UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2013
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-12019
QUAKER CHEMICAL CORPORATION
(Exact name of Registrant as specified in its charter)
| A Pennsylvania Corporation | No. 23-0993790 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
One Quaker Park, 901 E. Hector Street, Conshohocken, Pennsylvania | 19428-2380 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (610) 832-4000
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each Exchange on which registered |
| Common Stock, $1.00 par value | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files) Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer x | Accelerated filer ¨ | |
Non-accelerated filer ¨ (Do not check if smaller reporting company) | Smaller reporting company ¨ | |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
State the aggregate market value of voting and non-voting common equity held by non-affiliates of the Registrant. (The aggregate market value is computed by reference to the last reported sale on the New York Stock Exchange on June 30, 2013): $805,019,401
Indicate the number of shares outstanding of each of the Registrant’s classes of common stock as of the latest practicable date: 13,199,043 shares of Common Stock, $1.00 Par Value, as of January 31, 2014.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement relating to the Annual Meeting of Shareholders to be held on May 7, 2014 are incorporated by reference into Part III.
PART I
As used in this Report, the terms “Quaker,” the “Company,” “we” and “our” refer to Quaker Chemical Corporation, its subsidiaries, and associated companies, unless the context otherwise requires.
Item 1. Business.
General Description
Quaker develops, produces, and markets a broad range of formulated chemical specialty products and offers chemical management services (“CMS”) for various heavy industrial and manufacturing applications in a global portfolio throughout its four regions: the North America region, the Europe, Middle East and Africa (“EMEA”) region, the Asia/Pacific region and the South America region. The principal products and services in Quaker’s global portfolio include: (i) rolling lubricants (used by manufacturers of steel in the hot and cold rolling of steel and by manufacturers of aluminum in the hot rolling of aluminum); (ii) corrosion preventives (used by steel and metalworking customers to protect metal during manufacture, storage, and shipment); (iii) metal finishing compounds (used to prepare metal surfaces for special treatments such as galvanizing and tin plating and to prepare metal for further processing); (iv) machining and grinding compounds (used by metalworking customers in cutting, shaping, and grinding metal parts which require special treatment to enable them to tolerate the manufacturing process, achieve closer tolerance, and improve tool life); (v) forming compounds (used to facilitate the drawing and extrusion of metal products); (vi) hydraulic fluids (used by steel, metalworking, and other customers to operate hydraulically activated equipment); (vii) chemical milling maskants for the aerospace industry and temporary and permanent coatings for metal and concrete products; (viii) construction products, such as flexible sealants and protective coatings, for various applications; (ix) specialty greases; (x) die casting lubricants; (xi) technology for the removal of hydrogen sulfide in various industrial applications; and (xii) programs to provide chemical management services. Individual product lines representing more than 10% of consolidated revenues for any of the past three years are as follows:
| 2013 | 2012 | 2011 | |||||||||
| Rolling Lubricants | 20.7 | % | 20.7 | % | 22.0 | % | |||||
| Machining and grinding compounds | 17.7 | % | 17.6 | % | 18.8 | % | |||||
| Hydraulic fluids | 12.9 | % | 13.5 | % | 12.9 | % | |||||
| Corrosion preventives | 12.5 | % | 12.4 | % | 11.5 | % |
A substantial portion of Quaker’s sales worldwide are made directly through its own employees and its CMS programs with the balance being handled through distributors and agents. Quaker employees visit the plants of customers regularly and, through training and experience, identify production needs which can be resolved or alleviated either by adapting Quaker’s existing products or by applying new formulations developed in Quaker’s laboratories. Quaker relies less on the use of advertising, and more heavily upon its reputation in the markets which it serves. Generally, separate manufacturing facilities of a single customer are served by different personnel. As part of the Company’s chemical management services, certain third-party product sales to customers are managed by the Company. Where the Company acts as principal, revenues are recognized on a gross reporting basis at the selling price negotiated with its customers. Where the Company acts as an agent, such revenue is recorded using net reporting as service revenues at the amount of the administrative fee earned by the Company for ordering the goods. Third-party products transferred under arrangements resulting in net reporting totaled $41.6 million, $39.3 million and $50.9 million for 2013, 2012 and 2011, respectively. The Company recognizes revenue in accordance with the terms of the underlying agreements, when title and risk of loss have been transferred, when collectability is reasonably assured, and when pricing is fixed or determinable. This generally occurs for product sales when products are shipped to customers or, for consignment-type arrangements, upon usage by the customer and, for services, when they are performed. License fees and royalties are included in other income when recognized in accordance with agreed-upon terms, when performance obligations are satisfied, when the amount is fixed or determinable, and when collectability is reasonably assured.
In 2013, the Company acquired a chemical milling maskants distribution network for net consideration of approximately $0.7 million and a business that primarily related to tin plating for net consideration of approximately $1.8 million. In July 2012, the Company acquired NP Coil Dexter Industries, S.r.l., for approximately $2.7 million. NP Coil Dexter is a European manufacturer and supplier of metal surface treatment products.
Competition
The chemical specialty industry comprises a number of companies of similar size as well as companies larger and smaller than Quaker. Quaker cannot readily determine its precise position in every industry it serves. Based on information available to Quaker, however, it is estimated that Quaker holds a leading global position (among a group in excess of 25 other suppliers) in the market for process fluids to produce sheet steel. It is also believed that Quaker holds significant global positions in the markets for process fluids in portions of the automotive and industrial markets. The offerings of many of our competitors differ from Quaker, with some who offer a broad portfolio of fluids, including general lubricants, to those who have a more specialized product range, and all of whom provide different levels of technical services to individual customers. Competition in the industry is based primarily on the ability to provide products that meet the needs of the customer, render technical services and laboratory assistance to the customer and, to a lesser extent, on price.
1
Major Customers and Markets
In 2013, Quaker’s five largest customers (each composed of multiple subsidiaries or divisions with semi-autonomous purchasing authority) accounted for approximately 18% of our consolidated net sales, with the largest customer (Arcelor-Mittal Group) accounting for approximately 9% of our consolidated net sales. A significant portion of Quaker’s revenues are realized from the sale of process fluids and services to manufacturers of steel, automobiles, appliances, and durable goods, and, therefore, Quaker is subject to the same business cycles as those experienced by these manufacturers and their customers. Furthermore, steel customers typically have limited manufacturing locations as compared to metalworking customers and generally use higher volumes of products at a single location. Accordingly, the loss or closure of a steel mill or other major customer site can have a material adverse effect on Quaker’s business.
Raw Materials
Quaker uses over 1,000 raw materials, including mineral oils and derivatives, animal fats and derivatives, vegetable oils and derivatives, ethylene derivatives, solvents, surface active agents, chlorinated paraffinic compounds, and a wide variety of other organic and inorganic compounds. In 2013, three raw material groups (mineral oils and derivatives, animal fats and derivatives, and vegetable oils and derivatives) each accounted for at least 10% of the total cost of Quaker’s raw material purchases. The price of mineral oil can be affected by the price of crude oil and its refining capacity. In addition, animal fat and vegetable oil prices are impacted by increased biodiesel consumption. Accordingly, significant fluctuations in the price of crude oil can have a material effect upon the Company’s business. Many of the raw materials used by Quaker are “commodity” chemicals, and, therefore, Quaker’s earnings can be affected by market changes in raw material prices. Reference is made to the disclosure contained in Item 7A of this Report.
Patents and Trademarks
Quaker has a limited number of patents and patent applications, including patents issued, applied for, or acquired in the United States and in various foreign countries, some of which may prove to be material to its business. Principal reliance is placed upon Quaker’s proprietary formulae and the application of its skills and experience to meet customer needs. Quaker’s products are identified by trademarks that are registered throughout its marketing area.
Research and Development—Laboratories
Quaker’s research and development laboratories are directed primarily toward applied research and development since the nature of Quaker’s business requires continual modification and improvement of formulations to provide chemical specialties to satisfy customer requirements. Quaker maintains quality control laboratory facilities in each of its manufacturing locations. In addition, Quaker maintains facilities in Conshohocken, Pennsylvania; Santa Fe Springs, California; Batavia, New York; Uithoorn, The Netherlands; Rio de Janiero, Brazil; and Qingpu, China that are devoted primarily to applied research and development.
Research and development costs are expensed as incurred. Research and development expenses during 2013, 2012 and 2011 were $21.6 million, $20.0 million and $18.8 million, respectively.
Most of Quaker’s subsidiaries and associated companies also have laboratory facilities. Although not as complete as the Conshohocken, Santa Fe Springs, Batavia, Uithoorn, Rio de Janiero or Qingpu laboratories, these facilities are generally sufficient for the requirements of the customers being served. If problems are encountered which cannot be resolved by local laboratories, such problems may be referred to the laboratory staff in Conshohocken or Uithoorn.
Regulatory Matters
In order to facilitate compliance with applicable Federal, state, and local statutes and regulations relating to occupational health and safety and protection of the environment, the Company has an ongoing program of site assessment for the purpose of identifying capital expenditures or other actions that may be necessary to comply with such requirements. The program includes periodic inspections of each facility by Quaker and/or independent experts, as well as ongoing inspections and training by on-site personnel. Such inspections address operational matters, record keeping, reporting requirements and capital improvements. Capital expenditures directed solely or primarily to regulatory compliance amounted to approximately $0.6 million, $1.0 million and $1.0 million in 2013, 2012 and 2011, respectively. In 2014, the Company expects to incur approximately $1.4 million for capital expenditures directed primarily to regulatory compliance.
Number of Employees
On December 31, 2013, Quaker’s consolidated companies had 1,783 full-time employees of whom 563 were employed by the parent company and its U.S. subsidiaries and 1,220 were employed by its non-U.S. subsidiaries. Associated companies of Quaker (in which it owns less than 50% and has significant influence) employed 74 people on December 31, 2013.
2
Company Segmentation
The Company’s reportable operating segments evidence the structure of the Company’s internal organization, the method by which the Company’s resources are allocated and the manner by which the Company assesses its performance. During 2013, certain internal shifts in the Company’s management and changes to the structure of internally reported information occurred. The Company currently believes its structure, its resource allocation and its performance assessment are now more closely aligned with its four geographical regions: North America, EMEA, Asia/Pacific and South America. Therefore, the Company changed its reportable operating segments from those categorized by product nature to those organized by geography and recast all prior period information to reflect the four regions as the Company’s new reportable operating segments. See Note 3 of Notes to Consolidated Financial Statements included in Item 8 of this Report.
Non-U.S. Activities
Since significant revenues and earnings are generated by non-U.S. operations, Quaker’s financial results are affected by currency fluctuations, particularly between the U.S. Dollar and the E.U. Euro, the Brazilian Real, the Chinese Renminbi and the Indian Rupee, and the impact of those currency fluctuations on the underlying economies. Incorporated by reference is (i) the foreign exchange risk information contained in Item 7A of this Report, (ii) the geographic information in Note 3 of Notes to Consolidated Financial Statements included in Item 8 of this Report and (iii) information regarding risks attendant to foreign operations included in Item 1A of this Report.
Quaker on the Internet
Financial results, news and other information about Quaker can be accessed from the Company’s Web site at http://www.quakerchem.com. This site includes important information on the Company’s locations, products and services, financial reports, news releases and career opportunities. The Company’s periodic and current reports on Forms 10-K, 10-Q and 8-K, including exhibits and supplemental schedules filed therewith, and amendments to those reports, filed with the Securities and Exchange Commission (“SEC”) are available on the Company’s Web site, free of charge, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. Information contained on, or that may be accessed through, the Company’s Web site is not incorporated by reference in this Report and, accordingly, you should not consider that information part of this Report.
Factors that May Affect Our Future Results
(Cautionary Statements under the Private Securities Litigation Reform Act of 1995)
Certain information included in this Report and other materials filed or to be filed by Quaker with the SEC (as well as information included in oral statements or other written statements made or to be made by us) contain or may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements can be identified by the fact that they do not relate strictly to historical or current facts. We have based these forward-looking statements on our current expectations about future events. These forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, intentions, financial condition, results of operations, future performance, and business, including:
| • | statements relating to our business strategy; |
| • | our current and future results and plans; and |
| • | statements that include the words “may,” “could,” “should,” “would,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan” or similar expressions. |
Such statements include information relating to current and future business activities, operational matters, capital spending, and financing sources. From time to time, oral or written forward-looking statements are also included in Quaker’s periodic reports on Forms 10-K, 10-Q and 8-K, press releases, and other materials released to, or statements made to, the public.
Any or all of the forward-looking statements in this Report, in Quaker’s Annual Report to Shareholders for 2013, and in any other public statements we make may turn out to be wrong. This can occur as a result of inaccurate assumptions or as a consequence of known or unknown risks and uncertainties. Many factors will be important in determining our future performance. Consequently, actual results may differ materially from those that might be anticipated from our forward-looking statements.
We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. However, any further disclosures made on related subjects in Quaker’s subsequent reports on Forms 10-K, 10-Q and 8-K should be consulted. These forward-looking statements are subject to risks, uncertainties and assumptions about us and our operations that are subject to change based on various important factors, some of which are beyond our control. A major risk is that the demand for the Company’s products and services is largely derived from the demand for its customers’ products, which subjects the Company to uncertainties related to downturns in a customer’s business and unanticipated customer production shutdowns. Other major risks and uncertainties include, but are not limited to, significant increases in raw material costs, worldwide economic and
3
political conditions, foreign currency fluctuations, terrorist attacks and other acts of violence, each of which is discussed in greater detail in Item 1A of this Report. Furthermore, the Company is subject to the same business cycles as those experienced by steel, automobile, aircraft, appliance, and durable goods manufacturers. These risks, uncertainties, and possible inaccurate assumptions relevant to our business could cause our actual results to differ materially from expected and historical results. Other factors beyond those discussed in this Report could also adversely affect us. Therefore, we caution you not to place undue reliance on our forward-looking statements. This discussion is provided as permitted by the Private Securities Litigation Reform Act of 1995.
Item 1A. Risk Factors.
Changes to the industries and markets that Quaker serves could have a material adverse effect on the Company’s liquidity, financial position and results of operations.
The chemical specialty industry comprises a number of companies of similar size as well as companies larger and smaller than Quaker. It is estimated that Quaker holds a leading and significant global position in the markets for process fluids to produce sheet steel and significant global positions in portions of the automotive and industrial markets. The industry is highly competitive, and a number of companies with significant financial resources and/or customer relationships compete with us to provide similar products and services. Our competitors may be positioned to offer more favorable pricing and service terms, resulting in reduced profitability and a loss of market share for us. In addition, several competitors could potentially consolidate their businesses to gain scale to better position their product offerings, which could have a negative impact to our profitability and market share. Historically, competition in the industry has been based primarily on the ability to provide products that meet the needs of the customer and render technical services and laboratory assistance to the customer and, to a lesser extent, on price. Factors critical to the Company’s business include successfully differentiating the Company’s offering from its competition, operating efficiently and profitably as a globally integrated whole, and increasing market share and customer penetration through internally developed business programs and strategic acquisitions.
The business environment in which the Company operates remains uncertain. The Company is subject to the same business cycles as those experienced by steel, automobile, aircraft, appliance, and durable goods manufacturers. A major risk is that the Company’s demand is largely derived from the demand for its customers’ products, which subjects the Company to uncertainties related to downturns in our customers’ business and unanticipated customer production shutdowns or curtailments. The Company has limited ability to adjust its cost level contemporaneously with changes in sales and gross margins. Thus, a significant downturn in sales or gross margins due to weak end-user markets, loss of a significant customer, and/or rising raw material costs could have a material adverse effect on the Company’s liquidity, financial position, and results of operations.
Our business depends on attracting and retaining qualified management personnel.
The unanticipated departure of any key member of our management team could have an adverse effect on our business. Given the relative size of the Company and the breadth of its global operations, there are a limited number of qualified management personnel to assume the responsibilities of management level employees should there be management turnover. In addition, because of the specialized and technical nature of our business, our future performance is dependent on the continued service of, and our ability to attract and retain, qualified management, commercial and technical personnel. Competition for such personnel is intense, and we may be unable to continue to attract or retain such personnel. In an effort to mitigate such risks, the Company utilizes retention bonuses, offers competitive pay and maintains continued succession planning, but there can be no assurance that these mitigating factors will be adequate to attract or retain qualified management personnel.
Inability to obtain sufficient price increases or contract concessions to offset increases in the costs of raw material could have a material adverse effect on the Company’s liquidity, financial position and results of operations. Price increases implemented could result in the loss of sales.
Quaker uses over 1,000 raw materials, including mineral oils and derivatives, animal fats and derivatives, vegetable oils and derivatives, ethylene derivatives, solvents, surface active agents, chlorinated paraffinic compounds, and a wide variety of other organic and inorganic compounds. In 2013, three raw material groups (mineral oils and derivatives, animal fats and derivatives, and vegetable oils and derivatives) each accounted for at least 10% of the total cost of Quaker’s raw material purchases. The price of mineral oil can be affected by the price of crude oil and its refining capacity. In addition, many of the raw materials used by Quaker are “commodity” chemicals. Accordingly, Quaker’s earnings can be affected by market changes in raw material prices.
In the past, Quaker experienced significant volatility in its raw material costs, particularly crude oil derivatives. In addition, refining capacity can be constrained by various factors, which can further contribute to volatile raw material costs and negatively impact margins. Animal fat and vegetable oil prices also can be impacted by increased biodiesel consumption. Although the Company has been successful in the past in recovering a substantial amount of the raw material cost increases while retaining customers, there can be no assurance that the Company can continue to recover raw material costs or retain customers in the future. As a result of the Company’s past pricing actions, customers may become more likely to consider competitors’ products, some of which may be available at a lower cost. A significant loss of customers could result in a material adverse effect on the Company’s results of operations.
4
Availability of raw materials, including sourcing from some single suppliers and some suppliers in volatile economic environments, could have a material adverse effect on the Company’s liquidity, financial position and results of operations.
The chemical specialty industry can experience some tightness of supply for certain raw materials. In addition, in some cases, we choose to source from a single supplier and/or suppliers in economies that have experienced instability. Any significant disruption in supply could affect our ability to obtain raw materials, which could have a material adverse effect on our liquidity, financial position and results of operations. In addition, the Company’s raw materials are subject to various regulatory laws, and a change in the ability to legally use such raw materials may impact Quaker’s liquidity, financial position and results of operations.
Loss of a significant manufacturing facility may materially and adversely affect the Company’s liquidity, financial position and results of operations.
Quaker has multiple manufacturing facilities throughout the world. In certain countries such as Brazil and China, there is only one such facility. If one of the Company’s facilities was damaged to such extent that production was halted for an extended period, the Company may not be able to timely supply affected customers. This could result in a loss of sales over an extended period or permanently. The Company does take steps to mitigate against this risk, including contingency planning and procuring property and casualty insurance (including business interruption insurance). Nevertheless, the loss of sales in any one region over any extended period of time could have a significant material adverse effect on Quaker’s liquidity, financial position and results of operations.
Bankruptcy of a significant customer could have a material adverse effect on our liquidity, financial position and results of operations.
A significant portion of Quaker’s revenues is derived from sales to customers in the steel and automotive industries; including some of our larger customers, where a number of bankruptcies have occurred in the past and companies have experienced financial difficulties. As part of the bankruptcy process, the Company’s pre-petition receivables may not be realized, customer manufacturing sites may be closed or contracts voided. The bankruptcy of a major customer could have a material adverse effect on the Company’s liquidity, financial position, and results of operations. Steel customers typically have limited manufacturing locations as compared to metalworking customers and generally use higher volumes of products at a single location. The loss or closure of a steel mill or other major site of a significant customer could have a material adverse effect on Quaker’s business.
During 2013, our five largest customers (each composed of multiple subsidiaries or divisions with semi-autonomous purchasing authority) together accounted for approximately 18% of our consolidated net sales, with the largest customer (Arcelor-Mittal Group) accounting for approximately 9% of our consolidated net sales.
Failure to comply with any material provision of our credit facility or other debt agreements could have a material adverse effect on our liquidity, financial position and results of operations.
The Company maintains a $300.0 million unsecured credit facility (the “Credit Facility”) with a group of lenders, which can be increased to $400.0 million at the Company’s option if lenders agree to increase their commitments and the Company satisfies certain conditions. The Credit Facility, which matures in 2018, provides the availability of revolving credit borrowings. In general, the borrowings under the Credit Facility bear interest at either a base rate or LIBOR rate plus a margin based on the Company’s consolidated leverage ratio.
The Credit Facility contains certain limitations on investments, acquisitions and liens, as well as default provisions customary for facilities of its type. While these covenants and restrictions are not currently considered to be overly restrictive, they could become more difficult to comply with as our business or financial conditions change. In addition, deterioration in the Company’s results of operations or financial position could significantly increase borrowing costs.
Quaker is exposed to market rate risk for changes in interest rates, due to the variable interest rate applied to the Company’s borrowings under its Credit Facility. Accordingly, if interest rates rise significantly, the cost of debt to Quaker will increase, perhaps significantly, depending on the extent of Quaker’s borrowings under the Credit Facility. At December 31, 2013, the Company had no outstanding borrowings under the Credit Facility.
Environmental laws and regulations and pending legal proceedings may materially and adversely affect the Company’s liquidity, financial position and results of operations.
The Company is a party to proceedings, cases, and requests for information from, and negotiations with, various claimants and Federal and state agencies relating to various matters, including environmental matters. An adverse result in one or more matters or any potential future matter of a similar nature could materially and adversely affect the Company’s liquidity, financial position and results of operations. Incorporated herein by reference is the information concerning pending asbestos-related litigation against an inactive subsidiary and amounts accrued associated with certain environmental non-capital remediation costs in Note 22 of Notes to Consolidated Financial Statements which appears in Item 8 of this Report.
5
Compliance with a complex global regulatory environment could have an impact on the Company’s public perception and/or a material adverse effect on the Company’s liquidity, financial position and results of operations.
Changes in the Company’s regulatory environment, particularly, but not limited to, the United States, Brazil, China and the European Union, could lead to heightened regulatory scrutiny, could adversely impact our ability to continue selling certain products in our domestic or foreign markets and could increase the cost of doing business. For instance, the European Union’s Registration, Authorization and Restriction of Chemicals (“REACH” and analogous non-E.U. laws and regulations), or other similar laws and regulations, could result in fines, ongoing monitoring and other future business activity restrictions, which could have a material adverse effect on the Company’s liquidity, financial position and results of operations. In addition, non-compliance with the U.S. Foreign Corrupt Practices Act (“FCPA”), the UK Bribery Act and other similar laws and regulations, could result in a negative impact to the Company’s reputation, potential fines or ongoing monitoring, which could also have an adverse effect on the Company.
Climate change and greenhouse gas restrictions may materially affect the Company’s liquidity, financial position and results of operations.
The Company is subject to various regulations regarding its emission of greenhouse gases in its manufacturing facilities. In addition, a number of countries have adopted, or are considering the adoption of regulatory frameworks to reduce greenhouse gas emissions. These include adoption of cap and trade regimes, carbon taxes, increased efficiency standards and incentives or mandates for renewable energy. These requirements could make our products more expensive and reduce demand for our products. Current and pending greenhouse gas regulations may also increase our compliance costs.
Potential product, service or other related liability claims could have a material adverse effect on the Company’s liquidity, financial position and results of operations.
The development, manufacture and sale of specialty chemical products and other related services involve inherent exposure to potential product liability claims, service level claims, product recalls and related adverse publicity. Any of these potential product or service risks could also result in substantial and unexpected expenditures and affect customer confidence in our products and services, which could have a material adverse effect on the Company’s liquidity, financial position and results of operations. Although the Company maintains product and other general liability insurance, there can be no assurance that this type or the level of coverage would be adequate to cover these potential risks. In addition, the Company may not be able to continue to maintain its existing insurance or obtain comparable insurance at a reasonable cost, if at all, in the event a significant product or service claim arises.
We may be unable to adequately protect our proprietary rights, which may limit the Company’s ability to compete in its markets.
Quaker has a limited number of patents and patent applications, including patents issued, applied for, or acquired in the United States and in various foreign countries, some of which may prove to be material to its business. Principal reliance is placed upon Quaker’s proprietary formulae and the application of its skills and experience to meet customer needs. Quaker’s products are identified by trademarks that are registered throughout its marketing area. Despite our efforts to protect such proprietary information through patent and trademark filings and through the use of appropriate trade secret protections and the inability of certain products to be effectively replicated by others, it is possible that competitors and other unauthorized third parties may obtain, copy, use or disclose our technologies, products, and processes. In addition, the laws and/or judicial systems of foreign countries in which we design, manufacture, market and sell our products may afford little or no effective protection of our proprietary technology. These potential risks to our proprietary information could subject the Company to increased competition and negative impacts to our liquidity, financial position and results of operations.
We might not be able to timely develop, manufacture and gain market acceptance of new and enhanced products required to maintain or expand our business.
We believe that our continued success depends on our ability to continuously develop and manufacture new products and product enhancements on a timely and cost-effective basis, in response to customers’ demands for higher performance process chemicals, coatings and other chemical products. Our competitors may develop new products or enhancements to their products that offer performance, features and lower prices that may render our products less competitive or obsolete and, as a consequence, we may lose business and/or significant market share. The development and commercialization of new products require significant expenditures over an extended period of time, and some products that we seek to develop may never become profitable. In addition, we may not be able to develop and introduce products incorporating new technologies in a timely manner that will satisfy our customers’ future needs or achieve market acceptance.
6
An inability to appropriately capitalize on Company growth, including prior or future acquisitions, may adversely affect the Company’s liquidity, financial position and results of operations.
Quaker has completed several acquisitions in the past and may continue to seek acquisitions to grow its business. In addition, the Company continues to grow organically through increased end market growth and incremental market share. The success of the Company’s growth depends on its ability to successfully integrate such opportunities, including, but not limited to, the following:
| • | successfully execute the integration or consolidation of the acquired or additional business into existing processes and operations, | |
| • | develop or modify financial reporting, information systems and other related financial tools to ensure overall financial integrity and adequacy of internal control procedures, | |
| • | identify and take advantage of potential cost reduction opportunities, while maintaining legacy business and other related attributes, and | |
| • | further penetrate existing markets with the product capabilities acquired in new acquisitions. |
The Company may fail to derive significant benefits or may not create the appropriate infrastructure to support such additional business, which could have a material adverse effect on liquidity, financial position and results of operations. Also, if the Company fails to achieve sufficient financial performance from an acquisition, certain long-lived assets, such as property, plant and equipment and goodwill and other intangible assets, could become impaired and result in the recognition of an impairment loss.
The scope of our international operations subjects the Company to risks, including risks from changes in trade regulations, currency fluctuations, and political and economic instability.
Since significant revenues and earnings are generated by non-U.S. operations, Quaker’s financial results are affected by currency fluctuations, particularly between the U.S. Dollar and the E.U. Euro, the Brazilian Real, the Chinese Renminbi and the Indian Rupee, and the impact of those currency fluctuations on the underlying economies. During the past three years, sales by non-U.S. subsidiaries accounted for approximately 60% to 65% of our annual consolidated net sales. All of these operations use the local currency as their functional currency. The Company generally does not use financial instruments that expose it to significant risk involving foreign currency transactions; however, the size of non-U.S. activities has a significant impact on reported operating results and attendant net assets. Therefore, as exchange rates vary, Quaker’s results can be materially affected. Incorporated by reference is the foreign exchange risk information contained in Item 7A of this Report and the geographic information in Note 3 of Notes to Consolidated Financial Statements included in Item 8 of this Report.
The Company often sources inventory among its worldwide operations. This practice can give rise to foreign exchange risk resulting from the varying cost of inventory to the receiving location, as well as from the revaluation of intercompany balances. The Company mitigates this risk through local sourcing efforts.
Additional risks associated with the Company’s international operations include, but are not limited to, the following:
| • | changes in economic conditions from country to country, similar to the past instability in certain European economies, | ||||||||||
| • | changes in a country’s political condition, such as the current political unrest in the Middle East, | ||||||||||
| • | trade protection measures, | ||||||||||
| • | longer payment cycles, | ||||||||||
| • | licensing and other legal requirements, | ||||||||||
| • | restrictions on the repatriation of our assets, including cash, | ||||||||||
| • | the difficulties of staffing and managing dispersed international operations, | ||||||||||
| • | less protective foreign intellectual property laws, | ||||||||||
| • | legal systems that may be less developed and predictable than those in the United States, and | ||||||||||
| • | local tax issues. | ||||||||||
The breadth of Quaker’s international operations subjects the Company to various local non-income taxes, including value-added-taxes (“VAT”). With VAT, the Company essentially operates as an agent for various jurisdictions by collecting VAT from customers and remitting those amounts to the taxing authorities on the goods it sells. The laws and regulations regarding VAT can be complex and vary widely among countries as well as among individual jurisdictions within a given country for the same products, making full compliance difficult. As VAT is often charged as a percentage of the selling price of the goods sold, the amounts involved can be material. Should there be non-compliance by the Company, it may need to remit funds to the tax authorities prior to collecting the appropriate amounts from the customers or jurisdictions which may have been incorrectly paid. In addition, the Company may choose for commercial reasons not to seek repayment from certain customers. This could have a material adverse effect on the Company’s
7
liquidity, financial position and results of operations. See Note 22 of Notes to Consolidated Financial Statements, included in Item 8 of this Report, which is incorporated herein by this reference, for further discussion.
Terrorist attacks, other acts of violence or war, natural disasters, cybersecurity incidents or other uncommon global events may affect the markets in which we operate and our profitability.
Terrorist attacks, other acts of violence or war, natural disasters, cybersecurity incidents or other uncommon global events may negatively affect our operations. There can be no assurance that there will not be further terrorist attacks against the U.S. or other locations where we do business. Also, other uncommon global events, such as earthquakes, fires and tsunami, cannot be predicted. Terrorist attacks, other acts of violence or armed conflicts, and natural disasters may directly impact our physical facilities or those of our suppliers or customers. Additional terrorist attacks or natural disasters may disrupt the global insurance and reinsurance industries with the result that we may not be able to obtain insurance at historical terms and levels, if at all, for all of our facilities. Furthermore, any of these events may make travel and the transportation of our supplies and products more difficult and more expensive and ultimately affect the sales of our products. In addition, a failure to effectively prevent, detect and recover from breaches in the Company’s cybersecurity infrastructure could also negatively impact the Company’s results of operation through the loss of Company assets, business disruptions or other misuses of the Company’s information technology. The consequences of terrorist attacks, other acts of violence or armed conflicts, natural disasters, cybersecurity incidents or other uncommon global events can be unpredictable, and we may not be able to foresee events, such as these, that could have an adverse effect on our business.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Quaker’s corporate headquarters and a laboratory facility are located in the North American segment’s Conshohocken, Pennsylvania office. The Company’s other principal facilities in the North American segment are located in Detroit, Michigan; Middletown, Ohio; Santa Fe Springs, California; Batavia, New York; Dayton, Ohio; and Monterrey, N.L., Mexico. The Company’s EMEA segment has principal facilities in Uithoorn, The Netherlands; Santa Perpetua de Mogoda, Spain; Tradate, Italy; and Gorgonzola, Italy. The Company’s Asia/Pacific segment operates out of its principal facilities located in Qingpu, China and Kolkata, India, while its South American segment operates out of its principal facility in Rio de Janeiro, Brazil. With the exception of the Conshohocken, Santa Fe Springs and Gorgonzola sites, which are leased, all of these principal facilities are owned by Quaker and, as of December 31, 2013, were mortgage free. Quaker also leases sales, laboratory, manufacturing, and warehouse facilities in other locations.
