SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
Annual Report Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2007
Commission File Number 1-4858
INTERNATIONAL FLAVORS & FRAGRANCES INC.
(Exact name of Registrant as specified in its charter)
| NEW YORK | 13-1432060 | ||
| (State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) | ||
| 521 WEST 57TH STREET, NEW YORK, N.Y. | 10019 | ||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code (212) 765-5500
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| TITLE OF EACH CLASS | NAME OF EACH EXCHANGE ON WHICH REGISTERED | ||
| Common Stock, par value 12½¢ per share | New York Stock Exchange |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ![]() No
No ![]()
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months, and (2) has been subject to such filing requirements for the past 90 days. Yes ![]() No
No ![]()
Indicate by check mark whether the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ![]() No
No ![]()
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendments to this Form 10-K. ![]()
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of ‘‘accelerated filer’’, ‘‘large accelerated filer’’ and ‘‘smaller reporting company’’ in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | Accelerated filer | Non-accelerated filer | Smaller reporting company |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12B-2 of the Exchange Act). Yes ![]() No
No ![]()
For the purpose of reporting the following market value of Registrant’s outstanding common stock, the term ‘‘affiliate’’ refers to persons, entities or groups which directly or indirectly control, are controlled by, or are under common control with the Registrant and does not include individual executive officers, directors or less than 10% shareholders. The aggregate market value of Registrant’s common stock not held by affiliates as of June 30, 2007 was $4,655,751,202.
Indicate the number of shares outstanding of each of the Registrant’s classes of common stock, as of February 11, 2008: 81,020,808 shares of common stock, par value 12½¢ per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement to be sent to shareholders in connection with the 2008 Annual Meeting (the ‘‘IFF 2008 Proxy Statement’’) are incorporated by reference in Part III of this Form 10-K.
INTERNATIONAL FLAVORS & FRAGRANCES INC.
TABLE OF CONTENTS
| PAGE | |||||||||
| PART I | |||||||||
| ITEM 1. | Business | 3 | |||||||
| ITEM 1A. | Risk Factors | 7 | |||||||
| ITEM 1B. | Unresolved Staff Comments | 8 | |||||||
| ITEM 2. | Properties | 8 | |||||||
| ITEM 3. | Legal Proceedings | 10 | |||||||
| ITEM 4. | Submission of Matters to a Vote of Security Holders | 11 | |||||||
| PART II | |||||||||
| ITEM 5. | Market for the Registrant’s Common Equity and Related Stockholder Matters and Issuer Purchases of Equity Securities | 12 | |||||||
| ITEM 6. | Selected Financial Data | 14 | |||||||
| ITEM 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 16 | |||||||
| ITEM 7A. | Quantitative and Qualitative Disclosures About Market Risk | 32 | |||||||
| ITEM 8. | Financial Statements and Supplementary Data | 34 | |||||||
| ITEM 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 34 | |||||||
| ITEM 9A. | Controls and Procedures | 34 | |||||||
| ITEM 9B. | Other Information | 35 | |||||||
| PART III | |||||||||
| ITEM 10. | Directors, Executive Officers and Corporate Governance | 36 | |||||||
| ITEM 11. | Executive Compensation | 36 | |||||||
| ITEM 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 36 | |||||||
| ITEM 13. | Certain Relationships and Related Transactions, and Director Independence | 36 | |||||||
| ITEM 14. | Principal Accounting Firm Fees and Services | 37 | |||||||
| PART IV | |||||||||
| ITEM 15. | Exhibits and Financial Statement Schedules | 38 | |||||||
| SIGNATURES | 71 | ||||||||
Table of Contents
PART I
| ITEM 1. | BUSINESS. |
International Flavors & Fragrances Inc., incorporated in New York in 1909, and its subsidiaries (the ‘‘Registrant’’, ‘‘IFF’’, ‘‘we’’, ‘‘us’’, and ‘‘our’’), is a leading creator and manufacturer of flavor and fragrance products used by other manufacturers to impart or improve flavor or fragrance in a wide variety of consumer products. Fragrance products are sold principally to manufacturers of perfumes, cosmetics, personal care products, hair care products, deodorants, soaps, detergents, fabric care and air care products; our flavor products are sold principally to manufacturers of prepared foods, beverages, dairy foods, pharmaceuticals and confectionery products as well as the food service industry.
We currently have 31 manufacturing facilities with the major manufacturing facilities located in the United States, Great Britain, Ireland, the Netherlands, Spain, Argentina, Brazil, Mexico, Australia, China, India, Indonesia, Japan and Singapore. The remaining manufacturing facilities are located in 8 other countries. We maintain our own sales and distribution facilities in 31 countries and are represented by sales agents and distributors in other countries. Our principal executive offices are located at 521 West 57th Street, New York, New York 10019 (212-765-5500).
MARKETS
Our fragrance products are used by customers in the manufacture of consumer products such as soaps, detergents, fabric care, cosmetic creams, lotions and powders, lipsticks, after-shave lotions, deodorants, hair preparations, candles, air fresheners and all-purpose cleaners as well as in other consumer products designed solely to appeal to the sense of smell, such as perfumes and colognes. The cosmetics industry, including perfume and toiletries manufacturers, is one of our two largest fragrance customer groups. Most of the major United States companies in this industry are our customers, and five of the largest United States cosmetics companies are among principal customers. The household products industry, including soaps, detergents and fabric care, is the other important fragrance customer group. Four of the largest United States household product manufacturers are our major customers. In the three years ended December 31, 2007, sales of fragrance produ cts accounted for 56%, 57% and 57%, respectively, of our total sales.
Our flavor products are sold principally to the food and beverage industries for use in consumer products such as soft drinks, candies, baked goods, desserts, prepared foods, dietary foods, dairy products, drink powders, pharmaceuticals, snack foods and alcoholic beverages. Two of our largest customers for flavor products are major producers of prepared foods and beverages in the United States. In the three years ended December 31, 2007, sales of flavor products accounted for 44%, 43% and 43%, respectively, of our total sales.
See Note 12, Segment Information, for information concerning the two business segments, Flavors and Fragrances, and our geographic regions, which is incorporated by reference.
PRODUCTS
Our principal fragrance and flavor products consist of compounds of large numbers of ingredients blended in proprietary formulas created by our perfumers and flavorists. Most of these compounds contribute the total fragrance or flavor to the consumer products in which they are used. This fragrance or flavor characteristic is often a major factor in the consumer selection and acceptance of the consumer end product. A smaller number of compounds are sold to manufacturers who further blend them to achieve the finished fragrance or flavor in their products. We produce thousands of compounds, and new compounds are constantly being created in order to meet the many and changing characteristics of our customers’ end products. Most of the fragrance and flavor compounds are created and produced for the exclusive use of particular customers. Our products are sold in solid, powder and liquid forms and in amounts ranging from a few pounds to many tons, depending upon the nature of the product.
The ingredients used by us in our compounds are both synthetic and natural. We manufacture a substantial portion of the synthetic ingredients. While a majority of our synthetic ingredients production is used in our compounds, a substantial portion is also sold to others. Natural ingredients are derived from flowers, fruits and other botanical products as well as from animal products. They contain varying numbers of organic chemicals,
3
Table of Contents
which are responsible for the fragrance or flavor of the natural product. The natural products are purchased in processed or semi-processed form. Some are used in compounds in the state in which they are purchased and others after further processing. Natural products, together with various chemicals, are also used as raw materials for the manufacture of synthetic ingredients by chemical processes. Our flavor products also include extracts and seasonings derived from various fruits, vegetables, nuts, herbs and spices as well as microbiologically-derived ingredients.
MARKET DEVELOPMENTS
The demand for consumer products utilizing flavors and fragrances has been stimulated and broadened by changing social habits resulting from various factors such as increases in personal income, dual-earner households, teenage population, leisure time, urbanization, health and wellness concerns, including increased demand for nature based products and by the continued growth in world population. In the fragrance field, these developments have expanded the market for hair care, candles and air care products and deodorant and personal wash products with finer fragrance quality, as well as the market for colognes, toilet waters, men’s toiletries and other products beyond traditional luxury items such as perfumes. In the flavor field, similar market characteristics have stimulated the demand for products such as convenience foods, soft drinks and low-fat and organic food products that must conform to expected tastes. New and improved methods of packaging, appl ication and dispensing have been developed for many consumer products that utilize some of our flavor or fragrance products. These developments have called for the creation of new compounds and ingredients compatible with the newly introduced materials and methods of application.
PRODUCT DEVELOPMENT AND RESEARCH
The development of new flavors and fragrances is a complex technical and artistic process calling upon the combined knowledge and skill of our creative perfumers and flavorists, and our scientists. With extensive experience, the perfumers and flavorists continuously advance their skills for creating fragrances or flavors best suited to the market requirements of the customers’ products.
Scientists from various disciplines work in project teams with the perfumers and flavorists to develop fragrance and flavor products with consumer preferred performance characteristics. Scientific expertise includes: natural products research, plant science, organic chemistry, analytical chemistry, biochemistry, microbiology, process engineering, food science, material science and sensory science. Analytical and sensory science is applied to understand the complex interactions of the many ingredients in a consumer product in order to optimize the flavor or fragrance performance at all points of use. Material science technology is applied to create controlled release and delivery systems to enhance flavor and fragrance performance in consumer products. An important contribution to the creation of new fragrances and flavors is the discovery and development of new ingredients having improved fragrance or flavor value. The ingredients research program discovers mole cules found in natural substances and creates new molecules that are subsequently tested for their fragrance or flavor value. The new molecules that meet rigorous requirements for commercial development are subsequently transferred to manufacturing operations for production.
Creative and technical product development is conducted in 31 fragrance and flavor laboratories in 23 countries. We maintain a research and development center at Union Beach, New Jersey. We spent $199 million in 2007, $186 million in 2006 and $180 million in 2005 on our research and development activities or about 9% of our revenues each year. We expect these expenditures to remain at approximately 9% of our revenues in 2008. Of the amount expended in 2007 on such activities, 65% was for fragrances and the balance was for flavors. We employed 1,132 persons in 2007 and 1,065 persons in 2006 in such activities.
Our business is not materially dependent upon any patents, trademarks or licenses.
DISTRIBUTION
Distribution for both the flavors and fragrances business units is similar in that most of our sales are through our own sales force. The flavors business operates from two sales offices in the United States and 38 sales offices in 29 foreign countries; while the fragrances business operates from two sales offices in the United States and 36 sales offices in 28 foreign countries. Sales in additional countries are made through agents and distributors. For the year ended December 31, 2007, 28% of our sales were to customers in North America, 37% in Europe, Africa and Middle East (‘‘EAME’’), 22% in Greater Asia and 13% in Latin America.
4
Table of Contents
During 2007, our 30 largest customers accounted for 57% of our sales. Sales to one customer accounted for 11% of our sales in 2007. These sales were largely in the fragrances business unit. No single customer accounted for more than 10% of our sales in 2006 and 2005.
GOVERNMENTAL REGULATION
The manufacture and sale of our products are subject to regulation in the United States by the Food and Drug Administration, the Agriculture Department, the Bureau of Alcohol, Tobacco and Firearms, the Environmental Protection Agency, the Occupational Safety and Health Administration, the Drug Enforcement Administration and state authorities. Foreign subsidiaries are subject to similar regulation in a number of countries. In particular, the European Union in December 2006 adopted legislation requiring extensive chemical registration and testing over the next 11 years. Compliance with existing governmental requirements regulating the discharge of materials into the environment has not materially affected our operations, earnings or competitive position. In 2008, we expect to spend approximately $8 million in capital projects and $20 million in operating expenses and governmental charges for the purpose of complying with such requirements.
RAW MATERIAL PURCHASES
We purchase roughly 10,000 different raw materials from many sources all over the world. The principal natural raw materials consist of essential oils, extracts and concentrates derived from fruits, vegetables, flowers, woods and other botanicals, animal products and raw fruits. The principal synthetic raw material purchases consist of organic chemicals. We believe that alternate materials or alternate sources of materials are available to enable us to maintain our competitive position in the event of any interruption in the supply of raw materials from present sources.
COMPETITION
We have more than 50 competitors in the United States and world markets; two leading competitors have made significant acquisitions in the last two years. While no single factor is responsible, our competitive position is based principally on the creative skills of our perfumers and flavorists, the technological advances resulting from our research and development activities, the quality of our customer service, the support provided by our marketing and application groups, and our understanding of consumers. We believe that we are one of the largest companies producing and marketing on an international basis a wide range of fragrance and flavor products for sale to manufacturers of consumer products. In particular countries and localities, we face competition from numerous companies specializing in certain product lines, among which are some companies larger than us and some more important in a particular product line or lines. Most of our customers do not buy a ll of their fragrance or flavor products from the same supplier, and some customers make their own fragrance or flavor compounds with ingredients supplied by us or others.
EMPLOYEE RELATIONS
At December 31, 2007, we employed approximately 5,300 persons, of whom approximately 1,400 were employed in the United States. We have never experienced a work stoppage or strike and consider our employee relations to be satisfactory.
5
Table of Contents
EXECUTIVE OFFICERS OF REGISTRANT:
| Name | Office and Other Business Experience (1) | Age | Year First Became Officer | ||||||
| Robert M. Amen | Chairman of the Board and Chief Executive Officer since July 2006; President, International Paper from 2003 to March 2006; Executive Vice President, International Paper, prior thereto. | 58 | 2006 | ||||||
| Nicolas Mirzayantz | Group President, Fragrances since January 2007; Senior Vice President, Fine Fragrance and Beauty Care and Regional Manager, North America Region from April, 2005 to December 2006; Senior Vice President, Fine Fragrance and Beauty Care from October 2004 to March 2005; Vice President, Global Business Development, Fine Fragrance and Toiletries, prior thereto. | 45 | 2002 | ||||||
| Hernan Vaisman | Group President, Flavors since January 2007; Vice President, Latin America Region from October 2004 to December 2006; Regional Finance Director, Latin America Region, prior thereto. | 49 | 2004 | ||||||
| Steven J. Heaslip | Senior Vice President, Human Resources since December 2002; Vice President Human Resources, prior thereto. | 50 | 2001 | ||||||
| Dennis M. Meany | Senior Vice President, General Counsel and Secretary since January 2004; Associate General Counsel, prior thereto. | 60 | 2004 | ||||||
| Douglas J. Wetmore | Senior Vice President, Chief Financial Officer and Treasurer since October 2007; Senior Vice President and Chief Financial Officer, prior thereto. | 50 | 1992 | ||||||
| Joseph Faranda | Vice President and Chief Marketing Officer since March 2005; Vice President, Strategic Marketing, The Home Depot, Inc., prior thereto. | 54 | 2005 | ||||||
| Kimberly A. Hendricks | Controller since July 2007; Vice President, Finance, JLG Industries, Inc. from January 2006 to February 2007; Vice President, Finance, Bristol-Myers Squibb Company, prior thereto. | 44 | 2007 |
| (1) | Employed by us or an affiliated company for the last five years, except as otherwise indicated. |
AVAILABLE INFORMATION
We make available free of charge on or through the Investor Relations link on our website, www.iff.com, all materials that we file electronically with the SEC, including our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports, filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after electronically filing such materials with, or furnishing them to, the SEC. During the period covered by this Form 10-K, we made all such materials available through our website as soon as reasonably practicable after filing such materials with the SEC.
You may also read and copy any materials filed by us with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549, and you may obtain information on the operation of the Public
6
Table of Contents
Reference Room by calling the SEC in the U.S. at 1-800-SEC-0330. In addition, the SEC maintains an Internet website, www.sec.gov, that contains reports, proxy and information statements and other information that we file electronically with the SEC.
A copy of our Corporate Governance Guidelines, Code of Business Conduct and Ethics, and the charters of the Audit Committee, Compensation Committee, and Nominating and Governance Committee of the Board of Directors are posted on the Investor Relations section of our website, www.iff.com and are available in print to any shareholder who requests copies by contacting Dennis M. Meany, Senior Vice President, General Counsel and Secretary, at our principal executive office set forth above.
| Item 1A. | Risk Factors. |
Competitive factors may negatively impact our sales and marketability.
The market for flavor and fragrance products is fragmented and highly competitive. IFF competes with many companies and some of our competitors specialize in one or more of our product lines while others sell many of the same product lines. In addition, some of our competitors may have greater financial and technical resources. Increased competition by existing or future competitors, including aggressive price competition, could result in the need for us to reduce prices or increase spending and this could have an impact on sales and profitability.
We are subject to economic and social changes which may impact sales.
Demand for consumer products using flavors and fragrances has been stimulated and broadened by changing social habits resulting from factors such as increases in personal income, dual-earner households, teenage population, leisure time, health concerns and urbanization and by the continued growth in world population. Changes in any number of external economic factors, or changes in social or consumer preferences, could adversely impact our results of operations. The current macro-economic environment in the United States and Western Europe may adversely impact consumer spending on products for which we supply the flavor or fragrance.
Results may be negatively impacted by the price, quality and availability of raw materials.
Raw materials are purchased from many sources from all over the world, including essential oils, extracts and concentrates derived from fruits, vegetables, flowers, woods and other botanicals, animal products, raw fruits and organic chemicals. Disruptions in the supply or quality of ingredients or rising prices for ingredients purchased could adversely impact our results of operations and profitability.
Results may be negatively impacted by the inability to implement our business strategy, including the achievement of anticipated cost savings, profitability or growth targets.
We are committed to those particular business strategies which have been identified as likely to drive profitable future growth and improve operations and customer service. If we are unable to successfully and timely implement these strategies, it would adversely impact our financial condition and results of operations.
Results may be negatively affected by the impact of currency fluctuation or devaluation in principal foreign markets and the effectiveness of hedging and risk management strategies.
Our operations are conducted in many countries, the results of which are reported in the local currency and then translated into U.S. dollars at applicable exchange rates. The exchange rates between these currencies and the U.S. dollar have fluctuated and may continue to do so in the future. We employ a variety of techniques to reduce the impact of exchange rate fluctuations, including foreign currency hedging activities. However, volatility in currency exchange rates may adversely impact our reported results of operations, financial condition or liquidity.
Results may be negatively impacted by the outcome of uncertainties related to litigation.
We are involved in a number of legal claims. While we believe that related insurance coverage is adequate with respect to such claims, we cannot predict the ultimate outcome of such litigation. In addition, we cannot provide assurance that future events will not require an increase in the amount accrued for any such claims, or require accrual for one or more claims that has not been previously accrued.
7
Table of Contents
Results and cash flows may be negatively impacted by future pension funding and other postretirement obligations.
We establish assumptions concerning discount rates and actuarial assumptions regarding pension funding and other postretirement benefit obligations based on current market conditions, plan participants, asset returns, interest rates and other factors. Changes in pension and other postretirement benefits, and associated expenses, may occur in the future due to changes in demographics and assumptions. These changes may adversely impact our financial condition, results of operations or liquidity.
Results may be negatively impacted by the effect of legal and regulatory requirements, as well as restrictions imposed on operations by foreign and domestic governmental entities.
The manufacture and sale of our products are subject to regulation in the United States by the Food and Drug Administration, the Agriculture Department, the Bureau of Alcohol, Tobacco and Firearms, the Environmental Protection Agency, the Occupational Safety and Health Administration, the Drug Enforcement Administration and state authorities. Our foreign operations are subject to similar substantial governmental regulation in a number of countries, including extensive new requirements within the European Union. Compliance with existing governmental requirements and future governmental regulations may adversely impact financial condition, results of operations or liquidity.
We may face risks associated with events which may affect the world economy.
World events such as terrorist attacks, or regional conflicts have and may in the future weaken world economies. Any resulting weaknesses in these economies may adversely affect our business or the businesses of our customers, with a resultant negative impact on our financial condition, results of operations or liquidity.
| ITEM 1B. | Unresolved Staff Comments. |
None.
| ITEM 2. | PROPERTIES. |
Our principal properties are as follows:
| Location | Operation | ||
| United States | |||
| Augusta, GA | Production of fragrance ingredients. | ||
| Carrollton, TX(1) | Production of flavor compounds; flavor laboratories. | ||
| Hazlet, NJ(1) | Production of fragrance compounds; fragrance laboratories. | ||
| Jacksonville, FL | Production of fragrance ingredients. | ||
| New York, NY(1) | Fragrance laboratories. | ||
| South Brunswick, NJ(1) | Production of flavor compounds and ingredients; flavor laboratories. | ||
| Union Beach, NJ | Research and development center. | ||
| France | |||
| Neuilly(1) | Fragrance laboratories. | ||
| Grasse | Production of flavor and fragrance ingredients; fragrance laboratories. | ||
| Great Britain | |||
| Haverhill | Production of flavor compounds and ingredients, and fragrance ingredients; flavor laboratories. | ||
| Ireland | |||
| Drogheda | Production of fragrance compounds. |
8
Table of Contents
| Location | Operation | ||
| Netherlands | |||
| Hilversum | Flavor and fragrance laboratories. | ||
| Tilburg | Production of flavor compounds and ingredients, and fragrance compounds. | ||
| Spain | |||
| Benicarlo | Production of fragrance ingredients. | ||
| Argentina | |||
| Garin | Production of flavor compounds and ingredients, and fragrance compounds; flavor laboratories. | ||
| Brazil | |||
| Rio de Janeiro | Production of fragrance compounds. | ||
| São Paulo | Fragrance laboratories. | ||
| Taubate | Production of flavor compounds and ingredients; flavor laboratories. | ||
| Mexico | |||
| Tlalnepantla | Production of flavor and fragrance compounds; flavor and fragrance laboratories. | ||
| India | |||
| Chennai(2) | Production of flavor compounds and ingredients and fragrance compounds; flavor laboratories. | ||
| Australia | |||
| Dandenong | Production of flavor compounds and flavor ingredients. | ||
| China | |||
| Guangzhou(4) | Production of flavor and fragrance compounds. | ||
| Shanghai(6) | Flavor and fragrance laboratories. | ||
| Xin’anjiang(5) | Production of fragrance ingredients. | ||
| Zhejiang | Production of fragrance ingredients. | ||
| Indonesia | |||
| Jakarta(3) | Production of flavor compounds and ingredients, and fragrance compounds and ingredients; flavor and fragrance laboratories. | ||
| Japan | |||
| Gotemba | Production of flavor compounds. | ||
| Tokyo | Flavor and fragrance laboratories. | ||
| Singapore | |||
| Jurong | Production of flavor and fragrance compounds. | ||
| Science Park(1) | Flavor and fragrance laboratories. |
| (1) | Leased. |
| (2) | We have a 93.4% interest in the subsidiary company that owns this facility. |
| (3) | Land is leased and building is partially leased and partially owned. |
| (4) | Land is leased and building and machinery and equipment are owned. |
| (5) | We have a 90% interest in the subsidiary company that leases the land and owns the buildings and machinery. |
| (6) | Building is leased and machinery and equipment are owned. |
Our principal executive offices and New York laboratory facilities are located at 521 West 57th Street, New York City.
9
Table of Contents
| ITEM 3. | LEGAL PROCEEDINGS. |
We are subject to various claims and legal actions in the ordinary course of our business. For purposes of reporting these actions, Bush Boake Allen (‘‘BBA’’) a wholly owned subsidiary of IFF and/or IFF are referred to as the ‘‘Company’’.
In September 2001, the Company was named as a defendant in a purported class action brought against it in the Circuit Court of Jasper County, Missouri, on behalf of employees of a plant owned and operated by Gilster-Mary Lee Corp. in Jasper, Missouri (‘‘Benavides case’’). The plaintiffs alleged that they sustained respiratory injuries in the workplace due to the use by Gilster-Mary Lee of a BBA, and/or IFF flavor. We are subject to various claims and legal actions in the ordinary course of our business.
In January 2004, the Court ruled that class action status was not warranted. As a result of this decision, each of the 47 plaintiff cases was to be tried separately. Subsequently, 8 cases were tried to a verdict, 4 verdicts resulted for the plaintiffs and 4 verdicts resulted for the Company, all of which were appealed by the losing party. Subsequently all plaintiff cases related to the Benavides case, including those on appeal, were settled.
Sixteen actions based on similar claims of alleged respiratory illness due to workplace exposure to flavor ingredients are currently pending against the Company and other flavor suppliers and related companies.
In May 2004, the Company and another flavor supplier were named defendants, and subsequently a number of third party defendants were added, in a lawsuit by 4 former workers and their spouses at a Ridgeway, Illinois factory in an action brought in the Circuit Court for the Second Judicial Circuit, Gallatin County, Illinois (Barker case) and another concerning 8 other workers and 5 spouses at this same plant was filed in July 2004 and is pending in this same Court against the same defendants (Batteese case). In June 2004, the Company and 2 other flavor suppliers were named defendants in a lawsuit by 1 former worker and spouse at a Northlake, Illinois facility in an action brought in the Circuit Court of Cook County, Illinois. Nine third party defendants have been added (Lopez case). In March 2005, the Company and 8 other companies were named defendants in a lawsuit by 1 former employee and spouse of Bell Flavors and Fragrances, Inc. in an action brought in the Circuit Court of Cook County, Illinois (Robinson case). The Company was dismissed from this lawsuit in November 2007. In July 2005, the Company and 11 other flavor and chemical suppliers were named defendants in a lawsuit by 1 former worker and spouse of Brach’s Confections, Inc. in an action brought in the Circuit Court of Cook County, Illinois. Brach’s has been added as a third party defendant (Campbell case). In August 2005, the Company and 16 other companies were named defendants in a lawsuit by 3 former employees of the Gilster-Mary Lee facility in McBride, Missouri in the Missouri Circuit Court, 32nd Judicial Circuit (Fults case). In August 2006, the Company and 3 other flavor and chemical suppliers were named defendants in a lawsuit by 34 current and former employees and/or a neighbor of the Gilster-Mary Lee facility in Jasper, Missouri in the Missouri Circuit Court of Jasper County (Arles case) and 5 other current and former employees in the same Court (Bowan case). A similar case involving 5 former employees, originally plaintiffs in the Arles case, was filed in the same Court in August 2006 and then removed to the U.S. District Court, Western District of Missouri, Southwest Division (Parker case). In November 2006, the Company, 15 other flavor and chemical suppliers, a trade association and a third party defendant company were named defendants in a lawsuit filed in the Circuit Court of Cook County, Illinois by 1 plaintiff allegedly injured by exposure to butter flavor and other substances at various facilities in which he worked (Solis case). In January 2007, the Company and another flavor supplier were named defendants in a lawsuit filed in Hamilton County, Ohio Court of Common Pleas by approximately 245 current and former employees of two separate Marion, Ohio factories and 92 spouses of such employees (Aldrich case). In May 2007, the Company and 13 other companies were named defendants in a lawsuit filed in Circuit Court of Cook County, Illinois by 5 former employees of Brach’s Confections, Inc. in Chicago, Illinois (Williams case). In June 2007, the Company and another flavor supplier were named defendants in a lawsuit filed in Hamilton County, Ohio Court of Common Pleas by 58 current and former employees of a M arion, Ohio facility and 18 spouses of such employees (Arnold case). In June 2007, the Company and 22 other companies were named defendants in a lawsuit in the Missouri Circuit Court, 32nd Judicial Circuit by 7 former employees of a McBride, Missouri facility (Geile case). In July 2007, the Company and another flavor manufacturer were named defendants in a lawsuit filed in Hamilton County, Ohio Court of Common Pleas by 128 current and former workers of two Ohio facilities and 52 spouses of such employees (Ad amson case). In July 2007, the Company was joined as a defendant in a case filed in June 2005 against 7 companies and a trade association in the 8th Judicial District Court of Montana by the widow of the former owner/operator of a popcorn business in Montana (Yatsko case).
