Many of our products are subject to regulation by U.S. and foreign regulatory agencies. The following is a description of the principal regulations affecting our businesses.
Our veterinary diagnostic instrument systems are medical devices regulated by the U.S. Food and Drug Administration (“FDA”) under the Food, Drug and Cosmetics Act (the “FDC Act”). While the sale of these products does not require premarket approval by FDA and does not subject us to the FDA’s current Good Manufacturing Practices regulations (“cGMP”), these products must not be adulterated or misbranded under the FDC Act.
Any acquisitions of new products and technologies may subject us to additional areas of government regulation. These may involve food, drug, medical device and water-quality regulations of the FDA, the EPA and the USDA, as well as state, local and foreign governments. See “Part I, Item 1A. Risk Factors.”
At December 31, 2006, we had approximately 3,900 full-time and part-time employees. We are not a party to any collective bargaining agreement and we believe that relations with our employees are good.
Our future operating results involve a number of risks and uncertainties. Actual events or results may differ materially from those discussed in this report. Factors that could cause or contribute to such differences include, but are not limited to, the factors discussed below, as well as those discussed elsewhere in this report.
Our ability to maintain our growth rate depends on our successful implementation of various strategies, including:
However, we may not be able to successfully implement some or all of these strategies and increase or sustain our rate of growth or profitability.
One of our veterinary pharmaceutical products is sold under the FDA’s regulatory discretion and we believe that the FDA would require us to discontinue sales of this product within a short period if and when the FDA approves another product to treat the same condition, whether such new product was our product or that of another commercial supplier. In addition, we have a finite inventory of the raw materials used in the manufacture of the product, and these raw materials are no longer commercially available. We believe that our remaining inventory of raw materials will be adequate to satisfy existing market demand until late 2008 or early 2009. We have, in advanced development and clinical trials, a new product based on different raw materials and we intend to seek FDA approval of this product. FDA approval of this new product would fully mitigate the commercial risk that we would be required to stop selling our current product due either to FDA approval of another manufacturer’s product or to the full depletion of our inventory of raw materials. While we hope to smoothly transition to our new product, we cannot predict when or if the FDA will approve our new product or any product that treats the same condition from another manufacturer. Revenues from sales of this pharmaceutical product were $11.9 million in 2006.
Many of our rapid assay and production animal diagnostic products are biologics, which are products that are comprised of materials from living organisms, such as antibodies, cells and sera. Manufacturing biologic products is highly complex. Unlike products that rely on chemicals for efficacy (such as most pharmaceuticals), biologics are difficult to characterize due to the inherent variability of biological input materials. Difficulty in characterizing biological materials or their interactions creates greater risk in the manufacturing process. There can be no assurance that we will be able to maintain adequate sources of biological materials or that biological materials that we maintain in inventory will yield finished products that satisfy applicable product release criteria. Our inability to obtain necessary biological materials or to successfully manufacture biologic products that incorporate such materials could have a material adverse effect on our results of operations.
We rely on a combination of patent, trade secret, trademark and copyright laws to protect our proprietary rights. If we do not have adequate protection of our proprietary rights, our business may be affected by competitors who develop substantially equivalent technologies that compete with us.
We cannot ensure that we will obtain issued patents, that any patents issued or licensed to us will remain valid, or that any patents owned or licensed by us will provide protection against competitors with similar technologies. Even if our patents cover products sold by our competitors, the time and expense of litigating to enforce our patent rights could be substantial, and could have a material adverse effect on our results of operations. In addition, expiration of patent rights could result in substantial new competition in the markets for products previously covered by those patent rights.
In the past, we have received notices claiming that our products infringe third-party patents and we may receive such notices in the future. Patent litigation is complex and expensive, and the outcome of patent litigation can be difficult to predict. We cannot ensure that we will win a patent litigation case or negotiate an acceptable resolution of such a case. If we lose, we may be stopped from selling certain products and/or we may be required to pay damages and/or ongoing royalties as a result of the lawsuit. Any such adverse result could have a material adverse effect on our results of operations.
We sell many of our products, including substantially all of the rapid assays and instrument consumables sold in the U.S., through distributors. Distributor purchasing patterns can be unpredictable and may be influenced by factors unrelated to the end-user demand for our products. In addition, our agreements with distributors may generally be terminated by the distributors for any reason on 60 days notice. Because significant product sales are made to a limited number of distributors, the loss of a distributor or unanticipated changes in the frequency, timing or size of distributor purchases, could have a negative effect on our results of operations. Our financial performance, therefore, is subject to an unexpected downturn in product demand and may be unpredictable.
Distributors of veterinary products have entered into business combinations resulting in fewer distribution companies. Consolidation within distribution channels would increase our customer concentration level, which could increase the risks described in the preceding paragraph.
| | Increased Competition and Technological Advances by Our Competitors Could Negatively Affect Our Operating Results |
We face intense competition within the markets in which we sell our products and services. We expect that future competition will become even more intense, and that we will have to compete with changing and improving technologies. Some of our competitors and potential competitors, including large pharmaceutical and diagnostic companies, have substantially greater capital, manufacturing, marketing, and research and development resources than we do.
| | Changes in Testing Could Negatively Affect Our Operating Results |
The market for our companion and production animal diagnostic tests and our dairy and water testing products could be negatively impacted by a number of factors. The introduction or broad market acceptance of vaccines or preventatives for the diseases and conditions for which we sell diagnostic tests and services could result in a decline in testing. Eradication or substantial declines in the prevalence of certain diseases also could lead to a decline in diagnostic testing for such diseases. Our production animal products business in particular is subject to fluctuations resulting from changes in disease prevalence. In addition, changes in government regulations could negatively affect sales of our products that are driven by compliance testing, such as our dairy and water products. Declines in testing for any of the reasons described could have a material adverse effect on our results of operations.
On December 29, 2006, the Drinking Water Inspectorate in the U.K. published a proposal to discontinue the regulation that requires testing water supplies forCryptosporidia effective as of December 22, 2007 or, if approved by the regulator, at an earlier date. If this proposal is adopted, the Company believes that it will lose a substantial portion of its sales of Filta-Max® products in England and Wales, which were $2.9 million in the year ended December 31, 2006.
| | Consolidation of Veterinary Hospitals in the U.S. Could Negatively Affect Our Business |
An increasing percentage of veterinary hospitals in the U.S. are owned by corporations that are in the business of acquiring veterinary hospitals and/or opening new veterinary hospitals nationally or regionally. Major corporate hospital owners include VCA/Antech, Inc. and Banfield, The Pet Hospital, both of whom are currently customers of IDEXX. Corporate owners of veterinary hospitals could attempt to improve profitability by leveraging the buying power they derive from their scale to obtain favorable pricing from suppliers, which could have a negative impact on our results. In addition, VCA/Antech is our primary competitor in the U.S. market for reference laboratory services, and hospitals acquired by VCA/Antech will use its laboratory services almost exclusively. Therefore, hospitals acquired by VCA/Antech generally will cease to be customers or potential customers of our reference laboratories business.
| | Our Inexperience in the Human Point-of-Care Market Could Inhibit Our Success in this Market |
Upon acquiring the Critical Care Division of Osmetech plc, we entered the human point-of-care medical diagnostics market for the first time with the sale of the OPTI® line of electrolyte and blood gas analyzers. The human point-of-care medical diagnostics market differs in many respects from the veterinary medical market. Significant differences include the impact of third party reimbursement on diagnostic testing, more extensive regulation, greater product liability risks, larger competitors, and more rapid technological innovation. There can be no assurance that we will be successful in achieving growth and profitability in the human point-of-care medical diagnostics market comparable to the results we have achieved in the veterinary medical market.
| | Risks Associated with Doing Business Internationally Could Negatively Affect Our Operating Results |
For the year ended December 31, 2006, 35% of our revenue was attributable to sales of products and services to customers outside the U.S. Various risks associated with foreign operations may impact our international sales. Possible risks include fluctuations in the value of foreign currencies, disruptions in transportation of our products, the differing product and service needs of foreign customers, difficulties in building and managing foreign operations, import/export duties and quotas, and unexpected regulatory, economic or political changes in foreign markets. Prices that we charge to foreign customers may be different than the prices we charge for the same products in the U.S. due to competitive, market or other factors. As a result, the mix of domestic and international sales in a particular period could have a material impact on our results for that period. In addition, many of the products for which our selling price may be denominated in foreign currencies are manufactured, sourced, or both, in the U.S. and our costs are incurred in U.S. dollars. We utilize non-speculative forward currency exchange contracts to mitigate foreign currency exposure. However, an appreciation of the U.S. dollar relative to the foreign currencies in which we sell these products would reduce our operating margins.
13
| | The Loss of Our President, Chief Executive Officer and Chairman Could Adversely Affect Our Business |
We rely on the management and leadership of Jonathan W. Ayers, our President, Chief Executive Officer and Chairman. We do not maintain key man life insurance coverage for Mr. Ayers. The loss of Mr. Ayers could have a material adverse impact on our business.
| | We Could Be Subject to Class Action Litigation Due to Stock Price Volatility, which, if it Occurs, Could Result in Substantial Costs or Large Judgments Against Us |
The market for our common stock may experience extreme price and volume fluctuations, which may be unrelated or disproportionate to our operating performance or prospects. In the past, securities class action litigation has often been brought against companies following periods of volatility in the market prices of their securities. We may be the target of similar litigation in the future. Securities litigation could result in substantial costs and divert our management’s attention and resources, which could have a negative effect on our business, operating results and financial condition.
| | If Our Quarterly Results of Operations Fluctuate, This Fluctuation May Cause Our Stock Price to Decline, Resulting in Losses to You |
Our prior operating results have fluctuated due to a number of factors, including seasonality of certain product lines; changes in our accounting estimates; the impact of acquisitions; timing of distributor purchases, product launches, research and development expenditures, litigation and claim-related expenditures; changes in competitors’ product offerings; and other matters. Similarly, our future operating results may vary significantly from quarter to quarter due to these and other factors, many of which are beyond our control. If our operating results or projections of future operating results do not meet the expectations of market analysts or investors in future periods, our stock price may fall.
| | Future Operating Results Could Be Negatively Affected By the Resolution of Various Uncertain Tax Positions and by Potential Changes to Tax Incentives |
In the ordinary course of our business, there are many transactions and calculations where the ultimate tax determination is uncertain. Significant judgment is required in determining our worldwide provision for income taxes and our income tax filings are regularly under audit by tax authorities. The final determination of tax audits could be materially different than that which is reflected in historical income tax provisions and accruals. Additionally, we benefit from certain tax incentives offered by various jurisdictions. If we are unable to meet the requirements of such incentives, our inability to use these benefits could have a material negative effect on future earnings.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
In May 2006, we acquired the Westbrook, Maine facility in which we previously leased space. We currently occupy 350,000 square feet of this facility for manufacturing, research and development, and corporate headquarters functions. We plan to renovate and expand this facility during 2007 through 2009, which will provide an additional 200,000 square feet of space. We lease collectively approximately 110,000 square feet of additional office space in Scarborough and Westbrook, Maine under leases expiring in 2009 and 2013, respectively. We lease approximately 97,500 square feet of industrial space in Memphis, Tennessee for use as a distribution facility under a lease expiring in 2013; approximately 40,000 square feet of office and manufacturing space in Eau Claire, Wisconsin for our practice information management systems business under leases expiring in 2008 and 2009; approximately 60,000 square feet of office and manufacturing space in Roswell, Georgia for our OPTI Medical Systems business under a lease expiring in 2010; approximately 16,000 square feet of office and manufacturing space in Switzerland for our European production animal products manufacturing activities under a lease expiring in 2013; and approximately 48,000 square feet of warehouse and office space in the Netherlands for use as our headquarters for European operations under a lease expiring in 2008.
