United States
Securities and Exchange Commission
Washington, D.C. 20549
Form 10-K
Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
for the fiscal year ended December 31, 2008
Commission file number001-06351
Eli Lilly and Company
An Indiana corporation I.R.S. employer identification no. 35-0470950
Lilly Corporate Center, Indianapolis, Indiana 46285
(317) 276-2000
Securities registered pursuant to Section 12(b) of the Act:
| | | |
Title of Each Class | | Name of Each Exchange On Which Registered |
| |
| Common Stock (no par value) | | New York Stock Exchange |
| 6.57% Notes Due January 1, 2016 | | New York Stock Exchange |
7-1/8% Notes Due June 1, 2025 | | New York Stock Exchange |
| 6.77% Notes Due January 1, 2036 | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No þ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months, and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in the definitive proxy statement incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” inRule 12b-2 of the Exchange Act. (Check one):
| | | |
| Large accelerated filer þ | Accelerated filer o | Non-accelerated filer o | Smaller reporting company o |
(Do not check if a smaller reporting company)
Indicate by check mark whether the Registrant is a shell company as defined inRule 12b-2 of the Act: Yes o No þ
Aggregate market value of the common equity held by non-affiliates computed by reference to the price at which the common equity was last sold as of the last business day of the Registrant’s most recently completed second fiscal quarter (Common Stock): approximately $46,687,100,000
Number of shares of common stock outstanding as of February 13, 2009: 1,149,015,882
Portions of the Registrant’s Proxy Statement to be filed on or about March 9, 2009 have been incorporated by reference into Part III of this report.
Part I
Eli Lilly and Company (the “Company” or “Registrant”, which may be referred to as “we”, “us”, or “our”) was incorporated in 1901 in Indiana to succeed to the drug manufacturing business founded in Indianapolis, Indiana, in 1876 by Colonel Eli Lilly. We discover, develop, manufacture, and sell products in one significant business segment − pharmaceutical products. We also have an animal health business segment, whose operations are not material to our financial statements. We manufacture and distribute our products through owned or leased facilities in the United States, Puerto Rico, and 25 other countries. Our products are sold in approximately 135 countries.
Most of the products we sell today were discovered or developed by our own scientists, and our success depends to a great extent on our ability to continue to discover and develop innovative new pharmaceutical products. We direct our research efforts primarily toward the search for products to prevent and treat human diseases. We also conduct research to find products to treat diseases in animals and to increase the efficiency of animal food production.
Products
Our products include:
Neurosciences products,our largest-selling product group, including:
| | |
| | • | Zyprexa®,for the treatment of schizophrenia, acute mixed or manic episodes associated with bipolar I disorder, and bipolar maintenance |
| |
| | • | Cymbalta®, for the treatment of major depressive disorder, diabetic peripheral neuropathic pain, generalized anxiety disorder, and in the United States for the management of fibromyalgia |
| |
| | • | Strattera®,for the treatment of attention-deficit hyperactivity disorder in children, adolescents and adults |
| |
| | • | Prozac®,for the treatment of major depressive disorder, obsessive-compulsive disorder, bulimia nervosa and panic disorder |
| |
| | • | Symbyax®,for the treatment of bipolar depression |
Endocrinology products,including:
| | |
| | • | Humalog®,Humalog Mix 75/25®,andHumalog Mix 50/50tm, for the treatment of diabetes |
| |
| | • | Humulin®,for the treatment of diabetes |
| |
| | • | Byetta®, for the treatment of type 2 diabetes |
| |
| | • | Actos®,for the treatment of type 2 diabetes |
| |
| | • | Evista®,for the prevention and treatment of osteoporosis in postmenopausal women and for the reduction of the risk of invasive breast cancer in postmenopausal women with osteoporosis and postmenopausal women at high risk for invasive breast cancer |
| |
| | • | Forteo®,for the treatment of osteoporosis in postmenopausal women and men at high risk for fracture |
| |
| | • | Humatrope®,for the treatment of human growth hormone deficiency and idiopathic short stature |
Oncology products,including:
| | |
| | • | Gemzar®,for the treatment of pancreatic cancer; in combination with other agents, for the treatment of metastatic breast cancer, non-small cell lung cancer and advanced or recurrent ovarian cancer; and in the European Union for the treatment of bladder cancer |
-1-
| | |
| | • | Alimta®,for the first-line treatment, in combination with another agent, of non-small cell lung cancer for patients with non-squamous histology; for the second-line treatment of non-small cell lung cancer; and in combination with another agent, for the treatment of malignant pleural mesothelioma |
| |
| | • | Erbitux®,a product of ImClone Systems Incorporated, joined our oncology product portfolio upon our acquisition of ImClone in late November 2008. Erbitux is indicated both as a single agent and with other chemotherapy agents for the treatment of certain types of colorectal cancers and as a single agent or in combination with radiation therapy for head and neck cancers. |
Cardiovascular products, including:
| | |
| | • | Cialis®,for the treatment of erectile dysfunction |
| |
| | • | Efient®,for the prevention of atherothrombotic events in patients with acute coronary syndromes undergoing percutaneous coronary invention, was approved in February 2009 in the European Union. The drug is undergoing final regulatory review in the United States, where it would be marketed asEffient®. |
| |
| | • | ReoPro®,for use as an adjunct to percutaneous coronary intervention (“PCI”), including patients undergoing angioplasty, atherectomy or stent placement |
| |
| | • | Xigris®,for the treatment of adults with severe sepsis at high risk of death |
Animal health products, including:
| | |
| | • | Rumensin®,a cattle feed additive that improves feed efficiency and growth and also controls and prevents coccidiosis |
| |
| | • | Tylan®,an antibiotic used to control certain diseases in cattle, swine, and poultry |
| |
| | • | Micotil®,Pulmotil®,andPulmotil AC®,antibiotics used to treat respiratory disease in cattle, swine, and poultry, respectively |
| |
| | • | Paylean®andOptaflexx®,leanness and performance enhancers for swine and cattle, respectively |
| |
| | • | Posilac®, a protein supplement to improve milk productivity in dairy cows. We acquired the worldwide rights to Posilac from Monsanto Company in August 2008. |
| |
| | • | Coban®, Monteban®,andMaxiban®, anticoccidial agents for use in poultry |
| |
| | • | Apralan®, an antibiotic used to control enteric infections in calves and swine |
| |
| | • | Surmax®(sold asMaxus®in some countries), a performance enhancer for swine and poultry |
| |
| | • | Elector®, a parasiticide for use on cattle and premises |
| |
| | • | Two products for dogs:Comfortistm, the first FDA-approved, chewable tablet that kills fleas and prevents flea infestations on dogs; andReconciletm, for treatment of canine separation anxiety in conjunction with behavior modification training |
Other pharmaceuticals, including:
| | |
| | • | Vancocin®HCl, used primarily to treat staphylococcal infections |
| |
| | • | Ceclor®,for the treatment of a wide range of bacterial infections. |
Marketing
We sell most of our products worldwide. We adapt our marketing methods and product emphasis in various countries to meet local needs.
-2-
Pharmaceuticals − United States
In the United States, we distribute pharmaceutical products principally through independent wholesale distributors, with some sales directly to pharmacies. Our marketing policy is designed to assure that products and relevant medical information are immediately available to physicians, pharmacies, hospitals, public and private payers, and appropriate health care professionals throughout the country. Three wholesale distributors in the United States − AmerisourceBergen Corporation, Cardinal Health, Inc., and McKesson Corporation − each accounted for between 12 and 16 percent of our worldwide consolidated net sales in 2008. No other distributor accounted for more than 10 percent of consolidated net sales. We also sell pharmaceutical products directly to the United States government and other manufacturers, but those sales are not material.
We promote our major pharmaceutical products in the United States through sales representatives who call upon physicians and other health care professionals. We advertise in medical and drug journals, distribute literature and samples of certain products to physicians, and exhibit at medical meetings. In addition, we advertise certain products directly to consumers in the United States and we maintain web sites with information about all our major products. Divisions of our sales force are assigned to therapeutic areas, such as neuroscience, diabetes, osteoporosis, and oncology. We supplement our employee sales force with contract sales organizations as appropriate to leverage our own resources and the strengths of our partners in various markets.
Large purchasers of pharmaceuticals, such as managed-care groups, government agencies, and long-term care institutions, account for a significant portion of total pharmaceutical purchases in the United States. We maintain special business groups to service wholesalers, managed-care organizations, government and long-term care institutions, hospitals, and certain retail pharmacies. In response to competitive pressures, we have entered into arrangements with a number of these organizations providing for discounts or rebates on one or more Lilly products.
Pharmaceuticals − Outside the United States
Outside the United States, we promote our pharmaceutical products primarily through sales representatives. While the products marketed vary from country to country, neuroscience products constitute the largest single group in total sales. Distribution patterns vary from country to country. In most countries, we maintain our own sales organizations. In some countries, however, we market our products through independent distributors.
Pharmaceutical Marketing Collaborations
We market certain of our significant products in collaboration with other pharmaceutical companies:
| | |
| | • | Cymbalta is co-promoted in the United States by Quintiles Transnational Corp. and is co-promoted or co-marketed outside the U.S. (except Japan) by Boehringer Ingelheim GmbH. |
| |
| | • | Evista is marketed in major European markets by Daiichi Sankyo Europe GmbH, a subsidiary of Daiichi Sankyo Co., Ltd. of Japan. |
| |
| | • | We co-promote Byetta with Amylin Pharmaceuticals, Inc. in the United States and Puerto Rico, and we have exclusive marketing rights in other territories. |
| |
| | • | Erbitux is marketed in North America by Bristol-Myers Squibb. We co-promote Erbitux in North America. Outside North America, Erbitux is commercialized by Merck KGaA. We receive royalties from Bristol-Myers Squibb and Merck KGaA. |
| |
| | • | Efient will beco-promoted with us in major European markets by Daiichi Sankyo Europe GmbH. Assuming regulatory approvals, Daiichi Sankyo will alsoco-promote the product with us in the United States, Brazil, Mexico, China and several other Asian countries. Daiichi Sanko retains sole marketing rights in Japan, and we retain sole marketing rights in Canada, Australia, Russia and certain other countries. |
-3-
Animal Health Products
Our Elanco animal health business unit employs field salespeople throughout the United States to market animal health products. Elanco also has an extensive sales force outside the United States. Elanco sells its products primarily to wholesale distributors.
Competition
Our pharmaceutical products compete with products manufactured by many other companies in highly competitive markets throughout the world. Our animal health products compete on a worldwide basis with products of animal health care companies as well as pharmaceutical, chemical, and other companies that operate animal health divisions or subsidiaries.
Important competitive factors include product efficacy, safety, and ease of use, price and demonstrated cost-effectiveness, marketing effectiveness, service, and research and development of new products and processes. If competitors introduce new products or delivery systems with therapeutic or cost advantages, our products can be subject to progressive price reductions, decreased volume of sales, or both. Most new products that we introduce must compete with other products already on the market or products that are later developed by competitors. Manufacturers of generic pharmaceuticals invest far less in research and development than research-based pharmaceutical companies and therefore can price their products significantly lower than branded products. Accordingly, when a branded pharmaceutical loses its market exclusivity, it normally faces intense price competition from generic forms of the product. In many countries outside the United States, patent protection is weak or nonexistent and we must compete with generic versions of our products. Increasingly, to obtain favorable reimbursement and formulary positioning with government payers, managed care and pharmacy benefits management organizations, we must demonstrate that our products offer not only medical benefits but also cost advantages as compared with other forms of care.
We believe our long-term competitive position depends upon our success in discovering and developing (either alone or in collaboration with others) innovative, cost-effective products that serve unmet medical needs, together with our ability to continuously improve the productivity of our discovery, development, manufacturing, marketing and support operations in a highly competitive environment. There can be no assurance that our research and development efforts will result in commercially successful products or that our products or processes will not become uncompetitive from time to time as a result of products or processes developed by our competitors.
Patents, Trademarks, and Other Intellectual Property Rights
Overview
Intellectual property protection is, in the aggregate, material to our ability to successfully commercialize our life sciences innovations. We own, have applied for, or are licensed under, a large number of patents, both in the United States and in other countries, relating to products, product uses, formulations, and manufacturing processes. There is no assurance that the patents we are seeking will be granted or that the patents we have been granted would be found valid and enforceable if challenged. Moreover, patents relating to particular products, uses, formulations, or processes do not preclude other manufacturers from employing alternative processes or from marketing alternative products or formulations that might successfully compete with our patented products. In addition, from time to time, competitors or other third parties assert claims that our activities infringe patents or other intellectual property rights held by them, or allege a third-party right of ownership in our existing intellectual property.
Outside the United States, the adequacy and effectiveness of intellectual property protection for pharmaceuticals varies widely. Under the Trade-Related Aspects of Intellectual Property Agreement (TRIPs) administered by the World Trade Organization (WTO), over 140 countries have now agreed to provide non-discriminatory protection for most pharmaceutical inventions and to assure that adequate and effective rights are available to all patent owners. Because of TRIPs transition provisions, dispute resolution mechanisms, and substantive
-4-
limitations, it is still too soon to assess when and how much, if at all, we will benefit commercially from these changes.
When a product patent expires, the patent holder often loses effective market exclusivity for the product. This can result in a severe and rapid decline in sales of the formerly patented product, particularly in the United States. However, in some cases the innovator company may achieve exclusivity beyond the expiry of the product patent through manufacturing trade secrets, later-expiring patents on methods of use or formulations, or data-based exclusivity that may be available under pharmaceutical regulatory laws.
Some of our products, including Erbitux, Forteo, ReoPro, and Xigris, are biological products, or biologics. Additionally, many of the potential products in our research pipeline are biologics. Currently, generic versions of biologics cannot be approved under U.S. law. Competitors seeking approval of biologics must file their own safety and efficacy data, and address the challenges of biologics manufacturing, which involves more complex and costly processes than those of traditional pharmaceutical operations. However, the law could change in the future to allow generic biologics. Even in the absence of new legislation, the U.S. Food and Drug Administration (FDA) is taking steps toward allowing generic versions of certain biologics.
Our Intellectual Property Portfolio
We consider intellectual property protection for certain products, processes, and uses − particularly those products discussed below − to be important to our operations. For many of our products, in addition to the compound patent we hold other patents on manufacturing processes, formulations, or uses that may extend exclusivity beyond the expiration of the product patent.
The most relevant U.S. patent protection, together with expected expiration, for our major marketed products is as follows:
| | |
| | • | Alimta is protected by a compound patent (2016). |
| |
| | • | Byettais protected by a patent covering its use in treating type 2 diabetes (2017). |
| |
| | • | Cialisis protected by compound and use patents (2017). |
| |
| | • | Cymbaltais protected by a compound patent (2013). |
| |
| | • | Evistais protected by patents on the treatment and prevention of osteoporosis (2012 and 2014), and its dosage form (2017). Evista for use in breast cancer risk reduction is protected by orphan drug exclusivity (2014). |
| |
| | • | Gemzaris protected by a compound patent (2010) and a patent covering its antineoplastic use (2013). |
| |
| | • | Humalogis protected by a compound patent (2013). |
| |
| | • | Stratterais protected by a patent covering its use in treating attention deficit-hyperactivity disorder (2016). |
| |
| | • | Zyprexais protected by a compound patent (2011). |
Worldwide, we sell all of our major products under trademarks that we consider in the aggregate to be important to our operations. Trademark protection varies throughout the world, with protection continuing in some countries as long as the mark is used, and in other countries as long as it is registered. Registrations are normally for fixed but renewable terms.
Patent Licenses
Most of our important products were discovered in our own laboratories and are not subject to significant license agreements. Two of our larger products, Cialis and Alimta, are subject to patent assignments or licenses granted to us by others.
| |
| • | The compound patent for Cialis is the subject of a license agreement with Glaxo SmithKline which assigns to us exclusively all rights in the compound. The agreement calls for royalties of a single-digit percentage |
-5-
| |
| of net sales. The agreement is not subject to termination by Glaxo for any reason other than a material breach by Lilly of the royalty obligation, after a substantial cure period. |
| |
| • | The compound patent for Alimta is the subject of a license agreement with Princeton University, granting us an irrevocable exclusive worldwide license to the compound patents for the lives of the patents in the respective territories. The agreement calls for royalties of a single-digit percentage of net sales. The agreement is not subject to termination by Princeton for any reason other than a material breach by Lilly of the royalty obligation, after a substantial cure period. Alimta is also the subject of a worldwide, nonexclusive license to certain compound and process patents owned by Takeda Pharmaceutical Company Limited. The agreement calls for royalties of a single-digit percentage of net sales in countries covered by a relevant patent. The agreement is subject to termination for material default and failure to cure by Lilly and in the event that Lilly becomes bankrupt or insolvent. |
Patent Challenges
In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as “Hatch-Waxman,” made a complex set of changes to both patent and new-drug-approval laws. Before Hatch-Waxman, no drug could be approved without providing the FDA complete safety and efficacy studies,i.e., a complete New Drug Application (NDA). Hatch-Waxman authorizes the FDA to approve generic versions of innovative pharmaceuticals (other than biologics) without such information by filing an Abbreviated New Drug Application (ANDA). In an ANDA, the generic manufacturer must demonstrate only “bioequivalence” between the generic version and the NDA-approved drug − not safety and efficacy.
Absent a patent challenge, the FDA cannot approve an ANDA until after the innovator’s patents expire. However, after the innovator has marketed its product for four years, a generic manufacturer may file an ANDA alleging that one or more of the patents listed in the innovator’s NDA are invalid or not infringed. This allegation is commonly known as a “Paragraph IV certification.” The innovator must then file suit against the generic manufacturer to protect its patents. The FDA is then prohibited from approving the generic company’s application for a 30- to42-month period (which can be shortened or extended by the trial court judge hearing the patent challenge). If one or more of the NDA-listed patents are challenged, the first filer of a Paragraph IV certification may be entitled to a180-day period of market exclusivity over all other generic manufacturers.
In recent years, generic manufacturers have used Paragraph IV certifications extensively to challenge patents on a wide array of innovative pharmaceuticals, and we expect this trend to continue. In addition, generic companies have shown an increasing willingness to launch “at risk,” i.e., after receiving ANDA approval but before final resolution of their patent challenge. We are currently in litigation with numerous generic manufacturers arising from their Paragraph IV certifications on Alimta, Cymbalta, Evista, Gemzar, and Strattera. For more information on these, see Part II, Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters.”
Outside the United States, the legal doctrines and processes by which pharmaceutical patents can be challenged vary widely. In recent years, we have experienced an increase in patent challenges from generic manufacturers in many countries outside the United States, and we expect this trend to continue. For more information on significant patent challenges outside the United States, see Part II, Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters.”
Government Regulation
Regulation of Our Operations
Our operations are regulated extensively by numerous national, state and local agencies. The lengthy process of laboratory and clinical testing, data analysis, manufacturing development, and regulatory review necessary for required governmental approvals is extremely costly and can significantly delay product introductions in a given market. Promotion, marketing, manufacturing, and distribution of pharmaceutical and animal health products are extensively regulated in all major world markets. We are required to conduct extensive post-marketing surveillance of the safety of the products we sell. In addition, our operations are subject to complex
-6-
federal, state, local, and foreign laws and regulations concerning the environment, occupational health and safety, and privacy. The laws and regulations affecting the manufacture and sale of current products and the discovery, development and introduction of new products will continue to require substantial scientific and technical effort, time, and expense and significant capital investment.
Of particular importance is the FDA in the United States. Pursuant to the Federal Food, Drug, and Cosmetic Act, the FDA has jurisdiction over all of our products and administers requirements covering the testing, safety, effectiveness, manufacturing, quality control, distribution, labeling, marketing, advertising, dissemination of information and post-marketing surveillance of our pharmaceutical products. The FDA, along with the U.S. Department of Agriculture (USDA), also regulates our animal health products. The U.S. Environmental Protection Agency also regulates some animal health products. In 2007, Congress passed the Food and Drug Administration Amendments Act (FDAAA) of 2007, which imposes additional requirements for drug development and commercialization and provides the FDA with further authorities and resources, particularly in the area of drug safety.
The FDA extensively regulates all aspects of manufacturing quality under its current Good Manufacturing Practices (cGMP) regulations. In recent years, we have made, and we continue to make, substantial investments of capital and operating expenses to implement comprehensive, company-wide improvements in our manufacturing, product and process development, and quality operations to ensure sustained cGMP compliance. However, in the event we fail to adhere to cGMP requirements in the future, we could be subject to interruptions in production, fines and penalties, and delays in new product approvals.
Outside the United States, our products and operations are subject to similar regulatory requirements, notably by the European Medicines Agency (EMEA) in the European Union and the Ministry of Health, Labor and Welfare (MHLW) in Japan. Specific regulatory requirements vary from country to country.
The marketing, promotional, and pricing practices of pharmaceutical manufacturers, as well as the manner in which manufacturers interact with purchasers and prescribers, are subject to various other federal and state laws, including the federal anti-kickback statute and the False Claims Act and state laws governing kickbacks, false claims, unfair trade practices, and consumer protection. These laws are administered by, among others, the Department of Justice, the Office of Inspector General of the Department of Health and Human Services, the Federal Trade Commission, the Office of Personnel Management and state attorneys general. Over the past several years, the FDA, the Department of Justice, and many of these other agencies have increased their enforcement activities with respect to pharmaceutical companies and increased the inter-agency coordination of enforcement activities. Over this period, several claims brought by these agencies against Lilly and other companies under these and other laws have resulted in corporate criminal sanctions and very substantial civil settlements. See Part I, Item 3, “Legal Proceedings,” and Part II, Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters,” for information about currently pending and recently resolved marketing and promotional practices investigations involving Lilly, including information regarding a Corporate Integrity Agreement entered into by Lilly in connection with the resolution of a U.S. federal marketing practices investigation and certain related state investigations involving Zyprexa.
It is possible that we could become subject to additional administrative and legal proceedings and actions, which could include claims for civil penalties (including treble damages under the False Claims Act), criminal sanctions, and administrative remedies, including exclusion from federal health care programs. It is possible that an adverse outcome in pending or future actions could have a material adverse impact on our consolidated results of operations, liquidity, and financial position.
Regulations Affecting Pharmaceutical Pricing and Reimbursement
In the United States, we are required to provide rebates to state governments on their purchases of certain of our products under state Medicaid programs. Other cost containment measures have been adopted or proposed by federal, state, and local government entities that provide or pay for health care. In most international markets, we operate in an environment of government-mandated cost containment programs, which may include price controls, reference pricing, discounts and rebates, restrictions on physician prescription levels, restrictions on reimbursement, compulsory licenses, health economic assessments, and generic substitution.
-7-
In the U.S., the Medicare Prescription Drug Improvement and Modernization Act of 2003 (MMA), took effect in 2006, providing a prescription drug benefit for seniors under the Medicare program, known as Medicare Part D. Pricing to manufacturers for drugs covered by the program is currently established through competitive negotiations between the manufacturers and private payers. However, various measures have been proposed that would allow or require the federal government to negotiate Medicare Part D drug prices directly with manufacturers. In addition, various proposals have been introduced that would increase the rebates we pay to the government. See Part II, Item 7, “Management’s Discussion and Analysis − Executive Overview − Legal, Regulatory, and Other Matters,” for more discussion of MMA and other federal healthcare cost containment measures. At the state level, budget pressures are causing various states to impose cost-control measures such as higher rebates and more restrictive formularies.
International operations are also generally subject to extensive price and market regulations, and there are many proposals for additional cost-containment measures, including proposals that would directly or indirectly impose additional price controls, limit access to or reimbursement for our products, or reduce the value of our intellectual property protection.
We cannot predict the extent to which our business may be affected by these or other potential future legislative or regulatory developments. However, we expect that pressures on pharmaceutical pricing will become more severe.
Research and Development
Our commitment to research and development dates back more than 100 years. Our research and development activities are responsible for the discovery and development of most of the products we offer today. We invest heavily in research and development because we believe it is critical to our long-term competitiveness. At the end of 2008, we employed approximately 8,600 people in pharmaceutical and animal health research and development activities, including a substantial number of physicians, scientists holding graduate or postgraduate degrees, and highly skilled technical personnel. Our research and development expenses were $3.13 billion in 2006, $3.49 billion in 2007, and $3.84 billion in 2008.
Our pharmaceutical research and development focuses on four therapeutic categories: central nervous system and related diseases; endocrine diseases, including diabetes, obesity and musculoskeletal disorders; cancer; and cardiovascular diseases. However, we remain opportunistic, selectively pursuing promising leads in other therapeutic areas. We are actively engaged in a strong biotechnology research program including therapeutic proteins, antibodies and antisense oligonucleotides as well as genomics (the development of therapeutics through identification of disease-causing genes and their cellular function), biomarkers, and targeted therapeutics. In addition to discovering and developing new chemical entities, we look for ways to expand the value of existing products through new uses, formulations and therapeutic approaches that can provide additional benefits to patients. We also conduct research in animal health, including animal nutrition and physiology, control of parasites, and veterinary medicine (both food and companion animal).
To supplement our internal efforts, we collaborate with others, including educational institutions and research-based pharmaceutical and biotechnology companies, and we contract with others for the performance of research in their facilities. We use the services of physicians, hospitals, medical schools, and other research organizations worldwide to conduct clinical trials to establish the safety and effectiveness of our products. We actively seek out investments in external research and technologies that hold the promise to complement and strengthen our own research efforts. These investments can take many forms, including licensing arrangements, co-development and co-marketing agreements, co-promotion arrangements, joint ventures, and acquisitions.
Drug development is time-consuming, expensive, and risky. On average, only one out of many thousands of chemical compounds discovered by researchers proves to be both medically effective and safe enough to become an approved medicine. The process from discovery to regulatory approval can take 12 to 15 years or longer. Drug candidates can fail at any stage of the process, and even late-stage drug candidates sometimes fail to receive regulatory approval or commercial success. Even after approval and launch of a product, we expend considerable resources on post-marketing surveillance and clinical studies. We believe our investments in research, both internally and in collaboration with others, have been rewarded by the number of new
-8-
compounds and new indications for existing compounds that we have in all stages of development. Among our new investigational compounds in the later stages of development are potential therapies for acute coronary syndromes, diabetes, osteoporosis, and cancer. Further, we are studying many other drug candidates in the earlier stages of development, including compounds targeting cancers, diabetes, obesity, musculoskeletal disorders, lipid abnormalities, Alzheimer’s disease, schizophrenia, multiple sclerosis, depression, sleep disorders, pain and migraine, attention-deficit hyperactivity disorder (ADHD), alcoholism, and autoimmune disorders including rheumatoid arthritis. At present we have approximately 60 drug candidates across all stages of clinical development. We are also developing new uses and formulations for many of these compounds as well as our currently marketed products, such as Alimta, Byetta, Cialis, Cymbalta, Erbitux, Forteo, Gemzar, and Zyprexa.
Raw Materials and Product Supply
Most of the principal materials we use in our manufacturing operations are available from more than one source. We obtain certain raw materials principally from only one source. In addition, Byetta is manufactured by third-party suppliers to Amylin. In the event one of these suppliers was unable to provide the materials or product, we generally have sufficient inventory to supply the market until an alternative source of supply can be implemented. However, in the event of an extended failure of a supplier, it is possible that we could experience an interruption in supply until we established new sources or, in some cases, implemented alternative processes.
Our primary bulk manufacturing occurs at three sites in Indiana as well as locations in Ireland, Puerto Rico, and the United Kingdom. Finishing operations, including labeling and packaging, take place at a number of sites throughout the world.
We seek to design and operate our manufacturing facilities and maintain inventory in a way that will allow us to meet all expected product demand while maintaining flexibility to reallocate manufacturing capacity to improve efficiency and respond to changes in supply and demand. However, pharmaceutical production processes are complex, highly regulated, and vary widely from product to product. Shifting or adding manufacturing capacity can be a very lengthy process requiring significant capital expenditures and regulatory approvals. Accordingly, if we were to experience extended plant shutdowns or extraordinary unplanned increases in demand, we could experience an interruption in supply of certain products or product shortages until production could be resumed or expanded.
Quality Assurance
Our success depends in great measure upon customer confidence in the quality of our products and in the integrity of the data that support their safety and effectiveness. Product quality arises from a total commitment to quality in all parts of our operations, including research and development, purchasing, facilities planning, manufacturing, and distribution. We have implemented quality-assurance procedures relating to the quality and integrity of scientific information and production processes.
Control of production processes involves rigid specifications for ingredients, equipment, facilities, manufacturing methods, packaging materials, and labeling. We perform tests at various stages of production processes and on the final product to assure that the product meets all regulatory requirements and our standards. These tests may involve chemical and physical chemical analyses, microbiological testing, testing in animals, or a combination. Additional assurance of quality is provided by a corporate quality-assurance group that monitors existing pharmaceutical and animal health manufacturing procedures and systems in the parent company, subsidiaries and affiliates, and third-party suppliers.
-9-
Executive Officers of the Company
The following table sets forth certain information regarding our executive officers. All executive officers except Mr. Azar have been employed by the Company in executive positions during the last five years.
The term of office for each executive officer expires on the date of the annual meeting of the Board of Directors, to be held on April 20, 2009, or on the date his or her successor is chosen and qualified. No director or executive officer of the Company has a “family relationship” with any other director or executive officer of the Company, as that term is defined for purposes of this disclosure requirement. There is no understanding between any executive officer and any other person pursuant to which the executive officer was selected.
| | | | | | | |
| Name | | Age | | Offices |
| |
|
| John C. Lechleiter, Ph.D. | | | 55 | | | Chairman (since January 2009), President (since October 2005), Chief Executive Officer (since April 2008) and a Director |
| Robert A. Armitage | | | 60 | | | Senior Vice President and General Counsel (since January 2003) |
| Alex M. Azar II | | | 41 | | | Senior Vice President, Corporate Affairs and Communications (since June 2007). From 2005 to 2007, Azar served as Deputy Secretary of the U.S. Department of Health and Human Services (HHS). From 2001 to 2005, he served HHS as General Counsel. |
| Bryce D. Carmine | | | 57 | | | Executive Vice President, Marketing and Sales (since April 2008) |
| Frank M. Deane, Ph.D. | | | 59 | | | President, Manufacturing Operations (since June 2007) |
| Anthony J. Murphy, Ph.D. | | | 58 | | | Senior Vice President, Human Resources (since June 2005) |
| Steven M. Paul, M.D. | | | 58 | | | Executive Vice President, Science and Technology (since July 2003) |
| Derica W. Rice | | | 44 | | | Senior Vice President and Chief Financial Officer (since May 2006) |
| Gino Santini | | | 52 | | | Senior Vice President, Corporate Strategy and Business Development (since June 2007) |
Employees
At the end of 2008, we employed approximately 40,500 people, including approximately 19,600 employees outside the United States. A substantial number of our employees have long records of continuous service.
Financial Information Relating to Business Segments and Classes of Products
You can find financial information relating to our business segments and classes of products in Part II, Item 8 of thisForm 10-K, “Segment Information.” That information is incorporated here by reference.
The relative contribution of any particular product to our consolidated net sales changes from year to year. This is due to several factors, including the introduction of new products by us and by other manufacturers and the introduction of generic pharmaceuticals upon patent expirations. In addition, margins vary for our different products due to various factors, including differences in the cost to manufacture and market the products, the value of the products to the marketplace, and government restrictions on pricing and reimbursement. Our major product sales are generally not seasonal.
Financial Information Relating to Foreign and Domestic Operations
You can find financial information relating to foreign and domestic operations in Part II, Item 8 of thisForm 10-K, “Segment Information.” That information is incorporated here by reference.
-10-
To date, our overall operations abroad have not been significantly deterred by local restrictions on the transfer of funds from branches and subsidiaries located abroad, including the availability of dollar exchange. We cannot predict what effect these restrictions or the other risks inherent in foreign operations, including possible nationalization, might have on our future operations or what other restrictions may be imposed in the future. In addition, changing currency values can either favorably or unfavorably affect our financial position and results of operations. We actively manage foreign exchange risk through various hedging techniques including the use of foreign currency contracts.
Available Information on Our Web Site
We make available through our company web site, free of charge, our company filings with the Securities and Exchange Commission (SEC) as soon as reasonably practicable after we electronically file them with, or furnish them to, the SEC. The reports we make available include our annual reports onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K, proxy statements, registration statements, and any amendments to those documents. The company web site link to our SEC filings ishttp://investor.lilly.com/edgar.cfm.
In addition, the Corporate Governance portion of our web site includes our corporate governance guidelines, board and committee information (including committee charters), and our articles of incorporation and by-laws. The link to our corporate governance information ishttp://investor.lilly.com/corp-gov.cfm.
We will provide paper copies of our SEC filings and corporate governance documents free of charge upon request to the company’s secretary at the address listed on the front of thisForm 10-K.
| |
| Item 1A: | Risk Factors; Cautionary Statement Regarding Forward Looking Statements |
In addition to the other information contained in thisForm 10-K, the following risk factors should be considered carefully in evaluating our company. It is possible that our business, financial condition, liquidity or results of operations could be materially adversely affected by any of these risks.
