As filed with the U.S. Securities and Exchange Commission on August 9, 2024
Registration No. 333-279801
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 to
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ASEP MEDICAL HOLDINGS INC.
(Exact Name of Registrant as Specified in Its Charter)
Not Applicable
(Translation of Registrant’s Name into English)
| British Columbia | | 541710 | | Not Applicable |
(State or other Jurisdiction of Incorporation or Organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification Number) |
420 – 730 View Street
Victoria, BC V8W 3Y7
Canada
Tel: 778-600-0509
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Clark Wilson LLP
Suite 900 - 885 West Georgia St
Vancouver, BC, V6C 3H1
604-687-5700
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copy to:
Darrin Ocasio, Esq. Glenn Burlingame, Esq. Sichenzia Ross Ference Carmel LLP 1185 Avenue of the Americas, 31st Floor New York, NY 10036 Tel: (212) 930-9700 Fax: (212) 930 9725 | | Anthony W. Basch, Esq. Yan (Natalie) Wang, Esq. Chenxi Lu, Esq. Kaufman & Canoles, P.C. 1021 East Cary Street, Suite 1400 Richmond, VA 23219 Tel: (804) 771-5700 Fax: (888) 360-9092 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission acting pursuant to said Section 8(a) may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | | SUBJECT TO COMPLETION | | DATED AUGUST 9, 2024 |
Units, each Unit consisting of
One Common Share or One Pre-Funded Warrant to Purchase One Common Share, and
One Common Warrant to purchase One Common Share
Up to Common Shares underlying the Pre-Funded Warrants, and
Up to Common Shares underlying the Common Warrants
This is an initial public offering in the United States of up to units (“Units”) of ASEP Medical Holdings Inc., with each Unit consisting of one common share no par value (“common share”), and one warrant to purchase one common share (each a “common warrant”), based on an assumed initial public offering price of $ per Unit, the midpoint of the range discussed below. Our common shares are currently quoted on the Canadian Stock Exchange under the trading symbol “ASEP” and on the OTC Pink operated by the OTC Markets Group Inc. (the “OTC Pink”) under the trading symbol “SEPSF”. On May 28, 2024, the closing price of our common shares on CSE was CAD 0.1850 and the last reported sale price for our common shares on the OTC Pink was $0.05.
We anticipate that the initial public offering price of each Unit will be between $[__] and $[__] per Unit. We are offering all Units offered by this prospectus. This prospectus also covers the common shares issuable upon exercise of the Pre-Funded Warrants, and the common warrants.
We are also offering to each purchaser of Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our common shares outstanding immediately following the consummation of this offering, the opportunity to purchase Units consisting of one pre-funded warrant (in lieu of one common share, each a “Pre-Funded Warrant”), and one common warrant. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such limit may be increased to up to 9.99%) of the number of common shares outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one common share. The purchase price of each Unit including a Pre-Funded Warrant will be equal to the price per Unit that includes one common share, minus $0.0001, and the remaining exercise price of each Pre-Funded Warrant will equal $0.0001 per share. The Pre-Funded Warrants will be immediately exercisable (subject to the beneficial ownership cap) and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Unit including a Pre-Funded Warrant we sell (without regard to any limitation on exercise set forth therein), the number of Units including a share of common stock we are offering will be decreased on a one-for-one basis. The Pre-Funded Warrant may be exercised on a cashless basis.
The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The common shares and Pre-Funded Warrants can each be purchased in this offering only with the accompanying common warrants, as part of a Unit, but the components of the Unit are immediately separable and will be issued separately in this offering. See “Description of Securities” in this prospectus for more information.
We have applied to list the common shares on The Nasdaq Stock Market LLC under the symbol “SEPS”. No assurance can be given that our application will be approved. Completion of this offering is contingent on the approval of our listing application for trading of our common shares on the Nasdaq Capital Market. There is no established trading market for any of our common shares, Pre-Funded Warrants, or common warrants. We do not expect a market to develop for our Pre-Funded Warrants or our common warrants, neither do we intend to apply for a listing of any Pre-Funded Warrants, or common warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants and the common warrants will be limited.
We are both an “emerging growth company” and a “foreign private issuer” as defined under the U.S. federal securities laws and, as such, may elect to comply with certain reduced public company reporting requirements for this and future filings. See “Prospectus Summary — Implications of Being an Emerging Growth Company” and “Prospectus Summary — Implications of Being a Foreign Private Issuer.”
An investment in our securities is highly speculative, involves a high degree of risk and should be considered only by persons who can afford the loss of their entire investment. See “Risk Factors” beginning on page 9 of this prospectus.
| | | Per Unit comprising Common Share and common warrant | | | Per Unit comprising Pre-Funded Warrant and common warrant | | | Total Without Over- Allotment Option | | | Total With Over- Allotment Option | |
| Initial public offering price | | $ | | | | $ | | | | $ | | | | $ | | |
| Underwriting discounts and commissions (8%) (1) | | $ | | | | $ | | | | $ | | | | $ | | |
| Proceeds, before expenses, to us | | $ | | | | $ | | | | $ | | | | $ | | |
| (1) | We have also agreed to pay a non-accountable expense allowance to Aegis Capital Corp. (“Aegis”) as the underwriter, of one percent (1%) of the gross proceeds received in this offering and to reimburse the underwriter for other out-of-pocket expenses related to the offering. In addition, Aegis will receive warrants to purchase up to a total of [__] common shares (equal to five percent (5%) of the aggregate number of common shares included in the Units and issuable upon exercise of the Pre-Funded Warrants sold in this offering, excluding the exercise of the over-allotment option described in the paragraph below) and exercisable at a price per share equal to 125% of the offering price (the “Underwriter’s Warrants”), which are also being registered under this registration statement. For a description of compensation to be received by the underwriter, see “Underwriting.” |
We have granted the underwriter an option, exercisable for forty-five (45) days from the closing of the offering, to purchase up to an additional [__] common shares and/or Pre-Funded Warrants and [ ] common warrants (equal to fifteen percent (15%) of the total number of Units to be offered by us in this offering) on the same terms as the common shares, Pre-Funded Warrants, and common warrants being purchased by the underwriter from us. For a description of the other compensation to be received by the underwriter, see “Underwriting.”
The underwriter expects to deliver the securities comprising the Units against payment in U.S. dollars on or about [___], 2024.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SECURITIES AND EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION NOR HAS THE SECURITIES AND EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Sole Book – Running Manager
Aegis Capital corp.
The date of this prospectus is , 2024.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form F-1 that we filed with the U.S. Securities and Exchange Commission (the “SEC”). You should read this prospectus and the information and documents incorporated herein by reference carefully. Such documents contain important information you should consider when making your investment decision. See “Where You Can Find Additional Information” and “Incorporation of Certain Documents by Reference” in this prospectus.
You should rely only on the information contained in or incorporated by reference into this prospectus. We have not authorized anyone to provide you with information different from, or in addition to, that contained in or incorporated by reference into this prospectus. This prospectus is an offer to sell only the securities offered hereby but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in or incorporated by reference into this prospectus is current only as of their respective dates or on the date or dates that are specified in those documents. Our business, financial condition, results of operations and prospects may have changed since those dates.
Unless the context otherwise requires the terms “ASEP Medical Holdings Inc.,” “ASEP,” “we,” “us,” “our,” the “Company,” and “our business” refer to ASEP Medical Holdings Inc., a Canadian public company together with its subsidiaries.
We are not offering to sell or seeking offers to purchase these securities in any jurisdiction where the offer or sale is not permitted. We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the jurisdiction of the United States who come into possession of this prospectus are required to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus applicable to that jurisdiction.
TRADEMARKS AND TRADENAMES
All service marks, trademarks and trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ®, © and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
PRESENTATION OF FINANCIAL INFORMATION
We publish our consolidated financial statements in Canadian dollars as of April 29, 2024. In this prospectus, unless otherwise specified, all references to “C$” and “CAD$” means Canadian dollars and all references to “$”and “dollars” mean United States dollars.
This prospectus includes our audited annual consolidated financial statements, or the “Financial Statements.” Our audited consolidated financial statements for the years ended December 31, 2023 and December 31, 2022 were prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, the independent, private-sector body that develops and approves IFRS, and Interpretations issued by the International Financial Reporting Interpretations Committee, or IFRIC. None of the financial statements were prepared in accordance with generally accepted accounting principles in the United States.
INDUSTRY AND MARKET DATA
This prospectus includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable. Although we are responsible for all of the disclosures contained in this prospectus, including such statistical, market and industry data, we have not independently verified any of the data from third-party sources, nor have we ascertained the underlying economic assumptions relied upon therein. In addition, while we believe the market opportunity information included in this prospectus is generally reliable and is based on reasonable assumptions, such data involves risks and uncertainties, including those discussed under the heading “Risk Factors.”
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
We have made statements in this prospectus, including under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Our Business” and elsewhere that constitute forward-looking statements. Forward-looking statements involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “should,” “will,” “could” and similar expressions denoting uncertainty or an action that may, will or is expected to occur in the future. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements. Examples of forward-looking statements include, but are not limited to, statements about the following:
● our prospects, including our future business, revenues, expenses, net income, earnings per share, gross margins, profitability, cash flows, cash position, liquidity, financial condition and results of operations, backlog of orders and revenue, our targeted growth rate, our goals for future revenues and earnings, and our expectations about realizing the revenues in our backlog and in our sales pipeline;
● the potential impact of COVID-19 on our business and results of operations;
● the effects on our business, financial condition and results of operations of current and future economic, business, market and regulatory conditions, including the current economic and market conditions and their effects on our customers and their capital spending and ability to finance purchases of our products, services, technologies and systems;
● the effects of fluctuations in sales on our business, revenues, expenses, net income, earnings per share, margins, profitability, cash flows, capital expenditures, liquidity, financial condition and results of operations;
● our products, services, technologies and systems, including their quality and performance in absolute terms and as compared to competitive alternatives, their benefits to our customers and their ability to meet our customers’ requirements, and our ability to successfully develop and market new products, services, technologies and systems;
● our markets, including our market position and our market share;
● our ability to successfully develop, operate, grow and diversify our operations and businesses;
● our business plans, strategies, goals and objectives, and our ability to successfully achieve them;
● the sufficiency of our capital resources, including our cash and cash equivalents, funds generated from operations, availability of borrowings under our credit and financing arrangements and other capital resources, to meet our future working capital, capital expenditure, lease and debt service and business growth needs;
● the value of our assets and businesses, including the revenues, profits and cash flows they are capable of delivering in the future;
● the effects on our business operations, financial results, and prospects of business acquisitions, combinations, sales, alliances, ventures and other similar business transactions and relationships;
● industry trends and customer preferences and the demand for our products, services, technologies and systems; and
● the nature and intensity of our competition, and our ability to successfully compete in our markets.
These statements are necessarily subjective, are based upon our current plans, intentions, objectives, goals, strategies, beliefs, projections and expectations, and involve known and unknown risks, uncertainties and other important factors that could cause our actual results, performance or achievements, or industry results, to differ materially from any future results, performance or achievements described in or implied by such statements. Actual results may differ materially from expected results described in our forward-looking statements, including with respect to correct measurement and identification of factors affecting our business or the extent of their likely impact, the accuracy and completeness of the publicly-available information with respect to the factors upon which our business strategy is based, or the success of our business. Furthermore, industry forecasts are likely to be inaccurate, especially over long periods of time.
Forward-looking statements should not be read as a guarantee of future performance or results and will not necessarily be accurate indications of whether, or the times by which, our performance or results may be achieved. Forward-looking statements are based on information available at the time those statements are made and management’s belief as of that time with respect to future events and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that may cause actual results, our performance or achievements, or industry results to differ materially from those contemplated by such forward-looking statements include, without limitation, those discussed under the caption “Risk Factors” in this prospectus as well as other risks and factors identified from time to time in our SEC filings.
PROSPECTUS SUMMARY
This summary highlights information that we present more fully in the rest of this prospectus. This summary does not contain all of the information you should consider before buying common shares in this offering. This summary contains forward-looking statements that involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “should,” “will,” “could,” and similar expressions denoting uncertainty or an action that may, will or is expected to occur in the future. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements. You should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and the notes to those statements.
Business Overview
We are focused on combating global health issues through diagnostic and therapeutic solutions by acquiring assets, technologies and/or businesses in the areas of life sciences and medical diagnostics. Currently, we are focused on the issue of antibiotic failure, which encompasses the widespread and deadly crises of sepsis and biofilm infections. Antibiotic failure occurs when an infection is not responsive to antibiotics. Per year, there are estimated to be over 49 million cases of sepsis and more than 11 million deaths due to sepsis1. Since the major front line treatment for sepsis is potent antibiotics, this high death rate represents antibiotic failure. Furthermore, severe COVID-19, caused by infection with a coronavirus (SARS-CoV-2), is severe sepsis and caused a further 6-18 million deaths to date. In the U.S., 65% of all infections are due to biofilms that are highly resistant to antibiotics, which is estimated to cost global health care $368 billion dollars annually.2
Sepsis and antibiotic resistant infections impose significant burdens on healthcare systems and create the need for new diagnostics and therapeutics. In 2020, the global sepsis diagnostic market was valued at $569.49 million and is projected to reach $1.2 billion by 2030, based on a compound annual growth rate (“CAGR”) of 7.8% from 2021 to 2030.3 Our therapeutic anti-biofilm products are directed at wound care, oral health care and sinusitis, which have global markets of $22.5 billion (CAGR 4.1%), 2.8 billion (CAGR 3.5%) and $33.7 billion (CAGR 6.4%) respectively.5 The global antibiotic market is $40.9 billion and has a CAGR of 3.7%.5
Our fight against sepsis and antibiotic failure has two fronts, diagnostic and therapeutic. Our diagnostic product consists of a novel assay that is intended to provide earlier, faster risk assessment/diagnosis to enable informed treatment of sepsis. Based on data from published clinical research studies with more than 700 patients our diagnostic technology is designed to detect sepsis based on whether a patient has unique molecular signatures of sepsis specific to their immune response instead of detecting the presence of a pathogen, and, as such, does not differentiate between bacterial and viral sepsis. Furthermore, although sepsis is the body’s response to an infection, not all patients with sepsis have an identifiable pathogen associated with sepsis development. Conventional sepsis diagnoses can take approximately 13 to 48 hours, but our technology has the potential to provide results in approximately 1 hour after a blood draw in the emergency room or intensive care unit. Our diagnostic technology has been developed using data acquired in a large clinical study performed by our founding scientists.
Our therapeutic product consists of patented pharmaceutical peptides that target presently untreatable biofilm infections and the associated pathology caused by inflammation. Currently, 65% of all infections are caused by biofilms and there are no approved drugs to fight them. Our technology involves small potent broad-spectrum anti-biofilm and anti-inflammatory peptides6. Our therapeutic products are directed against wound, oral and sinusitis biofilm infections. Our peptides have been optimized using a combination of conventional structure-activity relationship studies and artificial intelligence (AI) aided discovery. The AI studies have employed neural networks to relate structural descriptors, based on the primary sequences of the peptides, to activity, and predict the activities of computationally-generated peptide sequences6a. We are increasingly employing generative and explainable AI methods to generate further improved peptides. Proceeds from this offering will be applied to further develop therapeutic product candidates in these areas in addition to sepsis diagnostics.
Our peptides have not been shown to be safe and effective in humans. Statements related to safety and efficacy are within the sole authority of the FDA. Indeed, we have not performed human clinical studies to formally demonstrate a safety profile for our therapeutic candidates that is consistent with our animal model results, which are not pre-clinical studies and cannot support an investigational new drug (“IND”) application, and play no part in the regulatory approval process. Pre-clinical studies must be conducted to enable the Company to perform human clinical trials. Currently, we do not have any commercial products that compete in the markets for sepsis diagnostics and anti-infective therapeutics. Further, none of our products have yet been approved by the Food and Drug Administration or other regulators as safe and effective. Almost all funding obtained by the Company to date has gone towards product development and associated activities and thus represents a loss of $5,757,286 for the year ended December 31, 2022, and $2,343,563 for the period from January 20, 2021 (incorporation) to December 31, 2021; we anticipate that we will require future funding for these activities. As of the date of this prospectus, our auditor has included an explanatory paragraph in their opinion that accompanies our audited consolidated financial statements as of and for the year ended December 31, 2022, indicating that our current liquidity position raises substantial doubt about our ability to continue as a going concern. Please see “Risks Associated with Our Business” in this summary on page 6 and the section entitled “Risk Factors” for a description of the risks and limitations we may face.
1 Rudd, K.E., Johnson, S.C., Agesa, K.M., Shackelford, K.A., Tsoi, D., Kievlan, D.R., Colombara, D.V., Ikuta, K.S., Kissoon, N., Finfer, S., Fleischmann-Struzek, C (2020). Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet. 395(10219), 200-11.
2 Cámara et al. (2022) Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. NPJ Biofilms and Microbiomes 8:42.
3 https://www.globenewswire.com/news-release/2022/10/18/2536561/0/en/Global-Sepsis-Therapeutics-Market-to-Reach-435-9-Billion-by-2027.html
4 https://www.globenewswire.com/news-release/2022/10/18/2536561/0/en/Global-Sepsis-Therapeutics-Market-to-Reach-435-9-Billion-by-2027.html
5 https://www.globenewswire.com/en/news-release/2023/04/06/2642247/0/en/Antibiotics-Market-Size-58-4-Bn-by-2032-at-3-7-CAGR-Global-Analysis-by-Market-us.html; https://www.grandviewresearch.com/industry-analysis/wound-care-market; https://www.businessresearchinsights.com/market-reports/sinusitis-drugs-market-101686; https://www.grandviewresearch.com/industry-analysis/oral-care-market
6 Fuente-NúñezC, REW Hancock et al. 2014. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathogens 10(5):e1004152; Fuente-Núñez C, REW Hancock et al. 2015. D-enantiomeric peptides that eradicate wild-type and multi-drug resistant bacterial biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 22:196–205; Pletzer, D., S.C. Mansour, and R.E.W. Hancock. 2018. Synergy between conventional antibiotics and anti-biofilm peptides in a murine,sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathogens 14(6):e1007084; Reffuveille F, C. REW Hancock et al. 2014. A broad-spectrum anti-biofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 58:5363-5371; Zhang, T., L.Xia, Z. Wang, R.E.W. Hancock, and M. Haapasalo. 2021. Recovery of oral in vitro biofilms after exposure to peptides and chlorhexidine. J. Endodontics 47:466-471; Alford, M.A., K.-Y.G. Choi, M.J. Trimble, H. Masoudi, P. Kalsi, D. Pletzer, R.E.W. Hancock. 2021. Murine models of sinusitis infection for screening antimicrobial and immunomodulatory therapies. Frontiers Cell. Infect. Microbiol. 11:621081; Wu, B.C., A.H. Lee, and R.E.W. Hancock. 2017. Mechanisms of the innate defense regulator peptide-1002 anti-inflammatory activity in a sterile inflammation mouse model. J. Immunol. 199:3592-3603.
6a. Cherkasov, A., K. Hilpert, H. Jenssen, C.D. Fjell, M. Waldbrook, S.C. Mullaly, R. Volkmer and R.E.W. Hancock. 2009. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic resistant Superbugs. ACS Chemical Biol. 4:65-74.
Haney, E.F., Y. Brito-Sánchez, M.J. Trimble, S.C. Mansour, A. Cherkasov, and R.E.W. Hancock. 2018. Computer-aided discovery of peptides that specifically attack bacterial biofilms. Sci. Reports 8:1871 .

Company History and Development
On January 20, 2021, we were incorporated under the British Columbia Business Corporations Act (“BCBCA”) under the name Trenchant Life Sciences Investment Corp. On November 9, 2021, we changed our name to ASEP Medical Holdings Inc.
On November 22, 2021, we commenced trading on the Canadian Securities Exchange (“CSE”) as a life sciences issuer under the trading symbol “ASEP”. On April 19, 2022, we commenced trading on the OTCQB under the trading symbol “SEPSF”, although we currently trade on the OTC Pink. On November 10, 2022, we commenced trading on the Frankfurt Stock Exchange (“FSE”) under the trading symbol “FSX:JJ8”.
Since our inception in 2021, ASEP has been a committed innovator within the medical industry. We strive to mitigate the global crisis of antibiotic failure by improving patients’ odds of survival and quality of life. Currently, we are developing therapeutic and diagnostic products through two of our subsidiaries. As of the date of this prospectus, we have the following subsidiaries:
| | | Principal Activity | | Location | | Percentage | |
| Asep Medical Inc. | | Life Sciences | | British Columbia, Canada | | | 100 | % |
| ABT Innovations Inc. (“ABT”)# | | Life Sciences | | British Columbia, Canada | | | 50.1 | % |
| Sepset Biosciences Inc. (“Sepset”) | | Life Sciences | | British Columbia, Canada | | | 50.1 | % |
| SafeCoat Medical Inc. (“SafeCoat”)(1) | | Life Sciences | | British Columbia, Canada | | | 100 | % |
| OHP Innovations Inc. (“OHP”)* | | Life Sciences | | British Columbia, Canada | | | 100 | % |
(1) Pursuant to the Start-Up License Agreement (as defined herein), UBC and certain inventors will collectively own 12% of the total issued and outstanding common shares of SafeCoat following the issuance of the consideration shares. As at the date of this Registration Statement, the consideration shares have not yet been issued and ASEP owns 100% of SafeCoat. ASEP will own 88% of SafeCoat, with UBC and inventors receiving a total of 12% of shares as a requirement of the licensing agreement with UBC.
# Our subsidiary ABT owns 100% of ABT Peptides Inc, an inactive company incorporated in British Columbia, Canada.
* Our subsidiary OHP is inactive.
ABT Innovations Inc.
ABT was incorporated on July 3, 2015 pursuant to the provisions of the BCBCA under the name “ABT Innovations Inc.” for the purpose of ensuring the commercialization of the broad peptide technology developed by its founder, Dr. Robert E.W. Hancock. ABT’s peptide technology covers a broad range of therapeutic applications including bacterial biofilm infections. Bacterial biofilms are serious global health concern due to their abilities to shelter bacteria from antibiotics and host defense during infection. Therefore, biofilms contribute to persistent chronic infections; they play a significant role in human infections as diverse as medical device infections, chronic infections, lung, bladder, wound, dental, skin, ear-nose and throat, sinusitis, orthopedic, etc., representing two thirds of all infections.7 Our technology also includes anti-inflammatories, anti-infective immune-modulators and vaccine adjuvants.
Sepset Biosciences Inc.
Sepset was incorporated on April 23, 2015 pursuant to the provisions of the BCBCA under the name “Sepset Biosciences Inc.” for the purpose of ensuring the commercialization of a diagnostic kit for predicting the onset of severe sepsis and organ failure that was developed by its founder, Dr. Robert E.W. Hancock. The Sepset diagnostic technology involves a patient gene expression signature predictive of the development of severe sepsis and organ failure. The SepsetER test involves drawing a small amount of a patient’s blood and determining whether the patient presents with the sepsis markers in their gene expression profiles as assessed by nucleic acid amplification technologies. The SepsetER diagnostic technology differs from current diagnostic tests by enabling diagnosis of severe sepsis within 1-2 hours of first clinical presentation (i.e., in the emergency room), while other diagnostics only provide definitive diagnosis after 13-48 hours of first clinical presentation. We believe this will enable critical early decisions to be made by physicians regarding appropriate therapies leading to reduced overall morbidity and mortality due to sepsis.
SafeCoat Medical Inc.
On December 1, 2022, we entered into a definitive Earn-In and Option Agreement with SafeCoat Medical Inc. (“SafeCoat”) and the security holders of SafeCoat, whereby we acquired a 50.1% equity interest of SafeCoat and were granted the option to acquire the remaining common shares of SafeCoat from the securityholders, such that, subject to any future ownership interest of the University of British Columbia (“UBC”), we would own 100% of common shares of SafeCoat, free and clear of all liens. On December 12, 2022, we exercised the option and completed our 100% acquisition of SafeCoat. On July 31, 2023, ASEP, through SafeCoat, entered into an exclusive worldwide license agreement with UBC for the use, development and commercialization of medical device coating technology. Through this agreement, UBC and certain inventors of the technology will hold 12% of SafeCoat while ASEP will hold 88% following the issuance of the consideration shares. Pursuant to the Start-Up License Agreement (as defined herein), UBC and certain non-waiving inventors (namely Dr. Jayachandran Kizhakkedathu, Dr. Dirk Lange, Dr. Robert Hancock, and Dr. Kai Yu) will collectively own 12% (8% to UBC and 1% to each of the non-waiving inventors) of the total issued and outstanding common shares of SafeCoat following the issuance of the consideration shares. As at the date of this Registration Statement, the consideration shares have not yet been issued and ASEP owns 100% of SafeCoat. Following such issuance, ASEP will own 88% of SafeCoat, with UBC and the non-waiving inventors receiving a total of 12% of shares (as further described above) as a requirement of the licensing agreement with UBC.
SafeCoat’s technology provides coatings for medical devices that are non-fouling (i.e. do not readily bind live or dead bacteria) and are protected by a peptide coating that kills bacteria. This product addresses the definitive risk of infection of medical devices including prosthetics, implants, catheters and stents, etc. Indeed, over half of the nearly 2 million healthcare-associated infections in the U.S. annually can be attributed to indwelling medical devices.8
ASEP’s head office is located at 420 – 730 View Street, Victoria, BC V8W 3Y7 and its registered office is located at 800 – 885 West Georgia Street, Vancouver, BC V6C 3H1. Our main telephone number is 778-600-0509.
Emerging Growth Company Status
As a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012, and may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| | ● | being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in our SEC filings; |
| | | |
| | ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| | | |
| | ● | reduced disclosure obligations regarding executive compensation in periodic reports, proxy statements and registration statements; and |
| | | |
| | ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval. |
7 Sharma, D., Misba, L. & Khan, A.U. 2019. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 8, 76.
8 VanEpps J.S. & Younger J.G. 2016. Implantable device related infection. Shock. 46(6):597-608.
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended (the “Securities Act”). However, if certain events occur before the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.235 billion or we issue more than $1.00 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company before the end of such five-year period.
In addition, Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and acknowledge such election is irrevocable pursuant to Section 107 of the JOBS Act.
We have elected to take advantage of certain of the reduced disclosure obligations regarding executive compensation in this prospectus and, as long as we continue to qualify as an emerging growth company, we may elect to take advantage of this and other reduced burdens in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
Competitive Market Conditions
The pharmaceutical and biotechnology industries are intensely competitive. We compete directly and indirectly with other pharmaceutical companies, biotechnology companies and academic and research organizations in developing therapies to treat diseases and antibiotic resistance. There is no strong evidence that any of our competitors in the therapeutics space are developing products that work against a broad spectrum of bacterial biofilms or have conjoint anti-inflammatory activity. As for the sepsis diagnostics, our competitive edge lies in the timeframe required to identify patients who might develop severe sepsis as well as our ability to provide a binary (sepsis or not) risk assessment. As mentioned earlier, conventional sepsis diagnoses can take approximately 13 to 48 hours whereas SepsetER results should be available within approximately 1 hour.
Foreign Private Issuer Status
We are a “foreign private issuer,” as defined in Rule 405 under the Securities Act and Rule 3b-4(c) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As a result, we are not subject to the same requirements as U.S. domestic issuers. Under the Exchange Act, we will be subject to reporting obligations that, to some extent, are more lenient and less frequent than those of U.S. domestic reporting companies. For example, we will not be required to issue quarterly reports or proxy statements. We will not be required to disclose detailed individual executive compensation information. Furthermore, our directors and executive officers will not be required to report equity holdings under Section 16 of the Exchange Act and will not be subject to the insider short-swing profit disclosure and recovery regime.
As an exempted Canadian company to be listed on The Nasdaq Stock Market (“Nasdaq”), we will be subject to Nasdaq’s corporate governance listing standards. However, Nasdaq rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in Canada, which is our home country, may differ significantly from Nasdaq corporate governance listing standards.
Currently, as a Canadian company, we intend to rely on home country practice with respect to our corporate governance. A description of the significant ways in which our governance practices currently differ from those followed by domestic companies pursuant to the Rule 5600 series of the Nasdaq Stock Market Rules is set out below:
| ● | Quorum Requirement —Nasdaq Listing Rule 5620(c) provides that the minimum quorum requirement for a meeting of shareholders is 33 1/3 % of the outstanding common voting shares. We do not follow this Nasdaq Listing Rule. Instead, we follow our articles which provide that the quorum for the transaction of business at a meeting of shareholders is one or more persons, present in person or by proxy. |
| | |
| ● | Shareholder Approval Requirements —In certain instances, Nasdaq Listing Rule 5635 requires each issuer to obtain shareholder approval before an issuance of securities in connection with: (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; and (iii) transactions other than public offerings. We do not follow this Nasdaq Listing Rule. Instead, we comply with the laws, rules and regulations of Canada and the Province of British Columbia and the policies of the Canadian Securities Exchange, which have different requirements for shareholder approval (including, in certain instances, not requiring any shareholder approval) in connection with issuances of securities in the circumstances listed above. |
Risks Associated with Our Business
Our ability to execute our business strategy is subject to numerous risks, as more fully described in the section captioned “Risk Factors” immediately following this prospectus summary. You should read these risks before you invest in our securities. In particular, risks associated with our business include, but are not limited to, the following:
| | ● | We have a relatively limited operating history and have not generated revenue to date and thus are subject to risks of business development and you have no basis on which to evaluate our ability to achieve our business objective. |
| | ● | Future acquisitions or strategic investments could disrupt our business and harm our business, results of operations or financial condition. |
| | ● | We will require additional funding for our growth plans, and such funding may result in a dilution of your investment. |
| | ● | We may not have sufficient capital to fund our ongoing operations, effectively pursue our strategy or sustain our growth initiatives. |
| | ● | If we fail to keep up with technological developments, our business, results of operations and financial condition may be materially and adversely affected. |
| | ● | If we cannot continue to develop, acquire, market and offer new products and services or enhancements to existing products and services that meet customer requirements, our operating results could suffer. |
| | ● | We could make significant investments in new products and services that may not achieve expected returns. |
| | ● | Changes in laws or regulations, or a failure to comply with any laws and regulations, may adversely affect our business, investments and results of operations. |
| | ● | Public health epidemics or outbreaks, such as COVID-19, could materially and adversely impact our business. |
THE OFFERING
| Units offered | | Up to [__] Units, based on an assumed initial public offering price of $[ ] per Unit, the midpoint of the initial public offering price range reflected on the cover page. Each Unit consists of one common share (or a Pre-Funded Warrant to purchase one common share in lieu thereof) and one common warrant. The common shares and Pre-Funded Warrants, if any, can each be purchased in this offering only with the accompanying common warrant, as part of a Unit, but the components of the Units are immediately separable and will be issued separately in this offering. |
| | | |
| Units, comprising Pre-Funded Warrants, offered by us in this offering | | We are offering to each purchaser whose purchase of Units consisting of common shares in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common shares immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, Units, that include Pre-Funded Warrants, in lieu of common shares. Subject to limited exceptions, a holder of a Pre-Funded Warrant will not have the right to exercise any portion of its Pre-Funded Warrant if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of common shares outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one common share. The purchase price of each Unit that includes a Pre-Funded Warrant will equal the price per Unit which comprises a common share and a common warrant, minus $0.0001, and the exercise price of each Pre-Funded Warrant will be $0.0001 per share. The Pre-Funded Warrants are immediately exercisable and may be exercised at any time until all the Pre-Funded Warrants are exercised in full. This offering also relates to the common shares issuable upon exercise of any Pre-Funded Warrants sold in this offering. For each Pre-Funded Warrant we sell, the number of common shares we are offering will be decreased on a one-for-one basis. See “Description of Securities” in this prospectus for more information. |
| | | |
| Common Warrants being offered by us | | Each Unit being offered includes one common share, or one Pre-Funded Warrant in lieu of one common share, and a common warrant. Each common warrant is exercisable at a price of $ per share, or pursuant to an alternate cashless exercise option. The common warrants will be immediately exercisable upon issuance and will expire five (5) years from the closing date of this offering. This prospectus also relates to the offering of the common shares issuable upon exercise of the common warrants. See “Description of Securities” in this prospectus for more information. |
| | | |
Over-allotment Option to purchase additional common shares from us | | We have granted the underwriter an option, exercisable for forty-five (45) days after the closing of this offering, to purchase up to an additional [__] common shares and/or Pre-Funded Warrants and [ ] common warrants (equal to fifteen percent (15%) of the total number of Units to be offered by us in this offering) on the same terms as the other Units being purchased by the underwriter from us. |
| | | |
| Common shares outstanding before this offering | | 76,059,147 common shares. |
| | | |
| Common shares outstanding after this offering | | [__] common shares (assuming no sale of any Pre-Funded Warrants and assuming none of the common warrants issued in this offering are exercised). |
| | | |
| Use of Proceeds | | We intend to use the proceeds from this offering for working capital and general corporate purpose. Additionally, we may use a portion of the net proceeds from this offering to acquire or invest in complementary products or assets. However, we have no current plans, commitments or obligations to do so. See “Use of Proceeds” for more information. |
| | | |
| Underwriter | | Aegis Capital Corp. |
| | | |
| Underwriter’s Warrants | | We have agreed to issue to Aegis warrants to purchase up to a total of [__] common shares (equal to five percent (5%) of the aggregate number of common shares included in the Units and issuable upon exercise of the Pre-Funded Warrants sold in this offering, excluding the exercise of the over-allotment option), which are exercisable at a price per share of [__] (equal to 125% of the offering price) during the four- year-and-six-month period commencing six months after the commencement of sales in the offering. |
| | | |
| Lockup Agreements | | Our executive officers, directors, employees and shareholders holding ten percent (10%) or more of our common shares prior to the offering, collectively, have agreed with the underwriter not to sell, transfer or dispose of any common shares or similar securities for a period of 90 days following the closing of this offering. |
| | | |
| Trading symbol | | We have applied for listing of our common shares on The Nasdaq Stock Market under the symbol “SEPS.” The offering will not proceed unless the Company is accepted for listing on Nasdaq. |
| | | |
Listing of Pre-Funded Warrants and common warrants | | We do not intend to list the Pre-Funded Warrants or the common warrants on any securities exchange or nationally recognized trading system. Without a trading market, the liquidity of the Pre-Funded Warrants and common warrants will be extremely limited. |
| | | |
| Risk Factors | | Investing in these securities involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section of this prospectus before deciding to invest in our common shares. |
SUMMARY CONSOLIDATED FINANCIAL DATA
The following tables summarize our historical financial data. The summary consolidated statements of operation for the year ended December 31, 2023 and the summary consolidated balance sheets as of December 31, 2023 have been derived from our consolidated financial statements included elsewhere in this prospectus. The following summary financial data should be read in conjunction with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future.
The following table presents our summary consolidated statements of operation for the year ended December 31, 2023:
| | | For the year ended December 31, 2023 | |
| Revenue | | $ | 21,140 | |
| Total revenue | | | 21,140 | |
| Expenses | | | | |
| Amortization | | | 1,294,266 | |
| Board advisory fees | | | 230,673 | |
| Compensation | | | 782,301 | |
| Consulting | | | 514,478 | |
| General and administrative | | | 222,903 | |
| Investor relations | | | 1,110,409 | |
| Patent fees | | | 134,880 | |
| Professional fees | | | 760,006 | |
| Research and development costs | | | 678,543 | |
| Share-based compensation | | | 1,952,054 | |
| Transfer agent and filing fees | | | 61,890 | |
| Total expenses | | | 7,742,403 | |
| Operating loss | | | (7,721,263 | ) |
| Other income (expenses) | | | | |
| Borrowing costs | | | (270 | ) |
| Foreign exchange loss | | | (4,116 | ) |
| Loss on debt settlement | | | (429,864 | ) |
| SafeCoat Medical Inc. acquisition expense | | | (1,275,000 | ) |
| Net loss and comprehensive loss for period | | $ | (9,430,513 | ) |
| Net loss attributable to: | | | | |
| Shareholders of ASEP | | $ | (8,227,328 | ) |
| Non-controlling interest | | | (1,203,185 | ) |
| | | $ | (9,430,513 | ) |
| Loss per share – basic and fully diluted | | $ | (0.13 | ) |
| Weighted average number of common shares outstanding – basic and fully diluted | | | 62,235,372 | |
The following table presents our summary consolidated balance sheet data as of December 31, 2023:
| | | December 31, 2023 | |
| ASSETS | | | | |
| Current assets | | | | |
| Cash | | $ | 64,721 | |
| GST receivable | | | 25,423 | |
| Prepaids and deposits | | | 160,799 | |
| | | | 250,943 | |
| Non-current assets | | | | |
| Equipment | | | 37,183 | |
| Intangible assets | | | 27,949,560 | |
| Investment in Hunan Sanway Sepsmart Ltd. | | | 2,375,000 | |
| | | | 25,361,743 | |
| TOTAL ASSETS | | $ | 25,612,686 | |
| LIABILITIES | | | | |
| Current liabilities | | | | |
| Accounts payable and accrued liabilities | | $ | 677,731 | |
| Deferred revenue | | | 297,586 | |
| Loan payable | | | 20,270 | |
| | | | 995,587 | |
| Non-current liabilities | | | | |
| Deferred revenue | | | 2,056,274 | |
| TOTAL LIABILITIES | | | 3,051,861 | |
| EQUITY | | | | |
| Share capital | | | 24,045,616 | |
| Other components of equity | | | 3,550,141 | |
| Deficit | | | (14,983,767 | ) |
| Total equity attributable to ASEP shareholders | | | 12,611,990 | |
| Non-controlling interest | | | 9,948,835 | |
| TOTAL EQUITY | | | 22,560,825 | |
| TOTAL LIABILITIES AND EQUITY | | $ | 25,612,686 | |
RISK FACTORS
Investing in our securities is speculative and involves a high degree of risk. You should consider carefully the following risk factors, as well as the other information in this prospectus, including our consolidated financial statements and notes thereto, before you decide to purchase our securities. If any of the following risks actually occur, our business, financial condition, results of operations and prospects could be materially adversely affected, the value of our common shares could decline, and you may lose all or part of your investment. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of a number of factors, including the risks described below. See “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to Our Business
If ABT or Sepset experience delays or difficulties in the enrollment of volunteers or patients in the clinical trials, receipt of necessary regulatory approvals could be delayed or prevented.
Clinical trials for treatment candidates require identification and enrollment of a large number of volunteers or eligible patients. ABT or Sepset may not be able to enroll sufficient volunteers or eligible patients to complete clinical trials in a timely manner or at all. Patient enrollment is a function of many factors, including the following: design of the protocol, size of the patient population, eligibility criteria for the study in question, perceived risks and benefits of the product under study, availability of competing therapies, efforts to facilitate timely enrollment in clinical trials, patient referral practices of physicians, and availability of clinical trial sites. If ABT or Sepset have difficulty enrolling sufficient volunteers or patients to conduct its clinical trials as planned, they may need to delay, forego or terminate ongoing clinical trials, which could have a material adverse effect on the Company’s financial condition or results of operations.
The commercialization of certain technology is dependent on the Hancock License Agreement and the UBC License Agreement.
The SepsetER technology is covered by two separate filed and issued patents. The first patent is owned by Dr. Robert E.W. Hancock together with other inventors. Dr. Robert E.W. Hancock is also one of three inventors for the technology underlying the second patent. However, the second patent has been assigned to UBC, who provided an exclusive license to Sepset. Sepset has been granted an exclusive and worldwide license for the use and sublicense of the Sepset technology as well as any improvements, variations, updates, modifications, and enhancements made and/or acquired thereon, and to manufacture, have made, distribute and sell products made from or based upon the Sepset technology pursuant to the terms of the Hancock License Agreement. In the event the Hancock License Agreement is terminated prior to the expiration of its term, the ability of Sepset to achieve its stated business objectives and milestones would be severely impacted.
UBC has licensed to ABT pursuant to the terms and conditions of the University of British Columbia License Agreement the patents found in “Business- Intellectual Property- ABT Innovations Inc” of this prospectus. UBC may, at its option, immediately terminate the UBC License Agreement by giving notice to ABT under certain circumstances, including insolvency, breach of contract, winding up, liquidation or other termination of the existence of ABT. Either party may terminate this Agreement for any breach which is not remedied after proving notice to the party in breach. In the event the UBC License Agreement is terminated prior to the expiration of its term, the ability of ABT to achieve its stated business objectives and milestones could be impacted.
Probable lack of business diversification.
Because the Company will be focused on developing its business ancillary to the life sciences industry, and potentially directly in the life sciences industry, the prospects for the Company’s success will be dependent upon the future performance and market acceptance of the Company’s intended products, processes, and services. Unlike certain entities that have the resources to develop and explore numerous product lines, operating in multiple industries or multiple areas of a single industry, the Company does not anticipate the ability to immediately diversify or benefit from the possible spreading of risks or offsetting of losses. Again, the prospects for the Company’s success may become dependent upon the development or market acceptance of a very limited number of products, processes or services.
The Company’s officers and directors may be engaged in a range of business activities that could result in conflicts of interest.
Certain of the directors and officers of the Company may also serve as directors and/or officers of other companies involved in the industries in which the Company may operate and consequently there exists the possibility for such directors and officers to be in a position of conflict. For example, Dr. Hancock currently holds a position as a faculty member at UBC, where he runs a research and development laboratory. Additionally, Dr. Hancock holds 23% of ABT, 31.5% of Sepset and holds several other positions with other life sciences companies. Dr. Hancock is one of the non-waiving University of British Columbia (UBC) inventors of the technology in-licensed by SafeCoat Medical. He was not involved in any of the discussions regarding the licencing. However, as a result of his involvement as an inventor, he will receive one quarter of the inventor’s shares, representing 12.5% of the SafeCoat shares to be issued to UBC and Inventors. He will receive 1.5% of total SafeCoat shares. Any decision made by any of such directors and officers will be made in accordance with their duties and obligations to deal fairly and in good faith with a view to the best interests of the Company and its shareholders. In addition, each director is required to declare and refrain from voting on any matter in which such director may have a conflict of interest in accordance with the procedures set forth in applicable laws.
Competition.
An increase in other companies competing in the industry could limit the ability of the Company’s potential of expanding its operations. Current and new competitors may have better capitalization, a longer operating history, more expertise and able to develop higher quality equipment or products, at the same or a lower cost. The Company will not be able to provide assurances that we will be able to compete successfully against current and future competitors. Competitive pressures that the Company may face could have a material adverse effect on our business, operating results and financial condition.
Risks Related to Government Regulation
If the Company fails to comply with extensive regulations of United States and foreign regulatory agencies, the commercialization of our products could be delayed or prevented entirely.
The Company’s future product candidates and research and development activities are subject to extensive government regulations related to its development, testing, manufacturing and commercialization in the United States and other countries. The determination of when and whether a product is ready for large-scale purchase and potential use in the United States will be made by the United States government through consultation with a number of governmental agencies, including the Food and Drug Administration (“FDA”), the National Institutes of Health and the Centers for Disease Control and Prevention. The Company’s products have not received regulatory approval from the FDA, or any foreign regulatory agencies, to be commercially marketed and sold. The process of obtaining and complying with FDA and other governmental regulatory approvals and regulations in the United States and in foreign countries is costly, time consuming, uncertain and subject to unanticipated delays. Obtaining such regulatory approvals, if any, can take several years. Despite the time and expense exerted, regulatory approval is never guaranteed. The Company is also subject to the following risks and obligations, among others:
| | ● | the FDA (and/or other competent regulatory agency, as applicable) may refuse to approve an application if it believes that applicable regulatory criteria are not satisfied; |
| | | |
| | ● | the FDA (and/or other regulatory authority) may require additional testing for safety and effectiveness; |
| | | |
| | ● | the FDA (and/or other regulatory authority) may not agree with the Company’s interpretation of data from pre-clinical testing and clinical trials; |
| | | |
| | ● | if regulatory approval of a product is granted, the approval may be limited to specific indications or limited with respect to its distribution; and |
| | | |
| | ● | the FDA (and/or other regulatory authority) may change its approval policies and/or adopt new regulations. |
Failure to comply with these or other regulatory requirements may subject the Company to administrative or judicially imposed sanctions, including:
| | ● | warning letters, untitled letters or other written notice of violations; |
| | | |
| | ● | civil penalties; |
| | | |
| | ● | criminal penalties; |
| | | |
| | ● | injunctions; |
| | | |
| | ● | product seizure or detention; |
| | | |
| | ● | product recalls; and |
| | | |
| | ● | total or partial suspension of productions. |
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in other jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or to receive applicable marketing approvals, our future target market could be reduced and our ability to realize the full market potential of our future product candidates could be harmed.
If the Company fails to comply with laws and regulations, it could be subject to regulatory actions, which could affect its ability to develop, market and sell its product candidates and any other future product candidates and may harm its reputation.
If the Company fails to comply with applicable federal, state or foreign laws or regulations, the Company could be subject to regulatory actions, which could affect its ability to successfully develop, market and sell any future product candidates under development and could harm its reputation and lead to reduced or non-acceptance of its proposed product candidates by the market. Technical recommendations or evidence provided by the FDA through letters, site visits, and overall recommendations to academia or biotechnology companies may also make the manufacturing of a clinical product extremely labor intensive or expensive, making the product candidate no longer viable to manufacture in a cost-efficient manner. The mode of administration or the required testing of the product candidate may make that candidate no longer commercially viable. The FDA, or a clinical trial site’s Institutional Review Board or Institutional Biosafety Committee, may not agree with the proposed clinical trials, which may delay or make impossible the clinical testing of a product candidate. For example, the Institutional Review Board for a clinical trial may request to stop a trial or deem a product candidate unsafe to continue testing. This would have a material adverse effect on the value of the product candidate and the Company’s business, results of operations and financial condition.
United States legislative or FDA regulatory reforms may make it more difficult and costly for the Company to obtain regulatory approval of its product candidates and to manufacture, market and distribute its products after approval is obtained.
From time to time, legislation is drafted and implemented that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect the Company’s business and its products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future products or impose changes on other products already on the market. It is impossible to predict whether legislative changes will be enacted, or FDA regulations, guidance or interpretations will be changed, and what the impact of such changes, if any, may be on the Company’s new product development efforts.
Current and future legislation may increase the difficulty and cost of commercializing our drug candidates and may affect the prices we may obtain if our drug candidates are approved for commercialization.
In the U.S. and some foreign jurisdictions, there have been a number of adopted and proposed legislative and regulatory changes regarding the healthcare system that could prevent or delay regulatory approval of our drug candidates, restrict or regulate post-marketing activities and affect our ability to profitably sell any of our drug candidates for which we obtain regulatory approval.
In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. Cost reduction initiatives and other provisions of this legislation could limit the coverage and reimbursement rate that we receive for any of our future approved products. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively the PPACA, was enacted. The PPACA was intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The PPACA increased manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate amount for both branded and generic drugs and revised the definition of “average manufacturer price,” or AMP, which may also increase the amount of Medicaid drug rebates manufacturers are required to pay to states. The legislation also expanded Medicaid drug rebates and created an alternative rebate formula for certain new formulations of certain existing products that is intended to increase the rebates due on those drugs. The Centers for Medicare & Medicaid Services, or CMS, which administers the Medicaid Drug Rebate Program, also has proposed to expand Medicaid rebates to the utilization that occurs in the territories of the U.S., such as Puerto Rico and the Virgin Islands. Further, beginning in 2011, the PPACA imposed a significant annual fee on companies that manufacture or import branded prescription drug products and required manufacturers to provide a discount, equal to 70% off, effective as of 2019, the negotiated price of prescriptions filled by beneficiaries in the Medicare Part D coverage gap, referred to as the “donut hole.” Legislative and regulatory proposals have been introduced at both the state and federal level to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under government payor programs, and review the relationship between pricing and manufacturer patient programs. We also expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our future drug candidates, if approved for commercialization.
In Europe, the United Kingdom withdrew from the European Union on January 31, 2020, and entered into a transition period that expired on December 31, 2020. A significant portion of the previous regulatory framework in the United Kingdom was derived from the regulations of the European Union. In 2021, the United Kingdom’s Medicines and Healthcare products Regulatory Agency, or MHRA, and the European Medicines Agency, or EMA, released guidance explaining the new regulatory framework. We cannot predict the consequences or impact that the new regulatory framework would have on our future operations, if any, in these jurisdictions.
In addition, on August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, which, among other things, includes policies that are designed to have a direct impact on drug prices and reduce drug spending by the federal government, which shall take effect in 2023. Under the Inflation Reduction Act, Congress authorized Medicare beginning in 2026 to negotiate lower prices for certain costly single-source drug and biologic products that do not have competing generics or biosimilars. This provision is limited in terms of the number of pharmaceuticals whose prices can be negotiated in any given year and it only applies to drug products that have been approved for at least 9 years and biologics that have been licensed for 13 years. Drugs and biologics that have been approved for a single rare disease or condition are categorically excluded from price negotiation. Further, the new legislation provides that if pharmaceutical companies raise prices in Medicare faster than the rate of inflation, they must pay rebates back to the government for the difference. The new law also caps Medicare out-of-pocket drug costs at an estimated $4,000 a year in 2024 and, thereafter beginning in 2025, at $2,000 a year.
Our future clinical trials may fail to demonstrate substantial evidence of the safety and effectiveness of future product candidates that we may identify and pursue, which would prevent, delay or limit the scope of regulatory approval and commercialization.
In the United States, we would be able to begin marketing a product after we receive approval from the FDA, or, in any foreign countries, when we receive the requisite approval from those countries. Before obtaining regulatory approvals for the commercial sale of any product candidate for a target indication, we must demonstrate with evidence gathered in preclinical and well-controlled clinical trials, and, with respect to approval in the United States, to the satisfaction of the FDA and, with respect to approval in other countries, similar regulatory authorities in those countries, that the product candidate is safe and effective for use for that target indication and that the manufacturing facilities, processes and controls are adequate. Our failure to adequately demonstrate the safety and effectiveness of any of our products or product candidates for the treatment of particular diseases may delay or prevent our receipt of the FDA’s and foreign regulatory authorities’ approval and, ultimately, may prevent commercialization of our products and product candidates for those diseases. The FDA and foreign regulatory authorities have substantial discretion in deciding whether, based on the benefits and risks in a particular disease, any of our products or product candidates should be granted approval for the treatment of that particular disease. Even if we believe that a clinical trial or trials has demonstrated the safety and statistically significant efficacy of any of our products or product candidates for the treatment of a disease, the results may not be satisfactory to the FDA or foreign regulatory authorities. Preclinical and clinical data can be interpreted by the FDA and foreign regulatory authorities in different ways, which could delay, limit or prevent regulatory approval.
Additionally, clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Most product candidates that begin clinical trials are never approved by regulatory authorities for commercialization. We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support marketing approval.
We cannot be certain that any clinical trials will be successful. In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same therapeutic candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the clinical trial protocols and the rate of dropout among clinical trial participants.
Even if any of our future products obtain regulatory approval, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expenses. Additionally, any such products, if approved, could be subject to labeling and other restrictions and market withdrawal, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with any of our future products.
If Health Canada (“HC”), the FDA, the European Commission (“EC”) and the European Medicines Agency (“EMA”) or a comparable foreign regulatory authority approves any of our future products, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with current good manufacturing practice (“cGMP”) and with good clinical practice (“GCP”) for any clinical trials that we conduct post-approval, all of which may result in significant expense and limit our ability to commercialize such therapies. Later discovery of previously unknown problems with any approved product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| | ● | restrictions on the labeling, distribution, marketing or manufacturing of our future products, withdrawal of the product from the market, or product recalls; |
| | | |
| | ● | untitled and warning letters, or holds on clinical trials; |
| | | |
| | ● | refusal by the HC, the FDA, the EC, the EMA or other foreign regulatory body to approve pending applications, supplements or modifications to approved applications we filed or suspension or revocation of license approvals; |
| | | |
| | ● | requirements to conduct post-marketing studies or clinical trials; |
| | | |
| | ● | restrictions on coverage by third-party payors; |
| | | |
| | ● | fines, restitution or disgorgement of profits or revenue; |
| | | |
| | ● | suspension or withdrawal of marketing approvals; |
| | | |
| | ● | product seizure or detention, or refusal to permit the import or export of the product; and |
| | | |
| | ● | injunctions or the imposition of civil or criminal penalties. |
In addition, any regulatory approvals that we receive for our future products may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including clinical trials, and surveillance to monitor the safety and efficacy of such products.
If there are changes in the application of legislation, regulations or regulatory policies or if we or one of our distributors, licensees or co-marketers fails to comply with regulatory requirements, the regulatory agencies/bodies could take various actions. In such a case, the Company may be subject to fines, manufacturing or distribution restrictions or limitations, product recalls or removal of the product from the market, including marketing authorization suspension or withdrawal. The Company may also be subject to additional clinical trial requirements, product labeling changes or marketing authorization resubmissions. If any of these events occurs, our ability to sell such therapy may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could materially adversely affect our business, financial condition and results of operations.
The results of preclinical studies and early-stage clinical trials of our products may not be predictive of the results of later stage clinical trials. Initial success in our ongoing clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials.
Our products in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. Furthermore, there can be no assurance that any of our clinical trials will ultimately be successful or support further clinical development of any of our future products. There is a high failure rate for products proceeding through clinical trials. A number of companies in the medical industry have suffered significant setbacks in clinical development even after achieving promising results in earlier studies.
The commercial success of our products will depend on the degree of market access and acceptance of our products among healthcare professionals, patients, healthcare payors, health technology assessment bodies and the medical community at large.