14
We also lease a total of approximately 35,000 square feet of smaller office, manufacturing and warehouse space in the U.S. and elsewhere in the world under leases having expiration dates up to the year 2021. In addition, we own or lease approximately 300,000 square feet of space in the U.S., Australia, Canada, France, Germany, Switzerland, South Africa, and the United Kingdom for use as veterinary reference laboratories and office space for our veterinary consulting services. Of this space, 73,000 square feet is owned by us and the remaining amount is leased, under leases having expiration dates up to the year 2019.
We consider that the properties are generally in good condition, are well-maintained, and are generally suitable and adequate to carry on our business.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we are subject to litigation in the ordinary course of business. However, we do not believe that we are party to any material legal proceedings.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to a vote of security holders during the fourth quarter of the fiscal year covered by this report.
EXECUTIVE OFFICERS OF THE COMPANY
Our executive officers as of February 23, 2007 were as follows:
Name
| Age
| | Title
|
|---|
| Jonathan W. Ayers | | 50 | | Chairman of the Board of Directors, President and Chief Executive Officer | |
| William C. Wallen, PhD | | 63 | | Senior Vice President and Chief Scientific Officer | |
| Conan R. Deady | | 45 | | Corporate Vice President, General Counsel and Secretary | |
| Thomas J. Dupree | | 38 | | Corporate Vice President, Companion Animal Group | |
| S. Sam Fratoni, PhD | | 59 | | Corporate Vice President and Chief Information Officer | |
| Robert S. Hulsy | | 62 | | Corporate Vice President, Reference Laboratories and Digital | |
| Irene C. Kerr | | 57 | | Corporate Vice President, Worldwide Operations | |
| Ali Naqui, PhD | | 53 | | Corporate Vice President Water, Dairy, Asia Pacific and Latin America Operations | |
| James F. Polewaczyk | | 43 | | Corporate Vice President, Rapid Assay | |
| Merilee Raines | | 51 | | Corporate Vice President, Chief Financial Officer and Treasurer | |
| Quentin J. Tonelli, PhD | | 58 | | Corporate Vice President, Production Animal Segment | |
| Michael J. Williams, PhD | | 39 | | Corporate Vice President, Instrument Diagnostics | |
Mr. Ayers has been Chairman of the Board, Chief Executive Officer and President of IDEXX since January 2002. Prior to joining IDEXX, from 1999 to 2001, Mr. Ayers was President of Carrier Corporation, the then-largest business unit of United Technologies Corporation, and from 1997 to 1999, he was President of Carrier’s Asia Pacific Operations. From 1995 to 1997, Mr. Ayers was Vice President, Strategic Planning at United Technologies. Before joining United Technologies, from 1986 to 1995, Mr. Ayers held various positions at Morgan Stanley & Co. in mergers and acquisitions and corporate finance. Prior to Morgan Stanley, Mr. Ayers was a strategy consultant for Bain & Company from 1983 to 1986 and was in the field sales organization of IBM’s Data Processing Division from 1978 to 1981. Mr. Ayers holds an undergraduate degree in molecular biophysics and biochemistry from Yale University and graduated from Harvard Business School in 1983.
15
Dr. Wallen has been Senior Vice President and Chief Scientific Officer of the Company and has been leading the Pharmaceutical Products business since September 2003. Prior to joining IDEXX, Dr. Wallen held various positions with Bayer Corporation, most recently as Senior Vice President, Research and Development, and Head, Office of Technology for the Diagnostics Division of Bayer Healthcare. From 2001 to 2003, Dr. Wallen served as Senior Vice President and Head of Research, Nucleic Acid Diagnostics Segment; from 1999 to 2001, as Senior Vice President of Research and Development Laboratory Testing Segment; and from 1993 to 1999, as Vice President of Research and Development, Immunodiagnostic and Clinical Chemistry Business Units. Before joining Bayer Corporation, from 1990 to 1993, Dr. Wallen was Vice President, Research and Development at Becton Dickinson Advanced Diagnostics.
Mr. Deady has been Corporate Vice President and General Counsel of the Company since 1999 and has been leading the Company’s business development activities since April 2005. Mr. Deady was Deputy General Counsel of the Company from 1997 to 1999. Before joining the Company in 1997, Mr. Deady was Deputy General Counsel of Thermo Electron Corporation (now Thermo Fisher Scientific, Inc.), a manufacturer of technology-based instruments. Previously, Mr. Deady was a partner at Hale and Dorr LLP (now WilmerHale).
Mr. Dupree has been Corporate Vice President of the Company since September 2006 and has been leading the Companion Animal Group Customer Facing Organization in North America since January 2007. Mr. Dupree was General Manager of the Company’s Rapid Assay business from April 2005 to January 2007. Prior to that, Mr. Dupree was Vice President, Business Development. Before joining the Company in 2003, Mr. Dupree was employed at the Boston Consulting Group, a business strategy consulting firm, where he spent seven years leading project teams in the firm’s technology and health care practices. Prior to that, Mr. Dupree held various management positions at Bath Iron Works Corporation.
Dr. Fratoni has been Corporate Vice President of the Company since May 1997 and Chief Information Officer since November 2000 and has been leading the Practice Information Management Systems business since November 2000. He led the Company’s Food and Environmental Group from July 1999 to December 2000. From May 1997 to July 1999, Dr. Fratoni was Vice President of Human Resources of the Company, and from October 1996 to May 1997, he was Director of Business Development for the Food and Environmental Group. Before joining the Company in October 1996, Dr. Fratoni held various positions with Hewlett-Packard Company.
Mr. Hulsy has been Corporate Vice President of the Company since February 1999 and has been leading the Company’s Reference Laboratory and Consulting Services business since August 1998 and the Digital Radiography business since its launch in December 2000. Before joining the Company in August 1998, Mr. Hulsy was President of American Environmental Network, Inc., a network of environmental laboratories, from 1992 to 1998.
Ms. Kerr joined IDEXX as Corporate Vice President, Worldwide Operations in December 2006. Prior to joining IDEXX, Ms. Kerr led strategic initiatives and investments at MDS, Inc., Canada’s largest health and life sciences company. From 1993 to 1999, Ms. Kerr was employed at Bayer Diagnostics, most recently as Senior Vice President of Group Development, and, prior to that, as Senior Vice President of the Clinical Chemistry and Immunodiagnostics Business Units. Ms. Kerr was employed by Abbott Laboratories from 1983 to 1993, initially in Corporate Planning and subsequently as General Manager of Drugs and Drug Delivery Systems in the Hospital Products Division and then as Vice President and General Manager of several global business units and sectors in the Diagnostics Division. Prior to joining Abbott, Ms. Kerr was a general management consultant with Booz Allen & Hamilton.
Dr. Naqui became Corporate Vice President of the Company in January 2006 and oversees the Company’s Water and Dairy Testing businesses, as well as the Company’s Asia Pacific and Latin American operations. Dr. Naqui served as Vice President, Water and Dairy from January 2000 to December 2005, General Manager, Water from September 1997 to January 2000, and Director of Research and Development from February 1993 to September 1997. Dr. Naqui joined the Company in 1993 as a result of the acquisition of Environetics, where he was the Director of Research and Development. Prior to joining Environetics, he was a research and development manager with Becton, Dickinson and Company.
16
Mr. Polewaczyk joined IDEXX as Corporate Vice President and General Manager of the Company’s Rapid Assay business in February 2007. Prior to joining IDEXX, Mr. Polewaczyk was employed with Philips Medical Systems, a subsidiary of Royal Philips Electronics, The Netherlands for fifteen years in various senior marketing and general management positions, most recently as General Manager, Medical Consumables and Sensor Business.
Ms. Raines has been Chief Financial Officer of the Company since October 2003 and Corporate Vice President, Finance of the Company since May 1995. Ms. Raines served as Vice President, Finance from March 1995 to May 1995, Director of Finance from 1988 to March 1995 and Controller from 1985 to 1988.
Dr. Tonelli has been Corporate Vice President of the Company since June 2001 and oversees the Company’s Production Animal Segment and infectious disease research and development activities. Previously he held various positions with the Company, including Vice President for Research and Development and Vice President, Business Development. Before joining the Company in 1984, he was a Group Leader of Research and Development for the Hepatitis and AIDS Business Unit within the diagnostic division of Abbott Laboratories, Inc.
Dr. Williams has been Corporate Vice President of the Company since September 2006 and General Manager of the Companion Animal Instrument and Consumables business since 2004. Effective February 1, 2007, Dr. Williams also oversees the OPTI Medical Systems business. Dr. Williams was Vice President and General Manager of the Company’s chemistry instruments and consumables business from 2003 to 2004. Prior to joining the Company in 2003, Dr. Williams was a healthcare strategy consultant at McKinsey & Company from 1995 to 2002 and a senior research associate at the Scripps Research Institute from 1992 to 1995.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is quoted on the NASDAQ Global Market under the symbol IDXX. The table below shows the high and low sale prices per share of our common stock as reported on the NASDAQ Global Market for the years 2006 and 2005.
Calendar Year
| High
| | Low
| |
|---|
| 2006 | | | | | | | | |
| First Quarter | | | $ | 86.36 | | $ | 71.00 | |
| Second Quarter | | | | 85.50 | | | 74.14 | |
| Third Quarter | | | | 94.36 | | | 72.69 | |
| Fourth Quarter | | | | 95.17 | | | 79.19 | |
| | | | | | | | | |
| 2005 | | | | | | | | |
| First Quarter | | | $ | 58.23 | | $ | 52.18 | |
| Second Quarter | | | | 63.00 | | | 52.94 | |
| Third Quarter | | | | 67.95 | | | 60.16 | |
| Fourth Quarter | | | | 75.14 | | | 61.11 | |
| | | | | | | | | |
As of February 27, 2007, there were 909 holders of record of our common stock.
We have never paid any cash dividends on our common stock. From time to time our Board of Directors may consider the declaration of a dividend. However, we have no present intention to pay a dividend.
17
During the three months ended December 31, 2006, we repurchased our shares as described below:
Period
| Total Number of
Shares Purchased
(a)
| | Average
Price Paid
per Share
(b)
| | Total Number of
Shares Purchased
as Part of
Publicly
Announced Plans
or Programs
(c)
| | Maximum Number of
Shares that May Yet
Be Purchased Under
the Plans or
Programs
(d)
| |
|---|
| October 1, 2006 to October 31, 2006 | | | | - | | $ | - | | | - | | | 857,430 | |
| November 1, 2006 to November 30, 2006 | | | | 69,300 | | | 83.86 | | | 69,300 | | | 788,130 | |
| December 1, 2006 to December 31, 2006 | | | | 73,500 | | | 82.54 | | | 73,500 | | | 714,630 | (1) |
|
| | |
| | |
| Total | | | | 142,800 | | $ | 83.18 | | | 142,800 | | | 714,630 | (1) |
|
| | |
| | |
| (1) | Represents the number of shares remaining at December 31, 2006 exclusive of the subsequent amendment on February 14, 2007 whereby our Board of Directors approved an increase to the repurchase authorization of 2,000,000 shares. |
Our Board of Directors has approved the repurchase of up to 18,000,000 shares of our common stock in the open market or in negotiated transactions. The plan was approved and announced on August 13, 1999, and subsequently amended on October 4, 1999, July 21, 2000, October 20, 2003, October 12, 2004, October 12, 2005, and February 14, 2007 and does not have a specified expiration date. There were no other repurchase plans outstanding during the year ended December 31, 2006, and no repurchase plans expired during the period. Repurchases of approximately 1,338,000 shares were made during the year ended December 31, 2006 in open market transactions.
