Issuer Free Writing Prospectus dated January 21, 2025
Filed Pursuant to Rule 433 of the Securities Act of 1933, as amended
Relating to Preliminary Prospectus dated January 10, 2024
Registration Statement File No. 333-284106

Anbio Improving Prognosis by Decentralizing Diagnostics Anbio

Forward - Looking Statements Anbio Biotechnology Inc., or the Company, has filed a registration statement on Form F - 1 (File No. 333 - 284106) with the U.S. Securities and Exchange Commission, or the SEC, for the offering to which this free writing prospectus relates. Before you invest, you should read the prospectus in the regi stration statement and other documents the Company has filed with the SEC for more complete information about the Company and this offering. Investors should rely upon the prospectus and any relevant free - writing prospectus for complete details. You may obtain these documents for free by visiting EDGAR on the SEC website at www.sec.gov. Alternatively, the Company, any underwriter, or any dealer participating in the offering will arrange to send you the prospectus if you request it by contacting AC Sunshi ne Securities LLC. at 200 E. Robinson Street, Suite 295 Orlando, FL 32801 , via email: ycui@acsunshine.com. You may also access the Company’s most recent prospectus dated 1/10/2025, which is included in Amendment No. 1 to the Company’s registration statement on Form F - 1 filed with the SEC on 1/10/2025, by visiting EDGAR on the SEC website at: https:// www.sec.gov/Archives/edgar/data/1982708/000121390025002551/ea0200357 - 11.htm. This presentation contains forward - looking statements, including statements about the Company’s business outlook, strategy and market opportunities, and statements that may suggest trends for its business. All statements contained in this presentation other than statements of historical facts are individually and collectively forward - looking statements within the meaning of Section 27A of the Securities Act of 1933. The words “believe,” “may,” “should,” “aim,” “est imate,” “continue,” “anticipate,” “intend,” “will,” “expect” and similar words are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. The Company may not actually achieve the plans, intentions and expectations disclosed in these forward - looking statements. These forward - looking statements made in reliance on the safe harbor provisions of this presentation are based on estimates and information available to the Company at the time of this presentation. These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict and beyond the Company’s control. Therefore, prospective investors are cautioned that actual results may differ materially from those expressed or implied in the forward - looking statements herein. We urge you to review the risk factors discussed in the “Risk Factors” section of the Company’s registration statement on Form F - 1 for a discussion of the risks and uncertainties. There can be no assurance that the forward - looking statements made during this presentation will in fact be realized. Except as otherwise required by applicable securities law, the Company and its representatives undertake no duty to update or revise forward - looking statements, whether as a result of new information, future events or otherwise. Considering these limitations, you should not make any investment decision in reliance on forward - looking statements contained in this prospectus. This presentation and any oral statements made in connection with this presentation do no constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities of the Company in any jurisdiction in which such offer, solicitation or sale would be unlawful prior t o registration or qualification under the securities laws of such jurisdiction. The Company’s securities may only be sold pursuant to an effective registration statement filed with the SEC. See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss.
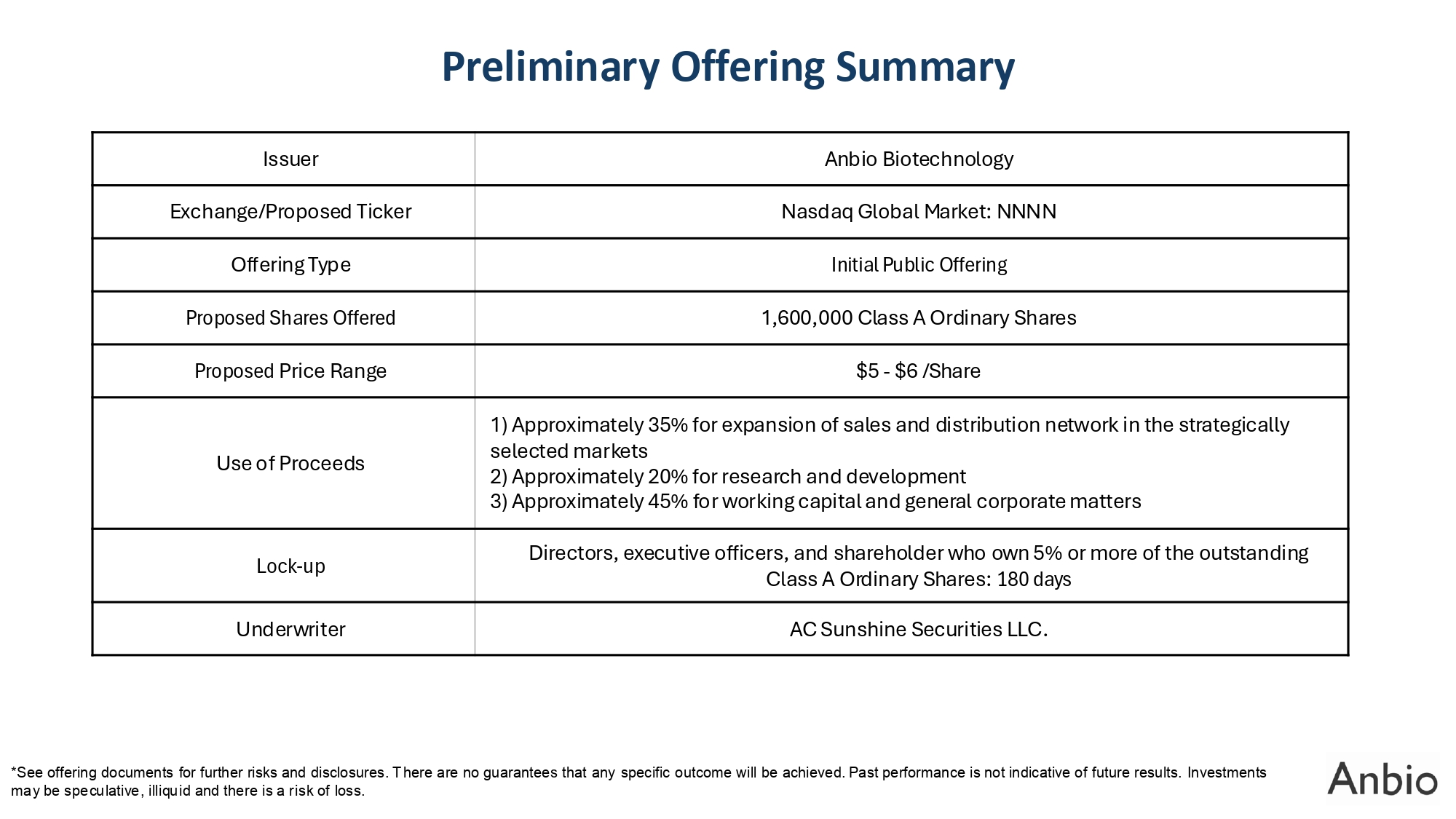
Preliminary Offering Summary *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Anbio Biotechnology Issuer Nasdaq Global Market: NNNN Exchange/Proposed Ticker Initial Public Offering Offering Type 1,600,000 Class A Ordinary Shares Proposed Shares Offered $5 - $6 /Share Proposed Price Range 1) Approximately 35% for expansion of sales and distribution network in the strategically selected markets 2) Approximately 20% for research and development 3) Approximately 45% for working capital and general corporate matters Use of Proceeds Directors, executive officers, and shareholder who own 5% or more of the outstanding Class A Ordinary Shares: 180 days Lock - up AC Sunshine Securities LLC. Underwriter