Quaker’s principal facilities (excluding Conshohocken) consist of various manufacturing, administrative, warehouse, and laboratory buildings. Substantially all of the buildings (including Conshohocken) are of fire-resistant construction and are equipped with sprinkler systems. All facilities are primarily of masonry and/or steel construction and are adequate and suitable for Quaker’s present operations. The Company has a program to identify needed capital improvements that are implemented as management considers necessary or desirable. Most locations have various numbers of raw material storage tanks ranging from 2 to 58 at each location with a capacity ranging from 1,000 to 82,000 gallons and processing or manufacturing vessels ranging in capacity from 7 to 16,000 gallons.
Each of Quaker’s non-U.S. associated companies (in which it owns a less than 50% interest and has significant influence) owns or leases a plant and/or sales facilities in various locations, with the exception of Primex, Ltd.
Item 3. Legal Proceedings.
The Company is a party to proceedings, cases, and requests for information from, and negotiations with, various claimants and Federal and state agencies relating to various matters, including environmental matters. For information concerning pending asbestos-related litigation against an inactive subsidiary, amounts accrued associated with certain environmental non-capital remediation costs and the Company’s value-added-tax dispute settlements, reference is made to Note 22 of Notes to Consolidated Financial Statements, included in Item 8 of this Report, which is incorporated herein by this reference. The Company is a party to other litigation which management currently believes will not have a material adverse effect on the Company’s results of operations, cash flow or financial condition.
Item 4. Mine Safety Disclosures.
Not Applicable
8
Item 4(a). Executive Officers of the Registrant.
Set forth below is information regarding the executive officers of the Company, each of whom (with the exception of Ms. Loebl, Mr. Steeples and Mr. Hostetter) has been employed by the Company for more than five years, including the respective positions and offices with the Company held by each over the respective periods indicated. Each of the executive officers, with the exception of Mr. Hostetter, is elected annually to a one-year term. Mr. Hostetter is considered an executive officer in his capacity as principal accounting officer for purposes of this item.
Name, Age, and Present Position with the Company | Business Experience During the Past Five Years and Period Served as an Officer | ||
Michael F. Barry, 55 Chairman of the Board, Chief Executive Officer and President and Director | Mr. Barry, who has been employed by the Company since 1998, has served as Chairman of the Board since May 2009, in addition to his position as Chief Executive Officer and President held since October 2008. He served as Senior Vice President and Managing Director – North America from January 2006 to October 2008. He served as Senior Vice President and Global Industry Leader – Metalworking and Coatings from July 2005 through December 2005. He served as Vice President and Global Industry Leader – Industrial Metalworking and Coatings from January 2004 through June 2005 and Vice President and Chief Financial Officer from 1998 to August 2004. | ||
Margaret M. Loebl, 54 Vice President, Chief Financial Officer and Treasurer | Ms. Loebl, has served as Vice President, Chief Financial Officer and Treasurer since she joined the Company in June 2012. Prior to joining the Company, Ms. Loebl, from August 2011 to December 2011, provided senior executive-level financial consulting services in Paris, France, for Constellium, a leader in the manufacturing of high-quality aluminum products and solutions. Prior to joining Constellium, she served from October 2008 through December 2010 as Corporate Vice President, Chief Financial Officer and Treasurer of TechTeam Global, Inc., a provider of information technology and business process outsourcing services. Ms. Loebl served as an Executive in Residence at the University of Illinois in support of the University’s Finance Academy from August 2007 to December 2008. | ||
D. Jeffry Benoliel, 55 Vice President and Global Leader – Metalworking, Can and Corporate Secretary | Mr. Benoliel, who has been employed by the Company since 1995, has served as Vice President and Global Leader – Metalworking, Can and Corporate Secretary since July 1, 2013. He served as Vice President – Global Metalworking and Fluid Power and Corporate Secretary from June 2011 through June 2013, and until March 2012 also held the position of General Counsel. He served as Vice President – Global Strategy, General Counsel and Corporate Secretary from October 2008 until mid-June 2011 and Vice President, Secretary and General Counsel from 2001 through September 2008. | ||
Joseph A. Berquist, 42 Vice President and Managing Director – North America | Mr. Berquist, who has been employed by the Company since 1997, has served as Vice President and Managing Director – North America since April 2010. He served as Senior Director, North America Commercial from October 2008 through March 2010. | ||
Ronald S. Ettinger, 61 Vice President – Human Resources | Mr. Ettinger, who has been employed by the Company since 2002, has served as Vice President-Human Resources since December 2011. He served as Director-Global Human Resources from August 2005 through November 2011. | ||
9
Name, Age, and Present Position with the Company | Business Experience During the Past Five Years and Period Served as an Officer | ||
Shane W. Hostetter, 32 Corporate Controller | Mr. Hostetter, who has been employed by the Company since July 2011, has served in his current position since May 2013. He served as Assistant Global Controller from July 2011 through May 2013. Prior to joining the Company, Mr. Hostetter led the financial reporting department for Pulse Electronics Corporation (formerly Technitrol, Inc.) from May 2008 to June 2011. | ||
Dieter Laininger, 51 Vice President and Managing Director – South America and Global Leader – Primary Metals | Mr. Laininger, who has been employed by the Company since 1991, has served as Vice President and Managing Director – South America, since January 2013, in addition to his position as Vice President and Global Leader – Primary Metals, to which he was appointed in June 2011. He served as Industry Business Manager for Steel and Metalworking – EMEA from March 2001 through July 2011. | ||
Joseph F. Matrange, 72 Vice President and Global Leader – Coatings | Mr. Matrange, who has been employed by the Company since 2000, has served as Vice President and Global Leader – Coatings since October 2008. He has also served as President of AC Products, Inc., a California subsidiary, since October 2000, and Epmar Corporation, a California subsidiary, since April 2002. | ||
Jan F. Nieman, 53 Vice President and Global Leader – Grease, Fluid Power and Mining | Mr. Nieman, who has been employed by the Company since 1992, was appointed to his current position, effective August 2013. He served as Vice President and Managing Director – Asia/Pacific from February 2005 through July 2013. | ||
Wilbert Platzer, 52 Vice President and Managing Director – Europe | Mr. Platzer, who has been employed by the Company since 1995, has served in his current position since January 2006. | ||
Adrian Steeples, 53 Vice President and Managing Director – Asia/Pacific | Mr. Steeples, who has been employed by the Company since 2010, has served as Vice President and Managing Director – Asia/Pacific since July 1, 2013. He served as Industry Business Director – Metalworking from March 2011 through June 2013, and Manager, European and Global Special Projects, from May 2010 through February 2011. Prior to joining the Company, he worked for the BP Group serving as BP/Castrol European and Asian Pacific Sales Director in Industrial Lubricants and Services from January 2009 through December 2009. | ||
10
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
The Company’s common stock is listed on the New York Stock Exchange (“NYSE”) under the trading symbol KWR. The following table sets forth, for the calendar quarters during the two most recent fiscal years, the range of high and low sales prices for the common stock as reported on the NYSE composite tape (amounts rounded to the nearest penny), and the quarterly dividends declared and paid:
| Price Range | Dividends | Dividends | ||||||||||||||||||||||
| 2013 | 2012 | Declared | Paid | |||||||||||||||||||||
| High | Low | High | Low | 2013 | 2012 | 2013 | 2012 | |||||||||||||||||
| First quarter | $ | 63.50 | $ | 54.24 | $ | 48.15 | $ | 35.82 | $ | 0.245 | $ | 0.24 | $ | 0.245 | $ | 0.24 | ||||||||
| Second quarter | 67.27 | 53.54 | 46.59 | 37.86 | 0.25 | 0.245 | 0.245 | 0.24 | ||||||||||||||||
| Third quarter | 73.41 | 61.67 | 50.55 | 40.21 | 0.25 | 0.245 | 0.25 | 0.245 | ||||||||||||||||
| Fourth quarter | 81.52 | 70.02 | 54.00 | 45.07 | 0.25 | 0.245 | 0.25 | 0.245 | ||||||||||||||||
There are no restrictions that currently materially limit the Company’s ability to pay dividends or that the Company believes are likely to materially limit the payment of future dividends. If a default under the Company’s primary credit facility were to occur and continue, the payment of dividends would be prohibited. Reference is made to the “Liquidity and Capital Resources” disclosure contained in Item 7 of this Report.
As of January 17, 2014, there were 948 shareholders of record of the Company’s common stock, its only outstanding class of equity securities.
Every holder of Quaker common stock is entitled to one vote or ten votes for each share held of record on any record date depending on how long each share has been held. As of January 17, 2014, 13,197,638 shares of Quaker common stock were issued and outstanding. Based on the information available to the Company on January 17, 2014, as of that date the holders of 808,653 shares of Quaker common stock would have been entitled to cast ten votes for each share, or approximately 39% of the total votes that would have been entitled to be cast as of that record date and the holders of 12,388,985 shares of Quaker common stock would have been entitled to cast one vote for each share, or approximately 61% of the total votes that would have been entitled to be cast as of that date. The number of shares that are indicated as entitled to one vote includes those shares presumed to be entitled to only one vote. Because the holders of these shares may rebut this presumption, the total number of votes entitled to be cast as of January 17, 2014 could be more than 20,475,515.
Reference is made to the information in Item 12 of this Report under the caption “Equity Compensation Plans,” which is incorporated herein by this reference.
11
The following graph compares the cumulative total return (assuming reinvestment of dividends) from December 31, 2008 to December 31, 2013 for (i) Quaker’s common stock, (ii) the S&P SmallCap 600 Index (the “SmallCap Index”), and (iii) the S&P 600 Materials Group Index (the “Materials Group Index”). The graph assumes the investment of $100 on December 31, 2008 in each of Quaker’s common stock, the stocks comprising the SmallCap Index and the stocks comprising the Materials Group Index.
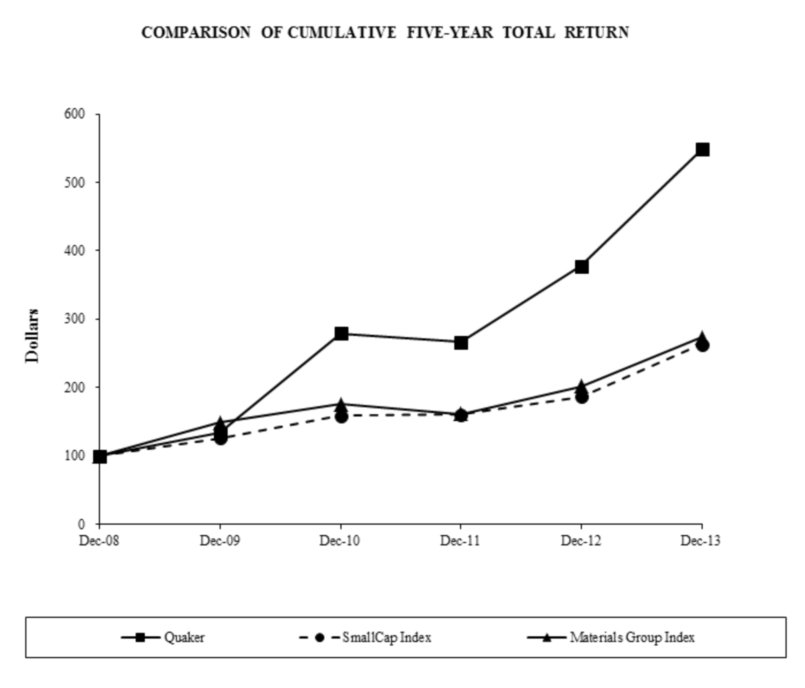
| 12/31/2008 | 12/31/2009 | 12/31/2010 | 12/31/2011 | 12/31/2012 | 12/31/2013 | |||||||||||||||||||
| Quaker | $ | 100.00 | $ | 133.66 | $ | 279.02 | $ | 266.75 | $ | 377.28 | $ | 548.20 | ||||||||||||
| SmallCap Index | 100.00 | 125.57 | 158.60 | 160.22 | 186.37 | 263.37 | ||||||||||||||||||
| Materials Group Index | 100.00 | 148.18 | 175.26 | 160.77 | 201.45 | 273.56 | ||||||||||||||||||
12
Item 6. Selected Financial Data.
The following table sets forth selected financial data for the Company and its consolidated subsidiaries (in thousands, except dividends and per share data):
| Year Ended December 31, | ||||||||||||||||||||
| 2013 (1) | 2012 (2) | 2011 (3) | 2010 (4) | 2009 (5) | ||||||||||||||||
| Summary of Operations: | ||||||||||||||||||||
| Net sales | $ | 729,395 | $ | 708,226 | $ | 683,231 | $ | 544,063 | $ | 451,490 | ||||||||||
| Income before taxes and equity in net income of associated companies | 72,826 | 62,948 | 59,377 | 46,213 | 23,692 | |||||||||||||||
| Net income attributable to Quaker Chemical Corporation | 56,339 | 47,405 | 45,892 | 32,120 | 16,058 | |||||||||||||||
| Per share: | ||||||||||||||||||||
| Net income attributable to Quaker Chemical Corporation | ||||||||||||||||||||
| Common Shareholders - basic | $ | 4.28 | $ | 3.64 | $ | 3.71 | $ | 2.85 | $ | 1.46 | ||||||||||
| Net income attributable to Quaker Chemical Corporation | ||||||||||||||||||||
| Common Shareholders - diluted | $ | 4.27 | $ | 3.63 | $ | 3.66 | $ | 2.80 | $ | 1.45 | ||||||||||
| Dividends declared | 0.995 | 0.975 | 0.955 | 0.935 | 0.92 | |||||||||||||||
| Dividends paid | 0.99 | 0.97 | 0.95 | 0.93 | 0.92 | |||||||||||||||
| Financial Position | ||||||||||||||||||||
| Working capital | $ | 197,991 | $ | 170,018 | $ | 152,900 | $ | 114,291 | $ | 98,994 | ||||||||||
| Total assets | 584,146 | 536,634 | 511,152 | 452,868 | 398,183 | |||||||||||||||
| Long-term debt | 17,321 | 30,000 | 46,701 | 73,855 | 63,685 | |||||||||||||||
| Total equity | 345,031 | 289,676 | 261,357 | 190,537 | 159,186 | |||||||||||||||
Notes to the above table (in thousands):
| (1 | ) | The results of operations for 2013 include equity income from a captive insurance company of $5,451 after tax; an increase to other income of $2,540 related to a mineral oil excise tax refund; and an increase to other income of $497 related to a change in an acquisition-related earnout liability; partially offset by an after-tax charge of $357 related to a currency devaluation at the Company’s 50% owned affiliate in Venezuela; $1,419 of charges related to cost streamlining initiatives in the Company’s EMEA and South American segments; and a $796 net charge related to a non-income tax contingency. | |
| (2 | ) | The results of operations for 2012 include equity income from a captive insurance company of $1,812 after tax; and an increase to other income of $1,737 related to a change in an acquisition-related earnout liability; partially offset by a charge of $1,254 related to the bankruptcy of certain customers in the U.S.; and a charge of $609 related to CFO transition costs. | |
| (3 | ) | The results of operations for 2011 include equity income from a captive insurance company of $2,323 after tax; an increase to other income of $2,718 related to the revaluation of the Company’s previously held ownership interest in Tecniquimia Mexicana S.A de C.V. to its fair value; and an increase to other income of $595 related to a change in an acquisition-related earnout liability. | |
| (4 | ) | The results of operations for 2010 include equity income from a captive insurance company of $313 after tax; offset by a final charge of $1,317 related to the retirement of the Company’s former Chief Executive Officer in 2008; a net charge of $4,132 related to a non-income tax contingency; a $322 after-tax charge related to a currency devaluation at the Company’s 50% owned affiliate in Venezuela; and a $564 after-tax charge related to an out-of-period adjustment at the Company’s 40% owned affiliate in Mexico. | |
| (5 | ) | The results of operations for 2009 include other income of $1,193 from the disposition of land in Europe; offset by a charge for restructuring and related activities of $2,289; a charge of $2,443 related to the retirement of the Company’s former Chief Executive Officer in 2008; and an equity loss from a captive insurance company of $162 after tax. |
13
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Executive Summary
Quaker Chemical Corporation is a leading global provider of process fluids, chemical specialties, and technical expertise to a wide range of industries, including steel, aluminum, automotive, mining, aerospace, tube and pipe, cans, and others. For nearly 100 years, Quaker has helped customers around the world achieve production efficiency, improve product quality, and lower costs through a combination of innovative technology, process knowledge, and customized services. Headquartered in Conshohocken, Pennsylvania USA, Quaker serves businesses worldwide with a network of dedicated and experienced professionals whose mission is to make a difference.
The Company performed very well in terms of net sales, earnings and cash flow in 2013. The Company’s performance was driven by an increase of 3% in net sales from 2012, which was primarily the result of increased product volumes. In addition, the Company’s gross profit increased 9% from 2012, with gross margin improving to 35.8% from 33.7% in 2012, which reflects the return of product margins to more acceptable levels. The Company achieved the improvements in sales and margin through market share gains and acquisitions, despite a market that was generally down due to several challenges in the global economic environment. Due to selling and other costs related to the Company’s improved performance and costs added with its recent acquisitions, along with higher labor related costs on general year-over-year merit increases, there was an 8% increase in selling, general and administrative expenses (“SG&A”) from 2012. In addition, the Company’s performance in 2013 and 2012 included certain uncommon costs in SG&A, other income and other expense, which are further discussed in the Company’s non-GAAP section and operations section below.
The net result was earnings per diluted share of $4.27 in 2013 compared to earnings per diluted share of $3.63 for 2012, with non-GAAP earnings per diluted share increasing approximately 10% to $3.84 in 2013 compared to $3.49 in 2012 and adjusted EBITDA increasing 11% to $89.6 million for 2013 from $80.9 million for 2012. See the Non-GAAP Measures section in this Item, below.
Net cash flows provided by operating activities were $73.8 million in 2013, increasing by $10.9 million, or 17%, from 2012 on higher net income and improved working capital management. In addition, the Company’s balance sheet remains very strong with no borrowings on its credit facility and its cash position exceeding its debt at December 31, 2013, which will provide opportunities for the Company to pursue strategic growth opportunities, including acquisitions, in the future. In addition, the Company enhanced its financial flexibility during 2013 by revising its credit facility, expanding the amount available for borrowing under this facility from $175.0 million to $300.0 million.
As the Company looks to 2014, it expects to see modest market growth in all regions of the world. In addition, the Company expects market share gains from its strategic initiatives and its recent acquisitions, which will build upon anticipated end market growth. However, the Company continues to operate in a highly competitive market with challenging economic conditions over various parts of the world. Also, the Company could experience increases in raw material costs from their current levels in the near term. On balance, the Company remains confident in its future and expects 2014 to be another good year for Quaker as we strive to increase revenue and earnings for the fifth consecutive year.
Critical Accounting Policies and Estimates
Quaker’s discussion and analysis of its financial condition and results of operations are based upon Quaker’s consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires Quaker to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, Quaker evaluates its estimates, including those related to customer sales incentives, product returns, bad debts, inventories, property, plant and equipment, investments, goodwill, intangible assets, income taxes, financing operations, restructuring, incentive compensation plans (including equity-based compensation), pensions and other postretirement benefits, and contingencies and litigation. Quaker bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Quaker believes the following critical accounting policies describe the more significant judgments and estimates used in the preparation of its consolidated financial statements:
1. Accounts receivable and inventory exposures—Quaker establishes allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments. If the financial condition of Quaker’s customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. As part of its terms of trade, Quaker may custom manufacture products for certain large customers and/or may ship product on a consignment basis. Further, a significant portion of Quaker’s revenues is derived from sales to customers where a number of bankruptcies have occurred during recent years and companies have experienced financial difficulties. When a bankruptcy occurs, Quaker must judge the amount of proceeds, if any, that may ultimately be received through the bankruptcy or liquidation process. These matters may increase the Company’s exposure, should a bankruptcy occur, and may require a write down or a disposal of certain inventory due to its estimated obsolescence or limited marketability. Reserves for customers filing for bankruptcy protection are generally established at 75-100% of
14
the amount outstanding at the bankruptcy filing date, dependent on the Company’s evaluation of likely proceeds from the bankruptcy process. Large and/or financially distressed customers are generally reserved for on a specific review basis, while a general reserve is maintained for other customers based on historical experience. The Company’s consolidated allowance for doubtful accounts was $7.1 million and $6.4 million at December 31, 2013 and December 31, 2012, respectively. Further, the Company recorded provisions for doubtful accounts of $1.1 million, $2.1 million and $0.9 million in 2013, 2012 and 2011, respectively. An increase of 10% to the recorded provisions would have decreased the Company’s pre-tax earnings by approximately $0.1 million, $0.2 million and $0.1 million in 2013, 2012 and 2011, respectively.
2. Environmental and litigation reserves—Accruals for environmental and litigation matters are recorded when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. Accrued liabilities are exclusive of claims against third parties and are not discounted. Environmental costs and remediation costs are capitalized if the costs extend the life, increase the capacity or improve the safety or efficiency of the property from the date acquired or constructed, and/or mitigate or prevent contamination in the future. Estimates for accruals for environmental matters are based on a variety of potential technical solutions, governmental regulations and other factors, and are subject to a large range of potential costs for remediation and other actions. A considerable amount of judgment is required in determining the most likely estimate within the range of total costs, and the factors determining this judgment may vary over time. Similarly, reserves for litigation and similar matters are based on a range of potential outcomes and require considerable judgment in determining the most probable outcome. If no amount within the range is considered more probable than any other amount, the Company accrues the lowest amount in that range in accordance with generally accepted accounting principles. See Note 22 of Notes to Consolidated Financial Statements which appears in Item 8 of this Report.
3. Realizability of equity investments—Quaker holds equity investments in various foreign companies, whereby it has the ability to influence, but not control, the operations of the entity and its future results. Quaker records an impairment charge to an investment when it believes a decline in value that is other than temporary has occurred. Future adverse changes in market conditions, poor operating results of underlying investments, devaluation of foreign currencies or other events or circumstances could result in losses or an inability to recover the carrying value of the investments. These indicators may result in an impairment charge in the future. The carrying amount of the Company’s equity investments at December 31, 2013 was $19.4 million, which included four investments of $12.1 million, or a 32.8% interest, in Primex, Ltd. (Barbados), $5.3 million, or a 50% interest, in Nippon Quaker Chemical, Ltd. (Japan), $1.6 million, or a 50% interest, in Kelko Quaker Chemical, S.A. (Venezuela) and $0.4 million, or a 50% interest, in Kelko Quaker Chemical, S.A. (Panama), respectively. See Note 12 of Notes to Consolidated Financial Statements which appears in Item 8 of this Report.
4. Tax exposures, valuation allowances and uncertain tax positions—Quaker records expenses and liabilities for taxes based on estimates of amounts that will be ultimately determined to be deductible in tax returns filed in various jurisdictions. The filed tax returns are subject to audit, which often occur several years subsequent to the date of the financial statements. Disputes or disagreements may arise during audits over the timing or validity of certain items or deductions, which may not be resolved for extended periods of time. Quaker applies the provisions of FASB’s guidance regarding uncertain tax positions. The guidance applies to all income tax positions taken on previously filed tax returns or expected to be taken on a future tax return. The FASB’s guidance regarding accounting for uncertainty in income taxes prescribes the recognition threshold and measurement attributes for financial statement recognition and measurement of tax positions taken or expected to be taken on a tax return. The guidance further requires the determination of whether the benefits of tax positions will be more likely than not sustained upon audit based upon the technical merits of the tax position. For tax positions that are determined to be more likely than not sustained upon audit, a company recognizes the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement in the financial statements. For tax positions that are not determined to be more likely than not sustained upon audit, a company does not recognize any portion of the benefit in the financial statements. Additionally, the guidance provides for derecognition, classification, penalties and interest, accounting in interim periods, disclosure and transition. The guidance also requires that the amount of interest expense and income to be recognized related to uncertain tax positions be computed by applying the applicable statutory rate of interest to the difference between the tax position recognized, including timing differences, and the amount previously taken or expected to be taken in a tax return. The Company’s continuing practice is to recognize interest and/or penalties related to income tax matters in income tax expense. The guidance also requires that an entity net its liability for unrecognized tax benefits against deferred tax assets related to net operating losses or other tax credit carryforwards that would apply if the uncertain tax position were settled for the presumed amount at the balance sheet date. Quaker also records valuation allowances when necessary to reduce its deferred tax assets to the amount that is more likely than not to be realized. While Quaker has considered future taxable income and employs prudent and feasible tax planning strategies in assessing the need for a valuation allowance, in the event Quaker were to determine that it would be able to realize its deferred tax assets in the future in excess of its net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made. Likewise, should Quaker determine that it would not be able to realize all or part of its net deferred tax assets in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination was made. Both determinations could have a material adverse impact on the Company’s financial statements. U.S. income taxes have not been provided on the undistributed earnings of non-U.S. subsidiaries since it is the Company’s intention to continue to reinvest these earnings in those foreign subsidiaries for working capital needs and growth initiatives. The amount of such undistributed earnings at December 31, 2013 was approximately $188.0 million. U.S. and foreign income taxes that would be payable if such earnings were distributed may be lower than the amount computed at the U.S. statutory rate due to the availability of foreign tax credits.
15
5. Goodwill and other intangible assets — During 2013, certain internal shifts in the Company’s management and changes to the structure of internally reported information occurred. The Company currently believes its structure, its resource allocation and its performance assessment are now more closely aligned with its four geographical regions: North America, EMEA, Asia/Pacific and South America. Therefore, the Company changed its reportable operating segments from those categorized by product nature to those organized by geography. See Note 3 of Notes to Consolidated Financial Statements in Item 8 of this Report for further information. Similarly, the Company reassessed and changed its reporting units for goodwill testing purposes during the third quarter of 2013 to adhere to its geographical orientation.
The Company records goodwill and intangible assets at fair value as of the acquisition date and amortizes definite-lived intangible assets on a straight-line basis over the useful lives of the intangible assets based on third-party valuations of the assets. Goodwill and intangible assets, which have indefinite lives, are not amortized and are required to be assessed at least annually for impairment. The Company compares the assets’ fair value to their carrying value, primarily based on future discounted cash flows, in order to determine if an impairment charge is warranted. The estimates of future cash flows involve considerable management judgment and are based upon assumptions about expected future operating performance. Assumptions used in these forecasts are consistent with internal planning, but the actual cash flows could differ from management’s estimates due to changes in business conditions, operating performance, and economic conditions. The Company’s assumption of weighted average cost of capital (“WACC”) and estimated future net operating profit after tax (“NOPAT”) are particularly important in determining estimated future cash flows. In addition, the Company revised its calculation for its step one impairment model during 2013, which now includes an estimate of future growth and cash flows in perpetuity, among other, less significant, changes.
Based on its revised reporting units, the Company completed its annual impairment assessment as of the end of the third quarter of 2013, and no impairment charge was warranted. Furthermore, the estimated fair value of each of the Company’s reporting units substantially exceeded its carrying value, with none of the Company’s reporting units at risk for failing step one of the goodwill impairment test. The Company’s consolidated goodwill and indefinite-lived intangible assets at December 31, 2013 and December 31, 2012 were $59.3 million and $60.3 million, respectively. The Company currently uses a WACC of 12% and, at September 30, 2013, this assumption would have had to increase by more than 73.2 percentage points before any of the Company’s reporting units would fail step one of the impairment analysis. Further, at September 30, 2013, the Company’s estimate of future NOPAT would have had to decrease by more than 70.3% before any of the Company’s reporting units would be considered potentially impaired.
6. Postretirement benefits—The Company provides certain pension and other postretirement benefits to employees and retirees. Independent actuaries, in accordance with accounting principles generally accepted in the United States, perform the required valuations to determine benefit expense and, if necessary, non-cash charges to equity for additional minimum pension liabilities. Critical assumptions used in the actuarial valuation include the weighted average discount rate, rates of increase in compensation levels, and expected long-term rates of return on assets. If different assumptions were used, additional pension expense or charges to equity might be required. The Company’s U.S. pension plan year-end is November 30, and the measurement date is December 31. The following table highlights the potential impact on the Company’s pre-tax earnings, due to changes in assumptions with respect to the Company’s pension plans, based on assets and liabilities at December 31, 2013:
| 1/2 Percentage Point Increase | 1/2 Percentage Point Decrease | |||||||||||||||||
| Foreign | Domestic | Total | Foreign | Domestic | Total | |||||||||||||
| (Dollars in millions) | ||||||||||||||||||
| Discount rate | $ | (0.6 | ) | $ | (0.1 | ) | $ | (0.7 | ) | $ | 0.7 | $ | 0.1 | $ | 0.8 | |||
| Expected rate of return on plan assets | (0.3 | ) | (0.2 | ) | (0.5 | ) | 0.3 | 0.2 | 0.5 | |||||||||
Liquidity and Capital Resources
Quaker’s cash and cash equivalents increased to $68.5 million at December 31, 2013 from $32.5 million at December 31, 2012. The $36.0 million increase was primarily the result of a strong amount of cash provided by operating activities of $73.8 million, net of cash used in investing activities of $12.4 million, cash used in financing activities of $26.2 million and an increase due to foreign exchange rate translation of $0.8 million. At December 31, 2013, the Company held approximately $48.6 million of its total cash and cash equivalents among its foreign subsidiaries, which is subject to possible limitations on repatriation to the United States.
Net cash flows provided by operating activities were $73.8 million in 2013, compared with $62.9 million provided by operating activities in 2012. The $10.9 million increase in net operating cash flow was led by the Company’s higher net income and improved working capital management. Affecting the working capital comparison from 2012 to 2013 were higher cash inflows in 2013 related to accounts payable and accrued liabilities due to increased business activity and timing of such expenses and lower cash outflows in 2013 for payments on estimated taxes, which were partially offset by significant cash outflows in 2013 from accounts receivable due to the timing of sales in the final months of the year compared to lower sales in the comparable months of 2012. In addition, the Company’s first dividend distribution from its captive insurance equity affiliate of $2.0 million, the mineral oil excise tax refund, discussed below and lower pension plan contributions during 2013, partially offset by fees related to the Company’s 2013 revision to its credit facility discussed below, also affected the net operating cash flow comparisons.
16
Net cash flows used in investing activities decreased from $16.7 million in 2012 to $12.4 million in 2013. The $4.3 million decrease of cash used in investing activities was primarily caused by lower payments for acquisitions and lower investments in property, plant and equipment. In 2013, the Company acquired a business that primarily related to tin plating and a chemical milling maskant distribution network for its North American segment for net consideration of approximately $2.5 million. Whereas, the Company acquired NP Coil Dexter Industries, S.r.l. for approximately $2.7 million for its EMEA segment and, also, paid hold-backs of consideration that were assumed in the past acquisitions of Tecniquimia Mexicana, S.A. de C.V. and G.W. Smith and Sons, Inc. for approximately $3.0 million during 2012. As it relates to the Company’s lower investments in property, plant and equipment, the primary changes were higher payments for the Company’s technology infrastructure during 2012, which were partially offset by an increased investment in its Asia/Pacific facilities during 2013.
Net cash flows used by financing activities were $26.2 million in 2013, compared with $30.6 million used by financing activities during 2012. In both years, the Company was able to leverage strong operating cash flow to fund its investing and financing activities, while also making payments on its revolving credit line. However, there were lower repayments of debt in 2013 compared to 2012, as the Company had repaid all outstanding borrowings on its credit line as of the third quarter of 2013. In addition, higher dividend payments and changes in stock option exercise activity in 2013 also affected the financing cash flow comparisons.
The Company completed its annual goodwill impairment assessment as of the end of the third quarter of 2013 and the estimated fair value of each of the Company’s reporting units substantially exceeded their carrying value, so no impairment charge was warranted.