During the year ended December 31, 2006, we received 227 shares of our common stock that were surrendered by employees in payment for the minimum required withholding taxes due on the vesting of restricted stock units and settlement of deferred stock units. These shares do not reduce the number of shares that may yet be purchased under the repurchase plan.
18
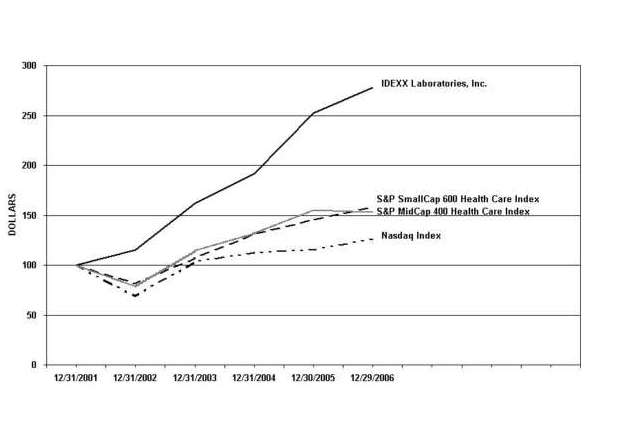
STOCK PERFORMANCE GRAPH
This graph compares our total stockholder returns, the Standard & Poor’s (“S&P”) MidCap 400 Health Care Index, the S&P SmallCap 600 Health Care Index and the Total Return Index for the NASDAQ Stock Market (U.S. Companies) prepared by the Center for Research in Security Prices (the “NASDAQ Index”). This graph assumes the investment of $100 on December 31, 2001 in IDEXX’s common stock, the S&P MidCap 400 Health Care Index, the S&P SmallCap 600 Health Care Index and the NASDAQ Index and assumes dividends, if any, are reinvested. Measurement points are the last trading days of the years ended December 2001, 2002, 2003, 2004, 2005 and 2006.
| 12/31/2001
| | 12/31/2002
| | 12/31/2003
| | 12/31/2004
| | 12/30/2005
| | 12/29/2006
| |
|---|
| IDEXX Laboratories, Inc. | | | $ | 100.00 | | $ | 115.22 | | $ | 162.33 | | $ | 191.48 | | $ | 252.47 | | $ | 278.15 | |
| S&P MidCap 400 Health Care Index | | | | 100.00 | | | 78.88 | | | 114.40 | | | 113.49 | | | 154.88 | | | 153.09 | |
| S&P SmallCap 600 Health Care Index | | | | 100.00 | | | 81.48 | | | 106.99 | | | 130.97 | | | 145.32 | | | 157.72 | |
| NASDAQ Index | | | | 100.00 | | | 69.13 | | | 103.36 | | | 112.49 | | | 114.88 | | | 126.22 | |
19
ITEM 6. SELECTED FINANCIAL DATA
The following table sets forth selected consolidated financial data of the Company for each of the five years ending with December 31, 2006. The selected consolidated financial data presented below have been derived from the Company’s consolidated financial statements. These financial data should be read in conjunction with the consolidated financial statements, related notes and other financial information appearing elsewhere in this Form 10-K.
| For the Years Ended December 31,
(in thousands, except per share data)
| |
|---|
| 2006
| | 2005
| | 2004
| | 2003
| | 2002
| |
|---|
| INCOME STATEMENT DATA: | | | | | | | | | | | | | | | | | |
| Revenue | | | $ | 739,117 | | $ | 638,095 | | $ | 549,181 | | $ | 475,992 | | $ | 412,670 | |
| Cost of revenue | | | | 359,588 | | | 315,195 | | | 270,164 | | | 245,688 | | | 219,945 | |
|
| |
| |
| |
| |
| |
| Gross profit | | | | 379,529 | | | 322,900 | | | 279,017 | | | 230,304 | | | 192,725 | |
| Expenses: | | | | | | | | | | | | | | | | | |
| Sales and marketing | | | | 115,882 | | | 101,990 | | | 85,710 | | | 71,846 | | | 56,794 | |
| General and administrative | | | | 82,097 | | | 64,631 | | | 49,870 | | | 45,752 | | | 40,787 | |
| Research and development | | | | 53,617 | | | 40,948 | | | 35,402 | | | 32,319 | | | 29,329 | |
|
| |
| |
| |
| |
| |
| Income from operations | | | | 127,933 | | | 115,331 | | | 108,035 | | | 80,387 | | | 65,815 | |
| Interest income, net | | | | 2,817 | | | 3,141 | | | 3,068 | | | 2,867 | | | 2,955 | |
|
| |
| |
| |
| |
| |
| Income before provision for income | | | | | | | | | | | | | | | | | |
| taxes and partner's interest | | | | 130,750 | | | 118,472 | | | 111,103 | | | 83,254 | | | 68,770 | |
| Provision for income taxes | | | | 37,224 | | | 40,670 | | | 33,165 | | | 26,278 | | | 23,381 | |
| Partner's interest in loss of subsidiary | | | | (152 | ) | | (452 | ) | | (394 | ) | | (114 | ) | | -- | |
|
| |
| |
| |
| |
| |
| Net income | | | $ | 93,678 | | $ | 78,254 | | $ | 78,332 | | $ | 57,090 | | $ | 45,389 | |
|
| |
| |
| |
| |
| |
| Earnings per share: | | | | | | | | | | | | | | | | | |
| Basic | | | $ | 2.98 | | $ | 2.41 | | | 2.29 | | | 1.67 | | | 1.35 | |
| Diluted | | | | 2.84 | | | 2.30 | | | 2.19 | | | 1.59 | | | 1.30 | |
| Weighted average shares outstanding: | | | | | | | | | | | | | | | | | |
| Basic | | | | 31,433 | | | 32,521 | | | 34,214 | | | 34,271 | | | 33,622 | |
| Diluted | | | | 32,954 | | | 34,055 | | | 35,800 | | | 35,931 | | | 35,043 | |
| Dividends paid | | | $ | -- | | $ | -- | | | -- | | | -- | | | -- | |
| | | | | | | | | | | | | | | | | | |
| BALANCE SHEET DATA: | | | | | | | | | | | | | | | | | |
| Cash and investments | | | $ | 96,666 | | $ | 132,731 | | | 156,959 | | | 255,787 | | | 162,763 | |
| Working capital | | | | 177,520 | | | 192,679 | | | 201,640 | | | 270,244 | | | 217,740 | |
| Total assets | | | | 559,560 | | | 490,676 | | | 514,237 | | | 521,875 | | | 417,426 | |
| Total debt | | | | 7,125 | | | 551 | | | 1,810 | | | 494 | | | 973 | |
| Stockholders' equity | | | | 409,861 | | | 369,010 | | | 397,660 | | | 413,292 | | | 340,973 | |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSES OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
During 2006, we operated primarily through three business segments: products and services for the veterinary market, which we refer to as our Companion Animal Group (“CAG”), water quality products (“Water”) and products for production animal health, which we refer to as the Production Animal Segment (“PAS”). We also operate a smaller segment that comprises products for dairy quality, which we refer to as Dairy. Financial information about the Dairy operating segment is presented in an “Other” category. As of January 2007, we have added an operating segment, OPTI Medical, in connection with our acquisition of the Critical Care Division of Osmetech plc, which is presented in an “Other” category. CAG develops, designs, manufactures, and distributes products and performs services for veterinarians. Water develops, designs, manufactures and distributes products to detect contaminants in water. PAS develops, designs, manufactures and distributes products to detect diseases in production animals. Dairy develops, designs, manufactures and distributes products to detect contaminants in dairy products. OPTI Medical develops, manufactures, and sells point-of-care electrolyte and blood gas analyzers and related consumable products for the human medical diagnostics market. Unallocated items that are not allocated to our operating segments are comprised primarily of share-based compensation costs (effective January 1, 2006), corporate research and development expenses, interest income and expense, and income taxes. The segment information for the years ended December 31, 2005 and 2004 has been restated to conform to our presentation of reportable segments for the year ended December 31, 2006. Previously, PAS and Dairy were aggregated into a single reportable segment, which we referred to as the Food Diagnostics Group. See Note 16 to the consolidated financial statements for the year ended December 31, 2006 included in this Form 10-K for financial information about our segments, including geographic information, and about our product and service categories.
20
The following is a discussion of the strategic and operating factors that we believe have the most significant effect on the performance of our business.
Companion Animal Group
In the CAG segment, we believe we have developed a strategic advantage over companies with more narrow product or service offerings. The breadth of our products and services gives us scale in sales and distribution, permits us to offer integrated disease-management solutions that leverage the advantages of both point-of-care and outside laboratory testing, and facilitates the flow of medical and business information in the veterinary practice by connecting practice information software systems, including connecting the electronic health record with laboratory test data, in-clinic test data from our IDEXX VetLab® suite of analyzers, and radiographic data in the IDEXX-PACS™ software taken by our digital radiography systems.
In the U.S., we sell instrument consumables, rapid assay products and pharmaceutical products primarily through distributors, and, therefore, our reported sales of these products are sales made to distributors, rather than sales to veterinarians, the end users. Because distributors’ inventory levels and purchasing patterns may fluctuate, sales of a particular product line in a particular period may not always be representative of the underlying end-user demand for the product. Therefore, we closely track sales of these products by our U.S. distributors to the veterinarians (“practice-level sales”), which we think provide a more accurate picture of the real growth rate for these products.
Instruments and Consumables. Our instrument strategy is to provide veterinarians with an integrated set of instruments that, individually and together, provide superior diagnostic information in the clinic, enabling veterinarians to practice better medicine and, in doing so, achieve their practice economic objectives, including growth and profitability. We derive substantial revenues and margins from the sale of consumables that are used in these instruments. During the early stage of an instrument’s life cycle, we derive relatively greater revenues from instrument placements, while consumable sales become relatively more significant in later stages as the installed base of instruments increases and instrument placement revenues begin to decline.
We have a large installed base of VetTest® Chemistry Analyzers, and substantially all of our revenues from that product line are now derived from consumables sales, although we continue to place instruments through sales, lease, rental and other programs. Our long-term success in this area of our business is dependent upon new customer acquisition, customer retention and increased customer utilization of existing and new assays introduced on these instruments. To increase utilization, we seek to educate veterinarians about best medical practices that emphasize the importance of blood and urine chemistry testing for a variety of diagnostic purposes.
We purchase the consumables used in VetTest® Chemistry Analyzers from Ortho under a supply agreement that continues through 2020. This supply agreement provides us with a long-term source of slides at costs that improve annually through 2010, and also improve over the term of the agreement as a result of increasing volume. Under this agreement, we are developing and expect to introduce a next-generation chemistry analyzer, named Catalyst Dx™, for the veterinary market based primarily on the Ortho dry-slide technology and secondarily on OPTI® electrolyte technology, and Ortho will supply us with slide consumables used in both the new instrument and the VetTest® Chemistry Analyzer. We plan to launch Catalyst Dx™ in January 2008.
