Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | |
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 27, 2009 Commission file number 000-14742
|
CANDELA CORPORATION
(Exact name of registrant as specified in its charter)
| | |
Delaware
(State or other jurisdiction of
incorporation or organization) | | 04-2477008
(I.R.S. Employer
Identification No.) |
530 Boston Post Road, Wayland, Massachusetts |
|
01778 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant's telephone number, including area code:(508) 358-7400 |
Securities registered pursuant to Section 12(b) of the Act:
| | |
| Title of each class | | Name of exchange on which registered |
|---|
| Common Stock, $.01 par value per share | | The NASDAQ Stock Market LLC |
Securities registered pursuant to section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period than the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer (as defined in Rule12b-2 of the Exchange Act)
| | | | | | |
| Large accelerated filer o | | Accelerated filer o | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule12b-2 of the Exchange Act) Yes o No ý
The aggregate market value of our voting stock held by non-affiliates was approximately $10,784,350 on December 29, 2008 based on the last reported sale price of our common stock on the NASDAQ Stock Market on that day. As of September 30, 2009, 22,913,036 shares of the registrant's common stock, $.01 par value, were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates by reference certain information from the registrant's definitive Proxy Statement relating to the 2009 meeting of stockholders, which the registrant expects to file prior to October 25, 2009.
Table of Contents
CANDELA CORPORATION
2009 Form 10-K Annual Report
Table of Contents
| | | | |
| |
| | Begins on
Page |
|---|
PART I |
Item 1. | | Business | | 3 |
Item 1A. | | Risk Factors | | 20 |
Item 1B. | | Unresolved Staff Comments | | 26 |
Item 2. | | Properties | | 26 |
Item 3. | | Legal Proceedings | | 27 |
Item 4. | | Submission of Matters to a Vote of Security Holders | | 29 |
PART II |
Item 5. | | Market for the Registrant's Common Equity; Related Stockholder Matters and Issuer Purchases of Equity Securities | | 29 |
Item 6. | | Selected Financial Data | | 31 |
Item 7. | | Management's Discussion and Analysis of Financial Condition and Results of Operations | | 32 |
Item 8. | | Financial Statements and Supplementary Data | | 42 |
Item 9. | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | | 77 |
Item 9A(T). | | Controls and Procedures | | 77 |
Item 9B. | | Other Information | | 77 |
PART III |
Item 10. | | Directors, Executive Officers, and Corporate Governance | | 78 |
Item 11. | | Executive Compensation | | 78 |
Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | | 78 |
Item 13. | | Certain Relationships and Related Transactions; and Director Independence | | 78 |
Item 14. | | Principal Accountant Fees and Services | | 79 |
PART IV |
Item 15. | | Exhibits and Financial Statement Schedules | | 81 |
2
Table of Contents
PART I
Item 1. Business.
Introduction
Candela Corporation ("Candela" or the "Company") is a pioneer in the development and commercialization of advanced aesthetic lasers that allow physicians and personal care practitioners to treat a wide variety of cosmetic and medical conditions including:
- •
- permanent hair reduction on all skin types;
- •
- skin rejuvenation, skin tightening and wrinkle reduction;
- •
- vascular lesions such as rosacea, facial spider veins, leg veins, port wine stains, angiomas and hemangiomas;
- •
- all-color tattoo removal;
- •
- skin resurfacing scars, stretch marks and warts;
- •
- removal of benign pigmented lesions such as sun spots, age spots, freckles, and Nevus of Ota/Ito;
- •
- acne and acne scars;
- •
- sebaceous hyperplasia;
- •
- pseudofolliculitis barbae (beard bumps or "PFB");
- •
- psoriasis; and
- •
- other cosmetic skin treatments.
Since our founding nearly 40 years ago, we have continuously developed and enhanced applications for laser technology. In the mid-1980's we began developing laser technology for medical applications, and since that time have shipped approximately 14,500 systems to over 85 countries. Since the early 1990's we have focused our organizational resources on developing laser and light-based technology for use solely in cosmetic, dermatological, and aesthetic applications. Below is a sampling of Candela's innovations in the laser and light-based technologies market:
- •
- first laser system based on selective photothermolysis to treat cutaneous vascular lesions with minimal skin injury;
- •
- first laser system to treat vascular lesions approved by the FDA for use on children;
- •
- first Q-switched alexandrite laser for pigmented lesions and tattoos;
- •
- first effective pulsed dye laser treatment of hemangiomas, scars and warts;
- •
- first multi-wavelength long-pulse dye laser for treatment of leg telangiectasia (spider veins);
- •
- first laser with a clearance for non-invasive treatment of acne and acne scars;
- •
- first hair removal laser with integrated Dynamic Cooling Device ("DCD");
- •
- first pulsed dye laser with DCD for wrinkles;
- •
- first DCD for epidermal protection in vascular lesion treatment;
- •
- first to introduce "laser-pumped-laser" hand-piece technology for more efficacious pigmented lesion and all color tattoo removal treatments (for which we have applied for a patent); and
- •
- first pain reduction device for aesthetic lasers and intense pulse light ("IPL") systems.
3
Table of Contents
Candela's current product line offers comprehensive and technologically sophisticated cosmetic and aesthetic lasers and light-based systems used by physicians and personal care practitioners to treat a wide variety of cosmetic and medical conditions. Candela's product line includes the following innovative products:
- •
- GentleMax™—Multi-wavelength technology integrated aesthetic treatment workstation.
- •
- AlexTriVantage™—The total pigmented lesion and tattoo solution using multi-wavelength technology in conjunction with our "laser-pumped-laser" hand piece technology.
- •
- GentleLASE® family of alexandrite lasers which provide the highest power, speed, efficacy and ease-of-use in permanent hair reduction, vascular lesions, wrinkle reduction and the treatment of pigmented lesions.
- •
- GentleYAG® family of Nd: YAG lasers which provide fast, effective permanent hair reduction for every skin type including tanned or dark skin, treatment of beard bumps, leg and facial veins, and skin tightening.
- •
- Vbeam®—The gold standard pulsed-dye technology which eliminates vascular lesions including port wine stain birthmarks, rosacea, leg and facial veins, post-operative bruising and provides skin rejuvenation by the reduction of diffuse redness and pigmentation, scars, warts, psoriasis and hemangiomas.
- •
- Smoothbeam® diode laser, for treatment of acne and acne scars, sebaceous hyperplasia and wrinkle reduction.
- •
- SmoothPeel™—Erbium: YAG laser, the simple choice for superior laser peels.
- •
- QuadraLASE™—A complete solution to Fractionated and traditional C02 skin resurfacing.
There were nearly 10.3 million surgical and non-surgical cosmetic procedures performed in the United States in 2008, as reported by the American Society for Aesthetic Plastic Surgery ("ASAPS"). While there was a 12% decrease in total number of cosmetic procedures from 2007 to 2008, there still has been an overall increase of over 160% in the number of cosmetic procedures performed since 1997. Women represented 92% of this total market or nearly 9.3 million procedures. The growth in the market for using aesthetic laser and light-based products is currently a worldwide phenomenon being driven by an increase in discretionary income of aging baby-boomers, which continues to create new opportunities for Candela. This market demographic places a premium on good health and personal appearance, and has demonstrated a willingness to pay for health and cosmetic products and services.
In addition, growth in the popularity of laser and light-based treatments among the general population is also spurring demand for Candela's products. In calendar 2008, according to The American Society for Aesthetic Plastic Surgery, Americans spent an estimated $11.8 billion on cosmetic procedures. In particular, laser and light-based systems are proving an attractive alternative for eliminating unwanted hair. Of the top five non-surgical cosmetic procedures performed in 2008, laser hair removal was the second most popular and is the most common procedures performed on patients aged 18 and under. According to a 2007 report published by the Millennium Research Group, it is estimated that by 2011 the U.S. market for laser hair removal will reach approximately $238.5 million, representing a CAGR of 10.8%.
Candela is dedicated to developing safe and effective products. Our aesthetic laser and light-based systems are further distinguished by being among the fastest, smallest and most affordable in their respective markets.
Candela was incorporated in Massachusetts on October 22, 1970 and subsequently reincorporated in Delaware on July 1, 1985.
4
Table of Contents
Proposed Merger
On September 9, 2009, Candela Corporation and Syneron Medical Ltd. ("Syneron") announced that they had entered into an Agreement and Plan of Merger (the "Merger Agreement"), pursuant to which Candela and Syneron will combine their businesses through a merger of Candela and a newly formed, wholly owned subsidiary of Syneron. Existing stockholders of Candela will exchange each of their shares of Candela common stock for 0.2911 ordinary shares of Syneron (the "Merger"). The Merger is discussed more fully below under the caption "Proposed Merger with Syneron." If the merger is not consummated on or before June 1, 2010, either party may terminate the Merger Agreement.
Industry Overview
Lasers and light-based devices use the unique characteristics of light to achieve precise and efficacious therapeutic effects, often in a non-invasive manner. The precise color, concentration and controllability of different types of light provide for the delivery of a wide range of specialized treatments. First introduced in the 1960's, the use of lasers for medical applications grew rapidly in the 1990's as technical advances made medical lasers more effective and reliable. Medical lasers and light-based devices are today used for numerous types of procedures falling into four broad categories: ophthalmic surgery, aesthetic and cosmetic procedures, general surgery, and dental procedures. Candela competes solely within the growing market for lasers and light-based devices for performing aesthetic, dermatological, and cosmetic procedures including:
- •
- removal of unwanted hair;
- •
- treatment of rosacea, facial veins and leg veins, red birthmarks, scars, stretch marks and warts;
- •
- facial rejuvenation and skin tightening to reduce the appearance of fine lines and wrinkles;
- •
- removal of pigmented lesions such as sun spots, age spots and freckles;
- •
- treatment of acne and acne scars;
- •
- treatment of psoriasis;
- •
- treatment of Nevus of Ota and melasma;
- •
- tattoo removal; and
- •
- skin resurfacing.
Lasers produce intense bursts of highly focused light to treat skin tissues. A laser's wavelength (color), energy level, spot size and pulse width (exposure time) are optimized for the specific treatment. Light-based devices commonly referred to as intense pulsed light ("IPL"), feature a broad range of wavelength of light designed to target vascular and pigmentary targets for facial rejuvenation procedures. Hair removal and the treatment of various leg vein malformations require the deepest laser penetration for successful treatment while scars and red birthmarks (port wine stains and hemangiomas) require less laser penetration. Pigmented lesions such as sun spots, age spots and tattoos are typically surface conditions that require the least amount of penetration. Different conditions may require the use of different types of lasers with specific power requirements and optimized operating parameters. An active aesthetic practice addressing a broad range of cosmetic procedures may need multiple lasers.
We believe the aesthetic and cosmetic laser industry, while in a temporary downturn, remains a sector poised for long-term growth. The major factors converging to drive this anticipated growth are:
- •
- medical practitioners desire to drive additional revenue into their practices due to the increasing reduction in insurance reimbursement;
5
Table of Contents
- •
- discretionary income of aging baby boomers;
- •
- increased general acceptance of aesthetic and cosmetic laser treatments.
Aesthetic and cosmetic device vendors, who are able to deliver lasers that are efficacious, cost effective, reliable, and easy to use, will be well positioned to take advantage of such broad-based industry growth.
Managed care and reimbursement restrictions in the United States, and similar constraints outside the United States, such as socialized medicine, are motivating practitioners to emphasize aesthetic and cosmetic procedures that are delivered on a private, fee-for-service basis. While cosmetic procedures were once the domain of plastic surgeons and dermatologists, reimbursement reductions coupled with the reliable revenue stream from private-pay procedures have piqued the interest of other specialties, including general practitioners, family care practitioners, obstetricians, gynecologists and general and vascular surgeons.
Key technical developments required for the broader cosmetic laser and light-based treatment markets relate to ease-of-use, versatility, speed, lower costs, safety and effective elimination of undesirable side effects. These factors are critical for broader segments of practitioners who wish to build aesthetic and cosmetic laser and light-based treatment practices. These factors are also important for minimizing the disruption of a patient's normal routine and for building demand for procedures addressing very large patient populations.
Business Strategy
Candela believes that a convergence of price, performance and technology is occurring in the aesthetic and cosmetic laser industry which is driving market expansion. We believe we have the necessary infrastructure in place to capitalize on this expansion. Candela's objective is to continue to be the leading provider of aesthetic and cosmetic lasers by using its proprietary technology and expertise in light and tissue interaction, as well as by developing market-oriented products that utilize related technologies. Our business strategy is focused on the following goals:
- •
- increase penetration of our traditional customer base;
- •
- expand beyond our traditional customer base;
- •
- reduce product costs;
- •
- expand and leverage our international distribution channels;
- •
- introduce new technologies or expand existing technologies that improve current treatment procedures and outcomes and provide our customers with unique differentiation;
- •
- develop home use products that enable consumers to leverage the advantages of light based technologies at home; and
- •
- continue investing in research and development to develop new applications that are efficacious, cost-effective, reliable and easy to use and have a high return on investment for our customers.
Increase Penetration of Our Traditional Customer Base. Our traditional customer base consists of dermatologists and plastic and cosmetic surgeons. We believe that the continued innovation that results in the introduction of devices and applications that enable our traditional customers to deliver new services to their patients is the key to growth in this segment. In addition, more versatile devices, differentiated features and increased efficacy and speed will enable Candela to continue to grow our share in this market.
Expand beyond our traditional customer base. As reimbursement rates have dropped over the past several years, many primary care physicians (family and general practice, and obstetrics and gynecology
6
Table of Contents
physicians) have begun to seek new services to offer to their patients. Cosmetic laser and light-based procedures represent an ideal new service that is appealing to their patients, and adds a new "cash-pay" revenue stream to their practices. As more primary care physicians realize that these procedures are safe and easy to perform and that the devices are versatile and affordable, awareness of cosmetic laser and light-based procedures will continue to grow.
Reduce Product Costs. We apply bottom-up engineering, focusing on each component to improve the performance of each device while reducing its size, complexity, and cost. We believe our approach leads to devices with fewer parts and greater manufacturing efficiency, resulting in lower production costs. This enables us to offer more reliable products at more affordable prices.
Expand and Leverage Our Distribution Channels. North America presently is the largest single geographic market for our products. We continue to upgrade our United States sales force to better address the needs of our traditional core markets. Outside of the Unites States, we continue to strengthen our long-standing positions in Europe and Japan and are seeking to expand our markets in Asia-Pacific and Latin America. We currently have direct sales offices in Madrid, Lisbon, Rome, Frankfurt, Paris, Cwmbran (U.K.), Osaka, Fukuoka, Nagoya, Tokyo, Prospect (Australia), and strong distribution partners covering 76 countries worldwide including China. Over the past year we increased the number and improved the quality of our international independent distributor channels.
Introduce New Technologies or Expand Existing Technologies that Improve Current Treatment Procedures. As the market for non-invasive cosmetic procedures continues to expand, Candela has a strong focus on how to improve existing treatments which enables our customers to improve results and the overall experience for their customers. For example, improving patient outcomes for post-surgical bruising is an expanded treatment capability for the Vbeam.
Develop Home Use Products. Candela continues to evaluate opportunities to leverage technologies and application knowledge from our professional business and apply it to the Home Use Market. Despite the current economic environment we have continued to fund proof of concept activities focused on the Home Use market.
Continue Investing in Research & Development. We believe that continued investment in research and development is necessary to maintain our position as a leader in the aesthetic laser and light-based device market. Our research and development approach is to develop high quality, reliable, and affordable products that address our existing markets and also allow us to expand into larger, growing markets. In support of this approach, our research and development staff works closely with our marketing and operations teams to identify new clinical applications that serve our markets. We have numerous research and development arrangements with leading physicians, hospitals and academic laboratories in the United States and throughout the world. In a parallel effort, our research and development and engineering teams continue to optimize our existing products by adding new technologies, increasing the speed of treatment and ease of use for our customers as well as reduce the overall size of our products.
The Market for Aesthetic and Cosmetic Laser and Light-Based Systems
Our traditional customer base for aesthetic and cosmetic devices consists of dermatologists and plastic and cosmetic surgeons. In addition, other practitioner groups are emerging as potential customers, including general and family practitioners, obstetricians, gynecologists, and general and vascular surgeons. In the United States, according to the American Medical Association and various professional societies, there are approximately 15,580 dermatologists, 10,000 plastic and cosmetic surgeons and 13,600 ear, nose and throat specialists. Practitioners in other specialties that are beginning to buy aesthetic and cosmetic laser and light-based systems include 101,000 general and family practitioners, 40,433 obstetricians and gynecologists, and 28,000 general and vascular surgeons. In
7
Table of Contents
addition, the aesthetic and cosmetic laser and light-based system market includes non-medical practitioners, notably electrologists, of which there are an estimated 6,000 in the United States and approximately 4,000 med spa owners in the United States.
The end markets for cosmetic procedures encompass broad and growing patient groups, including aging "baby boomers" as well as younger age groups. There are approximately 80 million baby boomers, representing approximately 29% of the total United States population. This large population group has exhibited a strong demand for aesthetic and cosmetic procedures. We believe that as the cost of treatments decreases and the popularity of cosmetic procedures such as hair removal increases, the target market for these procedures will expand beyond the baby boomers to include a broad range of women and men aged 17 to 65. We believe that similar demographics will help support the growth of the aesthetic and cosmetic market outside of the United States as well.
Hair Removal. We believe that the great majority of the 108 million women over the age of 16 in the United States employ one or more techniques for temporary hair removal from various parts of the body. Also, a growing number of men are removing hair by means other than their daily shaving routine. A number of techniques are used to pull hair from the follicle including waxing, depilatories, and tweezing. In the waxing process, a lotion, generally beeswax-based, is spread on the area to be treated and is then rapidly peeled off, pulling out the entrapped hairs. Depilatories employ rotating spring coils or slotted rubber rolls to trap and pull out the hairs. Tweezing involves removing individual hairs with a pair of tweezers. Pulling hair from the follicle produces temporary results, but is often painful and may cause skin irritation. Depilatory creams, which contain chemicals to dissolve hair, frequently leave a temporary unpleasant odor and may also cause skin irritation. Shaving is the most widely used method of hair removal, especially for legs and underarms but produces the shortest-term results. Hair bleaches do not remove hair, but instead lighten the color of hair so that it is less visible. A principal drawback of all of these methods is that they require frequent treatment.
We believe the market for laser and light-based hair removal is growing as the customer compares laser and light-based treatments to the other hair removal methods currently available. The benefits of a laser and light-based system treatment include:
- •
- significantly reduced procedure time and number of treatments;
- •
- significantly longer-term cosmetic improvement;
- •
- treatment of larger areas in each treatment session;
- •
- less discomfort during and immediately following procedures;
- •
- reduced risk of scarring and infection; and
- •
- non-invasive in nature.
Vascular Lesions. Benign vascular lesions are abnormal, generally enlarged and sometimes proliferating blood vessels that appear on the surface of the skin as splotches, dots, bulges, and spider shapes in a variety of colors ranging from red to purple. Different types of benign vascular lesions include the following:
- •
- stretch marks and scars;
- •
- varicose veins, which are large veins greater than 1mm in diameter and often bulge above the skin surface;
- •
- rosacea, which is the dilation of capillaries in the cheeks, nose, forehead and chin;
- •
- telangiectasias, more commonly referred to as spider veins, appearing on the face and other parts of the body;
8
Table of Contents
- •
- leg telangiectasias, which are smaller spider veins up to 1mm in diameter appearing as single strands;
- •
- port wine stains, which are vascular birthmarks characterized by a red or purple discoloration of the skin; and
- •
- hemangiomas, which are protuberances that consist of dilated vessels, which often appear on newborns within one month of birth.
Pigmented Lesions/Tattoos. Benign pigmented lesions can be both epidermal, on the outer layer of skin, and dermal, on the innermost layer of skin, natural or man-made (tattoos) and can constitute a significant cosmetic problem to those who have them. There has been a significant increase, in recent years, in the number of people getting tattoos and it is currently estimated that approximately 24% of the U.S. population has one or more of them. This figure is expected to grow to as much as 40% of the population in the next several years.
Skin Rejuvenation. Skin rejuvenation is one of the fastest growing segments of the aesthetic laser and light-based system market. A significant percentage of the population suffers from fine lines and wrinkles or older looking skin as a result of the normal aging process. This is the primary group of candidates for non-ablative laser and skin tightening treatments. While the market for skin rejuvenation is greatest in the United States, significant opportunities abound in international markets where there is an aging demographic, such as Japan, or a high prevalence of photo-damaged skin, such as Australia and Latin America.
Acne. Patients have expressed dissatisfaction with existing therapies for acne and acne scarring. These therapies include the following: drug therapy, dermabrasion, ablation, excision, chemical peeling and injections of filler materials. These therapies have minimal efficacy, a significant side-effect profile, require long recovery periods and, in most cases, do not meet patient expectations. For many of these patients, laser treatment can represent a better treatment alternative. Laser therapy can effectively target the sebaceous gland which is the root cause of acne.
Acne Scars. The majority of existing acne scar patients that initially seek traditional treatments decide not to undertake a procedure due to the side-effects and social down-time. A more acceptable alternative for acne scar treatment is laser therapy. In the treatment of acne scars, the laser initiates deposition of new collagen to raise depressions in the skin, reducing the appearance of acne scars. Over a series of treatments, new collagen can fill in and soften the appearance of acne scars.
Psoriasis. According to the National Institutes of Health (NIH), as many as 7.5 million Americans and 125 million people worldwide have psoriasis.
Skin Resurfacing. In calendar 2008, there were approximately 571,000 skin resurfacing procedures performed in the U.S., most using non-ablative skin resurfacing technologies. In addition to these procedures, there were also approximately 592,000 chemical peels and 557,000 microdermabrasion procedures conducted in the U.S, respectively.
Candela's Products
We research, develop, manufacture, market, sell, distribute, and service laser systems used to perform procedures addressing patients' aesthetic medical and cosmetic concerns. We offer a comprehensive range of products based on proprietary technologies focusing on the major aesthetic and cosmetic laser applications.
9
Table of Contents
Our objective is to remain a leading provider of aesthetic and cosmetic lasers by continually striving to develop effective, versatile, smaller, faster, and less expensive devices. Candela has been, and continues to be, a pioneer in the laser industry. From the start, our mission has been to lead the way in the development of innovative laser products which include:
Dynamic Cooling Device ("DCD"). The DCD cools the top layer of the skin, while leaving the targeted underlying hair follicle, vein or other structure at normal temperature. As a result, higher levels of laser energy can be delivered during treatment, while minimizing thermal injury, pain, and the inconvenience associated with anesthetics. The design of the hand-held DCD enables the practitioner to clearly see the area being treated, and the combined efficiency of the lasers and DCD reduces the risks of over treatment. The DCD delivers just the right amount of cooling quickly and consistently. Currently, DCD is available as an option on several Candela laser systems.
GentleMax®
- •
- Integrated aesthetic treatment workstation with multiple wavelength capability;
- •
- The fastest and most powerful Nd:YAG 1064 laser and 755 nm laser in one;
- •
- Superior results in less time and in fewer treatments;
- •
- Touch-screen technology for user-friendly interface;
- •
- Unlimited potential with complete treatment versatility offering unmatched permanent hair reduction to skin tightening and skin rejuvenation to treating leg and facial veins- as many as 36 different applications;
- •
- Offers a choice of skin cooling options—from chilled air technology to Candela's integrated and patented DCD that utilizes bursts of cryogen before and after the laser pulse to offer unparalleled patient comfort.
SmoothPeel®
- •
- Erbium:YAG laser for precise and predictable peels without the chemicals;
- •
- Superior ablative characteristics for skin;
- •
- Offering patients multiple treatment options from simple freshening to a peel comparable to a glycolic acid peel with just a few days of downtime.
AlexTriVantage®™
- •
- The total all-color tattoo and pigmented lesion solution;
- •
- New laser-pumped laser hand pieces (patent pending) offer a unique and elegant design;
- •
- Effective removal of even the most difficult tattoo colors;
- •
- New long pulse mode offers advanced rejuvenation treatments for a wider variety of pigmented lesions;
- •
- Candela's fastest, gentlest, most powerful q-switched alexandrite laser;
- •
- Touch-screen technology for user friendly interface.
QuadraLASE™
- •
- The complete solution to fractional and traditional CO2 skin resurfacing;
- •
- Provides a unique QuadraSCAN™ scanning system for enhanced patient comfort;
10
Table of Contents
- •
- Dual fractionated (300 and 180m) hand pieces and surgical hand pieces provide variable depth therapy and full range of treatment options;
- •
- No disposables and value-pricing mean lower cost of ownership and therefore greater profitability for users.
GentleLASE®
- •
- One of the largest spot size on any hair removal laser (18mm) resulting in faster treatment times, more patients seen, more revenue generated , greater profit ability;
- •
- Treats most skin types;
- •
- Longer delivery system: treat head-to-toe without repositioning laser;
- •
- Lightweight, compact design;
- •
- Multiple spot sizes and applications;
- •
- DCD epidermal cooling—no messy gels required.
GentleYAG®
- •
- One of the fastest, most powerful Nd:YAG laser on the market today;
- •
- Provides treatment versatility, including skin tightening and leg vein removal;
- •
- One of the largest spot (18mm) on any Nd:YAG laser; multiple spot sizes for multiple applications;
- •
- Optimized for all skin types, including dark and tanned skin;
- •
- Multiple configurations, upgradeable;
- •
- Advanced yet affordable;
- •
- DCD epidermal cooling—no messy gels required.
Vbeam®
- •
- Gold standard pulsed dye laser offering vascular and skin rejuvenation treatments with no downtime;
- •
- Reduces bruising associated with injectables and other cosmetic procedures;
- •
- Micro-pulse technology for purpura-free results;
- •
- Smart user interface provides pre-set treatment parameters with a touch of the screen;
- •
- DCD epidermal cooling—no messy gels required.
Smoothbeam®
- •
- Clinically proven leader in acne therapy with clearance up to 24 months;
- •
- 1450 nm wavelength stimulates new collagen growth to treat fine lines and wrinkles;
- •
- Quick, effective, non-invasive treatment for patients;
- •
- DCD epidermal cooling—no messy gels required;
- •
- Convenient table top size and simple set-up.
11
Table of Contents
Sales and Distribution
We market and sell our products worldwide. Executives in North America, Latin America, Japan, Asia-Pacific, and Europe manage our marketing, selling, and service activities through a combination of direct personnel and a network of independent distributors.
The mix of direct sales and distributor sales varies by region. Generally, our distributors enter into 2-3 year exclusive agreements during which they typically agree not to sell our competitors' products. Our sales strategy is to choose the most productive and practicable distribution channel within each of our geographic markets.
We sell products in the United States primarily through our direct sales force to our traditional customer base of dermatologists and plastic and cosmetic surgeons. Candela's products are distributed to our non-traditional markets through an exclusive arrangement with McKesson's Medical Surgical Division. McKesson has approximately 450 dedicated sales representatives that showcase a wide range of products to family and general practice, and obstetrics and gynecology, physicians.
Outside the United States, we sell our products in Europe, Japan, Latin America, the Middle East, and Asia-Pacific through direct sales offices and distribution relationships. We have a total of 130 employees in our direct sales offices in Madrid, Lisbon, Rome, Frankfurt, Paris, Tokyo, Nagoya, Fukuoka, Osaka, Cwmbran, and Sydney. We also have established distribution relationships in China, Europe, Japan, Africa, Latin America, and the Middle East. Outside the United States, we are utilizing approximately 60 distributors. Refer to Note 13 of our Consolidated Financial Statements for additional financial information about segments and geographical areas.
The following chart shows data relating to Candela's international activities during each of the last three fiscal years by geographic region. Revenue generated from regions other than the United States includes sales from our German, Spanish, Italian, United Kingdom, French, Japanese, and Australian subsidiaries, as well as sales shipped directly to international locations from the United States.
| | | | | | | | | | |
Revenues: | | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | |
|---|
(in thousands)
| |
| |
| |
| |
|---|
United States and Canada | | $ | 39,050 | | $ | 55,077 | | $ | 64,397 | |
Europe | | | 43,814 | | | 55,521 | | | 42,344 | |
Japan and Asia-Pacific | | | 29,717 | | | 31,330 | | | 29,935 | |
Latin America & all others | | | 3,999 | | | 4,689 | | | 11,101 | |
| | | | | | | | |
| | $ | 116,580 | | $ | 146,617 | | $ | 147,777 | |
| | | | | | | | |
Service and Support
Candela believes that quick and effective delivery of service is important to our customers. Our principal service center and parts depot is located at our Wayland, Massachusetts headquarters. Parts depots are also located at our sales offices in Japan, Australia, the U.K., Spain, Portugal, Italy, Germany, and France. Independent distributors also maintain parts depots.
