UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 10-K
| R | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
| For the fiscal year ended December 31, 2012 | |
| £ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
Commission File Number: 001-32360
AKORN, INC.
(Exact name of registrant as specified in its charter)
| LOUISIANA | 72-0717400 |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) |
| incorporation or organization) |
1925 W. Field Court, Suite 300, Lake Forest, Illinois 60045
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (847) 279-6100
Title of each class | Name of each exchange on which registered | |
| Common Stock, No Par Value | The NASDAQ Stock Market LLC |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
(None)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes R No £
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes £ No R
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes R No £
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes R No £
Indicate by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. £
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (check one):
Large accelerated filer: R | Accelerated filer: £ | Non-accelerated filer: £ | Smaller reporting company: £ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes £ No R
The aggregate market value of the voting stock of the registrant held by non-affiliates (affiliates being, for these purposes only, directors, executive officers and holders of more than 5% of the registrant’s common stock) of the registrant as of June 30, 2012 was approximately $934,248,000 based on the closing market price of $15.77 reported on the Nasdaq Stock Market LLC on Friday, June 29, 2012.
The number of shares of the registrant’s common stock, no par value per share, outstanding as of February 25, 2013 was 95,921,212.
Documents incorporated by reference: Definitive Proxy Statement for the 2013 Annual Meeting incorporated by reference into Part III, Items 10-14 of this Form 10-K.
Forward-Looking Statements and Factors Affecting Future Results
Certain statements in this Form 10-K constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act. When used in this document, the words “anticipate,” “believe,” “estimate” and “expect” and similar expressions are generally intended to identify forward-looking statements. Any forward-looking statements, including statements regarding our intent, belief or expectations are not guarantees of future performance. These statements are subject to risks and uncertainties and actual results may differ materially from those in the forward-looking statements as a result of various factors, including but not limited to:
| ● | Our ability to continue to comply with all of the requirements of the Food and Drug Administration, including current Good Manufacturing Practices regulations; |
| ● | Our ability to obtain additional funding or financing to operate and grow our business; |
| ● | The effects of federal, state and other governmental regulation on our business; |
| ● | Our ability to obtain and maintain regulatory approvals for our products; |
| ● | Our success in developing, manufacturing, acquiring and marketing new products; |
| ● | Our ability to generate cash from operations sufficient to meet our working capital requirements; |
| ● | The success of our strategic partnerships for the development and marketing of new products; |
| ● | Our ability to bring new products to market and the effects of sales of such products on our financial results; |
| ● | Our ability to successfully integrate acquired businesses and products; |
| ● | The effects of competition from other generic pharmaceuticals and from other pharmaceutical companies; |
| ● | Availability of raw materials needed to produce our products; and |
| ● | Other factors referred to in this Form 10-K and our other Securities and Exchange Commission filings. |
See “Item 1A. Risk Factors”. You should read this report completely with the understanding that our actual results may differ materially from what we expect. Unless required by law, we undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
2
FORM 10-K TABLE OF CONTENTS
Page | ||
| 4 | ||
| 9 | ||
| 20 | ||
| 20 | ||
| 20 | ||
| 21 | ||
| 23 | ||
| 24 | ||
| 38 | ||
| 38 | ||
| 76 | ||
| 76 | ||
| 77 | ||
| 78 | ||
| 78 | ||
| 78 | ||
| 78 | ||
| 78 | ||
| 79 | ||
3
We manufacture and market a full line of diagnostic and therapeutic ophthalmic pharmaceuticals as well as niche hospital drugs and injectable pharmaceuticals. In addition, we market and distribute vaccines purchased from outside sources. Our customers include physicians, optometrists, hospitals, wholesalers, group purchasing organizations, retail pharmacy chains and other pharmaceutical companies. Akorn, Inc. is a Louisiana corporation founded in 1971 in Abita Springs, Louisiana. In 1997, we relocated our corporate headquarters to the Chicago, Illinois area and currently maintain our corporate offices in Lake Forest, Illinois. We operate pharmaceutical manufacturing facilities in Decatur, Illinois and Somerset, New Jersey, and in Paonta Sahib, Himachal Pradesh, India. We also operated a Research and Development (“R&D”) center in Skokie, Illinois and a distribution warehouse in Gurnee, Illinois.
In this annual report, we have reported results for three operating segments: ophthalmic; hospital drugs & injectables; and contract services. These three segments are described in greater detail below. For information regarding revenues and gross profit for each of our segments, see Item 8. Financial Statements and Supplementary Data, Note 13 — “Segment Information.”
Ophthalmic Segment. We market a full line of diagnostic and therapeutic ophthalmic pharmaceutical products. Diagnostic products, primarily used in the office setting, include mydriatics and cycloplegics, anesthetics, topical stains, gonioscopic solutions, angiography dyes and others. Therapeutic products, sold primarily to wholesalers, chain drug stores and other national account customers, include antibiotics, steroids, steroid combinations, glaucoma medications, decongestants/antihistamines and anti-edema medications. Non-pharmaceutical products include various artificial tear solutions, preservative-free lubricating ointments and eyelid cleansers.
We also market a line of over-the-counter (“OTC”) dry eye and other eye health products principally under the TheraTears® brand name. These products are sold through major chain drug stores and big box retailers, as well as directly to optometrists, ophthalmologists and other eye care practitioners and clinics.
Hospital Drugs & Injectables Segment. We market a line of niche hospital drug and injectable pharmaceutical products, including antidotes, anti-infectives, controlled substances for pain management and anesthesia, and other selected pharmaceutical products. These products are predominately sold to hospitals through the wholesale distribution channel. We target products with limited competition due to difficulty in manufacturing and/or the product’s market size.
We also market Td vaccine from time to time. We ceased the distribution of Td vaccine in early 2010, and restarted their distribution at the end of 2012. In 2010 and prior years, we included Td vaccine sales within the biologics & vaccines segment, which was discontinued in 2010. Due to the non-materiality of Td vaccine sales in 2010 and 2012 and the fact that they are marketed in a similar way and to the same customers as many of our injectable drugs, we are now including Td vaccine sales within the hospital drugs & injectables segment.
Contract Services Segment. We manufacture ophthalmic and injectable pharmaceutical products for third party pharmaceutical customers based on their specifications.
Manufacturing. We operate domestic U.S. manufacturing facilities in Decatur, Illinois and Somerset, New Jersey, and a foreign manufacturing facility in Paonta Sahib, Himachal Pradesh, India. (See Item 2. Properties, for more information.) Through these manufacturing facilities, we manufacture a diverse group of sterile pharmaceutical products, including dye products, liquid injectables, lyophilized injectables, gels, and ophthalmic solutions and ointments for our ophthalmic, hospital drugs & injectables and contract services segments. Our Somerset facility manufactures ophthalmic solutions and ointment products for our ophthalmic and contract services segments, and gels for our hospital drugs & injectables segment. Our Decatur manufacturing facility manufactures dye products, liquid injectables, lyophilized injectables and ophthalmic solutions for our ophthalmic, hospital drugs & injectables and contract services segments. The manufacturing complex in Paonta Sahib, Himachal Pradesh, India manufactures liquid generic pharmaceutical injectables, injectable and oral cephalosporins, sterile injectable carbapenems, hormones and oncology products. The Paonta Sahib plant currently manufactures product for Indian contract customers and for export to Africa, Asia and other unregulated markets. We are working toward obtaining approval from the U.S. Food and Drug Administration (“FDA”) to manufacture various products from this plant for export to the U.S. and other regulated markets.
4
Sales and Marketing. We rely on our sales and marketing teams to help us maintain and, where possible, increase our market share in our predominantly non-proprietary product offering. Our sales organization consists of multiple teams, including: (1) regional outside sales teams focused on (a) ophthalmic sales and (b) injectable and other acute care sales; (2) an inside sales team focused on customers in smaller markets; and (3) a national accounts sales team focused on wholesale, retail pharmacy chain and group purchasing organization (“GPO”) markets. Our outside sales representatives sell ophthalmic products directly to retinal surgeons and ophthalmologists, and sell hospital drugs & injectables directly to local hospitals in order to support compliance and pull-through against GPO contracts. Inside sales augments our outside sales teams in the sale of ophthalmic and hospital drugs & injectables products in markets where outside sales would not be cost effective. Our national accounts sales team seeks to establish and maintain contracts with wholesalers, retail pharmacy chains and GPOs that represent hospitals in the United States. To support our sales efforts, we have a customer service team, as well as a marketing department focused on educating current and future customers about our product offerings and manufacturing capabilities.
Research and Development. We seek to continually grow our business by developing new products, either internally or with the assistance of external partners. We have operated an R&D facility in Skokie, Illinois since early 2010, and are relocating to a new, larger facility in Vernon Hills, Illinois during the first quarter of 2013. The majority of our internal product development will take place at the Vernon Hills facility, while our manufacturing plants in Decatur, IL and Somerset, NJ will provide support for the latter phases of product development. We believe that having our own centralized and dedicated R&D facility allows us to significantly increase the size of our product pipeline as well as shorten the time from project start to filing for approval with the FDA. We also continue to work with strategic partners for the external development of certain products. As of December 31, 2012, we had 36 full-time employees directly involved in product research and development activities.
R&D costs are expensed as incurred. Such costs amounted to $15.9 million, $11.6 million and $7.0 million for the years ended December 31, 2012, 2011 and 2010, respectively, and includes both internal R&D expenses and milestone fees paid to our strategic partners. Our strategic partnerships are discussed further in “Business Development.”
We received five Abbreviated New Drug Application (“ANDA”) product approvals from the FDA in 2012, one approval in 2011 and four in 2010. During 2012, we submitted 25 new ANDA filings to the FDA, increasing to 55 the number of our ANDA product filings currently under review by the FDA Office of Generic Drugs: 51 from internal development and four from various strategic agreements with other external partners. In most but not all instances, we own, or will own, the ANDAs that are produced by our strategic partnerships. We plan to continue to file ANDAs on a regular basis in anticipation of selected pharmaceutical products coming off patent, thereby allowing us to compete by marketing generic equivalents. For more information, see “Government Regulation.”
No assurance can be given as to: (1) whether we will file New Drug Applications (“NDAs”) or ANDAs when anticipated; (2) whether or when such NDAs or ANDAs will be approved by the FDA; (3) whether or not we will ultimately develop marketable products based on any filings we do make; (4) the actual size of the market for any such products or (5) whether our participation in such market would be profitable. See “Government Regulation” and Item 1A. Risk Factors – “Our growth depends on our ability to timely develop and successfully integrate new pharmaceutical products.”
Mergers and Acquisitions. We actively seek to expand and enhance our business through strategic acquisitions. We may seek to acquire ANDAs and NDAs from other pharmaceutical companies or pursue acquisition of independent businesses that we believe would complement our existing business and provide us opportunities for growth. During 2011 and early 2012, we completed three significant acquisitions.
On May 3, 2011, we acquired AVR Business Trust and its subsidiaries, Advanced Vision Research, Inc. and Advanced Vision Pharmaceuticals, LLC (collectively, "AVR") for $26.0 million in cash. AVR is a developer and marketer of a line of OTC eye care products marketed primarily under the TheraTears® brand name. AVR products are carried by major drug retailers throughout the United States and are being marketed in various foreign countries.
On December 22, 2011, we acquired three NDAs from H. Lundbeck A/S (“Lundbeck”). On the date of closing, of the acquisition (the “Lundbeck Acquisition”), we made an initial payment of $45.0 million and will likely owe a subsequent milestone payment of $15.0 million in cash on the third anniversary of the closing date. The initial purchase price and the subsequent milestone payment are subject to a reduction if certain sales targets are not met in the first three years and the subsequent three years post closing. The acquired portfolio consists of Nembutal®, a Schedule II controlled drug, Diuril® and Cogentin®. In addition, we signed a transition services agreement with Lundbeck to ensure product availability, and separately paid approximately $4.6 million for Lundbeck’s existing inventory of the three acquired products. This acquisition provided us with three branded, hospital injectables to add to our portfolio.
On February 28, 2012, we acquired selected assets of Kilitch Drugs (India) Limited (“Kilitch”) pursuant to a Business Transfer Agreement (“BTA”) between our subsidiary, Akorn India Private Limited (“AIPL”), and Kilitch signed on October 6, 2011 (the “Kilitch Acquisition”). We paid approximately $60.1 million in cash at closing, which included consideration of $55.2 million and acquisition related costs of $4.9 million. The primary assets acquired were Kilitch’s pharmaceutical manufacturing complex in Paonta Sahib, Himachal Pradesh, India and its ongoing book of business. We also acquired pursuant to the BTA selected assets of NBZ Pharma Limited, a company affiliated with Kilitch, from which we acquired the rights to manufacture and distribute certain pharmaceuticals products. The Paonta Sahib plant currently manufactures pharmaceutical products primarily for contract customers in India and for export to unregulated markets. We plan to obtain FDA and other international certification so that we can manufacture product for export to the U.S. and other regulated markets. See Item 1A. Risk Factors — “Failure to obtain regulatory certification of our manufacturing plant in India for production of pharmaceutical products for export to the United States, as well as other regulated world markets, could impair our ability to grow and adversely affect our business, financial condition and results of operations” and “Failure to comply with the U.S. Foreign Corrupt Practices Act could subject us to, among other things, penalties and legal expenses that could harm our reputation and have a material adverse effect on our business, financial condition and operating results” for more information.
5
Business Development. In additional to our internal research and development, we also maintain a business development program that identifies potential product acquisition or product licensing opportunities. We have strategically focused our business development efforts on products that complement our existing product lines and which are expected to have few competitors.
In 2004, we entered into a 50/50 strategic partnership with Strides Arcolab Limited (“Strides”) in a new company named Akorn-Strides LLC (the “Joint Venture Company”) for the development and marketing of a number of injectable ANDA products for the hospital and alternate site markets in the United States. Each partner funded the Joint Venture Company with $1,500,000 for initial development projects. See Item 8. Financial Statements and Supplementary Data, Note 18 – “Unconsolidated Joint Venture” for more information. Strides was responsible for developing, manufacturing and supplying the injectable products, while Akorn was responsible for sales and marketing of these products within the United States. The Joint Venture Company launched its first products in the second half of 2008. To supplement Strides’ manufacturing capabilities, during 2010 Akorn began manufacturing one Joint Venture Company product in our Decatur, Illinois plant. The Joint Venture Company product pipeline was limited to those products identified at the founding of the Joint Venture Company and placed into development shortly thereafter.
On December 29, 2010, the Joint Venture Company entered into a purchase agreement with Pfizer, Inc. (“Pfizer”) to sell all of its ANDAs to Pfizer for a purchase price of $63.2 million (the “Pfizer Sale Agreement”). Ownership of dormant products and those in development transferred as of the purchase date, while ownership of the actively-marketed ANDAs transferred in the second quarter of 2011. Pursuant to the terms of the Pfizer Sale Agreement, the Joint Venture Company was allowed to sell its actively-marketed ANDA products through April 30, 2011. Subsequent agreement between the parties allowed for the continued sale of one product into June 2011. We recognized $34.9 million in pre-tax income related to the Pfizer Sale Agreement, of which $21.5 million was recognized in the fourth quarter of 2010 and $13.4 million was recognized in the second quarter of 2011. For the years 2011 and 2010, the Joint Venture Company generated net sales of $6.4 million and $16.3 million, respectively.
Patents, Trademarks and Proprietary Rights. We consider the protection of our patents, trademarks and proprietary rights important to maintaining and growing our business. Through our acquisitions, we have increased the number and importance of trademarks related to our products and product lines. One of our acquired companies, AVR, maintains a line of OTC eye care products sold under trade names such as TheraTears® and SteriLid®, among others. We are committed to maintaining and defending the trade names of AVR’s products, as they are important in supporting the success and growth of this business. In addition, we maintain and defend trademarks related to a number of internally-developed products, as well as those acquired or licensed from other companies.
We have sought, and intend to continue to seek, patent protection in the United States and selected foreign countries where deemed appropriate and advantageous to us. The importance of these patents does not vary among our business segments. We currently have five patents, none of which expire within the next three years.
We also rely upon trade secrets, unpatented proprietary know-how and continuing technological innovation to maintain and develop our competitive position. We enter into confidentiality agreements with certain of our employees pursuant to which such employees agree to assign to us any inventions relating to our business made by them while in our employ. However, there can be no assurance that others may not acquire or independently develop similar technology or, if patents are not issued with respect to products arising from research, that we will be able to maintain information pertinent to such research as proprietary technology or trade secrets. See Item 1A. Risk Factors — “Our patents and proprietary rights may not adequately protect our products and processes” and “Third parties may claim that we infringe their proprietary rights and may prevent or delay us from manufacturing and selling some of our new products” for more information.
Employee Relations. As of December 31, 2012, we had 759 permanent, full-time employees and eight part-time or temporary employees in the United States and 217 employees working for our subsidiary in India. Of our full-time employees working in the U.S., 409 worked at our manufacturing facilities in Decatur, Illinois, 142 worked at our manufacturing facility in Somerset, New Jersey, 57 were field-based salespersons, 25 worked at our R&D facility in Skokie, Illinois, and the remaining 126 worked in corporate support functions, either at our corporate offices in Lake Forest, Illinois or Ann Arbor, Michigan, or at our distribution facility in Gurnee, Illinois. We believe we have good relations with our employees. None of our employees are represented by a collective bargaining agreement.
6
Competition. The marketing and manufacturing of pharmaceutical products is highly competitive, with many established manufacturers, suppliers and distributors actively engaged in all phases of the business. Many of our competitors have substantially greater financial and other resources, including greater sales volume, larger sales forces and greater manufacturing capacity. See Item 1A. Risk Factors — “Our industry is very competitive. Additionally, changes in technology could render our products obsolete” for more information.
The companies that compete with our ophthalmic segment include Allergan Pharmaceuticals, Inc., Novartis, Bausch & Lomb, Inc., Apotex and Sun Pharmaceuticals, among others. The ophthalmic segment competes primarily on the basis of price and service.
The companies that compete with our hospital drugs & injectables segment include both generic and name brand companies such as Hospira, Inc., Teva Pharmaceutical Industries, Pfizer, Sagent Pharmaceuticals, Novartis, Fresenius-Kabi, American Regent, Inc., Hikma and Bedford. The hospital drugs & injectables segment competes primarily on the basis of price.
Suppliers and Customers. No supplier represented 10% or more of our purchases in 2012 or 2011. In 2010, purchases from Massachusetts Biologic Laboratories (“MBL”) represented 14% of our total purchases. MBL was the sole supplier of Td vaccine for our former biologics & vaccines segment. Aside from MBL, no other suppliers represented 10% or more of our purchases in 2010.
We require a supply of quality raw materials and components to manufacture and package pharmaceutical products for ourselves and for third parties with which we have contracted. The principal components of our products are active and inactive pharmaceutical ingredients and certain packaging materials. Many of these components are available from only a single source and, in the case of many of our ANDAs and NDAs, only one supplier of raw materials has been identified. Because FDA approval of drugs requires manufacturers to specify their proposed suppliers of active ingredients and certain packaging materials in their applications, FDA approval of any new supplier would be required if active ingredients or such packaging materials were no longer available from the specified supplier. The qualification of a new supplier could delay our development and marketing efforts. If for any reason we are unable to obtain sufficient quantities of any of the raw materials or components required to produce and package our products, we may not be able to manufacture our products as planned. See Item 1A. Risk Factors – “Many of the raw materials and components used in our products come from a single source” for more information.
In 2012, 2011 and 2010, a high percentage of our sales were to the three large wholesale drug distributors noted below. These three large wholesale drug distributors account for a large portion of our gross sales, net revenues and accounts receivable in all our business segments except for contract services. The three distributors are:
| ● | AmerisourceBergen Corporation (“AmerisourceBergen”); |
| ● | Cardinal Health, Inc. (“Cardinal”); and |
| ● | McKesson Drug Company (“McKesson”). |
On a combined basis, these three wholesale drug distributors accounted for approximately 58% of our total gross sales and 42% of our net revenue in 2012, and 73% of our gross accounts receivable as of December 31, 2012. The difference between gross sales and net revenue is that gross sales is calculated before allowances for chargebacks, rebates, promotions and product returns (See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — “Critical Accounting Policies” – “Allowance for Chargebacks and Rebates” for more information).
The table below presents the percentages of our total gross sales, net revenue and gross trade accounts receivable attributed to each of these three wholesale drug distributors as of and for the years ended December 31, 2012, 2011 and 2010:
| 2012 | 2011 | 2010 | ||||||||||||||||||||||||||||||||||
Gross Sales | Net Revenue | Gross Accounts Receivable | Gross Sales | Net Revenue | Gross Accounts Receivable | Gross Sales | Net Revenue | Gross Accounts Receivable | ||||||||||||||||||||||||||||
| AmerisourceBergen | 19 | % | 14 | % | 29 | % | 23 | % | 23 | % | 29 | % | 24 | % | 17 | % | 32 | % | ||||||||||||||||||
| Cardinal | 23 | % | 17 | % | 30 | % | 27 | % | 25 | % | 34 | % | 25 | % | 17 | % | 31 | % | ||||||||||||||||||
| McKesson | 16 | % | 11 | % | 14 | % | 16 | % | 15 | % | 9 | % | 15 | % | 11 | % | 7 | % | ||||||||||||||||||
| Combined Total | 58 | % | 42 | % | 73 | % | 66 | % | 63 | % | 72 | % | 64 | % | 45 | % | 70 | % | ||||||||||||||||||
AmerisourceBergen, Cardinal and McKesson are key distributors of our products, as well as a broad range of health care products for many other companies. None of these distributors is an end user of our products. If sales to any one of these distributors were to diminish or cease, we believe that the end users of our products would likely find little difficulty obtaining our products either directly from us or from another distributor. However, the loss of one or more of these distributors, together with a delay or inability to secure an alternative distribution source for end users, could have a material negative impact on our revenue, business, financial condition and results of operations. We consider our business relationships with these three wholesalers to be in good standing and have fee for services contracts with each of them. A change in purchasing patterns, a decrease in inventory levels, an increase in returns of our products, delays in purchasing products and delays in payment for products by one or more of these distributors could have a material negative impact on our revenue, business, financial condition and results of operations. See Item 1A Risk factors – “We depend on a small number of distributors, the loss of any of which could have a material adverse effect” for more information.
7
Backorders. As of December 31, 2012, we had approximately $0.8 million of products on backorder as compared to approximately $2.9 million of backorders as of December 31, 2011. We anticipate filling all open backorders during 2013.
Government Regulation. Pharmaceutical manufacturers and distributors are subject to extensive regulation by government agencies, including the FDA, the Drug Enforcement Administration (“DEA”), the Federal Trade Commission (“FTC”) and other federal, state and local agencies. The Federal Food, Drug and Cosmetic Act (the “FDC Act”), the Controlled Substance Act and other federal statutes and regulations govern or influence the development, testing, manufacture, labeling, storage and promotion of products that we manufacture and market. The FDA inspects drug manufacturers and storage facilities to determine compliance with its current Good Manufacturing Practices (“cGMP”) regulations, non-compliance with which can result in fines, recall and seizure of products, total or partial suspension of production, refusal to approve NDAs and ANDAs and criminal prosecution. The FDA also has the authority to revoke approval of drug products.
FDA approval is required before any application drug product can be manufactured and marketed. New drugs require the application filing of an NDA, including clinical studies demonstrating the safety and efficacy of the drug. Generic drugs, which are equivalents of existing, off-patent brand name drugs, require the application filing of an ANDA. An ANDA does not, for the most part, require clinical studies since safety and efficacy have already been demonstrated by the product originator. However, the ANDA must, for example, provide data demonstrating the equivalency of the generic formulation in terms of bioavailability. The time required by the FDA to review and approve NDAs and ANDAs is variable and, to a large extent, beyond our control.
We are subject to periodic inspections by the FDA and the DEA. Throughout the five year period ended December 31, 2012, there have been no product interruptions associated with regulatory inspection or review activities. The most recent inspections conducted during January/February 2013 at our Somerset, New Jersey plant and August 2012 at our Decatur, Illinois plant, resulted in no significant observations.
Product Recalls. There were no recalls of any of our products during 2012, 2011 or 2010.
DEA Regulation. We also manufacture and distribute several controlled-drug substances, the distribution and handling of which are regulated by the DEA. Failure to comply with DEA regulations can result in fines or seizure of product. There were no DEA citations issued to us in 2012, 2011 or 2010.
Environment. We do not anticipate any material adverse effect from compliance with federal, state and local provisions that have been enacted or adopted regulating the discharge of materials into the environment, or otherwise relating to the protection of the environment.
Foreign Sales. During 2012, 2011 and 2010, approximately $29.4 million, $5.3 million, and $1.1 million of our net revenue, respectively, was related to sales to customers in foreign countries. The 2012 sales figure includes $16.7 million in sales generated by AIPL, our subsidiary in India, which exclusively sells product to customers in India and other unregulated world markets.
Seasonality and other Cyclical Sales Fluctuations. Most of our business segments do not experience significant seasonality. We do market certain allergy products that typically generate higher sales volume in the warmer months, but these products do not materially impact our overall sales trends. Additionally, we market various antidote products through our hospital drugs & injectables segment, the sales of which are largely timed to the expiration of existing stock held by our ongoing customers. In addition, late in 2012 we restarted the distribution of Td vaccines, which tends to generate higher sales in spring through fall.
Government Contracts. None of our business segments is generally subject to renegotiation of profits or termination of contracts at the election of the Federal government.
Available Information. We file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission (“SEC”). Materials filed with the SEC can be read and copied at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet web site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings are available to the public at the website maintained by the SEC, http://www.sec.gov. We also make available, free of charge, through our web site at www.akorn.com, our reports on Forms 10-K, 10-Q, and 8-K, and amendments to those reports, as soon as reasonably practicable after they are filed with or furnished to the SEC. The information contained on our web site is not a part of this document.
8
An investment in our common stock involves a high degree of risk. In addition to the other information included in this Annual Report on Form 10-K, you should carefully consider each of the risks described below before purchasing shares of our common stock. The risk factors set forth below are not the only risks that may affect our business. Our business could also be affected by additional risks not currently known to us or that we currently deem to be immaterial. If any of the following risks actually occur, our business, financial condition and results of operations could materially suffer. As a result, the trading price of our common stock could decline, and you may lose all or part of your investment.
Our growth depends on our ability to timely develop and successfully integrate new pharmaceutical products.
Our strategy for growth is dependent upon our ability to develop products that can be promoted through current marketing and distributions channels and, when appropriate, the enhancement of such marketing and distribution channels. We may fail to meet our anticipated time schedule for the filing of ANDAs and NDAs or may decide not to pursue ANDAs or NDAs that we have submitted or anticipate submitting. Our internal development of new pharmaceutical products is dependent upon the research and development capabilities of our personnel and our strategic business alliance infrastructure. There can be no assurance that our strategic business alliance partners or we will successfully develop new pharmaceutical products or, if developed, successfully integrate new products into our existing product lines. In addition, there can be no assurance that we will receive all necessary FDA approvals or that such approvals will not involve delays, which may adversely, affect the marketing and sale of our products. Our failure to develop new products or to receive FDA approval of ANDAs or NDAs could have a material adverse effect on our business, financial condition and results of operations.
Generic and off-patent pharmaceutical products are particularly susceptible to competition, substitution policies and reimbursement policies.
Our success depends, in part, on our ability to identify suitable branded pharmaceutical products to target for development of generic equivalents, determine or anticipate the dates when these branded pharmaceuticals are expected to come off patent, and time our product development activities accordingly so that we will be ready to manufacture and market our generic equivalent products at the most advantageous times. Generic pharmaceuticals must meet the same quality standards as branded pharmaceuticals, even though these equivalent pharmaceuticals are sold at prices that are significantly lower than branded pharmaceuticals. Generic substitution is regulated by the federal and state governments, as is reimbursement for generic drug dispensing. There can be no assurance that substitution will be permitted for newly approved generic drugs or that such products will be subject to government reimbursement. In addition, generic products developed by other third parties may render our generic products noncompetitive or obsolete, or may glut the market with competing products resulting in a reduction in sale price or market share for the generic products we sell. There can be no assurance that we will be able to consistently bring generic pharmaceutical products to market quickly and efficiently in the future. An increase in competition in the sale of generic pharmaceutical products or our failure to bring such products to market before our competitors could have a material adverse effect on our business, financial condition and results of operations.
Further, there is no proprietary protection for most of the branded pharmaceutical products that either we or other pharmaceutical companies sell. In addition, governmental and cost-containment pressures regarding the dispensing of generic equivalents will likely result in generic substitution and competition generally for our branded pharmaceutical products. We attempt to mitigate the effect of this substitution through, among other things, creation of strong brand-name recognition and product-line extensions for our branded pharmaceutical products, but there can be no assurance that we will be successful in these efforts.
We depend on a small number of distributors, the loss of any of which could have a material adverse effect.
A small number of large wholesale drug distributors account for a significant portion of our gross sales, net revenues and accounts receivable. The following three wholesalers –AmerisourceBergen, Cardinal and McKesson – accounted for approximately 58% of total gross sales and 42% of total net revenues in 2012, and 73% of gross trade receivables as of December 31, 2012. In addition to acting as distributors of our products, these three companies also distribute a broad range of health care products on behalf of many other companies. The loss of our relationship with one or more of these wholesalers, together with a delay or inability to secure an alternative distribution source for end users, could have a material adverse impact on our revenue and results of operations. A change in purchasing patterns or inventory levels, an increase in returns of our products, penalties assessed against us for failure to supply or failure to maintain service levels, delays in purchasing products and delays in payment for products by one or more of these distributors also could have a material adverse impact on our revenue, results of operations and cash flows.
9
Sales of our products may be adversely affected by the continuing consolidation of our customer base.
A significant proportion of our sales is made to relatively few retail drug chains, wholesalers, and managed care purchasing organizations. These customers are continuing to undergo significant consolidation. Such consolidation has provided and may continue to provide them with additional purchasing leverage, and consequently may increase the pricing pressures that we face. Additionally, the emergence of large buying groups representing independent retail pharmacies, and the prevalence and influence of managed care organizations and similar institutions, enable those groups to extract price discounts on our products.
Our net sales and quarterly growth comparisons may also be affected by fluctuations in the buying patterns of retail chains, major distributors and other trade buyers, whether resulting from seasonality, pricing, wholesaler buying decisions or other factors. In addition, since such a significant portion of our revenues is derived from relatively few customers, any financial difficulties experienced by a single customer, or any delay in receiving payments from a single customer, could have a material adverse effect on our business, results of operations and financial condition.
We are subject to extensive government regulations that increase our costs and could subject us to fines, prevent us from selling our products or prevent us from operating our facilities.
Federal and state government agencies regulate virtually all aspects of our business. The development, testing, manufacturing, processing, quality, safety, efficacy, packaging, labeling, record keeping, distribution, storage and advertising of our products, and disposal of waste products arising from such activities, are subject to regulation by the FDA, DEA, FTC, the Consumer Product Safety Commission, the Occupational Safety and Health Administration and the Environmental Protection Agency. Similar state and local agencies also have jurisdiction over these activities. Noncompliance with applicable United States and/or state or local regulatory requirements can result in fines, injunctions, penalties, mandatory recalls or seizures, suspensions of production, recommendations by the FDA against governmental contracts and criminal prosecution. Any of these could have a material adverse effect on our business, financial condition and results of operations. New, modified and additional regulations, statutes or legal interpretation, if any, could, among other things, require changes to manufacturing methods, expanded or different labeling, recall, replacement or discontinuation of certain products, additional record keeping procedures and expanded documentation of the properties of certain products and additional scientific substantiation. Such changes or new legislation could have a material adverse effect on our business, financial condition and results of operations.
We are subject to regulation by the FDA. All pharmaceutical manufacturers, including us, are subject to regulation by the FDA under the authority of the FDC Act. Under the FDC Act, the federal government has extensive administrative and judicial enforcement authority over the activities of finished drug product manufacturers to ensure compliance with FDA regulations. This authority includes, but is not limited to, the authority to initiate judicial action to seize unapproved or non-complying products, to enjoin non-complying activities, to halt manufacturing operations that are not in compliance with cGMP, to recall products, to seek civil and monetary penalties and to criminally prosecute violators. Other enforcement activities include refusal to approve product applications, withdrawal of previously approved applications or prohibition on marketing of certain grandfathered products. Any such enforcement activities, especially the restriction or prohibition on sales of products we market or the halting of our manufacturing operations, could have a material adverse effect on our business, financial condition and results of operations. In addition, the FDA or other government agencies having regulatory authority over pharmaceutical products may request us to voluntarily or involuntarily conduct product recalls due to disputed labeling claims, manufacturing issues, quality defects or for other reasons. Restriction or prohibition on sales, halting of manufacturing operations, recalls of our pharmaceutical products or other enforcement actions could have a material adverse effect on our business, financial condition and results of operations. Further, such actions, in certain circumstances, may constitute an event of default under the terms of our various financing arrangements.
We must obtain approval from the FDA for each pharmaceutical product that we market. The FDA approval process is typically lengthy, and approval is never certain. Our new products could take a significantly longer time than we expect to gain regulatory approval and may never gain approval. Even if the FDA or another regulatory agency approves a product, the approval may limit the indicated uses for a product, may otherwise limit our ability to promote, sell and distribute a product or may require post-marketing studies or impose other post-marketing obligations, which could have a material adverse effect on marketability and profitability of the new products.
We and our third-party manufacturers are subject to periodic inspection by the FDA to assure regulatory compliance regarding the manufacturing, distribution, and promotion of pharmaceutical products. The FDA imposes stringent mandatory requirements on the manufacture and distribution of pharmaceutical products to ensure their safety and efficacy. The FDA also regulates drug labeling and the advertising of prescription drugs. A finding by a governmental agency or court that we are not in compliance with FDA requirements could have a material adverse effect on our business, financial condition and results of operations.
10
If the FDA changes its regulatory position, it could force us to delay or suspend our manufacturing, distribution or sales of certain products. FDA interpretations of existing or pending regulations and standards may change over time with the advancement of associated technologies, industry trends, and/or prevailing scientific rationale. If the FDA changes its regulatory position due to such factors, it could result in delay or suspension of the manufacturing, distribution or sales of certain of our products. In addition, modifications or enhancements of approved products are in many circumstances subject to additional FDA approvals which may or may not be granted and which may be subject to a lengthy application process. Any change in the FDA’s enforcement policy or any decision by the FDA to require an approved NDA or ANDA for one of our products not currently subject to the approved NDA or ANDA requirements or any delay in the FDA approving an NDA or ANDA for one of our products could have a material adverse effect on our business, financial condition and results of operations.
We are subject to extensive DEA regulation, which could result in our being fined or otherwise penalized. We also manufacture and sell drugs which are “controlled substances” as defined in the federal Controlled Substances Act and similar state laws, which impose, among other things, certain licensing, security and record keeping requirements administered by the DEA and similar state agencies, as well as quotas for the manufacture, purchase and sale of controlled substances. The DEA could limit or reduce the amount of controlled substances which we are permitted to manufacture and market or issue fines and penalties against us for purported non-compliance with DEA regulations, which could have a material adverse effect on our business, financial condition and results of operations.
Recently enacted and future healthcare law and policy changes may adversely affect our business.
In March 2010, the U.S. Congress enacted the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act. This health care reform legislation is intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. As examples, the current legislation include measures that would (i) significantly increase Medicaid rebates through both the expansion of the program and significant increases in rebates; (ii) substantially expand the Public Health System (340B) program to allow other entities to purchase prescription drugs at substantial discounts; (iii) extend the Medicaid rebate rate to a significant portion of Managed Medicaid enrollees; (iv) assess a 50% rebate on Medicaid Part D spending in the coverage gap for branded and authorized generic prescription drugs; and (v) levy a significant excise tax on the industry to fund the healthcare reform.