Anbio Biotechnology’s unwavering commitment lies in transforming the diagnostics landscape on a global scale, fostering a paradigm shift towards personalized and decentralized diagnostic solutions. By doing so, we aim to significantly enhance patient prognosis and contribute to the betterment of healthcare worldwide. *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss.

Table of Contents 1. Company Overview 2. Our Products 3. Industry Overview 4. Growth Strategies 5. Financials *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss.

Key Facts Company Founded in Incorporated in *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials Technology IVD Platforms Patent Applications Trademarks

Leadership Team Significant leadership experience in the diagnostic space for developing and commercializing IVD instruments and consumables globally MICHAEL LAU, PhD, MBA - Chief Executive Officer • Ph.D. from University of California, Riverside • M.B.A. from University of Wisconsin • More than 10 years of experience in the life sciences industry SUKI SONG, CPA - Chief Financial Officer • Extensive financial expertise with 20+ years of experience. • Proven leadership in audits and financial management roles. • Highly qualified with CPA and multiple professional certifications. CHRIS TIAN - Chief Business Officer *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials • Expert in business strategies. • Business Director at VIKBAY AB prior to joining Anbio. • Researcher for Amway prior to joining Anbio.

Anbio is a Global Diagnostic Company *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

THE HEALTHCARE LANDSCAPE IS EVOLVING Patient Healthcare Increasing importance of Chronic Disease Management Declining Population Health More focus on Precision Medicine High Demand for In - Vitro Diagnostics *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials
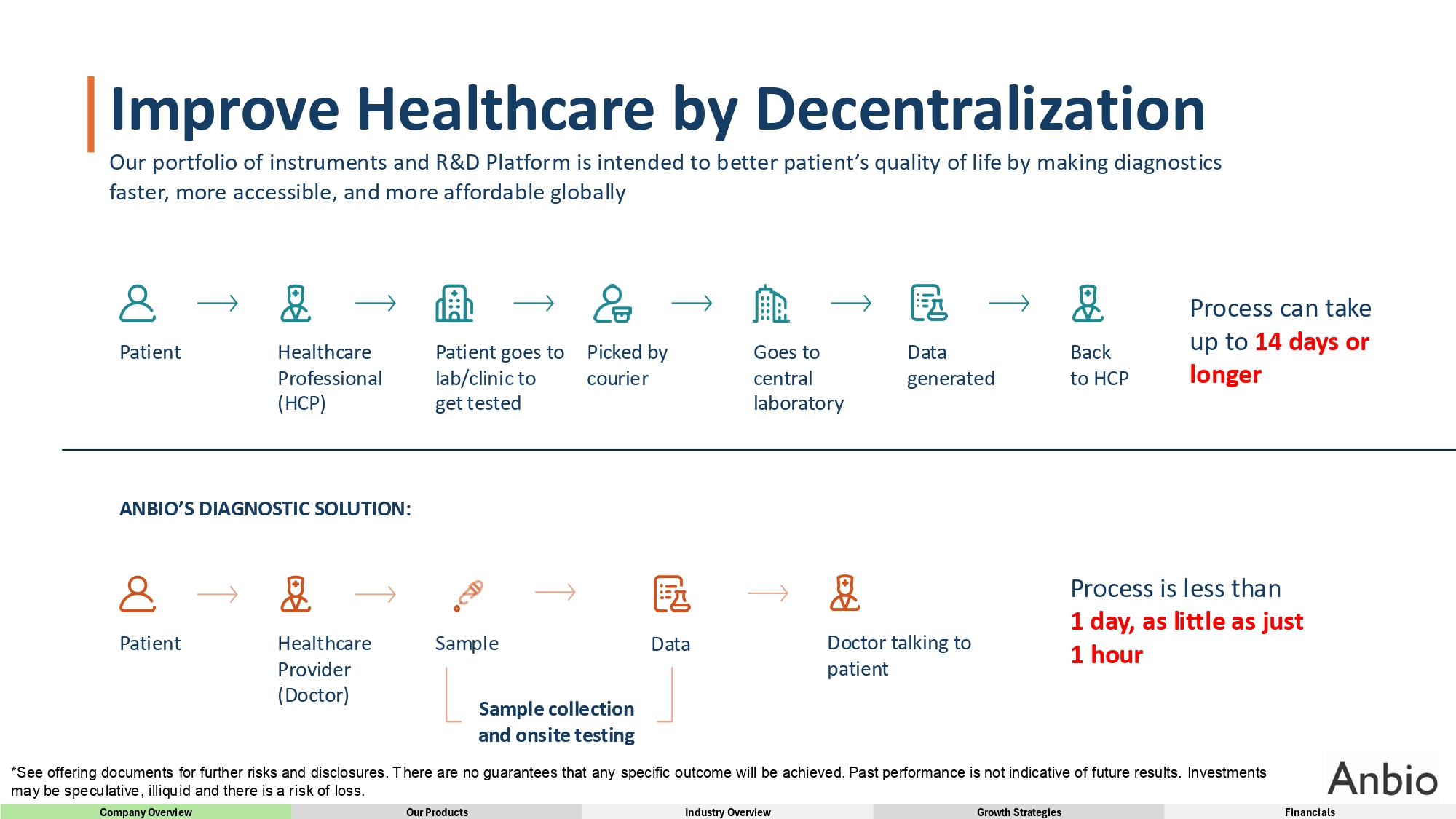
Improve Healthcare by Decentralization Our portfolio of instruments and R&D Platform is intended to better patient’s quality of life by making diagnostics faster, more accessible, and more affordable globally Process can take up to 14 days or longer Back Data Goes to Picked by Patient goes to Healthcare Patient to HCP generated central laboratory courier lab/clinic to get tested Professional (HCP) Process is less than 1 day, as little as just 1 hour Data Sample Healthcare Provider (Doctor) Doctor talking to patient Patient Sample collection and onsite testing ANBIO’S DIAGNOSTIC SOLUTION: *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