We also believe a highly trained and qualified service staff is important to product reliability. Distributors and subsidiaries have the primary responsibility of servicing systems within their territories. Their service personnel are required to attend formal training to become authorized. We provide service training in Asia, Europe and the United States. In addition, we have service and technical support staff in each of our markets worldwide.
Post-warranty product maintenance and repair provides an additional recurring source of revenue. Customers may elect to purchase a service contract or purchase service on a time-and-materials basis.
12
Table of Contents
Candela's service contracts offer a range of service levels and options, including additional clinical support. The contract terms generally run in 12 or 24-month intervals (See Item 8).
Candela emphasizes education and support of its customers. Periodic device calibration/verification, coupled with the continuous training of our service providers, helps to ensure product reliability. We extend prompt and courteous technical and clinical support to our customers when needed.
Manufacturing and Raw Materials
We design, assemble, and test our branded products at our Wayland, Massachusetts and South Plainfield, New Jersey facilities. Ensuring adequate and flexible production capacity, applying lean manufacturing techniques, continually reducing costs, and maintaining superior product quality are top priorities of our manufacturing organization. We achieve our goals by:
- •
- working closely with the research and development organization, including significant early involvement in product design;
- •
- continually improving our just-in-time manufacturing and inventory processes;
- •
- effectively managing and working closely with a limited number of the most qualified suppliers; and
- •
- applying the latest cell manufacturing techniques.
Our facility has ISO 13485 certification and has established and is maintaining a quality system that meets the requirements of ISO 13485:2003 from both the EC Directive 93/42/EEC and Canadian Medical Devices Regulation. The ISO 13485 certification provides evidence that Candela conforms to quality system requirements for the design, development, production, servicing and distribution of medical lasers and accessories.
Our products are manufactured with standard components and subassemblies supplied by third party manufacturers to our specifications. We purchase certain components and subassemblies from a limited number of suppliers.
If our suppliers are unable to meet our requirements on a timely basis, our production could be interrupted until we obtain an alternative source of supply. To date, we have not experienced significant delays in obtaining dyes, optical and electro-optical components, electronic components, Alexandrite rods and other raw materials for our products. We believe that over time alternative component and subassembly manufacturers and suppliers can be identified if our current third party manufacturers and suppliers fail to fulfill our requirements.
Research and Development
We believe that our advanced research and engineering activities are crucial to maintaining and enhancing our business, and we are currently conducting research on a number of applications. We believe that our in-house research and development (R&D) staff has demonstrated its ability to develop innovative new products that meet evolving market needs. Our core competencies include:
- •
- applied laser physics and technology;
- •
- tissue optics;
- •
- photochemistry;
- •
- light-tissue interaction;
- •
- clinical research;
- •
- aesthetic laser applications;
13
Table of Contents
- •
- engineering and design of medical laser devices; and
- •
- collaborative research with leading academic and medical institutions.
As we discover new technologies or applications with commercial potential, we assemble a team to develop the new product or application in cooperation with leading physicians and medical and research institutions. In the United States in particular, we must receive United States Food and Drug Administration ("FDA") clearance before marketing new products or applications.
Our research and development team works with our operations group to design our products for ease of manufacturing and assembly and with our marketing group to create and respond to market opportunities. We believe this interaction between functional groups facilitates the introduction of new products with the right balance of features, clinical benefits, performance, quality, and cost. Historically, our research and development effort has relied primarily on internal development building on our core technologies. In addition to our internal team, we regularly engage the services of independent engineering and development firms to assist in the acceleration of new product development.
Candela also conducts joint research with physicians affiliated with various medical and research institutions. One example of technology developed through joint research is our Dynamic Cooling Device which was developed in conjunction with the Beckman Laser Institute at the University of California, Irvine. We anticipate continuing joint research and licensing arrangements with reputable medical research institutions in future periods.
Our expenditures on research and development are set forth in Item 7.
Backlog
We typically have not had, and do not anticipate having, significant backlog in the future. Accordingly, we do not believe that our backlog at any particular date is necessarily an accurate predictor of revenue for any succeeding period.
Customers
Our sales are not dependent on any single customer or distributor, and we continue to expand our distribution channel worldwide through direct and indirect sales forces. We experience some seasonal reduction of our product sales in our first fiscal quarter due to the summer holiday schedule of physicians and their patients.
Competition
Competition in the aesthetic and cosmetic laser industry is intense and technological developments are expected to continue at a rapid pace. Although there are several manufacturers of aesthetic and cosmetic lasers, we believe Candela is one of only a few companies that offer a broad range of technologies and products able to address multiple clinical applications. Unlike Candela, few of our competitors focus exclusively on the cosmetic and aesthetic laser market and several rely on a single technology which can be limiting. We compete on the basis of proprietary technology, product features, performance, service, price, and reputation. Some of our competitors have greater financial, marketing, and technical resources than we do. Moreover, some competitors have developed, and others may attempt to develop, products with applications similar to ours. We believe that many factors will affect our competitive position in the future, including our ability to:
- •
- develop and manufacture new products that meet the needs of our markets;
- •
- respond to competitive developments and technological changes;
- •
- manufacture our products at lower cost;
14
Table of Contents
- •
- retain a highly qualified research and engineering staff;
- •
- provide sales and service to a worldwide customer base; and
- •
- maintain and improve product reliability.
Proprietary Rights
We have numerous patents and have a number of patent applications pending to protect our rights in certain technical aspects of our hair removal, wrinkle removal, acne treatment, benign vascular lesion, pigmented lesion, and other laser systems.
In addition to our portfolio of issued patents and patent applications, we license patented technology from third parties. We use our patented DCD under a license agreement to patent rights owned by the Regents of the University of California ("Regents").
In August 2000, we obtained exclusive license rights to the DCD, subject to certain limited license rights of Cool Touch, Inc. ("Cool Touch"), in the following fields of use:
- •
- procedures that involve skin resurfacing and rejuvenation,
- •
- vascular skin lesions, and
- •
- laser hair removal.
Cool Touch obtained a license to the DCD on a co-exclusive basis with Candela, in certain narrower fields of use. Cool Touch is restricted in its ability to assign its license rights to certain existing competitors of Candela. Candela is entitled to one-half of all royalty income payable to the Regents from Cool Touch. Under the agreement, Candela no longer is required by the Regents to negotiate sublicenses to third parties. However, Candela is entitled to one-half of all royalties due from any other entity that licenses the DCD technology from the Regents in other fields of use.
We rely primarily on a combination of patent, copyright, and trademark laws to establish and protect our proprietary rights. We also rely on trade secret laws, confidentiality procedures and licensing arrangements to establish and protect our technology rights. In addition, we seek to protect our proprietary rights by using confidentiality agreements with employees, consultants, advisors, and others. We cannot be certain that these agreements will adequately protect our proprietary rights in the event of any unauthorized use or disclosure, that our employees, consultants, advisors or others will maintain the confidentiality of such proprietary information, or that our competitors will not otherwise learn about or independently develop such proprietary information.
Despite our efforts to protect our intellectual property, unauthorized third parties may attempt to copy aspects of our products, to violate our patents, or to obtain and use our proprietary information. In addition, the laws of some foreign countries do not protect our intellectual property to the same extent as do the laws of the United States. The loss of any material patent, trademark, trade name, trade secret or copyright could hurt our business, results of operations and financial condition.
We believe that our products do not infringe the rights of third parties, although two parties are presently asserting that our products infringe their patents (see Item 3). We cannot be certain that third parties will not assert infringement claims against us in the future or that any such assertions will not result in costly litigation or require us to obtain a license to third party intellectual property. In addition, we cannot be certain that such licenses will be available on reasonable terms or at all, which could hurt our business, results of operations and financial condition.
15
Table of Contents
Government Regulation
FDA's Pre-market Clearance and Approval ("PMA") Requirements. Unless an exemption applies, each medical device that we wish to market in the U.S. must receive either "510(k) clearance" or PMA in advance from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act. The FDA's 510(k) clearance process usually takes from three to twelve months, but it can last longer. The process of obtaining PMA approval is much more costly, lengthy, and uncertain and generally takes from one to three years or even longer. We cannot be sure that 510(k) clearance or PMA approval will ever be obtained for any product we propose to market.
The FDA decides whether a device must undergo either the 510(k) clearance or PMA approval process based upon statutory criteria. These criteria include the level of risk that the agency perceives is associated with the device and a determination whether the product is a type of device that is similar to devices that are already legally marketed. Devices deemed to pose relatively less risk are placed in either class I or II, which requires the manufacturer to submit a pre-market notification requesting 510(k) clearance, unless an exemption applies. The pre-market notification must demonstrate that the proposed device is "substantially equivalent" in intended use and in safety and effectiveness to a legally marketed "predicate device" that is either in class I, class II, or is a "pre-amendment" class III device (i.e., one that was in commercial distribution before May 28, 1976) for which the FDA has not yet decided to require PMA approval.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with a manufacturer's decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to submit a pre-market notification requiring 510(k) clearance. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance is obtained. We have modified some of our 510(k) cleared devices but have determined that, in our view, new 510(k) clearances are not required. We cannot be certain that the FDA would agree with any of our decisions not to seek 510(k) clearance. If the FDA requires us to seek 510(k) clearance for any modification, we also may be required to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance.
Devices deemed by the FDA to pose the greatest risk such as life-sustaining, life-supporting or implantable devices, or deemed not substantially equivalent to a legally marketed predicate device, are placed in class III. Such devices are required to undergo the PMA approval process in which the manufacturer must prove the safety and effectiveness of the device to the FDA's satisfaction. A PMA application must provide extensive pre-clinical and clinical trial data and also information about the device and its components regarding, among other things, manufacturing, labeling, and promotion. After approval of a PMA, a new PMA or PMA supplement is required in the event of a modification to the device, it's labeling, or its manufacturing process.
A clinical trial may be required in support of a 510(k) submission or PMA application. Such trials generally require an Investigational Device Exemption ("IDE") application approved in advance by the FDA for a limited number of patients, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results. Clinical trials may begin once the IDE application is approved by the FDA and the appropriate institutional review boards are at the clinical trial sites.
To date, the FDA has deemed our products to be class II devices eligible for the 510(k) clearance process. We believe that most of our products in development will receive similar treatment. However, we cannot be certain that the FDA will not deem one or more of our future products to be a class III device and impose the more burdensome PMA approval process.
16
Table of Contents
Pervasive and Continuing FDA Regulation. A host of regulatory requirements apply to marketed devices such as our laser and light-based products, including labeling regulations, the Quality System Regulation (which requires manufacturers to follow design, testing, control, documentation, and other quality assurance procedures), the Medical Device Reporting regulation (which requires that manufacturers report to the FDA certain types of adverse events involving their products), and the FDA's general prohibition against promoting products for unapproved or "off label" uses. Our Class II devices can have special controls such as performance standards, post-market surveillance, patient registries, and FDA guidelines that do not apply to class I devices. Unanticipated changes in existing regulatory requirements or adoption of new requirements could hurt our business, financial condition, and results of operations.
We are subject to inspection and market surveillance by the FDA for compliance with regulatory requirements. If the FDA finds that we have failed to comply with applicable requirements, the agency can institute a wide variety of enforcement actions. The FDA sometimes issues a public warning letter but also may pursue more drastic remedies, such as refusing our requests for 510(k) clearance or PMA approval of new products, withdrawing product approvals already granted to us, requiring us to recall products, or asking a court to require us to pay civil penalties or criminal fines, adhere to operating restrictions, or close down our operations. Ultimately, criminal prosecution is available to the FDA as punishment for egregious offenses. Any FDA enforcement action against us could hurt our business, financial condition, and results of operation.
Other United States Regulation. We also must comply with numerous federal, state, and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and hazardous substance disposal. We cannot be sure that we will not be required to incur significant costs to comply with such laws and regulations in the future or that such laws or regulations will not hurt our business, financial condition, and results of operations.
Foreign Regulation. International sales are subject to foreign government regulation, the requirements of which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. Companies are now required to obtain the CE Mark on a product prior to sale of those products within the European Union. During this process, the sponsor must demonstrate conformance to quality system requirements; i.e., ISO13485:2003.
Candela and its products may also be subject to other federal, state, local, or foreign regulations relating to health and safety, environmental matters, quality assurance, and the like. To date, Candela's compliance with laws that regulate the discharge of materials into the environment or otherwise relate to the protection of the environment has not had a material effect on its ongoing operations. Candela has not made any material expenditure for environmental control facilities. However, we cannot be certain that we will not be required to incur significant costs to comply with such laws and regulations in the future.
Product Liability and Warranties
Our products are generally covered by a standard warranty, with an option to purchase extended warranty contracts at the time of product sale or service contracts after the time of sale. We maintain a reserve based on anticipated standard warranty claims. We believe such reserves to be adequate, but in the event of a major product problem or recall, such reserves may be inadequate to cover all costs, and such an event could have a material adverse effect on our business, financial condition, and results of operations.
Our business involves the inherent risk of product liability claims. We believe that we maintain appropriate product liability insurance with respect to our products. We cannot be certain that with respect to our current or future products, such insurance coverage will continue to be available on
17
Table of Contents
terms acceptable to us or that such coverage will be adequate for liabilities that may actually be incurred.
Applied Optronics
In 2003, Candela acquired substantially all of the assets of Applied Optronics, the diode division of Schwartz Electro-Optics, Inc. Applied Optronics was a leading manufacturer of high-powered, pulsed and CW diode lasers, and was a component supplier to the OEM market that serves a variety of industries including the military, medical, industrial, research and robotics fields. Applied Optronics was the lead supplier of the diodes for our Smoothbeam® diode laser system. The Applied Optronics division of Candela, located in South Plainfield, New Jersey, generates revenue from diode sales to third-party customers.
Inolase
�� On March 6, 2007, we acquired Inolase (2002) Ltd. ("Inolase") a non-public company engaged in the development and manufacture of proprietary pneumatic skin flattening PSF devices for the aesthetic light-based treatment industry as well as the development of intellectual property related to medical devices and light sources.
In the fiscal quarter ended December 27, 2008, in connection with our plan to focus on our core products, we initiated a plan to close the Inolase subsidiary. Accordingly, we classified the operating results of this business as discontinued operations in the statements of operations.
The Company determined that there was no future cash value inherent to its Inolase subsidiary and, accordingly, shut down this reporting unit as its fair value was zero. As part of that process the Company recognized a 100% impairment loss associated with the goodwill, other intangible assets, inventory, and fixed assets. The Company also recorded restructuring charges of approximately $0.4 million at December 27, 2008 associated with future occupancy costs, minimum termination benefits, and professional service fees. See Note 15 of our Consolidated Financial Statements for additional information about discontinued operations.
Employees
As of June 27, 2009, we employed 363 employees, 233 in the United States and 130 internationally. None of our employees are subject to collective bargaining agreements.
Available Information
Access to our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to these reports filed with or furnished to the Securities and Exchange Commission may be obtained through the Investor Relations section of our website at www.candelalaser.com/ir_corp.asp as soon as reasonably practical after we electronically file or furnish these reports. We do not charge for access to and viewing of these reports. Information on our Investor Relations page and on our website is not part of this Annual Report on Form 10-K or any of our other securities filings unless specifically incorporated herein by reference. In addition, our filings with the Securities and Exchange Commission may be accessed through the Securities and Exchange Commission's Electronic Data Gathering, Analysis and Retrieval system at www.sec.gov. All statements made in any of our securities filings, including all forward-looking statements or information, are made as of the date of the document in which the statement is included, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
18
Table of Contents
Proposed Merger with Syneron
On September 8, 2009 we entered into an Agreement and Plan of Merger with Syneron Medical under which our business would be combined with that of Syneron through a merger of Candela and a newly formed, wholly owned subsidiary of Syneron.
The completion of the merger is subject to various closing conditions, including adoption and approval of the merger agreement by Candela's stockholders and receipt of certain regulatory and antitrust approvals. The merger is intended to qualify as a reorganization for federal income tax purposes.
At the effective time of the Merger, by virtue of the merger and without any action on the part of any stockholder, each share of our common stock then issued and outstanding will be converted into the right to receive 0.2911 shares of common stock of Syneron.
Mr. Louis P. Scafuri, currently chief executive officer of Syneron, will remain chief executive officer of the combined company and Mr. Gerard E. Puorro, currently chief executive officer of Candela, will join Syneron's Board of Directors.
The merger agreement contains certain termination rights both for us and for Syneron. If the merger agreement is terminated under circumstances specified in the merger agreement due to action by us, we will be required to pay Syneron a termination fee of $2.6 million.
Our board of directors and the board of directors of Syneron have each approved the merger.
The merger agreement contains representations and warranties which the parties thereto made to, and solely for, the benefit of each other. The assertions embodied in those representations and warranties are qualified by information in confidential disclosure schedules that the parties have exchanged in connection with signing the merger agreement and that modify, qualify and create exceptions to the representations and warranties contained in the merger agreement. We and Syneron each have made covenants in the merger agreement about continuing our respective businesses in the ordinary course.
Satisfaction of the closing conditions could take several months or longer. There can be no assurance that the conditions to completion of the merger will be met, or that the merger will be completed. If the merger is not consummated on or before June 1, 2010, either party may terminate the merger agreement.
Forward-looking and Cautionary Statements
Certain statements contained in this Form 10-K may constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 ("Reform Act"). We may also make forward-looking statements in other reports filed with the Securities and Exchange Commission, in materials delivered to stockholders and in press releases. In addition, our representatives may from time to time make oral forward-looking statements. Forward-looking statements provide current expectations of future events based on certain assumptions and include any statement that does not directly relate to any historical or current fact. Words such as "anticipates," "believes," "expects," "estimates," "intends," "plans," "projects," and similar expressions, may identify such forward-looking statements. Such forward looking statements include but are not limited to: that we have the necessary infrastructure in place to capitalize on expansion; the affordability of our products will allow for expansion; that we can lower production costs; or that the market will expand beyond baby boomers. Candela assumes no obligation to update or revise any forward-looking statements. In accordance with the Reform Act, set forth under Item 1A "Risk Factors", are cautionary statements that accompany those forward-looking statements. Readers should carefully review such cautionary statements as they identify certain important factors that could cause actual results to differ materially from those in the forward-looking statements and from historical trends. Those cautionary
19
Table of Contents
statements are not exclusive and are in addition to other factors discussed elsewhere in this Annual Report, in our filings with the Securities and Exchange Commission, or in materials incorporated therein by reference.
Candela GentleLASE, Vbeam, GentleYAG and Smoothbeam are registered trademarks and GentleMax, AlexTriVantage, SmoothPeel, Dynamic Cooling Device, DCD and the Candela flame are trademarks of Candela Corporation. Serenity, Pneumatic Skin Flattening, and, PSF are also trademarks of Candela Corporation.
Item 1A. Risk Factors.
The following important factors, among others, could cause our actual operating results to differ materially from those indicated or suggested by forward-looking statements made in this Form 10-K or presented elsewhere by management from time to time.
The ability to complete the merger is subject to the receipt of consents and approvals from government entities, which may impose conditions that could have an adverse effect on us or could cause either party to abandon the merger.
In deciding whether to grant regulatory or antitrust approvals, the relevant governmental entities will consider the effect of the merger on competition within their relevant jurisdictions. The terms and conditions of the approvals that are granted may impose requirements, limitations or costs or place restrictions on the conduct of the combined company's business.
The merger agreement may require us to accept significant conditions from regulatory bodies before either of us may refuse to close the merger on the basis of those regulatory conditions. We cannot provide any assurance that either party will obtain the necessary approvals or that any other conditions, terms, obligations or restrictions imposed by these regulatory bodies will not have a material adverse effect on the combined company following the merger. In addition, we can provide no assurance that these conditions, terms, obligations or restrictions will not result in the delay or abandonment of the merger.
Any delay in completion of the merger may significantly reduce the benefits expected to be obtained from the merger.
In addition to the required regulatory clearances and approvals, the merger is subject to a number of other conditions beyond our control that may prevent, delay or otherwise materially adversely affect its completion. We cannot predict whether and when these other conditions will be satisfied. Further, the requirements for obtaining the required clearances and approvals could delay the completion of the merger for a significant period of time or prevent it from occurring. Any delay in completing the merger may significantly reduce the synergies and other benefits that we expect to achieve if we successfully complete the merger within the expected timeframe and integrate our and Syneron's businesses.
The anticipated benefits of the merger may not be realized fully or at all or may take longer to realize than expected.
The merger involves the integration of two companies that have previously operated independently with principal offices in two distinct locations. Due to legal restrictions, we and Syneron have conducted only limited planning regarding the integration of the two companies. The combined company will be required to devote significant management attention and resources to integrating the two companies. Delays in this process could adversely affect the combined company's business, financial results, financial condition and stock price. Even if we were able to integrate our business operations successfully, there can be no assurance that this integration will result in the realization of the full
20
Table of Contents
benefits of synergies, cost savings, innovation and operational efficiencies that may be possible from this integration or that these benefits will be achieved within a reasonable period of time.
Additionally, as a condition to their approval of the merger, regulatory agencies may impose requirements, limitations or costs or require divestitures or place restrictions on the conduct of the combined company's business. If we and Syneron agree to these requirements, limitations, costs, divestitures or restrictions, the ability to realize the anticipated benefits of the merger may be impaired.
Failure to complete the merger for regulatory or other reasons could adversely affect our stock price and our future business and financial results.
Completion of the merger is conditioned upon, among other things, the receipt of certain regulatory and antitrust approvals and the approval of our stockholders. There is no assurance that we will receive the necessary approvals or satisfy the other conditions to the completion of the merger. Failure to complete the proposed merger would prevent us from realizing the anticipated benefits of the merger. We also will remain liable for significant transaction costs, including legal, accounting and financial advisory fees, regardless of whether the merger is completed. In addition, the market price of our common stock may reflect various market assumptions as to whether the merger will occur. Consequently, the failure to complete the merger could result in a significant change in the market price of our common stock.
Unfavorable results in our intellectual property litigation with Palomar Medical Technologies may result in significant decline to our stock price or have a material affect on results of operations or on cash flows.
On August 9, 2006, one of our competitors, Palomar Medical Technologies ("Palomar"), alleged that the manufacture, use and sale of our products for laser hair removal infringe a certain United States patent. Public announcements concerning this litigation that are unfavorable to us may result in significant declines in our stock price. An adverse ruling or judgment in this matter, including the possible assessment of material historical infringement damages, could cause our stock price to decline significantly.
Litigation with Palomar will be expensive and protracted, and our intellectual property position may be weakened as a result of an adverse ruling or judgment. Whether or not we are successful in the pending lawsuits, litigation consumes substantial amounts of our financial resources and diverts management's attention away from our core business. See Item 3—"Legal Proceedings."
We depend on sales from outside the United States that could be adversely affected by changes in international markets.
We sell more than half of our products and services outside the United States. International sales accounted for approximately 67% of our revenue for fiscal year 2009 and we expect that they will continue to be significant. Accordingly, a major part of our revenues and operating results could be adversely affected by risks associated with international commerce. Significant fluctuations in the exchange rates between the United States dollar and foreign currencies could cause us to lower our prices and thus reduce our profitability, or could cause prospective customers to push out orders to later dates because of the increased relative cost of our products in the aftermath of a currency devaluation or currency fluctuation. Other risks associated with international business include:
- •
- longer payment cycles common in foreign markets;
- •
- changes in tax and other laws;
- •
- regulatory practices and tariffs; and
- •
- difficulties in staffing and managing our foreign operations.
21
Table of Contents
The failure to obtain Alexandrite rods for the GentleLASE® and the AlexTriVantage™ systems from our sole supplier would impair our ability to manufacture and sell these systems.
We use Alexandrite rods to manufacture the GentleLASE® and the AlexTriVantage™ systems, which accounts for a significant portion of our total revenues. We depend exclusively on our contract manufacturer to supply these rods, for which no alternative supplier meeting our quality standards exists. We cannot be certain that our contract manufacturer will be able to meet our future requirements at current prices or at all. To date, we have been able to obtain adequate supplies of Alexandrite rods in a timely manner, but any extended interruption in our supplies could hurt our results.
Disappointing quarterly revenue or operating results could cause the price of our common stock to fall.
Our quarterly revenue and operating results are difficult to predict and may swing sharply from quarter to quarter. Historically, our first fiscal quarter has typically had the least amount of revenue in any quarter of our fiscal year. The results of the first quarter are directly impacted by the seasonality of the purchasing cycle.
If our quarterly revenue or operating results fall below the expectations of investors or public market analysts, the price of our common stock could fall substantially. Our quarterly revenue is difficult to forecast for many reasons, some of which are outside of our control, including the following:
Market supply and demand
- •
- potential increases in the level and intensity of price competition between our competitors and us;
- •
- potential decrease in demand for our products; and
- •
- possible delays in market acceptance of our new products.
Customer behavior
- •
- changes in or extensions of our customers' budgeting and purchasing cycles; and
- •
- changes in the timing of product sales in anticipation of new product introductions or enhancements by us or our competitors.
Company operations
- •
- absence of significant product backlogs;
- •
- our effectiveness in our manufacturing process;
- •
- unsatisfactory performance of our distribution channels, service providers, or customer support organizations; and
- •
- timing of any acquisitions and related costs.
Our failure to respond to rapid changes in technology and intense competition in the laser industry could make our lasers obsolete.
The aesthetic and cosmetic laser equipment industry is subject to rapid and substantial technological development and product innovations. To be successful, we must be responsive to new developments in laser technology and new applications of existing technology. Our financial condition and operating results could be hurt if our products fail to compete favorably in response to such technological developments, or we are not agile in responding to competitors' new product introductions or product price reductions. In addition, we compete against numerous companies
22
Table of Contents
offering products similar to ours, some of which have greater financial, marketing, and technical resources than we do. We cannot be sure that we will be able to compete successfully with these companies and our failure to do so could hurt our business, financial condition, and results of operations.
Like other companies in our industry, we are subject to a regulatory review process and our failure to receive necessary government clearances or approvals could affect our ability to sell our products and remain competitive.
The types of medical devices that we seek to market in the United States generally must receive either "510(k) clearance" or "PMA approval" in advance from the United States Food and Drug Administration ("FDA") pursuant to the Federal Food, Drug, and Cosmetic Act. The FDA's 510(k) clearance process usually takes from three to twelve months, but it can last longer. The process of obtaining PMA approval is much more costly and uncertain and generally takes from one to three years or even longer. To date, the FDA has deemed our products eligible for the 510(k) clearance process. We believe that most of our products in development will receive similar treatment. However, we cannot be sure that the FDA will not impose the more burdensome PMA approval process upon one or more of our future products, nor can we be sure that 510(k) clearance or PMA approval will ever be obtained for any product we propose to market.
Many foreign countries in which we market or may market our products have regulatory bodies and restrictions similar to those of the FDA. We cannot be certain that we will be able to obtain (or continue to obtain) any such government approvals or successfully comply with any such foreign regulations in a timely and cost-effective manner, if at all, and our failure to do so could adversely affect our ability to sell our products.
We have modified some of our products without FDA clearance. The FDA could retroactively decide the modifications were improper and require us to cease marketing and/or recall the modified products.
Any modification to one of our 510(k) cleared devices that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance. The FDA requires every manufacturer to make this determination in the first instance, but the FDA can review any such decision. We have modified some of our marketed devices, but we believe that new 510(k) clearances are not required. We cannot be certain that the FDA would agree with any of our decisions not to seek 510(k) clearance. If the FDA requires us to seek 510(k) clearance for any modification, we also may be required to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance.
