Use these links to rapidly review the document
TABLE OF CONTENTS
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the Fiscal Year Ended December 31, 2012 | ||
or | ||
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from to | ||
Commission file number 001-10865
AMAG Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) | 04-2742593 (I.R.S. Employer Identification No.) | |
100 Hayden Avenue Lexington, Massachusetts (Address of Principal Executive Offices) | 02421 (Zip Code) |
(617) 498-3300
(Registrant's Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:Common Stock, par value $0.01 per share, NASDAQ Global Select Market
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of "accelerated filer," "large accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller Reporting Company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The aggregate market value of the registrant's voting stock held by non-affiliates as of June 30, 2012 was approximately $329,200,000 based on the closing price of $15.40 of the Common Stock of the registrant as reported on the NASDAQ Global Select Market on such date. As of February 15, 2013, there were 21,541,891 shares of the registrant's Common Stock, par value $0.01 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Proxy Statement to be filed in connection with the solicitation of proxies for the Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
AMAG PHARMACEUTICALS, INC.
FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2012
TABLE OF CONTENTS
PART I | ||||
Item 1. | Business | 2 | ||
Item 1A. | Risk Factors | 28 | ||
Item 1B. | Unresolved Staff Comments | 55 | ||
Item 2. | Properties | 55 | ||
Item 3. | Legal Proceedings | 56 | ||
Item 4. | Mine Safety Disclosures | 57 | ||
PART II | ||||
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 58 | ||
Item 6. | Selected Financial Data | 61 | ||
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | 62 | ||
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 91 | ||
Item 8. | Financial Statements and Supplementary Data | 93 | ||
Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 136 | ||
Item 9A. | Controls and Procedures | 136 | ||
Item 9B. | Other Information | 136 | ||
PART III | ||||
Item 10. | Directors, Executive Officers and Corporate Governance | 137 | ||
Item 11. | Executive Compensation | 137 | ||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 137 | ||
Item 13. | Certain Relationships and Related Transactions, and Director Independence | 137 | ||
Item 14. | Principal Accountant Fees and Services | 137 | ||
PART IV | ||||
Item 15. | Exhibits and Financial Statement Schedules | 138 |
Except for the historical information contained herein, the matters discussed in this Annual Report on Form 10-K may be deemed to be forward-looking statements that involve risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. In this Annual Report on Form 10-K, words such as "may," "will," "expect," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements.
Examples of forward-looking statements contained in this report include statements regarding the following: our expectation to expand our portfolio through the in-license or purchase of additional specialty pharmaceutical products, our expectation that we may receive a decision from the U.S. Food and Drug Administration on our supplemental New Drug Application sometime in the fourth quarter of 2013, our expectation that Takeda Pharmaceutical Company Limited plans to file a Type II Variation with the European Medicines Agency in 2013 for the treatment of iron deficiency anemia in adult patients, our expectation that the enrollment of our ongoing pediatric studies will take several years to complete, our intention to commence a pediatric iron deficiency anemia study once the appropriate dose is determined, our plan to begin enrollment in the second quarter of 2013 for a post-approval trial to assess the safety and efficacy of repeat doses of Feraheme for the treatment of iron deficiency anemia, our expectation that 3SBio, Inc. will begin a clinical trial if approved by the Chinese State Food and Drug Administration, our expectation of costs to be incurred in connection with and revenue sources to fund our future operations, our expectation that the majority of all Feraheme utilization in the U.S. will be in the non-dialysis chronic kidney disease patient population, our expectation that final data from IDA-303 will be available in 2013, our expectations regarding the success of our collaboration with Takeda Pharmaceutical Company Limited, including any potential milestone payments, product sales or royalties we may receive, our intention to no longer commercially manufacture or sell GastroMARK after completion of our obligations to our licensees in the first quarter of 2013, our expectation that we will sell our Cambridge, Massachusetts manufacturing facility in the near future, our expectations regarding the manufacture of all Feraheme/Rienso drug substance and drug product at our third-party manufacturers, our expectations regarding the timing of regulatory approval by the European Medicines Agency on our new assay, our expectations regarding the validity of our European patent and timing of the appeals process, our expectation that dialysis sales will not be significant in 2013, our expectation that our reserves as a percentage of gross sales will increase slightly in 2013, our expectation that increases in the Branded Drug Fee under the Health Care Reform Act will not be material to our results of operations or financial condition, our expectation that our license fee and other collaboration revenues will decrease in 2013, our expectation that we will not achieve new milestones under the Amended Takeda Agreement in 2013, our expectation that our costs of product sales as a percentage of net product sales and royalties will decrease in 2013, our expectation that our research and development expenses will decrease in 2013, our expectations regarding the amount of external expenses we expect to incur and the timing of our planned research and development projects, our expectation that selling, general and administrative expenses will remain relatively stable in 2013, our expectation regarding our dividend and interest income, our expectations regarding our short- and long-term liquidity and capital requirements and our ability to finance our operations, our expectations regarding our future cash flows, our belief regarding the potential impact of the adoption of newly issued and future accounting guidance on our financial statements, our expectations that the aggregate of our cash, cash equivalents and investments balances will decrease in 2013, and information with respect to any other plans and strategies for our business. Our actual results and the timing of certain events may differ materially from the results discussed, projected, anticipated or indicated in any forward-looking statements. Any forward-looking statement should be considered in light of the factors discussed in Part I, Item 1A below under "Risk Factors" and elsewhere in this Annual Report on Form 10-K. We caution readers not to place undue reliance on any such forward-looking statements, which speak only as of the date they are made. We disclaim any obligation, except as specifically required by law and the rules of the United States Securities and Exchange Commission to publicly update or revise any such statements to reflect any change in company expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
1
Overview
AMAG Pharmaceuticals, Inc., a Delaware corporation, was founded in 1981. We are a specialty pharmaceutical company focused on the development and commercialization of Feraheme® (ferumoxytol) Injection for Intravenous, or IV, use to treat iron deficiency anemia, or IDA. Currently, our principal source of revenue is from the sale ofFeraheme, which was approved for marketing in the U.S. in June 2009 by the U.S. Food and Drug Administration, or the FDA, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with chronic kidney disease, or CKD. We began commercial sale ofFeraheme in the U.S. in July 2009 through our own commercial organization, including a specialty sales force. We sellFeraheme to authorized wholesalers and specialty distributors, who in turn, sellFeraheme to healthcare providers who administerFeraheme primarily within hospitals, hematology and oncology centers, and nephrology clinics.
We are working to continue to growFeraheme in the U.S. CKD market and to drive additional growth ofFeraheme through both international and label expansion. To further build our business, we intend to expand our portfolio through the in-license or purchase of additional marketed specialty pharmaceutical products. We are seeking complementary products that will leverage our commercial infrastructure and focus on hematology and oncology centers, hospital infusion centers or other sites of care where IV iron is administered or where IDA patients are diagnosed or treated. We may also pursue more strategic transactions which complement our future market expansion goals forFeraheme.
International Expansion
Outside of the U.S., ferumoxytol has been granted marketing approval in Canada, Switzerland and the European Union, or EU, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. The European marketing authorization is valid in the current EU member states as well as in Iceland and Norway. Under our amended agreement with Takeda Pharmaceutical Company Limited, or Takeda, Takeda has an exclusive license to market and sell ferumoxytol in Canada, the EU and Switzerland, as well as certain other geographic territories. In Canada, Takeda promotes ferumoxytol under the trade nameFeraheme and in the EU and Switzerland, Takeda promotes ferumoxytol under the trade name Rienso® 30mg/ml solution for Injection.
Label Expansion
We believe that a significant opportunity exists in the U.S. forFeraheme beyond the treatment of IDA in adult patients with CKD. In the U.S. in 2012, approximately 800,000 grams of IV iron were administered for the treatment of non-dialysis patients with IDA. We believe that approximately half, or 400,000 grams, of the IV iron administered in the U.S. is for the treatment of non-dialysis patients with CKD and the other half is for non-CKD patients with IDA due to other causes, including patients with gastrointestinal diseases or disorders, abnormal uterine bleeding, or AUB, inflammatory diseases, and chemotherapy-induced anemia.
In 2012, we completed a phase III clinical program forFeraheme in patients with IDA who had failed to or could not use oral iron. The IDA program consisted of two controlled, multi-center phase III clinical trials, or IDA-301 and IDA-302, including more than 1,400 patients, which evaluated the safety and efficacy of ferumoxytol for the treatment of IDA in this broader patient population. Both studies met the primary efficacy endpoints related to improvements in hemoglobin. In these studies no new safety signals were observed withFeraheme treatment and the types of reported adverse events, or AEs, were consistent with those seen in previous studies and those contained in the approved U.S. package insert forFeraheme. In addition, patients from IDA-301 were eligible to enroll in an open-label extension study, or IDA-303, and receive treatment withFeraheme, as defined in the protocol.
2
In December 2012, we submitted a supplemental new drug application, or sNDA, to the FDA, seeking approval forFeraheme for the treatment of IDA in adult patients who have failed to or could not use oral iron. The sNDA submission was primarily based on the data from IDA-301 and IDA-302. In addition, the sNDA included data from an interim analysis of IDA-303 and a previously completed post-approval clinical study evaluatingFeraheme treatment compared to treatment with another IV iron. We believe that approval forFeraheme for this expanded indication would effectively double the market opportunity forFeraheme, by allowing us to access the half of the IV iron market that is beyond our current approved indication. Assuming a normal review cycle, we expect a decision from the FDA on our sNDA sometime in the fourth quarter of 2013.
We expect that Takeda will file a Type II Variation, which is the EU equivalent of a U.S. sNDA, with the European Medicines Agency, or EMA, in 2013 seeking marketing approval forRienso for the treatment of IDA in adult patients.
Takeda Collaboration
In March 2010, we entered into a License, Development and Commercialization Agreement, or the Takeda Agreement, with Takeda under which we granted exclusive rights to Takeda to develop and commercializeFeraheme/Rienso as a therapeutic agent in Europe, certain Asia-Pacific countries (excluding Japan, China and Taiwan), the Commonwealth of Independent States, Canada, India and Turkey. In June 2012, we entered into an amendment to the Takeda Agreement, or the Amended Takeda Agreement, which removed the Commonwealth of Independent States from the territories under which Takeda has the exclusive rights to develop and commercializeFeraheme/Rienso. In addition, the Amended Takeda Agreement modified the timing and pricing arrangements for a supply agreement to be entered into between us and Takeda in the future, the terms related to primary and secondary manufacturing for drug substance and drug product, certain patent related provisions, and the re-allocation of certain of the agreed-upon milestone payments. In 2012, we received a total of $33.0 million in milestone payments from Takeda associated with the EU approval and the commercial launches ofFeraheme/Rienso in Canada and the EU. In addition, in connection with the commercial launches ofFeraheme/Rienso by Takeda, we recorded revenue from product sales to Takeda and royalties on sales by Takeda of $0.1 million in 2012.
Clinical Development of Feraheme
We have initiated two randomized, active-controlled pediatric studies ofFeraheme for the treatment of IDA in pediatric CKD patients to meet our FDA post-approval Pediatric Research Equity Act requirement to support pediatric labeling ofFeraheme in the U.S. One study covers dialysis-dependent CKD pediatric patients, and the other covers CKD patients not on dialysis. Each study will assess the safety and efficacy ofFeraheme treatment as compared to oral iron in approximately 144 pediatric patients. Both of these pediatric studies are currently open for enrollment with enrollment expected to take several years to complete.
Our pediatric investigation plan, which was a requirement for submission of the Marketing Authorization Application, or MAA, for ferumoxytol, was approved by the EMA in December 2009 and amended in 2012, and includes the two pediatric studies needed to meet the requirements of the Pediatric Research Equity Act in the U.S. described above, and two additional pediatric studies requested by the EMA. These studies include a rollover study in pediatric CKD patients and a study in pediatric patients with IDA regardless of the underlying cause. The rollover study is open for enrollment. The pediatric IDA study will commence once the appropriate dose ofFeraheme is determined from the study data resulting from the two ongoing pediatric studies ofFeraheme for the treatment of IDA in pediatric CKD patients, described above. The amendment to our pediatric investigation plan in 2012 was intended to increase the rate of enrollment for these studies through modifications to the patient entry criteria.
3
As part of our obligations under the Amended Takeda Agreement and as part of our post-approval commitments to the EMA, we are planning to initiate a multi-center clinical trial to determine the safety and efficacy of repeat doses of ferumoxytol for the treatment of IDA in patients with hemodialysis dependent CKD. As part of the post-approval commitment we made to the EMA as a condition of the approval of the MAA for ferumoxytol in the EU this study includes a treatment arm with iron sucrose as well as a magnetic resonance imaging, or MRI, study which will evaluate the potential for iron to accumulate in the body following treatment with IV iron, specifically in the heart and liver, and, where possible, other major organs following repeated IV iron administration over a two year period. We currently expect enrollment to begin in the second quarter of 2013. The costs related to the MRI portion of this study are subject to our established cost sharing arrangement with Takeda.
From time to time, we or our licensees may sponsor pilot clinical studies or collaborate with investigators on their research ideas to evaluate the safety and efficacy of Feraheme in new indications or alternative dosing regimens.
In addition, certain clinical trials may be necessary to secure desired pricing in various European markets. If so, the cost of any future trials may be allocated between us and Takeda according to the Amended Takeda Agreement.
In December 2009, our licensee in China, 3SBio Inc., or 3SBio, filed an application with the Chinese State Food and Drug Administration, or the SFDA, to obtain approval to begin a clinical trial necessary to file for marketing approval ofFeraheme in China. If approved by the SFDA, 3SBio plans to commence a multi-center randomized efficacy and safety study ofFeraheme in China involving approximately 200 CKD patients.
Other information
Prior to the 2009 U.S. approval and commercial launch ofFeraheme, we devoted substantially all of our resources to our research and development programs. Since then, we have incurred substantial costs related to the commercialization and development ofFeraheme. We expect to continue to incur significant expenses as we continue to manufacture, market and sellFeraheme/Rienso as an IV iron replacement therapeutic for use in adult CKD patients in the U.S., to seek marketing approval forFeraheme for the treatment of IDA in a broad range of patients, and to continue to obtain marketing approval forFeraheme in countries whereFeraheme/Rienso has yet to receive approval. Prior to the U.S. commercial launch ofFeraheme, we financed our operations primarily from the sale of our equity securities, cash generated by our investing activities, and payments from our licensees. Since 2009, our revenues have been primarily attributable to product sales ofFeraheme/Rienso, along with milestone and license fee payments from Takeda. We currently expect to fund our future operations from cash from sales ofFeraheme in the U.S., milestone payments we expect to earn from Takeda, product sales and royalties we may receive with respect to sales ofFeraheme/Rienso outside of the U.S., cash generated by our investing activities, and the sale of our equity or debt securities, if necessary. As of December 31, 2012, we had an accumulated deficit of approximately $456.7 million and a cash, cash equivalents and investments balance of approximately $227.0 million.
Our common stock trades on the NASDAQ Global Select Market, or NASDAQ, under the trading symbol "AMAG."
4
Our Core Technology
Our core technology is based on coated superparamagnetic iron oxide particles and their characteristic properties. Our core competencies include the ability to design such particles for particular applications and to manufacture the particles in controlled sizes. Our technology and expertise enable us to synthesize, sterilize and stabilize these iron oxide particles in a manner necessary for use in pharmaceutical products such as IV iron replacement therapeutics.
Our iron oxide particles are composed of bioavailable iron that is easily utilized by the body and incorporated into the body's iron stores. As a result, our core technology is well suited for use as an IV iron replacement therapy product. Our rights to our technology are derived from and/or protected by license agreements, patents, patent applications and trade secret protections. See "Patents and Trade Secrets."
Products
The following table summarizes the uses and potential uses of ferumoxytol, the names of our principal licensees, the current U.S. and foreign regulatory status, and the primary markets for ferumoxytol.
Product | Uses/Potential Uses | Licensees | U.S. Regulatory Status | Foreign Regulatory Status | ||||
|---|---|---|---|---|---|---|---|---|
| Feraheme® (ferumoxytol) Injection | IV iron replacement therapeutic agent for the treatment of IDA in adult patients with CKD. | Takeda (Europe, certain Asia-Pacific countries (excluding Japan, China and Taiwan), Canada, India and Turkey). 3SBio (China). | Approved and marketed. | Approved and marketed asFeraheme in Canada. Approved and marketed asRienso in the European Union and Switzerland. Filed for CKD registrational trial with the SFDA in China, December 2009. | ||||
Feraheme® (ferumoxytol) Injection | IV iron replacement therapeutic agent in patients with IDA, regardless of the underlying cause. | Takeda (Europe, certain Asia-Pacific countries (excluding Japan, China and Taiwan), Canada, India and Turkey). 3SBio (China) (option to extend license into additional therapeutic indications). | sNDA filed in December 2012. | Type II Variation expected to be filed with the EMA by Takeda in 2013. |
For a discussion of the substantive regulatory requirements applicable to the development and regulatory approval process in the U.S. and other countries, see "Government Regulation."
Feraheme for the treatment of IDA in patients with CKD
Overview
In June 2009,Feraheme was approved for marketing in the U.S. by the FDA for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. In July 2009, we began to market and sellFeraheme in the U.S. in both the dialysis and non-dialysis CKD markets, including to nephrologists, hematologists, dialysis organizations, hospitals and other end-users who treat patients with CKD. Beginning in 2010, due to changes in the way the federal government reimburses providers
5
for the care of dialysis patients, the utilization ofFeraheme shifted from primarily dialysis patients to non-dialysis patients. Accordingly, we have since focused our commercial efforts entirely on buildingFeraheme utilization in non-dialysis CKD patients. We anticipate the majority of allFeraheme utilization in the U.S. will continue to be in the non-dialysis CKD patient population until, and if, the Company achieves a broader label to include non-CKD patients.
In December 2011, ferumoxytol was granted marketing approval in Canada, under the trade nameFeraheme, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. In June 2012, the European Commission granted marketing authorization for ferumoxytol, under the trade nameRienso, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. The marketing authorization is valid in the current EU member states as well as in Iceland and Norway. In August 2012, ferumoxytol was granted marketing approval in Switzerland under the trade nameRienso for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. Under our amended agreement with Takeda, Takeda has an exclusive license to market and sell ferumoxytol in Canada, the EU and Switzerland, as well as certain other geographic territories.
Chronic kidney disease, anemia, and iron deficiency
Based on data contained in a 2007 publication in theJournal of the American Medical Association, it is estimated that approximately 10 to 15% of the U.S. adult population is affected by CKD, a condition generally characterized by damaged kidneys, or a reduction in kidney function below 50% of normal. Anemia, a common condition among CKD patients, is associated with cardiovascular complications, decreased quality of life, hospitalizations, and increased mortality. Anemia develops early during the course of CKD and worsens with advancing kidney disease. Iron deficiency is a common cause of anemia in CKD patients and can result from multiple blood draws, hospitalizations and interventional procedures, gastrointestinal bleeding, or poor nutritional intake. Regardless of the cause of anemia, iron replacement therapy is essential to increase iron stores and raise hemoglobin levels. Iron is also essential for effective treatment with erythropoiesis stimulating agents, or ESAs, which are commonly used in anemic patients to stimulate red blood cell production. Based on data contained in a 2009 publication in theJournal of the American Society of Nephrology, we estimate there are approximately 1.6 million adults in the U.S. with IDA and stages 3 through 5 CKD, who are patients in the later stages of CKD but not yet on dialysis and could therefore benefit from receiving iron.
Currently there are two methods used to treat IDA in CKD patients: oral iron supplements and IV iron. Oral iron is currently the first line iron replacement therapy of choice of most physicians in both the U.S. and abroad. However, oral iron supplements are often not absorbed well by the gastrointestinal tract and frequently have unpleasant side effects, such as constipation, diarrhea, and cramping, which can cause patients to stop taking their medication. In addition, it can take an extended time for hemoglobin levels to improve following the initiation of oral iron treatment, and even then may not reach the targeted hemoglobin levels. Conversely, iron given intravenously allows larger amounts of iron to be provided to patients while avoiding many of the side effects and treatment compliance issues associated with oral iron, and can result in faster rises in hemoglobin levels. The administration of IV iron has been shown to be effective in treating anemia either when used alone and also in combination with an ESA. Current U.S. treatment guidelines indicate that treating first with iron alone may delay, reduce or eliminate the need for ESA therapy. We believe that a small fraction of non-dialysis CKD patients in the U.S. with IDA are currently being treated with IV iron, and thus a significant opportunity remains to grow the market for IV iron in this patient population.
Feraheme for the treatment of IDA in a broad range of patients
IDA not associated with CKD is widely prevalent in many different patient populations. For many of these patients, treatment with oral iron is unsatisfactory. These patients include patients with
6
gastrointestinal diseases or disorders, women with AUB, patients with inflammatory diseases, and cancer patients. It is estimated that more than 4 million patients in the U.S. have IDA (CKD and non-CKD). Currently, we estimate that approximately 5 to 10% of these patients are treated with IV iron.
In December 2012, we submitted a sNDA to the FDA forFeraheme to expand the approved indication for ferumoxytol beyond the current indication for treatment of IDA in adult patients with CKD to adult IDA patients who have failed or could not use oral iron. The sNDA included data from two controlled, multi-center phase III clinical trials, IDA-301 and IDA-302, including more than 1,400 patients, which served as the primary data supporting the safety and efficacy of ferumoxytol for the treatment of IDA in this target patient population. In addition, the sNDA included data from an interim analysis of the IDA open-label extension study, IDA-303, and a previously completed post-approval clinical study evaluatingFeraheme treatment compared to treatment with another IV iron. Assuming a standard review cycle, we expect a decision from the FDA on our sNDA sometime in the fourth quarter of 2013.
We expect that Takeda will file a Type II Variation, which is the EU equivalent of a U.S. sNDA, with the EMA in 2013 seeking marketing approval forRienso for the treatment of IDA in adult patients.
IDA-301 was a double-blind, placebo-controlled trial designed to compare the safety and efficacy of two doses of 510 milligrams each ofFeraheme to that of placebo in a total of 808 patients with IDA at 136 sites in the U.S., Canada, India, Latvia, Hungary, and Poland. The patients enrolled in this study had a history of unsatisfactory response to, or could otherwise not use, oral iron and had IDA associated with various conditions including gastrointestinal diseases or disorders, AUB, inflammatory diseases, and chemotherapy-induced anemia.
Patients in this study were randomized to receive a one gram IV course of eitherFeraheme or saline as placebo and the study was designed to demonstrate superiority on efficacy. Of the 808 patients enrolled in this study, 608 patients were randomly assigned to receiveFeraheme and 200 were randomly assigned to receive placebo. The demographics and all baseline parameters of patients who participated in this study were well balanced between the two treatment groups. The primary efficacy endpoint of the study for the FDA was the proportion of patients who achieved an increase in hemoglobin of greater than 2.0 grams per deciliter at any time from the date of determination of their baseline hemoglobin level, or baseline, to the fifth week following administration of the study drug, or week five. The primary efficacy endpoint of the study for the EU regulatory authorities was the mean change in hemoglobin from baseline to week five. Patients enrolled in this study were eligible to enter IDA-303, a recently completed open-label extension study to evaluate repeat dosing withFeraheme. We have closed enrollment in this extension study with 637 patients. These patients were followed for six months and were eligible to receive two doses of 510 milligrams each ofFeraheme whenever they met treatment criteria. Final data from IDA-303 is expected to be available in 2013.
In the IDA-301 study,Feraheme achieved both primary efficacy endpoints evaluated. Patients treated withFeraheme in the IDA-301 trial achieved a statistically significant mean increase in hemoglobin at week five of 2.7 grams per deciliter, as compared to a mean increase of 0.1 grams per deciliter in patients who received placebo. In addition, a greater than 2.0 grams per deciliter increase in hemoglobin at any time from baseline to week five was achieved in a statistically significantly greater proportion of patients treated withFeraheme in this study, 81.1%, as compared with 5.5% of patients who received placebo. Further, data from IDA-301 also showed a direct correlation between a rise in hemoglobin and improvement in fatigue, as assessed by patient reported outcome measures.
No new safety signals were observed withFeraheme in the IDA-301 trial and the types of reported AEs were consistent with those seen in our previous studies and those contained in the approved U.S. package insert forFeraheme. Overall, AEs were reported in 49.2% ofFeraheme-treated patients as
7
compared to 43.0% of patients who received placebo. Patients in both treatment groups experienced protocol-defined AEs of special interest, including mild to severe hypotension or hypersensitivity reactions, ranging from fever alone to an anaphylactoid reaction. Of theFeraheme-treated patients, 3.6% experienced protocol-defined AEs of special interest as compared to 1.0% of patients who received placebo. Cardiovascular AEs were reported in 0.8% ofFeraheme-treated patients, all of which were considered unrelated to study drug by the investigators, and none were reported in the placebo group. Serious adverse events, or SAEs, were reported at a comparable frequency in both treatment groups, with SAEs reported in 2.6% ofFeraheme-treated patients and 3.0% of patients who received placebo. Four of the SAEs inFeraheme-treated patients, or 0.7%, were reported as related to study drug by investigators.
IDA-302 was a multi-center, open-label, active-controlled international clinical trial designed to compare treatment withFeraheme to treatment with IV iron sucrose in a total of 605 patients with IDA at 74 sites in Europe, Asia Pacific and Australia. The patients enrolled in the study had a history of unsatisfactory response to, or could not otherwise use, oral iron therapy and had IDA associated with various conditions including gastrointestinal diseases or disorders, AUB, inflammatory diseases, and chemotherapy-induced anemia.
Patients in IDA-302 were randomized to receive a one gram IV course of eitherFeraheme or iron sucrose and the study was designed to demonstrate non-inferiority on efficacy. Of the 605 patients enrolled in the study, 406 patients were randomly assigned to receiveFeraheme and 199 were randomly assigned to receive iron sucrose. The demographics and all baseline parameters of patients who participated in this study were well balanced between the two treatment groups. The primary efficacy endpoint of the study for the FDA was the proportion of patients who achieved a greater than or equal to 2.0 grams per deciliter increase in hemoglobin at any time from baseline to week five. The primary efficacy endpoint of the study for EU regulatory authorities was the mean change in hemoglobin from baseline to week five.
In the IDA-302 study,Feraheme achieved both primary efficacy endpoints evaluated. Patients treated withFeraheme in the IDA-302 trial achieved a mean increase in hemoglobin at week five of 2.7 grams per deciliter as compared to a mean increase of 2.4 grams per deciliter in patients treated with IV iron sucrose. In addition, an increase of 2.0 grams per deciliter or more in hemoglobin at any time from baseline to week five was achieved in 84% of patients treated withFeraheme as compared to 81% of patients treated with IV iron sucrose.
No new safety signals were observed withFeraheme in the IDA-302 trial and the types of reported AEs were consistent with those seen in our previous studies and those contained in the approved U.S. package insert forFeraheme. Overall, AEs experienced by patients in the two treatment groups were comparable, with AEs reported in 41.4% ofFeraheme-treated patients as compared to 44.2% of patients treated with IV iron sucrose. Patients in both treatment groups experienced protocol-defined AEs of special interest, including moderate to severe hypotension or hypersensitivity reactions, ranging from fever alone to an anaphylactoid reaction. Of theFeraheme-treated patients, 2.7% experienced protocol-defined AEs of special interest as compared to 5.0% of patients who received IV iron sucrose. Cardiovascular AEs were comparable between the two treatment groups, with cardiovascular AEs reported in 1.0% of both theFeraheme-treated patients and the patients in the IV iron sucrose group. SAEs were reported in 4.2% ofFeraheme-treated patients as compared to 2.5% of patients treated with IV iron sucrose. Two of the SAEs inFeraheme-treated patients, or 0.5%, were reported as related to study drug by the investigators.
8
Multiple underlying conditions are associated with the development of IDA including gastrointestinal diseases or disorders, AUB, inflammatory diseases, and chemotherapy-induced anemia. IDA in patients with gastrointestinal diseases or disorders is likely caused by blood loss and/or the inadequate intake or absorption of iron due primarily to bariatric surgeries, inflammatory bowel disease, chronic gastrointestinal bleeding and certain malabsorption disorders. Based on market research, we estimate that more than 500,000 patients who have gastrointestinal diseases or disorders in the U.S. also have IDA. Oral iron has been used to treat IDA in patients with gastrointestinal diseases or disorders, but its efficacy is variable due to inconsistent bioavailability and absorption, the high incidence of gastrointestinal side effects and patient noncompliance.
AUB is defined as chronic, heavy, or prolonged uterine bleeding that can result from multiple causes, including uterine abnormalities, blood disorders, pregnancy, intrauterine devices, medications, and heavy menstrual bleeding. IDA is commonly associated with AUB, and based on market research, we estimate that approximately 1 million women in the U.S. have both IDA and AUB and are treated with a variety of surgical and/or medical management techniques. IDA in patients with AUB, regardless of the cause, requires treatment with iron supplementation, either by oral or IV administration.
IDA is also common in patients with cancer, and based on market research, we estimate that nearly 400,000 cancer patients in the U.S. have IDA. Iron supplementation through both oral and IV administration plays an important role in treating anemia in cancer patients. While there may be some differences in the underlying causes of anemia and iron deficiency in cancer patients who are receiving chemotherapy and those who are not, patients in both categories may develop IDA due to blood loss and/or the inadequate intake or absorption of iron. Oral iron has been used to treat IDA in cancer patients, but its efficacy is variable due to inconsistent bioavailability and poor absorption, a high incidence of gastrointestinal side effects, potential interactions with other treatments, and patient noncompliance. IV iron has been shown in small clinical trials to be well tolerated in the cancer patient population in both patients who are receiving chemotherapy and those who are not.
Currently, only INFeD® and Dexferrum® are approved in the U.S. for the treatment of a broader group of patients with IDA in whom oral iron is unsatisfactory or impossible. All of the other currently marketed IV iron products, includingFeraheme, are only approved in the U.S. for either the treatment of IDA in CKD patients or CKD patients on hemodialysis. We believe that a new entrant into the broader IDA U.S. market could significantly increase the number of patients who will be treated with IV iron.
GastroMARK
GastroMARK®, which is marketed and sold under the trade name Lumirem® outside of the U.S, is our oral contrast agent used for delineating the bowel during MRI and is approved and marketed in the U.S., Europe and other countries through our licensees. In the second quarter of 2012, we terminated our commercial license agreements forGastroMARK. Following the completion of our obligations under these agreements in the first quarter of 2013, we intend to cease commercially manufacturing or sellingGastroMARK. Pursuant to the terms of the respective termination agreements, in June 2012, we paid our licensees aggregate termination fees of $1.6 million, which we recorded in selling, general and administrative expenses in our consolidated statement of operations.
Licensing, Marketing and Distribution Arrangements
Although we are commercializingFeraheme in the U.S. through our own commercial organization, our commercial strategy also includes the formation of collaborations with other companies to facilitate
9
the development, manufacture, sale and distribution of our products in the U.S. and abroad. At present we are parties to the following collaborations:
Takeda
In March 2010, we entered into the Takeda Agreement, under which we granted exclusive rights to Takeda to develop and commercializeFeraheme/Rienso as a therapeutic agent in certain agreed-upon territories. In June 2012, we entered into the Amended Takeda Agreement, which removed the Commonwealth of Independent States from the territories under which Takeda has the exclusive rights to develop and commercializeFeraheme/Rienso. In addition, the Amended Takeda Agreement modified the timing and pricing arrangements for a supply agreement to be entered into between us and Takeda in the future, the terms related to primary and secondary manufacturing for drug substance and drug product, certain patent related provisions, and the re-allocation of certain of the agreed-upon milestone payments.
In December 2011, ferumoxytol was granted marketing approval in Canada, under the trade nameFeraheme, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. In June 2012, the European Commission granted marketing authorization for ferumoxytol under the trade nameRienso for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. The marketing authorization is valid in the current EU member states as well as in Iceland and Norway. In August 2012, ferumoxytol was granted marketing approval in Switzerland under the trade nameRienso for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. During 2012, we received $33.0 million in milestone payments related to the EU regulatory approval and the commercial launches ofFeraheme/Rienso in Canada and the EU. In addition, in connection with the commercial launches ofFeraheme/Rienso by Takeda, we recorded revenue from product sales to Takeda and royalties on sales by Takeda of $0.1 million in 2012.
Under the Amended Takeda Agreement, except under limited circumstances, we have retained the right to manufactureFeraheme/Rienso and, accordingly, are responsible for supply ofFeraheme/Rienso to Takeda at a fixed price per unit, which is capped for a certain period of time. We are also responsible for conducting, and bearing the costs related to, certain pre-defined clinical studies with the costs of future modifications or additional studies to be allocated between the parties according to an agreed-upon cost-sharing mechanism. In connection with the execution of the original Takeda Agreement, we received a $60.0 million upfront payment from Takeda in April 2010. We have received and may also receive additional regulatory approval and performance-based milestone payments, reimbursement of certain out-of-pocket regulatory and clinical supply costs, defined payments for supply ofFeraheme/Rienso, and tiered double-digit royalties on net product sales in the agreed-upon territories. The remaining milestone payments we may be entitled to receive under the agreement could over time equal up to $186.0 million. We can make no assurances as to the amount of milestone payments, if any, we will actually receive under the agreement.
Packaging Coordinators, Inc.
In May 2009, we entered into a commercial packaging services agreement with Packaging Coordinators, Inc. (formerly Catalent Pharma Solutions, LLC), or PCI, as amended in January 2013, or the PCI Agreement. Under the provisions of the PCI Agreement, PCI provides certain labeling, packaging and storage services for final U.S.Feraheme drug product and storage services for Canadian and SwissFeraheme/Rienso drug product. This agreement will renew automatically for successive established time periods unless either party provides written notice of its desire not to renew within certain time constraints. In addition, either party has the right to immediately terminate the agreement based on certain bankruptcy-related conditions or if the other party materially breaches any provision of this agreement and such breach is not cured within a certain period of time. Further, we may terminate the PCI Agreement for any reason or no reason with ninety days' written notice to PCI. PCI
10
has two qualified facilities in the U.S., which we can utilize for our labeling, packaging and storage needs.Rienso labeling and packaging for sale in the EU and Switzerland is currently conducted in Italy and is the responsibility of Takeda.
Integrated Commercialization Services, Inc.
In October 2008, we entered into a commercial outsourcing services agreement with Integrated Commercialization Services, Inc., or ICS, as amended, or the ICS Agreement. Under the provisions of the ICS Agreement, ICS agreed to be our exclusive third-party logistics provider to perform a variety of functions related to the sale and distribution ofFeraheme in the U.S., including services related to warehousing and inventory management, distribution, chargeback processing, accounts receivable management and customer service call center management. This agreement, as amended, will continue in effect until January 31, 2014, unless terminated earlier. The term of the agreement may be extended upon written mutual agreement of the parties six months prior to the expiration of the term. In addition, the ICS Agreement may be terminated under certain conditions such as non-payment of amounts due, failure to perform any material obligations under the agreement, or upon the occurrence of certain bankruptcy-related events.
3SBio
In 2008, we entered into a Collaboration and Exclusive License Agreement, or the 3SBio License Agreement, and a Supply Agreement, or the 3SBio Supply Agreement, with 3SBio for the development and commercialization ofFeraheme as an IV iron replacement therapeutic agent in China. The 3SBio License Agreement grants 3SBio an exclusive license for an initial term of thirteen years to develop and commercializeFeraheme as a therapeutic agent in China for an initial indication for the treatment of IDA in patients with CKD and an option to expand into additional therapeutic indications. In consideration of the grant of the license, we received an upfront payment of $1.0 million. We are eligible to receive certain other specified milestone payments upon regulatory approval ofFeraheme in China for CKD and other indications. We are also entitled to receive tiered royalties of up to 25% based on net sales ofFeraheme by 3SBio in China. We retained all manufacturing rights forFeraheme under these agreements. In addition, pursuant to the 3SBio Supply Agreement, 3SBio has agreed to purchase from us, and we have agreed to supply to 3SBio,Feraheme at a predetermined per unit price for use in connection with 3SBio's development and commercialization obligations for so long as the 3SBio License Agreement is in effect. If approved by the SFDA, 3SBio currently plans to begin a clinical trial necessary to file for marketing approval ofFeraheme in China.
Manufacturing
In the third quarter of 2012, we ceased our manufacturing operations at our Cambridge, Massachusetts manufacturing facility, where we previously manufacturedFeraheme for U.S. commercial sale and for use in human clinical trials. We currently rely solely on third parties for the manufacture ofFeraheme/Rienso for our commercial and clinical requirements of ferumoxytol in the U.S., the EU and Switzerland. Our third-party contract manufacturing facilities are subject to current good manufacturing practices, or cGMP, regulations enforced by the FDA and equivalent foreign regulatory agencies through periodic inspections to confirm such compliance. Although we and Takeda are currently working to establish and qualify alternative manufacturing facilities for both drug substance and finished drug product ofFeraheme/Rienso, we do not currently have an alternative manufacturer for ourFeraheme/Rienso drug substance and finished drug product. We target to maintain sufficient inventory levels of our projected U.S. near-term demand ofFeraheme drug product in order to minimize risks of supply disruption at points in our single source supply chain. We intend to continue to outsource the manufacture and distribution ofFeraheme/Rienso for the foreseeable future, and we
11
believe this manufacturing strategy will enable us to direct our financial resources to the commercialization ofFeraheme.
Prior to ceasing our manufacturing operations in 2012, we manufacturedFeraheme drug substance and drug product for use in the Canadian market at our Cambridge facility. Although we and Takeda are working to obtain regulatory approval of the manufacturing facilities at our current third-party contract manufacturers to produceFeraheme for sale in Canada, we do not currently have manufacturing facilities for this geography. Prior to closing our Cambridge manufacturing facility, we produced what we believe to be sufficient inventory to satisfy Takeda's Canadian supply needs until we have obtained regulatory approval at our third-party manufacturing facilities.
We have also established certain testing and release specifications with the FDA and other foreign regulatory agencies. This release testing must be performed in order to allow the finished product to be used for commercial sale. In addition, variations in the regulatory approval ofFeraheme/Rienso in the currently approved territories require that our third-party manufacturers follow different manufacturing processes and analytical testing methods. In late 2012, we produced a batch ofRienso which did not meet our release specifications in the EU. As a result, we are incurring additional costs associated with the development, validation and technology transfer to Takeda of a more accurate assay in order to be able to release this batch and any future batches produced for sale in the EU. This new assay will require review and approval by the EMA, which we expect will occur in the first half of 2013.
Sigma-Aldrich, Inc.
In August 2012, we entered into a Commercial Supply Agreement, or the SAFC Agreement, with Sigma-Aldrich, Inc., or SAFC, pursuant to which SAFC agreed to manufacture and we agreed to purchase from SAFC, the active pharmaceutical ingredient, or API, or the drug product intermediate, or DPI, for use in the finished product of ferumoxytol for U.S. commercial sale, for sale outside of the U.S. by Takeda, as well as for use in clinical trials. Subject to certain conditions, the SAFC Agreement provides that we purchase from SAFC certain minimum quantities of API or DPI each year, but we are not obligated to use SAFC as our sole supplier of API or DPI. In addition, the prices for each batch will decline as batches are produced in greater quantities throughout each year of the agreement. The SAFC Agreement will continue in force until June 22, 2015 and may be extended thereafter for additional two year periods, unless cancelled by us or SAFC within an agreed-upon notice period. The SAFC Agreement may also be terminated by either party at any time in the event of a material breach of the agreement by the other party provided that the breaching party fails to cure such breach within an agreed-upon notice period.
DSM Pharmaceuticals, Inc.
In January 2010, we entered into a Pharmaceutical Manufacturing and Supply Agreement, or the DSM Agreement, with DSM Pharmaceuticals, Inc., or DSM, pursuant to which DSM agreed to manufacture ferumoxytol finished drug product for U.S. commercial sale, for sale outside of the U.S., as well as for use in clinical trials at a fixed price per vial. The DSM Agreement will continue in force until January 13, 2015. The DSM Agreement may be terminated at any time upon mutual written agreement by us and DSM or at any time by us subject to certain notice requirements and early termination fees. In addition, the DSM Agreement may be terminated by either us or DSM in the event of a material breach of the agreement by the other party provided that the breaching party fails to cure such breach within an agreed-upon notice period.
To support the global commercialization ofFeraheme/Rienso, we have developed a fully integrated manufacturing support system, including quality assurance, quality control, regulatory affairs and inventory control policies and procedures. These support systems are intended to enable us to maintain high standards of quality for our products.
12
Raw Materials
We and our third-party manufacturers currently purchase certain raw and other materials used to manufactureFeraheme/Rienso from third-party suppliers and at present do not have any long-term supply contracts with these third parties. Although certain of our raw or other materials are readily available, others may be obtained only from qualified suppliers. Certain materials used inFeraheme/Rienso may from time to time be procured from a single source without a qualified alternative supplier. The qualification of an alternative source may require repeated testing of the new materials and generate greater expenses to us or our third-party manufacturers if materials that we or they test do not perform in an acceptable manner. In addition, we or our third-party manufacturers sometimes obtain raw or other materials from one vendor only, even where multiple sources are available, to maintain quality control and enhance working relationships with suppliers, which could make us or our third-party manufacturers susceptible to price inflation by the sole supplier, thereby increasing our production costs. As a result of the high quality standards imposed on our raw and other materials used to manufactureFeraheme/Rienso, we or our third-party manufacturers may not be able to obtain such materials of the quality required to manufactureFeraheme/Rienso from an alternative source on commercially reasonable terms, or in a timely manner, if at all.
Patents and Trade Secrets
We consider the protection of our technology to be material to our business. Because of the substantial length of time and expense associated with bringing new products through development and regulatory approval to the marketplace, we place considerable importance on obtaining patent and trade secret protection for our products. Our success depends, in large part, on our ability to maintain the proprietary nature of our technology and other trade secrets. To do so, we must prosecute and maintain existing patents, obtain new patents and ensure trade secret protection. We must also operate without infringing the proprietary rights of third parties or allowing third parties to infringe our rights.
Our policy is to aggressively protect our competitive technology position by a variety of means, including applying for patents in the U.S. and in appropriate foreign countries. We currently hold a number of U.S. and foreign patents, which expire at various times through 2020. OurFeraheme patents currently expire in 2020, however, our primary U.S. patent forFeraheme may be subject to an extension to 2023 under U.S. patent law and FDA regulations. Our foreign patents may also be eligible for extension in accordance with applicable law in certain countries.
We also have patent applications pending in the U.S. and have filed counterpart patent applications in certain foreign countries. Although further patents may be issued on pending applications, we cannot be sure that any such patents will be issued on a timely basis, if at all. In addition, any issued patents may not provide us with competitive advantages or may be challenged by others, and the existing or future patents of third parties may limit our ability to commercializeFeraheme/Rienso. For example, in July 2010, Sandoz GmbH, or Sandoz, filed with the European Patent Office, or the EPO, an opposition to our previously issued patent that covers ferumoxytol in the EU. In October 2012, at an oral hearing, the Opposition Division of the EPO revoked our European ferumoxytol patent. In December 2012, our notice of appeal was recorded with the EPO, which suspends the revocation of our patent. We will continue to defend the validity of this patent throughout the appeals process, which we expect to take two to three years. In the event the appeals process is unfavorable to us, it could result in a loss of proprietary rights in the EU and may allow other companies in the EU to use our proprietary technology without a license from us, and may also result in a loss of future royalty or milestone payments to us from Takeda. We cannot predict the outcome of our appeal of the EPO decision. In the event that we do not experience a successful outcome from the appeals process, under EU regulations ferumoxytol would still be entitled to eight years of data protection and ten years of market exclusivity from the date of approval, which we believe would create
13
barriers to entry for any generic version of ferumoxytol into the EU market until sometime between 2020 and 2022.
We also rely on the benefits of market exclusivity in protecting our intellectual property rights forFeraheme in the U.S. The FDA previously determined that ferumoxytol did not qualify as a new chemical entity, or NCE, and instead grantedFeraheme a three-year "new use" market exclusivity, which expired in June 2012. In March 2010 and December 2012, we formally requested that the FDA reconsider its determination with respect toFeraheme's NCE status, which, if granted, would provideFeraheme with exclusivity until June 2014, or five years from the date ofFeraheme's U.S. approval. We cannot give any assurances as to whether the FDA will accept our most recent request for reconsideration, that the FDA will make this reconsideration in a timely manner, or thatFeraheme will be granted NCE exclusivity. The regulatory approval process for NCE status is discussed in more detail below under the heading "U.S. Approval Process—Marketing Exclusivity" and the associated risks are discussed in more detail in Part I, Item 1A below under "Risk Factors" under the heading, "Competitors could file applications seeking a path to U.S. approval of a generic ferumoxytol."
Frequently, the unpredictable nature and significant costs of patent litigation leads the parties to settle to remove any uncertainty related to the status of their patents. Settlement agreements between branded companies and generic applicants may allow, among other things, a generic product to enter the market prior to the expiration of any or all of the applicable patents covering the branded product, either through the introduction of an authorized generic or by providing a license to the applicant for the patents in suit.
Competition
The pharmaceutical and biopharmaceutical industry is intensely competitive and subject to rapid technological change. We and Takeda compete in the marketing and sale ofFeraheme/Rienso and many of our competitors are large, well-known pharmaceutical companies. One or more of our competitors may benefit from significantly greater financial, sales and marketing capabilities, greater technological or competitive advantages, and other resources. Our competitors may develop products that are more widely accepted than ours and may receive patent protection that dominates, blocks or adversely affects our product development or business.
The iron replacement therapy market is highly sensitive to several factors including, but not limited to, the actual or perceived safety and efficacy profile of the available products, the ability to obtain appropriate insurance coverage and reimbursement rates and terms, price competitiveness, and product characteristics such as convenience of administration and dosing regimens.
AlthoughFeraheme is approved in the U.S. for the treatment of IDA in adult patients with CKD, including both dialysis and non-dialysis CKD patients, our U.S. commercial strategy is now entirely focused on growing the utilization ofFeraheme in non-dialysis dependent adult CKD patients with IDA. We believe there is a significant opportunity in the U.S. forFeraheme for the treatment of IDA in CKD patients not yet on dialysis. The U.S. non-dialysis IV iron market is comprised primarily of three sites of care where a substantial number of CKD patients are treated: hematology and oncology centers, hospitals, and nephrology clinics.
There are currently two iron replacement options for treating IDA in CKD patients: oral iron supplements and IV iron. The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guidelines recommend either oral or IV iron for peritoneal dialysis patients and non-dialysis patients with stages 1 through 5 CKD. Oral iron is currently the first-line iron replacement therapy of choice of most physicians in both the U.S. and abroad. However, oral iron supplements are poorly absorbed by many patients, which may adversely impact their effectiveness, and are associated with certain side effects that may adversely affect patient compliance in using such products. The alternative to oral iron for the treatment of IDA is IV iron.
14
Feraheme currently competes with the following IV iron replacement therapies in the U.S. for the treatment of IDA in CKD patients:
- •
- Venofer®, an iron sucrose complex, which is approved for use in hemodialysis, peritoneal dialysis, non-dialysis dependent CKD patients and pediatric CKD patients and is marketed in the U.S. by Fresenius Medical Care North America and American Regent Laboratories, Inc., or American Regent, a subsidiary of Luitpold Pharmaceuticals, Inc., or Luitpold;
- •
- Ferrlecit®, a sodium ferric gluconate, which is marketed by Sanofi-Aventis U.S. LLC, is approved for use only in hemodialysis patients;
- •
- A generic version of Ferrlecit® marketed by Watson Pharmaceuticals, Inc., or Watson;
- •
- INFeD®, an iron dextran product marketed by Watson, which is approved in the U.S. for the treatment of patients with documented iron deficiency in whom oral iron administration is unsatisfactory or impossible; and
- •
- Dexferrum®, an iron dextran product marketed by American Regent, which is approved in the U.S. for the treatment of patients with documented iron deficiency in whom oral iron administration is unsatisfactory or impossible.
In addition to the currently marketed products described above,Feraheme may also compete in the U.S. with Injectafer®, which is known as Ferinject® in Europe and which is discussed below, which is in development in the U.S. for the treatment of IDA. In September 2011, Luitpold submitted an NDA to the FDA seeking marketing approval for Injectafer® for the treatment of IDA. In July 2012, Luitpold received a Complete Response letter from the FDA withholding approval of Injectafer®. If approved in the U.S., Injectafer® is expected to be marketed by American Regent, the current distributor of Venofer®. Pharmacosmos A/S, or Pharmacosmos, the producer of another IV iron, Monofer® (iron isomaltoside 1000), which is approved in Europe, is also conducting clinical trials in the U.S. and may try to gain regulatory approval in the U.S. for Monofer®.
Outside of the U.S.,Feraheme/Rienso also competes with a number of branded IV iron replacement products, including Venofer®, Ferrlecit®, Monofer®, Ferinject® (ferric carboxymaltose injection) (the brand name for Injectafer® outside the U.S.) and certain other iron dextran and iron sucrose products. Monofer® is an injectable iron preparation developed by Pharmacosmos, which is currently approved for marketing in approximately 23 countries for the treatment of IDA. Ferinject® is an IV iron replacement therapy developed by Vifor Pharma, the pharmaceuticals business unit of the Galenica Group, and is currently approved for marketing in approximately 43 countries worldwide, including 29 countries within Europe, for the treatment of iron deficiency where oral iron is ineffective or cannot be used. In December 2010, Vifor Pharma and Fresenius Medical Care North America announced that they had created a new company which will hold the commercialization rights to Venofer® and Ferinject® outside of the U.S. Venofer® and Ferrlecit® have been marketed in many countries throughout the world, including most of Europe and Canada, for many years.Feraheme/Rienso competes primarily with Venofer®, Ferinject® and Ferrlecit® in both the Canadian and European markets. Currently, all other IV iron products currently approved and marketed in the EU are approved for marketing to a broader group of patients with IDA.Feraheme/Rienso was approved only for use in CKD patients, which could putFeraheme/Rienso at a competitive disadvantage unless and until it receives approval for a broader indication outside of the U.S.