While the aforementioned healthcare reform legislation may increase the number of patients who have insurance coverage for our products, such insurance mandate does not commence until January 2014, and the healthcare reform legislation also restructures payments to Medicare managed care plans and reduces reimbursements to many third-party payers. Accordingly, the timing on the insurance mandate, the change in the Medicaid rebate levels, the additional fees imposed on us to the extent we market branded drugs, other compliance obligations, and the reduced reimbursement levels to institutional customers may result in a loss of revenue and could adversely affect our business. While we will not know the full effects of this health care reform legislation until applicable federal and state agencies issue regulations or guidance under the new law and the new law has been fully implemented, it appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and to increase our regulatory burdens and operating costs.
The sales of our products depend in part on the availability of reimbursement from third-party payers such as government health administration authorities, private health insurers, health maintenance organizations including pharmacy benefit managers (“PBMs”) and other health care-related organizations. We expect both federal and state governments in the U.S. and foreign governments to continue to propose and pass new legislation, rules and regulations designed to contain or reduce the cost of healthcare while expanding individual healthcare benefits. Existing regulations that affect the price of pharmaceutical and other medical products may also change before any of our products are approved for marketing. Cost control initiatives could decrease the price that we receive for any product we develop in the future. In addition, PBMs and other third-party payers are increasingly challenging the price and cost-effectiveness of medical products and services. Significant uncertainty exists as to the reimbursement status of newly approved pharmaceutical products. Our products may not be considered cost effective, or adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize a return on our investments. All of these may harm our ability to market our products and generate profits.
Our growth and profitability is dependent on our ability to successfully utilize our existing cash reserves and operating cash flows to complete strategic acquisitions or to identify, acquire or develop, new products to market and distribute.
We continue to seek growth opportunities, either by completing strategic acquisitions or by developing and introducing new pharmaceutical products. Continued improvement in our financial performance is dependent on our ability to introduce new products on an ongoing basis, whether developed internally or by third party partners, or acquired from other companies. Any delays or an inability to successfully identify suitable acquisition targets, or acquire or develop, and market and distribute new products, or acquisition or development of new products that do not yield sufficient margins, may result in adverse financial consequences to our business.
11
We may not achieve the anticipated benefits from our acquisitions and may face difficulties in integrating them, which could adversely affect our operating results, increase costs and place a significant strain on our management.
If we fail to manage the integration of our domestic and international acquisitions and achieve expected synergies, our business could be disrupted and our operating results could be negatively impacted. The operating success of both our domestic and international acquisitions involves the integration of products, processes and personnel into our existing model. In addition, the integration of international acquisitions requires both establishing and training a local management team and overseeing the operations remotely, and can involve cultural, monetary and systems challenges. Our personnel, systems, procedures, or controls may not be adequate to support both our ongoing business and the acquired businesses. If our newly-acquired businesses require a disproportionate share of our resources and management’s attention, our overall financial results may suffer.
We have entered into several strategic business alliances that may not result in marketable products.
We have entered into several strategic business alliances that have been formed to supply us with low cost finished dosage form products. We have entered into various purchase and supply agreements and license agreements that are all designed to provide finished dosage form products that can be marketed through our distribution pipeline. There can be no assurance that these agreements will result in additional FDA-approved ANDAs or NDAs, or that we will be able to market any such additional products at a profit. In addition, any clinical trial expenses that we may incur in connection with these strategic business alliances may negatively impact our financial results.
Failure to obtain regulatory certification of our manufacturing plant in India for production of pharmaceutical products for export to the United States, as well as other regulated world markets, could impair our ability to grow and adversely affect our business, financial condition and results of operations.
We operate a manufacturing campus in Paonta Sahib, India, which we acquired through a business combination in 2012. The manufacturing units within this campus were built to the standards of regulated markets, including the United States, but they are not currently approved by the FDA to manufacture products for export to the United States. It is our intention to obtain certification from the FDA and other regulatory authorities to allow this facility to manufacture products for export to the United States and other regulated world markets. Obtaining such certification in a timely manner is critical to our sustaining our growth. An inability to obtain or maintain such certification could restrict our ability to achieve our growth objectives, which would adversely affect our business, financial condition and results of operations.
Further, our operations in India may be adversely affected by general economic conditions and economic and fiscal policy in India, including changes in exchange rates and controls, interest rates and taxation policies; any reversal of India’s recent economic liberalization and deregulation policies; as well as social stability and political, economic or diplomatic developments affecting India in the future. In addition, India is known to have experienced governmental corruption to some degree and, in some circumstances, anti-bribery laws may conflict with some local customs and practices. As a result of our policy to comply with the U.S. Foreign Corrupt Practices Act (“FCPA”) and similar anti-bribery laws, we may be at a competitive disadvantage to competitors that are not subject to, or do not comply with, such laws.
We may not generate cash flow sufficient to pay interest on our outstanding convertible senior notes or repurchase the notes upon a fundamental change.
In June 2011, we issued $120.0 million aggregate principal amount of 3.50% Convertible Senior Notes due 2016 (the “Notes”). The Notes require us to make semi-annual coupon interest payments of $2.1 million on June 1 and December 1 of each year until the Notes mature on June 1, 2016. If we do not generate sufficient operating cash flows to fund these payments or obtain additional funding from external sources at acceptable terms, we may not have sufficient funds to satisfy our interest payment obligations when those obligations are due which would place us in default under the Indenture (as defined below). If a fundamental change (as defined in the Indenture) occurs, holders of the Notes may require us to repurchase their Notes. If we fail to repurchase the Notes when required, we will be in default under the Indenture.
Availability under our Credit Agreement may be restricted if we fail to meet our covenant requirements.
We are party to a revolving Credit Agreement with Bank of America, N.A., (the “Agent”) and other financial institutions (collectively with the Agent, the “BoA Lenders”) through which we obtained a $20.0 million revolving line of credit (the “BoA Credit Facility”), which includes a $2.0 million letter of credit facility. We may request expansion of the BoA Credit Facility from time to time in increments of at least $5.0 million up to a maximum commitment of $35.0 million, so long as no default or event of default has occurred and is continuing. As of December 31, 2012, no amounts or letters of credit were outstanding under the BoA Credit Facility.
12
Availability under the BoA Credit Facility is equal to the lesser of (a) $20.0 million reduced by outstanding letter of credit obligations or (b) the amount of a Borrowing Base (as defined in the BoA Credit Agreement) determined by reference to the value of the borrowers’ eligible accounts receivable, eligible inventory and fixed assets as of the closing date and the end of each calendar month thereafter. The BoA Credit Agreement contains representations and warranties, and affirmative and negative covenants customary for financings of this type, including, but not limited to, limitations on: distributions; additional borrowings, liens and guarantees; additional investments and asset sales; and fundamental changes to corporate structure or organization documents. The financial covenants require the Borrowers to maintain a fixed charge coverage ratio of at least 1.1 to 1.0 during any period commencing on the date that an event of default occurs or availability under the BoA Credit Agreement is less than 15% of the aggregate BoA Lenders’ commitments under the BoA Credit Agreement. In addition, we must periodically provide to the Agent financial statements, compliance certificates and budget projections. Should we fail to maintain compliance with these covenants, availability under the Credit Agreement could be restricted which would negatively impact our liquidity and may require us to seek additional sources of capital in order to maintain our continuing operations or to fund growth opportunities.
We may need to obtain additional capital to continue to grow our business.
We may require additional funds in order to materially grow our business. We require substantial liquidity to implement long-term cost savings and productivity improvement plans, continue capital spending to improve our manufacturing plants to increase capacity and support product development programs, meet scheduled term debt and lease maturities, and run our normal business operations. We may seek additional funds through public and private financing, including equity and debt offerings. However, adequate funds through the financial markets or from other sources may not be available to us when needed or on favorable terms. Without sufficient additional capital funding, we may be required to delay, scale back or abandon some or all of our product development, manufacturing, acquisition, licensing and marketing initiatives, or operations. Further, such additional financing, if obtained, may require the granting of rights, preferences or privileges senior to those of the common stock and result in substantial dilution of the existing ownership interests of the common stockholders and could include covenants and restrictions that limit our ability to operate or expand our business in a manner that we deem to be in our best interest.
Our industry is very competitive. Additionally, changes in technology could render our products obsolete.
We face significant competition from other pharmaceutical companies, including major pharmaceutical companies with financial resources substantially greater than ours, in developing, acquiring, manufacturing and marketing pharmaceutical products. The selling prices of pharmaceutical products typically decline as competition increases. Further, other products now in use, under development or acquired by other pharmaceutical companies, may be more effective or offered at lower prices than our current or future products. The industry is characterized by rapid technological change that may render our products obsolete, and competitors may develop their products more rapidly than we can. Competitors may also be able to complete the regulatory process sooner, and therefore, may begin to market their products in advance of ours. We believe that competition in sales of our products is based primarily on price, service and technical capabilities. There can be no assurance that: (i) we will be able to develop or acquire commercially attractive pharmaceutical products; (ii) additional competitors will not enter the market; (iii) our existing products will not be rendered obsolete by the introduction or switch to generic of competing products; or (iv) competition from other pharmaceutical companies will not have a material adverse effect on our business, financial condition and results of operations.
Unstable market and economic conditions may have serious adverse consequences on our business.
Our general business strategy may be adversely affected by general economic conditions, a volatile business environment and continued unpredictable and unstable market conditions. If equity and credit market conditions prove unfavorable, we may have difficulty obtaining desired debt or equity financing, or obtaining such financing may be more difficult, more costly, and more dilutive. A prolonged or profound economic downturn could result in adverse changes to product reimbursement, pricing or sales levels, which would harm our operating results. There is a risk that one or more of our current service providers, manufacturers and other partners may not survive difficult economic times, which would directly affect our ability to attain our operating goals on schedule and on budget. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon development plans. Moreover, our stock price may decline due to the volatility of the stock market and general economic conditions.
13
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results, timely file our periodic reports, maintain our reporting status or prevent fraud.
In connection with the audit of our financial statements as of and for the year ended December 31, 2012, we concluded there is a material weakness in internal control over financial reporting related to deficiencies in the financial statement close process. Under standards established by the Public Company Oversight Board, a material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected or corrected on a timely basis.
We are working to remediate the material weakness. We have begun taking steps and plan to take additional measures to remediate the underlying causes of the material weakness, primarily through the continued development and implementation of formal policies, improved processes and documented procedures, as well as the hiring of additional finance personnel. The actions that we are taking are subject to ongoing senior management review, as well as audit committee oversight. Although we plan to complete this remediation as quickly as possible, we cannot at this time estimate how long it will take, and our initiatives may not prove to be successful in remediating this material weakness. If our remedial measures are insufficient to address the material weakness, or if additional material weaknesses or significant deficiencies in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain material misstatements and we could be required to restate our financial results.
Our management or our independent registered public accounting firm may identify other material weaknesses in our internal control over financial reporting in the future. The existence of internal control material weaknesses may result in current and potential stockholders losing confidence in our financial reporting, which could harm our business, the market price of our common stock, and our ability to retain our current, or obtain new, alliance and collaboration agreements’ partners.
In addition, the existence of material weaknesses in our internal control over financial reporting may affect our ability to timely file periodic reports under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The inability to timely file periodic reports could result in the SEC revoking the registration of our common stock, which would prohibit us from listing or having our stock quoted on any public market. This would have an adverse effect on our business and stock price by limiting the publicly available information regarding us and greatly reducing the ability of our stockholders to sell or trade our common stock.
We may become involved in legal proceedings from time to time which may result in losses, damage to our business and reputation and place a strain on our internal resources.
In the ordinary course of our business, we may be involved in legal proceedings with both private parties and certain government agencies, including FDA. Litigation may result in verdicts against us, which may include significant monetary awards, judgments that certain of our intellectual property rights are invalid or unenforceable and injunctions preventing the manufacture, marketing and sale of our products. If disputes are resolved unfavorably, our business, financial condition and results of operations may be adversely affected.
Any litigation, whether or not successful, may damage our reputation. Furthermore, we are likely to incur substantial expense in defending these lawsuits and the time demands of such lawsuits could divert management’s attention from ongoing business concerns and interfere with our normal operations.
In the normal course of business, we periodically enter into employment agreements, legal settlements, and other agreements which incorporate indemnification provisions. We maintain insurance coverage which we believe will effectively mitigate our obligations under these indemnification provisions. However, should our obligation under an indemnification provision exceed our coverage or should coverage be denied, it could have a material adverse effect on our business, financial position and results of operations.
Third parties may claim that we infringe their proprietary rights and may prevent or delay us from manufacturing and selling some of our new products.
The manufacture, use and sale of new products that are the subject of conflicting patent rights have been the subject of substantial litigation in the pharmaceutical industry. Pharmaceutical companies with patented brand products frequently sue companies that file applications to produce generic equivalents of their patented brand products for alleged patent infringement or other violations of intellectual property rights, which may delay or prevent the entry of such generic products into the market. Generally, a generic drug may not be marketed until the applicable patent(s) on the brand name drug expire or are held to be not infringed, invalid, or unenforceable. When we or our development partners submit an ANDA to the FDA for approval of a generic drug, we and/or our development partners must certify either (1) that there is no patent listed by the FDA as covering the relevant brand product, (2) that any patent listed as covering the brand product has expired, (3) that the patent listed as covering the brand product will expire prior to the marketing of the generic product, in which case the ANDA will not be finally approved by the FDA until the expiration of such patent, or (4) that any patent listed as covering the brand drug is invalid or will not be infringed by the manufacture, sale or use of the generic product for which the ANDA is submitted.
14
Under any circumstance in which an act of infringement is alleged to occur, there is a risk that a brand pharmaceutical company may sue us for alleged patent infringement or other violations of intellectual property rights. Also, competing pharmaceutical companies may file lawsuits against us or our strategic partners alleging patent infringement or may file declaratory judgment actions of non-infringement, invalidity, or unenforceability against us relating to our own patents. We have been sued for patent infringement related to several of our current ANDA filings and we anticipate that we will be sued once we file ANDAs for other products in our pipeline. Such litigation is often costly and time-consuming and could result in a substantial delay in, or prevent, the introduction and/or marketing of our products, which could have a material adverse effect on our business, financial condition and results of operations.
Although the parties to patent and intellectual property disputes in the pharmaceutical industry have often settled their disputes through licensing or similar arrangements, the costs associated with these arrangements may be substantial and could include ongoing royalties. Furthermore, we cannot be certain that the necessary licenses would be available to us on terms we believe to be acceptable.
Our patents and proprietary rights may not adequately protect our products and processes.
The patent and proprietary rights position of competitors in the pharmaceutical industry generally is highly uncertain, involves complex legal and factual questions, and is the subject of much litigation. There can be no assurance that any patent applications or other proprietary rights, including licensed rights, relating to our potential products or processes will result in patents being issued or other proprietary rights secured, or that the resulting patents or proprietary rights, if any, will provide protection against competitors who: (i) successfully challenge our patents or proprietary rights; (ii) obtain patents or proprietary rights that may have an adverse effect on our ability to conduct business; or (iii) are able to circumvent our patent or proprietary rights position. It is possible that other parties have conducted or are conducting research and could make discoveries of pharmaceutical formulations or processes that would precede any discoveries made by us, which could prevent us from obtaining patent or other protection for these discoveries or marketing products developed therefrom. Consequently, there can be no assurance that others will not independently develop pharmaceutical products similar to or rendering obsolete those that we are planning to develop, or duplicate any of our products. Our inability to obtain patents for, or other proprietary rights in, our products and processes or the ability of competitors to circumvent or obsolete our patents or proprietary rights could have a material adverse effect on our business, financial condition and results of operations.
Further, virtually all the drug products that we market are generics, with essentially no patent or proprietary rights attached. While this fact allowed us the opportunity to develop or to purchase and obtain FDA approval to market our generic products, it also allows competing drug companies to do the same. Should multiple additional drug companies choose to develop and market the same generic products that we actively market, our profit margins could decline, which would have a material adverse effect on our business, financial condition and results of operations.
The Chairman of our Board of Directors is subject to conflicts of interest, and through his stock ownership and position as Chairman has substantial influence over our business strategies and policies.
John N. Kapoor, Ph.D., the Chairman of our Board of Directors and a principal shareholder, is the President of EJ Financial Enterprises, Inc. (“EJ Financial”), a health care consulting investment company. EJ Financial is involved in the management of health care companies in various fields, and Dr. Kapoor is involved in various capacities with the management and operation of these companies. The John N. Kapoor Trust dated 9/20/89 (the “Kapoor Trust”), the beneficiary and sole trustee of which is Dr. Kapoor, is a principal shareholder of each of these companies. As a result, Dr. Kapoor does not devote his full time to our business. Although such companies do not currently compete directly with us, certain companies with which EJ Financial is involved are in the pharmaceutical business. Discoveries made by one or more of these companies could render our products less competitive or obsolete. Potential conflicts of interest could have a material adverse effect on our business, financial condition and results of operations.
As of December 31, 2012, Dr. Kapoor beneficially owns approximately 31% of our common stock. As a result, Dr. Kapoor can strongly influence, and potentially control, the outcome of our corporate actions, including the election of our directors and transactions involving a change of control. This concentrated control limits other shareholders’ ability to influence corporate matters and, as a result, the Company may take actions that other shareholders do not view as beneficial. Further, decisions made by Dr. Kapoor with respect to his and his related parties’ ownership or trading of our common stock could have an adverse effect on the market value of our common stock and an adverse effect on our business.
We depend on key executive officers and must continue to attract and retain key personnel in order to compete successfully.
Our success will depend, in part, on our ability to attract and retain key executive officers. The loss of one or more of our key executive officers could have a material adverse effect on our business, financial condition and results of operations.
15
Further, our performance depends, to a large extent, on the continued service of our key research and development personnel, other technical employees, managers and sales personnel and our ability to continue to attract and retain such personnel. Competition for such personnel is intense, particularly for highly motivated and experienced research and development and other technical personnel. We are facing increasing competition from companies with greater financial resources for such personnel. There can be no assurance that we will be able to attract and retain sufficient numbers of highly skilled personnel in the future, and the inability to do so could have a material adverse effect on our business, and on our results of operations and financial condition.
We may implement product recalls and could be exposed to significant product liability claims; we may have to pay significant amounts to those harmed and may suffer from adverse publicity as a result.
The manufacturing and marketing of pharmaceuticals involves an inherent risk that our products, or items within our products, may prove to be defective and cause a health risk. In that event, we may voluntarily implement a recall or market withdrawal or may be required to do so by a regulatory authority. We have recalled products in the past and, based on this experience, believe that the occurrence of a recall could result in significant costs to us, potential disruptions in the supply of our products to our customers and adverse publicity, all of which could harm our ability to market our products. For example, we were prompted to initiate one product recall of our Cyanide Antidote Kit during 2008 due to recall notification by Becton, Dickinson and Company of their 60ml syringe. Our recall of the Cyanide Antidote Kit was monitored by FDA and has resulted in no patient impact and no shortage of product supply to the marketplace. There were no product recalls during 2010, 2011 or 2012.
Although we are not currently subject to any material product liability proceedings, we may incur material liabilities relating to product liability claims in the future. Even meritless claims could subject us to adverse publicity, hinder us from securing insurance coverage in the future and require us to incur significant legal fees and divert the attention of the key employees from running our business. Successful product liability claims brought against us could have a material adverse effect on our business, financial condition and results of operations.
We currently have product liability insurance in the amount of $10,000,000 for aggregate annual claims with a $100,000 deductible per incident and a $500,000 aggregate annual deductible. However, there can be no assurance that such insurance coverage will be sufficient to fully cover potential claims. Additionally, there can be no assurance that adequate insurance coverage will be available in the future at acceptable costs, if at all, or that a product liability claim would not have a material adverse effect on our business, financial condition and results of operations.
FDA may require us to stop marketing certain grandfathered drugs.
We market several generic prescription products which do not have formal FDA approvals because these products have been grandfathered. These products are non-application drugs that are manufactured and marketed without FDA-issued ANDAs or NDAs on the basis of their having been marketed by industry prior to the 1962 Amendment of the FDC Act. We marketed eight such products during 2012, generating net sales revenue of $20.8 million. Following enactment of the FDC Act in 1938, drugs on the market prior to that time were exempted or “grandfathered” and manufacturers were not required to file an NDA. Recently, FDA has increased its efforts to force companies to file and seek FDA approval for grandfathered products. Efforts have included issuing notices to companies currently manufacturing these products to cease its distribution of said products.
On October 2, 2012, we received a warning letter from FDA citing that we were manufacturing Pilocarpine Hydrochloride Ophthalmic Solution (“PHOS”), a long grandfathered drug, without an approved NDA. We fully cooperated with the FDA and discontinued selling PHOS. No enforcement action was initiated and no fines were assessed by FDA against us and the loss of revenue associated with the discontinuation of PHOS is expected to be insignificant. Further, in the second quarter of 2012, we filed an ANDA for PHOS, which has been granted expedited review.
If FDA issues additional warning letters with respect one or more of our grandfathered products, we may be forced to discontinue marketing the affected products, which could have an adverse effect on our revenues and results of operations.
The FDA may authorize sales of some prescription pharmaceuticals on a non-prescription basis, which would reduce the profitability of our prescription products.
From time to time, the FDA elects to permit sales of some pharmaceuticals currently sold on a prescription basis, without a prescription. FDA approval of the sale of our products without a prescription would reduce demand for our competing prescription products and, accordingly, reduce our profits.
16
Our revenues depend on sale of products manufactured by third parties, which we cannot control.
We rely on external third parties to manufacture certain of the products we sell. Currently, this risk is limited to several of our products. However, we expect this risk to become more significant as we receive approvals for new products to be manufactured through our strategic partnerships and as we seek additional growth opportunities beyond the capacity and capabilities of our current manufacturing facilities. If we are unable to obtain or retain third-party manufacturers for these products on commercially acceptable terms, we may not be able to distribute such products as planned. Further, no assurance can be given that the manufacturers we use will be able to provide us with sufficient quantities of our products to meet our needs or that the products supplied to us will meet our specifications. Any delays or difficulties with third-party manufacturers could adversely affect the marketing and distribution of certain of our products, which could have a material adverse effect on our business, financial condition and results of operations.
Many of the raw materials and components used in our products come from a single source.
We require a supply of quality raw materials and components to manufacture and package pharmaceutical products for ourselves and for third parties with which we have contracted. Many of the raw materials and components used in our products come from a single source and interruptions in the supply of these raw materials and components could disrupt our manufacturing of specific products and cause our sales and profitability to decline. Further, in the case of many of our ANDAs and NDAs, only one supplier of raw materials has been identified. Because FDA approval of drugs requires manufacturers to specify their proposed suppliers of active pharmaceutical ingredients and certain packaging materials in their applications, FDA approval of any new supplier would be required if active ingredients or such packaging materials were no longer available from the specified supplier. The qualification of a new supplier could delay our development and marketing efforts. If for any reason we are unable to obtain sufficient quantities of any of the raw materials or components required to produce and package our products, we may not be able to manufacture our products as planned, which could have a material adverse effect on our business, financial condition and results of operations.
We could experience business interruptions at our manufacturing facilities, which may have a material adverse effect on our business, financial position and results of operations.
We manufacture drug products at one international and two domestic manufacturing facilities. Any one or more of these facilities may be forced to shut down or may be unable to operate at full capacity as a result of hurricanes, tornadoes, earthquakes, storms and other extreme weather events as well as strikes, war, violent upheavals, terrorist acts and other force majeure events. For example, our manufacturing plant in Somerset, New Jersey was shut down for approximately two weeks in October/November 2012 as a result of power outages and related business disruptions caused by Superstorm Sandy. A significant disruption at any of these facilities, even on a short-term basis, could impair our ability to produce and ship drug products to the market on a timely basis, which may have a material adverse effect on our business, financial position and results of operations.
The testing required for the regulatory approval of our products is conducted by independent third parties. Any failure by any of these third parties to perform this testing properly and in a timely manner may have an adverse effect upon our ability to obtain regulatory approvals.
Our applications for the regulatory approval of our products incorporate the results of testing and other information that is conducted or gathered by independent third parties (including, for example, manufacturers of raw materials, testing laboratories, contract research organizations or independent research facilities). Our ability to obtain regulatory approval of the products being tested is dependent upon the quality of the work performed by these third parties, the quality of the third parties’ facilities, and the accuracy of the information provided by third parties. We have little or no control over any of these factors. If this testing is not performed properly, our ability to obtain regulatory approvals could be restricted or delayed.
We may be subject to disruptions or failures in our information technology systems and network infrastructures that could have a material adverse effect on our business.
We rely on the efficient and uninterrupted operation of complex information technology systems and network infrastructures to operate our business. We also hold data in various data center facilities upon which our business depends. A disruption, infiltration or failure of our information technology systems or any of our data centers as a result of software or hardware malfunctions, system implementations or upgrades, computer viruses, third-party security breaches, employee error, theft or misuse, malfeasance, power disruptions, natural disasters or accidents could cause breaches of data security, loss of intellectual property and critical data and the release and misappropriation of sensitive competitive information. Any of these events could result in the loss of key information, impair our production and supply chain processes, harm our competitive position, cause us to incur significant costs to remedy any damages and ultimately materially and adversely affect our business, results of operations and financial condition. While we have implemented a number of protective measures, such measures may not be adequate or implemented properly to prevent or fully address the adverse effect of such events.
17
Failure to comply with the U.S. Foreign Corrupt Practices Act could subject us to, among other things, penalties and legal expenses that could harm our reputation and have a material adverse effect on our business, financial condition and operating results.
Our U.S. operations are currently subject to the FCPA. We are required to comply with the FCPA, which generally prohibits covered entities and their intermediaries from engaging in bribery or making other prohibited payments to foreign officials for the purpose of obtaining or retaining business or other benefits. In addition, the FCPA imposes accounting standards and requirements on publicly traded U.S. corporations and their foreign affiliates, which are intended to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. If our employees, third-party sales representatives or other agents are found to have engaged in such practices, we could suffer severe penalties, including criminal and civil penalties, disgorgement and other remedial measures, including further changes or enhancements to our procedures, policies and controls, as well as potential personnel changes and disciplinary actions.
Any failure to comply with the complex reporting and payment obligations under Medicare, Medicaid and other government programs may result in litigation or sanctions.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback, false claims, marketing and pricing laws. We are also subject to Medicaid and other government reporting and payment obligations that are highly complex and somewhat ambiguous. Violations of these laws and reporting obligations are punishable by criminal and/or civil sanctions and exclusion from participation in federal and state healthcare programs such as Medicare and Medicaid. The recent healthcare reform legislation made several changes to the federal anti-kickback statute, false claims laws, and health care fraud statute such as increasing penalties and making it easier for the government to bring sanctions against pharmaceutical companies. If our past, present or future operations are found to be in violation of any of the laws described above or other similar governmental regulations, we may be subject to the applicable penalty associated with the violation which could adversely affect our ability to operate our business and negatively impact our financial results. Further, if there is a change in laws, regulations or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which could materially adversely affect our business, financial position and results of operations.
The requirements of being a public company may strain our resources and distract management.
As a public company, we are subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). These requirements are extensive. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, significant resources and management oversight is required. This may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition and results of operations.
Exercise of warrants and options, or issuance of shares pursuant to our convertible debt, may have a substantial dilutive effect on our common stock.
If the price per share of our common stock at the time of exercise or conversion of any warrants or stock options is in excess of the various exercise or conversion prices of such convertible securities, exercise or conversion of such convertible securities would have a dilutive effect on our common stock. As of December 31, 2012, holders of our outstanding warrants and options would receive 16,919,342 shares of our common stock at a weighted average exercise price of $2.91 per share. Any additional financing that we secure likely will require the granting of rights, preferences or privileges senior to those of our common stock which may result in substantial dilution of the existing ownership interests of our common shareholders.
Our earnings per share will be diluted if the average closing price of our common stock exceeds the conversion price (currently $8.76 per share) on our convertible Notes. In addition, the Notes become convertible if the closing trading price of our common stock exceeds 130% of the Conversion Price for 20 of the last 30 trading days of any calendar quarter through the remaining term of the Notes. If the Notes become convertible and are surrendered for conversion, we have the option of satisfying all or a portion of our obligation in shares of our common stock, which could result in substantial dilution of the existing ownership interests of our common shareholders.
18
We may issue preferred stock and the terms of such preferred stock may reduce the market value of our common stock.
We are authorized to issue up to a total of 5,000,000 shares of preferred stock in one or more series. Our board of directors may authorize issuance of additional shares of preferred stock and the terms of such preferred stock without further action by holders of our common stock. If we issue additional shares of preferred stock, it could affect the rights or reduce the market value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with or sell our assets to a third party. These terms may include voting rights, preferences as to dividends and liquidation, conversion and redemption rights, and sinking fund provisions. We continue to seek capital for the growth of our business, and this additional capital may be raised through the issuance of additional preferred stock.
We experience significant quarterly fluctuation of our results of operations, which may increase the volatility of our stock price.
Our results of operations may vary from quarter to quarter due to a variety of factors including, but not limited to, the timing of the development and marketing of new pharmaceutical products, the failure to develop such products, delays in obtaining government approvals, including FDA approval of NDAs or ANDAs for our products, expenditures to comply with governmental requirements for manufacturing facilities, expenditures incurred to acquire and promote pharmaceutical products, changes in our customer base, a customer’s termination of a substantial account, the availability and cost of raw materials, interruptions in supply by third-party manufacturers, seasonal or cyclical fluctuations in the sales of certain of our products, the introduction of new products or technological innovations by our competitors, loss of key personnel, changes in the mix of products sold by us, changes in sales and marketing expenditures, competitive pricing pressures, expenditures incurred to pursue or contest pending or threatened legal action and our ability to meet our financial covenants. There can be no assurance that we will be successful in avoiding losses in any future period. Such fluctuations may result in volatility in the price of our common stock.
Further, concentrated ownership of our common stock creates a risk of sudden changes in our share price. As such, the sale by any of our large shareholders of a significant portion of that shareholder’s holdings could have a material adverse effect on the market price of our common stock.
19
None.
We have two company-owned facilities in Decatur, Illinois. The Wyckles Road facility, which consists of 76,000 square feet of building space located on 15 acres of land, is used for packaging, warehousing, distribution, and office space. The Grand Avenue facility is a 65,000 square-foot manufacturing facility. Our Decatur facilities support our ophthalmic, hospital drugs & injectables, and contract services segments.
Our wholly-owned subsidiary, Akorn (New Jersey) Inc. leases a 50,000 square-foot facility in Somerset, New Jersey pursuant to a seven-year lease agreement that commenced on August 1, 2010. This lease allows us the option to renew for up to four additional 5-year periods beyond the initial expiration date of July 31, 2017. The Somerset facility is used for drug manufacturing, research and development and administrative activities related to our ophthalmic and hospital drugs & injectables segments.
Our current space in Decatur is considered adequate to accommodate our current manufacturing needs, and at Somerset we have expanded our manufacturing space and continue to make capital improvements to accommodate both current demand and anticipated future growth opportunities.
Our corporate headquarters and administrative offices consist of 34,000 square feet of leased space in an office building in Lake Forest, Illinois. We maintain a leased space in Gurnee, Illinois, consisting of 74,000 square feet, to accommodate our product warehousing and distribution needs. Both the Lake Forest lease and the Gurnee lease extend through March 2018. We are in the process of relocating our R&D operations to a 19,000-square foot leased facility in Vernon Hills, Illinois pursuant to an 89-month lease expiring April 30, 2020. We anticipate moving into this new facility in March 2013 and vacating our previous R&D facility in Skokie, Illinois shortly thereafter. The lease on our Skokie, Illinois R&D facility has been shortened by two years and will now expire on January 31, 2014. Our subsidiary, AVR, maintains their corporate offices in a 3,200-square foot leased facility in Ann Arbor, Michigan.
Our wholly-owned subsidiary, AIPL, owns and operates approximately 245,000 square feet of pharmaceutical manufacturing, warehousing and distribution facilities situated on approximately 14 acres of land in Paonta Sahib, Himachal Pradesh, India. This facility manufactures drugs primarily for contract customers in India and for export to various unregulated world markets.
On September 12, 2012, Fera Pharmaceuticals, LLC (“Fera”) filed a civil complaint against the Company and certain individual defendants (together, the “Defendants”) in the Supreme Court of New York (the “Fera lawsuit”). The complaint alleges, among other things, breach of manufacturing and confidentiality agreements and misappropriation of the plaintiff’s trade secrets. On October 15, 2012, the case was removed to the Federal District Court for the Southern District of New York. Fera filed an amended complaint on December 21, 2012. The Defendants filed a motion to dismiss portions of the amended complaint on January 25, 2013. The Company intends to vigorously defend these allegations. However, no assurance may be given regarding the ultimate outcome of this lawsuit.
In April 2012, Allergan Sales (“Allergan”) filed a lawsuit alleging patent infringement claims against the Company relating to the 0.4% ketorolac tromethamine formulation. Allergan seeks unspecified monetary damages in this case. The Company has asserted invalidity and non-infringement. The Company intends to vigorously defend these allegations. However, no assurance may be given regarding the ultimate outcome of this lawsuit.
We are party to legal proceedings and potential claims arising in the ordinary course of our business. The amount, if any, of ultimate liability with respect to such matters cannot be determined. Despite the inherent uncertainties of litigation, we at this time do not believe that such proceedings will have a material adverse impact on our financial condition, results of operations, or cash flows.
20
The following table sets forth, for the fiscal periods indicated, the high and low sales prices for our common stock for the two most recent fiscal years and for the first quarter of our current fiscal year. On February 7, 2007, our common stock was listed on the NASDAQ Global Market under the symbol “AKRX” and continues to be listed there as of the date hereof. Previously, from November 24, 2004 until February 6, 2007, our common stock was listed on the American Stock Exchange under the symbol “AKN.”
High | Low | |||||||
| Year Ending December 31, 2013 | ||||||||
| 1st Quarter (through February 25, 2013) | $ | 14.70 | $ | 12.44 | ||||
| Year Ended December 31, 2012 | ||||||||
| 4th Quarter | $ | 13.77 | $ | 11.73 | ||||
| 3rd Quarter | 16.87 | 11.99 | ||||||
| 2nd Quarter | 16.09 | 10.53 | ||||||
| 1st Quarter | 13.09 | 10.52 | ||||||
| Year Ended December 31, 2011 | ||||||||
| 4th Quarter | $ | 11.77 | $ | 7.10 | ||||
| 3rd Quarter | 9.50 | 6.63 | ||||||
| 2nd Quarter | 7.15 | 5.66 | ||||||
| 1st Quarter | 6.20 | 4.87 | ||||||
As of February 25, 2013, there were 95,921,212 shares of our common stock outstanding, held by approximately 385 stockholders of record. This number does not include stockholders for which shares are held in a “nominee” or “street” name. The closing price of our common stock on February 25, 2013 was $12.56 per share.
We did not pay cash dividends in 2012, 2011 or 2010 and do not expect to pay dividends on our common stock in the foreseeable future. Moreover, we may be restricted from making dividend payments pursuant to the terms of our $20.0 million revolving Loan and Security Agreement with Bank of America, N.A., and other financial institutions (see Note 6, Financing Arrangements).
We did not repurchase any shares of our common stock during the fourth quarter of the fiscal year covered by this report.
21
PERFORMANCE GRAPH
The following Stock Performance Graph and related information shall not be deemed “soliciting material” or “filed” with the Securities and Exchange Commission, nor should such information be incorporated by reference into any future filings under the Securities Act of 1933 or the Securities Exchange Act of 1934, each as amended, except to the extent that we specifically incorporate it by reference in such filing.
The graph below compares the cumulative shareholder return on our common stock with the NASDAQ Stock Market (U.S.) Index, and the Nasdaq Health Care Index (ticker symbol: ^IXHC) over the last five years through December 31, 2012. The graph assumes $100 was invested in our common stock, and also the two indices presented, at the end of December 2007 and that all dividends were reinvested during the subsequent five-year period.
