UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE |
| | SECURITIES EXCHANGE ACT OF 1934 |
| | For the fiscal year ended: November 30, 2005 |
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE |
| | SECURITIES EXCHANGE ACT OF 1934 |
| | For the transition period from to |
Commission file number: 000-11868
CARDIODYNAMICS INTERNATIONAL CORPORATION
(Exact name of registrant as specified in its charter)
| | |
| California | | 95-3533362 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
| 6175 Nancy Ridge Drive, San Diego, California | | 92121 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code:(858) 535-0202
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, no par value
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Act. (Check one):
Large accelerated filer ¨ Accelerated Filer x Non-accelerated filer ¨
Indicate by check mark whether the registrant is a shell company(as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of the registrant’s voting and non-voting common equity held by non-affiliates of the registrant was approximately $95,669,000 based on the average bid and asked price of common stock as reported on the Nasdaq Stock Market on May 31, 2005, which was the last business day of the registrant’s most recently completed second fiscal quarter. For purposes of this disclosure, shares of Common Stock held by persons who hold more than 5% of the outstanding shares of Common Stock and shares held by directors and officers of the registrant have been excluded because such persons may be deemed to be affiliates.
At January 31, 2006, 48,803,410 shares of registrant’s Common Stock were outstanding.
Documents Incorporated by Reference: None
TABLE OF CONTENTS
This Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements include statements regarding our plans, goals, strategies, intent, beliefs or current expectations. These statements are expressed in good faith and based upon reasonable assumptions when made, but there can be no assurance that these expectations will be achieved or accomplished. Sentences in this document containing verbs such as “believe”, “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect,” and the like, and/or future-tense or conditional constructions (“will,” “may,” “could,” “should,” etc.) constitute forward-looking statements that involve risks and uncertainties. Items contemplating, or making assumptions about, actual or potential future sales, market size, collaborations, trends or operating results also constitute such forward-looking statements. These statements are only predictions and actual results could differ materially. Certain factors that might cause such a difference are discussed throughout this Annual Report on Form 10-K, including the section entitled “Risk Factors”. Any forward-looking statement speaks only as of the date we made the statement, and we do not undertake to update the disclosures contained in this document or reflect events or circumstances that occur subsequently or the occurrence of unanticipated events.
2
PART I
CardioDynamics International Corporation (“CardioDynamics” or “the Company”) is the innovator and market leader of an important medical technology called impedance cardiography (ICG). We develop, manufacture and market noninvasive ICG diagnostic and monitoring devices, proprietary ICG sensors and a broad array of medical device electrodes.
The Company was incorporated as a California corporation in June 1980 and changed its name to CardioDynamics International Corporation in October 1993. On March 22, 2004, the Company completed the acquisition of substantially all of the assets and certain liabilities (the “Acquired Assets”) of the Vermed Division (“Vermed”) of Vermont Medical, Inc. Vermed is a manufacturer of electrodes and related supplies used in electrocardiograms (ECG) and other diagnostic procedures for cardiology, electrotherapy, sleep testing, neurology and general purpose diagnostic testing. On June 2, 2004, the Company completed the acquisition of 80% of all outstanding shares of Medis Medizinische Messtechnik GmbH (“Medis”). Medis is a manufacturer of diagnostic and monitoring devices, which uses impedance cardiography (ICG) technology for cardiovascular diagnostics sold internationally.
In December 2004, the Company received 510(k) clearance by the U.S. Federal Drug Administration (FDA) for our new lead product, the BioZ ICG Dx. The BioZ Dx is the result of a co-development partnership and OEM Agreement between the Company and Philips Medical Systems, a division of Royal Philips Electronics (Philips), a worldwide leader in clinical measurement and diagnostic solutions for the healthcare industry. This partnership leverages each company’s technology and expertise. The BioZ Dx also carries the CE mark, which is a required certification of essential environmental and safety compliance by the European Community for sale of electronic equipment. In June 2005, the Company received FDA 510(k) clearance for 12 lead diagnostic ECG capabilities integrated into the BioZ Dx product platform, which provided the world’s first product with the ability to assess mechanical function with ICG and electrical function with 12 lead ECG.
We continue to sell our previous lead product, the BioZ® ICG Monitor (previously known as the BioZ.com®), which has FDA 510(k) clearance and carries the CE mark. We sell to physicians and hospitals in the United States through our own direct sales force and distribute our products to domestic and targeted international markets through a network of distributors. In November 1998, Health Care Finance Administration (HCFA), now known as the Center for Medicare & Medicaid Services (CMS), mandated Medicare reimbursement for our BioZ® procedures and, in January 2001, implemented national uniform pricing throughout the United States. CMS reevaluated reimbursement of our ICG technology and issued a policy clarification in 2004 that restricted the availability of Medicare reimbursement for hypertension patients. To date, we have sold over 5,900 ICG systems and modules to over 3,500 physician offices and hospital sites throughout the world.
Our proprietary and patented ICG technology noninvasively quantifies themechanical functioning of the heart and monitors the heart’s ability to deliver blood to the body. Our systems provide hemodynamic (blood flow) parameters, the most familiar of which is cardiac output, or the amount of blood pumped by the heart each minute. Our products help physicians assess, diagnose, and treat patients with heart failure, hypertension (high blood pressure), and shortness of breath. It is estimated that there are over 5 million heart failure patients in the United States and over 65 million patients with high blood pressure. Our technology complements ECG (electrical characteristics) and supplements information obtained through the five vital signs—heart rate, respiration rate, body temperature, blood pressure and oxygen saturation—quickly, safely and cost effectively.
Currently, the primary method used to measure blood flow (hemodynamic) parameters is pulmonary artery catheterization (PAC), which is an invasive procedure that requires insertion of a catheter (plastic tube) into the heart itself. Complications associated with this procedure occur in as many as one in four reported cases and typically include irregular heartbeats or infection, but in some cases, pulmonary artery rupture or even death. The PAC procedure is a diagnostic procedure with a catheter inserted into the right side of the heart and should not be
3
confused with the diagnostic and therapeutic procedures involving the left side of the heart, which are used to assess whether coronary artery blockages exist and then intervene to prevent the further occlusion of coronary arteries.
Because of the high risk of complications, physicians generally prescribe PAC only for critically ill patients. In the non-sterile environment of a physician’s office or outpatient clinic, PAC is simply unavailable. As a result, in the majority of situations, a physician seeking to assess hemodynamics normally must do so through indirect means, such as by measuring blood pressure, checking the pulse, looking at neck veins and employing subjective examination techniques that are prone to human error. A compelling need exists for objective, noninvasive measurement tools, such as our BioZ® ICG Systems.
During ICG monitoring using our BioZ ICG Systems, an undetectable electrical signal is sent through our proprietary sensors placed on the patient’s neck and chest. Our DISQ® (Digital Impedance Signal Quantifier) and AERIS™ (Adaptive Extraction and Recognition of Impedance Signals) processing analyzes ICG waveforms and the Z MARC® Algorithm is used to calculate significant hemodynamic parameters. Based on this data, a physician can quickly and safely assess and diagnose the underlying cardiovascular disorder, customize and target treatment, monitor the effectiveness of prescribed medications and more accurately identify potential complications.
Our objective is to enhance patient lives through pioneering a new approach to drug management and to make a genuine contribution to healthcare economics with our noninvasive technologies. Key elements of our strategy include efforts to:
| | • | | aggressively market and sell ICG products through our direct sales force; |
| | • | | broaden our product offerings and distribution channels through strategic relationships; |
| | • | | grow recurring revenue through increased use of our proprietary disposable sensors; |
| | • | | expand evidence of our technology’s validity and clinical application in our target markets; |
| | • | | maintain market leadership through product improvements and extensions; and |
| | • | | target new market opportunities through acquisition and technology development. |
Investors wishing to obtain more information about CardioDynamics may access our annual, quarterly and other reports and information filed with the SEC. Investors can read and copy any information the Company has filed with the SEC at the SEC’s Public Reference Room at 450 Fifth Street, NW, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us. The Company also maintains an Internet site (www.cdic.com) where we make available, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and any amendments to those reports, as well as Section 16 filings, as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. The information on our website is not incorporated by reference into this annual report on Form 10-K.
Industry Overview and Company History
Our proprietary technology provides medical professionals in the hospital and physician’s office with noninvasive access to objective patient data to effectively assess, diagnose and treat heart failure, high blood pressure, shortness of breath, emergency, pacemaker and dialysis patients.
In the hospital setting, the BioZ is a noninvasive, cost-effective and safe alternative to the invasive PAC procedure and may also be used in many situations in which PAC is not feasible. However, the advantages of our proprietary technology are not limited to the hospital or the critically ill. We believe that the greatest current potential for the BioZ product line lies in the use of noninvasive hemodynamic measurements in the physician office. We believe that the worldwide market opportunity is over five billion dollars for our BioZ product line.
4
We envision expanding the domestic market from the current 1.2 million annual invasive PAC procedures to over 72 million annual noninvasive ICG procedures, as summarized in the chart below. Assuming total worldwide procedures are approximately double those in the United States, there would be over 140 million potential procedures worldwide. These figures do not include home healthcare monitoring, which we believe could, if implemented, significantly increase demand for our technology.
Potential Annual Noninvasive Monitoring Procedures
Patients in the United States
| | | | | | |
Disease State
| | US Patients
| | Potential Annual
Monitoring
Procedures/Patient
| | Total Annual
Procedures
|
Heart Failure | | 5,000,000 | | 4.0 | | 20,000,000 |
Hypertension | | 50,000,000 | | 0.5 | | 25,000,000 |
Emergency Departments | | 20,000,000 | | 1.0 | | 20,000,000 |
Pacemaker | | 1,100,000 | | 2.0 | | 2,200,000 |
Dialysis | | 450,000 | | 12.0 | | 5,400,000 |
TOTAL | | | | | | 72,600,000 |
Strategy
Our objective is to enhance patient lives through pioneering a new approach to drug management and to make a genuine contribution to healthcare economics with our noninvasive technologies. Our objective will be achieved if and when noninvasive ICG technology becomes a cardiovascular standard of care. We intend to position BioZ ICG technology as a key diagnostic and monitoring tool for assessing and treating heart failure, hypertension, shortness of breath, pacemaker, emergency, critically ill, surgical, high-risk obstetric, dialysis and home healthcare patients. Our corporate strategy includes:
Aggressively market and sell ICG products through our direct sales force.
We intend to leverage our direct sales force to capitalize on our first-to-market position in the United States to further penetrate the physician office market. We believe that a strong direct sales force supplemented by distributor partners to increase access to physicians, and our clinical application specialists to clinically train is best suited to educate the medical community about how our technology can improve patient outcomes and decrease costs. We currently have 46 domestic direct sales representatives who sell our products, as well as four regional sales managers, and a vice president of sales. In addition, we have 18 clinical application specialists, two regional clinical managers and a national clinical applications director to supplement our field sales team by enhancing customer understanding, usage and satisfaction. By improving device utilization, we believe we can strengthen customer loyalty and increase recurring revenue from our proprietary sensors.
Broaden our distribution channels through strategic relationships.
We plan to establish strategic relationships with major patient monitoring and diagnostic cardiology companies, pacemaker manufacturers and other medical products and technology companies to increase the availability of our proprietary technology. We believe that strategic relationships can accelerate market penetration of BioZ ICG technology in markets not served by our direct sales team and provide us with access to the large installed bases of patient monitoring, cardiology and other complementary medical equipment.
Grow recurring revenue through increased use of our proprietary disposable sensors.
During fiscal 2000, we successfully developed and received FDA 510(k) clearance on our patented BioZtect® sensor technology that provides notable improvements in performance and features. Its unique shape and chemical composition, adhesion characteristics and more user-friendly design optimize signal transmission
5
and detection sensitivity. Our proprietary sensor and cable systems provide enhanced features to our customers and promote the exclusive use of our proprietary sensors with our equipment to ensure optimal product performance and accuracy. During fiscal 2004, we acquired Vermed, an ECG electrode manufacturer that accounted for 25% of our net sales in 2005. The Vermed acquisition provided vertical integration allowing in-house advanced product research and development and manufacturing of our ICG sensors. As our installed base of BioZ products grows, and our ECG sensor business grows, we expect that the disposable sensor revenue stream could account for more than half of our total net sales within the next several years.
Expand evidence of the technology’s validity and clinical application in our target markets.
While a significant amount of evidence substantiating ICG’s validity and clinical application is now available, we will continue to invest in supporting clinical trials to further expand this evidence and provide prospective customers with data regarding the efficacy of ICG. Several multi-center clinical trials including studies in outpatient heart failure (PREDICT) and emergency department shortness of breath (ED-IMPACT) were presented in late 2004. A multi-center trial in hypertension (CONTROL) was presented in May 2005. All three of these studies have been submitted by the investigators to leading peer-reviewed medical journals and we anticipate they will be published in 2006.
Maintain market leadership through product improvements and extensions.
We intend to advance the development of our core algorithms to provide physicians with improved cardiac function measurement capabilities on a broad class of patients. We believe that continued advances in our ICG technology will increase physician usage and loyalty and strengthen our industry position. We will capitalize on our expertise in ICG signal processing and sensor technology to improve system performance in the presence of signal noise and patient movement thereby leading to additional applications for cardiovascular disease management.
In 2001, we released the BioZ® ICG Module for the GE Medical Systems Information Technologies (GEMS-IT) bedside monitoring systems. This product is distributed worldwide by GEMS-IT for their Solar® 7000, 8000, 8000M, and DASH 3000, 4000 patient monitors. In July 2002, we signed a Co-Development and OEM Agreement with Philips, a worldwide leader in clinical measurement and diagnostic solutions for the healthcare industry. As a result of the Co-Development, in December 2004, CardioDynamics received 510(k) clearance by the U.S. Federal Drug Administration (FDA) for our new product the BioZ Dx ICG Diagnostics device. We released this product in the beginning of 2005. In June 2005, we received FDA 510(k) clearance for 12 lead diagnostic ECG capabilities on the Dx ICG platform. Philips has the right to purchase ICG modules from us and sell the combined ECG/ICG product in their key markets.
Target new market opportunities through acquisitions of complementary technologies and technology development.
In 2004, we acquired two companies, Medis (a German ICG device design and manufacturer) and Vermed (a Vermont-based electrode manufacturer). The Medis acquisition strengthens our core ICG technology development capabilities and provides us with a European partner for market development opportunities in that region. The Vermed acquisition provides vertical integration of our disposable sensor business, protecting and increasing the margin on that important revenue source.
We will continue to focus on new applications for our core technology. Advances in ICG technology could be applied in the areas of sensor technologies, pacemaker optimization, fluid management (including dialysis), oncology, drug noncompliance, and pharmaceutical development and testing. Pharmaceutical companies such as GlaxoSmithKline, Eli Lilly and Co. and Pfizer Inc. are currently using our technology to document the cardiovascular effects of their pharmaceutical agents in both animals and humans.
6
Continued innovation and commercialization of new proprietary products are essential elements in our long-term growth strategy. We intend to maintain our competitive advantage by acquiring complementary technologies and additional patents and other proprietary rights, as we deem appropriate.
ICG Technology
While ECG technology noninvasively measures the heart’selectrical characteristics, our ICG technology makes it possible to measure the heart’smechanical, or blood flow, characteristics. By using our products, physicians have an easy, noninvasive, safe, painless and cost-effective way to monitor the heart’s ability to deliver blood to the body.
In order to measure this conductivity change, our BioZ products use four dual sensors (two on the neck and two on the chest) to deliver a high frequency (70 kHz), low magnitude (4 mA), alternating current through the chest that is not felt by the patient. Our BioZ ICG Monitor uses proprietary DISQ® and AERIS™ processing which measures the changes in impedance to the electrical signal. The changes in impedance are then applied to the Z MARC® Algorithm to provide cardiac output, the amount of blood pumped by heart in one minute. Additional parameters that are provided include those indicating; blood flow from the heart, the resistance the heart is pumping against, the force the heart is contracting, and the amount of fluid in the chest. These parameters are printed on a report that allows the doctor to customize and optimize treatment for a particular patient.
Some physical and medical conditions may diminish the accuracy of the measurements provided by our products; therefore, use of our BioZ ICG products in such cases is not appropriate. We believe that inaccuracies are most likely to occur in patients who are experiencing severe septic shock, severe aortic valve regurgitation, severe irregular ventricular heartbeats or heart rates greater than 180 beats per minute. In addition, there is inadequate data demonstrating the accuracy of our products in patients who are shorter than 47 inches or who weigh less than 66 pounds or more than 342 pounds, as well as in patients who move excessively during the BioZ procedure.
Pricing
Our products have established list prices and we discount the list prices of our products in some circumstances based primarily upon volume commitments or marketing promotions. We also provide discounts on the purchase of refurbished equipment and to distributors who perform sales and customer service functions for us.
Segments
We focus our business on two principal operating segments: Impedance Cardiography (“ICG”) and Electrocardiography (“ECG”). Note 3 “Segment Reporting,” to our financial statements describes these segments. The principal products in each of these segments consist of the following:
· ICG Segment
BioZ® Dx ICG Diagnostics
In December 2004, we received FDA 510(k) clearance on the BioZ Dx, which resulted from a co-development partnership and OEM Agreement with Philips. The BioZ Dx has significant improvements with AERIS™ processing and optional 12-lead ECG capability. It also features an integrated full-page thermal printer, color display screen, a standard five-year warranty and a new Thera-Trak™ reporting function that allows physicians to automatically compare a patient’s last ICG report to the current ICG report. Commercial shipments of the BioZ Dx commenced in the first quarter of 2005.
7
BioZ® ICG Monitor
Our noninvasive cardiac function monitoring device, the BioZ® ICG Monitor, features a portable design, transportable battery and integrated blood pressure. BioZ ICG Monitors are sold with a pole cart, printer and keyboard for end user data entry and include a standard one-year warranty.
BioZ® ICG Module
The BioZ ICG Module was jointly developed with GEMS-IT. The module integrates our proprietary BioZ ICG technology into GE’s Solar® and DASH patient monitoring systems and includes a standard one-year warranty.
BioZtect® Sensors
We market disposable sensors designed specifically for use with our BioZ® products. Four of our patented dual sensors are used in each monitoring session. Our proprietary sensor and cable systems provide enhanced features to our customers and promote the exclusive use of our proprietary sensors with our equipment to ensure optimal product performance and accuracy.
Niccomo ICG Monitor
The Medis Niccomo ICG monitor is sold through our international sales force outside the United States. It incorporates a color touch screen and integrated strip printer and includes a standard one-year warranty. Medis also manufactures and sells the Cardioscreen and Rheoscreen product lines of venous blood flow products that are sold internationally. None of these Medis products have FDA clearance for sale in the U.S.
· ECG segment
ECG Electrodes
Vermed manufactures and markets a large number of ECG electrodes for resting, stress, and ambulatory applications and other diagnostic procedures for cardiology, electrotherapy, sleep testing, neurology and general purpose diagnostic testing. Vermed also manufactures private label ECG electrodes for distributors and medical device companies.
Backlog
We do not generally carry order backlog in our ICG business, however order backlog in our ECG business was $1,025,000 and $1,118,000 as of November 30, 2005 and 2004 respectively. The orders included in our backlog are generally credit approved customer purchase orders expected to ship within the next 12 months.
Sales and Distribution
We view the United States ICG marketplace as two distinct segments: the outpatient (physician) market and the hospital market. In the outpatient market, we target physician offices and hospital-based and freestanding outpatient facilities for our stand-alone BioZ® products through our direct sales force and distributors. In 2004, we initiated distributor sales efforts with Physician Sales and Service (PSS) and Caligor Medical (Henry Schein, Inc.) however, at the end of 2005 we determined once again to put primary emphasis on our internal sales force to drive product acceptance. In contrast to the hospital market, there are few, if any, formal capital equipment budget processes in the outpatient market and purchasing decisions can therefore be made more quickly. Consequently, our direct and distributor sales force is focused primarily on the outpatient markets.
We continue to believe that the hospital market represents a large and viable market for our products. In 2004, we piloted a non-capital, usage-based program targeted to increase hospital market penetration but due to
8
the complex sale involving numerous decision makers and 24 hour support required, the investment did not generate an adequate return compared to the outpatient market. Therefore, we decided that for the present time the vast majority of our focus should continue on the outpatient market.
Internationally, we sell our products through local medical distributors. Currently, we have distribution partners and end-users in more than 30 countries around the world. Additionally, our international sales team supports GEMS-IT sales teams in selling our ICG Module that interfaces with the GEMS-IT Solar and DASH monitoring systems. We do not offer product return rights to our distributors.
Strategic Relationships
During the fourth quarter of fiscal 2000, we entered into an agreement with GEMS-IT for the development of a custom plug-in module for the GEMS-IT Solar® and DASH series of bedside monitors. This product was introduced to the market in June 2001 and extends the capabilities of the GEMS-IT Solar product family to provide all of the hemodynamic parameters of the BioZ ICG Monitor to GEMS-IT’s installed customer base of over 30,000 units. This product is distributed worldwide by CardioDynamics and GEMS-IT for their Solar® 7000, 8000, 8000M, and DASH 3000, 4000 patient monitors. We believe that other patient monitoring companies could benefit from the addition of similar modules to their estimated installed base of over 200,000 modular bedside monitors.
In July 2002, we signed a Co-Development and OEM Agreement with Philips Medical Systems. The joint product development combines CardioDynamics’ proprietary ICG technology with Philips’ diagnostic 12-lead ECG. We released both an ICG-only device with the jointly developed platform in the beginning of 2005, and a combined ICG/ECG device in mid-2005. The Co-Development and OEM agreement allows both companies to market the combined ICG/ECG product, although only CardioDynamics is doing so at the current time.
Medis entered into a technology licensing relationship with Analogic Corporation in March 2001. Under the agreement, Medis licensed their ICG circuit board and software design to Analogic as a key component to their own ICG monitor. This product, called the LifeGard Monitor, was released in 2004, and is also sold by Philips as a stand-alone ICG monitor under the Philips brand. We receive a licensing fee each time an Analogic or Philips ICG device is sold.
Medicare and Other Third-Party Reimbursement
In the outpatient market, most medical procedures are reimbursed by a variety of insurance sources, including Medicare, Medicaid and private insurers. CMS, which is the governmental body that approves medical services for financial reimbursement under Medicare and Medicaid, determines whether to reimburse for a given procedure and assigns an amount allowed. In September 1998, the CMS mandated Medicare coverage of Electrical Bioimpedance services, such as the CardioDynamics BioZ, on a national basis. The established Medicare coverage for BioZ ICG Systems has improved our ability to penetrate the outpatient market, as Medicare provides health insurance to approximately 50 million people in the United States.
In November 2000, CMS established a uniform national pricing level for the use of our equipment. In January 2002, the American Medical Association issued a formal Level I HCPCS procedure code, (also referred to as a CPT Code) for BioZ ICG technology. The code is 93701.
In December 2002, CMS initiated a reconsideration of ICG’s indications for use. In January 2004, CMS issued an updated national coverage determination. Of the six indications previously indicated, five are substantially unchanged. One indication, “suspected or known cardiovascular disease,” has been revised to specifically allow CMS carrier discretion in the coverage of resistant hypertension. Resistant hypertension is defined by CMS to include patients with uncontrolled blood pressure (greater than or equal to 140 mm Hg systolic blood pressure and/or 90 mm Hg diastolic blood pressure) on three or more anti-hypertensive
9
medications. This change served to restrict the number of hypertensive patients eligible for CMS reimbursement for ICG monitoring. The other current CMS indications are as follows:
| | • | | Optimization of fluid management in patients with heart failure. |
| | • | | Differentiation of cardiogenic from pulmonary causes of acute dyspnea. |
| | • | | Optimization of atrioventricular (A/V) interval for patients with A/V sequential cardiac pacemakers. |
| | • | | Monitoring of continuous inotropic therapy for patients with terminal heart failure, when those patients have chosen to die with comfort at home, or for patients waiting at home for a heart transplant. |
| | • | | Evaluation for rejection in patients with a heart transplant as a predetermined alternative to a myocardial biopsy. |
Some private insurers cover the BioZ ICG test, including Aetna, Humana, BlueCross, BlueShield and others (in select states). We continue active discussions with CMS and private insurers to maintain and expand reimbursement indications for ICG.
Marketing
Our primary prospects in the outpatient market include cardiologists, internal medicine physicians and family practitioners caring for heart failure, hypertension, shortness of breath, and pacemaker patients. Patients in the United States who may benefit from our technology include the 65 million hypertension patients, five million heart failure patients, over one million pacemaker patients, and 20 million patients with a sudden onset of shortness of breath. Our marketing strategy is designed to:
| | • | | increase physician and hospital personnel knowledge of ICG technology; |
| | • | | demonstrate the ability of the BioZ ICG Systems to assist physicians in the objective identification and appropriate pharmacological treatment of heart failure, hypertension, and shortness of breath patients; |
| | • | | show the ability of the BioZ ICG Systems to assist physicians in the optimization of pacemakers; |
| | • | | demonstrate cost savings of providing ICG monitoring to patients through more efficient care and reimbursement through CMS-mandated Medicare and private insurers; and |
| | • | | educate physicians and hospital staff of the importance of hemodynamics in the treatment of patients who would normally not be monitored with a PAC due to practice setting, costs and complications. |
Our marketing promotion strategy is based on key medical conference participation, direct mail programs, internet-based product and clinical information, and live and direct mail clinical education literature.
Research and Development
Our research and development team, which consists of both scientific and engineering professionals, has extensive experience in the areas of ICG, physiologic signal processing, hardware and software development, and regulatory compliance. The team is responsible for on-going product engineering, new product development and basic research into ICG technology and additional noninvasive monitoring applications.
Our team continues to investigate the physiologic mechanisms underlying our ICG signal as a means of developing new diagnostic parameters. In addition, we are continuously researching the application of digital signal processing methodologies to improve the quality of signal acquisition and analysis algorithms. Some of this research has resulted in several U.S. patents issued and patents pending. Our expenditures that have been accounted for as research and development were $2,487,000 (6.7% of net sales) in 2005, $4,353,000 (10.6% of net sales) in 2004, $3,272,000 (10.8% of net sales) in 2003.
To supplement our internal development team, we retain Rivertek Medical Systems, Inc. (Rivertek) as an adjunct to our development efforts, of which the Chief Technology Officer of the Company is a 100% beneficial
10
owner. See Item 13. Certain Relationships and Related Transactions in this Report on Form 10-K. Rivertek is located in Minneapolis, Minnesota and serves as an engineering consulting firm for medical device manufacturers, including Guidant Corporation and Medtronic, Inc., as well as emerging medical technology companies. Subsequent to fiscal year end, in December 2005, Dennis Hepp retired from services as CardioDynamics’ Chief Technology Officer.
Intellectual Property
Our success, to some extent, depends on our ability to maintain patent protection for our products and processes, to preserve our trade secrets and proprietary technology and to operate without infringing upon the patents or proprietary rights of others. We have developed proprietary software for which we have not filed patents. We generally file patent applications in the United States and foreign countries where patent protection for our technology is appropriate and available. We have filed nine U.S. utility patent applications and three design patent applications, six of which were issued in 2003. Of the three utility patents that were issued, a key patent isU.S. Patent Number 6,561,986, “Method and Apparatus for Hemodynamic Assessment Including Fiducial Point Detection,” which contains 46 claims and is a strategic patent underlying the Company’s novel AERIS™ (Adaptive Extraction & Recognition of Impedance Signals) processing. AERIS utilizes breakthrough techniques in time-scale signal processing to filter and accurately determine key ICG and ECG waveform characteristics, known as “fiducial points.” ICG and ECG fiducial points form the core measurements from which BioZ parameters are determined. AERIS processing provides enhanced stability, accuracy, and reproducibility in a broader range of patient monitoring conditions.
Another utility patent, U.S. 6,636,754, relates to our electrode technology protection. Our three design patents that were issued in 2003 cover various design aspects of the Company’s BioZtect sensors. The intellectual property protection applies to sensors for use with both its BioZ ICG Monitor and BioZ ICG Module. The BioZtect sensors offer notable improvements in safety and signal transmission and detection, which are critical for device performance. During 2005 we became aware of a company that was selling non-FDA cleared ICG sensors for use with our BioZ systems. We believe their sensors are in violation of our patent and have filed a patent infringement suit. They have in turn countersued to have our patent declared invalid and for tortious interference with contracts, unlawful tying and unlawful restraint of trade. While we are attempting to resolve the matter amicably, if it cannot be resolved, we intend to vigorously defend our intellectual property rights. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect trade secrets and other proprietary technology.
Clinical Studies
We are committed to supporting well-designed clinical research studies utilizing ICG technology that demonstrate validity, reproducibility, clinical utility and cost-effectiveness. Our clinical research team participates in monitoring and analysis of company-sponsored clinical trials, for which we employ clinical research associates, and the support of multiple investigator-initiated trials. Each of these individuals has a strong clinical research background and is responsible for monitoring clinical studies and assisting in research and customer training.
Previous generation technology
Several hundred research papers on ICG technology have been published since 1993. In general, these studies reported mostly favorable results when comparing cardiac output measurements with those of other techniques, such as PAC.
The previous generation technology we acquired in 1993 worked reasonably well in a select group of patients. However, significant technological limitations became evident when monitoring ventilated patients and those with increasing heart rates, high heart rates, abnormal heartbeats, high respiration rates and pacemakers. These limitations related to both hardware and software inadequacies. As a result of intense research and
11
development focus and concerted effort, combined with advances in computer processing power, CardioDynamics has addressed these limitations by improving the electronics, digital signal processing, and parameter computation algorithms.
New technology
As studies are conducted with our new technology, their results are summarized first as abstracts, and then as manuscripts that move through the peer review process towards publication. This process can take two years or more to complete. The results of several major studies addressing each of these areas have been released with positive results.
In May 2002, the results of a significant Mayo Clinic study were published in the peer-review journal, Hypertension. The results of the study demonstrated 70% superiority in effectively treating previously-uncontrolled hypertension patients when our BioZ® ICG was used as compared to traditional management by high blood pressure specialist physicians.
In September 2004, the results of the ED-IMPACT trial (Impedance Cardiography-Aided Assessment Changes Therapy in Emergent Dyspnea) were presented at the Heart Failure Society of America. The study demonstrated the impact of ICG data upon diagnosis and treatment in patients short of breath in the emergency department. The results demonstrated a 39% change in therapeutic plan and 12% change in diagnosis, which were considered very significant findings.