The market opportunity forFeraheme/Rienso in the U.S. and abroad could also be negatively affected by approved generic IV iron replacement therapy products that achieve commercial success. For example, in 2011, Watson launched a generic version of Ferrlecit® in the U.S. which is approved for marketing in the U.S. for the treatment of IDA in adult patients and in pediatric patients age six years and older undergoing chronic hemodialysis who are receiving supplemental epoetin therapy. Sagent Pharmaceuticals, Inc. has also indicated its intention to introduce a generic iron sucrose in the
15
U.S. in the future. There are also a number of approved generic IV iron products in countries outside the U.S. which directly compete withFeraheme/Rienso, including a generic version of Venofer®.
The Drug Price Competition and Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, requires an applicant whose subject drug is a drug with an FDA listed patent to notify the patent-holder of their application and potential infringement of their patent rights. If an applicant for ferumoxytol notifies us of such application, we would have 45 days upon receipt of that notice to bring a patent infringement suit in federal district court against the applicant seeking approval of a product. If such a suit is commenced, the FDA is generally prohibited from granting approval of an application until the earliest of 30 months from the date the FDA accepted the application for filing, the conclusion of litigation in the generic's favor, or expiration of the patent(s). If the litigation is resolved in favor of the applicant or the challenged patent expires during the 30-month stay period, the stay is lifted and the FDA may thereafter approve the application based on the applicable standards for approval.
A generic version ofFeraheme can be marketed only with the approval of the FDA of the respective application for such generic version. In December 2012, the FDA issued draft guidance making recommendations regarding establishing bioequivalence withFeraheme, pursuant to which a party could seek approval of a generic version of that product through an abbreviated new drug application, or ANDA. The FDA generally publishes product-specific bioequivalence guidance after it has received an inquiry from a generic drug manufacturer about submitting an ANDA for the product in question; thus, it is possible that a generic drug manufacturer has approached the FDA requesting guidance about submitting an ANDA for ferumoxytol, the active ingredient inFeraheme, and that such an ANDA may be filed in the near future. The ANDA process is discussed in more detail below under the heading "U.S. Approval Process—Marketing Exclusivity."
Companies that manufacture generic products typically invest far fewer resources in research and development than the manufacturers of branded products and can therefore price their products significantly lower than those branded products already on the market. Therefore, competition from generic IV iron products could limit our U.S. sales and any royalties we may receive from Takeda on sales outside of the U.S. Please see the discussion in Part I, Item 1A below under "Risk Factors" under the heading, "Competitors could file applications seeking a path to U.S. approval of a generic ferumoxytol."
For IV iron replacement therapy in patients with CKD, the total therapeutic course of iron typically used in clinical practice is 1,000 milligrams, or one gram. Venofer® is typically administered as a slow intravenous injection over two to five minutes in doses of 100 to 200 milligrams, thus requiring five to ten physician visits to reach a standard one gram therapeutic course. The recommended dose of Ferrlecit® and the generic version of Ferrlecit® is 125 milligrams administered by intravenous infusion over one hour per dialysis session or undiluted as a slow intravenous injection per dialysis session, thus requiring eight physician visits to reach a standard one gram therapeutic course. The recommended dose of INFeD® and Dexferrum® is a slow push in 100 milligram doses and require up to ten physician visits to receive a standard one gram therapeutic course.Feraheme/Rienso is administered as a 510 milligram injection followed by a second 510 milligram injection three to eight days later, each of which can be administered in less than one minute at a regular office visit without the use of infusion equipment or prolonged medical intervention. In 2011, the FDA required changes to the product labels of Venofer®, Ferrlecit® andFeraheme, to include a 30 minute observation period to monitor patients for signs and symptoms of hypersensitivity during and following the administration of these products. There is no observation period for the iron dextran products.
We believe that our and Takeda's ability to successfully compete with other IV iron products in the U.S. and internationally depends on a number of factors, including the actual or perceived safety and efficacy profile ofFeraheme/Rienso as compared to alternative iron replacement therapeutics, our ability to obtain and maintain favorable pricing, insurance coverage and reimbursement rates and terms for
16
Feraheme/Rienso, the timing and scope of regulatory approval ofFeraheme/Rienso for the broad IDA indication and of products or additional indications by our competitors, our ability to implement effective marketing campaigns, the effectiveness of our sales force, our ability to maintain favorable patent protection forFeraheme/Rienso, market acceptance ofFeraheme/Rienso, and our ability to manufacture sufficient quantities ofFeraheme/Rienso at commercially acceptable costs. In addition, our ability to effectively compete with these products in the U.S. non-dialysis CKD market depends in part upon our ability to gain formulary access in hospitals and effectively promoteFeraheme within group purchasing organizations, or GPOs, and to physicians who treat non-dialysis CKD patients.
Based on sales data provided to us in February 2013 by IMS Health Incorporated, or IMS, we estimate that the size of the total 2012 U.S. non-dialysis IV iron replacement therapy market was approximately 806,000 grams, which represented an increase of approximately 2% over 2011. Based on this IMS data, the following represents the 2012 and 2011 U.S. market share allocation of the total non-dialysis IV iron market based on the volume of IV iron administered:
| | 2012 U.S. Non-dialysis IV Iron Market (806,000 grams) | 2011 U.S. Non-dialysis IV Iron Market (792,000 grams) | |||||
|---|---|---|---|---|---|---|---|
Venofer® | 46 | % | 48 | % | |||
INFeD® | 20 | % | 20 | % | |||
Feraheme | 14 | % | 12 | % | |||
Generic sodium ferric gluconate | 10 | % | 5 | % | |||
Ferrlecit® | 7 | % | 11 | % | |||
Dexferrum® | 3 | % | 4 | % | |||
The market share data listed in the table above is not necessarily indicative of the market shares in dollars due to the variations in prices among the IV iron products.
Sales, Marketing and Distribution
In July 2009, we began U.S. commercial sale ofFeraheme, which is being marketed and sold in the U.S. through our own commercial organization, including a specialty sales force. We sellFeraheme to authorized wholesalers and specialty distributors who, in turn, sellFeraheme to healthcare providers who administerFeraheme primarily within hospitals, hematology and oncology centers and nephrology clinics. Since many hospitals and hematology, oncology and nephrology practices are members of GPOs, which leverage the purchasing power of a group of entities to obtain discounts based on the collective bargaining power of the group, we also routinely enter into pricing agreements with GPOs in these markets so the members of the GPOs have access toFeraheme and the related discounts. In addition, we outsource a number of our product supply chain services to ICS, our third-party logistics provider, including services related to warehousing and inventory management, distribution, chargeback processing, accounts receivable management and customer service call center management.
Our sales and marketing teams use a variety of common pharmaceutical marketing strategies to promoteFeraheme including sales calls to purchasing entities, such as hospitals, hematology and oncology centers and nephrology practices in addition to individual physicians or other healthcare professionals, medical education symposia, personal and non-personal promotional materials, local and national educational programs, scientific meetings and conferences and informational websites. In addition, we provide customer service and other related programs forFeraheme including physician reimbursement support services, a patient assistance program for uninsured or under-insured patients and a customer service call center.
Our commercial strategy currently focuses on the non-dialysis dependent CKD market in the U.S. Based on data contained in a 2009 publication in theJournal of the American Society of Nephrology, we estimate there are 1.6 million adults in the U.S. with stages 3 through 5 CKD and IDA, and we believe
17
that a small fraction of those patients are currently being treated with IV iron. We believe there is a significant opportunity in this market to provide IV iron to non-dialysis CKD patients, and our sales team has been working to educate physicians who treat CKD patients on the benefits of IV iron and the dosing profile ofFeraheme in order to change existing treatment paradigms and expand the IV iron use in physicians' offices, clinics, and hospitals where CKD patients are treated for IDA.
Feraheme/Rienso has been granted marketing approval in Canada, the EU, Iceland, Norway and Switzerland for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD and was commercially launched in Canada, Switzerland and the EU in late 2012. Under our amended agreement with Takeda, Takeda is solely responsible forFeraheme/Rienso commercialization efforts in these areas including the deployment of a specialized sales force, pricing and reimbursement negotiations with national, provincial or local health authorities and customers, and development of market access strategies.
The following table sets forth customers who represented 10% or more of our total revenues for the years ended December 31, 2012, 2011, and 2010. Revenues from Takeda include collaboration revenue recognized in connection with the Amended Takeda Agreement, milestone payments we received in 2012 and revenues from product sales to Takeda and royalties received from Takeda in 2012.
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
AmerisourceBergen Drug Corporation | 34 | % | 41 | % | 36 | % | ||||
Takeda Pharmaceuticals Company Limited | 31 | % | 13 | % | <10 | % | ||||
McKesson Corporation | 17 | % | 21 | % | <10 | % | ||||
Cardinal Health, Inc. | 12 | % | 13 | % | <10 | % | ||||
Metro Medical Supply, Inc. | <10 | % | <10 | % | 21 | % | ||||
Government Regulation
Overview
The development, manufacture and commercialization of pharmaceutical products are subject to extensive regulation by numerous governmental authorities in the U.S. and abroad. In the U.S., the Federal Food, Drug and Cosmetic Act, or the FDC Act, and other federal and state statutes and regulations govern, among other things, the research and development, manufacturing, quality control (testing), labeling, record-keeping, approval, storage, distribution, and advertising and promotion of pharmaceutical products. In addition, many of our activities in the U.S. are subject to the jurisdiction of various other federal regulatory and enforcement departments and agencies, such as the Department of Health and Human Services, the Federal Trade Commission and the Department of Justice. Individual states, acting through their attorneys general, have become active as well, seeking to regulate the marketing of prescription drugs under state consumer protection and false advertising laws. A number of states, along with the federal government, have also enacted, or are considering enacting, legislation to control pharmaceutical marketing activities, such as the federal Physician Payment Sunshine Act, or the Sunshine Act.
Our activities outside of the U.S. are also subject to regulatory requirements governing the testing, approval, safety, effectiveness, manufacturing, labeling and marketing ofFeraheme/Rienso. These regulatory requirements vary from country to country. The approval process may be more or less rigorous from country to country and the time required for approval may also vary from country to country. In Europe, Canada and some other international markets, the government provides healthcare at low direct cost to consumers and regulates pharmaceutical prices or patient reimbursement levels to control costs for the government-sponsored healthcare system.
18
Failure to comply with any of the applicable U.S. or foreign regulatory requirements may result in a variety of administrative or judicially imposed sanctions including, among other things, the regulatory agency's refusal to approve pending applications, withdrawals of approval, clinical holds, warning letters, product recalls, product seizures, total or partial suspension of operations, injunctions, fines, civil penalties or criminal prosecution.
The development and approval of a product candidate requires a significant number of years of work and the expenditure of substantial resources, and is often subject to unanticipated delays and may be subject to new legislation or regulations. In addition to complying with requirements as they currently exist, a sponsor could be negatively impacted by changes in the regulatory framework. From time to time, legislation is introduced that could significantly alter laws pertaining to the approval, manufacturing, pricing, and/or marketing of drug products. Even without changes to relevant laws, U.S. and foreign regulatory agencies could release new guidance or revise its implementation of current regulations in a manner that significantly affects us and our products, including our ability to receive marketing approval for new indications forFeraheme/Rienso. It is impossible to predict whether legislative changes will be enacted, or whether regulations or guidance will be amended or supplemented, or the potential impact of such changes.
U.S. Approval Process
Clinical Development
Before new human pharmaceutical products may be marketed or sold commercially in the U.S., the FDA requires the following steps: (a) pre-clinical laboratory tests, pre-clinical safety and efficacy studies and formulation studies; (b) the submission to the FDA of an Investigational New Drug Application, or IND, for human clinical testing, which must become effective before human clinical trials may commence; (c) adequate and well-controlled human clinical trials under current good clinical practices to establish the safety and efficacy of the drug for its intended use; (d) submission of an NDA to the FDA; (e) approval and validation of manufacturing facilities used in production of the pharmaceutical product under cGMP; and (f) review and approval of the NDA by the FDA.
Pre-clinical studies include the laboratory evaluation of product chemistry and animal studies to assess the potential safety and efficacy of a product and its formulation. The results of such laboratory tests and animal studies are submitted to the FDA as a part of an IND and are reviewed by the FDA prior to and during human clinical trials. If there are no objections from the FDA within 30 days of filing an IND, a sponsor may proceed with initial studies in human volunteers, also known as clinical trials.
Clinical trials are typically conducted in the following three sequential phases, which may overlap in some instances:
- •
- Phase I: Clinical trials which involve the initial administration of the study drug to a small group of healthy human volunteers (or, more rarely, to a group of selected patients with the targeted disease or disorder) under the supervision of a principal investigator selected by the sponsor. These Phase I trials are designed to test for safety, dosage tolerance, absorption, distribution, metabolism, excretion and clinical pharmacology and, if possible, early indications of effectiveness.
- •
- Phase II: Clinical trials which involve a small sample of the actual intended patient population and aim to: (a) provide a preliminary assessment of the efficacy of the investigational drug for a specific clinical indication; (b) ascertain dose tolerance and optimal dose range; and (c) collect additional clinical information relating to safety and potential adverse effects.
- •
- Phase III: If an investigational drug is found through Phase I and Phase II studies to have some efficacy and an acceptable clinical safety profile in the targeted patient population, Phase III
19
studies can be initiated. Phase III studies are well-controlled comparative studies designed to gather additional information within an expanded patient population in geographically dispersed clinical trial sites in order to further establish safety and efficacy in conditions that the drug will be used if approved for marketing.
The FDA may suspend clinical trials at any point in this process if it concludes that patients are being exposed to an unacceptable health risk. In addition, clinical trial results are frequently susceptible to varying interpretations by scientists, medical personnel, regulatory personnel, statisticians and others, which may delay, limit or prevent further clinical development or regulatory approvals of a product candidate.
Submission and FDA Review of an NDA
Following the successful completion of adequate and well-controlled human clinical trials, the results of the trials, together with the results of pre-clinical tests and studies, are submitted to the FDA as part of an NDA. The NDA must also include information related to the preparation and manufacturing of the new drug, analytical methods, and proposed product packaging and labeling. When the NDA is submitted, the FDA has 60 days from receipt to determine whether the application is sufficiently complete to merit a substantive review and should therefore be "filed." If the FDA determines that the application is incomplete, it must notify the sponsor through a "refusal-to-file" letter, and the sponsor then has the option to resubmit the NDA after addressing the concerns raised by the FDA. If the FDA accepts the NDA for filing, the NDA undergoes a series of reviews intended to confirm and validate the sponsor's conclusion that the drug is safe and effective for its proposed use.
Under the Food and Drug Administration Modernization Act, an NDA is designated for either Standard Review or Priority Review. A Priority Review designation may be given if a new drug offers major advancements in treatment or provides a treatment where no adequate therapy exists. In July 2012, the Food and Drug Administration Safety and Innovation Act of 2012, or FDASIA, was enacted. The FDASIA includes the reauthorization of the Prescription Drug User Fee Act, or PDUFA, that provides FDA with the necessary resources to maintain a predictable and efficient review process for human drug and biologic products. The FDA has, pursuant to PDUFA, as reauthorized by FDASIA, set a goal that it review and act upon 90% of NDAs with a Standard Review designation within ten months of the FDA's acceptance of the filing and 90% of NDAs with a Priority Review designation within six months of the filing date. However, whether an NDA is designated for a Standard or Priority Review, there is no guarantee that any single submission will be acted on within these time frames, and the FDA's goals are subject to change from time to time. In addition, FDA review of a drug development program may proceed under its "Fast Track" programs, which are intended for a combination of a product and a claim that addresses an unmet medical need. Fast Track is designed to facilitate the development and expedite the review of new drugs that are intended to treat serious or life threatening conditions and demonstrate the potential to address unmet medical needs. A Fast Track designation provides the sponsor the benefits of scheduling meetings when needed to receive FDA input into development plans, the option of submitting an NDA in sections rather than all components simultaneously, or a rolling review, and the option of requesting evaluation of studies using surrogate endpoints. Fast Track status does not, however, necessarily lead to a Priority Review, as described above, or Accelerated Approval designation, which provides earlier approval of drugs to treat serious diseases and that fill an unmet medical need.
20
If the FDA's evaluations of the NDA and the sponsor's manufacturing facilities are favorable, the FDA will issue an approval letter, and the sponsor may begin marketing the drug in the U.S. for the approved indications, subject to certain universal post-approval requirements described further below. The FDA may also impose drug-specific conditions on its approval, such as requirements for additional post-marketing testing or surveillance. If the FDA determines that it cannot approve the NDA in its current form, it will issue a complete response letter to indicate that the review cycle for an application is complete and that the application will not be approved in its current form. The complete response letter usually describes the specific deficiencies that the FDA identified in the application and may require additional clinical or other data or impose other conditions that must be met in order to obtain final approval of the NDA. Addressing the deficiencies noted by the FDA could be impractical or costly and may result in significant delays prior to final approval.
Adverse Event Reporting
The FDA requires a sponsor to submit reports of certain information on side effects and AEs associated with its products that occur either during clinical trials or after marketing approval. These requirements include specific and timely notification of certain serious, unexpected and/or frequent AEs, as well as regular periodic reports summarizing adverse drug experiences. Failure to comply with these FDA safety reporting requirements may result in FDA regulatory action that may include civil action or criminal penalties. In addition, as a result of these reports, the FDA could create a Tracked Safety Issue for a product in the FDA's Document Archiving, Reporting and Regulatory Tracking System, place additional limitations on an approved product's use, such as through labeling changes, or, potentially, could require withdrawal or suspension of the product from the market.
FDA Post-Approval Requirements
Even if initial approval of an NDA is granted, such approval is subject to a wide-range of regulatory requirements, any or all of which may adversely impact a sponsor's ability to effectively market and sell the approved product. Furthermore, the FDA may require the sponsor to conduct Phase IV clinical trials, also known as post-marketing requirements or post-marketing commitments, to provide additional information on safety and efficacy. The results of such post-market studies may be negative and could lead to limitations on the further marketing of a product. Also, under the Pediatric Research Equity Act, the FDA may require pediatric assessment of certain drugs unless waived or deferred due to the fact that necessary studies are impossible or highly impractical to conduct or where there is strong evidence that suggests the drug would be ineffective or unsafe or that the drug does not represent a meaningful therapeutic benefit over existing therapies and is not likely to be used in a substantial number of pediatric patients. In addition, the FDA may require a sponsor to implement a Risk Evaluation Mitigation Strategy, or REMS, a strategy to manage a known or potential serious risk associated with the product. The FDA may, either prior to approval or subsequent to approval if new safety data arises, require a REMS if it determines that a REMS is necessary to ensure that the benefits of the product outweigh its risks. A REMS may include a medication guide, patient package insert, a plan for communication with healthcare providers, elements to ensure safe use of the product, and an implementation system. A REMS must also include a timetable for submission of assessments of the strategy at specified time intervals. Failure to comply with a REMS, including submission of a required assessment, may result in substantial civil penalties.
Where a sponsor wishes to expand the originally approved prescribing information, such as adding a new indication, it must submit and obtain approval of a sNDA. Changes to an indication generally require additional clinical studies, which can be time-consuming and require the expenditure of substantial additional resources. Under PDUFA, the target timeframe for the review of a sNDA to add a new clinical indication is ten months from the date of filing.
21
Marketing Exclusivity
Market exclusivity provisions under the FDC Act can delay the submission or the approval of certain applications. Under Sections 505(c)(3)(E)(ii) and 505(j)(5)(F)(ii) of the FDC Act, as amended by the Hatch-Waxman Act, an NCE that is granted regulatory approval may be eligible for five years of marketing exclusivity in the U.S. following regulatory approval. A drug is an NCE if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a Section 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an ANDA may be submitted after four years if it contains a certification of patent invalidity or non-infringement, or the Paragraph IV certification. An ANDA differs from the typical NDA described above in that it is an application containing information to demonstrate that the proposed product is identical to a previously approved product. ANDA applicants are not required to conduct costly and time-consuming clinical trials to establish the safety and efficacy of their products; rather, they are permitted to rely on the innovator's data regarding safety and efficacy, and an applicant usually needs to only submit data demonstrating that its product has the same active ingredient(s) and is bioequivalent to the branded product, in addition to any data necessary to establish that any difference in strength, dosage form, inactive ingredients, or delivery mechanism does not result in different safety or efficacy profiles, as compared to the reference drug. Likewise, a Section 505(b)(2) NDA differs from the typical NDA in that it allows a sponsor to rely, at least in part, on the FDA's findings of safety and/or effectiveness for a previously approved drug. Thus, generic manufacturers can sell their products at prices much lower than those charged by the innovative pharmaceutical or biotechnology companies which have incurred substantial expenses associated with the research and development of a new drug.
The FDC Act also provides three years of marketing exclusivity for an NDA, Section 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application (for example, for new indications, dosages, or strengths of an existing drug). This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or tentative approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
FDA Regulation of Product Marketing and Promotion
The FDA also regulates all advertising and promotional activities for products, both prior to and after approval, including but not limited to direct-to-consumer advertising, sales representative communications to healthcare professionals, promotional programming, and promotional activities involving the internet, publications, radio and TV as well as other media. Approved drug products must be promoted in a manner consistent with their terms and conditions of approval, including the scope of their approved use. The FDA may take enforcement action against a company for promoting unapproved uses of a product, or off-label promotion, or for other violations of its advertising and labeling laws and regulations. Failure to comply with these requirements could lead to, among other things, adverse publicity, product seizures, civil or criminal penalties, or regulatory letters, which may include warnings and require corrective advertising or other corrective communications to healthcare professionals.
22
FDA Regulation of Manufacturing Facilities
Manufacturing procedures and quality control for approved drugs must conform to cGMP, which practices are described in the FDC Act and FDA guidance. cGMP requirements must be followed at all times, and domestic manufacturing establishments are subject to periodic inspections by the FDA in order to assess, among other things, cGMP compliance. In addition, prior to approval of an NDA or sNDA, the FDA will perform a pre-approval inspection of the sponsor's manufacturing facility, including its equipment, facilities, laboratories and processes, to determine the facility's compliance with cGMP and other rules and regulations. Vendors that supply finished products or components to the sponsor that are used to manufacture, package and label products are subject to similar regulation and periodic inspections. If the FDA identifies deficiencies during an inspection, it may issue notices on FDA Form 483, which may be followed by warning letters if observations are not addressed satisfactorily listing conditions the FDA investigators believe may violate cGMP or other FDA regulations. FDA guidelines specify that a warning letter be issued only for violations of "regulatory significance" for which the failure to adequately and promptly achieve correction may be expected to result in an enforcement action.
Product approval may be delayed or denied due to cGMP non-compliance or other issues at the sponsor's manufacturing facilities or contractor sites or suppliers included in the NDA or sNDA, and the complete resolution of these inspectional findings may be beyond the sponsor's control. If after a successful completion of an FDA inspection of a sponsor's manufacturing facilities, the sponsor makes a material change in manufacturing equipment, location or process, additional regulatory review may be required. Re-inspection of the sponsor's manufacturing facilities or contractor sites or suppliers may also occur. If the FDA determines that the sponsor's equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against the sponsor, including suspension of its manufacturing operations.
To supply products for use outside of the U.S., our third-party manufacturers must comply with cGMP and are subject to periodic inspection by the FDA or by regulatory authorities in certain other countries. In complying with these requirements, manufacturers, including a drug sponsor's third-party contract manufacturers, must continue to expend time, money and effort in the area of production and quality to ensure compliance. Failure to maintain compliance with cGMP regulations and other applicable manufacturing requirements of various regulatory agencies could result in fines, unanticipated compliance expenditures, recall, total or partial suspension of production, suspension of the FDA's review of future sNDAs, enforcement actions, injunctions or criminal prosecution.
Fraud and abuse regulation
Our general operations, and the research, development, manufacture, sale and marketing of our products, are subject to extensive federal and state regulation, including but not limited to FDA regulations, the Federal Anti-Kickback Statute, the Federal False Claims Act, and the Foreign Corrupt Practices Act, and their state analogues. Anti-kickback laws make it illegal to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug, that is reimbursed by a state or federal program. False claims laws prohibit anyone from knowingly presenting, or causing to be presented for payment to third-party payers, including Medicare and Medicaid, false or fraudulent claims for reimbursed drugs or services, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. The Foreign Corrupt Practices Act and similar foreign anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Our activities relating to the sale and marketing of our products may be subject to scrutiny under these laws. If we or our representatives fail to comply with any of these laws or regulations, a range of fines, penalties and/or other sanctions could be imposed on
23
us, including, but not limited to, restrictions on how we market and sellFeraheme, significant fines, exclusions from government healthcare programs, including Medicare and Medicaid, litigation, or other sanctions.
Other U.S. Regulatory Requirements
We are also subject to regulation under local, state and federal law regarding occupational safety, laboratory practices, handling of chemicals, environmental protection and hazardous substances control. We possess a Byproduct Materials License from the Commonwealth of Massachusetts for receipt, possession, manufacturing and distribution of radioactive materials and Registration Certificates from the federal Drug Enforcement Agency and the Commonwealth of Massachusetts Department of Public Health for handling controlled substances. We are also registered with the federal Environmental Protection Agency, or EPA, as a generator of hazardous waste. All hazardous waste disposals must be made in accordance with EPA and Commonwealth of Massachusetts requirements. We are subject to the regulations of the Occupational Safety and Health Act and have a safety program in effect to assure compliance with all of these regulations. We believe our procedures for handling and disposing of hazardous materials used in our research and development activities comply with all applicable federal, state and local requirements. Nevertheless, the risk of accidental contamination or injury from these materials cannot be completely eliminated and, in the event of an accident or injury, we could be held liable for any damages that result.
Certain states also require that we obtain licenses or permits as an out-of-state distributor or manufacturer in order to market, sell and/or ship our pharmaceutical products into their state. We have obtained licenses and permits in some states and, depending on our future activities, may also need to obtain additional licenses or permits in other areas where we decide to manufacture, market or sell our products. New laws, regulations or judicial decisions, or new interpretations of existing laws and regulations, may require us to modify our development programs, revise the way we manufacture, market and sell our products, require additional clinical trials or post-approval safety studies, or limit coverage or reimbursement rates and terms for our products.
In recent years, several states have enacted legislation requiring pharmaceutical companies operating within the state to establish marketing and promotional compliance programs or codes of conduct and/or file periodic reports with the state or make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities. Similar legislation is being considered by additional states and by Congress. In addition, as part of The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the Health Care Reform Act, the federal government has enacted the Sunshine Act provisions. Beginning in August 2013, manufacturers of drugs are required to publicly report gifts and payments made to physicians and teaching hospitals. Many of these requirements are new and uncertain, and the penalties for failure to comply with these requirements are unclear. Failure to comply with any of these laws could result in a range of fines, penalties and/or other sanctions.
Foreign Regulatory Process
In our efforts to market and sellFeraheme/Rienso outside of the U.S., we and our licensees are subject to foreign regulatory requirements. Approval of a drug by applicable regulatory agencies of foreign countries must be secured prior to the marketing of such drug in those countries. The regulatory approval process in countries outside of the U.S. vary widely from country to country and may in some cases be more rigorous than requirements in the U.S. Certain foreign regulatory authorities may require additional studies or studies designed with different clinical endpoints and/or comparators than those which we are conducting or have already completed. In addition, any adverse regulatory action taken by the FDA with respect to an approved product in the U.S. may affect the
24
regulatory requirements or decisions made by certain foreign regulatory bodies with regard to the regulatory approval of products outside of the U.S.
To obtain regulatory approval of a drug in the EU, marketing authorizations may be submitted under a centralized, mutual recognition or decentralized procedure or national procedure (single country). Under the centralized procedure, the sponsor can submit a single application to the EMA which, if approved, permits the marketing of a product in all EU Member States and certain non-member states, including Iceland and Norway. Under the mutual recognition procedure, the sponsor applies for national marketing authorization in one state, and upon approval can then seek simultaneous approval in all other EU Member States. Under the decentralized procedure, the sponsor can file simultaneously to several EU Member States, identifying a single reference member state to act as the primary reviewer of the application. Upon approval, the product will be licensed only in the reference member state and the other countries to which it applied. Once an applicant receives marketing authorization in an EU Member State, through any application route, the applicant is then required to engage in pricing discussions and negotiations with a separate pricing authority in that country. In certain countries, commercial sales are only able to commence once pricing approval has been received. In addition, all products, irrespective of the method of filing, are afforded 10 years of market exclusivity and eight years of data protection upon approval. In June 2012,Rienso was granted marketing approval in the EU for the treatment of IDA in CKD patients and commercially launched in late 2012.
The Canadian pharmaceutical industry is subject to federal regulation by Health Canada, the public health department of the Canadian government charged with overseeing healthcare-related regulatory matters, pursuant to the Canadian federal Food and Drugs Act. Health Canada's criteria for obtaining and maintaining marketing approval is generally similar to that of the FDA. In December 2011,Feraheme was granted marketing approval by Health Canada for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD and commercially launched in late 2012.
The pharmaceutical industry in Switzerland is subject to federal regulation by Swissmedic, the Swiss Agency for Therapeutic Products. In August 2012,Rienso was granted marketing approval by Swissmedic and commercially launched in late 2012.
Reimbursement
In both the U.S. and foreign markets, our and Takeda's ability to successfully commercializeFeraheme/Rienso is dependent, in significant part, on the availability and extent of reimbursement to end-users from third-party payors for the use ofFeraheme/Rienso, including governmental payors, managed care organizations, and private health insurers. Reimbursement by third-party payors may depend on a number of factors, including the third-party's determination that the product is competitively priced, safe and effective, appropriate for the specific patient, and cost-effective. Third-party payors are increasingly challenging the prices charged for pharmaceutical products and have instituted and continue to institute cost containment measures to control or significantly influence the purchase of pharmaceutical products. For example, to reduce expenditures associated with pharmaceutical products, many third-party payors use cost containment methods, including: (a) formularies, which limit coverage for drugs not included on a predetermined list; (b) variable co-payments, which may make a certain drug more expensive for patients as compared with a competing drug; (c) utilization management controls, such as requirements for prior authorization before the payor will cover the drug; and (d) other coverage policies that limit access to certain drugs for certain uses based on the payor-specific coverage policy.
In addition, U.S. and many foreign governments continue to attempt to curb health care costs through legislation, including legislation aimed at reducing the pricing and reimbursement of pharmaceutical products. The Health Care Reform Act was enacted in the U.S. in March 2010 and
25
includes certain cost containment measures including an increase to the minimum rebates for products covered by Medicaid programs, the extension of such rebates to drugs dispensed to Medicaid beneficiaries enrolled in Medicaid managed care organizations and the expansion of the 340B Drug Discount Program under the Public Health Service Act. In addition, the heightened focus on the health care industry by the federal government could result in the implementation of significant federal spending cuts including cuts in Medicare and other health related spending in the near term, such as a potential 2% across the board sequestration of Medicare expenditures. In recent years, some states have also passed legislation to control the prices of drugs as well as begun a move toward managed care to relieve some of their Medicaid cost burden. These and any future changes in government regulation or private third-party payors' reimbursement policies may reduce the extent of reimbursement forFeraheme/Rienso and adversely affect our future operating results.
Currently, in U.S. physician clinic settings, Medicare Part B generally reimburses for physician-administered drugs at a rate of 106% of the drug's average selling price, or ASP. ASP is defined by statute based on certain historical sales and sales incentive data, including rebates and chargebacks, for a defined period of time. Manufacturers submit the required information to the Centers for Medicare and Medicaid Services, or CMS, on a quarterly basis. In advance of the quarter in which the payment limit for drugs reimbursed under Medicare Part B program will go into effect, CMS confirms and publishes the payment limit. Under this methodology, payment rates change on a quarterly basis, and significant downward fluctuations in ASP, and therefore reimbursement rates, could negatively impact sales of a product. Because ASP is defined by statute, and changes to Medicare payment methodologies require legislative change, it is unclear if and when ASP reimbursement methodology will change. While Medicare is the predominant payor for treatment of patients with CKD, Medicare payment policy, in time, can also influence pricing and reimbursement in the non-Medicare markets, as private third-party payors and state Medicaid plans frequently adopt Medicare principles in setting reimbursement methodologies. We cannot predict the impact that any changes in reimbursement policies may have on our ability to compete effectively.
In January 2011, a prospective payment system for dialysis services provided to Medicare beneficiaries who have end-stage renal disease, or ESRD, became effective under which all costs of providing dialysis services are bundled together into a single prospective payment per treatment. This bundled approach to reimbursement has and will likely continue to alter the utilization of physician-administered drugs in the ESRD market as well as put downward pressure on the prices pharmaceutical companies can charge ESRD facilities for such drugs, particularly where alternative products are available. In the U.S.,Feraheme is sold at a price that is substantially higher than alternative IV iron products in the dialysis setting, and as a result, the demand forFeraheme in the dialysis setting has largely disappeared. In addition, it is also possible that this "bundled" approach may be applied to specific disease states other than ESRD. For example, one large insurer in the U.S has attempted to bundle certain costs related to the treatment of cancer patients.
In addition, in the U.S. hospital in-patient setting,Feraheme is reimbursed by Medicare under a diagnosis related group payment system, which provides a per discharge reimbursement based on the diagnosis and/or procedure rather than actual costs incurred in patient treatments, thereby increasing the incentive for a hospital to limit or control expenditures. As a result,Feraheme has not been nor do we expect it to be broadly used in the hospital in-patient setting.
In countries outside of the U.S., market acceptance may also depend, in part, upon the availability of reimbursement within existing healthcare payment systems. Generally, in Europe and other countries outside of the U.S., the government sponsored healthcare system is the primary payor of healthcare costs of patients and therefore enjoys significant market power. Some foreign countries also set prices for pharmaceutical products as part of the regulatory process, and we cannot guarantee that the prices set by such governments will be sufficient to generate substantial revenues or allow sales ofFeraheme/Rienso to be profitable in those countries.
26
If adequate reimbursement levels are not maintained by government and other third-party payors forFeraheme/Rienso, our and Takeda's ability to sellFeraheme/Rienso may be limited and/or our and Takeda's ability to establish acceptable pricing levels forFeraheme/Rienso may be impaired, thereby reducing anticipated revenues and our prospects of achieving profitability.
Backlog
We had a $1.7 million and $0.1 million product sales backlog as of December 31, 2012 and 2011, respectively. The $1.7 million backlog as of December 31, 2012 was largely due to increased orders from certain of our licensees and to the timing of orders received from our third-party logistics provider. Generally, product orders from our customers are fulfilled within a relatively short time of receipt of a customer order.
Employees
As of February 15, 2013, we had 129 employees. We also utilize consultants and independent contractors on a regular basis to assist in the development and commercialization ofFeraheme. Our success depends in part on our ability to attract, retain and motivate qualified executive, sales, technical operations, managerial, scientific and medical personnel. Although we believe we have been relatively successful to date in obtaining and retaining such personnel, we may not be successful in the future.
None of our employees is represented by a labor union, and we consider our relationship with our employees to be good.
Foreign Operations
We have no foreign operations. Revenues from customers outside of the U.S. amounted to approximately 32%, 14% and 10% of our total revenues for the years ended December 31, 2012, 2011 and 2010, respectively, and were principally related to collaboration revenues recognized in connection with our agreement with Takeda, which is based in Japan. During 2012, our revenues from customers outside of the U.S included approximately $20.0 million related to milestone payments we received from Takeda.
Research and Development
We have dedicated a significant portion of our resources to our efforts to develop our products and product candidates, particularlyFeraheme. We incurred research and development expenses of $33.3 million, $58.1 million and $54.5 million during the years ended December 31, 2012, 2011 and 2010, respectively. We expect our research and development expenses to decrease in 2013 due to the completion in 2012 of our Phase III clinical program forFeraheme in patients with IDA regardless of the underlying cause. However, we will continue to incur significant expenses in 2013 and beyond related to our pediatric clinical studies and our clinical trial to determine the safety and efficacy of repeat doses ofFeraheme for the treatment of IDA in patients with hemodialysis dependent CKD.
Code of Ethics
Our Board of Directors has adopted a code of ethics that applies to our officers, directors and employees. We have posted the text of our code of ethics on our website athttp://www.amagpharma.com in the "Investors" section. In addition, should any changes be made to our code of ethics, we intend to disclose within four business days, on our website (or in any other medium required by law or the NASDAQ): (1) the date and nature of any amendment to our code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver,
27
from a provision of our code of ethics that is granted to one of these specified officers, the name of such person who is granted the waiver, and the date of the waiver.
Available Information
Our internet website address ishttp://www.amagpharma.com. Through our website, we make available, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy and registration statements, and all of our insider Section 16 reports (and any amendments to such filings), as soon as reasonably practicable after such material is electronically filed with, or furnished to, the U.S. Securities and Exchange Commission, or the SEC. These SEC reports can be accessed through the "Investors" section of our website. The information found on our website is not part of this or any other report we file with, or furnish to, the SEC. Paper copies of our SEC reports are available free of charge upon request in writing to Investor Relations, AMAG Pharmaceuticals, Inc., 100 Hayden Avenue, Lexington, MA 02421. The content on any website referred to in this Form 10-K is not incorporated by reference into this Form 10-K unless expressly noted.
For additional information regarding our segments, refer to Note L of the Notes to Financial Statements included in Part II, Item 8 "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K.
The following information sets forth material risks and uncertainties that may affect our business, including our future financial and operational results and could cause our actual results to differ materially from those contained in forward-looking statements we have made in this Annual Report on Form 10-K and elsewhere as discussed in the introduction to Part I above. You should carefully consider the risks described below, in addition to the other information in this Annual Report on Form 10-K, before making an investment decision. The risks and uncertainties described below are not the only ones we face. Additional risks not presently known to us or other factors not perceived by us to present material risks to our business at this time also may impair our business operations.
We are solely dependent on the success of Feraheme/Rienso.
We currently derive and expect to continue to derive substantially all of our revenue from sales ofFeraheme/Rienso by us in the U.S. and by our licensees, including Takeda Pharmaceutical Company Limited, or Takeda, outside of the U.S. and, therefore, our ability to become profitable is solely dependent on our and our licensees' successful commercialization and development ofFeraheme/Rienso. Accordingly, if we are unable to generate sufficient revenues from sales ofFeraheme/Rienso, or from milestone payments and royalties we may receive related toFeraheme/Rienso, we may never be profitable, our financial condition will be materially adversely affected, and our business prospects will be limited.
We intend to continue to dedicate significant resources to the development and commercialization ofFeraheme/Rienso. However, we or Takeda may not be successful in our efforts to successfully commercializeFeraheme/Rienso in its current chronic kidney disease, or CKD, indication or to expand the approved indication ofFeraheme/Rienso to include additional indications. Although we filed a supplemental New Drug Application, or sNDA, in the U.S. for our global registration program forFeraheme in patients with iron deficiency anemia, or IDA, who had failed to or could not use oral iron, the U.S. Food and Drug Administration, or the FDA, may not accept or approve our sNDA, or may require that we narrow the scope of our proposed indication. In addition, we expect that Takeda will file a Type II Variation, which is the European Union, or EU, equivalent of a U.S. sNDA, with the European Medicines Agency, or EMA, in 2013 seeking marketing approval forRienso for the treatment of IDA in adult patients. However, we have no control over Takeda's process, timeline or interactions
28
with the European regulatory agencies, Takeda may not be successful in filing a Type II Variation in a timely manner, or at all, and we cannot be assured that the EMA will accept and approve the filing. We are not currently conducting or sponsoring research to expand our product development pipeline beyondFeraheme. Therefore, our revenues and operations will not be as diversified as some of our competitors which have multiple products or product candidates. Any failure by us or Takeda to gain marketing approval forFeraheme/Rienso for the treatment of IDA regardless of the underlying cause could limit long-term shareholder value and adversely affect the future prospects of our business.
Competitors could file applications seeking a path to U.S. approval of a generic ferumoxytol.
Under Sections 505(c)(3)(E)(ii) and 505(j)(5)(F)(ii) of the Federal Food, Drug, and Cosmetic Act, or FDC Act, as amended by The Drug Price Competition and Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, a new chemical entity, or NCE, that is granted regulatory approval may be eligible for five years of marketing exclusivity in the U.S. following regulatory approval. A drug can be classified as an NCE if the FDA has not previously approved any other drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. The FDA previously determined that ferumoxytol did not qualify as an NCE and instead grantedFeraheme a three-year "new use" market exclusivity, which expired in June 2012. In March 2010 and December 2012, we formally requested that the FDA reconsider its determination with respect toFeraheme's NCE status. The FDA may deny our request for reconsideration of NCE status forFeraheme, in which caseFeraheme may be subjected to early generic competition.
NCE status, if granted, would preclude approval during the exclusivity period of certain applications made under Section 505(b)(2) of the FDC Act, as amended by the Hatch-Waxman Act, or a Section 505(b)(2) new drug application, or NDA, and abbreviated new drug application, or ANDA, submitted by another company for another version of the subject drug; however, under governing law an application may be submitted four years after approval of the subject drug (even with a five year exclusivity period prohibiting approval) if it contains a certification of patent invalidity or non-infringement pursuant to Paragraph IV of the Hatch-Waxman Act, or the "Paragraph IV" certification procedure. In recent years, generic manufacturers have used Paragraph IV certifications extensively to challenge the applicability of Orange Book-listed patents on a wide array of innovative pharmaceuticals, and we expect this trend to continue. If we are not able to gain or exploit marketing exclusivity beyond the initial three year exclusivity period that expired in June 2012, we may face significant future competitive threats to our commercialization ofFeraheme from other manufacturers, including the manufacturers of generic alternatives through the submission of Section 505(b)(2) NDAs and ANDAs. Further, even ifFeraheme is granted NCE status and we are able to gain marketing exclusivity until June 2014, another company could challenge that decision and seek to overturn the FDA's determination. Although costly, another company could also gain such marketing exclusivity under the provisions of the FDC Act, as amended by the Hatch-Waxman Act, if such company can, under certain circumstances, complete a human clinical trial process and obtain regulatory approval of its product.
In addition, in December 2012, the FDA published draft guidance regarding new draft product-specific bioequivalence for drug products containing ferumoxytol. The FDA generally publishes product-specific bioequivalence guidance after it has received an inquiry from a generic drug manufacturer about submitting an ANDA for the product in question; thus, it is possible that a generic drug manufacturer has approached the FDA requesting guidance about submitting an ANDA for ferumoxytol, the active ingredient inFeraheme, and that such an ANDA may be filed in the near future. Because the FDA may deny our request for reconsideration of NCE status forFeraheme and because the published bioequivalence guidance could encourage a generic entrant seeking a path to approval of a generic ferumoxytol to file an ANDA, we could face generic competition in the near-term or have to engage in extensive litigation with a generic competitor to protect our patent rights, either
29
of which could adversely affect our business and results of operations. Companies that manufacture generic products typically invest far fewer resources in research and development than the manufacturers of branded products and can therefore price their products significantly lower than those branded products already on the market. Therefore, competition from generic intravenous, or IV, iron products could limit our U.S. sales and any royalties we may receive from Takeda, which would have an adverse impact on our business and results of operations.
We are completely dependent on third parties to manufacture Feraheme/Rienso and any difficulties, disruptions or delays in the Feraheme/Rienso manufacturing process, including our transition to alternative source manufacturing facilities, could increase our costs, or adversely affect our profitability and future business prospects.
In the third quarter of 2012, we ceased our manufacturing operations at our Cambridge, Massachusetts manufacturing facility. Consequently, we currently rely solely on our third-party contract manufacturers to manufactureFeraheme/Rienso for our commercial and clinical use in the U.S., the EU and Switzerland. We do not currently have an alternative manufacturer for ourFeraheme/Rienso drug substance and finished drug product and we may not be able to enter into agreements with manufacturers whose facilities and procedures comply with current good manufacturing practices, or cGMP, regulations and other regulatory requirements on terms that are favorable to us, if at all. Prior to ceasing our manufacturing operations in 2012, we manufacturedFeraheme drug substance and drug product for use in the Canadian market at our Cambridge facility. Although we and Takeda are working to obtain regulatory approval of the manufacturing facilities at our current third-party contract manufacturers to produceFeraheme for sale in Canada, we do not currently have manufacturing facilities for this geography.
Our ability to haveFeraheme/Rienso manufactured in sufficient quantities and at acceptable costs to meet our commercial demand and clinical development needs is dependent on the uninterrupted and efficient operation of our third-party contract manufacturing facilities. Any difficulties, disruptions or delays in theFeraheme/Rienso manufacturing process could result in product defects or shipment delays, recall or withdrawal of product previously shipped for commercial or clinical purposes, inventory write-offs or the inability to meet commercial demand forFeraheme/Rienso in a timely and cost-effective manner. Our current third-party manufacturer does not manufacture for us exclusively and may exhaust some or all of its resources meeting the demand of other customers. Insufficient manufacturing capacity due to scheduling conflicts at our third-party manufacturers to produce sufficient quantities ofFeraheme/Rienso to meet our demand forecasts or any potential manufacturing delays resulting from our efforts to gain regulatory approval of a new assay for the production ofRienso for sale in the EU, could result in our inability to meet our commercial demand forFeraheme/Rienso.
In addition, the transition of the manufacturing processes to third-party contract manufacturers and the oversight of such third parties could take a significant amount of time and may increase the risk of certain problems, including cost overruns, process reproducibility, stability issues, the inability to deliver required quantities of product that conform to specifications in a timely manner, or the inability to manufactureFeraheme/Rienso in accordance with cGMP. If we are unable to haveFeraheme/Rienso manufactured on a timely or sufficient basis because of these or other factors, we may not be able to meet commercial demand or our clinical development needs forFeraheme/Rienso or may not be able to manufactureFeraheme/Rienso in a cost-effective manner, particularly in light of the fixed price at which we are required to supplyFeraheme/Rienso to Takeda under our License, Development and Commercialization Agreement, as most recently amended in June 2012, or the Amended Takeda Agreement. As a result, we may lose sales, fail to generate increased revenues, suffer regulatory setbacks and/or we may lose money on our supply ofFeraheme/Rienso to Takeda, any of which could have an adverse impact on our potential profitability and future business prospects.
30
Significant safety or drug interaction problems could arise with respect to Feraheme/Rienso, which could result in restrictions in the Feraheme/Rienso label, recalls, withdrawal of Feraheme/Rienso from the market, an adverse impact on Feraheme/Rienso sales, or our need to alter or terminate current or future Feraheme development programs, any of which would adversely impact our future business prospects.
Significant safety or drug interaction problems could arise with respect toFeraheme/Rienso, including an increase in the severity or frequency of known problems or the discovery of previously unknown problems, and may result in a variety of adverse regulatory actions. In the U.S., under the Food and Drug Administration Amendments Act of 2007, the FDA has broad authority to force drug manufacturers to take any number of actions if safety or drug interaction problems arise, including, but not limited to the following:
- •
- Requiring manufacturers to conduct post-approval clinical studies to assess known risks or signals of serious risks, or to identify unexpected serious risks;
- •
- Mandating labeling changes to a product based on new safety information; or
- •
- Requiring manufacturers to implement a Risk Evaluation Mitigation Strategy where necessary to assure safe use of the drug.
Similar laws and regulations exist in countries outside of the U.S. In addition, previously unknown safety or drug interaction problems could result in product recalls, restrictions on the product's permissible uses, or withdrawal of the product from the U.S. and/or foreign markets.
For example, in November 2010, following discussions with the FDA, we revised theFeraheme package insert, which includes essential information regarding the FDA-approved use ofFeraheme, including, among other things, the approved indication, side effects, and dosage instructions, to include bolded warnings and precautions that describe events that have been reported during post-marketing review afterFeraheme administration, including life-threatening hypersensitivity reactions and clinically significant hypotension. We directly alerted healthcare providers of the changes to theFeraheme package insert. During June 2011, we made further changes to theFeraheme package insert based on additional post-marketing data. These or any future changes to theFeraheme package insert could adversely impact our or Takeda's ability to successfully compete in the IV iron market and could have an adverse impact on potential sales ofFeraheme and our future business prospects.
The data submitted to both the FDA as part of our NDA and to the EMA as part of the Marketing Authorization Application forFeraheme/Rienso in the CKD indication was obtained in controlled clinical trials of limited duration. New safety or drug interaction issues may arise asFeraheme/Rienso is used over longer periods of time by a wider group of patients, some of whom may be taking numerous other medicines or by patients with additional underlying health problems. In addition, as we conduct and complete other clinical trials forFeraheme, new safety issues may be identified which could negatively impact our ability to successfully complete these studies, the use and/or regulatory status ofFeraheme/Rienso for the treatment of IDA in patients with CKD in the U.S., EU or other territories, and the prospects for approval of future sNDAs, such as our December 2012 sNDA submission forFeraheme for the treatment of IDA regardless of the underlying cause. For example, the FDA may determine that our sNDA for our IDA global registrational program does not establish a sufficiently acceptable safety profile for the approval of a broaderFeraheme label.
As more data become available and an increased number of patients are treated withFeraheme/Rienso, new safety or drug interaction issues may arise and require us to, among other things, provide additional warnings and/or restrictions on theFeraheme/Rienso package insert, including a boxed warning in the U.S. or similar warnings outside of the U.S., directly alert healthcare providers of new safety information, narrow our approved indications, alter or terminate current or future trials for additional uses ofFeraheme, or even removeFeraheme/Rienso from the market, any of which could
31
have a significant adverse impact on potential sales ofFeraheme/Rienso or require us to expend significant additional funds.
Our and Takeda's ability to grow revenues from sales of Feraheme/Rienso could be limited if we or Takeda do not obtain approval, or if we or Takeda experience significant delays in our or Takeda's efforts to obtain approval to market and sell Feraheme/Rienso for the treatment of IDA in a broad range of patients.
In December 2012, we submitted a sNDA to the FDA forFeraheme for the treatment of IDA in a broad range of patients. In addition, we expect that Takeda will file a Type II Variation, which is the EU equivalent of a U.S. sNDA, with the EMA in 2013 seeking marketing approval forFeraheme/Rienso for the treatment of IDA in adult patients. Before applying for regulatory approval in the U.S. or foreign countries for the commercial marketing and sale ofFeraheme/Rienso for the broad IDA indication, we have to demonstrate, through extensive human clinical trials, thatFeraheme/Rienso is safe and effective for use in this broader patient population. Conducting these and other clinical trials is a complex, time-consuming and expensive process that requires adherence to a wide range of regulatory requirements. The FDA and foreign regulatory agencies have substantial discretion in the approval process and may decide that the results of our recently completed clinical trials are insufficient for approval or thatFeraheme/Rienso is not effective or safe in indications other than IDA in adult patients with CKD. For example, in our Phase III clinical trial in the broader patient population,Feraheme-treated patients experienced a 0.6% rate of related serious adverse events, or SAEs, as compared to a 0.2% rate of related SAEs from our currentFeraheme label for treatment of IDA patients with CKD. Clinical and other data is often susceptible to varying interpretations, and many companies that have believed their product candidates performed satisfactorily in clinical trials have nonetheless failed to obtain FDA or EMA approval for their products. There is no guarantee that the FDA or EMA will determine that the results of our clinical trials in our global registrational program forFeraheme/Rienso in a broad range of patients with IDA will adequately demonstrate thatFeraheme/Rienso is safe and effective in such a patient population to grant approval.
