overall performance. |
In addition to its use by Allurion Balloon patients, we believe the VCS can potentially be a platform for optimal long-term follow up after other medical and surgical weight loss interventions in the future. For example, VCS includes a Treatment Tracking and Clinic-Led Onboarding feature that enables seamless onboarding and management of patients undergoing one or multiple weight loss treatments, including gastric balloons such as the Allurion Balloon, surgery, or medications. In addition, in connection with our collaboration with Medtronic, we may incorporate the VCS in onboarding and managing Medtronic’s patients.
108
Table of Contents
Our products are currently sold in Europe, the Middle East, Africa, Latin America, Canada and the Asia-Pacific region. The FDA has approved the investigational device exemption, or IDE, for Allurion’s AUDACITY clinical trial, a 48-week, prospective, randomized, open-label trial. We received approval of the IDE from the FDA in November 2021 to initiate the AUDACITY clinical trial in the United States. The first patient in the trial was treated in July 2022. During the third quarter of 2023, we completed the enrollment of 550 patients in the trial across 17 sites in the United States. The results of the trial are expected to support a premarket approval submission to the FDA.
Since our inception, we have incurred significant operating losses. Our ability to generate revenue and achieve cost improvements sufficient to achieve profitability will depend on the successful further development and commercialization of our products and receipt and maintenance of regulatory approvals. We generated revenue of $9.4 million and $14.1 million for the three months ended March 31, 2024 and 2023, respectively, and had net income of $5.6 million and incurred a net loss of $17.8 million for those same periods. We expect to continue to incur net losses as we focus on obtaining regulatory approvals for our products in new markets, refining our sales and marketing strategies, and continuing research and development efforts to further enhance our existing products. Further, following the closing of the Business Combination described below in “Recent Developments,” we have incurred, and expect to continue to incur, additional costs associated with operating as a public company. As a result, we will need substantial additional funding for expenses related to our operating activities, including selling, marketing, general and administrative expenses and research and development expenses.
Because of the numerous risks and uncertainties associated with regulatory approval, market acceptance of our product, product development and enhancement, and commercialization, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability. Until such time, if ever, as we can generate substantial revenue sufficient to achieve profitability, we expect to finance our operations through a combination of equity offerings and debt financings. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we are unable to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back or discontinue the further development and commercialization efforts of one or more of our products, or may be forced to reduce or terminate our operations. See the subsections titled — “Liquidity and Capital Resources” and “Recent Developments” below.
Recent Developments
Business Combination Agreement
On February 9, 2023, Legacy Allurion and Allurion Technologies, Inc. (formerly Allurion Technologies Holdings, Inc.) entered into the Business Combination Agreement with Compute Health, Merger Sub I and Merger Sub II. Pursuant to the Business Combination Agreement, on the Closing Date, the Mergers (as defined below) were consummated in three steps: (a) the CPUH Merger, with Allurion surviving the CPUH Merger as a publicly listed entity at the CPUH Merger Effective Time and becoming the sole owner of the Merger Subs; (b) three hours following the consummation of the CPUH Merger, Merger Sub I merged with and into Legacy Allurion in the Intermediate Merger, with Legacy Allurion surviving the Intermediate Merger and becoming a direct, wholly-owned subsidiary of Allurion; and (c) thereafter, Legacy Allurion merged with and into Merger Sub II in the Final Merger, with Merger Sub II surviving the Final Merger and remaining a direct, wholly-owned subsidiary of Allurion at the Final Merger Effective Time. Allurion shares began trading on the NYSE under the ticker symbol “ALUR” on August 2, 2023. Upon completion of the Business Combination, Legacy Allurion’s business operations continued as our business operations.
The Business Combination was accounted for as a reverse capitalization in accordance with GAAP. Under this method of accounting, Compute Health, which was the legal acquirer, was treated as the “acquired” company for financial reporting purposes and Legacy Allurion was the accounting “acquirer”. Accordingly, the Business
109
Table of Contents
Combination was treated as the equivalent of our company issuing stock for the net assets of Compute Health, accompanied by a recapitalization. Our net assets and the net assets of Compute Health are stated at historical costs, with no goodwill or other intangible assets recorded. This determination is primarily based on the fact that, immediately following the Business Combination, Legacy Allurion Stockholders had a majority of the voting power of Allurion, Legacy Allurion controlled the majority of the board seats of Allurion, and Legacy Allurion senior management comprised all of the senior management of Allurion. The equity structure has been restated in all comparative periods up to the Closing Date to reflect the number of shares of the Company’s common stock, $0.0001 par value per share (“Allurion Common Stock” or the “Company’s Common Stock”), issued to Legacy Allurion Stockholders in connection with the Business Combination. As such, the shares and corresponding capital amounts and earnings per share related to Legacy Allurion’s convertible preferred stock and Legacy Allurion common stock prior to the Business Combination have been retroactively restated as shares reflecting the exchange ratio of approximately 0.9780 (the “Exchange Ratio”) established in the Business Combination. As a result of this retrospective application, certain prior period balances within the condensed consolidated financial statements have changed. Refer to Note 3, Business Combination in the notes to our unaudited interim condensed consolidated financial statements for further discussion regarding the closing of the Business Combination with Compute Health.
Note Purchase Agreement
On April 14, 2024, Allurion, RTW, as agent for the the Purchasers party thereto from time to time (RTW in such capacity, the Principal Purchaser), and Acquiom, as collateral agent for the Purchasers and the Principal Purchaser, entered into the Original Note Purchase Agreement. Subsequently, on April 16, 2024, the Company, the Principal Purchaser, the Purchasers and Acquiom entered into the First Amendment to the Original Note Purchase Agreement (the “Amendment”) and the Amended Note Purchase Agreement.
Pursuant to the Amended Note Purchase Agreement, the Company issued and sold the Notes to the Purchasers in a private placement transaction. The Company used the proceeds from the issuance of the Notes to refinance its outstanding obligations under the Fortress Credit Agreement in full and to pay fees and expenses in connection therewith and in connection with the transactions contemplated by the Amended Note Purchase Agreement. The Company has terminated and repaid in full the outstanding borrowings and other obligations under the Fortress Credit Agreement (defined below).
The Notes bear interest at the annual rate of 6.0%, which interest is payable quarterly in cash or, at the Company’s option, in kind for the first three years. The maturity date for the Notes is April 16, 2031. The Notes are guaranteed by Allurion Opco, and certain other current and future subsidiaries of the Company, and are secured by substantially all the assets of the Company and the guarantors.
The Notes are convertible into shares of our common stock, at a Purchaser’s election at any time after the earliest of (i) the date on which Stockholder Approval (as defined below) is obtained, (ii) December 31, 2025, (iii) the date of a Fundamental Change Company Notice (as defined in the Amended Note Purchase Agreement), and (iv) the Make-Whole Fundamental Change Effective Date (as defined in the Amended Note Purchase Agreement), subject to certain terms and limitations in the Amended Note Purchase Agreement, based on the higher of (x) an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of Notes (equivalent to an initial conversion price of approximately $3.26 per share, which represents a 35% premium to the trailing 30 day VWAP of the Company’s common stock on the NYSE as of the close of business on April 12, 2024) and (y) a 35% conversion premium to the lowest price per share in the Next Equity Financing. If certain corporate events that constitute a “Make-Whole Fundamental Change” (as defined in the Amended Note Purchase Agreement) occur, then the conversion rate will, in certain circumstances, be increased for a specified period of time up to a maximum rate of an additional 321.9182 shares of common stock per $1,000 principal amount of Notes. In addition, calling any note for redemption will also constitute a Make-Whole Fundamental Change with respect to that Note, in which case the conversion rate applicable to the conversion of that Note will be increased in certain circumstances if it is converted after it is called for redemption. Although the initial
110
Table of Contents
conversion price based on clause (x) above for the Notes is above the NYSE’s “Minimum Price” (as such term is defined in Section 312.03 of the NYSE Listed Company Manual), it is subject to a reset provision at the time of the Next Equity Financing that could result in the conversion price falling below such Minimum Price. Therefore, the Amended Note Purchase Agreement provides that unless and until the Company obtains Stockholder Approval, the Company will not deliver common stock upon conversion of the Notes in excess of 1% of the number of shares of the Company’s common stock outstanding as of April 14, 2024. The Company is required to include a proposal in its definitive proxy statement on Schedule 14A seeking Stockholder Approval no later than December 31, 2025. If the Company does not obtain Stockholder Approval at such meeting, it shall call a special meeting of stockholders each 90-day period thereafter at least two times, and thereafter at each subsequent annual meeting until Stockholder Approval is obtained or the Notes are no longer outstanding; provided, that shares of common stock issued upon conversion of the Notes prior to obtaining Stockholder Approval shall not be entitled to vote in favor of Stockholder Approval.
Amendment to Revenue Interest Financing Agreement
On February 9, 2023, Allurion Opco entered into the Original RIFA with the Original RIFA Investors.
On April 14, 2024, the Original RIFA was amended pursuant to the RIFA Amendment by and among the Company, Allurion Opco, Allurion Australia Pty Ltd, a proprietary limited company organized under the laws of Australia and a wholly-owned subsidiary of the Company, the Original RIFA Investors (as defined therein) and RTW, to reflect certain modifications agreed between the parties thereto in connection with the Purchasers’ purchase of the Notes and the refinancing of the Fortress Credit Agreement. Among other things, the RIFA Amendment waived the existing event of default under the Original RIFA, increased the Royalty Rate for net sales less than or equal to $100 million prior to December 31, 2026 from 6% to 12%, and increased the Royalty Rate on net sales less than or equal to $100 million on or after January 1, 2027 from 10% to 12%, subject to the terms and conditions of the RIFA Amendment.
Amendment to RTW Side Letter
On April 14, 2024, the Company, Allurion Opco and the Additional RIFA Investors entered into the First Amendment to Amended and Restated Letter Agreement (the “Side Letter Amendment”) to reflect certain modifications to the Original RTW Side Letter in connection with the Purchasers’ purchase of the Notes. The Side Letter Amendment provides, among other things, that the Additional RIFA Investors may make a single election in certain circumstances to convert up to $7,500,000 of the purchase price that the Additional RIFA Investors paid for certain equity interests in the Company into an amount of financing provided by the Additional RIFA Investors to Allurion Opco pursuant to an additional revenue interest financing agreement with Allurion Opco.
Termination of Fortress Term Loan
On April 16, 2024, the Company terminated and repaid in full all outstanding borrowings and fees under the Fortress Credit Agreement, including the release of all guarantees and liens related thereto.
Key Factors Affecting Our Operating Results
We believe that our performance and future success depend on many factors that present significant opportunities but also pose risks and challenges, including those discussed below and in the “Risk Factors” section of this prospectus.
| • | Market Acceptance. The growth of our business depends on our ability to gain broader acceptance of our current products by continuing to make health care providers aware of the benefits of our products to generate increased demand and frequency of use, and thus increase our sales. Our ability to grow our business will also depend on our ability to expand our customer base in existing or new target markets. Although we have increased the number of patients treated with our products through our established relationships and focused sales efforts, we cannot provide assurance that our efforts will continue to increase the use of our products. |
111
Table of Contents
| • | Regulatory approval and timing and efficiency of new product introductions. We must successfully obtain timely approvals and introduce new products that gain acceptance with health care providers. For our sales to grow, we will also need to obtain regulatory approval of our existing product and any new products or modifications/enhancements to our existing products in the markets that we operate in and new markets as applicable. |
| • | Sales force size and effectiveness. The speed at which newly hired salespeople become effective can impact our revenue growth or our costs incurred in anticipation of such growth. We intend to continue to improve and increase performance in our sales and marketing organization, and expand our international programs to help facilitate further adoption of our products as well as broaden awareness of our products to new customers. |
| • | Product and geographic mix; timing. Our financial results, including our gross margins, may fluctuate from period to period based on the timing of orders, fluctuations in foreign currency exchange rates and the number of available selling days in a particular period, which can be impacted by a number of factors, such as holidays or days of severe inclement weather in a particular geography, the mix of products sold and the geographic mix of where products are sold. |
Operating Segments
We operate our business in a single segment and as one reporting unit, which is how our chief operating decision maker (“CODM”), our chief executive officer, reviews financial performance and allocates resources. The CODM reviews financial information presented on a regular basis at the consolidated level for purposes of allocating resources and evaluating financial performance. Since we operate as one operating segment, all required financial segment information can be found in the consolidated financial statements.
Components of Our Results of Operations
Revenue
We derive revenue from the sale of the Allurion Balloon to customers, which are either distributors or health care providers. The Allurion Balloon is the foundation of the Allurion Program, a holistic weight loss program that offers patients the opportunity to receive, and clinic and other health care providers the ability to deliver, behavior change assistance through their use of our remote patient support and monitoring tools.
Cost of Revenue
Cost of revenue consists primarily of costs that are closely correlated or directly related to the delivery of our products, including material costs, labor costs, and depreciation expense for fixed assets.
Operating Expenses
Sales and Marketing Expenses
Sales and marketing expenses consist primarily of salaries and related expenses (including commissions) for our sales and marketing personnel. Marketing programs consist of advertising, training events, brand building, product marketing activities and shipping costs.
Research and Development Expenses
Our research and development expenses consist of costs associated with performing research and development activities such as registering our products in various jurisdictions and performing clinical trials. These costs include salaries and benefits, stock-based compensation, non-capitalizable software development costs, product development costs, materials and supplies, clinical trial activities, registration expenses, depreciation of equipment and other outside services.
112
Table of Contents
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and personnel-related costs, including stock-based compensation, for our personnel in executive, information technology, finance and accounting, human resources and other administrative functions. General and administrative expenses also include legal fees relating to corporate matters; professional fees paid for accounting, auditing, consulting and tax services; insurance costs; travel expenses; office and information technology costs; and facilities, depreciation and other expenses related to general and administrative activities, which include direct or allocated expenses for rent and maintenance of facilities and utilities.
Other Income (Expense), Net
Interest Expense
Interest expense consists of interest expense associated with outstanding borrowings under our debt obligations as well as the amortization of debt issuance costs and discounts associated with such borrowings.
Change in Fair Value of Warrants
The change in fair value of warrants consists of the expense recognized upon the mark to market of our warrant liabilities.
Change in Fair Value of Revenue Interest Financing and PIPE Conversion Option
The change in fair value of Revenue Interest Financing and PIPE Conversion Option (as defined below) consists of the expense recognized upon the mark to market of the Revenue Interest Financing with RTW and the issuance and mark to market of the PIPE Conversion Option. See Note 10, Fair Value Measurements for further information.
Change in Fair Value of Earn-out Liabilities
The change in fair value of earn-out liabilities consists of the gain or loss recognized upon mark to market of the contingent earn-out consideration. See Note 10, Fair Value Measurements for further information.
Other Income (Expense), net
Other income (expense), net consists of interest earned on our invested cash balances, which primarily consist of deposit accounts and money market funds, foreign currency transaction gains and losses and expense associated with our success fee associated with Legacy Allurion’s November 2019 loan and security agreement with Western Alliance Bank (the “2019 Term Loan” and such fee, the “Success Fee”) and derivative liability and Fortress Term Loan derivative liability. See Note 10, Fair Value Measurements for further information.
113
Table of Contents
Results of Operations
Comparison of the Three Months Ended March 31, 2024 and 2023 (unaudited)
The following table summarizes our results of operations for the three months ended March 31, 2024 and 2023 (in thousands):
| Three Months Ended March 31, | ||||||||||||
| 2024 | 2023 | Change | ||||||||||
Revenue | $ | 9,386 | $ | 14,071 | $ | (4,685 | ) | |||||
Cost of revenue | 2,520 | 2,940 | (420 | ) | ||||||||
|
|
|
|
|
| |||||||
Gross profit | 6,866 | 11,131 | (4,265 | ) | ||||||||
|
|
|
|
|
| |||||||
Operating expenses: | ||||||||||||
Sales and marketing | 6,145 | 11,864 | (5,719 | ) | ||||||||
Research and development | 5,725 | 7,852 | (2,127 | ) | ||||||||
General and administrative | 6,386 | 5,306 | 1,080 | |||||||||
|
|
|
|
|
| |||||||
Total operating expenses: | 18,256 | 25,022 | (6,766 | ) | ||||||||
|
|
|
|
|
| |||||||
Loss from operations | (11,390 | ) | (13,891 | ) | 2,501 | |||||||
|
|
|
|
|
| |||||||
Other (expense) income: | ||||||||||||
Interest expense | (1,931 | ) | (2,237 | ) | 306 | |||||||
Changes in fair value of warrants | 3,131 | (1,475 | ) | 4,606 | ||||||||
Changes in fair value of Revenue Interest Financing and PIPE conversion option | 1,490 | — | 1,490 | |||||||||
Changes in fair value of earn-out liabilities | 14,190 | — | 14,190 | |||||||||
Other (expense) income, net | 172 | (164 | ) | 336 | ||||||||
|
|
|
|
|
| |||||||
Total other (expense) income: | 17,052 | (3,876 | ) | 20,928 | ||||||||
|
|
|
|
|
| |||||||
Income (loss) before income taxes: | 5,662 | (17,767 | ) | 23,429 | ||||||||
Provision for income taxes: | (76 | ) | (34 | ) | (42 | ) | ||||||
|
|
|
|
|
| |||||||
Net income (loss) | $ | 5,586 | $ | (17,801 | ) | $ | 23,387 | |||||
|
|
|
|
|
| |||||||
Revenue
Revenue decreased $4.7 million, or 33%, to $9.4 million for the three months ended March 31, 2024, compared to the same period in 2023. The decrease in revenue was primarily the result of decreased gastric balloon units sold due to lower re-order rates as distributors and certain accounts adjusted their inventory levels. The decrease in revenue was also attributable to selling less or no product to certain distributors and accounts to manage our credit risk.
Cost of Revenue
Cost of revenue decreased $0.4 million, or 14%, to $2.5 million for the three months ended March 31, 2024, compared to the same periods in 2023. The decrease in cost of revenue was a direct result of decreased gastric balloon units sold, partially offset by an increase in manufacturing expense, as less labor and overhead was absorbed due to lower production volumes.
Gross Profit
Gross profit decreased $4.3 million, or 38%, to $6.9 million for the three months ended March 31, 2024, compared to the same period in 2023. The decrease in gross profit was primarily the result of an increase in our manufacturing expense, as less labor and overhead was absorbed due to lower production volumes, as well as a decrease in revenue and sales volume of our gastric balloon system.
114
Table of Contents
Operating Expenses
Sales and Marketing Expenses
Sales and marketing expenses decreased $5.7 million, or 48%, to $6.2 million for the three months ended March 31, 2024, compared to the same period in 2023. The decrease in sales and marketing expenses was the result of a $2.7 million decrease in marketing spend driven by a reorganization of our selling and marketing spend on more efficient channels and geographies, a $1.4 million decrease in shipping and logistics expenses driven by a decrease in sales during the comparable periods, a $0.5 million decrease in sales meetings, and a $0.5 million decrease in salaries, related benefits, and consulting costs.
Research and Development Expenses
Research and development expenses decreased $2.1 million, or 27%, to $5.7 million for the three months ended March 31, 2024, compared to the same period in 2023. The decrease in research and development expenses was primarily the result of a $1.3 million decrease in costs related to the AUDACITY clinical trial as it nears completion, a $0.6 million decrease attributable to salaries and related benefit costs due to lower headcount, and a $0.2 million decrease in outside consulting costs. We expect research and development expenses to decrease in 2024 as our AUDACITY trial nears completion.
General and Administrative Expenses
General and administrative expenses increased $1.1 million, or 20%, to $6.4 million for the three months ended March 31, 2024, compared to the same period in 2023. The increase in general and administrative expenses was primarily the result of a $0.9 million increase attributable to salaries, benefits, and related costs, a $0.7 million increase in legal and professional fees, a $0.3 million increase in insurance expense related to director and officer insurance, and a $0.2 million increase in stock-based compensation expense. These increases were partially offset by a $1.0 million decrease in bad debt expense.
Other expense (income)
Interest Expense
Interest expense decreased $0.3 million, or 14%, to $1.9 million for the three months ended March 31, 2024, compared to the same period in 2023. The decrease in interest expense was due to a reduction in principal of our Fortress Term Loan as compared to our 2021 Term Loan during the comparable period.
Change in Fair Value of Warrants
The $4.6 million gain attributable to the change in fair value of warrants for the three months ended March 31, 2024, compared to the same period in 2023, was due to mark to market fluctuations in our warrant liabilities due to the decline in value of our common stock during the period.
Change in Fair Value of Revenue Interest Financing and PIPE Conversion Option
The $1.5 million gain attributable to the change in fair value of Revenue Interest Financing and PIPE Conversion Option for the three months ended March 31, 2024 was primarily due to a $3.4 million gain related to the change in fair value in the Revenue Interest Financing from December 31, 2023 to March 31, 2024. This gain was partially offset by a $1.9 million loss in the change in fair value of the PIPE Conversion Option from December 31, 2023 to March 31, 2024 due to a decrease in the Company’s stock price during the period.
Change in Fair Value of Earn-Out Liabilities
The $14.2 million gain attributable to the change in the fair value of earn-out liabilities for the three months ended March 31, 2024 was due to the decrease in the Company’s stock price from December 31, 2023 to March 31, 2024.
115
Table of Contents
Other (Expense) Income, Net
The change in Other (expense) income, net for the three months ended March 31, 2024 compared to the same period in 2023, was $0.3 million of income primarily driven by interest income of $0.5 million, partially offset by a $0.2 million loss related to fluctuations in exchange rates of foreign currencies.
Provision for Income Taxes
We recorded a provision for income taxes of $0.1 million and less than $0.1 million for the three months ended March 31, 2024 and 2023, respectively. These provisions for income taxes are due to net income in certain foreign jurisdictions.
Comparison of the Years Ended December 31, 2023 and 2022
The following table summarizes our results of operations for the years ended December 31, 2023 and 2022 (in thousands):
| Years Ended December 31, | ||||||||||||
| 2023 | 2022 | Change | ||||||||||
Revenue | $ | 53,467 | $ | 64,211 | $ | (10,744 | ) | |||||
Cost of revenue | 11,970 | 13,485 | (1,515 | ) | ||||||||
|
|
|
|
|
| |||||||
Gross profit | 41,497 | 50,726 | (9,229 | ) | ||||||||
|
|
|
|
|
| |||||||
Operating expenses: | ||||||||||||
Sales and marketing | 46,857 | 50,405 | (3,548 | ) | ||||||||
Research and development | 27,694 | 16,966 | 10,728 | |||||||||
General and administrative | 46,024 | 15,365 | 30,659 | |||||||||
|
|
|
|
|
| |||||||
Total operating expenses: | 120,575 | 82,736 | 37,839 | |||||||||
|
|
|
|
|
| |||||||
Loss from operations | (79,078 | ) | (32,010 | ) | (47,068 | ) | ||||||
|
|
|
|
|
| |||||||
Other income (expense): | ||||||||||||
Interest expense | (10,566 | ) | (4,426 | ) | (6,140 | ) | ||||||
Changes in fair value of warrants | 8,364 | (821 | ) | 9,185 | ||||||||
Changes in fair value of debt | (3,751 | ) | — | (3,751 | ) | |||||||
Changes in fair value of Revenue Interest Financing and PIPE conversion option | (2,192 | ) | — | (2,192 | ) | |||||||
Changes in fair value of earn-out liabilities | 29,050 | — | 29,050 | |||||||||
Termination of convertible note side letters | (17,598 | ) | — | (17,598 | ) | |||||||
Loss on extinguishment of debt | (3,929 | ) | — | (3,929 | ) | |||||||
Other income (expense), net | (643 | ) | (344 | ) | (299 | ) | ||||||
|
|
|
|
|
| |||||||
Total other income (expense): | (1,265 | ) | (5,591 | ) | 4,326 | |||||||
|
|
|
|
|
| |||||||
Loss before income taxes: | (80,343 | ) | (37,601 | ) | (42,742 | ) | ||||||
Provision for income taxes: | (264 | ) | (143 | ) | (121 | ) | ||||||
|
|
|
|
|
| |||||||
Net loss | $ | (80,607) | $ | (37,744 | ) | $ | (42,863 | ) | ||||
|
|
|
|
|
| |||||||
Revenue
Revenue decreased $10.7 million, or 17%, to $53.5 million for the year ended December 31, 2023, compared to the same period in 2022. The decrease in revenue was primarily the result of a delay in closing of the Business Combination, which led to decreased investment in certain markets, leading to temporarily lower
116
Table of Contents
re-order rates as distributors and certain accounts adjusted their inventory levels during the second half of 2023. The decrease in revenue was also attributable to selling less or no product to certain distributors and accounts to manage our credit risk.
Cost of Revenue
Cost of revenue decreased $1.5 million, or 11%, to $12.0 million for the year ended December 31, 2023, compared to the same period in 2022. The decrease in cost of revenue was primarily a direct result of decreased gastric balloons sold.
Gross Profit
Gross profit decreased $9.2 million, or 18%, to $41.5 million for the year ended December 31, 2023, compared to the same period in 2022. The decrease in gross profit was primarily the result of fewer gastric balloons sold, an increase in our direct manufacturing costs and a higher percentage of sales in lower-margin geographies.
Operating Expenses
Sales and Marketing Expenses
Sales and marketing expenses decreased $3.5 million, or 7%, to $46.9 million for the year ended December 31, 2023, compared to the same period in 2022. The decrease in sales and marketing expenses was primarily the result of a $3.9 million decrease in marketing spend driven by our decision to delay investment in sales and marketing while completing the Business Combination with Compute Health, and a $1.8 million decrease related to changes in our distribution model, which were partially offset by a $2.3 million increase attributable to salaries and related benefit costs due to higher headcount. We expect sales and marketing costs to decrease in 2024 as we have implemented cost reduction initiatives, including a reduction in force, and shift our focus of selling and marketing spend on more efficient channels and geographies.
Research and Development Expenses
Research and development expenses increased $10.7 million, or 63%, to $27.7 million for the year ended December 31, 2023, compared to the same period in 2022. The increase in research and development expenses was the result of an increase of $9.8 million in costs related to the AUDACITY clinical trial, and a $0.9 million increase attributable to salaries due to higher headcount to support new product development and clinical trials. We expect research and development expenses to decrease in 2024 as our AUDACITY trial nears completion.
General and Administrative Expenses
General and administrative expenses increased $30.6 million, or 199%, to $46.0 million for the year ended December 31, 2023, compared to the same period in 2022. The increase in general and administrative expenses was primarily the result of a $12.0 million increase in accounts receivable reserves resulting primarily from challenges entering new markets and transitioning certain markets from a distributor model to a direct model, a $7.8 million increase in stock-based compensation, including $4.9 million of one-time charges as a result of the Business Combination, a $3.6 million increase in insurance expense as a result of the Business Combination, a $4.5 million increase in professional, legal, and consulting fees, including $1.7 million of one-time charges as a result of the Business Combination, and a $2.2 million increase attributable to salaries and related benefit costs due to higher headcount. We expect general and administrative expenses to decrease in 2024, as we have implemented cost reduction initiatives. Further, we had incurred a significant increase in accounting, audit, legal, regulatory, compliance, and investor and public relations expenses associated with the Business Combination for the year ended December 31, 2023. We also incurred significant bad debts expense in 2023 which is not expected to recur.
117
Table of Contents
Other expense (income)
Interest Expense
Interest expense increased $6.1 million, or 139%, to $10.6 million for the year ended December 31, 2023, compared to the same period in 2022. The increase in interest expense was primarily due to a $4.9 million increase in interest expense associated with our Fortress Term Loan signed in August 2023, a $0.8 million increase in interest on our 2021 Term Loan, and a $0.5 million increase in interest expense associated with our 2023 convertible notes that were converted into shares of our common stock at the closing of the Business Combination in August 2023.
Change in Fair Value of Warrants
The change in fair value of warrants for the year ended December 31, 2023 compared to the same period in
2022 was a gain of $9.2 million, primarily driven by a $7.8 million gain attributable to the change in fair value of the CPUH public warrants and a $0.5 million gain attributable to the change in fair value of our private warrants due to the decline in value of our common and preferred stock during those periods.
Change in Fair Value of Debt
The $3.8 million loss attributable to the change in fair value of debt for the year ended December 31, 2023 was driven by mark to market fluctuations in our convertible debt as a result of the closing of the Business Combination and automatic conversion of the notes to our common stock.
Change in Fair Value of Revenue Interest Financing and PIPE Conversion Option
The $2.2 million loss attributable to the change in fair value of Revenue Interest Financing and PIPE conversion option for the year ended December 31, 2023 was primarily due to the initial recognition of the PIPE Conversion Option of $3.3 million on August 1, 2023 and an additional $2.3 million loss in the fair value from August 1, 2023 to December 31, 2023 due to a decrease in the Company’s stock price during that period. This expense was partially offset by a $3.4 million gain in the fair value in the Revenue Interest Financing from when the liability was established on August 1, 2023 to December 31, 2023.
Change in Fair Value of Earn-Out Liabilities
The $29.1 million gain attributable to the change in the fair value of earn-out liabilities for the year ended December 31, 2023 was due to the decrease in our stock price between the establishment of the liability on August 1, 2023 and December 31, 2023.
Termination of convertible note side letters
The $17.6 million loss attributable to the termination of convertible note side letters for the year ended December 31, 2023 was primarily due to a $13.4 million loss related to the Base PubCo Shares and Backstop Shares (each as defined in our consolidated financial statements included elsewhere in this prospectus) liabilities driven by the use of the full backstop, a $2.7 million loss on the PubCo Additional Shares liability, and a $1.5 million prepayment penalty related to the repayment of the Company’s Legacy Allurion convertible note with HVL.
Loss on Extinguishment of Debt
The $3.9 million loss on extinguishment of debt for the year ended December 31, 2023 was due to the loss on extinguishment of debt related to the repayment of our 2021 Term Loan in connection with the entrance into the Fortress Term Loan on August 1, 2023.
118
Table of Contents
Other (Expense) Income, Net
The change in Other (expense) income, net for the year ended December 31, 2023 compared to the same period in 2022 was $0.3 million of expense, primarily driven by interest expense of $1.7 million due to the establishment of the Fortress Term Loan derivative liability and mark to market fluctuations of the 2019 Term Loan Success Fee derivative liability. This was partially offset by $1.2 million of interest income during the period.
Liquidity and Capital Resources
Since our inception, we have primarily obtained cash to fund our operations through the sale of Allurion preferred stock, issuance of term loans and issuance of convertible debt instruments. As of March 31, 2024, we had $29.7 million in cash and cash equivalents. We had net income of $5.6 million and incurred a net loss of $17.8 million for the three months ended March 31, 2024 and 2023, respectively. We incurred cash outflows from operating activities of $8.6 million and $10.3 million during the three months ended March 31, 2024 and 2023, respectively. As of March 31, 2024, we had an accumulated deficit of $207.2 million. As of December 31, 2023, we had $38.0 million in cash and cash equivalents. We incurred net losses of $80.6 million and $37.7 million for the years ended December 31, 2023 and 2022, respectively. We incurred cash outflows from operating activities of $64.0 million and $47.0 million during the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $212.8 million. We expect to continue to generate significant operating losses for the foreseeable future.