Achieving complete compliance with FDA regulations is difficult, and if we fail to comply, we could be subject to FDA enforcement action.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. The FDA's regulatory scheme is complex, especially the Quality System Regulation ("QSR"), which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures. This complexity makes complete compliance difficult to achieve. Also, the determination as to whether a QSR violation has occurred is often subjective. If the FDA finds that we have failed to comply with the QSR or other applicable requirements, the agency can institute a wide variety of enforcement actions, including a public warning letter or other stronger remedies, such as:
- •
- fines, injunctions and civil penalties against us;
- •
- recall or seizure of our products;
- •
- operating restrictions, partial suspension or total shutdown of our production;
23
Table of Contents
- •
- refusing our requests for 510(k) clearance or PMA approval of new products;
- •
- withdrawing product approvals already granted; and
- •
- criminal prosecution.
We may also be subject to state regulations. State regulations, and changes to state regulations, may prevent sales to particular end users or may restrict use of the products to particular end users or under particular supervision which may decrease revenues or prevent growth of revenues.
Our products may also be subject to state regulations. Federal regulation allows our products to be sold to and used by licensed practitioners as determined on a state-by-state basis, which complicates monitoring compliance. As a result, in some states non-physicians may purchase and operate our products. In most states, it is within a physician's discretion to determine whom they can supervise in the operation of our products and the level of supervision. However, some states have specific regulations as to appropriate supervision and who may be supervised. A state could disagree with our decision to sell to a particular type of end user or change regulations to prevent sales to particular types of end users or change regulations as to supervision requirements. In several states applicable regulations are in flux. Thus, state regulations and changes to state regulations may decrease revenues or prevent growth of revenues.
Claims by others that our products infringe their patents or other intellectual property rights could prevent us from manufacturing and selling some of our products or require us to incur substantial costs from litigation or development of non-infringing technology.
Our industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Patent applications are maintained in secrecy in the United States until such patents are issued and are maintained in secrecy for a period of time outside the United States. Accordingly, we can conduct only limited searches to determine whether our technology infringes any patents or patent applications of others. Any claims of patent infringement would be time-consuming and could:
- •
- result in costly litigation;
- •
- divert our technical and management personnel;
- •
- cause product shipment delays;
- •
- require us to develop non-infringing technology; and
- •
- require us to enter into royalty or licensing agreements.
Although patent and intellectual property disputes in the laser industry have often been settled through licensing or similar arrangements, costs associated with such arrangements may be substantial and often require the payment of ongoing royalties, which could hurt our gross margins. In addition, we cannot be sure that the necessary licenses would be available to us on satisfactory terms, or that we could redesign our products or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from manufacturing and selling some of our products, which could hurt our business, results of operations, and financial condition. On the other hand, we may have to start costly and time consuming litigation to enforce our patents, to protect trade secrets and know-how owned by us or to determine the enforceability, scope, and validity of the proprietary rights of others.
We could incur substantial costs as a result of product liability claims.
There are various risks of physical injury to the patient when using our lasers for aesthetic and cosmetic treatments. Injuries often result in product liability or other claims being brought against the practitioner utilizing the device and us. The costs and management time we would have to spend in
24
Table of Contents
defending or settling any such claims, or the payment of any award in connection with such claims, could hurt our business, results of operations, and financial condition. Although we maintain product liability insurance, we cannot be certain that our policy will provide sufficient coverage for any claim or claims that may arise, or that we will be able to maintain such insurance coverage on favorable economic terms.
We may be unable to attract and retain management and other personnel we need to succeed.
The loss of any of our senior management or other key research, development, sales, and marketing personnel, particularly if lost to competitors, could hurt our future operating results. Our future success will depend in large part upon our ability to attract, retain, and motivate highly skilled employees. We cannot be certain that we will attract, retain, and motivate sufficient numbers of such personnel.
Our failure to manage future acquisitions and joint ventures effectively may divert management attention from our core business and cause us to incur additional debt, liabilities or costs.
We may acquire businesses, products, and technologies that complement or expand our business. We may also consider joint ventures and other collaborative projects. We may not be able to:
- •
- identify appropriate acquisition or joint venture candidates;
- •
- successfully negotiate, finance, or integrate any businesses, products, or technologies that we acquire; and
- •
- successfully manage any joint ventures or collaborations.
Furthermore, the integration of any acquisition or joint venture may divert management time and resources. If we fail to manage these acquisitions or joint ventures effectively, we may incur debts or other liabilities or costs that could harm our operating results or financial condition. While we from time to time evaluate potential acquisitions of businesses, products, and technologies, consider joint ventures and other collaborative projects, and anticipate continuing to make these evaluations and considerations, we have no present understandings, commitments, or agreements with respect to any acquisitions or joint ventures.
We face risks associated with product warranties.
We could incur substantial costs as a result of product failures for which we are responsible under warranty obligations.
The expense and potential unavailability of insurance coverage for our customers could adversely affect our ability to sell our products and negatively impact our financial condition.
Some of our customers and prospective customers have had difficulty in procuring or maintaining liability insurance to cover their operation and use of our products. Medical malpractice carriers are withdrawing coverage in certain states or substantially increasing premiums. If this trend continues or worsens, our customers may discontinue using our products and, industry-wide, potential customers may opt against purchasing laser and other light-based products due to the cost of or inability to procure insurance coverage.
25
Table of Contents
Failure to maintain effective internal control over financial reporting could have a material adverse effect on our business, operating results and stock price.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to include a report by our management on our internal control over financial reporting. Such report must contain an assessment by management of the effectiveness of our internal control over financial reporting as of the end of our fiscal year and a statement as to whether or not such internal control is effective.
Effective internal controls are necessary for us to provide reasonable assurance with respect to our financial reports and to effectively prevent fraud. If we cannot provide reasonable assurance with respect to our financial reports and effectively prevent fraud, our brand and operating results could be harmed. Internal control over financial reporting may not prevent or detect misstatements because of its inherent limitations, including the possibility of human error, the circumvention or overriding of controls, or fraud. Therefore, even effective internal controls cannot provide absolute assurance with respect to the preparation and fair presentation of financial statements. In addition, projections of any evaluation of effectiveness of internal control over financial reporting to future periods are subject to the risk that the control may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. If we fail to maintain the adequacy of our internal controls, including any failure to implement required new or improved controls, or if we experience difficulties in their implementation, our business and operating results could be harmed, and we could fail to meet our reporting obligations, which could have a material adverse effect on our business.
Our efforts to comply with Section 404 have resulted in, and are likely to continue to result in, significant costs, the commitment of time and operational resources and the diversion of management's attention.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
We lease two facilities totaling approximately 38,000 square feet for our operations in Wayland, Massachusetts, which is located approximately 20 miles west of Boston. The leases on these facilities expire December 2011. We also lease a 12,000 square foot facility in South Plainfield, New Jersey for our diode operation. This lease expires in October 2011. Our management believes that the current facilities are suitable and adequate for our near-term needs.
Our subsidiaries currently lease the following facilities:
- •
- Candela KK.—We lease 4 offices in Japan in Tokyo, Osaka, Nagoya, and Fukuoka. These leases expire between November 14, 2009 and September 16, 2012.
- •
- Candela Iberica, S.A. We lease two locations in Lisbon, Portugal and Madrid, Spain. These leases expire on December 12, 2010 and December 31, 2011, respectively.
- •
- Candela Deutschland GmbH. The lease on this facility in Neu-Isenberg, Germany expires on October 14, 2011.
- •
- Candela France SARL. The lease on this facility in Villebou Sur Yvette, France expires on June 30, 2016.
- •
- Candela Italia. The lease on this facility in Formello, Italy expires on November 3, 2011.
- •
- Candela (U.K.) Limited. The lease on this facility in Cwmbran, U.K. expires on September 18, 2012.
26
Table of Contents
- •
- Candela Corporation Australia PTY, Ltd. The lease on the facility in Heidelberg West, Australia expires on March 4, 2010.
- •
- Inolase. The lease on this facility in Netanya, Israel expires on April 30, 2010. As described in Note 15 of "Notes to Consolidated Financial Statements" lease commitments on unused facilities in excess of expected sublease income have been included in our restructuring provision.
Item 3. Legal Proceedings.
The Company is currently involved in two litigation matters with Palomar Medical Technologies, Inc. ("Palomar").
- •
- Palomar asserts that it is the exclusive licensee from The General Hospital Corporation ("Mass. General") of U.S. Patent No. 5,735,844 (the "'844 Patent") and U.S. Patent No. 5,595,568, which is related to the '844 Patent (the "'568 Patent"). On August 9, 2006, Palomar filed suit against the Company in the United States District Court for the District of Massachusetts, asserting willful infringement by the Company of the '844 Patent. Palomar seeks compensatory and treble damages, as well as attorneys' fees and injunctive relief. In November 2006, the Company answered the complaint by denying Palomar's allegations and asserting a variety of affirmative defenses and counterclaims against Palomar. This response included a counterclaim by the Company against Palomar seeking a declaratory judgment that the '568 Patent is either invalid, or products manufactured by the Company do not infringe the '568 Patent, or both. In November 2006, Palomar filed an answer denying the Company's counterclaim. In February 2007, Palomar moved to amend its Complaint to add The General Hospital Corporation as a party, to add a claim for infringement by Candela of the '568 Patent, and to allege that additional Candela products infringe the '844 Patent. Palomar's motion to amend its Complaint was granted in August 2007. In February 2007, Candela moved to amend its Answer and Counterclaims to add allegations of inequitable conduct, double-patenting and violation of Mass. Gen. Laws Ch. 93A by Palomar. The Company's motion to amend its Answer and Counterclaims was granted in March 2007. Palomar filed a general denial response to Candela's Amended Answer and Counterclaim in March 2007. In August 2007, the parties had a Markman hearing before the U.S. District Court Judge presiding over the above-described legal proceeding. In a Markman hearing, the Court interprets the definitions of the disputed claim terms of a patent. On October 31, 2008, the Company requested a stay of the case with the Court, which was granted on November 17, 2008. The Company did so based upon a third party's re-examination request that was granted by the United States Patent and Trademark Office on August 29, 2008. The re-examination is with respect to both the '568 and '844 Patents, including many of the Claims at issue in this case. In November 2008, the Court stayed the case until the United States Patent and Trademark Office rules on the re-examination. On December 9, 2008, Candela filed requests for re-examination of the '568 and '844 Patents with the United States Patent & Trademark Office asserting that all claims currently in dispute with Palomar be held invalid. On January 7, 2009, the United States Patent and Trademark Office granted the Company's re-examination request of the '844 Patent and the '568 Patent. The United States Patent and Trademark Office stated that there are substantial new questions of patentability for all claims asserted against the Company by Palomar. On June 5, 2009, the PTO issued an office action confirming as patentable the majority of the claims of the '844 patent in re-examination as patentable. On June 19, 2009, Palomar moved to lift the stay based on responses by the PTO in the '844 re-examination proceeding, which the Company opposed on July 13, 2009. In July 2009, the PTO issued a notice of intent to issue a re-examination certificate for the '844 patent. In August 2009, the PTO issued a notice of intent to issue a
27
Table of Contents
On August 10, 2006, the Company filed suit against Palomar in the United States District Court for the District of Massachusetts, asserting willful infringement by Palomar of U.S. Patent Nos. 6,659,999, 5,312,395 (the "395 Patent"), and 6,743,222 (the "'222 Patent"). The Company seeks compensatory and treble damages for the patent infringement, as well as attorneys' fees and an injunction against Palomar to prevent Palomar's continuing infringement. The Company served its complaint on Palomar in August 2006. In October 2006, the Company amended the Company's suit against Palomar by removing from the suit allegations that Palomar infringes Patent 6,659,999. In October 2006, Palomar answered the Company's amended complaint by denying the Company's allegations and asserting an affirmative defense of inequitable conduct with respect to the '395 Patent. In addition, Palomar filed a demand for a declaratory judgment seeking a judicial determination that Palomar products either do not infringe the '395 Patent and '222 Patent or that such patents are invalid. In November 2006, the Company answered the counterclaim by denying Palomar's allegations. In February 2008, Palomar filed a request for re-examination of the Company's '222 Patent with the United States Patent and Trademark Office ("PTO"). In March 2008, the PTO granted re-examination. In June 2008, the Court stayed the case until the PTO rules on the re-examination. To avoid the necessity of repeated status conferences, the Company requested administrative closure of the case on November 26, 2008, subject to reopening when the '222 Patent emerged from re-examination. The Court granted this request on December 1, 2008. While the Company intends to vigorously contest Palomar's allegations, and to pursue its own claims against Palomar, each lawsuit is inherently uncertain and unpredictable as to its ultimate outcome. An adverse outcome in Palomar's suit against the Company would materially hurt the Company's business, financial condition, results of operations and cash flows.
- •
- On September 15, and 24, 2009, after the announcement of the Company's proposed merger with Syneron, two putative class action lawsuits were filed in the Superior Courts of Massachusetts against Candela and the members of Candela's Board of Directors, among others. The plaintiffs in the two actions purport to sue on behalf of a class of Candela stockholders, and allege that members of the board breached fiduciary duties owed to Candela stockholders through, among other things, the failure to maximize shareholder value and the use of unfair process in connection with the proposed merger between Candela and Syneron, and that Candela, with others, aided and abetted the purported breaches of fiduciary duties. The complaints seek, among other relief: rescission of the merger agreement entered into on September 8, 2009; an injunction against the merger; an order requiring disclosure of all material information necessary for shareholders to make an informed vote on the proposed merger; and an accounting for damages caused by defendants as well as an award of all costs and disbursements of the actions, including reasonable attorneys' and expenses. Candela denies the allegations in the complaints and intends to defend these lawsuits vigorously.
On February 19, 2008, Cardiofocus, Inc. ("Cardiofocus") filed suit against the Company and eight other companies in the United States District Court for the District of Massachusetts, asserting willful infringement by the Company of U.S. Patents 6,457, 780, 6,159,203 and 5,843,073. Cardiofocus seeks compensatory and treble damages, as well as attorneys' fees and injunctive relief. On April 21, 2008, the Company answered Cardiofocus' complaint and asserted a variety of counterclaims against Cardiofocus. The Company intends to vigorously defend against the lawsuit. Based upon Candela's request, on July 23, 2008, the United States Patent & Trademark Office ("PTO") agreed to re-examine the validity of the Cardiofocus patents. On October 14, 2008, the Court agreed to stay this matter for a minimum of one year, up to a maximum of two years, but no later than the PTO's decision with respect to the re-examination proceedings.
28
Table of Contents
While we believe that the likelihood of loss in each of the disclosed proceedings is reasonably possible, the possibility of loss or range of loss for each matter is not estimable, except with regard to Palomar. In accordance with SFAS 5,Accounting for Contingencies, as of October 1, 2009, we estimate the range of the loss contingency associated with the Palomar litigation claims to be in the range of $0 to $46 million. As we have assessed that contingent losses related to these proceedings are only reasonably possible, pursuant to SFAS 5, an accrual has not been recorded for any of these loss contingencies.
From time to time, the Company is party to various legal proceedings incidental to its business. The Company believes that none of the legal proceedings, other than the lawsuit initiated by Palomar, that are presently pending, if adversely decided against us, will have a material adverse effect upon the Company's financial position, results of operations, or liquidity. While we believe that the likelihood of loss in each of the disclosed proceedings is reasonably possible, the possibility of loss or range of loss for each matter is not estimable.
Item 4. Submission of Matters to a Vote of Security Holders.
Not applicable.
PART II
Item 5. Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Candela's common stock trades on The NASDAQ Global Select Market under the symbol "CLZR."
At September 28, 2009, there were approximately 308 holders of record of our common stock and the closing sale price of our common stock was $3.28.
Market Price of Common Stock
The following table sets forth quarterly high and low sales prices of the common stock for the indicated fiscal periods:
| | | | | | | | |
| | High | | Low | |
|---|
Fiscal 2008 | | | | | | | |
| | First Quarter | | $ | 11.65 | | $ | 7.14 | |
| | Second Quarter | | | 8.76 | | | 5.44 | |
| | Third Quarter | | | 5.58 | | | 3.01 | |
| | Fourth Quarter | | | 3.40 | | | 2.12 | |
Fiscal 2009 | | | | | | | |
| | First Quarter | | $ | 3.17 | | $ | 2.20 | |
| | Second Quarter | | | 2.49 | | | 0.35 | |
| | Third Quarter | | | 0.68 | | | 0.29 | |
| | Fourth Quarter | | | 1.28 | | | 0.39 | |
Repurchase of our Equity Securities
We did not repurchase any of our common stock during the three-month period ended June 27, 2009.
Dividend Policy
We have never paid a cash dividend and have no present intention to pay cash dividends in the foreseeable future. We intend to retain any future earnings for use in our business.
29
Table of Contents
Stock Performance Graphs
The following performance graph shall not be deemed "filed" for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any filing of Candela under the Securities Act or the Exchange Act.
The following graph compares the cumulative 5-year total return to shareholders on Candela Corporation's common stock relative to the cumulative total returns of the S&P 500 index, and SIC Code 3845 index of sixty-nine companies listed in footnote (a) below. The graph assumes that the value of the investment in the company's common stock, in each index, and in the peer group (including reinvestment of dividends) was $100 on 7/3/2004 and tracks it through 6/27/2009.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Candela Corporation, The S&P 500 Index
And SIC Code 3845
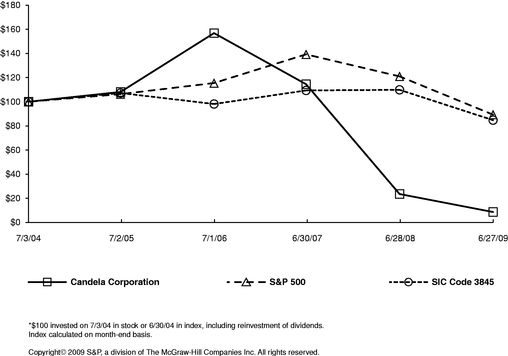
| | | | | | | | | | | | | | | | | | | |
| | 7/3/04 | | 7/2/05 | | 7/1/06 | | 6/30/07 | | 6/28/08 | | 6/27/09 | |
|---|
Candela Corporation | | | 100.00 | | | 108.11 | | | 156.87 | | | 114.54 | | | 23.54 | | | 8.61 | |
S&P 500 | | | 100.00 | | | 106.32 | | | 115.50 | | | 139.28 | | | 121.01 | | | 89.29 | |
SIC Code 3845 | | | 100.00 | | | 107.24 | | | 98.13 | | | 109.37 | | | 109.88 | | | 84.74 | |
- (a)
- The sixty-nine companies included are: 4-D Neuroimaging, Abiomed Inc, Arrhythmia Research Technology, Aspect Medical Systems Inc, Biofield Corp., BSD Medical Corp., Cambridge Heart Inc, Candela Corp., Cardiodynamics International Corp, Cardiogenesis Corp., Celsion Corp., China Yibai United Guarantee, Clarient Inc, Covidien PLC, Curon Medical Inc, Cutera Inc, Cyberonics Inc, Cybex International Inc, Cynosure Inc, Dexcom Inc, Digirad Corp., Dynatronics Corp., Edwards Lifesciences Corp., Embryo Development Corp., Encision Inc, Enteromedics Inc, Fischer Imaging Corp., Fonar Corp., Healthtronics Inc, Heart Tronics Inc, Hypertension Diagnostics Inc, Imaging Diagnostic Systems Inc, Ingen Technologies Inc, International Isotopes Inc, Iris International Inc, Ivivi Technologies Inc, Lectec Corp., Longport Inc, Magna-LAB Inc, Masimo Corp., Medtronic Inc, Millipore Corp., Misonix Inc, Nano Global Inc, Natus Medical Inc, Noble Medical Technologies Inc, Non Invasive Monitoring Systems Inc, Nxstage Medical Inc, Ophthalmic Imaging Systems Inco, Orthometrix Inc, Palomar Medical Technologies Inc, Paradigm Medical Industries Inc, Performance Health Technologies Inc, PLC Systems Inc, Positron Corp., Precision Optics Corp. Inc, Sirona Dental Systems Inc, Solta Medical Inc, Somanetics Corp., St. Jude Medical Inc, Stereotaxis Inc, The Spectranetics Corp., Tomotherapy Inc, Varian Medical Systems Inc, Vasamed Inc, Viking Systems Inc, Vision-Sciences Inc, Zoll Medical Corp. and Zynex Inc.
30
Table of Contents
Item 6. Selected Financial Data.
The table set forth below contains certain consolidated financial data for each of the last five fiscal years of Candela. This data should be read in conjunction with the detailed information, financial statements and related notes, as well as Management's Discussion and Analysis of Financial Condition and Results of Operations included elsewhere herein.
| | | | | | | | | | | | | | | | | | |
| | For the Year Ended | |
|---|
(in thousands, except per share data)
Consolidated Statement of Operations Data: | | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | | July 1,
2006 | | July 2,
2005 | |
|---|
Revenue: | | | | | | | | | | | | | | | | |
Lasers and other products | | $ | 74,955 | | $ | 104,583 | | $ | 112,445 | | $ | 121,838 | | $ | 102,316 | |
Product-related service | | | 41,625 | | | 42,034 | | | 35,332 | | | 27,628 | | | 21,585 | |
| | | | | | | | | | | | |
Total revenue | | | 116,580 | | | 146,617 | | | 147,777 | | | 149,466 | | | 123,901 | |
| | | | | | | | | | | | |
Cost of sales: | | | | | | | | | | | | | | | | |
Lasers and other products | | | 40,740 | | | 49,545 | | | 49,188 | | | 54,748 | | | 45,235 | |
Product related service | | | 31,279 | | | 31,559 | | | 24,191 | | | 20,869 | | | 17,918 | |
Litigation-related charges | | | — | | | — | | | — | | | — | | | 4,829 | |
| | | | | | | | | | | | |
Total cost of sales | | | 72,019 | | | 81,104 | | | 73,379 | | | 75,617 | | | 67,982 | |
| | | | | | | | | | | | |
Gross profit: | | | 44,561 | | | 65,513 | | | 74,398 | | | 73,849 | | | 55,919 | |
| | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | |
Selling, general and administrative | | | 51,514 | | | 68,075 | | | 52,753 | | | 44,297 | | | 40,165 | |
Research and development | | | 10,555 | | | 10,104 | | | 17,489 | | | 8,879 | | | 6,890 | |
Litigation-related charges | | | — | | | — | | | — | | | — | | | 773 | |
| | | | | | | | | | | | |
Total operating expenses | | | 62,069 | | | 78,179 | | | 70,242 | | | 53,176 | | | 47,828 | |
| | | | | | | | | | | | |
(Loss) income from operations: | | | (17,508 | ) | | (12,666 | ) | | 4,156 | | | 20,673 | | | 8,091 | |
| | | | | | | | | | | | |
Other income (expense): | | | | | | | | | | | | | | | | |
Interest income | | | 511 | | | 1,628 | | | 2,719 | | | 1,748 | | | 640 | |
Other income (expense), net | | | (361 | ) | | (1,841 | ) | | 3,725 | | | (19 | ) | | (73 | ) |
| | | | | | | | | | | | |
Total other income (expense) | | | 150 | | | (213 | ) | | 6,444 | | | 1,729 | | | 567 | |
| | | | | | | | | | | | |
(Loss) income from continuing operations before income tax: | | | (17,358 | ) | | (12,879 | ) | | 10,600 | | | 22,402 | | | 8,658 | |
(Benefit from) provision for income taxes | | | (6,769 | ) | | (5,769 | ) | | 3,568 | | | 7,468 | | | 2,194 | |
| | | | | | | | | | | | |
(Loss) income from continuing operations | | | (10,589 | ) | | (7,110 | ) | | 7,032 | | | 14,934 | | | 6,464 | |
Discontinued operations: | | | | | | | | | | | | | | | | |
Loss from discontinued operations, net of tax benefit of $615, $720 and $25 in 2009, 2008 and 2007, respectively | | | (885 | ) | | (1,961 | ) | | (776 | ) | | — | | | — | |
Net loss on disposal of discontinued operations, net of tax benefit of $7,687 | | | (11,057 | ) | | — | | | — | | | — | | | — | |
Gain on revision of the skin care center's leasehold obligations net of income tax expense of $145 and $515 in 2009 and 2005, respectively | | | 249 | | | — | | | — | | | — | | | 859 | |
| | | | | | | | | | | | |
Net (loss) income | | $ | (22,282 | ) | $ | (9,071 | ) | $ | 6,256 | | $ | 14,934 | | $ | 7,323 | |
| | | | | | | | | | | | |
Net (loss) income per share of common stock | | | | | | | | | | | | | | | | |
| | Basic: | | | | | | | | | | | | | | | | |
| | | Income (loss) from continuing operations | | $ | (0.47 | ) | $ | (0.31 | ) | $ | 0.30 | | $ | 0.65 | | $ | 0.29 | |
| | | Income (loss) from discontinued operations | | | (0.51 | ) | | (0.09 | ) | | (0.03 | ) | | — | | | 0.04 | |
| | | | | | | | | | | | |
| | | Net (loss) income | | $ | (0.98 | ) | $ | (0.40 | ) | $ | 0.27 | | $ | 0.65 | | $ | 0.33 | |
| | Diluted: | | | | | | | | | | | | | | | | |
| | | Income (loss) from continuing operations | | $ | (0.47 | ) | $ | (0.31 | ) | $ | 0.30 | | $ | 0.62 | | $ | 0.28 | |
| | | Income (loss) from discontinued operations | | | (0.51 | ) | | (0.09 | ) | | (0.03 | ) | | — | | | 0.04 | |
| | | | | | | | | | | | |
| | | Net (loss) income | | $ | (0.98 | ) | $ | (0.40 | ) | $ | 0.27 | | $ | 0.62 | | $ | 0.32 | |
Basic weighted average shares outstanding | | | 22,714 | | | 22,673 | | | 23,086 | | | 23,017 | | | 22,388 | |
Diluted weighted average shares outstanding | | | 22,714 | | | 22,673 | | | 23,525 | | | 23,948 | | | 23,073 | |
31
Table of Contents
| | | | | | | | | | | | | | | | |
| | For the Year Ended | |
|---|
Consolidated Balance Sheet Data, in thousands: | | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | | July 1,
2006 | | July 2,
2005 | |
|---|
Cash, restricted cash, and cash equivalents | | $ | 25,707 | | $ | 21,059 | | $ | 27,200 | | $ | 40,360 | | $ | 56,383 | |
Marketable securities | | | 3,100 | | | 12,131 | | | 11,773 | | | 27,332 | | | — | |
Marketable securities (long-term portion) | | | — | | | 3,512 | | | 12,260 | | | 11,953 | | | — | |
Working capital | | | 56,421 | | | 68,462 | | | 66,775 | | | 81,910 | | | 70,378 | |
Total assets | | | 123,170 | | | 158,095 | | | 150,230 | | | 149,656 | | | 116,816 | |
Long-term debt | | | — | | | — | | | — | | | — | | | — | |
Total stockholders' equity | | | 77,444 | | | 99,789 | | | 101,510 | | | 100,012 | | | 74,339 | |
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
All statements, trend analysis and other information contained in the following discussion relative to markets for our products and trends in revenue, gross margins and anticipated expense levels, as well as other statements including words such as "anticipate," "believe," "plan," "estimate," "expect," "intend" and other similar expressions, constitute forward-looking statements. These forward-looking statements are subject to business and economic risks and uncertainties, and our actual results of operations may differ materially from those contained in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in Item1A. "Risk Factors" as well as other risks and uncertainties referenced in this Annual Report on Form 10-K.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent liabilities in the consolidated financial statements and accompanying notes. Estimates are used for, but not limited to, receivables valuation, valuation of investments, inventory valuation, depreciable lives of fixed assets, valuation of goodwill and acquired intangibles, income taxes, stock-based compensation, and accrued warranties. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies and the related judgments and estimates affect the preparation of our consolidated financial statements.
Revenue Recognition. We generally recognize revenue upon shipment of product to customers and the fulfillment of all contractual terms and conditions, pursuant to the guidance provided by SAB No. 104, "Revenue Recognition." Credit is not extended to customers and revenue is not recognized until collectibility is reasonably assured. Revenue from the sale of service contracts is deferred and recognized on a straight-line basis over the contract period. Revenue from service administered by us that is not covered by a service contract is recognized as the services are provided. In certain instances, we may sell products together with extended warranties or maintenance contracts. The revenue recognized per element is determined by allocating the total sales price to each element, based on the relative fair values in accordance with Emerging Issues Task Force ("EITF") 00-21, "Revenue Arrangements with Multiple Deliverables." Separately priced extended warranty and maintenance products are accounted for over the life of the contract. Unearned revenue is reported on the balance sheet as deferred revenue.