10
Table of Contents
In October 2007, the Company and 23 other companies were named defendants in a lawsuit in the Missouri Circuit Court, 32nd Judicial Circuit by the widow and daughter of a former worker at a McBride, Missouri facility (Wibbenmeyer case).
The Company believes that all IFF and BBA flavors at issue in these matters meet the requirements of the U.S. Food and Drug Administration and are safe for handling and use by workers in food manufacturing plants when used according to specified safety procedures. These procedures are detailed in instructions that IFF and BBA provided to all their customers for the safe handling and use of their flavors. It is the responsibility of IFF’s customers to ensure that these instructions, which include the use of appropriate engineering controls, such as adequate ventilation, prior handling procedures and respiratory protection for workers, are followed in the workplace.
At each balance sheet date, or more frequently as conditions warrant, we review the status of each pending claim, as well as our insurance coverage for such claims with due consideration given to potentially applicable deductibles, retentions and reservation of rights under its insurance policies with respect to all these matters. While the ultimate outcome of any litigation cannot be predicted, management believes that adequate provision has been made with respect to all known claims. Based on information presently available and in light of the merits of its defenses and the availability of insurance, we do not expect the outcome of the above cases, singly or in the aggregate, to have a material adverse effect on our financial condition, results of operation or liquidity. There can be no assurance that future events will not require us to increase the amount we have accrued for any matter or accrue for a matter that has not been previously accrued. See Note 16 to the Consolidated Financial Statements.
Over the past 20 years, various federal and state authorities and private parties have claimed that we are a Potentially Responsible Party (‘‘PRP’’) as a generator of waste materials for alleged pollution at a number of waste sites operated by third parties located principally in New Jersey and have sought to recover costs incurred and to be incurred to clean up the sites.
We have been identified as a PRP at nine facilities operated by third parties at which investigation and/or remediation activities may be ongoing. We analyze our liability on a regular basis. We accrue for environmental liabilities when they are probable and estimable. At December 31, 2007, we estimated our share of the total future cost for these sites to be less than $5 million.
While joint and several liability is authorized under federal and state environmental laws, we believe the amounts we have paid and anticipate paying in the future for clean-up costs and damages at all sites are not and will not be material to our financial condition, results of operations or liquidity. This conclusion is based upon, among other things, the involvement of other PRPs at most sites, the status of proceedings, including various settlement agreements and consent decrees, the extended time period over which payments will likely be made and an agreement reached in July 1994 with three of our liability insurers pursuant to which defense costs and indemnity amounts payable by us in respect of the sites will be shared by the insurers up to an agreed amount.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS. |
None.
11
Table of Contents
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information.
Our common stock is traded principally on the New York Stock Exchange. The high and low stock prices for each quarter during the last two years were:
| 2007 | 2006 | |||||||||||||||||||||||
| Quarter | High | Low | High | Low | ||||||||||||||||||||
| First | $50.77 | $46.00 | $36.03 | $32.53 | ||||||||||||||||||||
| Second | 52.75 | 47.14 | 38.84 | 33.46 | ||||||||||||||||||||
| Third | 53.93 | 47.45 | 39.96 | 34.32 | ||||||||||||||||||||
| Fourth | 54.20 | 47.32 | 49.88 | 39.19 | ||||||||||||||||||||
Approximate Number of Equity Security Holders.
| (A) Title of Class | (B) Number of shareholders of record as of December 31, 2007 | |||||
| Common stock, par value 12 ½¢ per share | 3,248 | |||||
Dividends.
Cash dividends declared per share for each quarter ending after January 1, 2006 were as follows:
| Quarter | 2007 | 2006 | ||||||||||
| First | $ | 0.210 | $ | 0.185 | ||||||||
| Second | 0.210 | 0.185 | ||||||||||
| Third | 0.230 | 0.185 | ||||||||||
| Fourth | 0.230 | 0.210 | ||||||||||
Performance graph.
Total Return To Shareholders(1)
(Includes reinvestment of dividends)
| ANNUAL RETURN PERCENTAGE Years Ending | ||||||||||||||||||||||||||||||
| Company Name / Index | 2003 | 2004 | 2005 | 2006 | 2007 | |||||||||||||||||||||||||
| International Flavors & Fragrances | 1.42 | 24.89 | -20.21 | 49.64 | -0.36 | |||||||||||||||||||||||||
| S&P 500 Index | 28.68 | 10.88 | 4.91 | 15.79 | 5.49 | |||||||||||||||||||||||||
| Peer Group | 15.93 | 8.71 | 4.43 | 18.29 | 22.26 | |||||||||||||||||||||||||
| INDEXED RETURNS Years Ending | ||||||||||||||||||||||||||||||||||||
| Company Name / Index | Base Period 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | ||||||||||||||||||||||||||||||
| International Flavors & Fragrances | $100 | $101.42 | $126.65 | $101.06 | $151.23 | $150.69 | ||||||||||||||||||||||||||||||
| S&P 500 Index | 100 | 128.68 | 142.69 | 149.70 | 173.34 | 182.86 | ||||||||||||||||||||||||||||||
| Peer Group | 100 | 115.93 | 126.03 | 131.61 | 155.69 | 190.34 | ||||||||||||||||||||||||||||||
| Peer Group Companies(2) | ||||||
| Alberto Culver Company | Hormel Foods Corp. | Unilever NV | ||||
| Avon Products | Kellogg Co. | W.M. Wrigley Jr Com. | ||||
| Campbell Soup Co. | Estee Lauder Companies, Inc. | YUM Brands, Inc. | ||||
| Church & Dwight Inc. | McCormick & Company, Inc. | |||||
| Clorox Company | McDonald’s Corp. | |||||
| Coca-Cola Company | Nestle SA | |||||
| Colgate-Palmolive Co. | Pepsico Inc. | |||||
| ConAgra Foods, Inc. | Procter & Gamble Co. | |||||
| General Mills Inc. | Revlon Inc. | |||||
| H.J. Heinz Co. | Sara Lee Corp. | |||||
| Hershey Company | Sensient Technologies Corp. |
12
Table of Contents

| (1) | The Cumulative Shareholder Return assumes that the value of an investment in our Common Stock and each index was $100 on December 31, 2002, and that all dividends were reinvested. |
| (2) | Due to the international scope and breadth of our business, we believe that a Peer Group comprised of international public companies, which are representative of the customer group to which we sell our products, with market capitalizations ranging from approximately $589 million to approximately $205 billion, is the most appropriate group against which to compare shareholder returns. |
Issuer Purchases of Equity Securities.
During the first six months of 2007, under a share repurchase program of $300 million authorized in October 2006 (the ‘‘October 2006 Plan’’), we repurchased approximately 1.6 million shares at a cost of $81 million; at June 30, 2007, we had approximately $125 million remaining under the October 2006 Plan. In July 2007, the October 2006 Plan was terminated and superseded by a new program authorized by our Board of Directors (‘‘Board’’) to repurchase up to 15% or $750 million worth of our outstanding common stock, whichever is less (the ‘‘July 2007 Plan’’).
In September 2007, under the July 2007 Plan, we entered into two agreements to purchase shares of our common stock under a $450 million accelerated share repurchase (‘‘ASR’’) program. On September 28, 2007, we paid $450 million in exchange for an initial delivery of 7.6 million shares under the ASR, representing 90% of the shares that could have been purchased, based on the average trading price of IFF stock, on that date. The remaining 10%, or $45 million, not used in the initial settlement will be included in the determination of the cost of the shares purchased upon completion of the ASR and is reflected in the accompanying Consolidated Balance Sheet as a reduction to Capital in excess of par value.
As a result of the ASR, we did not purchase any of our equity securities in the quarter ended December 31, 2007.
13
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA. |
INTERNATIONAL FLAVORS & FRAGRANCES INC.
QUARTERLY FINANCIAL DATA (UNAUDITED)
(DOLLARS IN THOUSANDS EXCEPT PER SHARE AMOUNTS)
| Net Income Per Share(b) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net Sales | Gross Profit | Net Income(a) | Basic | Diluted | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quarter | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| First | $ | 566,101 | $ | 511,432 | $ | 236,719 | $ | 216,614 | $ | 62,689 | $ | 53,690 | $0.70 | $0.59 | $0.69 | $0.58 | ||||||||||||||||||||||||||||||||||||||||||||
| Second | 573,726 | 530,505 | 246,058 | 227,616 | 78,372 | 61,182 | 0.88 | 0.67 | 0.87 | 0.67 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Third | 583,313 | 539,135 | 244,138 | 228,986 | 58,844 | 63,646 | 0.68 | 0.71 | 0.67 | 0.70 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Fourth | 553,498 | 514,318 | 225,299 | 210,915 | 47,223 | 47,982 | 0.59 | 0.54 | 0.58 | 0.53 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| $ | 2,276,638 | $ | 2,095,390 | $ | 952,214 | $ | 884,131 | $ | 247,128 | $ | 226,500 | $2.86 | $2.50 | $2.82 | $2.48 | |||||||||||||||||||||||||||||||||||||||||||||
| (a) | Net Income in the 2007 second and fourth quarter includes the after-tax benefit of gains on sale of assets of ($3,686) and ($4,033), respectively; the third quarter includes the after-tax effects of a pension curtailment charge of $3,685. Net Income in the 2007 second quarter also includes a tax benefit of $9,718; see Note 9 to the Consolidated Financial Statements for further discussion. Net Income in the 2006 first, second, third and fourth quarter includes the after-tax effects of restructuring charges (credits) of $461, ($200), $210, and $1,405, respectively. The 2006 third and fourth quarters also include after-tax benefits of gains on sale of assets of ($5,325) and ($4,743), respectively; and the benefit of an insurance recovery of ($2,496) in the third quarter. Net income in the 2006 fourth quarter also includes a ta x benefit of $3,511. |
| (b) | The sum of the 2007 and 2006 quarters’ net income per share does not equal the earnings per share for the full year due to changes in average shares outstanding. |
14
Table of Contents
INTERNATIONAL FLAVORS & FRAGRANCES INC.
FIVE-YEAR SUMMARY
(DOLLARS IN THOUSANDS EXCEPT PER SHARE AMOUNTS)
| 2007 | 2006 | 2005 | 2004 | 2003 | ||||||||||||||||||||||||||
| Consolidated Statement of Income Data | ||||||||||||||||||||||||||||||
| Net sales | $ | 2,276,638 | $ | 2,095,390 | $ | 1,993,393 | $ | 2,033,653 | $ | 1,901,520 | ||||||||||||||||||||
| Cost of goods sold(b) | 1,324,424 | 1,211,259 | 1,168,992 | 1,160,235 | 1,092,456 | |||||||||||||||||||||||||
| Research and development expenses(b) | 199,023 | 185,692 | 179,812 | 175,173 | 159,286 | |||||||||||||||||||||||||
| Selling and administrative expenses(b) | 375,287 | 351,923 | 339,323 | 341,306 | 308,951 | |||||||||||||||||||||||||
| Amortization of intangibles | 12,878 | 14,843 | 15,071 | 14,830 | 12,632 | |||||||||||||||||||||||||
| Curtailment loss | 5,943 | — | — | — | — | |||||||||||||||||||||||||
| Restructuring and other charges, net(a) | — | 2,680 | 23,319 | 31,830 | 42,421 | |||||||||||||||||||||||||
| Interest expense | 41,535 | 25,549 | 23,956 | 24,002 | 28,477 | |||||||||||||||||||||||||
| Other (income) expense, net | (11,136 | ) | (9,838 | ) | (3,268 | ) | 5,275 | 5,437 | ||||||||||||||||||||||
| 1,947,954 | 1,782,108 | 1,747,205 | 1,752,651 | 1,649,660 | ||||||||||||||||||||||||||
| Income before taxes | 328,684 | 313,282 | 246,188 | 281,002 | 251,860 | |||||||||||||||||||||||||
| Taxes on income | 81,556 | 86,782 | 53,122 | 84,931 | 79,263 | |||||||||||||||||||||||||
| Net income | $ | 247,128 | $ | 226,500 | $ | 193,066 | $ | 196,071 | $ | 172,597 | ||||||||||||||||||||
| Percentage of net sales | 10.9 | 10.8 | 9.7 | 9.6 | 9.1 | |||||||||||||||||||||||||
| Percentage of average shareholders’ equity | 32.5 | 24.9 | 21.1 | 23.7 | 26.2 | |||||||||||||||||||||||||
| Net income per share – basic | $ | 2.86 | $ | 2.50 | $ | 2.06 | $ | 2.08 | $ | 1.84 | ||||||||||||||||||||
| Net income per share – diluted | $ | 2.82 | $ | 2.48 | $ | 2.04 | $ | 2.05 | $ | 1.83 | ||||||||||||||||||||
| Average number of shares (thousands) | 86,541 | 90,443 | 93,584 | 94,143 | 93,718 | |||||||||||||||||||||||||
| Consolidated Balance Sheet Data | ||||||||||||||||||||||||||||||
| Cash and short-term investments | $ | 152,075 | $ | 115,112 | $ | 272,897 | $ | 32,995 | $ | 12,555 | ||||||||||||||||||||
| Receivables, net | 450,579 | 405,302 | 368,519 | 358,361 | 339,725 | |||||||||||||||||||||||||
| Inventories | 484,222 | 446,606 | 430,794 | 457,204 | 454,631 | |||||||||||||||||||||||||
| Property, plant and equipment, net | 508,820 | 495,124 | 499,145 | 501,334 | 510,612 | |||||||||||||||||||||||||
| Goodwill and intangible assets, net | 732,836 | 745,716 | 772,651 | 789,676 | 799,413 | |||||||||||||||||||||||||
| Total assets(d) | 2,726,788 | 2,478,904 | 2,638,196 | 2,363,294 | 2,306,892 | |||||||||||||||||||||||||
| Bank borrowings, overdrafts and current portion of long-term debt | 152,473 | 15,897 | 819,392 | 15,957 | 194,304 | |||||||||||||||||||||||||
| Long-term debt | 1,060,168 | 791,443 | 131,281 | 668,969 | 690,231 | |||||||||||||||||||||||||
| Shareholders’ equity(b)(c)(d) | 617,197 | 905,168 | 915,347 | 910,487 | 742,631 | |||||||||||||||||||||||||
| Other Data | ||||||||||||||||||||||||||||||
| Current ratio(e) | 2.2 | 2.4 | 1.0 | 2.4 | 1.7 | |||||||||||||||||||||||||
| Gross additions to property, plant and equipment | $ | 65,614 | $ | 58,282 | $ | 93,433 | $ | 70,607 | $ | 65,955 | ||||||||||||||||||||
| Depreciation and amortization expense | 82,788 | 89,733 | 91,928 | 90,996 | 86,721 | |||||||||||||||||||||||||
| Cash dividends declared | 76,465 | 68,956 | 68,397 | 64,789 | 59,032 | |||||||||||||||||||||||||
| per share | $ | 0.880 | $ | 0.765 | $ | 0.730 | $ | 0.685 | $ | 0.630 | ||||||||||||||||||||
| Number of shareholders of record at year-end | 3,248 | 3,393 | 3,207 | 3,419 | 3,655 | |||||||||||||||||||||||||
| Number of employees at year-end | 5,315 | 5,087 | 5,160 | 5,212 | 5,454 | |||||||||||||||||||||||||
| (a) | Restructuring and other charges ($1,982 after tax) in 2006, ($15,857 after tax) in 2005, ($20,370 after tax) in 2004, and ($27,514 after tax) in 2003 were the result of various reorganization programs of the Company. |
| (b) | 2006 and 2007 amounts include equity compensation expense in accordance with FAS123(R). See Note 11 to the Consolidated Financial Statements for additional details. |
| (c) | The 2006 amounts reflect adoption of FAS158. See Note 13 to the Consolidated Financial Statements for additional details. |
| (d) | The 2007 amounts reflect adoption of FIN 48. See Note 9 to the Consolidated Financial Statements for additional details. |
| (e) | Current ratio is equal to current assets divided by current liabilities. |
15
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
(UNLESS INDICATED OTHERWISE, DOLLARS IN MILLIONS EXCEPT PER SHARE AMOUNTS)
Organization of Information
Management’s Discussion and Analysis provides a narrative on our operating performance, financial condition and liquidity and should be read in conjunction with the accompanying financial statements. It includes the following sections:
| • | Executive Overview |
| • | Sales Commentary |
| • | Consolidated Operating Results |
| • | Goodwill and Intangible Assets |
| • | Restructuring and Other Charges |
| • | Income Taxes |
| • | Retirement Benefits |
| • | Financial Condition |
| • | Critical Accounting Policies and Use of Estimates |
| • | New Accounting Standards |
| • | Non-GAAP Financial Measures |
| • | Cautionary Statement Under the Private Securities Litigation Reform Act of 1995 |
Executive Overview
We are a leading creator and manufacturer of flavor and fragrance compounds used to impart or improve the flavor or fragrance in a wide variety of consumer products. The precise size of the global market for flavors and fragrances is difficult to determine because the industry is highly fragmented, both geographically and along product lines; there are a limited number of publicly traded companies in the industry; certain customers maintain in-house capabilities fulfilling a portion of their flavor or fragrance needs; and the quality and depth of market information in developing regions of the world is limited. Analysts generally estimate the global market to be $11 - $12 billion of which IFF represents 17-19%; the largest competitor in the industry has approximately a 25% market share. Currently, the largest companies in the industry combined represent approximately 65% of the global market.
Effective January 1, 2007, IFF reorganized into two units that reflect our flavor and fragrance businesses. Approximately 44% of our 2007 net sales were flavor compounds. Flavor compounds are sold to the food and beverage industries for use in consumer products such as prepared foods, beverages, dairy, food and confectionery products. The remaining 56% of sales, representing the fragrances business unit, were in three fragrance categories: functional fragrances, including fragrance compounds for personal care (e.g., soaps) and household products (e.g., detergents and cleaning agents); fine fragrance and beauty care, including perfumes, colognes and toiletries; and ingredients, consisting of synthetic ingredients that can be combined with other materials to create unique functional and fine fragrance compounds. Major fragrance customers include the cosmetics industry, including perfume and toiletries manufacturers, and the household products industry, i ncluding manufacturers of soaps, detergents, fabric care, household cleaners and air fresheners. Approximately 55% of our ingredient production is consumed internally; the balance is sold to third party customers.
Changing social habits resulting from such factors as changes in disposable income, leisure time, health concerns, urbanization and population growth stimulate demand for consumer products utilizing flavors and fragrances. These developments expand the market for products with finer fragrance quality, as well as the
16
Table of Contents
market for colognes and toiletries. Such developments also stimulate demand for convenience foods, soft drinks and low-fat and organic food products that must conform to expected tastes. These developments necessitate the creation and development of flavors and fragrances and ingredients that are compatible with newly introduced materials and methods of application used in consumer products.
Flavors and fragrances are generally:
| • | created for the exclusive use of a specific customer; |
| • | sold in solid, powder or liquid form, in amounts ranging from a few pounds to several tons depending on the nature of the end product in which they are used; |
| • | a small percentage of the volume and cost of the end product sold to the consumer; and |
| • | a major factor in consumer selection and acceptance of the product. |
The flavor and fragrance industry is impacted by macroeconomic factors in all product categories and geographic regions. Such factors include the impact of currency on the price of raw materials and operating costs as well as on translation of reported results. In addition, pricing pressure placed on our customers by large and powerful retailers and distributors is inevitably passed along to us, and our competitors. Leadership in innovation and creativity mitigates the impact of pricing pressure. Success and growth in the industry is dependent upon creativity and innovation in meeting the many and varied needs of the customers’ products in a cost-efficient and effective manner, and with a consistently high level of timely service and delivery.
We produce more than 33,000 unique compounds, of which approximately 60% are flavors and 40% fragrances. We continually create new compounds to meet the changing characteristics and needs of our customers’ end products. No single compound represents more than 1.5% of net sales. Development of fragrances and flavors is a complex artistic and technical process calling upon the combined knowledge and talents of creative perfumers and flavorists, and application and research chemists. An important element of creation is the development of new ingredients. We bear essentially all costs incurred in connection with the creation and development of new flavors and fragrances and such formulae are generally protected under trade secrecy. We are not materially dependent on any patents, trademarks or licenses.
Our strategic direction is defined by the following:
| • | Be a global leader in fragrances and flavors. |
| • | Provide our customers with differentiated solutions. |
Our plan to achieve this strategy is to:
| • | Execute on our business unit focus that will align management and resources with the needs of our strategic customers and provide greater accountability; this will drive improved results. |
| • | Focus our research and development efforts on projects considered most likely to drive future profitable growth. We anticipate much of this research will be conducted internally, but such efforts may be augmented by joint research undertakings and through acquisition of technology. |
| • | Provide quality, safe and suitable products, for inclusion in our customers’ end products; an essential element is the consistent assurance of the quality and safety of raw materials through a combination of steps including but not limited to vendor certification and quality assurance testing. |
| • | Continuously improving our operations and customer service, and related initiatives. |
| • | Build a culture that attracts, retains and develops the best talent in the world. Our customers, stakeholders and employees expect the best. |
As implementation of the strategy progresses, setting strategic initiatives requires regular establishment and reassessment of priorities and necessitates choices in order to provide the best opportunity for continuous improvement in shareholder value.
17
Table of Contents
Sales Commentary
A breakdown of sales by principal product category is depicted in the graph below.
2007 Sales by Category

Our five largest customers comprise 32% of consolidated sales and our top 30 customers 57%; these percentages have remained fairly constant for several years although sales to larger customers are trending higher. We have one customer that accounts for 11% of our sales. A key factor for commercial success is inclusion on the strategic customers’ core supplier lists, opening opportunities to win new business. We are on the core supplier lists of a majority of our strategic customers.
Net sales by business unit for 2007, 2006 and 2005 were as follows:
| Net Sales | 2007 | Percent Change | 2006 | Percent Change | 2005 | |||||||||||||||||||||||||
| Flavors | $ | 1,006 | 12 | % | $ | 895 | 4 | % | $ | 858 | ||||||||||||||||||||
| Fragrances | 1,271 | 6 | % | 1,200 | 6 | % | $ | 1,135 | ||||||||||||||||||||||
| Total net sales | $ | 2,277 | 9 | % | $ | 2,095 | 5 | % | $ | 1,993 | ||||||||||||||||||||
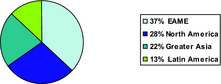
2007 Sales by Destination
We currently manage our operations by business unit but consider destination sales a supplemental performance measure. Although reported sales and earnings are affected by the weakening or strengthening of the U.S. dollar, this has not had a long-term effect on the underlying strength of our business.
18
Table of Contents
Net sales by destination for 2007, 2006 and 2005 were as follows:
| Sales by Destination | 2007 | Percent Change | 2006 | Percent Change | 2005 | |||||||||||||||||||||||||
| EAME(1) | $ | 850 | 12 | % | $ | 758 | 3 | % | $ | 739 | ||||||||||||||||||||
| North America | 630 | 3 | % | 612 | 7 | % | 572 | |||||||||||||||||||||||
| Greater Asia | 491 | 12 | % | 439 | 5 | % | 420 | |||||||||||||||||||||||
| Latin America | 306 | 7 | % | 286 | 9 | % | 262 | |||||||||||||||||||||||
| Total net sales, as reported | $ | 2,277 | 9 | % | $ | 2,095 | 5 | % | $ | 1,993 | ||||||||||||||||||||
| (1) | Europe, Africa and Middle East |
2007 in Comparison to 2006
Sales totaled $2,277 million, up 9% from 2006; flavor and fragrance sales increased 12% and 6%, respectively. 2007 sales benefited from the generally weaker U.S. dollar and at comparable exchange rates would have increased 5% over the prior year.
Flavors Business Unit
Flavors delivered strong sales performance across all regions – most notably in Latin America, Greater Asia and Europe – and in virtually all categories, particularly beverages and savory.
Fragrances Business Unit
Total Fragrance sales increased by 6% for the year and were driven by continued growth in Fine and Beauty Care of 8% and Ingredients of 9%, despite a decline in Ingredients pricing. Foreign exchange accounted for 4% of the sales increase.