In November of 2004, the results of the PREDICT trial (Prospective Evaluation and Identification of Cardiac Decompensation in Patients with Heart Failure by Impedance Cardiography Test) were presented at the American Heart Association by principal investigator, Dr. Milton Packer, who led 21 top U.S. heart failure centers in the study. The study was designed to show whether ICG variables could predict whether a heart failure patient would die or be hospitalized. The results showed that of all the variables measured in the study, ICG was the most powerful predictor of death or hospitalization. A patient with a high risk ICG test was over 8 times more likely to die or be hospitalized in the short-term (2 weeks) than a patient with a low risk ICG test.
In May of 2005, the results of the 11 center multi-center CONTROL trial (Consideration of Noninvasive Hemodynamic Monitoring to Target Reduction of Blood Pressure Levels) were presented at the American Society of Hypertension 20th Annual Scientific Meeting. This study was designed to evaluate the community-based treatment of mild to moderate hypertension patients (vs. the Mayo Clinic Study that was conducted in more severe hypertension treated by specialists). We evaluated the reduction of blood pressure and the achievement of blood pressure control in patients treated with and without BioZ ICG. The results of the study demonstrated that use of BioZ® ICG achieved significantly greater reductions in blood pressure (8 mm Hg systolic and 7 mm Hg diastolic) more than two times better than standard care for achievement of blood pressure control to 130/85 mm Hg.
In addition, multiple other ICG studies have been published in journals such asAcademic Emergency Medicine, Chest, American Journal of Cardiology, andCongestive Heart Failure.
Strategic Future Trial
We are sponsoring an international multi-center trial in heart failure patients which will evaluate whether the predictive power of ICG as demonstrated in PREDICT study can be used to change medical managements and subsequently reduce heart failure hospitalizations, as compared to standard care without the use of ICG. The study is called PREVENT—Prevention of Heart Failure Events with Impedance Cardiography Testing and is expected to commence by mid-2006.
12
Manufacturing
Our products are manufactured in San Diego, California; Bellows Falls, Vermont; and Illmenau, Germany. The CardioDynamics headquarters in San Diego includes the manufacturing and service facility for the CardioDynamics BioZ ICG systems. The Vermed subsidiary in Bellows Falls is a wholly owned subsidiary that manufactures and packages disposable electrodes and related supplies utilized in ECG, ICG and other diagnostic procedures. The Medis subsidiary in Illmenau is a majority owned subsidiary that manufactures ICG and venous blood flow products, including the Rheoscreen product line, Cardioscreen and Niccomo ICG monitors.
Each location has established procedures and controls intended to ensure that both products and purchased parts are designed and manufactured to meet customers’ requirements. We purchase the components and raw materials used in manufacturing our products from various suppliers. Our suppliers are evaluated, qualified and monitored to assure continuity of supply while maintaining high quality and reliability. We have systems and procedures in place to ensure timely and effective corrective and preventive actions are taken if we, or our customers, identify non-conformities.
Warranty and Repair
We warrant that our stand-alone BioZ Dx System will be free from defects for a period of 60 months from the date of shipment on each new system sold in the United States, and for 12 months on BioZ Dx systems sold internationally. Medis warrants that the Niccomo ICG monitor will be free from defects for 12 months. We warrant that new stand-alone BioZ Monitors and factory certified refurbished BioZ Dx and BioZ Monitor systems will be free from defects for a period of 12 months from the date of shipment. The warranty includes all options and accessories purchased with the system, except for the external patient cables, the external printer, power cords, and inflatable blood pressure cuffs that are covered for a period of 90 days. When warranty repairs are necessary, we generally perform them at our San Diego facility. In some cases, our distributors perform repairs in authorized service centers. In 2004, we added an additional International service center in Dubai, United Arab Emirates to fulfill the needs of the growing Middle-eastern market for ICG.
We provide on-call technical support and, on occasion, offer field clinical support specialists. In addition to our standard warranty, we offer Z Care® extended warranty agreements for maintenance beyond the standard warranty period. We repair equipment that is out of warranty on a time and materials basis.
Competition
Direct competition
To date, we have experienced very limited direct competition. Through our recently acquired German subsidiary, Medis, we inherited a licensing agreement and relationship with Analogic Corporation, which manufactures a stand-alone ICG device for Philips as well as an Analogic-manufactured device, the Lifegard Monitor, which is distributed through a small medical device manufacturer and distributor, Advanced Cardiac Systems (ACS). The Philips stand-alone device is primarily sold into the hospital market where we have not traditionally focused. The Lifegard Monitor is primarily sold in the physician office market and has a suggested retail price that is substantially lower than our BioZ ICG Monitors. The Lifegard Monitor represents the most significant form of competition we have experienced to-date, however, we believe that since its introduction in late 2004, we have lost fewer than ten competitive accounts in the U.S. market to the Lifegard Monitor. We are also aware of at least two domestic and one international manufacturer of ICG monitors. None of these companies have direct sales or clinical teams, and thus far, have had very little visibility in the market. We believe that our BioZ products provide the most advanced ICG monitoring and commercially attractive designs, at prices that are competitive.
13
Indirect competition
PAC
Also known as thermodilution, right heart catheterization or Swan-Ganz™ catheterization, the PAC procedure was introduced in the early 1970’s. Despite its limitations, costs and risks, PAC remains the most commonly used technology for monitoring hemodynamic status. Medical Data International estimates that PAC procedures are used nearly two million times per year worldwide. Edwards Lifesciences, Abbott Laboratories and Datex-Ohmeda produce the majority of right heart catheters used in the United States.
ICG technology may eliminate PAC-caused complications, lower costs, reduce procedure time, expand clinical applications and offer immediate availability of vital, real-time, continuous hemodynamic data.
Echocardiography
Echocardiography (echo) is a diagnostic tool utilizing ultrasound frequency waves to detect anatomical abnormalities of the heart and blood vessels. Echo technology was developed during the 1970’s and has advanced through the years with the addition of sophisticated electronics and digitalization for acquisition of better images. A continuous wave suprasternal Doppler echo measures cardiac output noninvasively by placing a Doppler transducer on the chest, aiming it toward the ascending aorta and measuring aortic blood flow velocity. Specifically, echo measures the aortic diameter and the movement of red blood cells to determine the velocity and direction of blood flow to calculate stroke volume and thus calculate cardiac output. While it is possible, echo is not routinely used to measure cardiac output because of its technological limitations, cost, time, and lack of reimbursement for this purpose.
Trans-esophageal echo
Trans-esophageal echo is an ultrasound advancement that is used to obtain closer images of the heart. It is useful in patients for whom examination from the usual external position is technically impossible or for hospitalized patients undergoing cardiac surgery. Trans-esophageal echo is performed with the ultrasound transducer placed in the esophagus through the mouth. Although this procedure enables more direct, accurate images of the heart, disadvantages include its invasive nature, increased patient discomfort and the requirement for patient sedation to promote procedure tolerance. In addition, patient airway complications may result, therefore emergency equipment, such as oxygen, intubation equipment and ECG monitoring must be immediately available. The procedure is customarily performed with several attendants, including an echo technician, a nurse and a physician.
Direct and Indirect Fick
Direct Fick was the original method conceived in the late 1800’s to measure cardiac output. It is based on calculating the oxygen difference between the arterial and venous blood, along with oxygen inhalation and expiration. The direct Fick method is seldom used because it is time consuming, costly and complicated. A variation of the direct Fick method is called CO2 Re-breathing, or Indirect Fick. It was introduced in the 1980’s to the hospital surgical market. Because CO2 Re-breathing method is limited to patients who are mechanically ventilated, the number of patients who are candidates for the procedure is severely limited.
Government Regulation
Our products are classified as medical devices subject to regulation in the United States by the Food and Drug Administration (FDA). New products generally required FDA clearance under a procedure known as 510(k) pre-market notification. A 510(k) pre-market notification clearance indicates FDA agreement with an applicant’s determination that the product is substantially equivalent to another marketed medical device. Our products generally are Class II products with the FDA. Delays in receipt of, or failure to obtain or maintain, regulatory clearances and approvals, or any failure to comply with regulatory requirements, could delay or prevent our ability to market our product line.
14
The Federal Food, Drug and Cosmetic Act, its subsequent amendments and modernization acts, and similar foreign regulations, require that medical devices be manufactured in accordance with good manufacturing practices and quality system requirements. Our manufacturing processes and facilities are subject to periodic on-site inspections and continuing review by applicable regulatory bodies to ensure compliance with Quality System regulations. We believe that our products currently meet applicable standards for the countries in which they are marketed.
We are required to report to the FDA and international agencies information that a device has or may contribute to a death or a serious injury. We also may be subject to product recalls. No such report or recall has had a material effect on our financial condition or prospects, but there can be no assurance that regulatory issues may not have a material adverse effect in the future.
We are subject to various environmental laws and regulations. Like other medical device companies, our operations involve the use of substances regulated under environmental laws, primarily in manufacturing processes. While it is difficult to quantify the potential impact of compliance with environmental protection laws, we believe that we are in material compliance with current environmental standards and that continued compliance will not have a material impact on our financial position or prospects, results of operations or liquidity.
Failure to comply with applicable governmental regulations can result in various penalties, including fines, recalls or seizure of product, total or partial suspension of production, refusal or delay in product approvals or clearances, increased quality control costs or criminal prosecution. Any change in existing federal, state or foreign laws or regulations, or in the interpretation or enforcement thereof, of the promulgation of any additional laws or regulations could have an adverse effect on our business, financial condition, prospects, results of operations or cash flows.
In order to sell our products within the European community, we must comply with the European Commission’s medical device directive. In late 1998, we received authorization from TUV Rhineland of North America to place the CE mark on our BioZ ICG Monitor. The CE mark is recognized worldwide as an essential European regulatory approval and enabled us to expand our sales and distribution of the BioZ ICG Monitor throughout Europe. Future regulatory changes could limit our ability to use the CE mark, and any new products we develop may not qualify for the CE mark. If we fail to obtain authorization to use the CE mark or lose this authorization, we will not be able to sell our products in the European community. In December 2005, we had our annual compliance review and we passed without any significant issues.
In May 2000, we received approval from the State Drug Administration of the People’s Republic of China, and in November 2000, we received a Canadian Medical Device License. In February 2002, our distribution partners received MHLW and KFDA approvals, enabling our products to be sold in Japan and Korea.
Employees
As of November 30, 2005, we had 233 employees, none of whom are covered by a collective bargaining agreement. We consider our employee relations with our employees to be good.
15
In addition to the other information contained in this Form 10-K, you should consider the following risk factors which could affect our business, financial condition and results of operations.
We depend upon on our BioZ product line, which is in its early stages of market acceptance.
Our future is dependent upon the success of the BioZ product line and similar products that are based on the same core technology. The market for these products is in a relatively early stage of development and may never fully develop as we expect. The long-term commercial success of the BioZ product line requires widespread acceptance of our products as safe, efficient and cost-effective. Widespread acceptance would represent a significant change in medical practice patterns. In the past, some medical professionals have hesitated to use ICG products because of limitations experienced with older, analog-based monitors. Invasive procedures, such as PAC, are generally accepted in the medical community and have a long history of use.
We have sponsored and intend to continue to sponsor or support clinical trials. We cannot be certain that clinical trials will be completed, that they will have a positive outcome or that a positive outcome in these trials will be sufficient to promote widespread acceptance of our products within the medical community.
Our success depends in part upon the availability of adequate third-party reimbursement.
Our success will depend in part on the availability of adequate reimbursement for our customers from third-party healthcare payers, such as Medicare, private health insurers and managed care organizations. Third-party payers increasingly are challenging the pricing of medical products and services. Reimbursement may not be at, or remain at, price levels adequate to allow medical professionals to realize an appropriate return on the purchase of our products. In addition, third-party payers may not cover all or a portion of the cost of our products and related services, or they may place significant restrictions on the circumstances in which coverage will be available. In January 2004, CMS issued an updated national coverage determination which served to restrict the number of hypertensive patients eligible for Medicare reimbursement for ICG monitoring.
We must maintain and develop strategic relationships with third parties to increase market penetration of our product lines.
We distribute our products to targeted international markets through our strategic alliance with GEMS-IT and a network of regional distributors and to the US market through our direct sales force and several regional and national distributors. We may enter into similar agreements with other companies and establish technology partnerships with other medical product, distribution and technology companies. Successfully managing the interaction of our direct sales force and strategic distribution partners is a complex process. Moreover, since each distribution method has distinct risks, gross margins and operation costs, our failure to implement and maintain the most advantageous balance in the delivery model for our products could adversely affect our revenues, gross margins and profitability. Widespread acceptance of our BioZ products may be dependent on our establishing and maintaining these strategic relationships with third parties and on the successful distribution efforts of third parties.
Many aspects of our relationships with third parties, and the success with which third parties promote distribution of our products, are beyond our control. We may be unsuccessful in maintaining our existing strategic relationships and in identifying and entering into future development and distribution agreements with third parties.
We may not be able to make future acquisitions or successfully integrate our acquisitions.
As part of our growth strategy, we intend to continue to pursue opportunities to acquire or make investments in other technologies, products and businesses that could enhance our technical capabilities, complement our current products or expand the breadth of our markets or customer base.
16
Potential and completed acquisitions and strategic investments involve numerous risks, including:
| | • | | problems assimilating the purchased technologies, products or business operations; |
| | • | | unanticipated cost associated with the acquisition, including accounting charges and transaction expenses; |
| | • | | problems implementing and maintaining adequate standards, procedures, controls and policies; |
| | • | | diversion of management’s attention from our core business; |
| | • | | adverse effects on existing business relationships with suppliers and customers; |
| | • | | risks associated with entering markets in which we have no or limited prior experience; and |
| | • | | potential loss of key employees of acquired organizations. |
If we fail to properly evaluate and execute acquisitions and strategic investments, our management team may be distracted from our day-to-day operations, our business may be disrupted, and our operating results may suffer. In addition, if we finance acquisitions by incurring debt or issuing equity, our existing shareholders would be diluted or we would have to generate additional profits to cover the interest expense.
Our future financial results could be adversely impacted by asset impairments or other charges.
Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” requires that we cease amortization of goodwill and other intangible assets determined to have indefinite lives, and establish a method testing these assets for impairment on an annual or on an interim basis if certain events occur or circumstances change that would reduce the fair value of a reporting unit below its carrying value or if the fair value of intangible assets with indefinite lives falls below their carrying value.
We evaluate intangible assets determined to have finite lives for recoverability whenever events or changes in circumstances indicate that their carrying amounts may not be recoverable. These events or circumstances could include a significant change in the business climate, legal factors, operating performance indicators, competition, sales or disposition of a significant portion of the business, or other factors such as a decline in our market value below our book value for an extended period of time. In the case of intangible assets with indefinite lives, we evaluate whether events or circumstances continue to support an indefinite useful life.
We also evaluate the estimated lives of all intangible assets on an annual basis, including those with indefinite lives, to determine if events and circumstances continue to support an indefinite useful life or the remaining useful life, as applicable, or if a revision in the remaining period of amortization is required. The amount of any such annual or interim impairment charge could be significant, and could have a material adverse effect on reported financial results for the period in which the charge is taken.
Utilization of our deferred tax assets may be limited and is dependant on future taxable income.
We have federal operating loss carryforwards of approximately $28,400,000 for federal income tax purposes. The Tax Reform Act of 1986 contains provisions under section 382 of the Internal Revenue Code that limit the federal net operating loss carryforwards that may be used in any given year in the event of specified occurrences, including significant ownership changes. If these specified events occur we may lose some or all of the tax benefits of these carryforwards.
We determined, based on our assessment of both positive and negative evidence and objective and subjective evidence, which takes into consideration our forecasted taxable income, that it is more likely than not that we will benefit from the use of the deferred tax assets, and therefore we reduced our valuation allowance on the existing deferred tax assets related to these NOL’s to zero in the fourth quarter of fiscal 2004 as required by SFAS No. 109 “Accounting for Income Taxes”. Upon reducing the valuation allowance and recognizing the
17
deferred tax assets, we recognized the tax benefit of deferred tax assets. The favorable impact of the tax benefit distorts the trends in our operating results and impacts the comparability of our current period results of operations with other periods. At November 30, 2005, in light of recent losses, we determined, based on our assessment of both positive and negative evidence and objective and subjective evidence, which takes into consideration our forecasted taxable income, that it is more likely than not that we will not realize all or a portion of the deferred tax assets, and recorded a valuation allowance for the full amount of the deferred tax assets which resulted in recognition of income tax expense and lower earnings in 2005. In calculating our tax provision and assessing the likelihood that we will be able to use the deferred tax assets we will continue to evaluate our ability to realize these deferred tax assets. As we generate taxable income for book purposes, we will realize the benefit of the deferred tax assets to offset the tax expense associated with pre-tax income, resulting in a reduced net tax expense for the period and greater net income than would normally be expected.
Technological change is difficult to predict and new product transitions are difficult to manage.
Our product line has required, and any future products will require, substantial development efforts and compliance with governmental clearance or approval requirements. We may encounter unforeseen technological or scientific problems that force abandonment or substantial change in the development of a specific product or process. In addition, as we introduce new products and product enhancements, we may not be able to effectively segregate or transition from existing products which could negatively impact revenue, gross margin and overall profitability. Among the risks associated with the introduction of new products are delays in development or manufacturing, variations in cost, delays in customer purchases in anticipation of new introductions, difficulty in predicting customer demand for the new and existing offerings and effectively managing inventory levels and the risks that new products may have quality or other defects. Furthermore, sales of our new products may replace sales, or result in discounting of our current offerings.
We depend on management and other key personnel.
We are dependent on a limited number of key management and technical personnel. The loss of one or more of our key employees may hurt our business if we are unable to identify other individuals to provide us with similar services. We do not maintain “key person” insurance on any of our employees.
We face intense competition in our recruiting activities and may not be able to attract or retain qualified personnel. We have historically used stock options as key components of our total employee compensation program. In recent periods, many of our employee stock options have had exercise prices in excess of our stock price, which reduces their value to employees and could affect our ability to retain and attract present and prospective employees. In addition, the implementation of FAS 123R will require us to record a charge to earnings for employee stock option grants and other equity incentives which will change our compensation strategy. Our ability to retain our existing personnel and attract additional highly qualified personnel will impact our future success.
We are subject to stock exchange and government regulation.
The Sarbanes-Oxley Act of 2002 and recent SEC and stock exchange regulations have increased financial reporting and disclosure requirements, corporate governance and internal control requirements, including compliance of our acquired businesses that have significantly increased the administrative costs of documenting and auditing internal processes, gathering data, and reporting information. In 2004 and 2005, costs related to compliance with these regulations amounted to approximately $500,000 and $1.2 million, respectively. In addition, the reporting timeframes have been compressed. The need to commit substantial resources and management attention in these areas could impact our ability to deploy those same resources to other areas of our business. If we are unable to comply with the requirements, it could significantly impact our market valuation.
18
We may not have adequate intellectual property protection.
Our patents and proprietary technology may not be sufficient to protect our intellectual property rights. In addition, we have received communications from third parties asserting that our ‘754 patent is invalid. For example, during 2005, we became aware of a company that was selling non-FDA cleared ICG sensors for use with our BioZ systems. We believe their sensors are in violation of our patent and have filed a patent infringement suit. They have in turn countersued to invalidate the patent as well as for tortious interference with contracts, unlawful tying and unlawful restraint of trade. We intend to vigorously defend against these claims and protect our intellectual property rights. However, if we do not prevail, we are at risk of losing the ‘754 patent. Any claims resulting in intellectual property litigation, whether defensive or offensive would have no certain outcome and would be costly and time-consuming. In addition, if our actions to enforce our patents are found to be a violation of laws related to unlawful tying or restraint of trade, we may be required to pay damages to third parties, which could be costly and could harm our business.
The validity and breadth of claims in medical technology patents involve complex legal and factual questions. There can be no assurance that pending patent applications will result in issued patents, that future patent applications will be issued, that patents issued to or licensed by us will not be challenged or circumvented by competitors or that such patents will be found to be valid or sufficiently broad to protect our technology or to provide us with a competitive advantage. Our patents may be found to be invalid and other companies may claim rights in or ownership of the patents and other proprietary rights held or licensed by us. Also, our existing patents may not cover products that we develop in the future. Moreover, when our patents expire, the inventions will enter the public domain.
We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect trade secrets and other proprietary technology. There can be no assurance that these agreements will not be breached, that we will have adequate remedies for any breach, that others will not independently develop equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets and proprietary knowledge.
We face competition from other companies and technologies.
We compete with other companies that are developing and marketing noninvasive hemodynamic monitors. In mid-2004, Analogic released the LifeGard ICG Monitor, which is also available to be sold by Philips. We are also subject to competition from companies that support invasive technologies. Many of these companies have more established and larger marketing and sales organizations, significantly greater financial and technical resources and a larger installed base of customers than we do.
The introduction by others of products embodying new technologies and the emergence of new industry standards may render our products obsolete and unmarketable. In addition, other technologies or products may be developed that have an entirely different approach or means of accomplishing the intended purposes of our products. Accordingly, the life cycles of our products are difficult to estimate. To compete successfully, we must develop and introduce new products that keep pace with technological advancements, respond to evolving consumer requirements and achieve market acceptance. We may be unable to develop new products that address our competition.
Our business plan contemplates an income stream from sales of disposable sensors that are compatible with an installed base of our monitors. We may be subject to price competition from other sensor manufacturers whose products are also compatible with our monitors. In addition, our current and potential competitors may establish cooperative relationships with large medical equipment companies to gain access to greater research and development or marketing resources. Competition may result in price reductions, reduced gross margins and loss of market share.
19
If market conditions cause us to reduce the selling price of our products or sensors, our margins and operating results will decrease.
The selling price of our products and sensors are subject to market conditions. Market conditions that could impact these aspects of our operations include:
| | • | | Changes in the reimbursement policies of government and third-party payers; |
| | • | | Hospital or physician practice budgetary constraints; |
| | • | | The introduction of competing products; |
| | • | | Price reductions by our competitors; |
| | • | | Development of more effective products by our competitors; and |
| | • | | Lengthening of buying or selling cycles; |
If such conditions force us to sell our products and systems at lower prices, or if we are unable to effectively develop and market competitive products, our market share, margins and operating results will likely decrease.
Effective December 1, 2005, we will be required to account for stock options under our employee stock plans as a compensation expense, our net income and earnings per share could be significantly reduced.
For employee option grants, we calculate compensation expense and disclose the impact on net income (loss) and net income (loss) per share in a footnote to our financial statements. Recent accounting pronouncements will require us to record compensation expense for employee stock option grants in our statement of operations. Note 1, “Stock-Based Compensation,” to our financial statements, reflects the impact that adoption of FAS 123 would have had on our net income (loss) and net income (loss) per share had we adopted this provision during the past three years. We have not yet determined the specific impact of FAS 123R on future periods.
We have a history of losses and may experience continued losses.
With the exception of 2002 through 2004, we have experienced losses every year because we have expended more money in the course of researching, developing and enhancing our technology and products and establishing our sales, marketing and administrative organizations than we have generated in revenues. We expect that our operating expenses will continue to increase in the foreseeable future as we increase our sales and marketing activities, expand our operations and continue to develop our technology. It is possible that we will not be able to achieve the revenue levels required to achieve and sustain profitability.
We may need additional capital, which may be unavailable.
The commercialization of our current product line, acquisition of complimentary technologies and the development and commercialization of any additional products may require greater expenditures than expected in our current business plan. Our capital requirements will depend on numerous factors, including:
| | • | | our rate of sales growth—fast growth may actually increase our need for additional capital to hire additional staff, purchase additional inventory, and finance the increase in accounts receivable; |
| | • | | our progress in marketing-related clinical evaluations and product development programs, all of which will require additional capital; |
| | • | | our receipt of, and the time required to obtain, regulatory clearances and approvals—the longer regulatory approval takes, the more working capital we will need to support our regulatory and development efforts in advance of sales; |
| | • | | the level of resources that we devote to the development, manufacture and marketing of our products—any decision we make to improve, expand, acquire complimentary technologies or simply change our process, products or technology will require increased funds; |
20
| | • | | facilities requirements—as we grow we need additional manufacturing, warehousing and administration facilities and the costs of the facilities will be borne before substantially increased revenue from growth would occur; |
| | • | | market acceptance and demand for our products—although growth may increase our capital needs, the lack of growth and continued losses would also increase our need for capital; |
| | • | | customer financing strategies—our attempt to accelerate the purchasing processes by offering internal leasing programs as an alternative to outright purchasing and by providing purchasers with extended payment terms and financing options will consume additional capital; and |
| | • | | large electrode contracts—we intend to bid on several large electrode manufacturing contracts, any one of which, if awarded would require substantial capital investment in equipment at our Vermed division. |
We may be unable to predict accurately the timing and amount of our capital requirements. We may be required to raise additional funds through public or private financing, bank loans, collaborative relationships or other arrangements earlier than expected. It is possible that banks, venture capitalists and other investors may perceive our capital structure, our history of losses or the need to achieve widespread acceptance of our technology as too great a risk to bear. As a result, additional funding may not be available at attractive terms, or at all. If we cannot obtain additional capital when needed, we may be forced to agree to unattractive financing terms, to change our method of operation or to curtail our operations.
We may default on our credit agreements and have to immediately repay all amounts outstanding.
Our credit agreement contains certain reporting, profitability and liquidity covenants which if not met, could result in an event of default under the credit agreement under which the lenders could elect to declare all amounts outstanding under the credit agreement, together with accrued interest, to be immediately due and payable. If we were unable to repay those amounts, the lenders could proceed against the collateral guaranteed to them to secure such indebtedness. In addition, the operating and financial restrictions and covenants in our credit agreement, and any future financing agreements may adversely affect our ability to finance future operations and meet our capital needs. Our current credit agreement restricts our ability to incur additional indebtedness, create liens, sell assets, make certain investments, pay dividends or make distributions, repurchase equity interests, redeem subordinated indebtedness, enter into certain transactions with affiliates, or enter into mergers or acquisitions.
We may not be able to manage growth successfully.
If successful, we will experience a period of growth that could place a significant strain upon our managerial, financial and operational resources. Our infrastructure, procedures, controls and information systems may not be adequate to support our operations and to achieve the rapid execution necessary to successfully market our products. Our future operating results will also depend on our ability to continually upgrade our information systems, expand our direct sales force and our internal sales, marketing and support staff. If we are unable to manage future expansion effectively, our business, results of operations and financial condition will suffer, our senior management will be less effective, and our revenues and product development efforts may decrease.
Our international sales expose us to unique risks.
In fiscal 2005, international sales accounted for approximately 9% of our net sales. We believe that international sales could represent a meaningful portion of our revenue in the future. We rely on regional distributors and GEMS-IT to assist us with our international sales efforts. In June of 2004, we purchased 80% of the outstanding common stock of Medis, which is located in Ilmenau, Germany. As such, we are exposed to risks from international sales, which include unexpected changes in regulatory requirements, tariffs and other barriers and restrictions and reduced protection for intellectual property rights. Moreover, fluctuations in the rates of exchange may subject us to foreign currency losses related to the Medis deferred acquisition payments or increase the price in local currencies of our products in foreign markets and make our products relatively more expensive than competitive products.
21
We may not receive approvals by foreign regulators that are necessary for international sales.
Sales of medical devices outside the United States are subject to foreign regulatory requirements that vary from country to country. If we or our international distributors fail to obtain or maintain required pre-market approvals or fail to comply with foreign regulations, foreign regulatory authorities may require us to file revised governmental notifications, cease commercial sales of our products in the applicable countries or otherwise cure the problem. Such enforcement action by regulatory authorities may be costly. In order to sell our products within the European community, we must comply with the European community’s medical device directive. The CE marking on our products attests to this compliance. Future regulatory changes may limit our ability to use the CE mark, and any new products we develop may not qualify for the CE mark. If we lose this authorization or fail to obtain authorization on future products, we will not be able to sell our products in the European community.
Our quarterly operating results frequently vary due to factors outside our control.
We have experienced and expect to continue to experience fluctuations in quarterly operating results due to a number of factors. We cannot control many of these factors, which include the following:
| | • | | the timing and number of new product introductions; |
| | • | | the number of selling days in a given quarter; |
| | • | | the mix of sales of higher and lower margin products in a quarter; |
| | • | | the market acceptance of, and changes in demand for our products; |
| | • | | the impact of any changes in generally accepted accounting principles; |
| | • | | the loss of any of our key distributors; |
| | • | | the impact of acquisitions, divestitures, strategic alliances, and other significant corporate events; |
| | • | | development and promotional expenses relating to the introduction of new products or enhancements of existing products; |
| | • | | product returns or bad debt write-offs; |
| | • | | changes in pricing policies by our competitors; |
| | • | | the timing of regulatory compliance audits; |
| | • | | the impact of weather related disasters such as hurricane Katrina; |
| | • | | the timing of orders from major customers and distributors; |
| | • | | delays in shipment of products or components to us by our vendors; and |
| | • | | The timing of customer orders and shipments. Our quarterly sales have reflected a pattern in which a disproportionate percentage of our total quarterly sales occur toward the end of each of our fiscal quarters. This uneven sales pattern makes prediction of revenue, earnings and working capital for each financial period difficult, increases the risk of unanticipated variations in quarterly results and financial condition and places pressure on our inventory management, staffing, and logistics systems. |
Accordingly, you should not rely on period-to-period comparisons of our financial results as indications of future results.
We depend on third parties for development and manufacturing services.
Our strategy for development and commercialization of some of our products depends upon entering into various arrangements with third parties and upon the subsequent success of these parties in performing their obligations. We may not be able to negotiate acceptable arrangements in the future, and our existing arrangements may not be successful. We rely on contracted engineering services, particularly from Rivertek. Also, we currently
22
assemble our products from components manufactured by a limited number of manufacturers. Therefore, we are dependent on component and subassembly manufacturers. If we experience a termination, modification or disruption of any of our development or manufacturing arrangements, we may be unable to deliver products to our customers on a timely basis, which may lead to customer dissatisfaction and damage to our reputation.
Our limited order backlog makes it difficult to predict sales and plan manufacturing requirements, which can lead to lower revenues, higher expenses and reduced margins.