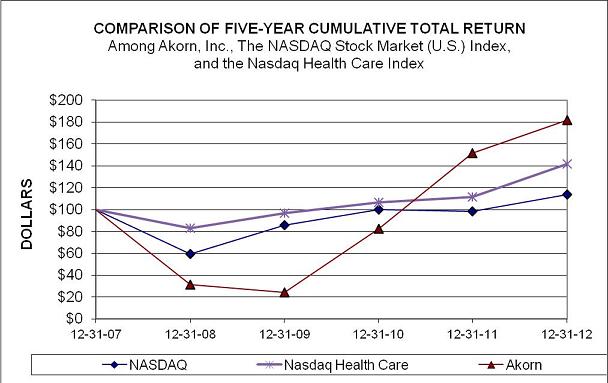
| Total Return Chart | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | ||||||||||||
| NASDAQ Stock Market (U.S.) Index | 100 | 59 | 86 | 100 | 98 | 114 | ||||||||||||
| NASDAQ Health Care Index (^IXHC) | 100 | 83 | 97 | 107 | 112 | 142 | ||||||||||||
| Akorn, Inc. (AKRX) | 100 | 31 | 24 | 83 | 151 | 182 |
22
The following table sets forth selected summary historical financial data. We have prepared this table using our consolidated financial statements for the five years ended December 31, 2012. Our consolidated financial statements during this period have been audited by Ernst & Young LLP, independent registered public accounting firm. This summary should be read in conjunction with our audited Consolidated Financial Statements and Notes thereto, and "Item 7 -- Management's Discussion and Analysis of Financial Condition and Results of Operations" and other financial information included herein.
| Years Ended December 31, | ||||||||||||||||||||
| 2012 | 2011 | 2010 | 2009 | 2008 | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
| Revenues | $ | 256,158 | $ | 136,920 | $ | 86,409 | $ | 75,891 | $ | 93,598 | ||||||||||
| Gross profit | 148,692 | 79,689 | 42,465 | 15,672 | 26,592 | |||||||||||||||
| Operating income (loss) | 68,756 | 33,266 | 11,272 | (19,512 | ) | (7,183 | ) | |||||||||||||
| Interest and other non-operating income (expense) | (11,256 | ) | 8,040 | 10,704 | (5,792 | ) | (752 | ) | ||||||||||||
| Pretax income (loss) | 57,500 | 41,306 | 21,976 | (25,304 | ) | (7,935 | ) | |||||||||||||
| Income tax provision (benefit) | 22,122 | (1,707 | ) | 152 | 2 | 4 | ||||||||||||||
| Net income (loss) | $ | 35,378 | $ | 43,013 | $ | 21,824 | $ | (25,306 | ) | $ | (7,939 | ) | ||||||||
| Weighted average shares outstanding: | ||||||||||||||||||||
| Basic | 95,189 | 94,549 | 92,801 | 90,253 | 89,209 | |||||||||||||||
| Diluted | 110,510 | 103,912 | 99,250 | 90,253 | 89,209 | |||||||||||||||
| PER SHARE: | ||||||||||||||||||||
| Equity, per diluted share | $ | 1.82 | $ | 1.52 | $ | 0.87 | $ | 0.43 | $ | 0.69 | ||||||||||
| Net income (loss): | ||||||||||||||||||||
| Basic | 0.37 | 0.45 | 0.24 | (0.28 | ) | (0.09 | ) | |||||||||||||
| Diluted | 0.32 | 0.41 | 0.22 | (0.28 | ) | (0.09 | ) | |||||||||||||
| Share Price: High | 16.87 | 11.77 | 6.50 | 2.69 | 8.19 | |||||||||||||||
| Low | 10.52 | 4.87 | 1.27 | 0.73 | 1.11 | |||||||||||||||
| BALANCE SHEET DATA: | ||||||||||||||||||||
| Current assets | $ | 158,707 | $ | 155,949 | $ | 73,613 | $ | 26,069 | $ | 40,746 | ||||||||||
| Net property, plant & equipment | 80,679 | 44,389 | 32,731 | 31,473 | 34,223 | |||||||||||||||
| Total assets | 369,565 | 307,145 | 111,116 | 68,759 | 82,329 | |||||||||||||||
| Current liabilities, including debt in default | 43,291 | 28,289 | 21,940 | 21,666 | 18,103 | |||||||||||||||
| Long-term obligations, less current installments | 125,193 | 120,648 | 2,424 | 8,456 | 2,783 | |||||||||||||||
| Shareholders’ equity | 201,081 | 158,208 | 86,752 | 38,637 | 61,443 | |||||||||||||||
| CASH FLOW DATA: | ||||||||||||||||||||
| Cash provided by (used in) operating activities | $ | 26,244 | $ | 19,657 | $ | 12,282 | $ | (1,038 | ) | $ | (5,420 | ) | ||||||||
| Cash (used in) provided by investing activities | (75,501 | ) | (95,034 | ) | 31,555 | (1,397 | ) | (3,787 | ) | |||||||||||
| Cash provided by (used in) financing activities | 6,366 | 117,716 | (3,831 | ) | 2,989 | 2,322 | ||||||||||||||
| (Decrease)/increase in cash and cash equivalents | (43,181 | ) | 42,339 | 40,006 | 554 | (6,885 | ) | |||||||||||||
The Company’s consolidated statement of cash flows for the year ended December 31, 2011 has been restated to correct a classification error which resulted in overstatement of cash provided by operating activities in the amount of $3,346,000 and overstatement of cash used by investing activities by that same amount. The error was related to capital expenditures that were accrued but unpaid.
23
OVERVIEW
We manufacture and market a full line of diagnostic and therapeutic ophthalmic pharmaceuticals as well as niche hospital drugs and injectable pharmaceuticals. We manufacture and/or offer products in various specialty areas, including ophthalmology, antidotes, anti-infectives, controlled substances for pain management and anesthesia, and vaccines, among others. We also manufacture and market a line of over-the-counter dry eye and eye health products under the brand name TheraTears®.
We have three identified operating segments:
Active segments:
| ■ | Ophthalmic – sales of diagnostic and therapeutic ophthalmic drugs and over-the-counter eye care products |
| ■ | Hospital Drugs & Injectables – sales of diagnostic and therapeutic injectables and other hospital drugs, as well as biologics and vaccines |
| ■ | Contract Services – sales of various drugs that we manufacture for others to be sold under their own brand names |
Revenue:
The year 2012 was marked by continued growth in revenue and profitability, as we continued to execute on our strategic objectives. Revenue grew to $256.2 million in 2012 compared to $136.9 million in 2011, and we generated operating income of $68.8 million compared to $33.3 million in the prior year. This growth was the result of new product launches, the re-launch of dormant products and acquisitions.
New Product Development:
During 2012, we continued the expansion of our R&D efforts and began to reap the benefits of prior FDA filings by receiving a number of ANDA approvals. Among our product approvals in 2012 were vancomycin hydrochloride capsules and progesterone capsules. R&D expenses increased from $11.6 million in 2011 to $15.9 million in 2012, and we are in the process of relocating our R&D center to a new, larger facility to support future growth.
Re-launch of Dormant Products:
During 2011, we re-launched a number of dormant products in response to market shortages and/or changes in their overall market. During 2012, we sought to establish an ongoing market presence for these products, as well as filling any market shortage needs. New products and re-launched products launched since the start of 2011 combined to account for 37.8% of our sales growth in 2012 over the prior year.
Acquisitions:
On February 28, 2012, we completed the acquisition of the business assets and principal manufacturing plant of Kilitch Drugs (India) Limited which operated a pharmaceutical manufacturing plant in Paonta Sahib, India. This facility currently manufactures drugs for contract customers in India and for export to unregulated worldwide markets. Our goal is for this facility to gain FDA approval for the manufacture of various drugs for distribution to the United States, which will supplement our existing manufacturing capacity and provide opportunity for expansion of our product menu as well as provide additional capabilities.
The Kilitch Acquisition followed our acquisition of three branded, injectable drugs from Lundbeck in December 2011 and our acquisition of AVR, the makers of the TheraTears® line of over-the-counter eye care products, in May 2011. Business combinations and product acquisitions combined to account for 56.3% of our sales growth in 2012 over the prior year.
24
RESULTS OF OPERATIONS
For the years 2012, 2011 and 2010, we have identified and reported operating results for three distinct business segments: Ophthalmic; Hospital drugs & injectables; and Contract services. Our reported results by segment are based upon various internal financial reports that disaggregate certain operating information. Our chief operating decision maker, as defined in Accounting Standards Codification (“ASC”) Topic 280, Segment Reporting, is our CEO. Our CEO oversees operational assessments and resource allocations based upon the results of our reportable segments, all of which have available discrete financial information.
In prior years, we had reported a fourth segment, Biologics & vaccines, which consisted of our sale of Td vaccine and various influenza vaccines manufactured by others and marketed by us. We ceased the distribution of vaccines in early 2010, and restarted their distribution at the end of 2012. Due to the non-material nature of vaccine sales in 2010 and 2012, and the similarities in customers and distribution methods to our hospital drugs & injectables segment, we have included sales of biologics and vaccines in our Hospital drugs & injectables segment for all years presented below.
The following table sets forth amounts and percentages of total revenue for certain items from our Consolidated Statements of Operations and our segment reporting information for the years ended December 31, 2012, 2011 and 2010 (dollar amounts in thousands):
| 2012 | 2011 | 2010 | ||||||||||||||||||||||
| Amount | % of Revenue | Amount | % of Revenue | Amount | % of Revenue | |||||||||||||||||||
| Revenues: | ||||||||||||||||||||||||
| Hospital drugs & injectables | $ | 129,723 | 50.6 | % | $ | 55,077 | 40.2 | % | $ | 34,053 | 39.4 | % | ||||||||||||
| Ophthalmic | 103,765 | 40.5 | % | 68,591 | 50.1 | % | 32,750 | 37.9 | % | |||||||||||||||
| Contract services | 22,670 | 8.9 | % | 13,252 | 9.7 | % | 19,606 | 22.7 | % | |||||||||||||||
| Total revenues | 256,158 | 100.0 | % | 136,920 | 100.0 | % | 86,409 | 100.0 | % | |||||||||||||||
| Gross profit and gross margin percentage: | ||||||||||||||||||||||||
| Hospital drugs & injectables | 83,413 | 64.3 | % | 30,057 | 54.6 | % | 15,768 | 46.3 | % | |||||||||||||||
| Ophthalmic | 58,785 | 56.7 | % | 43,054 | 62.8 | % | 19,453 | 59.4 | % | |||||||||||||||
| Contract services | 6,494 | 28.6 | % | 6,578 | 49.6 | % | 7,244 | 36.9 | % | |||||||||||||||
| Total gross profit | 148,692 | 58.0 | % | 79,689 | 58.2 | % | 42,465 | 49.1 | % | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||
| Selling, general & administrative expenses | 48,053 | 18.8 | % | 32,392 | 23.7 | % | 22,721 | 26.3 | % | |||||||||||||||
| Research and development expenses | 15,858 | 6.2 | % | 11,555 | 8.4 | % | 6,975 | 8.1 | % | |||||||||||||||
| Amortization & write-down of intangibles | 6,870 | 2.7 | % | 1,733 | 1.3 | % | 1,497 | 1.7 | % | |||||||||||||||
| Acquisition-related costs | 9,155 | 3.6 | % | 743 | 0.5 | % | — | 0.0 | % | |||||||||||||||
| Operating income | $ | 68,756 | 26.8 | % | $ | 33,266 | 24.3 | % | $ | 11,272 | 13.0 | % | ||||||||||||
| Net income | $ | 35,378 | 13.8 | % | $ | 43,013 | 31.4 | % | $ | 21,824 | 25.3 | % | ||||||||||||
COMPARISON OF TWELVE MONTHS ENDED DECEMBER 31, 2012 AND 2011
Our revenues were $256.2 million in 2012, an increase of $119.2 million, or 87.1%, compared to 2011. This increase in revenue was related to a number of factors, including acquisitions, sales of new and revived products and increased sales of existing products. Of the $119.2 million increase in revenues, $67.1 million was related to business combinations and product acquisitions completed since the start of 2011, $45.1 million was from a combination of newly-approved products and re-launched products in response to more favorable market conditions, and $9.3 million was related to sales volume increases for continuing products, partially offset by a $2.3 million reduction attributable to price changes on continuing products.
In terms of reportable segments, 2012 revenues from our hospital drugs & injectables segment were $129.7 million, an increase of $74.6 million, or 135.5%, over the prior year. This increase was principally attributable to sales of products acquired through the Lundbeck acquisition, and sales of newly-approved and revived products. Ophthalmic segment revenues were $103.8 million, an increase of $35.2 million, or 51.3%, over the prior year. The three main factors contributing to this increase were sales from new and revived products, a full year’s revenue from the AVR acquisition, and sales increases from existing ophthalmic products. Contract services revenue was $22.7 million in 2012, an increase of $9.4 million, or 71.1%, over the prior year. This increase was related to the $16.7 million revenue generated from the Kilitch Acquisition, partially offset by a decline in U.S. contract business of $7.3 million due to a shift in manufacturing toward Akorn products.
25
Our 2012 revenues of $256.2 million was net of adjustments totaling $130.8 million for chargebacks, rebates, administration fees, returns, discounts and allowances, and coupons and advertising. Chargeback and rebate expense for 2012 was $112.2 million, or 29.0% of gross revenue, compared to $68.1 million, or 31.5% of gross revenue, in 2011. The $44.1 million increase in chargeback and rebate expense was due to higher gross sales volume in 2012. The slight decrease in chargeback and rebate expense as a percentage of gross sales was attributable to increases in sales outside the wholesale channel. Our products returns provision in 2012 was $3.8 million, or 1.0% of gross sales, compared to $2.7 million, or 1.3% of gross sales, in 2011. The slight decrease in percentage was due to favorable historical product return trends and a higher percentage of sales of non-returnable products.
Our consolidated gross profit for 2012 was $148.7 million, or 58.0% of revenue, compared to $79.7 million, or 58.2% of revenue, in 2011. This gross profit increase of $69.0 million, or 86.6%, was principally due to our revenue growth from acquisitions, new product introductions and product revivals. The slight decrease in overall profit margin was due to lower margin business, such as the contract revenue of Akorn India, which offset higher-margin business, such as the sales of products acquired through the Lundbeck Acquisition. The gross profit margin on ophthalmic segment sales was 56.7% in 2012 compared to 62.8% in 2011, this decline being primarily attributable to increased sales of over-the-counter ophthalmic products by our subsidiary, AVR, which was acquired in May 2011. The gross profit margin on hospital drugs & injectables increased to 64.3% in 2012 from 54.6% in 2011 primarily due to the higher gross margin on the Lundbeck products. The gross profit margin on contract services decreased to 28.6% in 2012 from 49.6% in 2011 primarily due to lower margin business from our Indian subsidiary.
Selling, general and administrative (“SG&A”) expenses were $48.1 million in 2012, an increase of $15.7 million, or 48.3%, over the prior year SG&A expenses of $32.4 million. This increase was due primarily to compensation-related costs resulting from higher headcount supporting our growth, operating expenses associated with our India operations that were acquired during the first quarter of 2012 and marketing costs associated with our AVR business. As a percentage of sales, SG&A expenses was 18.8% down from 23.7% in 2011.
Research and development (“R&D”) expenses were $15.9 million in 2012 compared to $11.6 million in 2011. This increase of $4.3 million was the result of continued increases in R&D activities, both internally and through strategic partnerships and includes $1.2 million of expenses associated with the FDA’s backlog and new filing fees that went into effect in the fourth quarter of 2012.
Amortization of intangibles consists of the amortization of NDA and ANDA drug acquisition costs over the anticipated market lives of the acquired products, as well as the amortization of other intangible assets acquired through business combinations. Amortization of intangibles was $6.9 million in 2012 compared to $1.7 million in 2011. This increase of $5.2 million was primarily due to amortization of the product rights acquired through the Lundbeck Acquisition, and amortization of intangible assets acquired through the Kilitch Acquisition.
Amortization of deferred financing costs totaled $0.8 million in 2012 compared to $1.9 million in 2011. The 2012 expense was related to amortizing the financing costs on our Notes and our BoA credit facility. The 2011 expense included a $1.2 million write-off of the unamortized deferred financing costs to our EJ Credit Facility, which we elected to early terminate in June 2011. Our 2011 expense also included $0.4 million in amortization of deferred financing costs related to our Notes.
In 2012, we recorded non-cash interest expense of $6.4 million compared to $2.1 million in the prior year. Our non-cash interest expense was related to the debt discount on our Notes and to the change in fair value of our contingent consideration payable related to the acquisition of Lundbeck products.
Interest expense was $4.0 million in 2012 compared to $2.3 million in 2011. Our interest expense in each year was principally related to the Notes, which were issued effective June 1, 2011.
We are a 50% partner in the Joint Venture Company, which we account for using the equity method. During 2011, we recorded $14.6 million of equity in income from this unconsolidated joint venture, of which $13.4 million was related to our share of the gain from the Joint Venture Company’s sale of its ANDAs to Pfizer on December 29, 2010, and the remaining $1.2 million was from the Joint Venture Company’s operations. The Joint Venture Company ceased operations in 2011 and no income was recorded in 2012.
In 2011, we recorded a non-operating expense of $0.2 million related to an option agreement we entered into to protect ourselves from a negative movement in the foreign exchange rate between U.S. dollars and Indian rupees. We entered into this option agreement in October 2011 following our entry into an agreement to buy certain assets from Kilitch in India, as the purchase price for the Kilitch Acquisition was established in Indian rupees. We incurred no similar expense in 2012.
26
COMPARISON OF TWELVE MONTHS ENDED DECEMBER 31, 2011 AND 2010
Our revenues were $136.9 million in 2011, an increase of $50.5 million, or 58.5%, compared to 2010. This increase in revenue was related to a number of factors, including the acquisition of AVR, increased sales of existing products through sales efforts and share gains from market shortages, introduction of new products, and price increases for certain products. Of the $50.5 million increase in revenues, $18.0 million was related to new products and the re-launch of dormant products to capitalize on market opportunities, $17.8 million was due to increased sales of existing products, $5.0 million was related to selected price increases, and $14.9 million was related to acquisitions, partially offset by a decline of $5.2 million related to our strategic decision to cease the distribution of biologics & vaccines effective March 2010.
As it relates to our reportable segments, the increase in revenue for our ophthalmic segment was primarily due to the acquisition of AVR and sales volume increases for our existing products. The increase in revenue in our hospital drugs & injectables segment was primarily due to re-launches and new products, along with volume increases for existing products. The decline in contract segment revenues was due to refocusing our manufacturing plants on producing Akorn-branded products, along with the loss of AVR as a contract customer upon our acquisition of this business in May 2011.
The market shortages are related to a number of factors, including cGMP issues experienced by various competing drug companies and competitors’ strategic decisions to cease manufacturing various products. We monitor market conditions and attempt to respond to market opportunities, such as those provided by market shortages. However, it is difficult to predict the duration and severity of market shortage for most drugs, and our revenues and gross profit margins may fluctuate accordingly.
Our 2011 revenues of $136.9 million was net of adjustments totaling $79.1 million for chargebacks, rebates, administration fees, returns, discounts and allowances, and coupons and advertising. Chargeback and rebate expense for 2011 was $68.1 million or 31.5% of gross revenue, compared to 2010 expense of $45.0 million, or 32.9% of gross revenue. The $23.1 million increase in chargeback expense was due to higher gross sales volume in 2011. As a percentage of gross sales, the decrease in chargeback and rebate expenses is attributable to the AVR business, which is subject to minimal chargebacks. Our products returns provision was $2.7 million in 2011 compared to $1.5 million in 2010. This $1.2 million increase was due to higher sales volume in 2011.
Our consolidated gross profit for 2011 was $79.7 million, or 58.2% of revenue, compared to $42.5 million, or 49.1% of revenue, in 2010. This gross profit increase of $37.2 million, or 87.5%, was due to several factors, including revenue growth from our introduction of new products carrying higher profit margins, increased sales and selected price increases for existing products, improved plant utilization, and improved inventory management. The gross profit margin on ophthalmic segment sales increased to 62.8% in 2011 compared to 59.4% in 2010, and the gross profit margin on hospital drugs & injectables increased to 54.6% in 2011 compared to 46.3% in the prior year. These increases were primarily due to improved utilization of our manufacturing facilities, as well as a number of lesser factors, such as selected price increases for certain products. The gross profit margin on contract services increased to 49.6% in 2011 compared to 36.9% in the prior year, this increase being primarily attributable to improved plant utilization, price increases for certain products, and the elimination of lower margin contract revenue from AVR upon our acquisition of that business.
Selling, general and administrative (“SG&A”) expenses were $32.4 million in 2011, an increase of $9.7 million, or 42.6%, from the prior year. This increase was due primarily to SG&A expenses for AVR, increases in wages and salaries for additional headcount to support our growth, and increases in non-cash stock compensation expense and management bonuses in accordance with our improved financial performance.
Research and development (“R&D”) expenses were $11.6 million in 2011 compared to $7.0 million in 2010. This increase of $4.6 million was the result of our commitment to enhancing our internal R&D infrastructure, increased R&D activity at our dedicated facility in Skokie, Illinois, and the establishment of a $1.7 million reserve against inventory of products pending FDA approval.
Amortization of intangibles consists of the amortization of NDA and ANDA drug acquisition costs over the anticipated market lives of the acquired products, as well as the amortization of other intangible assets acquired through business combinations. Amortization of intangibles was $1.7 million in 2011 compared to $1.5 million in 2010. This increase was due to amortization expense of products acquired in 2011, including the AVR TheraTears® trademark.
27
Write-off and amortization of deferred financing costs totaled $1.9 million in 2011 compared to $2.8 million in 2010. In each year, the majority of the expense was related to write-offs. In June 2011, we elected to early terminate our EJ Credit Facility and wrote off $1.2 million in remaining unamortized deferred financing costs. In December 2010, we early paid the balance due under our Subordinated Note, writing off $1.2 million of unamortized deferred financing costs and $0.6 million of early payment fee. In 2011, we also recorded $0.4 million in amortization of deferred financing costs related to our Notes.
In 2011, we recorded non-cash interest expense of $2.1 million related to the debt discount of our Notes. We incurred no similar expense in 2010.
Interest expense was $2.3 million in 2011 compared to $0.9 million in the prior year. This increase was related to our Notes, which were issued effective June 1, 2011. Interest expense related to the Notes was $2.5 million and was partially offset by interest earned on the proceeds from the offering. The lower interest expense in 2010 was related primarily to our Subordinated Note with EJ Funds.
We are a 50% partner in the Joint Venture Company, which we account for using the equity method. During 2011, we recorded $14.6 million of equity in income from this unconsolidated joint venture, compared to $23.4 million in the prior year. Of the $14.6 million income in 2011, $13.4 million was related to our share of the gain from the Joint Venture Company’s sale of its ANDAs to Pfizer on December 29, 2010, and the remaining $1.2 million was from the Joint Venture Company’s operations. Of the $23.4 million income in 2010, $21.6 million was related to our share of the gain. The Joint Venture Company entered into an Asset Purchase Agreement to sell the rights to all of its ANDAs to Pfizer for $63.2 million in cash. The Asset Purchase Agreement contained two closing dates, with some ANDAs having been transferred on the initial close date of December 29, 2010 and the rest transferred on the final closing date of May 1, 2011. The gains from this sale were allocated between the two closing dates based on the relative fair value of the ANDAs transferred to Pfizer on each date. The Joint Venture Company essentially ceased operations in the second quarter of 2011.
During 2010, we incurred non-cash expenses of $8.9 million related to the change in fair value of warrants we granted at various dates in 2009 to companies controlled by our Chairman, Dr. John Kapoor (the “Kapoor Warrants”). We classified the Kapoor Warrants as current liabilities from their grant dates until June 28, 2010, and adjusted their book values quarterly to reflect changes in their fair values. As a result of an amendment entered into on June 28, 2010 to the registration rights agreement associated with these warrants, we reclassified the Kapoor Warrants from current liabilities to a component of shareholders’ equity on June 28, 2010 and made no subsequent fair value adjustments beyond that date. Accordingly, there was no similar expense recorded in 2011.
In 2011, we recorded a non-operating expense of $0.2 million related to an option agreement we entered into to protect ourselves from a negative movement in the foreign exchange rate between U.S. dollars and Indian rupees. We entered into this option agreement in October 2011 following our entry into an agreement to buy certain assets from Kilitch in India, as the purchase price for the Kilitch Acquisition was established in Indian rupees. We incurred no similar expenses in 2010.
FINANCIAL CONDITION AND LIQUIDITY
Cash Flow
As of December 31, 2012, we had cash and cash equivalents of $40.8 million, which is $43.2 million lower than our cash and cash equivalents balance of $84.0 million as of December 31, 2011. This decrease in cash and cash equivalents was primarily due to the $54.2 million we used to complete the Kilitch Acquisition on February 28, 2012 and $20.5 million used to acquire property, plant and equipment, partially offset by $26.2 million in positive cash flow from operations. Our net working capital was $115.4 million at December 31, 2012 compared to $127.7 million at December 31, 2011. This decrease of $12.3 million was primarily attributable to the decline in our cash and cash equivalents balance, partially offset by increases in accounts receivable and inventory, which grew in step with our overall business growth during 2012.
For the year 2012, we generated $26.2 million in cash flow from operations. This positive operating cash flow was primarily the result of our net income of $35.4 million and non-cash expenses of $20.6 million, partially offset by a $23.9 million increase in accounts receivable and a $15.4 million increase in inventory. In the prior year of 2011, we generated $19.7 million in positive cash flow from operations. This positive operating cash flow was primarily due to the combination of $43.0 million of net income and $14.5 million of non-cash expenses, partially offset by $14.6 million equity in earnings of the Joint Venture Company and a combined increase of $22.9 million in accounts receivable and inventory.
In 2012, we used $75.5 million cash in investing activities. Of this total, $54.2 million was used to complete the Kilitch Acquisition in February and $20.5 million was used to acquire property, plant and equipment, principally as part of the expansion project at our Somerset, New Jersey manufacturing plant. In the prior year, we used $95.0 million in investing activities, of which $77.4 million was used for business and product acquisitions, $11.5 million was used to purchase property, plant and equipment, and $10.0 million was used to make an equity method investment in Aciex, offset by $3.9 million generated from distributions from the Joint Venture Company.
28
Financing activities generated $6.4 million in cash during 2012, all of which was related to stock option exercises and participation in the employee stock purchase plan. During 2011, we generated $117.7 million in cash through financing activities, with the most significant source being the net $115.3 million generated through our $120.0 million offering of 3.5% Convertible Senior Notes due 2016, partially offset by $5.1 million in financing fees related to the Notes and the BoA Credit Facility. Additional financing cash flow of $1.7 million was generated from PIPE Warrant exercises in the first quarter of 2011, while stock option exercises and participation in the employee stock purchase plan generated a combined $1.1 million during the year.
We believe that our cash reserves, operating cash flows and availability under our BoA Credit Facility will be sufficient to meet our cash needs for the foreseeable future.
Liquidity and Capital Needs
We require certain capital resources in order to maintain and expand our business. Specifically, we anticipate investing in the range of $25.0 million in capital projects during 2013, which includes approximately $15.0 million to continue the expansion and upgrade of our manufacturing facility in Paonta Sahib, India. As of December 31, 2012, we had $40.8 million in cash and cash equivalents. We believe that our cash reserves, operating cash flows and availability under our Credit Facility will be sufficient to meet our cash needs for the foreseeable future.
We continue to evaluate opportunities to grow and expand our business through the acquisition of new businesses, manufacturing facilities, or pharmaceutical product rights. Such acquisitions may require us to obtain additional sources of capital. We cannot predict the amount of capital that may be required to complete such acquisitions, and there is no assurance that sufficient financing for these activities would be available on terms acceptable to us, if at all.
Convertible Notes
On June 1, 2011, we completed our offering of $120.0 million aggregate principal amount of 3.50% Convertible Senior Notes due 2016 (the “Notes”), which includes $20.0 million of Notes issued in connection with the full exercise by the initial purchasers of their over-allotment option. The Notes are governed by our indenture with Wells Fargo Bank, National Association, as trustee (the “Indenture”). The Notes were offered and sold only to qualified institutional buyers. The net proceeds from the sale of the Notes were approximately $115.3 million, after deducting underwriting fees and other related expenses.
The Notes have a maturity date of June 1, 2016 and pay interest at an annual rate of 3.50% semiannually in arrears on June 1 and December 1 of each year, beginning on December 1, 2011. The Notes are convertible into our common stock, cash or a combination thereof at an initial conversion price of $8.76 per share, which is equivalent to an initial conversion rate of approximately 114.1553 shares per $1,000 principal amount of Notes. The conversion price is subject to adjustment for certain events described in the Indenture, including certain corporate transactions which will increase the conversion rate and decrease the conversion price for a holder that elects to convert its Notes in connection with such corporate transaction.
The Notes may be converted at any time prior to the close of business on the business day immediately preceding December 1, 2015 only under the following circumstances: (1) during any calendar quarter commencing after September 30, 2011, if the closing sale price of the our common stock, for at least 20 trading days (whether or not consecutive) during the period of 30 consecutive trading days ending on the last trading day of the calendar quarter immediately preceding the calendar quarter in which the conversion occurs, is more than 130% of the conversion price in effect on each applicable trading day; (2) during the five consecutive trading-day period following any five consecutive trading-day period in which the trading price for the Notes per $1,000 principal amount of Notes for each such trading day was less than 98% of the closing sale price of our common stock on such date multiplied by the then-current conversion rate; or (3) upon the occurrence of specified corporate events. On or after December 1, 2015 until the close of business on the business day immediately preceding the stated maturity date, holders may surrender all or any portion of their Notes for conversion at any time, regardless of the foregoing circumstances. Upon conversion, we will pay or deliver, at our option, cash, shares of our common stock, or a combination thereof. We may not redeem the Notes prior to the maturity date. If a fundamental change (as defined in the Indenture) occurs prior to the stated maturity date, holders may require us the purchase for cash all or a portion of their Notes.
29
During the year 2012, the Company recorded the following expenses in relation to the Notes (in thousands):
| Interest expense at 3.50% coupon rate | $ | 4,200 | ||
| Debt discount amortization | 3,828 | |||
| Deferred financing cost amortization | 692 | |||
| $ | 8,720 |
Credit Facilities:
Bank of America Credit Facility
On October 7, 2011, the Company and its domestic subsidiaries (the “Borrowers”) entered into a Loan and Security Agreement (the “BoA Credit Agreement”) with Bank of America, N.A. (the “Agent”) and other financial institutions (collectively with the Agent, the “BoA Lenders”) through which we obtained a $20.0 million revolving line of credit (the “Facility”), which includes a $2.0 million letter of credit facility. We may request expansion of the Facility from time to time in increments of at least $5.0 million up to a maximum commitment of $35.0 million, so long as no default or event of default has occurred and is continuing. The facility matures in March 2016. We may early terminate the BoA Lenders’ commitments under the Facility upon 90 days’ notice to the Agent at any time after the first year.
Under the terms of the BoA Credit Agreement, amounts outstanding will bear interest at our election at (a) LIBOR or (b) the bank’s Base Rate (which is the greatest of: (i) the prime rate, (ii) the federal funds rate plus 0.50%, or (iii) LIBOR plus 1.0%), plus an applicable margin, which margin is based on the consolidated fixed charge coverage ratio of Akorn, Inc. and its subsidiaries from time to time. Additionally, the Borrowers will pay an unused line fee of 0.250% per annum on the unused portion of the Facility. Interest and unused line fees will be accrued and paid monthly. In addition, with respect to any letters of credit that may be issued, the Borrowers will pay: (i) a fee equal to the applicable margin times the average amount of outstanding letters of credit, (ii) a fronting fee equal to 0.125% per annum on the stated amount of each letter of credit, and (iii) any additional fees incurred by the applicable issuer in connection with issuing the letter of credit. During an event of default, any interest or fees payable will be increased by 2% per annum.
Availability under the revolving credit line is equal to the lesser of (a) $20.0 million reduced by outstanding letter of credit obligations or (b) the amount of a Borrowing Base (as defined in accordance with the terms of the BoA Credit Agreement) determined by reference to the value of the Borrowers’ eligible accounts receivable, eligible inventory and fixed assets as of the closing date and the end of each calendar month thereafter.
Obligations under the BoA Credit Agreement are secured by substantially all of the assets of each of the Borrowers and a pledge by the Borrowers of their respective equity interest in each of our domestic subsidiaries and 65% of their respective equity interests in any foreign subsidiaries. The BoA Credit Agreement contains representations and warranties, and affirmative and negative covenants customary for financings of this type, including, but not limited to, limitations on: distributions while we have any outstanding commitments or obligations under the BoA Credit Agreement; additional borrowings and liens; additional investments and asset sales; and fundamental changes to corporate structure or organization documents. The financial covenants require the Borrowers to maintain a fixed charge coverage ratio of at least 1.1 to 1.0 during any period commencing on the date that an event of default occurs or availability under the BoA Credit Agreement is less than 15% of the aggregate BoA Lenders’ commitments under the BoA Credit Agreement. During the term of the agreement, we must provide the Agent with monthly, quarterly and annual financial statements, monthly compliance certificates, annual budget projections and copies of press releases and SEC filings.
As of December 31, 2012, we had availability on our line of credit of $19.7 million and there were no outstanding borrowings or letters of credit.
EJ Funds Credit Facility
On January 7, 2009, we entered into a Credit Agreement (the “GE/EJ Credit Agreement”) with General Electric Capital Corporation (“GE Capital”) as agent for several financial institutions (the “Lenders”) to replace our previous credit agreement with Bank of America which expired on January 1, 2009. (As more fully discussed below, the GE/EJ Credit Agreement was subsequently assigned to EJ Funds LP.) Pursuant to the GE/EJ Credit Agreement, the Lenders agreed to extend loans to us under a revolving credit facility up to an aggregate principal amount of $25.0 million (the “Credit Facility”). The Credit Facility was scheduled to terminate, and all amounts outstanding thereunder were to become due and payable, on January 7, 2013, or on an earlier date as specified in the GE/EJ Credit Agreement. On June 17, 2011, we elected to early terminate the GE/EJ Credit Agreement. There was no early termination fee upon termination of the GE/EJ Credit Agreement.
30
On February 19, 2009, GE Capital informed us that it was applying a reserve against availability which effectively restricted our borrowings under the GE/EJ Credit Agreement to the balance outstanding as of February 19, 2009, which was $5.5 million. On March 31, 2009, we consented to an Assignment Agreement (“Assignment”) between GE Capital and EJ Funds LP (“EJ Funds”) which transferred to EJ Funds all of GE Capital’s rights and obligations under the GE/EJ Credit Agreement. Pursuant to the Assignment, EJ Funds became the agent and lender under the GE/EJ Credit Agreement. Dr. Kapoor is the President of EJ Financial Enterprises, Inc., a healthcare consulting investment company (“EJ Financial”) and EJ Financial is the general partner of EJ Funds.
In connection with the Assignment, on April 13, 2009, we entered into a Modification, Warrant and Investor Rights Agreement (the “Modification Agreement”) with EJ Funds that, among other things, (i) reduced the revolving loan commitment under the GE/EJ Credit Agreement to $5.7 million, and (ii) set the interest rate for all amounts outstanding under the GE/EJ Credit Agreement at an annual rate of 10% with interest payable monthly. The Modification Agreement also granted EJ Funds the right to require us to nominate two directors to serve on our Board of Directors. The Kapoor Trust is entitled to require us to nominate a third director under our Stock Purchase Agreement dated November 15, 1990 with the Kapoor Trust. Pursuant to the Modification Agreement, on April 13, 2009, we granted EJ Funds a warrant (the “Modification Warrant”) to purchase 1,939,639 shares of our common stock at an exercise price of $1.11 per share, subject to certain adjustments. The Modification Warrant expires five years after its issuance and is exercisable upon payment of the exercise price in cash or by means of a cashless exercise yielding a net share figure.
On August 17, 2009, we completed negotiations with EJ Funds for additional capacity on our Credit Facility, increasing the loan commitment from $5.7 million to $10.0 million. In consideration of this amendment, EJ Funds was granted a warrant to acquire 1,650,806 shares of our common stock at $1.16 per share, the closing market price on August 14, 2009 (the “Restatement Warrants”). The estimated fair value of the Restatement Warrants, using a Black-Scholes valuation model, was $1.2 million on the date of grant. The Credit Facility was secured by our assets and per the terms of this amendment was not subject to debt covenants until April 1, 2010.