Our future capital requirements will depend on many factors, including:
| • | the emergence of competing innovative weight loss experiences and other adverse marketing developments; |
| • | the timing and extent of our sales and marketing and research and development expenditures; and |
| • | any investments or acquisitions we may choose to pursue in the future. |
Our revenue for the three months ended March 31, 2024 was $9.4 million, which represented a quarter-over-quarter decrease of 33%. The decrease in revenue was primarily the result of lower re-order rates as distributors and certain accounts adjusted their inventory levels. The decrease in revenue was also attributable to selling less or no product to certain distributors and accounts to manage our credit risk. Our revenue for the year ended December 31, 2023 was $53.5 million, which represented a year-over-year decrease of 17%. The decline in revenue for the year ended December 31, 2023 was primarily the result of a delay in closing of the Business Combination, which led to decreased investment in certain markets leading to temporarily lower re-order rates as distributors and certain accounts adjusted their inventory levels during the second half of 2023. The decrease in revenue was also attributable to selling less or no product to certain distributors and accounts to manage our credit risk. If our current cash and anticipated revenue and resulting cash flows from operations are insufficient to satisfy our liquidity requirements, due to increased expenditures, lower demand for our gastric balloon system, the occurrence of other events or the realization of the risks described in this prospectus, we may be required to raise additional capital through the issuances of public or private equity or debt financing or other capital sources earlier than expected.
Until such time as we can generate significant revenue to fund operations, we expect to use proceeds from the issuance of equity, debt financings, or other capital transactions to fund our operations and satisfy our liquidity requirements. We may be unable to increase our revenue, raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, scale back or discontinue our operations or the development and commercialization of one or more of our product candidates and other strategic initiatives. As of March 31, 2024, based on our recurring losses from operations incurred since inception, the expectation of continuing operating losses for the foreseeable future, and the potential need to raise
119
Table of Contents
additional capital to finance its future operations and debt service payments, we have concluded that there is substantial doubt about our ability to continue as a going concern for a period of one year from the date that the consolidated financial statements included in this prospectus are issued.
Financing Arrangements
Note Purchase Agreement
On April 16, 2024, we received $48 million in gross proceeds from the Amended Note Purchase Agreement with RTW, which proceeds were used to repay all outstanding principal, accrued and unpaid interest and other obligations with respect to the Fortress Term Loan.
Chardan Purchase Agreement
On December 18, 2023, we entered into the Purchase Agreement with Chardan. Pursuant to the Purchase Agreement, we have the right from time to time at our option to sell to Chardan up to the lesser of (i) $100,000,000 in aggregate gross purchase price of newly issued shares of our common stock, and (ii) the Exchange Cap.
As of March 31, 2024, we have received $0.4 million in proceeds from sales of shares of our common stock pursuant to the Purchase Agreement with Chardan.
Fortress Credit Agreement
On August 1, 2023, we received $57.6 million in net proceeds from the Fortress Credit Agreement that was to mature in June 2027. We entered into the Fortress Term Loan pursuant to the Fortress Credit Agreement on August 1, 2023 in connection with the closing of the Business Combination. In December 2023, we repaid $20.0 million of outstanding principal under the Fortress Term Loan. On April 16, 2024, we received $48 million in net proceeds from the Amended Note Purchase Agreement with RTW, which proceeds were used to repay all outstanding principal, accrued and unpaid interest and other obligations with respect to the Fortress Term Loan. The Fortress Term Loan is included in current portion of term loan on our consolidated balance sheet as of March 31, 2024. See Note 8, Debt in the notes to our unaudited interim condensed consolidated financial statements for the three months ended March 31, 2024 and 2023 included within this prospectus for additional details regarding the Fortress Term Loan.
Revenue Interest Financing Agreement
On August 1, 2023, we received $40.0 million in proceeds from the Revenue Interest Financing Agreement with RTW, which matures in December 2030. We entered into the Revenue Interest Financing Agreement on February 9, 2023 and received the proceeds at the closing of the Business Combination. The Revenue Interest Financing Agreement is included in Revenue Interest Financing liability on our consolidated balance sheet as of March 31, 2024. On April 14, 2024, the Revenue Interest Financing Agreement was amended to, among other things, increase the rate of revenue interest payments to be paid to RTW. See Note 9, Revenue Interest Financing, Side Letter, and PIPE Conversion Option and Note 19, Subsequent Events in the notes to our unaudited interim condensed consolidated financial statements for the three months ended March 31, 2024 and 2023 included within this prospectus for additional details regarding the Revenue Interest Financing Agreement.
PIPE Investment
On August 1, 2023, we received $37.9 million proceeds from the PIPE Subscription Agreement for which the PIPE Investors received 5,386,695 shares of Allurion Common Stock at a price of $7.04 per share.
120
Table of Contents
2021 Term Loan
On August 1, 2023, in connection with the closing of the Business Combination, we repaid all outstanding principal, accrued and unpaid interest and other obligations with respect to the 2021 Term Loan, which has been terminated. See Note 8, Debt in the notes to our unaudited interim condensed consolidated financial statements for the three months ended March 31, 2024 and 2023 for additional details regarding the 2021 Term Loan.
Convertible Notes
On August 1, 2023, all outstanding Allurion Convertible Notes and related interest expense were converted into shares of Allurion Common Stock in connection with the closing of the Business Combination. As of March 31, 2024, there were no outstanding convertible notes. See Note 8, Debt in the notes to our unaudited interim condensed consolidated financial statements for the three months ended March 31, 2024 and 2023 for additional details related to our convertible notes.
Material Cash Requirements for Known Contractual and Other Obligations
Leases
We have entered into various non-cancellable operating leases for our corporate office, manufacturing facilities, research and development labs, management office space and certain equipment. The leases have varying terms expiring between 2024 and 2028. See Note 16, Commitments and Contingencies of the notes to our unaudited interim condensed consolidated financial statements for the three months ended March 31, 2024 and 2023 for additional details related to our noncancelable operating leases.
Term Loan and Financing Strategy
We had $43.1 million of outstanding debt under the Fortress Term Loan as of March 31, 2024. On April 16, 2024, we received $48 million in gross proceeds from the Amended Note Purchase Agreement with RTW, which proceeds were used to repay all outstanding principal, accrued and unpaid interest and other obligations with respect to the Fortress Term Loan.
Revenue Interest Financing
We received $40.0 million in proceeds from the Revenue Interest Financing Agreement with RTW on August 1, 2023. In exchange, we are obligated to remit to RTW certain revenue interest payments on all current and future products, digital solutions and services developed, imported, manufactured, marketed, offered for sale, promoted, sold, tested or otherwise distributed by Allurion and its subsidiaries at certain specified rates. On April 14, 2024, the Revenue Interest Financing Agreement was amended to, among other things, increase the rate of revenue interest payments to be paid to RTW for net sales less than or equal to $100 million prior to December 31, 2026 from 6% to 12%, and increase the royalty rate on net sales less than or equal to $100 million on or after January 1, 2027 from 10% to 12%.
If RTW has not received aggregate revenue interest payments equal to at least 100% of the Investment Amount by December 31, 2027, we must make a cash payment in an amount sufficient to catch RTW up to 100% of the Investment Amount. If RTW has not received revenue interest payments equal to at least 240% of the Investment Amount by December 31, 2030, we must make a cash payment in an amount sufficient to catch RTW up to 240% of the Investment Amount.
Research and Development Costs
We are continuing to invest in our U.S. FDA AUDACITY clinical trial and have entered into contractual obligations with each clinical trial site. Each contract shall continue until the completion of the trial at that site,
121
Table of Contents
which is approximately 48 weeks from the start of each contract. Our clinical trial costs are dependent on, among other things, the size, number, and length of our clinical trial. We also incur research and development costs related to the enhancement of our existing products.
Other Capital Requirements
We enter into agreements in the normal course of business with various vendors, which are generally cancelable upon notice. Payments due upon cancellation typically consist only of payments for services provided or expenses incurred, including non-cancelable obligations of service providers, up to the date of cancellation.
Cash Flows
The following table sets forth a summary of cash flows for the periods presented:
| Three Months Ended March 31, | Years Ended December 31, | |||||||||||||||
| (In thousands) | 2024 | 2023 | 2023 | 2022 | ||||||||||||
Net cash used in operating activities | $ | (8,636 | ) | $ | (10,318 | ) | $ | (63,982 | ) | $ | (46,981 | ) | ||||
Net cash used in investing activities | (104 | ) | (277 | ) | (1,606 | ) | (1,550 | ) | ||||||||
Net cash provided by financing activities | 387 | 12,866 | 95,986 | 30,537 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net increase (decrease) in cash and cash equivalents, and restricted cash | $ | (8,353 | ) | $ | 2,271 | $ | 30,938 | $ | (17,994 | ) | ||||||
|
|
|
|
|
|
|
| |||||||||
Net Cash Used in Operating Activities
Three Months Ended March 31, 2024 and 2023
During the three months ended March 31, 2024, net income of $5.6 million and net cash provided by changes in our operating assets and liabilities of $2.6 million was more than offset by non-cash income of $16.8 million, resulting in cash used from operating activities of $8.6 million.
Non-cash income consisted of $14.2 million of income related to the change in fair value of our earn-out liabilities, $3.1 million of mark to market adjustments related to our warrant liabilities, and $1.5 million of income related to the change in fair value of the Revenue Interest Financing and PIPE Conversion Option. This non-cash income was partially offset by $0.6 million of stock-based compensation expenses, $0.4 million of depreciation and amortization expense, $0.3 million of non-cash interest expense primarily related to the accretion of debt discount associated with our Fortress Term Loan, $0.3 million of unrealized loss on foreign exchange, a $0.2 million provision for inventory, and $0.2 million of non-cash lease expense.
Net cash provided by changes in our operating assets and liabilities consisted of a $1.7 million decrease in accounts receivable, a $0.3 million decrease in inventory, a $0.2 million decrease in prepaid expenses, other current and long-term assets, and a net $0.6 million increase in accounts payable, accrued expenses and other current liabilities.
The decrease in accounts receivable was the result of an increase in cash collections. The decrease in inventory was primarily related to a decrease in raw materials and work in progress. The decrease in prepaid expenses, other current and long-term assets was primarily related to a decrease in payroll deposits. The net increase in accounts payable, accrued expenses and other current liabilities was primarily related to timing of payments.
During the three months ended March 31, 2023, operating activities used $10.3 million of cash, resulting from a net loss of $17.8 million, partially offset by net cash provided by changes in our operating assets and liabilities of $3.9 million and non-cash charges of $3.6 million.
122
Table of Contents
Non-cash charges consisted of $1.5 million of mark to market adjustments related to our warrant and derivative liabilities, a $1 million provision for uncollectible accounts, $0.5 million of depreciation and amortization expense, $0.4 million of stock-based compensation expense, and $0.3 million of non-cash interest expense primarily related to the accretion of debt discount associated with our outstanding debt arrangements.
Net cash provided by changes in our operating assets and liabilities consisted of a net $4.9 million increase in accounts payable, accrued expenses and other current liabilities, partially offset by a $0.4 million increase in inventory, and a $0.4 million increase in accounts receivable.
The net increase in accounts payable, accrued expenses and other current liabilities was primarily related to increased expenses as well as timing of payments. The increase in inventory was primarily related to an increase in finished goods and raw materials. The increase in accounts receivable was primarily related to growth in sales.
Years Ended December 31, 2023 and 2022
During the year ended December 31, 2023, operating activities used $64.0 million of cash, resulting from a net loss of $80.6 million, partially offset by net cash provided by changes in our operating assets and liabilities of $0.2 million and non-cash charges of $16.4 million.
Non-cash charges consisted of $16.1 million for termination of convertible note side letters, a $12.7 million provision for uncollectible accounts, $8.4 million of stock-based compensation expense, a $3.9 million loss on extinguishment of debt for our 2021 Term Loan, $3.8 million related to the change in fair value of our convertible debt, $2.2 million related to the change in fair value of the Revenue Interest Financing and PIPE Conversion Option, $2.1 million of non-cash interest expense primarily related to the amortization of debt discount associated with our outstanding debt arrangements, $1.7 million of mark to market adjustments related to our derivative liabilities, $1.2 million of debt issuance costs associated with the Revenue Interest Financing, $0.8 million of lease expense, and $0.7 million of depreciation and amortization expense. These charges were partially offset by $29.1 million of income related to the change in fair value of our earn-out liabilities, $8.4 million of mark to market adjustments related to our warrant liabilities, and $1.1 million of interest paid on debt recorded at fair value.
Net cash provided by changes in our operating assets and liabilities consisted of a net $5.7 million increase in accounts payable, accrued expenses and other current liabilities and a $0.3 million decrease in prepaid expenses, other current and long-term assets, partially offset by a $3.7 million increase in inventory, a $1.3 million increase in accounts receivable, and a $0.8 million decrease in lease liabilities.
The net increase in accounts payable, accrued expenses and other current liabilities was primarily related to increased expenses as well as timing of payments. The increase in accounts receivable was primarily related to the timing of cash collections. The decrease in prepaid expenses, other current and long-term assets was primarily related to the settlement of deferred deal costs related to the Business Combination. The increase in inventory was primarily related to an increase in work in progress and raw materials.
During the year ended December 31, 2022, operating activities used $47.0 million of cash, resulting from a net loss of $37.7 million and net cash used by changes in our operating assets and liabilities of $13.8 million, offset by non-cash charges of $4.6 million.
Non-cash charges primarily consisted of $2.0 million of depreciation and amortization expense and non-cash lease expense, $1.0 million of interest expense primarily related to the accretion of debt discount associated with our outstanding debt arrangements, a $0.8 million change in fair value of warrant liabilities, a $0.4 million of stock-based compensation expense and a $0.4 million provision for uncollectible accounts.
Net cash used by changes in our operating assets and liabilities consisted of a $22.8 million increase in accounts receivable, a $1.2 million increase in inventory, a $0.7 million decrease in operating lease liabilities and
123
Table of Contents
a $0.6 million increase in prepaid expenses, other current assets and long-term assets, partially offset by a net $11.5 million increase in accounts payable, accrued expenses and other current liabilities.
The increase in accounts receivable was primarily related to growth in sales. The increase in prepaid expenses and other current assets was primarily related to increases in prepaid inventory and additional software subscription costs. The increase in inventory was primarily related to an increase in finished goods and raw materials. The decrease in lease liabilities was primarily due to payment of rent for our leased property. The net increase in accounts payable, accrued expenses and other current liabilities was primarily related to an increase in sales and marketing and general and administrative expenses.
Net Cash Used in Investing Activities
Three Months Ended March 31, 2024 and 2023
During the three months ended March 31, 2024 and March 31, 2023, cash used in investing activities was $0.1 million and $0.3 million, respectively, consisting of purchases of property and equipment.
Years Ended December 31, 2023 and 2022
During the years ended December 31, 2023 and December 31, 2022, cash used in investing activities was $1.6 million, consisting of purchases of property and equipment.
Net Cash Provided by Financing Activities
Three Months Ended March 31, 2024 and 2023
During the three months ended March 31, 2024, cash provided by financing activities was $0.4 million, consisting of $0.4 million of proceeds from our equity line financing.
During the three months ended March 31, 2023, cash provided by financing activities was $12.9 million, consisting of $13.6 million from the issuance of our 2023 Convertible Notes, net of issuance costs, partially offset by $0.8 million of payments of deferred financing costs.
Years Ended December 31, 2023 and 2022
During the year ended December 31, 2023, cash provided by financing activities was $96.0 million, consisting of $61.7 million of proceeds from the Business Combination, net of transaction costs, $57.6 million from the issuance of our Fortress Term Loan net of debt issuance costs, $38.8 million from the issuance of our Revenue Interest Financing Agreement with RTW net of issuance costs, and $28.7 million from the issuance of our 2023 Convertible Notes, net of issuance costs, partially offset by the $57.7 million repayment of our 2021 Term Loan, $20.0 million repayment of our Fortress Term Loan, $10.8 million repayment of our 2023 Convertible Notes and $2.5 million repayment of a promissory note assumed from Compute Health in the Business Combination.
During the year ended December 31, 2022, cash provided by financing activities was $30.5 million, consisting of $29.6 million from the issuance of our 2021 Term Loan, net of issuance costs, $1.1 million from the issuance of our 2022 Convertible Notes, net of issuance costs and $0.1 million from the exercise of stock options, partially offset by $0.3 million payment of deferred financing costs.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States. The preparation of our consolidated financial statements and related disclosures
124
Table of Contents
requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are described in more detail in Note 2 to our audited consolidated financial statements included in this prospectus, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We account for revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (“Topic 606” or “ASC 606”). In accordance with ASC 606, we recognize revenue when control of our products is transferred to our customers in an amount that reflects the consideration we expect to receive in exchange for those products. Our revenue recognition process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the transaction price, allocating the transaction price to the distinct performance obligations in the contract, and recognizing revenue as performance obligations are satisfied.
Revenue is generated primarily from the sale of our gastric balloon system, which includes the Allurion Balloon and related accessories. We have provided customers purchasing the Allurion Balloon with an implied license for access to our VCS software. This implied software license was given to customers for no additional consideration and was not negotiated as part of the customer’s contracts. As such, it has been deemed an immaterial promise in the context of the contract, and we do not consider the license as a separate performance obligation. In the future, if and when VCS services are determined to be a performance obligation, we expect the associated consideration will be deferred and recognized over the license period.
We sell our products directly to customers through our direct sales personnel and indirectly through independent distributors. For distributor sales, we sell our products to our distributors, who subsequently resell the products to health care providers, among others. For direct sales, our products are sold directly to our customers, which are typically health care providers. Generally, customer contracts contain Free on Board or Ex-Works shipping point incoterms. We recognize revenue when the customer obtains control of our product, which typically occurs upon shipment, in return for agreed-upon, fixed-price consideration.
Additionally, from time to time, we offer certain incentives to our customers, which are recorded as a reduction of revenue in the period the related product revenue is recognized. Any discounts we offer are outlined in our customer agreements. Payments to the customer for a distinct good or service that reasonably estimates the fair value of the distinct benefit received, such as marketing programs and shipping and logistics services, are recorded as a selling and marketing expense.
Our payment terms are consistent with prevailing practice in the respective markets in which we do business, which are not affected by contingent events that could impact the transaction price. Our contracts with customers do not provide general rights of return unless certain product quality standards are not met.
Valuation of Earn-Out Liabilities
In connection with the Business Combination, holders of Legacy Allurion common stock and Legacy Allurion preferred stock and holders of vested options, warrants and restricted stock units exercisable or convertible into Earn-Out Shares upon the achievement of certain earn-out targets. As the contingent earn-out consideration contains a settlement provision that precludes it from being indexed to our stock, it is classified as
125
Table of Contents
a liability under ASC 480, as defined in Note 2, Summary of Significant Accounting Policies. The fair value of contingent earn-out consideration is estimated as of the acquisition date at the present value of the expected contingent payments using a Monte Carlo Simulation Method (“MCSM”). The MCSM utilizes a combination of observable (Level 2) and unobservable (Level 3) inputs, which include the trading price and volatility of the underlying common stock, expected term, risk-free interest rates, and expected date of a qualifying event. The determination of the fair value of these financial instruments is complex and highly judgmental due to the significant estimation required. In particular, the fair value estimate is sensitive to certain assumptions, such as the volatility of underlying shares.
Changes in the estimated fair value of the contingent earn-out consideration are recorded in Other income (expense) in the condensed consolidated statements of operations and are reflected in the period in which they are identified. Changes in the estimated fair value of the continent earn-out consideration may materially impact or cause volatility in our operating results.
Valuation of Revenue Interest Financing and PIPE Conversion Option
In connection with the Business Combination, we entered into the Revenue Interest Financing Agreement with RTW, under which the Company received $40.0 million upfront. In exchange, we are obligated to remit to RTW certain revenue interest payments on all current and future products, digital solutions and services developed, imported, manufactured, marketed, offered for sale, promoted, sold, tested or otherwise distributed by Allurion and its subsidiaries until December 31, 2030. We account for the Revenue Interest Financing Agreement under the fair value option election of ASC 825. The Revenue Interest Financing Agreement accounted for under the fair value option (“FVO”) election is a debt host financial instrument containing embedded features wherein the entire financial instrument is initially measured at its issue-date estimated fair value and then subsequently remeasured at estimated fair value on a recurring basis at each reporting period date. The fair value of the Revenue Interest Financing is calculated using a discounted cash flow method under the income approach utilizing future revenue projections and a discount rate. Changes in the estimated fair value of the Revenue Interest Financing Agreement are recorded as a component of Other income (expense) in the condensed consolidated statements of operations. A portion of the estimated change in fair value must be reported in other comprehensive loss to the extent that it is attributable to instrument-specific credit risk. As a result of electing the FVO, direct costs and fees related to the Revenue Interest Financing are expensed as incurred.
In connection with the Company entering in the Revenue Interest Financing, we and RTW entered into the RTW Side Letter under which RTW may elect to convert up to $7.5 million of its initial PIPE subscription into an additional revenue interest financing by forfeiting a number of shares of our common stock acquired by the RTW in its PIPE Investment (the “PIPE Conversion Option”). We account for the PIPE Conversion Option as a freestanding financial instrument that qualifies for derivative liability accounting in accordance with ASC 815, Derivatives and Hedging. The fair value of the PIPE Conversion Option is measured using a MCSM using a combination of observable (Level 2) and unobservable (Level 3) inputs, which include the number of shares convertible, the stock price of the underlying common stock, volatility, risk-free rates, and expected term. The PIPE Conversion Option is initially measured at its fair value within Other liabilities on the condensed consolidated balance sheets with corresponding recognition of expense at inception as there is no right received by the Company that meets the definition of an asset and the transaction did not involve a distribution or a dividend. Subsequent changes in fair value of the derivative liability are recognized as a gain or loss as a component of Other income (expense) in the condensed consolidated statements of operations.
Determination of Fair Value of Legacy Allurion Preferred Stock, Common Stock and Warrants
The estimated fair value of our Legacy Allurion shares has been determined by the Legacy Allurion board of directors, with input from management, considering the most recently available third-party valuations then
126
Table of Contents
available and the Legacy Allurion board of directors’ assessment of additional objective and subjective factors that it believed were relevant. These factors included, but were not limited to:
| • | the prices at which we sold shares of Legacy Allurion preferred stock and the superior rights and preferences of the Legacy Allurion preferred stock relative to the Legacy Allurion common stock at the time of each grant; |
| • | our stage of development and business strategy; |
| • | external market conditions and trends affecting our industry; |
| • | our financial position, including cash on hand, and our historical and forecasted performance and operating results; |
| • | the lack of an active public market for our common stock and our preferred stock; |
| • | the likelihood of achieving a liquidity event, such as an initial public offering, deSPAC transaction, or sale of our company in light of prevailing market conditions; and |
| • | the analysis of initial public offerings or other financing transactions and market performance of comparable companies in the industry. |
The fair value of the underlying Legacy Allurion Preferred Stock, common stock, and warrants was determined by the Legacy Allurion board of directors until we were listed on the NYSE on August 2, 2023. The fair value of the Legacy Allurion shares is utilized in the determination of stock-based compensation expense, common stock warrant liability expense, preferred stock recorded at fair value and the convertible notes conversion price. The assumptions underlying these valuations represented management’s best estimates, which involved inherent uncertainties and the application of management’s judgment. As a result, if we had used significantly different assumptions or estimates, the fair value of the Legacy Allurion preferred stock issued in 2021 and Legacy Allurion Convertible Notes converted in 2021 could be materially different. Significantly different assumptions or estimates could also impact the fair value of the Legacy Allurion stock options and stock-based compensation and fair value of the Legacy Allurion Warrants, but these have not been material to date.
Recent Accounting Pronouncements
See Note 2, Summary of Significant of Accounting Policies in the accompanying notes to the consolidated financial statements included in this prospectus for a description of recently issued accounting pronouncements that may potentially impact our financial position, results of operations or cash flows.
Emerging Growth Company and Smaller Reporting Company
We are an emerging growth company, as defined in the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that (i) we are no longer an emerging growth company or (ii) we affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, these financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates. We may choose to early adopt any new or revised accounting standards whenever such early adoption is permitted for private companies.
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K, which allows us to take advantage of certain exemptions from disclosure requirements including exemption from compliance with the auditor attestation requirements of Section 404. We will remain a smaller reporting
127
Table of Contents
company until the last day of the fiscal year in which (i) the market value of the shares of our common stock held by non-affiliates exceeds $250 million as of the prior June 30, and (ii) our annual revenue exceeded $100 million during such completed fiscal year or the market value of the shares of our common stock held by non-affiliates exceeds $700 million as of the prior June 30. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
We had cash and cash equivalents totaling $29.7 million at March 31, 2024. Cash equivalents were invested primarily in money market funds. Our investment policy is focused on the preservation of capital and supporting our liquidity needs. Under our investment policy, we invest in highly rated securities, issued by the U.S. government or liquid money market funds. We do not invest in financial instruments for trading or speculative purposes, nor do we use leveraged financial instruments. We utilize external investment managers who adhere to the guidelines of our investment policy. A hypothetical 10% change in interest rates would not have a material impact on the value of our cash, cash equivalents, net loss or cash flows.
We have exposure to interest rate risk from our variable rate debt. We do not hedge our exposure to changes in interest rates. As of March 31, 2024, we had $43.1 million in variable rate debt outstanding with Fortress. Interest on our debt is payable monthly during the term of the loan and is calculated as 6.44% plus the greater of (i) the Wall Street Journal Prime Rate and (ii) 3.0% (14.94% as of March 31, 2024 in the aggregate). Changes in the Wall Street Journal Prime Rate may therefore affect our interest expense associated with the loan. A 10% change in interest rates would increase expense by approximately $0.4 million annually based on the amounts outstanding at March 31, 2024 and would not materially affect our results of operations.
Foreign Currency Exchange Risk
We are exposed to foreign currency risks that arise from normal business operations. These risks include transaction gains and losses associated with transactions denominated in currencies other than a location’s functional currency and the remeasurement of foreign currencies to our U.S. dollar reporting currency. As such, we have exposure to adverse changes in exchange rates associated with operating expenses of our foreign operations. Transaction gains or losses are included in other income (expense), net in the consolidated statements of operations, as incurred.
We believe that a 10% increase or decrease in current exchange rates between the U.S. dollar and our foreign currencies could have a material impact on our business, financial condition or results of operations. Our primary exposures related to foreign currency denominated sales and expenses are in Europe and we also have exposure in the Middle East and the Asia-Pacific region, and are monitoring potential developing exposure in the Latin American, Canadian and African markets.
To date, we have not engaged in any foreign currency hedging activities. As our international operations grow, we will continue to reassess our approach to managing the risks relating to fluctuations in foreign currency exchange rates. During the three months ended March 31, 2024, the effect of an immediate 10% adverse change in foreign exchange rates on foreign-denominated accounts would have had an impact of approximately 7% on revenues and 7% on expenses and would have impacted our net income by approximately 6%. During the three months ended March 31, 2023, the effect of an immediate 10% adverse change in foreign exchange rates on foreign-denominated accounts would have had an impact of approximately 6% on revenues and 2% on expenses and would have impacted our net loss by approximately 1%.
128
Table of Contents
Internal Control Over Financial Reporting
In connection with the audits of our consolidated financial statements as of and for the years ended December 31, 2022 and 2021, we identified material weaknesses in our internal control over financial reporting that we are currently working to remediate, which relate to: (a) insufficient segregation of duties in the financial statement close process; (b) a lack of sufficient levels of staff with public company and technical accounting experience to maintain proper control activities and perform risk assessment and monitoring activities; and (c) insufficient information systems controls, including access and change management controls. We have concluded that these material weaknesses in our internal control over financial reporting occurred because we did not have the necessary business processes, personnel and related internal controls to operate in a manner to satisfy the accounting and financial reporting timeline requirements of a public company.
We are focused on designing and implementing effective internal controls measures to improve our evaluation of disclosure controls and procedures, including internal control over financial reporting, and remediating the material weaknesses. In order to remediate these material weaknesses, we have taken and plan to take the following actions:
| • | the hiring and planned continued hiring of additional accounting staff with public company experience, |
| • | implemented a new enterprise resource planning system to replace the prior enterprise resource planning system, |
| • | implementation of additional review controls and processes requiring timely account reconciliation and analyses of certain transactions and accounts, and |
| • | hired a national accounting firm to assist in the design and implementation of controls and remediation of controls gaps. |
While significant progress has been made to enhance our internal control over financial reporting, we are still in the process of building and enhancing our processes, procedures, and controls. Additional time is required to complete the remediation of these material weaknesses and the assessment to ensure the sustainability of these remediation actions. We believe the above actions, when complete, will be effective in the remediation of the material weakness described above.
This prospectus does not include a report of management’s assessment regarding our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) or an attestation report of our independent registered accounting firm due to a transition period established by rules of the SEC for newly public companies. Additionally, our independent registered accounting firm will not be required to opine on the effectiveness of our internal control over financial reporting pursuant to Section 404 until we are no longer an “emerging growth company” as defined in the JOBS Act.
129
Table of Contents
MANAGEMENT
Directors and Executive Officers
The following table sets forth information regarding our directors and executive officers, including their ages, as of May 1, 2024.
Name | Age | Position | ||
Directors | ||||
Dr. Shantanu Gaur | 37 | Chief Executive Officer and Director | ||
Krishna Gupta | 36 | Co-Chairman and Director | ||
Dr. Omar Ishrak | 68 | Co-Chairman and Lead-Independent Director | ||
Michael Davin | 66 | Director | ||
Douglas Hudson | 55 | Director | ||
Nicholas Lewin | 47 | Director | ||
Milena Alberti-Perez | 51 | Director | ||
Executive Officers | ||||
Christopher Geberth | 53 | Chief Financial Officer | ||
Dr. Ram Chuttani | 64 | Chief Medical Officer | ||
Brendan Gibbons | 48 | Chief Legal Officer |
Directors
Dr. Shantanu Gaur has served as our Chief Executive Officer and as a member of our Board since September 2009. Dr. Gaur founded Allurion in 2009 while at Harvard Medical School. Dr. Gaur graduated summa cum laude with a B.S. in Biology from Harvard College and with an M.D. from Harvard Medical School, where he was a Paul Revere Frothingham Scholar and Paul & Daisy Soros Fellow. Dr. Gaur is qualified to serve on our Board because of his experience founding Allurion and serving as our Chief Executive Officer.
Krishna Gupta has served as a member of our Board since January 2017. He is the founder of REMUS Capital and Romulus Capital (collectively referred to herein as “REMUS Capital”), technology-focused venture capital firms he initially founded in 2008. Mr. Gupta serves as a director on the boards of several privately held companies, including: Ceres Imaging, Inc., which provides computer vision-analytics in agriculture; Cogito Corporation, which provides voice artificial intelligence for large enterprises; Cohealo, Inc., which provides equipment sharing services for health systems; Spotta Ltd., which develops smart insect and pest monitoring solutions; and ZeroCater, Inc., which provides catering technology for enterprises. He was the first investor in Ginger, which was a leader in mental health software and merged with Headspace, Inc. Mr. Gupta has also served as a member of the board of directors of Presto Automation, Inc. (Nasdaq: PRST) since 2017, where he served as chairman of the board from September 2022 to March 2024. Prior to REMUS Capital, Mr. Gupta held roles at McKinsey & Company, a management consulting firm, and JPMorgan Chase & Co., an investment banking firm, where he advised several Fortune 100 clients on technology M&A deals. Mr. Gupta holds B.S. degrees in materials science and engineering, as well as in management sciences, both from the Massachusetts Institute of Technology. Mr. Gupta is qualified to serve on our Board due to his experience investing in technology and healthcare businesses.