Our products may not achieve an adequate level of acceptance by payors, health technology assessment bodies, healthcare professionals, patients and the medical community at large, and we may not become profitable. The level of acceptance we ultimately achieve may be affected by negative public perceptions. Because of this history, efforts to educate the medical community and third-party payors and health technologies assessment bodies on the benefits of our products may require significant resources and may never be successful, which would prevent us from generating significant revenue or becoming profitable. Market acceptance of our products by healthcare professionals, patients, healthcare payors and health technology assessment bodies will depend on a number of factors, many of which are beyond our control, including, but not limited to, the following:
| | ● | acceptance by healthcare professionals, patients and healthcare payors of each product as safe, effective and cost-effective; |
| | | |
| | ● | changes in the standard of care for the targeted indications; |
| | | |
| | ● | the strength of sales, marketing and distribution support; |
| | | |
| | ● | potential product liability claims; |
| | | |
| | ● | the product’s relative convenience, ease of use, ease of administration and other perceived advantages over alternative products; |
| | ● | the prevalence and severity of adverse events or publicity; |
| | | |
| | ● | limitations, precautions or warnings listed in the summary of therapeutic characteristics, patient information leaflet, package labeling or instructions for use; |
| | | |
| | ● | the cost of treatment with our products in relation to alternative treatments; |
| | | |
| | ● | the ability to manufacture our product in sufficient quantities and yields; |
| | | |
| | ● | the availability and amount of coverage and reimbursement from healthcare payors, and the willingness of patients to pay out of pocket in the absence of healthcare payor coverage or adequate reimbursement; |
| | | |
| | ● | the willingness of the target patient population to try, and of healthcare professionals to use, the products; |
| | | |
| | ● | any potential unfavorable publicity, including negative publicity; |
| | | |
| | ● | the extent to which products are approved for inclusion and reimbursed on formularies of hospitals and managed care organizations; and |
| | | |
| | ● | whether our products are designated under physician treatment guidelines or under reimbursement guidelines as a first-line, second-line, third-line or last-line therapy. |
If our future products fail to gain market access and acceptance, this will have a material adverse impact on our ability to generate revenue to provide a satisfactory, or any, return on our investments. Even if some products achieve market access and acceptance, the market may prove not to be large enough to allow us to generate significant revenue.
We must comply with Health Information Privacy and Security Standards.
The privacy regulations Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) contains detailed requirements concerning the use and disclosure of individually identifiable patient health information by various healthcare providers, such as medical groups. HIPAA covered entities must implement certain administrative, physical, and technical security standards to protect the integrity, confidentiality and availability of certain electronic health information received, maintained, or transmitted. HIPAA also implemented standard transaction code sets and standard identifiers that covered entities must use when submitting or receiving certain electronic healthcare transactions, including billing and claim collection activities. Violations of the HIPAA privacy and security rules may result in civil and criminal penalties, including a tiered system of civil money penalties that range from $100 to $50,000 per violation, with a cap of $1.5 million per year for identical violations. A HIPAA covered entity must also promptly notify affected individuals where a breach affects more than 500 individuals and report breaches affecting fewer than 500 individuals annually. State attorneys general may bring civil actions on behalf of state residents for violations of the HIPAA privacy and security rules, obtain damages on behalf of state residents, and enjoin further violations.
Failure to comply with health and data protection laws and regulations could lead to U.S. federal and state government enforcement actions, including civil or criminal penalties, private litigation, and adverse publicity and could negatively affect our operating results and business.
We and any potential collaborators may be subject to U.S. federal and state data protection laws and regulations, such as laws and regulations that address privacy and data security. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws, govern the collection, use, disclosure, and protection of health-related and other personal information. In addition, we may obtain health information from third parties, including research institutions from which we obtain clinical trial data, which are subject to privacy and security requirements under HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”). To the extent that we act as a business associate to a healthcare provider engaging in electronic transactions, we may also be subject to the privacy and security provisions of HIPAA, as amended by HITECH, which restricts the use and disclosure of patient-identifiable health information, mandates the adoption of standards relating to the privacy and security of patient-identifiable health information, and requires the reporting of certain security breaches to healthcare provider customers with respect to such information. Additionally, many states have enacted similar laws that may impose more stringent requirements on entities like ours. Depending on the facts and circumstances, we could be subject to significant civil, criminal, and administrative penalties if we obtain, use, or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
Compliance with U.S. and foreign privacy and data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Failure to comply with these laws and regulations could result in government enforcement actions (which could include civil, criminal and administrative penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects, employees and other individuals about whom we or our potential collaborators obtain personal information, as well as the providers who share this information with us, may limit our ability to collect, use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business. Please also refer to risk factor, “A failure of one or more of our key IT systems, networks, processes, associated sites or service providers could have a material adverse impact on business operations, and if the failure is prolonged, our financial condition.”
We will be subject to environmental, health and safety laws and regulations, and we may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities which may adversely affect our business and financial condition.
Our operations, including our research, development, testing and manufacturing activities, will be subject to numerous foreign, federal, state and local environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, manufacture, handling, release and disposal of and the maintenance of a registry for, hazardous materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds and compounds that have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens.
We may incur significant costs to comply with these current or future environmental and health and safety laws and regulations. Furthermore, if we fail to comply with such laws and regulations, we could be subject to fines or other sanctions.
Risks Related to Our Financial Position and Need for Capital
We have only a limited history upon which an evaluation of our prospects and future performance can be made and have no history of profitable operations.
We commenced operations in 2021 and have a limited history upon which an evaluation of our prospects and future performance can be made. We may sustain losses in the future as we implement our business plan. We do not have a history of profitable operations and we may not achieve profitable operations.
We will require additional financing in the future to fund our operations.
We will need additional capital in the future to continue to execute our business plan. Therefore, we will be dependent upon additional capital in the form of either debt or equity to continue our operations. Our ability to obtain additional financing will be subject to a number of factors, including market conditions, our operating performance and investor sentiment. We cannot assure that additional financing will be available to us on acceptable terms or at all. If additional capital is either unavailable or cost prohibitive, our operations and growth may be limited, and we may need to change our business strategy to slow the rate of, or eliminate, our expansion or to reduce or curtail our operations. Also, any additional financing we undertake could impose covenants upon us that restrict our operating flexibility, and, if we issue equity securities to raise capital, our existing shareholders may experience dilution and the new securities may have rights, preferences and privileges senior to those of our common shares.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish certain rights.
We may seek additional capital through a combination of equity offerings, debt financings, strategic collaborations and alliances or licensing arrangements. To the extent that we raise additional capital through the sale of equity, convertible debt securities or other equity-based derivative securities, your ownership interest will be diluted and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Any indebtedness we incur could involve restrictive covenants, such as limitations on our ability to incur additional debt, acquire or license intellectual property rights, declare dividends, make capital expenditures and other operating restrictions that could adversely impact our ability to conduct our business. Furthermore, the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline. If we raise additional funds through strategic collaborations and alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to future candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Adequate additional financing may not be available to us on acceptable terms, or at all. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our future therapeutic candidates that we would otherwise prefer to develop and market ourselves.
We may not be able to consummate proposed acquisitions or divestitures successfully or integrate acquired businesses successfully.
From time to time, we acquire businesses, assets or securities of companies that we believe will provide a strategic fit with our business. We integrate acquired businesses with our existing operations; our overall internal control over financial reporting processes; and our financial, operations and information systems. If the financial performance of our business, as supplemented by the assets and businesses acquired, does not meet our expectations, it may make it more difficult for us to service our debt obligations and our results of operations may fail to meet market expectations. We may not effectively assimilate the business or product offerings of acquired companies into our business or within the anticipated costs or timeframes, retain key customers and suppliers or key employees of acquired businesses or successfully implement our business plan for the combined business. In addition, our final determinations and appraisals of the estimated fair value of assets acquired and liabilities assumed in our acquisitions may vary materially from earlier estimates and we may fail to realize fully anticipated cost savings, growth opportunities or other potential synergies. We cannot assure that the fair value of acquired businesses or investments will remain constant.
We may also divest ourselves of businesses, assets or securities of companies that we believe no longer provide a strategic fit with our business. We may provide various indemnifications in connection with the divestiture of businesses or assets. Divestitures of portions of our business may also result in costs stranded in our remaining business. Delays in developing or implementing plans to address such costs could delay or prevent the accomplishment of our financial objectives.
In addition, our continued success depends, in part, on our ability to develop new products. The launch and ongoing success of new products are inherently uncertain, especially with respect to consumer appeal. A new product launch can give rise to a variety of costs. An unsuccessful launch, among other things, can affect consumer perception of existing brands and our reputation. Unsuccessful implementation or short-lived popularity of our product innovations may result in inventory write-offs and other costs.
We cannot assure that we will realize the expected benefits of acquisitions, divestitures or investments. We also cannot assure that our acquisitions, investments, or joint ventures will be profitable, that forecasts regarding our acquisitions, divestitures, or investment activities will be accurate or that the internal control over financial reporting of entities which we must consolidate as a result of our investment activities will be as robust as the internal control over financial reporting for our wholly-owned entities. Our failure to adequately manage the risks associated with acquisitions or divestitures, or the failure of an entity in which we have an equity or membership interest, could have a material adverse effect on our business, liquidity, financial condition and/or results of operations.
We may not be able to retain and recruit executive management or build morale and performance amongst our workforce.
Our success depends upon the efforts and abilities of our executive management team, key senior management, and a high-quality employee base, as well as our ability to attract, motivate, reward and retain them. If one of our executive officers or critical senior management terminates his or her employment, we may not be able to replace their expertise, fully integrate new personnel or replicate the prior working relationships. The loss of critical employees might significantly delay or prevent the achievement of our business objectives. Qualified individuals with the breadth of skills and experience in our industry that we require are in high demand and we may incur significant costs to attract them. Difficulties in hiring or retaining key executive or employee talent or the unexpected loss of experienced employees could have an adverse impact on our business performance. In addition, we could experience business disruption and/or increased costs related to organizational changes, reductions in workforce, or other cost-cutting measures.
If we are not able to successfully execute on our future operating plans and objectives, our financial condition, and results of operation may be materially adversely affected, and we may not be able to continue as a going concern.
It is important that we meet our future sales goals and increase sales going forward. If we do not meet our sales goals, our available cash and working capital will decrease and our financial condition will be negatively impacted.
In order to be successful, we believe that we must, among other things:
| | ● | commence the sales volume and gross margins for our products; |
| | | |
| | ● | maintain efficiencies in operations; |
| | | |
| | ● | manage our operating expenses to sufficiently support operating activities; |
| | | |
| | ● | maintain fixed costs at or near current levels; and |
| | | |
| | ● | avoid significant increases in variable costs relating to production, marketing and distribution. |
We may not be able to meet these objectives, which could have a material adverse effect on our results of operations. We have incurred significant operating expenses in the past and may do so again in the future and, as a result, will need to commence revenues in order to improve our results of operations. Our ability to increase sales will depend primarily on success in expanding our current markets, improving our distribution base, entering into depository trust company (“DTC”) arrangements with national accounts, and introducing new products or product extensions to the market. Our ability to successfully enter new distribution areas and obtain new accounts will, in turn, depend on various factors, many of which are beyond our control, including, but not limited to, the continued demand for our brands and products in target markets, the ability to price our products at competitive levels, the ability to establish and maintain relationships with distributors in each geographic area of distribution and the ability in the future to create, develop and successfully introduce one or more new brands, products, and product extensions.
Although our financial statements have been prepared on a going concern basis, we must raise additional capital to fund our operations in order to continue as a going concern.
Manning Elliott LLP, our independent registered public accounting firm for the fiscal year ended December 31, 2023, has included an explanatory paragraph in their opinion that accompanies our audited consolidated financial statements as of and for the year ended December 31, 2023, indicating that our current liquidity position raises substantial doubt about our ability to continue as a going concern. If we are unable to improve our liquidity position we may not be able to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result if we are unable to continue as a going concern and, therefore, be required to realize our assets and discharge our liabilities other than in the normal course of business which could cause investors to suffer the loss of all or a substantial portion of their investment.
Based on the Company’s financial position as at December 31, 2023, the available funds are not considered adequate to meet requirements for the estimated operations and development activities on the Company’s technologies in the coming twelve-month period. These requirements may be adversely impacted by an absence of normal available financing due to the continued uncertainty in the markets. To address its financing requirements, the Company will seek financing through and not limited to debt financing, equity financing and strategic alliances. However, there is no assurance that such financing will be available. This material uncertainty casts significant doubt upon the Company’s ability to continue as a going concern. If the going concern assumption were not appropriate for these financial statements, then adjustments would be necessary to the carrying values of assets, liabilities, the reported income and expenses and the consolidated statement of financial position classifications used. Such adjustments could be material.
We may be unable to adequately protect our proprietary and intellectual property rights
Our ability to compete may depend on the superiority, uniqueness and value of any intellectual property and technology that it may develop or license. To the extent we are able to do so, to protect any proprietary rights of the Company, we intend to rely on a combination of patent, trademark, copyright and trade secret laws, confidentiality agreements with its employees and third parties, and protective contractual provisions. Despite these efforts, any of the following occurrences may reduce the value of any of the Company’s intellectual property:
| | ● | issued patents, trademarks and registered copyrights may not provide the Company with competitive advantages; the Company’s efforts to protect the current intellectual property rights of ABT and Sepset may not be effective in preventing misappropriation of any its products or intellectual property; |
| | | |
| | ● | the Company’s efforts may not prevent the development and design by others of products or marketing strategies similar to or competitive with, or superior to those the Company develops; |
| | | |
| | ● | another party may assert a blocking patent and the Company would need to either obtain a license or design around the patent in order to continue to offer the contested feature or service in its products; or |
| | | |
| | ● | the expiration of patent or other intellectual property protections for any assets owned or licensed by the Company, Sepset or ABT could result in significant competition, potentially at any time and without notice, resulting in a significant reduction in sales. The effect of the loss of these protections on the Company and its financial results will depend, among other things, upon the nature of the market and the position of the Company’s products in the market from time to time, the growth of the market, the complexities and economics of manufacturing a competitive product and regulatory approval requirements but the impact could be material and adverse. |
Volatility in the price or availability of the inputs we depend on, including raw materials, packaging, energy, and labor, could adversely impact our financial results upon commercialization.
The principal raw materials we use include peptides, nucleic acids and enzymes. These costs are subject to fluctuation. Substantial increases in the prices of our raw materials and packaging materials, to the extent that they cannot be recouped through increases in the prices of finished products, would increase our operating costs and could reduce our profitability upon commercialization. If our supply of these raw materials is impaired or if prices increase significantly, it could affect the affordability of our products and reduce sales.
If we are unable to secure sufficient ingredients or raw materials and other key supplies, we might not be able to satisfy demand on a short-term basis.
Operational disruptions could cause delays in our production.
As our operations are concentrated in a limited number of production and distribution facilities, we are more likely to experience a significant operational disruption or catastrophic loss in any one location from acts of war or terrorism, fires, floods, earthquakes, severe winter storms, hurricanes, pandemics, labor strike, or other labor activities, cyber-attacks, and other attempts to penetrate our information technology systems or the information technology used by our employees who work from home during the COVID-19 pandemic, unavailability of raw or packaging materials, or other natural or man-made events. If a significant operational disruption or catastrophic loss were to occur, we could breach agreements, our reputation could be harmed, and our business, liquidity, financial condition, and/or results of operations could be adversely affected due to higher maintenance charges, unexpected capital spending, or product supply constraints.
Our insurance policies do not cover certain types of catastrophes and may not cover certain events such as pandemics. Economic conditions and uncertainties in global markets may adversely affect the cost and other terms upon which we are able to obtain property damage and business interruption insurance. If our insurance coverage is adversely affected, or to the extent we have elected to self-insure, we may be at greater risk that we may experience an adverse impact to our business, liquidity, financial condition, and/or results of operations.
A security incident may allow unauthorized access to our systems, networks, or data, harm our reputation, create additional liability, and harm our financial results.
Increasingly, companies are subject to a wide variety of attacks on their systems on an ongoing basis. In addition to threats from traditional computer “hackers,” malicious code (such as malware, viruses, worms, and ransomware), employee theft or misuse, password spraying, phishing, credential stuffing, and denial-of-service attacks, we may also face threats from sophisticated organized crime, nation-state, and nation-state supported actors who engage in attacks (including advanced persistent threat intrusions) that add to the risks to our internal systems. Third parties may attempt to fraudulently induce employees, users, or organizations into disclosing sensitive information or otherwise compromise the security of our internal electronic systems, networks, and/or physical facilities in order to gain access to our data, which could result in significant legal and financial exposure, a loss of confidence in us, interruptions or malfunctions in our operations, and, ultimately, harm to our future business prospects and revenue. The Company is in the process of engaging an information technology company, which will ensure appropriate security systems are in place to mitigate the risk of attacks on the Company’s system.
Failure to obtain satisfactory performance from our suppliers or loss of our existing suppliers could harm our business and as a result, our operations could suffer upon commercialization
Currently, we do not have long-term, written agreements with any of our suppliers. Upon commercialization, the termination of our relationships or an adverse change in the terms of these arrangements could have a negative impact on our business. If our suppliers increase their prices, we may not be able to secure alternative suppliers, and may not be able to raise the prices of our products to cover all or even a portion of the increased costs. Also, our future suppliers’ failure to perform satisfactorily or handle increased orders, delays in shipments of products from suppliers or the loss of our existing suppliers, especially our key suppliers, could cause us to fail to meet orders for our products, lose sales, incur additional costs and/or expose us to product quality issues. In turn, upon commercialization, this could cause us to lose credibility in the marketplace and damage our relationships with distributors, ultimately leading to a decline in our business and results of operations. If we are not able to renegotiate these contracts on acceptable terms or find suitable alternatives, our business, financial condition, or results of operations could be negatively impacted.
If our third-party service providers and business partners do not satisfactorily fulfill their commitments and responsibilities, our financial results could suffer.
In the conduct of our business, we rely on relationships with third parties, including manufacturers, consultants, service providers, and other external business partners, for certain functions or for services in support of key portions of our operations. These third-party service providers and business partners are subject to similar risks as we are, relating to cybersecurity, privacy violations, business interruption, and systems and employee failures, and are subject to legal, regulatory, and market risks of their own. Our third-party service providers and business partners may not fulfill their respective commitments and responsibilities in a timely manner and in accordance with the agreed-upon terms. In addition, while we have procedures in place for selecting and managing our relationships with third-party service providers and other business partners, we do not have control over their business operations or governance and compliance systems, practices and procedures, which increases our financial, legal, reputational, and operational risk. If we are unable to effectively manage our third-party relationships, or for any reason our third-party service providers or business partners fail to satisfactorily fulfill their commitments and responsibilities, our financial results could suffer.
Pandemics, such as the current global COVID-19 pandemic, outbreaks of communicable infections or diseases, or other public health concerns in the markets in which our employees live and/or in which we or our distributors, retailers, and suppliers operate may damage our business and disrupt our operations.
Disease outbreaks and other public health conditions could result in disruptions and damage to our business caused by potential disruption to our supply chains, production processes, and operations. Supply disruption may result from restrictions on the ability of employees and others in the supply chain to travel and work, caused by quarantine or individual illness, or which may result from border closures imposed by governments to deter the spread of communicable infection or disease, or determinations by us or our suppliers or distributors to temporarily suspend operations in affected areas, or other actions which restrict the ability to distribute our products or which may otherwise negatively impact our ability to produce, bottle, and ship our product, for our distributors to distribute our products, or for our suppliers to provide us our raw materials. Ports or channels of entry may be closed or operate at only a portion of capacity, or transportation of products within a region or country may be limited, if workers are unable to report to work due to travel restrictions or personal illness. Our operations and the operations of our suppliers may become less efficient or otherwise become negatively impacted if our executive leaders or other personnel critical to our operations are unable to work or if a significant percentage of the workforce is unable to work or is required to work from home. Our cyber-security could be compromised if persons who are forced to work from home do not maintain adequate information security. A prolonged quarantine or border closure could result in temporary or longer-term disruptions of sales patterns, consumption and trade patterns, supply chains, production processes, and operations. A widespread health crisis, such as the COVID-19 pandemic, could negatively affect the economies and financial markets of many countries resulting in a global economic downturn, which could negatively impact demand for our products and our ability to borrow money. Any of these events could have a material adverse effect on our business, liquidity, financial condition, and/or results of operations.
A failure of one or more of our key IT systems, networks, processes, associated sites or service providers could breach HIPAA, patient privacy, and/or have a material adverse impact on business operations, and if the failure is prolonged, our financial condition.
We rely on IT systems, networks, and services, including internet sites, data hosting and data processing facilities and tools, hardware (including laptops and mobile devices), software and technical applications and platforms, some of which are managed, hosted, provided and used by third-parties or their vendors, to assist us in the management of our business. The various uses of these IT systems, networks and services include, but are not limited to: hosting our internal network and communication systems; collecting and storing employee, shareholder, and other data; processing transactions; summarizing and reporting results of operations; hosting, processing and sharing confidential and proprietary research, business plans and financial information; complying with regulatory, legal or tax requirements; providing data security; and handling other processes necessary to manage our business.
The proper functioning of our own information technology (“IT”) infrastructure is critical to the efficient operation and management of our business. We may not have the necessary financial resources to update and maintain our IT infrastructure, and any failure or interruption of our IT system could adversely impact our operations. In addition, our IT is vulnerable to cyber-attacks, computer viruses, worms and other malicious software programs, physical and electronic break-ins, sabotage and similar disruptions from unauthorized tampering with our computer systems. We believe that we have adopted appropriate measures to mitigate potential risks to our technology infrastructure and our operations from these IT-related and other potential disruptions. However, given the unpredictability of the timing, nature, and scope of any such IT failures or disruptions, we could potentially be subject to downtimes, transactional errors, processing inefficiencies, operational delays, other detrimental impacts on our operations or ability to provide products to our customers, the compromising of confidential or personal information, destruction or corruption of data, security breaches, other manipulation or improper use of our systems and networks, financial losses from remedial actions, loss of business or potential liability, and/or damage to our reputation, any of which could have a material adverse effect on our cash flows, competitive position, financial condition, or results of operations. In any of these events, we could be required to spend significant financial and other resources to remedy the damage caused by a security breach or to repair or replace networks and IT systems.
Our operations, products, data and intellectual property are inherently at risk of loss, inappropriate access, or tampering by both insider threats and external bad actors. In particular, our operations face various cyber and other security threats, including attempts to gain unauthorized access to sensitive information, intellectual property, mission operations, systems and networks. Our systems (internal, customer and partner systems) and assets may also be subject to damage or interruption from natural and other disaster events or disruptions including hurricanes, floods, earthquakes, fires, other extreme weather conditions, epidemics or pandemics, acts of terrorism, power shortages and blackouts, aging infrastructures and telecommunications failures. In addition, insider threats, threats to the safety of our directors and employees, threats to the security of our facilities, infrastructure and supply chain and threats from terrorist acts or other acts of aggression could have a material adverse impact on our business.
Our partners (including our supply chain) face similar threats. Partner proprietary, classified, or sensitive data and information transmitted to, from, or stored on our networks are at risk. Assets and intellectual property and products in partner environments are also at risk. We also have risk where we have access to partner networks and face risks of breach, disruption or loss as well.
While we have implemented reasonable measures consistent with government regulations aimed at reducing the risk of cyber threats as well as to help thwart bad actors and protect our data and our systems and assets, the techniques used to gain unauthorized access are constantly evolving, and we may be unable to anticipate or prevent all unauthorized access, disruption, loss, or harm. Because of our desired data and intellectual property we (and/or partners we use) may be an attractive target for such attacks. We cannot offer assurances, however, that future attacks will not materially adversely affect our business or reputation.
Litigation and litigation risks may have an adverse impact on our operations, business, and reputation.
From time to time, we may become involved in various litigation matters and claims, including employment, regulatory proceedings, administrative proceedings, governmental investigations, and contract disputes. We could face potential claims or liability for, among other things, breach of contract, defamation, libel, fraud, or negligence. We may also face employment-related litigation, including claims of age discrimination, sexual harassment, gender discrimination, immigration violations, or other local, state, and federal labor law violations. Because of the uncertain nature of litigation and insurance coverage decisions, the outcome of such actions and proceedings cannot be predicted with certainty and an unfavorable resolution of one or more of them could have a material adverse effect on our business, financial condition, results of operations, cash flows, reputation, brand identity, and the trading price of our securities. Any such litigation, with or without merit, could also result in substantial expenditures of time and money, and divert attention of our management team from other tasks important to the success of our business.
In the future, if we were to encounter product recalls or other product quality issues, our business may suffer.
Any future product quality issues, real or imagined, or allegations of product contamination, even when false or unfounded, could tarnish our image and could cause consumers to choose other products. In addition, because of changing government regulations or implementation thereof, or allegations of product contamination, we could be required from time to time to recall products entirely or from specific markets. Any future product recalls could affect our profitability and could negatively affect brand image.
International operations, worldwide and domestic economic trends, and financial market conditions, geopolitical uncertainty, changes to international trade agreements and tariffs, import and excise duties, other taxes, or other governmental rules and regulations could have a material adverse effect on our business and operations.
Risks associated with international operations, any of which could have a material adverse effect on our business, liquidity, financial condition, and/or results of operations, include:
| | ● | changes in local political, economic, social, and labor conditions; |
| | | |
| | ● | potential disruption from socio-economic violence, including terrorism and drug-related violence; |
| | | |
| | ● | restrictions on foreign ownership and investments or on repatriation of cash earned in countries outside the U.S.; |
| | | |
| | ● | import and export requirements and border accessibility; |
| | | |
| | ● | currency exchange rate fluctuations; |
| | | |
| | ● | a less developed and less certain legal and regulatory environment in some countries, which, among other things, can create uncertainty regarding contract enforcement, intellectual property rights, privacy obligations, real property rights, and liability issues; and |
| | | |
| | ● | inadequate levels of compliance with applicable anti-bribery laws, including the Foreign Corrupt Practices Act. |
Unfavorable global or regional economic conditions, including economic slowdown and the disruption, volatility, and tightening of credit and capital markets, as well as unemployment, tax increases, governmental spending cuts, or a return of high levels of inflation, could affect consumer spending patterns and purchases of our products. These could also create or exacerbate credit issues, cash flow issues, and other financial hardships for us and our suppliers, distributors, retailers, and consumers. The inability of suppliers, distributors, and retailers to access liquidity could impact our ability to produce and distribute our products.
We are also exposed to risks associated with interest rate fluctuations. We could experience changes in our ability to manage fluctuations in interest rates and, accordingly, there can be no assurance that we will be successful in reducing those risks.
We could also be affected by nationalization of our international operations, unstable governments, unfamiliar or biased legal systems, intergovernmental disputes or animus against the U.S. Any determination that our operations or activities did not comply with applicable U.S. or foreign laws or regulations could result in the imposition of fines and penalties, interruptions of business, terminations of necessary licenses and permits, and other legal and equitable sanctions.
We will be subject to risks inherent in sales of products in international markets.
There can be no assurance that future products that we manufacture, distribute, or sell will be accepted or be successful in any particular foreign market, due to local or global competition, product price, cultural differences, consumer preferences, or otherwise. There are many factors that could adversely affect demand for our products in foreign markets, including our inability to attract and maintain key distributors in these markets; volatility in the economic growth of certain of these markets; changes in economic, political or social conditions; the status and renegotiations of the North American Free Trade Agreement; imposition of new or increased labeling, product or production requirements, or other legal restrictions; restrictions on the import or export of our products or ingredients or substances used in our products; inflationary currency, devaluation or fluctuation; and increased costs of doing business due to compliance with complex foreign and U.S. laws and regulations. If we are unable to effectively operate or manage the risks associated with operating in international markets, our business, financial condition, or results of operations could be adversely affected.
Damage to our reputation could harm our future business and cause a decline in our future sales.
Our future sales’ success, in part, depends upon the positive image that consumers have of our products and maintaining a good reputation is critical to selling our future products. Our reputation could also be impacted negatively by public perception, adverse publicity (whether or not valid, such as the similarity of the name of certain of our brands or trademarks and a type of virus), negative comments in social media, or our responses relating to:
| | ● | a perceived failure to maintain high ethical and environmental, social, and governance standards and practices for all our operations and activities; |
| | | |
| | ● | a perceived failure to address concerns relating to the quality, safety, or integrity of our products, whether arising accidentally or through deliberate third-party action; |
| | | |
| | ● | allegations that we, or persons associated with us or formerly associated with us, have violated applicable laws or regulations, including but not limited to those related to safety, employment, discrimination, harassment, whistle-blowing, privacy, corporate citizenship, improper business practices, or cyber-security; and |
| | | |
| | ● | our environmental impact, including packaging, water and energy use, and waste management. |
Failure to comply with federal, state, or local laws and regulations, maintain an effective system of internal controls, provide accurate and timely financial statement information, or protect our information systems against service interruptions, misappropriation of data, or breaches of security, could also hurt our reputation. Damage to our reputation or loss of consumer confidence in our future products for any of these or other reasons could result in decreased demand for our future products and could have a material adverse effect on our business, liquidity, financial condition, and/or results of operations, as well as require additional resources to rebuild our reputation, competitive position, and brand equity and renew investor confidence.
Our intangible assets, such as goodwill and trademarks, could have a material adverse effect in the event of a write-down of the assets.
We have a significant amount of intangible assets such as goodwill and trademarks and may acquire more intangible assets in the future. Intangible assets are subject to a periodic impairment evaluation under applicable accounting standards. The write-down of any of these intangible assets could have a material adverse effect on our business, liquidity, financial condition, and/or results of operations.
Any changes to existing accounting pronouncements or taxation rules or practices may affect how we conduct business.
New accounting pronouncements, taxation rules and varying interpretations of accounting pronouncements or taxation rules have occurred in the past and may occur in the future. The change to existing rules, future changes, if any, or the need for the Company to modify a current tax position may adversely affect the way we conduct business.
We may be required in the future to record a significant charge to earnings if our intangible assets become impaired.
Under International Financial Reporting Standards (“IFRS”), we are required to review our intangible assets for impairment when events or changes in circumstances indicate the carrying value may not be recoverable. Factors that may be considered a change in circumstances indicating that the carrying value of our intangible assets may not be recoverable include, declining or slower than anticipated growth rates for certain of our existing products, a decline in stock price and market capitalization, and slower growth rates in our industry. We may be required in the future to record a significant charge to earnings during the period in which we determine that our intangible assets have been impaired. Any such charge would adversely impact our results of operations. As of December 31, 2023, our intangible assets totaled C$22,949,560.
Changes in accounting standards and subjective assumptions, estimates and judgments by management related to complex accounting matters could significantly affect our financial results.
IFRS with regard to a wide variety of matters that are relevant to our business, such as, but not limited to, fair value measurement for financial instruments and the recoverability, share-based compensation and deferred tax assets are highly complex and involve many subjective assumptions, estimates, and judgments by our management. Changes to these standards or changes in underlying assumptions, estimates or judgments by our management could significantly change our reported results.
If we are unable to maintain effective disclosure controls and procedures and internal control over financial reporting, our stock price and investor confidence could be materially and adversely affected.
We are required to maintain both disclosure controls and procedures and internal control over financial reporting that are effective. Because of their inherent limitations, internal control over financial reporting, however well designed and operated, can only provide reasonable, and not absolute, assurance that the controls will prevent or detect misstatements. Because of these and other inherent limitations of control systems, there is only the reasonable assurance that our controls will succeed in achieving their goals under all potential future conditions. The failure of controls by design deficiencies or absence of adequate controls could result in a material adverse effect on our business and financial results, which could also negatively impact our stock price and investor confidence.
Our business operations may be adversely affected by social, political, and economic conditions affecting market risks and the demand for and pricing of our products.
These risks include:
| | ● | Unfavorable economic conditions and related low consumer confidence, high unemployment, weak credit or capital markets, sovereign debt defaults, sequestrations, austerity measures, higher interest rates, political instability, higher inflation, deflation, lower returns on pension assets, or lower discount rates for pension obligations; |
| | | |
| | ● | Changes in laws, regulations, or policies surrounding regulatory approval, manufacture and marketing of regulated healthcare products; |
| | | |
| | ● | Tax rate changes (including excise, sales, tariffs, duties, corporate, individual income, dividends, capital gains), or changes in related reserves, changes in tax rules or accounting standards, and the unpredictability and suddenness with which they can occur; |
| | | |
| | ● | Dependence upon the continued growth of brand names; |
| | | |
| | ● | Changes in consumer preferences, consumption, or purchase patterns, and our ability to anticipate and react to them; |
| | | |
| | ● | Production facility or supply chain disruption; |
| | | |
| | ● | Imprecision in supply/demand forecasting; |
| | | |
| | ● | Higher costs, lower quality, or unavailability of energy, input materials, labor, or finished goods; |
| | | |
| | ● | Direct-to-consumer changes that affect the timing of our sales, temporarily disrupt the marketing or sale of our products, or result in higher implementation—related or fixed costs; |
| | | |
| | ● | Inventory fluctuations in our products by distributors, wholesalers, or retailers; |
| | | |
| | ● | Competitors’ consolidation or other competitive activities, such as pricing actions (including price reductions, promotions, discounting, couponing, or free goods), marketing, category expansion, product introductions, or entry or expansion in our geographic markets; |
| | | |
| | ● | Insufficient protection of our intellectual property rights; |
| | | |
| | ● | Product recalls or other product liability claims; |
| | | |
| | ● | Product counterfeiting, tampering, or product quality issues; |
| | | |
| | ● | Significant legal disputes and proceedings; |
| | | |
| | ● | Government investigations (particularly of industry or company business, trade or marketing practices); |
| | | |
| | ● | Failure or breach of key information technology systems; |
| | | |
| | ● | Negative publicity related to our company, brands, marketing, personnel, operations, business performance or prospects; and |
| | | |
| | ● | Business disruption, decline, or costs related to organizational changes, reductions in workforce, or other cost-cutting measures, or our failure to attract or retain key executive or employee talent. |
Uncertainty in the financial markets and other adverse changes in general economic or political conditions in any of the major countries in which we do business could adversely affect our industry, business and results of operations.
Global economic uncertainties, including foreign currency exchange rates, affect businesses such as ours in a number of ways, making it difficult to accurately forecast and plan our future business activities. There can be no assurance that economic improvements will occur, or that they would be sustainable, or that they would enhance conditions in markets relevant to us.
An investment in our common shares, Pre-Funded Warrants and common warrants is speculative and there can be no assurance of any return on any such investment.
An investment in our common shares, Pre-Funded Warrants and common warrants is speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
Future sales of common shares, or the perception of such future sales, by some of our existing shareholders could cause our share price to decline.
The market price of our common shares could decline as a result of sales of a large number of our common shares in the market or the perception that these sales may occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell shares in the future at a time and at a price that we deem appropriate.
From time to time, certain of our shareholders may be eligible to sell all or some of their common shares by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act of 1933, as amended (the “Securities Act”), subject to certain limitations. In general, pursuant to Rule 144, non-affiliate shareholders may sell freely after six months subject only to the current public information requirement. Affiliates may sell after six months subject to the Rule 144 volume, manner of sale (for equity securities), and current public information and notice requirements.
We do not expect to pay dividends and investors should not buy our common shares expecting to receive dividends.
We do not anticipate that we will declare or pay any dividends in the foreseeable future. Consequently, you will only realize an economic gain on your investment in our common shares if the price appreciates. You should not purchase our common shares expecting to receive cash dividends. Since we do not pay dividends, and if we are not successful in establishing an orderly trading market for our shares, then you may not have any manner to liquidate or receive any payment on your investment. Therefore, our failure to pay dividends may cause you to not see any return on your investment even if we are successful in our business operations. In addition, because we do not anticipate that we will pay dividends, we may have trouble raising additional funds which could affect our ability to expand our business operations.
There can be no assurances that our common shares once listed on Nasdaq will not be subject to potential delisting if we do not continue to maintain the listing requirements of Nasdaq.
We intend to apply to list the shares of our common shares on The Nasdaq Stock Market, under the symbol “SEPS.” An approval of our listing application by Nasdaq will be subject to, among other things, our fulfilling all of its listing requirements. In addition, Nasdaq has rules for continued listing, including, without limitation, minimum market capitalization and other requirements. Failure to maintain our listing (i.e., being de-listed from Nasdaq), would make it more difficult for shareholders to sell our common shares and more difficult to obtain accurate price quotations on our common shares. This could have an adverse effect on the price of our common shares. Our ability to issue additional securities for financing or other purposes, or otherwise to arrange for any financing we may need in the future, may also be materially and adversely affected if our common shares is not traded on a national securities exchange.
Our common shares could be further diluted as the result of the issuance of additional common shares, convertible securities, options, or warrants.
Our issuance of additional common shares, convertible securities, options, and warrants could affect the rights of our shareholders, result in a reduction in the overall percentage holdings of our shareholders, could put downward pressure on the market price of our common shares, could result in adjustments to conversion and exercise prices of outstanding notes and warrants, and could obligate us to issue additional common shares to certain of our shareholders.
Our formation documents permit us to issue an unlimited number of the common shares without additional shareholder approval.
Our formation documents permit us to issue an unlimited number of common shares. The Company may, from time to time, issue additional Common Shares in the future. Subject to the requirements of Nasdaq and the TSX, the Company will not be required to obtain the approval of shareholders for the issuance of additional common shares. Any further issuances of common shares will result in immediate dilution to existing shareholders and may have an adverse effect on the value of their shareholdings.
There is no established public trading market for the Pre-Funded Warrants or the common warrants being offered in this offering, and we do not expect a market to develop for either the Pre-Funded Warrants or the common warrants.
There is no established public trading market for the Pre-Funded Warrants, or the common warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list either the Pre-Funded Warrants or the common warrants on any national securities exchange or other nationally recognized trading system. Without an active market, the liquidity of the Pre-Funded Warrants and the common warrants will be limited. Further, the existence of the Pre-Funded Warrants or the common warrants may act to reduce both the trading volume and the trading price of our common shares.
Except as otherwise provided in the Pre-Funded Warrants or the common warrants, holders of the Pre-Funded Warrants or the common warrants purchased in this offering will have no rights as shareholders until such holders exercise their Pre-Funded Warrants or the common warrants and acquire our common shares.
Except as otherwise provided in the Pre-Funded Warrants or the common warrants, until holders of the Pre-Funded Warrants or the common warrants acquire common shares upon exercise of the Pre-Funded Warrants or the common warrants, holders of the respective warrants will have no rights with respect to the common shares underlying such Pre-Funded Warrants or common warrants. Upon exercise of the Pre-Funded Warrants or the common warrants, the holders will be entitled to exercise the rights of a holder of our common shares only as to matters for which the record date occurs after the exercise date.
Since the common warrants are executory contracts, they may have no value in a bankruptcy or reorganization proceeding.
In the event a bankruptcy or reorganization proceeding is commenced by or against us, a bankruptcy court may hold that any unexercised common warrants are executory contracts that are subject to rejection by us with the approval of the bankruptcy court. As a result, holders of the common warrants may, even if we have sufficient funds, not be entitled to receive any consideration for their common warrants or may receive an amount less than they would be entitled to if they had exercised their common warrants prior to the commencement of any such bankruptcy or reorganization proceeding.
Our articles of incorporation provide that a quorum for the transaction of business at a meeting of shareholders is one or more persons present in person or by proxy and we do not require shareholder approval before certain issuances of shares other than public offerings.
Our articles of incorporation provide that a quorum for the transaction of business at a meeting of shareholders is one or more persons present in person or by proxy and we do not require shareholder approval before an issuance of securities in connection with the acquisition of the stock or assets of another company; equity-based compensation of officers, directors, employees, or consultants; and transactions other than public offerings. This could result in dilution to existing shareholders and may have an adverse effect on shareholder value.
We are an “emerging growth company,” and any decision on our part to comply with certain reduced disclosure requirements applicable to emerging growth companies could make the common shares less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act, and, for as long as we continue to be an emerging growth company, we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, not being required to comply with any new requirements adopted by the Public Company Accounting Oversight Board, or the PCAOB, requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer, not being required to comply with any new audit rules adopted by the PCAOB after April 5, 2012 unless the SEC determines otherwise, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could remain an emerging growth company until the earlier of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.235 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities pursuant to an effective registration statement; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer. We cannot predict if investors will find our common shares less attractive if we choose to rely on these exemptions. If some investors find our common shares less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common shares and our share price may be more volatile. Further, as a result of these scaled regulatory requirements, our disclosure may be more limited than that of other public companies and you may not have the same protections afforded to shareholders of such companies.
Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”), for complying with new or revised accounting standards. We have opted for taking advantage of the extended transition period for complying with new or revised accounting standards pursuant to Section 107(b) of the Jobs Act.
Unstable market and economic conditions caused by the ongoing conflict between the Ukraine and Russia, as well as the ongoing COVID-19 pandemic, could have adverse consequences on our business, financial condition and results of operations.
The global economy, including credit and financial markets, has experienced extreme volatility and disruptions as a result of the ongoing conflict between the Ukraine and Russia, as well as challenges arising from the ongoing COVID-19 pandemic, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, increases in inflation rates and uncertainty about economic stability. We could suffer inflationary pressure in our business such as through the increased costs of the supplies that we use to manufacture our products and distributing our products to all our customers where we do business. Any such volatility and disruptions could have adverse consequences on us or the third parties upon whom we rely.
You should consult your independent tax advisor regarding any tax matters arising with respect to our common shares, including the possibility that we may be classified as a passive foreign investment company (as discussed below under “Certain Material United States Federal Income Tax Considerations”).
All prospective purchasers of our common shares are advised to consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. tax consequences relevant to the purchase, ownership, and disposition of our common shares.
CURRENCY AND EXCHANGE RATE INFORMATION
The following table sets forth, for each period indicated, the period-end and the high and low exchange rate for U.S. dollars expressed in Canadian dollars, and the average exchange rate for the periods indicated. These rates are based on the noon buying rate certified for custom purposes by the U.S. Federal Reserve Bank of New York set forth in the H.10 statistical release of the Federal Reserve Board. These rates are provided solely for your convenience and are not necessarily the exchange rates that we used in this prospectus or will use in the preparation of any other reports or information to be provided to you. We make no representation that any Canadian dollar or U.S. dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars or Canadian dollars, as the case may be, at any particular rate or at all. We have presented our results of operations in Canadian dollars.
On February 26, 2024, the noon buying rate was US$1.00 = C$1.352422.
| | | Period End | | | Period Average | | | Low | | | High | |
| | | (C$ per US$) | |
| Year Ended December 31: | | | | | | | | | | | | |
| 2021 | | | 1.2777 | | | | 1.2533 | | | | 1.2031 | | | | 1.2941 | |
| 2022 | | | 1.3532 | | | | 1.3013 | | | | 1.2452 | | | | 1.3873 | |
| 2023 | | | 1.3202 | | | | 1.3549 | | | | 1.3199 | | | | 1.3881 | |
USE OF PROCEEDS
We estimate that the net proceeds to us from the sale of our [__] Units in this offering will be approximately [$_________], after deducting the underwriting discounts and commissions and the estimated offering expenses payable by us (including the offering expenses that have been committed to be paid), and assuming no exercise of the over-allotment option by the Underwriter, and no exercise of the common warrants.
We intend to use net proceeds from this offering for working capital and general corporate purposes, including product development other than as set forth, we do not anticipate requiring any material amounts of other funds to accomplish the specified purposes. Of the net proceeds from this offering, 85% will go towards product development and associated activities, with 35% going to Sepset, 40% to ABT and 10% to SafeCoat. The majority of Sepset funding will go towards completing 510(k) filings for clearance of early diagnostics for severe sepsis and severe Covid with follow-up filings for regulatory approval in Europe, China and Bahrain. ABT funding will focus on completing a clinical study of high-end dental mouth rinse and obtaining regulatory clearance in Europe and China. The SafeCoat funding will be used for development and testing of a prototype medical device (e.g., contact lenses) with our proprietary coating. Other activities will depend on the amount of proceeds raised. If sufficient, we will further develop and test our diagnostic for sepsis mechanism (endotypes) in patients enabling personalized treatments in preparation, and perform advance development of enhanced bandages, treatments for chronic rhinosinusitis and other oral therapies/preventatives, preparing for device or Investigational New Drug applications. We believe that the net proceeds from this offering and our existing cash will be sufficient to fund our operations through at least the next 24 months. This expected use of the net proceeds from the offering represents our intentions based upon our current plans and business conditions. We cannot specify with certainty all of the particular uses of the net proceeds that we will receive from this offering, or the amounts that we will actually spend on the uses set forth above. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in using these proceeds. Investors will be relying on our judgment regarding the use of the net proceeds from this offering.
The expected use of net proceeds of this offering represents our current intentions based upon our present plan and business conditions. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. We will have broad discretion in the application of the net proceeds in the category of “for general corporate purposes,” and investors will be relying on our judgment regarding the application of the proceeds of this offering. Depending on the outcome of our business activities and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this offering in different proportions than we currently anticipate.
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
Market Information for Common Shares
Our common shares are currently quoted on the Canadian Stock Exchange (“CSE”) under the trading symbol “ASEP”, the OTC Pink under the trading symbol “SEPSF” and on the Frankfurt Stock Exchange (“FSE”) under the trading symbol “FSX:JJ8”. On May 28, 2024, the closing price of our common shares on CSE was CAD 0.1850 and the last reported sale price on the OTC Pink was $0.05. We do not intend to apply for the listing of the Pre-Funded Warrants or the common warrants that are part of this offering on any national securities exchange.
Holders
As at December 31, 2023, the registrar and transfer agent for our common shares reported that there were 74,184,087 common shares issued and outstanding. Of these, 71,093,882 were registered to Canadian residents, including 60,538,587 shares registered to CDS & Co., which is a nominee of the Canadian Depository for Securities Limited. The 71,093,882 shares were registered to 145 shareholders in Canada, one of which is CDS & Co. 3,090,205 of our shares were registered to residents of the United States, including 1,000 shares registered to CEDE & Co., which is a nominee of Depository Trust Company. The 3,090,205 shares were registered to 9 shareholders in the United States, one of which is CEDE & Co. None of our shares were registered to residents of other foreign countries.
Dividends
We have not declared any common share dividends to date. We have no present intention of paying any cash dividends on our common shares in the foreseeable future, as we intend to use earnings, if any, to generate growth. The payment by us of dividends, if any, in the future, is within the discretion of our board of directors and will depend upon, among other things, our earnings, capital requirements and financial condition, as well as other relevant factors. There are no material restrictions in our articles that restrict us from declaring dividends.
DILUTION
If you invest in our common shares and/or Pre-Funded Warrants, your interest will be diluted for each common share you purchase to the extent of the difference between the public offering price per common share, included as part of the Units (without assigning any value to the common warrants) and our net tangible book value per common share after this offering. Dilution results from the fact that the public offering price per common share is substantially in excess of the net tangible book value per common share attributable to the existing shareholders for our presently outstanding common shares.
Our net tangible book value as of December 31, 2023, was [___], or [__] per common share. Net tangible book value represents the amount of our total consolidated tangible assets, less the amount of our total consolidated liabilities. Dilution is determined by subtracting the net tangible book value per common share (as adjusted for the offering) from the initial public offering price per common share and after deducting the estimated underwriting discounts and the estimated offering expenses payable by us.
After giving effect to our sale of [__] Units offered in this offering based on the assumed public offering price of $[__] per Unit, which is the midpoint of the estimated range of the public offering price shown on the front cover of this prospectus, after deduction of the estimated underwriting discounts and the estimated offering expenses payable by us, and assuming no Pre-Funded Warrants are issued, our as adjusted net tangible book value as of [__], would have been $[__], or $[__] per outstanding common share. This represents an immediate increase in net tangible book value of $[__] per common share to the existing shareholders, and an immediate dilution in net tangible book value of $[__] per common share to investors purchasing common shares included in the Units sold in this offering. The as adjusted information discussed above is illustrative only.
A $1.00 change in the assumed public offering price of $[__] per Unit would, in the case of an increase, increase and, in the case of a decrease, decrease our pro forma net tangible book value after giving effect to the offering by $[__] million, the pro forma net tangible book value per common share after giving effect to this offering by $[__] and the dilution in pro forma net tangible book value per common share included in the Units sold to new investors in this offering by $[__] assuming no change to the number of common share included in the Units offered by us as set forth on the cover page of this prospectus, and after deducting underwriting discounts and estimated offering expenses. The pro forma information discussed above is illustrative only. Our net tangible book value following the completion of this offering is subject to adjustment based on the actual initial public offering price of our common shares included in the Units and other terms of this offering determined at pricing.
The following table illustrates the estimated net tangible book value per share after this offering and the per share dilution to persons purchasing common shares included in the Units in this offering based on the foregoing offering assumptions:
| Assumed public offering price per share per Unit comprising a common share and common warrant | | $ | | |
| Historical net tangible book value per share as of [____] | | $ | | |
| Pro forma net tangible book value per share as of [____] | | $ | | |
| Increase in per share attributable to this offering | | $ | | |
| Pro forma as adjusted net tangible book value per share after this offering | | $ | | |
| Dilution per share to new investors | | $ | | |
The following tables summarize, on a pro forma as adjusted basis as of [___], the differences between existing shareholders and the new investors with respect to the number of common shares included in the Units purchased from us, the total consideration paid and the average price per common share before deducting the estimated underwriting discounts and the estimated offering expenses payable by us. The table below assumes no exercise of the Underwriter’s over-allotment option
| | | Common Shares | | | Total Consideration | | | Average
Price
Per
Common | |
| | | Number | | | Percent | | | Amount | | | Percent | | | Share | |
| Existing shareholders | | | | | | | | | | $ | | | | | | | | $ | | |
| New investors | | | | | | | | | | $ | | | | | | | | $ | | |
| Total | | | | | | | | | | $ | | | | | | | | $ | | |
To the extent that our outstanding options, warrants or subscription investment units are exercised, investors in this offering may suffer additional dilution.
The pro forma as adjusted information as discussed above is illustrative only. Our net tangible book value following the completion of this offering is subject to adjustment based on the actual initial public offering price of our common shares and other terms of this offering determined at the pricing.
CAPITALIZATION
The following table sets forth our cash and our capitalization as of December 31, 2023:
| | ● | On an actual basis; and |
| | | |
| | ● | On a pro forma basis [____]. |
The pro forma information below is illustrative only and our capitalization following the completion of this offering is subject to adjustment based on the public offering price of our common shares and other terms of this offering determined at pricing. You should read this capitalization table together with our consolidated financial statements and the related notes appearing elsewhere in this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other financial information included elsewhere in this prospectus.
| | | December 31, 2023 | |
| | | Actual | | | Pro Forma | |
| | | | | | | |
| Cash and cash equivalents | | $ | 64,721 | | | | | |
| Shareholders’ Equity: | | | | | | | | |
| Common shares, no par value; no authorization limit; 74,184,087 ordinary shares issued and outstanding; [___] ordinary shares issued and outstanding, as adjusted assuming the over-allotment option is not exercised | | | 24,045,616 | | | | | |
| Other components of equity | | | 3,550,141 | | | | | |
| Accumulated deficit | | | (14,983,767 | ) | | | | |
| Total Shareholders’ Equity | | | 12,611,990 | | | | | |
| Total Capitalization | | $ | 12,676,711 | | | $ | | |
A $1.00 increase (decrease) in the assumed public offering price of $[__] per Unit would increase (decrease) the pro forma net tangible book value per share by approximately $[__] and the dilution in pro forma net tangible book value per share to investors participating in this offering by $[__] per share, assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions, non-accountable expense allowance, and offering expenses payable by us.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition should be read in conjunction with our audited consolidated financial statements for the years ended December 31, 2023 and December 31, 2022 (our “Audited Financial Statements”), which are included elsewhere in this registration statement. Our financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) and all amounts are expressed in Canadian dollars unless otherwise noted. Our accounting policies are described in note 3 of our Audited Financial Statements.
Our Business
ASEP is in the business of acquiring assets, technologies and/or businesses in area of life sciences and medical diagnostics.
Year ended December 31, 2023 compared to year ended December 31, 2022
A summary of the Company’s operating results for the years ended December 31, 2023 and December 31, 2022 are as follows:
| | | For the year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | CA$ | | | CA$ | |
| Revenue | | | 21,140 | | | | - | |
| | | | 21,140 | | | | - | |
| Operating expenses | | | | | | | | |
| Amortization | | | 1,294,266 | | | | 1,282,394 | |
| Board advisory fees | | | 230,673 | | | | 79,887 | |
| Compensation | | | 782,301 | | | | 556,550 | |
| Consulting | | | 514,478 | | | | 246,946 | |
| General & administrative | | | 222,903 | | | | 139,718 | |
| Investor relations | | | 1,110,409 | | | | 845,950 | |
| Patent fees | | | 134,880 | | | | 155,401 | |
| Professional fees | | | 760,006 | | | | 284,606 | |
| Research & development costs | | | 678,543 | | | | 615,040 | |
| Share-based compensation | | | 1,952,054 | | | | 1,119,072 | |
| Transfer agent & filing fees | | | 61,890 | | | | 117,088 | |
| | | | 7,742,403 | | | | 5,442,652 | |
| Operating loss | | | (7,721,263 | ) | | | (5,442,653 | ) |
| Borrowing costs | | | (270 | ) | | | (814 | ) |
| Foreign exchange gain (loss) | | | (4,116 | ) | | | (2,399 | ) |
| Loss on debt settlement | | | (429,864 | ) | | | - | |
| SafeCoat Medical Inc. acquisition expense | | | (1,275,000 | ) | | | (400,521 | ) |
| | | | | | | | | |
| Loss before deferred tax recovery | | | (9,430,513 | ) | | | (5,846,386 | ) |
| Deferred tax recovery | | | - | | | | 89,100 | |
| | | | | | | | | |
| Loss and comprehensive loss for year | | | (9,430,513 | ) | | | (5,757,286 | ) |
| | | | | | | | | |
| Net loss and comprehensive loss for year | | | | | | | | |
| Attributable to shareholders | | | (8,227,328 | ) | | | (4,524,981 | ) |
| Attributable to non-controlling shareholders | | | (1,203,185 | ) | | | (1,232,305 | ) |
| | | | | | | | | |
| | | | (9,430,513 | ) | | | (5,757,286 | ) |
The net loss attributable to the shareholders for the year ended December 31, 2023 amounted to $8,227,328 compared to a net loss for the comparative period of $4,524,981 . Current year results included borrowing costs of $270 (2022: $814), a foreign exchange loss of $4,116 (2022: $2,399 ), loss on debt settlement of $429,864 (2022: $Nil) and SafeCoat Medical Inc. acquisition expense of $1,275,000 (2022: $400,521 ) and a deferred tax recovery of $Nil (2022: $89,100).
The loss on debt settlement resulted from the issue of 2,105,000 shares and 2,105,000 warrants with an estimated fair value of $850,864 to settle debt totaling $421,000.