Our customers typically order products on a purchase order basis, and we do not generally have long-term purchase contracts. In limited circumstances, customer orders may be cancelled, changed or delayed on short notice. Lack of significant order backlog makes it difficult for us to forecast future sales with certainty. Varying sales cycles with our customers make it difficult to accurately forecast component and product requirements. These factors expose us to a number of risks:
| | • | | If we overestimate our requirements we may be obligated to purchase more components or third-party products than is required; |
| | • | | If we underestimate our requirements, our third-party manufacturers and suppliers may have an inadequate product or product component inventory, which could interrupt manufacturing of our products and result in delays in shipments and revenues; |
| | • | | We may also experience shortages of product components from time to time, which also could delay the manufacturing of our products; and |
| | • | | Over or under production can lead to higher expense, lower than anticipated revenues, and reduced margins. |
Our common stock is subject to price volatility.
The market price of our common stock has been and is likely to continue to be highly volatile. Our stock price could be subject to wide fluctuations in response to various factors beyond our control, including:
| | • | | actual or anticipated quarterly variations in operating results; |
| | • | | announcements of technological innovations, new products or pricing by our competitors; |
| | • | | changes in, or failure to meet, financial estimates of securities analysts; |
| | • | | the rate of adoption by physicians of ICG technology in targeted markets; |
| | • | | the timing and extent of technological advancements, patent and regulatory approvals; |
| | • | | the impact of acquisitions, divestitures, strategic alliances, and other significant corporate events; |
| | • | | anything other than unqualified reports by our outside auditors; |
| | • | | results of clinical studies; |
| | • | | changes in reimbursement policies of third-party payers; |
| | • | | the sales of our common stock by affiliates or other shareholders with large holdings; and |
| | • | | general economic and market conditions. |
Our future operating results may fall below the expectations of securities industry analysts or investors. Any such shortfall could result in a significant decline in the market price of our common stock. In addition, the stock market has at times experienced significant price and volume fluctuations that have affected the market prices of the stock of many medical device companies that often have been unrelated to the operating performance of such companies. These broad market fluctuations may directly influence the market price of our common stock.
23
We may not continue to receive necessary FDA or other regulatory clearances or approvals.
Our products and activities are subject to extensive, ongoing regulation by the Food and Drug Administration and other governmental authorities. Delays in receipt of, or failure to obtain or maintain, regulatory clearances and approvals, or any failure to comply with regulatory requirements, could delay or prevent our ability to market or distribute our product line.
We are subject to product liability claims and product recalls that may not be covered by insurance.
The nature of our business exposes us to risks of product liability claims and product recalls. Medical devices as complex as ours frequently experience errors or failures, especially when first introduced or when new versions are released. Our products are sometimes used in procedures where there is a high risk of serious injury or death. These risks will exist even with respect to those products that have received, or may in the future receive, regulatory clearance for commercial sale.
We did not carry product liability insurance during some periods before May 15, 1995. We currently maintain product liability insurance, however our product liability insurance may not be adequate. In the future, insurance coverage may not be available on commercially reasonable terms, or at all. In addition, product liability claims or product recalls could damage our reputation even if we have adequate insurance coverage.
A low stock price could result in our being de-listed from the NASDAQ Market and subject us to regulations that could reduce our ability to raise funds.
If our stock price were to drop below $1.00 per share and remain below $1.00 per share for an extended period of time, or if we fail to maintain other NASDAQ criteria, NASDAQ may de-list our common stock from the Nasdaq National Market. In such an event, our shares could only be traded on over-the-counter bulletin board system. This method of trading could significantly impair our ability to raise new capital.
In the event that our common stock was de-listed from the Nasdaq National Market due to low stock price, we may become subject to special rules, called penny stock rules that impose additional sales practice requirements on broker-dealers who sell our common stock. The rules require, among other things, the delivery, prior to the transaction, of a disclosure schedule required by the SEC relating to the market for penny stocks. The broker-dealer also must disclose the commissions payable both to the broker-dealer and the registered representative and current quotations for the securities, and monthly statements must be sent disclosing recent price information.
In the event that our common stock becomes characterized as a penny stock, our market liquidity could be severely affected. The regulations relating to penny stocks could limit the ability of broker-dealers to sell our common stock and thus the ability of purchasers in this offering to sell their common stock in the secondary market.
Corporate scandals involving accounting irregularities have resulted in unavailability of or significantly higher premiums for director and officer liability insurance.
As a result of well-publicized corporate business failures involving improper acts by executives and accounting irregularities, director and officer liability insurance has become more difficult to obtain. If we are unable to obtain sufficient director and officer liability insurance at rates that are reasonable or at all or if the possibility of director and officer liability is expanded by regulatory or judicial developments, we may not be able to retain our current officers and directors or attract qualified directors and officers in the future.
We do not know the effects of healthcare reform proposals.
The healthcare industry is undergoing fundamental changes resulting from political, economic and regulatory influences. In the United States, comprehensive programs have been suggested that would increase
24
access to healthcare for the uninsured, control the escalation of healthcare expenditures within the economy and use healthcare reimbursement policies to balance the federal budget.
We expect that the U.S. Congress and state legislatures will continue to review and assess various healthcare reform proposals, and public debate of these issues will likely continue. We cannot predict which, if any, of such reform proposals will be adopted and when they might be effective. Other countries also are considering healthcare reform. Significant changes in healthcare systems could have a substantial impact on the manner in which we conduct our business and could require us to revise our strategies.
We do not intend to pay dividends in the foreseeable future.
We do not intend to pay any cash dividends on our common stock in the foreseeable future. Payment of such cash dividends would, in any event, be prohibited or limited under the terms of our bank line of credit.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
We are headquartered in San Diego, California and operate manufacturing locations in San Diego, California; Bellow Falls, Vermont; and Ilmenau, Germany as described below. We believe that our properties are adequate and suitable for our current and foreseeable business needs.
| | | | | | | | |
Location
| | Use
| | Owned/Leased
| | Lease Termination
Date
| | Size (Sq. Feet)
|
| | | | |
San Diego, California | | Corporate Headquarters Sales & Marketing Research & Development Manufacturing & Distribution | | Leased | | December 2009 | | 32,779 |
| | | | |
Bellow Falls, Vermont | | General & Administrative Sales & Marketing Research & Development Manufacturing & Distribution | | Owned | | N/A | | 45,972 |
| | | | |
Ilmenau, Germany | | General & Administrative Sales & Marketing Research & Development Manufacturing & Distribution | | Owned (80% owned subsidiary) | | N/A | | 7,173 |
The Company is from time to time subject to legal proceedings and claims, which arise in the ordinary course of our business, none of which is required to be disclosed under this Item 3. Management believes that resolution of these matters will not have a material adverse effect on our results of operations, financial condition or cash flows.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
None.
25
EXECUTIVE OFFICERS OF THE REGISTRANT
The following table sets forth information with respect to our executive officers:
| | | | |
Name
| | Age
| | Position(s)
|
Michael K. Perry | | 45 | | Chief Executive Officer and Director |
Rhonda F. Rhyne | | 45 | | President |
Steve P. Loomis | | 45 | | Chief Financial Officer |
Russell H. Bergen | | 58 | | Vice President of Operations |
Dennis G. Hepp | | 56 | | Chief Technology Officer |
Patrick W. Bradley, Ph.D. | | 52 | | Vice President of Business Development |
Richard L. Kalich | | 59 | | President of Vermed |
Richard E. Trayler | | 55 | | Vice President of International Operations |
Michael K. Perry, age 45, has been the Chief Executive Officer and Director of CardioDynamics since April 1998. From 1994 to 1997, Mr. Perry was Vice President of Operations at Pyxis Corporation, and in 1995 assumed additional responsibility for Research and Development. Pyxis Corporation was a pioneer of healthcare automation and information management services, in addition to pharmacy management services to hospitals and outpatient facilities. Mr. Perry was part of the executive team that successfully acquired and integrated three businesses into Pyxis, and in 1996, sold the company to Cardinal Health, Inc. for $980 million. Prior to joining Pyxis, Mr. Perry served in several increasingly responsible management assignments with Hewlett-Packard Company’s Medical Products Group in manufacturing and finance. Additionally, he was Director of Quality for a division of Hewlett-Packard’s DeskJet Printer Group. In 2003, Mr. Perry was named San Diego Entrepreneur of the Year for Medical Products and Technology. Mr. Perry holds a Master’s degree in Business Administration from Harvard University and a Bachelor’s degree in Mechanical Engineering from General Motors Institute. Mr. Perry serves on the Advisory Board of the University of California San Diego Cardiovascular Center and the Board of Directors for Junior Achievement of San Diego.
Rhonda F. Rhyne, age 45, has been our president since June 1997, previously serving as chief operating officer from 1996 to 1997 and as vice president of operations from 1995 to 1996. From 1992 until 1995, Ms. Rhyne was president, chief executive officer and vice president of sales and marketing for Culture Technology, Inc. Ms. Rhyne has also held sales positions at GE Medical Systems and Quinton Instrument Company, both medical device subsidiaries of publicly held companies. Ms. Rhyne holds a bachelor’s degree in pharmacy from Washington State University and a master’s degree in business administration, executive program, from University of California Los Angeles, Anderson School of Business.
Steve P. Loomis, age 45, joined the Company in September 1996 as vice president of finance and has held the positions of chief financial officer and corporate secretary since April 1997. From 1993 until 1996, he served as director of financial reporting at Kinko’s Inc. From 1988 to 1993, Mr. Loomis was chief financial officer for Terminal Data Corporation, a publicly traded company. He earned his bachelor’s degree in business administration from California State University at Northridge. Mr. Loomis is a certified public accountant.
Russell H. Bergen, age 58, has served as our vice president of operations since joining us in September 1998. From 1971 to 1998, Mr. Bergen held management positions in the instrument group, peripheral products group and inkjet business unit of Hewlett Packard Company. Previously, Mr. Bergen was employed at Honeywell, Inc. as a procurement engineer. Mr. Bergen earned bachelor degrees in aerospace engineering and manpower management from the University of Colorado at Boulder.
Dennis G. Hepp, age 56, served as served as a consultant to us since July 1995 and as our chief technology officer from June 1997 until his retirement from CardioDynamics in December 2005. From 1974 to 1986, Mr. Hepp held engineering and management positions at Medtronic, Inc. In 1989, Mr. Hepp founded, and remains a key employee and managing director of Rivertek Medical Systems, Inc., an engineering consulting firm to medical device manufacturers. Mr. Hepp has a bachelor’s degree in electrical engineering from the University of Detroit.
26
Patrick W. Bradley, Ph.D., age 52, joined CardioDynamics in September 2000 as vice president of business development. Dr. Bradley served as director of new technologies for Cardiac Science, Inc. from 1999 to 2000 and from 1993 to 1999; Dr. Bradley was associate vice president for OEM Technologies at VitalCom, Inc. Prior to that, he was director of product management at Quinton Instrument Company. Dr. Bradley has served on the Scientific Peer Review Committee for the American Heart Association and on the Sports Science Research Group for the United States Olympic Committee. He has a bachelor’s degree in biology from Texas A&M University and a master’s degree and Ph.D. in physiology from University of Houston.
Richard L. Kalich, age 59, is President of our Vermed division where he has served as president since 1999. Between 1993 and 1999 he was President and CEO of Imtec, Inc. and prior to that, from 1983 to 1993, Mr. Kalich held progressively responsible management positions with Matthews International, Inc. leading to Vice President and Division Manager. Between 1978 and 1983, Mr. Kalich held various positions with Sears, Roebuck Inc. leading to National Marketing Manager of the Hardware Department and Craftsman tools. He holds a bachelor’s degree in economics from Penn State and a master’s degree in management from Michigan State.
Richard E. Trayler, age 54, is our vice president of international operations and served as our chief operating officer from July 1997 to January 2003. From 1982 to 1997, Mr. Trayler held sales management positions at Quinton Instrument Company. He has also held positions at the Heart Institute for CARE, the University of Washington and the Boeing Company. Mr. Trayler earned a bachelor’s degree from Texas A&M University and a master’s degree from the University of Washington.
27
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS |
Our common stock trades on the Nasdaq Stock Market under the symbol “CDIC.” The following table provides the high and low sales prices per share of our common stock as reported by the Nasdaq Stock Market.
| | | | | | |
| | | Market Price
|
| | | High
| | Low
|
Year Ended November 30, 2005: | | | | | | |
Fourth Quarter | | $ | 1.49 | | $ | 1.04 |
Third Quarter | | | 2.31 | | | 1.36 |
Second Quarter | | | 4.47 | | | 2.03 |
First Quarter … | | | 5.59 | | | 4.02 |
| | |
Year Ended November 30, 2004: | | | | | | |
Fourth Quarter | | $ | 5.67 | | $ | 3.62 |
Third Quarter | | | 6.80 | | | 3.72 |
Second Quarter | | | 6.85 | | | 5.81 |
First Quarter … | | | 6.98 | | | 5.19 |
On January 16, 2006 there were approximately 450 holders of record of our common stock. The Company has not declared or paid any cash dividends on shares of our common stock and does not anticipate paying any cash dividends in the foreseeable future. The Company currently intends to retain any future earnings for use in the operation of the business.
28
| ITEM 6. | SELECTED FINANCIAL DATA |
The following table summarizes certain selected financial data and has been derived from our audited financial statements and should be read in conjunction with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited financial statements and related Notes appearing elsewhere in this Annual Report on Form 10-K.
| | | | | | | | | | | | | | | | | |
| | | Years Ended November 30,
| |
| | | 2005 (2)
| | | 2004 (1)
| | 2003
| | 2002
| | 2001
| |
| | | (In thousands, except per share data) | |
Statements of Operations Data: | | | | | | | | | | | | | | | | | |
Net sales | | $ | 37,005 | | | $ | 40,988 | | $ | 30,332 | | $ | 23,523 | | $ | 19,598 | |
Income (loss) from operations | | | (3,832 | ) | | | 2,924 | | | 2,328 | | | 10 | | | (1,162 | ) |
Net income (loss) | | | (14,945 | ) | | | 10,123 | | | 2,452 | | | 310 | | | (659 | ) |
Net income (loss) per common share: | | | | | | | | | | | | | | | | | |
Basic | | $ | (.31 | ) | | $ | .21 | | $ | .05 | | $ | .01 | | $ | (.01 | ) |
| | |
|
|
| |
|
| |
|
| |
|
| |
|
|
|
Diluted | | $ | (.31 | ) | | $ | .21 | | $ | .05 | | $ | .01 | | $ | (.01 | ) |
| | |
|
|
| |
|
| |
|
| |
|
| |
|
|
|
| | | | | |
Balance Sheet Data (at end of period): | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and investments | | $ | 3,615 | | | $ | 6,801 | | $ | 9,345 | | $ | 6,879 | | $ | 6,394 | |
Total assets | | | 39,998 | | | | 58,030 | | | 26,648 | | | 23,566 | | | 21,461 | |
Revolving line of credit-bank | | | 2,200 | | | | — | | | — | | | — | | | — | |
Long-term debt, including current portion | | | 1,751 | | | | 5,730 | | | — | | | — | | | — | |
Total long-term liabilities | | | 2,777 | | | | 5,338 | | | 719 | | | 560 | | | 327 | |
Total shareholders’ equity | | | 29,763 | | | | 44,734 | | | 23,020 | | | 19,724 | | | 18,423 | |
| (1) | 2004 includes the results of operations of Vermed and Medis businesses acquired March 22, 2004 and June 2, 2004, respectively, which affect the comparability of the Selected Financial Data. See note 2 to the Notes to Consolidated Financial Statements. Also includes an income tax benefit of $7.4 million resulting from elimination of the valuation allowance on our deferred tax assets (see Note 13 to the Notes to Consolidated Financial Statements). |
| (2) | 2005 includes an income tax expense of $12.7M resulting from the re-establishment of the valuation allowance on our deferred tax assets (see Note 13 to the Notes to Consolidated Financial Statements). |
29
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following management’s discussion and analysis of financial condition and results of operations should be read in conjunction with the consolidated financial statements and related Notes, as well as the other financial information included in this Form 10-K. Some of our discussion is forward-looking and involves risks and uncertainties. For information regarding risk factors that could have a material adverse effect on our business, refer to Part I, Item 1A of this Form 10-K, “Business—Risk Factors.”
Results of Operations
CardioDynamics is the innovator and market leader of an important medical technology called Impedance Cardiography (ICG). We develop, manufacture and market noninvasive ICG diagnostic and monitoring devices, proprietary ICG sensors and a broad array of medical device electrodes. Unlike other traditional cardiac function monitoring technologies, our monitors are noninvasive (without cutting into the body). Our BioZ ICG Systems obtain data in a safe, efficient and cost-effective manner not previously available in the physician’s office and many hospital settings.
Just as electrocardiography noninvasively measures the heart’s electrical characteristics, ICG makes it possible to noninvasively measure the heart’s mechanical characteristics by monitoring the heart’s ability to deliver blood to the body. Our ICG products measure 12 hemodynamic (blood flow) parameters, the most significant of which is cardiac output, or the amount of blood pumped by the heart each minute.
Our lead products, the BioZ Dx Diagnostics, BioZ ICG Monitor and the BioZ ICG Module for GE Healthcare patient monitoring systems, have been cleared by the FDA and carry the CE Mark, which is a required certification of environmental and safety compliance by the European Community for sale of electronic equipment.
The aging of the worldwide population along with continued cost containment pressures on healthcare systems and the desire of clinicians and administrators to use less invasive (or noninvasive) procedures are important trends that are helping drive adoption of our BioZ ICG Systems. These trends are likely to continue into the foreseeable future and should provide continued growth prospects for our Company.
There is often a slow adoption of new technologies in the healthcare industry, even technologies that ultimately become widely accepted. Making physicians aware of the availability and benefits of a new technology, changing physician habits, the time required to secure adequate reimbursement levels, and the malpractice and legal issues that overlay the healthcare industry are all factors that tend to slow the rate of adoption for new medical technologies. We have invested a significant amount of our resources in clinical trials, which, if results prove successful, should contribute to further physician acceptance and market adoption of our technology. As with any clinical trial, there is no assurance of achieving the desired positive outcome.
We continue to invest in our partnerships to increase the presence and adoption of ICG technology. Our principal strategic partners include GE Healthcare and Philips, both of which are among the premier medical technology companies in the world and have a substantial installed base of medical devices. We are currently selling the BioZ ICG Module through GE Healthcare and co-developed the BioZ Dx with Philips, the latest generation ICG monitor. These strategic relationships further validate the importance of our technology to the clinical community and provide additional distribution channels for our systems. We intend to seek additional strategic partnerships over time accelerate the validation, distribution, and adoption of our technology.
We believe that the greatest risks in executing our business plan in the near term include: an adverse change in U.S. reimbursement policies for our technology, negative clinical trial results, competition from emerging ICG companies or other new technologies that could yield similar or superior clinical outcomes at reduced cost, or the inability to hire, train, and retain the necessary sales and clinical personnel to meet our growth objectives. Our
30
management team devotes a considerable amount of time to mitigating these and other risks, described in the risk factor section in Part I, Item 1A of this Form 10-K, to the greatest extent possible.
Following is a list of some of the key milestones we achieved in 2005:
| | • | | Received FDA 510(k) clearance on BioZ Dx and began shipping in December, 2004; |
| | • | | Obtained FDA 510(k) marketing clearance for the second phase of the BioZ Dx with 12-lead ECG capability and began shipping in the second quarter of 2005; |
| | • | | Made significant progress towards paying down the Comerica bank term loan; |
| | • | | Continued our investment in ECG electrode sensor production capabilities at our Vermed division to improve productivity and capacity to meet current and future supply demands related to anticipated ICG sensor growth and more aggressive pursuit of GPO, private label and OEM medical sensor opportunities; |
| | • | | Published several articles supporting use of ICG in hypertension management in theAmerican Journal of Hypertension; |
| | • | | Broadened the use of domestic distributors working with our direct sales team; |
| | • | | Leveraged our international sales team to expand the sales growth of Medis ICG products; |
| | • | | Vermed was awarded a three-year, multi-source contract with Premier Purchasing Partners LLP, the group purchasing division of Premier, Inc. and one of the largest healthcare alliances in the United States; |
| | • | | Presented the multi-center CONTROL study demonstrating that use of the BioZ ICG was more than two times better than standard care for achievement of blood pressure control in mild to moderate hypertensive patients; and |
| | • | | Presentation of a study conducted by researchers at the University of Texas Southwestern Medical Center at Dallas showing use of the BioZ reduced the incidence of low blood pressure in women undergoing cesarean section by 52%. |
| | • | | Continued growth of our recurring sensor revenue; and |
| | • | | Reduced operating expense structure in light of lower revenue levels. |
We also faced some significant challenges during 2005:
| | • | | Effects of CMS ICG policy clarification that served to restrict reimbursement for the use of ICG for hypertension in all but resistant hypertensive patients; |
| | • | | Lengthened sales cycle and decreased average selling prices due to the emergence of lower-priced ICG competition; and |
| | • | | Lack of incremental direct sales force productivity from our emphasis during the year on a domestic distribution team selling approach. |
Operating Segments—Our business consists of the following two principal operating segments:
ICG Segment
The ICG segment consists primarily of the development, manufacture and sales of the BioZ Dx ICG Diagnostics, BioZ ICG Monitor, BioZ ICG Module and associated BioZtect sensors. These devices use ICG technology to noninvasively measure the heart’s mechanical characteristics by monitoring the heart’s ability to deliver blood to the body. These products are used principally by physicians to assess, diagnose, and treat cardiovascular disease and are sold to physicians and hospitals throughout the world. With the acquisition of
31
Medis in June 2004, the ICG segment now also includes the Medis diagnostic and monitoring devices such as the Niccomo, Cardioscreen monitor and the Rheoscreen family of measurement devices. We sell Medis products internationally to physicians, researchers and equipment manufacturers.
We derive our ICG segment revenue primarily from the sale of our BioZ ICG Monitors and associated disposable sensors, which are consumed each time a patient test is performed. During 2005, 24% of our ICG revenue came from our disposable ICG sensors, and that percentage has increased each year from approximately 6% in 2000, to 9% in 2001, 12% in 2002, 17% in 2003 and 19% in 2004. We have now shipped nearly 4.3 million ICG sensor sets to customers since introducing the BioZ in 1997. We employ a workforce of clinical application specialists (CAS) who are responsible for driving customer satisfaction and use of the BioZ ICG Systems. We believe our CAS investment is important to drive the growth of our ICG sensor business, which should improve the predictability of our revenue, earnings, and cash flow.
In early 2004, CMS issued an updated national coverage determination. Of the six indications previously indicated, five are substantially unchanged. One indication, “suspected or known cardiovascular disease,” has been revised to specifically allow CMS carrier discretion in the coverage of resistant hypertension. Resistant hypertension is defined by CMS to include patients with uncontrolled blood pressure on three or more anti-hypertensive medications. While this development was positive in those states that did not have previous hypertension coverage, the clarification has severely limited ICG Medicare reimbursement for hypertension patients in a number of states that previously had more liberal hypertension coverage. This coverage limitation has contributed to the downturn in ICG system sales and slowed ICG sensor revenue growth. In the near-term, we are focusing our sales efforts on cardiologists and larger physician practices and on better clinical education and usage of our products for heart failure and shortness of breath patients. In addition, we are driving toward the publication of our multi-center CONTROL study (which showed that clinician use of BioZ technology helped patients reach targeted blood pressure levels twice as effectively as standard clinical practice) in a leading medical journal by early 2006. Based on the results of this study, we have asked CMS to open the reconsideration review process regarding our request to broaden ICG hypertension coverage.
ECG Segment
The ECG segment, also referred to as the Medical Sensor segment, designs, manufactures and sells proprietary ICG sensors and a broad array of medical device electrodes and related supplies through our Vermed division acquired in March 2004. Revenue is generated primarily by ECG sensor sales that are used principally in electrocardiogram and other diagnostic procedures for cardiology, electrotherapy, sleep testing, neurology and general purpose diagnostic testing. The products are sold to a diverse client base of medical suppliers, facilities and physicians. We have begun to more aggressively pursue GPO, private label and OEM medical sensor opportunities. Customers expect a steady, uninterrupted flow of top quality sensors, and we have invested in capital equipment to ensure we can fulfill that requirement. We believe that the acquisition will improve on our predictability of operating results by increasing the recurring sensor revenue mix of our business. When combined with our ICG sensor sales, the disposable sensor revenue stream comprised 43% and 32% of our overall Company revenue in 2005 and 2004, respectively.
Net Sales of ICG Segment—Net sales were $27,686,000, $34,260,000, and $30,332,000 for 2005, 2004, and 2003, respectively. The 19% sales decrease in 2005 was due primarily to 17% fewer BioZ® placements by our domestic direct sales force and 10% lower average selling prices of our BioZ Systems. The sales growth in 2004 and 2003 was 13% and 29%, respectively, and was achieved primarily through higher BioZ placements by our domestic direct sales force and distributors and increasing disposable BioZtect® sensor revenue from our growing installed base of ICG monitors. In addition, the Medis acquisition contributed about two percentage points of growth in 2004 over 2003.
In March 1998, we received 510(k) marketing clearance for our BioZ ICG Monitor. The BioZ ICG Monitor features a portable design, transportable battery, integrated blood pressure and incorporates our Z MARC®
32
algorithm. In December 2004, we received FDA 510(k) clearance on the BioZ Dx ICG Diagnostics. The BioZ Dx has significant improvements with AERIS™ processing and optional 12-lead ECG capability. It also features an integrated full-page thermal printer, color display screen, a standard five-year warranty and a new Thera-Trak™ reporting function that allows physicians to automatically compare a patient’s last ICG report to the current ICG report. Commercial shipments of the BioZ Dx commenced in the first quarter of 2005.
Together, these BioZ ICG Monitors accounted for 69% of our overall ICG sales in 2005, compared with 75% and 80% of our sales in 2004 and 2003, respectively. The fluctuation in the percentage of BioZ sales is due to increasing sales of our disposable sensors and BioZ ICG Modules, as well as the addition of Medis ICG product sales during the second half of 2004.
The BioZ ICG Module is a custom plug-in non-invasive cardiac function monitoring device for the GE Healthcare Solar® and Dash® patient monitors. During 2005, 2004 and 2003 we sold 243, 233 and 8 BioZ ICG Modules, respectively. The significant increase in 2004 over 2003 is primarily due to higher BioZ ICG Module placements and sell-through of previously purchased stocking inventory by our strategic partner GE Healthcare
Although we have increased our investment in our domestic direct sales force and broadened the use of U.S. distributors, we experienced a decline in our domestic sales force productivity. Altogether, we sold 1,012 (including 450 of our new BioZ Dx systems) ICG Monitors and Modules in 2005, compared with 1,124 in 2004 and 805 in 2003, increasing the total number of BioZ Monitors and ICG Modules sold to nearly 6,000. Net sales by our domestic direct sales force, which targets physician offices and hospitals, decreased 22% in 2005 with sales of $24,007,000, from $30,889,000 in 2004, which were 8% higher than domestic direct sales in 2003 of $28,542,000.
We believe that the decline in 2005 is the result of several factors including:
| | • | | Retargeting of our sales force toward cardiologists, congestive heart failure clinics and larger physician practices as a result of the CMS clarification issued in 2004 limiting hypertension ICG coverage to patients on three or more drugs; |
| | • | | A relatively new sales associate base with approximately 42% hired in 2005; |
| | • | | Significant time commitment of our sales personnel as they worked with two large physician office distributors (Physician Sales and Service and the Caligor Division of Henry Schein); |
| | • | | Disruption caused by new sales management, realignment of our sales regions, and a Company-wide restructuring; and |
| | • | | Lengthened sales cycles and lower average selling prices of BioZ Systems due to the emergence of competition. |
While we believe that we are addressing these factors, we also believe that both the CMS clarification and the emergence of competition will continue to have an impact on our results in subsequent periods.
Net sales of our ICG products internationally increased by $309,000 in 2005 to $3,148,000, up 11% from 2004. The increase in 2005 is partially due to growing sales of our Medis products of $771,000, offset partially by a $462,000 reduction in the number of BioZ systems sold internationally. We acquired Medis on June 2, 2004, therefore the prior year to date period does not include a full year of Medis sales as in 2005. In 2004, international sales totaled $2,839,000, an increase of $1,280,000 or 82% over international sales of $1,559,000 in 2003. In 2004, Medis contributed $730,000 of this increase.
Each time our BioZ ICG products are used, disposable sets of four BioZtect sensors are required. This recurring ICG sensor revenue continued to grow in 2005 as a percentage of our overall ICG sales, up 2% to $6,675,000, representing 24% of ICG net sales (18% of consolidated net sales). ICG sensor sales for 2004 were $6,520,000, representing 19% of ICG net sales (16% of consolidated net sales), up from $5,170,000 or 17% of net sales in 2003. We offer a Discount Sensor Program to our domestic outpatient customers that provides
33
considerable discounts and a fixed price on sensor purchases in exchange for minimum monthly sensor purchase commitments. As the installed base of BioZ equipment grows, we expect the revenue generated by our disposable BioZtect sensors will continue to increase as well.
Included in ICG net sales is revenue derived from extended warranty contracts, spare parts, accessories and non-warranty repairs of our BioZ systems of $435,000 in 2005, $739,000 in 2004 and $764,000 in 2003.
Net Sales of ECG Segment—Net sales of medical sensors by our Vermed division in 2005 and 2004 were $9,319,000 and $6,728,000, respectively. Due to the acquisition of Vermed on March 22, 2004, the prior year period does not include a comparable number of days to 2005. During the second half of 2005, the ECG segment experienced a slowdown in sales which we believe is due to a short-term general softening of the market, and impacted somewhat by the hurricanes in Louisiana and Texas that occurred in 2005. We believe that this impact will likely continue to have a negative effect on sensor sales in those regions in 2006.
Gross Margin of ICG Segment—Gross margin was $17,878,000, $26,665,000 and $23,210,000 for the years ended 2005, 2004 and 2003, respectively. As a percentage of net sales, gross margins in 2005, 2004 and 2003 were 64.6%, 77.8% and 76.5%, respectively. In 2005, as compared to 2004, the decrease in gross margin was primarily due to higher raw material costs associated with the Philips transfer price of the BioZ Dx, lower average selling prices of the BioZ systems, increases to our warranty accrual of $458,000 and inventory provision for potential excess BioZ.com demo systems of $281,000, as well as higher scrap and rework costs of $282,000.
The improved gross margin percentage in 2004 over 2003 resulted from several factors including improved manufacturing efficiencies, reduced manufacturing engineering expenses related to supporting newly released products, an increase of 10% in the average sales price of our disposable sensor in 2004 and lower warranty costs per unit. In 2004, we commenced the transition of the BioZtect ICG disposable sensor manufacturing to our Vermed division which allows us to benefit from the synergies of the efficiencies in sensor manufacturing and reduce our cost of disposable sensors.