The FDA or EMA could also determine that our clinical trials and/or our manufacturing processes were not properly designed, were not conducted in accordance with applicable laws and regulations, or were otherwise not properly managed. In addition, under the FDA's current good clinical practices regulations, or cGCP, we are responsible for conducting, recording and reporting the results of clinical trials to ensure that the data and results are credible and accurate and that the trial participants are adequately protected. The FDA may conduct inspections of clinical investigator sites which are involved in our clinical development programs to ensure their compliance with cGCP regulations. If the FDA determines that we, our clinical research organizations or our study sites fail to comply with applicable cGCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may disqualify certain data generated from those sites or require us to perform additional clinical trials before approving our marketing application, which could adversely impact our ability to obtain marketing approval in the U.S. forFeraheme/Rienso in the broad IDA indication. Any such deficiency in the design, implementation or oversight of our clinical development programs could cause us to incur significant additional costs, experience significant delays or prevent us from obtaining marketing approval forFeraheme/Rienso for the broad IDA indication. In addition, any failure by us or Takeda to obtain approval for the broad IDA indication could adversely affect the commercialization ofFeraheme/Rienso in its current indication. If, for any of these or other reasons, we or Takeda do not obtain approval, or if we or Takeda experience significant delays in our or Takeda's efforts to obtain approval to market and sellFeraheme/Rienso for the treatment of IDA in a broad range of patients, our cash position, our ability to increase revenues, our ability to achieve profitability, and the future prospects of our business could be materially adversely affected.
32
We may not be able to expand our product portfolio by entering into business development transactions, such as in-licensing arrangements, acquisitions, or collaborations or if such arrangements are entered into they could disrupt our business, decrease our profitability, result in dilution to our stockholders or cause us to incur debt or significant additional expense.
As part of our business strategy to expand our product portfolio and achieve profitability, we are seeking to acquire or in-license other products that we believe would be complementary to our existing business. We have limited experience with respect to these business development activities and there can be no assurance that we will be able to identify or complete any such transaction in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated financial benefits of any such transaction. We may not be successful in acquiring or in-licensing a product or product candidate that will provide us with commercial, development and/or financial synergies withFeraheme and our current organization such that we will be able to eliminate expenses either from our existing operations or from the cost structure of the acquired product.
In addition, proposing, negotiating and implementing collaborations, in-licensing arrangements or acquisition agreements may be a lengthy and complex process. Other companies, including those with substantially greater financial, marketing and sales resources, may compete with us for these arrangements, and we may not be able to enter into such arrangements on acceptable terms or at all. Further, any such strategic transactions by us could result in large and immediate write-offs or the incurrence of debt and contingent liabilities, any of which could adversely impact our operating results. Management of a license arrangement, collaboration, or other strategic arrangement and/or integration of an acquired asset or company may also disrupt our ongoing business, require management resources that otherwise would be available for ongoing development of our existing business and our U.S. commercialization ofFeraheme. In addition, to finance any such strategic transactions, we may choose to issue shares of our common or preferred stock as consideration, which would result in dilution to our stockholders. Alternatively, it may be necessary for us to raise additional funds through public or private financings, and such additional funds may not be available on terms that are favorable to us, if at all. If we are unable to successfully obtain rights to suitable products or if any acquisition or in-license arrangement we make is not successful, our business, financial condition and prospects for growth could suffer.
The success of Feraheme in the U.S. depends on our ability to maintain the proprietary nature of our technology.
We rely on a combination of patents, trademarks and copyrights in the conduct of our business. The patent positions of pharmaceutical and biopharmaceutical firms are generally uncertain and involve complex legal and factual questions. We may not be successful or timely in obtaining any patents for which we submit applications. The breadth of the claims obtained in our patents may not provide sufficient protection for our technology. The degree of protection afforded by patents for proprietary or licensed technologies or for future discoveries may not be adequate to preserve our ability to protect or commercially exploit those technologies or discoveries. The patents issued to us may provide us with little or no competitive advantage. In addition, there is a risk that others will independently develop or duplicate similar technology or products or circumvent the patents issued to us.
Our U.S. ferumoxytol patents are currently scheduled to expire in 2020. These and any other patents issued to us may be contested or invalidated. There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. We may become a party to patent litigation and other proceedings, including interference and reexamination proceedings declared by the United States Patent and Trademark Office.
33
In addition, claims of infringement or violation of the proprietary rights of others may be asserted against us. If we are required to defend against such claims or to protect our own proprietary rights against others, it could result in substantial financial and business costs, including the business cost attributable to the resulting distraction of our management. An adverse ruling in any litigation or administrative proceeding could prevent us from marketing and sellingFeraheme, increase the risk that a generic version ofFeraheme could enter the market to compete withFeraheme, limit our development and commercialization ofFeraheme, or otherwise harm our competitive position and result in additional significant costs. In addition, any successful claim of infringement asserted against us could subject us to monetary damages or an injunction, preventing us from making or sellingFeraheme. We also may be required to obtain licenses to use the relevant technology. Such licenses may not be available on commercially reasonable terms, if at all. Frequently, the unpredictable nature and significant costs of patent litigation leads the parties to settle to remove this uncertainty. Settlement agreements between branded companies and generic applicants may allow, among other things, a generic product to enter the market prior to the expiration of any or all of the applicable patents covering the branded product, either through the introduction of an authorized generic or by providing a license to the applicant for the patents in suit.
We also rely upon unpatented trade secrets and improvements, unpatented know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our corporate licensees, collaborators, contract manufacturers, employees and consultants. These agreements, however, may be breached. We may not have adequate remedies for any such breaches, and our trade secrets might otherwise become known or might be independently discovered by our competitors. In addition, we cannot be certain that others will not independently develop substantially equivalent or superseding proprietary technology, or that an equivalent product will not be marketed in competition withFeraheme, thereby substantially reducing the value of our proprietary rights. Our inability to protectFeraheme through our patents and other intellectual property rights prior to their expiration could have a material adverse effect on our business, financial condition and prospects.
The success of Feraheme/Rienso abroad depends on our ability to protect our intellectual property rights and the laws of foreign countries may not provide the same level of protection as do the laws of the U.S.
The laws of foreign countries may not protect our intellectual property rights to the same extent as do the laws of the U.S. and therefore, in addition to similar risks to those describe above under the heading "The success of Feraheme in the U.S. depends on our ability to maintain the proprietary nature of our technology" our intellectual property rights may be subject to increased risk abroad, including opposition proceedings before the patent offices for other countries, such as the European Patent Office, or the EPO, or similar adversarial proceedings, regarding intellectual property rights with respect toFeraheme/Rienso. For example, in July 2010, Sandoz GmbH, or Sandoz, filed with the EPO an opposition to one of our previously issued patents which covers ferumoxytol in the EU. In October 2012, at an oral hearing, the Opposition Division of the EPO revoked our European ferumoxytol patent. In December 2012, our notice of appeal was recorded with the EPO. The appeals process is costly and time-consuming and if it results in an unfavorable outcome to us, it could result in a loss of proprietary rights in the EU and may allow Sandoz or other companies to use our proprietary technology without a license from us, which may also result in a loss of future royalty or milestone payments to us, as well as the possibility that Takeda may determine that the terms of our agreement are no longer viable. We cannot predict the outcome of our appeal of the EPO decision. This or any future patent interference proceedings involving our patents may result in substantial costs to us, distract our management from day-to-day business operations and responsibilities, prevent us or Takeda from marketing and sellingFeraheme/Rienso or increase the risk that a generic version ofFeraheme/Rienso could enter the market to compete withFeraheme/Rienso. In countries where we do not have or have not applied for patents for ferumoxytol, such as in China, where we license certain development
34
and commercial rights toFeraheme to 3SBio, Inc., we may be unable to prevent others from developing or selling similar products. In addition, in jurisdictions outside the U.S. where we have patent rights, we may be unable to prevent unlicensed parties from selling or importing products or technologies derived elsewhere using our proprietary technology. Any such limitation on our intellectual property rights would cause substantial harm to our competitive position and to our ability to develop and commercializeFeraheme/Rienso. Our inability to protectFeraheme/Rienso through our patents and other intellectual property rights in any territory prior to their expiration could have a material adverse effect on our business, financial condition and prospects.
Competition in the pharmaceutical and biopharmaceutical industries is intense. If we fail to compete effectively, our business and market position will suffer.
The pharmaceutical and biopharmaceutical industry is intensely competitive and subject to rapid technological change. We and Takeda compete in the marketing and sale ofFeraheme/Rienso and many of our competitors are large, well-known pharmaceutical companies. One or more of our competitors may benefit from significantly greater financial, sales and marketing capabilities, greater technological or competitive advantages, and other resources. Our competitors may develop products that are more widely accepted than ours and may receive patent protection that dominates, blocks or adversely affects our product development or business.
The iron replacement therapy market is highly sensitive to several factors including, but not limited to the following:
- •
- the actual and perceived safety and efficacy profile of the available products;
- •
- the ability to obtain appropriate insurance coverage and reimbursement rates and terms;
- •
- price competitiveness; and
- •
- product characteristics such as convenience of administration and dosing regimens.
The introduction by our competitors of alternatives toFeraheme/Rienso that would be, or are perceived to be, more efficacious, safer, cheaper, easier to administer, or more favorable insurance coverage or reimbursement could reduce our revenues and the value of our product development efforts.
Feraheme/Rienso may not receive the same level of market acceptance as competing iron replacement therapy products, in part because most of these products have been on the market longer and are currently widely used by physicians in the U.S. and abroad. In addition, certain of the IV iron products that we compete with are approved for the treatment of IDA in a broader group of patients thanFeraheme/Rienso. We or Takeda may not be able to convince physicians and other healthcare providers or payers to switch from using the other IV iron therapeutic products toFeraheme/Rienso. If we or Takeda are not able to differentiateFeraheme/Rienso from other marketed IV iron products, our ability to maintain a premium price, our ability to generate revenues and achieve and maintain profitability, and our long-term business prospects could be adversely affected.
Feraheme currently competes with several IV iron replacement therapies in the U.S., If these or other iron replacement products are approved for marketing and sale in the U.S. or are approved for a broader IDA indication thanFeraheme, our efforts to market and sellFeraheme in the U.S. and our ability to generate additional revenues and achieve profitability could be adversely affected.
Feraheme/Rienso also competes with a number of branded IV iron replacement and certain other iron dextran and iron sucrose products outside of the U.S. If Takeda is unable to convince physicians and other healthcare providers to switch from using the competing IV iron products toFeraheme/Rienso, our ability to generate revenues from royalties we may receive from Takeda will be limited and our operating results will be negatively affected. In addition, all other IV iron products currently
35
approved and marketed and sold in the EU are approved for marketing to a broader group of patients with IDA.Feraheme/Rienso was approved only for use in adult CKD patients, which could putFeraheme/Rienso at a competitive disadvantage unless and until it receives approval for a broader indication outside of the U.S.
Feraheme/Rienso may not be widely adopted by physicians, hospitals, patients, or healthcare payors, which would adversely impact our potential profitability and future business prospects.
The commercial success ofFeraheme/Rienso in the U.S. and in other territories depends upon its level of market adoption by physicians, hospitals, patients, and healthcare payors, including managed care organizations and group purchasing organizations, or GPOs. IfFeraheme/Rienso does not achieve or maintain an adequate level of market adoption for any reason, our potential profitability and our future business prospects will be severely adversely impacted.Feraheme/Rienso represents an alternative to other products and might not be adopted if perceived to be no safer, less safe, no more effective, less effective, no more convenient, or less convenient than currently available products. In addition, the pricing and/or reimbursement rates and terms forFeraheme/Rienso may not be viewed as advantageous to potential prescribers and payors as the pricing and/or reimbursement rates and terms of alternative IV iron products. The degree of market acceptance ofFeraheme/Rienso in the U.S. and abroad depends on a number of factors, including but not limited to the following:
- •
- Our and Takeda's ability to demonstrate to healthcare providers, particularly hematologists, oncologists, hospitals, nephrologists, and others who may purchase or prescribeFeraheme/Rienso, the clinical efficacy and safety ofFeraheme/Rienso as an alternative to currently marketed IV iron products which treat IDA in CKD patients;
- •
- Our and Takeda's ability to convince physicians and other healthcare providers to use IV iron, andFeraheme/Rienso in particular, rather than oral iron, which is the current treatment of choice of most physicians for treating IDA in CKD patients;
- •
- The actual or perceived safety and efficacy profile ofFeraheme/Rienso as compared to alternative iron replacement therapeutic agents, particularly if unanticipated adverse reactions toFeraheme/Rienso result in further changes to or restrictions in theFeraheme/Rienso package insert and/or otherwise create safety concerns among potential prescribers;
- •
- The relative level of available reimbursement in the U.S. forFeraheme from payors, including government payors, such as Medicare and Medicaid, and private payors as compared to the level of available reimbursement for alternative IV iron products;
- •
- The relative price and/or level of reimbursement ofFeraheme/Rienso outside of the U.S. as compared to alternative iron replacement therapeutic agents;
- •
- The actual or perceived convenience and ease of administration ofFeraheme/Rienso as compared to alternative iron replacement therapeutic agents, including iron administered orally; and
- •
- The effectiveness of our and Takeda's commercial organizations and distribution networks in marketing, selling and supplyingFeraheme/Rienso.
The key component of our U.S. commercialization strategy is to market and sellFeraheme for use in non-dialysis adult CKD patients. The current U.S. non-dialysis CKD market is comprised primarily of three sites of care where a substantial number of CKD patients are treated: hematology and oncology centers, hospitals, and nephrology clinics. IV iron therapeutic products are not currently widely used by certain physicians who treat non-dialysis CKD patients in the U.S., particularly nephrologists, due to safety concerns and the inconvenience and often impracticability of administering IV iron therapeutic products in their offices. It is often difficult to change physicians' existing treatment paradigms even when supportive clinical data is available. In addition, our ability to effectively market
36
and sellFeraheme in the U.S. hospital market depends in part upon our ability to achieve acceptance ofFeraheme onto hospital formularies. Since many hospitals and hematology, oncology and nephrology practices are members of GPOs, which leverage the purchasing power of a group of entities to obtain discounts based on the collective bargaining power of the group, our ability to attract customers in these sites of care also depends in part on our ability to effectively promoteFeraheme to and enter into pricing agreements with GPOs. If we are not successful in capturing a significant share of the U.S. non-dialysis CKD market or if we are not successful in securing and maintaining formulary coverage forFeraheme, our potential profitability as well as our long-term business prospects could be adversely affected.
We derive a substantial amount of our revenue from a limited number of customers and the loss of one or more of these customers or a decline in revenue from one or more of these customers could have an adverse impact on our results of operations and financial condition.
In the U.S., we sellFeraheme primarily to wholesalers and specialty distributors and therefore a significant portion of our revenues is generated by a small number of customers. Four customers accounted for 94% of our total revenues during the year ended December 31, 2012, and three customers accounted for 94% of our accounts receivable balance as of December 31, 2012. We pay these wholesalers and specialty distributors a fee for the services that they provide to us. Because our business is concentrated with such a small number of wholesalers and specialty distributors, we could be forced to accept increases in their fees in order to maintain the current distribution networks through whichFeraheme is sold. Any increase in fees could have a negative impact on our current and future sales ofFeraheme in the U.S. and could have a negative impact on the reimbursement rate an individual physician, hospital or clinic would realize upon usingFeraheme. In addition, a significant portion of our U.S.Feraheme sales are generated through a small number of contracts with GPOs. For example, approximately 32% of our end-user demand in the year ended December 31, 2012 was generated by members of a single GPO with which we have contracted. As a result of the significant percentage of our end-user demand being generated by a single GPO, we may be at a disadvantage in future contract or price negotiations with such GPO and that GPO may be able to influence the demand forFeraheme from its members in a particular quarter through communications they make to their customers. In addition, the loss of, material reduction in sales volume to, or a significant adverse change in our relationship with any of our key wholesalers, distributors or GPOs could have a material adverse effect on our revenue in any given period and may result in significant annual or quarterly revenue fluctuations.
We depend, to a significant degree, on the availability and extent of reimbursement from third-party payors for the use of Feraheme/Rienso, and a reduction in the availability or extent of reimbursement could adversely affect our Feraheme/Rienso sales revenues and results of operations.
In both the U.S. and foreign markets, our and Takeda's ability to successfully commercializeFeraheme/Rienso is dependent, in significant part, on the availability and extent of reimbursement to end-users from third-party payors for the use ofFeraheme/Rienso, including governmental payors, managed care organizations and private health insurers. Reimbursement by third-party payors depends on a number of factors, including the third-party's determination that the product is competitively priced, safe and effective, appropriate for the specific patient, and cost-effective. Third-party payors are increasingly challenging the prices charged for pharmaceutical products and have instituted and continue to institute cost containment measures to control or significantly influence the purchase of pharmaceutical products. If these entities do not provide coverage and reimbursement forFeraheme/Rienso or provide an insufficient level of coverage and reimbursement, physicians and other healthcare providers may choose to use alternative IV iron replacement products, which would have an adverse effect on our ability to generate revenues.
37
In addition, U.S. and many foreign governments continue to propose and pass legislation designed to reduce the cost of health care for patients. In the U.S., the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the Health Care Reform Act, was enacted in March 2010 and includes certain cost containment measures including an increase to the minimum rebates for products covered by Medicaid programs, the extension of such rebates to drugs dispensed to Medicaid beneficiaries enrolled in Medicaid managed care organizations and the expansion of the 340B Drug Discount Program under the Public Health Service Act. In addition, the heightened focus on the health care industry by the federal government could result in the implementation of significant federal spending cuts, including cuts in Medicare and other health related spending in the near-term, such as a potential 2% across-the-board sequestration of Medicare expenditures. The full impact of these laws on our business is uncertain. In recent years some states have also passed legislation to control the prices of drugs as well as begun a move toward managed care to relieve some of their Medicaid cost burden. While Medicare is the predominant payor for treatment of patients with CKD, Medicare payment policy, in time, can also influence pricing and reimbursement in the non-Medicare markets, as private third-party payors and state Medicaid plans frequently adopt Medicare principles in setting reimbursement methodologies. These and any future changes in government regulation or private third-party payors' reimbursement policies may reduce the extent of reimbursement forFeraheme/Rienso and adversely affect our future operating results.
In January 2011, a prospective payment system for dialysis services provided to Medicare beneficiaries who have end-stage renal disease, or ESRD, became effective under which all costs of providing dialysis services are bundled together into a single prospective payment per treatment. This bundled approach to reimbursement has and will likely continue to alter the utilization of physician-administered drugs in the ESRD market as well as put downward pressure on the prices pharmaceutical companies can charge ESRD facilities for such drugs, particularly where alternative products are available. In the U.S.,Feraheme is sold at a price that is substantially higher than alternative IV iron products in the dialysis setting, and as a result, the demand forFeraheme in the dialysis setting has largely disappeared. In addition, it is also possible that this "bundled" approach may be applied to specific disease states other than ESRD. For example, one large insurer in the U.S has attempted to bundle certain costs related to the treatment of cancer patients. Further changes in the Medicare reimbursement rate, which result in lower payment rates from payors, including Medicare payors, would further limit our ability to successfully market and sellFeraheme in the U.S. In addition, in the U.S. hospital in-patient setting,Feraheme is reimbursed by Medicare under a diagnosis-related group payment system, which provides a per discharge reimbursement based on the diagnosis and/or procedure rather than actual costs incurred in patient treatments, thereby increasing the incentive for a hospital to limit or control expenditures. As a result,Feraheme has not been nor do we expect it to be broadly used in the hospital in-patient setting.
In countries outside of the U.S., market acceptance may also depend, in part, upon the availability of reimbursement within existing healthcare payment systems. Generally, in Europe and other countries outside of the U.S., the government sponsored healthcare system is the primary payor of healthcare costs of patients and therefore enjoys significant market power. Some foreign countries also set prices for pharmaceutical products as part of the regulatory process, and we cannot guarantee that the prices set by such governments will be sufficient to generate substantial revenues or allow sales ofFeraheme/Rienso to be profitable in those countries. Any such limitations on the reimbursement forFeraheme/Rienso in countries outside of the U.S. would have an adverse impact on Takeda's ability to generate product sales ofFeraheme/Rienso in such territories, which would, in turn, limit the amount of royalties we may receive under our amended agreement with Takeda.
38
We are substantially dependent upon our collaboration with Takeda to commercialize Feraheme/Rienso in certain regions outside of the U.S., including Canada, Switzerland and the EU, and if Takeda fails to successfully fulfill its obligations, or is ineffective in its commercialization of Feraheme/Rienso in the licensed territories, or if our collaboration is terminated, our plans to commercialize Feraheme/Rienso outside of the U.S. may be adversely affected.
In March 2010, we entered into our initial agreement with Takeda, which was amended in June 2012, under which we granted exclusive rights to Takeda to develop and commercializeFeraheme/Rienso as a therapeutic agent in Europe, certain Asia-Pacific countries (excluding Japan, China and Taiwan), Canada, India and Turkey. We are highly dependent on Takeda for certain regulatory filings outside of the U.S. with respect toFeraheme/Rienso and the commercialization ofFeraheme/Rienso outside of the U.S., including in Canada, Switzerland and the EU. If Takeda fails to perform its obligations under the Amended Takeda Agreement or is ineffective in its commercialization ofFeraheme/Rienso in the agreed-upon territories, or if we fail to effectively manage our relationship with Takeda, our ability to and the extent to which we obtain regulatory approvals forFeraheme/Rienso and ourFeraheme/Rienso commercialization efforts outside of the U.S. would be significantly harmed, which would have an adverse effect on milestone payments and royalties we may receive under the Amended Takeda Agreement. Further, if we fail to fulfill certain of our obligations under the Amended Takeda Agreement, Takeda has the right to assume the responsibility of clinical development and manufacturing ofFeraheme/Rienso in the agreed-upon territories, which would increase the cost of and delay theFeraheme/Rienso development program outside of the U.S.
Takeda has the unilateral right to terminate the Amended Takeda Agreement under certain conditions, including without cause. If Takeda terminates the agreement and we chose to continue to commercializeFeraheme/Rienso in Takeda's territories, we would be required to either enter into alternative arrangements with third parties to commercializeFeraheme/Rienso in Takeda's territories, which we may be unable to do, or to increase our internal infrastructure, both of which would likely result in significant additional expense and the disruption or failure of commercial efforts outside of the U.S. In addition, such a termination would prevent us from receiving the milestone payments and royalties we may receive under the Amended Takeda Agreement.
Our contract manufacturers may not be able to operate their manufacturing facilities in compliance with current good manufacturing practices, release specifications and other FDA and equivalent foreign regulations, which could result in a suspension of our contract manufacturers' ability to manufacture Feraheme/Rienso, the loss of Feraheme/Rienso inventory, an inability to manufacture sufficient quantities of Feraheme/Rienso to meet U.S. or foreign demand, or other unanticipated compliance costs.
Our third-party contract manufacturing facilities are subject to cGMP regulations enforced by the FDA and equivalent foreign regulatory regulations and agencies through periodic inspections to confirm such compliance. Our contract manufacturers must continually expend time, money and effort in production, record-keeping and quality assurance and control to ensure that these manufacturing facilities meet applicable regulatory requirements. Failure to maintain ongoing compliance with cGMP or similar regulations and other applicable manufacturing requirements of various U.S. or foreign regulatory agencies could result in, among other things, the issuance of warning letters, fines, the withdrawal or recall ofFeraheme/Rienso from the marketplace, total or partial suspension ofFeraheme/Rienso production, the loss ofFeraheme/Rienso inventory, suspension of the review of any future sNDAs or equivalent foreign filings, enforcement actions, injunctions or criminal prosecution. A government-mandated recall or a voluntary recall could divert managerial and financial resources, could be difficult and costly to correct, could result in the suspension of sales ofFeraheme/Rienso, and could have a severe adverse impact on our potential profitability and the future prospects of our business. If any U.S. or foreign regulatory agency inspects any of these manufacturing facilities and determines that they are not in compliance with cGMP or similar regulations or our contract manufacturers otherwise determine that they are not in compliance with these regulations, our contract manufacturers could experience an inability to manufacture sufficient quantities ofFeraheme/Rienso to meet U.S. or foreign demand or incur unanticipated compliance expenditures.
39
We have also established certain testing and release specifications with the FDA and other foreign regulatory agencies. This release testing must be performed in order to allow the finished product to be used for commercial sale. If our finished product does not meet these release specifications or if the release testing is variable, we may not be able to supply product to meet our projected demand. In addition, variations in the regulatory approval ofFeraheme/Rienso in the currently approved territories require that our third-party manufacturers follow different manufacturing processes and analytical testing methods. For example, in late 2012, we produced a batch ofRienso which did not meet our release specifications in the EU. As a result, we are incurring additional costs associated with the development, validation and technology transfer to Takeda of a more accurate assay in order to be able to release this batch and any future batches produced for sale in the EU. This new assay will require review and approval by the EMA. If we are unable to develop, validate, transfer or gain regulatory approval for the new release test, our ability to supply product to the EU will be adversely affected. Such setbacks could have an adverse impact onFeraheme/Rienso sales, our potential profitability and the future prospects of our business.
Our inability to obtain raw and other materials used in the manufacture of Feraheme/Rienso could adversely impact our ability to manufacture sufficient quantities of Feraheme/Rienso, which would have an adverse impact on our business.
We and our third-party manufacturers currently purchase certain raw and other materials used to manufactureFeraheme/Rienso from third-party suppliers and at present do not have any long-term supply contracts with these third parties. These third-party suppliers may cease to produce the raw or other materials used inFeraheme/Rienso or otherwise fail to supply these materials to us or our third-party manufacturers or fail to supply sufficient quantities of these materials to us or our third-party manufacturers in a timely manner for a number of reasons, including but not limited to the following:
- •
- Unexpected demand for or shortage of raw or other materials;
- •
- Adverse financial developments at or affecting the supplier;
- •
- Regulatory requirements or action;
- •
- An inability to provide timely scheduling and/or sufficient capacity;
- •
- Manufacturing difficulties;
- •
- Labor disputes or shortages; or
- •
- Import or export problems.
If any of our third-party suppliers cease to supply certain raw or other materials to us or our third-party manufacturers for any reason we could be unable to manufactureFeraheme/Rienso in sufficient quantities, on a timely basis, or in a cost-effective manner until we are able to qualify an alternative source. For example, one of the key components in ferumoxytol is produced specifically for us by a third-party supplier and if our third-party supplier is no longer able to supply it to us we will be unable to manufactureFeraheme/Rienso until we are able to identify and qualify an alternative supplier. This or any other interruption in our third-party supply chain could adversely affect our ability to satisfy commercial demand and our clinical development needs forFeraheme/Rienso.
The qualification of an alternative source may require repeated testing of the new materials and generate greater expenses to us if materials that we test do not perform in an acceptable manner. In addition, we or our third-party manufacturers sometimes obtain raw or other materials from one vendor only, even where multiple sources are available, to maintain quality control and enhance working relationships with suppliers, which could make us susceptible to price inflation by the sole supplier, thereby increasing our production costs. As a result of the high quality standards imposed on our raw or other materials, we or our third-party manufacturers may not be able to obtain such
40
materials of the quality required to manufactureFeraheme/Rienso from an alternative source on commercially reasonable terms, or in a timely manner, if at all.
Even if we are able to obtain raw or other materials from an alternative source, if these raw or other materials are not available in a timely manner or on commercially reasonable terms, we would be unable to manufactureFeraheme/Rienso, both for commercial sale and for use in our clinical trials, on a timely and cost-effective basis, which could cause us to lose money. Any such difficulty in obtaining raw or other materials could severely hinder our ability to manufactureFeraheme/Rienso and could have a material adverse impact on our ability to generate additional revenues and to achieve profitability.
We have a history of net losses, and we may not be able to generate sufficient revenues to achieve and maintain profitability in the future.
We have a history of significant operating losses, we may not be profitable in the future, and if we do attain profitability, such profitability may not be sustainable. In the past, we have financed our operations primarily from the sale of our equity securities, cash from sales ofFeraheme/Rienso, cash generated by our investing activities, and payments from our licensees. As of December 31, 2012, we had an accumulated deficit of approximately $456.7 million. Our losses were primarily the result of costs incurred in our efforts to manufacture, market and sellFeraheme/Rienso, including costs associated with maintaining our commercial infrastructure and marketing and promotion costs, research and development costs, such as costs associated with our clinical trials, and selling, general and administrative costs. We expect to continue to incur significant expenses as we continue to manufacture, market and sellFeraheme as an IV iron replacement therapeutic for use in adult CKD patients in the U.S., and as we further develop and seek marketing approval forFeraheme for the treatment of IDA in a broad range of patients. As a result, we will need to generate sufficient revenues in future periods to achieve and maintain profitability. We anticipate that the majority of any revenue we generate in the next twelve months will be from sales ofFeraheme/Rienso as an IV iron replacement therapeutic agent for use in adult CKD patients in the U.S. and royalties we may receive with respect to sales ofFeraheme/Rienso in Canada and the EU under the Amended Takeda Agreement, which we originally entered into with Takeda in 2010. We have never independently marketed or sold any products prior toFeraheme, and we or Takeda may not be successful in marketing or sellingFeraheme/Rienso. If we or Takeda are not successful in marketing and sellingFeraheme/Rienso, if revenues grow more slowly than we anticipate or if our operating expenses exceed our expectations, or if we are otherwise unable to achieve, maintain or increase profitability on a quarterly or annual basis, our business, results of operations and financial condition could be materially adversely affected and the market price of our common stock may decline.
We have limited experience independently commercializing a pharmaceutical product, and any failure on our part to effectively execute our Feraheme commercial plans in the U.S. would have an adverse impact on our business.
Prior to our commercialization ofFeraheme in the U.S., we had never independently marketed or sold a drug product as we had relied on our licensees to market and sell our previously approved products. We have an internal commercial infrastructure to market and sellFeraheme in the U.S., and if we are unsuccessful in maintaining an effective commercial function or experience a high level of employee turnover, then the commercialization ofFeraheme could be severely impaired. For example, we reduced our workforce in 2011 as part of an overall corporate restructuring, including certain positions within our commercial function, with further restructuring occurring in 2012. These workforce reductions or any future reductions or departures, could harm our ability to attract and retain qualified personnel, which could prevent us from successfully commercializingFeraheme in the U.S., impair our ability to maintain sales levels and/or impair our ability to support potential sales growth and sales ofFeraheme for any additional indications we may commercialize in the future. Any failure by us to
41
successfully commercializeFeraheme in the U.S. could have a material adverse impact on our ability to generate revenues, our ability to achieve profitability, and the future prospects for our business.
Our success depends on our ability to attract and retain key employees, and any failure to do so may be disruptive to our operations.
Because of the specialized nature of our business, our success depends to a significant extent on the continued service of our executive officers and on our ability to continue to attract, retain and motivate qualified executive, sales, technical operations, managerial, scientific, and medical personnel. We have entered into employment agreements with most of our current senior executives, but such agreements do not guarantee that these executives will remain employed by us for any significant period of time, or at all. There is intense competition for qualified personnel in the areas of our activities, and we may not be able to continue to attract and retain the qualified personnel necessary for the development of our business.
Previously implemented workforce reductions could residually harm our ability to attract and retain qualified personnel. In addition, any restructuring plans we may initiate in the future may be disruptive to our operations and could harm our ability to attract and retain qualified key personnel. For example, cost saving measures may distract management from our core business, harm our reputation, or yield unanticipated consequences, such as attrition beyond planned reductions in workforce, increased difficulties in our day-to-day operations, reduced employee productivity and a deterioration of employee morale. Any workforce reductions could also harm our ability to attract and retain qualified executive, sales, technical operations, managerial, scientific, and medical personnel who are critical to our business. Furthermore, because we are currently operating with fewer employees and service providers, any further turnover, whether occurring as part of a restructuring plan or otherwise, could cause significant disruption if we are unable to implement or maintain a sufficient succession plan for certain personnel or departments. Any failure to attract, retain or replace qualified personnel could prevent us from successfully commercializing and developingFeraheme, impair our ability to maintain sales levels and/or support potential sales growth.
Moreover, although we believe it is necessary to reduce the cost of our operations to improve our performance, these initiatives may preclude us from making potentially significant expenditures that could improve our competitiveness over the longer term. We cannot guarantee that any cost reduction measures, or other measures we may take in the future, will result in the expected cost savings, or that any cost savings will be unaccompanied by these or other unintended consequences.
We have limited experience independently distributing a pharmaceutical product, and our Feraheme/Rienso commercialization plans could suffer if we fail to effectively manage and maintain our supply chain and distribution network.
We do not have significant experience in managing and maintaining a supply chain and distribution network, and we are placing substantial reliance on third parties to perform product supply chain services for us. Such services include packaging, warehousing, inventory management, storage and distribution ofFeraheme/Rienso. We have contracted with Packaging Coordinators, Inc. (formerly Catalent Pharma Solutions, LLC), or PCI, to provide certain labeling, packaging and storage services for final U.S. and CanadianFeraheme drug product. In addition, we have contracted with Integrated Commercialization Services, Inc., or ICS, to be our exclusive third-party logistics provider to perform a variety of functions related to the sale and distribution ofFeraheme in the U.S., including services related to warehousing and inventory management, distribution, chargeback processing, accounts receivable management and customer service call center management. If ICS or PCI are unable to provide uninterrupted supply chain services or labeling, packaging and storage services, respectively, we may incur substantial losses of sales to wholesalers or other purchasers ofFeraheme.
42
In addition, the packaging, storage and distribution ofFeraheme/Rienso in the U.S. and abroad requires significant coordination among our and Takeda's manufacturing, sales, marketing and finance organizations and multiple third parties including our third-party logistics provider, packaging, labeling and storage provider, distributors, and wholesalers. In most cases, we do not currently have back-up suppliers or service providers to perform these tasks. If any of these third parties experience significant difficulties in their respective processes, fail to maintain compliance with applicable legal or regulatory requirements, fail to meet expected deadlines or otherwise do not carry out their contractual duties to us, or encounter physical or natural damages at their facilities, our ability to deliverFeraheme/Rienso to meet U.S. or foreign commercial demand could be significantly impaired. The loss of any of our third-party providers, together with a delay or inability to secure an alternate distribution source for end-users in a timely manner, could cause the distribution ofFeraheme/Rienso to be delayed or interrupted, which would have an adverse effect on our business, financial condition and results of operations.
We rely on third parties in the conduct of our business, including our clinical trials and manufacturing, and if they fail to fulfill their obligations, our commercialization and development plans may be adversely affected.
We rely and intend to continue to rely on third parties, including clinical research organizations, third-party manufacturers, third-party logistics providers, packaging and labeling providers, wholesale distributors and certain other important vendors and consultants in the conduct of our business. As a result of the current volatile and unpredictable global economic situation, there may be a disruption or delay in the performance or satisfaction of commitments to us by our third-party contractors or suppliers. For example, our distributors, customers or suppliers may experience difficulty in obtaining the financial resources necessary to purchase inventory or raw or other materials, may begin to maintain lower inventory levels or may become insolvent. If such third parties are unable to adequately satisfy their contractual commitments to us in a timely manner, our business could be severely adversely affected.
In addition, we have contracted and plan to continue to contract with certain third parties to provide certain services, including site selection, enrollment, monitoring, data management and other services, in connection with the conduct of our clinical trials and the preparation and filing of our regulatory applications. We have limited experience conducting clinical trials outside the U.S., and, therefore, we are also largely relying on third parties such as clinical research organizations to manage, monitor and carry out these clinical trials. Although we depend heavily on these parties, we do not control them and, therefore, we cannot be assured that these third parties will adequately perform all of their contractual obligations to us. If our third-party service providers cannot adequately fulfill their obligations to us in a timely and satisfactory manner, if the quality and accuracy of our clinical trial data or our regulatory submissions are compromised due to poor quality or failure to adhere to our protocols or regulatory requirements or if such third parties otherwise fail to adequately discharge their responsibilities or meet deadlines, our development plans and planned regulatory submissions both in and outside of the U.S may be delayed or terminated, which would adversely impact our ability to generate revenues fromFeraheme/Rienso sales in additional indications and/or outside of the U.S.
Our operating results will likely fluctuate so you should not rely on the results of any single quarter to predict how we will perform over time.
Our future operating results will likely vary from quarter to quarter depending on a number of factors, some of which we cannot control, including but not limited to:
- •
- The magnitude of U.S.Feraheme sales;
- •
- The loss of a key customer or GPO;
43
- •
- The impact of any pricing strategies we have implemented or may implement related toFeraheme, including the magnitude of rebates and/or discounts we may offer, or changes in pricing by our competitors or a new entrant into the market;
- •
- The introduction of new competitive products in the iron replacement therapeutic market, such as Injectafer®, if approved or generic versions of new or currently available drug therapies;
- •
- Any expansion or contraction of the overall size of the IV iron market, which could result from a number of factors including but not limited to changes in treatment guidelines or practices related to IDA;
- •
- Any changes to the mix of our business;
- •
- Changes in buying patterns and inventory levels of our wholesalers or distributors;
- •
- The timing and magnitude of milestone payments, product sales revenues and royalties we may receive from Takeda under the Amended Takeda Agreement;
- •
- The initiation or outcome of any material litigation or patent challenges to which we are or become a party and the magnitude of costs associated with such litigation;
- •
- The timing and magnitude of costs associated with the commercialization ofFeraheme in the U.S., including costs associated with maintaining our commercial infrastructure and executing our promotional and marketing strategy;
- •
- The magnitude of costs incurred in connection with business development activities or business development transactions into which we enter;
- •
- Changes in accounting estimates related to reserves on revenue, returns, or other accruals or changes in the timing and availability of government or customer discounts, rebates and incentives;
- •
- Changes in the actual or perceived safety or efficacy profile ofFeraheme/Rienso, or products that compete withFeraheme/Rienso that could cause customers to increase, reduce or discontinue their use ofFeraheme/Rienso;
- •
- The timing and magnitude of costs associated with commercial-scale manufacturing ofFeraheme/Rienso, including costs of raw and other materials and costs associated with maintaining commercial inventory and qualifying additional manufacturing capacities and alternative suppliers;
- •
- The timing and magnitude of costs associated with our ongoing and planned clinical studies ofFeraheme/Rienso in connection with our pediatric program, our post-marketing commitments for the EMA and other regulatory agencies, our pursuit of additional indications and our development ofFeraheme/Rienso in countries outside of the U.S;
- •
- The costs associated with manufacturing batch failures or inventory write-offs due to out-of-specification release testing or ongoing stability testing that results in a batch no longer meeting specifications;
- •
- Changes in reimbursement practices and laws and regulations affectingFeraheme/Rienso from federal, state and foreign legislative and regulatory authorities, government health administration authorities, private health insurers and other third-party payors; and
- •
- The implementation of new or revised accounting or tax rules or policies.
As a result of these and other factors, our quarterly operating results could fluctuate, and this fluctuation could cause the market price of our common stock to decline. Results from one quarter should not be used as an indication of future performance.
44
In the U.S. there have been, and we expect there will continue to be, a number of federal and state legislative initiatives implemented to reform the healthcare system in ways that could adversely impact our business and our ability to sell Feraheme profitably.
In the U.S., there have been, and we expect there will continue to be, a number of legislative and regulatory proposals aimed at changing the U.S. healthcare system. For example, the Health Care Reform Act contains a number of provisions that significantly impact the pharmaceutical industry and may negatively affect our potentialFeraheme revenues. Among other things, the Health Care Reform Act increased the minimum Medicaid drug rebates for pharmaceutical companies, extended the rebate provisions to Medicaid managed care organizations, and expanded the 340B Drug Discount Program under the Public Health Service Act. For example, the percentage ofFeraheme business sold to 340B institutions has grown from 5% in 2010 to 14% in 2012. Since these institutions are granted lower prices than those offered to our other customers, any further growth in the 340B business may have a negative impact on our sales price per gram and gross margins. Substantial new provisions affecting compliance have also been added, which may require us to modify our business practices with healthcare providers and potentially incur additional costs. While we are continuing to evaluate this legislation and its potential impact on our business, this legislation may adversely affect the demand forFeraheme in the U.S. or cause us to incur additional expenses and therefore adversely affect our financial position and results of operations.
In addition, various healthcare reform proposals have emerged at the state level in the U.S. We cannot predict the impact that newly enacted laws or any future legislation or regulation will have on us. We expect that there will continue to be a number of U.S. federal and state proposals to implement governmental pricing controls and limit the growth of healthcare costs. These efforts could adversely affect our business by, among other things, limiting the prices that can be charged forFeraheme or the amount of reimbursement rates and terms available from governmental agencies or third-party payors, limiting the profitability ofFeraheme, increasing our rebate liability or limiting the commercial opportunity forFeraheme, including its acceptance by healthcare payors.
Wholesaler, distributor and GPO buying patterns and other factors may cause our quarterly results to fluctuate, and these fluctuations may adversely affect our short-term results.
Our results of operations, including, in particular, product sales revenues, may vary from period to period due to a variety of factors, including the buying patterns of our U.S. wholesalers and distributors, which vary from quarter to quarter. In addition, our contracts with GPOs require certain performance from the members of the GPOs such as growth over prior periods or certain market share attainment goals in order to qualify for discounts off the list price ofFeraheme and a GPO may be able to influence the demand forFeraheme from its members in a particular quarter through communications they make to their customers. In the event wholesalers and distributors with whom we do business in the U.S. determine to limit their purchases ofFeraheme, sales ofFeraheme could be adversely affected. For example, in advance of an anticipated price increase or a reduction in expected rebates or discounts, customers may orderFeraheme in larger than normal quantities which could cause sales ofFeraheme to be lower in subsequent quarters than they would have been otherwise. Further, any changes in purchasing patterns, inventory levels, increases in returns ofFeraheme, delays in purchasing products or delays in payment for products by one of our distributors or GPOs could also have a negative impact on our revenue and results of operations.
45
If the estimates we make, or the assumptions on which we rely, in preparing our consolidated financial statements prove inaccurate, our actual results may vary from those reflected in our projections and accruals.
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, revenues and expenses, the amounts of charges accrued by us, and the related disclosure of contingent assets and liabilities. On an ongoing basis, our management evaluates our critical and other significant estimates and judgments, including among others those associated with revenue recognition related to product sales and collaboration agreements, product sales allowances and accruals, assessing investments for potential other-than-temporary impairment and determining the values of investments, estimates used to measure the fair value of our held for sale assets, accrued expenses, income taxes and equity-based compensation expense. We base our estimates on market data, our observance of trends in our industry, and various other assumptions that we believe to be reasonable under the circumstances. If actual results differ from these estimates, there could be a material adverse effect on our financial results and the performance of our stock.
As part of our revenue recognition policy, our estimates of product returns, rebates and chargebacks, fees and other discounts require subjective and complex judgments due to the need to make estimates about matters that are inherently uncertain. Any significant differences between our actual results and our estimates could materially affect our financial position and results of operations. For example during the years ended December 31, 2012 and 2011, we revised our estimated Medicaid reserve rate, which resulted in a reduction of our estimated Medicaid rebate reserve related to priorFeraheme sales of $0.6 million and $2.5 million, respectively. Further, during the year ended December 31, 2012, we reduced our reserve for product returns by approximately $2.2 million due to a lower than expected actual returns rate since the 2009 launch ofFeraheme as well as a reduction in our expected rate of product returns in the future.
In addition, to determine the required quantities ofFeraheme and the related manufacturing schedule, we also need to make significant judgments and estimates based on inventory levels, current market trends, anticipated sales, forecasts from our licensees, including Takeda, and other factors. Because of the inherent nature of estimates, there could be significant differences between our and Takeda's estimates and the actual amount of product need. For example, the level of our access to wholesaler and distributor inventory levels and sales data in the U.S., which varies based on the wholesaler or distributor, affects our ability to accurately estimate certain reserves included in our financial statements. Any difference between our estimates and the actual amount of product demand could result in unmet demand or excess inventory, each of which would adversely impact our financial results and results of operations.
Our stock price has been and may continue to be volatile, and your investment in our stock could decline in value or fluctuate significantly.
The market price of our common stock has been, and may continue to be, volatile, and your investment in our stock could decline in value or fluctuate significantly. Our stock price has ranged between $12.43 and $18.50 in the fifty-two week period through February 15, 2013. The stock market has from time to time experienced extreme price and volume fluctuations, particularly in the biotechnology and pharmaceuticals sectors, which have often been unrelated to the operating performance of particular companies. Various factors and events, many of which are beyond our control, may have a significant impact on the market price of our common stock. Factors which may affect the market price of our common stock include, among others:
- •
- Our ability to successfully commercializeFeraheme in the U.S. and Takeda's ability to successfully commercializeFeraheme/Rienso in territories outside of the U.S.;
46
- •
- The timing and magnitude ofFeraheme/Rienso revenue and actual or anticipated fluctuations in our operating results;
- •
- Changes in or our failure to meet financial estimates published by securities analysts or our own publicly disclosed financial guidance;
- •
- Increases or decreases in our operating expenses or our gross margin onFeraheme/Rienso;
- •
- Developments in patents or other proprietary rights by or for the benefit of us or our competitors, such as the recent decision by the EPO regarding our European ferumoxytol patent or decisions regardingFeraheme's NCE status or an ANDA filing by a generic entrant;
- •
- The availability of reimbursement coverage forFeraheme/Rienso or changes in the reimbursement policies of U.S. or foreign governmental or private payors;
- •
- Public announcements of U.S. or foreign regulatory actions with respect toFeraheme/Rienso or products or product candidates of our competitors;
- •
- Actual or perceived safety concerns related toFeraheme/Rienso or products or product candidates of our competitors, including any actions taken by U.S. or foreign regulatory authorities in connection with such concerns;
- •
- The status or results of clinical trials forFeraheme or products or product candidates of our competitors;
- •
- The acquisition, development or regulatory approvals of technologies, product candidates or products by us or our competitors;
- •
- Cash milestones earned, if any, under the Amended Takeda Agreement;
- •
- The initiation or outcome of any material litigation or patent challenges to which we are or may become a party;
- •
- Significant collaboration, product or business acquisitions, joint venture or similar agreements by us or our competitors;
- •
- Shareholder activism and attempts to disrupt our strategy by activist investors;
- •
- General market conditions; and
- •
- Sales of large blocks of our common stock.
Thus, as a result of events both within and beyond our control, our stock price could fluctuate significantly or lose value rapidly.
If securities analysts downgrade our stock, cease coverage of us, or if our operating results do not meet analysts' forecasts and expectations, our stock price could decline.
The trading market for our common stock relies in part on the research and reports that industry or financial analysts publish about us and our business. Currently, seven financial analysts publish reports about us and our business. We do not control these or any other analysts. Furthermore, there are many large, well-established, publicly traded companies active in our industry and market, which may mean that it is less likely that we will receive widespread analyst coverage. In addition, our future operating results are subject to substantial uncertainty, and our stock price could decline significantly if we fail to meet or exceed analysts' forecasts and expectations. If any of the analysts who cover us downgrade our stock or issue commentary or observations about us or our stock that are perceived by the market as negative, our stock price would likely decline rapidly. In addition, if these analysts cease coverage of our company, we could lose visibility in the market, which in turn could also cause our stock price to decline.
47
If our operating results do not meet our own publicly disclosed financial guidance our stock price could decline.
In 2013, we publicly provided financial guidance, including expected 2013Feraheme/Rienso product revenue, total revenue, estimated operating expenses, estimated cost of goods sold as a percent of sales, quarterly cash flow trajectory throughout 2013 and estimated year-end cash and cash equivalents balance. If, for any reason, we are unable to realize our expected revenue growth in 2013 and beyond, including as the result of a lower-than-anticipated impact of our 2013 price increases, we may fail to realize our publicly announced revenue and year-end cash and cash equivalents balance guidance. If we fail to realize or if we change or update any element of our publicly disclosed financial guidance or other expectations about our business, our stock price could decline in value.
We may need additional capital to achieve our business objectives.
We have expended and will continue to expend substantial funds to successfully commercialize and developFeraheme. Our long-term capital requirements will depend on many factors, including, but not limited to:
- •
- Our ability to successfully commercializeFeraheme in the U.S. and Takeda's ability to successfully commercializeFeraheme/Rienso in its licensed territories outside of the U.S.;
- •
- The magnitude of U.S.Feraheme sales;
- •
- The magnitude ofFeraheme/Rienso sales and royalties we may receive from Takeda outside of the U.S.;
- •
- Our ability to obtain regulatory approval forFeraheme/Rienso to treat IDA regardless of the underlying cause both within the U.S. and outside of the U.S., particularly in the EU;
- •
- The success, costs and structure of any business or corporate development initiatives to bring additional products into our portfolio;
- •
- The outcome of and costs associated with any material litigation or patent challenges to which we are or may become a party;
- •
- Our ability to achieve the various milestones and receive the associated payments under the Amended Takeda Agreement;
- •
- Costs associated with the U.S. commercialization ofFeraheme, including costs associated with maintaining our commercial infrastructure, executing our promotional and marketing strategy forFeraheme, and conducting our required pediatric clinical studies and any post-marketing clinical studies;
- •
- The timing and magnitude of costs associated with qualifying additional manufacturing capacities and alternative suppliers;
- •
- Costs associated with our development ofFeraheme for the treatment of IDA in a broad range of patients in the U.S.;
- •
- Our ability to maintain successful collaborations with our licensees and/or to enter into additional alternative strategic relationships, if necessary; and
- •
- Our ability to raise additional capital on terms and within a timeframe acceptable to us, if necessary.
We estimate that our cash resources as of December 31, 2012, combined with cash we currently expect to receive from sales ofFeraheme/Rienso, from earnings on our investments, and potential royalty payments we may receive from Takeda will be sufficient to finance our currently planned
48
operations for at least the next twelve months. We may require additional funds or need to establish additional alternative strategic arrangements to execute a business development transaction. We may at any time seek funding through additional arrangements with collaborators through public or private equity or debt financings. We may not be able to obtain financing or to secure alternative strategic arrangements on acceptable terms or within an acceptable timeframe, if at all.