In the fourth quarter of 2002, we introduced the LaserCyte® Hematology Analyzer, which provides more extensive hematological diagnostic information than our original platform, the VetAutoread™ Hematology Analyzer. A substantial portion of LaserCyte® placements have been made at veterinary clinics that already own our VetAutoread™ Hematology Analyzers. Although we have experienced growth in sales of hematology consumables, LaserCyte® consumable sales have been partially offset by declines in sales of VetAutoread™ consumables. Because the gross margin percentage of LaserCyte®consumables exceeds the gross margin percentage of the VetAutoread™ consumables, gross margin from hematology consumables is expected to increase with continued penetration of the LaserCyte® Hematology Analyzer.
21
With all of our instrument lines, we seek to differentiate our products based on breadth of diagnostic menu, flexibility of menu selection, accuracy, reliability, ease of use, ability to handle compromised samples, time to result, analytical capability of software, integration with the IDEXX VetLab® Station, education and training, and superior sales and customer service. Our instruments and consumables typically are sold at a premium price to competitive offerings. Our success depends, in part, on our ability to differentiate our products in a way that justifies premium pricing.
Rapid Assay Products. Our rapid assay business consists primarily of single-use kits for point-of-care testing and, to a limited degree, microwell-based kits for laboratory testing for canine and feline diseases and conditions. Our rapid assay strategy is to develop, manufacture, market and sell proprietary tests that address important medical needs for particular diseases prevalent in the companion animal population. We seek to differentiate our tests through superior performance, including by providing our customers with proprietary combination tests that test a single sample for multiple analytes. Where alternative point-of-care offerings exist, we seek to differentiate our tests with superior performance. As in our other lines of business, we also seek to differentiate our products through superior customer service. These products carry price premiums over competitive products that we believe do not offer equivalent performance and diagnostic capabilities, and which we believe do not include a similar level of support. We further augment our product development and customer service efforts with sales and marketing programs that enhance medical awareness and understanding regarding our target diseases and the importance of diagnostic testing. In 2008, we plan to introduce a new IDEXX VetLab® analyzer, SNAPshot Dx™, that, later that year, will include the capability to interpret the test results from all SNAP assays and log the tests and their results in the IDEXX VetLab® Station database and, therefore, in the medical and financial records of the practice.
Reference Laboratory and Consulting Services. We believe that more than half of all diagnostic testing by U.S. veterinarians is done at outside reference laboratories such as our IDEXX Reference Laboratories. In markets outside the U.S., in-clinic testing is less prevalent and an even greater percentage of diagnostic testing is done in reference laboratories. We attempt to differentiate our laboratory testing services from those of our competitors primarily on the basis of quality, customer service, technology employed and specialized test menu. Revenue growth in this business is achieved both through increased sales at existing laboratories and through the acquisition of new customers, including through laboratory acquisitions and opening new laboratories. In 2004, we acquired a laboratory in Columbus, Ohio, opened a laboratory in Seattle, Washington, and acquired Vet Med Lab, which is based in Germany and is the largest European veterinary reference laboratory. In 2005, we acquired laboratories in Switzerland, the United Kingdom, and France and acquired veterinary laboratory customer lists in the U.S. and Germany. In 2006, we acquired laboratories in Clearwater, Florida, South Africa, and Canada and acquired a veterinary laboratory customer list in the U.S. Profitability of this business is largely the result of our ability to achieve efficiencies from both volume and operational improvements. New laboratories that we open typically will operate at a loss until testing volumes reach a level that permits profitability. Acquired laboratories frequently operate less profitably than our existing laboratories and those laboratories may not achieve profitability comparable to our existing laboratories for several years while we implement operating improvements and efficiencies. Therefore, in the short term, new and acquired reference laboratories generally will have a negative effect on the operating margin of the laboratory and consulting services business.
Practice Information Management Systems and Digital Radiography. These businesses consist of veterinary practice information management systems (“PIMS”) including hardware and software and veterinary-specific digital radiography systems. Our strategy in the PIMS business is to provide superior total software and hardware integrated information solutions, backed by superior customer support and education, to allow the veterinarian to practice better medicine and achieve the practice’s business objectives. We differentiate our software systems through enhanced functionality and ease of use. Our veterinary-specific digital radiography systems allow veterinarians to capture digital radiographs with ease and without the use of hazardous chemicals. The digital radiography systems also incorporate IDEXX-PACS™ picture archiving and communication software developed by IDEXX that allows for image enhancement, manipulation, storage and retrieval, and integration with the practice information software. Our strategy in digital radiography is to offer a system that provides superior image quality and software capability at a competitive price, backed by the same customer support provided for our other products and services in the Companion Animal Group.
22
Pharmaceutical Products. We currently offer pharmaceutical products to regulate feline diabetes, eradicate internal parasites, and treat lameness in horses. Our pharmaceutical strategy is to develop, register and sell proprietary pharmaceutical products for the veterinary market. We seek to differentiate our pharmaceutical products through ease of use, which in turn enhances customers’ compliance with prescribed treatment programs. Our product development efforts are focused on applying superior and proprietary delivery technologies to existing pharmaceutical compounds.
Water
Our strategy in the water testing business is to develop, manufacture, market and sell proprietary products with superior performance, supported by exceptional customer service. Our customers are primarily water utilities to whom strong relationships and customer support are very important. Over the past several years, the rate of growth of this product line has slowed as a result of market penetration by competitors and increased competition. International sales of water testing products represented 42% of total water product sales in 2006, and we expect that future growth in this business will be significantly dependent on our ability to increase international sales. Growth also will be dependent on our ability to enhance and broaden our product line. Most water microbiological testing is driven by regulation, and, in many countries, a test may not be used for regulatory testing unless it has been approved by the applicable regulatory body. As a result, we maintain an active regulatory program under which we are seeking regulatory approvals in a number of countries, primarily in Europe.
Production Animal Segment
We develop, manufacture, market and sell a broad range of tests for various poultry, cattle and swine diseases and conditions, and have an active research and development and in-licensing program in this area. Our strategy is to offer proprietary tests with superior performance characteristics. Disease outbreaks are episodic and unpredictable, and certain diseases that are prevalent at one time may be substantially contained or eradicated at a later time. In response to outbreaks, testing initiatives may lead to exceptional demand for certain products in certain periods. Conversely, successful eradication programs may result in significantly decreased demand for certain products. The performance of this business, therefore, can be subject to fluctuation. In 2006, approximately 79% of our sales in this business were international. Because of the significant dependence of this business on international sales, the performance of the business is particularly subject to the various risks described below that are associated with doing business internationally.
In 2004, we received USDA approval of our postmortem test for BSE (mad cow disease) and, in February 2005, we were informed that this test was approved by the European Commission for sale in EU member countries. While BSE testing is very limited in the U.S., a larger market for BSE testing exists in Europe.
Other
Dairy. Our strategy in the dairy testing business is to develop, manufacture and sell antibiotic residue testing products that satisfy applicable regulatory requirements for testing of bulk milk by producers and provide reliable field performance. The manufacture of these testing products leverage, almost exclusively, the SNAP® platform as well as the production equipment and lines of our rapid assay business, incorporating customized reagents for antibiotic detection. Sales of dairy testing products have declined slightly over the last several years largely as a result of increased competition in the domestic market. To increase sales of dairy testing products, we look to increase penetration in geographies outside the U.S. and in the farm segment of the dairy market, and to develop product line enhancements and extensions.
OPTI Medical Systems. Our strategy in the OPTI Medical Systems business is to develop, manufacture, and sell electrolyte and blood gas analyzers and related consumable products for the medical point-of-care diagnostics market worldwide, with a focus on small- to mid-sized hospitals. We seek to differentiate our products based on ease of use, menu, convenience, international distribution and service, and instrument reliability. Similar to our veterinary instruments and consumables strategy, a substantial portion of the revenues from this product line are derived from the sale of consumables for use on the installed base of electrolyte and blood gas analyzers. During the early stage of an instrument’s life cycle, relatively greater revenues are derived from instrument placements, while consumable sales become relatively more significant in later stages as the installed base of instruments increases and instrument placement revenues begin to decline. Our long-term success in this area of our business is dependent upon new customer acquisition, customer retention and increased customer utilization of existing and new assays introduced on these instruments.
23
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue recognition, inventory, goodwill and other intangible assets, share-based compensation, income taxes, and contingencies. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. Note 2 to the consolidated financial statements for the year ended December 31, 2006 included in this Form 10-K describes the significant accounting policies used in preparation of these financial statements.
We believe the following critical accounting policies reflect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We recognize revenue when four criteria are met: (i) persuasive evidence that an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the sales price is fixed or determinable, and (iv) collectibility is reasonably assured.
| | • | We recognize revenue at the time of shipment to distributors for substantially all products sold through distributors, as title and risk of loss pass to these customers on delivery to the common carrier. Our distributors do not have the right to return products. We recognize revenue for the remainder of our customers when the product is delivered, except as noted below. |
| | • | We recognize revenue from the sales of instruments, noncancelable software licenses and hardware systems upon installation (and completion of training if applicable) and the customer’s acceptance of the instrument or system because at this time we have no significant further obligations. |
| | • | We recognize service revenue at the time the service is performed. |
| | • | We recognize revenue associated with extended maintenance agreements over the life of the contracts. Amounts collected in advance of revenue recognition are recorded as a current or long-term liability based on the time from the balance sheet date to the future date of revenue recognition. |
| | • | We recognize revenue on certain instrument systems under rental programs over the life of the rental agreement. Amounts collected in advance of revenue recognition are recorded as a current or long-term liability based on the time from the balance sheet date to the future date of revenue recognition. |
Certain diagnostic instruments and practice information management systems offered for sale may include software that is considered more than incidental to the utility and value of the product. Sales arrangements may provide for software update rights or postcontract customer support. Judgment is required to determine whether sales arrangements include multiple elements.
When multiple products and/or services are sold together, we generally allocate the total consideration received amongst the elements based on their relative fair values, which is determined by amounts charged separately for the delivered and undelivered elements to other customers. When there is objective and reliable evidence of the fair value of the undelivered elements but no such evidence for the delivered elements, the fair value of the undelivered elements is deferred and the residual revenue is allocated to the delivered elements. The delivered elements are recognized as revenue when appropriate under the policies described above. If there is not sufficient evidence of the fair value of the undelivered elements, no revenue is allocated to the delivered elements and the total consideration received is deferred until delivery of those elements for which objective and reliable evidence of the fair value is not available. Shipping costs reimbursed by the customer are included in revenue.
24
We record estimated reductions to revenue in connection with customer programs and incentive offerings, which may give customers credits, award points, or trade-in rights. Awards points may be applied to trade receivables owed to us and/or toward future purchase of our products and services. We estimate these reductions based on our experience with similar customer programs in prior years. Revenue reductions are recorded on a quarterly basis based on issuance of credits, points actually awarded, and estimates of points to be awarded in the future based on current revenue. For the SNAP Up the Savings™ program, estimates of future points are revised quarterly and finalized annually in the third quarter of each year upon the issuance of points to customers. For our Practice Developer™ volume discount program, we have reduced revenue assuming all points granted will result in future credits because the historical forfeitures have been de minimus. On November 30 of each year, unused points awarded before January 1 of the prior year expire.