As the market matures and penetration increases, we believe that prices will naturally decline, as ICG technology becomes more of a standard of care, potentially driving our gross margin to lower levels. The commercial release of our multi-function second phase BioZ Dx should serve to support our BioZ System average sales prices in the near term, however, because we purchase a portion of the system from Philips, the manufactured cost of the BioZ Dx is higher than the BioZ Monitor.
Gross Margin of ECG Segment—Gross margin for 2005 and 2004 was $3,609,000 and $2,686,000, respectively. As a percentage of net ECG sales, gross margin was 38.7% in 2005 as compared with 39.9% in 2004. The decrease in gross margin percentage is due to increased discounts to large purchasing organizations, lower margins associated with a greater mix of distributor sales, increased inventory provisions of $70,000 and depreciation costs resulting from increased investment in automated sensor manufacturing equipment of $75,000. Since Vermed was acquired on March 22, 2004, the 2004 fiscal year does not include a comparable number of business days to 2005.
Research and Development of ICG Segment—Our investment in research and development for the years ended 2005, 2004 and 2003 were $2,334,000, $4,268,000 and $3,272,000, respectively. Research and development expenses in the ICG segment decreased 45% in 2005 as compared with 2004. The decrease in 2005 is primarily attributed to reduced investments required for the development and testing of the Phase I BioZ Dx and the Phase II ICG/ECG product capability co-developed with Philips, which was substantially complete in 2004. This was partially offset by $316,000 of Medis research and development spending in 2005. Also contributing to lower research and development expenses was the categorization of approximately $516,000 of spending on clinical studies in sales and marketing as these studies from assisting in the development and validation of ICG technology to assisting in market development and clinical applications for ICG.
34
Research and development expenses in the ICG segment increased 30% in 2004 over 2003, primarily due to the hiring of four additional engineering and research personnel which provided us with the capacity to focus increased attention on the development and testing of the joint ICG/ECG BioZ Dx product development with Philips which received FDA 510(k) clearance in December 2004.
Research and Development for ECG Segment—Research and development expenses for the ECG segment primarily relate to research, design and testing of Vermed product enhancements and extensions as well as modifications for private label products. ECG segment R&D expenses were $153,000 and $85,000 in 2005 and 2004, respectively. Due to the acquisition of Vermed on March 22, 2004, the prior year period does not include a comparable number of business days to 2005.
Selling and Marketing for ICG Segment—Selling and marketing expenses for the ICG segment for 2005 were $16,790,000, compared with $17,514,000 in 2004, a decrease of 4%. The decrease in selling and marketing expenses in 2005 is primarily attributed to a decrease in commissions and bonuses earned of approximately $900,000, decreased recruitment costs of $148,000, and lower bad debt expense of $833,000 resulting from lower accounts receivable balances and improved collections from our customers. These decreases were largely offset by increased personnel costs due to an increase in field sales personnel of $945,000 and consulting costs of $79,000.
As a percentage of ICG net sales, selling and marketing expenses were 61% in 2005, 51% in 2004, and 50% in 2003. One of our primary objectives is to increase field sales productivity and to reduce selling and marketing expenses as a percentage of net sales. In order to better manage our sales associates and serve our customer base, during fiscal 2005 we restructured the sales regions and brought in new sales management. In addition, we made a concerted effort to broaden our sales reach through distribution partners. These initiatives did not improve our ability to generate increased equipment and recurring sensor revenue in 2005.
Selling and marketing expenses include approximately $516,000 of clinical development spending on clinical studies. This investment is expected to increase as we launch the PREVENT-HF study in 2006. PREVENT-HF is an international, prospective, randomized study intended to demonstrate that ICG can help determine which heart failure patients are at high risk to be hospitalized or die within the next two to four weeks and, with that knowledge, doctors can intervene with treatment to prevent hospitalizations or deaths.
Selling and marketing expenses for the ICG segment increased 15% to $17,514,000 in 2004 from $15,249,000 in 2003. The ICG segment expense growth in 2004 was primarily due to an overall 20% increase in the average number of field sales personnel, higher commissions earned on increased sales by our direct sales force and additional provision for doubtful accounts resulting from higher accounts receivable balances and slower payments by our customers.
Selling and Marketing for ECG Segment—Selling and marketing expenses for the ECG segment are primarily for Vermed’s telemarketing, customer service and the OEM sales team. ECG segment selling and marketing expenses in 2005 and 2004 were $874,000 and $582,000, respectively. Because Vermed was acquired on March 22, 2004, the prior year period does not include a comparable number of days to 2005. As a percentage of net ECG sales, selling and marketing expenses in 2005 and 2004 were 9.4% and 8.7%, respectively.
Selling and Marketing for Corporate Unallocated—Corporate unallocated selling and marketing expenses totaled $317,000, $261,000 and $299,000 in 2005, 2004 and 2003, respectively. These expenses include general corporate expenses of a non-segment related nature such as sales related Sarbanes-Oxley expenses and costs for the corporate business development function, which assists in targeting new market opportunities and complementary technologies through acquisitions or strategic relationships.
General and Administrative for ICG Segment—General and administrative expenses for the ICG segment were $1,696,000, $1,654,000 and $1,545,000 in 2005, 2004 and 2003, respectively. As a percentage of net sales, general and administrative expenses were 6% in 2005 and 5% in both 2004 and 2003. The overall
35
expense increase in 2005 is primarily due to increased annual financial audit fees and Medis administrative expenses. The overall expense increase in 2004 as compared to 2003 is primarily due to additional headcount and the inclusion of Medis administrative expenses. Due to the acquisition of Medis on June 2, 2004, the prior year period does not include a comparable number of business days to 2005. We continue to focus on ongoing cost containment in all areas of our business with specific emphasis in the areas that are not directly related to sales growth.
General and Administrative for ECG Segment—General and administrative expenses for the ECG segment in 2005 and 2004 were $691,000 and $412,000, respectively. Because we did not acquire Vermed until March 22, 2004, the prior year period does not include a comparable number of business days to 2005. As a percentage of ECG net sales, general and administrative expenses in 2005 and 2004 were 7% and 6%, respectively. The overall expense increase in 2005 is primarily due to additional provision for bad debts of $66,000.
General and Administrative for Corporate Unallocated—Corporate unallocated items consist of general corporate expenses of a non-segment related nature. These unallocated expenses in 2005, 2004 and 2003 were $2,000,000, $1,317,000 and $517,000, respectively. In 2005, the increase was primarily due to increased external audit fees related to compliance with Section 404 internal control requirements provisions of the Sarbanes-Oxley Act of 2002. In 2004, the significant increase was due to additional headcount, outside consulting and accounting service fees related to compliance with the provisions of the Sarbanes-Oxley Act of 2002, including Section 404 relating to internal controls.
Also included in the 2004 amount are tax consulting fees related to the IRS Section 382 analysis of our net operating loss carry-forwards. We anticipate that these increased regulatory compliance requirements will continue to negatively impact our general and administrative costs in future periods.
Amortization of Intangible Assets for ICG Segment—In 2005 and 2004, the ICG segment had $77,000 and $66,000 of amortization expense. The second half of 2005 reflects reduced amortization expense based on the lower finalized valuation of identified intangible assets purchased in the acquisition of Medis. Because the acquisition of Medis occurred on June 2, 2004, the prior year cumulative results do not include a comparable number of business days to 2005.
Amortization of Intangible Assets for ECG Segment—Amortization expense for intangible assets for the ECG segment was $387,000 and $268,000 in 2005 and 2004, respectively. Because the acquisition of Vermed occurred on March 22, 2004, the prior year cumulative results do not include a comparable number of days to 2005.
Other Income for the ICG segment—Other ICG income was $177,000, $171,000, and $230,000 in 2005, 2004 and 2003, respectively. In 2005, other income included interest income of $193,000 and foreign translation gain of $61,000 resulting from the revaluation of the Medis deferred acquisition liability, partially offset by interest expense of $70,000 primarily related to interest on the Medis deferred acquisition liability and capital leases. In 2005, interest income was lower than 2004 due to less interest earned on internally financed equipment leases. In 2004, other income included interest income of $255,000, partially offset by a $56,000 foreign currency translation loss and $27,000 of interest expense. Foreign currency translation gain or losses are a result of the revaluation of the Medis deferred acquisition liability which can fluctuate depending on foreign exchange rates in effect as of the current reporting period.
Other Income (Expense) for Corporate Unallocated—Corporate unallocated net other income (expense) in 2005, 2004 and 2003 was ($240,000), ($147,000) and $121,000. The increased net other expense in both 2005 and 2004 was primarily the result of interest expense on the Comerica bank term loan in connection with the Vermed acquisition. In addition, interest income in 2005 and 2004 is due to lower cash balances as a result of cash used to fund the 2004 acquisitions. Because the acquisitions of Vermed and Medis occurred on March 22, 2004 and June 2, 2004, respectively, the prior year does not include a comparable number of days as what is included in 2005.
36
Income Tax Benefit (Provision)—Income tax benefit (provision) for 2005, 2004 and 2003 was ($11,003,000), $7,209,000 and ($227,000), respectively. The 2005 income tax provision includes a $12.5 million adjustment to re-establish the valuation allowance on our deferred tax assets as a result of the net tax losses incurred in 2005. In 2004, based on a growing history of profitability and projections of future taxable earnings and other factors, we determined that it is more likely than not that we will benefit from the use of our net operating loss carryforwards and therefore, eliminated our valuation allowance on our related deferred tax assets by $13.8 and realized a net income tax benefit.
Minority Interest in Income of Subsidiary—Minority interest in income of Medis in 2005 and 2004 was $54,000 and $37,000, respectively, and represents the 20% minority share interests retained by the sellers.
Liquidity and Capital Resources
Net cash provided by (used in) operating activities was ($138,000), $4,365,000 and $2,229,000 in 2005, 2004 and 2003, respectively. In 2005, net cash used in operations was due primarily to the net loss reported for the year and higher payments of accounts payable, accrued expenses and accrued compensation. This is offset by a significant decrease in accounts receivable balances due to strong cash collections. In 2004, the net cash provided by operations was due to an increase in net income, higher accounts payable and accrued compensation and expense balances and the tax benefit from the exercise of stock options and warrants, offset by deferred income tax and increased accounts receivable balances. In addition, the increase in deferred rent in both 2004 and 2005 is due to tenant improvement allowances received as reimbursement of leasehold improvements in accordance with the amended lease of our San Diego facility that was accounted for as a lease incentive.
In 2005, 2004 and 2003, we invested $1,244,000, $1,392,000 and $510,000, respectively, in property, plant and equipment. The higher property, plant and equipment investment levels in 2005 and 2004 were primarily due to acquisition of new manufacturing equipment at our Vermed division, leasehold improvements due to the expansion of our San Diego facility, and investments in computer software and equipment to continually upgrade our network, information system safeguarding and security capabilities in order to comply with the more stringent requirements of the Sarbanes-Oxley Act.
In June 1997, we entered into a five-year lease for an 18,000 square-foot manufacturing facility that also houses our research, development, marketing, sales and administrative activities. In June 2004, the lease was amended to include an additional 15,000 square-feet of expansion space and the term was extended through December 31, 2007. In March 2005, the lease was again amended to extend the lease an additional two years through December 31, 2009 and to provide for an additional $197,000 tenant improvement allowance. The lease payments on the original space are $20,000 per month through July 31, 2007, increasing to $22,000 per month through July 31, 2008 with annual increases of 3% each anniversary thereafter. The lease payments on the expansion space commenced on November 1, 2004 at $7,000 per month and then increased to $14,000 per month on November 1, 2005 with a 3% annual increase on each anniversary thereafter.
Cash provided (used) in financing activities in 2005, 2004 and 2003 was ($1,709,000), $8,203,000 and $767,000, respectively. This includes the exercise of stock options and warrants of $221,000, $2,937,000 and $757,000 in 2005, 2004 and 2003, respectively. In 2004, we borrowed $7,000,000 on a Comerica bank term loan for the Vermed acquisition in March 2004, and paid the balance down by $1,767,000, which included fixed principal repayments of $1,167,000 and pre-payments of $600,000. In 2005, the Comerica term loan payments totaled $2,050,000, which includes fixed principal payments of $1,750,000 and pre-payments of $300,000.
In November 2005, we restructured and amended certain provisions of the Comerica Bank term loan to extend the maturity date to November 1, 2008 and shift $2.2 million from term loan to the revolving credit line in order to lower our monthly installment payments. The interest rate on both the term loan and revolving line of credit were adjusted to one percent above the prime rate (subject to adjustment on a monthly basis), the minimum tangible net worth, liabilities to tangible net worth and debt service coverage ratios were eliminated, a maximum loss covenant was added and the minimum liquidity coverage was lowered to $1 million.
37
As of November 30, 2005, the Company’s revolving credit line availability is $2,087,000. In fiscal 2005, we had $2,200,000 of borrowings under the revolving credit line to pay down a portion of the term loan and had no other borrowings during the year. During fiscal 2004 and at November 30, 2004 there were no outstanding borrowings under the revolving credit line. At November 30, 2005, our consolidated quarterly loss exceeded the maximum net loss covenant of $450,000 for the fourth quarter of 2005 and as a result we were not in compliance with this covenant. On February 23, 2006, we entered into an amendment and waiver agreement with Comerica Bank which waived the fourth quarter covenant violation and amended the covenants, including the maximum loss covenants. We do not believe that the revised covenants are reasonably likely to materially limit our ability to borrow on the credit line.
There is an unused commitment fee of one half percent of the difference between the available revolving credit line and the average daily revolving credit balance, reduced by one-quarter percent based on a stated level of cash on deposit with the bank. The obligations of the Company under the revolving credit line and the term loan are secured by a pledge of all of the Company’s assets. In 2004, we issued letters of credit relating to the acquisition of Medis to secure the deferred acquisition payments due to the minority shareholders of Medis to be paid annually over five years through 2009. As of November 30, 2005, our outstanding letters of credit was 608,000 Euros ($713,000) which reduces credit available under our revolving credit line.
In March 2005, the Company’s Vermed subsidiary entered into a loan and promissory note agreement subject to a maximum loan availability of $480,000 with the Vermont Economic Development Authority (VEDA) to assist with the purchase and installation of custom designed manufacturing equipment. The interest rate is adjustable at 0.75% less than the tax exempt rate and the loan matures in January 2012 (4.25% at November 30, 2005). Under the terms of the loan, Vermed is required to maintain certain debt coverage levels and current ratios. We do not believe that the covenants are reasonably likely to materially limit our ability to borrow on the loan and promissory note agreement. The note payable is guaranteed by CardioDynamics and is secured by the manufacturing equipment which will approximate $1.2 million upon project completion.
At November 30, 2005, we have net operating loss carryforwards of approximately $28.4 million for federal income tax purposes that begin to expire in 2011. The Tax Reform Act of 1986 contains provisions that limit the amount of federal net operating loss carryforwards that can be used in any given year in the event of specified occurrences, including significant ownership changes. In 2004, we retained independent tax specialists to perform an analysis to determine the applicable annual limitation applied to the utilization of the net operating loss carryforwards due to ownership changes as defined in Internal Revenue Code (IRC) Section 382 that may have occurred. As a result of this study, and managements’ consideration of subsequent share ownership activity, we do not believe that the ownership change limitations would impair our ability to use our net operating losses against our current forecasted taxable income.
Prior to the fourth quarter of 2004, a valuation allowance was maintained for the full amount of the deferred tax asset created by the carryforwards. However, based on historical and forecasted taxable earnings, the conclusion of identified uncertainties such as the integration of recent acquisitions, manufacturing transition of our ICG sensors to Vermed, conclusion of multi-year clinical studies, and completion of the development and FDA approval of our BioZ Dx, the valuation allowance was removed, resulting in a significant income tax benefit in 2004.At November 30, 2005, in light of recent losses and other factors, we determined, based on our assessment of both positive and negative evidence and objective and subjective evidence, which takes into consideration our forecasted taxable income, that it is more likely than not that we will not realize all or a portion of the deferred tax assets, and therefore recorded a valuation allowance for the full amount of the deferred tax assets.
We believe that over the next 12 months our current cash and cash equivalents, operating cash flows and availability under our revolving line of credit will be sufficient to support our ongoing operating and investing requirements, capital expenditures and to meet the working capital requirements of anticipated future growth. As we continue to pursue opportunities to acquire or make investments in other technologies, products and businesses, we may choose to finance such acquisitions or investments by incurring debt or issuing equity. Our
38
long-term liquidity will depend on our ability to commercialize the BioZ and other diagnostic products and may require us to raise additional funds through public or private financing, bank loans, collaborative relationships or other arrangements.
Contractual Obligations
The following table summarizes our contractual obligations at November 30, 2005,(in thousands):
| | | | | | | | | | | | | | | |
| | | Total
| | Less than
1 year
| | 1-3
years
| | 3-5
years
| | More
than 5
years
|
Long-term debt obligations | | $ | 1,687 | | $ | 404 | | $ | 812 | | $ | 178 | | $ | 293 |
Capital lease obligations | | | 64 | | | 27 | | | 28 | | | 9 | | | — |
Non-cancelable operating lease obligations | | | 1,861 | | | 434 | | | 913 | | | 514 | | | — |
Deferred acquisition payments | | | 602 | | | 162 | | | 300 | | | 140 | | | — |
Warranty obligations | | | 578 | | | 132 | | | 138 | | | 308 | | | — |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Total | | $ | 4,792 | | $ | 1,159 | | $ | 2,191 | | $ | 1,149 | | $ | 293 |
| | |
|
| |
|
| |
|
| |
|
| |
|
|
Off-Balance Sheet Arrangements
We are not a party to off-balance sheet arrangements other than operating leases, and have not engaged in trading activities involving non-exchange traded contracts, and are not a party to any transaction with persons or activities that derive benefits, except as disclosed herein, from their non-independent relationships with the Company.
Critical Accounting Policies
The methods, estimates, and judgments we use in applying our most critical accounting policies have a significant impact on the results that we report in our consolidated financial statements. The SEC considers an entity’s most critical accounting policies to be those policies that are both most important to the portrayal of a company’s financial condition and results of operations, and those that require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about matters that are inherently uncertain at the time of the estimation. We believe the following critical accounting policies require significant judgments and estimates used in the preparation of our consolidated financial statements and this discussion and analysis of our financial condition and results of operations:
Revenue Recognition—We recognize revenue from the sale of products to end-users, distributors and strategic partners when persuasive evidence of a sale exists, the product is complete, tested and has been shipped which coincides with transfer of title and risk of loss, the sales price is fixed and determinable and collection of the resulting receivable is reasonably assured and there are no material contingencies or rights of return and the Company does not have significant obligations for future performance. Provisions for estimated future product returns and allowances are recorded in the period of the sale based on the historical and anticipated future rate of returns. Revenue is reduced for any discounts or trade-in allowances given to the buyer.
We sell some products under long-term financing arrangements and recognize the present value of the minimum payments using the rate implicit in the financing agreement as revenue at the time of sale and recognize interest income over the term of the contract. Revenue for extended warranty contracts beyond our standard warranty is recognized evenly over the life of the contract. Amounts received for warranty contracts that have not yet been earned, are recorded as deferred revenue.
Allowance for Doubtful Accounts and Sales Returns—We maintain an allowance for doubtful accounts to cover estimated losses resulting from the inability of our customers to make required payments. We determine
39
the adequacy of this allowance by regularly reviewing the accounts receivable aging and historical write-off rates. If customer payment timeframes were to deteriorate, additional allowances for doubtful accounts would be required.
Also included in the allowance for doubtful accounts is an estimate of potential future product returns related to current period sales recorded as a reduction of revenue. We analyze the rate of historical returns when evaluating the adequacy of the allowance for product returns.
Inventory Obsolescence—We maintain a reserve for excess, slow moving, and obsolete inventory as well as inventory with a carrying value in excess of its net realizable value. We value our inventory at the lower of cost, using the first-in, first-out method, or market. We review inventory on hand quarterly and record provisions for excess and obsolete inventory based on several factors, including our current assessment of future product demand, historical experience, and product expiration.
Valuation of Goodwill and Other Indefinite Lived Intangible Assets—We are required to perform an annual review for impairment of goodwill in accordance with Statement of Financial Accounting Standards No. 142 (SFAS No. 142), “Goodwill and Other Intangible Assets”. Goodwill is considered to be impaired if we determine that the carrying value of the reporting unit exceeds its fair value.
In addition to the annual review, an interim review is required if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. Examples of such events or circumstances include:
| | • | | a significant adverse change in legal factors or in the business climate; |
| | • | | a significant decline in our projected revenue or cash flows; |
| | • | | an adverse action or assessment by a regulator; |
| | • | | unanticipated competition; |
| | • | | a loss of key personnel; |
| | • | | a more-likely-than-not expectation that a reporting unit or a significant portion of a reporting unit will be sold or otherwise disposed of; and |
| | • | | the testing for recoverability under Statement 144 of a significant asset group within a reporting unit. |
There were no such impairments in the current year. If the assets are considered to be impaired, the impairment we recognize is the amount by which the carrying value of the assets exceeds the fair value of the assets. If a change were to occur in any of the above-mentioned factors or estimates, the likelihood of a material change in our reported results would increase.
Valuation of Long-Lived Assets—We assess the impairment of long-lived assets, consisting of property, plant and equipment and finite lived intangible assets, whenever events or circumstances indicate that the carrying value may not be recoverable. Examples of such events or circumstances include:
| | • | | the asset’s ability to continue to generate income from operations and positive cash flow in future periods; |
| | • | | loss of legal ownership or title to the asset; |
| | • | | significant changes in our strategic business objectives and utilization of the asset(s); and |
| | • | | the impact of significant negative industry or economic trends. |
There were no such impairments in the current year. If the assets are considered to be impaired, the impairment we recognize is the amount by which the carrying value of the assets exceeds the fair value of the
40
assets. In addition, we base useful lives and amortization or depreciation expense on our estimate of the period that the assets will generate revenue or otherwise be used by us.
We also periodically review the lives assigned to our intangible assets to ensure that our initial estimates do not exceed any revised estimated periods from which we expect to realize cash flows from the technologies. If a change were to occur in any of the above-mentioned factors or estimates, the likelihood of a material change in our reported results would increase.
Warranty Cost—We maintain a provision for product warranties. Estimates for warranty costs are calculated based primarily upon historical warranty experience and are evaluated on a quarterly basis to determine the appropriateness of such assumptions. Warranty provisions are adjusted from time to time when actual warranty claim experience differs from our estimates.
Income Taxes—We use the asset and liability approach to account for income taxes. This methodology recognizes deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax base of assets and liabilities and operating loss and tax credit carryforwards. We then record a valuation allowance to reduce deferred tax assets to an amount that more likely than not will be realized. We consider future taxable income in assessing the need for the valuation allowance, which requires the use of estimates. If we determine during any period that we could realize a larger net deferred tax asset than the recorded amount, we would adjust the deferred tax asset to record a charge to income for the period.
Recent Accounting Pronouncements
In December 2004, the Financial Accounting Standards Board (FASB) issued Statement of Accounting Standard (SFAS) 123R,Share-Based Payment.This statement is a revision of SFAS 123,Accounting for Stock-Based Compensation and supersedes APB 25,Accounting for Stock Issued to Employees. SFAS 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based at their fair values beginning with the first fiscal year beginning after June 15, 2005, with early adoption encouraged. Under the current rules, the Company will be required to adopt SFAS 123R in the first quarter of fiscal 2006, beginning December 1, 2005.
Under SFAS 123R, the pro forma disclosures previously permitted will no longer be an alternative to financial statement recognition. CardioDynamics must determine the appropriate fair value model to be used for valuing share-based payments to employees, the amortization method for compensation cost and the transition method to be used at the date of adoption. The transition methods include modified prospective and modified retrospective adoption options. Under the modified retrospective options, prior periods may be restated either as of the beginning of the year of adoption or for all periods presented. The modified prospective method requires that compensation expense be recorded for all unvested stock options and restricted stock at the beginning of the first quarter of adoption of SFAS 123R, while the modified retrospective method would record compensation expense for all unvested stock options and restrictive stock beginning with the first period restated.
Additionally, SFAS 123R clarifies the timing for recognizing compensation expense for awards subject to acceleration of vesting on retirement. This compensation expense must be recognized over the period from the date of grant to the date retirement eligibility is met if it is shorter than the vesting term. The effect on future earnings is dependent upon a number of factors including the number of options granted, the vesting period, the exercise price of the options, the Company stock price and volatility, and other factors and therefore cannot be determined. However, while the adoption of FAS 123R will have no effect on the Company’s cash flows or financial position, the Company believes that it will have an adverse effect on future results of operations.
In March 2005, the U.S. Securities and Exchange Commission, or SEC, released Staff Accounting Bulletin 107,Share-Based Payments, (“SAB 107”). The interpretations in SAB 107 express views of the SEC staff, or staff, regarding the interaction between SFAS 123R and certain SEC rules and regulations, and provide the
41
staff’s views regarding the valuation of share-based payment arrangements for public companies. SAB 107 provides guidance related to share-based payment transactions with non-employees, the transition from nonpublic to public entity status, valuation methods (including assumptions such as expected volatility and expected term), the accounting for certain redeemable financial instruments issued under share-based payment arrangements, the classification of compensation expense, non-GAAP financial measures, first-time adoption of SFAS 123R in an interim period, capitalization of compensation cost related to share-based payment arrangements, the accounting for income tax effects of share-based payment arrangements upon adoption of SFAS 123R, the modification of employee share options prior to adoption of SFAS 123R and disclosures in Management’s Discussion and Analysis subsequent to adoption of SFAS 123R. SAB 107 requires stock-based compensation be classified in the same expense lines as cash compensation is reported for the same employees. We will apply the principles of SAB 107 in conjunction with our adoption of SFAS 123R.
In May 2005, the FASB issued SFAS 154,Accounting Changes and Error Corrections, a replacement of APB 20,Accounting Changes and SFAS 3Reporting Accounting Changes in Interim Financial Statements. SFAS 154 requires retrospective application to prior periods’ financial statements of a voluntary change in accounting principle unless it is impracticable. SFAS 154 is intended to improve financial reporting by enhancing the consistency of financial information between periods. SFAS 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The Company is required to adopt the provisions of SFAS 154 in its first quarter of fiscal 2006. We do not expect the adoption of this statement will have a material impact on our financial position or results of operations.
The adoption of SFAS 151, Inventory Costs, an amendment of ARB No. 43, Chapter 4; had no effect on the Company’s financial position or results of operations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Sensitivity
The primary objective of our investment activities is to preserve principal, while at the same time, maximize the income we receive from our investments without significantly increasing risk. In the normal course of business, we employ established policies and procedures to manage our exposure to changes in the fair value of our investments. Under our current policies, we do not use interest rate derivative instruments to manage exposure to interest rate changes. We attempt to ensure the safety and preservation of our invested principal funds by limiting default risks, market risk and reinvestment risk. We mitigate default risk by investing in investment grade securities. Some of the securities that we have invested in may be subject to market risk. This means that a change in prevailing interest rates may cause the principal amount of the investment to fluctuate. To minimize this risk, we maintain substantially our entire portfolio of cash equivalents in commercial paper, certificates of deposit, money market and mutual funds. Our interest income is sensitive to changes in the general level of U.S. interest rates; however, due to the nature of our short-term investments, we have concluded that there is no material market risk exposure. As of November 30, 2005, we do not have any short-term investments.
During 2005, our primary exposure to market risk was interest rate risk associated with our variable rate debt. See “Item 7. Management’s Discussion and Analysis—Liquidity and Capital Resources” for further description of this debt instrument. A 1% change in interest rates on variable rate debt would have resulted in interest expense fluctuating by approximately $41,000 in fiscal 2005.
Foreign Currency Exchange Rate Risk
We are exposed to market risks related to foreign currency exchange rates, and have concluded that the market risk exposure is not material at this time.
42
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
CardioDynamics International Corporation
We have audited the accompanying consolidated balance sheet of CardioDynamics International Corporation and subsidiaries (the “Company”) as of November 30, 2005, and the related consolidated statements of operations, shareholders’ equity and comprehensive income (loss), and cash flows for the year then ended. Our audit also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of the Company as of November 30, 2005, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the financial statement schedule, presents fairly, in all material respects, the information set forth therein.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) the effectiveness of the Company’s internal control over financial reporting as of November 30, 2005, based on the criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 28, 2006 expressed an unqualified opinion thereon.
/s/ Mayer Hoffman McCann P.C.
San Diego, California
February 28, 2006
43
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
CardioDynamics International Corporation:
We have audited the accompanying consolidated balance sheet of CardioDynamics International Corporation and subsidiaries as of November 30, 2004, and the related consolidated statements of operations, shareholders’ equity and comprehensive income, and cash flows for each of the years in the two-year period ended November 30, 2004. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of CardioDynamics International Corporation and subsidiaries as of November 30, 2004, and the results of their operations and their cash flows for each of the years in the two-year period ended November 30, 2004, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of the Company’s internal control over financial reporting as of November 30, 2004, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 28, 2005, not included herein, expressed an unqualified opinion on management’s assessment of, and an adverse opinion on the effective operation of, internal control over financial reporting.
