UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark one)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 000-09165
STRYKER CORPORATION
(Exact name of registrant as specified in its charter)
| | |
| Michigan | | 38-1239739 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| |
| 2825 Airview Boulevard, Kalamazoo, Michigan | | 49002 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (269) 385-2600
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class | | Name of each exchange on which registered |
| Common Stock, $.10 par value | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
YES x NO ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
YES ¨ NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
YES x NO ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
YES x NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of large “accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| | |
Large accelerated filer x | | Accelerated filer ¨ |
| Non-accelerated filer ¨ | | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
YES ¨ NO x
Based on the closing sales price of June 30, 2010, the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $18,806,045,091.
The number of shares outstanding of the registrant’s common stock, $.10 par value, was 391,246,163 at January 31, 2011.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the proxy statement to be filed with the U.S Securities and Exchange Commission relating to the 2011 Annual Meeting of Shareholders (the 2011 proxy statement) are incorporated by reference into Part III.
FORWARD-LOOKING STATEMENTS
This report contains information that includes or is based on forward-looking statements within the meaning of the federal securities law that are subject to various risks and uncertainties that could cause actual results of Stryker Corporation (the Company) to differ materially from those expressed or implied in such statements. Such factors include, but are not limited to: weakening of economic conditions that could adversely affect the level of demand for the Company’s products; pricing pressures generally, including cost-containment measures that could adversely affect the price of or demand for the Company’s products; changes in foreign exchange markets; legislative and regulatory actions; unanticipated issues arising in connection with clinical studies and otherwise that affect U.S. Food and Drug Administration approval of new products; changes in reimbursement levels from third-party payors; a significant increase in product liability claims; unfavorable resolution of tax audits; changes in financial markets; changes in the competitive environment; and the Company’s ability to integrate acquisitions.
While the Company believes the assumptions underlying such forward-looking statements are reasonable, there can be no assurance that future events or developments will not cause such statements to be inaccurate. All forward-looking statements contained in this report are qualified in their entirety by this cautionary statement.
REGISTERED TRADEMARKS AND TRADEMARKS
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks in this Report: 3-Chip, ABG, Accolade, ADM, Ascent Healthcare Solutions, Asnis, AxSOS, AVS, BackSmart, Big Wheel, CentPillar, CerviCore, Chaperone, Colorado, Dall-Miles, Dynatran, Exeter, FlexiCore, Formula, Flyte, Gamma3, GMRS, Hoffmann, Howmedica, HydroSet, iBed, IDEAL EYES, iSuite, Leibinger, LFIT, Maestro, Mantis, Mobile Bearing Hip, Monotube, NRG, OASYS, Omnifit, OP-1, OtisKnee, PainPump, Power-PRO, PureFix, Radius, Reflex, Rejuvenate, RemB, Restoration, S3, Scorpio, Scorpio ClassiQ, Secur-Fit, Sightline, Simplex, Sonopet, SpineCore, Stair-PRO, Stair-TREAD, Stryker, Stryker Orthopaedics, Switchpoint Infinity, System 6, T2, TenXor, THOR, TMZF, Triathlon, Trident, Tritanium, UHR, VariAx, VLIFT, WiSe, X3, Xia, Zoom. Cormet is a registered trademark of Corin Limited. All other trademarks or service marks are trademarks or service marks of their respective owners or holders.
Not all products referenced in this report are approved or cleared for sale, distribution or use in the United States.
TABLE OF CONTENTS
PART I
GENERAL
Stryker Corporation (the Company or Stryker) is one of the world’s leading medical technology companies and is dedicated to helping healthcare professionals perform their jobs more efficiently while enhancing patient care. The Company provides innovative orthopaedic implants as well as state-of-the-art medical and surgical equipment to help people lead more active and more satisfying lives. The Company’s products include implants used in joint replacement, trauma and spinal surgeries; surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment as well as other medical device products used in a variety of medical specialties. Stryker was incorporated in Michigan in 1946 as the successor company to a business founded in 1941 by Dr. Homer H. Stryker, a leading orthopaedic surgeon and the inventor of several orthopaedic products.
Stryker’s filings with the U.S. Securities and Exchange Commission (SEC), including its annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, are accessible free of charge at www.stryker.com within the “Investor—SEC Filings & Ownership Reports” link.
In 2010 the Company completed several acquisitions in all cash transactions, including the Sonopet Ultrasonic Aspirator assets from Mutoh Co., Ltd. and Synergetics USA, Inc., Gaymar Industries, Inc. and the bioimplantable implants product line and related assets from Porex Surgical, Inc. In December 2009 the Company acquired Ascent Healthcare Solutions, Inc. (Ascent), also in an all cash transaction.
In December 2010 the Company announced a definitive agreement to sell its OP-1 product family for use in orthopaedic bone applications and its manufacturing facility based in West Lebanon, NH. This transaction was completed on February 1, 2011 for total consideration of $60.0 million.
In October 2010 the Company announced a definitive agreement to acquire the assets of the Neurovascular division of Boston Scientific Corporation (Neurovascular) in an all cash transaction of up to $1.5 billion. This transaction was completed on January 3, 2011 for $1.45 billion in cash plus an additional $50.0 million to be paid upon the completion of certain milestones. The acquisition of Neurovascular is expected to substantially enhance the Company’s presence in the neurovascular market, allowing it to offer a comprehensive portfolio of products in both neurosurgical and neurovascular devices.
PRODUCT SALES
The Company segregates its operations into two reportable business segments: Orthopaedic Implants and MedSurg Equipment. The Orthopaedic Implants segment sells orthopaedic reconstructive (hip and knee), trauma and spinal implant systems and other related products. The MedSurg Equipment segment sells surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment; as well as other medical device products used in a variety of medical specialties.
1
The following amounts and percentages represent domestic/international and business segment net sales during each of the three years ended December 31 (dollars in millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | $ | | | % | | | $ | | | % | | | $ | | | % | |
Domestic/international sales: | | | | | | | | | | | | | | | | | | | | | | | | |
Domestic | | $ | 4,792.8 | | | | 65 | % | | $ | 4,317.4 | | | | 64 | % | | $ | 4,282.2 | | | | 64 | % |
International | | | 2,527.2 | | | | 35 | % | | | 2,405.7 | | | | 36 | % | | | 2,436.0 | | | | 36 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total net sales | | $ | 7,320.0 | | | | 100 | % | | $ | 6,723.1 | | | | 100 | % | | $ | 6,718.2 | | | | 100 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Business segment sales: | | | | | | | | | | | | | | | | | | | | | | | | |
Orthopaedic Implants | | $ | 4,308.4 | | | | 59 | % | | $ | 4,119.7 | | | | 61 | % | | $ | 3,967.5 | | | | 59 | % |
MedSurg Equipment | | | 3,011.6 | | | | 41 | % | | | 2,603.4 | | | | 39 | % | | | 2,750.7 | | | | 41 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total net sales | | $ | 7,320.0 | | | | 100 | % | | $ | 6,723.1 | | | | 100 | % | | $ | 6,718.2 | | | | 100 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Additional financial information regarding the Company’s operating segments and geographic areas can be found under the captionResults of Operations in Item 7 of this report and Note 13 to the Consolidated Financial Statements in Item 8 of this report.
Approximately 76% of the Company’s sales in 2010, 77% in 2009 and 74% in 2008 consisted of products with short lives, such as reconstructive, trauma, craniomaxillofacial (CMF) and spinal implant systems (while implants have a long useful life to the patient, they have a one-time use to the hospital); disposables and expendable tools; and parts and service revenues, including service and repair charges. The balance of sales in each of the years came from products that could be considered capital equipment, having useful lives in excess of one year.
The Company’s backlog of firm orders is not considered material to an understanding of its business.
Orthopaedic Implants
Orthopaedic Implants consist of such products as implants used in joint replacement, trauma, craniomaxillofacial and spinal surgeries; bone cement; and the bone growth factor OP-1. Artificial joints are made of cobalt chromium, titanium alloys, ceramics or ultrahigh molecular weight polyethylene and are implanted in patients whose natural joints have been damaged by arthritis, osteoporosis, other diseases or injury. Many of Stryker’s technologically advanced reconstructive implants are suited to minimally invasive surgery (MIS) procedures that are intended to reduce soft-tissue damage and pain while hastening return to function. The Company supports surgeons with technology, procedural development and specialized instrumentation as they develop new MIS techniques.
Hip Implant Systems
The Company offers a variety of hip implant systems for the global reconstructive market. Total hip replacement surgeries involve the replacement of both the head of the femur as well as the acetabulum (socket) of the pelvis. Stryker offers hip replacement products to address first time (primary) hip surgeries as well as revision systems. The ABG Hip System, Partnership Hip System, Secur-Fit Hip System, Omnifit Hip System, Accolade Hip System, CentPillar Hip System, Trident Acetabular Hip System, ADM Mobile Bearing Hip System, Rejuvenate Modular Primary Hip System, Cormet Hip Resurfacing System and Restoration Hip System are all comprehensive systems of hip implants and associated instrumentation designed to provide physicians and patients with reliable clinical results across the continuum of care, while enhancing value and operating room efficiency for the hospital.
2
Stryker is committed to following clinical outcomes and recognizes that long-term clinical results are an important factor in the Company’s ability to market hip implants. Significant clinical milestones for the Company include more than 40 years of clinical history with the Exeter Hip System, more than 25 years of clinical history with the Dall-Miles Cable System, more than 20 years of clinical history with the Omnifit HA stem and more than 10 years clinical history with the Accolade TMZF Hip System.
Stryker was the first company to receive clearance from the U.S. Food and Drug Administration (the FDA) to commercially release for sale in the United States a hip implant with hydroxylapatite (HA) surface treatment. HA is a naturally occurring calcium phosphate material that demonstrates a high level of biocompatibility due to its resemblance to bone. The Company’s global clinical experience with HA-coated hip stems now extends over 20 years, and reported clinical performance continues to equal or exceed that of comparable hip stems reported in the scientific literature.
Primary Femoral Hip Systems:
In 2009 Stryker introduced the Rejuvenate Modular Primary Hip System, the latest evolution in the Company’s OmniFit and Secur-Fit hip systems. The Rejuvenate Modular Primary Hip System offers surgeons unparalleled options for personalizing the implant to each patient’s anatomy. The Rejuvenate System is designed to optimize anatomic restoration by providing options that offer enhanced stability, proven modularity and intraoperative flexibility. The modular design enables the surgeon to independently manage stem size, leg length and offset to recreate the patient’s anatomy, restore biomechanics and, consequently, minimize the risk of dislocation.
The Accolade TMZF Hip System has demonstrated strong clinical results for more than 10 years, with 2009 marking the first introduction of the product into Japan. The Accolade TMZF System is a tapered wedge implant, based on a broach only technique and is recognized for its simplicity and flexibility to accommodate all surgical approaches and navigation.
The ABG II Modular Hip System represents the next generation of design based on the ABG monolithic stem that has had positive clinical experience for more than 10 years. This modular primary hip stem provides the opportunity to recreate patient anatomy through independent sizing of the stem and neck. Versatile instrumentation also accommodates surgeon preference for a navigated procedure or direct anterior surgical approach.
The Company’s Exeter Total Hip System is based on a collarless, highly polished, double-tapered femoral design that reduces shear stresses and increases compression at the cement/bone interface.
Primary Acetabular Systems:
The Company’s advanced bearing system, Low Friction Ion Treatment (LFIT) Anatomic Femoral Heads with X3 polyethylene liners represents a significant advance in hip-bearing technology through the combination of Stryker’s LFIT technology and X3 advanced bearing technology. The femoral heads are anatomically sized for more natural hip performance. In 2010 the Company introduced the ADM X3 Mobile Bearing Acetabular System, a next-generation technology for hip replacement surgery designed to minimize the risk associated with total hip replacement surgery by offering a large diameter bearing without a metal-on-metal articulation. X3 advanced bearing technology is based on the Company’s highly crosslinked polyethylene, which demonstrates enhanced material characteristics in laboratory testing, including improved strength, reduced wear and oxidation resistance. This second generation bearing option offers a significant technological advance for both hip and knee replacements. The Company also offers the Biolox Delta Ceramic Anatomic head for further options to reduce wear and potentially increase implant longevity.
3
The Company has a premarket approval (PMA) from the FDA for its ceramic-on-ceramic hip replacement system, the Trident Ceramic Acetabular Insert, for patients in the United States. Stryker Orthopaedics has successfully launched the Trident ceramic insert in the United States, Europe, Australia and Canada. The Trident insert has demonstrated low wear clinically, and it is protected and strengthened by a patented titanium sleeve.
In 2009 the Company introduced the Tritanium Primary Acetabular System in Latin America, Australia, and Europe following a U.S. launch in 2008. This system is the only Advanced Fixation Technology manufactured from a commercially-pure Titanium matrix. Introduction of this highly porous surface into the primary hip market provides a biologically-inspired enhanced fixation acetabular solution that was previously not available for primary use.
Hip Fracture Hip Systems:
Stryker offers a broad array of femoral stem options and bearings to accommodate the hip fracture patient including the Accolade HFx stem and the UHR bipolar head.
Revision Hip Systems:
The Restoration Modular Revision Hip System offers surgeons performing revision surgeries flexibility in treating complex hip stem revisions and restoring patient biomechanics. The Restoration Modular Revision Hip System also takes advantage of Stryker’s long clinical history with HA by incorporating PureFix HA coating on many components. The Restoration Modular Revision Hip System complements the Company’s existing Restoration HA and Restoration plasma spray (PS) monolithic revision systems.
The Company’s Trident Tritanium Acetabular Shell contains a highly porous surface that closely resembles the structure of bone. This shell is designed for revision surgery and contains multiple screw holes to achieve bone fixation and initial stability.
Knee Implant Systems
The Company offers three major knee implant systems: Triathlon, Scorpio and the Global Modular Replacement System (GMRS).
The Triathlon Knee System utilizes the Company’s evolutionary design that more closely reproduces natural knee motion and provides mobility with stability through more than 150 degrees of flexion. In 2008, Stryker continued to expand the Triathlon brand with the Triathlon Partial Knee Resurfacing (PKR) offering in the uni-condylar market segment and the Triathlon Total Stabilizer (TS) offering in the fast growing revision knee market. Both products incorporate the single radius design to provide the potential for better ligament balancing. The Company also offers the X3 advanced bearing technology for use with the Triathlon Knee System.
The Scorpio knee implant design is based on Stryker’s patented single radius; this approach to total knee replacement addresses significant clinical issues, such as improved patient rehabilitation and midflexion stability. The Scorpio NRG is the high flexion evolution to the Scorpio line. This design includes increased rotational allowance, an articulating design for deeper flexion and greater extension allowance without impingement. The Scorpio NRG with X3 advanced bearing technology is designed to lower wear rates compared to standard inserts. Scorpio ClassiQ was developed for the emerging global markets and is based on the Scorpio System to offer a high-quality and clinically proven affordable technology.
The GMRS is a global product that offers a comprehensive solution for severe bone loss in oncology, trauma and revision surgery patients. GMRS has tibial and femoral components, including a total femur, and a modular rotating hinge knee. The system employs both titanium and cobalt chrome alloys for strength and lightness of weight, together with the superior flexibility of the hinge.
4
Bone Cement
Simplex bone cement, a material used to secure cemented implants to bone, was first approved for orthopaedic use in the United States in 1971 and is the most widely used bone cement in the world. The Company manufactures and provides several variations of Simplex bone cement to meet specific patient needs. Simplex has nearly 50 years of clinical history, the longest of any bone cement, with more than 600 published clinical papers.
Trauma Implant Systems
The Company develops, manufactures and markets trauma, extremities and deformity correction systems. These systems include Intramedullary (IM) and cephlomedullary nails, locked and non-locked plating, hip fracture solutions and external fixation systems, as well as bone substitutes that are used primarily for the treatment of traumatic injuries.
The Company’s internal fixation portfolio includes a full array of IM & cephlomedullary nails; hip fracture solutions, including compression hip screws, cannulated screws as well as anatomically designed plates and screws in both titanium and stainless steel. These products provide a possible restorative option prior to joint reconstruction. These products are marketed worldwide as: Gamma3, Asnis III, AxSOS, VariAx, HydroSet, and T2.
The Company’s external fixation portfolio includes products such as Hoffmann II MRI, Hoffmann Xpress, Monotube Triax mono-lateral, as well as the Hoffmann II Hybrid (TenXor) circular fixation systems. These systems are used to construct frames for bone stabilization that are either definitive or as a temporary step in the treatment process associated with damage control orthopaedics. Hoffmann systems have been defined by their ease of assembly with “snap-fit” couplers. The use of a proprietary Vectran coating on the bars makes Hoffmann II MRI an MRI conditional solution.
The Company also offers a product portfolio for the treatment of fractures and injuries of the extremities. These products include fracture specific locked plating for the wrist, shoulder, elbow, fibula and foot, as well as bone substitutes and external fixation systems. These are all designed to treat the unique nature of upper extremity and foot and ankle injuries. These products are marketed worldwide under the brands VariAx, AxSOS and Hoffmann. New products launched in 2010 include the VariAx Fibula and VariAx Elbow Systems.
Spinal Implant Systems
The Company develops, manufactures and markets spinal implant products including cervical, thoracolumbar and interbody systems used in spinal injury, deformity and degenerative therapies. Spinal implant products include plates, rods, screws, connectors, spacers and cages, along with proprietary implant instrumentation.
In 2009 the Company introduced the Xia 3 Sacral Iliac system that completes the thoracolumbar system and makes it one of the most comprehensive platforms on the market. Also in 2009, the Company introduced the Dynatran-Dynamic/Translational Anterior Cervical Plate, which expands the Company’s presence in the cervical space with its unique locking mechanism. In 2008 the Company introduced the Radius Thoracolumbar Spinal Implant System. The Radius system provides a non-threaded wedgelock locking mechanism designed to reduce the potential for false locking and cross-threading and to increase the speed, ease and reliability of connecting rods to screws. Also in 2008, the Company launched Xia 3, the next generation of its thoracolumbar spinal implant system, and THOR, its anterior lumbar plating system that incorporates a proprietary screw locking technology. The Company also offers the Mantis minimally invasive access system for posterior instrumented spinal fusion and the Reflex Zero Profile anterior cervical plating system. In addition the Company offers the VLIFT vertebral body replacement system consisting of a preassembled, cylindrically shaped titanium
5
cage with a distractible or retractable center. The hollow core of the cage allows for packing bone graft. The Company’s AVS AS and AL Spacers are used as vertebral body support devices in anterior procedures. Other product lines include the OASYS fixation system that serves the posterior cervical fusion market, the Reflex Hybrid anterior cervical plate and the AVS PL and TL vertebral spacer systems.
Craniomaxillofacial (CMF) Implant Systems
The Company develops, manufactures and markets plating systems and related implants and products for craniomaxillofacial surgery, including dura substitutes, bone substitutes, electrosurgical microdissection needles and surgical instruments. They are primarily used in the fixation of fractures due to sudden injury as well as in the correction of congenital deformities. These products are marketed under such names as the Universal Fixation System, Colorado Needle, DuraMatrix-Onlay, Leibinger Instruments and HydroSet.
In 2010 the Company acquired the bioimplantable porous polyethylene (PPE) product line from Porex Surgical, Inc. for use primarily in reconstructive surgery of the head and face. These PPE products have a long history with considerable clinical data that is supported by over 350 references in peer-reviewed journals.
OP-1/BMP-7
Stryker’s OP-1 Implant is composed of recombinant human OP-1 and a bioresorbable collagen matrix. Stryker has received two approvals for a Humanitarian Device Exemption (HDE) from the FDA. An HDE, as defined by the FDA, is for a product intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 4,000 individuals per year in the United States. OP-1 is currently used for orthopaedic bone applications, including the use of OP-1 Implant as an alternative to autograft in recalcitrant long-bone nonunions where use of autograft is not feasible and alternative treatments have failed. A second application is for revision posterolateral spine fusion using a new formulation of OP-1 known as OP-1 Putty. In 2010 the Company announced that it had entered into a definitive agreement with Olympus Corporation for the sale of its OP-1 product family as used in orthopaedic bone applications and completed this transaction on February 1, 2011.
The Company continues to conduct research and development efforts directed toward exploring the cartilage regeneration properties of BMP-7 for potential use in osteoarthritis as well as other non-orthopaedic applications. The Company has successfully completed preclinical studies showing that BMP-7 can stimulate new cartilage formation and increase disc height in animal models of degenerative disc disease. In 2008 the Company completed enrollment in a Phase I dose-ranging clinical safety study for the first time use of BMP-7 to treat the disc. Stryker has also filed an Investigational New Drug application with the FDA to treat osteoarthritis in the knee with the injectable form of BMP-7. Following FDA concurrence in 2007, the Company proceeded with patient enrollment in the Phase I clinical study, which was completed in 2008. Based on the results of that study, a Phase II protocol was submitted to and approved by the FDA. The Company began enrollment in the Phase II study in 2010. Given the early stage of these clinical efforts and the expected scope of data to be required by the FDA, commercialization of BMP-7 is not expected for at least five years.
MedSurg Equipment
MedSurg Equipment products include surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment; as well as other medical device products used in a variety of medical specialties.
Surgical Equipment and Surgical Navigation Systems
The Company offers a broad line of surgical, neurologic, ENT and interventional spine equipment that is used in surgical specialties for drilling, burring, rasping or cutting bone in small-bone orthopaedic, neurosurgical, spine and ENT procedures; wiring or pinning bone fractures; and preparing hip or knee surfaces for the placement of artificial implants. Stryker also manufactures an array of different attachments and cutting accessories for use by orthopaedic, neurologic and small-bone specialists.
6
In 2010 the Company acquired the assets used to produce the Sonopet Ultrasonic Aspirator control consoles, handpieces and accessories. The Sonopet Ultrasonic Aspirator is used in the fields of neurosurgery as well as for orthopedic, general, laparoscopic and plastic surgeries. The device is used by surgeons to fragment soft and hard tissue and for tumor removal and bone cutting.
The System 6 heavy duty, large-bone power system represents the Company’s primary heavy-duty, cordless product offering. The System 6 Rotary Handpieces provide multiple options to surgeons by allowing both high-speed drilling and high-torque reaming in one handpiece.
In 2009 Stryker introduced the RemB micro electric system, combining the Company’s Consolidated Operating Room Equipment (CORE) platform with lightweight, specialized handpieces, allowing surgeons to work effectively with greater precision and control. This versatile system is an evolution in the Company’s offering of powered surgical instruments designed to remove and reshape bone in a wide variety of medical specialties including hand surgery, podiatry, orthopaedic foot and ankle surgery and extremity trauma surgery. The Maestro drill represents Stryker’s line of micro powered instruments for spine, neurology and ENT applications. The Maestro drill leverages the Company’s Total Performance System (TPS) and CORE platforms by using the same cutting attachments. The Stryker Bone Mill, launched in 2008, further leverages the CORE platform and is designed for use in spine, orthognathic and orthopaedic primary and revision joint procedures.
To promote safety for patients and medical staff, Stryker works closely with hospitals and other healthcare organizations to develop a broad product portfolio. In 2009 the Company introduced the Flyte personal protection system, the latest version of Stryker’s Sterishield line of personal protection products combining improved comfort and support with higher levels of protection against contamination, exposure to infectious bodily fluids and transfer of microorganisms and particulate matter. Additionally, Flyte’s integrated helmet with illumination represents enhancements aimed towards improving the surgical environment.
The Company also offers a broad line of surgical navigation systems that give surgeons in several specialties the ability to use electronic imaging to see more clearly, better align instruments and more accurately track where the instruments are relative to a patient’s anatomy during surgical procedures. The Company offers the Navigation System II Cart, the eNlite suitcase system and the Navigation iSuite. All of these product offerings are either image based or imageless platforms, incorporating intuitive Smart hardware and software functionality, and a highly accurate digital infrared camera that result in greater ease of use and less invasive procedures.
Endoscopic and Communications Systems
The Company develops, manufactures and markets medical video and communications equipment and instruments for arthroscopy, general surgery and urology. Stryker has established a position of leadership in the production of medical video technology and accessories for minimally invasive surgery, as well as communications equipment to facilitate local and worldwide sharing of medical information among operating rooms, doctors’ offices and teaching institutions. Products include medical video cameras, digital documentation equipment, arthroscopes, laparoscopes, powered surgical instruments, radio frequency ablation systems, irrigation fluid management systems, i-Suite operating room solutions and state-of-the-art equipment for telemedicine and enterprise-wide connectivity. Stryker’s line of rigid scopes, which range in diameter from 1.9 millimeters to 10 millimeters, contains a series of precision lenses as well as fiber optics that, when combined with Stryker’s high-definition (HD) camera systems, allow the physician to view internal anatomy with a high degree of clarity.
In 2010 Stryker continued to expand its wireless platform by launching the WiSe 1:3 Transmitter, which allows simultaneous transmission to three high-definition wireless WiSe monitors. The Switchpoint Infinity 3 video router provides surgeons and operating room staff with an integrated platform tailored specifically to each individual operating room. This router increases efficiency and flexibility during procedures.
7
In 2009 Stryker introduced the 1288 HD Camera, the next generation Stryker 3-Chip HD medical video camera. This latest version has HD 1080p resolution with wireless high definition transmission to the new Stryker WiSe wide screen monitor system. This new camera system provides superior image quality compared to previous camera systems and ease of use through customized programmable buttons. This product provides surgical teams with improved visibility during endoscopic procedures, which can improve overall surgical and patient outcomes. In conjunction with the launch of the 1288 HD Camera, Stryker also introduced the L-9000 lightsource. This new lightsource includes proprietary LED technology that provides the customer with a cooler and longer lasting bulb. Also introduced in 2009 was the IDEAL EYES line of HD arthroscopes and laparoscopes. To accommodate the recording of HD images, the Company offers the SDC HD digital documentation system. The Company also offers its Formula shaver system, which is small, light and equipped with radio frequency identification (RFID), facilitating communication between the blade and console.
Patient Handling and Emergency Medical Equipment
Stryker is a leader in the patient handling equipment segment, offering a wide variety of stretchers customized to fit the needs of acute care and specialty surgical care facilities with a focus on providing a safe and comfortable surface for patients while reducing the risk of back injury for hospital staff.
In 2010 the Company acquired Gaymar Industries, which specializes in support surfaces and pressure ulcer management solutions as well as the temperature management segment of the healthcare industry. The acquisition expands the Company’s product offerings while also providing a complementary product offering to its existing customer base through its temperature management technology platform.
In 2010 Stryker introduced the Prime series of stretchers, designed to ensure caregiver safety and efficiency while enhancing patient comfort. Patients can adjust their own positions without calling a caregiver for assistance. The Prime stretchers incorporate Glideaway siderails with Zero Transfer Gap; the Zoom Motorized Drive System, virtually eliminating push force; Big Wheel technology, reducing start-up force by up to 50 percent and increasing maneuverability; and a 700-pound weight capacity.
Stryker also develops and manufactures beds and accessories that are designed to meet the unique needs of specialty departments within the acute care environment. In 2008 the Company introduced the redesigned S3 Med/Surg Hospital Bed, the first redesign since its original 1994 introduction, combining a retractable frame with the Company’s BackSmart ergonomically designed side rails and featuring an open architecture to accept any standard support surface. The S3 offers the Chaperone center-of-gravity bed-exit system with Zone Control to help prevent patient falls, as well as iBed Awareness, an exclusive technology that monitors safe patient bed positions and alerts caregivers in the event that the desired safe bed configuration is altered. In 2009 the Company introduced the Impression non-powered support surface designed to improve pressure redistribution, enhance patient comfort and provide enhanced moisture management similar to that achieved by powered support surfaces. Stryker has a complete line of intensive care unit (ICU) beds for critical care and step-down units. The beds incorporate advanced features that facilitate patient care, such as in-bed scales that accurately weigh the patient regardless of bed position and a radiolucent surface that facilitates chest x-rays without moving the patient from the bed. Stryker’s XPRT support surface, with low air loss, percussion and rotational therapy, aids in the prevention and treatment of certain skin ulcers and pulmonary care.
To serve the worldwide pre-hospital market, the Company offers a line of manually operated and powered ambulance cots and cot-to-ambulance fastening systems. In addition, Stryker offers the Stair-PRO stair chairs with Stair-TREAD track systems that facilitate patient transport up and down stairs. The Company’s Power-PRO ambulance cot incorporates an advanced battery-powered hydraulic lift system that enables emergency medical professionals to raise and lower the cot with the press of a button. The use of Stair-PRO and the Power-PRO helps prevent caregiver back injuries. Stryker expanded the Power-PRO line with a version customized for streamlined incubator transport on both inter-facility and intra-facility transports and with a version customized for ambulances that use hydraulic tail lifts or ramps that are popular in the United Kingdom.
8
Medical Device Reprocessing
In December 2009 the Company acquired Ascent, the market leader in the medical device reprocessing industry. Ascent’s reprocessing of medical devices includes collecting recyclable medical devices from hospitals and cleaning, function testing and sterilizing the medical devices for reuse in subsequent procedures. The reprocessed medical device is equivalent to a new medical device in terms of function, quality and effectiveness. Extending the life of these medical devices beyond a single use enables the Company to partner with health care providers in managing costs without compromising the quality of patient care and diverts thousands of pounds of medical waste from landfills.
PRODUCT DEVELOPMENT
Most of the Company’s products and product improvements have been developed internally. The Company maintains close working relationships with physicians and medical personnel in hospitals and universities who assist in product research and development. New and improved products play a critical role in the Company’s sales growth. The Company continues to place emphasis on the development of proprietary products and product improvements to complement and expand its existing product lines. The Company has a decentralized research and development focus, with manufacturing locations responsible for new product development and product improvements. Research, development and engineering personnel at the various manufacturing locations maintain relationships with staff at distribution locations and with customers to understand changes in the market and product needs.
Total expenditures for product research, development and engineering were $393.9 million in 2010, $336.2 million in 2009 and $367.8 million in 2008. Research, development and engineering expenses represented 5.4% of sales in 2010, compared to 5.0% in 2009 and 5.5% in 2008. The spending level in 2010 increased due to the Company’s increased focus on new product development for anticipated future product launches and investments in new technologies. Recent new product introductions in the Orthopaedic Implants and MedSurg Equipment segments are more fully described under the captionProduct Sales.