Dr. Omar Ishrak has served as a member of our Board since August 2023, and previously served as chairman of the board of directors of Compute Health Acquisition Corp. (formerly NYSE: CPUH) (“Compute Health”) from October 2020 to August 2023. Dr. Ishrak also serves on the board of directors at Intel Corporation (Nasdaq: INTC), where he served as independent chairman of the board of directors from January 2020 to January 2023. Dr. Ishrak has also served on the board of directors of Amgen, Inc. (Nasdaq: AMGN) since July 2021, of Cargill, Inc., a private company, where he has served since January 2021, and of Insightec, Ltd., a private company, where he has served since May 2022. Dr. Ishrak was the chief executive officer of Medtronic
130
Table of Contents
plc (Nasdaq: MDT) from June 2011 to April 2020 and served as executive chairman and chairman of the board of directors until he stepped down in December 2020. Prior to joining Medtronic, Dr. Ishrak was the president and chief executive officer of GE Healthcare Systems. Earlier in his career, Dr. Ishrak amassed 13 years of technology development and business management experience, holding leadership positions at Diasonics Vingmed Ultrasound Ltd., and various product development and engineering positions at Philips Ultrasound Inc. He was inducted into the American Institute for Medical and Biological Engineering College of Fellows in 2016 and was elected to the National Academy of Engineering in 2020. Dr. Ishrak serves on the board of directors of the Cleveland Clinic, a nonprofit academic medical center. He is also a member of the Board of Trustees of the Asia Society, a leading educational organization dedicated to promoting mutual understanding and strengthening partnerships among peoples, leaders, and institutions of Asia and the United States in a global context. In addition, he is member of the Minnesota Public Radio Board of Trustees. He earned a B.S. degree and Ph.D. in electrical engineering from the University of London, King’s College. He is also a fellow of King’s College. Dr. Ishrak is qualified to serve on our Board based on his extensive experience on boards of various healthcare and technology companies.
Michael Davin has served as a member of our Board since October 2017. Mr. Davin has also served as a director of several privately-held companies, including: Follica, Inc. since December 2020, Amsel Medical Corporation since August 2020, Cynosure, LLC since January 2020, and Inkbit Corporation since June 2018. Previously, Mr. Davin served as chairman, president, and chief executive officer of Cynosure Inc. (formerly Nasdaq: CYNO), from 2003 until March 2017, when it was acquired by Hologic Inc. Mr. Davin received his B.S. and B.A. from Southern New Hampshire University. Mr. Davin is qualified to serve on our Board due to his experience as a director of health care and technology companies, as well as his familiarity with Allurion as a director.
Douglas Hudson has served as a member of our Board since August 2023. Mr. Hudson has also served as the founder, chief executive officer and a director of Noho Dental, Inc. since July 2018, and as a director of Modern Age since October 2020. Previously, Mr. Hudson served on the board of directors of Relode.com, LLC from 2017 until August 2022. Mr. Hudson also served as the founder and chief executive officer of SmileDirectClub Inc. (formerly Nasdaq: SDC) from 2013 to 2017, and as chairman and chief executive officer of Simplex Healthcare from 2007 until 2013, when it was acquired by Arriva Medical LLC. Earlier in his career, Mr. Hudson served as founder, chief executive officer and director of HearingPlanet, Inc. from 1999 until 2007. Mr. Hudson received his B.S. in organizational behavior from Eckerd College and his M.B.A. from Vanderbilt University, and he completed the executive education programs at Harvard Business School. Mr. Hudson is qualified to serve on our Board due to his experience as an entrepreneur and an executive of health care companies.
Nicholas Lewin has served as a member of our Board since August 2023. Mr. Lewin has also served as a director of Establishment Labs Holding, Inc. (Nasdaq: ESTA) since September 2015, and as its chairman since December 2017. He has been a managing member and head of investments at Crown Predator Holdings LLC since 2008 and has been a private investor since 2000. Mr. Lewin has also served as a director of Cutera Inc. (Nasdaq: CUTR) since May 2023, as a director of FaZe Holding Inc. (Nasdaq: FAZE) since July 2022, and as a director of Halo Maritime Defense Systems, a private company, since 2007. Mr. Lewin holds a bachelor’s degree in political science from Johns Hopkins University. Mr. Lewin is qualified to serve on our Board due to his experience as an investor in, and director of, innovative companies, including health care and medical device companies.
Milena Alberti-Perez has served on our Board since March 2024. Ms. Alberti-Perez has also served on the board of directors of Digimarc Corporation (Nasdaq: DMRC) since February 2022, where she serves as the chair of the audit committee and as a member of the compensation and talent management committee. She was most recently the chief financial officer of Getty Images, Inc., the world’s leading visual content company, from December 2020 to January 2022. Prior to her work with Getty, in 2020, she served as the chief financial officer of MediaMath, Inc., a demand-side platform for programmatic marketing and advertising. Ms. Alberti-Perez
131
Table of Contents
worked in a variety of financial and publishing roles from 2001 to 2017 at Penguin Random House LLC, the world’s largest book publisher, serving as the Global and US chief financial officer of Penguin Random House LLC from 2015 to 2017 and, as management, served as a non-voting member of its Board and its audit committee. Ms. Alberti-Perez also worked in financial analyst and research roles at Lehman Brothers Holdings Inc. and Morgan Stanley. She also currently serves on several private company boards of directors, including RBmedia and Overdrive, Inc., and has previously served on the private company boards of Companhia das Letras and Flatworld/Sagence Group, Inc. She has served on not-for-profit boards of directors, including The University of Pennsylvania Executive Fund, Jumpstart, and the Wild Bird Fund. Ms. Alberti-Perez holds a B.A. from The University of Pennsylvania, with Distinction in Economics and minors in Math and Latin American Studies. She received her Master’s degree in Business Administration, with Distinction, from the Harvard Business School. Ms. Alberti-Perez is qualified to serve on our Board due to her experience as a chief financial officer and as a director at various public companies.
Executive Officers
Christoper Geberth has served as our Chief Financial Officer since September 2020. Prior to joining us, Mr. Geberth was chief financial officer at Lutronic Inc., a medical equipment manufacturer, from December 2017 to September 2020. From June 2008 until December 2017, Mr. Geberth was the executive vice president of finance at Cynosure, Inc., another medical equipment manufacturer. Mr. Geberth holds a B.B.A. in Business from Pace University.
Dr. Ram Chuttani has served as our Chief Medical Officer since November 2017 and is a founder of Allurion. Prior to joining us, Dr. Chuttani was the Director of Endoscopy and Chief, Interventional Gastroenterology at Beth Israel Deaconess Medical Center and was on the faculty of Harvard Medical School for 20 years. Dr. Chuttani holds a M.B. and a B.S. in medicine from Maulana Azad Medical College. Dr. Chuttani is internationally recognized as a leader in digestive disease care and has pioneered several innovative endoscopic treatments. He co-invented an endoscopic treatment for gastroesophageal reflux disease and co-developed a novel treatment for obesity. He has published over 100 original scientific articles, in addition to several reviews and book chapters. He has lectured on, and demonstrated, novel and advanced endoscopic procedure at innumerable international conferences and workshops all over the world. Dr. Chuttani completed a residency at Norwalk Hospital, Yale University School of Medicine in Internal Medicine in 1987, and a fellowship in gastroenterology at Harvard Medical School in 1990.
Brendan Gibbons has served as our Chief Legal Officer and Secretary since January 2024. Prior to joining Allurion, Mr. Gibbons served as a chief legal officer at several public companies. From December 2017 to December 2021, he served as executive vice president, chief legal officer, and corporate secretary for Acushnet Company (NYSE: GOLF), the world’s largest golf ball, club, footwear and gear company. From 2014 to 2017, Mr. Gibbons was senior vice president, general counsel and corporate secretary at Wolverine Worldwide, Inc. (NYSE: WWW), a leading international footwear and apparel company. From 2004 to 2013, Mr. Gibbons was senior vice president of legal and corporate affairs, general counsel and secretary at Carter’s, Inc. (NYSE: CRI), the world’s largest baby and young children’s apparel and accessory company. Mr. Gibbons began his career at Ropes & Gray LLP. He received his B.A., magna cum laude, from The University of Pennsylvania and his J.D., magna cum laude, from Boston College Law School.
Family Relationships
There are no family relationships among our directors and executive officers. There is no arrangement or understanding between any of our directors or executive officers and any other person or persons pursuant to which he or she was or is to be selected as a director or an executive officer. There are no material legal proceedings to which any of our directors or executive officers is a party adverse to us or any of our subsidiaries or in which any such person has a material interest adverse to us or any of our subsidiaries.
132
Table of Contents
Corporate Governance
General
We believe that good corporate governance is important to ensure that our company is managed for the long-term benefit of our stockholders. We periodically review our corporate governance policies and practices and compare them to those suggested by various authorities in corporate governance and the practices of other public companies. As a result, we have adopted policies and procedures that we believe are in the best interests of our company and our stockholders. Key information regarding our corporate governance initiatives can be found on our corporate website at https://investors.allurion.com under “Governance Documents,” including our Corporate Governance Guidelines, our Code of Business Conduct and Ethics (our “Code of Conduct”), and the charters for the committees of our Board, described below. Information contained on, or that can be accessed through, our website is not incorporated by reference into and does not form a part of this prospectus.
Corporate Governance Guidelines
Our corporate governance guidelines assist our Board in the exercise of its duties and responsibilities and to serve the best interests of our company and our stockholders. These guidelines, which provide a framework for the conduct of Board business, provide that:
| • | at least a majority of the members of the Board shall be independent directors as defined by NYSE rules; |
| • | the independent directors shall meet at least quarterly, and at other times at the request of any independent director, in executive session; |
| • | directors shall have full and free access to management and, as necessary and appropriate, independent advisors; and |
| • | at least annually, the Nominating and Corporate Governance Committee (the “Nominating and Corporate Governance Committee”) oversees a self-evaluation by the Board to assess the effectiveness of the Board and its committees. |
Our Board is responsible for managing or supervising the management of our business and affairs. This includes appointing our Chief Executive Officer, advising management on strategic issues, approving our business and other plans, and monitoring our performance against those plans and against our operating and capital budgets. In addition, our Board also receives and considers recommendations from its various committees with respect to matters such as:
| • | the compensation of our executive officers and directors; |
| • | criteria for Board and committee membership; |
| • | persons to be nominated for election as members of the Board and to each of the committees of the Board; and |
| • | matters relating to our corporate governance, including to our Code of Conduct and Corporate Governance Guidelines. |
Board Composition
Our Board is comprised of seven directors, divided into three classes with staggered three-year terms. The three classes are as follows:
| • | Class I directors: Krishna Gupta, Dr. Shantanu Gaur, and Nicholas Lewin, whose terms will expire at the annual meeting of stockholders to be held in 2024; |
| • | Class II directors: Omar Ishrak and Larson Douglas Hudson, whose terms will expire at the annual meeting of stockholders to be held in 2025; and |
133
Table of Contents
| • | Class III directors: Michael Davin and Milena Alberti-Perez, whose terms will expire at the annual meeting of stockholders to be held in 2026. |
Directors in a particular class will be elected for three-year terms at the annual meeting of stockholders in the year in which their terms expire. As a result, only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Each director’s term continues until the election and qualification of his or her successor, or the earlier of his or her death, resignation or removal. Pursuant to the Investor Rights Agreement, the following persons have the following nomination rights with respect to our Board, subject to the limitations set forth in the Investor Rights Agreement:
(i) one director and one independent director nominated by Dr. Shantanu Gaur, which are currently Dr. Gaur and Michael Davin;
(ii) one director and one independent director nominated by Remus Group Management, LLC, which are currently Krishna Gupta and Larson Douglas Hudson;
(iii) one director nominated by Compute Health Sponsor LLC, which is currently Omar Ishrak; and
(iv) two independent directors nominated by Allurion, one of which shall be designated by RTW, which are currently Milena Alberti-Perez and Nicholas Lewin, respectively.
Pursuant to the Amended Note Purchase Agreement, we agreed to include an additional nominee in our proxy statement for the election of Class I directors at the 2024 annual meeting of stockholders, which additional nominee will go through our director nomination process, described below. RTW has the right to approve such nominee, such approval not to be unreasonably withheld.
Our organizational documents provide that only our Board can fill vacant directorships, including newly created seats. Any additional directorships resulting from an increase in the authorized number of directors would be distributed pro rata among the three classes so that, as nearly as possible, each class would consist of one-third of the authorized number of directors.
Board Diversity
Our Corporate Governance Guidelines provide that diversity of background and experience should be considered in determining director candidates as well as other factors such as a candidate’s character, judgment, skills, education, expertise, and absence of conflicts of interest. Based on our commitment to diversity, our corporate governance guidelines require that the Nominating and Corporate Governance Committee include in its initial list of director candidates for consideration in filling any vacancy on our Board at least one or more qualified candidates who reflect diverse backgrounds, including diversity of gender and race or ethnicity. However, we do not have a formal policy concerning the diversity of the Board.
Our priority in the selection of directors is identification of members who will further the interests of our stockholders through their established records of professional accomplishment, their ability to contribute positively to the collaborative culture among board members, their knowledge of our business and understanding of the competitive landscape in which we operate, and their adherence to high ethical standards. Although we do not have a formal diversity policy and do not follow any ratio or formula with respect to diversity in order to determine the appropriate composition of the Board, the Nominating and Corporate Governance Committee and the Board are committed to creating a board of directors that promotes our strategic objectives and fulfills its responsibilities to our stockholders, and they consider diversity of gender, race, national origin, education, professional experience, and differences in viewpoints and skills when evaluating proposed director candidates.
134
Table of Contents
Board Committees
Our Board has established an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. The Board may establish other committees to facilitate the management of our business. The Board and its committees set schedules for meeting throughout the year and can also hold special meetings and act by written consent from time to time, as appropriate. The Board has delegated various responsibilities and authority to its committees as generally described below. The committees regularly report on their activities and actions to the full Board. Members will serve on these committees until their resignation or until otherwise determined by the Board.
Audit Committee
The members of our Audit Committee include Milena Alberti-Perez (Chair), Omar Ishrak, and Michael Davin each of whom can read and understand fundamental financial statements. The Board has concluded each member of the Audit Committee is independent under the rules and regulations of the SEC and the listing standards of the NYSE applicable to Audit Committee members. Each of Milena Alberti-Perez and Michael Davin qualify as an audit committee financial expert within the meaning of SEC regulations and meet the financial literacy requirements of the NYSE.
Our Audit Committee assists the Board with its oversight of the following:
| • | the integrity of our financial statements; |
| • | our compliance with legal and regulatory requirements; |
| • | the qualifications, independence, and performance of our independent registered public accounting firm; and |
| • | the design and implementation of our internal audit function, and our risk assessment and risk management activities. |
The Audit Committee also is responsible for reviewing and discussing with management the adequacy and effectiveness of our disclosure controls and procedures, and it discusses with management and our independent registered public accounting firm the annual audit plan, the scope and timing of audit activities and the results of the audit. The Audit Committee also reviews of our financial statements quarterly. As appropriate, the Audit Committee may initiate inquiries into certain aspects of our financial affairs.
Further, the Audit Committee is responsible for establishing and overseeing procedures for the receipt, retention, and treatment of any complaints regarding accounting, internal accounting controls, or auditing matters, as well as for the confidential and anonymous submissions by our employees of concerns regarding questionable accounting or auditing matters. In addition, the Audit Committee has direct responsibility for the appointment, compensation, retention, and oversight of the work of our independent registered public accounting firm. This includes sole authority to approve the hiring and discharging of our independent registered public accounting firm, all audit engagement terms and fees, and all permissible non-audit engagements with the independent auditor. Finally, the Audit Committee reviews and oversees all related-person transactions in accordance with our policies and procedures.
Our Audit Committee operates under a written charter that satisfies the applicable rules and regulations of the SEC and the listing standards of the NYSE. A copy of the charter of our Audit Committee is available on our website at https://investors.allurion.com.
Compensation Committee
The members of our Compensation Committee include Michael Davin (Chair), Nicholas Lewin, and Larson Douglas Hudson. The Board has concluded each member of the Compensation Committee is considered
135
Table of Contents
independent under the rules and regulations of the SEC and the listing standards of the NYSE applicable to Compensation Committee members. The primary objective of the Compensation Committee is to develop and implement compensation policies and plans that ensure the attraction and retention of key management personnel, the motivation of management to achieve our goals and strategies, and the alignment of management’s interests with the long-term interests of our stockholders.
Our Compensation Committee’s responsibilities include, among other things:
| • | reviewing and evaluating our Chief Executive Officer’s performance against established goals and objectives, and determining and approving the Chief Executive Officer’s compensation (including long-term incentives) based on such evaluation; |
| • | reviewing, approving, and determining the compensation of our executive officers other than the CEO and, at the discretion of the committee, other members of senior management; |
| • | reviewing and recommending to the Board the compensation of our non-employee directors; |
| • | reviewing and making recommendations to the Board with regard to incentive-based compensation plans and the policies and procedures for awards thereunder, including making grants pursuant to such plans; |
| • | overseeing the administration of all incentive compensation and equity-based plans for employees; and |
| • | administering our Compensation Recovery Policy, discussed in the section entitled “—Compensation Recovery Policy” below. |
Our Compensation Committee operates under a written charter that satisfies the applicable rules and regulations of the SEC and the listing standards of the NYSE. A copy of the charter of our Compensation Committee is available on our website at https://investors.allurion.com.
Nominating and Corporate Governance Committee
The members of our Nominating and Corporate Governance Committee include Larson Douglas Hudson (Chair), Omar Ishrak, and Krishna Gupta. The Board has concluded each member of the Nominating and Corporate Governance Committee is considered independent under the rules and regulations of the SEC and the listing standards of the NYSE.
The Nominating and Corporate Governance Committee assists the Board with its identification and evaluation of individuals qualified to become members of the Board (including those recommended by stockholders), consistent with criteria approved by the Board, and recommends that the Board select the director nominees for election at annual stockholder meetings. The Nominating and Corporate Governance Committee also develops and recommends to the Board a set of corporate governance guidelines, monitors compliance with our Code of Conduct, and oversees the evaluation of the Board, its committees and management.
Our Nominating and Corporate Governance Committee’s responsibilities include, among other things:
| • | establishing and recommending to the Board criteria for Board and committee membership, and establishing policies and processes to evaluate nominees – including those of stockholders – against such criteria; |
| • | identifying, evaluating, and making recommendations to our Board regarding nominees for election to the Board and its committees, including to fill vacancies; |
| • | evaluating the performance of our Board, our committees, and of individual directors; |
| • | considering and making recommendations to our Board regarding the composition of our Board and its committees; |
136
Table of Contents
| • | overseeing an annual evaluation of our Board, its committees, and management; |
| • | developing and recommending to the Board corporate governance guidelines and periodically reviewing those guidelines and recommending any changes; |
| • | monitoring compliance with our Code of Conduct; and |
| • | reviewing and assessing the adequacy of the corporate governance guidelines and the Code of Conduct and recommending any changes to the Board for approval. |
Our Nominating and Corporate Governance Committee operates under a written charter that satisfies the applicable listing standards of the NYSE. A copy of the charter of our Nominating and Corporate Governance Committee is available on our website at https://investors.allurion.com.
Director Nomination Process
As noted above, our Nominating and Corporate Governance Committee is responsible for identifying individuals qualified to serve as directors, consistent with criteria approved by our Board, and recommending to the Board qualified individuals to be nominated for election as directors at each annual meeting of stockholders.
In identifying prospective director candidates, the Nominating and Corporate Governance Committee may consider all facts and circumstances that it deems appropriate or advisable, including, among other things, the skills of the prospective candidate, his or her depth and breadth of business experience or other background characteristics, his or her independence, and the needs of the Board. At a minimum, the Nominating and Corporate Governance Committee must be satisfied that each recommended nominee meets the following minimum qualifications:
(i) relevant experience and expertise to enable him or her to be able to offer germane advice and guidance to management;
(ii) proven achievement and competence in his or her field;
(iii) the ability to exercise sound business judgment;
(iv) an understanding of the fiduciary responsibilities required of a director;
(v) commitment to devoting time and energy to our affairs;
(vi) a diverse personal background, perspective, and experience; and
(vii) commitment to vigorously represent the long-term interests of our stockholders.
In addition to any other standards the Nominating and Corporate Governance Committee deems appropriate from time to time for the overall structure and composition of the Board, the Nominating and Corporate Governance Committee may consider whether the candidate, if elected, assists in achieving a mix of board members that represents a diversity of background and experience.
Board and Committee Meetings Attendance
During 2023, following the closing of the Business Combination with Compute Health on August 1, 2023, the Board of Directors met five times, the Audit Committee met three times, the Compensation Committee met five times and acted by written consent once, and the Nominating and Corporate Governance Committee met one time.
137
Table of Contents
During 2023, following the closing of the Business Combination, each member of the Board attended in person or participated in 75% or more of the aggregate of (i) the total number of meetings of the Board (held during the period for which such person has been a director) and (ii) the total number of meetings held by all committees of the Board on which such person served (during the periods that such person served).
Director Attendance at Annual Meeting of Stockholders
Directors are encouraged to attend the annual meeting of stockholders to the extent practicable.
Policy on Insider Trading, Pledging, and Hedging
Our Insider Trading Policy prohibits our directors (including non-employee directors), officers, employees, consultants, and their “affiliated persons” (as defined in our Insider Trading Policy) from engaging in the following transactions:
| • | trading in our securities, whether for their own account or for the account of another, while in the possession of material, nonpublic information about us; |
| • | disclosing material, nonpublic information about us to others who may trade on the basis of that information (“tipping”). |
| • | selling any of our securities that they do not own at the time of the sale (referred to as a “short sale”); |
| • | buying or selling puts, calls, other derivative securities of our securities, or any derivative securities that provide the economic equivalent of ownership of any of our securities or an opportunity, direct or indirect, to profit from any change in the value of our securities or engaging in any other hedging transaction with respect to our securities; |
| • | using our securities as collateral in a margin account; and |
| • | pledging our securities as collateral for a loan (or modifying an existing pledge). |
Compensation Recovery Policy
In light of the SEC’s adoption of final clawback rules in October 2022 and the NYSE’s adoption of final listing standards consistent with the SEC rules in June 2023, we adopted a Compensation Recovery Policy effective as of October 2, 2023. If we are required to prepare an accounting restatement due to material non-compliance with any financial reporting requirements under applicable securities laws, the Compensation Recovery Policy requires (subject to certain limited exceptions described in the policy and permitted by the final clawback rules) that we recover erroneously awarded compensation received by any current or former executive officer in the three fiscal years prior to the date we were required to restate our financial statements that is in excess of the amount that would have been received based on the restated financial statements.
Compensation Committee Interlocks and Insider Participation
During 2023, the members of our Compensation Committee included Michael Davin, Nicholas Lewin, and Larson Douglas Hudson. None of the members of our Compensation Committee has ever been an officer or employee of our company, or had any other relationship requiring disclosure herein. None of our executive officers serve, or have served during the last fiscal year, as a member of the Board or Compensation Committee of any other entity that has or has had one or more executive officers serving as a member of our Board or Compensation Committee.
Code of Conduct
We have adopted our Code of Conduct, which applies to all of our directors, officers, employees, consultants, and certain designated agents in connection with their work for us. The full text of our Code of
138
Table of Contents
Conduct is posted on our website at https://investors.allurion.com. We intend to disclose future amendments to, or waivers of, our Code of Conduct, as and to the extent required by SEC regulations, at the same location on our website identified herein or in public filings. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider information contained on our website to be part of this prospectus.
Board Leadership Structure and Board’s Role in Risk Oversight
Krishna Gupta and Omar Ishrak are co-chairmen of the Board, and Mr. Ishrak is our lead independent director. Our co-chairmen preside at all meetings of our Board. Currently, the role of the co-chairmen of the Board is separated from the role of Chief Executive Officer. Separating these positions allows our Chief Executive Officer to focus on our day-to-day business, while allowing the co-chairmen to lead the Board in its fundamental role of providing advice to and independent oversight of management.
The Board has not adopted a position description for the co-chairmen. However, there is a shared understanding on the Board of the co-chairmen’s responsibilities. The co-chairmen’s primary role is to provide leadership to the Board and its committees, including chairing meetings in a manner that facilitates open discussions and expressions of competing views. The co-chairmen are also responsible for, among other things, assisting the Board in obtaining information required for the performance of its duties, retaining appropriately qualified and independent advisors as needed, working with the Board to support board development and to ensure a proper committee structure is in place, providing a link between the Board and management, and acting in an advisory capacity to the Chief Executive Officer in all matters concerning the interests and management of Allurion.
Our Board recognizes the time, effort, and energy that the Chief Executive Officer must devote to his position in the current business environment, as well as the commitment required to serve as the chairman of the Board. Our Board also believes this leadership structure ensures a greater role for the non-management directors in the oversight of our company and active participation of the independent directors in setting agendas and establishing priorities and procedures for the work of our Board.
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. As a medical device manufacturer, we face a number of risks, including risks related to patient safety, our financial condition, development and commercialization activities, operations, strategic direction, and intellectual property.
Management is responsible for the day-to-day management of risks we face, while our Board, as a whole and through its committees, has responsibility for the oversight of risk management. In its risk oversight role, our Board has the responsibility to satisfy itself that the risk management processes designed and implemented by management are adequate and functioning as designed.
The role of the Board in overseeing the management of our risks is conducted primarily through committees of the Board, as disclosed in the descriptions of each of the committees above and in the charters of each of the committees. The full Board (or the appropriate committee in the case of risks that are under the purview of a particular committee) discusses with management our major risk exposures, their potential impact on us, and the steps we take to manage them. When a committee is responsible for evaluating and overseeing the management of a particular risk or risks, the chairperson of the relevant committee reports on the discussion to the full Board during the committee reports portion of the next Board meeting. This enables the Board and its committees to coordinate the risk oversight role, particularly with respect to risk interrelationships.
Enterprise Risk Management Policy
In support of our risk management strategy, we have adopted an Enterprise Risk Management Policy (the “ERM Policy”). The ERM Policy governs our risk management practices and establishes responsibilities and
139
Table of Contents
processes for the identification, classification, analysis, treatment/mitigation, prevention and governance of all risks to us – defined as anything that could reasonably be expected to have a material adverse impact on the achievement of our strategic objectives and operational goals. The goal of the ERM Policy is to ensure that risks and related exposures are aligned with our strategic objectives, as well as risk tolerances set by the Board. The ERM policy is implemented through a framework guided by internal key principles as well as those outlined by the Committee of Sponsoring Organizations of the Treadway Commission.
140
Table of Contents
EXECUTIVE COMPENSATION
We have opted to comply with the executive compensation disclosure rules applicable to “smaller reporting companies” and “emerging growth companies” as such terms are defined in the rules promulgated under the Securities Act. As a result of the Business Combination completed on August 1, 2023, share amounts presented for periods prior to the Business Combination have been retroactively converted by application of the exchange ratio of 0.9780.
This section discusses the compensation awarded to, earned by, or paid to Allurion’s chief executive officer and two other most highly compensated executive officers who were serving as executive officers as of December 31, 2023 (our “Named Executive Officers” or “NEOs”). Our Named Executive Officers for the fiscal year ended December 31, 2023 were:
| • | Shantanu Gaur, M.D., our Chief Executive Officer; |
| • | Christopher Geberth, our Chief Financial Officer; and |
| • | Benoit Chardon, our former Chief Commercial Officer. |
2023 Summary Compensation Table
The following table presents information regarding the compensation awarded to, earned by or paid to our NEOs for services rendered during the fiscal years ended December 31, 2023 and December 31, 2022.
Name and Principal | Year | Salary ($) | Bonus ($) (1) | Option Awards ($) (2) | Non-equity Incentive Plan Compensation ($) (3) | All Other Compensation ($) (4) | Total ($) | |||||||||||||||||||||
Dr. Shantanu Gaur | 2023 | 354,875 | (5) | 296,751 | — | — | 9,600 | 661,226 | ||||||||||||||||||||
Chief Executive Officer | 2022 | 295,000 | — | 2,605,904 | 79,761 | 9,100 | 2,989,765 | |||||||||||||||||||||
Christopher Geberth | 2023 | 280,650 | (5) | 255,342 | — | — | 6,600 | 542,592 | ||||||||||||||||||||
Chief Financial Officer | 2022 | 319,087 | — | 844,510 | 83,760 | 6,100 | 1,253,457 | |||||||||||||||||||||
Benoit Chardon (6) | 2023 | 176,676 | (7) | 280,037 | — | 37,026 | 367,778 | 861,517 | ||||||||||||||||||||
Former Chief Commercial Officer | 2022 | 358,153 | 9,389 | — | 291,450 | — | 658,992 | |||||||||||||||||||||
| (1) | Amounts reported in this column include the following amounts: (i) for Dr. Gaur, a $200,000 bonus in connection with our Business Combination, and a $96,751 retention bonus, (ii) for Mr. Geberth, a $150,000 bonus in connection with our Business Combination, and a $105,342, and (iii) for Mr. Chardon, a $280,037 retention bonus. Retention bonuses represent incentive awards made to executives for their continued employment through a target date. |
| (2) | Amounts shown reflect the grant date fair value of stock options granted during such fiscal year, calculated in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718, excluding any estimates of forfeitures related to service-based vesting conditions. For information regarding assumptions underlying the valuation of equity awards, see Note 14 to our consolidated financial statements included in this prospectus. The amounts reported in this column reflect the accounting cost for the option awards, and do not correspond to the actual economic value that may be recognized by holders upon the vesting or exercise of the applicable awards. |
| (3) | For Dr. Gaur and Mr. Geberth, amounts reflect performance-based cash bonuses. For Mr. Chardon, amount reflects commissions earned as a member of our commercial team. See “Narrative to the Summary Compensation Table – Non-equity Incentive Plan Compensation” below for a description of the material terms pursuant to which this compensation was awarded. |
| (4) | For the 2023 fiscal year, amounts reported in this column include the following amounts: (i) for Dr. Gaur, $3,000 in payments in lieu of medical coverage, and $6,600 of 401(k) plan employer contributions, (ii) for Mr. Geberth, $6,600 of 401(k) plan employer contributions, and (iii) for Mr. Chardon, a €156,740 termination fee and €183,260 paid pursuant to a settlement agreement. For the 2022 fiscal year, amounts |
141
Table of Contents
| reported in this column include the following: (i) for Dr. Gaur, $3,000 in payments in lieu of medical coverage, and $6,100 of 401(k) plan employer contributions, and (ii) for Mr. Geberth, $6,100 of 401(k) plan employer contributions. |
| (5) | To preserve our cash prior to the Business Combination, Dr. Gaur’s and Mr. Geberth’s annual base salary was reduced to $36,000 from April 15, 2023 to July 31, 2023, and Mr. Chardon’s consulting fees were adjusted from January 1, 2023 to July 31, 2023. |
| (6) | For Mr. Chardon, amounts reported in the “Salary”, “Bonus” and “All Other Compensation” columns have been converted to USD at an exchange rate of 1 Euro to 1.0817 USD, which represents the average annual exchange rate published by the Federal Reserve Bank for 2023. |
| (7) | Amount represents annual base consulting fees of €340,000. |
Narrative to Summary Compensation Table
Base Salaries
We use base salaries to recognize the experience, skills, knowledge, and responsibilities required of all our employees, including our Named Executive Officers. Base salaries are reviewed annually and adjusted from time to time to align salaries with market levels after taking into account individual responsibilities, performance, and experience. For the year ended December 31, 2023, the annual base salaries for each of Dr. Gaur and Mr. Geberth were initially $303,850 and $328,660, respectively, and were reduced to $36,000 from April 15, 2023 to July 31, 2023 in order to preserve our cash prior to the consummation of the Business Combination. Effective as of the closing of the Business Combination, the annual base salaries of Dr. Gaur and Mr. Geberth were increased to $620,000 and $425,000, respectively. For the year ended December 31, 2023, Mr. Chardon was paid base annual consulting fees equal to €340,000.