We have made certain forward-looking statements in thisForm 10-K, and company spokespeople may make such statements in the future based on then-current expectations of management. Where possible, we try to identify forward-looking statements by using such words as “expect,” “plan,” “will,” “estimate,” “forecast,” “project,” “believe,” “anticipate,” and similar expressions. Forward-looking statements do not relate strictly to historical or current facts. They are likely to address our growth strategy, sales of current and anticipated products, financial results, the results of our research and development programs, the status of product approvals, and the outcome of contingencies such as litigation and investigations. All forward-looking statements made by us are subject to risks and uncertainties, including those summarized below.
| |
| • | Pharmaceutical research and development is very costly and highly uncertain. There are many difficulties and uncertainties inherent in new product research and development and the introduction of new products. There is a high rate of failure inherent in the research to develop new drugs. To bring a pharmaceutical compound from the discovery phase to market typically takes a decade or more and costs over $1 billion. Failure can occur at any point in the process, including late in the process after significant funds have been invested. As a result, there is a significant risk that funds invested in research programs will not generate financial returns. New product candidates that appear promising in development may fail to reach the market or may have only limited commercial success because of efficacy or safety concerns, inability to obtain necessary regulatory approvals, limited scope of approved uses, difficulty or excessive costs to manufacture, or infringement of the patents or intellectual property rights of others. Delays and uncertainties in the FDA approval process and the approval processes in other countries can result in delays in product launches and lost market opportunity. In recent years, FDA review times have increased substantially and fewer new drugs are being approved. In addition, it can be very difficult to predict sales growth rates of new products. |
-11-
| |
| • | We face intense competition. We compete with large number of multinational pharmaceutical companies, biotechnology companies and generic pharmaceutical companies. To compete successfully, we must continue to deliver to the market innovative, cost-effective products that meet important medical needs. Our product sales can be adversely affected by the introduction by competitors of branded products that are perceived as superior by the marketplace, by generic versions of our branded products, and by generic versions of other products in the same therapeutic class as our branded products. See Item 1, “Business − Competition,” for more details. |
| |
| • | Our long-term success depends on intellectual property protection. Our long-term success depends on our ability to continually discover, develop, and commercialize innovative new pharmaceutical products. Without strong intellectual property protection, we would be unable to generate the returns necessary to support the enormous investments in research and development, capital, and other expenditures required to bring new drugs to the market. Several major products will lose intellectual property protection in the U.S. in the next decade beginning in late 2011. Several of these products will lose intellectual property protection in various countries outside the U.S. even before then. See Item 1, “Business − Patents, Trademarks, and Other Intellectual Property Protection,” for more details. |
Intellectual property protection varies throughout the world and is subject to change over time. In the U.S., the Hatch-Waxman Act provides generic companies powerful incentives to seek to invalidate our patents; as a result, we expect that our U.S. patents on major products will be routinely challenged, and there can be no assurance that our patents will be upheld. See Item 1, “Business − Patents, Trademarks, and Other Intellectual Property Protection,” for more details. We are increasingly facing generic manufacturer challenges to our patents outside the U.S. as well. In addition, competitors or other third parties may claim that our activities infringe patents or other intellectual property rights held by them. If successful, such claims could result in our being unable to market a product in a particular territory or being required to pay damages for past infringement or royalties on future sales.
| |
| • | Our business is subject to increasing government price controls and other health care cost containment measures. Government health care cost-containment measures can significantly affect our sales and profitability. In many countries outside the United States, government agencies strictly control, directly or indirectly, the prices at which our products are sold. In the United States, we are subject to substantial pricing pressures from state Medicaid programs and private insurance programs and pharmacy benefit managers, including those operating under the Medicare Part D pharmaceutical benefit. Many federal and state legislative proposals would further negatively affect our pricingand/or reimbursement for our products. We expect pricing pressures from both governments and private payers inside and outside the United States to become more severe. See Item I, “Business − Regulations Affecting Pharmaceutical Pricing and Reimbursement,” for more details. |
| |
| • | Pharmaceutical products can develop unexpected safety or efficacy concerns. Unexpected safety or efficacy concerns can arise with respect to marketed products, leading to product recalls, withdrawals, or declining sales, as well as costly product liability claims. |
| |
| • | We depend on key products for most of our revenues, cash flows, and earnings. Zyprexa sales of $4.70 billion represented 23 percent of our revenues in 2008, and Cymbalta sales of $2.70 billion constituted 13 percent of our 2008 revenues. Six other products − Humalog, Gemzar, Cialis, Alimta, Evista, and Humulin − each contributed more than $1 billion in revenues in 2008. If these or other key products were to become subject to a problem such as loss of patent protection, materially adverse changes in prescription growth rates, unexpected side effects, regulatory proceedings, material product liability litigation, publicity affecting doctor or patient confidence, or pressure from competitive products, the adverse impact on our revenues, cash flows and earnings could be significant. |
| |
| • | Regulatory compliance problems could be damaging to the company. The marketing, promotional, and pricing practices of pharmaceutical manufacturers, as well as the manner in which manufacturers interact with purchasers, prescribers, and patients, are subject to extensive regulation. Many companies, including Lilly, have been subject to claims related to these practices asserted by federal and state governmental authorities and private payers and consumers. These claims have resulted in substantial expense and other |
-12-
| | |
| | | significant consequences to the company. It is possible other products could become subject to investigation and that the outcome of these matters could include criminal charges and fines, penalties, or other monetary or nonmonetary remedies. In particular, See Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters,” for the discussions of the U.S. sales and marketing practices investigations. In addition, regulatory issues concerning compliance with current Good Manufacturing Practice (cGMP) regulations for pharmaceutical products can lead to product recalls and seizures, interruption of production leading to product shortages, and delays in the approvals of new products pending resolution of the cGMP issues. We are now operating under a Corporate Integrity Agreement with the Office of Inspector General of the U.S. Department of Health and Human Services that requires us to maintain comprehensive compliance programs governing our research, manufacturing, and sales and marketing of pharmaceuticals. Material failures to comply with the Agreement could result in severe sanctions to the company. See Item 1, “Business − Regulation of our Operations,” for more details. |
| |
| • | We face many product liability claims today, and future claims will be largely self-insured. We are subject to a substantial number of product liability claims involving primarily Zyprexa, DES, and thimerosal, and because of the nature of pharmaceutical products, it is possible that we could become subject to large numbers of product liability claims for other products in the future. See Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters,” and Item 3, “Legal Proceedings,” for more information on our current product liability litigation. In the past few years, we have experienced difficulties in obtaining product liability insurance due to a very restrictive insurance market. Therefore, for substantially all our currently marketed products we have been and expect that we will continue to be largely self-insured for future product liability losses. In addition, there is no assurance that we will be able to fully collect from our insurance carriers on past claims. |
| |
| • | Manufacturing difficulties could lead to product supply problems. Pharmaceutical manufacturing is complex and highly regulated. Manufacturing difficulties can result in product shortages, leading to lost sales. See Item 1, “Business − Raw Materials and Product Supply,” for more details. |
| |
| • | The current volatility in financial markets could adversely affect the cost and availability of financing. Although the current contraction of the credit markets has not yet materially affected our borrowing costs or flexibility, if there is additional significant contraction of the markets, it could adversely affect our ability to obtain short-term or long-term financing at reasonable rates. |
| |
| • | A prolonged economic downturn could adversely affect our business and operating results. While pharmaceuticals have not generally been sensitive to overall economic cycles, a prolonged economic downturn coupled with rising unemployment (and a corresponding increase in the uninsured and underinsured population) could lead to decreased utilization of drugs, affecting our sales volume. Declining tax revenues attributable to the downturn may increase the pressure on governments to reduce healthcare spending, leading to increasing government efforts to control drug prices. In addition, a prolonged economic downturn could have an adverse impact on our investment portfolio, which could lead to the recognition of losses on our corporate investments and increased benefit expense related to our pension investments. Also, if our customers, suppliers or collaboration partners experience financial difficulties, we could experience slower customer collections, greater bad debt expense, and performance defaults by suppliers or collaboration partners. |
| |
| • | We face other risks to our business and operating results. Our business is subject to a number of other risks and uncertainties, including: |
| | |
| | • | Economic factors over which we have no control, including changes in inflation, interest rates and foreign currency exchange rates can affect our results of operations. |
| |
| | • | Changes in tax laws, including laws related to the remittance of foreign earnings or investments in foreign countries with favorable tax rates, and settlements of federal, state, and foreign tax audits, can affect our net income. |
-13-
| | |
| | • | Changes in accounting standards promulgated by the Financial Accounting Standards Board, the Securities and Exchange Commission, and the Emerging Issues Task Force can affect reported results. |
| |
| | • | Our results can also be affected by internal factors, such as changes in business strategies and the impact of restructurings, asset impairments, technology acquisition and disposition transactions, and business combinations. |
We undertake no duty to update forward-looking statements.
| |
| Item 1B. | Unresolved Staff Comments |
None.
Our principal domestic and international executive offices are located in Indianapolis. At December 31, 2008, we owned 15 production and distribution facilities in the United States and Puerto Rico. Together with the corporate administrative offices, these facilities contain an aggregate of approximately 15.6 million square feet of floor area dedicated to production, distribution, and administration. Major production sites include Indianapolis, Clinton, and Lafayette, Indiana; two sites in Puerto Rico; Branchburg, New Jersey; and Augusta, Georgia.
We own production and distribution facilities in 15 countries outside the United States and Puerto Rico, containing an aggregate of approximately 3.9 million square feet of floor space. Major production sites include facilities in France, Ireland, Spain, Brazil, Italy, and the United Kingdom. We lease production and warehouse facilities in Puerto Rico and several countries outside the United States.
Our research and development facilities in the United States consist of approximately 3.4 million square feet and are located primarily in Indianapolis, with smaller sites in San Diego and New York City. In October 2008 we sold our Greenfield, Indiana research facility to Covance Inc. Our major research and development facilities abroad are located in United Kingdom, Canada, Singapore, and Spain, and contain an aggregate of approximately 350,000 square feet.
We believe that none of our properties is subject to any encumbrance, easement, or other restriction that would detract materially from its value or impair its use in the operation of the business. The buildings we own are of varying ages and in good condition.
| |
| Item 3. | Legal Proceedings |
We are a party to various currently pending legal actions, government investigations, and environmental proceedings, and we anticipate that such actions could be brought against us in the future. The most significant of these matters are described below or, as noted, in Part II, Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters.” While it is not possible to determine the outcome of the legal actions, investigations and proceedings brought against us, we believe that, except as otherwise specifically noted in Part II, Item 7, the resolution of all such matters will not have a material adverse effect on our consolidated financial position or liquidity, but could possibly be material to our consolidated results of operations in any one accounting period.
Legal Proceedings Described in Management’s Discussion and Analysis
See Part II, Item 7, “Management’s Discussion and Analysis − Legal and Regulatory Matters,” for information on various legal proceedings, including but not limited to:
| | |
| | • | The U.S. patent litigation involving Alimta, Cymbalta, Evista, Gemzar, and Xigris |
| |
| | • | The patent litigation outside the U.S. involving Zyprexa |
-14-
| | |
| | • | The investigations by the U.S. Attorney for the Eastern District of Pennsylvania and various state attorneys general relating to our U.S. sales, marketing, and promotional practices |
| |
| | • | The Zyprexa product liability and related litigation, including claims brought on behalf of state Medicaid agencies and private healthcare payers |
That information is incorporated into this Item by reference.
Other Patent Litigation
Strattera: Actavis Elizabeth LLC (Actavis), Glenmark Pharmaceuticals Inc., USA (Glenmark), Sun Pharmaceutical Industries Limited (Sun), Sandoz Inc. (Sandoz), Mylan Pharmaceuticals Inc. (Mylan), Teva Pharmaceuticals USA, Inc. (Teva), Apotex Inc. (Apotex), Aurobindo Pharma Ltd. (Aurobindo), Synthon Laboratories, Inc. (Synthon), and Zydus Pharmaceuticals, USA, Inc. (Zydus) each submitted an ANDA seeking permission to market generic versions of Strattera prior to the expiration of our relevant U.S. patent (expiring in 2017), and alleging that this patent is invalid. We filed a lawsuit against Actavis in the United States District Court for the District of New Jersey in August 2007, and added Glenmark, Sun, Sandoz, Mylan, Teva, Apotex, Aurobindo, Synthon, and Zydus as defendants in September 2007. In December 2007, Zydus agreed to entry of a consent judgment in which Zydus conceded the validity and enforceability of the patent and agreed to a permanent injunction. In June 2008, Glenmark agreed to entry of a permanent injunction, enjoining it from selling a generic product prior to the expiration of the U.S. patent. Also in June 2008, Synthon notified us that it has withdrawn its ANDA and agreed to a stipulated dismissal of all outstanding claims. For the remaining defendants, trial is anticipated as early as December 2009.
Evista: In June 2005, Dr. Alan Schreiber filed a lawsuit against us in the United States District Court for the Eastern District of Pennsylvania raising a number of claims, including patent infringement, misappropriation of trade secrets, breach of contract, and unjust enrichment, and seeking a declaration for inventorship of Lilly’s Evista method-of-use patents. After the original lawsuit was filed, the University of Pennsylvania was added as a plaintiff. This matter was settled in December 2008. The settlement did not have a material impact on our consolidated results of operations, liquidity, or financial position.
Cialis: In July 2005, Vanderbilt University filed a lawsuit in the United States District Court in Delaware against ICOS Corporation seeking to add three of its scientists as co-inventors on the Cialis compound and method-of-use patents. In January 2009, the district court judge ruled in our favor, declining to add any of these scientists as an inventor on either patent. The plaintiff may appeal this ruling. We believe these claims are without legal merit and expect to prevail in any appeal of this litigation; however, it is not possible to determine the outcome. An unfavorable final outcome could have a material adverse impact on our consolidated results of operations, liquidity, and financial position.
In October 2002, Pfizer Inc. was issued a method-of-use patent in the United States and commenced a lawsuit in the United States District Court in Delaware against us, Lilly ICOS LLC, and ICOS Corporation (both now subsidiaries of Lilly) alleging that the marketing of Cialis for erectile dysfunction infringed this patent. This litigation has been stayed pending the outcome of a reexamination of the patent by the U.S. Patent and Trademark Office. The Office has now made a final rejection of the relevant patent claims which Pfizer is appealing. We believe Pfizer’s claims are without merit and expect to prevail. However, it is not possible to determine the outcome of this litigation.
Other Product Liability Litigation
We are currently a defendant in a variety of product liability lawsuits in the United States involving primarily Zyprexa, diethylstilbestrol (“DES”) and thimerosal.
In approximately 50 U.S. actions involving approximately 75 claimants, plaintiffs seek to recover damages on behalf of children or grandchildren of women who were prescribed DES during pregnancy.
We have been named as a defendant in approximately 210 actions in the U.S., involving approximately 285 claimants, brought in various state courts and federal district courts on behalf of children with autism or other
-15-
neurological disorders who received childhood vaccines (manufactured by other companies) that contained thimerosal, a generic preservative used in certain vaccines in the U.S. beginning in the 1930s. We purchased patents and conducted research pertaining to thimerosal in the 1920s. We have been named in the suits even though we discontinued manufacturing the raw material in 1974 and discontinued selling it in the United States to vaccine manufacturers in 1992. The lawsuits typically name the vaccine manufacturers as well as Lilly and other distributors of thimerosal, and allege that the children’s exposure to thimerosal-containing vaccines caused their autism or other neurological disorders. We strongly deny any liability in these cases. There is no credible scientific evidence establishing a causal relationship between thimerosal-containing vaccines and autism or other neurological disorders. In addition, we believe the majority of the cases should not be prosecuted in the courts in which they have been brought because the underlying claims are subject to the National Childhood Vaccine Injury Act of 1986. Implemented in 1988, the Act established a mandatory, federally administered no-fault claims process for individuals who allege that they were harmed by the administration of childhood vaccines. Under the Act, claims must first be brought before the U.S. Court of Claims for an award determination under the compensation guidelines established pursuant to the Act. Claimants who are unsatisfied with their awards under the Act may reject the award and seek traditional judicial remedies.
Other Marketing Practices Investigations
In November 2008, we received a subpoena from the U.S. Department of Health and Human Services Office of Inspector General in coordination with the U.S. Attorney for the Western District of New York seeking production of a wide range of documents and information relating to reimbursement of Alimta. We are cooperating in this investigation.
In February 2006, we reached a settlement of an investigation by the Office of Consumer Litigation, Department of Justice, related to our marketing and promotional practices and physician communications with respect to Evista. As part of the settlement, we agreed to plead guilty to one misdemeanor violation of the Food, Drug, and Cosmetic Act. The plea was for the off-label promotion of Evista during 1998. The government did not charge the company with any unlawful intent, nor do we acknowledge any such intent. In connection with the overall settlement, we paid a total of $36.0 million. In addition, as part of the settlement, a civil consent decree requires us to continue to have a compliance program and to undertake a set of defined corporate integrity obligations related to Evista for five years.
In August 2003, we received notice that the staff of the SEC is conducting an investigation into the compliance by Polish subsidiaries of certain pharmaceutical companies, including Lilly, with the U.S. Foreign Corrupt Practices Act of 1977. The staff has issued subpoenas to us requesting production of documents related to the investigation. In connection with that matter, staffs of the SEC and the Department of Justice (DOJ) have asked us to voluntarily provide additional information related to certain activities of Lilly affiliates in a number of other countries. We are cooperating with the SEC and the DOJ in this investigation.
Shareholder Derivative Litigation
In 2007, the company received two demands from shareholders that the board of directors cause the company to take legal action against current and former directors and others for allegedly causing damage to the company through improper marketing of Evista, Prozac, and Zyprexa. In accordance with procedures established under the Indiana Business Corporation Law (Ind. Code§ 23-1-32), the board has appointed a committee of independent persons to consider the demands and determine what action, if any, the company should take in response. Since January 2008, we have been served with seven shareholder derivative lawsuits:Lambrecht, et al. v. Taurel, et al., filed January 17, 2008, in the United States District Court for the Southern District of Indiana;Staehr et al. v. Eli Lilly and Company et al.,filed March 27, 2008, in Marion County Superior Court in Indianapolis, Indiana;Waldman et al., v. Eli Lilly and Company et al., filed February 11, 2008, in the United States District Court for the Eastern District of New York;Solomon v. Eli Lilly and Company et al., filed March 27, 2008, in Marion County Superior Court in Indianapolis, Indiana;Robbins v. Taurel, et al., filed April 9, 2008, in the United States District Court for the Eastern District of New York;City of Taylor General Employees Retirement System v. Taurel, et al., filed April 15, 2008, in the United States
-16-
District Court for the Eastern District of New York; andZemprelli v. Taurel, et al., filed June 24, 2008, in the United States District Court for the Southern District of Indiana. Two of these lawsuits were filed by the shareholders who served the demands described above. All seven lawsuits are nominally filed on behalf of the company, against various current and former directors and officers and allege that the named officers and directors harmed the company through the improper marketing of Zyprexa, and in certain suits, Evista and Prozac. The Zemprelli suit also claims that certain defendants violated sections 10(b) and 20(a) of the Securities Exchange Act of 1934. We believe these lawsuits are without merit and are prepared to defend against them vigorously.
Employee Litigation
In April 2006, three former employees and one current employee filed a putative class action against the company in the U.S. District Court for the Southern District of Indiana (Welch, et al. v. Eli Lilly and Company, filed April 20, 2006) alleging racial discrimination. Plaintiffs have since amended their complaint twice, adding to the lawsuit a total of 154 individual plaintiffs as well as the national and local chapters of the National Association for the Advancement of Colored People (NAACP). Under the current schedule, the plaintiffs are to file their class certification motion in March 2009. We believe this lawsuit is without merit and are prepared to defend against it vigorously.
We have also been named as a defendant in a lawsuit filed in the U.S. District Court for the Northern District of New York (Schaefer-LaRose, et al., filed November 14, 2006) claiming that our pharmaceutical sales representatives should have been categorized as “non-exempt” rather than “exempt” employees, and claiming that the company owes them back wages for overtime worked, as well as penalties, interest, and attorneys fees. Other pharmaceutical industry participants face identical lawsuits. The case was transferred to the U.S. District Court for the Southern District of Indiana in August 2007. In February 2008, the Indianapolis court conditionally certified a nationwide opt-in collective action under the Fair Labor Standards Act of all current and former employees who served as a Lilly pharmaceutical sales representative at any time from November 2003 to the present. As of the close of the opt-in period, fewer than 400 of the over 7,500 potential plaintiffs elected to participate in the lawsuit. We believe this lawsuit is without merit and are prepared to defend against it vigorously.
We have been named in a lawsuit brought by the Labor Attorney for 15th Region in the Labor Court of Paulinia, State of Sao Paulo, alleging possible harm to employees and former employees caused by exposure to heavy metals. We have also been named in approximately 50 lawsuits filed in the same court by individual former employees making similar claims. We believe these lawsuits are without merit and are prepared to defend against them vigorously.
Other Matters
In October 2005, the U.S. Attorney’s office for the Eastern District of Pennsylvania advised that it is conducting an inquiry regarding certain rebate agreements we entered into with a pharmacy benefit manager covering Axid, Evista, Humalog, Humulin, Prozac, and Zyprexa. The inquiry includes a review of our Medicaid best price reporting related to the product sales covered by the rebate agreements. We are cooperating in this matter.
In October 2005, we received a subpoena from the U.S. Attorney’s office for the District of Massachusetts for the production of documents relating to our business relationship with a long-term care pharmacy organization concerning Actos, Evista, Humalog, Humulin, and Zyprexa. We are cooperating in this matter.
Between 2003 and 2005, various municipalities in New York sued us and many other pharmaceutical manufacturers, claiming in general that as a result of alleged improprieties by the manufacturers in the calculation and reporting of average wholesale prices for purposes of Medicaid reimbursement, the municipalities overpaid their portion of the cost of pharmaceuticals. The suits seek monetary and other relief, including civil penalties and treble damages. Similar suits were filed against us and many other manufacturers by the States of Mississippi, Iowa, Utah, and Kansas. These suits are pending either in the U.S. District Court for the District of Massachusetts or in various state courts. All of these suits are in early stages or discovery is ongoing.
-17-
During 2004 we, along with several other pharmaceutical companies, were named in a consolidated lawsuit in California state court brought on behalf of consumers alleging that the conduct of pharmaceutical companies in preventing commercial importation of prescription drugs from outside the United States violated antitrust laws. The case sought restitution for alleged overpayments for pharmaceuticals and an injunction against the allegedly violative conduct. Summary judgment was granted to us and the other defendants. In July 2008, the California Court of Appeals affirmed that decision. The California Supreme Court has accepted plaintiff’s appeal, and we expect it to be heard later this year.
In July 2008, we received a request from the Civil Division of the United States Department of Justice requesting the production of documents related to nominal pricing. We are cooperating in this matter.
We previously received requests for information about Zyprexa from the offices of Representative Henry Waxman, former Chair of the House Committee on Oversight and Government Reform, and Senator Charles Grassley, ranking member of the Senate Finance Committee. We also received a request from Representative Waxman’s office for information about drug pricing under Medicare Part D. We are cooperating with these requests.
Along with over 100 other pharmaceutical companies operating in Europe, we have received a questionnaire from the European Commission as part of its ongoing inquiry into whether pharmaceutical companies have improperly blocked or created artificial barriers to pharmaceutical innovation or market entry of medicines through the misuse of patent rights, settlement of patent claims, litigation, or other means. We are cooperating with this request.
Under the Comprehensive Environmental Response, Compensation, and Liability Act, commonly known as Superfund, we have been designated as one of several potentially responsible parties with respect to the cleanup of fewer than 10 sites. Under Superfund, each responsible party may be jointly and severally liable for the entire amount of the cleanup.
We are also a defendant in other litigation and investigations, including product liability, patent, employment, and premises liability litigation, of a character we regard as normal to our business.
| |
| Item 4. | Submission of Matters to a Vote of Security Holders |
During the fourth quarter of 2008, no matters were submitted to a vote of security holders.
Part II
| |
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
You can find information relating to the principal market for our common stock and related stockholder matters at Part II, Item 8 under “Selected Quarterly Data (unaudited)” and “Selected Financial Data (unaudited).” That information is incorporated here by reference.
The following table summarizes the activity related to repurchases of our equity securities during the fourth quarter ended December 31, 2008:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Total Number of Shares
| | | Approximate Dollar Value
| |
| | | | | | | | | Purchased as Part of
| | | of Shares that May Yet Be
| |
| | | Total Number of
| | | Average Price Paid
| | | Publicly Announced
| | | Purchased Under the
| |
| | | Shares Purchased
| | | per Share
| | | Plans or Programs
| | | Plans or Programs
| |
| Period | | (a) | | | (b) | | | (c) | | | (d) | |
| |
| | | (in thousands) | | | | | | | | | (Dollars in millions) | |
| |
| October 2008 | | | 4 | | | $ | 33.47 | | | | — | | | | $419.2 | |
| November 2008 | | | 2 | | | | 32.34 | | | | — | | | | 419.2 | |
| December 2008 | | | — | | | | | | | | — | | | | 419.2 | |
| | | | | | | | | | | | | | | | | |
| Total | | | 6 | | | | | | | | — | | | | | |
| | | | | | | | | | | | | | | | | |
-18-
The amounts presented in columns (a) and (b) above represent purchases of common stock related to employee stock option exercises. The amounts presented in columns (c) and (d) in the above table represent activity related to our $3.00 billion share repurchase program announced in March 2000. As of December 31, 2008, we have purchased $2.58 billion related to this program.
| |
| Item 6. | Selected Financial Data |
You can find selected financial data for each of our five most recent fiscal years in Part II, Item 8 under “Selected Financial Data (unaudited).” That information is incorporated here by reference.
| |
| Item 7. | Management’s Discussion and Analysis of Results of Operations and Financial Condition |
Review of Operations
EXECUTIVE OVERVIEW
This section provides an overview of our financial results, recent product and late-stage pipeline developments, significant business development, and legal, regulatory, and other matters affecting our company and the pharmaceutical industry.
Financial Results
We achieved worldwide sales growth of 9 percent, which was primarily driven by volume increases in several key products. The favorable impact of foreign exchange rates on cost of sales contributed to an improvement in gross margin. Marketing, selling, and administrative expenses grew at the same rate as sales, driven by pre-launch activities associated with prasugrel, marketing costs associated with Cymbalta and Evista, the impact of foreign exchange rates, and increased litigation-related expenses; while our investment in research and development grew 10 percent. We completed our acquisition of ImClone Systems Inc. (ImClone), resulting in a significant charge of $4.69 billion for acquired in-process research and development (IPR&D) and reached resolution on government investigations related to our past U.S. marketing and promotional practices for Zyprexa, resulting in an additional charge of $1.48 billion. We incurred tax expense of $764.3 million, despite a loss before income taxes of $1.31 billion, primarily caused by the non-deductibility of the ImClone IPR&D charge and the partial deductibility of the Zyprexa investigation settlements. Accordingly, earnings decreased $5.02 billion, to a net loss of $2.07 billion, and earnings per share decreased $4.60, to a loss of $1.89 per share, in 2008 as compared with net income of $2.95 billion, or earnings per share of $2.71 in 2007. Net income comparisons between 2008 and 2007 are affected by the impact of the following significant items (see Notes 3, 5, 12, and 14 to the consolidated financial statements for additional information):
2008
Acquisitions (Note 3)
| |
| • | We recognized charges totaling $4.73 billion (pretax) associated with the acquisition of ImClone, which decreased earnings per share by $4.46. These amounts include an IPR&D charge of $4.69 billion (pretax). The remaining net expenses are related to ImClone’s operating results subsequent to the acquisition, incremental interest costs, and amortization of the intangible asset associated with Erbitux. We also incurred IPR&D charges of $28.0 million (pretax) associated with the acquisition of SGX Pharmaceuticals, Inc. (SGX), which decreased earnings per share by $.03. |
| |
| • | We incurred IPR&D charges associated with licensing arrangements with BioMS Medical Corp. (BioMS) and TransPharma Medical Ltd. totaling $122.0 million (pretax), which decreased earnings per share by $.07. |
-19-
Asset Impairments and Related Restructuring and Other Special Charges (Notes 5 and 14)
| |
| • | We recognized asset impairments, restructuring, and other special charges totaling $497.0 million (pretax), which decreased earnings per share by $.30. A similar charge of $57.1 million (pretax), which decreased earnings per share by $.04, was included in cost of sales. These charges were primarily associated with the sale of our Greenfield, Indiana site, the termination of the AIR® Insulin program, and strategic exit activities related to manufacturing operations. |
| |
| • | We recorded charges of $1.48 billion (pretax) related to the federal and state Zyprexa investigations led by the U.S. Attorney for the Eastern District of Pennsylvania (EDPA), as well as the resolution of a multi-state investigation regarding Zyprexa involving 32 states and the District of Columbia, which decreased earnings per share by $1.20. |
Other (Note 12)
| |
| • | We recognized a discrete income tax benefit of $210.3 million as a result of the resolution of a substantial portion of the IRS audit of our federal income tax returns for the years 2001 through 2004, which increased earnings per share by $.19. |
2007
Acquisitions (Note 3)
| |
| • | We incurred IPR&D charges associated with the acquisitions of ICOS Corporation (ICOS), Hypnion, Inc. (Hypnion), and Ivy Animal Health, Inc. (Ivy), totaling $631.6 million (pretax), which decreased earnings per share by $.57. |
| |
| • | We incurred IPR&D charges associated with our licensing arrangements with Glenmark Pharmaceuticals Limited India, MacroGenics, Inc., and OSI Pharmaceuticals, totaling $114.0 million (pretax), which decreased earnings per share by $.06. |
-20-
Asset Impairments and Related Restructuring and Other Special Charges (Notes 5 and 14)
| |
| • | We recognized asset impairments, restructuring, and other special charges of $190.6 million (pretax), which decreased earnings per share by $.12. These charges were primarily associated with previously announced strategic decisions affecting manufacturing and research facilities. |
| |
| • | We incurred a special charge following a settlement with one of our insurance carriers over Zyprexa product liability claims, which led to a reduction of our expected product liability insurance recoveries, and other product liability charges. This resulted in a charge totaling $111.9 million (pretax), which decreased earnings per share by $.09. |
Late-Stage Pipeline Developments and Business Development Activity
Our long-term success depends, to a great extent, on our ability to continue to discover and develop innovative pharmaceutical products and acquire or collaborate on compounds currently in development by other biotech-nology or pharmaceutical companies. There were a number of late-stage pipeline developments and business development transactions within the past year, including:
Pipeline
| |
| • | We, along with our partner Daiichi Sankyo Company Limited, are seeking from the U.S. Food and Drug Administration (FDA) approval for prasugrel as a treatment for patients with acute coronary syndrome being managed with percutaneous coronary intervention. The Cardiovascular and Renal Drugs Advisory Committee of the FDA reviewed prasugrel during a hearing and unanimously recommended it for approval. The FDA will consider the recommendation as it continues its review and makes its final decision. |
| |
| • | Prasugrel was approved for marketing by the European Commission under the trade name Efient in February 2009 for the prevention of atherothrombotic events in patients with acute coronary syndromes undergoing percutaneous coronary intervention. |
| |
| • | We received a complete response letter from the FDA for olanzapine long-acting injection (LAI) for acute and maintenance treatment of schizophrenia in adults. We are continuing to work with the agency on the new drug application (NDA). The FDA does not require any additional clinical trials for the continued review of the NDA. Per the agency’s request, we are preparing a proposed Risk Evaluation and Mitigation Strategy, which will be submitted in the near future. In addition, olanzapine long-acting injection was approved by the European Commission under the trade name Zypadheratm. |
| |
| • | We withdrew our supplemental NDA from the FDA for Cymbalta for the management of chronic pain. We plan to resubmit the application in the first half of 2009, adding data from a recently completed study in chronic osteoarthritis pain of the knee. |
| |
| • | The FDA approved Alimta, in combination with cisplatin, as a first-line treatment for locally advanced and metastatic non-small cell lung cancer (NSCLC) for patients with nonsquamous histology. The European health authorities also approved Alimta, in combination with cisplatin, as a first-line treatment for non-small cell lung cancer patients with other than predominantly squamous cell histology. |
| |
| • | We submitted tadalafil as a treatment for pulmonary arterial hypertension (PAH) to regulatory authorities in the U.S., Europe, and Japan. |
| |
| • | The FDA approved Cymbalta for the management of fibromyalgia, a chronic pain disorder. In addition, the European Commission approved Cymbalta for the treatment of generalized anxiety disorder (GAD). |
| |
| • | We, along with our partner Amylin Pharmaceuticals, Inc. (Amylin), submitted Byetta as a monotherapy treatment for type 2 diabetes to the FDA. |
| |
| • | The European Commission approved a new indication for Forsteo® for the treatment of osteoporosis associated with sustained, systemic glucocorticoid therapy in women and men at increased risk for fracture. We have also received an approvable letter from the FDA for Forteo for the same indication. |
-21-
| |
| • | We terminated development of our AIR Insulin program, which was being conducted in collaboration with Alkermes, Inc. The program had been in Phase III clinical development as a potential treatment for type 1 and type 2 diabetes. This decision was not a result of any observations during AIR Insulin trials relating to the safety of the product, but rather was a result of increasing uncertainties in the regulatory environment and a thorough evaluation of the evolving commercial and clinical potential of the product compared to existing medical therapies. |
Business Development
| |
| • | We acquired all of the outstanding shares of ImClone for a total purchase price of approximately $6.5 billion. This strategic combination will offer both targeted therapies and oncolytic agents along with an oncology pipeline spanning all phases of clinical development. It also expands our biotechnology capabilities. |
| |
| • | We entered into a license and a supply arrangement with United Therapeutics Corporation related to the U.S. commercialization rights for the PAH indication of tadalafil. We received an upfront payment of $150.0 million in exchange for exclusive rights to commercialize tadalafil for PAH in the U.S., as well as for a product manufacturing and supply arrangement. As part of this arrangement, we acquired a $150.0 million equity position in the company. The indication is currently under review by the FDA. |
| |
| • | We acquired the worldwide rights to the dairy cow supplement Posilac, as well as the product’s supporting operations, from Monsanto Company (Monsanto) for an upfront payment of $300.0 million, as well as contingent consideration based on future Posilac sales. The acquisition of Posilac provides us with a product that complements those of our animal health product line. |
| |
| • | We sold our Greenfield Laboratories site in Greenfield, Indiana, to Covance Inc. We also signed a10-year service agreement, under which Covance will assume responsibility for our toxicology testing and other R&D support activities at the site. |
| |
| • | We acquired SGX for approximately $64 million in cash. The acquisition allows us to integrate SGX’s structure-guided drug discovery platform into our drug discovery efforts. It also gives us access to FASTtm, SGX’s fragment-based, protein structure guided drug discovery technology, and to a portfolio of preclinical oncology compounds focused on a number of kinase targets. |
| |
| • | We entered into a licensing and development agreement with TransPharma Medical Ltd. (TransPharma) to acquire rights to its product and related drug delivery system for the treatment of osteoporosis. The product, which is administered transdermally using TransPharma’s proprietary technology, is currently in Phase II clinical testing. |
| |
| • | We entered into an agreement with an affiliate of TPG-Axon Capital (TPG) for the Phase III development of our two lead molecules for the treatment of Alzheimer’s disease. This agreement provides TPG with success-based milestones and royalties in exchange for clinical trial funding. |
| |
| • | We entered into a licensing and development agreement with BioMS whereby we acquired exclusive worldwide rights to a multiple sclerosis (MS) compound. The compound is currently being evaluated in two pivotal Phase III clinical trials in secondary progressive MS. |
Legal, Regulatory, and Other Matters
In March 2004, we were notified by the U.S. Attorney’s office for the EDPA that it had commenced an investigation relating to our U.S. marketing and promotional practices for Zyprexa, Prozac, and Prozac Weeklytm. In October 2008, we announced that we were in advanced discussions to resolve the ongoing investigations led by the EDPA, and we recorded a charge of $1.42 billion. In January 2009, we announced that the discussions had been successfully concluded, and that we settled the Zyprexa-related federal claims, as well as similar Medicaid-related claims of states which decide to participate in the settlement.
Beginning in August 2006, we received civil investigative demands or subpoenas from the attorneys general of a number of states under various state consumer protection laws seeking documents pertaining to Zyprexa. In
-22-
October 2008, we reached a settlement with 32 states and the District of Columbia, under which we paid $62.0 million.
In December 2008, the Federal Supreme Court (BGH) in Germany re-established our Zyprexa patent that had been declared invalid in 2007 by the German Federal Patent Court. As a result of this ruling, generic olanzapine has been withdrawn from the German market as of the beginning of 2009.
We continue to reach agreements with claimants’ attorneys involved in U.S. Zyprexa product liability litigation to settle claims against us relating to the medication. Approximately 120 claims remain.
In the third quarter of 2008, we initiated a strategic review of our Tippecanoe manufacturing facility in Lafayette, Indiana. Options being considered for this site include continuing operations with a revised site mission, exploring opportunities to sell the facility, and ceasing operations altogether. The review is expected to last six to twelve months. No final decisions have been made at this time; however, depending on the decision, we could record significant charges.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) continues to provide an effective prescription drug benefit under the Medicare program (known as Medicare Part D). Various measures have been discussedand/or passed in both the U.S. House of Representatives and U.S. Senate that would impose additional pricing pressures on our products, including proposals to legalize the importation of prescription drugs and either allow, or require, the Secretary of Health and Human Services to negotiate drug prices within Medicare Part D directly with pharmaceutical manufacturers. Additionally, various proposals have been introduced that would increase the rebates we pay on sales to Medicaid patients or impose additional rebates on sales to patients who receive their medicines through Medicare Part D. Uncertainty exists surrounding the new administration and Congress and the impact any government decisions or programs will have on the pharmaceutical industry. In addition, many states are facing substantial budget difficulties due to the downturn in the economy and are expected to seek aggressive cuts or other offsets in healthcare spending. We expect pricing pressures at the federal and state levels to become more severe, which could have a material adverse effect on our consolidated results of operations.
International operations also are generally subject to extensive price and market regulations, and there are many proposals for additional cost-containment measures, including proposals that would directly or indirectly impose additional price controls or reduce the value of our intellectual property protection.
The following table summarizes our net sales activity in 2008 compared with 2007:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Year Ended
| | | | |
| | | Year Ended
| | | December 31,
| | | Percent
| |
| | | December 31, 2008 | | | 2007
| | | Change
| |
| Product | | U.S.1 | | | Outside U.S. | | | Total | | | Total | | | from 2007 | |
| | |
| (Dollars in millions) | | | | | | | | | | | | | | | |
| | |
| |
| Zyprexa | | $ | 2,202.5 | | | $ | 2,493.6 | | | $ | 4,696.1 | | | $ | 4,761.0 | | | | (1 | ) |
| Cymbalta | | | 2,253.8 | | | | 443.3 | | | | 2,697.1 | | | | 2,102.9 | | | | 28 | |
| Humalog | | | 1,008.4 | | | | 727.4 | | | | 1,735.8 | | | | 1,474.6 | | | | 18 | |
| Gemzar | | | 734.8 | | | | 985.0 | | | | 1,719.8 | | | | 1,592.4 | | | | 8 | |
Cialis2 | | | 539.0 | | | | 905.5 | | | | 1,444.5 | | | | 1,143.8 | | | | 26 | |
| Alimta | | | 561.9 | | | | 592.8 | | | | 1,154.7 | | | | 854.0 | | | | 35 | |
| Animal health products | | | 537.3 | | | | 556.0 | | | | 1,093.3 | | | | 995.8 | | | | 10 | |
| Evista | | | 700.5 | | | | 375.1 | | | | 1,075.6 | | | | 1,090.7 | | | | (1 | ) |
| Humulin | | | 380.9 | | | | 682.3 | | | | 1,063.2 | | | | 985.2 | | | | 8 | |
| Forteo | | | 489.9 | | | | 288.8 | | | | 778.7 | | | | 709.3 | | | | 10 | |
| Strattera | | | 437.8 | | | | 141.7 | | | | 579.5 | | | | 569.4 | | | | 2 | |
| Other pharmaceutical products | | | 1,087.6 | | | | 1,252.1 | | | | 2,339.7 | | | | 2,354.4 | | | | (1 | ) |
| | | | | | | |
| | | | | | | |
| Total net sales | | $ | 10,934.4 | | | $ | 9,443.6 | | | $ | 20,378.0 | | | $ | 18,633.5 | | | | 9 | |
| | | |
| | | |
| | |
| 1 | | U.S. sales include sales in Puerto Rico. |
-23-
| | |
| 2 | | Prior to the acquisition of ICOS in late January 2007, the Cialis sales shown do not include sales in the joint-venture territories of Lilly ICOS LLC (North America, excluding Puerto Rico, and Europe). Our share of the joint-venture territory sales for January 2007, net of expenses and income taxes, is reported in other — net in our consolidated statements of operations. Subsequent to the acquisition, all Cialis product sales are reported in our net sales. Worldwide 2008 sales for Cialis grew 19 percent from 2007 sales of $1.22 billion. |
OPERATING RESULTS — 2008
Sales
Our worldwide sales for 2008 increased 9 percent, to $20.38 billion, driven primarily by growth of Cymbalta, Cialis, Alimta, Humalog, and Gemzar. Worldwide sales volume increased 5 percent, while foreign exchange rates contributed 3 percent, and selling prices contributed 2 percent. (Numbers do not add due to rounding.) Sales in the U.S. increased 8 percent, to $10.93 billion, driven primarily by increased sales of Cymbalta, Humalog, Cialis, and Alimta. Sales outside the U.S. increased 11 percent, to $9.44 billion, driven primarily by the sales growth of Alimta, Cialis, Cymbalta, and Humalog.