We may offer customers the right to trade in instruments for credit against the purchase price of other instruments acquired in the future. For trade-in rights, we have reduced revenue using estimates regarding the percentage of qualifying instruments that will be traded in and the average trade-in value.
We recognize revenue only in those situations where collection from the customer is reasonably assured. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We base our estimates on detailed analysis of specific customer situations and a percentage of our accounts receivable by aging category. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances might be required.
Inventory Valuation
We write down inventory for estimated obsolescence when warranted by estimates of future demand and market conditions. If actual market conditions are less favorable than those we estimated, additional inventory write-downs may be required, which would have a negative effect on results of operations. Certain major components of inventory for which we have made critical valuation judgments are discussed in more detail below.
LaserCyte® Hematology Analyzer. At December 31, 2006, our net inventories included $11.5 million of component parts and finished goods associated with our LaserCyte® hematology instrument. In addition, we had firm purchase commitments for an additional $4.2 million of component parts as of December 31, 2006. At December 31, 2006, $1.7 million of the net LaserCyte® inventory required rework before it could be used to manufacture finished goods, which was net of $0.9 million write-downs for inventory estimated to be obsolete. We expect to fully realize our net investment in inventory and purchase commitments. However, if we alter the design of this product, we may be required to write off some or all of the remaining associated inventory.
Nitazoxanide. Our nitazoxanide product, Navigator®, for the treatment of equine protozoal myeloencephalitis (“EPM”) was approved by the FDA in November 2003. At December 31, 2006, our inventories included $9.3 million of inventory associated with Navigator®, consisting of $0.2 million of finished goods and $9.1 million of active ingredient and other raw materials. We have an agreement with our supplier of nitazoxanide under which the supplier agreed until 2017 to replace any expiring inventory of nitazoxanide with longer-dated material. We paid $0.9 million in January 2005 for consideration for this agreement and capitalized this payment as inventory cost. We believe that this agreement has substantially mitigated the risk that we would be required to write down nitazoxanide inventory due to its anticipated expiration prior to sale. However, if actual market conditions or our market share through 2022 are less than we estimate, we may be required to write off some of the associated inventory. For example, if we sell approximately 50% fewer units through 2025 than we estimate, we would have approximately $5 million of excess inventory.
Valuation of Goodwill and Other Intangible Assets
A significant portion of the purchase prices of our business acquisitions is assigned to intangible assets. Intangible assets other than goodwill are initially valued at the lesser of fair value or, if applicable, fair value proportionately reduced by the excess of the fair value of acquired net assets over cost, (collectively, “fair value”) when acquired. If a market value is not readily available, the fair value of the intangible asset is estimated based on discounted cash flows using market participant assumptions. The estimation of discounted cash flows requires significant assumptions about the timing and amounts of future cash flows, risks, the cost of capital, and the useful lives of intangible assets. When the fair values of acquired intangible assets are significant, we utilize independent valuation experts to advise and assist us in allocating the purchase prices of acquisitions to the fair values of the identified intangible assets and in determining appropriate amortization methods and periods for those intangible assets. Goodwill is initially valued based on the excess of the purchase price of a business combination over the fair values of acquired net assets.
25
We assess the impairment of identifiable intangible assets and other long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important that could trigger an impairment review include, but are not limited to, the following:
| | • | Significant under-performance relative to historical or projected future operating results; |
| | • | Failure to obtain regulatory approval of certain products; |
| | • | Significant changes in regulations; |
| | • | Significant changes in the manner of our use of the acquired assets or the strategy for our overall business; |
| | • | Significant increase in the discount rate assumed to calculate the present value of future cash flow; |
| | • | Significant negative industry or economic trends; |
| | • | Significant advancements or changes in technology; and |
| | • | Cancellation or significant changes in contractual relationships. |
We continually assess the realizability of intangible assets other than goodwill in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS No. 144”). If an impairment review is triggered, we evaluate the carrying value of long-lived assets by determining if impairment exists based on estimated undiscounted future cash flows over the remaining useful life of the assets and comparing that value to the carrying value of the assets. If the carrying value of the asset is greater than the estimated future cash flows, the asset is written down to its estimated fair value. In determining expected future cash flows, assets are grouped at the lowest level for which cash flows are identifiable and independent of cash flows from other asset groups. The cash flow estimates that are used contain our best estimates, using appropriate and customary assumptions and projections at the time.
We assess goodwill for impairment annually and whenever events or circumstances indicate an impairment may exist, in accordance with SFAS No. 142, “Goodwill and Other Intangible Assets” (“SFAS No. 142”). For impairment testing, we identify our reporting units, allocate assets and liabilities (including goodwill) to the reporting units and compare the reporting units’ net book value to their estimated fair value. The fair value of the reporting units is estimated using a discounted cash flow approach. The cash flow estimates used contain our best estimates, using appropriate and customary assumptions and projections at the time. If a reporting unit’s net book value exceeds its fair value, then the implied fair value of goodwill is determined. If the net book value of goodwill exceeds the implied fair value of goodwill, a goodwill impairment loss is recognized in an amount equal to that excess. No impairments have been identified as a result of the annual or event-driven reviews during the years ended December 31, 2006, 2005 or 2004.
The determination of the fair value of our pharmaceutical products business unit requires significant assumptions about the timing and amounts of the unit’s future cash flows, including assumptions about the markets for our products and proprietary technologies, the future success of research and development activities, the attainment and timing of regulatory approvals to manufacture and sell new products, the introduction and success of competitive products by other market participants, and other business risks. We believe that the goodwill attributable to our pharmaceutical business of $13.7 million is not impaired at December 31, 2006. However, changes in our assumptions and estimates due to new information, or actual results that are below expectations, could result in an impairment in the future of some or all of the goodwill attributable to our pharmaceutical products business.
26
Share-based Compensation
We adopted the provisions of SFAS No. 123(R), “Share-Based Payment” (“SFAS No. 123(R)”) on January 1, 2006. SFAS No. 123(R) requires all share-based compensation to employees, including grants of stock options, to be valued at fair value on the date of grant, and to be expensed over the requisite service period (generally the vesting period). Prior to January 1, 2006, we measured costs related to employee share-based compensation plans in accordance with Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”). Accordingly, no employee compensation cost was recognized for these plans prior to January 1, 2006.
Effective January 1, 2006, under the modified prospective method of transition, share-based compensation expense includes expense for unvested awards at December 31, 2005 and all awards granted subsequent to December 31, 2005. Share-based compensation expense for the unvested awards outstanding at December 31, 2005 is based on the grant-date fair value previously calculated in developing the pro forma disclosures in accordance with the provisions of SFAS No. 123.
Beginning in 2006, we modified our share-based employee compensation programs to shift from the grant of stock options and employee stock purchase rights only to the grant of a mix of restricted stock units and stock options, along with employee stock purchase rights. There were no modifications to the terms of outstanding options during 2006 or 2005.
In connection with the adoption of SFAS No. 123(R), we adopted the straight-line method to prospectively expense share-based awards granted subsequent to December 31, 2005. The graded-vesting, or accelerated, method has been used to calculate the expense for stock options granted prior to January 1, 2006. If the total fair value of share-based compensation awards, as well as other features that impact expense, including forfeitures and capitalization of costs, was consistent from year-to-year in each of the last five years and through 2010, this change in expense method from graded-vesting to straight-line expensing would yield decreasing annual expense through 2010 until awards granted prior to January 1, 2006 were fully expensed. However, the total fair value of future awards may vary significantly from past awards based on a number of factors, including our share-based award practices. Therefore, share-based compensation expense is likely to fluctuate, possibly significantly, from year to year.
Selected financial impacts of share-based compensation, excluding the impact of deferred stock units issued under our Director Deferred Compensation Plan or our Executive Deferred Compensation Plan that do not have vesting conditions (which are described below), are presented in the table below (in thousands, except per share amounts):
| For the
Year Ended
December 31,
2006
| |
|---|
| Share-based compensation expense included in cost of revenue | | | $ | 1,671 | |
| Share-based compensation expense included in operating expense | | | | 8,986 | |
|
| |
| Total share-based compensation expense | | | | 10,657 | |
|
| |
| Income tax benefit in net income for share-based compensation expense | | | | (1,845 | ) |
| Income tax benefit in net income for employees' disqualifying dispositions of shares | | | | | |
| acquired through the exercise of stock options and employee stock purchase rights | | | | (57 | ) |
|
| |
| Total income tax benefit | | | | (1,902 | ) |
|
| |
| Net impact of share-based compensation on net income | | | $ | 8,755 | |
|
| |
| Net impact of share-based compensation on: | | | | | |
| Earnings per share, basic | | | $ | 0.28 | |
| Earnings per share, diluted | | | | 0.27 | |
Share-based compensation costs are classified in costs of sales and operating expenses consistently with the classification of cash compensation paid to the employees receiving such share-based compensation. Capitalized share-based employee compensation cost at December 31, 2006 was $0.2 million, which was included in inventory on the consolidated balance sheet.
27
The fair value of options, restricted stock units, deferred stock units with vesting conditions, and employee stock purchase rights awarded during the years ended December 31, 2006, 2005 and 2004 totaled $11.9 million, $15.7 million and $13.4 million, respectively. The total unrecognized compensation cost for unvested share-based compensation awards outstanding at December 31, 2006, net of estimated forfeitures, was $14.6 million. Approximately $6.3 million is expected to be recognized in the year ending December 31, 2007 for outstanding awards and decreasing amounts of the total expense are expected to be recognized over the subsequent five years, resulting in a weighted average remaining expense recognition period of approximately 1.5 years.
We use the Black-Scholes-Merton option-pricing model to determine the fair value of options granted. Option-pricing models require the input of highly subjective assumptions, particularly for the expected stock price volatility and the expected term of options. Changes in the subjective input assumptions can materially affect the fair value estimate. Our expected stock price volatility assumptions are based on the historical volatility of our stock over periods that are similar to the expected terms of grants, and other relevant factors. Lower estimated volatility reduces the fair value of an option. To develop the expected term assumption for 2006 option awards, we elected to use the simplified method described in the Securities and Exchange Commission Staff Accounting Bulletin No. 107, which is based on vesting and contractual terms. The application of the simplified method is allowable for options granted through December 31, 2007. We will transition to developing expected term assumptions for future awards based on historical experience and other relevant factors concerning expected employee behavior with regards to option exercise. Longer expected term assumptions increase the fair value of option awards, and therefore increase the expense recognized per award.
The weighted average valuation assumptions used to determine the fair value of each option grant on the date of grant and the weighted average estimated fair values were as follows:
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Expected stock price volatility | | | | 30 | % | | 40 | % | | 40 | % |
| Expected term, in years | | | | 5.0 | | | 5.8 | | | 5.8 | |
| Risk-free interest rate | | | | 4.6 | % | | 4.2 | % | | 3.1 | % |
Share-based compensation expense is based on the number of awards ultimately expected to vest and is, therefore, reduced for an estimate of the number of awards that are expected to be forfeited. The forfeiture estimate is based on historical data and other factors, and compensation expense is adjusted for actual results. Changes in estimated forfeiture rates and differences between estimated forfeiture rates and actual experience may result in significant, unanticipated increases or decreases in share-based compensation expense from period to period. The termination of employment by certain employees who hold large numbers of share-based compensation instruments may also have a significant, unanticipated impact on forfeiture experience and, therefore, on share-based compensation expense.