San Diego, California
February 28, 2005
44
CARDIODYNAMICS INTERNATIONAL CORPORATION
Consolidated Balance Sheets
(In thousands)
| | | | | | | | |
| | | November 30,
| |
| | | 2005
| | | 2004
| |
| Assets (Pledged) | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 3,615 | | | $ | 6,801 | |
Accounts receivable, net of allowance for doubtful accounts of $1,667 in 2005 and $2,400 in 2004 | | | 7,359 | | | | 11,674 | |
Inventory, net | | | 5,379 | | | | 4,647 | |
Current portion of long-term and installment receivables | | | 1,452 | | | | 1,518 | |
Deferred tax assets, current portion | | | — | | | | 2,046 | |
Other current assets | | | 398 | | | | 571 | |
| | |
|
|
| |
|
|
|
Total current assets | | | 18,203 | | | | 27,257 | |
| | |
Long-term receivables, net | | | 1,171 | | | | 1,248 | |
Property, plant and equipment, net | | | 5,508 | | | | 5,123 | |
Intangible assets, net | | | 3,711 | | | | 4,550 | |
Goodwill | | | 11,346 | | | | 11,186 | |
Deferred tax assets | | | — | | | | 8,595 | |
Other assets | | | 59 | | | | 71 | |
| | |
|
|
| |
|
|
|
Total assets | | $ | 39,998 | | | $ | 58,030 | |
| | |
|
|
| |
|
|
|
| Liabilities and Shareholders’ Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Revolving line of credit—bank | | $ | 2,200 | | | $ | — | |
Accounts payable, including amounts due to related party of $65 at November 30, 2005 and $145 at November 30, 2004 | | | 2,022 | | | | 2,781 | |
Accrued expenses and other current liabilities | | | 351 | | | | 606 | |
Accrued compensation | | | 1,612 | | | | 2,009 | |
Income taxes payable | | | 111 | | | | 37 | |
Current portion deferred revenue | | | 196 | | | | 256 | |
Current portion of deferred acquisition payments | | | 162 | | | | 191 | |
Provision for warranty repairs—current | | | 132 | | | | 99 | |
Current portion long-term debt | | | 431 | | | | 1,785 | |
| | |
|
|
| |
|
|
|
Total current liabilities | | | 7,217 | | | | 7,764 | |
| | |
|
|
| |
|
|
|
Long-term portion of deferred revenue | | | 65 | | | | 90 | |
Long-term portion of deferred rent | | | 506 | | | | 323 | |
Long-term portion of deferred acquisition payments | | | 440 | | | | 670 | |
Provision for warranty repairs—long-term | | | 446 | | | | 310 | |
Long-term debt, less current portion | | | 1,320 | | | | 3,945 | |
| | |
|
|
| |
|
|
|
Total long-term liabilities | | | 2,777 | | | | 5,338 | |
| | |
|
|
| |
|
|
|
Total liabilities | | | 9,994 | | | | 13,102 | |
| | |
|
|
| |
|
|
|
Minority interest | | | 241 | | | | 194 | |
Commitments and contingencies | | | — | | | | — | |
| | |
Shareholders’ equity: | | | | | | | | |
| | |
Preferred stock, 18,000 shares authorized, no shares issued or outstanding at November 30, 2005 or 2004 | | | — | | | | — | |
Common stock, no par value, 100,000 shares authorized, issued and outstanding 48,803 shares at November 30, 2005 and 48,721 shares at November 30, 2004 | | | 62,284 | | | | 62,063 | |
Accumulated other comprehensive income (loss) | | | (98 | ) | | | 149 | |
Accumulated deficit | | | (32,423 | ) | | | (17,478 | ) |
| | |
|
|
| |
|
|
|
Total shareholders’ equity | | | 29,763 | | | | 44,734 | |
| | |
|
|
| |
|
|
|
Total liabilities and shareholders’ equity | | $ | 39,998 | | | $ | 58,030 | |
| | |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements.
45
CARDIODYNAMICS INTERNATIONAL CORPORATION
Consolidated Statements of Operations
(In thousands, except per share data)
| | | | | | | | | | | | |
| | | For the years ended November 30,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Net sales | | $ | 37,005 | | | $ | 40,988 | | | $ | 30,332 | |
Cost of sales | | | 15,518 | | | | 11,637 | | | | 7,122 | |
| | |
|
|
| |
|
|
| |
|
|
|
Gross margin | | | 21,487 | | | | 29,351 | | | | 23,210 | |
| | |
|
|
| |
|
|
| |
|
|
|
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 2,487 | | | | 4,353 | | | | 3,272 | |
Selling and marketing | | | 17,981 | | | | 18,357 | | | | 15,548 | |
General and administrative | | | 4,387 | | | | 3,383 | | | | 2,062 | |
Amortization of intangible assets | | | 464 | | | | 334 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total operating expenses | | | 25,319 | | | | 26,427 | | | | 20,882 | |
| | |
|
|
| |
|
|
| |
|
|
|
Income (loss) from operations | | | (3,832 | ) | | | 2,924 | | | | 2,328 | |
| | |
|
|
| |
|
|
| |
|
|
|
Other income (expense): | | | | | | | | | | | | |
Interest income | | | 227 | | | | 339 | | | | 366 | |
Interest expense | | | (336 | ) | | | (255 | ) | | | (15 | ) |
Foreign currency gain (loss) | | | 61 | | | | (56 | ) | | | — | |
Other, net | | | (7 | ) | | | (1 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Other income (expense), net | | | (55 | ) | | | 27 | | | | 351 | |
| | |
|
|
| |
|
|
| |
|
|
|
Income (loss) before income taxes and minority interest | | | (3,887 | ) | | | 2,951 | | | | 2,679 | |
Minority interest in income of subsidiary | | | (55 | ) | | | (37 | ) | | | — | |
Income tax benefit (provision) | | | (11,003 | ) | | | 7,209 | | | | (227 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net income (loss) | | $ | (14,945 | ) | | $ | 10,123 | | | $ | 2,452 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net income (loss) per common share: | | | | | | | | | | | | |
Basic | | $ | (.31 | ) | | $ | .21 | | | $ | .05 | |
| | |
|
|
| |
|
|
| |
|
|
|
Diluted | | $ | (.31 | ) | | $ | .21 | | | $ | .05 | |
| | |
|
|
| |
|
|
| |
|
|
|
Weighted-average number of shares used in per share calculation: | | | | | | | | | | | | |
Basic | | | 48,787 | | | | 47,668 | | | | 46,248 | |
| | |
|
|
| |
|
|
| |
|
|
|
Diluted | | | 48,787 | | | | 49,164 | | | | 47,607 | |
| | |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements.
46
CARDIODYNAMICS INTERNATIONAL CORPORATION
Consolidated Statements of Shareholders’ Equity and Comprehensive Income (Loss)
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock
| | | Accumulated Other
Comprehensive Income (Loss)
| | | Accumulated Deficit
| | | Total Shareholders’ Equity
| | | Comprehensive Income (loss)
| |
| | | Shares
| | Amount
| | | | | |
Balance at November 30, 2002 | | 46,171 | | $ | 49,774 | | | $ | 3 | | | $ | (30,053 | ) | | $ | 19,724 | | | $ | 313 | |
Compensatory stock options granted | | — | | | 50 | | | | — | | | | — | | | | 50 | | | | — | |
Issuance of common stock, net of issuance costs | | — | | | 26 | | | | — | | | | — | | | | 26 | | | | — | |
Issuance of common stock—upon exercise of stock options and warrants | | 347 | | | 757 | | | | — | | | | — | | | | 757 | | | | — | |
Stock options income tax benefit | | — | | | 31 | | | | — | | | | — | | | | 31 | | | | — | |
Unrealized loss on marketable securities | | — | | | — | | | | (20 | ) | | | — | | | | (20 | ) | | | (20 | ) |
Net income | | — | | | — | | | | — | | | | 2,452 | | | | 2,452 | | | | 2,452 | |
| | |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at November 30, 2003 | | 46,518 | | | 50,638 | | | | (17 | ) | | | (27,601 | ) | | | 23,020 | | | | 2,432 | |
| | | | | | | | | | | | | | | | | | | | |
|
|
|
Compensatory stock options granted | | — | | | 12 | | | | — | | | | — | | | | 12 | | | | — | |
Issuance of common stock, net of issuance costs | | — | | | (22 | ) | | | — | | | | — | | | | (22 | ) | | | — | |
Issuance of common stock—upon exercise of stock options and warrants | | 1,357 | | | 2,937 | | | | — | | | | — | | | | 2,937 | | | | — | |
Issuance of common stock for purchase of businesses | | 846 | | | 5,136 | | | | — | | | | — | | | | 5,136 | | | | — | |
Stock options income tax benefit | | — | | | 3,362 | | | | — | | | | — | | | | 3,362 | | | | — | |
Unrealized gain on marketable securities | | — | | | — | | | | 17 | | | | — | | | | 17 | | | | 17 | |
Foreign currency translation adjustment, net of deferred taxes of $95 | | — | | | — | | | | 149 | | | | — | | | | 149 | | | | 149 | |
Net income | | — | | | — | | | | — | | | | 10,123 | | | | 10,123 | | | | 10,123 | |
| | |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at November 30, 2004 | | 48,721 | | | 62,063 | | | $ | 149 | | | | (17,478 | ) | | | 44,734 | | | | 10,289 | |
| | | | | | | | | | | | | | | | | | | | |
|
|
|
Issuance of common stock—upon exercise of stock options and warrants | | 82 | | | 221 | | | | — | | | | — | | | | 221 | | | | — | |
Foreign currency translation adjustment, net of deferred taxes of $0 | | — | | | — | | | | (247 | ) | | | — | | | | (247 | ) | | | (247 | ) |
Net loss | | — | | | — | | | | — | | | | (14,945 | ) | | | (14,945 | ) | | | (14,945 | ) |
| | |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
Balance at November 30, 2005 | | 48,803 | | $ | 62,284 | | | $ | (98 | ) | | $ | (32,423 | ) | | $ | 29,763 | | | $ | (15,192 | ) |
| | |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements
47
CARDIODYNAMICS INTERNATIONAL CORPORATION
Consolidated Statements of Cash Flows
(In thousands)
| | | | | | | | | | | | |
| | | For the years ended
November 30,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Cash flows from operating activities: | | | | | | | | | | | | |
Net income (loss) | | $ | (14,945 | ) | | $ | 10,123 | | | $ | 2,452 | |
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
Minority interest in income of subsidiary | | | 55 | | | | 37 | | | | — | |
Provision (benefit) for deferred income taxes | | | 10,736 | | | | (10,736 | ) | | | — | |
Tax benefit from exercise of stock options and warrants | | | — | | | | 3,362 | | | | 31 | |
Depreciation | | | 733 | | | | 484 | | | | 290 | |
Amortization of intangible assets | | | 464 | | | | 334 | | | | — | |
Provision for (reduction in) warranty repairs, net | | | 281 | | | | (237 | ) | | | 301 | |
Provision for doubtful accounts | | | 2,545 | | | | 2,904 | | | | 3,003 | |
Provision for (reduction in) doubtful long-term receivables | | | 23 | | | | (201 | ) | | | (7 | ) |
Stock based compensation expense | | | — | | | | 12 | | | | 50 | |
Other non-cash items, net | | | (48 | ) | | | 73 | | | | — | |
Changes in operating assets and liabilities, excluding the effects of acquisitions in 2004: | | | | | | | | | | | | |
Accounts receivable | | | 1,770 | | | | (4,420 | ) | | | (3,947 | ) |
Inventory | | | (732 | ) | | | (392 | ) | | | 311 | |
Long-term and installment receivables and note receivable | | | 120 | | | | 939 | | | | 412 | |
Other current assets | | | 173 | | | | (185 | ) | | | (100 | ) |
Other assets | | | 3 | | | | (56 | ) | | | (68 | ) |
Accounts payable | | | (759 | ) | | | 1,404 | | | | (733 | ) |
Accrued expenses and other current liabilities | | | (333 | ) | | | 376 | | | | 7 | |
Accrued compensation | | | (397 | ) | | | 472 | | | | 226 | |
Income taxes payable | | | 74 | | | | (67 | ) | | | 44 | |
Deferred revenue | | | (85 | ) | | | (74 | ) | | | (31 | ) |
Deferred rent | | | 184 | | | | 213 | | | | (12 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Net cash provided by (used) in operating activities | | | (138 | ) | | | 4,365 | | | | 2,229 | |
| | |
|
|
| |
|
|
| |
|
|
|
Cash flows from investing activities: | | | | | | | | | | | | |
Proceeds from sale of short-term investments | | | — | | | | 4,588 | | | | 2,000 | |
Purchases of short-term investments | | | — | | | | — | | | | (2,099 | ) |
Purchases of property, plant and equipment | | | (1,244 | ) | | | (1,392 | ) | | | (510 | ) |
Purchase of businesses, net of cash acquired | | | (56 | ) | | | (13,750 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Net cash used in investing activities | | | (1,300 | ) | | | (10,554 | ) | | | (609 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Cash flows from financing activities: | | | | | | | | | | | | |
Proceeds from issuance of debt | | | 2,602 | | | | 7,069 | | | | — | |
Repayment of debt | | | (4,334 | ) | | | (1,781 | ) | | | (16 | ) |
Payment of deferred acquisition costs | | | (198 | ) | | | — | | | | — | |
Exercise of stock options and warrants | | | 221 | | | | 2,937 | | | | 757 | |
Issuance of common stock, net | | | — | | | | (22 | ) | | | 26 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net cash provided by (used in) financing activities | | | (1,709 | ) | | | 8,203 | | | | 767 | |
| | |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements.
48
CARDIODYNAMICS INTERNATIONAL CORPORATION
Consolidated Statements of Cash Flows—(Continued)
(In thousands)
| | | | | | | | | | | | |
| | | For the years ended
November 30,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Effect of exchange rate changes on cash and cash equivalents | | | (39 | ) | | | 25 | | | | — | |
Net increase (decrease) in cash and cash equivalents | | | (3,186 | ) | | | 2,039 | | | | 2,387 | |
Cash and cash equivalents at beginning of year | | | 6,801 | | | | 4,762 | | | | 2,375 | |
| | |
|
|
| |
|
|
| |
|
|
|
Cash and cash equivalents at end of year | | $ | 3,615 | | | $ | 6,801 | | | $ | 4,762 | |
| | |
|
|
| |
|
|
| |
|
|
|
Supplemental disclosures of cash flow information: | | | | | | | | | | | | |
Cash payments during the year for: | | | | | | | | | | | | |
Interest | | $ | 307 | | | $ | 276 | | | $ | 15 | |
Income taxes | | $ | 225 | | | $ | 223 | | | $ | 150 | |
Supplemental disclosures of non-cash investing and financing activities: | | | | | | | | | | | | |
Unrealized holding gain (loss) on available-for-sale securities, less deferred tax effect | | $ | — | | | $ | 17 | | | $ | (20 | ) |
Equipment acquired under capital lease | | $ | — | | | $ | 44 | | | $ | — | |
Supplemental non-cash disclosure of purchase of businesses: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | — | | | $ | 127 | | | $ | — | |
Accounts receivable, net | | | — | | | | 598 | | | | — | |
Inventory | | | — | | | | 1,092 | | | | — | |
Other current assets | | | — | | | | 64 | | | | — | |
Property, plant and equipment | | | — | | | | 3,517 | | | | — | |
Goodwill | | | — | | | | 11,055 | | | | — | |
Intangible assets | | | — | | | | 4,721 | | | | — | |
Accounts payable | | | — | | | | (582 | ) | | | — | |
Accrued expenses and other current liabilities | | | — | | | | (50 | ) | | | — | |
Accrued compensation | | | — | | | | (116 | ) | | | — | |
Income taxes payable | | | — | | | | (60 | ) | | | — | |
Provision for warranty repairs | | | — | | | | (12 | ) | | | — | |
Long-term debt, including current portion | | | — | | | | (386 | ) | | | — | |
Minority interest | | | — | | | | (149 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total purchase price | | | — | | | | 19,819 | | | | — | |
Less cash paid | | | — | | | | (13,877 | ) | | | — | |
Less deferred acquisition payments | | | — | | | | (806 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Common stock issued | | $ | — | | | $ | 5,136 | | | $ | — | |
| | |
|
|
| |
|
|
| |
|
|
|
See accompanying notes to consolidated financial statements.
49
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements
(1) Organization and Summary of Significant Accounting Policies
Description of Business
CardioDynamics International Corporation (“CardioDynamics” or “the Company”) is an innovator of an important medical technology called Impedance Cardiography (ICG). CardioDynamics develops, manufactures and markets noninvasive ICG diagnostic and monitoring devices, proprietary ICG sensors and a broad array of medical device electrodes. The Company was incorporated as a California corporation in June 1980 and changed its name to CardioDynamics International Corporation in October 1993.
Principles of Consolidation
The consolidated financial statements include the accounts of CardioDynamics International Corporation and its majority and wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. These estimates and assumptions include, but are not limited to, assessing the following: the valuation of accounts receivables, inventory and long-term receivables, valuation allowance of deferred tax assets, the ability to estimate warranty obligations, provisions for returns and allowances and the determination of whether collection of revenue arrangements is probable or reasonably assured.
Revenue Recognition
The Company recognizes revenue from the sale of its products to end-users, distributors and strategic partners when persuasive evidence of a sale exists including; the product is complete, tested and has physically shipped, the sales price is fixed and determinable, the buyer is obligated to pay the total purchase price, title for the product has transferred to the buyer, collection of the resulting receivable is reasonably assured, there are no material contingencies or rights of return and the Company does not have significant obligations for future performance.
The Company also sells products under long-term financing arrangements and recognizes the present value of the minimum payments, based on the interest rate implicit in the financing agreement, as revenue at the time of sale. Deferred interest income is recognized on a monthly basis over the term of the financing arrangement. Revenue for separately priced extended warranty contracts is recognized evenly over the life of the contract. Amounts received for warranty contracts that have not yet been earned, are recorded as deferred revenue.
Provisions for estimated future product returns and allowances are recorded in the period of the sale based on the historical and anticipated future rate of returns. The Company records shipping and handling charged to customers as revenue and shipping and handling costs to cost of sales as incurred. Revenue is reduced for any discounts or trade in allowances given to the buyer.
Fair Value of Financial Instruments
The carrying amounts of financial instruments such as cash and cash equivalents, accounts receivable, other current assets, long-term receivables, revolving line of credit, accounts payable, accrued expenses and other current liabilities and accrued compensation, are reasonable estimates of their fair value because of the short-term
50
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
nature of these financial instruments. The fair value of each below-market, long-term receivable was estimated by discounting the future cash flows based on the interest rate implicit in the financing agreement. Long-term receivable financing arrangements include a market rate of interest and the carrying value approximates fair value. Long-term debt, which is based on borrowing rates currently available to the Company for loans with similar terms and maturities, is reported at its carrying value, which the Company believes approximates the fair value.
Cash Equivalents
Cash equivalents are short-term, highly liquid investments with maturities of three months or less at the time of purchase. These investments generally consist of money market funds and commercial paper and are stated at cost, which approximates fair market value.
Accounts Receivable
The Company provides allowances against trade receivables for estimated losses resulting from our customers’ inability to pay. The adequacy of this allowance is determined by regularly reviewing the accounts receivable aging and historical write-off rates. If customer payment timeframes were to deteriorate, additional allowances for doubtful accounts would be required. Also included in the allowance for doubtful accounts is an estimate of potential future product returns related to product sales. We analyze the rate of historical returns when evaluating the adequacy for product returns which is recorded as a reduction of current period revenue.
Inventory
Inventory is stated at the lower of cost (first-in, first-out method) or market. The Company evaluates inventory on hand against historical and planned usage to determine appropriate provisions for obsolete, slow-moving and demonstration inventory. Inventory includes material, labor and overhead costs.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost. Property, plant and equipment acquired under capital leases are recorded at the present value of future minimum lease payments. Leasehold improvements are amortized using the straight-line method over the shorter of the remaining lease term or the estimated useful life of the improvement. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is recorded.
Goodwill and Other Intangible Assets
In accordance with SFAS No. 142,Goodwill and Other Intangible Assets, goodwill and other intangible assets with indefinite lives are tested for impairment annually or more frequently if an event or circumstances indicates that impairment has occurred. We perform impairment reviews at a reporting unit level and used both the guideline company method and a discounted cash flow model, based on management’s judgment and assumptions, to determine the estimated fair value of each reporting unit.
An impairment loss generally would be recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit. Impairment testing indicated that the estimated fair value of each reporting unit exceeded its corresponding carrying amount, including goodwill and, as such, no impairment exists as of November 30, 2004 or 2005.
51
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
Long-Lived Assets
In accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, long-lived assets with finite lives are tested for impairment whenever events or changes in circumstances indicate that its carrying value may not be recoverable. A significant decrease in the fair value of a long-lived asset, an adverse change in the extent or manner in which a long-lived asset is being used or in its physical condition or an expectation that a long-lived asset will be sold or disposed of significantly before the end of its previously estimated life are among several of the factors that could result in an impairment charge.
Recoverability of assets to be held and used in operations is measured by a comparison of the carrying amount of an asset to the future net cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less selling costs. As of November 30, 2005 and 2004, there were no known events or circumstances that have occurred which would indicate that impairment has occurred.
Warranty Cost
The Company records a provision for warranty repairs on all stand-alone BioZ systems sold, which is included in cost of sales in the consolidated statements of operations and is recorded in the same period the related revenue is recognized. The warranty provision is calculated using historical data to determine the percentage of systems that may require repairs during the warranty period and the average cost to repair a system. This financial model is then used to calculate the future probable expenses related to warranty and the required warranty provision. The historical data used in this model are reviewed and updated as circumstances change over the product’s life cycle. If actual warranty expenditures differ substantially from our estimates, revisions to the warranty provision would be required. Actual warranty expenditures are recorded against the warranty provision as they are incurred.
Research and Development
Research and development costs are expensed in the period incurred.
Advertising
Advertising costs are expensed in the period incurred. Advertising costs, including trade show expenses, amounted to $1,011,000 in 2005, $1,198,000 in 2004 and $1,019,000 in 2003.
Comprehensive Income (Loss)
Comprehensive income (loss) is the change in the Company’s equity (net assets) during each period from transactions and other events and circumstances from non-owner sources. It includes net income (loss), unrealized gains and losses on short-term investments and foreign currency translation adjustments. Marketable securities generally consist of investments in debt instruments of financial institutions and corporations with strong credit ratings, and in U.S. government obligations.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit
52
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. To the extent that available evidence about future taxable earnings indicates that it is more likely than not that the tax benefit associated with the deferred tax assets will not be realized, a valuation allowance is established.
Foreign Currency Translation
Foreign currency translation adjustments are a result of translating assets and liabilities of our foreign subsidiary from its functional currency into the reporting currency, U.S. dollars, using the period-end exchange rate. The average exchange rate of each reporting period is used to translate revenue and expenses. The cumulative translation adjustments are included in accumulated other comprehensive income (loss) reported as a separate component of shareholders’ equity.
We have a payable relating to the Medis acquisition that is denominated in a foreign currency. This payable is reported as deferred acquisition payments in the consolidated balance sheet. The carrying amount of this payable is recorded at net present value and is subject to changes in currency exchange rates and the unrealized gains or losses are included in the determination of net income (loss) in the consolidated statements of operations as foreign currency loss.
Stock-Based Compensation
The Company accounts for stock options granted to employees in accordance with the provisions of Accounting Principles Board (“APB”) Opinion No. 25,Accounting for Stock Issued to Employees, as amended, and related interpretations.As such, compensation expense is recorded on the date of grant only if the current market price of the underlying stock exceeds the exercise price. The Company recognizes any resulting compensation expense over the associated service period, which is generally the option vesting term. The Company has implemented the disclosure provisions of SFAS No. 148 Accounting for Stock-Based Compensation—Transition and Disclosure. SFAS No. 148 amends SFAS No. 123,Accounting for Stock-Based Compensation. SFAS 148 amended the disclosure requirements of FAS 123 to require more prominent disclosures in both annual and interim f/s regarding the method of accounting for stock-based compensation and the effect of the method used on reported results.
At November 30, 2005, the Company had two stock-based employee compensation plans. No compensation cost has been recognized in the consolidated financial statements for the stock options issued to employees since they were all issued at fair market value on the date of grant. Awards under the plan vest over periods of up to four years.
In October 2005, the Board of Directors of the Company accelerated the vesting of all unvested, out-of-the-money, employee explicit service period stock options granted under the Company’s Stock Option Plans. The Board took this action with the belief that it is in the best interests of the Company’s stockholders as it will reduce the Company’s reported compensation expense in future periods that the Company would otherwise recognize in its Statement of Operations with respect to these accelerated stock options upon the adoption by the Company of SFAS No. 123R,Revised 2004, Share-Based Payment.
A stock option was considered “out-of-the money” if the stock option exercise price was $2.00 or higher. As a result of this action, stock options to purchase 1.4 million shares of the Company’s common stock became immediately exercisable, including 0.5 million stock options held by Company executive officers. The weighted average exercise price of all the accelerated stock options was $4.46. FAS 123R will be effective commencing
53
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
with the Company’s first quarter in 2006 and will require that compensation cost related to share-based payment transactions, including stock options, be recognized in the Company’s financial statements.
The weighted-average fair value of options granted during fiscal 2005, 2004 and 2003, was $1.24, $2.97 and $1.98, respectively, using a Black-Scholes option pricing model with the following assumptions:
| | | | | | | | | |
| | | 2005
| | | 2004
| | | 2003
| |
Expected volatility | | 60.9 | % | | 68.1 | % | | 88.1 | % |
Expected dividend yield | | 0 | % | | 0 | % | | 0 | % |
Risk-free interest rate | | 3.8 | % | | 2.3 | % | | 2.1 | % |
Expected life | | 3.5 years | | | 3.7 years | | | 3.1 years | |
The following table illustrates the effect on net income (loss) and net income (loss) per common share as if the Company had applied the fair value recognition provisions of SFAS No. 123 to all outstanding and unvested awards in each period.
| | | | | | | | | | | | |
| | | (In thousands, except per share data) | |
| | | |
| | | 2005
| | | 2004
| | | 2003
| |
Net income (loss) as reported | | $ | (14,945 | ) | | $ | 10,123 | | | $ | 2,452 | |
Add: Stock-based employee compensation expense included in reported net income (loss), net of tax | | | — | | | | — | | | | — | |
Deduct: Total stock-based employee compensation expense determined under fair value based method for all awards, net of tax | | | (5,500 | ) | | | (1,847 | ) | | | (2,205 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Pro forma net income (loss) | | $ | (20,445 | ) | | $ | 8,276 | | | $ | 247 | |
| | |
|
|
| |
|
|
| |
|
|
|
Earnings (loss) per common share: | | | | | | | | | | | | |
As reported—basic | | $ | (.31 | ) | | $ | .21 | | | $ | .05 | |
| | |
|
|
| |
|
|
| |
|
|
|
As reported—diluted | | $ | (.31 | ) | | $ | .21 | | | $ | .05 | |
| | |
|
|
| |
|
|
| |
|
|
|
Pro forma—basic | | $ | (.42 | ) | | $ | .17 | | | $ | .01 | |
| | |
|
|
| |
|
|
| |
|
|
|
Pro forma—diluted | | $ | (.42 | ) | | $ | .17 | | | $ | .01 | |
| | |
|
|
| |
|
|
| |
|
|
|
Net Income (Loss) Per Common Share
Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted-average number of common shares outstanding during the period. Diluted income (loss) per share is calculated by including the additional shares of common stock issuable upon exercise of outstanding options and warrants that are not anti-dilutive, in the weighted-average share calculation. The following table lists the potentially dilutive equity instruments, each convertible into one share of common stock.(In thousands)
| | | | | | |
| | | For the years ended
November 30,
|
| | | 2005
| | 2004
| | 2003
|
Weighted average common shares outstanding—basic | | 48,787 | | 47,668 | | 46,248 |
Effect of dilutive securities: | | | | | | |
Stock options | | — | | 1,169 | | 1,332 |
Warrants | | — | | 327 | | 27 |
| | |
| |
| |
|
Dilutive potential shares | | — | | 1,496 | | 1,359 |
| | |
| |
| |
|
Weighted average common shares outstanding—dilutive | | 48,787 | | 49,164 | | 47,607 |
| | |
| |
| |
|
54
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
The following potentially dilutive instruments were not included in the per share calculation for 2005, 2004 or 2003 as their effect was antidilutive.
| | | | | | |
| | | (In thousands) |
| |
| | | For the years ended
November 30,
|
| | | 2005
| | 2004
| | 2003
|
Stock options | | 4,345 | | 1,317 | | 1,378 |
Warrants | | — | | — | | 2,000 |
| | |
| |
| |
|
Total | | 4,345 | | 1,317 | | 3,378 |
| | |
| |
| |
|
Reclassifications
Financial presentations for certain prior years account balances have been reclassified in order to conform to current year presentation.
Recent Accounting Pronouncements
In December 2004, the Financial Accounting Standards Board (FASB) issued Statement of Accounting Standard (SFAS) 123R,Share-Based Payment.This statement is a revision of SFAS 123,Accounting for Stock-Based Compensation and supersedes APB 25,Accounting for Stock Issued to Employees. SFAS 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based at their fair values beginning with the first fiscal year beginning after June 15, 2005, with early adoption encouraged. Under the current rules, the Company will be required to adopt SFAS 123R in the first quarter of fiscal 2006, beginning December 1, 2005.
Under SFAS 123R, the pro forma disclosures previously permitted will no longer be an alternative to financial statement recognition. CardioDynamics must determine the appropriate fair value model to be used for valuing share-based payments to employees, the amortization method for compensation cost and the transition method to be used at the date of adoption. The transition methods include modified prospective and modified retrospective adoption options. Under the modified retrospective options, prior periods may be restated either as of the beginning of the year of adoption or for all periods presented. The modified prospective method requires that compensation expense be recorded for all unvested stock options and restricted stock at the beginning of the first quarter of adoption of SFAS 123R, while the modified retrospective method would record compensation expense for all unvested stock options and restrictive stock beginning with the first period restated.
Additionally, SFAS 123R clarifies the timing for recognizing compensation expense for awards subject to acceleration of vesting on retirement. This compensation expense must be recognized over the period from the date of grant to the date retirement eligibility is met if it is shorter than the vesting term. The effect on future earnings is dependent upon a number of factors including the number of options granted, the vesting period, the exercise price of the options, the Company stock price and volatility, and other factors and therefore cannot be determined. However, while the adoption of FAS 123R will have no effect on the Company’s cash flows or financial position, the Company believes that it will have an adverse effect on future results of operations.
In March 2005, the U.S. Securities and Exchange Commission, or SEC, released Staff Accounting Bulletin 107,Share-Based Payments, (“SAB 107”). The interpretations in SAB 107 express views of the SEC staff, or staff, regarding the interaction between SFAS 123R and certain SEC rules and regulations, and provide the staff’s views regarding the valuation of share-based payment arrangements for public companies. SAB 107 provides guidance related to share-based payment transactions with non-employees, the transition from
55
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
nonpublic to public entity status, valuation methods (including assumptions such as expected volatility and expected term), the accounting for certain redeemable financial instruments issued under share-based payment arrangements, the classification of compensation expense, non-GAAP financial measures, first-time adoption of SFAS 123R in an interim period, capitalization of compensation cost related to share-based payment arrangements, the accounting for income tax effects of share-based payment arrangements upon adoption of SFAS 123R, the modification of employee share options prior to adoption of SFAS 123R and disclosures in Management’s Discussion and Analysis subsequent to adoption of SFAS 123R. SAB 107 requires stock-based compensation be classified in the same expense lines as cash compensation is reported for the same employees. We will apply the principles of SAB 107 in conjunction with our adoption of SFAS 123R.