As discussed in the Current Report on Form 8-K filed on June 17, 2013, the Company entered into a revised syndicated multicurrency credit facility on June 14, 2013, which amended and replaced the Company’s previous credit facility with Bank of America, N.A. and certain other major financial institutions. The revised facility increased the maximum principal amount available for revolving credit borrowings under this facility from $175.0 million to $300.0 million, which can be increased to $400.0 million at the Company’s option if the lenders agree and the Company satisfies certain conditions. This facility matures in June 2018. In addition, the revised facility amended certain financial, acquisition and other covenants, but the consolidated leverage ratio calculation, on which access to credit under the former facility largely depended, remains relatively consistent and cannot exceed 3.50 to 1. At December 31, 2013 and December 31, 2012, the consolidated leverage ratio was below 1.0 to 1 and the Company was also in compliance with all of the current and former facilities’ other covenants, respectively. At December 31, 2013, the Company had no outstanding borrowings under this revised facility. At December 31, 2012, the Company had approximately $12.2 million outstanding under its former facility.
During 2002 and 2003, the Company’s Netherlands and Italian subsidiaries paid excise taxes on mineral oil sales in Italy in the total amount of approximately $2.0 million. Alleging that the mineral oil excise tax was contrary to European Union directives, the subsidiaries filed with the Customs’ Authority of Milan (“Customs Office” or “Office”) requests to obtain a refund of the above-mentioned amount. The parties appealed rulings to various levels of tax courts up through the Supreme Court of Italy. In March 2012, the Supreme Court rejected the appeal of the Customs Office, ruling in favor of the subsidiaries and granting a refund for the amounts requested. After filing an enforcement action, the Company ultimately collected the $2.0 million, along with approximately $0.5 million of interest, in the second quarter of 2013. This amount was recorded as other income on the Company’s 2013 Consolidated Statement of Income.
At December 31, 2013, the Company’s gross liability for uncertain tax positions, including interest and penalties, was $16.8 million. The Company cannot determine a reliable estimate of the timing of cash flows by period related to its uncertain tax position liability. However, should the entire liability be paid, the amount of the payment may be reduced by up to $11.4 million as a result of offsetting benefits in other tax jurisdictions.
The Company believes it is capable of supporting its operating requirements, including pension plan contributions, payments of dividends to shareholders, possible acquisitions and other business opportunities, capital expenditures and possible resolution of contingencies, through internally generated funds supplemented with debt or equity, as needed.
17
The following table summarizes the Company’s contractual obligations at December 31, 2013, and the effect such obligations are expected to have on its liquidity and cash flows in future periods. Pension and other postretirement plan contributions beyond 2014 are not determinable since the amount of any contribution is heavily dependent on the future economic environment and investment returns on pension trust assets. The timing of payments related to other long-term liabilities, which consist primarily of deferred compensation agreements, also cannot be readily determined due to their uncertainty. Interest obligations on the Company’s short and long-term debt are included and assume the debt levels will be outstanding for the entire respective period and apply the interest rates in effect at December 31, 2013. The contingent acquisition consideration is included based on management’s estimate of the earnout liability at December 31, 2013:
| Payments due by period | ||||||||||||||||||||||||||||
| 2019 and | ||||||||||||||||||||||||||||
| Contractual Obligations (Amounts in millions) | Total | 2014 | 2015 | 2016 | 2017 | 2018 | Beyond | |||||||||||||||||||||
| Short-term debt | $ | 0.945 | $ | 0.945 | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||
| Long-term debt | 26.550 | 1.173 | 1.175 | 1.174 | 1.173 | 6.148 | 15.707 | |||||||||||||||||||||
| Capital lease obligations | 0.361 | 0.141 | 0.096 | 0.065 | 0.059 | — | — | |||||||||||||||||||||
| Non-cancelable operating leases | 14.468 | 4.991 | 4.154 | 3.507 | 1.811 | 0.005 | — | |||||||||||||||||||||
| Purchase obligations | 6.836 | 5.489 | 1.347 | — | — | — | — | |||||||||||||||||||||
| Pension and other postretirement plan | ||||||||||||||||||||||||||||
| contributions | 6.779 | 6.779 | — | — | — | — | — | |||||||||||||||||||||
| Contingent acquisition consideration | 4.697 | 4.697 | — | — | — | — | — | |||||||||||||||||||||
| Other long-term liabilities (See Note 17 of Notes | ||||||||||||||||||||||||||||
| to Consolidated Financial Statements) | 6.144 | — | — | — | — | — | 6.144 | |||||||||||||||||||||
| Total contractual cash obligations | $ | 66.780 | $ | 24.215 | $ | 6.772 | $ | 4.746 | $ | 3.043 | $ | 6.153 | $ | 21.851 | ||||||||||||||
Operations
CMS Discussion
The Company currently has numerous CMS contracts around the world. Under its traditional CMS approach, the Company effectively acts as an agent, and the revenues and costs from these sales are reported on a net sales or “pass-through” basis. Under an alternative structure for certain contracts, the contracts are structured differently in that the Company’s revenue received from the customer is a fee for products and services provided to the customer, which are indirectly related to the actual costs incurred. Profit is dependent on how well the Company controls product costs and achieves product conversions from other third-party suppliers’ products to its own products. As a result, under the alternative structure, the Company recognizes in reported revenue the gross revenue received from the CMS site customer and in cost of goods sold the third-party product purchases, which substantially offset each other until the Company achieves significant product conversions. This may result in a decrease in reported gross margin as a percentage of sales.
The Company has maintained a mix of CMS contracts with both the traditional product pass-through structure and the alternative structure, including fixed price contracts that cover all services and products. Since the global economic downturn and its impact on the automotive sector, the Company has experienced shifts in customer requirements and business circumstances, but the Company’s offerings continue to include both approaches to CMS.
Non-GAAP Measures
Included in this Form 10-K filing are non-GAAP financial measures of non-GAAP earnings per diluted share and non-GAAP adjusted EBITDA. The Company believes these non-GAAP financial measures provide meaningful supplemental information as they enhance a reader’s understanding of the financial performance of the Company, are more indicative of the future performance of the Company and facilitate a better comparison among fiscal periods, as the non-GAAP financial measures exclude items that are not considered core to the Company’s operations. Non-GAAP results are presented for supplemental informational purposes only, and should not be considered a substitute for the financial information presented in accordance with GAAP.
18
The following is a reconciliation between the non-GAAP (unaudited) financial measure of non-GAAP earnings per diluted share to its most directly comparable GAAP financial measure:
| For the years ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| GAAP earnings per diluted share attributable to Quaker Chemical Corporation | ||||||||||||
| Common Shareholders | $ | 4.27 | $ | 3.63 | $ | 3.66 | ||||||
| Equity income in a captive insurance company per diluted share | (0.41 | ) | (0.14 | ) | (0.19 | ) | ||||||
| Mineral oil excise tax refund per diluted share | (0.14 | ) | — | — | ||||||||
| Change in acquisition-related earnout liability | ||||||||||||
| per diluted share | (0.03 | ) | (0.09 | ) | (0.03 | ) | ||||||
| Revaluation of a previously held ownership interest in an equity affiliate per | ||||||||||||
| diluted share | — | — | (0.22 | ) | ||||||||
| Cost streamlining initiatives per diluted share | 0.08 | — | — | |||||||||
| Devaluation of the Venezuelan Bolivar Fuerte per diluted share | 0.03 | — | — | |||||||||
| Non-income tax contingency charge per diluted share | 0.04 | — | — | |||||||||
| Customer bankruptcy costs per diluted share | — | 0.06 | — | |||||||||
| CFO transition costs per diluted share | — | 0.03 | — | |||||||||
| Non-GAAP earnings per diluted share | $ | 3.84 | $ | 3.49 | $ | 3.22 | ||||||
The following is a reconciliation of the non-GAAP (unaudited) financial measure of adjusted EBITDA to its most directly comparable GAAP financial measure:
| For the years ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net income attributable to Quaker Chemical Corporation | $ | 56,339 | $ | 47,405 | $ | 45,892 | ||||||
| Depreciation and amortization | 15,784 | 15,358 | 13,793 | |||||||||
| Interest expense | 2,922 | 4,283 | 4,666 | |||||||||
| Taxes on income before equity in net income of associated companies | 20,489 | 15,575 | 14,256 | |||||||||
| Equity income in a captive insurance company | (5,451 | ) | (1,812 | ) | (2,323 | ) | ||||||
| Mineral oil excise tax refund | (2,540 | ) | — | — | ||||||||
| Change in acquisition-related earnout liability | (497 | ) | (1,737 | ) | (595 | ) | ||||||
| Revaluation of a previously held ownership interest in an equity affiliate | — | — | (2,718 | ) | ||||||||
| Cost streamlining initiatives | 1,419 | — | — | |||||||||
| Devaluation of the Venezuelan Bolivar Fuerte | 357 | — | — | |||||||||
| Non-income tax contingency charge | 796 | — | — | |||||||||
| Customer bankruptcy costs | — | 1,254 | — | |||||||||
| CFO transition costs | — | 609 | — | |||||||||
| Adjusted EBITDA | $ | 89,618 | $ | 80,935 | $ | 72,971 | ||||||
Out-of-Period Adjustment
As previously disclosed in the Company’s 2012 Annual Report on Form 10-K, the Company had reassessed its ability to significantly influence the operating and financial policies of its captive insurance equity affiliate, Primex. Based on its ownership percentage and other factors, the Company determined that, during 2012, the Company obtained the ability to significantly influence Primex and, as a result, changed its method of accounting from the cost to equity method. During the first quarter of 2013, the Company identified errors in Primex’s estimated 2012 financial statements, which primarily related to a reinsurance contract held by Primex. The identified errors resulted in a cumulative $1.0 million understatement of the Company’s equity in net income from associated companies for the year ended December 31, 2012. The Company corrected the errors related to Primex in the first quarter of 2013, which had the net effect of increasing equity in net income from associated companies by $1.0 million for the three months ended March 31, 2013 and the year ended December 31, 2013. The Company does not believe this adjustment is material to its consolidated financial statements for the year ended December 31, 2012 or to the Company’s results for the year ended December 31, 2013 and, therefore, has not restated any prior period amounts. See Note 2 of Notes to Consolidated Financial Statements in Item 8 of this Report.
19
Consolidated Operations Review – Comparison of 2013 with 2012
Net sales for 2013 of $729.4 million increased approximately 3% from $708.2 million in 2012. The increase in the Company’s net sales from the prior year was primarily due to a 3% increase in product volumes, including acquisitions, generally across all regions, partially offset by a decrease of $3.1 million, or less than 1%, due to foreign exchange rate translation. The effects on net sales related to price and product mix were generally consistent in 2013 compared to 2012.
Gross profit increased by approximately $22.4 million, or approximately 9%, from 2012, which was primarily the result of an improvement in gross margin to 35.8% in 2013 from 33.7% in 2012. The increase in gross margin reflects the return of the Company’s product margins to more acceptable levels.
SG&A increased approximately $14.3 million from 2012, which was primarily driven by higher labor related costs on general year-over-year merit increases, increased selling and other related costs on improved Company performance and costs added with our recent acquisitions. In addition, non-operating SG&A expenses increased due to certain uncommon costs. For instance, 2013 SG&A includes a non-income tax contingency charge of approximately $0.8 million, or $0.04 per diluted share, and, also, costs related to streamlining certain operations in the Company’s EMEA and South American segments of approximately $1.2 million, or $0.07 per diluted share. Whereas, in 2012, there were costs associated with the bankruptcies of certain U.S customers of $1.3 million, or $0.06 per diluted share, the prior year costs associated with the Company’s CFO transition of $0.6 million, or $0.03 per diluted share, and lower translation due to changes in foreign exchange rates.
Other income for 2013 was approximately $3.5 million, which was primarily driven by a refund of $2.5 million, or $0.14 per diluted share, related to past excise taxes paid on certain mineral oil sales, income of $0.5 million, or $0.03 per diluted share, related to a change in an acquisition-related earnout liability and earnings from third-party license fees, partially offset by foreign exchange losses and $0.2 million, or $0.01 per diluted share, of costs associated with the streamlining initiatives mentioned above. Other income for 2012 was approximately $3.4 million, which was primarily driven by income of $1.7 million, or $0.09 per diluted share, related to a change in the acquisition-related earnout liability noted above, earnings from third-party license fees and income from a change in a separate acquisition-related liability, partially offset by foreign exchange losses.
The decrease in interest expense from 2012 to 2013 was primarily due to lower average borrowings and lower interest rates. The increase in interest income from 2012 to 2013 was primarily due to a higher level of the Company’s cash on hand.
The Company’s effective tax rates for 2013 and 2012 of 28.1% and 24.7%, respectively, reflect decreases in reserves for uncertain tax positions due to the expiration of applicable statutes of limitations for certain years of approximately $0.15 and $0.17 per diluted share, respectively. In addition, the Company had certain one-time discrete items in the prior year that lowered its 2012 effective tax rate, which were partially offset by a change in the mix of income to lower tax jurisdictions in 2013. The Company has experienced and expects to further experience volatility in its effective tax rates due to the varying timing of tax audits and the expiration of applicable statutes of limitations as they relate to uncertain tax positions, among other factors.
Equity in net income of associated companies increased due to higher earnings related to the Company’s equity interest in a captive insurance company in 2013 compared to 2012. Earnings attributable to this equity interest increased from approximately $1.8 million, or $0.14 per diluted share, for 2012 to approximately $5.5 million, or $0.41 per diluted share, for 2013, which includes a non-cash out-of-period adjustment of approximately $1.0 million recorded in 2013. See the Out-of-Period Adjustment section in this Item, above. Partially offsetting this increase in equity in net income of associated companies was a charge of approximately $0.4 million, or $0.03 per diluted share, related to the devaluation of the Venezuelan Bolivar Fuerte in 2013.
Changes in foreign exchange rates negatively impacted 2013 net income by approximately $0.7 million, or $0.05 per diluted share.
Consolidated Operations Review – Comparison of 2012 with 2011
Net sales for 2012 were $708.2 million, an increase of 4% from $683.2 million in 2011. Product volumes, including acquisitions, increased revenues by approximately 5% and price and product mix increased revenues by approximately 3%, while foreign exchange rate translation decreased revenues by approximately $26.8 million, or 4%.
Gross profit increased by approximately $16.1 million, or 7%, from 2011, with gross margin improving to 33.7% from 32.6%, for 2011, reflecting some stabilization in raw material costs experienced primarily at the end of 2012, allowing margins to return to more acceptable levels.
SG&A increased by approximately $10.7 million, or 7%, compared to 2011, primarily related to acquisitions and higher selling, inflationary and other costs on increased business activity, which were partially offset by decreases due to foreign exchange rate translation and lower incentive compensation. Also, SG&A for 2012 included charges of $1.3 million, or $0.06 per diluted share, for certain customer bankruptcies in the U.S. and $0.6 million, or $0.03 per diluted share, related to the Company’s CFO transition.
The decrease in interest expense was primarily due to lower average borrowings and lower interest rates in 2012 as compared to 2011, and the decrease in interest income from 2011 to 2012 was primarily caused by lower cash levels invested in higher interest rate jurisdictions.
20
Other income for 2012 of $3.4 million decreased from $5.1 million in 2011. Other income increased in 2012 due to changes in the fair value of an acquisition-related earnout liability of $1.7 million, or $0.09 per diluted share and, also, a separate acquisition-related liability, compared to lower other income in 2011 included from a similar change in the acquisition-related earnout liability of $0.6 million, or $0.03 per diluted share. However, other income for 2011 also included $2.7 million, or $0.22 per diluted share, related to the revaluation of the Company’s previously held ownership interest in its Mexican affiliate to its fair value, caused by the Company’s 2011 purchase of the remaining ownership interest in this entity. In addition, the Company experienced higher foreign exchange losses in 2012 and, also, received lower third party license fees in 2012, primarily as a result of the prior year purchase of the remaining ownership interest in the Company’s Mexican affiliate.
The Company’s 2012 and 2011 effective tax rates were generally consistent at 24.7% and 24.0%, respectively. The effective tax rates reflect decreases in reserves for uncertain tax positions due to the expiration of applicable statutes of limitations for certain tax years of approximately $0.17 and $0.16 per diluted share in 2012 and 2011, respectively.
The decrease in equity in net income of associated companies was caused by lower income from the Company’s equity investment in a captive insurance company, which was $1.8 million, or $0.14 per diluted share, in 2012 compared to $2.3 million, or $0.19 per diluted share, in 2011. This decrease from 2011 to 2012 in equity in net income of associated companies was partially offset by improved performance over the majority of the Company’s other equity affiliates during 2012, in particular in our Japanese affiliate.
Earnings per diluted share for 2012 of $3.63 reflected an approximate $0.11 per share dilutive effect as a result of the Company’s equity offering in May of 2011. Changes in foreign exchange rates negatively impacted the 2012 net income by approximately $1.7 million, or $0.13 per diluted share.
Reportable Operating Segment Review – Comparison of 2013 with 2012
The Company’s reportable operating segments evidence the structure of the Company’s internal organization, the method by which the Company’s resources are allocated and the manner by which the Company assesses its performance. During 2013, certain internal shifts in the Company’s management and changes to the structure of internally reported information occurred. The Company currently believes its structure, its resource allocation and its performance assessment are now more closely aligned with its four geographical regions: North America, EMEA, Asia/Pacific and South America. Therefore, the Company changed its reportable operating segments from those categorized by product nature to those organized by geography. All prior period information has been recast to reflect these four regions as the Company’s new reportable operating segments. See Note 3 of Notes to Consolidated Financial Statements in Item 8 of this Report for further information.
The Company continues to offer its industrial process fluids, chemical specialties and technical expertise to a wide range of industries in a global product portfolio. Overall, the Company experienced improved product margins in each of its four reportable operating segments in 2013 compared to 2012, as product margins have returned to more acceptable levels globally, within each regional segment. The following is further analysis by reportable operating segment for each respective period.
North America
North America represented approximately 42% of the Company’s consolidated net sales in 2013, which decreased approximately $1.8 million, or approximately 1%, from 2012. The decrease in net sales was primarily due to a 2% decrease in base product volumes, partially offset by an increase of 1% due to acquisitions. The impact on net sales from price and product mix remained comparable to 2012. This reportable segment’s operating earnings, excluding indirect expenses, increased approximately $2.7 million, or approximately 5%, from 2012, which reflects the increase in the reportable segment’s product margins, noted above.
EMEA
EMEA represented approximately 26% of the Company’s consolidated net sales in 2013, which increased approximately $13.0 million, or approximately 7%, from 2012. The increase in net sales was primarily due to a 5% increase in base product volumes, a 3% increase from acquisitions and a 3% increase from foreign currency exchange rate translation, partially offset by a 3% decrease in price and product mix. The foreign currency translation impact was primarily due to the E.U. Euro to U.S. Dollar exchange rate, which averaged 1.33 in 2013 compared to 1.29 in 2012. This reportable segment’s operating earnings, excluding indirect expenses, increased approximately $5.0 million, or approximately 20%, from 2012, which reflects its increased net sales and the increase in the reportable segment’s product margins, noted above.
Asia/Pacific
Asia/Pacific represented approximately 23% of the Company’s consolidated net sales in 2013, which increased approximately $12.4 million, or approximately 8%, from 2012. The increase in net sales was primarily due to a 7% increase in base product volumes and a 2% increase in price and product mix, partially offset by a 1% decrease in foreign currency exchange rate translation. The foreign currency translation impact was primarily due to decreases in the Indian Rupee and Australian Dollar to U.S. Dollar exchange rates, which averaged 0.017 and 0.97 in 2013 compared to 0.019 and 1.04 in 2012, respectively. These foreign exchange decreases were partially offset by an increase in the Chinese Renminbi to U.S. Dollar exchange rate, which averaged 0.161 in 2013 compared to 0.158 in 2012. This reportable segment’s operating earnings, excluding indirect expenses, increased approximately $5.3 million, or
21
approximately 14%, from 2012, which reflects its increased net sales and the increase in the reportable segment’s product margins, noted above.
South America
South America represented approximately 9% of the Company’s consolidated net sales in 2013, which decreased approximately $2.5 million, or approximately 4%, from 2012. The decrease in net sales was driven by an 11% decrease due to foreign currency exchange rate translation, partially offset by a 2% increase in base product volumes and a 5% increase in price and product mix. The foreign currency translation impact was primarily due to decreases in the Brazilian Real and Argentinian Peso to U.S. Dollar exchange rates, which averaged 0.47 and 0.18 in 2013 compared to 0.51 and 0.22 in 2012, respectively. This reportable segment’s operating earnings, excluding indirect expenses, increased approximately $2.4 million, or approximately 36%, from 2012, which reflects the increase in the reportable segment’s product margins, noted above, and the favorable impact of cost streamlining initiatives implemented in 2013.
Reportable Operating Segment Review – Comparison of 2012 with 2011
Similar to the comparison between 2013 and 2012, the Company experienced improved product margins generally over each of its four reportable operating segments in 2012 compared to 2011. This improvement was primarily caused by raw material costs stabilizing toward the end of 2012, which began the return of the Company’s product margins to more acceptable levels that continued into 2013. The following is further analysis by reportable operating segment for each respective period.
North America
North America represented approximately 44% of the Company’s consolidated net sales in 2012, which increased approximately $41.6 million, or approximately 15%, from 2011. The increase in net sales was primarily due to a 7% increase in price and product mix and an increase of 8% due to acquisitions. The impact on net sales from base product volumes was comparable with 2011. This reportable segment’s operating earnings, excluding indirect expenses, increased approximately $10.2 million, or approximately 21%, from 2011, which reflects its increased net sales and the increase in the reportable operating segment’s product margins, noted above.
EMEA
EMEA represented approximately 25% of the Company’s consolidated net sales in 2012, which decreased approximately $9.3 million, or approximately 5%, from 2011. The decrease in net sales was primarily due to an 8% decrease in foreign currency exchange rate translation and a 1% decrease in base product volumes, partially offset by a 3% increase due to acquisitions and a 1% increase in price and product mix. The foreign currency translation impact was primarily due to the E.U. Euro to U.S. Dollar exchange rate, which averaged 1.29 in 2012 compared to 1.39 in 2011. This reportable segment’s operating earnings, excluding indirect expenses, decreased approximately $2.7 million, or approximately 10%, from 2011, which reflects its decreased net sales, partially offset by the increase in the reportable operating segment’s product margins, noted above.
Asia/Pacific
Asia/Pacific represented approximately 22% of the Company’s consolidated net sales in 2012, which increased approximately $5.6 million, or approximately 4%, from 2011. The increase in net sales was primarily due to a 3% increase in base product volumes and a 2% increase in price and product mix, partially offset by a 1% decrease in foreign currency exchange rate translation. The foreign currency translation impact was primarily due to a decrease in the Indian Rupee to U.S. Dollar exchange rate, which averaged 0.019 in 2012 compared to 0.021 in 2011, partially offset by an increase in the Chinese Renminbi to U.S. Dollar exchange rate, which averaged 0.158 in 2012 compared to 0.155 in 2011. This reportable segment’s operating earnings, excluding indirect expenses, increased approximately $4.9 million, or approximately 15%, from 2011, which reflects its increased net sales and the increase in the reportable operating segment’s product margins, noted above.
South America
South America represented approximately 9% of the Company’s consolidated net sales in 2012, which decreased approximately $12.9 million, or approximately 16%, from 2011. The decrease in net sales was primarily driven by a 13% decrease in foreign currency exchange rate translation and a 2% decrease in base product volumes, while price and product mix remained consistent with 2011. The foreign currency translation impact was primarily due to decreases in the Brazilian Real and Argentinian Peso to U.S. Dollar exchange rates, which averaged 0.51 and 0.22 in 2012 compared to 0.60 and 0.24 in 2011, respectively. This reportable segment’s operating earnings, excluding indirect expenses, decreased approximately $4.9 million, or approximately 42%, from 2011, which reflects the decrease in its net sales and a change in the mix of those sales to lower margin products during 2012.
Environmental Clean-up Activities
The Company is involved in environmental clean-up activities in connection with an existing plant location and former waste disposal sites. In April of 1992, the Company identified certain soil and groundwater contamination at AC Products, Inc. (“ACP”), a wholly owned subsidiary. In voluntary coordination with the Santa Ana California Regional Water Quality Board (“SACRWQB”), ACP is remediating the contamination. Effective October 17, 2007, ACP agreed to operate the two existing groundwater treatment systems associated with the extraction wells P-2 and P-3 so as to hydraulically contain groundwater contamination emanating from
22
ACP’s site until such time as the concentrations of contaminants are below the current Federal maximum contaminant level for four consecutive quarterly sampling events. On September 11, 2012, ACP received a letter from the SACRWQB advising that no further action is required to remediate the soil contamination on site. At December 31, 2013, the Company believes that the remaining potential-known liabilities associated with the ACP contamination, namely estimated future cost of the water remediation program, is approximately $0.4 million to $0.8 million, for which the Company has sufficient reserves. Notwithstanding the foregoing, the Company cannot be certain that liabilities in the form of remediation expenses and damages will not be incurred in excess of the amount reserved. See Note 22 of Notes to Consolidated Financial Statements which appears in Item 8 of this Report.
General
The Company generally does not use financial instruments that expose it to significant risk involving foreign currency transactions; however, the size of our non-U.S. activities has a significant impact on reported operating results and the attendant net assets. During the past three years, sales by non-U.S. subsidiaries accounted for approximately 60% to 65% of our consolidated net annual sales. See Note 3 of Notes to Consolidated Financial Statements which appears in Item 8 of this Report and the Foreign Exchange Risk section in Item 7A of this Report.
Factors that May Affect Our Future Results
(Cautionary Statements Under the Private Securities Litigation Reform Act of 1995)
Certain information included in this Report and other materials filed or to be filed by Quaker with the SEC (as well as information included in oral statements or other written statements made or to be made by us) contain or may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements can be identified by the fact that they do not relate strictly to historical or current facts. We have based these forward-looking statements on our current expectations about future events. These forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, intentions, financial condition, results of operations, future performance, and business, including:
| • | statements relating to our business strategy; | ||||
| • | our current and future results and plans; and | ||||
| • | statements that include the words “may,” “could,” “should,” “would,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan” or similar expressions. | ||||
Such statements include information relating to current and future business activities, operational matters, capital spending, and financing sources. From time to time, oral or written forward-looking statements are also included in Quaker’s periodic reports on Forms 10-Q and 8-K, press releases and other materials released to, or statements made to, the public.
Any or all of the forward-looking statements in this Report, in Quaker’s Annual Report to Shareholders for 2013 and in any other public statements we make may turn out to be wrong. This can occur as a result of inaccurate assumptions or as a consequence of known or unknown risks and uncertainties. Many factors discussed in this Report will be important in determining our future performance. Consequently, actual results may differ materially from those that might be anticipated from our forward-looking statements.
We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. However, any further disclosures made on related subjects in Quaker’s subsequent reports on Forms 10-K, 10-Q and 8-K should be consulted. These forward-looking statements are subject to risks, uncertainties and assumptions about us and our operations that are subject to change based on various important factors, some of which are beyond our control. A major risk is that the Company’s demand is largely derived from the demand for its customers’ products, which subjects the Company to uncertainties related to downturns in a customer’s business and unanticipated customer production shutdowns. Other major risks and uncertainties include, but are not limited to, significant increases in raw material costs, worldwide economic and political conditions, foreign currency fluctuations, and terrorist attacks and other acts of violence, each of which is discussed in greater detail in Item 1A of this Report. Furthermore, the Company is subject to the same business cycles as those experienced by steel, automobile, aircraft, appliance, and durable goods manufacturers. These risks, uncertainties, and possible inaccurate assumptions relevant to our business could cause our actual results to differ materially from expected and historical results. Other factors beyond those discussed in this Report could also adversely affect us. Therefore, we caution you not to place undue reliance on our forward-looking statements. This discussion is provided as permitted by the Private Securities Litigation Reform Act of 1995.
23
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Quaker is exposed to the impact of interest rates, foreign currency fluctuations, changes in commodity prices, and credit risk.
Interest Rate Risk. Quaker’s exposure to market rate risk for changes in interest rates relates primarily to its short and long-term debt. Most of Quaker’s debt is negotiated at market rates. Accordingly, if interest rates rise significantly, the cost of debt to Quaker will increase. This can have an adverse effect on Quaker, depending on the extent of Quaker’s borrowings throughout a given year. As of December 31, 2013, Quaker had no borrowings under its credit facility, but had weighted average borrowings of $25.1 million in 2012 and 2013 at a weighted average borrowing rate of approximately 1.66% (LIBOR plus a spread). If interest rates had changed by 10%, the Company’s interest expense would have correspondingly increased or decreased by less than $0.1 million. The Company previously used derivative financial instruments primarily for the purposes of hedging exposures to fluctuations in interest rates. Specifically, the Company had previously entered into interest rate swaps in order to fix a portion of its variable rate debt. The swaps matured during 2012. The Company does not enter into derivative contracts for trading or speculative purposes. See the information included under the caption “Derivatives” in Note 1 and the information in Note 21 of Notes to Consolidated Financial Statements which appears in Item 8 of this Report and is incorporated herein by reference.
Foreign Exchange Risk. A significant portion of Quaker’s revenues and earnings is generated by its foreign operations. These foreign operations also represent a significant portion of Quaker’s assets and liabilities. All such operations use the local currency as their functional currency. Accordingly, Quaker’s financial results are affected by risks typical of global business such as currency fluctuations, particularly between the U.S. Dollar and the E.U. Euro, the Brazilian Real, the Chinese Renminbi and the Indian Rupee. As exchange rates vary, Quaker’s results can be materially affected. If the E.U. Euro, the Brazilian Real, the Chinese Renminbi and the Indian Rupee had each changed by 10% against the U.S. Dollar, the Company’s 2013 revenues and pre-tax earnings would have correspondingly increased or decreased approximately $35.7 million and $5.5 million, respectively.
The Company generally does not use financial instruments that expose it to significant risk involving foreign currency transactions; however, the size of non-U.S. activities has a significant impact on reported operating results and the attendant net assets. During the past three years, sales by non-U.S. subsidiaries accounted for approximately 60% to 65% of consolidated net annual sales.
In addition, the Company often sources inventory among its worldwide operations. This practice can give rise to foreign exchange risk resulting from the varying cost of inventory to the receiving location, as well as from the revaluation of intercompany balances. The Company mitigates this risk through local sourcing efforts.
Commodity Price Risk. Many of the raw materials used by Quaker are commodity chemicals, and, therefore, Quaker’s earnings can be materially affected by market changes in raw material prices. In certain cases, Quaker has entered into fixed-price purchase contracts having a term of up to two years. These contracts provide protection to Quaker if the prices for the contracted raw materials rise; however, in certain limited circumstances, Quaker will not realize the benefit if such prices decline. If the Company’s gross margin had changed by one percentage point, the Company’s 2013 pre-tax earnings would have correspondingly increased or decreased by approximately $7.3 million.
Credit Risk. Quaker establishes allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments. If the financial condition of Quaker’s customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. Downturns in the overall economic climate may also exacerbate specific customer financial issues. A significant portion of Quaker’s revenues is derived from sales to customers in the U.S. steel and automotive industries, including some of our larger customers, where a number of bankruptcies have occurred during recent years and companies have experienced financial difficulty. When a bankruptcy occurs, Quaker must judge the amount of proceeds, if any, that may ultimately be received through the bankruptcy or liquidation process. In addition, as part of its terms of trade, Quaker may custom manufacture products for certain large customers and/or may ship product on a consignment basis. These practices may increase the Company’s exposure should a bankruptcy occur, and may require a write-down or disposal of certain inventory due to its estimated obsolescence or limited marketability. Customer returns of products or disputes may also result in similar issues related to the realizability of recorded accounts receivable or returned inventory. The Company recorded provisions for doubtful accounts of $1.1 million, $2.1 million and $0.9 million in 2013, 2012 and 2011, respectively. A change of 10% to the recorded provisions would have increased or decreased the Company’s pre-tax earnings by approximately $0.1 million, $0.2 million and $0.1 million in 2013, 2012 and 2011, respectively.