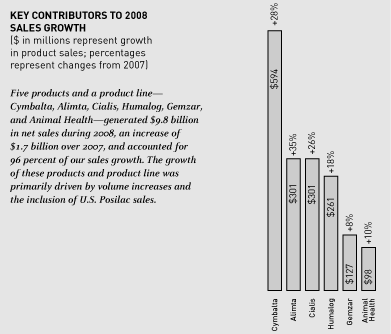
Zyprexa, our top-selling product, is a treatment for schizophrenia, acute mixed or manic episodes associated with bipolar I disorder, and bipolar maintenance. Zyprexa sales in the U.S. decreased 1 percent in 2008, driven by lower demand, partially offset by higher prices. Sales outside the U.S. decreased 1 percent, driven by decreased demand and to a lesser extent, lower prices, partially offset by the favorable impact of foreign exchange rates. Demand outside the U.S. was unfavorably impacted by generic competition in Germany and Canada. As noted previously, generic olanzapine has been withdrawn from the German market as of the beginning of 2009.
Sales of Cymbalta, a product for the treatment of major depressive disorder, diabetic peripheral neuropathic pain, generalized anxiety disorder, and fibromyalgia, increased 23 percent in the U.S., driven by increased demand and, to a lesser extent, higher prices. Sales outside the U.S. increased 66 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates and higher prices. Higher demand outside the U.S. reflects increased demand in established markets as well as recent launches in new markets.
Sales of Humalog, our injectable human insulin analog for the treatment of diabetes, increased 14 percent in the U.S., driven by increased demand and higher prices. Sales outside the U.S. increased 24 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates.
-24-
Sales of Gemzar, a product approved to fight various cancers, increased 10 percent in the U.S., driven by increased demand and higher prices. Sales outside the U.S. increased 7 percent, driven primarily by the favorable impact of foreign exchange rates and, to a lesser extent, increased demand, partially offset by lower prices. We will likely face increased generic competition in certain markets outside the U.S. in 2009.
Our sales of Cialis, a treatment for erectile dysfunction, increased 27 percent in the U.S., driven by increased demand and higher prices. Sales outside the U.S. increased 26 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates and higher prices. Total worldwide sales of Cialis increased 19 percent to $1.44 billion in 2008 as compared to $1.22 billion in 2007. This includes $72.7 million of sales in the Lilly ICOS joint-venture territories for the 2007 period prior to the acquisition of ICOS.
Sales of Alimta, a treatment for various cancers, increased 25 percent in the U.S., driven by increased demand and, to a lesser extent, higher prices. Sales outside the U.S. increased 46 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates.
Sales of Evista, a product for the prevention and treatment of osteoporosis in postmenopausal women and for risk reduction of invasive breast cancer in postmenopausal women with osteoporosis and postmenopausal women at high risk for invasive breast cancer, decreased 1 percent in the U.S., driven by decreased demand, partially offset by higher prices. Sales outside the U.S. decreased 2 percent, driven by lower demand and lower prices, partially offset by the favorable impact of foreign exchange rates. As described in Legal and Regulatory Matters, Evista is the subject of a Hatch-Waxman patent challenge by Teva Pharmaceuticals USA, Inc. (Teva), which has received tentative approval of its Abbreviated New Drug Application (ANDA) from the FDA. Unless the current stay on Teva’s approved ANDA remains in force or Teva is preliminarily enjoined from markets if the stay is lifted, it is possible that Teva could choose to launch before the current action against Teva is concluded. Such a launch could have a material adverse impact on our future consolidated results of operations.
Sales of Humulin, an injectable human insulin for the treatment of diabetes, increased 4 percent in the U.S., driven by higher prices. Sales outside the U.S. increased 10 percent, driven by the favorable impact of foreign exchange rates and increased demand.
Sales of Forteo, an injectable treatment for osteoporosis in postmenopausal women and men at high risk for fracture, decreased 1 percent in the U.S., driven by decreased demand, partially offset by higher prices. Sales outside the U.S. increased 34 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates.
Sales of Strattera, a treatment for attention-deficit hyperactivity disorder in children, adolescents, and adults, decreased 6 percent in the U.S., driven by decreased demand, partially offset by higher prices. Sales outside the U.S. increased 35 percent, driven primarily by increased demand.
Worldwide sales of Byetta, an injectable product for the treatment of type 2 diabetes that we market with Amylin, increased 16 percent to $751.4 million during 2008. We report as revenue our 50 percent share of Byetta’s gross margin in the U.S., 100 percent of Byetta sales outside the U.S., and our sales of Byetta pen delivery devices to Amylin. Our revenues increased 20 percent to $396.1 million in 2008.
Animal health product sales in the U.S. increased 12 percent, driven by the inclusion of U.S. Posilac sales since the date of acquisition. Sales outside the U.S. increased 8 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates.
Gross Margin, Costs, and Expenses
The 2008 gross margin increased to 78.5 percent of sales compared with 77.2 percent for 2007. This increase was primarily due to the favorable impact of foreign exchange rates.
-25-
Marketing, selling, and administrative expenses increased 9 percent in 2008, to $6.63 billion. This increase was due to increased marketing and selling expenses, including prelaunch expenses for prasugrel and marketing costs associated with Cymbalta and Evista; the impact of foreign exchange rates; and increased litigation-related expenses. Investment in research and development increased 10 percent, to $3.84 billion, due to increased late-stage clinical trial and discovery research costs.
Acquired IPR&D charges related to the acquisitions of ImClone and SGX, as well as our in-licensing arrangements with BioMS and TransPharma, were $4.84 billion in 2008 as compared to $745.6 million in
-26-
2007. We recognized asset impairments, restructuring, and other special charges of $1.97 billion in 2008, as compared to $302.5 million in 2007. The 2008 charges were primarily associated with the resolution of Zyprexa investigations with the U.S. Attorney for the EDPA and multiple states. See Notes 3, 5 and 14 to the consolidated financial statements for additional information.
Other — net decreased $148.1 million, to a net expense of $26.1 million. This line item consists of interest expense, interest income, the after-tax operating results of the Lilly ICOS joint venture, and all other miscellaneous income and expense items.
| |
| • | Interest expense for 2008 was essentially flat at $228.3 million. The impact of lower interest rates on our debt was substantially offset by lower capitalized interest due to lowerconstruction-in-progress balances and increased interest expense due to the financing of the ImClone acquisition. |
| |
| • | Interest income for 2008 decreased $4.6 million, to $210.7 million, as lower interest rates were partially offset by higher cash balances. |
| |
| • | The Lilly ICOS joint venture income prior to the 2007 acquisition was $11.0 million. Subsequent to the acquisition, all activity related to ICOS is included in our consolidated financial results. |
| |
| • | Net other miscellaneous items decreased $132.5 million to a loss of $8.5 million, primarily as a result of lower outlicensing income and increased net losses on investment securities in 2008 (the majority of which consisted of unrealized losses). |
We incurred tax expense of $764.3 million in 2008, despite having a loss before income taxes of $1.31 billion. Our net loss was driven by the $4.69 billion acquired IPR&D charge for ImClone and the $1.48 billion Zyprexa investigation settlements. The IPR&D charge was not tax deductible, and only a portion of the Zyprexa investigation settlements was deductible. In addition, we recorded tax expense associated with the ImClone acquisition, as well as a discrete income tax benefit of $210.3 million for the resolution of the IRS audit. The effective tax rate was 23.8 percent in 2007. See Note 12 to the consolidated financial statements for additional information.
OPERATING RESULTS — 2007
Financial Results
We achieved worldwide sales growth of 19 percent. This growth was primarily driven by volume increases in a number of key products, with a significant portion of this increase in volume resulting from the acquisition of ICOS. Our additional investments in marketing and selling expenses in support of key products, primarily Cymbalta and the diabetes care products, contributed to this sales growth and enabled us to increase our investment in research and development 11 percent in 2007. While cost of sales and operating expenses in the aggregate grew at approximately the same rate as sales, other — net decreased and the effective tax rate increased. As a result, net income and earnings per share increased 11 percent, to $2.95 billion, or $2.71 per share, in 2007 as compared with $2.66 billion, or $2.45 per share, in 2006. Net income comparisons between 2007 and 2006 are affected by the impact of significant items that are reflected in our financial results. The significant items for 2007 are summarized in the Executive Overview. The 2006 items are summarized as follows (see Notes 5 and 14 to the consolidated financial statements for additional information):
| |
| • | We recognized asset impairments, restructuring, and other special charges of $450.3 million (pretax) in the fourth quarter, which decreased earnings per share by $.31 (Note 5). |
| |
| • | In the fourth quarter, we incurred a charge related to Zyprexa product liability litigation matters of $494.9 million (pretax), or $.42 per share (Notes 5 and 14). |
Sales
Our worldwide sales for 2007 increased 19 percent, to $18.63 billion, driven primarily by the inclusion of Cialis since our January 29, 2007 acquisition of ICOS and sales growth of Cymbalta, Zyprexa, Alimta, Gemzar, and Humalog. Worldwide sales volume increased 12 percent, while selling prices and foreign
-27-
exchange rates each increased sales by 3 percent. (Numbers do not add due to rounding.) Sales in the U.S. increased 18 percent, to $10.15 billion, driven primarily by increased sales of Cymbalta, Zyprexa, Alimta, and Byetta, and the inclusion of Cialis. Sales outside the U.S. increased 20 percent, to $8.49 billion, driven primarily by the inclusion of Cialis, and sales growth of Zyprexa, Alimta, Gemzar, and Cymbalta.
The following table summarizes our net sales activity in 2007 compared with 2006:
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended
| | | Year Ended
| | | Percent
| |
| | | December 31, 2007 | | | December 31, 2006
| | | Change
| |
| Product | | U.S.1 | | | Outside U.S. | | | Total | | | Total | | | from 2006 | |
| | |
| (Dollars in millions) | | | | | | | | | | | | | | | |
| | |
| |
| Zyprexa | | $ | 2,236.0 | | | $ | 2,525.0 | | | $ | 4,761.0 | | | $ | 4,363.6 | | | | 9 | |
| Cymbalta | | | 1,835.6 | | | | 267.3 | | | | 2,102.9 | | | | 1,316.4 | | | | 60 | |
| Gemzar | | | 670.0 | | | | 922.4 | | | | 1,592.4 | | | | 1,408.1 | | | | 13 | |
| Humalog | | | 888.0 | | | | 586.6 | | | | 1,474.6 | | | | 1,299.5 | | | | 13 | |
Cialis2 | | | 423.8 | | | | 720.0 | | | | 1,143.8 | | | | 215.8 | | | | NM | |
| Evista | | | 706.1 | | | | 384.6 | | | | 1,090.7 | | | | 1,045.3 | | | | 4 | |
| Animal health products | | | 480.9 | | | | 514.9 | | | | 995.8 | | | | 875.5 | | | | 14 | |
| Humulin | | | 365.2 | | | | 620.0 | | | | 985.2 | | | | 925.3 | | | | 6 | |
| Alimta | | | 448.0 | | | | 406.0 | | | | 854.0 | | | | 611.8 | | | | 40 | |
| Forteo | | | 494.1 | | | | 215.2 | | | | 709.3 | | | | 594.3 | | | | 19 | |
| Strattera | | | 464.6 | | | | 104.8 | | | | 569.4 | | | | 579.0 | | | | (2 | ) |
| Humatrope | | | 213.6 | | | | 227.2 | | | | 440.8 | | | | 415.6 | | | | 6 | |
| Actos | | | 150.8 | | | | 219.8 | | | | 370.6 | | | | 448.5 | | | | (17 | ) |
| Byetta | | | 316.5 | | | | 14.2 | | | | 330.7 | | | | 219.0 | | | | 51 | |
| Other pharmaceutical products | | | 452.3 | | | | 760.0 | | | | 1,212.3 | | | | 1,373.3 | | | | (12 | ) |
| | | | | | | |
| | | | | | | |
| Total net sales | | $ | 10,145.5 | | | $ | 8,488.0 | | | $ | 18,633.5 | | | $ | 15,691.0 | | | | 19 | |
| | | |
| | | |
NM — Not meaningful
| | |
| 1 | | U.S sales include sales in Puerto Rico. |
| |
| 2 | | Prior to the acquisition of ICOS, the Cialis sales shown in the table above represent results only in the territories in which we marketed Cialis exclusively. The remaining sales relate to the joint-venture territories of Lilly ICOS LLC (North America, excluding Puerto Rico, and Europe). Our share of the joint-venture territory sales, net of expenses and income taxes, is reported in other — net in our consolidated statements of operations. Subsequent to the acquisition, all Cialis product sales are reported in our net sales. |
Zyprexa sales in the U.S. increased 6 percent in 2007, driven by higher net selling prices, partially offset by lower demand. Sales outside the U.S. increased 12 percent, driven by the favorable impact of foreign exchange rates and increased demand.
Sales of Cymbalta increased 58 percent in the U.S., driven primarily by strong demand. Sales outside the U.S. increased 70 percent, driven by increased demand and the favorable impact of foreign exchange rates.
Sales of Gemzar increased 10 percent in the U.S., driven by higher prices and increased demand. Sales outside the U.S. increased 16 percent, driven by increased demand and the favorable impact of foreign exchange rates.
Sales of Humalog increased 9 percent in the U.S., driven by higher prices and increased demand. Sales outside the U.S. increased 20 percent, driven by increased demand and the favorable impact of foreign exchange rates, partially offset by declining prices.
Total worldwide sales of Cialis were $1.22 billion and $971.0 million during 2007 and 2006, respectively. This includes $72.7 million of sales in the Lilly ICOS joint-venture territories for the 2007 period prior to the acquisition of ICOS. Worldwide sales grew 25 percent in 2007. U.S. sales increased 20 percent in 2007, driven by increased demand and higher prices. Sales outside the U.S. increased 28 percent in 2007, driven by increased demand, the favorable impact of foreign exchange rates, and higher prices.
-28-
Sales of Evista increased 6 percent in the U.S., driven by higher prices. Sales outside the U.S. increased 1 percent, driven by the favorable impact of foreign exchange rates, partially offset by lower prices and lower demand.
Sales of Humulin decreased 1 percent in the U.S., driven by lower demand, partially offset by higher prices. Sales outside the U.S. increased 11 percent, driven by increased demand and the favorable impact of foreign exchange rates, partially offset by lower prices.
Sales of Alimta increased 28 percent in the U.S., driven by increased demand and, to a lesser extent, higher prices. Sales outside the U.S. increased 55 percent, driven by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates.
Sales of Forteo increased 19 percent in the U.S., driven by higher net selling prices. U.S. sales growth benefited from access to medical coverage through the Medicare Part D program and decreased utilization of our U.S. patient assistance program and, to a lesser extent, increased demand. Sales outside the U.S. increased 21 percent, driven by increased demand and the favorable impact of foreign exchange rates.
Sales of Strattera decreased 9 percent in the U.S., as a result of decreased demand. Sales outside the U.S. increased 50 percent, driven by increased demand and the favorable impact of foreign exchange rates.
Our revenues from Actos decreased 46 percent in the U.S. Sales outside the U.S. increased 30 percent, driven primarily by increased demand and, to a lesser extent, the favorable impact of foreign exchange rates.
Worldwide sales of Byetta increased 51 percent to $650.2 million during 2007. Our revenues increased 51 percent to $330.7 million in 2007.
Animal health product sales in the U.S. increased 18 percent, driven by increased demand, the acquisition of Ivy Animal Health, and new companion-animal product launches. Sales outside the U.S. increased 10 percent, driven by the favorable impact of foreign exchange rates and increased demand.
Gross Margin, Costs, and Expenses
The 2007 gross margin decreased to 77.2 percent of sales compared with 77.4 percent for 2006. This decrease was primarily due to the expense resulting from the amortization of the intangible assets acquired in the ICOS acquisition, the unfavorable impact of foreign exchange rates, and production volumes growing at a slower rate than sales, offset partially by manufacturing expenses growing at a slower rate than sales.
Operating expenses (the aggregate of research and development and marketing, selling, and administrative expenses) increased 19 percent in 2007. Investment in research and development increased 11 percent, to $3.49 billion. In addition to the acquisition of ICOS, this increase was due to increases in discovery research and late-stage clinical trial costs. Marketing, selling, and administrative expenses increased 25 percent in 2007, to $6.10 billion. This increase was largely due to the impact of the ICOS acquisition, as well as increased marketing and selling expenses in support of key products, primarily Cymbalta and the diabetes care products, and the unfavorable impact of foreign exchange rates.
Acquired IPR&D charges were $745.6 million in 2007 and related to the acquisitions of ICOS, Hypnion, and Ivy, as well as our licensing arrangements with OSI, MacroGenics, and Glenmark. We incurred asset impairments, restructuring, and other special charges of $302.5 million in 2007 as compared to $945.2 million in 2006. See Notes 3, 5 and 14 to the consolidated financial statements for additional information.
Other — net decreased $115.8 million, to income of $122.0 million. This line item consists of interest expense, interest income, the after-tax operating results of the Lilly ICOS joint venture, and all other miscellaneous income and expense items.
| |
| • | Interest expense for 2007 decreased $9.8 million, to $228.3 million. This decrease is a result of lower average debt balances in 2007 compared to 2006. |
| |
| • | Interest income for 2007 decreased $46.6 million, to $215.3 million, due to lower cash balances in 2007 compared to 2006. |
-29-
| |
| • | The Lilly ICOS joint-venture income was $11.0 million in 2007 as compared to $96.3 million in 2006, due to the acquisition of ICOS on January 29, 2007. |
| |
| • | Net other miscellaneous income items increased $6.3 million to $124.0 million. |
We incurred tax expense of $923.8 million in 2007, resulting in an effective tax rate of 23.8 percent, compared with 22.1 percent for 2006. The effective tax rates for 2007 and 2006 were affected primarily by the nondeductible ICOS and Hypnion IPR&D charges of $594.6 million in 2007, and the product liability charges of $494.9 million in 2006. The tax effect of the product liability charge was less than our effective tax rate, as the tax benefit was calculated based upon existing tax laws in the countries in which we reasonably expect to deduct the charge. See Note 12 to the consolidated financial statements for additional information.
FINANCIAL CONDITION
As of December 31, 2008, cash, cash equivalents, and short-term investments totaled $5.93 billion compared with $4.83 billion at December 31, 2007. Cash flow from operations in 2008 of $7.30 billion and net proceeds from the issuance of debt of $4.41 billion exceeded the total of the net cash paid for corporate acquisitions of $6.08 billion, dividends paid of $2.06 billion, purchases of property and equipment of $947.2 million, and net purchases of noncurrent investments of $815.1 million.
Capital expenditures of $947.2 million during 2008 were $135.2 million less than in 2007. We expect 2009 capital expenditures to be approximately $1.1 billion as we invest in our biotechnology capabilities, continue to upgrade our manufacturing and research facilities to enhance productivity and quality systems, and invest in the long-term growth of our diabetes care products.
Total debt as of December 31, 2008 increased $5.45 billion, to $10.46 billion, reflecting the commercial paper we issued in November 2008 primarily to finance our acquisition of ImClone, offset by long-term debt repayments and paydown of commercial paper with cash and cash equivalents on hand. Our current debt ratings from Standard & Poor’s and Moody’s are at AA and A1, respectively.
Dividends of $1.88 per share were paid in 2008, an increase of 11 percent from 2007. In the fourth quarter of 2008, effective for the first-quarter dividend in 2009, the quarterly dividend was increased to $.49 per share (a 4.3 percent increase), resulting in an indicated annual rate for 2009 of $1.96 per share. The year 2008 was the
-30-
124th consecutive year in which we made dividend payments and the 41st consecutive year in which dividends have been increased.
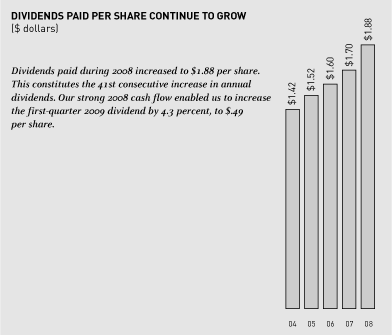
In recent months, global economic conditions have deteriorated. Triggered by the liquidity crisis in the capital markets, the implications have become more widespread, resulting in higher unemployment and declines in real consumer spending. In addition, many financial institutions have tightened lines of credit, reducing funding available for near-term economic growth. Pharmaceutical consumption has traditionally been relatively unaffected by economic downturns; however, an extended downturn could lead to a decline in overall prescriptions corresponding with the growth of the uninsured and underinsured population in the U.S. In addition, both private and public health care payers are facing heightened fiscal challenges due to the economic slowdown and are taking aggressive steps to reduce the costs of care, including pressures for increased pharmaceutical discounts and rebates and efforts to drive greater use of generic drugs. We continue to monitor the potential near-term impact of prescription trends, the credit worthiness of our wholesalers and other customers and suppliers, the decline of health insurance coverage in the overall population, and the federal government’s involvement in the economic crisis.
We believe that cash generated from operations, along with available cash and cash equivalents, will be sufficient to fund our normal operating needs, including debt service, capital expenditures, costs associated with litigation and government investigations, and dividends in 2009. We believe that amounts accessible through existing commercial paper markets should be adequate to fund short-term borrowings. Our access to credit markets has not been adversely affected by the recent illiquidity in the market because of the high credit quality of our short- and long-term debt. In 2009, we intend to fund payments required in connection with the EDPA settlements, and to further reduce outstanding commercial paper with cash and cash equivalents on hand, cash generated from operations, and the issuance of longterm debt. We currently have $1.24 billion of unused committed bank credit facilities, $1.20 billion of which backs our commercial paper program. Additionally, in November 2008, we obtained a one-year short-term revolving credit facility in the amount of $4.00 billion asback-up, alternative financing. Various risks and uncertainties, including those discussed in the Financial Expectations for 2009 section, may affect our operating results and cash generated from operations.
-31-
In the normal course of business, our operations are exposed to fluctuations in interest rates and currency values. These fluctuations can vary the costs of financing, investing, and operating. We address a portion of these risks through a controlled program of risk management that includes the use of derivative financial instruments. The objective of controlling these risks is to limit the impact on earnings of fluctuations in interest and currency exchange rates. All derivative activities are for purposes other than trading.
Our primary interest rate risk exposure results from changes in short-term U.S. dollar interest rates. In an effort to manage interest rate exposures, we strive to achieve an acceptable balance between fixed and floating rate debt positions and may enter into interest rate derivatives to help maintain that balance. Based on our overall interest rate exposure at December 31, 2008 and 2007, including derivatives and other interest rate risk-sensitive instruments, a hypothetical 10 percent change in interest rates applied to the fair value of the instruments as of December 31, 2008 and 2007, respectively, would have no material impact on earnings, cash flows, or fair values of interest rate risksensitive instruments over a one-year period.
Our foreign currency risk exposure results from fluctuating currency exchange rates, primarily the U.S. dollar against the euro and the Japanese yen, and the British pound against the euro. We face transactional currency exposures that arise when we enter into transactions, generally on an intercompany basis, denominated in currencies other than the local currency. We also face currency exposure that arises from translating the results of our global operations to the U.S. dollar at exchange rates that have fluctuated from the beginning of the period. We may use forward contracts and purchased options to manage our foreign currency exposures. Our policy outlines the minimum and maximum hedge coverage of such exposures. Gains and losses on these derivative positions offset, in part, the impact of currency fluctuations on the existing assets, liabilities, commitments, and anticipated revenues. Considering our derivative financial instruments outstanding at December 31, 2008 and 2007, a hypothetical 10 percent change in exchange rates (primarily against the U.S. dollar) as of December 31, 2008 and 2007, respectively, would have no material impact on earnings, cash flows, or fair values of foreign currency rate risk-sensitive instruments over a one-year period. These calculations do not reflect the impact of the exchange gains or losses on the underlying positions that would be offset, in part, by the results of the derivative instruments.
Off-Balance Sheet Arrangements and Contractual Obligations
We have no off-balance sheet arrangements that have a material current effect or that are reasonably likely to have a material future effect on our financial condition, changes in financial condition, revenues or expenses,
-32-
results of operations, liquidity, capital expenditures, or capital resources. We acquire and collaborate on assets still in development and enter into research and development arrangements with third parties that often require milestone and royalty payments to the third party contingent upon the occurrence of certain future events linked to the success of the asset in development. Milestone payments may be required contingent upon the successful achievement of an important point in the development life cycle of the pharmaceutical product (e.g., approval of the product for marketing by the appropriate regulatory agency or upon the achievement of certain sales levels). If required by the arrangement, we may have to make royalty payments based upon a percentage of the sales of the pharmaceutical product in the event that regulatory approval for marketing is obtained. Because of the contingent nature of these payments, they are not included in the table of contractual obligations.
Individually, these arrangements are not material in any one annual reporting period. However, if milestones for multiple products covered by these arrangements would happen to be reached in the same reporting period, the aggregate charge to expense could be material to the results of operations in any one period. These arrangements often give us the discretion to unilaterally terminate development of the product, which would allow us to avoid making the contingent payments; however, we are unlikely to cease development if the compound successfully achieves clinical testing objectives. We also note that, from a business perspective, we view these payments as positive because they signify that the product is successfully moving through development and is now generating or is more likely to generate cash flows from sales of products.
Our current noncancelable contractual obligations that will require future cash payments are as follows (in millions):
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | | | | Less Than
| | | 1-3
| | | 3-5
| | | More Than
| |
| | | Total | | | 1 Year | | | Years | | | Years | | | 5 Years | |
| |
Long-term debt, including interest payments1 | | $ | 8,205.5 | | | $ | 595.8 | | | $ | 387.0 | | | $ | 881.2 | | | $ | 6,341.5 | |
| Capital lease obligations | | | 41.3 | | | | 13.1 | | | | 17.0 | | | | 5.2 | | | | 6.0 | |
| Operating leases | | | 335.3 | | | | 90.8 | | | | 141.4 | | | | 73.6 | | | | 29.5 | |
Purchase obligations2 | | | 7,923.0 | | | | 5,976.3 | | | | 723.5 | | | | 388.5 | | | | 834.7 | |
Other long-term liabilities reflected on our balance sheet3 | | | 1,088.8 | | | | — | | | | 316.7 | | | | 185.0 | | | | 587.1 | |
Other4 | | | 157.1 | | | | 157.1 | | | | — | | | | — | | | | — | |
| | | |
| | | |
| Total | | $ | 17,751.0 | | | $ | 6,833.1 | | | $ | 1,585.6 | | | $ | 1,533.5 | | | $ | 7,798.8 | |
| | | |
| | | |
| | |
| 1 | | Our long-term debt obligations include both our expected principal and interest obligations and our interest rate swaps. We used the interest rate forward curve at December 31, 2008 to compute the amount of the contractual obligation for interest on the variable rate debt instruments and swaps. |
| |
| 2 | | We have included the following: |
| | |
| | • | Purchase obligations, consisting primarily of all open purchase orders at our significant operating locations as of December 31, 2008. Some of these purchase orders may be cancelable; however, for purposes of this disclosure, we have not distinguished between cancelable and noncancelable purchase obligations. |
| |
| | • | Contractual payment obligations with each of our significant vendors, which are noncancelable and are not contingent. |
| | |
| 3 | | We have included long-term liabilities consisting primarily of our nonqualified supplemental pension funding requirements and deferred compensation liabilities. We excluded liabilities for unrecognized tax benefits of $906.2 million, as we cannot reasonably estimate the timing of future cash outflows associated with those liabilities. |
| |
| 4 | | This category comprises primarily minimum pension funding requirements. |
The contractual obligations table is current as of December 31, 2008. We expect the amount of these obligations to change materially over time as new contracts are initiated and existing contracts are completed, terminated, or modified.
-33-
APPLICATION OF CRITICAL ACCOUNTING POLICIES
In preparing our financial statements in accordance with generally accepted accounting principles (GAAP), we must often make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses, and related disclosures. Some of those judgments can be subjective and complex, and consequently actual results could differ from those estimates. For any given individual estimate or assumption we make, it is possible that other people applying reasonable judgment to the same facts and circumstances could develop different estimates. We believe that, given current facts and circumstances, it is unlikely that applying any such other reasonable judgment would cause a material adverse effect on our consolidated results of operations, financial position, or liquidity for the periods presented in this report. Our most critical accounting policies have been discussed with our audit committee and are described below.
Revenue Recognition and Sales Return, Rebate, and Discount Accruals
We recognize revenue from sales of products at the time title of goods passes to the buyer and the buyer assumes the risks and rewards of ownership. For more than 90 percent of our sales, this is at the time products are shipped to the customer, typically a wholesale distributor or a major retail chain. The remaining sales, which are outside the U.S., are recorded at the point of delivery. Provisions for returns, rebates, and discounts are established in the same period the related sales are recorded.
We regularly review the supply levels of our significant products sold to major wholesalers in the U.S. and in major markets outside the U.S., primarily by reviewing periodic inventory reports supplied by our major wholesalers and available prescription volume information for our products, or alternative approaches. We attempt to maintain wholesaler inventory levels at an average of approximately one month or less on a consistent basis across our product portfolio. Causes of unusual wholesaler buying patterns include actual or anticipated product supply issues, weather patterns, anticipated changes in the transportation network, redundant holiday stocking, and changes in wholesaler business operations. In the U.S., the current structure of our arrangements eliminates the incentive for speculative wholesaler buying and provides us improved data on inventory levels at our wholesalers. When we believe wholesaler purchasing patterns have caused an unusual increase or decrease in the sales of a major product compared with underlying demand, we disclose this in our product sales discussion if we believe the amount is material to the product sales trend; however, we are not always able to accurately quantify the amount of stocking or destocking. Wholesaler stocking and destocking activity historically has not caused any material changes in the rate of actual product returns.
We establish sales return accruals for anticipated product returns. We record the return amounts as a deduction to arrive at our net sales. Once the product is returned, it is destroyed. Consistent with SFAS 48, Revenue Recognition When Right of Return Exists, we estimate a reserve when the sales occur for future product returns related to those sales. This estimate is primarily based on historical return rates as well as specifically identified anticipated returns due to known business conditions and product expiry dates. Actual product returns have been approximately one percent of our net sales over the past three years and have not fluctuated significantly as a percent of sales.
We establish sales rebate and discount accruals in the same period as the related sales. The rebate and discount amounts are recorded as a deduction to arrive at our net sales. Sales rebates and discounts that require the use of judgment in the establishment of the accrual include Medicaid, managed care, Medicare, chargebacks, long-term-care, hospital, patient assistance programs, and various other government programs. We base these accruals primarily upon our historical rebate and discount payments made to our customer segment groups and the provisions of current rebate and discount contracts.
The largest of our sales rebate and discount amounts are rebates associated with sales covered by Medicaid. In determining the appropriate accrual amount, we consider our historical Medicaid rebate payments by product as a percentage of our historical sales as well as any significant changes in sales trends, an evaluation of the current Medicaid rebate laws and interpretations, the percentage of our products that are sold to Medicaid recipients, and our product pricing and current rebate and discount contracts. Although we accrue a liability for Medicaid rebates at the time we record the sale (when the product is shipped), the Medicaid rebate related
-34-
to that sale is typically paid up to six months later. Because of this time lag, in any particular period our rebate adjustments may incorporate revisions of accruals for several periods.
Most of our rebates outside the U.S. are contractual or legislatively mandated and are estimated and recognized in the same period as the related sales. In some large European countries, government rebates are based on the anticipated pharmaceutical budget deficit in the country. A best estimate of these rebates, updated as governmental authorities revise budgeted deficits, is recognized in the same period as the related sale. If our estimates are not reflective of the actual pharmaceutical budget deficit, we adjust our rebate reserves.
We believe that our accruals for sales returns, rebates, and discounts are reasonable and appropriate based on current facts and circumstances. Sales returns, federally mandated Medicaid rebate and state pharmaceutical assistance programs (Medicaid) and Medicare rebates reduced sales by $1.03 billion, $738.8 million, and $704.8 million in 2008, 2007, and 2006, respectively. A 5 percent change in the sales return, Medicaid, and Medicare rebate amounts we recognized in 2008 would lead to an approximate $52 million effect on our income before income taxes. As of December 31, 2008, our sales returns, Medicaid, and Medicare rebate liability was $618.5 million.
Our global rebate and discount liabilities are included in sales rebates and discounts on our consolidated balance sheet. Our global sales return liability is included in other current liabilities and other noncurrent liabilities on our consolidated balance sheet. Approximately 80 percent and 78 percent of our global sales return, rebate, and discount liability resulted from sales of our products in the U.S. as of December 31, 2008 and 2007, respectively. The following represents a roll-forward of our most significant U.S. returns, rebate, and discount liability balances, including Medicaid (in millions):
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| |
| Sales return, rebate, and discount liabilities, beginning of year | | $ | 693.5 | | | $ | 614.5 | |
Reduction of net sales due to sales returns, discounts, and rebates1 | | | 1,864.9 | | | | 1,404.0 | |
| Cash payments of discounts and rebates | | | (1,751.8 | ) | | | (1,325.0 | ) |
| | | |
| | | |
| Sales return, rebate, and discount liabilities, end of year | | $ | 806.5 | | | $ | 693.5 | |
| | | |
| | | |
| | |
| 1 | | Adjustments of the estimates for these returns, rebates, and discounts to actual results were less than 0.1 percent of net sales for each of the years presented. |
Product Litigation Liabilities and Other Contingencies
Product litigation liabilities and other contingencies are, by their nature, uncertain and are based upon complex judgments and probabilities. The factors we consider in developing our product litigation liability reserves and other contingent liability amounts include the merits and jurisdiction of the litigation, the nature and the number of other similar current and past litigation cases, the nature of the product and the current assessment of the science subject to the litigation, and the likelihood of settlement and current state of settlement discussions, if any. In addition, we accrue for certain product liability claims incurred, but not filed, to the extent we can formulate a reasonable estimate of their costs. We estimate these expenses based primarily on historical claims experience and data regarding product usage. We accrue legal defense costs expected to be incurred in connection with significant product liability contingencies when probable and reasonably estimable.
We also consider the insurance coverage we have to diminish the exposure for periods covered by insurance. In assessing our insurance coverage, we consider the policy coverage limits and exclusions, the potential for denial of coverage by the insurance company, the financial condition of the insurers, and the possibility of and length of time for collection. In the past few years, we have experienced difficulties in obtaining product liability insurance due to a very restrictive insurance market. Therefore, for substantially all of our currently marketed products, we have been and expect that we will continue to be completely self-insured for future product liability losses. In addition, there is no assurance that we will be able to fully collect from our insurance carriers in the future.
-35-
The litigation accruals and environmental liabilities and the related estimated insurance recoverables have been reflected on a gross basis as liabilities and assets, respectively, on our consolidated balance sheets.
We believe that the accruals and related insurance recoveries we have established for product litigation liabilities and other contingencies are appropriate based on current facts and circumstances.
Pension and Retiree Medical Plan Assumptions
Pension benefit costs include assumptions for the discount rate, retirement age, and expected return on plan assets. Retiree medical plan costs include assumptions for the discount rate, retirement age, expected return on plan assets, and health-care-cost trend rates. These assumptions have a significant effect on the amounts reported. In addition to the analysis below, see Note 13 to the consolidated financial statements for additional information regarding our retirement benefits.
Periodically, we evaluate the discount rate and the expected return on plan assets in our defined benefit pension and retiree health benefit plans. In evaluating these assumptions, we consider many factors, including an evaluation of the discount rates, expected return on plan assets, and health-care-cost trend rates of other companies; our historical assumptions compared with actual results; an analysis of current market conditions and asset allocations (approximately 88 percent to 92 percent of which are growth investments); and the views of leading financial advisers and economists. We use an actuarially determined, company-specific yield curve to determine the discount rate. In evaluating our expected retirement age assumption, we consider the retirement ages of our past employees eligible for pension and medical benefits together with our expectations of future retirement ages.