In May 2005, the FASB issued SFAS 154, Accounting Changes and Error Corrections, a replacement of APB 20, Accounting Changes and SFAS 3 Reporting Accounting Changes in Interim Financial Statements. SFAS 154 requires retrospective application to prior periods’ financial statements of a voluntary change in accounting principle unless it is impracticable. SFAS 154 improves financial reporting because its requirements enhance the consistency of financial information between periods. SFAS 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The Company is required to adopt the provisions of SFAS 154 in its first fiscal quarter of fiscal 2006. We do not expect the adoption of this statement to have a material impact on our financial position or results of operations.
The adoption of SFAS 151, Inventory Costs, an amendment of ARB No. 43, Chapter 4; had no effect on the Company’s financial position or results of operations.
(2) Business Combinations
Vermed Acquisition
On March 22, 2004, the Company acquired substantially all of the assets and certain liabilities of Vermed. Vermed is a manufacturer of electrodes and related supplies used in electrocardiograms and other diagnostic procedures for cardiology, electrotherapy, sleep testing, neurology and general purpose diagnostic testing. Vermed is located in Bellow Falls, Vermont. The final purchase price consisted of $12 million in cash, $533,000 of acquisition costs, and the issuance to Vermont Medical, Inc. of 745,733 shares of the Company’s common stock valued at $4.5 million.
The Vermed acquisition was accounted for using the purchase method of accounting whereby the total purchase price was allocated to tangible and identifiable intangible assets based on their fair values as of the date of acquisition. The excess of the purchase price over the fair value of net tangible and identifiable intangible assets has been recorded as goodwill.
The results of Vermed’s operations have been included in the accompanying consolidated financial statements from the date of acquisition. The final cost of the acquisition is as follows(In thousands):
| | | |
Cash paid for net assets and acquisition costs | | $ | 12,533 |
Issuance of common stock | | | 4,500 |
| | |
|
|
Total purchase price | | $ | 17,033 |
| | |
|
|
56
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
The allocation of the Vermed purchase price is as follows:
| | | | |
Accounts receivable, net | | $ | 468 | |
Inventory | | | 1,065 | |
Property, plant and equipment | | | 2,551 | |
Goodwill | | | 9,521 | |
Identifiable intangible assets | | | 4,000 | |
Accounts payable | | | (572 | ) |
| | |
|
|
|
Total Vermed purchase price | | $ | 17,033 | |
| | |
|
|
|
Identifiable intangible assets acquired in the Vermed transaction consist of the following (In thousands):
| | | | | |
| | | | | Estimated Life
(years)
|
Customer lists | | $ | 2,900 | | 10 |
OEM relationships | | | 900 | | 10 |
Proprietary gel formulas | | | 100 | | 15 |
Trademark and trade name | | | 100 | | Indefinite |
| | |
|
| | |
| | | $ | 4,000 | | |
| | |
|
| | |
The following unaudited pro forma consolidated information is presented as if the March 2004 acquisition of Vermed occurred on December 1, 2003. These unaudited pro forma consolidated results have been prepared for comparative purposes only and do not purport to be indicative of the results of operations that would have actually resulted had the acquisition been in effect in the periods indicated above, or of the future results of operation. The unaudited pro forma consolidated results for year ended November 30 are as follows(In thousands):
| | | |
| | | 2004
|
Net sales | | $ | 43,559 |
Net income | | | 10,307 |
Earnings per share: | | | |
Basic | | $ | 0.22 |
Diluted | | $ | 0.21 |
Medis Acquisition
On June 2, 2004, the Company acquired 80% of all outstanding shares of Medis, a privately held European cardiology and vascular device company. Medis is located in Ilmenau, Germany and develops, manufactures and sells ICG and venous blood flow products. Medis operates as a majority-owned subsidiary of CardioDynamics and Dr. Olaf Solbrig, co-founder of Medis, continues as Managing Director. Dr. Solbrig and his partner retain a 20% minority interest in Medis. The final purchase price consisted of Euros 800,000 ($985,000) in cash at the date of acquisition, Euros 760,000 (at present value of $806,000 using a 5% discount rate) to be paid over five years in equal installments, the issuance of 100,000 shares of the Company’s common stock valued at $636,000 and $415,000 of acquisition costs. The financial results of Medis are included in our consolidated financial statements from the date of acquisition.
57
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
The Medis acquisition was accounted for using the purchase method of accounting whereby the total purchase price was allocated to tangible and identifiable intangible assets based on their fair values as of the date of acquisition. The excess of the purchase price over the fair value of net tangible and identifiable intangible assets has been recorded as goodwill. The Company funded the cash portion of the transaction with available cash. The acquisition was not considered material to the overall consolidated financial statements, or pro forma financial statements. As of November 30, 2005 the Company had $1,770,000 of goodwill related to Medis, an increase of $356,000 over the prior year balance based on the final valuation of Medis’ amortizable intangible assets and additional acquisition related costs.
The total cost of the acquisition is as follows(In thousands):
| | | |
Cash paid for net assets and acquisition costs | | $ | 1,400 |
Issuance of common stock | | | 636 |
Deferred acquisition payment to be paid over five years | | | 806 |
| | |
|
|
Total purchase price | | $ | 2,842 |
| | |
|
|
The allocation of the purchase price, which was finalized in March 2005, is as follows:
| | | | |
Total current assets | | $ | 348 | |
Property, plant and equipment | | | 966 | |
Goodwill | | | 1,911 | |
Identifiable intangible assets | | | 400 | |
Total current liabilities | | | (248 | ) |
Total long-term liabilities | | | (386 | ) |
Minority interest | | | (149 | ) |
| | |
|
|
|
Total purchase price | | $ | 2,842 | |
| | |
|
|
|
(3) Geographic and Segment Information
Significant Customers
During the year ended November 30, 2005, the Company had a single major customer, Physician Sales and Services (PSS), that exceeded 10% of total net sales. The net revenues from PSS included in both ICG and ECG segment net sales for the year ended November 30, 2005 totaled $7,144,000. For the years ended November 30, 2004 and 2003, the Company did not have any individual customer or distributor that accounted for 10% or more of total net sales.
Geographic Information
Net sales for domestic and international for the years ended November 30 were as follows(In thousands):
| | | | | | | | | |
| | | 2005
| | 2004
| | 2003
|
United States | | $ | 33,567 | | $ | 37,868 | | $ | 28,773 |
International (1) | | | 3,438 | | | 3,120 | | | 1,559 |
| | |
|
| |
|
| |
|
|
Total consolidated net sales | | $ | 37,005 | | $ | 40,988 | | $ | 30,332 |
| | |
|
| |
|
| |
|
|
58
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
Net long-lived assets by geographic area at November 30 were as follows(In thousands):
| | | | | | | | | |
| | | 2005
| | 2004
| | 2003
|
United States | | $ | 17,564 | | $ | 17,432 | | $ | 634 |
Europe | | | 3,001 | | | 3,427 | | | — |
| | |
|
| |
|
| |
|
|
Net long-lived assets (2) | | $ | 20,565 | | $ | 20,859 | | $ | 634 |
| | |
|
| |
|
| |
|
|
| (1) | Sales to customers attributed to geographical areas other than the United States are not material for purposes of separate disclosure. |
| (2) | Net long-lived assets include property, plant and equipment, goodwill and intangible assets. |
Segment Information
We classify our businesses principally through two reportable operating segments as follows:
Impedance Cardiography (“ICG”)
The ICG segment consists primarily of the development, manufacture and sales of the BioZ ICG Monitor, BioZ ICG Module and associated BioZtect sensors. These devices use ICG technology to noninvasively measure the heart’s mechanical characteristics by monitoring the heart’s ability to deliver blood to the body and are used principally by physicians to assess, diagnose, and treat cardiovascular disease and are sold through our direct sales force and distributors to physicians and hospitals throughout the world. Following the acquisition of Medis in June 2004, the ICG segment also includes diagnostic and monitoring devices such as the Niccomo and Cardioscreen monitors and the Rheoscreen family of measurement devices.
In December 2004, the Company received FDA 510(k) clearance on the first phase of the BioZ Dx. The BioZ Dx has improved signal processing and features an integrated full-page thermal printer, color display screen, and a new reporting function that allows physicians to automatically compare a patient’s last ICG report to the current ICG report. Commercial shipments of the BioZ Dx commenced in the first fiscal quarter of 2005. In June 2005, the Company received FDA 510(k) clearance on the second phase of the BioZ Dx. The second phase of the BioZ Dx is the combined ICG/ECG device that includes 12-lead ECG capability which provides physicians the ability to assess the patient’s electrical and mechanical cardiovascular status in one efficient platform. Shipments of the BioZ Dx commenced in the third fiscal quarter of 2005. Existing BioZ Dx customers will be able to add the 12-lead diagnostic ECG capability with a convenient field upgrade.
Electrocardiography (“ECG”)
The ECG segment, (Medical Sensor segment), designs, manufactures and sells electrocardiogram electrodes and related supplies through the Company’s Vermed subsidiary acquired in March 2004. These products are used principally in electrocardiogram and other diagnostic procedures for cardiology, electrotherapy, sleep testing, neurology and general purpose diagnostic testing. The products are sold to a diverse client base of medical suppliers, facilities and physicians.
59
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
Segment Profit and Assets
Segment information for the Company’s reporting segments for years ended November 30 is as follows. The Corporate unallocated items are comprised of general corporate expenses of a non-segment related nature. “Other”, includes elimination of intersegment sales(In thousands):
| | | | | | | | | | | | |
| | | For the years ended November 30,
| |
| | | 2005
| | | 2004
| | | 2003
| |
Net external sales: | | | | | | | | | | | | |
ICG | | $ | 27,686 | | | $ | 34,260 | | | $ | 30,332 | |
ECG | | | 9,319 | | | | 6,728 | | | | — | |
Intersegment | | | 1,098 | | | | — | | | | — | |
Other | | | (1,098 | ) | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Consolidated net external sales | | | 37,005 | | | | 40,988 | | | | 30,332 | |
| | |
|
|
| |
|
|
| |
|
|
|
Gross margin: | | | | | | | | | | | | |
ICG | | | 17,878 | | | | 26,665 | | | | 23,210 | |
ECG | | | 3,609 | | | | 2,686 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Consolidated gross margin | | | 21,487 | | | | 29,351 | | | | 23,210 | |
| | |
|
|
| |
|
|
| |
|
|
|
Gross margin as a percentage of sales: | | | | | | | | | | | | |
ICG | | | 64.6 | % | | | 77.8 | % | | | 76.5 | % |
ECG | | | 38.7 | % | | | 39.9 | % | | | — | |
Consolidated gross margin as a percentage of sales | | | 58.1 | % | | | 71.6 | % | | | 76.5 | % |
| | | |
Income (loss) before income taxes and minority interest: | | | | | | | | | | | | |
ICG | | | (2,741 | ) | | | 3,404 | | | | 3,374 | |
ECG | | | 1,410 | | | | 1,271 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Income (loss) before income taxes and minority interest of reportable segments | | | (1,331 | ) | | | 4,675 | | | | 3,374 | |
| | | |
Corporate unallocated | | | (2,556 | ) | | | (1,724 | ) | | | (695 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Consolidated income (loss) before income taxes and minority interest | | $ | (3,887 | ) | | $ | 2,951 | | | $ | 2,679 | |
| | |
|
|
| |
|
|
| |
|
|
|
| | | |
| | | 2005
| | | 2004
| | | | |
Total assets: | | | | | | | | | | | | |
ICG | | $ | 22,672 | | | $ | 28,470 | | | | | |
ECG | | | 21,687 | | | | 19,266 | | | | | |
| | |
|
|
| |
|
|
| | | | |
Total assets of reportable segments | | | 44,359 | | | | 47,736 | | | | | |
Corporate unallocated | | | (4,361 | ) | | | 10,294 | | | | | |
| | |
|
|
| |
|
|
| | | | |
Consolidated total assets | | $ | 39,998 | | | $ | 58,030 | | | | | |
| | |
|
|
| |
|
|
| | | | |
Goodwill included above for the ICG segment in 2005 and 2004 was $1,825,000 and $1,686,000, respectively, and for the ECG segment in 2005 and 2004 was $9,521,000 and $9,500,000, respectively.
60
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
(4) Inventory
Inventory consists of the following at November 30(In thousands):
| | | | | | | | |
| | | 2005
| | | 2004
| |
Electronic components and subassemblies | | $ | 2,450 | | | $ | 2,182 | |
Finished goods | | | 2,121 | | | | 1,705 | |
Demonstration units | | | 1,766 | | | | 1,369 | |
Less provision for obsolete and slow-moving inventory | | | (628 | ) | | | (350 | ) |
Less provision for demonstration inventory | | | (330 | ) | | | (259 | ) |
| | |
|
|
| |
|
|
|
Inventory, net | | $ | 5,379 | | | $ | 4,647 | |
| | |
|
|
| |
|
|
|
(5) Long-Term Receivables
In fiscal 2000, the Company offered its customers no-interest financing with maturities ranging from 24 to 60 months. Revenue is recorded on these contracts at the time of sale based on the present value of the minimum payments using market interest rates or the rate implicit in the financing arrangement.
Interest income is deferred and recognized on a monthly basis over the term of the contract. In fiscal 2001, the Company established a similar program through a third party financing company to replace the internal equipment-financing program. Under certain circumstances, the Company continues to provide in-house financing to its customers, although the contracts now include market rate interest provisions. The long-term receivables resulting from internal financing are collateralized by the individual systems. Long-term receivables consist of the following at November 30(In thousands):
| | | | | | | | |
| | | 2005
| | | 2004
| |
Long-term receivables, net of deferred interest | | $ | 2,928 | | | $ | 2,748 | |
Less allowance for doubtful long-term receivables | | | (381 | ) | | | (359 | ) |
| | |
|
|
| |
|
|
|
| | | | 2,547 | | | | 2,389 | |
Less current portion of long-term receivables | | | (1,376 | ) | | | (1,141 | ) |
| | |
|
|
| |
|
|
|
Long-term receivables and note receivable, net | | $ | 1,171 | | | $ | 1,248 | |
| | |
|
|
| |
|
|
|
(6) Property, Plant and Equipment
Property, plant and equipment at November 30 consist of the following(In thousands):
| | | | | | | | | | |
| | | Estimated
Useful Life
(In years)
| | 2005
| | | 2004
| |
Land | | — | | $ | 181 | | | $ | 192 | |
Buildings and improvements | | 5-35 | | | 2,618 | | | | 2,514 | |
Computer software and equipment | | 3-5 | | | 1,653 | | | | 1,546 | |
Manufacturing, lab equipment and fixtures | | 3-20 | | | 2,766 | | | | 2,162 | |
Office furniture and equipment | | 3-8 | | | 341 | | | | 351 | |
Sales equipment and exhibit booth | | 3-5 | | | 73 | | | | 73 | |
Auto | | 5 | | | 18 | | | | 21 | |
Construction in progress | | — | | | 265 | | | | — | |
| | | | |
|
|
| |
|
|
|
| | | | | | 7,915 | | | | 6,859 | |
Accumulated depreciation | | | | | (2,407 | ) | | | (1,736 | ) |
| | | | |
|
|
| |
|
|
|
Property, plant and equipment, net | | | | $ | 5,508 | | | $ | 5,123 | |
| | | | |
|
|
| |
|
|
|
61
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
(7) Goodwill and Intangible Assets
The Company accounts for goodwill under the provisions of SFAS No. 142. Goodwill and intangible assets with indefinite lives are not subject to amortization, but are tested for impairment annually or whenever events or changes in circumstances indicate that the asset might be impaired. Goodwill is considered to be impaired if the Company determines that the carrying value of the reporting unit exceeds its fair value. In addition to the annual review, an interim review is required if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. In 2005, the first year following the acquisition of Vermed and Medis, the Company performed an annual impairment review of goodwill. Based on the analysis, there was no impairment of goodwill for the year ended November 30, 2005.
Identifiable intangible assets with finite lives are subject to amortization, and any impairment is determined in accordance with SFAS 144,Accounting for the Impairment or Disposal of Long-Lived Assets. The company recorded amortization expense of $464,000 in 2005 and $334,000 in 2004. Estimated amortization expense for the years ending November 30, 2006, 2007, 2008, 2009 and 2010 is $490,000, $490,000, $487,000, $424,000 and $403,000, respectively.
The Company recorded $11,432,000 of goodwill related to the Vermed and Medis acquisitions. The identifiable intangible assets consist of the following at November 30(In thousands):
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | 2005
| | 2004
|
| | | Estimated
Life (in
years)
| | Cost
| | Accumulated
Amortization
| | | Net
| | Cost
| | Accumulated
Amortization
| | | Net
|
Customer lists | | 10 | | $ | 2,900 | | $ | (491 | ) | | $ | 2,409 | | $ | 2,900 | | $ | (201 | ) | | $ | 2,699 |
OEM Relationships | | 10 | | | 900 | | | (153 | ) | | | 747 | | | 900 | | | (62 | ) | | | 838 |
Proprietary gel Formulas | | 15 | | | 100 | | | (11 | ) | | | 89 | | | 100 | | | (5 | ) | | | 95 |
Trademark and trade name | | Indefinite | | | 100 | | | — | | | | 100 | | | 100 | | | — | | | | 100 |
Developed technology | | 4 – 5 | | | 383 | | | (121 | ) | | | 262 | | | 781 | | | (68 | ) | | | 713 |
Patents | | 5 | | | 120 | | | (16 | ) | | | 104 | | | 108 | | | (3 | ) | | | 105 |
| | | | |
|
| |
|
|
| |
|
| |
|
| |
|
|
| |
|
|
| | | | | $ | 4,503 | | $ | (792 | ) | | $ | 3,711 | | $ | 4,889 | | $ | (339 | ) | | $ | 4,550 |
| | | | |
|
| |
|
|
| |
|
| |
|
| |
|
|
| |
|
|
(8) Guarantees
Product Warranties
The Company warrants that its stand-alone BioZ Systems shall be free from defects for a period of 60 months (on the BioZ Dx) and 12 months (on the BioZ.com) from the date of shipment on each new system sold in the United States, 12 months on factory certified refurbished or demonstration systems and for 13 months on systems sold by CardioDynamics or Medis internationally. Additional years of warranty can be purchased on the BioZ Systems. Options and accessories purchased with the system are covered for a period of 90 days. The Company records a provision for warranty repairs on all systems sold, which is included in cost of sales in the consolidated statements of operations and is recorded in the same period the related revenue is recognized.
The warranty provision is calculated using historical data to estimate the percentage of systems that will require repairs during the warranty period and the average cost to repair a system. This financial model is then used to calculate the future probable expenses related to warranty and the required warranty provision. The estimates used in this model are reviewed and updated as actual warranty expenditures change over the product’s life cycle. If actual warranty expenditures differ substantially from our estimates, revisions to the warranty provision would be required.
62
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
The following table summarizes information related to our warranty provision at November 30(In thousands):
| | | | | | | | |
| | | 2005
| | | 2004
| |
Beginning balance | | $ | 409 | | | $ | 634 | |
Provision for warranties issued | | | 281 | | | | 234 | |
Warranty expenditures incurred | | | (112 | ) | | | (147 | ) |
Adjustments and expirations | | | — | | | | (312 | ) |
| | |
|
|
| |
|
|
|
Ending balance | | $ | 578 | | | $ | 409 | |
| | |
|
|
| |
|
|
|
(9) Financing Agreements
In November 2005, the Company amended certain provisions of the Comerica Bank term loan to extend the maturity date to November 1, 2008 and take an advance of $2.2 million from the revolving credit line to reduce the outstanding principal balance of the term loan which lowered future monthly installment payments. The interest rate on both the term loan and revolving line of credit increased to one percent above the prime rate, the minimum tangible net worth, liabilities to tangible net worth and debt service coverage ratios were eliminated, a maximum loss covenant was added and the minimum liquidity coverage was lowered to $1 million.
The Company’s revolving credit line availability is $2,087,000 as of November 30, 2005. In fiscal 2005, the Company had $2,200,000 of borrowings under the revolving credit line related to the advance to pay down a portion of the term loan. During fiscal 2004 and at November 30, 2004 there were no outstanding borrowings under the revolving credit line. At November 30, 2005, our fourth quarter consolidated loss exceeded the maximum net loss covenant of $450,000 and as a result the Company was not in compliance with this covenant. On February 23, 2006, we entered into an amendment and waiver agreement with Comerica Bank which waived the fourth quarter covenant violation and amended the covenants, including the maximum loss covenants. We do not believe that the revised covenants are reasonably likely to materially limit our ability to borrow on the credit line.
Both the revolving credit line and the term loan bear interest at a rate of one percent above the bank’s monthly prime rate and are subject to adjustment on a monthly basis (8% as of November 30, 2005). There is an unused commitment fee of one half percent of the difference between the available revolving credit line and the average daily revolving credit balance, reduced by one-quarter percent based on a stated level of cash on deposit with the bank. The obligations of the Company under the revolving credit line and the term loan are secured by a pledge of all of the Company’s assets. In 2004, the Company issued letters of credit relating to the acquisition of Medis to secure the deferred acquisition payments due to the minority shareholders of Medis to be paid annually over five years through 2009. As of November 30, 2005, our outstanding letters of credit was $713,000 which reduces credit available under our revolving credit line.
Also in connection with the acquisition of Medis in 2004, the Company assumed two bank loans with the Sparkasse Arnstadt-Ilmenau bank. Under the terms of the loan agreement, the loans are secured by a pledge of the building valued at 760,000 Euros ($891,000) as of November 30, 2005. One of the loans bears interest at a fixed rate of 5.5% through July 2006 and then the bank has the option to adjust the rate. The other loan bears a fixed rate of 5.9% through July 2011 and then the bank has the right to adjust the rate. Both loans mature in August 2021.
In March 2005, the Company’s Vermed subsidiary entered into a loan and promissory note agreement subject to a maximum loan availability of $480,000 with the Vermont Economic Development Authority
63
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
(VEDA) to assist with the purchase and installation of custom designed manufacturing equipment. The interest rate is adjustable at 0.75% less than the tax exempt rate and the loan matures in January 2012 (4.25% as of November 30, 2005). Under the terms of the loan, Vermed is required to maintain certain debt coverage levels and current ratios. The note payable is guaranteed by CardioDynamics and is secured by the manufacturing equipment which will approximate $1.2 million upon project completion.
(10) Long-term Debt
Long-term debt consists of the following at November 30(In thousands):
| | | | | | | | |
| | | 2005
| | | 2004
| |
Secured bank loan payable to Comerica Bank at 8% in 2005 and 5.5% in 2004 (matures November 2008)(See Note 9) | | $ | 983 | | | $ | 5,233 | |
Secured bank loans payable to Sparkasse Arnstadt-Ilmenau at 5.5% and 5.9% (mature August 2021)(See Note 9) | | | 351 | | | | 412 | |
Note payable to Vermont Economic Development Authority at 4.25% (matures January 2012)(See Note 9) | | | 353 | | | | — | |
Capital leases | | | 64 | | | | 85 | |
| | |
|
|
| |
|
|
|
Long-term debt | | | 1,751 | | | | 5,730 | |
Less current portion | | | (431 | ) | | | (1,785 | ) |
| | |
|
|
| |
|
|
|
| | | $ | 1,320 | | | $ | 3,945 | |
| | |
|
|
| |
|
|
|
Maturities of the long-term debt at November 30, 2005 are as follows(In thousands):
| | | | | | | | | | |
Years Ending November 30,
| | Gross
Maturities
| | Imputed Interest On
Minimum
Lease Payment Under
Capital Leases
| | | Net Long-
Term Debt
|
2006 | | $ | 435 | | $ | (4 | ) | | $ | 431 |
2007 | | | 417 | | | (2 | ) | | | 415 |
2008 | | | 427 | | | (1 | ) | | | 426 |
2009 | | | 96 | | | (1 | ) | | | 95 |
2010 | | | 91 | | | — | | | | 91 |
Thereafter | | | 293 | | | — | | | | 293 |
| | |
|
| |
|
|
| |
|
|
Total | | $ | 1,759 | | $ | (8 | ) | | $ | 1,751 |
| | |
|
| |
|
|
| |
|
|
Capital Leases
The Company leases certain equipment under capital leases where the lessors retain a security interest in the equipment until the capital lease obligation is concluded. Capital leases included in property, plant and equipment are as follows at November 30(In thousands):
| | | | | | | | |
| | | 2005
| | | 2004
| |
Office furniture and equipment | | $ | 74 | | | $ | 74 | |
Less accumulated amortization | | | (28 | ) | | | (16 | ) |
| | |
|
|
| |
|
|
|
| | | $ | 46 | | | $ | 58 | |
| | |
|
|
| |
|
|
|
64
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
(11) Shareholders’ Equity
On August 25, 1999, the Company issued 2,000,000 common stock warrants to GEMS-IT. One million of the warrants were granted to GEMS-IT to obtain access to their technology. The remaining 1,000,000 performance based warrants vested during November 2000, as a result of GEMS-IT meeting their minimum sales objectives. This resulted in a non-cash charge included in sales and marketing expense in fiscal 1999 of $3,382,000 based on the fair market value of the warrants. Each warrant represents the right to purchase one share of the Company’s stock at an exercise price of $4.10, until the expiration date of August 25, 2004. Just prior to the expiration date, in accordance with their original terms, these warrants were exercised in a cashless net exercise transaction whereby the Company issued 340,753 shares of common stock equivalent to the “in the money” value of the warrants at the exercise date.
In August, 2004, 350,000 premium priced warrants that had been granted to an institutional investor in August, 1999, as part of an equity financing, were exercised at $3.54 per share.
(12) Stock Options
In 2004, the shareholders approved the 2004 Stock Incentive Plan (the 2004 Plan), which replaced the 1995 Stock Option/Issuance Plan (the 1995 Plan). Although the 1995 plan remains in effect for outstanding options, no new options may be granted under this plan.
The 2004 Plan authorizes awards of the following types of equity-based compensation: incentive stock options (ISO), nonqualified stock options (NSO), stock appreciation rights, stock units and restricted stock. The total number of shares reserved and available under the 2004 Plan is 2,000,000 plus any shares remaining available for grant under the 1995 Plan on the effective date, including shares subject to outstanding options that are subsequently forfeited or terminate for any other reason before being exercised.
The exercise price of an ISO shall not be less than 100% of the fair market value of a share on the date of grant, and the exercise price of an NSO shall not be less 85% of the fair market value of a share on the date of grant. The Compensation Committee, at its sole discretion, shall determine the option exercise price. Under the Option Plan, the exercise price of each option equals the market price of the Company’s stock on the date of grant and an option’s maximum term is ten years.
The 2004 Plan provides for annual grants to each outside director who was not an employee of the Company within the preceding two years. Each director who will continue to serve on the Company’s Board of Director’s shall receive a nonstatutory option to purchase 12,000 shares following the conclusion of the annual shareholder meeting. In addition, at their election, they also receive either a cash fee of $2,000 per month or an annual stock option grant at fair market value for their services on the board. In 2006, the elected grant was for 24,000 stock options and in 2005 the elected grant was for 12,000 stock options. The options vest monthly over 12 months and expire upon the earlier of ten years from the date of grant or two years after the director terminates their position on the Board. In July 2003, the Company’s Board of Directors granted 10,000 options to a new board member. During fiscal 2005, 2004 and 2003, 198,000, 102,000, and 82,000 options, respectively, were granted to the Board of Directors at fair market value on the date of grant. Individual Board members who elected to receive stock options in lieu of cash compensation for 2006, were granted their option in November 2005, therefore the 2005 stock option grant amount listed above represents option compensation for more than one year.
The Option Plans also provided for grants of options and issuances of stock in exchange for professional services or incentives. During fiscal year 2005, there were no options granted in exchange for services. During fiscal 2004 and 2003, options of 665 and 17,510, respectively, were granted in exchange for services or as an incentive resulting in expense in the amount of $2,000 and $17,000, respectively. The compensation expense is
65
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
calculated using the Black-Scholes option pricing model and recorded during the period the services were provided or, in the case of options granted for services already provided, the period when the option was granted. At November 30, 2005, there were 762,716 shares available for grant under the Option Plan. Stock option activity during the periods indicated is as follows:
| | | | | | |
| | | Number of
shares
| | | Weighted-average
exercise price
|
Options outstanding at November 30, 2002 | | 3,160,107 | | | $ | 3.79 |
Granted | | 1,056,469 | | | | 3.50 |
Exercised | | (229,509 | ) | | | 2.40 |
Forfeited | | (157,618 | ) | | | 3.91 |
Expired | | (81,441 | ) | | | 4.12 |
| | |
|
| |
|
|
Options outstanding at November 30, 2003 | | 3,748,008 | | | | 3.78 |
Granted | | 1,223,315 | | | | 5.67 |
Exercised | | (509,343 | ) | | | 2.83 |
Forfeited | | (152,361 | ) | | | 3.94 |
Expired | | (157,286 | ) | | | 4.66 |
| | |
|
| |
|
|
Options outstanding at November 30, 2004 | | 4,152,333 | | | | 4.39 |
Granted | | 2,019,453 | | | | 2.61 |
Exercised | | (72,288 | ) | | | 2.83 |
Forfeited | | (376,236 | ) | | | 4.37 |
Expired | | (314,286 | ) | | | 4.57 |
| | |
|
| |
|
|
Options outstanding at November 30, 2005 | | 5,408,976 | | | $ | 3.73 |
| | |
|
| |
|
|
At November 30, 2005, 2004 and 2003, the number of options exercisable was 4,938,411, 2,661,653 and 2,385,456, respectively, and the weighted-average exercise prices of those options were $3.96, $4.00 and $3.77, respectively.