Non-equity Incentive Plan Compensation
Each of Dr. Gaur and Mr. Geberth are eligible to earn a performance-based annual cash bonus based on achievement of pre-established company and individual performance criteria established by our Board or Compensation Committee in their discretion. For 2023, the target annual bonus for each of Dr. Gaur and Mr. Geberth was initially equal to 30% of annual base salary, and was increased to 80% of Dr. Gaur’s annual base salary and 50% of Mr. Geberth’s annual base salary in connection with the closing of the Business Combination. No annual performance bonuses were paid for fiscal 2023. Mr. Chardon was eligible to earn quarterly commission compensation based on achievement of specified company performance criteria established by our Board or Directors in its discretion. For 2023, Mr. Chardon’s target commission compensation was equal to 40% of his annual base consulting fees.
Equity Compensation
We believe that equity grants provide our executives with a strong link to our long-term performance, create an ownership culture and help to align the interests of our executives with our stockholders. In addition, we believe that equity grants with a time-based vesting feature promote executive retention because this feature incentivizes our executive officers to remain in our employment during the vesting period.
Independent Compensation Consultant
In assessing and setting the compensation of our Named Executive Officers, the Compensation Committee has engaged Pearl Meyer & Partner LLC (“Pearl Meyer”) as its independent compensation consultant. Pearl Meyer advises the Compensation Committee on best practices in executive compensation and provides the Compensation Committee with market data in an effort to ensure our compensation program is competitive and designed to attract, retain, and incentivize our Named Executive Officers.
142
Table of Contents
Employment Arrangements with our Named Executive Officers
Effective upon the closing of the Business Combination, we entered into new employment agreements with each of Dr. Gaur and Mr. Geberth and a new corporate officer agreement with Mr. Chardon, which set forth the terms and conditions of each executive’s employment or consulting relationship. The new agreements supersede and replace each NEO’s prior employment or consulting arrangement and provide for specified payments and benefits in connection with a termination of the NEO’s employment or consulting relationship in certain circumstances. Our goal in providing these severance payments and benefits is to offer sufficient cash continuity protection such that the NEOs will focus their full time and attention on the requirements of the business rather than the potential implications for their respective positions. We prefer to have certainty regarding the potential severance amounts payable to the NEOs, rather than negotiating severance at the time that a NEO’s employment or other service relationship terminates. We have also determined that accelerated vesting provisions with respect to outstanding equity awards in connection with a qualifying termination of employment in certain circumstances are appropriate to encourage our NEOs to stay focused on the business in those circumstances, rather than focusing on the potential implications for them personally. The material terms of each NEO’s employment or consulting arrangements are summarized below.
Employment Agreement with Dr. Shantanu Gaur
We have entered into an employment agreement with Dr. Gaur, effective August 1, 2023, pursuant to which we employ Dr. Gaur as our Chief Executive Officer on an “at will” basis. Dr. Gaur’s employment agreement provides that his initial annual base salary is $620,000, and is subject to periodic review by our Board or Compensation Committee. In addition, the employment agreement provides that Dr. Gaur is eligible to receive annual cash bonuses, which the target annual amount shall be 80% of his annual base salary. Dr. Gaur is also eligible to participate in the employee benefit plans generally available to our employees, subject to the terms of such plans.
In the event of a termination of Dr. Gaur’s employment by Allurion without “cause” (as defined in his employment agreement) or by his resignation for “good reason” (as defined in his employment agreement), subject to Dr. Gaur’s execution and non-revocation of a separation agreement containing, among other things, a release of claims in favor of Allurion and its affiliates, Dr. Gaur will be entitled to receive (i) base salary continuation for 12 months following his termination date, and (ii) subject to Dr. Gaur’s election to receive continued health benefits under COBRA, payment of the full cost of such continuation coverage plus any administration fee until the earliest of (A) 12 months following termination; (B) the date he becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the expiration of Dr. Gaur’s COBRA health continuation period.
In addition, in lieu of the payments and benefits described above, in the event that Dr. Gaur’s employment is terminated by us without “cause” or by him for “good reason,” in each case, within three months prior to or 12 months following a “sale event” (as defined in 2023 Plan), and subject to Dr. Gaur’s execution and non-revocation of a separation agreement containing, among other things, a release of claims in favor of Allurion and its affiliates, Dr. Gaur will be entitled to receive (i) an amount in cash equal to 1.5 times the sum of (x) Dr. Gaur’s then-current base salary (or, his base salary in effect immediately prior to the sale event, if higher) and (y) Dr. Gaur’s target annual bonus for the then-current year (or, his target annual bonus in effect immediately prior to the sale event, if higher); (ii) full acceleration of vesting of all outstanding time-based equity awards held by Dr. Gaur; and (iii) subject to Dr. Gaur’s election to receive continued health benefits under COBRA, payment of the full cost of such continuation coverage plus any administration fee until the earliest of (A) 18 months following termination; (B) the date he becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the expiration of Dr. Gaur’s COBRA health continuation period. The cash severance payable to Dr. Gaur upon a termination of employment is generally payable in lump sum within 60 days following the date of termination, subject to limited exceptions.
143
Table of Contents
Employment Agreement with Mr. Christopher Geberth
We have entered into an employment agreement with Mr. Geberth effective August 1, 2023, which provides that his initial annual base salary is $425,000, and is subject to periodic review by our Board or Compensation Committee. In addition, the employment agreement provides that Mr. Geberth is eligible to receive annual cash bonuses, which target annual amount shall be 50% of his annual base salary. Mr. Geberth is also eligible to participate in the employee benefit plans generally available to our employees, subject to the terms of such plans.
In the event of a termination of Mr. Geberth’s employment by Allurion without “cause” (as defined in his employment agreement) or by his resignation for “good reason” (as defined in his employment agreement), subject to Mr. Geberth’s execution and non-revocation of a separation agreement containing, among other things, a release of claims in favor of Allurion and its affiliates, Mr. Geberth will be entitled to receive (i) base salary continuation for nine months following his termination date, and (ii) subject to Mr. Geberth’s election to receive continued health benefits under COBRA, payment of the full cost of such continuation coverage plus any administration fee until the earliest of (A) 12 months following termination; (B) the date he becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the expiration of Mr. Geberth’s COBRA health continuation period.
In addition, in lieu of the payments and benefits described above, in the event that Mr. Geberth’s employment is terminated by us without “cause” or by him for “good reason,” in each case, within three months prior to or 12 months following a “sale event” (as defined in our 2023 Plan), and subject to Mr. Geberth’s execution and non-revocation of a separation agreement containing, among other things, a release of claims in favor of Allurion and its affiliates, Mr. Geberth will be entitled to receive (i) an amount in cash equal to 1.0 times the sum of (x) Mr. Geberth’s then-current base salary (or, his base salary in effect immediately prior to the sale event, if higher) and (y) Mr. Geberth’s target annual bonus for the then-current year (or, his target annual bonus in effect immediately prior to the sale event, if higher); (ii) full acceleration of vesting of all outstanding time-based equity awards held by Mr. Geberth; and (iii) subject to Mr. Geberth’s election to receive continued health benefits under COBRA, payment of the full cost of such continuation coverage plus any administration fee until the earliest of (A) 12 months following termination; (B) the date he becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the expiration of Mr. Geberth’s COBRA health continuation period. The cash severance payable to Mr. Geberth upon a termination of employment is generally payable in lump sum within 60 days following the date of termination, subject to limited exceptions.
Termination Agreement and Settlement Agreement with Mr. Benoit Chardon
Effective as of September 1, 2023, we entered into a corporate officer agreement with Mr. Chardon and Benoit Chardon Consulting, a French société à responsabilité limitée that was solely owned by Mr. Chardon (“BCC”), pursuant to which BCC agreed to serve as Managing Director of Allurion France, a French société par actions simplifiée and wholly-owned subsidiary of Allurion (“Allurion France”). The corporate officer agreement provided that BCC would receive base consulting fees of €28,333.33 per month and additional variable compensation subject to the incentive plan terms issued annually by Allurion and conditional on meeting Allurion France and personal performance goal attainment as defined each year by Allurion.
The corporate officer agreement was terminated as of December 31, 2023, pursuant to a Termination Agreement (as defined herein), which provided for payment to BCC of a lump-sum termination fee of €156,740. Additionally, in connection with his departure, we entered into a settlement agreement with Mr. Chardon, pursuant to which we agreed to pay Mr. Chardon €183,260, which amount was used by Mr. Chardon in payment of the exercise price of his vested options.
144
Table of Contents
Outstanding Equity Awards at 2023 Fiscal Year End
The following table sets forth information concerning outstanding equity awards held by each of our NEOs as of December 31, 2023:
| Option Awards | ||||||||||||||||||||||||
| Name | Grant Date | Vesting Commencement Date | Number of Securities Underlying Unexercised Options (#) (Exercisable) (1) | Number of Securities Underlying Unexercised Options (#) (Unexercisable) (1) | Option Exercise | Option Expiration Date | ||||||||||||||||||
| Dr. Shantanu Gaur | | 08/03/2017 03/05/2020 12/20/2022 | (2) (4) | | — 01/01/2020 12/08/2022 |
| | 171,149 47,880 536,316 |
| | — 1,019 429,052 |
| | 1.13 1.17 4.51 |
| | 08/02/2027 03/04/2030 12/19/2032 |
| ||||||
| Christopher Geberth | | 02/11/2021 12/07/2021 12/20/2022 | (3) (3) (4) | | 11/16/2020 01/01/2022 12/08/2022 |
| | 94,234 35,146 173,807 |
| | 28,015 38,203 139,045 |
| | 0.95 1.88 4.51 |
| | 02/10/2031 12/06/2031 12/19/2032 |
| ||||||
| Benoit Chardon | | 03/05/2020 12/31/2020 12/07/2021 12/07/2020 | (2) (3) (3) (3) | | 01/01/2020 07/01/2020 01/01/2022 01/01/2023 |
| | — — — — |
| | 510 4,279 12,734 7,641 | (5) (5) (5) (5) | | 1.17 0.95 1.88 1.88 |
| | 03/04/2030 12/28/2030 12/06/2031 12/06/2031 |
| ||||||
| (1) | Amounts reported have been adjusted to reflect the closing of the Business Combination. |
| (2) | This option vests in 48 equal monthly installments following the vesting commencement date, subject to the executive’s continuing service relationship on each vesting date. |
| (3) | This option vests with respect to 25% of the shares on the first anniversary of the vesting commencement date, with the remaining shares vesting in 36 equal monthly installments thereafter, subject to the executive’s continuing service relationship on each vesting date. |
| (4) | This option vests in 36 equal monthly installments beginning on the last date of each one-month period following the vesting commencement date, subject to the executive’s continuing service relationship on each vesting date. Upon the consummation of the Business Combination, one-third of the then unvested shares subject to the option accelerated and vested. |
| (5) | Each of Mr. Chardon’s unvested option awards terminated on December 31, 2023 in connection with his departure. |
Additional Narrative Disclosure
Employee Benefits
Our Named Executive Officers who are employees are eligible to participate in our welfare benefit plans, including medical, dental, vision, basic life and accidental death & dismemberment, and short-term and long- term disability insurance benefits, in each case on the same basis as all of our other employees.
401(k) Plan
Allurion participates in the ADP TotalSource Retirement Savings Plan (the “401(k) Plan”), which provides eligible U.S. employees (including our Named Executive Officers who are employees) with an opportunity to save for retirement on a tax advantaged basis. Under the 401(k) Plan, eligible employees may defer eligible compensation subject to applicable annual contribution limits imposed by the Code. Allurion’s employees’ contributions are allocated to each participant’s individual account and employees may begin participating after an initial 90 day waiting period. Under the provisions of the 401(k) Plan, Allurion makes 2% matching contributions and may make discretionary non-matching contributions, as determined by our Board. The 401(k) Plan is intended to be qualified under Section 401(a) of the Code with the 401(k) Plan’s related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) Plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) Plan.
145
Table of Contents
Director Compensation Table
The following table sets forth information regarding the compensation awarded to, earned by, or paid to Allurion’s non-employee directors for service on our Board during the year ended December 31, 2023. Dr. Gaur, who is our Chief Executive Officer, also served on our Board, but did not receive any additional compensation for his service as a director and therefore is not included in the table below. Dr. Gaur’s compensation for his service, as our Chief Executive Officer, is set forth above under “Executive Compensation-Summary Compensation Table.”
Name | Fees Earned or Paid in Cash ($) | RSU Awards ($) (1)(4) | Option Awards ($) (2)(4) | Total Compensation ($) | ||||||||||||
Omar Ishrak | 43,750 | 195,415 | — | 239,165 | ||||||||||||
Krishna Gupta | 539,583 | (3) | 195,415 | — | 734,998 | |||||||||||
Michael Davin | 33,333 | 195,415 | 111,810 | 340,558 | ||||||||||||
Douglas Hudson | 26,042 | 195,415 | — | 221,457 | ||||||||||||
Nicholas Lewin | 21,875 | 195,415 | — | 217,290 | ||||||||||||
| (1) | Amounts shown reflect the grant date fair value of restricted stock units granted in 2023, calculated in accordance with FASB ASC Topic 718, excluding any estimates of forfeitures related to service-based vesting conditions. For information regarding assumptions underlying the valuation of equity awards, see Note 14 to our consolidated financial statements included in this prospectus. The amounts reported in this column reflect the accounting cost for the restricted stock unit awards, and do not correspond to the actual economic value that may be recognized by holders upon the vesting or settlement of the applicable awards. |
| (2) | Amounts shown reflect the grant date fair value of stock options granted in 2023, calculated in accordance with FASB ASC Topic 718, excluding any estimates of forfeitures related to service-based vesting conditions. For information regarding assumptions underlying the valuation of equity awards, see Note 14 to our consolidated financial statements included in this prospectus. The amounts reported in this column reflect the accounting cost for the option awards, and do not correspond to the actual economic value that may be recognized by holders upon the vesting or exercise of the applicable awards. |
| (3) | Amount shown for Mr. Gupta includes $500,000 of fees paid to KKG Enterprises and Remus Group Management (as defined below); Mr. Gupta is an affiliate of both. Refer to the section entitled “Certain Relationships and Related Party Transactions—Consulting Agreements with KKG Enterprises, LLC and Remus Group Management, LLC” below for further information. |
| (4) | The following table provides information regarding the number of shares of our common stock underlying stock options and restricted stock units held by our non-employee directors that were outstanding as of December 31, 2023: |
| Name | RSU Awards Outstanding at 2023 Year End (number of shares) | Option Awards Outstanding at 2023 Year End (number of shares) (1) | ||||||
Omar Ishrak | 45,235 | — | ||||||
Krishna Gupta | 462,695 | — | ||||||
Michael Davin | 45,235 | 161,367 | ||||||
Douglas Hudson | 45,235 | — | ||||||
Nicholas Lewin | 45,235 | — | ||||||
| (1) | Amount reflects outstanding stock options awarded to Mr. Davin in his capacity as a non-employee director, all of which are vested. |
Non-Employee Director Compensation Policy
Effective as of August 2023, our Board adopted a non-employee director compensation policy (the “Director Compensation Policy”) designed to enable us to attract and retain, on a long-term basis, highly
146
Table of Contents
qualified non-employee directors. Under the policy, our non-employee directors are eligible to receive cash retainers (which are payable quarterly in arrears and prorated for partial years of service) and equity awards as set forth below. In addition, we reimburse non-employee directors for all reasonable out-of-pocket expenses incurred in attending meetings of our Board or committees. We do not pay additional compensation for attending individual meetings of our Board.
Annual Retainer for Board Membership | $ | 45,000 | ||
Additional Annual Retainer for Non-Executive Chair | 45,000 | |||
Additional Annual Retainer for Committee Membership | ||||
Audit Committee Chairperson | 20,000 | |||
Audit Committee Member (other than Chairperson) | 10,000 | |||
Compensation Committee Chairperson | 15,000 | |||
Compensation Committee Member (other than Chairperson) | 7,500 | |||
Nominating and Corporate Governance Committee Chairperson | 10,000 | |||
Nominating and Corporate Governance Committee Member (other than Chairperson) | 5,000 |
Initial Award: The Director Compensation policy provides that each non-employee director serving on our Board on August 1, 2023 and each new non-employee director later elected or appointed to our Board will be granted an initial, one-time restricted stock unit award with a value of $225,000 that will vest in equal annual installments over three years, subject to continued service as a director through each vesting date.
Annual Award: On the date of each annual meeting of stockholders, each continuing non-employee director (excluding any non-employee director that was initially elected or appointed to our Board within six months prior to such annual meeting) will receive an annual restricted stock unit award with a value of $150,000 that will vest in full on the earlier of the first anniversary of the date of grant and the date of the next annual meeting of stockholders, subject to continued service as a director through such vesting date unless the Board determines otherwise. The vesting of all outstanding initial restricted stock unit awards and annual restricted stock unit awards held by non-employee directors will fully accelerate upon a “sale event” (as defined in our 2023 Plan).
For purposes of determining the size of each restricted stock unit award, the Director Compensation Policy defines “value” as the product of (A) the average closing market price on the NYSE (or such other market on which our common stock is then principally listed) of one share of our common stock over the trailing 30-day period ending on the last day of the month immediately prior to the month of the grant date, and (B) the aggregate number of shares of our common stock underlying such award.
In addition, on October 18, 2017, the board of directors of Legacy Allurion granted Mr. Davin an option to purchase 90,000 shares of Legacy Allurion common stock under our 2010 Plan (as defined below), which was converted into an option to purchase 88,019 shares of common stock upon consummation of the Business Combination and which was fully vested as of October 18, 2021. On March 5, 2020, the board of directors of Legacy Allurion also granted Mr. Davin an option to purchase 45,000 shares of Legacy Allurion common stock under our 2010 Plan, which was converted into an option to purchase 44,009 shares of common stock upon consummation of the Business Combination, and which vested with respect to twenty-five percent (25%) of the shares underlying such option on the one-year anniversary of the date of grant, and then vested in 36 equally monthly installments thereafter. On December 7, 2021, the board of directors of Legacy Allurion also granted Mr. Davin an option to purchase 30,000 shares of Legacy Allurion common stock under our 2020 Plan (as defined below), which was converted into an option to purchase 29,339 shares of common stock upon consummation of the Business Combination, and which vested in equal monthly installments over two years.
147
Table of Contents
PRINCIPAL STOCKHOLDERS
The following table sets forth information regarding the beneficial ownership of shares of our common stock, as of May 1, 2024, in each case, by:
| • | each person who is known to us to be the beneficial owner of more than 5% of the issued and outstanding shares of our common stock; |
| • | each of our directors and Named Executive Officers; and |
| • | all of our directors and executive officers as a group. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options, restricted stock units, and warrants that are currently exercisable or releasable or exercisable or releasable within 60 days.
Unless otherwise indicated, we believe that all persons named in the table below have sole voting and investment power with respect to all shares of voting shares beneficially owned by them. Unless otherwise noted, the business address of each of those listed in the table is c/o Allurion Technologies, Inc., 11 Huron Drive, Natick, MA 01760.
The beneficial ownership of the shares of our common stock is based on 47,943,568 shares of our common stock issued and outstanding as of May 1, 2024.
Name and Address of Beneficial Owner | Number of Shares | % of Ownership | ||||||
Directors and Named Executive Officers: | ||||||||
Shantanu Gaur(1) | 2,414,395 | 4.9 | % | |||||
Krishna Gupta(2) | 5,754,470 | 12.0 | % | |||||
Omar Ishrak(3)(10) | 3,972,939 | 8.3 | % | |||||
Chris Geberth(4) | 363,930 | * | ||||||
Benoit Chardon(5) | 199,183 | * | ||||||
Michael Davin(6) | 226,554 | * | ||||||
Larson Doug Hudson | — | — | ||||||
Nicholas Lewin | — | — | ||||||
Milena Alberti-Perez | — | — | ||||||
All Current Directors and Executive Officers as a Group (Ten Persons) (7) | 14,581,001 | 29.3 | % | |||||
Five Percent Holders: | ||||||||
Romulus Growth Allurion L.P.(8) | 4,151,846 | 8.7 | % | |||||
Omar Ishrak(3)(10) | 3,972,939 | 8.3 | % | |||||
RTW(9) | 3,936,903 | 8.1 | % | |||||
Compute Health Sponsor LLC(10) | 3,262,711 | 6.8 | % | |||||
Jean Nehme (10) | 3,262,711 | 6.8 | % | |||||
Joshua Fink(10) | 3,262,711 | 6.8 | % | |||||
Citadel Advisors LLC(11) | 2,563,726 | 5.4 | % | |||||
Amarat Investments Limited(12) | 2,386,405 | 5.0 | % | |||||
| * | Less than one percent. |
| (1) | Consists of (i) 1,003,090 shares of common stock held by The Shantanu K. Gaur Revocable Trust Of 2021, of which Shantanu K. Gaur and Neha Gaur serve as trustees, (ii) 547,679 shares of common stock held by The Gaur Family Irrevocable Trust Of 2021, of which Steven M. Burke, Esq. and Neha Gaur serve as trustees and which Ms. Gaur has voting and dispositive control, and (iii) 863,626 shares of common stock issuable upon exercise of options within 60 days of May 1, 2024. |
| (2) | Consists of (i) 3,124,244 shares of common stock held by Romulus Growth Allurion L.P., (ii) 72,953 shares of common stock held by Romulus Capital I, L.P., (iii) 73,349 shares of common stock issuable upon exercise of a warrant held by Romulus Capital I, L.P., (iv) 881,300 shares of common stock held by |
148
Table of Contents
| Romulus Allurion Special Opportunity L.P., (v) 538,101 shares of common stock held by Samin Capital LLC, (vi) 939,285 shares of common stock held by Krishna Gupta, and (vii) 125,238 shares of common stock issuable upon vesting of restricted stock units held by Krishna Gupta within 60 days of May 1, 2024. Krishna Gupta is the general partner of Romulus Allurion Special Opportunity L.P., Romulus Growth Allurion L.P., and Romulus Capital I, L.P., and the manager of Samin Capital LLC. |
| (3) | Consists of (i) 710,228 shares held by Omar & Helen Ishrak Living Trust and (ii) 3,262,711 shares held by the Sponsor. |
| (4) | Consists of 363,930 shares of common stock issuable upon exercise of options within 60 days of May 1, 2024. |
| (5) | Consists of 199,183 shares of common stock. |
| (6) | Consists of (i) 65,187 shares of common stock and (ii) 161,367 shares of common stock issuable upon exercise of options within 60 days of May 1, 2024. |
| (7) | See footnotes 1 through 6 above. Includes (i) 1,662,896 shares of common stock and (ii) 185,817 shares of common stock issuable upon exercise of options within 60 days of May 1, 2024, each held by Ram Chuttani. |
| (8) | Consists of (i) 3,124,244 shares of common stock held by Romulus Growth Allurion L.P., (ii) 72,953 shares of common stock held directly by Romulus Capital I, L.P. and 73,349 shares of common stock issuable upon exercise of a warrant held by Romulus Capital I, L.P., and (iii) 881,300 shares of common stock held by Romulus Allurion Special Opportunity L.P. Krishna Gupta is the general partner of Romulus Allurion Special Opportunity L.P., Romulus Growth Allurion L.P., and Romulus capital I, L.P. The address of each of the entities is 151 Tremont Street, Suite 6F, Boston, MA 02111. |
| (9) | Beneficial ownership is as of April 16, 2024, based solely on a Schedule 13D/A filed jointly on April 17, 2024 with the SEC by RTW and Roderick Wong, M.D., which consists of 3,936,903 shares of common stock beneficially owned by RTW, including 479,196 shares of common stock underlying the Notes issuable to certain RTW entities and RTW, as agent to the Purchasers. RTW, in its capacity as the investment manager of those certain RTW entities, has the power to vote and the power to direct the disposition of the shares held by RTW. Accordingly, RTW may be deemed to be the beneficial owner of such securities. Roderick Wong, M.D., as the Managing Partner of RTW, has the power to direct the vote and disposition of the securities held by RTW. Dr. Wong disclaims beneficial ownership of the shares held by RTW, except to the extent of his pecuniary interest therein. The address and principal office of RTW is 40 10th Avenue, Floor 7, New York, NY 10014, and the address of Dr. Wong and each of the RTW entities is c/o RTW Investments, LP, 40 10th Avenue, Floor 7, New York, NY 10014. |
| (10) | Consists of 3,262,711 shares of common stock held by the Sponsor. The Sponsor is managed by each of Drs. Nehmé and Ishrak and Mr. Fink. As a result of the foregoing, each of Drs. Nehmé and Ishrak and Mr. Fink may be deemed to beneficially own shares held by the Sponsor. |
| (11) | Beneficial ownership is as of December 31, 2023, based solely on a Schedule 13G/A filed jointly on February 14, 2024 with the SEC by Citadel Advisors LLC, a Delaware limited liability company (“Citadel Advisors”), Citadel Advisors Holdings LP, a Delaware limited partnership (“CAH”), Citadel GP LLC, a Delaware limited liability company (“CGP”), Citadel Securities LLC, a limited liability company (“Citadel Securities”), Citadel Securities Group LP (“CALC4”), a Delaware limited partnership, Citadel Securities GP LLC (“CSGP”), a Delaware limited liability company, and Kenneth Griffin, a U.S. citizen, with respect to shares owned by Citadel Multi-Strategy Equities Master Fund Ltd., a Cayman Islands company (“CM”), and Citadel Securities. Citadel Advisors is the portfolio manager for CM. CAH is the sole member of Citadel Advisors. CGP is the general partner of CAH. CALC4 is the non-member manager of Citadel Securities. CSGP is the general partner of CALC4. Mr. Griffin is the President and Chief Executive Officer of CGP and owns a controlling interest in CGP and CSGP. Mr. Griffin has shared voting and dispositive power over 2,563,726 shares of common stock, Citadel Advisors, CAH, and CGP have shared voting and dispositive power over 2,555,436 shares of common stock, and Citadel Securities, CALC4, and CSGP have shared voting and dispositive power over 8,290 shares of common stock. The address of each of these persons is Southeast Financial Center, 200 S. Biscayne Blvd., Suite 3300, Miami, Florida 33131. |
| (12) | Consists of 2,386,405 shares of common stock held by Amarat Investments Limited. The address of Amarat Investments Limited is 44 Esplande, St. Helier, Jersey, JE49WG. |
149
Table of Contents
SELLING SECURITYHOLDER
Unless the context otherwise requires, as used in this prospectus, “selling securityholder” includes the selling securityholder listed below and donees, pledgees, transferees or other successors-in-interest selling shares of common stock received after the date of this prospectus from the selling securityholder as a gift, pledge or other non-sale related transfer.
In accordance with the Amended Note Purchase Agreement, this prospectus covers the resale of up to 30,191,900 shares of common stock issued or issuable pursuant to the conversion of the Notes, determined as if the outstanding Notes were converted at an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of Notes, subject to Stockholder Approval for such issuance and exclusive of any accrued but unpaid interest on the Notes that may be added to the outstanding principal amount of such Notes at the time of conversion. Because the conversion price of the Notes may be adjusted pursuant to the Amended Note Purchase Agreement, the number of shares of common stock that will actually be issued may be more or less than the number of shares being offered by this prospectus. In particular, the Amended Note Purchase Agreement provides that unless and until Stockholder Approval is obtained, we will not deliver common stock upon conversion of the Notes in excess of 479,196 shares of common stock (the “Conversion Cap”), or 1% of the number of shares of the common stock outstanding as of April 14, 2024. We are required to include a proposal in our definitive proxy statement on Schedule 14A seeking Stockholder Approval no later than December 31, 2025. If we do not obtain Stockholder Approval at such meeting, we shall call a special meeting of stockholders each 90-day period thereafter at least two times, and thereafter at each subsequent annual meeting until Stockholder Approval is obtained or the Notes are no longer outstanding; provided, that shares of common stock issued upon conversion of the Notes prior to obtaining Stockholder Approval shall not be entitled to vote in favor of Stockholder Approval. In addition, the number of shares of common stock issuable upon conversion of the Notes may increase as interest on the Notes accrues. For additional information regarding the issuance of the Notes, see “Private Placement of Notes” above.
In addition to being a party to the Amended Note Purchase Agreement and a Purchaser of Notes, the selling securityholder is party to the Revenue Interest Financing Agreement, the Amended and Restated RTW Side Letter and the PIPE Subscription Agreement. Pursuant to the Amended and Restated RTW Side Letter, the selling securityholder has the right to designate an independent director nominee to be elected by our stockholders. Pursuant to the Amended Note Purchase Agreement, until the Notes are converted or repaid in full, RTW will be entitled to designate one representative who will serve as a non-voting board observer to the Board. We also must include an additional director nominee in our proxy statement for the election of the Class I directors at our 2024 annual meeting of stockholders, with the recommendation of the Board to vote in favor of such additional nominee as well as the “RTW Designated Director” (as defined in the Investor Rights Agreement). Such additional director nominee shall go through our director nomination process led by the Nominating and Corporate Governance Committee. RTW shall have the right to approve such additional nominee, with such approval not to be unreasonably withheld. For additional information regarding the relationships between us and RTW, see “Certain Relationships and Related Party Transactions” below. Except for the foregoing relationships, the selling securityholder has not had any material relationship with us within the past three years.
The table below lists the selling securityholder and other information regarding the beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder) of the shares of common stock held by the selling securityholder. The second column lists the number of shares of common stock beneficially owned by the selling securityholder, based on its beneficial ownership of shares of common stock, including shares of common stock issuable upon conversion of the Notes, based on 47,943,568 shares of common stock outstanding as of May 1, 2024 and assuming conversion of the Notes held by the selling securityholder on that date but taking account of the Conversion Cap. The third column lists the shares of common stock being offered by this prospectus by the selling securityholder and does not take in account any limitations on conversion of the Notes set forth therein. The fourth column assumes the
150
Table of Contents
sale of all of the shares of common stock offered by the selling securityholder up to the Conversion Cap pursuant to this prospectus, and further assumes that the selling securityholder will not acquire beneficial ownership of any additional securities during the offering. In addition, the selling securityholder may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time and from time to time, our securities in transactions exempt from the registration requirements of the Securities Act after the date on which the information in the table is presented.
Subject to specified conditions, on or after April 16, 2028, the Notes are redeemable by us at a redemption price equal to 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date. Pursuant to the terms of the Amended Note Purchase Agreement, each Purchaser is subject to a beneficial ownership conversion limitation such that no Purchaser shall be permitted to convert Notes to the extent it would result in such purchaser and its affiliates beneficially owning more than the beneficial ownership limitation, currently set at 9.99% of our common stock.