ANBIO IS PAVING THE WAY OF THE FUTURE FOR DECENTRALIZED HEALTHCARE TRADITIONAL CARE DIAGNOSTICS ◗ Long time for test results ◗ Samples are sent to central laboratories ◗ Doctors are limited to tests that can be reimbursed by insurance OUR DIAGNOSTICS SOLUTION ◗ Rapid results within 20 minutes at the site of care ◗ Samples are processed at the site of care ◗ HCP can interpret test results and prescribe treatment to the patient in just one visit ◗ Tests can be conducted by HCP and interpreted together with the patient *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

Our Products We are a Diagnostics Solutions Provider Anbio’s Fluorescent Immunochromatographic Solution (FIA) Portable reader with available assays suitable for laboratory, wellness, and point - of - care applications. Anbio’s Chemiluminescent Immunoassay Solution (CLIA) Automated and compact analyzer with available assays suitable for laboratory and wellness applications. Anbio’s RT - PCR Solution (MDx) Assays suitable for laboratory application. Anbio’s Lateral Flow Assay (Colloidal Gold) Solution (LFIA) Solution for visually detecting specific biomarker(s) in samples Anbio’s Loop - Mediated Isothermal Amplification Solution (LAMP) Molecular diagnostic technique used to amplify and detect specific DNA or RNA sequences. *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

Anbio’s Comprehensive Portfolio of Diagnostic Assays Assays for Detection of Disease Biomarkers INFECTIOUS DISEASES ◗ Infectious diseases assays available for FIA, CLIA, MDx, and LFIA platforms CANCER ◗ Cancer assays available for FIA, CLIA, and LFIA platforms Molecular Diagnostics ◗ Molecular diagnostic assays suitable for both pharmacogenetics and disease detection CARDIOVASCULAR ◗ Cardiovascular assays available for FIA, LFIA, and CLIA platforms HORMONES ◗ Hormone assays available for FIA, LFIA, and CLIA platforms DRUG OF ABUSE ◗ Drug of abuse assays available for FIA and LFIA platforms *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

SPEED ◗ We focus on rapid test results for faster health solutions. AFFORDABLE INNOVATION ◗ We provide affordable diagnostic solutions and eliminate financial barriers often seen in our current system. ◗ We focus on diagnostic technology driven by mobility and ease of use. OUR PRODUCT DEVELOPMENT PHILOSOPHY Anbio adheres to three key principles when developing our diagnostic products *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