Details of the SafeCoat acquisition expense are as follows:
| | | For the year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | CA$ | | | CA$ | |
| Consideration given: | | | | | | | | |
| Common shares | | | 1,275,000 | | | | 375,000 | |
| Services | | | - | | | | 150,752 | |
| | | | 1,275,000 | | | | 525,752 | |
| Net (assets) liabilities acquired | | | - | | | | (125,231 | ) |
| | | | | | | | | |
| SafeCoat acquisition expense | | | 1,275,000 | | | | 400,521 | |
Revenue
The Company recorded an investment in SepSMART of $2,375,000 on the vend in of certain patents licensed to the associate. The revenue from the license is being recognized over the license term of 8 years commencing December 5, 2023. In the current year, revenues of $21,140 have been recorded and the balance of the revenues of $2,353,860 to be recognized over the remaining term of the licenses are disclosed as deferred revenues in the consolidated statement of financial position.
Operating expenses
Operating expenses totaled $7,742,403 for the year ended December 31, 2023 compared to $5,442,653 for the comparative prior period.
Significant factors that contributed to the variances are discussed below:
The Company recorded amortization of $1,294,266 for the year ended December 31, 2023 (2022: $1,282,394) on intangible assets, lab equipment and computer equipment. During the year ended December 31, 2023, the Company acquired an additional license ($596,591) included in Intellectual Property resulting in an increase in the amortization expense in the current financial year.
Breakdown of amortization expenses for the respective years are as follows:
| | | For Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | CA$ | | | CA$ | |
| Category | | | | | | |
| Intellectual property | | | 1,262,761 | | | | 1,251,454 | |
| Website | | | 16,468 | | | | 16,468 | |
| Trademarks | | | 786 | | | | 633 | |
| Equipment | | | 14,251 | | | | 13,839 | |
| Totals | | | 1,294,266 | | | | 1,282,394 | |
The Company incurred board advisory fees of $230,673 for the year ended December 31, 2023 (2022: $79,887). In prior comparative period, the Company had one board advisor who was receiving monthly compensation of US$5,000. In March 2023, the Company appointed new members to the advisory board, three of whom are receiving monthly compensation of $5,000 for their services, which are being settled through the issue of shares on a quarterly basis.
The Company recorded compensation expense of $782,301 for 2 employees of Asep, 3 employees of ASEP Medical Inc. and 2 employees of Sepset for the year ended December 31, 2023 (2022: $556,550). One full time employee was hired October 1, 2022 at a monthly rate of $15,000 and an additional employee was hired January 1, 2024 at a monthly rate of $9,200.
The Company incurred consulting costs of $514,478 (2022: $246,946) for the year ended December 31, 2023, which is related to the services provided by the Company’s COO, CFO, Medical Director and external consultants for regulatory advisory services. In the prior year, consulting fees included the CEO up to the date of his resignation in June 2022, the COO from date of his appointment in July 2022 and the CFO from date of appointment in October 2022 and financial advisory services.
The Company incurred $222,903 of general & administrative costs during the year ended December 31, 2023 compared to $139,718 for the year ended December 31, 2022. The current year’s expense includes travel, occupancy, IT support services and insurance costs. In the prior comparative period, there were no external IT support services and lower corporate activity.
For the year ended December 31, 2023, the Company has incurred investor relations costs of $1,110,409 (2022: $845,950). The Company has engaged the services of numerous consultants to assist in
| | i. | managing communication between Asep’s corporate management and current and potential investors; and, |
| | ii. | development of an investor relations program. |
The Company incurred 134,880 (2022: $155,401) in patent fees related to patent filings and maintenance fees incurred by ABT and Sepset.
The Company incurred $760,006 in professional fees for the year ended December 31, 2023, which included legal, audit and accounting fees. Professional fees for the prior comparative period totalled $284,606. The increase is a result of increased corporate activity (SafeCoat License Agreement, Joint Venture Contract and supplemental agreements SepSMART, RSU Plan, Life Offering, Debt Settlement, general corporate matters and preparation of the draft registration statement on Form F-1 filed on a confidential basis with the U.S. Securities and Exchange Commission (July 2023).
The Company incurred research & development costs of $678,543 for the year ended December 31, 2023 (2022: $615,040) related to the collaborative research agreements with the University of British Columbia (UBC). The current period expense is net of a SR&ED refund of $45,439 received by ABT for the claim made for the period from January 1, 2021 to November 9, 2021.
Details are as follows:
| | | For the year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | CA$ | | | CA$ | |
| ABT Innovations Inc. | | | | | | | | |
| UBC Licensing fees | | | 10,000 | | | | 10,000 | |
| Collaborative Research Agreement (UBC) | | | 351,298 | | | | 271,744 | |
| Project services & supplies | | | 59,733 | | | | 14,924 | |
| Less - SR&ED refund | | | (45,439 | ) | | | - | |
| | | | 375,592 | | | | 296,668 | |
| Sepset Biosciences Inc. | | | | | | | | |
| Collaborative Research Agreement (UBC) | | | 239,237 | | | | 309,857 | |
| Project services & supplies | | | 63,714 | | | | 8,515 | |
| | | | 302,951 | | | | 318,372 | |
| Consolidated research & development expense | | | 678,543 | | | | 615,040 | |
The collaborative research agreement between UBC and ABT Innovations Inc. is for activities related to the development of the Company’s proprietary peptide technology. These include evaluating the antibiofilm and immunomodulatory activity of several lead peptide molecules as they relate to the various biofilm-associated infections being targeted by the company such as chronic sinusitis, biofilm-infected wounds and oral health.
The collaborative research agreement between UBC and Sepset Biosciences Inc. is to experimentally test and validate the components of the diagnostic assays for sepsis that are designed and developed by Sepset employees. This includes activities related to the nucleic acid isolation procedures as well as testing the performance of the various components of the quantitative polymerase chain reaction assay.
The Company recognized share-based compensation of $1,952,054 (2022: $1,119,072) related to stock options and restricted stock units (“RSUs) that were granted to certain directors, officers, employees and consultants and vested during the year ended December 31, 2023. Stock options are granted to directors, officers, employees and consultants pursuant to employment, consulting or board advisory agreements, collectively referred to as service agreements, as part of the remuneration package to the respective party, as set out in their respective service agreement. RSUs are rewarded to directors, officers, employees, consultants and advisory board members to promote the long-term success of the Company and the creation of shareholder value by: (i) encouraging the attraction and retention; (ii) encouraging such to directors, officers, employees and consultants to focus on critical long-term objectives; and (iii) promoting greater alignment of the interests of such to directors, officers, employees and consultants with the interests of the Company.
Details are as follows:
| | | For the year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | CA$ | | | CA$ | |
| Share-based compensation | | | | | | | | |
| Stock options | | | 314,690 | | | | 1,119,072 | |
| RSUs | | | 1,637,364 | | | | - | |
| | | | 1,952,054 | | | | 1,119,072 | |
The following table details the Company’s quarterly results:
| Quarter Ended | | Net revenues | | | Net income (loss)
attributable to
shareholders | | | Net income (loss)
attributable to
non-controlling
interest | | | Net income (loss) | | | Income (loss) per
share - basic &
fully diluted | |
| | | $’s | | | $’s | | | $’s | | | $’s | | | $’s | |
| 31-Dec-23 | | | 21,140 | | | | (2,487,683 | ) | | | (259,968 | ) | | | (2,747,651 | ) | | | (0.04 | ) |
| 30-Sep-23 | | | - | | | | (1,315,498 | ) | | | (323,222 | ) | | | (1,638,720 | ) | | | (0.02 | ) |
| 31-Jun-23 | | | - | | | | (2,482,137 | ) | | | (323,990 | ) | | | (2,806,127 | ) | | | (0.04 | ) |
| 31-Mar-23 | | | - | | | | (1,942,010 | ) | | | (296,005 | ) | | | (2,238,015 | ) | | | (0.03 | ) |
| 31-Dec-22 | | | - | | | | (1,218,249 | ) | | | (324,234 | ) | | | (1,542,483 | ) | | | (0.02 | ) |
| 30-Sep-22 | | | - | | | | (751,861 | ) | | | (395,089 | ) | | | (1,146,950 | ) | | | (0.03 | ) |
| 30-Jun-22 | | | - | | | | (1,153,033 | ) | | | (354,069 | ) | | | (1,507,102 | ) | | | (0.03 | ) |
| 31-Mar-22 | | | - | | | | (1,401,838 | ) | | | (158,913 | ) | | | (1,560,751 | ) | | | (0.03 | ) |
There are no meaningful trends evident from analysis of the summary of quarterly financial information.
Factors that can cause significant fluctuations in the Company’s quarterly results include share-based compensation:
| Quarter Ended | | Professional fees | | | Share-based
compensation | |
| | | $’s | | | $’s | |
| 31-Dec-23 | | | 397,650 | | | | 691,592 | |
| 30-Sep-23 | | | 63,011 | | | | 428,809 | |
| 30-Jun-23 | | | 196,523 | | | | 501,231 | |
| 31Mar-23 | | | 102,822 | | | | 330,423 | |
| 31-Dec-22 | | | 20,148 | | | | 169,332 | |
| 30-Sep-22 | | | 65,377 | | | | 184,803 | |
| 30-Jun-22 | | | 118,625 | | | | 278,584 | |
| 31-Mar-22 | | | 80,456 | | | | 486,353 | |
Significant items contributing to the loss incurred during the fourth quarter ended December 31, 2023 and 2022 are as follows
| | ● | Amortization of $327,234 (2022-$320,505) on intangible assets, lab equipment and computer equipment. The Company acquired an additional license in July 2023 with a fair value of $596,591 that is included as an addition to intangible assets and being amortized on a straight line basis over 20 years resulting in an increase in amortization in 2023 compared to the prior comparative period. |
| | | |
| | ● | Investor relations fees and expenses of $348,188 (2022: $177,774) for the current quarter. Increase in expense in 2023 compared to prior comparative period is the result of entering into a contract in November 2023 for approximately $300,000 that was expensed in the quarter as the timing of when services were to be provided could not reasonably be determined. |
| | | |
| | ● | Operating expenses (board advisory fees, compensation, general & administration, patent fees and transfer agent and filing fees) of $394,292 (2022 - $365,305); |
| | ● | Consulting fees of 2023- $127,209 (2022: $(78,039)), prior year’s quarterly results includes a consulting fee received for the negotiation of the term sheet with UBC to acquire the for the use, development and commercialization of a peptide medical device coating technology license; |
| | | |
| | ● | Professional fees primarily legal of $397,650 (2022-$20,148). Fees in current quarter relate to legal fees for services provided in connection with the negotiation and closing of the joint venture agreement with Sansure, Life Offering, debt settlement and general corporate services. |
| | | |
| | ● | Research & development expenses of $80,905 (2022 - $253,273). Increase in activity level in 2022 compared to prior comparative period that reflects the results of operations of the companies’ subsidiaries, ABT Innovations Inc. and Seset Biosciences Inc., from date of acquisition in November 2022. |
Details are as follows:
| | | For the three month period ended
December 31, 2023 | | | For the three month period ended
December 31, 2022 | |
| ABT Innovations Inc. | | | | | | | | |
| UBC Licensing fees | | $ | 4,234 | | | $ | 2,500 | |
| Collaborative Research Agreement (UBC) | | | - | | | | 126,893 | |
| Project services & supplies | | | 26,562 | | | | 11,174 | |
| | | | 30,796 | | | | 140,567 | |
| Sepset Biosciences Inc. | | | | | | | | |
| Collaborative Research Agreement (UBC) | | | 39,400 | | | | 175,397 | |
| Project services & supplies | | | 10,709 | | | | 1,847 | |
| | | | 50,109 | | | | 177,244 | |
| | | | | | | | | |
| Consolidated research & development expense | | $ | 80,905 | | | $ | 317,811 | |
The collaborative research agreement between UBC and ABT Innovations Inc. is for activities related to the development of the company’s proprietary peptide technology. These include evaluating the antibiofilm and immunomodulatory activity of several lead peptide molecules as they relate to the various biofilm-associated infections being targeted by the company such as chronic sinusitis, biofilm-infected wounds and oral health.
The collaborative research agreement between UBC and Sepset Biosciences Inc. is to experimentally test and validate the components of the diagnostic assays for sepsis that are designed and developed by Sepset employees. This includes activities related to the nucleic acid isolation procedures as well as testing the performance of the various components of the quantitative polymerase chain reaction assay.
| | ● | Share-based compensation of $221,476 (2022 - $169,332) for vested options and RSUs in the respective quarters. |
Details are as follows:
| | | For the three month period ended
December 31, 2023 | | | For the three month period ended
December 31, 2022 | |
| | | CA$ | | | CA$ | |
| Share-based compensation | | | | | | | | |
| Stock options | | | 15,725 | | | | 169,332 | |
| RSUs | | | 205,751 | | | | - | |
| | | | 221,476 | | | | 169,332 | |
Liquidity and Capital Resources
The following table summarizes our cash flow data as of:
| | | For the year ended
December 31, 2023 | | | For the year ended
December 31, 2022 | |
| | | CA$ | | | CA$ | |
| Net cash provided by (used in) | | | | | | | | |
| Operating activities | | | (2,712,598 | ) | | | (3,614,476 | ) |
| Investing activities | | | (4,471 | ) | | | 124,796 | |
| Financing activities | | | 651,400 | | | | 330,000 | |
| | | | | | | | | |
| Net increase (decrease) | | | (2,065,669 | ) | | | (3,159,680 | ) |
| Cash and cash equivalents, beginning of year | | | 2,130,390 | | | | 5,290,070 | |
| | | | | | | | | |
| Cash and cash equivalents, end of year | | | 64,721 | | | | 2,130,390 | |
Operating Activities
Net cash used in operating activities consists of net loss adjusted for non cash items, including amortization, borrowing costs, consulting fees, loss on debt settlement, revenue, SafeCoat acquisition expense, shares issued for services and share-based compensation and for impact of changes in working capital. Net cash used in operations for the year ended December 31, 2023 was $2,712,598 representing a decrease of $901,878 compared to net cash used in operation of $3,614,476 for the year ended December 31, 2022.
Investing Activities
During the year ended December 31, 2023, the Company purchased computer equipment ($1,227) and incurred costs in registering trademarks ($3,244). Net cash provided by investing during the year ended December 31, 2022 was provided by the cash acquired on the acquisition of SafeCoat ($125,231) offset by the cost incurred in registering trademarks ($435).
Financing Activities
During the year ended December 31, 2023, the Company completed a Life Offering for gross proceeds of $587,000 and received loan proceeds of $20,000. Additionally, 200,000 stock options were exercised for proceeds of $60,000. During the year ended December 31, 2022, the Company completed a debt financing for proceeds of $330,000.
Cash Resources and Capital Resources
We have no revenue generating operations from which we can internally generate funds. To date the Company’s ongoing operations have been funded through debt and equity financings. The Company believes that it will be able to secure additional debt financings, private placements and public financings in the future, although it can not predict the size or pricing of any such financings. This situation is unlikely to change until such time as the Company commences profitable operations from the sale of its products. When acquiring technologies through purchase or option, the Company will sometimes issue common shares to the vendor or optioned of the technology as partial or full consideration for the technology in order to preserve cash.
As at December 31, 2023, the Company had cash and cash equivalents of $64,721, other current assets of $186,222 and current liabilities of $995,587, compared to cash and cash equivalents of $2,130,390, other current assets of $1,268,255 and current liabilities of $528,161as at December 31, 2022.
As of December 31, 2023, the Company had working capital (deficit) of $(744,644) (December 31, 2022 –$2,870,484 ). The Company estimates that it does not have available funds to meet requirements for the coming twelve months based on current estimated expenditures for operations and development of its technologies. These uncertainties cast significant doubt on the ability of the Company to continue as a going concern. As at December 31, 2023, the consolidated financial statements were prepared on a going concern basis which contemplates the realization of assets and the settlement of liabilities in the normal course of operations. Management is working with its financial advisors to determine the best manner of raising capital over the next number of months. Management is considering an equity raise as well as, in the right circumstances and the right terms, a debt financing.
The ability of the Company to carry out its planned business objectives is dependent on its ability to raise adequate capital funds including and not limited to grants, strategic alliances, debt financing and equity financing. If adequate financing is not available, the Company may be required to delay, reduce the scope of, or eliminate one or more development activities. There is no assurance that Company will be able to obtain financing in the future or that such financing will be on terms acceptable to Company.
Material Accounting Policies
A summary of material accounting policies of Asep Medical Holdings Inc. is presented in note 3 of our Audited Financial Statements. The financial statements and notes are representations of the Company’s management, which is responsible for their integrity and objectivity. These accounting policies conform too accounting principles under IFRS and have been consistently applied in the preparation of financial statements.
Basis of presentation
These consolidated financial statements have been prepared on an accrual basis and on a historical cost basis. The preparation of the consolidated financial statements in compliance with IFRS requires management to make certain critical accounting estimates. It also requires management to exercise judgment in applying the Company’s accounting policies. The areas involving a higher degree of judgment of complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in note 3. These consolidated financial statements are prepared in Canadian dollars, with all amounts rounded to the nearest dollar, unless otherwise stated.
Consolidation
These consolidated financial statements include the accounts of the Company and its subsidiaries. Subsidiaries are all entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are consolidated from the date on which control is transferred to the Company and are deconsolidated from the date that control ceases.
All intercompany transactions, balances and unrealized gains and losses from intercompany transactions in Canada are eliminated on consolidation. The functional and presentation currency of the Company and its subsidiaries is the Canadian dollar. The principal subsidiaries of ASEP and their geographic locations at December 31, 2023, are as follows:
| | | Principal Activity | | Location | | Percentage ownership | |
| Asep Medical Inc. (“ASEP Medical”) | | Life Sciences | | Canada | | | 100 | % |
| ABT Innovations Inc. (“ABT”) | | Life Sciences | | Canada | | | 50.1 | % |
| Sepset Biosciences Inc. (“Sepset”) | | Life Sciences | | Canada | | | 50.1 | % |
| OHP Innovations Inc. (“OHP”)* | | Life Sciences | | Canada | | | 100 | % |
| SafeCoat Medical Inc. (“SafeCoat”) | | Life Sciences | | Canada | | | 88 | % |
* Inactive
Non-controlling interest
Non-controlling interest (“NCI”) represents equity interests in subsidiaries owned by outside parties. The share of net assets of subsidiaries attributable to non-controlling interest is presented as a component of equity. The loss and each component of other comprehensive income are attributed to non-controlling interests where applicable.
Associates
An associate is an entity over whose operating and financial policies the Company exercises significant influence. Significant influence is presumed to exist where the Company has between 20 percent and 50 percent of the voting rights but can also arise where the Company holds less than 20 percent of the voting rights but has the power to be actively involved and influential in policy decisions affecting the entity. The Company’s share of the net assets, post-tax results and reserves of the associate are included in the consolidated financial statements using the equity accounting method. This involves recording the investment initially at cost to the Company, and then, in subsequent periods, adjusting the carrying amount of the investment to reflect the Company’s share of the associate’s results. Unrealized gains on transactions between the Company and an associate are eliminated to the extent of the Company’s interest in the associate.
Financial instruments
Classification
The Company classifies its financial instruments in the following categories: at fair value through profit and loss (“FVTPL”), at fair value through other comprehensive income (loss) (“FVTOCI”) or at amortized cost. The Company determines the classification of financial assets at initial recognition. The classification of debt instruments is driven by the Company’s business model for managing the financial assets and their contractual cash flow characteristics. Equity instruments that are held for trading are classified as FVTPL. For other equity instruments, on the day of acquisition the Company can make an irrevocable election (on an instrument-by-instrument basis) to designate them as at FVTOCI. Financial liabilities are measured at amortized cost, unless they are required to be measured at FVTPL (such as instruments held for trading or derivatives) or if the Company has opted to measure them at FVTPL.
The following table shows the classification of the Company’s financial instruments under IFRS 9:
| Financial assets/liabilities | | Classification - IFRS 9 |
| Cash and cash equivalents | | FVTPL |
| Accounts payable and accrued liabilities | | Amortized cost |
| Loans payable | | Amortized cost |
Measurement
Financial assets and liabilities at amortized cost
Financial assets and liabilities at amortized cost are initially recognized at fair value plus or minus transaction costs, respectively, and subsequently carried at amortized cost less any impairment.
Financial assets and liabilities at FVTPL
Financial assets and liabilities carried at FVTPL are initially recorded at fair value and transaction costs are expensed in the statements of comprehensive loss. Realized and unrealized gains and losses arising from changes in the fair value of the financial assets and liabilities held at FVTPL are included in the statement of comprehensive loss in the period in which they arise.
Impairment of financial assets at amortized cost
The Company recognizes a loss allowance for expected credit losses on financial assets that are measured at amortized cost. At each reporting date, the Company measures the loss allowance for the financial asset at an amount equal to the lifetime expected credit losses if the credit risk on the financial asset has increased significantly since initial recognition. If at the reporting date, the credit risk of the financial asset has not increased significantly since initial recognition, the Company measures the loss allowance for the financial asset at an amount equal to the twelve month expected credit losses. The Company shall recognize in the statements of comprehensive loss, as an impairment gain or loss, the amount of expected credit losses (or reversal) that is required to adjust the loss allowance at the reporting date to the amount that is required to be recognized.
Derecognition
Financial assets
The Company derecognizes financial assets only when the contractual rights to cash flows from the financial assets expire, or when it transfers the financial assets and substantially all of the associated risks and rewards of ownership to another entity.
Financial liabilities
The Company derecognizes a financial liability when its contractual obligations are discharged or cancelled or expire. The Company also derecognizes a financial liability when the terms of the liability are modified such that the terms and / or cash flows of the modified instrument are substantially different, in which case a new financial liability based on the modified terms is recognized at fair value. Gains and losses on derecognition are recognized in the statements of comprehensive loss.
Impairment of Non-Financial Assets
At the end of each reporting period, the Company reviews the carrying amounts of its tangible and intangible assets to determine whether there is an indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss, if any. Where it is not possible to estimate the recoverable amount of an individual asset, the Company estimates the recoverable amount of the cash-generating unit (“CGU”) to which the assets belong.
Recoverable amount is the higher of fair value less costs to sell and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
If the recoverable amount of an asset or CGU is estimated to be less than its carrying amount, the carrying amount of the asset or CGU is reduced to its recoverable amount. An impairment loss is recognized immediately in the statement of comprehensive loss.
Where an impairment loss subsequently reverses, the carrying amount of the asset or CGU is increased to the revised estimate of its recoverable amount, however the increased carrying amount cannot exceed the carrying amount that would have been determined had no impairment loss been recognized for the asset or CGU in prior years.
Financing Costs
The costs related to equity transactions are deferred until the closing of the equity transactions. These costs are accounted for as a deduction from equity. Transaction costs of abandoned equity transactions are recognized in the statement of comprehensive loss.
Equipment
Equipment is stated at historical cost less accumulated amortization and accumulated impairment losses. Cost includes costs paid to acquire assets from third parties as well as costs incurred in internally constructed assets.
Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost of the item can be measured reliably. The carrying amount of the replaced part is derecognized. All other repairs and maintenance are charged to the statement of comprehensive loss during the financial period in which they are incurred.
Gains and losses on disposals are determined by comparing the proceeds with the carrying amount and are recognized in profit or loss. Amortization is calculated as follows:
Computer equipment is amortized on a straight-line basis over its estimated useful lives of 36 months starting when the asset is available for use. Lab equipment is amortized on a straight-line basis over its estimated useful lives of 60 months starting when the asset is available for use. No amortization is recorded where an asset is in development and not yet ready for its intended use.
Intangible assets
Intangible assets are recorded at cost less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Amortization of definite life intangible assets is recognized on a straight-line basis over their 20-year estimated useful lives.
Income taxes
Current income tax:
Current income tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted, at the reporting date, in the countries where the Company operates and generates taxable income.
Current income tax relating to items recognized directly in other comprehensive income or equity is recognized in other comprehensive income or equity and not in profit or loss. Management periodically evaluates positions taken in the tax returns with respect to situations in which applicable tax regulations are subject to interpretation and establishes provisions where appropriate.
Deferred income tax:
Deferred income tax is provided using the asset and liability method on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred income tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized.
Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. Deferred income tax assets and deferred income tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred income taxes relate to the same taxable entity and the same taxation authority.
Research and development
Research costs are expensed when incurred. Internally-generated technology costs are capitalized as intangible assets when the Company can demonstrate that the technical feasibility of the project has been established; the Company intends to complete the asset for use or sale and has the ability to do so; the asset can generate probable future economic benefits; the technical and financial resources are available to complete the development; and the Company can reliably measure the expenditure attributable to the intangible asset during its development. After initial recognition, internally generated intangible assets are recorded at cost less accumulated amortization and accumulated impairment losses.
The Company did not have any development costs that met the capitalization criteria for the years ended December 31, 2023 and 2022.
Share capital
The proceeds from the exercise of stock options and warrants, in addition to the estimated fair value attributable to these equity instruments, are recorded as share capital when exercised. Warrants issued are recorded at the estimated fair value using the Black-Scholes pricing model.
On unit offerings, the Company prorates the proceeds between the relative fair values of the shares issued and the Black-Scholes value of the warrants issued.
Share-based compensation
The Company grants stock options to directors, officers, employees and service providers. Each tranche in an award is considered a separate award with its own vesting period and fair values. The Company applies the fair-value method of accounting for share-based compensation. The fair value is calculated using the Black-Scholes option-pricing model.
Share-based compensation for employees and others providing similar services are determined based on the grant date fair value. Share-based compensation for non-employees is determined based on the fair value of the goods or services received or option granted measured at the date on which the Company obtains such goods or services.
Share-based compensation expense is recognized over each tranche’s vesting period in the consolidated statements of loss or capitalized as appropriate, based on the number of awards that vest less the estimated forfeitures. The number of forfeitures likely to occur is estimated on grant date. If and when stock options are exercised, the applicable amounts of contributed surplus are transferred to share capital.
Restricted share units
The Company has established a restricted share plan under which share units are granted to directors, officers, key executive and non-executive employees, consultants and advisory board members. The restricted share units are considered equity-settled and are measured using the grant date fair value. Share-based compensation expense is recognized over the vesting period in the consolidated statement of loss with a corresponding amount recognized in equity.
Earnings (loss) per share
Basic earnings (loss) per share is calculated by dividing the earnings (loss) attributable to common shareholders of ASEP by the weighted average number of common shares outstanding in the period. Contingently returnable shares are excluded from the calculation of weighted average number of common shares outstanding. Diluted loss per share is calculated by the treasury stock method. Under the treasury stock method, the weighted average number of common shares outstanding for the calculation of diluted loss per share assumes that the proceeds to be received on the exercise of dilutive share options and warrants are used to repurchase common shares at the average market price during the period.
Foreign currency translation
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transaction. Foreign currency monetary items are translated at the period-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined.
Exchange differences arising on the translation of monetary items or on settlement of monetary items are recognized in profit or loss in the statement of comprehensive loss in the period in which they arise, except where deferred in equity as a qualifying cash flow or net investment hedge.
Exchange differences arising on the translation of non-monetary items are recognized in other comprehensive loss in the statement of comprehensive loss to the extent that gains and losses arising on those non-monetary items are also recognized in other comprehensive loss. Where the non-monetary gain or loss is recognized in profit or loss, the exchange component is also recognized in profit or loss.
Revenue recognition
Revenue is derived from licensing of Intellectual Property and providing technical support and other services.
Revenue is recognized when control of these licenses and services are transferred to the customer, in an amount that reflects the consideration the Company expects to be entitled in exchange for services.
Revenue recognition is determined through the following five steps:
| | 1. | Identify the contract with a customer |
| | 2. | Identify the performance obligations in the contract |
| | 3. | Determine the transaction price |
| | 4. | Allocate the transaction price to the performance obligations in the contract |
| | 5. | Recognize revenue when (or as) the entity satisfies a performance obligation |
Revenue may be earned over time as the performance obligations are satisfied or at a point in time which is when the entity has earned a right to payment, the customer has possession of the asset and the related significant risks and rewards of ownership, and the customer has accepted the asset or service. Royalty revenue is recognized as the underlying sales occur.
The estimated transaction price, which includes variable consideration, may be subject to constraint and is included in the transaction price only to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. At the end of each reporting period, the Company updates the estimated transaction price, including updating its assessment of whether an estimate of variable consideration is constrained, to represent faithfully the circumstances present at the end of the reporting period and the changes in circumstances during the reporting period. Actual amounts may ultimately differ from the Company’s estimates. If actual results vary, the Company will adjust these estimates, which could have an effect on earnings in the period of adjustment.
Patent costs
Patent costs include patent filing fees and patent maintenance fees and are expensed when incurred.
Significant estimates and assumptions
The preparation of the Company’s consolidated financial statements in conformity with IFRS requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and contingent liabilities at the date of the consolidated financial statements and reported amounts of revenues and expenses during the reporting period. Estimates and assumptions are continuously evaluated and are based on management’s experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. However, actual outcomes can differ from these estimates.
Estimates and assumptions where there is significant risk of material adjustments to assets and liabilities in future accounting periods include the useful lives of intangible assets, market multiples utilized in the determination of recoverable amounts of intangible assets, fair value measurements for financial instruments, share-based payments and measurement of deferred tax assets.
Significant judgements
The preparation of financial statements in accordance with IFRS requires the Company to make judgments, apart from those involving estimates, in applying accounting policies. The most significant judgments in applying the Company’s accounting policies in these financial statements were:
| | ○ | Whether performance obligations in the contract are distinct. |
| | ○ | Whether it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the contingency is resolved. |
| | ○ | Whether the expected value method or most likely amount method better predicts the amount of consideration to which the Company will be entitled. |
| | ○ | Whether the Company satisfies a performance obligation over time or at a point in time. |
| | ○ | The determination of the method to measure the progress towards complete satisfaction of a performance obligation satisfied over time. |
| ● | Evaluating whether or not costs incurred by the Company meet the criteria for capitalization as intangible assets. |
| | |
| ● | The Company assesses the carrying values of its tangible and intangible assets at the end of each reporting period for indicators of impairment. If it is determined that there are indicators of impairment, the Company determines the recoverable amount of the related cash generating units. Recoverability is dependent upon assumptions and judgments regarding historical R&D costs and market multiples. Other assumptions used in the calculation of recoverable amounts are discount rates and future cash flows. A material change in assumptions may significantly impact the potential impairment of these assets. During the year ended December 31, 2023, no impairment indicators were identified by management. |
| | |
| ● | Management determines whether assets acquired and liabilities assumed constitute a business. A business consists of inputs and processes applied to those inputs that have the ability to create outputs. The Company completed the acquisition of SafeCoat Medical Inc. (“SafeCoat”) on December 12, 2022 and concluded that the acquired entity did not qualify as a business combination under IFRS 3, “Business Combinations”. Accordingly, the acquisition has been accounted for as an asset acquisition. |
Other significant judgments in applying the Company’s accounting policies relate to the assessment of the Company’s ability to continue as a going concern (Note 1), the recoverability of deferred tax assets, functional currency determinations and the classification of its financial instruments.
BUSINESS
Overview
We are a company focused on discovering and developing diagnostic and therapeutic solutions to mitigate the global crisis of antibiotic failure by improving patients’ odds of survival and quality of life through acquiring assets, technologies and/or businesses in the areas of life sciences and medical diagnostics.
The sepsis and antibiotic resistance crises are widespread and deadly. Per year, there are approximately 49 million cases of sepsis.9 Of these, 11 million people die from sepsis per year10. The front-line treatment for all-cause sepsis, after effective diagnosis, is antibiotics, so, the 23% death rate represents an example of antibiotic failure. Regarding COVID-19, severe COVID-19 is severe sepsis and largely responsible for COVID-19 deaths, with 6.86M people having died as of February 2023.11 Currently, 65% of all infections are biofilms. Biofilm infections are 10 to 1000 times more resistant to most antibiotics. Appallingly, there are no approved drugs to combat biofilms.
Sepsis and antibiotic resistant infections pose severe burdens on healthcare systems and create excellent investment potential. Combined, the market is $179 billion. Globally, sepsis has a $1 billion market and a compound annual growth rate (“CAGR”) of 8.5%. The global antibiotic market is $41.9 billion and has a CAGR of 3%. More specifically, (1) the Methicillin-resistant Staphylococcus aureus (“MRSA”) market is $1 billion and has a CAGR of 4.4%; (2) the global device infection market is $2 billion and has a CAGR of 3.6%; (3) the global CRS market is $2.1 billion and has a CAGR of 7.4%; (4) the global ear infection market is $2.8 billion and has a CAGR of 5%; (5) the global bacterial vaginosis market is $4.5 billion and has a CAGR of 9%; (6) the global urinary tract infection market is $9.5 billion and has a CAGR of 3.6%; (7) the global wound care market is $20 billion and has a CAGR of 4.1%.
We fight antibiotic failure on two fronts, diagnostic and therapeutic. For our diagnostic product, we are developing a novel assay that is intended to provide earlier, faster risk assessment to enable targeted treatment of sepsis. Our therapeutic product consists of patented pharmaceutical peptides that target currently untreatable biofilm infections and associated inflammation.
Our technologies are novel in the early diagnosis of sepsis and treatment of biofilm infections. Conventional sepsis diagnoses can take approximately 13 to 48 hours. Our technology can yield results significantly faster than conventional methods, by providing results in approximately 1 hour after patient sample collection. On the therapeutic side, there are currently no approved drugs that treat biofilm infections.
As near-term revenue models, we have prioritized development of our sepsis diagnostic and oral health and wound anti-biofilm therapeutics. In addition to our own activities in pursuing approval of our diagnostic in the next 12 months through the 510(k) process we have formed a joint venture in Bahrain and signed a term sheet with a company in China to collaboratively develop our diagnostic for these markets. As such, we have signed a definitive agreement for a joint venture with Bahrain-based international investment consultancy firm, Seaspring W.L.L., to advance regulatory approval and commercialization of the SepsetER in the Kingdom of Bahrain and the Middle East and North Africa. The terms of the definitive agreement include the formation of a 50/50 joint venture whereby Seaspring will contribute the capital required by the joint venture to conduct its business operations (regulatory approval, sales and distribution) and ASEP through its subsidiary Sepset Biosciences, will provide the licensing rights for the use of the SepsetER technology in the Kingdom of Bahrain, Algeria, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Qatar, Saudi Arabia, Syria, Tunisia, United Arab Emirates and Yemen. The license is a sole license for each country to: a) use, develop and commercialize and otherwise exploit, in whole or in part, for clinical purposes, SepsetER and related patents; b) copy, translate, modify, enhance, upgrade or add to SepsetER; c) practice or otherwise reduce to practice SepsetER (including making improvements); and d) grant sublicenses on written terms and conditions in compliance with and not inconsistent with the terms and conditions set forth in the license agreement.
We have a term sheet in place with Sansure Biotech for the Chinese market, providing a road map for a joint venture in preparation for the research, development, use, sublicensing and commercialization of SepsetER in this market, and are in the concluding stages of negotiating a final agreement.
On the therapeutic side, we have also advanced an oral health care solution and have signed a Feasibility Study Agreement (the “Feasibility Study Agreement”) with the Chinese firm Bohai Biomedical Technology Development (“Bohai”). The agreement permits Bohai Biomedical to conduct a feasibility study using one of our proprietary anti-biofilm peptides as an active ingredient. The peptide will be studied as an agent to mitigate and prevent erosive dental biofilm infections in an oral rinse product. The peptide mouth rinse has already undergone testing at the UBC Department of Dentistry under the supervision of Dr. Ya Shen and Dr. Markus Haapasalo in close collaboration with the ASEP team. In the Feasibility Study Agreement, we have granted to Bohai a non-transferable, non-exclusive license to use the proprietary anti-bacterial peptides, any peptide created by exchanging any single amino acid in the peptide for one with similar properties, and/or any substances created by Bohai that constitute an unmodified functional subunit or product expressed by the anti-biofilm peptide (the “Material”) and any and all information, know-how, techniques or practices ASEP discloses to Bohai in writing and identified as confidential, subject to certain exclusions in the Feasibility Study Agreement, only for research purposes as set out in the Feasibility Study, for a period commencing on the date the Feasibility Study Agreement is signed and ending 6 months thereafter, unless terminated earlier or extended pursuant to the agreement. Bohai will provide ASEP with a quarterly written report on the progress of the feasibility study and upon the culmination of the feasibility study, Bohai will provide a copy of all data obtained to ASEP. Bohai has granted ASEP an irrevocable, royalty-free, worldwide license to use such data for any research or business purposes. If the feasibility study results in the creation of any modifications, subject to certain exclusions, that are considered Materials under the Feasibility Study Agreement, the parties will be deemed to jointly own all rights, title and interest in and to such modifications. Upon successful completion of the feasibility study, Bohai may negotiate a commercial license with ASEP for the Material pursuant to an exclusive license or joint venture for distribution and sales rights in the territory of China.
9 https://www.who.int/news-room/fact-sheets/detail/sepsis
10 https://www.who.int/news-room/fact-sheets/detail/sepsis
11COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, accessible at https://ourworldindata.org/grapher/cumulative-covid-deaths-region; The study is found in Baghela, A., R.E.W. Hancock et al. 2022. Predicting sepsis severity at first clinical presentation: the role of endotypes and mechanistic signatures. eBiomedicine 75:103776. See also Baghela, A., R.E.W. Hancock et al. 2023. Predicting severity in COVID-19 disease using sepsis blood gene expression signatures. Sci. Reports 13:1247.
Discussion of Diagnostic Business—Diagnostic Technology
Sepsis is caused by the body’s inappropriate and dysfunctional immune response to infection. We have developed a diagnostic technology based on the early detection of unique molecular signatures of sepsis specific to the immune response, rather than the presence of a pathogen. When treating sepsis, every hour counts with a 7.6% increased risk of death for every hour of delayed diagnosis; however, current sepsis diagnostic tests can take between 13-48 hours to deliver a result.12 The ASEP SepsetER rapid diagnostic test works with human blood samples and is intended to deliver results in approximately 1 hour, which we believe based on historical data should translate into significantly improved sepsis survival rates. This is depicted in the Figure below.13
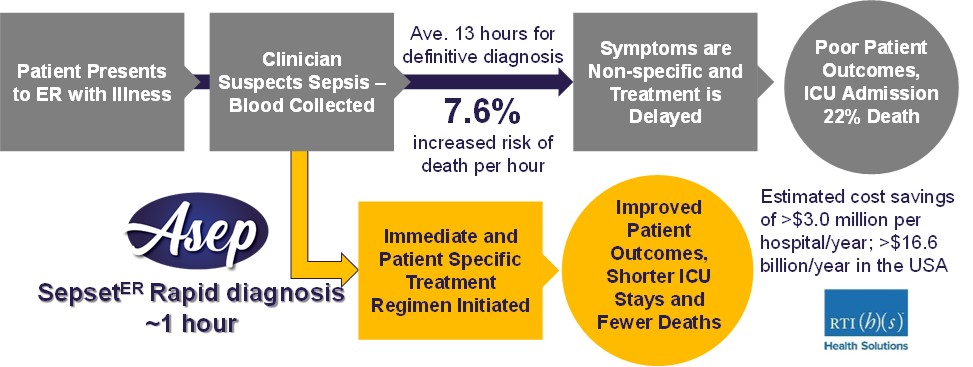
Based on Sepset’s internal research data, there are several likely advantages to the ASEP SepsetER rapid diagnostic test:
| | ● | Greater sensitivity and specificity than the systemic inflammatory response syndrome (“SIRS”) criteria used today. |
| | | |
| | ● | Earlier recognition of sepsis, at a time when patients are admitted to the emergency room. |
| | | |
| | ● | Reliable risk assessment for determining the most appropriate treatment to improve survival. |
| | | |
| | ● | Next-generation molecular diagnostic focusing on the patient’s specific immune response. |
ASEP has undertaken a clinical genomics study of early sepsis to validate our diagnostic technology14. Prospective multicenter clinical studies with 647 patient blood samples confirm that the SepsetER gene expression signature is highly statistically significant (p<10-752) with strong predictive power (AUC = 0.85) in predicting progression to sepsis or not, by one day after patient enrollment at the time of recruitment, based on the international standardized Sepsis 3 definition16 of a Sequential Organ Failure Assessment (“SOFA”) score >2, and confirmed or physician-suspected infection. Furthermore, these studies helped to refine the larger list of signature genes that we found to be predictive, to a smaller number (namely 6 genes).15 Of these 647 patients, 373 (57.6%) were found to have sepsis based on the Sepsis 3 definition16. We did not compare the SepsetER diagnosis to traditional diagnosis methods, but large studies have shown that early diagnosis of sepsis using the Sepsis 3 recommended abbreviated quick SOFA (qSOFA)17 or assessment of systemic infection18 using conventional or PCR-Based methods18 are only about 50% accurate. NB. “Prospective” is an official term for clinical studies/trails. In prospective studies, individuals are followed over time and data about them is collected as their characteristics or circumstances change. The study, performed in 4 different countries/continents (Australia/Australia; Netherlands/Europe; Canada /North America; Colombia/South America), tested the level of expression of our diagnostic signature in the blood of patients with a wide range of symptoms; the patients were recruited in either the emergency room or early after entry into the intensive care unit. The number of all-cause sepsis patient samples tested were 338 emergency room/ER samples from one study and 309 Intensive Care Unit/ICU samples from that plus one other study. The studies correlate clinical outcomes (namely organ dysfunction as revealed by SOFA score) with the expression of our SepsetER signature that reports on immune status (dysfunctional in sepsis). It is worth noting that the study was designed to enumerate both sensitivity (true positives) and specificity (true negatives), as well as the smaller number of false positives and false negatives.
Our other studies looking at overexpression of our SepsetER gene expression signature, that forms the basis of the SepsetER test, using the same study design as reported in the preceding paragraph, has allowed extrapolation of our technology to additional patient populations including, among others, perforated appendicitis19b (71 patients with pediatric appendicitis), severe pancreatitis19a (87 patients with acute pancreatitis) and severe COVID-19 sepsis (357 samples from 124 hospitalized COVID patients)19, potentially enabling physicians to make informed decisions as to whether to treat with relevant anti-viral agents or monoclonal antibodies). In each of the additional patient populations, analysis of the whole blood transcriptomes revealed that the SepsetER test was able to diagnose severely afflicted patients with >85% accuracy.
Discussion of Therapeutic Business
Current treatment options have limited efficacy against biofilm infections and separate drugs are required to address inflammation.
12 Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-96.
13 Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-96.
14 The study is found in Baghela, A., O.M. Pena, A.H. Lee, B. Baquir, R. Falsafi, A. An, S.W. Farmer, A. Hurlburt, A. Mondragon-Cardona, J.D. Rivera, A. Baker, U. Trahtemberg, M. Shojaei, C.E. Jimenez-Canizales, C.C. dos Santos, B. Tang, H.R. Bouma, G.V. Cohen Freue, and R.E.W. Hancock. 2022. Predicting sepsis severity at first clinical presentation: the role of endotypes and mechanistic signatures. eBiomedicine 75:103776.
15 The study is found in Baghela, A., R.E.W. Hancock et al. 2022. Predicting sepsis severity at first clinical presentation: the role of endotypes and mechanistic signatures. eBiomedicine 75:103776; Baghela, A., A. R.E.W. Hancock et al. 2023. Predicting severity in COVID-19 disease using sepsis blood gene expression signatures. Sci. Reports 13:1247.
16 Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016).
17 Wang C, Xu R, Zeng Y, Zhao Y, Hu X. A comparison of qSOFA, SIRS and NEWS in predicting the accuracy of mortality in patients with suspected sepsis: A meta-analysis. PLoS One. (2022) 17(4):e0266755. doi:10.1371/journal.pone.0266755.
18 Gupta, S. et al. Culture-negative severe sepsis: nationwide trends and outcomes. Chest 150, 1251–1259 (2016); Tsalik, E. L. et al. Multiplex PCR to diagnose bloodstream infections in patients admitted from the emergency department with sepsis. J Clin Microbiol 48, 26–33 (2010).
19 Dhillon, B.K., Kortbeek, S. Kortbeek, A. Baghela, M. Brindle, D.-A. Martin, C.N. Jenne, HJ. Vogel, A.H.Y. Lee, G.C. Thompson, Hancock, and R.E.W. Hancock. 2024. Gene expression profiling in pediatric appendicitis. JAMA Pediatrics, In press.
19a. Nesvaderani, M., B.K. Dhillon, T. Chew, B. Tang, A. Baghela, R.E.W. Hancock, G. Eslick, and M. Cox. 2022. Gene expression profiling identifies novel pathways and potential biomarkers in severe acute pancreatitis. J. Amer. Coll. Surgeons, 234:803-815.
19b. The study is found in Baghela, A., O.M. Pena, A.H. Lee, B. Baquir, R. Falsafi, A. An, S.W. Farmer, A. Hurlburt, A. Mondragon-Cardona, J.D. Rivera, A. Baker, U. Trahtemberg, M. Shojaei, C.E. Jimenez-Canizales, C.C. dos Santos, B. Tang, H.R. Bouma, G.V. Cohen Freue, and R.E.W. Hancock. 2022. Predicting sepsis severity at first clinical presentation: the role of endotypes and mechanistic signatures. eBiomedicine 75:103776; Baghela, A., A. An, P. Zhang, E. Acton, J. Gauthier, E. Brunet-Ratnasingham, T. Blimkie, G. Cohen Freue, D. Kaufmann, A.H.Y. Lee, R.C. Levesque, and R.E.W. Hancock. 2023. Predicting severity in COVID-19 disease using sepsis blood gene expression signatures. Sci. Reports 13:1247.
Biofilms are surface associated communities of bacteria encased in a protective polymeric sugar matrix. They are very resistant to essentially all antibiotics. Biofilm infections are very common (nearly two thirds of all infections) and include growth on medical devices, chronic infections, and lung, bladder, wound, dental, skin, ear, nose and throat, sinusitis, orthopedic, infections etc. In lab studies of biofilms formed on plastic surfaces, Asep peptides eliminated or reduced the number of viable biofilm bacteria formed by the most antibiotic resistant ESKAPEE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter cloacae and Escherichia coli) pathogens based on confocal microscopy and live/dead staining20,21. Conventional minimal biofilm inhibition concentration (MBIC) assays revealed 50% or more bacteria were killed by a peptide at 0.9 to 4 µg/ml21. When biofilms of the virulent antibiotic resistant bacteria S. aureus and P. aeruginosa were formed on human skin organoid cultures, as little as 0.12 milligrams of peptide killed 99.99% of bacteria22. In lab studies in combination with conventional antibiotics, peptides showed strong synergy (Fractional Inhibitory Concentration of less than 0.5, indicating that each agent demonstrated a 4-fold lower active concentration in combination)6, while murine wound20 (and sinusitis23) animal model studies confirmed that the peptides were active against ESKAPEE pathogens20,24, and demonstrated in synergy with conventional antibiotics an ability to reduce bacterial numbers by 90-99.9%. The peptides demonstrated an ability to suppress inflammation by 2-3 fold in both sterile inflammation25 and sinusitis inflammation23 murine animal models. It is important to note that such studies cannot be part of an IND application and it will require formal pre-clinical studies thorough a Contract Research Organization in a format acceptable to the FDA, confirming safety, in order for clinical trials to be initiated.
Up to this point, drug development and clinical research studies have been carried in academic research labs and, while indicative, are not “pre-clinical studies” per se and, as such, cannot be part of a investigational new drug (“IND”) application to the FDA or equivalent in other countries. It is also worth noting that statements related to safety and efficacy are within the sole authority of the FDA. This year we plan to initiate formal pre-clinical studies to pursue clinical validation in the treatment of organisms associated with sinusitis pathogens pursuant to filing an IND application. We have other drug development results, including: (1) in vitro and in vivo toxicity study requirements have been identified and initiated; (2) in vitro and in vivo anti-inflammatory and immunomodulatory activities have been extensively characterized; (3) peptides have demonstrated excellent activity profiles in relevant biofilm infection models of sinusitis as well as infected wounds; and (4) new formulations as well as initial pharmacokinetics and pharmacodynamics studies have been defined and/or developed. Wound dressings have been prepared with funding from the US military and shown to be effective in skin organoid (as per the figure under Business Overview) and animal model wound studies and will be the subject of separate clinical studies. We have also shown the activity of our peptides against oral biofilms that are the root cause of dental issues including gingivitis. We have performed a pilot human study demonstrating, with a formulation utilizing our peptides, the efficacy as a mouth rinse and plan this year to pursue validation in a large clinical study.
Antibiotic failure happens when bacteria develop the genetic ability to defeat the drugs designed to kill them (termed antimicrobial resistance, AMR) or when bacteria, by virtue of their growth state (e.g. biofilms), develop so-called “adaptive” resistance to many antibiotics (i.e. resistance dependent on their growth mode or environment). As a result of their resistance or recalcitrance, the bacteria are not killed and continue to grow in a person’s body. These resistant infections can be difficult, and sometimes impossible, to treat. Genetically-encoded antimicrobial resistance is undoubtedly an urgent global public health threat, killing an estimated 1.27 million people worldwide and associated with nearly 5 million deaths in 2019. However the antibiotic failure issues that we are addressing are even more prominent, with sepsis killing 11 million people annually worldwide, while biofilms that are adaptively multiple antibiotic resistant, cause 65% of all infections and 80% of chronic infections. By addressing very common, recalcitrant biofilm infections for which not a single specific drug has been approved, we are able to discriminate our approach from many other companies.
Our subsidiaries, ABT and Sepset, are the arms by which we develop our therapeutic and diagnostic solutions, together with our new acquisition, SafeCoat, which is addressing an area related to biofilm infections, namely prevention of medical device-related infections. On January 20, 2021, we were incorporated under the British Columbia Business Corporations Act (“BCBA”) under the name Trenchant Life Sciences Investment Corp. On November 9, 2021, we changed our name to ASEP Medical Holdings Inc. On November 22, 2021, we commenced trading on the Canadian Securities Exchange (“CSE”) as a life sciences issuer under the trading symbol “ASEP”. On April 19, 2022, we commenced trading on the OTCQB under the trading symbol “SEPSF”, although we currently trade on the OTC Pink. On November 10, 2022, we commenced trading on the Frankfurt Stock Exchange (“FSE”) under the trading symbol “FSX:JJ8”. Since our inception in 2021, ASEP has been a committed innovator within the medical industry.
20 Pletzer, D., S.C. Mansour, and R.E.W. Hancock. 2018. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathogens 14(6):e1007084.
21 de la Fuente-Núñez, C., F. Reffuveille, S.C. Mansour, S.L. Reckseidler-Zenteno, D. Hernández, G. Brackman, T. Coenye and R.E.W. Hancock. 2015. D-enantiomeric peptides that eradicate wild-type and multi-drug resistant bacterial biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 22:196–205.
22 Wu, B.C., E.F. Haney, N. Akhoundsadegh, D. Pletzer, M.J. Trimble, A.E. Adriaans, P.H. Nibbering and R.E.W. Hancock. 2021. Human organoid biofilm model for assessing antibiofilm activity of novel agents. NPJ Biofilms and Microbiomes 7:8.
23 Alford, M.A., K.-Y.G. Choi, M.J. Trimble, H. Masoudi, P. Kalsi, D. Pletzer, R.E.W. Hancock. 2021. Murine models of sinusitis infection for screening antimicrobial and immunomodulatory therapies. Frontiers Cell. Infect. Microbiol. 11:621081.
24 Pletzer, D., S.C. Mansour, K. Wuerth, N. Rahanjam, and R.E.W. Hancock. 2017. New mouse model for chronic infections by Gram-negative bacteria enabling the study of anti-infective efficacy and host-microbe interactions. mBio 8:e00140-17.
25 Wu, B.C., A.H. Lee, and R.E.W. Hancock. 2017. Mechanisms of the innate defense regulator peptide-1002 anti-inflammatory activity in a sterile inflammation mouse model. J. Immunol. 199:3592-3603.
Growth Strategy
We have a business development team that has a focused outreach program to identify potential clinical, manufacturing and commercialization relationships. We have identified a targeted list of companies that can potentially help us achieve our business goals as well as the ones that would achieve synergistic benefits from our technologies.
Currently, our major focus has been on potential partnerships for our SepsetER technology, described below, because molecular diagnostics have a shorter development cycle and can result in clear overall savings. Our discussions thus far have contemplated stages of involvement and key territories including the United States, European Union, the Middle East and Asia.
We are exploring opportunities to commercialize our peptide technologies to address specific areas where biofilms are of concern. We have identified a potential opportunity in the oral health sector since dental plaque is a well-known and ubiquitous natural biofilm that can contribute to infections within the oral cavity. There are few products on the market that effectively address issues related to plaque biofilms and most strategies involve physical removal and scraping of the plaque from the tooth surface. Other areas we are addressing include sinusitis and wound biofilm infections.
ASEP has also recently acquired SafeCoat that has a technology that complements ASEP’s biofilm therapeutics. SafeCoat’s technology relates to medical device coatings with anti-fouling and antimicrobial/anti-biofilm activity that can both prevent binding of and kill bacteria through conjugated antimicrobial or antibiofilm peptides, as well as the methods for making such coatings. In particular, the technology may be applied to virtually any device surface and has particularly intriguing applications for medical devices and implants. Our first target market for the SafeCoat technology is the contact lens market. The global optical coating market was valued at $11.8 billion in 2020, and is projected to reach $24.0 billion by 2030, growing at a CAGR of 7.41% from 2021 to 203026.
Corporate Structure
We currently have the following subsidiaries:
| | | Principal Activity | | Location | | Percentage | |
| | | | | | | | |
| Asep Medical Inc. (“Asep”) | | Life Sciences | | British Columbia, Canada | | | 100 | % |
| ABT Innovations Inc. (“ABT”) | | Life Sciences | | British Columbia, Canada | | | 50.1 | % |
| Sepset Biosciences Inc. (“Sepset”) | | Life Sciences | | British Columbia, Canada | | | 50.1 | % |
| OHP Innovations Inc. (“OHP”) | | Life Sciences | | British Columbia, Canada | | | 100 | %(2) |
| SafeCoat Medical Inc. (“SafeCoat”) | | Life Sciences | | British Columbia, Canada | | | 100 | %(1) |
| | (1) | Pursuant to the Start-Up License Agreement (as defined herein), UBC and certain inventors will collectively own 12% of the total issued and outstanding common shares of SafeCoat following the issuance of the consideration shares. As at the date of this Registration Statement, the consideration shares have not yet been issued and ASEP owns 100% of SafeCoat. ASEP will own 88% of SafeCoat, with UBC and inventors receiving a total of 12% of shares as a requirement of the licensing agreement with UBC. |
| | (2) | Inactive. |
The remaining 49.9% of ABT and Sepset is owned in part by Dr. Robert Hancock, who owns 32% of ABT and 31.5% of Sepset. The other holders are minority holders, including 5% held by UBC and other inventors of the technology. Our subsidiary, ABT, owns 100% of ABT Peptides Inc, an inactive company incorporated in British Columbia, Canada.