On January 13, 2010, the parties entered into an amendment to the GE/EJ Credit Agreement which, among other things, reduced the number of financial covenants to two: (1) a limit on capital expenditures of $7.5 million in 2010, $5.0 million in 2011, and $5.0 million in 2012 and (2) a requirement to have positive liquidity throughout the life of the GE/EJ Credit Agreement. Subsequently, on January 27, 2011, EJ Funds and the Company signed a Waiver and Consent that waived our obligation to comply with the capital expenditure limit for 2011.
On June 17, 2011, we elected to early terminate our $10.0 million revolving GE/EJ Credit Agreement with EJ Funds. We had not borrowed against the GE/EJ Credit Agreement since repaying its outstanding balance in the first quarter of 2010. Upon terminating the GE/EJ Credit Agreement, we expensed $1.2 million in remaining unamortized deferred financing costs related to the GE/EJ Credit Agreement. We incurred no fees or penalties related to the early termination of the GE/EJ Credit Agreement.
Subordinated Note Payable
On July 28, 2008, we borrowed $5.0 million from the Kapoor Trust dated September 20, 1989, the sole trustee and sole beneficiary of which is Dr. John N. Kapoor, the Chairman of our Board of Directors and the holder of a significant stock position in the Company, in return for issuing the trust a Subordinated Note (the “Subordinated Note”). The Subordinated Note accrued interest at a rate of 15% per year and was due and payable on July 28, 2009. On August 17, 2009, the Subordinated Note was refinanced, with the principal amount increased to $5.9 million to include interest accrued through August 16, 2009, and the term of the Subordinated Note extended by an additional five years to August 17, 2014. The interest rate remained unchanged at 15% per year, and interest on the refinanced note was payable monthly. As part of this refinancing agreement, we issued to the Kapoor Trust an additional 2,099,935 warrants (the “Subordinated Note Warrants”) to purchase our common stock at an exercise price of $1.16, the closing price of the our stock on August 14, 2009. The fair value of these warrants on August 17, 2009, as calculated using a Black-Scholes valuation model, was $1.6 million. This amount, along with $28,000 in legal fees, was capitalized as deferred financing costs and was being amortized over the term of the subordinated debt.
On December 16, 2010, we voluntarily prepaid the principal of the Subordinated Note, along with a 10% early payment fee and all accrued interest. Our total cash payment on December 16, 2010, including principal, accrued interest, and the early payment fee, was $6.5 million. Upon completing this early payment we expensed the remaining $1.2 million unamortized balance of the $1.6 million in deferred financing costs incurred when we refinanced the Subordinated Note.
31
Preferred Stock and Warrants
Kapoor Warrants
During 2009, in connection with modifications to our Subordinated Note, Credit Agreement and MBL Distribution Agreement, we granted various warrants to acquire our common stock (the “Kapoor Warrants”) to EJ Funds and the Kapoor Trust, companies controlled by the Chairman of our Board of Directors, Dr. John N. Kapoor. Each of the Kapoor Warrants will expire five years after its grant date, if not exercised.
The fair value of each of the Kapoor Warrants was calculated at their grant dates using the Black-Scholes option pricing model. From their grant dates until June 28, 2010, the Kapoor Warrants were classified as current liabilities on our consolidated balance sheets and adjusted quarterly to reflect changes in their calculated fair values. Increases in fair value, or decreases in fair value to, but not below, their initial calculated fair values, were recorded as non-operating expenses or income in our condensed consolidated statements of operations for the applicable periods. We classified the fair value of the Kapoor Warrants as a current liability in accordance with ASC 815-40-15-3, Derivatives and Hedging, (formerly EITF 00-19, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock) . This is a result of a requirement in the Registration Rights Agreement – entered into among the Kapoor Trust, EJ Funds and us on August 17, 2009 – that the shares to be issued upon exercise of the warrants be registered shares, which cannot be absolutely assured.
On June 28, 2010, we entered into an Amended and Restated Registration Rights Agreement (the “Amended Agreement”) with Dr. Kapoor which modified certain terms related to our obligation to obtain and maintain registration of any shares issued pursuant to exercise of the Kapoor Warrants. The Amended Agreement still requires us to use “commercially reasonable efforts” to file a registration statement pursuant to Rule 415 of the Securities Act of 1933 (“Registration Statement”) for any shares of common stock that may be issued under the applicable warrant agreements, and to maintain the continuous effectiveness of such Registration Statement until the earliest of: (i) the date no shares of the our common stock qualify as registrable securities, (ii) the date on which all of the registrable securities may be sold in a single transaction by the holder to the public pursuant to Rule 144 or a similar rule, or (iii) the date upon which the John N. Kapoor Trust dated September 20, 1989 (the “Kapoor Trust”) and EJ Funds have transferred all of the registrable securities. However, the Registration Rights Agreement has been amended to explicitly state that in the event that we, after using good faith commercially reasonable efforts, are not able to obtain or maintain registration of the common stock, delivery of unregistered shares upon exercise of the Kapoor Warrants will be deemed acceptable and a net cash settlement will not be required. The Amended Agreement further provides that the term “commercially reasonable efforts” in such instance shall not mean an absolute obligation of ours to obtain and maintain registration.
On June 28, 2010, upon entering into the Amended Agreement, we completed a final Black-Scholes calculation of the fair value of the Kapoor Warrants and adjusted their book value accordingly, then reclassified the Kapoor Warrants from a current liability to a component of shareholders’ equity. After reclassifying the Kapoor Warrants to shareholders’ equity, no subsequent fair value adjustments were required.
The increases in fair value of the Kapoor Warrants were recorded as expenses under the caption “Change in fair value of warrants liability” in our consolidated statement of operations for the years ended December 31, 2010 and 2009. We recorded expense of $8.9 million during 2010 related to the increase in fair value of the Kapoor Warrants.
The assumptions used in estimating the fair value of the warrants at June 28, 2010 and December 31, 2009 were as follows:
June 28, 2010 | December 31, 2009 | ||
| Expected Volatility | 79.7% | 79.5% | |
| Expected Life (in years) | 3.8 – 4.1 | 4.3 – 4.6 | |
| Risk-free interest rate | 1.8% | 2.3% | |
| Dividend yield | — | — |
The following table provides summarized information about the Kapoor Warrants:
| Fair Values ($000s) | ||||||||||||||||||||||
Granted To: | Warrant Identification | Grant Date | Warrants Granted | Exercise Price | At Grant Dates | As of 12/31/09 | As of 6/28/10 | |||||||||||||||
| EJ Funds | Modification Warrants | Apr.13, 2009 | 1,939,639 | $ | 1.11 | $ | 1,358 | $ | 2,425 | $ | 4,829 | |||||||||||
| Kapoor Trust | Reimbursement Warrants | Apr.13, 2009 | 1,501,933 | $ | 1.11 | 1,051 | 1,877 | 3,740 | ||||||||||||||
| EJ Funds | Credit Facility Warrants | Aug.17, 2009 | 1,650,806 | $ | 1.16 | 1,238 | 2,096 | 4,127 | ||||||||||||||
| Kapoor Trust | Subordinated Note Warrants | Aug.17, 2009 | 2,099,935 | $ | 1.16 | 1,575 | 2,667 | 5,250 | ||||||||||||||
| 7,192,313 | $ | 5,222 | $ | 9,065 | $ | 17,946 | ||||||||||||||||
32
Footnotes:
| 1 | The Modification Warrants were granted to EJ Funds on April 13, 2009 when we signed the Modification Agreement with EJ Funds related to modifications made to our Credit Agreement following its assignment from GE Capital to EJ Funds on March 31, 2009. Those modifications included resetting the maximum loan commitment to $5.7 million and setting the interest rate at a fixed 10% per annum, among others. |
| 2 | The Reimbursement Warrants were granted to the Kapoor Trust on April 13, 2009 when we entered into a Reimbursement and Warrant Agreement (the “Reimbursement Agreement”) with EJ Funds and the Kapoor Trust pursuant to which the Kapoor Trust agreed to provide the L/C as security for our payment obligations to MBL under the MBL Letter Agreement and the MBL Settlement Agreement. |
| 3 | The Credit Facility Warrants were granted to EJ Funds on August 17, 2009 in connection with the negotiated modification to the Credit Agreement increasing the total loan commitment from $5.7 million to $10.0 million. |
| 4 | The Subordinated Note Warrants were issued to the Kapoor Trust on August 17, 2009 in connection with refinancing the Subordinated Note to extend its term for an additional five years and increase the principal from $5.0 million to $5.9 million to include accrued interest through August 17, 2009. |
PIPE Warrants
On March 8, 2006, we issued 4,311,669 shares of our common stock in a private placement with various investors at a price of $4.50 per share which included warrants to purchase 1,509,088 additional shares of common stock. The warrants were exercisable for a five year period ended March 8, 2011 at an exercise price of $5.40 per share and may be exercised by cash payment of the exercise price or by means of a cashless exercise. Holders submitted 77,779 warrants for cashless exercise during 2010, leaving 1,431,309 remaining outstanding as of December 31, 2010. Subsequently, during the period from January 1, 2011 through March 8, 2011, holders submitted 1,197,975 of the warrants for exercise. The remaining 233,334 warrants expired unexercised on March 8, 2011.
CONTRACTUAL OBLIGATIONS
In order to support the continued increase in the number of relevant and marketable pharmaceutical products that we market and sell, we will from time to time partner with outside firms for the development of selected products. These development agreements frequently call for the payment of “milestone payments” as various steps in the process are completed in relation to product development and submission to the FDA for approval. The dollar amount of these payments is generally fixed contractually, assuming that the required milestones are achieved. However, the timing of such payments is contingent based on a variety of factors and is therefore subject to change. The amounts disclosed in the below table under the caption “Strategic Partners – Contingent Payments” represents our best estimate of the amount and expected timing of the “milestone payments” and other fees we expect to pay to outside development partners based on our current contractual agreements with them. These milestone payments are accrued as liabilities on the our balance sheets once the milestones have been achieved.
On December 22, 2011, we entered into the Lundbeck Agreement through which we acquired the NDA rights to three branded, off-patent drugs. In addition to an initial cash payment of $45.0 million, the Lundbeck Agreement committed us to paying additional consideration of $15.0 million in cash on the third anniversary of the agreement date, assuming that subsequent sales of the applicable products achieved certain targets. We believe that there is a strong likelihood that these targets will be reached and that the additional consideration will be paid when due. This liability has been recorded on our books at the initial discounted value of $11.3 million, which considers both the time value of money and the slight possibility that less than the full amount will ultimately become due. At December 31, 2012 the liability was $14.2 million.
As more fully described under Item 2. Properties, we lease the facilities that we occupy in Gurnee, Lake Forest, Skokie and Vernon Hills, Illinois, Ann Arbor, Michigan, and in Somerset, New Jersey. We also lease various pieces of office equipment at these facilities, as well as at our manufacturing plant in Decatur, Illinois. Our remaining obligations under these leases are summarized in the table below.
As of December 31, 2012, our principal financial obligation was related to our Notes. We had no balance outstanding under our BoA Credit Agreement at December 31, 2012 or any time since we entered into this agreement on October 7, 2011.
33
The following table details our future contractual obligations as of December 31, 2012 (in thousands):
Description | Total | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 and beyond | |||||||||||||||||||||
| 3.5% convertible senior notes due 2016 | $ | 120,000 | — | — | — | 120,000 | — | — | ||||||||||||||||||||
| Interest payable – 3.5% convertible notes | 14,700 | 4,200 | 4,200 | 4,200 | 2,100 | — | — | |||||||||||||||||||||
| Contingent consideration – acquisitions | 15,000 | — | 15,000 | — | — | — | — | |||||||||||||||||||||
| Inventory purchase commitments | 25,666 | 10,081 | 12,368 | 3,217 | — | — | — | |||||||||||||||||||||
| Leases | 10,628 | 2,084 | 1,973 | 1,972 | 2,002 | 1,793 | 804 | |||||||||||||||||||||
Strategic partners – contingent payments 1 | 10,222 | 8,524 | 1,698 | — | — | — | — | |||||||||||||||||||||
| Total: | $ | 196,216 | $ | 24,889 | $ | 35,239 | $ | 9,389 | $ | 124,102 | $ | 1,793 | $ | 804 | ||||||||||||||
| 1 | Note the Strategic Partner Payments are estimates which assume that various contingencies and market opportunities occur in 2012 and beyond |
OFF BALANCE SHEET ARRANGEMENTS
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our shareholders.
CRITICAL ACCOUNTING POLICIES
Revenue Recognition
We recognize product sales for our ophthalmic and hospital drugs & injectables business segments upon the shipment of goods or upon the delivery of goods, depending on the sales terms. The contract services segment, which produces products for third party customers based upon their specification at a pre-determined price, also recognizes sales upon the shipment of goods or upon delivery of the product or service as appropriate. Revenue is recognized when all of our obligations have been fulfilled and collection of the related receivable is probable. Provision for estimated doubtful accounts, chargebacks, rebates, discounts and product returns is made at the time of sale and is analyzed and adjusted, if necessary, at each balance sheet date.
Allowance for Chargebacks and Rebates
We enter into contractual agreements with certain third parties such as hospitals and GPOs to sell certain products at predetermined prices. The parties have elected to have these contracts administered through wholesalers that buy the product from us and subsequently sell it to those third parties. When a wholesaler sells products to one of the third parties that are subject to a contractual price agreement, the difference between the price paid to us by the wholesaler and the price under the specific contract is charged back to us by the wholesaler. We track sales and submitted chargebacks by product number and contract for each wholesaler. Utilizing this information, we estimate a chargeback percentage for each product. We reduce gross sales and increase the chargeback allowance by the estimated chargeback amount for each product sold to a wholesaler. We reduce the chargeback allowance when we process a request for a chargeback from a wholesaler. Actual chargebacks processed can vary materially from period to period based upon actual sales volume through the wholesalers. However, our provision for chargebacks is fully reserved for at the time when sales revenues are recognized.
We obtain certain wholesaler inventory reports to aid in analyzing the reasonableness of the chargeback allowance that will be paid out in the future. We assess the reasonableness of our chargeback allowance by applying the product chargeback percentage based on historical activity to the quantities of inventory on hand per the wholesaler inventory reports and an estimate of in-transit inventory that is not reported on the wholesaler inventory reports at the end of the period. In accordance with our accounting policy, our estimate of the percentage amount of wholesaler inventory that will ultimately be sold to a third party that is subject to a contractual price agreement is based on a six-quarter trend of such sales through wholesalers. We use this percentage estimate until historical trends or new information indicates that a revision should be made. On an ongoing basis, we evaluate our actual chargeback rate experience and new trends are factored into our estimates each quarter as market conditions change.
34
The historical percentages that we have used during 2010, 2011 and 2012 are as follows:
Period Start Date | Period End Date | Estimated % of wholesaler inventory that will be subject to contractual price agreements | |||
| July 1, 2009 | - | June 30, 2010 | 97.0% | ||
| July 1, 2010 | - | June 30, 2012 | 98.5% | ||
| July 1, 2012 | - | September 30, 2012 | 95.0% | ||
| October 1, 2012 | - | Current | 90.0% |
We will continue to use the 90.0% estimate in future periods until trends indicate that a revision should be made.
Similarly, we maintain an allowance for rebates related to fee for service contracts and other programs with certain customers. Rebate percentages vary by product and by volume purchased by each eligible customer. We track sales by product number for each eligible customer and then apply the applicable rebate percentage, using both historical trends and actual experience to estimate our rebate allowance. We reduce gross sales and increase the rebate allowance by the estimated rebate amount when we sell our products to our rebate-eligible customers. We reduce the rebate allowance when we process a customer request for a rebate. At each balance sheet date, we analyze the allowance for rebates against actual rebates processed and make necessary adjustments as appropriate. Actual rebates processed can vary materially from period to period. However, our provision for rebates is fully reserved for at the time when sales revenues are recognized.
The recorded allowances reflect our current estimate of the future chargeback and rebate liability to be paid or credited to our wholesaler and other customers under the various contracts and programs. For the years ended December 31, 2012, 2011, and 2010, we recorded chargeback and rebate expense of $112.2 million, $68.1 million and $45.2 million, respectively. The allowances for chargebacks and rebates were $13.5 million and $5.9 million as of December 31, 2012 and 2011, respectively. The current year increase within our allowance for chargebacks and rebates was primarily due to the increases in overall sales volume in 2012 compared to 2011.
Allowance for Product Returns
Certain of our products are sold with the customer having the right to return the product within specified periods and guidelines for a variety of reasons, including but not limited to pending expiration dates. Provisions are made at the time of sale based upon tracked historical experience, by customer in some cases. We estimate our required product returns reserve based on historical percentage of returns to sales by product, considering actual returns processed to date, the expected impact of product recalls and current wholesaler inventory levels of our products to assess the magnitude of unconsumed product that may result in future product returns. We also consider one-time historical return events or pending new developments that would impact the expected level of future returns. For new products, we assess the market dynamics for that product and consider our past returns experience for similar products in our portfolio. Our sales returns level can be impacted by factors such as overall market demand and market competition and availability for substitute products which can increase or decrease the end-user pull through for sales of our products and ultimately impact the level of sales returns. Actual returns experience and trends are factored into our estimates each quarter as market conditions change. Actual returns processed can vary materially from period to period.
For the years ended December 31, 2012, 2011 and 2010, we recorded a net provision for product returns of $3.8 million, $2.7 million and $1.5 million, respectively. The increase in returns provision from 2010 to 2011 was in line with the increase in revenue from year to year. The increase from $2.7 million in 2011 to $3.8 million in 2012 was at a pace lower than our increase in sales, largely due to adjustments to our required reserve level based on evaluation of historical product returns trends. As of December 31, 2012 and 2011, our allowances for product returns were $8.4 million and $6.8 million, respectively.
Allowance for Coupons and Promotions
We issue coupons from time to time redeemable against our TheraTears® eye care products. Upon release of coupons into the market, we record an estimate of the dollar value of coupons we expect to be redeemed. This estimate is based on historical experience and is adjusted as needed based on actual redemptions. In addition to couponing, from time to time we authorize various retailers to run in-store promotional sales of our products. Upon confirmation that a promotion was run, we accrue an estimate of the dollar amount we expect to owe back to the retailer. This estimate is trued up upon receipt of invoice from the retailer.
For the years ended December, 31, 2012 and 2011, we recorded provisions for coupons and promotions totaling $3.0 million and $1.9 million, respectively. As of December 31, 2012 and 2011, the balance in our reserve for coupons and promotions was $0.8 million and $1.0 million, respectively.
35
Allowance for Doubtful Accounts
Provisions for doubtful accounts, which reflect trade receivable balances owed to us that are believed to be uncollectible, are recorded as a component of SG&A expenses. In estimating the allowance for doubtful accounts, we consider our historical experience with collections and write-offs, the credit quality of our customers and any recent or anticipated changes thereto, and the outstanding balances and past due amounts from our customers.
For the years ended December 31, 2012, 2011 and 2010, we recorded net provisions for doubtful accounts of ($82,000), $25,000 and $92,000, respectively. The recovery of $82,000 recorded in 2012 was related the recovery of accounts previously thought to be uncollectible. Our allowance for doubtful accounts was $30,000 and $99,000 as of December 31, 2012 and 2011, respectively. As of December 31, 2012, we had a total of $2.5 million of past due gross accounts receivable, of which $1.0 million was more than 60 days past due. On a monthly basis, we perform a detailed analysis of the receivables due from our wholesaler customers and provide a specific reserve against known uncollectible items for each of the wholesaler customers. We also include in the allowance for doubtful accounts an amount that we estimate to be uncollectible for all other customers based on a percentage of the past due receivables. The percentage we reserve increases as the age of the receivables increases.
Allowance for Slow-Moving and Obsolete Inventory
Inventories are stated at the lower of cost (average cost method) or market. We maintain an allowance for slow-moving and obsolete inventory as well as inventory with a carrying value in excess of its net realizable value (“NRV”). For finished goods inventory, we estimate the amount of inventory that may not be sold prior to its expiration or is slow-moving based upon recent sales activity by unit and wholesaler inventory information. We also analyze our raw material and component inventory for slow moving items. For the years ended December 31, 2012, 2011 and 2010, we recorded a provision for inventory obsolescence in cost of sales of $2.4 million, $0.6 million, and $0.7 million, respectively. The allowance for inventory obsolescence/NRV was $2.2 million and $1.2 million as of December 31, 2012 and 2011, respectively.
We capitalize inventory costs associated with our products prior to regulatory approval when, based on management judgment, future commercialization is considered probable and the future economic benefit is expected to be realized. We assess the regulatory approval process and where the product stands in relation to that approval process including any known constraints or impediments to approval. We consider the shelf life of the product in relation to the product timeline for approval.
Business Combinations
Business combinations are accounted for under ASC 805, Business Combinations, using the acquisition method of accounting. The acquisition method of accounting requires an acquirer to recognize the assets acquired and the liabilities assumed at the acquisition date measured at their fair values as of that date. Fair value determinations are based on discounted cash flow analyses or other valuation techniques. In determining the fair value of the assets acquired and liabilities assumed in a material acquisition, we may utilize appraisals from third party valuation firms to determine fair values of some or all of the assets acquired and liabilities assumed, or may complete some or all of the valuations internally. In either case, we take full responsibility for the determination of the fair value of the assets acquired and liabilities assumed. The value of goodwill will be determined as the excess of the fair value of the consideration conveyed to the seller over the fair value of the net assets received. Under the acquisition method of accounting, we will identify the acquirer and the closing date and apply applicable recognition principles and conditions.
Acquisition-related costs are costs incurred by us to effect a business combination. We account for acquisition-related costs as expenses in the periods in which the costs are incurred and the services are received.
Warranty Liability
The product warranty liability primarily relates to a ten-year expiration guarantee on DTPA Products sold to HHS in 2006. We are performing yearly stability studies for this product and, if the annual stability does not support the ten-year product life, we will replace the product at no charge. Our supplier, Hameln Pharmaceuticals, will also share one-half of this cost if the product does not meet the stability requirement. If the ongoing product testing confirms the ten-year stability for the DTPA Products we will not incur a replacement cost and this reserve will be eliminated with a corresponding reduction to cost of sales after the ten-year period.
36
Income Taxes
Deferred income tax assets and liabilities are determined based on differences between the tax and book basis assets and liabilities, as well as net operating loss and other tax credit carry-forwards. Our deferred tax assets and liabilities are measured using the enacted tax rates that will likely be in effect when the book-to-tax differences are expected to reverse. We record a valuation allowance to reduce deferred income tax assets to the amount that is more likely than not to be realized.
Intangibles
Our intangible assets consist primarily of goodwill, trademarks and customer relationships acquired through business acquisitions and product licensing rights obtained through our acquisition of ANDAs and NDAs from other pharmaceutical companies. Goodwill is deemed to be an indefinite-lived asset and is not amortized. Our other intangible assets are deemed to be finite-lived and are amortized on a straight-line basis over estimated useful lives, which range from 6 to 30 years. We regularly test all of our intangibles for impairment based on several factors, including estimated fair values and anticipated future cash flows from each asset.
We recorded amortization expense of $6.9 million, $1.7 million, and $1.5 million for the years ended December 31, 2012, 2011, and 2010, respectively, in relation to our intangibles. Accumulated amortization was $31.9 million and $25.0 million at December 31, 2012 and 2011, respectively.
Goodwill is tested for impairment annually or more frequently if changes in circumstances or the occurrence of events suggest that impairment may exist. We use widely accepted valuation techniques to determine the fair value of our reporting units used in our annual goodwill impairment analysis. Our valuation is primarily based on qualitative and quantitative assessments regarding the fair value of goodwill relative to its carrying value. We modeled the fair value of the reporting unit based on actual projected earnings and cash flows of the reporting unit. We performed our annual impairment test on October 1, 2012 and determined that the fair value of this reporting unit exceeded its carrying value and, therefore, no impairment charge was necessary.
Stock-Based Compensation
Stock-based compensation cost is estimated at the grant date based on the fair value of the award, and this cost is recognized as expense ratably over the vesting period. We use the Black-Scholes model for estimating the grant date fair value of the stock options we grant. Determining the assumptions that enter into the model is subjective and requires a certain amount of judgment. We use an expected volatility that is based on the historical volatility of our stock. The expected life assumption is based on historical employee exercise patterns and employee post-vesting terminations experience. The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. treasury securities in effect during the quarter in which the options were granted. The dividend yield has historically been set at zero, reflecting the fact that we have not historically issued dividends and do not anticipate issuing dividends in the foreseeable future. We estimate forfeitures at the time of grant and revise our estimates in subsequent periods, when necessary, if actual forfeitures differ from those estimates.
RECENT ACCOUNTING PRONOUNCEMENTS
In June 2011, the Financial Accounting Standard Board (“FASB”) issued ASU 2011-05, Comprehensive Income (Topic 220), Presentation of Comprehensive Income, which converges the presentation of other comprehensive income (OCI) in financial statements prepared under US GAAP and International Financial Reporting Standards (IFRS). This guidance would require disclosure of reclassification adjustments from OCI to net income. In December 2011, the FASB issued ASU 2011-12, Comprehensive Income (Topic 220), Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05 , which deferred the effective date of this guidance to fiscal years beginning after December 15, 2011, with early election permitted. We adopted ASU 2011-05 during fiscal year 2012. The adoption of this guidance did not have a material impact on our financial position or operating results.
In September 2011, the FASB issued ASU 2011-08, Goodwill and Other (ASC Topic 350), Testing Goodwill for Impairment, which added a simplified alternative method for performing annual goodwill impairment tests. Companies now have the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the assessment indicates that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company no longer has to perform the two-step impairment test. ASU 2011-08 is effective for fiscal years beginning after December 15, 2011. The adoption of this guidance did not have a material impact on our financial position or operating results.
37
In May 2011, the FASB issued ASU 2011-4, Fair Value Measurement (ASC Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS, which was intended by the FASB and the International Accounting Standards Board (IASB) to develop common requirements for measuring fair value and for disclosing information about fair value measurements in accordance with GAAP and International Financial Reporting Standards (IFRSs). Additional disclosures required by this amendment include information about transfers between Level 1 and Level 2 instruments, information regarding the sensitivity of Level 3 instruments, and categorization by level of items that are not measured at fair value in the statement of financial position (but for which disclosure of fair value is still required). The guidance is effective prospectively for fiscal years beginning after December 15, 2011 and interim periods within those years. The adoption of this guidance did not have a material impact on our financial position or operating results.
In July 2012, the FASB issued ASU No. 2012-02, Intangibles – Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment. The amendments in this update aim to simplify the impairment test for indefinite-lived intangible assets by permitting an entity the option to first assess qualitative factors to determine whether it is more likely than not (defined as having a likelihood of more than 50 percent) that an indefinite-lived intangible asset is impaired as a basis for determining whether the quantitative impairment test included in Accounting Standards Codification Subtopic 350-30, Intangibles – Goodwill and Other – General Intangibles Other than Goodwill must be performed. The amendment is effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012. Adoption of this amendment is not expected to have a material effect on our financial position or operating results.
As of December 31, 2012, our principal debt was related to our Notes. Interest accrues at a fixed rate of 3.50% on the outstanding principal amount of the Notes and is paid semi-annually every June 1st and December 1st until the notes mature on June 1, 2016. Since the interest rate is fixed, we have no market risk related to the Notes.
Our $20.0 million revolving Credit and Security Agreement with Bank of America, N.A. calls for interest to accrue based on a premium above either the current prime rate or current LIBOR rates. Therefore, borrowings pursuant to this revolving credit facility would be subject to market risk. However, as of December 31, 2012, we had no outstanding balance and therefore no market risk related to this revolving credit facility.
We acquired the principal manufacturing facility and ongoing business of Kilitch, an Indian pharmaceutical company on February 28, 2012. Accordingly, we are subject to foreign exchange risk based on changes in the exchange rate between U.S. dollars and Indian rupees. Additionally, the business we acquired from Kilitch is itself subject to foreign exchange risk related to certain of its export sales to unregulated markets in Africa, Asia and elsewhere to the extent such sales are to be transacted in the local currency rather than in Indian rupees.
Our financial instruments include cash and cash equivalents, accounts receivable, accounts payable and the Notes. The fair values of cash and cash equivalents, accounts receivable and accounts payable approximate book value because of the short maturity of these instruments. Likewise, as of December 31, 2012, the fair value of the Notes approximates their book value.
At December 31, 2012, the bulk of our cash and cash equivalents balance was invested in overnight instruments, the interest rates of which may change daily. Accordingly, these overnight investments are subject to market risk.
The following financial statements are included in Part II, Item 8 of this Form 10-K.
INDEX:
Reports of Independent Registered Public Accounting Firm
Consolidated Balance Sheets as of December 31, 2012 and 2011
Consolidated Statements of Comprehensive Income for the years ended December 31, 2012, 2011 and 2010
Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2012, 2011 and 2010
Consolidated Statements of Cash Flows for the years ended December 31, 2012, 2011 and 2010
Notes to Consolidated Financial Statements
38
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Akorn, Inc.
We have audited the accompanying consolidated balance sheets of Akorn, Inc. as of December 31, 2012 and 2011, and the related consolidated statements of comprehensive income, shareholders' equity and cash flows for each of the three years in the period ended December 31, 2012. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Akorn, Inc. at December 31, 2012 and 2011, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2012, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Akorn Inc.’s internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2013, expressed an adverse opinion thereon.
/s/Ernst & Young LLP
Chicago, Illinois
March 1, 2013
39
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Akorn, Inc.
We have audited Akorn, Inc.’s internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Akorn, Inc. management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As indicated in the accompanying Management’s Report on Internal Control Over Financial Reporting, management’s assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of Akorn India Private Limited, which is included in the 2012 consolidated financial statements of Akorn, Inc. and constituted $61,393,000 and $57,673,000 of total and net assets, respectively, as of December 31, 2012 and $16,704,000 and $7,025,000 of revenues and net loss, respectively, for the year then ended. Our audit of internal control over financial reporting of Akorn, Inc. also did not include an evaluation of the internal control over financial reporting of Akorn India Private Limited.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness has been identified and included in management’s assessment. Management has identified a material weakness in controls related to the company’s financial statement close process. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), consolidated balance sheets of Akorn, Inc. as of December 31, 2012 and 2011 and the related consolidated statements of comprehensive income, shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2012. This material weakness was considered in determining the nature, timing and extent of audit tests applied in our audit of the December 31, 2012, financial statements, and this report does not affect our report dated March 1, 2013, which expressed an unqualified opinion on those financial statements.
In our opinion, because of the effect of the material weakness described above on the achievement of the objectives of the control criteria, Akorn, Inc. has not maintained effective internal control over financial reporting as of December 31, 2012, based on the COSO criteria.
/s/ Ernst & Young LLP
Chicago, Illinois
March 1, 2013
40
AKORN, INC.
CONSOLIDATED BALANCE SHEETS
(In Thousands,
Except Share Data)
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 40,781 | $ | 83,962 | ||||
| Trade accounts receivable, net | 51,017 | 25,307 | ||||||
| Inventories, net | 52,495 | 35,456 | ||||||
| Deferred taxes, current | 9,190 | 8,153 | ||||||
| Prepaid expenses and other current assets | 5,224 | 3,071 | ||||||
| TOTAL CURRENT ASSETS | 158,707 | 155,949 | ||||||
| PROPERTY, PLANT AND EQUIPMENT, NET | 80,679 | 44,389 | ||||||
| OTHER LONG-TERM ASSETS | ||||||||
| Goodwill | 32,159 | 11,863 | ||||||
| Product licensing rights, net | 63,654 | 67,822 | ||||||
| Other intangibles, net | 16,731 | 13,016 | ||||||
| Deferred financing costs, net | 3,078 | 3,864 | ||||||
| Long-term investments | 10,299 | 10,137 | ||||||
| Deferred taxes, non current | 930 | — | ||||||
| Other | 3,328 | 105 | ||||||
| TOTAL OTHER LONG-TERM ASSETS | 130,179 | 106,807 | ||||||
| TOTAL ASSETS | $ | 369,565 | $ | 307,145 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Trade accounts payable | $ | 21,784 | $ | 17,874 | ||||
| Accrued compensation | 7,533 | 5,094 | ||||||
| Accrued royalties | 5,768 | 206 | ||||||
| Accrued administration fees | 2,204 | 1,154 | ||||||
| Accrued expenses and other liabilities | 6,002 | 3,961 | ||||||
| TOTAL CURRENT LIABILITIES | 43,291 | 28,289 | ||||||
| LONG-TERM LIABILITIES | ||||||||
| Long-term debt | 104,637 | 100,808 | ||||||
| Purchase consideration payable | 16,113 | 13,841 | ||||||
| Deferred taxes – non-current | 1,991 | 3,742 | ||||||
| Product warranty liability | 1,299 | 1,299 | ||||||
| Lease incentive obligations and other long-term liabilities | 1,153 | 958 | ||||||
| TOTAL LONG-TERM LIABILITIES | 125,193 | 120,648 | ||||||
| TOTAL LIABILITIES | 168,484 | 148,937 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
Common stock, no par value — 150,000,000 shares authorized; 95,844,012 and 94,936,282 shares issued and outstanding at December 31, 2012 and 2011 | 226,035 | 212,636 | ||||||
| Warrants to acquire common stock | 17,946 | 17,946 | ||||||
| Accumulated deficit | (36,996 | ) | (72,374 | ) | ||||
| Accumulated other comprehensive loss | (5,904 | ) | — | |||||
| TOTAL SHAREHOLDERS’ EQUITY | 201,081 | 158,208 | ||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | $ | 369,565 | $ | 307,145 | ||||
See notes to the consolidated financial statements.
41
AKORN, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In Thousands, Except Per Share Data)
| Year ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| REVENUES | $ | 256,158 | $ | 136,920 | $ | 86,409 | ||||||
Cost of sales (exclusive of amortization of intangibles, included within operating expenses below) | 107,466 | 57,231 | 43,944 | |||||||||
| GROSS PROFIT | 148,692 | 79,689 | 42,465 | |||||||||
| Selling, general and administrative expenses | 48,053 | 32,392 | 22,721 | |||||||||
| Research and development expenses | 15,858 | 11,555 | 6,975 | |||||||||
| Amortization of intangibles | 6,870 | 1,733 | 1,497 | |||||||||
| Acquisition-related costs | 9,155 | 743 | — | |||||||||
| TOTAL OPERATING EXPENSES | 79,936 | 46,423 | 31,193 | |||||||||
| OPERATING INCOME | 68,756 | 33,266 | 11,272 | |||||||||
| Amortization of deferred financing costs | (782 | ) | (1,948 | ) | (2,841 | ) | ||||||
| Non-cash interest expense | (6,436 | ) | (2,109 | ) | — | |||||||
| Interest expense, net | (4,038 | ) | (2,283 | ) | (942 | ) | ||||||
| Equity in earnings of unconsolidated joint venture | — | 14,550 | 23,368 | |||||||||
| Change in fair value of warrants liability | — | — | (8,881 | ) | ||||||||
| Other non-operating expenses | — | (170 | ) | — | ||||||||
| INCOME BEFORE INCOME TAXES | 57,500 | 41,306 | 21,976 | |||||||||
| Income tax provision (benefit) | 22,122 | (1,707 | ) | 152 | ||||||||
| CONSOLIDATED NET INCOME | $ | 35,378 | $ | 43,013 | $ | 21,824 | ||||||
CONSOLIDATED NET INCOME PER COMMON SHARE: | ||||||||||||
| BASIC | $ | 0.37 | $ | 0.45 | $ | 0.24 | ||||||
| DILUTED | $ | 0.32 | $ | 0.41 | $ | 0.22 | ||||||
SHARES USED IN COMPUTING CONSOLIDATED NET INCOME PER COMMON SHARE: | ||||||||||||
| BASIC | 95,189 | 94,549 | 92,801 | |||||||||
| DILUTED | 110,510 | 103,912 | 99,250 | |||||||||
| COMPREHENSIVE INCOME: | ||||||||||||
| Consolidated net income | $ | 35,378 | $ | 43,013 | $ | 21,824 | ||||||
| Foreign currency translation loss | (5,904 | ) | — | — | ||||||||
| COMPREHENSIVE INCOME | $ | 29,474 | $ | 43,013 | $ | 21,824 | ||||||
See notes to the consolidated financial statements.