The tax benefit related to the option fair value is recognized when disqualifying dispositions of incentive stock options occur as either a reduction of the current period tax provision or an increase in additional paid-in capital, as required by SFAS No. 123(R) transitional accounting rules, depending on the vesting status of awards at the SFAS No. 123(R) adoption date, and the amounts previously expensed under SFAS No. 123(R). Employees’ exercise of vested options and disposition of shares acquired is influenced by the market price of the common stock and other factors outside of our control. The timing and volume of disqualifying dispositions; the vesting status of such exercised options at the date of our adoption of SFAS No. 123(R); and the relationship between the sale price of the common stock, the option exercise price and the option fair value may have a significant, unpredictable impact on our effective tax rate. As the aggregate fair value of outstanding options that has been expensed under SFAS No. 123(R) grows, we expect to recognize increasing tax benefits in net income related to disqualifying dispositions. However, the growth of the aggregate fair value of outstanding options that has been expensed under SFAS No. 123(R) will be limited in future years as a result of changes implemented in 2006 in our share-based compensation programs, under which we have shifted from the grant of stock options only to the grant of a mix of stock options and restricted stock unit awards that have a lower aggregate fair value than was awarded in prior years. Reductions in the fair value of options outstanding are expected to reduce the variability in our effective tax rate.
28
Income Taxes
We account for income taxes under SFAS No. 109, “Accounting for Income Taxes.” This statement requires that we recognize a current tax liability or asset for current taxes payable or refundable, respectively; and a deferred tax liability or asset, as the case may be, for the estimated future tax effects of temporary differences between book and tax treatment of assets and liabilities and carryforwards to the extent they are realizable. We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we consider future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for a valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of the net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination was made. Significant judgment is required in determining our worldwide provision for income taxes and our income tax filings are regularly under audit by tax authorities. We periodically assess our exposures related to our worldwide provision for income taxes and believe that we have appropriately accrued taxes for contingencies. Any reduction of these contingent liabilities or additional assessment would increase or decrease income, respectively, in the period such determination was made.
We consider the operating earnings of non-United States subsidiaries to be indefinitely invested outside the United States, the cumulative amount of which was $111.4 million at December 31, 2006. No provision has been made for United States federal and state, or international taxes that may result from future remittances of undistributed earnings of non-United States subsidiaries. Should we repatriate non-United States earnings in the future, we would have to adjust the income tax provision in the period in which the decision to repatriate earnings is made.
Estimates for Certain Contingencies
Under our workers’ compensation insurance policies for U.S. employees for the years ended December 31, 2006, 2005, 2004 and 2003, we retain the first $250,000 in claim liability per incident and $3.1 million, $2.8 million, $3.0 million and $1.4 million, respectively, in aggregate claim liability. We entered into a similar workers’ compensation insurance policy effective January 1, 2007. The insurance company provides insurance for claims above the individual occurrence and aggregate limits. We estimate claim liability based on claims incurred and the estimated ultimate cost to settle the claims. Based on this analysis, we have recognized cumulative expenses of $1.3 million, $0.6 million, $0.7 million and $0.8 million for claims incurred during the years ended December 31, 2006, 2005, 2004 and 2003, respectively.
Under our employee health care insurance policy, we retain claims liability risk up to $125,000 per incident and an aggregate claim limit based on the number of employees enrolled in the plan per month. We estimate our liability for the uninsured portion of employee health care obligations based on individual and aggregate coverage, our claims experience, the number of employees enrolled in the program, and the average time from when a claim is incurred to the time it is reported. Should actual employee health care claims liability exceed estimates, we are liable for up to an additional $1.5 million for potential uninsured obligations at December 31, 2006. We have insurance coverage of $1.0 million for claims above the aggregate limit. Should employee health insurance claims exceed this coverage, we would have further obligations for the amount in excess of such coverage.
We are subject to claims that arise in the ordinary course of business, including with respect to actual and threatened litigation and other matters. We accrue contingent liabilities when it is probable that future expenditures will be made and such expenditures can reasonably be estimated. However, our actual losses with respect to these contingencies could exceed our accruals.
29
RESULTS OF OPERATIONS
Twelve Months Ended December 31, 2006 Compared to Twelve Months Ended December 31, 2005
Revenue
Total Company. Revenue increased $101.0 million, or 16%, to $739.1 million from $638.1 million for the prior year. The following table presents revenue by reportable operating segment:
For the Twelve Months Ended December 31,
|
|---|
Net Revenue
(dollars in thousands)
| 2006
| | 2005
| | Dollar
Change
| | Percentage
Change
| | Percentage
Change from
Currency
(1)
| | Percentage
Change from
Acquisitions
(2)
| | Percentage
Change Net of
Acquisitions
and Currency
Effect
| |
|---|
| CAG | | | $ | 606,319 | | $ | 520,830 | | $ | 85,489 | | | 16.4 | % | | 0.3 | % | | 2.4 | % | | 13.7 | % |
| Water | | | | 58,466 | | | 56,760 | | | 1,706 | | | 3.0 | % | | 0.8 | % | | -- | | | 2.2 | % |
| PAS | | | | 58,940 | | | 44,945 | | | 13,995 | | | 31.1 | % | | 0.9 | % | | -- | | | 30.2 | % |
| Other | | | | 15,392 | | | 15,560 | | | (168 | ) | | (1.1 | %) | | (0.1 | %) | | -- | | | (1.0 | %) |
|
| |
| |
| | | | | |
| Total | | | $ | 739,117 | | $ | 638,095 | | $ | 101,022 | | | 15.8 | % | | 0.4 | % | | 1.8 | % | | 13.6 | % |
|
| |
| |
| | | | | |
| (1) | Represents the percentage change in revenue attributed to the effect of changes in currency rates from the twelve months ended December 31, 2005 to the twelve months ended December 31, 2006. |
| (2) | Represents the percentage change in revenue attributed to incremental revenues from businesses acquired since January 2005 during the twelve months ended December 31, 2005 compared to the twelve months ended December 31, 2006. |
Companion Animal Group. Revenue for CAG increased $85.5 million, or 16%, to $606.3 million from $520.8 million for the prior year. Incremental sales from businesses acquired since January 2005, consisting primarily of veterinary reference laboratories, a digital radiography business, and intellectual property and distribution rights of a veterinary diagnostics business, contributed 2% to CAG revenue growth. The following table presents revenue by product and service categories for CAG:
For the Twelve Months Ended December 31,
|
|---|
Net Revenue
(dollars in thousands)
| 2006
| | 2005
| | Dollar
Change
| | Percentage
Change
| | Percentage
Change from
Currency
(1)
| | Percentage
Change from
Acquisitions
(2)
| | Percentage
Change Net of
Acquisitions
and Currency
Effect
| |
|---|
| Instruments and consumables | | | $ | 242,312 | | $ | 217,537 | | $ | 24,775 | | | 11.4 | % | | 0.5 | % | | -- | | | 10.9 | % |
| Rapid assay products | | | | 114,536 | | | 100,255 | | | 14,281 | | | 14.2 | % | | (0.1 | %) | | 1.6 | % | | 12.7 | % |
| Laboratory and consulting | | | | | | | | | | | | | | | | | | | | | | | |
| services | | | | 187,114 | | | 156,425 | | | 30,689 | | | 19.6 | % | | 0.2 | % | | 4.5 | % | | 14.9 | % |
| Practice information | | | | | | | | | | | | | | | | | | | | | | | |
| management systems | | | | | | | | | | | | | | | | | | | | | | | |
| and digital radiography | | | | 44,427 | | | 32,589 | | | 11,838 | | | 36.3 | % | | 0.9 | % | | 11.5 | % | | 23.9 | % |
| Pharmaceutical products | | | | 17,930 | | | 14,024 | | | 3,906 | | | 27.9 | % | | -- | | | -- | | | 27.9 | % |
|
| |
| |
| | | | | |
| Net CAG Revenue | | | $ | 606,319 | | $ | 520,830 | | $ | 85,489 | | | 16.4 | % | | 0.3 | % | | 2.4 | % | | 13.7 | % |
|
| |
| |
| | | | | |
| (1) | Represents the percentage change in revenue attributed to the effect of changes in currency rates from the twelve months ended December 31, 2005 to the twelve months ended December 31, 2006. |
| (2) | Represents the percentage change in revenue attributed to incremental revenues from businesses acquired since January 2005 during the twelve months ended December 31, 2005 compared to the twelve months ended December 31, 2006. |
The following revenue analysis reflects the results of operations net of the impact of currency exchange rates on sales outside the U.S.
30
Because our instrument consumables, rapid assay products, and pharmaceutical products are sold in the U.S. and certain other geographies by distributors, distributor purchasing dynamics have an impact on our reported sales of these products. Distributors purchase products from us and sell them to veterinary practices, who are the end users. Distributor purchasing dynamics may be affected by many factors and may be unrelated to underlying end-user demand for our products. As a result, fluctuations in distributors’ inventories may cause reported results in a period not to be representative of underlying end-user demand. Therefore, we believe it is important to track distributor sales to end users and to distinguish between the impact of end-user demand and the impact of distributor purchasing dynamics on reported revenue growth.
Where growth rates are affected by changes in end-user demand, we refer to the impact of practice-level sales on growth. Where growth rates are affected by distributor purchasing dynamics, we refer to the impact of changes in distributors’ inventories. If during the comparable period of the prior year, distributors’ inventories grew by more than those inventories grew in the current year, then changes in distributors’ inventories have a negative impact on our reported sales growth in the current period. Conversely, if during the comparable period of the prior year, distributors’ inventories grew by less than those inventories grew in the current year, then distributors’ inventories have a positive impact on our reported sales growth in the current period.
The increase in sales of instruments and consumables from 2005 to 2006 was due mainly to higher unit sales volume of both instruments and of consumables and, to a lesser extent, to higher average unit sales prices for slides that are sold for use in VetTest® Chemistry Analyzers. Higher consumables sales volumes were attributable primarily to higher worldwide practice-level sales of slides and, to a lesser extent, to increased U.S. practice-level sales of tubes used with our hematology analyzers, with all consumables categories benefiting from the continued growth of our installed base of instruments. Higher instrument sales volume resulted mainly from sales of VetStat® Electrolyte and Blood Gas Analyzers and, to a lesser extent, LaserCyte® Hematology Analyzers and SNAP® Readers. The impact from changes in distributors’ inventory levels had no significant impact on reported revenue growth of instruments and consumables.
The increase in sales of rapid assay products from 2005 to 2006 was due primarily to increased sales volume of canine products, including sales of SNAP®4Dx®, which was launched in the U.S. in September 2006. The impact from changes in distributors’ inventory levels increased reported rapid assay revenue growth by 3%. To a lesser extent, higher average unit sales prices of canine products, in part due to less promotional discounting and higher relative sales of combination test products, also contributed to rapid assay revenue growth. These increases were partly offset by lower average unit sales prices of feline products, partly due to greater promotional discounting. Incremental sales of rapid assay products for which we acquired distribution rights in the second quarter of 2006 contributed 2% to rapid assay revenue growth.
The increase in sales of laboratory and consulting services from 2005 to 2006 resulted primarily from higher testing volume and, to a lesser extent, the impact of price increases and incremental sales attributable to acquisitions since January 2005. Businesses acquired since January 2005 contributed 4% to laboratory and consulting services revenue growth.