Please see the section entitled “Plan of Distribution” for further information regarding RTW’s method of distributing these securities.
| Common Stock Beneficially Owned Prior to this Offering | Maximum Number of Shares of Common Stock to be Offered Pursuant to this Prospectus | Common Stock Owned After this Offering (3) | ||||||||||||||||||
Name of Selling Securityholder | Number of Shares | Percent | Number of Shares | Percent | ||||||||||||||||
Entities affiliated with RTW Investments, LP | 3,936,903 | (1) | 8.1 | % | 30,191,900 | (2) | 3,457,707 | (3) | 7.2 | % | ||||||||||
| (1) | Consists of 3,936,903 shares of common stock beneficially owned by RTW, including 479,196 shares of common stock underlying the Notes up to the Conversion Cap issuable to RTW and certain RTW entities who are Original RIFA Investors and Purchasers, including RTW Master Fund, Ltd., RTW Innovation Master Fund, Ltd., and RTW Biotech Opportunities Operating Ltd. RTW, in its capacity as the investment manager of such Original RIFA Investors and Purchasers, has the power to vote and the power to direct the disposition of the shares held by RTW and such Original RIFA Investors and Purchasers. Accordingly, RTW may be deemed to be the beneficial owner of such securities. Roderick Wong, M.D., as the Managing Partner of RTW, has the power to direct the vote and disposition of the securities held by RTW. Dr. Wong disclaims beneficial ownership of the shares held by RTW, except to the extent of his pecuniary interest therein. The address and principal office of RTW is 40 10th Avenue, Floor 7, New York, NY 10014, and the address of Dr. Wong and each of the RTW entities is c/o RTW Investments, LP, 40 10th Avenue, Floor 7, New York, NY 10014. |
| (2) | This table assumes the conversion of the Notes at an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of Notes, plus an increase in the conversion rate of an additional 321.9182 shares of common stock per $1,000 principal amount of Notes assuming the maximum adjustment upon consummation of a Make-Whole Fundamental Change, subject to Stockholder Approval for such issuance and exclusive of any accrued but unpaid interest on the Notes that may be added to the outstanding principal amount of such Notes at the time of conversion. Because the conversion price of the Notes may be adjusted, the number of shares that will actually be issued may be more or less than the number of shares being offered by this prospectus. We do not know when or in what amounts the selling securityholder may offer shares of common stock for sale following conversion of the Notes. The selling securityholder may choose not to sell any of the shares offered by this prospectus. |
| (3) | Assumes the sale of all shares of common stock being offered pursuant to this prospectus. The selling securityholder may choose not to sell any of the shares of common stock offered by this prospectus up to the Conversion Cap. The selling securityholder is not obligated to sell all or any portion of the shares of our common stock offered pursuant to this prospectus. This column represents the number of shares that will be held by the selling securityholder after completion of this offering based on 47,943,568 shares of our |
151
Table of Contents
| common stock outstanding as of May 1, 2024, and based on the assumptions that (a) all 479,196 shares of common stock issuable upon conversion of the Notes registered for resale by the registration statement of which this prospectus is part of will be sold, up to the Conversion Cap, and (b) no other shares of common stock are acquired or sold by the selling securityholder prior to completion of this offering. |
152
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than the compensation agreements and other arrangements described under “Executive Compensation” and “Director Compensation” in this prospectus and the transactions described below, since January 1, 2023, there has not been and there is not currently proposed any transaction or series of similar transactions to which we were, or will be, a party in which the amount involved exceeded, or will exceed, $120,000 (or, if less, 1% of the average of our total assets at year end for the last two completed fiscal years) and in which any director, executive officer, holder of five percent or more of any class of our capital stock or any member of the immediate family of, or entities affiliated with, any of the foregoing persons, had, or will have, a direct or indirect material interest.
PIPE Investment
PIPE Investors related to Legacy Allurion entered into PIPE Subscription Agreements with Compute Health and us, pursuant to which they subscribed for shares of our common stock in connection with a private placement pursuant. The PIPE Investors made a private investment in the aggregate amount of $37.9 million on the terms and conditions set forth in the PIPE Subscription Agreements. Such PIPE Investors who participated in the PIPE Investment include Michael Davin (30,824 shares), who is one of our directors, Omar Ishrak (710,228 shares), who is one of our directors, and RTW (2,130,681 shares), who has the right to designate an independent director nominee to be elected by our stockholders. See the “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Recent Developments — PIPE Investment” for additional information.
Gaur Contribution Agreement
On May 2, 2023, the Gaur Trust and we entered into the Gaur Contribution Agreement, pursuant to which, among other things, upon the terms and subject to the conditions set forth therein, the Gaur Trust agreed to contribute, as a contribution of capital, the Gaur Trust Contributed Shares. The Gaur Trust’s contribution of the Gaur Trust Contributed Shares was effective immediately following the consummation of the Business Combination between us and Compute Health and the transactions contemplated thereby. See the subsection entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Recent Developments — Gaur Contribution Agreement” for additional information.
RSU Forfeiture Agreement
On May 2, 2023, Krishna Gupta, a member of our Board, entered into the RSU Forfeiture Agreement, pursuant to which, among other things, upon the terms and subject to the conditions set forth therein, Mr. Gupta agreed to forfeit the Forfeited RSUs. The Forfeited RSUs were terminated and cancelled without consideration therefor immediately following the consummation of the transactions contemplated by the Business Combination Agreement between Allurion and Compute Health and other parties thereto, dated as of February 9, 2023, as amended from time to time. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Recent Developments—RSU Forfeiture Agreement” for additional information.
2023 Convertible Note Incremental Financing
On February 15, 2023, we entered into the Initial Financing. In connection with the refinancing of the Initial Financing, we entered into the HVL Termination Agreement on May 2, 2023.
The HVL Termination Agreement provided us, upon the terms and subject to the conditions set forth therein, the right to prepay, in one or more transactions, all or a portion of the outstanding principal amount, plus accrued interest, under the HVL Bridge Note, including by way of (a) a $2 million prepayment, $1.5 million of which was deemed a prepayment penalty and (b) immediately prior to the consummation of the Business Combination, an additional payment of at least $6 million under the HVL Bridge Note by way of (i) payment in
153
Table of Contents
cash by us and/or (ii) the sale and transfer of all or any portion of the HVL Bridge Note, equivalent in value to the portion of the additional payment to be repaid pursuant to this clause (b)(ii), to any person or persons designated in writing by us.
In addition, under the HVL Termination Agreement, upon the terms and subject to the conditions set forth therein, we agreed to issue to HVL 387,696 additional shares of our common stock.
For more information about the 2023 Convertible Note Incremental Financing and the HVL Bridge Note, see the subsection entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations— 2023 Convertible Note Incremental Financing.”
Revenue Interest Financing Agreement, Side Letter and PIPE Conversion Option
On February 9, 2023, concurrently with the execution of the Business Combination Agreement, we entered into the Revenue Interest Financing Agreement with certain entities that engaged RTW, pursuant to which, at the closing of the Business Combination, we assumed all obligations of Legacy Allurion under the Revenue Interest Financing Agreement, RTW paid us an aggregate of $40.0 million (the “Investment Amount”). In exchange for the Investment Amount, We will remit revenue interest payments on all current and future products, digital solutions and services developed, imported, manufactured, marketed, offered for sale, promoted, sold, tested or otherwise distributed by Allurion and its subsidiaries.
On April 14, 2024, the Revenue Interest Financing Agreement was amended pursuant to the Omnibus Amendment to the Revenue Interest Financing Agreement to reflect certain modifications agreed between the parties thereto in connection with the Amended Note Purchase Agreement and the refinancing of the Fortress Credit Agreement.
Additionally, in connection with our entry into the Revenue Interest Financing Agreement, we, Compute Health, Legacy Allurion, Merger Sub II and RTW entered into the RTW Side Letter under which RTW may elect to exercise the PIPE Conversion Option. Refer to Note 9, Revenue Interest Financing, Side Letter, and PIPE Conversion Option, in the notes to our annual consolidated financial statements for the years ended December 31, 2023 and 2022 included within prospectus for further discussion on the Revenue Interest Financing.
On May 2, 2023, the parties amended and restated the RTW Side Letter in connection with the Backstop Agreement, pursuant to which, among other things, Allurion issued 250,000 shares of common stock to RTW immediately prior to the Intermediate Merger Effective Time. On April 14, 2024, the parties entered into the Side Letter Amendment to reflect certain modifications to the Amended and Restated RTW Side Letter in connection with the Amended Note Purchase Agreement. The Side Letter Amendment provides, among other things, that RTW may make a single election in certain circumstances to convert up to $7,500,000 of the purchase price that RTW paid for certain equity interests in Allurion into an amount of financing provided by RTW to Allurion pursuant to an additional revenue interest financing agreement with Legacy Allurion.
Amended Note Purchase Agreement
Pursuant to the Amended Note Purchase Agreement, on April 16, 2024, we issued and sold $48 million of Notes to RTW in a private placement. Until the Notes are converted or repaid in full, RTW will be entitled to designate one representative who will serve as a non-voting board observer to the Board. We also must include an additional director nominee in our proxy statement for the election of the Class I directors at our 2024 annual meeting of stockholders, with the recommendation of the Board to vote in favor of such additional nominee as well as the “RTW Designated Director” (as defined in the Investor Rights Agreement). Such additional director nominee shall go through our director nomination process led by the Nominating and Corporate Governance Committee. RTW shall have the right to approve such additional nominee, with such approval not to be
154
Table of Contents
unreasonably withheld. For more information about the Amended Note Purchase Agreement, see the subsection entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—RTW Note Purchase Agreement..”
Investment by Former Chief Commercial Officer
On June 24, 2023, Legacy Allurion sold a $200,000 Bridge Note to its former Chief Commercial Officer, Benoit Chardon.
Investor Rights Agreement
Immediately prior to the closing of the Business Combination, we entered into the Investor Rights Agreement. For more information about the Investor Rights Agreement, see the section entitled “Description of Securities—Investor Rights Agreement.”
Consulting Agreements with KKG Enterprises, LLC and Remus Group Management, LLC
In the first quarter of 2023, we entered into consulting agreements with KKG Enterprises, LLC (“KKG Enterprises”) and Remus Group Management, LLC (“Remus Group Management”) to assist us in building out our AI platform, augment our AI advisory board, and provide advisory services related to the Business Combination. These agreements were tied to board-related work by Krishna Gupta, who is a member of our Board, CEO of Remus Group Management, principal at KKG Enterprises, and affiliated with Romulus Capital, a stockholder. The agreements included payments of $0.2 million to KKG Enterprises and $0.3 million to Remus Group Management, and were terminated in June 2023.
Allurion Middle East Medical Instruments Trading, LLC
Allurion Middle East Medical Instruments Trading, LLC (“Allurion Middle East”) is Allurion’s subsidiary in the United Arab Emirates (the “UAE”). Per the law of the UAE, the majority owner of a UAE limited liability company must be a UAE entity. Pursuant to the Second Restated Memorandum of Association of Allurion Middle East Medical Instruments Trading (the “MoA”), Shuraa Management & Consultancy LLC is a 51% owner of Allurion Middle East; Allurion owns the remaining 49%.
Allurion Middle East was established to carry on the business of medical, surgical equipment, and instruments trading in the Middle East on behalf of Allurion. Allurion Middle East is permitted to enter into agreements and act as an agent on Allurion’s behalf. Allurion Middle East’s capital is AED 300,000 divided into 300 non-divisible shares, par value AED 1,000. The capital is fully paid in cash and divided as follows: Shuraa Management & Consultancy LLC received 153 shares at a value of AED 153,000 and holds 51% in capital. Allurion holds 147 shares for a value of AED 147,000 and holds 49% in capital. New shares may be issued at any time to increase capital or by transferring the available reserve to share capital by a resolution of the board. Shares are assignable. Under the MoA, transfers are not permitted where they would reduce the UAE national partner’s hold in the capital below 51% unless the number of partners is reduced to below 2 or increased to above 50. Profits from Allurion Middle East are distributed 20% to Shuraa Management and Consultancy LLC and 80% to Allurion.
On February 2, 2022, Allurion Middle East entered into a one-year lease agreement with Shuraa Business Centre Branch (“SBCB”), an affiliate of Shuraa Management & Consultancy LLC. On August 29, 2022, Allurion Middle East entered into a second one-year lease agreement with SBCB. Under each lease, Allurion Middle East pays to SBCB AED 41,500 and AED 35,000 per year, respectively, plus a AED 3,000 refundable security deposit and 5% VAT. These leases automatically renew after one year as agreed to by the parties. On February 2, 2024, Allurion Middle East entered into a six-month lease agreement with SBCB in lieu of the second annual renewal of its one-year lease agreement entered into on February 2, 2022. Cancellation of these contracts is not permitted until the seventh month and requires a two month notice period. Standard indemnity provisions exist.
155
Table of Contents
Corporate Officer Agreement and Termination Agreement
Effective as of September 1, 2023, we entered into a corporate officer agreement with Benoit Chardon, then our Chief Commercial Officer, and BCC, pursuant to which BCC served as Managing Director of Allurion France. The corporate officer agreement provided that BCC would receive base consulting fees of €28,333.33 per month and additional variable compensation subject to the incentive plan terms issued annually by Allurion and conditional on meeting Allurion France and personal performance goal attainment defined each year by Allurion.
On December 12, 2023, Mr. Chardon, BCC and Allurion France entered into a Termination Agreement (the “Termination Agreement”), pursuant to which the parties agreed to terminate the Corporate Officer Agreement by and among BCC, Mr. Chardon and Allurion France (the “Corporate Officer Agreement”). By virtue of the Termination Agreement, the parties mutually agreed to terminate the Corporate Officer Agreement as of December 31, 2023 (the “Departure Date”). Pursuant to the Termination Agreement, BCC resigned from its duties as managing director of Allurion France effective as of the Departure Date and Allurion paid BCC all amounts due to it under the Corporate Officer Agreement for monthly consulting fees through the Departure Date and its variable compensation due for the third quarter of 2023. In addition, Allurion paid BCC a lump-sum termination fee of €156,740. The Termination Agreement contains a mutual release and non-disparagement provision as well as a non-solicitation provision by BCC in favor of Allurion France.
Paris Lease Agreement
LNMP JPBC Investment, a French entity, was the lessor under the 56 Rue des Petites Ecuries, Paris Lease (the “56 Rue des Petites Ecuries Lease”), pursuant to which we leased certain space for company purposes. LNMP JPBC Investment is partially owned by Benoit Chardon, our former Chief Commercial Officer. Under the 56 Rue des Petites Ecuries Lease, Allurion paid monthly rent of €9,284. The 56 Rue des Petites Ecuries Lease had a three year term, which began in August 2022, and was renewable by agreement between Allurion and LNMP JPBC Investment. This lease was terminated in February 2024.
Indemnification Agreements
We entered into indemnification agreements with our directors and executive officers. The indemnification agreements and our Charter and Bylaws require us to indemnify our directors and executive officers to the fullest extent permitted by law.
Executive Officer and Director Compensation Arrangements
See the sections entitled “Executive Compensation” and “Director Compensation” above for information regarding compensation arrangements with our executive officers and directors, which include, among other things, employment, termination of employment and change in control arrangements, stock awards and certain other benefits.
Related Person Transaction Policy
We have adopted a written related person transaction policy that sets forth the following policies and procedures for the review and approval or ratification of related person transactions.
A “Related Person Transaction” is any transaction in which Allurion or any of its subsidiaries was, is or will be a participant, the amount of which involved exceeds $120,000, and in which any related person had, has or will have a direct or indirect material interest. A “Related Person” means:
| • | any person who is, or at any time during the applicable period was, one of Allurion’s executive officers or a member of our Board; |
| • | any person who is known by Allurion to be the beneficial owner of more than 5% of our voting stock; and |
156
Table of Contents
| • | any immediate family member of any of the foregoing persons, which means any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law or sister-in-law or any other person (other than a tenant or employee) sharing the household of such related person of a director, executive officer or a beneficial owner of more than 5% of our voting stock. |
We also have policies and procedures designed to minimize potential conflicts of interest arising from any dealings we may have with our affiliates and to provide appropriate procedures for the disclosure of any real or potential conflicts of interest that may exist from time to time. Specifically, the Audit Committee will have the responsibility to review related person transactions.
Director Independence
Our Board has determined that each member of the Board, other than Dr. Gaur, qualifies as independent, as defined under the listing standards of the NYSE. In addition, our Board has determined that each of the members of the Audit, Nominating and Corporate Governance, and Compensation Committees is independent in accordance with the rules of the NYSE and, in the case of the members of the Audit Committee, the rules of the SEC. In determining the independence of its members, the Board considered all the facts and circumstances it deemed relevant in determining their independence, including, but not limited to, the director’s commercial, industrial, banking, consulting, legal, accounting, charitable, and familial relationships. We are subject to the rules of the SEC and NYSE relating to the memberships, qualifications, and operations of the Audit Committee.
157
Table of Contents
DESCRIPTION OF SECURITIES
The following description summarizes certain important terms of our capital stock as of the date of this prospectus as specified in our Charter and Bylaws. Because the following description is only a summary, it does not contain all the information that may be important to you. For a complete description of the matters set forth in this section entitled “Description of Securities,” you should refer to the Charter, the Bylaws, which are included as exhibits to the registration statement of which this prospectus is a part, and to the applicable provisions of Delaware law.
Authorized and Outstanding Stock
Our Charter authorizes the issuance of 1.1 billion shares, consisting of 1 billion shares of common stock, $0.0001 par value per share, and 100 million shares of Preferred Stock, $0.0001 par value. As of May 1, 2024, there were 47,943,568 shares of our common stock outstanding. No shares of our Preferred Stock are currently outstanding.
Common Stock
Our Charter provides the following with respect to the rights, powers, preferences and privileges of our common stock.
Voting Power
Except as otherwise required by law or as otherwise provided in any certificate of designation for any series of Preferred Stock, the holders of our common stock will possess all voting power for the election of Allurion’s directors and all other matters requiring stockholder action. Holders of our common stock will be entitled to one vote per share on matters to be voted on by stockholders.
Dividends
Holders of our common stock will be entitled to receive such dividends, if any, as may be declared from time to time by our Board in its discretion out of funds legally available therefor. We do not intend to pay cash dividends in the foreseeable future. Any payment of cash dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements and general financial conditions. In no event will any stock dividends or stock splits or combinations of stock be declared or made on our common stock unless all shares of our common stock at the time outstanding are treated equally and identically.
Liquidation, Dissolution and Winding Up
In the event of our voluntary or involuntary liquidation, dissolution or winding-up, our net assets will be distributed pro rata to the holders of our common stock, subject to the rights of the holders of Preferred Stock, if any.
Preemptive or Other Rights
There are no sinking fund provisions applicable to our common stock.
Preferred Stock
Our Charter provides that shares of Preferred Stock may be issued from time to time in one or more series. Our Board is authorized to fix designations, powers, including voting powers, full or limited, or no voting powers, preferences and the relative, participating, optional or other special rights of the shares of each series of
158
Table of Contents
Preferred Stock and any qualifications, limitations and restrictions thereof. Our Board is able to, without stockholder approval, issue Preferred Stock with voting and other rights that could adversely affect the voting power and other rights of the holders of our common stock and could have anti-takeover effects. The ability of our Board to issue Preferred Stock without stockholder approval could have the effect of delaying, deferring or preventing a change of control of Allurion or the removal of existing management.
Warrants
Public Warrants
Each whole Public Warrant entitles the registered holder to purchase 1.420455 shares of our common stock at a price of $8.10 per share, subject to adjustment as discussed below, at any time commencing on the date that is 30 days after the completion of the Business Combination. Pursuant to the Warrant Agreement, a Public Warrant holder may exercise its Public Warrants only for a whole number of shares of common stock. This means that only a whole Public Warrant may be exercised at any given time by a Public Warrant holder. The Public Warrants will expire seven years after the completion of the Business Combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
Redemption of Public Warrants when the price per share of our common stock equals or exceeds $12.67
Not less than all of the Public Warrants may be redeemed for cash or for shares of common stock after a date that is ninety (90) days after Closing Date of the Business Combination. Once the Public Warrants become exercisable, we may redeem the outstanding Public Warrants:
| • | in whole and not in part; |
| • | at a price of $0.01 per Public Warrant; |
| • | upon a minimum of 30 days’ prior written notice of redemption to each Public Warrant holder; and |
| • | if, and only if, the closing price of our common stock equals or exceeds $12.67 per share (as adjusted for adjustments to the number of shares issuable upon exercise or the exercise price of a Public Warrant as described under the heading “—Anti-dilution adjustments”) for any 20 trading days within a 30-trading day period ending three trading days before we send the notice of redemption to the Public Warrant holders. |
We will not redeem the Public Warrants as described above unless a registration statement under the Securities Act covering the issuance of the common stock issuable upon exercise of the Public Warrants is then effective and a current prospectus relating to those shares of common stock is available throughout the 30-day redemption period. If and when the Public Warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws.
We have established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the Public Warrant exercise price. If the foregoing conditions are satisfied and we issue a notice of redemption of the Public Warrants, each Public Warrant holder will be entitled to exercise his, her or its Public Warrant prior to the scheduled redemption date. Any such exercise would not be done on a “cashless” basis and would require the exercising Public Warrant holder to pay the exercise price for each Public Warrant being exercised. However, the price of the common stock may fall below the $12.67 redemption trigger price (as adjusted for adjustments to the number of shares issuable upon exercise or the exercise price of a Public Warrant as described under the heading “— Anti-dilution Adjustments”) as well as the $8.10 (for whole shares) Public Warrant exercise price after the redemption notice is issued.
159
Table of Contents
Redemption of Public Warrants when the price per share of common stock equals or exceeds $7.04
Once the Public Warrants become exercisable, we may redeem the outstanding Public Warrants:
| • | in whole and not in part; |
| • | at $0.10 per Public Warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their Public Warrants on a cashless basis prior to redemption and receive that number of shares of common stock to be determined by reference to the table below, based on the redemption date and the “fair market value” (as defined below) of our common stock except as otherwise described below; and |
| • | if, and only if, the closing price of our common stock equals or exceeds $7.04 per share (as adjusted for adjustments to the number of shares issuable upon exercise or the exercise price of a Public Warrant as described under the heading “—Anti-dilution adjustments”) for any 20 trading days within a 30-trading day period ending three trading days before we send the notice of redemption to the Public Warrant holders. |
Beginning on the date the notice of redemption is given until the Public Warrants are redeemed or exercised, holders may elect to exercise their Public Warrants on a cashless basis. The numbers in the table below represent the number of shares of common stock that a Public Warrant holder will receive upon such cashless exercise in connection with a redemption by us pursuant to this redemption feature, based on the “fair market value” of our common stock on the corresponding redemption date (assuming holders elect to exercise their Public Warrants and such Public Warrants are not redeemed for $0.10 per Public Warrant), determined for these purposes based on the volume-weighted average price of the common stock during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of Public Warrants, and the number of months that the corresponding redemption date precedes the expiration date of the Public Warrants, each as set forth in the table below.
We will provide our Public Warrant holders with the final fair market value no later than one business day after the 10-day trading period described above ends. The stock prices set forth in the column headings of the table below will be adjusted as of any date on which the number of shares issuable upon exercise of a Public Warrant or the exercise price of a Public Warrant is adjusted as set forth under the heading “Anti-dilution Adjustments” below. If the number of shares issuable upon exercise of a Public Warrant is adjusted, the adjusted stock prices in the column headings will equal the stock prices immediately prior to such adjustment, multiplied by a fraction, the numerator of which is the number of shares deliverable upon exercise of a Public Warrant immediately prior to such adjustment and the denominator of which is the number of shares deliverable upon exercise of a Public Warrant as so adjusted. The number of shares in the table below shall be adjusted in the same manner and at the same time as the number of shares issuable upon exercise of a Public Warrant.
If the exercise price of a Public Warrant is adjusted, (a) in the case of an adjustment pursuant to the fifth paragraph under the heading “Anti-dilution Adjustments” below, the adjusted share prices in the column headings will equal the unadjusted share price multiplied by a fraction, the numerator of which is the higher of the market value and the newly issued price as set forth under the heading “Anti-dilution Adjustments” and the denominator of which is $10.00 and (b) in the case of an adjustment pursuant to the second paragraph under the heading “Anti-dilution Adjustments” below, the adjusted share prices in the column headings will equal the unadjusted share price less the decrease in the exercise price of a Public Warrant pursuant to such exercise price adjustment.
| Redemption Fair Market Value of shares of Common Stock | ||||||||||||||||||||||||||||||||||||
Redemption Date | ≤$4.96 | $5.45 | $5.95 | $6.45 | $6.94 | $7.44 | $7.93 | $8.43 | ≥$8.93 | |||||||||||||||||||||||||||
84 months | 0.412 | 0.432 | 0.449 | 0.464 | 0.476 | 0.487 | 0.497 | 0.506 | 0.513 | |||||||||||||||||||||||||||
81 months | 0.409 | 0.429 | 0.447 | 0.462 | 0.475 | 0.486 | 0.496 | 0.505 | 0.513 | |||||||||||||||||||||||||||
78 months | 0.405 | 0.426 | 0.444 | 0.460 | 0.473 | 0.485 | 0.496 | 0.505 | 0.513 | |||||||||||||||||||||||||||
75 months | 0.400 | 0.422 | 0.441 | 0.458 | 0.472 | 0.484 | 0.495 | 0.505 | 0.513 | |||||||||||||||||||||||||||
160
Table of Contents
| Redemption Fair Market Value of shares of Common Stock | ||||||||||||||||||||||||||||||||||||
Redemption Date | ≤$4.96 | $5.45 | $5.95 | $6.45 | $6.94 | $7.44 | $7.93 | $8.43 | ≥$8.93 | |||||||||||||||||||||||||||
72 months | 0.396 | 0.419 | 0.438 | 0.455 | 0.470 | 0.483 | 0.494 | 0.504 | 0.513 | |||||||||||||||||||||||||||
69 months | 0.391 | 0.415 | 0.435 | 0.453 | 0.468 | 0.482 | 0.494 | 0.504 | 0.513 | |||||||||||||||||||||||||||
66 months | 0.386 | 0.411 | 0.432 | 0.450 | 0.466 | 0.480 | 0.493 | 0.503 | 0.513 | |||||||||||||||||||||||||||
63 months | 0.381 | 0.406 | 0.428 | 0.447 | 0.464 | 0.479 | 0.492 | 0.503 | 0.513 | |||||||||||||||||||||||||||
60 months | 0.375 | 0.401 | 0.424 | 0.444 | 0.462 | 0.477 | 0.491 | 0.503 | 0.513 | |||||||||||||||||||||||||||
57 months | 0.369 | 0.396 | 0.420 | 0.441 | 0.459 | 0.475 | 0.490 | 0.502 | 0.513 | |||||||||||||||||||||||||||
54 months | 0.362 | 0.391 | 0.415 | 0.437 | 0.457 | 0.474 | 0.488 | 0.501 | 0.513 | |||||||||||||||||||||||||||
51 months | 0.355 | 0.385 | 0.411 | 0.434 | 0.454 | 0.472 | 0.487 | 0.501 | 0.513 | |||||||||||||||||||||||||||
48 months | 0.347 | 0.378 | 0.405 | 0.429 | 0.451 | 0.469 | 0.486 | 0.500 | 0.513 | |||||||||||||||||||||||||||
45 months | 0.339 | 0.371 | 0.399 | 0.425 | 0.447 | 0.467 | 0.484 | 0.499 | 0.513 | |||||||||||||||||||||||||||
42 months | 0.330 | 0.363 | 0.393 | 0.420 | 0.443 | 0.464 | 0.482 | 0.499 | 0.513 | |||||||||||||||||||||||||||
39 months | 0.320 | 0.355 | 0.386 | 0.414 | 0.439 | 0.461 | 0.481 | 0.498 | 0.513 | |||||||||||||||||||||||||||
36 months | 0.309 | 0.345 | 0.378 | 0.408 | 0.434 | 0.458 | 0.478 | 0.497 | 0.513 | |||||||||||||||||||||||||||
33 months | 0.297 | 0.335 | 0.370 | 0.401 | 0.429 | 0.454 | 0.476 | 0.496 | 0.513 | |||||||||||||||||||||||||||
30 months | 0.284 | 0.324 | 0.360 | 0.393 | 0.423 | 0.450 | 0.473 | 0.494 | 0.513 | |||||||||||||||||||||||||||
27 months | 0.269 | 0.311 | 0.349 | 0.384 | 0.416 | 0.445 | 0.470 | 0.493 | 0.513 | |||||||||||||||||||||||||||
24 months | 0.253 | 0.296 | 0.337 | 0.374 | 0.408 | 0.439 | 0.467 | 0.491 | 0.513 | |||||||||||||||||||||||||||
21 months | 0.234 | 0.279 | 0.322 | 0.362 | 0.399 | 0.433 | 0.463 | 0.489 | 0.513 | |||||||||||||||||||||||||||
18 months | 0.214 | 0.260 | 0.306 | 0.349 | 0.389 | 0.425 | 0.458 | 0.487 | 0.513 | |||||||||||||||||||||||||||
15 months | 0.190 | 0.238 | 0.286 | 0.332 | 0.376 | 0.415 | 0.452 | 0.484 | 0.513 | |||||||||||||||||||||||||||
12 months | 0.162 | 0.212 | 0.262 | 0.312 | 0.360 | 0.404 | 0.444 | 0.481 | 0.513 | |||||||||||||||||||||||||||
9 months | 0.130 | 0.180 | 0.234 | 0.288 | 0.340 | 0.389 | 0.435 | 0.476 | 0.513 | |||||||||||||||||||||||||||
6 months | 0.092 | 0.142 | 0.197 | 0.256 | 0.315 | 0.371 | 0.423 | 0.470 | 0.513 | |||||||||||||||||||||||||||
3 months | 0.046 | 0.091 | 0.149 | 0.214 | 0.282 | 0.348 | 0.409 | 0.464 | 0.513 | |||||||||||||||||||||||||||
0 months | — | — | 0.060 | 0.163 | 0.254 | 0.331 | 0.399 | 0.459 | 0.513 | |||||||||||||||||||||||||||
The exact fair market value and redemption date may not be set forth in the table above, in which case, if the fair market value is between two values in the table or the redemption date is between two redemption dates in the table, the number of shares of common stock to be issued for each Public Warrant exercised will be determined by a straight-line interpolation between the number of shares set forth for the higher and lower fair market values and the earlier and later redemption dates, as applicable, based on a 365 or 366-day year, as applicable. For example, if the volume weighted average price of our common stock during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of the Public Warrants is $5.45 per share, and at such time there are 57 months until the expiration of the Public Warrants, holders may choose to, in connection with this redemption feature, exercise their Public Warrants for 0.396 shares of common stock for each whole Public Warrant. For an example where the exact fair market value and redemption date are not as set forth in the table above, if the volume weighted average price of our common stock during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of the Public Warrants is $5.70 per share, and at such time there are 58 months until the expiration of the Public Warrants, holders may choose to, in connection with this redemption feature, exercise their Public Warrants for 0.410 shares of common stock for each whole Public Warrant. In no event will the Public Warrants be exercisable on a cashless basis in connection with this redemption feature for more than 0.513 shares of common stock per Public Warrant (subject to adjustment). Finally, as reflected in the table above, if the Public Warrants are out of the money and about to expire, they cannot be exercised on a cashless basis in connection with a redemption by us pursuant to this redemption feature, since they will not be exercisable for any shares of common stock.
As stated above, we can redeem the Public Warrants when our common stock is trading at a price starting at $7.04, which is below the exercise price of $8.10, because it will provide certainty with respect to our capital
161
Table of Contents
structure and cash position while providing Public Warrant holders with the opportunity to exercise their Public Warrants on a cashless basis for the applicable number of shares. If we choose to redeem the Public Warrants when our common stock is trading at a price below the exercise price of the Public Warrants, this could result in the Public Warrant holders receiving fewer common stock than they would have received if they had chosen to wait to exercise their Public Warrants for common stock if and when such common stock was trading at a price higher than the exercise price of $8.10.