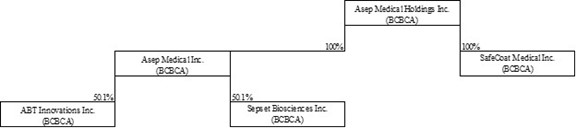
Our ownership of ABT and Sepset is discussed in greater detail in the Material Contracts section below.
26 https://www.alliedmarketresearch.com
ABT Innovations Inc.
ABT was incorporated on July 3, 2015 pursuant to the provisions of the BCBCA under the name “ABT Innovations Inc.” for the purpose of pursuing the commercialization of the broad peptide technology developed by its founder, Dr. Robert E.W. Hancock.
Antibiotic resistance occurs when bacteria change genetically, or grow in such a way in patients so as to induce adaptive multiple antibiotic resistance (based on their growth state, e.g. biofilms). ABT’s peptide technology addresses a broad range of therapeutic applications particularly bacterial biofilm infections (medical device infections, chronic infections, lung, bladder, wound, dental, skin, ear-nose and throat, sinusitis, orthopedic, etc.), representing nearly two thirds of all infections, as well as anti-inflammatories, anti-infective immune-modulators and vaccine adjuvants. Biofilms in particular are ten- to one thousand-fold more resistant to almost all antibiotics, and although combinations of antibiotics are used for such infections, therapeutic failure occurs frequently and the alternative (surgically scraping out the biofilm and infected tissue) causes substantial damage and does not prevent regrowth of any biofilm that remains at the infection site.
Based on our drug development activities to date, including many animal model experiments, we believe our technology has broad applications in these areas. Our peptide technology includes small, potent, broad-spectrum anti-biofilm and anti-inflammatory peptides, that have been documented through filed patents, patent applications and published scientific papers to be highly active (at ≥1 to 4 μg/ml) against most bacterial pathogens growing as biofilms. The mechanisms of these peptides have been substantially defined. For example, against biofilm infections these peptides demonstrate multiple mechanisms including inhibiting a bacterial signaling molecule required for biofilm formation; their multi-target mechanism of action reduces the chances of resistance development. While the peptides are safe and effective in animal models, they have not been shown to be safe and effective in humans. Statements related to safety and efficacy are within the sole authority of the FDA.
More simply, ABT’s therapeutic solution treats antibiotic resistant infections by suppressing biofilm regrowth and reducing inflammation through the use of potent cationic antimicrobial peptides. Cationic antimicrobial peptides, found in most species of life, represent a good template for a new generation of antimicrobials and through work performed by our Founders, were found to selectively work against bacterial biofilms. These peptides kill bacteria growing as biofilms and most notably in drug development studies work against biofilms formed by the most common clinically-resistant bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug resistant Gram-negative pathogens (collectively termed the ESKAPE pathogens). Moreover, they show synergy with conventional antibiotics enabling combination treatments, and have activity in established and newly-developed models of biofilm infections, including animal and human tissue models. These peptides also have a general ability to modulate the immune system to improve protective immunity while counteracting inflammation, an important feature since one of the major harms caused by biofilms is mediated through inflammation that causes local tissue damage and pain.
Recently, ABT has continued to make progress advancing the synthetic antibiofilm and immunomodulatory peptide technology towards clinical trials. ABT has a collaboration, involving its proprietary peptides to which it retains all rights, with iFyber, which was funded by US Army Medical Research, to develop peptide loaded wound dressings and are particularly engaged in identifying biocompatible and resorbable materials that facilitate sustained peptide release and potent antibiofilm activity. The novel technology (peptides) are owned by ABT and there is no licensing agreement. The US military does not have any rights to our technology. For more information, see “iFyber LLC and ABT Innovations collaboration letter of intent dated December 6, 2016 and July 17, 2018” on page 60. Prototype dressings have been evaluated in mouse and pig models of efficacy, demonstrating better antimicrobial activity than other commercially available wound care products. In addition, ABT scientists have identified a range of antimicrobials that work in synergy with (that is improve the activity of) ABT’s proprietary peptide technology that can be applied together in dressings for wound care. Moreover, the presence of the peptides prevented the development of resistance which addresses concerns related to the growing problem of antibiotic resistance. ABT has also been engaged in detailed in vivo toxicity studies in mice. This information was essential to inform ongoing formulation studies and will reveal the optimal route of delivery for the peptides to achieve peak activity while avoiding toxicity issues.
Regarding human clinical trials, ABT plans to first seek regulatory approval for the therapeutic peptide technology for chronic rhinosinusitis in the U.S. Chronic rhinosinusitis (“CRS”) refers to a condition where the lining of the sinuses gets infected or irritated, becomes swollen, and creates extra mucus that lasts at least 12 weeks, despite attempts to treat it.27 CRS, responsible for 4 million ambulatory physician visits annually, is a very painful condition caused by a biofilm infection that leads to local inflammation. Current treatments are inadequate, antibiotics do not work well, surgical removal (500,000 surgeries annually) usually does not prevent recurrence, and very expensive monoclonal antibodies ($10,000 to $50,000 per year) do not target bacteria biofilms but rather inflammation and have fairly low success rates (~30%). Our peptides have shown promise in animal models of sinusitis infections and associated inflammation.
ABT is exploring opportunities beyond clinical applications. One such opportunity is in the oral care sector. Dental plaque is a known and pervasive natural biofilm that can contribute to infections within the oral cavity including gingivitis, dental cavities and periodontal disease. ABT believes that there are few products on the market that can adequately address issues related to plaque biofilms. A majority of the current strategies involve physical removal and scraping of the plaque from the tooth surface. Collaboration with dental researchers at UBC has demonstrated excellent anti-biofilm activity for the peptides against oral plaque bacteria cultures on hydroxyapatite (teeth and bone material) surfaces and demonstrated the potential to develop this technology as a high-end oral rinse. An important aspect of developing mouthwashes and oral rinses is that they likely do not require clinical trials to demonstrate their efficacy which makes the regulatory approval process less burdensome. ABT has made significant progress in this area and has recently entered into an agreement with Bohai Biomedical to perform a feasibility study to test the efficacy of a peptide-containing oral rinse in preventing plaque biofilm growth in humans.
Sepset Biosciences Inc.
Sepset was incorporated on April 23, 2015 pursuant to the provisions of the BCBCA under the name “Sepset Biosciences Inc.” for the purpose of ensuring the commercialization of a diagnostic kit for predicting the onset of severe sepsis and organ failure that was developed by its founder Dr. Robert E.W. Hancock.
Sepsis is the body’s dysfunctional response to an infection where the immune system causes harm. It is a common life-threatening medical emergency causing 19.7% of all deaths in 2017, and is responsible for virtually all deaths in patients with severe COVID-19 disease.28 Patients with suspected sepsis typically receive initial evaluations including basic laboratory tests, cultures, imaging studies, and analysis of sepsis biomarkers, such as procalcitonin and lactate levels found in blood samples. Typical blood cultures can take an average of 13, up to 48, hours for results, and although there are now ways of identifying infections in 5 hours, an infection per se does not predict sepsis and in around a half of patients who go on to sepsis blood cultures often return a negative result. Critically, every hour’s delay in diagnosis and appropriate treatment increases the probability of death by 7.6%. A commissioned economic study by RTI Health Solutions, indicated that early diagnosis of severe sepsis has the potential to generate estimated annual hospital cost savings of $3.0 to $3.9 million per hospital and $16.6 to $22.0 billion overall in the USA. These costs were estimated according to a model taking into account public data on numbers of patients per hospital and total numbers of patients in USA, average costs of medical care while in hospital, time at which the SepsetER test would be performed in the ER and deliver a result to physicians, and the average time at which antibiotic therapy is initiated in the absence of a SepsetER test. The reduced number of average days in hospital due to an earlier diagnosis were modelled to be 5.85 days (12.59 vs. 18.44 days) and the reduction in ICU stay was 3.16 days (5.78 vs. 8.46 days).
ASEP’s Chief Executive Officer, along with a team of scientists, hypothesized and confirmed by an initial single-blinded prospective observational clinical study, and through extensive testing of more than 700 patients, including 150 with severe COVID-19, that sepsis patients possess a gene expression signature characteristic of a biological phenomenon known as cellular reprogramming (“CR”) which is a type of immunosuppression.29 CR involves the reprogramming of immune cells to a state where they are unable to respond to microbial signatures in a way that triggers protective immune responses. The CR signature is present in patients suspected of sepsis at first clinical presentation (in the emergency room) and is able to identify which patients do or do not go on to develop severe sepsis, which includes organ failure. Most importantly, the strong presence of the signature was associated with worse outcomes, including multiple organ dysfunction and intensive care unit admission.
27 https://www.uptodate.com/contents/chronic-rhinosinusitis-beyond-the-basics
28 Rudd, K.E., Johnson, S.C., Agesa, K.M., Shackelford, K.A., Tsoi, D., Kievlan, D.R., Colombara, D.V., Ikuta, K.S., Kissoon, N., Finfer, S., Fleischmann-Struzek, C (2020). Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet. 395(10219), 200-11.
29 Pena, O.M., D.G. Hancock, N.H. Lyle, A. Linder, J.A. Russell, J. Xia, C.D. Fjell, J.H. Boyd, and R.E.W. Hancock. 2014. An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. eBiomedicine 1:64–71; Baghela, A., O.M. Pena, A.H. Lee, B. Baquir, R. Falsafi, A. An, S.W. Farmer, A. Hurlburt, A. Mondragon-Cardona, J.D. Rivera, A. Baker, U. Trahtemberg, M. Shojaei, C.E. Jimenez-Canizales, C.C. dos Santos, B. Tang, H.R. Bouma, G.V. Cohen Freue, and R.E.W. Hancock. 2022. Predicting sepsis severity at first clinical presentation: the role of endotypes and mechanistic signatures. eBiomedicine 75:103776; Baghela, A., A. An, P. Zhang, E. Acton, J. Gauthier, E. Brunet-Ratnasingham, T. Blimkie, G. Cohen Freue, D. Kaufmann, A.H.Y. Lee, R.C. Levesque, and R.E.W. Hancock. 2023. Predicting severity in COVID-19 disease using sepsis blood gene expression signatures. Sci. Reports 13:1247.
Specifically, the Sepset diagnostic technology differs from most traditional evaluations because it directly assesses the patient’s dysfunctional immune response. It measures a patient’s CR gene expression signature, which is identified in the blood and assessed by nucleic acid amplification technologies that are already available in hospital labs. The output of this assessment of gene expression is then fed into a proprietary machine learning (artificial intelligence) algorithm that quickly informs the physician of the probability that the patient will acquire severe sepsis, enabling critical treatment decisions. Critically, it also will inform the physician of the high probability that a patient will not go on to severe sepsis thus sparing antibiotic usage and reducing ICU entry. Our diagnostic technology is executed through our SepsetER test, which measures the overexpression of a particular subset of CR signature genes that are diagnostic of the immune dysfunction that is a feature of severe sepsis. The test predicts whether a patient is likely to go on to (multi) organ failure or not and develop severe sepsis.
Sepset’s diagnostic technology enables diagnosis of severe sepsis within 1 to 2 hours of first clinical presentation and blood sample collection (i.e., in the emergency room). This is novel since current methods either do not work in the emergency room or are directed towards predicting sepsis in the ICU. We believe our sepsis diagnostic technology will enable critical early decisions to be made by physicians regarding appropriate therapies, which can reduce mortality and morbidity, and save potent antibiotics so they will be used only in patients who need them. Since our test also predicts patients who will not go on to severe sepsis, it will likely be important in preventing the rise of antimicrobial resistant (“AMR”) organisms that are consistently eroding the effectiveness of antibiotics, which are among the most effective human medicines and are an essential part of clinical care worldwide.
An in-house prototype of the SepsetER test kit has been prepared and its ability to quantify the gene expression levels from RNA samples isolated from healthy donors has been assessed. Furthermore, Sepset has been working on establishing various performance criteria for the diagnostic kit including examining test precision, reproducibility, kit storage conditions, sample requirements and detection limits. Current testing and validation are being performed on the 7500 Fast Dx Real-Time PCR Instrument from Applied Biosystems, which is a widely used diagnostic platform found in many clinical diagnostic labs. Additionally, an accelerated RNA isolation procedure has also been developed that provides high-quality purified RNA to serve as the input for the gene expression test. Lastly, the company is in the process of procuring clinical samples from sepsis patients to enable evaluation of performance of the diagnostic test on representative patient samples.
Sepset plans to seek regulatory approval for its molecular diagnostic test for severe sepsis first in the U.S. Once that approval is secured and Sepset begins to generate revenues, regulatory approvals will be sought in Canada and other countries.
In anticipation of engaging with the FDA for a pre-submission meeting as well as initiating a prospective clinical trial to evaluate the performance of SepsetER in a clinical setting, a regulatory strategy assessment of the SepsetER development progress was performed by a leading contract research organization (“CRO”), IQVIA, that specializes in regulatory approval of medical devices. Following this assessment, Sepset engaged the same CRO to prepare for a pre-submission meeting with the FDA, as well as write a clinical study protocol that will evaluate the performance of the SepsetER test in a real-world situation. In addition, Sepset is implementing an electronic quality management system (QMS) to ensure compliance with the regulatory requirements for medical devices. These two activities are key steps to ensuring compliance with regulatory requirements, as the SepsetER test approaches its pivotal clinical trial for validation.
SafeCoat Medical Inc.
On December 1, 2022, ASEP entered into a definitive Earn-In and Option Agreement with SafeCoat Medical Inc. (“SafeCoat”) and the security holders of SafeCoat, pursuant to which ASEP earned a 50.1% equity interest in SafeCoat in consideration for assisting SafeCoat in its negotiations with UBC which resulted in SafeCoat and UBC entering into a term sheet for the grant of a license by UBC to SafeCoat for the use, development and commercialization of a peptide medical device coating technology. In addition, the Earn-In and Option Agreement granted ASEP the option to purchase the remaining 49.9% equity interest in SafeCoat in exchange for the issuance of 6,000,000 ASEP common shares to the shareholders of SafeCoat. On December 12, 2022, ASEP exercised the option to purchase the remaining 49.9% equity interest in SafeCoat such that, ASEP then owned 100% of common shares of SafeCoat, free and clear of all liens.
SafeCoat’s technology provides coatings for medical devices that are non-fouling (i.e. do not readily bind live or dead bacteria) and are protected by an anti-biofilm/antimicrobial peptide coating that kills bacteria. This product addresses the definitive risk of infection of medical devices including prosthetics, implants, catheters and stents, etc. Indeed, over half of the nearly 2 million healthcare-associated infections in the USA annually can be attributed to indwelling medical devices30. As this is a recent acquisition, we are currently preparing a Business Plan for this company.
On July 21, 2023, ASEP, through SafeCoat, entered into an exclusive license agreement (the “Start-Up License Agreement”) with UBC for the use, development and commercialization of a medical device coating technology. Pursuant to the Start-Up License Agreement, 12% of the securities of SafeCoat will be owned by UBC and inventors of the technology who have the right to purchase up to their then current pro-rata share of the securities issued in each future offering on the same terms and conditions as are offered to other purchasers in each such financing, up to July 21, 2027. As at the date of this Registration Statement, the consideration shares have not yet been issued and ASEP owns 100% of SafeCoat. The consideration shares will be issued when UBC gives its consent to the deemed issue price of shares to be issued to non waiving inventors. The licensing agreement ends on the later of 20 years from the start date of the agreement, being July 20, 2043, or the expiry of the last patent licensed under the agreement. In addition to the share consideration, SafeCoat will pay certain annual license fees and royalty payments to UBC, when and if they become payable.
30 VanEpps J.S. & Younger J.G. 2016. Implantable device related infection. Shock. 46(6):597-608.
Property, Plants, and Equipment
Asep, Sepset and ABT operate as virtual companies without central facilities, and own no property or plants. It occasionally purchases equipment to further its developmental goals but most research is performed under contract with CROs or University labs. The advantage of being virtual is more efficient use of funding and shareholders’ equity, preventing the very large overheads that occur when companies operate their own facilities, and avoiding substantial capital depreciation and outdating when equipment is purchased.
Market Opportunities
Bacteria growing in a biofilm have an altered stress-adapted growth state compared to free swimming bacteria leading to the altered expression of thousands of genes. This altered growth state makes biofilms 10- to 1,000-fold more (adaptively) resistant to multiple conventional antibiotics and also prevents their clearance by the immune system. Consequently, biofilm-associated infections are exceedingly difficult to treat which is troubling since biofilms cause 65% of all infections (80% of chronic infections); strikingly, no biofilm specific therapies are approved to date. Common biofilm-associated infections include skin and soft tissue infections, periodontitis, dental plaque, rhinosinusitis, ear, nose, throat and eye infections, and chronic wounds. The most common treatment for biofilm-associated infections is to surgically remove (debride) the biofilm infected tissues and administer high doses of (multiple) potent antibiotics. Surgical removal can be traumatic and painful and often does not prevent biofilm regrowth, while antibiotics, although highly used, are poorly effective and their use exacerbates the antimicrobial resistance problem. Thus, there is an urgent need to develop alternative therapies that can directly target bacteria growing within a biofilm to address this therapeutic gap for patients. Since biofilms are highly resistant to multiple antibiotics, our technologies also address the urgent need described by the World Health Organization and the Centers for Disease Control and Prevention (“CDC”) to find solutions to the growing crisis of antimicrobial resistance. Our solution is quite novel in addressing biofilm infections and addresses an unmet medical need since no existing therapies have been approved to date.
Our primary clinical target, chronic rhinosinusitis (CRS), is a chronic inflammatory condition of the paranasal sinuses affecting 1-5% of the US population and 3-6.4% of the European population.31 A subgroup, CRS with polyposis (~20-30% of patients), has fleshy swellings (nasal polyps) in the nose and paranasal sinuses.32 This causes moderate to severe inflammation and is frequently associated with biofilms growing in the mucosa, which contributes to the debilitating inflammation of the sinuses. Costs associated with management and treatment of adult CRS are substantial, with an estimated annual economic burden of US$11-22 billion in the US alone.33 Critically, treatments for CRS are known to be largely ineffective with frequent recurrence of infection.34 The only therapeutic options available are recently approved monoclonal antibodies (omalizumab, mepolizumab, benralizumab, and dupilumab) that are indicated for chronic rhinosinusitis with nasal polyposis. These drugs are massively expensive (up to $50,000/year) and have quite modest efficacy (only 17-35% of treatments deemed successful with some partial successes).35 Preventing wound infections ($20 billion) and oral health care (US$33.7 billion) are also enormous markets with unmet medical needs.36
Sepsis is a deadly medical emergency that happens when an infection someone already has triggers a chain reaction throughout their body and one’s own immune system begins to fight itself instead of the bacteria. Infections that lead to sepsis most often start in the lungs, urinary tract, skin, or gastrointestinal tract. Without timely treatment, sepsis can rapidly lead to tissue damage, organ failure, and death. Almost any infection, including COVID-19, can lead to sepsis. Each year, (i) approximately 1,700,000 adult Americans develop sepsis; (ii) at least 350,000 adults who develop sepsis die during their hospitalization or are discharged to hospice; and (iii) 1 in 3 patients who die in a hospital had sepsis during their hospitalization.37 Worldwide, sepsis causes at least 20% of all deaths, including most of those due to severe COVID-19.38 To combat sepsis, the CDC recommends healthcare practitioners know its signs and symptoms so that practitioners can identify and treat patients early. Our SepsetER sepsis diagnostic test can assist practitioners with early detection of severe sepsis (sepsis with organ failure), which is critical to saving the patient’s life. As mentioned above, a commissioned economic study by RTI Health Solutions (who have consented to be identified in this prospectus) determined that early diagnosis of severe sepsis has the potential to generate estimated annual cost savings of $3.0 to $3.9 million per hospital and $16.6 to $22.0 billion in the USA. This study only concerned patients newly arriving in hospital and did not consider the average number of days in the hospital due to an earlier diagnosis.
31 Sedaghat, AR Chronic Rhinosinusitis. AFP 96:500–506 (2017); Rui L & T Okeyode. 2016. National Ambulatory Medical Care Survey: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf.; Loos, DD et al. 2019. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. Journal of Allergy and Clinical Immunology 143:1207–1214.
32 https://acaai.org/allergies/allergic-conditions/chronic-rhinosinusitis-with-nasal-polyps/
33 Smith KA, RR Orlandi, & R Ludmik. 2015. Cost of adult chronic rhinosinusitis: A systematic review. Laryngoscope 125:1547–1556; Albu S. 2020. Chronic rhinosinusitis-an update on epidemiology, pathogenesis and management. J Clin Med. 9(7):2285.; Schleimer RP. 2017. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 12:331–357.
34 Kennedy JL, L Borish. 2013. Chronic rhinosinusitis and antibiotics: the good, the bad, and the ugly. Am J Rhinol Allergy. 27(6):467-72; Desrosiers M, et al. 2011. Canadian clinical practice guidelines for acute and chronic rhinosinusitis. Allergy, Asthma & Clinical Immunology, 7(1):2.; Patel GB, Kern RC, Bernstein JA, Hae-Sim P, Peters AT. 2020. Current and future treatments of rhinitis and sinusitis. J Allergy Clin Immunol Pract. 8(5):1522-1531
35 Meier EC, et al. 2021. Real-life experience of monoclonal antibody treatments in chronic rhinosinusitis with nasal polyposis. Int Arch Allergy Immunol. 182, 736–743.
36 https://www.grandviewresearch.com/industry-analysis/wound-care-market);https://www.grandviewresearch.com/industry-analysis/oral-care-market
37 https://www.grandviewresearch.com/industry-analysis/oral-care-market; https://medicalcountermeasures.gov/newsroom/2023/cytovale/#:~:text=According%20to%20the%20U.S.%20Centers,hospital%20had%20sepsis%20during%20hospitalization
38 Rudd, K.E., et al. (2020). Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet. 395(10219), 200-11; https://www.global-sepsis-alliance.org/news/2020/4/7/update-can-covid-19-cause-sepsis-explaining-the-relationship-between-the-coronavirus-disease-and-sepsis-cvd-novel-coronavirus; Baghela, A et al. 2023. Predicting severity in COVID-19 disease using sepsis blood gene expression signatures. Sci. Reports 13:1247
Pharmaceutical Coverage, Pricing and Reimbursement
Our ability to commercialize our product candidates successfully will depend in part on the extent to which the U.S. and foreign governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our product candidates and related treatments. The company believes the seriousness and unmet needs of the diseases for which it is developing products and especially the major projected health care savings to US hospitals will enable it to mount a credible campaign for reimbursement. In many of the markets where we would commercialize a product following regulatory approval, the prices of pharmaceutical products are subject to direct price controls (by law) and to drug reimbursement programs with varying price control mechanisms. Public and private health care payors control costs and influence drug pricing through a variety of mechanisms, including through negotiating discounts with the manufacturers and through the use of tiered formularies and other mechanisms that provide preferential access to certain drugs over others within a therapeutic class. Payors also set other criteria to govern the uses of a drug that will be deemed medically appropriate and therefore reimbursed or otherwise covered. In particular, many public and private health care payors limit reimbursement and coverage to the uses of a drug that are either approved by the FDA or that are supported by other appropriate evidence (for example, published medical literature) and appear in a recognized drug compendium. Drug compendia are publications that summarize the available medical evidence for particular drug products and identify which uses of a drug are supported or not supported by the available evidence, whether or not such uses have been approved by the FDA.
In the U.S., there have been a number of legislative and regulatory changes to the healthcare system in ways that could affect our future revenues and profitability and the future revenues and profitability of our potential customers. For example, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (the “MMA”) revised the payment methodologies for many drugs, which resulted in reduced reimbursement to providers. Additionally, the MMA created an outpatient prescription drug benefit which became effective on January 1, 2006. This benefit is administered by private pharmacy benefit managers and other managed care organizations and is putting increased pressure on the pharmaceutical industry to reduce prices.
Government Regulation
ABT and Sepset’s planned technologies are contingent upon receipt of various regulatory approvals. Such receipt may be obtained directly by ABT and Sepset, or through contract partners that may perform specific tasks on behalf of ABT or Sepset, that are required for those regulatory approvals. ABT and Sepset plan to conduct their trials and studies first in the United States and Canada. As such, ABT and Sepset (or their applicable contractual partners) require approvals under FDA and Health Canada regulations in the near-term. We are currently implementing a quality management system (QMS) to ensure compliance with the regulatory requirements for medical devices and plan the same for therapeutics, a process that is being overseen by our Head of Quality Assurance and Regulatory Affairs. As of the date of this offering, the table below contains a list of government and regulatory approvals required by ABT and Sepset to conduct various activities in the United States and Canada.
Sepset and ABT plan to seek clearance for the molecular diagnostic test and approval for the peptide therapeutic for CRS, respectively, in the U.S. first. Once this is secured and ABT and Sepset start to generate revenues, market approvals will be sought in Canada. As of the date of this prospectus, ABT is moving towards its pre-clinical phase and Sepset has contracted with a CRO to help assemble the necessary documents and plan the clinical study for its 510(k) application with the FDA.
Government and Regulatory Approvals Required by ABT |
Study technology/ trial for which approval is required | | Jurisdiction | | Type of Approval | | Cost of Obtaining Approval | | Timeline |
| R&D | | Canada | | Biosafety Environmental Animal Safety and Health | | Included in contracting out costs. Borne by the specific agency to which services are contracted | | Usually days to weeks |
| Preclinical research | | Location of the CRO | | As for R&D | | Included in contracting out costs. Borne by the specific agency to which services are contracted | | Blanket approval usually in place |
| Clinical Trials | | USA | | Investigation New Drugs (IND) | | $200,000 including fees and costs for preparing IND submission | | 3 months |
| Regulatory approval for sales of products | | USA | | New Drug Approval (NDA) | | $120,000 | | 10 months |
| Government and Regulatory Approvals Required by Sepset |
Study technology/ trial for which approval is required [if applicable] | | Jurisdiction | | Type of Approval | | Cost of Obtaining Approval | | Timeline |
| R&D | | Canada, Netherlands, Australia, Colombia and USA | | Human Ethics, Environmental, Biosafety | | Included in contracting out costs. Borne by the specific agency to which services are contracted. Many ethics approvals already obtained | | Completed |
| Validation research | | Canada | | As for R&D | | Included in contracting out costs. Borne by the specific agency (University or Hospital) to which services are contracted | | Blanket approval usually in place |
| Regulatory clearance for sales of products | | USA | | 510(k) (Unless exempt anyone who wants to sell in the U.S. a device intended for human use, for which a Premarket Approval application (PMA) is not required, is required to make a 510(k) submission at least 90 days prior to offering the device for sale (FDA) | | $4,967 per 510(k) application (2023 FDA 510(k) fees for small businesses) plus an Annual Establishment Registration Fee of $6,493. | | Final decision is expected within 90 days of filing (FDA decisions) |
FDA Regulation
After a new drug is formulated, the regulatory strategy adopted, and the clinical trial designs defined, a pre- Investigational New Drug (“IND”) meeting is scheduled with the FDA to discuss planned studies. The pre-IND process will commonly take one month and once the application is submitted to the FDA, an additional 3-12 months. Delays usually relate to insufficient information, which can be corrected usually with the assistance of the regulatory agency, or concerning toxicity or efficacy data. The latter considerations are usually prevented by performing the appropriate preclinical studies, and either more detailed studies or altering the formulation. An established manufacturing process is also required for the drug.
As part of the clinical trials process, it is required that all prospective medicines, such as ABT’s peptides, be tested first in pre-clinical studies to determine safety/toxicity in two animal species, efficacy in relevant animal models, consistency of manufacture of the product under Good Laboratory Practice (“GLP”) rules and analytical testing methods to ensure this. GLP covers the organizational process and the conditions under which non-clinical laboratory studies are planned, conducted, monitored, recorded and reported. It is intended to promote the quality and validity of test data and improve the international acceptance of data generated in adherence to its principles. Analytical testing is a term used to describe various techniques that are used to identify the chemical makeup or characteristics of a particular sample. In the case of pharmaceuticals, analytical testing is used to detect and identify contaminants. Pharmacokinetics, the time course of drug absorption, distribution, metabolism, and excretion, also needs to be established to enable appropriate choices of dosing regimens. This information is then bundled with the results of the pre-IND meeting into an IND application that is submitted to the FDA. When an IND application is granted, a company may start human clinical trials that generally fall into 3 phases: Phase I, which involves testing safety using small numbers of uninfected individuals (or healthy volunteers); Phase II to establish appropriate dosing; and Phase III to test efficacy in the condition that the medicine is intended to treat. This process can be amended under rare drug legislation to enable efficacy to be established in Phase II and companies often design Phase I or II trials to gain preliminary evidence of efficacy. Numbers of patients and costs increase as these clinical trials progress, and the process is monitored by the FDA which has the ability to require trials to be terminated if major issues of safety arise. The costs to an applicant to complete each of the three phases varies greatly, up to a total of approximately $80,000,000.
At the end of this three-phase clinical trials process, the data is analyzed and forms the basis of a New Drug Application (“NDA”). Approval of the NDA by the FDA, based on non-equivalence with existing treatments, is required before any drug may be sold.
The FDA application process differs greatly for diagnostics/medical devices and will depend on the classification of the device. The FDA classifies devices in Class I, II, or III, depending upon the level of control necessary to provide reasonable assurance of their safety and effectiveness. The class into which a device is placed determines the requirements that a medical device manufacturer must meet prior to distributing a device in interstate commerce. According to section 513(a)(1) of the U.S. Food, Drug, and Cosmetic Act (“FDCA”), the three device classes are defined as follows:
● Class I: Devices are subject to a comprehensive set of regulatory authorities called general controls that are applicable to all classes of devices.
● Class II: Devices for which general controls, by themselves, are insufficient to provide reasonable assurance of the safety and effectiveness of the device, and for which there is sufficient information to establish special controls to provide such assurance.
● Class III: Devices for which general controls, by themselves, are insufficient and for which there is insufficient information to establish special controls to provide reasonable assurance of the safety and effectiveness of the device. Class III devices typically require premarket approval.
Based on the classification criteria above, as well as similar in vitro diagnostic tests on the market (specifically RT-qPCR Assays for mRNA Transcript Immune Biomarkers), it is anticipated that SepsetER will be classified as a Class II medical device in the US.
510(k) Application
Each person who wants to market in the U.S., a Class I, II, and III device intended for human use, for which a Premarket Approval application (PMA) is not required, must submit a 510(k) to FDA unless the device is exempt from 510(k) requirements of the Federal Food, Drug, and Cosmetic Act (the FD&C Act) and does not exceed the limitations of exemptions in .9 of the device classification regulation chapters (e.g., 21 CFR 862.9, 21 CFR 864.9).
Section 510(k) of the FDCA requires device manufacturers to notify the FDA of their intent to market a medical device at least 90 days in advance. This is known as Premarket Notification - also called PMN or 510(k). A 510(k) enables the FDA to determine that the new device to be marketed is “substantially equivalent” to a legally marketed device that has already been placed into one of the three classes described above. The term “substantially equivalent” or “substantial equivalence” means, with respect to a device being compared to a predicate device, that the device has the same intended use as the predicate device and any differences in technological characteristics do not raise different questions of safety and effectiveness.
Through this process, “new” devices that have not been classified can be properly identified. Specifically, medical device manufacturers are required to submit a premarket notification if they intend to introduce a device into commercial distribution for the first time or reintroduce a device that will be significantly changed or modified to the extent that its safety or effectiveness could be affected. Such change or modification could relate to the design, material, chemical composition, energy source, manufacturing process, or intended use. Successfully demonstrating that the new device to be marketed is “substantially equivalent” to a legally marketed device may result in shorter time and/or reduced costs to obtain approval for the new device.
Expedited Review and Approval Programs
The FDA has various programs, including Fast Track Designation, accelerated approval, priority review, and breakthrough therapy designation, which are intended to expedite the process for the development and FDA review of drugs that are intended for the treatment of serious or life-threatening diseases or conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
To be eligible for a Fast Track Designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. The FDA may review sections of the NDA for a fast-track product on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
The FDA may give a priority review designation to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A priority review means that the goal for the FDA to review an application is six months, rather than the standard review of ten months under current Prescription Drug User Fee Act (“PDUFA”) guidelines. Under the new PDUFA agreement, these six- and ten- month review periods are measured from the “filing” date rather than the receipt date for NDAs for new molecular entities, which typically adds approximately two months to the timeline for review and decision from the date of submission. Most products that are eligible for Fast Track Designation are also likely to be considered appropriate to receive a priority review.
In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require a sponsor of a drug receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the drug may be subject to accelerated withdrawal procedures.
Moreover, under the provisions of the U.S. Food and Drug Administration Safety and Innovation Act, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs designated as breakthrough therapies are also eligible for accelerated approval. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. ABT may explore some of these opportunities for its product candidates as appropriate.
With CRS having such high unmet medical needs, ABT believes it may qualify for fast-track designation and potentially other designations such as preferred status rules for drugs addressing anti-microbial resistance.
However, there is no guarantee that the FDA would agree with our belief and there is no guarantee that such a qualification would result in an approval or a faster review process.
Intellectual Property
Trademarks
We currently hold three trademarks.
| Number | | File/Serial No. | | Location | | Mark | | Mark Drawing Type | | Active/Expired | | Expiration Date |
| 1 | | 2142043 | | Canada | | The mark consists of the word Sepset and four chevrons forming a cross appearing to the upper right of the letter t. | | Design | | Active | | N/A |
| 2 | | 97145586 | | United States | | The mark consists of the word Sepset and four chevrons forming a cross appearing to the upper right of the letter t. | | 42- Scientific and technological services and research and design relating thereto; industrial analysis, industrial research and industrial design services; quality control and authentication services; design and development of computer hardware and software. | | Inactive | | N/A |
| 3 | | 2142047 | | Canada | | SEPSET ER | | 5-pharmaceuticals and other items and preparations for either veterinary or medical purposes 42- Scientific and technological services and research and design relating thereto; industrial analysis, industrial research and industrial design services; quality control and authentication services; design and development of computer hardware and software | | Active | | N/A |
ABT Innovations Inc.
The following table discloses certain intellectual property owned by UBC and licensed to ABT pursuant to the terms and conditions of the UBC License Agreement (collectively, the “UBC Intellectual Property”). The term of the UBC License Agreement ends on the later of April 24, 2037 and the expiry of the last patent licensed under the UBC License Agreement:
| Country | | Serial Number | | Patent Number | | File Date | | Issue Date | | Status |
| Patent Family 1: Small Cationic Antimicrobial Peptides |
| Australia | | 2007288080 | | 2007288080 | | 8-21-2007 | | 10-17-2013 | | Issued |
| Canada | | 2,660,668 | | 2,660,668 | | 8-21-2007 | | 06-28-2016 | | Issued |
| European | | 07800481.9 | | 2061886 | | 8-21-2007 | | 5-7-2014 | | Issued |
| France | | 07800481.9 | | 2061886 | | 8-21-2007 | | 5-7-2014 | | Issued |
| Germany | | 07800481.9 | | 206186 | | 8-21-2007 | | 5-7-2014 | | Issued |
| Italy | | 07800481.9 | | 206186 | | 8-21-2007 | | 5-7-2014 | | Issued |
| Spain | | 07800481.9 | | 206186 | | 8-21-2007 | | 5-7-2014 | | Issued |
| United Kingdom | | 07800481.9 | | 206186 | | 8-21-2007 | | 5-7-2014 | | Issued |
| United States | | 12/438,055 | | 8,343,475 | | 2-19-2009 | | 1-1-2013 | | Issued |
| United States | | 13/725,327 | | 9,017,656 | | 12-21-2012 | | 4-28-2015 | | Issued |
| United States | | 14/684,136 | | 9,707,282 | | 4-10-2015 | | 7-18-2017 | | Issued |
| Patent Family 2: Cationic Peptides with Immunomodulatory and/or Anti-Biofilm Activities |
| Canada | | 3,089,485 | | | | 1-25-2019 | | | | Filed |
| United States | | 16/964,566 | | | | 1-25-2019 | | | | Filed |
| European(1) | | 19743997.9 | | | | 1-25-2019 | | | | Filed |
(1) The European Patent Convention (“EPC”) is a multilateral treaty instituting the European Patent Organisation and providing an autonomous legal system according to which European patents are granted. The term “European” patent is used to refer to patents granted under the EPC. A European patent is not a unitary right, but a group of essentially independent nationally enforceable, nationally revocable patents. The EPC provides a legal framework for the granting of European patents, via a single, harmonized procedure before the European Patent Office.
All patents licensed to ABT noted above are governed by the UBC License Agreement. All issued patents are subject to annual maintenance fees and an expiry date that is twenty (20) years following the date of first issuance. None of the expired or abandoned patents noted above are material to ABT and these patents were abandoned to save on patent maintenance fees. At this time there is no intention to renew or refile these expired or abandoned patents.
The UBC License Agreement terminates automatically and immediately without notice if any proceeding under the Bankruptcy and Insolvency Act of Canada is started by or against ABT. UBC may, at its option, immediately terminate the UBC License Agreement by giving notice to ABT under certain circumstances, including insolvency, breach of contract, winding up, liquidation or other termination of the existence of ABT. Either party may terminate this Agreement for any breach which is not remedied after proving notice to the party in breach. If the UBC License Agreement is terminated, ABT will make all outstanding payments to UBC and within 5 days of termination, ABT will cease to use the technology.
Sepset Biosciences Inc.
The following table discloses certain patents, as well as any improvements, variations, updates, modifications and enhancements made thereto by Dr. Robert E.W. Hancock, are either owned by Dr. Robert E.W. Hancock and coinventors, or licensed to Sepset on an exclusive and worldwide basis pursuant to the terms and conditions of the Hancock License Agreement (Patent Family 1). The term of the Hancock License Agreement ends on the later of March 13, 2037 and the expiry of the patents relating to the below intellectual property. Patent family 2 was licensed to Sepset pursuant to the terms and conditions of the UBC License Agreement (collectively, the “UBC Intellectual Property”). The term of the UBC License Agreement ends on the later of April 24, 2037 and the expiry of the last patent licensed under the UBC License Agreement:
Country | | Application Number # | | Continuity | | Application Publication or Patent No. # | | Status * |
| Patent Family 1: Diagnostic for sepsis |
| Australia | | 2015230632 | | National phase of PCT/CA2015/000160 | | AU 2015230632 | | Refiled as 2020201564 |
| Australia | | 2020201564 | | Divisional of AU2015230632 | | AU2020201564 A1 | | Granted |
| Belgium | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| Canada | | 2942577 | | National phase of PCT/CA2015/000160 | | CA2942577 A1 | | Pending |
| China | | 201580020498.0 | | National phase of PCT/CA2015/000160 | | CN106661765 B | | Granted |
| Denmark | | NA | | Country endorsement of EP15761149.2 | | DK3117030T3 | | Granted |
| European(1) | | 15761149.2 | | National phase of PCT/CA2015/000160 | | EP3117030 A1 | | Pending |
| Finland | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| France | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| Germany | | NA | | Country endorsement of EP15761149.2 | | DE602015078514.2 | | Granted |
| Hong Kong | | 17111280.7 | | Via CN201580020498.0 | | HK1237382 | | Granted |
| Ireland | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| Italy | | NA | | Country endorsement of EP15761149.2 | | IT502022000039650 | | Granted |
| Japan | | 2016-557954 | | National phase of PCT/CA2015/000160 | | JP 2017-514459A | | Refiled as 2020-101999 |
| Japan | | 2020-101999 | | Divisional of JP2016-557954 | | JP 2020-162615A | | Pending |
| Netherlands | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| Norway | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| PCT(1) | | PCT/CA2015/000160 | | Priority from US61/953,458 | | WO 2015/135071 A1 | | Converted into National phase filings |
| Spain | | NA | | Country endorsement of EP15761149.2 | | ES2918777T3 | | Granted |
| Sweden | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| Switzerland | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| UK | | NA | | Country endorsement of EP15761149.2 | | NA | | Granted |
| United States | | 61/953,458 | | Priority application | | N/A | | Converted into PCT/CA2015/000160 |
| United States | | 15/124,333 | | National phase of PCT/CA2015/000160 | | US 2017/0073734 A1 | | Refiled as 16/279,788 |
| United States | | 16/279,788 | | Continuation of US15/124,333 | | US 11,851,717 | | Granted |
| Patent Family 2: Diagnostic for sepsis endotypes and/or severity |
| PCT(1) | | PCT/CA2022/050831 | | - | | WO 2022/246553 A1 | | Pending conversion into National phase filings |
| US | | 63/192,746 | | Priority application | | N/A | | Converted into PCT/CA2022/050831 |
*In Europe, individual countries, when requested, endorse the European grant of patent. This does not always result in a unique country patent number or application number. Therefore NA indicates not available or not applicable.
*Status indicated only for pending applications, allowed applications and granted patents. Remaining patent explained where they were converted. All patents except for US61/953,458 have exactly the same body of the patent.
| (1) | The EPC is a multilateral treaty instituting the European Patent Organisation and providing an autonomous legal system according to which European patents are granted. The term “European” patent is used to refer to patents granted under the EPC. A European patent is not a unitary right, but a group of essentially independent nationally enforceable, nationally revocable patents. The EPC provides a legal framework for the granting of European patents, via a single, harmonized procedure before the European Patent Office. |
| (2) | PCT is the patent cooperation treaty administered by WIPO, the World Intellectual property Organization and. These are legal frameworks whereby patents prosecuted in multiple countries are published after a certain time so they become a matter of public record. |
All patents licensed to Sepset as noted above are governed by the Hancock License Agreement. All issued patents are subject to annual maintenance fees and an expiry date that is twenty (20) years following the date of first issuance.
| SafeCoat |
| Title | | Serial Number | | Country | | File Date | | Status |
| Polymeric Antifouling Coating with Antimicrobial Peptides | | PCT/CA2022/050883 | | PCT | | 6-2-2022 | | Filed. Will convert to National Phase Dec 2, 2023. |
Material Contracts
Except for contracts entered into in the ordinary course of business, the only contracts which have been entered into by ASEP and its subsidiaries since its inception and which are regarded presently as material are:
iFyber LLC and ABT Innovations collaboration letter of intent dated December 6, 2016 and July 17, 2018
On December 6, 20216, ABT and iFyber, LLC (“iFyber”) entered into a letter of intent (the “LOI”) where ABT and iFyber agreed to sub-license the proprietary peptides to iFyber for use in developing novel peptide-based wound dressings. iFyber and ABT agreed to collaboratively apply for non-dilutive funding from governmental agencies. Any funds received would be used to fund technical, regulatory and market feasibility studies. Any further and broader collaborations with iFyber is subject to (a) successful preclinical efficacy and safety studies and (b) viable business case for product development in the peptides. ABT holds an exclusive license to the peptides used in the area of antimicrobial peptides as part of a medical device intended for wound healing, including solid and semisolid wound dressings (the “Background IP”), and has the right to and interest in sub-licensing. Pursuant to a confidential disclosure agreement entered into on September 22, 2016, all confidential information provided in the letter of intent will be the property of the disclosing party.
On July 17, 2018, ABT sent a letter to iFyber noting that given successful preclinical efficacy and safety studies and a viable business case for product development, ABT has provided a waiver to use its licensed intellectual property from UBC in the project. ABT authorized the use of all intellectual property, technology, patents etc. covered in the ABT/UBC license in the iFyber project, that will enable UBC to meet all requirements of the sub-award and all applicable United States government regulations governing the grant. ABT also provided a waiver on behalf of UBC to notify iFyber of any inventions achieved through the use of ABT-licensed, as long as ABT is notified conjointly.
On May 18, 2021, the parties entered into a first amendment to the confidential disclosure agreement executed on September 22, 2016, where the term of the confidential disclosure agreement was extended to May 17, 2026.
The Michael Smith Foundation Commitment Letter dated April 29, 2020 between ABT and the Michael Smith Foundation for Health Research
On April 29, 2020, ABT signed a commitment letter to the Michael Smith Foundation for Health Research pursuant to which ABT committed to providing not less than $50,000, comprising $30,000 of direct support for purchasing peptide for necessary research activities and $20,000 in-kind support for IND planning. Michael Smith Foundation for Health Research provided funding for drug development research being performed at UBC, using ABT’s licensed technology. All rights were retained by UBC and its licensee ABT. The funding from the Michael Smith Foundation was provided as a research grant to UBC for advanced drug development work on ABT’s in-licensed peptides, with ABT providing peptides to support the study. The Michael Smith Foundation is funded by the government of British Columbia and has no affiliation with UBC. The Michael Smith Foundation has no rights to any discoveries made under this grant, nor does the Foundation perform research. Since the peptides have been in-licensed from UBC, any new discoveries would fall under the definition of “improvements” and thus available for license by ABT.
The Burton Engagement Letter dated January 18, 2021 between Sepset and Burton
Burton Financial Inc. (“Burton”) agreed to act, as exclusive transaction advisor to Sepset in consideration for a success fee equal to a 10% equity interest in Sepset, calculated prior to the closing of a potential acquisition, merger, sale, divestiture, or similar transaction(s) involving all or a material part of Sepset or its assets. Burton was issued 1,111,111 shares as payment of its fee. The payment of this fee was triggered by the Sepset Option exercise. The Burton engagement letter has no defined term, however either party retained the right to terminate the Burton Engagement Letter upon 30 days’ written notice to the other party. If, during term of the Burton engagement letter, Sepset enters into or publicly announces a transaction but is not completed and Sepset receives a break-up fee, lock-up option, topping fee or other termination fee of any kind (collectively, a “Termination Fee”), Sepset is required pay Burton a fee equal to 20% of such Termination Fee at such time as such Termination Fee is received by Sepset. The Burton engagement letter also provides that Sepset will indemnify and hold harmless Burton from any and all claims, actions, suits or otherwise arising out of or are based upon, directly or indirectly, the engagement of Burton by ASEP Medical, Inc. in connection with the Burton engagement letter. Upon completing the Sepset Option exercise, Burton fulfilled its duties under the Burton Engagement Letter and is no longer engaged as an advisor to Sepset, and as such, Burton has no further role in future transactions involving Sepset.
Employment agreement dated March 1, 2021 (“Saad Employment Agreement”) between ASEP Medical, Inc. and Dr. Fadia Saad
On March 1, 2021, ASEP Medical, Inc. entered into the Saad Employment Agreement with Dr. Fadia Saad, pursuant to which Dr. Saad agreed to provide certain management services to ASEP, including but not limited to acting as Chief Business Development Officer of ASEP Medical, Inc. As consideration for the services to be provided by Dr. Saad, ASEP Medical, Inc. agreed to pay a monthly fee of $15,000, less statutory deductions. Dr. Saad is also eligible for an annual performance bonus up to 20% of base salary determinable at the absolute discretion of the ASEP Medical, Inc. board.
The Saad Employment Agreement may be terminated: (a) at any time by Dr. Saad by giving a minimum of thirty (30) days written notice to ASEP Medical, Inc.; (b) without notice or payment in lieu of notice, for sufficient cause by ASEP at any time; or (c) at any time by ASEP without the requirement to show cause, provided ASEP pays Dr. Saad an amount equal to six (6) months’ salary in the event of termination before the end of a 3-year period. Thereafter, severance will be additional to six (6) month’s salary and commensurate to years of service and consistent with legislation as may be in effect at the time of termination.
Employment agreement dated March 1, 2021 (“Gretchen Employment Agreement”) between ASEP Medical, Inc. and Jennifer Gretchen
On March 1, 2021, ASEP Medical, Inc. entered into the Gretchen Employment Agreement with Jennifer Gretchen, pursuant to which Ms. Gretchen agreed to provide certain management services to ASEP Medical, Inc., including but not limited to acting as CFO of ASEP Medical, Inc. As consideration for the services to be provided by Ms. Gretchen, ASEP Medical, Inc. agreed to pay a monthly fee of $12,500. Ms. Gretchen is also eligible for an annual performance bonus up to 25% of base salary determinable at the absolute discretion of the ASEP Medical, Inc. board.
The Gretchen Employment Agreement may be terminated: (a) at any time by Ms. Gretchen by giving a minimum of thirty (30) days written notice to ASEP Medical, Inc.; (b) without notice or payment in lieu of notice, for sufficient cause by ASEP Medical, Inc. at any time; or (c) at any time by ASEP Medical, Inc. without the requirement to show cause, provided ASEP Medical, Inc. pays Ms. Gretchen an amount equal to six (6) months’ salary in the event of termination before the end of a 3-year period. Thereafter, severance will be additional to six (6) month’s salary and commensurate to years of service and consistent with legislation as may be in effect at the time of termination. On October 1, 2022, Ms. Gretchen resigned as CFO of the Company. Ms. Gretchen provided the minimum thirty days written notice to ASEP Medical Inc, resulting in no termination obligations for the Company.
Consulting agreement dated March 1, 2021 (“RAM Consulting Agreement”) between ASEP Medical, Inc. and RAM Advisors, Inc.
On March 1, 2021, ASEP Medical, Inc. entered into the RAM Consulting Agreement with RAM Advisors, Inc., pursuant to which RAM Advisors, Inc. agreed to provide, through its principal Rudy Mazzocchi, certain management services to ASEP Medical, Inc., including but not limited to acting as CEO and Executive Chairman of ASEP Medical, Inc. As consideration for the services to be provided by RAM Advisors, Inc., ASEP Medical, Inc. agreed to pay a monthly fee of $20,000. RAM Advisors, Inc. is also eligible for an annual performance bonus up to 25% of base salary determinable at the absolute discretion of the ASEP Medical, Inc. board.
The RAM Consulting Agreement could be terminated: (a) at any time by RAM Advisors, Inc. by giving a minimum of thirty (30) days written notice to ASEP Medical, Inc.; or (b) at any time by ASEP Medical, Inc. by giving a minimum of six (6) months written notice to RAM Advisors, Inc. The RAM Consulting Agreement was terminated on June 30, 2022.
Consulting agreement dated April 1, 2021 (“Burton Consulting Agreement”) between ASEP Medical, Inc. and Burton
On April 1, 2021, ASEP Medical, Inc. entered into the Burton Consulting Agreement, pursuant to which Burton agreed to provide certain management services to ASEP Medical, Inc., including but not limited to financial and operational expertise. The Burton Consulting Agreement had a three-month term commencing April 1, 2021 and ending June 30, 2021. As consideration for the services to be provided by Burton, ASEP agreed to pay a monthly fee of $25,000. In addition, ASEP Medical, Inc. paid Burton a success fee of $150,000 payable in cash in relation to the Transaction.
ABT Second UBC Collaborative Research Agreement dated June 2, 2021 between ABT and UBC
The ABT Second UBC Collaborative Research Agreement (“ABT Second UBC Collaborative Research Agreement”) was entered into on June 2, 2021 with a commitment of $250,000 to be paid to UBC. The Second UBC Collaborative Research Agreement aims to conduct necessary experiments to identify the most promising molecules for development of ABT’s peptide technology and move ABT towards the regulatory approval stages and an IND application. Pursuant to the ABT Second UBC Collaborative Research Agreement, UBC retains intellectual property ownership over any and all inventions made jointly by UBC and ABT, as well as all inventions made solely by UBC. ABT retains intellectual property ownership over any and all inventions made solely by ABT.
The first payment of $124,114.20 was paid on July 23, 2021. The second installment was invoiced by UBC on December 12, 2021 in the amount of $124,114.20 and was paid on December 30, 2021. The ABT Second UBC Collaborative Research Agreement had a start date of June 2, 2021 with a contract period of 8 months, resulting in a contract end date of March 30, 2022. In October 2022, the contract was extended to August 31, 2023.
Sepset UBC Collaborative Research Agreement dated August 6, 2021 between Sepset and UBC
On August 6, 2021, Sepset entered into the Sepset UBC Collaborative Research Agreement with UBC. The Sepset UBC Collaborative Research Agreement is a collaborative research program aimed at testing the rapid quantitative measurement of a carefully selected set of Sepsis signature markers developed by Dr. Hancock and licensed to Sepset. This testing will be done by using quantitative reversed transcription PCR (“RT-qPCR”) to obtain an accurate outcome prediction within an hour or less. Pursuant to the Sepset UBC Collaborative Research Agreement, UBC retains intellectual property ownership over any and all inventions made jointly by UBC and ABT, as well as all inventions made solely by UBC. ABT retains intellectual property ownership over any and all inventions made solely by ABT. Ten already identified housekeeping genes will be the first part of the proposed RT-PCR validation to establish an expression baseline. The two best performing genes will be selected using bioinformatic strategies. On the other hand, an accurate quantitative assessment of 12 already identified diagnostic cellular reprogramming signature markers, coupled with an expression baseline measured with housekeeping genes, will be carried out, as it is critical for reliable prediction for Sepsis status and severity. Once the quantitative assessment of signature markers is validated, the process of quantitative measurement can be further improved by employing automated, high-throughout, microfluidic-based technologies, such as Power-Blade, which would allow us to rapidly survey the expression of signature markers. Research funding of $93,258 in total was provided by Sepset to UBC, but no other consideration was transferred. The term of the Sepset UBC Collaborative Research Agreement ends on December 31, 2023.
The Sepset UBC Collaborative Research Agreement also includes an option (“Sepset UBC Collaborative Research Agreement Option”) whereby Sepset may acquire a royalty-bearing license to use and exploit certain intellectual property developed by UBC during the term of the Sepset UBC Collaborative Research Agreement for a period of 6 months following the disclosure by UBC of such intellectual property in writing to Sepset. If Sepset wishes to exercise the Sepset UBC Collaborative Research Agreement Option, the parties will negotiate in good faith the specific terms and conditions on which a license will be granted by UBC.