The increase in sales of practice information management systems and digital radiography from 2005 to 2006 resulted primarily from an increase in the number of digital radiography systems sold. Digital radiography sales volume growth was due primarily to sales attributable to a business acquired in the third quarter of 2005, which contributed 12% to practice information management systems and digital radiography revenue growth; higher sales volumes of existing products; and sales of the IDEXX-DR™ 1417 Digital Radiography System, which became commercially available during the third quarter of 2006. To a lesser extent, revenue growth was also due to the impact of price increases for support services for our practice information management systems, higher sales of computer hardware to practice information management systems customers, and a shift in sales mix to larger practice information management systems.
The increase in sales of pharmaceutical products from 2005 to 2006 resulted primarily from increased practice-level demand and, to a lesser extent, from price increases, both impacts related largely to PZI VET®, our insulin product for the treatment of diabetic cats.
Water. Revenue for Water increased $1.7 million, or 3%, to $58.5 million from $56.8 million for the prior year. The increase resulted primarily from higher sales volume in the Americas and Europe and, to a lesser extent, to higher average unit sales prices. The favorable impact of currency exchange rates contributed an aggregate of $0.5 million, or 1%, to the increase in Water revenue.
31
Production Animal Segment. Revenue for PAS increased $14.0 million, or 31%, to $58.9 million from $44.9 million for the prior year. The increase resulted primarily from higher worldwide livestock diagnostics sales volume, including, notably, sales in Europe of our HerdChek®products that test for transmissible spongiform encephalopathies. To a lesser extent, increased average unit sales prices in certain geographies, higher relative sales in geographies where products are sold at higher unit prices, and higher poultry diagnostics sales volume in the Americas also contributed to production animal products revenue growth. The favorable impact of currency exchange rates contributed an aggregate of $0.4 million, or 1%, to the increase in PAS revenue.
Gross Profit
Total Company. Gross profit increased $56.6 million, or 18%, to $379.5 million from $322.9 million for the prior year. As a percentage of total revenue, gross profit was approximately constant at 51%.
We adopted the provisions of SFAS No. 123(R) and began expensing share-based compensation beginning on January 1, 2006, which had a negative impact on our gross profit percentages and on operating margins.Share-based compensation expense is not allocated to our operating segments and therefore has been categorized as “unallocated amounts.” The following table presents gross profit and gross profit percentage by reportable segment:
For the Twelve Months Ended December 31,
| |
|---|
Gross Profit(dollars in thousands)
| 2006
| | Percent of
Revenue
| | 2005
| | Percent of
Revenue
| | Dollar
Change
| | Percentage
Change
| |
|---|
| CAG | | | $ | 297,999 | | | 49.1% | | $ | 250,409 | | | 48.1% | | $ | 47,589 | | | 19.0% | |
| Water | | | | 38,441 | | | 65.7% | | | 38,277 | | | 67.4% | | | 165 | | | 0.4% | |
| PAS | | | | 38,654 | | | 65.6% | | | 27,788 | | | 61.8% | | | 10,866 | | | 39.1% | |
| Other | | | | 6,106 | | | 39.7% | | | 6,426 | | | 41.3% | | | (320 | ) | | (5.0% | ) |
| Unallocated amounts | | | | (1,671 | ) | | N/A | | | -- | | | N/A | | | (1,671 | | | N/A | |
|
| | |
| | |
| | |
| Total Company | | | $ | 379,529 | | | 51.3% | | $ | 322,900 | | | 50.6% | | $ | 56,629 | | | 17.5% | |
|
| | |
| | |
| | |
Companion Animal Group. Gross profit for CAG increased $47.6 million, or 19%, to $298.0 million from $250.4 million for the prior year due primarily to increased sales volume across the CAG product lines and, to a lesser extent, to an increase in the gross profit percentage to 49% from 48% for the prior year. The increase in the gross profit percentage was largely due to lower cost of slides that are sold for use in VetTest® Chemistry Analyzers under the agreement with our supplier and higher average selling prices. The increase in the gross profit percentage was partly offset by greater relative sales of lower margin products and services such as laboratory and consulting services.
Water. Gross profit for Water increased $0.2 million to $38.4 million from $38.3 million for the prior year due to higher sales volume, partly offset by a decrease in the gross profit percentage to 66% from 67%. The gross profit percentage was unfavorably impacted by increased freight and distribution costs and higher relative sales of lower margin products.
Production Animal Segment. Gross profit for PAS increased $10.9 million, or 39%, to $38.7 million from $27.8 million for the prior year due primarily to increased sales volume and, to a lesser extent, to an increase in the gross profit percentage to 66% from 62%. The gross profit percentage was favorably impacted by higher relative sales of higher margin livestock products, the absence in 2006 of certain discrete costs that occurred in 2005, and higher average unit sales prices. Discrete costs in 2005 comprised integration costs and the impacts of purchase accounting that were associated with an acquisition in December 2004.
Operating Expenses and Operating Income
Total Company. Total operating expenses increased $44.0 million to $251.6 million from $207.6 million for the prior year. As a percentage of revenue, operating expenses increased to 34% from 33% for the prior year. The change in accounting for share-based compensation beginning January 1, 2006 resulted in an increase of $9.0 million, or 4%, in total company operating expenses for 2006. Share-based compensation expense is not allocated to our operating segments and therefore has been categorized as “unallocated amounts.”
32
Operating income increased $12.6 million to $127.9 million from $115.3 million for the prior year. As a percentage of revenue, operating income decreased to 17% from 18%. The change in accounting for share-based compensation beginning January 1, 2006 had a negative impact of 1% on reported operating income as a percentage of total company revenue.
The following tables present operating expenses and operating income by reportable segment:
For the Twelve Months Ended December 31,
| |
|---|
Operating Expenses
(dollars in thousands)
| 2006
| | Percent of
Revenue
| | 2005
| | Percent of
Revenue
| | Dollar
Change
| | Percentage
Change
| |
|---|
| CAG | | | $ | 197,239 | | | 32.5% | | $ | 167,439 | | | 32.1% | | $ | 29,800 | | | 17.8% | |
| Water | | | | 12,679 | | | 21.7% | | | 12,303 | | | 21.7% | | | 376 | | | 3.1% | |
| PAS | | | | 22,482 | | | 38.1% | | | 20,471 | | | 45.5% | | | 2,011 | | | 9.8% | |
| Other | | | | 4,254 | | | 27.6% | | | 3,849 | | | 24.7% | | | 405 | | | 10.5% | |
| Unallocated amounts | | | | 14,942 | | | N/A | | | 3,507 | | | N/A | | | 11,435 | | | 326.1% | |
|
| | |
| | |
| | |
| Total Company | | | $ | 251,596 | | | 34.0% | | $ | 207,569 | | | 32.5% | | $ | 44,027 | | | 21.2% | |
|
| | |
| | |
| | |
Operating Income
(dollars in thousands)
| 2006
| | Percent of
Revenue
| | 2005
| | Percent of
Revenue
| | Dollar
Change
| | Percentage
Change
| |
|---|
| CAG | | | $ | 100,760 | | | 16.6% | | $ | 82,970 | | | 15.9% | | $ | 17,790 | | | 21.4% | |
| Water | | | | 25,762 | | | 44.1% | | | 25,974 | | | 45.8% | | | (212 | ) | | (0.8% | ) |
| PAS | | | | 16,172 | | | 27.4% | | | 7,317 | | | 16.3% | | | 8,855 | | | 121.0% | |
| Other | | | | 1,852 | | | 12.0% | | | 2,577 | | | 16.6% | | | (725 | ) | | (28.1% | ) |
| Unallocated amounts | | | | (16,613 | ) | | N/A | | | (3,507 | ) | | N/A | | | (13,106 | ) | | (373.7% | ) |
|
| | |
| | |
| | |
| Total Company | | | $ | 127,933 | | | 17.3% | | $ | 115,331 | | | 18.1% | | $ | 12,602 | | | 10.9% | |
|
| | |
| | |
| | |
Companion Animal Group. Operating expenses for CAG increased $29.8 million, or 18%, to $197.2 million from $167.4 million for the prior year and, as a percentage of revenue, increased to 33% from 32%. The increase was attributable to a 13% ($11.3 million) increase in sales and marketing expense, a 20% ($10.1 million) increase in general and administrative expense, and a 28% ($8.4 million) increase in research and development expense. The increase in sales and marketing expense resulted primarily from higher personnel-related costs due, in part, to expanded worldwide sales, marketing and customer service headcount and to higher sales commissions as a result of revenue performance. To a lesser extent, incremental activities associated with businesses acquired since January 2005 also contributed to the increase in sales and marketing expense. The increase in general and administrative expense resulted primarily from higher spending on facilities, information technology and other general support functions. To a lesser extent, incremental expenses associated with businesses acquired since January 2005, comprised mainly of amortization expense for intangible assets acquired and general and administrative expenses of a recurring nature to support the acquired businesses, also contributed to the increase in general and administrative expense. Increases in general and administrative expenses were partly offset by the favorable impact of net transaction gains on foreign currency denominated expenses in 2006 compared to transaction losses in 2005. The increase in research and development expense resulted primarily from increased product development spending related primarily to IDEXX VetLab® instrumentation and, to a lesser extent, rapid assay and digital radiography products.
Water. Operating expenses for Water increased $0.4 million, or 3%, to $12.7 million from $12.3 million for the prior year and, as a percentage of revenue, were constant at 22%. The increase was attributable to a 7% ($0.3 million) increase in general and administrative expense and a 3% ($0.1 million) increase in sales and marketing expense, partly offset by 5% ($0.1 million) decrease in research and development expense. The increase in general and administrative expense resulted primarily from higher spending on facilities, information technology and other general support functions; higher bad debt expense; higher compensation; and costs incurred to consolidate our office and production facilities based in the United Kingdom into a single facility. The increase in sales and marketing expense resulted primarily from higher personnel-related costs. The decrease in research and development expense resulted primarily from lower spending following the launch of the IDEXX Filta-Maxxpress™ system, aCryptosporidium andGiardia testing product, in the second quarter of 2006.
33
Production Animal Segment. Operating expenses for PAS increased $2.0 million, or 10%, to $22.5 million from $20.5 million for the prior year. As a percentage of revenue, PAS operating expenses decreased to 38% from 46%. The increase in operating expenses resulted primarily from a 14% ($1.1 million) increase in general and administrative expense and a 12% ($0.9 million) increase in sales and marketing expense. The increase in general and administrative expense resulted primarily from higher spending on facilities, information technology and other general support functions and, to a lesser extent, from a write-down of an equity investment in a technology licensor. Increases in general and administrative expenses were partly offset by the absence in 2006 of certain discrete costs that occurred in 2005 and the favorable impact of net transaction gains on foreign currency denominated expenses in 2006 compared to transaction losses in 2005. Discrete costs in 2005 were associated with the cessation of production in our Sweden-based facility in connection with the centralization of our European production animal diagnostics operations in Bern, Switzerland. The increase in sales and marketing expense resulted primarily from higher personnel-related costs. Increases in research and development expense from higher personnel-related costs due, in part, to expanded headcount and from higher patent-related costs were substantially offset by lower facilities and overhead costs in Europe as a result of the consolidation of our European production animal business, including research and development activities, during the second half of 2005.
Unallocated Amounts. Operating expenses that are not allocated to our operating segments, consisting primarily of the company-wide share-based compensation expense and corporate research and development, increased $11.4 million to $14.9 million from $3.5 million for the prior year. This increase is primarily due to the inclusion of share-based compensation expense of $9.0 million in 2006 due to the adoption of SFAS No. 123(R) on January 1, 2006. Corporate research and development expense grew mainly due to personnel additions in 2005 and 2006 to support increased long-term product development activities.