No fractional common stock will be issued upon exercise. If, upon exercise, a holder would be entitled to receive a fractional interest in a share, we will round down to the nearest whole number of the number of common stock to be issued to the holder. If, at the time of redemption, the Public Warrants are exercisable for a security other than the shares of common stock pursuant to the Warrant Agreement (for instance, if we are not the surviving company in our initial business combination), the Public Warrants may be exercised for such security. At such time as the Public Warrants become exercisable for a security other than the common stock, the company (or surviving company) will use its commercially reasonable efforts to register under the Securities Act the security issuable upon exercise of the Public Warrants.
Redemption Procedures
A holder of a Public Warrant may notify us in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such Public Warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the Public Warrant agent’s actual knowledge, would beneficially own in excess of 4.9% or 9.8% (as specified by the holder) of the shares of common stock outstanding immediately after giving effect to such exercise.
Anti-Dilution Adjustments
If the number of outstanding shares of common stock is increased by a stock capitalization or share dividend payable in shares of common stock, or by a split-up of shares of common stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of common stock issuable on exercise of each Public Warrant will be increased in proportion to such increase in the outstanding shares of common stock. A rights offering made to all, or substantially all, holders of common stock entitling holders to purchase shares of common stock at a price less than the “historical fair market value” (as defined below) will be deemed a stock dividend of a number of shares of common stock equal to the product of (i) the number of shares of common stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for common stock) and (ii) one (1) minus the quotient of (x) the price per share of common stock paid in such rights offering and (y) the historical fair market value. For these purposes, (i) if the rights offering is for securities convertible into or exercisable for common stock, in determining the price payable for common stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) “historical fair market value” means the volume weighted average price of common stock as reported during the ten (10) trading day period ending on the trading day prior to the first date on which the shares of common stock trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if we, at any time while the Public Warrants are outstanding and unexpired, pay a dividend or make a distribution in cash, securities or other assets to all or substantially all of the holders of the common stock on account of such common stock (or other securities into which the Public Warrants are convertible), other than (a) as described above, (b) any cash dividends or cash distributions which, when combined on a per share basis with all other cash dividends and cash distributions paid on the common stock during the 365-day period ending on the date of declaration of such dividend or distribution does not exceed $0.50 (as adjusted to appropriately reflect any other adjustments and excluding cash dividends or cash distributions that resulted in an adjustment to the exercise price or to the number of shares of common stock issuable on exercise of each Public Warrant) but
162
Table of Contents
only with respect to the amount of the aggregate cash dividends or cash distributions equal to or less than $0.50 per share, (c) to satisfy the redemption rights of the holders of common stock in connection with a proposed initial business combination, (d) to satisfy the redemption rights of the holders of common stock in connection with a stockholder vote to amend our amended and restated certificate of incorporation (A) to modify the substance or timing of our obligation to provide holders of our common stock the right to have their shares redeemed in connection with our initial business combination or to allow redemption in connection with our initial business combination or to redeem 100% of our public shares if we do not complete our initial business combination within 24 months from the closing of this offering or (B) with respect to any other provisions relating to stockholders’ rights or pre-initial business combination activity, or (e) in connection with the redemption of our public shares upon our failure to complete our initial business combination, then the Public Warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of common stock in respect of such event.
If the number of outstanding shares of our common stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of common stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of common stock issuable on exercise of each Public Warrant will be decreased in proportion to such decrease in outstanding shares of common stock.
Whenever the number of shares of common stock purchasable upon the exercise of the Public Warrants is adjusted, as described above, the Public Warrant exercise price will be adjusted by multiplying the Public Warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of common stock purchasable upon the exercise of the Public Warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of common stock so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding shares of common stock (other than those described above or that solely affects the par value of such shares of common stock), or in the case of any merger or consolidation of us with or into another corporation (other than a consolidation or merger in which we are the continuing corporation and that does not result in any reclassification or reorganization of our outstanding shares of common stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of us as an entirety or substantially as an entirety in connection with which we are dissolved, the holders of the Public Warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the Public Warrants and in lieu of the shares of our common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of common stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the Public Warrants would have received if such holder had exercised their Public Warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of common stock in such a transaction is payable in the form of common stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the Public Warrant properly exercises the Public Warrant within thirty (30) days following public disclosure of such transaction, the Public Warrant exercise price will be reduced as specified in the Warrant Agreement based on the Black-Scholes Public Warrant Value (as defined in the Warrant Agreement) of the Public Warrant.
The purpose of such exercise price reduction is to provide additional value to holders of the Public Warrants when an extraordinary transaction occurs during the exercise period of the Public Warrants pursuant to which the holders of the Public Warrants otherwise do not receive the full potential value of the Public Warrants.
The Public Warrants were issued in registered form under the Warrant Agreement between Continental Stock Transfer & Trust Company, as Public Warrant agent, and us. The Warrant Agreement provides that the
163
Table of Contents
terms of the Public Warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, and that all other modifications or amendments will require the vote or written consent of the holders of at least 50% of the then outstanding Public Warrants. You should review a copy of the Warrant Agreement, as amended, which is filed as an exhibit to the registration statement of which this prospectus is a part, for a complete description of the terms and conditions applicable to the Public Warrants.
The Public Warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to us, for the number of Public Warrants being exercised. The Public Warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise their Public Warrants and receive common stock. After the issuance of common stock upon exercise of the Public Warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
No fractional shares will be issued upon exercise of the Public Warrants. If, upon exercise of the Public Warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round down to the nearest whole number the number of shares of common stock to be issued to the Public Warrant holder.
We have agreed that, subject to applicable law, any action, proceeding or claim against us arising out of or relating in any way to the Warrant Agreement will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and we irrevocably submit to such jurisdiction, which jurisdiction will be the exclusive forum for any such action, proceeding or claim. This provision applies to claims under the Securities Act but does not apply to claims under the Exchange Act or any claim for which the federal district courts of the United States of America are the sole and exclusive forum.
Registration Rights
Investor Rights Agreement
In connection with the Closings, we and the Investors entered into the Investor Rights Agreement. Pursuant to the Investor Rights Agreement, upon the terms and subject to the conditions set forth therein, each Investor was granted certain registration rights with respect to his, her, or its shares of our common stock.
The Investor Rights Agreement restricts the ability of certain Investors to transfer all or a portion of their respective shares of our common stock (or any securities convertible into or exercisable or exchangeable for shares of our common stock), subject to certain permitted transfers, for a period of either 18 months or 12 months following the Closing Date, as applicable. The foregoing lock-up restrictions do not apply to: (a) any shares of our common stock purchased pursuant to the PIPE Subscription Agreements, (b) 100 shares of our common stock held by each Investor, (c) shares issued to the Sponsor in the Sponsor Loan Equity Issuance, (d) certain incremental shares of PIPE Investors who were existing Allurion Stockholders or existing holders of Allurion Convertible Notes or shares issued upon conversion of securities issued in the Incremental Financing and (e) the Backstop Shares, the Sponsor Contributed Shares, the Gaur Trust Contributed Shares or the shares of our common stock issued to each of HVL, RTW, Fortress and the other holders of Bridge Notes.
Additionally, pursuant to the Investor Rights Agreement, upon the terms and subject to the conditions set forth therein, the Board must consist of seven directors, a majority of which shall be “independent” directors for purposes of NYSE rules, and the following persons have the following nomination rights with respect to our Board, subject to the limitations set forth in the Investor Rights Agreement: (i) one director and one independent director nominated by Shantanu Gaur; (ii) one director and one independent director nominated by Remus Capital; (iii) one director nominated by the Sponsor; and (iv) two independent directors nominated by Allurion (one of which shall be designated by RTW).
164
Table of Contents
The Investor Rights Agreement will terminate upon the earlier of (i) the seventh anniversary of the Closing Date, (ii) a Change of Control (as defined in the Business Combination Agreement) or (iii) the date as of which there shall be no registrable securities outstanding; provided, that with respect to any Investor, such Investor will have no rights under the Investor Rights Agreement and all of our obligations to such Investor shall terminate upon the earlier of (x) the date at least one year after the Closing Date that such Investor ceases to hold at least one percent of the registrable securities outstanding on the Closing Date or (y) if such Investor is a one of our directors or executive officers, the date such Investor no longer serves as one of our directors or an executive officers. Notwithstanding the foregoing, (a) the piggy-back registration rights provided for in the Investor Rights Agreement shall terminate no later than the third anniversary of the Closing Date and (b) the obligations regarding the nomination of directors shall survive until the earlier of a termination of the Investor Rights Agreement in accordance with clauses (i) or (ii) above or with respect to any one Investor, at such time as such Investor is no longer entitled to nominate a director to our Board under the terms of the Investor Rights Agreement.
PIPE Subscription Agreements
The PIPE Subscription Agreements also provide that no later than 45 calendar days after the Closing Date, we were required to file a registration statement covering the resale of the PIPE Shares and to use our commercially reasonable efforts to have such registration statement declared effective as soon as practicable after the filing thereof but no later than the earlier of (i) 90 calendar days after the Closing Date (or 120 calendar days after the Closing Date if the SEC notifies us that it will “review” such registration statement) and (ii) the 10th business day after the date we are notified (orally or in writing, whichever is earlier) by the SEC that such Resale Registration Statement will not be “reviewed” or will not be subject to further review. Such resale registration statement was declared effective by the SEC on December 15, 2023.
Chardan Registration Rights Agreement
The Chardan Registration Rights Agreement provides for the registration of the offer and sale of the shares of our common stock issuable pursuant to the Chardan Purchase Agreement on a resale registration statement on Form S-1. Such registration statement was declared effective by the SEC on December 29, 2023.
Amended Note Purchase Agreement
Pursuant to the Amended Note Purchase Agreement, we issued and sold $48 million of Notes to RTW in a private placement. Until the Notes are converted or repaid in full, RTW will be entitled to designate one representative who will serve as a non-voting board observer to the Board of Directors. We also must include an additional director nominee in our proxy statement for the election of the Class I directors at our 2024 annual meeting of stockholders, with the recommendation of the Board of Directors to vote in favor of such additional nominee as well as the “RTW Designated Director” (as defined in the Investor Rights Agreement). Such additional director nominee shall go through our director nomination process led by the Nominating and Corporate Governance Committee. RTW shall have the right to approve such additional nominee, with such approval not to be unreasonably withheld. In addition, the Amended Note Purchase Agreement contains a covenant that we not increase the size of our Board of Directors to more than seven members prior to December 31, 2024 without RTW’s prior written consent, which may not be unreasonably withheld.
The Amended Note Purchase Agreement also provides that in no event later than 45 days after April 16, 2024, we must file with the SEC a registration statement covering the resale of the full amount of the shares of common stock underlying the Notes sold under the Amended Note Purchase Agreement. We must use commercially reasonable efforts to have such registration statement declared effective by the SEC as soon as practicable, but in no event later than the date which shall be either (a) in the event that the SEC does not review such registration statement, 90 days after April 16, 2024, or (b) in the event that the SEC reviews such registration statement, 120 days after the April 16, 2024 (but in any event, no later than three business days following the SEC indicating that it has no further comments on the registration statement).
165
Table of Contents
Anti-Takeover Provisions
Allurion Charter and Bylaws
Among other things, our Charter and Bylaws (as amended from time to time):
| • | permit our Board to issue up to 100 million shares of Preferred Stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change of control; |
| • | provide that the number of directors may be changed only by resolution of our Board; |
| • | provide that, subject to the rights of any series of preferred stock to elect directors, directors may be removed only with cause by the holders of at least 66 2/3% of all of our then- outstanding shares of the capital stock entitled to vote generally at an election of directors; |
| • | provide that all vacancies, subject to the rights of any series of Preferred Stock, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| • | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | provide that special meetings of stockholders may only be called by our Board pursuant to a resolution adopted by a majority of our Board, by the chairman of our Board or by the chief executive officer; |
| • | provide that our Board will be divided into three classes of directors, with the directors serving staggered three-year terms (see the section entitled “Management”), therefore making it more difficult for stockholders to change the composition of the Board; and |
| • | not provide for cumulative voting rights, therefore allowing the holders of a majority of the shares of our common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose. |
The combination of these provisions will make it more difficult for the existing stockholders to replace our Board as well as for another party to obtain control of Allurion by replacing our Board. Because our Board will have the power to retain and discharge its officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated Preferred Stock will make it possible for our Board to issue Preferred Stock with voting or other rights or preferences that could impede the success of any attempt to change the control of Allurion.
These provisions are intended to enhance the likelihood of continued stability in the composition of our Board and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our common stock.
Certain Anti-Takeover Provisions of Delaware Law
We are subject to the provisions of Section 203 of the DGCL. This statute prevents certain Delaware corporations, under certain circumstances, from engaging in a “business combination” with:
| • | a stockholder who owns 15% or more of our outstanding voting stock (otherwise known as an “interested stockholder”); |
| • | an affiliate of an interested stockholder; or |
166
Table of Contents
| • | an associate of an interested stockholder, for three years following the date that the stockholder became an interested stockholder. |
A “business combination” includes a merger or sale of more than 10% of a corporation’s assets. However, the above provisions of Section 203 would not apply if:
| • | the relevant Board approves the transaction that made the stockholder an “interested stockholder,” prior to the date of the transaction; |
| • | after the completion of the transaction that resulted in the stockholder becoming an interested stockholder, that stockholder owned at least 85% of the corporation’s voting stock outstanding at the time the transaction commenced, other than statutorily excluded shares of common stock; or |
| • | on or subsequent to the date of the transaction, the initial business combination is approved by the Board and authorized at a meeting of the corporation’s stockholders, and not by written consent, by an affirmative vote of at least two-thirds of the outstanding voting stock not owned by the interested stockholder. |
These provisions may have the effect of delaying, deferring, or preventing changes in control of Allurion.
Exclusive Forum
Our Charter provides that, unless we consent in writing to the selection of an alternative forum, the Chancery Court of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction the federal district court for the District of Delaware or other state courts of the State of Delaware) and any appellate court thereof shall, to the fullest extent permitted by law, be the sole and exclusive forum for: (1) any derivative action, suit or proceeding brought on our behalf; (2) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any of our current or former directors, officers, stockholders or employees of ours or our stockholders; (3) any action, suit or proceeding asserting a claim against us arising pursuant to any provision of the DGCL, our Bylaws or our Charter (as either may be amended from time to time); or (4) any action, suit or proceeding asserting a claim against us or any current or former director, officer or stockholder governed by the internal affairs doctrine.
Our Charter provides that the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. If any such foreign action is filed in a court other than the courts in the State of Delaware in the name of any stockholder, such stockholder shall be deemed to have consented to (a) the personal jurisdiction of the state and federal courts in the State of Delaware in connection with any action brought in any such court to enforce such actions and (b) having service of process made upon such stockholder in any such action by service upon such stockholder’s counsel in the foreign action as agent for such stockholder. Our Charter also provides that any person or entity purchasing or otherwise acquiring any interest in any of our securities shall be deemed to have notice of and consented to this choice of forum provision. It is possible that a court of law could rule that the choice of forum provision contained in our Charter is inapplicable or unenforceable if it is challenged in a proceeding or otherwise.
This choice of forum provision has important consequences for our stockholders. These provisions may limit or increase the difficultly of a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors and officers, or may increase the associated costs for such stockholder to bring a claim, both of which may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any applicable action brought against us, a court could find the choice of forum provisions contained in our Bylaws to be inapplicable or unenforceable in such action. If a court were to find the choice of forum provision inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm its business, results of operations, and financial condition.
167
Table of Contents
Transfer Agent and Warrant Agent
The Transfer Agent for our common stock and the warrant agent for our Public Warrants is Continental Stock Transfer & Trust Company.
Listing of our Common Stock and Public Warrants
Our common stock and Public Warrants are listed on the NYSE under the symbols “ALUR” and “ALUR WS,” respectively.
168
Table of Contents
SECURITIES ACT RESTRICTIONS ON RESALE OF OUR SECURITIES
Rule 144
Pursuant to Rule 144 under the Securities Act (“Rule 144”), a person who has beneficially owned restricted shares of our common stock or restricted Warrants for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of Allurion’s affiliates at the time of, or at any time during the three months preceding, a sale, and (ii) we are subject to the Exchange Act periodic reporting requirements for at least three months before the sale and have filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months (or such shorter period as we were required to file reports) preceding the sale.
Persons who have beneficially owned restricted shares of our common stock or restricted Warrants for at least six months but who are affiliates of Allurion at the time of, or at any time during the three months preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of:
| (1) | 1% of the total number of shares of our common stock or Warrants then outstanding, as applicable; and |
| (2) | the average weekly reported trading volume of our common stock or our Warrants, as applicable, during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales by Allurion’s affiliates under Rule 144 are also limited by manner of sale provisions and notice requirements and to the availability of current public information about Allurion.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for the resale of securities initially issued by shell companies (other than business-combination related shell companies) or issuers that have been at any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
| • | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| • | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| • | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials) other than Form 8-K reports; and |
| • | at least one year has elapsed from the time that the issuer filed current Form 10-type information with the SEC reflecting its status as an entity that is not a shell company. |
We are no longer a shell company and, as a result, once the conditions set forth in the exceptions listed above are satisfied, Rule 144 will become available for the resale of shares of common stock.
Registration Rights
Each of the Investor Rights Agreement, the PIPE Subscription Agreements, the Chardan Registration Rights Agreement and the Amended Note Purchase Agreement provide for certain registration rights. For additional information, see the subsections entitled “Description of Securities—Registration Rights.”
169
Table of Contents
PLAN OF DISTRIBUTION
The selling securityholder, which shall include donees, pledgees, transferees or other successors-in-interest selling shares of our common stock or interests in shares of our common stock received after the date of this prospectus from a selling securityholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their shares of common stock or interests in shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The selling securityholder may use any one or more of the following methods when disposing of shares or interests therein:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its own account; |
| • | to or through underwriters or broker-dealers; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | short sales effected after the date the registration statement of which this prospectus is a part is declared effective by the SEC; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| • | through agreements between broker-dealers and the selling securityholder to sell a specified number of such shares at a stipulated price per share; |
| • | a combination of any such methods of sale; and |
| • | any other method permitted by applicable law. |
The selling securityholder may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b) or other applicable provision of the Securities Act amending the list of selling securityholder to include the pledgee, transferee or other successors in interest as selling securityholder under this prospectus. The selling securityholder also may transfer the shares of common stock in other circumstances, in which case the pledgees, transferees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the sale of shares of our common stock or interests therein, the selling securityholder may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the shares of our common stock in the course of hedging the positions they assume. The selling securityholder may also sell shares of our common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling securityholder may also enter into options or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to each such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
170
Table of Contents
The aggregate proceeds to the selling securityholder from the sale of the shares of common stock offered by them will be the purchase price of the shares of common stock less discounts or commissions, if any. The selling securityholder reserves the right to accept and, together with its agents from time to time, to reject, in whole or in part, any proposed purchase of shares of common stock to be made directly or through agents. We will not receive any of the proceeds from this offering.
The selling securityholder also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act, provided that they meet the criteria and conform to the requirements of that rule.
The selling securityholder and any underwriters, broker-dealers or agents that participate in the sale of the shares of our common stock or interests therein may be “underwriters” within the meaning of Section 2(11) of the Securities Act. Any discounts, commissions, concessions or profit they earn on any resale of the shares may be underwriting discounts and commissions under the Securities Act. A selling securityholder who is an “underwriter” within the meaning of Section 2(11) of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act.
To the extent required, the shares of common stock to be sold, the name of the selling securityholder, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, and any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
In order to comply with the securities laws of some states, if applicable, the shares of common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states, the shares of common stock may not be sold unless they have been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have advised the selling securityholder that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the selling securityholder and its affiliates. In addition, to the extent applicable we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling securityholder for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The selling securityholder may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the selling securityholder against liabilities, including liabilities under the Securities Act and state securities laws, relating to the registration of the shares offered by this prospectus.
We have agreed with the selling securityholder to keep the registration statement of which this prospectus constitutes a part effective until the earlier of (1) such time as all of the shares covered by this prospectus have been disposed of pursuant to and in accordance with the registration statement and (2) the date on which all of the shares may be sold without restriction pursuant to Rule 144 of the Securities Act.
171
Table of Contents
LEGAL MATTERS
Goodwin Procter LLP has passed upon the validity of the shares of our common stock issued and issuable upon conversion of the Notes.
EXPERTS
The financial statements of Allurion Technologies, Inc. as of December 31, 2023 and 2022, and for each of the two years in the period ended December 31, 2023, included in this prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are included in reliance upon the report of such firm given their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1, including exhibits, under the Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus constitutes only a part of the registration statement. Some items are contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our securities, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or document referred to are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit.
In addition, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public on a website maintained by the SEC located at www.sec.gov. We also maintain a website at investors.allurion.com. Through our website, we make available, free of charge, annual, quarterly and current reports, proxy statements and other information as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained on, or that may be accessed through, our website is not part of, and is not incorporated into, this prospectus. Information contained on our website is not a part of or incorporated by reference into this prospectus and the inclusion of our website and investor relations website addresses in this prospectus is an inactive textual reference only.
172
Table of Contents
Page | ||||
Audited Consolidated Financial Statements as of December 31, 2023 | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
Interim Unaudited Financial Statements as of March 31, 2024 | ||||
| F-51 | ||||
| F-52 | ||||
| F-53 | ||||
| F-54 | ||||
| F-55 | ||||
| F-57 | ||||
December 31, | ||||||||
2023 | 2022 | |||||||
Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 38,037 | $ | 7,685 | ||||
| Accounts receivable, net of allowance of doubtful accounts of $12,671 and $741, respectively | 18,194 | 29,346 | ||||||
| Inventory, net | 6,171 | 3,865 | ||||||
| Prepaid expenses and other current assets | 2,414 | 2,487 | ||||||
| Total current assets | 64,816 | 43,383 | ||||||
| Property and equipment, net | 3,381 | 2,382 | ||||||
Right-of-use | 3,010 | 2,899 | ||||||
| Other long-term assets | 505 | 2,706 | ||||||
Total assets | $ | 71,712 | $ | 51,370 | ||||
Liabilities and Stockholders’ Deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 10,379 | $ | 5,809 | ||||