42
AKORN, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2010, 2011 AND 2012
(In Thousands)
| Warrants | Retained | |||||||||||||||||||||||
| to acquire | Earnings | Other | ||||||||||||||||||||||
| Common Stock | Common | (Accumulated | Comprehensive | |||||||||||||||||||||
| Shares | Amount | Stock | Deficit) | Loss | Total | |||||||||||||||||||
| BALANCES AT DECEMBER 31, 2009 | 90,390 | $ | 174,027 | $ | 1,821 | $ | (137,211 | ) | $ | — | $ | 38,637 | ||||||||||||
| Consolidated net income | — | — | — | 21,824 | — | 21,824 | ||||||||||||||||||
Net proceeds from common stock and warrant offering | 3,243 | 4,969 | — | — | — | 4,969 | ||||||||||||||||||
Reclassification of warrants from current liability to shareholders’ equity | — | — | 17,946 | — | — | 17,946 | ||||||||||||||||||
| Exercise of stock warrants | 9 | 94 | (94 | ) | — | — | — | |||||||||||||||||
| Exercise of stock options | 256 | 452 | — | — | — | 452 | ||||||||||||||||||
| Employee stock purchase plan issuances | 47 | 187 | — | — | — | 187 | ||||||||||||||||||
| Restricted stock awards | 30 | 60 | — | — | — | 60 | ||||||||||||||||||
| Stock-based compensation expense | — | 2,677 | — | — | — | 2,677 | ||||||||||||||||||
| BALANCES AT DECEMBER 31, 2010 | 93,975 | $ | 182,466 | $ | 19,673 | $ | (115,387 | ) | $ | — | $ | 86,752 | ||||||||||||
| Consolidated net income | — | — | — | 43,013 | — | 43,013 | ||||||||||||||||||
| Exercise of stock warrants | 365 | 3,454 | (1,727 | ) | — | — | 1,727 | |||||||||||||||||
| Exercise of stock options | 454 | 867 | — | — | — | 867 | ||||||||||||||||||
| Employee stock purchase plan issuances | 129 | 220 | — | — | — | 220 | ||||||||||||||||||
| Restricted stock awards | 15 | 17 | — | — | — | 17 | ||||||||||||||||||
| Equity portion of convertible notes offering | — | 20,470 | — | — | — | 20,470 | ||||||||||||||||||
| Stock-based compensation expense | — | 5,142 | — | — | — | 5,142 | ||||||||||||||||||
| BALANCES AT DECEMBER 31, 2011 | 94,938 | $ | 212,636 | $ | 17,946 | $ | (72,374 | ) | $ | — | $ | 158,208 | ||||||||||||
| Consolidated net income | — | — | — | 35,378 | — | 35,378 | ||||||||||||||||||
| Exercise of stock options | 806 | 1,511 | — | — | — | 1,511 | ||||||||||||||||||
| Employee stock purchase plan issuances | 71 | 368 | — | — | — | 368 | ||||||||||||||||||
| Restricted stock awards | 29 | 351 | — | — | — | 351 | ||||||||||||||||||
| Stock-based compensation expense | — | 6,681 | — | — | — | 6,681 | ||||||||||||||||||
| Foreign currency translation loss | — | — | — | — | (5,904 | ) | (5,904 | ) | ||||||||||||||||
| Excess tax benefit – stock compensation | — | 4,488 | — | — | — | 4,488 | ||||||||||||||||||
| BALANCES AT DECEMBER 31, 2012 | 95,844 | $ | 226,035 | $ | 17,946 | $ | (36,996 | ) | $ | (5,904 | ) | $ | 201,081 | |||||||||||
See notes to the consolidated financial statements.
43
AKORN, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In Thousands)
| Year ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| (restated) | ||||||||||||
| OPERATING ACTIVITIES: | ||||||||||||
| Consolidated net income | $ | 35,378 | $ | 43,013 | $ | 21,824 | ||||||
Adjustments to reconcile consolidated net income to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 11,455 | 5,246 | 5,030 | |||||||||
| Amortization of deferred financing fees | 782 | 1,948 | 2,841 | |||||||||
| Amortization of unfavorable contract liability | (635 | ) | — | — | ||||||||
| Non-cash stock compensation expense | 7,032 | 5,159 | 2,737 | |||||||||
| Non-cash change in fair value of warrants liability | — | — | 8,881 | |||||||||
| Non-cash interest expense | 6,436 | 2,109 | — | |||||||||
| Deferred tax assets, net | 67 | ( 4,411 | ) | — | ||||||||
| Excess tax benefit from stock compensation | (4,488 | ) | — | — | ||||||||
| Equity in earnings of unconsolidated joint venture | — | ( 14,550 | ) | ( 23,368 | ) | |||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Trade accounts receivable | ( 23,856 | ) | ( 13,581 | ) | ( 2,045 | ) | ||||||
| Inventories | ( 15,447 | ) | (9,307 | ) | ( 5,750 | ) | ||||||
| Prepaid expenses and other current assets | ( 5,689 | ) | ( 183 | ) | 233 | |||||||
| Supply agreement termination liabilities | — | — | ( 1,500 | ) | ||||||||
| Trade accounts payable | 4,489 | 2,546 | 1,608 | |||||||||
| Accrued expenses and other liabilities | 10,720 | 1,668 | 1,791 | |||||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | 26,244 | 19,657 | 12,282 | |||||||||
| INVESTING ACTIVITIES: | ||||||||||||
| Payments for acquisitions and equity investments | ( 55,047 | ) | ( 87,412 | ) | — | |||||||
| Purchases of property, plant and equipment | ( 20,454 | ) | ( 11,503 | ) | ( 4,710 | ) | ||||||
| Distributions from unconsolidated joint venture | — | 3,881 | 36,265 | |||||||||
| NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES | ( 75,501 | ) | ( 95,034 | ) | 31,555 | |||||||
| FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from issuance of convertible notes | — | 120,000 | — | |||||||||
| Debt financing costs | — | ( 5,098 | ) | — | ||||||||
| Net proceeds from common stock offering and warrant exercises | — | 1,727 | 4,969 | |||||||||
| Repayments of subordinated debt – related party | — | — | ( 6,439 | ) | ||||||||
| Repayments of revolving line of credit | — | — | ( 3,000 | ) | ||||||||
| Excess tax benefit from stock compensation | 4,488 | — | — | |||||||||
| Proceeds under stock option and stock purchase plans | 1,878 | 1,087 | 639 | |||||||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | 6,366 | 117,716 | ( 3,831 | ) | ||||||||
| Effect of changes in exchange rates on cash and cash equivalents | (290 | ) | — | — | ||||||||
| (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (43,181 | ) | 42,339 | 40,006 | ||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR | 83,962 | 41,623 | 1,617 | |||||||||
| CASH AND CASH EQUIVALENTS AT END OF YEAR | $ | 40,781 | $ | 83,962 | $ | 41,623 | ||||||
See notes to the consolidated financial statements.
44
AKORN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 — Business and Basis of Presentation
Business: Akorn, Inc. and its wholly-owned subsidiaries (collectively, the “Company”) manufacture and market a full line of diagnostic and therapeutic ophthalmic pharmaceuticals as well as niche hospital drugs and injectable pharmaceuticals. In addition, through its subsidiary Advanced Vision Research, Inc. (“AVR”), the Company manufactures and markets a line of over-the-counter (“OTC”) ophthalmic products for the treatment of dry eye, eyelid hygiene and macular degeneration primarily under the TheraTears® brand name. The Company is a manufacturer and/or marketer of diagnostic and therapeutic pharmaceutical products in various specialty areas, including ophthalmology, antidotes, anti-infectives, vaccines, and controlled substances for pain management and anesthesia, among others. The Company operates pharmaceutical manufacturing plants in the U.S. at Decatur, Illinois and Somerset, New Jersey, and internationally at Paonta Sahib, Himachal Pradesh, India, as well as a central distribution warehouse in Gurnee, Illinois, R&D centers in Skokie and Vernon Hills, Illinois and corporate offices in Lake Forest, Illinois. Customers of the Company’s products include physicians, optometrists, wholesalers, chain drug stores, group purchasing organizations and their member hospitals, alternate site providers, wholesalers, distributors, and other pharmaceutical companies.
Restatement: The Company’s consolidated statement of cash flows for the year ended December 31, 2011 has been restated to correct a classification error which resulted in overstatement of cash provided by operating activities in the amount of $3,346,000 and overstatement of cash used by investing activities by that same amount. The error was related to capital expenditures that were accrued but unpaid. The following table sets forth the numbers in the consolidated statement of cash flows that needed to be restated to correct the error (in thousands):
| Year ended | ||||||||
| December 31, 2011 | ||||||||
| As Filed | Restated | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Trade accounts payable | $ | 5,892 | $ | 2,546 | ||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | $ | 23,003 | $ | 19,657 | ||||
| Purchase of property, plant and equipment | $ | (14,849 | ) | $ | (11,503 | ) | ||
| NET CASH USED IN INVESTING ACTIVITIES | $ | (98,380 | ) | $ | (95,034 | ) | ||
Note 2 — Summary of Significant Accounting Policies
Consolidation: The accompanying consolidated financial statements include the accounts of Akorn, Inc and its wholly owned domestic and foreign subsidiaries, which include Akorn (New Jersey) Inc., Advanced Vision Research, Inc. (“AVR”), World Akorn Pharma Mauritius, Akorn India Private Limited (“AIPL”), Akorn Ophthalmics, Inc. and Oak Pharmaceuticals, Inc. All inter-company transactions and balances have been eliminated in consolidation, and the financial statements of AIPL have been translated from Indian rupees to U.S. dollars based on the currency translation rates in effect during the period or as of the date of consolidation, as applicable.
The Company is a 50% owner of a strategic joint venture, Akorn-Strides, LLC (the “Joint Venture Company”) (See Note 18.). The Company and its strategic partner each have equal voting rights and shared operational control. Accordingly, the Company accounts for its investment in the Joint Venture Company using the equity method of accounting. The Company’s proportionate share of the Joint Venture Company’s income has been recorded under the caption “Equity in earnings of unconsolidated joint venture” in the Company’s consolidated statements of operations. The Joint Venture Company sold all of its abbreviated new drug application (“ANDA”) rights to Pfizer, Inc. in December 2010 and ceased operations during 2011.
Use of Estimates: The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates. Significant estimates and assumptions for the Company relate to the allowances for doubtful accounts, chargebacks, rebates, product returns and coupons and promotions, and the reserve for slow-moving and obsolete inventories, the carrying value of intangible assets and the carrying value of deferred income tax assets.
45
Revenue Recognition: The Company recognizes product sales for its ophthalmic and hospital drugs & injectables business segments upon the shipment of goods or upon the delivery of the product or service as appropriate. Revenue is recognized when all obligations of the Company have been fulfilled and collection of the related receivable is probable.
The contract services segment, which manufactures products for third party customers based upon their specification and sells those products at a pre-determined price, also recognizes sales upon the shipment of goods or upon delivery of the product or service as appropriate. Revenue is recognized when all obligations of the Company have been fulfilled and collection of the related receivable is probable.
Provision for estimated doubtful accounts, chargebacks, coupon redemption, rebates, discounts and product returns is made at the time of sale and is analyzed and adjusted, if necessary, at each balance sheet date.
Freight: The Company records amounts billed to customers for shipping and handling as revenue, and records shipping and handling expense related to product sales as cost of sales.
Cash and Cash Equivalents: The Company considers all unrestricted, highly liquid investments with maturity of three months or less when purchased to be cash and cash equivalents. At December 31, 2012 approximately $3.2 million of cash held by our India operations were restricted and recorded as other long term assets. There was no restricted cash at December 31, 2011.
Accounts Receivable: The nature of the Company’s business involves, in the ordinary course, significant amounts and substantial volumes of transactions and estimates relating to chargebacks, coupon redemption, product returns, rebates, discounts given to customers and allowances for doubtful accounts. This is a normal circumstance within the pharmaceutical distribution industry which inherently lengthens and complicates the process of settling sales. Depending on the products, the end-user customers, the specific terms of national supply contracts and the particular arrangements with the Company’s wholesaler customers, certain rebates, chargebacks and other credits are deducted from the Company’s accounts receivable, or may be requested as refunds after the initial accounts receivable has been paid. The process of claiming these deductions depends on wholesalers reporting to the Company the amount of deductions that were earned under the respective terms with end-user customers (which, in turn, depends on which end-user customer with different pricing arrangements might be entitled to a particular deduction). This process can lead to “partial payments” against outstanding invoices as the wholesalers take the claimed deductions at the time of payment.
Unless otherwise noted, the provisions and allowances for the following customer deductions are reflected in the accompanying financial statements as reductions of revenues and trade accounts receivable, respectively.
Chargebacks and Rebates: The Company enters into contractual agreements with certain third parties such as hospitals and group-purchasing organizations to sell certain products at predetermined prices. The parties have elected to have these contracts administered through wholesalers that buy the product from the Company and subsequently sell it to these third parties. When a wholesaler sells products to one of these third parties that are subject to a contractual price agreement, the difference between the price paid to the Company by the wholesaler and the price under the specific contract is charged back to the Company by the wholesaler. The Company tracks sales and submitted chargebacks by product number and contract for each wholesaler. Utilizing this information, the Company estimates a chargeback percentage for each product. The Company reduces gross sales and increases the chargeback allowance by the estimated chargeback amount for each product sold to a wholesaler. The Company reduces the chargeback allowance when it processes a request for a chargeback from a wholesaler. Actual chargebacks processed by the Company can vary materially from period to period based upon actual sales volume through the wholesalers. However, the Company’s provision for chargebacks is fully reserved for at the time when sales revenues are recognized.
Management obtains certain wholesaler inventory reports to aid in analyzing the reasonableness of the chargeback allowance. The Company assesses the reasonableness of its chargeback allowance by applying the product chargeback percentage based on historical activity to the quantities of inventory on hand per the wholesaler inventory reports and an estimate of in-transit inventory that is not reported on the wholesaler inventory reports at the end of the period. In accordance with its accounting policy, the Company estimates the percentage amount of wholesaler inventory that will ultimately be sold to third parties that are subject to contractual price agreements based on a six-quarter trend of such sales through wholesalers. The Company uses this percentage estimate until historical trends indicate that a revision should be made. On an ongoing basis, the Company evaluates its actual chargeback rate experience, and new trends are factored into its estimates each quarter as market conditions change.
46
Period Start Date | Period End Date | Estimated % of wholesaler inventory that will be subject to contractual price agreements | |||
| July 1, 2009 | - | June 30, 2010 | 97.0% | ||
| July 1, 2010 | - | June 30, 2012 | 98.5% | ||
| July 1, 2012 | - | September 30, 2012 | 95.0% | ||
| October 1, 2012 | - | Current | 90.0% |
The Company will continue to use the 90.0% estimate in future periods until trends indicate that a revision should be made.
Similarly, the Company maintains an allowance for rebates related to contract and other programs with certain customers. Rebate percentages vary by product and by volume purchased by each eligible customer. The Company tracks sales by product number for each eligible customer and then applies the applicable rebate percentage, using both historical trends and actual experience to estimate its rebate allowance. The Company reduces gross sales and increases the rebate allowance by the estimated rebate amount when the Company sells its products to its rebate-eligible customers. The Company reduces the rebate allowance when it processes a customer request for a rebate. At each balance sheet date, the Company analyzes the allowance for rebates against actual rebates processed and makes necessary adjustments as appropriate. Actual rebates processed can vary materially from period to period. However, the Company fully records its provision for rebates at the time when sales revenues are recognized.
The recorded allowances reflect the Company’s current estimate of the future chargeback and rebate liability to be paid or credited to its wholesaler and other customers under the applicable contracts and programs. For the years ended December 31, 2012, 2011 and 2010, the Company recorded chargeback and rebate expense of $112.2 million, $68.1 million, and $45.2 million, respectively. The allowance for chargebacks and rebates was $13.5 million and $5.9 million as of December 31, 2012 and 2011, respectively.
Sales Returns: Certain of the Company’s products are sold with the customer having the right to return the product within specified periods and guidelines for a variety of reasons, including but not limited to, pending expiration dates. Provisions are made at the time of sale based upon tracked historical experience, by customer in some cases. One-time historical factors or pending new developments that would impact the expected level of returns are taken into account to determine the appropriate reserve estimate at each balance sheet date. As part of the evaluation of the balance required, the Company considers actual returns to date that are in process, the expected impact of any product recalls and the wholesaler’s inventory information to assess the magnitude of unconsumed product that may result in a sales return to the Company in the future. The sales returns level can be impacted by factors such as overall market demand and market competition and availability for substitute products which can increase or decrease the end-user pull through for sales of the Company’s products and ultimately impact the level of sales returns. Actual returns experience and trends are factored into the Company’s estimates each quarter as market conditions change. Actual returns processed can vary materially from period to period.
For the years ended December 31, 2012, 2011 and 2010, the Company recorded a net provision for product returns of $3.8 million, $2.7 million and $1.5 million, respectively. The increase in returns provision from 2010 to 2011 was in line with the increase in revenue from year to year. The increase in returns provision from $2.7 million to $3.8 million in 2012 was at a pace lower than our increase in sales, largely due to declining historical returns rates. The Company’s allowance for potential product returns was $8.4 million and $6.8 million at December 31, 2012 and 2011, respectively.
Allowance for Coupons and Promotions: We issue coupons from time to time redeemable against our TheraTears® eye care products. Upon release of coupons into the market, we record an estimate of the dollar value of coupons we expect to be redeemed. This estimate is based on historical experience and is adjusted as needed based on actual redemptions. In addition to couponing, from time to time we authorize various retailers to run in-store promotional sales of our products. Upon confirmation that a promotion was run, we accrue an estimate of the dollar amount we expect to owe back to the retailer. This estimate is trued up upon receipt of invoice from the retailer.
For the years ended December, 31, 2012 and 2011, we recorded provisions for coupons and promotions totaling $3.0 million and $1.9 million, respectively. As of December 31, 2012 and 2011, the balance in our reserve for coupons and promotions was $0.8 million and $1.0 million, respectively.
47
Doubtful Accounts: Provisions for doubtful accounts, which reflect trade receivable balances owed to the Company that are believed to be uncollectible, are recorded as a component of SG&A expenses. In estimating the allowance for doubtful accounts, the Company considers its historical experience with collections and write-offs, the credit quality of its customers and any recent or anticipated changes thereto, and the outstanding balances and past due amounts from its customers. Accounts are considered past due when they remain uncollected beyond the due date specified in the applicable contract or on the applicable invoice, whichever is deemed to take precedence.
For the years ended December 31, 2012, 2011 and 2010, the Company recorded net provisions for doubtful accounts of $(82,000), $25,000 and $92,000, respectively. The recovery of $82,000 recorded in 2012 was related to change in estimated reserves and collections related to accounts previously thought to be uncollectible. The allowance for doubtful accounts was $30,000 and $99,000 as of December 31, 2012 and 2011, respectively. As of December 31, 2012, the Company had a total of $2.5 million of past due gross accounts receivable, of which $1.0 million was more than 60 days past due. The Company performs monthly a detailed analysis of the receivables due from its wholesaler customers and provides a specific reserve against known uncollectible items for each of the wholesaler customers. The Company also includes in the allowance for doubtful accounts an amount that it estimates to be uncollectible for all other customers based on a percentage of the past due receivables. The percentage reserved increases as the age of the receivables increases. Accounts are written off once all reasonable collections efforts have been exhausted and/or when facts or circumstances regarding the customer (i.e. bankruptcy filing) indicate that the chance of collection is remote.
Advertising and Promotional Allowances to Customers: The Company routinely sells its non-prescription ophthalmic and other drug products to major retail drug chains. From time to time, the Company may arrange for these retailers to run in-store promotional sales of the Company’s products. The Company reserves an estimate of the dollar amount owed back to the retailer, recording this amount as a reduction to revenue at the later of the date on which the revenue is recognized or the date the sales incentive is offered. When the actual invoice for the sales promotion is received from the retailer, the Company adjusts its estimate accordingly.
For our treatment of advertising and promotional expenses paid to customers, we referred to guidance contained within ASC 605-50, Customer Payments and Incentives.
Inventories: Inventories are stated at the lower of cost (average cost method) or market (see Note 4 — “Inventories”). The Company maintains an allowance for slow-moving and obsolete inventory as well as inventory with a carrying value in excess of its net realizable value (“NRV”). For finished goods inventory, the Company estimates the amount of inventory that may not be sold prior to its expiration or is slow moving based upon recent sales activity by unit and wholesaler inventory information. The Company also analyzes its raw material and component inventory for slow moving items. For the years ended December 31, 2012, 2011 and 2010, the Company recorded a provision for inventory obsolescence/NRV of $2.4 million, $0.6 million and $0.7 million, respectively. The allowances for inventory obsolescence were $2.2 million and $1.2 million as of December 31, 2012 and 2011, respectively.
The Company capitalizes inventory costs associated with its products prior to regulatory approval when, based on management judgment, future commercialization is considered probable and the future economic benefit is expected to be realized. The Company assesses the regulatory approval process and where the product stands in relation to that approval process including any known constraints or impediments to approval. The Company considers the shelf life of the product in relation to the product timeline for approval.
At December 31, 2012, the Company had approximately $0.8 million in inventory for generic drugs under development which have not yet received FDA approval. The Company has reserved $0.8 million of this inventory related to products that may not receive FDA approval far enough in advance of expiration to be sellable.
At December 31, 2011, the Company had approximately $4.0 million in inventory for generic drugs which have not yet received FDA approval. The Company has reserved $1.6 million of this inventory related to products that may not receive FDA approval far enough in advance of expiration to be sellable. FDA approval is deemed probable for the remaining $2.4 million and the Company is expecting to fully recover the costs of this inventory upon FDA approval.
Intangibles: Intangibles consist primarily of goodwill, which is carried at its initial value, subject to evaluation for impairment, and product licensing costs, trademarks and other such costs, which are capitalized and amortized on a straight-line basis over their useful lives, ranging from four (4) years to thirty (30) years. Accumulated amortization was $31.9 million and $25.0 million at December 31, 2012 and 2011, respectively. Amortization expense was $6.9 million, $1.7 million and $1.5 million for the years ended December 31, 2012, 2011 and 2010, respectively. The Company regularly assesses its intangibles for impairment based on several factors, including estimated fair value and anticipated cash flows. If the Company incurs additional costs to renew or extend the life of an intangible asset, such costs are added to the remaining unamortized cost of the asset, if any, and the sum is amortized over the extended remaining life of the asset.
48
Goodwill is tested for impairment annually or more frequently if changes in circumstances or the occurrence of events suggest that impairment may exist. The Company uses widely accepted valuation techniques to determine the fair value of its reporting units used in its annual goodwill impairment analysis. The Company’s valuation is primarily based on qualitative and quantitative assessments regarding the fair value of goodwill relative to its carrying value. The Company modeled the fair value of the reporting unit based on actual projected earnings and cash flows of the reporting unit. The Company performed its annual impairment test on October 1, 2012 and determined that the fair value of its reporting unit exceeded its carrying value and, therefore, no impairment charge was necessary.
Movement in goodwill over the year was as follows (in thousands):
| Goodwill | ||||||
| January 1, 2012 | $ | 11,863 | ||||
| Acquisitions | 22,613 | |||||
| Impairments | - | |||||
| Foreign Currency Translation | (2,317 | ) | ||||
| December 31, 2012 | $ | 32,159 | ||||
The following table sets forth the major categories of the Company’s intangible assets and the weighted-average remaining amortization period as of December 31, 2012 for those assets that are not already fully amortized (dollar amounts in thousands):
| Gross | Net | Weighted Average | |||||||||||
| Carrying | Accumulated | Carrying | Remaining | ||||||||||
| Amount | Amortization | Amount | Amortization Period | ||||||||||
| Product licensing rights | $ | 93,534 | $ | (29,880 | ) | $ | 63,654 | 13.8 years | |||||
| Trademarks | 9,500 | (528 | ) | 8,972 | 28.3 years | ||||||||
| Customer relationships | 6,460 | (865 | ) | 5,595 | 9.8 years | ||||||||
| Non-Compete | 2,743 | (579 | ) | 2,164 | 3.2 years | ||||||||
| $ | 112,237 | $ | (31,852 | ) | $ | 80,385 | |||||||
Movement in intangible assets over the year was as follows (in thousands):
| Product licensing rights | Trademarks | Customer Relationships | Non-Compete | |||||||||||||
| January 1, 2012 | $67,822 | $9,289 | $3,727 | - | ||||||||||||
| Acquisitions | 1,100 | - | 2,560 | 2,743 | ||||||||||||
| Amortization | (5,268) | (317) | (705) | (580) | ||||||||||||
| Foreign Currency Translation | - | - | 6 | 8 | ||||||||||||
| December 31, 2012 | $63,654 | $8,972 | $5,588 | 2,171 | ||||||||||||
The amortization expense of acquired intangible assets for each of the following five years will be as follows (in thousands):
Year ending December 31, | Amortization Expense | ||||
| 2013 | $ | 6,606 | |||
| 2014 | 6,359 | ||||
| 2015 | 6,184 | ||||
| 2016 | 5,605 | ||||
| 2017 | 5,066 | ||||
Property, Plant and Equipment: Property, plant and equipment is stated at cost, less accumulated depreciation. Depreciation is provided using the straight-line method in amounts considered sufficient to amortize the cost of the assets to operations over their estimated useful lives or lease terms. Depreciation expense was $4.6 million, $3.5 million and $3.5 million for the years ended December 31, 2012, 2011 and 2010, respectively. The amortization of assets under capital leases is included within depreciation expense. The following table sets forth the average estimated useful lives of the Company’s property, plant and equipment, by asset category:
| Asset category | Depreciable Life | |
| Buildings | 30 years | |
| Leasehold improvements | 10 years | |
| Furniture and equipment | 10 years | |
| Automobiles | 5 years |
49
Net Income Per Common Share: Basic net income per common share is based upon weighted average common shares outstanding. Diluted net income per common share is based upon the weighted average number of common shares outstanding, including the dilutive effect, if any, of stock options, warrants and convertible securities using the treasury stock and if converted methods. Anti-dilutive shares excluded from the computation of diluted net income per share for 2012, 2011 and 2010 include 581,000, 1,560,000 and 2,859,000 shares, respectively, related to options, warrants and convertible securities.
Income Taxes: Deferred income tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities, and net operating loss and other tax credit carry-forwards. These items are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company records a valuation allowance to reduce the deferred income tax assets to the amount that is more likely than not to be realized.
Fair Value of Financial Instruments: The Company applies ASC Topic 820, which establishes a framework for measuring fair value and clarifies the definition of fair value within that framework. ASC Topic 820 defines fair value as an exit price, which is the price that would be received for an asset or paid to transfer a liability in the Company’s principal or most advantageous market in an orderly transaction between market participants on the measurement date. The fair value hierarchy established in ASC Topic 820 generally requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Observable inputs reflect the assumptions that market participants would use in pricing the asset or liability and are developed based on market data obtained from sources independent of the reporting entity. Unobservable inputs reflect the entity’s own assumptions based on market data and the entity’s judgments about the assumptions that market participants would use in pricing the asset or liability, and are to be developed based on the best information available in the circumstances.
The valuation hierarchy is composed of three categories. The classification within the valuation hierarchy is based on the lowest level of input that is significant to the fair value measurement. The categories within the valuation hierarchy are described below:
| - | Level 1—Assets and liabilities with unadjusted, quoted prices listed on active market exchanges. Inputs to the fair value measurement are observable inputs, such as quoted prices in active markets for identical assets or liabilities. The carrying value of the Company‘s cash and cash equivalents are considered Level 1 assets. |
| - | Level 2—Inputs to the fair value measurement are determined using prices for recently traded assets and liabilities with similar underlying terms, as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals. The Company does not have any Level 2 assets or liabilities. |
| - | Level 3—Inputs to the fair value measurement are unobservable inputs, such as estimates, assumptions, and valuation techniques when little or no market data exists for the assets or liabilities. The purchase consideration payable related to the Company’s acquisition on December 22, 2011 of three branded, injectable drug products from the U.S. subsidiary of H. Lundbeck A/S (the “Lundbeck Acquisition”) is a Level 3 liability. |
The following table summarizes the basis used to measure the fair values of the company’s financial instruments (amounts in thousands):
| Fair Value Measurements at Reporting Date, Using: | ||||||||||||||||
| Quoted Prices | Significant | |||||||||||||||
| in Active | Other | Significant | ||||||||||||||
| Markets for | Observable | Unobservable | ||||||||||||||
| December 31, | Identical Items | Inputs | Inputs | |||||||||||||
| Description | 2012 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
| Cash and cash equivalents | $ | 40,781 | $ | 40,781 | $ | — | $ | — | ||||||||
| Total assets | $ | 40,781 | $ | 40,781 | $ | — | $ | — | ||||||||
| Purchase consideration payable | $ | 14,208 | $ | — | $ | — | $ | 14,208 | ||||||||
| Total liabilities | $ | 14,208 | $ | — | $ | — | $ | 14,208 | ||||||||
50
| Quoted Prices | Significant | |||||||||||||||
| in Active | Other | Significant | ||||||||||||||
| Markets for | Observable | Unobservable | ||||||||||||||
| December 31, | Identical Items | Inputs | Inputs | |||||||||||||
| Description | 2011 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
| Cash and cash equivalents | $ | 83,962 | $ | 83,962 | $ | — | $ | — | ||||||||
| Total assets | $ | 83,962 | $ | 83,962 | $ | — | $ | — | ||||||||
| Purchase consideration payable | $ | 11,300 | $ | — | $ | — | $ | 11,300 | ||||||||
| Total liabilities | $ | 11,300 | $ | — | $ | — | $ | 11,300 | ||||||||
The carrying amount of the purchase consideration payable was initially determined based on the terms of the underlying contracts and the Company’s subjective evaluation of the likelihood of the additional purchase consideration becoming payable. The purchase consideration payable is related to the Company’s obligation to pay additional consideration related to the acquisition of selected assets from H. Lundbeck A/S (“Lundbeck”) on December 22, 2011. The underlying obligations are long-term in nature, and therefore were discounted to present value based on an assumed discount rate. The additional consideration of $15.0 million, contingently payable to Lundbeck on December 22, 2014, was initially discounted to $11.3 million based on a discount rate of 10.0%, and subsequently adjusted in final acquisition accounting to $11.6 million based on applying a 9.0% discount rate. At December 31, 2012, the Company performed an evaluation of the fair value of this liability based on utilizing significant unobservable inputs to derive a discount rate of 2.75%, and determined that the appropriate discounted value was $14.2 million. Accordingly, the Company recorded non-cash interest expense of $2.6 million during 2012 to accrue the carrying value of the contingent payment liability to $14.2 million as of December 31, 2012. The fair value of the liability is based upon the likelihood of achieving the underlying revenue targets and a derived cost of debt based on the remaining term. Therefore, the liability is sensitive to changes in the market rate.
The Company initially determined that there was a 100% likelihood of the purchase consideration ultimately becoming payable, and has reaffirmed that this is still the Company’s determination as of December 31, 2012. Should subjective and objective evidence lead the Company to change this assessment, an adjustment to the carrying value of the liability would be recorded as “other income” in the Company’s condensed consolidated statements of comprehensive income.
As of December 31, 2012 and 2011, the Company was carrying long-term investments valued at $10,299,000 and $10,137,000, respectively. The underlying assets are cost-basis investments for which fair value is not readily determinable.
Warrants: The Company issued various warrants during 2009 to entities controlled by John N. Kapoor, Ph.D., the Chairman of the Company’s Board of Directors (the “Kapoor Warrants”). The Company had classified the fair value of these warrants as a current liability in accordance with ASC 815-40-15-3, Derivatives and Hedging, (formerly EITF 00-19, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock). This classification was made as a result of the requirement that the shares to be issued upon exercise of the Kapoor Warrants be registered shares, which could not be absolutely assured. The Kapoor Warrants were adjusted to fair value at the end of each quarter through Black-Scholes calculations which considered changes in the market price of the Company’s common stock, the remaining contractual life of the Kapoor Warrants, and other factors. Any change in the fair value of the Kapoor Warrants was recorded as income or expense on the Company’s consolidated statements of operations for the applicable period.
On June 28, 2010, the Company and Dr. Kapoor entered into an Amended and Restated Registration Rights Agreement (the “Amended Agreement”) which modified certain terms related to the Company’s obligation to obtain and maintain registration of any shares issued pursuant to exercise of the Kapoor Warrants. The Amended Agreement still requires the Company to use “commercially reasonable efforts” to file a registration statement pursuant to Rule 415 of the Securities Act of 1933 (“Registration Statement”) for any shares of common stock that may be issued under the applicable warrant agreements, and to maintain the continuous effectiveness of such Registration Statement until the earliest of: (i) the date no shares of the Company’s common stock qualify as registrable securities, (ii) the date on which all of the registrable securities may be sold in a single transaction by the holder to the public pursuant to Rule 144 or a similar rule, or (iii) the date upon which the John N. Kapoor Trust Dated September 20, 1989 (the “Kapoor Trust”) and EJ Funds, LP (“EJ Funds”) have transferred all of the registrable securities. However, the Registration Rights Agreement has been amended to explicitly state that in the event the Company, after using its good faith commercially reasonable efforts, is not able to obtain or maintain registration of the common stock, delivery of unregistered shares upon exercise of the Kapoor Warrants will be deemed acceptable and a net cash settlement will not be required. The Amended Agreement further provides that the term “commercially reasonable efforts” in such instance shall not mean an absolute obligation of the Company to obtain and maintain registration.
51
As a result of the changes effected through the Amended Agreement, on June 28, 2010 the Company changed its accounting treatment of the Kapoor Warrants, no longer classifying them as a current liability with periodic adjustments to fair value but instead classifying them as a component of shareholders’ equity in accordance with ASC 815-40. Accordingly, the fair value of the Kapoor Warrants, which was $17.9 million on June 28, 2010, was reclassified from a current liability to a component of shareholders’ equity on that date. Following this change in classification, no future fair value adjustments are required.
The liability at June 28, 2010 for the Kapoor Warrants was estimated using a Black-Scholes valuation model with the fair value per warrant ranging from $2.49 to $2.50. The $8.9 million increase in fair value of the Kapoor Warrants from January 1 to June 28, 2010 was recorded as a non-operating expense under the caption “Change in fair value of warrants liability” in the Company’s consolidated statements of operations for 2010. During 2009, the Company recorded an expense of $3.8 million reflecting the increase in fair value of the Kapoor Warrants from their grant dates to December 31, 2009.
The expected volatility of the Kapoor Warrants was based on the historical volatility of the Company’s common stock. The expected life assumption was based on the remaining life of the Kapoor Warrants. The risk-free interest rate for the expected term of the Kapoor Warrants was based on the average market rate on U.S. treasury securities in effect during the applicable quarter. The dividend yield reflected historical experience as well as future expectations over the expected term of the Kapoor Warrants.