ANBIO’S FIA SOLUTION – ANALYZER Easy to Use Portable Reader for Quantitative Biomarker Detection ANBIO BIOTECHNOLOGY AF - 100S Handheld Fluorescent Chromatographic Immunoassay (FIA) Solution The Anbio AF - 100S is a handheld, easy - to - use, affordable, and rapid point - of - care FIA that utilizes an LED light source from the reader for excitation of fluorescent dye for immunochromatographic qualitative or quantitative testing of analytes in whole blood and urine samples, including hormones detection, myocardial disease detection, infectious disease detection, and tumor - related antigens detection. Compatible with many sample types Mobile and High Throughput *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials
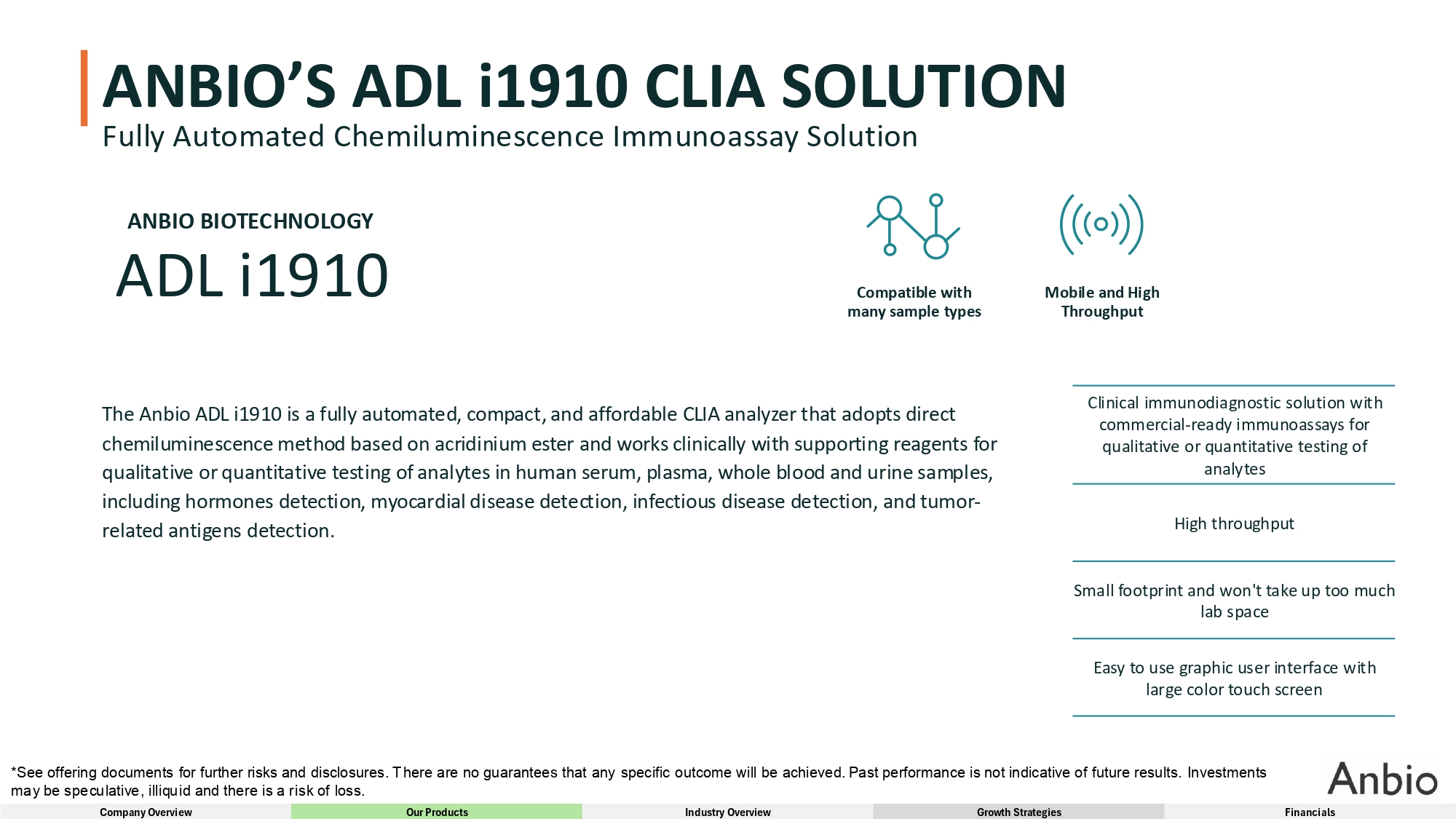
ANBIO’S ADL i1910 CLIA SOLUTION Fully Automated Chemiluminescence Immunoassay Solution ANBIO BIOTECHNOLOGY ADL i1910 The Anbio ADL i1910 is a fully automated, compact, and affordable CLIA analyzer that adopts direct chemiluminescence method based on acridinium ester and works clinically with supporting reagents for qualitative or quantitative testing of analytes in human serum, plasma, whole blood and urine samples, including hormones detection, myocardial disease detection, infectious disease detection, and tumor - related antigens detection. Clinical immunodiagnostic solution with commercial - ready immunoassays for qualitative or quantitative testing of analytes High throughput Small footprint and won't take up too much lab space Easy to use graphic user interface with large color touch screen Compatible with many sample types *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials Mobile and High Throughput
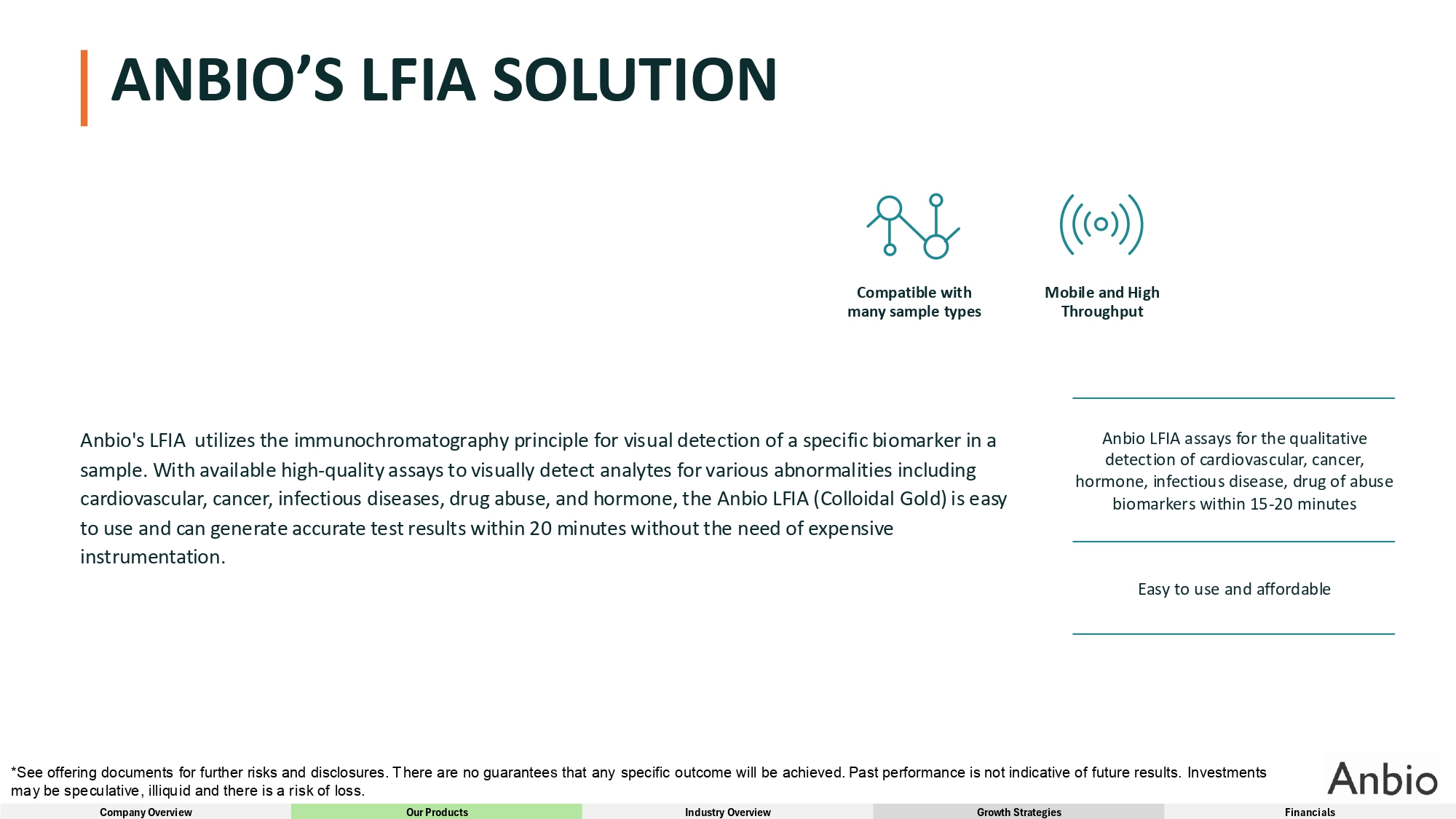
ANBIO’S LFIA SOLUTION Anbio's LFIA utilizes the immunochromatography principle for visual detection of a specific biomarker in a sample. With available high - quality assays to visually detect analytes for various abnormalities including cardiovascular, cancer, infectious diseases, drug abuse, and hormone, the Anbio LFIA (Colloidal Gold) is easy to use and can generate accurate test results within 20 minutes without the need of expensive instrumentation. Compatible with many sample types Mobile and High Throughput Anbio LFIA assays for the qualitative detection of cardiovascular, cancer, hormone, infectious disease, drug of abuse biomarkers within 15 - 20 minutes Easy to use and affordable *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials
ANBIO’S LAMP SOLUTION Easy to Use, Portable MDx Testing Anbio's LAMP Solution, or Loop - Mediated Isothermal Amplification, is a diagnostic technique used to amplify and detect specific DNA or RNA sequences . The LAMP assay is used for various applications, including the detection of infectious diseases, genetic disorders, and foodborne pathogens . Anbio’s LAMP solution is suitable for point - of - care diagnostics . Compatible with many sample types Mobile and High Throughput Anbio LAMP solution for the quantitative detection molecular biomarkers within 20 minutes For Point - of - Care Application Ready to use, all in one LAMP kit *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