| Current portion of term loan | 38,643 | 53,360 | ||||||
| Current portion of lease liabilities | 908 | 905 | ||||||
| Accrued expenses and other current liabilities | 15,495 | 15,793 | ||||||
| Total current liabilities | 65,425 | 75,867 | ||||||
| Convertible notes payable, net of discounts | — | 3,103 | ||||||
| Public warrant liabilities | 5,943 | — | ||||||
| Revenue Interest Financing liability | 36,200 | — | ||||||
Earn-out liabilities | 23,990 | — | ||||||
| Lease liabilities, net of current portion | 2,306 | 2,163 | ||||||
| Other liabilities | 8,335 | 2,551 | ||||||
Total liabilities | 142,199 | 83,684 | ||||||
| Commitments and Contingencies (Note 16) | ||||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $0.0001 par value — 100,000,000 shares authorized as of December 31, 2023; and no shares issued and outstanding as of December 31, 2023 and December 31, 2022 | — | — | ||||||
Common stock, $0.0001 par value — 1,000,000,000 shares authorized as of December 31, 2023; 47,688,096 and 27,079,856 shares issued and outstanding as of December 31, 2023 and 2022, respectively | 5 | 3 | ||||||
Additional paid-in capital | 143,007 | 99,875 | ||||||
| Accumulated other comprehensive loss | (700 | ) | — | |||||
| Accumulated deficit | (212,799 | ) | (132,192 | ) | ||||
Total stockholders’ deficit | (70,487 | ) | (32,314 | ) | ||||
Total liabilities and stockholders’ deficit | $ | 71,712 | $ | 51,370 | ||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Revenue | $ | 53,467 | $ | 64,211 | ||||
| Cost of revenue | 11,970 | 13,485 | ||||||
| Gross profit | 41,497 | 50,726 | ||||||
| Operating expenses: | ||||||||
| Sales and marketing | 46,857 | 50,405 | ||||||
| Research and development | 27,694 | 16,966 | ||||||
| General and administrative | 46,024 | 15,365 | ||||||
| Total operating expenses: | 120,575 | 82,736 | ||||||
| Loss from operations | (79,078 | ) | (32,010 | ) | ||||
| Other income (expense): | ||||||||
| Interest expense | (10,566 | ) | (4,426 | ) | ||||
| Changes in fair value of warrants | 8,364 | (821 | ) | |||||
| Changes in fair value of debt | (3,751 | ) | — | |||||
| Changes in fair value of Revenue Interest Financing and PIPE Conversion Option | (2,192 | ) | — | |||||
Changes in fair value of earn-out liabilities | 29,050 | — | ||||||
| Termination of convertible note side letters | (17,598 | ) | — | |||||
| Loss on extinguishment of debt | (3,929 | ) | — | |||||
| Other income (expense), net | (643 | ) | (344 | ) | ||||
| Total other income (expense): | (1,265 | ) | (5,591 | ) | ||||
| Loss before income taxes | (80,343 | ) | (37,601 | ) | ||||
| Provision for income taxes | (264 | ) | (143 | ) | ||||
| Net loss | (80,607 | ) | (37,744 | ) | ||||
| Cumulative undeclared preferred dividends | (1,697 | ) | (2,907 | ) | ||||
| Net loss attributable to common shareholders | $ | (82,304 | ) | $ | (40,651 | ) | ||
| Net loss per share | ||||||||
| Basic and diluted | $ | (2.31 | ) | $ | (1.51 | ) | ||
| Weighted-average shares outstanding | ||||||||
| Basic and diluted | 35,581,656 | 26,918,484 | ||||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Net loss | (80,607 | ) | (37,744 | ) | ||||
| Other comprehensive loss: | ||||||||
| Change in fair value of Revenue Interest Financing due to change in credit risk | (700 | ) | — | |||||
| Comprehensive loss | $ | (81,307 | ) | $ | (37,744 | ) | ||
Common Sock | Additional Paid-in Capital | Accumulated Other Comprehensive Loss | Accumulated Deficit | Stockholders’ Deficit | ||||||||||||||||||||
Shares | Amount | |||||||||||||||||||||||
Balance as of December 31, 2021 | 26,807,365 | 3 | 99,282 | — | (94,448 | ) | 4,837 | |||||||||||||||||
| Exercise of stock options | 128,567 | — | 128 | — | — | 128 | ||||||||||||||||||
| Stock-based compensation expense | — | — | 437 | — | — | 437 | ||||||||||||||||||
Issuance of Legacy Series A-1 convertible preferred stock for the exercise of warrants | 5,611 | — | 24 | — | — | 24 | ||||||||||||||||||
| Issuance of Legacy Series B convertible preferred stock for the exercise of warrants | 1,028 | — | 4 | — | — | 4 | ||||||||||||||||||
| Issuance of common stock for the exercise of warrants | 137,285 | — | — | — | — | — | ||||||||||||||||||
| Net loss | — | — | — | — | (37,744 | ) | (37,744 | ) | ||||||||||||||||
Balance as of December 31, 2022 | 27,079,856 | 3 | 99,875 | — | (132,192 | ) | (32,314 | ) | ||||||||||||||||
| Exercise of stock options | 298,562 | — | 145 | — | — | 145 | ||||||||||||||||||
| Issuance of Series B convertible preferred stock for the exercise of warrants | 8,530 | — | 89 | — | — | 89 | ||||||||||||||||||
Issuance of Series A-1 convertible preferred stock for the exercise of warrants | 492 | — | 6 | — | — | 6 | ||||||||||||||||||
| Reverse recapitalization, net of transaction costs (Note 3) | 13,735,872 | 1 | 58,572 | — | — | 58,573 | ||||||||||||||||||
| Recognition of warrant liabilities in connection with the Merger (Note 3) | — | — | (13,762 | ) | — | — | (13,762 | ) | ||||||||||||||||
| Issuance of common stock in connection with vesting of RSU awards | 918,412 | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock for the conversion of convertible notes | 3,301,222 | 1 | 25,569 | — | — | 25,570 | ||||||||||||||||||
Recognition of earn-out liabilities (Note 3) | — | — | (53,040 | ) | — | — | (53,040 | ) | ||||||||||||||||
| Reclassification of Legacy Allurion liability classified warrants to equity classification | — | — | 929 | — | — | 929 | ||||||||||||||||||
| Derecognition of liabilities associated with the Backstop Shares, Hunter shares, and the additional RTW and Fortress shares and issuance of related shares | 2,287,696 | — | 16,098 | — | — | 16,098 | ||||||||||||||||||
| Issuance of common stock for the exercise of warrants | 21,943 | — | 46 | — | — | 46 | ||||||||||||||||||
| Stock-based compensation expense | — | — | 8,357 | — | — | 8,357 | ||||||||||||||||||
| Issuance of common stock for commitment shares for equity line financing (Note 12) | 35,511 | — | 123 | — | — | 123 | ||||||||||||||||||
| Other comprehensive loss | — | — | (700 | ) | — | (700 | ) | |||||||||||||||||
| Net loss | — | — | — | — | (80,607 | ) | (80,607 | ) | ||||||||||||||||
Balance as of December 31, 2023 | 47,688,096 | $ | 5 | $ | 143,007 | $ | (700 | ) | $ | (212,799 | ) | $ | (70,487 | ) | ||||||||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
Operating Activities: | ||||||||
| Net loss | $ | (80,607 | ) | $ | (37,744 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Non-cash lease expense | 824 | 1,104 | ||||||
| Depreciation and amortization | 746 | 895 | ||||||
| Stock-based compensation | 8,357 | 437 | ||||||
| Provision for uncollectible accounts | 12,675 | 436 | ||||||
| Unrealized exchange gain | (180 | ) | (113 | ) | ||||
| Provision for inventory | 1,399 | — | ||||||
| Change in fair value of warrant liabilities | (8,364 | ) | 820 | |||||
| Change in fair value of derivative liabilities | 1,730 | 19 | ||||||
| Change in fair value of debt | 3,751 | — | ||||||
| Change in fair value of Revenue Interest Financing and PIPE Conversion Option | 2,192 | — | ||||||
Change in fair value of earn-out liabilities | (29,050 | ) | — | |||||
| Interest paid on debt recorded at fair value | (1,092 | ) | — | |||||
Non-cash interest expense | 2,083 | 953 | ||||||
Non-cash termination of convertible note side letters | 16,098 | — | ||||||
| Loss on extinguishment of debt | 3,929 | — | ||||||
Non-cash issuance of common stock for commitment shares | 123 | — | ||||||
| Debt issuance costs associated with debt recorded at fair value | 1,210 | — | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (1,318 | ) | (22,817 | ) | ||||
| Inventory | (3,705 | ) | (1,150 | ) | ||||
| Prepaid expenses, other current and long-term assets | 285 | (577 | ) | |||||
| Lease liabilities | (789 | ) | (733 | ) | ||||
| Accounts payable | 4,664 | 3,324 | ||||||
| Accrued expenses and other current liabilities | 1,057 | 8,165 | ||||||
| Net cash used in operating activities | $ | (63,982 | ) | $ | (46,981 | ) | ||
Investing Activities: | ||||||||
| Purchases of property and equipment | (1,606 | ) | (1,550 | ) | ||||
| Net cash used in investing activities | $ | (1,606 | ) | $ | (1,550 | ) | ||
Financing Activities: | ||||||||
| Proceeds from issuance of convertible notes - net | 28,700 | 1,103 | ||||||
| Proceeds from term loan - net | 59,780 | 29,850 | ||||||
| Payment of debt issuance costs | (3,450 | ) | (262 | ) | ||||
| Proceeds from Business Combination, net of transaction costs | 61,652 | — | ||||||
| Proceeds from Revenue Interest Financing | 40,000 | — | ||||||
| Repayment of 2021 Term Loan | (57,659 | ) | — | |||||
| Repayment of Fortress Term Loan | (20,000 | ) | — | |||||
| Repayment of promissory note assumed in Business Combination | (2,500 | ) | — | |||||
| Proceeds from option and warrant exercises | 213 | 132 | ||||||
| Repayment of convertible notes | (10,750 | ) | — | |||||
| Payment of deferred financing costs | — | (286 | ) | |||||
| Net cash provided by financing activities | $ | 95,986 | $ | 30,537 | ||||
| Net increase (decrease) in cash and cash equivalents and restricted cash | 30,398 | (17,994 | ) | |||||
| Cash and cash equivalents and restricted cash at beginning of period | 8,023 | 26,017 | ||||||
| Cash and cash equivalents and restricted cash at end of period | $ | 38,421 | $ | 8,023 | ||||
Supplemental disclosure of cash flow information | ||||||||
| Cash paid for interest | $ | 8,035 | $ | 3,476 | ||||
Supplemental cash flow information on non-cash investing and financing activities | ||||||||
| Purchase of property and equipment included in accounts payable | 134 | 13 | ||||||
| Issuance of warrants in connection with financing | — | 834 | ||||||
| Deferred financing costs in accounts payable and accrued expenses | 580 | 1,919 | ||||||
| Recognition of assumed warrant liability | 13,762 | — | ||||||
Recognition of earn-out liabilities | 53,040 | — | ||||||
| Issuance of common stock upon conversion of convertible notes | 25,569 | — | ||||||
| Change in fair value of Revenue Interest Financing through OCI | (700 | ) | — | |||||
1. | Organization and Basis of Presentation |
2. | Summary of Significant Accounting Policies |
Revenue | Accounts Receivable | |||||||||||||||
Years Ended December 31 | December 31, | |||||||||||||||
2023 | 2022 | 2023 | 2022 | |||||||||||||
| Customer A | 10 | % | N/A | 16 | % | N/A | ||||||||||
| Customer B | N/A | 11 | % | N/A | N/A | |||||||||||
| Customer C | N/A | N/A | N/A | 13 | % | |||||||||||
| Customer D | N/A | N/A | N/A | 12 | % | |||||||||||
December 31, | ||||||||
2023 | 2022 | |||||||
| Cash and cash equivalents | $ | 38,037 | $ | 7,685 | ||||
Restricted cash included in other long-term assets | 384 | 338 | ||||||
| Cash and cash equivalents and restricted cash shown in the statement of cash flows | $ | 38,421 | $ | 8,023 | ||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Balance at beginning of period | $ | (741 | ) | $ | (354 | ) | ||
| Provision for uncollectible accounts | (12,675 | ) | (436 | ) | ||||
| Uncollectible accounts written off | 745 | 49 | ||||||
| Balance at end of period | $ | (12,671 | ) | $ | (741 | ) | ||
3. | Business Combination |
December 31, 2023 | ||||
| Cash – CPUH trust (net of redemptions) | $ | 38,395 | ||
| Cash – PIPE Investors | 37,922 | |||
| Gross Proceeds | 76,317 | |||
| Less: transaction costs paid | (14,665 | ) | ||
| Net proceeds from the Business Combination (1) | 61,652 | |||
| Less: warrant liabilities assumed (2) | (13,762 | ) | ||
| Less: repayment of note assumed in the Business Combination (1) | (2,500 | ) | ||
| Less: accrued transaction costs at December 31, 2023 (1) | (580 | ) | ||
| Business Combination, net of transaction costs | $ | 44,810 | ||
| (1) | The Net proceeds from the Business Combination, less the repayment of note assumed in the Business Combination, less the accrued transaction costs at December 31, 2023 are presented net in the consolidated statements of stockholders deficit within line “Reverse recapitalization, net of transaction costs (Note 3)”. |
| (2) | The warrant liabilities assumed are presented separately from the “Reverse recapitalization, net of transaction costs (Note 3)” line within the consolidated statements of stockholders deficit. |
Common Stock | ||||
| Legacy Allurion Equityholders (1) | 27,897,387 | |||
| CPUH Stockholders (2) | 5,165,698 | |||
| Shares Issued to PIPE Investors (2) | 5,386,695 | |||
| Shares issued to RTW and Fortress (3) | 1,900,000 | |||
| Shares issued to convertible note holders | 3,301,222 | |||
| CPUH Sponsor Shares (2) | 3,262,711 | |||
| Side Letter Termination Shares (3) | 387,696 | |||
| Total shares of Common Stock immediately after Business Combination | 47,301,409 | |||
| (1) | Consists of Legacy Allurion common stock and Legacy Allurion preferred stockholders, plus the issuance of common stock in connection with the vesting of RSUs at closing, less the Gaur Contributed Shares (as defined below). |
| (2) | The CPUH Stockholders shares, PIPE shares, and CPUH Sponsor shares are presented combined within the consolidated statements of redeemable convertible preferred stock and stockholders deficit on the “Reverse recapitalization, net of transaction costs” line, which is less the Gaur Contributed Shares (as defined below). |
| (3) | The shares issued to RTW and Fortress and the Side Letter Termination shares are presented combined within the consolidated statements of redeemable convertible preferred stock and stockholders deficit on the “Derecognition of liabilities associated with the Backstop Shares, Hunter shares, and additional RTW and Fortress shares and issuance of related shares” line. |
4. | Revenue |
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| France | $ | 5,569 | $ | 6,032 | ||||
| Turkey | 5,494 | 4,079 | ||||||
| Spain | 4,618 | 6,852 | ||||||
| Chile | 2,708 | 5,008 | ||||||
| All other countries | 35,078 | 42,240 | ||||||
| Total Revenues | $ | 53,467 | $ | 64,211 | ||||
5. | Inventory |
December 31, | ||||||||
2023 | 2022 | |||||||
| Finished goods | $ | 3,427 | $ | 2,096 | ||||
| Work in progress | 967 | 213 | ||||||
| Raw materials | 1,777 | 1,556 | ||||||
| Total Inventory | $ | 6,171 | $ | 3,865 | ||||
6. | Property and Equipment, net |
Estimates Useful Life (in Years) | December 31, | |||||||||
2023 | 2022 | |||||||||
| Computers and purchased software | 3 | $ | 618 | $ | 575 | |||||
| Leasehold improvements | Shorter of useful life or lease term | 1,943 | 1,822 | |||||||
| Furniture and fixtures | 5 | 291 | 251 | |||||||
| Machinery and equipment | 3-5 | 2,893 | 2,002 | |||||||
| Property and equipment—at cost | 5,745 | 4,650 | ||||||||
| Less accumulated depreciation and amortization | (3,559 | ) | (2,851 | ) | ||||||
| Construction in progress | 1,195 | 583 | ||||||||
| Property and equipment—net | $ | 3,381 | $ | 2,382 | ||||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Cost of revenue | $ | 367 | $ | 568 | ||||
| Research and development | 179 | 90 | ||||||
| General and administrative | 138 | 160 | ||||||
| Sales and marketing | 62 | 57 | ||||||
| Total depreciation and amortization expense | $ | 746 | $ | 875 | ||||
7. | Accrued Expenses and Other Current Liabilities |
December 31, | ||||||||
2023 | 2022 | |||||||
| Distributor fees and marketing reimbursements | $ | 2,834 | $ | 6,348 | ||||
| Accrued compensation | 1,687 | 3,453 | ||||||
| Accrued clinical trials and R&D | 3,694 | 228 | ||||||
| Accrued selling and marketing | 1,110 | 481 | ||||||
| Accrued professional fees | 1,505 | 2,105 | ||||||
| Accrued interest | — | 489 | ||||||
| Accrued warranty | 44 | 48 | ||||||
| Accrued restructuring | 655 | — | ||||||
| Other accrued expenses | 3,966 | 2,641 | ||||||
| Total accrued expenses and other current liabilities | $ | 15,495 | $ | 15,793 | ||||
8. | Debt |
December 31, | ||||||||
2023 | 2022 | |||||||
| Fortress Term Loan | $ | 43,100 | $ | — | ||||
| 2021 Term Loan | — | 55,000 | ||||||
| Convertible Notes | — | 3,103 | ||||||
| Total principal amounts of debt | 43,100 | 58,103 | ||||||
| Plus: Accretion | 148 | 213 | ||||||
| Less: current portion of long-term debt, net of discounts | (38,643 | ) | (53,360 | ) | ||||
| Less: unamortized deferred financing costs and debt discounts | (4,605 | ) | (1,853 | ) | ||||
| Long-term debt, net of current portion and discounts | $ | — | $ | 3,103 | ||||
| December 31, 2024 | — | |||
| December 31, 2025 | — | |||
| December 31, 2026 | 8,979 | |||
| December 31, 2027 | 34,121 | |||
| $ | 43,100 |
9. | Revenue Interest Financing, Side Letter, and PIPE Conversion Option |
December 31, 2023 | ||||
| Revenue Interest Financing liability | $ | 40,000 | ||
| Total principal amounts of debt | 40,000 | |||
| Less: Royalty payments | (1,092 | ) | ||
| Less: Change in fair value of debt | (2,708 | ) | ||
| Long-term Revenue Interest Financing liability | $ | 36,200 | ||
10. | Fair Value Measurements |
Fair Value Measurement as of December 31, 2023 | ||||||||||||||||
Total Carrying Value | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets: | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 30,582 | $ | 30,582 | $ | — | $ | — | ||||||||
| Total assets | $ | 30,582 | $ | 30,582 | $ | — | $ | — | ||||||||
Liabilities: | ||||||||||||||||
| Legacy Allurion Common Stock Warrant Liabilities | $ | 821 | $ | — | $ | — | $ | 821 | ||||||||
| Public Warrants | 5,943 | 5,943 | — | — | ||||||||||||
| Revenue Interest Financing | 36,200 | — | — | 36,200 | ||||||||||||
| PIPE Conversion Option | 5,600 | — | — | 5,600 | ||||||||||||
Earn-out Liability | 23,990 | — | — | 23,990 | ||||||||||||
| Term Loan Derivative Liability | 1,895 | — | — | 1,895 | ||||||||||||
| Success Fee Derivative Liability | 14 | — | — | 14 | ||||||||||||
| Total Liabilities | $ | 74,463 | $ | 5,943 | $ | — | $ | 68,520 | ||||||||
Fair Value Measurement as of December 31, 2022 | ||||||||||||||||
Total Carrying Value | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets: | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 4,925 | $ | 4,925 | $ | — | $ | — | ||||||||
| Total assets | $ | 4,925 | $ | 4,925 | $ | — | $ | — | ||||||||
Liabilities: | ||||||||||||||||
| Legacy Allurion Series C Common Stock Warrant Liability | $ | 340 | $ | — | $ | — | $ | 340 | ||||||||
| Legacy Allurion Series B Preferred Stock Warrant Liability | 303 | — | — | 303 | ||||||||||||
Legacy Allurion Series A-1 Preferred Stock Warrant Liability | 82 | — | — | 82 | ||||||||||||
| Other Common Stock Warrant Liabilities | 255 | — | — | 255 | ||||||||||||
| Legacy Allurion Series C Preferred Stock Warrant Liability | 684 | — | — | 684 | ||||||||||||
| Derivative Liability-Success Fee | 180 | — | — | 180 | ||||||||||||
Legacy Allurion Series D-1 Preferred Stock Warrant Liability | 707 | — | — | 707 | ||||||||||||
| Total Liabilities | $ | 2,551 | $ | — | $ | — | $ | 2,551 | ||||||||
Measurement Date | Interest Rate | Exercise Price | Estimated Fair Value of Underlying Share Price | Expected Volatility | Expected Life (Years) | |||||||||||||||||||
| Legacy Allurion Series C Preferred Stock warrants (as converted to Common) | December 31, 2023 | 3.88 | % | 6.73 | 3.74 | 100 | % | 7.25 | ||||||||||||||||
| Other Common Stock | December 31, 2023 | 3.95 | % | 1.05 | 3.74 | 100 | % | 3.69 | ||||||||||||||||
Legacy Allurion Series D-1 Preferred Stock warrants (as converted to Common) | December 31, 2023 | 3.88 | % | 12.14 | 3.74 | 100 | % | 7.25 - 8.71 | ||||||||||||||||
Legacy Allurion Series A-1 Preferred Stock warrants (as converted to Rollover warrants) | December 31, 2022 | 4.42 | % | $ | 1.90 | $ | 6.75 | 69 | % | 0.25 | ||||||||||||||
| Legacy Allurion Series B Preferred Stock warrants (as converted to Rollover warrants) | December 31, 2022 | 4.41 | % | 2.38 | 6.91 | 65 | % | 2.00 | ||||||||||||||||
| Legacy Allurion Series C Common Stock warrants (as converted to Rollover warrants) | December 31, 2022 | 4.11 | % | 0.01 | 4.54 | 63 | % | 4.00 | ||||||||||||||||
| Legacy Allurion Series C Preferred Stock warrants (as converted to Rollover warrants) | December 31, 2022 | 3.92 | % | 6.58 | 7.24 | 63 | % | 8.20 | ||||||||||||||||
| Other Common Stock Warrants | December 31, 2022 | 3.99 | % | 1.02 | 4.54 | 63 | % | 4.6 - 4.7 | ||||||||||||||||
Legacy Allurion Series D-1 Preferred Stock warrants (as converted to Rollover warrants) | December 31, 2022 | 3.88 -3.92 | % | 11.87 | 11.31 | 63 | % | 8.2 - 9.7 | ||||||||||||||||
Preferred Stock Warrants (as converted to Common) | Common Stock Warrants | Total | ||||||||||
| Balance – January 1, 2022 | $ | 510 | $ | 231 | $ | 741 | ||||||
| Fair value upon issuance | 834 | — | 834 | |||||||||
Change in fair value | 456 | 365 | 821 | |||||||||
| Exercise of warrants | (23 | ) | — | (23 | ) | |||||||
| Balance – December 31, 2022 | $ | 1,777 | $ | 596 | $ | 2,373 | ||||||
Change in fair value | (720 | ) | 172 | (548 | ) | |||||||
| Exercise of warrants | (75 | ) | — | (75 | ) | |||||||
| Derecognition of liability to equity | (340 | ) | (589 | ) | (929 | ) | ||||||
| Balance – December 31, 2023 | $ | 642 | $ | 179 | $ | 821 | ||||||
December 31, 2023 | ||||
| Stock Price | 3.74 | |||
| Risk-free interest rate | 4.46 | % | ||
| Expected term (in years) | 1.6 | |||
| Expected volatility | 82.5 | % | ||
December 31, 2023 | ||||
| Stock Price | 3.74 | |||
| Risk-free interest rate | 3.9 | % | ||
| Expected term (in years) | 4.6 | |||
| Expected volatility | 87.0 | % | ||
Success Fee Derivative Liability | 2023 Convertible Notes | PubCo Share Liability | Base PubCo & Backstop Share Liability | Revenue Interest Financing | PIPE Conversion Derivative | Earn-Out Liability | Term Loan Derivative Liability | Total | ||||||||||||||||||||||||||||
| Balance – January 1, 2022 | $ | 159 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 159 | ||||||||||||||||||
| Fair value upon issuance | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Change in fair value | 19 | — | — | — | — | — | — | — | 19 | |||||||||||||||||||||||||||
| Exercise of warrants | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Balance – December 31, 2022 | $ | 178 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 178 | ||||||||||||||||||
| Fair value upon issuance | — | $ | 28,700 | 3,370 | 3,264 | 40,000 | 3,340 | 53,040 | 1,895 | 133,609 | ||||||||||||||||||||||||||
| Change in fair value | (164 | ) | 3,751 | (642 | ) | 10,106 | (3,408 | ) | 2,260 | (29,050 | ) | — | (17,147 | ) | ||||||||||||||||||||||
| Change in fair value – OCI | — | — | — | — | 700 | — | — | — | 700 | |||||||||||||||||||||||||||
| Payments | — | (10,750 | ) | — | — | (1,092 | ) | — | — | — | (11,842 | ) | ||||||||||||||||||||||||
| Derecognition of liability to equity | — | (21,701 | ) | (2,728 | ) | (13,370 | ) | — | — | — | — | (37,799 | ) | |||||||||||||||||||||||
| Balance – December 31, 2023 | $ | 14 | $ | — | $ | — | $ | — | $ | 36,200 | $ | 5,600 | $ | 23,990 | $ | 1,895 | $ | 67,699 | ||||||||||||||||||
11. | Income Taxes |
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| U.S. | $ | (81,259 | ) | $ | (38,267 | ) | ||
| Foreign | 916 | 666 | ||||||
| Loss before income taxes | $ | (80,343 | ) | $ | (37,601 | ) | ||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| U.S. statutory federal income tax rate | 21.0 | % | 21.0 | % | ||||
| State income taxes, net of federal income tax benefit | 7.1 | % | 6.9 | % | ||||
| Change in fair value of financial instruments | 3.0 | % | 0.0 | % | ||||
| Tax credits | 0.4 | % | 1.0 | % | ||||
| Valuation allowance | (30.5 | )% | (29.0 | )% | ||||
Non-deductible expenses | (0.9 | )% | 0.0 | % | ||||
| Other | (0.4 | )% | (0.2 | )% | ||||
| Effective tax rate | (0.3 | )% | (0.3 | )% | ||||
December 31, | ||||||||
2023 | 2022 | |||||||
| U.S. federal and state net operating loss carryforwards | $ | 36,092 | $ | 25,051 | ||||
Capitalized start-up and research and development expenses | 10,868 | 5,377 | ||||||
| Research and development tax credits | 2,275 | 1,780 | ||||||
| Interest expense | 3,918 | — | ||||||
| Lease liability | 659 | 838 | ||||||
| Depreciation | 203 | 236 | ||||||
| Bad debt reserve | 3,461 | — | ||||||
| Other temporary differences | 2,145 | 994 | ||||||
| Total deferred tax assets | 59,621 | 34,276 | ||||||
| Valuation allowance | (57,985 | ) | (33,484 | ) | ||||
| Net deferred tax assets | 1,636 | 792 | ||||||
| Right of use asset | (623 | ) | (792 | ) | ||||
| Other deferred tax liability | (1,013 | ) | — | |||||
| Total deferred tax liabilities | (1,636 | ) | (792 | ) | ||||
| Net deferred tax asset | $ | — | $ | — | ||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Beginning balances | $ | 33,484 | $ | 22,579 | ||||
| Additions charged to net loss | 24,501 | 10,905 | ||||||
| Ending balances | $ | 57,985 | $ | 33,484 | ||||
12. | Capital Stock and Stockholders’ Deficit |
Preferred Stock Authorized (1) | Preferred Stock Issued and Outstanding | Carrying Value | ||||||||||
| Series A Preferred Stock | 2,276,786 | 2,276,786 | $ | 1,542 | ||||||||
Series A-1 Preferred Stock | 1,513,028 | 1,486,048 | 2,842 | |||||||||
| Series B Preferred Stock | 2,298,929 | 2,245,515 | 5,253 | |||||||||
| Series C Preferred Stock | 8,113,616 | 7,927,446 | 39,122 | |||||||||
Series D-1 Preferred Stock | 1,684,565 | 842,283 | 9,614 | |||||||||
Series D-2 Preferred Stock | 3,644,616 | 3,644,616 | 24,054 | |||||||||
Series D-3 Preferred Stock | 1,498,348 | 1,498,348 | 14,789 | |||||||||
Total | 21,029,888 | 19,921,042 | $ | 97,216 | ||||||||
| Outstanding options to purchase common stock | 3,886,038 | |||
| Restricted Stock Units | 643,635 | |||
| Warrants to purchase preferred stock (as converted to warrants to purchase common stock) | 138,857 | |||
| Warrants to purchase common stock | 264,801 | |||
| Public warrants to purchase common stock | 18,759,838 | |||
Earn-Out shares | 9,000,000 | |||
| Total | 32,693,169 | |||
December 31, 2023 | ||||||||||||||||||
Issuance Date | Remaining Contractual Term (in years) | Underlying Equity Instrument | Balance Sheet Classification | Shares Issuable Upon Exercise of Warrant | Weighted Average Exercise Price | |||||||||||||
| 12/1/2014 | 0.9 | Common stock | Equity | 44,272 | $ | 2.44 | ||||||||||||
| 3/30/2021 | 7.2 | Common stock | Liability | 130,053 | 6.73 | |||||||||||||
| 9/15/2022 | 8.7 | Common stock | Liability | 45,238 | 12.14 | |||||||||||||
| 6/4/2022 | 8.4 | Common stock | Liability | 45,238 | 12.14 | |||||||||||||
| 1/17/2017 | 3.0 | Common stock | Equity | 73,349 | 0.02 | |||||||||||||
| 8/3/2017 | 3.6 | Common stock | Equity | 9,779 | 1.13 | |||||||||||||
| 9/8/2017 | 3.7 | Common stock | Liability | 28,764 | 1.05 | |||||||||||||
| 6/19/2018 | 4.5 | Common stock | Liability | 17,977 | 1.05 | |||||||||||||
| 6/25/2019 | 5.5 | Common stock | Liability | 8,988 | 1.05 | |||||||||||||
| 403,658 | ||||||||||||||||||
December 31, 2022 | ||||||||||||||||||
Issuance Date | Remaining Contractual Term (in years) | Underlying Equity Instrument | Balance Sheet Classification | Shares Issuable Upon Exercise of Warrant | Weighted Average Exercise Price | |||||||||||||
| 4/1/2013 | 0.2 | Series A-1 Preferred Stock | Liability | 16,557 | $ | 1.95 | ||||||||||||
| 12/1/2014 | 1.9 | Series B Preferred Stock | Liability | 60,768 | 2.44 | |||||||||||||
| 3/30/2021 | 8.2 | Series C Preferred Stock | Liability | 130,053 | 6.73 | |||||||||||||
| 9/15/2022 | 9.7 | Series D-1 Preferred Stock | Liability | 45,238 | 12.14 | |||||||||||||
| 6/4/2022 | 9.4 | Series D-1 Preferred Stock | Liability | 45,238 | 12.14 | |||||||||||||
| 1/17/2017 | 4.0 | Common stock | Liability | 73,349 | 0.02 | |||||||||||||
| 8/3/2017 | 4.6 | Common stock | Liability | 9,779 | 1.13 | |||||||||||||
| 9/8/2017 | 4.7 | Common stock | Liability | 28,764 | 1.05 | |||||||||||||
| 6/19/2018 | 5.5 | Common stock | Liability | 17,977 | 1.05 | |||||||||||||
| 6/25/2019 | 6.5 | Common stock | Liability | 8,988 | 1.05 | |||||||||||||
| 436,711 | ||||||||||||||||||
13. | Net Loss per Share |
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Numerator: | ||||||||
| Net loss | $ | (80,607 | ) | $ | (37,744 | ) | ||
| Cumulative undeclared dividends to participating securities (Legacy Series D convertible preferred stock) | (1,697 | ) | (2,907 | ) | ||||
| Net loss attributable to common stockholders | $ | (82,304 | ) | $ | (40,651 | ) | ||
| Denominator: | ||||||||
| Basic and diluted weighted-average common stock outstanding | 35,581,656 | 26,918,484 | ||||||
| Net loss per share attributable to common stockholders, basic and diluted (1) | $ | (2.31 | ) | $ | (1.51 | ) | ||
| (1) | The weighted-average common shares and thus net loss per share calculations and potentially dilutive security amounts for all periods prior to the Business Combination have been retrospectively adjusted to the equivalent number of shares outstanding immediately after the Business Combination to effect the reverse capitalization. See Note 3 for further information. |
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Outstanding options to purchase common stock | 3,886,038 | 4,301,960 | ||||||
| Restricted Stock Units | 643,635 | 1,415,104 | ||||||
| Warrants to purchase preferred stock (as converted to warrants to purchase common stock) | 138,857 | 297,854 | ||||||
| Warrants to purchase common stock | 264,801 | 138,857 | ||||||
| Shares of Common Stock issued upon the exercise of Public Warrants | 18,759,838 | — | ||||||
Earn-Out Shares | 9,000,000 | — | ||||||
| Convertible notes (as converted to common stock) | — | 171,256 | ||||||
| Total | 32,693,169 | 6,325,031 | ||||||
14. | Stock Based Compensation |
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Cost of revenue | $ | 32 | $ | — | ||||
| Selling, general, and administrative | 8,198 | 400 | ||||||
| Research and development | 127 | 37 | ||||||
| Total stock-based compensation expense | $ | 8,357 | $ | 437 | ||||
Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||||
(per option) | (in years) | (in thousands) | ||||||||||||||
| Outstanding—January 1, 2023 | 4,301,968 | $ | 2.35 | 7.7 | $ | 9,437 | ||||||||||
| Granted | 257,683 | $ | 5.41 | |||||||||||||
| Cancellations and forfeitures | (375,051 | ) | $ | 2.00 | ||||||||||||
| Exercised | (298,562 | ) | $ | 1.15 | ||||||||||||
| Outstanding—December 31, 2023 | 3,886,038 | $ | 2.67 | 6.9 | $ | 5,565 | ||||||||||
| Exercisable at December 31, 2023 | 2,735,884 | $ | 2.23 | 6.2 | $ | 4,812 | ||||||||||
Year Ended December 31, | ||||||||
2023 | 2022 | |||||||
| Expected volatility | 85.8 | % | 63 | % | ||||
| Risk-free interest rate | 4.5 | % | 3.56 | % | ||||
| Expected dividend yield | — | % | — | % | ||||
| Expected term (in years) | 5.8 | 5.8 | ||||||
Number of RSUs | Weighted Average Grant Date Fair Value | |||||||
(per share) | ||||||||
| Outstanding—January 1, 2023 | 1,415,104 | $ | 4.51 | |||||
| Granted | 226,175 | 4.32 | ||||||
| Cancellations and forfeitures | (79,232 | ) | 4.51 | |||||
| Vested | (918,412 | ) | 4.51 | |||||
| Outstanding—December 31, 2023 | 643,635 | 4.45 | ||||||
15. | Employee Benefit Plan |
16. | Commitments and Contingencies |
December 31, 2023 | December 31, 2022 | |||||||
| Operating lease costs | $ | 1,123 | $ | 918 | ||||
| Short-term lease costs | 12 | 14 | ||||||
| Variable operating lease costs | 187 | 191 | ||||||
| Operating cash flows paid for amounts in the measurement of lease liabilities | 1,084 | 807 | ||||||
| Operating lease assets obtained in exchange for lease obligations | 936 | 1,677 | ||||||
| 2024 | $ | 1,173 | ||
| 2025 | 1,114 | |||
| 2026 | 737 | |||
| 2027 | 645 | |||
| 2028 | 108 | |||
| Total lease payments | $ | 3,777 | ||
| Less: present value adjustment | (563 | ) | ||
| Present value of total lease liabilities | 3,214 | |||
| Less: current lease liability | (908 | ) | ||
| Long-term lease liabilities | $ | 2,306 | ||
2023 | 2022 | |||||||
| Weighted-average remaining lease term (in years) | 3.5 | 3.9 | ||||||
| Weighted-average discount rate | 9.9 | % | 9.5 | % | ||||
17. | Geographic Information |
December 31, | ||||||||
2023 | 2022 | |||||||
| United States | $ | 5,381 | $ | 3,999 | ||||
| France | 1,010 | 1,282 | ||||||
| All other countries | — | — | ||||||
| Long-lived assets | $ | 6,391 | $ | 5,281 | ||||
18. | Related-party Transactions |
March 31, 2024 | December 31, 2023 | |||||||
Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 29,682 | $ | 38,037 | ||||
| Accounts receivable, net of allowance of doubtful accounts of $12,671 and $12,671, respectively | 16,159 | 18,194 | ||||||
| Inventory, net | 5,631 | 6,171 | ||||||
| Prepaid expenses and other current assets | 2,167 | 2,414 | ||||||
| Total current assets | 53,639 | 64,816 | ||||||
| Property and equipment, net | 3,180 | 3,381 | ||||||
Right-of-use | 2,659 | 3,010 | ||||||
| Other long-term assets | 510 | 505 | ||||||
Total assets | $ | 59,988 | $ | 71,712 | ||||
Liabilities and Stockholders’ Deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 11,944 | $ | 10,379 | ||||