In addition to internally developed products, the Company invests in technologies developed by third parties that have the potential to expand the markets in which the Company operates. In 2010 the Company acquired the Sonopet Ultrasonic Aspirator control consoles, handpieces and accessories from Mutoh Co., Ltd. and Synergetics USA, Inc., Gaymar Industries, a manufacturer of specialized support surface and pressure ulcer management solutions, and the bioimplantable porous polyethylene (PPE) product line from Porex Surgical, Inc. for use primarily in reconstructive surgery of the head and face. In December 2009 the Company acquired Ascent, the market leader in the reprocessing and remanufacturing of medical devices in the U.S. During 2010 and 2009, the Company acquired certain additional companies all of which are expected to enhance the Company’s product offerings to its customers within its Orthopaedic Implants and MedSurg Equipment business segments.
MARKETING
Domestic sales accounted for 65% of total revenues in 2010. Most of the Company’s products are marketed directly to doctors, hospitals and other healthcare facilities through dedicated sales forces for each of its principal product lines to provide focus and a high level of expertise to each medical specialty served. International sales accounted for 35% of total revenues in 2010. The Company’s products are sold in approximately 100 countries through local dealers and direct sales efforts. Additional information regarding the Company’s international and domestic operations and sales appears in Note 13 to the Consolidated Financial Statements in Item 8 of this report.
The Company’s business is generally not seasonal in nature; however, the number of orthopaedic implant surgeries is lower during the summer months.
9
COMPETITION
The Company is one of five leading competitors in the United States for orthopaedic reconstructive products. The four other leading competitors are DePuy Orthopaedics, Inc. (a subsidiary of Johnson & Johnson), Zimmer Holdings, Inc., Biomet, Inc., and Smith & Nephew plc. While competition abroad varies from area to area, the Company believes it is also a leading player in the international markets with these same companies as its principal competitors.
In the trauma implant segment, Stryker is one of five leaders competing principally with Synthes, Inc., Smith & Nephew Orthopaedics (a division of Smith & Nephew plc), Zimmer Holdings, Inc., and DePuy Orthopaedics, Inc.
In the spinal implant segment, the Company is one of four leaders, competing principally with Medtronic Sofamor Danek, Inc. (a subsidiary of Medtronic, Inc.), DePuy Spine, Inc. (a subsidiary of Johnson & Johnson), and Synthes, Inc.
In the craniomaxillofacial implant segment, Stryker is one of four leaders, competing principally with Synthes, Inc., Biomet Microfixation, LLC (a subsidiary of Biomet, Inc.), and KLS Martin L.P.
In the surgical equipment segment, Stryker is one of three leaders, competing principally with Medtronic, Inc. and Conmed Linvatec, Inc. (a subsidiary of CONMED Corporation). These companies are also competitors in the international segments, along with Aesculap-Werke AG (a division of B. Braun Melsungen AG), a large European manufacturer.
In the surgical navigation segment, Stryker is one of six principal competitors, including Medtronic Surgical Navigation Technologies (a division of Medtronic, Inc.), BrainLAB Inc. (a subsidiary of BrainLAB AG), AESCULAP AG & Co. KG (a division of B. Braun Melsungen AG), Radionics, Inc. (a subsidiary of Integra LifeSciences Corporation), and GE Medical Systems Navigation and Visualization, Inc. (a subsidiary of General Electric Company).
In the arthroscopy segment, the Company is one of four leaders, together with Smith & Nephew Endoscopy (a division of Smith & Nephew plc), ConMed Linvatec, Inc., and Arthrex, Inc. In the laparoscopic imaging products segment, the Company is one of three leaders, together with Karl Storz GmbH & Co. (a German company) and Olympus Optical Co. Ltd. (a Japanese company).
The Company’s primary competitor in the patient handling segment is Hill-Rom Holdings, Inc. In the specialty stretcher segment, the primary competitors are Hausted, Inc. (a subsidiary of STERIS Corporation), Hill-Rom Holdings, Inc., and Midmark Hospital Products Group (a subsidiary of Ohio Medical Instrument Company, Inc.). In the emergency medical services segment, Ferno-Washington, Inc. is the Company’s principal competitor.
The Company’s primary competitor in the U.S. market for reprocessing and remanufacturing of medical devices is SteriMed Inc.
The principal factors that the Company believes differentiate it in the highly competitive market segments in which it operates and enable it to compete effectively are innovation, reliability, service and reputation. The Company believes that its competitive position in the future will depend to a large degree on its ability to develop new products and make improvements to existing products. While the Company does not consider patents a major factor in its overall competitive success, patents and trademarks are significant to the extent that a product or an attribute of a product represents a unique design or process. Patent protection of such products restricts competitors from duplicating these unique designs and features. Stryker seeks to obtain patent protection on its products whenever appropriate for protecting its competitive advantage. As of December 31, 2010, the Company owns approximately 1,125 United States patents and 1,945 international patents.
10
MANUFACTURING AND SOURCES OF SUPPLY
The Company’s manufacturing processes consist primarily of precision machining, metal fabrication and assembly operations; the forging and investment casting of cobalt chrome; and the finishing of cobalt chrome and titanium. Approximately 12% of the Company’s cost of sales in 2010 represented finished products that were purchased complete from outside suppliers. The Company also purchases parts and components, such as forgings, castings, gears, bearings, casters and electrical components, and uses outside sources for certain finishing operations, such as plating, hardening and coating of machined components and sterilization of certain products. The principal raw materials used by the Company are stainless steel, aluminum, cobalt chrome and titanium alloys. In all, purchased parts and components from outside sources were approximately 38% of the total cost of sales in 2010.
While the Company relies on single sources for certain purchased materials and services, it believes alternate sources are available if needed. The Company has not experienced any significant difficulty in the past in obtaining the materials necessary to meet its production schedules.
Substantially all products manufactured by the Company are stocked in inventory, while certain products manufactured within the Company’s MedSurg Equipment segment are assembled to order.
REGULATION AND PRODUCT QUALITY
The Medical Device Amendments of 1976 to the federal Food, Drug and Cosmetic Act and the Safe Medical Devices Act of 1990, together with regulations issued or proposed thereunder, provide for regulation by the FDA of the design, manufacture and marketing of medical devices, including most of the Company’s products.
The FDA’s Quality System regulations set forth standards for the Company’s product design and manufacturing processes, require the maintenance of certain records and provide for inspections of the Company’s facilities by the FDA. There are also certain requirements of state, local and foreign governments that must be complied with in the manufacturing and marketing of the Company’s products.
In 2009 the Company received a warning letter from the FDA related to compliance issues for one of its CMF implant products that was previously sold through its CMF distribution facility in Portage, Michigan. In 2008 the Company received a warning letter from the FDA related to quality systems and compliance issues at its OP-1 implant manufacturing facility in Hopkinton, Massachusetts. In 2007 the Company received two warning letters from the FDA regarding compliance with certain quality system specifications at its reconstructive implant manufacturing facilities: one letter for its facility in Cork, Ireland and another for its facility in Mahwah, New Jersey. In October 2009, the FDA informed the Company that the warning letter related to its OP-1 implant manufacturing facility had been resolved following a productive reinspection earlier in 2009. In March 2010, the FDA informed the Company that the warning letter related to its Mahwah manufacturing facility had been resolved following a re-inspection in 2009 and additional corrective actions. In May 2010, the FDA informed the Company that the warning letters related to its Cork, Ireland and CMF facilities had been resolved following FDA re-inspection of the Cork, Ireland facility and additional corrective actions at both the Cork and CMF facilities.
Most of the Company’s new products fall into FDA classifications that require notification of and review by the FDA before marketing, submitted as a 510(k). Certain of the Company’s products require extensive clinical testing, consisting of safety and efficacy studies, followed by PMA applications for specific surgical indications.
Stryker also is subject to the laws that govern the manufacture and distribution of medical devices of each country in which the Company manufactures or sells products. The member states of the European Union (EU) have adopted the European Medical Device Directives, which create a single set of medical device regulations
11
for all EU member countries. These regulations require companies that wish to manufacture and distribute medical devices in EU member countries to obtain CE Marking for their products. Stryker has authorization to apply the CE Marking to substantially all of its products. The Company’s OP-1 product has been considered a drug under the regulations for Europe, Australia and Japan.
Initiatives sponsored by government agencies, legislative bodies and the private sector to limit the growth of healthcare expenses generally and hospital costs in particular, including price regulation and competitive pricing, are ongoing in markets where the Company does business. It is not possible to predict at this time the long-term impact of such cost-containment measures on the Company’s future business.
EMPLOYEES
At December 31, 2010, the Company had 20,036 employees worldwide. Certain international employees are covered by collective bargaining agreements that are updated annually. The Company believes that its employee relations are satisfactory.
EXECUTIVE OFFICERS OF THE REGISTRANT
Information regarding the executive officers of the Company appears under the caption “Directors, Executive Officers and Corporate Governance” in Item 10 of this report.
The following information discusses specific risks that could potentially impact the Company’s business, financial condition or operating results. The Company may be subject to additional risks that are not currently known to the Company or those which the Company deems immaterial that may also impact its business operations.
The Company’s operating results could be negatively impacted by economic, political or other developments in countries in which the Company does business.
The Company distributes its products throughout the world. As a result, the Company’s future operating results could be negatively impacted by unstable economic, political and social conditions, including but not limited to fluctuations in foreign currency exchange rates, political instability or changes in the interpretation or creation of laws and regulations, including tax laws and regulations, in each of the countries where the Company conducts business, including the United States.
Stricter pricing guidelines for the medical technology industry could have a negative impact on the Company’s future operating results.
Initiatives sponsored by government agencies, legislative bodies and the private sector to limit the growth of healthcare costs, including price regulation and competitive pricing, are ongoing in markets where the Company does business. The Company could experience a negative impact on its operating results due to increased pricing pressure in the United States, Europe, Japan and certain other markets. Governments, hospitals and other third party payers could reduce the amount of approved reimbursements for the Company’s products. Reductions in reimbursement levels or coverage or other cost-containment measures could unfavorably affect the Company’s future operating results.
The Company’s operating results could be negatively impacted by future product liability claims, unfavorable court decisions, regulatory compliance or legal settlements.
The Company is a defendant in various proceedings, legal actions and claims arising in the normal course of business, including product liability and other matters. Such matters are subject to many uncertainties and
12
outcomes are not predictable with assurance. In addition, the Company may incur significant legal expenses regardless of whether it is found to be liable. To partially mitigate losses arising from unfavorable outcomes in such matters, the Company purchases third-party insurance coverage subject to certain deductibles and loss limitations. While the Company believes its current insurance coverage is adequate to mitigate losses arising from such matters, its future operating results may be unfavorably impacted by any settlement payments or losses beyond the amounts of insurance carried. In addition, such product liability matters may negatively impact the Company’s ability to obtain cost-effective third-party insurance coverage in future periods.
Substantially all of the Company’s products are subject to regulation by the FDA and other governmental authorities both inside and outside of the United States. If the Company were to fail to comply with the applicable regulatory requirements, it may be subject to a range of sanctions including, but not limited to, warning letters, monetary fines, product recalls and the suspension of product manufacturing. Such sanctions, if implemented, could have a material unfavorable impact on the Company’s future operating results.
The Company’s inability to maintain adequate working relationships with healthcare professionals could have a negative impact on the Company’s future operating results.
The Company maintains close working relationships with respected physicians and medical personnel in hospitals and universities who assist in product research and development. The Company continues to place emphasis on the development of proprietary products and product improvements to complement and expand its existing product lines. If the Company is unable to maintain these good relationships, its ability to develop, market and sell new and improved products could decrease and future operating results could be unfavorably affected.
The Company’s operating results could be negatively impacted by future changes in the allocation of income to each of the income tax jurisdictions in which the Company operates.
The Company operates in multiple income tax jurisdictions both inside and outside the United States. Accordingly, management must determine the appropriate allocation of income to each of these jurisdictions based on current interpretations of complex income tax regulations. Income tax authorities in these jurisdictions regularly perform audits of the Company’s income tax filings. Income tax audits associated with the allocation of this income and other complex issues, including inventory transfer pricing and cost sharing, product royalty and foreign branch arrangements, may require an extended period of time to resolve and may result in significant income tax adjustments. If changes to the income allocation are required between jurisdictions with different income tax rates, such adjustments could have a material unfavorable impact on the Company’s income tax expense and net earnings in future periods.
The Company’s operating results could be negatively impacted if it is unable to capitalize on research and development spending.
The Company has spent a significant amount of time and resources on research and development projects in order to develop and validate new and innovative products. The Company believes these projects will result in the commercialization of new products and will create additional future sales. However, factors including regulatory delays, safety concerns or patent disputes could delay the introduction or marketing of new products. Additionally, unanticipated issues may arise in connection with current and future clinical studies that could delay or terminate a product’s development prior to regulatory approval. The Company may experience an unfavorable impact on its operating results if it is unable to capitalize on those efforts by attaining the proper FDA approval or to successfully market new products.
13
The Company’s operating results could be negatively impacted by changes in its excess and obsolete inventory reserves.
The Company maintains reserves for excess and obsolete inventory resulting from the potential inability to sell its products at prices in excess of current carrying costs. The markets in which the Company operates are highly competitive, and new products and surgical procedures are introduced on an ongoing basis. Such marketplace changes may cause some of the Company’s products to become obsolete. The Company makes estimates regarding the future recoverability of the costs of these products and records a provision for excess and obsolete inventories based on historical experience, expiration of sterilization dates and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write-downs may be required, which could unfavorably affect future operating results.
The Company’s operating results could be negatively impacted if it is unable to capitalize on previous or future acquisitions.
In addition to internally developed products, the Company relies upon investment in new technologies through acquisitions. Investments in medical technology are inherently risky and the Company cannot guarantee that any of its previous or future acquisitions will be successful or will not have a material unfavorable impact on the Company’s future operating results. Such risks include the activities required to integrate new businesses into the Company and may result in the need to allocate more resources to integration and product development activities than originally anticipated and involve significant amounts of management’s time, which could adversely affect management’s ability to focus on other projects and could have a material unfavorable impact on the Company’s financial condition, consolidated results of operations or cash flows. In addition, the Company cannot be certain that the businesses it acquires will become profitable or remain so, which may result in unexpected impairment charges.
The Company’s inability to continue to hire and retain key employees could have a negative impact on the Company’s future operating results.
The talent and drive of the Company’s employees are key factors in the success of its business. The Company’s sales, technical and other key personnel play an integral role in the development, marketing and selling of new and existing products. If the Company is unable to recruit, hire, develop and retain a talented, competitive work force, it may not be able to meet its strategic business objectives.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. |
Not applicable.
14
The Company has the following properties as of December 31, 2010:
| | | | | | | | | | | | |
Location | | Segment | | Use | | Square
Feet | | | Owned/
Leased | |
Mahwah, New Jersey | | Orthopaedic Implants | | Manufacturing of reconstructive implants | | | 531,000 | | | | Owned | |
Limerick, Ireland | | Orthopaedic Implants | | Manufacturing of reconstructive implants and OP-1 | | | 130,000 | | | | Owned | |
Kiel, Germany | | Orthopaedic Implants | | Manufacturing of trauma implants | | | 174,000 | | | | Owned | |
Selzach, Switzerland | | Orthopaedic Implants | | Manufacturing of trauma implants | | | 78,000 | | | | Owned | |
Neuchâtel, Switzerland | | Orthopaedic Implants | | Manufacturing of spinal implants | | | 88,000 | | | | Owned | |
Bordeaux, France | | Orthopaedic Implants | | Manufacturing of spinal implants | | | 79,000 | | | | Owned | |
Bordeaux, France | | Orthopaedic Implants | | Manufacturing of spinal implants | | | 35,000 | | | | Leased | |
Carrigtwohill, Ireland | | Orthopaedic Implants and MedSurg Equipment | | Manufacturing of reconstructive implants and surgical equipment | | | 154,000 | | | | Owned | |
Freiburg, Germany | | Orthopaedic Implants and MedSurg Equipment | | Manufacturing of craniomaxillofacial implants and surgical navigation systems | | | 106,000 | | | | Owned | |
Stetten, Germany | | Orthopaedic Implants | | Manufacturing of craniomaxillofacial implants | | | 33,000 | | | | Owned | |
| West Lebanon, New Hampshire | | Orthopaedic Implants | | Manufacturing of OP-1 | | | 140,000 | | | | Owned | |
Hopkinton, Massachusetts | | Orthopaedic Implants | | Manufacturing of OP-1 | | | 69,000 | | | | Leased | |
Suzhou, China | | Orthopaedic Implants | | Manufacturing of reconstructive, trauma and spinal implants | | | 155,000 | | | | Owned | |
Newnan, Georgia | | Orthopaedic Implants | | Manufacturing of reconstructive implants | | | 54,000 | | | | Leased | |
Portage, Michigan | | MedSurg Equipment | | Manufacturing of surgical equipment and patient-handling and emergency medical equipment | | | 1,034,000 | | | | Owned | |
Arroyo, Puerto Rico | | MedSurg Equipment | | Manufacturing of surgical equipment and endoscopic systems | | | 220,000 | | | | Leased | |
San Jose, California | | MedSurg Equipment | | Manufacturing of endoscopic systems | | | 165,000 | | | | Leased | |
Flower Mound, Texas | | MedSurg Equipment | | Manufacturing of communications systems | | | 114,000 | | | | Leased | |
Lakeland, Florida | | MedSurg Equipment | | Reprocessing and remanufacturing of medical devices | | | 112,000 | | | | Leased | |
Phoenix, Arizona | | MedSurg Equipment | | Reprocessing and remanufacturing of medical devices | | | 51,000 | | | | Leased | |
Buffalo, New York | | MedSurg Equipment | | Manufacturing of pressure ulcer and temperature management products | | | 112,000 | | | | Owned | |
Kalamazoo, Michigan | | Other | | Corporate headquarters | | | 75,000 | | | | Owned | |
In addition to the above, the Company maintains administrative and sales offices and warehousing and distribution facilities in multiple countries. The Company believes that its properties are suitable and adequate for the manufacture and distribution of the Company’s products.
15
| ITEM 3. | LEGAL PROCEEDINGS. |
The Company is involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product, labor and intellectual property, and other matters that are more fully described in Note 17 to the Consolidated Financial Statements in Item 8 of this report. The outcomes of these matters will generally not be known for prolonged periods of time. In certain of the legal proceedings, the claimants seek damages, as well as other compensatory relief, which could result in the payment of significant claims and settlements. In legal matters for which management has sufficient information to reasonably estimate the Company’s future obligations, a liability representing management’s best estimate of the probable cost, or the minimum of the range of probable losses when a best estimate within the range is not known, for the resolution of these legal matters is recorded. The estimates are based on consultation with legal counsel, previous settlement experience and settlement strategies.
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
The Company’s common stock is traded on the New York Stock Exchange under the symbol SYK. Quarterly stock prices and dividend information for the years ended December 31, 2010 and 2009 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2010 Quarter Ended | | | 2009 Quarter Ended | |
| | | Mar. 31 | | | June 30 | | | Sept. 30 | | | Dec. 31 | | | Mar. 31 | | | June 30 | | | Sept. 30 | | | Dec. 31 | |
Dividends declared per share of common stock | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.15 | | | $ | 0.18 | | | $ | 0.0 | | | $ | 0.0 | | | $ | 0.0 | | | $ | 0.25 | |
Market price of common stock: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
High | | | 58.49 | | | | 59.72 | | | | 53.29 | | | | 55.00 | | | | 44.46 | | | | 41.73 | | | | 48.10 | | | | 52.66 | |
Low | | | 49.85 | | | | 48.76 | | | | 42.74 | | | | 48.13 | | | | 30.96 | | | | 32.34 | | | | 37.14 | | | | 42.74 | |
The Company’s Board of Directors considers payment of a cash dividend at each of its quarterly meetings.
In the fourth quarter of 2010, the Company issued 230 shares of common stock as performance incentive awards to certain employees. The shares were not registered under the Securities Act of 1933 (the Act) based on the conclusion that the awards would not be events of sale within the meaning of Section 2(a)(3) of the Act.
On January 31, 2011, there were 4,748 shareholders of record of the Company’s common stock.
In December 2010 the Company announced that its Board of Directors had authorized the Company to purchase up to $500.0 million of its common stock from time to time in the open market, in privately negotiated transactions or otherwise. The Company did not make any repurchase pursuant to the $500.0 million repurchase program in 2010.
16
In December 2009 the Company announced that its Board of Directors had authorized the Company to repurchase up to $750.0 million of its common stock from time to time in the open market, in privately negotiated transactions or otherwise. During the fourth quarter of 2010, the Company repurchased 6.1 million shares of its common stock in the open market at a cost of $314.5 million, as follows (in millions, except per share amounts):
| | | | | | | | | | | | | | | | |
Period | | (a)
Total Number
of Shares
Purchased | | | (b)
Average Price
Paid
Per Share | | | (c)
Total Number of
Shares Purchased as
Part of Publicly
Announced Plans | | | (d)
Maximum
Dollar Value
of Shares that may
yet be Purchased
Under the Plans | |
$750.0 million repurchase program | | | | | | | | | | | | | | | | |
Month #1 | | | | | | | | | | | | | | | | |
October 1, 2010—October 31, 2010 | | | — | | | $ | — | | | | — | | | $ | — | |
Month #2 | | | | | | | | | | | | | | | | |
November 1, 2010—November 30, 2010 | | | 3.9 | | | $ | 51.53 | | | | 3.9 | | | $ | 478.7 | |
Month #3 | | | | | | | | | | | | | | | | |
December 1, 2010—December 31, 2010 | | | 2.2 | | | $ | 51.19 | | | | 2.2 | | | $ | 324.4 | |
| | | | | | | | | | | | | | | | |
Total | | | 6.1 | | | $ | 51.41 | | | | 6.1 | | | | | |
| | | | | | | | | | | | | | | | |
$500.0 million repurchase program | | | | | | | | | | | | | | | | |
Month #3 | | | | | | | | | | | | | | | | |
December 1, 2010—December 31, 2010 | | | — | | | $ | — | | | | — | | | $ | 500.0 | |
| | | | | | | | | | | | | | | | |
Total | | | — | | | $ | — | | | | — | | | | | |
| | | | | | | | | | | | | | | | |
17
PERFORMANCE GRAPH (UNAUDITED)
Set forth below is a graph comparing the total returns (including reinvestments of dividends) of the Company, the Standard & Poor’s (S&P) 500 Composite Stock Price Index and the S&P Health Care (Medical Products and Supplies) Index. The graph assumes $100 invested on December 31, 2005 in the Company’s Common Stock and each of the indices.
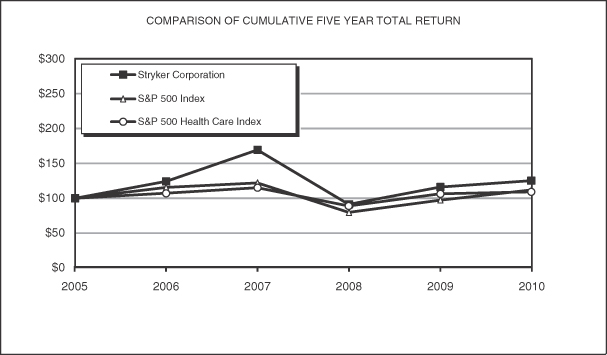
| | | | | | | | | | | | | | | | | | | | | | | | |
Company / Index | | 2005 | | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | |
Stryker Corporation | | | 100 | | | | 124.53 | | | | 169.59 | | | | 91.58 | | | | 116.04 | | | | 125.20 | |
S&P 500 Index | | | 100 | | | | 115.79 | | | | 122.16 | | | | 76.96 | | | | 97.33 | | | | 111.99 | |
S&P 500 Health Care Index | | | 100 | | | | 107.53 | | | | 115.22 | | | | 88.94 | | | | 106.46 | | | | 109.55 | |
18
| ITEM 6. | SELECTED FINANCIAL DATA. |
The financial information for each of the five years in the period ended December 31, 2010 is set forth below (dollars in millions, except per share amounts):
| | | | | | | | | | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
Net sales | | $ | 7,320.0 | | | $ | 6,723.1 | | | $ | 6,718.2 | | | $ | 6,000.5 | | | $ | 5,147.2 | |
Cost of sales | | | 2,285.7 | | | | 2,183.7 | | | | 2,131.4 | | | | 1,865.2 | | | | 1,616.6 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 5,034.3 | | | | 4,539.4 | | | | 4,586.8 | | | | 4,135.3 | | | | 3,530.6 | |
| | | | | |
Research, development and engineering expenses | | | 393.9 | | | | 336.2 | | | | 367.8 | | | | 375.3 | | | | 324.6 | |
Selling, general and administrative expenses | | | 2,707.3 | | | | 2,506.3 | | | | 2,625.1 | | | | 2,391.5 | | | | 2,047.0 | |
Intangibles amortization | | | 58.2 | | | | 35.5 | | | | 40.0 | | | | 41.4 | | | | 42.7 | |
Other (a) | | | 123.5 | | | | 67.0 | | | | 34.9 | | | | 19.8 | | | | 52.7 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 3,282.9 | | | | 2,945.0 | | | | 3,067.8 | | | | 2,828.0 | | | | 2,467.0 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income | | | 1,751.4 | | | | 1,594.4 | | | | 1,519.0 | | | | 1,307.3 | | | | 1,063.6 | |
Other income (expense) | | | (21.8 | ) | | | 29.5 | | | | 61.2 | | | | 62.8 | | | | 30.2 | |
| | | | | | | | | | | | | | | | | | | | |
Earnings from continuing operations before income taxes | | | 1,729.6 | | | | 1,623.9 | | | | 1,580.2 | | | | 1,370.1 | | | | 1,093.8 | |
Income taxes | | | 456.2 | | | | 516.5 | | | | 432.4 | | | | 383.4 | | | | 322.4 | |
| | | | | | | | | | | | | | | | | | | | |
Net earnings from continuing operations | | | 1,273.4 | | | | 1,107.4 | | | | 1,147.8 | | | | 986.7 | | | | 771.4 | |
Net earnings and gain on sale of discontinued operations | | | — | | | | — | | | | — | | | | 30.7 | | | | 6.3 | |
| | | | | | | | | | | | | | | | | | | | |
Net earnings | | $ | 1,273.4 | | | $ | 1,107.4 | | | $ | 1,147.8 | | | $ | 1,017.4 | | | $ | 777.7 | |
| | | | | | | | | | | | | | | | | | | | |
Net earnings from continuing operations per share of common stock: | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 3.21 | | | $ | 2.79 | | | $ | 2.81 | | | $ | 2.41 | | | $ | 1.90 | |
Diluted | | $ | 3.19 | | | $ | 2.77 | | | $ | 2.78 | | | $ | 2.37 | | | $ | 1.87 | |
| | | | | |
Net earnings per share of common stock: | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 3.21 | | | $ | 2.79 | | | $ | 2.81 | | | $ | 2.48 | | | $ | 1.91 | |
Diluted | | $ | 3.19 | | | $ | 2.77 | | | $ | 2.78 | | | $ | 2.44 | | | $ | 1.89 | |
| | | | | |
Dividends declared per share of common stock | | $ | 0.63 | | | $ | 0.25 | | | $ | 0.40 | | | $ | 0.33 | | | $ | 0.22 | |
| | | | | |
Average number of shares outstanding—in millions: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 396.4 | | | | 397.4 | | | | 408.1 | | | | 409.7 | | | | 406.5 | |
Diluted | | | 399.5 | | | | 399.4 | | | | 413.6 | | | | 417.2 | | | | 411.8 | |
| (a) | Includes restructuring charges, property, plant and equipment impairment, intangible asset impairment and purchased in-process research and development charges. |
19
FINANCIAL AND STATISTICAL DATA
| | | | | | | | | | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
Cash and current marketable securities | | | 4,380.1 | | | | 2,954.8 | | | | 2,195.6 | | | | 2,410.8 | | | | 1,414.8 | |
Working capital | | | 6,026.4 | | | | 4,410.2 | | | | 3,517.2 | | | | 3,571.9 | | | | 2,182.8 | |
Current ratio | | | 4.8 | | | | 4.1 | | | | 3.4 | | | | 3.7 | | | | 2.6 | |
Property, plant and equipment—net | | | 798.3 | | | | 947.6 | | | | 963.8 | | | | 991.6 | | | | 914.9 | |
Capital expenditures | | | 182.1 | | | | 131.3 | | | | 155.2 | | | | 187.7 | | | | 209.4 | |
Depreciation and amortization | | | 410.2 | | | | 385.3 | | | | 387.6 | | | | 366.6 | | | | 324.1 | |
Total assets | | | 10,895.1 | | | | 9,071.3 | | | | 7,603.3 | | | | 7,354.0 | | | | 5,873.8 | |
Long-term debt, including current maturities | | | 1,021.8 | | | | 18.0 | | | | 20.5 | | | | 16.8 | | | | 14.8 | |
Shareholders’ equity | | | 7,173.6 | | | | 6,595.1 | | | | 5,406.7 | | | | 5,378.5 | | | | 4,191.0 | |
Return on average equity | | | 18.5 | % | | | 18.5 | % | | | 21.3 | % | | | 21.3 | % | | | 20.8 | % |
Net cash provided by operating activities | | | 1,547.4 | | | | 1,460.7 | | | | 1,175.9 | | | | 1,028.3 | | | | 867.3 | |
Number of shareholders of record | | | 4,586 | | | | 4,607 | | | | 4,500 | | | | 4,373 | | | | 4,091 | |
Number of employees | | | 20,036 | | | | 18,582 | | | | 17,594 | | | | 16,026 | | | | 18,806 | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
Throughout this discussion, references are made to the following financial measures: “constant currency,” “adjusted net earnings,” “adjusted basic net earnings per share,” and “adjusted diluted net earnings per share.” These financial measures are an alternative representation of Stryker Corporation’s (the Company or Stryker) past and potential future operational performance and do not replace the presentation of the Company’s reported financial results under U.S. generally accepted accounting principles (GAAP). The Company has provided these supplemental non-GAAP financial measures because they provide meaningful information regarding the Company’s results on a consistent and comparable basis for the periods presented. Management uses these non-GAAP financial measures for reviewing the operating results of its business segments, for analyzing potential future business trends in connection with its budget process and bases certain annual bonus plans on these non-GAAP financial measures. In order to measure the Company’s sales performance on a constant currency basis, it is necessary to remove the impact of changes in foreign currency exchange rates which affects the comparability and trend of sales. Constant currency results are calculated by translating current year results at prior year average foreign currency exchange rates. In order to measure earnings performance on a consistent and comparable basis, the Company excludes the impairment of property, plant and equipment and gain on sale of certain assets recorded in 2010, the patent litigation gain recorded in 2009, the income tax effect associated with the repatriation of foreign earnings recorded in 2010 and 2009 and the restructuring charges recorded in 2009 and 2008, each of which affects the comparability of operating results and the trend of earnings. Additional details regarding the nature, determination and financial statement impact of these items are included inResults of Operations. In addition, the Company believes investors will utilize this information to evaluate period-to-period results on a comparable basis and to better understand potential future operating results. The Company encourages investors and other users of these financial statements to review its Consolidated Financial Statements and other publicly filed reports in their entirety and not to rely solely on any single financial measure.