Sales By Region and Category
Regional and product category sales performance for 2007 compared to the prior year, in reported dollars and local currency, was as follows:
| 2007 vs. 2006 | |||||||||||||||||||||||||||||||||||||||
| Percent Change in Sales by Region of Destination | |||||||||||||||||||||||||||||||||||||||
| Fine & Beauty Care | Functional | Ingredients | Total Frag. | Flavors | Total | ||||||||||||||||||||||||||||||||||
| North America | Reported | 4 | % | -1 | % | 1 | % | 2 | % | 4 | % | 3 | % | ||||||||||||||||||||||||||
| EAME | Reported | 8 | % | 12 | % | 17 | % | 12 | % | 13 | % | 12 | % | ||||||||||||||||||||||||||
| Local Currency | 0 | % | 4 | % | 8 | % | 3 | % | 5 | % | 4 | % | |||||||||||||||||||||||||||
| Latin America | Reported | 11 | % | -7 | % | 3 | % | -1 | % | 27 | % | 7 | % | ||||||||||||||||||||||||||
| Greater Asia | Reported | 16 | % | 2 | % | 4 | % | 6 | % | 16 | % | 12 | % | ||||||||||||||||||||||||||
| Local Currency | 13 | % | 1 | % | 4 | % | 4 | % | 12 | % | 9 | % | |||||||||||||||||||||||||||
| Total | Reported | 8 | % | 3 | % | 9 | % | 6 | % | 12 | % | 9 | % | ||||||||||||||||||||||||||
| Local Currency | 4 | % | 0 | % | 5 | % | 2 | % | 9 | % | 5 | % | |||||||||||||||||||||||||||
| • | North America fine fragrance growth was driven by new product introductions of $20 million partially offset by volume declines. Ingredients volume growth was partially offset by pricing declines. The decline in Functional fragrances was mainly volume related. Flavors sales growth was driven by new product introductions of $20 million mainly in the beverage and savory categories. |
| • | EAME flavor sales growth resulted mainly from new product introductions of $25 million. Functional fragrance growth was strong primarily due to new product introductions of $23 million partially offset by volume declines and to a lesser extent lower pricing. The growth in fine fragrance related to new product introductions of $28 million was offset by volume declines. Ingredients sales growth was volume related, partially offset by lower pricing. |
19
Table of Contents
| • | Latin America sales growth reflects strong performances in both flavors and fine fragrances. Flavors growth was driven by new product introductions of $21 million. Fine fragrances sales growth is largely attributable to new product introductions of $9 million. Functional fragrance performance was primarily volume related in the fabric care category; we saw some reversal of this trend in the fourth quarter as a result of new product introductions. Ingredients sales performance was largely due to volume increases partially offset by price declines. |
| • | Greater Asia sales growth was driven by new product introductions of $35 million in flavors. Fragrance sales growth was driven by fine fragrances as a result of new product introductions of $4 million. |
2006 in Comparison to 2005
In 2006, sales increased 5% in both reported dollars and local currency compared to 2005. Foreign exchange had no impact on sales growth over 2005.
Flavors Business Unit
Flavors sales grew by 4% driven by new product introductions and volume growth most notably in Latin America.
Fragrances Business Unit
Total Fragrance sales increased by 6% for the year and were driven by continued growth in Fine and Beauty Care of 13% primarily as a result of new product introductions.
Sales By Region and Category
Regional and product category sales performance for 2006 compared to the prior year, in reported dollars and local currency, was as follows:
| 2006 vs. 2005 | |||||||||||||||||||||||||||||||||||||||
| % Change in Sales by Region of Destination | |||||||||||||||||||||||||||||||||||||||
| Fine & Beauty Care | Functional | Ingredients | Total Frag. | Flavors | Total | ||||||||||||||||||||||||||||||||||
| North America | Reported | 20 | % | 2 | % | 10 | % | 10 | % | 3 | % | 7 | % | ||||||||||||||||||||||||||
| EAME | Reported | 7 | % | 5 | % | -4 | % | 4 | % | 1 | % | 3 | % | ||||||||||||||||||||||||||
| Local Currency | 8 | % | 5 | % | -3 | % | 4 | % | 1 | % | 3 | % | |||||||||||||||||||||||||||
| Latin America | Reported | 25 | % | 2 | % | 6 | % | 8 | % | 11 | % | 9 | % | ||||||||||||||||||||||||||
| Greater Asia | Reported | 8 | % | 2 | % | 7 | % | 4 | % | 5 | % | 4 | % | ||||||||||||||||||||||||||
| Local Currency | 7 | % | 1 | % | 10 | % | 4 | % | 6 | % | 5 | % | |||||||||||||||||||||||||||
| Total | Reported | 13 | % | 3 | % | 3 | % | 6 | % | 4 | % | 5 | % | ||||||||||||||||||||||||||
| Local Currency | 13 | % | 3 | % | 3 | % | 6 | % | 4 | % | 5 | % | |||||||||||||||||||||||||||
| • | North America fine fragrance and flavors increased primarily from new product introductions of $25 million and $21 million, respectively. Functional fragrances new product introductions totaled $14 million, the benefit of which was partially offset by declines in volume. Ingredient sales growth was due to a combination of both volume and price. |
| • | EAME growth was strongest in Eastern Europe, Middle East, France, Italy and Spain. Fine and functional fragrance growth resulted from new product introductions of $33 million, in each category, while the decline in ingredients was volume related. Flavor growth was the result of new product introductions of $11 million, partially offset by declines in volume. |
| • | Latin America fine fragrance sales growth resulted from new product introductions of $14 million while functional fragrance product introductions of $9 million were partially offset by volume decreases. Ingredient sales growth resulted from new product introductions. Flavor sales were strong throughout the region, driven mainly by new product introductions of $8 million. |
| • | Greater Asia fragrance sales growth resulted mainly from new product introductions of $4 million and volume growth in India; ingredients sales growth was mainly volume related. Flavor sales growth resulted from new product introductions of $11 million and volume growth. |
20
Table of Contents
Consolidated Operating Results
The percentage relationship of cost of goods sold and other operating expenses to reported sales is detailed as follows:
| 2007 | 2006 | 2005 | ||||||||||||||||
| Cost of goods sold | 58.2 | % | 57.8 | % | 58.6 | % | ||||||||||||
| Research and development expenses | 8.7 | % | 8.9 | % | 9.0 | % | ||||||||||||
| Selling and administrative expenses | 16.5 | % | 16.8 | % | 17.0 | % | ||||||||||||
Cost of goods sold includes the cost of materials and manufacturing expenses; raw materials generally constitute 70% of the total. Research and development expenses are for the development of new and improved products, technical product support, compliance with governmental regulations, and help in maintaining relationships with customers who are often dependent on technological advances. Selling and administrative expenses support our sales and operating levels.
2007 in Comparison to 2006
Cost of goods sold, as a percentage of sales, was 58.2% compared with 57.8% in 2006. This increase was mainly as a result of product mix, notably higher sales of fragrance ingredients and flavor compounds. Lower selling prices for fragrance ingredients, some impact of higher material costs and under absorption of manufacturing costs at a new fragrance ingredient facility in China, which scaled up production in 2007, also contributed to the increase. The average cost of raw materials increased 2-3% over the prior year.
Research and Development expense, as a percentage of sales, was 8.7%, comparable to the prior year levels.
Selling and administrative expenses, as a percentage of sales, were 16.5% in the current period compared to 16.8% in the prior year period, reflecting good cost control and the benefit of headcount reductions that occurred in the first half of 2006. Selling and administrative expenses in 2007 include $4 million of business transformation costs to enable us to better leverage our global SAP software platform; such costs are not expected to recur in 2008. The 2006 results also included the benefit of a $3 million insurance recovery related to a 2005 product contamination matter; excluding the insurance recovery, 2006 Selling and administrative expenses would have been 16.9% of sales for the year.
Interest Expense
In 2007, interest expense totaled $42 million, increasing 63% compared to 2006, due to higher borrowings incurred in connection with share repurchase activities. See Note 10 to the Consolidated Financial Statements for further discussion of the share repurchase activities. Average cost of debt was 4.5% for 2007 compared to 3.3% in 2006.
Other (Income) Expense, Net
Other (income) expense, net in 2007 increased $1 million over the prior year, mainly due to favorable exchange results and higher interest income as a result of higher interest rates. Other income included $11 million and $15 million, in 2007 and 2006, respectively, primarily related to gains on asset sales.
Income Taxes
The effective tax rate was 24.8% in 2007 as compared to a rate of 27.7% in the prior year. Both the 2007 and 2006 rates benefited from favorable tax rulings with respect to prior years; excluding the benefit of these rulings from both years, the 2007 effective tax rate would have been 27.8% compared to a rate of 28.8% for 2006. The lower effective tax rate for the current year was largely the result of a greater percentage of consolidated pre-tax earnings in lower tax jurisdictions.
Operating Results by Business Unit
We evaluate the performance of business units based on operating profit before gains/losses on the disposition of assets, interest expense, other income (expense), net and income taxes. See Note 12 to our Consolidated Financial Statements for the reconciliation to Income before taxes.
21
Table of Contents
Flavors Business Unit
In 2007, Flavors operating profit of $187 million or 18.6%, as a percentage of sales, increased as compared to $153 million or 17.1% in 2006. The amount reported in 2006 benefited from a $3 million insurance recovery related to a 2005 product contamination matter; excluding the insurance recovery from the prior year comparative, Flavors profitability would have increased an additional 30 basis points over 2006. This profitability improvement was primarily driven by margin improvement enabled by strong sales growth, favorable product mix, increased absorption of manufacturing expenses and good cost control.
Fragrances Business Unit
In 2007, Fragrance operating profit of $210 million or 16.5%, as a percentage of sales, declined from the $212 million or 17.7% reported in 2006. Profitability was negatively impacted by lower selling prices of fragrance ingredients, higher material costs and underabsorption of manufacturing costs related to scaling up the China facility in 2007.
Global Expenses
Global expenses represent corporate and headquarters-related expenses which include legal, finance, human resources and other administrative expenses that are not allocated to an individual business unit, as well as gains on asset sales and a pension curtailment charge. In 2007, Global expenses increased $9 million to $27 million from $18 million incurred in 2006. In 2007, Global expenses included approximately $6 million of curtailment loss as a result of changes to the U.S. defined benefit pension plan and approximately $11 million related to gains on asset sales. The 2006 expenses included approximately $15 million of other income primarily related to gains on asset sales.
2006 in Comparison to 2005
Cost of goods sold, as a percentage of sales, decreased 80 basis points compared with 2005, mainly as a result of the improved sales performance leading to better absorption of manufacturing expenses, and favorable product mix. We also benefited from the elimination of 69 manufacturing positions in 2005, mainly in North America and Europe, which resulted in savings of $4 million. The average cost of raw materials increased 2-3% over the prior year.
Research and development expenses were 8.9% of sales, in-line with our objective of spending approximately 9% of revenues on research and development annually.
Selling and administrative expenses were 16.8% of sales compared to 17.0% in 2005. The 2005 results included $8 million relating to a product contamination matter; 2006 results reflect the benefit of a $3 million insurance recovery related to this contamination matter. We benefited from the elimination of 129 positions, mainly in North America and Europe, which resulted in savings of $8 million. The 2006 period also included $31 million in incentive compensation expense driven by improved sales and operating performance; the 2005 results included $5 million of such expense.
Interest Expense
Interest expense totaled $26 million and $24 million in 2006 and 2005, respectively. The average interest rate remained at 3.3% for both years. The interest rate for each period reflects our debt and interest rate management plans. Additional details are contained in Note 8 of the Notes to the Consolidated Financial Statements.
Other (Income) Expense, Net
Other (income) expense, net was $10 million of income in 2006 and $3 million of expense in 2005. In 2006, income resulted from gains on asset sales of $18 million, partially offset by higher exchange losses and other non-operating expenses. In 2005, income resulted primarily from exchange gains and higher levels of interest income earned on higher cash balances. Exchange (losses) or gains were $(7) million and $3 million in 2006 and 2005, respectively. The exchange losses in 2006 were mainly the result of having U.S. dollar positions in Europe and Latin America which resulted in exchange losses upon the weakening of the U.S. dollar in comparison to the Euro and other currencies.
22
Table of Contents
Income Taxes
The effective tax rate for 2006 was 27.7% and 21.6% for 2005. Our effective tax rate fluctuates as a result of earnings in the countries in which we operate. The 2005 rate was significantly reduced as a result of a tax benefit associated with the American Jobs Creation Act (‘‘AJCA’’), which provided for a special one-time tax deduction of 85% of dividends received on eligible repatriated foreign earnings. Tax expense in 2005 reflects a benefit of $25 million relating to our repatriation of $242 million of dividends under the provisions of AJCA. Excluding the benefit of AJCA, the effective tax rate for 2005 would have been 31.6%.
Operating Results by Business Unit
Flavors Business Unit
Flavors operating profit of $153 million or 17.1%, as a percentage of sales, increased as compared to $108 million or 12.6% in 2005. The 2005 results included $8 million relating to a product contamination matter; 2006 results reflect the benefit of a $3 million insurance recovery related to this contamination matter. The 2005 results also included approximately $6 million of restructuring charges. This profitability improvement was partially the result of increased sales volume leading to better absorption of manufacturing expenses, and favorable product mix. Selling and administrative expenses declined substantially from 2005 largely as a result of a reduction in headcount related to the restructuring more fully described below, while research and development expenses remained constant with 2005. Offsetting the benefit of the headcount reduction was approximately a $6 million increase in incentive compensation as a result of impro ved operating results.
Fragrances Business Unit
Fragrance operating profit of $212 million increased from the $187 million reported in 2005, with a corresponding increase in operating profit, as a percentage of sales, to 17.7% in 2006 from 16.4% in 2005. The 2005 results included approximately $10 million in restructuring charges compared to $3 million in 2006. Higher sales volume led to increased absorption of manufacturing expenses, which was partially offset by higher raw material costs, resulting in improved profitability. Spending on research and development remained fairly constant between the two years while selling and administrative expenses declined as a result of the headcount reduction related to the restructuring discussed below. Offsetting the benefit of the headcount reduction was approximately a $10 million increase in incentive compensation as a result of improved operating results.
Global Expenses
The Global expense caption represents corporate and headquarters-related expenses which include legal, finance, human resources and other administrative expenses that are not allocated to individual business unit, as well as gains on asset sales. Global expenses increased by $9 million in 2006 over 2005, primarily due to approximately $11 million of additional incentive compensation in 2006. 2005 Global expenses included $7 million in restructuring charges.
23
Table of Contents
Goodwill and Intangible Assets
At December 31, 2007 and 2006, goodwill and other intangible assets, net of accumulated amortization, totaled $733 million and $746 million, respectively. We completed our annual assessments in 2007 and 2006 concluding there was no impairment of goodwill or other intangible assets. Additional details are contained in Note 4 to the Consolidated Financial Statements.
We perform a goodwill impairment test on at least an annual basis or more frequently in certain circumstances, by assessing the fair value of our reporting units based upon both discounted cash flow and comparison of multiples of comparable companies. We deem goodwill to be impaired if the carrying amount of the reporting unit exceeds the estimated fair value. We completed our annual assessments in 2007 and 2006, concluding there was no impairment of goodwill.
Other intangible assets include patents, trademarks and other intellectual property, valued at acquisition, primarily through independent appraisals, which are amortized on a straight-line basis over periods ranging from 7 to 20 years. We review our other intangible assets for impairment when events or changes in business conditions indicate that their full carrying value may not be recovered.
Restructuring and Other Charges
With respect to the restructuring and other charges:
| • | Separation costs for employees relate primarily to severance, outplacement and other benefit costs; |
| • | Asset write-down charges relate to establishment of the new carrying value for assets held for sale or disposal; and |
| • | Other costs include lease termination costs and other reorganization expenses incurred to effect either the employee separation or location closure. |
In 2005, we undertook to eliminate 300 positions in manufacturing, selling, research and administration functions, principally in our European and North American operating regions. The majority of affected positions involve employee separation while the balance related to open positions that are not expected to be filled. We recorded pre-tax restructuring charges relating primarily to employee separation expenses of $23 million in 2005 and $3 million in 2006. This plan is essentially complete. Annual savings from these actions approximated $16-$18 million.
Positions eliminated, including those not replaced by the 2005 program, and charges by business segment in 2006 and 2005 are detailed in the table below; there were no such actions undertaken in 2007 nor charges incurred.
Restructuring Charges
| ( In Thousands) | Positions Eliminated | |||||||||||||||||||||||
| 2006 | 2005 | 2006 | 2005 | |||||||||||||||||||||
| Flavors | $ | (463 | ) | $ | 6,293 | 19 | 148 | |||||||||||||||||
| Fragrances | 2,639 | 9,617 | 29 | 155 | ||||||||||||||||||||
| Global | 504 | 7,409 | 10 | 126 | ||||||||||||||||||||
| Total | $ | 2,680 | $ | 23,319 | 58 | 429 | ||||||||||||||||||
24
Table of Contents
Movements in related accruals in each of the three years in the period ended December 31, 2007 were:
| (In Millions) | Employee- Related | Asset- Related and Other | Total | |||||||||||||||
| Balance January 1, 2005 | $ | 29 | $ | 14 | $ | 43 | ||||||||||||
| Additional charges | 22 | 1 | 23 | |||||||||||||||
| Cash and other costs | (21 | ) | (11 | ) | (32 | ) | ||||||||||||
| Balance December 31, 2005 | 30 | 4 | 34 | |||||||||||||||
| Additional charges | 4 | (1 | ) | 3 | ||||||||||||||
| Cash and other costs | (21 | ) | (1 | ) | (22 | ) | ||||||||||||
| Balance December 31, 2006 | 13 | 2 | 15 | |||||||||||||||
| Cash and other costs | (10 | ) | (2 | ) | (12 | ) | ||||||||||||
| Balance December 31, 2007 | $ | 3 | $ | — | $ | 3 | ||||||||||||
The remaining employee-related liabilities are expected to be utilized in 2008 as obligations are satisfied.
Income Taxes
In September 2006, the FASB issued Interpretation No. 48 (‘‘FIN 48’’), Accounting for Uncertainty in Income Taxes, which clarifies the application of FAS 109 by prescribing the minimum threshold a tax position must meet before being recognized in the financial statements. The adoption of FIN 48 did not have a material impact on the Consolidated Financial Statements. See Note 9 to the Consolidated Financial Statements for more information.
Retirement Benefits
In 2007, we amended our U.S. salaried qualified and non-qualified pension plans under which accrual of future benefits was suspended for all participants that did not meet the rule of 70 (age plus years of service equal at least 70) at December 31, 2007. As a result of this suspension, we recorded a curtailment loss of $5.9 million to recognize a portion of the unrecognized prior service costs associated with years of service no longer expected to be rendered and credited as service under the plans. We also introduced an enhanced defined contribution plan for those employees affected by the pension curtailment that will become effective January 1, 2008. As a result of these events, we expect to realize a net annual cost savings of approximately $4 million beginning in 2008.
Financial Condition
Cash, cash equivalents and short-term investments totaled $152 million at December 31, 2007 compared to $115 million and $273 million at December 31, 2006 and 2005, respectively. Working capital totaled $652 million at year-end 2007 compared to $633 million and ($11) million at December 31, 2006 and 2005, respectively. The increase in working capital was primarily related to increases in accounts receivable and inventories partially offset by an increase of $137 million in current debt largely as a result of borrowings that mature in November 2008. The 2005 working capital balance resulted from the classification of certain debt as current due to its maturity in May 2006; such debt was refinanced during 2006. Gross additions to property, plant and equipment were $66 million, $58 million and $93 million in 2007, 2006 and 2005, respectively, and are expected to approximate $90&nbs p;million in 2008.
Our financial condition continues to be of high quality, as evidenced by substantial cash flow from operations and ready access to capital markets at competitive rates. Operating cash flow provides the primary source of funds for operating and capital needs as well as dividends paid to shareholders and share repurchase activities. We anticipate that cash flows from operations and availability under our existing credit facilities are expected to be sufficient to fund our currently anticipated capital spending and other currently expected cash requirements for at least the next eighteen months.
Operating cash flow in 2007 was $314 million compared to $282 million and $177 million in 2006 and 2005, respectively. Operating cash flows in 2006 were revised from the $263 million previously reported. Amounts
25
Table of Contents
previously reported had been reduced by the purchase of investments of $18 million and deferred financing costs totaling $1 million that were determined to be more appropriately classified as investing and financing activities, respectively; 2005 operating cash flows were unaffected by the revision. The increase in operating cash flow in 2007 compared to the prior year was primarily the result of higher net income; net earnings adjusted for non-cash items (primarily depreciation, amortization, and equity-based compensation) was the primary source of operating cash flows.
Operating cash flow increased in 2006 as compared to 2005 as a result of improved sales and operating results; net earnings adjusted for non-cash items (primarily depreciation, amortization and equity-based compensation) was the primary source of operating cash flows. Improvements in inventory and current payables were sufficient to offset an increase in receivables; the increases in receivables and payables supported the business growth achieved in 2006.
Net investing activities in 2007 utilized $51 million compared to $48 million and $91 million in 2006 and 2005, respectively. The 2006 cash used in investing activities increased $18 million as a result of revising the cash flow for certain investing and financing activities as discussed above. Investing cash outflows increased primarily due to purchase of investments of $13 million partially offset by proceeds from investment sales of $10 million.
In 2007 and 2006, we realized cash proceeds from the sale of assets. In both years, the proceeds related to the sale of land and buildings; to the extent such assets had been written down in connection with previous restructuring activities, any gain or loss on disposition was accounted for as part of the restructuring activities. All transactions were with third party investors and we retained no ownership interest in any of the disposed assets.
Compliance with existing governmental requirements regulating the discharge of materials into the environment has not materially affected our operations, earnings or competitive position. In 2007, we spent $3 million on capital projects and $18 million in operating expenses and governmental charges for the purpose of complying with such regulations. Expenditures for these purposes will continue for the foreseeable future. In addition, we are party to a number of proceedings brought under the Comprehensive Environmental Response, Compensation and Liability Act or similar state statutes. It is expected that the impact of any judgments in or voluntary settlements of such proceedings will not be material to our financial condition, results of operations or liquidity.
The dividend paid per share in 2007, 2006 and 2005 was $.86, $.74 and $.72, respectively. In January, April and July 2007, we paid a quarterly cash dividend of $.21 per share to shareholders. In July 2007, we announced a 10% increase in our quarterly dividend rate to $.23 per share effective with the dividend payable in October 2007. We paid dividends totaling $77 million, $67 million and $68 million in 2007, 2006 and 2005. Our current intention is to pay dividends approximating 30 − 35% of yearly earnings; however, the payment of dividends is determined by the Board at its discretion based on various factors, and no assurance can be provided as to future dividends.
We supplement short-term liquidity with access to capital markets, mainly through the issuance of commercial paper, and with bank credit facilities. In 2005, we, including certain subsidiaries, entered into a revolving credit agreement (the ‘‘Facility’’) with certain banks. The Facility provides for a five-year U.S. $350 million (‘‘Tranche A’’) and Euro 400 million (‘‘Tranche B’’) multi-currency revolving credit facility. Tranche A is available to IFF for commercial paper backstop and general corporate purposes; Tranche B is available to both IFF and the European subsidiaries for general corporate purposes. Borrowings under the Facility bear interest at an annual rate of LIBOR (London Inter Bank Offer Rate) (or in relation to any Euro-denominated loans, EURIBOR, European Inter Bank Offer Rate) plus a margin, currently 20 basis points, linked to our credit rating. We pay a commitmen t fee on the aggregate unused commitments and a utilization fee based on amounts outstanding under the Facility; such fees are not material. As permitted by the Facility, in 2007, the termination dates were extended by one year until November 23, 2012. The Facility contains various affirmative and negative covenants customary in a facility of this type, including a covenant requiring us to maintain, at the end of each fiscal quarter, a ratio of net debt for borrowed money to EBITDA (Earnings Before Interest, Taxes, Depreciation and Amortization) in respect of the previous 12-month period of not more than 3.25 to 1. We have complied with this covenant at all times.
In addition, including our subsidiaries, we had unused lines of credit totaling $846 million at December 31, 2007 compared to $701 million at December 31, 2006.
26
Table of Contents
At December 31, 2007, we had $1,213 million of debt outstanding. On September 27, 2007, we issued $500 million of Senior Unsecured Notes (‘‘Senior Notes − 2007’’) in four series under the Note Purchase Agreement (‘‘NPA’’): (i) $250 million in aggregate principal amount of 6.25% Series A Senior Notes due September 27, 2017, (ii) $100 million in aggregate principal amount of 6.35% Series B Notes due September 27, 2019, (iii) $50 million in aggregate principal amount of 6.50% Series C Notes due September 27, 2022 and (iv) $100 million in aggregate principal amount of 6.79% Series D Notes due September 27, 2027. Proceeds were used primarily to fund repurchases of IFF stock (discussed below). Under the NPA, we may at any time, with notice, prepay all or a portion equal to or greater than 10% of the Notes, for an amount equal to the principal, accrued interest and a ‘‘make-whole’’ prepayment premium as calculated under the NPA. We may also prepay the Senior Notes − 2007 solely for the principal and accrued interest thereon in connection with certain asset sales. We will be required to make an offer to prepay the Senior Notes − 2007 following a change in control (as defined in the NPA) for an amount equal to the principal and accrued interest but without a ‘‘make-whole’’ or other premium.
In contemplation of this debt issuance, we entered into interest rate hedge contracts, with a notional amount of $400 million to manage our exposure to changes in interest rates. The contracts were designated as hedges of the variability of the cash flows due to changes in the long-term benchmark interest rates and the credit spread. We recorded a $1.6 million gain on the settlement of these interest rate hedge contracts, which coincided with the issuance of the long-term fixed-rate debt. For additional information regarding this transaction, see Note 8 to the Consolidated Financial Statements.
Pursuant to the NPA, we are required to maintain a consolidated net debt to consolidated EBITDA ratio of 3.5:1 (as calculated under the NPA), provided that such ratio may exceed 3.5:1 (but not 4:1) upon the payment of certain additional interest and provided further that such ratio does not exceed 3.5:1 for more than eight consecutive quarters. In addition, we are required not to permit the aggregate amount of our subsidiaries’ debt to exceed 20% of our consolidated total assets as calculated under the NPA, with certain exceptions. We, including our subsidiaries, will not incur any liens, subject to certain permitted categories of liens and a general lien allowance of $120 million. We also made certain affirmative and negative covenants relating to the operation of our business; material events such as asset sales and mergers, and the pari passu ranking of the Senior Notes-2007.
As a result of the additional debt, Moody’s and Standard & Poor’s (‘‘S&P’’) affirmed their short-term credit rating for IFF at P2 and A2, respectively, and Moody’s and S&P adjusted their long-term rating to Baa1 and BBB, respectively, with a stable outlook.
During the first six months of 2007, under a share repurchase program of $300 million authorized in October 2006 (the ‘‘October 2006 Plan’’), we repurchased approximately 1.6 million shares at a cost of $81 million; at June 30, 2007, we had approximately $125 million remaining under the October 2006 Plan. In July 2007, the October 2006 Plan was terminated and superseded by a new program authorized by our Board to repurchase up to 15% or $750 million worth of our outstanding common stock, whichever is less (the ‘‘July 2007 Plan’’).