The following table sets forth information regarding options outstanding and exercisable under the Option Plans at November 30, 2005:
| | | | | | | | | | | | |
| | | Options Outstanding
| | Options Exercisable
|
Range of exercise prices
| | Number
outstanding
| | Weighted-
average
remaining
contractual
life
| | Weighted-
average
exercise
price
| | Number
exercisable
| | Weighted-
average
exercise
price
|
$0.00 – 1.18 | | 139,250 | | 8.6 | | $ | 1.09 | | 116,000 | | $ | 1.08 |
1.19 – 2.37 | | 1,424,636 | | 7.1 | | | 1.56 | | 979,321 | | | 1.67 |
2.38 – 3.56 | | 1,163,050 | | 5.9 | | | 3.00 | | 1,162,050 | | | 3.00 |
3.57 – 4.75 | | 716,527 | | 5.9 | | | 4.19 | | 716,527 | | | 4.19 |
4.76 – 5.94 | | 959,431 | | 7.2 | | | 5.22 | | 958,431 | | | 5.22 |
5.95 – 7.12 | | 937,564 | | 6.6 | | | 6.16 | | 937,564 | | | 6.16 |
7.13 – 8.31 | | 62,518 | | 4.4 | | | 8.00 | | 62,518 | | | 8.00 |
8.32 – 9.50 | | 5,000 | | 4.3 | | | 8.77 | | 5,000 | | | 8.77 |
11.88 | | 1,000 | | 4.3 | | | 11.88 | | 1,000 | | | 11.88 |
| | |
| | | | | | |
| | | |
| | | 5,408,976 | | 6.7 | | $ | 3.73 | | 4,938,411 | | $ | 3.96 |
| | |
| | | | | | |
| | | |
66
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
As part of an employment agreement with the Company’s then chief executive officer, Richard Otto, 250,000 non-transferable stock options were granted in June 1995 (outside the Option Plans) to Mr. Otto at an exercise price of $0.50 per share. The options vest if the quoted market price of the Company’s common stock attains specified levels. During fiscal 2000, 100,000 of these options vested and were exercised. The remaining 150,000 options expired on June 15, 2005.
On March 23, 1998, the Company entered into an employment agreement with Michael K. Perry, who succeeded Mr. Otto as chief executive officer. Under the terms of the agreement, Mr. Perry was granted 1,295,000 non-transferable stock options (outside the Option Plans) at the grant date fair market value exercise price of $1.625 per share. The options vest over a four-year period, which commenced on October 16, 1998. During fiscal 2005, 2004 and 2003, Mr. Perry exercised 10,000, 157,000 and 108,000 options, respectively. At November 30, 2005, 603,000 of the options are outstanding and exercisable. The options expire on October 15, 2008.
(13) Income Taxes
Income tax benefit (provision) in the accompanying consolidated statements of operations is comprised of the following for the years ended November 30(In thousands):
| | | | | | | | | | | | |
| | | 2005
| | | 2004
| | | 2003
| |
Current: | | | | | | | | | | | | |
Federal | | $ | — | | | $ | (106 | ) | | $ | — | |
State | | | (50 | ) | | | (60 | ) | | | (227 | ) |
Foreign | | | (217 | ) | | | (47 | ) | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total current | | | (267 | ) | | | (213 | ) | | | (227 | ) |
Deferred: | | | | | | | | | | | | |
Federal | | | (9,692 | ) | | | 6,696 | | | | — | |
State | | | (1,044 | ) | | | 726 | | | | — | |
Foreign | | | — | | | | — | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total deferred | | | (10,736 | ) | | | 7,422 | | | | — | |
| | |
|
|
| |
|
|
| |
|
|
|
Total benefit (provision) | | $ | (11,003 | ) | | $ | 7,209 | | | $ | (227 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
The difference between the income tax benefit (provision) and income taxes computed using the U.S. federal income tax rate was as follows for the years ended November 30(In thousands):
| | | | | | | | | | | | |
| | | 2005
| | | 2004
| | | 2003
| |
Computed “expected” tax provision | | $ | 1,321 | | | $ | (1,003 | ) | | $ | (904 | ) |
State and local taxes, net of federal benefit | | | (1,224 | ) | | | 798 | | | | (167 | ) |
Change in federal valuation allowance | | | (11,098 | ) | | | 8,279 | | | | 902 | |
Adjustment for prior year and expiring net operating losses | | | 170 | | | | (40 | ) | | | — | |
Deferred compensation | | | (2 | ) | | | (752 | ) | | | — | |
Other | | | (170 | ) | | | (73 | ) | | | (58 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
Benefit (provision) for income taxes | | $ | (11,003 | ) | | $ | 7,209 | | | $ | (227 | ) |
| | |
|
|
| |
|
|
| |
|
|
|
At November 30, 2005 the Company had federal net operating loss carryforwards of approximately $28,400,000, which begin to expire in 2011.
67
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
The Tax Reform Act of 1986 contains provisions that limit the federal net operating loss carryforwards that may be used in any given year in the event of specified occurrences, including significant ownership changes. If these specified events occur, the Company may lose some or all of the tax benefits of these carry forwards. During fiscal 2004, the Company performed a §382 study and determined that the extent of such limitations for prior years had no effect on the availability of the current net operating losses. The Company believes that the above conclusion remains consistent for fiscal 2005.
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets as of November 30 are as follows(In thousands):
| | | | | | | | |
| | | 2005
| | | 2004
| |
Current deferred tax assets: | | | | | | | | |
Allowance for doubtful accounts and returns | | $ | 794 | | | $ | 1,061 | |
Inventory reserves | | | 427 | | | | 273 | |
Deferred compensation | | | 55 | | | | 56 | |
Accrued expenses | | | 812 | | | | 589 | |
Deferred revenue | | | 104 | | | | 136 | |
Other | | | 43 | | | | 26 | |
| | |
|
|
| |
|
|
|
Net current deferred tax assets | | | 2,235 | | | | 2,141 | |
Current deferred tax liability: | | | | | | | | |
Foreign currency translation gain | | | (2 | ) | | | (95 | ) |
| | |
|
|
| |
|
|
|
Net current deferred tax liability | | | (2 | ) | | | (95 | ) |
| | |
|
|
| |
|
|
|
Subtotal deferred tax assets, current portion | | | 2,233 | | | | 2,046 | |
Long-term deferred tax assets: | | | | | | | | |
Net operating loss carryforwards | | | 10,874 | | | | 8,691 | |
Other | | | (24 | ) | | | 128 | |
| | |
|
|
| |
|
|
|
Net long-term deferred tax assets | | | 10,850 | | | | 8,819 | |
Long-term deferred tax liability: | | | | | | | | |
Intangible assets | | | (431 | ) | | | (224 | ) |
| | |
|
|
| |
|
|
|
Net long-term deferred tax liability | | | (431 | ) | | | (224 | ) |
| | |
|
|
| |
|
|
|
Subtotal deferred tax assets, long-term | | | 10,419 | | | | 8,595 | |
| | |
|
|
| |
|
|
|
Total deferred tax assets | | | 12,652 | | | | 10,641 | |
Valuation allowance | | | (12,652 | ) | | | — | |
| | |
|
|
| |
|
|
|
Net deferred tax assets | | $ | — | | | $ | 10,641 | |
| | |
|
|
| |
|
|
|
In assessing the realizability of deferred income tax assets, management follows the guidance contained within SFAS No. 109 “Accounting for Income Taxes,” which requires that deferred income tax assets or liabilities be reduced by a valuation allowance, if based on weight of available evidence, considering all relevant positive and negative, objective and subjective evidence, it is “more likely than not” that some portion or all of the deferred income tax assets will not be realized. The realization of the gross deferred tax assets is dependent on several factors, including the generation of sufficient taxable income prior to the expiration of the loss carry forwards. In order to realize the benefit associated with net operating losses (NOL), the Company must earn cumulative Federal taxable income of at least $28,400,000 prior to the expiration of those NOL’s. The Federal NOL’s will begin to expire in 2011 and will fully expire by 2025. Additionally, at November 30, 2005, the Company had California net operating loss carryforwards of approximately $11,900,000 that begin to expire in fiscal 2006 and will fully expire by 2017.
68
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
Under provisions of SFAS No, 109, forming a conclusion that a valuation allowance is not needed is difficult when there is negative evidence such as historical losses, uncertainty of future profitability and determination of exact net operating losses subject to section 382 limitations. The Company has experienced taxable losses during the majority of its reporting periods including its most recent period. Despite the Company’s forecasts for future taxable income, it is difficult to predict with certainty future taxable income due to business uncertainties and because of the tax expense from the exercise of stock options and warrants which are not in the control of management. The Company’s historical operating losses, particularly the loss incurred in the most recent period make it very difficult to rely on projections beyond a relatively short forecast window to meet the “more likely than not” standard required to conclude that its deferred tax assets will be fully utilized. Therefore, management has concluded that it is appropriate to record a valuation allowance equal to the total deferred income tax assets at November 30, 2005.
(14) Commitments and Contingencies
Letters of Credit
The Company had outstanding letters of credit at November 30, 2005 of $713,000 (Euros 608,000), which expire on June 3, 2009, to support deferred acquisition payments associated with the Medis acquisition to be paid annually through 2009. The deferred acquisition payments are reported in the current and long-term liabilities in the consolidated balance sheet.
Operating Leases
In June 2004, the Company amended the operating lease for the existing 18,000 square-foot facility in San Diego, California to extend the terms of the lease from July 31, 2007 to December 31, 2007. The amended lease terms provide for additional expansion space of approximately 15,000 square-feet effective November 1, 2004, and included a tenant improvement allowance of $225,000 for the construction of building improvements.
In March 2005, the Company amended the operating lease for both the existing and original space in the San Diego, California facility to extend the terms of the lease from December 31, 2007, to December 31, 2009, and to provide for an additional $197,000 of tenant improvement allowance for the construction of building improvements. The lease payments on the original space are $20,000 per month through July 31, 2007, increasing to $22,000 per month through July 31, 2008 with annual increases of 3% each anniversary thereafter. The lease payments on the expansion space commenced on November 1, 2004 at $7,000 per month and then increased to $14,000 per month on November 1, 2005 with a 3% annual increase on each anniversary thereafter. The total future lease commitments on the amended lease through December 31, 2009 approximate $1,745,000. The lease terms provide for rent incentives and escalations for which the Company has recorded a deferred rent liability which is recognized evenly over the entire period. The difference between the base rent paid, which also includes triple net costs, and the straight-line rent expense, as well as rent incentives is $507,000 as of November 30, 2005 and is recorded as deferred rent on the accompanying consolidated balance sheets.
Assets Pledged on Bank Revolving Credit Line and Term Loan
In March 2004, the revolving line of credit was modified to increase the amount available to $5 million and the Company borrowed $7 million on a term loan in connection with the Vermed acquisition. The Company has pledged all assets as collateral and security in connection with the bank term loan and revolving credit line agreement.
In March 2005, the Company’s Vermed subsidiary entered into a loan and promissory note agreement subject to a maximum loan availability of $480,000 with VEDA to assist with the purchase and installation of custom designed manufacturing equipment. The note payable is guaranteed by CardioDynamics and secured by the manufacturing equipment.
69
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
Rent expense, including triple net building lease, under operating leases was $406,000, $373,000 and $326,000 for the years ended November 30, 2005, 2004 and 2003, respectively. Future minimum lease payments for operating leases as of November 30, 2005 are as follows(In thousands):
| | | |
Years ending November 30,
| | Lease Commitments
|
2006 | | $ | 434 |
2007 | | | 445 |
2008 | | | 468 |
2009 | | | 476 |
2010 | | | 38 |
Thereafter | | | — |
| | |
|
|
| | | $ | 1,861 |
| | |
|
|
Contingent Obligation
As part of the acquisition of Medis, the Company assumed a contingent obligation to repay a German government for public grant subsidies of $364,000 (310,800 Euros, which represents the Company’s 80% share) if it does not meet certain conditions through December 31, 2007. The minority shareholders are personally liable for the other 20% share of the contingent obligation.
The grant subsidies were used to assist with the construction of the building now occupied and used for Medis’ business operations. The following conditions must be maintained:
| | • | | Number of employees must be retained at a minimum level. |
| | • | | Medis must manufacture at least 50% of it sales volume in medical or comparable devices. |
| | • | | The Medis business is not allowed to be discontinued or transferred to another owner without transferring the aforementioned conditions and contingent liability associated with the government grant provisions. |
Legal Proceedings
The Company is from time to time subject to legal proceedings and claims, which arise in the ordinary course of our business, none of which is required to be disclosed under this Item 3. Management believes that resolution of these matters will not have a material adverse effect on our results of operations, financial condition or cash flows.
(15) Employee Benefit Plan
In 1996, the Company established a qualified savings plan under section 401(k) of the Internal Revenue Code of 1986. Employees who are at least 21 years of age are eligible to participate in the plan at the first calendar quarterly entry date after 90 days of service. The Company may make discretionary contributions to the plan. Employer matching contributions were $229,000, $207,000 and $161,000 for the fiscal years ended November 30, 2005, 2004 and 2003, respectively.
(16) Related Party Transactions
The Company receives certain engineering, development and consulting services from Rivertek Medical Systems, Inc., of which the Chief Technology Officer of the Company during fiscal year 2005 was a 100%
70
CARDIODYNAMICS INTERNATIONAL CORPORATION
Notes to Consolidated Financial Statements—(Continued)
beneficial owner. The Company paid $230,000, $259,000 and $269,000 for services in fiscal 2005, 2004 and 2003, respectively. Amounts payable to Rivertek at November 30, 2005, 2004 and 2003 were $65,000, $145,000 and $77,000, respectively.
(17) Supplementary Financial Data(Unaudited)
The following table presents selected unaudited financial results for each of the eight quarters in the two-year period ended November 30, 2005. In the opinion of management, this unaudited information has been prepared on the same basis as the audited information and includes all adjustments (consisting of only normal recurring adjustments) necessary for the fair statement of the financial information for the periods presented(In thousands, except for per share data):
| | | | | | | | | | | | | | | | |
| | | First
Quarter
| | | Second
Quarter
| | | Third
Quarter
| | | Fourth
Quarter (2)
| |
Year Ended November 30, 2005 | | | | | | | | | | | | | | | | |
Net sales | | $ | 9,678 | | | $ | 9,370 | | | $ | 8,770 | | | $ | 9,187 | |
Gross margin | | | 6,331 | | | | 5,293 | | | | 5,270 | | | | 4,593 | |
Loss from operations | | | (984 | ) | | | (827 | ) | | | (1,004 | ) | | | (1,017 | ) |
Net loss | | | (644 | ) | | | (682 | ) | | | (474 | ) | | | (13,145 | ) |
Net loss per share—basic and diluted | | | (.01 | ) | | | (.01 | ) | | | (.01 | ) | | | (.27 | ) |
| | | | |
| | | First
Quarter
| | | Second
Quarter
| | | Third
Quarter
| | | Fourth
Quarter (1)
| |
Year Ended November 30, 2004 | | | | | | | | | | | | | | | | |
Net sales | | $ | 8,015 | | | $ | 10,270 | | | $ | 11,075 | | | $ | 11,628 | |
Gross margin | | | 6,160 | | | | 7,390 | | | | 7,600 | | | | 8,201 | |
Income from operations | | | 737 | | | | 1,032 | | | | 1,059 | | | | 96 | |
Net income | | | 761 | | | | 958 | | | | 992 | | | | 7,412 | |
Net income per share—basic and diluted | | | .02 | | | | .02 | | | | .02 | | | | .15 | |
| (1) | Fourth quarter 2004 net income was impacted by an income tax benefit of $7.4 million due to the elimination of the valuation allowance on our deferred tax assets. |
| (2) | Fourth quarter 2005 net loss was impacted by an income tax provision of $12.7 million due to the re-establishment of the valuation allowance on our deferred tax assets. |
71
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
On April 21, 2005, the Company informed KPMG LLP (“KPMG”) of its decision not to extend the engagement of KPMG as the Company’s independent registered public accounting firm to audit its 2005 consolidated financial statements. The change is the result of a competitive bidding process initiated by the Company as part of its ongoing efforts to reduce expenses. The decision to change independent accountants was recommended by the Company’s management and approved unanimously by the Company’s Audit Committee and Board of Directors.
During the two years ended November 30, 2004 and through April 21, 2005, there were no (1) disagreements between the Company and KPMG on any matters of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the Company or KPMG, would have caused the Company or KPMG to make reference thereto in their report on the financial statements, or (2) reportable events, except that KPMG advised the Company of material weaknesses in internal control over financial reporting related to the Company’s accounting for income taxes and the calculation of the Company’s allowance for doubtful accounts. KPMG’s report on the Company’s financial statements for the two years ended November 30, 2004 did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles. KPMG’s report on management’s assessment of the effectiveness of internal control over financial reporting as of November 30, 2004, expressed an unqualified opinion on management’s assessment of, and an adverse opinion on the effective operation of internal control over financial reporting.
| ITEM 9A. | CONTROLS AND PROCEDURES |
(a) Evaluation of Disclosure Controls and Procedures
The Company’s management has evaluated, under the supervision and with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, the effectiveness of the design and operations of the Company’s disclosure controls and procedures (as defined in Securities Exchange Act Rule 13a-15(e)), as of the end of the period covered by this annual report. Based on that evaluation, the Company’s Chief Executive Officer and Chief Financial Officer have concluded that the Company’s disclosure controls and procedures were effective as of the end of the period covered by this annual report.
(b) Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining an adequate system of internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Further, because of changes in conditions, effectiveness of internal control over financial reporting may vary over time.
A significant deficiency is a control deficiency, or combination of control deficiencies, that adversely affects the company’s ability to initiate, authorize, record, process, or report external financial data reliably in
72
accordance with generally accepted accounting principles such that there is more than a remote likelihood that a misstatement of the company’s annual or interim financial statements that is more than inconsequential will not be prevented or detected. An internal control material weakness is a significant deficiency, or combination of significant deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected.
Management of the Company conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting based on theInternal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, the Company’s management concluded that its internal control over financial reporting was effective as of November 30, 2005. Mayer Hoffman McCann P.C., an independent registered public accounting firm, has issued a report on management’s assessment of the Company’s internal control over financial reporting, which is included herein.
(c) Changes in Internal Control Over Financial Reporting
The Company has made no changes in its internal control over financial reporting in connection with its fourth quarter evaluation that would materially affect, or are reasonably likely to materially affect, its internal control over financial reporting.
73
Report of Independent Registered Public Accounting Firm on Internal Controls Over Financial Reporting
To the Shareholders and the Board of Directors of CardioDynamics International Corporation
We have audited management’s assessment, included in the accompanying Management’s Report on Internal Control over Financial Reporting, that CardioDynamics International Corporation (the “Company”) maintained effective internal control over financial reporting as of November 30, 2005, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment about the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that the Company maintained effective internal control over financial reporting as of November 30, 2005, is fairly stated, in all material respects, based on the COSO criteria. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of November 30, 2005, based on the COSO criteria.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of the Company as of November 30, 2005 and the related consolidated statements of operations, shareholders’ equity and comprehensive income (loss), and cash flows for the period ended November 30, 2005, and our report dated February 28, 2006 expressed an unqualified opinion thereon.
/s/ Mayer Hoffman McCann P.C.
San Diego, California
February 28, 2006
| ITEM 9B. | OTHER INFORMATION |
None.
74
PART III
| ITEM 10. | DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT |
Directors and Executive Officers
Our directors and executive officers as of January 15, 2006 are as follows:
| | | | |
Name
| | Age
| | Position(s)
|
James C. Gilstrap | | 69 | | Chairman of the Board of Directors |
Michael K. Perry | | 45 | | Chief Executive Officer and Director |
Rhonda F. Rhyne | | 45 | | President |
Steve P. Loomis | | 45 | | Chief Financial Officer |
Russell H. Bergen | | 58 | | Vice President of Operations |
Patrick W. Bradley, Ph.D. | | 52 | | Vice President of Business Development |
Richard L. Kalich | | 59 | | President of Vermed |
Richard E. Trayler | | 54 | | Vice President of International Operations |
Connie R. Curran, Ed.D., RN | | 58 | | Director |
Robert W. Keith | | 48 | | Director |
Richard O. Martin, Ph.D. | | 65 | | Director |
B. Lynne Parshall | | 51 | | Director |
James C. Gilstrap, age 69, has served as the chairman or co-chairman of our board of directors since May 1995. Mr. Gilstrap is retired from Jefferies & Company, where he served as senior executive vice president, partner, board member, and a member of the executive committee. Mr. Gilstrap serves on the board of Corautus Genetics, Inc. Mr. Gilstrap is past president of the Dallas Securities Dealers, as well as a past member of the board of governors of the National Association of Securities Dealers, Inc.
Michael K. Perry, See description under “Executive Officers” in Part I of this Form 10-K.
Rhonda F. Rhyne, See description under “Executive Officers” in Part I of this Form 10-K.
Steve P. Loomis, See description under “Executive Officers” in Part I of this Form 10-K.
Russell H. Bergen,See description under “Executive Officers” in Part I of this Form 10-K.
Patrick W. Bradley, Ph.D., See description under “Executive Officers” in Part I of this Form 10-K.
Richard L. Kalich, See description under “Executive Officers” in Part I of this Form 10-K.
Richard E. Trayler, See description under “Executive Officers” in Part I of this Form 10-K.
Connie R. Curran, RN, Ed.D., age 58, has served on our board of directors since February 2000. Dr. Curran is the Executive Director of C-Change, a collaboration to conquer cancer, in Washington, D.C. Dr. Curran was president and chief executive officer of CurranCare, LLC from 1995 to 2000. She has held a variety of executive positions in academia and multi-system healthcare operations and serves as vice president of the American Hospital Association, as national director of patient care for APM, Inc. and as Dean at the Medical College of Wisconsin. Dr. Curran serves on the boards of IDX, DeVry, The National Student Nurses Association and Silver Cross Hospital. Dr. Curran holds a master’s degree in medical-surgical nursing from De Paul University, a doctorate in educational psychology from Northern Illinois University and is also a graduate of the Harvard Business School program for company owners and presidents.
75
Robert W. Keith, age 48, has served as a director since July 2005. Mr. Keith is President and Chief Operating Officer of Verus Pharmaceuticals, Inc. and has held various executive management positions since co-founding the company in November 2002. Prior to that, he was a Partner at Advantage Management Solutions, L.L.C., a commercial strategy and operations consulting firm for life science companies. Prior to that, Mr. Keith was a member of the senior management team at Dura Pharmaceuticals, Inc. and Elan Corporation, plc from 1996 to 2001; during his tenure he served in several positions, including Vice President of sales, Vice President of commercial strategies and marketing and Vice President of managed care and national accounts. Prior to that, he held an executive management position at Abbott Laboratories. Mr. Keith has more than 20 years of experience in the life sciences industry and management consulting. Mr. Keith earned master degrees from both the Wharton School of the University of Pennsylvania and the University of Pennsylvania and a bachelor’s degree from The George Washington University.
Richard O. Martin, Ph.D., age 65, has served as a director since July 1997. From 1998 until 2001, Dr. Martin served as president of Medtronic Physio-Control Corporation, a medical device company that designs, manufactures and sells external defibrillators and heart monitors. Prior to its acquisition by Medtronic in 1998, Dr. Martin was chairman and chief executive officer of Physio-Control Corporation. Prior to that, he was vice president of cardiovascular business development with Sulzer Medica and has held management positions at Intermedics, Inc. and Medtronic, Inc. Dr. Martin serves on the boards of directors of Endographic Solutions, Inc., Cardiac Dimensions, Inc., Encore Medical Corp., Inovise Medical Inc., MDdatacor Inc. and Prescient Medical, Inc. Dr. Martin earned a bachelor’s degree in electrical engineering from Christian Brothers College, a master’s degree in electrical engineering from Notre Dame University and a doctorate in electrical/biomedical engineering from Duke University.
B. Lynne Parshall, age 51, has served as a director since July 2005. Ms. Parshall has been Executive Vice President of Isis Pharmaceuticals, Inc. since December 1995 and Chief Financial Officer since June 1994. She has held various positions with Isis Pharmaceuticals, Inc. since 1991. Prior to that, she was a Partner at Cooley Godward, LLP from 1986 to 1991. Ms. Parshall serves on the board for Corautus Genetics, Inc. and is on the Board of Trustees of the Bishop’s School. She is also a member of the American, California and San Diego bar associations. Ms. Parshall earned a bachelor’s degree in government and economics from Harvard University and a J.D. degree from Stanford Law School.
Section 16(a) Beneficial Ownership Reporting Compliance
The Company believes that each person who, at any time during the fiscal year ended November 30, 2004, was a director, officer, or beneficial owner of more than 10% of a class of registered equity securities of CardioDynamics, filed on a timely basis all reports required by Section 16(a) of the Securities Exchange Act.
76
| ITEM 11. | EXECUTIVE COMPENSATION |
The following table provides information regarding the annual and long-term compensation earned for services rendered in all capacities to CardioDynamics for the fiscal years ended November 30, 2005, 2004 and 2003 of those persons who were, at November 30, 2005 (i) the Chief Executive Officer and (ii) the four most highly compensated executive officers of CardioDynamics whose aggregate direct remuneration from the Company during the fiscal year ended November 30, 2005 exceeded $100,000 (collectively, the “Named Officers”).
SUMMARY COMPENSATION TABLE
| | | | | | | | | | |
| | | | | | | Long Term
Compensation
Awards
|
| | | | | Annual Compensation(1)
| | Securities Underlying
Options/
SARs (#)
|
Name and Principal Position
| | Year
| | Salary($)
| | Bonus($)
| | Other Annual
Compensation($)
| |
Michael K. Perry | | 2005 | | 279,588 | | -0- | | -0- | | 95,000 |
Chief Executive Officer | | 2004 | | 262,237 | | 103,575 | | -0- | | 60,000 |
| | | 2003 | | 239,400 | | 123,575 | | -0- | | 60,600 |
Rhonda F. Rhyne | | 2005 | | 230,504 | | -0- | | -0- | | 81,200 |
President | | 2004 | | 218,214 | | 62,425 | | -0- | | 50,000 |
| | | 2003 | | 211,348 | | 85,500 | | -0- | | 50,000 |
Steve P. Loomis | | 2005 | | 184,712 | | -0- | | -0- | | 70,000 |
Chief Financial Officer | | 2004 | | 171,130 | | 53,925 | | -0- | | 40,000 |
| | | 2003 | | 163,393 | | 67,750 | | -0- | | 40,000 |
Russell H. Bergen | | 2005 | | 169,116 | | -0- | | -0- | | 70,000 |
Vice President of Operations | | 2004 | | 160,465 | | 52,200 | | -0- | | 40,000 |
| | | 2003 | | 155,386 | | 65,200 | | -0- | | 40,600 |
Richard E. Trayler | | 2005 | | 174,032 | | -0- | | -0- | | 50,000 |
Vice President of International Operations | | 2004 | | 166,680 | | 52,950 | | -0- | | 20,000 |
| | | 2003 | | 161,000 | | 59,525 | | -0- | | 20,000 |
| (1) | Employee benefits provided to each of the Named Officers under various Company programs do not exceed the disclosure thresholds established under the SEC rules and are therefore not included. |
77
The following table provides information regarding option grants during the fiscal year ended November 30, 2005 to the Named Officers in fiscal 2005. One grant was made in January of 2005 based on executive achievements in fiscal 2004 and a second grant was made in October of 2005 based on executive achievements thus far in fiscal 2005. Therefore the stock option grant amounts listed below represent option compensation for more than one year. The Company has not granted any stock appreciation rights.
OPTION/SAR GRANTS IN LAST FISCAL YEAR
| | | | | | | | | | | | | | |
| | | Individual Grants
| | |
Name
| | Number of
Securities
Underlying
Options
Granted (#)
| | Percent of
Total Options
Granted to
Employees In
Fiscal Year
2005
| | | Exercise
Price Per
Share
($/Sh.)(1)
| | Expiration
Date
| | Potential Realizable
Value at Assumed
Annual Rates of Stock
Appreciation for the
Option Term (2)
|
| | | | | | 5% ($)
| | 10% ($)
|
Michael K. Perry | | 45,000 | | 2.2 | % | | $ | 4.89 | | 1/18/2015 | | 138,389 | | 350,703 |
| | | 50,000 | | 2.5 | % | | $ | 1.19 | | 10/20/2015 | | 37,419 | | 94,828 |
Rhonda F. Rhyne | | 35,000 | | 1.7 | % | | $ | 4.89 | | 1/18/2015 | | 107,635 | | 272,769 |
| | | 1,200 | | 0.1 | % | | $ | 1.81 | | 8/1/2015 | | 1,366 | | 3,462 |
| | | 45,000 | | 2.2 | % | | $ | 1.19 | | 10/20/2015 | | 33,677 | | 85,345 |
Steve P. Loomis | | 30,000 | | 1.5 | % | | $ | 4.89 | | 1/18/2015 | | 92,259 | | 233,802 |
| | | 40,000 | | 2.0 | % | | $ | 1.19 | | 10/20/2015 | | 29,935 | | 75,862 |
Russell H. Bergen | | 30,000 | | 1.5 | % | | $ | 4.89 | | 1/18/2015 | | 92,259 | | 233,802 |
| | | 40,000 | | 2.0 | % | | $ | 1.19 | | 10/20/2015 | | 29,935 | | 75,862 |
Richard E. Trayler | | 30,000 | | 1.5 | % | | $ | 4.89 | | 1/18/2015 | | 92,259 | | 233,802 |
| | | 20,000 | | 1.0 | % | | $ | 1.19 | | 10/20/2015 | | 14,968 | | 37,931 |
| (1) | All options were granted at fair market value (closing sale price for our common stock on the Nasdaq Stock Market on the date of grant). |
| (2) | The potential realizable value is calculated based on the ten-year term of the options at the time of grant; the assumption that the closing price for the common stock on the grant date appreciates at the indicated annual rate compounded annually for the entire term of the option; and the assumption that the option is exercised and sold on the last day of its term. |
The following table provides further information regarding the Named Officers’ exercises and outstanding stock options as of November 30, 2005. No stock appreciation rights were granted or exercised.
AGGREGATED OPTION/SAR EXERCISES IN LAST
FISCAL YEAR AND FISCAL YEAR END OPTION/SAR VALUES
| | | | | | | | | |
Name
| | Shares Acquired
On Exercise (#)
| | Value Realized ($)
| | Number of Securities
Underlying
Unexercised
Options/SARs at
FY-End (#)
Exercisable/
Unexercisable
| | Value (1) of Unexercised In-the-Money Options/SARs at
FY-End ($)
Exercisable/ Unexercisable(2)
|
Michael K. Perry | | 10,000 | | $ | 9,950 | | 944,939/23,961 | | -0- |
Rhonda F. Rhyne | | 5,000 | | $ | 8,100 | | 515,535/21,565 | | -0- |
Steve P. Loomis | | 2,694 | | $ | 9,389 | | 209,334/19,169 | | -0- |
Russell H. Bergen | | 4,000 | | $ | 5,720 | | 259,731/19,169 | | -0- |
Richard E. Trayler | | -0- | | | -0- | | 172,316/9,584 | | -0- |
| (1) | Represents the difference between the closing sale price of our common stock on the Nasdaq Stock Market of $1.04 on November 30, 2005 and the exercise price of the options. |
78
| (2) | The respective Named Officers as of November 30, 2005 could not exercise these options and future exercisability is subject to certain vesting provisions including specific stock price thresholds and/or remaining in the employ of the Company for up to four additional years. |
Employment Agreements
The Company is not party to any employment agreements.