The ABT Option Agreement dated May 14, 2021 among ASEP Medical, Inc., ABT and all of the shareholders of ABT other than UBC
On May 14, 2021 (the “ABT Effective Date”), ABT granted ASEP Medical, Inc. the ABT option (the “ABT Option”) to acquire 50.1% of the common shares of ABT (the “ABT Shares”), in exchange for aggregate cash consideration of $2,500,000. ASEP agreed to subscribe for, and ABT agreed to issue, unsecured convertible notes of ABT (the “ABT Notes”) in the aggregate principal amount of up to $2,500,000 in five equal tranches of $500,000 on or prior to each of the dates (the “ABT Note Subscription Date”) set forth below: (a) $500,000 on the ABT Effective Date; (b) $500,000 on the four month anniversary of the ABT Effective Date; (c) $500,000 on the eight month anniversary of the ABT Effective Date; (d) $500,000 on the twelve month anniversary of the ABT Effective Date; and (e) $500,000 on the last Business Day prior to the sixteen month anniversary of the ABT Effective Date. The ABT Notes are unsecured, non-interest bearing and repayable on the earlier of September 14, 2022 (the “Expiry Date”) or the date on which the ABT Option Agreement is breached due to the Company’s failure to fund the ABT Notes on the ABT Note Subscription Dates (the “Breach Date”).
The ABT Notes can be converted by ASEP at any time up to the maturity date and will be automatically converted, subject to there not being an occurrence of an event of default, into such number of ABT Shares on the maturity date as determined in accordance with the ABT Automatic Conversion Calculation (as defined below), and the ABT Note(s) shall be terminated, and the total aggregate principal amount shall be automatically applied towards satisfaction of the Company’s payment for such shares. The number of ABT Shares to be determined by ABT on the Expiry Date, Breach Date or the date upon which ABT converts any principal amount outstanding under the Notes into Shares pursuant to Section 9.2 of the Notes (the “Default Date”), as applicable, is calculated by dividing (A) by (B), and then rounding the quotient of such equation down to the nearest whole number, where (A) is the product obtained by multiplying: (a) the aggregate number of ABT Shares that are issued and outstanding, as of the Expiry Date, Breach Date or Default Date, as applicable, on a fully diluted basis assuming conversion of all outstanding convertible securities of ABT other than the ABT Notes, with (b) the product obtained by 10.02 multiplied by the number of ABT Notes outstanding as of the Expiry Date, Breach Date, or Default Date, as applicable, and where (B) is the product obtained by subtracting: (c) the product obtained by 10.02 multiplied by the number of ABT Notes outstanding as of the Expiry Date, Breach Date, or Default Date, as applicable, from (d) 100 (the “ABT Automatic Conversion Calculation”). On May 14, 2021, the Company subscribed for the $500,000 note referred to in (a) above and on September 14, 2021, the Company subscribed for the $500,000 note referred to in (b) above. On November 10, 2021, the Company exercised the ABT Option to acquire a 50.1% equity interest in ABT. Following the exercise of the ABT Option, the remaining ABT Notes were extinguished pursuant to the ABT Option Agreement.
In addition, pursuant to the terms of the ABT Option Agreement, all of the shareholders of ABT (excluding UBC, that holds 3.53% of shares) granted ASEP, subject to the exercise of the ABT Option, an option (the “ABT Additional Option”) to acquire the remaining 49.9% equity interest in the capital of ABT from each of the shareholders of ABT (less the equity interest held by each of ASEP and UBC), resulting in ASEP holding a 100% equity interest in the capital of ABT after exercise of the ABT Additional Option and the UBC Option (as defined below). ASEP may exercise the ABT Additional Option at any time prior to the third anniversary of the exercise by ASEP of the ABT Option upon payment of an aggregate $20,000,000 (the “ABT Additional Option Exercise Price”), less the amount payable to UBC pursuant to the UBC Option Agreement (as defined below), payable pro rata to each of the shareholders of ABT (excluding the Company and UBC). The ABT Additional Option Exercise Price is payable as follows:
| | a. | if ASEP’s shares (including any shares of any assignee of ASEP, including, without limitation, TLS (as defined below) upon completion of the transactions contemplated in the Amalgamation Agreement) are listed on a recognized stock exchange, the ABT Additional Option Exercise Price, less the amount payable to UBC pursuant to the UBC Option Agreement, shall be payable to the shareholders of ABT (excluding ASEP and UBC), on a pro rata basis: (A) in cash, as to an aggregate minimum of $5,000,000; and (B) in shares of ASEP (or its assignees) as to the balance of the ABT Additional Option Exercise Price remaining after deduction of the cash portion advance under part (A), with such shares to be issued based on the 20-day volume-weighted average trading price of ASEP (or its assignees) ending on the trading day preceding the date on which ASEP provides notice to ABT that it is exercising the ABT Additional Option; or |
| | b. | if ASEP’s shares are not listed on a recognized stock exchange, the ABT Additional Option Exercise Price shall be payable in cash, on a pro rata basis, as to the full $20,000,000, less the amount payable to UBC pursuant to the UBC Option Agreement. |
At the time of entry of the ABT Option Agreement, Michael Graw was the sole officer and director of ASEP Medical, Inc. and a director of ABT. Mr. Graw, as a director of ASEP Medical, Inc., disclosed his interest as a director of ABT to the board of directors of ASEP Medical, Inc. in accordance with the requirements set forth in the BCBCA. At the time, Dr. Robert E.W. Hancock, our CEO and a director, was also a director of ABT. The ABT Additional Option has not been exercised and remains outstanding for three years following the exercise of the ABT Option, which occurred on November 10, 2021.
The Sepset Option Agreement dated May 14, 2021 among ASEP, Sepset and all of the shareholders of Sepset
On May 14, 2021 (the “Sepset Effective Date”), ASEP entered into an option agreement with Sepset wherein Sepset granted ASEP Medical, Inc. an option (the “Sepset Option”) to acquire 50.1% of common shares of Sepset (the “Sepset Shares”), in exchange for aggregate cash consideration of $2,500,000.
ASEP agreed to subscribe for, and Sepset agreed to issue, unsecured convertible notes of Sepset (the “Sepset Notes”) in the aggregate principal amount of up to $2,500,000 in five equal tranches of $500,000 on or prior to each of the dates (the “Sepset Note Subscription Date”) set forth below: (a) $500,000 on the ABT Effective Date; (b) $500,000 on the four month anniversary of the ABT Effective Date; (c) $500,000 on the eight month anniversary of the ABT Effective Date; (d) $500,000 on the twelve month anniversary of the ABT Effective Date; and (e) $500,000 on the last Business Day prior to the sixteen month anniversary of the ABT Effective Date. The ABT Notes are unsecured, non-interest bearing and repayable on the maturity date, which is the earlier of September 14, 2022 (the “Expiry Date”) or the date on which the ABT Option Agreement is breached due to the Company’s failure to fund the ABT Notes on the ABT Note Subscription Dates (the “Breach Date”).
The Sepset Notes can be converted by ASEP at any time up to the Maturity Date, being the earlier of the Expiry Date, September 14, 2022 and the Breach Date, the date on which the Sepset Option Agreement is breached due to the Company’s failure to fund the Sepset Notes on the Sepset Note Subscription Dates. and will be automatically converted, subject to there not being an occurrence of an event of default, into such number of shares on the Maturity Date as determined in accordance with the Sepset Automatic Conversion Calculation (as defined below), and the Sepset Note(s) shall be terminated, and the total aggregate principal amount shall be automatically applied towards satisfaction of ASEP’s payment for such shares. The number of Sepset Shares to be determined by Sepset on the Expiry Date, Breach Date or the date upon which Sepset converts any principal amount outstanding under the Notes into Shares pursuant to Section 9.2 of the Notes (the “Default Date”), as applicable, is calculated by dividing (A) by (B), and then rounding the quotient of such equation down to the nearest whole number, where (A) is the product obtained by multiplying: (a) the aggregate number of Sepset Shares that are issued and outstanding, as of the Expiry Date, Breach Date or Default Date, as applicable, on a fully diluted basis assuming conversion of all outstanding convertible securities of the Sepset other than the Sepset Notes, with (b) the product obtained by 10.02 multiplied by the number of Sepset Notes outstanding as of the Expiry Date, Breach Date, or Default Date, as applicable, and where (B) is the product obtained by subtracting: (c) the product obtained by 10.02 multiplied by the number of Sepset Notes outstanding as of the Expiry Date, Breach Date, or Default Date, as applicable, from (d) 100 (the “Sepset Automatic Conversion Calculation”). On May 14, 2021, ASEP subscribed for the $500,000 note referred to in (a) above and on September 14, 2021, ASEP subscribed for the $500,000 note referred to in (b). ASEP exercised the Sepset Option on November 10, 2021.
In addition, pursuant to the terms of the Sepset Option Agreement, all of the shareholders of Sepset granted ASEP, subject to the exercise of the Sepset Option, an option (the “Sepset Additional Option”) to acquire the remaining 49.9% equity interest in the capital of Sepset from each of the shareholders of Sepset (excluding ASEP), resulting in ASEP, after exercise of the Sepset Additional Option, holding a 100% equity interest in the capital of Sepset. ASEP may exercise the Sepset Additional Option at any time prior to the third anniversary of the exercise by ASEP of the Sepset Option upon payment of an aggregate $20,000,000 (the “Sepset Additional Option Exercise Price”) payable pro rata to each of the shareholders of Sepset (excluding ASEP). The Sepset Additional Option Exercise Price is payable as follows:
| | a. | if ASEP’s shares (including any shares of any assignee of ASEP, including, without limitation, TLS upon completion of the transactions contemplated in the Amalgamation Agreement) are listed on a recognized stock exchange, the Sepset Additional Option Exercise Price shall be payable to the shareholders of Sepset (excluding ASEP), on a pro rata basis: (A) in cash, as to an aggregate minimum of $5,000,000; and (B) in shares of ASEP (or its assignees) as to the balance of the Sepset Additional Option Exercise Price remaining after deduction of the cash portion advance under part (A), with such shares to be issued based on the 20-day volume-weighted average trading price of ASEP (or its assignees) ending on the trading day preceding the date on which ASEP provides notice to Sepset that it is exercising the Sepset Additional Option; or |
| | b. | if ASEP’s shares are not listed on a recognized stock exchange, the Sepset Additional Option Exercise Price shall be payable in cash, on a pro rata basis, as to the full $20,000,000. |
At the time, Dr. Robert E.W. Hancock, our CEO and a director, was also a director of Sepset. The Sepset Additional Option has not been exercised and remains outstanding for three years following the exercise of the Sepset Option, which occurred on November 10, 2021.
The UBC Share Purchase Option Agreement dated May 14, 2021 among ASEP Medical, Inc., ABT and UBC
UBC granted ASEP Medical, Inc. an option (the “UBC Option”) to purchase 143,388 Class A Shares, together with any Class A Shares or other equity interest in ABT that UBC might have acquired following the contract’s effective date (the “UBC Shares”), in consideration for twenty million CDN$20,000,000 multiplied by a percentage equal to the quotient of the number of UBC Shares divided by (i) the total number of issued and outstanding Class A Shares less (ii) any Class A Shares then held by ASEP Medical, Inc. (“UBC’s Pro Rata Percentage).
ASEP Medical, Inc. can exercise the UBC Option by providing notice in writing to UBC of its intention to exercise the UBC Option, together with the full payment of the UBC Purchase Price as follows:
| | (a) | if ASEP Medical, Inc.’s shares, or its assigns including without limitation the Company on completion of the transaction contemplated by the Amalgamation Agreement) are listed on a recognized stock exchange, the UBC Purchase Price shall be payable by ASEP to UBC as follows: |
| | (i) | a cash amount equal to UBC’s Pro Rata Percentage, defined above, of an aggregate minimum of CDN$5,000,000, and |
| | (ii) | in ASEP shares, as to the balance of the UBC Purchase Price remaining after deduction of the cash portion advanced, issued based on the 20-day volume weighted average trading price of such shares ending on the trading day preceding the date on which ASEP provides notice in writing to UBC of its intention to exercise the UBC Option, subject to applicable securities laws; and |
| | (b) | if the ASEP Shares (including the common shares) are not listed on a recognized stock exchange, the UBC Purchase Price shall be payable by ASEP to UBC in cash. |
The UBC Option expires on the Additional Option Expiry Date, which is 5:00 pm PST on the date that both the Exercise and Conversion Notice and the payment of the Exercise Price are delivered by ASEP Medical Inc. to UBC.
At the time, Dr. Robert E.W. Hancock, was the COO and director of the Company, and was also a director of ABT.
Employment Agreement dated June 1, 2021 (“Haney Employment Agreement”) between ASEP Medical, Inc. and Dr. Evan Haney
On June 1, 2021, ASEP Medical, Inc. entered into the Haney Employment Agreement with Dr. Evan Haney, pursuant to which Dr. Haney agreed to provide certain management services to ASEP, including but not limited to acting as Chief Scientific Officer of ASEP Medical, Inc. As consideration for the services to be provided by Dr. Haney, ASEP Medical, Inc. agreed to pay an annual salary of CDN$70,000.
The Haney Employment Agreement may be terminated: (a) at any time by Dr. Haney by giving a minimum of thirty (30) days written notice to ASEP Medical, Inc.; (b) without notice or payment in lieu of notice, for sufficient cause by ASEP at any time; or (c) at any time by ASEP without the requirement to show cause, provided ASEP pays Dr. Haney an amount equal to six (6) months’ salary in the event of termination before the end of a 3-year period. Thereafter, severance will be additional to six (6) month’s salary and commensurate to years of service and consistent with legislation as may be in effect at the time of termination.
The Amalgamation Agreement dated June 3, 2021 among the Company, ASEP Medical, Inc. and NewCo
On June 3, 2021, the Company and NewCo, pursuant to the terms of the amalgamation agreement (“Amalgamation Agreement”), the Company, ASEP Medical, Inc. and NewCo agreed to combine their respective businesses by way of a three-concerned amalgamation (“Amalgamation”) under the provisions of the BCBCA. Upon completion of the Amalgamation, ASEP Medical, Inc, the resulting entity of the Amalgamation, became a wholly-owned subsidiary of the Company and ASEP Medical Holdings, Inc. carried on the business of ASEP Medical, Inc.
Escrow Agreement dated November 9, 2021 among the Company, Odyssey Trust Company and the holders of the Escrowed Securities
On November 9, 2021, ASEP Medical Holdings, Inc. entered into an escrow agreement (the “Escrow Agreement”) with Odyssey Trust Company (“Odyssey”), wherein Odyssey agreed to act as escrow agent, for a term of 36 months beginning on November 23, 2021, in order to comply with the policies of the CSE and National Policy 46-201 Escrow for Initial Public Offerings, in connection with listing the Company’s common shares on the CSE. Pursuant to the Escrow Agreement, common shares held by key personnel, including directors and officers of the Company, are held in escrow for a period of 3 years from the date of listing the common shares on the CSE. The escrowed shares are released on a pre-determined schedule as set forth in the Escrow Agreement, with the final common shares being released on November 23, 2024. Either Odyssey or ASEP may terminate the Escrow Agreement with written notice to the other party. The resignation or termination will be effective 60 days after receipt of written notice terminating the Escrow Agreement. The parties may agree to another resignation date, provided that the resignation or terminate date will not be less than ten business days before an escrow release date. Pursuant to the Escrow Agreement, ASEP has agreed to pay Odyssey reasonable remuneration for its services, which remuneration is paid monthly when shares are released from escrow, and ASEP has agreed to reimburse Odyssey for its expenses and disbursements.
Employment agreement dated November 23, 2022 (“November Gretchen Employment Agreement”) between ASEP and Jennifer Gretchen
On November 23, 2022, ASEP entered into the November Gretchen Employment Agreement with Jennifer Gretchen, pursuant to which Ms. Gretchen agreed to act as the Company’s executive assistant and perform other necessary actions as described in the November Gretchen Employment Agreement. As consideration for the services to be provided by Ms. Gretchen, ASEP agreed to pay an annual, base salary of CAD $60,000. The base salary is payable on a semi-monthly basis. Ms. Gretchen is also eligible for an annual performance bonus determinable at the discretion of the Company’s board. Upon execution of the November Gretchen Employment Agreement, the Company issued Ms. Gretchen 50,000 options to purchase common shares of the Company exercisable at a price of $0.30 per share. The shares were fully vested on the date of the execution of the agreement. The Gretchen Employment Agreement will continue in full force and effect unless it is terminated earlier by the Company or the employee based on the termination provisions set forth in the November Gretchen Employment Agreement.
Collaborative Research Agreement dated April 8, 2022 between the University of British Columbia and Sepset
On April 8, 2022, UBC and Sepset entered into a collaborative research agreement wherein UBC agreed to test and optimize the RNA extraction protocol from human blood or cells collected non-invasively using nasal or oral swabs (the “Project”). The isolated RNA will then be evaluated using an RT-qPCR protocol to quantify the expression levels of the genes present within the sepsis gene expression signature. In exchange, Sepset agreed to pay UBC CAD$549,094 within 30 days of April 8, 2022. Sepset will pay interest on all amounts owing to UBC not paid on the due date, at the rate of 12.68% per annum. The interest accrues on the outstanding balance from the due date. On May 2, 2022, a sum of $549,094 was paid. The term of this collaborative research agreement was for 12 months, following the payment of $549, 094 on May 2, 2022.
Sepset retains all ownership and rights to its patents and intellectual property, while UBC retains all ownership and rights to its patents and intellectual property. Any joint patents and intellectual property will be owned jointly by Sepset and UBC. To date, no disclosures of new intellectual property have been made and we anticipate no new requirements for licensing and commercialization of technology resulting from the arrangement. While UBC is not restricted from presenting at symposia, national or regional professional meetings, or from publishing in journals or other publications, results from the Project, provided that Sepset is provided with copies of the proposed disclosure at least 30 calendar days before the presentation or publication date and does not, within 15 calendar days after delivery of the proposed disclosure, give notice to UBC indicating that it objects to the proposed disclosure. Sepset can object to the proposed disclosure on the grounds that (i) it contains confidential information that was disclosed to UBC by Sepset; or (ii) that it discloses patentable subject matter which needs protection. If Sepset makes objection on the grounds of the inclusion of Sepset’s confidential information, UBC will remove such confidential information immediately from the proposed disclosure, after which UBC is free to present and/or publish the proposed disclosure. If Sepset makes an objection on the grounds of protection of patentable subject matter: (i) it will be deemed to be a direction to UBC to file a patent application as described in the agreement; and (ii) UBC will delay the proposed disclosure until UBC has filed one or more patent applications with one or more patent offices directed to such patentable subject matter (the “Delay”). A provisional patent application will be considered to be a patent application in the United States of America for the purposes of this Agreement. The Delay will be no longer than six (6) months from the date UBC delivered the proposed disclosure to Sepset, after which UBC is free to present and/or publish the proposed disclosure.
Sepset can direct that UBC file patent applications in the name of UBC for certain intellectual property of UBC’s and/or in joint names of UBC and Sepset for any new and useful art, process, machine, manufacture or composition of matter, or any new and useful improvement in any art, process, machine, manufacture or composition of matter made jointly by UBC and Sepset during the term of the contract in the performance of the Project (“Joint Intellectual Property”). Sepset will bear all costs incurred in connection with the preparation, filing, prosecution and maintenance of the patent applications. Within 30 calendar days of UBC’s written request, Sepset will pay to UBC a reasonable payment as an advance against expected patent expenses. In the event that the Sponsor wishes to discontinue the financial support for prosecution or maintenance of any patents or patent applications for Joint Intellectual Property (the “Event”), the Sponsor will notify UBC in writing at least 30 calendar days prior to the Event (the “Notice to Discontinue”) and UBC will be free to continue the prosecution or maintenance of any such patents or patent applications for Joint Intellectual Property. The Sponsor will then promptly execute and deliver to UBC any assignment or documents UBC may deem necessary or desirable to vest in UBC all right, title and interest in the patents and patent applications. Sponsor will pay for all expenses incurred in connection with the patents and patent applications prior to the Event and for 30 calendar days from UBC’s receipt of the Notice to Discontinue.
Consulting agreement dated June 30, 2022 (“Murphy Consulting Agreement”) between ASEP Medical, Inc. and Murphy Enterprises Inc.
On June 30, 2022, ASEP Medical, Inc. entered into the Murphy Consulting Agreement, pursuant to which Murphy Enterprises Inc. (“Murphy”) agreed to provide certain management services to ASEP Medical, Inc. as the Chief Operating Officer. The Murphy Consulting Agreement can be terminated at any time by Murphy by giving a thirty days written notice to the Company or by the Company by giving a minimum of 6 months written notice to Murphy. Otherwise, the Murphy Consulting Agreement will remain in full force and effect. As consideration for the services to be provided by Murphy, ASEP agreed to pay a monthly fee of CAD$10,000, plus GST. In addition, ASEP Medical, Inc. will grant Murphy 200,000 stock options exercisable at $0.25 per option.
Consulting agreement dated October 1, 2022 (“Tucker Consulting Agreement”) between ASEP Medical, Inc. and J.M. Tucker Professional Corporation
On October 1, 2022, ASEP Medical, Inc. entered into the Tucker Consulting Agreement, pursuant to which J.M. Tucker Professional Corporation (“Tucker”) agreed to provide certain management services to ASEP Medical, Inc. as the Chief Financial Officer. The Tucker Consulting Agreement can be terminated at any time by Tucker by giving a thirty days written notice to the Company or by the Company by giving a thirty days written notice to Tucker. As consideration for the services to be provided by Tucker, ASEP agreed to pay a monthly fee of CAD$7,500, which shall be paid in the following manner: (i) $6,000 plus GST, and (ii) $1,500 as a transfer or all of, or a fraction of, a particular security or share of the Company, or as a contribution to the purchase of such a particular security of share, up to a maximum of $60,000 and will automatically apply at the end of each calendar year towards the purchase of all vested options and the underlying security or share. In addition, ASEP Medical, Inc. granted Tucker 200,000 stock options exercisable at $0.30 per option.
Employment Agreement dated October 1, 2022 (“Hancock Employment Agreement”) between ASEP Medical, Inc. and Dr. Robert E.W. Hancock
On October 1, 2022, ASEP Medical, Inc. entered into the Hancock Employment Agreement with Dr. Robert E.W. Hancock, pursuant to which Dr. Hancock agreed to provide certain management services to ASEP, including but not limited to acting as Chief Executive Officer of ASEP Medical, Inc. As consideration for the services to be provided by Dr. Hancock, ASEP Medical, Inc. agreed to pay an annual salary of CAD$180,000 and until the Company completes a financing of at least CAD$2,000,000, the Company has the right to defer payment of up to 100% of the salary. One day after the end of the deferred salary, the Company shall pay the full salary plus a management bonus of $45,000. Dr. Hancock is also eligible for a bonus, the amount of which will be determined by the Board in its discretion. In addition, the Company will grant 300,000 options at a price of $0.30 per option.
The Hancock Employment Agreement may be terminated: (a) at any time by Dr. Hancock by giving a minimum of four weeks written notice to ASEP Medical, Inc.; (b) without notice or payment in lieu of notice, for sufficient cause by ASEP at any time; or (c) at any time by ASEP without the requirement to show cause, provided ASEP pays Dr. Hancock an amount equal to six (6) months’ salary in the event of termination.
The Earn-In and Option Agreement dated December 1, 2022 among SafeCoat Medical Inc., SafeCoat Medical Inc.’s shareholders, and ASEP
Effective as of December 1, 2022, SafeCoat Medical Inc. (“SafeCoat”), SafeCoat’s Shareholders, and ASEP entered into an Earn-In and Option Agreement (the “SafeCoat Agreement”) wherein SafeCoat, in consideration for ASEP facilitating negotiations with UBC in respect of a license of the peptide medical device coating technology (the “Technology”) to SafeCoat (the “License”) and agreeing to provide certain scientific advisory, management and administrative services to SafeCoat in connection with the granting of the License and the development of the Technology (collectively, the “Services”), agreed to allot and issue to ASEP, conditional upon the granting of the License to SafeCoat, an aggregate of 6,526,052 SafeCoat shares (the “SafeCoat Shares”), or such other number of SafeCoat Shares such that ASEP, at the time of the grant of the License, will own 50.1% of the outstanding SafeCoat Shares (collectively, the “Earn-In Shares”) and the SafeCoat Shareholders will collectively own the other 49.9% of the outstanding SafeCoat Shares (the “Earn-In Transaction”). Also in connection therewith, it was proposed that the SafeCoat Shareholders grant to ASEP an option to acquire a 100% legal and beneficial interest in and to the SafeCoat Shares owned by the SafeCoat Shareholders (collectively, with any additional SafeCoat Shares that may be issued to SafeCoat Shareholders or New SafeCoat Securityholders prior to the grant of the License, the (“Optioned Shares”); SafeCoat shareholders granted to ASEP, and ASEP accepted, the option to purchase the remaining whereby the SafeCoat Shareholders would sell and ASEP would purchase the Optioned Shares such that following the Exercise Date, ASEP would hold a 100% fully-diluted equity interest in SafeCoat.
On December 12, 2022, we acquired the remaining 49.9% SafeCoat shares by paying C$1,200,000 to the SafeCoat Shareholders through the allotment and issuance on a pro rata basis of an aggregate of 6,000,000 common shares of the Company at a deemed price of $0.20 per share.
The option exercise shares are subject to a voluntary escrow and released from escrow in tranches of 25% upon each of the following milestones being satisfied: (a) exercise of the Option, which has been completed, (b) upon SafeCoat and UBC entering into a definitive license agreement, (c) the date a patent in respect of the technology is published on google patents, which has been completed and (d) the date ASEP Medical confirms that SafeCoat has reasonably demonstrated activity of antifouling on surfaces.
On July 21, 2023, SafeCoat entered into the Start-Up License Agreement pursuant to which UBC and certain non-waiving inventors were granted a 12% equity interest in SafeCoat, with the right to purchase additional shares on a pro-rata basis in future financings of SafeCoat. See “Business – SafeCoat Medical Inc.” for more details regarding the Start-Up License Agreement.
Joint Venture and License Agreement dated May 15, 2023 among ASEP Medical Holdings Inc., Sepset Biosciences Inc., and Seaspring W.L.L.
On May 15, 2023, ASEP Medical Holdings Inc., Sepset Biosciences Inc. and Seaspring W.L.L. entered into a joint venture and license agreement for a term of 10 years, unless terminated earlier. Within 30 days of the execution of the license agreement, the parties agreed to form a joint venture entity in the Kingdom of Bahrain (the “Licensee”). Sepset has agreed, for the term, to grant to Licensee a sole, non-transferable, royalty-free license to and under the technology developed by Dr. Robert Hancock and certain co-inventors, referred to as: “Sepset ER” (the “Licensed Invention”), including the know-how related to the technology, and all intellectual property rights in Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Qatar, Saudi Arabia, State of Palestine, Sudan, Syrian Arab Republic, Tunisia, United Arab Emirates, and Yemen (the “Territory”) in the field of sepsis diagnostic testing in the Territory to: (i) commercialize and use a product, process or service that makes use of the Licensed Invention in any country in the Territory to predict the likelihood that patients will acquire severe sepsis, and includes any combination of either products or services sold together as a single product in the field of sepsis diagnostic testing or provided as a service in the sepsis diagnostic testing for a single invoiced price that includes a Licensed Product, and one or more additional components (whether products or services) that are not Licensed Products; (ii) copy, translate or add to any or all of the Licensed Invention, all know-how related to the Licensed Invention, and all intellectual property rights in the Territory of Sepset and its affiliates therein, including any patents and patent applications of Sepset or its affiliates that claim or cover the Licensed Invention and are filed in the Territory, including any provisionals, substitutions, divisions, continuations, continuations-in-part, reissues, renewals, registrations, confirmations, reexaminations, extensions, or supplementary protection certificates, anywhere in the Territory, that claim, cover or derive priority from, or involve the Licensed Invention for purposes of commercializing and using the product, process or service for sepsis diagnostics in the Territory; (iii) obtain regulatory approvals for the product, process or service for sepsis diagnostics in the Territory; and (iv) grant sublicenses of all rights set out in the license agreement on written terms and conditions in compliance with and not inconsistent with the terms and conditions set forth in the license agreement. A sublicense can only be granted with the prior written approval of Sepset.
Any improvements made the Licensee, Sepset, and joint improvements will be owned by Sepset. Any sublicensee improvements and any intellectual property rights associated therewith will be owned by the sublicensee and Sepset jointly, with Sepset having an irrevocable, royalty-free, world-wide right and license to make, use, sell, offer for sale, disclose and otherwise exploit the sublicensee’s improvements as Sepset sees fit (including through the granting of sublicenses) and without any duty to account for any royalties or other fees or consideration to sublicensee.
The license agreement may be terminated without cause by either Sepset or the Licensee with no less than twelve months’ prior written notice to the other, unless a shorter period has been agreed upon. In certain instances, Sepset may terminate the license agreement immediately by giving written notice to the Licensee. As of the date of the Licensee’s establishment, ASEP is to receive payment of a one-time license fee for the license granted herein by the issuance to ASEP of shares equal to 50% of the Licensee.
Start-Up License Agreement dated July 21, 2023 among SafeCoat Medical Ltd. and the University of British Columbia
On July 21, 2023, ASEP, through SafeCoat, entered into an exclusive license agreement with UBC for the use, development and commercialization of a medical device coating technology. Pursuant to the Start-Up License Agreement, 12% of the securities of SafeCoat will be owned by UBC and inventors of the technology. UBC and the inventors also have the right to purchase up to their then current pro-rata share of the securities issued in any future offering on the same terms and conditions as are offered to other purchasers in each such financing, up to July 21, 2027. The Start-Up License Agreement ends on the later of 20 years from the start date of the agreement, which would be on July 20, 2043, or the expiry of the last patent licensed under the agreement. In addition to the share consideration, SafeCoat will pay certain annual license fees and royalty payments to UBC, when and if they become payable pursuant to the terms and conditions of the Start-Up License Agreement.
As at the date of this Registration Statement, the consideration shares have not yet been issued and ASEP owns 100% of SafeCoat. The consideration shares will be issued when UBC gives its consent to the deemed issue price of shares to be issued to non waiving inventors.
Joint Venture Agreement with Sansure Biotech Fund, L.P.
On October 27, 2023, Sepset signed a definitive joint venture agreement (the “JV Agreement”) with Sansure Biotech Fund, L.P. (the “Sansure Fund”), a subsidiary of Sansure Biotech Inc. (“Sansure”). Sansure Fund is an investment fund formed by Sansure, Changsha Sanway Spring Venture Capital CO., Ltd. (“Sanway Spring”) and certain other investors. Formation of the joint venture and closing of the Transaction have subsequently received formal approval of the Chinese government.
Under the JV Agreement, the registered capital of the joint venture entity, Hunan Sanway SepSMART Ltd. (“SepSMART”), which will be based in Changsha, China, will be RMB 50,000,000 (the “Total Registered Capital”) with (a) the Sansure Fund subscribing for RMB 37,500,000.00 (~CAD$ 7 million) thereof, representing seventy-five percent (75%) of the Total Registered Capital and (b) Sepset deemed to be subscribing for RMB 12,500,000 (~CAD$ 2.4 million) thereof, representing twenty-five percent (25%) thereof through the contribution of certain patent rights to Sepset’s first generation rapid sepsis test, SepsetER, pursuant to a technology license and collaboration agreement concurrently entered into on October 27, 2023 between Sepset and SepSMART (the “License Agreement” and together with the JV Agreement, the “Transaction”). The License Agreement grants SepSMART the exclusive right to commercialize SepsetER in Mainland China, the Hong Kong Special Administrative Region, the Macao Special Administrative Region and the Taiwan area for a period of eight (8) years and requires SepSMART to pay periodic royalty payments to Sepset on a performance basis.
In connection with entry into the JV Agreement, ASEP, Sepset, Sanway Spring and the Sansure Fund entered into a side letter (the “Side Letter”) to provide certain additional representations, warranties, agreements and terms in relation to the joint venture. Pursuant to the Side Letter, ASEP and Sansure entered into a performance warrant issuance agreement whereby ASEP agreed to provide 3,000,000 performance warrants (the “Performance Warrants”) to Sansure or its designated nominees, subject to the required approvals and compliance with applicable securities laws and stock exchange policies. Each Performance Warrant is exercisable into one common share of ASEP at an exercise price of $1.00 per common share until the first anniversary of the date of issuance. The Performance Warrants and underlying common shares are subject to a hold period expiring four months and one day from the date of issuance.
Competition
The pharmaceutical and biotechnology industries are intensely competitive. We compete directly and indirectly with other pharmaceutical companies, biotechnology companies and academic and research organizations in developing therapies to treat diseases and antibiotic resistance. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. We compete with companies that have products on the market or in development for the same indications as our product candidates. We may also compete with organizations that are developing similar technology platforms. Specific competitors of ABT and Sepset are as follows:
| ABT’S COMPETITORS |
| Brand | | Stock Symbol | | Clinical Indications | | Differentiation from ABT | | Phase of Development |
| ContraFect (USA) | | CAPISTAN (CFRX: NASDAQ) | | Bacteremia, including endocarditis Prosthetic joint infections MRSA bacteremia in COVID-19 patients Hospital-acquired or ventilator-acquired bacterial pneumonia, cystic fibrosis- associated infections, complicated urinary tract infections, blood stream infections | | Developing protein-based lysins that dissolve cell walls of bacteria, largely for topical treatment. Uses different technology to ABT but targeting similar infections, though not biofilms or inflammation. | | Phase III Phase I Phase I Lead Optimization |
| PolyPhor (Switzer-land) | | AG (POLN: SWX) | | Pseudomonas aeruginosa infections in CF | | Developing multiple antibiotic and cancer targeted drugs, including a peptide-like antimicrobial drug and an outer membrane targeting anti- bacterial drug both of which are pre-clinical. Does not target biofilms or inflammation. | | Pre-Clinical |
| Peptilogics (USA) | | - | | Prosthetic joint infections | | Has an engineered, Cationic Antibiotic Peptide in Phase I/II. No evidence Peptides work against biofilms or have anti-inflammatory activity, at least not mentioned on the company website. | | Phase II |
| Omnix Medical (Israel) | | - | | Hospital-acquired or Ventilator-acquired bacterial pneumoniae | | Developing antimicrobial peptides. Preclinical. No evidence peptides work against biofilms or have anti- inflammatory activity, at least not mentioned on the company website | | Pre-Clinical |
| SEPSET’S COMPETITORS |
| Brand | | Stock Symbol | | Clinical Indications | | Differentiation from Sepset | | Phase of Development |
| ImmunExpress/ Septicyte® (USA) | | - | | Distinguish between Sepsis and non- infections systemic inflammation | | Uses a different patented gene expression biomarker set. No published evidence it is directed towards emergency room patients. In Sepset studies these biomarkers do not perform as well as the Sepset biomarkers. | | Approved (Septicyte) |
| Molzym (Germany) | | - | | Detection and identification of a broad range of Gram- positive bacteria, Gram-negative bacteria and fungi within a working day | | Uses 16S and 18S ribosomal RNA sequencing to define underlying pathogens causing Sepsis. Not all patients with Sepsis have an identifiable pathogen associated with Sepsis development and this test takes a day so is significantly slowerthan Sepset’s test. Also the presence of a pathogen does not per se reveal sepsis as non-septic infections occur. | | Approved in EU (SeptiTest) |
| Abbott Laboratories (USA) | | (ABT: NYSE) | | Identify infection- causing pathogens directly from a patient’s sample, without the need for culture | | Uses mass tag PCR to identify underlying pathogens causing Sepsis. Not all patients with Sepsis have an identifiable pathogen associated with Sepsis development and this test takes at least 6 hours so is significantly slower than Sepset’s test. | | Approved (IRIDICA) |
| Roche Holding AG | | (RO: SWX) (RHHBY: OTC) | | Detection of 25 common blood pathogens considerably faster than conventional blood culture | | Uses multiplex PCR to identify 25 of the more common underlying pathogens causing Sepsis. Not all patients with Sepsis have an identifiable pathogen associated with Sepsis development and many do not have the pathogens revealed by this test. Also, the presence of a pathogen does not per se reveal sepsis as non-septic infections occur. | | Approved (SeptiFast) |
| Seegene Inc. (South Korea) | | (096530: KOSDAQ) | | Detection and identification of more than 90 Sepsis-causing pathogens | | Uses multiplex PCR to identify 25 of the more common underlying pathogens causing Sepsis. Not all patients with Sepsis have an identifiable pathogen associated with Sepsis development and many do not have the pathogens revealed by this test. Also, the presence of a pathogen does not per se reveal sepsis as non-septic infections occur. | | Approved in EU (MagicPlex Sepsis) |
| Biomerieux (France) | | (BMXMF: OTC) | | Tests for a variety of pathogens that cause viral respiratory, pneumonia, bloodstream, and gastrointestinal infections and meningitis- encephalitis as well as antimicrobial resistance genes | | Uses a culture methodology. Not all patients with Sepsis have an identifiable pathogen associated with Sepsis development and this test takes at least 24 hours so is significantly slower than Sepset’s test. Also, the presence of a pathogen does not per se reveal sepsis as non-septic infections occur. | | Approved |
Cytovale Intellisep® (US) | | - | | Categorizes patients into three bands according to their probability of sepsis based on a standard blood draw and analysis of blood cell morphology | | | | Approved |
MANAGEMENT
The following table sets forth the name, age and position of each of our directors and executive officers as of the date of this prospectus. The address for our directors and executive officers is c/o ASEP Medical Holdings Inc., 420 – 730 View Street Victoria, BC V8W 3Y7 Canada.
| Name | | Age | | Position |
| Dr. Robert E.W. Hancock | | 74 | | Chief Executive Officer, Executive Chair, Director |
| Jacqueline Tucker | | 66 | | Chief Financial Officer |
| Timothy Murphy | | 52 | | Chief Operating Officer, Corporate Secretary, Director |
| Derrold Norgaard | | 62 | | Director |
| Dr. Richard Heinzl | | 60 | | Director |
| Dr. Fadia Saad | | 60 | | Chief Business Development Officer |
| Dr. Evan Haney | | 40 | | Chief Science Officer |
Dr. Robert E.W. Hancock has served as our Chief Executive Officer since June 30, 2022. He has served as a director since November 9, 2021. Dr. Hancock has been a faculty member at UBC since 1978 where he runs a large Research & Development laboratory. He is a co-founder of several companies, both private and public, including Migenix Inc., Inimex Pharmaceuticals Inc., ABT Innovations Inc., Sepset Biosciences Inc. and the Centre for Drug Research and Development and is the Canada Research Chair (Tier 1) in microbiology. From 2021 to January 2023, Dr. Hancock served as a director of Core One Labs Inc., a life sciences biotechnology company listed on the Canadian Securities Exchange (the “CSE”) and the OTCQB and, since 2020, has been the CEO of Vocan Biotechnologies Inc. He holds 72 patents and is an Officer of the Order of Canada. Dr. Hancock received his BSc, with First Class Honors, and PhD in microbiology from the University of Adelaide. We believe that Dr. Hancock is qualified to serve as our Chief Executive Officer and a member of our board of directors because of his extensive medical background and knowledge of the company.
Jacqueline Tucker has served as our Chief Financial Officer (“CFO”) since October 1, 2022. Ms. Tucker is a Fellow of the Chartered Professional Accountants of British Columbia. Ms. Tucker is the president of J. M. Tucker Professional Corporation, which provides CFO advisory services to public companies. Established in 1995, J. M. Tucker Professional Corporation was initially a Vancouver based chartered accounting firm whose primary business was focused on providing audit and related services to various development stage and emerging enterprises in the resource, bio-medical and high-tech sectors. Upon Ms. Tucker’s relocation to Calgary, Alberta late in 2005, the firm realigned its business to provide advisory services to Canadian public companies on financial reporting and disclosure issues and compliance with securities legislation. Ms. Tucker is a member of the Institute of Chartered Professional Accountants of Alberta and the Chartered Professional Accountants of British Columbia. Ms. Tucker has an outstanding track record of financial leadership, having held senior positions at many leading Canadian corporations. Currently, she is the CFO of East Africa Metal Inc. (May 2019 to present) and True North Gems Inc. (July 2017 to present). Previously, she was CFO and Director at Canamax Energy Ltd. (October 1998 to April 2011), CEO and Director at Canadian Imperial Venture Corp. (October 2018 to April 2021) and CFO and Director at IGC Resources, Inc. (August 15, 2015 to January 2019). She received an undergraduate degree from the University of Western Ontario. We believe that Ms. Tucker is qualified to serve as our Chief Financial Officer because of her background in accounting and extensive experience serving as a CFO of public companies.
Timothy Murphy has served as the Chief Operating Officer since June 30, 2022 and as a director since November 9, 2021. Mr. Murphy is an experienced business executive and lawyer. Mr. Murphy sits on numerous boards as both a Director and Advisory Director and also has experience as a CEO. Since January 1, 2011, he has been the Founding Partner of Coal Harbour Law (formerly Murphy & Company LLP), a business law firm based in Vancouver, British Columbia. Between January 1, 2018 and April 30, 2021, Mr. Murphy acted as a board member and officer with the Rival Group Inc. From October 1, 2018 to August 31, 2019, Mr. Murphy acted as CEO of Casting Workbook Global Ltd. From October 1, 2019 and October 1, 2020, Mr. Murphy served as a Director with Geyser Brands Inc., a health and wellness company listed on the NEX board of the TSX Venture Exchange. Since August 1, 2018, he has served as a Director with the Angus Reid Institute. Mr. Murphy holds a bachelor of laws (LL.B) degree from the University of Saskatchewan, master of laws (LL.M) degree from McGill University and is a member of the law societies of British Columbia and England & Wales. We believe that Mr. Murphy is qualified to serve as our Chief Operating Officer, corporate secretary and a member of our board of directors because of his extensive legal experience, board and prior chief executive officer experience.
Derrold Norgaard has served as a director since November 9, 2021. Mr. Norgaard is a Fellow of the Chartered Professional Accountants of British Columbia. Mr. Norgaard worked for Peat Markwick Mitchell (which later became known as KPMG LLP) in Vancouver, BC from September of 1984 to September 1996. In September of 1996, he transferred to KPMG LLP’s Victoria, BC office and took up a role as Tax Partner and Office Managing Partner, in later years, with KPMG’s firm there. In May of 2008, Mr. Norgaard left KPMG LLP and began private equity investing in Western Canada, including making investments in selected health care and skin care companies. In April of 2011, he started Norgaard Kratofil Professional Group, a registered CPA accountancy firm, that specializes in tax and accounting services for the mid-market sector of clients in Victoria, BC. He has acted as an investor, director and board member of a number of private and public companies. Mr. Norgaard received his BBA in Business Administration, Management and Operations from Simon Fraser University. We believe that Mr. Norgaard is qualified to serve as a member of our board of directors because of his experience in accounting and corporate taxation.
Dr. Richard Heinzl has served as a director since September 29, 2022. Dr. Heinzl is a physician, humanitarian, entrepreneur and author whose current focus is genomics, artificial intelligence and healthcare worldwide. Based in New York and Toronto, he has served as the CEO of My Next Health Inc., a next-generation functional genomics company, since 2021. Dr. Heinzl was the founder of the Canadian chapter of Médecins Sans Frontières/Doctors Without Borders (MSF Canada), which won the Nobel Peace Prize in 1999. From 2015 to 2021, he was Global Medical Director for WorldCare Inc., a Boston-based, Harvard-affiliated virtual medicine company. He graduated from McMaster University’s Michael G. DeGroote School of Medicine and completed postgraduate degrees related to global health at Harvard University and the University of Oxford. He is an Emeritus Fellow of the American College of Preventive Medicine. His work and travels have taken him to over 80 countries, and he speaks widely in North America and abroad. In 2000 he received an Honorary Doctorate (LLD) from his alma mater McMaster University and was named one of the “Hundred People Who Make a Difference” in Canada by Penguin Books. In September 2016, he received the Harvard T.H. Chan School of Public Health Alumni Award of Merit, the School’s highest award. We believe that Dr. Heinzl is qualified to serve as a member of our board of directors because of his experience in the medical field.
Dr. Fadia Saad has served as Chief Business Development Officer since November 9, 2021. Dr. Saad, PhD, MBA, previously worked as Head of Business Development at Aspreva Pharmaceuticals (2003-2007), Chief Business Development Officer at Green Sky Labs (2014-2017), President at Pebble Labs (2017-2018) and CEO & Founder, ArthroSolutions, Victoria, BC (2017-present). She also has consulted for a number of biotechnology and pharmaceutical companies (e.g., Eupraxia Pharmaceuticals (2021-2023) and Psilobrain Therapeutics (2021-2022)). Dr. Saad has a PhD in Microbiology from McGill University and an MBA from l’École des Hautes Études Commerciales. We believe that Dr. Saad is qualified to serve as our Chief Business Development Officer because of Dr. Saad’s experiences in consulting and previous positions as head of business development.
Dr. Evan Haney has served as Chief Science Officer since November 9, 2021. Prior to this, Dr. Haney was a Post Doctoral Fellow (2012-2017) and a Research Associate (2017-2021) in Dr. Hancock’s academic research lab at the University of British Columbia. Dr. Haney is a scientist and a research coordinator. He is an inventor on the peptide patents that are core to ABT’s therapeutic technology. Dr. Haney holds a PhD in Biochemistry from the University of Calgary. We believe that Dr. Haney is qualified to serve as our Chief Science Officer because of Dr. Haney’s experience as an inventor on core patents to ABT’s therapeutic technology.
2021 Stock Option Plan
ASEP adopted a stock option plan on July 21, 2021 (the “Plan”) to attract and retain directors, officers, employees and consultants of the Company and to motivate them to advance the interest of the Company by affording them with the opportunity to acquire an equity interest in the Company through the grant of stock options under the Plan. The below is a summary of the Plan’s terms. As of the date of this prospectus, 5,820,000 awards have been granted under the Plan.
Shares Subject to the Equity Incentive Plan
The Plan provides that the number of Shares available for issuance is subject to the restrictions imposed under applicable securities laws or CSE policies and, in any case, shall not exceed 10% of the total number of issued Shares (calculated on a non-diluted basis) at the time any stock option is granted. As of December 31, 2022, there were 5,820,000 options outstanding under the Plan.
Administration of the Equity Incentive Plan
The Plan is administered by the Board. The Board will have full and final authority with respect to the granting of all options thereunder.
Participation
Stock Options may be granted under the Plan to such directors, officers, employees, or consultants of the Company and its affiliates, if any, as the Board may from time to time designate.
Types of Awards
Awards that may be granted under the plan include: (a) incentive stock options, (b) non-qualified stock options, (c) stock appreciation rights, (d) restricted awards, (e) performance share awards, (f) cash awards, and (g) other equity-based awards.
2023 Long Term Incentive Plan
The Board adopted a long term incentive plan (the “LTIP”) on January 11, 2023, and it is subject to shareholder approval. The purpose of the LTIP is to align the interests of those directors, employees and consultants designated by the Board as being eligible to participate in the LTIP with those of the Company and its shareholders and to assist in attracting, retaining and motivating key employees by making a portion of the incentive compensation of participating employees directly dependent upon the achievement of key strategic, financial and operational objectives that are critical to ongoing growth and increasing the long-term value of the Company. In particular, the LTIP is designed to promote the long-term success of the Company and the creation of shareholder value by: (a) encouraging the attraction and retention of directors, key employees and consultants of the Company and its subsidiaries; (b) encouraging such directors, key employees and consultants to focus on critical long-term objectives; and (c) promoting greater alignment of the interests of such directors, key employees and consultants with the interests of the Company. The LTIP remains subject to approval of the shareholders of the Company at the next annual meeting.
Shares Subject to the Equity Incentive Plan
The Plan provides that the number of Shares available for issuance is subject to the restrictions imposed under applicable securities laws or CSE policies, subject to shareholder approval, and, in any case, shall not exceed 10% of the total number of issued Shares (calculated on a non-diluted basis) at the time any stock option is granted.
Administration of the Equity Incentive Plan
The Plan is administered by the Board. The Board will have full and final authority with respect to the granting of all options thereunder.
Participation
Awards may be granted under the Plan to such directors, officers, employees, or consultants of the Company and its affiliates, if any, as the Board may from time to time designate.
Types of Awards
Awards that may be granted under the plan include: (a) restricted share units, (b) performance share units, and (c) deferred share units.
Executive Compensation of Directors and Officers
The following table sets forth information regarding the compensation paid to our directors and our executive officers during the years ended December 31, 2022 and December 31, 2023.
| | | | | | CAD$ | | | | | | | |
| | | | | | Short Term Employment Benefits | | | Post-Employment Benefits | | | Long Term Benefits | | | Share Based Payments | | | CAD$ | |
| Directors | | Year | | | Salary & Fees | | | Cash Bonus | | | Non- monetary benefits | | | Super- annuation | | | Retirement Benefits | | | Cash Incentives | | | Long Service Leave | | | Options/ Warrants | | | Shares | | | Termination payments | | | Total | |
| Richard Heinzl | | | 2023 | | | | 72,000, | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 61,000 | | | | - | | | | 133,000 | |
| | | | 2022 | | | | 24,000 | (1) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 33,971 | | | | - | | | | - | | | | 57,971 | |
| Derrold Norgaard | | | 2023 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 73,500 | | | | - | | | | 73,500 | |
| | | | 2022 | | | | -l | (2) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Robert Hancock | | | 2023 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | - | | | | - | | | | - | | | | - | |
| | | | 2022 | | | | - | (3) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | | | | | - | | | | - | |
| Timothy Murphy | | | 2023 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | 2022 | | | | -l | (4) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | | | | | - | | | | - | |
| Total Directors (4 persons) | | | 2023 | | | | 72,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 134,500 | | | | - | | | | 206,500 | |
| Total Directors (4 persons) | | | 2022 | | | | 24,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 33,971 | | | | - | | | | - | | | | 57,971 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Officers | | | Year | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Robert Hancock, CEO | | | 2023 | | | | 180,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 97,304 | | | | 128,625 | | | | - | | | | 405,929 | |
| | | | 2022 | | | | 45,000 | (3) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Jacqueline Tucker, CFO | | | 2023 | | | | 90,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 91,875 | | | | | | | | 181,875 | |
| | | | 2022 | | | | 22,500 | (5) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 27,466 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Timothy Murphy, COO | | | 2023 | | | | 120,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 91,875 | | | | | | | | 211,875 | |
| | | | 2022 | | | | 60,000 | (4) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 35,173 | | | | - | | | | - | | | | - | |
| Evan Haney, CSO | | | 2023 | | | | 73,500 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 53,125 | | | | - | | | | 126,625 | |
| | | | 2022 | | | | 72,041 | (6) | | | 5,201 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fadia Saad, CBO | | | 2023 | | | | 180,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 91,875 | | | | - | | | | 271,875 | |
| | | | 2022 | | | | 180,000 | (7) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Rudy Mazzocchi, Former CEO | | | 2023 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | - | | | | - | | | | - | | | | - | |
| | | | 2022 | | | | 140,000 | (8) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Jennifer Gretchen, Former CFO | | | 2023 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | 2022 | | | | 112,500 | (9) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Total Officers (5 persons) | | | 2023 | | | | 643,500 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 97,304 | | | | 457,375 | | | | - | | | | 1,198,179 | |
| Total Officers (7 persons) | | | 2022 | | | | 632,041 | | | | 5,201 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 62,639 | | | | - | | | | - | | | | 700,181 | |
| Totals | | | 2023 | | | | 715,500 | | | | - | | | | - | | | | - | | | | - | | | | , | | | | , | | | | 97,304 | | | | 591,875 | | | | - | | | | 1,404,679 | |
| Totals | | | 2022 | | | | 656,041 | | | | 5,201 | | | | - | | | | - | | | | - | | | | | | | | | | | | 96,610 | | | | - | | | | - | | | | 758,152 | |
(1) Mr. Heinzl was appointed as director of the Company on September 1, 2022.
(2) Mr. Norgaard was appointed as director of the Company on November 9, 2021.
(3) Dr. Hancock was appointed as director of the Company on November 9, 2021 and as the chief executive officer of the Company on June 30, 2022.
(4) Mr. Murphy was appointed as director of the Company on November 9, 2021 and as the corporate secretary of the Company on October 1, 2022.
(5) Ms. Tucker was appointed as the chief financial officer of the Company on October 1, 2022.
(6) Mr. Haney was appointed as the chief scientific officer of the Company on November 9, 2021.
(7) Ms. Saad was appointed as the chief business development officer of the Company on November 9, 2021. Pursuant to the Saad Employment Agreement, Ms. Saad received an annual fee of CAD $180,000.
(8) Mr. Mazzocchi was appointed as the chief executive officer of the Company on November 9, 2021 and resigned in June 2022.
(9) Ms. Gretchen was appointed as the chief financial officer of the Company on November 9, 2021 and resigned on September 30, 2022.
Dr. Hancock has agreed to defer his salary of CAD$180,000 per year, beginning October 1, 2022, until the Company completes a financing of at least CAD$2,000,000.
Employment Agreements
We have entered into employment agreements with certain of our executive officers and directors. We have entered into employment agreements with (i) Dr. Robert E.W. Hancock, our Chief Executive Officer and Executive Chair of the Board of Directors; (ii) Dr. Fadia Saad, our Chief Business Development Officer; and (iii) Dr. Evan Haney, our Chief Science Officer. For details of their employment agreements see “Business — Material Contracts”, starting on page 60.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information regarding the outstanding equity awards for each of our named executive officers for the year ended December 31, 2023.
| | | Option awards | | | Stock awards | |
| Name | | Number of securities underlying unexercised options (#) exercisable | | | Number of securities underlying unexercised options (#) unexercisable | | | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | | | Option exercise price ($) | | | Option expiration date | | Number of shares or units of stock that have not vested (#) | | | Market value of shares of units of stock that have not vested ($) | | | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) | | | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | |
| Robert Hancock, CEO | | | 900,000 | | | | - | | | | - | | | $ | 0.50 | | | 18-Nov-31 | | | - | | | | - | | | | - | | | | - | |
| | | | 300,000 | | | | - | | | | - | | | $ | 0.36 | | | 19-Jan-33 | | | - | | | | - | | | | | | | | | |
| Timothy Murphy, COO | | | 300,000 | | | | - | | | | - | | | $ | 0.50 | | | 18-Nov-31 | | | - | | | | - | | | | - | | | | - | |
| | | | 200,000 | | | | - | | | | - | | | $ | 0.30 | | | 24-Nov-32 | | | - | | | | - | | | | | | | | | |
| Jacqueline Tucker, CFO | | | 20,000 | | | | | | | | - | | | $ | 0.50 | | | 18-Nov-31 | | | - | | | | - | | | | - | | | | - | |
| | | | 200,000 | | | | 100,000 | | | | - | | | $ | 0.30 | | | 01-Oct-32 | | | 100,000 | | | $ | 21,000 | | | | - | | | | - | |
| Fadia Saad, CBO | | | 400,000 | | | | - | | | | - | | | $ | 0.50 | | | 18-Nov-31 | | | - | | | | - | | | | - | | | | - | |
| Evan Haney, CSO | | | 300,000 | | | | - | | | | - | | | $ | 0.50 | | | 18-Nov-31 | | | - | | | | - | | | | - | | | | - | |
Family Relationships
There are no family relationships among our officers or directors as defined in Item 401 of Regulation S-K.