Executive Level Overview
Stryker is one of the world’s leading medical technology companies and is dedicated to helping healthcare professionals perform their jobs more efficiently while enhancing patient care. The Company provides innovative orthopaedic implants as well as state-of-the-art medical and surgical equipment to help people lead more active and more satisfying lives. The Company’s products include implants used in joint replacement, trauma and spinal surgeries; surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment as well as other medical device products used in a variety of medical specialties.
20
Domestic sales accounted for 65% of total revenues in 2010. Most of the Company’s products are marketed directly to doctors, hospitals and other healthcare facilities by approximately 4,600 sales and marketing personnel in the United States. Stryker primarily maintains separate and dedicated sales forces for each of its principal product lines to provide focus and a high level of expertise to each medical specialty served.
International sales accounted for 35% of total revenues in 2010. The Company’s products are sold in approximately 100 countries through Company-owned sales subsidiaries and branches as well as third-party dealers and distributors.
The Company’s business is generally not seasonal in nature; however, the number of orthopaedic implant surgeries is lower during the summer months.
In 2010 the Company completed several acquisitions in all cash transactions, including the Sonopet Ultrasonic Aspirator control consoles, handpieces and accessories from Mutoh Co., Ltd. and Synergetics USA, Inc., Gaymar Industries Inc, and the bioimplantable implants product line and related assets from Porex Surgical, Inc.
In December 2010 the Company announced a definitive agreement to sell its OP-1 product family for use in orthopaedic bone applications and its manufacturing facility based in Lebanon, NH and the transaction was completed on February 1, 2011. Additional details, including the financial statement impact of this transaction, are included inResults of Operations.
In October 2010 the Company announced a definitive agreement to acquire the assets of the Neurovascular division of Boston Scientific Corporation (Neurovascular) in an all cash transaction of up to $1.5 billion. The transaction was completed on January 3, 2011. The acquisition of Neurovascular is expected to substantially enhance the Company’s presence in the neurovascular market, allowing it to offer a comprehensive portfolio of products in both neurosurgical and neurovascular devices.
During the third quarter of 2010, the Company sold its Orthopaedics Implants manufacturing facility based in Caen, France. In addition, during the third quarter of 2010, the Company recorded a favorable income tax adjustment of $7.4 million to reduce the income tax liability originally recorded in the fourth quarter of 2009 associated with the repatriation of foreign earnings to the United States. Additional details, including the financial statement impact of these transactions, are included inResults of Operations.
In August 2010 the Company refinanced its credit facility with a new $1,000.0 million Senior Unsecured Revolving Credit Facility due August 2013 (the 2010 Facility). The 2010 Facility replaced the previously outstanding $1,000.0 million Unsecured Credit Facility due in November 2010 (the 2005 Facility). Additional details are included inLiquidity and Capital Resources.
In January 2010 the Company sold $500.0 million of senior unsecured notes due January 15, 2015 (the 2015 Notes) and $500.0 million of senior unsecured notes due January 15, 2020 (the 2020 Notes). The net proceeds from the offering have been and will continue to be available for working capital and other general corporate purposes, including acquisitions, stock repurchases and other business opportunities. Additional details are included inLiquidity and Capital Resources.
21
Results of Operations
The table below outlines the components of net earnings from continuing operations from the Consolidated Statements of Earnings as a percentage of net sales and the year-to-year percentage change in dollar amounts:
| | | | | | | | | | | | | | | | | | | | |
| | | Percentage of Net Sales | | | Percentage Change | |
| | | 2010 | | | 2009 | | | 2008 | | | 2010/2009 | | | 2009/2008 | |
Net sales | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % | | | 9 | % | | | 0 | % |
Cost of sales | | | 31.2 | | | | 32.5 | | | | 31.7 | | | | 5 | | | | 2 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 68.8 | | | | 67.5 | | | | 68.3 | | | | 11 | | | | (1 | ) |
Research, development and engineering expenses | | | 5.4 | | | | 5.0 | | | | 5.5 | | | | 17 | | | | (9 | ) |
Selling, general and administrative expenses | | | 37.0 | | | | 37.3 | | | | 39.1 | | | | 8 | | | | (5 | ) |
Intangibles amortization | | | 0.8 | | | | 0.5 | | | | 0.6 | | | | 64 | | | | (11 | ) |
Property, plant and equipment impairment | | | 1.7 | | | | — | | | | — | | | | — | | | | — | |
Restructuring charges | | | — | | | | 1.0 | | | | 0.5 | | | | (100 | ) | | | 92 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income | | | 23.9 | | | | 23.7 | | | | 22.6 | | | | 10 | | | | 5 | |
Other income (expense) | | | (0.3 | ) | | | 0.4 | | | | 0.9 | | | | — | | | | (52 | ) |
| | | | | | | | | | | | | | | | | | | | |
Earnings before income taxes | | | 23.6 | | | | 24.2 | | | | 23.5 | | | | 7 | | | | 3 | |
Income taxes | | | 6.2 | | | | 7.7 | | | | 6.4 | | | | (12 | ) | | | 19 | |
| | | | | | | | | | | | | | | | | | | | |
Net earnings | | | 17.4 | % | | | 16.5 | % | | | 17.1 | % | | | 15 | | | | (4 | ) |
| | | | | | | | | | | | | | | | | | | | |
The Company segregates its operations into two reportable business segments: Orthopaedic Implants and MedSurg Equipment. The Orthopaedic Implants segment includes orthopaedic reconstructive (hip and knee), trauma and spinal implant systems and other related products. The MedSurg Equipment segment includes surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment; as well as other medical device products used in a variety of medical specialties.
The table below sets forth domestic/international and business segment net sales information (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage Change | |
| | | Net Sales | | | 2010/2009 | | | 2009/2008 | |
| | | | Reported | | | Constant
Currency | | | Reported | | | Constant
Currency | |
| | | 2010 | | | 2009 | | | 2008 | | | | | |
Domestic/international sales: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Domestic | | $ | 4,792.8 | | | $ | 4,317.4 | | | $ | 4,282.2 | | | | 11 | % | | | 11 | % | | | 1 | % | | | 1 | % |
International | | | 2,527.2 | | | | 2,405.7 | | | | 2,436.0 | | | | 5 | | | | 2 | �� | | | (1 | ) | | | 3 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total net sales | | $ | 7,320.0 | | | $ | 6,723.1 | | | $ | 6,718.2 | | | | 9 | | | | 8 | | | | 0 | | | | 2 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Business segment sales: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Orthopaedic Implants | | $ | 4,308.4 | | | $ | 4,119.7 | | | $ | 3,967.5 | | | | 5 | | | | 3 | | | | 4 | | | | 6 | |
MedSurg Equipment | | | 3,011.6 | | | | 2,603.4 | | | | 2,750.7 | | | | 16 | | | | 15 | | | | (5 | ) | | | (4 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total net sales | | $ | 7,320.0 | | | $ | 6,723.1 | | | $ | 6,718.2 | | | | 9 | | | | 8 | | | | 0 | | | | 2 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
22
The tables below set forth additional geographical sales growth information for significant products within the Company’s Orthopaedic Implants and MedSurg Equipment segments on both a reported basis and a constant currency basis:
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, 2010 | |
| | | Percentage Change | |
| | | Domestic | | | International | | | Total | |
| | | Reported | | | Reported | | | Constant
Currency | | | Reported | | | Constant
Currency | |
Orthopaedic Implants sales: | | | | | | | | | | | | | | | | | | | | |
Hips | | | 5 | | | | 5 | | | | 1 | | | | 5 | | | | 3 | |
Knees | | | 6 | | | | 1 | | | | (2 | ) | | | 4 | | | | 3 | |
Trauma | | | 11 | | | | 5 | | | | 5 | | | | 8 | | | | 7 | |
Spine | | | (1 | ) | | | 8 | | | | 6 | | | | 2 | | | | 1 | |
Total Orthopaedic Implants | | | 5 | | | | 4 | | | | 1 | | | | 5 | | | | 3 | |
| | | | | |
MedSurg Equipment sales: | | | | | | | | | | | | | | | | | | | | |
Surgical equipment and surgical navigation systems | | | 9 | | | | 5 | | | | 3 | | | | 8 | | | | 8 | |
Endoscopic and communications systems | | | 6 | | | | 9 | | | | 7 | | | | 7 | | | | 6 | |
Patient handling and emergency medical equipment | | | 24 | | | | 5 | | | | 0 | | | | 20 | | | | 18 | |
Total MedSurg Equipment | | | 19 | | | | 7 | | | | 4 | | | | 16 | | | | 15 | |
| |
| | | Year Ended December 31, 2009 | |
| | | Percentage Change | |
| | | Domestic | | | International | | | Total | |
| | | Reported | | | Reported | | | Constant
Currency | | | Reported | | | Constant
Currency | |
Orthopaedic Implants sales: | | | | | | | | | | | | | | | | | | | | |
Hips | | | 6 | | | | (2 | ) | | | 4 | | | | 2 | | | | 5 | |
Knees | | | 10 | | | | (5 | ) | | | 0 | | | | 4 | | | | 6 | |
Trauma | | | 10 | | | | 2 | | | | 4 | | | | 5 | | | | 6 | |
Spine | | | 11 | | | | 9 | | | | 12 | | | | 10 | | | | 11 | |
Total Orthopaedic Implants | | | 7 | | | | (1 | ) | | | 3 | | | | 4 | | | | 6 | |
| | | | | |
MedSurg Equipment sales: | | | | | | | | | | | | | | | | | | | | |
Surgical equipment and surgical navigation systems | | | 2 | | | | (2 | ) | | | 3 | | | | 1 | | | | 2 | |
Endoscopic, communications and digital imaging systems | | | (5 | ) | | | 6 | | | | 11 | | | | (2 | ) | | | (1 | ) |
Patient handling and emergency medical equipment | | | (23 | ) | | | (17 | ) | | | (12 | ) | | | (22 | ) | | | (20 | ) |
Total MedSurg Equipment | | | (7 | ) | | | (2 | ) | | | 3 | | | | (5 | ) | | | (4 | ) |
2010 Compared to 2009
The Company’s net sales increased 9% to $7,320.0 million in 2010 from $6,723.1 million in 2009. Net sales grew by 7% as a result of increased unit volume and changes in product mix, 1% due to the favorable impact of foreign currency exchange rates on net sales and 3% due to acquisitions, which were partially offset by an unfavorable impact of 2% due to changes in price.
The Company’s domestic sales were $4,792.8 million for 2010, representing an increase of 11%, as a result of higher shipments of Orthopaedic Implants and MedSurg Equipment. International sales were $2,527.2 million for 2010, representing an increase of 5%. The impact of foreign currency exchange rate movements to the dollar value of international sales was favorable by $69.7 million for 2010. On a constant currency basis, international sales increased 2% in 2010 as a result of higher shipments of Orthopaedic Implants and MedSurg Equipment.
23
Worldwide sales of Orthopaedic Implants were $4,308.4 million for 2010, representing an increase of 5%. On a constant currency basis, sales of Orthopaedic Implants increased 3% in 2010 as a result of higher shipments of hips, knees, trauma and spinal implant systems.
Hip Implant Systems:Sales of hip implant systems increased 5% in 2010 (3% on a constant currency basis). In the United States, sales growth of hip products was driven by sales of X3 Polyethylene, Rejuvenate hip products, Accolade cementless hip products and Restoration Modular Hip System revision hip products. Sales growth in X3 Polyethylene hip products in Europe, Canada, the Pacific and Latin America regions as well as sales growth in Trident hip products in Japan and the Pacific and Latin America regions also contributed to the Company’s constant currency sales growth in 2010. Sales growth in Accolade cementless hip products in Europe, Japan and the Latin America region also contributed to the Company’s constant currency sales growth in 2010.
Knee Implant Systems:Sales of knee implant systems increased 4% in 2010 (3% on a constant currency basis) due to due to worldwide sales growth in the Triathlon knee system as well as sales growth in Scorpio knee products in the Latin America region.
Trauma Implant Systems:Sales of trauma implant systems increased 8% in 2010 (7% on a constant currency basis) as a result of sales growth in the Gamma3 Hip Fracture System in the United States, Europe, Canada and the Pacific region as well as sales growth in the Company’s T2 Nailing System in the United States, Europe and the Pacific region. Sales growth in VariAx distal radius products in the United States, Europe, Canada, Japan and the Latin America region also contributed to the Company’s constant currency sales growth.
Spinal Implant Systems:Sales of spinal implant systems increased 2% in 2010 (1% on a constant currency basis). The increase was driven by sales growth of thoracolumbar implant systems in the United States, Europe, Japan, Canada and the Latin America region, interbody device products in Japan and Canada as well as cervical implants in the United States and Canada.
Worldwide sales of MedSurg Equipment were $3,011.6 million in 2010, representing an increase of 16%. On a constant currency basis, sales of MedSurg Equipment increased 15% in 2010 as a result of higher shipments of surgical equipment and surgical navigation systems, endoscopic and communications systems as well as patient handling and emergency medical equipment. Sales of MedSurg Equipment were also positively impacted by 7% from acquisitions and 1% from a one-time shipment of patient handling equipment.
Surgical Equipment and Surgical Navigation Systems:Sales of surgical equipment and surgical navigation systems increased 8% in 2010 (8% on a constant currency basis) due to worldwide sales growth in operating room equipment as well as sales growth in powered surgical products in the United States, Europe, Canada, the Pacific and Latin America regions and sales growth in interventional pain products in the United States, Japan, Canada and the Latin America region.
Endoscopic and Communications Systems:Sales of endoscopic and communications systems increased 7% in 2010 (6% on a constant currency basis) due to worldwide sales growth in general surgery products and sales growth in communications products in Europe, Japan and the Latin America region. Sales growth in medical video equipment in the United States, Japan, Canada, and the Pacific and Latin America regions also contributed to the Company’s constant currency sales growth.
Patient Handling and Emergency Medical Equipment:Sales of patient handling and emergency medical equipment increased 20% in 2010 (18% on a constant currency basis) due to higher sales of hospital bed products and stretchers in the United States and the Latin America region. Strong sales growth of EMS products in the United States and the Pacific region also contributed to the Company’s constant currency sales growth. Sales of patient handling and emergency medical equipment in 2010 were also positively impacted by 3% from acquisitions and 3% from a one-time shipment of patient handling equipment.
24
Cost of sales represented 31.2% of sales in 2010 compared to 32.5% in 2009. The decrease in the cost of sales percentage is primarily due to lower excess and obsolete inventory charges, higher absorption due to higher production levels as well as a favorable impact from the effect of foreign currency exchange rates on costs from the Company’s Euro based manufacturing sites.
Research, development and engineering expenses represented 5.4% of sales in 2010 compared to 5.0% in 2009. The spending level in 2010 increased by 17% to $393.9 million. The higher spending level is the result of the Company’s increased focus on new product development for anticipated future product launches and continued investments in new technologies. New product introductions in 2010 for the Orthopaedic Implants segment included the ADM X3 Mobile Bearing Acetabular system. Within the MedSurg equipment segment, new product introductions in 2010 included the Prime series of stretchers and the WiSe 1:3 Transmitter.
Selling, general and administrative expenses increased 8% in 2010 and represented 37.0% of sales compared to 37.3% in 2009. In 2010 the Company sold its Orthopaedic Implant manufacturing facility in Caen, France and recorded a gain of $24.3 million, which is included in selling, general and administrative expenses. In 2009 the Company settled an outstanding patent infringement lawsuit and received $62.5 million pursuant to a confidential settlement agreement. This gain also represented a reduction to selling, general and administrative expenses. The remaining decrease in selling, general and administrative expenses as a percentage of sales in 2010 is primarily due to management of discretionary spending levels.
In 2010 the Company announced a definitive agreement to sell its OP-1 product family for use in orthopaedic bone applications and its manufacturing facility based in Lebanon, NH. As a result of the announcement, the Company recorded a $76.6 million (net of $46.9 million income tax expense) non-cash impairment charge to reflect the reduction of the carrying amount of the associated assets to their fair value.
Interest and marketable securities income, which is included in other income (expense), decreased to $48.7 million in 2010 from $53.9 million in 2009 primarily as a result of lower average yields on the Company’s investments. Interest expense, which is also included in other income (expense), increased to $77.5 million in 2010 from $23.2 million in 2009 primarily as a result of the interest cost on the debt issued in January 2010.
The Company’s effective income tax rate on earnings for the year ended December 31, 2010 was 26.4% compared to an effective income tax rate for the year ended December 31, 2009 of 31.8%. The effective income tax rate for the year ended December 31, 2010 reflects the property, plant and equipment impairment charge of $76.6 million (net of $46.9 million income tax benefit), the gain on sale of the Caen facility of $13.4 million (net of $10.9 million income tax expense) and the impact of the favorable income tax expense adjustment of $7.4 million associated with the repatriation of foreign earnings to the United States completed in the fourth quarter of 2009. The effective income tax rate for the year ended December 31, 2009 reflects the impact of restructuring charges of $48.4 million (net of $18.6 million income tax benefits), the patent litigation gain of $42.9 million (net of $19.6 million income tax expenses) and the impact of the $67.1 million income tax expenses associated with the repatriation of foreign earnings of $787.0 million. The Company’s effective income tax rate on earnings for the fourth quarter of 2010 was 22.2% compared to the fourth quarter of 2009 of 41.5%. The effective income tax rate for the fourth quarter of 2010 reflects the property, plant and equipment impairment charge of $76.6 million (net of $46.9 million income tax benefit). The effective income tax rate for the fourth quarter of 2009 reflects the patent litigation gain of $42.9 million (net of $19.6 million income tax expenses) and the impact of the $67.1 million income tax expenses associated with the repatriation of foreign earnings of $787.0 million. In addition to these factors, the Company’s reported effective income tax rates for the years ended December 31, 2010 and 2009 are lower than the U.S. statutory income tax rate primarily as a result of manufacturing in lower income tax jurisdictions.
Net earnings increased 15% in 2010 to $1,273.4 million from $1,107.4 million in 2009. Basic net earnings per share increased 15% in 2010 to $3.21 from $2.79 in 2009, and diluted net earnings per share increased 15% to $3.19 in 2010 from $2.77 in 2009.
25
Excluding the impact of the property, plant and equipment impairment charge and gain on sale of certain assets recorded in 2010, the patent litigation gain recorded in 2009, the income tax adjustment associated with the repatriation of foreign earnings recorded in 2010 and 2009 and the restructuring charges recorded in 2009, adjusted net earnings increased 13% in 2010 to $1,329.2 million from $1,180.0 million in 2009. Adjusted basic net earnings per share increased 13% in 2010 to $3.35 from $2.97 in 2009 and adjusted diluted net earnings per share increased 13% in 2010 to $3.33 from $2.95 in 2009.
The reconciliations of these non-GAAP financial measures are as follows (in millions, except per share amounts):
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | Percentage
Change | |
Reported net earnings | | $ | 1,273.4 | | | $ | 1,107.4 | | | | 15 | |
Restructuring charges | | | — | | | | 48.4 | | | | (100 | ) |
Patent litigation gain | | | — | | | | (42.9 | ) | | | (100 | ) |
Gain on sale of property, plant and equipment | | | (13.4 | ) | | | — | | | | — | |
Income taxes on repatriation of foreign earnings | | | (7.4 | ) | | | 67.1 | | | | — | |
Impairment of property, plant and equipment | | | 76.6 | | | | — | | | | — | |
| | | | | | | | | | | | |
Adjusted net earnings | | $ | 1,329.2 | | | $ | 1,180.0 | | | | 13 | |
| | | | | | | | | | | | |
Basic net earnings per share of common stock: | | | | | | | | | | | | |
Reported basic net earnings per share | | $ | 3.21 | | | $ | 2.79 | | | | 15 | |
Restructuring charges | | | — | | | $ | 0.12 | | | | (100 | ) |
Patent litigation gain | | | — | | | $ | (0.11 | ) | | | (100 | ) |
Gain on sale of property, plant and equipment | | $ | (0.03 | ) | | | — | | | | — | |
Income taxes on repatriation of foreign earnings | | $ | (0.02 | ) | | $ | 0.17 | | | | — | |
Impairment of property, plant and equipment | | $ | 0.19 | | | | — | | | | — | |
Adjusted basic net earnings per share | | $ | 3.35 | | | $ | 2.97 | | | | 13 | |
| | | |
Weighted-average basic shares outstanding | | | 396.4 | | | | 397.4 | | | | | |
| | | |
Diluted net earnings per share of common stock: | | | | | | | | | | | | |
Reported diluted net earnings per share | | $ | 3.19 | | | $ | 2.77 | | | | 15 | |
Restructuring charges | | | — | | | $ | 0.12 | | | | (100 | ) |
Patent litigation gain | | | — | | | $ | (0.11 | ) | | | (100 | ) |
Gain on sale of property, plant and equipment | | $ | (0.03 | ) | | | — | | | | — | |
Income taxes on repatriation of foreign earnings | | $ | (0.02 | ) | | $ | 0.17 | | | | — | |
Impairment of property, plant and equipment | | $ | 0.19 | | | | — | | | | — | |
Adjusted diluted net earnings per share | | $ | 3.33 | | | $ | 2.95 | | | | 13 | |
| | | |
Weighted-average diluted shares outstanding | | | 399.5 | | | | 399.4 | | | | | |
The weighted-average basic and diluted shares outstanding used in the calculation of these non-GAAP financial measures are the same as the weighted-average shares outstanding used in the calculation of the reported per share amounts.
2009 Compared to 2008
The Company’s net sales increased to $6,723.1 million in 2009 from $6,718.2 million in 2008. Net sales grew by 2% as a result of increased unit volume and changes in product mix partially offset by unfavorable changes in foreign currency exchange rates.
The Company’s domestic sales were $4,317.4 million for 2009, representing an increase of 1%, as a result of higher shipments of Orthopaedic Implants partially offset by lower shipments of MedSurg Equipment.
26
International sales were $2,405.7 million for 2009, representing a decrease of 1%. The impact of foreign currency comparisons to the dollar value of international sales was unfavorable by $110.1 million for 2009. On a constant currency basis, international sales increased 3% in 2009 as a result of higher shipments of Orthopaedic Implants and MedSurg Equipment.
Worldwide sales of Orthopaedic Implants were $4,119.7 million for 2009, representing an increase of 4%. On a constant currency basis, sales of Orthopaedic Implants increased 6% in 2009 as a result of higher shipments of hips, knees, trauma, craniomaxillofacial and spinal implant systems.
Hip Implant Systems:Sales of hip implant systems increased 2% in 2009 (5% on a constant currency basis). In the United States, sales growth was driven by Trident hip products, X3 Polyethylene hip products, Accolade cementless hip products and Restoration Modular Hip System revision hip products. Sales growth in several hip systems, including X3 Polyethylene and Accolade cementless hip products in Europe, Canada and the Latin America and Pacific regions and Trident hip products in Japan also contributed to the Company’s constant currency sales growth in 2009.
Knee Implant Systems:Sales of knee implant systems increased 4% in 2009 (6% on a constant currency basis) due to strong sales growth in the Triathlon Knee System in the United States, Europe, Japan, Canada and the Pacific region and solid sales growth in the Scorpio Knee System in the Latin America region.
Trauma Implant Systems:Sales of trauma implant systems increased 5% in 2009 (6% on a constant currency basis) as a result of sales growth in the Gamma 3 Hip Fracture System and the SPS Calcaneal Foot Plating System in the United States, Europe, Canada and the Latin America and Pacific regions as well as sales growth in the Company’s VariAx Distal Radius System in Europe, Canada and the Latin America region. Strong sales growth of the HydroSet injectable bone substitute product in the United States, Canada and the Pacific region also contributed to the Company’s constant currency sales growth in 2009.
Spinal Implant Systems:Sales of spinal implant systems increased 10% in 2009 (11% on a constant currency basis). The increase was driven by strong worldwide sales growth of thoracolumbar implant systems, interbody devices and cervical implants in the United States, Europe and the Latin America and Pacific regions.
Worldwide sales of MedSurg Equipment were $2,603.4 million for 2009, representing a decrease of 5%. The general economic slowdown in the United States resulted in a significant and rapid contraction in hospital capital budgets that depressed demand for certain MedSurg Equipment products. The severe weakening of the economy caused the Company’s hospital customers to reduce capital purchases, which generates the majority of sales within the MedSurg Equipment segment, to a degree not previously experienced in prior recessionary periods. On a constant currency basis, sales of MedSurg Equipment decreased 4% in 2009 as higher shipments of surgical equipment and surgical navigation systems were offset by lower shipments of endoscopic and communication systems as well as patient handling and emergency medical equipment.
Surgical Equipment and Surgical Navigation Systems:Sales of surgical equipment and surgical navigation systems increased 1% in 2009 (2% on a constant currency basis) due to worldwide sales growth in operating room equipment as well as sales growth in powered surgical products in the United States, Europe, Japan and the Latin America region and sales growth in interventional pain products in the United States, Japan and the Pacific region.
Endoscopic and Communications Systems:Sales of endoscopic and communications systems decreased 2% in 2009 (1% decrease on a constant currency basis) due to lower sales of medical video imaging equipment products and image portal products in the United States partially offset by worldwide sales growth in arthroscopy and general surgery products, sales growth in communications products in Japan and the Latin America and Pacific regions and medical video imaging equipment in Europe, Japan and the Latin America and Pacific regions.
27
Patient Handling and Emergency Medical Equipment:Sales of patient handling and emergency medical equipment decreased 22% in 2009 (20% decrease on a constant currency basis) due to lower worldwide sales of hospital bed products and stretchers in the United States, Europe, Canada and the Pacific and Latin America regions, partially offset by sales growth in stretchers in Japan and emergency medical equipment in the United States.
Cost of sales represented 32.5% of sales in 2009 compared to 31.7% in 2008. The increase in the cost of sales percentage is primarily due to increased spending for compliance initiatives, higher excess and obsolete inventory costs associated with the Orthopaedic Implants businesses as well as higher unabsorbed costs due to lower production levels.
Research, development and engineering expenses represented 5.0% of sales in 2009 compared to 5.5% in 2008. The spending level in 2009 decreased by 9% to $336.2 million due to tight control on discretionary spending as well as the Company’s continued focus of certain research and development resources on compliance initiatives. New product introductions in 2009 for the Orthopaedic Implants segment included the Rejuvenate Modular Primary Hip system, the VariAx elbow plating system and the Xia Uniplanar Titanium Spinal System. Within the MedSurg equipment segment, new product introductions in 2009 included the 1288 HD Camera, the Impression non-powered support surface, the Flyte Suit personal protection system and the RemB micro electric system.
Selling, general and administrative expenses decreased 5% in 2009 and represented 37.3% of sales compared to 39.1% in 2008. In 2009 the Company settled an outstanding patent infringement lawsuit and received $62.5 million pursuant to a confidential settlement agreement. This gain represented a reduction to selling, general and administrative expenses. The remaining decrease in selling, general and administrative expenses as a percent of sales in 2009 is due to tight control on discretionary spending that more than offset increased legal settlement costs, net of insurance recoveries, recorded for certain product liability claims.
In 2009 the Company recorded $67.0 million ($48.4 million net of income taxes) in restructuring charges related to decisions to terminate certain third-party agent agreements at the Company’s EMEA Division, to simplify the organization structure at its Biotech, EMEA, Japan and Canada divisions and to discontinue selling certain products within its Orthopaedic Implants and MedSurg Equipment segments. In 2008 the Company recorded $34.9 million ($21.7 million net of income taxes) in restructuring charges related to the decisions to simplify the structure of the Company’s Japanese distribution business and to substantially reduce development efforts associated with Sightline product technologies acquired in 2006.
Interest and marketable securities income, which is included in other income (expense), decreased to $53.9 million in 2009 from $97.7 million in 2008 primarily as a result of lower average yields on the Company’s investments.
The Company’s effective income tax rate on earnings for the year ended December 31, 2009 was 31.8% compared to an effective income tax rate for the year ended December 31, 2008 of 27.4%. The effective income tax rate for the year ended December 31, 2009 reflects the impact of restructuring charges of $48.4 million (net of $18.6 million income tax benefits), the patent litigation gain of $42.9 million (net of $19.6 million income tax expenses) and the impact of the $67.1 million income tax expenses associated with the repatriation of foreign earnings of $787.0 million. The effective income tax rate for the year ended December 31, 2008 reflects the impact of the restructuring charges of $21.7 million (net of $13.2 million income tax benefits). In addition to these factors, the Company’s reported effective income tax rates for the years ended December 31, 2009 and 2008 are lower than the U.S. statutory income tax rate primarily as a result of manufacturing in lower income tax jurisdictions.
Net earnings decreased 4% in 2009 to $1,107.4 million from $1,147.8 million in 2008. Basic net earnings per share decreased 1% in 2009 to $2.79 from $2.81 in 2008, and diluted net earnings per share decreased to $2.77 in 2009 from $2.78 in 2008.