Any additional equity financings or alternative strategic arrangements would be dilutive to our stockholders. In addition, the terms of any debt financing could greatly restrict our ability to raise additional capital and may provide rights and preferences to the investors in any such financing which are senior to those of, and not available to, current stockholders. Our inability to raise additional capital on terms and within a timeframe acceptable to us when needed could force us to dramatically reduce our expenses and delay, scale back or eliminate certain of our activities and operations, including our commercialization and development activities, any of which would have a material adverse effect on our business, financial condition and future business prospects.
The investment of our cash is subject to risks, which may cause losses or adversely affect the liquidity of these investments and our results of operations, liquidity and financial condition.
As of December 31, 2012, we had $46.3 million in cash and cash equivalents and $180.8 million in short-term investments. These investments are subject to general credit, liquidity, market and interest rate risks, which have been and may, in the future, be exacerbated by a U.S. and/or global financial crisis. We may realize losses in the fair value of certain of our investments or a complete loss of these investments if the credit markets tighten, which would have an adverse effect on our results of operations, liquidity and financial condition.
The condition of the credit markets remains unpredictable. As a result, we may experience a reduction in value or loss of liquidity with respect to our investments. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. Further, as part of our determination of the fair value of our investments, we consider credit ratings provided by independent investment rating agencies as of the valuation date. These ratings are subject to change. These market risks associated with our investment portfolio may have an adverse effect on our results of operations, cash position, liquidity and overall financial condition.
We are subject to increasingly complex corporate governance, public disclosure and accounting requirements that could adversely affect our business and financial results.
We are subject to changing rules and regulations of U.S. federal and state government as well as the stock exchange on which our common stock is listed. These entities, including the Public Company Accounting Oversight Board, the NASDAQ Stock Market, or NASDAQ, and the U.S. Securities and Exchange Commission, or the SEC, have issued a significant number of new and increasingly complex requirements and regulations over the last several years and continue to develop additional regulations and requirements in response to laws enacted by Congress. Our efforts to comply with these requirements have resulted in, and are likely to continue to result in, an increase in our expenses and a diversion of management's time from other business activities.
Our ability to use net operating loss carryforwards and tax credit carryforwards to offset future taxable income may be limited as a result of future transactions involving our common stock.
In general, under Section 382 of the Internal Revenue Code of 1986, as amended, a corporation that undergoes an "ownership change" is subject to limitations on its ability to utilize its pre-change net operating losses and certain other tax assets to offset future taxable income. In general, an ownership change occurs if the aggregate stock ownership of certain stockholders increases by more than 50 percentage points over such stockholders' lowest percentage ownership during the testing period,
49
which is generally three years. An ownership change could limit our ability to utilize our net operating loss and tax credit carryforwards for taxable years including or following such "ownership change." Limitations imposed on the ability to use net operating losses and tax credits to offset future taxable income could require us to pay U.S. federal income taxes earlier than we have estimated would otherwise be required if such limitations were not in effect and could cause such net operating losses and tax credits to expire unused, in each case reducing or eliminating the benefit of such net operating losses and tax credits and potentially adversely affecting our financial position. Similar rules and limitations may apply for state income tax purposes.
If we fail to comply with our reporting and payment obligations under U.S. governmental pricing programs, we could be required to reimburse government programs for underpayments and could pay penalties, sanctions and fines which could have a material adverse effect on our business, financial condition and results of operations.
As a condition of reimbursement by various U.S. federal and state healthcare programs forFeraheme, we are required to calculate and report certain pricing information to U.S. federal and state healthcare agencies. For example, we are required to provide average selling price information to the Centers for Medicare and Medicaid Services on a quarterly basis in order to compute Medicare Part B payment rates. Price reporting and payment obligations are highly complex and vary among products and programs. The calculation of average selling price includes a number of inputs from our contracts with wholesalers, specialty distributors, GPOs and other customers. It also requires us to make an assessment of whether these agreements are deemed to be for bona fide services and that the services are deemed to be at fair market value in our industry and for our products. Our processes for estimating amounts due under these governmental pricing programs involve subjective decisions. As a result, our price reporting calculations remain subject to the risk of errors and our methodologies for calculating these prices could be challenged under the Federal False Claims Act or other laws. In addition, the Health Care Reform Act modified the rules related to certain price reports and expanded the scope of pharmaceutical product sales to which Medicaid rebates apply, among other things. Presently, uncertainty exists as many of the specific determinations necessary to implement this new legislation have yet to be decided and communicated to industry participants. This uncertainty in the interpretation of the legislation increases the chances of an error in price reporting, which could in turn lead to a legal challenge, restatement or investigation. If we become subject to investigations, restatements, or other inquiries concerning our compliance with price reporting laws and regulations, we could be required to pay or be subject to additional reimbursements, penalties, sanctions or fines, which could have a material adverse effect on our business, financial condition and results of operations.
We and/or Takeda are subject to ongoing U.S. and foreign regulatory obligations and oversight of Feraheme/Rienso, and any failure by us to maintain compliance with applicable regulations may result in several adverse consequences including the suspension of the manufacturing, marketing and sale of Feraheme/Rienso, the incurrence of significant additional expense and other limitations on our ability to commercialize Feraheme/Rienso.
We and/or Takeda are subject to ongoing regulatory requirements and review both in the U.S. and in foreign jurisdictions pertaining toFeraheme/Rienso's manufacture, labeling, packaging, adverse event reporting, storage, marketing, promotion and record keeping. Failure to comply with such regulatory requirements or the later discovery of previously unknown problems withFeraheme/Rienso or our third-party contract manufacturing facilities or processes by which we manufactureFeraheme/Rienso may result in restrictions on our ability to manufacture, market or sellFeraheme/Rienso, including its withdrawal from the market. Any such restrictions could result in a decrease inFeraheme/Rienso sales, damage to our reputation or the initiation of lawsuits against us, Takeda, or our third-party contract
50
manufacturers. We and/or Takeda may also be subject to additional sanctions, including but not limited to:
- •
- Warning letters;
- •
- Civil or criminal penalties;
- •
- Suspension or withdrawal of regulatory approvals;
- •
- Temporary or permanent closing of the facilities of our third-party contract manufacturers;
- •
- Requirements to communicate with physicians and other customers about concerns related to actual or potential safety, efficacy, or other issues involvingFeraheme/Rienso;
- •
- Changes to theFeraheme/Rienso package insert, such as potential limitations on the current dosage and administration ofFeraheme/Rienso or IV irons as a class;
- •
- Implementation of risk mitigation programs;
- •
- Restrictions on our continued manufacturing, marketing or sale ofFeraheme/Rienso; or
- •
- Recalls or a refusal by regulators to consider or approve applications for additional indications.
Any of the above sanctions could have a material adverse impact on our ability to generate revenues and to achieve profitability and cause us to incur significant additional expenses.
If we or Takeda market or distribute Feraheme/Rienso in a manner that violates federal, state or foreign healthcare fraud and abuse laws, marketing disclosure laws or other federal, state or foreign laws and regulations, we may be subject to civil or criminal penalties.
In addition to FDA and related regulatory requirements in the U.S. and abroad, we are subject to extensive additional federal, state and foreign healthcare regulation, which includes but is not limited to, the Federal False Claims Act, the Federal Anti-Kickback Statute, the Foreign Corrupt Practices Act, and their state analogues, and similar laws in countries outside of the U.S., and government price reporting laws. False claims laws prohibit anyone from knowingly presenting, or causing to be presented for payment to third-party payors, including Medicare and Medicaid, false or fraudulent claims for reimbursed drugs or services, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Anti-kickback laws make it illegal to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug, that is reimbursed by a state or federal program. The Foreign Corrupt Practices Act and similar foreign anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Similar laws and regulations exist in many other countries throughout the world in which we intend to commercializeFeraheme/Rienso through Takeda and our other licensees. We have developed and implemented a corporate compliance program based on what we believe are current best practices in the pharmaceutical industry, but we cannot guarantee that we, our employees, our consultants or our contractors are or will be in compliance with all federal, state and foreign regulations. If we, our representatives, or our licensees, including Takeda, fail to comply with any of these laws or regulations, a range of fines, penalties and/or other sanctions could be imposed on us and/or Takeda, including, but not limited to, restrictions on how we and/or Takeda market and sellFeraheme/Rienso, significant fines, exclusions from government healthcare programs, including Medicare and Medicaid, litigation, or other sanctions. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could also have an adverse effect on our business, financial condition and results of operations.
51
In recent years, several U.S. states have enacted legislation requiring pharmaceutical companies to establish marketing and promotional compliance programs or codes of conduct and/or to file periodic reports with the state or make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities. Similar legislation is being considered by additional states and foreign governments. In addition, as part of the Health Care Reform Act, the federal government has enacted the Physician Payment Sunshine Act and related regulations. Beginning in August 2013, manufacturers of drugs are required to publicly report gifts and payments made to physicians and teaching hospitals. Many of these requirements are new and uncertain, and the penalties for failure to comply with these requirements are unclear. Compliance with these laws is difficult, time consuming and costly, and if we are found to not be in full compliance with these laws, we may face enforcement actions, fines and other penalties, and we could receive adverse publicity which could have an adverse effect on our business, financial condition and results of operations.
If we fail to comply with any federal, state or foreign laws or regulations governing our industry, we could be subject to a range of regulatory actions that could adversely affect our ability to commercializeFeraheme/Rienso, harm or prevent sales ofFeraheme/Rienso, or substantially increase the costs and expenses of commercializing and marketingFeraheme/Rienso, all of which could have a material adverse effect on our business, financial condition and results of operations.
The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products. If we are found to have improperly promoted off-label uses, we may become subject to significant fines and other liability.
The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product's approved labeling. If we are found to have promoted such off-label uses, we may become subject to significant government fines and other related liability. For example, the U.S. government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The government has also required companies to enter into complex corporate integrity agreements and/or non-prosecution agreements that can impose significant restrictions and other burdens on the affected companies.
In addition, incentives exist under applicable U.S. law that encourage employees and physicians to report violations of rules governing promotional activities for pharmaceutical products. These incentives could lead to so called whistleblower lawsuits as part of which such persons seek to collect a portion of moneys allegedly overbilled to government agencies due to, for example, promotion of pharmaceutical products beyond labeled claims. Such lawsuits, whether with or without merit, are typically time consuming and costly to defend. Such suits may also result in related shareholder lawsuits, which are also costly to defend.
Our business could be negatively affected as a result of the actions of activist shareholders.
Proxy contests have been waged against many companies in the biopharmaceutical industry over the last few years. For example, in 2011, MSMB Capital Management LLC, or MSMB Capital, filed a preliminary consent solicitation statement with the SEC seeking to remove and replace most of our then current directors with MSMB Capital's nominees. The review, consideration and response to efforts by activist shareholders may require the expenditure of significant time and resources by us and may be a significant distraction for our management and employees. The impact of activist shareholders' efforts due to these or other factors may undermine our business and have a material adverse effect on our results of operations. If faced with a proxy contest, we may not be able to successfully defend against the contest, which would be disruptive to our business.
52
If we identify a material weakness in our internal controls over financial reporting, our ability to meet our reporting obligations and the trading price of our stock could be negatively affected.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Accordingly, a material weakness increases the risk that the financial information we report contains material errors.
We regularly review and update our internal controls, disclosure controls and procedures, and corporate governance policies. In addition, we are required under the Sarbanes-Oxley Act of 2002 to report annually on our internal control over financial reporting. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. If we, or our independent registered public accounting firm, determine that our internal controls over our financial reporting are not effective, or we discover areas that need improvement in the future, or we experience high turnover of our personnel in our financial reporting functions, these shortcomings could have an adverse effect on our business and financial results, and the price of our common stock could be negatively affected.
If we cannot conclude that we have effective internal control over our financial reporting, or if our independent registered public accounting firm is unable to provide an unqualified opinion regarding the effectiveness of our internal control over financial reporting, investors could lose confidence in the reliability of our financial statements, which could lead to a decline in our stock price. Failure to comply with reporting requirements could also subject us to sanctions and/or investigations by the SEC, NASDAQ or other regulatory authorities.
An adverse determination in any current or future lawsuits in which we are a defendant, including the class action lawsuit to which we are currently a party, could have a material adverse effect on us.
A purported class action complaint was originally filed on March 18, 2010 in the United States District Court for the District of Massachusetts, entitled Silverstrand Investments et. al. v. AMAG Pharm., Inc., et. al., Civil Action No. 1:10-CV-10470-NMG, and was amended on September 15, 2010 and on December 17, 2010. The second amended complaint, or SAC, filed on December 17, 2010 alleged that we and our former President and Chief Executive Officer, former Executive Vice President and Chief Financial Officer, the then members of our Board of Directors, or Board, and certain underwriters in our January 2010 offering of common stock violated certain federal securities laws by making certain alleged false and misleading statements and omissions in a registration statement filed in January 2010. The plaintiff sought unspecified damages on behalf of a purported class of purchasers of our common stock pursuant to our common stock offering on or about January 21, 2010. On August 11, 2011, the District Court issued an Opinion and Order dismissing the SAC in its entirety for failure to state a claim upon which relief could be granted. On September 14, 2011, the plaintiffs filed a Notice of Appeal to the United States Court of Appeals for the First Circuit, or the Court of Appeals. After briefing was completed by all parties, the Court of Appeals heard oral argument on May 11, 2012, and took the matter under advisement. On February 4, 2013, the Court of Appeals affirmed in part and reversed in part the District Court's Opinion and Order, and remanded the case to the District Court. On February 18, 2013, we filed a Petition for Panel Hearing or RehearingEn Banc, asking the Court of Appeals to reconsider its decision. Whether or not the plaintiff's appeal is successful, this type of litigation is often expensive and diverts management's attention and resources, which could adversely affect the operation of our business. If we are ultimately required to pay significant defense costs, damages or settlement amounts, such payments could adversely affect our operations.
53
We may also be the target of similar litigation in the future. Any future litigation could result in substantial costs and divert our management's attention and resources, which could cause serious harm to our business, operating results and financial condition. Though we maintain liability insurance, if any costs or expenses associated with this or any other litigation exceed our insurance coverage, we may be forced to bear some or all of these costs and expenses directly, which could be substantial.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of Feraheme/Rienso.
The administration ofFeraheme/Rienso to humans, whether in clinical trials or after approval for commercial use, may expose us to liability claims, whether or notFeraheme/Rienso is actually at fault for causing an injury. AsFeraheme/Rienso is used over longer periods of time by a wider group of patients taking numerous other medicines or by patients with additional underlying health problems, the likelihood of adverse drug reactions or unintended side effects, including death, may increase. Although we maintain product liability insurance coverage for claims arising from the use of our products in clinical trials and commercial use, coverage is expensive, and we may not be able to maintain sufficient insurance at a reasonable cost, if at all. Product liability claims, whether or not they have merit, could also decrease demand forFeraheme/Rienso, subject us to product recalls or harm our reputation, cause us to incur substantial costs, and divert management's time and attention.
Our shareholder rights plan, certain provisions in our charter and by-laws, certain contractual relationships and certain Delaware law provisions could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current members of our Board.
In 2009, we adopted a shareholder rights plan, the provisions of which are intended to deter a hostile takeover by making any proposed hostile acquisition of us more expensive and less desirable to a potential acquirer by enabling our stockholders (other than the potential hostile acquiror) to purchase significant amounts of additional shares of our common stock at dilutive prices. The rights issued pursuant to our shareholder rights plan become exercisable generally upon the earlier of 10 days after a person or group acquires 20% or more of our outstanding common stock or 10 business days after the announcement by a person or group of an intention to acquire 20% of our outstanding common stock via tender offer or similar transaction. The shareholder rights plan could delay or discourage transactions involving an actual or potential change in control of us or our management, including transactions in which stockholders might otherwise receive a premium for their shares over then current prices.
In addition, certain provisions in our certificate of incorporation and our by-laws may discourage, delay or prevent a change of control or takeover attempt of our company by a third-party as well as substantially impede the ability of our stockholders to benefit from a change of control or effect a change in management and our Board. These provisions include:
- •
- The ability of our Board to increase or decrease the size of the Board without stockholder approval;
- •
- Advance notice requirements for the nomination of candidates for election to our Board and for proposals to be brought before our annual meeting of stockholders;
- •
- The authority of our Board to designate the terms of and issue new series of preferred stock without stockholder approval;
- •
- Non-cumulative voting for directors; and
- •
- Limitations on the ability of our stockholders to call special meetings of stockholders.
54
As a Delaware corporation, we are subject to the provisions of Section 203 of the Delaware General Corporation Law, or Section 203, which prevents us from engaging in any business combination with any "interested stockholder," which is defined generally as a person that acquires 15% or more of a corporation's outstanding voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in the manner prescribed in Section 203. These provisions could have the effect of delaying or preventing a change of control, whether or not it is desired by, or beneficial to, our stockholders.
In addition to the above factors, an acquisition of our company could be made more difficult by employment agreements we have in place with our executive officers, as well as a company-wide change of control policy, which provide for severance benefits as well as the full acceleration of vesting of any outstanding options or restricted stock units in the event of a change of control and subsequent termination of employment. Further, our Second Amended and Restated 2007 Equity Incentive Plan generally permits our Board to provide for the acceleration of vesting of options granted under that plan in the event of certain transactions that result in a change of control.
We are subject to environmental laws and potential exposure to environmental liabilities.
Because we use certain hazardous materials in the production of our products, we are subject to various federal, state and local environmental laws and regulations that govern our operations, including the import, handling and disposal of non-hazardous and hazardous wastes, and emissions and discharges into the environment. Failure to comply with these laws and regulations could result in costs for corrective action, penalties or the imposition of other liabilities. We also are subject to laws and regulations that impose liability and clean-up responsibility for releases of hazardous substances into the environment. Under certain of these laws and regulations, a current or previous owner or operator of property may be liable for the costs of remediating the release or spill of hazardous substances or petroleum products on or from its property, without regard to whether the owner or operator knew of, or caused, the contamination, and such owner or operator may incur liability to third parties impacted by such contamination. The presence of, or failure to remediate properly the release or spill of, these substances could adversely affect the value of, and our ability to transfer or encumber, our real property.
ITEM 1B. UNRESOLVED STAFF COMMENTS:
None.
In May 2008, we entered into a lease agreement for certain real property located at 100 Hayden Avenue, Lexington, Massachusetts for use as our principal executive offices. The term of the lease began on May 22, 2008 and will continue until August 31, 2016 with two successive five year extension terms at our option. The aggregate size of rentable floor area for the offices is 55,924 square feet, and the rent for the initial term commenced in February 2009.
During any extension term, the base rent will be an amount agreed upon by us and the landlord. In addition to base rent, we are also required to pay a proportionate share of the landlord's annual operating costs. On May 20, 2008, in connection with our facility lease, we delivered to the landlord a security deposit of approximately $0.5 million in the form of an irrevocable letter of credit. The cash securing this letter of credit is classified on our balance sheet as a long-term asset and is restricted in its use.
Our manufacturing and quality operations were located in a building we own comprised of approximately 25,000 square feet located at 61 Mooney Street, Cambridge, Massachusetts. In the third quarter of 2012, we ceased our manufacturing operations at our Cambridge, Massachusetts facility and
55
moved to a fully outsourced manufacturing supply chain and intend to sell the land and building in the near future. Employees who manage the contract manufacturers and quality operations were moved to our headquarters in Lexington, Massachusetts.
We accrue a liability for legal contingencies when we believe that it is both probable that a liability has been incurred and that we can reasonably estimate the amount of the loss. We review these accruals and adjust them to reflect ongoing negotiations, settlements, rulings, advice of legal counsel and other relevant information. To the extent new information is obtained and our views on the probable outcomes of claims, suits, assessments, investigations or legal proceedings change, changes in our accrued liabilities would be recorded in the period in which such determination is made. For the matters referenced below, the liability is not probable or the amount cannot be reasonably estimated and, therefore, accruals have not been made. In addition, in accordance with the relevant authoritative guidance, for any matters in which the likelihood of material loss is at least reasonably possible, we will provide disclosure of the possible loss or range of loss. If a reasonable estimate cannot be made, however, we will provide disclosure to that effect.
A purported class action complaint was originally filed on March 18, 2010 in the United States District Court for the District of Massachusetts, entitled Silverstrand Investments et. al. v. AMAG Pharm., Inc., et. al., Civil Action No. 1:10-CV-10470-NMG, and was amended on September 15, 2010 and on December 17, 2010. The second amended complaint, or SAC, filed on December 17, 2010 alleged that we and our former President and Chief Executive Officer, former Executive Vice President and Chief Financial Officer, the then members of our Board of Directors, and certain underwriters in our January 2010 offering of common stock violated certain federal securities laws, specifically Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended, and that our former President and Chief Executive Officer and former Executive Vice President and Chief Financial Officer violated Section 15 of such Act, respectively, by making certain alleged false and misleading statements and omissions in a registration statement filed in January 2010. The plaintiff sought unspecified damages on behalf of a purported class of purchasers of our common stock pursuant to our common stock offering on or about January 21, 2010. On August 11, 2011, the District Court issued an Opinion and Order dismissing the SAC in its entirety for failure to state a claim upon which relief could be granted. A separate Order of Dismissal was filed on August 15, 2011. On September 14, 2011, the plaintiffs filed a Notice of Appeal to the United States Court of Appeals for the First Circuit, or the Court of Appeals. After briefing was completed by all parties, the Court of Appeals heard oral argument on May 11, 2012, and took the matter under advisement. On February 4, 2013, the Court of Appeals affirmed in part and reversed in part the District Court's Opinion and Order, and remanded the case to the District Court. On February 18, 2013, we filed a Petition for Panel Hearing or RehearingEn Banc, asking the Court of Appeals to reconsider its decision. We are currently unable to predict the outcome or reasonably estimate the range of potential loss associated with this matter, if any, and have therefore not recorded any potential estimated liability as we do not believe that such a liability is probable nor do we believe that a range of loss is currently estimable.
In July 2010, Sandoz GmbH, or Sandoz, filed with the European Patent Office, or the EPO, an opposition to our previously issued patent which covers ferumoxytol in the EU. In October 2012, at an oral hearing, the Opposition Division of the EPO revoked our European ferumoxytol patent. In December 2012, our notice of appeal was recorded with the EPO, which suspends the revocation of our patent. We will continue to defend the validity of this patent throughout the appeals process, which we expect to take two to three years. However, in the event that we do not experience a successful outcome from the appeals process, under EU regulations ferumoxytol would still be entitled to eight years of data protection and ten years of market exclusivity from the date of approval, which we believe would create barriers to entry for any generic version of ferumoxytol into the EU market until
56
sometime between 2020 and 2022. This decision had no impact on our revenues for the year ended December 31, 2012. However, any future unfavorable outcome in this matter could negatively affect the magnitude and timing of future revenues, including royalties and milestone payments we may receive from Takeda pursuant to our collaboration agreement with Takeda. We continue to believe the patent is valid and intend to vigorously appeal the decision.
We may periodically become subject to legal proceedings and claims arising in connection with ongoing business activities, including claims or disputes related to patents that have been issued or that are pending in the field of research on which we are focused. Other than the above actions, we are not aware of any material claims against us as of December 31, 2012.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
57
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES:
Market Information
Our common stock trades on the NASDAQ Global Select Market, or NASDAQ, under the trading symbol "AMAG." On February 15, 2013, the closing price of our common stock, as reported on the NASDAQ, was $16.91 per share. The following table sets forth, for the periods indicated, the high and low sale prices per share for our common stock as reported on the NASDAQ.
| | High | Low | |||||
|---|---|---|---|---|---|---|---|
Year Ended December 31, 2012 | |||||||
First quarter | $ | 19.24 | $ | 14.98 | |||
Second quarter | $ | 16.45 | $ | 12.43 | |||
Third quarter | $ | 17.95 | $ | 14.11 | |||
Fourth quarter | $ | 18.50 | $ | 13.85 | |||
Year Ended December 31, 2011 | |||||||
First quarter | $ | 19.47 | $ | 15.93 | |||
Second quarter | $ | 19.40 | $ | 15.18 | |||
Third quarter | $ | 19.48 | $ | 12.65 | |||
Fourth quarter | $ | 19.62 | $ | 13.05 | |||
Stockholders
On February 15, 2013, we had approximately 100 stockholders of record of our common stock, and we believe that the number of beneficial holders of our common stock was approximately 23,000 based on responses from brokers to a search conducted by Broadridge Financial Solutions, Inc. on our behalf.
Dividends
We have never declared or paid a cash dividend on our common stock. We currently anticipate that we will retain all of our earnings for use in the development of our business and do not anticipate paying any cash dividends in the foreseeable future.
58
Repurchases of Equity Securities
The following table provides certain information with respect to our purchases of shares of our stock during the fourth quarter of 2012.
Period | Total Number of Shares Purchased(1) | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs(2) | Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs(2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
October 1, 2012 through October 31, 2012 | — | — | — | — | |||||||||
November 1, 2012 through November 30, 2012 | 628 | $ | 14.34 | — | — | ||||||||
December 1, 2012 through December 31, 2012 | 2,426 | $ | 15.03 | — | — | ||||||||
Total | 3,054 | $ | 14.89 | — | — | ||||||||
- (1)
- Represents shares of our common stock withheld by us to satisfy the minimum tax withholding obligations in connection with the vesting of restricted stock units held by our employees.
- (2)
- We do not currently have any publicly announced repurchase programs or plans.
Securities Authorized for Issuance Under Equity Compensation Plans
See Part III, Item 12 for information regarding securities authorized for issuance under our equity compensation plans. Such information is incorporated by reference to our definitive proxy statement pursuant to Regulation 14A, to be filed with the U.S. Securities and Exchange Commission, or the SEC, not later than 120 days after the close of our year ended December 31, 2012.
Five-Year Comparative Stock Performance Graph
The following graph compares the yearly percentage change in the cumulative total stockholder return on our common stock with the cumulative total return on the NASDAQ Global Market Composite Index and NASDAQ Biotechnology Index over the past five years. The comparisons assume
59
$100 was invested on December 31, 2007 in our common stock, in the NASDAQ Global Market and the NASDAQ Biotechnology Index, and assumes reinvestment of dividends, if any.
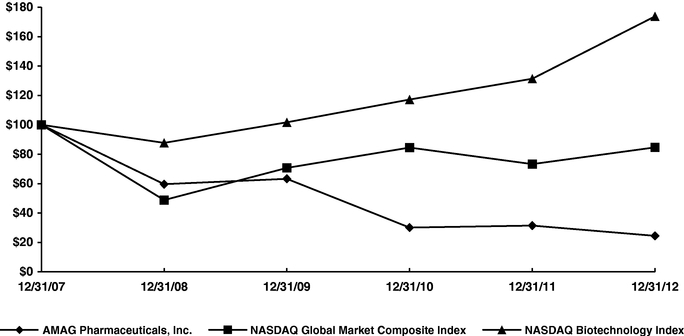
| | 12/31/2007 | 12/31/2008 | 12/31/2009 | 12/31/2010 | 12/31/2011 | 12/31/2012 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
AMAG Pharmaceuticals, Inc. | 100.00 | 59.62 | 63.25 | 30.10 | 31.45 | 24.46 | |||||||||||||
NASDAQ Global Market Composite Index | 100.00 | 48.80 | 70.67 | 84.51 | 73.26 | 84.64 | |||||||||||||
NASDAQ Biotechnology Index | 100.00 | 87.70 | 101.70 | 117.18 | 131.34 | 173.75 | |||||||||||||
The stock price performance shown in this performance graph is not necessarily indicative of future price performance. Information used in the graph was obtained from Zach's Investment Research, Inc., a source we believe is reliable. However, we are not responsible for any errors or omissions in such information.
The material in this section is being furnished and shall not be deemed "filed" with the SEC for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall the material in this section be deemed to be incorporated by reference in any registration statement or other document filed with the SEC under the Securities Act of 1933, except to the extent we specifically and expressly incorporate it by reference into such filing.
60
ITEM 6. SELECTED FINANCIAL DATA:
The following table sets forth selected financial data as of and for the years ended December 31, 2012, 2011, 2010, 2009 and 2008. The selected financial data set forth below has been derived from our audited financial statements. This information should be read in conjunction with the financial statements and the related notes thereto included in Part II, Item 8 of this Annual Report on Form 10-K and Management's Discussion and Analysis of Financial Condition and Results of Operations included in Part II, Item 7 of this Annual Report on Form 10-K.
| | Years Ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||
| | (in thousands, except per share data) | |||||||||||||||
Statement of Operations Data | ||||||||||||||||
Revenues: | ||||||||||||||||
U.S. product sales, net | $ | 58,287 | $ | 52,097 | $ | 59,339 | $ | 15,774 | $ | — | ||||||
International product sales and royalties | 120 | — | — | — | — | |||||||||||
License fee and other collaboration revenues | 26,475 | 8,321 | 6,132 | 516 | 959 | |||||||||||
Other product sales and royalties | 496 | 831 | 774 | 888 | 979 | |||||||||||
Total revenues | 85,378 | 61,249 | 66,245 | 17,178 | 1,938 | |||||||||||
Costs and expenses: | ||||||||||||||||
Cost of product sales | 14,220 | 10,531 | 7,606 | 1,013 | 292 | |||||||||||
Research and development expenses | 33,296 | 58,140 | 54,462 | 36,273 | 31,716 | |||||||||||
Selling, general and administrative expenses | 53,071 | 68,863 | 84,939 | 77,829 | 49,536 | |||||||||||
Restructuring expenses | 2,215 | 3,508 | 2,224 | — | — | |||||||||||
Total costs and expenses | 102,802 | 141,042 | 149,231 | 115,115 | 81,544 | |||||||||||
Other income (expense): | ||||||||||||||||
Interest and dividend income, net | 1,286 | 1,747 | 1,741 | 3,154 | 9,139 | |||||||||||
(Losses) gains on investments, net | (1,466 | ) | (193 | ) | 408 | 942 | (3,024 | ) | ||||||||
Fair value adjustment of settlement rights | — | — | (788 | ) | (778 | ) | 1,566 | |||||||||
Total other income (expense) | (180 | ) | 1,554 | 1,361 | 3,318 | 7,681 | ||||||||||
Net loss before income taxes | (17,604 | ) | (78,239 | ) | (81,625 | ) | (94,619 | ) | (71,925 | ) | ||||||
Income tax benefit | 854 | 1,170 | 472 | 1,268 | 278 | |||||||||||
Net loss | $ | (16,750 | ) | $ | (77,069 | ) | $ | (81,153 | ) | $ | (93,351 | ) | $ | (71,647 | ) | |
Net loss per share—basic and diluted: | $ | (0.78 | ) | $ | (3.64 | ) | $ | (3.90 | ) | $ | (5.46 | ) | $ | (4.22 | ) | |
Weighted average shares outstanding used to compute net loss per share: | ||||||||||||||||
Basic and diluted | 21,392 | 21,189 | 20,806 | 17,109 | 16,993 | |||||||||||
| | December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||
| | (in thousands) | |||||||||||||||
Balance Sheet Data | ||||||||||||||||
Working capital (current assets less current liabilities) | $ | 221,423 | $ | 201,037 | $ | 254,073 | $ | 85,168 | $ | 149,918 | ||||||
Total assets | $ | 258,137 | $ | 267,224 | $ | 336,076 | $ | 184,619 | $ | 231,955 | ||||||
Long-term liabilities | $ | 52,383 | $ | 47,634 | $ | 54,079 | $ | 4,081 | $ | 4,149 | ||||||
Stockholders' equity | $ | 172,797 | $ | 180,596 | $ | 245,286 | $ | 142,977 | $ | 213,414 | ||||||
61
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS:
Overview
AMAG Pharmaceuticals, Inc., a Delaware corporation, was founded in 1981. We are a specialty pharmaceutical company focused on the development and commercialization of Feraheme® (ferumoxytol) Injection for Intravenous, or IV, use to treat iron deficiency anemia, or IDA. Currently, our principal source of revenue is from the sale ofFeraheme, which was approved for marketing in the U.S. in June 2009 by the U.S. Food and Drug Administration, or the FDA, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with chronic kidney disease, or CKD. We began commercial sale ofFeraheme in the U.S. in July 2009 through our own commercial organization, including a specialty sales force. We sellFeraheme to authorized wholesalers and specialty distributors, who in turn, sellFeraheme to healthcare providers who administerFeraheme primarily within hospitals, hematology and oncology centers, and nephrology clinics.
We are working to continue to growFeraheme in the U.S. CKD market and to drive additional growth ofFeraheme through both international and label expansion. To further build our business, we intend to expand our portfolio through the in-license or purchase of additional marketed specialty pharmaceutical products. We are seeking complementary products that will leverage our commercial infrastructure and focus on hematology and oncology centers, hospital infusion centers or other sites of care where IV iron is administered or where IDA patients are diagnosed or treated. We are also looking at the potential addition of products outside of our current sales force's call points, which could be synergistic with ourFeraheme goal of expanding the IV iron market through increased referrals from certain physician specialties, such as gastroenterologists.
International Expansion
Outside of the U.S., ferumoxytol has been granted marketing approval in Canada, Switzerland and the European Union, or EU, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. The European marketing authorization is valid in the current EU member states as well as in Iceland and Norway. Under our amended agreement with Takeda Pharmaceutical Company Limited, or Takeda, Takeda has an exclusive license to market and sell ferumoxytol in Canada, the EU and Switzerland, as well as certain other geographic territories. In Canada, Takeda promotes ferumoxytol under the trade nameFeraheme and in the EU and Switzerland, Takeda promotes ferumoxytol under the trade name Rienso® 30mg/ml solution for Injection.
Label Expansion
We believe that a significant opportunity exists in the U.S. forFeraheme beyond the treatment of IDA in adult patients with CKD. In the U.S. in 2012, approximately 800,000 grams of IV iron were administered for the treatment of non-dialysis patients with IDA. We believe that approximately half, or 400,000 grams, of the IV iron administered in the U.S. is for the treatment of non-dialysis patients with CKD and the other half is for non-CKD patients with IDA due to other causes, including patients with gastrointestinal diseases or disorders, abnormal uterine bleeding, inflammatory diseases, and chemotherapy-induced anemia.
In 2012, we completed a phase III clinical program forFeraheme in patients with IDA who had failed to or could not use oral iron. The IDA program consisted of two controlled, multi-center phase III clinical trials, or IDA-301 and IDA-302, including more than 1,400 patients, which evaluated the safety and efficacy of ferumoxytol for the treatment of IDA in this broader patient population. Both studies met the primary efficacy endpoints related to improvements in hemoglobin. In these studies no new safety signals were observed withFeraheme treatment and the types of reported adverse events were consistent with those seen in previous studies and those contained in the approved U.S.
62
package insert forFeraheme. In addition, patients from IDA-301 were eligible to enroll in an open-label extension study, or IDA-303, and receive treatment withFeraheme, as defined in the protocol.
In December 2012, we submitted a supplemental new drug application, or sNDA, to the FDA, seeking approval forFeraheme for the treatment of IDA in adult patients who have failed to or could not use oral iron. The sNDA submission was primarily based on the data from IDA-301 and IDA-302. In addition, the sNDA included data from an interim analysis of IDA-303 and a previously completed post-approval clinical study evaluatingFeraheme treatment compared to treatment with another IV iron. We believe that approval forFeraheme for this expanded indication would effectively double the market opportunity forFeraheme, by allowing us to access the half of the IV iron market outside of dialysis that is beyond our current approved indication. Assuming a standard review cycle, we expect a decision from the FDA on our sNDA sometime in the fourth quarter of 2013.
We expect that Takeda will file a Type II Variation, which is the EU equivalent of a U.S. sNDA, with the European Medicines Agency, or EMA, in 2013 seeking marketing approval forRienso for the treatment of IDA in adult patients.
Takeda Collaboration
In March 2010, we entered into a License, Development and Commercialization Agreement, or the Takeda Agreement, with Takeda under which we granted exclusive rights to Takeda to develop and commercializeFeraheme/Rienso as a therapeutic agent in Europe, certain Asia-Pacific countries (excluding Japan, China and Taiwan), the Commonwealth of Independent States, Canada, India and Turkey. In June 2012, we entered into an amendment to the Takeda Agreement, or the Amended Takeda Agreement, which removed the Commonwealth of Independent States from the territories under which Takeda has the exclusive rights to develop and commercializeFeraheme/Rienso. In addition, the Amended Takeda Agreement modified the timing and pricing arrangements for a supply agreement to be entered into between us and Takeda in the future, the terms related to primary and secondary manufacturing for drug substance and drug product, certain patent related provisions, and the re-allocation of certain of the agreed-upon milestone payments. In 2012, we received a total of $33.0 million in milestone payments from Takeda associated with the EU approval and the commercial launches ofFeraheme/Rienso in Canada and the EU. In addition, in connection with the commercial launches ofFeraheme/Rienso by Takeda, we recorded revenue from product sales to Takeda and royalties on sales by Takeda of $0.1 million in 2012.
Clinical Development of Feraheme
We have initiated two randomized, active-controlled pediatric studies ofFeraheme for the treatment of IDA in pediatric CKD patients to meet our FDA post-approval Pediatric Research Equity Act requirement to support pediatric labeling ofFeraheme in the U.S. One study covers dialysis-dependent CKD pediatric patients, and the other covers CKD patients not on dialysis. Each study will assess the safety and efficacy ofFeraheme treatment as compared to oral iron in approximately 144 pediatric patients. Both of these pediatric studies are currently open for enrollment with enrollment expected to take several years to complete.
Our pediatric investigation plan, which was a requirement for submission of the Marketing Authorization Application, or MAA, for ferumoxytol, was approved by the EMA in December 2009 and amended in 2012, and includes the two pediatric studies needed to meet the requirements of the Pediatric Research Equity Act in the U.S. described above, and two additional pediatric studies requested by the EMA. These studies include a rollover study in pediatric CKD patients and a study in pediatric patients with IDA regardless of the underlying cause. The rollover study is open for enrollment. The pediatric IDA study will commence once the appropriate dose ofFeraheme is determined from the study data resulting from the two ongoing pediatric studies ofFeraheme for the
63
treatment of IDA in pediatric CKD patients, described above. The amendment to our pediatric investigation plan in 2012 was intended to increase the rate of enrollment for these studies through modifications to the patient entry criteria.
As part of our obligations under the Amended Takeda Agreement and as part of our post-approval commitments to the EMA, we are planning to initiate a multi-center clinical trial to determine the safety and efficacy of repeat doses of ferumoxytol for the treatment of IDA in patients with hemodialysis dependent CKD. As part of the post-approval commitment we made to the EMA as a condition of the approval of the MAA for ferumoxytol in the EU this study includes a treatment arm with iron sucrose as well as a magnetic resonance imaging, or MRI, study which will evaluate the potential for iron to accumulate in the body following treatment with IV iron, specifically in the heart and liver, and, where possible, other major organs following repeated IV iron administration over a two year period. We currently expect enrollment to begin in the second quarter of 2013. The costs related to the MRI portion of this study are subject to our established cost sharing arrangement with Takeda.
From time to time, we or our licensees may sponsor pilot clinical studies or collaborate with investigators on their research ideas to evaluate the safety and efficacy ofFeraheme in new indications or alternative dosing regimens.
In addition, certain clinical trials may be necessary to secure desired pricing in various European markets. If so, the cost of any future trials may be allocated between us and Takeda according to the Amended Takeda Agreement.
In December 2009, our licensee in China, 3SBio Inc., or 3SBio, filed an application with the Chinese State Food and Drug Administration, or the SFDA, to obtain approval to begin a clinical trial necessary to file for marketing approval ofFeraheme in China. If approved by the SFDA, 3SBio plans to commence a multi-center randomized efficacy and safety study ofFeraheme in China involving approximately 200 CKD patients with IDA.
Critical Accounting Policies
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make certain estimates and assumptions that affect the reported amount of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. The most significant estimates and assumptions are used in, but are not limited to, revenue recognition related to product sales and collaboration agreements, product sales allowances and accruals, assessing investments for potential other-than-temporary impairment and determining values of investments, estimates used to measure the fair value of our held for sale assets, accrued expenses, income taxes and equity-based compensation expense. Actual results could differ materially from those estimates. In making these estimates and assumptions, management employs critical accounting policies. Our critical accounting policies include revenue recognition and related sales allowances and accruals, valuation of investments and equity-based compensation.
1. Revenue Recognition and Related Sales Allowances and Accruals
We recognize revenue from the sale ofFeraheme/Rienso as well as license fee and other collaboration revenues, including milestone payments, other product sale revenues, and royalties we receive from our licensees. We recognize revenue in accordance with current accounting guidance related to the recognition, presentation and disclosure of revenue in financial statements, which
64
outlines the basic criteria that must be met to recognize revenue and provides guidance for disclosure of revenue in financial statements. We recognize revenue when:
- •
- Persuasive evidence of an arrangement exists;
- •
- Delivery of product has occurred or services have been rendered;
- •
- The sales price charged is fixed or determinable; and
- •
- Collection is reasonably assured.
U.S. Product Sales, Net
We record product sales allowances and accruals related to prompt payment discounts, chargebacks, government and other rebates, distributor, wholesaler and group purchasing organization, or GPO, fees, and product returns as a reduction of revenue in our consolidated statement of operations at the time product sales are recorded. Calculating these gross-to-net sales adjustments involves estimates and judgments based primarily on actualFeraheme sales data, forecasted customer buying patterns and market research data related to utilization rates by various end-users. In addition, we also monitor our distribution channel to determine whether additional allowances or accruals are required based on inventory in our sales channel.
Classification of U.S. Product Sales Allowances and Accruals
Product sales allowances and accruals are primarily comprised of both direct and indirect fees, discounts and rebates, and provisions for estimated product returns. Direct fees, discounts and rebates are contractual fees and price adjustments payable to wholesalers, specialty distributors and other customers that purchase products directly from us. Indirect fees, discounts and rebates are contractual price adjustments payable to healthcare providers and organizations, such as certain physicians, clinics, hospitals, GPOs, and dialysis organizations that typically do not purchase products directly from us but rather from wholesalers and specialty distributors. In accordance with guidance related to accounting for fees and consideration given by a vendor to a customer, including a reseller of a vendor's products, these fees, discounts and rebates are presumed to be a reduction of the selling price ofFeraheme. Product sales allowances and accruals are based on definitive contractual agreements or legal requirements (such as Medicaid laws and regulations) related to the purchase and/or utilization of the product by these entities and are recorded in the same period that the related revenue is recognized. We estimate product sales allowances and accruals using either historical, actual and/or other data, including estimated patient usage, applicable contractual rebate rates, contract performance by the benefit providers, other current contractual and statutory requirements, historical market data based upon experience ofFeraheme and other products similar toFeraheme, specific known market events and trends such as competitive pricing and new product introductions, current and forecasted customer buying patterns and inventory levels, and the shelf life ofFeraheme. As part of this evaluation, we also review changes to federal and other legislation, changes to rebate contracts, changes in the level of discounts, and changes in product sales trends. Although allowances and accruals are recorded at the time of product sale, certain rebates are typically paid out, on average, up to six months or longer after the sale.
Allowances against receivable balances primarily relate to prompt payment discounts, provider chargebacks and certain government agency rebates and are recorded at the time of sale, resulting in a reduction in product sales revenue and the reporting of product sales receivables net of allowances. Accruals related to Medicaid and provider volume rebates, wholesaler and distributor fees, GPO fees, other discounts to healthcare providers and product returns are recorded at the time of sale, resulting in a reduction in product sales revenue and the recording of an increase in accrued expenses.
65
Discounts
We typically offer a 2% prompt payment discount to our customers as an incentive to remit payment in accordance with the stated terms of the invoice, generally thirty days. Because we anticipate that those customers who are offered this discount will take advantage of the discount, we accrue 100% of the prompt payment discount, at the time of sale, based on the gross amount of each invoice. We adjust the accrual quarterly to reflect actual experience.
Chargebacks
Chargeback reserves represent our estimated obligations resulting from the difference between the prices at which we sellFeraheme to wholesalers and the sales price ultimately paid to wholesalers under fixed price contracts by third-party payors, including governmental agencies. We determine our chargeback estimates based on actualFeraheme sales data and forecasted customer buying patterns. Actual chargeback amounts are determined at the time of resale to the qualified healthcare provider, and we generally issue credits for such amounts within several weeks of receiving notification from the wholesaler. Estimated chargeback amounts are recorded at the time of sale, and we adjust the allowance quarterly to reflect actual experience.
Government and Other Rebates
Government and other rebate reserves relate to our reimbursement arrangements with state Medicaid programs or performance rebate agreements with certain classes of trade. We determine our estimates for Medicaid rebates based on actualFeraheme sales data, forecasted customer buying patterns and market research data related to utilization rates by various end-users blended with historical experience of products similar toFeraheme sold by others. We currently have limited actual claims payment data, and therefore are not able to solely rely on our actualFeraheme claims experience. In estimating these reserves, we provide for a Medicaid rebate associated with both those expected instances where Medicaid will act as the primary insurer as well as in those instances where we expect Medicaid will act as the secondary insurer. For rebates associated with reaching defined performance goals, we determine our estimates using actualFeraheme sales data and forecasted customer buying patterns. Rebate amounts generally are invoiced quarterly and are paid in arrears, and we expect to pay such amounts within several weeks of notification by the Medicaid or provider entity. Estimated government and other rebates are recorded at the time of sale and, with the exception of Medicaid as discussed below, we adjust the accrual quarterly to reflect actual experience.
During 2012, we revised our estimated Medicaid utilization rate based on actual rebate claims received since the 2009 launch ofFeraheme, our expectations of state level activity, and estimated rebate claims not yet submitted, which resulted in a $0.6 million reduction of our estimated Medicaid rebate reserve related to prior periodFeraheme sales. This change in estimate was reflected as an increase in our net product sales in 2012. As a result, our gross to net percentage for 2012 was slightly lower than it otherwise would have been had we not reduced our Medicaid rebate reserve. We regularly assess our Medicaid reserve balance and the rate at which we accrue for claims against product sales. If we determine in future periods that our actual rebate experience is not indicative of expected claims or if other factors affect estimated claims rates, we may be required to change our estimated Medicaid reserve and/or the current rate at which we estimate our Medicaid claims, which would affect our earnings in the period of the change in estimate and such change could be significant. A 1.0% increase in our estimate of our Medicaid utilization rate for the year ended December 31, 2012 would have resulted in approximately a $0.2 million decrease in net product sales.
66
Distributor/Wholesaler and Group Purchasing Organization Fees
Fees under our arrangements with distributors and wholesalers are usually based upon units ofFeraheme purchased during the prior month or quarter and are usually paid by us within several weeks of our receipt of an invoice from the wholesaler or distributor, as the case may be. Fees under our arrangements with GPOs are usually based upon member purchases during the prior quarter and are generally billed by the GPO within 30 days after period end. Current accounting standards related to consideration given by a vendor to a customer, including a reseller of a vendor's products, specify that cash consideration given by a vendor to a customer is presumed to be a reduction of the selling price of the vendor's products or services and therefore should be characterized as a reduction of revenue. Consideration should be characterized as a cost incurred if we receive, or will receive, an identifiable benefit (goods or services) in exchange for the consideration and we can reasonably estimate the fair value of the benefit received. Because the fees we pay to wholesalers do not meet the foregoing conditions to be characterized as a cost, we have characterized these fees as a reduction of revenue. We generally pay such amounts within several weeks of our receipt of an invoice from the distributor, wholesaler, or GPO. Accordingly, we accrue the estimated fee due at the time of sale, based on the contracted price invoiced to the customer. We adjust the accrual quarterly to reflect actual experience.
Product Returns
Consistent with industry practice, we generally offer our distributors and wholesaler customers a limited right to return product purchased directly from us principally based upon the product's expiration date which, once packaged, is currently four or five years in the U.S. We estimate product returns based upon historical experience since the 2009 launch ofFeraheme and trends of products similar toFeraheme sold by others. We currently have limited actual returns data, and therefore are not able to solely rely on our actual returns experience. We track actual returns by individual production lots. Returns on lots eligible for credits under our returned goods policy are monitored and compared with historical return trends and rates.
We consider several additional factors in our product return estimation process, including our internal sales forecasts and inventory levels in the distribution channel. We expect that wholesalers and healthcare providers will not stock significant inventory due toFeraheme's cost and expense to store. Based on the level of inventory in the wholesale distribution channel, we determine whether an adjustment to the sales return reserve is appropriate.
We record an estimate of returns at the time of sale. If necessary, our estimated rate of returns may be adjusted for actual return experience as it becomes available and for known or expected changes in the marketplace. During 2012, we reduced our reserve for product returns by approximately $2.2 million, primarily as a result of a lower than expected rate of product returns as well as the lapse of the product return period on certain manufacturedFeraheme lots that carried a two year expiration. As a result, the product returns provision applied to gross product sales for the year ended December 31, 2012 was a credit of $1.5 million, resulting in an increase to net product sales for the year. The reduction of our estimated product returns reserve had a positive impact of $0.10 per basic and diluted share for year ended December 31, 2012. We did not significantly adjust our reserve for product returns during 2011 or 2010.Feraheme is still early in its product lifecycle and returns experience may change over time. A future revision to our product returns estimate would result in a corresponding change to our net product sales in the period in which the change is made and could be significant. A 1.0% increase in our returns as a percentage of gross sales for the year ended December 31, 2012 would have resulted in approximately a $0.8 million decrease in net product sales.
67
International Product Sales and Royalties
We record all international product sales and royalties forFeraheme/Rienso sold to Takeda in deferred revenues in our consolidated balance sheet. We recognize these deferred revenues, and the associated cost of product sales, in our consolidated statement of operations at the time Takeda reports to us that sales have been made to its customers.
Milestone Payments under Multiple Element Arrangements
From time to time, we may enter into collaborative license and development agreements with biotechnology and pharmaceutical companies for the development and commercialization of our products or product candidates. The terms of the agreements may include non-refundable license fees, payments based on the achievement of certain milestones and performance goals, reimbursement of certain out-of-pocket costs, payments for manufacturing services, and royalties on product sales.