In September 2007, under the July 2007 Plan, we entered into two agreements to purchase shares of our common stock under a $450 million accelerated share repurchase (‘‘ASR’’) program. On September 28, 2007, we paid $450 million in exchange for an initial delivery of 7.6 million shares under the ASR, representing 90% of the shares that could have been purchased, based on the prior closing price of our stock. The remaining 10%, or $45 million, not used in the initial settlement will be included in the determination of the cost of the shares purchased on completion of the ASR and was reflected in the accompanying Consolidated Balance sheet as a reduction to Capital in excess of par value.
On completion of the ASR, which is expected to be by the end of the second quarter of 2008, we may receive additional shares or may be required to pay cash or additional shares at our option, based on the volume-weighted average price (‘‘VWAP’’) of the common stock during the agreement term. For additional information regarding these transactions, see Note 10 to the Consolidated Financial Statements. Proceeds from the $500 million Senior Notes — 2007 described above funded the payment.
In the year ended December 31, 2007, we purchased 10.2 million shares at a cost of $532 million. In the year ended December 31, 2006, we purchased 6.9 million shares at a cost of $271 million.
27
Table of Contents
At December 31, 2007, we have contractual payment obligations due within the time periods as specified in the following table:
| Payments Due | ||||||||||||||||||||||||||||||
| Contractual Obligations | Total | 2008 | 2009-2010 | 2011-2012 | 2013 and thereafter | |||||||||||||||||||||||||
| Borrowings(1) | $ | 1,213 | $ | 153 | $ | 50 | $ | 276 | $ | 734 | ||||||||||||||||||||
| Interest on borrowings(1) | 571 | 63 | 120 | 109 | 279 | |||||||||||||||||||||||||
| Operating leases(2) | 293 | 24 | 41 | 34 | 194 | |||||||||||||||||||||||||
| Purchase commitments(3) | 19 | 19 | — | — | — | |||||||||||||||||||||||||
| Pension funding obligations(4) | 20 | 16 | 1 | 1 | 2 | |||||||||||||||||||||||||
| Post-retirement obligations(4) | 49 | 4 | 8 | 9 | 28 | |||||||||||||||||||||||||
| Total | $ | 2,165 | $ | 279 | $ | 220 | $ | 429 | $ | 1,237 | ||||||||||||||||||||
| (1) | See Note 8 to the Consolidated Financial Statements for a further discussion of our various borrowing facilities. |
| (2) | Operating leases include facility and other lease commitments executed in the normal course of the business. Additional details concerning the United States facilities are contained in Note 7 of the Notes to the Consolidated Financial Statements and further details concerning worldwide aggregate operating leases are contained in Note 16 of the Notes to the Consolidated Financial Statements. |
| (3) | Purchase obligations and capital project commitments are not recorded on our consolidated balance sheet. |
| (4) | See Note 13 to the Consolidated Financial Statements for a further discussion of our retirement plans. Anticipated funding obligations are based on current actuarial assumptions. Minimum funding requirements reported in the above table do not extend beyond 2016. |
Critical Accounting Policies and Use of Estimates
Our accounting policies are more fully described in Note 1 to the Consolidated Financial Statements. As disclosed in Note 1, the preparation of financial statements in conformity with U.S. generally accepted accounting principles (‘‘GAAP’’) requires management to make estimates and assumptions that affect reported amounts and accompanying disclosures. These estimates are based on management’s best judgment of current events and actions that we may undertake in the future. Actual results may ultimately differ from estimates.
Those areas requiring the greatest degree of management judgment or deemed most critical to our financial reporting involve:
The periodic assessment of potential impairment of intangible assets acquired in business combinations. We currently have net intangible assets, including goodwill, of $733 million. Goodwill is evaluated for impairment annually. In assessing goodwill, management uses the most current actual and forecasted operating data available, and current market based assumptions. A two-step approach is employed. The first step involves estimating the value of reporting units based on the present value of estimated future cash flows. The second step, if necessary, is to measure the value of the impairment loss, if any. Management’s most subjective assumptions relate to the estimated/projected sales and operating growth values employed in the forecast.
The analysis and evaluation of income taxes. We account for taxes in accordance with Statement of Financial Accounting Standard No. 109, ‘‘Accounting for Income Taxes’’ (‘‘FAS 109’’). Under FAS 109, deferred tax assets and liabilities are determined based on temporary differences between financial reporting and tax bases of assets and liabilities, based on tax laws as currently enacted. The provision for income taxes is based on statutory income taxes rates and planning opportunities available in the various tax jurisdictions where we operate. Significant judgment is required in determining income tax provisions and tax positions. We may be challenged upon review by the applicable taxing authority and positions taken by us may not be sustained. We regularly update these accr uals in light of changing facts and circumstances.
In 2007, we adopted SFAS No. 48, ‘‘Accounting for Uncertainty in Income Taxes’’, an interpretation of FAS 109 (‘‘FIN 48’’). As a result of adopting FIN 48, we recognized a $1 million increase in Other liabilities for unrecognized tax benefits and a corresponding cumulative effect adjustment to Retained earnings. Also as prescribed by FIN 48, certain tax related amounts in the Consolidated Balance Sheet are classified differently than in prior periods. Amounts receivable from various tax jurisdictions are now included in Other current assets and tax reserves previously classified as accrued taxes on income are now included in Other liabilities.
28
Table of Contents
The evaluation of potential legal and environmental liabilities, where changing circumstances, rules and regulations require regular reassessment of related practices and anticipated costs. We are subject to certain legal claims regarding products and other matters, as well as environmental-related matters. Significant management judgment is involved in determining when it is probable that a liability has been incurred and the extent to which it can be reasonably estimated.
We regularly assesses potential liabilities with respect to all legal claims based on the most recent available information, in consultation with outside counsel handling the defense of such matters. To the extent a liability is deemed to have been incurred and can be reasonably estimated, we recognize a corresponding liability; if the reasonably estimated liability is a range, we recognize that amount considered most likely, or in the absence of such a determination, the minimum reasonably expected liability. To the extent such claims are covered by various insurance policies, we separately evaluate the likelihood of recovery and account for any related insurance receivable. Management judgments involve determination as to whether a liability has been incurred, the reasonably estimated amount of that liability, and any potential insurance recovery.
We regularly evaluate potential environmental exposure in terms of total estimated cost and the viability of other potentially responsible parties (‘‘PRP’s’’) associated with our exposure. Recorded liabilities are adjusted periodically as remediation efforts progress and additional information becomes available. Critical management assumptions relate to expected total costs to remediate and the financial viability of PRP’s to share such costs.
Determination of the various assumptions employed in the valuation of pension and retiree health care expense and associated obligations. Amounts recognized in the Consolidated Financial Statements related to pension and other postretirement benefits are determined from actuarial valuations. Inherent in such valuations are assumptions including expected return on plan assets, discount rates at which the liabilities could be settled, rates of increase in future compensation levels, mortality rates and health care cost trend rates. These assumptions are updated annually and are disclosed in Note 13 to the Consolidated Financial Statements. In accordance with GAAP, actual results that differ from the assumptions are accumulated and amortized over future periods and, therefore, affect expense recognized and obligations record ed in future periods.
With respect to the U.S. plans, the expected return on plan assets was determined based on an asset allocation model using the current benchmark allocation, real rates of return by asset class and an anticipated inflation rate. The benchmark asset allocation was: 10 − 20% in cash and fixed income investments expected to yield 1.0%; 10 − 20% employed in corporate and government bonds expected to yield 2.1%; and 65 − 75% in equity investments with a long-term expected yield of 8.5 − 9.3%. The inflation rate assumed in the model was 2.5%. The plan has achieved a compounded annual rate of return of 9.6% over the previous 20 years. The expected annual rate of return for the non-U.S. plans employs a similar set of criteria adapted for local investments, inflation rates and in certain cases specific government requirements. The discount rate used for determining future pension obligations for each individual plan is based on a review of long-ter m bonds that receive a high rating from a recognized rating agency. Additionally, for the U.S. plans, the discount rate was based on the internal rate of return for a portfolio of Moody’s Aa3-rated high quality bonds with maturities that are consistent with the projected future benefit payment obligations of the plan. The rate of compensation increase for all plans and the medical cost trend rate for the applicable U.S. plans are based on plan experience.
Management establishes the assumptions concerning discount rates and actuarial assumptions based on current market conditions, including asset returns and other factors applicable under the circumstances. Changes in pension and other post-employment benefits, and associated expenses, may occur in the future due to changes in these assumptions. The impact that a .25% decrease in the discount rate or a 1% change in the medical cost trend rate would have on our pension and other post-employment benefit expense, as applicable, is discussed in Note 13 to the Consolidated Financial Statements.
The ongoing assessment of the valuation of inventory, given the large number of natural ingredients employed, the quality of which may be diminished over time. We maintain between 40% and 55% of our inventory as raw materials, providing the greatest degree of flexibility in manufacture and use. Materials are evaluated based on shelf life, known uses and anticipated demand based on forecasted customer order activity and changes in product/sales mix. Management policy provides for an ongoing assessment of inventory with adjustments recorded when an item is deemed to be slow moving or obsolete.
29
Table of Contents
Determination of various assumptions employed in the calculation of equity compensation expense. Amounts recognized in the Consolidated Financial Statements related to equity compensation are determined based on the number of awards and type of award as well as specific assumptions regarding expected life, stock price volatility, risk free interest rate, termination rates, exercise multiple and the dividend yield. These assumptions are employed in the Binomial model used to value certain awards. Management establishes the assumptions based on current market conditions and historical trends related to the equity awards.
We changed our valuation model used for estimating the fair value of options granted after January 1, 2006, from a Black-Scholes option-pricing model to a Binomial lattice-pricing model. This change was made in order to provide a better estimate of fair value since the Binomial model is a more flexible method for valuing employee stock options than the Black-Scholes model. The flexibility of the simulated Binomial model stems from the ability incorporate inputs that change over time, such as volatility and interest rates, and to allow for actual exercise behavior of option holders. We are using an average of implied and historical volatility while the expected term assumption was determined based on historical patterns.
Developing the assumptions used in the Binomial model requires significant judgment on our part and, generally, may involve analyzing available historical data, considering whether historical data is relevant to predicting future behavior, making appropriate adjustments to historical data for future expectations, supplementing or replacing company-specific historical data with data from other supportable sources and appropriately weighting each of the inputs. These assumptions are evaluated at each grant date. If factors change and we employ different assumptions for estimating share-based compensation expense in future periods or if we decide to use a different valuation model, the future periods may differ significantly from what we have recorded in the current period and could materially affect operating income, net income and net income per share.
We believe that we have considered relevant circumstances that we may be currently subject to, and the financial statements accurately reflect our best estimate of the results of our operations, financial condition and cash flows for the years presented. We have discussed the decision process and selection of these critical accounting policies with the Audit Committee of the Board of Directors.
New Accounting Standards
In September 2006, the FASB issued SFAS No. 157 (‘‘FAS 157’’), ‘‘Fair Value Measurements.’’ This Statement defines fair value, establishes a framework for measuring fair value and expands disclosures regarding fair value measurements. FAS 157 is effective for years beginning after December 15, 2007. We are currently assessing the impact of this interpretation, but we do not believe its adoption will have a material impact on our results or financial condition.
In February 2007, the FASB issued SFAS No. 159 ‘‘The Fair Value Option for Financial Assets and Liabilities — Including an amendment of FASB No. 115’’ (‘‘FAS 159’’). This standard allows companies to elect, at specific election dates, to measure eligible financial assets and liabilities at fair value that are not otherwise required to be measured at fair value. If a company elects the fair value option, subsequent changes in that item’s fair value must be recognized in current earnings. FAS 159 is effective for years beginning after November 15, 2007. We are currently evaluating the impact of this standard.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), ‘‘Business Combinations’’ (‘‘FAS 141(R)’’). In FAS 141(R), the FASB retained the fundamental requirements of Statement No. 141 to account for all business combinations using the acquisition method (formerly the purchase method) and for an acquiring entity to be identified in all business combinations. However, the new standard requires the acquiring entity in a business combination to recognize the assets acquired and liabilities assumed in the transaction; establishes the acquisition-date fair value as the measurement objective for all assets acquired and liabilities assumed; and requires the acquirer to disclose to investors and other users all of the information they need to evaluate and understand the nature and financial effect of the business combination. FAS 141(R) is effective for annual periods beginning on or after December 15, 2008. We have not yet determined the impact, if any, that FAS 141(R) will have on our results of operations or financial position.
Non-GAAP Financial Measures
The discussion of our historical results include and, where indicated, exclude the benefit of a 2006 insurance recovery relating to a product contamination issue, certain favorable tax rulings with respect to prior years and the
30
Table of Contents
impact of repatriation of certain dividends from foreign subsidiaries under the AJCA, as well as the effects of exchange rate fluctuations. Such information is supplemental to information presented in accordance with GAAP and is not intended to represent a presentation in accordance with GAAP. In discussing our historical and expected future results and financial condition, we believe it is meaningful for investors to be made aware of and to be assisted in a better understanding of, on a period-to-period comparative basis, such matters as the 2006 insurance recovery relating to a product contamination issue, repatriation of certain dividends from foreign subsidiaries under AJCA, certain favorable tax rulings with respect to prior years as well as the impact of exchange rate fluctuations on operating results and financial condition. We believe such additional non-GAAP information provides investors with an overall perspective of the period-to-period performance of our core business. In addition, management in ternally reviews each of these non-GAAP measures to evaluate performance on a comparative period-to-period basis in terms of absolute performance, trends and expected future performance with respect to its core continuing business. A material limitation of these non-GAAP measures is that such measures do not reflect actual GAAP amounts, an insurance recovery includes actual cash inflows, and the benefit from favorable tax rulings and AJCA reflect actual accounting and cash benefits realized; and we compensate for such limitations by presenting the accompanying reconciliation to the most directly comparable GAAP measure. These non-GAAP measures may not be comparable to similarly titled measures used by other companies.
Cautionary Statement Under the Private Securities Litigation Reform Act of 1995
Statements in this Annual Report, which are not historical facts or information, are ‘‘forward-looking statements’’ within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements are based on management’s current assumptions, estimates and expectations. Certain of such forward-looking information may be identified by such terms as ‘‘expect,’’ ‘‘believe,’’ ‘‘outlook,’’ ‘‘guidance,’’ ‘‘may,’’ and similar terms or variations thereof. All information concerning future revenues, tax rates or benefits, interest savings, earnings and other future financial results or financial position, constitutes forward-looking information. Such forward-looking statements involve significant risks, uncertainties and other factors. Actual results of the Company may differ materially from any future results expressed or implied by such forward-looking statements. Such factors include, among others, the following: general economic and business conditions in the Company’s markets, including economic, population health and political uncertainties; interest rates; the price, quality and availability of raw materials; the Company’s ability to implement its business strategy, including the achievement of anticipated cost savings, profitability and growth targets; the impact on cash and the impact of increased borrowings related to the July 2007 share repurchase program; the impact of currency fluctuation or devaluation in the Company’s principal foreign markets and the success of the Company’s hedging and risk management strategies; the outcome of uncertainties related to litigation; the impact of possible pension funding obligations and increased pension expense on the Company’s cash flow and results of operations; and the effect of legal and regulatory proceedings, as well as restrictions imposed on the Company, its operations or its representatives by U.S. and foreign governments. The Company intends its forward-looking statements to speak only as of the time of such statements and does not undertake or plan to update or revise them as more information becomes available or to reflect changes in expectations, assumptions or results.
Any public statements or disclosures by IFF following this report that modify or impact any of the forward-looking statements contained in or accompanying this report will be deemed to modify or supersede such outlook or other forward-looking statements in or accompanying this report.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
We operate on a global basis and are exposed to currency fluctuation related to the manufacture and sale of our products in currencies other than the U.S. dollar. The major foreign currencies involve the markets in the European Union, Great Britain, Mexico, Brazil, China, Indonesia, Australia and Japan, although all regions are subject to foreign currency fluctuations versus the U.S. dollar. We actively monitor our foreign currency exposures in all major markets in which we operate, and employ a variety of techniques to mitigate the impact of exchange rate fluctuations, including foreign currency hedging activities. We enter into foreign currency forward contracts with the objective of reducing exposure to cash flow volatility associated with foreign currency receivables and payables, and with anticipated purchases of certain raw materials used in operations. These contracts, the counterparties to which are major international financial institutions, generally i nvolve the exchange of one
31
Table of Contents
currency for a second currency at a future date, and have maturities not exceeding six months. The notional amount and maturity dates of such contracts match those of the underlying transactions. The gain or loss on the hedging instrument is recorded in earnings at the same time as the transaction being hedged is recorded in earnings. In February 2003, we executed a 10-year Yen − U.S. dollar currency swap related to the monthly sale and purchase of products between the U.S. and Japan, which is designated as a cash flow hedge. The annual notional value of this swap is approximately $5 million. The associated asset or liability on the open hedge instrument is recorded in Current Assets or Current Liabilities, as applicable. Gains and losses related to this swap are recorded currently, and the mark-to-market adjustment related to the value of the swap is reflected as a component of Accumulated Other Comprehensive Income (‘‘AOCI’’).
In addition to the foreign exchange forward contracts noted above, we use non-U.S. dollar borrowings and foreign exchange swap agreements, to hedge the foreign currency exposures of our net investment in certain foreign affiliates, primarily in the European Union. These foreign exchange swap agreements are designated as hedges of net investments. In September 2007 and January 2006, we entered into a $250 million and a $300 million Cross Currency Interest Rate Swap, respectively, to hedge a portion of our consolidated EUR net investment. The derivative was structured as a swap of floating USD LIBOR to floating EURIBOR, with both floating interest rates reset and paid semi-annually. Because the derivative is structured as a floating to floating swap, the only value, other than that derived from changes in the spot Foreign Exchange (FX) rate, are interest rate accruals which are reset semi-annually. These accruals are netted and booked in curren t earnings. Mark-to-market changes due to differences in the underlying FX rate are recorded in AOCI and provide an offset to the cumulative translation adjustment of the underlying Euro net investments being hedged. Effectiveness is assessed quarterly. At maturity, or if the swaps are terminated early, any gain or loss will not be recorded to earnings, but will be recorded to AOCI until the EUR net investment is divested.
We use derivative instruments as part of our interest rate risk management strategy. The derivative instruments used are comprised principally of fixed to floating rate interest rate swaps. In September 2007, we entered into a $250 million interest rate swap agreement effectively converting the fixed rate on our long-term U.S. dollar borrowings to a variable short-term rate based on USD LIBOR rate plus markup. In addition, in 2005, we entered into certain interest rate swap agreements effectively converting the fixed rate on our long-term Japanese Yen borrowings to a variable short-term rate based on the Japanese Yen LIBOR rate plus markup. These swaps are designated as qualified fair value hedges. Swap contracts are generally held to maturity and are intended to create an appropriate balance of fixed and floating rate debt. To the extent we have not received cash or otherwise amended or settled any swap agreements, any applicable mark-to-market adjust ment relating to that swap is included as a separate component of debt.
The following table summarizes our derivatives outstanding as of December 31, 2007. These swaps were all effective under FAS 133.
| Notional Amount | 300,000,000 | 250,000,000 | 15,150,000,000 | 50,000,000 | 250,000,000 | ||||||||||
| Currency | USD | USD | JPY | USD | USD | ||||||||||
| Description | Cross Currency Interest Rate Swap (USD/EUR) | Cross Currency Interest Rate Swap (USD/EUR) | Interest Rate Swap | 10 Year Cross Currency Swap (JPY/USD) | Interest Rate Swap | ||||||||||
| Hedged risk | Foreign Exchange Risk(1) | Foreign Exchange Risk(1) | The risk of changes in fair value attributable to interest rate risk(2) | The variability of cash flows attributable to foreign exchange risk(3) | The risk of changes in fair value attributable to interest rate risk(2) |
Method used to test for effectiveness:
| (1) | Quarterly hedge effectiveness is tested using spot-to-spot methodology. We use a hypothetical derivative on an after-tax basis, and ensure that the hedged amount is less then the designated Euro net investment. We ensure that the terms of the hedging instrument have not changed and perform a review of counterparty creditworthiness. |
| (2) | Changes in fair value attributable to the risk being hedged are expected to be completely offset by the |
32
Table of Contents
| hedging derivative because the critical terms of the Interest Rate Swap and the hedged debt match (i.e., the currency, notional amount, timing and date of interest payments). Quarterly hedge effectiveness testing includes ensuring the terms of the hedging instrument and the debt have not changed and reviewing counterparty creditworthiness. |
| (3) | Changes in cash flow attributable to the risk being hedged are expected to be completely offset by the hedging derivative because the critical terms of the Cross Currency Swap and the forecasted transaction match (i.e., the currency, notional amount and timing). Quarterly hedge effectiveness testing includes reviewing counterparty creditworthiness, ensuring the probability of hedged forecasted transactions has not changed and ensuring the terms of the hedging instrument have not changed. |
We have established a centralized reporting system to evaluate the effects of changes in interest rates, currency exchange rates and other relevant market risks. Our risk management procedures include the monitoring of interest rate and foreign exchange exposures and hedge positions utilizing statistical analyses of cash flows, market value and sensitivity analysis. However, the use of these techniques to quantify the market risk of such instruments should not be construed as an endorsement of their accuracy or the accuracy of the related assumptions. Market exposures are evaluated using a sensitivity model that is intended to measure the potential 10% loss in interest rate and foreign exchange financial instruments, assuming adverse market conditions occur. Historical interest rates and foreign exchange rates are used to estimate the volatility and correlation of future rates.
The estimated maximum potential one-day loss in fair value of interest rate or foreign exchange rate instruments, calculated using the sensitivity model, is not material to our consolidated financial position, results of operations or cash flows in 2007, 2006 and 2005. The estimated maximum yearly loss in earnings due to interest rate or foreign exchange rate instruments, calculated utilizing the sensitivity model, is not material to our results of operations in 2007, 2006 and 2005. Actual results in the future may differ materially from these projected results due to actual developments in the global financial markets.
The foreign currency and interest rate swap contracts existing during 2007 and 2006 were entered into for the purpose of seeking to mitigate the risk of certain specific adverse currency and interest rate risks. As a result of these financial instruments, we reduced financial risk in exchange for foregoing any gain (reward) that might have occurred if the markets moved favorably. In using these contracts, management exchanged the risks of the financial markets for counterparty risk. Counterparty risk arises from the inability of a counterparty to meet its obligations. To mitigate counterparty risk, we entered into derivative contracts with major leading financial institutions that have credit ratings equal to or better than our credit rating.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
See index to Consolidated Financial Statements on page 38. See Item 6 for supplemental quarterly data on page 14.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures and Changes in Internal Control over Financial Reporting.
Our Chief Executive Officer and Chief Financial Officer, with the assistance of other members of our management, have evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective as of the end of the period covered by this Annual Report on Form 10-K.
We have established controls and procedures designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Commission’s rules and forms and is accumulated and communicated to management, including the principal executive officer and the principal financial officer, to allow timely decisions regarding required disclosure.
33
Table of Contents
Our Chief Executive Officer and Chief Financial Officer have concluded that there have not been any changes in our internal control over financial reporting during the fourth quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
The Report of Independent Registered Public Accounting Firm is contained in Part IV of this Annual Report on Form 10-K and is incorporated herein by reference.
Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2007. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (‘‘COSO’’) in Internal Control − Integrated Framework.
Based on this assessment, management determined that, as of December 31, 2007, our internal control over financial reporting was effective.
PricewaterhouseCoopers LLP, our independent registered public accounting firm, has audited the effectiveness of our internal control over financial reporting as of December 31, 2007 as stated in their report which is included herein.
34
Table of Contents
Certifications to NYSE and SEC
Our Chief Executive Officer certification was timely filed with the NYSE as required by NYSE Rule 303A(12). Our Chief Executive Officer and Chief Financial Officer have each filed with the Securities and Exchange Commission the required certifications regarding the quality of our public disclosures.
| ITEM 9B. | OTHER INFORMATION. |
None.
35
Table of Contents
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
The information relating to our directors and nominees are set forth under the caption ‘‘Election of Directors’’ in the IFF 2008 Proxy Statement and is incorporated by reference herein. The information under the caption ‘‘Section 16(a) Beneficial Ownership Reporting Compliance’’ that appears in the IFF 2008 Proxy Statement is also incorporated by reference herein. See Part I, Item 1 of this Form 10-K for information relating to our Executive Officers.
We have adopted a Code of Business Conduct and Ethics (the ‘‘Code of Ethics’’) that applies to our Chief Executive Officer, principal financial officer, principal accounting officer, and to all of our other directors, officers and employees. The Code of Ethics is available at the Investor Relations / Corporate Governance section on our website www.iff.com. A waiver from any provision of the Code of Ethics in favor of a director or Executive Officer may only be granted by the Board or the Audit Committee of the Board and any such waiver will be publicly disclosed. We will disclose substantive amendments to and any waivers from the Code of Ethics provided to our Chief Executive Officer, principal financial officer or principal accounting officer, as well as any other executive officer or directo r, at the Investor Relations / Corporate Governance section on our Internet website: www.iff.com. On August 15, 2007, we initiated an anonymous worldwide Hotline to address the serious concerns of employees. We contracted with Global Compliance Services to assist our employees in identifying issues that might compromise our health, safety, or reputation, employees or shareholders.
The information regarding our Audit Committee and designated audit committee financial expert is set forth under the captions ‘‘Board and Committee Memberships’’ and ‘‘Audit Committee’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein.
The information concerning procedures by which shareholders may recommend director nominees is set forth under ‘‘Director Candidates’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein.
| ITEM 11. | EXECUTIVE COMPENSATION. |
The information relating to executive compensation is set forth under the captions ‘‘Executive Compensation’’ and ‘‘Directors’ Compensation’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein; except that the information under the caption ‘‘Compensation Committee Report’’ shall be deemed ‘‘furnished’’ with this report and shall not be deemed ‘‘filed’’ with this report, not deemed incorporated by reference into any filing under the Securities Act of 1933 except only as may be expressly set forth in any such filing by specific reference.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information relating to security ownership of management and certain beneficial owners is set forth under the caption ‘‘Beneficial Ownership Table’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein. The information relating to our equity plans is set forth under the caption ‘‘Equity Compensation Plans’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
The information regarding certain relationships and related party transactions and director independence is set forth under the caption ‘‘Independence of Directors and Committee Members and Related Person Matters’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein.