Anbio simplifies the challenges faced in pharmacogenomics with our real - time PCR (RT - PCR) solutions approach that caters to the needs of both new and experienced users in quantitative reverse transcriptase PCR (qRT - PCR) and pharmacogenomics. Anbio’s portfolio of pharmacogenomics assays supports HCP’s analysis of genetic variations that affect drug metabolism, efficacy, and toxicity to determine personalized treatment options. Our reagent kits are compatible with most RT - PCR readers and can support user customization and optimization for even the most demanding assays. TARGET APPLICATIONS Anbio RT - PCR has fast cycling protocols and throughput Optimized and customizable for your sample type Compatible with most RT - PCR readers Customizable ANBIO’S PHARMACOGENOMICS SOLUTION RT - PCR Assay to Enhance HCP Analysis *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials
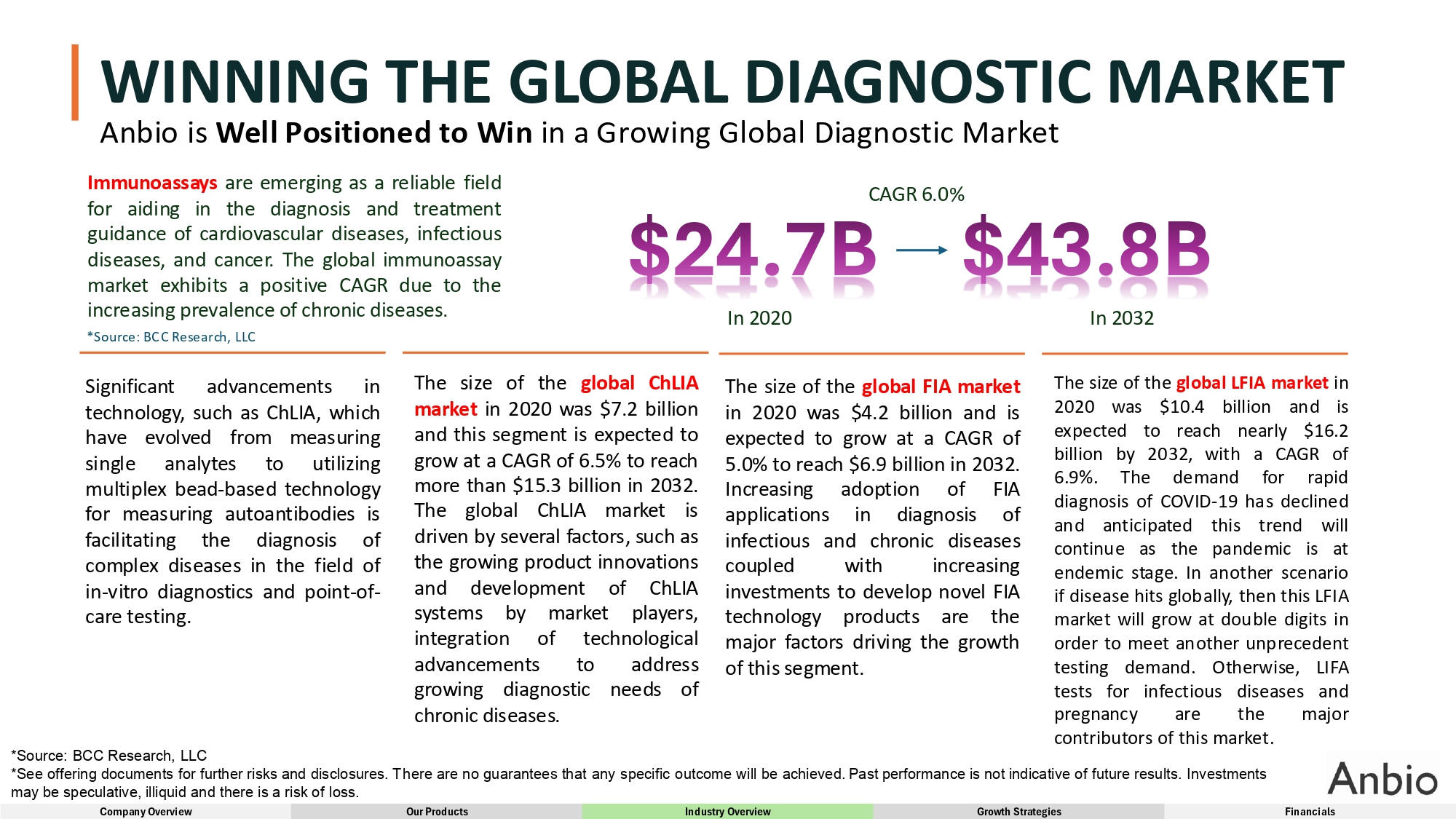
WINNING THE GLOBAL DIAGNOSTIC MARKET Anbio is Well Positioned to Win in a Growing Global Diagnostic Market Immunoassays are emerging as a reliable field for aiding in the diagnosis and treatment guidance of cardiovascular diseases, infectious diseases, and cancer . The global immunoassay market exhibits a positive CAGR due to the increasing prevalence of chronic diseases . *Source : BCC Research, LLC Significant advancements in technology, such as ChLIA, which have evolved from measuring single analytes to utilizing multiplex bead - based technology for measuring autoantibodies is facilitating the diagnosis of complex diseases in the field of in - vitro diagnostics and point - of - care testing . In 2020 In 2032 CAGR 6.0% The size of the global ChLIA market in 2020 was $ 7 . 2 billion and this segment is expected to grow at a CAGR of 6 . 5 % to reach more than $ 15 . 3 billion in 2032 . The global ChLIA market is driven by several factors, such as the growing product innovations and development of ChLIA systems by market players, integration of technological advancements to address growing diagnostic needs of chronic diseases . The size of the global FIA market in 2020 was $ 4 . 2 billion and is expected to grow at a CAGR of 5 . 0 % to reach $ 6 . 9 billion in 2032 . Increasing adoption of FIA applications in diagnosis of infectious and chronic diseases coupled with increasing investments to develop novel FIA technology products are the major factors driving the growth of this segment. The size of the global LFIA market in 2020 was $10.4 billion and is expected to reach nearly $ 16 . 2 billion by 2032 , with a CAGR of 6 . 9 % . The demand for rapid diagnosis of COVID - 19 has declined and anticipated this trend will continue as the pandemic is at endemic stage . In another scenario if disease hits globally, then this LFIA market will grow at double digits in order to meet another unprecedent testing demand . Otherwise, LIFA tests for infectious diseases and pregnancy are the major contributors of this market . *Source: BCC Research, LLC *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