The assumptions used in estimating the fair value of the warrants at June 28, 2010 and December 31, 2009 were as follows:
June 28, 2010 | December 31, 2009 | |
| Expected Volatility | 79.7% | 79.5% |
| Expected Life (in years) | 3.8 – 4.1 | 4.3 – 4.6 |
| Risk-free interest rate | 1.8% | 2.3% |
| Dividend yield | — | — |
The following table summarizes the terms of the Kapoor Warrants:
| Fair Value ($000s) | ||||||||||||||||||||||
Granted To: | Warrant Identification | Grant Date | Warrants Granted | Exercise Price | At Grant Dates | As of 12/31/09 | As of 6/28/10 | |||||||||||||||
| EJ Funds | Modification Warrants | Apr.13, 2009 | 1,939,639 | $ | 1.11 | $ | 1,358 | $ | 2,425 | $ | 4,829 | |||||||||||
| Kapoor Trust | Reimbursement Warrants | Apr.13, 2009 | 1,501,933 | $ | 1.11 | 1,051 | 1,877 | 3,740 | ||||||||||||||
| EJ Funds | Credit Facility Warrants | Aug.17, 2009 | 1,650,806 | $ | 1.16 | 1,238 | 2,096 | 4,127 | ||||||||||||||
| Kapoor Trust | Subordinated Note Warrants | Aug.17, 2009 | 2,099,935 | $ | 1.16 | 1,575 | 2,667 | 5,250 | ||||||||||||||
| 7,192,313 | $ | 5,222 | $ | 9,065 | $ | 17,946 | ||||||||||||||||
Stock-Based Compensation: Stock-based compensation cost is estimated at the grant date based on the fair value of the award, and the cost is recognized as expense ratably over the vesting period. The Company uses the Black-Scholes model for estimating the fair value of stock options. Determining the assumptions that enter into the model is highly subjective and requires judgment. The Company uses an expected volatility that is based on the historical volatility of its stock. The expected life assumption is based on historical employee exercise patterns and employee post-vesting termination behavior. The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. treasury securities in effect during the quarter in which the options were granted. The dividend yield reflects historical experience as well as future expectations over the expected term of the option. The Company estimates forfeitures at the time of grant and revises in subsequent periods, if necessary, if actual forfeitures differ from those estimates.
Warranty Liability: The product warranty liability relates to a ten year expiration guarantee on DTPA Products sold to the United States Department of Health and Human Services (“HHS”) in 2006. The Company is performing yearly stability studies for the DTPA Products and, if the annual stability does not support the ten-year product life, it will replace the product at no charge. The Company’s supplier, Hameln Pharmaceuticals (“Hameln”), will also share one-half of this cost if the product does not meet the stability requirement. If the ongoing product testing confirms the ten-year stability for the DTPA Products, the Company will not incur a replacement cost and this reserve will be eliminated with a corresponding reduction to cost of sales after the ten-year period.
Reclassifications: Certain amounts in the prior years’ consolidated financial statements have been reclassified to conform to the current year presentation.
52
Note 3 — Allowance for Customer Deductions
The annual activity in the Company’s allowance for customer deductions accounts for the three years ended December 31, 2012 is as follows (in thousands):
| Returns | Chargebacks & Rebates | Discounts | Doubtful Accounts | Advert. & Promotions | TOTAL | |||||||||||||||||||
| Balance at December 31, 2009 | $ | 3,192 | $ | 3,234 | $ | 336 | $ | 4 | $ | — | $ | 6,766 | ||||||||||||
| Provision | 1,535 | 45,209 | 1,994 | 92 | — | 48,830 | ||||||||||||||||||
| Charges processed | (1,264 | ) | (45,921 | ) | (1,985 | ) | (93 | ) | — | (49,263 | ) | |||||||||||||
| Balance at December 31, 2010 | 3,463 | 2,522 | 345 | 3 | — | 6,333 | ||||||||||||||||||
| Provision | 2,687 | 68,067 | 3,431 | 25 | 1,135 | 75,345 | ||||||||||||||||||
| Additions from business combinations | 1,845 | — | 50 | 187 | 132 | 2,214 | ||||||||||||||||||
| Charges processed | (1,149 | ) | (64,640 | ) | (3,083 | ) | (116 | ) | (881 | ) | (69,869 | ) | ||||||||||||
| Balance at December 31, 2011 | 6,846 | 5,949 | 743 | 99 | 386 | 14,023 | ||||||||||||||||||
| Provision | 3,783 | 112,243 | 6,074 | (82 | ) | 2,063 | 124,081 | |||||||||||||||||
| Charges processed | (2,220 | ) | (104,740 | ) | (5,455 | ) | 13 | (1,864 | ) | (114,266 | ) | |||||||||||||
| Balance at December 31, 2012 | $ | 8,409 | $ | 13,452 | $ | 1,362 | $ | 30 | $ | 585 | $ | 23,838 | ||||||||||||
Note 4 — Inventories
The components of inventories, net of allowances, are as follows (in thousands):
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Finished goods | $ | 24,657 | $ | 11,588 | ||||
| Work in process | 3,743 | 5,841 | ||||||
| Raw materials and supplies | 24,095 | 18,027 | ||||||
| $ | 52,495 | $ | 35,456 | |||||
The Company maintains an allowance for excess and obsolete inventory, as well as inventory with a carrying value in excess of its net realizable value. The activity in the allowance for excess and obsolete inventory account for the three years ended December 31, 2012 was as follows (in thousands):
Years Ended December 31, | ||||||||||||
2012 | 2011 | 2010 | ||||||||||
| Balance at beginning of year | $ | 1,239 | $ | 1,612 | $ | 1,780 | ||||||
| Provision | 2,385 | 598 | 725 | |||||||||
| Charges | (1,380 | ) | (971 | ) | (893 | ) | ||||||
| Balance at end of year | $ | 2,244 | $ | 1,239 | $ | 1,612 | ||||||
Note 5 – Property, Plant and Equipment
Property, plant and equipment consist of the following (in thousands):
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Land | $ | 2,715 | $ | 396 | ||||
| Buildings and leasehold improvements | 43,190 | 20,337 | ||||||
| Furniture and equipment | 70,874 | 50,833 | ||||||
| 116,779 | 71,566 | |||||||
| Accumulated depreciation | (47,635 | ) | (43,060 | ) | ||||
| 69,144 | 28,506 | |||||||
| Construction in progress | 11,535 | 15,883 | ||||||
| $ | 80,679 | $ | 44,389 | |||||
At December 31, 2012, property plant and equipment totaling $23.7 million was located outside the United States.
53
Note 6 — Financing Arrangements
Convertible Notes
On June 1, 2011, the Company closed on its offering of $120.0 million aggregate principal amount of 3.50% Convertible Senior Notes due 2016 (the “Notes”) which includes $20.0 million in aggregate principal amount of the Notes issued in connection with the full exercise by the initial purchasers of their over-allotment option. The Notes are governed by the Company’s indenture with Wells Fargo Bank, National Association, as trustee (the “Indenture”). The Notes were offered and sold only to qualified institutional buyers. The net proceeds from the sale of the Notes were approximately $115.3 million, after deducting underwriting fees and other related expenses.
The Notes have a maturity date of June 1, 2016 and pay interest at an annual rate of 3.50% semiannually in arrears on June 1 and December 1 of each year, beginning on December 1, 2011. The Notes are convertible into the Company’s common stock, cash or a combination thereof at an initial conversion price of $8.76 per share, which is equivalent to an initial conversion rate of approximately 114.1553 shares per $1,000 principal amount of Notes. The conversion price is subject to adjustment for certain events described in the Indenture, including certain corporate transactions which will increase the conversion rate and decrease the conversion price for a holder that elects to convert its Notes in connection with such corporate transaction.
The Notes are not listed on any securities exchange or on any automated dealer quotation system, but are traded on a secondary market made by the initial purchasers. The initial purchasers of the Notes advised the Company of their intent to make a market in the Notes following the offering, though they are not obligated to do so and may discontinue any market making at any time.
As of December 31, 2012, the Notes were trading at approximately 167.6% of their face value, resulting in a total market value of $201.1 million compared to their face value of $120.0 million. The actual conversion value of the Notes is based on the product of the conversion rate and the market price of the Company's common stock at conversion, as defined in the Indenture. As of December 31, 2012, the Company's common stock closed at $13.36 per share, resulting in a pro forma conversion value for the Notes of approximately $183.0 million. Increases in the market value of the Company’s common stock increase the Company’s obligation accordingly. There is no upper limit placed on the possible conversion value of the Notes.
The Notes may be converted at any time prior to the close of business on the business day immediately preceding December 1, 2015 only under the following circumstances: (1) during any calendar quarter commencing after September 30, 2011, if the closing sale price of the Company’s common stock, for at least 20 trading days (whether or not consecutive) during the period of 30 consecutive trading days ending on the last trading day of the calendar quarter immediately preceding the calendar quarter in which the conversion occurs, is more than 130% of the conversion price in effect on each applicable trading day; (2) during the five consecutive trading-day period following any five consecutive trading-day period in which the trading price for the Notes per $1,000 principal amount of Notes for each such trading day was less than 98% of the closing sale price of the Company’s common stock on such date multiplied by the then-current conversion rate; or (3) upon the occurrence of specified corporate events. On or after December 1, 2015 until the close of business on the business day immediately preceding the stated maturity date, holders may surrender all or any portion of their Notes for conversion at any time, regardless of the foregoing circumstances. Upon conversion, the Company will pay or deliver, at the Company’s option, cash, shares of the Company’s common stock, or a combination thereof. If a fundamental change (as defined in the Indenture) occurs prior to the stated maturity date, holders may require the Company to purchase for cash all or a portion of their Notes.
The Notes become convertible for the quarter starting on April 1, 2012 and ending on June 30, 2012 as a result of the Company’s stock trading at or above the required price of $11.39 per share for 20 of the last 30 trading days in the quarter ended March 31, 2012. The Notes have remained convertible for each successive quarter as a result of meeting the trading price requirement at the end of each prior quarter.
The Notes are accounted for in accordance with ASC 470-20, Debt with Conversion and Other Options. Under ASC 470-20, issuers of convertible debt instruments that may be settled in cash upon conversion, including partial cash settlement, are required to separately account for the liability (debt) and equity (conversion option) components. The application of ASC 470-20 resulted in the recognition of $21.3 million as the value for the equity component. This amount was offset by $0.8 million of equity issuance costs, as described below. At the dates indicated, the net carrying amount of the liability component and the remaining unamortized debt discount were as follows (in thousands):
54
December 31, 2011 | December 31, 2012 | |||||||
| Carrying amount of equity component | $ | 20,470 | 20,470 | |||||
| Carrying amount of the liability component | 100,808 | 104,637 | ||||||
| Unamortized discount of the liability component | 19,192 | 15,363 | ||||||
| Unamortized debt financing costs | 3,470 | 2,778 | ||||||
The Company incurred debt issuance costs of $4.7 million related to its issuance of the Notes. In accordance with ASC 470-20, the Company allocated this debt issuance cost ratably between the liability and equity components of the Notes, resulting in $3.9 million of debt issuance costs allocated to the liability component and $0.8 million allocated to the equity component. The portion allocated to the liability component was classified as deferred financing costs and is being amortized by the effective interest method through the earlier of the maturity date of the Notes or the date of conversion, while the portion allocated to the equity component was recorded as an offset to additional paid-in capital upon issuance of the Notes.
During the years ended December 31, 2012 and 2011, the Company recorded the following expenses in relation to the Notes (in thousands):
| 2012 | 2011 | |||||||
| Interest expense at 3.50% coupon rate | $ | 4,200 | $ | 2,450 | ||||
| Debt discount amortization | 3,828 | 2,109 | ||||||
| Deferred financing cost amortization | 692 | 382 | ||||||
| $ | 8,720 | $ | 4,941 | |||||
Upon issuing the Notes, the Company established a deferred tax liability of $8.6 million related to the debt discount of $21.3 million, with an offsetting debit of $8.6 million to Common stock. The deferred tax liability was established because the amortization of the debt discount generates non-cash interest expense that is not deductible for income tax purposes. Since the Company’s net deferred tax assets were fully reserved by valuation allowance at the time the Notes were issued, the Company reduced its valuation allowance by $8.6 million upon recording the deferred tax liability related to the debt discount with an offsetting credit of $8.6 million to Common stock. As a result, the net impact of these entries was a debit of $8.6 million to the valuation reserve against the Company’s deferred tax assets and a credit of $8.6 million to deferred tax liability. The deferred tax liability is being amortized monthly as the Company records non-cash interest from its amortization of the debt discount on the Notes.
Bank of America Credit Facility
On October 7, 2011, the Company and its domestic subsidiaries (the “Borrowers”) entered into a Loan and Security Agreement (the “BoA Credit Agreement”) with Bank of America, N.A. (the “Agent”) and other financial institutions (collectively with the Agent, the “BoA Lenders”) through which it obtained a $20.0 million revolving line of credit (the “Facility”), which includes a $2.0 million letter of credit facility. The Company may request expansion of the Facility from time to time in increments of at least $5.0 million up to a maximum commitment of $35.0 million, so long as no default or event of default has occurred and is continuing. The facility matures in March 2016. The Company may early terminate the BoA Lenders’ commitments under the Facility upon 90 days’ notice to the Agent at any time after the first year.
Under the terms of the BoA Credit Agreement, amounts outstanding will bear interest at the Company’s election at (a) LIBOR or (b) the bank’s Base Rate (which is the greatest of: (i) the prime rate, (ii) the federal funds rate plus 0.50%, or (iii) LIBOR plus 1.0%), plus an applicable margin, which margin is based on the consolidated fixed charge coverage ratio of the Company and its subsidiaries from time to time. Additionally, the Borrowers will pay an unused line fee of 0.250% per annum on the unused portion of the Facility. Interest and unused line fees will be accrued and paid monthly. In addition, with respect to any letters of credit that may be issued, the Borrowers will pay: (i) a fee equal to the applicable margin times the average amount of outstanding letters of credit, (ii) a fronting fee equal to 0.125% per annum on the stated amount of each letter of credit, and (iii) any additional fees incurred by the applicable issuer in connection with issuing the letter of credit. During an event of default, any interest or fees payable will be increased by 2% per annum.
Availability under the revolving credit line is equal to the lesser of (a) $20.0 million reduced by outstanding letter of credit obligations or (b) the amount of a Borrowing Base (as defined in accordance with the terms of the BoA Credit Agreement) determined by reference to the value of the Borrowers’ eligible accounts receivable, eligible inventory and fixed assets as of the closing date and the end of each calendar month thereafter.
55
Obligations under the BoA Credit Agreement are secured by substantially all of the assets of each of the Borrowers and a pledge by the Borrowers of their respective equity interest in each domestic subsidiary of the Company and 65% of their respective equity interests in any foreign subsidiary of the Company. The BoA Credit Agreement contains representations and warranties, and affirmative and negative covenants customary for financings of this type, including, but not limited to, limitations on: distributions while we have any outstanding commitments or obligations under the BoA Credit Agreement; additional borrowings and liens; additional investments and asset sales; and fundamental changes to corporate structure or organization documents. The financial covenants require the Borrowers to maintain a fixed charge coverage ratio of at least 1.1 to 1.0 during any period commencing on the date that an event of default occurs or availability under the BoA Credit Agreement is less than 15% of the aggregate BoA Lenders’ commitments under the BoA Credit Agreement. During the term of the agreement, the Company must provide the Agent with monthly, quarterly and annual financial statements, monthly compliance certificates, annual budget projections and copies of press releases and SEC filings.
As of December 31, 2012, we had availability on our line of credit of $19.7 million and there were no outstanding borrowings.
EJ Funds Credit Facility
On January 7, 2009, the Company entered into a Credit Agreement (the “GE/EJ Credit Agreement”) with General Electric Capital Corporation (“GE Capital”) as agent for several financial institutions (the “Lenders”). Effective March 31, 2009, the GE/EJ Credit Agreement was assigned to EJ Funds, a company controlled by Dr. Kapoor, the Chairman of the Company’s board of directors. Pursuant to the GE/EJ Credit Agreement, the Lenders agreed to extend loans to the Company under a revolving credit facility up to an aggregate principal amount of $25.0 million (the “Credit Facility”). The maximum loan commitment was decreased to $5.7 million upon assignment of the GE/EJ Credit Agreement to EJ Funds, and was subsequently increased on August 17, 2009 to $10.0 million. The Credit Facility was scheduled to terminate, and all amounts outstanding thereunder were to become due and payable, on January 7, 2013, or on an earlier date as specified in the GE/EJ Credit Agreement. The Company elected to early terminate the GE/EJ Credit Agreement on June 17, 2011. It had not borrowed against the Credit Facility since the first quarter of 2010. A more detailed timeline of events regarding the GE/EJ Credit Agreement follows.
On February 19, 2009, GE Capital informed the Company that it was applying a reserve against availability which effectively restricted the Company’s borrowings under the GE/EJ Credit Agreement to the balance outstanding as of February 19, 2009, which was $5.5 million. GE Capital advised that it had applied this reserve due to concerns about financial performance, including the Company’s prospective compliance with certain covenants in the GE/EJ Credit Agreement for the quarter ended March 31, 2009.
On March 31, 2009, the Company consented to an Assignment Agreement (“Assignment”) between GE Capital and EJ Funds which transferred to EJ Funds all of GE Capital’s rights and obligations under the GE/EJ Credit Agreement. Pursuant to the Assignment, EJ Funds became the agent and lender under the GE/EJ Credit Agreement. Dr. Kapoor is the President of EJ Financial Enterprises, Inc., a healthcare consulting investment company (“EJ Financial”) and EJ Financial is the general partner of EJ Funds. In connection with the Assignment, on April 13, 2009, the Company entered into a Modification, Warrant and Investor Rights Agreement (the “Modification Agreement”) with EJ Funds that, among other things, (i) reduced the revolving loan commitment under the GE/EJ Credit Agreement to $5.7 million, and (ii) set the interest rate for all amounts outstanding under the GE/EJ Credit Agreement at an annual rate of 10% with interest payable monthly. The Modification Agreement also granted EJ Funds the right to require the Company to nominate two directors to serve on its Board of Directors. The Kapoor Trust is entitled to require the Company to nominate a third director under its Stock Purchase Agreement dated November 15, 1990 with the Kapoor Trust.
Pursuant to the Modification Agreement, on April 13, 2009, the Company granted EJ Funds a warrant (the “Modification Warrant”) to purchase 1,939,639 shares of its common stock at an exercise price of $1.11 per share, subject to certain adjustments. The Modification Warrant expires five years after its issuance and is exercisable upon payment of the exercise price in cash or by means of a cashless exercise yielding a net share figure.
On August 17, 2009, the Company completed negotiations with EJ Funds for additional capacity on its Credit Facility, increasing the loan commitment from $5.7 million to $10.0 million. The Credit Facility was secured by the assets of the Company and was not subject to debt covenants until April 1, 2010. In consideration of this amendment, EJ Funds was granted a warrant to acquire 1,650,806 shares of the Company’s common stock at $1.16 per share, the closing market price on August 14, 2009 (the “Restatement Warrants”). The estimated fair value of the Restatement Warrants, using a Black-Scholes valuation model, was $1.2 million on date of grant. This amount plus $7,000 in other associated costs was capitalized as financing costs and was being amortized. Upon termination of the Credit Facility on June 17, 2011, the remaining unamortized cost was written off.
56
On January 13, 2010, the parties entered into an amendment to the GE/EJ Credit Agreement which, among other things, reduced the number of financial covenants to two: (1) a limit on capital expenditures of $7.5 million in 2010, $5.0 million in 2011, and $5.0 million in 2012 and (2) a requirement to have positive liquidity throughout the life of the GE/EJ Credit Agreement. Positive liquidity was defined as the revolving line of credit borrowing base (up to $10.0 million) plus cash and cash equivalents less the outstanding principal on the revolving line of credit, the total of which must be greater than zero. The capital expenditures limit allowed that any unused portion from one year may be carried over and added to the next year’s limit. On January 27, 2011, EJ Funds and the Company signed a Waiver and Consent that waived the Company’s obligation to comply with the capital expenditure limit for 2011.
On June 17, 2011, the Company elected to early terminate its $10.0 million revolving GE/EJ Credit Agreement with EJ Funds. The Company had not borrowed against the GE/EJ Credit Agreement since repaying its outstanding balance in the first quarter of 2010. Upon terminating the GE/EJ Credit Agreement, the Company expensed $1.2 million in remaining unamortized deferred financing costs related to the GE/EJ Credit Agreement. The Company incurred no fees or penalties related to its early termination of the GE/EJ Credit Agreement.
Subordinated Note Payable
On July 28, 2008, the Company borrowed $5.0 million from the Kapoor Trust, the sole trustee and sole beneficiary of which is Dr. John N. Kapoor, the Company’s Chairman of the Board of Directors and the holder of a significant stock position in the Company, in return for issuing the trust a Subordinated Promissory Note (“Subordinated Note”). The Subordinated Note accrued interest at a rate of 15% per year and was due and payable on July 28, 2009.
On August 17, 2009, the Company refinanced its $5.0 million subordinated debt payable to the Kapoor Trust. The principal amount of $5.0 million was increased to $5.9 million to include accrued interest through August 16, 2009. The annual interest rate remained unchanged at 15% with interest on the refinanced note due and payable monthly. The term of the Subordinated Note was extended by an additional five years and was due and payable on August 17, 2014. As part of this refinancing agreement, the Company issued the Kapoor Trust an additional 2,099,935 warrants to purchase the Company’s common stock at an exercise price of $1.16 per share, the closing market price of the Company’s stock on August 14, 2009.
On December 16, 2010, the Company voluntarily prepaid the principal of the Subordinated Note, along with a 10% early payment fee and all accrued interest. The Company’s total cash payment on December 16, 2010, including principal, accrued interest, and the early payment fee, was $6.5 million. Upon completing this early payment, the Company expensed the remaining $1.2 million unamortized balance of the $1.6 million in deferred financing costs that it incurred when the Subordinated Note was refinanced.
Note 7 — Common Stock
Private Placement with Serum Institute of India Ltd.
On March 11, 2010, the Company entered into an agreement to issue and sell 1,838,235 shares of the Company’s common stock to Serum Institute of India Ltd. (“Serum”) at a price of $1.36 per share, resulting in aggregate proceeds of $2,500,000 (the “Serum Stock Purchase Agreement”). The purchase price represented a discount of 15% to the closing price of the Company’s common stock on March 5, 2010. As part of the Serum Stock Purchase Agreement, Serum was granted a warrant to purchase 1,404,494 shares of the Company’s common stock at an exercise price of $1.78 per share (the “Serum Warrants”). The net proceeds, after payment of $31,000 in expenses, were allocated based on the relative fair values of the common stock and warrants, with $2,060,000 allocated to the common stock and $409,000 allocated to the warrants. There were no commissions paid in connection with this private placement.
The Serum Warrants were to become exercisable beginning on the fifth consecutive trading day that the Company’s common stock closed at $2.22 per share or above, and were to expire upon the earlier of 30 days after becoming exercisable or on March 10, 2013. The Serum Warrants became exercisable on May 10, 2010 and were exercised by Serum on May 24, 2010 upon delivery of the $2,500,000 cash purchase price to the Company.
The initial 1,838,235 common shares issued to Serum and the subsequent 1,404,494 shares issued upon exercise of the Serum Warrants are restricted securities (the “Restricted Securities”). Serum has agreed that it will not sell, dispose of or otherwise deal in the Restricted Securities for 180 days from date of purchase. If at any time during which the Restricted Securities may be sold without restriction pursuant to Securities and Exchange Commission (“SEC”) Rule 144, the Company fails to satisfy the current public information requirement under SEC Rule 144(c), then the Company shall pay to Serum cash in an amount equal to 1.0% of the aggregate purchase price of the Restricted Securities per month for each month until such failure is cured, up to a maximum liability of 6.0% of the total purchase price. Serum’s right to receive such cash payment would be subordinated to obligations under the Credit Facility.
57
Under the Serum Stock Purchase Agreement, Serum relinquished all right that it and any of its affiliates had to appoint a nominee for election to the Company’s Board of Directors. Prior to relinquishing such right, Dr. Subhash Kapre, Executive Director of Serum, served on the Company’s Board of Directors from 2007 until his resignation on March 8, 2010. Serum retains the right to appoint a representative to attend all meetings of the Company’s Board of Directors and all committees thereof as a nonvoting observer, and to receive copies of all notices, minutes, consents and other materials that the Company provides to its directors. The appointed representative is subject to the Company’s consent, not to be unreasonably withheld, and will be required to enter into a non-disclosure agreement with the Company. This right to an observer continues as long as Serum owns one of the following: (i) at least 1,000,000 shares of Akorn, Inc. common stock of the 1,838,235 acquired on March 11, 2010; (ii) at least 1,000,000 unexercised Serum Warrants, or (iii) at least 1,000,000 shares purchased through exercise of the Serum Warrants.
In connection with the Serum Stock Purchase Agreement, on March 10, 2010 the Company entered into a Waiver and Consent with EJ Funds as lender under the Credit Agreement. Under the Waiver and Consent, EJ Funds consented to the Serum Stock Purchase Agreement and waived compliance with certain of the Company’s covenants under the Credit Agreement with respect to the Serum Stock Purchase Agreement, the shares issued thereunder and the Serum Warrants that were granted.
PIPE Warrants
On March 8, 2006, the Company issued 4,311,669 shares of its common stock in a private placement with various investors at a price of $4.50 per share which included warrants to purchase 1,509,088 additional shares of common stock (the “PIPE Warrants”). The PIPE Warrants were exercisable for a five-year period ended March 8, 2011 at an exercise price of $5.40 per share and could be exercised by cash payment of the exercise price or by means of a cashless exercise. The total price of the private placement was approximately $19,402,000 and the net proceeds to the Company, after payment of approximately $1,324,000 of commissions and expenses, was approximately $18,078,000. The net proceeds were allocated based on the relative fair values of the common stock and warrants, with $16,257,000 allocated to the common stock and $1,821,000 allocated to the warrants.
In December 2010, holders submitted 77,779 of the PIPE Warrants for cashless exercise, resulting in the Company issuing 9,195 shares of its common stock. Of the 1,431,309 PIPE Warrants that remained outstanding as of December 31, 2010, 1,197,975 including shares issued were exercised during the first quarter and the remaining 233,334 expired unexercised on March 8, 2011.
Note 8 — Earnings per Common Share
Basic net income per common share is based upon the weighted average common shares outstanding during the period. Diluted net income per common share is based upon the weighted average number of common shares outstanding, including the dilutive effect, if any, of stock options, warrants and the conversion feature of convertible notes using the treasury stock method.
The Company’s potentially dilutive shares consist of: (i) vested and unvested stock options that are in-the-money, (ii) unvested RSAs, (iii) warrants that are in-the-money, and (iv) shares potentially issuable upon conversion of the Notes. The Company calculates and includes in dilutive securities incremental shares issuable related to the Notes to the extent that the conversion value of each note exceeds $1,000.
A reconciliation of the earnings per share data from a basic to a fully diluted basis is detailed below (amounts in thousands, except per share data):
| 2012 | 2011 | 2010 | ||||||||||
| Net income | $ | 35,378 | $ | 43,013 | $ | 21,824 | ||||||
| Net income per share: | ||||||||||||
| Basic | $ | 0.37 | $ | 0.45 | $ | 0.24 | ||||||
| Diluted | $ | 0.32 | $ | 0.41 | $ | 0.22 | ||||||
| Shares used in computing net income per share: | ||||||||||||
| Weighted average basic shares outstanding | 95,189 | 94,549 | 92,801 | |||||||||
| Dilutive securities: | ||||||||||||
| Stock options and unvested RSAs | 4,289 | 3,281 | 1,684 | |||||||||
| Stock warrants | 6,564 | 6,082 | 4,765 | |||||||||
| Shares issuable on conversion of the Notes | 4,468 | — | — | |||||||||
| Total dilutive securities | 15,321 | 9,363 | 6,449 | |||||||||
| Weighted average diluted shares outstanding | 110,510 | 103,912 | 99,250 | |||||||||
58
Note 9 — Leasing Arrangements
The Company leases real and personal property in the normal course of business under various operating leases, including non-cancelable and month-to-month agreements. Rental expense under these leases was $2,312,000, $2,361,000 and $1,985,000 for the years ended December 31, 2012, 2011 and 2010, respectively.
Landlord incentives are recorded as deferred rent and amortized on a straight-line basis over the lease term. Rent escalations are recorded on a straight-line basis over the lease term. The Company’s main operating leases for its Lake Forest and Gurnee facilities have original terms of ten years. The Lake Forest facility lease allows for a five-year renewal at the option of the Company.
The following is a schedule, by year, of future minimum rental payments required under non-cancelable operating and capital leases in place as of December 31, 2012 (in thousands):
| Year ending December 31, | ||||||
| 2013 | $ | 2,084 | ||||
| 2014 | 1,973 | |||||
| 2015 | 1,972 | |||||
| 2016 | 2,002 | |||||
| 2017 | 1,793 | |||||
| 2018 and thereafter | 804 | |||||
Total | $ | 10,628 | ||||
On July 27, 2010, the Company, through its wholly-owned subsidiary, Akorn (New Jersey), Inc., an Illinois corporation, entered into a seven-year building lease agreement (the “Somerset Lease”) with Veronica Development Associates, a New Jersey general partnership, extending the Company’s occupancy of its 50,000 square foot manufacturing facility located at 72-6 Veronica Avenue, Somerset, New Jersey. This lease commenced on August 1, 2010 and continues through July 31, 2017. Under terms of the new lease, base rent was initially set at $38,801 per month, subject to periodic cost of living adjustments. In addition to base rent, the Company is obligated to pay its proportionate share of estimated property taxes, assessments and maintenance costs. The lease agreement contains a renewal provision allowing the Company the option to renew for up to four additional five-year periods upon providing written notice of its intention to renew at least six months prior to termination of the original lease or any renewal period.
Effective February 1, 2010, the Company entered into a six-year building lease for an R&D facility within the Illinois Science & Technology Park in Skokie, Illinois. The Company’s total base rent commitment over the six-year life of this lease was to be approximately $1,041,000. Late in 2012, the Company provided the landlord with notice of its intention to shorten the lease term by two years and paid the applicable early termination fee. The Company anticipates abandoning this lease during 2013.
On December 1, 2012, the Company entered into a lease for a new R&D center in Vernon Hills, Illinois. This lease extends through April 30, 2020, and obligates the Company to pay total base rent of $1,324,000, plus proportionate real estate taxes and common area maintenance, over the life of the agreement.
On March 3, 2010, the Company entered into an eight-year sub-lease agreement with a related party, EJ Financial, for their sub-lease of a portion of the Company’s corporate offices in Lake Forest, Illinois. John N. Kapoor, Ph.D., Chairman of the Company’s Board of Directors, is the President of EJ Financial. This sub-lease commenced on April 1, 2010. Per the terms of the sub-lease agreement, EJ Financial will pay monthly base rent plus a proportionate share of common area maintenance costs. The Company and EJ Financial agreed to early terminate this agreement, and the sub-lease was terminated in July 2012 at which time the space was retrofitted for corporate purposes. EJ Financial paid the Company approximately $240,000 in rent and common area maintenance fees during the shortened term of this sub-lease.
59
Note 10 — Stock Options, Employee Stock Purchase Plan and Restricted Stock
Stock Option Plan
The Company maintains stock options plans that allow the Company’s Board of Directors to grant stock options to eligible employees, officers and directors. The Akorn, Inc. 2003 Stock Option Plan (“2003 Stock Option Plan”) was approved by the Company’s Board of Directors on November 6, 2003 and approved by its stockholders on July 8, 2004. Under the 2003 Stock Option Plan, 2,519,000 options were granted, none of which remained outstanding as of December 31, 2011. On March 29, 2005, the Company’s Board of Directors approved the Amended and Restated Akorn, Inc. 2003 Stock Option Plan (the “Amended 2003 Plan”), effective as of April 1, 2005, and this was subsequently approved by its stockholders on May 27, 2005. The Amended 2003 Plan is an amendment and restatement of the 2003 Stock Option Plan and provides the Company with the ability to grant other types of equity awards to eligible participants besides stock options. Starting on May 27, 2005, all new awards have been granted under the Amended 2003 Plan. The aggregate number of shares of the Company’s common stock initially approved for issuance pursuant to awards granted under the Amended 2003 Plan was 5,000,000. On August 7, 2009, the Company’s stockholders voted to increase this figure to 11,000,000 at the recommendation of the Company’s Board of Directors, and on December 31, 2011 voted to increase the available shares by another 8,000,000, to a final total of 19,000,000 shares. Under the Amended 2003 Plan, 15,507,000 options have been granted to employees and directors, 1,673,000 options have been exercised, 4,106,000 options have been canceled, and 9,727,000 remain outstanding as of December 31, 2012. Options granted under the 2003 Stock Option Plan and the Amended 2003 Plan have exercise prices equivalent to the market value of the Company’s common stock on the date of grant and generally vest ratably on each grant date anniversary over a three-year period and expire five years from date of issuance.
The Company accounts for stock-based compensation in accordance with ASC Topic 718, Compensation – Stock Compensation (formerly SFAS No. 123 (revised 2004), Share Based Payment (SFAS 123(R))). Accordingly, stock-based compensation cost is estimated at the grant date based on the fair value of the award, and the cost is recognized as expense ratably over the vesting period. The Company uses the Black-Scholes model for estimating the grant date fair value of stock options. Determining the assumptions that enter into the model is highly subjective and requires judgment. The Company uses an expected volatility that is based on the historical volatility of its stock. The expected life assumption is based on historical employee exercise patterns and employee post-vesting termination behavior. The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. treasury securities in effect during the quarter in which the options were granted. The dividend yield reflects historical experience as well as future expectations over the expected term of the option. The Company estimates forfeitures at the time of grant and revises in subsequent periods, if necessary, if actual forfeitures differ from those estimates.
The Company recorded stock option compensation expense of $6,436,000, $4,947,000 and $2,677,000 during the years ended December 31, 2012, 2011 and 2010, respectively. The Company uses the single-award method for allocating the compensation cost to each period.
The Company uses the Black-Scholes model to determine the grant-date value of stock options. Expected volatility is based on the historical volatility of the Company’s common stock. The expected life assumption is based on historical employee exercise patterns and employee post-vesting termination behavior. The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. treasury securities in effect during the quarter in which the options were granted. The dividend yield reflects historical experience as well as future expectations over the expected term of the option. The Company estimates forfeitures at the time of grant and revises those estimates subsequently based on actual forfeitures.
The assumptions used in estimating the fair value of the stock options granted during the period, along with the weighted-average grant date fair values, were as follows:
| 2012 | 2011 | 2010 | |||
| Expected Volatility | 77% -85% | 75% -76% | 78% - 80% | ||
| Expected Life (in years) | 4.0 | 3.8 | 3.9 | ||
| Risk-free interest rate | 0.7% - 0.8% | 1.3% - 2.0% | 1.2% - 2.4% | ||
| Dividend yield | — | — | — | ||
| Fair value per stock option | $7.76 | $3.71 | $1.62 |
A summary of stock option activity within the Company’s stock-based compensation plans for the years ended December 31, 2012, 2011 and 2010 is as follows:
Number of Shares (in thousands) | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at December 31, 2009 | 5,157 | 1.49 | ||||||||||||||
| Granted | 3,264 | 2.56 | ||||||||||||||
| Exercised | (257 | ) | 1.76 | |||||||||||||
| Forfeited | (204 | ) | 3.36 | |||||||||||||
| Outstanding at December 31, 2010 | 7,960 | 1.87 | ||||||||||||||
| Granted | 2,030 | 6.63 | ||||||||||||||
| Exercised | (454 | ) | 1.93 | |||||||||||||
| Forfeited | (137 | ) | 2.30 | |||||||||||||
| Outstanding at December 31, 2011 | 9,399 | 2.89 | ||||||||||||||
| Granted | 1,221 | 12.96 | ||||||||||||||
| Exercised | (806 | ) | 1.87 | |||||||||||||
| Forfeited | (87 | ) | 4.42 | |||||||||||||
| Outstanding at December 31, 2012 | 9,727 | 4.22 | 2.55 | $ | 88,918,000 | |||||||||||
| Exercisable at December 31, 2012 | 6,039 | 2.40 | 2.07 | $ | 66,215,000 | |||||||||||
60
The aggregate intrinsic value for stock options outstanding and exercisable is defined as the difference between the market value of the Company’s common stock as of the end of the period and the exercise price of the in-the-money stock options. The total intrinsic value of stock options exercised during the years ended December 31, 2012, 2011 and 2010 was $9,111,000, $3,061,000 and $692,000, respectively. As a result of the stock options exercised, the Company received cash and recorded additional paid-in-capital of $1,511,000, $867,000 and $452,000 during the years ended December 31, 2012, 2011 and 2010, respectively.