We evaluate revenue from arrangements that have multiple elements to determine whether the components of the arrangement represent separate units of accounting as defined in the accounting guidance related to revenue arrangements with multiple deliverables. Under current accounting guidance, which governs any agreements that contain multiple elements that are either entered into or materially modified subsequent to January 1, 2011, companies are required to establish the fair value of undelivered products and services based on a separate revenue recognition process using management's best estimate of the selling price for an undelivered item when there is no other means to determine the fair value of that undelivered item. Agreements entered into prior to January 1, 2011, that have not been materially modified, including our agreements with Takeda and 3SBio, are accounted for under previous accounting guidance, which provides that an element of a contract can be accounted for separately if the delivered elements have standalone value and the fair value of all undelivered elements is determinable. If an element is considered to have standalone value but the fair value of any of the undelivered items cannot be determined, all elements of the arrangement are recognized as revenue as a single unit of accounting over the period of performance for such undelivered items or services. Significant management judgment is required in determining what elements constitute deliverables and what deliverables or combination of deliverables should be considered units of accounting.
When multiple deliverables are combined and accounted for as a single unit of accounting, we base our revenue recognition pattern on the last to be delivered element. Revenue is recognized using either a proportional performance or straight-line method, depending on whether we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and whether such performance obligations are provided on a best-efforts basis. To the extent we cannot reasonably estimate our performance obligations, we recognize revenue on a straight-line basis over the period we expect to complete our performance obligations. Significant management judgment is required in determining the level of effort required under an arrangement and the period over which we are expected to complete our performance obligations under an arrangement. We may have to revise our estimates based on changes in the expected level of effort or the period we expect to complete our performance obligations.
Our collaboration agreements may entitle us to additional payments upon the achievement of performance-based milestones. If a milestone involves substantive effort on our part and its achievement is not considered probable at the inception of the collaboration, we recognize the milestone consideration as revenue in the period in which the milestone is achieved only if it meets the following additional criteria: (1) the milestone consideration received is commensurate with either the level of effort required to achieve the milestone or the enhancement of the value of the item delivered as a result of a specific outcome resulting from our performance to achieve the milestone; (2) the milestone is related solely to past performance; and (3) the milestone consideration is reasonable
68
relative to all deliverables and payment terms in the arrangement. There is significant judgment involved in determining whether a milestone meets all of these criteria. For milestones that do not meet the above criteria and are therefore not considered substantive milestones, we recognize that portion of the milestone payment equal to the percentage of the performance period completed at the time the milestone is achieved and the above conditions are met. The remaining portion of the milestone will be recognized over the remaining performance period using a proportional performance or straight-line method.
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in our consolidated balance sheets. Amounts not expected to be recognized within the next 12 months are classified as long-term deferred revenue.
Takeda Agreement
In March 2010, we entered into the Takeda Agreement which, as discussed above, was amended in June 2012 to, among other things, modify the timing and pricing arrangements for a supply agreement to be entered into between us and Takeda in the future, the terms related to primary and secondary manufacturing for drug substance and drug product, certain patent related provisions, and the re-allocation of certain of the agreed-upon milestone payments. We analyzed the Amended Takeda Agreement and determined that the amended terms did not result in a material modification of the original Takeda Agreement based on the fact that there were no changes to the deliverables under the original Takeda Agreement as a result of the amendment, and the change in arrangement consideration as a result of the amendment was not quantitatively material in relation to the total arrangement consideration.
Under the Amended Takeda Agreement, except under limited circumstances, we have retained the right to manufactureFeraheme/Rienso and, accordingly, are responsible for supply ofFeraheme/Rienso to Takeda at a fixed price per unit, which is capped for a certain period of time. We are also responsible for conducting, and bearing the costs related to, certain pre-defined clinical studies with the costs of future modifications or additional studies to be allocated between the parties according to an agreed-upon cost-sharing mechanism. We have determined that our obligations under the Amended Takeda Agreement have not changed from those under the original Takeda Agreement and include the following four deliverables: the license, access to future know-how and improvements to theFeraheme/Rienso technology, regulatory and clinical research activities, and the manufacturing and supply of product. Pursuant to the accounting guidance in effect in 2010, when we signed the original Takeda Agreement and which governed revenue recognition on multiple element arrangements, we evaluated the four deliverables under the original Takeda Agreement and determined that our obligation to provide manufacturing supply of product meets the criteria for separation and is therefore treated as a single unit of accounting, which we refer to as the supply unit of accounting. Further, we concluded that the license is not separable from the undelivered future know-how and technological improvements or the undelivered regulatory and clinical research activities. Accordingly, these deliverables are being combined and also treated as a single unit of accounting, which we refer to as the combined unit of accounting.
With respect to the combined unit of accounting, our obligation to provide access to our future know-how and technological improvements is the final deliverable and is an obligation which exists throughout the term of the Amended Takeda Agreement. In connection with the execution of the original Takeda Agreement, we received a $60.0 million upfront payment from Takeda in April 2010, which we recorded as deferred revenue, as well as approximately $1.0 million reimbursed to us during 2010 for certain expenses incurred prior to entering the agreement, which we considered an additional upfront payment. Because we cannot reasonably estimate the total level of effort required to complete the obligations under the combined deliverable, we are recognizing the entire $60.0 million upfront payment, the $1.0 million reimbursed to us in 2010, as well as any non-substantive milestone payments
69
that are achieved into revenues on a straight-line basis over a period of ten years from March 31, 2010, the date on which we originally entered the Takeda Agreement, which represented the then current patent life ofFeraheme/Rienso and our best estimate of the period over which we will substantively perform our obligations. We continue to believe that the then current patent life ofFeraheme/Rienso is our best estimate of the period over which we will substantively perform our obligations under this agreement. Any potential non-substantive milestone payments that may be received in the future will be recognized as revenue on a cumulative catch up basis when they become due and payable.
Under the terms of the Amended Takeda Agreement, Takeda is responsible for reimbursing us for certain out-of-pocket regulatory and clinical trial supply costs associated with carrying out our regulatory and clinical research activities under the collaboration agreement. Because we are acting as the principal in carrying out these services, any reimbursement payments received from Takeda will be recorded in license fee and other collaboration revenues in our consolidated statement of operations to match the costs that we incur during the period in which we perform those services.
2. Valuation of investments
We generally invest in corporate debt securities, U.S. treasury and government agency securities, commercial paper and, in 2011, auction rate securities, or ARS. All of our investments are classified as "available-for-sale" and are recorded at their estimated fair value. The fair value of our investments is generally determined from quoted market prices received from independent pricing services based upon market transactions. Independent pricing services normally derive security prices from recently reported trades for identical or similar securities, making adjustments based upon other significant observable market transactions. At the end of each reporting period, we perform quantitative and qualitative analyses of prices received from third parties to determine whether prices are reasonable estimates of fair value.
We also analyze when the volume and level of activity for an asset or liability have significantly decreased and when circumstances indicate that a transaction may not be considered orderly. In order to determine whether the volume and level of activity for an asset or liability have significantly decreased, we assess current activity as compared to normal market activity for the asset or liability. We rely on many factors such as trading volume, trading frequency, the levels at which market participants indicate their willingness to buy and sell our securities, as reported by market participants, and current market conditions. Using professional judgment and experience, we evaluate and weigh the relevance and significance of all applicable factors to determine if there has been a significant decrease in the volume and level of activity for an asset, group of similar assets or liabilities. Similarly, in order to identify transactions that are not orderly, we take into consideration the activity in the market which can influence the determination and occurrence of an orderly transaction. Also, we inquire as to whether there may have been restrictions on the marketing of the security to a single or limited number of participants. Where possible, we assess the financial condition of the seller to determine whether observed transactions may have been forced. If there is a significant disparity between the trading price for a security held by us as compared to the trading prices of similar recent transactions, we consider whether this disparity is an indicator of a disorderly trade. Using professional judgment and experience, we evaluate and weigh the relevance and significance of all applicable factors to determine if the evidence suggests that a transaction or group of similar transactions is not orderly. Based upon these procedures, we determined that market activity for our non-ARS assets appeared normal and that transactions did not appear disorderly as of December 31, 2012 and 2011.
In order to assess whether our investments in debt securities which experience a decline in fair value below amortized cost basis are other-than-temporarily impaired, we evaluate whether (i) we have the intent to sell the security or (ii) it is more likely than not that we will be required to sell the security prior to recovery of its amortized cost basis. If either of these conditions is met, we recognize the difference between the amortized cost of the security and its fair value at the impairment
70
measurement date in our consolidated statement of operations. If neither of these conditions is met, we must perform additional analyses to evaluate whether there could be a credit loss associated with the security. Factors we consider in making this judgment include, but are not limited to:
- •
- The extent to which the market value is less than the cost basis;
- •
- The length of time that the market value has been less than the cost basis;
- •
- Whether the unrealized loss is event-driven, credit-driven or a result of changes in market interest rates or risk premium;
- •
- The investment's rating and whether the investment is investment-grade and/or has been downgraded since its purchase;
- •
- Whether the issuer is current on all payments in accordance with the contractual terms of the investment and is expected to meet all of its obligations under the terms of the investment;
- •
- Any underlying collateral and the extent to which the recoverability of the carrying value of our investment may be affected by changes in such collateral;
- •
- Whether we have a favorable history in redeeming similar securities at prices at or above fair value;
- •
- Unfavorable changes in expected cash flows; and
- •
- Other subjective factors.
If we determine from this analysis that we do not expect to receive cash flows sufficient to recover the entire amortized cost of the security, a credit loss exists, and the impairment is considered other-than-temporary and is recognized in our consolidated statement of operations. Our assessment of whether unrealized losses are other-than-temporary requires significant judgment.
3. Equity-Based Compensation
Under the fair value recognition guidance of equity-based compensation accounting rules, equity-based compensation cost is generally required to be measured at the grant date (based upon an estimate of the fair value of the compensation granted) and recorded to expense over the requisite service period, which generally is the vesting period. Because equity-based compensation expense is based on awards ultimately expected to vest, we must make certain judgments about whether employees and directors will complete the requisite service period. Accordingly, we have reduced the compensation expense being recognized for estimated forfeitures. Under current accounting guidance, forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based upon historical experience, adjusted for unusual events such as the corporate restructurings in 2012, 2011 and 2010, which resulted in higher than expected turnover and forfeitures in those years. If factors change and we employ different assumptions in future periods, the compensation expense that we record in the future may differ significantly from what we have recorded in the current period.
We estimate the fair value of equity-based compensation involving stock options based on the Black-Scholes option pricing model. We estimate the fair value of our restricted stock units whose vesting is contingent upon market conditions using the Monte-Carlo simulation method. These models require the input of several factors such as the expected option term, the expected risk-free interest rate over the expected option term, the expected volatility of our stock price over the expected option term, and the expected dividend yield over the expected option term and are subject to various assumptions. The fair value of awards whose fair values are calculated using the Black-Scholes option pricing model is generally being amortized on a straight-line basis over the requisite service period and is recognized based on the proportionate amount of the requisite service period that has been rendered
71
during each reporting period. The fair value of awards with market conditions is being amortized based upon the estimated derived service period. We believe our valuation methodologies are appropriate for estimating the fair value of the equity awards we grant to our employees and directors. Our equity award valuations are estimates and thus may not be reflective of actual future results or amounts ultimately realized by recipients of these grants. These amounts, and the amounts applicable to future quarters, are also subject to future quarterly adjustments based upon a variety of factors, which include, but are not limited to, changes in estimated forfeiture rates and the issuance of new equity-based awards. The fair value of restricted stock units granted to our employees and directors is determined based upon the quoted closing market price per share on the date of grant, adjusted for estimated forfeitures. As with any accounting policy that applies judgments and estimates, actual results could significantly differ from those estimates or our estimates could change in the future.
Impact of Recently Issued and Proposed Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board, or FASB, issued amended guidance on the presentation of comprehensive income in financial statements. This amendment provides companies the option to present the components of net income and other comprehensive income either as one continuous statement of comprehensive income or as two separate but consecutive statements. This guidance eliminates the option to present components of other comprehensive income as part of the statement of changes in stockholders' equity. The provisions of this guidance became effective in 2012. We have adopted all provisions of this pronouncement by including other comprehensive income as part of our consolidated statements of comprehensive loss and such adoption did not have a significant impact on our consolidated financial statements.
In May 2011, the FASB issued an amendment to the accounting guidance for fair value measurements and related disclosures. This amendment clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable inputs, or Level 3 measurements. This guidance became effective for interim and annual periods beginning after December 15, 2011. We have adopted all provisions of this pronouncement and such adoption did not have a significant impact on our consolidated financial statements.
Results of Operations—Years Ended December 31, 2012, 2011 and 2010
Revenues
Our total revenues for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
U.S. product sales, net | $ | 58,287 | $ | 52,097 | $ | 59,339 | $ | 6,190 | 12 | % | $ | (7,242 | ) | -12 | % | |||||||
International product sales and royalties | 120 | — | — | 120 | N/A | — | — | |||||||||||||||
License fee and other collaboration revenues | 26,475 | 8,321 | 6,132 | 18,154 | >100 | % | 2,189 | 36 | % | |||||||||||||
Other product sales and royalties | 496 | 831 | 774 | (335 | ) | -40 | % | 57 | 7 | % | ||||||||||||
Total | $ | 85,378 | $ | 61,249 | $ | 66,245 | $ | 24,129 | 39 | % | $ | (4,996 | ) | -8 | % | |||||||
72
Our total revenues in 2012 increased by $24.1 million as compared to 2011, primarily as the result of a $6.2 million increase in U.S. net product sales and a $18.2 million increase in our license fee and other collaboration revenues associated with our collaboration agreement with Takeda, as described in further detail below.
The $5.0 million decrease in our total revenues in 2011 as compared to 2010 was primarily attributable to a $7.2 million decrease in U.S. net product sales, partially offset by a $2.2 million increase in our license fee and other collaboration revenues associated with our collaboration agreement with Takeda, as described in further detail below.
The following table sets forth customers who represented 10% or more of our total revenues for the years ended December 31, 2012, 2011 and 2010.
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
AmerisourceBergen Drug Corporation | 34 | % | 41 | % | 36 | % | ||||
Takeda Pharmaceuticals Company Limited | 31 | % | 13 | % | <10 | % | ||||
McKesson Corporation | 17 | % | 21 | % | <10 | % | ||||
Cardinal Health, Inc. | 12 | % | 13 | % | <10 | % | ||||
Metro Medical Supply, Inc. | <10 | % | <10 | % | 21 | % | ||||
U.S. Product Sales, Net
Net U.S. product sales for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
Feraheme | $ | 58,287 | $ | 52,097 | $ | 59,339 | $ | 6,190 | 12 | % | $ | (7,242 | ) | -12 | % | |||||||
Total | $ | 58,287 | $ | 52,097 | $ | 59,339 | $ | 6,190 | 12 | % | $ | (7,242 | ) | -12 | % | |||||||
Our total net U.S. product sales increased by $6.2 million, or 12%, during 2012 as compared to 2011 primarily as the result of an increase inFeraheme provider demand in 2012 and to a lesser extent, the impact of our 2012Feraheme price increases, and changes in our estimated reserves, described below. The $6.2 million increase in our net U.S. product sales was the result of a $15.5 million increase in our gross U.S. product sales in 2012 as compared to 2011, partially offset by higher allowances related to customer discounts and chargebacks in 2012. During 2012, we recorded allowances of $33.2 million as compared to $23.6 million in 2011. These allowances do not include the aggregate of changes in estimated Medicaid and product return reserves of $2.8 million and $2.5 million, as described below, that we recorded during the years ended December 31, 2012 and 2011, respectively.
During 2012 and 2011, we revised our estimated Medicaid reserve rate based on actual rebate claims received since the 2009 launch ofFeraheme, our expectations of state level activity, and estimated rebate claims not yet submitted, which resulted in a reduction of our estimated Medicaid rebate reserve related to priorFeraheme sales of $0.6 million and $2.5 million, respectively. Further, during 2012, we reduced our reserve for product returns by approximately $2.2 million primarily as a result of a lower than expected rate of product returns as well as the lapse of the product return period on certain manufacturedFeraheme lots that carried a two year expiration. There was no significant adjustment of our reserve for product returns in 2011. These changes in estimates were reflected as an increase in our net product sales for the years ended December 31, 2012 and 2011 and resulted in reductions to our gross to net percentage in these respective periods. We regularly assess our Medicaid and product return reserve balances and accrual rates. If we determine in future periods that our actual
73
rebate or returns experience is not indicative of expected claims or returns, if our actual claims or returns experience changes, or if other factors affect estimated claims or returns rates, we may be required to change our Medicaid reserve or product return reserve estimates and/or the current rates at which we estimate Medicaid reserves or returns, which would affect our earnings in the period of the change and could be significant.
Our total net U.S. product sales decreased by $7.2 million, or 12%, in 2011 as compared to 2010. The $7.2 million decrease was primarily due to decreased sales ofFeraheme to dialysis providers during 2011 as compared to 2010, including a decrease of $6.8 million in net sales related to a launch incentive program which we initiated in 2009 and under which we recognized revenues of $7.0 million during 2010 as compared to $0.2 million during 2011. OurFeraheme net product sales to dialysis customers in 2011 were de minimis relative to our dialysis sales during 2010 principally as a result of the January 2011 implementation of the Medicare prospective payment system, which made it unlikely that dialysis providers would choose to useFeraheme. The decreasedFeraheme net product sales in the dialysis market was only partially offset by increasedFeraheme net product sales in the non-dialysis market in 2011 as compared to 2010. In addition, during 2011, we revised our estimated Medicaid utilization rate based on actual rebate claims received since the 2009 launch ofFeraheme, our expectations of state level activity, and estimated rebate claims not yet submitted, which resulted in a $2.5 million reduction of our 2011 estimated Medicaid rebate reserve related to prior yearFeraheme sales as compared to a $0.6 million reduction in our 2010 estimated Medicaid rebate reserve. We also offered higher average customer discounts, chargebacks and rebates to our end-users during 2011 as compared to 2010. During 2011, we reduced our gross product sales by recording allowances of $23.6 million, excluding the $2.5 million Medicaid rebate reserve reduction, as compared to allowances of $22.8 million recorded during 2010, excluding the $0.6 million Medicaid rebate reserve reduction.
Our U.S. net product sales may fluctuate from period to period due to the enactment of or changes in legislation that impact third-party reimbursement coverage and pricing. For example, in January 2011, the implementation of the Medicare prospective payment system had the effect of significantly diminishing the utilization ofFeraheme in the dialysis market and as a result, beginning in 2010,Feraheme sales in the dialysis setting began to significantly decline and were de minimis in 2012 and 2011. We expect that dialysis sales will continue to be insignificant in 2013 and beyond.
An analysis of our product sales allowances and accruals for the years ended December 31, 2012, 2011 and 2010 is as follows (in thousands):
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Provision for U.S. product sales allowances and accruals | ||||||||||
Discounts and chargebacks | $ | 26,517 | $ | 13,851 | $ | 5,113 | ||||
Government and other rebates | 6,058 | 8,544 | 16,374 | |||||||
Medicaid rebate reserve adjustment | (621 | ) | (2,532 | ) | (599 | ) | ||||
Returns | (1,516 | ) | 1,259 | 1,334 | ||||||
Total provision for U.S. product sales allowances and accruals | $ | 30,438 | $ | 21,122 | $ | 22,222 | ||||
Total gross U.S. product sales | $ | 88,725 | $ | 73,219 | $ | 81,561 | ||||
Total provision for U.S. product sales allowances and accruals as a percent of total gross U.S. product sales | 34 | % | 29 | % | 27 | % | ||||
Total discounts and chargebacks for 2012 were $26.5 million, or 30% of total gross product sales, as compared to $13.9 million, or 19% of total gross product sales, in 2011. The 11% increase in total discounts and chargebacks as a percentage of total gross product sales in 2012 as compared to 2011 was primarily due to higher discounts offered to customers off the gross sales price as well as a change in pricing strategy from offering rebates for purchases ofFeraheme above a certain minimum volume
74
threshold to entering into commercial contracts which provide increased upfront discounts on the purchase price ofFeraheme. Total government and other rebates (excluding any changes in estimates related to Medicaid rebate reserves) were $6.1 million, or 7% of total gross product sales, in 2012 as compared to $8.5 million, or 12% of gross product sales, in 2011. The decrease in total government and other rebates as a percentage of gross product sales was related primarily to lower volume rebates offered in 2012 as compared to 2011.
Total discounts and chargebacks for 2011 were $13.9 million, or 19% of total gross product sales, as compared to $5.1 million, or 6% of total gross sales in 2010. Total government and other rebates (excluding any changes in estimates related to Medicaid rebate reserves) were $8.5 million, or 12% of gross product sales, in 2011 as compared to $16.4 million, or 20% of gross product sales, in 2010. The increase in total discounts and chargebacks as a percentage of total gross product sales and the corresponding decrease in government and other rebates as a percentage of total gross product sales were primarily due to a change in our customer mix and pricing strategy. Beginning in January 2011, with the implementation of the Medicare prospective payment system, the utilization ofFeraheme in the dialysis market significantly decreased. As a result, our U.S. commercial strategy shifted to focus on growing the utilization ofFeraheme in non-dialysis CKD patients with IDA, specifically in hematology, oncology, nephrology, and hospital sites of care, many of which are members of GPOs, which leverage the purchasing power of a group of customers to obtain lower prices based on the collective bargaining power of the group. These lower prices are typically obtained through contractually arranged discounting programs. Additionally, as end user experience withFeraheme became more established, particularly in these non-dialysis sites of care, during 2011, we entered into commercial contracts which provided discounts on the purchase price ofFeraheme and gradually decreased our volume rebate programs. These changes resulted in a decrease to our net selling price per unit ofFeraheme in 2011 as compared to 2010.
We are subject to reimbursement arrangements with state Medicaid programs for which we estimate and record rebate reserves. We determine our estimates for Medicaid rebates based on actualFeraheme sales data, forecasted customer buying patterns and market research data related to utilization rates by various end-users blended with historical experience of products similar toFeraheme sold by others. During 2012, 2011 and 2010, we revised our estimated Medicaid reserve rate based on actual rebate claims received since the launch ofFeraheme in July 2009, our expectations of state level activity, and estimated rebate claims not yet submitted, which resulted in a reduction of our estimated Medicaid rebate reserve related to prior periodFeraheme sales of $0.6 million, $2.5 million and $0.6 million, respectively. These changes in estimates were reflected as an increase in our net product sales for the years ended December 31, 2012, 2011 and 2010 and resulted in reductions to our gross to net percentage in these respective periods. Actual claims to date have been limited. In future periods, we may be required to adjust our estimates based on additional experience or other changes in expectations, and such adjustments may be significant. Any such adjustments would be reflected as a change to our sales allowances and, accordingly, an increase or decrease to net product sales in that period. If actual future results vary from any of our estimates, we may need to adjust our previous estimates, which would also affect our earnings in the period of the adjustment.
We generally offer our wholesalers, specialty distributors and other customers a limited right to return product purchased directly from us, principally based on the product's expiration date which, once packaged, is currently four or five years in the U.S. Reserves for product returns for U.S. sales are recorded in the period the related revenue is recognized, resulting in a reduction to product sales. Currently, sales to our licensees are recognized as revenue when product is sold to our licensees' customers and therefore no return reserve is required at the time of sale to our licensees. We evaluate our estimated product returns rate each period based on the historical return patterns and known or expected changes in the marketplace. During 2012, we reduced our reserve for product returns by approximately $2.2 million, primarily as a result of a lower than expected rate of product returns as
75
well as the lapse of the product return period on certain manufacturedFeraheme lots that carried a two year expiration. As a result, the product returns provision applied to gross product sales for 2012 was a credit of $1.5 million, resulting in an increase to product sales, as compared to a $1.3 million charge in both 2011 and 2010, resulting in decreases to product sales. Actual returns to date have been limited. In future periods, we may be required to adjust our estimates based on additional experience or other changes in expectations, which would result in a corresponding change to our net product sales in the period in which the change is made and could be significant. If actual future results vary from any of our estimates, we may need to adjust our previous estimates, which would also affect our earnings in the period of the adjustment.
An analysis of the amount of, and change in, reserves for the years ended December 31, 2012, 2011 and 2010 is as follows (in thousands):
| | Discounts and Chargebacks | Government and Other Rebates | Returns | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at January 1, 2010 | $ | 499 | $ | 5,194 | $ | 463 | $ | 6,156 | |||||
Current provisions relating to sales in current year | 5,113 | 16,374 | 1,405 | 22,892 | |||||||||
Other provisions relating to deferred revenue | — | (1,085 | ) | — | (1,085 | ) | |||||||
Adjustments relating to sales in prior year | — | (599 | ) | (71 | ) | (670 | ) | ||||||
Payments/returns relating to sales in current year | (3,965 | ) | (8,540 | ) | — | (12,505 | ) | ||||||
Payments/returns relating to sales in prior year | (499 | ) | (3,126 | ) | — | (3,625 | ) | ||||||
Balance at December 31, 2010 | $ | 1,148 | $ | 8,218 | $ | 1,797 | $ | 11,163 | |||||
Current provisions relating to sales in current year | 14,074 | 8,605 | 1,259 | 23,938 | |||||||||
Other provisions relating to deferred revenue | — | (18 | ) | — | (18 | ) | |||||||
Adjustments relating to sales in prior years | (223 | ) | (2,593 | ) | — | (2,816 | ) | ||||||
Payments/returns relating to sales in current year | (12,251 | ) | (6,195 | ) | (55 | ) | (18,501 | ) | |||||
Payments/returns relating to sales in prior years | (926 | ) | (4,916 | ) | (159 | ) | (6,001 | ) | |||||
Balance at December 31, 2011 | $ | 1,822 | $ | 3,101 | $ | 2,842 | $ | 7,765 | |||||
Current provisions relating to sales in current year | 26,517 | 6,152 | 577 | 33,246 | |||||||||
Adjustments relating to sales in prior years | — | (715 | ) | (2,093 | ) | (2,808 | ) | ||||||
Payments/returns relating to sales in current year | (24,739 | ) | (4,511 | ) | — | (29,250 | ) | ||||||
Payments/returns relating to sales in prior years | (1,859 | ) | (1,597 | ) | (308 | ) | (3,764 | ) | |||||
Balance at December 31, 2012 | $ | 1,741 | $ | 2,430 | $ | 1,018 | $ | 5,189 | |||||
During 2012, 2011 and 2010, we decreased our product sales allowances and accruals by approximately $2.8 million, $2.8 million and $0.7 million, respectively, for changes in estimates relating to sales in prior years. The $2.8 million adjustments made during 2012 were primarily due to a net reduction of our reserve for product returns of $2.2 million as a result of a lower than expected rate of product returns as well as the lapse of the return period on certain manufacturedFeraheme lots that carried a two year expiration and a $0.6 million change to our estimated Medicaid rebate reserve. The adjustments made during 2011 and 2010 were primarily due to changes in our estimated Medicaid utilization rate based on actual rebate claims received since the 2009 launch ofFeraheme, our expectations of state level activity, and estimated rebate claims not yet submitted. This resulted in $2.5 million and $0.6 million reductions of our estimated Medicaid rebate reserve for 2011 and 2010, respectively.
Overall, we expect that our reserves as a percentage of gross sales will increase slightly during 2013 as compared to 2012 due primarily to our efforts to continue to increase adoption and utilization ofFeraheme, our efforts to address continuing reimbursement and competitive pricing pressures, the expected customer mix and utilization rates, and the fact that our reserves as percentage of gross
76
product sales were positively impacted by changes in our estimated Medicaid rebate and product return reserves during 2012. During 2012, we implemented gross price increases forFeraheme, some of which was discounted back to customers under performance-based contracts. We anticipate that the effect of these price increases will offset the impact of the widening gross to net adjustment and that the average net revenue per gram ofFeraheme will increase in future periods.
There are a number of factors that make it difficult to predict the magnitude of future U.S.Feraheme sales, including but not limited to, the following:
- •
- The magnitude and timing of adoption ofFeraheme by physicians, hospitals and other healthcare payors and providers;
- •
- Any expansion or contraction of the overall size of the IV iron market, which could result from a number of factors including but not limited to, changes in treatment guidelines or practices related to IDA;
- •
- The introduction of new competitive products in the iron replacement therapeutic market, such as Injectafer®, if approved or generic versions of new or currently available drug therapies;
- •
- The effect of federal and other legislation such as the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the Health Care Reform Act, and the Budget Control Act of 2011;
- •
- The inventory levels maintained byFeraheme wholesalers, distributors and other customers;
- •
- The frequency of re-orders by existing customers;
- •
- The impact of any actual or perceived safety or efficacy issues withFeraheme; and
- •
- The impact of and any actions taken by us or our competitors to address pricing and reimbursement considerations related toFeraheme or products that compete withFeraheme.
As a result of these and other factors, futureFeraheme sales could vary significantly from quarter to quarter and, accordingly, ourFeraheme net product revenues in current or previous quarters may not be indicative of futureFeraheme net product revenues.
Recent Healthcare Reform Legislation
The Health Care Reform Act was enacted in the U.S. in March 2010 and includes certain cost containment measures including an increase to the minimum rebates for products covered by Medicaid programs and the extension of such rebates to drugs dispensed to Medicaid beneficiaries enrolled in Medicaid managed care organizations as well as the expansion of the 340B Drug Discount Program under the Public Health Service Act. This legislation contains provisions that can affect the operational results of companies in the pharmaceutical industry, including us, and other healthcare related industries by imposing on them additional costs. In the first quarter of 2010, an increase from 15.1% to 23.1% in the minimum statutory Medicaid rebate to states participating in the Medicaid program became effective. Given the relatively small portion of our sales that are subject to Medicaid claims, this increase in the minimum Medicaid rebate did not materially reduce our product revenues in 2012, 2011 and 2010.
The Health Care Reform Act also requires pharmaceutical manufacturers to pay a prorated share of the overall Branded Drug Fee, based on the dollar value of its branded prescription drug sales to certain federal programs identified in the legislation. The amount of our annual share of the Branded Drug Fee for 2012 was $0.1 million and was non-deductible for income tax purposes. We have included this amount in selling, general and administrative expense in our consolidated statement of operations. We were not assessed and therefore did not record any Branded Drug Fee in any period during 2011 or 2010. The amount of this annual payment could increase in future years due to both higher eligible
77
Feraheme sales and the increasing amount of the overall fee assessed across manufacturers, but any such increases are not expected to be material to our results of operations or financial condition.
In addition, the number of entities covered by the 340B Drug Discount Program under the Public Health Service Act, which provides drugs at reduced rates, was expanded by the Health Care Reform Act to include additional hospitals. The expansion of 340B eligible entities did not materially impact our discounts and chargebacks as a percentage of gross product sales in 2012 as compared to 2011 or in 2011 as compared to 2010. However, the amount ofFeraheme business in 340B eligible entities is growing faster than other customers to which we sell. Because of the federal pricing discounts granted to these 340B institutions, the revenue realized per unit ofFeraheme sold to 340B institutions is lower than from other customers and this change in the mix of our business contributed to our increase in discounts in 2012 as compared to 2011.
We were not materially impacted by recent healthcare reform legislation during 2012, 2011 or 2010. Presently, we have not identified any provisions that could materially impact our business but we will continue to monitor future developments related to this legislation. The potential long-term impact on our business is inherently difficult to predict as many details regarding the implementation of this legislation have not yet been determined.
International Product Sales and Royalties
We record all international product sales and royalties forFeraheme/Rienso sold to Takeda in deferred revenues in our consolidated balance sheet. We recognize these deferred revenues, and the associated cost of product sales, in our consolidated statement of operations at the time Takeda reports to us that sales have been made its customers. During 2012, we recognized $0.1 million in product sales and royalty revenue related to the Amended Takeda Agreement and we have included this revenue in international product sales and royalties in our consolidated statement of income. Takeda launchedFeraheme/Rienso in Canada, Switzerland and the EU in the fourth quarter of 2012. As of December 31, 2012, we have $1.0 million in deferred revenue related to product shipped to Takeda, but not yet sold through to Takeda's customers.
License Fee and Other Collaboration Revenues
License fee and other collaboration revenues for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
Milestone revenues recognized from Takeda | $ | 19,950 | $ | — | $ | — | $ | 19,950 | N/A | $ | — | — | ||||||||||
Deferred license fee revenues recognized from Takeda | 6,096 | 6,096 | 4,572 | — | — | 1,524 | 33 | % | ||||||||||||||
Reimbursement revenues primarily from Takeda | 429 | 2,225 | 1,560 | (1,796 | ) | -81 | % | 665 | 43 | % | ||||||||||||
Total | $ | 26,475 | $ | 8,321 | $ | 6,132 | $ | 18,154 | >100 | % | $ | 2,189 | 36 | % | ||||||||
78
Our license fee and other collaboration revenues in 2012 increased by $18.2 million as compared to 2011 and increased by $2.2 million in 2011 as compared to 2010. During 2012, we received a $15.0 million milestone payment from Takeda associated with the regulatory approval ofRienso in the EU, which we deemed a substantive milestone and recorded in its entirety in revenues in our license fee and other collaboration revenues in our consolidated statement of operations. In addition, during 2012, we received an aggregate of $18.0 million of milestone payments related to the commercial launches ofFeraheme/Rienso in Canada and the EU, which we deemed non-substantive milestones and are amortizing into revenue on a cumulative catch up basis using the proportional performance method extended over the original life of the Takeda Agreement. As a result, we have included $5.0 million of the $18.0 million in our license fee and other collaboration revenues in 2012. We did not receive any milestone payments in 2011 or 2010.
In addition, during 2012, 2011 and 2010 we recorded $6.1 million, $6.1 million, and $4.6 million, respectively, of revenues associated with the amortization of $61.0 million of deferred revenues recorded in connection with the original Takeda Agreement. The $1.5 million increase in 2011 as compared to 2010 was the result of recognizing a full year of the upfront payment from Takeda during 2011 as compared to nine months during 2010. The $61.0 million of deferred revenues was comprised of a $60.0 million upfront payment which we received from Takeda in April 2010, as well as approximately $1.0 million reimbursed to us during 2010 for certain expenses incurred prior to entering the agreement, which we considered an additional upfront payment. As of December 31, 2012, we had approximately $44.2 million remaining in deferred revenues related to the $61.0 million upfront payments received from Takeda.
Under the terms of the Amended Takeda Agreement, Takeda is responsible for reimbursing us for certain out-of-pocket regulatory and clinical trial supply costs we incur in the conduct of certain regulatory and clinical research activities we manage under the agreement. Because we are acting as the principal in carrying out these activities, any reimbursement payments received from Takeda are recorded in license fee and other collaboration revenues in our consolidated statement of operations and offset the costs that we incur during the period in which we perform those services. During 2012, 2011 and 2010, we recorded $0.4 million, $2.0 million, and $1.6 million, respectively, of revenues associated with the reimbursement of out-of pocket regulatory and clinical supply costs in connection with the Amended Takeda Agreement.
We anticipate that our license fee and other collaboration revenues will decrease in 2013 as compared to 2012 given the non-recurring $15.0 million substantive milestone payment and the $5.0 million cumulative catch up associated with the $18.0 million non-substantive milestone payments we received from Takeda in 2012 and recognized in our 2012 revenues, as discussed above. We do not expect any new milestones to be achieved under the Amended Takeda Agreement in 2013.
Other Product Sales and Royalties
Our other product sales and royalties include product sales ofGastroMARK to our licensees as well as royalties received from our licensees' sales ofGastroMARK. The $0.3 million decrease in our other product sales and royalties in 2012 as compared to 2011 was due to decreased sales as a result of our 2012 terminations of our agreements with ourGastroMARK licensees.
79
Costs and Expenses
Cost of Product Sales
Cost of product sales for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
Cost of Product Sales | $ | 14,220 | $ | 10,531 | $ | 7,606 | $ | 3,689 | 35 | % | $ | 2,925 | 38 | % | ||||||||
Percentage of Net Product Sales and Royalties | 24 | % | 20 | % | 13 | % | ||||||||||||||||
Our cost of product sales are primarily comprised of manufacturing costs, costs of managing our contract manufacturers, and costs for quality assurance and quality control associated with our sales ofFeraheme in the U.S., international sales ofFeraheme/Rienso, andGastroMARK sales to our licensees. During 2012, our cost of product sales increased by $3.7 million, or 35%, as compared to 2011. Included in our cost of product sales for 2012 was $2.3 million in accelerated depreciation and impairment costs associated with our ongoing divestiture of our Cambridge, Massachusetts manufacturing facility. During the third quarter of 2012, we determined that our manufacturing facility and related assets were considered held for sale, based on an analysis of current accounting guidance. This $2.3 million charge during 2012 reflects an adjustment to reduce the carrying value of these assets to fair value less the cost to sell based on what we believe is the best estimate of the net realizable value of the assets upon divestiture. In addition, the increase in our cost of product sales during 2012 as compared to 2011 was due to $0.9 million of additionalFeraheme vials sold and a $0.6 million write-off of commercial inventory deemed no longer saleable.
Our cost of product sales increased by $2.9 million, or 38%, during 2011 as compared to 2010 primarily due to higher idle capacity costs at our Cambridge, Massachusetts manufacturing facility. The high idle capacity costs resulted from reduced production activity due to our alignment of production volumes and inventory with our then current and expectedFeraheme sales. Idle capacity costs are included in cost of product sales in the period incurred.
We expect our cost of product sales as a percentage of net product sales and royalties to decrease during 2013 as compared to 2012 because we do not expect to record any significant costs related to our manufacturing facility during 2013.
Research and Development Expenses
Research and development expenses include external expenses, such as costs of clinical trials, contract research and development expenses, certain manufacturing research and development costs, regulatory filing fees, consulting and professional fees and expenses, and internal expenses, such as compensation of employees engaged in research and development activities, the manufacture of product needed to support research and development efforts, related costs of facilities, and other general costs related to research and development. Where possible, we track our external costs by major project. To the extent that external costs are not attributable to a specific project or activity, they are included in other external costs. Prior to the initial regulatory approval of our products or development of new manufacturing processes, costs associated with manufacturing process development and the manufacture of drug product are recorded as research and development expenses. Subsequent to initial regulatory approval, costs associated with the manufacture of our products for commercial sale are capitalized in inventory and recorded as cost of product sales when sold.
80
Research and development expenses for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
External Research and Development Expenses | ||||||||||||||||||||||
Feraheme to treat IDA regardless of the underlying cause | $ | 12,357 | $ | 27,405 | $ | 17,132 | $ | (15,048 | ) | -55 | % | $ | 10,273 | 60 | % | |||||||
Feraheme to treat IDA in CKD patients | 3,226 | 9,385 | 11,003 | (6,159 | ) | -66 | % | (1,618 | ) | -15 | % | |||||||||||
Feraheme as a therapeutic agent, general | 1,033 | 917 | 799 | 116 | 13 | % | 118 | 15 | % | |||||||||||||
Feraheme manufacturing process development and materials | 2,297 | 2,752 | 3,059 | (455 | ) | -17 | % | (307 | ) | -10 | % | |||||||||||
Feraheme as an imaging agent | — | — | 2,483 | — | — | (2,483 | ) | -100 | % | |||||||||||||
Other external costs | 152 | 263 | 763 | (111 | ) | -42 | % | (500 | ) | -66 | % | |||||||||||
Total | $ | 19,065 | $ | 40,722 | $ | 35,239 | $ | (21,657 | ) | -53 | % | $ | 5,483 | 16 | % | |||||||
Internal Research and Development Expenses | ||||||||||||||||||||||
Compensation, payroll taxes, benefits and other expenses | 12,237 | 15,544 | 15,715 | (3,307 | ) | -21 | % | (171 | ) | -1 | % | |||||||||||
Equity-based compensation expense | 1,994 | 1,874 | 3,508 | 120 | 6 | % | (1,634 | ) | -47 | % | ||||||||||||
Total | $ | 14,231 | $ | 17,418 | $ | 19,223 | $ | (3,187 | ) | -18 | % | $ | (1,805 | ) | -9 | % | ||||||
Total Research and Development Expenses | $ | 33,296 | $ | 58,140 | $ | 54,462 | $ | (24,844 | ) | -43 | % | $ | 3,678 | 7 | % | |||||||
Total research and development expenses incurred in 2012 decreased by $24.8 million, or 43%, as compared to 2011. The decrease was primarily due to reduced external research and development costs of $21.7 million in 2012. In addition, 2012 internal research and development costs decreased by $3.2 million as compared to 2011.
The $21.7 million, or 53%, decrease in our external research and development expenses in 2012 as compared to 2011, was due primarily to a $15.0 million decrease in costs incurred in connection with our Phase III clinical development program forFeraheme to treat IDA regardless of the underlying cause, which was completed in 2012. In addition, costs associated with our global clinical program to support the MAA in the EU for the treatment of IDA in CKD patients, which was completed in 2012, our post-approval clinical study evaluatingFeraheme treatment as compared to treatment with another IV iron, which was completed in 2011, and the current pace of enrollment in our on-going pediatric studies ofFeraheme, decreased by $6.2 million.
The $3.2 million, or 18%, decrease in our internal research and development expenses in 2012 as compared to 2011 was primarily attributable to the decrease in compensation and related benefits following our 2012 and 2011 corporate restructurings, which resulted in lower headcount in our research and development departments.
Total research and development expenses incurred in 2011 increased by $3.7 million, or 7%, from 2010 due to increased external research and development expenses of $5.5 million in 2011, partially offset by a $1.8 million decrease in our internal research and development costs in 2011 as compared to 2010.
81
The $5.5 million, or 16%, increase in our external research and development expenses in 2011 as compared to 2010, was due primarily to an increase of $10.3 million in costs incurred in connection with our Phase III clinical development program forFeraheme to treat IDA regardless of the underlying cause, which was initiated in June 2010, and costs incurred related to certain of our pediatric studies ofFeraheme. This increase was partially offset by a $1.6 million reduction in costs associated with our global clinical program to support the MAA in the EU for the treatment of IDA in CKD patients, which was completed in 2012, as well as $2.5 million in certain costs incurred in 2010 in connection with a clinical trial forFeraheme as an imaging agent, which was discontinued in 2010.
The $1.8 million, or 9%, decrease in our internal research and development expenses in 2011 as compared to 2010, was primarily attributable to a $1.6 million reduction of equity-based compensation expense and the net decrease in compensation and related benefits principally due to restructurings that took place in 2011 and 2010, which resulted in lower headcount in our research and development departments during 2011.
Research and Development Activities
We expect research and development expenses to decrease in 2013 as compared to 2012 primarily due to the completion of our clinical development program ofFeraheme for the treatment of IDA regardless of the underlying cause and the reduction of costs related to the preparation and the related December 2012 submission of our U.S.Feraheme sNDA to treat IDA regardless of the underlying cause, partially offset by costs associated with certainFeraheme clinical studies we have committed to conduct as a condition of approval of theRienso MAA by the EMA, such as our post-approval commitment discussed above, as well as other miscellaneous research and development related activities in support of ourFeraheme/Rienso development programs.
We do not track our internal costs by project since our research and development personnel work on a number of projects concurrently and much of our fixed costs benefit multiple projects or our operations in general. We track our external costs on a major project basis, in most cases through the later of the completion of the last trial in the project or the last submission of a regulatory filing to the FDA or applicable foreign regulatory body. The following two major research and development projects are currently ongoing:
- •
- Feraheme to treat IDA regardless of the underlying cause. This project currently includes: (1) a completed Phase III clinical study evaluatingFeraheme treatment as compared to treatment with placebo; (2) a completed Phase III clinical study evaluatingFeraheme treatment as compared to treatment with another IV iron; and (3) a completed extension study.
- •
- Feraheme to treat IDA in CKD patients. This project currently includes: (1) a completed clinical study evaluatingFeraheme treatment as compared to treatment to another IV iron to support the MAA submission; (2) two ongoing pediatric studies that are being conducted as part of our post-approval Pediatric Research Equity Act requirement to support pediatric CKD labeling ofFeraheme; (3) two additional pediatric studies to be completed in accordance with our approved pediatric investigation plan to support the MAA submission; and (4) a multi-center clinical trial to be conducted to determine the safety and efficacy of repeat doses ofFeraheme for the treatment of IDA in patients with hemodialysis dependent CKD, including a treatment arm with iron sucrose as well as a MRI study to evaluate the potential for iron to accumulate in the body following repeated IV iron administration for the treatment of IDA in patients with CKD over a two year period.
Through December 31, 2012, we have incurred aggregate external research and development expenses of approximately $57.7 million related to our current program for the development ofFeraheme to treat IDA regardless of the underlying cause. We currently estimate that the total
82
remaining external costs associated with the efforts needed to complete this development project will be less than $3.0 million, which will be incurred in 2013.
Through December 31, 2012, we have incurred aggregate external research and development expenses of approximately $23.9 million related to our current program for the development ofFeraheme to treat IDA in CKD patients. We currently estimate that the total remaining external costs associated with this development project will be in the range of approximately $23.0 to $33.0 million over the next several years.
Conducting clinical trials involves a number of uncertainties, many of which are out of our control. Our estimates of external costs associated with our research and development projects could therefore vary from our current estimates for a variety of reasons including but not limited to the following:
- •
- Delays in our clinical trials due to slow enrollment;
- •
- Unexpected results from our clinical sites that affect our ability to complete the studies in a timely manner;
- •
- Unanticipated adverse reactions toFeraheme either in commercial use or in a clinical trial setting;
- •
- Inadequate performance or errors by third-party service providers;
- •
- Any deficiencies in the design or oversight of these studies by us;
- •
- The need to conduct additional clinical trials; or
- •
- Any adverse regulatory action or delay in the submission of any applicable regulatory filing.
As a result, we are unable to reasonably estimate the specific timing of any expected net cash inflows resulting from these projects, provided however, as the result of recent regulatory decisions on our marketing applications in the EU and the respective commercial launches forFeraheme/Rienso in the CKD indication in the EU and Canada, we have received $33.0 million in milestone payments and we have begun receiving product sales revenues and royalty payments in accordance with the Amended Takeda Agreement.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses include costs related to our commercial personnel, including our specialty sales force, medical education professionals, pharmacovigilance and safety monitoring and other commercial support personnel, costs related to our administrative personnel, including our legal, finance, business development and executive personnel, external and facilities costs required to support the marketing and sale ofFeraheme and other costs associated with our corporate activities.
Selling, general and administrative expenses for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
Compensation, payroll taxes and benefits | $ | 23,273 | $ | 29,553 | $ | 35,274 | $ | (6,280 | ) | -21 | % | $ | (5,721 | ) | -16 | % | ||||||
Sales and marketing consulting, professional fees, and other expenses | 12,133 | 16,859 | 27,593 | (4,726 | ) | -28 | % | (10,734 | ) | -39 | % | |||||||||||
General and administrative consulting, professional fees and other expenses | 12,860 | 14,903 | 11,498 | (2,043 | ) | -14 | % | 3,405 | 30 | % | ||||||||||||
Equity-based compensation expense | 4,805 | 7,548 | 10,574 | (2,743 | ) | -36 | % | (3,026 | ) | -29 | % | |||||||||||
Total | $ | 53,071 | $ | 68,863 | $ | 84,939 | $ | (15,792 | ) | -23 | % | $ | (16,076 | ) | -19 | % | ||||||
83
Total selling, general and administrative expenses incurred in 2012 decreased by $15.8 million, or 23%, as compared to 2011 for the following reasons:
- •
- A $6.3 million decrease in compensation, payroll taxes and benefits during 2012 as compared to 2011 due primarily to reduced headcount resulting from our 2012 and 2011 corporate restructurings;
- •
- A $4.7 million decrease in sales and marketing consulting, professional fees, and other expenses during 2012 as compared to 2011 primarily due to reduced costs related to advertising and marketing materials, and certain other general marketing costs;
- •
- A $2.0 million decrease in general and administrative consulting, professional fees and other expenses during 2012 as compared to 2011 primarily due to a decrease in our professional fees, specifically $4.5 million of costs incurred in 2011 in connection with our then proposed merger with Allos Therapeutics, Inc., or Allos, including a $2.0 million expense reimbursement fee paid to Allos in connection with the termination of the merger agreement. These increased costs were partially offset by $1.6 million in termination fee payments made in 2012 to ourGastroMARK licensees in connection with the termination of our commercial license agreements with them, costs incurred in 2012 in connection with our intention to expand our product portfolio and the 2012 closure of our Cambridge, Massachusetts manufacturing facility; and
- •
- A $2.7 million decrease in equity-based compensation expenses for 2012 due primarily to a $3.3 million reduction of equity-based compensation expense associated with the 2011 departures of certain of our executive officers, including our former chief financial officer, our chief executive officer and our chief commercial officer, and the impact of our 2012 and 2011 corporate workforce reductions, partially offset by the expense associated with equity awards to new employees in 2012, including our current chief executive officer, and additional equity awards to existing employees.
Total selling, general and administrative expenses incurred in 2011 decreased by $16.1 million, or 19%, as compared to 2010 for the following reasons:
- •
- A $5.7 million decrease in compensation, payroll taxes and benefits during 2011 as compared to 2010 primarily as a result of reduced headcount resulting from our 2010 restructuring;
- •
- A $10.7 million decrease in sales and marketing consulting, professional fees, and other expenses during 2011 as compared to 2010 due to reduced costs related to the reduction or elimination of field-based contract nurses, advertising and marketing materials, and certain other general marketing costs;
- •
- A $3.4 million increase in our general and administrative consulting, professional fees and other expenses during 2011 as compared to 2010 primarily due to $4.5 million of costs incurred in connection with our then proposed merger with Allos; and
- •
- A $3.0 million decrease in equity-based compensation expense during 2011 as compared to 2010 due primarily to a $1.6 million reduction of equity-based compensation expense associated with the 2011 departures of certain of our executive officers, and the expected impact on our equity compensation forfeitures resulting from our 2011 corporate workforce reduction, partially offset by the expense associated with equity awards to new employees and additional equity awards to existing employees. This $1.6 million includes a reduction of expense of approximately $0.7 million previously recorded for certain of our former chief executive officer's outstanding equity awards as the result of the modification of the terms of such awards pursuant to his November 2011 separation agreement.
We expect total selling, general and administrative expenses will remain relatively stable during 2013 as compared to 2012.
84
Restructuring Expense
During 2012, we initiated a corporate restructuring, including a workforce reduction plan. The majority of the workforce reduction plan was associated with our manufacturing and development infrastructure, including our decision to divest our Cambridge, Massachusetts manufacturing facility. As a result of the restructuring, we recorded charges of approximately $2.2 million in 2012. Of the $2.2 million in restructuring expense, approximately $1.5 million was related to employee severance and benefits, and approximately $0.7 million was related to the write-down of primarily raw material inventory that was no longer usable due to the closure of the facility. The workforce reduction was substantially completed by the end of 2012, and the majority of the related expenses were paid by the end of 2012.