We believe our pension and retiree medical plan assumptions are appropriate based upon the above factors. If the health-care-cost trend rates were to be increased by one percentage point each future year, the aggregate of the service cost and interest cost components of the 2008 annual expense would increase by approximately $27 million. A one-percentage-point decrease would lower the aggregate of the 2008 service cost and interest cost by approximately $21 million. If the 2008 discount rate for the U.S. defined benefit pension and retiree health benefit plans (U.S. plans) were to be changed by a quarter percentage point, income before income taxes would change by approximately $26 million. If the 2008 expected return on plan assets for U.S. plans were to be changed by a quarter percentage point, income before income taxes would change by approximately $17 million. If our assumption regarding the 2008 expected age of future retirees for U.S. plans were adjusted by one year, our income before income taxes would be affected by approximately $28 million. The U.S. plans represent approximately 83 percent of the total accumulated postretirement benefit obligation and approximately 84 percent of total plan assets at December 31, 2008.
Impairment of Long-Lived Assets
We review the carrying value of long-lived assets (both intangible and tangible) for potential impairment on a periodic basis and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. We determine impairment by comparing the projected undiscounted cash flows to be generated by the asset to its carrying value. If an impairment is identified, a loss is recorded equal to the excess of the asset’s net book value over its fair value, and the cost basis is adjusted. The estimated future cash flows, based on reasonable and supportable assumptions and projections, require management’s judgment. Actual results could vary from these estimates.
Income Taxes
We prepare and file tax returns based on our interpretation of tax laws and regulations and record estimates based on these judgments and interpretations. In the normal course of business, our tax returns are subject to examination by various taxing authorities, which may result in future tax, interest, and penalty assessments by these authorities. Inherent uncertainties exist in estimates of many tax positions due to changes in tax law resulting from legislation, regulation,and/or as concluded through the various jurisdictions’ tax court systems. We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the
-36-
position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate resolution. The amount of unrecognized tax benefits is adjusted for changes in facts and circumstances. For example, adjustments could result from significant amendments to existing tax law and the issuance of regulations or interpretations by the taxing authorities, new information obtained during a tax examination, or resolution of an examination. We believe that our estimates for uncertain tax positions are appropriate and sufficient to pay assessments that may result from examinations of our tax returns. We recognize both accrued interest and penalties related to unrecognized tax benefits in income tax expense.
We have recorded valuation allowances against certain of our deferred tax assets, primarily those that have been generated from net operating losses and tax credit carryforwards in certain taxing jurisdictions. In evaluating whether we would more likely than not recover these deferred tax assets, we have not assumed any future taxable income or tax planning strategies in the jurisdictions associated with these carryforwards where history does not support such an assumption. Implementation of tax planning strategies to recover these deferred tax assets or future income generation in these jurisdictions could lead to the reversal of these valuation allowances and a reduction of income tax expense.
We believe that our estimates for the uncertain tax positions and valuation allowances against the deferred tax assets are appropriate based on current facts and circumstances. A 5 percent change in the amount of the uncertain tax positions and the valuation allowance would result in a change in net income of approximately $43.2 million and $42.3 million, respectively.
FINANCIAL EXPECTATIONS FOR 2009
For the full year of 2009, we expect earnings per share to be in the range of $4.00 to $4.25. We expect volume growth in sales again in 2009, driven by Cymbalta, Alimta, Cialis, Humalog, and the anticipated launches of prasugrel, as well as by the Elanco animal health division. However, the negative impact of weaker foreign currencies, worldwide pricing pressures, and the impact of generic competition in certain markets for Gemzar are anticipated to partially offset these positive impacts. As a result, we expect mid-single digit sales growth. We expect gross margin as a percent of net sales to increase, driven by the strengthening dollar. This increase could be more pronounced in the first half of 2009. Marketing, selling, and administrative expenses are expected to show flat to low-single digit growth. Research and development expenses are projected to grow in the low-double digits. Other — net is expected to be a net loss of between $200 million and $250 million. Capital expenditures are expected to be approximately $1.1 billion, and we expect continued strong operating cash flow.
Actual results could differ materially and will depend on, among other things, the continuing growth of our currently marketed products; developments with competitive products; the timing and scope of regulatory approvals and the success of our new product launches; asset impairments, restructurings, and acquisitions of compounds under development resulting in acquired in-process research and development charges; foreign exchange rates and global macroeconomic conditions; changes in effective tax rates; wholesaler inventory changes; other regulatory developments, litigation, and government investigations; and the impact of governmental actions regarding pricing, importation, and reimbursement for pharmaceuticals. We undertake no duty to update these forward-looking statements.
LEGAL AND REGULATORY MATTERS
We are a party to various legal actions and government investigations. The most significant of these are described below. While it is not possible to determine the outcome of these matters, we believe that, except as specifically noted below, the resolution of all such matters will not have a material adverse effect on our consolidated financial position or liquidity, but could possibly be material to our consolidated results of operations in any one accounting period.
-37-
Patent Litigation
We are engaged in the following patent litigation matters brought pursuant to procedures set out in the Hatch-Waxman Act (the Drug Price Competition and Patent Term Restoration Act of 1984):
| |
| • | Cymbalta: Sixteen generic drug manufacturers have submitted ANDAs seeking permission to market generic versions of Cymbalta prior to the expiration of our relevant U.S. patents (the earliest of which expires in 2013). Of these challengers, all allege non-infringement of the patent claims directed to the commercial formulation, and eight allege invalidity of the patent claims directed to the active ingredient duloxetine. Of the eight challengers to the compound patent claims, one further alleges invalidity of the claims directed to the use of Cymbalta for treating fibromyalgia, and one alleges the patent having claims directed to the active ingredient is unenforceable. Lawsuits have been filed in U.S. District Court for the Southern District of Indiana against Activis Elizabeth LLC; Aurobindo Pharma Ltd.; Cobalt Laboratories, Inc.; Impax Laboratories, Inc.; Lupin Limited; Sandoz Inc.; Sun Pharma Global, Inc.; and Wockhardt Limited, seeking rulings that the patents are valid, infringed, and enforceable. Answers to the complaints are pending. |
| |
| • | Gemzar: Sicor Pharmaceuticals, Inc. (Sicor), Mayne Pharma (USA) Inc. (Mayne), and Sun Pharmaceutical Industries Inc. (Sun) each submitted an ANDA seeking permission to market generic versions of Gemzar prior to the expiration of our relevant U.S. patents (compound patent expiring in 2010 and method-of-use patent expiring in 2013), and alleging that these patents are invalid. We filed lawsuits in the U.S. District Court for the Southern District of Indiana against Sicor (February 2006) and Mayne (October 2006 and January 2008), seeking rulings that these patents are valid and are being infringed. The suit against Sicor has been scheduled for trial in July 2009. Sicor’s ANDAs have been approved by the FDA; however, Sicor must provide 90 days notice prior to marketing generic Gemzar to allow time for us to seek a preliminary injunction. Both suits against Mayne have been administratively closed, and the parties have agreed to be bound by the results of the Sicor suit. In November 2007, Sun filed a declaratory judgment action in the United States District Court for the Eastern District of Michigan, seeking rulings that our method-of-use and compound patents are invalid or unenforceable, or would not be infringed by the sale of Sun’s generic product. This trial is scheduled for December 2009. |
| |
| • | Alimta: Teva Parenteral Medicines, Inc. (Teva) and APP Pharmaceuticals, LLC (APP) each submitted ANDAs seeking approval to market generic versions of Alimta prior to the expiration of the relevant U.S. patent (licensed from the Trustees of Princeton University and expiring in 2016), and alleging the patent is invalid. We, along with Princeton, filed lawsuits in the U.S. District Court for the District of Delaware against Teva and APP, seeking rulings that the compound patent is valid and infringed. Trial is scheduled for November 8, 2010. |
| |
| • | Evista: Barr Laboratories, Inc. (Barr) submitted an ANDA in 2002 seeking permission to market a generic version of Evista prior to the expiration of our relevant U.S. patents (expiring in2012-2017) and alleging that these patents are invalid, not enforceable, or not infringed. In November 2002, we filed a lawsuit against Barr in the U.S. District Court for the Southern District of Indiana, seeking a ruling that these patents are valid, enforceable, and being infringed by Barr. Teva Pharmaceuticals USA, Inc. (Teva) has also submitted an ANDA seeking permission to market a generic version of Evista. In June 2006, we filed a similar lawsuit against Teva in the U.S. District Court for the Southern District of Indiana. The lawsuit against Teva is currently scheduled for trial beginning March 9, 2009, while no trial date has been set in the lawsuit against Barr. In April 2008, the FDA granted Teva tentative approval of its ANDA, but Teva’s ability to market a generic product is subject to a statutory stay, which has been extended to expire on March 9, 2009. If the stay expires and the company cannot obtain preliminary relief from the court, Teva can launch its generic product, regardless of the status of the current litigation, but subject to our right to recover damages, should we prevail at trial. |
We believe each of these Hatch-Waxman challenges is without merit and expect to prevail in this litigation. However, it is not possible to determine the outcome of this litigation, and accordingly, we can provide no assurance that we will prevail. An unfavorable outcome in any of these cases could have a material adverse impact on our future consolidated results of operations, liquidity, and financial position.
-38-
We have received challenges to Zyprexa patents in a number of countries outside the U.S.:
| |
| • | In Canada, several generic pharmaceutical manufacturers have challenged the validity of our Zyprexa compound and method-of-use patent (expiring in 2011). In April 2007, the Canadian Federal Court ruled against the first challenger, Apotex Inc. (Apotex), and that ruling was affirmed on appeal in February 2008. In June 2007, the Canadian Federal Court held that an invalidity allegation of a second challenger, Novopharm Ltd. (Novopharm), was justified and denied our request that Novopharm be prohibited from receiving marketing approval for generic olanzapine in Canada. Novopharm began selling generic olanzapine in Canada in the third quarter of 2007. We sued Novopharm for patent infringement, and the trial began in November 2008. We expect the trial to run through the first quarter of 2009, with a decision in the second half of 2009. In November 2007, Apotex filed an action seeking a declaration of the invalidity of our Zyprexa compound and method-of-use patents, and no trial date has been set. We have brought similar actions against Pharmascience (August 2007), Sandoz (July 2007), Nu-Pharm (June 2008), Genpharm (June 2008) and Cobalt (January 2009); none of these suits has been scheduled for trial. Pharmascience has agreed to be bound by the outcome of the Novopharm suit, and, pending the outcome of the lawsuit, we have agreed not to take any further steps to prevent the company from coming to market with generic olanzapine tablets, subject to a contingent damages obligation should we be successful against Novopharm. |
| |
| • | In Germany, generic pharmaceutical manufacturers Egis-Gyogyszergyar and Neolab Ltd. challenged the validity of our Zyprexa compound and method-of-use patent (expiring in 2011). In June 2007, the German Federal Patent Court held that our patent is invalid. Generic olanzapine was launched by competitors in Germany in the fourth quarter of 2007. We appealed the decision to the German Federal Supreme Court and following a hearing in December 2008, the Supreme Court reversed the Federal Patent Court and found the patent to be valid. Following the decision of the Supreme Court, the generic companies either agreed to withdraw from the market or were subject to preliminary injunction. We are pursuing these companies for damages arising from infringement. |
| |
| • | We have received challenges in a number of other countries, including Spain, the United Kingdom (U.K.), France, and several smaller European countries. In Spain, we have been successful at both the trial and appellate court levels in defeating the generic manufacturers’ challenges, but further legal challenge is now pending before the Commercial Court in Madrid. In the U.K., the generic pharmaceutical manufacturer Dr. Reddy’s Laboratories (UK) Limited has challenged the validity of our Zyprexa compound and method-of-use patent (expiring in 2011). In October 2008, the Patents Court in the High Court, London ruled that our patent was valid. Dr. Reddy’s appealed this decision, and a hearing date for the appeal has not been set. |
We are vigorously contesting the various legal challenges to our Zyprexa patents on acountry-by-country basis. We cannot determine the outcome of this litigation. The availability of generic olanzapine in additional markets could have a material adverse impact on our consolidated results of operations.
Xigris and Evista: In June 2002, Ariad Pharmaceuticals, Inc., the Massachusetts Institute of Technology, the Whitehead Institute for Biomedical Research, and the President and Fellows of Harvard College in the U.S. District Court for the District of Massachusetts sued us, alleging that sales of two of our products, Xigris and Evista, were inducing the infringement of a patent related to the discovery of a natural cell signaling phenomenon in the human body, and seeking royalties on past and future sales of these products. On May 4, 2006, a jury in Boston issued an initial decision in the case that Xigris and Evista sales infringe the patent. The jury awarded the plaintiffs approximately $65 million in damages, calculated by applying a 2.3 percent royalty to all U.S. sales of Xigris and Evista from the date of issuance of the patent through the date of trial. In addition, a separate bench trial with the U.S. District Court of Massachusetts was held in August 2006, on our contention that the patent is unenforceable and impermissibly covers natural processes. In June 2005, the United States Patent and Trademark Office (USPTO) commenced a reexamination of the patent, and in August 2007 took the position that the Ariad claims at issue are unpatentable, a position that Ariad continues to contest. In September 2007, the Court entered a final judgment indicating that Ariad’s claims are patentable, valid, and enforceable, and finding damages in the amount of $65 million plus a 2.3 percent royalty on net U.S. sales of Xigris and Evista since the time of the jury decision. However, the Court deferred the requirement to pay any damages until after all rights to appeal have been exhausted. We have appealed this
-39-
judgment. The Court of Appeals for the Federal Circuit heard oral arguments on the appeal on February 6, 2009. We believe that these allegations are without legal merit, that we will ultimately prevail on these issues, and therefore that the likelihood of any monetary damages is remote.
Government Investigations and Related Litigation
In March 2004, the Office of the U.S. Attorney for the EDPA advised us that it had commenced an investigation related to our U.S. marketing and promotional practices, including our communications with physicians and remuneration of physician consultants and advisors, with respect to Zyprexa, Prozac, and Prozac Weekly. In addition, the State Medicaid Fraud Control Units of more than 30 states coordinated with the EDPA in its investigation of any Medicaid-related claims relating to our marketing and promotion of Zyprexa. In January 2009, we announced that we reached resolution of this matter. As part of the resolution, we pled guilty to one misdemeanor violation of the Food, Drug, and Cosmetic Act and agreed to pay $615.0 million. The misdemeanor plea is for the off-label promotion of Zyprexa in elderly populations as treatment for dementia, including Alzheimer’s dementia, between September 1999 and March 2001. We have also entered into a settlement agreement resolving the federal civil claims, under which we will pay approximately $438.0 million, although we do not admit to the allegations. We have also agreed to settle the civil investigations brought by the State Medicaid Fraud Control Units of the states that have coordinated with the EDPA in its investigation, and will make available a maximum amount of approximately $362.0 million for payment to those states that agree to settle. The charge we recorded for this matter in the third quarter of $1.42 billion will be sufficient to cover these payments. Also, as part of the settlement, we have entered into a corporate integrity agreement with the Office of Inspector General (OIG) of the U.S. Department of Health and Human Services (HHS). This agreement will require us to maintain our compliance program and to undertake a set of defined corporate integrity obligations for five years. The agreement also provides for an independent third-party review organization to assess and report on the company’s systems, processes, policies, procedures and practices.
In June 2005, we received a subpoena from the Office of the Attorney General, Medicaid Fraud Control Unit, of the State of Florida, seeking production of documents relating to sales of Zyprexa and our marketing and promotional practices with respect to Zyprexa. In September 2006, we received a subpoena from the California Attorney General’s Office seeking production of documents related to our efforts to obtain and maintain Zyprexa’s status on California’s formulary, marketing and promotional practices with respect to Zyprexa, and remuneration of health care providers. We expect these matters to be resolved if Florida and California participate in the state component of the EDPA resolution.
Beginning in August 2006, we received civil investigative demands or subpoenas from the attorneys general of a number of states under various state consumer protection laws. Most of these requests became part of a multistate investigative effort coordinated by an executive committee of attorneys general. In October 2008, we reached a settlement with 32 states and the District of Columbia. While there is no finding that we have violated any provision of the state laws under which the investigations were conducted, we paid $62.0 million and agreed to undertake certain commitments regarding Zyprexa for a period of six years, through consent decrees filed in the settling states. The 32 states participating in the settlement are: Alabama, Arizona, California, Delaware, Florida, Hawaii, Illinois, Indiana, Iowa, Kansas, Maine, Maryland, Massachusetts, Michigan, Missouri, Nebraska, Nevada, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Dakota, Tennessee, Texas, Vermont, Washington, and Wisconsin.
Product Liability and Related Litigation
We have been named as a defendant in a large number of Zyprexa product liability lawsuits in the U.S. and have been notified of many other claims of individuals who have not filed suit. The lawsuits and unfiled claims (together the “claims”) allege a variety of injuries from the use of Zyprexa, with the majority alleging that the product caused or contributed to diabetes or high blood-glucose levels. The claims seek substantial compensatory and punitive damages and typically accuse us of inadequately testing for and warning about side effects of Zyprexa. Many of the claims also allege that we improperly promoted the drug. Almost all of the
-40-
federal lawsuits are part of a Multi-District Litigation (MDL) proceeding before The Honorable Jack Weinstein in the Federal District Court for the Eastern District of New York (MDL No. 1596).
Since June 2005, we have entered into agreements with various claimants’ attorneys involved in U.S. Zyprexa product liability litigation to settle a substantial majority of the claims. The agreements cover a total of approximately 32,670 claimants, including a large number of previously filed lawsuits and other asserted claims. The two primary settlements were as follows:
| |
| • | In June 2005, we reached an agreement in principle (and in September 2005 a final agreement) to settle more than 8,000 claims for $690.0 million plus $10.0 million to cover administration of the settlement. |
| |
| • | In January 2007, we reached agreements with a number of plaintiffs’ attorneys to settle more than 18,000 claims for approximately $500 million. |
The 2005 settlement totaling $700.0 million was paid during 2005. The January 2007 settlements were paid during 2007.
We are prepared to continue our vigorous defense of Zyprexa in all remaining claims. The U.S. Zyprexa product liability claims not subject to these agreements include approximately 105 lawsuits in the U.S. covering approximately 120 plaintiffs, of which about 80 cases covering about 90 plaintiffs are part of the MDL. No trials have been scheduled related to these claims.
In early 2005, we were served with four lawsuits seeking class action status in Canada on behalf of patients who took Zyprexa. One of these four lawsuits has been certified for residents of Quebec, and a second has been certified in Ontario and includes all Canadian residents except for residents of Quebec and British Columbia. The allegations in the Canadian actions are similar to those in the litigation pending in the U.S.
Since the beginning of 2005, we have recorded aggregate net pretax charges of $1.61 billion for Zyprexa product liability matters. The net charges, which take into account our actual insurance recoveries, covered the following:
| |
| • | The cost of the Zyprexa product liability settlements to date; and |
| |
| • | Reserves for product liability exposures and defense costs regarding the known Zyprexa product liability claims and expected future claims to the extent we could formulate a reasonable estimate of the probable number and cost of the claims. |
In December 2004, we were served with two lawsuits brought in state court in Louisiana on behalf of the Louisiana Department of Health and Hospitals, alleging that Zyprexa caused or contributed to diabetes or high blood-glucose levels, and that we improperly promoted the drug. These cases have been removed to federal court and are now part of the MDL proceedings in the Eastern District of New York (EDNY). In these actions, the Department of Health and Hospitals seeks to recover the costs it paid for Zyprexa through Medicaid and other drug-benefit programs, as well as the costs the department alleges it has incurred and will incur to treat Zyprexa-related illnesses. We have been served with similar lawsuits filed by the states of Alaska, Arkansas, Connecticut, Idaho, Minnesota, Mississippi, Montana, New Mexico, Pennsylvania, South Carolina, Utah, and West Virginia in the courts of the respective states. The Connecticut, Louisiana, Minnesota, Mississippi, Montana, New Mexico, and West Virginia cases are part of the MDL proceedings in the EDNY. The Alaska case was settled in March 2008 for a payment of $15.0 million, plus terms designed to ensure, subject to certain limitations and conditions, that Alaska is treated as favorably as certain other states that may settle with us in the future over similar claims. The following cases have been set for trial in 2009: Connecticut in the EDNY in June, Pennsylvania in November, and South Carolina in August, in their respective states.
In 2005, two lawsuits were filed in the EDNY purporting to be nationwide class actions on behalf of all consumers and third-party payors, excluding governmental entities, which have made or will make payments for their members or insured patients being prescribed Zyprexa. These actions have now been consolidated into a single lawsuit, which is brought under certain state consumer protection statutes, the federal civil RICO statute, and common law theories, seeking a refund of the cost of Zyprexa, treble damages, punitive damages, and attorneys’ fees. Two additional lawsuits were filed in the EDNY in 2006 on similar grounds. In September
-41-
2008, Judge Weinstein certified a class consisting of third-party payors, excluding governmental entities and individual consumers. We appealed the certification order, and Judge Weinstein’s order denying our motion for summary judgment, in September 2008. In 2007, The Pennsylvania Employees Trust Fund brought claims in state court in Pennsylvania as insurer of Pennsylvania state employees, who were prescribed Zyprexa on similar grounds as described in the New York cases. As with the product liability suits, these lawsuits allege that we inadequately tested for and warned about side effects of Zyprexa and improperly promoted the drug. The Pennsylvania case is set for trial in October 2009.
We cannot determine with certainty the additional number of lawsuits and claims that may be asserted. The ultimate resolution of Zyprexa product liability and related litigation could have a material adverse impact on our consolidated results of operations, liquidity, and financial position.
In addition, we have been named as a defendant in numerous other product liability lawsuits involving primarily diethylstilbestrol (DES) and thimerosal. The majority of these claims are covered by insurance, subject to deductibles and coverage limits.
Because of the nature of pharmaceutical products, it is possible that we could become subject to large numbers of product liability and related claims for other products in the future. In the past few years, we have experienced difficulties in obtaining product liability insurance due to a very restrictive insurance market. Therefore, for substantially all of our currently marketed products, we have been and expect that we will continue to be completely self-insured for future product liability losses. In addition, there is no assurance that we will be able to fully collect from our insurance carriers in the future.
PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 — A CAUTION CONCERNING FORWARD-LOOKING STATEMENTS
Under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, we caution investors that any forward-looking statements or projections made by us, including those made in this document, are based on management’s expectations at the time they are made, but they are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Economic, competitive, governmental, technological, legal, and other factors that may affect our operations and prospects are discussed earlier in this section and our most recent report onForms 10-Q and10-K filed with the Securities and Exchange Commission. We undertake no duty to update forward-looking statements.
| |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
You can find quantitative and qualitative disclosures about market risk (e.g., interest rate risk) in Part II, Item 7 at “Review of Operations − Financial Condition.” That information is incorporated in this report by reference.
-42-
| |
| Item 8. | Financial Statements and Supplementary Data |
Consolidated Statements of Operations
| | | | | | | | | | | | | |
| | | Year Ended December 31 | |
ELI LILLY AND COMPANY AND SUBSIDIARIES | | 2008 | | | 2007 | | | 2006 | |
| | | (Dollars in millions, except per-share data) | |
| |
| Net sales | | $ | 20,378.0 | | | $ | 18,633.5 | | | $ | 15,691.0 | |
| Cost of sales | | | 4,382.8 | | | | 4,248.8 | | | | 3,546.5 | |
| Research and development | | | 3,840.9 | | | | 3,486.7 | | | | 3,129.3 | |
| Marketing, selling, and administrative | | | 6,626.4 | | | | 6,095.1 | | | | 4,889.8 | |
| Acquired in-process research and development (Note 3) | | | 4,835.4 | | | | 745.6 | | | | — | |
| Asset impairments, restructuring, and other special charges (Note 5) | | | 1,974.0 | | | | 302.5 | | | | 945.2 | |
| Other — net, expense (income) | | | 26.1 | | | | (122.0 | ) | | | (237.8 | ) |
| | | |
| | | |
| | | | 21,685.6 | | | | 14,756.7 | | | | 12,273.0 | |
| | | |
| | | |
| Income (loss) before income taxes | | | (1,307.6 | ) | | | 3,876.8 | | | | 3,418.0 | |
| Income taxes (Note 12) | | | 764.3 | | | | 923.8 | | | | 755.3 | |
| | | |
| | | |
| Net income (loss) | | $ | (2,071.9 | ) | | $ | 2,953.0 | | | $ | 2,662.7 | |
| | | |
| | | |
| Earnings (loss) per share — basic and diluted (Note 11) | | $ | (1.89 | ) | | $ | 2.71 | | | $ | 2.45 | |
| | | |
| | | |
See notes to consolidated financial statements.
-43-
Consolidated Balance Sheets
| | | | | | | | | |
| | | December 31 | |
ELI LILLY AND COMPANY AND SUBSIDIARIES | | 2008 | | | 2007 | |
| | | (Dollars in millions)} | |
| |
Assets | | | | | | | | |
Current Assets | | | | | | | | |
| Cash and cash equivalents | | $ | 5,496.7 | | | $ | 3,220.5 | |
| Short-term investments | | | 429.4 | | | | 1,610.7 | |
| Accounts receivable, net of allowances of $97.4 (2008) and $103.1(2007) | | | 2,778.8 | | | | 2,673.9 | |
| Other receivables (Note 9) | | | 498.5 | | | | 1,030.9 | |
| Inventories | | | 2,493.2 | | | | 2,523.7 | |
| Deferred income taxes (Note 12) | | | 382.1 | | | | 642.8 | |
| Prepaid expenses | | | 374.6 | | | | 613.6 | |
| | | |
| | | |
| Total current assets | | | 12,453.3 | | | | 12,316.1 | |
Other Assets | | | | | | | | |
| Prepaid pension (Note 13) | | | — | | | | 1,670.5 | |
| Investments (Note 6) | | | 1,544.6 | | | | 577.1 | |
| Goodwill and other intangibles — net (Note 3) | | | 4,054.1 | | | | 2,455.4 | |
| Sundry (Note 9) | | | 2,534.3 | | | | 1,280.6 | |
| | | |
| | | |
| | | | 8,133.0 | | | | 5,983.6 | |
Property and Equipment, net | | | 8,626.3 | | | | 8,575.1 | |
| | | |
| | | |
| | | $ | 29,212.6 | | | $ | 26,874.8 | |
| | | |
| | | |
Liabilities and Shareholders’ Equity | | | | | | | | |
Current Liabilities | | | | | | | | |
| Short-term borrowings and current maturities of long-term debt (Note 7) | | $ | 5,846.3 | | | $ | 413.7 | |
| Accounts payable | | | 885.8 | | | | 924.4 | |
| Employee compensation | | | 771.0 | | | | 823.8 | |
| Sales rebates and discounts | | | 873.4 | | | | 706.8 | |
| Dividends payable | | | 536.8 | | | | 513.6 | |
| Income taxes payable (Note 12) | | | 229.2 | | | | 238.4 | |
| Other current liabilities (Note 9) | | | 3,967.2 | | | | 1,816.1 | |
| | | |
| | | |
| Total current liabilities | | | 13,109.7 | | | | 5,436.8 | |
| | | |
| | | |
Other Liabilities | | | | | | | | |
| Long-term debt (Note 7) | | | 4,615.7 | | | | 4,593.5 | |
| Accrued retirement benefit (Note 13) | | | 2,387.6 | | | | 1,145.1 | |
| Long-term income taxes payable (Note 12) | | | 906.2 | | | | 1,196.7 | |
| Deferred income taxes (Note 12) | | | 74.7 | | | | 287.5 | |
| Other noncurrent liabilities (Note 9) | | | 1,383.4 | | | | 711.3 | |
| | | |
| | | |
| | | | 9,367.6 | | | | 7,934.1 | |
| Commitments and contingencies (Note 14) | | | | | | | | |
| Shareholders’ Equity (Notes 8 and 10) | | | | | | | | |
| Common stock — no par value | | | | | | | | |
| Authorized shares: 3,200,000,000 | | | | | | | | |
| Issued shares: 1,136,948,610 (2008) and 1,135,212,894 (2007) | | | 711.1 | | | | 709.5 | |
| Additional paid-in capital | | | 3,976.6 | | | | 3,805.2 | |
| Retained earnings | | | 7,654.9 | | | | 11,806.7 | |
| Employee benefit trust | | | (2,635.0 | ) | | | (2,635.0 | ) |
| Deferred costs — ESOP | | | (86.3 | ) | | | (95.2 | ) |
| Accumulated other comprehensive income (loss) (Note 15) | | | (2,786.8 | ) | | | 13.2 | |
| | | |
| | | |
| | | | 6,834.5 | | | | 13,604.4 | |
| Less cost of common stock in treasury | | | | | | | | |
| 2008 — 888,998 shares | | | | | | | | |
| 2007 — 899,445 shares | | | 99.2 | | | | 100.5 | |
| | | | 6,735.3 | | | | 13,503.9 | |
| | | |
| | | |
| | | $ | 29,212.6 | | | $ | 26,874.8 | |
| | | |
| | | |
See notes to consolidated financial statements.
-44-
Consolidated Statements of Cash Flows
| | | | | | | | | | | | | |
| | | Year Ended December 31 | |
ELI LILLY AND COMPANY AND SUBSIDIARIES | | 2008 | | | 2007 | | | 2006 | |
| | | (Dollars in millions) | |
| |
Cash Flows From Operating Activities | | | | | | | | | | | | |
| Net income (loss) | | $ | (2,071.9 | ) | | $ | 2,953.0 | | | $ | 2,662.7 | |
Adjustments To Reconcile Net Income To Cash Flows | | | | | | | | | | | | |
From Operating Activities | | | | | | | | | | | | |
| Depreciation and amortization | | | 1,122.6 | | | | 1,047.9 | | | | 801.8 | |
| Change in deferred taxes | | | 442.6 | | | | 60.7 | | | | 346.8 | |
| Stock-based compensation expense | | | 255.3 | | | | 282.0 | | | | 359.3 | |
| Acquired in-process research and development, net of tax | | | 4,792.7 | | | | 692.6 | | | | — | |
| Other, net | | | 406.5 | | | | 172.1 | | | | 600.6 | |
| | | |
| | | |
| | | | 4,947.8 | | | | 5,208.3 | | | | 4,771.2 | |
| Changes in operating assets and liabilities, net of acquisitions Receivables — (increase) decrease | | | 799.1 | | | | (842.7 | ) | | | 243.9 | |
| Inventories — (increase) decrease | | | 84.8 | | | | 154.3 | | | | (60.2 | ) |
| Other assets — (increase) decrease | | | 1,648.6 | | | | (355.8 | ) | | | (43.0 | ) |
| Accounts payable and other liabilities — increase (decrease) | | | (184.7 | ) | | | 990.4 | | | | (936.0 | ) |
| | | |
| | | |
| | | | 2,347.8 | | | | (53.8 | ) | | | (795.3 | ) |
| | | |
| | | |
Net Cash Provided by Operating Activities | | | 7,295.6 | | | | 5,154.5 | | | | 3,975.9 | |
| | | |
| | | |
Cash Flows From Investing Activities | | | | | | | | | | | | |
| Purchases of property and equipment | | | (947.2 | ) | | | (1,082.4 | ) | | | (1,077.8 | ) |
| Disposals of property and equipment | | | 25.7 | | | | 32.3 | | | | 65.2 | |
| Net change in short-term investments | | | 957.6 | | | | (376.9 | ) | | | 1,247.5 | |
| Proceeds from sales and maturities of noncurrent investments | | | 1,597.3 | | | | 800.1 | | | | 1,507.7 | |
| Purchases of noncurrent investments | | | (2,412.4 | ) | | | (750.7 | ) | | | (1,313.2 | ) |
| Purchases of in-process research and development | | | (122.0 | ) | | | (111.0 | ) | | | — | |
| Cash paid for acquisitions, net of cash acquired | | | (6,083.0 | ) | | | (2,673.2 | ) | | | — | |
| Other, net | | | (284.8 | ) | | | (166.3 | ) | | | 179.0 | |
| | | |
| | | |
Net Cash Provided by (Used for) Investing Activities | | | (7,268.8 | ) | | | (4,328.1 | ) | | | 608.4 | |
| | | |
| | | |
Cash Flows From Financing Activities | | | | | | | | | | | | |
| Dividends paid | | | (2,056.7 | ) | | | (1,853.6 | ) | | | (1,736.3 | ) |
| Net change in short-term borrowings | | | 5,060.5 | | | | (468.5 | ) | | | (8.4 | ) |
| Proceeds from issuance of long-term debt | | | 0.1 | | | | 2,512.6 | | | | — | |
| Repayments of long-term debt | | | (649.8 | ) | | | (1,059.5 | ) | | | (2,781.5 | ) |
| Purchases of common stock | | | — | | | | — | | | | (122.1 | ) |
| Issuances of common stock under stock plans | | | — | | | | 24.7 | | | | 59.6 | |
| Other, net | | | (8.1 | ) | | | (0.6 | ) | | | 9.9 | |
| | | |
| | | |
Net Cash Provided by (Used for) Financing Activities | | | 2,346.0 | | | | (844.9 | ) | | | (4,578.8 | ) |
| | | |
| | | |
| Effect of exchange rate changes on cash and cash equivalents | | | (96.6 | ) | | | 129.7 | | | | 97.1 | |
| | | |
| | | |
| Net increase in cash and cash equivalents | | | 2,276.2 | | | | 111.2 | | | | 102.6 | |
| Cash and cash equivalents at beginning of year | | | 3,220.5 | | | | 3,109.3 | | | | 3,006.7 | |
| | | |
| | | |
Cash and Cash Equivalents at End of Year | | $ | 5,496.7 | | | $ | 3,220.5 | | | $ | 3,109.3 | |
| | | |
| | | |
See notes to consolidated financial statements.
-45-
Consolidated Statements of Comprehensive Income (Loss)
| | | | | | | | | | | | | |
| | | Year Ended December 31 | |
ELI LILLY AND COMPANY AND SUBSIDIARIES | | 2008 | | | 2007 | | | 2006 | |
| | | (Dollars in millions) | |
| |
| Net income (loss) | | $ | (2,071.9 | ) | | $ | 2,953.0 | | | $ | 2,662.7 | |
| Other comprehensive income (loss) | | | | | | | | | | | | |
| Foreign currency translation gains (losses) | | | (766.1 | ) | | | 756.6 | | | | 542.4 | |
| Net unrealized losses on securities | | | (190.6 | ) | | | (11.4 | ) | | | (3.2 | ) |
| Minimum pension liability adjustment (Note 13) | | | — | | | | — | | | | (18.8 | ) |
| Defined benefit pension and retiree health benefit plans (Note 13) | | | (2,941.2 | ) | | | 943.8 | | | | — | |
| Effective portion of cash flow hedges | | | 23.2 | | | | (0.1 | ) | | | 143.3 | |
| | | |
| | | |
| Other comprehensive income (loss) before income taxes | | | (3,874.7 | ) | | | 1,688.9 | | | | 663.7 | |
| Provision for income taxes related to other comprehensive income (loss) items | | | 1,074.7 | | | | (287.0 | ) | | | (43.1 | ) |
| | | |
| | | |
| Other comprehensive income (loss) (Note 15) | | | (2,800.0 | ) | | | 1,401.9 | | | | 620.6 | |
| | | |
| | | |
| Comprehensive income (loss) | | $ | (4,871.9 | ) | | $ | 4,354.9 | | | $ | 3,283.3 | |
| | | |
| | | |
See notes to consolidated financial statements
-46-
Segment Information
We operate in one significant business segment — human pharmaceutical products. Operations of the animal health business segment are not material and share many of the same economic and operating characteristics as human pharmaceutical products. Therefore, they are included with pharmaceutical products for purposes of segment reporting.
| | | | | | | | | | | | | |
| | | Year Ended December 31 | |
| ELI LILLY AND COMPANY AND SUBSIDIARIES | | 2008 | | | 2007 | | | 2006 | |
| | | (Dollars in millions) | |
| |
| Net sales — to unaffiliated customers | | | | | | | | | | | | |
| Neurosciences | | $ | 8,371.5 | | | $ | 7,851.0 | | | $ | 6,728.5 | |
| Endocrinology | | | 5,890.7 | | | | 5,479.6 | | | | 5,014.5 | |
| Oncology | | | 2,874.5 | | | | 2,446.4 | | | | 2,020.2 | |
| Cardiovascular | | | 1,882.7 | | | | 1,624.1 | | | | 730.4 | |
| Animal health | | | 1,093.3 | | | | 995.8 | | | | 875.5 | |
| Other pharmaceuticals | | | 265.3 | | | | 236.6 | | | | 321.9 | |
| | | |
| | | |
| Net sales | | $ | 20,378.0 | | | $ | 18,633.5 | | | $ | 15,691.0 | |
| | | |
| | | |
Geographic Information | | | | | | | | | | | | |
Net sales — to unaffiliated customers1 | | | | | | | | | | | | |
| United States | | $ | 10,934.4 | | | $ | 10,145.5 | | | $ | 8,599.2 | |
| Europe | | | 5,334.9 | | | | 4,731.8 | | | | 3,804.0 | |
| Other foreign countries | | | 4,108.7 | | | | 3,756.2 | | | | 3,287.8 | |
| | | |
| | | |
| | | $ | 20,378.0 | | | $ | 18,633.5 | | | $ | 15,691.0 | |
| | | |
| | | |
| Long-lived assets | | | | | | | | | | | | |
| United States | | $ | 5,750.0 | | | $ | 5,905.4 | | | $ | 6,207.4 | |
| Europe | | | 2,119.0 | | | | 2,057.7 | | | | 1,733.8 | |
| Other foreign countries | | | 1,753.0 | | | | 1,768.6 | | | | 1,718.4 | |
| | | |
| | | |
| | | $ | 9,622.0 | | | $ | 9,731.7 | | | $ | 9,659.6 | |
| | | |
| | | |
| |
| 1 | Net sales are attributed to the countries based on the location of the customer. |
The largest category of products is the neurosciences group, which includes Zyprexa, Cymbalta, Strattera, and Prozac. Endocrinology products consist primarily of Humalog, Humulin, Byetta, Actos, Evista, Forteo, and Humatrope. Oncology products consist primarily of Gemzar and Alimta. Cardiovascular products consist primarily of Cialis, ReoPro, and Xigris. Animal health products include Posilac, Tylan, Rumensin, Coban, and other products for livestock and poultry, and Comfortis and other products for companion animals. The other pharmaceuticals category includes anti-infectives, primarily Ceclor and Vancocin, and other miscellaneous pharmaceutical products and services.