36
Table of Contents
| ITEM 14. | PRINCIPAL ACCOUNTING FIRM FEES AND SERVICES. |
The information regarding fees and services of the independent registered public accounting firm (‘‘independent accountant’’) and our pre-approval policies and procedures for audit and non-audit services provided by our independent accountant are set forth under the captions ‘‘Principal Accountant Fees and Services’’ and ‘‘Audit Committee Pre-Approval Policies and Procedures’’ in the IFF 2008 Proxy Statement and such information is incorporated by reference herein.
37
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
(a)(1) FINANCIAL STATEMENTS: The following consolidated financial statements, related notes, and independent registered public accounting firm’s report are included in this report on Form 10-K:
All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
38
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of International Flavors & Fragrances Inc.:
In our opinion, the consolidated financial statements listed in the index appearing under item 15(a)(1) present fairly, in all material respects, the financial position of International Flavors & Fragrances Inc. and its subsidiaries at December 31, 2007 and December 31, 2006, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria establi shed in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Managements’ Report on Internal Control Over Financial Reporting, appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and wh ether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 9 to the Consolidated Financial Statements, the Company changed the manner in which it accounts for uncertain tax positions in 2007. As discussed in Note 11 to the Consolidated Financial Statements, the Company changed the manner in which it accounts for share-based compensation in 2006. As discussed in Note 13 to the Consolidated Financial Statements, the Company changed the manner in which it accounts for defined benefit pension and other postretirement plans effective December 31, 2006.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
39
Table of Contents
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
New York, New York
February 27, 2008
40
Table of Contents
International Flavors & Fragrances Inc.
CONSOLIDATED STATEMENT OF INCOME
| Year Ended December 31, | ||||||||||||||||||
| (DOLLARS IN THOUSANDS EXCEPT PER SHARE AMOUNTS) | 2007 | 2006 | 2005 | |||||||||||||||
| Net sales | $ | 2,276,638 | $ | 2,095,390 | $ | 1,993,393 | ||||||||||||
| Cost of goods sold | 1,324,424 | 1,211,259 | 1,168,992 | |||||||||||||||
| Research and development expenses | 199,023 | 185,692 | 179,812 | |||||||||||||||
| Selling and administrative expenses | 375,287 | 351,923 | 339,323 | |||||||||||||||
| Amortization of intangibles | 12,878 | 14,843 | 15,071 | |||||||||||||||
| Curtailment loss | 5,943 | — | — | |||||||||||||||
| Restructuring and other charges, net | — | 2,680 | 23,319 | |||||||||||||||
| Interest expense | 41,535 | 25,549 | 23,956 | |||||||||||||||
| Other (income) expense, net | (11,136 | ) | (9,838 | ) | (3,268 | ) | ||||||||||||
| 1,947,954 | 1,782,108 | 1,747,205 | ||||||||||||||||
| Income before taxes | 328,684 | 313,282 | 246,188 | |||||||||||||||
| Taxes on income | 81,556 | 86,782 | 53,122 | |||||||||||||||
| Net income | 247,128 | 226,500 | 193,066 | |||||||||||||||
| Other comprehensive income: | ||||||||||||||||||
| Foreign currency translation adjustments | (1,136 | ) | 15,515 | (55,596 | ) | |||||||||||||
| Accumulated losses (gains) on derivatives qualifying as hedges | 622 | 141 | 3,088 | |||||||||||||||
| Minimum pension liability adjustment | — | 92,831 | 10,325 | |||||||||||||||
| Pension and Post-retirement liability adjustment | 53,039 | — | — | |||||||||||||||
| Comprehensive income | $ | 299,653 | $ | 334,987 | $ | 150,883 | ||||||||||||
| 2007 | 2006 | 2005 | ||||||||||||||||
| Net income per share – basic | $ | 2.86 | $ | 2.50 | $ | 2.06 | ||||||||||||
| Net income per share – diluted | $ | 2.82 | $ | 2.48 | $ | 2.04 | ||||||||||||
Notes to Consolidated Financial Statements
41
Table of Contents
International Flavors & Fragrances Inc.
CONSOLIDATED BALANCE SHEET
| December 31, | ||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| ASSETS | ||||||||||||
| Current Assets: | ||||||||||||
| Cash and cash equivalents | $ | 151,471 | $ | 114,508 | ||||||||
| Short-term investments | 604 | 604 | ||||||||||
| Receivables: | ||||||||||||
| Trade | 412,221 | 369,870 | ||||||||||
| Allowance for doubtful accounts | (11,694 | ) | (12,715 | ) | ||||||||
| Other | 50,052 | 48,147 | ||||||||||
| Inventories | 484,222 | 446,606 | ||||||||||
| Deferred income taxes | 77,572 | 89,448 | ||||||||||
| Prepaid expenses | 26,030 | 23,335 | ||||||||||
| Total Current Assets | 1,190,478 | 1,079,803 | ||||||||||
| Property, Plant and Equipment, net | 508,820 | 495,124 | ||||||||||
| Goodwill | 665,582 | 665,582 | ||||||||||
| Other Intangible Assets, net | 67,254 | 80,134 | ||||||||||
| Other Assets | 294,654 | 158,261 | ||||||||||
| Total Assets | $ | 2,726,788 | $ | 2,478,904 | ||||||||
| December 31, | ||||||||||||
| 2007 | 2006 | |||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||
| Current Liabilities: | ||||||||||||
| Bank borrowings, overdrafts and current portion of long-term debt | $ | 152,473 | $ | 15,897 | ||||||||
| Accounts payable | 130,992 | 111,661 | ||||||||||
| Accrued payrolls and bonuses | 64,271 | 71,976 | ||||||||||
| Dividends payable | 18,628 | 18,764 | ||||||||||
| Income taxes | — | 45,251 | ||||||||||
| Restructuring and other charges | 2,654 | 15,288 | ||||||||||
| Other current liabilities | 169,878 | 167,934 | ||||||||||
| Total Current Liabilities | 538,896 | 446,771 | ||||||||||
| Other Liabilities: | ||||||||||||
| Long-term debt | 1,060,168 | 791,443 | ||||||||||
| Deferred gains | 61,659 | 64,686 | ||||||||||
| Retirement liabilities | 171,991 | 170,719 | ||||||||||
| Other liabilities | 276,877 | 100,117 | ||||||||||
| Total Other Liabilities | 1,570,695 | 1,126,965 | ||||||||||
| Commitments and Contingencies (Note 16) | ||||||||||||
| Shareholders’ Equity: | ||||||||||||
| Common stock 12 ½¢ par value; authorized 500,000,000 shares; issued 115,761,840 shares | 14,470 | 14,470 | ||||||||||
| Capital in excess of par value | 54,995 | 96,635 | ||||||||||
| Retained earnings | 2,078,937 | 1,909,599 | ||||||||||
| Accumulated other comprehensives (loss) income: | ||||||||||||
| Cumulative translation adjustment | (32,990 | ) | (31,854 | ) | ||||||||
| Accumulated losses on derivatives qualifying as hedges | (1,843 | ) | (2,465 | ) | ||||||||
| Pension and Postemployment liability adjustment | (109,514 | ) | (162,553 | ) | ||||||||
| 2,004,055 | 1,823,832 | |||||||||||
| Treasury stock, at cost -34,766,612 shares in 2007 and 26,344,638 shares in 2006 | (1,386,858 | ) | (918,664 | ) | ||||||||
| Total Shareholders’ Equity | 617,197 | 905,168 | ||||||||||
| Total Liabilities and Shareholders’ Equity | $ | 2,726,788 | $ | 2,478,904 | ||||||||
See Notes to Consolidated Financial Statements
42
Table of Contents
International Flavors & Fragrances Inc.
CONSOLIDATED STATEMENT OF CASH FLOWS
| Year Ended December 31, | ||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| Cash flows from operating activities: | ||||||||||||||||||
| Net income | $ | 247,128 | $ | 226,500 | $ | 193,066 | ||||||||||||
| Adjustments to reconcile to net cash provided by operating activities | ||||||||||||||||||
| Depreciation and amortization | 82,788 | 89,733 | 91,928 | |||||||||||||||
| Deferred income taxes | (6,343 | ) | (12,423 | ) | (32,882 | ) | ||||||||||||
| Gain on disposal of assets | (13,791 | ) | (22,836 | ) | (2,108 | ) | ||||||||||||
| Equity based compensation | 18,168 | 18,185 | 7,350 | |||||||||||||||
| Curtailment loss | 5,943 | — | — | |||||||||||||||
| Changes in assets and liabilities: | ||||||||||||||||||
| Current receivables | (32,974 | ) | (27,153 | ) | (1,897 | ) | ||||||||||||
| Inventories | (12,406 | ) | 9,492 | (117 | ) | |||||||||||||
| Current payables | 22,298 | 38,087 | (6,369 | ) | ||||||||||||||
| Changes in other assets | (2,088 | ) | (20,396 | ) | (46,225 | ) | ||||||||||||
| Changes in other liabilities | 5,339 | (17,570 | ) | (25,586 | ) | |||||||||||||
| Net cash provided by operations | 314,062 | 281,619 | 177,160 | |||||||||||||||
| Cash flows from investing activities: | ||||||||||||||||||
| Additions to property, plant and equipment | (65,614 | ) | (58,282 | ) | (93,433 | ) | ||||||||||||
| Proceeds from disposal of assets | 16,959 | 27,235 | 2,787 | |||||||||||||||
| Proceeds from investments | 10,471 | — | — | |||||||||||||||
| Purchase of investments | (13,170 | ) | (17,355 | ) | — | |||||||||||||
| Net cash used in investing activities | (51,354 | ) | (48,402 | ) | (90,646 | ) | ||||||||||||
| Cash flows from financing activities: | ||||||||||||||||||
| Cash dividends paid to shareholders | (76,600 | ) | (67,381 | ) | (67,779 | ) | ||||||||||||
| Net change in bank borrowings and overdrafts | (129,648 | ) | (48,714 | ) | 312,094 | |||||||||||||
| Net proceeds from long-term debt | 498,569 | 373,637 | — | |||||||||||||||
| Repayments of long-term debt | — | (499,300 | ) | (11,653 | ) | |||||||||||||
| Proceeds from issuance of stock under stock plans | 50,116 | 110,867 | 23,015 | |||||||||||||||
| Excess tax benefits on stock options exercised | 6,568 | 4,653 | — | |||||||||||||||
| Purchase of treasury stock | (577,001 | ) | (270,998 | ) | (98,319 | ) | ||||||||||||
| Net cash (used in) provided by financing activities | (227,996 | ) | (397,236 | ) | 157,358 | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | 2,251 | 5,982 | (3,923 | ) | ||||||||||||||
| Net change in cash and cash equivalents | 36,963 | (158,037 | ) | 239,949 | ||||||||||||||
| Cash and cash equivalents at beginning of year | 114,508 | 272,545 | 32,596 | |||||||||||||||
| Cash and cash equivalents at end of year | $ | 151,471 | $ | 114,508 | $ | 272,545 | ||||||||||||
| Non-Cash investing activity: | ||||||||||||||||||
| Interest paid | $ | 36,017 | $ | 31,701 | $ | 37,012 | ||||||||||||
| Taxes paid | $ | 60,405 | $ | 79,442 | $ | 66,668 | ||||||||||||
See Notes to Consolidated Financial Statements
43
Table of Contents
International Flavors & Fragrances Inc.
CONSOLIDATED STATEMENT OF SHAREHOLDERS’ EQUITY
| (DOLLARS IN THOUSANDS) | Common stock | Capital in excess of par value | Retained earnings | Accumulated other comprehensive (loss) income | Treasury stock | |||||||||||||||||||||||||||||||
| Shares | Cost | |||||||||||||||||||||||||||||||||||
| Balance at December 31, 2004 | $14,470 | $79,498 | $1,627,386 | $(108,172 | ) | (21,088,993 | ) | $(701,825 | ) | |||||||||||||||||||||||||||
| Net Income | 193,066 | |||||||||||||||||||||||||||||||||||
| Cumulative translation adjustment | (55,596 | ) | ||||||||||||||||||||||||||||||||||
| Accumulated losses on derivatives qualifying as hedges; net of tax: $674 | 3,088 | |||||||||||||||||||||||||||||||||||
| Minimum pension liability adjustment; net of tax: $(2,262) | 10,325 | |||||||||||||||||||||||||||||||||||
| Total comprehensive income | ||||||||||||||||||||||||||||||||||||
| Cash dividends declared | (68,397 | ) | ||||||||||||||||||||||||||||||||||
| Stock options | (7,604 | ) | 828,644 | 34,094 | ||||||||||||||||||||||||||||||||
| Reacquired shares | (2,587,000 | ) | (98,319 | ) | ||||||||||||||||||||||||||||||||
| Restricted stock award | (200,000 | ) | (6,667 | ) | ||||||||||||||||||||||||||||||||
| Amortization | ||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2005 | 14,470 | 71,894 | 1,752,055 | (150,355 | ) | (23,047,349 | ) | (772,717 | ) | |||||||||||||||||||||||||||
| Net Income | 226,500 | |||||||||||||||||||||||||||||||||||
| Cumulative translation adjustment | 15,515 | |||||||||||||||||||||||||||||||||||
| Accumulated losses on derivatives qualifying as hedges | 141 | |||||||||||||||||||||||||||||||||||
| Minimum pension liability adjustment; net of tax: $42,469 | 92,831 | |||||||||||||||||||||||||||||||||||
| Adoption of FAS 158 minimum pension liability adjustment; net of tax: $4,276 | 7,549 | |||||||||||||||||||||||||||||||||||
| Pension and postemployment liability adjustment; net of tax: $(76,742) | (162,553 | ) | ||||||||||||||||||||||||||||||||||
| Cash dividends declared | (68,956 | ) | ||||||||||||||||||||||||||||||||||
| Stock options | 598 | 3,346,326 | 116,050 | |||||||||||||||||||||||||||||||||
| Reacquired shares | (6,891,152 | ) | (270,998 | ) | ||||||||||||||||||||||||||||||||
| Vested restricted stock units | 29,632 | 1,597 | ||||||||||||||||||||||||||||||||||
| Restricted stock award | 24,143 | 217,905 | 7,404 | |||||||||||||||||||||||||||||||||
| Balance at December 31, 2006 | 14,470 | 96,635 | 1,909,599 | (196,872 | ) | (26,344,638 | ) | (918,664 | ) | |||||||||||||||||||||||||||
| Net Income | 247,128 | |||||||||||||||||||||||||||||||||||
| FIN 48 adoption adjustment | (1,325 | ) | ||||||||||||||||||||||||||||||||||
| Cumulative translation adjustment | (1,136 | ) | ||||||||||||||||||||||||||||||||||
| Accumulated losses on derivatives qualifying as hedges | 622 | |||||||||||||||||||||||||||||||||||
| Pension liability and post-retirement adjustment; net of tax: $25,758 | 53,039 | |||||||||||||||||||||||||||||||||||
| Cash dividends declared | (76,465 | ) | ||||||||||||||||||||||||||||||||||
| Stock options | 4,121 | 1,332,595 | 48,483 | |||||||||||||||||||||||||||||||||
| Reacquired shares | (45,000 | ) | (10,198,877 | ) | (532,001 | ) | ||||||||||||||||||||||||||||||
| Vested restricted stock units | (16,126 | ) | 234,388 | 7,874 | ||||||||||||||||||||||||||||||||
| Stock based compensation | 15,365 | 209,920 | 7,450 | |||||||||||||||||||||||||||||||||
| Balance at December 31, 2007 | $14,470 | $54,995 | $2,078,937 | $(144,347 | ) | (34,766,612 | ) | $(1,386,858 | ) | |||||||||||||||||||||||||||
See Notes to Consolidated Financial Statements
44
Table of Contents
INTERNATIONAL FLAVORS & FRAGRANCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| NOTE 1. | NATURE OF OPERATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Nature of Operations We are a leading creator and manufacturer of flavor and fragrance compounds used to impart or improve flavor or fragrance in a wide variety of consumer products. Our products are sold principally to manufacturers of perfumes and cosmetics, hair and other personal care products, soaps and detergents, cleaning products, dairy, meat and other processed foods, beverages, snacks and savory foods, confectionery, sweet and baked goods, and pharmaceutical and oral care products.
Use of Estimates The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts and accompanying disclosures. These estimates are based on management’s best knowledge of current events and actions we may undertake in the future. Actual results may ultimately differ from estimates.
Principles of Consolidation The consolidated financial statements include our accounts and those of our subsidiaries. Significant intercompany balances and transactions have been eliminated. To the extent a subsidiary is not wholly-owned, any related minority interest is included in Other liabilities; applicable (income) expense attributable to the minority interest is included in Other (income) expense, net.
Revenue Recognition We recognize revenue when the earnings process is complete. This generally occurs when (i) products are shipped to the customer in accordance with the terms of sale, (ii) title and risk of loss have been transferred and (iii) collectibility is reasonably assured. Net sales are reduced, at the time revenue is recognized, by accruing for applicable discounts, rebates and sales allowances based on historical experience. Related accruals are included in accrued liabilities.
Foreign Currency Translation We translate the assets and liabilities of non-U.S. subsidiaries into U.S. dollars at year-end exchange rates. Income and expense items are translated at average exchange rates during the year. Cumulative translation adjustments are shown as a separate component of Shareholders’ Equity.
Research and Development All research and development costs are expensed as incurred.
Cash Equivalents Cash equivalents include highly liquid investments with maturities of three months or less at date of purchase.
Other Receivables Other receivables consist primarily of Value Added Tax (VAT) receivables in the various countries in which we operate and insurance recoveries receivable. VAT receivables are recorded when goods are received and are normally recoverable within 30 days. Insurance recoveries receivable are recorded, on an undiscounted basis, when it is probable that recovery will be realized; such recoveries are accounted for as a component of the income statement caption in which the original expense was recognized.
Inventories Inventories are stated at the lower of cost (on weighted average basis) or market. Our inventories consisted of the following:
| December 31, | ||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Raw materials | $ | 237,943 | $ | 213,675 | ||||||||
| Work in process | 10,707 | 12,686 | ||||||||||
| Finished goods | 235,572 | 220,245 | ||||||||||
| Total | $ | 484,222 | $ | 446,606 | ||||||||
45
Table of Contents
Property, Plant and Equipment Property, plant and equipment are recorded at cost. Depreciation is calculated on a straight-line basis, principally over the following estimated useful lives: buildings and improvements, 10 to 40 years; machinery and equipment, 3 to 10 years; information technology hardware and software, 3 to 7 years; and leasehold improvements which are included in buildings and improvements, the estimated life of the improvements or the remaining term of the lease, whichever is shorter. We periodically evaluate whether current events or circumstances indicate that the carrying value of our depreciable assets may not be recoverable.
Goodwill and Other Intangible Assets Goodwill represents the difference between the total purchase price and the fair value of identifiable assets and liabilities acquired in business acquisitions.
We perform a goodwill impairment test on at least an annual basis or more frequently in certain circumstances, by assessing the fair value of our reporting units based upon both discounted cash flow and comparison of multiples of comparable companies. We deem goodwill to be impaired if the carrying amount of the reporting unit exceeds the estimated fair value. We completed our goodwill impairment assessment, which indicated no impairment of goodwill.
Other intangible assets include patents, trademarks and other intellectual property valued at acquisition, and amortized on a straight-line basis over periods ranging from 7 to 20 years.
We review our long-lived assets for impairment when events or changes in business conditions indicate that their full carrying value may not be recovered. An estimate of undiscounted future cash flows produced by an asset or group of assets is compared to the carrying value to determine whether impairment exists. If assets are determined to be impaired, the loss is measured based on an estimate of fair value using various valuation techniques, including a discounted estimate of future cash flows.
Income Taxes Deferred income taxes reflect the impact of temporary differences between the amount of assets and liabilities recognized for financial reporting purposes and such amounts recognized for tax purposes, based on tax laws as currently enacted. Additional taxes which would result from distributions by subsidiary companies to the parent are provided to the extent anticipated. No provision is made for additional taxes on undistributed earnings of subsidiary companies that are intended to be indefinitely invested in such subsidiaries. No income tax benefit is attributed to the currency translation component of Accumulated other comprehensive income (‘‘AOCI’’).
Retirement Benefits Current service costs of retirement plans and postretirement health care and life insurance benefits are accrued currently. Prior service costs resulting from plan improvements are amortized over periods ranging from 10 to 20 years.
Financial Instruments We use derivative financial instruments to manage interest and foreign currency exposures. The gain or loss on the hedging instrument is recorded in earnings at the same time as the transaction being hedged is recorded in earnings. The associated asset or liability related to the open hedge instrument is recorded in Current Assets or Current Liabilities, as applicable.
We record all derivative instruments on the balance sheet at fair value. Changes in a derivative’s fair value are recognized in earnings unless specific hedge criteria are met. If the derivative is designated as a fair value hedge, the changes in the fair value of the derivative and of the hedged item attributable to the hedged risk are recognized in the consolidated statement of earnings. If the derivative is designated as a cash flow hedge, the effective portions of changes in the fair value of the derivative are recorded in other comprehensive income (loss) and are subsequently recognized in the consolidated statement of earnings when the hedged item affects earnings. Ineffective portions of changes in the fair value of cash flow hedges, if any, are recognized as a charge or credit to earnings.
Software Costs We capitalize direct internal and external development costs for certain significant projects associated with internal-use software and amortize these costs over 7 years. Neither preliminary evaluation costs nor costs associated with the software after implementation are capitalized. Costs related to projects that are not significant are expensed as incurred.
Shipping and Handling Costs Net sales include shipping and handling charges billed to customers. Cost of goods sold includes all costs incurred in connection with shipping and handling.
46
Table of Contents
Net Income Per Share Net income per share is based on the weighted average number of shares outstanding. A reconciliation of shares used in the computations of basic and diluted net income per share is as follows:
| Number of Shares | ||||||||||||||||||
| (SHARES IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| Basic | 86,541 | 90,443 | 93,584 | |||||||||||||||
| Dilution under stock plans | 1,092 | 926 | 1,242 | |||||||||||||||
| Diluted | 87,633 | 91,369 | 94,826 | |||||||||||||||
Net income used in the computation of net income per share is unaffected by the assumed issuance of stock under our stock plans.
Options to purchase 248,000, 309,000 and 1,905,000 shares were outstanding at December 31, 2007, 2006, and 2005, respectively, but not included in the computation of diluted net income per share because the exercise prices were greater than the average market price of the common shares in the respective years.
Stock-Based Compensation We have stock-based compensation plans which are described more fully in Note 11 to the Consolidated Financial Statements. We applied the recognition and measurement principles of APB 25 in accounting for these plans prior to 2006. Under APB 25, no compensation expense for employee stock options was recognized, as all options granted had an exercise price not less than the market value of the common stock on the date of the grant. We adopted the provisions of SFAS No. 123(R) ‘‘Share-Based Payment’’ (‘‘FAS 123R’’) using the modified prospective method, which requires measurement of compensation cost of all stock-based awards at fair value on the date of grant and recognition of compensation expense over the service periods for awards expected to vest. Under this transition method, 2006 compensation cost includes the portion vesting in the year for (1) all share-based payments granted prior to, but not vested, as of January 1, 2006, based on the grant date fair value estimated in accordance with the original provisions of FAS 123 ‘‘Accounting for Stock-Based Compensation,’’(‘‘FAS 123’’), and (2) all share-based payments granted subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of FAS 123R. The cost of employee stock options will be recognized on a straight-line attribution basis over their respective vesting periods, net of estimated forfeitures. Results for prior periods have not been restated.
The following table illustrates the effect on net income and net income per share if we had applied the fair value recognition provisions of FAS 123, for the year ended December 31, 2005:
| (DOLLARS IN THOUSANDS, EXCEPT PER SHARE AMOUNTS) | 2005 | |||||
| Net income, as reported | $ | 193,066 | ||||
| Deduct: | ||||||
| Total stock-based employee compensation expense determined under fair value method for all stock option awards, net of related tax effects | 6,698 | |||||
| Pro-forma net income | $ | 186,368 | ||||
| Net income per share: | ||||||
| Basic − as reported | $ | 2.06 | ||||
| Basic − pro-forma | $ | 1.99 | ||||
| Diluted − as reported | $ | 2.04 | ||||
| Diluted − pro-forma | $ | 1.97 | ||||
These pro-forma amounts may not be representative of future results because the estimated fair value of stock options is amortized to expense over the vesting period, and additional options may be granted in future years.
47
Table of Contents
New Accounting Standards
In September 2006, Financial Accounting Standard Board (‘‘FASB’’) issued SFAS No. 157 ‘‘Fair Market Value Measurements’’ (‘‘FAS 157’’). This standard defines fair value, establishes a framework for measuring fair value and expands disclosures regarding fair value measurements. FAS 157 is effective for fiscal years beginning after December 15, 2007. We are currently assessing the impact of this interpretation, but we do not believe its adoption will have a material impact on our results or financial condition.
In February 2007, the FASB issued SFAS No. 159 ‘‘The Fair Value Option for Financial Assets and Liabilities − including an amendment of FASB No. 115’’ (‘‘FAS 159’’). This standard allows companies to elect at specific election dates, to measure eligible financial assets and liabilities at fair value that are not otherwise required to be measured at fair value. If a company elects the fair value option, subsequent changes in that item’s fair value must be recognized in current earnings. FAS 159 is effective for years beginning after November 15, 2007. We are currently evaluating the impact of this standard.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), ‘‘Business Combinations’’ (‘‘FAS 141(R)’’). In FAS 141(R), the FASB retained the fundamental requirements of Statement No. 141 to account for all business combinations using the acquisition method (formerly the purchase method) and for an acquiring entity to be identified in all business combinations. However, the new standard requires the acquiring entity in a business combination to recognize all (and only) the assets acquired and liabilities assumed in the transaction; establishes the acquisition-date fair value as the measurement objective for all assets acquired and liabilities assumed; and requires the acquirer to disclose to investors and other users all of the information they need to evaluate and understand the nature and financial effect of the business combination. FAS 141(R) is effective for annual periods beginning on or after December 15, 2008. We have not yet determined the impact, if any, that FAS 141(R) will have on our results of operations or financial position.