Involvement in Certain Legal Proceedings
To the Company’s knowledge, none of our directors or executive officers has, during the past 10 years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Board of Directors and Board Committees
Corporate Governance
We are incorporated under the laws of the Province of British Columbia. Our governing documents consist of our Notice of Articles, our Articles, our Incorporation Agreement, and our Certificate of Incorporation.
We qualify as a “foreign private issuer” as defined in Section 405 of the Securities Act. As a foreign private issuer, we are exempt from certain rules under the Exchange Act that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, the members of our board of directors and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the Exchange Act. They will, however, be subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules, to the extent applicable.
The foreign private issuer exemption will also permit us to follow home country corporate governance practices or requirements instead of certain Nasdaq listing requirements, including the following:
| ● | Quorum Requirement —Nasdaq Listing Rule 5620(c) provides that the minimum quorum requirement for a meeting of shareholders is 331/3% of the outstanding common voting shares. We do not follow this Nasdaq Listing Rule. Instead, we follow our articles which provide that the quorum for the transaction of business at a meeting of shareholders is one or more persons, present in person or by proxy. |
| ● | Shareholder Approval Requirements —In certain instances, Nasdaq Listing Rule 5635 requires each issuer to obtain shareholder approval before an issuance of securities in connection with: (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; and (iii) transactions other than public offerings. We do not follow this Nasdaq Listing Rule. Instead, we comply with the laws, rules and regulations of Canada and the Province of British Columbia and the policies of the TSX Venture Exchange, which have different requirements for shareholder approval (including, in certain instances, not requiring any shareholder approval) in connection with issuances of securities in the circumstances listed above. |
Board Composition and Election of Directors
Our board of directors currently consists of four (4) directors. A retiring director remains in office until the relevant shareholder meeting and will be eligible for re-election at that meeting.
Meetings of Directors
Our board of directors may meet together for the conduct of business, adjourn and otherwise regulate their meetings as they think fit, and meetings of the board held at regular intervals may be held at the place and at the time that the board may by resolution from time to time determine.
Remuneration
Our directors are entitled to the remuneration, if any, for acting as directors as the directors may from time to time determine. If the directors so decide, the remuneration of the directors will be determined by the shareholders. That remuneration may be in addition to any salary or other remuneration paid to a director in such director’s capacity as an officer or employee of the Company.
Board Committees
Our directors may, by resolution, appoint committees consisting of the directors they consider appropriate.
Audit Committee
The members of our Audit Committee are Timothy Murphy, Richard Heinzl, and Derrold Norgaard. Our board of directors has determined that it will satisfy the independence requirements under Nasdaq listing standards and Rule 10A-3(b)(1) of the Exchange Act. Derrold Norgaard is the chairperson of our Audit Committee. Our board of directors has determined that Derrold Norgaard is an “audit committee financial expert” within the meaning of SEC regulations. Each member of our Audit Committee can read and understand fundamental financial statements in accordance with applicable requirements. In arriving at these determinations, our board of directors has examined each member’s scope of experience and the nature of his or her employment.
The Audit Committee’s duties are specified in our Audit Committee Charter, and include, but are not limited to:
| | ● | appointing the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| | ● | reviewing with the independent auditors any audit problems or difficulties and management’s response; |
| | ● | discussing the annual audited financial statements with management and the independent auditors; and |
| | ● | reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any steps taken to monitor and control major financial risk exposures. |
Compensation Committee
Currently, the Company does not have a compensation committee or a formal compensation policy. The Company relies solely on the directors to determine the compensation of any named executive officers. In determining compensation, the directors will consider industry standards and the Company’s financial situation, but the Company will not have any formal objectives or criteria. The performance of each executive officer will be informally monitored by the directors, having in mind the business strengths of the individual and the purpose of originally appointing the individual as an officer.
In establishing compensation for executive officers, the Board as a whole seeks to accomplish the following goals:
| | ● | to recruit and subsequently retain highly qualified executive officers by competitive offering overall compensation; |
| | ● | to motivate executives to achieve important corporate and personal performance objectives and reward them when such objectives are met; and |
| | ● | to align the interests of executive officers with the long-term interests of Shareholders through participation in the Plan. |
When considering the appropriate executive compensation to be paid to our officers, the Board will have regard to a number of factors including: (i) recruiting and retaining executives critical to the success of the Company and the enhancement of shareholder value; (ii) providing fair and competitive compensation; (iii) balancing the interests of management and the Shareholders; (iv) rewarding performance, both on an individual basis and with respect to operations generally; and (v) available financial resources.
Nominating Committee
Currently, the Board as a whole will be responsible for annually identifying and recommending to the Board an annual slate of nominees for membership on the Board. In recommending the annual slate of nominees, the Board will identify and screen individuals to determine potential candidates, taking into account the number of directors required to carry out the Board’s duties effectively and to maintain a diversity of views and experience.
Code of Conduct
Currently, the Company has not adopted a Code of Conduct applicable to all of our directors, officers and employees.
Interests of Directors
A director who has a material interest in a matter before our board of directors or any committee on which he or she serves is required to disclose such interest as soon as the director becomes aware of it. In situations where a director has a material interest in a matter to be considered by our board of directors or any committee on which he or she serves, such director may be required to excuse himself or herself from the meeting while discussions and voting with respect to the matter are taking place.
RELATED PARTY TRANSACTIONS
The aggregate value of transactions and outstanding balances relating to key management personnel and entities over which they have control or significant influence were as follows:
| | | Year ended December 31, 2022 | |
| Consulting fees (a) | | $ | 267,500 | |
| Directors fees (b) | | | 24,000 | |
| Management salaries (c) | | | 369,742 | |
| Share-based compensation (d) | | | 1,051,798 | |
| | | $ | 1,713,040 | |
| Share-based compensation expense | | $ | 1,051,798 | |
| | (a) | Consulting fees consist of the following: |
| | | |
| | | $140,000 paid to Rudy Mazzocchi, the Company’s former Chief Executive Officer and former director. |
| | | |
| | | $60,000 paid to Timothy Murphy, the Company’s current Chief Operating Officer and director. |
| | | |
| | | $18,000 paid and $4,500 accrued to Jacqueline M. Tucker, the Company’s current Chief Financial Officer. |
| | (b) | Directors fees consist of the following: |
| | | |
| | | $24,000 paid to Richard Heinzl, a director as part of the Board Services Agreement. |
| | (c) | Management salaries consist of the following: |
| | | |
| | | $45,000 accrued to Dr. Robet Hancock, the Company’s current Chief Executive Officer and director. |
| | | |
| | | $112,500 paid to Jennifer Gretchen, the Company’s former Chief Financial Officer for the period January 1, 2022–October 1, 2022. |
| | | |
| | | $180,000 paid to Dr. Fadia Saad, the current Chief Business Development Officer of the Company. |
| | | |
| | | $77,242 paid to Dr. Evan Haney, the current Chief Scientific Officer of the Company. |
| | (d) | Share-based compensation consists of the following: |
| | | |
| | | During the period ended December 31, 2022, the Company had recognized $1,051,798 of share-based compensation. |
| | | Year ended December 31, 2023 | |
| Consulting fees (a) | | $ | 210,000 | |
| Directors fees (b) | | | 72,000 | |
| Management salaries (c) | | | 433,500 | |
| Share-based compensation (d) | | | 1,090,133 | |
| | | $ | 1,805,633 | |
| Share-based compensation expense | | $ | 1,090,133 | |
| | (a) | Consulting fees consist of the following: |
| | | |
| | | $110,000 paid and $10,000 accrued to the Company’s current Chief Operating Officer and director. |
| | | |
| | | $82,500 paid and $7,500 accrued to the Company’s current Chief Financial Officer. |
| | (b) | Directors fees consist of the following: |
| | | |
| | | $66,000 paid and $6,000 accrued to a director as part of the Board Services Agreement. |
| | (c) | Management salaries consist of the following: |
| | | |
| | | $180,000 paid to the current Chief Business Development Officer of the Company. |
| | | |
| | | $73,500 paid to the current Chief Scientific Officer of the Company. |
| | | |
| | | $180,000 accrued for the current CEO of the Company. |
| | (d) | Share-based compensation consists of the following: |
| | | |
| | | During the year ended December 31, 2023, the Company had recognized $1,090,133 of share-based compensation. |
SECURITY OWNERSHIP OF BENEFICIAL OWNERS AND MANAGEMENT
The following tables set forth certain information with respect to the beneficial ownership of our common shares and as adjusted to reflect the sale of the common shares offered by us in our initial public offering, for:
| | ● | each shareholder known by us to be the beneficial owner of more than 5% of our outstanding common shares, |
| | ● | each of our named executive officers, and |
| | ● | all of our directors and executive officers as a group. |
We have determined beneficial ownership in accordance with the rules of the SEC. Under such rules, beneficial ownership includes any common shares over which the individual has sole or shared voting power or investment power as well as any common shares that the individual has the right to subscribe for within 60 days of March 1, 2023, through the exercise of any warrants or other rights. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power or the power to receive the economic benefit with respect to all common shares that they beneficially own, subject to applicable community property laws. None of the shareholders listed in the table are a broker-dealer or an affiliate of a broker dealer.
Applicable percentage ownership prior to the offering is based on 76,059,147 common shares outstanding at May 28, 2024. The table also lists the percentage ownership after this offering based on 74,184,087 common shares outstanding immediately after the completion of this offering, assuming no exercise of the underwriter’s option to purchase additional common shares from us in this offering. For purposes of the table below, we deem common shares issuable pursuant to options and warrants that are currently exercisable or exercisable within 60 days of May 28, 2024 to be outstanding and to be beneficially owned by the person holding the options for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o ASEP Medical Holdings Inc., 420 – 730 View Street Victoria, BC V8W 3Y7 Canada.
| | | Beneficial Ownership Prior to Offering | | | Beneficial Ownership After Offering | |
| Name of Beneficial Owner | | Common shares | | | Percentage | | | Common shares | | | Percentage | |
| 5% Shareholders | | | | | | | | | | | | | | | | |
| Michael Graw (1) | | | 7,006,500 | | | | 6.85 | % | | | | | | | | |
| 2394997 Ontario Inc | | | 4,898,000 | | | | 6.44 | % | | | | | | | | |
| Tom English (2) | | | 8,768,692 | | | | 6.52 | % | | | | | | | | |
| Officers and Directors | | | | | | | | | | | | | | | | |
| Dr. Robert E.W. Hancock, Chief Executive Officer (3) | | | 1,600,000 | | | | .92 | % | | | | | | | | |
| Timothy Murphy, Chief Operating Officer and Director (4) | | | 915,085 | | | | .55 | % | | | | | | | | |
| Jacqueline Tucker, Chief Financial Officer (5) | | | 830,000 | | | | .80 | % | | | | | | | | |
| Derrold Norgaard, Director (6) | | | 1,143,732 | | | | 1.11 | % | | | | | | | | |
| Dr. Richard Heinzl, Director (7) | | | 600,000 | | | | .53 | % | | | | | | | | |
| Dr. Fadia Saad, Chief Business Development Officer (8) | | | 992,910 | | | | .78 | % | | | | | | | | |
| Dr. Evan Haney, Chief Science Officer (9) | | | 800,000 | | | | .66 | % | | | | | | | | |
| All officers and directors as a group | | | 6,881,727 | | | | 5.33 | % | | | | | | | | |
| 5% or greater beneficial owners | | | 20,673,192 | | | | 19.80 | % | | | | | | | | |
* Represents beneficial ownership of less than one percent (1%) of the outstanding common shares.
Notes:
| (1) | Includes 5,206,500 common shares, 1,200,000 warrants exercisable as of December 27, 2023 for $0.26 per share, 600,000 options to purchase common shares, of which 400,000 options were exercisable as of November 18, 2021 for $0.50 per share and 200,000 options were exercisable as of November 24, 2022 for $0.30 per share. |
| (2) | Includes 4,957,762 common shares, 1,000,000 warrants exercisable as of December 19, 2023 for $0.26 per share and 2,410,930 warrants exercisable as of December 27, 2023 at $0.26 per share and 400,000 options exercisable as of November 18, 2021 for $0.50 per. |
| (3) | Includes 700,000 common shares, 900,000 options to purchase common shares, granted on and exercisable as of November 18, 2021 for $0.50 per share and 300,000 options to purchase common shares, granted on and exercisable as of January 19, 2023 for $0.36 per share. |
| (4) | Includes 415,085 common shares, 300,000 options to purchase common shares granted on and exercisable as of November 18, 2021 for $0.50 per share, and 200,000 options to purchase common shares granted on and exercisable as of November 24, 2022 for $0.30 per. |
| (5) | Includes 605,000 common shares, 105,000 warrants exercisable as of December 27, 2023 for $0.26 per share, 20,000 options to purchase common shares granted on and exercisable as of November 18, 2021 for $0.50 per share, and 150,000 options to purchase common shares granted on October 1, 2022 and exercisable as of May 28, 2024 for $0.30 per share. |
| (6) | Includes 843,732 common shares and 300,000 options to purchase common shares granted on and exercisable as of November 18, 2021 for $0.50 per share. |
| (7) | Includes 400,000 common shares and 200,000 options to purchase common shares granted on and exercisable as of September 29, 2022 for $0.30 per share. |
| (8) | Includes 592,910 common shares and 400,000 options to purchase common shares, granted on November 18, 2021 exercisable for $0.50 per share. |
| (9) | Includes 500,000 common shares and 300,000 options to purchase common shares granted on November 18, 2021 exercisable for $0.50 per share. |
As of May 28, 2024 , there were 148 holders of record entered in our share register. The number of individual holders of record is based exclusively upon our share register and does not address whether common shares may be held by the holder of record on behalf of more than one person or institution who may be deemed to be the beneficial owner of common shares in our company.
To our knowledge, other than as set forth above, no other shareholder beneficially owns more than 5% of our common shares. Our company is not owned or controlled directly or indirectly by any government or by any corporation or by any other natural or legal person severally or jointly. Our major shareholders do not have any special voting rights.
DESCRIPTION OF SECURITIES
Description of Share Capital
The Company is authorized to issue an unlimited number of common shares without par value. As of May 24, 2024, we had 76,059,147 issued and outstanding common shares.
The following description of our common shares, Pre-Funded Warrants and accompanying common warrants and provisions of our articles are summaries of material terms and provisions and are qualified by reference to our articles, a copy of which has been filed with the SEC as an exhibit to the registration statement of which this prospectus is a part.
Common Shares
The holders of our common shares who are present and entitled to vote at a meeting are entitled to one vote. Subject to the rights of shareholders, if any, holding shares with special rights as to dividends, all dividends on shares of any class or series of shares must be declared and paid according to the number of shares held.
Common warrants being offered in this offering
The following summary of certain terms and provisions of the common warrants (the “Warrants”) offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of the Warrant, which is filed as Exhibit 4.3 to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of the Warrant.
Exercisability. The Warrants are exercisable at any time after their original issuance up to five years from the original issuance date. Each of the Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full in immediately available funds for the number of our common shares subscribed for upon such exercise (except in the case of a cashless exercise as discussed below). If a registration statement registering the issuance of the shares of our common shares underlying the Warrants under the Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the Warrants through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of our common shares determined according to the formula set forth in the Warrants. No fractional shares will be issued in connection with the exercise of a Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Exercise Limitation. A holder will not have the right to exercise any portion of the Warrants if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or, upon election by a holder prior to the issuance of any warrants, 9.99%) of the number of common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, upon at least 61 days’ prior notice from the holder to us with respect to any increase in such percentage.
Exercise Price. The exercise price of the Warrants is $[*]. The exercise price and number of common shares issuable upon exercise will adjust in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications, dilutive issuances or similar events.
Adjustments for Subsequent Offerings. If, at any time while the Warrants are outstanding, the Company issues, sells, enters into an agreement to sell, or grants any option to purchase, or sells, enters into an agreement to sell, or grants any right to reprice, or otherwise disposes of or issues (or announces any offer, sale, grant or any option to purchase or other disposition) any common shares or common shares equivalents for a consideration per share at an effective price per share that is less than the exercise price of the Warrants then in effect (such lower price, the “Base Share Price” and such issuances collectively, a “Dilutive Issuance”), then the exercise price then in effect shall be reduced to the Base Share Price, provided that the Base Share Price shall not be less than the greater of (i) a specified price in the Warrant or (ii) the price of the Dilutive Issuance ((i) and (ii) together, the “Floor Price”) (subject to adjustment for reverse and forward share splits, recapitalizations and similar transactions following the date of the Underwriting Agreement). If the Company enters into a Variable Rate Transaction, the Company shall be deemed to have issued common shares or common share equivalents at the lowest possible price, conversion price or exercise price at which such securities may be issued, converted or exercised. Notwithstanding the foregoing, no adjustments shall be made in respect of an Exempt Issuance (defined in the Warrant).
Voluntary Adjustment by Company. Subject to the rules and regulations of the trading market and the consent of the holder, the Company may at any time during the term of the Warrants reduce the then current exercise price to any amount and for any period of time deemed appropriate by the board of directors of the Company.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Warrants and generally including, with certain exceptions, any reorganization, recapitalization or reclassification of our common shares, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common shares, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common shares, the holders of the Warrants will be entitled to receive upon exercise thereof the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. Additionally, as more fully described in the Warrant, in the event of such fundamental transaction, the Company will at the option of the holder of the Warrant purchase the Warrant at a price equal to the Black Scholes value of the remaining unexercised portion of the Warrants on the date of consummation of such fundamental transaction.
Rights as a Shareholder. Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of our common shares, the holder of a Warrant does not have the rights or privileges of a holder of our common shares, including any voting rights, until the holder exercises the Warrant.
Warrant Agent. The Warrants will be issued in registered form under a warrant agent agreement between [*], as warrant agent, and us. The Warrants shall initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Transferability. Subject to applicable laws, the Warrants may be offered for sale, sold, transferred or assigned without our consent.
Governing Law. The Warrants are governed by New York law.
Pre-Funded Warrants Offered in this Offering
The following summary of certain terms and provisions of the Pre-Funded Warrants offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of Pre-Funded Warrant, which is filed as Exhibit 4.2 to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of Pre-Funded Warrant.
The term “pre-funded” refers to the fact that the purchase price of our common shares in this offering includes almost the entire exercise price that will be paid under the Pre-Funded Warrants, except for a nominal remaining exercise price of $0.0001. The purpose of the Pre-Funded Warrants is to enable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, upon election of the holder, 9.99%) of our outstanding common shares following the consummation of this offering the opportunity to invest capital into our Company without triggering their ownership restrictions, by receiving Pre-Funded Warrants in lieu of our common shares which would result in such ownership of more than 4.99% (or 9.99%), and receive the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at such nominal price at a later date.
Duration. The Pre-Funded Warrants offered hereby will entitle the holders thereof to purchase shares of our common shares at a nominal exercise price of $0.0001 per share, at any time after its original issuance until exercised in full.
Exercise Limitation. A holder will not have the right to exercise any portion of the Pre-Funded Warrant if the holder (together with its affiliates and certain related parties) would beneficially own in excess of 4.99% (or, upon election of the holder, 9.99%) of the number of our common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. However, any holder may increase, but not in excess of 9.99%, or decrease such percentage, provided that any increase will not be effective until the sixty-first (61st) day after such election.
Exercise Price. The Pre-Funded Warrants will have an exercise price of $0.0001 per share. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common shares and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Warrant Agent. The Warrants will be issued in registered form under a warrant agent agreement between [*], as warrant agent, and us. The Warrants shall initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Transferability. Subject to applicable laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. There is no established public trading market for the Pre-Funded Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Pre-Funded Warrants on any national securities exchange or other nationally recognized trading system, including The Nasdaq Capital Market. Without an active trading market, the liquidity of Pre-Funded Warrants will be limited.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including, with certain exceptions, any reorganization, recapitalization or reclassification of our shares of Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common shares, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common shares, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise thereof the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. Additionally, as more fully described in the Pre-Funded Warrants, in the event of such fundamental transaction, the Company will at the option of the holder of the Pre-Funded Warrants purchase the Pre-Funded Warrants at a price equal to the Black Scholes value of the remaining unexercised portion of the Pre-Funded Warrants on the date of consummation of such fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of our common shares, the holder of a Pre-Funded Warrant does not have the rights or privileges of a holder of our common shares, including any voting rights, until the holder exercises the Pre-Funded Warrant.
Underwriter’s Warrants to be issued in this Offering
We have agreed to issue warrants to the underwriter, upon the closing of this offering, which entitle it to purchase up to 5.0% of the total number of securities, excluding any securities sold to cover over-allotments, if any, sold in this offering (the “Underwriter’s Warrants”). The exercise price of these warrants is equal to 125% of the public offering price of the Units offered hereby. The Underwriter’s Warrants will be exercisable after six months from the commencement of sales of the offering, at a per common share exercise price equal to 125% of the public offering price per Unit in the offering, and will expire five years from the commencement of sales of the offering. The terms of the Underwriter’s Warrants are substantially similar to the common warrants being issued as part of this offering.
Transfer Agent and Registrar
The U.S. transfer agent and registrar for our common shares is Odyssey Trust Company, located at United Kingdom Building, 350 – 409 Granville Street, Vancouver, British Columbia V6C 1T2. The telephone number is 1-855-584-2880.
Articles of Association
Key Provisions of our Notice of Articles, Articles and the Business Corporations Act (British Columbia)
The following is a summary of certain key provisions of our charter documents and certain related sections of the Business Corporations Act (British Columbia) (the “BCBCA”). This is only a summary and is not intended to be exhaustive. For further information please refer to the full version of our notice of articles.
Stated Objects or Purposes
Our articles do not contain stated objects or purposes and do not place any limitations on the business that we may carry on.
Directors
Power to vote on matters in which a director is materially interested. Under the BCBCA a director who has a material interest in a contract or transaction, whether made or proposed, that is material to us, must disclose such interest to us, subject to certain exceptions such as if the contract or transaction: (i) is an arrangement by way of security granted by us for money loaned to, or obligations undertaken by, the director for our benefit or for one of our affiliates’ benefit; (ii) relates to an indemnity or insurance permitted under the BCBCA; (iii) relates to the remuneration of the director in his or her capacity as director, officer, employee or agent of our company or of one of our affiliates; (iv) relates to a loan to our company while the director is the guarantor of some or all of the loan; or (v) is with a corporation that is affiliated to us while the director is also a director or senior officer of that corporation or an affiliate of that corporation.
A director who holds such disclosable interest in respect of any material contract or transaction into which we have entered or propose to enter may be required to absent himself or herself from the meeting while discussions and voting with respect to the matter are taking place. Directors are also required to comply with certain other relevant provisions of the BCBCA regarding conflicts of interest.
Directors’ power to determine the remuneration of directors. The remuneration of our directors is determined by our directors subject to our articles. The remuneration may be in addition to any salary or other remuneration paid to any of our employees (including executive officers) who are also directors.
Number of shares required to be owned by a director. Neither our articles nor the BCBCA provide that a director is required to hold any of our shares as a qualification for holding his or her office.
Shareholder Meetings
Subject to applicable stock exchange requirements, we must hold a general meeting of our shareholders at least once every year at a time and place determined by our board of directors, provided that the meeting must not be held later than 15 months after the preceding annual general meeting. A meeting of our shareholders may be held anywhere in or outside British Columbia.
A notice to convene a meeting, specifying the date, time and location of the meeting, and, where a meeting is to consider special business, the general nature of the special business must be sent to each shareholder entitled to attend the meeting and to each director not less than 21 days prior to the meeting for so long as we are a public company. The accidental omission to send notice of any meeting of shareholders to, or the non-receipt of any notice by, any person entitled to notice does not invalidate any proceedings at that meeting.
Subject to the special rights and restrictions attached to the shares or any class or series of shares, the quorum for the transaction of business at a meeting of shareholders is one or more persons, present in person or by proxy. If a quorum is not present within one-half hour of the time set for the holding of a meeting of shareholders, (1) in the case of a general meeting convened by requisition of shareholders, the meeting di dissolved; and (2) in the case of any other meeting of shareholders, the shareholders entitled to vote at the meeting who are present, in person or by proxy, at the meeting may adjourn the meeting to a set time and place.
Shareholder Proposals and Advance Notice Procedures
Under the BCBCA, qualified shareholders holding at least one percent (1%) of our issued voting shares or whose shares have a fair market value in excess of CAD$2,000 may make proposals for matters to be considered at the annual general meeting of shareholders. Such proposals must be sent to us in advance of any proposed meeting by delivering a timely written notice in proper form to our registered office in accordance with the requirements of the BCBCA. The notice must include information on the business the shareholder intends to bring before the meeting in the prescribed form. To be a qualified shareholder, a shareholder must currently be and have been a registered or beneficial owner of at least one share of the company for at least two years before the date of signing the proposal.
The Company’s Articles contain a notice provision (the “Notice Provision”) of the nomination of directors in certain circumstances. To be timely, the notice by the nominating shareholder (the “Nominating Shareholder”) must be made:
| | (a) | in the case of an annual meeting of Shareholders, not less than 30 and not more than 65 days prior to the date of the annual meeting of Shareholders; provided, however, that in the event that the annual meeting of Shareholders is to be held on a date that is less than 50 days after the date (the “Notice Date”) on which the first public announcement of the date of the annual meeting was made, notice by the Nominating Shareholder is to be made not later than the close of business on the 10th day after the Notice Date in respect of such meeting; and |
| | (b) | in the case of a special meeting (which is not also an annual meeting) of shareholders called for the purpose of electing directors (whether or not called for other purposes), not later than the close of business on the 15th day following the day on which the first public announcement of the date of the special meeting of shareholders was made. |
Limitation of Liability and Indemnification
Under the BCBCA, a company may indemnify: (i) a current or former director or officer of that company; (ii) a current or former director or officer of another corporation if, at the time such individual held such office, the corporation was an affiliate of the company, or if such individual held such office at the company’s request; or (iii) an individual who, at the request of the company, held, or holds, an equivalent position in another entity, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment actually and reasonably incurred by him or her in respect of any legal proceeding or investigative action (whether current, threatened, pending or completed) in which he or she is involved because of that person’s position as an indemnifiable person, unless: (i) the individual did not act honestly and in good faith with a view to the best interests of such company or the other entity, as the case may be; or (ii) in the case of a proceeding other than a civil proceeding, the individual did not have reasonable grounds for believing that the individual’s conduct was lawful. A company cannot indemnify an indemnifiable person if it is prohibited from doing so under its articles or by applicable law. A company may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an indemnifiable person in respect of that proceeding only if the indemnifiable person has provided an undertaking that, if it is ultimately determined that the payment of expenses was prohibited, the indemnifiable person will repay any amounts advanced. Subject to the aforementioned prohibitions on indemnification, a company must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by an indemnifiable person in respect of such eligible proceeding if such indemnifiable person has not been reimbursed for such expenses, and was wholly successful, on the merits or otherwise, in the outcome of such eligible proceeding or was substantially successful on the merits in the outcome of such eligible proceeding. On application from us or from an indemnifiable person, a court may make any order the court considers appropriate in respect of an eligible proceeding, including the indemnification of penalties imposed or expenses incurred in any such proceedings and the enforcement of an indemnification agreement. As permitted by the BCBCA, our articles require us to indemnify our directors, former directors or alternate directors (and such individual’s respective heirs and legal representatives) and permit us to indemnify any person to the extent permitted by the BCBCA.
Ownership and Exchange Controls
There is no limitation imposed by Canadian law or by our articles on the right of a non-resident to hold or vote our common shares, other than discussed below.
Competition Act
Limitations on the ability to acquire and hold our common shares may be imposed by the Competition Act (Canada). This legislation permits the Commissioner of Competition, or Commissioner, to review any acquisition or establishment, directly or indirectly, including through the acquisition of shares, of control over or of a significant interest in us. This legislation grants the Commissioner jurisdiction, for up to one year after the acquisition has been substantially completed, to challenge this type of acquisition by seeking a remedial order, including an order to prohibit the acquisition or require divestitures, from the Canadian Competition Tribunal, which may be granted where the Competition Tribunal finds that the acquisition substantially prevents or lessens, or is likely to substantially prevent or lessen, competition.
This legislation also requires any person or persons who intend to acquire more than 20% of our voting shares or, if such person or persons already own more than 20% of our voting shares prior to the acquisition, more than 50% of our voting shares, to file a notification with the Canadian Competition Bureau if certain financial thresholds are exceeded. Where a notification is required, unless an exemption is available, the legislation prohibits completion of the acquisition until the expiration of the applicable statutory waiting period, unless the Commissioner either waives or terminates such waiting period or issues an advance ruling certificate. The Commissioner’s review of a notifiable transaction for substantive competition law considerations may take longer than the statutory waiting period.
Investment Canada Act
The Investment Canada Act requires each “non Canadian” (as defined in the Investment Canada Act) who acquires “control” of an existing “Canadian business,” to file a notification in prescribed form with the responsible federal government department or departments not later than 30 days after closing, provided the acquisition of control is not a reviewable transaction under the Investment Canada Act. Subject to certain exemptions, a transaction that is reviewable under the Investment Canada Act may not be implemented until an application for review has been filed and the responsible Minister of the federal cabinet has determined that the investment is likely to be of “net benefit to Canada” taking into account certain factors set out in the Investment Canada Act. Under the Investment Canada Act, an investment in our common shares by a non-Canadian who is a World Trade Organization member country investor that is not a state-owned enterprise, including a United States investor would be reviewable only if it were an investment to acquire control of us pursuant to the Investment Canada Act and our enterprise value (as determined pursuant to the Investment Canada Act and its regulations) was equal to or greater than $1.287 billion (as of January 23, 2023). The enterprise value threshold for “trade agreement investors” that are not state-owned enterprises is $1.931 billion (as of January 23, 2023).
The Investment Canada Act contains various rules to determine if there has been an acquisition of control. Generally, for purposes of determining whether an investor has acquired control of a corporation by acquiring shares, the following general rules apply, subject to certain exceptions: the acquisition of a majority of the voting interests or a majority of the undivided ownership interests in the voting shares of the corporation is deemed to be acquisition of control of that corporation; the acquisition of less than a majority, but one-third or more, of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of voting shares; and the acquisition of less than one third of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is deemed not to be acquisition of control of that corporation.
Under the national security review regime in the Investment Canada Act, review on a discretionary basis may also be undertaken by the federal government with respect to a much broader range of investments by a non-Canadian to “acquire, in whole or part, or to establish an entity carrying on all or any part of its operations in Canada.” No financial threshold applies to a national security review. The relevant test is whether such investment by a non-Canadian could be “injurious to national security.” Review on national security grounds is at the discretion of the responsible ministers and may occur on a pre- or post-closing basis.
Certain transactions relating to our common shares will generally be exempt from the Investment Canada Act, subject to the federal government’s prerogative to conduct a national security review, including:
| | ● | the acquisition of our common shares by a person in the ordinary course of that person’s business as a trader or dealer in securities; |
| | | |
| | ● | the acquisition of control of us in connection with the realization of security granted for a loan or other financial assistance and not for any purpose related to the provisions of the Investment Canada Act if the acquisition is subject to approval under Canadian legislation relating to financial institutions; and |
| | | |
| | ● | the acquisition of control of us by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of us, through ownership of our common shares, remains unchanged. |
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our common shares in the public market could adversely affect market prices prevailing from time to time. Furthermore, because only a limited number of common shares will be available for sale shortly after this offering due to existing contractual and legal restrictions on resale as described below, there may be sales of substantial amounts of our common shares in the public market after such restrictions lapse. This may adversely affect the prevailing market price of our common shares and our ability to raise equity capital in the future.
Upon completion of this offering, we will have [__] common shares outstanding, or [__] common shares outstanding if the underwriter exercises their option in full to purchase additional common shares, in each case excluding common shares issuable upon the exercise of the Pre-Funded Warrants or the common warrants sold in this offering. Of these, [__] common shares, or [__] common shares if the underwriter exercises their option in full, in each case excluding common shares issuable upon the exercise of the Pre-Funded Warrants or the common warrants sold in this offering to purchase additional common shares, sold in this offering will be freely transferable without restriction or registration under the Securities Act, except for any shares purchased by one of our existing “affiliates,” as that term is defined in Rule 144 under the Securities Act. The remaining common shares are “restricted shares” as defined in Rule 144. Restricted shares may be sold in the public market only if registered or if they qualify for an exemption from registration under Rules 144 or 701 of the Securities Act. As a result of the contractual 90-day lock-up period described below and the provisions of Rules 144 and 701, these shares will be available for sale in the public market as follows:
| Number of Shares | | Date |
| 76,059,147 | | On the date of this prospectus. |
| | | After 91 days from the date of this prospectus (subject, in some cases, to volume limitations). |
| | | After 180 days from the date of this prospectus (subject, in some cases, to volume limitations). |
Lock-up Restrictions
We and each of our directors, executive officers, and certain of our shareholders, have agreed, without the prior written consent of the underwriter, not to directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common shares, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of our common shares, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any common shares or securities convertible into or exercisable or exchangeable for common shares or any other securities of our company or publicly disclose the intention to do any of the foregoing for a period of 90 days after the date of this prospectus. The lock-up restrictions and specified exceptions are described in more detail under “Underwriting.”
Rule 144
In general, under Rule 144, any person who is not our affiliate and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, subject to the availability of current public information about us. In addition, under Rule 144, any person who is not our affiliate and has not been our affiliate at any time during the preceding three months and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares without regard to whether current public information about us is available.
A person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of common shares within any three-month period that does not exceed the greater of: (i) 1% of the number of our shares outstanding; and (ii) the average weekly trading volume of our common shares on [___] during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions, notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701 under the Securities Act, any of our employees, directors, officers, consultants or advisors who acquired common shares from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 prior to our IPO is entitled to sell such shares in reliance on Rule 144 but without compliance with certain of the requirements contained in Rule 144. Accordingly, subject to any applicable lock-up restrictions, under Rule 701 persons who are not our affiliates may resell those shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our affiliates may resell those shares without compliance with Rule 144’s minimum holding period requirements.
Canadian Resale Restrictions
Any sale of any of our shares which constitutes a “control distribution” under Canadian securities laws (generally a sale by a person or a group of persons holding more than 20% of our outstanding voting securities) will be subject to restrictions under Canadian securities laws in addition to those restrictions noted above, unless the sale is qualified under a prospectus filed with Canadian securities regulatory authorities, or if prior notice of the sale is filed with the Canadian securities regulatory authorities at least seven days before any sale and there has been compliance with certain other requirements and restrictions regarding the manner of sale, payment of commissions, reporting and availability of current public information about us and compliance with applicable Canadian securities laws.
Equity Incentive Plans
We may file with the SEC a registration statement on Form S-8 under the Securities Act covering the common shares that are subject to outstanding options and other awards that may be granted pursuant to our equity incentive plans. Shares covered by such registration statement will be available for sale in the open market following its effective date, subject to certain Rule 144 limitations applicable to affiliates and the terms of lock-up restrictions applicable to those shares.
Canadian Federal Income Tax Considerations For United States Residents
The following is a summary of the principal Canadian federal income tax considerations generally applicable to the holding and disposition of our common shares acquired by a holder who, at all relevant times, (a) for the purposes of and within the meanings applicable under the Income Tax Act (Canada) (the “Tax Act”), including by virtue of any applicable deeming rules, (i) is not resident in Canada, (ii) deals at arm’s length with us, and is not affiliated with us, (iii) holds our common shares as capital property, (iv) does not use or hold the common shares in the course of carrying on, or otherwise in connection with, a business carried on in Canada and (v) is not a “registered non-resident insurer” or “authorized foreign bank” (each as defined in the Tax Act), and (b) for the purposes of the Canada-U.S. Tax Convention, is a resident of the United States, has never been a resident of Canada, does not have and has not had, at any time, a permanent establishment or fixed base in Canada, for purposes of the Tax Act, and who otherwise qualifies for the full benefits of the Canada-U.S. Tax Convention. The common shares will generally be considered to be capital property to a holder unless such common shares are held in the course of carrying on a business of buying or selling securities, or as part of an adventure or concern in the nature of trade. Holders who meet all the criteria in clauses (a) and (b) are referred to herein as “Non-Canadian Holders”.
This summary does not deal with special situations, such as the particular circumstances of traders or dealers, tax exempt entities, insurers or financial institutions. Such holders and other holders who do not meet the criteria in clauses (a) and (b) should consult their own tax advisers.
This summary is based upon the current provisions of the Tax Act, the regulations thereunder in force at the date hereof (“Regulations”), the current provisions of the Canada-U.S. Tax Convention and our understanding of the administrative and assessing practices published by the Canada Revenue Agency (“CRA”) prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and assumes that such Proposed Amendments will be enacted in the form proposed. However, such Proposed Amendments might not be enacted in the form proposed or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative or assessing practices, whether by legislative, governmental or judicial decision or action, nor does it take into account tax laws of any province or territory of Canada or of any other jurisdiction outside Canada, which may differ from those discussed in this summary. For the purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of our common shares must generally be expressed in Canadian dollars. Amounts denominated in any other currency generally must be converted into Canadian dollars at applicable market rates of exchange, which rates should be determined taking into account applicable views of CRA with respect to the determination of such rates.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Non-Canadian Holder and no representation with respect to the Canadian federal income tax consequences to any particular Non-Canadian Holder or prospective Non-Canadian Holder is made. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, prospective purchasers should consult with their own tax advisors for advice with respect to their own particular circumstances.
Withholding Tax on Dividends
Amounts paid or credited or deemed to be paid or credited as, on account or in lieu of payment, or in satisfaction of, dividends on our common shares to a Non Canadian Holder will be subject to Canadian withholding tax. The general statutory rate of Canadian withholding tax is 25%. Under the Canada-U.S. Tax Convention, the rate of Canadian withholding tax on dividends paid or credited by us to a Non-Canadian Holder that beneficially owns such dividends is generally 15% unless the beneficial owner is a company which owns at least 10% of our voting stock at that time, in which case the rate of Canadian withholding tax is reduced to 5%.
Dispositions
A Non-Canadian Holder will not be subject to tax under the Tax Act on any capital gain realized on a disposition or deemed disposition of a common share unless the common shares are “taxable Canadian property” to the Non-Canadian Holder for purposes of the Tax Act and the Non-Canadian Holder is not entitled to relief under an applicable income tax convention between Canada and the country of which the Non-Canadian Holder is resident.
Generally, the common shares will not constitute “taxable Canadian property” to a Non-Canadian Holder at a particular time unless, at any time during the 60-month period immediately preceding the disposition, more than 50% of the fair market value of the common shares was derived, directly or indirectly, from one or any combination of (i) real or immoveable property situated in Canada, (ii) “Canadian resource properties” (as defined in the Tax Act), (iii) “timber resource properties” (as defined in the Tax Act), and (iv) options in respect of, or interests in, or for civil law rights in, property in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, common shares could be deemed to be “taxable Canadian property”. Non-Canadian Holders whose common shares may constitute “taxable Canadian property” should consult their own tax advisors.
Under the Canada-U.S. Tax Convention, the gains derived by a Non-Canadian Holder from the disposition of common shares would generally not be taxable in Canada unless the value of the common shares is derived principally from real property situated in Canada. We believe that the value of our common shares is not currently, and has not at any time in the preceding 60 months been, derived principally from real property situated in Canada or other relevant assets described above, and we do not expect this to change in the foreseeable future.
Certain Material United States Federal Income Tax Considerations
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from the acquisition, ownership and disposition of our common share. This summary applies only to U.S. Holders that acquire our common shares pursuant to this prospectus and does not apply to any subsequent U.S. Holder of our common shares.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder as a result of the acquisition, ownership and disposition of our common shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including specific tax consequences to a U.S. Holder under an applicable tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any particular U.S. Holder. In addition, this summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. Medicare contribution, U.S. state and local, or non-U.S. tax consequences of the acquisition, ownership or disposition of our common shares. Except as specifically set forth below, this summary does not discuss applicable tax reporting requirements. Each U.S. Holder should consult its own tax advisor regarding all U.S. federal, U.S. state and local and non-U.S. tax consequences of the acquisition, ownership and disposition of our common shares.
No opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership or disposition of our common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, or contrary to, any position taken in this summary. In addition, because the authorities upon which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of This Disclosure
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date hereof. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis, which could affect the U.S. federal income tax considerations described in this summary. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of our common shares that is for U.S. federal income tax purposes:
| | ● | an individual who is a citizen or resident of the U.S.; |
| | | |
| | ● | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the U.S., any state thereof or the District of Columbia; |
| | | |
| | ● | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| | | |
| | ● | a trust that (a) is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions or (b) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Transactions Not Addressed
This summary does not address the tax consequences of transactions effected prior or subsequent to, or concurrently with, any purchase of our common shares pursuant to this prospectus (whether or not any such transactions are undertaken in connection with the purchase of our common shares pursuant to this prospectus).
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations of the acquisition, ownership or disposition of our common shares by U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following: (a) tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) broker-dealers, dealers, or traders in securities or currencies that elect to apply a “mark-to-market” accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that own our common shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquire our common shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold our common shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); and (h) U.S. Holders that own directly, indirectly, or by attribution, 10% or more, by voting power, of our outstanding stock. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Income Tax Act (Canada); (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold our common shares in connection with carrying on a business in Canada; (d) persons whose common shares in our company constitutes “taxable Canadian property” under the Income Tax Act (Canada); or (e) persons that have a permanent establishment in Canada for purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described immediately above, should consult their own tax advisors regarding all U.S. federal, U.S. state and local, and non-U.S. tax consequences (including the potential application and operation of any income tax treaties) relating to the acquisition, ownership or disposition of our common shares.
If an entity or arrangement that is classified as a partnership (or other “pass-through” entity, or as a passive foreign investment company (through which a U.S. Holder invests in our shares) for U.S. federal income tax purposes holds our common shares, the U.S. federal income tax consequences to such partnership and the partners (or other owners) of such partnership of the acquisition, ownership or disposition of our common shares generally will depend on the activities of the partnership and the status of such partners (or other owners). This summary does not address the U.S. federal income tax considerations for any such partner or partnership (or other “pass-through” entity or its owners). Owners of entities and arrangements that are classified as partnerships (or other “pass-through” entities), or as passive foreign investment companies, for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences of the acquisition, ownership or disposition of our common shares.
Acquisition of Our Common Shares
A U.S. Holder generally will not recognize gain or loss upon the acquisition of our common shares for cash pursuant to this prospectus. A U.S. Holder’s initial tax basis in our common shares acquired pursuant to this prospectus will be equal to the U.S. Holder’s U.S. dollar cost for the common shares. A U.S. Holder’s holding period for such common shares will begin on the day after the acquisition.
Ownership and Disposition of Our Common Shares
Distributions on Our Common Shares
Subject to the “passive foreign investment company” (“PFIC”) rules discussed below (see “Tax Consequences if the Company is a PFIC”), a U.S. Holder that receives a distribution, including a constructive distribution, with respect to our common shares will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the current or accumulated “earnings and profits” of the Company, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Company, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in our common shares and thereafter as gain from the sale or exchange of such common shares (see “Sale or Other Taxable Disposition of Our Common Shares” below). However, the Company may not maintain calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by the Company with respect to our common shares will constitute a dividend. Dividends received on our common shares generally will not be eligible for the “dividends received deduction” available to U.S. corporate shareholders receiving dividends from U.S. corporations. If the Company is eligible for the benefits of the Canada-U.S. Tax Convention or our common shares are readily tradable on an established securities market in the U.S., dividends paid by the Company to non-corporate U.S. Holders generally will be eligible for the preferential tax rates applicable to long-term capital gains, provided certain holding period and other conditions are satisfied, including that the Company not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Our Common Shares
Subject to the PFIC rules discussed below, upon the sale or other taxable disposition of our common shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount of cash plus the fair market value of any property received and such U.S. Holder’s tax basis in the common shares sold or otherwise disposed of. Such capital gain or loss will be long-term capital gain or loss if, at the time of the sale or other taxable disposition, the U.S. Holder’s holding period for our common shares is more than one year. Preferential tax rates apply to long-term capital gains of non-corporate U.S. Holders. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
PFIC Status of the Company
If the Company is or becomes a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the ownership and disposition of our common shares. The U.S. federal income tax consequences of owning and disposing of our common shares if the Company is or becomes a PFIC are described below under the heading “Tax Consequences if the Company is a PFIC.”
A non-U.S. corporation is a PFIC for each tax year in which (i) 75% or more of its gross income is passive income (as defined for U.S. federal income tax purposes) (the “income test”) or (ii) on average for such tax year, 50% or more (by value) of its assets are assets that either produce or are held for the production of passive income (the “asset test”).
Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business) and gains from the disposition of passive assets. Assets used in the operation of a business are not passive assets, but cash is treated as a passive asset even if it is held as working capital.
In determining whether or not it is a PFIC, a non-U.S. corporation is required to take into account its pro rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value).
Under certain attribution and indirect ownership rules, if the Company is a PFIC, U.S. Holders will generally be deemed to own their proportionate shares of the Company’s direct or indirect equity interest in any company that is also a PFIC (a “Subsidiary PFIC”).
The Company has not determined whether it may have been a PFIC during prior years or will be a PFIC during the current year; Moreover, based on current business plans and financial projections, the Company cannot determine with certainty whether it will be classified as a PFIC in subsequent tax years. The determination of PFIC status is inherently factual, is subject to a number of uncertainties, and can be determined only annually after the close of the tax year in question. Additionally, the analysis of PFIC status depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. We might be determined to be a PFIC for the current tax year or any future tax year, and no opinion of legal counsel or ruling from the IRS concerning the status of the Company as a PFIC has been obtained or will be requested. U.S. Holders should consult their own U.S. tax advisors regarding the PFIC status of the Company.
Tax Consequences if the Company is a PFIC
If the Company is a PFIC for any tax year during which a U.S. Holder owns our common shares, special rules may increase such U.S. Holder’s U.S. federal income tax liability with respect to the ownership and disposition of such common shares. If the Company meets the income test or the asset test for any tax year during which a U.S. Holder owns our common shares, the Company will be treated as a PFIC with respect to such U.S. Holder for that tax year and for all subsequent tax years, regardless of whether the Company meets the income test or the asset test for such subsequent tax years, unless the U.S. Holder makes a timely and effective QEF Election or, if applicable, a Mark-to-Market Election, or a “Purging Election”, all as discussed below
Under the default PFIC rules:
| | ● | any gain realized on the sale or other disposition (including dispositions and certain other events that would not otherwise be treated as taxable events) of our common shares (including an indirect disposition of the stock of any Subsidiary PFIC) and any “excess distribution” (defined as a distribution to the extent it, together with all other distributions received in the relevant tax year, exceeds 125% of the average annual distribution received during the preceding three years) received on our common shares or with respect to the stock of a Subsidiary PFIC will be allocated ratably to each day of such U.S. Holder’s holding period for our common shares; |
| | | |
| | ● | the amount allocated to the current tax year and any year prior to the first year in which the Company was a PFIC will be taxed as ordinary income in the current year; |
| | | |
| | ● | the amount allocated to each of the other tax years (the “Prior PFIC Years”) will be subject to tax at the highest ordinary income tax rate in effect for the applicable class of taxpayer for that year; |
| | | |
| | ● | an interest charge will be imposed with respect to the resulting tax attributable to each Prior PFIC Year, which interest charge is not deductible by non-corporate U.S. Holders; and |
| | | |
| | ● | any loss realized on the disposition of our common shares generally will not be recognized. |
A U.S. Holder that makes a timely and effective election to treat the Company and each Subsidiary PFIC as a “qualified electing fund” (a “QEF”) under Section 1295 of the Code (a “QEF Election”) or a timely and effective “mark-to-market” election under Section 1296 of the Code (a “Mark-to-Market Election”) , or a “Purging Election” may generally mitigate or avoid the PFIC consequences described above with respect to our common shares.
QEF election. If a U.S. Holder makes a timely and effective QEF Election, the U.S. Holder must include currently in gross income each year its pro rata share of the Company’s ordinary income and net capital gains (if any), regardless of whether such income and gains are actually distributed. Thus, a U.S. Holder could have a tax liability with respect to such ordinary income or gains without a corresponding receipt of cash from the Company. If the Company is a QEF with respect to a U.S. Holder, the U.S. Holder’s basis in our common shares will be increased to reflect the amount of the taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in our common shares and will not be taxed again as a distribution to a U.S. Holder. Taxable gains on the disposition of our common shares by a U.S. Holder that has made a timely and effective QEF Election are generally capital gains. A U.S. Holder must make a QEF Election for the Company and each Subsidiary PFIC if it wishes to have this treatment. To make a QEF Election, a U.S. Holder will need to receive an annual information statement from the Company setting forth the ordinary income and net capital gains for the year. In the event that the Company is a PFIC for any tax year, it will endeavor to satisfy the recordkeeping requirements that apply to a QEF and to supply U.S. Holders with information that they require in order to report under the QEF rules. However, there is no assurance that we will be able to do so.
In general, a U.S. Holder must make a QEF Election on or before the due date for filing its income tax return for the first year to which the QEF Election applies. Under applicable Treasury Regulations, a U.S. Holder will be permitted to make retroactive elections in particular circumstances, including if it had a reasonable belief that the Company was not a PFIC and filed a protective election. If a U.S. Holder owns PFIC stock indirectly through another PFIC, separate QEF Elections must be made for the PFIC in which the U.S. Holder is a direct shareholder and the Subsidiary PFIC for the QEF rules to apply to both PFICs. Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective QEF Election for the Company and any Subsidiary PFIC.
Mark-to-Market election. A Mark-to-Market Election may be made with respect to stock in a PFIC if such stock is “regularly traded” on a “qualified exchange or other market” (within the meaning of the Code and the applicable Treasury Regulations). We are currently listed on the Canadian Stock Exchange and trade on the OTC Pink, and we will apply for a listing on The Nasdaq Stock Market LLC, all of which are qualified exchanges. A class of stock that is traded on one or more qualified exchanges or other markets is considered to be “regularly traded” for any calendar year during which such class of stock is traded in other than de minimis quantities on at least 15 days during each calendar quarter. If our common shares are considered to be “regularly traded” within this meaning, then a U.S. Holder generally will be eligible to make a Mark-to-Market Election with respect to our common shares but not with respect to a Subsidiary PFIC.
Should our common shares become “regularly traded,” a U.S. Holder that makes a timely and effective Mark-to-Market Election with respect to our common shares generally will be required to recognize as ordinary income in each tax year in which the Company is a PFIC an amount equal to the excess, if any, of the fair market value of such stock as of the close of such taxable year over the U.S. Holder’s adjusted tax basis in such stock as of the close of such taxable year. A U.S. Holder’s adjusted tax basis in our common shares generally will be increased by the amount of ordinary income recognized with respect to such stock. If the U.S. Holder’s adjusted tax basis in our common shares as of the close of a tax year exceeds the fair market value of such stock as of the close of such taxable year, the U.S. Holder generally will recognize an ordinary loss, but only to the extent of net mark-to-market income recognized with respect to such stock for all prior taxable years. A U.S. Holder’s adjusted tax basis in our common shares generally will be decreased by the amount of ordinary loss recognized with respect to such stock. Any gain recognized upon a disposition of our common shares generally will be treated as ordinary income, and any loss recognized upon a disposition generally will be treated as ordinary loss to the extent of the net mark-to-market income recognized for all prior taxable years. Any loss recognized in excess thereof will be taxed as a capital loss. Capital losses are subject to significant limitations under the Code. Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective Mark-to-Market Election with respect to our common shares.
“Purging Election.” If a U.S. Holder does not make a QEF election or a Mark-to-Market election (as described above), and if we were a PFIC at any time during the period that the U.S. Holder holds our common shares, then such common shares will continue to be treated as stock of a PFIC with respect to the U.S. Holder even if we cease to be a PFIC in a future year, unless the U.S. Holder makes a “Purging Election” for the year we cease to be a PFIC. A Purging Election creates a deemed sale of the U.S. Holder’s common shares at their fair market value on the last day of the last year in which we are treated as a PFIC. The gain recognized by the Purging Election will be subject to the special tax and interest charge rules, described above, that apply to excess distributions. But as long as we are not thereafter a PFIC, dividends distributed by us (or gains from the sale of our common shares), following a Purging Election will no longer be subject to the rules (described above) that apply to excess distributions. As a result of the Purging Election, a U.S. Holder will have a new basis (equal to the fair market value of the common shares on the last day of the last year in which we are treated as a PFIC) and a new holding period (which will begin the day after such last day) in its common shares for tax purposes. If we become a PFIC, we will endeavor to notify our U.S. Holders if and when we cease to be a PFIC, but we cannot be certain that we will be able to do so.
U.S. Holders should be aware that, as stated above, a foreign company that becomes a PFIC while a U.S. Holder owns stock in the company remains a PFIC with respect to that shareholder for as long as the shareholder holds the stock (even if the company is at some point no longer classified as a PFIC), unless the shareholder had made an appropriate election or a Purging Election. Accordingly, even though our PFIC status may have no immediate impact on your U.S. tax liability (if we do not make any distributions or if we do not have any net earnings or capital gains), your future tax liability as a U.S. Holder of shares in our company may be affected by elections that you make or do not make on a timely basis. For this reason, it is important for U.S. Holders to consult their own tax advisors about the consequences of PFIC status.