24
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
To the Shareholders and Board of Directors
of Quaker Chemical Corporation:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, comprehensive income, changes in equity, and cash flows present fairly, in all material respects, the financial position of Quaker Chemical Corporation and its subsidiaries at December 31, 2013 and December 31, 2012, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2013 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Philadelphia, PA
February 28, 2014
CONSOLIDATED STATEMENT OF INCOME
(In thousands, except per share amounts)
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net sales | $ | 729,395 | $ | 708,226 | $ | 683,231 | ||||||
| Costs and expenses | ||||||||||||
| Cost of goods sold | 468,320 | 469,515 | 460,581 | |||||||||
| Selling, general and administrative expenses | 189,832 | 175,487 | 164,738 | |||||||||
| 658,152 | 645,002 | 625,319 | ||||||||||
| Operating income | 71,243 | 63,224 | 57,912 | |||||||||
| Other income, net | 3,519 | 3,415 | 5,050 | |||||||||
| Interest expense | (2,922 | ) | (4,283 | ) | (4,666 | ) | ||||||
| Interest income | 986 | 592 | 1,081 | |||||||||
| Income before taxes and equity in net income of associated companies | 72,826 | 62,948 | 59,377 | |||||||||
| Taxes on income before equity in net income of associated companies | 20,489 | 15,575 | 14,256 | |||||||||
| Income before equity in net income of associated companies | 52,337 | 47,373 | 45,121 | |||||||||
| Equity in net income of associated companies | 6,514 | 2,867 | 3,102 | |||||||||
| Net income | 58,851 | 50,240 | 48,223 | |||||||||
| Less: Net income attributable to noncontrolling interest | 2,512 | 2,835 | 2,331 | |||||||||
| Net income attributable to Quaker Chemical Corporation | $ | 56,339 | $ | 47,405 | $ | 45,892 | ||||||
| Earnings per common share data: | ||||||||||||
| Net income attributable to Quaker Chemical Corporation Common Shareholders – basic | $ | 4.28 | $ | 3.64 | $ | 3.71 | ||||||
| Net income attributable to Quaker Chemical Corporation Common Shareholders – diluted | $ | 4.27 | $ | 3.63 | $ | 3.66 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net income | $ | 58,851 | $ | 50,240 | $ | 48,223 | ||||||
| Other comprehensive income (loss), net of tax | ||||||||||||
| Currency translation adjustments | (3,490 | ) | (1,510 | ) | (9,734 | ) | ||||||
| Defined benefit retirement plans | ||||||||||||
| Net gain (loss) arising during the period, other | 6,614 | (14,582 | ) | (9,119 | ) | |||||||
| Amortization of actuarial loss | 2,748 | 1,852 | 1,230 | |||||||||
| Amortization of prior service cost | 119 | 76 | 77 | |||||||||
| Current period change in fair value of derivatives | — | 272 | 395 | |||||||||
| Unrealized (loss) gain on available-for-sale securities | (142 | ) | 867 | (138 | ) | |||||||
| Other comprehensive income (loss) | 5,849 | (13,025 | ) | (17,289 | ) | |||||||
| Comprehensive income | 64,700 | 37,215 | 30,934 | |||||||||
| Less: comprehensive income attributable to noncontrolling interest | (1,206 | ) | (2,698 | ) | (1,256 | ) | ||||||
| Comprehensive income attributable to Quaker Chemical Corporation | $ | 63,494 | $ | 34,517 | $ | 29,678 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED BALANCE SHEET
(In thousands, except par value and share amounts)
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 68,492 | $ | 32,547 | ||||
| Accounts receivable, net | 165,629 | 154,197 | ||||||
| Inventories, net | 71,557 | 72,471 | ||||||
| Current deferred tax assets | 7,826 | 6,401 | ||||||
| Prepaid expenses and other current assets | 15,343 | 12,194 | ||||||
| Total current assets | 328,847 | 277,810 | ||||||
| Property, plant and equipment, net | 85,488 | 85,112 | ||||||
| Goodwill | 58,151 | 59,169 | ||||||
| Other intangible assets, net | 31,272 | 32,809 | ||||||
| Investments in associated companies | 19,397 | 16,603 | ||||||
| Non-current deferred tax assets | 24,724 | 30,673 | ||||||
| Other assets | 36,267 | 34,458 | ||||||
| Total assets | $ | 584,146 | $ | 536,634 | ||||
| LIABILITIES AND EQUITY | ||||||||
| Current liabilities | ||||||||
| Short-term borrowings and current portion of long-term debt | $ | 1,395 | $ | 1,468 | ||||
| Accounts payable | 72,281 | 67,586 | ||||||
| Dividends payable | 3,299 | 3,208 | ||||||
| Accrued compensation | 20,801 | 16,842 | ||||||
| Accrued pension and postretirement benefits | 1,438 | 2,188 | ||||||
| Current deferred tax liabilities | 1,057 | 253 | ||||||
| Other current liabilities | 30,585 | 16,247 | ||||||
| Total current liabilities | 130,856 | 107,792 | ||||||
| Long-term debt | 17,321 | 30,000 | ||||||
| Non-current deferred tax liabilities | 6,394 | 6,383 | ||||||
| Non-current accrued pension and postretirement benefits | 37,006 | 49,916 | ||||||
| Other non-current liabilities | �� | 47,538 | 52,867 | |||||
| Total liabilities | 239,115 | 246,958 | ||||||
| Equity | ||||||||
| Common stock $1 par value; authorized 30,000,000 shares; issued and outstanding | ||||||||
| 2013 – 13,196,140 shares; 2012 – 13,094,901 shares | 13,196 | 13,095 | ||||||
| Capital in excess of par value | 99,038 | 94,470 | ||||||
| Retained earnings | 258,620 | 215,390 | ||||||
| Accumulated other comprehensive loss | (34,700) | (41,855) | ||||||
| Total Quaker shareholders’ equity | 336,154 | 281,100 | ||||||
| Noncontrolling interest | 8,877 | 8,576 | ||||||
| Total equity | 345,031 | 289,676 | ||||||
| Total liabilities and equity | $ | 584,146 | $ | 536,634 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENT OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Net income | $ | 58,851 | $ | 50,240 | $ | 48,223 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
| Depreciation | 12,339 | 12,252 | 11,455 | |||||||||
| Amortization | 3,445 | 3,106 | 2,338 | |||||||||
| Equity in undistributed earnings of associated companies, net of dividends | (4,162 | ) | (2,350 | ) | (2,365 | ) | ||||||
| Deferred income taxes | (30 | ) | 2,354 | 2,431 | ||||||||
| Uncertain tax positions (non-deferred portion) | (1,826 | ) | (1,407 | ) | 3,673 | |||||||
| Acquisition-related fair value adjustments | 200 | (1,909 | ) | (2,624 | ) | |||||||
| Deferred compensation and other, net | (259 | ) | (156 | ) | 566 | |||||||
| Stock-based compensation | 4,161 | 3,807 | 3,513 | |||||||||
| Loss (gain) on disposal of property, plant and equipment | 200 | (108 | ) | (86 | ) | |||||||
| Insurance settlements realized | (988 | ) | (1,391 | ) | (1,840 | ) | ||||||
| Pension and other postretirement benefits | 862 | (1,427 | ) | (4,239 | ) | |||||||
| (Decrease) increase in cash from changes in current assets and current liabilities, net of acquisitions: | ||||||||||||
| Accounts receivable | (11,837 | ) | 779 | (31,558 | ) | |||||||
| Inventories | 406 | 3,228 | (9,281 | ) | ||||||||
| Prepaid expenses and other current assets | (743 | ) | 504 | (2,505 | ) | |||||||
| Accounts payable and accrued liabilities | 11,301 | (2,562 | ) | 4,442 | ||||||||
| Estimated taxes on income | 1,881 | (2,067 | ) | (2,477 | ) | |||||||
| Net cash provided by operating activities | 73,801 | 62,893 | 19,666 | |||||||||
| Cash flows from investing activities | ||||||||||||
| Investments in property, plant and equipment | (11,439 | ) | (12,735 | ) | (12,117 | ) | ||||||
| Payments related to acquisitions, net of cash acquired | (2,478 | ) | (5,635 | ) | (25,477 | ) | ||||||
| Proceeds from disposition of assets | 513 | 245 | 393 | |||||||||
| Interest earned on an insurance settlement | 52 | 69 | 80 | |||||||||
| Change in restricted cash, net | 936 | 1,322 | 1,760 | |||||||||
| Net cash used in investing activities | (12,416 | ) | (16,734 | ) | (35,361 | ) | ||||||
| Cash flows from financing activities | ||||||||||||
| Net decrease in short-term borrowings | — | (315 | ) | (254 | ) | |||||||
| Repayment of long-term debt | (12,791 | ) | (17,632 | ) | (27,364 | ) | ||||||
| Dividends paid | (13,018 | ) | (12,616 | ) | (11,586 | ) | ||||||
| Stock options exercised, other | (307 | ) | (924 | ) | 1,105 | |||||||
| Excess tax benefit related to stock option exercises | 815 | 2,045 | 109 | |||||||||
| Proceeds from sale of common stock, net of related expenses | — | — | 48,143 | |||||||||
| Dividends paid to noncontrolling shareholders | (905 | ) | (1,099 | ) | (1,000 | ) | ||||||
| Net cash (used in) provided by financing activities | (26,206 | ) | (30,541 | ) | 9,153 | |||||||
| Effect of exchange rate changes on cash | 766 | 20 | (2,315 | ) | ||||||||
| Net increase (decrease) in cash and cash equivalents | 35,945 | 15,638 | (8,857 | ) | ||||||||
| Cash and cash equivalents at beginning of period | 32,547 | 16,909 | 25,766 | |||||||||
| Cash and cash equivalents at end of period | $ | 68,492 | $ | 32,547 | $ | 16,909 | ||||||
| Supplemental cash flow disclosures: | ||||||||||||
| Cash paid during the year for: | ||||||||||||
Income taxes | $ | 17,744 | $ | 13,190 | $ | 9,110 | ||||||
Interest | 1,776 | 2,809 | 3,298 | |||||||||
| Non-cash activities: | ||||||||||||
Accrued purchases of property, plant and equipment | $ | 1,287 | $ | — | $ | — | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
(In thousands, except per share amounts)
��
| Accumulated | ||||||||||||||||||||||||
| Capital in | other | Non- | ||||||||||||||||||||||
| Common | excess of | Retained | comprehensive | controlling | ||||||||||||||||||||
| stock | par value | earnings | loss | interest | Total | |||||||||||||||||||
| Balance at December 31, 2010 | $ | 11,492 | $ | 38,275 | $ | 146,802 | $ | (12,753 | ) | $ | 6,721 | $ | 190,537 | |||||||||||
| Net income | — | — | 45,892 | — | 2,331 | 48,223 | ||||||||||||||||||
| Amounts reported in other comprehensive loss | — | — | — | (16,214 | ) | (1,075 | ) | (17,289 | ) | |||||||||||||||
| Dividends ($0.955 per share) | — | — | (11,984 | ) | — | — | (11,984 | ) | ||||||||||||||||
| Dividends paid to noncontrolling interests | — | — | — | — | (1,000 | ) | (1,000 | ) | ||||||||||||||||
| Stock offering, net of related expenses | 1,265 | 46,878 | — | — | — | 48,143 | ||||||||||||||||||
| Shares issued upon exercise of stock options and other | 47 | 811 | — | — | — | 858 | ||||||||||||||||||
| Shares issued for employee stock purchase plan | 8 | 239 | — | — | — | 247 | ||||||||||||||||||
| Equity based compensation plans | 100 | 3,413 | — | — | — | 3,513 | ||||||||||||||||||
| Excess tax benefit from stock option exercises | — | 109 | — | — | — | 109 | ||||||||||||||||||
| Balance at December 31, 2011 | 12,912 | 89,725 | 180,710 | (28,967 | ) | 6,977 | 261,357 | |||||||||||||||||
| Net income | — | — | 47,405 | — | 2,835 | 50,240 | ||||||||||||||||||
| Amounts reported in other comprehensive loss | — | — | — | (12,888 | ) | (137 | ) | (13,025 | ) | |||||||||||||||
| Dividends ($0.975 per share) | — | — | (12,725 | ) | — | — | (12,725 | ) | ||||||||||||||||
| Dividends paid to noncontrolling interests | — | — | — | — | (1,099 | ) | (1,099 | ) | ||||||||||||||||
| Shares issued upon exercise of stock options and other | 102 | (1,296 | ) | — | — | — | (1,194 | ) | ||||||||||||||||
| Shares issued for employee stock purchase plan | 7 | 263 | — | — | — | 270 | ||||||||||||||||||
| Equity based compensation plans | 74 | 3,733 | — | — | — | 3,807 | ||||||||||||||||||
| Excess tax benefit from stock option exercises | — | 2,045 | — | — | — | 2,045 | ||||||||||||||||||
| Balance at December 31, 2012 | 13,095 | 94,470 | 215,390 | (41,855 | ) | 8,576 | 289,676 | |||||||||||||||||
| Net income | — | — | 56,339 | — | 2,512 | 58,851 | ||||||||||||||||||
| Amounts reported in other comprehensive income | — | — | — | 7,155 | (1,306 | ) | 5,849 | |||||||||||||||||
| Dividends ($0.995 per share) | — | — | (13,109 | ) | — | — | (13,109 | ) | ||||||||||||||||
| Dividends paid to noncontrolling interests | — | — | — | — | (905 | ) | (905 | ) | ||||||||||||||||
| Shares issued upon exercise of stock options and other | 24 | (668 | ) | — | — | — | (644 | ) | ||||||||||||||||
| Shares issued for employee stock purchase plan | 6 | 331 | — | — | — | 337 | ||||||||||||||||||
| Equity based compensation plans | 71 | 4,090 | — | — | — | 4,161 | ||||||||||||||||||
| Excess tax benefit from stock option exercises | — | 815 | — | — | — | 815 | ||||||||||||||||||
| Balance at December 31, 2013 | $ | 13,196 | $ | 99,038 | $ | 258,620 | $ | (34,700 | ) | $ | 8,877 | $ | 345,031 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
31
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands except per share amounts)
Note 1 – Significant Accounting Policies
Principles of consolidation: All majority-owned subsidiaries are included in the Company’s consolidated financial statements, with appropriate elimination of intercompany balances and transactions. Investments in associated companies (less than majority-owned and in which the Company has significant influence) are accounted for under the equity method. The Company’s share of net income or losses in these investments in associated companies is included in the Consolidated Statement of Income. The Company periodically reviews these investments for impairments and, if necessary, would adjust these investments to their fair value when a decline in market value or other impairment indicators are deemed to be other than temporary.
The Financial Accounting Standards Board’s (“FASB’s”) guidance regarding the consolidation of certain Variable Interest Entities (“VIEs”) generally requires that assets, liabilities and results of the activities of a VIE be consolidated into the financial statements of the enterprise that is considered the primary beneficiary. The consolidated financial statements include the accounts of the Company and all of its subsidiaries in which a controlling interest is maintained and would include any VIEs if the Company was the primary beneficiary pursuant to the provisions of the applicable guidance.
Translation of foreign currency: Assets and liabilities of non-U.S. subsidiaries and associated companies are translated into U.S. Dollars at the respective rates of exchange prevailing at the end of the year. Income and expense accounts are translated at average exchange rates prevailing during the year. Translation adjustments resulting from this process are recorded directly in equity as accumulated other comprehensive income (loss) and will be included as income or expense only upon sale or liquidation of the underlying investment. All non-U.S. subsidiaries use their local currency as their functional currency.
Cash and cash equivalents: The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents.
Inventories: Inventories are valued at the lower of cost or market value, and are valued using the first-in, first-out (“FIFO”) method. See also Note 9 of Notes to Consolidated Financial Statements.
Long-lived assets: Property, plant and equipment are stated at cost. Depreciation is computed using the straight-line method on an individual asset basis over the following estimated useful lives: buildings and improvements, 10 to 45 years; and machinery and equipment, 1 to 15 years. The carrying value of long-lived assets is periodically evaluated whenever changes in circumstances or current events indicate the carrying amount of such assets may not be recoverable. An estimate of undiscounted cash flows produced by the asset, or the appropriate group of assets, is compared with the carrying value to determine whether an impairment exists. If necessary, the Company recognizes an impairment loss for the difference between the carrying amount of the assets and their estimated fair value. Fair value is based on current and anticipated future undiscounted cash flows. Upon sale or other dispositions of long-lived assets, the applicable amounts of asset cost and accumulated depreciation are removed from the accounts and the net amount, less proceeds from disposals, is recorded in income. Expenditures for renewals or improvements that increase the estimated useful life or capacity of the assets are capitalized, whereas expenditures for repairs and maintenance are expensed when incurred.
Capitalized software: The Company capitalizes certain costs incurred in connection with developing or obtaining software for internal use. These costs are amortized over a period of three to five years once the assets are ready for their intended use. In connection with the implementations and upgrades to the Company’s global transaction, consolidation and other related systems, approximately $1,198 and $2,395 of net costs were capitalized in property, plant and equipment on the Company’s December 31, 2013 and December 31, 2012 Consolidated Balance Sheets, respectively.
Goodwill and other intangible assets: The Company records goodwill, definite-lived intangible assets and indefinite-lived intangible assets at fair value at the date of acquisition. Goodwill and indefinite-lived intangible assets are not amortized, but tested for impairment at least annually. These tests will be performed more frequently if triggering events indicate potential impairment. Definite-lived intangible assets are amortized over their estimated useful lives, generally for periods ranging from 5 to 20 years. The Company continually evaluates the reasonableness of the useful lives of these assets, consistent with the discussion of long-lived assets, above. See Note 11 of Notes to Consolidated Financial Statements.
Revenue recognition: The Company recognizes revenue in accordance with the terms of the underlying agreements, when title and risk of loss have been transferred, when collectability is reasonably assured, and when pricing is fixed or determinable. This generally occurs when products are shipped to customers or, for consignment-type arrangements, upon usage by the customer and when services are performed. License fees and royalties are included in other income when recognized in accordance with their agreed-upon terms, when performance obligations are satisfied, when the amount is fixed or determinable, and when collectability is reasonably assured. As part of the Company’s chemical management services, certain third-party product sales to customers are managed by the Company. Where the Company acts as a principal, revenues are recognized on a gross reporting basis at the selling price negotiated with its customers. Where the Company acts as an agent, such revenue is recorded using net reporting as service
32
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
revenue at the amount of the administrative fee earned by the Company for ordering the goods. Third-party products transferred under arrangements resulting in net reporting totaled $41,553, $39,299 and $50,893 for 2013, 2012 and 2011, respectively.
Accounts receivable and allowance for doubtful accounts: Trade accounts receivable are recorded at the invoiced amount and generally do not bear interest. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses with its existing accounts receivable. Reserves for customers filing for bankruptcy protection are generally established at 75-100% of the amount owed at the filing date, dependent on the Company’s evaluation of likely proceeds from the bankruptcy process. Large and/or financially distressed customers are generally reserved for on a specific review basis while a general reserve is established for other customers based on historical experience. The Company performs a formal review of its allowance for doubtful accounts quarterly. Account balances are charged off against the allowance when the Company feels it is probable the receivable will not be recovered. The Company does not have any off-balance-sheet credit exposure related to its customers. During 2013, the Company’s five largest customers accounted for approximately 18% of its consolidated net sales with the largest customer (Arcelor-Mittal Group) accounting for approximately 9% of the Company’s consolidated net sales.
During 2012, the Company recorded charges of $1,254 to its allowance for doubtful accounts and selling, general and administrative expenses (“SG&A”) due to the bankruptcies of two U.S. customers. See Note 8 of Notes to Consolidated Financial Statements.
Research and development costs: Research and development costs are expensed as incurred and are included in SG&A. Research and development expenses were $21,578, $19,993 and $18,812 in 2013, 2012 and 2011, respectively.
Concentration of credit risk: Financial instruments, which potentially subject the Company to a concentration of credit risk, principally consist of cash equivalents, short-term investments and trade receivables. The Company invests temporary and excess funds in money market securities and financial instruments having maturities typically within 90 days. The Company has not experienced losses from the aforementioned investments.
Environmental liabilities and expenditures: Accruals for environmental matters are recorded when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. If there is a range of estimated liability and no amount in that range is considered more probable than another, then the Company records the lowest amount in the range in accordance with generally accepted accounting principles. Accrued liabilities are exclusive of claims against third parties and are not discounted. Environmental costs and remediation costs are capitalized if the costs extend the life, increase the capacity or improve safety or efficiency of the property from the date acquired or constructed, and/or mitigate or prevent contamination in the future.
Asset retirement obligations: The Company follows the FASB’s guidance regarding asset retirement obligations, which addresses the accounting and reporting for obligations associated with the retirement of tangible long-lived assets and the associated retirement costs. Also, the Company follows the FASB’s guidance for conditional asset retirement obligations (“CARO”), which relates to legal obligations to perform an asset retirement activity in which the timing and (or) method of settlement are conditional on a future event that may or may not be within the control of the entity. In accordance with this guidance, the Company records a liability when there is enough information regarding the timing of the CARO to perform a probability-weighted discounted cash flow analysis. At December 31, 2013 and December 31, 2012, the Company had limited exposure to such obligations and had immaterial liabilities recorded for such on its Consolidated Balance Sheets.
Pension and other postretirement benefits: The Company maintains various noncontributory retirement plans, the largest of which is in the U.S., covering substantially all of its employees in the U.S. and certain other countries. The plans of the Company’s subsidiaries in The Netherlands, the United Kingdom and Mexico are subject to the provisions of FASB’s guidance regarding employers’ accounting for defined benefit pension plans. The plans of the remaining non-U.S. subsidiaries are, for the most part, either fully insured or integrated with the local governments’ plans and are not subject to the provisions of the guidance. The guidance requires that employers recognize on a prospective basis the funded status of their defined benefit pension and other postretirement plans on their consolidated balance sheet and, also, recognize as a component of other comprehensive income, net of tax, the gains or losses and prior service costs or credits that arise during the period but are not recognized as components of net periodic benefit cost. The Company’s U.S. pension plan year ends on November 30 and the measurement date is December 31. The measurement date for the Company’s other postretirement benefits plan is December 31.
The Company’s pension investment policy is designed to ensure that pension assets are invested in a manner consistent with meeting the future benefit obligations of the pension plans and maintaining compliance with various laws and regulations including the Employee Retirement Income Security Act of 1974 (“ERISA”). The Company establishes strategic asset allocation percentage targets and appropriate benchmarks for significant asset classes with the aim of achieving a prudent balance between return and risk. The Company’s investment horizon is generally long term, and, accordingly, the target asset allocations encompass a long-term
33
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
perspective of capital markets, expected risk and return and perceived future economic conditions while also considering the profile of plan liabilities. To the extent feasible, the short-term investment portfolio is managed to immunize the short-term obligations, the intermediate portfolio duration is immunized to reduce the risk of volatility in intermediate plan distributions, and the total return portfolio is expected to maximize the long-term real growth of plan assets. The critical investment principles of diversification, assessment of risk and targeting the optimal expected returns for given levels of risk are applied. The Company’s investment guidelines prohibit use of securities such as letter stock and other unregistered securities, commodities or commodity contracts, short sales, margin transactions, private placements (unless specifically addressed by addendum), or any derivatives, options or futures for the purpose of portfolio leveraging.
The target asset allocation is reviewed periodically and is determined based on a long-term projection of capital market outcomes, inflation rates, fixed income yields, returns, volatilities and correlation relationships. The interaction between plan assets and benefit obligations is periodically studied to assist in establishing such strategic asset allocation targets. Asset performance is monitored with an overall expectation that plan assets will meet or exceed benchmark performance over rolling five-year periods. The Company’s pension committee, as authorized by the Company’s Board of Directors, has discretion to manage the assets within established asset allocation ranges approved by senior management of the Company. As of December 31, 2013, the plan’s investments were in compliance with all approved ranges of asset allocations. See Note 16 of Notes to Consolidated Financial Statements.
Comprehensive income (loss): The Company presents other comprehensive income (loss) in its Statement of Comprehensive Income. During 2013, the Company adopted the FASB’s guidance regarding the disclosure of reclassifications from Accumulated Other Comprehensive Income (Loss) (“AOCI”). The guidance requires the disclosure of significant amounts reclassified from each component of AOCI, the related tax amounts and the income statement line items affected by the reclassifications, either parenthetically on the Consolidated Statement of Comprehensive Income or in the Notes to the Consolidated Financial Statements. The Company elected to present the information in the Notes to the Consolidated Financial Statements, and the adoption of this guidance did not have a material impact on the Company’s results or financial condition. See Note 18 of Notes to Consolidated Financial Statements.
Income taxes and uncertain tax positions: The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. The provision for income taxes represents income taxes paid or payable for the current year and the change in deferred taxes during the year. Deferred taxes result from differences between the financial and tax bases of the Company’s assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. The FASB’s guidance regarding accounting for uncertainty in income taxes prescribes the recognition threshold and measurement attributes for financial statement recognition and measurement of tax positions taken or expected to be taken on a tax return. The guidance further requires the determination of whether the benefits of tax positions will be more likely than not sustained upon audit based upon the technical merits of the tax position. For tax positions that are determined to be more likely than not sustained upon audit, a company recognizes the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement in the financial statements. For tax positions that are not determined to be more likely than not sustained upon audit, a company does not recognize any portion of the benefit in the financial statements. Additionally, the guidance provides for derecognition, classification, penalties and interest, accounting in interim periods, disclosure and transition. The guidance also requires that the amount of interest expense and income to be recognized related to uncertain tax positions be computed by applying the applicable statutory rate of interest to the difference between the tax position recognized, including timing differences, and the amount previously taken or expected to be taken in a tax return. The Company’s continuing practice is to recognize interest and/or penalties related to income tax matters in income tax expense. During 2013, the Company adopted guidance that requires an entity to net its liability for unrecognized tax benefits against deferred tax assets related to net operating losses or other tax credit carryforwards that would apply if the uncertain tax position were settled for the presumed amount at the balance sheet date, which had an immaterial impact on the Company’s current year results and financial condition. See Note 6 of Notes to Consolidated Financial Statements.
Derivatives: The Company is exposed to the impact of changes in interest rates, foreign currency fluctuations, changes in commodity prices and credit risk. The Company is currently not using derivative instruments to mitigate the risks associated with foreign currency fluctuations, changes in commodity prices or credit risk, but has used derivative financial instruments primarily for purposes of hedging exposures to fluctuations in interest rates in the past. The Company recognized all derivatives on its balance sheet at fair value. For derivative instruments designated as cash flow hedges, the effective portion of any hedge would be reported in Accumulated Other Comprehensive Income (Loss) until it was cleared to earnings during the same period in which the hedged item affected earnings. The Company currently uses no derivative instruments designated as fair value hedges and has not entered into derivative contracts for trading or speculative purposes. See Note 21 of Notes to Consolidated Financial Statements.
34
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Fair value measurements: The Company utilizes the FASB’s guidance regarding fair value measurements, which establishes a common definition for fair value to be applied to guidance requiring use of fair value, establishes a framework for measuring fair value and expands disclosure about such fair value measurements. Specifically, the guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
| • | Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. | |
| • | Level 2: Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. | |
| • | Level 3: Unobservable inputs that reflect the reporting entity's own assumptions. |
Stock-based compensation: The Company applies the FASB’s guidance regarding share-based payments, which requires the recognition of the fair value of stock-based compensation as a component of expense. The Company has a long-term incentive program (“LTIP”) for key employees which provides for the granting of options to purchase stock at prices not less than its market value on the date of the grant. Most options become exercisable between one and three years after the date of the grant for a period of time determined by the Company, but not to exceed seven years from the date of grant. Common stock awards and Restricted Stock Units (“RSU”) issued under the LTIP program are subject only to time vesting over a three to five-year period. In addition, as part of the Company’s Global Annual Incentive Plan (“GAIP”), nonvested shares may be issued to key employees, which generally vest over a two to five-year period. Based on historical experience, the Company has generally assumed a forfeiture rate of 13% on its nonvested stock awards. The Company will record additional expense if the actual forfeiture rate is lower than estimated, and will record a recovery of prior expense if the actual forfeiture is higher than estimated. See Note 4 of Notes to Consolidated Financial Statements.
Earnings per share: The Company follows FASB’s guidance regarding the calculation of earnings per share (“EPS”) for nonvested stock awards with rights to non-forfeitable dividends. The guidance requires nonvested stock awards with rights to non-forfeitable dividends to be included as part of the basic weighted average share calculation under the two-class method. See Note 7 of Notes to Consolidated Financial Statements.
Segments: The Company’s reportable operating segments evidence the structure of the Company’s internal organization, the method by which the Company’s resources are allocated and the manner by which the Company assesses its performance. During 2013, certain internal shifts in the Company’s management and changes to the structure of internally reported information occurred. The Company currently believes its structure, its resource allocation and its performance assessment are now more closely aligned with its four geographical regions: the North America region, the Europe, Middle East and Africa (“EMEA”) region, the Asia/Pacific region and the South America region. Therefore, the Company changed its reportable operating segments from those categorized by product nature to those organized by geography. All prior period information has been recast to reflect these four regions as the Company’s new reportable operating segments. See Note 3 of Notes to Consolidated Financial Statements.
Reclassifications: Certain information has been reclassified to conform to the current year presentation.
Accounting estimates: The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and disclosure of contingencies at the date of the financial statements and the reported amounts of net sales and expenses during the reporting period. Actual results could differ from such estimates.
Note 2 – Out-of-Period Adjustment
As previously disclosed in the Company’s 2012 Annual Report on Form 10-K, the Company had reassessed its ability to significantly influence the operating and financial policies of its captive insurance equity affiliate, Primex. Based on its ownership percentage and other factors, the Company determined that, during 2012, the Company obtained the ability to significantly influence Primex and, as a result, changed its method of accounting from the cost to equity method. During the first quarter of 2013, the Company identified errors in Primex’s estimated 2012 financial statements, which primarily related to a reinsurance contract held by Primex. The identified errors resulted in a cumulative $1,038 understatement of the Company’s equity in net income from associated companies for the year ended December 31, 2012. The Company corrected the errors related to Primex in the first quarter of 2013,
35
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
which had the net effect of increasing equity in net income from associated companies by $1,038 for the three months ended March 31, 2013 and the year ended December 31, 2013. The Company does not believe this adjustment is material to its consolidated financial statements for the year ended December 31, 2012 or to the Company’s results for the year ended December 31, 2013 and, therefore, did not restate any prior period amounts.
Note 3 – Business Segments
The Company’s reportable operating segments evidence the structure of the Company’s internal organization, the method by which the Company’s resources are allocated and the manner by which the Company assesses its performance. During 2013, certain internal shifts in the Company’s management and changes to the structure of internally reported information occurred. The Company currently believes its structure, its resource allocation and its performance assessment are now more closely aligned with its four geographical regions: North America, EMEA, Asia/Pacific and South America. Therefore, the Company changed its reportable operating segments from those categorized by product nature to those organized by geography. All prior period information has been recast to reflect these four regions as the Company’s new reportable operating segments.
Though the Company changed its reportable operating segments in the third quarter of 2013, the calculation of the reportable segment’s measure of earnings remains relatively consistent with past practices. Operating earnings, excluding indirect operating expenses, for the Company’s reportable segments are comprised of revenues less costs of goods sold and SG&A directly related to the respective regions’ product sales. The Company’s indirect operating expenses consist of SG&A related expenses that are not directly attributable to the product sales of each respective reporting segment. Other items not specifically identified with the Company’s reportable operating segments include interest expense, interest income, license fees from non-consolidated affiliates and other income (expense).