During 2011, we initiated a corporate restructuring, including a workforce reduction plan for which we recorded $3.5 million of restructuring related costs, primarily related to employee severance and benefits. The workforce reduction was substantially completed by the end of 2011 and the majority of the related expenses were paid by the end of 2012.
During 2010, we also initiated a corporate restructuring, including a workforce reduction plan, for which we recorded $2.2 million of restructuring related costs, primarily related to employee severance and benefits. These expenses were substantially paid by the end of 2011. The majority of the workforce reduction was completed during 2010 and the remaining positions were eliminated by the end of 2011.
Other Income (Expense)
Other income (expense) for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | 2012 to 2011 change | 2011 to 2010 change | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | $ Change | % Change | $ Change | % Change | |||||||||||||||
Interest and dividend income, net | $ | 1,286 | $ | 1,747 | $ | 1,741 | $ | (461 | ) | -26 | % | $ | 6 | — | ||||||||
(Losses) gains on investments, net | (1,466 | ) | (193 | ) | 408 | (1,273 | ) | >100 | % | (601 | ) | <(100 | %) | |||||||||
Fair value adjustment of settlement rights | — | — | (788 | ) | — | — | 788 | -100 | % | |||||||||||||
Total | $ | (180 | ) | $ | 1,554 | $ | 1,361 | $ | (1,734 | ) | <(100 | %) | $ | 193 | 14 | % | ||||||
Other income (expense) for 2012 decreased by $1.7 million as compared to 2011 primarily due to the $1.5 million loss we realized on the June 2012 sale of our then remaining ARS portfolio. In addition, there was a $0.5 million decrease in our interest and dividend income as the result of lower average cash balances in 2012 as compared to 2011.
Other income (expense) in 2011 remained relatively constant as compared to 2010 and we expect interest and dividend income to remain relatively constant in 2013 as compared to 2012.
Income Tax Benefit
We recognized an income tax benefit of $0.9 million, $1.2 million and $0.5 million during the years ended December 31, 2012, 2011 and 2010, respectively, as the result of our recognition of a corresponding income tax expense associated with the increase in value of certain securities as a result of their redemption at prices higher than the fair market value at which they were recorded. This income tax expense was recorded in other comprehensive income.
Net Loss
For the reasons stated above, we incurred a net loss of $16.8 million, $77.1 million and $81.2 million, or $0.78, $3.64 and $3.90 per basic and diluted share, for the years ended December 31, 2012, 2011 and 2010, respectively.
85
Liquidity and Capital Resources
General
We finance our operations primarily from the sale ofFeraheme/Rienso, including payments from our licensees and cash generated from our investing activities and the sale of our common stock. We expect to continue to incur significant expenses as we continue to manufacture, market and sellFeraheme/Rienso as an IV iron replacement therapeutic for use in adult CKD patients in the U.S., Canada, Switzerland and the EU, and as we further develop and seek regulatory approval forFeraheme/Rienso for the treatment of IDA in a broad range of patients in and outside of the U.S.
Our long-term capital requirements will depend on many factors, including, but not limited to, the following:
- •
- Our ability to successfully commercializeFeraheme in the U.S. and Takeda's ability to successfully commercializeFeraheme/Rienso in its licensed territories outside of the U.S.;
- •
- The magnitude of U.S.Feraheme sales;
- •
- Our ability to obtain U.S. and EU regulatory approval for ferumoxytol to treat IDA regardless of the underlying cause;
- •
- Our ability to achieve the various milestones and receive the associated payments under the Amended Takeda Agreement;
- •
- The magnitude ofFeraheme/Rienso product sales and royalties we may receive from Takeda outside of the U.S.;
- •
- Costs associated with the U.S. commercialization ofFeraheme, including costs associated with maintaining our commercial infrastructure, executing our promotional and marketing strategy forFeraheme and conducting our required pediatric clinical trials and our post-marketing clinical studies;
- •
- Costs associated with qualifying additional manufacturing capacities and alternative suppliers;
- •
- The outcome of and costs associated with any material litigation or patent challenges to which we are or may become a party;
- •
- The success, costs and structure of any business or corporate development initiatives to bring additional products into our portfolio;
- •
- Our ability to maintain successful collaborations with our licensees and/or to enter into additional strategic relationships or acquisitions, if necessary; and
- •
- Our ability to raise additional capital on terms and within a timeframe acceptable to us, if necessary.
As of December 31, 2012, our investments consisted of corporate debt securities, U.S. treasury and government agency securities and commercial paper. We place our cash, cash equivalents and investments in instruments that meet high credit quality and diversification standards, as specified in our investment policy. Our investment policy also limits the amount of our credit exposure to any one issue or issuer, excluding U.S. government entities, and seeks to manage these assets to achieve our goals of preserving principal, maintaining adequate liquidity at all times, and maximizing returns.
86
Cash, cash equivalents and investments as of December 31, 2012 and 2011 consisted of the following (in thousands):
| | December 31, | | | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | $ Change | % Change | |||||||||
Cash and cash equivalents | $ | 46,293 | $ | 63,474 | $ | (17,181 | ) | -27 | % | ||||
Short-term investments | 180,750 | 148,703 | 32,047 | 22 | % | ||||||||
Long-term investments | — | 17,527 | (17,527 | ) | -100 | % | |||||||
Total | $ | 227,043 | $ | 229,704 | $ | (2,661 | ) | -1 | % | ||||
The $2.7 million decrease in cash, cash equivalents and investments as of December 31, 2012 as compared to December 31, 2011 was primarily due to cash expended to fund our operations and working capital, partially offset by cash received fromFeraheme sales, milestone payments, and product sales and royalty payments from Takeda and interest income.
We expect that our cash, cash equivalents and investments balances, in the aggregate, will decrease in 2013. Our expectation assumes our continued investment in the development and commercialization ofFeraheme, and the continued realignment of our cost structure following our 2012 and 2011 corporate restructurings. We believe that our cash, cash equivalents and investments as of December 31, 2012 and the cash we currently expect to receive from sales ofFeraheme, earnings on our investments, and potential product sales and royalty payments from Takeda will be sufficient to satisfy our cash flow needs for at least the next twelve months, including projected operating expenses related to our ongoing development and commercialization programs forFeraheme.
During 2012, we initiated a corporate restructuring, including a workforce reduction plan. The majority of the workforce reduction plan was associated with our manufacturing and development infrastructure, including our decision to divest our Cambridge, Massachusetts manufacturing facility. As a result of the restructuring, we recorded charges of approximately $2.2 million in 2012. Of the $2.2 million in restructuring expense, approximately $1.5 million was related to employee severance and benefits, and approximately $0.7 million was related to the write-down of primarily raw material inventory that was no longer usable due to the closure of the facility. The workforce reduction was substantially completed by the end of 2012, and the majority of the related expenses were paid by the end of 2012.
During 2011, we initiated a corporate restructuring, including a workforce reduction plan for which we recorded $3.5 million of restructuring related costs, primarily related to employee severance and benefits. The workforce reduction was substantially completed by the end of 2011 and the related expenses were substantially paid by the end of 2012.
In June 2012, we sold our remaining ARS portfolio, with a par value of $19.8 million, for proceeds of $18.3 million and recognized a loss of approximately $1.5 million in other income (expense) in our 2012 consolidated statement of income. All of the ARS we held consisted of municipal bonds with an auction reset feature and were classified as available-for-sale.
The ongoing uncertainty in the global financial markets has had an adverse impact on financial market activities world-wide, resulting in, among other things, volatility in security prices, periodic diminished liquidity and credit availability, ratings downgrades of certain investments and declining valuations of others. Although we invest our excess cash in investment grade securities, there can be no assurance that changing circumstances will not affect our future financial position, results of operations or liquidity.
87
Year Ended December 31, 2012
Cash flows from operating activities
During 2012, our use of $1.2 million in cash in operations was attributable principally to our net loss of approximately $16.8 million, adjusted for the following:
- •
- Non-cash operating items of $14.6 million including equity-based compensation expense, depreciation, amortization of premium/discount on purchased securities, net losses (gains) on investments, and other non-cash items;
- •
- An increase in deferred revenues and other long-term liabilities of $7.5 million, primarily from the deferral of a portion of the milestones received from Takeda in 2012;
- •
- A combined decrease of $5.6 million in accounts receivable, prepaid assets and inventories; and
- •
- A decrease of $12.1 million in accounts payable and accrued expenses.
Our net loss of $16.8 million was primarily the result of compensation and other expenses, commercialization expenses, including marketing and promotion costs, research and development costs, including costs associated with our clinical trials and general and administrative costs, partially offset by net product and collaboration revenues, including the recognition of approximately $20.0 million in milestone payments from Takeda.
Cash flows from investing activities
Cash used in investing activities in 2012 was $16.4 million and was primarily attributable to the purchases of investments, partially offset by proceeds from the sales and maturities of our investments, including the June 2012 sale of our remaining ARS portfolio.
Year Ended December 31, 2011
Cash flows from operating activities
During 2011, our use of $63.8 million of cash in operations was attributable principally to our net loss of approximately $77.1 million, adjusted for the following:
- •
- Non-cash operating items of $15.2 million including equity-based compensation expense, depreciation, income tax benefit, and other non-cash items;
- •
- A decrease in deferred revenues and other long-term liabilities of $6.7 million, which reflects timing differences between the receipt and payment of cash associated with certain transactions and the recognition of such amounts in our results of operations;
- •
- A combined decrease of $3.1 million in accounts receivable, prepaid assets and inventories; and
- •
- An increase of $1.7 million in accounts payable and accrued expenses.
Our net loss of $77.1 million in 2011 was primarily the result of commercialization expenses, including marketing and promotion costs, compensation and other expenses, research and development costs, including costs associated with our clinical trials, and general and administrative costs, partially offset by net product and collaboration revenues.
Cash flows from investing activities
Cash provided by investing activities in 2011 was $14.0 million during 2011 and was primarily attributable to proceeds from the sales and maturities of our investments partially offset by purchases of investments.
88
Contractual Obligations
We currently have no long-term debt obligations or capital lease obligations. Our contractual obligations primarily consist of our obligations under non-cancellable operating leases and other purchase obligations. Future lease obligations and purchase commitments, as of December 31, 2012, are as follows (in thousands):
| | Payment due by period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||
Facility lease obligations | $ | 7,945 | $ | 2,080 | $ | 4,309 | $ | 1,556 | $ | — | ||||||
Purchase commitments | 3,710 | 3,550 | 100 | 60 | — | |||||||||||
Operating lease obligations, excluding facility lease | 161 | 101 | 60 | — | — | |||||||||||
Total | $ | 11,816 | $ | 5,731 | $ | 4,469 | $ | 1,616 | $ | — | ||||||
Operating and Facility Lease Obligations
We have entered into certain operating leases, including leases of certain automobiles and certain office equipment which expire through 2014. We lease approximately 76 automobiles for our field-based employees. These leases require an initial minimum lease commitment of 12 months per automobile, after which we are responsible for certain disposal costs in the event of termination of the lease. As of December 31, 2012, all of our leased automobiles have been held beyond the initial 12 month commitment period.
In May 2008, we entered into a lease agreement for certain real property located at 100 Hayden Avenue, Lexington, Massachusetts for use as our principal executive offices. The term of the lease began on May 22, 2008 and will continue until August 31, 2016 with two successive five year extension terms at our option. The aggregate size of rentable floor area for the offices is 55,924 square feet, and the rent for the initial term commenced in February 2009. The lease requires us to pay rent as follows (in thousands):
Period | Minimum Lease Payments | |||
|---|---|---|---|---|
Year Ended December 31, 2013 | $ | 2,071 | ||
Year Ended December 31, 2014 | 2,127 | |||
Year Ended December 31, 2015 | 2,183 | |||
Year Ended December 31, 2016 | 1,556 | |||
Total | $ | 7,937 | ||
During any extension term, the base rent will be an amount agreed upon by us and the landlord. In addition to base rent, we are also required to pay a proportionate share of the landlord's annual operating costs.
Purchase Commitments
During 2012, we entered into various agreements with third parties for which we had remaining purchase commitments of approximately $3.7 million as of December 31, 2012. These agreements principally related to certain purchase orders for the production ofFeraheme/Rienso, outsourced commercial activities, manufacturing commitments, our information technology infrastructure and other operational activities.
89
Other Funding Commitments
As of December 31, 2012, we had several ongoing clinical studies in various clinical trial stages. Our most significant clinical trial expenditures were to clinical research organizations, or CROs. The contracts with CROs are generally cancellable, with notice, at our option. We have recorded accrued expenses in our consolidated balance sheet of approximately $0.7 million representing expenses incurred with these organizations as of December 31, 2012, net of any amounts prepaid to these CROs. As a result of our cancellation rights, we have not included these CRO contracts in the contractual obligations table above.
Severance Arrangements
We have entered into employment agreements or other arrangements with most of our executive officers and certain other employees, which provide for salary continuation payments and, in certain instances, the acceleration of the vesting of certain equity awards to such individuals in the event that the individual is terminated other than for cause, as defined in the applicable employment agreements or arrangements.
Indemnification Agreements
In the course of operating our business, we have entered into a number of indemnification arrangements under which we may be required to make payments to or on behalf of certain third parties including our directors, officers, and certain employees as well as certain other third parties with whom we enter into agreements. For further discussion of how this may affect our business, refer to Note M of the Notes to Financial Statements included in Part II, Item 8 "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K.
Legal Proceedings
We accrue a liability for legal contingencies when we believe that it is both probable that a liability has been incurred and that we can reasonably estimate the amount of the loss. We review these accruals and adjust them to reflect ongoing negotiations, settlements, rulings, advice of legal counsel and other relevant information. To the extent new information is obtained and our views on the probable outcomes of claims, suits, assessments, investigations or legal proceedings change, changes in our accrued liabilities would be recorded in the period in which such determination is made. For the matters referenced below, the liability is not probable or the amount cannot be reasonably estimated and, therefore, accruals have not been made. In addition, in accordance with the relevant authoritative guidance, for any matters in which the likelihood of material loss is at least reasonably possible, we will provide disclosure of the possible loss or range of loss. If a reasonable estimate cannot be made, however, we will provide disclosure to that effect.
A purported class action complaint was originally filed on March 18, 2010 in the United States District Court for the District of Massachusetts, entitled Silverstrand Investments et. al. v. AMAG Pharm., Inc., et. al., Civil Action No. 1:10-CV-10470-NMG, and was amended on September 15, 2010 and on December 17, 2010. The second amended complaint, or SAC, filed on December 17, 2010 alleged that we and our former President and Chief Executive Officer, former Executive Vice President and Chief Financial Officer, the then members of our Board of Directors, and certain underwriters in our January 2010 offering of common stock violated certain federal securities laws, specifically Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended, and that our former President and Chief Executive Officer and former Executive Vice President and Chief Financial Officer violated Section 15 of such Act, respectively, by making certain alleged false and misleading statements and omissions in a registration statement filed in January 2010. The plaintiff sought unspecified damages on behalf of a purported class of purchasers of our common stock pursuant to our common stock offering
90
on or about January 21, 2010. On August 11, 2011, the District Court issued an Opinion and Order dismissing the SAC in its entirety for failure to state a claim upon which relief could be granted. A separate Order of Dismissal was filed on August 15, 2011. On September 14, 2011, the plaintiffs filed a Notice of Appeal to the United States Court of Appeals for the First Circuit, or the Court of Appeals. After briefing was completed by all parties, the Court of Appeals heard oral argument on May 11, 2012, and took the matter under advisement. On February 4, 2013, the Court of Appeals affirmed in part and reversed in part the District Court's Opinion and Order, and remanded the case to the District Court. On February 18, 2013, we filed a Petition for Panel Hearing or RehearingEn Banc, asking the Court of Appeals to reconsider its decision.
In July 2010, Sandoz GmbH, or Sandoz, filed with the European Patent Office, or the EPO, an opposition to our previously issued patent which covers ferumoxytol in the EU. In October 2012, at an oral hearing, the Opposition Division of the EPO revoked our European ferumoxytol patent. In December 2012, our notice of appeal was recorded with the EPO, which suspends the revocation of our patent. We will continue to defend the validity of this patent throughout the appeals process, which we expect to take two to three years. However, in the event that we do not experience a successful outcome from the appeals process, under EU regulations ferumoxytol would still be entitled to eight years of data protection and ten years of market exclusivity from the date of approval, which we believe would create barriers to entry for any generic version of ferumoxytol into the EU market until sometime between 2020 and 2022. This decision had no impact on our revenues for the year ended December 31, 2012. However, any future unfavorable outcome in this matter could negatively affect the magnitude and timing of future revenues, including royalties and milestone payments we may receive from Takeda pursuant to our collaboration agreement with Takeda. We continue to believe the patent is valid and intend to vigorously appeal the decision.
For additional information on our Legal Proceedings, please see the discussion under Part I, Item 3—Legal Proceedings.
Off-Balance Sheet Arrangements
As of December 31, 2012, we did not have any off-balance sheet arrangements as defined in Regulation S-K, Item 303(a)(4)(ii).
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK:
As of December 31, 2012 and 2011, our investments equaled $180.8 million and $166.2 million, respectively, and were invested in corporate debt securities, U.S. treasury and government agency securities, commercial paper and, as of December 31, 2011, the amount also included auction rate securities, or ARS. Our investments meet high credit quality and diversification standards, as specified in our investment policy. Our investment policy also limits the amount of our credit exposure to any one issue or issuer, excluding U.S. government entities, and seeks to manage these assets to achieve our goals of preserving principal, maintaining adequate liquidity at all times, and maximizing returns. These investments are subject to interest rate risk. The modeling technique used measures the change in fair values arising from an immediate hypothetical shift in market interest rates and assumes that ending fair values include principal plus accrued interest. If market interest rates for comparable investments were to increase immediately and uniformly by 50 basis points, or one-half of a percentage point, from levels as of December 31, 2012 and 2011, this would have resulted in a hypothetical decline in fair value of our investments, excluding our ARS, of approximately $1.0 million and $0.6 million, respectively, and if market interest rates for comparable investments were to decrease immediately and uniformly by 50 basis points, or one-half of a percentage point, from levels as of December 31, 2012 and 2011, this would have resulted in a hypothetical increase in fair value of our investments, excluding our ARS, of approximately $0.9 million and $0.5 million, respectively. These amounts are determined by considering the impact of the hypothetical interest rate movements on our available-for-sale
91
investment portfolios. This analysis does not consider the effect of credit risk as a result of the changes in overall economic activity that could exist in such an environment.
As of December 31, 2011, we held a total of $17.5 million in fair market value of ARS, reflecting an impairment of approximately $2.4 million as compared to the par value of these securities of $19.9 million. These securities were sold in 2012 for $18.3 million and we recognized a loss of $1.5 million on the sale.
92
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA:
Index To Consolidated Financial Statements
Management's Annual Report on Internal Control over Financial Reporting | 94 | ||
Report of Independent Registered Public Accounting Firm | 95 | ||
Consolidated Balance Sheets—as of December 31, 2012 and 2011 | 96 | ||
Consolidated Statements of Operations—for the years ended December 31, 2012, 2011 and 2010 | 97 | ||
Consolidated Statements of Comprehensive Loss—for the years ended December 31, 2012, 2011 and 2010 | 98 | ||
Consolidated Statements of Stockholders' Equity—as of December 31, 2012, 2011 and 2010 | 99 | ||
Consolidated Statements of Cash Flows—for the years ended December 31, 2012, 2011 and 2010 | 100 | ||
Notes to Consolidated Financial Statements | 101 |
93
MANAGEMENT'S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining adequate internal controls over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities and Exchange Act of 1934, as amended. Our internal control over financial reporting is a process designed under the supervision of our principal executive officer and principal financial officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we assessed the effectiveness of our internal control over financial reporting as of December 31, 2012 based on the framework inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, management concluded that our internal control over financial reporting was effective as of December 31, 2012.
The effectiveness of our internal control over financial reporting as of December 31, 2012 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included herein.
94
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of AMAG Pharmaceuticals, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, comprehensive loss, stockholders' equity, and cash flows present fairly, in all material respects, the financial position of AMAG Pharmaceuticals, Inc. and its subsidiaries at December 31, 2012 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2012 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2012, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
![]()
Boston, Massachusetts
March 1, 2013
95
AMAG Pharmaceuticals, Inc.
Consolidated Balance Sheets
(in thousands, except share and per share data)
| | As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 46,293 | $ | 63,474 | |||
Short-term investments | 180,750 | 148,703 | |||||
Accounts receivable, net | 6,410 | 5,932 | |||||
Inventories | 12,451 | 15,206 | |||||
Receivable from collaboration | 263 | 428 | |||||
Assets held for sale | 2,000 | — | |||||
Prepaid and other current assets | 6,213 | 6,288 | |||||
Total current assets | 254,380 | 240,031 | |||||
Property, plant and equipment, net | 3,297 | 9,206 | |||||
Long-term investments | — | 17,527 | |||||
Restricted cash | 460 | 460 | |||||
Total assets | $ | 258,137 | $ | 267,224 | |||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 3,515 | $ | 3,732 | |||
Accrued expenses | 20,338 | 28,916 | |||||
Deferred revenues | 9,104 | 6,346 | |||||
Total current liabilities | 32,957 | 38,994 | |||||
Long-term liabilities: | |||||||
Deferred revenues | 50,350 | 45,196 | |||||
Other long-term liabilities | 2,033 | 2,438 | |||||
Total liabilities | 85,340 | 86,628 | |||||
Commitments and contingencies (Notes M & N) | |||||||
Stockholders' equity: | |||||||
Preferred stock, par value $0.01 per share, 2,000,000 shares authorized; none issued | — | — | |||||
Common stock, par value $0.01 per share, 58,750,000 shares authorized; 21,506,754 and 21,306,413 shares issued and outstanding at December 31, 2012 and 2011, respectively | 215 | 213 | |||||
Additional paid-in capital | 632,487 | 625,133 | |||||
Accumulated other comprehensive loss | (3,247 | ) | (4,842 | ) | |||
Accumulated deficit | (456,658 | ) | (439,908 | ) | |||
Total stockholders' equity | 172,797 | 180,596 | |||||
Total liabilities and stockholders' equity | $ | 258,137 | $ | 267,224 | |||
The accompanying notes are an integral part of these consolidated financial statements.
96
AMAG Pharmaceuticals, Inc.
Consolidated Statements of Operations
(in thousands, except per share data)
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Revenues: | ||||||||||
U.S. product sales, net | $ | 58,287 | $ | 52,097 | $ | 59,339 | ||||
International product sales and royalties | 120 | — | — | |||||||
License fee and other collaboration revenues | 26,475 | 8,321 | 6,132 | |||||||
Other product sales and royalties | 496 | 831 | 774 | |||||||
Total revenues | 85,378 | 61,249 | 66,245 | |||||||
Costs and expenses: | ||||||||||
Cost of product sales | 14,220 | 10,531 | 7,606 | |||||||
Research and development expenses | 33,296 | 58,140 | 54,462 | |||||||
Selling, general and administrative expenses | 53,071 | 68,863 | 84,939 | |||||||
Restructuring expenses | 2,215 | 3,508 | 2,224 | |||||||
Total costs and expenses | 102,802 | 141,042 | 149,231 | |||||||
Other income (expense): | ||||||||||
Interest and dividend income, net | 1,286 | 1,747 | 1,741 | |||||||
(Losses) gains on investments, net | (1,466 | ) | (193 | ) | 408 | |||||
Fair value adjustment of settlement rights | — | — | (788 | ) | ||||||
Total other income (expense) | (180 | ) | 1,554 | 1,361 | ||||||
Net loss before income taxes | (17,604 | ) | (78,239 | ) | (81,625 | ) | ||||
Income tax benefit | 854 | 1,170 | 472 | |||||||
Net loss | $ | (16,750 | ) | $ | (77,069 | ) | $ | (81,153 | ) | |
Net loss per share: | ||||||||||
Basic and diluted | $ | (0.78 | ) | $ | (3.64 | ) | $ | (3.90 | ) | |
Weighted average shares outstanding used to compute net loss per share: | ||||||||||
Basic and diluted | 21,392 | 21,189 | 20,806 | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
97
AMAG Pharmaceuticals, Inc.
Consolidated Statements of Comprehensive Loss
(in thousands)
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Net loss | $ | (16,750 | ) | $ | (77,069 | ) | $ | (81,153 | ) | |
Other comprehensive income (loss): | ||||||||||
Unrealized gains (losses) on securities: | ||||||||||
Holding gains (losses) arising during period, net of tax | 129 | 1,980 | 497 | |||||||
Reclassification adjustment for (gains) losses included in net loss | 1,466 | 206 | 400 | |||||||
Net unrealized gains (losses) on securities | 1,595 | 2,186 | 897 | |||||||
Total comprehensive loss | $ | (15,155 | ) | $ | (74,883 | ) | $ | (80,256 | ) | |
The accompanying notes are an integral part of these consolidated financial statements.
98
AMAG Pharmaceuticals, Inc.
Consolidated Statements of Stockholders' Equity
(in thousands)
| | Common Stock | | | Accumulated Other Comprehensive Income (Loss) | | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders' Equity | ||||||||||||||||
| | Shares | Amount | |||||||||||||||||
Balance at December 31, 2009 | 17,363 | $ | 174 | $ | 432,414 | $ | (281,686 | ) | $ | (7,925 | ) | $ | 142,977 | ||||||
Net shares issued in connection with the exercise of stock options and restricted stock units | 132 | 1 | 1,336 | — | — | 1,337 | |||||||||||||
Shares issued in connection with employee stock purchase plan | 42 | — | 892 | — | — | 892 | |||||||||||||
Non-cash equity-based compensation | — | — | 14,777 | — | — | 14,777 | |||||||||||||
Unrealized gains on securities, net of tax of $0.5 million | — | — | — | — | 897 | 897 | |||||||||||||
Shares issued in connection with financing, net of financing costs of $8.1 million | 3,600 | 36 | 165,523 | — | — | 165,559 | |||||||||||||
Net loss | — | — | — | (81,153 | ) | — | (81,153 | ) | |||||||||||
Balance at December 31, 2010 | 21,137 | 211 | 614,942 | (362,839 | ) | (7,028 | ) | 245,286 | |||||||||||
Net shares issued in connection with the exercise of stock options and restricted stock units | 132 | 1 | 120 | — | — | 121 | |||||||||||||
Shares issued in connection with employee stock purchase plan | 37 | 1 | 507 | — | — | 508 | |||||||||||||
Non-cash equity-based compensation | — | — | 9,564 | — | — | 9,564 | |||||||||||||
Unrealized gains on securities, net of tax of $1.2 million | — | — | — | — | 2,186 | 2,186 | |||||||||||||
Net loss | — | — | — | (77,069 | ) | — | (77,069 | ) | |||||||||||
Balance at December 31, 2011 | 21,306 | 213 | 625,133 | (439,908 | ) | (4,842 | ) | 180,596 | |||||||||||
Net shares issued in connection with the exercise of stock options and restricted stock units | 178 | 2 | 98 | — | — | 100 | |||||||||||||
Shares issued in connection with employee stock purchase plan | 23 | — | 270 | — | — | 270 | |||||||||||||
Non-cash equity-based compensation | — | — | 6,986 | — | — | 6,986 | |||||||||||||
Unrealized gains on securities, net of tax of $0.9 million | — | — | — | — | 1,595 | 1,595 | |||||||||||||
Net loss | — | — | — | (16,750 | ) | — | (16,750 | ) | |||||||||||
Balance at December 31, 2012 | 21,507 | $ | 215 | $ | 632,487 | $ | (456,658 | ) | $ | (3,247 | ) | $ | 172,797 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
99
AMAG Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Cash flows from operating activities: | ||||||||||
Net loss | $ | (16,750 | ) | $ | (77,069 | ) | $ | (81,153 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||
Depreciation | 3,084 | 2,536 | 2,405 | |||||||
Impairment loss on assets held for sale | 1,100 | — | — | |||||||
Non-cash equity-based compensation expense | 7,024 | 10,038 | 14,523 | |||||||
Non-cash income tax benefit | (854 | ) | (1,170 | ) | (481 | ) | ||||
Amortization of premium/discount on purchased securities | 2,808 | 3,639 | 1,679 | |||||||
Fair value adjustment of settlement rights | — | — | 788 | |||||||
Losses (gains) on investments, net | 1,466 | 193 | (408 | ) | ||||||
Changes in operating assets and liabilities: | ||||||||||
Accounts receivable, net | (478 | ) | (147 | ) | 21,565 | |||||
Inventories | 5,891 | 1,506 | (6,675 | ) | ||||||
Receivable from collaboration | 165 | 13 | (441 | ) | ||||||
Prepaid and other current assets | 75 | 1,661 | (2,477 | ) | ||||||
Accounts payable and accrued expenses | (12,195 | ) | 1,698 | 2,745 | ||||||
Deferred revenues | 7,912 | (6,353 | ) | 46,697 | ||||||
Other long-term liabilities | (405 | ) | (349 | ) | (294 | ) | ||||
Total adjustments | 15,593 | 13,265 | 79,626 | |||||||
Net cash used in operating activities | (1,157 | ) | (63,804 | ) | (1,527 | ) | ||||
Cash flows from investing activities: | ||||||||||
Proceeds from sales or maturities of investments | 133,061 | 141,095 | 160,079 | |||||||
Purchase of investments | (149,406 | ) | (126,585 | ) | (262,597 | ) | ||||
Capital expenditures | (47 | ) | (507 | ) | (1,223 | ) | ||||
Net cash (used in) provided by investing activities | (16,392 | ) | 14,003 | (103,741 | ) | |||||
Cash flows from financing activities: | ||||||||||
Proceeds from the exercise of stock options | 98 | 121 | 1,337 | |||||||
Proceeds from the issuance of common stock, net of underwriting discounts and other expenses | — | — | 165,559 | |||||||
Proceeds from the issuance of common stock under ESPP | 270 | 508 | 892 | |||||||
Net cash provided by financing activities | 368 | 629 | 167,788 | |||||||
Net (decrease) increase in cash and cash equivalents | (17,181 | ) | (49,172 | ) | 62,520 | |||||
Cash and cash equivalents at beginning of the year | 63,474 | 112,646 | 50,126 | |||||||
Cash and cash equivalents at end of the year | $ | 46,293 | $ | 63,474 | $ | 112,646 | ||||
Supplemental data: | ||||||||||
Non-cash investing activities: | ||||||||||
Accrued construction in progress | $ | 228 | $ | — | $ | — | ||||
The accompanying notes are an integral part of these consolidated financial statements.
100
Notes to Consolidated Financial Statements
A. Description of Business
AMAG Pharmaceuticals, Inc., a Delaware corporation, was founded in 1981. We are a specialty pharmaceutical company focused on the development and commercialization of Feraheme® (ferumoxytol) Injection for Intravenous, or IV, use to treat iron deficiency anemia, or IDA. Currently, our principal source of revenue is from the sale ofFeraheme, which was approved for marketing in the U.S. in June 2009 by the U.S. Food and Drug Administration, or the FDA, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with chronic kidney disease, or CKD. We began commercial sale ofFeraheme in the U.S. in July 2009 through our own commercial organization, including a specialty sales force. We sellFeraheme to authorized wholesalers and specialty distributors, who in turn, sellFeraheme to healthcare providers who administerFeraheme primarily within hospitals, hematology and oncology centers, and nephrology clinics.
Outside of the U.S., ferumoxytol has been granted marketing approval in Canada, Switzerland and the European Union, or EU, for use as an IV iron replacement therapy for the treatment of IDA in adult patients with CKD. The European marketing authorization is valid in the current EU member states as well as in Iceland and Norway. Under our amended agreement with Takeda Pharmaceutical Company Limited, or Takeda, Takeda has an exclusive license to market and sell ferumoxytol in Canada, the EU and Switzerland, as well as certain other geographic territories. In Canada, Takeda promotes ferumoxytol under the trade nameFeraheme and in the EU and Switzerland, Takeda promotes ferumoxytol under the trade name Rienso® 30mg/ml solution for Injection. In 2012, we received a total of $33.0 million in milestone payments from Takeda associated with the EU approval and the commercial launches ofFeraheme/Rienso in Canada and the EU. In addition, in connection with the commercial launches ofFeraheme/Rienso by Takeda, we recorded revenue from product sales to Takeda and royalties on sales by Takeda of $0.1 million in 2012.
GastroMARK®, which is marketed and sold under the trade name Lumirem® outside of the U.S, is our oral contrast agent used for delineating the bowel during magnetic resonance imaging and is approved and marketed in the U.S., Europe and other countries through our licensees. In the second quarter of 2012, we terminated our commercial license agreements forGastroMARK. Following the completion of our obligations under these agreements in the first quarter of 2013, we intend to cease commercially manufacturing or sellingGastroMARK. Pursuant to the terms of the respective termination agreements, in June 2012, we paid our licensees aggregate termination fees of $1.6 million, which we recorded in selling, general and administrative expenses in our consolidated statement of operations.
We are subject to risks common to companies in the pharmaceutical industry including, but not limited to, our sole dependence on the success ofFeraheme/Rienso, uncertainties related to the protection of our proprietary technology, our dependence on third parties to manufactureFeraheme/Rienso, the potential development of significant safety or drug interaction problems with respect toFeraheme/Rienso, uncertainty of the regulatory approval process for the broaderFeraheme/Rienso indication or for potential alternative manufacturing facilities and processes, uncertainties related to potential collaborations, in-licensing arrangements or acquisition agreements, competition in our industry, uncertainties regarding market acceptance ofFeraheme/Rienso, our reliance on a limited number of customers, uncertainties related to patient insurance coverage and third-party reimbursement rates and terms forFeraheme/Rienso, our reliance on Takeda to commercializeFeraheme/Rienso in certain territories outside of the U.S., the potential inability of our third-party manufacturers to operate their facilities in compliance with current good manufacturing practices and manufacture sufficient quantities ofFeraheme/Rienso, our or our third-party manufacturers' potential inability to obtain raw or other materials, our potential inability to become profitable in the future, our limited experience commercializing and distributing a pharmaceutical product, our dependence on key personnel, the potential fluctuation of our operating results, uncertainties related to the impact of
101
current and future healthcare initiatives and legislation, potential differences between actual future results and the estimates or assumptions used by us in preparation of our consolidated financial statements, the volatility of our stock price, our potential inadvertent failure to comply with reporting and payment obligations under government pricing programs, our potential inadvertent failure to comply with the regulations of the FDA or other federal, state or foreign government agencies, uncertainties related to the actions of activist stockholders, potential product liability, potential legislative and regulatory changes, and potential costs and liabilities associated with pending or future litigation or patent challenges.
Throughout this Annual Report on Form 10-K, AMAG Pharmaceuticals, Inc. and our consolidated subsidiaries are collectively referred to as "the Company," "we," "us," or "our."
B. Summary of Significant Accounting Policies
Use of Estimates and Assumptions
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. The most significant estimates and assumptions are used in, but are not limited to, revenue recognition related to product sales and collaboration agreements, product sales allowances and accruals, assessing investments for potential other-than-temporary impairment and determining values of investments, estimates used to measure the fair value of our held for sale assets, accrued expenses, income taxes and equity-based compensation expense. Actual results could differ materially from those estimates.
Principles of Consolidation
The accompanying consolidated financial statements include our accounts and the accounts of our wholly-owned subsidiaries, AMAG Europe Limited, and AMAG Securities Corporation. AMAG Europe Limited was incorporated in October 2009 in London, England. AMAG Securities Corporation is a Massachusetts corporation which was incorporated in August 2007. All intercompany account balances and transactions between the companies have been eliminated.
Cash and Cash Equivalents
Cash and cash equivalents consists principally of cash held in commercial bank accounts, money market funds and U.S. Treasury securities having an original maturity of less than three months. At December 31, 2012, substantially all of our cash and cash equivalents were held in either commercial bank accounts or money market funds.
Investments
We account for and classify our investments as either "available-for-sale," "trading," or "held-to-maturity," in accordance with current guidance related to the accounting and classification of certain investments in debt and equity securities. The determination of the appropriate classification by us is based on a variety of factors, including management's intent at the time of purchase. As of December 31, 2012 and 2011, all of our investments were classified as available-for-sale securities.
Available-for-sale securities are those securities which we view as available for use in current operations, if needed. We generally classify our available-for-sale securities as short-term investments, even though the stated maturity date may be one year or more beyond the current balance sheet date. Available-for-sale investments are stated at fair value with their unrealized gains and losses included as a separate component of stockholders' equity entitled "Accumulated other comprehensive loss," until such gains and losses are realized or until an unrealized loss is considered other-than-temporary.
102
We recognize and report other-than-temporary impairments of our debt securities in accordance with current accounting guidance, which requires that for debt securities with a decline in fair value below amortized cost basis, an other-than-temporary impairment exists if (i) we have the intent to sell the security or (ii) it is more likely than not that we will be required to sell the security prior to recovery of its amortized cost basis. If either of these conditions is met, we recognize the difference between the amortized cost of the security and its fair value at the impairment measurement date in our consolidated statement of operations. If neither of these conditions is met, we must perform additional analyses to evaluate whether the unrealized loss is associated with the creditworthiness of the security rather than other factors, such as interest rates or market factors. These factors include evaluation of the security, issuer and other factors such as the duration of the period that, and extent to which, the fair value was less than cost basis, the financial health of and business outlook for the issuer, including industry and sector performance, operational and financing cash flow factors, overall market conditions and trends, underlying collateral, whether we have a favorable history in redeeming similar securities at prices at or above fair value, and credit ratings with respect to our investments provided by investments ratings agencies. If we determine from this analysis that we do not expect to receive cash flows sufficient to recover the entire amortized cost of the security, a credit loss exists. In this situation, the impairment is considered other-than-temporary and is recognized in our consolidated statement of operations.
Fair Value of Financial Instruments
Under current accounting standards, fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs.
Current accounting guidance establishes a hierarchy used to categorize how fair value is measured and which is based on three levels of inputs, of which the first two are considered observable and the third unobservable, as follows:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Assets Measured at Fair Value on a Recurring Basis
We hold certain assets that are required to be measured at fair value on a recurring basis, including our cash equivalents and short- and long-term investments. The following tables represent the
103
fair value hierarchy as of December 31, 2012 and 2011 for those assets that we measure at fair value on a recurring basis (in thousands):
| | Fair Value Measurements at December 31, 2012 Using: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Total | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||
Money market funds | $ | 24,058 | $ | 24,058 | $ | — | $ | — | |||||
Corporate debt securities | 111,690 | — | 111,690 | — | |||||||||
U.S. treasury and government agency securities | 59,569 | — | 59,569 | — | |||||||||
Commercial paper | 9,491 | — | 9,491 | — | |||||||||
| $ | 204,808 | $ | 24,058 | $ | 180,750 | $ | — | ||||||
| | Fair Value Measurements at December 31, 2011 Using: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Total | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||
Money market funds | $ | 55,995 | $ | 55,995 | $ | — | $ | — | |||||
Corporate debt securities | 94,626 | — | 94,626 | — | |||||||||
U.S. treasury and government agency securities | 48,086 | — | 48,086 | — | |||||||||
Commercial paper | 5,991 | — | 5,991 | — | |||||||||
Auction rate securities | 17,527 | — | — | 17,527 | |||||||||
| $ | 222,225 | $ | 55,995 | $ | 148,703 | $ | 17,527 | ||||||
With the exception of our money market funds, and previously, our auction rate securities, or ARS, which we sold in June 2012, and which were valued using Level 3 inputs, the fair value of our investments is primarily determined from independent pricing services which use Level 2 inputs to determine fair value. Independent pricing services normally derive security prices from recently reported trades for identical or similar securities, making adjustments based upon other significant observable market transactions. At the end of each reporting period, we perform quantitative and qualitative analyses of prices received from third parties to determine whether prices are reasonable estimates of fair value. After completing our analyses, we did not adjust or override any fair value measurements provided by our pricing services as of either December 31, 2012 or 2011. In addition, there were no transfers or reclassifications of any securities between Level 1 and Level 2 during either of the years ended December 31, 2012 or 2011.
We also analyze when the volume and level of activity for an asset or liability have significantly decreased and when circumstances indicate that a transaction may not be considered orderly. In order to determine whether the volume and level of activity for an asset or liability have significantly decreased, we assess current activity as compared to normal market activity for the asset or liability. We rely on many factors such as trading volume, trading frequency, the levels at which market participants indicate their willingness to buy and sell our securities, as reported by market participants, and current market conditions. Using professional judgment and experience, we evaluate and weigh the relevance and significance of all applicable factors to determine if there has been a significant decrease in the volume and level of activity for an asset, group of similar assets or liabilities. Similarly, in order to identify transactions that are not orderly, we take into consideration the activity in the market which can influence the determination and occurrence of an orderly transaction. Also, we inquire as to whether there may have been restrictions on the marketing of the security to a single or limited
104
number of participants. Where possible, we assess the financial condition of the seller to determine whether observed transactions may have been forced. If there is a significant disparity between the trading price for a security held by us as compared to the trading prices of similar recent transactions, we consider whether this disparity is an indicator of a disorderly trade. Using professional judgment and experience, we evaluate and weigh the relevance and significance of all applicable factors to determine if the evidence suggests that a transaction or group of similar transactions is not orderly. Based upon these procedures, we determined that market activity for our non-ARS assets appeared normal and that transactions did not appear disorderly as of December 31, 2012 and 2011.
In June 2012, we sold our remaining ARS portfolio, with a par value of $19.8 million, for proceeds of $18.3 million.
The following table provides a rollforward of Level 3 assets for the years ended December 31, 2012 and 2011 (in thousands):
| | December 31, 2012 | December 31, 2011 | |||||
|---|---|---|---|---|---|---|---|
Balance at beginning of period | $ | 17,527 | $ | 33,597 | |||
Transfers to Level 3 | — | — | |||||
Total gains (losses) (realized or unrealized): | |||||||
Included in earnings | (1,471 | ) | (210 | ) | |||
Included in other comprehensive income (loss) | 2,373 | 3,790 | |||||
Purchases, issuances, sales and settlements: | |||||||
Purchases | — | — | |||||
Issuances | — | — | |||||
Sales | (18,329 | ) | — | ||||
Settlements | (100 | ) | (19,650 | ) | |||
Balance at end of period | $ | — | $ | 17,527 | |||
The amount of total gains (losses) for the | |||||||
period included in earnings attributable to | |||||||
the change in unrealized gains (losses) | |||||||
relating to assets still held at end of period | $ | — | $ | — | |||
Assets Held for Sale
During 2012, we determined that certain assets related to our Cambridge, Massachusetts manufacturing facility, including the related land, building and certain equipment, met the criteria established by current accounting guidance for classifying assets as held for sale. As a result, in 2012, we reclassified these assets from property, plant and equipment to assets held for sale in our consolidated balance sheet. In anticipation of a future sale, we have valued these assets at the lower of their carrying amount or fair value less cost to sell to arrive at the estimated fair value of $2.0 million as of December 31, 2012. Prior to our determination that our Cambridge, Massachusetts manufacturing facility and related assets met the requirements to be classified as assets held for sale, we accelerated the depreciation on such assets to reflect our then estimated fair value. In doing so, we recorded $1.4 million of accelerated depreciation in our consolidated statement of operations for the year ended December 31, 2012. Upon determination that these assets met the criteria for held for sale, we recognized an impairment loss to decrease the carrying value of the assets to our best estimate of fair value and continue to evaluate the estimate of fair value on an ongoing basis. As a result, we have recognized an aggregate impairment loss of $1.1 million to decrease the carrying value of the assets to our best estimate of fair value as of December 31, 2012. Of these $2.5 million of non-cash charges, we recorded $2.3 million in cost of product sales and $0.2 million in research and development expenses in our 2012 consolidated statement of operations. The fair values of the land, building, and equipment were estimated using offers received from potential purchasers, real estate appraisals and other estimates from third-parties and accordingly, these assets have been classified as Level 3 assets.
105
Inventories
Inventories are stated at the lower of cost or market (net realizable value), with approximate cost being determined on a first-in, first-out basis.
Prior to initial approval from the FDA or other regulatory agencies, we expense costs relating to the production of inventory in the period incurred. After such time as the product receives initial regulatory approval, we begin to capitalize the inventory costs related to the product. Prior to the June 2009 FDA approval ofFeraheme for commercial sale in the U.S., all production costs related toFeraheme were expensed to research and development. Subsequent to receiving FDA approval, costs related to the production ofFeraheme are capitalized to inventory, including the costs of converting previously existing raw or other materials to inventory and vialing, labeling, and packaging inventory manufactured prior to approval whose costs had already been recorded as research and development expense. We continue to expense costs associated with clinical trial material as research and development expense.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost and depreciated when placed into service using the straight-line method, based on the following estimated useful lives: buildings—40 years; building improvements—over the shorter of the remaining useful life of the building or the life of the improvement; laboratory and production equipment—5 years; and furniture and fixtures—5 years. The furniture, fixtures, and leasehold improvements associated with our facility lease are being depreciated over the shorter of their useful lives or the remaining life of the original lease (excluding optional lease renewal terms).
Costs for capital assets not yet placed in service are capitalized on our balance sheets, and the cost of maintenance and repairs is expensed as incurred. Upon sale or other disposition of property and equipment, the cost and related depreciation are removed from the accounts and any resulting gain or loss is charged to our consolidated statement of operations. Currently, our long-lived assets consist entirely of property and equipment. Long-lived assets to be held and used are evaluated for impairment whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset (asset group) and its eventual disposition. In the event such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. Assets classified as held for sale are no longer subject to depreciation and are recorded at the lower of carrying value or estimated net realizable value.
Patents
We expense all patent-related costs as incurred.
Research and Development Expenses
Research and development expenses include external expenses, such as costs of clinical trials, contract research and development expenses, certain manufacturing research and development costs, regulatory filing fees, consulting and professional fees and expenses, and internal expenses, such as compensation of employees engaged in research and development activities, the manufacture of product needed to support research and development efforts, related costs of facilities, and other general costs related to research and development. Manufacturing costs are expensed as incurred until a product has received the necessary initial regulatory approval.
106
Advertising Costs
Advertising costs are expensed as incurred and are included in selling, general and administrative expenses in our consolidated statement of operations. Advertising costs, including promotional expenses and costs related to trade shows were $1.8 million, $3.1 million and $7.4 million for the years 2012, 2011 and 2010, respectively.
Revenue Recognition and Related Sales Allowances and Accruals
We recognize revenue from the sale ofFeraheme/Rienso as well as license fee and other collaboration revenues, including milestone payments, other product sale revenues, and royalties we receive from our licensees. We recognize revenue in accordance with current accounting guidance related to the recognition, presentation and disclosure of revenue in financial statements, which outlines the basic criteria that must be met to recognize revenue and provides guidance for disclosure of revenue in financial statements. We recognize revenue when:
- •
- Persuasive evidence of an arrangement exists;
- •
- Delivery of product has occurred or services have been rendered;
- •
- The sales price charged is fixed or determinable; and
- •
- Collection is reasonably assured.
U.S. Product Sales, Net
We record product sales allowances and accruals related to prompt payment discounts, chargebacks, government and other rebates, distributor, wholesaler and group purchasing organization, or GPO, fees, and product returns as a reduction of revenue in our consolidated statement of operations at the time product sales are recorded. Calculating these gross-to-net sales adjustments involves estimates and judgments based primarily on actualFeraheme sales data, forecasted customer buying patterns, and market research data related to utilization rates by various end-users. In addition, we also monitor our distribution channel to determine whether additional allowances or accruals are required based on inventory in our sales channel. An analysis of our product sales allowances and accruals for the years ended December 31, 2012, 2011 and 2010 is as follows (in thousands):
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Provision for U.S. product sales allowances and accruals | ||||||||||
Discounts and chargebacks | $ | 26,517 | $ | 13,851 | $ | 5,113 | ||||
Government and other rebates | 6,058 | 8,544 | 16,374 | |||||||
Medicaid rebate reserve adjustment | (621 | ) | (2,532 | ) | (599 | ) | ||||
Returns | (1,516 | ) | 1,259 | 1,334 | ||||||
Total provision for U.S. product sales allowances and accruals | $ | 30,438 | $ | 21,122 | $ | 22,222 | ||||
Total gross U.S. product sales | $ | 88,725 | $ | 73,219 | $ | 81,561 | ||||
Total provision for U.S. product sales allowances and accruals as a percent of total gross U.S. product sales | 34 | % | 29 | % | 27 | % | ||||
Classification of U.S. Product Sales Allowances and Accruals
Product sales allowances and accruals are primarily comprised of both direct and indirect fees, discounts and rebates and provisions for estimated product returns. Direct fees, discounts and rebates are contractual fees and price adjustments payable to wholesalers, specialty distributors and other customers that purchase products directly from us. Indirect fees, discounts and rebates are contractual
107
price adjustments payable to healthcare providers and organizations, such as certain physicians, clinics, hospitals, GPOs, and dialysis organizations that typically do not purchase products directly from us but rather from wholesalers and specialty distributors. In accordance with guidance related to accounting for fees and consideration given by a vendor to a customer, including a reseller of a vendor's products, these fees, discounts and rebates are presumed to be a reduction of the selling price ofFeraheme. Product sales allowances and accruals are based on definitive contractual agreements or legal requirements (such as Medicaid laws and regulations) related to the purchase and/or utilization of the product by these entities and are recorded in the same period that the related revenue is recognized. We estimate product sales allowances and accruals using either historical, actual and/or other data, including estimated patient usage, applicable contractual rebate rates, contract performance by the benefit providers, other current contractual and statutory requirements, historical market data based upon experience ofFeraheme and other products similar toFeraheme, specific known market events and trends such as competitive pricing and new product introductions, current and forecasted customer buying patterns and inventory levels, and the shelf life ofFeraheme. As part of this evaluation, we also review changes to federal and other legislation, changes to rebate contracts, changes in the level of discounts, and changes in product sales trends. Although allowances and accruals are recorded at the time of product sale, certain rebates are typically paid out, on average, up to six months or longer after the sale.