Most of our pharmaceutical products are distributed through wholesalers that serve pharmacies, physicians and other health care professionals, and hospitals. In 2008, our three largest wholesalers each accounted for between 12 percent and 16 percent of consolidated net sales. Further, they each accounted for between 10 percent and 15 percent of accounts receivable as of December 31, 2008. Animal health products are sold primarily to wholesale distributors.
Our business segments are distinguished by the ultimate end user of the product: humans or animals. Performance is evaluated based on profit or loss from operations before income taxes. The accounting policies of the individual segments are substantially the same as those described in the summary of significant accounting policies in Note 1 to the consolidated financial statements. Income before income taxes for the animal health business was approximately $192 million, $173 million, and $184 million in 2008, 2007, and 2006, respectively.
-47-
The assets of the animal health business are intermixed with those of the pharmaceutical products business. Long-lived assets disclosed above consist of property and equipment and certain sundry assets.
We are exposed to the risk of changes in social, political, and economic conditions inherent in foreign operations, and our results of operations and the value of our foreign assets are affected by fluctuations in foreign currency exchange rates.
-48-
Selected Quarterly Data (unaudited)
| | | | | | | | | | | | | | | | | |
| ELI LILLY AND COMPANY AND SUBSIDIARIES | | Fourth | | | Third | | | Second | | | First | |
| | | (Dollars in millions, except per-share data) | |
| |
2008 | | | | | | | | | | | | | | | | |
| Net sales | | $ | 5,210.5 | | | $ | 5,209.5 | | | $ | 5,150.4 | | | $ | 4,807.6 | |
| Cost of sales | | | 915.4 | | | | 1,155.2 | | | | 1,200.9 | | | | 1,111.3 | |
| Operating expenses | | | 2,785.9 | | | | 2,602.2 | | | | 2,651.6 | | | | 2,427.6 | |
| Acquired in-process research and development | | | 4,685.4 | | | | 28.0 | | | | 35.0 | | | | 87.0 | |
| Asset impairments, restructuring, and other special charges | | | 80.0 | | | | 1,659.4 | | | | 88.9 | | | | 145.7 | |
| Other — net, expense (income) | | | 81.2 | | | | (2.5 | ) | | | (32.3 | ) | | | (20.3 | ) |
| Income (loss) before income taxes | | | (3,337.4 | ) | | | (232.8 | ) | | | 1,206.3 | | | | 1,056.3 | |
Net income (loss)1 | | | (3,629.4 | ) | | | (465.6 | ) | | | 958.8 | | | | 1,064.3 | |
| Earnings (loss) per share — basic and diluted | | | (3.31 | ) | | | (.43 | ) | | | .88 | | | | .97 | |
| Dividends paid per share | | | .47 | | | | .47 | | | | .47 | | | | .47 | |
| Common stock closing prices | | | | | | | | | | | | | | | | |
| High | | | 43.69 | | | | 49.25 | | | | 53.06 | | | | 57.18 | |
| Low | | | 29.91 | | | | 43.92 | | | | 45.61 | | | | 47.81 | |
| | | | | | | | | | | | | | | | | |
| | | Fourth | | | Third | | | Second | | | First | |
2007 | | | | | | | | | | | | | | | | |
| Net sales | | $ | 5,189.6 | | | $ | 4,586.8 | | | $ | 4,631.0 | | | $ | 4,226.1 | |
| Cost of sales | | | 1,272.8 | | | | 1,054.6 | | | | 998.9 | | | | 922.5 | |
| Operating expenses | | | 2,709.4 | | | | 2,322.3 | | | | 2,379.1 | | | | 2,171.0 | |
| Acquired in-process research and development | | | 89.0 | | | | — | | | | 328.1 | | | | 328.5 | |
| Asset impairments, restructuring, and other special charges | | | 98.2 | | | | 81.3 | | | | — | | | | 123.0 | |
| Other — net, expense (income) | | | (32.1 | ) | | | (49.8 | ) | | | (1.8 | ) | | | (38.3 | ) |
| Income before income taxes | | | 1,052.3 | | | | 1,178.4 | | | | 926.7 | | | | 719.4 | |
| Net income | | | 854.4 | | | | 926.3 | | | | 663.6 | | | | 508.7 | |
| Earnings per share — basic and diluted | | | .78 | | | | .85 | | | | .61 | | | | .47 | |
| Dividends paid per share | | | 425 | | | | .425 | | | | .425 | | | | .425 | |
| Common stock closing prices | | | | | | | | | | | | | | | | |
| High | | | 59.47 | | | | 58.44 | | | | 60.56 | | | | 54.99 | |
| Low | | | 49.09 | | | | 54.09 | | | | 54.39 | | | | 51.63 | |
Our common stock is listed on the New York, London, and Swiss stock exchanges.
| |
| 1 | We incurred tax expense of $764.3 million in 2008, despite having a loss before income taxes of $1.31 billion. Our net loss was driven by the $4.69 billion acquired IPR&D charge for ImClone in the fourth quarter and the $1.48 billion Zyprexa investigation settlements recorded in the third quarter. The IPR&D charge was not tax deductible, and only a portion of the Zyprexa investigation settlements was deductible. In addition, we recorded tax expense associated with the ImClone acquisition in the fourth quarter, as well as a discrete income tax benefit of $210.3 million in the first quarter for the resolution of the IRS audit. |
-49-
Selected Financial Data (unaudited)
| | | | | | | | | | | | | | | | | | | | | |
ELI LILLY AND COMPANY AND SUBSIDIARIES | | 2008 | | | 20072 | | | 2006 | | | 2005 | | | 2004 | |
| | | | |
| | | (Dollars in millions, except net sales per employee and per-share data) | |
| |
Operations | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 20,378.0 | | | $ | 18,633.5 | | | $ | 15,691.0 | | | $ | 14,645.3 | | | $ | 13,857.9 | |
| Cost of sales | | | 4,382.8 | | | | 4,248.8 | | | | 3,546.5 | | | | 3,474.2 | | | | 3,223.9 | |
| Research and development | | | 3,840.9 | | | | 3,486.7 | | | | 3,129.3 | | | | 3,025.5 | | | | 2,691.1 | |
| Marketing, selling, and administrative | | | 6,626.4 | | | | 6,095.1 | | | | 4,889.8 | | | | 4,497.0 | | | | 4,284.2 | |
| Other | | | 6,835.5 | 4 | | | 926.1 | | | | 707.4 | | | | 931.1 | | | | 716.8 | |
| Income (loss) before income taxes and cumulative effect of a change in accounting principle | | | (1,307.6 | ) | | | 3,876.8 | | | | 3,418.0 | | | | 2,717.5 | | | | 2,941.9 | |
| Income taxes | | | 764.3 | | | | 923.8 | | | | 755.3 | | | | 715.9 | | | | 1,131.8 | |
| Net income (loss) | | | (2,071.9 | ) | | | 2,953.0 | | | | 2,662.7 | | | | 1,979.6 | 1 | | | 1,810.1 | |
| Net income as a percent of sales | | | NM | | | | 15.8 | % | | | 17.0 | % | | | 13.5 | % | | | 13.1 | % |
| Net income (loss) per share — diluted | | | (1.89 | ) | | | 2.71 | | | | 2.45 | | | | 1.81 | | | | 1.66 | |
| Dividends declared per share | | | 1.90 | | | | 1.75 | | | | 1.63 | | | | 1.54 | | | | 1.45 | |
| Weighted-average number of shares outstanding — diluted (thousands) | | | 1,094,499 | | | | 1,090,750 | | | | 1,087,490 | | | | 1,092,150 | | | | 1,088,936 | |
| | | |
| | | |
Financial Position | | | | | | | | | | | | | | | | | | | | |
| Current assets | | $ | 12,453.3 | | | $ | 12,316.1 | | | $ | 9,753.6 | | | $ | 10,855.0 | | | $ | 12,895.0 | |
| Current liabilities | | | 13,109.7 | | | | 5,436.8 | | | | 5,254.0 | | | | 5,884.8 | | | | 7,762.2 | |
| Property and equipment — net | | | 8,626.3 | | | | 8,575.1 | | | | 8,152.3 | | | | 7,912.5 | | | | 7,550.9 | |
| Total assets | | | 29,212.6 | | | | 26,874.8 | | | | 22,042.4 | | | | 24,667.8 | | | | 24,954.0 | |
| Long-term debt | | | 4,615.7 | | | | 4,593.5 | | | | 3,494.4 | | | | 5,763.5 | | | | 4,491.9 | |
| Shareholders’ equity | | | 6,735.3 | | | | 13,503.9 | | | | 10,820.2 | | | | 10,631.4 | | | | 10,759.4 | |
| | | |
| | | |
Supplementary Data | | | | | | | | | | | | | | | | | | | | |
| Return on shareholders’ equity | | | (16.3 | )% | | | 24.3 | % | | | 24.8 | % | | | 18.5 | % | | | 17.8 | % |
| Return on assets | | | (7.5 | )% | | | 12.1 | % | | | 11.1 | % | | | 8.2 | % | | | 7.8 | % |
| Capital expenditures | | $ | 947.2 | | | $ | 1,082.4 | | | $ | 1,077.8 | | | $ | 1,298.1 | | | $ | 1,898.1 | |
| Depreciation and amortization | | | 1,122.6 | | | | 1,047.9 | | | | 801.8 | | | | 726.4 | | | | 597.5 | |
| Effective tax rate | | | NM | 3 | | | 23.8 | % | | | 22.1 | % | | | 26.3 | % | | | 38.5 | % |
| Net sales per employee | | $ | 504,000 | | | $ | 459,000 | | | $ | 378,000 | | | $ | 344,000 | | | $ | 311,000 | |
| Number of employees | | | 40,450 | | | | 40,600 | | | | 41,500 | | | | 42,600 | | | | 44,500 | |
| Number of shareholders of record | | | 39,800 | | | | 41,700 | | | | 44,800 | | | | 50,800 | | | | 52,400 | |
| | | |
| | | |
NM — Not Meaningful
| | |
| 1 | | Reflects the impact of a cumulative effect of a change in accounting principle in 2005 of $22.0 million, net of income taxes of $11.8 million. The diluted earnings per share impact of this cumulative effect of a change in accounting principle was $.02. The net income per diluted share before the cumulative effect of a change in accounting principle was $1.83. |
| |
| 2 | | Reflects the ICOS acquisition, effective January 29, 2007. See Note 3 for additional information. |
| |
| 3 | | We incurred tax expense of $764.3 million in 2008, despite having a loss before income taxes of $1.31 billion. Our net loss was driven by the $4.69 billion acquired IPR&D charge for ImClone and the $1.48 billion |
-50-
| | |
| | Zyprexa investigation settlements. The IPR&D charge was not tax deductible, and only a portion of the Zyprexa investigation settlements was deductible. In addition, we recorded tax expense associated with the ImClone acquisition, as well as a discrete income tax benefit of $210.3 million for the resolution of the IRS audit. |
| |
| 4 | | The increase reflects the in-process research and development expense of $4.69 billion associated with the ImClone acquisition and $1.48 billion associated with the Zyprexa investigation settlements. |
-51-
Notes to Consolidated Financial Statements
ELI LILLY AND COMPANY AND SUBSIDIARIES
(Dollars in millions, except per-share data)
| |
| Note 1: | Summary of Significant Accounting Policies |
Basis of presentation: The accompanying consolidated financial statements have been prepared in accordance with accounting practices generally accepted in the United States (GAAP). The accounts of all wholly owned and majority-owned subsidiaries are included in the consolidated financial statements. Where our ownership of consolidated subsidiaries is less than 100 percent, the outside shareholders’ interests are reflected in other noncurrent liabilities. All intercompany balances and transactions have been eliminated.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses, and related disclosures at the date of the financial statements and during the reporting period. Actual results could differ from those estimates.
All per-share amounts, unless otherwise noted in the footnotes, are presented on a diluted basis, that is, based on the weighted-average number of outstanding common shares plus the effect of dilutive stock options and other incremental shares.
Cash equivalents: We consider all highly liquid investments with a maturity of three months or less from the date of purchase to be cash equivalents. The cost of these investments approximates fair value. Included in cash equivalents at December 31, 2008, is restricted cash of $339.0 million related to the debt assumed with the ImClone acquisition, which is expected to be paid in the first quarter of 2009.
Inventories: We state all inventories at the lower of cost or market. We use thelast-in, first-out (LIFO) method for the majority of our inventories located in the continental United States, or approximately 45 percent of our total inventories. Other inventories are valued by thefirst-in, first-out (FIFO) method. FIFO cost approximates current replacement cost. Inventories at December 31 consisted of the following:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | |
| |
| Finished products | | $ | 771.0 | | | $ | 653.4 | |
| Work in process | | | 1,657.1 | | | | 1,803.0 | |
| Raw materials and supplies | | | 236.3 | | | | 202.7 | |
| | | |
| | | |
| | | | 2, 664.4 | | | | 2,659.1 | |
| Reduction to LIFO cost | | | (171.2 | ) | | | (135.4 | ) |
| | | |
| | | |
| | | $ | 2,493.2 | | | $ | 2,523.7 | |
| | | |
| | | |
Investments: Substantially all of our investments in debt and marketable equity securities are classified as available-for-sale. Available-for-sale securities are carried at fair value with the unrealized gains and losses, net of tax, reported in other comprehensive income. Unrealized losses considered to be other-than-temporary are recognized in earnings. Factors we consider in making this evaluation include company-specific drivers of the decrease in fair value, status of projects in development, near-term prospects of the issuer, the length of time the value has been depressed, and the financial condition of the industry. We do not evaluate cost-method investments for impairment unless there is an indicator of impairment. We review these investments for indicators of impairment on a regular basis. Realized gains and losses on sales of available-for-sale securities are computed based upon specific identification of the initial cost adjusted for any other-than-temporary declines in fair value. Investments in companies over which we have significant influence but not a controlling interest are accounted for using the equity method with our share of earnings or losses reported in other — net. We own no investments that are considered to be trading securities.
Risk-management instruments: Our derivative activities are initiated within the guidelines of documented corporate risk-management policies and do not create additional risk because gains and losses on derivative
-52-
contracts offset losses and gains on the assets, liabilities, and transactions being hedged. As derivative contracts are initiated, we designate the instruments individually as either a fair value hedge or a cash flow hedge. Management reviews the correlation and effectiveness of our derivatives on a quarterly basis.
For derivative contracts that are designated and qualify as fair value hedges, the derivative instrument is marked to market with gains and losses recognized currently in income to offset the respective losses and gains recognized on the underlying exposure. For derivative contracts that are designated and qualify as cash flow hedges, the effective portion of gains and losses on these contracts is reported as a component of other comprehensive income and reclassified into earnings in the same period the hedged transaction affects earnings. Hedge ineffectiveness is immediately recognized in earnings. Derivative contracts that are not designated as hedging instruments are recorded at fair value with the gain or loss recognized in current earnings during the period of change.
We may enter into foreign currency forward and option contracts to reduce the effect of fluctuating currency exchange rates (principally the euro, the British pound, and the Japanese yen). Foreign currency derivatives used for hedging are put in place using the same or like currencies and duration as the underlying exposures. Forward contracts are principally used to manage exposures arising from subsidiary trade and loan payables and receivables denominated in foreign currencies. These contracts are recorded at fair value with the gain or loss recognized in other — net. The purchased option contracts are used to hedge anticipated foreign currency transactions, primarily intercompany inventory activities expected to occur within the next year. These contracts are designated as cash flow hedges of those future transactions and the impact on earnings is included in cost of sales. We may enter into foreign currency forward contracts and currency swaps as fair value hedges of firm commitments. Forward and option contracts generally have maturities not exceeding 12 months.
In the normal course of business, our operations are exposed to fluctuations in interest rates. These fluctuations can vary the costs of financing, investing, and operating. We address a portion of these risks through a controlled program of risk management that includes the use of derivative financial instruments. The objective of controlling these risks is to limit the impact of fluctuations in interest rates on earnings. Our primary interest rate risk exposure results from changes in short-term U.S. dollar interest rates. In an effort to manage interest rate exposures, we strive to achieve an acceptable balance between fixed and floating rate debt and investment positions and may enter into interest rate swaps or collars to help maintain that balance. Interest rate swaps or collars that convert our fixed-rate debt or investments to a floating rate are designated as fair value hedges of the underlying instruments. Interest rate swaps or collars that convert floating rate debt or investments to a fixed rate are designated as cash flow hedges. Interest expense on the debt is adjusted to include the payments made or received under the swap agreements.
Goodwill and other intangibles: Goodwill is not amortized. All other intangibles arising from acquisitions and research alliances have finite lives and are amortized over their estimated useful lives, ranging from 5 to 20 years, using the straight-line method. The weighted-average amortization period for developed product technology is approximately 12 years. Amortization expense for 2008, 2007, and 2006 was $193.4 million, $172.8 million, and $7.6 million before tax, respectively. The estimated amortization expense for each of the five succeeding years approximates $280 million before tax, per year. Substantially all of the amortization expense is included in cost of sales. See Note 3 for further discussion of goodwill and other intangibles acquired in 2008 and 2007.
-53-
Goodwill and other intangible assets at December 31 were as follows:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | |
| |
| Goodwill | | $ | 1,167.5 | | | $ | 745.7 | |
| Developed product technology — gross | | | 3,035.4 | | | | 1,767.5 | |
| Less accumulated amortization | | | (346.6 | ) | | | (162.6 | ) |
| | | |
| | | |
| Developed product technology — net | | | 2,688.8 | | | | 1,604.9 | |
| Other intangibles — gross | | | 243.2 | | | | 142.8 | |
| Less accumulated amortization | | | (45.4 | ) | | | (38.0 | ) |
| | | |
| | | |
| Other intangibles — net | | | 197.8 | | | | 104.8 | |
| | | |
| | | |
| Total intangibles — net | | $ | 4,054.1 | | | $ | 2,455.4 | |
| | | |
| | | |
Goodwill and net other intangibles are reviewed to assess recoverability at least annually and when certain impairment indicators are present. No significant impairments occurred with respect to the carrying value of our goodwill or other intangible assets in 2008, 2007, or 2006.
Property and equipment: Property and equipment is stated on the basis of cost. Provisions for depreciation of buildings and equipment are computed generally by the straight-line method at rates based on their estimated useful lives (12 to 50 years for buildings and 3 to 18 years for equipment). We review the carrying value of long-lived assets for potential impairment on a periodic basis and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. Impairment is determined by comparing projected undiscounted cash flows to be generated by the asset to its carrying value. If an impairment is identified, a loss is recorded equal to the excess of the asset’s net book value over its fair value, and the cost basis is adjusted.
At December 31, property and equipment consisted of the following:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | |
| |
| Land | | $ | 219.0 | | | $ | 180.0 | |
| Buildings | | | 5,953.4 | | | | 5,543.7 | |
| Equipment | | | 8,045.2 | | | | 7,454.9 | |
| Construction in progress | | | 1,098.3 | | | | 1,662.7 | |
| | | |
| | | |
| | | | 15,315.9 | | | | 14,841.3 | |
| Less allowances for depreciation | | | (6,689.6 | ) | | | (6,266.2 | ) |
| | | |
| | | |
| | | $ | 8,626.3 | | | $ | 8,575.1 | |
| | | |
| | | |
Depreciation expense for 2008, 2007, and 2006 was $731.7 million, $682.3 million, and $627.4 million, respectively. Approximately $48.2 million, $95.3 million, and $106.7 million of interest costs were capitalized as part of property and equipment in 2008, 2007, and 2006, respectively. Total rental expense for all leases, including contingent rentals (not material), amounted to approximately $327.4 million, $294.2 million, and $293.6 million for 2008, 2007, and 2006, respectively. Assets under capital leases included in property and equipment in the consolidated balance sheets, capital lease obligations entered into, and future minimum rental commitments are not material.
Litigation and environmental liabilities: Litigation accruals and environmental liabilities and the related estimated insurance recoverables are reflected on a gross basis as liabilities and assets, respectively, on our consolidated balance sheets. With respect to the product liability claims currently asserted against us, we have accrued for our estimated exposures to the extent they are both probable and estimable based on the information available to us. We accrue for certain product liability claims incurred but not filed to the extent we can formulate a reasonable estimate of their costs. We estimate these expenses based primarily on historical claims experience and data regarding product usage. Legal defense costs expected to be incurred in connection with significant product liability loss contingencies are accrued when probable and reasonably estimable. A
-54-
portion of the costs associated with defending and disposing of these suits is covered by insurance. We record receivables for insurance-related recoveries when it is probable they will be realized. These receivables are classified as a reduction of the litigation charges on the statement of income. We estimate insurance recoverables based on existing deductibles, coverage limits, our assessment of any defenses to coverage that might be raised by the carriers, and the existing and projected future level of insolvencies among the insurance carriers. However, for substantially all of our currently marketed products, we are completely self-insured for future product liability losses.
Revenue recognition: We recognize revenue from sales of products at the time title of goods passes to the buyer and the buyer assumes the risks and rewards of ownership. For more than 90 percent of our sales, this is at the time products are shipped to the customer, typically a wholesale distributor or a major retail chain. The remaining sales are recorded at the point of delivery. Provisions for returns, discounts, and rebates are established in the same period the related sales are recorded.
We also generate income as a result of collaboration agreements. Revenue from co-promotion services is based upon net sales reported by our co-promotion partners and, if applicable, the number of sales calls we perform. Initial fees we receive from the partnering of our compounds under development are amortized through the expected product approval date. Initial fees received from out-licensing agreements that include both the sale of marketing rights to our commercialized products and a related commitment to supply the products are generally recognized as net sales over the term of the supply agreement. We immediately recognize the full amount of milestone payments due to us upon the achievement of the milestone event if the event is substantive, objectively determinable, and represents an important point in the development life cycle of the pharmaceutical product. Milestone payments earned by us are generally recorded in other — net.
Royalty revenue from licensees, which are based on third-party sales of licensed products and technology, are recorded as earned in accordance with the contract terms when third-party sales can be reasonably measured and collection of the funds is reasonably assured. This royalty revenue is included in net sales.
Acquired research and development: We recognize as incurred the cost of directly acquiring assets to be used in the research and development process that have not yet received regulatory approval for marketing and for which no alternative future use has been identified. Once the product has obtained regulatory approval, we capitalize the milestones paid and amortize them over the period benefited. Milestones paid prior to regulatory approval of the product are generally expensed when the event requiring payment of the milestone occurs.
Other — net: Other — net consisted of the following:
| | | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | |
| |
| Interest expense | | $ | 228.3 | | | $ | 228.3 | | | $ | 238.1 | |
| Interest income | | | (210.7 | ) | | | (215.3 | ) | | | (261.9 | ) |
| Joint venture income | | | — | | | | (11.0 | ) | | | (96.3 | ) |
| Other | | | 8.5 | | | | (124.0 | ) | | | (117.7 | ) |
| | | |
| | | |
| | | $ | 26.1 | | | $ | (122.0 | ) | | $ | (237.8 | ) |
| | | |
| | | |
The joint venture income represents our share of the Lilly ICOS LLC joint venture results of operations, net of income taxes. We acquired the outstanding ownership of the joint venture in January 2007 as a result of our acquisition of ICOS. See Note 3 for further discussion.
Income taxes: Deferred taxes are recognized for the future tax effects of temporary differences between financial and income tax reporting based on enacted tax laws and rates. Federal income taxes are provided on the portion of the income of foreign subsidiaries that is expected to be remitted to the United States and be taxable.
We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate resolution.
-55-
Earnings per share: We calculate basic earnings per share based on the weighted-average number of outstanding common shares and incremental shares. We calculate diluted earnings per share based on the weighted-average number of outstanding common shares plus the effect of dilutive stock options and other incremental shares. See Note 11 for further discussion.
Stock-based compensation: We recognize the fair value of stock-based compensation as expense over the requisite service period of the individual grantees, which generally equals the vesting period. Under our policy all stock-based awards are approved prior to the date of grant. The Compensation Committee of the Board of Directors approves the value of the award and date of grant. Stock-based compensation that is awarded as part of our annual equity grant is made on a specific grant date scheduled in advance.
Reclassifications: Certain reclassifications have been made to the December 31, 2007 and 2006 consolidated financial statements and accompanying notes to conform with the December 31, 2008 presentation.
| |
| Note 2: | Implementation of New Financial Accounting Pronouncements |
In March 2008, the Financial Accounting Standards Board (FASB) issued Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities, an amendment of FASB Statement No. 133 (SFAS 161). SFAS 161 applies to all derivative instruments and related hedged items accounted for under FASB Statement No. 133, Accounting for Derivative Instruments and Hedging Activities. This Statement requires entities to provide enhanced disclosures about how and why an entity uses derivative instruments, how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations, and how derivative instruments and related hedged items affect an entity’s financial position, results of operations, and cash flows. This Statement is effective for us January 1, 2009.
We adopted the provisions of Emerging Issues Task Force (EITF) IssueNo. 07-3(EITF 07-3), Accounting for Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and Development Activities, on January 1, 2008. Pursuant toEITF 07-3, nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities should be deferred and capitalized. Such amounts should be recognized as an expense when the related goods are delivered or services are performed, or when the goods or services are no longer expected to be received. This Issue is to be applied prospectively for contracts entered into on or after the effective date.
We adopted the provisions of FASB Statement No. 157 (SFAS 157), Fair Value Measurements, on January 1, 2008. SFAS 157 defines fair value, establishes a framework for measuring fair value in GAAP, and expands disclosures about fair value measurements. The implementation of this Statement was not material to our consolidated financial position or results of operations.
In December 2007, the FASB revised and issued Statement No. 141, Business Combinations (SFAS 141(R)). SFAS 141(R) changes how the acquisition method is applied in accordance with SFAS 141. The primary revisions to this Statement require an acquirer in a business combination to measure assets acquired, liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date, at their fair values as of that date, with limited exceptions specified in the Statement. This Statement also requires the acquirer in a business combination achieved in stages to recognize the identifiable assets and liabilities, as well as the noncontrolling interest in the acquiree, at the full amounts of their fair values (or other amounts determined in accordance with the Statement). Assets acquired and liabilities assumed arising from contractual contingencies as of the acquisition date are to be measured at their acquisition-date fair values, and assets or liabilities arising from all other contingencies as of the acquisition date are to be measured at their acquisition-date fair value, only if it is more likely than not that they meet the definition of an asset or a liability in FASB Concepts Statement No. 6, Elements of Financial Statements. This Statement significantly amends other Statements and authoritative guidance, including FASB Interpretation No. 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method, and now requires the capitalization of research and development assets acquired in a business combination at their acquisition-date fair values, separately from goodwill. SFAS No. 109, Accounting for Income Taxes, was also amended by this Statement to require the acquirer to recognize changes in the amount of its deferred tax benefits that are recognizable because of a business combination either in income from continuing operations in the period of the combination or directly
-56-
in contributed capital, depending on the circumstances. This Statement is effective for us for business combinations for which the acquisition date is on or after January 1, 2009.
In December 2007, in conjunction with SFAS 141(R), the FASB issued Statement No. 160, Accounting for Noncontrolling Interests. This Statement amends Accounting Research Bulletin No. 51, Consolidated Financial Statements (ARB 51), by requiring companies to report a noncontrolling interest in a subsidiary as equity in its consolidated financial statements. Disclosure of the amounts of consolidated net income attributable to the parent and the noncontrolling interest will be required. This Statement also clarifies that transactions that result in a change in a parent’s ownership interest in a subsidiary that do not result in deconsolidation will be treated as equity transactions, while a gain or loss will be recognized by the parent when a subsidiary is deconsolidated. This Statement is effective for us January 1, 2009, and we do not anticipate the implementation will be material to our consolidated financial position or results of operations.
In December 2007, the FASB ratified the consensus reached by the EITF on IssueNo. 07-1(EITF 07-1), Accounting for Collaborative Arrangements.EITF 07-1 defines collaborative arrangements and establishes reporting requirements for transactions between participants in a collaborative arrangement and between participants in the arrangement and third parties. This Issue is effective for us beginning January 1, 2009 and will be applied retrospectively to all prior periods presented for all collaborative arrangements existing as of the effective date. The implementation of this Issue will not be material to our consolidated financial position or results of operations.
We adopted the provisions of FASB Interpretation (FIN) No. 48, Accounting for Uncertainty in Income Taxes, on January 1, 2007. FIN 48 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. See Note 12 for further discussion of the impact of adopting this Interpretation.
During 2008 and 2007, we acquired several businesses. These acquisitions were accounted for as business combinations under the purchase method of accounting. Under the purchase method of accounting, the assets acquired and liabilities assumed were recorded at their respective fair values as of the acquisition date in our consolidated financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. The excess of the purchase price over the fair value of the acquired net assets, where applicable, has been recorded as goodwill. The results of operations of these acquisitions are included in our consolidated financial statements from the date of acquisition.
Most of these acquisitions included in-process research and development (IPR&D), which represented compounds, new indications, or line extensions under development that had not yet achieved regulatory approval for marketing. There are several methods that can be used to determine the estimated fair value of the IPR&D acquired in a business combination. We utilized the “income method,” which applies a probability weighting to the estimated future net cash flows that are derived from projected sales revenues and estimated costs. These projections are based on factors such as relevant market size, patent protection, historical pricing of similar products, and expected industry trends. The estimated future net cash flows are then discounted to the present value using an appropriate discount rate. This analysis is performed for each project independently. In accordance with FIN 4, Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method, these acquired IPR&D intangible assets totaling $4.71 billion and $340.5 million in 2008 and 2007, respectively, were expensed immediately subsequent to the acquisition because the products had no alternative future use. The ongoing activities with respect to each of these products in development are not material to our research and development expenses.
In addition to the acquisitions of businesses, we also acquired several products in development. The acquired IPR&D related to these products of $122.0 million and $405.1 million in 2008 and 2007, respectively, was also written off by a charge to income immediately upon acquisition because the products had no alternative future use.
-57-
ImClone Acquisition
On November 24, 2008, we acquired all of the outstanding shares of ImClone Systems Inc. (ImClone), a biopharmaceutical company focused on advancing oncology care, for a total purchase price of approximately $6.5 billion, which was financed through borrowings. This strategic combination will offer both targeted therapies and oncolytic agents along with a pipeline spanning all phases of clinical development. The combination also expands our biotechnology capabilities.
The acquisition has been accounted for as a business combination under the purchase method of accounting, resulting in goodwill of $419.5 million. No portion of this goodwill is expected to be deductible for tax purposes.
Allocation of Purchase Price
We are currently determining the fair values of a significant portion of these net assets. The purchase price has been preliminarily allocated based on an estimate of the fair value of assets acquired and liabilities assumed as of the date of acquisition. The final determination of these fair values will be completed as soon as possible but no later than one year from the acquisition date. Although the final determination may result in asset and liability fair values that are different than the preliminary estimates of these amounts included herein, it is not expected that those differences will be material to our financial results.
| | | | | |
| Estimated Fair Value at November 24, 2008 | | | |
| | |
| |
| Cash and short-term investments | | $ | 982.9 | |
| Inventories | | | 136.2 | |
Developed product technology (Erbitux)1 | | | 1,057.9 | |
| Goodwill | | | 419.5 | |
| Property and equipment | | | 339.8 | |
| Debt assumed | | | (600.0 | ) |
| Deferred taxes | | | (315.0 | ) |
| Deferred income | | | (127.7 | ) |
| Other assets and liabilities — net | | | (72.1 | ) |
| Acquired in-process research and development | | | 4,685.4 | |
| | | | | |
| Total purchase price | | $ | 6,506.9 | |
| | | | | |
| | |
| 1 | | This intangible asset will be amortized on a straight-line basis through 2023 in the U.S. and 2018 in the rest of the world. |
All of the estimated fair value of the acquired IPR&D is attributable to oncology-related products in development, including $1.33 billion to line extensions for Erbitux. A significant portion (81 percent) of the remaining value of acquired IPR&D is attributable to two compounds in Phase III clinical testing and one compound in Phase II clinical testing, all targeted to treat various forms of cancers. The discount rate we used in valuing the acquired IPR&D projects was 13.5 percent, and the charge for acquired IPR&D of $4.69 billion recorded in the fourth quarter of 2008, was not deductible for tax purposes.
-58-
Pro Forma Financial Information
The following unaudited pro forma financial information presents the combined results of our operations with ImClone as if the acquisition and the financing for the acquisition had occurred as of the beginning of each of the years presented. We have adjusted the historical consolidated financial information to give effect to pro forma events that are directly attributable to the acquisition. The unaudited pro forma financial information is not necessarily indicative of what our consolidated results of operations actually would have been had we completed the acquisition at the beginning of each year. In addition, the unaudited pro forma financial information does not attempt to project the future results of operations of our combined company.
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | | | |
| |
| Net sales | | $ | 20,801.8 | | | $ | 19,051.4 | |
Net income1 | | | 2,356.2 | | | | 2,704.1 | |
| Earnings per share: | | | | | | | | |
| Basic and diluted | | | 2.15 | | | | 2.48 | |
| | |
| 1 | | The unaudited pro forma financial information above excludes the non-recurring charge incurred for acquired IPR&D of $4.69 billion and other merger-related costs. |
The unaudited pro forma financial information above reflects the following:
| |
| • | a reduction of the amortization of ImClone’s deferred income of $86.2 million (2008) and $98.4 million (2007); |
| |
| • | the increase of amortization expense of $78.8 million in 2008 and 2007 related to the estimated fair value of identifiable intangible assets from the purchase price allocation which are being amortized over their estimated useful lives through 2023 in the U.S. and through 2018 in the rest of the world. The change in depreciation expense related to the change in the estimated fair value of property and equipment from the book value at the time of the acquisition was not material; |
| |
| • | the adjustment to increase interest expense related to the debt incurred to finance the acquisition and the adjustment to decrease interest income related to the lost interest income on the cash used to purchase ImClone by a total of $301.0 million in 2008 and 2007; |
| |
| • | the reduction of ImClone’s income tax expense to provide for income taxes at the statutory tax rate and the adjustment to income taxes for pro forma adjustments at the statutory tax rate, totaling $139.3 million (2008) and $189.5 million (2007). This excludes the acquired IPR&D charge of $4.69 billion, which was not tax deductible; |
| |
| • | certain reclassifications to conform to accounting policies and classifications that are consistent with our practices (e.g., ImClone’s license fees and milestones were classified as other — net, rather than net sales). |
Posilac
On October 1, 2008, we acquired the worldwide rights to the dairy cow supplement Posilac, as well as the product’s supporting operations, from Monsanto Company (Monsanto). The acquisition of Posilac provides us with a product that complements those of our animal health business. Under the terms of the agreement, we acquired the rights to the Posilac brand, as well as the product’s U.S. sales force and manufacturing facility, for an aggregate purchase price of $403.9 million, which includes a $300.0 million upfront payment, transaction costs, and an accrual for contingent consideration to Monsanto based on estimated future Posilac sales for which payment is considered likely beyond a reasonable doubt.
This acquisition has been accounted for as a business combination under the purchase method of accounting. We allocated $204.3 million to identifiable intangible assets related to Posilac, $167.6 million to inventories, and $99.5 million of the purchase price to property and equipment. We also assumed $67.5 million of liabilities. Substantially all of the identifiable intangible assets are being amortized over their estimated remaining useful lives of 20 years. The amount allocated to each of the intangible assets acquired is deductible for tax purposes.
