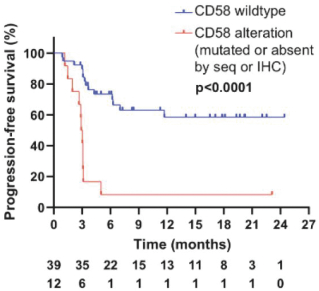
In a Phase 3 clinical trial of ZUMA-7, a randomized, open-label, multi-center trial, Yescarta was administered to 180 patients and supported the initial treatment in adults with 2L R/R LBCL. After 14.7 months of follow-up, the ORR, CR rate and PFS were 83%, 65% and 50%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 7% and 25% of patients, respectively. After 4 years of follow-up, the ORR, CR rate and PFS were 83%, 65% and 42%, respectively.
Tisagenlecleucel (Kymriah)
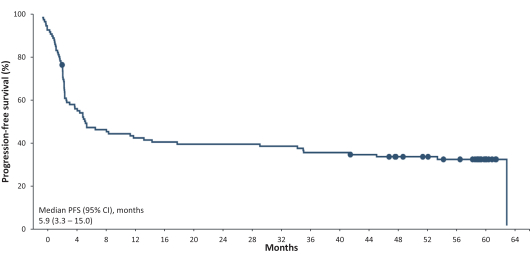
In a Phase 2 clinical trial of JULIET, an open-label, multi-center, single-arm trial, Kymriah was administered to 68 patients. At 9.4 months, the ORR and CR rate were 50% and 32%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 22% and 12% of patients, respectively. At 24 months of follow-up, the ORR and CR rate were 52% and 38%, respectively, as compared to after 40.3 months of follow-up, where the ORR and CR rate were 53% and 39%, respectively. After 36 months of follow-up, the PFS was 31%.
Lisocabtagene maraleucel (Breyanzi)
In the pivotal TRANSCEND NHL 001 clinical trial, Breyanzi was administered to 192 patients. After 18.8 months of follow-up, the ORR and CR rate were 73% and 54%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 2% and 10% of patients, respectively. After 2 years of follow-up, the ORR, CR rate and PFS were 73%, 53% and 41%, respectively.
In the pivotal Phase 3 TRANSFORM clinical trial, Breyanzi was administered to 92 patients and supported the initial treatment in adults with 2L R/R LBCL. At 6.2 months, the ORR and CR rate were 84% and 66%, respectively, and Grade 3 or higher CRS and neurologic events occurred in 1% and 7% of patients, respectively. After 17.5 months of follow-up, the ORR, CR rate and PFS were 87%, 74% and 58%, respectively.
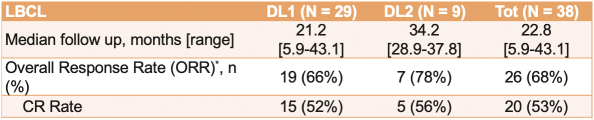
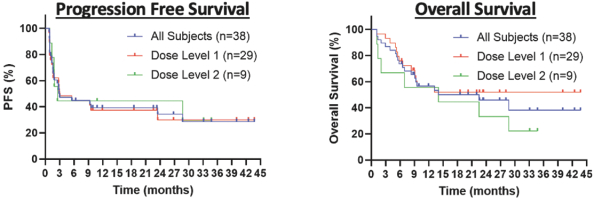
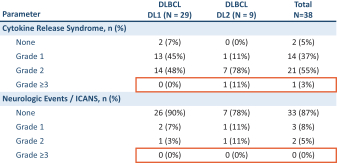
ORR and CR rate
The following reflects the published data on ORR and CR rates of CD19 CAR T-cell therapies for the treatment of 3L+ LBCL after 2 \years of follow-up: Yescarta (83% ORR, 54% CR rate), Kymriah (52% ORR, 38% CR rate) and Breyanzi (73% ORR, 53% CR rate).
Intellectual property
Intellectual property rights are important to the success of our business. We rely on a combination of patent, trademark and trade secret laws in the United States and in jurisdictions outside of the United States, including Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Japan, Korea, Mexico, New Zealand, Russia, Singapore, South Africa and the United Kingdom, as well as license agreements, confidentiality procedures, non-disclosure agreements with third parties, and other contractual protections, to protect our intellectual property rights, including our proprietary technology, solutions, know-how and brands.