28
Excluding the impact of the patent litigation gain and the income tax charge associated with the repatriation of foreign earnings recorded in 2009 and the restructuring charges recorded in 2009 and 2008, adjusted net earnings increased 1% in 2009 to $1,180.0 million from $1,169.5 million in 2008. Adjusted basic net earnings per share increased 4% in 2009 to $2.97 from $2.87 in 2008 and adjusted diluted net earnings per share increased 4% in 2009 to $2.95 from $2.83 in 2008.
The reconciliations of these non-GAAP financial measures are as follows (in millions, except per share amounts):
| | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | Percentage
Change | |
Reported net earnings | | $ | 1,107.4 | | | $ | 1,147.8 | | | | (4 | ) |
Restructuring charges | | | 48.4 | | | | 21.7 | | | | 123 | |
Patent litigation gain | | | (42.9 | ) | | | — | | | | — | |
Income taxes on repatriation of foreign earnings | | | 67.1 | | | | — | | | | — | |
| | | | | | | | | | | | |
Adjusted net earnings | | $ | 1,180.0 | | | $ | 1,169.5 | | | | 1 | |
| | | | | | | | | | | | |
Basic net earnings per share of common stock: | | | | | | | | | | | | |
Reported basic net earnings per share | | $ | 2.79 | | | $ | 2.81 | | | | (1 | ) |
Restructuring charges | | $ | 0.12 | | | $ | 0.05 | | | | 140 | |
Patent litigation gain | | $ | (0.11 | ) | | | — | | | | — | |
Income taxes on repatriation of foreign earnings | | $ | 0.17 | | | | — | | | | — | |
Adjusted basic net earnings per share | | $ | 2.97 | | | $ | 2.87 | | | | 4 | |
| | | |
Weighted-average basic shares outstanding | | | 397.4 | | | | 408.1 | | | | | |
| | | |
Diluted net earnings per share of common stock: | | | | | | | | | | | | |
Reported diluted net earnings per share | | $ | 2.77 | | | $ | 2.78 | | | | — | |
Restructuring charges | | $ | 0.12 | | | $ | 0.05 | | | | 140 | |
Patent litigation gain | | $ | (0.11 | ) | | | — | | | | — | |
Income taxes on repatriation of foreign earnings | | $ | 0.17 | | | | — | | | | — | |
Adjusted diluted net earnings per share | | $ | 2.95 | | | $ | 2.83 | | | | 4 | |
| | | |
Weighted-average diluted shares outstanding | | | 399.4 | | | | 413.6 | | | | | |
The weighted-average basic and diluted shares outstanding used in the calculation of these non-GAAP financial measures are the same as the weighted-average shares outstanding used in the calculation of the reported per share amounts.
Liquidity and Capital Resources
The Company’s working capital at December 31, 2010 increased $1,616.2 million to $6,026.4 million from $4,410.2 million at December 31, 2009. The increase in working capital was primarily due to the issuance of $1,000.0 million of senior unsecured notes in January 2010 as well as increases in accounts receivable, inventories and prepaid expenses, partially offset by the use of cash to fund dividend payments and acquisitions. Accounts receivable days sales outstanding was 56 days at December 31, 2010 and 2009. Days sales in inventory increased by 9 days to 154 days at December 31, 2010 from 145 days at December 31, 2009. Days sales in inventory at December 31, 2010 is higher than the December 31, 2009 level primarily due to higher levels of inventory in support of anticipated 2011 sales growth.
The Company generated cash of $1,547.4 million from operations in 2010 compared to $1,460.7 million in 2009. The increase in cash from operations in 2010 is primarily due to increased earnings partially offset by increased inventory levels and higher tax payments.
29
In 2010 the Company used cash of $425.5 million for the repurchase of common stock, $238.3 million for the payment of dividends, $182.1 million for capital expenditures and $265.4 million for acquisitions. On January 15, 2010, the Company sold $500.0 million of the 2015 Notes and $500.0 million of the 2020 Notes. The 2015 Notes bear interest at 3.00% per year and, unless previously redeemed, will mature on January 15, 2015. The 2020 Notes bear interest at 4.375% per year and, unless previously redeemed, will mature on January 15, 2020. The Company received net proceeds of $996.1 million, net of an offering discount of $3.9 million. The 2015 Notes and 2020 Notes carry effective interest rates of 3.02% and 4.46%, respectively. Debt issuance costs of $10.5 million were incurred in connection with the sale of the senior unsecured notes. These costs were capitalized and are amortized to interest expense over the lives of the related senior unsecured notes. The Company also purchased and sold marketable securities, including exercising its Auction Rate Securities (ARS) Rights agreement, which are classified as available-for-sale investments and trading marketable securities, respectively, in accordance with the provisions of theInvestments-Debt and Equity Securities Topic of the Financial Accounting Standard Board (FASB) Accounting Standards Codification (Codification).
The Company had $1,757.6 million in cash and cash equivalents and $2,622.5 million in current marketable securities at December 31, 2010. The Company had outstanding borrowings totaling $1,021.8 million at December 31, 2010. On January 3, 2011 the Company completed the acquisition of the Neurovascular division from Boston Scientific Corporation. The initial payment of $1.45 billion was funded from the Company’s cash position. An addition $50.0 million will be payable upon completion of certain milestones. The Company believes its cash and current marketable securities on hand, anticipated future cash flows from operations and additional borrowing capacity under existing credit facilities will be sufficient to fund future operating capital requirements; future manufacturing facility construction and other capital expenditures; loaner instrumentation for surgical implants in support of new product launches; future debt service requirements; and the payment of dividends.
In August 2010 the Company refinanced its credit facility with a new $1,000.0 million Senior Unsecured Revolving Credit Facility due August 2013 (the 2010 Facility). The 2010 Facility replaces the previously outstanding $1,000.0 million Unsecured Credit Facility due in November 2010 (the 2005 Facility). The 2010 Facility includes an increase option permitting the Company to increase the size of the facility up to an additional $500.0 million, a $500.0 million multicurrency sublimit (with no sublimit for euro borrowings), a $100.0 million letter of credit sublimit and other terms, conditions and covenants substantially the same as the 2005 Facility. The 2010 Facility has an annual facility fee ranging from 10 to 45 basis points and bears interest at LIBOR, as defined in the 2010 Facility agreement, plus an applicable margin ranging from 65 to 205 basis points, both of which are dependent on the Company’s credit rating. Based on the Company’s current credit ratings, the 2010 Facility has an annual facility fee of 12.5 basis points and an interest margin of 87.5 basis points.
Should additional funds be required, the Company had $1,027.5 million of additional borrowing capacity available under all of its existing credit facilities as of December 31, 2010, including the 2010 Facility.
The Company’s additional borrowing capacity, along with the expected expiration period of the commitments, is summarized as follows (in millions):
| | | | | | | | | | | | |
| | | Total
Amount
Committed | | | Amount of
Commitment
Expiration Per Period | |
| | | Less Than
1 Year | | | In Excess
of 1 Year | |
Unsecured Credit Facility and other lines of credit | | $ | 1,027.5 | | | $ | 117.1 | | | $ | 910.4 | |
The Company reviews declines in the fair value of its investments classified as available-for-sale for impairment in accordance with the provisions of the Investments-Debt and Equity Securities Topic of the FASB Codification in order to determine whether the decline in fair value is an other-than-temporary impairment. Other-than-temporary impairments of available-for-sale marketable securities are recorded in earnings.
30
In June 2010 the Company exercised the ARS Rights agreement (ARS Rights) it had entered into in 2008 with UBS Financial Services Inc. (UBS), one of its investment providers, whereby the Company received the right to sell its ARS at par value to UBS at any time during the period from June 30, 2010 through July 2, 2012. Pursuant to this agreement, the Company redeemed its entire remaining outstanding ARS investment of $139.9 million par value in the second quarter. Prior to the exercise of the ARS Rights, the Company had applied the fair value option to its ARS Rights pursuant to the provisions of theFair Value Option for Financial Assets and Financial Liabilities Topic of the FASB Codification. As a result of this election, in the twelve-month period ended December 31, 2010, the Company recorded a loss of $17.0 million in other income (expense) to recognize the change in fair value estimate of its ARS Rights. These losses were offset by corresponding gains in the fair value estimate of the related ARS investment.
The Company’s future contractual obligations for agreements with initial terms greater than 1 year, including agreements to purchase materials in the normal course of business, are summarized as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Payment Period | |
| | | 2011 | | | 2012 | | | 2013 | | | 2014 | | | 2015 | | | After 2015 | | | Total | |
Balance sheet obligations: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Long-term debt | | $ | 25.3 | | | $ | — | | | $ | — | | | $ | — | | | $ | 499.6 | | | $ | 496.9 | | | $ | 1,021.8 | |
Contributions to defined benefit plans | | | 18.7 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 18.7 | |
Other | | | 8.6 | | | | 2.2 | | | | 2.7 | | | | 1.9 | | | | 1.7 | | | | 39.8 | | | | 56.9 | |
| | | | | | | |
Off-balance sheet arrangements: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Unconditional purchase obligations | | | 548.8 | | | | 181.0 | | | | 140.6 | | | | 120.1 | | | | 91.5 | | | | — | | | | 1,082.0 | |
Operating leases | | | 48.2 | | | | 36.0 | | | | 24.8 | | | | 17.4 | | | | 12.3 | | | | 10.2 | | | | 148.9 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | $ | 649.6 | | | $ | 219.2 | | | $ | 168.1 | | | $ | 139.4 | | | $ | 605.1 | | | $ | 546.9 | | | $ | 2,328.3 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
As further described in Note 11 to the Consolidated Financial Statements, as of December 31, 2010 the Company’s defined benefit pension plans are in an underfunded status of $107.7 million. Due to the rules affecting tax-deductible contributions in the jurisdictions in which the plans are offered and the impact of future plan asset performance, changes in interest rates and the potential for changes in legislation in the United States and other foreign jurisdictions, the Company is not able to reasonably estimate the future periods, beyond 2011, in which contributions to fund defined benefit pension plans will be made. As further described in Note 12 to the Consolidated Financial Statements, as of December 31, 2010, the Company has recorded a liability for uncertain income tax positions of $365.6 million. Due to uncertainties regarding the ultimate resolution of income tax audits, the Company is not able to reasonably estimate the future periods in which income tax payments to settle these uncertain income tax positions will be made.
Critical Accounting Policies and Estimates
The preparation of the Company’s Consolidated Financial Statements requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Management evaluates these estimates and assumptions on an ongoing basis. Estimates are based on historical experience, when available, and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Management believes that, of its significant accounting policies (see Note 1 to the Consolidated Financial Statements), an understanding of the following critical accounting policies is important in obtaining an overall understanding of the Consolidated Financial Statements.
31
Allowance for Doubtful Accounts
The Company maintains an allowance for doubtful accounts for estimated losses in the collection of accounts receivable. The Company makes estimates regarding the future ability of its customers to make required payments based on historical credit experience and expected future trends. If actual customer financial conditions are less favorable than projected by management, additional accounts receivable write offs may be necessary, which could unfavorably affect future operating results.
Inventory Reserves
The Company maintains reserves for excess and obsolete inventory resulting from the potential inability to sell its products at prices in excess of current carrying costs. The markets in which the Company operates are highly competitive and new products and surgical procedures are introduced on an ongoing basis. These marketplace changes may cause some of the Company’s products to become obsolete. The Company makes estimates regarding the future recoverability of the costs of these products and records a provision for excess and obsolete inventories based on historical experience, expiration of sterilization dates and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write downs may be required, which could unfavorably affect future operating results.
Income Taxes
The Company operates in multiple income tax jurisdictions both inside and outside the United States. Accordingly, management must determine the appropriate allocation of income to each of these jurisdictions. Income tax audits associated with the allocation of this income and other complex issues, including inventory transfer pricing and cost sharing, product royalty and foreign branch arrangements, may require an extended period of time to resolve and may result in income tax adjustments if changes to the income allocation are required between jurisdictions with different income tax rates. Because income tax adjustments in certain jurisdictions can be significant, the Company records accruals representing management’s best estimate of the probable resolution of these matters. To the extent additional information becomes available, such accruals are adjusted to reflect the revised estimated probable outcome.
Legal and Other Contingencies
The Company is involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product, labor and intellectual property, and other matters that are more fully described in Note 17 to the Consolidated Financial Statements. The outcomes of these matters will generally not be known for prolonged periods of time. In certain of the legal proceedings, the claimants seek damages, as well as other compensatory relief, which could result in the payment of significant claims and settlements. For legal matters for which management has sufficient information to reasonably estimate the Company’s future obligations, a liability representing management’s best estimate of the probable cost, or the minimum of the range of probable losses when a best estimate within the range is not known, for the resolution of these legal matters is recorded. The estimates are based on consultation with legal counsel, previous settlement experience and settlement strategies. If actual outcomes are less favorable than those projected by management, additional expense may be incurred, which could unfavorably affect future operating results.
To partially mitigate losses arising from unfavorable outcomes in such matters, the Company purchases third-party insurance coverage subject to certain deductibles and loss limitations. Future operating results may be unfavorably impacted by any settlement payments or losses beyond the amounts of insurance carried. In addition, such matters may negatively impact the Company’s ability to obtain cost-effective third-party insurance coverage in future periods.
32
Recently Adopted Accounting Standards
In 2010 the Company adopted the provisions of theImprovement to Financial Reporting by Enterprises Involved with Variable Interest Entities Topic of the FASB Codification. The topic requires a qualitative approach to identifying a controlling financial interest in a variable interest entity (VIE) and requires ongoing assessment of whether an entity is a VIE and whether an interest in a VIE makes the holder the primary beneficiary of the VIE. There was no impact to the Consolidated Financial Statements as a result of the adoption of this topic of the FASB Codification.
In 2010 the Company adopted the provisions of theFair Value Measurements and Disclosures Topic“Improving Disclosures About Fair Value Measurements” of the FASB Codification. This topic requires companies to make new disclosures about recurring and nonrecurring fair value measurements, including significant transfers into and out of Level 1 and Level 2 fair value measurements, and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair value measurements. The enhanced disclosures about recurring and nonrecurring fair value measurements are included in Note 2 to the Consolidated Financial Statements.
In 2009 the Company adopted the provisions of theBusiness Combinations Topic of the FASB Codification.This topic significantly changes the principles and requirements for how an acquisition is recognized and measured in financial statements, including the identifiable assets acquired and the liabilities assumed. This topic also provides guidance for recognizing and measuring goodwill acquired in a business combination and requires disclosures to enable users of the financial statements to evaluate the nature and financial effects of the business combination. The additional disclosure requirements regarding the Business Combinations Topic of the FASB Codification are included in Note 5 to the Consolidated Financial Statements.
Other Matters
The Company distributes its products throughout the world. As a result, the Company’s financial results could be significantly affected by factors such as weak economic conditions or changes in foreign currency exchange rates. The Company’s operating results are primarily exposed to changes in exchange rates among the U.S. dollar, European currencies, in particular the euro and the British pound, the Japanese yen, the Australian dollar and the Canadian dollar. When the U.S. dollar weakens against foreign currencies, the dollar value of sales denominated in foreign currencies increases. When the U.S. dollar strengthens, the opposite situation occurs. The Company develops and manufactures its products in the United States, China, France, Germany, Ireland, Puerto Rico and Switzerland and incurs costs in the applicable local currencies. This worldwide deployment of facilities serves to partially mitigate the impact of currency exchange rate changes on the Company’s cost of sales.
The Company enters into forward currency exchange contracts to mitigate the impact of currency fluctuations on transactions denominated in nonfunctional currencies, thereby limiting risk to the Company that would otherwise result from changes in exchange rates. These nonfunctional currency exposures principally relate to intercompany receivables and payables arising from intercompany purchases of manufactured products as well as, in 2010, nonfunctional cash balances associated with the anticipated acquisition of the Neurovascular division from Boston Scientific and, in 2009, intercompany loans associated with the repatriation of foreign earnings. The periods of the forward currency exchange contracts correspond to the periods of the exposed transactions, with realized gains and losses included in the measurement and recording of transactions denominated in the nonfunctional currencies. All forward currency exchange contracts are recorded at their fair value each period, with resulting gains (losses) included in other income (expense) in the Consolidated Statements of Earnings.
At December 31, 2010, the Company had outstanding forward currency exchange contracts to purchase $1,011.9 million and sell $366.9 million of various currencies (principally U.S. dollars and euros) with maturities ranging from 7 to 99 days. At December 31, 2009, the Company had outstanding forward currency exchange
33
contracts to purchase $2,041.1 million and sell $280.5 million of various currencies (principally U.S. dollars and euros) with maturities ranging from 4 to 106 days. The estimated fair value of forward currency exchange contracts represents the measurement of the contracts at month-end spot rates as adjusted by current forward points. A hypothetical 10% change in foreign currencies relative to the U.S. dollar would change the December 31, 2010 fair value by approximately $63.9 million. The Company is exposed to credit loss in the event of nonperformance by counterparties on its outstanding forward currency exchange contracts but does not anticipate nonperformance by any of the counterparties.
The Company has certain investments in net assets in international locations that are not hedged. These investments are subject to translation gains and losses due to changes in foreign currency exchange rates. For the year ended December 31, 2010, the strengthening of U.S. dollar relative to foreign currencies decreased the value of these investments in net assets and the related foreign currency translation adjustment loss in shareholders’ equity by $81.0 million to $196.5 million from $277.5 million at December 31, 2009.
In the third quarter of 2010, the Company received separate subpoenas from the U.S. Department of Justice related to (i) the sales, marketing and regulatory matters related to the Stryker PainPump and (ii) sales, marketing and regulatory matters related to the OtisKnee device. The Company is in the process of responding to these subpoenas.
In March 2010 a shareholder’s derivative action complaint against certain current and former Directors and Officers of the Company was filed in the United States District Court for the Western District of Michigan Southern Division. This lawsuit was brought by the Westchester Putnam Counties Heavy and Highway Laborers Local 60 Benefit Funds and Laborers Local 235 Benefit Funds. The complaint alleges claims for breach of fiduciary duties and gross mismanagement in connection with certain product recalls, FDA warning letters, government investigations relating to physician compensation and the criminal proceeding brought against the Company’s Biotech division. The case has been stayed while a Special Committee of the Board of Directors evaluates the claims.
In January 2010 a purported class action lawsuit against the Company was filed in the United States District Court for the Southern District of New York on behalf of those who purchased the Company’s common stock between January 25, 2007 and November 13, 2008, inclusive. The lawsuit seeks remedies under the Securities Exchange Act of 1934. In May 2010 the lawsuit was transferred to the United States District Court for the Western District of Michigan Southern Division. The Company intends to defend itself vigorously.
In 2009 a federal grand jury in the District of Massachusetts returned an indictment charging Stryker Biotech LLC and certain current and former employees of Stryker Biotech with wire fraud, conspiracy to defraud the FDA, distribution of a misbranded device and false statements to the FDA. The Company still hopes to be able to reach a fair and just resolution of this matter. The ultimate resolution of this matter is not reasonably estimable at this time, however, a conviction on the charges described above could result in significant monetary fines. Because Stryker Biotech is not presently involved in the sale of health care products and services (see additional information in Note 18 to the Consolidated Financial Statements), any conviction on these charges resulting in exclusion from participating in federal and state health care programs would not be expected to have a material effect on Stryker Biotech’s present business operations. Certain former Stryker Biotech employees have pled guilty to charges in connection with this matter.
In 2009 the Company received a warning letter from the FDA related to compliance issues for one of its craniomaxillofacial (CMF) implant products that was previously sold through its CMF distribution facility in Portage, Michigan. In 2007 the Company received two warning letters from the FDA regarding compliance with certain quality system specifications at its reconstructive implant manufacturing facilities: one letter for its facility in Cork, Ireland and another for its facility in Mahwah, New Jersey. In March 2010 the FDA informed the Company that the warning letter related to its Mahwah manufacturing facility had been resolved following a re-inspection in 2009 and additional corrective actions. In May 2010 the FDA informed the Company that the
34
warning letters related to its Cork, Ireland and CMF facilities had been resolved following FDA re-inspection of the Cork, Ireland facility and additional corrective actions at both the Cork and CMF facilities.
In 2007 the Company announced that it reached a resolution with the U.S. Attorney’s office for the District of New Jersey in connection with an investigation relating to “any and all consulting contracts, professional service agreements, or remuneration agreements between Stryker Corporation and any orthopedic surgeon, orthopedic surgeon in training, or medical school graduate using or considering the surgical use of hip or knee joint replacement/reconstruction products manufactured or sold by Stryker Corporation.” The resolution was in the form of a non-prosecution agreement, which included oversight by a federal monitor, for an 18-month period that ended on March 27, 2009. Subsequent to entering into the non-prosecution agreement, the U.S. Department of Health and Human Services, Office of Inspector General (HHS) issued a civil subpoena to the Company in seeking to determine whether the Company violated various laws by paying consulting fees and providing other things of value to orthopedic surgeons and healthcare and educational institutions as inducements to use Stryker’s orthopedic medical devices in procedures paid for in whole or in part by Medicare. The investigation is ongoing and the Company has produced numerous documents and other materials to HHS in response to the subpoena.
In 2007 the Company disclosed that the U.S. Securities and Exchange Commission (SEC) made an informal inquiry of the Company regarding possible violations of the Foreign Corrupt Practices Act in connection with the sale of medical devices in certain foreign countries. Subsequently, in 2008, the Company received a subpoena from the U.S. Department of Justice, Criminal Division, requesting certain documents for the period since January 1, 2000 in connection with the SEC inquiry. The Company is fully cooperating with the U.S. Department of Justice and the SEC regarding these matters.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Quantitative and qualitative disclosures about market risk are included in the “Results of Operations, Liquidity and Capital Resources” and “Other Matters” sections of the Company’s Management’s Discussion and Analysis of Financial Condition in Item 7 of this report.
35
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON CONSOLIDATED FINANCIAL STATEMENTS
The Board of Directors and Shareholders of Stryker Corporation:
We have audited the accompanying consolidated balance sheets of Stryker Corporation and subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of earnings, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Stryker Corporation and subsidiaries at December 31, 2010 and 2009, and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
As discussed in Note 1 to the consolidated financial statements, in 2009 the Company changed its method of accounting for business combinations with the adoption of the guidance originally issued in FASB Statement No. 141(R),Business Combinations(codified in FASB ASC Topic 805,Business Combinations).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Stryker Corporation’s internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 18, 2011 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
Grand Rapids, Michigan
February 18, 2011
36
Stryker Corporation and Subsidiaries
CONSOLIDATED BALANCE SHEETS
(in millions, except per share amounts)
| | | | | | | | |
| | | December 31 | |
| | 2010 | | | 2009 | |
ASSETS | | | | | | | | |
Current Assets | | | | | | | | |
Cash and cash equivalents | | $ | 1,757.6 | | | $ | 658.7 | |
Marketable securities | | | 2,622.5 | | | | 2,296.1 | |
Accounts receivable, less allowance of $57.0 ($66.3 in 2009) | | | 1,251.9 | | | | 1,147.1 | |
Inventories | | | 1,056.8 | | | | 943.0 | |
Deferred income taxes | | | 653.2 | | | | 602.2 | |
Prepaid expenses and other current assets | | | 289.4 | | | | 204.1 | |
| | | | | | | | |
Total current assets | | | 7,631.4 | | | | 5,851.2 | |
| | |
Property, Plant and Equipment | | | | | | | | |
Land, buildings and improvements | | | 553.8 | | | | 693.4 | |
Machinery and equipment | | | 1,296.1 | | | | 1,270.3 | |
| | | | | | | | |
Total Property, Plant and Equipment | | | 1,849.9 | | | | 1,963.7 | |
Less allowance for depreciation | | | 1,051.6 | | | | 1,016.1 | |
| | | | | | | | |
Net Property, Plant and Equipment | | | 798.3 | | | | 947.6 | |
| | |
Other Assets | | | | | | | | |
Goodwill | | | 1,072.3 | | | | 956.8 | |
Other intangibles, less accumulated amortization of $465.3 ($421.0 in 2009) | | | 703.0 | | | | 634.7 | |
Loaner instrumentation, less accumulated amortization of $684.1 ($771.3 in 2009) | | | 290.5 | | | | 285.4 | |
Deferred income taxes | | | 248.3 | | | | 258.9 | |
Other | | | 151.3 | | | | 136.7 | |
| | | | | | | | |
Total assets | | $ | 10,895.1 | | | $ | 9,071.3 | |
| | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
Current Liabilities | | | | | | | | |
Accounts payable | | $ | 291.7 | | | $ | 200.2 | |
Accrued compensation | | | 417.5 | | | | 354.1 | |
Income taxes | | | 47.4 | | | | 134.7 | |
Dividend payable | | | 70.4 | | | | 59.7 | |
Accrued expenses and other liabilities | | | 752.7 | | | | 674.3 | |
Current maturities of long-term debt | | | 25.3 | | | | 18.0 | |
| | | | | | | | |
Total current liabilities | | | 1,605.0 | | | | 1,441.0 | |
Long-Term Debt, excluding current maturities | | | 996.5 | | | | — | |
| | |
Other Liabilities | | | 1,120.0 | | | | 1,035.2 | |
| | |
Shareholders’ Equity | | | | | | | | |
Common stock, $0.10 par value: | | | | | | | | |
Authorized—1,000.0 shares, Outstanding -391.1 shares (397.9 in 2009) | | | 39.1 | | | | 39.8 | |
Additional paid-in capital | | | 964.1 | | | | 899.9 | |
Retained earnings | | | 6,016.9 | | | | 5,397.4 | |
Accumulated other comprehensive gain | | | 153.5 | | | | 258.0 | |
| | | | | | | | |
Total shareholders’ equity | | | 7,173.6 | | | | 6,595.1 | |
| | | | | | | | |
Total liabilities & shareholders’ equity | | $ | 10,895.1 | | | $ | 9,071.3 | |
| | | | | | | | |
See accompanying notes to Consolidated Financial Statements.
37
Stryker Corporation and Subsidiaries
CONSOLIDATED STATEMENTS OF EARNINGS
(in millions, except per share amounts)
| | | | | | | | | | | | |
| | | Years Ended December 31 | |
| | | 2010 | | | 2009 | | | 2008 | |
Net sales | | $ | 7,320.0 | | | $ | 6,723.1 | | | $ | 6,718.2 | |
Cost of sales | | | 2,285.7 | | | | 2,183.7 | | | | 2,131.4 | |
| | | | | | | | | | | | |
Gross profit | | | 5,034.3 | | | | 4,539.4 | | | | 4,586.8 | |
Research, development and engineering expenses | | | 393.9 | | | | 336.2 | | | | 367.8 | |
Selling, general and administrative expenses | | | 2,707.3 | | | | 2,506.3 | | | | 2,625.1 | |
Intangible asset amortization | | | 58.2 | | | | 35.5 | | | | 40.0 | |
Property, plant and equipment impairment | | | 123.5 | | | | — | | | | — | |
Restructuring charges | | | — | | | | 67.0 | | | | 34.9 | |
| | | | | | | | | | | | |
Total operating expenses | | | 3,282.9 | | | | 2,945.0 | | | | 3,067.8 | |
| | | | | | | | | | | | |
Operating income | | | 1,751.4 | | | | 1,594.4 | | | | 1,519.0 | |
Other income (expense) | | | (21.8 | ) | | | 29.5 | | | | 61.2 | |
| | | | | | | | | | | | |
Earnings before income taxes | | | 1,729.6 | | | | 1,623.9 | | | | 1,580.2 | |
Income taxes | | | 456.2 | | | | 516.5 | | | | 432.4 | |
| | | | | | | | | | | | |
Net earnings | | $ | 1,273.4 | | | $ | 1,107.4 | | | $ | 1,147.8 | |
| | | | | | | | | | | | |
Net earnings per share of common stock: | | | | | | | | | | | | |
Basic net earnings per share of common stock | | $ | 3.21 | | | $ | 2.79 | | | $ | 2.81 | |
Diluted net earnings per share of common stock | | $ | 3.19 | | | $ | 2.77 | | | $ | 2.78 | |
See accompanying notes to Consolidated Financial Statements.
38
Stryker Corporation and Subsidiaries
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in millions, except per share amounts)
| | | | | | | | | | | | | | | | | | | | |
| | | Common
Stock | | | Additional
Paid-In
Capital | | | Retained
Earnings | | | Accumulated
Other
Comprehensive
Gain (Loss) | | | Total | |
Balances at January 1, 2008 | | $ | 41.1 | | | $ | 711.9 | | | $ | 4,364.7 | | | $ | 260.8 | | | $ | 5,378.5 | |
Net earnings for 2008 | | | — | | | | — | | | | 1,147.8 | | | | — | | | | 1,147.8 | |
Unrealized gains on securities, including $0.7 income tax benefit | | | — | | | | — | | | | — | | | | 0.8 | | | | 0.8 | |
Unfunded pension losses, net of $8.3 income tax benefit | | | — | | | | — | | | | — | | | | (28.2 | ) | | | (28.2 | ) |
Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | (68.6 | ) | | | (68.6 | ) |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive earnings for 2008 | | | | | | | | | | | | | | | | | | | 1,051.8 | |
Issuance of 2.8 shares of common stock under stock option and benefit plans, including $33.7 excess income tax benefit | | | 0.2 | | | | 69.3 | | | | — | | | | — | | | | 69.5 | |
Share-based compensation | | | — | | | | 65.5 | | | | — | | | | — | | | | 65.5 | |
Cash dividend declared of $0.40 per share of common stock | | | — | | | | — | | | | (158.6 | ) | | | — | | | | (158.6 | ) |
Repurchase and retirement of 17.4 shares of common stock | | | (1.7 | ) | | | (33.9 | ) | | | (964.4 | ) | | | — | | | | (1,000.0 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2008 | | | 39.6 | | | | 812.8 | | | | 4,389.5 | | | | 164.8 | | | | 5,406.7 | |
Net earnings for 2009 | | | — | | | | — | | | | 1,107.4 | | | | — | | | | 1,107.4 | |
Unrealized gains on securities, including $1.4 income tax expense | | | — | | | | — | | | | — | | | | 2.7 | | | | 2.7 | |
Unfunded pension gains, net of $8.2 income tax expense | | | — | | | | — | | | | — | | | | 16.7 | | | | 16.7 | |
Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | 73.8 | | | | 73.8 | |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive earnings for 2009 | | | | | | | | | | | | | | | | | | | 1,200.6 | |
Issuance of 1.4 shares of common stock under stock option and benefit plans, including $6.9 excess income tax benefit | | | 0.2 | | | | 24.8 | | | | — | | | | — | | | | 25.0 | |
Share-based compensation | | | — | | | | 62.3 | | | | — | | | | — | | | | 62.3 | |
Cash dividends declared of $0.25 per share of common stock | | | — | | | | — | | | | (99.5 | ) | | | — | | | | (99.5 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2009 | | | 39.8 | | | | 899.9 | | | | 5,397.4 | | | | 258.0 | | | | 6,595.1 | |
Net earnings for 2010 | | | — | | | | — | | | | 1,273.4 | | | | — | | | | 1,273.4 | |
Unrealized loss on securities, including $0.3 income tax benefit | | | — | | | | — | | | | — | | | | (2.0 | ) | | | (2.0 | ) |
Unfunded pension loss, net of $14.0 income tax benefit | | | — | | | | — | | | | — | | | | (21.5 | ) | | | (21.5 | ) |
Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | (81.0 | ) | | | (81.0 | ) |
| | | | | | | | | | | | | | | | | | | | |
Comprehensive earnings for 2010 | | | | | | | | | | | | | | | | | | | 1,168.9 | |
Issuance of 1.5 shares of common stock under stock option and benefit plans, including $11.2 excess income tax benefit | | | 0.1 | | | | 15.2 | | | | — | | | | — | | | | 15.3 | |
Share-based compensation | | | — | | | | 68.8 | | | | — | | | | — | | | | 68.8 | |
Cash dividends declared of $0.63 per share of common stock | | | — | | | | — | | | | (249.0 | ) | | | — | | | | (249.0 | ) |
Repurchase and retirement of 8.3 shares of common stock | | | (0.8 | ) | | | (19.8 | ) | | | (404.9 | ) | | | — | | | | (425.5 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2010 | | $ | 39.1 | | | $ | 964.1 | | | $ | 6,016.9 | | | $ | 153.5 | | | $ | 7,173.6 | |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes to Consolidated Financial Statements.