Reclassifications and revisions Certain reclassifications have been made to the prior years’ financial statements to conform to the 2007 presentation. In addition, Operating cash flows in 2006 were revised to $282 million from the $263 million reported in 2006 to property reflect the inclusion of the purchase of investments of $18 million in investing activities and $1 million of deferred financing costs in financing activities; 2005 cash flows were unaffected.
| NOTE 2. | RESTRUCTURING AND OTHER CHARGES |
In 2005, we eliminated positions in manufacturing, selling, research and administration functions, principally in our European and North American operating regions. As a result of these actions, restructuring charges, relating primarily to employee separation expenses, of $23 million and $3 million were recognized in 2005 and 2006, respectively. This plan is essentially complete.
Movements in related accruals were:
| (DOLLARS IN THOUSANDS) | Employee- Related | Asset-Related and Other | Total | |||||||||||||||
| Balance January 1, 2005 | $ | 28,218 | $ | 14,908 | $ | 43,126 | ||||||||||||
| Additional charges | 21,979 | 1,340 | 23,319 | |||||||||||||||
| Cash and other costs | (20,681 | ) | (11,315 | ) | (31,996 | ) | ||||||||||||
| Balance December 31, 2005 | 29,516 | 4,933 | 34,449 | |||||||||||||||
| Additional charges (credits) | 3,840 | (1,160 | ) | 2,680 | ||||||||||||||
| Cash and other costs | (20,495 | ) | (1,346 | ) | (21,841 | ) | ||||||||||||
| Balance December 31, 2006 | 12,861 | 2,427 | 15,288 | |||||||||||||||
| Cash and other costs | (10,273 | ) | (2,361 | ) | (12,634 | ) | ||||||||||||
| Balance December 31, 2007 | $ | 2,588 | $ | 66 | $ | 2,654 | ||||||||||||
The employee-related liabilities are expected to be utilized in 2008 as obligations are satisfied.
48
Table of Contents
| NOTE 3. | PROPERTY, PLANT AND EQUIPMENT, NET |
| Cost | ||||||||||||
| Asset Type | December 31, | |||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Land | $ | 27,998 | $ | 24,859 | ||||||||
| Buildings and Improvements | 242,181 | 225,231 | ||||||||||
| Machinery and Equipment | 641,902 | 594,914 | ||||||||||
| Information Technology | 213,936 | 203,890 | ||||||||||
| CIP | 39,065 | 25,878 | ||||||||||
| Total | $ | 1,165,082 | $ | 1,074,772 | ||||||||
| Accumulated Depreciation | ||||||||||||
| Asset Type | December 31, | |||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Buildings and Improvements | $ | 107,960 | $ | 92,525 | ||||||||
| Machinery and Equipment | 391,880 | 341,838 | ||||||||||
| Information Technology | 156,422 | 145,285 | ||||||||||
| Total | $ | 656,262 | $ | 579,648 | ||||||||
| Net | ||||||||||||
| Asset Type | December 31, | |||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Land | $ | 27,998 | $ | 24,859 | ||||||||
| Buildings and Improvements | 134,221 | 132,706 | ||||||||||
| Machinery and Equipment | 250,022 | 253,076 | ||||||||||
| Information Technology | 57,514 | 58,605 | ||||||||||
| CIP | 39,065 | 25,878 | ||||||||||
| Total | $ | 508,820 | $ | 495,124 | ||||||||
| NOTE 4. | GOODWILL AND OTHER INTANGIBLE ASSETS, NET |
Goodwill by business segment for 2007 and 2006 is as follows:
| DOLLARS IN THOUSANDS | Amount | |||||
| Flavors | $ | 319,479 | ||||
| Fragrances | 346,103 | |||||
| Total | $ | 665,582 | ||||
Trademark and other intangible assets consist of the following amounts:
| December 31, | ||||||||||||
| DOLLARS IN THOUSANDS | 2007 | 2006 | ||||||||||
| Gross carrying value | $ | 165,406 | $ | 165,406 | ||||||||
| Accumulated amortization | 98,152 | 85,272 | ||||||||||
| Total | $ | 67,254 | $ | 80,134 | ||||||||
Amortization expense for the year ended December 31, 2007 was $13 million; estimated annual amortization is $6 million in 2008 and $6 million in each year from 2009 to 2012.
49
Table of Contents
| NOTE 5. | OTHER ASSETS |
Other assets consist of the following amounts:
| December 31, | ||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Pension assets | $ | 111,400 | $ | 33,886 | ||||||||
| Deferred tax asset − noncurrent | 107,028 | 82,385 | ||||||||||
| Other | 76,226 | 41,990 | ||||||||||
| Total | $ | 294,654 | $ | 158,261 | ||||||||
| NOTE 6. | OTHER CURRENT LIABILITIES |
Other current liabilities consist of the following amounts:
| December 31, | ||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Workers compensation and general liability | $ | 28,900 | $ | 23,500 | ||||||||
| Interest payable | 19,764 | 11,069 | ||||||||||
| Current pension and other retiree accruals | 15,531 | 12,576 | ||||||||||
| Rebates and incentives | 11,822 | 11,989 | ||||||||||
| Commissions and professional fees payable | 9,825 | 8,567 | ||||||||||
| Other | 84,036 | 100,233 | ||||||||||
| Total | $ | 169,878 | $ | 167,934 | ||||||||
| NOTE 7. | SALE AND LEASEBACK TRANSACTIONS |
In connection with the disposition of certain real estate in prior years, we entered into long-term operating leases covering the facilities disposed of. The leases are classified as operating leases in accordance with SFAS No. 13, ‘‘Accounting for Leases,’’ and the gains realized have been deferred and are being credited to income over the initial lease term. Such deferred gains totaled $65 million and $68 million at December 31, 2007 and 2006, respectively, of which $62 million and $65 million, respectively, are reflected in the accompanying balance sheet under the caption Deferred gains, with the remainder included as a component of Other current liabilities.
| NOTE 8. | BORROWINGS |
Debt consists of the following at December 31:
| (DOLLARS IN THOUSANDS) | Rate | Maturities | 2007 | 2006 | ||||||||||||||||||||
| Bank borrowings and overdrafts | $ | 35,671 | $ | 15,897 | ||||||||||||||||||||
| Current portion of long-term debt | 2.40 | % | 2008 | 116,802 | — | |||||||||||||||||||
| Total current debt | 152,473 | 15,897 | ||||||||||||||||||||||
| Senior Notes-2007 | 6.38 | % | 2017-27 | 500,000 | — | |||||||||||||||||||
| Senior Notes-2006 | 5.94 | % | 2009-16 | 375,000 | 375,000 | |||||||||||||||||||
| Bank borrowings | 4.77 | % | various | 169,057 | 287,904 | |||||||||||||||||||
| Japanese Yen notes | 2.81 | % | 2011 | 15,927 | 127,684 | |||||||||||||||||||
| Other | 2011 | 33 | 38 | |||||||||||||||||||||
| Deferred realized gains on interest rate swaps | 151 | 817 | ||||||||||||||||||||||
| Total long-term debt | 1,060,168 | 791,443 | ||||||||||||||||||||||
| Total debt | $ | 1,212,641 | $ | 807,340 | ||||||||||||||||||||
Commercial paper issued by us generally has terms of 30 days or less; there were no outstanding commercial paper borrowings at December 31, 2007 or 2006.
50
Table of Contents
In 2005, we, including certain subsidiaries, entered into a revolving credit agreement (the ‘‘Facility’’) with certain banks. The Facility provides for a five-year US $350 million (‘‘Tranche A’’) and Euro 400 million (‘‘Tranche B’’) multi-currency revolving credit facility. Tranche A is available to IFF for commercial paper backstop and general corporate purposes; Tranche B is available to both IFF and the European subsidiaries for general corporate purposes. Borrowings under the Facility bear interest at an annual rate of LIBOR (London Inter Bank Offer Rate) (or in relation to any Euro-denominated loans, EURIBOR, European Inter Bank Offer Rate) plus a margin, currently 20 basis points, linked to our credit rating. We pay a commitment fee on the aggregate unused commitments and a utilization fee based on amounts outstanding under the Facility; such fees are not material. As permitt ed by the Facility, in 2007, the termination dates were extended by one year until November 23, 2012. The Facility contains various affirmative and negative covenants, including the requirement for us to maintain, at the end of each fiscal quarter, a ratio of net debt for borrowed money to EBITDA (Earnings Before Interest, Taxes, Depreciation and Amortization) in respect of the previous 12-month period of not more than 3.25 to 1. We have complied with this covenant at all times. As the Facility is a multi-year revolving credit agreement, we classify it as long-term debt. At December 31, 2007, approximately $160 million of bank borrowings on the Euro revolver was classified as long-term debt as we expect it to remain outstanding for longer than the next twelve months. At December 31, 2006, approximately $288 million of bank borrowings was classified as long-term debt.
Short-term bank loans primarily in the form of overdrafts, in addition to the Facility, were outstanding in several countries and averaged $26 million in 2007, compared with $16 million in 2006. The highest levels were $66 million in 2007, $31 million in 2006, and $315 million in 2005. The 2007 weighted average interest rate of these bank loans, based on balances outstanding at the end of each month, was 4.6% and the average rate on balances outstanding at December 31, 2007 was 5.1%. These rates compare with 3.9% and 4.7%, respectively, in 2006, and 3.4% and 2.8%, respectively, in 2005.
On September 27, 2007, we issued $500 million of Senior Unsecured Notes (‘‘Senior Notes − 2007’’) in four series under the Note Purchase Agreement (‘‘NPA’’): (i) $250 million in aggregate principal amount of 6.25% Series A Senior Notes due September 27, 2017, (ii) $100 million in aggregate principal amount of 6.35% Series B Notes due September 27, 2019, (iii) $50 million in aggregate principal amount of 6.50% Series C Notes due September 27, 2022, and (iv) $100 million in aggregate principal amount of 6.79% Series D Notes due September 27, 2027. Proceeds of the offering were used primarily to fund an accelerated repurchase of IFF stock.
In 2006, we issued $375 million of Senior Unsecured Notes (‘‘Senior Notes − 2006’’) in four series under another NPA: (i) $50 million in aggregate principal amount of 5.89% Series A Senior Notes due July 12, 2009, (ii) $100 million in aggregate principal amount of 5.96% Series B Notes due July 12, 2011, (iii) $100 million in aggregate principal amount of 6.05% Series C Notes due July 12, 2013, and (iv) $125 million in aggregate principal amount of 6.14% Series D Notes due July 12, 2016. Proceeds of the offering were used primarily to repay commercial paper borrowings used to fund our maturing debt.
Maturities on debt outstanding at December 31, 2007 are: 2008, $153 million; 2009, $50 million; 2011 and thereafter, $1,010 million. There is no debt maturing in 2010. The estimated fair value of the long-term debt at December 31, 2007 and 2006, based on interest rates currently available with similar terms and maturities, approximated the recorded value.
In 2002, we entered into certain interest rate swap agreements effectively converting the fixed rate on our long-term Japanese Yen borrowings to a variable short-term rate based on the Japanese Yen LIBOR rate plus a markup. These swaps were designated as qualified fair value hedges. During 2003 and 2005, we amended the swaps and the counterparty paid us $3 million and $1 million, respectively, including accrued interest. Such gains have been deferred and are being amortized over the remaining term of the debt.
51
Table of Contents
| NOTE 9. | INCOME TAXES |
Earnings before income taxes consisted of the following:
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| U.S. loss before taxes | $ | (53,159 | ) | $ | (31,309 | ) | $ | (52,471 | ) | |||||||||
| Foreign income before taxes | 381,843 | 344,591 | 298,659 | |||||||||||||||
| Total income before taxes | $ | 328,684 | $ | 313,282 | $ | 246,188 | ||||||||||||
The income tax provision consisted of the following:
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| Current | ||||||||||||||||||
| Federal | $ | 9,315 | $ | 2,869 | $ | 5,642 | ||||||||||||
| State and local | (1,417 | ) | 4,240 | (1,612 | ) | |||||||||||||
| Foreign | 80,001 | 92,096 | 81,974 | |||||||||||||||
| 87,899 | 99,205 | 86,004 | ||||||||||||||||
| Deferred | ||||||||||||||||||
| Federal | (13,648 | ) | (10,080 | ) | (25,618 | ) | ||||||||||||
| State and local | (1,047 | ) | (747 | ) | 778 | |||||||||||||
| Foreign | 8,352 | (1,596 | ) | (8,042 | ) | |||||||||||||
| (6,343 | ) | (12,423 | ) | (32,882 | ) | |||||||||||||
| Total income taxes | $ | 81,556 | $ | 86,782 | $ | 53,122 | ||||||||||||
A reconciliation between the U.S. Federal statutory income tax rate to our actual effective tax rate follows:
| 2007 | 2006 | 2005 | ||||||||||||||||
| Statutory tax rate | 35.0 | % | 35.0 | % | 35.0 | % | ||||||||||||
| Difference in effective tax rate on foreign earnings and remittances | (9.2 | ) | (8.4 | ) | (2.8 | ) | ||||||||||||
| State and local taxes | (0.6 | ) | 1.1 | (0.2 | ) | |||||||||||||
| AJCA benefit | — | — | (10.0 | ) | ||||||||||||||
| Other, net | (0.4 | ) | — | (0.4 | ) | |||||||||||||
| Effective tax rate | 24.8 | % | 27.7 | % | 21.6 | % | ||||||||||||
Our effective tax rate reflects the benefit from having significant operations outside the U.S. that are taxed at rates that are lower than the U.S. federal rate of 35%. The 2007 and 2006 effective tax rates were also favorably impacted by the reversal of previously established tax accruals of $10 million and $4 million, respectively; such accruals were no longer required based on rulings obtained from applicable non-U.S. tax jurisdictions.
52
Table of Contents
The components of the deferred tax assets and liabilities included on the balance sheet are as follows:
| December 31, | ||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| ASSETS | ||||||||||||
| Inventory | $ | 13,700 | $ | 10,200 | ||||||||
| Employee and retiree benefits | 83,500 | 101,900 | ||||||||||
| Credit and net operating loss carryforwards | 181,400 | 168,400 | ||||||||||
| Property, plant and equipment | 21,800 | 25,000 | ||||||||||
| Other, net | 30,800 | 26,400 | ||||||||||
| Gross deferred tax assets | 331,200 | 331,900 | ||||||||||
| Valuation allowance | (171,600 | ) | (141,200 | ) | ||||||||
| Total deferred tax assets | 159,600 | 190,700 | ||||||||||
| LIABILITIES | ||||||||||||
| Trademarks and other | (14,900 | ) | (18,900 | ) | ||||||||
| Total net deferred tax assets | $ | 144,700 | $ | 171,800 | ||||||||
In 2007, the FASB issued Interpretation No. 48 (‘‘FIN 48’’), ‘‘Accounting for Uncertainty in Income Taxes’’ which clarifies the application of FAS 109 by prescribing the minimum threshold a tax position must meet before being recognized in the financial statements. Under FIN 48, the financial statement effect of a tax position is initially recognized when it is more likely than not the position will be sustained upon examination. A tax position that meets the ‘‘more likely than not’’ recognition threshold is initially and subsequently measured as the largest amount of benefit, determined on a cumulative probability basis, that is more likely than not to be realized upon ultimate settlement with the taxing authority.
As a result of adopting FIN 48, we recognized a $1 million increase in other liabilities for unrecognized tax benefits and a corresponding cumulative effect adjustment to Retained earnings. Also as prescribed by FIN 48, certain tax related amounts in the Consolidated Balance Sheet are classified differently than in prior periods. Amounts receivable from various tax jurisdictions are now included in Other current assets and tax reserves previously classified as accrued taxes on income are now included in Other liabilities.
A reconciliation of the total of unrecognized tax benefits at the beginning and end of the year is as follows:
| (DOLLARS IN THOUSANDS) | ||||||
| Balance of unrecognized benefits at January 1, 2007 | $ | 71,968 | ||||
| Gross amount of increases in unrecognized tax benefits as a result of positions taken during a prior year | 2,185 | |||||
| Gross amount of increases in unrecognized tax benefits as a result of positions taken during the current year | 13,117 | |||||
| The amounts of decreases in unrecognized benefits relating to settlements with taxing authorities | (6,549 | ) | ||||
| Reduction in unrecognized tax benefits due to the lapse of applicable statute of limitation | (76 | ) | ||||
| Balance of unrecognized tax benefits at December 31, 2007 | $ | 80,645 | ||||
The amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate is $79 million.
We have consistently recognized interest and penalties related to unrecognized tax benefits as a component of income tax expense. At December 31, 2007, we had accrued $9 million of interest and penalties classified as Other liabilities.
Net operating loss carryforwards were $169 million and $144 million at December 31, 2007 and 2006, respectively. If unused, $2 million will expire between 2008 and 2027. The remainder, totaling $167 million, may be carried forward indefinitely. Tax credit carry forwards were $12 million and $24 million at December 31, 2007 and December 31, 2006, respectively. If unused, $11 million will expire between 2008 and 2027. The remainder, totaling $1 million, may be carried forward indefinitely.
53
Table of Contents
Of the $181 million deferred tax asset for credit and net operating loss carryforwards at December 31, 2007, we consider it unlikely that a portion of the tax benefit will be realized. Accordingly, a $167 million and $5 million valuation allowance has been established against these deferred tax assets, respectively.
Tax benefits credited to Shareholders’ equity totaled $7 million and $11 million for 2007 and 2006, respectively.
U.S. income taxes and foreign withholding taxes associated with the repatriation of earnings of foreign subsidiaries were not provided on a cumulative total of $432 million of undistributed earnings of foreign subsidiaries. We intend to reinvest these earnings indefinitely in our foreign subsidiaries. Any additional U.S. taxes payable on the remaining foreign earnings, if remitted, would be substantially offset by credits for foreign taxes already paid.
We have several tax audits in process and have open tax years with various significant taxing jurisdictions that range primarily from 2001 to 2006. Based on currently available information, we do not believe the ultimate outcome of these tax audits and other tax positions related to open tax years, when finalized, will have a material adverse effect on our financial position, results of operations or cash flows.
| NOTE 10. | SHAREHOLDERS’ EQUITY |
During the first six months of 2007, under a share repurchase program of $300 million authorized in October 2006 (the ‘‘October 2006 Plan’’), we repurchased approximately 1.6 million shares at a cost of $81 million; at June 30, 2007, we had approximately $125 million remaining under the October 2006 Plan. In July 2007, the October 2006 Plan was terminated and superseded by a new program authorized by our Board to repurchase up to 15% or $750 million worth of our outstanding common stock, whichever is less (the ‘‘July 2007 Plan’’).
In September 2007, under the July 2007 Plan, we entered into two agreements to purchase shares of our common stock under a $450 million accelerated share repurchase (‘‘ASR’’) program. On September 28, 2007, we paid $450 million in exchange for an initial delivery of 7.6 million shares under the ASR, representing 90% of the shares that could have been purchased, based on the average trading price of our stock on that date. The remaining 10%, or $45 million, not used in the initial settlement will be included in the determination of the cost of the shares purchased on completion of the ASR. This amount was reflected in the accompanying Consolidated Balance sheet as a reduction to Capital in excess of par value.
Under the first agreement (the ‘‘Collared ASR’’), $112.5 million of share purchases are subject to collar provisions that establish minimum number of shares based on the volume-weighted average price of our stock (‘‘VWAP’’) over an initial hedge period. Under the Collared ASR, the minimum number of shares was set at 1.9 million and the maximum number of shares was set at 2.2 million shares when the hedge period ended on October 3, 2007.
Under the second agreement (the ‘‘Uncollared ASR’’), the number of shares to be repurchased is based on the VWAP of the common stock during the term of the Uncollared ASR.
On completion of the ASR, we may receive shares or may be required to pay cash or additional shares at our option, based on the VWAP of the common stock during the agreement term. Proceeds from the $500 million Senior Notes − 2007 funded the ASR payment as described in Note 8 to the Consolidated Financial Statements.
On March 9, 2000, we adopted a shareholder protection rights agreement (the ‘‘Rights Agreement’’) and declared a dividend of one right on each share of common stock outstanding on March 24, 2000 or issued thereafter.
Under the Rights Agreement, as amended, until a person or group acquires 15% or more of our common stock or commences a tender offer that would result in such person’s or group’s owning 15% or more, the rights are evidenced by the common stock certificates, automatically trade with the common stock and are not exercisable.
Thereafter, if we are involved in a merger or sell more than 50% of our assets or earnings power, each right entitles its holder to purchase a certain number of shares for a specified exercise price. Also, under certain circumstances, our Board has the option to redeem or exchange one share of common stock for each right. Finally,
54
Table of Contents
in the event a new Board is elected in a successful proxy contest, (i) the rights may not be redeemed and no business combination with us can be effected for 180 days thereafter unless certain procedures are followed to ensure (A) that steps are taken to maximize shareholder value, or (B) that any decision to redeem the rights, if challenged, would meet an ‘‘entire fairness’’ test; and (ii) the Rights Agreement may not be amended during such 180-day period. To establish ‘‘entire fairness’’ in connection with a redemption, the new Board must be able to demonstrate that all aspects of the redemption decision were fair, including the redemption procedure and the financial terms of the redemption. The Rights Agreement expires in March 2010.
| NOTE 11. | STOCK COMPENSATION PLANS |
We have various equity plans under which our officers, senior management, other key employees and directors may be granted options to purchase IFF common stock or other forms of equity-based awards. Beginning in 2004, we granted Restricted Stock Units (‘‘RSU’s’’) as the principal element of our equity compensation for all eligible U.S. − based employees and a majority of eligible overseas employees. Vesting of the RSU’s for officers and senior management has been performance and time based, and for the remainder of eligible employees, vesting is solely time based; the vesting period is primarily three years from date of grant. For a small group of employees, primarily overseas, we continue to grant stock options.
On January 1, 2006, we adopted FAS 123(R), and thereby recognized the cost of all employee stock options on a straight-line attribution basis over their respective vesting periods, net of estimated forfeitures. Total stock-based compensation expense included in our Consolidated Statement of Income for 2007, 2006 and 2005 was $18 million ($12 net of tax), $18 million ($12 net of tax), $7 million ($4 net of tax), respectively.
Under our 2000 Stock Award and Incentive Plan (‘‘2000 SAIP’’) the issuance of 9 million shares were authorized by the Board and approximately 2 million shares available under a previous plan were rolled into the 2000 SAIP making the total number of available shares approximately 11 million to satisfy awards. At December 31, 2007, 3,165,883 shares were subject to outstanding awards and 1,993,948 shares remained available for future awards.
In 2006, our Board approved a Long-Term Incentive Plan (‘‘LTIP’’) for senior management under our 2000 SAIP for the years 2006 − 2008 (‘‘Cycle VI’’). Under Cycle VI, each participant has a range of awards that would be paid out 50% in cash and 50% in equity at the end of the three-year cycle. The portion that would be paid in equity would not be determined until the end of the LTIP cycle. Because the number of shares is not fixed at the time of the award we account for these awards as liability based awards and recognize compensation expense on a straight-line basis over the three-year period based on the fair value of our stock at the end of each year and the expected payout based on the percent of performance achieved to date.
In 2007, our Board approved a LTIP for senior management under our 2000 SAIP for the years 2007 — 2009 (‘‘Cycle VII’’). Under Cycle VII, each participant has a range of awards that would be paid out 50% in cash and 50% in equity at the end of the three-year cycle and provides for segmentation in which one-fourth of the award vests during each twelve-month period, with the final one-fourth segment vesting over the full three-year period. These awards are earned based on the achievement of defined EPS targets and our performance ranking of total shareholder return as a percentile of the S&P 500. When the award is granted, 50% of the target dollar value of the award is converted to a number of ‘‘notional’’ shares based on the closing price on the date the grant is approved by the Board. Because the award vests over the three-year cycle, the graded-vesting attribution method is used to recognize compensa tion expense over the cycle.
In 2006, our Board approved a Long Term Incentive Choice program (the ‘‘Program’’) for senior management under our 2000 SAIP. Under the Program, eligible employees can choose from among three equity alternatives and will be granted such equity awards up to certain dollar awards depending on the participant’s grade level. A participant may choose among (1) Purchase Restricted Stock (‘‘PRS’’), (2) Stock Settled Appreciation Rights (‘‘SSAR’s ’’) or (3) RSU’s. The balance of employees who are not eligible under the Program receive RSU’s or, as noted above, options.
55
Table of Contents
Purchase Restricted Stock
PRS provides for the participant to purchase restricted shares of IFF stock at 50% of the fair market value on the grant date of the award. The shares vest on the third anniversary of the grant date, are subject to employment and other specified conditions, and pay dividends if and when paid by us. Holders of PRS have, in most instances, all of the rights of stockholders, except that they may not sell, assign, pledge or otherwise encumber such shares. RSU’s provide no such rights. We issued 209,920 shares of PRS in 2007 for an aggregate purchase price of $5 million and 217,905 shares in 2006 for $4 million.
Stock Options and SSAR’s
Stock options granted vest in periods ranging from one to three years and have a maximum term of ten years. SSAR’s become exercisable on the third anniversary of the grant date and have a maximum term of seven years. We awarded stock options and SSAR’s in 2007 of 188,000 and 66,493, respectively.
Prior to 2006, we applied the recognition and measurement principles of APB 25 and provided the pro forma disclosures required by FAS 123. Under APB 25, no compensation expense for employee or director stock options was reflected in net earnings.
In January 2006, we began using a Binomial lattice-pricing model instead of the Black-Scholes model, as our valuation model for estimating the fair value of option granted. The Binomial model can accommodate a broader array of inputs, such as stock price volatility and interest rates; the Black-Scholes model does not allow for incorporation of changes in such variables.
In applying the Binomial model, we utilize historical information to estimate expected term and forfeitures within the model. The expected term of an option is based on historical employee exercise behavior, vesting terms and a contractual life of primarily ten years for options and seven years for SSAR’s. The risk-free interest rate for periods within the expected term of the award is based on the U.S. Treasury yield curve in effect at the time of grant. Expected volatility is based on an average of implied and historical volatility of the price of our common stock over the calculated expected term. We anticipate paying cash dividends in the future and therefore use an expected dividend yield in the valuation model; the cash dividend in effect at the time of grant was employed in this calculation.