PFIC Reporting requirements. In addition to the reporting requirements discussed below (see “Information Reporting; Backup Withholding”, below, if you hold our common shares during any taxable year in which we are a PFIC, you will in all likelihood be required to file IRS Form 8621 for each such year and provide certain annual information regarding your common shares, including information on distributions received on the common shares and any gain realized on the disposition of the common shares. U.S. Holders should consult their own tax advisors about the requirement to file Form 8621.
Foreign Tax Credit
A U.S. Holder that pays (whether directly or through withholding) Canadian income tax in connection with the ownership or disposition of our common shares may be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all creditable foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year.
Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source.” Generally, dividends paid by a non-U.S. corporation should be treated as foreign source for this purpose, and gains recognized on the sale of securities of a non-U.S. corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to our common shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Special rules apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution, including a constructive distribution, from a PFIC. Subject to such special rules, non-U.S. taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult its own tax advisor regarding their application to the U.S. Holder.
Receipt of Foreign Currency
The amount of any distribution or proceeds paid in Canadian dollars to a U.S. Holder in connection with the ownership, sale or other taxable disposition of our common shares, will be included in the gross income of a U.S. Holder as translated into U.S. dollars calculated by reference to the exchange rate prevailing on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars at that time. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in Canadian dollars and engages in a subsequent conversion or other disposition of the Canadian dollars may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method with respect to foreign currency. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of Canadian dollars.
Information Reporting; Backup Withholding
Under U.S. federal income tax law, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of “specified foreign financial assets” includes not only financial accounts maintained in non-U.S. financial institutions, but also, if held for investment and not in an account maintained by certain financial institutions, any stock or security issued by a non-U.S. person, any financial instrument or contract that has an issuer or counterparty other than a U.S. person and any interest in a non-U.S. entity. A U.S. Holder may be subject to these reporting requirements unless such U.S. Holder’s shares of our common shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns on IRS Form 8938 for specified foreign financial assets, filing obligations relating to the PFIC rules including possible reporting on IRS Form 8621, and any other applicable reporting requirements.
Payments made within the U.S. or by a U.S. payor or U.S. middleman of (a) distributions on our common shares, and (b) proceeds arising from the sale or other taxable disposition of our common shares generally will be subject to information reporting. In addition, backup withholding, currently at a rate of 28%, may apply to such payments if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (“TIN”) (generally on Form W-9), (b) furnishes an incorrect U.S. TIN, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. TIN and that the IRS has not notified such U.S. Holder that it is subject to backup withholding. Certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules are allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. The information reporting and backup withholding rules may apply even if, under the Canada-U.S. Tax Convention, payments are exempt from dividend withholding tax or otherwise eligible for a reduced withholding rate.
The discussion of reporting requirements set forth above is not intended to constitute an exhaustive description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and, under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL U.S. TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP OR DISPOSITION OF OUR COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR PARTICULAR CIRCUMSTANCES.
CANADIAN TAX IMPLICATIONS FOR NON-CANADIAN HOLDERS
The following summary describes, as of the date hereof, the principal Canadian federal income tax considerations generally applicable to a purchaser who acquires, as a beneficial owner, common shares, pursuant to this offering and who, at all relevant times, for the purposes of the application of the Income Tax Act (Canada) and the Income Tax Regulations (Canada) (collectively, the “Canadian Tax Act”), (1) is not, and is not deemed to be, resident in Canada, (2) deals at arm’s length with the Company, (3) is not affiliated with the Company, (4) does not use or hold, and is not deemed to use or hold, common shares or warrants in a business carried on in Canada, (5) holds the common shares as capital property, and (6) has not entered into, with respect to the common shares, a “derivative forward agreement” (as defined in the Canadian Tax Act) (a “Non-Canadian Holder”). Special rules, which are not discussed in this summary, may apply to a Non-Canadian Holder that is an insurer carrying on an insurance business in Canada and elsewhere or an “authorized foreign bank” (as defined in the Canadian Tax Act). For the purposes of this summary, unless otherwise indicated, references to common shares include common shares acquired pursuant to this offering.
This summary is based on the current provisions of the Canadian Tax Act, and an understanding of the current administrative policies of the Canada Revenue Agency (the “CRA”) published in writing prior to the date hereof. This summary takes into account all specific proposals to amend the Canadian Tax Act and the Canada-United States Tax Convention (1980), as amended (the “Canada-U.S. Tax Treaty”) publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and assumes that all Proposed Amendments will be enacted in the form proposed. However, no assurances can be given that the Proposed Amendments will be enacted as proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative policy or assessing practice whether by legislative, regulatory, administrative or judicial action nor does it take into account tax legislation or considerations of any province, territory or foreign jurisdiction, which may differ from those discussed herein.
This summary is of a general nature only and is not, and is not intended to be, legal or tax advice to any particular purchaser. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, you should consult your own tax advisor with respect to your particular circumstances.
Generally, for purposes of the Canadian Tax Act, all amounts relating to the acquisition, holding, or disposition of the common shares must be converted into Canadian dollars based on the exchange rates as determined in accordance with the Canadian Tax Act. The amount of any dividends required to be included in the income of, and capital gains or capital losses realized by, a Non-Canadian Holder may be affected by fluctuations in the Canadian exchange rate.
Allocation of Cost
Non-Canadian Holders will be required to allocate on a reasonable basis their cost of each common share in order to determine their respective costs for purposes of the Canadian Tax Act. For its purposes, the Company intends to allocate $[ ] of each common share. Although the Company believes that its allocation is reasonable, it is not binding on the CRA or a Non-Canadian Holder and the CRA may not agree with such allocation.
The adjusted cost base to a Non-Canadian Holder of each common share acquired pursuant to this offering will be determined by averaging the cost of such common share with the adjusted cost base to such Non-Canadian Holder of all other common shares in the capital stock of the Company, if any, held by the Non-Canadian Holder as capital property immediately prior to the acquisition.
Dividends
Dividends paid or credited on the common shares or deemed to be paid or credited on the common shares to a Non-Canadian Holder will be subject to Canadian withholding tax at the rate of 25%, subject to any reduction in the rate of withholding to which the Non-Canadian Holder is entitled under any applicable income tax convention between Canada and the country in which the Non-Canadian Holder is resident. For example, under the Canada-U.S. Tax Treaty, where dividends on the common shares are considered to be paid to or derived by a Non-Canadian Holder that is a beneficial owner of the dividends and is a U.S. resident for the purposes of, and is entitled to benefits of, the Canada-U.S. Tax Treaty, the applicable rate of Canadian withholding tax is generally reduced to 15%.
Dispositions of Common Shares
A Non-Canadian Holder will not be subject to tax under the Canadian Tax Act on any capital gain realized on a disposition or deemed disposition of a common share, nor will capital losses arising therefrom be recognized under the Canadian Tax Act, unless the common share is “taxable Canadian property” to the Non-Canadian Holder for purposes of the Canadian Tax Act and the Non-Canadian Holder is not entitled to relief under an applicable income tax convention between Canada and the country in which the Non-Canadian Holder is resident.
Generally, a common share will not constitute “taxable Canadian property” to a Non-Canadian Holder at a particular time provided that the common shares are listed at that time on a “designated stock exchange” (as defined in the Canadian Tax Act), which currently includes the Nasdaq, unless at any particular time during the 60-month period that ends at that time: (i) one or any combination of (a) the Non-Canadian Holder, (b) persons with whom the Non-Canadian Holder does not deal at arm’s length, and (c) partnerships in which the Non-Canadian Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships, has owned 25% or more of the issued shares of any class or series of the capital stock of the Company; and (ii) more than 50% of the fair market value of the common shares was derived, directly or indirectly, from one or any combination of (a) real or immoveable property situated in Canada, (b) “Canadian resource properties” (as defined in the Canadian Tax Act), (c) “timber resource properties” (as defined in the Canadian Tax Act), or (d) options in respect of, or interests in, or for civil law rights in, any of the foregoing property, whether or not such property exists. Notwithstanding the foregoing, in certain circumstances set out in the Canadian Tax Act, common shares could be deemed to be “taxable Canadian property.”
Non-Canadian Holders whose common shares may constitute “taxable Canadian property” should consult their own tax advisors.
UNDERWRITING
We have entered into an underwriting agreement, dated [______], with Aegis Capital Corp., or Aegis, acting as the underwriter in this offering. Subject to the terms and conditions of the underwriting agreement with the underwriter, we have agreed to sell to the underwriter and the underwriter has agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of Units as listed next to its name in the following table:
| Underwriter | | Number of
Units comprising one common share and common warrant | | | Number of
Units comprising one Pre-Funded Warrant and common warrant | |
| Aegis Capital Corp. | | | | | | | | |
| Total | | | | | | | | |
The underwriter is committed to purchase all the securities offered by us other than those covered by the over-allotment option described below, if any, are purchased. The underwriter is not obligated to purchase the securities covered by the underwriter’s over-allotment option described below. The underwriter is offering the securities, subject to prior sale, when, as and if issued to and accepted by it, subject to approval of legal matters by its counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriter of officer’s certificates and legal opinions. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Underwriting Discounts and Expenses
The underwriter proposes initially to offer the securities to the public at the public offering price set forth on the cover page of this prospectus and to dealers at those prices less a concession not in excess of $[___] per Unit. If all of the securities offered by us are not sold at the public offering price, the underwriter may change the offering price and other selling terms by means of a supplement to this prospectus by filing of a post-effective amendment to the registration statement of which this prospectus forms a part.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise of the over-allotment option we granted to the underwriter.
| | | Per Unit comprising one common share and common warrant | | | Per Unit comprising one Pre-Funded Warrant and common warrant | | | Total with no Over- Allotment | | | Total with Full
Over- Allotment | |
| Public offering price | | $ | | | | | | | | $ | | | | $ | | |
| Underwriting discount (8.0%) | | $ | | | | | | | | $ | | | | $ | | |
| Proceeds, before expenses, to us | | $ | | | | | | | | $ | | | | $ | | |
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount, will be approximately $[______]. We have also agreed to pay (i) a non-accountable expense allowance to the underwriter equal to 1.0% of the gross proceeds of the offering, and (ii) the underwriter’s accountable expenses relating to this offering, including the underwriter’s reasonable out-of-pocket costs and expenses incident to the performance of its obligations under the underwriting agreement (including, without limitation, the reasonable fees and expenses of the underwriter’s outside legal counsel up to $125,000 in the aggregate, unless we have agreed in advance to reimburse such costs and expenses in excess of $125,000).
Over-Allotment Option
We have granted the underwriter an over-allotment option. This option, which is exercisable for up to 45 days after the date of the closing of this offering, permits the underwriter to purchase up to additional common shares and/or Pre-Funded Warrants in lieu thereof, and up to additional common warrants from us, to cover over-allotments. If the underwriter exercises all or part of this option, they will purchase common shares or Pre-Funded Warrants in lieu thereof, and common warrants at the public offering price per Unit that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
Underwriter’s Warrants
We have agreed to issue to the underwriter or its designees warrants to purchase up to ____ common shares in this offering, excluding the over-allotment option. The Underwriter’s Warrants are exercisable after six months from the commencement of sales of the offering, at a per common share exercise price equal to 125% of the public offering price per Unit in the offering, and will expire five years from the commencement of sales of the offering.
The Underwriter’s Warrants and the underlying shares may be deemed to be compensation by FINRA, and therefore will be subject to FINRA Rule 5110(g)(1), which provides that neither the underwriter’s warrants nor any of our shares issued upon exercise of the underwriter’s warrants may be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities by any person, for a period of 180 days immediately following the date of commencement of sales of the offering pursuant to which the underwriter’s warrants are being issued, subject to certain exceptions. In addition, pursuant to FINRA Rule 5110(g)(8)(A), the underwriter’s warrants are not exercisable more than five years from the commencement of sales of the public offering. The underwriter’s warrants will have anti-dilution terms that are consistent with FINRA Rule 5110(g)(8)(E) and (F). The underwriter’s warrants will also specify that the piggyback registration right shall not exceed seven years from the commencement of sales of the offering and the demand registration right shall not be more than five years, in compliance with FINRA Rule 5110(g)(8)(C)-(D).
Lock-Up Agreements
Pursuant to “lock-up” agreements, our executive officers and directors, employees and certain holders of at least 10% of our common shares and securities exercisable or exchangeable for or convertible into common shares outstanding immediately before the closing of this offering, have agreed, without the prior written consent of the underwriter, not to directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common shares, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of our common shares, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any common shares or securities convertible into or exercisable or exchangeable for common shares or any other securities of our company or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of 90 days from the closing date of this offering.
Company Standstill
We have agreed, for a period of ninety (90) days from the closing date of the offering, that without the prior written consent of the underwriter, we will not (a) offer, sell, issue, or otherwise transfer or dispose of, directly or indirectly, any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of the Company; (b) file or caused to be filed any registration statement with the Commission relating to the offering of any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of the Company; or (c) enter into any agreement or announce the intention to effect any of the actions described in subsections (a) or (b) hereof (all of such matters, the “Standstill”). So long none of such equity securities shall be saleable in the public market until the expiration of the ninety (90) day period described above, the following matters shall not be prohibited by the Standstill: (i) the adoption of an equity incentive plan and the grant of awards or equity pursuant to any equity incentive plan, and the filing of a registration statement on Form S-8; (ii) the issuance of securities of the Company upon exercise, vesting, settlement or conversion of securities or contracts of the Company outstanding on the closing date and (iii) the issuance of equity securities in connection with an acquisition or a strategic relationship, which may include the sale of equity securities. In no event should any equity transaction during the Standstill period result in the sale of equity at an offering price to the public less than that of the offering referred herein.
Right of First Refusal
We have agreed that, for the period beginning on the closing date of the offering and ending eighteen (18) months after the closing date of the offering, the Company or any of its subsidiaries (a) decides to finance or refinance any indebtedness, the underwriter (or any affiliate designated by the underwriter) shall have the right to act as sole book-runner, sole manager, sole placement agent or sole agent with respect to such financing or refinancing; or (b) decides to raise funds by means of a public offering (including at-the-market facility) or a private placement or any other capital raising financing of equity, equity-linked or debt securities, the underwriter (or any affiliate designated by the underwriter) shall have the right to act as sole book-running manager, sole underwriter or sole placement agent for such financing. If the underwriter or one of its affiliates decides to accept any such engagement, the agreement governing such engagement (each, a “Subsequent Transaction Agreement”) will contain, among other things, provisions for customary fees for transactions of similar size and nature, but in no event will the fees be less than those outlined in the underwriting agreement for this offering, and the provisions of the underwriting agreement for this offering, including indemnification, which are appropriate to such a transaction. Notwithstanding the foregoing, the decision to accept the Company’s engagement under this right shall be made by the underwriter or one of its affiliates, by a written notice to the Company, within ten (10) days of the receipt of the Company’s notification of its financing needs.
Tail Financing
The underwriter will be entitled to compensation from the Company with respect to any public or private offering or other financing or capital raising transaction of any kind by the Company to the extent that such financing or capital is provided to the Company by investors whom the underwriter had contacted or introduced to the Company during the engagement period, if such tail financing is consummated at any time within the six (6) month period following the expiration or termination of the letter of engagement between the underwriter and the Company dated October 7, 2022.
Indemnification
We have agreed to indemnify the underwriter, its affiliates and each person controlling the underwriter against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriter may be required to make for these liabilities.
Stabilization
In connection with this offering, the underwriter may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
● Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the securities while the offering is in progress.
● Over-allotment transactions involve sales by the underwriter of securities in excess of the number of securities that underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriter is not greater than the number of securities that it may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriter may close out any short position by exercising its over-allotment option and/or purchasing securities in the open market.
● Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared with the price at which they may purchase securities through exercise of the over-allotment option. If the underwriter sells more securities than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying securities in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing there could be downward pressure on the price of the securities in the open market that could adversely affect investors who purchase in the offering.
● Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of our securities. As a result, the price of our securities in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our securities. These transactions may be effected on Nasdaq, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive Market Making
In connection with this offering, the underwriter and selling group members may also engage in passive market making transactions in our common shares. Passive market making consists of displaying bids limited by the prices of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation M promulgated by the SEC limits the amount of net purchases that each passive market maker may make and the displayed size of each bid. Passive market making may stabilize the market price of the common shares at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members. The underwriter may agree to allocate a number of securities to underwriter and selling group members for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriter and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should not be relied upon by investors.
Other Relationships
The underwriter and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they may in the future receive customary fees.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who come into possession of this prospectus are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
LEGAL MATTERS
The validity of our common shares and certain other matters of Canadian law will be passed upon for us by Clark Wilson LLP. Certain matters of U.S. federal law will be passed upon for us by Sichenzia Ross Ference Carmel LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriter by Kaufman & Canoles P.C., Richmond, Virginia with respect to U.S. law.
EXPERTS
Our audited consolidated financial statements as of and for the year ended December 31, 2023 and December 31, 2022 are included in this prospectus and have been so included in reliance upon the report of Manning Elliott LLP, Chartered Professional Accountants, independent registered public accountants, upon the authority of the said firm as experts in accounting and auditing.
EXPENSES
The following table sets forth the expenses expected to be incurred by us in connection with the issuance and distribution of the Securities registered hereby, all of which expenses, except for the Securities and Exchange Commission registration fee, are estimates:
| Description | | Amount | |
| SEC Filing Fee | | $ | | |
| FINRA Filing Fee | | | | |
| Printing Expenses | | | | |
| Accounting Fees and Expenses | | | | |
| Legal Fees and Expenses | | | | |
| Miscellaneous | | | | |
| Total | | $ | | |
ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated under the laws of the Province of British Columbia. Some of our directors and officers, and some of the experts named in this prospectus, are residents of Canada or otherwise reside outside of the United States, and all or a substantial portion of their assets, and all or a substantial portion of our assets, are located outside of the United States. We have appointed an agent for service of process in the United States, but it may be difficult for shareholders who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for shareholders who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. There can be no assurance that U.S. investors will be able to enforce against us, members of our board of directors, officers or certain experts named herein who are residents of Canada or other countries outside the United States, any judgments in civil and commercial matters, including judgments under the federal securities laws.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act with respect to the securities being offered by this prospectus. This prospectus does not contain all of the information in the registration statement of which this prospectus is a part and the exhibits to such registration statement. For further information with respect to us and the securities offered by this prospectus, we refer you to the registration statement of which this prospectus is a part and the exhibits to such registration statement. Statements contained in this prospectus as to the contents of any contract or any other document are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement of which this prospectus is a part. Each of these statements is qualified in all respects by this reference.
The registration statement of which this prospectus is a part is available at the SEC’s website at http://www.sec.gov.
Immediately upon the completion of this offering, we will be subject to the information and reporting requirements of the Exchange Act, as applicable to foreign private issuers. Accordingly, we will be required to file periodic reports (including an annual report on Form 20-F, which we will be required to file within 120 days from the end of each fiscal year), and other information with the SEC pursuant to the Exchange Act. The registration statement, reports and other information so filed are available at the SEC’s website referred to above. We also maintain a website at www.asepmedical.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
Units, each Unit consisting of
One Common Share or One Pre-Funded Warrant to Purchase One Common Share, and
One Common Warrant to purchase One Common Share
Up to [___] Common Shares underlying the Pre-Funded Warrants and the Common Warrants
ASEP MEDICAL HOLDINGS INC.
Sole Book-Running Manager
Aegis Capital Corp.
PRELIMINARY PROSPECTUS
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of directors and officers
Sections 159 to 164 of the BCBCA authorize companies to indemnify past and present directors, officers and certain other individuals for the liabilities incurred in connection with their services as such (including costs, expenses and settlement payments) unless such individual did not act honestly and in good faith with a view to the best interests of the company and, in the case of a criminal or administrative proceeding, if such individual did not have reasonable grounds for believing his or her conduct was lawful. In the case of a suit by or on behalf of the corporation, a court must approve the indemnification.
Our articles require us to indemnify directors and officers to the extent required by law.
We have entered into agreements with our directors and certain officers, or an Indemnitee, to indemnify the Indemnitee, to the fullest extent permitted by law and subject to certain limitations, against all liabilities, costs, charges and expenses reasonably incurred by an Indemnitee in an action or proceeding to which the Indemnitee was made a party by reason of the Indemnitee being an officer or director of (i) our company or (ii) an organization of which we are a shareholder or creditor if the Indemnitee serves such organization at our request.
We maintain insurance policies relating to certain liabilities that our directors and officers may incur in such capacity.
Item 7. Recent sales of unregistered securities
During the past three years, we have issued securities in the below transactions, each of which was exempt from the registration requirements of the Securities Act. Except for the common shares that were issued upon the exercise of our warrants, all of the below-referenced securities were issued pursuant to the exemption from registration under Section 4(a)(2) of the Securities Act and are deemed to be restricted securities for purposes of the Securities Act. There were no underwriters or placement agents employed in connection with any of these transactions. Use of the exemption provided in Section 4(a)(2) for transactions not involving a public offering is based on the following facts:
● Neither we nor any person acting on our behalf solicited any offer to buy or sell securities by any form of general solicitation or advertising.
● The recipients were either accredited or otherwise sophisticated individuals who had such knowledge and experience in business matters that they were capable of evaluating the merits and risks of the prospective investment in our securities.
● The recipients had access to business and financial information concerning our company.
● All securities issued were issued with a restrictive legend and may only be disposed of pursuant to an effective registration or exemption from registration in compliance with federal and state securities laws.
The common shares that were issued upon the exercise of our warrants were issued pursuant to the exemption from registration under Section 3(a)(9) of the Securities Act and are deemed to be restricted securities for purposes of the Securities Act.
| | (a) | On January 20, 2021, the Company issued 2,500,000 common shares at $0.001 per share for proceeds of $2,500. |
| | | |
| | (b) | On April 14, 2021, the Company issued 8,000,000 common shares at $0.001 per share for proceeds of $8,000. |
| | | |
| | (c) | On April 16, 2021, the Company issued 6,500,000 common shares at $0.02 per share for proceeds of $130,000. |
| | | |
| | (d) | On May 25, 2021, the Company closed a private placement of unsecured convertible debentures in the aggregate amount of $500,000. |
| | | |
| | (e) | On November 9, 2021, in connection with the amalgamation, the Company issued 25,540,626 common shares with a value of $12,770,313 to the shareholders of Asep Medical Inc. |
| | | |
| | (f) | On November 10, 2021, the Company converted 11,731,500 special warrants that were issued for total proceeds of $5,865,750 to 11,731,500 common shares. |
| | | |
| | (g) | On November 10, 2021, the Company converted the total outstanding convertible debentures of $500,000 at a conversion price of $0.269 resulting in 1,858,218 common shares being issued (see Note 8 to our annual financial statements). |
| | | |
| | (h) | On November 18, 2021, the Company granted 4,540,000 stock options (the “November 2021 Options”), with each November 2021 Option, once vested, exercisable into one common share of the Company at an exercise price of $0.50 per common share until November 18, 2031. To date, 4,052,500 November 2021 Options have vested and 287,500 November 2021 Options will vest by November 18, 2023. On June 24, 2022, 200,000 November 2021 Options were cancelled pursuant to their terms. To date, none of the November 2021 Options have been exercised. |
| | | |
| | (i) | On September 29, 2022, the Company granted 200,000 stock options (the “September 2022 Options”), with each September 2022 Option, once vested, exercisable into one common share of the Company at an exercise price of $0.30 per common share until September 29, 2032. All 200,000 September 2022 Options have vested but none have been exercised. |
| | | |
| | (j) | On October 1, 2022, the Company granted 200,000 stock options (the “October 2022 Options”), with each October 2022 Option, once vested, exercisable into one common share of the Company at an exercise price of $0.30 per common share until October 1, 2032. To date, 100,000 October 2022 Options have vested, with the remaining October 2022 Options vesting in 25% increments every six months starting from April 1, 2024. To date, none of the October 2022 Options have been exercised. |
| | | |
| | (k) | On November 24, 2022, the Company granted 680,000 stock options (the “November 2022 Options”), with each November 2022 Option exercisable into one common share of the Company at an exercise price of $0.30 per common share until November 24, 2032. All 680,000 November 2022 Options have vested but none have been exercised to date. |
| | | |
| | (l) | On December 15, 2022, the Company issued 6,000,000 common shares to acquire the non- controlling interest in SafeCoat (see note 5 to our financial statements). Of the common shares, 1,500,000 are being held in escrow and are considered contingently returnable shares. |
| | (m) | On December 22, 2022, the Company issued unsecured convertible debentures in the aggregate principal amount of CAD$300,000. The debentures mature on December 22, 2024, bear interest at a rate of 10% per annum, are unsecured and are convertible into common shares of the company at a conversion price of CAD$0.33 per share until the maturity date. On December 27, 2023, the convertible debenture plus accrued interest thereon ($362,186) were debt settled. |
| | | |
| | (n) | On January 19, 2023, the Company granted 400,000 stock options (the “January 2023 Options”), with each January 2023 Option, once vested, exercisable into one common share of the Company at an exercise price of $0.36 per common share until January 19, 2033. To date, 362,500 January 2023 Options have vested and the remaining will vest in equal parts of 6250 every quarter starting from September 1, 2023, with the last amount vesting on December 1, 2024. To date, none of the January 2023 Options have been exercised. |
| | | |
| | (o) | On March 1, 2023, the Company granted 300,000 stock options (the “March 2023 Options”), with each March 2023 Option, once vested, exercisable into one common share of the Company at an exercise price of $0.30 per common share until March 1, 2033. To date, 100,000 of the March 2023 Options have vested, with the remaining 200,000 March 2023 Options vesting in 25% increments every six months starting on September 2, 2023. To date, none of the March 2023 Options have been exercised. |
| | | |
| | (p) | On March 1, 2023, the Company granted 6,200,000 Restricted Share Units (“March 2023 RSUs”), with each RSU representing the right to receive, once vested, and in accordance with the RSU award agreement under which it is granted and the Company’s Long Term Incentive Plan, one common share in the capital of the Company. To date, 6,200,000 March 2023 RSUs have vested. |
| | | |
| | (q) | On June 20, 2023 the Company granted 93,034 stock options (the “June 2023 Options”), with each June 2023 Option exercisable into one common share of the Company at an exercise price of $0.39 per common share until June 20, 2033. All 93,034 June 2023 Options have vested but none have been exercised to date. |
| | | |
| | (r) | On June 20, 2023, the Company also granted 13,034 RSUs (the “June 2023 RSUs”), with each June 2023 RSU representing the right to receive, once vested, and in accordance with the RSU award agreement under which it is granted and the Company’s Long Term Incentive Plan, one common share in the capital of the Company. To date, 9,777 June 2023 RSUs have vested, with the remaining 3,257 RSUs vesting on June 20, 2024. |
| | | |
| | (s) | On July 19, 2023, the Company issued 145,161 common shares to certain members of the Advisory Board to compensate them for consulting services provided totalling $45,000 for the period March 1, 2023 to May 31, 2023. |
| | | |
| | (t) | On July 21, 2023, stock options entitling the holder to acquire 200,000 common shares were exercised for proceeds of $60,000. |
| | | |
| | (u) | On September 12, 2023, the Company issued 249,999 common shares to certain members of the Advisory Board to compensate them for consulting fees services provided totalling $45,000 for the period June 1, 2023 to August 31, 2023. |
| | | |
| | (v) | On December 1, 2023, the Company issued 163,635 common shares to certain members of the Advisory Board to compensate them for consulting fees services provided totalling $45,000 for the period September 1, 2023 to November 30, 2023. |
| | | |
| | (w) | On December 19, 2023, the Company completed a unit offering at $0.20 per unit for gross proceeds of $587,000 and issued 2,935,000 shares ($333,481) and 2,935,000 warrants ($253,519). Each warrant entitles the holder to acquire one common share for a period of two years at a price of $0.26 per share. In connection with the unit offering, the Company paid cash finder’s fees of $15,600 and issued 128,000 finder’s warrants ($23,772). |
| | | |
| | (x) | On December 27, the Company settled debts totalling $783,186 through the issuance of 3,915,930 shares (445,640) and 3,915,930 warrants ($337,546). Each warrant entitles the holder to acquire one common share for a period of two years at a price of $0.26 per share. |
| | | |
| | (y) | On March 1, 2024, the Company issued 209,301 common shares to certain members of the Advisory Board to compensate them for consulting fees services provided totalling $45,000 for the period December 1, 2023 to February 29, 2024. |
| | | |
| | (z) | On March 7, 2024, the Company issued 1,662,500 common shares on settlement of the fourth tranche of the RSUs granted March 1, 2023 and the estimated fair value of these shares ($457,188) was transferred from reserves to capital stock on date of issue. |
| | | |
| | (aa) | On March 27, 2024, the Company issued 3,259 common shares on settlement of the third tranche of the RSUs granted June 20, 2023 and the estimated fair value of these shares ($1,173) was transferred from reserves to capital stock on date of issue. |
As at May 28, 2024, there were 76,059,147 issued and outstanding common shares.
Item 8. Exhibits and Financial Statement Schedules
EXHIBIT INDEX
The following documents are filed as part of this registration statement:
Exhibit Number | | Exhibit Description |
| | | |
| 1.1** | | Form of Underwriting Agreement. |
| 3.1+ | | Notice of Articles dated November 9, 2021. |
| 3.2+ | | Notice of Articles dated July 12, 2022. |
| 3.3+ | | Notice of Articles dated October 6, 2022. |
| 3.4+ | | Notice of Articles dated November 14, 2022. |
| 3.5+ | | Articles of ASEP Medical Inc. |
| 4.1 | | Specimen Stock Certificate evidencing common shares. |
| 4.2** | | Form of Pre-Funded Warrant |
4.3** | | Form of common warrant |
| 4.4** | | Form of Underwriter’s Warrant. |
| 4.5** | | Form of Warrant Agent Agreement for common warrants |
| 4.6** | | Form of Warrant Agent Agreement for Pre-Funded Warrants |
| 5.1** | | Opinion of Sichenzia Ross Ference Carmel LLP. |
| 5.2** | | Opinion of Clark Wilson LLP. |
| 10.1 | | ASEP Medical Holdings Inc. Long-Term Performance Incentive Plan |
| 10.2+ | | License Agreement between the University of British Columbia and ABT Innovation Inc. dated April 25, 2017. |
| 10.3+ | | Hancock-Sepset License Agreement dated March 13, 2017. |
| 10.4 | | The Michael Smith Foundation Commitment Letter dated April 29, 2020 between ABT and the Michael Smith Foundation for Health Research. |
| 10.5+ | | The Burton Engagement Letter dated January 18, 2021. |
| 10.6+ | | Employment agreement dated March 1, 2021 between ASEP Medical, Inc. and Dr. Fadia Saad. |
| 10.7+ | | Consulting agreement dated April 1, 2021 between ASEP Medical, Inc. and Burton Financial Inc. |
| 10.8 | | Amendment No. 1 dated July 7, 2022 to Collaborative Research Agreement dated June 2, 2021 between ABT Innovations Inc. and the University of British Columbia. |
| 10.9+ | | Collaborative Research Agreement between the University of British Columbia and ABT Innovations Inc. dated June 2, 2021. |
| 10.10+ | | Collaborative Research Agreement between Sepset Biosciences Inc. and University of British Columbia dated August 6, 2021. |
| 10.11+ | | Option Agreement between ASEP Medical Inc., ABT Innovations Inc. and all of the shareholders of ABT other than UBC dated May 14, 2021. |
| 10.12+ | | The Share Purchase Option Agreement dated May 14, 2021 among ASEP Medical, Inc., ABT Innovations Inc. and the University of British Columbia. |
| 10.13+ | | Option Agreement between ASEP Medical Inc., Sepset Biosciences Inc., and all of the shareholders of Sepset Bisciences Inc. dated May 14, 2021. |
| 10.14+ | | Employment Agreement dated June 1, 2021 between ASEP Medical, Inc. and Dr. Evan Haney. |
| 10.15+ | | Employment Agreement dated November 23, 2022 between ASEP Medical Holdings Inc. and Jennifer Gretchen. |
| 10.16+ | | Escrow Agreement dated November 9, 2021 among the Company, Odyssey Trust Company and the holders of the Escrowed Securities. |
| 10.17+ | | The Amalgamation Agreement dated June 3, 2021 among ASEP Medical Inc., Trenchant Life Sciences Investment Corp. and 1295277 B.C. LTD. |
| 10.18+ | | Consulting agreement dated October 1, 2022 between ASEP Medical Inc. and J.M. Tucker Professional Corporation. |
| 10.19+ | | Consulting agreement dated June 30, 2022 between ASEP Medical, Inc. and Murphy Enterprises Inc. |
| 10.20+ | | The Earn-In and Option Agreement dated December 1, 2022 among SafeCoat Medical Inc., SafeCoat Medical Inc.’s shareholders, and ASEP. |
| 10.21+ | | Joint Venture and License Agreement dated May 15, 2023 among ASEP Medical Holdings Inc., Sepset Biosciences Inc., and Seaspring W.L.L. |
| 10.22+ | | Feasibility Study Agreement entered into with Bohai Biomedical Technology Development Co., Ltd. on October 3, 2022. |
| 10.23+ | | Letter of Intent between iFyber LLC and ABT Innovations collaboration letter of intent dated July 17, 2018. |
| 10.24+ | | Employment Agreement dated October 1, 2022 between ASEP Medical, Inc. and Dr. Robert E.W. Hancock. |
| 10.25+ | | Collaborative Research Agreement dated April 8, 2022 between the University of British Columbia and Sepset BioSciences Inc. |
| 10.26+ | | Letter of Intent between iFyber LLC and ABT Innovations collaboration letter of intent dated September 22, 2016. |
| 10.27+ | | First Amendment dated May 18, 2021 to Confidential Disclosure Agreement dated September 22, 2016 between iFyber LLC and ABT Innovations Inc. |
| 10.28+ | | Letter of Intent between iFyber LLC and ABT Innovations collaboration letter of intent dated December 6, 2016. |
| 10.29+ | | Start-Up License Agreement dated July 21, 2023 among SafeCoat Medical Ltd. and the University of British Columbia. |
| 10.30+ | | Material terms of the Joint Venture Agreement dated October 27, 2023 between Sepset BioSciences, Inc. and Hunan Xiang Jiang Sansure BioTech Fund, LP. |
| 21.1 | | List of Subsidiaries |
| 23.1 | | Consent of Independent Registered Public Accounting Firm. |
| 23.3** | | Consent Sichenzia Ross Ference Carmel LLP (included in Exhibit 5.1). |
| 23.4 | | Consent of RTI Health Solutions |
| 23.5** | | Consent of Clark Wilson LLP |
| 24.1* | | Power of Attorney |
| 107* | | Filing Fee Table |
* Previously filed
**To be filed by amendment.
+ Pursuant to Item 601(b)(10)(iv) of Regulation S-K promulgated by the U.S. Securities and Exchange Commission, certain portions of this exhibit have been omitted because it is both not material and the type of information that the company treats as private or confidential.
Item 9. Undertakings
The undersigned registrant hereby undertakes to provide to the Underwriter at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the Underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding)is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by Section 10(a)(3) of the Securities Act;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement(or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective Registration Statement;
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering;
(4) To file a post-effective amendment to the registration statement to include any financial statements required by “Item 8.A.of Form 20-F (17 CFR 249.220f)” at the start of any delayed offering or throughout a continuous offering.
(5) That, for the purpose of determining liability under the Securities Act to any purchaser: if the registrant is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use
(6) For the purposes of determining liability under the Securities Act of 1933 to any purchaser in the initial distributions of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
i. Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
ii. Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
iii. The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
iv. Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(7) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the undersigned, the duly authorized representative of ASEP Medical Holdings Inc., in the United States, has signed this amended registration statement on August 9, 2024.
| | ASEP MEDICAL HOLDINGS INC. |
| | |
| | By: | /s/ Robert E.W. Hancock |
| | | Robert E.W. Hancock |
| | | Chief Executive Officer
(Principal Executive Officer) |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Name | | Position | | Date |
| | | | | |
| /s/ Robert E.W. Hancock | | Chairman and Chief Executive Officer | | August 9, 2024 |
| Robert E.W. Hancock | | (Principal Executive Officer) | | |
| | | | | |
| /s/ * | | Chief Financial Officer | | August 9, 2024 |
| Jacqueline Tucker | | (Principal Financial and Accounting Officer) | | |
| | | | | |
| /s/ * | | Chief Operating Officer and Director | | August 9, 2024 |
| Timothy Murphy | | | | |
| | | | | |
| /s/ * | | Director | | August 9, 2024 |
| Derrold Norgaard | | | | |
| | | | | |
| /s/ * | | Director | | August 9, 2024 |
| Richard Heinzl | | | | |
| *By: | /s/ Robert E.W. Hancock | |
| | Robert E.W. Hancock | |
| | Attorney-in-fact | |
ASEP MEDICAL HOLDINGS INC.
Consolidated Financial Statements
For the Years Ended December 31, 2023 and 2022
Expressed in Canadian Dollars

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of ASEP Medical Holdings Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of ASEP Medical Holdings Inc. and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of comprehensive loss, changes in equity and cash flows for the years ended December 31, 2023 and 2022, and the related notes (collectively referred to as the “consolidated financial statements”).
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as at December 31, 2023 and 2022, and its financial performance and its cash flows for the years ended December 31, 2023 and 2022, in conformity with IFRS Accounting Standards as issued by the International Accounting Standards Board.
Explanatory Paragraph – Going Concern
The consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 1, for the year ended December 31, 2023, the Company incurred a net loss of $9,430,513 and used cash in operating activities of $2,712,598. As at December 31, 2023, the Company had an accumulated deficit of $14,983,767 and a working capital deficiency of $744,644. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
“/s/ Manning Elliott LLP”
CHARTERED PROFESSIONAL ACCOUNTANTS
Vancouver, Canada
April 26, 2024
We have served as the Company’s auditor since 2022.
ASEP Medical Holdings Inc.
Consolidated Statements of Financial Position
(Expressed in Canadian Dollars)
| | | Notes | | December 31,
2023 | | | December 31,
2022 | |
| | | | | | | | | |
| Assets | | | | | | | | | | |
| Current assets | | | | | | | | | | |
| Cash | | | | $ | 64,721 | | | $ | 2,130,390 | |
| GST receivable | | | | | 25,423 | | | | 114,619 | |
| Deposits and prepaid expenses | | | | | 160,799 | | | | 1,153,636 | |
| | | | | | 250,943 | | | | 3,398,645 | |
| Non-current assets | | | | | | | | | | |
| Equipment | | 6 | | | 37,183 | | | | 50,204 | |
| Intangible assets | | 7 | | | 22,949,560 | | | | 23,629,740 | |
| Investment in Hunan Sanway Sepsmart Ltd. | | 8 | | | 2,375,000 | | | | - | |
| | | | | | 25,361,743 | | | | 23,679,944 | |
| TOTAL ASSETS | | | | $ | 25,612,686 | | | $ | 27,078,589 | |
| | | | | | | | | | | |
| Liabilities | | | | | | | | | | |
| Current liabilities | | | | | | | | | | |
| Accounts payable and accrued liabilities | | 13 | | $ | 677,731 | | | $ | 528,161 | |
| Deferred revenue | | 8 | | | 297,586 | | | | - | |
| Loan payable | | 9,13 | | | 20,270 | | | | - | |
| | | | | | 995,587 | | | | 528,161 | |
| Non-current liabilities | | | | | | | | | | |
| Deferred revenue | | 8 | | | 2,056,274 | | | | - | |
| | | | | | 3,051,861 | | | | 528,161 | |
| TOTAL LIABILITIES | | | | | | | | | | |
| Equity | | | | | | | | | | |
| Share capital | | 5,9 | | | 24,045,616 | | | | 19,842,132 | |
| Other components of equity | | 8,9 | | | 3,550,141 | | | | 2,352,934 | |
| Deficit | | | | | (14,983,767 | ) | | | (6,725,067 | ) |
| | | | | | 12,611,990 | | | | 15,469,998 | |
| Non-controlling interests | | 10 | | | 9,948,835 | | | | 11,080,429 | |
| TOTAL EQUITY | | | | | 22,560,825 | | | | 26,550,427 | |
| TOTAL LIABILITIES AND EQUITY | | | | $ | 25,612,686 | | | $ | 27,078,589 | |
Nature of operations and going concern (Note 1)
Commitments (Note 16)
Subsequent events (Note 17)
On behalf of the board:
| “/s/ Derrold Norgaard” | | “/s/ Richard Heinzl” |
| Derrold Norgaard, Chairman of the Audit Committee and Independent Director | | Dr. Richard Heinzl, Independent Director |
The accompanying notes are an integral part of these consolidated financial statements
ASEP Medical Holdings Inc.
Consolidated Statements of Comprehensive Loss
(Expressed in Canadian Dollars)
| | | Notes | | For the year ended December 31,
2023 | | | For the year ended December 31,
2022 | |
| | | | | | | | | |
| Revenue | | 8 | | $ | 21,140 | | | $ | - | |
| | | | | | 21,140 | | | | | |
| Expenses | | | | | | | | | | |
| Amortization | | | | | 1,294,266 | | | | 1,282,394 | |
| Board advisory fees | | | | | 230,673 | | | | | |
| Compensation | | 13 | | | 782,301 | | | | 556,550 | |
| Consulting | | 13 | | | 514,478 | | | | 246,946 | |
| General & administrative | | | | | 222,903 | | | | 336,693 | |
| Investor relations | | | | | 1,110,409 | | | | 845,950 | |
| Patent fees | | | | | 134,880 | | | | 155,401 | |
| Professional fees | | | | | 760,006 | | | | 284,606 | |
| Research & development costs | | | | | 678,543 | | | | 615,040 | |
| Share-based compensation | | 11,13 | | | 1,952,054 | | | | 1,119,072 | |
| Transfer agent & filing fees | | | | | 61,890 | | | | - | |
| | | | | | 7,742,403 | | | | 5,442,653 | |
| Operating loss | | | | | (7,721,263 | ) | | | (5,442,653 | ) |
| Other income (expenses) | | | | | | | | | | |
| Borrowing costs | | 9,10 | | | (270 | ) | | | (814 | ) |
| Foreign exchange gain (loss) | | | | | (4,116 | ) | | | (2,399 | ) |
| Loss on debt settlement | | | | | (429,864 | ) | | | | |
| SafeCoat Medical Inc. acquisition expense | | 5 | | | (1,275,000 | ) | | | (400,521 | |
| Loss before deferred tax recovery | | | | | (9,430,513 | ) | | | (5,846,386 | ) |
| Deferred tax recovery | | | | | - | | | | 89,100 | |
| Loss and comprehensive loss for period | | | | $ | (9,430,513 | ) | | $ | (5,757,286 | ) |
| | | | | | | | | | | |
| Net loss and comprehensive loss attributable to: | | | | | | | | | | |
| Shareholders | | | | $ | (8,227,329 | ) | | $ | (4,524,981 | ) |
| Non-controlling interests | | | | | (1,203,185 | ) | | | (1,232,305 | ) |
| | | | | $ | (9,430,513 | ) | | $ | (5,757,286 | ) |
| | | | | | | | | | | |
| Loss per share - basic and fully diluted | | | | $ | (0.13 | ) | | $ | (0.08 | ) |
| | | | | | | | | | | |
| Weighted average number of common shares - basic and fully diluted | | | | | 62,235,372 | | | | 56,196,097 | |
The accompanying notes are an integral part of these consolidated financial statements
ASEP Medical Holdings Inc.
Consolidated Statement of Changes in Equity
(Expressed in Canadian Dollars)
| | | Share Capital | | | Other components of equity | | | | | | Equity Attributable | | | Non- | | | | |
| | | Shares | | | Amount | | | Convertible Debenture | | | Warrants | | | Contributed Surplus | | | Deficit | | | to Shareholders | | | Controlling Interest | | | Total Equity | |
| Balance - December 31, 2021 | | | 56,130,344 | | | $ | 19,467,132 | | | $ | - | | | $ | 35,921 | | | $ | 956,227 | | | $ | (2,200,086 | ) | | $ | 18,259,194 | | | $ | 12,312,734 | | | $ | 30,571,928 | |
| Issuance of common shares for non cash consideration | | | 6,000,000 | | | | 375,000 | | | | - | | | | - | | | | - | | | | - | | | | 375,000 | | | | - | | | | 375,000 | |
| Issance of convertible debenture for cash | | | | | | | - | | | | 330,000 | | | | - | | | | - | | | | - | | | | 330,000 | | | | - | | | | 330,000 | |
| Deferred tax liability | | | | | | | | | | | (89,100 | ) | | | - | | | | - | | | | - | | | | (89,100 | ) | | | - | | | | (89,100 | ) |
| Borrowing costs | | | | | | | | | | | 814 | | | | - | | | | - | | | | - | | | | 814 | | | | - | | | | 814 | |
| Expiration of warrants | | | | | | | - | | | | - | | | | (35,921 | ) | | | 35,921 | | | | - | | | | - | | | | - | | | | - | |
| Share- based compensation | | | | | | | - | | | | - | | | | - | | | | 1,119,072 | | | | - | | | | 1,119,072 | | | | - | | | | 1,119,072 | |
| Net loss and comprehensive loss for year | | | | | | | - | | | | - | | | | - | | | | - | | | | (4,524,981 | ) | | | (4,524,981 | ) | | | (1,232,305 | ) | | | (5,757,286 | ) |
| Balance - December 31, 2022 | | | 62,130,344 | | | | 19,842,132 | | | | 241,714 | | | | - | | | | 2,111,220 | | | | (6,725,067 | ) | | | 15,469,998 | | | | 11,080,429 | | | | 26,550,427 | |
| Debt settlement | | | 3,915,930 | | | | 639,539 | | | | (273,086 | ) | | | 484,412 | | | | - | | | | - | | | | 850,865 | | | | - | | | | 850,865 | |
| Issue of common shares for services | | | 558,795 | | | | 135,000 | | | | - | | | | - | | | | - | | | | - | | | | 135,000 | | | | - | | | | 135,000 | |
| Issue of common shares on exercise of stock options | | | 200,000 | | | | 95,173 | | | | - | | | | - | | | | (35,173 | ) | | | - | | | | 60,000 | | | | - | | | | 60,000 | |
| Issue of common shares on vesting of RSUs | | | 4,444,018 | | | | 1,222,659 | | | | - | | | | - | | | | - | | | | (1,222,659 | ) | | | - | | | | - | | | | - | |
| Life offering – unit placement | | | 2,935,000 | | | | 333,481 | | | | - | | | | 253,519 | | | | - | | | | - | | | | 587,000 | | | | - | | | | 587,000 | |
| Life offering – finder’s warrants | | | | | | | - | | | | - | | | | 23,772 | | | | - | | | | - | | | | 23,772 | | | | - | | | | 23,772 | |
| Life offering – finder’s fees & finder’s warrants | | | | | | | (22,368 | ) | | | - | | | | (17,004 | ) | | | - | | | | - | | | | (39,372 | ) | | | - | | | | (39,372 | ) |
| Release of contingently returnable shares | | | | | | | 1,800,000 | | | | - | | | | - | | | | - | | | | - | | | | 1,800,000 | | | | - | | | | 1,800,000 | |
| Borrowing costs | | | | | | | - | | | | 31,372 | | | | - | | | | - | | | | (31,372 | ) | | | - | | | | - | | | | - | |
| Share-based compensation | | | | | | | - | | | | - | | | | - | | | | 1,952,054 | | | | - | | | | 1,952,054 | | | | - | | | | 1,952,054 | |
| Net loss and comprehensive loss for year | | | | | | | - | | | | - | | | | - | | | | - | | | | (8,227,328 | ) | | | (8,227,328 | ) | | | (1,203,185 | ) | | | (9,430,513 | ) |
| Balance – December 31, 2023 | | | 74,184,087 | | | $ | 24,045,616 | | | $ | - | | | $ | 744,699 | | | $ | 2,805,442 | | | $ | (14,983.767 | ) | | $ | 12,611,990 | | | $ | 9,948,835 | | | $ | 20,560,825 | |
The accompanying notes are an integral part of these consolidated financial statements
ASEP Medical Holdings Inc.
Consolidated Statements of Cash Flows
(Expressed in Canadian Dollars)
| | | Notes | | For the year ended
December 31,
2023 | | | For the year ended
December 31,
2022 | |
| | | | | | | | | |
| Cash provided by (used for) operating activities | | | | | | | | | | |
| Loss for year | | | | $ | (9,430,513 | ) | | $ | (5,757,286 | ) |
| Items not involving cash | | | | | | | | | | |
| Amortization | | | | | 1,294,266 | | | | 1,282,394 | |
| Borrowing costs | | | | | 270 | | | | 814 | |
| Consulting | | | | | - | | | | (150,752 | ) |
| Deferred tax recovery | | | | | - | | | | (89,100 | ) |
| Loss on debt settlement | | 11 | | | 429,864 | | | | - | |
| Revenue | | | | | (21,140 | ) | | | | |
| SafeCoat acquisition expenses | | 5 | | | 1,275,000 | | | | 400,521 | |
| Shares issued for services | | 11 | | | 135,000 | | | | - | |
| Share-based compensation | | | | | 1,952,054 | | | | 1,119,072 | |
| Changes in operating assets and liabilities | | | | | | | | | | |
| GST receivable | | | | | 89,196 | | | | (41,220 | ) |
| Deposits and prepaid expenses | | | | | 992,838 | | | | (762,875 | ) |
| Accounts payable and accrued liabilities | | | | | 570,567 | | | | 383,956 | |
| | | | | | (2,712,598 | ) | | | (3,614,476 | ) |
| Cash flows provided by (used for) investing activities | | | | | | | | | | |
| Net assets acquired from acquisition of SafeCoat Medical Inc. | | | | | - | | | | 125,231 | |
| Purchase of equipment | | | | | (1,227 | ) | | | - | |
| Trademark costs | | | | | (3,244 | ) | | | (435 | ) |
| | | | | | (4,471 | ) | | | 124,796 | |
| Cash flows provided by (used for) financing activities | | | | | | | | | | |
| Loan advances | | | | | 20,000 | | | | - | |
| Convertible debenture | | | | | - | | | | 330,000 | |
| Life offering – cash proceeds | | | | | 587,000 | | | | - | |
| Life offering – finder’s fees paid | | | | | (15,600 | ) | | | - | |
| Exercise of stock options | | | | | 60,000 | | | | - | |
| | | | | | 651,400 | | | | 330,000 | |
| Increase (decrease) in cash and cash equivalents | | | | | (2,065,669 | ) | | | (3,159,680 | ) |
| Cash and cash equivalents- beginning of period | | | | | 2,130,390 | | | | 5,290,070 | |
| Cash and cash equivalents- end of period | | | | $ | 64,721 | | | $ | 2,130,390 | |
| | | | | | | | | | | |
| Non cash investing and financing activities | | | | | | | | | | |
| Consideration given to acquire SafeCoat Medical Inc. | | | | $ | 1,800,000 | | | $ | 525,752 | |
| Debt settlement of accrued liabilities | | | | $ | 850,865 | | | $ | - | |
| Debt settlement of convertible debenture | | | | $ | 273,086 | | | $ | - | |
| Investment in Hunan Sanway Sepsmart Ltd. | | | | $ | 2,375,000 | | | $ | - | |
| Life offering – finder’s warrants issued | | | | $ | 23,772 | | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 1. | Nature of operations and going concern |
ASEP Medical Holdings Inc. (the “Company” or “ASEP”) was incorporated under the British Columbia Business Corporations Act on January 20, 2021. On November 22, 2021, the Company commenced trading on the Canadian Securities Exchange (the “CSE”) as a life sciences issuer under the trading symbol “ASEP”. The Company’s head office is located at Unit 420, 730 View Street, Victoria, BC V8W 3Y7. ASEP is in the business of acquiring research and development assets, technologies and/or businesses in the area of life sciences and medical diagnostics.
These consolidated financial statements are prepared on a going concern basis, which contemplates that the Company will continue in operation for at least the next twelve months from December 31, 2023 and will be able to realize on its assets and discharge its liabilities in the normal course of business. For the year ended December 31, 2023, the Company incurred a net loss of $9,430,513 and used cash in operating activities of $2,712,598. As at December 31, 2023, the Company had an accumulated deficit of $14,973,218 and working capital deficit of $744,644.
Based on the Company’s financial position as at December 31, 2023, the available funds are not considered adequate to meet requirements for the estimated operations and development activities on the Company’s technologies in the coming twelve-month period. These requirements may be adversely impacted by an absence of normal available financing due to the continued uncertainty in the markets. To address its financing requirements, the Company will seek financing through and not limited to debt financing, equity financing and strategic alliances. However, there is no assurance that such financing will be available. This material uncertainty casts significant doubt upon the Company’s ability to continue as a going concern. If the going concern assumption were not appropriate for these financial statements, then adjustments would be necessary to the carrying values of assets, liabilities, the reported income and expenses and the consolidated statement of financial position classifications used. Such adjustments could be material.
| 2. | Statement of compliance with IFRS Accounting Standards |
These consolidated financial statements of the Company and its subsidiaries are prepared in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (“IFRS”). These consolidated financial statements were approved by the Board of Directors on April 26, 2024.
| 3. | Material accounting policies |
Basis of presentation
These consolidated financial statements have been prepared on an accrual basis and on a historical cost basis. The preparation of the consolidated financial statements in compliance with IFRS requires management to make certain critical accounting estimates. It also requires management to exercise judgment in applying the Company’s accounting policies. The areas involving a higher degree of judgment of complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in note 3. These consolidated financial statements are prepared in Canadian dollars, with all amounts rounded to the nearest dollar, unless otherwise stated.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies (con’t) |
Consolidation
These consolidated financial statements include the accounts of the Company and its subsidiaries. Subsidiaries are all entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are consolidated from the date on which control is transferred to the Company and are deconsolidated from the date that control ceases.