32
Table of Contents
Allowance for Doubtful Accounts. Our policy is to maintain allowances for estimated losses resulting from the inability of our customers to make required payments. Credit limits are established through a process of reviewing the financial history and stability of each customer. Where appropriate, we obtain credit rating reports and financial statements of customers when determining or modifying their credit limits. We regularly evaluate the collectibility of our trade receivable balances based on a combination of factors. When a customer's account balance becomes past due, we initiate dialogue with the customer to determine the cause. If it is determined that the customer will be unable to meet its financial obligation to us, such as in the case of a bankruptcy filing, deterioration in the customer's operating results or financial position or other material events impacting their business, we record a specific allowance to reduce the related receivable to the amount we expect to recover given all information presently available. We will also provide a general reserve based on the aging of the accounts and notes receivable based on historical experiences of write-offs.
As of June 27, 2009, our accounts receivable balance of $34.1 million is reported net of allowances for doubtful accounts of $2.8 million. We believe our reported allowances at June 27, 2009, are adequate. If the financial conditions of those customers were to deteriorate, however, resulting in their inability to make payments, we may need to record additional allowances that would result in additional selling, general and administrative expenses being recorded for the period in which such determination is made.
Inventory Reserves. As a designer and manufacturer of high technology equipment, we are exposed to a number of economic and industry factors that could result in portions of our inventory becoming either obsolete or in excess of anticipated usage. These factors include, but are not limited to, technological changes in our markets, our ability to meet changing customer requirements, competitive pressures in products and prices, and the availability of key components from our suppliers. Our policy is to establish inventory reserves when conditions exist that suggest that our inventory may be in excess of anticipated demand or is obsolete based upon our assumptions about future demand for our products and market conditions. We regularly evaluate the ability to realize the value of our inventory based on a combination of factors including the following: historical usage rates, forecasted sales or usage, product end of life dates, estimated current and future market values and new product introductions. Purchasing requirements and alternative usage avenues are explored within these processes to mitigate inventory exposure. When recorded, our reserves are intended to reduce the carrying value of our inventory to its net realizable value. As of June 27, 2009, our inventory of $27.6 million is stated net of inventory reserves of $2.4 million. If actual demand for our products deteriorates, or market conditions are less favorable than those that we project, additional inventory reserves may be required.
Fair Value of Financial Instruments. Carrying amounts of our financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximate their fair values due to the short term nature of these instruments. Held-to-maturity marketable securities, which are generally comprised of short-duration certificates of deposit or government-backed bonds, are valued at amortized cost which approximates market value. Available-for-sale securities are marked-to-market on a quarterly basis and the associated change is reflected as a component of other comprehensive income (loss), net of tax.
Product Warranties. Our products are sold with warranty provisions that require us to remedy deficiencies in quality or performance of our products over a specified period of time at no cost to our customers. Our policy is to establish warranty reserves at levels that represent our estimate of the costs that will be incurred to fulfill those warranty requirements at the time that revenue is recognized. We believe that our recorded liability at June 27, 2009, is adequate to cover our future cost of materials, labor and overhead for the servicing of our products sold through that date. If actual product failures
33
Table of Contents
or material or service delivery costs differ from our estimates, our warranty liability would need to be revised accordingly.
Stock based compensation. Effective July 3, 2005, we implemented the fair value recognition provisions of Statement of Financial Accounting Standards No. 123 revised ("SFAS No. 123R") and Staff Accounting Bulletin 107 ("SAB 107") for all share-based compensation that was not vested as of July 2, 2005. We adopted SFAS No. 123R using a modified prospective application, as permitted under SFAS No. 123R. Accordingly, prior period amounts have not been restated. Under this application, we were required to record compensation expense for all awards granted after the date of adoption and for the unvested portion of previously granted awards that remain outstanding at the date of adoption. Compensation cost is recognized over the period that an employee provides service in exchange for the award.
The fair value of each option/stock appreciation rights ("SARs") award is estimated on the date of grant using the Black-Scholes option-pricing model incorporating various assumptions. Expected volatility is determined using both current and historical implied volatilities of the underlying stock which is obtained from public data sources. The expected life of the options is based on historical observations adjusted for the estimated exercise dates of unexercised options/SARS. Additionally, separate groups of employees that have similar historical exercise behavior are considered separately for valuation purposes. The average risk-free interest rate is based on the U.S. treasury security rate with a term to maturity that approximates the option's expected life as of the grant date. The forfeiture rate is based on historical experience as well as future expectations. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Contingencies. We are subject to proceedings, lawsuits and other claims. We assess the likelihood of any adverse judgments or outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of reserves required, if any, for these contingencies is made after careful analysis of each individual issue. The required reserves may change in the future due to new developments in each matter or changes in approach such as a change in settlement strategy in dealing with these matters. We record charges for the costs we anticipate incurring in connection with litigation and claims against us when such costs are probable and can be reasonably estimated.
Restructuring. We record restructuring charges incurred in connection with consolidation or relocation of operations, exited business lines, or shutdowns of specific sites. These restructuring charges, which reflect our commitment to a termination or exit plan that will begin within twelve months, are based on estimates of the expected costs associated with site closure, legal matters, contract terminations, or other costs directly related to the restructuring. If the actual cost incurred exceeds the estimated cost, an additional charge to earnings will result. If the actual cost is less than the estimated cost, a credit to earnings will be recognized.
Income Taxes. The Company accounts for income taxes using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the financial statements or tax returns. In estimating future tax consequences, all expected future events are considered other than enactments of changes in tax laws or rates. Valuation allowances are established as necessary to reduce deferred tax assets in the event that realization of the assets is considered unlikely (see Note 12).
On July 1, 2007, we adopted Financial Accounting Standards Board ("FASB") Interpretation No. 48, ("FIN 48"), "Accounting for Uncertainty in Income Taxes", as discussed more fully below.
34
Table of Contents
FIN 48 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. FIN 48 also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
FIN 48 prescribes a two-step process to determine the amount of tax benefit to be recognized. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step requires us to estimate and measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement. It is inherently difficult and subjective to estimate such amounts, as this requires us to determine the probability of various possible outcomes. We re-evaluate these uncertain tax positions on a quarterly basis. This evaluation is based on factors including, but not limited to, changes in facts or circumstances, changes in tax law, effectively settled issues under audit, and new audit activity. Such a change in recognition or measurement would result in the recognition of a tax benefit or an additional charge to the tax provision in the period. Refer to Note 12 of our Consolidated Financial Statements for additional financial information about income taxes.
Overview
We research, develop, manufacture, market, sell, distribute, and service lasers and light-based products used to perform aesthetic and cosmetic procedures. We sell our lasers and light-based products principally to medical practitioners. We market our products directly and through a network of distributors to end users. Our traditional customer base includes plastic and cosmetic surgeons and dermatologists. More recently, we have expanded our sales to a broader group of practitioners consisting of general practitioners and certain specialists including obstetricians, gynecologists, and general and vascular surgeons. We generate our revenue from the sale of lasers, light-based devices, and other products, and product-related services.
We distribute products worldwide through our direct sales force and independent distributors.
We assemble substantially all of our products in our Wayland, Massachusetts and South Plainfield, New Jersey facilities in the quarter in which they are shipped, and backlog has not been significant. We experience some seasonal reduction of our product sales in our first fiscal quarter due to the summer holiday schedule of physicians and their patients.
Our products are generally covered by a standard warranty, with an option to purchase extended warranty contracts at the time of product sale or service contracts after the time of sale. Distributor sales generally include a parts warranty only. The anticipated cost associated with the standard warranty coverage is accrued at the time of shipment as a cost of sales. Any costs associated with product installation are also recognized as costs of sales. Both such anticipated and actual costs have no associated revenue and therefore reduce the gross profit from product-related service revenue.
Product-related service revenue consists of revenue from maintenance and repair services and the sale of spare parts and consumables. We derive revenue from extended service contracts, which are typically for a 12 or 24-month period, and the revenue is initially deferred and recognized over the life of the service contract. In addition, we provide on-site service worldwide on a time-and-materials basis directly or through our distributors.
International revenue consists of sales from our subsidiaries in the United Kingdom, Germany, France, Italy, Spain, Japan, and Australia, as well as sales of products shipped directly to international locations from the United States. During the fiscal years ended June 27, 2009, June 28, 2008, and June 30, 2007, international revenue represented 67%, 62%, and 56% of total sales, respectively.
35
Table of Contents
Our fiscal year consists of the 52 or 53-week period ending on the Saturday closest to June 30 of each year. The years ended June 27, 2009, June 28, 2008, and June 30, 2007 contained 52 weeks each, respectively.
Discontinued Operations
On March 6, 2007, we acquired Inolase (2002) Ltd. ("Inolase") a non-public company engaged in the development and manufacture of proprietary pneumatic skin flattening PSF devices for the aesthetic light-based treatment industry as well as the development of intellectual property related to medical devices and light sources.
In the fiscal quarter ended December 27, 2008, in connection with our plan to focus on our core products, we initiated a plan to close the Inolase subsidiary. Accordingly, we classified the operating results of this business as discontinued operations in the statements of operations.
The Company determined that there was no future cash value inherent to its Inolase subsidiary and, accordingly, shut down this reporting unit as its fair value was zero. As part of that process the Company recognized a 100% impairment loss associated with the goodwill, other intangible assets, inventory, and fixed assets. The components of the loss recognized during the year ended 2009 are as follows:
| | | | |
| | In thousands | |
|---|
Goodwill | | $ | 10,596 | |
Patents & Developed Technology | | | 5,162 | |
Inventory | | | 1,681 | |
Fixed Assets, net | | | 237 | |
| | | | |
| | $ | 17,676 | |
| | | | |
The Company also recorded restructuring charges of approximately $0.4 million at December 27, 2008 associated with future occupancy costs, minimum termination benefits, and professional service fees. During the six-month period ended June 27, 2009 we recognized certain residual revenues, incurred ongoing operating expenses, and made required adjustments to income taxes in the ordinary course of business.
As of June 27, 2009, the Company had no assets or liabilities held in connection with the disposal of Inolase other than the accrual for future occupancy costs and residual professional fees of approximately $0.1 million.
36
Table of Contents
Results of Operations
The following tables set forth selected financial data for the periods indicated, expressed as a percentage of total revenue.
| | | | | | | | | | | |
| | For the Year Ended | |
|---|
| | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | |
|---|
Revenue Mix: | | | | | | | | | | |
| | Lasers and other products | | | 64.3 | % | | 71.3 | % | | 76.1 | % |
| | Product-related services | | | 35.7 | % | | 28.7 | % | | 23.9 | % |
| | | | | | | | |
| | Total revenue | | | 100.0 | % | | 100.0 | % | | 100.0 | % |
| | | | | | | | |
Gross profit: | | | | | | | | | | |
| | Lasers and other product | | | 29.3 | % | | 37.6 | % | | 42.8 | % |
| | Product-related services | | | 8.9 | % | | 7.1 | % | | 7.5 | % |
| | | | | | | | |
| | Total gross profit | | | 38.2 | % | | 44.7 | % | | 50.3 | % |
| | | | | | | | |
Operating expenses: | | | | | | | | | | |
| | Selling, general & administrative | | | 44.2 | % | | 46.4 | % | | 35.7 | % |
| | Research and development | | | 9.0 | % | | 6.9 | % | | 11.8 | % |
| | | | | | | | |
| | Total operating expenses | | | 53.2 | % | | 53.3 | % | | 47.5 | % |
| | | | | | | | |
(Loss) income from operations | | |
- -15.0 |
% | |
- -8.6 |
% | |
2.8 |
% |
Other income, net | | |
0.1 |
% | |
- -0.1 |
% | |
4.4 |
% |
| | | | | | | | |
(Loss) income from continuing operations before income taxes | | |
- -14.9 |
% | |
- -8.8 |
% | |
7.1 |
% |
(Benefit from) Provision for income taxes | | |
- -5.8 |
% | |
- -4.1 |
% | |
2.4 |
% |
| | | | | | | | |
(Loss) income from continuing operations | | |
- -9.1 |
% | |
- -4.7 |
% | |
4.7 |
% |
Loss from discontinued operations, net of income taxes | | |
- -10.0 |
% | |
- -1.5 |
% | |
- -0.5 |
% |
| | | | | | | | |
Net (loss) income | | |
- -19.1 |
% | |
- -6.2 |
% | |
4.2 |
% |
| | | | | | | | |
Fiscal Year Ended June 27, 2009 Compared to Fiscal Year Ended June 28, 2008
Revenue. Revenue for the year ended June 27, 2009 was $116.6 million as compared to $146.6 million in the prior fiscal year. The decrease is primarily related to the current global economic slowdown which has resulted in decreased sales volume as well as a year-over-year decrease in the average selling price of our units. The economic turmoil impacted all geographic areas of our business.
Domestic revenue was impacted the most significantly and decreased approximately $16.0 million or 29%. Domestic revenue accounted for 33% of the worldwide total compared to approximately 38% the year earlier. International revenue decreased approximately $14.0 million over the prior year and accounted for approximately 67% of total revenue for fiscal 2009 as compared to approximately 62% for fiscal 2008. This $14.0 million decrease, inclusive of an approximate $0.7 million decreases in sales in Latin America, which represents approximately a 15% decrease in sales for that region. Sales in Europe decreased approximately $11.7 million, or 21%, as compared to fiscal year 2008. Sales in our Asia Pacific area declined approximately $1.2 million or 2%.
37
Table of Contents
Product-related service revenue decreased approximately 1% to $41.6 million in fiscal year 2009 from $42.0 million in fiscal year 2008. We believe this is primarily related to the economic situation which has reduced unit sales as well as actual usage of the existing units which, in turn, results in fewer sales of consumable and spare parts.
Gross Profit. Gross profit decreased approximately $21.0 million to $44.6 million, or 38.2% of revenue, in fiscal year 2009 from $65.5 million, or 44.7% of revenue, in fiscal year 2008. The decrease in gross profit results from a combination of factors such as the continuing shift in our revenue mix from domestic sales to more international. International sales increased to approximately 67% of our overall business in fiscal 2009 as compared to approximately 62% in fiscal year 2008. International sales typically have lower gross margins since a greater proportion of that volume is sold through distributors as compared to domestic sales which are primarily sold through our direct sales force. Average selling price of our units have decreased from year to year as a result of the economic situation and extreme competitive pressures. The price decrease has ranged from 3% to 5% over the product lines. Our product related services portion of our revenue increased to 35% of our overall revenue in the current fiscal year as compared to 33% in fiscal year 2008. The product-related services typically carry a lower gross profit than our product revenue. We also encountered certain product reliability problems during fiscal year 2009 that resulted in increased service-related costs, thus eroding such margins further. We believe we have identified the causes of these problems and have been implementing the necessary solutions and will continue to do so in future periods.
Research and Development Expense. Research and development spending increased approximately $0.5 million to $10.6 million in fiscal year 2009 as compared to $10.1 million for fiscal year 2008. The slight increase was attributable primarily to the increased amortization expense of approximately $1.0 million related to the write-off of certain R&D-related intangibles, offset by lower expenses in all other areas. As a percentage of revenue, research and development expenses were approximately 9.1% and 6.9% for fiscal years 2009 and 2008, respectively.
Selling, General and Administrative Expense. Selling, general and administrative expense decreased to approximately $51.5 million or 44.2% of revenue during fiscal year 2008 as compared to $68.1 million or 46.4% during fiscal year 2008. Approximately $7.8 million of this $16.6 million is attributable to decreased legal primarily related to the completion of one lawsuit with Palomar and the temporary stay of the other patent litigation. Selling related expenses were down approximately $4.2 million directly related to the lower sales activity. Overall marketing related expenses were down approximately $2.1 million as a result of a cut back on all marketing related programs throughout the world due to the slowdown in the global economy.
Other Income/Expense. Other expense, relating primarily to net exchange losses, was approximately $0.4 million for the fiscal year ended June 27, 2009, as compared to approximately $1.8 million for the same period ended June 28, 2008. The decrease in other expense is primarily due to the recognition of a $1.9 million net loss on the impairment of our holdings of common shares of OccuLogix, Inc. in the comparable fiscal period offset by miscellaneous other income.
The decrease in interest income earned during the fiscal year ended 2009, as compared to the fiscal year ended 2008, relates directly to a decrease in market interest rates combined with an overall decrease in cash and cash equivalents and in marketable securities. Refer to Note 18 of our Consolidated Financial Statements for additional financial information about other income.
Income Taxes. The provision for income taxes results from a combination of activities of both the domestic and foreign subsidiaries. The Company recorded a 39% effective tax rate for the fiscal year ended 2009 compared to a 45% effective tax rate for the fiscal year ended 2008. The provision for income taxes for the year ended June 27, 2009 includes the effect of the changing mix of foreign and
38
Table of Contents
U.S. pre-tax income (loss), the re-instatement of the research and experimentation credit, and other permanent items.
Fiscal Year Ended June 28, 2008 Compared to Fiscal Year Ended June 30, 2007
Revenue. Revenue for the year ended June 28, 2008 was $146.6 million as compared to $147.8 million in the prior fiscal year. The slight decrease is primarily related to the slowdown in our domestic market that is related to the current economic conditions which has been offset by growth in our international operations as well as our service area.
International revenue increased approximately $8.2 million over the prior year and accounted for approximately 62% of total revenue for fiscal 2008 as compared to approximately 56% for fiscal 2007. The increase of $8.2 million was net of an approximate $6.4 million decreases in sales in Latin America, which represents approximately a 58% decrease in sales for that region. Sales in Europe increased approximately 31%, representing a $13.2 million increase in Europe as compared to fiscal year 2007. Sales in our Asia Pacific area also had strong growth of approximately $3.9 million.
Product-related service revenue increased approximately 19% to $42.0 million in fiscal year 2008 from $35.3 million in fiscal year 2007. This increase was consistent with the increase in systems sold over the past few years and increase in renewal contracts.
Gross Profit. Gross profit decreased approximately $8.9 million to $65.5 million, or 44.7% of revenue, in fiscal year 2008 from $74.4 million, or 50.3% of revenue, in fiscal year 2007. The decrease in gross profit results from a shift in our revenue mix from domestic sales to more international. International sales increased to approximately 62% of our overall business in fiscal 2008 as compared to approximately 56% in fiscal year 2007. International sales typically have lower gross margins since a greater proportion of that volume is sold through distributors as compared to domestically which are primarily sold through our direct sales force. Our product related services portion of our revenue increased to 29% of our overall revenue in the current fiscal year as compared to 24% in fiscal year 2007. The product-related services typically carry a lower gross profit than our product revenue. We also encountered certain product reliability problems during fiscal year 2008 that resulted in increased service-related costs, thus eroding such margins further. We believe we have identified the causes of these problems and will be implementing the necessary solutions in future periods.
Research and Development Expense. Research and development spending decreased approximately $7.4 million to $10.1 million in fiscal year 2008 as compared to $17.5 million for fiscal year 2007. The decrease was attributable primarily to the completion of approximately $5.7 million of outsourced project-related work which was part of the new-product introduction initiative in the prior fiscal year. As a percentage of revenue, research and development expenses were approximately 6.9% and 11.8% for fiscal years 2008 and 2007, respectively.
Selling, General and Administrative Expense. Selling, general and administrative expense increased to approximately $68.1 million or 46.1% of revenue during fiscal year 2008 as compared to $52.8 million or 35.7% of revenue during fiscal year 2007. Legal expenses represent approximately $8.9 million of the $16.0 million increase due to our current lawsuits. Approximately $2.0 million was due to higher personnel expense resulting from increased headcount and salary.
Other Income/Expense. Other expense was approximately $1.8 million for the fiscal year ended June 28, 2008, as compared to other income of approximately $3.7 million for the same period ended June 30, 2007. The decrease is primarily due to the Company having recognized a $3.5 million gain on the initial exchange of common stock of Solx, Inc. for cash and common stock of OccuLogix, Inc. during the fiscal year ended 2007, as compared to the recognition of a $2.5 million loss on the other-than-temporary impairment of our holdings of common shares of OccuLogix, Inc. during the
39
Table of Contents
fiscal year 2008. Refer to Note 18 of our Consolidated Financial Statements for additional financial information about other income.
The decrease in interest income earned during the fiscal year ended 2008, as compared to the fiscal year ended 2007, relates to a decrease in market interest rates combined with an overall decrease in cash and cash equivalents and in marketable securities.
Income Taxes. The provision for income taxes results from a combination of activities of both the domestic and foreign subsidiaries. The Company recorded a 45% effective tax rate for the fiscal year ended 2008 compared to a 34% effective tax rate for the fiscal year ended 2007. The provision for income taxes for the year ended June 28, 2008 includes the effect of the changing mix of foreign and U.S. pre-tax income (loss), the sun-setting of the research and experimentation credit and other permanent items.
Liquidity and Capital Resources
Our cash and cash equivalents, and marketable securities at June 27, 2009 totaled approximately $28.8 million as compared to approximately $36.7 million at June 28, 2008. We continue to have no long-term debt. We believe that the combination of existing cash and cash equivalents, and marketable securities on hand, along with cash to be generated by future operations, will be sufficient to meet our ongoing operating and capital expenditure requirements for the foreseeable future. However, we cannot be sure that we will not require additional capital beyond the amounts currently forecasted by us, nor that any such required additional capital will be available on favorable terms, if at all, as it becomes required. Further, our merger agreement with Syneron restricts our ability to incur additional debt financing beyond existing credit facilities (or equivalent funding) and limits the amount of new equity we can issue, in each case without approval from Syneron. If the merger does not close during the fourth quarter of 2009, as currently expected, and we need to raise capital and Syneron is unwilling to give us approval to do so, these restrictions could adversely impact our financial condition.
Cash used by operating activities amounted to $6.1 million for the fiscal year ended 2009 as compared to $11.7 million for the fiscal year 2008. This decrease in cash used by operating activities was primarily due to the year-over-year decrease in inventory and accounts receivable, offset by changes in other operating assets and liabilities in the normal course of business.
Cash provided by investing activities amounted to approximately $12.3 million for fiscal 2009 as compared to $4.9 million for fiscal 2008. This increase in cash provided by investing activities primarily reflects the net maturities of held-to-maturity investments.
Cash provided by financing activities amounted to less than $0.1 million for the fiscal year 2009 as compared to cash used by financing activities of approximately $2.2 million for fiscal 2008. The decrease is directly related to the Company repurchasing less of its own equity shares in the fiscal year ended 2008 as compared to the fiscal year ended 2009.
Debt Instruments and Related Covenants
We maintain a renewable $10,000,000 revolving credit agreement with a major bank with interest at the bank's base rate of LIBOR plus 2.25%. Any borrowings outstanding under the line of credit are due on demand or according to a payment schedule established at the time funds are borrowed. The line of credit is secured by compensating cash balances. The agreement contains restrictive covenants limiting the establishment of new liens, and the purchase of margin stock. No amounts were outstanding under the line of credit as of June 27, 2009 or June 28, 2008.
40
Table of Contents
Off-Balance Sheet Arrangements
Our only off-balance sheet arrangements consist of non-cancelable operating leases entered into in the ordinary course of business and the license agreement with the Regents. The table below in the next section titled "Contractual Obligations" shows the amounts of our operating lease commitments and purchase commitments payable by year. For liquidity purposes, we choose to lease our facilities and automobiles instead of purchasing them.
Contractual Obligations
In August 2000, Candela obtained exclusive license rights to the DCD from The Regents, subject to certain limited license rights of Cool Touch, Inc. ("Cool Touch"), in the following fields of use: procedures that involve skin resurfacing and rejuvenation, vascular skin lesions, and laser hair removal. Cool Touch, a subsidiary of New Star Technology, Inc. obtained a license to the DCD on a co-exclusive basis with Candela, in certain narrower fields of use. Cool Touch is restricted in its ability to assign its license rights to certain existing competitors of Candela. Candela is entitled to one-half of all royalty income payable to The Regents from Cool Touch. Under an amendment to the license agreement, Candela no longer is required by The Regents to negotiate sublicenses to third parties. However, Candela is entitled to one-half of all royalties due from any other entity that licenses the DCD technology from the Regents in other fields of use.
Effective July 3, 2005, we amended certain portions of our agreement with The Regents whereby for the annual license fee of $0.3 million, our royalty obligation was reduced to 3% from its prior level of 6%. The annual fee of $0.3 million was paid by a lump sum of $3.0 million and is being amortized over the remaining life of the patent agreement, which as of June 27, 2009 was approximately six years. This reduced royalty rate and the amortization of the annual license fee payment is reflected in our financial statements as of June 27, 2009. The unamortized portion of the license fee payment is reflected in prepaid licenses in the June 27, 2009 balance sheet. We recognized royalty expense associated with this patent of approximately $2.5 million for fiscal year 2009.
Outstanding contractual obligations are reflected in the following table:
| | | | | | | | | | | | | | | | |
(in thousands) | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | After 5
years | |
|---|
Royalty commitments | | $ | 2,250 | | $ | 1,000 | | $ | 500 | | $ | 500 | | $ | 250 | |
Operating leases | | | 4,748 | | | 2,102 | | | 2,415 | | | 119 | | | 112 | |
| | | | | | | | | | | | |
Total contractual obligations | | $ | 6,998 | | $ | 3,102 | | $ | 2,915 | | $ | 619 | | $ | 362 | |
| | | | | | | | | | | | |
Recent Accounting Pronouncements
Information with respect to Recent Accounting Pronouncements may be found in Note 1 of the Notes to Consolidated Financial Statements in this Form 10-K, which information is incorporated herein by reference.
41
Table of Contents
Item 8. Financial Statements and Supplementary Data.
Candela Corporation
Index to Consolidated Financial Statements
| | | |
| | Page |
|---|
Report of Independent Registered Public Accounting Firm on Financial Statements | | 43 |
Financial Statements: | | |
| | Consolidated Balance Sheets—June 27, 2009 and June 28, 2008 | |
44 |
| | Consolidated Statements of Income (Loss) and Comprehensive Income (Loss)—Years ended June 27, 2009, June 28, 2008, and June 30, 2007 | |
45 |
| | Consolidated Statements of Stockholders' Equity—Years ended June 27, 2009, June 28, 2008, and June 30, 2007 | |
46 |
| | Consolidated Statements of Cash Flows—Years ended June 27, 2009, June 28, 2008, and June 30, 2007 | |
47 |
| | Notes to Consolidated Financial Statements | |
48 |
Supporting Financial Statement Schedule: | | |
| | Schedule II—Valuation and Qualifying Accounts | |
80 |
42
Table of Contents
Report of Independent Registered Public Accounting Firm on Financial Statements
Shareholders and Board of Directors
Candela Corporation
Wayland, Massachusetts
We have audited the accompanying consolidated balance sheets of Candela Corporation as of June 27, 2009 and June 28, 2008 and the related consolidated statements of income (loss) and comprehensive income (loss), stockholders' equity and cash flows for each of the three years in the period ended June 27, 2009. We have also audited the schedule listed in the accompanying index for each of the three years in the period ended June 27, 2009. These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements and schedule are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements and schedule, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements and schedule. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Candela Corporation at June 27, 2009 and June 28, 2008 and the results of its operations and its cash flows for each of the three years in the period ended June 27, 2009, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 12 to the consolidated financial statements, effective July 1, 2007, the Company adopted FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes—an interpretation of FASB No. 109."