The following tables present information about the performance of the Company’s reportable operating segments for the years ended December 31, 2013, December 31, 2012 and December 31, 2011:
| 2013 | 2012 | 2011 | ||||||||||
| Net sales | ||||||||||||
| North America | $ | 308,353 | $ | 310,127 | $ | 268,519 | ||||||
| EMEA | 187,794 | 174,799 | 184,063 | |||||||||
| Asia/Pacific | 169,505 | 157,062 | 151,468 | |||||||||
| South America | 63,743 | 66,238 | 79,181 | |||||||||
| Total net sales | $ | 729,395 | $ | 708,226 | $ | 683,231 | ||||||
| 2013 | 2012 | 2011 | ||||||||||
| Operating earnings, excluding indirect operating expenses | ||||||||||||
North America | $ | 61,307 | $ | 58,571 | $ | 48,342 | ||||||
EMEA | 29,643 | 24,640 | 27,376 | |||||||||
Asia/Pacific | 42,373 | 37,030 | 32,145 | |||||||||
South America | 9,177 | 6,730 | 11,642 | |||||||||
| Total operating earnings, excluding indirect operating expenses | 142,500 | 126,971 | 119,505 | |||||||||
| Non-operating charges | (67,145 | ) | (59,983 | ) | (58,689 | ) | ||||||
| Depreciation of corporate assets and amortization | (4,112 | ) | (3,764 | ) | (2,904 | ) | ||||||
| Consolidated operating income | 71,243 | 63,224 | 57,912 | |||||||||
| Other income, net | 3,519 | 3,415 | 5,050 | |||||||||
| Interest expense | (2,922 | ) | (4,283 | ) | (4,666 | ) | ||||||
| Interest income | 986 | 592 | 1,081 | |||||||||
| Consolidated income before taxes and equity in net income of associated companies | $ | 72,826 | $ | 62,948 | $ | 59,377 | ||||||
36
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
The following tables present information regarding the Company’s reportable segments’ assets as of December 31, 2013, December 31, 2012 and December 31, 2011:
| 2013 | 2012 | 2011 | ||||||||||
| Segment assets | ||||||||||||
North America | $ | 304,069 | $ | 284,362 | $ | 289,761 | ||||||
EMEA | 104,774 | 102,159 | 88,494 | |||||||||
Asia/Pacific | 129,894 | 101,760 | 81,086 | |||||||||
South America | 45,409 | 48,353 | 51,811 | |||||||||
Total segment assets | $ | 584,146 | $ | 536,634 | $ | 511,152 | ||||||
| 2013 | 2012 | 2011 | ||||||||||
| Segment long-lived assets | ||||||||||||
North America | $ | 91,464 | $ | 91,121 | $ | 90,800 | ||||||
EMEA | 20,863 | 20,056 | 19,243 | |||||||||
Asia/Pacific | 24,695 | 19,720 | 15,225 | |||||||||
South America | 4,130 | 5,276 | 6,577 | |||||||||
Total segment long-lived assets | $ | 141,152 | $ | 136,173 | $ | 131,845 | ||||||
The following tables present information regarding the Company’s reportable segments’ capital expenditures and depreciation as of December 31, 2013, December 31, 2012 and December 31, 2011:
| 2013 | 2012 | 2011 | ||||||||||
| Capital expenditures | ||||||||||||
North America | $ | 2,793 | $ | 3,262 | $ | 5,185 | ||||||
EMEA | 1,391 | 3,332 | 2,442 | |||||||||
Asia/Pacific | 6,386 | 5,451 | 2,614 | |||||||||
South America | 869 | 690 | 1,876 | |||||||||
Total segment capital expenditures | $ | 11,439 | $ | 12,735 | $ | 12,117 | ||||||
| 2013 | 2012 | 2011 | ||||||||||
| Depreciation | ||||||||||||
North America | $ | 5,236 | $ | 5,635 | $ | 4,739 | ||||||
EMEA | 3,145 | 2,906 | 2,922 | |||||||||
Asia/Pacific | 2,080 | 1,720 | 1,774 | |||||||||
South America | 1,211 | 1,333 | 1,454 | |||||||||
Total segment depreciation | $ | 11,672 | $ | 11,594 | $ | 10,889 | ||||||
The following table presents information regarding the Company’s product lines that represent more than 10% of consolidated revenues for December 31, 2013, December 31, 2012 and December 31, 2011, with the remaining product sales impractical to present:
| 2013 | 2012 | 2011 | |||||||
| Rolling Lubricants | 20.7 | % | 20.7 | % | 22.0 | % | |||
| Machining and grinding compounds | 17.7 | % | 17.6 | % | 18.8 | % | |||
| Hydraulic fluids | 12.9 | % | 13.5 | % | 12.9 | % | |||
| Corrosion preventives | 12.5 | % | 12.4 | % | 11.5 | % |
At December 31, 2013, December 31, 2012 and December 31, 2011, the North American segment had approximately $29,002, $27,125 and $12,818 of net sales and $3,649, $3,716 and $3,576 of long-lived assets, respectively, which were attributable to non-domestic operations.
37
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Inter-segment revenue for the years ended December 31, 2013, December 31, 2012 and December 31, 2011 was $8,984, $10,026 and $13,728 for North America, $20,135, $15,414 and $17,246 for EMEA, $504, $321 and $399 for Asia/Pacific, and zero in all periods for South America, respectively. However, all inter-segment transactions have been eliminated from each reportable operating segment’s net sales and earnings for all periods presented in the above tables.
Note 4 – Stock-Based Compensation
The Company recognized share-based compensation expense in SG&A in its Consolidated Statement of Income for the years ended December 31, 2013, December 31, 2012 and December 31, 2011, including the following:
| December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Stock options | $ | 517 | $ | 542 | $ | 482 | ||||||
| Nonvested stock awards and restricted stock units | 1,900 | 1,504 | 1,430 | |||||||||
| Employee stock purchase plan | 60 | 48 | 44 | |||||||||
| Non-elective and elective 401(k) matching contribution in stock | 1,612 | 1,653 | 1,497 | |||||||||
| Director stock ownership plan | 72 | 60 | 60 | |||||||||
| Total share-based compensation expense | $ | 4,161 | $ | 3,807 | $ | 3,513 | ||||||
As of December 31, 2013, December 31, 2012 and December 31, 2011, the Company recorded $815, $2,045 and $109, respectively, of excess tax benefits in capital in excess of par value on its Consolidated Balance Sheets related to stock option exercises. For 2013, 2012 and 2011, the Company also recognized these benefits as a cash inflow from financing activities in its Consolidated Statement of Cash Flows, which represents the Company’s estimate of cash savings during 2013, 2012 and 2011.
Stock option activity under all plans is as follows:
| 2013 | 2012 | ||||||||||||
| Weighted | Weighted | Weighted | Weighted | ||||||||||
| Average | Average | Average | Average | ||||||||||
| Exercise | Remaining | Exercise | Remaining | ||||||||||
| Number of | Price | Contractual | Number of | Price | Contractual | ||||||||
| Shares | per Share | Term (years) | Shares | per Share | Term (years) | ||||||||
| Options outstanding at January 1, | 107,455 | $ | 31.23 | 253,342 | $ | 16.43 | |||||||
| Options granted | 29,302 | 58.26 | 40,157 | 38.57 | |||||||||
| Options exercised | (57,137) | 27.12 | (177,574) | 11.87 | |||||||||
| Options forfeited | (3,601) | 37.81 | (8,470) | 29.32 | |||||||||
| Options expired | (768) | 37.37 | — | — | |||||||||
| Options outstanding at December 31, | 75,251 | $ | 44.49 | 5.2 | 107,455 | $ | 31.23 | 5.1 | |||||
| Options exercisable at December 31, | 11,840 | $ | 28.42 | 3.9 | 17,360 | $ | 28.81 | 4.4 | |||||
The total intrinsic value of options exercised during 2013 was approximately $2,237. Intrinsic value is calculated as the difference between the current market price of the underlying security and the strike price of a related option. As of December 31, 2013, the total intrinsic value of options outstanding was $2,462 and the total intrinsic value of exercisable options was approximately $578.
38
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
A summary of the Company’s outstanding stock options at December 31, 2013 is as follows:
| Number | Weighted | Weighted | Number | Weighted | |||||||||||
| Outstanding | Average | Average | Exercisable | Average | |||||||||||
| Range of | at | Contractual | Exercise | at | Exercise | ||||||||||
| Exercise Prices | 12/31/2013 | Life | Price | 12/31/2013 | Price | ||||||||||
| $ | 0.00 | - | $ | 10.00 | — | — | $ | — | — | $ | — | ||||
| $ | 10.01 | - | $ | 20.00 | 6,155 | 3.1 | 18.82 | 6,155 | 18.82 | ||||||
| $ | 20.01 | - | $ | 30.00 | — | — | — | — | — | ||||||
| $ | 30.01 | - | $ | 40.00 | 37,602 | 4.8 | 37.86 | 4,954 | 37.73 | ||||||
| $ | 40.01 | - | $ | 50.00 | 2,192 | 5.5 | 46.21 | 731 | 46.21 | ||||||
| $ | 50.01 | - | $ | 60.00 | 29,302 | 6.2 | 58.26 | — | — | ||||||
| 75,251 | 5.2 | 44.49 | 11,840 | 28.42 | |||||||||||
As of December 31, 2013, unrecognized compensation expense related to options granted in 2011 was $24, for options granted during 2012 was $240 and for options granted in 2013 was $464.
Consistent with prior years, the Company granted stock options under its LTIP plan that are subject only to time vesting over a three-year period in the first quarters of 2010, 2011, 2012 and 2013. Also, in connection with a transition of key employees in the Company during the second quarter of 2012, stock options were granted that are also subject only to time vesting over a three-year period. For the purposes of determining the fair value of stock option awards, the Company uses the Black-Scholes option pricing model and the assumptions set forth in the table below:
| For the Year Ended December 31, | June 30, | ||||||||||||||
| 2013 | 2012 | 2011 | 2010 | 2012 | |||||||||||
| Stock option awards | 29,302 | 37,965 | 36,835 | 110,939 | 2,192 | ||||||||||
| Dividend yield | 2.49 | % | 3.09 | % | 5.00 | % | 5.10 | % | 2.69 | % | |||||
| Expected volatility | 57.28 | % | 69.90 | % | 62.13 | % | 53.72 | % | 69.09 | % | |||||
| Risk-free interest rate | 0.63 | % | 0.61 | % | 1.99 | % | 2.85 | % | 0.58 | % | |||||
| Expected term (years) | 4.0 | 4.0 | 5.0 | 6.0 | 4.0 | ||||||||||
These awards are being amortized on a straight-line basis over the respective vesting period of each award. The compensation expense recorded on each award during 2013, 2012 and 2011, respectively, is as follows:
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| 2013 Stock option awards | $ | 174 | $ | — | $ | — | ||||||
| 2012 Stock option awards | $ | 189 | $ | 167 | $ | — | ||||||
| 2011 Stock option awards | $ | 138 | $ | 164 | $ | 139 | ||||||
| 2010 Stock option awards | $ | 16 | $ | 193 | $ | 224 | ||||||
39
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Activity of nonvested shares granted under the Company’s LTIP plan is shown below:
| Weighted | |||||
| Average Grant | |||||
| Number of | Date Fair Value | ||||
| Shares | (per share) | ||||
| Nonvested awards, December 31, 2012 | 122,944 | $ | 31.98 | ||
Granted | 51,659 | $ | 61.56 | ||
Vested | (51,981) | $ | 26.02 | ||
Forfeited | (6,638) | $ | 41.70 | ||
| Nonvested awards, December 31, 2013 | 115,984 | $ | 47.27 | ||
The fair value of the nonvested stock is based on the trading price of the Company’s common stock on the date of grant. The Company adjusts the grant date fair value for expected forfeitures based on historical experience for similar awards. As of December 31, 2013, unrecognized compensation expense related to these awards was $2,772, to be recognized over a weighted average remaining period of 2.32 years.
Activity of nonvested restricted stock units granted under the Company’s LTIP plan is shown below:
| Weighted | |||||
| Average Grant | |||||
| Number of | Date Fair Value | ||||
| Units | (per unit) | ||||
| Nonvested awards, December 31, 2012 | 2,100 | $ | 38.13 | ||
Granted | 1,918 | $ | 62.39 | ||
| Nonvested awards, December 31, 2013 | 4,018 | $ | 49.71 | ||
The fair value of the nonvested restricted stock units is based on the trading price of the Company’s common stock on the date of grant. The Company adjusts the grant date fair value for expected forfeitures based on historical experience for similar awards. As of December 31, 2013, unrecognized compensation expense related to these awards was $114 to be recognized over a weighted average remaining period of 2.16 years.
Employee Stock Purchase Plan
In 2000, the Board adopted an Employee Stock Purchase Plan (“ESPP”) whereby employees may purchase Company stock through a payroll deduction plan. Purchases are made from the plan and credited to each participant’s account at the end of each month, the “Investment Date.” The purchase price of the stock is 85% of the fair market value on the Investment Date. The plan is compensatory and the 15% discount is expensed on the Investment Date. All employees, including officers, are eligible to participate in this plan. A participant may withdraw all uninvested payment balances credited to a participant’s account at any time. An employee whose stock ownership of the Company exceeds five percent of the outstanding common stock is not eligible to participate in this plan.
2013 Director Stock Ownership Plan
In March 2013, the Company adopted the 2013 Director Stock Ownership Plan (the “Plan”), subject to the approval by the Company’s shareholders at the annual meeting, to encourage the Directors to increase their investment in the Company. The Plan was approved at the Company’s May 2013 shareholders’ meeting. The Plan authorizes the issuance of up to 75,000 shares of Quaker common stock in accordance with the terms of the Plan in payment of all or a portion of the annual cash retainer payable to each of the Company’s non-employee directors in 2013 and subsequent years during the term of the Plan. Under the Plan, each director who, on May 1st of the applicable calendar year, owns less than 400% of the annual cash retainer for the applicable calendar year, divided by the average of the closing price of a share of Quaker Common Stock as reported by the composite tape of the New York Stock Exchange for the previous calendar year (the “Threshold Amount”), is required to receive 75% of the annual cash retainer in Quaker common stock and 25% of the retainer in cash, unless the director elects to receive a greater percentage of Quaker common stock (up to 100%) of the annual cash retainer for the applicable year. Each director who owns more than the Threshold Amount may elect to receive common stock in payment of a percentage (up to 100%) of the annual cash retainer. The annual retainer is $50 and the
40
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
retainer payment date is June 1. The Plan was adopted in order to replace the 2003 Director Stock Ownership Plan, which expired in May 2013.
Note 5 – Other income (expense)
Other income (expense) includes:
| 2013 | 2012 | 2011 | ||||||||||
| Non-income tax refunds | $ | 2,876 | $ | 358 | $ | 244 | ||||||
| Change in fair value of acquisition-related liabilities | 497 | 2,770 | 595 | |||||||||
| Gain on revaluation of previously held ownership interest in equity affiliate | — | — | 2,718 | |||||||||
| Income from third party license fees | 1,027 | 1,264 | 1,652 | |||||||||
| Net foreign exchange losses | (1,076 | ) | (1,034 | ) | (482 | ) | ||||||
| Asset impairment related to a cost streamlining initiative | (211 | ) | — | — | ||||||||
| Other non-operating income | 629 | 362 | 591 | |||||||||
| Other non-operating expense | (223 | ) | (305 | ) | (268 | ) | ||||||
| Total other income, net | $ | 3,519 | $ | 3,415 | $ | 5,050 | ||||||
Note 6 – Taxes on Income and Uncertain Tax Positions
Taxes (benefit) on income consist of the following:
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Current: | ||||||||||||
| Federal | $ | 7,216 | $ | 3,318 | $ | 3,485 | ||||||
| State | 263 | (69 | ) | 385 | ||||||||
| Foreign | 13,040 | 9,972 | 7,955 | |||||||||
| 20,519 | 13,221 | 11,825 | ||||||||||
| Deferred: | ||||||||||||
| Federal | 155 | 4,409 | 2,022 | |||||||||
| State | 138 | (794 | ) | — | ||||||||
| Foreign | (323 | ) | (1,261 | ) | 409 | |||||||
| Total | $ | 20,489 | $ | 15,575 | $ | 14,256 | ||||||
The components of earnings before income taxes were as follows:
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Domestic | $ | 25,900 | $ | 26,520 | $ | 24,071 | ||||||
| Foreign | 46,926 | 36,428 | 35,306 | |||||||||
| Total | $ | 72,826 | $ | 62,948 | $ | 59,377 | ||||||
41
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Total deferred tax assets and liabilities are composed of the following at December 31:
| 2013 | 2012 | |||||||||||||||
| Current | Non-current | Current | Non-current | |||||||||||||
| Retirement benefits | $ | 585 | $ | 9,371 | $ | 614 | $ | 14,397 | ||||||||
| Allowance for doubtful accounts | 1,990 | — | 1,984 | — | ||||||||||||
| Insurance and litigation reserves | 677 | 126 | 580 | 260 | ||||||||||||
| Postretirement benefits | — | 1,951 | — | 2,543 | ||||||||||||
| Supplemental retirement benefits | — | 3,010 | — | 2,501 | ||||||||||||
| Performance incentives | 3,858 | 686 | 3,002 | 520 | ||||||||||||
| Equity-based compensation | 351 | 585 | 349 | 395 | ||||||||||||
| Insurance settlement | 6 | 9,071 | 10 | 9,425 | ||||||||||||
| Operating loss carryforward | — | 9,228 | — | 9,425 | ||||||||||||
| Uncertain tax positions | — | 5,806 | — | 7,700 | ||||||||||||
| Other | 975 | 888 | 679 | 1,039 | ||||||||||||
| 8,442 | 40,722 | 7,218 | 48,205 | |||||||||||||
| Valuation allowance | (924 | ) | (6,742 | ) | (710 | ) | (7,148 | ) | ||||||||
| Total deferred income tax assets, net | $ | 7,518 | $ | 33,980 | $ | 6,508 | $ | 41,057 | ||||||||
| Depreciation | — | 4,712 | — | 5,069 | ||||||||||||
| Europe pension and other | — | 2,343 | — | 2,552 | ||||||||||||
| Amortization and other | 749 | 8,595 | 360 | 9,146 | ||||||||||||
| Total deferred income tax liabilities | $ | 749 | $ | 15,650 | $ | 360 | $ | 16,767 | ||||||||
Following are the changes in the Company’s deferred tax asset valuation allowance for the years ended December 31, 2013, December 31, 2012 and December 31, 2011:
| Effect of | ||||||||||||||||||||
| Balance at | Additional | Allowance | Exchange | Balance | ||||||||||||||||
| Beginning | Valuation | Utilization | Rate | at End | ||||||||||||||||
| of Period | Allowance | and Other | Changes | of Period | ||||||||||||||||
| VALUATION ALLOWANCE | ||||||||||||||||||||
| Year ended December 31, 2013 | $ | 7,858 | $ | 26 | $ | (1 | ) | $ | (217 | ) | $ | 7,666 | ||||||||
| Year ended December 31, 2012 | $ | 1,377 | $ | 6,594 | $ | (34 | ) | $ | (79 | ) | $ | 7,858 | ||||||||
| Year ended December 31, 2011 | $ | 4,923 | $ | 348 | $ | (3,753 | ) | $ | (141 | ) | $ | 1,377 | ||||||||
The Company’s net deferred tax assets and liabilities are classified in the Consolidated Balance Sheet as follows:
| 2013 | 2012 | |||||||
| Current deferred tax assets | $ | 7,826 | $ | 6,401 | ||||
| Non-current deferred tax assets | 24,724 | 30,673 | ||||||
| Current deferred tax liabilities | 1,057 | 253 | ||||||
| Non-current deferred tax liabilities | 6,394 | 6,383 | ||||||
| Net deferred tax asset | $ | 25,099 | $ | 30,438 | ||||
42
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
The following is a reconciliation of income taxes at the Federal statutory rate with income taxes recorded by the Company for the years ended December 31, 2013, December 31, 2012 and December 31, 2011:
| 2013 | 2012 | 2011 | ||||||||||
| Income tax provision at the Federal statutory tax rate | $ | 25,489 | $ | 22,032 | $ | 20,782 | ||||||
| Differences in tax rates on foreign earnings and | ||||||||||||
| remittances | (2,487 | ) | (3,207 | ) | (3,692 | ) | ||||||
| Foreign dividends | 1,922 | 815 | 735 | |||||||||
| Excess foreign tax credit utilization | (3,664 | ) | (2,237 | ) | (2,493 | ) | ||||||
| Research and development activities credit utilization | (200 | ) | — | (1,348 | ) | |||||||
| Uncertain tax positions | (589 | ) | (1,196 | ) | 701 | |||||||
| Domestic production activities deduction | (560 | ) | (402 | ) | — | |||||||
| State income tax provisions, net | 171 | (45 | ) | 250 | ||||||||
| Non-deductible entertainment and business meals | ||||||||||||
| expense | 229 | 200 | 166 | |||||||||
| Non-taxable gain on acquisition | — | — | (951 | ) | ||||||||
| Miscellaneous items, net | 178 | (385 | ) | 106 | ||||||||
| Taxes on income | $ | 20,489 | $ | 15,575 | $ | 14,256 | ||||||
At December 31, 2013, the Company domestically had a net deferred tax asset of $12,695. In addition, the Company has foreign tax loss carryforwards of $12,160 of which $15 expires in 2014, $352 expires in 2015, $119 expires in 2016, $344 expires in 2017, $619 expires in 2018, $291 expires in 2019, $102 expires in 2020, $245 expires in 2021 and $258 expires in 2022; the remaining foreign tax losses have no expiration dates. A partial valuation allowance has been established with respect to the tax benefit of these losses for $2,104.
U.S. income taxes have not been provided on the undistributed earnings of non-U.S. subsidiaries because it is the Company’s intention to continue to reinvest these earnings in those subsidiaries to support growth initiatives. U.S. and foreign income taxes that would be payable if such earnings were distributed may be lower than the amount computed at the U.S. statutory rate due to the availability of tax credits. The amount of such undistributed earnings at December 31, 2013 was approximately $188,000. Any income tax liability, which might result from ultimate remittance of these earnings, is expected to be substantially offset by foreign tax credits. It is currently impractical to estimate any such incremental tax expense.
As of December 31, 2013, the Company’s cumulative liability for gross unrecognized tax benefits was $12,596. The Company had accrued $2,100 for cumulative penalties and $2,108 for cumulative interest at December 31, 2013. As of December 31, 2012, the Company’s cumulative liability for gross unrecognized tax benefits was $12,410. The Company had accrued $1,630 for cumulative penalties and $2,288 for cumulative interest at December 31, 2012.
The Company continues to recognize interest and penalties associated with uncertain tax positions as a component of taxes on income before equity in net income of associated companies in its Consolidated Statement of Income. The Company recognized $392 for penalties and ($247) for interest (net of expirations and settlements) on its 2013 Consolidated Statement of Income, $301 for penalties and ($26) for interest (net of expirations and settlements) on its 2012 Consolidated Statement of Income and $502 for penalties and $529 for interest (net of expirations and settlements) on its 2011 Consolidated Statement of Income.
The Company estimates that during the year ending December 31, 2014, it will reduce its cumulative liability for gross unrecognized tax benefits by approximately $1,700 to $1,800 due to the expiration of the statute of limitations with regard to certain tax positions. This estimated reduction in the cumulative liability for unrecognized tax benefits does not consider any increase in liability for unrecognized tax benefits with regard to existing tax positions or any increase in cumulative liability for unrecognized tax benefits with regard to new tax positions for the year ending December 31, 2014.
The Company and its subsidiaries are subject to U.S. Federal income tax, as well as the income tax of various state and foreign tax jurisdictions. Tax years that remain subject to examination by major tax jurisdictions include Brazil from 2000, The Netherlands from 2007, United Kingdom from 2008, Spain from 2009, the United States, China and Italy from 2010 and various domestic state tax jurisdictions from 1993.
��
43
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
In the first quarter of 2013, the Internal Revenue Service (“IRS”) initiated a limited scope audit of the Company’s 2010 Federal Income Tax Return. By letter dated March 25, 2013, the IRS notified the Company that it had completed the review of the Company’s 2010 Federal Income Tax Return without any changes to the reported tax.
During the second quarter of 2012, the Italian tax authorities initiated a transfer pricing audit of the Company’s Italian subsidiary. On July 7, 2012, the Company received a preliminary tax report related to this transfer pricing audit, which proposed several adjustments to the taxable income of the subsidiary. During the fourth quarter of 2012, the Company’s Italian subsidiary received an assessment for the tax year 2007, which the Company appealed during the first quarter of 2013. On June 24, 2013, a hearing was held before the Provincial Tax Court of Varese, Italy. On September 16, 2013, the Provincial Tax Court of Varese delivered a decision confirming the Italian tax authorities’ proposed adjustment to the taxable income of the subsidiary, but denying the proposed assessment of penalties. On January 24, 2014, Company’s Italian subsidiary appealed the decision of the Provincial Tax Court of Varese.
On November 29, 2013, the Italian tax authorities issued a tax assessment for the tax year 2008, raising identical issues as the assessment for 2007, noted above. The Company intends to file an appeal with the Provincial Tax Court of Varese and apply for competent authority relief between the Italian and Dutch tax authorities.
Related to each of the above events, the Company and outside counsel believe we should prevail on the merits of each case. Therefore, the Company does not believe it has any exposures warranting an uncertain tax position reserve as of December 2013.
A reconciliation of the beginning and ending amounts of unrecognized tax benefits for the years ended December 31, 2013, December 31, 2012 and December 31, 2011, respectively, is as follows:
| 2013 | 2012 | 2011 | ||||||||||
| Unrecognized tax benefits at January 1 | $ | 12,410 | $ | 12,719 | $ | 10,464 | ||||||
| Increase in unrecognized tax benefits taken in prior periods | 83 | — | 1,597 | |||||||||
| (Decrease) in unrecognized tax benefits taken in prior periods | — | (411 | ) | — | ||||||||
| Increase in unrecognized tax benefits taken in current period | 2,182 | 1,733 | 2,623 | |||||||||
| (Decrease) in unrecognized tax benefits due to lapse of statute of limitations | (2,485 | ) | (1,837 | ) | (1,578 | ) | ||||||
| Increase (decrease) due to foreign exchange rates | 406 | 206 | (387 | ) | ||||||||
| Unrecognized tax benefits at December 31 | $ | 12,596 | $ | 12,410 | $ | 12,719 | ||||||
The amount of unrecognized tax benefits above that, if recognized, would impact the Company’s tax expense and effective tax rate is $1,194, $1,652 and $2,966 in 2013, 2012 and 2011, respectively.
44
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 7 – Earnings Per Share
The following table summarizes EPS calculations for the years ended December 31, 2013, December 31, 2012 and December 31, 2011:
| December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Basic earnings per common share | ||||||||||||
| Net income attributable to Quaker Chemical Corporation | $ | 56,339 | $ | 47,405 | $ | 45,892 | ||||||
| Less: income allocated to participating securities | (481 | ) | (526 | ) | (825 | ) | ||||||
| Net income available to common shareholders | $ | 55,858 | $ | 46,879 | $ | 45,067 | ||||||
| Basic weighted average common shares outstanding | 13,044,842 | 12,871,703 | 12,159,958 | |||||||||
| Basic earnings per common share | $ | 4.28 | $ | 3.64 | $ | 3.71 | ||||||
| Diluted earnings per common share | ||||||||||||
| Net income attributable to Quaker Chemical Corporation | $ | 56,339 | $ | 47,405 | $ | 45,892 | ||||||
| Less: income allocated to participating securities | (481 | ) | (524 | ) | (817 | ) | ||||||
| Net income available to common shareholders | $ | 55,858 | $ | 46,881 | $ | 45,075 | ||||||
| Basic weighted average common shares outstanding | 13,044,842 | 12,871,703 | 12,159,958 | |||||||||
| Effect of dilutive securities | 24,770 | 58,798 | 158,215 | |||||||||
| Diluted weighted average common shares outstanding | 13,069,612 | 12,930,501 | 12,318,173 | |||||||||
| Diluted earnings per common share | $ | 4.27 | $ | 3.63 | $ | 3.66 | ||||||
The following number of stock options are not included in diluted earnings per share since the effect would have been anti-dilutive: 2,863 in 2013, 4,417 in 2012 and 11,683 in 2011.
Note 8 – Accounts Receivable and Allowance for Doubtful Accounts
At December 31, 2013 and December 31, 2012, the Company had gross trade accounts receivable totaling $172,762 and $160,596 with trade accounts receivable greater than 90 days past due of $11,345 and $9,401, respectively. The following are changes in the allowance for doubtful accounts during the years ended December 31, 2013, December 31, 2012 and December 31, 2011:
| Exchange | ||||||||||||||||||||
| Charged | Rate | |||||||||||||||||||
| Balance at | to Costs | Write-Offs | Changes | Balance | ||||||||||||||||
| Beginning | and | Charged to | And Other | at End | ||||||||||||||||
| of Period | Expenses | Allowance | Adjustments | of Period | ||||||||||||||||
| ALLOWANCE FOR DOUBTFUL ACCOUNTS | ||||||||||||||||||||
| Year ended December 31, 2013 | $ | 6,399 | $ | 1,136 | $ | (407 | ) | $ | 5 | $ | 7,133 | |||||||||
| Year ended December 31, 2012 | $ | 4,569 | $ | 2,072 | $ | (737 | ) | $ | 495 | $ | 6,399 | |||||||||
| Year ended December 31, 2011 | $ | 4,278 | $ | 855 | $ | (607 | ) | $ | 43 | $ | 4,569 | |||||||||
Included in exchange rate changes and other adjustments are allowance for doubtful accounts of $0, $416 and $146 acquired in 2013, 2012 and 2011 business acquisitions.
45
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 9 – Inventories
Total inventories comprise:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Raw materials and supplies | $ | 37,063 | $ | 40,417 | ||||
| Work in process and finished goods | 34,494 | 32,054 | ||||||
| $ | 71,557 | $ | 72,471 | |||||
Note 10 – Property, Plant and Equipment
Property, plant and equipment comprise:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Land | $ | 8,510 | $ | 8,346 | ||||
| Building and improvements | 80,644 | 72,292 | ||||||
| Machinery and equipment | 136,549 | 134,754 | ||||||
| Construction in progress | 8,162 | 9,785 | ||||||
| 233,865 | 225,177 | |||||||
| Less accumulated depreciation | (148,377 | ) | (140,065 | ) | ||||
| $ | 85,488 | $ | 85,112 | |||||
The Company leases certain equipment under capital leases in its North American, EMEA and South American segments. Gross property, plant and equipment includes $793 and $1,275 of capital leases with $380 and $569 of accumulated depreciation at December 31, 2013 and December 31, 2012, respectively. The following is a schedule by years of future minimum lease payments:
| For the year ended December 31, | ||||
| 2014 | $ | 141 | ||
| 2015 | 96 | |||
| 2016 | 65 | |||
| 2017 | 59 | |||
| 2018 | — | |||
| 2019 and beyond | — | |||
| Total net minimum lease payments | 361 | |||
| Less amount representing interest | (18 | ) | ||
| Present value of net minimum lease payments | $ | 343 | ||
Note 11 – Goodwill and Other Intangible Assets
During 2013, certain internal shifts in the Company’s management and changes to the structure of internally reported information occurred. The Company currently believes its structure, its resource allocation and its performance assessment are now more closely aligned with its four geographical regions: North America, EMEA, Asia/Pacific and South America. See Note 3 of Notes to Consolidated Financial Statements for further information. Similarly, the Company reassessed and changed its reporting units for goodwill testing purposes during 2013 to adhere to its geographical orientation. Based on its revised reporting units, the Company completed its annual impairment test as of the end of the third quarter of 2013 and no impairment charge was warranted. The estimated fair value of each of the Company’s reporting units substantially exceeded its carrying value, with none of the Company’s reporting units at risk for failing step one of the goodwill impairment test. In addition, the Company has recorded no impairment charges in the past.