-59-
SGX Pharmaceuticals, Inc.
On August 20, 2008, we acquired all of the outstanding common stock of SGX Pharmaceuticals, Inc. (SGX), a collaboration partner since 2003. The acquisition allows us to integrate SGX’s structure-guided drug discovery platform into our drug discovery efforts. It also gives us access to FASTtm, SGX’s fragment-based, protein structure guided drug discovery technology, and to a portfolio of preclinical oncology compounds focused on a number of kinase targets. Under the terms of the agreement, the outstanding shares of SGX common stock were redeemed for an aggregate purchase price, including transaction costs, of $66.8 million.
The acquisition has been accounted for as a business combination under the purchase method of accounting. We allocated $29.6 million of the purchase price to deferred tax assets and $28.0 million to acquired IPR&D. The acquired IPR&D charge of $28.0 million was recorded in the third quarter of 2008 and was not deductible for tax purposes.
ICOS Corporation
On January 29, 2007, we acquired all of the outstanding common stock of ICOS Corporation (ICOS), our partner in the Lilly ICOS LLC joint venture for the manufacture and sale of Cialis for the treatment of erectile dysfunction. The acquisition brought the full value of Cialis to us and enabled us to realize operational efficiencies in the further development, marketing, and selling of this product. The aggregate cash purchase price of approximately $2.3 billion was financed through borrowings.
The acquisition has been accounted for as a business combination under the purchase method of accounting, resulting in goodwill of $646.7 million. No portion of this goodwill was deductible for tax purposes.
We determined the following estimated fair values for the assets acquired and liabilities assumed as of the date of acquisition.
| | | | | |
| Estimated Fair Value at January 29, 2007 | | | |
| | |
| |
| Cash and short-term investments | | $ | 197.7 | |
Developed product technology (Cialis)1 | | | 1,659.9 | |
| Tax benefit of net operating losses | | | 404.1 | |
| Goodwill | | | 646.7 | |
| Long-term debt assumed | | | (275.6 | ) |
| Deferred taxes | | | (583.5 | ) |
| Other assets and liabilities — net | | | (32.1 | ) |
| Acquired in-process research and development | | | 303.5 | |
| | | | | |
| Total purchase price | | $ | 2,320.7 | |
| | | | | |
| | |
| 1 | | This intangible asset will be amortized over the remaining expected patent lives of Cialis in each country; patent expiry dates range from 2015 to 2017. |
New indications for and formulations of the Cialis compound in clinical testing at the time of the acquisition represented approximately 48 percent of the estimated fair value of the acquired IPR&D. The remaining value of acquired IPR&D represented several other products in development, with no one asset comprising a significant portion of this value. The discount rate we used in valuing the acquired IPR&D projects was 20 percent, and the charge for acquired IPR&D of $303.5 million recorded in the first quarter of 2007 was not deductible for tax purposes.
Other Acquisitions
During the second quarter of 2007, we acquired all of the outstanding stock of both Hypnion, Inc. (Hypnion), a privately held neuroscience drug discovery company focused on sleep disorders, and Ivy Animal Health, Inc. (Ivy), a privately held applied research and pharmaceutical product development company focused on the animal health industry, for $445.0 million in cash.
-60-
The acquisition of Hypnion provided us with a broader and more substantive presence in the area of sleep disorder research and ownership of HY10275, a novel Phase II compound with a dual mechanism of action aimed at promoting better sleep onset and sleep maintenance. This was Hypnion’s only significant asset. For this acquisition, we recorded an acquired IPR&D charge of $291.1 million, which was not deductible for tax purposes. Because Hypnion was a development-stage company, the transaction was accounted for as an acquisition of assets rather than as a business combination and, therefore, goodwill was not recorded.
The acquisition of Ivy provides us with products that complement those of our animal health business. This acquisition has been accounted for as a business combination under the purchase method of accounting. We allocated $88.7 million of the purchase price to other identifiable intangible assets, primarily related to marketed products, $37.0 million to acquired IPR&D, and $25.0 million to goodwill. The other identifiable intangible assets are being amortized over their estimated remaining useful lives of 10 to 20 years. The $37.0 million allocated to acquired IPR&D was charged to expense in the second quarter of 2007. Goodwill resulting from this acquisition was fully allocated to the animal health business segment. The amount allocated to each of the intangible assets acquired, including goodwill of $25.0 million and the acquired IPR&D of $37.0 million, was deductible for tax purposes.
Product Acquisitions
In June 2008, we entered into a licensing and development agreement with TransPharma Medical Ltd. (TransPharma) to acquire rights to its product and related drug delivery system for the treatment of osteoporosis. The product, which is administered transdermally using TransPharma’s proprietary technology, was in Phase II clinical testing, and had no alternative future use. Under the arrangement, we also gained non-exclusive access to TransPharma’s ViaDerm drug delivery system for the product. As with many development-phase products, launch of the product, if approved, was not expected in the near term. The charge of $35.0 million for acquired IPR&D related to this arrangement was included as expense in the second quarter of 2008 and is deductible for tax purposes.
In January 2008, our agreement with BioMS Medical Corp. to acquire the rights to its compound for the treatment of multiple sclerosis became effective. At the inception of this agreement, this compound was in the development stage (Phase III clinical trials) and had no alternative future use. As with many development-phase compounds, launch of the product, if approved, was not expected in the near term. The charge of $87.0 million for acquired IPR&D related to this arrangement was included as expense in the first quarter of 2008 and is deductible for tax purposes.
In October 2007, we entered into an agreement with Glenmark Pharmaceuticals Limited India to acquire the rights to a portfolio of transient receptor potential vanilloid sub-family 1 (TRPV 1) antagonist molecules, including a clinical-phase compound. The compound was in early clinical phase development as a potential next-generation treatment for various pain conditions, including osteoarthritic pain, and had no alternative future use. As with many development-phase compounds, launch of the product, if approved, was not expected in the near term. The charge of $45.0 million for acquired IPR&D was deductible for tax purposes and was included as expense in the fourth quarter of 2007. Development of this compound has been suspended.
In October 2007, we entered into a global strategic alliance with MacroGenics, Inc. (MacroGenics) to develop and commercialize teplizumab, a humanized anti-CD3 monoclonal antibody, as well as other potential next-generation anti-CD3 molecules for use in the treatment of autoimmune diseases. As part of the arrangement, we acquired the exclusive rights to the molecule, which was in the development stage (Phase II/III clinical trial for individuals with recent-onset type 1 diabetes) and had no alternative future use. As with many development-phase compounds, launch of the product, if approved, was not expected in the near term. The charge of $44.0 million for acquired IPR&D was deductible for tax purposes and was included as expense in the fourth quarter of 2007.
In January 2007, we entered into an agreement with OSI Pharmaceuticals, Inc. to acquire the rights to its compound for the treatment of type 2 diabetes. At the inception of this agreement, this compound was in the development stage (Phase I clinical trials) and had no alternative future use. As with many development-phase compounds, launch of the product, if approved, was not expected in the near term. The charge of $25.0 million
-61-
for acquired IPR&D related to this arrangement was included as expense in the first quarter of 2007 and was deductible for tax purposes.
In connection with these arrangements, our partners are generally entitled to future milestones and royalties based on sales should these products be approved for commercialization.
We often enter into collaborative arrangements to develop and commercialize drug candidates. Collaborative activities might include research and development, marketing and selling (including promotional activities and physician detailing), manufacturing, and distribution. These collaborations often require milestone and royalty or profit share payments, contingent upon the occurrence of certain future events linked to the success of the asset in development, as well as expense reimbursements or payments to the third party. Each collaboration is unique in nature and our more significant arrangements are discussed below.
Erbitux
Prior to our acquisition, ImClone entered into several collaborations with respect to Erbitux, a product approved to fight cancer, while still in its development phase. The most significant collaborations operate in these geographic territories: the U.S., Japan, and Canada (Bristol-Myers Squibb); and worldwide except the U.S. and Canada (Merck KGaA). The agreements are expected to expire in 2018, upon which all of the rights with respect to Erbitux in the U.S. and Canada return to us.
Bristol-Myers Squibb Company
Pursuant to a commercial agreement with Bristol-Myers Squibb Company and E.R. Squibb (collectively, BMS), relating to Erbitux, ImClone is co-developing and co-promoting Erbitux in North America with BMS, and is co-developing and co-promoting Erbitux in Japan with BMS. The companies had jointly agreed to expand the investment in the ongoing clinical development plan for Erbitux to further explore its use in additional tumor types. Under this arrangement, Erbitux research and development and other costs, up to threshold amounts, are the sole responsibility of BMS, with costs in excess of the thresholds shared by both companies according to a predetermined ratio.
Responsibilities associated with clinical and other ongoing studies are apportioned between the parties as determined pursuant to the agreement. Collaborative reimbursements received by ImClone for supply of product for research and development, for a portion of royalty expenses, and for a portion of marketing, selling, and administrative expenses, are recorded as a reduction to the respective expense line items on the consolidated statement of operations. Royalty expense paid to third parties is included in costs of sales. We receive a distribution fee in the form of a royalty from BMS, based on a percentage of net sales in the U.S. and Canada, which is recorded in net sales.
We are responsible for the manufacture and supply of all requirements of Erbitux in bulk-form active pharmaceutical ingredient (API) for clinical and commercial use in the territory, and BMS will purchase all of its requirements of API for commercial use from us, subject to certain stipulations per the agreement. Sales of Erbitux to BMS for commercial use are reported in net sales.
Merck KGaA
A development and license agreement between ImClone and Merck KGaA (Merck) with respect to Erbitux granted Merck exclusive rights to market Erbitux outside of North America and co-exclusive rights with BMS in Japan. Merck also has rights to manufacture Erbitux for supply in its territory. We manufacture and provide a portion of Merck’s requirements for API; we also receive a royalty on the sales of Erbitux outside of the U.S. and Canada, both of which are included in net sales as earned. Collaborative reimbursements received for supply of product for research and development, reimbursement of a portion of royalty expense, and marketing, selling, and administrative expenses are recorded as a reduction to the respective expense line items on the consolidated statement of operations. Royalty expense paid to third parties is included in cost of sales.
-62-
Exenatide
We are in a collaborative arrangement with Amylin Pharmaceuticals (Amylin) for the joint development, marketing, and selling of Byetta and other forms of exenatide such as exenatide once weekly. Byetta (exenatide injection) is presently approved as an adjunctive therapy to improve glycemic control in patients with type 2 diabetes who have not achieved adequate glycemic control using metformin, a sulfonylureaand/or a thiazolidinediene (U.S. only), three common oral therapies for type 2 diabetes. Lilly and Amylin are co-promoting exenatide in the U.S. Amylin is responsible for manufacturing and primarily utilizes third-party contract manufacturing organizations to supply Byetta. However, Lilly is manufacturing Byetta pen delivery devices for Amylin. Lilly is responsible for development and commercialization costs outside the U.S.
Under the terms of our collaboration with Amylin, we report as revenue our 50 percent share of gross margin on sales in the U.S., 100 percent of sales outside the U.S., and our sales of Byetta pen delivery devices to Amylin. We recorded revenues of $396.1 million, $330.7 million, and $219.0 million in 2008, 2007, and 2006, respectively, for Byetta. We pay Amylin a percentage of the gross margin of exenatide sales outside of the U.S., and these costs are recorded in cost of sales. Under the50/50 profit-sharing arrangement for the U.S., in addition to recording as revenue our 50 percent share of exenatide’s gross margin, we also report 50 percent of U.S. research and development costs, and marketing and selling costs in the research and development and marketing, selling, and administrative line items, respectively, on the consolidated statements of income.
Exenatide once weekly is presently in Phase III clinical trials and has not received regulatory approval. Amylin is constructing and will operate a manufacturing facility for exenatide once weekly, and we have entered into a supply agreement in which Amylin will supply exenatide once weekly product to us for sales outside the U.S. The estimated total cost of the facility is approximately $550 million. In 2008, we paid $125.0 million to Amylin, which we will amortize to cost of sales over the estimated life of the supply agreement beginning with product launch. We would be required to reimburse Amylin for a portion of any future impairment of this facility, recognized in accordance with GAAP. A portion of the $125.0 million payment we made to Amylin would be creditable against any amount we would owe as a result of impairment. We have also agreed to loan up to $165.0 million to Amylin at an indexed rate beginning December 1, 2009, and any borrowings have to be repaid by June 30, 2014.
Cymbalta
Boehringer Ingelheim
We are in a collaborative arrangement with Boehringer Ingelheim (BI) to market and promote Cymbalta, a product for the treatment of major depressive disorder, diabetic peripheral neuropathic pain, generalized anxiety disorder, and fibromyalgia, outside the U.S. Pursuant to the terms of the agreement, we generally share equally in development, marketing, and selling expenses, and pay BI a commission on sales in the co-promotional territories. We manufacture the product for all territories.
Collaborative reimbursements or payments for the cost sharing of marketing, selling, and administrative expenses are recorded in the respective expense line items in the consolidated statement of operations. The commission paid to BI is recognized in marketing, selling, and administrative expenses.
Quintiles
We are in a collaborative arrangement with Quintiles Transnational Corp. (Quintiles) to market and promote Cymbalta in the U.S. Pursuant to the terms of the agreement, Quintiles shares in the costs to co-promote Cymbalta with us. In exchange, Quintiles receives a payment based upon net sales. According to the current agreement, Quintiles’ obligation to promote Cymbalta expires in 2009, and we will pay a lower rate on net sales for three years post their promotion efforts. The royalties paid to Quintiles are recorded in marketing, selling, and administrative expenses.
-63-
Prasugrel
We are in a collaborative arrangement with Daiichi Sankyo Company, Limited (D-S) to develop, market, and promote prasugrel, an antiplatelet agent for the treatment of patients with acute coronary syndromes (ACS) who are being managed with an artery-opening procedure known as percutaneous coronary intervention (PCI). Prasugrel was approved for marketing by the European Commission under the tradename Efient in February 2009. We have submitted a new drug application to the FDA and are currently awaiting its decision. Within this arrangement, we have agreed to co-promote under the same trademark in certain territories (including the U.S. and five major European markets), while we have exclusive marketing rights in certain other territories. D-S has exclusive marketing rights in Japan. Pursuant to the terms of the agreement, we paid D-S an upfront license fee and agreed to pay future success milestones. Both parties share in the costs of the development and marketing in the co-promotion territories and share in the profits according to the terms specified in the agreement. D-S is responsible for supplying bulk product, but we will produce the finished product for our exclusive and co-promotion territories. Profits in the U.S. and other co-promotion territories will be shared according to the agreement. In our exclusive territories, we will pay D-S a royalty specific to those territories. Profit share payments made to D-S will be recorded as marketing, selling, and administrative expenses. All royalties paid to D-S will be recorded in cost of sales.
TPG-Axon Capital
In 2008, we entered into an agreement with an affiliate of TPG-Axon Capital (TPG) for the Phase III development of our gamma-secretase inhibitor and our A-beta antibody, our two lead molecules for the treatment of mild to moderate Alzheimer’s disease. Pursuant to the terms of the agreement, both we and TPG will provide funding for the Alzheimer’s clinical trials. Funding from TPG will not exceed $325 million and could extend into 2014. In exchange for their funding, TPG may receive success-based milestones totaling $330 million and mid- to high-single digit royalties that are contingent upon the successful development of the Alzheimer’s treatments. The royalties will be paid for approximately eight years after launch of a product. Reimbursements received from TPG for their portion of research and development costs incurred related to the Alzheimer’s treatments are recorded as a reduction to the research and development expense line item on the consolidated statement of operations. The reimbursement from TPG is not expected to be material in any period.
| |
| Note 5: | Asset Impairments, Restructuring, and Other Special Charges |
The components of the charges included in asset impairments, restructuring, and other special charges in our consolidated statements of income are described below.
Asset Impairments and Related Restructuring and Other Charges
We incurred asset impairment, restructuring, and other special charges of $80.0 million in the fourth quarter of 2008. These charges were the result of decisions approved by management in the fourth quarter as well as previously announced strategic decisions. The primary components of this charge include non-cash asset impairments of $35.1 million for the write down of impaired assets, all of which have no future use, and other charges of $44.9 million, primarily related to severance and environmental cleanup charges in connection with previously announced strategic decisions made in prior periods. We anticipate that substantially all of these costs will be paid during the first quarter of 2009.
As discussed further in Note 14, in the third quarter of 2008, we recorded a charge of $1.48 billion related to the Zyprexa investigations led by the U.S. Attorney for the Eastern District of Pennsylvania, as well as the resolution of a multi-state investigation regarding Zyprexa involving 32 states and the District of Columbia.
Further, in the third quarter of 2008, as a result of our previously announced agreements with Covance Inc. (Covance), Quintiles Transnational Corp. (Quintiles), and Ingenix Pharmaceutical Services, Inc., doing business as i3 Statprobe (i3), and as part of our efforts to transform into a more flexible organization, we recognized asset impairments, restructuring, and other special charges of $182.4 million. We sold our Greenfield, Indiana site to Covance, a global drug development services firm, and entered into a10-year service agreement under which Covance will provide preclinical toxicology work and perform additional
-64-
clinical trials for us as well as operate the site to meet our needs and those of other pharmaceutical industry clients. In addition, we signed agreements with Quintiles for clinical trial monitoring services and with i3 for clinical data management services. Components of the third-quarter restructuring charge include non-cash charges of $148.3 million primarily related to the loss on sale of assets sold to Covance, severance costs of $27.8 million, and exit costs of $6.3 million. Substantially all of these costs were paid in 2008.
In the second quarter of 2008, we recognized restructuring and other special charges of $88.9 million. In addition, we recognized non-cash charges of $57.1 million for the write down of impaired manufacturing assets that had no future use, which were included in cost of sales. In April 2008, we announced a voluntary exit program that was offered to employees primarily in manufacturing. Components of the second-quarter restructuring charge include total severance costs of $53.5 million related to these programs and $35.4 million related to exit costs incurred during the second quarter in connection with previously announced strategic decisions made in prior periods. Substantially all of these costs were paid by the end of July 2008.
In March 2008, we terminated development of our AIR Insulin program, which was being conducted in collaboration with Alkermes, Inc. The program had been in Phase III clinical development as a potential treatment for type 1 and type 2 diabetes. This decision was not a result of any observations during AIR Insulin trials relating to the safety of the product, but rather was a result of increasing uncertainties in the regulatory environment, and a thorough evaluation of the evolving commercial and clinical potential of the product compared to existing medical therapies. As a result of this decision, we halted our ongoing clinical studies and transitioned the AIR Insulin patients in these studies to other appropriate therapies. We implemented a patient program in the U.S., and other regions of the world where allowed, to provide clinical trial participants with appropriate financial support to fund their medications and diagnostic supplies through the end of 2008.
We recognized asset impairment, restructuring, and other special charges of $145.7 million in the first quarter of 2008. These charges were primarily related to the decision to terminate development of AIR Insulin. Components of these charges included non-cash charges of $40.9 million for the write down of impaired manufacturing assets that had no use beyond the AIR Insulin program, as well as charges of $91.7 million for estimated contractual obligations and wind-down costs associated with the termination of clinical trials and certain development activities, and costs associated with the patient program to transition participants from AIR Insulin. This amount includes an estimate of Alkermes’ wind-down costs for which we were contractually obligated. The wind-down activities and patient programs were substantially complete by the end of 2008. The remaining component of these charges, $13.1 million, is related to exit costs incurred in the first quarter of 2008 in connection with previously announced strategic decisions made in prior periods.
We incurred asset impairment, restructuring, and other special charges of $67.6 million in the fourth quarter of 2007. These charges were a result of decisions approved by management in the fourth quarter as well as previously announced strategic decisions. Components of this charge include non-cash charges of $42.5 million for the write down of impaired assets, all of which have no future use, and other charges of $25.1 million, primarily related to additional severance and environmental cleanup charges related to previously announced strategic decisions. The impairment charges were necessary to adjust the carrying value of the assets to fair value. These restructuring activities were substantially complete at December 31, 2007.
In connection with previously announced strategic decisions, we recorded asset impairment, restructuring, and other special charges of $123.0 million in the first quarter of 2007. These charges primarily related to a voluntary severance program at one of our U.S. plants and other costs related to this action as well as management actions taken in the fourth quarter of 2006 as described below. The component of these charges related to the non-cash asset impairment was $67.6 million, and were necessary to adjust the carrying value of the assets to fair value. These restructuring activities were substantially complete at December 31, 2007.
In the fourth quarter of 2006, management approved plans to close two research and development facilities and one production facility outside the U.S. Management also made the decision to stop construction of a planned insulin manufacturing plant in the U.S. in an effort to increase productivity in research and development operations and to reduce excess manufacturing capacity. These decisions, as well as other strategic changes, resulted in non-cash charges of $308.8 million for the write down of certain impaired assets, substantially all of which have no future use, and other charges of $141.5 million, primarily related to
-65-
severance and contract termination payments. The impairment charges were necessary to adjust the carrying value of the assets to fair value. These restructuring activities were substantially complete at December 31, 2007.
Product Liability and Other Special Charges
As a result of our product liability exposures, the substantial majority of which were related to Zyprexa, we recorded net pretax charges of $111.9 million and $494.9 million in 2007 and 2006, respectively. These charges, which are net of anticipated insurance recoveries, include the costs of product liability settlements and related defense costs, reserves for product liability exposures and defense costs regarding known product liability claims, and expected future claims to the extent we could formulate a reasonable estimate of the probable number and cost of the claims. See Note 14 for further discussion.
| |
| Note 6: | Financial Instruments and Investments |
Financial instruments that potentially subject us to credit risk consist principally of trade receivables and interest-bearing investments. Wholesale distributors of life-sciences products and managed care organizations account for a substantial portion of trade receivables; collateral is generally not required. The risk associated with this concentration is mitigated by our ongoing credit review procedures and insurance. We place substantially all of our interest-bearing investments with major financial institutions, in U.S. government securities, or with top-rated corporate issuers. At December 31, 2008, our investments in debt securities were comprised of 41 percent corporate securities, 34 percent asset-backed securities, and 25 percent U.S. government securities. In accordance with documented corporate policies, we limit the amount of credit exposure to any one financial institution or corporate issuer. We are exposed to credit-related losses in the event of nonperformance by counterparties to financial instruments but do not expect any counterparties to fail to meet their obligations given their high credit ratings.
Fair Value of Financial Instruments
The following table summarizes certain fair value information at December 31 for assets and liabilities measured at fair value on a recurring basis, as well as the carrying amount of certain other investments:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2008 | | | 2007 | |
| | | | | | Fair Value Measurements Using | | | | | | | | | | |
| | | | | | Quoted
| | | | | | | | | | | | | | | | |
| | | | | | Prices in
| | | | | | | | | | | | | | | | |
| | | | | | Active
| | | Significant
| | | | | | | | | | | | | |
| | | | | | Markets for
| | | Other
| | | Significant
| | | | | | | | | | |
| | | | | | Identical
| | | Observable
| | | Unobservable
| | | | | | | | | | |
| | | Carrying
| | | Assets
| | | Inputs
| | | Inputs
| | | Fair
| | | Carrying
| | | Fair
| |
| Description | | Amount | | | (Level 1) | | | (Level 2) | | | (Level 3) | | | Value | | | Amount | | | Value | |
| |
| Short-term investments | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Debt securities | | $ | 429.4 | | | $ | 212.3 | | | $ | 217.1 | | | $ | — | | | $ | 429.4 | | | $ | 1,610.7 | | | $ | 1,610.7 | |
| Long-term investments | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Debt securities | | $ | 1,194.9 | | | $ | 179.2 | | | $ | 1,004.6 | | | $ | 11.1 | | | $ | 1,194.9 | | | $ | 408.3 | | | $ | 408.3 | |
| Marketable equity | | | 221.9 | | | | 221.9 | | | | — | | | | — | | | | 221.9 | | | | 70.0 | | | | 70.0 | |
| Equity method and other investments | | | 127.8 | | | | | | | | | | | | | | | | NA | | | | 98.8 | | | | NA | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | $ | 1,544.6 | | | | | | | | | | | | | | | | | | | $ | 577.1 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Long-term debt, including current portion | | $ | (5,036.1 | ) | | | — | | | $ | (5,180.1 | ) | | | — | | | $ | (5,180.1 | ) | | $ | (4,988.6 | ) | | $ | (5,056.9 | ) |
| Risk-management instruments — asset | | | 455.0 | | | | — | | | | 455.0 | | | | — | | | | 455.0 | | | | 23.6 | | | | 23.6 | |
NA — Not available
-66-
We determine fair values based on a market approach using quoted market values, significant other observable inputs for identical or comparable assets or liabilities, or discounted cash flow analyses, principally for long-term debt. The fair value of equity method and other investments is not readily available. Approximately $1.1 billion of our investments in debt securities mature within five years.
A summary of the fair value of available-for-sale securities in an unrealized gain or loss position and the amount of unrealized gains and losses (pretax) in other comprehensive income at December 31 follows:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| |
| Unrealized gross gains | | $ | 69.9 | | | $ | 43.5 | |
| Unrealized gross losses | | | 239.0 | | | | 22.0 | |
| Fair value of securities in an unrealized gain position | | | 767.5 | | | | 921.7 | |
| Fair value of securities in an unrealized loss position | | | 1,046.1 | | | | 964.6 | |
The securities in an unrealized loss position are comprised of fixed-rate debt securities of varying maturities. The value of fixed income securities is sensitive to changes to the yield curve and other market conditions which led to the decline in value during 2008. Approximately 90 percent of the securities in a loss position are investment-grade debt securities. The majority of these securities first moved into an unrealized loss position during 2008. At this time, there is no indication of default on interest or principal payments for asset-backed securities. We have the intent and ability to hold the securities in a loss position until the market values recover or all of the underlying cash flows have been received and we have concluded that no other-than-temporary loss exists at December 31, 2008. The fair values of all of our auction rate securities and collateralized debt obligations held at December 31, 2008 were determined using Level 3 inputs. We do not hold securities issued by structured investment vehicles at December 31, 2008.
The net adjustment to unrealized gains and losses (net of tax) on available-for-sale securities increased (decreased) other comprehensive income by $(125.8) million, $(5.4) million, and $0.3 million in 2008, 2007, and 2006, respectively. Activity related to our available-for-sale investment portfolio was as follows:
| | | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | | |
| |
| Proceeds from sales | | $ | 1,876.4 | | | $ | 1,212.1 | | | $ | 2,848.4 | |
| Realized gross gains on sales | | | 45.7 | | | | 21.4 | | | | 63.5 | |
| Realized gross losses on sales | | | 8.7 | | | | 6.1 | | | | 9.0 | |
During the years ended December 31, 2008, 2007, and 2006, net losses related to ineffectiveness and net losses related to the portion of our risk-management hedging instruments, fair value and cash flow hedges, excluded from the assessment of effectiveness were not material.
We expect to reclassify an estimated $10.2 million of pretax net losses on cash flow hedges of the variability in expected future interest payments on floating rate debt from accumulated other comprehensive loss to earnings during 2009.
Available-for-sale investment securities are classified as long-term investments when they are likely to be held for more than one year because of our intent to hold securities in an unrealized loss position until the market values recover or all of the underlying cash flows have been received.
-67-
Long-term debt at December 31 consisted of the following:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | | | |
| |
| 4.50 to 7.13 percent notes (due 2012 — 2037) | | $ | 3,987.4 | | | $ | 3,987.4 | |
| Floating rate bonds (due 2037) | | | 400.0 | | | | 400.0 | |
| 2.90 percent notes (due 2008) | | | — | | | | 300.0 | |
| Other, including capitalized leases | | | 116.8 | | | | 222.0 | |
| SFAS 133 fair value adjustment | | | 531.9 | | | | 79.2 | |
| | | | | | | | | |
| | | | 5,036.1 | | | | 4,988.6 | |
| Less current portion | | | (420.4 | ) | | | (395.1 | ) |
| | | | | | | | | |
| | | $ | 4,615.7 | | | $ | 4,593.5 | |
| | | | | | | | | |
In March 2007, we issued $2.50 billion of fixed-rate notes ($1.00 billion at 5.20 percent due in 2017; $700.0 million at 5.50 percent due in 2027; and $800.0 million at 5.55 percent due in 2037).
The $400.0 million of floating rate bonds outstanding at December 31, 2008 are due in 2037 and have variable interest rates at LIBOR plus our six-month credit spread, adjusted semiannually (total of 4.10 percent at December 31, 2008). We pay interest monthly on this borrowing program. We expect to refinance the bonds in 2009 and have classified them as current at December 31, 2008.
The 6.55 percent Employee Stock Ownership Plan (ESOP) debentures are obligations of the ESOP but are shown on the consolidated balance sheet because we guarantee them. The principal and interest on the debt are funded by contributions from us and by dividends received on certain shares held by the ESOP. Because of the amortizing feature of the ESOP debt, bondholders will receive both interest and principal payments each quarter. The balance was $81.9 million and $90.6 million at December 31, 2008 and 2007, respectively, and is included in Other in the table above.
The aggregate amounts of maturities on long-term debt for the next five years are as follows: 2009, $420.4 million; 2010, $19.7 million; 2011, $13.1 million; 2012, $510.8 million; and 2013, $11.1 million.
At December 31, 2008 and 2007, short-term borrowings included $5.43 billion and $18.6 million, respectively, of notes payable to banks and commercial paper. Commercial paper was issued in late 2008 for the acquisition of ImClone. At December 31, 2008, we have $1.24 billion of unused committed bank credit facilities, $1.20 billion of which backs our commercial paper program. Additionally, in November 2008, we obtained a one-year short-term revolving credit facility in the amount of $4.00 billion asback-up, alternative financing. Compensating balances and commitment fees are not material, and there are no conditions that are probable of occurring under which the lines may be withdrawn.
We have converted approximately 50 percent of all fixed-rate debt to floating rates through the use of interest rate swaps. The weighted-average effective borrowing rates based on debt obligations and interest rates at December 31, 2008 and 2007, including the effects of interest rate swaps for hedged debt obligations, were 4.77 percent and 5.47 percent, respectively.
In 2008, 2007, and 2006, cash payments of interest on borrowings totaled $203.1 million, $159.2 million, and $305.7 million, respectively, net of capitalized interest.
In accordance with the requirements of SFAS 133, the portion of our fixed-rate debt obligations that is hedged is reflected in the consolidated balance sheets as an amount equal to the sum of the debt’s carrying value plus the fair value adjustment representing changes in fair value of the hedged debt attributable to movements in market interest rates subsequent to the inception of the hedge.
-68-
Stock-based compensation expense in the amount of $255.3 million, $282.0 million, and $359.3 million was recognized in 2008, 2007, and 2006, respectively, as well as related tax benefits of $88.6 million, $96.4 million, and $115.9 million, respectively. Our stock-based compensation expense consists primarily of performance awards (PAs), shareholder value awards (SVAs), and stock options. We recognize the stock-based compensation expense over the requisite service period of the individual grantees, which generally equals the vesting period. We provide newly issued shares and treasury stock to satisfy stock option exercises and for the issuance of PA and SVA shares. We classify tax benefits resulting from tax deductions in excess of the compensation cost recognized for exercised stock options as a financing cash flow in the consolidated statements of cash flows.
At December 31, 2008, additional stock options, PAs, SVAs, or restricted stock grants may be granted under the 2002 Lilly Stock Plan for not more than 88.0 million shares.
Performance Award Program
Performance awards (PAs) are granted to officers and management and are payable in shares of our common stock. The number of PA shares actually issued, if any, varies depending on the achievement of certain pre-establishedearnings-per-share targets over a one-year period. PA shares are accounted for at fair value based upon the closing stock price on the date of grant and fully vest at the end of the fiscal year of the grant. The fair values of performance awards granted in 2008, 2007, and 2006 were $51.22, $54.23, and $56.18, respectively. The number of shares ultimately issued for the performance award program is dependent upon the earnings achieved during the vesting period. Pursuant to this plan, approximately 2.5 million shares, 2.3 million shares, and 1.7 million shares were issued in 2008, 2007, and 2006, respectively. Approximately 2.8 million shares are expected to be issued in 2009.
Shareholder Value Award Program
In 2007, we implemented a shareholder value award (SVA) program, which replaced our stock option program. SVAs are granted to officers and management and are payable in shares of common stock at the end of a three-year period. The number of shares actually issued varies depending on our stock price at the end of the three-year vesting period compared to pre-established target stock prices. We measure the fair value of the SVA unit on the grant date using a Monte Carlo simulation model. The Monte Carlo simulation model utilizes multiple input variables that determine the probability of satisfying the market condition stipulated in the award grant and calculates the fair value of the award. Expected volatilities utilized in the model are based on implied volatilities from traded options on our stock, historical volatility of our stock price, and other factors. Similarly, the dividend yield is based on historical experience and our estimate of future dividend yields. The risk-free interest rate is derived from the U.S. Treasury yield curve in effect at the time of grant. The weighted-average fair values of the SVA units granted during 2008 and 2007 were $43.46 and $49.85, respectively, determined using the following assumptions:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | | | |
| |
| Expected dividend yield | | | 3.00% | | | | 2.75% | |
| Risk-free interest rate | | | 2.05% - 2.29% | | | | 4.81% - 5.16% | |
| Range of volatilities | | | 20.48% - 21.48% | | | | 22.54% - 23.90% | |
-69-
A summary of the SVA activity is presented below:
| | | | | |
| | | Units Attributable to SVAs
| |
| | | (In thousands) | |
| |
| Outstanding at January 1, 2007 | | | — | |
| Granted | | | 969 | |
| Issued | | | — | |
| Forfeited or expired | | | (47 | ) |
| | | | | |
| Outstanding at December 31, 2007 | | | 922 | |
| Granted | | | 1,282 | |
| Issued | | | — | |
| Forfeited or expired | | | (301 | ) |
| | | | | |
| Outstanding at December 31, 2008 | | | 1,903 | |
| | | | | |
The maximum number of shares that could ultimately be issued upon vesting of the SVA units outstanding at December 31, 2008, is 2.7 million. As of December 31, 2008, the total remaining unrecognized compensation cost related to nonvested SVAs amounted to $46.7 million, which will be amortized over the weighted-average remaining requisite service period of 21.6 months.
Stock Option Program
Stock options were granted in 2006 to officers and management at exercise prices equal to the fair market value of our stock price at the date of grant. No stock options were granted in 2008 or 2007. Options fully vest three years from the grant date and have a term of 10 years. We utilized a lattice-based option valuation model for estimating the fair value of the stock options. The lattice model allows the use of a range of assumptions related to volatility, risk-free interest rate, and employee exercise behavior. Expected volatilities utilized in the lattice model are based on implied volatilities from traded options on our stock, historical volatility of our stock price, and other factors. Similarly, the dividend yield is based on historical experience and our estimate of future dividend yields. The risk-free interest rate is derived from the U.S. Treasury yield curve in effect at the time of grant. The model incorporates exercise and post-vesting forfeiture assumptions based on an analysis of historical data. The expected life of the 2006 grants is derived from the output of the lattice model. The weighted-average fair values of the individual options granted during 2006 were $15.61, determined using the following assumptions:
| | | | | |
| | | 2006 | |
| |
| Dividend yield | | | 2.0% | |
| Weighted-average volatility | | | 25.0% | |
| Range of volatilities | | | 24.8%-27.0% | |
| Risk-free interest rate | | | 4.6%-4.8% | |
| Weighted-average expected life | | | 7 years | |
Stock option activity during 2008 is summarized below:
| | | | | | | | | | | | | | | | | |
| | | Shares of
| | | | | | Weighted-Average
| | | | |
| | | Common Stock
| | | Weighted-Average
| | | Remaining
| | | | |
| | | Attributable to Options
| | | Exercise
| | | Contractual Term
| | | Aggregate
| |
| | | (in thousands) | | | Price of Options | | | (in years) | | | Intrinsic Value | |
| | | | |
| |
| Outstanding at January 1, 2008 | | | 81,149 | | | $ | 69.57 | | | | | | | | | |
| Granted | | | — | | | | — | | | | | | | | | |
| Exercised | | | (145 | ) | | | 19.69 | | | | | | | | | |
| Forfeited or expired | | | (8,979 | ) | | | 72.31 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2008 | | | 72,025 | | | | 69.35 | | | | 3.6 | | | $ | 1.9 | |
| Exercisable at December 31, 2008 | | | 68,033 | | | | 70.04 | | | | 3.4 | | | | 1.9 | |
-70-
A summary of the status of nonvested options as of December 31, 2008, and changes during the year then ended, is presented below:
| | | | | | | | | |
| | | | | | Weighted-Average
| |
| | | Shares
| | | Grant Date
| |
| | | (in thousands) | | | Fair Value | |
| | | | |
| |
| Nonvested at January 1, 2008 | | | 9,049 | | | $ | 16.47 | |
| Granted | | | — | | | | — | |
| Vested | | | (5,045 | ) | | | 17.51 | |
| Forfeited | | | (12 | ) | | | 15.76 | |
| | | | | | | | | |
| Nonvested at December 31, 2008 | | | 3,992 | | | | 15.26 | |
| | | | | | | | | |
The intrinsic value of options exercised during 2008, 2007, and 2006 amounted to $4.8 million, $1.5 million, and $40.8 million, respectively. The total grant date fair value of options vested during 2008, 2007, and 2006 amounted to $84.1 million, $381.8 million, and $249.1 million, respectively. We received cash of $2.9 million, $15.2 million, and $66.2 million from exercises of stock options during 2008, 2007, and 2006, respectively, and recognized related tax benefits of $0.5 million, $0.4 million, and $11.3 million during those same years.