Principal assumptions used in applying the Binomial model in 2007 and 2006 and the Black-Scholes model in 2005 were:
| 2007 | 2006 | 2005 | ||||||||||||||||
| Risk-free interest rate | 4.7 | % | 5.0 | % | 4.2 | % | ||||||||||||
| Expected volatility | 21.7 | % | 21.7 | % | 26.9 | % | ||||||||||||
| Expected dividend yield | 1.6 | % | 2.1 | % | 1.7 | % | ||||||||||||
| Expected life, in years | 5 | 5 | 5 | |||||||||||||||
| Termination rate | 0.40 | % | 0.94 | % | NA | |||||||||||||
| Exercise multiple | 1.35 | 1.47 | NA | |||||||||||||||
NA − Not applicable under Black-Scholes valuation model
Stock option and SSAR activity was as follows:
| (SHARE AMOUNTS IN THOUSANDS) | Shares Subject to Options/SSARs | Weighted Average Exercise Price | Options/SSAR’s Exercisable | |||||||||||||||
| Balance at December 31, 2006 | 3,633 | $ | 33.56 | 2,851 | ||||||||||||||
| Granted | 254 | 51.47 | ||||||||||||||||
| Exercised | (1,302 | ) | 32.84 | |||||||||||||||
| Cancelled | (94 | ) | 39.67 | |||||||||||||||
| Balance at December 31, 2007 | 2,491 | $ | 35.66 | 1,725 | ||||||||||||||
56
Table of Contents
The weighted average exercise price of our options exercisable at December 31, 2007, 2006 and 2005 were $33.21, $32.75, and $32.21, respectively. The following tables summarize information concerning currently outstanding and exercisable options and SSAR’s:
Stock options and SSAR’s outstanding at December 31, 2007 were as follows:
| Price Range | Number Outstanding (in thousands) | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
| $15-$25 | 1 | 2.9 | $ | 17.94 | ||||||||||||||||||||
| $26-$30 | 591 | 4.8 | 29.26 | |||||||||||||||||||||
| $31-$35 | 1,178 | 5.4 | 33.64 | |||||||||||||||||||||
| $36-$40 | 310 | 7.2 | 37.45 | |||||||||||||||||||||
| $41-$45 | 79 | 6.3 | 42.24 | |||||||||||||||||||||
| $46-$55 | 332 | 7.1 | 51.01 | |||||||||||||||||||||
| 2,491 | $ | 35.66 | $ | 31,091 | ||||||||||||||||||||
Stock options and SSAR’s exercisable as of December 31, 2007 were as follows:
| Price Range | Number Outstanding (in thousands) | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
| $15-$25 | 1 | 2.9 | $ | 17.94 | ||||||||||||||||||||
| $26-$30 | 591 | 4.8 | 29.26 | |||||||||||||||||||||
| $31-$35 | 894 | 4.5 | 33.15 | |||||||||||||||||||||
| $36-$40 | 104 | 5.5 | 38.50 | |||||||||||||||||||||
| $41-$45 | 51 | 5.9 | 42.30 | |||||||||||||||||||||
| $46-$55 | 84 | 0.4 | 49.69 | |||||||||||||||||||||
| 1,725 | $ | 33.21 | $ | 25,754 | ||||||||||||||||||||
The weighted average grant date fair value of options and SSAR’s granted during 2007, 2006 and 2005 were $11.50, $7.66 and $10.57 respectively. The total intrinsic value of options exercised during 2007, 2006 and 2005 totaled $23 million, $35 million, and $7 million, respectively.
Stock option and SSAR activity for non-vested awards was as follows:
| (SHARE AMOUNTS IN THOUSANDS) | Shares | Weighted Average Exercise Price | ||||||||||
| Non-vested at December 31, 2006 | 782 | $ | 36.50 | |||||||||
| Granted | 254 | |||||||||||
| Vested | (215 | ) | ||||||||||
| Cancelled | (61 | ) | ||||||||||
| Non-vested at December 31, 2007 | 760 | $ | 41.13 | |||||||||
As of December 31, 2007, there was $4 million of total unrecognized compensation cost related to non-vested stock option and SSAR awards granted; such cost is expected to be recognized over a weighted average period of 1.8 years. The total fair value of outstanding vested shares during the years ended December 31, 2007, 2006 and 2005 was $16 million, $26 million and $49 million, respectively.
Restricted Stock and Units
We may grant restricted shares and RSU’s to eligible employees. Such restricted shares and RSU’s are subject to forfeiture if certain employment conditions are not met. RSU’s generally vest 100% at the end of three years with no performance criteria; however, RSU’s granted to all officers and senior management in 2005 contained a
57
Table of Contents
performance restriction since achieved and now vest at the end of their three-year term. The fair value of the RSU’s is equal to the market price of our stock at date of grant and is amortized to expense ratably over the vesting period.
Restricted stock and RSU activity was as follows:
| (SHARE IN THOUSANDS) | Number of Shares | Weighted-Average Grant Date Fair Value Per Share | ||||||||||
| Balance at December 31, 2006 | 1,346 | $ | 37.22 | |||||||||
| Granted | 445 | $ | 51.76 | |||||||||
| Vested | (439 | ) | $ | 50.81 | ||||||||
| Forfeited | (62 | ) | $ | 39.53 | ||||||||
| Balance at December 31, 2007 | 1,290 | $ | 42.81 | |||||||||
The total fair value of RSU’s which vested during the year ended December 31, 2007 was $22.3 million.
As of December 31, 2007, there was $19 million of total unrecognized compensation cost related to non-vested RSU awards granted under the equity incentive plans; such cost is expected to be recognized over a weighted average period of 2 years.
Prior to the adoption of FAS 123(R), we presented the tax benefits of deductions resulting from the exercise of stock options as cash flows from operating activities in the Consolidated Statement of Cash Flows. FAS 123(R) requires the cash flows from the excess tax benefits we realize on stock-based compensation to be presented as cash flows from financing activities. Pre-tax compensation expense for the year ended December 31, 2006 included a cumulative effect gain of $1 million from the adoption of FAS 123(R), which was recorded in operating expenses.
| NOTE 12. | SEGMENT INFORMATION |
On January 1, 2007, we reorganized into two business segments, Flavors and Fragrances; these segments align with the internal structure used to manage these businesses. Flavor compounds are sold to the food and beverage industries for use in consumer products such as prepared foods, beverages, dairy, food and confectionery products. The fragrances business unit, is comprised of three fragrance categories: functional fragrances, including fragrance compounds for personal care (e.g., soaps) and household products (e.g., detergents and cleaning agents); fine fragrance and beauty care, including perfumes, colognes and toiletries; and ingredients, consisting of synthetic ingredients that can be combined with other materials to create unique functional and fine fragrance compounds. Major fragrance customers include the cosmetics industry, including perfume and toiletries manufacturers, and the household products industry, including manufacturers of soap s, detergents, fabric care, household cleaners and air fresheners. Prior year segment information, which had been reported by major geographic region, has been reclassified to conform to the current presentation.
We evaluate the performance business units based on operating profit before interest expense, other income (expense), net and income taxes. The Global expense caption represents corporate and headquarters-related expenses which include legal, finance, human resources and other administrative expenses that are not allocated to individual business units. Unallocated assets are principally cash, short-term investments and other corporate and headquarters-related assets.
Our reportable segment information follows:
| Year Ended December 31, | ||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| Net sales | ||||||||||||||||||
| Flavors | $ | 1,005,544 | $ | 894,775 | $ | 857,724 | ||||||||||||
| Fragrances | 1,271,094 | 1,200,615 | 1,135,669 | |||||||||||||||
| Consolidated | $ | 2,276,638 | $ | 2,095,390 | $ | 1,993,393 | ||||||||||||
58
Table of Contents
| Year Ended December 31, | ||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| Operating income (expense) | ||||||||||||||||||
| Flavors | $ | 187,275 | $ | 153,099 | $ | 107,791 | ||||||||||||
| Fragrances | 209,812 | 212,240 | 186,752 | |||||||||||||||
| Global Expenses | (26,976 | ) | (18,485 | ) | (27,667 | ) | ||||||||||||
| Consolidated operating income | $ | 370,111 | $ | 346,854 | $ | 266,876 | ||||||||||||
| Interest expense | (41,535 | ) | (25,549 | ) | (23,956 | ) | ||||||||||||
| Miscellaneous other income (expense) (1) | 108 | (8,023 | ) | 3,268 | ||||||||||||||
| Income before taxes | $ | 328,684 | $ | 313,282 | $ | 246,188 | ||||||||||||
| (1) | Miscellaneous other income (expense) does not agree to the amounts on the Consolidated Statement of Income as gains on assets are included in the operating income of the business segments. |
| At December 31, | ||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | ||||||||||
| Segment assets | ||||||||||||
| Flavors | $ | 1,005,423 | $ | 972,814 | ||||||||
| Fragrances | 1,301,554 | 1,244,680 | ||||||||||
| Global Assets | 419,811 | 261,410 | ||||||||||
| Consolidated Segment assets | $ | 2,726,788 | $ | 2,478,904 | ||||||||
| Capital Expenditures | Depreciation and Amortization | |||||||||||||||||||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | 2007 | 2006 | 2005 | ||||||||||||||||||||||||||||||
| Flavors | $ | 24,794 | $ | 19,175 | $ | 27,820 | $ | 33,468 | $ | 35,785 | $ | 36,748 | ||||||||||||||||||||||||
| Fragrances | 36,335 | 34,689 | 58,566 | 47,668 | 52,652 | 54,146 | ||||||||||||||||||||||||||||||
| Unallocated assets | 4,485 | 4,418 | 7,047 | 1,652 | 1,296 | 1,034 | ||||||||||||||||||||||||||||||
| Consolidated | $ | 65,614 | $ | 58,282 | $ | 93,433 | $ | 82,788 | $ | 89,733 | $ | 91,928 | ||||||||||||||||||||||||
Sales to one customer accounted for 11% of total sales.
| Net Sales | ||||||||||||||||||
| Geographic Areas (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | |||||||||||||||
| EAME(1) | $ | 850 | $ | 758 | $ | 739 | ||||||||||||
| North America | 630 | 612 | 572 | |||||||||||||||
| Greater Asia | 491 | 439 | 420 | |||||||||||||||
| Latin America | 306 | 286 | 262 | |||||||||||||||
| Total | $ | 2,277 | $ | 2,095 | $ | 1,993 | ||||||||||||
| (1) | Europe, Africa and Middle East |
Net sales to external customers are attributed to individual regions based upon the destination of product delivery. Total long-lived assets consist of net property, plant and equipment and net intangible assets and amounted to $1,242 million, $1,241 million and $1,272 million at December 31, 2007, 2006 and 2005, respectively. Of this total $850 million, $867 million, and $946 million were located in the United States.
| NOTE 13. | RETIREMENT BENEFITS |
We, including some of our subsidiaries, have pension and/or other retirement benefit plans covering substantially all employees. Pension benefits are generally based on years of service and on compensation during the final years of employment. Plan assets consist primarily of equity securities and corporate and government
59
Table of Contents
fixed income securities. Substantially all pension benefit costs are funded as accrued; such funding is limited, where applicable, to amounts deductible for income tax purposes. Certain other retirement benefits are provided by balance sheet accruals.
We sponsor a qualified defined contribution plan covering substantially all U.S. employees. Under this plan, we match 100% of participants’ contributions up to 4% of compensation and 75% of participants’ contributions from over 4% to 8%. Employees that are still eligible to accrue benefits under the defined benefit plan are limited to a 50% match up to 6% of the participants’ contributions.
In addition to pension benefits, certain health care and life insurance benefits are provided to qualifying United States employees upon retirement from IFF. Such coverage is provided through insurance plans with premiums based on benefits paid. We do not generally provide health care or life insurance coverage for retired employees of foreign subsidiaries; such benefits are provided in most foreign countries by government-sponsored plans, and the cost of these programs is not significant to us.
The plan assets and benefit obligations of a majority of our pension plans are measured at December 31 of each year.
In 2006, we adopted SFAS No. 158, ‘‘Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans’’ which requires the recognition of the funded status of each defined pension benefit plan, retiree health care and other postretirement benefit plans and postemployment benefit plans on the balance sheet. Each overfunded plan is recognized as an asset and each underfunded plan is recognized as a liability. The initial impact of the standard due to unrecognized prior service costs or credits and net actuarial gains or losses as well as subsequent changes in the funded status is recognized as a component of accumulated other comprehensive income (‘‘AOCI’’) in shareowners’ equity. Additional minimum pension liabilities and related intangible assets were also eliminated upon adoption of the new standard.
| U.S. Plans | Non-U.S. Plans | |||||||||||||||||||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2005 | 2007 | 2006 | 2005 | ||||||||||||||||||||||||||||||
| Components of net periodic benefit cost | ||||||||||||||||||||||||||||||||||||
| Service cost for benefits earned | $ | 10,312 | $ | 9,941 | $ | 9,381 | $ | 11,633 | $ | 12,739 | $ | 10,317 | ||||||||||||||||||||||||
| Interest cost on projected benefit obligation | 23,953 | 21,716 | 21,218 | 35,595 | 29,391 | 28,701 | ||||||||||||||||||||||||||||||
| Expected return on plan assets | (24,266 | ) | (21,919 | ) | (21,406 | ) | (52,604 | ) | (39,767 | ) | (32,514 | ) | ||||||||||||||||||||||||
| Net amortization and deferrals | 6,590 | 7,436 | 5,357 | 6,071 | 8,753 | 8,457 | ||||||||||||||||||||||||||||||
| Settlement and curtailment | 5,943 | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Expense | 22,532 | 17,174 | 14,550 | 695 | 11,116 | 14,961 | ||||||||||||||||||||||||||||||
| Defined contribution and other retirement plans | 4,923 | 3,527 | 2,884 | 4,086 | 3,526 | 3,265 | ||||||||||||||||||||||||||||||
| Total pension expense | $ | 27,455 | $ | 20,701 | $ | 17,434 | $ | 4,781 | $ | 14,642 | $ | 18,226 | ||||||||||||||||||||||||
| Changes in plan assets and benefit obligations recognized in OCI | ||||||||||||||||||||||||||||||||||||
| Net actuarial gain | $ | (3,209 | ) | $ | (61,019 | ) | ||||||||||||||||||||||||||||||
| Actuarial loss | (5,746 | ) | (5,401 | ) | ||||||||||||||||||||||||||||||||
| Prior service cost | (6,787 | ) | (594 | ) | ||||||||||||||||||||||||||||||||
| Initial net asset | — | (76 | ) | |||||||||||||||||||||||||||||||||
| Currency translation adjustment | — | 9,675 | ||||||||||||||||||||||||||||||||||
| Total recognized in OCI (before tax effects) | $ | (15,742 | ) | $ | (57,415 | ) | ||||||||||||||||||||||||||||||
60
Table of Contents
The amounts expected to be recognized in net periodic cost in 2008 are:
| (DOLLARS IN THOUSANDS) | U.S. Plans | Non-U.S. Plans | Postretirement Benefits | |||||||||||||||
| Loss recognition | $ | 5,424 | $ | 2,491 | $ | 2,052 | ||||||||||||
| Prior service cost recognition | 419 | 576 | (2,262 | ) | ||||||||||||||
| Net initial obligation/(asset) recognition | — | 76 | — | |||||||||||||||
| U.S. Plans | Non-U.S. Plans | |||||||||||||||||||||||||||||||||||
| 2007 | 2006 | 2005 | 2007 | 2006 | 2005 | |||||||||||||||||||||||||||||||
| Weighted-average actuarial assumption used to determine expense | ||||||||||||||||||||||||||||||||||||
| Discount rate | 6.00 | % | 5.75 | % | 6.00 | % | 4.95 | % | 4.57 | % | 5.03 | % | ||||||||||||||||||||||||
| Expected return on plan assets | 8.25 | % | 8.25 | % | 8.25 | % | 7.06 | % | 6.99 | % | 6.86 | % | ||||||||||||||||||||||||
| Rate of compensation increase | 3.75 | % | 3.75 | % | 3.75 | % | 2.54 | % | 2.46 | % | 2.41 | % | ||||||||||||||||||||||||
Changes in the postretirement benefit obligation and plan assets, as applicable, are detailed in the following table:
| U.S. Plans | Non-U.S. Plans | Postretirement Benefits | ||||||||||||||||||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | ||||||||||||||||||||||||||||||
| Benefit obligation at beginning of year | $ | 378,670 | $ | 378,209 | $ | 680,950 | $ | 614,211 | $ | 104,495 | $ | 111,479 | ||||||||||||||||||||||||
| Service cost for benefits earned | 10,312 | 9,941 | 11,633 | 12,739 | 2,628 | 2,907 | ||||||||||||||||||||||||||||||
| Interest cost on projected benefit obligation | 23,953 | 21,716 | 35,595 | 29,391 | 5,869 | 5,487 | ||||||||||||||||||||||||||||||
| Actuarial (gain) loss | 16,525 | (12,213 | ) | (72,582 | ) | (32,001 | ) | (5,980 | ) | (6,509 | ) | |||||||||||||||||||||||||
| Plan amendments | — | — | — | (1,108 | ) | — | (5,149 | ) | ||||||||||||||||||||||||||||
| Plan participants’ contributions | — | — | 2,040 | 1,448 | 1,087 | 972 | ||||||||||||||||||||||||||||||
| Benefits paid | (19,903 | ) | (18,983 | ) | (27,925 | ) | (22,983 | ) | (5,402 | ) | (5,021 | ) | ||||||||||||||||||||||||
| Medicare Rx subsidy | — | — | — | — | 240 | 329 | ||||||||||||||||||||||||||||||
| Curtailments | (14,603 | ) | — | — | (339 | ) | — | — | ||||||||||||||||||||||||||||
| Translation adjustments | — | — | 31,661 | 79,592 | — | — | ||||||||||||||||||||||||||||||
| Benefit obligation at end of year | $ | 394,954 | $ | 378,670 | $ | 661,372 | $ | 680,950 | $ | 102,937 | $ | 104,495 | ||||||||||||||||||||||||
| Fair value of plan assets at beginning of year | $ | 336,057 | $ | 294,190 | $ | 694,295 | $ | 535,089 | ||||||||||||||||||||||||||||
| Actual return on plan assets | 29,397 | 37,487 | 39,120 | 45,749 | ||||||||||||||||||||||||||||||||
| Employer contributions | 3,680 | 23,363 | 18,680 | 59,214 | ||||||||||||||||||||||||||||||||
| Participants’ contributions | — | — | 2,040 | 1,448 | ||||||||||||||||||||||||||||||||
| Benefits paid | (19,903 | ) | (18,983 | ) | (27,925 | ) | (22,983 | ) | ||||||||||||||||||||||||||||
| Translation adjustments | — | — | 29,817 | 75,778 | ||||||||||||||||||||||||||||||||
| Fair value of plan assets at end of year | $ | 349,231 | $ | 336,057 | $ | 756,027 | $ | 694,295 | ||||||||||||||||||||||||||||
| Funded status at end of year | ($45,723 | ) | ($42,613 | ) | $ | 94,655 | $ | 13,345 | ||||||||||||||||||||||||||||
| U.S. Plans | Non-U.S. | |||||||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2007 | 2006 | ||||||||||||||||||||
| Amounts recognized in the balance sheet: | ||||||||||||||||||||||||
| Non-current assets | $ | — | $ | — | $ | 111,400 | $ | 33,886 | ||||||||||||||||
| Current liabilities | (2,586 | ) | (2,520 | ) | (667 | ) | (562 | ) | ||||||||||||||||
| Non-current liabilities | (43,137 | ) | (40,093 | ) | (16,078 | ) | (19,979 | ) | ||||||||||||||||
| Net amount recognized | $ | (45,723 | ) | $ | (42,613 | ) | $ | 94,655 | $ | 13,345 | ||||||||||||||
61
Table of Contents
| U.S. Plans | Non-U.S. Plans | Postretirement Benefits | ||||||||||||||||||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2007 | 2006 | 2007 | 2006 | ||||||||||||||||||||||||||||||
| Amounts Recognized in AOCI consist of: | ||||||||||||||||||||||||||||||||||||
| Net actuarial loss | $ | 41,682 | $ | 50,637 | $ | 97,437 | $ | 154,315 | $ | 38,033 | $ | 46,335 | ||||||||||||||||||||||||
| Prior service cost (credit) | 3,007 | 9,794 | 1,425 | 1,923 | (21,020 | ) | (23,682 | ) | ||||||||||||||||||||||||||||
| Unrecognized net initial obligation | — | — | (66 | ) | (27 | ) | — | — | ||||||||||||||||||||||||||||
| Total AOCI (before tax effects) | $ | 44,689 | $ | 60,431 | $ | 98,796 | $ | 156,211 | $ | 17,013 | $ | 22,653 | ||||||||||||||||||||||||
| U.S. Plans | Non-U.S. Plans | |||||||||||||||||||||||
| (DOLLARS IN THOUSANDS) | 2007 | 2006 | 2007 | 2006 | ||||||||||||||||||||
| Accumulated Benefit Obligation − end of year | $ | 374,732 | $ | 361,723 | $ | 628,728 | $ | 646,816 | ||||||||||||||||
| Information for Pension Plans with an ABO in excess of Plan Assets: | ||||||||||||||||||||||||
| Projected benefit obligation | $ | 48,550 | $ | 41,927 | $ | 22,370 | $ | 43,973 | ||||||||||||||||
| Accumulated benefit obligation | 44,422 | 41,823 | 19,876 | 38,524 | ||||||||||||||||||||
| Fair value of plan assets | 5,568 | 4,761 | 5,625 | 23,432 | ||||||||||||||||||||
| Weighted-average assumptions used to determine obligations at December 31 | ||||||||||||||||||||||||
| Discount rate | 6.10 | % | 6.00 | % | 5.78 | % | 4.95 | % | ||||||||||||||||
| Rate of compensation increase | 4.00 | % | 3.75 | % | 2.98 | % | 2.54 | % | ||||||||||||||||
| Percentage of assets invested in: | 2007 | 2006 | 2007 | 2006 | ||||||||||||||||||||
| Equities | 76 | % | 74 | % | 34 | % | 39 | % | ||||||||||||||||
| Bonds | 7 | % | 12 | % | 41 | % | 36 | % | ||||||||||||||||
| Property | n/a | n/a | 13 | % | 11 | % | ||||||||||||||||||
| Other investments | 17 | % | 14 | % | 12 | % | 14 | % | ||||||||||||||||
| (DOLLARS IN THOUSANDS) | U.S. Plans | Non-U.S. Plans | Postretirement Benefits | |||||||||||||||
| Estimated Future Benefit Payments | ||||||||||||||||||
| 2008 | $ | 19,852 | $ | 26,421 | $ | 3,680 | ||||||||||||
| 2009 | 20,524 | 27,699 | 3,879 | |||||||||||||||
| 2010 | 21,715 | 29,414 | 4,167 | |||||||||||||||
| 2011 | 22,825 | 33,045 | 4,420 | |||||||||||||||
| 2012 | 24,651 | 33,959 | 4,623 | |||||||||||||||
| 2013-2019 | 142,991 | 180,769 | 27,781 | |||||||||||||||
| Contributions | ||||||||||||||||||
| Expected Company Contributions in the Following Year (2008) | $ | 453 | $ | 15,809 | $ | 3,680 | ||||||||||||
62
Table of Contents
With respect to the U.S. plans, the expected return on plan assets was determined based on an asset allocation model using the current benchmark allocation, real rates of return by asset class and an anticipated inflation rate. The benchmark asset allocation was 10 − 20% employed in cash and fixed income investments expected to yield 1.0%; 10 − 20% employed in corporate and government bonds expected to yield 2.1%; and 65 − 75% in equity investments with a long-term expected yield of 8.5 − 9.3%. The inflation rate assumed in the model was 2.5%. The U.S. plan has employed a similar asset allocation strategy for the prior 20 years and has achieved a compounded annual return of 9.6% during this period. The expected annual rate of return for the non-U.S. plans employs a similar set of criteria adapted for local investments, inflation rates and in certain cases specific government requirements. The discount rate used for determining future pens ion obligations for each individual plan is based on a review of long-term bonds that receive a high rating from a recognized rating agency. Additionally, for the U.S. plans, the discount rate was based on the internal rate of return for a portfolio of Moody’s Aa3-rated bonds with maturities that are consistent with the projected future benefit payment obligations of the plan. The rate of compensation increase is based on plan experience.
In 2007, the percentage of assets held in equities increased primarily as a result of the performance of the equity investment portfolio. There has been no change in our long-term allocation.
Equity investments include our common stock valued at $14 million (4% of total plan assets) and $26 million (8% of total plan assets) at December 31, 2007 and 2006, respectively.
Total non-U.S. plan assets consist of a blend of various asset mixes defined by each plan’s liability profile and country or statutory requirements. Each plan maintains an investment policy appropriate to meet its benefit obligations.
The following weighted average assumptions were used to determine our postretirement benefit expense and obligation for the years ended December 31:
| Expense | Liability | |||||||||||||||||||||||
| 2007 | 2006 | 2007 | 2006 | |||||||||||||||||||||
| Discount rate | 6.00 | % | 5.75 | % | 6.10 | % | 6.00 | % | ||||||||||||||||
| Current medical cost trend rate | 9.00 | % | 8.00 | % | 8.00 | % | 9.00 | % | ||||||||||||||||
| Ultimate medical cost trend rate | 4.75 | % | 4.75 | % | 4.75 | % | 4.75 | % | ||||||||||||||||
| Medical cost trend rate decreases to ultimate rate in year | 2013 | 2011 | 2013 | 2013 | ||||||||||||||||||||
| Sensitivity of Disclosures to Changes in Selected Assumptions | ||||||||||||||||||||||||
| 25 BP Decrease in Discount Rate | 25 BP Decrease in Discount Rate | 25 BP Decrease in Long Term Rate of Return | ||||||||||||||||||||||
| Change in PBO | Change in ABO | Change in pension expense | Change in pension expense | |||||||||||||||||||||
| U.S. Pension Plans | $ | 10,213 | $ | 9,403 | $ | 1,008 | $ | 752 | ||||||||||||||||
| Non-U.S. Pension Plans | $ | 26,334 | $ | 24,049 | $ | 1,401 | $ | 1,880 | ||||||||||||||||
| Postretirement Benefit Plan | N/A | $ | 4,126 | $ | 419 | N/A | ||||||||||||||||||
The effect of a 1% increase in the medical cost trend rate would increase the accumulated postretirement benefit obligation, and the annual postretirement expense, by approximately $18 million and $2 million, respectively; a 1% decrease in the rate would decrease the obligation and expense by approximately $15 million and $1 million, respectively.