WINNING THE GLOBAL DIAGNOSTIC MARKET Anbio is Well Positioned to Win in a Growing Global Diagnostic Market Global RT - PCR Market CAGR 6.8% *Source: BCC Research **Source : Isothermal Nucleic Acid Amplification Technology Market Size, Share & Trends Analysis Report By Product, By Technology (NASBA, HDA, LAMP, SDA, SPIA, NEAR), By Application, By End - use, By Region, And Segment Forecasts, 2023 — 2030 . In 2023 In 2032 The global Loop - Mediated Isothermal Amplification (LAMP) market size was valued at USD 4235 . 34 million in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 12 . 3 % from 2023 to 2030 . The Loop - Mediated Isothermal Amplification (LAMP) technology is experiencing a notable surge in demand as a molecular testing technique, primarily attributed to the growing prevalence of infectious diseases . Tuberculosis, hepatitis, and influenza are among the leading causes of deaths caused by infectious diseases, especially in the developing countries . As a result, demand for rapid, user - friendly, and disease - specific testing options is expected to boost the adoption of LAMP offerings** . PCR testing is one of the most valuable techniques currently used in bioscience, diagnostics, and forensic science . In response to the pandemic, laboratories are equipped with high volume PCR testing for COVID - 19 in order to increase the testing capacity and preferring RT - PCR as gold standard technique to diagnosis infections such as COVID - 19 , influenza, HIV are major reasons driving this market* . *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