39
Stryker Corporation and Subsidiaries
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
| | | | | | | | | | | | |
| | | Years Ended December 31 | |
| | | 2010 | | | 2009 | | | 2008 | |
Operating Activities | | | | | | | | | | | | |
Net earnings | | $ | 1,273.4 | | | $ | 1,107.4 | | | $ | 1,147.8 | |
Adjustments to reconcile net earnings from continuing operations to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation | | | 164.6 | | | | 165.2 | | | | 155.4 | |
Amortization | | | 245.6 | | | | 220.1 | | | | 232.2 | |
Share-based compensation | | | 68.8 | | | | 62.3 | | | | 65.5 | |
Income tax benefit from exercise of stock options | | | 24.5 | | | | 12.7 | | | | 44.6 | |
Excess income tax benefit from exercise of stock options | | | (11.2 | ) | | | (6.9 | ) | | | (33.7 | ) |
Restructuring charges | | | — | | | | 67.0 | | | | 34.9 | |
Property, plant and equipment impairment | | | 123.5 | | | | — | | | | — | |
Payments of restructuring charges | | | (8.7 | ) | | | (47.4 | ) | | | (0.5 | ) |
(Gain) loss on sale of property, plant and equipment | | | (23.0 | ) | | | 2.9 | | | | (1.7 | ) |
Provision for losses on accounts receivable | | | 18.6 | | | | 27.9 | | | | 10.4 | |
Deferred income tax credit | | | (103.8 | ) | | | (72.7 | ) | | | (17.6 | ) |
Other | | | 5.9 | | | | 7.1 | | | | 1.9 | |
Changes in operating assets and liabilities, net of effects of acquisitions: | | | | | | | | | | | | |
Accounts receivable | | | (121.4 | ) | | | (9.8 | ) | | | (131.2 | ) |
Inventories | | | (131.2 | ) | | | 33.7 | | | | (180.2 | ) |
Loaner instrumentation | | | (192.6 | ) | | | (188.6 | ) | | | (181.8 | ) |
Accounts payable | | | 95.9 | | | | (80.5 | ) | | | 10.4 | |
Accrued expenses and other liabilities | | | 91.1 | | | | 66.1 | | | | 54.8 | |
Income taxes | | | (23.9 | ) | | | 192.0 | | | | (29.1 | ) |
Other | | | 51.3 | | | | (97.8 | ) | | | (6.2 | ) |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 1,547.4 | | | | 1,460.7 | | | | 1,175.9 | |
| | | |
Investing Activities | | | | | | | | | | | | |
Acquisitions, net of cash acquired | | | (265.4 | ) | | | (570.2 | ) | | | (14.2 | ) |
Purchases of marketable securities | | | (5,619.0 | ) | | | (4,602.1 | ) | | | (16,832.3 | ) |
Proceeds from sales of marketable securities | | | 5,210.1 | | | | 3,973.7 | | | | 17,303.2 | |
Purchases of property, plant and equipment | | | (182.1 | ) | | | (131.3 | ) | | | (155.2 | ) |
Proceeds from sales of property, plant and equipment | | | 60.9 | | | | 1.5 | | | | 8.6 | |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | (795.5 | ) | | | (1,328.4 | ) | | | 310.1 | |
| | | |
Financing Activities | | | | | | | | | | | | |
Proceeds from borrowings | | | 100.1 | | | | 16.9 | | | | 26.0 | |
Payments on borrowings | | | (80.8 | ) | | | (19.6 | ) | | | (19.3 | ) |
Proceeds from issuance of long-term debt, net | | | 996.1 | | | | — | | | | — | |
Issuance cost of long-term debt | | | (10.5 | ) | | | — | | | | — | |
Dividends paid | | | (238.3 | ) | | | (198.4 | ) | | | (135.6 | ) |
Proceeds from exercise of stock options | | | 3.6 | | | | 6.3 | | | | 50.1 | |
Excess income tax benefit from exercise of stock options | | | 11.2 | | | | 6.9 | | | | 33.7 | |
Repurchase and retirement of common stock | | | (425.5 | ) | | | — | | | | (1,000.0 | ) |
Other | | | 54.2 | | | | (5.4 | ) | | | (1.0 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 410.1 | | | | (193.3 | ) | | | (1,046.1 | ) |
Effect of exchange rate changes on cash and cash equivalents | | | (63.1 | ) | | | 18.6 | | | | (29.3 | ) |
| | | | | | | | | | | | |
Increase (decrease) in cash and cash equivalents | | | 1,098.9 | | | | (42.4 | ) | | | 410.6 | |
Cash and cash equivalents at beginning of year | | | 658.7 | | | | 701.1 | | | | 290.5 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of year | | $ | 1,757.6 | | | $ | 658.7 | | | $ | 701.1 | |
| | | | | | | | | | | | |
See accompanying notes to Consolidated Financial Statements.
40
Stryker Corporation and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
NOTE 1
SIGNIFICANT ACCOUNTING POLICIES
Business: Stryker Corporation (the Company or Stryker) is one of the world’s leading medical technology companies and is dedicated to helping healthcare professionals perform their jobs more efficiently while enhancing patient care. The Company provides innovative orthopaedic implants as well as state-of-the-art medical and surgical equipment to help people lead more active and more satisfying lives. The Company’s products include implants used in joint replacement, trauma, and spinal surgeries; surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment as well as other medical device products used in a variety of medical specialties.
Principles of Consolidation: The Consolidated Financial Statements include the accounts of the Company and its subsidiaries after elimination of intercompany accounts and transactions.
Use of Estimates: The preparation of these Consolidated Financial Statements in conformity with U.S. generally accepted accounting principles (GAAP) requires Company management to make estimates and assumptions that affect the amounts reported in the Consolidated Financial Statements and accompanying notes. Actual results could differ from those estimates.
Revenue Recognition: A significant portion of the Company’s Orthopaedic Implants revenue is generated from consigned inventory maintained at hospitals or with field representatives. The Company retains title to all inventory held on consignment at hospitals or with field locations until the Company receives appropriate notification that the product has been used or implanted at which time revenue is recognized. The Company records revenue from Orthopaedic Implants not held on consignment and MedSurg Equipment product sales when title and risk of ownership have been transferred to the customer, which is typically upon shipment to the customer. The Company records estimated sales returns, discounts, rebates and other sales incentives as a reduction of net sales in the same period revenue is recognized. Estimates of sales returns are recorded for anticipated product returns based on historical sales and returns information. Estimates of sales discounts, rebates and other sales incentives are recorded based on contractual terms, historical experience and trend analysis.
Shipping and Handling of Products: Amounts billed to customers for shipping and handling of products are included in net sales. Costs incurred related to shipping and handling of products are included in cost of sales.
Foreign Currency Translation: The functional currencies for substantially all of the Company’s international affiliates are their local currencies. Accordingly, the financial statements of these international affiliates are translated into U.S. dollars using current exchange rates for balance sheets and average exchange rates for statements of earnings and cash flows. Unrealized translation adjustments are included in accumulated other comprehensive gain (loss) in shareholders’ equity. Transaction gains and losses, such as those resulting from the settlement of nonfunctional currency receivables or payables, are included in net earnings.
Financial Instruments: The Company’s financial instruments consist of cash, cash equivalents, marketable securities, accounts receivable, other investments, accounts payable, debt and foreign currency exchange contracts. The Company’s estimates of fair value for financial instruments approximate their carrying amounts as of December 31, 2010 and 2009.
Cash equivalents are highly liquid investments with a maturity of three months or less when purchased. Marketable securities consist of marketable debt securities and certificates of deposit and mutual funds. Mutual funds are acquired to offset changes in certain liabilities related to deferred compensation arrangements and are
41
expected to be used to settle these liabilities. Pursuant to the Company’s investment policy, all individual marketable security investments must have a minimum credit quality of single A (per Standard & Poor’s or Fitch) or A2 (per Moody’s Corporation) at the time of acquisition, while the overall portfolio of marketable securities must maintain a minimum average credit quality of double A (per Standard & Poor’s or Fitch) or Aa (per Moody’s Corporation). In the event of a rating downgrade below the minimum credit quality subsequent to purchase, the marketable security investment is evaluated to determine the appropriate action to take to minimize the overall risk to the Company’s marketable security investment portfolio. As of December 31, 2010, only 1% of the Company’s investments in marketable securities had a credit quality rating of less than single A (per Standard & Poor’s or Fitch) and A2 (per Moody’s Corporation).
The Company follows the provisions of theInvestments-Debt and Equity Securities Topicof the Financial Accounting Standard Board (FASB) Accounting Standard Codification (Codification) in accounting for its marketable securities, which are classified as available-for-sale and trading securities. This topic requires the Company to recognize all marketable securities on the Consolidated Balance Sheets at fair value. The Company’s marketable securities are stated at fair value pursuant to the requirements of theFair Value Measurements and Disclosures Topicof the FASB Codification. Adjustments to the fair value of marketable securities that are classified as available-for-sale are recorded as increases or decreases, net of income taxes, within accumulated other comprehensive gain (loss) in shareholders’ equity and adjustments to the fair value of marketable securities that are classified as trading are recorded in earnings. The amortized cost of marketable debt securities is adjusted for amortization of premiums and discounts to maturity computed under the effective interest method. Such amortization is included in other income (expense) along with interest and realized gains and losses. The cost of securities sold is determined by the specific identification method.
The Company reviews declines in the fair value of its investments classified as available-for-sale for impairment in accordance with the provisions of theInvestments-Debt and Equity Securities Topicof the FASB Codification in order to determine whether the decline in fair value is an other-than-temporary impairment. Other-than-temporary impairments of available-for-sale marketable securities are recorded in earnings.
The Company follows the provisions of the Derivatives and Hedging Topic of the FASB Codification, which requires the Company to recognize all derivatives on the Consolidated Balance Sheets at fair value. The Company enters into forward currency exchange contracts to mitigate the impact of currency fluctuations on transactions denominated in nonfunctional currencies, thereby limiting risk to the Company that would otherwise result from changes in exchange rates. These nonfunctional currency exposures principally relate to intercompany receivables and payables arising from intercompany purchases of manufactured products. The periods of the forward currency exchange contracts correspond to the periods of the exposed transactions, with realized gains and losses included in the measurement and recording of transactions denominated in the nonfunctional currencies. All forward currency exchange contracts are recorded at their fair value each period, with resulting gains (losses) included in other income (expense) in the Consolidated Statements of Earnings.
Accounts Receivable: Accounts receivable consists of trade and other miscellaneous receivables. The Company maintains an allowance for doubtful accounts for estimated losses in the collection of accounts receivable. The Company makes estimates regarding the future ability of its customers to make required payments based on historical credit experience and expected future trends. Accounts receivable are written off when all reasonable collection efforts are exhausted.
Inventories: Inventories are stated at the lower of cost or market. Cost for approximately 78% of inventories is determined using the first-in, first-out (FIFO) cost method. Cost for certain domestic inventories is determined using the last-in, first-out (LIFO) cost method. The FIFO cost for all inventories approximates replacement cost.
The Company maintains reserves for excess and obsolete inventory resulting from the potential inability to sell its products at prices in excess of current carrying costs. The markets in which the Company operates are highly competitive, and new products and surgical procedures are introduced on an ongoing basis. Such
42
marketplace changes may cause some of the Company’s products to become obsolete. The Company makes estimates regarding the future recoverability of the costs of these products and records a provision for excess and obsolete inventories based on historical experience, expiration of sterilization dates and expected future trends.
Property, Plant and Equipment: Property, plant and equipment is stated at cost. Depreciation is computed by either the straight-line or declining-balance method over the estimated useful lives of 3 to 30 years for buildings and improvements and 3 to 10 years for machinery and equipment.
Goodwill and Other Intangible Assets:Goodwill represents the excess of purchase price over fair value of tangible net assets of acquired businesses after amounts allocated to other intangible assets. Other intangible assets include developed technology, customer relationships (which reflect expected continued customer patronage), trademarks and patents, which are amortized on a straight-line basis over 4 to 40 years (weighted-average life of 15 years for other intangible assets).
Goodwill and Long-Lived Assets Impairment Tests:The Intangibles-Goodwill and Other Topicof the FASB Codification requires companies to test goodwill for possible impairment on an annual basis. The Company performs the annual impairment test in the fourth quarter of each year using a discounted cash flow analysis that requires certain assumptions and estimates be made regarding market conditions and the Company’s future profitability. The Company also performs impairment tests of goodwill and other intangible and long-lived assets during interim periods upon the occurrence of certain events or changes in circumstance as defined in theProperty, Plant, and EquipmentandPresentation of Financial Statements Topics of the FASB Codification.
Loaner Instrumentation: Loaner instrumentation represents the net book value of loaner instruments for surgical implants provided to customers by the Company. Loaner instrumentation is amortized on a straight-line basis over a 3-year period. Amortization expense for loaner instrumentation is included in selling, general and administrative expenses.
Stock Options:At December 31, 2010, the Company had key employee and director stock option plans, which are described more fully in Note 9 to the Consolidated Financial Statements. Pursuant to the provisions of theCompensation-Stock Compensation Topic of the FASB Codification, the Company measures the cost of employee stock options based on the grant-date fair value and recognizes that cost over the period during which a recipient is required to provide services in exchange for the options, typically the vesting period. The weighted-average fair value per share of options granted during 2010, 2009 and 2008, estimated on the date of grant using the Black-Scholes option pricing model, was $15.87, $13.09 and $19.87, respectively. The fair value of options granted was estimated using the following weighted-average assumptions:
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
Risk-free interest rate | | | 3.0 | % | | | 2.5 | % | | | 3.2 | % |
Expected dividend yield | | | 1.4 | % | | | 0.7 | % | | | 0.5 | % |
Expected stock price volatility | | | 28.6 | % | | | 27.7 | % | | | 22.7 | % |
Expected option life | | | 6.8 years | | | | 6.8 years | | | | 6.7 years | |
The risk-free interest rate for periods within the expected life of options granted is based on the U.S. Treasury yield curve in effect at the time of grant. Expected stock price volatility is based on historical volatility of the Company’s stock. The expected option life, representing the period of time that options granted are expected to be outstanding, is based on historical option exercise and employee termination data. The Company recognizes the cost of stock options using the straight-line method over their vesting periods.
Income Taxes: The Company accounts for income taxes pursuant to the provisions of theIncome Taxes Topic of the FASB Codification. Deferred income tax assets and liabilities are determined based on differences between financial reporting and income tax bases of assets and liabilities and are measured using the enacted income tax rates in effect for the years in which the differences are expected to reverse. Deferred income tax
43
credit represents the change in net deferred income tax assets and liabilities during the year. Interest expense and penalties incurred associated with uncertain income tax positions are included in other income (expense) pursuant to the provisions of theIncome Taxes Topicof the FASB Codification.
The Company operates in multiple income tax jurisdictions both inside and outside the United States. Accordingly, management must determine the appropriate allocation of income to each of these jurisdictions based on current interpretations of complex income tax regulations. Income tax authorities in these jurisdictions regularly perform audits of the Company’s income tax filings. Income tax audits associated with the allocation of this income and other complex issues, including inventory transfer pricing and cost sharing, product royalty and foreign branch arrangements, may require an extended period of time to resolve and may result in significant income tax adjustments if changes to the income allocation are required between jurisdictions with different income tax rates.
Legal and Other Contingencies: The Company is involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product, labor and intellectual property, and other matters that are more fully described in Note 17 to the Consolidated Financial Statements. The outcomes of these matters will generally not be known for prolonged periods of time. In certain of the legal proceedings, the claimants seek damages, as well as other compensatory relief, which could result in the payment of significant claims and settlements. For legal matters for which management has sufficient information to reasonably estimate the Company’s future obligations, a liability representing management’s best estimate of the probable cost, or the minimum of the range of probable losses when a best estimate within the range is not known, for the resolution of these legal matters is recorded. The estimates are based on consultation with legal counsel, previous settlement experience and settlement strategies.
Accumulated Other Comprehensive Gain (Loss): The components of accumulated other comprehensive gain (loss) are as follows (in millions):
| | | | | | | | | | | | | | | | |
| | | Unrealized
Gains (Losses)
on Securities | | | Unfunded
Pension
Gains (Losses) | | | Foreign
Currency
Translation
Adjustments | | | Accumulated
Other
Comprehensive
Gain (Loss) | |
Balances at January 1, 2009 | | $ | 0.9 | | | $ | (39.8 | ) | | $ | 203.7 | | | $ | 164.8 | |
Other comprehensive gain for 2009 | | | 2.7 | | | | 16.7 | | | | 73.8 | | | | 93.2 | |
| | | | | | | | | | | | | | | | |
Balances at December 31, 2009 | | | 3.6 | | | | (23.1 | ) | | | 277.5 | | | | 258.0 | |
Other comprehensive loss for 2010 | | | (2.0 | ) | | | (21.5 | ) | | | (81.0 | ) | | | (104.5 | ) |
| | | | | | | | | | | | | | | | |
Balances at December 31, 2010 | | $ | 1.6 | | | $ | (44.6 | ) | | $ | 196.5 | | | $ | 153.5 | |
| | | | | | | | | | | | | | | | |
Recently Adopted Accounting Standards: In 2010 the Company adopted the provisions of theImprovement to Financial Reporting by Enterprises Involved with Variable Interest Entities Topic of the FASB Codification. The topic requires a qualitative approach to identifying a controlling financial interest in a variable interest entity (VIE) and requires ongoing assessment of whether an entity is a VIE and whether an interest in a VIE makes the holder the primary beneficiary of the VIE. There was no impact to the Consolidated Financial Statements as a result of the adoption of this topic of the FASB Codification.
In 2010 the Company adopted the provisions of theFair Value Measurements and Disclosures Topic“Improving Disclosures About Fair Value Measurements” of the FASB Codification. This topic requires companies to make new disclosures about recurring and nonrecurring fair value measurements, including significant transfers into and out of Level 1 and Level 2 fair value measurements, and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair value measurements. The enhanced disclosures about recurring and nonrecurring fair value measurements are included in Note 2 to the Consolidated Financial Statements.
44
In 2009 the Company adopted the provisions of theBusiness Combinations Topic of the FASB Codification.This topic significantly changes the principles and requirements for how an acquisition is recognized and measured in a company’s financial statements including the identifiable assets acquired and the liabilities assumed. This topic also provides guidance for recognizing and measuring goodwill acquired in a business combination and required disclosures to enable users of the financial statements to evaluate the nature and financial effects of the business combination. The additional disclosure requirements regarding the Business Combinations Topic of the FASB Codification are included in Note 5 to the Consolidated Financial Statements.
Recently Issued Accounting Standards: In 2009 the FASB amended the provisions of theRevenue Recognition for Multiple-Deliverable Revenue Arrangements Topicof the FASB Codification. This topic amends prior guidance and requires an entity to apply the relative selling price allocation method in order to estimate the selling price for all units of accounting, including delivered items, when vendor-specific objective evidence or acceptable third-party evidence does not exist. These provisions are effective for revenue arrangements entered into or which contain material modifications in fiscal years beginning on or after June 15, 2010, applied prospectively. This topic is effective for the Company beginning on January 1, 2011. The Company does not expect the adoption of the topic to have a material impact on its Consolidated Financial Statements.
Reclassifications: Certain prior year amounts have been reclassified to conform with the presentation used in 2010. In 2010 the Company separately disclosed the (gain) loss on sale of property, plant and equipment in its Consolidated Statements of Cash Flows.
NOTE 2
FINANCIAL INSTRUMENTS
The Company’s financial instruments consist of cash, cash equivalents, marketable securities, accounts receivable, other investments, accounts payable, debt and foreign currency exchange contracts. The Company’s estimates of fair value for financial instruments approximate their carrying amounts as of December 31, 2010.
Pursuant to the requirements of theFair Value Measurements and Disclosures Topicof the FASB Codification, the Company’s financial assets and liabilities measured at fair value on a recurring basis are classified and disclosed in one of the following three categories:
| | |
Level 1: | | Financial instruments with unadjusted, quoted prices listed on active market exchanges. |
| |
Level 2: | | Financial instruments lacking unadjusted, quoted prices from active market exchanges, including over-the-counter traded financial instruments. The prices for the financial instruments are determined using prices for recently traded financial instruments with similar underlying terms as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals. |
| |
Level 3: | | Financial instruments that are not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques. |
The following describes the methods the Company uses to estimate the fair value of the Company’s financial assets and liabilities:
Cash and cash equivalents:
The Company considers the carrying values of these financial instruments to approximate fair value for these financial instruments because of the short period of time between origination of the instruments and their expected realization.
45
Available-for-sale marketable securities:
The Company’s Level 2 available-for-sale marketable securities primarily include U.S. agency debt securities, foreign government debt securities, asset backed debt securities, corporate debt securities and certificates of deposit. The Company’s Level 2 available-for-sale marketable securities values are determined using prices for recently traded financial instruments with similar underlying terms as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals. The Company’s Level 3 available-for-sale marketable securities include corporate and foreign government debt securities. The Company’s Level 3 available-for-sale marketable securities valuations are based on the income approach, specifically, discounted cash flow analyses that utilize significant inputs based on the Company’s estimates and assumptions. Using this approach, estimates for timing and amount of cash flows and expected holding periods of the securities were used and the expected future cash flows were calculated over the expected life of each security and were discounted to a single present value using an estimated market required rate of return.
Trading marketable securities:
The Company’s Level 1 trading marketable securities consist of mutual funds and are valued using a market approach based on quoted prices for the specific mutual fund from transactions in active exchange markets.
Auction Rate Securities Rights:
In June 2010 the Company exercised the ARS Rights agreement (ARS Rights) it had entered into in 2008 with UBS Financial Services Inc. (UBS), one of its investment providers, whereby the Company received the right to sell its ARS at par value to UBS at any time during the period from June 30, 2010 through July 2, 2012. Pursuant to this agreement, the Company redeemed its entire remaining outstanding ARS investment of $139.9 million par value. Prior to the exercise of the ARS Rights, the Company had applied the fair value option to its ARS Rights pursuant to the provisions of theFair Value Option for Financial Assets and Financial Liabilities Topic of the FASB Codification. As a result of this election, in the twelve-month period ended December 31, 2010, the Company recorded a loss of $17.0 million in other income (expense) to recognize the change in fair value estimate of its ARS Rights. The loss was offset by corresponding gains in the fair value estimate of the related ARS investment. In the twelve-month period ended December 31, 2009 the Company recorded a loss of $11.0 million in other income (expense) to recognize the change in fair value estimate of its ARS Rights. This loss was offset by a corresponding gain in the fair value estimate of the related trading marketable securities.
Foreign currency exchange contracts:
The Company values foreign currency exchange contracts using a market approach based on foreign currency exchange rates obtained from active markets. The estimated fair value of forward currency exchange contracts represents the measurement of the contracts at month-end spot rates as adjusted by current forward points. At December 31, 2010, the fair value carrying amount of the Company’s forward currency exchange contracts assets and liabilities were $2.4 million and $1.1 million, respectively.