63
Table of Contents
The expected benefit payments shown above are net of the Medicare Part D subsidy. The following table shows the expected benefit payments prior to reflecting the subsidy, the expected subsidy and the expected benefit payments reflecting the subsidy.
| (DOLLARS IN THOUSANDS) | Not Reflecting Medicare Part D Subsidy | Medicare Part D Subsidy | Reflecting Medicare Part D Subsidy | |||||||||||||||
| Expected Benefit Payments | ||||||||||||||||||
| 2008 | $ | 4,219 | $ | (539 | ) | $ | 3,680 | |||||||||||
| 2009 | 4,472 | (593 | ) | 3,879 | ||||||||||||||
| 2010 | 4,807 | (640 | ) | 4,167 | ||||||||||||||
| 2011 | 5,104 | (684 | ) | 4,420 | ||||||||||||||
| 2012 | 5,361 | (738 | ) | 4,623 | ||||||||||||||
| 2013 − 2019 | 32,092 | (4,311 | ) | 27,781 | ||||||||||||||
We did not contribute to our qualified U.S. pension plans in 2007. In addition, $4 million of benefit payments were made with respect to the U.S. non-qualified plan. We contributed $15 million to our non-U.S. pension plans in 2007.
In 2007, we amended our U.S. salaried qualified and non-qualified pension plans under which accrual of future benefits was suspended for all participants that did not meet the rule of 70 (age plus years of service equal at least 70) at December 31, 2007. As a result of this suspension, we recorded a curtailment loss of $5.9 million to recognize a portion of the unrecognized prior service costs associated with years of service no longer expected to be rendered and credited as service under the plans.
| NOTE 14. | FINANCIAL INSTRUMENTS |
We enter into foreign currency forward contracts with the objective of reducing exposure to cash flow volatility associated with foreign currency receivables and payables, and with anticipated purchases of certain raw materials used in operations. These contracts, the counterparties to which are major international financial institutions, generally involve the exchange of one currency for a second currency at a future date, and have maturities not exceeding six months. The notional amount and maturity dates of such contracts match those of the underlying transactions. The gain or loss on the hedging instrument is recorded in earnings at the same time as the transaction being hedged is recorded in earnings. The associated asset or liability related to the open hedge instrument is recorded in Current Assets or Current Liabilities, as applicable.
We employ various interest rate swaps and debt issuances with the objective of managing and optimizing our interest rate exposure. In 2003, we executed a 10-year Yen − U.S. dollar currency swap related to the monthly sale and purchase of products between the U.S. and Japan. The annual notional value of this swap is approximately $5 million. As of December 31, 2007, the cash flow hedge experienced no ineffectiveness and therefore no net gain or loss is recognized in earnings during the reporting period. In addition, no component of the derivative instruments’ gain or loss is excluded from the assessment of hedge effectiveness. Interest income on the periodic settlement and the foreign exchange gain/loss on the closed out portion of the hedge is recorded in current income. Any gain or loss on the hedge is offset by a corresponding change in the receivable/revenue exchange rate. The gain or loss in the change in fair value of the remainin g hedge balance outstanding is marked to market in AOCI as a hedge of forecasted future cash flow and released month by month through earnings over the ten-year period of the hedge.
In September 2007, we entered into a $250 million interest rate swap agreement effectively converting the fixed rate on our long-term U.S. dollar borrowings to a variable short-term rate based on USD LIBOR rate plus markup. These swaps are designated as qualified fair value hedges. To the extent we have not received cash or otherwise amended or settled any swap agreements, any applicable mark-to-market adjustment relating to that swap is included as a separate component of debt. We had no ineffective fair value hedges at December 31, 2007.
In September 2007 and January 2006, we entered into a $250 million and a $300 million Cross Currency Interest Rate Swap, respectively, to hedge a portion of our consolidated EUR net investment. The derivative was structured as a swap of floating USD LIBOR to floating EURIBOR, with both floating interest rates reset and paid semi-annually. Because the derivatives are structured as a floating to floating swap, the only value, other than
64
Table of Contents
that derived from changes in the foreign exchange (FX) spot rate, are interest rate accruals which are reset semi-annually. These accruals are netted and booked in current earnings. Mark-to-market changes due to differences in the underlying FX rate are recorded in AOCI and provide an offset to the cumulative translation adjustment of the underlying Euro assets being hedged, which are also being valued based on changes in the FX spot rate. Effectiveness is assessed quarterly. At maturity, or if the swap is terminated early, any gain or loss will be recorded to AOCI until the Euro net investment is divested.
In 2002, we entered into certain interest rate swap agreements effectively converting the fixed rate on our long-term Japanese Yen borrowings to a variable short-term rate based on the Japanese Yen LIBOR rate plus an interest markup. These swaps are designated as qualified fair value hedges. During 2003 and 2005, we amended the swaps and the counterparty paid us $3 million and $1 million, respectively, including accrued interest. Such gains have been deferred, classified as a separate component of debt and are amortized over the remaining term of the debt. As of December 31, 2007, the fair value hedge experienced no ineffectiveness; therefore no net gain or loss is recognized in earnings during the reporting period. In addition, no component of the derivative instruments’ gain or loss is excluded from the assessment of hedge effectiveness. Interest income on the periodic settlement and reset of the floating interest rate is recorded in current income and the gain or loss in the change in fair value of the underlying debt attributable to the hedge risk adjusts the carrying amount of the hedged debt and is reflected as a component of income.
| NOTE 15. | CONCENTRATIONS OF CREDIT RISK |
We have no significant concentrations of risk in financial instruments. Temporary investments are made in a well-diversified portfolio of high-quality, liquid obligations of government, corporate and financial institutions. There are also limited concentrations of credit risk with respect to trade receivables because of the large number of customers spread across many industries and geographic regions.
| NOTE 16. | COMMITMENTS AND CONTINGENCIES |
Minimum rental commitments under non-cancelable operating leases are $24 million in 2008, $21 million in 2009, $20 million in 2010, $18 million in 2011, $16 million in 2012 and thereafter through 2031, the aggregate lease obligations are $194 million. The corresponding rental expense amounted to $27 million, $25 million and $21 million in 2007, 2006 and 2005, respectively. None of our leases contain step rent provisions or escalation clauses and they do not require capital improvement funding.
We are party to a number of lawsuits and claims related primarily to flavoring supplied by us to manufacturers of butter flavor popcorn. At each balance sheet date, or more frequently as conditions warrant, we review the status of each pending claim, as well as our insurance coverage for such claims with due consideration given to potentially applicable deductibles, retentions and reservation of rights under our insurance policies, and the advice of our outside legal counsel with respect to all these matters. While the ultimate outcome of any litigation cannot be predicted, management believes that adequate provision has been made with respect to all known claims. There can be no assurance that future events will not require us to increase the amount we have accrued for any matter or accrue for a matter that has not been previously accrued.
We recorded the expected liability with respect to these claims in Other liabilities and expected recoveries from our insurance carrier group in Other assets. We believe that realization of the insurance receivable is probable due to the terms of the insurance policies and the payment experience to date of the carrier group as it relates to these claims.
Over the past 20 years, various federal and state authorities and private parties have claimed that we are a Potentially Responsible Party (‘‘PRP’’) as a generator of waste materials for alleged pollution at a number of waste sites operated by third parties located principally in New Jersey and have sought to recover costs incurred and to be incurred to clean up the sites.
We have been identified as a PRP at nine facilities operated by third parties at which investigation and/or remediation activities may be ongoing. We analyze our liability on a regular basis and accrue for environmental liabilities when they are probable and estimable. At December 31, 2007, we estimated our share of the total future costs for these sites to be less than $5 million.
While joint and several liability is authorized under federal and state environmental laws, we believe that the amounts we have paid and anticipate paying in the future for clean-up costs and damages at all sites are not and
65
Table of Contents
will not be material to our financial condition, results of operations or liquidity. This conclusion is based upon, among other things, the involvement of other PRP’s at most sites, the status of the proceedings, including various settlement agreements and consent decrees, the extended time period over which payment will likely be made and an agreement reached in July 1994 with three of our liability insurers pursuant to which defense costs and indemnity amounts payable by us in respect of the sites will be shared by the insurers up to an agreed amount.
66
(a)(3) EXHIBITS
| Number | ||||||
| 3(i) | Restated Certificate of Incorporation of the Company, incorporated by reference to Exhibit 10(g) to Registrant’s Report on Form 10-Q filed on August 12, 2002 (SEC file number reference 001-04858). | |||||
| 3(ii) | By-laws of Registrant, incorporated by reference to Exhibit 3(ii) to Registrant’s Report on Form 8-K filed on July 16, 2007. | |||||
| 4 | .1 | Shareholder’s Protection Rights Agreement, dated as of March 21, 2000, between Registrant and The Bank of New York, as Rights Agent, incorporated by reference to Exhibit 4.1 to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| 4 | .1a | First Amendment dated September 26, 2000, to Shareholder Protection Rights Agreement, incorporated by reference to Exhibit 4.1a to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| 4 | .1b | Letter Agreement between the Registrant and Wachovia Bank, National Association (‘‘Wachovia’’), dated as of October 31, 2002, appointing Wachovia as Successor Rights Agent pursuant to the Shareholder Protection Rights Agreement, dated as of March 21, 2000 and amended as of September 26, 2000, incorporated by reference to Exhibit 4(a) to Registrant’s Report on Form 10-Q filed on November 12, 2002 (SEC file number reference 001-04858). | ||||
| 4 | .2 | Specimen Certificates of Registrant’s Common Stock bearing legend notifying of Shareholder Protection Rights Agreement, incorporated by reference to Exhibit 4(b) to Registrant’s Registration Statement on Form S-3 filed on September 29, 2000. (Reg. No. 333-46932). | ||||
| 4 | .3 | Note Purchase Agreement, dated as of July 12, 2006, by and among International Flavors & Fragrances Inc. and the various purchasers named therein, incorporated by reference to Exhibit 4.7 to Registrant’s Report on Form 8-K filed on July 13, 2006. | ||||
| 4 | .4 | Form of Series A, Series B, Series C and Series D Senior Notes incorporated by reference to Exhibit 4.8 of Registrant’s Report on Form 8-K filed on July 13, 2006. | ||||
| 4 | .5 | Note Purchase Agreement, dated as of September 27, 2007, by and among International Flavors & Fragrances Inc. and the various purchasers named therein, incorporated by reference to Exhibit 4.7 to Registrant’s Report on Form 8-K filed on October 1, 2007. | ||||
| 4 | .6 | Form of Series A, Series B, Series C and Series D Senior Notes incorporated by reference to Exhibit 4.8 of Registrant’s Report on Form 8-K filed on October 1, 2007. | ||||
| *10 | .1 | Letter Agreement, dated June 28, 2006, between Registrant and Robert M. Amen, Chairman of the Board of Directors and Chief Executive Officer, incorporated by reference to Registrant’s Report on Form 8-K filed on June 29, 2006. | ||||
| *10 | .2 | Letter Agreement dated as of November 8, 2007 between James H. Dunsdon, Senior Vice President and Chief Transition Officer, and the Company, incorporated by reference to Exhibit 10.1 to Registrant’s Report on Form 8-K filed on November 13, 2007. | ||||
| *10 | .3 | Compensation arrangements of Nicolas Mirzayantz, in connection with his appointment as Business Unit President, Fragrances, and Hernan Vaisman, in connection with his appointment as Business Unit President, Flavors, effective as of January 1, 2007, incorporated by reference to Registrant’s Report on Form 8-K filed on December 15, 2006; and further amended and effective on December 11, 2007 as incorporated by reference to Registrant’s Report on Form 8-K filed on December 13, 2007. | ||||
| *10 | .4 | 2007 Compensation Arrangements of Robert M. Amen, Douglas J. Wetmore, James H. Dunsdon, Nicolas Mirzayantz, Dennis Meany and Hernan Vaisman incorporated by reference to the Registrant’s Report on Form 8-K filed on March 12, 2007. | ||||
67
| Number | ||||||
| *10 | .5 | Supplemental Retirement Plan, adopted by the Registrant’s Board of Directors on October 29, 1986 as amended and restated through October 9, 2007. | ||||
| *10 | .6 | 2000 Stock Award and Incentive Plan, adopted by the Registrant’s Board of Directors on March 9, 2000 as amended and restated through October 9, 2007. | ||||
| *10 | .7 | 2000 Supplemental Stock Award Plan, adopted by the Registrant’s Board of Directors on November 14, 2000 as amended and restated through October 9, 2007. | ||||
| *10 | .8 | Registrant’s Executive Death Benefit Plan, effective July 1, 1990, incorporated by reference to Exhibit 10.6 to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| *10 | .9 | Registrant’s Vision 2001 Compensation Program, adopted by the Registrant’s Board of Directors on December 12, 2000 and amended in 2005, incorporated by reference to Exhibit 10.2 to Registrant’s Report on Form 8-K filed on January 28, 2005. | ||||
| *10 | .10 | Long Term Equity Choice Program Summary, incorporated by reference to Exhibit 10.3 to Registrant’s Report on Form 8-K filed on March 10, 2006. | ||||
| *10 | .11 | Performance Criteria for the Registrant’s 2005-2007 cycle, under the Company’s Long Term Incentive Plan, incorporated by reference to Exhibits 10.2 to Registrant’s Report on Form 8-K filed on March 11, 2005. | ||||
| *10 | .12 | Performance Criteria for the Registrant’s 2006-2008 cycle under the Company’s Long Term Incentive Plan, incorporated by reference to Exhibit 10.2 to Registrant’s Report on Form 8-K filed on March 10, 2006. | ||||
| *10 | .13 | Performance Criteria for the Registrant’s Annual Incentive Plan for 2007, incorporated by reference to Exhibit 10.1 to the Registrant’s Report on form 8-K filed on January 31, 2007. | ||||
| *10 | .14 | Performance Criteria for the 2007-2009 cycle under the Company’s Long Term Incentive Plan, incorporated by reference to Registrant’s Report on Form 8-K filed on March 12, 2007. | ||||
| *10 | .15 | Performance Criteria for the 2008-2010 cycle under the Company’s Long Term Incentive Plan, incorporated by reference to Registrant’s Report on Form 8-K filed on February 1, 2008. | ||||
| *10 | .16 | Form of Non-Employee Director’s Restricted Stock Unit Agreement under International Flavors & Fragrances Inc. 2000 Stock Award and Incentive Plan, incorporated by reference to Exhibit 10.7 to Registrant’s Report on Form 10-Q filed on October 31, 2007. | ||||
| *10 | .17 | Form of U.S. Restricted Stock Units Agreement under International Flavors & Fragrances Inc. 2000 Stock Award and Incentive Plan, incorporated by reference to Exhibit 10.5 to Registrant’s Report on Form 10-Q filed on October 31, 2007. | ||||
| *10 | .18 | Form of U.S. Purchased Restricted Stock Agreement under International Flavors & Fragrances Inc. 2000 Stock Award and Incentive Plan, incorporated by reference to Exhibit 10.4 to Registrant’s Report on Form 10-Q filed on October 31, 2007. | ||||
| *10 | .19 | Form of U.S. Stock Settled Appreciation Rights Agreement under International Flavors & Fragrances Inc. 2000 Stock Award and Incentive Plan, incorporated by reference to Exhibit 10.6 to Registrant’s Report on Form 10-Q filed on October 31, 2007. | ||||
| *10 | .20 | Non-Employee Director Compensation Arrangements, adopted by the Company’s Board of Directors on March 6, 2007, incorporated by reference to the Registrant’s Report on Form 8-K filed on March 12, 2007. | ||||
| *10 | .21 | Form of U.S. Performance-Based Restricted Stock Units Agreement under International Flavors & Fragrances Inc. 2000 Stock Award and Incentive Plan, incorporated by reference to Exhibit 10.8b to Registrant’s Report on Form 8-K filed on October 7, 2004. | ||||
68
| Number | ||||||
| *10 | .22 | Form of Employee Stock Option Agreement under International Flavors & Fragrances Inc. 2000 Stock Award and Incentive Plan, incorporated by reference to Exhibit 10.1 to Registrant’s Report on Form 10-Q filed on November 9, 2004. | ||||
| *10 | .23 | Form of International Flavors & Fragrances Inc. Stock Option Agreement under 2000 Stock Option Plan for Non-Employee Directors, incorporated by reference to Exhibit 10.2 to Registrant’s Report on Form 10-Q filed on November 9, 2004. | ||||
| *10 | .24 | Restated and Amended Executive Separation Policy as amended through and including December 31, 2007. | ||||
| *10 | .25 | 1997 Employee Stock Option Plan, incorporated by reference to Exhibit 10.18 to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| *10 | .26 | Amendment to 1997 Employee Stock Option Plan as amended by Registrant’s Board of Directors on February 8, 2000, incorporated by reference to Exhibit 10.19 to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| *10 | .27 | Resolutions Relating to Equity Awards as approved by the Board of Directors of the Registrant on January 29, 2007 incorporated by reference to Exhibit 10.25 to Registrant’s Report on Form 10-K filed on February 23, 2007. | ||||
| *10 | .28 | Deferred Compensation Plan adopted by Registrant’s Board of Directors on December 12, 2000, as amended and restated through October 9,2007. | ||||
| *10 | .29 | Trust Agreement dated October 4, 2000 among Registrant, First Union National Bank and Buck Consultants Inc. approved by Registrant’s Board of Directors on September 12, 2000, incorporated by reference to Exhibit 10.21 to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| *10 | .30 | Amendment dated August 2, 2005 to the Trust Agreement dated October 4, 2000 among Registrant, First Union National Bank and Buck Consultants Inc., incorporated by reference to Exhibit 10.1 to Registrant’s Report on Form 10-Q filed on August 4, 2005. | ||||
| *10 | .31 | 1990 Stock Option Plan for Non-Employee Directors, incorporated by reference to Exhibit 10.23 to Registrant’s Report on Form 10-K filed on March 13, 2006. | ||||
| *10 | .32 | 2000 Stock Option Plan for Non-Employee Directors as amended and restated as of December 15, 2004, incorporated by reference to Exhibit 10.2 to Registrant’s Report on Form 8-K filed on December 20, 2004. | ||||
| *10.33(a) | Director Charitable Contribution Program, adopted by the Board of Directors on February 14, 1995, incorporated by reference to Exhibit 10.25 to Registrant’s Report on Form 10-K filed on March 13, 2006. | |||||
| *10.33(b) | Summary of director charitable contribution arrangement between the Registrant and Arthur C. Martinez. | |||||
| *10 | .34 | Resolutions approving Non-Employee Directors’ Annual Stock Grant Program, adopted by Registrant’s Board of Directors on September 12, 2000, incorporated by reference to Exhibit 99(c) to Registrant’s Registration Statement on Form S-3 filed on September 29, 2000 (Reg. No. 333-46932). | ||||
| *10 | .35 | Retirement Agreement, dated April 3, 2006, between Registrant and Richard A. Goldstein, former Chairman of the Board of Directors and Chief Executive Officer, incorporated by reference to Exhibit 10.1 to Registrant’s Report on Form 8-K filed on April 3, 2006. | ||||
| *10 | .36 | Retirement Agreement dated November 30, 2006, between Registrant and Clint D. Brooks, former Senior Vice President, Research and Development, incorporated by reference to Registrant’s Report on Form 10-K filed on February 23, 2007. | ||||
69
| Number | ||||||
| *10 | .37 | Restricted Stock Units Agreement between Registrant and Arthur C. Martinez, dated July 25, 2006, incorporated by reference to Exhibit 10.1 to Registrant’s Report on Form 8-K filed on July 26, 2006. | ||||
| 10 | .38 | Multi-currency Revolving Credit Facility Agreement, dated November 23, 2005, among the Registrant, International Flavors & Fragrances (Luxembourg) S.A.R.L., certain subsidiaries, the banks named therein, including Citigroup Global Markets Limited, Fortis Bank S.A./N.V., Bank of America N.A., Bank of Tokyo-Mitsubishi Trust Company, BNP Paribas, ING Bank N.V., J.P. Morgan Chase and Wachovia Bank, National Association, as mandated lead arrangers, and Citibank International PLC, as Facility Agent, incorporated by reference to Exhibit 4.1 to Registrant’s Report on Form 8-K filed on Nov ember 29, 2005. | ||||
| 10 | .39 | Amendment Agreement dated September 17, 2007 to the Multicurrency Revolving Credit Facility Agreement dated November 23, 2005 among the Company, certain subsidiaries of the Company, and Citibank International PLC as agent on behalf of itself and others, incorporated by reference to Exhibit 10.1 to the Registrant’s Report on Form 10-Q filed on October 31, 2007. | ||||
| 10 | .40 | Amendment dated September 27, 2007 and confirmed on November 6, 2007, to the Multi-currency Revolving Credit Facility Agreement dated November 23, 2005; International Flavors and Fragrances Inc. requested an extension of the Termination Date for an additional period of 365 days until 2012. | ||||
| 10 | .41 | Confirmation, dated September 14, 2007, between International Flavors & Fragrances Inc. and Morgan Stanley & Co. Incorporated, incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed with the SEC on September 18, 2007. | ||||
| 10 | .42 | Confirmation, dated September 14, 2007, between International Flavors & Fragrances Inc. and Morgan Stanley & Co. Incorporated, incorporated by reference to Exhibit 10.2 to the Company’s Form 8-K filed with the SEC on September 18, 2007. | ||||
| 16 | .1 | Letter from PricewaterhouseCoopers LLP regarding change in Retirement Investment Fund Plan’s independent registered public accounting firm, incorporated by reference to Exhibit 16.1 on Registrant’s Report on Form 8-K filed on May 25, 2007. | ||||
| 21 | List of Principal Subsidiaries. | |||||
| 23 | Consent of PricewaterhouseCoopers LLP. | |||||
| 31 | .1 | Certification of Robert M. Amen pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||||
| 31 | .2 | Certification of Douglas J. Wetmore pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||||
| 32 | Certification of Robert M. Amen and Douglas J. Wetmore pursuant to 18 U.S.C. Section 1350 as adopted pursuant to the Sarbanes-Oxley Act of 2002. | |||||
| * | Management contract or compensatory plan or arrangement |
70
Table of Contents
Pursuant to the requirements of Section 13 of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| INTERNATIONAL FLAVORS & FRAGRANCES INC. |
| (Registrant) |
| By /s/ Douglas J. Wetmore Douglas J. Wetmore Senior Vice President, Chief Financial Officer and Treasurer |
Dated: February 27, 2008
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated:
Principal Executive Officer:
/s/ Robert M. Amen ��
Robert M. Amen
Chairman of the Board and
Chief Executive Officer
Principal Financial and Accounting Officer:
/s/ Douglas J. Wetmore
Douglas J. Wetmore
Senior Vice President,
Chief Financial Officer and Treasurer
Directors:
/s/ Robert M. Amen
ROBERT M. AMEN
/s/ Margaret Hayes Adame
MARGARET HAYES ADAME
/s/ Gunter Blobel
GUNTER BLOBEL
/s/ Marcello Bottoli
MARCELLO BOTTOLI
/s/ Linda B. Buck
LINDA B. BUCK
/s/ J. Michael Cook
J. MICHAEL COOK
/s/ Peter A. Georgescu
PETER A. GEORGESCU
/s/ Alexandra A. Herzan
ALEXANDRA A. HERZAN
/s/ Henry W. Howell, Jr.
HENRY W. HOWELL, JR.
/s/ Arthur C. Martinez
ARTHUR C. MARTINEZ
/s/ Burton M. Tansky
BURTON M. TANSKY
71
Table of Contents
INTERNATIONAL FLAVORS & FRAGRANCES INC. AND SUBSIDIARIES
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
(IN THOUSANDS)
| For the Year Ended December 31, 2007 | ||||||||||||||||||||||||||||||
| Balance at beginning of period | Additions charged to costs and expenses | Accounts written off | Translation adjustments | Balance at end of period | ||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 12,715 | $ | 1,369 | $ | 3,407 | $ | 1,017 | $ | 11,694 | ||||||||||||||||||||
| Valuation allowance on credit and operating loss carryforwards | 141,200 | 16,818 | — | 13,582 | 171,600 | |||||||||||||||||||||||||
| $ | 153,915 | $ | 18,187 | $ | 3,407 | $ | 14,599 | $ | 183,294 | |||||||||||||||||||||
| For the Year Ended December 31, 2006 | ||||||||||||||||||||||||||||||
| Balance at beginning of period | Additions charged to costs and expenses | Accounts written off | Translation adjustments | Balance at end of period | ||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 14,821 | $ | 894 | $ | 4,050 | $ | 1,050 | $ | 12,715 | ||||||||||||||||||||
| Valuation allowance on credit and operating loss carryforwards | 130,450 | (3,767 | ) | — | 14,517 | 141,200 | ||||||||||||||||||||||||
| $ | 145,271 | $ | (2,873 | ) | $ | 4,050 | $ | 15,567 | $ | 153,915 | ||||||||||||||||||||
| For the Year Ended December 31, 2005 | ||||||||||||||||||||||||||||||
| Balance at beginning of period | Additions charged to costs and expenses | Accounts written off | Translation adjustments | Balance at end of period | ||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 17,663 | $ | 910 | $ | 2,592 | $ | (1,160 | ) | $ | 14,821 | |||||||||||||||||||
| Valuation allowance on credit and operating loss carryforwards | 111,040 | 33,076 | — | (13,666 | ) | 130,450 | ||||||||||||||||||||||||
| $ | 128,703 | $ | 33,986 | $ | 2,592 | $ | (14,826 | ) | $ | 145,271 | ||||||||||||||||||||
S-1
INTERNATIONAL FLAVORS & FRAGRANCES INC.
INVESTOR INFORMATION
ANNUAL MEETING
The Annual Meeting of Shareholders will be held at the offices of the Company, 521 West 57th Street, New York, New York, on Tuesday, May 6, 2008 at 10:00 a.m., EDT.
A proxy statement and form of proxy will be mailed to each shareholder on or about March 21, 2008.
TRANSFER AGENT AND REGISTRAR
American Stock Transfer & Trust Company
59 Maiden Lane
New York, New York 10038
800-937-5449
www.amstock.com
LISTED
New York Stock Exchange

INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
PricewaterhouseCoopers LLP
WEB SITE
www.iff.com