| Current portion of term loan | 38,957 | 38,643 | ||||||
| Current portion of lease liabilities | 814 | 908 | ||||||
| Accrued expenses and other current liabilities | 14,506 | 15,495 | ||||||
| Total current liabilities | 66,221 | 65,425 | ||||||
| Public warrant liabilities | 3,329 | 5,943 | ||||||
| Revenue Interest Financing liability | 35,000 | 36,200 | ||||||
Earn-out liabilities | 9,800 | 23,990 | ||||||
| Lease liabilities, net of current portion | 2,011 | 2,306 | ||||||
| Other liabilities | 9,789 | 8,335 | ||||||
Total liabilities | 126,150 | 142,199 | ||||||
| Commitments and Contingencies (Note 16) | ||||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $0.0001 par value — 100,000,000 shares authorized as of March 31, 2024; and no shares issued and outstanding as of March 31, 2024 and December 31, 2023 | — | — | ||||||
| Common stock, $0.0001 par value — 1,000,000,000 shares authorized as of March 31, 2024; and 47,898,737 and 47,688,096 shares issued and outstanding as of March 31, 2024 and December 31, 2023, respectively | 5 | 5 | ||||||
Additional paid-in capital | 143,946 | 143,007 | ||||||
| Accumulated other comprehensive loss | (2,900 | ) | (700 | ) | ||||
| Accumulated deficit | (207,213 | ) | (212,799 | ) | ||||
Total stockholders’ deficit | (66,162 | ) | (70,487 | ) | ||||
Total liabilities and stockholders’ deficit | $ | 59,988 | $ | 71,712 | ||||
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
| Revenue | $ | 9,386 | $ | 14,071 | ||||
| Cost of revenue | 2,520 | 2,940 | ||||||
| Gross profit | 6,866 | 11,131 | ||||||
| Operating expenses: | ||||||||
| Sales and marketing | 6,145 | 11,864 | ||||||
| Research and development | 5,725 | 7,852 | ||||||
| General and administrative | 6,386 | 5,306 | ||||||
| Total operating expenses: | 18,256 | 25,022 | ||||||
| Loss from operations | (11,390 | ) | (13,891 | ) | ||||
| Other (expense) income: | ||||||||
| Interest expense | (1,931 | ) | (2,237 | ) | ||||
| Changes in fair value of warrants | 3,131 | (1,475 | ) | |||||
| Changes in fair value of Revenue Interest Financing and PIPE Conversion Option | 1,490 | — | ||||||
Changes in fair value of earn-out liabilities | 14,190 | — | ||||||
| Other income (expense), net | 172 | (164 | ) | |||||
| Total other income (expense): | 17,052 | (3,876 | ) | |||||
| Income (loss) before income taxes | 5,662 | (17,767 | ) | |||||
| Provision for income taxes | (76 | ) | (34 | ) | ||||
| Net income (loss) | 5,586 | (17,801 | ) | |||||
| Cumulative undeclared preferred dividends | — | (717 | ) | |||||
| Net income (loss) attributable to common shareholders | $ | 5,586 | $ | (18,518 | ) | |||
| Net income (loss) per share | ||||||||
| Basic | $ | 0.12 | $ | (0.68 | ) | |||
| Diluted | $ | 0.11 | $ | (0.68 | ) | |||
| Weighted-average shares outstanding | ||||||||
| Basic | 47,779,350 | 27,087,174 | ||||||
| Diluted | 49,190,474 | 27,087,174 | ||||||
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
| Net Income (loss) | $ | 5,586 | $ | (17,801 | ) | |||
| Other comprehensive loss: | ||||||||
| Change in fair value of Revenue Interest Financing due to change in credit risk | (2,200 | ) | — | |||||
| Comprehensive Income (loss) | $ | 3,386 | $ | (17,801 | ) | |||
Common Stock | Additional Paid-in Capital | Accumulated Other Comprehensive Loss | Accumulated Deficit | Stockholders’ Deficit | ||||||||||||||||||||
Shares | Amount | |||||||||||||||||||||||
Balance as of January 1, 2023 | 27,079,856 | 3 | 99,875 | — | (132,192 | ) | (32,314 | ) | ||||||||||||||||
| Exercise of stock options | 15,376 | — | 20 | — | — | 20 | ||||||||||||||||||
| Issuance of Legacy Allurion convertible preferred stock for the exercise of warrants | 3,554 | — | 29 | — | — | 29 | ||||||||||||||||||
| Stock-based compensation expense | — | — | 409 | — | — | 409 | ||||||||||||||||||
| Net loss | — | — | — | — | (17,801 | ) | (17,801 | ) | ||||||||||||||||
Balance as of March 31, 2023 | 27,098,786 | 3 | 100,333 | — | (149,993 | ) | (49,657 | ) | ||||||||||||||||
Balance as of January 1, 2024 | 47,688,096 | 5 | 143,007 | (700 | ) | (212,799 | ) | (70,487 | ) | |||||||||||||||
| Exercise of stock options | 4,646 | — | 9 | — | — | 9 | ||||||||||||||||||
| Issuance of common stock for the exercise of Public Warrants | 142 | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock from equity line financing (Note 12) | 143,234 | — | 378 | — | — | 378 | ||||||||||||||||||
| Issuance of common stock in connection with vesting of RSU awards | 62,619 | — | — | — | — | — | ||||||||||||||||||
| Stock-based compensation expense | — | — | 552 | — | — | 552 | ||||||||||||||||||
| Other comprehensive loss | — | — | — | (2,200 | ) | — | (2,200 | ) | ||||||||||||||||
| Net income | — | — | — | — | 5,586 | 5,586 | ||||||||||||||||||
Balance as of March 31, 2024 | 47,898,737 | 5 | 143,946 | (2,900 | ) | (207,213 | ) | (66,162 | ) | |||||||||||||||
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
Operating Activities: | ||||||||
| Net income (loss) | $ | 5,586 | $ | (17,801 | ) | |||
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | ||||||||
Non-cash lease expense | 199 | 209 | ||||||
| Depreciation and amortization | 367 | 245 | ||||||
| Stock-based compensation | 552 | 409 | ||||||
| Provision for uncollectible accounts | — | 999 | ||||||
| Unrealized exchange (gain) or loss | 314 | (88 | ) | |||||
| Provision for inventory | 209 | — | ||||||
| Change in fair value of warrant liabilities | (3,131 | ) | 1,475 | |||||
| Change in fair value of derivative liabilities | 62 | 29 | ||||||
| Change in fair value of Revenue Interest Financing and PIPE Conversion Option | (1,490 | ) | — | |||||
Change in fair value of earn-out liabilities | (14,190 | ) | — | |||||
Non-cash interest expense | 315 | 317 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | 1,673 | (427 | ) | |||||
| Inventory | 330 | (449 | ) | |||||
| Prepaid expenses, other current and long-term assets | 244 | 36 | ||||||
| Lease liabilities | (238 | ) | (175 | ) | ||||
| Accounts payable | 1,551 | 5,354 | ||||||
| Accrued expenses and other current liabilities | (989 | ) | (451 | ) | ||||
| Net cash used in operating activities | $ | (8,636 | ) | $ | (10,318 | ) | ||
Investing Activities: | ||||||||
| Purchases of property and equipment | (104 | ) | (277 | ) | ||||
| Net cash used in investing activities | $ | (104 | ) | $ | (277 | ) | ||
Financing Activities: | ||||||||
Proceeds from issuance of convertible notes - net | — | 13,600 | ||||||
| Proceeds from option and warrant exercises | 9 | 32 | ||||||
| Proceeds from equity line financing | 378 | — | ||||||
| Payment of deferred financing costs | — | (766 | ) | |||||
| Net cash provided by financing activities | $ | 387 | $ | 12,866 | ||||
| Net increase (decrease) in cash and cash equivalents and restricted cash | $ | (8,353 | ) | $ | 2,271 | |||
| Cash and cash equivalents and restricted cash at beginning of period | 38,421 | 8,023 | ||||||
| Cash and cash equivalents and restricted cash at end of period | $ | 30,068 | $ | 10,294 | ||||
Supplemental disclosure of cash flow information | ||||||||
| Cash paid for interest | $ | 1,831 | $ | 1,920 | ||||
Supplemental cash flow information on non-cash investing and financing activities | ||||||||
| Purchase of property and equipment included in accounts payable | $ | 73 | $ | 174 | ||||
| Deferred financing costs in accounts payable and accrued expenses | — | 2,634 | ||||||
| Change in fair value of Revenue Interest Financing through OCI | (2,200 | ) | — | |||||
March 31, 2024 | December 31, 2023 | |||||||
| Cash and cash equivalents | $ | 29,682 | $ | 38,037 | ||||
| Restricted cash included in other long-term assets | 386 | 384 | ||||||
| Cash and cash equivalents and restricted cash shown in the statement of cash flows | $ | 30,068 | $ | 38,421 | ||||
1. | Organization and Basis of Presentation |
2. | Summary of Significant Accounting Policies |
Revenue | Accounts Receivable | |||||||||||||||
Three Months Ended March 31, | March 31, | December 31, | ||||||||||||||
2024 | 2023 | 2024 | 2023 | |||||||||||||
| Customer A | N/A | N/A | 11 | % | 16 | % | ||||||||||
3. | Business Combination |
December 31, 2023 | ||||
| Cash – CPUH trust (net of redemptions) | $ | 38,395 | ||
| Cash – PIPE Investors | 37,922 | |||
| Gross Proceeds | 76,317 | |||
| Less: transaction costs paid | (14,665 | ) | ||
| Net proceeds from the Business Combination | 61,652 | |||
| Less: warrant liabilities assumed | (13,762 | ) | ||
| Less: repayment of note assumed in the Business Combination | (2,500 | ) | ||
| Less: accrued transaction costs at December 31, 2023 | (580 | ) | ||
| Business Combination, net of transaction costs | $ | 44,810 | ||
Common Stock | ||||
| Legacy Allurion Equityholders (1) | 27,897,387 | |||
| CPUH Stockholders (2) | 5,165,698 | |||
| Shares Issued to PIPE Investors (2) | 5,386,695 | |||
| Shares issued to RTW and Fortress (3) | 1,900,000 | |||
| Shares issued to convertible note holders | 3,301,222 | |||
| CPUH Sponsor Shares (2) | 3,262,711 | |||
| Side Letter Termination Shares (3) | 387,696 | |||
| Total shares of Common Stock immediately after Business Combination | 47,301,409 | |||
| (1) | Consists of Legacy Allurion common stock and Legacy Allurion preferred stock, plus the issuance of common stock in connection with the vesting of RSUs at closing, less the Gaur Contributed Shares (as defined below). |
| (2) | The CPUH Stockholders shares, PIPE shares, and CPUH Sponsor shares are presented combined within the condensed consolidated statements of stockholders deficit on the “Reverse recapitalization, net of transaction costs” line, which is less the Gaur Contributed Shares (as defined below). |
| (3) | The shares issued to RTW and Fortress and the Side Letter Termination shares are presented combined within the condensed consolidated statements of stockholders deficit on the “Derecognition of liabilities associated with the Backstop Shares, Hunter shares, and additional RTW and Fortress shares and issuance of related shares” line. |
4. | Revenue |
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
| France | $ | 1,671 | $ | 1,855 | ||||
| United Kingdom | 1,256 | 1,193 | ||||||
| Spain | 1,112 | 1,624 | ||||||
| All Other Countries | 5,347 | 9,399 | ||||||
| Total Revenues | $ | 9,386 | $ | 14,071 | ||||
5. | Inventory |
March 31, 2024 | December 31, 2023 | |||||||
| Finished goods | $ | 2,928 | $ | 3,427 | ||||
| Work in progress | 599 | 967 | ||||||
| Raw materials | 2,104 | 1,777 | ||||||
| Total Inventory | $ | 5,631 | $ | 6,171 | ||||
6. | Property and Equipment, net |
Estimated Useful Life (in Years) | March 31, 2024 | December 31, 2023 | ||||||||||
| Computers and purchased software | 3 | $ | 618 | $ | 618 | |||||||
| Leasehold improvements | Shorter of useful life or lease term | 1,943 | 1,943 | |||||||||
| Furniture and fixtures | 5 | 291 | 291 | |||||||||
| Machinery and equipment | 3-5 | 3,053 | 2,893 | |||||||||
| Property and equipment—at cost | 5,905 | 5,745 | ||||||||||
| Less accumulated depreciation and amortization | (3,814 | ) | (3,559 | ) | ||||||||
| Construction in progress | 1,089 | 1,195 | ||||||||||
| Property and equipment—net | $ | 3,180 | $ | 3,381 | ||||||||
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
| Cost of revenue | $ | 201 | $ | 159 | ||||
| Research and development | 49 | 37 | ||||||
| General and administrative | 64 | 35 | ||||||
| Sales and marketing | 53 | 14 | ||||||
| Total depreciation and amortization expense | $ | 367 | $ | 245 | ||||
7. | Accrued Expenses and Other Current Liabilities |
March 31, 2024 | December 31, 2023 | |||||||
| Marketing reimbursement | $ | 2,031 | $ | 2,834 | ||||
| Accrued compensation | 2,752 | 1,687 | ||||||
| Accrued clinical trials and R&D | 4,549 | 3,694 | ||||||
| Accrued selling and marketing | 845 | 1,110 | ||||||
| Accrued professional fees | 1,152 | 1,505 | ||||||
| Accrued warranty | 21 | 44 | ||||||
| Accrued restructuring | 8 | 655 | ||||||
| Other accrued expenses | 3,148 | 3,966 | ||||||
| Total accrued expenses and other current liabilities | $ | 14,506 | $ | 15,495 | ||||
8. | Debt |
March 31, 2024 | December 31, 2023 | |||||||
| Fortress Term Loan | $ | 43,100 | $ | 43,100 | ||||
| Total principal amount of debt | 43,100 | 43,100 | ||||||
| Plus: Accretion | 213 | 148 | ||||||
| Less: current portion of long-term debt, net of discounts | (38,957 | ) | (38,643 | ) | ||||
| Less: unamortized deferred financing costs and debt discounts | (4,356 | ) | (4,605 | ) | ||||
| Long-term debt, net of current portion and discounts | $ | — | $ | — | ||||
| December 31, 2024 | $ | — | ||
| December 31, 2025 | — | |||
| December 31, 2026 | 8,979 | |||
| December 31, 2027 | 34,121 | |||
| $ | 43,100 |
9. | Revenue Interest Financing, Side Letter, and PIPE Conversion Option |
10. | Fair Value Measurements |
Fair Value Measurement as of March 31, 2024 | ||||||||||||||||
Total Carrying Value | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets: | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 23,049 | $ | 23,049 | $ | — | $ | — | ||||||||
| Total assets | $ | 23,049 | $ | 23,049 | $ | — | $ | — | ||||||||
Liabilities: | ||||||||||||||||
| Legacy Allurion Common Stock Warrant Liabilities | $ | 304 | $ | — | $ | — | $ | 304 | ||||||||
| Public Warrants | 3,329 | 3,329 | — | — | ||||||||||||
| Revenue Interest Financing | 35,000 | — | — | 35,000 | ||||||||||||
| PIPE Conversion Option | 7,510 | — | — | 7,510 | ||||||||||||
Earn-out Liability | 9,800 | — | — | 9,800 | ||||||||||||
| Term Loan Derivative Liability | 1,957 | — | — | 1,957 | ||||||||||||
| Success Fee Derivative Liability | 14 | — | — | 14 | ||||||||||||
| Total Liabilities | $ | 57,914 | $ | 3,329 | $ | — | $ | 54,585 | ||||||||
Fair Value Measurement as of December 31, 2023 | ||||||||||||||||
Total Carrying Value | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets: | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 30,582 | $ | 30,582 | $ | — | $ | — | ||||||||
| Total assets | $ | 30,582 | $ | 30,582 | $ | — | $ | — | ||||||||
Liabilities: | ||||||||||||||||
| Legacy Allurion Common Stock Warrant Liabilities | $ | 821 | $ | — | $ | — | $ | 821 | ||||||||
| Public Warrants | 5,943 | 5,943 | — | — | ||||||||||||
| Revenue Interest Financing | 36,200 | — | — | 36,200 | ||||||||||||
| PIPE Conversion Option | 5,600 | — | — | 5,600 | ||||||||||||
Earn-out Liability | 23,990 | — | — | 23,990 | ||||||||||||
| Term Loan Derivative Liability | 1,895 | — | — | 1,895 | ||||||||||||
| Success Fee Derivative Liability | 14 | — | — | 14 | ||||||||||||
| Total Liabilities | $ | 74,463 | $ | 5,943 | $ | — | $ | 68,520 | ||||||||
Measurement Date | Interest Rate | Exercise Price | Estimated Fair Value of Underlying Share Price | Expected Volatility | Expected Life (Years) | |||||||||||||||||||
| Legacy Allurion Series C Preferred Stock warrants (as converted to Common) | March 31, 2024 | 4.20 | % | $ | 6.73 | $ | 1.75 | 90 | % | 7.00 | ||||||||||||||
| Other Common Stock | March 31, 2024 | 4.36 | % | 1.05 | 1.75 | 90 | % | 3.44 | ||||||||||||||||
Legacy Allurion Series D-1 Preferred Stock warrants (as converted to Common) | March 31, 2024 | 4.20 | % | 12.14 | 1.75 | 90 | % | 7.00 - 8.46 | ||||||||||||||||
| Legacy Allurion Series C Preferred Stock warrants (as converted to Common) | December 31, 2023 | 3.88 | % | 6.73 | $ | 3.74 | 100 | % | 7.25 | |||||||||||||||
| Other Common Stock | December 31, 2023 | 3.95 | % | 1.05 | 3.74 | 100 | % | 3.69 | ||||||||||||||||
Legacy Allurion Series D-1 Preferred Stock warrants (as converted to Common) | December 31, 2023 | 3.88 | % | 12.14 | 3.74 | 100 | % | 7.25 - 8.71 | ||||||||||||||||
Preferred Stock Warrants (as converted to Common) | Common Stock Warrants | Total | ||||||||||
| Balance – January 1, 2023 | $ | 1,777 | $ | 596 | $ | 2,373 | ||||||
Change in fair value | 826 | 649 | 1,475 | |||||||||
| Exercise of warrants | (18 | ) | — | (18 | ) | |||||||
| Balance – March 31, 2023 | $ | 2,585 | $ | 1,245 | $ | 3,830 | ||||||
| Balance – January 1, 2024 | $ | 642 | $ | 179 | $ | 821 | ||||||
Change in fair value | (409 | ) | (108 | ) | (517 | ) | ||||||
| Balance – March 31, 2024 | $ | 233 | $ | 71 | $ | 304 | ||||||
March 31, 2024 | ||||
| Stock Price | 1.75 | |||
| Risk-free interest rate | 4.9 | % | ||
| Expected term (in years) | 1.3 | |||
| Expected volatility | 100.0 | % | ||
March 31, 2024 | ||||
| Stock Price | 1.75 | |||
| Risk-free interest rate | 4.3 | % | ||
| Expected term (in years) | 4.3 | |||
| Expected volatility | 102.0 | % | ||
Success Fee Derivative Liability | 2023 Convertible Notes | Revenue Interest Financing | PIPE Conversion Derivative | Earn-Out Liability | Term Loan Derivative Liability | Total | ||||||||||||||||||||||
| Balance – January 1, 2023 | $ | 178 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 178 | ||||||||||||||
| Fair value upon issuance | — | 13,600 | $ | — | — | — | — | 13,600 | ||||||||||||||||||||
| Change in fair value | 29 | — | — | — | — | — | 29 | |||||||||||||||||||||
| Balance – March 31, 2023 | $ | 207 | $ | 13,600 | $ | — | $ | — | $ | — | $ | — | $ | 13,807 | ||||||||||||||
| Balance – January 1, 2024 | $ | 14 | $ | — | $ | 36,200 | $ | 5,600 | $ | 23,990 | $ | 1,895 | $ | 67,699 | ||||||||||||||
| Fair value upon issuance | — | — | — | — | — | — | — | |||||||||||||||||||||
| Change in fair value | — | — | (3,400 | ) | 1,910 | (14,190 | ) | 62 | (15,618 | ) | ||||||||||||||||||
| Change in fair value – OCI | — | — | 2,200 | — | — | — | 2,200 | |||||||||||||||||||||
| Repayments of debt | — | — | — | — | — | — | — | |||||||||||||||||||||
| Balance – March 31, 2024 | $ | 14 | $ | — | $ | 35,000 | $ | 7,510 | $ | 9,800 | $ | 1,957 | $ | 54,281 | ||||||||||||||
11. | Income Taxes |
12. | Capital Stock and Stockholders’ Deficit |
| Outstanding options to purchase common stock | 3,928,824 | |||
| Restricted Stock Units | 656,016 | |||
| Warrants to purchase preferred stock (as converted to warrants to purchase common stock) | 138,857 | |||
| Warrants to purchase common stock | 264,801 | |||
| Public warrants to purchase common stock | 18,759,696 | |||
Earn-Out Shares | 9,000,000 | |||
| Total | 32,748,194 |
March 31, 2024 | ||||||||||||||||||
Issuance Date | Remaining Contractual Term (in years) | Underlying Equity Instrument | Balance Sheet Classification | Shares Issuable Upon Exercise of Warrant | Weighted Average Exercise Price | |||||||||||||
| 12/1/2014 | 0.7 | Common stock | Equity | 44,272 | $ | 2.44 | ||||||||||||
| 3/30/2021 | 7.0 | Common stock | Liability | 130,053 | 6.73 | |||||||||||||
| 9/15/2022 | 8.5 | Common stock | Liability | 45,238 | 12.14 | |||||||||||||
| 6/4/2022 | 8.2 | Common stock | Liability | 45,238 | 12.14 | |||||||||||||
| 1/17/2017 | 2.8 | Common stock | Equity | 73,349 | 0.02 | |||||||||||||
| 8/3/2017 | 3.3 | Common stock | Equity | 9,779 | 1.13 | |||||||||||||
| 9/8/2017 | 3.4 | Common stock | Liability | 28,764 | 1.05 | |||||||||||||
| 6/19/2018 | 4.2 | Common stock | Liability | 17,977 | 1.05 | |||||||||||||
| 6/25/2019 | 5.2 | Common stock | Liability | 8,988 | 1.05 | |||||||||||||
| 403,658 | ||||||||||||||||||
December 31, 2023 | ||||||||||||||||||
Issuance Date | Remaining Contractual Term (in years) | Underlying Equity Instrument | Balance Sheet Classification | Shares Issuable Upon Exercise of Warrant | Weighted Average Exercise Price | |||||||||||||
| 12/1/2014 | 0.9 | Common stock | Equity | 44,272 | $ | 2.44 | ||||||||||||
| 3/30/2021 | 7.2 | Common stock | Liability | 130,053 | 6.73 | |||||||||||||
| 9/15/2022 | 8.7 | Common stock | Liability | 45,238 | 12.14 | |||||||||||||
| 6/4/2022 | 8.4 | Common stock | Liability | 45,238 | 12.14 | |||||||||||||
| 1/17/2017 | 3.0 | Common stock | Equity | 73,349 | 0.02 | |||||||||||||
| 8/3/2017 | 3.6 | Common stock | Equity | 9,779 | 1.13 | |||||||||||||
| 9/8/2017 | 3.7 | Common stock | Liability | 28,764 | 1.05 | |||||||||||||
| 6/19/2018 | 4.5 | Common stock | Liability | 17,977 | 1.05 | |||||||||||||
| 6/25/2019 | 5.5 | Common stock | Liability | 8,988 | 1.05 | |||||||||||||
| 403,658 | ||||||||||||||||||
13. | Net Income (Loss) per Share |
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
| Numerator: | ||||||||
| Net income (loss) | $ | 5,586 | $ | (17,801 | ) | |||
| Cumulative undeclared preferred dividends to participating securities (Legacy Series D convertible preferred stock) | — | (717 | ) | |||||
| Net income (loss) attributable to common shareholders—Basic | $ | 5,586 | $ | (18,518 | ) | |||
| Adjustment for change in fair value of liability classified warrants | (108 | ) | — | |||||
| Net income (loss) attributable to common shareholders—Diluted | $ | 5,478 | $ | (18,518 | ) | |||
| Basic weighted-average common shares outstanding | 47,779,350 | 27,087,174 | ||||||
| Effect of potentially dilutive securities: | ||||||||
| Stock options | 1,117,680 | — | ||||||
| Restricted stock units | 172,077 | — | ||||||
| Warrants | 121,367 | — | ||||||
| Diluted weighted-average common shares outstanding | 49,190,474 | 27,087,174 | ||||||
| Basic net income (loss) per common share | $ | 0.12 | $ | (0.68 | ) | |||
| Diluted net income (loss) per common share | $ | 0.11 | $ | (0.68 | ) | |||
As of March 31, | ||||||||
2024 | 2023 | |||||||
| Outstanding options to purchase Common Stock | 2,811,144 | 4,273,881 | ||||||
| Restricted Stock Units | 483,939 | 1,415,104 | ||||||
| Warrants to purchase preferred stock (as converted to warrants to purchase Common Stock) | 220,529 | 294,300 | ||||||
| Warrants to purchase Common Stock | — | 138,857 | ||||||
| Shares of Common Stock issued upon the exercise of Public Warrants | 18,759,696 | — | ||||||
Earn-Out Shares | 9,000,000 | — | ||||||
| Convertible Notes | — | 2,552,080 | ||||||
| Total | 31,275,308 | 8,674,222 | ||||||
14. | Stock-Based Compensation |
Three Months Ended March 31, | ||||||||
2024 | 2023 | |||||||
| Cost of revenue | $ | 5 | $ | 12 | ||||
| Selling, general and administrative | 550 | 386 | ||||||
| Research and development | (3 | ) | 11 | |||||
| Total stock-based compensation expense | $ | 552 | $ | 409 | ||||
Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||||
(per option) | (in years) | (in thousands) | ||||||||||||||
| Outstanding—January 1, 2024 | 3,886,038 | $ | 2.67 | 6.9 | $ | 5,565 | ||||||||||
| Granted | 226,244 | 2.96 | ||||||||||||||
| Cancellations and forfeitures | (178,812 | ) | 3.23 | |||||||||||||
| Exercised | (4,646 | ) | 1.74 | |||||||||||||
| Outstanding—March 31, 2024 | 3,928,824 | 2.67 | 6.5 | 1,087 | ||||||||||||
| Exercisable at March 31, 2024 | 2,814,622 | $ | 2.31 | 5.8 | $ | 1,028 | ||||||||||
| Expected volatility | 71 | % | ||
| Risk-free interest rate | 4.14 | % | ||
| Expected dividend yield | 0 | % | ||
| Expected term (in years) | 6.1 |
Number of RSUs | Weighted Average Grant Date Fair Value | |||||||
(per share) | ||||||||
| Outstanding—January 1, 2024 | 643,635 | $ | 4.45 | |||||
| Granted | 75,000 | 2.61 | ||||||
| Cancellations and forfeitures | — | |||||||
| Vested | (62,619 | ) | 4.51 | |||||
| Outstanding—March 31, 2024 | 656,016 | $ | 4.23 | |||||
15. | Employee Benefit Plan |
16. | Commitments and Contingencies |
Three Months Ended | ||||||||
March 31, 2024 | March 31, 2023 | |||||||
| Operating lease costs | $ | 278 | $ | 274 | ||||
| Short-term lease costs | 10 | 6 | ||||||
| Variable lease costs | 77 | 88 | ||||||
| Operating cash flows paid for amounts in the measurement of lease liabilities | 292 | 262 | ||||||
| Operating lease assets obtained in exchange for lease obligations | — | 874 | ||||||
| 2024 | $ | 785 | ||
| 2025 | 1,037 | |||
| 2026 | 732 | |||
| 2027 | 642 | |||
| Thereafter | 108 | |||
| Total lease payments | $ | 3,304 | ||
| Less: present value adjustment | (479 | ) | ||
| Total lease liabilities | 2,825 | |||
| Less: current lease liability | (814 | ) | ||
| Long-term operating lease liabilities | $ | 2,011 | ||
March 31, 2024 | March 31, 2023 | |||||||
| Weighted-average remaining lease term (in years) | 3.4 | 4.2 | ||||||
| Weighted-average discount rate | 9.9 | % | 9.5 | % | ||||
17. | Geographic Information |
March 31, 2024 | December 31, 2023 | |||||||
| United States | $ | 5,059 | $ | 5,381 | ||||
| France | $ | 780 | 1,010 | |||||
| All other countries | — | — | ||||||
| Long-lived assets | $ | 5,839 | $ | 6,391 | ||||
18. | Related-party Transactions |
19. | Subsequent Events |
Table of Contents
30,191,900 Shares of Common Stock

Allurion Technologies, Inc.
PROSPECTUS
May 31, 2024
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the estimated expenses to be borne by the registrant in connection with the securities being registered hereby.
Expense | Estimated Amount | |||
Securities and Exchange Commission registration fee | $ | 7,041.00 | ||
Accounting fees and expenses | * | |||
Legal fees and expenses | * | |||
Financial printing and miscellaneous expenses | * | |||
Total | $ | * | ||
| * | These fees are calculated based on the securities offered and the number of issuances and accordingly cannot be defined at this time. |
Item 14. Indemnification of Directors and Officers
Section 145(a) of the DGCL provides, in general, that a corporation may indemnify any person who was or is a party to or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation), because he or she is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding, if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section 145(b) of the DGCL provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification shall be made with respect to any claim, issue or matter as to which he or she shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, he or she is fairly and reasonably entitled to indemnity for such expenses that the Court of Chancery or other adjudicating court shall deem proper.
Section 145 of the DGCL further provides that to the extent a director or officer of a corporation has been successful on the merits or otherwise in the defense of any action, suit or proceeding referred to in Section 145(a) or (b) of the DGCL, or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith; that indemnification provided for by Section 145 of the DGCL shall not be deemed exclusive of any other rights to which the indemnified party may be entitled; and the indemnification provided for by Section 145 of the DGCL shall, unless otherwise provided when authorized or ratified, continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of such person’s heirs, executors and
II-1
Table of Contents
administrators. Section 145(g) of the DGCL provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation would have the power to indemnify the person against such liability under Section 145 of the DGCL.
Section 102(b)(7) of the DGCL provides that a corporation’s certificate of incorporation may contain a provision eliminating or limiting the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director; provided that such provision shall not eliminate or limit the liability of a director (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the DGCL or (iv) for any transaction from which the director derived an improper personal benefit.
Additionally, our Charter and Bylaws limit the liability of our (i) directors and (ii) officers, which includes each individual who has been duly appointed as an officer of Allurion and who, at the time of an act or omission as to which liability is asserted, is deemed to have consented to service of process to our registered agent as contemplated by Section 3114(b) of Title 10 of the DGCL, in each case, to the fullest extent permitted by the DGCL, and also provides that we indemnify our directors and officers to the fullest extent permitted by the DGCL.
In connection with the Business Combination, we entered into indemnification agreements with each of our directors and executive officers, a form of which is filed as Exhibit 10.1 to this prospectus. These agreements provide that we indemnify each of our directors and officers to the fullest extent permitted by law and our Charter and Bylaws, and provides for advancement of expenses incurred as a result of any proceeding against them as to which they could be indemnified.
We also maintain a general liability insurance policy, which covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers.
Item 15. Recent Sales of Unregistered Securities.
Pursuant to the Amended Note Purchase Agreement, on April 16, 2024, we issued and sold $48 million aggregate principal amount of convertible senior secured note to RTW in a private placement transaction, convertible into shares of our common stock based on the higher of (x) an initial conversion rate of 307.0797 shares of common stock per $1,000 principal amount of notes sold under the Amended Note Purchase Agreement and (y) a 35% conversion premium to the lowest price per share in an equity financing for capital raising purposes ending on the date on which we have raised aggregate gross offering proceeds of at least $15,000,000. The Notes bear interest at the annual rate of 6.0%, which interest is payable quarterly in cash or, at our option, in kind for the first three years. The maturity date for the Notes is April 16, 2031. The Notes are guaranteed by Allurion Opco and certain other current and future subsidiaries of the Company, and are secured by substantially all the assets of Allurion and the guarantors. We used the proceeds from the issuance of the Notes to refinance our outstanding obligations under the Fortress Credit Agreement in full and to pay fees and expenses in connection therewith and in connection with the transactions contemplated by the Amended Note Purchase Agreement.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. We believe each of these transactions was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act (and Regulation D promulgated thereunder) as transactions by an issuer not involving any public offering. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed on the share certificates issued in
II-2
Table of Contents
these transactions. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
Item 16. Exhibits and Financial Statements Schedules
(a) Exhibits.
II-3
Table of Contents
II-4
Table of Contents
II-5
Table of Contents
II-6
Table of Contents
| * | Filed herewith. |
| + | Certain of the exhibits and schedules to this exhibit have been omitted in accordance with Regulation S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of all omitted exhibits and schedules to the SEC upon its request. |
| ++ | Certain of the exhibits and schedules to this exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Registrant agrees to furnish supplementally a copy of all omitted exhibits and schedules to the SEC upon its request. |
| † | Portions of this exhibit have been redacted in accordance with Regulation S-K Item 601(a)(6). |
| # | Indicates a management contract or compensatory plan, contract or arrangement. |
II-7
Table of Contents
(b) Financial Statement Schedules.
See the index to the consolidated financial statements included on page F-1 for a list of the financial statements included in this registration statement. Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| Item 17. | Undertakings |
The undersigned registrant hereby undertakes:
| 1. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
| 2. | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 3. | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| 4. | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| 5. | That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
II-8
Table of Contents
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-9
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the town of Natick, Commonwealth of Massachusetts, on the 31st day of May, 2024.
| ALLURION TECHNOLOGIES, INC. | ||
| By: | /s/ Shantanu Gaur | |
Name: Shantanu Gaur Title: Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Shantanu Gaur and Christopher Geberth as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign one or more registration statements on Form S-1 and any and all amendments to such registration statements, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the United States Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his or her substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following person in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ Shantanu Gaur Shantanu Gaur | Chief Executive Officer, President and Director (Principal Executive Officer) | May 31, 2024 | ||
/s/ Christopher Geberth Christopher Geberth | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | May 31, 2024 | ||
/s/ Omar Ishrak Omar Ishrak | Director | May 31, 2024 | ||
/s/ Michael Davin Michael Davin | Director | May 31, 2024 | ||
/s/ Larson Douglas Hudson Larson Douglas Hudson | Director | May 31, 2024 | ||
/s/ Nicholas Lewin Nicholas Lewin | Director | May 31, 2024 | ||
/s/ Milena Alberti-Perez Milena Alberti-Perez | Director | May 31, 2024 | ||
II-10