46
The following table summarizes the valuation of the Company’s financial instruments by the aforementioned pricing categories (in millions):
| | | | | | | | | | | | | | | | |
| | | Total | | | Quoted Prices
in Active
Markets
(Level 1) | | | Prices with
Other
Observable
Inputs
(Level 2) | | | Prices with
Unobservable
Inputs
(Level 3) | |
At December 31, 2010 | | | | | | | | | | | | | | | | |
Assets: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 1,757.6 | | | $ | 1,757.6 | | | $ | — | | | $ | — | |
Available-for-sale marketable securities | | | | | | | | | | | | | | | | |
Corporate and asset backed debt securities | | | 1,620.0 | | | | — | | | | 1,619.3 | | | | 0.7 | |
Foreign government debt securities | | | 522.8 | | | | — | | | | 522.2 | | | | 0.6 | |
U.S. agency debt securities | | | 314.6 | | | | — | | | | 314.6 | | | | — | |
Certificates of deposit | | | 70.7 | | | | — | | | | 70.7 | | | | — | |
Other | | | 95.1 | | | | — | | | | 95.1 | | | | — | |
| | | | | | | | | | | | | | | | |
Total available-for-sale marketable securities | | | 2,623.2 | | | | — | | | | 2,621.9 | | | | 1.3 | |
Trading marketable securities | | | 48.2 | | | | 48.2 | | | | — | | | | — | |
Foreign currency exchange contracts | | | 2.4 | | | | — | | | | 2.4 | | | | — | |
| | | | | | | | | | | | | | | | |
| | $ | 4,431.4 | | | $ | 1,805.8 | | | $ | 2,624.3 | | | $ | 1.3 | |
| | | | | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | | | | | |
Deferred compensation arrangements | | $ | 48.2 | | | $ | 48.2 | | | $ | — | | | $ | — | |
Foreign currency exchange contracts | | | 1.1 | | | | — | | | | 1.1 | | | | — | |
| | | | | | | | | | | | | | | | |
| | $ | 49.3 | | | $ | 48.2 | | | $ | 1.1 | | | $ | — | |
| | | | | | | | | | | | | | | | |
At December 31, 2009 | | | | | | | | | | | | | | | | |
Assets: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 658.7 | | | $ | 658.7 | | | $ | — | | | $ | — | |
Available-for-sale marketable securities | | | | | | | | | | | | | | | | |
Corporate and asset backed debt securities | | | 1,047.8 | | | | — | | | | 1,047.1 | | | | 0.7 | |
Foreign government debt securities | | | 742.1 | | | | — | | | | 742.1 | | | | — | |
U.S. agency debt securities | | | 166.3 | | | | — | | | | 166.3 | | | | — | |
Certificates of deposit | | | 92.1 | | | | — | | | | 92.1 | | | | — | |
Other | | | 109.2 | | | | — | | | | 109.2 | | | | — | |
| | | | | | | | | | | | | | | | |
Total available-for-sale marketable securities | | | 2,157.5 | | | | — | | | | 2,156.8 | | | | 0.7 | |
Trading marketable securities | | | | | | | | | | | | | | | | |
Municipal debt securities (ARS) | | | 139.3 | | | | — | | | | — | | | | 139.3 | |
Mutual funds | | | 39.3 | | | | 39.3 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Total trading marketable securities | | | 178.6 | | | | 39.3 | | | | — | | | | 139.3 | |
ARS Rights | | | 17.0 | | | | — | | | | — | | | | 17.0 | |
Foreign currency exchange contracts | | | 8.3 | | | | — | | | | 8.3 | | | | — | |
| | | | | | | | | | | | | | | | |
Total | | $ | 3,020.1 | | | $ | 698.0 | | | $ | 2,165.1 | | | $ | 157.0 | |
| | | | | | | | | | | | | | | | |
Liabilities: | | | | | | | | | | | | | | | | |
Deferred compensation arrangements | | $ | 39.3 | | | $ | 39.3 | | | $ | — | | | $ | — | |
Foreign currency exchange contracts | | | 6.2 | | | | — | | | | 6.2 | | | | — | |
| | | | | | | | | | | | | | | | |
Total | | $ | 45.5 | | | $ | 39.3 | | | $ | 6.2 | | | $ | — | |
| | | | | | | | | | | | | | | | |
47
The following table presents a rollforward of the assets measured at fair value on a recurring basis using unobservable inputs (Level 3) (in millions):
| | | | | | | | |
| | | 2010 | | | 2009 | |
Balance as of January 1 | | $ | 157.0 | | | $ | 168.9 | |
Transfers into Level 3 | | | 0.6 | | | | — | |
Settlements | | | (156.3 | ) | | | (10.5 | ) |
Other | | | — | | | | (1.4 | ) |
| | | | | | | | |
Balance as of December 31 | | $ | 1.3 | | | $ | 157.0 | |
| | | | | | | | |
The following is a summary of the Company’s marketable securities (in millions):
| | | | | | | | | | | | | | | | |
| | | Amortized
Cost | | | Gross
Unrealized
Gains | | | Gross
Unrealized
(Losses) | | | Estimated
Fair Value | |
At December 31, 2010 | | | | | | | | | | | | | | | | |
Available-for-sale marketable securities: | | | | | | | | | | | | | | | | |
Corporate and asset backed debt securities | | $ | 1,618.4 | | | $ | 3.7 | | | $ | (2.1 | ) | | $ | 1,620.0 | |
Foreign government debt securities | | | 522.7 | | | | 0.6 | | | | (0.5 | ) | | | 522.8 | |
U.S. agency debt securities | | | 314.2 | | | | 0.6 | | | | (0.2 | ) | | | 314.6 | |
Certificates of deposit | | | 70.6 | | | | 0.1 | | | | — | | | | 70.7 | |
Other | | | 95.0 | | | | 0.1 | | | | — | | | | 95.1 | |
| | | | | | | | | | | | | | | | |
Total available-for-sale marketable securities | | $ | 2,620.9 | | | $ | 5.1 | | | $ | (2.8 | ) | | | 2,623.2 | |
| | | | | | | | | | | | | | | | |
Trading marketable securities | | | | | | | | | | | | | | | 48.2 | |
| | | | | | | | | | | | | | | | |
Total marketable securities | | | | | | | | | | | | | | $ | 2,671.4 | |
| | | | | | | | | | | | | | | | |
Reported as: | | | | | | | | | | | | | | | | |
Current assets-Marketable securities | | | | | | | | | | | | | | $ | 2,622.5 | |
Noncurrent assets-Other | | | | | | | | | | | | | | | 48.9 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | $ | 2,671.4 | |
| | | | | | | | | | | | | | | | |
| | | | |
| | | Amortized
Cost | | | Gross
Unrealized
Gains | | | Gross
Unrealized
(Losses) | | | Estimated
Fair Value | |
At December 31, 2009 | | | | | | | | | | | | | | | | |
Available-for-sale marketable securities: | | | | | | | | | | | | | | | | |
Corporate and asset backed debt securities | | $ | 1,043.6 | | | $ | 5.2 | | | $ | (1.0 | ) | | $ | 1,047.8 | |
Foreign government debt securities | | | 742.2 | | | | 0.9 | | | | (1.0 | ) | | | 742.1 | |
U.S. agency debt securities | | | 165.9 | | | | 0.5 | | | | (0.1 | ) | | | 166.3 | |
Certificates of deposit | | | 92.0 | | | | 0.1 | | | | — | | | | 92.1 | |
Other | | | 109.1 | | | | 0.1 | | | | — | | | | 109.2 | |
| | | | | | | | | | | | | | | | |
Total available-for-sale marketable securities | | $ | 2,152.8 | | | $ | 6.8 | | | $ | (2.1 | ) | | | 2,157.5 | |
| | | | | | | | | | | | | | | | |
Trading marketable securities: | | | | | | | | | | | | | | | | |
Municipal debt securities (ARS) | | | | | | | | | | | | | | | 139.3 | |
Mutual funds | | | | | | | | | | | | | | | 39.3 | |
| | | | | | | | | | | | | | | | |
Total trading marketable securities | | | | | | | | | | | | | | | 178.6 | |
| | | | | | | | | | | | | | | | |
Total marketable securities | | | | | | | | | | | | | | $ | 2,336.1 | |
| | | | | | | | | | | | | | | | |
Reported as: | | | | | | | | | | | | | | | | |
Current assets-Marketable securities | | | | | | | | | | | | | | $ | 2,296.1 | |
Noncurrent assets-Other | | | | | | | | | | | | | | | 40.0 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | $ | 2,336.1 | |
| | | | | | | | | | | | | | | | |
48
The cost and estimated fair value of available-for-sale marketable securities at December 31, 2010, by contractual maturity, are as follows (in millions):
| | | | | | | | |
| | | Cost | | | Estimated
Fair Value | |
Due in one year or less | | $ | 383.6 | | | $ | 383.7 | |
Due after one year through three years | | | 2,168.7 | | | | 2,170.8 | |
Due after three years | | | 68.6 | | | | 68.7 | |
| | | | | | | | |
| | $ | 2,620.9 | | | $ | 2,623.2 | |
| | | | | | | | |
The gross unrealized losses and fair value of the Company’s investments with unrealized losses that are not deemed to be other-than-temporarily impaired, aggregated by investment category and length of time that the individual securities have been in a continuous unrealized loss position, at December 31, 2010 and 2009 are as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Less Than 12 months | | | Total | |
| | | Number of
Investments | | | Estimated
Fair
Value | | | Gross
Unrealized
Losses | | | Number of
Investments | | | Estimated
Fair
Value | | | Gross
Unrealized
Losses | |
At December 31, 2010 | | | | | | | | | | | | | | | | | | | | | | | | |
Available-for-sale marketable securities: | | | | | | | | | | | | | | | | | | | | | | | | |
Corporate and asset backed debt securities | | | 216 | | | $ | 752.9 | | | $ | 2.1 | | | | 216 | | | $ | 752.9 | | | $ | 2.1 | |
Foreign government debt securities | | | 42 | | | | 224.0 | | | | 0.5 | | | | 42 | | | | 224.0 | | | | 0.5 | |
U.S. agency debt securities | | | 32 | | | | 99.1 | | | | 0.2 | | | | 32 | | | | 99.1 | | | | 0.2 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | 290 | | | $ | 1,076.0 | | | $ | 2.8 | | | | 290 | | | $ | 1,076.0 | | | $ | 2.8 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| | | Less Than 12 months | | | Total | |
| | | Number of
Investments | | | Estimated
Fair
Value | | | Gross
Unrealized
Losses | | | Number of
Investments | | | Estimated
Fair
Value | | | Gross
Unrealized
Losses | |
At December 31, 2009 | | | | | | | | | | | | | | | | | | | | | | | | |
Available-for-sale marketable securities: | | | | | | | | | | | | | | | | | | | | | | | | |
Corporate and asset backed debt securities | | | 102 | | | $ | 380.8 | | | $ | 1.0 | | | | 102 | | | $ | 380.8 | | | $ | 1.0 | |
Foreign government debt securities | | | 34 | | | | 380.2 | | | | 1.0 | | | | 34 | | | | 380.2 | | | | 1.0 | |
U.S. agency debt securities | | | 27 | | | | 54.3 | | | | 0.1 | | | | 27 | | | | 54.3 | | | | 0.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | 163 | | | $ | 815.3 | | | $ | 2.1 | | | | 163 | | | $ | 815.3 | | | $ | 2.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The unrealized losses on the Company’s investments in corporate and asset backed and U.S. agency debt securities were primarily caused by increases in interest yields as a result of continued challenging conditions in the global credit markets. While many of these investments have been downgraded by rating agencies since their initial purchase, less than 1% of the Company’s investments in corporate and asset-backed debt securities had a credit quality rating of less than single A (per Standard & Poor’s or Fitch). Because the Company does not intend to sell the investments and it is not more likely than not that the Company will be required to sell the investments before recovery of their amortized cost basis, which may be maturity, the Company does not consider those investments to be other-than-temporarily impaired at December 31, 2010.
The unrealized losses on the Company’s investments in foreign government debt securities were also caused by interest rate increases. Because the decline in market value is attributable to changes in interest rates and
49
because the Company does not intend to sell the investments and it is not more likely than not that the Company will be required to sell the investments before recovery of their amortized cost basis, which may be maturity, the Company does not consider those investments to be other-than-temporarily impaired at December 31, 2010.
Pursuant to the Company’s investment policy, all individual marketable security investments must have a minimum credit quality of single A (per Standard & Poor’s or Fitch) or A2 (per Moody’s Corporation) at the time of acquisition, while the overall portfolio of marketable securities must maintain a minimum average credit quality of double A (per Standard & Poor’s or Fitch) or Aa (per Moody’s Corporation). In the event of a rating downgrade below the minimum credit quality subsequent to purchase, the marketable security investment is evaluated to determine the appropriate action to take to minimize the overall risk to the Company’s marketable security investment portfolio. As of December 31, 2010, only 1% of the Company’s investments in marketable securities had a credit quality rating of less than single A (per Standard & Poor’s or Fitch) and A2 (per Moody’s Corporation). As of December 31, 2010, only 1% of the Company’s investments in marketable securities were held in asset backed debt securities. The majority of the Company’s asset backed debt securities relates to investments in U.S. agency-issued mortgage backed securities, where the recovery of the full amount by the investor is guaranteed by the issuing Federal agency.
Interest and marketable securities income, which is included in other income (expense), totaled $48.7 million in 2010, $53.9 million in 2009 and $97.7 million in 2008.
NOTE 3
DERIVATIVE INSTRUMENTS AND HEDGING STRATEGIES
The Company follows the provisions of theDerivatives and Hedging Topic of the FASB Codification, which requires the Company to recognize all derivatives on the Consolidated Balance Sheets at fair value.
The Company enters into forward currency exchange contracts to mitigate the impact of currency fluctuations on transactions denominated in nonfunctional currencies, thereby limiting risk to the Company that would otherwise result from changes in exchange rates. These currency exposures principally relate to intercompany receivables and payables arising from intercompany purchases of manufactured products as well as, in 2009, intercompany loans associated with the repatriation of foreign earnings. The duration of the forward currency exchange contracts correspond to the anticipated period the intercompany receivables and payables remain outstanding. The Company does not designate these contracts as hedges; therefore, all forward currency exchange contracts are recorded at their fair value each period, with resulting gains and losses included in other income (expense) in the Consolidated Statements of Earnings as an offset to the gains and losses recognized on the intercompany receivables and payables. For the twelve months ended December 31, 2010, 2009 and 2008, recognized foreign currency transaction gains (losses) included in other income (expense) in the Consolidated Statements of Earnings were $7.0 million, ($1.1) million and ($6.0) million, respectively.
At December 31, 2010, the Company had outstanding forward currency exchange contracts to purchase $1,011.9 million and sell $366.9 million of various currencies (principally U.S. dollars and euros) with original maturities ranging from 7 to 99 days. The maximum length of time over which the Company is limiting its exposure to the reduction in value of nonfunctional receivables and payables through foreign currency exchange contracts is through March 31, 2011. At December 31, 2009, the Company had outstanding forward currency exchange contracts to purchase $2,041.1 million and sell $280.5 million of various currencies (principally U.S. dollars and euros) with maturities ranging from 4 to 106 days.
At December 31, 2010, the fair value carrying amount of the Company’s forward currency exchange contracts asset and liabilities were $2.4 million and $1.1 million, respectively, and were included as a component of prepaid expenses and other current assets and accrued expenses and other liabilities, respectively, in the Consolidated Balance Sheets. The estimated fair value of forward currency exchange contracts represents the measurement of the contracts at month-end spot rates as adjusted by current forward points. The Company is exposed to credit loss in the event of nonperformance by counterparties on its outstanding forward currency exchange contracts but does not anticipate nonperformance by any of its counterparties.
50
NOTE 4
INVENTORIES
Inventories are summarized as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Finished goods | | $ | 834.3 | | | $ | 730.4 | |
Work-in-process | | | 65.4 | | | | 84.0 | |
Raw materials | | | 169.4 | | | | 140.1 | |
| | | | | | | | |
FIFO cost | | | 1,069.1 | | | | 954.5 | |
Less LIFO reserve | | | (12.3 | ) | | | (11.5 | ) |
| | | | | | | | |
| | $ | 1,056.8 | | | $ | 943.0 | |
| | | | | | | | |
NOTE 5
ACQUISITIONS
Business and product line acquisitions completed in the year ended December 31, 2010 for $265.4 million included the acquisition of assets used to produce the Sonopet Ultrasonic Aspirator control consoles, handpieces and accessories from Mutoh Co., Ltd. and Synergetics USA, Inc., the acquisition of Gaymar Industries, Inc. and the acquisition of the bioimplantable porous polyethylene (PPE) product line and related assets from Porex Surgical, Inc. for use primarily in reconstructive surgery of the head and face. The Sonopet Ultrasonic Aspirator is used in the fields of neurosurgery as well as for orthopedic, general, laparoscopic and plastic surgeries. Gaymar Industries specializes in support surfaces and pressure ulcer management solutions as well as the temperature management segment of the healthcare industry.
These acquisitions, which enhance the Company’s product offerings within its Orthopaedic Implants and MedSurg Equipment segments, did not have a material effect on the Company’s consolidated net sales or operating income for the year ended December 31, 2010. Pro forma consolidated results of operations would not differ significantly as a result of these acquisitions. The acquisitions described above were accounted for pursuant to the requirements of theBusiness Combinations Topic of the FASB Codification. The assets acquired and liabilities assumed as a result of the acquisitions were included in the Company’s Consolidated Balance Sheet as of the acquisition dates and did not have a material effect on the Company’s Consolidated Balance Sheet as of those dates. The purchase price for each of the acquisitions described above was primarily allocated to the tangible and identifiable intangible assets acquired and liabilities assumed based on their estimated fair values on the acquisition dates. The fair value assigned to identifiable intangible assets acquired was determined primarily by using the income approach. The excess purchase price over the value of the net tangible and identifiable intangible assets was recorded as goodwill. Purchased identifiable intangible assets are amortized on a straight-line basis over their respective estimated useful lives. The estimated useful lives range between 2 and 11 years.
51
The purchase price allocation in each case was based upon a preliminary valuation, and the Company’s estimates and assumptions are subject to change within the measurement period as valuations are finalized. The table below represents the allocation of the purchase price to the acquired net assets of acquisitions completed in 2010 (in millions):
| | | | |
Accounts receivable | | $ | 13.4 | |
Inventory | | | 17.3 | |
Other current assets | | | 5.0 | |
Identifiable intangible assets: | | | | |
Customer relationship | | | 48.0 | |
Developed technology | | | 26.3 | |
Trademarks | | | 7.9 | |
Other | | | 37.2 | |
Goodwill | | | 135.7 | |
Other assets | | | 29.3 | |
Current liabilities | | | (20.8 | ) |
Noncurrent liabilities | | | (33.9 | ) |
Goodwill associated with the acquisitions completed in 2010 was $135.7 million. The factors that contributed to the recognition of goodwill included securing synergies that are specific to the Company’s business and not available to other market participants, which are expected to increase revenues and profits; acquisition of a talented workforce; and cost savings opportunities.
In 2009 the Company completed several acquisitions including the acquisition of Ascent Healthcare Solutions, Inc. (Ascent), the market leader in the reprocessing and remanufacturing of medical devices in the United States, in an all cash transaction for $525.0 million. The acquisition of Ascent enhanced the Company’s product offerings as well as provided cost savings opportunities to customers in the MedSurg Equipment segment. Ascent’s purchase price valuation was completed in the fourth quarter of 2010 and did not differ from the preliminary valuation. The table below represents the allocation of the purchase price to the acquired net assets of acquisitions completed in 2009 (in millions):
| | | | | | | | | | | | |
| | | Ascent | | | All Other | | | Total | |
Accounts receivable | | $ | 10.6 | | | $ | 0.1 | | | $ | 10.7 | |
Inventory | | | 10.3 | | | | 0.2 | | | | 10.5 | |
Other current assets | | | 6.3 | | | | 2.9 | | | | 9.2 | |
Identifiable intangible assets: | | | | | | | | | | | | |
Customer relationship | | | 221.1 | | | | 9.1 | | | | 230.2 | |
Developed technology | | | 22.5 | | | | 18.5 | | | | 41.0 | |
In-process research and development | | | — | | | | 20.2 | | | | 20.2 | |
Trademarks | | | 5.0 | | | | — | | | | 5.0 | |
Other | | | — | | | | 1.7 | | | | 1.7 | |
Goodwill | | | 329.1 | | | | 58.4 | | | | 387.5 | |
Other assets | | | 22.7 | �� | | | 5.2 | | | | 27.9 | |
Current liabilities | | | (6.3 | ) | | | (2.0 | ) | | | (8.3 | ) |
Contingent consideration | | | — | | | | (45.1 | ) | | | (45.1 | ) |
Noncurrent deferred income tax liabilities | | | (96.7 | ) | | | (18.5 | ) | | | (115.2 | ) |
Other liabilities | | | — | | | | (0.5 | ) | | | (0.5 | ) |
52
In 2004 the Company acquired all of the outstanding stock of SpineCore, Inc. (SpineCore), a developer of artificial lumbar and cervical discs for an upfront payment of $120.0 million in cash plus certain transaction costs. Terms of the transaction also include potential milestone payments of $95 million upon commercialization of the FlexiCore lumbar artificial disc in the United States and $120 million upon commercialization of the CerviCore cervical artificial disc in the United States as well as royalty payments of up to an additional $25 million depending on the level of actual commercial sales of these devices, if any. The potential milestone payments are expected to be capitalized at their fair values as intangible assets at the time of payment, if they become due.
In October 2010 the Company made the decision to withdraw its application with the U.S. Food and Drug Administration (FDA) for the approval of the FlexiCore device. The CerviCore cervical artificial disc remains under development at this time; however, the Company continues to monitor the market, costs and approval process associated with the CerviCore device to determine whether the device will be made commercially available and result in the introduction of new products and additional future sales. In addition, unanticipated issues may arise that could further delay or terminate the development of the CerviCore device prior to regulatory approval or commercialization, which could have an unfavorable impact on the Company’s operating results.
NOTE 6
RESTRUCTURING CHARGES
The Company did not incur restructuring charges in 2010. In 2009 the Company initiated a number of restructuring projects focused on shifting resources to those areas where the Company has the greatest opportunities for growth and streamlining operations to drive operating leverage. In 2008 the Company made decisions to simplify the structure of its Japanese distribution business and to substantially reduce development efforts associated with product technologies purchased in the Sightline Technologies Ltd. acquisition in 2006. The Company recorded restructuring charges in 2009 and 2008 consisting of the following items (in millions):
| | | | | | | | |
| | | 2009 | | | 2008 | |
Agent conversion charges | | $ | 30.3 | | | $ | — | |
Asset impairment charges | | | 18.3 | | | | 22.3 | |
Severance and related costs | | | 12.9 | | | | 8.5 | |
Contractual obligations and other charges | | | 5.5 | | | | 4.1 | |
| | | | | | | | |
Total | | $ | 67.0 | | | $ | 34.9 | |
| | | | | | | | |
2009 Charges:
The $30.3 million agent conversion charges represent costs associated with the termination of certain third-party agent agreements at the Company’s Europe, Middle East, Africa (EMEA) Division. This initiative is intended to provide the Company greater control over its distribution channels as well as improve customer focus and selling efficiency. The $18.3 million asset impairment charges are associated with the Company’s decision to discontinue selling certain products within its Orthopaedic Implants and MedSurg Equipment business segments and relates primarily to identifiable intangible assets. The discontinued product lines are not expected to have a material impact on the Company’s future operating results. The $12.9 million severance and related costs charge represents workforce reduction employment-related severance costs for approximately 120 employees resulting from the Company’s decision to simplify the organization structure at its Biotech, EMEA, Japan and Canada divisions. The $5.5 million contractual obligations and other charges represent costs associated with the termination of various supplier contracts as well as other incidental costs related to the discontinued product lines.
53
2008 Charges:
The $22.3 million asset impairment charges represent the excess of net book value over fair market value for assets to be disposed of by sale, primarily related to sales offices and warehousing and distribution facilities in Japan. The $8.5 million charge represents employment-related severance costs for 84 employees. The $4.1 million contractual obligations and other charges represent costs associated with the termination of various supplier contracts as well as other incidental costs.
The following table provides a rollforward of the remaining liabilities, included within accrued expenses and other liabilities in the Consolidated Balance Sheet, related to the restructuring charges recorded by the Company in 2009 and 2008 (in millions):
| | | | | | | | | | | | |
| | | Agent
Conversions | | | Severance and
related costs | | | Contractual Obligations
and Other Charges | |
Balances at January 1, 2010 | | $ | 5.9 | | | $ | 3.4 | | | $ | 2.5 | |
Payments | | | (5.9 | ) | | | (1.9 | ) | | | (0.8 | ) |
Foreign currency translation effects | | | — | | | | (0.2 | ) | | | (0.3 | ) |
| | | | | | | | | | | | |
Balances at December 31, 2010 | | $ | 0.0 | | | $ | 1.3 | | | $ | 1.4 | |
| | | | | | | | | | | | |
The restructuring projects initiated in 2009 and 2008 are substantially complete. The Company expects the asset disposals to be completed and final severance payments to be made in 2011.
NOTE 7
GOODWILL AND OTHER INTANGIBLE ASSETS
The changes in the net carrying amount of goodwill by segment for the years ended December 31, 2010 and 2009 are as follows (in millions):
| | | | | | | | | | | | |
| | | Orthopaedic
Implants | | | MedSurg
Equipment | | | Total | |
Balance as of January 1, 2009 | | $ | 517.6 | | | $ | 49.9 | | | $ | 567.5 | |
Goodwill acquired | | | 36.3 | | | | 351.2 | | | | 387.5 | |
Foreign currency translation effects and other | | | (1.6 | ) | | | 3.4 | | | | 1.8 | |
| | | | | | | | | | | | |
Balance as of December 31,2009 | | | 552.3 | | | | 404.5 | | | | 956.8 | |
Goodwill acquired | | | 28.8 | | | | 106.9 | | | | 135.7 | |
Foreign currency translation effects and other | | | (19.5 | ) | | | (0.7 | ) | | | (20.2 | ) |
| | | | | | | | | | | | |
Balance as of December 31, 2010 | | $ | 561.6 | | | $ | 510.7 | | | $ | 1,072.3 | |
| | | | | | | | | | | | |
In the fourth quarters of 2010 and 2009, the Company completed the required annual impairment tests of goodwill as prescribed by theIntangibles-Goodwill and Other Topicof the FASB Codification and determined, in all instances, that recorded goodwill was not impaired and that no goodwill write down was necessary.
54
The following is a summary of the Company’s other intangible assets (in millions):
| | | | | | | | | | | | |
| | | Gross
Carrying
Amount | | | Less
Accumulated
Amortization | | | Net
Carrying
Amount | |
At December 31, 2010: | | | | | | | | | | | | |
Amortized intangible assets: | | | | | | | | | | | | |
Developed technology | | $ | 355.4 | | | $ | 160.5 | | | $ | 194.9 | |
Customer relationship | | | 446.3 | | | | 80.6 | | | | 365.7 | |
Patents | | | 233.0 | | | | 158.6 | | | | 74.4 | |
Trademarks | | | 56.0 | | | | 19.7 | | | | 36.3 | |
Other | | | 77.6 | | | | 45.9 | | | | 31.7 | |
| | | | | | | | | | | | |
| | $ | 1,168.3 | | | $ | 465.3 | | | $ | 703.0 | |
| | | | | | | | | | | | |
At December 31, 2009: | | | | | | | | | | | | |
Amortized intangible assets: | | | | | | | | | | | | |
Developed technology | | $ | 316.9 | | | $ | 160.3 | | | $ | 156.6 | |
Customer relationship | | | 410.9 | | | | 63.0 | | | | 347.9 | |
Patents | | | 241.4 | | | | 156.0 | | | | 85.4 | |
Trademarks | | | 37.8 | | | | 19.2 | | | | 18.6 | |
Other | | | 48.7 | | | | 22.5 | | | | 26.2 | |
| | | | | | | | | | | | |
| | $ | 1,055.7 | | | $ | 421.0 | | | $ | 634.7 | |
| | | | | | | | | | | | |
The estimated amortization expense for each of the five succeeding years is as follows (in millions):
| | | | |
2011 | | $ | 57.1 | |
2012 | | $ | 57.1 | |
2013 | | $ | 56.3 | |
2014 | | $ | 54.9 | |
2015 | | $ | 54.6 | |
NOTE 8
LONG-TERM DEBT AND CREDIT FACILITIES
The Company’s long-term debt is summarized as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
3.00% senior unsecured notes, due January 15, 2015 | | $ | 499.6 | | | $ | — | |
4.375% senior unsecured notes, due January 15, 2020 | | | 496.9 | | | | — | |
Other | | | 25.3 | | | | 18.0 | |
| | | | | | | | |
Total debt | | | 1,021.8 | | | | 18.0 | |
Less current maturities | | | (25.3 | ) | | | (18.0 | ) |
| | | | | | | | |
Long-term debt | | $ | 996.5 | | | $ | — | |
| | | | | | | | |
In August 2010 the Company refinanced its credit facility with a new $1,000.0 million Senior Unsecured Revolving Credit Facility due August 2013 (the 2010 Facility). The 2010 Facility replaced the previously outstanding $1,000.0 million Unsecured Credit Facility due in November 2010 (the 2005 Facility). The 2010 Facility includes an increase option permitting the Company to increase the size of the facility up to an additional $500.0 million, a $500.0 million multicurrency sublimit (with no sublimit for euro borrowings), a $100.0 million letter of credit sublimit and other terms, conditions and covenants substantially the same as the 2005 Facility.
55
The 2010 Facility has an annual facility fee ranging from 10 to 45 basis points and bears interest at LIBOR, as defined in the agreement, plus an applicable margin ranging from 65 to 205 basis points, both of which are dependent on the Company’s credit rating. Based on the Company’s current credit ratings, the 2010 Facility has an annual facility fee of 12.5 basis points and an interest margin of 87.5 basis points.
In January 2010 the Company sold $500.0 million of senior unsecured notes due January 15, 2015 (the 2015 Notes) and $500.0 million of senior unsecured notes due January 15, 2020 (the 2020 Notes). The 2015 Notes bear interest at 3.00% per year and, unless previously redeemed, will mature on January 15, 2015. The 2020 Notes bear interest at 4.375% per year and, unless previously redeemed, will mature on January 15, 2020. The Company received net proceeds of $996.1 million, net of an offering discount of $3.9 million. The 2015 Notes and 2020 Notes carry effective interest rates of 3.02% and 4.46%, respectively. The net proceeds from the offering have been and will continue to be available for working capital and other general corporate purposes, including acquisitions, stock repurchases and other business opportunities.
Debt issuance costs of $10.5 million were incurred in connection with the sale of the senior unsecured notes. These costs were capitalized and are being amortized to interest expense over the lives of the related senior unsecured notes. At December 31, 2010, total unamortized debt issuance costs were $9.0 million.
In addition to the senior unsecured notes, the Company had current debt outstanding under various debt instruments totaling $25.3 and $18.0 million at December 31, 2010 and 2009, respectively.
The weighted-average interest rate, excluding required fees, for all borrowings was 3.7% at December 31, 2010. The 2010 Facility requires the Company to comply with certain financial and other covenants. The Company was in compliance with all covenants at December 31, 2010. In addition to the 2010 Facility, the Company has lines of credit, issued by various financial institutions, available to fund the Company’s day-to-day operating needs. At December 31, 2010, the Company had $1,027.5 million of additional borrowing capacity available under all of its existing credit facilities.
The carrying amounts of the Company’s debt approximate their fair values, based on the quoted interest rates for similar types and amounts of borrowing agreements.
Interest expense, including required fees, incurred on outstanding debt and credit facilities, which is included in other income (expense), totaled $53.2 million in 2010, $14.0 million in 2009 and $12.7 million in 2008. Interest paid on debt, including required fees, was $39.2 million in 2010, $5.3 million in 2009 and $5.7 million in 2008.
NOTE 9
CAPITAL STOCK
In December 2010 and 2009 the Company announced that its Board of Directors had authorized the Company to purchase up to $500.0 million and $750.0 million, respectively, of the Company’s common stock from time to time in the open market, in privately negotiated transactions or otherwise. The Company had not made any stock repurchase pursuant to the $500.0 million repurchase program as of December 31, 2010. During 2010, the Company repurchased 8.3 million shares at a total cost of $425.5 million pursuant to the $750.0 million repurchase program.
The Company has 0.5 million authorized shares of $1 par value preferred stock, none of which is outstanding.
56
The Company’s stock based plans include stock options, restricted stock units and employee stock purchases. The following is a summary of the Company’s significant stock compensation plans:
Stock Options
The Company has key employee and director stock option plans under which options are granted at an exercise price not less than the fair market value of the underlying common stock at the date of grant. The options are granted for periods of up to 10 years and become exercisable in varying installments. A summary of stock option activity follows:
| | | | | | | | | | | | | | | | |
| | | Shares
(in millions) | | | Weighted
Average
Exercise Price | | | Weighted-Average
Remaining
Contractual Term
(in years) | | | Aggregate
Intrinsic Value
(in millions) | |
Options outstanding at January 1, 2010 | | | 26.3 | | | $ | 45.69 | | | | | | | | | |
Granted | | | 2.6 | | | | 53.09 | | | | | | | | | |
Exercised | | | (3.2 | ) | | | 30.27 | | | | | | | | | |
Cancelled | | | (0.9 | ) | | | 55.27 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Options outstanding at December 31, 2010 | | | 24.8 | | | $ | 48.06 | | | | 5.7 | | | $ | 201.9 | |
| | | | | | | | | | | | | | | | |
Exercisable at December 31, 2010 | | | 14.6 | | | $ | 45.77 | | | | 4.3 | | | $ | 146.7 | |
Options expected to vest | | | 9.7 | | | $ | 51.84 | | | | 7.7 | | | $ | 51.7 | |
The aggregate intrinsic value, which represents the cumulative difference between the fair market value of the underlying common stock and the option exercise prices, of options exercised during the years ended December 31, 2010, 2009 and 2008 was $72.9 million, $37.5 million and $135.4 million, respectively. Shares reserved for future compensation grants of Stryker common stock were 10.8 million at December 31, 2010 and 14.5 million at December 31, 2009. Exercise prices for options outstanding as of December 31, 2010 ranged from $23.30 to $67.80. At December 31, 2010, there was $106.1 million of unrecognized compensation cost related to nonvested stock options granted under the stock option plans; that cost is expected to be recognized over the following 4.9 years (weighted-average period of 1.5 years).
Restricted Stock Units (RSUs)
The Company grants RSUs to key employees. The fair value of RSUs is determined based on the number of shares granted and the closing quoted price of the Company’s common stock on the day prior to the date of grant less anticipated dividends. RSU generally vest in one-third increments over a three-year period and are settled in stock. A summary of RSU activity follows:
| | | | | | | | |
| | | Shares
(in millions) | | | Weighted
Average
Grant date
Fair value | |
Nonvested RSUs at January 1, 2010 | | | 0.1 | | | $ | 40.45 | |
Granted | | | 0.7 | | | | 51.06 | |
Vested | | | (0.0 | ) | | | 41.43 | |
Cancelled | | | (0.0 | ) | | | 51.09 | |
| | | | | | | | |
Nonvested RSUs at December 31, 2010 | | | 0.8 | | | $ | 49.89 | |
| | | | | | | | |
As of December 31, 2010, there was $25.9 million of unrecognized compensation cost related to nonvested RSUs; that cost is expected to be recognized as expense over the following 2.9 years (weighted-average period of 1.1 years). The weighted average grant date fair value per share of RSUs granted in 2010 and 2009 was $51.06 and $40.45, respectively. The fair value of RSUs vested in 2010 was $0.8 million.