Also, in our opinion, the schedule listed on the accompanying index, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein for the years ended June 27, 2009, June 28, 2008, and June, 30, 2007.
| | |
/s/ BDO SEIDMAN, LLP
BDO Seidman, LLP | | |
Boston, Massachusetts
October 1, 2009 |
|
|
43
Table of Contents
CANDELA CORPORATION
Consolidated Balance Sheets
(in thousands, except per-share data)
| | | | | | | | | |
| | June 27,
2009 | | June 28,
2008 | |
|---|
Assets | | | | | | | |
Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 24,694 | | $ | 21,030 | |
| | Restricted cash | | | 1,013 | | | 29 | |
| | Marketable securities | | | 3,100 | | | 12,131 | |
| | Accounts receivable, net of allowance for doubtful accounts of $2,828 and $2,322 at June 27 and June 28, respectively | | | 31,936 | | | 40,171 | |
| | Notes receivable | | | 382 | | | 728 | |
| | Inventories, net | | | 27,572 | | | 33,141 | |
| | Other current assets | | | 8,715 | | | 12,192 | |
| | | | | | |
| | | Total current assets | | | 97,412 | | | 119,422 | |
Property and equipment, net | | | 3,469 | | | 4,027 | |
Deferred tax assets | | | 20,565 | | | 8,065 | |
Goodwill | | | — | | | 13,225 | |
Acquired Intangible assets, net | | | 174 | | | 6,916 | |
Marketable securities, long-term | | | — | | | 3,512 | |
Other assets | | | 1,550 | | | 2,928 | |
| | | | | | |
| | | Total assets | | $ | 123,170 | | $ | 158,095 | |
| | | | | | |
Liabilities and Stockholders' Equity | | | | | | | |
Current liabilities: | | | | | | | |
| | Accounts payable | | $ | 11,887 | | $ | 16,429 | |
| | Accrued payroll and related expenses | | | 4,947 | | | 4,680 | |
| | Accrued warranty costs, current | | | 4,953 | | | 5,373 | |
| | Sales tax payable | | | 860 | | | 919 | |
| | Other accrued liabilities | | | 5,616 | | | 9,257 | |
| | Deferred revenue, current | | | 11,863 | | | 13,102 | |
| | Current liabilities of discontinued operations | | | 865 | | | 1,200 | |
| | | | | | |
| | | Total current liabilities | | | 40,991 | | | 50,960 | |
Deferred tax liability, long-term | | | — | | | 2,099 | |
Accrued warranty costs, long-term | | | 524 | | | 725 | |
Deferred revenue, long-term | | | 4,211 | | | 4,522 | |
| | | | | | |
| | | Total liabilities | | | 45,726 | | | 58,306 | |
| | | | | | |
Stockholders' equity: | | | | | | | |
| | Common stock, $.01 par value, 60,000,000 shares authorized;
26,175,000 and 26,123,000 issued at June 27 and June 28, respectively | | | 262 | | | 261 | |
| | Treasury stock, 3,450,000 common shares at
June 27 and June 28, respectively, at cost | | | (24,855 | ) | | (24,855 | ) |
| | Additional paid-in capital | | | 76,111 | | | 73,174 | |
| | Accumulated earnings | | | 23,306 | | | 45,588 | |
| | Accumulated other comprehensive income | | | 2,620 | | | 5,621 | |
| | | | | | |
| | | Total stockholders' equity | | | 77,444 | | | 99,789 | |
| | | | | | |
| | | Total liabilities and stockholders' equity | | $ | 123,170 | | $ | 158,095 | |
| | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
44
Table of Contents
CANDELA CORPORATION
Consolidated Statements of Income (Loss) and Comprehensive Income (Loss)
For the years ended June 27, 2009, June 28, 2008, and June 30, 2007
(in thousands, except per-share data)
| | | | | | | | | | | | |
| | 2009 | | 2008 | | 2007 | |
|---|
Revenue | | | | | | | | | | |
| | Lasers and other products | | $ | 74,955 | | $ | 104,583 | | $ | 112,445 | |
| | Product-related service | | | 41,625 | | | 42,034 | | | 35,332 | |
| | | | | | | | |
| | | Total revenue | | | 116,580 | | | 146,617 | | | 147,777 | |
Cost of sales | | | | | | | | | | |
| | Lasers and other products | | | 40,740 | | | 49,545 | | | 49,188 | |
| | Product-related service | | | 31,279 | | | 31,559 | | | 24,191 | |
| | | | | | | | |
| | | Total cost of sales | | | 72,019 | | | 81,104 | | | 73,379 | |
| | | | | | | | |
Gross profit | | | 44,561 | | | 65,513 | | | 74,398 | |
Operating expenses: | | | | | | | | | | |
| | Selling, general and administrative | | | 51,514 | | | 68,075 | | | 52,753 | |
| | Research and development | | | 10,555 | | | 10,104 | | | 17,489 | |
| | | | | | | | |
| | | Total operating expenses | | | 62,069 | | | 78,179 | | | 70,242 | |
| | | | | | | | |
(Loss) income from continuing operations | | | (17,508 | ) | | (12,666 | ) | | 4,156 | |
Other income (expense): | | | | | | | | | | |
| | Interest income | | | 511 | | | 1,628 | | | 2,719 | |
| | Other (expense) income, net | | | (361 | ) | | (1,841 | ) | | 3,725 | |
| | | | | | | | |
| | | Total other Income (expense) | | | 150 | | | (213 | ) | | 6,444 | |
| | | | | | | | |
(Loss) income from continuing operations before income taxes | | | (17,358 | ) | | (12,879 | ) | | 10,600 | |
(Benefit from) provision for income taxes | | | (6,769 | ) | | (5,769 | ) | | 3,568 | |
| | | | | | | | |
(Loss) income from continuing operations | | | (10,589 | ) | | (7,110 | ) | | 7,032 | |
Loss from discontinued operations, net of income taxes | | | (11,693 | ) | | (1,961 | ) | | (776 | ) |
| | | | | | | | |
Net (loss) income | | $ | (22,282 | ) | $ | (9,071 | ) | $ | 6,256 | |
| | | | | | | | |
Net (loss) income per share of common stock | | | | | | | | | | |
Basic | | | | | | | | | | |
| | (Loss) income from continuing operations | | $ | (0.47 | ) | $ | (0.31 | ) | $ | 0.30 | |
| | (Loss) income from discontinued operations | | | (0.51 | ) | | (0.09 | ) | | (0.03 | ) |
| | | | | | | | |
| | Net (loss) income | | $ | (0.98 | ) | $ | (0.40 | ) | $ | 0.27 | |
| | | | | | | | |
Diluted | | | | | | | | | | |
| | (Loss) income from continuing operations | | $ | (0.47 | ) | $ | (0.31 | ) | $ | 0.30 | |
| | (Loss) income from discontinued operations | | | (0.51 | ) | | (0.09 | ) | | (0.03 | ) |
| | | | | | | | |
| | Net (loss) income | | $ | (0.98 | ) | $ | (0.40 | ) | $ | 0.27 | |
| | | | | | | | |
Weighted average shares outstanding: | | | | | | | | | | |
| | Basic | | | 22,714 | | | 22,725 | | | 23,086 | |
| | Diluted | | | 22,714 | | | 22,725 | | | 23,525 | |
Net (loss) income | | $ | (22,282 | ) | $ | (9,071 | ) | $ | 6,256 | |
Other comprehensive (loss) income, net of tax: | | | | | | | | | | |
| | Foreign currency translation adjustment | | | (3,001 | ) | | 5,916 | | | 246 | |
| | Unrealized loss on available-for-sales securities, net of tax | | | — | | | 777 | | | (777 | ) |
| | | | | | | | |
Comprehensive (loss) income | | $ | (25,283 | ) | $ | (2,378 | ) | $ | 5,725 | |
| | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
45
Table of Contents
CANDELA CORPORATION
Consolidated Statements of Stockholders' Equity
For the years ended June 27, 2009, June 28, 2008, and June 30, 2007
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | |
| | Treasury Stock | |
| | Accumulated
Other
Comprehensive
Income (Loss) | |
| |
|---|
| | Additional
Paid-in
Capital | | Accumulated
Earnings | |
| |
|---|
(In thousands) | | Shares | | Amount | | Shares | | Amount | | Total | |
|---|
Balance July 1, 2006 | | | 25,914 | | | 259 | | | 64,234 | | | (2,250 | ) | | (12,997 | ) | | 48,280 | | | 236 | | | 100,012 | |
Sale of common stock under stock plans | | | 168 | | | 2 | | | 841 | | | | | | | | | | | | | | | 843 | |
Treasury stock activity—purchase of shares | | | | | | | | | | | | (875 | ) | | (9,461 | ) | | | | | | | | (9,461 | ) |
Net income | | | | | | | | | | | | | | | | | | 6,256 | | | | | | 6,256 | |
Stock-based compensation expense | | | | | | | | | 4,125 | | | | | | | | | | | | | | | 4,125 | |
Tax benefit of stock exercise | | | | | | | | | 266 | | | | | | | | | | | | | | | 266 | |
Unrealized loss on available-for-sales securities, net of tax | | | | | | | | | | | | | | | | | | | | | (777 | ) | | (777 | ) |
Currency translation adjustment | | | | | | | | | | | | | | | | | | | | | 246 | | | 246 | |
| | | | | | | | | | | | | | | | | | |
Balance June 30, 2007 | | | 26,082 | | | 261 | | | 69,466 | | | (3,125 | ) | | (22,458 | ) | | 54,536 | | | (295 | ) | | 101,510 | |
Sale of common stock under stock plans | | | 41 | | | | | | 194 | | | | | | | | | | | | | | | 194 | |
Treasury stock activity—purchase of shares | | | | | | | | | | | | (325 | ) | | (2,397 | ) | | | | | | | | (2,397 | ) |
Net income | | | | | | | | | | | | | | | | | | (9,071 | ) | | | | | (9,071 | ) |
Cumulative effect of adoption of FIN 48 | | | | | | | | | | | | | | | | | | 123 | | | | | | 123 | |
Stock-based compensation expense | | | | | | | | | 3,502 | | | | | | | | | | | | | | | 3,502 | |
Tax benefit of stock exercise | | | | | | | | | 12 | | | | | | | | | | | | | | | 12 | |
Unrealized loss on available-for-sales securities, net of tax | | | | | | | | | | | | | | | | | | | | | 777 | | | 777 | |
Currency translation adjustment | | | | | | | | | | | | | | | | | | | | | 5,139 | | | 5,139 | |
| | | | | | | | | | | | | | | | | | |
Balance June 28, 2008 | | | 26,123 | | $ | 261 | | $ | 73,174 | | | (3,450 | ) | $ | (24,855 | ) | $ | 45,588 | | $ | 5,621 | | $ | 99,789 | |
Sale of common stock under stock plans | | | 52 | | | 1 | | | 64 | | | | | | | | | | | | | | | 65 | |
Treasury stock activity—purchase of shares | | | | | | | | | | | | | | | | | | | | | | | | — | |
Net income | | | | | | | | | | | | | | | | | | (22,282 | ) | | | | | (22,282 | ) |
Stock-based compensation expense | | | | | | | | | 2,959 | | | | | | | | | | | | | | | 2,959 | |
Tax benefit of stock exercise | | | | | | | | | | | | | | | | | | | | | | | | — | |
Write-off previously recognized excess deferred tax assets upon stock exercise | | | | | | | | | (86 | ) | | | | | | | | | | | | | | (86 | ) |
Unrealized loss on available-for-sales securities, net of tax | | | | | | | | | | | | | | | | | | | | | | | | — | |
Currency translation adjustment | | | | | | | | | | | | | | | | | | | | | (3,001 | ) | | (3,001 | ) |
| | | | | | | | | | | | | | | | | | |
Balance June 27, 2009 | | | 26,175 | | $ | 262 | | $ | 76,111 | | | (3,450 | ) | $ | (24,855 | ) | $ | 23,306 | | $ | 2,620 | | $ | 77,444 | |
| | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
46
Table of Contents
CANDELA CORPORATION
Consolidated Statements of Cash Flows
For the years ended June 27, 2009, June 28, 2008, and June 30, 2007
(in thousands)
| | | | | | | | | | | | | |
| | 2009 | | 2008 | | 2007 | |
|---|
Cash flows from operating activities: | | | | | | | | | | |
| | Net (loss) income | | $ | (22,282 | ) | $ | (9,071 | ) | $ | 6,256 | |
| | | Adjustments to reconcile net (loss) income to net cash used by operating activities: | | | | | | | | | | |
| | | Non-cash items reported in discontinued operations | | | 11,057 | | | — | | | — | |
| | | Loss on other-than-temporary impairment of investment | | | — | | | 2,546 | | | — | |
| | | Gain on exchange of stock | | | — | | | — | | | (3,540 | ) |
| | | Share-based compensation expense | | | 2,959 | | | 3,502 | | | 4,125 | |
| | | Depreciation and amortization | | | 2,632 | | | 2,959 | | | 1,631 | |
| | | Provision for bad debts | | | 3,506 | | | 3,053 | | | 989 | |
| | | Provision for deferred taxes | | | (5,652 | ) | | (3,160 | ) | | 45 | |
| | | Change in restricted cash | | | (984 | ) | | 19 | | | 118 | |
| | | Other non-cash items | | | 127 | | | (71 | ) | | (144 | ) |
| | | Changes in operating assets and liabilities, net of effects of acquisitions: | | | | | | | | | | |
| | | | Accounts receivable | | | 4,003 | | | 4,500 | | | (4,943 | ) |
| | | | Notes receivable | | | 346 | | | (310 | ) | | 586 | |
| | | | Inventories | | | 4,122 | | | (10,892 | ) | | (4,702 | ) |
| | | | Other current assets | | | 459 | | | (2,828 | ) | | (266 | ) |
| | | | Other assets | | | 1,283 | | | 3 | | | (977 | ) |
| | | | Accounts payable | | | (3,843 | ) | | 1,723 | | | (10,376 | ) |
| | | | Accrued payroll and related expenses | | | 372 | | | (950 | ) | | (438 | ) |
| | | | Deferred revenue | | | (606 | ) | | 3,323 | | | 3,422 | |
| | | | Accrued warranty costs | | | (499 | ) | | (1,696 | ) | | (2,016 | ) |
| | | | Income taxes payable | | | 1,395 | | | 136 | | | (2,921 | ) |
| | | | Other accrued liabilities | | | (4,446 | ) | | (4,451 | ) | | 5,732 | |
| | | | | | | | |
Net cash used by operating activities | | | (6,051 | ) | | (11,665 | ) | | (7,419 | ) |
| | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | |
| | | Purchases of property and equipment | | | (800 | ) | | (1,780 | ) | | (763 | ) |
| | | Maturities of held-to-maturity marketable securities | | | 13,114 | | | 21,347 | | | 37,014 | |
| | | Cash proceeds from exchange of stock | | | — | | | — | | | 994 | |
| | | Purchases of held-to-maturity marketable securities | | | — | | | (14,250 | ) | | (17,706 | ) |
| | | Acquisition of business, net of cash acquired | | | — | | | — | | | (15,986 | ) |
| | | Acquisition of intangible assets | | | — | | | (324 | ) | | (1,380 | ) |
| | | | | | | | |
Net cash provided by investing activities | | | 12,314 | | | 4,993 | | | 2,173 | |
| | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | |
| | | Proceeds from the issuance of common stock | | | 65 | | | 194 | | | 843 | |
| | | Purchase of treasury stock | | | — | | | (2,397 | ) | | (9,461 | ) |
| | | Benefits of tax effects from exercise of stock options | | | — | | | 12 | | | 266 | |
| | | | | | | | |
Net cash provided by (used by) financing activities | | | 65 | | | (2,191 | ) | | (8,352 | ) |
| | | | | | | | |
Effect of exchange rates on cash and cash equivalents | | | (2,664 | ) | | 2,741 | | | 556 | |
| | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 3,664 | | | (6,122 | ) | | (13,042 | ) |
Cash and cash equivalents at beginning of period | | | 21,030 | | | 27,152 | | | 40,194 | |
| | | | | | | | |
Cash and cash equivalents at end of period | | $ | 24,694 | | $ | 21,030 | | $ | 27,152 | |
| | | | | | | | |
Cash paid during the year for: | | | | | | | | | | |
| | | Interest | | $ | 68 | | $ | 40 | | $ | 12 | |
| | | Income taxes | | | 491 | | | 1,115 | | | 5,451 | |
Noncash investing and financing activities: | | | | | | | | | | |
| | | Stock acquired on exchange of Solx, Inc. investment | | $ | — | | $ | — | | $ | 2,546 | |
The accompanying notes are an integral part of the consolidated financial statements.
47
Table of Contents
Notes to Consolidated Financial Statements
1. Description of Business and Summary of Significant Accounting Policies
Description of Business. Candela Corporation ("the Company" or "Candela") is involved in the development and commercialization of advanced aesthetic laser systems that allow physicians and personal care practitioners to treat a wide variety of cosmetic and medical conditions.
Basis of Consolidations. The financial statements include the accounts of the Company and its wholly owned subsidiaries. Inter-company transactions and balances have been eliminated. Investments in which we are not able to exercise significant influence over the investee and which do not have readily determinable fair values are accounted for under the cost method.
Subsequent Events. On September 9, 2009, Candela Corporation and Syneron Medical Ltd. ("Syneron") announced that they had entered into an Agreement and Plan of Merger (the "Merger Agreement"), pursuant to which Candela and Syneron will combine their businesses through a merger of Candela and a newly formed, wholly owned subsidiary of Syneron. Existing stockholders of Candela will exchange each of their shares of Candela common stock for 0.2911 ordinary shares of Syneron (the "Merger"). The Merger is discussed more fully below under the caption "Proposed Merger with Syneron." If the merger is not consummated on or before June 1, 2010, either party may terminate the Merger Agreement.
The date to which events occurring after June 27, 2009, the date of the most recent balance sheet, has been evaluated for possible adjustment to the financial statements or disclosure is October 1, 2009.
Reclassifications. Certain prior period amounts have been reclassified to conform to the current period presentation. There was no impact on operating income.
The following table details the effect of the reclassification on the June 28, 2008 balance sheet to conform to the June 27, 2009 presentation relative to:
| | | | | | | | | | |
(in thousands) | | June 28, 2008
Originally
Reported | | Reclassification | | June 28, 2008
Reclassified | |
|---|
Other current assets | | $ | 9,043 | | $ | 3,149 | | $ | 12,192 | |
Accounts receivable | | | 43,320 | | | (3,149 | ) | | 40,171 | |
Accounts payable | |
$ |
15,917 | |
$ |
512 | |
$ |
16,429 | |
Deferred revenue, current | | | 13,614 | | | (512 | ) | | 13,102 | |
Fiscal Years. The Company's fiscal year ends on the Saturday nearest June 30. The years ended June 27, 2009, June 28, 2008, and June 30, 2007 each contain 52 weeks.
Use of Estimates. The preparation of financial statements in conformity with accounting principles generally accepted in the United States ("GAAP") requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosures of contingent liabilities in the consolidated financial statements and accompanying notes. Estimates are used for, but not limited to, receivables valuation, valuation of investments, inventory valuation, depreciable lives of fixed assets, valuation of goodwill and acquired intangibles, income taxes, stock-based compensation, and accrued warranties. On an ongoing basis, management evaluates its estimates. Actual results could differ materially from those estimates.
Foreign Currency Translation. The functional currency of our foreign subsidiaries is generally the local currency. Assets and liabilities denominated in foreign currencies are translated using the exchange rate on the balance sheet dates. Revenues and expenses are translated using monthly average exchange rates prevailing during the year. The translation adjustments resulting from this process are
48
Table of Contents
included as a component of accumulated other comprehensive income. Foreign currency transaction gains and losses are included in other income, net. Deferred tax assets (liabilities) are established on the cumulative translation adjustment attributable to un-remitted foreign earnings that are not intended to be indefinitely reinvested.
Net exchange-related losses resulting from foreign currency transactions were approximately $0.4 million for the fiscal year 2009. Net exchange-related gains resulting from foreign currency transactions were approximately $0.2 million and $0.1 million for the fiscal years ended 2008 and 2007, respectively. The aforementioned net gains and losses are included in other income (expense) in the respective fiscal years.
Cash and Cash Equivalents. The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents. Substantially all cash and cash equivalents were invested in short duration certificates of deposit and overnight repurchase agreements with major financial institutions. These are valued at amortized cost which approximates market value.
Restricted Cash. The Company classifies as restricted cash all cash pledged as collateral to secure obligations and all cash whose use is otherwise limited by contractual provisions.
Marketable Securities. The Company accounts for marketable securities in accordance with Statement of Financial Accounting Standards No. 115,Accounting for Certain Investments in Debt and Equity Securities ("SFAS 115"). SFAS 115 establishes the accounting and reporting requirements for all debt securities and for investments in equity securities that have readily determinable fair values. All marketable securities must be classified as one of the following: held-to-maturity, available-for-sale, or trading.
The Company classifies substantially all of its marketable securities as held-to-maturity as the Company has the intent and ability to hold them to maturity. Held-to-maturity debt securities are reported at amortized cost which approximates fair value. The Company had classified its investment in Occulogix, Inc, (Note 18) as available-for-sale. As such, this investment was marked-to-market on a quarterly basis and the associated unrealized gain or loss reflected as a component of other comprehensive income (loss), net of tax.
Current held-to-maturity marketable securities include investments that are expected to mature in more than three months and up to twelve months. Long-term investments have remaining maturities of one to three years. Unrealized losses related to held-to-maturity securities are not considered a permanent decline in market value of such securities.
See Note 2 for disclosures relative to fair-value measurements.
Accounts Receivable and Notes Receivable. The Company's trade accounts receivable and notes receivable are primarily from sales to end users, leasing companies, and distributors servicing the medical device market, and reflect a broad domestic and international customer base. The Company does not require collateral and has not historically experienced significant credit losses related to receivables from individual customers or groups of customers in any particular industry or geographic area.
The Company's policy is to maintain reserves for potential losses resulting from the inability of our customers to make required payments. These reserves are charged to bad debts expense. The Company determines the adequacy of this allowance by regularly reviewing the aging of its accounts and notes receivable and evaluating the individual customer's balances considering the customer's financial condition, historical experience credit history and current economic conditions. Credit assessments are established through a process of reviewing the financial history and stability of each customer prior to inception of the sale. Where appropriate, the Company will obtain credit rating reports and financial statements of customers when determining or modifying their credit limits. When a customer's account
49
Table of Contents
balance becomes past due, the Company will initiate dialogue with the customer to determine the cause. If it is determined that the customer will be unable to meet its financial obligation, such as in the case of a bankruptcy filing, deterioration in the customer's operating results or financial position or other material events impacting their business, the Company will record an allowance to reduce the related receivable to the amount the Company expects to recover given all information presently available. The Company will also provide a general reserve based on the aging of the accounts and notes receivable based on historical experiences of write-offs.
Fair Value of Financial Instruments. Carrying amounts of the financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximate their fair values due to the short term nature of these instruments. Held-to-maturity marketable securities, which are generally comprised of short-duration certificates of deposit or government-backed bonds, are valued at amortized cost which approximates market value. Available-for-sale securities, if any, are marked-to-market on a quarterly basis and the associated unrealized gain or loss is reflected as a component of other comprehensive income (loss), net of tax.
Derivative Instruments and Hedging Activity. The Company enters into financial instruments to reduce its exposure to fluctuations in foreign exchange rates by creating offsetting positions through the use of foreign currency forward contracts. The Company recognizes all derivative instruments as either assets or liabilities in the statements of financial position and measures those instruments at fair value. Changes to the fair value of derivative contracts that do not qualify for hedge accounting are reported in earnings in the period of the change. For derivatives that qualify for hedge accounting, changes in the fair value of the derivatives are either recorded in shareholders' equity as a component of other comprehensive income (loss) or are recognized currently in earnings, along with an offsetting adjustment against the basis of the item being hedged.
The Company's foreign currency forward contracts may involve elements of credit and market risk in excess of the amounts recognized in the financial statements. The Company monitors its positions and the credit quality of counter-parties, consisting primarily of major financial institutions, and does not anticipate nonperformance by any counter-party. The Company does not use derivative financial instruments for trading or speculative purposes, nor is the Company a party to leveraged derivatives.
We have international subsidiaries that transact business in both local and foreign currencies and therefore we are exposed to foreign currency exchange risk resulting from fluctuations in foreign currencies. This risk could adversely impact our results and financial condition. From time to time, we may enter into foreign currency exchange and option contracts to reduce our exposure to foreign currency exchange risk and variability in operating results due to fluctuation in exchange rates underlying the value of current transactions and anticipated transactions denominated in foreign currencies. These contracts obligate us to exchange predetermined amounts of specified foreign currencies at specified exchange rates on specified dates. These contracts are denominated in the same currency in which the underlying transactions are denominated and bear a contract value and maturity date that approximate the value and expected settlement date, respectively, of the underlying transactions. Unrealized gains and losses on open contracts at the end of each accounting period, resulting from changes in the fair value of these contracts, are recognized in earnings generally in the same period as exchange gains and losses on the underlying foreign denominated receivables are recognized. We do not engage in foreign currency speculation.
We had no forward exchange contracts outstanding at June 27, 2009.
Inventories. Inventories are stated at the lower of cost (first-in, first-out method) or market, using a standard costing system which includes material, labor and manufacturing overhead Standard costs are monitored and updated as necessary, to reflect the changes in raw material costs and labor and overhead rates.
50
Table of Contents
As a designer and manufacturer of high technology equipment, the Company is exposed to a number of economic and industry factors that could result in portions of inventory becoming either obsolete or in excess of anticipated usage. These factors include, but are not limited to, technological changes in markets, the Company's ability to meet changing customer requirements, competitive pressures in products and prices, and the availability of key components from suppliers. The Company's policy is to establish inventory reserves when conditions exist that suggest inventory may be in excess of anticipated demand or is obsolete based upon assumptions about future demand for the Company's products and market conditions. The Company regularly evaluates the ability to realize the value of inventory based on a combination of factors including the following: historical usage rates, forecasted sales or usage, product end of life dates, estimated current and future market values and new product introductions. Purchasing requirements and alternative usage avenues are explored within these processes to mitigate inventory exposure. When recorded, the reserves are intended to reduce the carrying value of inventory to its net realizable value. Inventory of $27.6 million as of June 27, 2009 and $33.1 million as of June 28, 2008 is stated net of inventory reserves of $2.4 million in each year, respectively. If actual demand for the Company's products deteriorates, or market conditions are less favorable than those projected, additional inventory reserves may be required.
Property and Equipment. Purchased property and equipment is recorded at cost. Laser systems used for testing are capitalized at cost. Repairs and maintenance costs are expensed as incurred. Leasehold improvements are amortized over the shorter of the useful life or the life of the related lease. Depreciation is provided using the straight-line method over the estimated useful lives as follows:
| | | | |
| | Estimated Life or
Number of Years | |
|---|
Leasehold improvements | | | 2 to 15 | |
Office furniture, computer and other equipment | | | 2 to 10 | |
Prepaid license fees. Prepaid license fees are amortized over the term of the related contract (Note 10).
Goodwill, Acquired Intangible Assets and Long-Lived Assets. Goodwill is the amount by which the cost of acquired net assets in a business acquisition exceeded the fair values of net identifiable assets on the date of our purchase.
Intangible assets with indefinite useful lives, including goodwill, are evaluated for impairment annually or more frequently if events or changes in circumstances indicate that the asset might be impaired. Factors we consider important, on an overall company basis, that could trigger an impairment review include significant underperformance relative to historical or projected future operating results, significant changes in our use of the acquired assets or the strategy for our overall business, significant negative industry or economic trends, a significant decline in our stock price for a sustained period, or a reduction of our market capitalization relative to net book value. To conduct these tests of goodwill and indefinite-lived intangible assets, the fair value of the reporting unit is compared to its carrying value. If the reporting unit's carrying value exceeds its fair value, we record an impairment loss to the extent that the carrying value of goodwill exceeds its implied fair value. We estimate the fair values of our reporting units using discounted cash flow valuation models.
Long-lived assets primarily include property and equipment and intangible assets with finite lives. In accordance with SFAS 144,Accounting for the Impairment or Disposal of Long-Lived Assets, we periodically review long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of those assets are no longer appropriate. Each impairment test is based on a comparison of the undiscounted cash flows to the recorded value of the asset. If impairment is indicated, the asset
51
Table of Contents
is written down to its estimated fair value based on a discounted cash flow analysis. See Note 15—Discontinued Operations.
Revenue Recognition.