46
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Changes in the carrying amount of goodwill for the years ended December 31, 2013 and December 31, 2012 were as follows:
| North | South | |||||||||||||||||||
| America | EMEA | Asia/Pacific | America | Total | ||||||||||||||||
| Balance as of December 31, 2011 | $ | 28,128 | $ | 11,184 | $ | 15,018 | $ | 3,822 | $ | 58,152 | ||||||||||
| Goodwill additions | — | 1,786 | — | — | 1,786 | |||||||||||||||
| Currency translation adjustments | 407 | (1,559 | ) | 305 | 78 | (769 | ) | |||||||||||||
| Balance as of December 31, 2012 | $ | 28,535 | $ | 11,411 | $ | 15,323 | $ | 3,900 | $ | 59,169 | ||||||||||
| Goodwill additions | 277 | — | — | — | 277 | |||||||||||||||
| Currency translation adjustments | (685 | ) | (227 | ) | (305 | ) | (78 | ) | (1,295 | ) | ||||||||||
| Balance as of December 31, 2013 | $ | 28,127 | $ | 11,184 | $ | 15,018 | $ | 3,822 | $ | 58,151 | ||||||||||
Gross carrying amounts and accumulated amortization for definite-lived intangible assets as of December 31, 2013 and December 31, 2012 were as follows:
| Gross Carrying | Accumulated | |||||||||||||||
| Amount | Amortization | |||||||||||||||
| 2013 | 2012 | 2013 | 2012 | |||||||||||||
| Amortized intangible assets | ||||||||||||||||
Customer lists and rights to sell | $ | 33,559 | $ | 32,356 | $ | 10,221 | $ | 8,192 | ||||||||
Trademarks and patents | 6,838 | 6,760 | 3,202 | 2,548 | ||||||||||||
Formulations and product technology | 5,808 | 5,278 | 3,709 | 3,423 | ||||||||||||
Other | 5,544 | 5,467 | 4,445 | 3,989 | ||||||||||||
Total | $ | 51,749 | $ | 49,861 | $ | 21,577 | $ | 18,152 | ||||||||
The Company recorded $3,445, $3,106 and $2,338 of amortization expense in 2013, 2012 and 2011, respectively. Estimated annual aggregate amortization expense for the subsequent five years is as follows:
| For the year ended December 31, 2014 | $ | 3,254 | ||
| For the year ended December 31, 2015 | $ | 3,254 | ||
| For the year ended December 31, 2016 | $ | 2,775 | ||
| For the year ended December 31, 2017 | $ | 2,112 | ||
| For the year ended December 31, 2018 | $ | 2,089 |
The Company has two indefinite-lived intangible assets totaling $1,100 for trademarks at December 31, 2013 and December 31, 2012.
Note 12 – Investments in Associated Companies
As of December 31, 2013, the Company held a 50% investment in and had significant influence over Kelko Quaker Chemical, S.A. (Venezuela), Nippon Quaker Chemical, Ltd. (Japan) and Kelko Quaker Chemical S.A. (Panama) and held a 33% investment in and had significant influence over Primex, Ltd. (Barbados).
During the first quarter of 2013, the Company identified errors in Primex’s estimated 2012 financial statements, which primarily related to a reinsurance contract held by Primex. The identified errors resulted in a cumulative $1,038 understatement of the Company’s equity in net income from associated companies for the year ended December 31, 2012, which were corrected in the first quarter of 2013. See Note 2 of Notes to Consolidated Financial Statements for further information.
Venezuela’s economy is considered to be hyper inflationary under generally accepted accounting principles in the United States. Accordingly, all gains and losses resulting from the remeasurement of the Company’s Venezuelan equity affiliate (Kelko Quaker Chemical, S.A.) are required to be recorded directly to the Consolidated Statement of Income. In February 2013, the Venezuelan Government announced a devaluation of the Bolivar Fuerte. Accordingly, the Company recorded a charge of approximately $357, or $0.03 per diluted share, in equity in net income of associated companies during the first quarter of 2013.
47
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
In 2011, the Company purchased the remaining 60% ownership interest in Tecniquimia Mexicana, S.A. de C.V., the Company’s Mexican equity affiliate. As a result of the purchase, the Company only included six months of the affiliate’s 2011 results in its investments in associated companies, with the remaining six months after acquisition being reflected as a wholly owned subsidiary in the Company’s Consolidated Financial Statements.
The carrying amount of the Company’s equity investments at December 31, 2013 was $19,397, which included investments of $12,094 in Primex, Ltd. (Barbados), assigned to the Company’s North American segment; $5,267, in Nippon Quaker Chemical, Ltd. (Japan), assigned to the Company’s Asia/Pacific segment; $1,652 in Kelko Quaker Chemical, S.A. (Venezuela), assigned to the Company’s South American segment; and $384, in Kelko Quaker Chemical, S.A. (Panama), assigned to the Company’s South American segment.
Summarized financial information of Kelko Quaker Chemical, S.A. (Venezuela), Nippon Quaker Chemical, Ltd. (Japan) and Kelko Quaker Chemical S.A. (Panama), in the aggregate, is as follows:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Current Assets | $ | 28,363 | $ | 28,602 | ||||
| Noncurrent Assets | 717 | 2,402 | ||||||
| Current Liabilities | 13,974 | 15,158 | ||||||
| Noncurrent Liabilities | 501 | 248 | ||||||
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Net Sales | $ | 47,226 | $ | 55,963 | $ | 66,925 | ||||||
| Gross Margin | 16,096 | 18,480 | 22,092 | |||||||||
| Income Before Income Taxes | 3,687 | 3,170 | 3,788 | |||||||||
| Net Income | 2,142 | 2,118 | 1,696 | |||||||||
Summarized financial information of Primex, Ltd. is as follows:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Total Assets | $ | 106,450 | $ | 130,816 | ||||
| Total Liabilities | 63,938 | 97,754 | ||||||
| Year Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Revenue | $ | 20,895 | $ | 8,473 | $ | 11,523 | ||||||
| Income Before Income Taxes | 25,625 | 8,901 | 14,837 | |||||||||
| Net Income | 16,876 | 6,031 | 9,941 | |||||||||
As noted above, the Company identified errors in Primex’s estimated 2012 financial statements during the first quarter of 2013, which were corrected in the first quarter of 2013. The identified errors resulted in increases to Primex’s revenue of $4,905, income before taxes of $5,240 and net income of $3,422, which are included in the 2013 summarized financial information for Primex above.
During the first quarter of 2013, the Company received its first dividend distribution from Primex, Ltd. of approximately $2,000, which was accounted for as a reduction of the Company’s investment balance in this associated company.
48
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 13 – Other Assets
Other assets include:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Restricted insurance settlement | $ | 25,462 | $ | 26,398 | ||||
| Deferred compensation assets | 894 | 915 | ||||||
| Supplemental retirement income program | 1,885 | 1,653 | ||||||
| Uncertain tax positions | 4,677 | 3,058 | ||||||
| Other | 3,349 | 2,434 | ||||||
| Total | $ | 36,267 | $ | 34,458 | ||||
Previously, an inactive subsidiary of the Company executed separate settlement and release agreements with two of its insurance carriers for $35,000, of which $25,462 remains. The proceeds of both settlements are restricted and can only be used to pay claims and costs of defense associated with the subsidiary’s asbestos litigation. The proceeds of the settlement and release agreements have been deposited into interest bearing accounts which earned approximately $52 and $69 in 2013 and 2012, respectively, offset by $988 and $1,391 of payments in 2013 and 2012, respectively. Due to the restricted nature of the proceeds, a corresponding deferred credit was established in “Other non-current liabilities” for an equal and offsetting amount, and will remain until the restrictions lapse or the funds are exhausted via payments of claims and costs of defense. See Notes 17 and 22 of Notes to Consolidated Financial Statements.
Note 14 – Other Current Liabilities
Other current liabilities comprise:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Non-income taxes | $ | 7,658 | $ | 6,364 | ||||
| Acquisition-related consideration | 4,797 | — | ||||||
| Professional fees | 2,007 | 2,083 | ||||||
| Selling expenses | 4,266 | 2,205 | ||||||
| Legal | 960 | 1,018 | ||||||
| Freight | 1,914 | 1,120 | ||||||
| Income taxes payable | 5,216 | — | ||||||
| Other | 3,767 | 3,457 | ||||||
| Total | $ | 30,585 | $ | 16,247 | ||||
During 2013, the Company’s estimated acquisition-related earnout liability assumed in the 2010 purchase of Summit, Inc. was reclassified from non-current liabilities to other current liabilities, as the liability is expected to be settled during 2014. See also Notes 19 and 20 for more information. In 2012, the Company recorded net prepayments of income taxes, as compared to incurring income taxes payable in the current year.
49
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 15 – Debt
Debt includes the following:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Industrial development authority monthly 5.60% fixed rate demand bond maturing 2018 | $ | 5,000 | $ | 5,000 | ||||
| Industrial development authority monthly 5.26% fixed rate demand bond maturing 2028 | 10,000 | 10,000 | ||||||
| Credit facilities (1.66% weighted average borrowing rate at December 31, 2013) | — | 12,200 | ||||||
| Ohio Department of Development term loan (see below) | 2,428 | 2,754 | ||||||
| Other debt obligations (including capital leases) | 1,288 | 1,514 | ||||||
| 18,716 | 31,468 | |||||||
| Short-term debt | (945 | ) | (867 | ) | ||||
| Current portion of long-term debt | (450 | ) | (601 | ) | ||||
| $ | 17,321 | $ | 30,000 | |||||
During the next five years, payments on the Company’s debt, including capital lease maturities, are due as follows:
| 2014 | $ | 1,395 | ||
| 2015 | 418 | |||
| 2016 | 393 | |||
| 2017 | 396 | |||
| 2018 | 5,344 | |||
| 2019 and beyond | $ | 10,770 |
As discussed in the Current Report on Form 8-K filed on June 17, 2013, the Company entered into a revised syndicated multicurrency credit facility on June 14, 2013, which amended and replaced the Company’s previous credit facility with Bank of America, N.A. and certain other major financial institutions. The revised facility increased the maximum principal amount available for revolving credit borrowings under this facility from $175,000 to $300,000, which can be increased to $400,000 at the Company’s option if the lenders agree and the Company satisfies certain conditions. This facility matures in June 2018. In addition, the revised facility amended certain financial, acquisition and other covenants, but the consolidated leverage ratio calculation, for which access to credit under the former facility largely depended upon, remains relatively consistent and cannot exceed 3.50 to 1. As of December 31, 2013 and December 31, 2012, the Company’s consolidated leverage ratio was below 1.0 to 1 and the Company was also in compliance with all of the current and former facilities’ other covenants, respectively. At December 31, 2013 and December 31, 2012, the Company had approximately $0 and $12,200 outstanding on these credit lines at weighted average borrowing rates of 1.66% and 2.03% (LIBOR plus a spread) during each respective year.
As part of its Middletown, Ohio facility’s past expansion project, the Company agreed to a low interest rate $3,500 loan with the Ohio Department of Development. Principal repayment on this loan began in September 2010 with its final maturity being in 2021. The current interest rate of 1% will rise to 2% beginning January 1, 2014 and to 3% beginning January 1, 2019 until final maturity.
At December 31, 2013 and December 31, 2012, the amounts at which the Company’s debt is recorded are not materially different from their fair market value.
50
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 16 – Pension and Other Postretirement Benefits
The following table shows the Company’s plans’ funded status reconciled with amounts reported in the consolidated balance sheet as of December 31, 2013 and December 31, 2012:
| Other | ||||||||||||||||||||||||||||||||
| Postretirement | ||||||||||||||||||||||||||||||||
| Pension Benefits | Benefits | |||||||||||||||||||||||||||||||
| 2013 | 2012 | 2013 | 2012 | |||||||||||||||||||||||||||||
| Foreign | Domestic | Total | Foreign | Domestic | Total | Domestic | Domestic | |||||||||||||||||||||||||
| Change in benefit obligation | ||||||||||||||||||||||||||||||||
| Benefit obligation at beginning of year | $ | 81,280 | $ | 70,407 | $ | 151,687 | $ | 61,581 | $ | 66,226 | $ | 127,807 | $ | 7,317 | $ | 7,202 | ||||||||||||||||
| Service cost | 2,864 | 299 | 3,163 | 2,004 | 460 | 2,464 | 34 | 46 | ||||||||||||||||||||||||
| Interest cost | 3,150 | 2,437 | 5,587 | 3,020 | 2,803 | 5,823 | 185 | 283 | ||||||||||||||||||||||||
| Employee contributions | 111 | — | 111 | 101 | — | 101 | — | — | ||||||||||||||||||||||||
| Effect of plan amendments | (2,138 | ) | — | (2,138 | ) | — | — | — | — | — | ||||||||||||||||||||||
| Benefits paid | (1,853 | ) | (4,516 | ) | (6,369 | ) | (1,973 | ) | (4,668 | ) | (6,641 | ) | (566 | ) | (728 | ) | ||||||||||||||||
| Plan expenses and premiums paid | (367 | ) | (225 | ) | (592 | ) | (331 | ) | (225 | ) | (556 | ) | — | — | ||||||||||||||||||
| Actuarial (gain) loss | (566 | ) | (2,033 | ) | (2,599 | ) | 14,874 | 5,811 | 20,685 | (1,331 | ) | 514 | ||||||||||||||||||||
| Translation difference and other | 3,264 | — | 3,264 | 2,004 | — | 2,004 | — | — | ||||||||||||||||||||||||
| Benefit obligation at end of year | $ | 85,745 | $ | 66,369 | $ | 152,114 | $ | 81,280 | $ | 70,407 | $ | 151,687 | $ | 5,639 | $ | 7,317 | ||||||||||||||||
| Change in plan assets | ||||||||||||||||||||||||||||||||
| Fair value of plan assets at beginning of | ||||||||||||||||||||||||||||||||
| year | $ | 60,909 | $ | 45,991 | $ | 106,900 | $ | 54,968 | $ | 43,470 | $ | 98,438 | $ | — | $ | — | ||||||||||||||||
| Actual return on plan assets | 3,237 | 7,487 | 10,724 | 2,972 | 4,466 | 7,438 | — | — | ||||||||||||||||||||||||
| Employer contributions | 3,947 | 1,913 | 5,860 | 3,842 | 2,948 | 6,790 | 566 | 728 | ||||||||||||||||||||||||
| Employee contributions | 111 | — | 111 | 101 | — | 101 | — | — | ||||||||||||||||||||||||
| Benefits paid | (1,853 | ) | (4,516 | ) | (6,369 | ) | (1,973 | ) | (4,668 | ) | (6,641 | ) | (566 | ) | (728 | ) | ||||||||||||||||
| Plan expenses and premiums paid | (367 | ) | (225 | ) | (592 | ) | (331 | ) | (225 | ) | (556 | ) | — | — | ||||||||||||||||||
| Translation difference | 2,675 | — | 2,675 | 1,330 | — | 1,330 | — | — | ||||||||||||||||||||||||
| Fair value of plan assets at end of year | $ | 68,659 | $ | 50,650 | $ | 119,309 | $ | 60,909 | $ | 45,991 | $ | 106,900 | $ | — | $ | — | ||||||||||||||||
| Net amount recognized | $ | (17,086 | ) | $ | (15,719 | ) | $ | (32,805 | ) | $ | (20,371 | ) | $ | (24,416 | ) | $ | (44,787 | ) | $ | (5,639 | ) | $ | (7,317 | ) | ||||||||
| Amounts recognized in the balance sheet | ||||||||||||||||||||||||||||||||
| consist of: | ||||||||||||||||||||||||||||||||
| Current liabilities | $ | (242 | ) | $ | (589 | ) | $ | (831 | ) | $ | (892 | ) | $ | (577 | ) | $ | (1,469 | ) | $ | (607 | ) | $ | (719 | ) | ||||||||
| Non-current liabilities | (16,844 | ) | (15,130 | ) | (31,974 | ) | (19,479 | ) | (23,839 | ) | (43,318 | ) | (5,032 | ) | (6,598 | ) | ||||||||||||||||
| Net amount recognized | $ | (17,086 | ) | $ | (15,719 | ) | $ | (32,805 | ) | $ | (20,371 | ) | $ | (24,416 | ) | $ | (44,787 | ) | $ | (5,639 | ) | $ | (7,317 | ) | ||||||||
| Amounts not yet reflected in net periodic | ||||||||||||||||||||||||||||||||
| benefit costs and included in | ||||||||||||||||||||||||||||||||
| accumulated other comprehensive | ||||||||||||||||||||||||||||||||
| loss: | ||||||||||||||||||||||||||||||||
| Prior service credit (cost) | $ | 2,105 | $ | (311 | ) | $ | 1,794 | $ | (62 | ) | $ | (460 | ) | $ | (522 | ) | $ | — | $ | — | ||||||||||||
| Accumulated loss | (27,188 | ) | (27,593 | ) | (54,781 | ) | (29,227 | ) | (35,929 | ) | (65,156 | ) | (745 | ) | (2,107 | ) | ||||||||||||||||
| Accumulated other comprehensive | ||||||||||||||||||||||||||||||||
| loss (AOCI) | (25,083 | ) | (27,904 | ) | (52,987 | ) | (29,289 | ) | (36,389 | ) | (65,678 | ) | (745 | ) | (2,107 | ) | ||||||||||||||||
| Cumulative employer contributions | ||||||||||||||||||||||||||||||||
| in excess of net period benefit cost | 7,997 | 12,185 | 20,182 | 8,918 | 11,973 | 20,891 | (4,894 | ) | (5,210 | ) | ||||||||||||||||||||||
| Net amount recognized | $ | (17,086 | ) | $ | (15,719 | ) | $ | (32,805 | ) | $ | (20,371 | ) | $ | (24,416 | ) | $ | (44,787 | ) | $ | (5,639 | ) | $ | (7,317 | ) | ||||||||
The accumulated benefit obligation for all defined benefit pension plans was $150,374 ($66,369 Domestic and $84,005 Foreign) and $145,836 ($69,951 Domestic and $75,885 Foreign) at December 31, 2013 and December 31, 2012, respectively.
Information for pension plans with an accumulated benefit obligation in excess of plan assets:
| 2013 | 2012 | |||||||||||||||||||||||
| Foreign | Domestic | Total | Foreign | Domestic | Total | |||||||||||||||||||
| Projected benefit obligation | $ | 85,745 | $ | 66,369 | $ | 152,114 | $ | 81,280 | $ | 70,407 | $ | 151,687 | ||||||||||||
| Accumulated benefit obligation | 84,005 | 66,369 | 150,374 | 75,885 | 69,951 | 145,836 | ||||||||||||||||||
| Fair value of plan assets | 68,659 | 50,650 | 119,309 | 60,909 | 45,991 | 106,900 | ||||||||||||||||||
51
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Information for pension plans with a projected benefit obligation in excess of plan assets:
| 2013 | 2012 | |||||||||||||||||||||||
| Foreign | Domestic | Total | Foreign | Domestic | Total | |||||||||||||||||||
| Projected benefit obligation | $ | 85,745 | $ | 66,369 | $ | 152,114 | $ | 81,280 | $ | 70,407 | $ | 151,687 | ||||||||||||
| Fair value of plan assets | 68,659 | 50,650 | 119,309 | 60,909 | 45,991 | 106,900 | ||||||||||||||||||
Components of net periodic benefit costs – pension plans:
| 2013 | 2012 | |||||||||||||||||||||||
| Foreign | Domestic | Total | Foreign | Domestic | Total | |||||||||||||||||||
| Service cost | $ | 2,864 | $ | 299 | $ | 3,163 | $ | 2,004 | $ | 460 | $ | 2,464 | ||||||||||||
| Interest cost | 3,150 | 2,437 | 5,587 | 3,020 | 2,803 | 5,823 | ||||||||||||||||||
| Expected return on plan assets | (2,245 | ) | (3,664 | ) | (5,909 | ) | (1,995 | ) | (3,481 | ) | (5,476 | ) | ||||||||||||
| Actuarial loss amortization | 1,486 | 2,481 | 3,967 | 590 | 2,057 | 2,647 | ||||||||||||||||||
| Prior service cost amortization | 30 | 148 | 178 | 30 | 82 | 112 | ||||||||||||||||||
| Net periodic benefit cost | $ | 5,285 | $ | 1,701 | �� | $ | 6,986 | $ | 3,649 | $ | 1,921 | $ | 5,570 | |||||||||||
| 2011 | ||||||||||||
| Foreign | Domestic | Total | ||||||||||
| Service cost | $ | 1,890 | $ | 400 | $ | 2,290 | ||||||
| Interest cost | 3,037 | 3,145 | 6,182 | |||||||||
| Expected return on plan assets | (2,349 | ) | (3,592 | ) | (5,941 | ) | ||||||
| Actuarial loss amortization | 233 | 1,554 | 1,787 | |||||||||
| Prior service cost amortization | 32 | 82 | 114 | |||||||||
| Net periodic benefit cost | $ | 2,843 | $ | 1,589 | $ | 4,432 | ||||||
Other changes recognized in other comprehensive income:
| 2013 | 2012 | |||||||||||||||||||||||
| Foreign | Domestic | Total | Foreign | Domestic | Total | |||||||||||||||||||
| Net (gain) loss arising during the period | $ | (1,558 | ) | $ | (5,856 | ) | $ | (7,414 | ) | $ | 13,897 | $ | 4,826 | $ | 18,723 | |||||||||
| Effect of plan amendment | (2,138 | ) | — | (2,138 | ) | — | — | — | ||||||||||||||||
| Recognition of amortization in net periodic | ||||||||||||||||||||||||
| benefit cost | ||||||||||||||||||||||||
| Prior service cost | (30 | ) | (148 | ) | (178 | ) | (30 | ) | (82 | ) | (112 | ) | ||||||||||||
| Actuarial loss | (1,486 | ) | (2,481 | ) | (3,967 | ) | (590 | ) | (2,057 | ) | (2,647 | ) | ||||||||||||
| Effect of exchange rates on amounts included in | ||||||||||||||||||||||||
| AOCI | 1,007 | — | 1,007 | 809 | — | 809 | ||||||||||||||||||
| Total recognized in other comprehensive | ||||||||||||||||||||||||
| (income) loss | (4,205 | ) | (8,485 | ) | (12,690 | ) | 14,086 | 2,687 | 16,773 | |||||||||||||||
| Total recognized in net periodic benefit cost and | ||||||||||||||||||||||||
| other comprehensive (income) loss | $ | 1,080 | $ | (6,784 | ) | $ | (5,704 | ) | $ | 17,735 | $ | 4,608 | $ | 22,343 | ||||||||||
52
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
| 2011 | ||||||||||||
| Foreign | Domestic | Total | ||||||||||
| Net loss arising during period | $ | 5,164 | $ | 7,593 | $ | 12,757 | ||||||
| Recognition of amortization in net periodic benefit cost | ||||||||||||
| Prior service cost | (32 | ) | (82 | ) | (114 | ) | ||||||
| Actuarial loss | (233 | ) | (1,554 | ) | (1,787 | ) | ||||||
| Effect of exchange rates on amounts included in AOCI | (794 | ) | — | (794 | ) | |||||||
| Total recognized in other comprehensive loss | 4,105 | 5,957 | 10,062 | |||||||||
| Total recognized in net periodic benefit cost and other | ||||||||||||
| comprehensive loss | $ | 6,948 | $ | 7,546 | $ | 14,494 | ||||||
Components of net periodic benefit costs – other postretirement plan:
| 2013 | 2012 | 2011 | ||||||||||
| Interest cost | $ | 185 | $ | 283 | $ | 331 | ||||||
| Service cost | 34 | 46 | 16 | |||||||||
| Actuarial loss amortization | 32 | 115 | 75 | |||||||||
| Net periodic benefit costs | $ | 251 | $ | 444 | $ | 422 | ||||||
Other changes recognized in other comprehensive income – other postretirement benefit plans:
| 2013 | 2012 | 2011 | ||||||||||
| Net (gain) loss arising during period | $ | (1,331 | ) | $ | 514 | $ | (167 | ) | ||||
| Amortization of actuarial loss in net periodic benefit costs | (32 | ) | (115 | ) | (75 | ) | ||||||
| Total recognized in other comprehensive (income) loss | (1,363 | ) | 399 | (242 | ) | |||||||
| Total recognized in net periodic benefit cost and other | ||||||||||||
| comprehensive (income) loss | $ | (1,112 | ) | $ | 843 | $ | 180 | |||||
Estimated amounts that will be amortized from accumulated other comprehensive loss over the next fiscal year:
| Pension Plans | Other Postretirement | |||||||||||||||
| Foreign | Domestic | Total | Benefits | |||||||||||||
| Actuarial loss | $ | 1,351 | $ | 1,819 | $ | 3,170 | $ | 24 | ||||||||
| Prior service (credit) cost | (154 | ) | 63 | (91 | ) | — | ||||||||||
| $ | 1,197 | $ | 1,882 | $ | 3,079 | $ | 24 | |||||||||
53
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Weighted-average assumptions used to determine benefit obligations at December 31, 2013 and December 31, 2012:
| Other Postretirement | |||||||||||||
| Pension Benefits | Benefits | ||||||||||||
| 2013 | 2012 | 2013 | 2012 | ||||||||||
| U.S. Plans: | |||||||||||||
| Discount rate | 4.48% | 3.52% | 4.05% | 3.20% | |||||||||
| Rate of compensation increase | 3.63% | 3.40% | N/A | N/A | |||||||||
| Foreign Plans: | |||||||||||||
| Discount rate | 3.84% | 3.94% | N/A | N/A | |||||||||
| Rate of compensation increase | 3.05% | 3.60% | N/A | N/A | |||||||||
Weighted-average assumptions used to determine net periodic benefit costs for the years ended December 31, 2013 and December 31, 2012:
| Other Postretirement | |||||||||||||
| Pension Benefits | Benefits | ||||||||||||
| 2013 | 2012 | 2013 | 2012 | ||||||||||
| U.S. Plans: | |||||||||||||
| Discount rate | 3.52% | 4.41% | 3.20% | 4.15% | |||||||||
| Expected long-term return on plan assets | 8.25% | 8.25% | N/A | N/A | |||||||||
| Rate of compensation increase | 3.40% | 3.40% | N/A | N/A | |||||||||
| Foreign Plans: | |||||||||||||
| Discount rate | 3.94% | 4.99% | N/A | N/A | |||||||||
| Expected long-term return on plan assets | 3.57% | 3.51% | N/A | N/A | |||||||||
| Rate of compensation increase | 3.60% | 3.58% | N/A | N/A | |||||||||
The long-term rates of return on assets were selected from within the reasonable range of rates determined by (a) historical real returns for the asset classes covered by the investment policy and (b) projections of inflation over the long-term period during which benefits are payable to plan participants. See Note 1 for further information.
Assumed health care cost trend rates at December 31, 2013 and December 31, 2012:
| 2013 | 2012 | |||||
| Health care cost trend rate for next year | 7.10% | 7.30% | ||||
| Rate to which the cost trend rate is assumed to decline (the | ||||||
| ultimate trend rate) | 4.50% | 4.50% | ||||
| Year that the rate reaches the ultimate trend rate | 2027 | 2027 | ||||
Assumed health care cost trend rates have a significant effect on the amounts reported for the health care plans. A one-percentage-point change in assumed health care cost trend rates would have the following effects:
| 1% point | 1% point | |||||||
| Increase | Decrease | |||||||
| Effect on total service and interest cost | $ | 21 | $ | (18 | ) | |||
| Effect on postretirement benefit obligations | 456 | (401 | ) | |||||
54
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Plan Assets and Fair Value
The Company’s pension plan target asset allocation and the weighted-average asset allocations at December 31, 2013 and December 31, 2012 by asset category were as follows:
| Target | 2013 | 2012 | |||||||||
| Asset Category | |||||||||||
| U.S. Plans | |||||||||||
| Equity securities | 61% | 66% | 58% | ||||||||
| Debt securities | 32% | 32% | 40% | ||||||||
| Other | 7% | 2% | 2% | ||||||||
| Total | 100% | 100% | 100% | ||||||||
| Foreign Plans | |||||||||||
| Equity securities and other | 18% | 19% | 17% | ||||||||
| Debt securities | 82% | 81% | 83% | ||||||||
| Total | 100% | 100% | 100% | ||||||||
As of December 31, 2013 and December 31, 2012, “Other” consisted principally of cash and cash equivalents (approximately 2% of plan assets in each respective period).
The following is a description of the valuation methodologies used for the investments measured at fair value, including the general classification of such instruments pursuant to the valuation hierarchy:
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and money market funds and are classified as Level 1 investments.
Registered Investment Companies
The shares of registered investment companies, which represent the net asset values of shares held by the Plan, are valued at quoted market prices in an exchange and active market and are classified as Level 1 investments.
Common Stock
Common stock is valued at quoted market prices in an exchange and active market and is classified as Level 1 investments.
Corporate Fixed Income Securities
Corporate fixed income securities are valued at quoted market prices in an exchange and active market and are classified as Level 1 investments.
U.S. and Foreign Government Fixed Income Securities
U.S. and foreign government securities are valued at quoted market prices in an exchange and active market and are classified as Level 1 investments.
Pooled Separate Accounts
Pooled separate accounts consist of insurance annuity contracts and are valued based on the reported unit value at year end. Units of the pooled separate accounts are not traded in an active exchange or market; however, valuation is based on the underlying investments of the units and are classified as Level 2 investments.
Diversified Equity Securities of Registered Investment Companies
Investments in diversified equity securities of registered investment companies are based upon the quoted redemption value of shares in the fund owned by the plan at year end. The shares of the fund are not available in an exchange or active market; however, the fair value is determined based on the underlying investments in the fund as traded in an exchange and active market and are classified as Level 2 investments.
55
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Fixed Income Securities of Registered Investment Companies
Investments in fixed income securities of registered investment companies are based upon the quoted redemption value of shares in the fund owned by the plan at year end. The shares of the fund are not available in an exchange or active market; however, the fair value is determined based on the underlying investments in the fund as traded in an exchange and active market and are classified as Level 2 investments.
Insurance Contract
Investments in the foreign pension plan insurance contract are valued at reported cash surrender value of the contract at year end. Cash surrender value is determined based on unobservable inputs, which are contractually determined, regarding returns, fees, and the present value of the future cash flows of the contract. The contract is classified as a Level 3 investment.
Real Estate
The foreign pension plan’s investment in real estate consists of an investment in a property fund. The fund’s underlying investments consist of real property, which are valued using unobservable inputs. The property fund is classified as a Level 3 investment.