57
Employee Stock Purchase Plans (ESPP)
Full time and part time employees may participate in the Company’s ESPP, provided they meet certain eligibility requirements. Effective January 1, 2009, the purchase price for the Company’s common stock under the terms of the ESPP is defined as 95% of the closing stock price on the last trading day of a purchase. During 2010 and 2009, the Company issued 179,634 and 214,004 shares, respectively, under the ESPP.
NOTE 10
NET EARNINGS PER SHARE
The following table sets forth the computation of basic and diluted net earnings per share (in millions, except per share amounts):
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
Net earnings | | $ | 1,273.4 | | | $ | 1,107.4 | | | $ | 1,147.8 | |
Weighted-average shares outstanding for basic net earnings per share | | | 396.4 | | | | 397.4 | | | | 408.1 | |
Effect of dilutive employee stock options | | | 3.1 | | | | 2.0 | | | | 5.5 | |
| | | | | | | | | | | | |
Adjusted weighted-average shares outstanding for diluted net earnings per share | | | 399.5 | | | | 399.4 | | | | 413.6 | |
| | | | | | | | | | | | |
Net earnings per share of common stock: | | | | | | | | | | | | |
Basic | | $ | 3.21 | | | $ | 2.79 | | | $ | 2.81 | |
Diluted | | $ | 3.19 | | | $ | 2.77 | | | $ | 2.78 | |
Options to purchase an average of 7.5 million, 18.1 million and 5.7 million shares of common stock during the years ended December 31, 2010, 2009 and 2008, respectively, were outstanding but were not included in the computation of diluted net earnings per share because the exercise prices of the options were greater than the average market price of common stock for those periods.
58
NOTE 11
RETIREMENT PLANS
Certain of the Company’s subsidiaries have both funded and unfunded defined benefit pension plans covering some or all of their employees. Substantially all of the defined benefit pension plans have projected benefit obligations in excess of plan assets. A summary of the Company’s defined benefit pension plans is as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | 2010 | | | 2009 | |
Change in projected benefit obligations: | | | | | | | | |
Projected benefit obligations at beginning of year | | $ | 262.4 | | | $ | 252.1 | |
Service cost | | | 16.2 | | | | 15.8 | |
Interest cost | | | 12.3 | | | | 11.5 | |
Foreign exchange impact | | | (2.2 | ) | | | 3.7 | |
Employee contributions | | | 3.6 | | | | 3.4 | |
Actuarial gains | | | 25.9 | | | | (9.8 | ) |
Benefits paid | | | (10.6 | ) | | | (14.3 | ) |
| | | | | | | | |
Projected benefit obligations at end of year | | | 307.6 | | | | 262.4 | |
| | |
Change in plan assets: | | | | | | | | |
Fair value of plan assets at beginning of year | | | 177.3 | | | | 150.5 | |
Actual return | | | 8.3 | | | | 13.2 | |
Employer contributions | | | 18.2 | | | | 20.4 | |
Employee contributions | | | 3.6 | | | | 3.4 | |
Foreign exchange impact | | | 1.6 | | | | 3.2 | |
Benefits paid | | | (9.1 | ) | | | (13.4 | ) |
| | | | | | | | |
Fair value of plan assets at end of year | | | 199.9 | | | | 177.3 | |
| | | | | | | | |
Funded status | | $ | (107.7 | ) | | $ | (85.1 | ) |
| | | | | | | | |
The weighted-average discount rate used in the determination of the projected benefit obligations was 4.2% as of December 31, 2010 and 4.9% as of December 31, 2009.
The components of the amounts recognized in the Consolidated Balance Sheets are as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Noncurrent assets—Other | | $ | — | | | $ | 1.7 | |
Current liabilities—Accrued compensation | | | (1.0 | ) | | | (1.0 | ) |
Noncurrent liabilities—Other liabilities | | | (106.7 | ) | | | (85.8 | ) |
| | | | | | | | |
| | $ | (107.7 | ) | | $ | (85.1 | ) |
| | | | | | | | |
The components of the amounts recognized in accumulated other comprehensive gain (loss), before the effect of income taxes, are as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Unrecognized net actuarial loss | | $ | (60.6 | ) | | $ | (24.4 | ) |
Unrecognized prior service cost | | | (0.1 | ) | | | (0.1 | ) |
Unrecognized transition amount | | | 0.2 | | | | (0.5 | ) |
| | | | | | | | |
| | $ | (60.5 | ) | | $ | (25.0 | ) |
| | | | | | | | |
59
The accumulated benefit obligations for all of the defined benefit pension plans were $292.7 million and $238.2 million as of December 31, 2010 and 2009, respectively. Pension plans with an accumulated benefit obligation in excess of plan assets had projected benefit obligations, accumulated benefit obligations and fair value of plan assets of $288.3 million, $280.8 million and $186.4 million, respectively, as of December 31, 2010 and $248.9 million, $229.8 million and $165.0 million, respectively, as of December 31, 2009.
The components of net periodic benefit cost and other changes in plan assets and benefit obligations recognized in other comprehensive gain (loss) before the effect of income taxes are as follows (in millions):
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
Net periodic benefit cost: | | | | | | | | | | | | |
Service cost | | $ | (16.2 | ) | | $ | (15.8 | ) | | $ | (15.8 | ) |
Interest cost | | | (12.3 | ) | | | (11.5 | ) | | | (11.7 | ) |
Expected return on plan assets | | | 9.4 | | | | 8.4 | | | | 11.1 | |
Amortization of prior service cost and transition amount | | | — | | | | (0.1 | ) | | | (0.1 | ) |
Recognized actuarial loss | | | (0.8 | ) | | | (2.2 | ) | | | (0.2 | ) |
| | | | | | | | | | | | |
Net periodic benefit cost | | | (19.9 | ) | | | (21.2 | ) | | | (16.7 | ) |
| | | |
Other changes in plan assets and benefit obligations, recognized in other comprehensive gain (loss): | | | | | | | | | | | | |
Net actuarial gain (loss) | | | (37.0 | ) | | | 22.5 | | | | (36.5 | ) |
Recognized net actuarial loss | | | 0.8 | | | | 2.2 | | | | 0.2 | |
Prior service cost and transition amount | | | 0.7 | | | | 0.2 | | | | 0.3 | |
| | | | | | | | | | | | |
Total recognized in other comprehensive gain (loss) | | | (35.5 | ) | | | 24.9 | | | | (36.0 | ) |
| | | | | | | | | | | | |
Total recognized in net periodic benefit cost and other comprehensive gain (loss) | | $ | (55.4 | ) | | $ | 3.7 | | | $ | (52.7 | ) |
| | | | | | | | | | | | |
Weighted-average assumptions used in the determination of net periodic benefit cost: | | | | | | | | | | | | |
Discount rate | | | 4.9% | | | | 4.7% | | | | 4.7% | |
Expected return on plan assets | | | 5.2% | | | | 5.8% | | | | 5.5% | |
Rate of compensation increase | | | 2.8% | | | | 2.8% | | | | 2.9% | |
The estimated net actuarial loss for the defined benefit pension plans to be recognized from accumulated other comprehensive gain (loss) into net periodic benefit cost in the year ended December 31, 2011 is ($1.9) million. The Company estimates that an immaterial amount of amortization of prior service cost and transition amount for the defined benefit pension plans will be recognized from accumulated other comprehensive gain (loss) into net periodic benefit cost in the year ended December 31, 2011.
The Company has assumed an average long-term expected return on defined benefit plan assets of 5.2% as of December 31, 2010. The expected return is determined by applying the target allocation in each asset category of plan investments to the anticipated return for each asset category based on historical and projected returns.
The weighted-average allocation of plan assets by asset category is as follows:
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Equity securities | | | 39 | % | | | 57 | % |
Debt securities | | | 46 | | | | 26 | |
Other | | | 15 | | | | 17 | |
| | | | | | | | |
| | | 100 | % | | | 100 | % |
| | | | | | | | |
60
The investment strategy for the Company’s defined benefit pension plans is both to meet the liabilities of the plans as they fall due and to maximize the return on invested assets within appropriate risk tolerances.
Reflected below are weighted-average target investment allocation ranges for the plans at December 31, 2010:
| | | | | | | | |
| | | Low | | | High | |
Equity securities | | | 34 | % | | | 45 | % |
Debt securities | | | 46 | | | | 59 | |
Other | | | 3 | | | | 19 | |
The following table summarizes the valuation of the Company’s pension plan assets by pricing categories at December 31, 2010 and 2009 (in millions):
| | | | | | | | | | | | | | | | |
| | | Total | | | Quoted Prices
in Active Markets
(Level 1) | | | Prices with
Other
Observable
Inputs
(Level 2) | | | Prices with
Unobservable
Inputs
(Level 3) | |
At December 31, 2010 | | | | | | | | | | | | | | | | |
Assets: | | | | | | | | | | | | | | | | |
Cash and cash equivalent | | $ | 4.2 | | | $ | 4.2 | | | $ | — | | | $ | — | |
Equity securities: | | | | | | | | | | | | | | | | |
U.S companies | | | 57.6 | | | | 57.6 | | | | — | | | | — | |
International companies | | | 20.6 | | | | 20.6 | | | | — | | | | — | |
Corporate debt securities | | | 92.8 | | | | 91.3 | | | | 1.5 | | | | — | |
Other | | | 24.7 | | | | 6.4 | | | | — | | | | 18.3 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 199.9 | | | $ | 180.1 | | | $ | 1.5 | | | $ | 18.3 | |
| | | | | | | | | | | | | | | | |
At December 31, 2009 | | | | | | | | | | | | | | | | |
Assets: | | | | | | | | | | | | | | | | |
Cash and cash equivalent | | $ | 12.2 | | | $ | 8.5 | | | $ | 3.7 | | | $ | — | |
Equity securities: | | | | | | | | | | | | | | | | |
U.S companies | | | 34.8 | | | | 23.4 | | | | 11.4 | | | | — | |
International companies | | | 66.2 | | | | 31.7 | | | | 34.5 | | | | — | |
Mutual funds | | | 28.3 | | | | 28.3 | | | | — | | | | — | |
U.S Treasury securities | | | 12.4 | | | | — | | | | 12.4 | | | | — | |
Corporate debt securities | | | 5.7 | | | | 5.7 | | | | — | | | | — | |
Other | | | 17.7 | | | | 1.5 | | | | — | | | | 16.2 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 177.3 | | | $ | 99.1 | | | $ | 62.0 | | | $ | 16.2 | |
| | | | | | | | | | | | | | | | |
The Company’s Level 3 pension plan assets consist primarily of guaranteed investment contracts with insurance companies. The insurance contracts guarantee the Company principal repayment and a fixed rate of return. The Company’s valuation of its Level 3 assets is based on third party actuarial valuations which are an estimation of the surrender value of the guaranteed investment contract between the Company and the insurance company. The surrender value equals the actuarial value of the notional investments underlying the guaranteed investment contract, using the actuarial assumptions as stated in the guaranteed investment contract.
61
The following table presents a rollforward of pension plan assets measured at fair value on a recurring basis using unobservable inputs (Level 3) at December 31, 2010 and 2009 (in millions):
| | | | | | | | |
| | | 2010 | | | 2009 | |
Balance as of January 1 | | $ | 16.2 | | | $ | 14.9 | |
Actual return on plan assets held at the reporting date | | | (0.1 | ) | | | (0.3 | ) |
Purchases, sales, and settlements | | | 2.2 | | | | 1.6 | |
| | | | | | | | |
Balance as of December 31 | | $ | 18.3 | | | $ | 16.2 | |
| | | | | | | | |
The Company anticipates contributing $18.7 million to its defined benefit pension plans in 2011.
The following estimated future benefit payments, which reflect expected future service as appropriate, are expected to be paid in the years indicated (in millions):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2011 | | | 2012 | | | 2013 | | | 2014 | | | 2015 | | | 2016-2020 | |
Expected benefits payments | | $ | 10.4 | | | $ | 10.9 | | | $ | 11.2 | | | $ | 11.2 | | | $ | 11.2 | | | $ | 65.1 | |
Retirement plan expense under the Company’s defined contribution retirement plans totaled $101.6 million in 2010, $100.7 million in 2009 and $98.6 million in 2008. A portion of the Company’s retirement plan expense was funded with Stryker common stock totaling $11.3 million in 2010, $10.8 million in 2009 and $9.3 million in 2008. The use of Stryker common stock represents a noncash operating activity that is not reflected in the Consolidated Statements of Cash Flows. The amount of Stryker common stock held by the Company’s defined contribution retirement plans totaled $96.1 million (approximately 1.8 million shares) and $82.6 million (approximately 1.6 million shares) as of December 31, 2010 and 2009, respectively. The value of Stryker common stock as a percentage of total defined contribution retirement plan assets was 10% and 11% as of December 31, 2010 and 2009, respectively.
NOTE 12
INCOME TAXES
Earnings before income taxes consist of the following (in millions):
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
U.S. operations | | $ | 566.0 | | | $ | 709.5 | | | $ | 738.1 | |
Foreign operations | | | 1,163.6 | | | | 914.4 | | | | 842.1 | |
| | | | | | | | | | | | |
| | $ | 1,729.6 | | | $ | 1,623.9 | | | $ | 1,580.2 | |
| | | | | | | | | | | | |
The components of the provision for income taxes follow (in millions):
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
Current income tax expense | | | | | | | | | | | | |
Federal | | $ | 307.9 | | | $ | 382.4 | | | $ | 262.3 | |
State | | | 21.2 | | | | 26.6 | | | | 48.1 | |
Foreign | | | 230.9 | | | | 180.2 | | | | 139.6 | |
| | | | | | | | | | | | |
Total current income tax expense | | | 560.0 | | | | 589.2 | | | | 450.0 | |
Deferred income tax expense (benefit) | | | | | | | | | | | | |
Federal | | | (80.7 | ) | | | (17.3 | ) | | | (7.6 | ) |
State | | | (2.3 | ) | | | (2.2 | ) | | | 1.5 | |
Foreign | | | (20.8 | ) | | | (53.2 | ) | | | (11.5 | ) |
| | | | | | | | | | | | |
Total deferred income tax benefit | | | (103.8 | ) | | | (72.7 | ) | | | (17.6 | ) |
| | | | | | | | | | | | |
Total income tax expense | | $ | 456.2 | | | $ | 516.5 | | | $ | 432.4 | |
| | | | | | | | | | | | |
62
A reconciliation of the U.S. statutory income tax rate to the Company’s effective income tax rate from continuing operations follows:
| | | | | | | | | | | | |
| | | 2010 | | | 2009 | | | 2008 | |
U.S. statutory income tax rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
Add (deduct): | | | | | | | | | | | | |
State income taxes, less effect of federal deduction | | | 0.9 | | | | 2.6 | | | | 1.5 | |
International operations | | | (12.1 | ) | | | (13.8 | ) | | | (11.5 | ) |
Repatriation of foreign earnings | | | (0.4 | ) | | | 4.1 | | | | — | |
Other | | | 3.0 | | | | 3.9 | | | | 2.4 | |
| | | | | | | | | | | | |
| | | 26.4 | % | | | 31.8 | % | | | 27.4 | % |
| | | | | | | | | | | | |
Deferred income taxes reflect the net income tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Valuation allowances are recorded to reduce deferred income tax assets when it is more likely than not that an income tax benefit will not be realized.
The income tax effects of significant temporary differences, which comprise the Company’s deferred income tax assets and liabilities, are as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Deferred income tax assets: | | | | | | | | |
Inventories | | $ | 501.5 | | | $ | 471.7 | |
Other accrued expenses | | | 134.5 | | | | 130.7 | |
Depreciation and amortization | | | 30.7 | | | | 17.3 | |
State income taxes | | | 27.7 | | | | 29.0 | |
Share-based compensation | | | 109.0 | | | | 98.5 | |
Net operating loss carryforwards | | | 47.2 | | | | 59.7 | |
Other | | | 77.0 | | | | 81.8 | |
| | | | | | | | |
Total deferred income tax assets | | | 927.6 | | | | 888.7 | |
Less valuation allowances | | | (26.1 | ) | | | (27.6 | ) |
| | | | | | | | |
Total deferred income tax assets after valuation allowances | | | 901.5 | | | | 861.1 | |
| | |
Deferred income tax liabilities: | | | | | | | | |
Depreciation and amortization | | | (330.2 | ) | | | (347.4 | ) |
Other | | | (40.7 | ) | | | (59.3 | ) |
| | | | | | | | |
Total deferred income tax liabilities | | | (370.9 | ) | | | (406.7 | ) |
| | | | | | | | |
Total deferred income tax assets | | $ | 530.6 | | | $ | 454.4 | |
| | | | | | | | |
Reported as: | | | | | | | | |
Current assets—Deferred income taxes | | $ | 653.2 | | | $ | 602.2 | |
Noncurrent assets—Deferred income taxes | | | 248.3 | | | | 258.9 | |
Current liabilities—Accrued expenses and other liabilities | | | (26.9 | ) | | | (24.1 | ) |
Noncurrent liabilities—Other liabilities | | | (344.0 | ) | | | (382.6 | ) |
| | | | | | | | |
| | $ | 530.6 | | | $ | 454.4 | |
| | | | | | | | |
63
Net operating loss carryforwards totaling $160.9 million at December 31, 2010 are available to reduce future taxable earnings of certain domestic and foreign subsidiaries. U.S. loss carryforwards of $36.9 million expire between 2011 and 2026. The majority of the Company’s foreign loss carryforwards of $124.0 million do not expire. In addition, $102.1 million of the foreign loss carryforward is subject to a full valuation allowance.
During the fourth quarter of 2009, the Company repatriated $787.0 million of foreign earnings to the United States. The Company recorded tax expense of $67.1 million in 2009 and a tax benefit of $7.4 million in 2010 to recognize the tax liability and benefit associated with the repatriation. The repatriated cash was used to fund the acquisition of Ascent and previously announced initiatives, including the share repurchase authorization. Pursuant to the requirements of theAccounting for Income Taxes-Special Areas(included in the Income Taxes Topic of the FASB Codification), no provision has been made for U.S. federal and state income taxes or foreign income taxes that may result from future remittances of the undistributed earnings of foreign subsidiaries that are determined to be reinvested overseas indefinitely ($4,219.1 million at December 31, 2010). Determination of the amount of any unrecognized deferred income tax liability on these unremitted earnings is not practicable.
Total income taxes paid, net of refunds received, were $579.4 million in 2010, $406.4 million in 2009 and $478.5 million in 2008.
The changes in the amounts recorded for uncertain income tax positions are as follows (in millions):
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Balance at beginning of year | | $ | 292.7 | | | $ | 277.1 | |
Increases related to current year income tax positions | | | 32.5 | | | | 41.0 | |
Increases related to prior year income tax positions | | | 65.9 | | | | 64.7 | |
| | |
Decreases related to prior year income tax positions: | | | | | | | | |
Settlements and resolutions of income tax audits | | | (15.0 | ) | | | (66.9 | ) |
Statute of limitations expirations | | | (4.9 | ) | | | (3.7 | ) |
Other | | | (5.6 | ) | | | (19.5 | ) |
| | | | | | | | |
Balance at end of year | | $ | 365.6 | | | $ | 292.7 | |
| | | | | | | | |
Reported as: | | | | | | | | |
Current liabilities—Income taxes | | $ | 13.0 | | | $ | 0.5 | |
Noncurrent liabilities—Other liabilities | | | 352.6 | | | | 292.2 | |
| | | | | | | | |
| | $ | 365.6 | | | $ | 292.7 | |
| | | | | | | | |
The Company’s income tax expense could be reduced by $347.0 million and $274.3 million at December 31, 2010 and December 31, 2009, respectively, upon favorable resolution of these uncertain income tax positions.
At December 31, 2010, income tax authorities in several income tax jurisdictions both inside and outside the United States were conducting routine audits of the Company’s income tax returns filed in prior years. These audits are generally designed to determine if individual income tax authorities are in agreement with the Company’s interpretations of complex income tax regulations regarding the allocation of income to the various income tax jurisdictions. With few exceptions, the Company is no longer subject to audits by income tax authorities for tax years prior to 2003. Income tax years subsequent to 2002 are open to examination in many of the income tax jurisdictions in which the Company operates. In July 2010 the Company received an income tax assessment related to an income tax position the Company has taken for the allocation of profits within Europe in previously filed 2006 and 2007 income tax returns. The Company believes it followed the applicable tax laws and regulations and will vigorously defend this income tax position. If the Company were to ultimately lose with
64
respect to this income tax position it could have a material unfavorable impact on the Company’s income tax expense, results of operations and cash flows in future periods. In April 2009 the U.S. Internal Revenue Service (IRS) proposed adjustments to the Company’s previously filed 2003, 2004 and 2005 income tax returns related to income tax positions the Company has taken for its cost sharing arrangements with two wholly owned entities operating in Ireland. The Company believes it followed the applicable tax law and Treasury regulations and is vigorously defending these income tax positions. Ultimate resolution with respect to these proposed adjustments could have a material impact on the Company’s income tax expense, results of operations and cash flows in future periods.
Interest expense and penalties included in other income (expense) were $24.3 million, $9.2 million and $17.8 million for the year ended December 31, 2010, 2009 and 2008 respectively. Accrued interest and penalties included in accrued expenses and other liabilities were $83.0 million and $58.7 million at December 31, 2010 and December 31, 2009, respectively.
It is reasonably possible that the amount of unrecognized tax benefits will significantly change due to one or more of the following events in the next twelve months: expiring statutes, audit activity, tax payments, competent authority proceedings related to transfer pricing, or final decisions in matters that are the subject of controversy in various taxing jurisdictions in which we operate, including inventory transfer pricing and cost sharing, product royalty and foreign branch arrangements. The Company is not able to reasonably estimate the amount or the future periods in which changes in unrecognized tax benefits may be required.
NOTE 13
SEGMENT AND GEOGRAPHIC DATA
The Company segregates its operations into two reportable business segments: Orthopaedic Implants and MedSurg Equipment. The Orthopaedic Implants segment includes orthopaedic reconstructive (hip and knee), trauma and spinal implant systems and other related products. The MedSurg Equipment segment includes surgical equipment and surgical navigation systems; endoscopic and communications systems; patient handling and emergency medical equipment; as well as other medical device products used in a variety of medical specialties. The Other category includes corporate administration, interest expense, interest and marketable securities income and share-based compensation, which includes compensation related to both employee and director stock option and restricted stock grants.
The Company’s reportable segments are business units that offer different products and services and are managed separately because each business requires different manufacturing, technology and marketing strategies. The accounting policies of the segments are the same as those described in the summary of significant accounting policies in Note 1 to the Consolidated Financial Statements. The Company measures the financial results of its reportable segments using an internal performance measure that excludes the impairment of property, plant and equipment and gain on sale of certain assets recorded in 2010, the income tax adjustment associated with the repatriation of foreign earnings recorded in 2010 and 2009, the patent litigation gain recorded in 2009 and the restructuring charges recorded in 2009 and 2008. Identifiable assets are those assets used exclusively in the operations of each business segment or allocated when used jointly. Corporate assets are principally cash and cash equivalents, marketable securities and property, plant and equipment.
65
Sales and other financial information by business segment follows (in millions):
| | | | | | | | | | | | | | | | |
| | | Orthopaedic
Implants | | | MedSurg
Equipment | | | Other | | | Total | |
Year ended December 31, 2010: | | | | | | | | | | | | | | | | |
Net sales | | $ | 4,308.4 | | | $ | 3,011.6 | | | $ | — | | | $ | 7,320.0 | |
Interest and marketable securities income | | | — | | | | — | | | | 48.7 | | | | 48.7 | |
Interest expense | | | — | | | | — | | | | (77.5 | ) | | | (77.5 | ) |
Depreciation and amortization expense | | | 312.9 | | | | 87.5 | | | | 9.8 | | | | 410.2 | |
Income taxes (credit) | | | 391.3 | | | | 179.3 | | | | (71.0 | ) | | | 499.6 | |
Segment net earnings (loss) | | | 963.3 | | | | 524.0 | | | | (158.1 | ) | | | 1,329.2 | |
Less property, plant and equipment impairment, net of income tax benefit | | | | | | | | | | | | | | | (76.6 | ) |
Add income taxes on repatriation of foreign earnings | | | | | | | | | | | | | | | 7.4 | |
Add gain on sale of property, plant and equipment, net of income tax expense | | | | | | | | | | | | | | | 13.4 | |
| | | | | | | | | | | | | | | | |
Net earnings | | | | | | | | | | | | | | | 1,273.4 | |
Total assets | | | 3,955.3 | | | | 2,280.2 | | | | 4,659.6 | | | | 10,895.1 | |
Purchases of property, plant and equipment | | | 120.9 | | | | 48.9 | | | | 12.3 | | | | 182.1 | |
| | | | |
Year ended December 31, 2009: | | | | | | | | | | | | | | | | |
Net sales | | $ | 4,119.7 | | | $ | 2,603.4 | | | $ | — | | | $ | 6,723.1 | |
Interest and marketable securities income | | | — | | | | — | | | | 53.9 | | | | 53.9 | |
Interest expense | | | — | | | | — | | | | (23.2 | ) | | | (23.2 | ) |
Depreciation and amortization expense | | | 305.7 | | | | 68.8 | | | | 10.8 | | | | 385.3 | |
Income taxes (credit) | | | 327.0 | | | | 159.5 | | | | (38.2 | ) | | | 448.3 | |
Segment net earnings (loss) | | | 802.0 | | | | 462.9 | | | | (84.9 | ) | | | 1,180.0 | |
Less restructuring charges, net of income tax benefits | | | | | | | | | | | | | | | (48.4 | ) |
Less income taxes on repatriation of foreign earnings | | | | | | | | | | | | | | | (67.1 | ) |
Add patent litigation gain, net of income tax expense | | | | | | | | | | | | | | | 42.9 | |
| | | | | | | | | | | | | | | | |
Net earnings | | | | | | | | | | | | | | | 1,107.4 | |
Total assets | | | 3,830.7 | | | | 1,924.2 | | | | 3,316.4 | | | | 9,071.3 | |
Purchases of property, plant and equipment | | | 83.0 | | | | 43.4 | | | | 4.9 | | | | 131.3 | |
| | | | |
Year ended December 31, 2008: | | | | | | | | | | | | | | | | |
Net sales | | $ | 3,967.5 | | | $ | 2,750.7 | | | $ | — | | | | 6,718.2 | |
Interest and marketable securities income | | | — | | | | — | | | | 97.7 | | | | 97.7 | |
Interest expense | | | — | | | | — | | | | (30.5 | ) | | | (30.5 | ) |
Depreciation and amortization expense | | | 308.1 | | | | 72.2 | | | | 7.3 | | | | 387.6 | |
Income taxes (credit) | | | 310.6 | | | | 162.8 | | | | (27.8 | ) | | | 445.6 | |
Segment net earnings (loss) | | | 760.4 | | | | 471.2 | | | | (62.1 | ) | | | 1,169.5 | |
Less restructuring charges, net of income tax benefits | | | | | | | | | | | | | | | (21.7 | ) |
| | | | | | | | | | | | | | | | |
Net earnings | | | | | | | | | | | | | | | 1,147.8 | |
Total assets | | | 3,693.5 | | | | 1,319.6 | | | | 2,590.2 | | | | 7,603.3 | |
Purchases of property, plant and equipment | | | 95.3 | | | | 52.1 | | | | 7.8 | | | | 155.2 | |
66
The countries in which the Company has local revenue-generating operations have been combined into the following geographic areas: the United States (including Puerto Rico); Europe, Middle East, Africa (EMEA); and other foreign countries, which are comprised of Japan, Canada, the Pacific region and the Latin America region. Sales are attributable to a geographic area based upon the customer’s country of domicile. Long-lived assets include net property, plant and equipment, goodwill and other intangibles. Net property, plant and equipment are based upon physical location of the assets. Geographic information follows (in millions):
| | | | | | | | |
| | | Net Sales | | | Long-Lived
Assets | |
Year ended December 31, 2010: | | | | | | | | |
United States | | $ | 4,792.8 | | | $ | 2,084.4 | |
EMEA | | | 1,227.5 | | | | 752.6 | |
Other foreign countries | | | 1,299.7 | | | | 178.4 | |
| | | | | | | | |
| | $ | 7,320.0 | | | $ | 3,015.4 | |
| | | | | | | | |
Year ended December 31, 2009: | | | | | | | | |
United States | | $ | 4,317.4 | | | $ | 2,031.2 | |
EMEA | | | 1,254.2 | | | | 751.4 | |
Other foreign countries | | | 1,151.5 | | | | 178.6 | |
| | | | | | | | |
| | $ | 6,723.1 | | | $ | 2,961.2 | |
| | | | | | | | |
Year ended December 31, 2008: | | | | | | | | |
United States | | $ | 4,282.2 | | | $ | 1,440.1 | |
EMEA | | | 1,313.3 | | | | 784.1 | |
Other foreign countries | | | 1,122.7 | | | | 187.6 | |
| | | | | | | | |
| | $ | 6,718.2 | | | $ | 2,411.8 | |
| | | | | | | | |
NOTE 14
LEASES
The Company leases various manufacturing, warehousing and distribution facilities, administrative and sales offices as well as equipment under operating leases. Future minimum lease commitments under these leases are as follows (in millions):
| | | | |
2011 | | $ | 48.2 | |
2012 | | | 36.0 | |
2013 | | | 24.8 | |
2014 | | | 17.4 | |
2015 | | | 12.3 | |
Thereafter | | | 10.2 | |
| | | | |
| | $ | 148.9 | |
| | | | |
Rent expense totaled $80.9 million in 2010, $75.1 million in 2009 and $76.0 million in 2008.