As of December 31, 2008, there was no significant remaining unrecognized compensation cost related to non-vested stock options.
| |
| Note 9: | Other Assets and Other Liabilities |
Our other receivables include receivables from our collaboration partners and a variety of other items. The decrease in other receivables is primarily attributable to a decrease in income tax receivable, and lower insurance recoverables.
Our sundry assets primarily include our deferred tax assets (Note 12), capitalized computer software, and the fair value of our interest rate swaps. The increase in sundry assets is primarily attributable to an increase in deferred tax assets and an increase in the fair value of our interest rate swaps.
Our other current liabilities include product litigation, tax liabilities, and a variety of other items. The increase in other current liabilities is caused primarily by an increase in product litigation liabilities, specifically, the $1.42 billion related to the EDPA settlements discussed in Note 14, and an increase in current deferred taxes.
Our other noncurrent liabilities include deferred income from our collaboration and out-licensing arrangements, the long-term portion of our estimated product return liabilities, product litigation, and a variety of other items. The increase in other noncurrent liabilities is primarily due to an increase in deferred income attributable to our 2008 acquisitions and other business development arrangements.
-71-
| |
| Note 10: | Shareholders’ Equity |
Changes in certain components of shareholders’ equity were as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Additional
| | | Retained
| | | | | | Common Stock in Treasury | |
| | | Paid-in
| | | Earnings
| | | | | | Shares
| | | | |
| | | Capital | | | ESOP | | | Deferred Costs | | | (in thousands) | | | Amount | |
| | |
| |
| Balance at January 1, 2006 | | $ | 3,323.8 | | | $ | 9,866.7 | | | $ | (106.3 | ) | | | 934 | | | $ | 104.1 | |
| Net income | | | | | | | 2,662.7 | | | | | | | | | | | | | |
| Cash dividends declared per share: $1.63 | | | | | | | (1,763.2 | ) | | | | | | | | | | | | |
| Retirement of treasury shares | | | (129.1 | ) | | | | | | | | | | | (2,297 | ) | | | (130.6 | ) |
| Purchase for treasury | | | | | | | | | | | | | | | 2,145 | | | | 122.1 | |
| Issuance of stock under employee stock plans — net | | | 6.2 | | | | | | | | | | | | 128 | | | | 5.8 | |
| Stock-based compensation | | | 359.3 | | | | | | | | | | | | | | | | | |
| ESOP transactions | | | 11.7 | | | | | | | | 5.6 | | | | | | | | | |
| | | |
| | | |
| Balance at December 31, 2006 | | | 3,571.9 | | | | 10,766.2 | | | | (100.7 | ) | | | 910 | | | | 101.4 | |
| Net income | | | | | | | 2,953.0 | | | | | | | | | | | | | |
| Cash dividends declared per share: $1.75 | | | | | | | (1,903.9 | ) | | | | | | | | | | | | |
| Retirement of treasury shares | | | (3.9 | ) | | | | | | | | | | | (76 | ) | | | (3.9 | ) |
| Issuance of stock under employee stock plans — net | | | (55.2 | ) | | | | | | | | | | | 65 | | | | 3.0 | |
| Stock-based compensation | | | 282.0 | | | | | | | | | | | | | | | | | |
| ESOP transactions | | | 10.4 | | | | | | | | 5.5 | | | | | | | | | |
| FIN 48 implementation (Note 12) | | | | | | | (8.6 | ) | | | | | | | | | | | | |
| | | |
| | | |
| Balance at December 31, 2007 | | | 3,805.2 | | | | 11,806.7 | | | | (95.2 | ) | | | 899 | | | | 100.5 | |
| Net loss | | | | | | | (2,071.9 | ) | | | | | | | | | | | | |
| Cash dividends declared per share: $1.90 | | | | | | | (2,079.9 | ) | | | | | | | | | | | | |
| Retirement of treasury shares | | | (10.9 | ) | | | | | | | | | | | (170 | ) | | | (11.1 | ) |
| Issuance of stock under employee stock plans — net | | | (84.9 | ) | | | | | | | | | | | 160 | | | | 9.8 | |
| Stock-based compensation | | | 255.3 | | | | | | | | | | | | | | | | | |
| ESOP transactions | | | 11.9 | | | | | | | | 8.9 | | | | | | | | | |
| | | |
| | | |
| Balance at December 31, 2008 | | $ | 3,976.6 | | | $ | 7,654.9 | | | $ | (86.3 | ) | | | 889 | | | $ | 99.2 | |
| | | |
| | | |
As of December 31, 2008, we have purchased $2.58 billion of our announced $3.0 billion share repurchase program. We acquired approximately 2.1 million shares in 2006 under this program. No shares were repurchased in 2008 or 2007.
We have 5 million authorized shares of preferred stock. As of December 31, 2008 and 2007, no preferred stock has been issued.
We have funded an employee benefit trust with 40 million shares of Lilly common stock to provide a source of funds to assist us in meeting our obligations under various employee benefit plans. The funding had no net impact on shareholders’ equity as we consolidate the employee benefit trust. The cost basis of the shares held in the trust was $2.64 billion and is shown as a reduction in shareholders’ equity, which offsets the resulting
-72-
increases of $2.61 billion in additional paid-in capital and $25.0 million in common stock. Any dividend transactions between us and the trust are eliminated. Stock held by the trust is not considered outstanding in the computation of earnings per share. The assets of the trust were not used to fund any of our obligations under these employee benefit plans in 2008, 2007, or 2006. In the first quarter of 2009, we contributed an additional 10.0 million shares to the trust.
We have an ESOP as a funding vehicle for the existing employee savings plan. The ESOP used the proceeds of a loan from us to purchase shares of common stock from the treasury. The ESOP issued $200.0 million of third-party debt, repayment of which was guaranteed by us (see Note 7). The proceeds were used to purchase shares of our common stock on the open market. Shares of common stock held by the ESOP will be allocated to participating employees annually through 2017 as part of our savings plan contribution. The fair value of shares allocated each period is recognized as compensation expense.
| |
| Note 11: | Earnings (Loss) Per Share |
Following is a reconciliation of the denominators used in computing earnings (loss) per share:
| | | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | |
| | | (Shares in thousands) | |
| |
| Income (loss) available to common shareholders | | | $(2,071.9 | ) | | | $2,953.0 | | | | $2,662.7 | |
| | | |
| | | |
| Basic earnings (loss) per share | | | | | | | | | | | | |
| Weighted-average number of common shares outstanding, including incremental shares | | | 1,094,499 | | | | 1,090,430 | | | | 1,086,239 | |
| | | |
| | | |
| Basic earnings (loss) per share | | | $(1.89 | ) | | | $2.71 | | | | $2.45 | |
| | | |
| | | |
| Diluted earnings (loss) per share | | | | | | | | | | | | |
| Weighted-average number of common shares outstanding | | | 1,092,041 | | | | 1,088,929 | | | | 1,085,337 | |
| Stock options and other incremental shares | | | 2,458 | | | | 1,821 | | | | 2,153 | |
| | | |
| | | |
| Weighted-average number of common shares outstanding — diluted | | | 1,094,499 | | | | 1,090,750 | | | | 1,087,490 | |
| | | |
| | | |
| Diluted earnings (loss) per share | | | $(1.89 | ) | | | $2.71 | | | | $2.45 | |
| | | |
| | | |
Following is the composition of income tax expense:
| | | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | |
| |
| Current | | | | | | | | | | | | |
| Federal | | $ | (207.6 | ) | | $ | 489.5 | | | $ | 197.7 | |
| Foreign | | | 623.6 | | | | 412.1 | | | | 390.6 | |
| State | | | (44.6 | ) | | | 27.7 | | | | (25.2 | ) |
| | | |
| | | |
| | | | 371.4 | | | | 929.3 | | | | 563.1 | |
| Deferred | | | | | | | | | | | | |
| Federal | | | 363.0 | | | | 53.0 | | | | 78.3 | |
| Foreign | | | 23.7 | | | | (27.9 | ) | | | 113.5 | |
| State | | | 6.2 | | | | (30.6 | ) | | | 0.4 | |
| | | |
| | | |
| | | | 392.9 | | | | (5.5 | ) | | | 192.2 | |
| | | |
| | | |
| Income taxes | | $ | 764.3 | | | $ | 923.8 | | | $ | 755.3 | |
| | | |
| | | |
-73-
Significant components of our deferred tax assets and liabilities as of December 31 are as follows:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | |
| Deferred tax assets | | | | | | | | |
| Compensation and benefits | | $ | 1,154.6 | | | $ | 654.8 | |
| Tax credit carryforwards and carrybacks | | | 755.0 | | | | 361.5 | |
| Intercompany profit in inventories | | | 585.0 | | | | 810.5 | |
| Tax loss carryforwards and carrybacks | | | 562.3 | | | | 712.2 | |
| Contingencies | | | 345.2 | | | | 49.3 | |
| Asset purchases | | | 251.5 | | | | 174.6 | |
| Debt | | | 211.6 | | | | 27.7 | |
| Sale of intangibles | | | 117.9 | | | | 69.1 | |
| Product return reserves | | | 100.8 | | | | 110.0 | |
| Other | | | 313.6 | | | | 302.1 | |
| | | |
| | | |
| | | | 4,397.5 | | | | 3,271.8 | |
| Valuation allowances | | | (845.4 | ) | | | (354.2 | ) |
| | | |
| | | |
| Total deferred tax assets | | | 3,552.1 | | | | 2,917.6 | |
| Deferred tax liabilities | | | | | | | | |
| Intangibles | | | (860.2 | ) | | | (532.5 | ) |
| Property and equipment | | | (620.7 | ) | | | (662.2 | ) |
| Inventories | | | (542.7 | ) | | | (432.4 | ) |
| Unremitted earnings | | | (467.3 | ) | | | (65.3 | ) |
| Prepaid employee benefits | | | — | | | | (675.9 | ) |
| Other | | | (287.8 | ) | | | (133.0 | ) |
| | | |
| | | |
| Total deferred tax liabilities | | | (2,778.7 | ) | | | (2,501.3 | ) |
| | | |
| | | |
| Deferred tax assets — net | | $ | 773.4 | | | $ | 416.3 | |
| | | |
| | | |
At December 31, 2008, we had net operating losses and other carryforwards for international and U.S. income tax purposes of $1.24 billion: $84.3 million will expire within 10 years; $1.09 billion will expire between 10 and 20 years; and $63.1 million of the carryforwards will never expire. The primary component of the remaining portion of the deferred tax asset for tax loss carryforwards and carrybacks is related to net operating losses for state income tax purposes that are fully reserved. We also have tax credit carryforwards and carrybacks of $755.0 million available to reduce future income taxes; $295.1 million will be carried back; $84.1 million of the tax credit carryforwards will expire after 5 years; and $13.0 million of the tax credit carryforwards will never expire. The remaining portion of the tax credit carryforwards is related to federal tax credits of $97.4 million and state tax credits of $265.4 million, both of which are fully reserved.
Domestic and Puerto Rican companies generated the entire consolidated loss before income taxes in 2008 and contributed approximately 7 percent and 18 percent in 2007 and 2006, respectively, to consolidated income before income taxes. We have a subsidiary operating in Puerto Rico under a tax incentive grant. The current tax incentive grant will not expire prior to 2017.
At December 31, 2008, we had an aggregate of $13.31 billion of unremitted earnings of foreign subsidiaries that have been or are intended to be permanently reinvested for continued use in foreign operations and that, if distributed, would result in additional income tax expense at approximately the U.S. statutory rate.
Cash payments (refunds) of income taxes totaled $(52.0) million, $1.01 billion, and $864.0 million in 2008, 2007, and 2006, respectively.
-74-
Following is a reconciliation of the income tax expense (benefit) applying the U.S. federal statutory rate to income (loss) before income taxes to reported income tax expense:
| | | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | |
| |
| Income tax (benefit) at the U.S. federal statutory tax rate | | $ | (457.7 | ) | | $ | 1,356.9 | | | $ | 1,196.3 | |
| Add (deduct) | | | | | | | | | | | | |
| Acquisitions and non-deductible acquired in-process research and development | | | 1,819.4 | | | | 208.1 | | | | — | |
| International operations, including Puerto Rico | | | (641.3 | ) | | | (450.7 | ) | | | (229.9 | ) |
| Government investigation charges | | | 359.3 | | | | — | | | | — | |
| IRS audit conclusion | | | (210.3 | ) | | | — | | | | — | |
| General business credits | | | (58.0 | ) | | | (60.3 | ) | | | (47.6 | ) |
| Sundry | | | (47.1 | ) | | | (130.2 | ) | | | (163.5 | ) |
| | | |
| | | |
| Income tax expense | | $ | 764.3 | | | $ | 923.8 | | | $ | 755.3 | |
| | | |
| | | |
We adopted FIN 48 on January 1, 2007. FIN 48 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. As a result of the implementation of FIN 48, we recognized an increase of $8.6 million in the liability for unrecognized tax benefits, and an offsetting reduction to the January 1, 2007 balance of retained earnings. A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is as follows:
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| | |
| |
| Beginning balance at January 1 | | $ | 1,657.4 | | | $ | 1,470.8 | |
| Additions based on tax positions related to the current year | | | 115.6 | | | | 206.4 | |
| Additions for tax positions of prior years | | | 288.8 | | | | 35.6 | |
| Reductions for tax positions of prior years | | | (234.9 | ) | | | (53.1 | ) |
| Lapses of statutes of limitation | | | (216.2 | ) | | | — | |
| Settlements | | | (598.4 | ) | | | (2.3 | ) |
| | | |
| | | |
| Balance at December 31 | | $ | 1,012.3 | | | $ | 1,657.4 | |
| | | |
| | | |
The total amount of unrecognized tax benefits that, if recognized, would affect our effective tax rate was $863.8 million at December 31, 2008.
We file income tax returns in the U.S. federal jurisdiction and various state, local, andnon-U.S. jurisdictions. We are no longer subject to U.S. federal, state and local, ornon-U.S. income tax examinations in major taxing jurisdictions for years before 2002. During the first quarter of 2008, we completed and effectively settled our Internal Revenue Service (IRS) audit of tax years2001-2004 except for one matter for which we will seek resolution through the IRS administrative appeals process. As a result of the IRS audit conclusion, gross unrecognized tax benefits were reduced by approximately $618 million, and the consolidated results of operations were benefited by $210.3 million through a reduction in income tax expense. The majority of the reduction in gross unrecognized tax benefits related to intercompany pricing positions that were agreed with the IRS in a prior audit cycle for which a prepayment of tax was made in 2005. Application of the prepayment and utilization of tax carryovers resulted in a refund of approximately $50 million. The IRS began its examination of tax years2005-2007 during the third quarter of 2008. We do not believe it is reasonably possible that the total amount of unrecognized tax benefits will significantly increase or decrease within the next twelve months.
We recognize both accrued interest and penalties related to unrecognized tax benefits in income tax expense. During the years ended December 31, 2008, 2007, and 2006, we recognized income tax expense (benefit) of $(118.0) million, $66.6 million, and $51.2 million, respectively, related to interest and penalties. At December 31, 2008 and 2007, our accruals for the payment of interest and penalties totaled $177.6 million
-75-
and $364.2 million, respectively. Substantially all of the expense (benefit) and accruals relate to interest. The change in the 2008 accrual reflects the impact of the effective settlement of the IRS audit discussed above.
| |
| Note 13: | Retirement Benefits |
We use a measurement date of December 31 to develop the change in benefit obligation, change in plan assets, funded status, and amounts recognized in the consolidated balance sheets at December 31 for our defined benefit pension and retiree health benefit plans, which were as follows:
| | | | | | | | | | | | | | | | | |
| | | Defined Benefit Pension Plans | | | Retiree Health Benefit Plans | |
| | | 2008 | | | 2007 | | | 2008 | | | 2007 | |
| | |
| |
| Change in benefit obligation | | | | | | | | | | | | | | | | |
| Benefit obligation at beginning of year | | $ | 6,561.0 | | | $ | 6,480.3 | | | $ | 1,622.8 | | | $ | 1,740.7 | |
| Service cost | | | 260.1 | | | | 287.1 | | | | 62.1 | | | | 70.4 | |
| Interest cost | | | 409.8 | | | | 362.4 | | | | 105.7 | | | | 101.4 | |
| Actuarial (gain) loss | | | (257.4 | ) | | | (373.1 | ) | | | 101.6 | | | | 16.4 | |
| Benefits paid | | | (338.4 | ) | | | (311.0 | ) | | | (92.2 | ) | | | (81.6 | ) |
| Plan amendments | | | (2.4 | ) | | | 32.7 | | | | — | | | | (227.7 | ) |
| Foreign currency exchange rate changes and other adjustments | | | (279.0 | ) | | | 82.6 | | | | (3.7 | ) | | | 3.2 | |
| | | |
| | | |
| Benefit obligation at end of year | | | 6,353.7 | | | | 6,561.0 | | | | 1,796.3 | | | | 1,622.8 | |
| | | | | | | | | | | | | | | | | |
| Change in plan assets | | | | | | | | | | | | | | | | |
| Fair value of plan assets at beginning of year | | | 7,304.2 | | | | 6,519.0 | | | | 1,348.5 | | | | 1,157.3 | |
| Actual return on plan assets | | | (2,187.8 | ) | | | 833.8 | | | | (438.6 | ) | | | 147.4 | |
| Employer contribution | | | 223.7 | | | | 202.9 | | | | 87.9 | | | | 125.4 | |
| Benefits paid | | | (326.1 | ) | | | (301.4 | ) | | | (92.2 | ) | | | (81.6 | ) |
| Foreign currency exchange rate changes and other adjustments | | | (217.9 | ) | | | 49.9 | | | | — | | | | — | |
| | | |
| | | |
| Fair value of plan assets at end of year | | | 4,796.1 | | | | 7,304.2 | | | | 905.6 | | | | 1,348.5 | |
| | | |
| | | |
| Funded status | | | (1,557.6 | ) | | | 743.2 | | | | (890.7 | ) | | | (274.3 | ) |
| Unrecognized net actuarial loss | | | 3,474.8 | | | | 1,143.3 | | | | 1,409.6 | | | | 820.3 | |
| Unrecognized prior service cost (benefit) | | | 72.7 | | | | 88.4 | | | | (261.6 | ) | | | (297.7 | ) |
| | | |
| | | |
| Net amount recognized | | $ | 1,989.9 | | | $ | 1,974.9 | | | $ | 257.3 | | | $ | 248.3 | |
| | | |
| | | |
| | | | | | | | | | | | | | | | | |
| Amounts recognized in the consolidated balance sheet consisted of | | | | | | | | | | | | | | | | |
| Prepaid pension | | $ | — | | | $ | 1,670.5 | | | $ | — | | | $ | — | |
| Other current liabilities | | | (52.9 | ) | | | (47.9 | ) | | | (7.8 | ) | | | (8.6 | ) |
| Accrued retirement benefit | | | (1,504.7 | ) | | | (879.4 | ) | | | (882.9 | ) | | | (265.7 | ) |
| Accumulated other comprehensive loss before income taxes | | | 3,547.5 | | | | 1,231.7 | | | | 1,148.0 | | | | 522.6 | |
| | | |
| | | |
| Net amount recognized | | $ | 1,989.9 | | | $ | 1,974.9 | | | $ | 257.3 | | | $ | 248.3 | |
| | | |
| | | |
The unrecognized net actuarial loss and unrecognized prior service cost (benefit) have not yet been recognized in net periodic pension costs and are included in accumulated other comprehensive loss at December 31, 2008.
In 2009, we expect to recognize from accumulated other comprehensive loss as components of net periodic benefit cost, $97.5 million of unrecognized net actuarial loss and $8.7 million of unrecognized prior service cost related to our defined benefit pension plans, and $69.4 million of unrecognized net actuarial loss and
-76-
$35.9 million of unrecognized prior service benefit related to our retiree health benefit plans. We do not expect any plan assets to be returned to us in 2009.
The following represents our weighted-average assumptions as of December 31:
| | | | | | | | | | | | | | | | | |
| | | Defined Benefit Pension Plans | | | Retiree Health Benefit Plans | |
| (Percents) | | 2008 | | | 2007 | | | 2008 | | | 2007 | |
| | |
| |
| Weighted-average assumptions as of December 31 | | | | | | | | | | | | | | | | |
| Discount rate for benefit obligation | | | 6.7 | | | | 6.4 | | | | 6.9 | | | | 6.7 | |
| Discount rate for net benefit costs | | | 6.4 | | | | 5.7 | | | | 6.7 | | | | 6.0 | |
| Rate of compensation increase for benefit obligation | | | 4.1 | | | | 4.6 | | | | — | | | | — | |
| Rate of compensation increase for net benefit costs | | | 4.6 | | | | 4.6 | | | | — | | | | — | |
| Expected return on plan assets for net benefit costs | | | 9.0 | | | | 9.0 | | | | 9.0 | | | | 9.0 | |
In evaluating the expected return on plan assets, we have considered our historical assumptions compared with actual results, an analysis of current market conditions, asset allocations, and the views of leading financial advisers and economists. Our plan assets in our U.S. defined benefit pension and retiree health plans comprise approximately 84 percent of our worldwide benefit plan assets. Including the investment losses due to overall market conditions in 2001, 2002, and 2008, our20-year annualized rate of return on our U.S. defined benefit pension plans and retiree health benefit plan was approximately 8.2 percent as of December 31, 2008. Health-care-cost trend rates are assumed to increase at an annual rate of 8.5 percent in 2009, decreasing by approximately 0.6 percent per year to an ultimate rate of 5.5 percent by 2014.
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | 2013 | | | 2014-2018 | |
| | |
| |
| Defined benefit pension plans | | $ | 360.5 | | | $ | 378.6 | | | $ | 384.8 | | | $ | 392.4 | | | $ | 403.3 | | | $ | 2,234.0 | |
| | | |
| | | |
| Retiree health benefit plans — gross | | $ | 103.3 | | | $ | 106.0 | | | $ | 109.8 | | | $ | 110.3 | | | $ | 114.7 | | | $ | 599.0 | |
| Medicare rebates | | | (11.6 | ) | | | (7.9 | ) | | | (8.7 | ) | | | (10.0 | ) | | | (10.6 | ) | | | (69.0 | ) |
| | | |
| | | |
| Retiree health benefit plans — net | | $ | 91.7 | | | $ | 98.1 | | | $ | 101.1 | | | $ | 100.3 | | | $ | 104.1 | | | $ | 530.0 | |
| | | |
| | | |
The total accumulated benefit obligation for our defined benefit pension plans was $5.64 billion and $5.69 billion at December 31, 2008 and 2007, respectively. The projected benefit obligation and fair value of the plan assets for the defined benefit pension plans with projected benefit obligations in excess of plan assets were $6.35 billion and $4.80 billion, respectively, as of December 31, 2008, and $1.04 billion and $160.9 million, respectively, as of December 31, 2007. The accumulated benefit obligation and fair value of the plan assets for the defined benefit pension plans with accumulated benefit obligations in excess of plan assets were $4.98 billion and $4.06 billion, respectively, as of December 31, 2008, and $825.8 million and $46.9 million, respectively, as of December 31, 2007.
-77-
Net pension and retiree health benefit expense included the following components:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Defined Benefit
| | | Retiree Health
| |
| | | Pension Plans | | | Benefit Plans | |
| | | 2008 | | | 2007 | | | 2006 | | | 2008 | | | 2007 | | | 2006 | |
| | |
| |
| Components of net periodic benefit cost | | | | | | | | | | | | | | | | | | | | | | | | |
| Service cost | | $ | 260.1 | | | $ | 287.1 | | | $ | 280.0 | | | $ | 62.1 | | | $ | 70.4 | | | $ | 72.2 | |
| Interest cost | | | 409.8 | | | | 362.4 | | | | 343.5 | | | | 105.7 | | | | 101.4 | | | | 97.9 | |
| Expected return on plan assets | | | (603.0 | ) | | | (548.2 | ) | | | (494.8 | ) | | | (118.4 | ) | | | (102.1 | ) | | | (89.9 | ) |
| Amortization of prior service cost (benefit) | | | 8.2 | | | | 7.7 | | | | 8.3 | | | | (36.0 | ) | | | (15.7 | ) | | | (15.6 | ) |
| Recognized actuarial loss | | | 76.6 | | | | 130.0 | | | | 149.6 | | | | 62.7 | | | | 95.0 | | | | 107.9 | |
| | | |
| | | |
| Net periodic benefit cost | | $ | 151.7 | | | $ | 239.0 | | | $ | 286.6 | | | $ | 76.1 | | | $ | 149.0 | | | $ | 172.5 | |
| | | |
| | | |
If the health-care-cost trend rates were to be increased by one percentage point each future year, the December 31, 2008, accumulated postretirement benefit obligation would increase by $247.8 million (13.9 percent) and the aggregate of the service cost and interest cost components of the 2008 annual expense would increase by $26.9 million (16.0 percent). A one-percentage-point decrease in these rates would decrease the December 31, 2008, accumulated postretirement benefit obligation by $192.0 million (10.8 percent) and the aggregate of the 2008 service cost and interest cost by $20.7 million (12.3 percent).
The following represents the amounts recognized in other comprehensive income (loss) in 2008:
| | | | | | | | | |
| | | Defined Benefit
| | | Retiree Health
| |
| | | Pension Plans | | | Benefit Plans | |
| | |
| |
| Actuarial loss arising during period | | $ | 2,533.4 | | | $ | 658.6 | |
| Plan amendments during period | | | (2.4 | ) | | | — | |
| Amortization of prior service cost (benefit) included in net income | | | (8.2 | ) | | | 36.0 | |
| Amortization of net actuarial loss included in net income | | | (76.6 | ) | | | (62.7 | ) |
| Foreign currency exchange rate changes | | | (130.4 | ) | | | (6.5 | ) |
| | | |
| | | |
| Total other comprehensive loss during period | | $ | 2,315.8 | | | $ | 625.4 | |
| | | |
| | | |
We have defined contribution savings plans that cover our eligible employees worldwide. The purpose of these defined contribution plans is generally to provide additional financial security during retirement by providing employees with an incentive to save. Our contributions to the plan are based on employee contributions and the level of our match. Expenses under the plans totaled $114.1 million, $112.3 million, and $106.5 million, for the years 2008, 2007, and 2006, respectively.
We provide certain other postemployment benefits primarily related to disability benefits and accrue for the related cost over the service lives of employees. Expenses associated with these benefit plans in 2008, 2007, and 2006 were not significant.
Our U.S. defined benefit pension and retiree health benefit plan investment allocation strategy currently comprises approximately 88 percent to 92 percent growth investments and 8 percent to 12 percent fixed-income investments. Within the growth investment allocation, the plan asset strategy encompasses equity and equity-like instruments that are expected to represent approximately 75 percent of our plan asset portfolio of both public and private market investments. The largest component of these equity and equity-like instruments is public equity securities that are well diversified and invested in U.S. and international small-to-large companies. The remaining portion of the growth investment allocation includes alternative investments.
-78-
Our defined benefit pension plan and retiree health plan asset allocations as of December 31 are as follows:
| | | | | | | | | | | | | | | | | |
| | | Percentage of
| | | Percentage of
| |
| | | Pension Plan
| | | Retiree Health
| |
| | | Assets | | | Plan Assets | |
| (Percents) | | 2008 | | | 2007 | | | 2008 | | | 2007 | |
| | |
| |
| Asset Category | | | | | | | | | | | | | | | | |
| Equity securities and equity-like instruments | | | 70 | | | | 75 | | | | 74 | | | | 78 | |
| Debt securities | | | 12 | | | | 10 | | | | 14 | | | | 11 | |
| Real estate | | | 1 | | | | 1 | | | | — | | | | — | |
| Other | | | 17 | | | | 14 | | | | 12 | | | | 11 | |
| | | |
| | | |
| Total | | | 100 | | | | 100 | | | | 100 | | | | 100 | |
| | | |
| | | |
In 2009, we expect to contribute approximately $55 million to our defined benefit pension plans to satisfy minimum funding requirements for the year. In addition, we expect to contribute approximately $15 million of additional discretionary funding in 2009 to our defined benefit plans. We do not expect to make any contributions to our post-retirement health benefit plans during 2009.
We are a party to various legal actions, government investigations, and environmental proceedings. The most significant of these are described below. While it is not possible to determine the outcome of these matters, we believe that, except as specifically noted below, the resolution of all such matters will not have a material adverse effect on our consolidated financial position or liquidity, but could possibly be material to our consolidated results of operations in any one accounting period.
Patent Litigation
We are engaged in the following patent litigation matters brought pursuant to procedures set out in the Hatch-Waxman Act (the Drug Price Competition and Patent Term Restoration Act of 1984):
| |
| • | Cymbalta: Sixteen generic drug manufacturers have submitted Abbreviated New Drug Applications (ANDAs) seeking permission to market generic versions of Cymbalta prior to the expiration of our relevant U.S. patents (the earliest of which expires in 2013). Of these challengers, all allege non-infringement of the patent claims directed to the commercial formulation, and eight allege invalidity of the patent claims directed to the active ingredient duloxetine. Of the eight challengers to the compound patent claims, one further alleges invalidity of the claims directed to the use of Cymbalta for treating fibromyalgia, and one alleges the patent having claims directed to the active ingredient is unenforceable. Lawsuits have been filed in U.S. District Court for the Southern District of Indiana against Activis Elizabeth LLC; Aurobindo Pharma Ltd.; Cobalt Laboratories, Inc.; Impax Laboratories, Inc.; Lupin Limited; Sandoz Inc.; Sun Pharma Global, Inc.; and Wockhardt Limited, seeking rulings that the patents are valid, infringed, and enforceable. Answers to the complaints are pending. |
| |
| • | Gemzar: Sicor Pharmaceuticals, Inc. (Sicor), Mayne Pharma (USA) Inc. (Mayne), and Sun Pharmaceutical Industries Inc. (Sun) each submitted an ANDA seeking permission to market generic versions of Gemzar prior to the expiration of our relevant U.S. patents (compound patent expiring in 2010 and method-of-use patent expiring in 2013), and alleging that these patents are invalid. We filed lawsuits in the U.S. District Court for the Southern District of Indiana against Sicor (February 2006) and Mayne (October 2006 and January 2008), seeking rulings that these patents are valid and are being infringed. The suit against Sicor has been scheduled for trial in July 2009. Sicor’s ANDAs have been approved by the FDA; however, Sicor must provide 90 days notice prior to marketing generic Gemzar to allow time for us to seek a preliminary injunction. Both suits against Mayne have been administratively closed, and the parties have agreed to be bound by the results of the Sicor suit. In November 2007, Sun filed a declaratory judgment action in the United States District Court for the Eastern District of Michigan, seeking rulings that our method-of-use and |
-79-
| | |
| | | compound patents are invalid or unenforceable, or would not be infringed by the sale of Sun’s generic product. This trial is scheduled for December 2009. |
| |
| • | Alimta: Teva Parenteral Medicines, Inc. (Teva) and APP Pharmaceuticals, LLC (APP) each submitted ANDAs seeking approval to market generic versions of Alimta prior to the expiration of the relevant U.S. patent (licensed from the Trustees of Princeton University and expiring in 2016), and alleging the patent is invalid. We, along with Princeton, filed lawsuits in the U.S. District Court for the District of Delaware against Teva and APP, seeking rulings that the compound patent is valid and infringed. Trial is scheduled for November 8, 2010. |
| |
| • | Evista: Barr Laboratories, Inc. (Barr) submitted an ANDA in 2002 seeking permission to market a generic version of Evista prior to the expiration of our relevant U.S. patents (expiring in2012-2017) and alleging that these patents are invalid, not enforceable, or not infringed. In November 2002, we filed a lawsuit against Barr in the U.S. District Court for the Southern District of Indiana, seeking a ruling that these patents are valid, enforceable, and being infringed by Barr. Teva Pharmaceuticals USA, Inc. (Teva) has also submitted an ANDA seeking permission to market a generic version of Evista. In June 2006, we filed a similar lawsuit against Teva in the U.S. District Court for the Southern District of Indiana. The lawsuit against Teva is currently scheduled for trial beginning March 9, 2009, while no trial date has been set in the lawsuit against Barr. In April 2008, the FDA granted Teva tentative approval of its ANDA, but Teva’s ability to market a generic product is subject to a statutory stay, which has been extended to expire on March 9, 2009. If the stay expires and the company cannot obtain preliminary relief from the court, Teva can launch its generic product, regardless of the status of the current litigation, but subject to our right to recover damages, should we prevail at trial. |
We believe each of these Hatch-Waxman challenges is without merit and expect to prevail in this litigation. However, it is not possible to determine the outcome of this litigation, and accordingly, we can provide no assurance that we will prevail. An unfavorable outcome in any of these cases could have a material adverse impact on our future consolidated results of operations, liquidity, and financial position.
We have received challenges to Zyprexa patents in a number of countries outside the U.S.:
| |
| • | In Canada, several generic pharmaceutical manufacturers have challenged the validity of our Zyprexa compound and method-of-use patent (expiring in 2011). In April 2007, the Canadian Federal Court ruled against the first challenger, Apotex Inc. (Apotex), and that ruling was affirmed on appeal in February 2008. In June 2007, the Canadian Federal Court held that an invalidity allegation of a second challenger, Novopharm Ltd. (Novopharm), was justified and denied our request that Novopharm be prohibited from receiving marketing approval for generic olanzapine in Canada. Novopharm began selling generic olanzapine in Canada in the third quarter of 2007. We sued Novopharm for patent infringement, and the trial began in November 2008. We expect the trial to run through the first quarter of 2009, with a decision in the second half of 2009. In November 2007, Apotex filed an action seeking a declaration of the invalidity of our Zyprexa compound and method-of-use patents, and no trial date has been set. We have brought similar actions against Pharmascience (August 2007), Sandoz (July 2007), Nu-Pharm (June 2008), Genpharm (June 2008) and Cobalt (January 2009); none of these suits has been scheduled for trial. Pharmascience has agreed to be bound by the outcome of the Novopharm suit, and, pending the outcome of the lawsuit, we have agreed not to take any further steps to prevent the company from coming to market with generic olanzapine tablets, subject to a contingent damages obligation should we be successful against Novopharm. |
| |
| • | In Germany, generic pharmaceutical manufacturers Egis-Gyogyszergyar and Neolab Ltd. challenged the validity of our Zyprexa compound and method-of-use patent (expiring in 2011). In June 2007, the German Federal Patent Court held that our patent is invalid. Generic olanzapine was launched by competitors in Germany in the fourth quarter of 2007. We appealed the decision to the German Federal Supreme Court and following a hearing in December 2008, the Supreme Court reversed the Federal Patent Court and found the patent to be valid. Following the decision of the Supreme Court, the generic companies either agreed to withdraw from the market or were subject to preliminary injunction. We are pursuing these companies for damages arising from infringement. |
-80-
| |
| • | We have received challenges in a number of other countries, including Spain, the United Kingdom (U.K.), France, and several smaller European countries. In Spain, we have been successful at both the trial and appellate court levels in defeating the generic manufacturers’ challenges, but further legal challenge is now pending before the Commercial Court in Madrid. In the U.K., the generic pharmaceutical manufacturer Dr. Reddy’s Laboratories (UK) Limited has challenged the validity of our Zyprexa compound and method-of-use patent (expiring in 2011). In October 2008, the Patents Court in the High Court, London ruled that our patent was valid. Dr. Reddy’s appealed this decision, and a hearing date for the appeal has not been set. |
We are vigorously contesting the various legal challenges to our Zyprexa patents on acountry-by-country basis. We cannot determine the outcome of this litigation. The availability of generic olanzapine in additional markets could have a material adverse impact on our consolidated results of operations.