Long-Term Incentive Plans
The Company does not have any long-term incentive plans(as defined in the SEC rules and regulations).
Directors’ Compensation
Each non-employee director who has not been employed by us during the preceding two years receives 12,000 stock options. In addition, at their election, they also receive either a cash fee of $2,000 per month or an annual stock option grant at fair market value. In 2006, the elected grant was for 24,000 stock options and in 2005 the elected grant was for 12,000 stock options. The options vest monthly over 12 months and expire upon the earlier of ten years from the date of grant or two years after the director terminates their position on the Board. All directors are reimbursed for out-of-pocket expenses incurred in connection with their service on the Board.
During fiscal 2005, 2004 and 2003, 198,000, 102,000 and 82,000 options, respectively, were granted to the members of the Board of Directors. During fiscal 2005, the director compensation was as follows:
| | | | | |
| | | Cash Paid
| | Stock Options
|
James C. Gilstrap | | $ | — | | 48,000 |
Connie R. Curran, Ed.D., RN | | | — | | 48,000 |
Peter C. Farrell, Ph. D.(1) | | | 8,000 | | 6,000 |
Robert W. Keith(2) | | | 8,000 | | 12,000 |
Ronald A. Matricaria(1) | | | 16,000 | | — |
Richard O. Martin, Ph. D. | | | — | | 48,000 |
Ronald L. Merriman(1) | | | 17,667 | | — |
B. Lynne Parshall(2) | | | 8,000 | | 36,000 |
| (1) | Director’s Board term concluded at annual shareholder meeting on July 21, 2005. |
| (2) | Director’s Board term commenced at annual shareholder meeting on July 21, 2005. |
The stock option grant elected for 2006 in lieu of cash compensation was granted in November 2005, therefore the stock option grant amounts listed above represent option compensation for more than one year.
Director Independence
All of the members of our Board of Directors qualify as “independent” as defined by the rules of The NASDAQ National Market, except for Mr. Perry, by virtue of his position as Chief Executive Officer.
Relationships among Directors and Executive Officers
The Company is not aware of any family relationships among any of our directors or executive officers.
Code of Ethics
The Company has adopted (and filed as an exhibit to Form 10-K for the fiscal year ended November 30, 2003) a code of business conduct and ethics that applies to our principal executive officer, principal financial and accounting officer. The Code of Ethics is available on our website at www.cdic.com/ir/fr_govern.html.
79
BOARD COMPENSATION COMMITTEE REPORT ON EXECUTIVE
COMPENSATION
Our compensation programs and policies applicable to our executive officers are established and administered by the Compensation Committee of our Board of Directors. The Compensation Committee is comprised of three non-employee directors, none of whom have interlocking relationships as defined by the rules and regulations of the Securities and Exchange Commission.
It is the philosophy of the Compensation Committee that executive compensation be aligned with our overall business strategies, values and performance. These policies are designed to set its executive compensation, including salary, cash bonus awards and long-term stock-based incentive awards, at a level consistent with amounts paid to executive officers of companies of similar size, industry and geographic location.
Compensation Philosophy and Objectives
The Compensation Committee believes that compensation of our executive officers should:
| | • | | Encourage creation of shareholder value and achievement of strategic corporate objectives; |
| | • | | Integrate compensation with our annual and long-term corporate objectives, values and business strategies, and focus executive actions on the fulfillment of those objectives, values and strategies; |
| | • | | Provide a competitive total compensation package that enables us to attract and retain, on a long-term basis, high caliber personnel; |
| | • | | Provide a compensation opportunity that is competitive with companies in the medical device and biotechnology industries, taking into account relative company size, performance and geographic location as well as individual responsibilities and performance; and |
| | • | | Align the interests of management with the long-term interests of stockholders and enhance shareholder value by providing management with longer-term incentives through equity ownership. |
The Compensation Committee periodically reviews the approach to executive compensation and will make changes as competitive conditions and other circumstances warrant and will seek to ensure our compensation philosophy is consistent with our best interests.
Key Elements of Executive Compensation
The compensation of executive officers is based upon our financial performance as well as the achievement of certain business objectives, including: increased market penetration, broadening of distribution channels through strategic relationships, securing and growing recurring revenue, targeting new market opportunities, furthering ICG technology and developing ICG products for new medical applications, as well as the achievement of individual business objectives by each executive officer.
Executive compensation consists of three elements: (i) base salary, (ii) bonus awards and (iii) long-term stock-based incentive awards. The summary below describes in more detail the factors that the Compensation Committee considers in establishing each of the three primary components of the variable compensation package provided to the executive officers.
Base salary—Salary ranges are largely determined through comparisons with companies of similar revenues, market capitalization, headcount or complexity in the medical device and biotechnology industries. Actual salaries are based on individual performance contributions and other more qualitative and developmental criteria within a competitive salary range for each position that is established through evaluation of responsibilities, competitive, inflationary, internal equity and market considerations. The Compensation Committee, on the basis of its knowledge of executive compensation in the industry, believes that our salary
80
levels for the executive officers are at a level that the Compensation Committee, at the time such salary determinations were made, considered to be reasonable and necessary given our financial resources and stage of development. The Compensation Committee reviews executive salaries each October and sets the salaries for the following year.
Bonus awards—The Compensation Committee has established an incentive bonus program providing for the payment to each executive officer of quarterly bonuses that are directly related to the group and individual achievements of executive management and our revenues and earnings. The bonus pool is established based on quarterly revenues and earnings achieved and the pool is allocated to individual executives through a weighting, half of which is based on achievement of our revenue and earnings goals and half of which is based on CEO and Compensation Committee assessment of the executive’s individual quarterly goal achievement and overall contribution to CardioDynamics.
In fiscal 2005, the target bonuses for executive officers ranged from 42% to 53% of annual base salary, however there were no bonuses paid for the year. Incentive award amounts for each participant are based on his or her potential impact on our operating and financial results and on market competitive pay practices. Under the incentive bonus program, incentive awards are paid based on achievement of quarterly performance goals. These performance goals include individual performance goals, which are established by the CEO at the beginning of each quarter, and annual corporate performance goals. The corporate performance goals are based on our achievement of strategic objectives as well as specific revenue and earnings growth targets for each quarter and the fiscal year.
Long-term stock-based incentive awards—Our Stock Option Plan, which was approved by our shareholders in 2004, as well as the executive bonus plan, are structured to permit awards under such plans to qualify as performance-based compensation and to maximize the tax deductibility of such awards. The Committee intends that compensation paid to management, including stock options, be exempt from the limitations on deductibility under Section 162(m) of the Internal Revenue Code. Accordingly, the Compensation Committee administers grants under our Stock Option Plan to members of management.
The Compensation Committee believes that providing incentives that focus attention on managing us from the perspective of an owner with an equity stake in the business will closely align the interest of shareholders and executive officers. Therefore, all employees, and particularly those persons who have substantial responsibility for our management and growth, are eligible to receive annual stock option grants, although the Compensation Committee, at its discretion, may grant options at other times in recognition of exceptional achievements.
The number of shares underlying stock options granted to executive officers is based on competitive practices in the industry as determined by independent surveys and the Compensation Committee’s knowledge of industry practice. The Compensation Committee grants options under the Stock Option Plan with an exercise price equal to the fair market value on the date of grant. The grants are intended to be competitive in order to retain and encourage positive future performance and to provide a direct link with the interests of our shareholders.
The Compensation Committee, in making its determination as to grant levels, takes into consideration: (i) the executive’s historical and expected contribution toward our objectives, (ii) prior award levels, (iii) award levels necessary to replace particular executives, (iv) the executive’s direct ownership of our shares, (v) the number of options vested and unvested, (vi) anticipated accounting rule changes, and (vii) the number of options outstanding as a percentage of total shares outstanding. During fiscal 2005, 1,428,931 net new options were granted, representing 2.9% of our outstanding shares. Of those, 539,027 were granted to our executive officers. One grant was made in January of 2005 based on executive achievements in fiscal 2004, and a second grant was made in October of 2005 based on executive achievements thus far in fiscal 2005. Therefore, the stock option grant amounts in fiscal 2005 represent option compensation for more than one year. The Stock Option Plan limits the total number of shares subject to options that may be granted to a participant during the lifetime of the plan to 800,000 shares. The Stock Option Plan contains provisions providing for forfeiture of the options in the event the option holder engages in certain intentional misconduct contrary to our interests.
81
Chief Executive Officer Compensation
Michael K. Perry is our Chief Executive Officer. In October 2004, the Compensation Committee set Mr. Perry’s annual base salary for 2005 at $279,000. The Compensation Committee increased Mr. Perry’s base salary from $263,000 for the prior year to be competitive with the base salary levels in effect for chief executive officers at comparable companies within the industry. The salary increase was approved in recognition of his achievement of specific corporate objectives in 2004, which included: growing our revenues 35%, growing income from operations 26% and achieving $4,365,000 of positive operating cash flow. Additionally, he was recognized for the achievement of releasing the BioZ Dx product and for identifying, acquiring and integrating the Vermed and Medis operations. The Compensation Committee determined that these achievements were important to our future growth and could assist us in enhancing shareholder value. The Compensation Committee also considered Mr. Perry’s compensation relative to medical device and biotechnology industry norms within southern California.
As an incentive for future performance, the Compensation Committee granted Mr. Perry options under our Stock Option Plan, exercisable at the fair market value on the date of grant, to purchase 95,000 shares of our common stock.
Mr. Perry is a member of the Board of Directors, but did not participate in matters involving the evaluation of his own performance or the setting of his own compensation. The foregoing report has been furnished by our Compensation Committee.
Compensation Committee:
Richard O. Martin, Ph.D.—Chairman
James C. Gilstrap
Robert W. Keith
82
PERFORMANCE GRAPH
The following graph compares the five-year cumulative total return (assuming reinvestment of dividends) of an investment of $100 from November 30, 2000 to November 30, 2005 for: (i) our common stock, (ii) the Russell 2000 stock index and (iii) the Medical Equipment and Appliance Industry Group index. Our common stock has been included in both of these indices. The Russell 2000 is comprised of the smallest 2,000 companies in the Russell 3000 index, which contains the 3,000 largest United States companies, based on total market capitalization.
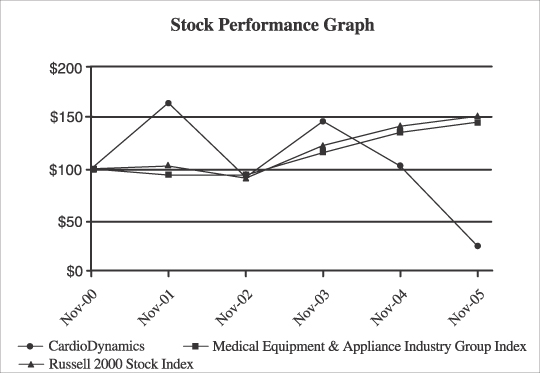
| | | | | | | | | | | | |
| (In dollars) | | 2000
| | 2001
| | 2002
| | 2003
| | 2004
| | 2005
|
CardioDynamics | | 100 | | 165 | | 92 | | 147 | | 103 | | 24 |
Medical Equipment & Appliance Group | | 100 | | 94 | | 94 | | 116 | | 136 | | 146 |
Russell 2000 Stock Index | | 100 | | 103 | | 91 | | 123 | | 142 | | 152 |
83
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Security Ownership of Certain Beneficial Holders
The following are the only persons known by us to own beneficially, 5% or more of the outstanding shares of our common stock as of January 13, 2006.
| | | | | |
| | | Shares Beneficially Owned.
| |
Name and Address of Beneficial Owner
| | Number (1)
| | Percentage (2)
| |
J. Michael Paulson(3) P.O. Box 9660 Rancho Santa Fe, CA 92067 | | 3,841,394 | | 7.9 | % |
| | |
Atlas Master Fund, Ltd.(4) c/o Walkers SPV Limited P.O. Box 908 GT George Town, Grand Cayman Cayman Islands, British West Indies | | 2,562,095 | | 5.0 | % |
| | |
Dr. Herbert A. Wertheim 191 Leucadendra Drive Coral Gables, FL 33156 | | 2,600,000 | | 5.0 | % |
| (1) | Except as indicated in the footnotes to this table, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws, where applicable. |
| (2) | Percentage of ownership is calculated pursuant to SEC Rule 13d-3(d)(1). |
| (3) | Includes 3,458,139 shares held in the Allen E. Paulson Living Trust dated December 23, 1986 of which J. Michael Paulson is the Trustee and executor. Also includes 7,000 shares beneficially owned by the Trust, by virtue of its right to acquire such shares from us under stock options now exercisable or exercisable within 60 days. Also includes 376,255 shares held by J. Michael Paulson, by virtue of his right to acquire such shares from us under stock options now exercisable or exercisable within 60 days. Excludes shares of common stock owned by Mr. Paulson’s brothers; Mr. Paulson disclaims beneficial ownership of such shares. |
| (4) | Includes shares beneficially held by Atlas Global, LLC, Atlas Global Investments, Ltd., Atlas Global Investments II, Ltd., Balyasny Asset Management L.P., Dmitry Balyasny |
84
Security Ownership of Management
The following table sets forth the beneficial ownership of our common stock as of January 13, 2006 by each of our Directors and each Named Officer, and by all of our Directors and executive officers as a group. Each such person has a business address, care of CardioDynamics.
| | | | | |
| | | Shares Beneficially Owned
| |
Name
| | Number(1)
| | Percent (2)
| |
Russell H. Bergen(3) | | 280,228 | | * | |
Connie R. Curran(3) | | 116,000 | | * | |
James C. Gilstrap(4) | | 2,360,427 | | 4.8 | % |
Robert W. Keith(3) | | 7,000 | | * | |
Steve P. Loomis(3) | | 228,831 | | * | |
Richard O. Martin, Ph.D.(3) | | 210,000 | | * | |
B. Lynne Parshall(3) | | 31,000 | | * | |
Michael K. Perry(3) | | 993,000 | | 2.0 | % |
Rhonda F. Rhyne(3) | | 545,219 | | 1.1 | % |
Richard E. Trayler(3) | | 241,564 | | * | |
All Directors and executive officers as a group (3)—(12 persons) | | 5,322,755 | | 10.3 | % |
| (1) | Except as indicated in the footnotes to this table, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws, where applicable. Share ownership in each case includes shares issuable on exercise of certain outstanding options as described in the footnotes below. |
| (2) | Percentage of ownership is calculated pursuant to SEC Rule 13d-3(d)(1). |
| (3) | Includes shares of common stock that may be acquired pursuant to stock options exercisable currently or exercisable within 60 days. |
| (4) | Includes 400,000 shares held by the Scripps Medical Charitable Limited Partnership of which Mr. Gilstrap is a general partner with a minority ownership interest. Mr. Gilstrap disclaims any pecuniary interest in 399,752 of the shares. Also includes 99,000 shares of common stock Mr. Gilstrap beneficially owns, by virtue of his right to acquire such shares from us under stock options now exercisable or exercisable within 60 days. Also includes 32,654 shares held by the James C. Gilstrap Trust dated January 16, 1995 and 71,000 shares held in his daughters trust’s, Mr. Gilstrap disclaims beneficial ownership of such shares. |
Equity Compensation Plan Information
The following table sets forth certain information concerning common stock to be issued in connection with our equity compensation plans.
| | | | | | | |
Plan Category
| | Number of securities
to be issued upon
exercise of outstanding
options, warrants and
rights (a)
| | Weighted-average
exercise price of
outstanding options,
warrants and rights (b)
| | Number of securities
remaining available
for issuance under
equity compensation
plans (excluding
securities reflected in
column (a)) (c)
|
Equity compensation plans approved by security holders | | 5,408,976 | | $ | 3.73 | | 762,716 |
Equity compensation plans not approved by security holders | | 603,000 | | $ | 1.625 | | None |
Total | | 6,011,976 | | $ | 3.52 | | 762,716 |
For a discussion of our equity compensation plans, See “Note 12 Stock Options” in the Notes to the Consolidated Financial Statements included in Part II, Item 8 of this Annual Report on Form 10-K.
85
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
The Company receives certain engineering, development and consulting services from Rivertek Medical Systems, Inc., of which Dennis Hepp, Chief Technology Officer of the Company is a 100% beneficial owner. The Company paid $230,000, $259,000 and $269,000 to Rivertek for engineering services in fiscal 2005, 2004 and 2003, respectively. Amounts payable to Rivertek at November 30, 2005, 2004 and 2003 were $65,000, $145,000 and $77,000, respectively. Subsequent to fiscal year end, in December 2005, Dennis Hepp retired from service as CardioDynamics’ Chief Technology Officer.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
On April 21, 2005, the Company changed its principal accountants from KPMG LLP to Mayer Hoffman McCann P.C. for purposes of the audit of the 2005 consolidated financial statements.
The following tables present fees billed, or expected to be billed, as of the Form 10-K filing date for professional audit services rendered by both KPMG LLP and Mayer Hoffman McCann P.C. during fiscal years 2005 and 2004 for the audit of the Company’s annual financial statements for the years ended November 30 and fees for other services rendered by KPMG LLP and Mayer Hoffman McCann P.C. during those periods.
| | | | | | |
| | | 2005
| | 2004
|
KPMG LLP: | | | | | | |
Audit Fees | | $ | 17,150 | | $ | 892,031 |
Tax Fees | | | — | | | 7,500 |
All Other Fees | | | 7,424 | | | 77,440 |
| | |
|
| |
|
|
Total | | $ | 24,574 | | $ | 976,971 |
| | |
|
| |
|
|
| | |
| | | 2005
| | 2004
|
Mayer Hoffman McCann P.C.: | | | | | | |
Audit Fees | | $ | 1,140,000 | | | — |
Tax Fees | | | — | | | — |
All Other Fees | | | — | | | — |
| | |
|
| |
|
|
Total | | $ | 1,140,000 | | | — |
| | |
|
| |
|
|
Audit fees. Audit fees relate to services rendered in connection with the annual audit of the Company’s financial statements and internal controls, SAS 100 quarterly review of our financial statements, and fees for SEC registration statement services.
Tax Fees. Tax services consisted of fees for tax consultation and tax compliance services.
All Other Fees. Other fees consisted primarily of acquisition related services.
The Audit committee considers whether the provisions of these services is compatible with maintaining our outside auditor’s independence, and has determined such services for fiscal 2005 and 2004 were compatible. All of the services described above were approved by the Audit Committee pursuant to paragraph (c)(7)(ii)(C) of Rule 2-01 of Regulation S-X under the Exchange Act, to the extent that the rule was applicable during 2004 and 2005.
86
AUDIT COMMITTEE REPORT
The Audit Committee assists the Board of Directors in fulfilling its oversight responsibilities relating to the Company’s financial reporting process, its systems of internal accounting and financial controls, and the independent audit of its financial statements. The Audit Committee consisted of three non-employee members. Each member of the Audit Committee is “independent” within the meaning of SEC rules and regulations and the listing standards of the National Association of Securities Dealers, and the Board has determined that Ms. Parshall qualifies as an audit committee financial expert within the meaning of SEC rules and regulations.
The Audit Committee has reviewed and discussed with management the Company’s audited financial statements for the fiscal year ended November 30, 2005, including a discussion of the quality, not just the acceptability, of the accounting principles, the reasonableness of significant judgments and the clarity of disclosures in the financial statements. In addition, the Audit Committee has discussed with the Company’s Independent Registered Public Accounting Firm, the auditors’ independence from management and the Audit Committee including the matters in the written disclosures required by the Independence Standards Board and considered the compatibility of non-audit services with the auditors’ independence.
The Audit Committee has reviewed and discussed with Mayer Hoffman McCann P.C., the Company’s Independent Registered Public Accounting Firm who are responsible for expressing an opinion on the conformity of those audited financial statements in accordance with accounting principles generally accepted in the United States of America, their judgment as to the quality, not just the acceptability, of the Company’s accounting practices and such other matters as are required to be discussed by the independent accountants with the Audit Committee under generally accepted auditing standards including the matters required to be discussed by Statement on Auditing Standards No. 61. The Audit Committee has also received the written disclosures from Mayer Hoffman McCann P.C. required by Independence Standards Board Standard No. 1 and the Audit Committee has discussed the independence of Mayer Hoffman McCann P.C. with that firm. Based on the Audit Committee’s review and discussions noted above, the Audit Committee recommended to the Board of Directors that the Company’s audited financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended November 30, 2005, for filing with the Securities and Exchange Commission.
Respectfully Submitted:
The Audit Committee
| | | | | | | | |
| | | | |
/s/ B. LYNNE PARSHALL
B. Lynne Parshall, Chairman | | | | /s/ CONNIE R. CURRAN
Connie R. Curran | | | | /s/ RICHARD O. MARTIN
Richard O. Martin |
87
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this report:
All other schedules are omitted because they are not applicable or because the required information is shown in the consolidated financial statements or the notes thereto.
3. Exhibits
The following exhibits are included with this report or incorporated herein by reference:
| | |
Exhibit
| | Title
|
| 2.1 | | Fourth Amended Plan of Reorganization. (Incorporated by reference from November 30, 1994 Form 10-KSB.) |
| |
| 3.1 | | Bylaws, as amended through May 15, 1995. (Incorporated by reference from May 31, 1995 Form 10-QSB.) |
| |
| 3.1.1 | | Amendment to Bylaws, dated June 2, 1999. (Incorporated by reference from August 31, 1999 Form 10-QSB.) |
| |
| 3.2 | | Restated Articles of Incorporation as filed July 24, 1998. (Incorporated by reference from August 31, 1998 Form 10-QSB.) |
| |
| 3.3 | | Charter of the Audit Committee of the Board of Directors. (Incorporated by reference from June 7, 2004 Form DEF 14A) |
| |
| 3.4 | | Code of Business Conduct and Ethics (Incorporated by reference from November 30, 2003 Form 10-K) |
| |
| 3.5 | | Charter of the Nominating Committee of the Board of Directors. (Incorporated by reference from June 7, 2005 Form DEF 14A) |
| |
| 10.1 | | Service Agreement among Rivertek Medical Systems, Inc., Dennis G. Hepp and the Company, dated January 1, 2002. (Incorporated by reference from May 31, 2002 Form 10-Q.) |
| |
| 10.2 | | Lease between AGBRI Nancy Ridge, LLC and the Company dated June 20, 1997. (Incorporated by reference from August 31, 1997 Form 10-QSB.) |
88
| | |
Exhibit
| | Title
|
| 10.2.1 | | Amendment to lease agreement between CRV Partners, L.P., and the Company, dated March 21, 2002. (Incorporated by reference from May 31, 2002 Form 10-Q.) |
| |
| 10.2.2 | | Second Amendment to Lease agreement between CarrAmerica Development Corp., LP and the Company, dated June 28, 2004 (Incorporated by reference from August 31, 2004 Form 10-Q) |
| |
| 10.2.3 | | Third Amendment to Lease agreement between CarrAmerica Development Corp., LP and the Company, dated March 15, 2005 (Incorporated by reference from May 31, 2005, Form 10-Q) |
| |
| 10.3 | | Employment Agreement, dated March 23, 1998, between the Company and Michael K. Perry. (Incorporated by reference from May 31, 1998 Form 10-QSB.) |
| |
| 10.4 | | 1995 Stock Option/Stock Issuance Plan, as amended June 10, 1997. (Incorporated by reference from August 31, 1997 Form 10-QSB.) |
| |
| 10.4.1 | | Amendment to 1995 Stock Option/Stock Issuance Plan dated May 20, 1998. (Incorporated by reference from August 31, 1998 Form 10-QSB.) |
| |
| 10.4.2 | | Amendment to 1995 Stock Option/Stock Issuance Plan dated April 12, 2001. (Incorporated by reference from October 3, 2001 Form S-8.) |
| |
| 10.4.3 | | Amendment to 1995 Stock Option/Stock Issuance Plan dated October 17, 2002. (Incorporated by reference from May 31, 2001 Form 10-Q.) |
| |
| 10.5 | | 2004 Stock Incentive Plan. (Incorporated by reference from September 16, 2004 Form S-8.) |
| |
| 10.6 | | Line of Credit Agreement with Imperial Bank, dated January 15, 1999. (Incorporated by reference from February 28, 1999 Form 10-QSB.) |
| |
| 10.6.1 | | Amended and restated Loan and Security Agreement with Comerica Bank, dated September 14, 2003. (Incorporated by reference from November 30, 2003 Form 10-K) |
| |
| 10.6.2 | | Second Amendment to Second Amended and Restated Loan and Security Agreement dated August 26, 2004. (Incorporated by reference from August 31, 2004 Form 10-Q) |
| |
| 10.6.3 | | Fifth Amendment to Second Amended and Restated Loan and Security Agreement dated November 13, 2005. (Incorporated by reference from November 29, 2005 Form 8-K) |
| |
| 10.7 | | Warrants to purchase 2,000,000 shares of the Company’s common stock to GE Marquette Medical Systems, Inc., dated August 25, 1999. (Incorporated by reference from August 31, 1999 Form 10-QSB.) |
| |
| 10.8* | | OEM Development and Purchase Agreement between the Company and GE Marquette Medical Systems, Inc., dated July 7, 2000. (Incorporated by reference from August 31, 2000 Form 10-QSB.) |
| |
| 10.8.1* | | Addendum to OEM Development and Purchase Agreement between the Company and GE Marquette Medical Systems, Inc., dated April 24, 2002. (Incorporated by reference from May 31, 2002 Form 10-Q.) |
| |
| 10.9* | | Omnibus Amendment between the Company and GE Medical Systems Information Technologies, Inc. (Incorporated by reference from November 30, 2000 Form 10-KSB.) |
| |
| 10.10 | | Co-Development and OEM Agreement between the Company and Philips Medical Systems, a division of PENAC dated July 17, 2002. (Incorporated by reference from August 31, 2002 Form 10-Q.) |
| |
| 16.1 | | Letter from KPMG to the Securities and Exchange commission dated April 29, 2005, regarding agreement with the statements made in the Form 8-K (Incorporated by reference from April 21, 2005 Form 8-K.) |
89
| | |
Exhibit
| | Title
|
| 21.1 | | List of Company’s Subsidiaries. |
| |
| 23.1 | | Consent of Independent Registered Public Accounting Firm. |
| |
| 23.2 | | Report and Consent of Independent Registered Public Accounting Firm. |
| |
| 31.1 | | Certification pursuant to Item 601 of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, by Michael K. Perry, the Registrant’s Chief Executive Officer. |
| |
| 31.2 | | Certification pursuant to Item 601 of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, by Steve P. Loomis, the Registrant’s Chief Financial Officer. |
| |
| 32.1 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, by Michael K. Perry, the Registrant’s Chief Executive Officer. |
| |
| 32.2 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, by Steve P. Loomis, the Registrant’s Chief Financial Officer. |
| * | Confidential treatment has been granted as to certain portions of this Exhibit pursuant to Rule 406 promulgated under the Securities Act. Such portions have been omitted and filed separately with the SEC. |
90
CARDIODYNAMICS INTERNATIONAL CORPORATION
Schedule II – Valuation and Qualifying Accounts
For the Years Ended November 30, 2005, 2004 and 2003
(In thousands)
| | | | | | | | | | | | | | | |
Column A
| | Column B
| | Column C
| | Column D
| | | | Column E
|
| | | | | Additions
| | | | | | |
Description
| | Balance at
Beginning of
Period
| | Charged to Costs
and Expenses
| | Charged to
Other Accounts-
Revenues
| | Deductions From
Reserves
| | Balance at End
of Period
|
Allowance for Doubtful Accounts | | | | | | | | | | | | | |
2003 | | $1,726 | | 1,122 | | 1,881 | | (2) | | 1,215 | | (1) | | $ | 1,940 |
| | | | | | | | | | | 1,574 | | (4) | | | |
| | | | | | | |
2004 | | $1,940 | | 1,387 | | 1,517 | | (2) | | 865 | | (1) | | $ | 2,400 |
| | | | | | | | | | | 1,579 | | (4) | | | |
| | | | | | | |
2005 | | $2,400 | | 567 | | 1,978 | | (2) | | 1,498 | | (1) | | $ | 1,667 |
| | | | | | | | | | | 1,780 | | (4) | | | |
| Provision for Excess, Obsolete and Slow Moving Inventory | | | | | | | | | | | | | |
2003 | | $ 129 | | 377 | | — | | | | 89 | | (3) | | $ | 417 |
| | | | | | | |
2004 | | $ 417 | | 15 | | — | | | | 82 | | (3) | | $ | 350 |
| | | | | | | |
2005 | | $ 350 | | 346 | | — | | | | 68 | | (3) | | $ | 628 |
| (1) | Represents the net write-off of uncollectible accounts (less recoveries). |
| (2) | Represents the net amounts charged against revenues for sales returns. |
| (3) | Represents the net amounts written off as excess, obsolete or slow-moving and adjustments to reserve requirement. |
| (4) | Represents the net amounts written off against the sales return reserve. |
See accompanying report of independent registered public accounting firm.
91
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| Dated: February 28, 2006 | | | | CARDIODYNAMICS INTERNATIONAL CORPORATION |
| | | | |
| | | | | | | By: | | /s/ MICHAEL K. PERRY |
| | | | | | | | | Michael K. Perry |
| | | | | | | | | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Signature
| | Title
| | Date
|
| | |
/s/ MICHAEL K. PERRY
Michael K. Perry | | Chief Executive Officer
(Principal Executive Officer) | | February 28, 2006 |
| | |
/s/ STEVE P. LOOMIS
Steve P. Loomis | | Vice President Finance,
Chief Financial Officer
(Principal Financial and Accounting Officer) | | February 28, 2006 |
| | |
/s/ CONNIE R. CURRAN
Connie R. Curran, Ed.D. RN | | Director | | February 28, 2006 |
| | |
/s/ JAMES C. GILSTRAP
James C. Gilstrap | | Director | | February 28, 2006 |
| | |
/s/ ROBERT W. KEITH
Robert W. Keith | | Director | | February 28, 2006 |
| | |
/s/ RICHARD O. MARTIN, Ph.D.
Richard O. Martin, Ph.D. | | Director | | February 28, 2006 |
| | |
/s/ B. LYNNE PARSHALL
B. Lynne Parshall | | Director | | February 28, 2006 |
92