Product sales—The Company generally recognizes revenue upon shipment of product to customers and the fulfillment of all contractual terms and conditions, pursuant to the guidance provided by Securities and Exchange Commission ("SEC") Staff Accounting Bulletin No. 104,Revenue Recognition ("SAB 104"). SAB 104 requires that at the time revenue is recognized persuasive evidence of an arrangement exists; delivery has occurred or services rendered; the fee is fixed and determinable; and collectibility is reasonably assured. Circumstances which generally preclude the immediate recognition of revenue include shipping terms of FOB destination. In these instances, revenue is deferred until adequate documentation is obtained to ensure that the delivery criteria have been fulfilled. The Company provides for the estimated cost to repair or replace products under warranty at the time of sale. Unearned revenue is reported on the balance sheet as deferred income.
Service—Revenue from the sale of service contracts is deferred and recognized on a straight-line basis over the contract period. Revenue from service administered by the Company that is not covered by a service contract is recognized as the services are provided.
Multiple-element arrangements—In certain instances, the Company may sell products together with extended warranties or maintenance contracts. The revenue recognized per element is determined by allocating the total sales price to each element, based on the relative fair values in accordance with Emerging Issues Task Force (EITF) 00-21, "Revenue Arrangements with Multiple Deliverables." Revenue for extended warranty and maintenance contracts are recognized on a straight line basis over the life of the contract. Unearned revenues are reported on the balance sheet as deferred revenue.
Product Warranty Costs. The Company's products generally carry a standard warranty. The Company maintains a reserve based on anticipated warranty claims at the time product revenue is recognized. Factors that affect the Company's product warranty liability include the number of installed units, the anticipated cost of warranty repairs and historical and anticipated rates of warranty claims.
Research and Development Costs. Research and development costs are expensed as incurred.
Advertising Costs. Advertising costs are expensed as incurred and are included as a component of selling, general and administrative expenses in the accompanying Consolidated Statements of Income (Loss) and Comprehensive Income (Loss). Advertising costs were approximately $0.4 million, $0.6 million and $0.7 million for each of the fiscal years ended 2009, 2008, and 2007, respectively.
Shipping and handling costs. The Company reports shipping and handling costs in both sales and the related costs as cost of sales to the extent they are billed to customers. In all other instances they are reflected as a component of cost of sales.
Stock-based compensation. We account for stock-based compensation under SFAS No. 123 (revised 2004), Share-Based Payment ("SFAS 123R"). SFAS 123R requires us to measure the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award using an option pricing model. That cost is recognized over the period during which an employee is required to provide service in exchange for the award.
The fair value of stock appreciation rights ("SARs") granted in 2009, 2008 and 2007 was based on the fair market value of our stock on the date of grant using the Black-Scholes option-pricing model. The weighted average grant-date fair value per share of SARs granted in 2009, 2008 and 2007 were $0.56, $2.27 and $6.77, respectively. Pre-vesting forfeiture rates for purposes of determining stock-based compensation for 2009, 2008 and 2007 were estimated by us to be 5% for each period, respectively.
52
Table of Contents
No options were granted during the years ended 2009, 2008, and 2007, respectively.
The assumptions we used to calculate the weighted average grant-date fair value per share of SARs under the Black-Scholes option-pricing model were as follows:
| | | | | | |
| | For the year
ended
June 27, 2009 | | For the year
ended
June 28, 2008 | | For the year
ended
June 30, 2007 |
|---|
Risk-free interest rate | | 0.93% to 2.38% | | 2.24% to 4.59% | | 4.59% to 5.125% |
Estimated volatility | | 57% to 87% | | 53% to 62% | | 63% to 73% |
Expected life for SARs (years) | | 2.75 to 4.7 | | 2.5 to 5.0 | | 2.5 to 5.0 |
Expected dividend yield | | — | | — | | — |
The following table shows total stock-based compensation expense recorded from our stock-based awards (see Note 11 for a description of the types of stock-based awards) as reflected in our consolidated statements of operations:
| | | | | | | | | | |
(in thousands) | | For the year
ended
June 27, 2009 | | For the year
ended
June 28, 2008 | | For the year
ended
June 30, 2007 | |
|---|
Selling, general and administrative | | | 2,308 | | $ | 3,191 | | $ | 3,155 | |
Research and development | | | 251 | | | (14 | ) | | 640 | |
Cost of sales | | | 400 | | | 325 | | | 330 | |
| | | | | | | | |
| | $ | 2,959 | | $ | 3,502 | | $ | 4,125 | |
| | | | | | | | |
We did not realize any tax benefit in the consolidated statement of operations relative to stock-based compensation during fiscal 2009.
The tax benefit realized relative to stock-based compensation totaled less than $0.1 million in fiscal 2008 and approximately $0.3 million in fiscal 2007, respectively.
As of June 27, 2009 total unrecognized compensation cost related to unvested SARs expected to vest was approximately $1.6 million. As of June 27, 2009, the weighted average remaining recognition period for unvested awards was 0.71 years.
Income Taxes. The Company accounts for income taxes using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the financial statements or tax returns. In estimating future tax consequences, all expected future events are considered other than enactments of changes in tax laws or rates. Valuation allowances are established as necessary to reduce deferred tax assets in the event that realization of the assets is considered unlikely (Note 12).
On July 1, 2007, we adopted Financial Accounting Standards Board ("FASB") Interpretation No. 48, ("FIN 48"), "Accounting for Uncertainty in Income Taxes", as discussed more fully below.
FIN 48 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. FIN 48 also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
FIN 48 prescribes a two-step process to determine the amount of tax benefit to be recognized. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step requires us to estimate and
53
Table of Contents
measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement. It is inherently difficult and subjective to estimate such amounts, as this requires us to determine the probability of various possible outcomes. We re-evaluate these uncertain tax positions on a quarterly basis. This evaluation is based on factors including, but not limited to, changes in facts or circumstances, changes in tax law, effectively settled issues under audit, and new audit activity. Such a change in recognition or measurement would result in the recognition of a tax benefit or an additional charge to the tax provision in the period (Note 12).
Earnings Per Share ("EPS"). Basic EPS is calculated by dividing net income (loss) by the weighted average number of shares outstanding during the period. Diluted EPS is calculated by dividing net income by the weighted average number of shares outstanding plus the dilutive effect, if any, of outstanding stock options, warrants, restricted shares, and SARs using the treasury stock method.
The following table provides share information used in the calculation of the Company's basic and diluted earnings per share:
| | | | | | | | | | |
| | For the years ended: | |
|---|
(in thousands, except per share data) | | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | |
|---|
Shares used in the calculation of Basic earnings per share | | | 22,714 | | | 22,725 | | | 23,086 | |
Effect of dilutive securities: Stock options/SAR's | | | — | | | — | | | 439 | |
| | | | | | | | |
Diluted shares used in the calculation of earnings per share | | | 22,714 | | | 22,725 | | | 23,525 | |
| | | | | | | | |
During the fiscal years ended June 27, 2009 and June 28, 2008, potential common shares consisting of all shares issuable upon exercise of stock options/SARs have been excluded from the computation of diluted loss per share as their effect would have been anti-dilutive.
During the year ended June 30, 2007, options/SARs to purchase 1.1 million shares of common stock were not included in the computation of diluted earnings per share because they would have had an anti-dilutive effect.
Comprehensive Income (Loss). The Company computes comprehensive income (loss) in accordance with SFAS No. 130 "Reporting Comprehensive Income" ("SFAS 130"). SFAS 130 establishes standards for the reporting and display of comprehensive income and its components in the financial statements. Other comprehensive income (loss), as defined, includes all changes in equity during a period from non-owner sources.
For the periods presented, accumulated other comprehensive income includes the effect of foreign currency translation and is summarized in the following table:
| | | | | | | |
(in thousands) | | June 27,
2009 | | June 28,
2008 | |
|---|
Foreign currency translation adjustment | | $ | 2,620 | | $ | 5,621 | |
| | | | | | |
Accumulated other comprehensive income | | $ | 2,620 | | $ | 5,621 | |
| | | | | | |
Concentration of Risk. Financial instruments that are subject to credit risk primarily consist of cash and cash equivalents, marketable securities, commercial paper and accounts receivable. The Company places its cash and cash equivalents and held-to-maturity marketable securities in established financial institutions. Deposits in the financial institutions may exceed the amount of insurance provided on such deposits. The Company believes the financial institutions are financially sound and, accordingly,
54
Table of Contents
minimal credit risk exists. The Company invests in high quality debt securities to minimize any adverse changes in interest rates.
The Company's sales are to end users, leasing companies, and distributors servicing the medical device market, and reflect a broad domestic and international customer base. The Company does not require collateral and has not historically experienced significant credit losses related to receivables from individual customers or groups of customers in any particular industry or geographic area. The Company maintains an allowance for potential losses. No single customer accounted for 10% of the Company's total net sales or accounts and notes receivable.
The Company is subject to risks common to companies in the aesthetic laser industry, including (i) the Company's ability to successfully complete preclinical and clinical development and obtain timely regulatory approval and adequate patent and other proprietary rights protection of its products and services, (ii) the content and timing of decisions made by the Food & Drug Administration and other agencies regarding the procedures for which the Company's products may be approved, (iii) the ability of the Company to manufacture adequate supplies of its products for development and commercialization activities, (iv) the accuracy of the Company's estimates of the size and characteristics of markets to be addressed by the Company's products and services, (v) market acceptance of the Company's products and services, (vi) the Company's ability to obtain reimbursement for its products from third-party payers, where appropriate, and (vii) the accuracy of the Company's information concerning the products and resources of competitors and potential competitors.
The Company depends on a single vendor for Alexandrite rods used to manufacture the GentleLASE® and AlexTriVantage™ systems. These products accounts for a significant portion of total revenues.
Recent Accounting Pronouncements.
In June 2009, the FASB issued SFAS No. 168,The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles. This standard replaces SFAS No. 162,The Hierarchy of Generally Accepted Accounting Principles, and establishes only two levels of U.S. GAAP, authoritative and non-authoritative. The FASB Accounting Standards Codification (the "Codification") will become the source of authoritative, non-governmental GAAP, except for rules and interpretive releases of the SEC, which are sources of authoritative GAAP for SEC registrants. All other non-grandfathered, non-SEC accounting literature not included in the Codification will become non-authoritative. This statement is effective for financial statements for interim or annual reporting periods ending after September 15, 2009. We will use the new guidelines and numbering system prescribed by the Codification when referring to U.S. GAAP beginning in the third quarter of fiscal 2009. As the Codification was not intended to change or alter existing U.S. GAAP, it will not have any impact on our financial position, results of operations or cash flows.
In June 2009, the FASB issued SFAS No. 167,Amendments to FASB Interpretation No. 46(R). This statement amends the consolidation guidance applicable to variable interest entities and is effective as of January 1, 2010. We are currently in the process of evaluating the impact of this pronouncement.
55
Table of Contents
In June 2009, the FASB issued SFAS No. 166,Accounting for Transfers of Financial Assets, an amendment to SFAS No. 140. This statement eliminates the concept of a qualifying special-purpose entity, changes the requirements for derecognizing financial assets, and requires additional disclosures in order to enhance information reported to users of financial statements by providing greater transparency about transfers of financial assets, including securitization transactions, and an entity's continuing involvement in and exposure to the risks related to transferred financial assets. This statement is effective for fiscal years beginning after November 15, 2009. We are currently in the process of evaluating the impact of this pronouncement.
Effective June 27, 2009, we adopted SFAS No. 165,Subsequent Events. This statement is intended to establish general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. In particular, this statement sets forth the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements, and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. The adoption of SFAS No. 165 did not have any impact on our financial position, results of operations or cash flows.
In June 2008, the FASB issued EITF Issue No. 07-5,Determining Whether an Instrument (or an Embedded Feature) Is Indexed to an Entity's Own Stock. EITF Issue No. 07-5 provides guidance on evaluating whether an equity-linked financial instrument (or embedded feature) is indexed to a company's own stock, including evaluating the instrument's contingent exercise and settlement provisions. EITF Issue No. 07-5 is effective for fiscal years beginning after December 15, 2008. We do not expect the adoption of EITF Issue No. 07-5 to have a material impact on our consolidated financial statements.
In April 2008, the FASB finalized FSP No. 142-3,Determination of the Useful Life of Intangible Assets. The position amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB SFAS No. 142. The position applies to intangible assets that are acquired individually or with a group of other assets and both intangible assets acquired in business combinations and asset acquisitions. FSP 142-3 is effective for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. We do not expect the adoption of FSP 142-3 to have a material impact on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations, which replaces SFAS No. 141. The statement retains the fundamental requirements in SFAS No. 141 that the acquisition method of accounting (previously referred to as the purchase method of accounting) be used for all business combinations, but requires a number of changes, including changes in the way assets and liabilities are recognized as a result of business combinations. It also requires the capitalization of in-process research and development at fair value and requires the expensing of acquisition-related costs as incurred. In April 2009, the FASB issued FSP FAS 141(R)-1 which amends SFAS No. 141(R) by establishing a model to account for certain pre-acquisition contingencies. Under the FSP, an acquirer is required to recognize at fair value an asset acquired or a liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, then the acquirer should follow the recognition criteria in SFAS No. 5,Accounting for Contingencies, and FASB Interpretation No. 14,Reasonable Estimation of the Amount of a Loss—an interpretation of FASB Statement No. 5. SFAS No. 141(R) and FSP FAS 141(R)-1 are effective for us beginning June 28, 2009, and will apply prospectively to business combinations completed on or after
56
Table of Contents
that date. The impact of the adoption of SFAS No. 141(R) and FSP FAS 141(R)-1 will depend on the nature of acquisitions completed after the date of adoption.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of SFAS No. 115. SFAS No. 159 permits companies to choose to measure certain financial instruments and certain other items at fair value and requires unrealized gains and losses on items for which the fair value option has been elected to be reported in earnings. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007. Effective March 29, 2008, we adopted SFAS 159, but we have not elected the fair value option for any eligible financial instruments as of June 27, 2009.
In April 2009, the FASB issued three related Staff Positions: (i) FSP 157-4,Determining Fair Value When the Volume and Level of Activity for the Asset or Liability have Significantly Decreased and Identifying Transactions That Are Not Orderly ("FSP 157-4"), (ii) SFAS 115-2 and SFAS 124-2,Recognition and Presentation of Other-Than-Temporary Impairment ("FSP 115-2" and "FSP 124-2"), and (iii) SFAS 107-1 and APB 28-1,Interim Disclosures about Fair Value of Financial Instruments ("FSP 107" and "APB 28-1"), which will be effective for interim and annual periods ending after June 15, 2009. FSP 157-4 provides guidance on how to determine the fair value of assets and liabilities under SFAS 157 in the current economic environment and reemphasizes that the objective of a fair value measurement remains an exit price. If we were to conclude that there has been a significant decrease in the volume and level of activity of the asset or liability in relation to normal market activities, quoted market values may not be representative of fair value and we may conclude that a change in valuation technique or the use of multiple valuation techniques may be appropriate. FSP 115-2 and FSP 124-2 modify the requirements for recognizing other-than-temporarily impaired debt securities and revise the existing impairment model for such securities, by modifying the current intent and ability indicator in determining whether a debt security is other-than-temporarily impaired. FSP 107 and APB 28-1 enhance the disclosure of instruments under the scope of SFAS 157 for both interim and annual periods. We are currently evaluating these Staff Positions and the impact, if any, that adoption will have on our financial position and results of operation.
2. Fair Value Measurements
We measure financial assets and liabilities at fair value based upon exit price, representing the amount that would be received on the sale of an asset or paid to transfer a liability, as the case may be, in an orderly transaction between market participants. As such, fair value may be based on assumptions that market participants would use in pricing an asset or liability. SFAS No. 157 (as impacted by FSP Nos. 157-1, 157-2 and 157-3) establishes a consistent framework for measuring fair value on either a recurring or nonrecurring basis whereby inputs, used in valuation techniques, are assigned a hierarchical level. The following are the hierarchical levels of inputs to measure fair value:
- •
- Level 1: Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
- •
- Level 2: Inputs reflect quoted prices for identical assets or liabilities in markets that are not active; quoted prices for similar assets or liabilities in active markets; inputs other than quoted prices that are observable for the assets or liabilities; or inputs that are derived principally from or corroborated by observable market data by correlation or other means.
- •
- Level 3: Unobservable inputs reflecting our own assumptions incorporated in valuation techniques used to determine fair value. These assumptions are required to be consistent with market participant assumptions that are reasonably available.
57
Table of Contents
The following table summarizes our financial assets and liabilities measured at fair value on a recurring basis, by level within the fair value hierarchy:
| | | | | | | | | | | | | |
| | As of June 27, 2009 | |
|---|
| | Level 1 | | Level 2 | | Level 3 | | Total | |
|---|
Cash equivalents | | $ | 6,214 | | $ | — | | $ | — | | $ | 6,214 | |
Short-term investments | | | 1,686 | | | — | | | — | | | 1,686 | |
| | | | | | | | | | |
| | $ | 7,900 | | $ | — | | $ | — | | $ | 7,900 | |
| | | | | | | | | | |
FSP FAS No. 157-2, which measures fair value of non-financial assets and liabilities which are recognized or disclosed at fair value on a nonrecurring basis, will be effective beginning in fiscal 2010. This deferral applies to us for such items as non-financial assets and liabilities initially measured at fair value in a business combination but not measured at fair value in subsequent periods, non-financial long-lived and intangible asset groups measured at fair value for an impairment assessment, and reporting units measured at fair value as part of a goodwill impairment test.
3. Marketable Securities
Marketable securities consist of the following:
| | | | | | | | | | | | | | | | | | | | |
| | June 27, 2009 | | June 28, 2008 | |
|---|
(in thousands) | | Net Carrying
Amount | | Fair
Value | | Unrealized
income
(loss) | | Net Carrying
Amount | | Fair
Value | | Unrealized
income
(loss) | |
|---|
Short-term (Held-to-maturity): | | | | | | | | | | | | | | | | | | | |
| | Government-backed securities | | $ | — | | $ | — | | $ | — | | $ | 9,000 | | $ | 9,055 | | $ | 55 | |
| | Certificates of deposit | | | 3,100 | | | 3,108 | | | 8 | | | 3,131 | | | 3,134 | | | 3 | |
| | | | | | | | | | | | | | |
| | | 3,100 | | | 3,108 | | | 8 | | | 12,131 | | | 12,189 | | | 58 | |
Short-term (Available-for-sale): | | | | | | | | | | | | | | | | | | | |
| | | — | | | — | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | | | |
Total marketable securities, short-term | | $ | 3,100 | | $ | 3,108 | | $ | 8 | | $ | 12,131 | | $ | 12,189 | | $ | 58 | |
| | | | | | | | | | | | | | |
Long-term (Held-to-maturity): | | | | | | | | | | | | | | | | | | | |
| | Government-backed securities | | $ | — | | $ | — | | $ | — | | $ | 2,000 | | $ | 2,014 | | $ | 14 | |
| | Certificates of deposit | | | — | | | — | | | — | | | 1,512 | | | 1,502 | | | (10 | ) |
| | | | | | | | | | | | | | |
Total marketable securities, long-term | | $ | — | | $ | — | | $ | — | | $ | 3,512 | | $ | 3,516 | | $ | 4 | |
| | | | | | | | | | | | | | |
Held-to-maturity investments at June 27, 2009 reach maturity in 2010.
4. Inventories
Inventories consist of the following:
| | | | | | | |
(in thousands) | | June 27,
2009 | | June 28,
2008 | |
|---|
Raw materials | | $ | 9,166 | | $ | 13,720 | |
Work in process | | | 381 | | | 1,635 | |
Finished goods | | | 18,025 | | | 17,786 | |
| | | | | | |
| | $ | 27,572 | | $ | 33,141 | |
| | | | | | |
58
Table of Contents
5. Property and Equipment
Property and equipment consist of the following:
| | | | | | | |
(in thousands) | | June 27,
2009 | | June 28,
2008 | |
|---|
Leasehold improvements | | $ | 388 | | $ | 1,014 | |
Office furniture | | | 409 | | | 718 | |
Computers, software, and other equipment | | | 7,467 | | | 11,845 | |
| | | | | | |
| | $ | 8,264 | | $ | 13,577 | |
Less: accumulated depreciation | | | (4,795 | ) | | (9,550 | ) |
| | | | | | |
Property and equipment, net | | $ | 3,469 | | $ | 4,027 | |
| | | | | | |
Depreciation expense was approximately $1.2 million, $1.2 million, and $0.9 million for the fiscal years ended 2009, 2008, 2007, respectively.
6. Intangible Assets
Goodwill and acquired intangible assets consist of the following:
| | | | | | | | |
(in thousands) | | June 27,
2009 | | June 28,
2008 | |
|---|
Goodwill | | $ | — | | $ | 13,225 | |
| | | | | | |
Intangible assets with finite lives: | | | | | | | |
| | Patents | | $ | 174 | | $ | 3,128 | |
| | Developed technology | | | — | | | 6,009 | |
| | | | | | |
| | | 174 | | | 9,137 | |
| | | | | | |
Accumulated amortization: | | | | | | | |
| | Patents | | | — | | | (886 | ) |
| | Developed technology | | | — | | | (1,335 | ) |
| | | | | | |
| | | — | | | (2,221 | ) |
| | | | | | |
Intangible assets with finite lives, net | | $ | 174 | | $ | 6,916 | |
| | | | | | |
Changes to the carrying amount of goodwill for the fiscal year ended 2009 were as follows (see Note 15—Discontinued Operations):
| | | | |
| | (in thousands) | |
|---|
Balance at June 28, 2008 | | $ | 13,225 | |
Effect of foreign currency adjustments | | | (2,629 | ) |
Impairment Charge | | | (10,596 | ) |
| | | | |
Balance at June 27, 2009 | | $ | — | |
| | | | |
Patents are generally amortized over periods ranging from five to six years. Developed technologies are generally amortized over 6 years.
Amortization expense on acquired intangible assets was approximately $1.5 million and $1.6 million for the fiscal years ended 2009 and 2008, respectively. There was no amortization expense associated with acquired intangible assets for the fiscal year ended 2007.
59
Table of Contents
7. Acquisitions
On March 6, 2007, we acquired Inolase (2002) Ltd. ("Inolase") a non-public company engaged in the development and manufacture of proprietary devices for the aesthetic light-based treatment industry as well as the development of intellectual property related to medical devices and light sources. The aggregate purchase price was approximately $16.8 million in cash, including $0.3 million of acquisition-related transaction costs. No Candela common stock was issued in the acquisition. The acquisition-related transactions costs included legal, accounting, and other external costs directly related to the acquisition.
This acquisition was accounted for as a business combination. Assets acquired and liabilities assumed were recorded at their fair values as of March 6, 2007. Fair-value of the intangible assets was based on valuations using an income approach with estimates and assumption provided by the management of Inolase and Candela. The excess of the purchase price over the fair value of tangible assets, identified intangible assets, and liabilities assumed was recorded as goodwill.
Based upon the valuations, the final purchase price was allocated as follows:
| | | | | |
| | (in thousands) | |
|---|
Goodwill | | $ | 10,596 | |
Identifiable intangible assets | | | 7,433 | |
Inventory | | | 214 | |
Accounts receivable & other assets | | | 390 | |
Accounts payable and accrued expenses | | | (248 | ) |
Deferred tax liability | | | (1,375 | ) |
Other liabilities | | | (201 | ) |
| | | | |
| | Total purchase price allocation | | $ | 16,809 | |
| | | | |
None of the goodwill or intangible assets acquired in the acquisition was determined to be deductible for income tax purposes. As a result, and in accordance with SFAS 109,Income Taxes, we recorded in the purchase accounting a deferred tax liability of approximately $1.4 million, equal to the tax effect of the amount of the acquired intangible assets other than goodwill.
In the fiscal quarter ended December 27, 2008, in connection with the Company's plan to focus on its core products, management initiated a plan to close its Inolase (2002) Ltd. ("Inolase") subsidiary. The closure was accounted for as a discontinued operation in accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets ("SFAS 144"), and SFAS No. 146,Accounting for Costs Associated with Exit or Disposal Activities ("SFAS 146"). See Note 15.
60
Table of Contents
8. Warranty Reserve
The following table reflects changes in the Company's accrued warranty account during the fiscal years ended June 27, 2009, June 28, 2008, and July 30, 2007:
| | | | | | | | | | | |
(in thousands) | | 2009 | | 2008 | | 2007 | |
|---|
Beginning balance | | $ | 6,098 | | $ | 7,613 | | $ | 9,629 | |
| | Incremental accruals on current sales | | | 5,225 | | | 6,764 | | | 5,722 | |
| | Amortization of prior-period accruals | | | (5,846 | ) | | (8,279 | ) | | (7,738 | ) |
| | | | | | | | |
Ending balance | | $ | 5,477 | | $ | 6,098 | | $ | 7,613 | |
| | | | | | | | |
9. Deferred Revenue
The Company offers extended service contracts that may be purchased after a standard warranty has expired. Service contracts may be purchased for periods from one to five years. The Company recognizes service contract revenue ratably over the life of the contract. Actual service contract expenses incurred and charged to costs of sales during an interim period may be more or less than the amount of amortized service contract revenue recognized in that period.
The following table reflects changes in the Company's deferred revenue during the fiscal years ended June 27, 2009 and June 28, 2008:
| | | | | | | | |
(in thousands) | | 2009 | | 2008 | |
|---|
Beginning balance | | $ | 18,136 | | $ | 13,751 | |
| | Deferral of new sales | | | 15,005 | | | 19,040 | |
| | Recognition of previously deferred revenue | | | (17,067 | ) | | (15,167 | ) |
| | | | | | |
Ending balance | | $ | 16,074 | | $ | 17,624 | |
| | | | | | |
The following table reflects the Company's current and long-term portions of deferred revenues for the fiscal years ended June 27, 2009 and June 28, 2008:
| | | | | | | |
(in thousands) | | 2009 | | 2008 | |
|---|
Total service contract revenue | | $ | 16,074 | | $ | 17,624 | |
Less: current portion | | | (11,863 | ) | | (13,102 | ) |
| | | | | | |
Long-term pertion of deferred revenue | | $ | 4,211 | | $ | 4,522 | |
| | | | | | |
10. Debt, Lease and Other Obligations
Line of Credit. The Company has a renewable $10,000,000 revolving credit agreement with a major bank with interest at the bank's base rate or LIBOR plus 2.25 percent. Any borrowings outstanding under the line of credit are due on demand or according to a payment schedule established at the time funds are borrowed. The line of credit is secured by compensating cash balances. The agreement contains restrictive covenants limiting the establishment of new liens, and the purchase of margin stock. No amounts were outstanding under the line of credit as of June 27, 2009 or June 28, 2008.
Operating Lease Commitments. The Company leases several facilities and automobiles under non-cancelable lease arrangements. The facility leases may be adjusted for increases in maintenance and insurance costs above specified levels. In addition, certain facility leases contain escalation provisions based on certain inflationary indices. These operating leases expire in various years through fiscal year 2016. These leases may be renewed for periods ranging from one to five years.
61
Table of Contents
Total outstanding lease commitments are reflected in the following table as of June 27, 2009:
| | | | |
(in thousands) | | Amount | |
|---|
2010 | | $ | 2,102 | |
2011 | | | 1,707 | |
2012 | | | 708 | |
2013 | | | 78 | |
2014 | | | 41 | |
Thereafter | | | 112 | |
| | | | |
Total minimum lease payments | | $ | 4,748 | |
| | | | |
Total facilities-related rent expense was approximately $1.5 million, $1.5 million, and $1.1 million in fiscal years 2009, 2008, and 2007, respectively.
Royalty. In August 2000, the Company obtained through an amended license agreement the with Regents of the University of California ("Regents"), the exclusive license rights to the Dynamic Cooling Device subject to certain limited license rights of Cool Touch, Inc. ("Cool Touch") in the following fields of use: procedures that involve skin resurfacing and rejuvenation, vascular skin lesions, and laser hair removal. Cool Touch, a subsidiary of New Star Technology, Inc. obtained a license to the DCD on a co-exclusive basis with the Company in certain narrower fields of use. Cool Touch is restricted in its ability to assign its license rights to certain existing competitors of the Company. The Company is entitled to one-half of all royalty income payable to the Regents from Cool Touch. Under the amended agreement, the Company no longer is required by the Regents to negotiate sublicenses to third parties. However, the Company is entitled to one-half of all royalties due from any other entity that licenses the DCD technology from the Regents in other fields of use.