Allowances against receivable balances primarily relate to prompt payment discounts, provider chargebacks and certain government agency rebates and are recorded at the time of sale, resulting in a reduction in product sales revenue and the reporting of product sales receivables net of allowances. Accruals related to Medicaid and provider volume rebates, wholesaler and distributor fees, GPO fees, other discounts to healthcare providers and product returns are recorded at the time of sale, resulting in a reduction in product sales revenue and the recording of an increase in accrued expenses.
Discounts
We typically offer a 2% prompt payment discount to our customers as an incentive to remit payment in accordance with the stated terms of the invoice, generally thirty days. Because we anticipate that those customers who are offered this discount will take advantage of the discount, we accrue 100% of the prompt payment discount, at the time of sale, based on the gross amount of each invoice. We adjust the accrual quarterly to reflect actual experience.
Chargebacks
Chargeback reserves represent our estimated obligations resulting from the difference between the prices at which we sellFeraheme to wholesalers and the sales price ultimately paid to wholesalers under fixed price contracts by third-party payors, including governmental agencies. We determine our chargeback estimates based on actualFeraheme sales data and forecasted customer buying patterns. Actual chargeback amounts are determined at the time of resale to the qualified healthcare provider, and we generally issue credits for such amounts within several weeks of receiving notification from the wholesaler. Estimated chargeback amounts are recorded at the time of sale, and we adjust the allowance quarterly to reflect actual experience.
Government and Other Rebates
Government and other rebate reserves relate to our reimbursement arrangements with state Medicaid programs or performance rebate agreements with certain classes of trade. We determine our estimates for Medicaid rebates based on actualFeraheme sales data, forecasted customer buying patterns and market research data related to utilization rates by various end-users blended with historical experience of products similar toFeraheme sold by others. We currently have limited actual claims payment data, and therefore are not able to solely rely on our actualFeraheme claims
108
experience. In estimating these reserves, we provide for a Medicaid rebate associated with both those expected instances where Medicaid will act as the primary insurer as well as in those instances where we expect Medicaid will act as the secondary insurer. For rebates associated with reaching defined performance goals, we determine our estimates using actualFeraheme sales data and forecasted customer buying patterns. Rebate amounts generally are invoiced quarterly and are paid in arrears, and we expect to pay such amounts within several weeks of notification by the Medicaid or provider entity. Estimated government and other rebates are recorded at the time of sale and, with the exception of Medicaid as discussed below, we adjust the accrual quarterly to reflect actual experience.
During the years ended December 31, 2012, 2011 and 2010, we revised our estimated Medicaid utilization rate based on actual rebate claims received since the 2009 launch ofFeraheme, our expectations of state level activity, and estimated rebate claims not yet submitted, which resulted in a reduction of our estimated Medicaid rebate reserve related to prior periodFeraheme sales of $0.6 million, $2.5 million and $0.6 million, respectively. These changes in estimates were reflected as an increase in our net product sales for the years ended December 31, 2012, 2011 and 2010. As a result, our gross to net percentages for 2012, 2011 and 2010 were lower than they otherwise would have been had we not reduced our Medicaid rebate reserve. The reduction of our estimated Medicaid rebate reserve had an impact of $0.03, $0.12 and $0.03 per basic and diluted share for the years ended December 31, 2012, 2011 and 2010, respectively. We regularly assess our Medicaid reserve balance and the rate at which we accrue for claims against product sales. If we determine in future periods that our actual rebate experience is not indicative of expected claims, or if other factors affect estimated claims rates, we may be required to change our estimated Medicaid reserve and/or the current rate at which we estimate our Medicaid claims, which would affect our earnings in the period of the change in estimate and such change could be significant.
Distributor/Wholesaler and Group Purchasing Organization Fees
Fees under our arrangements with distributors and wholesalers are usually based upon units ofFeraheme purchased during the prior month or quarter and are usually paid by us within several weeks of our receipt of an invoice from the wholesaler or distributor, as the case may be. Fees under our arrangements with GPOs are usually based upon member purchases during the prior quarter and are generally billed by the GPO within 30 days after period end. Current accounting standards related to consideration given by a vendor to a customer, including a reseller of a vendor's products, specify that cash consideration given by a vendor to a customer is presumed to be a reduction of the selling price of the vendor's products or services and therefore should be characterized as a reduction of revenue. Consideration should be characterized as a cost incurred if we receive, or will receive, an identifiable benefit (goods or services) in exchange for the consideration and we can reasonably estimate the fair value of the benefit received. Because the fees we pay to wholesalers do not meet the foregoing conditions to be characterized as a cost, we have characterized these fees as a reduction of revenue and have included them in government and other rebates in the table above. We generally pay such amounts within several weeks of our receipt of an invoice from the distributor, wholesaler, or GPO. Accordingly, we accrue the estimated fee due at the time of sale, based on the contracted price invoiced to the customer. We adjust the accrual quarterly to reflect actual experience.
Product Returns
Consistent with industry practice, we generally offer our distributors and wholesaler customers a limited right to return product purchased directly from us principally based upon the product's expiration date which, once packaged, is currently four or five years in the U.S. We estimate product returns based upon historical experience since the 2009 launch ofFeraheme and trends of products similar toFeraheme sold by others. We currently have limited actual returns data, and therefore are not able to solely rely on our actual returns experience. We track actual returns by individual production
109
lots. Returns on lots eligible for credits under our returned goods policy are monitored and compared with historical return trends and rates.
We consider several additional factors in our product return estimation process, including our internal sales forecasts and inventory levels in the distribution channel. We expect that wholesalers and healthcare providers will not stock significant inventory due toFeraheme's cost and expense to store. Based on the level of inventory in the wholesale distribution channel, we determine whether an adjustment to the sales return reserve is appropriate.
We record an estimate of returns at the time of sale. If necessary, our estimated rate of returns may be adjusted for actual return experience as it becomes available and for known or expected changes in the marketplace. During 2012, we reduced our reserve for product returns by approximately $2.2 million primarily as a result of a lower than expected rate of product returns as well as the lapse of the product return period on certain manufacturedFeraheme lots that carried a two year expiration. As a result, the product returns provision applied to gross product sales for the year ended December 31, 2012 was a credit of $1.5 million, resulting in an increase to net product sales for the year. The reduction of our estimated product returns reserve had a positive impact of $0.10 per basic and diluted share for year ended December 31, 2012. We did not significantly adjust our reserve for product returns during 2011 or 2010.Feraheme is still early in its product lifecycle and returns experience may change over time. A future revision to our product returns estimate would result in a corresponding change to our net product sales in the period in which the change is made and could be significant.
International Product Sales and Royalties
We record all international product sales forFeraheme/Rienso sold to Takeda in deferred revenues in our consolidated balance sheet. We recognize these deferred revenues, and the associated cost of product sales, in our consolidated statement of operations at the time Takeda reports to us that sales have been made to its customers.
License Fee and Other Collaboration Revenues
The terms of product development and commercialization agreements entered into between us and our collaborative licensees may include non-refundable license fees, payments based on the achievement of certain milestones and performance goals, reimbursement of certain out-of-pocket costs, payment for manufacturing services, and royalties on product sales. We recognize license fee and research and development revenue under collaborative arrangements over the term of the applicable agreements using a proportional performance model, if practical. Otherwise, we recognize such revenue on a straight-line basis. Under this model, revenue is generally recognized in an amount equal to the lesser of the amount due under the agreements or an amount based on the proportional performance to date. In cases where project costs or other performance metrics are not estimable but there is an established contract period, revenues are recognized on a straight-line basis over the term of the relevant agreement. In cases where we are reimbursed for certain research and development costs associated with our collaboration agreements and where we are acting as the principal in carrying out these services, any reimbursement payments are recorded in license fee and other collaboration revenues in our consolidated statement of operations to match the costs that we incur during the period in which we perform those services. Nonrefundable payments and fees are recorded as deferred revenue upon receipt and may require deferral of revenue recognition to future periods.
Multiple Element Arrangements and Milestone Payments
We evaluate revenue from arrangements that have multiple elements to determine whether the components of the arrangement represent separate units of accounting as defined in the accounting
110
guidance related to revenue arrangements with multiple deliverables. Under current accounting guidance, which governs any agreements that contain multiple elements that are either entered into or materially modified subsequent to January 1, 2011, companies are required to establish the fair value of undelivered products and services based on a separate revenue recognition process using management's best estimate of the selling price for an undelivered item when there is no other means to determine the fair value of that undelivered item. Agreements entered into prior to January 1, 2011, that have not been materially modified, including our agreements with Takeda and 3SBio, Inc., or 3SBio, are accounted for under previous accounting guidance, which provides that an element of a contract can be accounted for separately if the delivered elements have standalone value and the fair value of all undelivered elements is determinable. If an element is considered to have standalone value but the fair value of any of the undelivered items cannot be determined, all elements of the arrangement are recognized as revenue as a single unit of accounting over the period of performance for such undelivered items or services. Significant management judgment is required in determining what elements constitute deliverables and what deliverables or combination of deliverables should be considered units of accounting.
When multiple deliverables are combined and accounted for as a single unit of accounting, we base our revenue recognition pattern on the last to be delivered element. Revenue is recognized using either a proportional performance or straight-line method, depending on whether we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and whether such performance obligations are provided on a best-efforts basis. To the extent we cannot reasonably estimate our performance obligations, we recognize revenue on a straight-line basis over the period we expect to complete our performance obligations. Significant management judgment is required in determining the level of effort required under an arrangement and the period over which we are expected to complete our performance obligations under an arrangement. We may have to revise our estimates based on changes in the expected level of effort or the period we expect to complete our performance obligations.
Our collaboration agreements may entitle us to additional payments upon the achievement of performance-based milestones. If a milestone involves substantive effort on our part and its achievement is not considered probable at the inception of the collaboration, we recognize the milestone consideration as revenue in the period in which the milestone is achieved only if it meets the following additional criteria:
- •
- The milestone consideration received is commensurate with either the level of effort required to achieve the milestone or the enhancement of the value of the item delivered as a result of a specific outcome resulting from our performance to achieve the milestone;
- •
- The milestone is related solely to past performance; and
- •
- The milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement.
There is significant judgment involved in determining whether a milestone meets all of these criteria. For milestones that do not meet the above criteria and are therefore not considered substantive milestones, we recognize that portion of the milestone payment equal to the percentage of the performance period completed at the time the milestone is achieved and the above conditions are met. The remaining portion of the milestone will be recognized over the remaining performance period using a proportional performance or straight-line method.
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in our consolidated balance sheets. Amounts not expected to be recognized within the next 12 months are classified as long-term deferred revenue.
111
Shipping and Handling Costs
We utilize a third-party logistics provider, which is a subsidiary of one of our distribution customers, to provide us with various shipping and handling services related to sales ofFeraheme. Current accounting standards related to consideration given by a vendor to a customer, including a reseller of a vendor's products, specify that cash consideration given by a vendor to a customer is presumed to be a reduction of the selling price of the vendor's products or services and therefore should be characterized as a reduction of revenue. However, that presumption is overcome and the consideration should be characterized as a cost incurred if both of the following conditions are met:
- •
- We receive, or will receive, an identifiable benefit (goods or services) in exchange for the consideration; and
- •
- We can reasonably estimate the fair value of the benefit received.
Since both of the above conditions were met with respect to the costs we incurred for shipping and handling services incurred with our third-party logistics provider, we have recorded $0.2 million, $0.1 million and $0.2 million as a selling, general and administrative expense during the years ended December 31, 2012, 2011 and 2010, respectively.
Equity-Based Compensation
Under the fair value recognition guidance of equity-based compensation accounting rules, equity-based compensation cost is generally required to be measured at the grant date (based upon an estimate of the fair value of the compensation granted) and recorded to expense over the requisite service period, which generally is the vesting period. Because equity-based compensation expense is based on awards ultimately expected to vest, we must make certain judgments about whether employees and directors will complete the requisite service period. Accordingly, we have reduced the compensation expense being recognized for estimated forfeitures. Under current accounting guidance, forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based upon historical experience, adjusted for unusual events such as the corporate restructurings in 2012, 2011 and 2010, which resulted in higher than expected turnover and forfeitures in those years. If factors change and we employ different assumptions in future periods, the compensation expense that we record in the future may differ significantly from what we have recorded in the current period.
We estimate the fair value of equity-based compensation involving stock options based on the Black-Scholes option pricing model. We estimate the fair value of our restricted stock units whose vesting is contingent upon market conditions using the Monte-Carlo simulation model. These models require the input of several factors such as the expected option term, the expected risk-free interest rate over the expected option term, the expected volatility of our stock price over the expected option term, and the expected dividend yield over the expected option term and are subject to various assumptions. The fair value of awards whose fair values are calculated using the Black-Scholes option pricing model is generally being amortized on a straight-line basis over the requisite service period and is recognized based on the proportionate amount of the requisite service period that has been rendered during each reporting period. The fair value of awards with market conditions is being amortized based upon the estimated derived service period. We believe our valuation methodologies are appropriate for estimating the fair value of the equity awards we grant to our employees and directors. Our equity award valuations are estimates and thus may not be reflective of actual future results or amounts ultimately realized by recipients of these grants. These amounts, and the amounts applicable to future quarters, are also subject to future quarterly adjustments based upon a variety of factors, which include, but are not limited to, changes in estimated forfeiture rates and the issuance of new equity-based awards. The fair value of restricted stock units granted to our employees and directors is determined based upon the quoted closing market price per share on the date of grant, adjusted for estimated
112
forfeitures. As with any accounting policy that applies judgments and estimates, actual results could significantly differ from those estimates which could result in a material adverse impact to our financial results.
Income Taxes
Deferred tax assets and deferred tax liabilities are recognized based on temporary differences between the financial reporting and tax basis of assets and liabilities using future enacted rates. A valuation allowance is recorded against deferred tax assets if it is more likely than not that some or all of our deferred tax assets will not be realized.
Concentrations and Significant Customer Information
Financial instruments which potentially subject us to concentrations of credit risk consist principally of cash, investments, and accounts receivable. As of December 31, 2012, our cash, cash equivalents and investments amounted to approximately $227.0 million. We currently invest our excess cash primarily in U.S. government and agency money market funds, and investments in corporate debt securities, U.S. treasury and government agency securities and commercial paper. As of December 31, 2012 we had approximately $24.1 million of our total $46.3 million cash and cash equivalents balance invested in institutional money market funds, of which $16.3 million was invested in a single fund, which is collateralized solely by U.S. treasury and government agency securities.
Our operations are located solely within the U.S. We are focused principally on developing, manufacturing, and commercializingFeraheme/Rienso. We perform ongoing credit evaluations of our customers and generally do not require collateral. The following table sets forth customers who represented 10% or more of our total revenues for the years ended December 31, 2012, 2011 and 2010.
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
AmerisourceBergen Drug Corporation | 34 | % | 41 | % | 36 | % | ||||
Takeda Pharmaceuticals Company Limited | 31 | % | 13 | % | <10 | % | ||||
McKesson Corporation | 17 | % | 21 | % | <10 | % | ||||
Cardinal Health, Inc. | 12 | % | 13 | % | <10 | % | ||||
Metro Medical Supply, Inc. | <10 | % | <10 | % | 21 | % | ||||
In addition, approximately 32% of our end-user demand in 2012 was generated by members of a single GPO with which we have contracted. Revenues from customers outside of the U.S. amounted to approximately 32%, 14% and 10% of our total revenues for the years ended December 31, 2012, 2011 and 2010, respectively, and were principally related to collaboration revenue recognized in connection with our collaboration agreement with Takeda, which is based in Japan.
Comprehensive Income (Loss)
The current accounting guidance related to comprehensive income (loss) requires us to display comprehensive loss and its components as part of our consolidated financial statements. Our comprehensive loss consists of net loss and other comprehensive income (loss). Other comprehensive income (loss) includes changes in equity that are excluded from net loss, which for all periods presented related to unrealized holding gains and losses on available-for-sale investments, net of tax.
113
Net Loss per Share
We compute basic net loss per share by dividing net loss by the weighted average number of common shares outstanding during the relevant period. The components of basic and diluted net loss per share were as follows (in thousands, except per share data):
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Net loss | $ | (16,750 | ) | $ | (77,069 | ) | $ | (81,153 | ) | |
Weighted average common shares outstanding | 21,392 | 21,189 | 20,806 | |||||||
Net loss per share: | ||||||||||
Basic and diluted | $ | (0.78 | ) | $ | (3.64 | ) | $ | (3.90 | ) | |
The following table sets forth the potential common shares issuable upon the exercise of outstanding options and the vesting of restricted stock units (prior to consideration of the treasury stock method), the total of which was excluded from our computation of diluted net loss per share because such options and restricted stock units were anti-dilutive due to a net loss in the relevant periods (in thousands):
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Options to purchase shares of common stock | 2,190 | 1,817 | 2,411 | |||||||
Shares of common stock issuable upon the vesting of restricted stock units | 374 | 669 | 385 | |||||||
Total | 2,564 | 2,486 | 2,796 | |||||||
Reclassifications
Certain amounts in prior periods have been reclassified in order to conform to the current period presentation.
C. Investments
As of December 31, 2012 and 2011, our investments equaled $180.8 million and $166.2 million, respectively, and consisted of securities classified as available-for-sale in accordance with accounting standards which provide guidance related to accounting and classification of certain investments in debt and equity securities.
114
The following is a summary of our investments as of December 31, 2012 and 2011 (in thousands):
| | December 31, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | |||||||||
Corporate debt securities | �� | ||||||||||||
Due in one year or less | $ | 52,332 | $ | 88 | $ | (6 | ) | $ | 52,414 | ||||
Due in one to three years | 59,176 | 137 | (37 | ) | 59,276 | ||||||||
U.S. treasury and government agency securities | |||||||||||||
Due in one year or less | 24,795 | 86 | — | 24,881 | |||||||||
Due in one to three years | 34,606 | 84 | (2 | ) | 34,688 | ||||||||
Commercial paper | |||||||||||||
Due in one year or less | 9,494 | 1 | (4 | ) | 9,491 | ||||||||
Total investments | $ | 180,403 | $ | 396 | $ | (49 | ) | $ | 180,750 | ||||
| | December 31, 2011 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | |||||||||
Short-term investments: | |||||||||||||
Corporate debt securities | |||||||||||||
Due in one year or less | $ | 74,687 | $ | 81 | $ | (115 | ) | $ | 74,653 | ||||
Due in one to three years | 19,950 | 73 | (50 | ) | 19,973 | ||||||||
U.S. treasury and government agency securities | |||||||||||||
Due in one year or less | 26,770 | 67 | (7 | ) | 26,830 | ||||||||
Due in one to three years | 21,028 | 228 | — | 21,256 | |||||||||
Commercial paper | |||||||||||||
Due in one year or less | 5,997 | — | (6 | ) | 5,991 | ||||||||
Total short-term investments | $ | 148,432 | $ | 449 | $ | (178 | ) | $ | 148,703 | ||||
Long-term investments: | |||||||||||||
Auction rate securities | |||||||||||||
Due after five years | 19,900 | — | (2,373 | ) | 17,527 | ||||||||
Total long-term investments | $ | 19,900 | $ | — | $ | (2,373 | ) | $ | 17,527 | ||||
Total investments | $ | 168,332 | $ | 449 | $ | (2,551 | ) | $ | 166,230 | ||||
Auction Rate Securities
In June 2012, we sold our remaining ARS portfolio, with a par value of $19.8 million, for proceeds of $18.3 million and recognized a loss of approximately $1.5 million in other income (expense) in our consolidated statement of operations for the year ended December 31, 2012.
Impairments and Unrealized Gains and Losses on Investments
The following is a summary of the fair value of our investments with unrealized losses that are deemed to be temporarily impaired and their respective gross unrealized losses aggregated by
115
investment category and length of time that individual securities have been in a continuous unrealized loss position as of December 31, 2012 and 2011 (in thousands):
| | December 31, 2012 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Less than 12 Months | 12 Months or Greater | Total | ||||||||||||||||
| | Fair Value | Unrealized Losses | Fair Value | Unrealized Losses | Fair Value | Unrealized Losses | |||||||||||||
Corporate debt securities | $ | 37,036 | $ | (43 | ) | $ | — | $ | — | $ | 37,036 | $ | (43 | ) | |||||
U.S. treasury and government agency securities | 6,271 | (2 | ) | — | — | 6,271 | (2 | ) | |||||||||||
Commercial paper | 3,992 | (4 | ) | — | — | 3,992 | (4 | ) | |||||||||||
| $ | 47,299 | $ | (49 | ) | $ | — | $ | — | $ | 47,299 | $ | (49 | ) | ||||||
| | December 31, 2011 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Less than 12 Months | 12 Months or Greater | Total | ||||||||||||||||
| | Fair Value | Unrealized Losses | Fair Value | Unrealized Losses | Fair Value | Unrealized Losses | |||||||||||||
Corporate debt securities | $ | 34,097 | $ | (161 | ) | $ | 4,124 | $ | (4 | ) | $ | 38,221 | $ | (165 | ) | ||||
U.S. treasury and government agency securities | 8,841 | (7 | ) | — | — | 8,841 | (7 | ) | |||||||||||
Commercial paper | 5,991 | (6 | ) | — | — | 5,991 | (6 | ) | |||||||||||
Auction rate securities | — | — | 19,900 | (2,373 | ) | 19,900 | (2,373 | ) | |||||||||||
| $ | 48,929 | $ | (174 | ) | $ | 24,024 | $ | (2,377 | ) | $ | 72,953 | $ | (2,551 | ) | |||||
We did not recognize any unrealized other-than-temporary impairment losses in our consolidated statements of operations related to our securities during either of the years ended December 31, 2012 or 2011. Future events may occur, or additional information may become available, which may cause us to identify credit losses where we do not expect to receive cash flows sufficient to recover the entire amortized cost basis of a security and which may necessitate the recording of future realized losses on securities in our portfolio. Significant losses in the estimated fair values of our investments could have a material adverse effect on our earnings in future periods.
Realized Gains and Losses on Investments
Gains and losses are determined on the specific identification method. During 2012, we recorded realized losses of $1.5 million to our consolidated statement of operations related to the sale of our then remaining ARS portfolio, as discussed above.
D. Accounts Receivable, Net
Our net accounts receivable were $6.4 million and $5.9 million as of December 31, 2012 and 2011, respectively, and primarily represented amounts due from wholesalers and distributors to whom we sellFeraheme directly. Accounts receivable are recorded net of reserves for estimated chargeback obligations, prompt payment discounts and any allowance for doubtful accounts. Reserves for other sales-related allowances such as rebates, distribution and other fees, and product returns are included in accrued expenses in our consolidated balance sheets.
As part of our credit management policy, we perform ongoing credit evaluations of our customers, and we have not required collateral from any customer. To date, we have not experienced significant bad debts. Accordingly, we have not established an allowance for doubtful accounts at either December 31, 2012 or 2011. If the financial condition of any of our significant customers was to
116
deteriorate and result in an impairment of its ability to make payments owed to us, an allowance for doubtful accounts may be required which could have a material effect on earnings in the period of any such adjustment.
Customers which represented greater than 10% of our accounts receivable balances as of December 31, 2012 and 2011 were as follows:
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
AmerisourceBergen Drug Corporation | 48 | % | 44 | % | |||
McKesson Corporation | 28 | % | 33 | % | |||
Cardinal Health, Inc. | 18 | % | 15 | % | |||
E. Inventories
Our major classes of inventories were as follows as of December 31, 2012 and 2011 (in thousands):
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
Raw materials | $ | 2,652 | $ | 1,892 | |||
Work in process | 2,524 | 3,696 | |||||
Finished goods | 7,275 | 9,618 | |||||
Total inventories | $ | 12,451 | $ | 15,206 | |||
During 2012, we wrote-off $0.6 million of inventory which was initially produced to validate the manufacturing process at third-party suppliers and which we no longer believed was suitable for sale. We have recorded the $0.6 million write-off in research and development expenses. In addition, during 2012, we wrote-off $0.6 million of commercial inventory deemed no longer saleable, which we recorded in cost of goods sold. We reserved $0.7 million of additional inventory related to our ongoing divestiture of our Cambridge, Massachusetts manufacturing facility and have recorded the reserve in restructuring costs.
On a quarterly basis, we analyze our inventory levels to determine whether we have any obsolete, expired, or excess inventory. If any inventory is expected to expire prior to being sold, has a cost basis in excess of its net realizable value, is in excess of expected sales requirements as determined by internal sales forecasts, or fails to meet commercial sale specifications, the inventory is written-down through a charge to cost of goods sold. The determination of whether inventory costs will be realizable requires estimates by management. A critical input in this determination is future expected inventory requirements, based on internal sales forecasts and forecasts received from Takeda. Once packaged,Feraheme/Rienso currently has a shelf-life of four or five years in the U.S. and between two and three years outside of the U.S., and as a result of comparison to internal sales forecasts, we expect to fully realize the carrying value of our currentFeraheme/Rienso finished goods inventory. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required. Charges for inventory write-downs are not reversed if it is later determined that the product is saleable.
117
F. Property, Plant and Equipment, Net
Property, plant and equipment consisted of the following as of December 31, 2012 and 2011, respectively (in thousands):
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
Land | $ | — | $ | 360 | |||
Buildings and improvements | 5,373 | 11,308 | |||||
Laboratory and production equipment | 115 | 7,662 | |||||
Furniture and fixtures | 5,326 | 5,382 | |||||
Construction in process | 228 | 86 | |||||
| 11,042 | 24,798 | ||||||
Less—accumulated depreciation | (7,745 | ) | (15,592 | ) | |||
Property, plant and equipment, net | $ | 3,297 | $ | 9,206 | |||
During the third quarter of 2012, we determined that certain assets related to our Cambridge, Massachusetts manufacturing facility, including the related land, building and certain equipment, met the criteria established by current accounting guidance for classifying assets as held for sale. As a result, we reclassified these assets from property, plant and equipment to assets held for sale in our consolidated balance sheet during 2012. We have classified these assets as current as we expect to complete the sale within one year. Current accounting guidance requires us to record assets held for sale at the lower of the carrying amount or fair value less cost to sell and discontinue the recognition of depreciation. Based on such guidance, we recorded the value of these assets at $2.0 million, their estimated fair market value as of December 31, 2012. Prior to our determination that our Cambridge manufacturing facility and related assets met the requirements to be classified as assets held for sale, we accelerated the depreciation on such assets to reflect our then estimated fair value. In doing so, we recorded $1.4 million of accelerated depreciation in our consolidated statements of operations in 2012. Upon determination that these assets met the criteria for held for sale, we recognized an impairment loss to decrease the carrying value of the assets to our best estimate of fair value, and continue to evaluate the estimate of fair value on an ongoing basis. As a result, we have recognized an aggregate impairment loss of $1.1 million to decrease the carrying value of the assets to our best estimate of fair value as of December 31, 2012. The fair values of the land, building and equipment were estimated using offers received from potential purchasers, real estate appraisals and other estimates from third parties.
G. Current and Long-Term Liabilities
Accrued Expenses
Accrued expenses consisted of the following as of December 31, 2012 and 2011 (in thousands):
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
Clinical, manufacturing and regulatory consulting fees and expenses | $ | 7,737 | $ | 11,468 | |||
Salaries, bonuses, and other compensation | 5,236 | 5,924 | |||||
Commercial rebates, fees and returns | 3,448 | 5,943 | |||||
Professional, license, and other fees and expenses | 1,719 | 1,966 | |||||
Restructuring expense | 1,383 | 2,366 | |||||
Commercial consulting fees and expenses | 815 | 1,249 | |||||
Total accrued expenses | $ | 20,338 | $ | 28,916 | |||
118
Deferred Revenues
Deferred revenues consisted of the following as of December 31, 2012 and 2011 (in thousands):
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
Short-term deferred revenues: | |||||||
Takeda | $ | 8,854 | $ | 6,096 | |||
Other short-term deferred revenues | 250 | 250 | |||||
Total | $ | 9,104 | $ | 6,346 | |||
Long-term deferred revenues: | |||||||
Takeda | $ | 49,350 | $ | 44,196 | |||
3SBio | 1,000 | 1,000 | |||||
Total | $ | 50,350 | $ | 45,196 | |||
During 2010, under the terms of our License, Development and Commercialization Agreement, or the Takeda Agreement, we received certain payments, including a $60.0 million upfront fee and $1.0 million reimbursed to us for certain expenses incurred prior to entering the agreement. We have recorded such payments as deferred revenue which we are recognizing on a straight-line basis over a period of 10 years, which represents the current patent life ofFeraheme/Rienso and our best estimate of the period over which we will substantially perform our obligations. In addition, during 2012, we received an aggregate of $18.0 million in milestone payments from Takeda associated with the commercial launches ofFeraheme/Rienso in Canada and the EU. These milestone payments were considered non-substantive milestone payments and accounted for in accordance with our revenue attribution method, as described in more detail below in Note N. Therefore, we are amortizing the $18.0 million using the proportional performance method over the original life of the Takeda Agreement. During 2012, we recorded $5.0 million of the $18.0 million to license fee and other collaboration revenues in our consolidated statement of operations and have included the remaining $13.0 million in our deferred revenues in our consolidated balance sheet.
In consideration of the grant of the license to 3SBio in 2008, we received an upfront payment of $1.0 million, the recognition of which has been deferred and will be recognized under the proportional performance methodology over the remaining portion of the thirteen year initial term of the agreement once we begin to supplyFeraheme to 3SBio.
Other Long-Term Liabilities
Other long-term liabilities at both December 31, 2012 and 2011 consisted solely of deferred rent related to the lease of our principal executive offices in Lexington, Massachusetts.
H. Income Taxes
For the years ended December 31, 2012, 2011 and 2010, we recognized $0.9 million, $1.2 million and $0.5 million in current federal income tax benefits, respectively. These federal income tax benefits were the result of the recognition of corresponding income tax expense associated with the decrease in the unrealized loss on our investments, primarily related to our ARS, which we carried at fair market value during these respective periods. The corresponding income tax expense was recorded in other comprehensive income (loss). Due to the uncertainty surrounding the realization of favorable tax attributes in future tax returns, we have recorded a full valuation allowance against our otherwise recognizable net deferred tax assets.
119
The reconciliation of the statutory U.S. federal income tax rate to our effective income tax rate is as follows:
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Statutory U.S. federal tax rate | (34.0 | )% | (34.0 | )% | (34.0 | )% | ||||
State taxes, net of federal benefit | 4.2 | % | (3.4 | )% | (5.8 | )% | ||||
Equity-based compensation expense | 42.4 | % | 2.4 | % | 1.7 | % | ||||
Permanent items, net | 1.2 | % | 0.4 | % | 0.5 | % | ||||
Tax credits | 0.8 | % | (1.6 | )% | (2.2 | )% | ||||
Valuation allowance | (19.5 | )% | 34.7 | % | 39.2 | % | ||||
Total tax (benefit) expense | (4.9 | )% | (1.5 | )% | (0.6 | )% | ||||
Deferred tax assets and deferred tax liabilities are recognized based on temporary differences between the financial reporting and tax basis of assets and liabilities using future enacted rates. A valuation allowance is recorded against deferred tax assets if it is more likely than not that some or all of the deferred tax assets will not be realized. The components of our deferred tax assets and liabilities are as follows (in thousands):
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
Assets | |||||||
Net operating loss carryforwards | $ | 75,740 | $ | 75,738 | |||
Tax credit carryforwards | 12,403 | 12,560 | |||||
Deferred revenue | 22,315 | 19,321 | |||||
Equity-based compensation expense | 3,681 | 10,331 | |||||
Capitalized research & development | 45,137 | 43,463 | |||||
Other | 4,239 | 6,406 | |||||
Property, Plant, and Equipment Depreciation | 1,393 | — | |||||
Liabilities | |||||||
Property, Plant, and Equipment Depreciation | — | (130 | ) | ||||
| 164,908 | 167,689 | ||||||
Valuation allowance | (164,908 | ) | (167,689 | ) | |||
Net deferred taxes | $ | — | $ | — | |||
Due to the uncertainty surrounding the realization of favorable tax attributes in future tax returns, we have recorded a full valuation allowance against our otherwise recognizable net deferred tax assets. The valuation allowance decreased by approximately $2.8 million for the year ended December 31, 2012, primarily due to an increase in our net operating loss, or NOL, carryforwards, capitalized research and development expense, and offset by a decrease in our equity-based compensation expense. The valuation allowance increased by approximately $26.8 million and $27.9 million for the years ended December 31, 2011 and 2010, respectively, primarily due to an increase in our NOL carryforwards, capitalized research and development expense, and equity based compensation expense.
At December 31, 2012, we had federal NOL carryforwards of approximately $203.5 million and state NOL carryforwards of up to $132.7 million. We also had federal capital loss carryforwards of $3.3 million to offset future capital gains and an additional $24.4 million and $5.6 million of federal and state NOLs, respectively, not reflected above which were attributable to deductions from the exercise of equity awards. The benefit from these deductions will be recorded as a credit to additional paid-in capital if and when realized through a reduction of taxes paid in cash. Our federal NOLs and our most significant state NOLs expire at various dates through 2032. Our capital loss carryforwards will expire
120
through 2017. In addition, we have federal and state tax credits of approximately $9.2 million and $4.9 million, respectively, to offset future tax liabilities. Our tax credits will expire periodically through 2032 if not utilized.
Utilization of our NOLs and research and development, or R&D, credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future in accordance with Section 382 of the Internal Revenue Code of 1986, or Section 382, as well as similar state provisions. These ownership changes may limit the amount of NOL and R&D credit carryforwards that can be utilized annually to offset future taxable income and taxes, respectively. In general, an ownership change as defined by Section 382 results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. Since our formation, we have raised capital through the issuance of capital stock on several occasions. These financings, combined with the purchasing shareholders' subsequent disposition of those shares, may have resulted in a change of control as defined by Section 382 or could result in a change of control in the future upon subsequent disposition. In May 2011, we conducted an analysis under Section 382 to determine if historical changes in ownership through December 31, 2010 would limit or otherwise restrict our ability to utilize these NOL and R&D credit carryforwards. As a result of this analysis, we do not believe there are any significant limitations on our ability to utilize these carryforwards. However, changes in ownership after December 31, 2010 could affect the limitation in future years. Any limitation may result in expiration of a portion of the NOL or R&D credit carryforwards before utilization.
At December 31, 2012 and 2011, we had no unrecognized tax benefits. We have not, as yet, conducted a study of our R&D credit carryforwards. Such a study could result in an adjustment to our R&D credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against our R&D credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the consolidated balance sheet or statement of operations if an adjustment were required.
We would recognize both accrued interest and penalties related to unrecognized benefits in income tax expense. We have not recorded any interest or penalties on any unrecognized benefits since inception.
The statute of limitations for assessment by the Internal Revenue Service, or the IRS, and state tax authorities is closed for tax years prior to December 31, 2009, although carryforward attributes that were generated prior to tax year 2009 may still be adjusted upon examination by the IRS or state tax authorities if they either have been or will be used in a future period. We file income tax returns in the U.S. federal and various state jurisdictions. There are currently no federal or state audits in progress.
On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which reinstated, retroactive to January 1, 2012, certain tax benefits that had previously expired. In accordance with the financial accounting standards for income taxes, we are required to account for the effects of changes in tax law and rates on deferred tax balances in the period the legislation is enacted. As this legislation was enacted in January 2013, our 2012 financial statements were not affected by this legislation.
I. Equity-Based Compensation
We currently maintain several equity compensation plans, including our Second Amended and Restated 2007 Equity Incentive Plan, or the 2007 Plan, our Amended and Restated 2000 Stock Plan, or the 2000 Plan, and our 2010 Employee Stock Purchase Plan, or the 2010 ESPP. During 2012, we also granted equity to our chief executive officer through an inducement grant that was outside of these plans.
121
Second Amended and Restated 2007 Equity Incentive Plan
Our 2007 Plan was originally approved by our stockholders in November 2007. In each of May 2009 and May 2010, our stockholders approved proposals to amend and restate our 2007 Plan to, among other things, increase the number of shares authorized for issuance thereunder by 600,000 and 800,000 shares, respectively. In addition, the amendment approved by our stockholders in May 2009 replaced a limitation on the number of shares in the aggregate which could be issued under the 2007 Plan with respect to restricted stock units, restricted stock, stock and similar equity interests in our company with a fungible share reserve whereby the number of shares available for issuance under the 2007 Plan is reduced by one share of our common stock issued pursuant to an option or stock appreciation right and by 1.5 shares for each share of our common stock issued pursuant to a restricted stock unit award or other similar equity-based award.
The 2007 Plan provides for the grant of stock options, restricted stock units, restricted stock, stock, and other equity interests in our company to employees, officers, directors, consultants, and advisors of our company and our subsidiaries. We generally issue common stock from previously authorized but unissued shares to satisfy option exercises and restricted stock awards. The terms and conditions of each such grant, including, but not limited to, the number of shares, the exercise price, term of the option/award and vesting requirements, are determined by our Board of Directors, or Board, or the Compensation Committee of our Board. Our Board may award stock options in the form of nonqualified stock options or incentive stock options, or ISOs. ISOs may be granted at an exercise price no less than fair market value of a share of our common stock on the date of grant, as determined by our Board or the Compensation Committee of our Board, subject to certain limitations.
Our Board establishes the vesting schedule for stock options and the method of payment for the exercise price. In general, our equity-based awards are subject to three or four year vesting. Our standard stock option agreement allows for payment of the exercise price for vested stock options either through cash remittance of the exercise price to us in exchange for newly issued shares, or through a non-cash exchange of previously issued shares held by the recipient equal in value to the exercise price in exchange for newly issued shares. The latter method results in no cash being received by us, but also results in a lower number of total shares subsequently being outstanding (as compared to a cash exercise), as a direct result of previously issued shares being exchanged in return for the issuance of new shares. Shares returned to us in this manner are retired. In addition, under the 2007 Plan, participants may satisfy their tax obligations related to restricted stock unit vesting in whole or in part by transferring shares of common stock to us. Shares returned to us in this manner are retired.
As of December 31, 2012, we have granted options and restricted stock units covering 5,283,775 shares of common stock under our 2007 Plan, of which 2,273,686 stock options and 615,430 restricted stock units have expired or terminated, and of which 38,338 options have been exercised and 347,725 shares of common stock have been issued pursuant to restricted stock units that became fully vested. The number of options and restricted stock units outstanding under this plan as of December 31, 2012 was 1,734,920 and 273,676, respectively. The remaining number of shares available for future grants as of December 31, 2012 was 1,513,918, not including shares subject to outstanding awards under the 2000 Plan, which will be added to the total number of shares available for issuance under the 2007 Plan to the extent that such awards expire or terminate for any reason prior to exercise. All outstanding stock options granted under our 2007 Plan have an exercise price equal to the closing price of a share of our common stock on the grant date and have either a seven or ten-year term.
Amended and Restated 2000 Stock Plan
Our 2000 Plan provided for the grant of options and other equity-based awards to our directors, officers, employees and consultants. The terms and conditions of each such grant, including, but not limited to, the number of shares, the exercise price, term of the option/award and vesting requirements,
122
were determined by our Board or the Compensation Committee of our Board. As of December 31, 2012, we have granted stock options and restricted stock units covering 2,182,700 shares of common stock under the 2000 Plan, of which 946,977 stock options and 1,500 restricted stock units have expired or terminated, and of which 1,036,570 stock options have been exercised and 42,500 shares of common stock have been issued pursuant to restricted stock units that became fully vested. The remaining number of shares underlying outstanding stock options which were issued pursuant to our 2000 Plan as of December 31, 2012 was 155,153. There were no remaining restricted stock units which were issued pursuant to our 2000 Plan as of December 31, 2012. All outstanding stock options granted under the 2000 Plan have an exercise price equal to the closing price of our common stock on the grant date and have a ten year term. In November 2007, the 2000 Plan was succeeded by our 2007 Plan and, accordingly, no further grants may be made under this plan. Any shares that remained available for issuance under the 2000 Plan as of the date of adoption of the 2007 Plan are included in the number of shares that may be issued under the 2007 Plan. Any shares subject to outstanding awards granted under the 2000 Plan that expire or terminate for any reason prior to exercise will be added to the total number of shares available for issuance under the 2007 Plan.
Other Equity Compensation Grants
In May 2012, in connection with his entry into an employment agreement as our President and Chief Executive Officer, our Board granted William Heiden an option to purchase 300,000 shares of our common stock at an exercise price equal to the fair market value of a share of our common stock on the date of grant. The option will be exercisable in four equal annual installments beginning on the first anniversary of the grant date. Mr. Heiden was also granted 100,000 restricted stock units, which will vest in four equal annual installments beginning on the first anniversary of the grant date. The foregoing grants were made pursuant to an inducement grant outside of our 2007 Plan as permitted under the NASDAQ Global Market rules. We assessed the terms of these awards to Mr. Heiden and determined there was no possibility that we would have to settle these awards in cash and therefore, equity accounting was applied. In July 2012, we filed a Form S-8 registration statement with the Securities and Exchange Commission with respect to these equity compensation grants.
Equity-based compensation expense
Equity-based compensation expense, excluding amounts that have been capitalized into inventory, for the years ended December 31, 2012, 2011 and 2010 consisted of the following (in thousands):
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Cost of product sales | $ | 225 | $ | 616 | $ | 441 | ||||
Research and development | 1,994 | 1,874 | 3,508 | |||||||
Selling, general and administrative | 4,805 | 7,548 | 10,574 | |||||||
Total equity-based compensation expense | $ | 7,024 | $ | 10,038 | $ | 14,523 | ||||
We reduce the compensation expense being recognized to account for estimated forfeitures, which we estimate based primarily on historical experience, adjusted for unusual events such as the corporate restructurings in 2012, 2011 and 2010, which resulted in higher than expected turnover and forfeitures in those years. Under current accounting guidance, forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
In addition, during 2011, we reduced our equity-based compensation expense by approximately $0.7 million to reflect the modification of the terms of certain of our former chief executive officer's outstanding equity awards pursuant to his November 2011 separation agreement.
123
Due to the uncertainty surrounding the realization of the favorable tax attributes in future tax returns associated with operating losses we incurred in the past, we have not recognized any excess tax benefits from the exercise of options. Accordingly, there was no impact recorded in cash flows from financing activities or cash flows from operating activities as reported in the accompanying consolidated statements of cash flows.
The following table summarizes the weighted average assumptions we utilized for purposes of valuing grants of options to our employees and non-employee directors:
| | Years Ended December 31, | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | ||||||||||||||||
| | Employees | Non-Employee Directors | Employees | Non-Employee Directors | Employees | Non-Employee Directors | |||||||||||||
Risk free interest rate (%) | 0.66 | 0.68 | 1.67 | 1.36 | 2.47 | 1.61 | |||||||||||||
Expected volatility (%) | 57 | 56 | 51 | 51 | 58 | 53 | |||||||||||||
Expected option term (years) | 4.66 | 4.00 | 5.50 | 4.00 | 5.50 | 4.00 | |||||||||||||
Dividend yield | none | none | none | none | none | none | |||||||||||||
Risk free interest rates utilized are based upon published U.S. Treasury yields at the date of the grant for the expected option term. We estimate our expected stock price volatility by basing it on a blend of the historical volatility of our own common stock price and the historical volatility of other similar companies over the prior period equivalent to our expected option term to better reflect expected future volatility. To compute the expected option term, we estimate the calculated historical term of stock options.
The following table summarizes details regarding our stock option plans and any grants outside of the plans under an inducement grant for the year ended December 31, 2012 (excluding restricted stock units, which are presented separately below):
| | December 31, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value ($ in millions) | |||||||||
Outstanding at beginning of year | 1,817,027 | $ | 35.16 | ||||||||||
Granted | 1,500,800 | 14.72 | |||||||||||
Exercised | (9,188 | ) | 10.65 | ||||||||||
Expired and/or forfeited | (1,118,566 | ) | 31.60 | ||||||||||
Outstanding at end of year | 2,190,073 | $ | 23.07 | 6.7 | $ | 0.6 | |||||||
Outstanding at end of year—vested and unvested expected to vest | 1,981,028 | $ | 23.82 | 6.7 | $ | 0.6 | |||||||
Exercisable at end of year | 729,294 | $ | 36.59 | 6.0 | $ | 0.1 | |||||||
The weighted average grant date fair value of stock options granted during the years ended December 31, 2012, 2011 and 2010 was $6.90, $7.40, and $18.57, respectively. A total of 336,443 stock options vested during the year ended December 31, 2012. The total grant date fair value of options that vested during the years ended December 31, 2012, 2011 and 2010 was $5.5 million, $9.8 million, and $12.0 million, respectively. The aggregate intrinsic value of options exercised during the years ended December 31, 2012, 2011 and 2010, excluding purchases made pursuant to our employee stock purchase plans, measured as of the exercise date, was approximately $0.1 million, $0.1 million, and $1.1 million, respectively. The intrinsic value of a stock option is the amount by which the fair market value
124
of the underlying stock exceeds the exercise price of the common stock option on the last trading day of each year.
In the year ended December 31, 2012, we issued an aggregate of 247,050 restricted stock units to our employees and directors. In general, these grants vest on an annual basis over a three or four year period. The estimated fair value of restricted stock units granted was determined at the grant date based upon the quoted market price per share on the date of the grant. The estimated fair value of restricted stock unit awards issued during 2012 was approximately $3.9 million.
The following table summarizes details regarding restricted stock units granted under our equity incentive plans for the year ended December 31, 2012 and our May 2012 grant to our chief executive officer:
| | December 31, 2012 | ||||||
|---|---|---|---|---|---|---|---|
| | Unvested Restricted Stock Units | Weighted Average Grant Date Fair Value | |||||
Outstanding at beginning of year | 669,009 | $ | 21.16 | ||||
Granted | 247,050 | 15.64 | |||||
Vested | (171,182 | ) | 20.59 | ||||
Forfeited | (371,201 | ) | 21.91 | ||||
Outstanding at end of year | 373,676 | $ | 17.02 | ||||
Outstanding at end of year and expected to vest | 295,916 | $ | 16.54 | ||||
At December 31, 2012, the amount of unrecorded equity-based compensation expense, net of forfeitures, attributable to future periods was approximately $11.9 million. Of this amount, $8.2 million was associated with stock options and is expected to be amortized on a straight-line basis to expense over a weighted average period of approximately 3.1 years, and $3.7 million was associated with restricted stock units and is expected to be amortized to on a straight-line basis to expense over a weighted average period of approximately 2.9 years. Such amounts will be amortized primarily to research and development or selling, general and administrative expense. These future estimates are subject to change based upon a variety of future events, which include, but are not limited to, changes in estimated forfeiture rates, employee turnover, and the issuance of new stock options and other equity-based awards.
2010 Employee Stock Purchase Plan
In May 2010, our stockholders approved our 2010 ESPP as the successor to and continuation of the 2006 Employee Stock Purchase Plan, or 2006 ESPP. The 2010 ESPP authorizes the issuance of up to 100,000 shares of our common stock to eligible employees. Currently, eligible employees may purchase shares (subject to certain plan and/or income tax limitations) in semi-annual offerings through payroll deductions of up to an annual maximum of 10% of the employee's total compensation, as defined by our Board. The purchase price per share is the lesser of 85% of the fair market value of our common stock on the first or last day of the plan period. During 2012, we issued 23,025 shares under our 2010 ESPP.
125
The assumptions used for awards granted under our employee stock purchase plans were as follows:
| | Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | 2010 | |||||||
Risk free interest rate (%) | 0.12 | 0.09 | 0.22 | |||||||
Expected volatility (%) | 43 | 37 | 42 | |||||||
Expected option term (years) | 0.5 | 0.5 | 0.5 | |||||||
Dividend yield | none | none | none | |||||||
The weighted average fair value for purchase rights granted under our 2010 ESPP and our 2006 ESPP, during the years ended December 31, 2012, 2011 and 2010 was $4.48, $5.01, and $13.66, respectively, and was estimated using the Black-Scholes option-pricing model.
J. Employee Savings Plan
We provide a 401(k) Plan to our employees by which they may defer compensation for income tax purposes under Section 401(k) of the Internal Revenue Code. Each employee may elect to defer a percentage of his or her salary up to a specified maximum. Our 401(k) Plan provides, among other things, for a company contribution of 3% of each employee's combined salary and certain other compensation for the plan year. Salary deferred by employees and contributions by us to the 401(k) Plan are not taxable to employees until withdrawn from the 401(k) Plan and contributions are deductible by us when made. The amount of our company contribution for the 401(k) Plan was $0.8 million, $1.0 million, and $1.3 million for the years ended December 31, 2012, 2011 and 2010, respectively.
K. Stockholders' Equity
Preferred Stock
Our certificate of incorporation authorizes our Board to issue preferred stock from time to time in one or more series. The rights, preferences, restrictions, qualifications and limitations of such stock are determined by our Board. In September 2009, our Board adopted a shareholder rights plan, or Rights Plan. The terms of the Rights Plan provide for a dividend distribution of one preferred share purchase right, or Right, for each outstanding share of our common stock, par value $0.01 per share, to shareholders of record as of September 17, 2009, and for one such Right to attach to each newly issued share of common stock thereafter. Each Right entitles shareholders to purchase one one-thousandth of a share of Series A Junior Participating Preferred Stock for each outstanding share of our common stock. The Rights issued pursuant to our Rights Plan become exercisable generally upon the earlier of 10 days after a person or group, or an Acquiring Person, acquires 20% or more of our outstanding common stock or 10 business days after the announcement by a person or group of an intention to acquire 20% of our outstanding common stock via tender offer or similar transaction. In that event, each holder of a Right, other than the Acquiring Person, would for a period of 60 days be entitled to purchase, at the exercise price of the Right, such number of shares of our common stock having a current value of twice the exercise price of the Right. Once a person becomes an Acquiring Person, until such Acquiring Person acquires 50% or more of our common stock, our Board can exchange the Rights, in part or in whole, for our common stock at an exchange ratio of one share of common stock per Right. If we are acquired in a merger or other business combination transaction, each holder of a Right, other than the Acquiring Person, would then be entitled to purchase, at the exercise price of the Right, such number of shares of the acquiring company's common stock having a current value of twice the exercise price of the Right. The Board may redeem the Rights or terminate the Rights Plan at any time before a person or group becomes an Acquiring Person. The Rights will expire on September 17, 2019 unless the Rights are earlier redeemed or exchanged by us. In May 2012, we amended the
126
definition of an Acquiring Person in the Rights Plan to provide that Adage Capital Management, L.P., or Adage, would not be deemed an "Acquiring Person" unless Adage, together with its affiliates and associates, have acquired beneficial ownership of 25% or more of our outstanding common stock (other than solely from repurchases of stock by us which increases Adage's percentage ownership above 25%). Pursuant to the terms of the amendment, this provision terminated in the third quarter of 2012 and Adage returned to being subject to the 20% limit applied to our other stockholders.