All intercompany transactions, balances and unrealized gains and losses from intercompany transactions in Canada are eliminated on consolidation. The functional and presentation currency of the Company and its subsidiaries is the Canadian dollar. The principal subsidiaries of ASEP and their geographic locations at December 31, 2023, are as follows:
| | | Principal Activity | | Location | | | Percentage ownership | |
| Asep Medical Inc. (“ASEP Medical”) | | Life Sciences | | | Canada | | | | 100 | % |
| ABT Innovations Inc. (“ABT”) | | Life Sciences | | | Canada | | | | 50.1 | % |
| Sepset Biosciences Inc. (“Sepset”) | | Life Sciences | | | Canada | | | | 50.1 | % |
| OHP Innovations Inc. (“OHP”) | | Life Sciences | | | Canada | | | | 100 | % |
| SafeCoat Medical Inc. (“SafeCoat”) | | Life Sciences | | | Canada | | | | 88 | % |
Non-controlling interest
Non-controlling interest (“NCI”) represents equity interests in subsidiaries owned by outside parties. The share of net assets of subsidiaries attributable to non-controlling interest is presented as a component of equity. The loss and each component of other comprehensive income are attributed to non-controlling interests where applicable.
Associates
An associate is an entity over whose operating and financial policies the Company exercises significant influence. Significant influence is presumed to exist where the Company has between 20 percent and 50 percent of the voting rights but can also arise where the Company holds less than 20 percent of the voting rights but has the power to be actively involved and influential in policy decisions affecting the entity. The Company’s share of the net assets, post-tax results and reserves of the associate are included in the consolidated financial statements using the equity accounting method. This involves recording the investment initially at cost to the Company, and then, in subsequent periods, adjusting the carrying amount of the investment to reflect the Company’s share of the associate’s results. Unrealized gains on transactions between the Company and an associate are eliminated to the extent of the Company’s interest in the associate.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies (cont’d) |
Financial instruments
Classification
The Company classifies its financial instruments in the following categories: at fair value through profit and loss (“FVTPL”), at fair value through other comprehensive income (loss) (“FVTOCI”) or at amortized cost. The Company determines the classification of financial assets at initial recognition. The classification of debt instruments is driven by the Company’s business model for managing the financial assets and their contractual cash flow characteristics. Equity instruments that are held for trading are classified as FVTPL. For other equity instruments, on the day of acquisition the Company can make an irrevocable election (on an instrument-by-instrument basis) to designate them as at FVTOCI. Financial liabilities are measured at amortized cost, unless they are required to be measured at FVTPL (such as instruments held for trading or derivatives) or if the Company has opted to measure them at FVTPL.
The following table shows the classification of the Company’s financial instruments under IFRS 9:
| Financial assets/liabilities | | Classification - IFRS 9 |
| Cash and cash equivalents | | FVTPL |
| Accounts payable and accrued liabilities | | Amortized cost |
| Loans Payable | | Amortized cost |
Measurement
Financial assets and liabilities at amortized cost
Financial assets and liabilities at amortized cost are initially recognized at fair value plus or minus transaction costs, respectively, and subsequently carried at amortized cost less any impairment.
Financial assets and liabilities at FVTPL
Financial assets and liabilities carried at FVTPL are initially recorded at fair value and transaction costs are expensed in the statements of comprehensive loss. Realized and unrealized gains and losses arising from changes in the fair value of the financial assets and liabilities held at FVTPL are included in the statement of comprehensive loss in the period in which they arise.
Impairment of financial assets at amortized cost
The Company recognizes a loss allowance for expected credit losses on financial assets that are measured at amortized cost. At each reporting date, the Company measures the loss allowance for the financial asset at an amount equal to the lifetime expected credit losses if the credit risk on the financial asset has increased significantly since initial recognition. If at the reporting date, the credit risk of the financial asset has not increased significantly since initial recognition, the Company measures the loss allowance for the financial asset at an amount equal to the twelve month expected credit losses. The Company shall recognize in the statements of comprehensive loss, as an impairment gain or loss, the amount of expected credit losses (or reversal) that is required to adjust the loss allowance at the reporting date to the amount that is required to be recognized.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies (cont’d) |
Derecognition
Financial assets
The Company derecognizes financial assets only when the contractual rights to cash flows from the financial assets expire, or when it transfers the financial assets and substantially all of the associated risks and rewards of ownership to another entity.
Financial liabilities
The Company derecognizes a financial liability when its contractual obligations are discharged or cancelled or expire. The Company also derecognizes a financial liability when the terms of the liability are modified such that the terms and / or cash flows of the modified instrument are substantially different, in which case a new financial liability based on the modified terms is recognized at fair value. Gains and losses on derecognition are recognized in the statements of comprehensive loss.
Impairment of Non-Financial Assets
At the end of each reporting period, the Company applies judgment in assessing whether there are any indicators of impairment relating to intangible assets. If there are indicators of impairment, the recoverable amount of the related asset is estimated in order to determine the extent of impairment, if any. Indicators of impairment may include: (a) there are observable indications that the asset’s value has declined during the period significantly more than would be expected as a result of the passage of time or normal use; (b) significant changes with an adverse effect on the entity have taken place during the period, or will take place in the near future, in the technological, market, economic or legal environment in which the entity operates or in the market to which an asset is dedicated; (c) market interest rates or other market rates of return on investments have increased during the period, and those increases are likely to affect the discount rate used in calculating an asset’s value in use and decrease the asset’s recoverable amount materially; (d) the carrying amount of the net assets of the entity is more than its market capitalization; (e) evidence is available of obsolescence or physical damage of an asset; (f) significant changes with an adverse effect on the entity have taken place during the period, or are expected to take place in the near future, in the extent to which, or manner in which, an asset is used or is expected to be used. These changes include the asset becoming idle, plans to discontinue or restructure the operation to which an asset belongs, plans to dispose of an asset before the previously expected date, and reassessing the useful life of an asset as finite rather than indefinite; and (g) evidence is available from internal reporting that indicates that the economic performance of an asset is, or will be, worse than expected. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss, if any. Where it is not possible to estimate the recoverable amount of an individual asset, the Company estimates the recoverable amount of the cash-generating unit (“CGU”) to which the assets belong. As at December 31, 2023, no impairment indicators were identified by the Company.
Recoverable amount is the higher of fair value less costs to sell and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies (cont’d) |
If the recoverable amount of an asset or CGU is estimated to be less than its carrying amount, the carrying amount of the asset or CGU is reduced to its recoverable amount. An impairment loss is recognized immediately in the statement of comprehensive loss.
Where an impairment loss subsequently reverses, the carrying amount of the asset or CGU is increased to the revised estimate of its recoverable amount, however the increased carrying amount cannot exceed the carrying amount that would have been determined had no impairment loss been recognized for the asset or CGU in prior years.
Financing Costs
The costs related to equity transactions are deferred until the closing of the equity transactions. These costs are accounted for as a deduction from equity. Transaction costs of abandoned equity transactions are recognized in the statement of comprehensive loss.
Equipment
Equipment is stated at historical cost less accumulated amortization and accumulated impairment losses. Cost includes costs paid to acquire assets from third parties as well as costs incurred in internally constructed assets.
Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost of the item can be measured reliably. The carrying amount of the replaced part is derecognized. All other repairs and maintenance are charged to the statement of comprehensive loss during the financial period in which they are incurred.
Gains and losses on disposals are determined by comparing the proceeds with the carrying amount and are recognized in profit or loss. Amortization is calculated as follows:
Computer equipment is amortized on a straight-line basis over its estimated useful lives of 36 months starting when the asset is available for use. Lab equipment is amortized on a straight-line basis over its estimated useful lives of 60 months starting when the asset is available for use. No amortization is recorded where an asset is in development and not yet ready for its intended use.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies – continued |
Intangible assets
Intangible assets are recorded at cost less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Amortization of definite life intangible assets is recognized on a straight-line basis over their 20-year estimated useful lives.
Income taxes
Current income tax:
Current income tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted, at the reporting date, in the countries where the Company operates and generates taxable income.
Current income tax relating to items recognized directly in other comprehensive income or equity is recognized in other comprehensive income or equity and not in profit or loss. Management periodically evaluates positions taken in the tax returns with respect to situations in which applicable tax regulations are subject to interpretation and establishes provisions where appropriate.
Deferred income tax:
Deferred income tax is provided using the asset and liability method on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred income tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized.
Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. Deferred income tax assets and deferred income tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred income taxes relate to the same taxable entity and the same taxation authority.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies – continued |
Research and development
Research costs are expensed when incurred. Internally-generated technology costs are capitalized as intangible assets when the Company can demonstrate that the technical feasibility of the project has been established; the Company intends to complete the asset for use or sale and has the ability to do so; the asset can generate probable future economic benefits; the technical and financial resources are available to complete the development; and the Company can reliably measure the expenditure attributable to the intangible asset during its development. After initial recognition, internally generated intangible assets are recorded at cost less accumulated amortization and accumulated impairment losses.
The Company did not have any development costs that met the capitalization criteria for the years ended December 31, 2023 and 2022.
Share capital
The proceeds from the exercise of stock options and warrants, in addition to the estimated fair value attributable to these equity instruments, are recorded as share capital when exercised. Warrants issued are recorded at the estimated fair value using the Black-Scholes pricing model.
On unit offerings, the Company prorates the proceeds between the relative fair values of the shares issued and the Black-Scholes value of the warrants issued.
Share-based compensation
The Company grants stock options to directors, officers, employees and service providers. Each tranche in an award is considered a separate award with its own vesting period and fair values. The Company applies the fair-value method of accounting for share-based compensation. The fair value is calculated using the Black-Scholes option-pricing model.
Share-based compensation for employees and others providing similar services are determined based on the grant date fair value. Share-based compensation for non-employees is determined based on the fair value of the goods or services received. Share capital issued for non-monetary consideration is recorded at an amount based on estimated fair market value reduced by an estimate of transaction costs incurred when issuing shares for cash, unless the fair value of the shares cannot be estimated reliably. If the Company cannot estimate reliably the fair value of the goods or services received, the Company measures their value, and the corresponding increase in equity through the fair value of the equity instruments granted.
Share-based compensation expense is recognized over each tranche’s vesting period in the consolidated statements of loss or capitalized as appropriate, based on the number of awards that vest less the estimated forfeitures. The number of forfeitures likely to occur is estimated on grant date. If and when stock options are exercised, the applicable amounts of contributed surplus are transferred to share capital.
Restricted share units
The Company has established a restricted share plan under which share units are granted to directors, officers, key executive and non-executive employees, consultants and advisory board members. The restricted share units are considered equity-settled and are measured using the grant date fair value. Share-based compensation expense is recognized over the vesting period in the consolidated statement of loss with a corresponding amount recognized in equity.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies – continued |
Earnings (loss) per share
Basic earnings (loss) per share is calculated by dividing the earnings (loss) attributable to common shareholders of ASEP by the weighted average number of common shares outstanding in the period. Contingently returnable shares are excluded from the calculation of weighted average number of common shares outstanding. Diluted loss per share is calculated by the treasury stock method. Under the treasury stock method, the weighted average number of common shares outstanding for the calculation of diluted loss per share assumes that the proceeds to be received on the exercise of dilutive share options and warrants are used to repurchase common shares at the average market price during the period.
Foreign currency translation
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transaction. Foreign currency monetary items are translated at the period-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined.
Exchange differences arising on the translation of monetary items or on settlement of monetary items are recognized in profit or loss in the statement of comprehensive loss in the period in which they arise, except where deferred in equity as a qualifying cash flow or net investment hedge.
Exchange differences arising on the translation of non-monetary items are recognized in other comprehensive loss in the statement of comprehensive loss to the extent that gains and losses arising on those non-monetary items are also recognized in other comprehensive loss. Where the non-monetary gain or loss is recognized in profit or loss, the exchange component is also recognized in profit or loss.
Revenue recognition
Revenue is derived from licensing of Intellectual Property and providing technical support and other services.
Revenue is recognized when control of these licenses and services are transferred to the customer, in an amount that reflects the consideration the Company expects to be entitled in exchange for services.
Revenue recognition is determined through the following five steps:
| | 1. | Identify the contract with a customer |
| | 2. | Identify the performance obligations in the contract |
| | 3. | Determine the transaction price |
| | 4. | Allocate the transaction price to the performance obligations in the contract |
| | 5. | Recognize revenue when (or as) the entity satisfies a performance obligation |
Revenue may be earned over time as the performance obligations are satisfied or at a point in time which is when the entity has earned a right to payment, the customer has possession of the asset and the related significant risks and rewards of ownership, and the customer has accepted the asset or service. Royalty revenue is recognized as the underlying sales occur.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies – continued |
The estimated transaction price, which includes variable consideration, may be subject to constraint and is included in the transaction price only to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. At the end of each reporting period, the Company updates the estimated transaction price, including updating its assessment of whether an estimate of variable consideration is constrained, to represent faithfully the circumstances present at the end of the reporting period and the changes in circumstances during the reporting period. Actual amounts may ultimately differ from the Company’s estimates. If actual results vary, the Company will adjust these estimates, which could have an effect on earnings in the period of adjustment.
Patent costs
Patent costs include patent filing fees and patent maintenance fees and are expensed when incurred.
Significant estimates and assumptions
The preparation of the Company’s consolidated financial statements in conformity with IFRS requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and contingent liabilities at the date of the consolidated financial statements and reported amounts of revenues and expenses during the reporting period. Estimates and assumptions are continuously evaluated and are based on management’s experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. However, actual outcomes can differ from these estimates.
Estimates and assumptions where there is significant risk of material adjustments to assets and liabilities in future accounting periods include the useful lives of intangible assets, market multiples utilized in the determination of recoverable amounts of intangible assets, fair value measurements for financial instruments, share-based payments and measurement of deferred tax assets.
Significant judgements
The preparation of financial statements in accordance with IFRS requires the Company to make judgments, apart from those involving estimates, in applying accounting policies. The most significant judgments in applying the Company’s accounting policies in these financial statements were:
| | ○ | Whether performance obligations in the contract are distinct. |
| | ○ | Whether it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the contingency is resolved. |
| | ○ | Whether the expected value method or most likely amount method better predicts the amount of consideration to which the Company will be entitled. |
| | ○ | Whether the Company satisfies a performance obligation over time or at a point in time. |
| | ○ | The determination of the method to measure the progress towards complete satisfaction of a performance obligation satisfied over time. |
| | ● | Evaluating whether costs incurred by the Company meet the criteria for capitalization as intangible assets. |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 3. | Material accounting policies – continued |
| | ● | The Company assesses the carrying values of its tangible and intangible assets at the end of each reporting period for indicators of impairment. If it is determined that there are indicators of impairment, the Company determines the recoverable amount of the related cash generating units. Recoverability is dependent upon assumptions and judgments regarding historical R&D costs and market multiples. Other assumptions used in the calculation of recoverable amounts are discount rates and future cash flows. A material change in assumptions may significantly impact the potential impairment of these assets. During the year ended December 31, 2023, no impairment indicators were identified by management. |
| | ● | Management determines whether assets acquired and liabilities assumed constitute a business. A business consists of inputs and processes applied to those inputs that have the ability to create outputs. The Company completed the acquisition of SafeCoat Medical Inc. (“SafeCoat”) on December 12, 2022 and concluded that the acquired entity did not qualify as a business combination under IFRS 3, “Business Combinations”. Accordingly, the acquisition has been accounted for as an asset acquisition. |
Management determines whether assets acquired and liabilities assumed constitute a business. A business consists of inputs and processes applied to those inputs that have the ability to create outputs. The Company completed the acquisition of SafeCoat Medical Inc. (“SafeCoat”) on December 12, 2022 and concluded that the acquired entity did not qualify as a business combination under IFRS 3, “Business Combinations”. Accordingly, the acquisition has been accounted for as an asset acquisition.
Other significant judgments in applying the Company’s accounting policies relate to the assessment of the Company’s ability to continue as a going concern (Note 1), the recoverability of deferred tax assets, functional currency determinations and the classification of its financial instruments.
| 4. | Adoption of New Accounting Standards, Interpretations and Amendments |
New accounting standards adopted in the current year
Amendments to IAS 1: Classification of Liabilities as Current or Non – current:
In January 2020, the IASB issued amendments to paragraphs 69 to 76 of IAS 1 to specify the requirements for classifying liabilities as current or non-current. The amendments clarify:
| | ● | What is meant by a right to defer settlement |
| | ● | That a right to defer must exist at the end of the reporting period |
| | ● | That classification is unaffected by the likelihood that an entity will exercise its deferral right |
| | ● | That only if an embedded derivative in a convertible liability is itself an equity instrument would the terms of a liability not impact its classification. |
The amendments were effective for annual reporting periods beginning on or after January 1, 2023 and must be applied retrospectively. For the year ended December 31, 2023, these amendments did not affect the Company’s financial statements.
Amendment to IAS 1 and IFRS Practice Statement 2 – Disclosure of Accounting Policies
As part of the new amendments, the Company adopted Disclosure of Accounting Policies (Amendments to IAS 1 and IFRS Practice Statement 2) from January 1, 2023. Although the amendments did not result in any changes to the accounting policies themselves, they impacted the accounting policy information disclosed in the financial statements. The amendments require the disclosure of ‘material’, rather than ‘significant’, accounting policies. The amendments also provide guidance on the application of materiality to disclosure of accounting policies, assisting entities to provide useful, entity-specific accounting policy information that users need to understand other information in the financial statements. Management reviewed the accounting policies and made updates to the information disclosed in certain instances in line with the amendments.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 4. | Adoption of New Accounting Standards, Interpretations and Amendments – continued |
Standards issued but not yet effective
Accounting standards or amendments to existing accounting standards that have been issued but have future effective dates are either not applicable or are not expected to have a significant impact on the Company’s consolidated financial statements.
SafeCoat Medical Inc.
On December 1, 2022, the Company entered into an Earn-In and Option Agreement (the “Agreement”) with SafeCoat Medical Inc. (“SafeCoat”) and the securityholders of SafeCoat (the “Securityholders”). Pursuant to the terms of the Agreement, the Company will earn a 50.1% interest of the voting equity securities of SafeCoat in consideration for services the Company provides to SafeCoat in connection with the grant of a term sheet (the “Term Sheet”) by the University of British Columbia (“UBC”) to SafeCoat for the use, development and commercialization of a peptide medical device coating technology (the “Technology”).
The Securityholders also granted the Company the option to acquire their collective 49.9% equity interest in SafeCoat.
On December 8, 2022, the Company earned a 50.1% equity interest in SafeCoat in consideration of the services provided in connection with the grant of a License to SafeCoat by UBC for the use, development and commercialization of a peptide medical device coating technology. The estimated fair value of the 6,526,052 common shares issued to Asep being $150,752.
On December 12, 2022, the Company exercised its option to acquire the remaining 49.9% equity interest in SafeCoat from the Securityholders. The Option was exercised by the Company issuing 6,000,000 common shares from treasury of which 4,500,000 common shares are contingently returnable, as they are subject to a Voluntary Escrow Agreement. Release from escrow is based on certain milestones being met, as follows:
| | i. | 25% will be released on the date of the option exercise (released December 12, 2022); |
| | | |
| | ii. | 25% will be released on the date the Company and UBC enter into a definitive License Agreement (released July 21, 2023); |
| | | |
| | iii. | 25% will be released on the date that a patent with respect to the Technology is published in Google Patents (released January 18, 2023); and, |
| | iv. | 25% will be released on the date the Company confirms that SafeCoat has reasonably demonstrated activity of antifouling on surfaces (released June 5, 2023). |
As each milestone was achieved, the estimated fair value of the shares released from escrow were recognized and measured on the date the contingency was resolved.
The estimated fair value of the 1,500,000 shares issued on December 12, 2022 was $375,000. The estimated fair value of the 1,500,000 shares released from escrow on January 18, 2023 was $570,000, the estimated fair value of the 1,500,000 shares released from escrow on June 5, 2023 was $705,000 and the estimated fair value of the 1,500,000 shares released from escrow on July 21, 2023 was $525,000.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 5. | Acquisition – continued |
Amounts totalling $1,275,000 have been recorded in the consolidated financial statements for the year ended December 31, 2023 ($400,521 for the year ended December 31, 2022) as SafeCoat acquisition expense, as the shares were issued prior to the execution of the License Agreement. Prior to the execution of the License Agreement, the shares released from escrow did not meet the criteria in IAS 38, Intangible Assets, to be separable or arising from legal or contractual rights. An intangible asset of $596,591 has been recognized as intellectual property for the license to the use, development and commercialization of a peptide medical device coating technology granted to SafeCoat by the University of British Columbia. Upon execution of the license agreement, SafeCoat is obligated to issue 1,776,280 shares from treasury to the non-waiving parties such that they hold a 12% equity interest in SafeCoat. The value of the intangible asset was determined based on the consideration given by the Company represented by the 1,500,000 shares released from escrow on the date license was granted ($525,000) and the 1,776,280 shares of SafeCoat to be issued to the non-waiving parties ($71,591).
| | | December 31,
2023 | | | December 31,
2022 | |
| Cost | | $ | 66,641 | | | $ | 65,414 | |
| Accumulated amortization | | | (29,458 | ) | | | (15,210 | ) |
| | | $ | 37,183 | | | $ | 50,204 | |
| | | Intellectual
Property | | | Website | | | Trademarks | | | Total | |
| Cost | | | | | | | | | | | | | | | | |
| Balance-December 31, 2021 | | $ | 25,020,943 | | | $ | 49,404 | | | $ | 6,225 | | | $ | 25,076,572 | |
| Acquisitions | | | - | | | | - | | | | 435 | | | | 435 | |
| Balance -December 31, 2022 | | | 25,020,943 | | | | 49,404 | | | | 6,660 | | | | 25,077,007 | |
| Acquisitions | | | 596,591 | | | | - | | | | 3,244 | | | | 599,835 | |
| Balance -December 31, 2023 | | $ | 25,617,534 | | | $ | 49,404 | | | $ | 9,904 | | | $ | 25,676,842 | |
| Accumulated amortization | | | | | | | | | | | | | | | | |
| Balance-December 31, 2021 | | $ | 173,349 | | | $ | 5,269 | | | $ | 94 | | | $ | 178,712 | |
| Amortization | | | 1,251,454 | | | | 16,468 | | | | 633 | | | | 1,268,555 | |
| Balance -December 31, 2022 | | | 1,424,804 | | | | 21,737 | | | | 727 | | | | 1,447,267 | |
| Amortization | | | 1,262,761 | | | | 16,468 | | | | 786 | | | | 1,280,015 | |
| Balance -December 31, 2023 | | $ | 2,687,564 | | | $ | 38,205 | | | $ | 1,513 | | | $ | 2,727,282 | |
| | | | | | | | | | | | | | | | | |
| Net carrying value - December 31, 2022 | | $ | 23,596,140 | | | $ | 27,667 | | | $ | 5,933 | | | $ | 23,629,741 | |
| | | | | | | | | | | | | | | | | |
| Net carrying value - December 31, 2023 | | $ | 22,929,970 | | | $ | 11,199 | | | $ | 8,391 | | | $ | 22,949,560 | |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 8. | Investment in Hunan Sanway SepSMART Ltd. and Revenue |
ASEP holds a 25% interest in Hunan Sanway SepSMART Ltd.
| | | December 31,
2023 | | | December 31,
2022 | |
| Investment in Hunan Sanway SepSMART Ltd. | | $ | 2,375,000 | | | $ | - | |
On October 27, 2023, the Company through its subsidiary, Sepset Biosciences, Inc. (“Sepset”) entered into a joint venture agreement (the “JV Agreement”) with Sansure Biotech Inc.’s subisidary Hunan Xiang Jiang Sansure Biotech Fund, L.P. (the “Sansure Fund”). Under the JV Agreement, an equity joint venture entity, Hunan Sanway SepSMART Ltd. (“SepSMART”), was formed on December 5, 2023 in China, whereby the Sansure Fund subscribed for RMB 37,500,000 (CAD $7,125,000) of the registered capital (75%) and Sepset subscribed for RMB 12,500,000 (CAD $2,375,000) of the registered capital (25%) through the licensing of certain patent rights and know-how to Sepset’s first generation rapid sepsis test, SepSetER (“Intellectual Property”). In order to satisfy its subscribed registered capital, Sansure Fund is required to make a cash contribution of RMB 3,000,000 (CAD $570,000) upon the formation date of SepSMART (contributed on December 22, 2023) and to make additional cash contributions of RMB 24,500,000 (CAD $4,655,000) and RMB 10,000,000 (CAD $1,900,000), referred to as the second and third tranche, respectively. The second tranche of funding is due upon completion of 80 test samples based on a protocol mutually agreed by the Sansure Fund and Sepset regarding the sepsis kit and the third tranche is due upon completion of pre-clinical studies, trial design, and obtaining the approval to conduct the clinical study regarding the sepsis kit. Pursuant to the Technology License and Collaborative Agreement (the “Contract”), the term of the license is for a period of 8 years commencing on December 5, 2023 and includes the Company’s participation on a joint project team for the duration of the Contract. Under the Contract, the Company is also entitled to a royalty of 5% of future sales.
It was determined by management that SepSMART meets the definition of a customer and that granting it a license to the Company’s intellectual property represents an output of the Company’s ordinary activities in exchange for the 25% interest in SepSMART and the royalty of 5% of future sales. Management determined that the license of the Intellectual Property was not distinct from other promises in the contract and that the Company transfers control of the goods and services over time. Therefore, the performance obligation and revenue will be recognized over the Contract term of 8 years. As a result, revenue of $21,140 was recorded in the consolidated statement of comprehensive loss for the year ended December 31, 2023 and the balance of unearned revenues of $2,353,860 as at December 31, 2023 is recorded in the consolidated statement of financial position as deferred revenue and will be recognized over the life of the license on a straight-line basis. The estimated transaction price includes variable consideration of $2,185,000 for which management determined it was highly probable that a significant reversal in the amount of cumulative revenue will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 8. | Investment in Hunan Sanway SepSMART Ltd. and Revenue - continued |
Summary of Hunan Sanway SepSMART Ltd. statements of financial position
| | | December 31,
2023 | | | December 31,
2022 | |
| Current assets | | $ | 570,000 | | | $ | - | |
| Less – current liabilities | | | - | | | | - | |
| | | | 570,000 | | | | - | |
| Non-current assets | | | 8,930,000 | | | | - | |
| Less-non-current liabilities | | | - | | | | - | |
| | | | 8,930,000 | | | | - | |
| Net assets | | | 9,500,000 | | | | - | |
| ASEP’s share - percentage | | | 25 | % | | | 0 | % |
| ASEP’s share – net assets | | $ | 2,375,000 | | | $ | | |
Summary of Hunan Sanway SepSMART Ltd. statements of income (loss) and comprehensive income (loss)
| | | For the year ended December 31, | |
| | | 2023 | | | 2022 | |
| Revenues | | $ | - | | | $ | - | |
| Expenses | | | - | | | | - | |
| Net income (loss) | | | - | | | | - | |
| Other comprehensive income (loss) | | | - | | | | - | |
| Comprehensive income (loss) | | $ | - | | | $ | - | |
| | | December 31,
2023 | | | December 31,
2022 | |
| Demand loan | | | | | | | | |
| Principal | | $ | 20,000 | | | $ | - | |
| Interest | | | 270 | | | | - | |
| | | $ | 20,270 | | | $ | - | |
On November 20, 2023, REWH Consulting Inc. (“REWH”) provided a short-term unsecured loan to the Company for $20,000 with an interest rate of 10% per annum. The loan is repayable 5 days after the Company receives gross proceeds in excess of $250,000 from a financing. REWH waived this provision for repayment on closing of Life offering (see Note 11) and concurrently, the loan term was amended to be without stated terms of repayment.
REWH is an entity controlled by Robert Hancock, an officer and a director of the Company.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
On December 22, 2022, the Company issued a $330,000 convertible debenture (“Convertible Debenture”) to an unrelated party of the Company. The Convertible Debenture is unsecured, bears interest at a rate of 10% per annum payable at the earlier of maturity, payment of principal or conversion during the term and matures two years from the date of issuance. The Convertible Debenture and accrued interest thereon, may be converted at the option of the holder at a conversion price of $0.33 per common share until maturity. At maturity, the Company, at its option, may settle the Convertible Debenture and accrued interest thereon, in cash or common shares at a conversion price of $0.33 per common share. The Company may also, at its option, prepay any portion of the Convertible Debenture and accrued interest thereon prior to maturity.
The Company has evaluated the Convertible Debenture, embedded conversion option and embedded prepayment option and determined that all components of the compound financial instrument meet the criteria for classification as equity. The deferred tax liability on date of issuance was $89,100. On December 27, 2023, the convertible debenture plus accrued interest thereon ($273,086) were debt settled (note 11).
Authorized share capital
Unlimited number of common shares without par value and an unlimited number of preferred shares without par value.
Issued share capital
| | i. | On December 15, 2022, the Company issued 6,000,000 common shares to acquire the non-controlling interest in SafeCoat (note 5). At December 31, 2022, 4,500,000 common shares were held in escrow and considered contingently returnable shares. The fair value of the 1,500,000 unrestricted shares at date of issuance was $375,000. The fair value of the 4,500,000 shares released from escrow during the year ended December 31, 2023 was $1,800,000 (note 5). |
| | ii. | On June 15, 2023, the Company issued 1,200,000 common shares on settlement of the first tranche of the RSUs granted March 1, 2023 and the estimated fair value of these shares ($330,000) was transferred from reserves to capital stock on date of issue. |
| | iii. | On July 19, 2023, the Company issued 145,161 common shares to certain members of the Advisory Board to compensate them for consulting services provided totalling $45,000 for the period March 1, 2023 to May 31, 2023. |
| | iv. | On July 21, 2023, stock options entitling the holder to acquire 200,000 common shares were exercised for proceeds of $60,000. |
| | v. | On September 12, 2023, the Company issued 249,999 common shares to certain members of the Advisory Board to compensate them for consulting fees services provided totalling $45,000 for the period June 1, 2023 to August 31, 2023. |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 11. | Share capital - continued |
| | vi. | On September 12, 2023, the Company issued 1,675,000 common shares on settlement of the second tranche of the RSUs granted March 1, 2023 and the estimated fair value of these shares ($460,625) was transferred from reserves to capital stock on date of issue. |
| | vii. | On September 27, 2023, the Company issued 3,259 common shares on settlement of the first tranche of the RSUs granted June 20, 2023 and the estimated fair value of these shares ($1,173) was transferred from reserves to capital stock on date of issue. |
| | viii. | On December 1, 2023, the Company issued 163,635 common shares to certain members of the Advisory Board to compensate them for consulting fees services provided totalling $45,000 for the period September 1, 2023 to November 30, 2023. |
| | ix. | On December 5, 2023, the Company issued 1,562,500 common shares on settlement of the third tranche of the RSUs granted March 1, 2023 and the estimated fair value of these shares ($429,688) was transferred from reserves to capital stock on date of issue. |
| | x | On December 19, 2023, the Company completed a unit offering at $0.20 per unit for gross proceeds of $587,000 and issued 2,935,000 shares ($333,481) and 2,935,000 warrants ($253,519). Each warrant entitles the holder to acquire one common share for a period of two years at a price of $0.26 per share. In connection with the unit offering, the Company paid cash finder’s fees of $15,600 and issued 128,000 finder’s warrants ($23,772). |
| | xi. | On December 27, 2023, the Company settled debts totalling $783,186 through the issuance of 3,915,930 shares ($639,539) and 3,915,930 warrants ($484,412) and recognized a loss on debt settlement ($429,864). Each warrant entitles the holder to acquire one common share for a period of two years at a price of $0.26 per share. |
| | xii. | On December 29, 2023, the Company issued 3,259 common shares on settlement of the second tranche of the RSUs granted June 20, 2023 and the estimated fair value of these shares ($1,173) was transferred from reserves to capital stock on date of issue. |
As at December 31, 2023, there were 74,184,087 (2022 - 62,130,344) issued and outstanding common shares and nil issued and outstanding preferred shares (2022 – nil).
Escrowed shares
As at December 31, 2023, 889,366 (2022 – 1,778,731) shares are being held in escrow. The shares are being released over a 36-month term that commenced on January 23, 2022.
As at December 31, 2022, 4,500,000 shares were subject to a voluntary escrow agreement and were released during the year ended December 31, 2023.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 11. | Share capital - continued |
Warrants
| | | December 31, 2023 | | | December 31, 2022 | |
| | | Number of
warrants
outstanding | | | Amount | | | Number of
warrants
outstanding | | | Amount | |
| Opening balance | | | - | | | $ | - | | | | 187,200 | | | $ | 35,921 | |
| Granted | | | 6,978,930 | | | | 744,699 | | | | - | | | | - | |
| Expired | | | - | | | | - | | | | (187,200 | ) | | | (35,921 | ) |
| Closing balance | | | 6,978,930 | | | $ | 744,699 | | | | - | | | $ | - | |
The fair value of the 2,935,000 warrants issued in connection with the unit placement completed December 19, 2023 totaled $253,519. The fair value of the finder’s warrants issued in connection with the unit placement totaled $23,772. The fair value of the 3,915,930 warrants issued in connection with debt settlement completed December 27, 2023 totaled $484,412. The warrants were valued using the Black-Scholes valuation model, using the following assumptions:
Expected
life | | Volatility | | | Dividend
yield | | | Risk-free
interest rate | | | Warrants
issued | | | Estimated fair value | | | Warrant issue costs | | | Net | |
| 2 years | | | 167.29 | % | | | 0 | % | | | 3.85 | % | | | 2,935,000 | | | $ | 253,519 | | | $ | (17,004 | ) | | $ | 236.515 | |
| 2 years | | | 167.29 | % | | | 0 | % | | | 3.85 | % | | | 128,000 | | | | 23,772 | | | | - | | | | 23,772 | |
| 2 years | | | 167.29 | % | | | 0 | % | | | 3.76 | % | | | 3,915,930 | | | | 484,412 | | | | - | | | | 484,412 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 6,978,930 | | | $ | 761,703 | | | $ | (17,004 | ) | | $ | 744,699 | |
As at December 31, 2023, the following share purchase warrants are outstanding:
Number of
warrants
outstanding | | | Weighted average exercise price $ | | | Expiry date | | Weighted
average
contractual life |
| | 2,935,000 | | | | 0.26 | | | December 19, 2025 | | 0.83 years |
| | 128,000 | | | | 0.20 | | | December 19, 2025 | | 0.04 years |
| | 3,915,930 | | | | 0.26 | | | December 27, 2025 | | 1.12 Years |
| | 6,978,930 | | | | 0.26 | | | | | 1.98 years |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 11. | Share capital - continued |
Stock options
In July 2021, the Company adopted a stock option plan (“Plan”), which provides that the Board of Directors of the Company may from time to time, in its discretion, grant to directors, officers, employees and consultants of the Company stock options to purchase common shares, provided that the number of common shares reserved for issuance under the Plan shall not exceed 10% of the issued and outstanding common shares at the time of grant. The Board of Directors shall determine the exercise price and the term of the stock options at the time of grant. If the shares are listed on a stock exchange, then the exercise price for the options granted will not be less than the minimum prevailing price permitted by the stock exchange. If the shares are not listed, posted and trading on any stock exchange or quoted on any quotation system, the exercise price will be determined by the Board at the time of granting.
During the year ended December 31, 2023, the Company granted 793,034 stock options with an estimated fair value of $234,095. A total of 593,034 vested on grant date, 50,000 vest every three months over a twenty-four-month period and 200,000 vest every six months over a twenty-four-month period. The stock options were valued using the Black-Scholes model based on the following assumptions:
Expected
life | | Volatility | | | Dividend
yield | | | Risk-free
interest rate | | | Option
issued | | | Estimated fair
value | |
| 10 years | | | 100 | % | | | 0 | % | | | 2.75 | % | | | 400,000 | | | $ | 129,738 | |
| 10 years | | | 100 | % | | | 0 | % | | | 3.39 | % | | | 300,000 | | | | 74,227 | |
| 10 years | | | 100 | % | | | 0 | % | | | 3.30 | % | | | 93,034 | | | | 30,130 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 793,034 | | | $ | 234,095 | |
During the year ended December 31, 2022, the Company granted 1,080,000 stock options with an estimated fair value of $181,025. A total of 780,000 vested on grant date, 100,000 vest on the first anniversary from date of grant and 200,000 vest every six months over a twenty-four-month period. The stock options were valued using the Black-Scholes model based on the following assumptions:
Expected
life | | Volatility | | | Dividend
yield | | | Risk-free
interest rate | | | Option
issued | | | Estimated fair
value | |
| 10 years | | | 100 | % | | | 0 | % | | | 1.78 | % | | | 200,000 | | | $ | 33,970 | |
| 10 years | | | 100 | % | | | 0 | % | | | 1.78 | % | | | 200,000 | | | | 27,466 | |
| 10 years | | | 100 | % | | | 0 | % | | | 2.90 | % | | | 680,000 | | | | 119,589 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | 1,080,000 | | | $ | 181,025 | |
During the year ended December 31, 2023, the Company recognized $314,690 (2022 - $1,119,072) of share-based compensation for the vesting of stock options granted.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 11. | Share capital - continued |
A continuity of stock options for the years ended December 31, 2023 and 2022 is as follows:
| | | December 31, 2023 | | | December 31, 2022 | |
| | | Number of
options
outstanding | | | Weighted
average
exercise price | | | Number of
options
outstanding | | | Weighted
average
exercise price | |
| Opening balance | | | 5,420,000 | | | $ | 0.46 | | | | 4,540,000 | | | $ | 0.50 | |
| Granted | | | 793,034 | | | $ | 0.34 | | | | 1,080,000 | | | $ | 0.30 | |
| Exercised | | | (200,000 | ) | | $ | 0.30 | | | | - | | | | - | |
| Expired | | | (200,000 | ) | | $ | 0.50 | | | | - | | | | - | |
| Cancelled | | | - | | | $ | - | | | | (200,000 | ) | | $ | 0.50 | |
| Closing balance | | | 5,813,034 | | | $ | 0.45 | | | | 5,420,000 | | | $ | 0.46 | |
The following stock options are outstanding at December 31, 2023:
Number of
options
outstanding | | | Number of
options
outstanding
and exercisable | | | Weighted
average
exercise price
of options
exercisable | | | Weighted
average
remaining
contractual life | |
| | 4,140,000 | | | | 4,140,000 | | | $ | 0.37 | | | | 5.62 | |
| | 200,000 | | | | 200,000 | | | $ | 0.01 | | | | 0.30 | |
| | 200,000 | | | | 99,726 | | | $ | 0.01 | | | | 0.30 | |
| | 480,000 | | | | 480,000 | | | $ | 0.03 | | | | 0.74 | |
| | 400,000 | | | | 368,622 | | | $ | 0.02 | | | | 0.62 | |
| | 300,000 | | | | 158,276 | | | $ | 0.01 | | | | 0.47 | |
| | 93,034 | | | | 93,034 | | | $ | 0.01 | | | | 0.15 | |
| | 5,813,034 | | | | 5,539,658 | | | $ | 0.45 | | | | 8.20 | |
Restricted stock units (“RSUs)
During the year ended December 31, 2023, the Company adopted a Long-Term Performance Incentive Plan (the “Plan”). Under the terms of the Plan, the Company has the ability to issue restricted stock units (“RSUs), performance share units (“PSUs) or deferred share units up to a maximum of 10% of the shares issued and outstanding at date of grant to certain directors, officers, key executive and non-executive employees, consultants and advisory board members.
On March 1, 2023, the Company granted an aggregate of 6,200,000 RSUs with an estimated fair value of $1,705,000. The RSUs vest in stages, as follows: 25% on June 1, 2023, 25% on September 1, 2023, 25% on December 1, 2023 and 25% on March 1, 2024. On June 20, 2023, the Company granted 13,034 RSUs with an estimated fair value of $4,692. The RSUs vest in stages, as follows: 25% on September 20, 2023, 25% on December 20, 2023, 25% on March 20, 2024 and 25% on June 20, 2024. All of the RSUs are subject to a deferral right whereby the holder can defer any vesting date at their option, on five days prior written notice to the Company and in accordance with the terms of the RSU grant notice, to the earlier of the date of a change of control of the Company and the date the holder ceases to provide services to the Company and to be an eligible person. The RSUs and underlying common shares are subject to shareholder approval.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 11. | Share capital - continued |
During the year ended December 31, 2023, the Company issued 4,444,018 common shares on vesting of RSUs and the estimated fair value of these shares ($1,222,659) was transferred from reserves to capital stock on date of issue.
During the year ended December 31, 2023, the Company recognized $1,637,365 (2022 - $Nil) of share-based compensation over the vesting period of RSUs granted.
Continuity of the RSUs granted during the years ended December 31, 2023 and 2022 is as follows:
| | | December 31, 2023 | | | December 31, 2022 | |
| | | Number of
RSUs
outstanding | | | Estimated fair value | | | Number of
RSUs
outstanding | | | Estimated fair value | |
| Opening balance | | | - | | | $ | - | | | | - | | | $ | | |
| Granted | | | 6,213,034 | | | | 1,709,692 | | | | - | | | | - | |
| Settled | | | (4,444,018 | ) | | | (1,222,659 | ) | | | - | | | | - | |
| Closing balance | | | 1,769,016 | | | $ | 487,033 | | | | - | | | $ | - | |
| 12. | Non-controlling interests |
ASEP holds a 50.1% equity interest in ABT and Sepset with the remaining 49.9% held by various other parties and a 88% equity interest in SafeCoat with the remaining 12% held by the non-waiving investigators (note 5).
At December 31, 2023 and 2022, the NCI consisted of the following:
| | | December 31,
2023 | | | December 31,
2022 | |
| ABT | | $ | 5,060,259 | | | $ | 5,602,460 | |
| Sepset | | | 4,829,496 | | | | 5,477,969 | |
| SafeCoat | | | 59,080 | | | | - | |
| Total | | $ | 9,948,835 | | | $ | 11,080,429 | |
The below is the summarized financial information of ABT, Sepset and SafeCoat before inter-company eliminations:
Summary of statements of financial position
| | | December 31, 2023 | | | December 31, 2023 | | | December 31, 2023 | |
| | | ABT | | | Sepset | | | SafeCoat | |
| NCI percentage | | | 49.90 | % | | | 49.90 | % | | | 12 | % |
| Assets | | $ | 12,287,970 | | | $ | 14,046,405 | | | $ | 674,333 | |
| Less - liabilities | | | (48,353 | ) | | | (2,384,468 | ) | | | (62,059 | ) |
| Total net assets | | $ | 12,239,117 | | | $ | 11,661,937 | | | $ | 612,274 | |
| | | December 31, 2022 | | | December 31, 2022 | |
| | | ABT | | | Sepset | |
| NCI percentage | | | 49.90 | % | | | 49.90 | % |
| Assets | | $ | 13,347,551 | | | $ | 13,011,037 | |
| Less - liabilities | | | (21,859 | ) | | | (49,555 | ) |
| Total net assets | | $ | 13,325,692 | | | $ | 12,961,482 | |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 12. | Non-controlling interests - continued |
Summary statements of loss and comprehensive loss
| | | For the year ended December 31, 2023 | |
| | | ABT | | | Sepset | | | SafeCoat | | | Total | |
| Loss and comprehensive loss for year | | $ | (1,086,575 | ) | | $ | (1,299,546 | ) | | $ | (104,257 | ) | | $ | (2,490,378 | ) |
| | | For the year ended December 31, 2022 | |
| | | ABT | | | Sepset | | | Total | |
| Loss and comprehensive loss for year | | $ | (1,132,066 | ) | | $ | (1,337,483 | ) | | $ | (2,469,549 | ) |
Summary statements of cash flows
| | | For the year ended December 31, 2023 | |
| | | ABT | | | Sepset | | | SafeCoat | | | Total | |
| | | $ | | | $ | | | $ | | | $ | |
| Net cash provided by (used in) operations activities | | | (66,013 | ) | | | (486,197 | ) | | | (835 | ) | | | (552,211 | ) |
| Net cash provided by (used in) investing activities | | | - | | | | (4,470 | ) | | | - | | | | (4,470 | ) |
| Net cash provided by (used in) financing activities | | | (665,100 | ) | | | (399,844 | ) | | | - | | | | (1,064,944 | ) |
| | | For the year ended December 31, 2022 | |
| | | ABT | | | Sepset | | | Total | |
| | | $ | | | $ | | | $ | |
| Net cash provided by (used in) operations activities | | | (293,145 | ) | | | (295,335 | ) | | | (588,480 | ) |
| Net cash provided by (used in) investing activities | | | - | | | | (435 | ) | | | (435 | ) |
| Net cash provided by (used in) financing activities | | | (408,338 | ) | | | (10,000 | ) | | | (418,338 | ) |
Changes to NCI
| | | SafeCoat | | | ABT | | | Sepset | | | Total | |
| Balance – December 31, 2021 | | $ | - | | | $ | 6,167,361 | | | $ | 6,145,373 | | | $ | 12,312,734 | |
| NCI’s share of loss | | | - | | | | (564,901 | ) | | | (667,404 | ) | | | (1,232,305 | ) |
| Balance – December 31, 2022 | | | - | | | | 5,602,460 | | | | 5,477,969 | | | | 11,080,429 | |
| Recognition of NCI | | | 71,591 | | | | - | | | | - | | | | 71,591 | |
| Loss attributable to NCI | | | (12,511 | ) | | | (542,201 | ) | | | (648,473 | ) | | | (1,203,183 | ) |
| Balance – December 31, 2023 | | $ | 59,080 | | | $ | 5,060,259 | | | $ | 4,829,496 | | | $ | 9,948,835 | |
| 13. | Related party transactions |
Key management personnel compensation
Key management personnel include those persons having authority and responsibility for planning, directing and controlling the activities of the Company as a whole. The Company has determined that key management personnel consist of members of the Company’s Board of Directors and corporate officers.
| | | Year ended December 31, | |
| | | 2023 | | | 2022 | |
| Consulting fees | | $ | 210,000 | | | $ | 267,500 | |
| Directors fees | | | 72,000 | | | | 24,000 | |
| Management salaries | | | 433,500 | | | | 369,742 | |
| Share-based compensation | | | 1,090,133 | | | | 1,051,798 | |
| | | $ | 1,805,633 | | | $ | 1,713,040 | |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 13. | Related party transactions - continued |
| | | December 31, 2023 | | | December 31, 2022 | |
| Balances payable to key management personnel for compensation | | $ | 249,300 | | | $ | 49,500 | |
The balances payable are included in accounts payable and accrued liabilities.
Other amount included in the consolidated financial statements due to a related party, as disclosed in Note 9, is as follows:
| | | December 31, 2023 | | | December 31, 2022 | |
| Loan payable (note 9) | | $ | 20,270 | | | $ | - | |
Interest of $270 for the year ended December 31, 2023 (2022 - $Nil) has been accrued on the loan payable in these consolidated financial statements.
| 14. | Income tax recovery and deferred tax assets and liabilities |
A reconciliation of the expected income tax recovery to the actual income tax recovery is as follows:
| | | December 31,
2023 | | | December 31,
2022 | |
| | | | | | | |
| Expected rate | | | 27 | % | | | 27 | % |
| | | | | | | | | |
| Income tax recovery ( expense) computed at statutory rates | | $ | 2,546,239 | | | $ | 1,578,524 | |
| Share-based compensation and other permanent differences | | | (1,612,409 | ) | | | (704,395 | ) |
| Temporary income tax benefits not recognized | | | (933,830 | ) | | | (785,029 | ) |
| Recovery of (provision for) deferred income taxes | | $ | - | | | $ | 89,100 | |
At December 31, 2023 and 2022, the deferred tax assets are not recognized on the following temporary differences as it is not probable that sufficient future taxable profits will be available to utilize such differences:
| | | December 31,
2023 | | | December 31,
2022 | |
| Deferred tax assets | | | | | | | | |
| Financing costs | | $ | 21,848 | | | $ | 25,656 | |
| Non- capital losses | | | 2,361,172 | | | | 1,518,314 | |
| Equipment and intangible assets | | | 121,368 | | | | 115,688 | |
| Total gross deferred tax assets | | | 2,504,388 | | | | 1,659,658 | |
| Deferred tax assets not recognized | | | (2,504,388 | ) | | | (1,570,558 | ) |
| | | | | | | | 89,100 | |
| Deferred tax liabilities | | | - | | | | (89,100 | ) |
| | | $ | - | | | $ | - | |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 14. | Income tax recovery and deferred tax assets and liabilities - continued |
At December 31, 2023, the Company has Canadian non capital losses, which may be carried forward to apply against future year’s income for income tax purposes, subject to final determination by taxation authorities, as follows:
| Year of expiry | | Amount | |
| 2040 | | $ | 17,220 | |
| 2041 | | | 1,306,861 | |
| 2042 | | | 2,888,271 | |
| 2043 | | | 4,532,728 | |
| | | $ | 8,745,080 | |
| 15. | Financial risk and capital management |
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes, inclusive of documented investment policies, counterparty limits, and controlling and reporting structures. The type of risk exposure and the way in which such exposure is managed is provided as follows:
Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company’s primary exposure to credit risk is on its cash held in a bank account. The cash is deposited in a bank account held with a major bank in Canada. As the Company’s cash is held by one bank there is a concentration of credit risk. This risk is managed by using a major bank that is a high credit quality financial institution as determined by rating agencies. Credit risk is assessed as low. As at December 31, 2023, the Company’s maximum credit risk was $64,721.
Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they fall due. The Company has a planning and budgeting process in place to help determine the funds required to support the Company’s normal operating requirements on an ongoing basis. The Company ensures that there are sufficient funds to meet its short-term business requirements, taking into account its anticipated cash flows from operations and its holdings of cash.
Historically, the Company’s sole source of funding has been the issuance of equity and debenture securities for cash. The Company’s access to financing is always uncertain. There can be no assurance of continued access to significant equity and debt funding. Liquidity risk is assessed as high.
As of December 31, 2023, the Company had working capital deficit of $744,644 (note 1).
The Company’s contractual obligations at December 31, 2023 are as follows:
| | | Less than 1
year | | | Between 1 year
and 5 years | | | More than 5
years | | | Total | |
| Accounts payable and accrued liabilities | | $ | 677,731 | | | $ | - | | | $ | - | | | $ | 677,731 | |
| Loan payable | | | 20,270 | | | | - | | | | - | | | | 20,270 | |
| | | | 698,001 | | | | | | | | | | | | 698,001 | |
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
| 15. | Financial risk and capital management - continued |
Foreign exchange risk
Foreign currency risk is the risk that the fair values of future cash flows of a financial instrument will fluctuate because they are denominated in currencies that differ from the respective functional currency. The Company had no exposure to foreign exchange risk.
Interest rate risk
Interest rate risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company’s cash on hand is subject to minimal interest rate risk and the convertible debenture has a fixed interest rate. Interest rate risk is assessed as low.
Capital Management
The Company’s policy is to maintain a strong capital base to maintain investor and creditor confidence and to sustain future development of the business. The capital structure of the Company consists of equity, comprising share capital, reserves and deficit. There were no changes in the Company’s approach to capital management during the year. The Company is not subject to any externally imposed capital requirements.
Classification of financial instruments
Financial assets included in the statement of financial position are as follows:
| | | December 31,
2023 | | | December 31,
2022 | |
| Financial assets at FVTPL: | | | | | | | | |
| Cash | | $ | 64,721 | | | $ | 2,130,390 | |
Financial liabilities included in the statement of financial position are as follows:
| | | December 31,
2023 | | | December 31,
2022 | |
| Financial liabilities at amortized cost: | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 677,731 | | | $ | 528,161 | |
| Loan payable | | | 20,270 | | | | - | |
| | | $ | 698,001 | | | $ | 528,161 | |
Fair value
The fair values of the Company’s financial assets and liabilities approximate the carrying amounts due to their short-term nature.
Financial instruments measured at fair value are classified into one of three levels in the fair value hierarchy according to the relative reliability of the inputs used to estimate the fair values. The three levels of the fair value hierarchy are:
| | ● | Level 1 – Unadjusted quoted prices in active markets for identical assets or liabilities; |
| | | |
| | ● | Level 2 – Inputs other than quoted prices that are observable for the asset or liability either directly or indirectly; and |
| | | |
| | ● | Level 3 – Inputs that are not based on observable market data. |
Financial instruments measured at fair value on a recurring basis classified as level 1 – quoted prices in active markets include cash and cash equivalents.
ASEP Medical Holdings Inc.
Consolidated Notes to the Financial Statements
For the years ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
License Agreements
The SepsetER technology is covered by two separate filed and issued patents. The first patent is owned by Dr. Robert E.W. Hancock together with other inventors. Dr. Robert E.W. Hancock is also one of three inventors for the technology underlying the second patent. However, the second patent has been assigned to the University of British Columbia, who provided an exclusive license to Sepset. Both the license agreements require the Company to pay a royalty on revenues and sublicensing revenues derived from the sale of products.
Under the License Agreements with SafeCoat and ABT, the Company is obligated to pay royalties on revenues and sublicensing revenues generated from the sale of products.
Seaspring W.L.L. (“Seaspring”)
The Company has signed a definitive agreement to form a joint venture with Bahrain-based international investment consultancy firm, Seaspring, to advance regulatory approval and commercialization of the SepsetER technology in the Kingdom of Bahrain, the Middle East and North Africa. The terms of the definitive agreement include the formation of a 50/50 joint venture whereby Seaspring will contribute the capital required by the joint venture to conduct its business operations (regulatory approval, sales and distribution) and the Company through its subsidiary Sepset, will provide the licensing rights for the use of the SepsetER technology in the Kingdom of Bahrain, Algeria, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Qatar, Saudi Arabia, Syria, Tunisia, United Arab Emirates and Yemen.
The following events have occurred:
| | i. | On March 1, 2024, the Company issued 209,301 common shares to certain members of the Advisory Board to compensate them for consulting fees services provided totalling $45,000 for the period December 1, 2023 to February 29, 2024. |
| | ii. | On March 7, 2024, the Company issued 1,662,500 common shares on settlement of the fourth tranche of the RSUs granted March 1, 2023 and the estimated fair value of these shares ($429,688) was transferred from reserves to capital stock on date of issue. |
| | iii. | On March 27, 2024, the Company issued 3,259 common shares on settlement of the third tranche of the RSUs granted June 20, 2023 and the estimated fair value of these shares ($1,173) was transferred from reserves to capital stock on date of issue. |
| | iv. | On April 8, 2024, the Company issued 3,000,000 performance warrants (the “Performance Warrants”) to Sansure Biotech Inc. Each Performance Warrant is exercisable into one common share at an exercise price of $1.00 per common share for a period of one year from date of issuance. |