Xigris and Evista: In June 2002, Ariad Pharmaceuticals, Inc., the Massachusetts Institute of Technology, the Whitehead Institute for Biomedical Research, and the President and Fellows of Harvard College in the U.S. District Court for the District of Massachusetts sued us, alleging that sales of two of our products, Xigris and Evista, were inducing the infringement of a patent related to the discovery of a natural cell signaling phenomenon in the human body, and seeking royalties on past and future sales of these products. On May 4, 2006, a jury in Boston issued an initial decision in the case that Xigris and Evista sales infringe the patent. The jury awarded the plaintiffs approximately $65 million in damages, calculated by applying a 2.3 percent royalty to all U.S. sales of Xigris and Evista from the date of issuance of the patent through the date of trial. In addition, a separate bench trial with the U.S. District Court of Massachusetts was held in August 2006, on our contention that the patent is unenforceable and impermissibly covers natural processes. In June 2005, the United States Patent and Trademark Office (USPTO) commenced a reexamination of the patent, and in August 2007 took the position that the Ariad claims at issue are unpatentable, a position that Ariad continues to contest. In September 2007, the Court entered a final judgment indicating that Ariad’s claims are patentable, valid, and enforceable, and finding damages in the amount of $65 million plus a 2.3 percent royalty on net U.S. sales of Xigris and Evista since the time of the jury decision. However, the Court deferred the requirement to pay any damages until after all rights to appeal have been exhausted. We have appealed this judgment. The Court of Appeals for the Federal Circuit heard oral arguments on the appeal on February 6, 2009. We believe that these allegations are without legal merit, that we will ultimately prevail on these issues, and therefore that the likelihood of any monetary damages is remote.
Government Investigations and Related Litigation
In March 2004, the Office of the U.S. Attorney for the Eastern District of Pennsylvania (EDPA) advised us that it had commenced an investigation related to our U.S. marketing and promotional practices, including our communications with physicians and remuneration of physician consultants and advisors, with respect to Zyprexa, Prozac, and Prozac Weekly. In addition, the State Medicaid Fraud Control Units of more than 30 states coordinated with the EDPA in its investigation of any Medicaid-related claims relating to our marketing and promotion of Zyprexa. In January 2009, we announced that we reached resolution of this matter. As part of the resolution, we pled guilty to one misdemeanor violation of the Food, Drug, and Cosmetic Act and agreed to pay $615.0 million. The misdemeanor plea is for the off-label promotion of Zyprexa in elderly populations as treatment for dementia, including Alzheimer’s dementia, between September 1999 and March 2001. We have also entered into a settlement agreement resolving the federal civil claims, under which we will pay approximately $438.0 million, although we do not admit to the allegations. We have also agreed to settle the civil investigations brought by the State Medicaid Fraud Control Units of the states that have coordinated with the EDPA in its investigation, and will make available a maximum of approximately $362.0 million for payment to those states that agree to settle. The charge we recorded for this matter in the third quarter of $1.42 billion will be sufficient to cover these payments. Also, as part of the settlement, we have entered into a corporate integrity agreement with the Office of Inspector General (OIG) of the U.S. Department of Health and Human Services (HHS). This agreement will require us to maintain our compliance program and to undertake a set of defined corporate integrity obligations for five years. The agreement also provides for an independent third-party review organization to assess and report on the company’s systems, processes, policies, procedures and practices.
-81-
In June 2005, we received a subpoena from the Office of the Attorney General, Medicaid Fraud Control Unit, of the State of Florida, seeking production of documents relating to sales of Zyprexa and our marketing and promotional practices with respect to Zyprexa. In September 2006, we received a subpoena from the California Attorney General’s Office seeking production of documents related to our efforts to obtain and maintain Zyprexa’s status on California’s formulary, marketing and promotional practices with respect to Zyprexa, and remuneration of health care providers. We expect these matters to be resolved if Florida and California participate in the state component of the EDPA resolution.
Beginning in August 2006, we received civil investigative demands or subpoenas from the attorneys general of a number of states under various state consumer protection laws. Most of these requests became part of a multi-state investigative effort coordinated by an executive committee of attorneys general. In October 2008, we reached a settlement with 32 states and the District of Columbia. While there is no finding that we have violated any provision of the state laws under which the investigations were conducted, we paid $62.0 million and agreed to undertake certain commitments regarding Zyprexa for a period of six years, through consent decrees filed in the settling states. The 32 states participating in the settlement are: Alabama, Arizona, California, Delaware, Florida, Hawaii, Illinois, Indiana, Iowa, Kansas, Maine, Maryland, Massachusetts, Michigan, Missouri, Nebraska, Nevada, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Dakota, Tennessee, Texas, Vermont, Washington, and Wisconsin.
Product Liability and Related Litigation
We have been named as a defendant in a large number of Zyprexa product liability lawsuits in the U.S. and have been notified of many other claims of individuals who have not filed suit. The lawsuits and unfiled claims (together the “claims”) allege a variety of injuries from the use of Zyprexa, with the majority alleging that the product caused or contributed to diabetes or high blood-glucose levels. The claims seek substantial compensatory and punitive damages and typically accuse us of inadequately testing for and warning about side effects of Zyprexa. Many of the claims also allege that we improperly promoted the drug. Almost all of the federal lawsuits are part of a Multi-District Litigation (MDL) proceeding before The Honorable Jack Weinstein in the Federal District Court for the Eastern District of New York (MDL No. 1596).
Since June 2005, we have entered into agreements with various claimants’ attorneys involved in U.S. Zyprexa product liability litigation to settle a substantial majority of the claims. The agreements cover a total of approximately 32,670 claimants, including a large number of previously filed lawsuits and other asserted claims. The two primary settlements were as follows:
| |
| • | In June 2005, we reached an agreement in principle (and in September 2005 a final agreement) to settle more than 8,000 claims for $690.0 million plus $10.0 million to cover administration of the settlement. |
| |
| • | In January 2007, we reached agreements with a number of plaintiffs’ attorneys to settle more than 18,000 claims for approximately $500 million. |
The 2005 settlement totaling $700.0 million was paid during 2005. The January 2007 settlements were paid during 2007.
We are prepared to continue our vigorous defense of Zyprexa in all remaining claims. The U.S. Zyprexa product liability claims not subject to these agreements include approximately 105 lawsuits in the U.S. covering approximately 120 plaintiffs, of which about 80 cases covering about 90 plaintiffs are part of the MDL. No trials have been scheduled related to these claims.
In early 2005, we were served with four lawsuits seeking class action status in Canada on behalf of patients who took Zyprexa. One of these four lawsuits has been certified for residents of Quebec, and a second has been certified in Ontario and includes all Canadian residents except for residents of Quebec and British Columbia. The allegations in the Canadian actions are similar to those in the litigation pending in the U.S.
-82-
Since the beginning of 2005, we have recorded aggregate net pretax charges of $1.61 billion for Zyprexa product liability matters. The net charges, which take into account our actual insurance recoveries, covered the following:
| |
| • | The cost of the Zyprexa product liability settlements to date; and |
| |
| • | Reserves for product liability exposures and defense costs regarding the known Zyprexa product liability claims and expected future claims to the extent we could formulate a reasonable estimate of the probable number and cost of the claims. |
In December 2004, we were served with two lawsuits brought in state court in Louisiana on behalf of the Louisiana Department of Health and Hospitals, alleging that Zyprexa caused or contributed to diabetes or high blood-glucose levels, and that we improperly promoted the drug. These cases have been removed to federal court and are now part of the MDL proceedings in the Eastern District of New York (EDNY). In these actions, the Department of Health and Hospitals seeks to recover the costs it paid for Zyprexa through Medicaid and other drug-benefit programs, as well as the costs the department alleges it has incurred and will incur to treat Zyprexa-related illnesses. We have been served with similar lawsuits filed by the states of Alaska, Arkansas, Connecticut, Idaho, Minnesota, Mississippi, Montana, New Mexico, Pennsylvania, South Carolina, Utah, and West Virginia in the courts of the respective states. The Connecticut, Louisiana, Minnesota, Mississippi, Montana, New Mexico, and West Virginia cases are part of the MDL proceedings in the EDNY. The Alaska case was settled in March 2008 for a payment of $15.0 million, plus terms designed to ensure, subject to certain limitations and conditions, that Alaska is treated as favorably as certain other states that may settle with us in the future over similar claims. The following cases have been set for trial in 2009: Connecticut in the EDNY in June, Pennsylvania in November, and South Carolina in August, in their respective states.
In 2005, two lawsuits were filed in the EDNY purporting to be nationwide class actions on behalf of all consumers and third-party payors, excluding governmental entities, which have made or will make payments for their members or insured patients being prescribed Zyprexa. These actions have now been consolidated into a single lawsuit, which is brought under certain state consumer protection statutes, the federal civil RICO statute, and common law theories, seeking a refund of the cost of Zyprexa, treble damages, punitive damages, and attorneys’ fees. Two additional lawsuits were filed in the EDNY in 2006 on similar grounds. In September 2008, Judge Weinstein certified a class consisting of third-party payors, excluding governmental entities and individual consumers. We appealed the certification order, and Judge Weinstein’s order denying our motion for summary judgment, in September 2008. In 2007, The Pennsylvania Employees Trust Fund brought claims in state court in Pennsylvania as insurer of Pennsylvania state employees, who were prescribed Zyprexa on similar grounds as described in the New York cases. As with the product liability suits, these lawsuits allege that we inadequately tested for and warned about side effects of Zyprexa and improperly promoted the drug. The Pennsylvania case is set for trial in October 2009.
We cannot determine with certainty the additional number of lawsuits and claims that may be asserted. The ultimate resolution of Zyprexa product liability and related litigation could have a material adverse impact on our consolidated results of operations, liquidity, and financial position.
In addition, we have been named as a defendant in numerous other product liability lawsuits involving primarily diethylstilbestrol (DES) and thimerosal. The majority of these claims are covered by insurance, subject to deductibles and coverage limits.
Because of the nature of pharmaceutical products, it is possible that we could become subject to large numbers of product liability and related claims for other products in the future. In the past few years, we have experienced difficulties in obtaining product liability insurance due to a very restrictive insurance market. Therefore, for substantially all of our currently marketed products, we have been and expect that we will continue to be completely self-insured for future product liability losses. In addition, there is no assurance that we will be able to fully collect from our insurance carriers in the future.
-83-
Environmental Matters
Under the Comprehensive Environmental Response, Compensation, and Liability Act, commonly known as Superfund, we have been designated as one of several potentially responsible parties with respect to fewer than 10 sites. Under Superfund, each responsible party may be jointly and severally liable for the entire amount of the cleanup. We also continue remediation of certain of our own sites. We have accrued for estimated Superfund cleanup costs, remediation, and certain other environmental matters. This takes into account, as applicable, available information regarding site conditions, potential cleanup methods, estimated costs, and the extent to which other parties can be expected to contribute to payment of those costs. We have limited liability insurance coverage for certain environmental liabilities.
| |
| Note 15: | Other Comprehensive Income (Loss) |
The accumulated balances related to each component of other comprehensive income (loss) were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Unrealized
| | | Defined Benefit
| | | Effective
| | | Accumulated
| | | | |
| | | Foreign Currency
| | | Gains
| | | Pension and
| | | Portion of
| | | Other
| | | | |
| | | Translation
| | | (Losses)
| | | Retiree Health
| | | Cash Flow
| | | Comprehensive
| | | | |
| | | Gains (Losses) | | | on Securities | | | Benefit Plans | | | Hedges | | | Income (Loss) | | | | |
| | |
| |
| Beginning balance at January 1, 2008 | | $ | 1,317.0 | | | $ | 14.6 | | | $ | (1,151.6 | ) | | $ | (166.8 | ) | | $ | 13.2 | | | | | |
| Other comprehensive income (loss) | | | (766.1 | ) | | | (125.8 | ) | | | (1,924.8 | ) | | | 16.7 | | | | (2,800.0 | ) | | | | |
| | | |
| | | |
| Balance at December 31, 2008 | | $ | 550.9 | | | $ | (111.2 | ) | | $ | (3,076.4 | ) | | $ | (150.1 | ) | | $ | (2,786.8 | ) | | | | |
| | | |
| | | |
The amounts above are net of income taxes. The income taxes associated with the unrecognized net actuarial losses and prior service costs on our defined benefit pension and retiree health benefit plans (Note 13) were a benefit of $1.02 billion for 2008. The income taxes related to the other components of comprehensive income were not significant, as income taxes were not provided for foreign currency translation.
The unrealized gains (losses) on securities is net of reclassification adjustments of $1.7 million, $5.8 million, and $16.9 million, net of tax, in 2008, 2007, and 2006, respectively, for net realized gains on sales of securities included in net income. The effective portion of cash flow hedges is net of reclassification adjustments of $9.6 million, $8.8 million, and $2.3 million, net of tax, in 2008, 2007, and 2006, respectively, for realized losses on foreign currency options and $7.9 million, $11.6 million, and $17.1 million, net of tax, in 2008, 2007, and 2006, respectively, for interest expense on interest rate swaps designated as cash flow hedges.
Generally, the assets and liabilities of foreign operations are translated into U.S. dollars using the current exchange rate. For those operations, changes in exchange rates generally do not affect cash flows; therefore, resulting translation adjustments are made in shareholders’ equity rather than in income.
-84-
Management’s Reports
Management’s Report for Financial Statements — Eli Lilly and Company and Subsidiaries
Management of Eli Lilly and Company and subsidiaries is responsible for the accuracy, integrity, and fair presentation of the financial statements. The statements have been prepared in accordance with generally accepted accounting principles in the United States and include amounts based on judgments and estimates by management. In management’s opinion, the consolidated financial statements present fairly our financial position, results of operations, and cash flows.
In addition to the system of internal accounting controls, we maintain a code of conduct (known asThe Red Book) that applies to all employees worldwide, requiring proper overall business conduct, avoidance of conflicts of interest, compliance with laws, and confidentiality of proprietary information.The Red Bookis reviewed on a periodic basis with employees worldwide, and all employees are required to report suspected violations. A hotline number is published inThe Red Bookto enable employees to report suspected violations anonymously. Employees who report suspected violations are protected from discrimination or retaliation by the company. In addition toThe Red Book, the CEO, and all financial management must sign a financial code of ethics, which further reinforces their fiduciary responsibilities.
The consolidated financial statements have been audited by Ernst & Young LLP, an independent registered public accounting firm. Their responsibility is to examine our consolidated financial statements in accordance with generally accepted auditing standards of the Public Company Accounting Oversight Board (United States). Ernst & Young’s opinion with respect to the fairness of the presentation of the statements (see opinion on page 66) is included in our annual report. Ernst & Young reports directly to the audit committee of the board of directors.
Our audit committee includes five nonemployee members of the board of directors, all of whom are independent from our company. The committee charter, which is published in the proxy statement, outlines the members’ roles and responsibilities and is consistent with enacted corporate reform laws and regulations. It is the audit committee’s responsibility to appoint an independent registered public accounting firm subject to shareholder ratification, approve both audit and nonaudit services performed by the independent registered public accounting firm, and review the reports submitted by the firm. The audit committee meets several times during the year with management, the internal auditors, and the independent public accounting firm to discuss audit activities, internal controls, and financial reporting matters, including reviews of our externally published financial results. The internal auditors and the independent registered public accounting firm have full and free access to the committee.
We are dedicated to ensuring that we maintain the high standards of financial accounting and reporting that we have established. We are committed to providing financial information that is transparent, timely, complete, relevant, and accurate. Our culture demands integrity and an unyielding commitment to strong internal practices and policies. Finally, we have the highest confidence in our financial reporting, our underlying system of internal controls, and our people, who are objective in their responsibilities and operate under a code of conduct and the highest level of ethical standards.
-85-
Management’s Report on Internal Control Over Financial Reporting — Eli Lilly and Company and Subsidiaries
Management of Eli Lilly and Company and subsidiaries is responsible for establishing and maintaining adequate internal control over financial reporting as defined inRules 13a-15(f) and15d-15(f) under the Securities Exchange Act of 1934. We have global financial policies that govern critical areas, including internal controls, financial accounting and reporting, fiduciary accountability, and safeguarding of corporate assets. Our internal accounting control systems are designed to provide reasonable assurance that assets are safeguarded, that transactions are executed in accordance with management’s authorization and are properly recorded, and that accounting records are adequate for preparation of financial statements and other financial information. A staff of internal auditors regularly monitors, on a worldwide basis, the adequacy and effectiveness of internal accounting controls. The general auditor reports directly to the audit committee of the board of directors.
We conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under this framework, we concluded that our internal control over financial reporting was effective as of December 31, 2008. However, because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The internal control over financial reporting has been assessed by Ernst & Young LLP. Their responsibility is to evaluate whether internal control over financial reporting was designed and operating effectively.
| | | | | |
| John C. Lechleiter, Ph.D. | | | Derica W. Rice | |
Chairman, President, and
Chief Executive Officer | | | Senior Vice President and
Chief Financial Officer | |
February 16, 2009
-86-
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
Eli Lilly and Company
We have audited the accompanying consolidated balance sheets of Eli Lilly and Company and subsidiaries as of December 31, 2008 and 2007, and the related consolidated statements of operations, cash flows, and comprehensive income (loss) (pages 43 through 48 and pages 52 through 84) for each of the three years in the period ended December 31, 2008. These financial statements are the responsibility of the company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Eli Lilly and Company and subsidiaries at December 31, 2008 and 2007, and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Eli Lilly and Company and subsidiaries’ internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 16, 2009 expressed an unqualified opinion thereon.
As discussed in Note 12 to the financial statements, in 2007 Eli Lilly and Company and subsidiaries adopted a new accounting pronouncement for income taxes.
Indianapolis, Indiana
February 16, 2009
-87-
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
Eli Lilly and Company
We have audited Eli Lilly and Company and subsidiaries’ internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Eli Lilly and Company and subsidiaries’ management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Eli Lilly and Company and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the 2008 consolidated financial statements of Eli Lilly and Company and subsidiaries and our report dated February 16, 2009, expressed an unqualified opinion thereon.
Indianapolis, Indiana
February 16, 2009
-88-
| |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| |
| Item 9A. | Controls and Procedures |
Disclosure Controls and Procedures
Under applicable SEC regulations, management of a reporting company, with the participation of the principal executive officer and principal financial officer, must periodically evaluate the company’s “disclosure controls and procedures,” which are defined generally as controls and other procedures of a reporting company designed to ensure that information required to be disclosed by the reporting company in its periodic reports filed with the commission (such as thisForm 10-K) is recorded, processed, summarized, and reported on a timely basis.
Our management, with the participation of John C. Lechleiter, Ph.D., chairman, president, and chief executive officer, and Derica W. Rice, senior vice president and chief financial officer, evaluated our disclosure controls and procedures as of December 31, 2008, and concluded that they are effective.
Internal Control over Financial Reporting
Dr. Lechleiter and Mr. Rice provided a report on behalf of management on our internal control over financial reporting, in which management concluded that the company’s internal control over financial reporting is effective at December 31, 2008. In addition, Ernst & Young LLP, the company’s independent registered public accounting firm, provided an attestation report on the company’s internal control over financial reporting. You can find the full text of management’s report and Ernst & Young’s attestation report in Part II, Item 8, and both reports are incorporated by reference in this Item.
Changes in Internal Controls
During the fourth quarter of 2008, there were no changes in our internal control over financial reporting that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Not applicable.
Part III
| |
| Item 10. | Directors, Executive Officers and Corporate Governance |
Directors and Executive Officers
Information relating to our Board of Directors is found in our Proxy Statement to be dated on or about March 9, 2009 (the “Proxy Statement”) under “Board of Directors” at pages73-76, and is incorporated in this report by reference.
Information relating to our executive officers is found at Part I, Item 1 of thisForm 10-K under “Executive Officers of the Company.”
-89-
Code of Ethics
We have adopted a code of ethics that complies with the applicable SEC and New York Stock Exchange requirements. The code is set forth in:
| | |
| | • | The Red Book,a comprehensive code of ethical and legal business conduct applicable to all employees worldwide and to our Board of Directors; and |
| |
| | • | Code of Ethical Conduct for Lilly Financial Management, a supplemental code for our chief executive officer and all members of financial management that focuses on accounting, financial reporting, internal controls, and financial stewardship. |
Both documents are online on our web site athttp://investor.lilly.com/code business conduct.cfm. In the event of any amendments to, or waivers from, a provision of the code affecting the chief executive officer, chief financial officer, chief accounting officer, controller, or persons performing similar functions, we intend to post on the above web site within four business days after the event a description of the amendment or waiver as required under applicable SEC rules. We will maintain that information on our web site for at least 12 months. Paper copies of these documents are available free of charge upon request to the company’s secretary at the address on the front of thisForm 10-K.
Corporate Governance
In our proxy statements, we describe the procedures by which shareholders can recommend nominees to our board of directors. There have been no changes in those procedures since they were last published in our proxy statement of March 10, 2008.
The board has appointed an audit committee consisting entirely of independent directors in accordance with applicable SEC and New York Stock Exchange rules for audit committees. The members of the committee are Mr. J. Michael Cook (chairman), Michael L. Eskew, Dr. Martin S. Feldstein, Douglas R. Oberhelman, and Ms. Kathi P. Seifert. The board has determined that Messrs. Cook and Eskew are audit committee financial experts as defined in the SEC rules.
| |
| Item 11. | Executive Compensation |
Information on director compensation, executive compensation, and compensation committee matters can be found in the Proxy Statement under “Directors’ Compensation” at pages83-85, “Executive Compensation” at pages89-110, and “Compensation Committee Interlocks and Insider Participation” at page 89. That information is incorporated in this report by reference.
| |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Security Ownership of Certain Beneficial Owners and Management
Information relating to ownership of the Company’s common stock by management and by persons known by the Company to be the beneficial owners of more than five percent of the outstanding shares of common stock is found in the Proxy Statement under “Ownership of Company Stock,” at pages111-112. That information is incorporated in this report by reference.
Securities Authorized for Issuance Under Equity Compensation Plans
Information on securities authorized for issuance under our equity compensation plans can be found in the Proxy Statement under “Item 4 − Reapproval of Material Terms of Performance Goals for the Eli Lilly and Company Bonus Plan” at page 116. That information is incorporated in this report by reference.
-90-
| |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Related Person Transactions
Information relating to a now-terminated time-share arrangement between the company and Mr. Sidney Taurel, retired chairman and chief executive officer, relating to his personal use of the corporate aircraft can be found in the Proxy Statement under “Related Person Transaction” at pages 110, and information relating to the board’s policies and procedures for approval of related person transactions can be found in the Proxy Statement under “Highlights of the Company’s Corporate Governance Guidelines − Review and Approval of Transactions with Related Persons” at pages80-81. That information is incorporated in this report by reference.
Director Independence
Information relating to director independence can be found in the Proxy Statement under “Composition of the Board − Independence Determinations” at pages77-78, and is incorporated in this report by reference.
| |
| Item 14. | Principal Accountant Fees and Services |
Information related to the fees and services of our principal independent accountants, Ernst & Young LLP, can be found in the Proxy Statement under “Services Performed by the Independent Auditor” and “Independent Auditor Fees” at pages87-88. That information is incorporated in this report by reference.
| |
| Item 15. | Exhibits and Financial Statement Schedules |
(a)1. Financial Statements
The following consolidated financial statements of the Company and its subsidiaries are found at Part II, Item 8:
| | |
| | • | Consolidated Statements of Operations − Years Ended December 31, 2008, 2007, and 2006 |
| |
| | • | Consolidated Balance Sheets − December 31, 2008 and 2007 |
| |
| | • | Consolidated Statements of Cash Flows − Years Ended December 31, 2008, 2007, and 2006 |
| |
| | • | Consolidated Statements of Comprehensive Income (Loss) − Years Ended December 31, 2008, 2007, and 2006 |
| |
| | • | Segment Information |
| |
| | • | Notes to Consolidated Financial Statements |
(a)2. Financial Statement Schedules
The consolidated financial statement schedules of the Company and its subsidiaries have been omitted because they are not required, are inapplicable, or are adequately explained in the financial statements.
Financial statements of interests of 50 percent or less, which are accounted for by the equity method, have been omitted because they do not, considered in the aggregate as a single subsidiary, constitute a significant subsidiary.
(a)3. Exhibits
| | | | | |
| | 2 | | | Agreement and Plan of Merger dated October 6, 2008, among Eli Lilly and Company, Alaska Acquisition Corporation and ImClone Systems Incorporated |
| | 3 | .1 | | Amended Articles of Incorporation |
| | 3 | .2 | | By-laws, as amended |
| | 4 | .1 | | Form of Indenture with respect to Debt Securities dated as of February 1, 1991, between Eli Lilly and Company and Citibank, N.A., as Trustee |
-91-
| | | | | |
| | 4 | .2 | | Agreement dated September 13, 2007 appointing Deutsche Bank Trust Company Americas as Successor Trustee under the Indenture listed above |
| | 4 | .3 | | Form of Standard Multiple-Series Indenture Provisions dated, and filed with the Securities and Exchange Commission on, February 1, 1991 |
| | 4 | .4 | | Form of Indenture dated March 10, 1998, among The Lilly Savings Plan Master Trust Fund C, as issuer; Eli Lilly and Company, as guarantor; and The Chase Manhattan Bank, as Trustee, relating to ESOP Amortizing Debentures due 20171 |
| | 4 | .5 | | Form of Fiscal Agency Agreement dated May 30, 2001, between Eli Lilly and Company and Citibank, N.A., Fiscal Agent, relating to Resetable Floating Rate Debt Security due 20371 |
| | 4 | .6 | | Form of Resetable Floating Rate Debt Security due 20371 |
| | 10 | .1 | | 1998 Lilly Stock Plan, as amended2 |
| | 10 | .2 | | 2002 Lilly Stock Plan, as amended2 |
| | 10 | .3 | | Form of Performance Award under 2002 Lilly Stock Plan2 |
| | 10 | .4 | | Form of two-year Performance Award under 2002 Lilly Stock Plan2 |
| | 10 | .5 | | Form of Shareholder Value Award under 2002 Lilly Stock Plan2 |
| | 10 | .6 | | The Lilly Deferred Compensation Plan, as amended2 |
| | 10 | .7 | | The Lilly Directors’ Deferral Plan, as amended2 |
| | 10 | .8 | | The Eli Lilly and Company Bonus Plan, as amended2 |
| | 10 | .9 | | 2007 Change in Control Severance Pay Plan for Select Employees, as amended effective January 1, 20092 |
| | 10 | .10 | | 2007 Change in Control Severance Pay Plan for Select Employees, as amended effective October 20, 20102 |
| | 10 | .11 | | Letter agreement between the company and Charles E. Golden concerning retirement benefits2 |
| | 10 | .12 | | Letter agreement between the company and Steven M. Paul, M.D. concerning retirement benefits2 |
| | 10 | .13 | | Arrangement regarding retirement benefits for Robert A. Armitage2 |
| | 10 | .14 | | Time Sharing Agreement between the company and Sidney Taurel for use of corporate aircraft |
| | 10 | .15 | | Guilty Plea Agreement inThe United States District Court for the Eastern District of Pennsylvania, United States of America v. Eli Lilly and Company |
| | 10 | .16 | | Settlement Agreement among the company and the United States of America, acting through the United States Department of Justice, Civil Division, and the United States Attorney’s Office of the Eastern District of Pennsylvania, the Office of the Inspector General of the Department of Health and Human Services, TRICARE Management Activity, and the United States Office of Personnel Management, and certain individual relators |
| | 10 | .17 | | Corporate Integrity Agreement between the company and the Office of Inspector General of the Department of Health and Human Services |
| | 12 | | | Statement re: Computation of Ratio of Earnings (Loss) to Fixed Charges |
| | 21 | | | List of Subsidiaries |
| | 23 | | | Consent of Independent Registered Public Accounting Firm |
| | 31 | .1 | | Rule 13a-14(a) Certification of John C. Lechleiter, Ph.D., Chairman of the Board, President and Chief Executive Officer |
| | 31 | .2 | | Rule 13a-14(a) Certification of Derica W. Rice, Senior Vice President and Chief Financial Officer |
| | 32 | | | Section 1350 Certification |
1 This exhibit is not filed with this report. Copies will be furnished to the Securities and Exchange Commission upon request.
2 Indicates management contract or compensatory plan.
-92-
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Eli Lilly and Company
| | |
| By | /s/ John C. Lechleiter | |
John C. Lechleiter, Ph.D., Chairman of the Board, President and Chief Executive Officer
February 27, 2009
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below on February 27, 2009 by the following persons on behalf of the Registrant and in the capacities indicated.
| | | | | |
| Signature | | Title |
| |
| |
/s/ John C. Lechleiter
JOHN C. LECHLEITER, Ph.D. | | Chairman of the Board, Chief Executive Officer, and a Director (principal executive officer) |
| | | |
/s/ Derica W. Rice
DERICA W. RICE | | Senior Vice President and Chief Financial Officer
(principal financial officer) |
| | | |
/s/ Arnold C. Hanish
ARNOLD C. HANISH | | Vice President and Chief Accounting Officer
(principal accounting officer) |
| | | |
/s/ Sir Winfried Bischoff
SIR WINFRIED BISCHOFF | | Director |
| | | |
/s/ J. Michael Cook
J. MICHAEL COOK | | Director |
| | | |
/s/ Michael L. Eskew
MICHAEL L. ESKEW | | Director |
| | | |
/s/ Martin S. Feldstein
MARTIN S. FELDSTEIN, Ph.D. | | Director |
| | | |
/s/ J. Erik Fyrwald
J. ERIK FYRWALD | | Director |
| | | |
/s/ Karen N. Horn
KAREN N. HORN, Ph.D. | | Director |
| | | |
/s/ Alfred G. Gilman
ALFRED G. GILMAN, M.D., Ph.D. | | Director |
-93-
| | | | | |
| Signature | | Title |
| |
| |
/s/ Ellen R. Marram
ELLEN R. MARRAM | | Director |
| | | |
/s/ Douglas R. Oberhelman
DOUGLAS R. OBERHELMAN | | Director |
| | | |
/s/ Franklyn G. Prendergast
FRANKLYN G. PRENDERGAST, M.D., Ph.D. | | Director |
| | | |
/s/ Kathi P. Seifert
KATHI P. SEIFERT | | Director |
-94-
Trademarks Used In This Report
Trademarks or service marks owned by Eli Lilly and Company or its subsidiaries or affiliates, when first used in this report, appear with an initial capital and are followed by the symbol® ortm, as applicable. In subsequent uses of the marks in the report, the symbols are omitted.
Actos® is a trademark of Takeda Chemical Industries, Ltd.
Axid® is a trademark of Reliant Pharmaceuticals, LLC
Byetta® is a trademark of Amylin Pharmaceuticals, Inc.
-95-
Index to Exhibits
The following documents are filed as part of this report:
| | | | | | | |
| Exhibit | | | | Location |
| |
| | 2 | | | Agreement and Plan of Merger, dated as of October 6, 2008, among Eli Lilly and Company, Alaska Acquisition Corporation and ImClone Systems Incorporated | | Incorporated by reference from Exhibit 2.1 to the Company’s Report on Form 8-K filed October 10, 2008 |
| | 3 | .1 | | Amended Articles of Incorporation | | Incorporated by reference from Exhibit 3.1 to the Company’s Report on Form 10-Q for the quarter ended March 31, 2008 |
| | 3 | .2 | | By-laws, as amended | | Incorporated by reference from Exhibit 3.2 to the Company’s Report on Form 10-Q for the quarter ended March 31, 2008 |
| | 4 | .1 | | Form of Indenture with respect to Debt Securities dated as of February 1, 1991, between Eli Lilly and Company and Citibank, N.A., as Trustee | | Incorporated by reference from Exhibit 4.1 to the Company’s Registration Statement on Form S-3, Amendment No. 1, RegistrationNo. 333-106478 |
| | 4 | .2 | | Agreement dated September 13, 2007 appointing Deutsche Bank Trust Company Americas as Successor Trustee under the Indenture listed above | | Attached |
| | 4 | .3 | | Form of Standard Multiple-Series Indenture Provisions dated, and filed with the Securities and Exchange Commission on February 1, 1991 | | Incorporated by reference from Exhibit 4.2 to the Company’s Registration Statement on Form S-3, Amendment No. 1, RegistrationNo. 333-106478 |
| | 4 | .4 | | Form of Indenture dated March 10, 1998, among The Lilly Savings Plan Master Trust Fund C, as issuer; Eli Lilly and Company, as guarantor; and The Chase Manhattan Bank, as Trustee, relating to ESOP Amortizing Debentures due 2017 | | * |
| | 4 | .5 | | Form of Fiscal Agency Agreement dated May 30, 2001, between Eli Lilly and Company and Citibank, N.A., Fiscal Agent, relating to Resettable Floating Rate Debt Security due 2037 | | * |
| | 4 | .6 | | Form of Resettable Floating Rate Debt Security due 2037 | | * |
| | 10 | .1 | | 1998 Lilly Stock Plan, as amended | | Incorporated by reference from Exhibit 10.1 to the Company’s Report on Form 10-K for the year ended December 31, 2006 |
| | 10 | .2 | | 2002 Lilly Stock Plan, as amended | | Incorporated by reference from Exhibit 10.1 to the Company’s Report on Form 10-Q for the quarter ended September 30, 2008 |
| | 10 | .3 | | Form of Performance Award under 2002 Lilly Stock Plan | | Incorporated by reference from Exhibit 10 to the Company’s Report on Form 10-Q for the quarter ended September 30, 2004 |
| | 10 | .4 | | Form of two-year Performance Award under 2002 Lilly Stock Plan | | Incorporated by reference from Exhibit 10.1 to the Company’s Report on Form 8-K filed December 11, 2008 |
| | 10 | .5 | | Form of Shareholder Value Award under 2002 Lilly Stock Plan | | Incorporated by reference from Exhibit 10.1 to the Company’s Report on Form 10-Q for the quarter ended March 31, 2007 |
* Not filed with this report. Copies will be furnished to the Securities and Exchange Commission upon request.
-96-
| | | | | | | |
| Exhibit | | | | Location |
| |
| | 10 | .6 | | The Lilly Deferred Compensation Plan, as amended | | Incorporated by reference from Exhibit 10.3 to the Company’s Report on Form 10-Q for the quarter ended September 30, 2008 |
| | 10 | .7 | | The Lilly Directors’ Deferral Plan, as amended | | Incorporated by reference from Exhibit 10.2 to the Company’s Report on Form 10-Q for the quarter ended September 30, 2008 |
| | 10 | .8 | | The Eli Lilly and Company Bonus Plan, as amended | | Attached |
| | 10 | .9 | | 2007 Change in Control Severance Pay Plan for Select Employees, as amended effective January 1, 2009 | | Incorporated by reference from Exhibit 10.4 to the Company’s Report on Form 10-Q for the quarter ended September 30, 2008 |
| | 10 | .10 | | 2007 Change in Control Severance Pay Plan for Select Employees, as amended effective October 20, 2010 | | Incorporated by reference from Exhibit 10.5 to the Company’s Report on Form 10-Q for the quarter ended September 30, 2008 |
| | 10 | .11 | | Letter agreement between the Company and Charles E. Golden concerning retirement benefits | | Incorporated by reference from Exhibit 10.13 to the Company’s Report on Form 10-K for the year ended December 31, 2004 |
| | 10 | .12 | | Letter agreement between the Company and Steven M. Paul, M.D. concerning retirement benefits | | Incorporated by reference from Exhibit 10.14 to the Company’s Report on Form 10-K for the year ended December 31, 2004 |
| | 10 | .13 | | Arrangement regarding retirement benefits for Robert A. Armitage | | Incorporated by reference from Exhibit 10.15 to the Company’s Report on Form 10-K for the year ended December 31, 2004 |
| | 10 | .14 | | Time Sharing Agreement between the Company and Sidney Taurel for use of corporate aircraft | | Incorporated by reference from Exhibit 10.16 to the Company’s Report on Form 10-K for the year ended December 31, 2004 |
| | 10 | .15 | | Guilty Plea Agreement inThe United States District Court for the Eastern District of Pennsylvania, United States of America v. Eli Lilly and Company | | Attached |
| | 10 | .16 | | Settlement Agreement among the company and the United States of America, acting through the U. S. Department of Justice, Civil Division, and the U. S. Attorney’s Office of the Eastern District of Pennsylvania, the Office of the Inspector General of the Department of Health and Human Services, TRICARE Management Activity, and the U. S. Office of Personnel Management, and certain individual relators | | Attached |
| | 10 | .17 | | Corporate Integrity Agreement between the company and the Office of Inspector General of the Department of Health and Human Services | | Attached |
| | 12 | | | Statement re: Computation of Ratio of Earnings (Loss) to Fixed Charges | | Attached |
| | 21 | | | List of Subsidiaries | | Attached |
| | 23 | | | Consent of Registered Independent Public Accounting Firm | | Attached |
| | 31 | .1 | | Rule 13a-14(a) Certification of John C. Lechleiter, Ph.D., Chairman of the Board and Chief Executive Officer | | Attached |
| | 31 | .2 | | Rule 13a-14(a) Certification of Derica W. Rice, Senior Vice President and Chief Financial Officer | | Attached |
| | 32 | | | Section 1350 Certification | | Attached |
-97-