Common Stock Transactions
In January 2010, we sold 3,600,000 shares of our common stock, $0.01 par value per share, in an underwritten public offering at a price to the public of $48.25 per common share, resulting in gross proceeds of approximately $173.7 million. Net proceeds to us after deducting fees, commissions and other expenses related to the offering were approximately $165.6 million. The shares were issued pursuant to a shelf registration statement on Form S-3 which became effective upon filing.
L. Business Segments
We have determined that we conduct our operations in one business segment: the manufacture, development and commercialization of products derived from our proprietary technology for use in treating human diseases. Long-lived assets consist entirely of property and equipment and are located in the U.S. for all periods presented.
M. Commitments and Contingencies
Commitments
Operating and Facility Lease Obligations
We have entered into certain operating leases, including leases of certain automobiles, and certain office equipment which expire through 2014. Expense associated with these operating leases amounted to approximately $0.9 million, $0.8 million, and $1.0 million for the years ended December 31, 2012, 2011 and 2010, respectively. Future minimum lease payments associated with all noncancellable automobile, equipment, service and lease agreements, excluding facility-related leases are approximately $0.1 million for 2013. We lease 76 automobiles for our field-based employees. These leases require an initial minimum lease commitment of 12 months per automobile, after which we are responsible for certain disposal costs in the event of termination of the lease. As of December 31, 2012, all of our leased automobiles have been held beyond the initial 12 month commitment period.
In May 2008, we entered into a lease agreement for certain real property located at 100 Hayden Avenue, Lexington, Massachusetts for use as our principal executive offices. The term of the lease began on May 22, 2008 and will continue until August 31, 2016 with two successive five year extension terms at our option. In accordance with accounting guidance related to accounting for operating leases with scheduled rent increases, we recognize rent expense on this facility on a straight-line basis over the initial term of the lease. In addition, as provided for under the lease, we received approximately $2.2 million of tenant improvement reimbursements from the landlord. These reimbursements are being recorded as a deferred rent liability in our consolidated balance sheets and are amortized on a straight-line basis as a reduction to rent expense over the term of the lease. We have recorded all tenant improvements as leasehold improvements and are amortizing these improvements over the shorter of the estimated useful life of the improvement or the remaining life of the initial lease term. Amortization of leasehold improvements is included in depreciation expense.
127
The lease requires us to pay rent as follows (in thousands):
Period | Minimum Lease Payments | |||
|---|---|---|---|---|
Year Ended December 31, 2013 | $ | 2,071 | ||
Year Ended December 31, 2014 | 2,127 | |||
Year Ended December 31, 2015 | 2,183 | |||
Year Ended December 31, 2016 | 1,556 | |||
Total | $ | 7,937 | ||
During any extension term, the base rent will be an amount agreed upon by us and the landlord. In addition to base rent, we are also required to pay a proportionate share of the landlord's annual operating costs.
Facility-related rent expense was $1.7 million for each of the years ended December 31, 2012, 2011, and 2010.
In addition, in connection with our facility lease, in May 2008 we delivered to the landlord a security deposit of approximately $0.5 million in the form of an irrevocable letter of credit. The cash securing this letter of credit is classified on our balance sheets as a long-term asset and is restricted in its use.
Purchase Commitments
During 2012, we entered into various agreements with third parties for which we had remaining purchase commitments of approximately $3.7 million as of December 31, 2012. These agreements principally related to certain purchase orders for the production ofFeraheme/Rienso, certain outsourced commercial activities, manufacturing commitments, our information technology infrastructure, and other operational activities.
Severance Arrangements
We have entered into employment agreements or other arrangements with most of our executive officers and certain other employees, which provide for salary continuation payments and, in certain instances, the acceleration of the vesting of certain equity awards to such individuals in the event that the individual is terminated other than for cause, as defined in the applicable employment agreements or arrangements.
Indemnification Obligations
As permitted under Delaware law, pursuant to our certificate of incorporation, by-laws and agreements with all of our current directors, executive officers, and certain of our employees, we are obligated to indemnify such individuals for certain events or occurrences while the officer, director or employee is, or was, serving at our request in such capacity. The maximum potential amount of future payments we could be required to make under these indemnification obligations is not capped. Our director and officer insurance policy limits our initial exposure to $1.0 million and our policy provides significant coverage. As a result, we believe the estimated fair value of these indemnification obligations is likely to be immaterial.
128
We are also a party to a number of other agreements entered into in the ordinary course of business, which contain typical provisions and which obligate us to indemnify the other parties to such agreements upon the occurrence of certain events. Such indemnification obligations are usually in effect from the date of execution of the applicable agreement for a period equal to the applicable statute of limitations. Our aggregate maximum potential future liability under such indemnification provisions is uncertain. Except for expenses we incurred related to the ongoing class action lawsuit filed against us in March 2010, we have not incurred any expenses as a result of such indemnification provisions. Accordingly, we have determined that the estimated aggregate fair value of our potential liabilities under such indemnification provisions is not significant, and we have not recorded any liability related to such indemnification.
Contingencies
Legal Proceedings
We accrue a liability for legal contingencies when we believe that it is both probable that a liability has been incurred and that we can reasonably estimate the amount of the loss. We review these accruals and adjust them to reflect ongoing negotiations, settlements, rulings, advice of legal counsel and other relevant information. To the extent new information is obtained and our views on the probable outcomes of claims, suits, assessments, investigations or legal proceedings change, changes in our accrued liabilities would be recorded in the period in which such determination is made. For the matters referenced below, the liability is not probable or the amount cannot be reasonably estimated and, therefore, accruals have not been made. In addition, in accordance with the relevant authoritative guidance, for any matters in which the likelihood of material loss is at least reasonably possible, we will provide disclosure of the possible loss or range of loss. If a reasonable estimate cannot be made, however, we will provide disclosure to that effect.
A purported class action complaint was originally filed on March 18, 2010 in the United States District Court for the District of Massachusetts, entitled Silverstrand Investments et. al. v. AMAG Pharm., Inc., et. al., Civil Action No. 1:10-CV-10470-NMG, and was amended on September 15, 2010 and on December 17, 2010. The second amended complaint, or SAC, filed on December 17, 2010 alleged that we and our former President and Chief Executive Officer, former Executive Vice President and Chief Financial Officer, the then members of our Board, and certain underwriters in our January 2010 offering of common stock violated certain federal securities laws, specifically Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended, and that our former President and Chief Executive Officer and former Executive Vice President and Chief Financial Officer violated Section 15 of such Act, respectively, by making certain alleged false and misleading statements and omissions in a registration statement filed in January 2010. The plaintiff sought unspecified damages on behalf of a purported class of purchasers of our common stock pursuant to our common stock offering on or about January 21, 2010. On August 11, 2011, the District Court issued an Opinion and Order dismissing the SAC in its entirety for failure to state a claim upon which relief could be granted. A separate Order of Dismissal was filed on August 15, 2011. On September 14, 2011, the plaintiffs filed a Notice of Appeal to the United States Court of Appeals for the First Circuit, or the Court of Appeals. After briefing was completed by all parties, the Court of Appeals heard oral argument on May 11, 2012, and took the matter under advisement. On February 4, 2013, the Court of Appeals affirmed in part and reversed in part the District Court's Opinion and Order, and remanded the case to the District Court. On February 18, 2013, we filed a Petition for Panel Hearing or RehearingEn Banc, asking the Court of Appeals to reconsider its decision. We are currently unable to predict the outcome or reasonably estimate the range of potential loss associated with this matter, if any, and have therefore not recorded any potential estimated liability as we do not believe that such a liability is probable nor do we believe that a range of loss is currently estimable.
129
In July 2010, Sandoz GmbH, or Sandoz, filed with the European Patent Office, or the EPO, an opposition to our previously issued patent which covers ferumoxytol in the EU. In October 2012, at an oral hearing, the Opposition Division of the EPO revoked our European ferumoxytol patent. In December 2012, our notice of appeal was recorded with the EPO, which suspends the revocation of our patent. We will continue to defend the validity of this patent throughout the appeals process, which we expect to take two to three years. However, in the event that we do not experience a successful outcome from the appeals process, under EU regulations ferumoxytol would still be entitled to eight years of data protection and ten years of market exclusivity from the date of approval, which we believe would create barriers to entry for any generic version of ferumoxytol into the EU market until sometime between 2020 and 2022. This decision had no impact on our revenues for the year ended December 31, 2012. However, any future unfavorable outcome in this matter could negatively affect the magnitude and timing of future revenues, including royalties and milestone payments we may receive from Takeda pursuant to our collaboration agreement with Takeda. We do not expect to incur any related liability regardless of the outcome of the appeal and therefore have not recorded any liability as of December 31, 2012. We continue to believe the patent is valid and intend to vigorously appeal the decision.
We may periodically become subject to other legal proceedings and claims arising in connection with ongoing business activities, including claims or disputes related to patents that have been issued or that are pending in the field of research on which we are focused. Other than the above actions, we are not aware of any material claims against us at December 31, 2012. We expense legal costs as they are incurred.
N. Collaborative Agreements
Our commercial strategy includes the formation of collaborations with other pharmaceutical companies to facilitate the sale and distribution ofFeraheme/Rienso, primarily outside of the U.S. As of December 31, 2012, we were a party to the following collaborations:
Takeda
In March 2010, we entered into the Takeda Agreement with Takeda under which we granted exclusive rights to Takeda to develop and commercializeFeraheme/Rienso as a therapeutic agent in Europe, certain Asia-Pacific countries (excluding Japan, China and Taiwan), the Commonwealth of Independent States, Canada, India and Turkey. In June 2012, we entered into an amendment to the Takeda Agreement, or the Amended Takeda Agreement, which removed the Commonwealth of Independent States from the territories under which Takeda has the exclusive rights to develop and commercializeFeraheme/Rienso. In addition, the Amended Takeda Agreement modified the timing and pricing arrangements for a supply agreement to be entered into between us and Takeda in the future, the terms related to primary and secondary manufacturing for drug substance and drug product, certain patent related provisions, and the re-allocation of certain of the agreed-upon milestone payments. We analyzed the Amended Takeda Agreement and determined that the amended terms did not result in a material modification of the original Takeda Agreement (and thus did not require us to change our accounting model) based on the fact that there were no changes to the deliverables under the original Takeda Agreement as a result of the amendment, and the change in arrangement consideration as a result of the amendment was not quantitatively material in relation to the total arrangement consideration.
Under the Amended Takeda Agreement, except under limited circumstances, we have retained the right to manufactureFeraheme/Rienso and, accordingly, are responsible for supply ofFeraheme/Rienso to Takeda at a fixed price per unit, which is capped for a certain period of time. We are also responsible for conducting, and bearing the costs related to, certain pre-defined clinical studies with the costs of future modifications or additional studies to be allocated between the parties according to an
130
agreed-upon cost-sharing mechanism. We have determined that our obligations under the Amended Takeda Agreement have not changed from those under the original Takeda Agreement and include the following four deliverables: the license, access to future know-how and improvements to theFeraheme/Rienso technology, regulatory and clinical research activities, and the manufacturing and supply of product. Pursuant to the accounting guidance in effect in March 2010, when we signed the original Takeda Agreement and which governed revenue recognition on multiple element arrangements, we evaluated the four deliverables under the original Takeda Agreement and determined that our obligation to provide manufacturing supply of product meets the criteria for separation and is therefore treated as a single unit of accounting, which we refer to as the supply unit of accounting. Further, we concluded that the license is not separable from the undelivered future know-how and technological improvements or the undelivered regulatory and clinical research activities. Accordingly, these deliverables are being combined and also treated as a single unit of accounting, which we refer to as the combined unit of accounting. With respect to the combined unit of accounting, our obligation to provide access to our future know-how and technological improvements is the final deliverable and is an obligation which exists throughout the term of the Amended Takeda Agreement.
In connection with the execution of the original Takeda Agreement, we received a $60.0 million upfront payment from Takeda in April 2010, which we recorded as deferred revenue, as well as approximately $1.0 million reimbursed to us during 2010 for certain expenses incurred prior to entering the agreement, which we considered an additional upfront payment. Because we cannot reasonably estimate the total level of effort required to complete the obligations under the combined deliverable, we are recognizing the entire $60.0 million upfront payment, the $1.0 million reimbursed to us in 2010, as well as any non-substantive milestone payments that are achieved into revenues on a straight-line basis over a period of ten years from March 31, 2010, the date on which we originally entered the Takeda Agreement, which represented the then current patent life ofFeraheme/Rienso and our best estimate of the period over which we will substantively perform our obligations. We continue to believe that the then current patent life ofFeraheme/Rienso is our best estimate of the period over which we will substantively perform our obligations under this agreement. Revenues related to the combined unit of accounting and any reimbursement revenues are recorded in license fee and other collaboration revenues in our consolidated statement of operations. During the years ended December 31, 2012, 2011 and 2010, we recorded $6.1 million, $6.1 million and $4.6 million in revenues associated with the upfront payments. Any potential non-substantive milestone payments that may be received in the future will be recognized as revenue on a cumulative catch up basis when they become due and payable.
We have received and may also receive additional regulatory approval and performance-based milestone payments, reimbursement of certain out-of-pocket regulatory and clinical supply costs, defined payments for supply ofFeraheme/Rienso, and tiered double-digit royalties on net product sales in the agreed-upon territories under the Amended Takeda Agreement. During 2012, we received $33.0 million in milestone payments from Takeda associated with the EU approval and the commercial launches ofFeraheme/Rienso in Canada and the EU. The remaining milestone payments we may be entitled to receive under the agreement could over time equal approximately $186.0 million.
We have determined that any milestone payments which may become due upon approval by certain regulatory agencies will be deemed substantive milestones and, therefore, will be accounted for as revenue in the period in which they are achieved. In June 2012, we earned a $15.0 million milestone payment from Takeda based on the European Commission marketing authorization for ferumoxytol. We deemed this milestone payment to be a substantive milestone based on our analysis that the milestone consideration received was commensurate with our performance to achieve the milestone, was solely related to past performance, and was reasonable relative to all of the deliverables and payment terms, including other milestones, within the arrangement. Therefore, we recognized the $15.0 million milestone payment as revenue in the second quarter of 2012 in our consolidated statement of operations.
131
Additionally, we have determined that any non-substantive milestone payments will be accounted for in accordance with our revenue attribution method for the upfront payment, as described above. In the fourth quarter of 2012, we received an aggregate of $18.0 million in milestone payments from Takeda associated with the commercial launches ofFeraheme/Rienso in Canada and the EU. We deemed these milestone payments to be non-substantive milestone payments and accordingly, we recognized approximately $5.0 million of the $18.0 million on a cumulative catch up basis in the fourth quarter of 2012 in our consolidated statement of operations.
Under the terms of the Amended Takeda Agreement, Takeda is responsible for reimbursing us for certain out-of-pocket regulatory and clinical trial supply costs associated with carrying out our regulatory and clinical research activities under the collaboration agreement. Because we are acting as the principal in carrying out these services, any reimbursement payments received from Takeda will be recorded in license fee and other collaboration revenues in our consolidated statement of operations to match the costs that we incur during the period in which we perform those services. We recorded $0.4 million, $2.0 million and $1.6 million for the years ended December 31, 2012, 2011 and 2010, respectively, associated with other reimbursement revenues received from Takeda.
In accordance with current accounting guidance related to the recognition, presentation, and disclosure of revenue in the financial statements, we record all revenue forFeraheme/Rienso sold to our licensees in deferred revenues in our consolidated balance sheets. We will recognize revenues from product sales to our licensees, the related cost of goods sold, as well as any royalty revenues due from our licensees, in our consolidated statement of operations at the time our licensees report to us that sales have been made to its customers.
3SBio
In 2008, we entered into a Collaboration and Exclusive License Agreement, or the 3SBio License Agreement, and a Supply Agreement, or the 3SBio Supply Agreement, with 3SBio for the development and commercialization ofFeraheme as an IV iron replacement therapeutic agent in China. The 3SBio License Agreement grants 3SBio an exclusive license for an initial term of thirteen years to develop and commercializeFeraheme as a therapeutic agent in China for an initial indication for the treatment of IDA in patients with CKD, and an option to expand into additional therapeutic indications. In consideration of the grant of the license, we received an upfront payment of $1.0 million, the recognition of which has been deferred and will be recognized under the proportional performance methodology over the remaining portion of the thirteen year initial term of the agreement once we begin to supplyFeraheme to 3SBio. We are eligible to receive certain other specified milestone payments upon regulatory approval ofFeraheme in China for CKD and other indications. We are also entitled to receive tiered royalties of up to 25% based on net sales ofFeraheme by 3SBio in China. We retained all manufacturing rights forFeraheme under these agreements. In addition, pursuant to the 3SBio Supply Agreement, 3SBio has agreed to purchase from us, and we have agreed to supply to 3SBio,Feraheme at a predetermined supply price for use in connection with 3SBio's development and commercialization obligations described above for so long as the 3SBio License Agreement is in effect. To date, we have not provided 3SBio with any commercial product under this agreement.
O. Restructuring
During 2012, we initiated a corporate restructuring, including a workforce reduction plan. The majority of the workforce reduction plan was associated with our manufacturing and development infrastructure, including our decision to divest our Cambridge, Massachusetts manufacturing facility. As a result of the restructuring, we recorded charges of approximately $2.2 million in 2012. Of the $2.2 million in restructuring expense, approximately $1.5 million was related to employee severance and benefits, and approximately $0.7 million was related to the write-down of primarily raw material inventory that was no longer usable due to the closure of the facility. The workforce reduction was
132
substantially completed by the end of 2012, and the majority of the related expenses were paid by the end of 2012.
During 2011, we initiated a corporate restructuring, including a workforce reduction plan for which we recorded $3.5 million of restructuring related costs, primarily related to employee severance and benefits. The workforce reduction was substantially completed by the end of 2011 and the majority of the related expenses were paid by the end of 2012.
The following table outlines the components of our restructuring expenses which were recorded in operating expenses and current liabilities for the years ended December 31, 2012 and 2011 (in thousands):
| | December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2012 | 2011 | |||||
Accrued restructuring, beginning of period | $ | 2,366 | $ | 1,324 | |||
Employee severance, benefits and related costs | 1,624 | 3,697 | |||||
Payments | (2,674 | ) | (2,523 | ) | |||
Inventory and other adjustments | 67 | (132 | ) | ||||
Accrued restructuring, end of period | $ | 1,383 | $ | 2,366 | |||
P. Consolidated Quarterly Financial Data—Unaudited
The following tables provide unaudited consolidated quarterly financial data for the years ended December 31, 2012 and 2011 (in thousands, except per share data):
| | March 31, 2012 | June 30, 2012 | September 30, 2012 | December 31, 2012 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
U.S. product sales, net(a) | $ | 13,626 | $ | 14,094 | $ | 16,186 | $ | 14,381 | |||||
International product sales and royalties | — | 168 | (168 | ) | 120 | ||||||||
License fee and other collaboration revenues(b) | 1,753 | 16,592 | 1,566 | 6,564 | |||||||||
Other product sales and royalties | 101 | 158 | 158 | 79 | |||||||||
Total revenues | 15,480 | 31,012 | 17,742 | 21,144 | |||||||||
Cost of product sales | 2,646 | 3,224 | 4,323 | 4,027 | |||||||||
Operating expenses | 25,643 | 22,772 | 17,420 | 20,532 | |||||||||
Restructuring expenses(c) | — | 1,058 | 562 | 595 | |||||||||
Interest and dividend income, net | 393 | 338 | 295 | 260 | |||||||||
(Losses) gains on investments, net(d) | — | (1,471 | ) | 2 | 3 | ||||||||
Income tax benefit | — | 494 | 299 | 61 | |||||||||
Net income (loss) | $ | (12,416 | ) | $ | 3,319 | $ | (3,967 | ) | $ | (3,686 | ) | ||
Net income (loss) per share—basic | $ | (0.58 | ) | $ | 0.16 | $ | (0.19 | ) | $ | (0.17 | ) | ||
Net income (loss) per share—diluted | $ | (0.58 | ) | $ | 0.15 | $ | (0.19 | ) | $ | (0.17 | ) | ||
133
| | March 31, 2011 | June 30, 2011 | September 30, 2011 | December 31, 2011 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
U.S. product sales, net(a) | $ | 10,861 | $ | 12,846 | $ | 15,560 | $ | 12,830 | |||||
License fee and other collaboration revenues | 2,327 | 2,288 | 1,707 | 1,999 | |||||||||
Other product sales and royalties | 197 | 268 | 288 | 78 | |||||||||
Total revenues | 13,385 | 15,402 | 17,555 | 14,907 | |||||||||
Cost of product sales | 3,041 | 2,082 | 2,669 | 2,739 | |||||||||
Operating expenses | 33,200 | 33,521 | 32,124 | 28,158 | |||||||||
Restructuring expenses(c) | — | — | — | 3,508 | |||||||||
Interest and dividend income, net | 560 | 452 | 378 | 357 | |||||||||
Gains (losses) on investments, net | 1 | (209 | ) | 14 | 1 | ||||||||
Income tax benefit | — | 396 | 215 | 559 | |||||||||
Net loss | $ | (22,295 | ) | $ | (19,562 | ) | $ | (16,631 | ) | $ | (18,581 | ) | |
Net loss per share—basic and diluted | $ | (1.05 | ) | $ | (0.92 | ) | $ | (0.78 | ) | $ | (0.87 | ) | |
Quarterly loss per share totals differ from annual loss per share totals due to rounding.
(a) In the quarters ended September 30, 2012 and 2011, we revised our estimated Medicaid utilization rate, which resulted in a reduction of our estimated Medicaid rebate reserve related to prior yearFeraheme sales of $0.6 million and $2.5 million, respectively. In addition, in the first three quarters of 2012 we reduced our reserve for product returns by $2.2 million.
(b) During the quarters ended June 30, 2012 and December 31, 2012, we recognized $15.0 million and $5.0 million related to certain milestone payments we received from Takeda upon the EU marketing authorization ofRienso and the commercial launches ofFeraheme/Rienso in Canada and the EU, respectively.
(c) In 2012 and 2011 we carried out corporate restructurings pursuant to which we reduced our workforce and incurred charges related to employee severance and other related costs.
(d) In June 2012, we sold our then remaining ARS portfolio and recognized a loss of approximately $1.5 million.
Q. Valuation and Qualifying Accounts (in thousands)
| | Balance at Beginning of Period | Additions(a) | Deductions Charged to Reserves | Balance at End of Period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year ended December 31, 2012: | |||||||||||||
Accounts receivable allowances(b) | $ | 1,822 | $ | 26,517 | $ | (26,598 | ) | $ | 1,741 | ||||
Rebates, fees and returns reserves | $ | 5,943 | $ | 6,729 | $ | (9,224 | ) | $ | 3,448 | ||||
Year ended December 31, 2011: | |||||||||||||
Accounts receivable allowances(b) | $ | 1,148 | $ | 14,074 | $ | (13,400 | ) | $ | 1,822 | ||||
Rebates, fees and returns reserves | $ | 10,015 | $ | 9,864 | $ | (13,936 | ) | $ | 5,943 | ||||
Year ended December 31, 2010: | |||||||||||||
Accounts receivable allowances(b) | $ | 499 | $ | 5,113 | $ | (4,464 | ) | $ | 1,148 | ||||
Rebates, fees and returns reserves | $ | 5,657 | $ | 17,779 | $ | (13,421 | ) | $ | 10,015 | ||||
- (a)
- Additions to sales discounts, rebates, fees and returns reserves are recorded as a reduction of revenues.
- (b)
- We have not recorded an allowance for doubtful accounts in any of the years presented above. These accounts receivable allowances represent discounts and other chargebacks related to the provision for U.S. product sales.
134
R. Recently Issued and Proposed Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board, or FASB, issued amended guidance on the presentation of comprehensive income in financial statements. This amendment provides companies the option to present the components of net income and other comprehensive income either as one continuous statement of comprehensive income or as two separate but consecutive statements. This guidance eliminates the option to present components of other comprehensive income as part of the statement of changes in stockholders' equity. The provisions of this guidance became effective in 2012. We have adopted all provisions of this pronouncement by including other comprehensive income as part of our consolidated statements of comprehensive loss and such adoption did not have a significant impact on our consolidated financial statements.
In May 2011, the FASB issued an amendment to the accounting guidance for fair value measurements and related disclosures. This amendment clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable inputs, or Level 3 measurements. This guidance became effective for interim and annual periods beginning after December 15, 2011. We have adopted all provisions of this pronouncement and such adoption did not have a significant impact on our consolidated financial statements.
135
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE:
None.
ITEM 9A. CONTROLS AND PROCEDURES:
Managements' Evaluation of our Disclosure Controls and Procedures
Our principal executive officer and principal financial officer, after evaluating the effectiveness of our "disclosure controls and procedures" (as defined in the Exchange Act Rule 13a-15(e), or Rule 15d-15(e)), with the participation of our management, have each concluded that, as of December 31, 2012, the end of the period covered by this Annual Report on Form 10-K, our disclosure controls and procedures were designed and were effective to provide reasonable assurance that information we are required to disclose in the reports that we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms and that such information is accumulated and communicated to management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. It should be noted that any system of controls is designed to provide reasonable, but not absolute, assurances that the system will achieve its stated goals under all reasonably foreseeable circumstances.
Management's Annual Report on Internal Control Over Financial Reporting
Management's Report on Internal Control over Financial Reporting is contained in Part II, Item 8 "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K for the year ended December 31, 2012 and is incorporated herein by reference.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the fourth quarter of the year ended December 31, 2012 that materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
None.
136
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE:
The information required under this item is incorporated herein by reference to our definitive proxy statement pursuant to Regulation 14A, to be filed with the Securities and Exchange Commission, or the SEC, not later than 120 days after the close of our year ended December 31, 2012.
ITEM 11. EXECUTIVE COMPENSATION:
The information required under this item is incorporated herein by reference to our definitive proxy statement pursuant to Regulation 14A, to be filed with the SEC not later than 120 days after the close of our year ended December 31, 2012.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS:
The information required under this item is incorporated herein by reference to our definitive proxy statement pursuant to Regulation 14A, to be filed with the SEC not later than 120 days after the close of our year ended December 31, 2012.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE:
The information required under this item is incorporated herein by reference to our definitive proxy statement pursuant to Regulation 14A, to be filed with the SEC not later than 120 days after the close of our year ended December 31, 2012.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES:
The information required under this item is incorporated herein by reference to our definitive proxy statement pursuant to Regulation 14A, to be filed with the SEC not later than 120 days after the close of our year ended December 31, 2012.
137
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES:
- (a)
- The following documents are filed as part of this Annual Report on Form 10-K:
- 1.
- Financial Statements.
- 2.
- Financial Statement Schedules. No financial statement schedules have been submitted because they are not required, not applicable, or because the information required is included in the financial statements or the notes thereto.
- 3.
- Exhibit Index.
Management's Annual Report on Internal Control over Financial Reporting
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets—as of December 31, 2012 and 2011
Consolidated Statements of Operations—for the years ended December 31, 2012, 2011 and 2010
Consolidated Statements of Comprehensive Loss—for the years ended December 31, 2012, 2011 and 2010
Consolidated Statements of Stockholders' Equity—as of December 31, 2012, 2011 and 2010
Consolidated Statements of Cash Flows—for the years ended December 31, 2012, 2011 and 2010
Notes to Consolidated Financial Statements
| Exhibit Number | Description | |
|---|---|---|
| 3.1, 4.1 | Certificate of Incorporation of the Company, as restated (incorporated herein by reference to Exhibit 3.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, File No. 001-10865). | |
| 3.2, 4.2 | By-Laws of the Company, as amended (incorporated herein by reference to Exhibit 3.1 to the Company's Current Report on Form 8-K filed November 28, 2008, File No. 0-14732). | |
| 3.3, 4.3 | Certificate of Designation of Series A Junior Participating Preferred Stock (incorporated herein by reference to Exhibit 3.1 and 4.1 to the Company's Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | |
| 4.4 | Specimen certificate representing the Company's Common Stock (incorporated herein by reference to Exhibit 4.3 to the Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | |
| 4.5 | Rights Agreement dated as of September 4, 2009 by and among AMAG Pharmaceuticals, Inc. and American Stock Transfer & Trust Company, LLC (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | |
| 4.6 | Amendment to Rights Agreement, dated May 10, 2012, by and between the Company and American Stock Transfer & Trust Company LLC (incorporated herein by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed May 10, 2012). | |
| 4.7 | Form of Rights Certificate (incorporated herein by reference to Exhibit 4.3 to the Company's Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). |
138
| Exhibit Number | Description | |
|---|---|---|
| 10.1* | Representative Form of Indemnification Agreement (incorporated herein by reference to Exhibit 10.2 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009, File No. 001-10865). | |
| 10.2* | Summary of the Company's Change of Control Policy applicable to executive officers (incorporated herein by reference to Exhibit 10.5 to the Company's Current Report on Form 8-K filed February 13, 2006, File No. 0-14732). | |
| 10.3* | AMAG Pharmaceuticals, Inc. 2010 Employee Stock Purchase Plan (incorporated herein by reference to Appendix B to the Company's Definitive Proxy Statement on Schedule 14A, filed April 19, 2010, File No. 001-10865). | |
| 10.4* | AMAG Pharmaceuticals, Inc.'s Non-Employee Director Compensation Policy (incorporated herein by reference to Exhibit 10.4 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | |
| 10.5* | Employment Agreement, dated as of May 6, 2012, by and between the Company and William K. Heiden (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed May 10, 2012). | |
| 10.6* | Employment Agreement dated as of August 1, 2011 between the Company and Frank E. Thomas. (incorporated herein by reference to Exhibit 10.8 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | |
| 10.7* | Second Amendment to Employment Agreement dated as of November 30, 2011 between the Company and Frank E. Thomas (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed December 2, 2011, File No. 001-10865). | |
| 10.8* | Letter Agreement, dated as of May 9, 2012, by and between the Company and Frank E. Thomas (incorporated herein by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed May 10, 2012). | |
| 10.9* | Retention Agreement between the Company and Scott A. Holmes dated as of December 2, 2011 (incorporated herein by reference to Exhibit 10.11 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | |
| 10.10*+ | Second Amended and Restated Employment Agreement dated as of December 15, 2009, as amended February 1, 2011 and November 3, 2011 between the Company and Lee F. Allen, M.D., Ph.D. | |
| 10.11* | Retention Agreement dated as of August 27, 2012 between the Company and Lee F. Allen, M.D., Ph.D. (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed August 31, 2012, File No. 001-10865). | |
| 10.12*+ | Second Amended and Restated Employment Agreement dated as of December 15, 2009, as amended February 1, 2011 and November 3, 2011 between the Company and Christopher White. | |
| 10.13*+ | Employment Agreement dated as of August 15, 2012 between the Company and Scott B. Townsend. | |
| 10.14*+ | Employment Agreement dated as of January 1, 2013 between the Company and Greg Madison. | |
| 10.15* | Stockholder Agreement, May 9, 2012, by and between the Company and Adage Capital Management, L.P. (incorporated herein by reference to Exhibit 10.5 to the Company's Current Report on Form 8-K filed May 10, 2012). | |
| 10.16* | Advanced Magnetics, Inc. Amended and Restated 2000 Stock Plan (incorporated herein by reference to Appendix A to the Company's definitive proxy statement for the year ended September 30, 2005, File No. 0-14732). | |
| 10.17* | Form of Stock Option Grant under the Company's 2000 Stock Plan (employees) (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2005, File No. 0-14732). |
139
| Exhibit Number | Description | |
|---|---|---|
| 10.18* | Form of Stock Option Grant under the Company's 2000 Stock Plan (non-employees) (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2005, File No. 0-14732). | |
| 10.19* | AMAG Pharmaceuticals, Inc. Second Amended and Restated 2007 Equity Incentive Plan (incorporated herein by reference to Appendix A to the Company's Definitive Proxy Statement on Schedule 14A, filed April 19, 2010, File No. 001-10865). | |
| 10.20* | Form of Option Agreement (ISO) under the Company's 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed November 30, 2007, File No. 0-14732). | |
| 10.21* | Form of Option Agreement (Nonqualified Option) under the Company's 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed November 30, 2007, File No. 0-14732). | |
| 10.22* | Form of Restricted Stock Unit Agreement under the Company's 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed November 30, 2007, File No. 0-14732). | |
| 10.23* | Form of Option Agreement (Nonqualified Option) for Annual Director Grants under the Company's Second Amended and Restated 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, File No. 001-10865). | |
| 10.24* | Form of Restricted Stock Unit Agreement for Annual Director Grants under the Company's Second Amended and Restated 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.5 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, File No. 001-10865). | |
| 10.25* | Form of November 2011 Restricted Stock Unit Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and the Company's executive officers (incorporated herein by reference to Exhibit 10.21 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | |
| 10.26* | Form of November 2011 Restricted Stock Unit Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and each non-executive employee of the Company (incorporated herein by reference to Exhibit 10.22 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | |
| 10.27* | Form of Non-Plan Restricted Stock Unit Agreement, by and between the Company and William K. Heiden (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed May 10, 2012). | |
| 10.28* | Form of Non-Plan Stock Option Agreement, by and between the Company and William K. Heiden (incorporated herein by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed May 10, 2012). | |
| 10.29* | Option Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and Lee F. Allen, dated as of June 25, 2012 (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed June 29, 2012). | |
| 10.30* | Option Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and Christopher G. White, dated as of June 25, 2012 (incorporated herein by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed June 29, 2012). |
140
| Exhibit Number | Description | |
|---|---|---|
| 10.31 | Lease Agreement, dated as of May 27, 2008, by and between AMAG Pharmaceuticals, Inc. and Mortimer B. Zuckerman and Edward H. Linde, trustees of 92 Hayden Avenue Trust under Declaration of Trust dated August 18, 1983 (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed May 29, 2008, File No. 0-14732). | |
| 10.32 | Collaboration and Exclusive License Agreement between the Company and 3SBio Inc., dated as of May 25, 2008 (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2008, File No. 0-14732) (confidential treatment previously granted). | |
| 10.33 | Supply Agreement between the Company and 3SBio Inc., dated as of May 25, 2008 (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2008, File No. 0-14732) (confidential treatment previously granted). | |
| 10.34 | Commercial Outsourcing Services Agreement, dated October 2008, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed July 1, 2009, File No. 0-14732) (confidential treatment previously granted). | |
| 10.35 | First Amendment to Commercial Outsourcing Services Agreement, dated April 14, 2011, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2011, File No. 001-10865). | |
| 10.36 | Second Amendment to Commercial Outsourcing Services Agreement, dated effective as of December 1, 2011, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed December 22, 2011, File No. 001-10865) (confidential treatment previously granted). | |
| 10.37 | Third Amendment to Commercial Outsourcing Services Agreement, dated effective as of August 1, 2012, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q filed November 7, 2012, File No. 001-10865) (confidential treatment previously granted). | |
| 10.38 | Commercial Packaging Services Agreement, dated May 29, 2009, by and between the Company and Packaging Coordinators, LLC (formerly Catalent Pharma Solutions LLC) (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed July 1, 2009, File No. 0-14732) (confidential treatment previously granted). | |
| 10.39+ | Amendment No. 1 to Commercial Packaging Services Agreement, dated January 29, 2013, by and between the Company and Packaging Coordinators, LLC (formerly Catalent Pharma Solutions LLC) (Certain confidential information contained in this exhibit was omitted by means of redacting a portion of the text and replacing it with [***]. This exhibit has been filed separately with the SEC without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended). | |
| 10.40 | License, Development and Commercialization Agreement by and between the Company and Takeda Pharmaceutical Company Limited, dated March 31, 2010 (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, File No. 001-10865) (confidential treatment previously granted). |
141
| Exhibit Number | Description | |
|---|---|---|
| 10.41 | Amendment to the License, Development and Commercialization Agreement, dated June 25, 2012, by and between the Company and Takeda Pharmaceutical Company Limited (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed June 29, 2012, File No. 001-10865) (confidential treatment previously granted). | |
| 10.42 | Commercial Supply Agreement, dated effective as of August 29, 2012, by and between the Company and Sigma-Aldrich, Inc. (incorporated herein by reference to Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q filed November 7, 2012, File No. 001-10865) (confidential treatment previously granted). | |
| 10.43 | Pharmaceutical Manufacturing and Supply Agreement, dated effective as of January 8, 2010, by and between the Company and DSM Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q filed November 7, 2012, File No. 001-10865) (confidential treatment previously granted). | |
| 21.1+ | Subsidiaries of the Company. | |
| 23.1+ | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | |
| 24.1 | Power of Attorney (included on the signature page(s) hereto) | |
| 31.1+ | Certification Pursuant to Rule 13a-14(a)/15d-14(a) of the Exchange Act, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2+ | Certification Pursuant to Rule 13a-14(a)/15d-14(a) of the Exchange Act, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1++ | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2++ | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101++ | The following materials from AMAG Pharmaceuticals, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2012, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Loss, (iv) Consolidated Statements of Stockholders' Equity, (v) Consolidated Statements of Cash, and (vi) Notes to Consolidated Financial Statements. |
- +
- Exhibits marked with a plus sign ("+") are filed herewith.
- ++
- Exhibits marked with a double plus sign ("++") are furnished herewith.
- *
- Exhibits marked with a single asterisk reference management contracts, compensatory plans or arrangements, filed in response to Item 15(a)(3) of the instructions to Form 10-K.
The other exhibits listed have previously been filed with the SEC and are incorporated herein by reference, as indicated.
- (b)
- Exhibits. We hereby file or furnish as exhibits, as the case may be, to this Form 10-K those exhibits listed in Part IV, Item 15(a)(3) above.
- (c)
- Financial Statement Schedules. No financial statement schedules have been submitted because they are not required, not applicable, or because the information required is included in the financial statements or the notes thereto.
142
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AMAG PHARMACEUTICALS, INC. | ||||
By: | /s/ WILLIAM K. HEIDEN William K. Heiden President and Chief Executive Officer | |||
Date: | March 1, 2013 | |||
We, the undersigned officers and directors of AMAG Pharmaceuticals, Inc., hereby severally constitute and appoint William K. Heiden and Scott A. Holmes, and each of them singly, our true and lawful attorneys, with full power to them and each of them singly, to sign for us in our names in the capacities indicated below, all amendments to this report, and generally to do all things in our names and on our behalf in such capacities to enable AMAG Pharmaceuticals, Inc. to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all requirements of the Securities and Exchange Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Name | Title | Date | ||
|---|---|---|---|---|
| /s/ WILLIAM K. HEIDEN William K. Heiden | President and Chief Executive Officer (Principal Executive Officer) | March 1, 2013 | ||
/s/ SCOTT A. HOLMES Scott A. Holmes | Chief Accounting Officer, Vice President of Finance and Controller (Principal Financial and Accounting Officer) | March 1, 2013 | ||
/s/ JOSEPH V. BONVENTRE, MD, PHD Joseph V. Bonventre, MD, PhD | Director | March 1, 2013 | ||
/s/ RAJIV DE SILVA Rajiv De Silva | Director | March 1, 2013 | ||
/s/ MICHAEL NARACHI Michael Narachi | Director | March 1, 2013 | ||
/s/ ROBERT J. PEREZ Robert J. Perez | Director | March 1, 2013 | ||
/s/LESLEY RUSSELL, MB. CH.B., MRCP Lesley Russell, MB. Ch.B., MRCP | Director | March 1, 2013 | ||
/s/ GINO SANTINI Gino Santini | Director | March 1, 2013 | ||
/s/ DAVEY S. SCOON Davey S. Scoon | Director | March 1, 2013 |
143
| Exhibit Number | Description | ||
|---|---|---|---|
| 3.1, 4.1 | Certificate of Incorporation of the Company, as restated (incorporated herein by reference to Exhibit 3.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, File No. 001-10865). | ||
| 3.2, 4.2 | By-Laws of the Company, as amended (incorporated herein by reference to Exhibit 3.1 to the Company's Current Report on Form 8-K filed November 28, 2008, File No. 0-14732). | ||
| 3.3, 4.3 | Certificate of Designation of Series A Junior Participating Preferred Stock (incorporated herein by reference to Exhibit 3.1 and 4.1 to the Company's Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | ||
| 4.4 | Specimen certificate representing the Company's Common Stock (incorporated herein by reference to Exhibit 4.3 to the Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | ||
| 4.5 | Rights Agreement dated as of September 4, 2009 by and among AMAG Pharmaceuticals, Inc. and American Stock Transfer & Trust Company, LLC (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | ||
| 4.6 | Amendment to Rights Agreement, dated May 10, 2012, by and between the Company and American Stock Transfer & Trust Company LLC (incorporated herein by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed May 10, 2012). | ||
| 4.7 | Form of Rights Certificate (incorporated herein by reference to Exhibit 4.3 to the Company's Current Report on Form 8-K filed September 4, 2009, File No. 0-14732). | ||
| 10.1* | Representative Form of Indemnification Agreement (incorporated herein by reference to Exhibit 10.2 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009, File No. 001-10865). | ||
| 10.2* | Summary of the Company's Change of Control Policy applicable to executive officers (incorporated herein by reference to Exhibit 10.5 to the Company's Current Report on Form 8-K filed February 13, 2006, File No. 0-14732). | ||
| 10.3* | AMAG Pharmaceuticals, Inc. 2010 Employee Stock Purchase Plan (incorporated herein by reference to Appendix B to the Company's Definitive Proxy Statement on Schedule 14A, filed April 19, 2010, File No. 001-10865). | ||
| 10.4* | AMAG Pharmaceuticals, Inc.'s Non-Employee Director Compensation Policy (incorporated herein by reference to Exhibit 10.4 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | ||
| 10.5* | Employment Agreement, dated as of May 6, 2012, by and between the Company and William K. Heiden (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed May 10, 2012). | ||
| 10.6* | Employment Agreement dated as of August 1, 2011 between the Company and Frank E. Thomas. (incorporated herein by reference to Exhibit 10.8 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | ||
| 10.7* | Second Amendment to Employment Agreement dated as of November 30, 2011 between the Company and Frank E. Thomas (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed December 2, 2011, File No. 001-10865). | ||
| 10.8* | Letter Agreement, dated as of May 9, 2012, by and between the Company and Frank E. Thomas (incorporated herein by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed May 10, 2012). | ||
| 10.9* | Retention Agreement between the Company and Scott A. Holmes dated as of December 2, 2011 (incorporated herein by reference to Exhibit 10.11 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | ||
| 10.10*+ | Second Amended and Restated Employment Agreement dated as of December 15, 2009, as amended February 1, 2011 and November 3, 2011 between the Company and Lee F. Allen, M.D., Ph.D. | ||
144
| Exhibit Number | Description | ||
|---|---|---|---|
| 10.11* | Retention Agreement dated as of August 27, 2012 between the Company and Lee F. Allen, M.D., Ph.D. (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed August 31, 2012, File No. 001-10865). | ||
| 10.12*+ | Second Amended and Restated Employment Agreement dated as of December 15, 2009, as amended February 1, 2011 and November 3, 2011 between the Company and Christopher White. | ||
| 10.13*+ | Employment Agreement dated as of August 15, 2012 between the Company and Scott B. Townsend. | ||
| 10.14*+ | Employment Agreement dated as of January 1, 2013 between the Company and Greg Madison. | ||
| 10.15* | Stockholder Agreement, May 9, 2012, by and between the Company and Adage Capital Management, L.P. (incorporated herein by reference to Exhibit 10.5 to the Company's Current Report on Form 8-K filed May 10, 2012). | ||
| 10.16* | Advanced Magnetics, Inc. Amended and Restated 2000 Stock Plan (incorporated herein by reference to Appendix A to the Company's definitive proxy statement for the year ended September 30, 2005, File No. 0-14732). | ||
| 10.17* | Form of Stock Option Grant under the Company's 2000 Stock Plan (employees) (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2005, File No. 0-14732). | ||
| 10.18* | Form of Stock Option Grant under the Company's 2000 Stock Plan (non-employees) (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2005, File No. 0-14732). | ||
| 10.19* | AMAG Pharmaceuticals, Inc. Second Amended and Restated 2007 Equity Incentive Plan (incorporated herein by reference to Appendix A to the Company's Definitive Proxy Statement on Schedule 14A, filed April 19, 2010, File No. 001-10865). | ||
| 10.20* | Form of Option Agreement (ISO) under the Company's 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed November 30, 2007, File No. 0-14732). | ||
| 10.21* | Form of Option Agreement (Nonqualified Option) under the Company's 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed November 30, 2007, File No. 0-14732). | ||
| 10.22* | Form of Restricted Stock Unit Agreement under the Company's 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed November 30, 2007, File No. 0-14732). | ||
| 10.23* | Form of Option Agreement (Nonqualified Option) for Annual Director Grants under the Company's Second Amended and Restated 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, File No. 001-10865). | ||
| 10.24* | Form of Restricted Stock Unit Agreement for Annual Director Grants under the Company's Second Amended and Restated 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.5 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, File No. 001-10865). | ||
| 10.25* | Form of November 2011 Restricted Stock Unit Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and the Company's executive officers (incorporated herein by reference to Exhibit 10.21 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | ||
| 10.26* | Form of November 2011 Restricted Stock Unit Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and each non-executive employee of the Company (incorporated herein by reference to Exhibit 10.22 to the Company's Annual Report on Form 10-K filed March 8, 2012, File No. 001-10865). | ||
145
| Exhibit Number | Description | ||
|---|---|---|---|
| 10.27* | Form of Non-Plan Restricted Stock Unit Agreement, by and between the Company and William K. Heiden (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed May 10, 2012). | ||
| 10.28* | Form of Non-Plan Stock Option Agreement, by and between the Company and William K. Heiden (incorporated herein by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed May 10, 2012). | ||
| 10.29* | Option Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and Lee F. Allen, dated as of June 25, 2012 (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed June 29, 2012). | ||
| 10.30* | Option Agreement under the Company's Second Amended and Restated 2007 Equity Incentive Plan between the Company and Christopher G. White, dated as of June 25, 2012 (incorporated herein by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed June 29, 2012). | ||
| 10.31 | Lease Agreement, dated as of May 27, 2008, by and between AMAG Pharmaceuticals, Inc. and Mortimer B. Zuckerman and Edward H. Linde, trustees of 92 Hayden Avenue Trust under Declaration of Trust dated August 18, 1983 (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed May 29, 2008, File No. 0-14732). | ||
| 10.32 | Collaboration and Exclusive License Agreement between the Company and 3SBio Inc., dated as of May 25, 2008 (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2008, File No. 0-14732) (confidential treatment previously granted). | ||
| 10.33 | Supply Agreement between the Company and 3SBio Inc., dated as of May 25, 2008 (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2008, File No. 0-14732) (confidential treatment previously granted). | ||
| 10.34 | Commercial Outsourcing Services Agreement, dated October 2008, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed July 1, 2009, File No. 0-14732) (confidential treatment previously granted). | ||
| 10.35 | First Amendment to Commercial Outsourcing Services Agreement, dated April 14, 2011, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2011, File No. 001-10865). | ||
| 10.36 | Second Amendment to Commercial Outsourcing Services Agreement, dated effective as of December 1, 2011, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed December 22, 2011, File No. 001-10865) (confidential treatment previously granted). | ||
| 10.37 | Third Amendment to Commercial Outsourcing Services Agreement, dated effective as of August 1, 2012, by and between the Company and Integrated Commercialization Services, Inc. (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q filed November 7, 2012, File No. 001-10865) (confidential treatment previously granted). | ||
| 10.38 | Commercial Packaging Services Agreement, dated May 29, 2009, by and between the Company and Packaging Coordinators, LLC (formerly Catalent Pharma Solutions LLC) (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed July 1, 2009, File No. 0-14732) (confidential treatment previously granted). | ||
146
| Exhibit Number | Description | ||
|---|---|---|---|
| 10.39+ | Amendment No. 1 to Commercial Packaging Services Agreement, dated January 29, 2013, by and between the Company and Packaging Coordinators, LLC (formerly Catalent Pharma Solutions LLC) (Certain confidential information contained in this exhibit was omitted by means of redacting a portion of the text and replacing it with [***]. This exhibit has been filed separately with the SEC without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended). | ||
| 10.40 | License, Development and Commercialization Agreement by and between the Company and Takeda Pharmaceutical Company Limited, dated March 31, 2010 (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, File No. 001-10865) (confidential treatment previously granted). | ||
| 10.41 | Amendment to the License, Development and Commercialization Agreement, dated June 25, 2012, by and between the Company and Takeda Pharmaceutical Company Limited (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed June 29, 2012, File No. 001-10865) (confidential treatment previously granted). | ||
| 10.42 | Commercial Supply Agreement, dated effective as of August 29, 2012, by and between the Company and Sigma-Aldrich, Inc. (incorporated herein by reference to Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q filed November 7, 2012, File No. 001-10865) (confidential treatment previously granted). | ||
| 10.43 | Pharmaceutical Manufacturing and Supply Agreement, dated effective as of January 8, 2010, by and between the Company and DSM Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q filed November 7, 2012, File No. 001-10865) (confidential treatment previously granted). | ||
| 21.1+ | Subsidiaries of the Company. | ||
| 23.1+ | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | ||
| 24.1 | Power of Attorney (included on the signature page(s) hereto) | ||
| 31.1+ | Certification Pursuant to Rule 13a-14(a)/15d-14(a) of the Exchange Act, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||
| 31.2+ | Certification Pursuant to Rule 13a-14(a)/15d-14(a) of the Exchange Act, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||
| 32.1++ | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | ||
| 32.2++ | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | ||
| 101++ | The following materials from AMAG Pharmaceuticals, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2012, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Loss, (iv) Consolidated Statements of Stockholders' Equity, (v) Consolidated Statements of Cash, and (vi) Notes to Consolidated Financial Statements. | ||
- +
- Exhibits marked with a plus sign ("+") are filed herewith.
- ++
- Exhibits marked with a double plus sign ("++") are furnished herewith.
- *
- Exhibits marked with a single asterisk reference management contracts, compensatory plans or arrangements, filed in response to Item 15(a)(3) of the instructions to Form 10-K.
The other exhibits listed have previously been filed with the SEC and are incorporated herein by reference, as indicated.
147