Effective July 3, 2005, the Company amended certain portions of their agreement with the Regents whereby for the annual license fee of $0.3 million, the Company's royalty obligation was reduced to 3% from its prior level of 6%. The annual fee of $0.3 million was paid by a lump sum of $3.0 million and is being amortized over the remaining life of the patent agreement, which as of June 27, 2009 was approximately six years. This reduced royalty rate and the amortization of the annual license fee payment is reflected in the Company's financial statements for fiscal years 2009, 2008 and 2007, respectively. The unamortized portion of the license fee payment is reflected in both other current assets and in other assets in the June 27, 2009 and June 28, 2008 balance sheets, respectively. The Company recognized royalty expense of $3.4 million, $3.4 million and $3.6 million for the fiscal years ended 2009, 2008 and 2007, respectively.
The outstanding contractual obligations relating to future minimum royalties payable to the Regents are reflected in the following table as of June 27, 2009:
| | | | |
(in thousands) | | Amount | |
|---|
2010 | | $ | 1,000 | |
2011 | | | 500 | |
2012 | | | 500 | |
2013 | | | 250 | |
2014 | | | — | |
| | | | |
Total minimum royalty payments | | $ | 2,250 | |
| | | | |
62
Table of Contents
11. Equity Incentive Plan
General Discussion
Stock options provide the holder with the right to purchase common stock. The exercise price per share of "incentive stock options" within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended, may not be less than the fair market value per share of common stock on the date of grant. In the case of an incentive stock option granted to an employee possessing more than 10% of the total combined voting power of all classes of stock of the Company, the exercise price per share may not be less than 110% of the fair market value per share of common stock on the date of grant.
Stock Appreciation Rights ("SARs") provide the holder the right to receive an amount, payable in stock, cash or in any combination of these, as specified by a Committee established by the Board of Directors, equal to the excess of the market value of our common shares on the date of exercise over the grant price at the time of the grant. The grant price of a SAR may not be less than the fair market value per share of common stock on the date of grant, provided that the grant price of SARs granted in tandem with stock options shall equal the exercise price of the related stock option.
1990 Candela Corporation Employee Stock Purchase Plan
The 1990 Employee Stock Purchase Plan (the "Purchase Plan") provides for the sale of up to 1.5 million shares of common stock to eligible employees. The shares are issued at 85% of the market price on the last day of semi-annual periods. Substantially all full-time employees are eligible to participate in the Purchase Plan. At June 27, 2009 there were approximately 603,000 shares available for sale.
The following is a summary of shares issued under the Purchase Plan:
| | | | | |
Fiscal Year | | Number
of Shares | | Per-share
Price Range |
|---|
2007 | | | 33,978 | | $10.00 to $10.75 |
2008 | | | 21,142 | | $4.75 |
2009 | | | 37,396 | | $ 0.50 to $ 2.00 |
Pursuant to the terms of the Merger Agreement executed September 8, 2009, the Company's Board of Directors shall take action to amend the Purchase Plan to end the Payment Period, as defined by the Purchase Plan, no later than two (2) business days before the effective time and to terminate the Purchase Plan immediately thereafter.
1998 Candela Corporation Stock Option Plan
Options and rights granted under the 1998 Stock Plan become exercisable on the date of grant or in installments, as specified by a Committee established by the Board of Directors, and expire ten years from the date of the grant. Upon exercise of a SAR, only the net number of shares of common stock issued in connection with such exercise shall be deemed "issued" for this purpose. The Company may satisfy the awards upon exercise with either newly-issued or treasury shares.
There were approximately 0.6 million stock-based SARs granted under this plan during the fiscal year ended 2009. The SARs granted to employees become exercisable ratably over one to four years, while the SARs granted to directors become exercisable over two years.
There were approximately 4.1 million outstanding options/SARs at June 27, 2009 with a weighted-average exercise price of $8.24 per share, an aggregate intrinsic value of zero, and a weighted-average remaining contractual term of 7.28 years.
63
Table of Contents
Of the total 4.1 million outstanding options/SARs, there were approximately 2.6 million of exercisable options/SARs at June 27, 2009 with a weighted-average exercise price of $9.26 per share, an aggregate intrinsic value of zero, and a weighted-average remaining contractual term of 6.64 years.
The total intrinsic value of options exercised during the fiscal year ended 2009 was approximately $18,000.
The 1998 Stock Plan expired pursuant to its terms on September 18, 2008. As such, there were no options/SARs available for grant under this plan at June 27, 2009.
The following is a summary of stock option/SARs activity under the 1998 Stock Option Plan:
| | | | | | | | | |
| | Number of Shares | | Option Prices | | Weighted Exercise Price per Share | |
|---|
Balance at July 1, 2006 | | | 1,964,988 | | | | | | |
Granted | | �� | 1,115,000 | | $11.53 to $15.95 | | $ | 12.15 | |
Exercised | | | (133,238 | ) | $ 1.56 to $10.93 | | $ | 3.68 | |
Forfeited and expired | | | (71,467 | ) | $ 9.90 to $15.95 | | $ | 15.18 | |
| | | | | | | | | |
Balance at June 30, 2007 | | | 2,875,283 | | | | | | |
Granted | | | 1,455,930 | | $ 2.92 to $ 7.45 | | $ | 4.90 | |
Exercised | | | (20,180 | ) | $ 4.67 to $ 5.59 | | $ | 4.68 | |
Forfeited and expired | | | (511,351 | ) | $ 2.75 to $15.95 | | $ | 11.92 | |
| | | | | | | | | |
Balance at June 28, 2008 | | | 3,799,682 | | | | | | |
Granted | | | 575,881 | | $ 2.49 to $ 9.90 | | $ | 2.91 | |
Exercised | | | (15,000 | ) | $ 1.21 to $ 1.21 | | $ | 1.21 | |
Forfeited and expired | | | (238,551 | ) | $ 2.33 to $15.95 | | $ | 6.88 | |
| | | | | | | | | |
Balance at June 27, 2009 | | | 4,122,012 | | | | | | |
| | | | | | | | | |
2008 Candela Corporation Stock Plan
The Company's 2008 Stock Plan was adopted by the Board of Directors on October 27, 2008 and approved by the stockholders on December 12, 2008. The 2008 Stock Plan permits the Company to make grants of incentive stock options, non-qualified stock options, stock appreciation rights ("SARs"), restricted stock awards and restricted stock units ("RSUs"), which are referred to hereafter individually as a "Stock Right" and collectively as "Stock Rights."
The 2008 Stock Plan authorizes the issuance of up to 1.3 million shares of the Company's Common Stock. The shares of Common Stock subject to Stock Rights may be authorized but unissued shares of Common Stock or shares of Common Stock reacquired by the Company in any manner. Any shares of Common Stock subject to a Stock Right which for any reason expires or terminates unexercised or terminates without the delivery of shares of Common Stock may again be available for future grants under the 2008 Stock Plan. No employee may be granted options and/or SARs with respect to more than 700,000 shares of Common Stock in any one fiscal year. No employee may be granted restricted stock or RSUs having a fair market value in excess of $2,000,000 in any one fiscal year. The number of shares of Common Stock available under the 2008 Stock Plan, the award limits and a grantee's rights with respect to Stock Rights granted, unless otherwise specifically provided in the written instrument relating to the grant of such Stock Right, shall be adjusted upon the occurrence of any of the following: stock dividends, stock splits, consolidation, mergers, recapitalizations, reorganizations, dissolution or liquidation.
There were approximately 1.0 million stock-based SARs granted under this Plan during the fiscal year ended 2009. The SARs granted to employees become exercisable ratably over one to two years. No SARs were granted to directors during the fiscal year ended 2009.
64
Table of Contents
Due to forfeitures, there were approximately 0.9 million outstanding SARs under this Plan at June 27, 2009 with a weighted-average exercise price of $0.41 per share, an aggregate intrinsic value of approximately $0.4 million, and a weighted-average remaining contractual term of 9.56 years.
The Compensation Committee of the Board of Directors administers the 2008 Stock Plan. Subject to the provisions of the 2008 Stock Plan, the Compensation Committee has the authority to select the persons to whom Stock Rights are granted and determine the terms of each Stock Right. Subject to certain limitations set forth in the 2008 Stock Plan, the Board of Directors may appoint other committees to administer the 2008 Stock Plan and also may delegate to any officer of the Company certain authority under the 2008 Stock Plan, including the authority to grant Stock Rights.
Incentive stock options may be granted to employees of the Company. Non-Qualified options, SARs, restricted stock and RSUs may be granted to any employee, officer, Director or consultant of the Company.
The Compensation Committee may grant incentive stock options, nonqualified stock options and SARs in tandem with an option or on a free standing basis. Each option or SAR expires on the date specified by the Compensation Committee, but not more than (i) ten years from the date of grant in the case of options and SARs generally and (ii) five years from the date of grant in the case of incentive stock options granted to an employee possessing more than ten percent (10%) of the total combined voting power of all classes of stock of the Company. Options and SARs are subject to early termination in certain circumstances. The exercise price per share of Common Stock of an option may not be less than "fair market value" (as defined in the 2008 Stock Plan) per share of Common Stock on the date of grant. In the case of an incentive stock option to be granted to an employee possessing more than ten percent (10%) of the total combined voting power of all classes of stock of the Company, the exercise price per share of Common Stock may not be less than one hundred ten percent (110%) of the fair market value per share of Common Stock on the date of grant. The aggregate fair market value (determined on the date of grant) of the shares of Common Stock subject to incentive stock options granted to an employee and which first become exercisable during any calendar year cannot exceed $100,000. The grant price of SARs may not be less than the fair market value per share of Common Stock on the date of grant, provided that the grant price of SARs granted in tandem with an option shall equal the exercise price of the related option. Options and SARs may either be fully exercisable at the time of the grant or may become exercisable in such installments as the Compensation Committee may specify. The Compensation Committee has the right to accelerate the date of exercise of any installment of any option or SAR.
The Compensation Committee may grant restricted stock and RSUs. The instrument relating to the grant of restricted stock and RSUs will specify the restrictions that the Compensation Committee may impose, the number of shares of restricted Common Stock to be granted, and such other provisions as the Compensation Committee may determine. The restrictions may lapse separately or in combination at such times, under such circumstances, in such installments, upon the satisfaction of performance goals or otherwise, as the Compensation Committee determines at the time of the grant of the restricted stock or RSUs. Except as otherwise provided in the applicable instrument relating to the grant of the restricted stock or RSUs, a grantee shall have all of the rights of a stockholder with respect to the restricted stock, and such grantee shall have none of the rights of a stockholder with respect to RSUs until such time as shares of Common Stock are paid in settlement of the RSUs.
The Compensation Committee may take any action as may be necessary to ensure that Stock Rights granted under the 2008 Stock Plan qualify as "performance-based compensation" within the meaning of Section 162(m) of the Code. Any Stock Right granted under the 2008 Stock Plan which is intended to qualify as "performance-based compensation" may be conditioned on the attainment of one or more of the following performance goals: earnings, earnings before interest and taxes, earnings before interest, taxes, depreciation and amortization, earnings per share, economic value created,
65
Table of Contents
market share, net income (before or after taxes), operating income, adjusted net income after capital charge, return on assets, return on capital (based on earnings or cash flow), return on equity, return on investment, revenue, cash flow, operating margin, share price, total stockholder return, total market value, and strategic business criteria, consisting of one or more objectives based on meeting specified market penetration goals, productivity measures, geographic business expansion goals, cost targets, customer satisfaction or employee satisfaction goals, goals relating to merger synergies, management of employment practices and employee benefits, or supervision of litigation or information technology, and goals relating to acquisitions or divestitures of subsidiaries, affiliates or joint ventures.
Incentive stock options are not transferable by the optionee or grantee except by will or by the laws of descent and distribution. Non-Qualified options, SARs, restricted stock and RSUs are transferable to the extent determined by the Compensation Committee and as set forth in the instrument relating to the grant of any such non-qualified options, SARs, restricted stock and RSUs.
Unless otherwise specified in the instrument relating to the grant of an incentive stock options, if an incentive stock option optionee ceases to be employed other than reason of death or disability, all unvested incentive stock options shall terminate and the vested incentive stock options shall terminate on the earlier of three months after the date of termination of employment or the specified expiration date. If an incentive stock option optionee ceases to be employed by reason of death or disability, all unvested incentive stock options shall terminate and the vested incentive stock options shall terminate on the earlier of one hundred and eighty days after the date of termination of employment or the specified expiration date. The Compensation Committee shall specify in the instrument relating to the grant of nonqualified options, SARs, restricted stock and RSUs the treatment of such Stock Rights upon a grantee's termination of employment.
In the event of a merger, sale or consolidation of the Company, or a similar "acquisition," the administrator, or the board of directors of any successor, in its discretion, may either (i) provide for appropriate substitutions or assumptions of outstanding awards, (ii) provide that all option and stock appreciation rights must be exercised, to the extent exercisable, within a specified period following notice at the end of which period all outstanding options or stock appreciation rights will terminate, (iii) provide for the termination of all options and stock appreciation rights in exchange for a cash payment equal to the excess of the fair market value of the shares subject to such award over the exercise price of such award or (iv) terminate all awards, other than options and stock appreciation rights, on such terms and conditions as the administrator or the board of directors of any successor deems appropriate. The 2008 Stock Plan shall automatically expire on October 27, 2018, except as to options outstanding on that date.
The Board of Directors may adopt amendments to the 2008 Stock Plan, subject, in certain cases, to stockholder approval, and may terminate the 2008 Stock Plan at any time (although such action shall not affect Stock Rights previously granted). No Stock Rights may be granted under the 2008 Stock Plan after October 26, 2018.
The following is a summary of stock option/SARs activity under the 2008 Stock Option Plan:
| | | | | | | | | |
| | Number of
Shares | | Option Prices | | Weighted Exercise
Price per Share | |
|---|
Balance at June 28, 2008 | | | — | | | | | | |
Granted | | | 960,451 | | $0.41 to $0.41 | | $ | 0.41 | |
Exercised | | | — | | $ — to $ — | | $ | — | |
Forfeited and expired | | | (20,323 | ) | $0.41 to $0.41 | | $ | 0.41 | |
| | | | | | | | | |
Balance at June 27, 2009 | | | 940,128 | | | | | | |
| | | | | | | | | |
66
Table of Contents
12. Income Taxes
The components of our (benefit from) provision for income before income taxes consist of the following:
| | | | | | | | | | | |
| | For the Year Ended | |
|---|
| | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | |
|---|
(Loss) income from continuing operations before income taxes: | | | | | | | | | | |
| | Domestic | | $ | (19,449 | ) | $ | (15,776 | ) | $ | 8,388 | |
| | Foreign | | | 2,091 | | | 2,897 | | | 2,212 | |
| | | | | | | | |
| | $ | (17,358 | ) | $ | (12,879 | ) | $ | 10,600 | |
| | | | | | | | |
(Benefit from) provision for income taxes: | | | | | | | | | | |
Current provision: | | | | | | | | | | |
| | Federal | | $ | (1,953 | ) | $ | (4,129 | ) | $ | 2,510 | |
| | State | | | (285 | ) | | (190 | ) | | 246 | |
| | Foreign | | | 1,121 | | | 948 | | | 767 | |
| | | | | | | | |
Total current (benefit from) provision for income taxes | | | (1,117 | ) | | (3,371 | ) | | 3,523 | |
| | | | | | | | |
Deferred (benefit from) provision for income taxes | | | | | | | | | | |
| | Federal | | | (4,937 | ) | | (2,565 | ) | | 319 | |
| | State | | | (615 | ) | | (95 | ) | | 28 | |
| | Foreign | | | (100 | ) | | 262 | | | (302 | ) |
| | | | | | | | |
Total deferred (benefit from) provision for income taxes | | | (5,652 | ) | | (2,398 | ) | | 45 | |
| | | | | | | | |
Total (benefit from) provision for income taxes | | $ | (6,769 | ) | $ | (5,769 | ) | $ | 3,568 | |
| | | | | | | | |
The Company recorded a benefit for income taxes from discontinued operations of approximately $8.2 million, $0.7 million, and less than $0.1 million for the years ended 2009, 2008, and 2007, respectively.
67
Table of Contents
The components of the Company's deferred tax assets and liabilities consist of the following:
| | | | | | | | |
(in thousands) | | June 27,
2009 | | June 28,
2008 | |
|---|
Deferred Tax Asset—Current | | | | | | | |
| | Bad debt reserve | | $ | 572 | | $ | 771 | |
| | Inventory valuation reserves | | | 1,587 | | | 1,389 | |
| | Sales returns | | | 387 | | | | |
| | Deferred revenue | | | 259 | | | 404 | |
| | | | | | |
Total Deferred Tax Asset—Current | | $ | 2,805 | | $ | 2,564 | |
| | | | | | |
Deferred Tax Asset—Noncurrent | | | | | | | |
| | Accrued warranty reserve | | $ | 3,030 | | $ | 2,079 | |
| | Restructuring reserve | | | 17 | | | 131 | |
| | Share-based compensation | | | 4,586 | | | 3,447 | |
| | Depreciation & amortization | | | 397 | | | 180 | |
| | Federal and state tax credits | | | 1,311 | | | 549 | |
| | Federal and state net operating losses | | | 10,882 | | | 182 | |
| | Foreign net operating losses | | | 292 | | | 943 | |
| | Other | | | 48 | | | 554 | |
| | | | | | |
| | | 20,565 | | | 8,065 | |
| | Less: Valuation reserve | | | — | | | — | |
| | | | | | |
Total Deferred Tax Asset—Noncurrent | | $ | 20,565 | | $ | 8,065 | |
| | | | | | |
Deferred Tax Liability—Current | | | | | | | |
| | Prepaid expenses | | $ | 138 | | $ | — | |
| | | | | | |
Deferred Tax Liability—Noncurrent | | | | | | | |
| | Depreciation & amortization | | $ | — | | $ | 2,099 | |
| | | | | | |
In connection with the preparation of the financial statements, the Company performed an analysis to ascertain if it was more likely than not that it would be able to utilize its net deferred tax assets of approximately $23.2 million.
As part of that analysis, management considered the following as positive evidence outweighing the negative evidence of the recent pre-tax book losses:
- •
- The net temporary differences resulting in the deferred tax assets and in the deferred tax liabilities are expected to reverse in similar time periods.
- •
- The Company has a consistent taxpaying history.
- •
- The non-recurring nature of significant items contributing to the current year net operating loss.
- •
- Current projections of future taxable income, exclusive of reversing temporary differences, indicate a return to taxable income in a reasonable period of time.
Based upon the aforementioned, we have concluded that the positive evidence outweighs the negative evidence and, thus, that those deferred tax assets not otherwise subject to a valuation allowance are realizable on a "more likely than not" basis.
68
Table of Contents
The reconciliation from the federal statutory tax rate to the effective tax rate is as follows:
| | | | | | | | | | |
| | June 27,
2009 | | June 28,
2008 | | June 30,
2007 | |
|---|
Statutory rate | | | -35 | % | | -35 | % | | 35 | % |
State income taxes | | | -4 | % | | -1 | % | | 2 | % |
Difference between foreign and US tax rates | | | 1 | % | | 0 | % | | -3 | % |
Resolution of income tax audits and other adjustments for uncertain tax positions | | | 0 | % | | -4 | % | | 0 | % |
Benefit from foreign sales credits | | | 0 | % | | 0 | % | | -1 | % |
Other reconciling items | | | -1 | % | | -5 | % | | 1 | % |
| | | | | | | | |
Effective tax rate | | | -39 | % | | -45 | % | | 34 | % |
| | | | | | | | |
At June 27, 2009, the Company had available federal research and development tax credits of approximately $0.5 million which begin to expire in 2027 and other credits of approximately $0.2 million with no expiration. The Company had available state research and development tax credits of approximately $0.6 million which expire at various dates as indicated in the following table:
| | | | | | | |
(in thousands) | | Available | | Expires | |
|---|
Fiscal 2000 | | $ | 221 | | | June 2015 | |
Fiscal 2002 | | | 109 | | | June 2017 | |
Fiscal 2003 | | | 17 | | | June 2018 | |
Fiscal 2005 | | | 25 | | | June 2020 | |
Fiscal 2007 | | | 14 | | | June 2022 | |
various | | | 226 | | | No expiration | |
| | | | | | | |
Total | | $ | 612 | | | | |
| | | | | | | |
At June 27, 2009, the Company had federal net operating losses of $27.5 million which expire in June 2029. For state income tax purposes the Company has net operating losses of $17.1 million which begin to expire in June 2013. Foreign tax losses are as follows:
| | | | | |
(in thousands) | | Available | | Expires |
|---|
Germany | | $ | 614 | | No Expiration |
United Kingdom | | | 154 | | No Expiration |
Spain | | | 186 | | No Expiration |
Australia | | | 71 | | No Expiration |
| | | | | |
Total | | $ | 1,025 | | |
| | | | | |
No amount for U.S. income tax has been provided on undistributed earnings of the Company's foreign subsidiaries because the Company considers the approximately $12.4 million of accumulated foreign earnings to be indefinitely reinvested. In the event of distribution of those earnings in the form of dividends or otherwise, the Company would be subject to both U.S. income taxes, subject to an adjustment, if any, for foreign tax credits and foreign withholding taxes payable to certain foreign tax authorities. Determination of the amount of U.S income tax liability that would be incurred is not practicable because of the complexities associated with this hypothetical calculation; however, unrecognized foreign tax credit carry forwards may be available to reduce some portion of the U.S. tax liability, if any.
The Company adopted the provisions of FIN 48 effective July 1, 2007. FIN 48 addresses the accounting for and disclosure of uncertainty in income tax positions by prescribing a minimum
69
Table of Contents
recognition threshold that a tax position is required to satisfy before being recognized in the financial statements. FIN 48 also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
The cumulative effect of adopting FIN 48 was a decrease in tax reserves of approximately $0.1 million, resulting in an offsetting increase to the July 1, 2007 Retained Earnings balance.
Upon adoption, the gross liability for unrecognized tax benefits at July 1, 2007 was approximately $1.9 million, inclusive of interest and penalties and, if recognized, would favorably affect the Company's effective tax rate. In addition, consistent with the provisions of FIN 48, certain reclassifications were made to the balance sheet, including the reclassification of $0.4 million of income tax liabilities from current to non-current liabilities because payment of cash is not anticipated within one year of the balance sheet date.
The reconciliation of the total amounts of unrecognized tax benefits included in other accrued liabilities at June 27, 2009 and June 28, 2008 is as follows:
| | | | | | | |
(in thousands) | | June 27,
2009 | | June 28,
2008 | |
|---|
Balance, beginning of year | | $ | 1,400 | | $ | 1,853 | |
Additions based on tax positions related to the current year | | |
179 | | |
116 | |
Additions for tax positions of prior years | | | 28 | | | 10 | |
Reductions for positions of prior years | | | (207 | ) | | (554 | ) |
Settlements | | | — | | | (25 | ) |
| | | | | | |
Balance, end of year | | $ | 1,400 | | $ | 1,400 | |
| | | | | | |
The Company's policy to include interest and penalties related to gross unrecognized tax benefits within its provision for income taxes did not change upon the adoption of FIN 48. To the extent accrued interest and penalties do not ultimately become payable, amounts accrued will be reduced in the period that such determination is made and reflected as a reduction of the overall income tax provision. As of June 29, 2009 and June 28, 2008, the Company had approximately $0.1 million of accrued interest and penalties related to uncertain tax positions, respectively. In each of the fiscal years ended June 27, 2009 and June 28, 2008, the Company recognized approximately $0.1 million in interest and penalties, respectively.
The Company files income tax returns in the United States on a federal basis, in many U.S. state jurisdictions, and in certain foreign jurisdictions. The three most significant tax jurisdictions are the U.S., Spain, and Japan. The associated tax filings remain subject to examination by applicable tax authorities for a certain length of time following the tax year to which those filings relate. The 2006 through 2008 fiscal tax years remain subject to examination by the IRS for U.S. federal tax purposes, and the 2005 through 2008 fiscal tax years remain subject to examination by the appropriate governmental agencies for Spanish and Japanese tax purposes, respectively.
We do not anticipate experiencing any significant increases or decreases in our unrecognized tax benefits within the twelve months following June 27, 2009.
13. Segment, Geographic and Major Customer Information
The Company operates principally in one industry segment: the design, manufacture, sale, and service of medical devices and related equipment. The results in this segment have been presented in the Consolidated Statements of Operations for the appropriate periods. The Company has no single customer that represents a significant portion of revenue and accounts receivable during the periods presented.
70
Table of Contents
Geographic data
Geographic information for fiscal 2009, 2008, and 2007 is as follows (in thousands):
| | | | | | | | | | | | |
| | 2009 | | 2008 | | 2007 | |
|---|
Revenue: | | | | | | | | | | |
| | United States | | $ | 70,939 | | $ | 86,356 | | $ | 96,320 | |
| | Intercompany | | | 21,185 | | | 30,517 | | | 26,446 | |
| | | | | | | | |
| | | 92,124 | | | 116,873 | | | 122,766 | |
| | Japan | | | 15,410 | | | 17,598 | | | 20,318 | |
| | Spain | | | 14,234 | | | 25,583 | | | 21,323 | |
| | Germany | | | 3,068 | | | 3,893 | | | 3,162 | |
| | France | | | 6,364 | | | 6,606 | | | 4,488 | |
| | Italy | | | 1,704 | | | 1,691 | | | 2,033 | |
| | UK | | | 1,389 | | | 1,658 | | | — | |
| | Australia | | | 3,471 | | | 3,231 | | | 133 | |
| | | | | | | | |
| | | 137,765 | | | 177,133 | | | 174,223 | |
| | Elimination | | | (21,185 | ) | | (30,516 | ) | | (26,446 | ) |
| | | | | | | | |
| | | Consolidated total | | $ | 116,580 | | $ | 146,617 | | $ | 147,777 | |
| | | | | | | | |
Income (loss) from operations: | | | | | | | | | | |
| | United States | | $ | (20,511 | ) | $ | (15,350 | ) | $ | 3,559 | |
| | Europe | | | 1,373 | | | 1,324 | | | 991 | |
| | Japan | | | 986 | | | 1,085 | | | 1,245 | |
| | Australia | | | 176 | | | 170 | | | (19 | ) |
| | Elimination | | | 468 | | | 105 | | | (1,620 | ) |
| | | | | | | | |
| | | Consolidated total | | $ | (17,508 | ) | $ | (12,666 | ) | $ | 4,156 | |
| | | | | | | | |
Geographic identification of long-lived assets: | | | | | | | | | | |
| | United States | | $ | 2,840 | | $ | 3,322 | | $ | 3,002 | |
| | All others | | | 629 | | | 414 | | | 333 | |
| | | | | | | | |
| | | Consolidated total | | $ | 3,469 | | $ | 3,736 | | $ | 3,335 | |
| | | | | | | | |
United States revenue includes export sales to unaffiliated companies located principally in Europe, the Asia-Pacific region and Latin America, which approximated $31.9 million, $31.3 million, and $31.9 million in fiscal years 2009, 2008, 2007, respectively. The results of our Israeli subsidiary are reported as part of discontinued operations (Note 15).
14. Employee Benefit Plans
The Company offers a 401(k) Plan which allows eligible United States employees to make tax-deferred contributions, a portion of which are matched by the Company. The Company may make discretionary matching contributions to the Plan in an amount determined by its Board of Directors. Company contributions vest ratably with three years of employment and amounted to $0.2 million, $0.4 million, and $0.5 million for the fiscal years ended 2009, 2008, 2007, respectively.
71
Table of Contents
15. Discontinued Operations
Candela Skin Care Centers ("CSCC")
At June 27, 2009, CSCC had a net liability of approximately $0.8 million consisting primarily of deferred gift certificate revenue. We also recorded a gain during the year ended 2009 of approximately $0.2 million, net of tax, related to the settlement of the facility closure accrual for less than the Company's original estimate. The plan to close the only remaining skin care center was initiated by management during the fiscal quarter ended September, 2003.
Inolase (2002) Ltd.