NOTE 15
PROPERTY, PLANT AND EQUIPMENT
During the third quarter of 2010, the Company sold its Orthopaedics Implants manufacturing facility based in Caen, France for total consideration of $52.9 million in an all cash transaction and recorded a gain of $13.4 million (net of $10.9 million income tax expense). The transaction also included a 5-year supply agreement with the acquirer in volumes commensurate with the production levels achieved prior to the sale. The supply agreement is contingent, among other things, on the acquirer’s ability to provide products that meet quality standards, and may be terminated by Stryker if such a material breach occurs.
67
NOTE 16
ASSETS HELD FOR SALE
During the fourth quarter of 2010, the Company announced a definitive agreement to sell its OP-1 product family for use in orthopaedic bone applications and its manufacturing facility based in Lebanon, NH. As a result of the announcement, the Company recorded a $76.6 million (net of $46.9 million income tax expense) non cash impairment charge to reflect the reduction of the carrying amount of the associated assets to their fair value. At December 31, 2010 the assets held for sale included in current assets in the Consolidated Balance Sheet consisted of the following (in millions):
| | | | |
Accounts receivable, net | | $ | 1.0 | |
Inventories | | | 24.9 | |
Prepaid expenses and other current assets | | | 0.8 | |
Net property, plant and equipment | | | 29.0 | |
Other assets | | | 6.3 | |
| | | | |
Total | | $ | 62.0 | |
| | | | |
NOTE 17
CONTINGENCIES
In the third quarter of 2010, the Company received separate subpoenas from the U.S. Department of Justice related to (i) the sales, marketing and regulatory matters related to the Stryker PainPump and (ii) sales, marketing and regulatory matters related to the OtisKnee device. The Company is in the process of responding to these subpoenas.
In March 2010 a shareholder’s derivative action complaint against certain current and former Directors and Officers of the Company was filed in the United States District Court for the Western District of Michigan Southern Division. This lawsuit was brought by the Westchester Putnam Counties Heavy and Highway Laborers Local 60 Benefit Funds and Laborers Local 235 Benefit Funds. The complaint alleges claims for breach of fiduciary duties and gross mismanagement in connection with certain product recalls, FDA warning letters, government investigations relating to physician compensation and the criminal proceeding brought against the Company’s Biotech division. The case has been stayed while a Special Committee of the Board of Directors evaluates the claims.
In January 2010 a purported class action lawsuit against the Company was filed in the United States District Court for the Southern District of New York on behalf of those who purchased the Company’s common stock between January 25, 2007 and November 13, 2008, inclusive. The lawsuit seeks remedies under the Securities Exchange Act of 1934. In May 2010 the lawsuit was transferred to the United States District Court for the Western District of Michigan Southern Division. The Company intends to defend itself vigorously.
In 2009 a federal grand jury in the District of Massachusetts returned an indictment charging Stryker Biotech LLC and certain current and former employees of Stryker Biotech with wire fraud, conspiracy to defraud the FDA, distribution of a misbranded device and false statements to the FDA. The Company still hopes to be able to reach a fair and just resolution of this matter. The ultimate resolution of this matter is not reasonably estimable at this time, however, a conviction on the charges described above could result in significant monetary fines. Because Stryker Biotech is not presently involved in the sale of health care products or services (see additional information in Note 18), any conviction on these charges resulting in exclusion from participating in federal and state health care programs would not be expected to have a material effect on Stryker Biotech’s present business operations. Certain former Stryker Biotech employees have pled guilty to charges in connection with this matter.
In 2009 the Company received a warning letter from the FDA related to compliance issues for one of its craniomaxillofacial (CMF) implant products that was previously sold through its CMF distribution facility in
68
Portage, Michigan. In 2007 the Company received two warning letters from the FDA regarding compliance with certain quality system specifications at its reconstructive implant manufacturing facilities: one letter for its facility in Cork, Ireland and another for its facility in Mahwah, New Jersey. In March 2010 the FDA informed the Company that the warning letter related to its Mahwah manufacturing facility had been resolved following a re-inspection in 2009 and additional corrective actions. In May 2010 the FDA informed the Company that the warning letters related to its Cork, Ireland and CMF facilities had been resolved following FDA re-inspection of the Cork, Ireland facility and additional corrective actions at both the Cork and CMF facilities.
In 2007 the Company announced that it reached a resolution with the U.S. Attorney’s office for the District of New Jersey in connection with an investigation relating to “any and all consulting contracts, professional service agreements, or remuneration agreements between Stryker Corporation and any orthopedic surgeon, orthopedic surgeon in training, or medical school graduate using or considering the surgical use of hip or knee joint replacement/reconstruction products manufactured or sold by Stryker Corporation.” The resolution was in the form of a non-prosecution agreement, which included oversight by a federal monitor, for an 18-month period that ended on March 27, 2009. Subsequent to entering into the non-prosecution agreement, the U.S. Department of Health and Human Services, Office of Inspector General (HHS) issued a civil subpoena to the Company in seeking to determine whether the Company violated various laws by paying consulting fees and providing other things of value to orthopedic surgeons and healthcare and educational institutions as inducements to use Stryker’s orthopedic medical devices in procedures paid for in whole or in part by Medicare. The investigation is ongoing and the Company has produced numerous documents and other materials to HHS in response to the subpoena.
In 2007 the Company disclosed that the U.S. Securities and Exchange Commission (SEC) made an informal inquiry of the Company regarding possible violations of the Foreign Corrupt Practices Act in connection with the sale of medical devices in certain foreign countries. Subsequently, in 2008, the Company received a subpoena from the U.S. Department of Justice, Criminal Division, requesting certain documents for the period since January 1, 2000 in connection with the SEC inquiry. The Company is fully cooperating with the U.S. Department of Justice and the SEC regarding these matters.
Pursuant to certain of the Company’s credit and lease agreements, the Company has provided financial guarantees to third parties in the form of indemnification provisions. These provisions indemnify the third parties for costs, including but not limited to adverse judgments in lawsuits and the imposition of additional income taxes due to either a change in the tax law or an adverse interpretation of the tax law. The terms of the guarantees are equal to the terms of the related credit or lease agreements. The Company is not able to calculate the maximum potential amount of future payments it could be required to make under these guarantees, as any potential payment is dependent on the occurrence of future unknown events (e.g., changes in U.S. or foreign tax laws).
NOTE 18
SUBSEQUENT EVENTS
On February 1, 2011, the Company completed its previously announced sale of its OP-1 product family for use in orthopaedic bone applications and its manufacturing facility based in West Lebanon, NH for total consideration of $60.0 million.
On January 3, 2011, the Company completed the previously announced acquisition of assets of the Neurovascular division of Boston Scientific Corporation (Neurovascular) in an all cash transaction for $1.45 billion, with an additional $50.0 million payment to be made upon completion of certain milestones. The acquisition of Neurovascular is expected to substantially enhance the Company’s presence in the neurovascular market, allowing it to offer a comprehensive portfolio of products in both neurosurgical and neurovascular devices. The effect of the Neurovascular acquisition will be included in the Company’s consolidated results of operations prospectively from the date of acquisition.
69
The Company has evaluated subsequent events after December 31, 2010 and concluded that no material transactions occurred subsequent to that date that provided additional evidence about conditions that existed at or after December 31, 2010 that require adjustment to the Consolidated Financial Statements.
Stryker Corporation and Subsidiaries
SUMMARY OF QUARTERLY DATA (UNAUDITED)
(in millions, except per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2010 Quarter Ended | | | 2009 Quarter Ended | |
| | | Mar. 31 | | | June 30 | | | Sept. 30 | | | Dec. 31 | | | Mar. 31 | | | June 30 | | | Sept. 30 | | | Dec. 31 | |
Net sales | | $ | 1,799.1 | | | $ | 1,758.2 | | | $ | 1,767.6 | | | $ | 1,995.1 | | | $ | 1,601.3 | | | $ | 1,634.3 | | | $ | 1,653.3 | | | $ | 1,834.2 | |
Gross profit | | | 1,217.7 | | | | 1,218.9 | | | | 1,226.8 | | | | 1,370.9 | | | | 1,085.8 | | | | 1,098.0 | | | | 1,114.6 | | | | 1,241.0 | |
Earnings before income taxes | | | 445.8 | | | | 442.6 | | | | 461.9 | | | | 379.3 | | | | 386.4 | | | | 400.0 | | | | 314.4 | | | | 523.1 | |
Net earnings | | | 321.7 | | | | 319.0 | | | | 337.7 | | | | 295.0 | | | | 281.1 | | | | 291.3 | | | | 229.0 | | | | 306.0 | |
| | | | | | | | |
Net earnings per share of common stock: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.81 | | | | 0.80 | | | | 0.85 | | | | 0.75 | | | | 0.71 | | | | 0.73 | | | | 0.58 | | | | 0.77 | |
Diluted | | | 0.80 | | | | 0.80 | | | | 0.85 | | | | 0.74 | | | | 0.71 | | | | 0.73 | | | | 0.57 | | | | 0.76 | |
| | | | | | | | |
Market price of common stock: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
High | | | 58.49 | | | | 59.72 | | | | 53.29 | | | | 55.00 | | | | 44.46 | | | | 41.73 | | | | 48.10 | | | | 52.66 | |
Low | | | 49.85 | | | | 48.76 | | | | 42.74 | | | | 48.13 | | | | 30.96 | | | | 32.34 | | | | 37.14 | | | | 42.74 | |
The price quotations reported above were supplied by the New York Stock Exchange.
70
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
Not applicable.
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures—An evaluation of the effectiveness of the Company’s disclosure controls and procedures as of December 31, 2010 was carried out under the supervision and with the participation of the Company’s management, including the President and Chief Executive Officer and the Vice President and Chief Financial Officer (the Certifying Officers). Based on that evaluation, the Certifying Officers concluded that the Company’s disclosure controls and procedures are effective.
Changes in Internal Control Over Financial Reporting—There was no change to the Company’s internal control over financial reporting during the quarter ended December 31, 2010 that materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting—The management of Stryker Corporation is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Stryker Corporation’s internal control system was designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements.
Stryker Corporation’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2010. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission inInternal Control-Integrated Framework. Based on that assessment, management believes that, as of December 31, 2010, the Company’s internal control over financial reporting is effective.
Stryker Corporation’s independent registered public accounting firm, Ernst & Young LLP, has issued an attestation report on the effectiveness of the Company’s internal control over financial reporting. This report appears on the following page.
Other Matters—The Company is in the process of implementing new Enterprise Resource Planning (ERP) systems at certain of its divisions including its Europe division. An ERP system is a fully-integrated set of programs and databases that incorporate order processing, production planning and scheduling, purchasing, accounts receivable and inventory management and accounting. In connection with this ERP system implementation, the Company will update its internal controls over financial reporting, as necessary, to accommodate modifications to its business processes and accounting procedures. The Company does not believe that this ERP system implementation will have an adverse effect on the Company’s internal control over financial reporting.
71
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The Board of Directors and Shareholders of Stryker Corporation:
We have audited Stryker Corporation and subsidiaries’ internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Stryker Corporation and subsidiaries’ management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Stryker Corporation and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Stryker Corporation and subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of earnings, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010 of Stryker Corporation and subsidiaries, and our report dated February 18, 2011 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
Grand Rapids, Michigan
February 18, 2011
72
| ITEM 9B. | OTHER INFORMATION. |
Not applicable.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
Information regarding the directors of the Company and certain corporate governance and other matters appearing under the captions “Information About the Board of Directors and Corporate Governance Matters,” “Proposal 1—Election of Directors,” “Audit Committee” and “Additional Information—Section 16(a) Beneficial Ownership Reporting Compliance” in the 2011 proxy statement is incorporated herein by reference.
Information regarding the executive officers of the Company appears below. All officers are appointed annually. Reported ages are as of January 31, 2011.
Stephen P. MacMillan, age 47, was appointed President and Chief Operating Officer of the Company in June 2003 and Chief Executive Officer as of January 1, 2005. He was also appointed Chairman of the Board on January 1, 2010. Prior to joining the Company, he was most recently Sector Vice President, Global Specialty Operations for Pharmacia Corporation, which he joined in 1999. Prior to Pharmacia, he spent 11 years at Johnson & Johnson (“J&J”), most recently as President of Johnson & Johnson-Merck Consumer Pharmaceuticals, a joint venture between J&J and Merck. Prior to joining J&J, he held various marketing positions at Procter & Gamble.
Lonny J. Carpenter, age 49, was appointed Group President, Global Quality and Operations in September 2009 and was the Group President, Instruments and Medical since November 2008. He had previously been President, Stryker Medical since May 2008 and Vice President and General Manager, Stryker Medical since 2006. After joining the Company in 1989, Mr. Carpenter held various roles of increasing responsibility at Stryker Instruments before being promoted to Vice President, Global Operations, Stryker Instruments in 2004.
Andrew G. Fox-Smith, age 45, was appointed Group President, International in January 2008. He had previously been President, Pacific since 2005, Vice President and General Manager, Stryker Pacific since 2001 and Managing Director, UK/Ireland/South Africa since 1999. Prior to the acquisition of Howmedica in 1998, he held various sales positions with the Howmedica division of Pfizer since 1994.
Curtis E. Hall, age 54, was appointed Vice President and General Counsel of the Company in June 2004, and was also appointed Secretary of the Company in June, 2010. He had previously been General Counsel for the Company since 1994. Prior to joining the Company, he was a partner in the Michigan law firm of Miller, Canfield, Paddock and Stone, an Assistant United States Attorney in Washington, D.C. and an Assistant District Attorney in New York City.
Curt R. Hartman, age 47, was appointed Vice President and Chief Financial Officer in April 2009 and was the Vice President, Finance of the Company since November 2008. He had previously been President, Stryker Global Instruments since 2006 and President, Stryker Instruments since 2003. After joining the Company in 1990, Mr. Hartman held several functional leadership roles at Stryker Instruments before being promoted to Vice President and General Manager, Stryker Instruments in 1999.
Marcia S. Kaminsky, age 52, was appointed Vice President, Communications and Public Affairs in December 2010. Prior to joining the Company, she served as Vice President, Corporate Responsibility for United Airlines, Senior Vice President, Corporate Communications for USG Corporation, Senior Vice President, Public Affairs for Harris Bank/Bank of Montreal and various communication and public affairs roles at Nutrasweet and Quaker Oats.
73
Tony M. McKinney, age 41, was appointed Vice President, Chief Accounting Officer in November 2008. He had previously been the Vice President, Finance, International Group since 2006 and Group Controller, International Group since 2004. After joining the Company in 1995, Mr. McKinney held various roles of increasing responsibility in the Corporate Accounting department before becoming the Director, Finance for the Japan Division in 2002. Prior to joining the Company in 1995, Mr. McKinney was an Audit Senior Accountant with Ernst & Young LLP.
Michael P. Mogul, age 46, was appointed Group President, Orthopaedics in September 2009. He had previously been President, Stryker Orthopaedics since 2005 and Managing Director of Stryker’s businesses in Germany, Austria and Switzerland since 2000. After joining the Company in 1989, Mr. Mogul held various roles of increasing responsibility at Stryker Instruments before being promoted to Vice President of Sales for the now Orthopaedics division in 1994.
Katherine A. Owen, age 40, was appointed Vice President, Strategy and Investor Relations in February 2007. Prior to joining the Company, she served as a medical technology analyst at Merrill Lynch. Prior to that she held a similar position at Cowen & Co./SG Cowen and had been a corporate lending analyst at State Street Bank.
Michael W. Rude, age 49, was appointed Vice President, Human Resources of the Company in July 2000. Prior to joining the Company, he served as Vice President of Human Resources for the SCIMED Division of Boston Scientific Corporation. Prior to that he held various positions as Vice President, Human Resources within The Dun & Bradstreet Corporation and spent eight years in various Human Resources positions at Baxter International, Inc.
Timothy J. Scannell, age 46, was appointed Group President, MedSurg and Spine in September 2009 and was Group President, Spine and Endoscopy since November 2008. He had previously been President, Stryker Spine since 2005 and Vice President and General Manager, Stryker Spine since 2003. After joining the Company in 1990, Mr. Scannell held a variety of leadership roles at Stryker Endoscopy before being promoted to Vice President and General Manager, Stryker Biotech in 2001.
The Corporate Governance Guidelines adopted by the Company’s Board of Directors, as well as the charters of each of the Audit Committee, the Finance Committee, the Governance and Nominating Committee, the Compensation Committee and the Code of Ethics applicable to the principal executive officer, principal financial officer and principal accounting officer or controller or persons performing similar functions is available, free of charge, under the “Investors—Corporate Governance” section of the Company’s website at www.stryker.com. Print copies of such documents are available, free of charge, upon written request sent to the Secretary of the Company at 2825 Airview Boulevard, Kalamazoo, Michigan 49002.
| ITEM 11. | EXECUTIVE COMPENSATION. |
Information regarding the compensation of the management of the Company appearing under the captions “Compensation Discussion and Analysis,” “Compensation Committee Report,” “Executive Compensation” and “Compensation of Directors” in the 2011 proxy statement is incorporated herein by reference.
74
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information under the caption “Stock Ownership” in the 2011 proxy statement is incorporated herein by reference.
At December 31, 2010, the Company had key employee and director stock option plans under which options are granted at a price not less than fair market value at the date of grant. These stock option plans were previously submitted to and approved by the Company’s shareholders. Additional information regarding the Company’s stock option plans appears in Note 1 and Note 9 to the Consolidated Financial Statements in Item 8 of this report. At December 31, 2010, the Company also had a stock performance incentive award program pursuant to which shares of the Company’s common stock have been and may be issued to certain employees with respect to performance. The status of these plans as of December 31, 2010 follows:
| | | | | | | | | | | | |
Plan category | | Number of shares of common
stock to be issued upon
exercise of outstanding options,
warrants and rights | | | Weighted-average
exercise price of
outstanding options,
warrants and rights | | | Number of shares of common
stock remaining available for
future issuance under equity
compensation plans (excluding
shares reflected in the first column) | |
Equity compensation plans approved by shareholders | | | 25,532,552 | | | $ | 46.64 | | | | 16,026,270 | |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
The information under the caption “Information About the Board of Directors and Corporate Governance Matters—Independent Directors” and “Information About the Board of Directors and Corporate Governance Matters—Certain Relationships and Related Party Transactions” in the 2011 proxy statement is incorporated herein by reference.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES. |
The information under the caption “Proposal 2—Ratification of Appointment of Our Independent Registered Public Accounting Firm—Relationship with Ernst & Young LLP” in the 2011 proxy statement is incorporated herein by reference.
75
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES. |
| | | | | | |
| (a) 1. | | Financial Statements | | | | |
| | |
| | The following Consolidated Financial Statements of the Company and its subsidiaries are set forth in Part II, Item 8 of this report. | | | | |
| | |
| | Report of Independent Registered Public Accounting Firm on Financial Statements | | | 36 | |
| | Consolidated Balance Sheets as of December 31, 2010 and 2009 | | | 37 | |
| | Consolidated Statements of Earnings for the Years Ended December 31, 2010, 2009 and 2008 | | | 38 | |
| | Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2010, 2009 and 2008 | | | 39 | |
| | Consolidated Statements of Cash Flows for the Years Ended December 31, 2010, 2009 and 2008 | | | 40 | |
| | Notes to Consolidated Financial Statements | | | 41 | |
| | |
| (a) 2. | | Financial Statement Schedules | | | | |
| | |
| | The consolidated financial statement schedule (Schedule II) of the Company and its subsidiaries has been submitted as a separate section of this report following the signature page. All other schedules for which provision is made in the applicable accounting regulation of the U.S. Securities and Exchange Commission are not required under the related instructions or are inapplicable and, therefore, have been omitted. | | | | |
| | |
| (a) 3. | | Exhibits | | | | |
| | |
| | A list of exhibits required to be filed as part of this report is set forth in the Exhibit Index, which immediately precedes such exhibits, and is incorporated herein by reference. | | | | |
| | |
| (c) | | Financial Statement Schedules | | | | |
| | |
| | The consolidated financial statement schedule (Schedule II) of the Company and its subsidiaries has been submitted as a separate section of this report following the signature page. All other schedules for which provision is made in the applicable accounting regulation of the U.S. Securities and Exchange Commission are not required under the related instructions or are inapplicable and, therefore, have been omitted. | | | | |
76
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| | STRYKER CORPORATION |
| |
Date: February 18, 2011 | | /s/ CURT R. HARTMAN |
| | Curt R. Hartman, Vice President and
Chief Financial Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | |
/s/ STEPHEN P. MACMILLAN | | /s/ CURT R. HARTMAN |
Stephen P. MacMillan, Chairman, President and Chief Executive Officer (Principal Executive Officer) | | Curt R. Hartman, Vice President and Chief Financial Officer (Principal Financial Officer) |
| |
/s/ TONY M. MCKINNEY | | |
Tony M. McKinney, Vice President, Chief Accounting Officer (Principal Accounting Officer) | | |
| |
/s/ HOWARD E. COX, JR. | | /s/ SRIKANT M. DATAR |
| Howard E. Cox, Jr.—Director | | Srikant M. Datar, Ph.D.—Director |
| |
/s/ ROCH DOLIVEUX | | /s/ DONALD M. ENGELMAN |
| Roch Doliveux—Director | | Donald M. Engelman, Ph.D.—Director |
| |
/s/ LOUISE L. FRANCESCONI | | /s/ ALLAN C. GOLSTON |
| Louise L. Francesconi—Director | | Allan C. Golston—Director |
| |
/s/ HOWARD L. LANCE | | /s/ WILLIAM U. PARFET |
| Howard L. Lance—Director | | William U. Parfet—Director |
| |
/s/ RONDA E. STRYKER | | |
| Ronda E. Stryker—Director | | |
77
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
STRYKER CORPORATION AND SUBSIDIARIES
| | | | | | | | | | | | | | | | | | | | |
Column A | | Column B | | | Column C | | | Column D | | | Column E | | | Column F | |
| | | | | | Additions | | | Deductions | | | | |
Description | | Balance at
Beginning
of Period | | | Charged to
Costs &
Expenses | | | Describe (a) | | | Describe (b) | | | Balance
at End
of Period | |
DEDUCTED FROM ASSET ACCOUNTS | | | | | | | | | | | | | | | | | | | | |
Allowance for Doubtful Accounts (in millions): | | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2010 | | $ | 66.3 | | | $ | 18.6 | | | $ | 29.8 | | | $ | (1.9 | ) | | $ | 57.0 | |
| | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2009 | | $ | 44.5 | | | $ | 27.9 | | | $ | 6.6 | | | $ | (0.5 | ) | | $ | 66.3 | |
| | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2008 | | $ | 44.5 | | | $ | 10.4 | | | $ | 10.2 | | | $ | 0.2 | | | $ | 44.5 | |
| | | | | | | | | | | | | | | | | | | | |
| (a) | Uncollectible amounts written off, net of recoveries. |
| (b) | Effect of changes in foreign exchange rates. |
78
FORM 10-K—ITEM 15(a) 3. and ITEM 15(c)
STRYKER CORPORATION AND SUBSIDIARIES
EXHIBIT INDEX
| | |
Exhibit 1— | | Underwriting Agreements |
(i) | | Underwriting Agreement, dated January 12, 2010, between Stryker Corporation and Banc of America Securities LLC, Barclays Capital Inc. and Wells Fargo Securities, LLC, as representatives of the underwriters named therein—Incorporated by reference to Exhibit 1.1 to the Company’s Form 8-K dated January 15, 2010 (Commission File No. 000-09165). |
| |
Exhibit 3— | | Articles of Incorporation and By-Laws |
(i) | | Composite copy of Restated Articles of Incorporation as amended through April 19, 2000— Incorporated by reference to Exhibit 3(i) to the Company’s Form 10-K for the year ended December 31, 2000 (Commission File No. 000-09165). |
(ii) | | Certificate of Amendment of Restated Articles of Incorporation dated June 4, 2004— Incorporated by reference to Exhibit 3(i) to the Company’s Form 10-Q for the quarter ended June 30, 2004 (Commission File No. 000-09165). |
(iii) | | By-Laws-Incorporated by reference to Exhibit 3(ii) to the Company’s Form 8-K dated October 28, 2008 (Commission File No. 000-09165). |
| |
Exhibit 4— | | Instruments defining the rights of security holders, including indentures—The Company agrees to furnish to the Commission upon request a copy of each instrument pursuant to which long-term debt of the Company and its subsidiaries not exceeding 10% of the total assets of the Company and its consolidated subsidiaries is authorized. |
(i) | | Credit Agreement, dated August 5, 2010, among Stryker Corporation and certain subsidiaries, as designated borrowers; the lenders party thereto; and Bank of America, N.A., as administrative agent—Incorporated by reference to Exhibit 4.1 to the Company’s Form 10-Q for the quarter ended June 30, 2010 (Commission File No. 000-09165). |
(ii) | | Indenture, dated January 15, 2010, between Stryker Corporation and U.S. Bank National Association.—Incorporated by reference to Exhibit 4.1 to the Company’s Form 8-K dated January 15, 2010 (Commission File No. 000-09165). |
(iii) | | First Supplemental Indenture (including the form of 2015 note), dated January 15, 2010, between Stryker Corporation and U.S. Bank National Association.—Incorporated by reference to Exhibit 4.2 to the Company’s Form 8-K dated January 15, 2010 (Commission File No. 000-09165). |
(iv) | | Second Supplemental Indenture (including the form of 2020 note), dated January 15, 2010, between Stryker Corporation and U.S. Bank National Association.—Incorporated by reference to Exhibit 4.3 to the Company’s Form 8-K dated January 15, 2010 (Commission File No. 000-09165). |
| |
Exhibit 10— | | Material contracts |
(i)* | | 2006 Long-Term Incentive Plan (as amended effective February 8, 2011). |
(ii)* | | Form of grant notice and terms and conditions for stock options granted in 2011 under the 2006 Long-Term Incentive Plan. |
(iii)* | | Form of grant notice and terms and conditions for restricted stock units granted in 2011 under the 2006 Long-Term Incentive Plan. |
(iv)* | | Form of grant notice and terms and conditions for performance stock units granted in 2011 under the 2006 Long-Term Incentive Plan. |
(v)* | | Form of grant notice and terms and conditions for stock options granted in 2010 under the 2006 Long-Term Incentive Plan.—Incorporated by reference to Exhibit 10(ii) to the Company’s Form 10-K for the year ended December 31, 2009 (Commission File No.000-09165). |
(vi)* | | Form of grant notice and terms and conditions for restricted stock units granted in 2010 under the 2006 Long-Term Incentive Plan.—Incorporated by reference to Exhibit 10(iii) to the Company’s Form 10-K for the year ended December 31, 2009 (Commission File No.000-09165). |
79
| | |
(vii)* | | 1998 Stock Option Plan (as Amended Effective July 23, 2008)—Incorporated by reference to Exhibit 10.2 to the Company’s Form 10-Q for the quarter ended June 30, 2008 (Commission File No. 000-09165). |
(viii)* | | Supplemental Savings and Retirement Plan (as Amended Effective January 1, 1996)—Incorporated by reference to Exhibit 10(iii) to the Company’s Form 10-K for the year ended December 31, 1994 (Commission File No.000-09165). |
(ix)* | | Stock option agreement relating to special stock option award to Stephen P. MacMillan pursuant to the 1998 Stock Option Plan on February 7, 2006—Incorporated by reference to Exhibit 10.3 to the Company’s Form 8-K dated February 9, 2006 (Commission File No. 000-09165). |
(x)* | | Statement of Terms Relating to Employment dated as of December 4, 1998 between Stryker UK Limited and Andrew G. Fox-Smith as amended and restated through February 9, 2009—Incorporated by reference to Exhibit 10 (x) to the Company’s Form 10-K for the year ended December 31, 2008 (Commission File No. 000-09165). |
(xi)* | | Executive Management Agreement dated as of December 2, 2008 between Dean H. Bergy and Stryker Corporation- Incorporated by reference to Exhibit 10 (xii) to the Company’s Form 10-K for the year ended December 31, 2008 (Commission File No. 000-09165). |
(xii)* | | Stryker Corporation Executive Bonus Plan—Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K dated February 21, 2007 (Commission File No. 000-09165). |
(xiii) | | Form of Indemnification Agreement for Directors—Incorporated by reference to Exhibit 10 (xiv) to the Company’s Form 10-K for the year ended December 31, 2008 (Commission File No. 000-09165). |
(xiv) | | Form of Indemnification Agreement for Certain Officers—Incorporated by reference to Exhibit 10 (xv) to the Company’s Form 10-K for the year ended December 31, 2008 (Commission File No. 000-09165). |
(xv) | | Sale and Purchase Agreement, dated January 3, 2011, between Boston Scientific Corporation and Stryker Corporation. |
| |
Exhibit 11— | | Statement re: computation of per share earnings |
(i) | | Note 10 to the Consolidated Financial Statements in Item 8 of this report. |
| |
Exhibit 21— | | Subsidiaries of the registrant |
(i) | | List of Subsidiaries. |
| |
Exhibit 23— | | Consent of experts and counsel |
(i) | | Consent of Independent Registered Public Accounting Firm. |
| |
Exhibit 31— | | Rule 13a-14(a) Certifications |
(i) | | Certification of Principal Executive Officer of Stryker Corporation. |
(ii) | | Certification of Principal Financial Officer of Stryker Corporation. |
| |
Exhibit 32— | �� | 18 U.S.C. Section 1350 Certifications |
(i)** | | Certification by President and Chief Executive Officer of Stryker Corporation. |
(ii)** | | Certification by Vice President and Chief Financial Officer of Stryker Corporation. |
| |
Exhibit 99— | | Additional exhibits |
(i)* | | 2008 Employee Stock Purchase Plan as amended on February 10, 2009—Incorporated by reference to Exhibit 99 (i) to the Company’s Form 10-K for the year ended December 31, 2008 (Commission File No. 000-09165). |
| |
Exhibit 101— | | XBRL (Extensible Business Reporting Language) Documents |
101.INS** | | XBRL Instance Document |
101.SCH** | | XBRL Schema Document |
101.CAL** | | XBRL Calculation Linkbase Document |
101.DEF** | | XBRL Definition Linkbase Document |
101.LAB** | | XBRL Label Linkbase Document |
101.PRE** | | XBRL Presentation Linkbase Document |
| * | compensation arrangement |
| ** | furnished with this Form 10-K |
80