As of December 31, 2012, the total amount of unrecognized compensation cost related to non-vested stock options was $12,245,000 which is expected to be recognized as expense over a weighted-average period of 1.6 years.
Under the Amended 2003 Plan, the Company may grant restricted stock awards to certain employee and members of its Board of Directors. Restricted stock awards are valued at the closing market value of the Company’s common stock on the day of grant and the total value of the award is recognized as expense ratably over the vesting period of the grants. During 2012, the Company granted 35,000 shares of restricted stock valued at $512,000 to members of its Board of Directors, of which half vested immediately and half will vest on the one year anniversary of grant. No restricted stock awards were granted in 2011 or 2010. The Company recognized compensation expense of $351,000, $17,000 and $60,000 during the years ended December 31, 2012, 2011 and 2010, respectively, related to outstanding restricted stock awards.
The following is a summary of non-vested restricted stock activity:
Number of Shares (in thousands) | Weighted Average Grant Date Fair Value | |||||||
| Nonvested at December 31, 2009 | 108 | $ | 2.73 | |||||
| Granted | — | — | ||||||
| Vested | (25 | ) | 4.34 | |||||
| Canceled | (55 | ) | 2.43 | |||||
| Nonvested at December 31, 2010 | 28 | $ | 1.89 | |||||
| Granted | — | — | ||||||
| Vested | (15 | ) | 2.34 | |||||
| Canceled | — | — | ||||||
| Nonvested at December 31, 2011 | 13 | $ | 1.34 | |||||
| Granted | 35 | 14.63 | ||||||
| Vested | (30 | ) | 9.09 | |||||
| Canceled | — | — | ||||||
| Nonvested at December 31, 2012 | 18 | $ | 14.63 | |||||
Employee Stock Purchase Plan
The Akorn, Inc. Employee Stock Purchase Plan (the “ESPP”) permits eligible employees to acquire shares of the Company’s common stock through payroll deductions. The ESPP has been structured to qualify under Section 423 of the Internal Revenue Code (“IRC”). Employees who elect to participate in the ESPP may withhold from 1% to 15% of base wages toward the purchase of stock. Shares are purchased at a 15% discount off the lesser of the market price at the beginning or the ending of the applicable offering period. The ESPP has two offering periods each year, one running from January 1st to December 31st and the other running from July 1st to December 31st. In a given year, employees may enroll in either plan, but not both. Per IRC rules, annual purchases per employee are limited to $25,000 worth of stock, valued as of the beginning of the offering period. Accordingly, with the 15% discount, employees may withhold no more than $21,250 per year toward the purchase of stock under the ESPP.
A maximum of 2,000,000 shares of the Company’s common stock may be issued under the ESPP. A total of 1,280,459 shares have been issued thus far under the ESPP, leaving 719,541 shares available for future issuance. The 1,280,459 share total includes shares issued in early 2013 related to employee participation during 2012. The Company issued approximately 61,000, 71,000 and 128,000 shares of its common stock related to employee participation in the ESPP during 2012, 2011 and 2010, respectively. For the years ended December 31, 2012, 2011 and 2010, the Company recorded compensation expense of $244,000, $195,000 and $187,000, respectively, related to the ESPP.
61
Note 11 — Income Taxes
The income tax provision (benefit) consisted of the following (in thousands):
| Current | Deferred | Total | ||||||||||
| Year ended December 31, 2012 | ||||||||||||
| Federal | $ | 20,843 | $ | ( 504 | ) | $ | 20,339 | |||||
| State | 4,232 | (911 | ) | 3,321 | ||||||||
| Foreign | — | (1,538 | ) | (1,538 | ) | |||||||
| $ | 25,075 | $ | (2,953 | ) | $ | 22,122 | ||||||
| Year ended December 31, 2011 | ||||||||||||
| Federal | $ | — | $ | (460 | ) | $ | (460 | ) | ||||
| State | 2,704 | (3,951 | ) | (1,247 | ) | |||||||
| $ | 2,704 | $ | (4,411 | ) | $ | (1,707 | ) | |||||
| Year ended December 31, 2010 | ||||||||||||
| Federal | $ | (47 | ) | $ | — | $ | (47 | ) | ||||
| State | 199 | — | 199 | |||||||||
| $ | 152 | $ | — | $ | 152 | |||||||
Income tax expense differs from the “expected” tax expense (benefit) computed by applying the U.S. Federal corporate income tax rates of 35% in 2012 and 2011 and 34% in 2010 to income before income taxes, as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Computed “expected” tax expense | $ | 20,125 | $ | 14,457 | $ | 7,472 | ||||||
| Change in income taxes resulting from: | ||||||||||||
| State income taxes, net of federal income tax | 2,159 | 2,217 | 1,180 | |||||||||
| Foreign income tax expense (benefit) | 1,468 | — | — | |||||||||
| Deduction for domestic production activities | (1,277 | ) | — | — | ||||||||
| Other, net | (353 | ) | (876 | ) | 5,686 | |||||||
| Valuation allowance change | — | (17,505 | ) | (14,186 | ) | |||||||
| Income tax expense (benefit) | $ | 22,122 | $ | (1,707 | ) | $ | 152 | |||||
Net deferred income taxes at December 31, 2012 and 2011 include (in thousands):
| December 31, 2012 | December 31, 2011 | |||||||||||||||
| Current | Noncurrent | Current | Noncurrent | |||||||||||||
| Deferred tax assets: | ||||||||||||||||
| Net operating loss carry-forward | $ | — | $ | 4,328 | $ | 2,130 | $ | 2,612 | ||||||||
| Stock-based compensation | — | 4,912 | — | 2,954 | ||||||||||||
| Reserve for product returns | 2,787 | — | 1,972 | — | ||||||||||||
| Inventory | 3,980 | — | 3,545 | — | ||||||||||||
| Other | 2,974 | 1,349 | 506 | 312 | ||||||||||||
| Total deferred tax assets | 9,741 | 10,589 | 8,153 | 5,878 | ||||||||||||
| Deferred tax liabilities: | ||||||||||||||||
| Prepaid expenses | (551 | ) | — | — | — | |||||||||||
| Unamortized discount – convertible notes | — | (5,815 | ) | — | (7,455 | ) | ||||||||||
| Depreciation & amortization – tax over book | — | (5,835 | ) | — | (2,165 | ) | ||||||||||
| Total deferred tax liabilities | (551 | ) | (11,650 | ) | — | (9,620 | ) | |||||||||
| Net deferred income tax asset (liability) | $ | 9,190 | $ | (1,061 | ) | $ | 8,153 | $ | (3,742 | ) | ||||||
The Company records a valuation allowance to reduce net deferred income tax assets to the amount that is more likely than not to be realized. In performing its analysis of whether a valuation allowance to reduce the deferred income tax asset was necessary, the Company evaluated the data and determined that as of December 31, 2012 and 2011 its deferred income tax assets were more likely than not to be realized. Accordingly, no valuation allowance was in place as of either December 31, 2012 or December 31, 2011. The deferred tax balances have been reflected gross on the balance sheet, and are permitted to be netted only if in the same jurisdiction.
62
Due to historic operating losses, the Company had been carrying a 100% valuation allowance against its deferred tax assets in 2009 and maintained an allowance until reversing it in the quarter ended September 30, 2011. At that time, the Company determined that based on recent earnings history and future projections, it would be expected to realize the full net value of its deferred tax assets. Accordingly, the Company reversed its valuation allowances in that quarter. This reversal accounts for the Company’s net income tax benefit recorded for the year 2011.
Of the Company’s net operating loss (“NOL”) carry-forwards, $1.6 million relates to start-up costs at its Indian location. These losses can be carried forward indefinitely. The $2.7 million of domestic NOLs primarily relate to losses incurred during the decade of the 2000’s. In 2012, the Company fully utilized its remaining Federal NOL carry-forwards. The Company’s remaining unused state NOL carry-forwards are mainly due to suspension of the use of NOL carry-forwards for 2009, 2010 and 2011 in California and for 2010, 2011 and 2012 in Illinois. The state NOL carry-forwards do not begin to expire until 2014, and include $2.2 million in Illinois, all of which is due to expire from 2021 to 2025. Most of the Company’s remaining state NOL carry-forwards relate to New Jersey and expire as follows: $0.3 million in 2014 and 2015 combined; and $0.2 million from 2016 through 2024.
On January 2, 2013 President Obama signed the American Taxpayer Relief Act of 2012. The Act included an extension of the research and experimentation tax credit for periods ending in 2012 and 2013. Although not yet recognized for financial reporting purposes, the Company will include a research and experimentation tax credit in its 2012 Federal income tax return. The benefit of this credit, approximately $0.6 million, will be reflected in the Company’s first quarter 2013 financial statements.
The Company’s U.S. Federal income tax returns filed for years 2009 through 2011 are open for examination by the Internal Revenue Service. The majority of the Company’s state and local income tax returns filed for years 2009 through 2011 remain open for examination.
In accordance with ASC 740-10-25, Income Taxes – Recognition, the Company performs reviews of its tax positions to determine whether it is “more likely than not” that its tax positions will be sustained upon examination, and if any tax positions are deemed to fall short of that standard, the Company reserves based on the financial exposure and the likelihood of its tax positions not being sustained. Based on its review as of and for the year ended December 31, 2012, the Company determined that it would not recognize tax benefits as follows (in thousands):
| Balance at December 31, 2011 | $ — | ||
| Additions relating to current year | 1,265 | ||
| Additions relating to prior years | 220 | ||
| Balance at December 31, 2012 | $ 1,485 |
If recognized, $287,000 of the above positions will impact the Company’s effective rate, while the remaining $1,198,000 being temporary in nature, will have no impact on the Company’s financial statements. Due to the uncertainty of both timing and resolution of potential income tax examinations, the Company is unable to determine whether any amounts included in the December 31, 2012 balance of unrecognized tax benefits represent tax positions that could significantly change during the next twelve months. The Company accounts for interest and penalties as income tax expense. There were no uncertain positions prior to 2012.
Note 12 — Retirement Plan
All Akorn employees are eligible to participate in the Company’s 401(k) Plan. During the years ended December 31, 2012, 2011 and 2010, plan-related expense totaled $801,000, $545,000 and $138,000, respectively. The employer’s matching contribution is a percentage of the amount contributed by each employee and is funded on a current basis. The Company suspended its match on 401(k) contributions during 2009 and did not match 401(k) contributions through March 31, 2010. Effective April 1, 2010, the Company reinstituted a matching contribution at a rate of 25% of the first 6% contributed by employees. On January 1, 2011, the Company increased its matching contribution to 50% of the first 6% contributed, and has maintained this match rate through December 31, 2012. Company matching contributions vest 50% after two years of credited service and 100% after three years of credited service.
63
Note 13 — Segment Information
The Company is reporting its results of operations for the three-year period ended December 31, 2012 in three segments:
| - | Ophthalmic |
| - | Hospital Drugs & Injectables |
| - | Contract Services |
The ophthalmic segment manufactures, markets and distributes diagnostic and therapeutic pharmaceuticals. The hospital drugs & injectables segment manufactures, markets and distributes drugs and injectable pharmaceuticals, primarily in niche markets, as well as certain vaccines. The majority of the drug products included in this segment are injectables, though also included are a number of drugs administered to patients by other methods. The contract services segment manufactures products for third party pharmaceutical and biotechnology customers based on their specifications.
In 2010 and earlier years, the Company used to report results for a fourth segment, biologics & vaccines. However, the Company’s chief operating decision maker, who is our CEO, no longer views vaccine distribution as a separate segment, and the Company is therefore including Td vaccine distribution within the hospital drugs and injectables segment. Historical segment information has been adjusted to consolidate the biologics & vaccines revenues and gross profit data into the hospital drugs & injectables segment.
The Company’s reportable segments are based upon internal financial reports that aggregate certain operating information. The Company’s chief operating decision maker, as defined in ASC Topic 280, Segment Reporting, is its chief executive officer, or CEO. The Company’s CEO oversees operational assessments and resource allocations based upon the results of the Company’s reportable segments, all of which have available discrete financial information.
Selected financial information by segment is presented below (in thousands):
Years ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| REVENUES | ||||||||||||
| Hospital drugs & injectables | $ | 129,723 | $ | 55,077 | $ | 34,053 | ||||||
| Ophthalmic | 103,765 | 68,591 | 32,750 | |||||||||
| Contract services | 22,670 | 13,252 | 19,606 | |||||||||
| Total revenues | $ | 256,158 | $ | 136,920 | $ | 86,409 | ||||||
| GROSS PROFIT | ||||||||||||
| Hospital drugs & injectables | $ | 83,413 | $ | 30,057 | $ | 15,768 | ||||||
| Ophthalmic | 58,785 | 43,054 | 19,453 | |||||||||
| Contract services | 6,494 | 6,578 | 7,244 | |||||||||
| Total gross profit | $ | 148,692 | $ | 79,689 | $ | 42,465 | ||||||
The Company manages its business segments to the gross profit level and manages its operating and other costs on a company-wide basis. Inter-segment activity at the gross profit level is minimal. The Company does not have discrete assets by segment, as certain manufacturing and warehouse facilities support more than one segment, and therefore does not report assets by segment.
During 2012, 2011 and 2010, approximately $29.4 million, $5.3 million and $1.1 million of the Company’s net revenue, respectively, was from customers located in foreign countries. Of the 2012 figure, $16.7 million represented the net revenue generated by Akorn India Private Limited, the Company’s subsidiary in India. This entity currently sells exclusively to contract customers in India, and to export customers in unregulated world markets, outside the United States.
Note 14 — Commitments and Contingencies
(i) The Company has an outstanding product warranty reserve which relates to a ten year expiration guarantee on DTPA sold to HHS in 2006. The Company is performing yearly stability studies for this product and, if the annual stability does not support the ten-year product life, it will replace the product at no charge. The Company’s supplier, Hameln Pharmaceuticals, will also share one-half of this cost if the product does not meet the stability requirement. If the ongoing product testing confirms the ten-year stability for DTPA, the Company will not incur a replacement cost and this reserve will be eliminated with a corresponding reduction to cost of sales after the ten-year period.
64
(ii) On December 22, 2011, the Company acquired the rights to three NDA products from H. Lundbeck A/S ("Lundbeck"). The Company paid $45.0 million in initial consideration at closing and has a commitment to pay additional consideration of $15.0 million to Lundbeck on December 22, 2014, the third anniversary of the acquisition date. Both the initial $45.0 million paid at closing and the additional $15.0 million are subject to claw-back provisions if sales of the three underlying products fail to achieve certain minimum amounts specified in the Lundbeck Agreement. The discounted value of the additional consideration has been recorded by the Company as a long-term liability on its December 31, 2012 balance sheet. The liability was discounted at 9.0%, the Company’s approximate cost of long-term capital, to an initial value of $11.6 million. The accrual of non-cash interest expense during 2012 increased the carrying amount of this long-term liability to $14.2 million as of December 31, 2012.
As part of the Lundbeck Agreement, the Company assumed Lundbeck’s obligations under a supply agreement with the third party manufacturer of two of the three products acquired. The supply agreement committed the Company to purchase $13.3 million of product in total over the years 2012 through 2015. The Company determined that its committed purchase quantities exceeded the amount that it anticipated being able to sell. Accordingly, as part of the initial accounting for the Lundbeck Agreement, the Company recorded a long-term liability of $2.5 million, which equaled the estimated present value of the unfavorable contract terms and is subsequently being amortized over the supply term.
Also included within the Lundbeck Agreement is a commitment to pay royalties to Lundbeck based on the Company’s future sales of a generic form of one of the acquired products. This commitment is more fully described in Note 17 – Business Combinations and Other Strategic Investments. The dollar amount of this commitment is not estimable since it is subject to future sales volumes, prices and margins for the applicable product.
(iii) The Company has entered into strategic business agreements for the development and marketing of finished dosage form pharmaceutical products with various pharmaceutical development companies.
Each strategic business agreement includes a future payment schedule for contingent milestone payments and in certain strategic business agreements, minimum royalty payments. The Company will be responsible for contingent milestone payments and minimum royalty payments to these strategic business partners based upon the occurrence of future events. Each strategic business agreement defines the triggering event of its future payment schedule, such as meeting product development progress timelines, successful product testing and validation, successful clinical studies, various FDA and other regulatory approvals and other factors as negotiated in each agreement. None of the contingent milestone payments or minimum royalty payments is individually material to the Company. These costs, when realized, will be reported as part of research and development expense or as a component of cost of sales in the Company’s Consolidated Statement of Income.
The table below summarizes contingent potential milestone payments due to strategic partners in the years 2013 and beyond, assuming all such contingencies occur (in thousands):
Year ending December 31, | Milestone Payments | |||
| 2013 | $ | 8,524 | ||
| 2014 | 1,698 | |||
| Total | $ | 10,222 | ||
(iv) The Company is a party in other legal proceedings and potential claims arising in the ordinary course of its business. The amount, if any, of ultimate liability with respect to such matters cannot be determined. Despite the inherent uncertainties of litigation, management of the Company at this time does not believe that such proceedings will have a material adverse impact on the financial condition, results of operations, or cash flows of the Company.
(v) On October 17, 2012, the Company entered into an exclusive distribution agreement with MBL for the Company’s marketing of MBL-manufactured tetanus-diphtheria vaccine (“Td vaccine”) over an initial contract term of two years. The exclusive distribution agreement commits the Company to acquire $18.3 million in Td vaccine over the initial two-year term, which encompasses calendar years 2013 and 2014. The agreement contains a provision for automatic annual renewals if neither party delivers notice of non-renewal at least six months prior to the end of the initial two-year term or any subsequent annual term.
Note 15 — Supplemental Cash Flow Information (in thousands)
| Year ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Leasehold improvements funded by lessor | $ | — | $ | 22 | $ | — | ||||||
| Assets acquired through capital lease | — | — | 84 | |||||||||
| Interest and taxes paid: | ||||||||||||
| Interest paid | 4,200 | 2,125 | 1,008 | |||||||||
| Income taxes paid | 21,455 | 2,778 | 100 | |||||||||
65
Note 16 — Recent Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board (the “FASB”) issued ASU 2011-05, Comprehensive Income (Topic 220), Presentation of Comprehensive Income, which converges the presentation of other comprehensive income (OCI) in financial statements prepared under US GAAP and International Financial Reporting Standards (IFRS). This guidance would require disclosure of reclassification adjustments from OCI to net income. In December 2011, the FASB issued ASU 2011-12, Comprehensive Income (Topic 220), Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05 , which deferred the effective date of this guidance to fiscal years beginning after December 15, 2011, with early election permitted. We adopted ASU 2011-05 during fiscal year 2012. The adoption of this guidance did not have a material impact on our financial position or operating results.
In September 2011, the FASB issued ASU 2011-08, Goodwill and Other (ASC Topic 350), Testing Goodwill for Impairment, which added a simplified alternative method for performing annual goodwill impairment tests. Companies now have the option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the assessment indicates that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company no longer has to perform the two-step impairment test. ASU 2011-08 is effective for fiscal years beginning after December 15, 2011. The adoption of this guidance did not have a material impact on the Company’s financial position or operating results.
In May 2011, the FASB issued ASU 2011-4, Fair Value Measurement (ASC Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS, which was intended by the FASB and the International Accounting Standards Board (IASB) to develop common requirements for measuring fair value and for disclosing information about fair value measurements in accordance with GAAP and International Financial Reporting Standards (IFRSs). Additional disclosures required by this amendment include information about transfers between Level 1 and Level 2 instruments, information regarding the sensitivity of Level 3 instruments, and categorization by level of items that are not measured at fair value in the statement of financial position (but for which disclosure of fair value is still required). The guidance is effective prospectively for fiscal years beginning after December 15, 2011 and interim periods within those years. The adoption of this guidance did not have a material impact on the Company’s financial position or operating results.
In July 2012, the FASB issued ASU No. 2012-02, Intangibles – Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment. The amendments in this update aim to simplify the impairment test for indefinite-lived intangible assets by permitting an entity the option to first assess qualitative factors to determine whether it is more likely than not (defined as having a likelihood of more than 50 percent) that an indefinite-lived intangible asset is impaired as a basis for determining whether the quantitative impairment test included in Accounting Standards Codification Subtopic 350-30, Intangibles – Goodwill and Other – General Intangibles Other than Goodwill must be performed. The amendment is effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012. Adoption of this amendment is not expected to have a material effect on our financial position or operating results.
Note 17 – Business Combinations and Other Strategic Investments
Kilitch Acquisition
On February 28, 2012, Akorn India Private Limited (“AIPL”), a wholly owned subsidiary of the Company completed and closed on its previously announced acquisition of selected assets of Kilitch Drugs (India) Limited (“KDIL”). This acquisition (the “Kilitch Acquisition”) was pursuant to the terms of the Business Transfer Agreement (the “BTA”) entered into among the Company, KDIL and the members of the promoter group of KDIL on October 5, 2011. In accordance with terms contained in the BTA, the Company also closed on a related Product Transfer Agreement between the Company and NBZ Pharma Limited (“NBZ”), a company associated with KDIL. The primary asset transferred in the Kilitch Acquisition was KDIL’s manufacturing plant in Paonta Sahib, Himachal Pradesh, India, along with its existing book of business. KDIL was engaged in the manufacture and sale of pharmaceutical products for contract customers in India and for export to various unregulated world markets. While the Paonta Sahib manufacturing facility is not currently certified by the U.S. Food and Drug Administration (the “FDA”) for the exporting of drugs to the U.S., the facility was designed with future FDA certification in mind. Accordingly, the Kilitch Acquisition provided the Company with the potential for future expansion of its manufacturing capacity for products to be sold in the U.S., as well as the opportunity to expand the Company’s footprint into markets outside the U.S. The Company has determined that the assets acquired through the Kilitch Acquisition constitute a “business” as defined by Rule 11-01(d) of Regulation S-X and ASC 805, Business Combinations. Accordingly, the Company has accounted for the Kilitch Acquisition as a business combination.
66
AIPL paid the equivalent of approximately USD $60.1 million at closing. Total purchase consideration was approximately $55.2 million which consisted of approximately $51.2 million in base consideration and $4.0 million in reimbursement for capital expenditures made by KDIL from April 1, 2011 to the closing date. AIPL also paid $7.3 million related to compensation earned from the achievement of acquisition-related milestones, and $1.6 million in stamp duties paid to transfer title to the land and buildings at Paonta Sahib from Kilitch to AIPL. In addition, the Company expects to pay up to an additional $0.5 million for future services that would be expensed as the services are provided. The compensation for acquisition-related milestones and other acquisition costs have been recorded within “acquisition related costs” as part of operating expenses in the Company’s condensed consolidated statement of comprehensive income. The BTA also contains a working capital guarantee that calls for KDIL or AIPL to reimburse the other party for any shortfall or excess, respectively, in the actual acquired working capital compared to the target working capital as established in the BTA.
The following table sets forth the consideration paid for the Kilitch Acquisition, the acquisition-related costs incurred, and the fair values of the assets acquired and the liabilities assumed (U.S. dollar amounts in thousands):
| Consideration: | Initial Fair Valuation | Changes in Estimate | Adjusted Fair Valuation | |||||||||
| Cash paid | $ | 55,224 | $ | 55,224 | ||||||||
| Less working capital shortfall refunded by sellers | (890 | ) | (138 | ) | (1,028 | ) | ||||||
| $ | 54,334 | $ | (138 | ) | $ | 54,196 | ||||||
| Acquisition-related costs: | ||||||||||||
| Stamp duties paid for transfer of land and buildings | $ | 1,583 | $ | 1,583 | ||||||||
| Acquisition-related compensation expense | 6,741 | 511 | 7,252 | |||||||||
| Due diligence, legal, travel and other acquisition-related costs | 557 | 119 | 676 | |||||||||
| $ | 8,881 | $ | 630 | $ | 9,511 | |||||||
| Recognized amounts of identifiable assets acquired and liabilities assumed: | ||||||||||||
| Accounts receivable | $ | 2,130 | $ | 2,130 | ||||||||
| Inventory | 1,799 | 1,799 | ||||||||||
| Land | 3,714 | (1,131 | ) | 2,583 | ||||||||
| Buildings, plant and equipment | 8,474 | 8,474 | ||||||||||
| Construction in progress | 14,231 | 14,231 | ||||||||||
| Goodwill, deductible | 21,609 | 1,004 | 22,613 | |||||||||
| Other intangible assets, deductible | 5,806 | 102 | 5,908 | |||||||||
| Other assets | 38 | 38 | ||||||||||
| Assumed liabilities | (2,099 | ) | (779 | ) | (2,878 | ) | ||||||
| Deferred tax liabilities | (1,368 | ) | 666 | (702 | ) | |||||||
| $ | 54,334 | $ | (138 | ) | $ | 54,196 | ||||||
The Adjusted Fair Valuation presented above is expected to be final. The changes in estimate recorded subsequent to the initial accounting estimate were primarily related to refining the calculated fair value of certain acquired assets, adjustments to the working capital settlement amount due from the sellers to the Company, and final determination regarding the tax-deductibility of the acquired intangible assets. The acquisition-related compensation expense during 2012 was primarily related to pre-negotiated compensation paid to members of the sellers’ family based on achievement of various operational milestones.
Goodwill represents expected synergies and intangible assets that do not qualify for separate recognition. Based on a recent Indian Supreme Court ruling upholding the deductibility of goodwill for India tax purposes, the Company anticipates being able to deduct the value of goodwill for income tax purposes in India. A later Indian Supreme Court ruling raised doubt as to the tax deductibility of the cost of the non-compete agreement entered into between AIPL and the sellers. Accordingly, the Company amended its acquisition accounting to establish a deferred tax liability related to this intangible asset. The Company had initially recorded a deferred tax liability valued at $1.4 million and subsequently adjusted to $0.7 million related to intangible assets and other accrued liabilities that it does not believe will be amortizable for Indian tax purposes. This remaining deferred tax liability of $0.7 million was reversed against goodwill during 2012.
67
For book purposes, the other intangible assets acquired are being amortized over lives of four to five years. Goodwill is not amortized for book purposes but is subject to impairment testing, per Company policy. The tangible assets acquired consist primarily of construction in progress fair valued at $14.2 million buildings, plant and equipment fair valued at a combined $8.5 million land fair valued at $2.6 million accounts receivable fair valued at $2.1 million and inventory fair valued at $1.8 million
The unaudited pro forma results presented below reflect the consolidated results of operation of the Company as if the Kilitch Acquisition had taken place at the beginning of the period presented below. The pro forma results include amortization associated with the acquired intangible assets and interest on funds used for the acquisition. The unaudited pro forma financial information presented below does not reflect the impact of any actual or anticipated synergies expected to result from the acquisition. Accordingly, the unaudited pro forma financial information is not necessarily indicative of the results of operations as they would have been had the transaction been effected on the assumed date (amounts in thousands, except per share data):
| Year ended December 31, | ||||||
| 2011 | 2012 | |||||
| Revenue | $ | 162,427 | $ | 260,162 | ||
| Net income | $ | 34,106 | $ | 35,722 | ||
| Net income per diluted share | $ | 0.33 | $ | 0.32 | ||
The business acquired through the Kilitch Acquisition generated revenue of $16.7 million and a pre-tax loss of $8.6 million during the ten-month period from its acquisition through December 31, 2012. The pre-tax loss included the impact of acquisition-related costs of $8.8 million recorded on AIPL’s books in relation to the Kilitch Acquisition.
Lundbeck Products
On December 22, 2011, the Company entered into an Asset Sale and Purchase Agreement (the “Lundbeck Agreement”) to acquire the NDA rights to three branded, injectable drug products from the U.S. subsidiary of Lundbeck for an estimated purchase price of approximately $63.4 million (the “Lundbeck Acquisition”). Per terms of the Lundbeck Agreement, the Company made an upfront payment of $45.0 million. The Company also acquired inventory from Lundbeck for a price of $4.6 million, which was paid early in 2012. The Company will owe a subsequent milestone payment of $15.0 million in cash to Lundbeck on the third anniversary of closing of the Lundbeck Agreement, for which contingent consideration of $11.6 million was initially recorded. The initial purchase consideration and the subsequent milestone payment are subject to a reduction if certain sales targets are not met in the first three years and the subsequent three years post closing. The acquired products include Nembutal®, a Schedule II controlled drug, Diuril® and Cogentin®. This acquisition adds to the Company’s portfolio of injectable drug products, allowing the Company to leverage its existing sales infrastructure to promote sales of these products.
The Company has determined that the acquired assets do represent a “business” as defined per Rule 11-01(d) of Regulation S-X and ASC 805, Business Combinations. Accordingly, the Company has accounted for the Lundbeck Acquisition as a business combination.
68
During the post-acquisition period of December 22-31, 2011, sales of the products acquired from Lundbeck contributed revenue of $829,000 and gross profit of $745,000 to the Company’s consolidated financial results for 2011.
The following table sets forth the consideration paid related to the Lundbeck Acquisition, the total acquisition-related costs incurred by the Company, and the fair values of the assets acquired and the liabilities assumed (in thousands):
Initial Fair Value | Change in Estimate | Adjusted Fair Value | ||||||||||
| Consideration: | ||||||||||||
| Cash paid | $ | 49,559 | $ | 49,559 | ||||||||
| Present value of contingent consideration | 11,300 | 300 | 11,600 | |||||||||
| Total consideration | $ | 60,859 | $ | 300 | $ | 61,159 | ||||||
| Acquisition-related costs: | $ | 50 | $ | 50 | ||||||||
Recognized amounts of identifiable assets acquired and liabilities assumed: | ||||||||||||
| Product licensing rights | $ | 59,525 | $ | 300 | $ | 59,825 | ||||||
| Inventory | 3,825 | 3,825 | ||||||||||
| Fixed assets | 50 | 50 | ||||||||||
| Assumed liability – unfavorable contract | (2,541 | ) | (2,541 | ) | ||||||||
| $ | 60,859 | $ | 300 | $ | 61,159 | |||||||
The Company identified Product licensing rights as the only intangible assets acquired in the Lundbeck Acquisition, and expects to amortize this intangible asset straight-line over its anticipated useful life of 15 years for both book and tax purposes. The contingent consideration of $15.0 million was initially discounted to present value using a 9% discount rate, which takes into account the Company’s cost of long-term credit. The “Assumed liability – unfavorable contract” is in relation to a supply agreement assumed by the Company which obligates the Company to acquire more inventory of one of the three acquired products than the Company projects it would be able to sell based on historical sales volumes and future projections. This liability has been discounted in determining its fair value.
The tangible assets acquired consist of inventory of approximately $3.8 million and fixed assets of approximately $50,000. The acquired inventory has been valued below its acquisition cost based on the Company’s determination that not all of the acquired inventory can be sold at least six months prior to its expiration.
The Lundbeck Agreement commits the Company to paying royalties to Lundbeck based on the Company’s future sales of an authorized generic of one of the acquired products. There are currently generics of this product on the market. The Lundbeck Agreement calls for payment of royalties equal to 55% of the gross profit margin on sales of this authorized generic for a period of six years following the Company’s initial sale of this generic product. For purposes of the royalty calculation, brand sales in excess of a specified annual unit volume will be treated as generic sales and will therefore be subject to royalties. The dollar amount of this royalty commitment cannot be known, as it is subject to future sales prices, volumes and margins.
AVR Acquisition
On May 3, 2011, the Company purchased all the outstanding shares of stock of Advanced Vision Research, Inc. (“AVR”), paying $26,011,000 in cash, net of cash held by AVR. The acquisition of AVR is being accounted for as a business combination. The purchase price is subject to adjustment based on a working capital guarantee contained in the purchase agreement. During the quarter ended September 30, 2011, the Company paid an additional $723,000 to reimburse the sellers for the net cash balance in AVR’s bank accounts as of the acquisition date, as required by terms of the AVR purchase agreement. Akorn has further agreed to reimburse AVR Business Trust, Advanced Vision Research, Inc., Advanced Vision Pharmaceuticals, LLC , and the shareholders of AVR Business Trust (collectively, the “Sellers”) for any incremental income tax expense they should incur related to the parties making an Internal Revenue Code (“IRC”) Section 338(h)(10) election. In relation to this agreement, the Company paid $734,000 to the Sellers at closing, which represents the Seller’s initial estimate of their incremental income tax burden as a result of the IRC Section 338(h)(10) election.
The acquisition of AVR is a strategic extension of the Company’s ophthalmic business, allowing the Company to expand its presence in the OTC eye care product space. The Company is leveraging its existing operating infrastructure that markets products to ophthalmologists, optometrists, and retailers nationwide and also expects to attain cost savings and synergies as the Company continues to integrate the AVR business into its existing operations.
AVR markets a line of OTC eye care products under the TheraTears® brand name. Akorn had been a contract manufacturer of certain TheraTears® products since 2008. During 2011 and 2010, the Company generated revenues of $607,000 and $2,113,000, respectively, from the sale of TheraTears® products to AVR.
Subsequent to its acquisition, AVR generated revenues of $14.0 million and operating income of $2.0 million during 2011.
69
The following table summarizes the consideration paid for AVR, the total acquisition-related costs incurred by the Company during 2011 in connection with the acquisition, and the fair values of the assets acquired and liabilities assumed.
(Amounts in thousands)
| Consideration: | ||||
| Cash paid at closing | $ | 26,011 | ||
| Additional consideration paid | 723 | |||
| Fair value of total consideration transferred | $ | 26,734 | ||
| Acquisition-related costs: | $ | 246 | ||
Recognized amounts of identifiable assets acquired and liabilities assumed: | ||||
| Accounts receivable, net | $ | 611 | ||
| Inventories, net | 3,407 | |||
| Prepaid expenses and other current assets | 730 | |||
| Property and equipment | 250 | |||
| Goodwill, deductible | 11,863 | |||
| Trademarks and technology | 9,500 | |||
| Customer relationships | 3,900 | |||
| Estimated additional consideration due | (181) | |||
| Accounts payable & other assumed liabilities | (3,346) | |||
| $ | 26,734 |
The adjustment to purchase consideration and the fair valuation of acquired assets and assumed liabilities was primarily related to paying to the Sellers an additional cash amount of $723,000, which represented the net cash balance on AVR’s balance sheet on the acquisition date, as well as finalizing the valuation of the acquired intangible assets.
The Company identified Trademarks and technology and Customer relationships as finite-lived intangible assets acquired as part of the AVR acquisition. Both of these assets were valued based on the projected net present value of future cash flows to be generated from these assets. Goodwill represents both expected synergies and intangible assets that do not qualify for separate recognition and equals the excess of purchase price over the fair value of the identifiable tangible and intangible assets acquired. The net tangible assets acquired consist primarily of inventory of approximately $3.4 million, accounts receivable of $0.6 million, prepaid expenses and other currents assets of $0.7 million and property and equipment of $0.3 million. The fair value of inventory was determined based on its net realizable value which was adjusted to include all net costs allocable to the manufacturing effort.
For income tax purposes, the Company will be able to deduct the goodwill and other intangible assets resulting from the acquisition ratably over 15 years. Goodwill will not be amortized for book purposes but will be subject to impairment testing. Other intangible assets are being amortized straight-line over their estimated useful lives, which for Trademarks and technology is 30 years and for Customer relationships is 15 years.
Gross accounts receivable of $2,693,000 acquired as part of the AVR acquisition were recorded net of the following reserves: (i) product returns of $1,845,000; (ii) doubtful accounts of $187,000, and (iii) cash discounts of $50,000. The product returns reserve is an estimate of future returns of all products historically sold by AVR that are still potentially subject to return by the customer.