In the fiscal quarter ended December 27, 2008, in connection with the Company's plan to focus on its core products, management initiated a plan to close its Inolase (2002) Ltd. ("Inolase") subsidiary. The closure was accounted for as a discontinued operation in accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets ("SFAS 144"), and SFAS No. 146,Accounting for Costs Associated with Exit or Disposal Activities ("SFAS 146").
In determining the amount of the facilities closure accrual, we are required to estimate such factors as future vacancy rates. These estimates are reviewed quarterly and may result in revisions to established facility reserves on an ongoing basis.
The Company determined that there was no future cash value inherent to its Inolase subsidiary and, accordingly, shut down this reporting unit as its fair value was zero. As part of that process the Company recognized a 100% impairment loss associated with the goodwill, other intangible assets, inventory, and fixed assets. The components of the loss recognized during the year ended 2009 are as follows:
| | | | |
| | In thousands | |
|---|
Goodwill | | $ | 10,596 | |
Patents & Developed Technology | | | 5,162 | |
Inventory | | | 1,681 | |
Fixed Assets, net | | | 237 | |
| | | | |
| | $ | 17,676 | |
| | | | |
In accordance with SFAS 146, the Company also recorded restructuring charges of approximately $0.4 million associated with future occupancy costs, minimum termination benefits, and professional service fees. In determining the amount of the accrual for future occupancy costs, we are required to estimate such factors as future vacancy rates. These estimates are reviewed quarterly and may result in revisions to facility reserves in future periods.
A summary of the operating results of the discontinued operations are as follows:
| | | | | | | |
(in thousands) | | 2009 | | 2008 | |
|---|
Revenue | | $ | 438 | | $ | 1,601 | |
| | | | | | |
loss from discontinued operations | | | (1,106 | ) | | (2,681 | ) |
Benefit from Income tax from discontinued operations | | | (470 | ) | | (720 | ) |
Net loss on disposal of discontinued operations, net of tax benefit of $7,687 | | | (11,057 | ) | | — | |
| | | | | | |
Loss from discontinued operations, net | | $ | (11,693 | ) | $ | (1,961 | ) |
| | | | | | |
72
Table of Contents
During the six-month period since execution of the plan, for the year ended June 27, 2009, we recognized certain residual revenues, incurred ongoing operating expenses, and made required adjustments to income taxes in the ordinary course of business.
As of June 27, 2009, the Company had no assets or liabilities held in connection with the disposal of Inolase other than the accrual for future occupancy costs and residual professional fees of approximately $0.1 million.
16. Legal Proceedings
The Company is currently involved in two litigation matters with Palomar Medical Technologies, Inc. ("Palomar").
- •
- Palomar asserts that it is the exclusive licensee from The General Hospital Corporation ("Mass. General") of U.S. Patent No. 5,735,844 (the "'844 Patent") and U.S. Patent No. 5,595,568, which is related to the '844 Patent (the "'568 Patent"). On August 9, 2006, Palomar filed suit against the Company in the United States District Court for the District of Massachusetts, asserting willful infringement by the Company of the '844 Patent. Palomar seeks compensatory and treble damages, as well as attorneys' fees and injunctive relief. In November 2006, the Company answered the complaint by denying Palomar's allegations and asserting a variety of affirmative defenses and counterclaims against Palomar. This response included a counterclaim by the Company against Palomar seeking a declaratory judgment that the '568 Patent is either invalid, or products manufactured by the Company do not infringe the '568 Patent, or both. In November 2006, Palomar filed an answer denying the Company's counterclaim. In February 2007, Palomar moved to amend its Complaint to add The General Hospital Corporation as a party, to add a claim for infringement by Candela of the '568 Patent, and to allege that additional Candela products infringe the '844 Patent. Palomar's motion to amend its Complaint was granted in August 2007. In February 2007, Candela moved to amend its Answer and Counterclaims to add allegations of inequitable conduct, double-patenting and violation of Mass. Gen. Laws Ch. 93A by Palomar. The Company's motion to amend its Answer and Counterclaims was granted in March 2007. Palomar filed a general denial response to Candela's Amended Answer and Counterclaim in March 2007. In August 2007, the parties had a Markman hearing before the U.S. District Court Judge presiding over the above-described legal proceeding. In a Markman hearing, the Court interprets the definitions of the disputed claim terms of a patent. On October 31, 2008, the Company requested a stay of the case with the Court, which was granted on November 17, 2008. The Company did so based upon a third party's re-examination request that was granted by the United States Patent and Trademark Office on August 29, 2008. The re-examination is with respect to both the '568 and '844 Patents, including many of the Claims at issue in this case. In November 2008, the Court stayed the case until the United States Patent and Trademark Office rules on the re-examination. On December 9, 2008, Candela filed requests for re-examination of the '568 and '844 Patents with the United States Patent & Trademark Office asserting that all claims currently in dispute with Palomar be held invalid. On January 7, 2009, the United States Patent and Trademark Office granted the Company's re-examination request of the '844 Patent and the '568 Patent. The United States Patent and Trademark Office stated that there are substantial new questions of patentability for all claims asserted against the Company by Palomar. On June 5, 2009, the PTO issued an office action confirming as patentable the majority of the claims of the '844 patent in re-examination as patentable. On June 19, 2009, Palomar moved to lift the stay based on responses by the PTO in the '844 re-examination proceeding, which the Company opposed on July 13, 2009. In July 2009, the PTO issued a notice of intent to issue a re-examination certificate for the '844 patent. In August 2009, the PTO issued a notice of intent to issue a
73
Table of Contents
On August 10, 2006, the Company filed suit against Palomar in the United States District Court for the District of Massachusetts, asserting willful infringement by Palomar of U.S. Patent Nos. 6,659,999, 5,312,395 (the "395 Patent"), and 6,743,222 (the "'222 Patent"). The Company seeks compensatory and treble damages for the patent infringement, as well as attorneys' fees and an injunction against Palomar to prevent Palomar's continuing infringement. The Company served its complaint on Palomar in August 2006. In October 2006, the Company amended the Company's suit against Palomar by removing from the suit allegations that Palomar infringes Patent 6,659,999. In October 2006, Palomar answered the Company's amended complaint by denying the Company's allegations and asserting an affirmative defense of inequitable conduct with respect to the '395 Patent. In addition, Palomar filed a demand for a declaratory judgment seeking a judicial determination that Palomar products either do not infringe the '395 Patent and '222 Patent or that such patents are invalid. In November 2006, the Company answered the counterclaim by denying Palomar's allegations. In February 2008, Palomar filed a request for re-examination of the Company's '222 Patent with the United States Patent and Trademark Office ("PTO"). In March 2008, the PTO granted re-examination. In June 2008, the Court stayed the case until the PTO rules on the re-examination. To avoid the necessity of repeated status conferences, the Company requested administrative closure of the case on November 26, 2008, subject to reopening when the '222 Patent emerged from re-examination. The Court granted this request on December 1, 2008.
While the Company intends to vigorously contest Palomar's allegations, and to pursue its own claims against Palomar, each lawsuit is inherently uncertain and unpredictable as to its ultimate outcome. An adverse outcome in Palomar's suit against the Company would materially hurt the Company's business, financial condition, results of operations and cash flows.
- •
- On September 15, and 24, 2009, after the announcement of the Company's proposed merger with Syneron, two putative class action lawsuits were filed in the Superior Courts of Massachusetts against Candela and the members of Candela's Board of Directors, among others. The plaintiffs in the two actions purport to sue on behalf of a class of Candela stockholders, and allege that members of the board breached fiduciary duties owed to Candela stockholders through, among other things, the failure to maximize shareholder value and the use of unfair process in connection with the proposed merger between Candela and Syneron, and that Candela, with others, aided and abetted the purported breaches of fiduciary duties. The complaints seek, among other relief: rescission of the merger agreement entered into on September 8, 2009; an injunction against the merger; an order requiring disclosure of all material information necessary for shareholders to make an informed vote on the proposed merger; and an accounting for damages caused by defendants as well as an award of all costs and disbursements of the actions, including reasonable attorneys' and expenses. Candela denies the allegations in the complaints and intends to defend these lawsuits vigorously.
On February 19, 2008, Cardiofocus, Inc. ("Cardiofocus") filed suit against the Company and eight other companies in the United States District Court for the District of Massachusetts, asserting willful infringement by the Company of U.S. Patents 6,457, 780, 6,159,203 and 5,843,073. Cardiofocus seeks compensatory and treble damages, as well as attorneys' fees and injunctive relief. On April 21, 2008, the Company answered Cardiofocus' complaint and asserted a variety of counterclaims against Cardiofocus. The Company intends to vigorously defend against the lawsuit. Based upon Candela's request, on July 23, 2008, the United States Patent & Trademark Office ("PTO") agreed to re-examine the validity of the Cardiofocus patents. On October 14, 2008, the Court agreed to stay this matter for a minimum of one year, up to a maximum of two years, but no later than the PTO's decision with respect to the re-examination proceedings.
74
Table of Contents
While we believe that the likelihood of loss in each of the disclosed proceedings is reasonably possible, the possibility of loss or range of loss for each matter is not estimable, except with regard to Palomar. In accordance with SFAS 5,Accounting for Contingencies, as of September 30, 2009, we estimate the range of the loss contingency associated with the Palomar litigation claims to be in the range of $0 to $46 million. As we have assessed that contingent losses related to these proceedings are only reasonably possible, pursuant to SFAS 5, an accrual has not been recorded for any of these loss contingencies.
From time to time, the Company is party to various legal proceedings incidental to its business. The Company believes that none of the legal proceedings, other than the lawsuit initiated by Palomar, that are presently pending, if adversely decided against us, will have a material adverse effect upon the Company's financial position, results of operations, or liquidity. While we believe that the likelihood of loss in each of the disclosed proceedings is reasonably possible, the possibility of loss or range of loss for each matter is not estimable.
17. Derivative Instruments and Hedging Activity
The Company uses derivative instruments to reduce its exposure to fluctuations in foreign exchange rates by creating offsetting positions through the use of foreign currency forward contracts. Changes to the fair value of derivative contracts that do not qualify for hedge accounting are reported in other income (expense) in the period of the change. For derivatives that qualify for hedge accounting, the Company recognizes the instruments as either assets or liabilities in the statement of financial position and measures those instruments at fair value. Changes in the fair value of the derivatives are recorded in shareholders' equity as a component of other comprehensive income along with an offsetting adjustment against the basis of the derivative instrument itself.
We had no derivatives that qualified for hedge accounting during the fiscal year ended 2009.
During the fiscal year ended 2009, we recognized the following gains (losses) on derivatives and their related hedged items which were solely comprised of inter-company payables and receivables in the normal course of business.
| | | | |
(in thousands) | | 2009 | |
|---|
Other income (expense)—derivatives | | $ | (692 | ) |
Other income (expense)—Hedged items | | | 481 | |
| | | | |
Total | | $ | (211 | ) |
| | | | |
We did not enter into any other derivative or hedge contracts for any other risks during the fiscal year 2009. We did not have any outstanding contracts at June 27, 2009.
18. Other Income
During the fiscal year ended June 30, 2007, the Company recognized a $3.5 million gain on the exchange of common stock of Solx Inc. for cash and common stock of OccuLogix, Inc. (NasdaqGM: OCCX). The gain was a result of the acquisition of Solx Inc., a privately-held company, by OccuLogix, Inc., a publicly traded company. The Company held 19.99% of the outstanding common stock of Solx Inc. on an as-converted basis, prior to the merger with an associated book value of $0.
As a result of the acquisition of Solx, Inc., Candela received approximately $1.0 million in cash at the time of closing, future cash consideration of approximately $2.5 million, and approximately 1.3 million shares of common stock in OccuLogix, Inc.
The value of the shares of OccuLogix, Inc. was included in Candela's marketable securities at June 27, 2007 as they were considered available-for-sale securities in accordance with SFAS 115. The
75
Table of Contents
temporary differences between cost and fair value were presented in the consolidated financial statements in accumulated other comprehensive income within stockholders' equity, net of any related tax effect. At December 29, 2007, in the opinion of management, the value of the investment in the common shares of OccuLogix sustained an other-than-temporary impairment due to its consistent decline in value and public notification that the security was at risk of being de-listed from the NASDQ exchange. As such, the Company recognized an associated $2.5 million loss.
19. Quarterly Results of Operations (unaudited)
| | | | | | | | | | | | | | |
| | Quarter Ended | |
|---|
2009 (in thousands, except per-share data) | | First | | Second(2) | | Third | | Fourth(3) | |
|---|
Revenue | | $ | 26,665 | | $ | 28,757 | | $ | 29,765 | | $ | 31,393 | |
Gross Profit | | | 10,472 | | | 10,159 | | | 11,933 | | | 11,997 | |
Income (loss) from continuing operations | | | (6,606 | ) | | (9,262 | ) | | (1,360 | ) | | (131 | ) |
Net income (loss) | | | (4,009 | ) | | (24,945 | ) | | (465 | ) | | 7,137 | |
Earnings (loss) per common share: | | | | | | | | | | | | | |
| | Basic | | $ | (0.18 | ) | $ | (1.10 | ) | $ | (0.02 | ) | $ | 0.32 | |
| | | | | | | | | | |
| | Diluted | | $ | (0.18 | ) | $ | (1.10 | ) | $ | (0.02 | ) | $ | 0.31 | |
| | | | | | | | | | |
| | | | | | | | | | | | | | |
| | Quarter Ended | |
|---|
2008 (in thousands, except per-share data) | | First | | Second(1) | | Third | | Fourth | |
|---|
Revenue | | $ | 35,102 | | $ | 35,708 | | $ | 38,400 | | $ | 37,407 | |
Gross Profit | | | 17,060 | | | 16,503 | | | 16,789 | | | 15,161 | |
Income (loss) from continuing operations | | | 1,228 | | | (6,015 | ) | | (3,212 | ) | | (4,880 | ) |
Net income (loss) | | | 187 | | | (4,550 | ) | | (2,056 | ) | | (2,652 | ) |
Earnings (loss) per common share: | | | | | | | | | | | | | |
| | Basic | | $ | 0.01 | | $ | (0.20 | ) | $ | (0.09 | ) | $ | (0.12 | ) |
| | | | | | | | | | |
| | Diluted | | $ | 0.01 | | $ | (0.20 | ) | $ | (0.09 | ) | $ | (0.12 | ) |
| | | | | | | | | | |
- (1)
- Includes $2.5 million loss on the other-than-temporary impairment of its investment in OccuLogix, Inc. (Note 18).
- (2)
- Includes $18.4 million charge to discontinued operations relative to the closing of its Israeli subsidiary (Note 15).
- (3)
- Includes recognition of approximately $6.4 million in tax benefits relative to the closing of the Israeli subsidiary (Note 15).
76
Table of Contents
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
Not Applicable
Item 9A(T). Controls and Procedures.
- (a)
- EVALUATION OF DISCLOSURE CONTROLS AND PROCEDURES
Our principal executive officer and principal financial officer have evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended) as of June 27, 2009, the end of the period covered by this Annual Report on Form 10-K. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Management has designed such controls and procedures to provide reasonable assurance of achieving their objectives. Based on the evaluation, our principal executive officer and principal financial officer have concluded that, as of the end of the period covered by this report, the Company's disclosure controls and procedures were effective to provide reasonable assurance of achieving their objective.
- (b)
- MANAGEMENT'S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Under the supervision and with the participation of management, including our Chief Executive Officer and Principal Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of June 27, 2009, based on the criteria established in "Internal Control—Integrated Framework" issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO").
Based on our assessment, management determined that the Company's internal controls over financial reporting as of June 27, 2009 were effective.
This annual report does not include an attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the Company's independent registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management's report in this annual report.
- (c)
- CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
There was no change in our internal control over financial reporting (as defined under Rule 13a-15(f) under the Exchange Act) during our fourth quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| | |
/s/ GERARD PUORRO
Gerard Puorro
President and Chief Executive Officer | | /s/ ROBERT E. QUINN
Robert E. Quinn
Senior Vice President of Finance and
Chief Financial Officer |
October 1, 2009 |
|
|
Item 9B. Other Information
None.
77
Table of Contents
Part III
Notwithstanding anything to the contrary contained herein, in no event are the sections "Audit Committee Report" to be incorporated by reference herein from the Company's definitive proxy statement for the Company's 2009 annual meeting of stockholders.
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item concerning the directors and executive officers of the Company is incorporated by reference herein from the information contained in the sections entitled "Election of Directors", "Occupations of Directors" and "Executive Officers" in our definitive proxy statement (the "Definitive Proxy Statement") for the 2009 annual meeting of stockholders to be filed with the Securities and Exchange Commission within 120 days after the end of Candela's fiscal year ended June 27, 2009.
The information required by this item concerning compliance with Section 16(a) of the Exchange Act required under this item is incorporated herein by reference from the information contained in the section entitled "Section 16(a) Beneficial Ownership Reporting Compliance" in the Definitive Proxy Statement.
The information required by this item concerning the Company's Code of Ethics that applies to all directors, officers, and employees of the Company is incorporated by reference from the information contained in the section entitled "Code of Business Conduct and Ethics" in the Definitive Proxy Statement.
The information required by this item concerning the Company's audit committee is incorporated by reference from the information contained in the section entitled "Meetings of the Board of Directors and Committees Thereof" in the Definitive Proxy Statement.
Item 11. Executive Compensation.
The information required by this item concerning executive compensation is incorporated herein by reference from the information contained in the section entitled "Compensation and Other Information Concerning Directors and Officers" in the Definitive Proxy Statement.
The information required by this item concerning securities authorized for issuance under the equity compensation plans is incorporated by reference from the information contained in the section entitled "Equity Compensation Plan Information" in the definitive Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item concerning security ownership of certain beneficial owners and management is incorporated herein by reference from the information contained in the section entitled "Security Ownership of Certain Beneficial Owners and Management" in the Definitive Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item concerning certain relationships and related transactions is incorporated herein by reference from the information contained in the section entitled "Certain Relationships and Related Transactions" in the Definitive Proxy Statement.
The information required concerning director independence is incorporated by reference from the information contained in the section entitled "Meetings of the Board of Directors and Committees Thereof" in the Definitive Proxy Statement.
78
Table of Contents
Item 14. Principal Accountant Fees and Services.
The information required by this item concerning principal accountant fees and services is incorporated herein by reference from the information contained in the section entitled "Accounting Fees" in the Definitive Proxy Statement.
79
Table of Contents
SCHEDULE II
CANDELA CORPORATION
Valuation and Qualifying Accounts
For the years ended June 27, 2009, June 28, 2008, and July 30, 2007
| | | | | | | | | | | | | | |
(In thousands) | | Balance at
Beginning
of Period | | Additions
Charged to
Income | | Deductions
from
Reserves | | Balance at
End of
Period | |
|---|
Reserves deducted from assets to which they apply: | | | | | | | | | | | | | |
Allowance for doubtful accounts: | | | | | | | | | | | | | |
| | Year ended June 27, 2009 | | $ | 2,322 | | $ | 3,506 | | $ | 3,000 | | $ | 2,828 | |
| | Year ended June 28, 2008 | | $ | 1,412 | | $ | 3,053 | | $ | 2,143 | | $ | 2,322 | |
| | Year ended June 30, 2007 | | | 1,830 | | | 989 | | | 1,407 | | | 1,412 | |
Reserve for inventory | | | | | | | | | | | | | |
| | Year ended June 27, 2009 | | $ | 2,402 | | $ | 5,317 | | $ | 5,285 | | $ | 2,434 | |
| | Year ended June 28, 2008 | | $ | 2,088 | | $ | 3,225 | | $ | 2,911 | | $ | 2,402 | |
| | Year ended June 30, 2007 | | | 2,487 | | | 2,602 | | | 3,001 | | | 2,088 | |
Reserve for discontinued operations | | | | | | | | | | | | | |
| | Year ended June 27, 2009 | | $ | 361 | | $ | 514 | | $ | 722 | | $ | 153 | |
| | Year ended June 28, 2008 | | $ | 447 | | $ | — | | $ | 86 | | $ | 361 | |
| | Year ended June 30, 2007 | | | 447 | | | — | | | — | | | 447 | |
80
Table of Contents
Part IV
Item 15. Exhibits and Financial Statement Schedules.
- (a)
- The following Consolidated Financial Statements, Notes thereto, and Report of Independent Registered Public Accounting Firm are set forth under Item 8:
- (1)
- Consolidated Financial Statements:
| | | | |
Report of Registered Independent Public Accounting Firm | | | | |
Consolidated Balance Sheets—June 27, 2009 and June 28, 2008 | | | | |
Consolidated Statements of Income and Comprehensive Income
Years ended June 27, 2009, June 28, 2008 and June 30, 2007 | | | | |
Consolidated Statements of Stockholders' Equity—Years ended June 27, 2009, June 28, 2008, and June 30, 2007 | | | | |
Consolidated Statements of Cash Flows—Years ended June 27, 2009, June 28, 2008, and June 30, 2007 | | | | |
Notes to Consolidated Financial Statements | | | | |
- (2)
- Consolidated Financial Statement Schedules:
| | | | |
Schedule II—Valuation and Qualifying Accounts | | | | |
All other financial statements and schedules not listed have been omitted since the required information is included in the consolidated financial statements or the notes thereto, or is not applicable, material, or required.
(3) Exhibits: Except as otherwise noted, the following documents are incorporated by reference from the Company's Registration Statement on Form S-1 (File Number 333-78339) or filed herewith:
| | |
| 3.1(5) | | Certificate of Incorporation, as amended |
| 3.2(4) | | By-laws of the Company, as amended and restated |
| 10.1(1) | | 1990 Employee Stock Purchase Plan |
| 10.2(12) | | Amendment Number One to the Candela Corporation Employee Stock Purchase Plan |
| 10.3(5) | | 1998 Amended and Restated Stock Plan |
| 10.4(10) | | 1998 Second Amended and Restated Stock Plan |
| 10.5(13) | | 1998 Third Amended and Restated Stock Plan |
| 10.6(12) | | 2008 Stock Plan |
| 10.7(12) | | Form of Candela Corporation 2008 Stock Plan Notice of Stock Appreciation Right Grant |
| 10.8(2) | | Lease for premises at 526 Boston Post Road, Wayland, Massachusetts. |
| 10.9(2) | | Lease for premises at 530 Boston Post Road, Wayland, Massachusetts. |
| 10.10(3) | | Letter Agreement between the Company and Fleet Bank dated February 13, 1997. |
| 10.11(3) | | Amendment to Letter Agreement between the Company and Fleet Bank dated December 15, 1998. |
| 10.12(6)* | | Amended and Restated License Agreement between the Company and The Regents of the University of California for Dynamic Skin Cooling Method and Apparatus effective as of August 11, 2000. |
| 10.13(7)* | | Settlement Agreement dated August 11, 2000 by and among the Company, the Regents of the University of California, and Cool Touch, Inc. |
| 10.14(4) | | Form of Note delivered to the Company in the aggregate principal amount of $3,700,000 to Massachusetts Capital Resource Company, William D. Witter and Michael D. Witter. |
81
Table of Contents
| | |
| 10.15(4) | | Form of Common Stock Purchase Warrant to purchase an aggregate of 370,000 shares of the Company's Common Stock delivered to Massachusetts Capital Resource Company, William D. Witter and Michael D. Witter. |
| 10.16(10) | | Amended and Restated Employment Agreement dated as of April 5, 2007, between Candela Corporation and Gerard E. Puorro |
| 10.17(11) | | Executive Employment, Non-competition and Non-disclosure Agreement |
| 10.18(11) | | Senior Officer Executive Retention Agreement |
| 10.19(11) | | Amended and Restated Employment Agreement between Candela Corporation and Gerard E. Puorro |
| 10.20(11) | | Executive Retention Agreement with Gerard E. Puorro |
| 21.1** | | Subsidiaries of the Company |
| 23.1** | | Consent of BDO Seidman, LLP (Independent Registered Public Accounting Firm) |
| 31.1** | | Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer |
| 31.2** | | Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer |
| 32.1** | | Section 1350 Certification of Chief Executive Officer |
| 32.2** | | Section 1350 Certification of Chief Financial Officer |
| 99.1(8) | | McKesson Medical-Surgical Inc. Distribution Agreement |
| 99.2(9) | | First Amendment to the Amended and Restated License Agreement by and between The Regents of the University of California and Candela Corporation |
- *
- Portions of the exhibit have been omitted and filed separately with the SEC in reliance upon the Company's request for confidential treatment pursuant to Rule 24b-2.
- **
- Filed herewith
- (1)
- Previously filed as an exhibit to the Company's Annual Report on Form 10-K for the fiscal year ended June 30, 1990 (Commission file number 000-14742), and incorporated herein by reference.
- (2)
- Previously filed as an exhibit to the Company's Annual Report on Form 10-K for the fiscal year ended July 3, 1993 (Commission file number 000-14742) and incorporated herein by reference.
- (3)
- Previously filed as an S1 filed on May 12, 1999 (Commission file number 333-78339) and incorporated herein for reference.
- (4)
- Previously filed as an exhibit to the Company's Amended and Restated Annual Report on Form 10-K for the fiscal year ended June 27, 1998 (Commission file number 000-14742) and incorporated herein by reference.
- (5)
- Previously filed as Appendix A to the Company's Schedule 14A, filed December 28, 2002, and incorporated herein by reference.
- (6)
- Previously filed as an exhibit to Form 10-Q for the quarter ended March 31, 2001 (Commission file number 000-14742) and incorporated herein by reference.
- (7)
- Previously filed as an exhibit to Form 10-K for the fiscal year ended July 1, 2000 (Commission file number 000-14742) and incorporated by reference.
- (8)
- Previously filed as an exhibit to Form 10-K for the fiscal year ended July 2, 2005 (Commission file number 000-14742) and incorporated by reference
- (9)
- Previously filed as an exhibit to Form 10-Q for the fiscal quarter ended December 31, 2005 (Commission file number 000-14742) and incorporated by reference
(10)- Previously filed as an exhibit to Form 10-Q for the fiscal quarter ended March 31, 2007 (Commission file number 000-14742) and incorporated by reference
82
Table of Contents
(11)- Previously filed as an exhibit to Form 10-Q for the fiscal quarter ended December 29, 2007 (Commission file number 000-14742) and incorporated by reference
(12)- Previously filed as an exhibit to Form 10-Q for the fiscal quarter ended March 29, 2008 (Commission file number 000-14742) and incorporated by reference
(13)- Previously filed as an exhibit to the Company's Annual Report on Form 10-K for the fiscal year ended June 28, 2008 (Commission file number 000-14742) and incorporated by reference
- (c)
- The Company hereby files, as part of this Form 10-K, the exhibits listed in Item 15(a)(3) above, other than exhibits 32.1 and 32.2, which the Company hereby furnishes.
- (d)
- The Company hereby files, as part of this Form 10-K, the consolidated financial Statement schedules listed in Item 15(a)(2) above.
83
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| | CANDELA CORPORATION |
| | By: | | /s/ GERARD E. PUORRO
Gerard E. Puorro,
President, Chief Executive Officer and Director
October 1, 2009 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
|---|
|
|
|
|
|
/s/ GERARD E. PUORRO
Gerard E. Puorro | | President, Chief Executive
Officer, and Director
(Principal Executive Officer) | | October 1, 2009 |
/s/ ROBERT E. QUINN
Robert E. Quinn | | Senior Vice President, Finance,
and Chief Financial Officer
(Principal Financial Officer) | | October 1, 2009 |
/s/ KENNETH D. ROBERTS
Kenneth D. Roberts | | Chairman of the Board of Directors | | October 1, 2009 |
/s/ NANCY NAGER
Nancy Nager | | Director | | October 1, 2009 |
/s/ DOUGLAS W. SCOTT
Douglas W. Scott | | Director | | October 1, 2009 |
/s/ BEN BAILEY, III
Ben Bailey, III | | Director | | October 1, 2009 |
/s/ GEORGE ABE
George Abe | | Director | | October 1, 2009 |
84
Table of Contents
EXHIBIT INDEX
| | |
| 21.1 | | Subsidiaries of the Company |
| 23.1 | | Consent of BDO Seidman, LLP, (Independent Registered Public Accounting Firm) |
| 31.1 | | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | | Certification pursuant to 18 U.S.C. Section 1350 |
| 32.2 | | Certification pursuant to 18 U.S.C. Section 1350 |