As of December 31, 2013 and December 31, 2012, the U.S. and foreign plans’ investments measured at fair value on a recurring basis were as follows:
| Fair Value Measurements at December 31, 2013 | ||||||||||||||||
| Fair Value as of | Using Fair Value Hierarchy | |||||||||||||||
| U.S. Pension Assets | December 31, 2013 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Cash and cash equivalents | $ | 825 | $ | 825 | $ | — | $ | — | ||||||||
| Large capitalization common stock | 14,801 | 14,801 | — | — | ||||||||||||
| Large capitalization registered investment companies | 6,820 | 6,820 | — | — | ||||||||||||
| Small capitalization common stock | 771 | 771 | — | — | ||||||||||||
| Small capitalization registered investment companies | 2,384 | 2,384 | — | — | ||||||||||||
| International developed and emerging markets registered | ||||||||||||||||
| investment companies | 5,895 | 5,895 | — | — | ||||||||||||
| International developed and emerging markets common stock | 2,929 | 2,929 | — | — | ||||||||||||
| Fixed income corporate securities | 10,144 | 10,144 | — | — | ||||||||||||
| Fixed income registered investment companies | 4,486 | 4,486 | — | — | ||||||||||||
| U.S. and foreign government fixed income securities | 192 | 192 | — | — | ||||||||||||
| Pooled separate accounts | 1,403 | — | 1,403 | — | ||||||||||||
| Total U.S. pension plan assets | $ | 50,650 | $ | 49,247 | $ | 1,403 | $ | — | ||||||||
| Foreign Pension Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 5 | $ | 5 | $ | — | $ | — | ||||||||
| Insurance contract (underlying notional investments in | ||||||||||||||||
| debt and equity securities) | 57,175 | — | — | 57,175 | ||||||||||||
| Diversified equity securities - registered investment companies | 6,597 | — | 6,597 | — | ||||||||||||
| Fixed income registered investment companies | 4,448 | — | 4,448 | — | ||||||||||||
| Real estate registered investment companies | 434 | — | — | 434 | ||||||||||||
| Total foreign pension assets | $ | 68,659 | $ | 5 | $ | 11,045 | $ | 57,609 | ||||||||
| Total pension assets at fair value | $ | 119,309 | $ | 49,252 | $ | 12,448 | $ | 57,609 | ||||||||
56
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
| Fair Value Measurements at December 31, 2012 | ||||||||||||||||
| Fair Value as of | Using Fair Value Hierarchy | |||||||||||||||
| U.S. Pension Assets | December 31, 2012 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Cash and cash equivalents | $ | 905 | $ | 905 | $ | — | $ | — | ||||||||
| Large capitalization common stock | 12,195 | 12,195 | — | — | ||||||||||||
| Large capitalization registered investment companies | 6,551 | 6,551 | — | — | ||||||||||||
| Small capitalization common stock | 539 | 539 | — | — | ||||||||||||
| Small capitalization registered investment companies | 1,910 | 1,910 | — | — | ||||||||||||
| International developed and emerging markets registered | ||||||||||||||||
| investment companies | 3,107 | 3,107 | — | — | ||||||||||||
| International developed and emerging markets common stock | 2,527 | 2,527 | — | — | ||||||||||||
| Fixed income corporate securities | 10,297 | 10,297 | — | — | ||||||||||||
| Fixed income registered investment companies | 6,483 | 6,483 | — | — | ||||||||||||
| U.S. and foreign government fixed income securities | 12 | 12 | — | — | ||||||||||||
| Pooled separate accounts | 1,465 | — | 1,465 | — | ||||||||||||
| Total U.S. pension plan assets | $ | 45,991 | $ | 44,526 | $ | 1,465 | $ | — | ||||||||
| Foreign Pension Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 96 | $ | 96 | $ | — | $ | — | ||||||||
| Insurance contract (underlying notional investments in | ||||||||||||||||
| debt and equity securities) | 51,146 | — | — | 51,146 | ||||||||||||
| Diversified equity securities - registered investment companies | 5,072 | — | 5,072 | — | ||||||||||||
| Fixed income registered investment companies | 4,207 | — | 4,207 | — | ||||||||||||
| Real estate registered investment companies | 388 | — | — | 388 | ||||||||||||
| Total foreign pension assets | $ | 60,909 | $ | 96 | $ | 9,279 | $ | 51,534 | ||||||||
| Total pension assets at fair value | $ | 106,900 | $ | 44,622 | $ | 10,744 | $ | 51,534 | ||||||||
Changes in the fair value of the foreign plans’ Level 3 investments during the years ended December 31, 2013 and December 31, 2012 were as follows:
| Insurance | Real Estate | |||||||||||
| Contract | Fund | Total | ||||||||||
| Balance at December 31, 2011 | $ | 46,797 | $ | 363 | $ | 47,160 | ||||||
| Purchases | 2,997 | — | 2,997 | |||||||||
| Settlements | (1,466 | ) | — | (1,466 | ) | |||||||
| Unrealized gains | 1,854 | 10 | 1,864 | |||||||||
| Currency translation adjustment | 964 | 15 | 979 | |||||||||
| Balance at December 31, 2012 | 51,146 | 388 | 51,534 | |||||||||
| Purchases | 3,182 | — | 3,182 | |||||||||
| Settlements | (1,607 | ) | — | (1,607 | ) | |||||||
| Unrealized gains | 2,061 | 36 | 2,097 | |||||||||
| Currency translation adjustment | 2,393 | 10 | 2,403 | |||||||||
| Balance at December 31, 2013 | $ | 57,175 | $ | 434 | $ | 57,609 | ||||||
U.S. pension assets include Company common stock in the amounts of $771 (1% of total U.S. plan assets) and $539 (1% of total U.S. plan assets) at December 31, 2013 and December 31, 2012, respectively.
During 2013, it was discovered that the Company’s subsidiary in the United Kingdom did not appropriately amend a trust for a legacy change in its pension scheme, as it related to a past retirement age equalization law. Given the lack of an official deed to the pension trust, the effective date of the change to the subsidiary’s pension scheme will differ from the Company’s historical beliefs.
57
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
The Company is currently assessing the impact of such adjustment but, at December 31, 2013, cannot accurately estimate a potential exposure.
Cash Flows
Contributions
The Company expects to make minimum cash contributions of $6,172 to its pension plans ($1,989 Domestic and $4,183 Foreign) and $607 to its other postretirement benefit plan in 2014.
Estimated Future Benefit Payments
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid:
| Other | ||||||||||||||||
| Pension Benefits | Postretirement | |||||||||||||||
| Foreign | Domestic | Total | Benefits | |||||||||||||
| 2014 | $ | 1,968 | $ | 4,872 | $ | 6,840 | $ | 607 | ||||||||
| 2015 | 1,901 | 4,548 | 6,449 | 565 | ||||||||||||
| 2016 | 2,118 | 4,509 | 6,627 | 544 | ||||||||||||
| 2017 | 2,287 | 4,408 | 6,695 | 528 | ||||||||||||
| 2018 | 2,483 | 4,396 | 6,879 | 492 | ||||||||||||
| 2019 and beyond | 17,822 | 22,096 | 39,918 | 2,043 | ||||||||||||
The Company maintains a plan under which supplemental retirement benefits are provided to certain officers. Benefits payable under the plan are based on a combination of years of service and existing postretirement benefits. Included in total pension costs are charges of $811, $700 and $628 in 2013, 2012 and 2011, respectively, representing the annual accrued benefits under this plan.
Defined Contribution Plan
The Company has a 401(k) plan with an employer match covering substantially all domestic employees. The plan allows for and the Company has paid a nonelective contribution on behalf of participants who have completed one year of service equal to 3% of the eligible participants’ compensation in the form of Company common stock. Total Company contributions were $2,027, $1,703 and $1,624 for 2013, 2012 and 2011, respectively.
Note 17 – Other Non-Current Liabilities
Other non-current liabilities comprise:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Restricted insurance settlement | $ | 25,462 | $ | 26,398 | ||||
| Uncertain tax positions (includes interest and penalties) | 15,885 | 16,328 | ||||||
| Acquisition-related consideration | — | 4,651 | ||||||
| Deferred and other long-term compensation | 5,646 | 4,550 | ||||||
| Other | 545 | 940 | ||||||
| Total | $ | 47,538 | $ | 52,867 | ||||
See also Notes 13 and 22 of Notes to Consolidated Financial Statements.
58
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 18 – Equity and Accumulated Other Comprehensive Loss
The Company has 30,000,000 shares of common stock authorized, with a par value of $1, and 13,196,140 shares issued as of December 31, 2013.
Holders of record of the Company’s common stock for a period of less than 36 consecutive calendar months or less are entitled to one vote per share of common stock. Holders of record of the Company’s common stock for a period greater than 36 consecutive calendar months are entitled to 10 votes per share of common stock.
The Company is authorized to issue 10,000,000 shares of preferred stock, $1 par value, subject to approval by the Board of Directors. The Board of Directors may designate one or more series of preferred stock and the number of shares, rights, preferences, and limitations of each series. As of December 31, 2013, no preferred stock had been issued.
The Company filed a shelf registration statement on Form S-3 with the Securities and Exchange Commission (the “SEC”) in 2009. The registration statement was declared effective on January 29, 2010 and permitted the Company to offer and sell from time to time in one or more public offerings up to $100 million aggregate dollar amount of its securities, including shares of preferred stock (either separately or represented by depositary shares), common stock, debt securities and warrants to purchase the Company’s debt or equity securities, as well as units that include any of these securities, on terms, in each case, established at the time of the offering. The registration statement provided the Company with the ability to issue registered debt or equity securities on an accelerated basis. The Company sold 1,265,000 shares of its common stock during the second quarter of 2011. The Company received gross proceeds of $51,233 which were used to repay a portion of the Company’s revolving credit line during the second quarter of 2011. The shelf registration expired during 2013.
The following table shows the reclassifications from and resulting balances of accumulated other comprehensive loss (“AOCI”) for the years ended December 31, 2013, December 31, 2012 and December 31, 2011:
| Unrealized | ||||||||||||||||||||
| Currency | Defined | Change in | gain (loss) in | |||||||||||||||||
| translation | benefit | fair value of | available-for- | |||||||||||||||||
| adjustments | pension plans | derivatives | sale securities | Total | ||||||||||||||||
| Balance at December 31, 2010 | $ | 13,368 | $ | (26,448 | ) | $ | (667 | ) | $ | 994 | $ | (12,753 | ) | |||||||
| Other comprehensive (loss) income before reclassifications | (8,659 | ) | (12,783 | ) | (52 | ) | 1,235 | (20,259 | ) | |||||||||||
| Amounts reclassified from AOCI | — | 1,976 | 660 | (1,444 | ) | 1,192 | ||||||||||||||
| Related tax amounts | — | 2,995 | (213 | ) | 71 | 2,853 | ||||||||||||||
| Balance at December 31, 2011 | 4,709 | (34,260 | ) | (272 | ) | 856 | (28,967 | ) | ||||||||||||
| Other comprehensive (loss) income before reclassifications | (1,373 | ) | (20,045 | ) | 26 | 2,181 | (19,211 | ) | ||||||||||||
| Amounts reclassified from AOCI | — | 2,875 | 392 | (868 | ) | 2,399 | ||||||||||||||
| Related tax amounts | — | 4,516 | (146 | ) | (446 | ) | 3,924 | |||||||||||||
| Balance at December 31, 2012 | 3,336 | (46,914 | ) | — | 1,723 | (41,855 | ) | |||||||||||||
| Other comprehensive income (loss) before reclassifications | (2,184 | ) | 9,876 | — | 2,543 | 10,235 | ||||||||||||||
| Amounts reclassified from AOCI | — | 4,177 | — | (2,758 | ) | 1,419 | ||||||||||||||
| Related tax amounts | — | (4,572 | ) | — | 73 | (4,499 | ) | |||||||||||||
| Balance at December 31, 2013 | $ | 1,152 | $ | (37,433 | ) | $ | — | $ | 1,581 | $ | (34,700 | ) | ||||||||
Approximately 30% and 70% of the amounts reclassified from accumulated other comprehensive loss to the Consolidated Statement of Income for defined benefit retirement plans during the years ended December 31, 2013, December 31, 2012 and December 31, 2011 were recorded in cost of goods sold and SG&A, respectively. See Note 16 of Notes to Consolidated Financial Statements for further information. All reclassifications were recorded in interest expense for changes in fair value of derivatives and, also, reclassifications related to unrealized gain (loss) in available-for-sale securities primarily relate to the Company’s equity interest in a captive insurance company and, therefore, are recorded in equity in net income of associated companies. The amounts reported on the Consolidated Statement of Changes in Equity in other comprehensive income related to the Company’s non-controlling interests consist of currency translation adjustments.
59
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 19 – Business Acquisitions
In May 2013, the Company acquired a business that primarily related to tin plating for its North American reportable operating segment for net consideration of approximately $1,831. The Company allocated the purchase price to $830 of intangible assets, comprised of formulations, to be amortized over 10 years; a non-competition agreement, to be amortized over 4 years; and a customer list, to be amortized over 10 years. In addition, the Company recorded $277 of goodwill, all of which will be tax deductible. The remaining purchase price was allocated between the acquisition date fair value of inventory purchased of $454 and fixed assets purchased of $270.
In January 2013, the Company acquired a chemical milling maskants distribution network for net consideration of approximately $647, which was assigned to the North American reportable operating segment. The Company also assumed an additional $100 hold-back of consideration for potential indemnity obligations, which was paid to the former shareholders during January 2014. The acquired intangible was allocated to the Company’s customer lists and rights to sell intangible assets and will be amortized over 5 years.
In July 2012, the Company acquired NP Coil Dexter Industries, S.r.l. for approximately $2,748, and assumed short-term and long-term debt of approximately $1,186 and $854, respectively. NP Coil Dexter is a manufacturer and supplier of metal surface treatment products. The Company allocated the purchase price to $3,825 of intangible assets, comprised of trademarks and formulations, to be amortized over 10 years; two customer lists to be amortized over 8 and 4 years, respectively; and a non-competition agreement to be amortized over 5 years. In addition, the Company recorded $1,786 of goodwill, none of which will be tax deductible and was assigned to the EMEA reportable operating segment. Liabilities assumed included a hold-back of consideration to be paid to the former shareholders at 18 months from the acquisition date. During the fourth quarter of 2012, the Company recorded an increase to other income of approximately $1,033 on its Consolidated Statement of Income related to a change in the fair value of this hold-back of consideration liability.
In October 2011, the Company acquired G.W. Smith & Sons, Inc. for approximately $14,518. G.W. Smith manufactures and distributes high quality die casting lubricants, and also distributes metalworking fluids. The Company allocated the purchase price to $6,260 of intangible assets, comprised of trade names and formulations, to be amortized over 15 years; a trademark to be amortized over 5 years; a non-competition agreement to be amortized over 5 years; and customer lists to be amortized over 16 years. In addition, the Company recorded $1,120 of goodwill, all of which will be tax deductible and was assigned to the North American reportable operating segment. Liabilities assumed included a hold-back of consideration for potential indemnity obligations, which was settled during 2012 with a payment of approximately $1,000 to the former shareholder.
In July 2011, the Company acquired the remaining 60% ownership interest in Tecniquimia Mexicana, S.A. de C.V., the Company’s Mexican equity affiliate, for approximately $10,500. As part of the acquisition, the Company recorded a one-time increase to other income of approximately $2,718 to revalue the previously held ownership interest in Tecniquimia to its fair value. The acquisition of Tecniquimia allowed the Company to further capitalize on the growing Mexican market. The Company allocated the purchase price to $3,556 of intangible assets, comprised of trade names and trademarks, to be amortized over 5 years; and customer lists, to be amortized over 20 years. In addition, the Company recorded $6,773 of goodwill, none of which will be tax deductible, and was assigned to the North American reportable operating segment. Liabilities assumed included a hold-back of consideration for potential indemnity obligations, which was paid to the former shareholders during 2012 with a payment of approximately $2,000.
In December 2010, the Company completed the acquisition of Summit Lubricants, Inc., which manufactures and distributes specialty greases and lubricants, for approximately $29,116, subject to certain post-closing adjustments. During 2011, the Company paid an additional $717 to finalize the post-closing adjustments and recorded non-cash adjustments to fixed assets and goodwill to finalize its valuation of the assets acquired and liabilities assumed at the acquisition date. The Company allocated the purchase price to $17,100 of intangible assets, comprised of formulations, to be amortized over 15 years; customer lists, to be amortized over 20 years; a non-competition agreement, to be amortized over 5 years; and a trademark, which was assigned an indefinite life. In addition, the Company recorded $3,423 of goodwill, all of which will be tax deductible, and was assigned to the North American reportable operating segment. Liabilities assumed included an earnout to be paid to the former shareholders if certain earnings targets are met by the end of 2013, which is expected to be settled during 2014. During 2013, 2012 and 2011, the Company recorded net increases to other income of approximately $497, $1,737 and $595, respectively, in its Consolidated Statement of Income related to changes in the Company’s estimate of the fair value of the contingent consideration liability.
60
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
The following tables show the allocation of the purchase price of the assets and liabilities acquired during 2012 and 2011:
| NP Coil Dexter | ||||
| 2012 Acquisition | Industries, S.r.l. | |||
| Current assets | $ | 5,536 | ||
| Property, plant & equipment | 1,211 | |||
| Intangibles | 3,825 | |||
| Goodwill | 1,786 | |||
| Other long-term assets | 783 | |||
| Total assets purchased | 13,141 | |||
| Short-term debt | (1,186 | ) | ||
| Other current liabilities | (6,168 | ) | ||
| Long-term debt | (854 | ) | ||
| Other long-term liabilities | (1,258 | ) | ||
| Present value of a hold-back liability | (927 | ) | ||
| Total liabilities assumed | (10,393 | ) | ||
| Cash paid for an acquisition | $ | 2,748 | ||
| Quaker | GW Smith | |||||||||||
| 2011 Acquisitions | Tecniquimia | & Sons, Inc. | Total | |||||||||
| Current assets | $ | 8,946 | $ | 6,138 | $ | 15,084 | ||||||
| Property, plant and equipment | 4,308 | 2,869 | 7,177 | |||||||||
| Intangibles | 3,556 | 6,260 | 9,816 | |||||||||
| Goodwill | 6,773 | 1,120 | 7,893 | |||||||||
| Other long-term assets | 1,355 | 1 | 1,356 | |||||||||
| Total assets purchased | 24,938 | 16,388 | 41,326 | |||||||||
| Current liabilities | (2,224 | ) | (1,001 | ) | (3,225 | ) | ||||||
| Long-term liabilities | (6,869 | ) | — | (6,869 | ) | |||||||
| Present value of a hold-back liability | (1,754 | ) | (869 | ) | (2,623 | ) | ||||||
| Total liabilities assumed | (10,847 | ) | (1,870 | ) | (12,717 | ) | ||||||
| Additional minimum pension liability | 987 | — | 987 | |||||||||
| Total equity assumed | 987 | — | 987 | |||||||||
| Fair value of previously held equity interest | (4,578 | ) | — | (4,578 | ) | |||||||
| Cash paid for acquisitions | $ | 10,500 | $ | 14,518 | $ | 25,018 | ||||||
Included in the 2012 acquisition of NP Coil Dexter was approximately $113 of cash acquired, and, also, in the 2011 acquisitions of Tecniquimia Mexicana, S.A. de C.V. and G.W. Smith and Sons, Inc., cash acquired was approximately $236 and $22, respectively.
Certain pro forma and other disclosures have not been provided as of December 31, 2013 for the 2013, 2012 and 2011 acquisitions because the effects were not material.
61
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 20 – Fair Value Measures
The Company values company-owned life insurance policies, various deferred compensation assets and liabilities, acquisition-related consideration and an obligation related to a non-competition agreement at fair value. The Company’s assets and liabilities subject to fair value measurement are as follows:
| Fair Value Measurements at December 31, 2013 | ||||||||||||||||
| Fair Value | Using Fair Value Hierarchy | |||||||||||||||
| as of | ||||||||||||||||
| Assets | December 31, 2013 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Company-owned life insurance | $ | 1,885 | $ | — | $ | 1,885 | $ | — | ||||||||
| Company-owned life insurance - Deferred compensation assets | 409 | — | 409 | — | ||||||||||||
| Other deferred compensation assets | ||||||||||||||||
| Large capitalization registered investment companies | 74 | 74 | — | — | ||||||||||||
| Mid capitalization registered investment companies | 6 | 6 | — | — | ||||||||||||
| Small capitalization registered investment companies | 13 | 13 | — | — | ||||||||||||
| International developed and emerging markets registered investment | ||||||||||||||||
| companies | 40 | 40 | — | — | ||||||||||||
| Fixed income registered investment companies | 7 | 7 | — | — | ||||||||||||
| Total | $ | 2,434 | $ | 140 | $ | 2,294 | $ | — | ||||||||
| Fair Value Measurements at December 31, 2013 | ||||||||||||||||
| Fair Value | Using Fair Value Hierarchy | |||||||||||||||
| as of | ||||||||||||||||
| Liabilities | December 31, 2013 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Deferred compensation liabilities | ||||||||||||||||
| Large capitalization registered investment companies | $ | 405 | $ | 405 | $ | — | $ | — | ||||||||
| Mid capitalization registered investment companies | 109 | 109 | — | — | ||||||||||||
| Small capitalization registered investment companies | 95 | 95 | — | — | ||||||||||||
| International developed and emerging markets registered investment | ||||||||||||||||
| companies | 205 | 205 | — | — | ||||||||||||
| Fixed income registered investment companies | 43 | 43 | — | — | ||||||||||||
| Fixed general account | 167 | — | 167 | — | ||||||||||||
| Acquisition-related consideration | 4,876 | — | — | 4,876 | ||||||||||||
| Total | $ | 5,900 | $ | 857 | $ | 167 | $ | 4,876 | ||||||||
62
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
| Fair Value Measurements at December 31, 2012 | ||||||||||||||||
| Fair Value | Using Fair Value Hierarchy | |||||||||||||||
| as of | ||||||||||||||||
| Assets | December 31, 2012 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Company-owned life insurance | $ | 1,653 | $ | — | $ | 1,653 | $ | — | ||||||||
| Company-owned life insurance - Deferred compensation assets | 437 | — | 437 | — | ||||||||||||
| Other deferred compensation assets | ||||||||||||||||
| Large capitalization registered investment companies | 62 | 62 | — | — | ||||||||||||
| Mid capitalization registered investment companies | 6 | 6 | — | — | ||||||||||||
| Small capitalization registered investment companies | 9 | 9 | — | — | ||||||||||||
| International developed and emerging markets registered investment | ||||||||||||||||
| companies | 37 | 37 | — | — | ||||||||||||
| Fixed income registered investment companies | 8 | 8 | — | — | ||||||||||||
| Total | $ | 2,212 | $ | 122 | $ | 2,090 | $ | — | ||||||||
| Fair Value Measurements at December 31, 2012 | ||||||||||||||||
| Fair Value | Using Fair Value Hierarchy | |||||||||||||||
| as of | ||||||||||||||||
| Liabilities | December 31, 2012 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Deferred compensation liabilities | ||||||||||||||||
| Large capitalization registered investment companies | $ | 336 | $ | 336 | $ | — | $ | — | ||||||||
| Mid capitalization registered investment companies | 88 | 88 | — | — | ||||||||||||
| Small capitalization registered investment companies | 72 | 72 | — | — | ||||||||||||
| International developed and emerging markets registered investment | ||||||||||||||||
| ��companies | 187 | 187 | — | — | ||||||||||||
| Fixed income registered investment companies | 48 | 48 | — | — | ||||||||||||
| Fixed general account | 173 | — | 173 | — | ||||||||||||
| Acquisition-related consideration | 4,901 | — | — | 4,901 | ||||||||||||
| Total | $ | 5,805 | $ | 731 | $ | 173 | $ | 4,901 | ||||||||
The fair values of Company-owned life insurance (“COLI”) and COLI deferred compensation assets are based on quotes for like instruments with similar credit ratings and terms. The fair values of other deferred compensation assets and liabilities are based on quoted prices in active markets. The fair value of the Summit earnout has been based on unobservable inputs and is classified as Level 3. Significant inputs and assumptions were management’s estimate of the probability of the earnout ultimately being met/paid and the discount rate used to present value the liability. The fair value of the obligation related to a non-competition agreement is also based on unobservable inputs and is classified as Level 3. The significant inputs and assumptions for the obligation related to the non-competition agreement is management’s estimate of the discount rate used to present value the liability. A significant change in any Level 3 assumption in isolation would result in increases or decreases to the fair value measurements of the acquisition-related consideration.
63
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Changes in the fair value of the Level 3 liabilities during the year ended December 31, 2013 were as follows:
| Non-competition | ||||||||||||
| Earnout | Agreement | |||||||||||
| Summit | Obligation | Total | ||||||||||
| Balance at December 31, 2012 | $ | 4,497 | $ | 404 | $ | 4,901 | ||||||
| Interest accretion | 697 | 25 | 722 | |||||||||
| Change in fair value estimate | (497 | ) | — | (497 | ) | |||||||
| Payments | — | (250 | ) | (250 | ) | |||||||
| Balance at December 31, 2013 | $ | 4,697 | $ | 179 | $ | 4,876 | ||||||
During the first quarter of 2013, the Summit earnout liability became current and was reclassified from other non-current liabilities to other current liabilities on the Company’s Consolidated Balance Sheet, as the Company expects to settle the obligation within the next year.
Quantitative information about the Company’s Level 3 fair value measurements at December 31, 2013 were as follows:
Fair value at December 31, 2013 | Valuation technique | Unobservable input | Input value | ||||||
| Summit earnout | 4,697 | Discounted cash flow | Discount rate | 14.5% | |||||
| Non-competition agreement obligation | 179 | Discounted cash flow | Discount rate | 14.0% |
At December 31, 2013, the Summit earnout liability was formulated based on the former acquisition’s actual performance and is management’s estimate of the likely payout under the earnout criteria. Prior to December 31, 2013, the determination of the fair value of the Summit earnout was based on the weighted average probability of the outcome of different payout scenarios. The probabilities applied to the payout scenarios prior to the fourth quarter of 2013 ranged from 15% to 70%, depending on the Company’s estimate of the likelihood of each payout scenario.
Note 21 – Hedging Activities
The Company has utilized interest rate swaps to mitigate the impact of changes in interest rates by converting a portion of the Company’s variable interest rate debt to fixed interest rate debt. The interest rate swaps had a combined notional amount of $15,000 during 2012 until their maturity, which occurred during the third quarter of 2012. The Company had no derivatives designated as cash flow hedges as of December 31, 2012 and did not utilize any during the year ended December 31, 2013.
Information about the Company’s interest rate derivatives is as follows:
| Cash Flow Hedges | |||||||||||||
| Interest Rate Swaps | |||||||||||||
| For the Years Ended | |||||||||||||
| December 31, | |||||||||||||
| 2013 | 2012 | 2011 | |||||||||||
| Amount of Gain Recognized in | |||||||||||||
| Accumulated OCI on Derivative (Effective Portion) | $ | — | $ | 272 | $ | 395 | |||||||
| Amount and Location of Loss Reclassified from | |||||||||||||
| Accumulated OCI into Income (Effective Portion) | Interest Expense | $ | — | $ | (392 | ) | $ | (660 | ) | ||||
| Amount and Location of Loss Recognized in Income on Derivative | |||||||||||||
| (Ineffective Portion and Amount Excluded from Effectiveness Testing) | Other Income | $ | — | $ | — | $ | — | ||||||
64
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
Note 22 – Commitments and Contingencies
In April of 1992, the Company identified certain soil and groundwater contamination at AC Products, Inc. (“ACP”), a wholly owned subsidiary. In voluntary coordination with the Santa Ana California Regional Water Quality Board (“SACRWQB”), ACP has been remediating the contamination, the principal contaminant of which is perchloroethylene (“PERC”). On or about December 18, 2004, the Orange County Water District (“OCWD”) filed a civil complaint in Superior Court in Orange County, California against ACP and other parties potentially responsible for groundwater contamination. OCWD was seeking to recover compensatory and other damages related to the investigation and remediation of the contamination in the groundwater. Effective October 17, 2007, ACP and OCWD settled all claims related to this litigation. Pursuant to the settlement agreement with OCWD, ACP agreed to pay $2,000. In addition to the $2,000 payment, ACP agreed to operate the two existing groundwater treatment systems associated with its extraction wells P-2 and P-3 so as to hydraulically contain groundwater contamination emanating from ACP’s site until such time as the concentrations of PERC are below the current Federal maximum contaminant level for four consecutive quarterly sampling events. On September 11, 2012, ACP received a letter from the SACRWQB advising that no further action is required to remediate the soil contamination on site. As of December 31, 2013, the Company believes that the range of potential-known liabilities associated with the ACP water remediation program is approximately $395 to $800, for which the Company has sufficient reserves.
The low and high ends of the range are based on the length of operation of an offsite extraction well as determined by groundwater modeling with planned higher maintenance costs in later years if a longer treatment period is required. Costs of operation include the operation and maintenance of the extraction well, groundwater monitoring and program management. The duration of the well operation was estimated based on historical trends in concentrations in the monitoring well within the proximity of the applicable extraction well. Also factored into the model was the impact of water injected into the underground aquifer from a planned water treatment system to be installed by OCWD adjacent to P-2. Based on the modeling, it is estimated that P-2 will operate for another nine months to two years. The Company is in the process of closing P-3. Operation and maintenance costs were based on historical expenditures and estimated inflation. As mentioned above, a significantly higher maintenance expense was factored into the range if the system operates for the longer period.
The Company believes, although there can be no assurance regarding the outcome of other unrelated environmental matters, that it has made adequate accruals for costs associated with other environmental problems of which it is aware. Approximately $205 and $230 was accrued at December 31, 2013 and December 31, 2012, respectively, to provide for such anticipated future environmental assessments and remediation costs.
An inactive subsidiary of the Company that was acquired in 1978 sold certain products containing asbestos, primarily on an installed basis, and is among the defendants in numerous lawsuits alleging injury due to exposure to asbestos. The subsidiary discontinued operations in 1991 and has no remaining assets other than the proceeds from insurance settlements received. To date, the overwhelming majority of these claims have been disposed of without payment and there have been no adverse judgments against the subsidiary. Based on a continued analysis of the existing and anticipated future claims against this subsidiary, it is currently projected that the subsidiary’s total liability over the next 50 years for these claims is approximately $2,700 (excluding costs of defense). Although the Company has also been named as a defendant in certain of these cases, no claims have been actively pursued against the Company, and the Company has not contributed to the defense or settlement of any of these cases pursued against the subsidiary. These cases were handled by the subsidiary’s primary and excess insurers who had agreed in 1997 to pay all defense costs and be responsible for all damages assessed against the subsidiary arising out of existing and future asbestos claims up to the aggregate limits of the policies. A significant portion of this primary insurance coverage was provided by an insurer that is now insolvent, and the other primary insurers have asserted that the aggregate limits of their policies have been exhausted. The subsidiary challenged the applicability of these limits to the claims being brought against the subsidiary. In response, two of the three carriers entered into separate settlement and release agreements with the subsidiary in late 2005 and early 2007 for $15,000 and $20,000, respectively. The proceeds of both settlements are restricted and can only be used to pay claims and costs of defense associated with the subsidiary’s asbestos litigation. During the third quarter of 2007, the subsidiary and the remaining primary insurance carrier entered into a Claim Handling and Funding Agreement, under which the carrier will pay 27% of defense and indemnity costs incurred by or on behalf of the subsidiary in connection with asbestos bodily injury claims for a minimum of five years beginning July 1, 2007. The agreement continues until terminated and can only be terminated by either party by providing the other party with a minimum of two years prior written notice. As of December 31, 2013, no notice of termination has been given under this agreement. At the end of the term of the agreement, the subsidiary may choose to again pursue its claim against this insurer regarding the application of the policy limits. The Company also believes that, if the coverage issues under the primary policies with the remaining carrier are resolved adversely to the subsidiary and all settlement proceeds were used, the subsidiary may have limited additional coverage from a state guarantee fund established following the insolvency of one of the subsidiary’s primary insurers. Nevertheless, liabilities in respect of claims may exceed the assets and coverage available to the subsidiary.
65
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
If the subsidiary’s assets and insurance coverage were to be exhausted, claimants of the subsidiary may actively pursue claims against the Company because of the parent-subsidiary relationship. Although asbestos litigation is particularly difficult to predict, especially with respect to claims that are currently not being actively pursued against the Company, the Company does not believe that such claims would have merit or that the Company would be held to have liability for any unsatisfied obligations of the subsidiary as a result of such claims. After evaluating the nature of the claims filed against the subsidiary and the small number of such claims that have resulted in any payment, the potential availability of additional insurance coverage at the subsidiary level, the additional availability of the Company’s own insurance and the Company’s strong defenses to claims that it should be held responsible for the subsidiary’s obligations because of the parent-subsidiary relationship, the Company believes it is not probable that the Company will incur any material losses. The Company has been successful to date having claims naming it dismissed during initial proceedings. Since the Company may be in this early stage of litigation for some time, it is not possible to estimate additional losses or range of loss, if any.