ANBIO’S GO - TO - MARKET STRATEGY Strategic Approach to Expand Our IVD Market Share Market Expansion and Regulatory Approval •Focus on expanding global IVD market share through maturing and expanding sales, marketing, and distribution teams. •Target key regions: EU, APAC, North America, South America, and Africa. •U.S. business model includes authorized regulatory diagnostics and lab - developed tests (LDT) channels. Establish a Global Customer Base •Expansion across APAC, EU, Africa, and Americas. •Diversified customer portfolio to mitigate economic cycling impact. Promote Diagnostic Products with Global and Regional Market Conditions in Mind •Promote mature line of IVD products globally for continued growth. •Diverse product portfolio including FIA, LFIA, PCR, LAMP, and ChLIA. • Expanded customer portfolio to mitigate regional market recessions. Diversify Portfolio of IVD Products via Sales and Marketing • Concentric diversification approach in IVD sector. •Market research, product development, and cross - selling to existing clients. • New product developments targeting niches within IVD field. Provide Superior Quality Products and Customer Service •Collaborate with third - party laboratories for IVD product development. • Rigorous quality control testing for batch consistency. •Experienced Field Application Scientists (FAS) and sales team for customer service. •Fast product delivery through optimized logistics. Focus on Efficient Manufacturing and Cost Management • Strive for operational excellence to provide high - quality products at competitive prices. • Suppliers' personnel examine costs and profitability by product, plant, and region. •Leverage skilled manufacturing and supply chain management processes. *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials

Speed, Innovation and Low - Cost Given that global healthcare emphasis is shifting toward precision medicine, population health, and chronic disease management, Anbio develops and sell solutions to laboratory diagnostics and point - of - care technology in strategically selected markets. With a focus on low - cost, accessible IVD solutions, we offer a variety of mobile diagnostic instruments to detect diseases in patients who need treatment Large Scale Manufacturing and Supply Capability The supply chain we utilize to manufacturing our products may require a large amount of raw material consumption, allowing favorable financing terms, driving down the cost of goods, and exhibiting economies of scale. These factors promote maintain lean manufacturing processes, lowering the transfer price and maximizing our gross profit margin. Experienced and Proven Management Team Our management team has significant leadership experience in the diagnostic space for developing and commercializing IVD instruments and consumables globally. Competitive Advantages Our Advantages to Capture the Market Opportunities in the IVD Space *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials
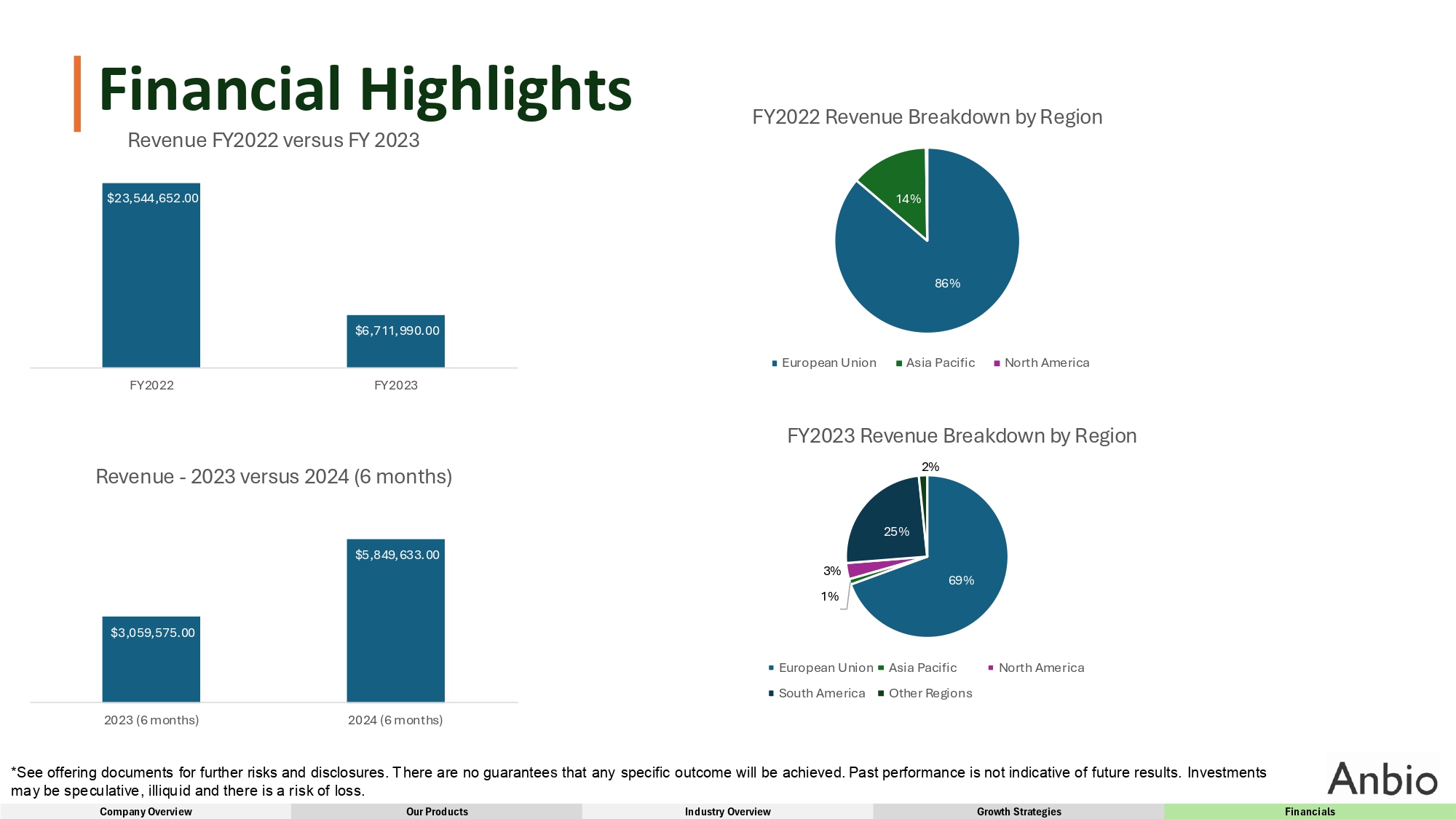
Financial Highlights Revenue FY2022 versus FY 2023 86% 14% FY2022 Revenue Breakdown by Region European Union Asia Pacific North America 69% 3% 1% 25% FY2023 Revenue Breakdown by Region 2% European Union South America Asia Pacific Other Regions North America $3,059,575.00 $5,849,633.00 2023 (6 months) 2024 (6 months) Revenue - 2023 versus 2024 (6 months) $23,544,652.00 *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials $6,711,990.00 FY2022 FY2023
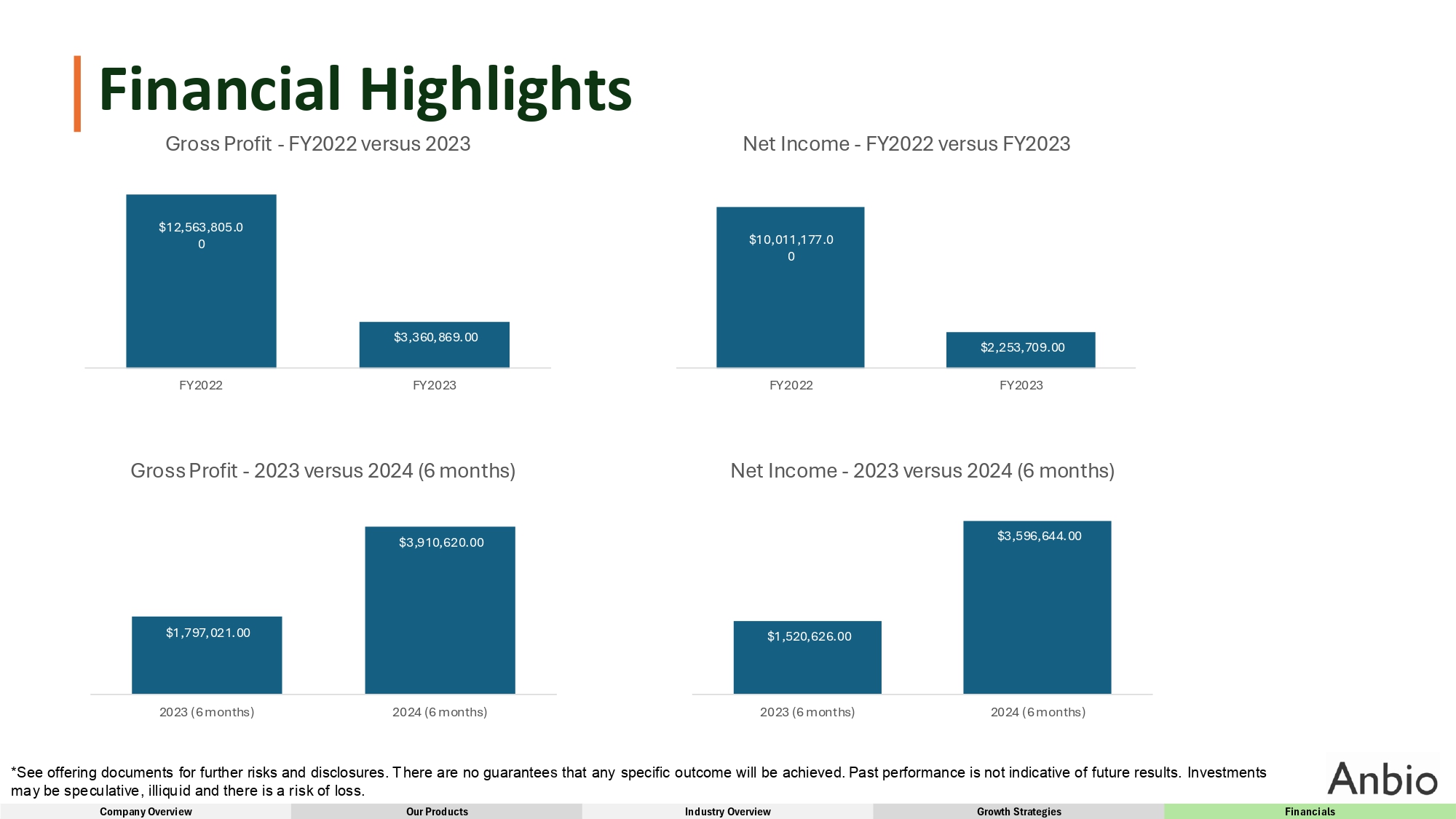
Financial Highlights Gross Profit - FY2022 versus 2023 $1,797,021.00 $3,910,620.00 2023 (6 months) 2024 (6 months) Gross Profit - 2023 versus 2024 (6 months) $12,563,805.0 0 $3,360,869.00 FY2022 FY2023 $10,011,177.0 0 $2,253,709.00 FY2022 FY2023 Net Income - FY2022 versus FY2023 $1,520,626.00 *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss. Company Overview Our Products Industry Overview Growth Strategies Financials $3,596,644.00 2023 (6 months) 2024 (6 months) Net Income - 2023 versus 2024 (6 months)

Anbio Improving Prognosis by Decentralizing Diagnostics Anbio info@acsunshine.co m +1 689 - 689 - 9686 www.acsunshine.com *See offering documents for further risks and disclosures. There are no guarantees that any specific outcome will be achieved. Past performance is not indicative of future results. Investments may be speculative, illiquid and there is a risk of loss.

