Pro Forma Results
The unaudited pro forma results presented below reflect the consolidated results of operations of the Company as if the Kilitch, Lundbeck and AVR acquisitions had taken place at the beginning of each period presented below. The pro forma results include amortization associated with the acquired intangible assets and interest on funds used for the acquisition. However, to better reflect the combined operating results, material non-recurring charges directly attributable to the transaction have been excluded. In addition, the unaudited pro forma financial information does not reflect the impact of any actual or anticipated synergies expected to result from the transaction. Accordingly, the unaudited pro forma financial information is not necessarily indicative of results of operations as they would have been had the transaction been effected on the assumed date (in thousands, except per share amounts):
70
| 2012 | 2011 | |||||||
| Revenues: | ||||||||
| Akorn | $ | 256,158 | $ | 136,920 | ||||
| Pro forma results of acquisitions: | ||||||||
| Kilitch Acquisition | 4,004 | 25,507 | ||||||
| Lundbeck Acquisition | — | 39,946 | ||||||
| AVR Acquisition | — | 6,351 | ||||||
| Pro forma revenue | $ | 260,162 | $ | 208,724 | ||||
| Net income: | ||||||||
| Akorn | $ | 35,378 | $ | 43,013 | ||||
| Pro forma results of acquisitions: | ||||||||
| Kilitch Acquisition | 344 | 234 | ||||||
| Lundbeck Acquisition | — | 17,405 | ||||||
| AVR Acquisition | — | 608 | ||||||
| Pro forma net income | $ | 35,722 | $ | 61,260 | ||||
| Net income per diluted share: | ||||||||
| Akorn | $ | 0.32 | $ | 0.41 | ||||
| Pro forma net income per diluted share | $ | 0.32 | $ | 0.59 | ||||
Other Strategic Investments
On August 1, 2011, the Company entered into a Series A-2 Preferred Stock Purchase Agreement (the “Aciex Agreement”) to acquire a minority ownership interest in Aciex Therapeutics Inc. (“Aciex”), based in Westborough, MA, for $8.0 million in cash. Subsequently, on September 30, 2011, the Company entered into Amendment No. 1 to Series A-2 Preferred Stock Purchase Agreement (the “Aciex Amendment”) to acquire additional shares of Series A-2 Preferred Stock in Aciex for approximately $2.0 million in cash. The Company’s investment in Aciex is being carried at cost on the Company’s Condensed Consolidated Balance Sheet as of December 31, 2011. Aciex is an ophthalmic drug development company focused on developing novel therapeutics to treat ocular diseases. Aciex’s pipeline consists of both clinical stage assets and pre-Investigational New Drug stage assets. The investments detailed above have provided the Company with an ownership interest in Aciex of below 20%. The Aciex Agreement and Aciex Amendment contain certain customary rights and preferences over the common stock of Aciex and further provide that the Company shall have the right to a seat on the Aciex board of directors.
During 2012 and 2011, the Company paid $800,000 and $5,678,000, respectively for the acquisition of drug product licensing rights (NDA and ANDA rights) from various entities. Along with the product rights acquired in 2011, the Company also acquired inventory valued at $347,000. In relation to the 2011 acquisitions, the Company has committed itself to paying an additional $875,000 upon the completion of certain milestones related to site transfer to a Company manufacturing facility of one of the acquired drug products. This future obligation has been recorded as a liability on the Company’s books at its net present value.
Note 18 — Unconsolidated Joint Venture
The Company has been a 50% partner in a joint venture agreement with an Indian drug development company since September 2004. This joint venture launched its first product in 2008 and generated revenue from 2008 until its business assets were sold and transferred in the second quarter of 2011. While the joint venture still exists legally, it ceased operations upon the completion of the sale and transfer of its operating assets to Pfizer, Inc. (“Pfizer”) in the second quarter of 2011.
On September 22, 2004, the Company entered into a joint venture agreement with Strides Arcolab Limited (“Strides”), a pharmaceutical manufacturer based in India, for the development, manufacturing and marketing of grandfathered products, patent-challenge products and ANDA products for the U.S. hospital and retail markets. The joint venture operated in the form of a Delaware limited liability company, Akorn-Strides, LLC (the “Joint Venture Company”). Strides was responsible for developing, manufacturing and supplying products under an Original Equipment Manufacturer Agreement between it and the Joint Venture Company. The Company was responsible for sales and marketing of the products under an exclusive Sales and Marketing Agreement with the Joint Venture Company. Under the terms of the joint venture agreement, the Company earned a fee from the Joint Venture Company equal to 7.5% of net sales for these services. The Joint Venture Company launched its first commercialized product in 2008. To supplement Strides’ manufacturing capabilities, during 2010 the Company began manufacturing one Joint Venture Company product in its Decatur, Illinois manufacturing plant. The Company recorded revenue of $830,000 and $1,854,000 in 2011 and 2010, respectively, related to sales of this product to the Joint Venture Company.
71
Strides and Akorn each own 50% of the Joint Venture Company with equal management representation. The Company accounts for the Joint Venture Company’s earnings and losses on the equity method of accounting in accordance with its 50% ownership interest. The Company’s share of the Joint Venture Company net income is reflected as “Equity in earnings of unconsolidated joint venture” on the Company’s consolidated statements of operations and consolidated statements of cash flows.
On December 29, 2010, the Joint Venture Company entered into an Asset Purchase Agreement with Pfizer, Inc. (“Pfizer”) to sell the rights to all of its ANDAs to Pfizer for $63.2 million in cash (the “Pfizer ANDA Sale”). In accordance with an amendment to the joint venture operating agreement, the Company and Strides agreed to an uneven split of the proceeds, with the Company receiving $35.0 million, or approximately 55.4% of the sale proceeds, and Strides receiving $28.2 million, or approximately 44.6%. Costs of $103,000 related to the sale were allocated to each partner in the same proportion as the sales proceeds. Transfer of ownership of the ANDAs took place in two steps. On the initial closing date of December 29, 2010, the ANDAs of all dormant and in-development products owned by the Joint Venture Company were transferred to Pfizer. On May 1, 2011, ownership of the ANDAs for products actively-marketed by the Joint Venture Company transferred to Pfizer. This arrangement allowed the Joint Venture Company time to liquidate existing inventory of the actively-marketed products and allowed Pfizer the opportunity to manufacture and label its own stock. No assets or liabilities of the Joint Venture Company other than its ANDA rights were transferred to Pfizer. The Joint Venture Company essentially ceased operations in the second quarter of 2011, though it will continue to exist legally for some time while its remaining assets and liabilities are liquidated and any potential future product returns have been processed. The impact of any future adjustments to the Joint Venture Company’s reserve for product returns or other accrued liabilities is not expected to be material to the Company’s results of operations in those future periods.
The Joint Venture Company recorded a total gain of approximately $63.1 million from the Pfizer ANDA Sale, of which $38.9 million, or 61.7%, was recognized in the fourth quarter of 2010 and the remaining $24.2 million, or 38.3%, was recognized in the second quarter of 2011. During the years ended December 31, 2011 and 2010, the Company recorded $14.6 million and $23.4 million, respectively, as its equity in earnings of the Joint Venture Company.
The following tables sets forth a condensed statements of income for the three years ended December 31, 2012 and condensed balance sheets as of December 31, 2012 and 2011 for Akorn-Strides, LLC, along with information regarding the amount of earnings allocated to each member-partner of the LLC (in thousands):
| CONDENSED STATEMENTS OF INCOME | ||||||||||||
| (Unaudited) | ||||||||||||
| Year ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| REVENUES | $ | — | $ | 6,364 | $ | 16,260 | ||||||
| Cost of sales | — | 3,562 | 11,200 | |||||||||
| GROSS PROFIT | — | 2,802 | 5,060 | |||||||||
| Operating expenses | — | 499 | 1,447 | |||||||||
| OPERATING INCOME | — | 2,303 | 3,613 | |||||||||
| Gain from Pfizer ANDA Sale | — | 24,160 | 38,937 | |||||||||
| INCOME BEFORE INCOME TAXES | — | 26,463 | 42,550 | |||||||||
| Income tax (benefit) / provision | — | (38 | ) | 4 | ||||||||
| NET INCOME | $ | — | $ | 26,501 | $ | 42,546 | ||||||
72
| CONDENSED BALANCE SHEETS | ||||||||
| (Unaudited) | ||||||||
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| ASSETS | ||||||||
| Cash | $ | 794 | $ | 859 | ||||
| Trade accounts receivable, net | — | — | ||||||
| Inventories, net | — | — | ||||||
| Other assets | — | 75 | ||||||
| TOTAL ASSETS | $ | 794 | $ | 934 | ||||
| LIABILITIES & MEMBERS’ EQUITY (DEFICIT) | ||||||||
| Trade accounts payable & other accrued liabilities | $ | 431 | $ | 543 | ||||
| Accounts payable - members | — | 28 | ||||||
| Deferred gain on Pfizer ANDA Sale | — | — | ||||||
| TOTAL LIABILITIES | 431 | 571 | ||||||
| Members’ equity, net of advances | 363 | 363 | ||||||
| TOTAL LIABILITIES & MEMBERS’ EQUITY (DEFICIT) | $ | 794 | $ | 934 | ||||
Trade accounts payable & other accrued liabilities as of December 31, 2012 primarily consists of accruals for potential product returns which may occur until sometime after the expiration dates of the applicable products.
Note 19 — Customer, Supplier and Product Concentration
Customer Concentration
In 2012, 2011 and 2010, a significant portion of the Company’s gross and net sales reported for its Ophthalmic and Hospital drugs & injectables segments were through three large wholesale drug distributors, and a significant portion of the Company’s accounts receivable as of December 31, 2012, 2011 and 2010 were due from these wholesale drug distributors as well. AmerisourceBergen Health Corporation (“Amerisource”), Cardinal Health, Inc. (“Cardinal”) and McKesson Drug Company (“McKesson”) are all distributors of the Company’s products, as well as suppliers of a broad range of health care products. Aside from these three wholesale drug distributors, no other customers accounted for more than 10% of gross sales, net revenues or gross trade receivables for the indicated dates and periods.
The following table sets forth the percentage of the Company’s gross and net sales and gross accounts receivable attributable to these three distributors for the periods indicated:
| 2012 | 2011 | 2010 | |||||||||||||||||
Gross Sales | Net Revenue | Gross Accounts Receivable | Gross Sales | Net Revenue | Gross Accounts Receivable | Gross Sales | Net Revenue | Gross Accounts Receivable | |||||||||||
| Amerisource | 19% | 14% | 29% | 23% | 23% | 29% | 24% | 17% | 32% | ||||||||||
| Cardinal | 23% | 17% | 30% | 27% | 25% | 34% | 25% | 17% | 31% | ||||||||||
| McKesson | 16% | 11% | 14% | 16% | 15% | 9% | 15% | 11% | 7% | ||||||||||
| Total | 58% | 42% | 73% | 66% | 63% | 72% | 64% | 45% | 70% | ||||||||||
If sales to Amerisource, Cardinal or McKesson were to diminish or cease, the Company believes that the end users of its products would find little difficulty obtaining the Company’s products either directly from the Company or from another distributor.
Supplier Concentration
During the years 2010, purchases of Tetanus-diphtheria vaccine (“Td vaccine”) from the Massachusetts Biological Laboratory of the University of Massachusetts (“MBL”) represented 14% of the Company’s purchases in that year. In 2010, MBL was the Company’s sole supplier of Td vaccine for its vaccine segment. The Company ceased distribution of Td vaccines in the first quarter of 2010 upon termination of the MBL Distribution Agreement. No other supplier represented 10% or more of the Company’s purchases in 2012, 2011 or 2010.
73
The Company requires a supply of quality raw materials and components to manufacture and package pharmaceutical products for its own use and for third parties with which it has contracted. The principal components of the Company’s products are active and inactive pharmaceutical ingredients and certain packaging materials. Many of these components are available from only a single source and, in the case of many of the Company’s ANDAs and NDAs, only one supplier of raw materials has been identified. Because FDA approval of drugs requires manufacturers to specify their proposed suppliers of active ingredients and certain packaging materials in their applications, FDA approval of any new supplier would be required if active ingredients or such packaging materials were no longer available from the specified supplier. The qualification of a new supplier could delay the Company’s development and marketing efforts. If for any reason the Company is unable to obtain sufficient quantities of any of the raw materials or components required to produce and package its products, it may not be able to manufacture its products as planned, which could have a material adverse effect on the Company’s business, financial condition and results of operations.
Product Concentration
During the year 2012, one of the Company’s injectable products represented 12.5% of the Company’s total net revenue for the year 2012 and during the year 2011, one of the Company’s ophthalmic products represented 10.4% of the Company’s total net revenue for the year. No other product represented 10% or more of the Company’s net revenue in 2012, 2011 or 2010. The Company attempts to minimize the risk associated with product concentrations by continuing to acquire and develop new products to add to its portfolio. The Company does not anticipate that any product will represent 10% or more of its revenues in 2013.
Note 20 — Related Party Transactions
The Company has engaged in various related party transactions with John N. Kapoor, Ph.D, Chairman of the Company’s Board of Directors and a significant holder of the Company’s common stock.
On March 3, 2010, the Company entered into an 8-year agreement with EJ Financial for their sub-lease of a portion of the Company’s corporate offices in Lake Forest, Illinois. This sub-lease commenced on April 1, 2010. Subsequently, the Company and EJ Financial agreed to early terminate this agreement, and the sub-lease was terminated in July 2012. EJ Financial paid the Company approximately $240,000 in rent and common area maintenance fees in total over the shortened term of this sub-lease.
From March 31, 2009 until June 17, 2011, the Company was party to a revolving credit facility for which the lender was EJ Funds, a company controlled by Dr. Kapoor. On March 31, 2009, the Company consented to an Assignment Agreement between GE Capital and EJ Funds which transferred all of GE Capital’s rights and obligations under its existing $25.0 million GE Credit Agreement to EJ Funds. Dr. Kapoor is the president of EJ Financial Enterprises, Inc., a healthcare consulting and investment company and EJ Financial is the general partner of EJ Funds. In connection with the Assignment Agreement, on April 13, 2009 the Company entered into a Modification, Warrant and Investor Rights agreement that reduced the loan commitment to $5.65 million. Subsequently, on August 17, 2009, the parties agreed to increase the loan commitment to $10.0 million. The Company repaid its outstanding balance under the facility in March 2010 and elected to early terminate the credit agreement on June 17, 2011.
From July 28, 2008 until December 16, 2010, the Company held a Subordinated Note payable to the Kapoor Trust, of which Dr. Kapoor is the sole trustee and beneficiary. On July 28, 2008, the Company borrowed $5,000,000 from the Kapoor Trust pursuant to a one-year Subordinated Note bearing interest at an annual rate of 15%. On August 17, 2009, the Subordinated Note was refinanced for an additional five years, establishing August 17, 2014 as the new due date. In addition to the term extension, the principal amount was increased to $5,853,267 to include accrued interest through August 16, 2009. The interest rate remained unchanged at 15% per year. On December 16, 2010, the Company early paid the balance due on the Subordinated Note, along with a 10% early payment fee, thereby canceling the note.
In connection with the various modifications agreed to during 2009 to the EJ Funds credit facility and the Subordinated Note, the Company issued various stock warrants to Dr. Kapoor (the “Kapoor Warrants”). See Note 2, Summary of Significant Accounting Policies / Warrants, for information about the Kapoor Warrants.
74
Note 21 — Severance Charges
Upon acquiring AVR on May 3, 2011, the Company entered into severance agreements with six of AVR’s employees. Each severance agreement promised a lump-sum payout in exchange for the employee remaining with the Company through a post-acquisition transition period, as defined in each agreement. Accordingly, severance expense was recorded ratably over the required transition period. Four out of the six employees received their severance payments in 2011 and the remaining two received their severance payments in 2012.
As of December 31, 2011, the accrued severance balance on the Company’s books was $317,000. As of December 31, 2012, the Company’s balance sheet included accrued severance of less than $10,000.
Note 22 – Selected Quarterly Financial Data (Unaudited)
Consolidated Net Income | ||||||||||||||||||||||||
(In thousands, except per share amounts) | Revenues | Gross Profit | Operating Income | Amount | Per Basic Share | Per Diluted Share | ||||||||||||||||||
| Year Ended December 31, 2012: | ||||||||||||||||||||||||
| 4th Quarter | $ | 71,520 | $ | 41,971 | $ | 19,715 | $ | 8,811 | $ | 0.09 | $ | 0.08 | ||||||||||||
| 3rd Quarter | 69,634 | 40,093 | 22,603 | 13,753 | 0.14 | 0.12 | ||||||||||||||||||
| 2nd Quarter | 63,287 | 35,727 | 18,776 | 9,706 | 0.10 | 0.09 | ||||||||||||||||||
| 1st Quarter | 51,717 | 30,901 | 7,662 | 3,108 | 0.03 | 0.03 | ||||||||||||||||||
| Year Ended December 31, 2011: | ||||||||||||||||||||||||
| 4th Quarter | $ | 42,625 | $ | 25,575 | $ | 11,528 | $ | 5,733 | $ | 0.06 | $ | 0.05 | ||||||||||||
| 3rd Quarter | 36,703 | 21,978 | 9,354 | 13,524 | 0.14 | 0.13 | ||||||||||||||||||
| 2nd Quarter | 32,148 | 17,883 | 6,676 | 17,946 | 0.19 | 0.17 | ||||||||||||||||||
| 1st Quarter | 25,444 | 14,253 | 5,708 | 5,810 | 0.06 | 0.06 | ||||||||||||||||||
Note 23 – Legal Proceedings.
On September 12, 2012, Fera Pharmaceuticals, LLC (“Fera”) filed a civil complaint against the Company and certain individual defendants (together, the “Defendants”) in the Supreme Court of New York (the “Fera lawsuit”). The complaint alleges, among other things, breach of manufacturing and confidentiality agreements and misappropriation of the plaintiff’s trade secrets. On October 15, 2012, the case was removed to the Federal District Court for the Southern District of New York. Fera filed an amended complaint on December 21, 2012. The Defendants filed a motion to dismiss portions of the amended complaint on January 25, 2013. The Company intends to vigorously defend these allegations. However, no assurance may be given regarding the ultimate outcome of this lawsuit.
In April 2012, Allergan Sales (“Allergan”) filed a lawsuit alleging patent infringement claims against the Company relating to the 0.4% ketorolac tromethamine formulation. Allergan seeks unspecified monetary damages in this case. The Company has asserted invalidity and non-infringement defenses. The Company intends to vigorously defend these allegations. However, no assurance may be given regarding the ultimate outcome of this lawsuit.
We are party to legal proceedings and potential claims arising in the ordinary course of our business. The amount, if any, of ultimate liability with respect to such matters cannot be determined. Despite the inherent uncertainties of litigation, we at this time do not believe that such proceedings will have a material adverse impact on our financial condition, results of operations, or cash flows.
75
None.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2012. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company's management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost benefit relationship of possible controls and procedures. Based on this evaluation, management concluded as of December 31, 2012 that our disclosure controls and procedures were not effective at the reasonable assurance level due to a material weakness in our internal control over financial reporting, which is described below.
In connection with the preparation of our financial statements for the year ended December 31, 2012, we concluded there is a material weakness in the design and operating effectiveness of our internal control over financial reporting as defined in SEC Regulation S-X. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The primary factors contributing to the material weakness, which relates to our financial statement close process, were:
| • | We did not maintain financial close process and procedures that were adequately designed, documented and executed to support the accurate and timely reporting of our financial results. As a result, we made a number of manual post-close adjustments necessary in order to prepare the financial statements included in this Form 10-K. |
| • | We did not maintain effective controls to provide reasonable assurance that accounts were complete and accurate and agreed to detailed support, and that account reconciliations were properly performed, reviewed and approved. While these activities should be performed in the ordinary course of our preparing our financial statements, we instead needed to undertake significant efforts to complete reconciliations and investigate items identified in those reconciliations during the course of the audit of our financial statements. |
With the oversight of senior management and our audit committee, we have begun taking steps and plan to take additional measures to remediate the underlying causes of the material weakness, which primarily relate to the development and implementation of formal policies, improved processes and documented procedures, as well as the hiring of additional finance personnel.
76
Management’s Report on Internal Control Over Financial Reporting
Company management is responsible for establishing and maintaining adequate internal control over financial reporting; as such term is defined in Rule 13a-15(f) under the Exchange Act. Under the supervision and with the participation of Company management, including the CEO and the CFO, an evaluation was performed of the effectiveness of the Company’s internal control over financial reporting. The evaluation was based on the framework in “Internal Control — Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
There are inherent limitations in the effectiveness of any internal control, including the possibility of human error and the circumvention or overriding of controls. Accordingly, even effective internal controls over financial reporting can provide only reasonable assurance with respect to financial statement preparation. Further, because of changes in conditions, the effectiveness of internal control may vary over time.
During the first quarter of fiscal 2012, the Company, through its wholly owned subsidiary, Akorn India Private Limited (“AIPL”), acquired selected assets of Kilitch Drugs (India) Limited (“KDIL”) (see Note 17 – Business Combinations and Other Strategic Investments). As permitted by Securities and Exchange Commission Staff interpretive guidance for newly acquired businesses, management excluded AIPL from its annual evaluation of internal control over financial reporting as of December 31, 2012. The Company will incorporate this acquisition into its annual report on internal control over financial reporting for its fiscal year end 2013. As of December 31, 2012, AIPL’s total assets represented approximately 17% of the Company's consolidated total assets and approximately 7% of the Company's consolidated net sales for the year ended December 31, 2012.
Based on our evaluation under the criteria set forth in Internal Control — Integrated Framework, our management concluded that, as of December 31, 2012, our internal control over financial reporting was not effective due to the identification of a material weakness related to our controls over our financial statement close process. More specifically, we did not maintain financial close process and procedures that were adequately designed, documented and executed to support the accurate and timely reporting of our financial results, and we did not maintain effective controls to provide reasonable assurance that accounts were complete and accurate and agreed to detailed support, and that account reconciliations were properly performed, reviewed and approved.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
The Company’s internal control over financial reporting as of December 31, 2012 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in its report which appears above.
Changes in Internal Control Over Financial Reporting
With the oversight of senior management and our audit committee, we have begun taking steps and plan to take additional measures to remediate the underlying causes of the material weakness, primarily through the development and implementation of formal policies, improved processes and documented procedures, as well as the hiring of additional finance personnel.
Notwithstanding the identified material weakness, management believes the consolidated financial statements included in this Annual Report on Form 10-K fairly represent in all material respects our financial condition, results of operations and cash flows at and for the periods presented in accordance with U.S. GAAP.
Item 9B. Other Information.
None
77
Incorporated by reference to the sections entitled “I. – Proposals – Proposal 1 – Elections of Directors”, “II. – Corporate Governance and Related Matters” and “IV. – Executive Compensation and Other Information – Executive Officers” in the definitive proxy statement for the 2013 annual meeting.
Incorporated by reference to the sections entitled “IV. – Executive Compensation and Other Information” in the definitive proxy statement for the 2013 annual meeting.
Incorporated by reference to the section entitled “III. – Security Ownership of Certain Beneficial Owners and Management” in the definitive proxy statement for the 2013 annual meeting.
Incorporated by reference to the section entitled “II. – Corporate Governance and Related Matters – Certain Relationships and Related Transactions” in the definitive proxy statement for the 2013 annual meeting.
Incorporated by reference to the section entitled “I. – Proposals – Proposal 2. Ratification of Selection of Independent Registered Public Accounting Firm” in the definitive proxy statement for the 2013 annual meeting.
78
| (a) | Documents filed as part of this report. |
| (1) | Financial Statements. The consolidated financial statements listed on the index to Item 8 of this Annual Report on Form 10-K are filed as a part of this Annual Report. |
| (2) | Financial Statement Schedules. All financial statement schedules have been omitted since the information is either not applicable or required or is included in the financial statements or notes thereof. |
| (3) | Exhibits. Those exhibits marked with a (*) refer to exhibits filed herewith. The other exhibits are incorporated herein by reference, as indicated in the following list. Those exhibits marked with a (†) refer to management contracts or compensatory plans or arrangements. Portions of the exhibits marked with a (Ω) are the subject of a Confidential Treatment Request under 17 C.F.R. §§ 200.80(b)(4), 200.83 and 240.24b-2. Omitted material for which confidential treatment has been requested has been filed separately with the SEC. |
Exhibit No. Description
| 2.1Ω | Asset Sale and Purchase Agreement dated December 22, 2011 between Oak Pharmaceuticals, Inc. and Lundbeck, Inc., incorporated by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on December 30, 2011. |
| 2.2Ω | Share Purchase Agreement, dated May 3, 2011, by and among Akorn, Inc., AVR Business Trust, Advanced Vision Research, Inc., Advanced Vision Pharmaceuticals, LLC, and the Shareholders of AVR Business Trust, incorporated by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on May 9, 2011. |
| 2.3 | Business Transfer Agreement dated as October 6, 2011 among Akorn, Inc., Akorn India Private Limited, Kilitch Drugs (India) Limited, and members of the promoter group of the Kilitch Drugs (India) Limited, incorporated by reference to Exhibit 2.1 to Akorn Inc.’s report on Form 8-K filed on October 6, 2011. |
| 3.1 | Restated Articles of Incorporation of Akorn, Inc. dated September 16, 2004, incorporated by reference to Exhibit 3.1 to Akorn, Inc.’s Registration Statement on Form S-1 filed on September 21, 2004(Commission file No. 001-32360). |
| 3.2* | Amended and Restated By-laws of Akorn, Inc., dated June 18, 2009. |
| 4.1 | Form of Securities Purchase Agreement dated March 1, 2006, between Akorn, Inc. and certain investors incorporated by reference to Exhibit 4.1 to Akorn, Inc.’s report on Form 8-K filed on March 7, 2006 (Commission file No. 001-32360). |
| 4.2 | Securities Purchase Agreement dated March 10, 2010, between Akorn, Inc. and Serum Institute of India Ltd, incorporated by reference to Exhibit 10.1 to our report on Form 8-K filed on March 16, 2010. |
| 4.3 | Akorn, Inc. Common Stock Purchase Warrant, dated April 13, 2009, in favor of EJ Funds LP, incorporated by reference to Exhibit 4.1 of Akorn, Inc.’s report on Form 8-K filed on April 17, 2009. |
| 4.4 | Modification, Warrant and Investor Rights Agreement, dated April 13, 2009, among Akorn, Inc., Akorn (New Jersey), Inc., and EJ Funds LP, incorporated by reference to Exhibit 4.2 to Akorn, Inc.’s report on Form 8-K filed on April 17, 2009. |
| 4.5 | Akorn, Inc. Common Stock Purchase Warrant, dated April 15, 2009, in favor of John N. Kapoor Trust dated 9/20/89, incorporated by reference to Exhibit 4.1 to Akorn, Inc.’s report on Form 8-K filed on April 21, 2009. |
| 4.6 | Common Stock Purchase Warrant dated August 17, 2009, in favor of EJ Funds LP, incorporated by reference to Exhibit 10.2 to Akorn, Inc.’s report on Form 8-K filed on August 21, 2009. |
| 4.7 | Common Stock Purchase Warrant dated August 17, 2009, in favor of John N. Kapoor Trust Dated 9/20/89, incorporated by reference to Exhibit 10.4 to Akorn, Inc.’s report on Form 8-K filed on August 21, 2009. |
| 4.8 | Warrant, dated March 10, 2010, granted by Akorn, Inc. to Serum Institute of India Ltd, incorporated by reference to Exhibit 4.1 to Akorn, Inc.’s report on Form 8-K filed on March 16, 2010. |
| 4.9 | Amended and Restated Registration Rights Agreement dated June 28, 2010, between Akorn, Inc. and The John N. Kapoor Trust Dated September 20, 1989 and EJ Funds LP, incorporated by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on July 2, 2010. |
| 4.10 | Indenture dated as of June 1, 2011 by and between Akorn, Inc. and Wells Fargo Bank, National Association, as trustee, including the form of 3.50% Convertible Senior Note due 2016 (included as Exhibit A to the Indenture), incorporated by reference to Exhibit 4.1 to Akorn, Inc.’s report on Form 8-K filed on June 2, 2011. |
79
| 10.1† | Form of Akorn, Inc. Non-Qualified Stock Option Agreement, incorporated by reference to Exhibit 10.36 to Akorn, Inc.’s report on Form 10-K for the fiscal year ended December 31, 2003, filed on March 30, 2004 (Commission file No. 001-32360). |
| 10.2† | Form of Akorn, Inc. Incentive Stock Option Agreement, incorporated by reference to Exhibit 10.37 to Akorn, Inc.’s report on Form 10-K for the fiscal year ended December 31, 2003, filed on March 30, 2004 (Commission file No. 001-32360). |
| 10.3† | Amended and Restated Akorn, Inc. 2003 Stock Option Plan, as amended, incorporated by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on March 8, 2012. |
| 10.4† | Amended and Restated Employee Stock Purchase Plan incorporated by reference to Appendix B to the Akorn, Inc. definitive proxy statement on Schedule 14A filed on July 24, 2009. |
| 10.5† | Form of Amendment to Executive Consulting Agreement between Akorn, Inc. and Raj Rai, dated December 8, 2009, incorporated by reference to Exhibit 10.1 to Akorn, Inc’s report on Form 8-K filed on December 22, 2009. |
| 10.6† | Form of Second Amendment to Executive Consulting Agreement between Akorn, Inc. and Raj Rai, its Chief Executive Officer, effective December 8, 2010, incorporated by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on December 28, 2010. |
| 10.7† | Form of Employment Agreement, dated December 22, 2010, between Akorn, Inc. and Timothy Dick, its Chief Financial Officer, incorporated by reference to Exhibit 10.2 to Akorn, Inc.’s report on Form 8-K filed on December 28, 2010. |
| 10.8† | Form of Employment Agreement, dated December 22, 2010, between Akorn, Inc. and Joe Bonaccorsi, its Secretary, incorporated by reference to Exhibit 10.3 to Akorn, Inc.’s report on Form 8-K filed on December 28, 2010. |
| 10.9 | Series A-2 Preferred Stock Purchase Agreement dated as of August 1, 2011 by and between Akorn, Inc. and Aciex Therapeutics, Inc., incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 10-Q filed on November 9, 2011. |
| 10.10 | Amendment #1 to Series A-2 Preferred Stock Purchase Agreement dated as of September 30, 2011 by and between Akorn, Inc. and Aciex Therapeutics, Inc., incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 10-Q filed on November 9, 2011. |
| 10.11 | Lease Agreement dated July 15, 2010, by and between Veronica Development Associates, a New Jersey general partnership, and Akorn (New Jersey), Inc., an Illinois corporation, for the Company’s 50,000 square foot manufacturing facility at 72-6 Veronica Avenue, Somerset, New Jersey, incorporate by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on July 30, 2010. |
| 10.12 | Commitment Letter dated November 2, 2008, by and between Akorn, Inc. and General Electric Capital Corporation, incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 8-K filed on November 5, 2008. |
| 10.13 | Subordinated Promissory Note dated July 28, 2008, issued by Akorn, Inc. to The John N. Kapoor Trust Dated September 20, 1989, in the principal amount of $5,000,000, incorporated by reference to Exhibit 10.1 to Akorn, Inc.’s report on Form 8-K filed on August 1, 2008. |
| 10.14 | Subordination and Intercreditor Agreement dated July 28, 2008, by and among Akorn, Inc., The John N. Kapoor Trust Dated September 20, 1989, LaSalle Bank National Association, as administrative agent for all senior lenders party to the senior credit agreement, and Akorn (New Jersey), Inc., incorporated by reference to Exhibit 10.2 to Akorn, Inc.’s report on Form 8-K filed on August 1, 2008. |
| 10.15 | Credit Agreement dated January 7, 2009, by and between Akorn, Inc., Akorn (New Jersey), Inc. and General Electric Capital Corporation, incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 8-K filed on January 9, 2009. |
| 10.16 | Memorandum of Agreement, dated March 31, 2009, among EJ Funds LP, Akorn Inc., and Akorn (New Jersey), Inc., incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 8-K filed on April 6, 2009. |
| 10.17 | Assignment, dated March 31, 2009, among General Electric Capital Corporation, EJ Funds LP, Akorn, Inc., and Akorn (New Jersey), Inc., incorporated by reference to Exhibit 10.2 to Akorn Inc.’s report on Form 8-K filed on April 6, 2009. |
| 10.18 | Reimbursement and Warrant Agreement, dated April 15, 2009, among Akorn, Inc. Akorn (New Jersey), Inc., John N. Kapoor Trust dated 09/20/89, and EJ Funds LP, incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 8-K filed on April 21, 2009. |
| 10.19 | Amended and Restated Credit Agreement dated August 17, 2009, by and among the Company, Akorn (New Jersey), Inc., a wholly owned subsidiary of the Company, other persons party thereto that are designated as credit parties, EJ Funds LP, and the other financial institution from time to time party thereto, incorporated by reference to Exhibit 10.1 of a Form 8-K filed on August 21, 2009. |
80
| 10.20 | Form of First Amendment to $10,000,000 Credit Facility, Amended and Restated Credit Agreement by and among Akorn, Inc. and Akorn (New Jersey), Inc., as Borrowers and EJ Funds, LP, as Lender, incorporated by reference to Exhibit 10.84 to the Akorn Inc. 2010 Annual Report on Form 10-K filed on March 14, 2011. |
| 10.21 | Amended and Restated Subordinated Note dated August 17, 2009, made by the Company and Akorn (New Jersey), Inc., in favor of John N. Kapoor Trust Dated 9/20/89, incorporated by reference to Exhibit 10.3 of a Form 8-K filed on August 21, 2009. |
| 10.22 | Amended and Restated Subordination Agreement dated as of August 17, 2009 by and among John N. Kapoor Trust Dated 9/20/89, the Company, Akorn (New Jersey), Inc. and EJ Funds LP, incorporated by reference to Exhibit 10.6 of a Form 8-K filed on August 21, 2009. |
| 10.23 | Loan and Security Agreement dated as of October 7, 2011 among Akorn, Inc., a Louisiana corporation, and its domestic subsidiaries, with certain financial institutions as lenders (Lenders), and Bank of America, N.A. as agent (Agent) for the Lenders, incorporated by reference to Exhibit 10.1 to Akorn Inc.’s report on Form 8-K filed on October 13, 2011. |
| 21.1 * | Listing of the Subsidiaries of Akorn, Inc. |
| 31.1* | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a). |
| 31.2* | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a). |
| 32.1* | Certification of the Chief Executive Officer pursuant to 18 USC Section 1350. |
| 32.2* | Certification of the Chief Financial Officer pursuant to 18 USC Section 1350. |
| 101 | The financial statements and footnotes from the Akorn, Inc. Annual Report on Form 10-K for the year ended December 31, 2012, filed on March 1, 2013, formatted in XBRL: (i) Condensed Consolidated Balance Sheets, (ii) Condensed Consolidated Statements of Operations, (iii) Condensed Consolidated Statement of Shareholders’ Equity, (iv) Condensed Consolidated Statements of Cash Flows and (v) Notes to Condensed Consolidated Financial Statements. |
81
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AKORN, INC. | ||
| By: | /s/ RAJAT RAI | |
Rajat Rai Chief Executive Officer | ||
Date: March 1, 2013
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant, and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ RAJAT RAI | Chief Executive Officer | March 1, 2013 | ||
| Rajat Rai | ||||
| /s/ TIMOTHY A. DICK | Chief Financial Officer (Principal Financial Officer | March 1, 2013 | ||
| Timothy A. Dick | and Principal Accounting Officer) | |||
| /s/ JOHN N. KAPOOR, PH.D. | Director, Chairman of the Board | March 1, 2013 | ||
| John N. Kapoor, Ph.D. | ||||
| /s/ KENNETH S. ABRAMOWITZ | Director | March 1, 2013 | ||
| Kenneth S. Abramowitz | ||||
| /s/ ADRIENNE L. GRAVES | Director | March 1, 2013 | ||
| Adrienne L. Graves | ||||
| /s/ RONALD M. JOHNSON | Director | March 1, 2013 | ||
| Ronald M. Johnson | ||||
| /s/ STEVEN J. MEYER | Director | March 1, 2013 | ||
| Steven J. Meyer | ||||
| /s/ BRIAN TAMBI | Director | March 1, 2013 | ||
| Brian Tambi | ||||
| /s/ ALAN WEINSTEIN | Director | March 1, 2013 | ||
| Alan Weinstein |
82