As initially disclosed in the Company’s second quarter 2010 Form 10-Q, one of the Company’s subsidiaries may have paid certain value-added-taxes (“VAT”) incorrectly and, in certain cases, may not have collected sufficient VAT from certain customers. The VAT rules and regulations at issue are complex, vary among the jurisdictions and can be contradictory, in particular as to how they relate to the subsidiary’s products and to sales between jurisdictions.
Since its inception, the subsidiary had been consistent in its VAT collection and remittance practices and had never been contacted by any tax authority relative to VAT. The subsidiary later determined that for certain products, a portion of the VAT was incorrectly paid and that the total VAT due exceeds the amount originally collected and remitted by the subsidiary. In response, the subsidiary modified its VAT invoicing and payment procedures to eliminate or mitigate future exposure. In 2010, three jurisdictions contacted the subsidiary and, since then, the subsidiary has either participated in an amnesty program or entered into a settlement whereby it paid a reduced portion of the amounts owed in resolution of those jurisdictions’ claims. In late 2013, an additional jurisdiction issued an assessment against the subsidiary for certain tax years. The subsidiary has filed an appeal of the assessment alleging certain errors by such jurisdiction related to the assessment.
In analyzing the subsidiary’s exposure, it is difficult to estimate both the probability and the amount of any potential liabilities due to a number of factors, including: the decrease in exposure over time due to applicable statutes of limitations and actions taken by the subsidiary, the joint liability of customers and suppliers for a portion of the VAT, the availability of a VAT refund for VAT incorrectly paid through an administrative process, any amounts which may have been or will be paid by customers, as well as the timing and structure of any tax amnesties or settlements. In addition, interest and penalties on any VAT due can be a multiple of the base tax. The subsidiary may contest any tax assessment administratively and/or judicially for an extended period of time, but may ultimately resolve its disputes through participation in tax amnesty programs, which are a common practice for settling tax disputes in the jurisdictions in question and which have historically occurred on a regular basis, resulting in significant reductions of interest and penalties. Also, the timing of payments and refunds of VAT may not be contemporaneous, and, if additional VAT is owed, it may not be fully recoverable from customers. As a result, this matter has the potential to have a material adverse impact on the Company’s financial position, liquidity and capital resources and the results of operations.
In 2010, the Company recorded a net charge of $4,132, which consisted of a net $3,901 charge related to two tax dispute settlements entered into by the subsidiary, as well as a net $231 charge representing management’s best estimate based on the information available to it, including the factors noted above, of the amount that ultimately may be paid related to the other jurisdiction that has made inquiries. At December 31, 2013 and December 31, 2012, the Company had no remaining accrual, related to the 2010 charges, for payments to be made under the tax dispute settlements entered into by the subsidiary, noted above. In the fourth quarter of 2013, the Company recorded a net charge of $796, representing the Company’s best estimate of the amount that ultimately may be paid related to the 2013 assessment referenced above.
The charges taken by the Company in 2010 and in the fourth quarter of 2013 assume a successful recovery of the VAT incorrectly paid, as well as reductions in interest and penalties from anticipated future amnesty programs or settlements. On a similar basis, if all other potentially impacted jurisdictions were to initiate audits and issue assessments, the remaining exposure, net of refunds, could be from $0 to $7,200 with one jurisdiction representing approximately 78 percent of this additional exposure, assuming the continued availability of future amnesty programs or settlements to reduce the interest and penalties. If there are future assessments but no such future amnesty programs or settlements, the potential exposure could be higher.
The Company is party to other litigation which management currently believes will not have a material adverse effect on the Company’s results of operations, cash flows or financial condition.
The Company leases certain manufacturing and office facilities and equipment under non-cancelable operating leases with various terms from 1 to 5 years expiring in 2018. Rent expense for 2013, 2012 and 2011 was $5,510, $5,189, and $5,282, respectively.
66
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
The Company’s minimum rental commitments under non-cancelable operating leases at December 31, 2013 for future years were approximately:
| 2014 | $ | 4,991 | ||
| 2015 | $ | 4,154 | ||
| 2016 | $ | 3,507 | ||
| 2017 | $ | 1,812 | ||
| 2018 | $ | 5 | ||
| 2019 and beyond | $ | — |
Note 23 – Quarterly Results (unaudited)
| First | Second | Third | Fourth | |||||||||||||
| Quarter (1) | Quarter (2) | Quarter (3) | Quarter (4) | |||||||||||||
| 2013 | ||||||||||||||||
| Net sales | $ | 176,193 | $ | 184,846 | $ | 184,059 | $ | 184,297 | ||||||||
| Gross profit | 62,608 | 67,314 | 65,990 | 65,163 | ||||||||||||
| Operating income | 17,411 | 19,793 | 18,807 | 15,232 | ||||||||||||
| Net income attributable to Quaker Chemical Corporation | 13,619 | 16,083 | 12,551 | 14,086 | ||||||||||||
| Net income attributable to Quaker Chemical Corporation | ||||||||||||||||
| Common Shareholders - Basic | $ | 1.04 | $ | 1.22 | $ | 0.95 | $ | 1.07 | ||||||||
| Net income attributable to Quaker Chemical Corporation | ||||||||||||||||
| Common Shareholders - Diluted | $ | 1.04 | $ | 1.22 | $ | 0.95 | $ | 1.07 | ||||||||
| 2012 | ||||||||||||||||
| Net sales | $ | 177,638 | $ | 176,797 | $ | 180,923 | $ | 172,868 | ||||||||
| Gross profit | 59,795 | 60,636 | 59,126 | 59,154 | ||||||||||||
| Operating income | 16,702 | 16,983 | 15,863 | 13,676 | ||||||||||||
| Net income attributable to Quaker Chemical Corporation | 12,365 | 11,108 | 10,925 | 13,007 | ||||||||||||
| Net income attributable to Quaker Chemical Corporation | ||||||||||||||||
| Common Shareholders - Basic | $ | 0.96 | $ | 0.86 | $ | 0.84 | $ | 0.99 | ||||||||
| Net income attributable to Quaker Chemical Corporation | ||||||||||||||||
| Common Shareholders - Diluted | $ | 0.95 | $ | 0.85 | $ | 0.83 | $ | 0.99 | ||||||||
| (1) | Net income attributable to Quaker Chemical Corporation for the first quarter of 2013 and the first quarter of 2012 include earnings from the Company’s equity interest in a captive insurance company of approximately $0.11 and $0.04 per diluted share, respectively. The first quarter of 2013 earnings from the Company’s equity interest in a captive insurance company includes an out-of-period adjustment of approximately $1,038, primarily related to a reinsurance contract held by the captive insurance company. Net income attributable to Quaker Chemical Corporation in the first quarter of 2013 includes a devaluation charge related to the Company’s 50% owned equity affiliate in Venezuela of $0.03 per diluted share. |
| (2) | Net income attributable to Quaker Chemical Corporation for the second quarter of 2013 and the second quarter of 2012 include earnings from the Company’s equity interest in a captive insurance company of approximately $0.13 and $0.04 per diluted share, respectively. Net income attributable to Quaker Chemical Corporation in the second quarter of 2013 also includes earnings per diluted share of approximately $0.14 related to a mineral oil excise tax refund. Net income attributable to Quaker Chemical Corporation in the second quarter of 2013 includes a charge related to a change in the fair value of an acquisition-earnout liability of approximately $0.03 per diluted share and costs related to streamlining certain operations in the Company’s South American segment of approximately $0.02 per diluted share. Net income attributable to Quaker Chemical Corporation for the second quarter of 2012 includes charges of approximately $0.06 per diluted share due to certain customer bankruptcies in the U.S. and approximately $0.03 per diluted share related to CFO transition costs. |
67
QUAKER CHEMICAL CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - Continued
(In thousands except per share amounts)
| (3) | Net income attributable to Quaker Chemical Corporation for the third quarter of 2013 and the third quarter of 2012 include earnings from the Company’s equity interest in a captive insurance company of approximately $0.09 and $0.03 per diluted share, respectively. Net income attributable to Quaker Chemical Corporation for the third quarter of 2013 also includes costs of approximately $0.05 per diluted share related to streamlining certain operations in the Company’s EMEA segment. |
| (4) | Net income attributable to Quaker Chemical Corporation for the fourth quarter of 2013 and the fourth quarter of 2012 include earnings from the Company’s equity interest in a captive insurance company of approximately $0.08 and $0.03 per diluted share, respectively. Net income attributable to Quaker Chemical Corporation also includes earnings per diluted share of approximately $0.06 and $0.09 in the fourth quarters of 2013 and 2012, respectively, related to changes in the fair value of an acquisition-related earnout liability. In addition, net income attributable to Quaker Chemical Corporation for the fourth quarter of 2013 includes costs of approximately $0.01 per diluted share related to streamlining certain operations in the Company’s EMEA segment and approximately $0.04 per diluted share related to a non-income tax contingency. |
68
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
Not Applicable.
Item 9A. Controls and Procedures.
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
As required by Rule 13a-15(b) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), our management, including our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Based on that evaluation, our principal executive officer and our principal financial officer have concluded that as of the end of the period covered by this report our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) were effective.
Management’s Report on Internal Control over Financial Reporting
The management of Quaker is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Rule 13a-15(f) promulgated under the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Our management, with the participation of our principal executive officer and principal financial officer, assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2013. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework (1992). Based on its assessment, Quaker’s management has concluded that as of December 31, 2013, the Company’s internal control over financial reporting is effective based on those criteria.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2013 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included in “Item 8. Financial Statements and Supplementary Data.”
Changes in Internal Controls Over Financial Reporting
As required by Rule 13a-15(d) under the Exchange Act, our management, including our principal executive officer and principal financial officer, has evaluated our internal control over financial reporting to determine whether any changes to our internal control over financial reporting occurred during the fourth quarter of the year ended December 31, 2013 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on that evaluation, no such changes to our internal control over financial reporting occurred during the fourth quarter of the year ended December 31, 2013.
Item 9B. Other Information.
None.
69
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Incorporated by reference is (i) the information beginning immediately following the caption “Proposal 1—Election of Directors and Nominee Biographies” in Quaker’s definitive Proxy Statement relating to the Annual Meeting of Shareholders to be held May 7, 2014, to be filed with the SEC no later than 120 days after the close of its fiscal year ended December 31, 2013 (the “2014 Proxy Statement”) to, but not including, the caption “Corporate Governance,” (ii) the information appearing in Item 4(a) of this Report, (iii) the information in the 2014 Proxy Statement beginning with and including the sub-caption, “Section 16(a) Beneficial Ownership Reporting Compliance” to, but not including, the caption “Certain Relationships and Related Transactions,” and (iv) the information in the 2014 Proxy Statement beginning with and including the sub-caption “Code of Conduct” to, but not including, the caption “Compensation Committee Interlocks and Insider Participation.”
Item 11. Executive Compensation.
Incorporated by reference is the information in the 2014 Proxy Statement (i) beginning with and including the caption “Compensation Committee Interlocks and Insider Participation” to, but not including, the caption “Proposal 2 – Advisory Vote on Compensation of Our Named Executive Officers,” and (ii) beginning with and including the caption “Director Compensation” to, but not including, the caption “Stock Ownership of Certain Beneficial Owners and Management.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Incorporated by reference is the information in the 2014 Proxy Statement beginning immediately following the caption “Stock Ownership of Certain Beneficial Owners and Management” to, but not including, the sub-caption “Section 16(a) Beneficial Ownership Reporting Compliance.”
Equity Compensation Plans
The following table sets forth certain information relating to the Company’s equity compensation plans as of December 31, 2013. Each number of securities reflected in the table is a reference to shares of Quaker common stock.
| Equity Compensation Plan Information | |||||||||||
| Number of securities | |||||||||||
| Number of securities | remaining available for | ||||||||||
| to be issued upon | Weighted-average | future issuance under | |||||||||
| exercise of | exercise price of | equity compensation plans | |||||||||
| outstanding options, | outstanding options, | (excluding securities | |||||||||
| Plan Category | warrants and rights | warrants and rights | reflected in column (a)) | ||||||||
| (a) | (b) | (c) | |||||||||
| Equity compensation plans approved | |||||||||||
| by security holders | 75,251 | $ | 44.49 | 845,926 (1) | |||||||
| Equity compensation plans not approved | |||||||||||
| by security holders | — | — | — | ||||||||
| Total | 75,251 | $ | 44.49 | 845,926 | |||||||
| (1) | As of December 31, 2013, 304,900 of these shares were available for issuance as restricted stock awards under the Company’s 2001 Global Annual Incentive Plan, 49,773 shares were available for issuance upon the exercise of stock options and/or as restricted stock awards under the Company’s 2006 Long-Term Performance Incentive Plan, 417,209 shares were available for issuance upon the exercise of stock options and/or as restricted stock awards and/or restricted stock unit awards under the Company’s 2011 Long-Term Performance Incentive Plan, and 74,044 shares were available for issuance under the 2013 Director Stock Ownership Plan. |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Incorporated by reference is the information in the 2014 Proxy Statement beginning immediately following the sub-caption “Certain Relationships and Related Transactions” to, but not including, the caption “Proposal 3—Ratification of Appointment of Independent Registered Public Accounting Firm,” and the additional information in the 2014 Proxy Statement beginning with and including the sub-caption “Director Independence” to, but not including, the sub-caption “Governance Committee Procedures for Selecting Director Nominees.”
70
Item 14. Principal Accountant Fees and Services.
Incorporated by reference is the information in the 2014 Proxy Statement beginning with and including the sub-caption “Audit Fees” to, but not including, the statement recommending a vote for ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the year ending December 31, 2014.
71
PART IV
Item 15. Exhibits and Financial Statement Schedules.
| (a) | Exhibits and Financial Statement Schedules |
| 1. | Financial Statements and Supplementary Data. |
| Page | ||||
| Financial Statements: | ||||
| 26 | ||||
| 27 | ||||
| 28 | ||||
| 29 | ||||
| 30 | ||||
| 31 | ||||
| 32 | ||||
| 2. | Financial Statement Schedules |
All schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. Financial statements of 50% or less owned companies have been omitted because none of the companies meets the criteria requiring inclusion of such statements.
3. Exhibits (numbered in accordance with Item 601 of Regulation S-K)
| 3(i) — | Articles of Incorporation (as amended through July 31, 2013). Incorporated by reference to Exhibit 3.1 as filed by Registrant with Form 8-K filed on July 31, 2013. | |
| 3(ii) — | By-laws (as amended effective October 4, 2008). Incorporated by reference to Exhibit 10.1 as filed by Registrant with Form 10-Q for the quarter ended September 30, 2008. | |
| 10.1 — | Deferred Compensation Plan as adopted by the Registrant dated December 17, 1999, effective July 1, 1997. Incorporated by reference to Exhibit 10(ff) as filed by Registrant with Form 10-K for the year 1999.* | |
| 10.2 — | Supplemental Retirement Income Program adopted by the Registrant on November 6, 1984, as amended November 8, 1989. Incorporated by reference to Exhibit 10(gg) as filed by Registrant with Form 10-K for the year 1999.* | |
| 10.3 — | 2001 Global Annual Incentive Plan as approved May 9, 2001, effective January 1, 2001. Incorporated by reference to Exhibit 10(hh) as filed by Registrant with Form 10-K for the year 2001.* | |
| 10.4 — | 2003 Director Stock Ownership Plan as approved May 14, 2003. Incorporated by reference to Exhibit 10(ww) as filed by the Registrant with Form 10-K for the year 2003.* | |
| 10.5 — | Credit Agreement between Registrant and Bank of America, N.A. and ABN AMRO Bank, N.V. and Banc of America Securities, in the amount of $100,000,000, dated October 14, 2005. Incorporated by reference to Exhibit 10(jjj) as filed by the Registrant with Form 10-Q for the quarter ended September 30, 2005. | |
| 10.6 — | Settlement Agreement and Release between Registrant, an inactive subsidiary of the Registrant, and Hartford Accident and Indemnity Company dated December 12, 2005. Incorporated by reference to Exhibit 10(nnn) as filed by the Registrant with Form 10-K for the year 2005. | |
| 10.7 — | Amendment to Registrant’s Deferred Compensation Plan for key officers dated December 20, 2005. Incorporated by reference to Exhibit 10 as filed by Registrant with Form 8-K filed on December 22, 2005.* | |
| 10.8 — | 2001 Global Annual Incentive Plan, as amended and restated. Incorporated by reference to Appendix D to the Registrant’s definitive proxy statement filed on March 31, 2006.* | |
| 10.9 — | 2006 Long-Term Performance Incentive Plan. Incorporated by reference to Appendix E to the Registrant’s definitive proxy statement filed on March 31, 2006.* | |
72
| 10.10 — | Form of Stock Option Agreement provided for associates under the Registrant’s 2006 Long-Term Performance Incentive Plan. Incorporated by reference to Exhibit 10.3 as filed by Registrant with Form 8-K filed on May 12, 2006.* | |
| 10.11 — | Form of Restricted Stock Award Agreement for executive officers and other employees under Registrant’s 2006 Long-Term Performance Incentive Plan. Incorporated by reference to Exhibit 10 as filed by Registrant with Form 8-K filed on June 27, 2006.* | |
| 10.12 — | Employment Agreement by and between L. Willem Platzer and Quaker Chemical B.V., a Netherlands corporation and a subsidiary of Registrant, dated August 21, 2006. Incorporated by reference to Exhibit 10 as filed by the Registrant with Form 8-K filed on August 22, 2006.* | |
| 10.13 — | First Amendment to Syndicated Multicurrency Credit Agreement between Registrant and Bank of America, N.A. and certain other financial institutions dated October 6, 2006. Incorporated by reference to Exhibit 10.30 as filed by the Registrant with Form 10-K for the year ended 2008. | |
| 10.14 — | 2006 Long-Term Performance Incentive Plan (amended and restated effective November 8, 2006). Incorporated by reference to Exhibit 10(www) as filed by the Registrant with Form 10-K for the year ended 2006.* | |
| 10.15 — | Financing Agreement by and among Montgomery County Industrial Development Authority and Registrant and Brown Brothers Harriman & Co. dated February 1, 2007. Incorporated by reference to Exhibit 10(yyy) as filed by the Registrant with Form 10-K for the year ended 2006. | |
| 10.16 — | Settlement Agreement and Release between Registrant, an inactive subsidiary of Registrant and Federal Insurance Company dated March 26, 2007. Incorporated by reference to Exhibit 10(zzz) as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2007. | |
| 10.17 — | Change in Control Agreement by and between Registrant and L. Willem Platzer dated April 2, 2007, effective January 1, 2007. Incorporated by reference to Exhibit 10(aaaa) as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2007.* | |
| 10.18 — | Change in Control Agreement by and between Registrant and Jan F. Nieman dated June 27, 2007, effective January 1, 2007. Incorporated by reference to Exhibit 10 (cccc) as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2007.* | |
| 10.19 — | Memorandum of Employment dated June 28, 2007 between Registrant and Mark A. Featherstone, effective April 9, 2007. Incorporated by reference to Exhibit 10 as filed by the Registrant with Form 8-K filed on July 2, 2007.* | |
| 10.20 — | Amendment No.1 to the Registrant’s Director Stock Ownership Plan (as amended March 7, 2007) approved on July 25, 2007. Incorporated by reference to Exhibit 10.37 as filed by the Registrant with Form 10-K for the year ended 2008.* | |
| 10.21 — | Second Amendment to Syndicated Multicurrency Credit Agreement between Registrant and Bank of America, N.A. and certain other financial institutions dated August 13, 2007. Incorporated by reference to Exhibit 10(eeee) as filed by the Registrant with Form 10-Q for the quarter ended September 30, 2007. | |
| 10.22 — | Claim Handling and Funding Agreement between SB Decking, Inc., an inactive subsidiary of Registrant, and Employers Insurance Company of Wausau dated September 25, 2007. Incorporated by reference to Exhibit 10(ffff) as filed by the Registrant with Form 10-Q for the quarter ended September 30, 2007. | |
| 10.23 — | Settlement Agreement and Mutual Release entered into between AC Products, Inc., wholly owned subsidiary of Registrant, and Orange County Water District, effective November 8, 2007. Incorporated by reference to Exhibit 10.47 as filed by the Registrant with Form 10-K for the year ended 2007. | |
| 10.24 — | Financing Agreement by and among Butler County Port Authority and Registrant and Brown Brothers Harriman & Co. dated May 15, 2008. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2008. | |
| 10.25 — | Engineering, Procurement and Construction Contract by and between Registrant and FMC Technologies, Inc., effective May 14, 2008. Incorporated by reference to Exhibit 10.2 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2008. |
73
| 10.26 — | Employment, Transition and Consulting Agreement by and between Registrant and Ronald J. Naples dated May 22, 2008, effective May 7, 2008. Incorporated by reference to Exhibit 10.3 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2008. * | |
| 10.27 — | Employment Agreeement by and between Registrant and Michael F. Barry dated July 1, 2008. Incorporated by reference to Exhibit 10.5 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2008. * | |
| 10.28 — | Change in Control Agreement by and between Registrant and Michael F. Barry dated July 1, 2008. Incorporated by reference to Exhibit 10.6 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2008. * | |
| 10.29 — | Butler County Port Authority Industrial Development Revenue Bond dated May 15, 2008. Incorporated by reference to Exhibit 10.7 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2008. | |
| 10.30 — | Expatriate Agreement by and between Jan F. Nieman and Quaker Chemical Limited (Hong Kong) and Quaker Chemical B.V., both subsidiaries of Registrant, dated June 3, 2003, effective August 1, 2003 and Amended Expatriate Agreement by and between Jan F. Nieman and Quaker Chemical (China) Co. Ltd., Quaker Chemical Limited (Hong Kong) and Quaker Chemical B.V., all subsidiaries of Registrant, dated July 27, 2008, effective August 1, 2008. Incorporated by reference to Exhibit 10.37 as filed by the Registrant with Form 10-K for the year ended December 31, 2009.* | |
| 10.31 — | Memorandum of Employment by and between Registrant and Joseph F. Matrange dated September 30, 2008. Incorporated by reference to Exhibit 10.48 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.32 — | Memorandum of Employment by and between Registrant and D. Jeffry Benoliel dated October 1, 2008. Incorporated by reference to Exhibit 10.49 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.33 — | Amendment to Memorandum of Employment by and between Mark A. Featherstone and Registrant dated November 19, 2008, effective January 1, 2008. Incorporated by reference to Exhibit 10.52 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.34 — | Change in Control Agreement by and between Registrant and Mark A. Featherstone dated November 19, 2008, effective January 1, 2008. Incorporated by reference to Exhibit 10.53 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.35 — | Change in Control Agreement by and between Registrant and D. Jeffry Benoliel dated November 19, 2008, effective January 1, 2008. Incorporated by reference to Exhibit 10.54 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.36 — | Change in Control Agreement by and between Registrant and Joseph F. Matrange dated November 19, 2008, effective October 1, 2008. Incorporated by reference to Exhibit 10.55 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.37 — | Change in Control Agreement by and between Registrant and Ronald S. Ettinger dated November 19, 2008, effective October 1, 2008. Incorporated by reference to Exhibit 10.56 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.38 — | Change in Control Agreement by and between Registrant and George H. Hill dated November 19, 2008, effective October 1, 2008. Incorporated by reference to Exhibit 10.57 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.39 — | Supplemental Retirement Income Program (as amended and restated effective January 1, 2008), approved November 19, 2008. Incorporated by reference to Exhibit 10.58 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.40 — | Amendment No. 1 to the 2001 Global Annual Incentive Plan (as amended and restated effective January 1, 2006), approved November 19, 2008. Incorporated by reference to Exhibit 10.60 as filed by the Registrant with Form 10-K for the year ended 2008. * | |
| 10.41 — | Amendment No. 1 to the 2006 Long-Term Performance Incentive Plan (as amended and restated effective November 8, 2006), approved November 19, 2008. Incorporated by reference to Exhibit 10.61 as filed by the Registrant with Form 10-K for the year ended 2008. * |
74
| 10.42 — | Third Amendment to Syndicated Multicurrency Credit Agreement between Registrant and Bank of America, N.A. and certain other financial institutions dated February 13, 2009, effective February 17, 2009. Incorporated by reference to Exhibit 10.62 as filed by the Registrant with Form 10-K for the year ended 2008. | |
| 10.43 — | Amendment No. 2 to the Quaker Chemical Corporation 2003 Director Stock Ownership Plan (As Amended March 7, 2007). Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2009. * | |
| 10.44 — | Amended Expatriate Agreement by and between Jan F. Nieman and Quaker Chemical (China) Ltd., Quaker Chemical Limited (Hong Kong) and Quaker Chemical B.V., all subsidiaries of Registrant, dated April 6, 2010, Effective March 1, 2010. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2010. * | |
| 10.45 — | Employment Agreement by and between Registrant and Joseph Berquist dated April 1, 2010. Incorporated by reference to Exhibit 10.2 as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2010. * | |
| 10.46 — | Change in Control Agreement by and between Registrant and Joseph Berquist dated April 1, 2010. Incorporated by reference to Exhibit 10.3 as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2010. * | |
| 10.47 — | Fourth Amendment to Syndicated Multicurrency Credit Agreement between Registrant and Bank of America, N.A. and certain other financial institutions dated June 21, 2010. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2010. | |
| 10.48 — | Stock Purchase Agreement by and among Registrant, Summit Lubricants Inc., Ronald Krol, Brian Caputi, Dale M. Perry and Anthony Musilli, dated December 31, 2010. Incorporated by reference to Exhibit 10.54 as filed by the Registrant with Form 10-K for the year ended 2010. | |
| 10.49 — | Amendment No. 3 to the Quaker Chemical Corporation 2003 Director Stock Ownership Plan (As Amended January 26, 2011). Incorporated by reference to Exhibit 10.55 as filed by the Registrant with Form 10-K for the year ended 2010.* | |
| 10.50 — | Employment Agreement by and between Carlos Claro and Quaker Chemical Industria e Comercio Ltda., a Brazilian corporation and a subsidiary of the Registrant, dated January 5, 2011. Incorporated by reference to Exhibit 10.56 as filed by the Registrant with Form 10-K for the year ended 2010.* | |
| 10.51 — | Employment Agreement by and between Dieter Laininger and Quaker Chemical B.V., a subsidiary of the registrant, dated June 1, 2011, effective June 15, 2011. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2011. * | |
| 10.52 — | Change in Control Agreement by and between Registrant and Dieter Laininger dated May 31, 2011, effective June 15, 2011. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2011. * | |
| 10.53 — | Global Annual Incentive Plan (as amended and restated effective May 11, 2011). Incorporated by reference to Appendix B to the Registrant’s definitive proxy statement filed on March 31, 2011. * | |
| 10.54 — | 2011 Long-Term Performance Incentive Plan. Incorporated by reference to Appendix C to the Registrant’s definitive proxy statement filed on March 31, 2011. * | |
| 10.55 — | Form of Restricted Stock Unit Agreement for executive officers and other employees under Registrant’s 2011 Long-Term Performance Incentive Plan. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2012.* | |
| 10.56 — | Memorandum of Employment by and between Registrant and Margaret M. Loebl, dated May 22, 2012, effective June 29, 2012. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2012.* | |
| 10.57 — | Change in Control Agreement by and between Registrant and Margaret M. Loebl, dated May 22, 2012, effective June 29, 2012. Incorporated by reference to Exhibit 10.2 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2012.* | |
| 10.58 — | Amendment to Employment Agreement by and between Jan Nieman and Quaker Chemical Limited (Hong Kong) and Quaker Chemical, B.V., both subsidiaries of Registrant, dated August 2, 2012. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended September 30, 2012.* |
75
| 10.59 — | Expatriate Agreement by and between the Registrant and Dieter Laininger, dated January 14, 2013, effective January 15, 2013. * | |
| 10.60 — | Expatriate Agreement by and between the Registrant and Adrian Steeples, dated January 29, 2013, effective July 1, 2013. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended March 31, 2013.* | |
| 10.61 — | Amended and Restated Multicurrency Credit Agreement by and between Registrant and Bank of America, N.A. and certain other lenders dated June 14, 2013. Incorporated by reference to Exhibit 10.1 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2013. | |
| 10.62 — | Memorandum of Employment and Addendum by and between Registrant and Jan F. Nieman, effective August 1, 2013. Incorporated by reference to Exhibit 10.2 as filed by the Registrant with Form 10-Q for the quarter ended June 30, 2013. * | |
| 21 — | Subsidiaries and Affiliates of the Registrant | |
| 23 — | Consent of Independent Registered Public Accounting Firm | |
| 31.1 — | Certification of Chief Executive Officer of the Company pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934. | |
| 31.2 — | Certification of Chief Financial Officer of the Company pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934. | |
| 32.1 — | Certification of Michael F. Barry pursuant to 18 U.S.C. Section 1350. | |
| 32.2 — | Certification of Margaret M. Loebl pursuant to 18 U.S.C. Section 1350. | |
| 101.INS — | XBRL Instance Document | |
101.SCH — | XBRL Extension Schema Document | |
101.CAL — | XBRL Calculation Linkbase Document | |
101.DEF — | XBRL Definition Linkbase Document | |
101.LAB — | XBRL Label Linkbase Document | |
101.PRE — | XBRL Presentation Linkbase Document |
| * | This exhibit is a management contract or compensation plan or arrangement required to be filed as an exhibit to this Report. |
(b) Exhibits required by Regulation 601 S-K
See (a) 3 of this Item 15.
(c) Financial Statement Schedules
See (a) 2 of this Item 15.
76
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| QUAKER CHEMICAL CORPORATION | ||
| Registrant | ||
| By: | /s/ MICHAEL F. BARRY | |
Michael F. Barry Chairman of the Board, Chief Executive Officer and President | ||
Date: February 28, 2014
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signatures | Capacity | Date | ||||
| /s/ MICHAEL F. BARRY | Principal Executive Officer and | February 28, 2014 | ||||
| Michael F. Barry | Director | |||||
| Chairman of the Board, Chief Executive Officer and President | ||||||
| /s/ MARGARET M. LOEBL | Principal Financial Officer | February 28, 2014 | ||||
| Margaret M. Loebl | ||||||
| Vice President, Chief Financial Officer and Treasurer | ||||||
| /s/ SHANE W. HOSTETTER | Principal Accounting Officer | February 28, 2014 | ||||
| Shane W. Hostetter | ||||||
| Corporate Controller | ||||||
| /s/ JOSEPH B. ANDERSON, JR. | Director | February 28, 2014 | ||||
| Joseph B. Anderson, Jr | ||||||
| /s/ PATRICIA C. BARRON | Director | February 28, 2014 | ||||
| Patricia C. Barron | ||||||
| /s/ DONALD R. CALDWELL | Director | February 28, 2014 | ||||
| Donald R. Caldwell | ||||||
| /s/ ROBERT E. CHAPPELL | Director | February 28, 2014 | ||||
| Robert E. Chappell | ||||||
| /s/ WILLIAM R. COOK | Director | February 28, 2014 | ||||
| William R. Cook | ||||||
| /s/ MARK A. DOUGLAS | Director | February 28, 2014 | ||||
| Mark A. Douglas | ||||||
| /s/ JEFFRY D. FRISBY | Director | February 28, 2014 | ||||
| Jeffry D. Frisby | ||||||
| /s/ ROBERT H. ROCK | Director | February 28, 2014 | ||||
| Robert H. Rock | ||||||
77