We seek to protect the intellectual property, or IP, and proprietary technology that we consider important to our business, including by pursuing patent applications that cover our technologies and product candidates and methods of using the same, as well as any other relevant inventions and improvements that are considered commercially important to the development of our business. We likewise seek to protect the IP to which we obtain rights through licenses and sublicenses and work collaboratively with our licensors to ensure patent prosecution and protection. We also rely on trademarks, copyrights, trade secrets, know- how and continuing technological innovation to develop and maintain our proprietary and IP positions. Our commercial success depends, in part, on our ability to obtain, maintain, enforce and protect our IP and other proprietary rights for the technology, inventions and improvements we consider important to our business, and to defend any patents we may own or in-license in the future, prevent others from infringing any patents we may own or in-license in the future, preserve the confidentiality of our trade secrets, and operate without infringing, misappropriating or otherwise violating the valid and enforceable IP and proprietary rights of third parties.
149

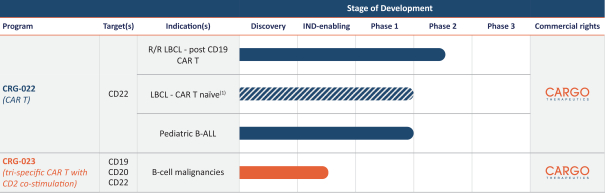
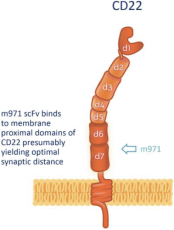
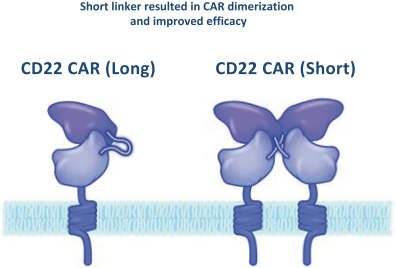
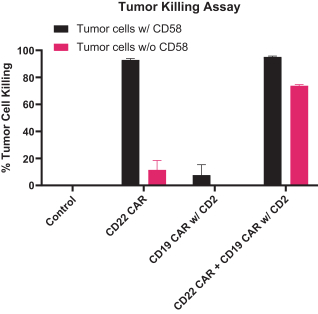
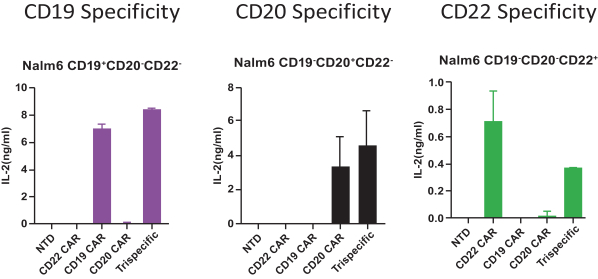
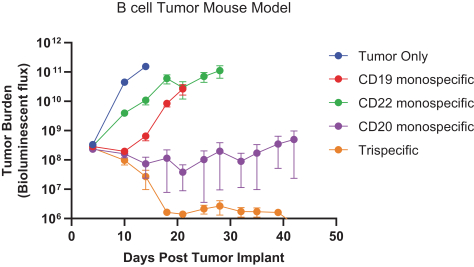
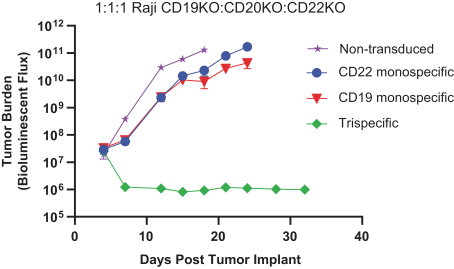
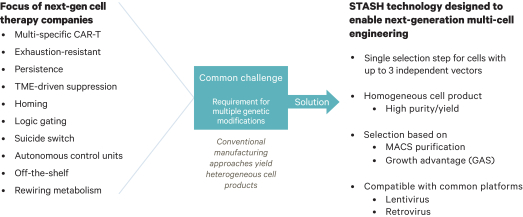
 .. It has been shown in a third-party study that the choice of costimulatory domain influences the persistence and memory phenotype of CAR T Cells. The inclusion of a 4-1BB costimulatory domain has been associated with reduced frequencies of serious adverse events and improved clinical outcomes in tumor models. The CD22 CAR used to create CRG-022 contains a 4-1BB costimulatory domain.
.. It has been shown in a third-party study that the choice of costimulatory domain influences the persistence and memory phenotype of CAR T Cells. The inclusion of a 4-1BB costimulatory domain has been associated with reduced frequencies of serious adverse events and improved clinical outcomes in tumor models. The CD22 CAR used to create CRG-022 contains a 4-1BB costimulatory domain.