Interest Income and Interest Expense
Interest income was $3.3 million for the year ended December 31, 2006 compared to $3.2 million for the year ended December 31, 2005. An increase in interest income from higher interest rates was largely offset by lower average invested cash balances.
Interest expense was $0.5 million for the year ended December 31, 2006 compared to $0.1 million for the year ended December 31, 2005. The increase in interest expense was primarily due to interest expense incurred on the mortgage assumed in connection with the Westbrook, Maine facility purchase in May 2006.
Provision for Income Taxes
Our effective income tax rate was 28.4% for the year ended December 31, 2006 compared with 34.2% for the year ended December 31, 2005. The majority of this rate differential resulted from the favorable impact of the resolution in 2006 of an IRS income tax audit for the years ended December 31, 2003 and 2004. As a result of completing this audit, we reduced previously accrued taxes and recognized a tax benefit of 3.7% of income before tax. Other items that decreased our effective tax rate for the year ended December 31, 2006 included a reduction of previously recorded international deferred tax liabilities as a result of obtaining certain multi-year tax incentives and the release of a valuation allowance on international deferred tax assets as a result of a subsidiary demonstrating consistent sustained profitability. In addition, the effective rate for the year ended December 31, 2006 was less than the effective rate for the year ended December 31, 2005 due to the incremental tax expense in 2005 on the repatriation of $30.0 million pursuant to theAmerican Jobs Creation Act of 2004. These rate reductions were partly offset by the nonrecognition, in 2006, of tax benefits on compensation expense for incentive stock options and employee stock purchase rights that were recorded in accordance with SFAS No. 123(R) effective January 1, 2006.
34
Twelve Months Ended December 31, 2005 Compared to Twelve Months Ended December 31, 2004
Revenue
Total Company. Revenue increased $88.9 million, or 16%, to $638.1 million from $549.2 million for the prior year. The following table presents revenue by reportable segment:
For the Twelve Months Ended December 31,
|
|---|
Net Revenue
(dollars in thousands)
| 2005
| | 2004
| | Dollar
Change
| | Percentage
Change
| | Percentage
Change from
Currency
(1)
| | Percentage
Change from
Acquisitions
(2)
| | Percentage
Change Net of
Acquisitions
and Currency
Effect
| |
|---|
| CAG | | | $ | 520,830 | | $ | 448,687 | | $ | 72,143 | | | 16.1 | % | | -- | | | 6.5 | % | | 9.6 | % |
| Water | | | | 56,760 | | | 53,098 | | | 3,662 | | | 6.9 | % | | 0.3 | % | | -- | | | 6.6 | % |
| PAS | | | | 44,945 | | | 31,690 | | | 13,255 | | | 41.8 | % | | (0.3 | %) | | 17.1 | % | | 25.0 | % |
| Other | | | | 15,560 | | | 15,706 | | | (146 | ) | | (0.9 | %) | | 0.3 | % | | -- | | | (1.2 | %) |
|
| |
| |
| | | | | |
| Total | | | $ | 638,095 | | $ | 549,181 | | $ | 88,914 | | | 16.2 | % | | -- | | | 6.3 | % | | 9.9 | % |
|
| |
| |
| | | | | |
| (1) | Represents the percentage change in revenue attributed to the effect of changes in currency rates from the 12 months ended December 31, 2004 to the 12 months ended December 31, 2005. |
| (2) | Represents the percentage change in revenue attributed to incremental revenues from businesses acquired since January 2004 during the twelve months ended December 31, 2004 compared to the twelve months ended December 31, 2005. |
Companion Animal Group. Revenue for CAG increased $72.1 million, or 16%, to $520.8 million from $448.7 million for the prior year. Incremental sales from businesses acquired during 2004 and 2005, consisting of veterinary reference laboratories and a digital radiography business, contributed approximately 7% to CAG revenue growth during the period. The following table presents revenue by product and service categories for CAG:
For the Twelve Months Ended December 31,
|
|---|
Net Revenue
(dollars in thousands)
| 2005
| | 2004
| | Dollar
Change
| | Percentage
Change
| | Percentage
Change from
Currency
(1)
| | Percentage
Change from
Acquisitions
(2)
| | Percentage
Change Net of
Acquisitions
and Currency
Effect
| |
|---|
| Instruments and consumables | | | $ | 217,537 | | $ | 197,939 | | $ | 19,598 | | | 9.9 | % | | -- | | | -- | | | 9.9 | % |
| Rapid assay products | | | | 100,255 | | | 93,506 | | | 6,749 | | | 7.2 | % | | 0.2 | % | | -- | | | 7.0 | % |
| Laboratory and consulting | | | | | | | | | | | | | | | | | | | | | | | |
| services | | | | 156,425 | | | 118,596 | | | 37,829 | | | 31.9 | % | | (0.3 | %) | | 22.9 | % | | 9.3 | % |
| Practice information | | | | | | | | | | | | | | | | | | | | | | | |
| management systems | | | | | | | | | | | | | | | | | | | | | | | |
| and digital radiography | | | | 32,589 | | | 28,163 | | | 4,426 | | | 15.7 | % | | 0.2 | % | | 7.0 | % | | 8.5 | % |
| Pharmaceutical products | | | | 14,024 | | | 10,483 | | | 3,541 | | | 33.8 | % | | -- | | | -- | | | 33.8 | % |
|
| |
| |
| | | | | |
| Net CAG Revenue | | | $ | 520,830 | | $ | 448,687 | | $ | 72,143 | | | 16.1 | % | | -- | | | 6.5 | % | | 9.6 | % |
|
| |
| |
| | | | | |
| (1) | Represents the percentage change in revenue attributed to the effect of changes in currency rates from the 12 months ended December 31, 2004 to the 12 months ended December 31, 2005. |
| (2) | Represents the percentage change in revenue attributed to incremental revenues from businesses acquired since January 2004 during the twelve months ended December 31, 2004 compared to the twelve months ended December 31, 2005. |
The following revenue analysis reflects the results of operations net of the impact of currency exchange rates on sales outside the U.S.
35
The increase in sales of instruments and consumables from 2004 to 2005 was due mainly to increased sales volume. The increased sales volume of consumables was due primarily to higher worldwide practice-level sales of VetTest® slides. To a lesser extent, increased domestic sales of consumables used with our VetLyte® Electrolyte Analyzers and higher practice-level sales of tubes used with our hematology instruments also resulted in increased sales volume of consumables. Increased VetTest® chemistry and hematology consumables sales volume was due primarily to an increase in our installed base of instruments throughout 2004 and 2005. The increase in sales of VetLyte® consumables was due, in part, to lower sales in the fourth quarter of 2004 due to product unavailability, which had a favorable impact of 1% on the growth rate for instruments and consumables during 2005. Increased instrument sales volume resulted mainly from higher sales of LaserCyte® Hematology Analyzers and, to a lesser extent, the launch of our VetStat® Electrolyte and Blood Gas Analyzer.
The increase in sales of rapid assay products from 2004 to 2005 was due primarily to increased domestic practice-level sales volume of our canine combination test, the SNAP® 3Dx®Canine Test, and to higher average unit sales prices for canine and feline products.
The increase in sales of laboratory and consulting services from 2004 to 2005 resulted primarily from the inclusion of sales from laboratories acquired in the fourth quarter of 2004 and in 2005 and, to a lesser extent, the impact of price increases and higher testing volume. Incremental sales from laboratories acquired in the fourth quarter of 2004 and in 2005 contributed approximately 23% to laboratory and consulting services revenue growth during 2005.
The increase in sales of practice information management and digital radiography systems from 2004 to 2005 resulted from increased sales volume of digital radiography instruments. The increase in digital radiography revenue was primarily due to an increase in the number of systems sold, including sales attributable to a business acquired in the third quarter of 2005. Incremental sales from this acquired business contributed approximately 7% to practice information management and digital radiography systems revenue growth during 2005.
The increase in sales of pharmaceutical products in 2005 resulted primarily from increased practice-level demand and, to a lesser extent, from price increases on certain products.
Water. Revenue for Water increased $3.7 million, or 7%, to $56.8 million from $53.1 million for the prior year. The increase resulted primarily from higher worldwide sales volume, partly offset by lower average unit sales prices attributable to both greater price competition in certain geographies and higher relative sales in geographies where products are sold at lower unit prices. The favorable impact of currency exchange rates contributed an aggregate of $0.2 million, or less than 1%, to the increase in Water revenue.
Production Animal Segment. Revenue for PAS increased $13.3 million, or 42%, to $44.9 million from $31.7 million for the prior year. The increase resulted primarily from higher worldwide sales volume of livestock and, to a lesser extent, poultry diagnostics, including sales attributable to acquisitions in 2004. Incremental sales from businesses acquired during 2004 contributed approximately 17% to production animal products revenue growth during the period. Changes in foreign currency rates did not have a significant impact on the PAS revenue growth rate.
Gross Profit
Total Company. Gross profit increased $43.9 million, or 16%, to $322.9 million from $279.0 million for the prior year and, as a percentage of total revenue, was approximately constant at 51%. The following table presents gross profit and gross profit percentage by reportable segment:
For the Twelve Months Ended December 31,
| |
|---|
Gross Profit(dollars in thousands)
| 2005
| | Percent of
Revenue
| | 2004
| | Percent of
Revenue
| | Dollar
Change
| | Percentage
Change
| |
|---|
| CAG | | | $ | 250,409 | | | 48.1% | | $ | 214,927 | | | 47.9% | | $ | 35,482 | | | 16.5% | |
| Water | | | | 38,277 | | | 67.4% | | | 35,885 | | | 67.6% | | | 2,392 | | | 6.7% | |
| PAS | | | | 27,788 | | | 61.8% | | | 21,221 | | | 67.0% | | | 6,567 | | | 30.9% | |
| Other | | | | 6,426 | | | 41.3% | | | 6,984 | | | 44.4% | | | (558 | ) | | (8.0% | ) |
|
| | |
| | |
| | |
| Total Company | | | $ | 322,900 | | | 50.6% | | $ | 279,017 | | | 50.8% | | $ | 43,883 | | | 15.7% | |
|
| | |
| | |
| | |
36
Companion Animal Group. Gross profit for CAG increased $35.5 million, or 17%, to $250.4 million from $214.9 million for the prior year due primarily to increased sales volume across the CAG product lines. As a percentage of revenue, CAG gross profit was approximately constant at 48%. The gross profit percentage was positively impacted by relatively higher selling prices, particularly for laboratory and consulting services and rapid assay products; lower product and service costs associated with the LaserCyte® Hematology Analyzer and lower product cost of slides sold for use in our VetTest® Chemistry Analyzers under the agreement with our supplier; and the favorable impact of foreign currency rates on sales denominated in those currencies, net of foreign exchange hedge contract losses. The increases in the gross profit percentage were largely offset by higher overall net product and service costs, apart from the favorable LaserCyte® and slide costs mentioned above; greater relative sales of lower margin products and services, mainly from higher sales growth of laboratory services; and write-downs of excess pharmaceutical product inventory.
Water. Gross profit for Water increased $2.4 million, or 7%, to $38.3 million from $35.9 million for the prior year due primarily to increased sales volume, partly offset by a slight decrease in the gross profit percentage to 67% from 68%. The gross profit percentage was unfavorably impacted by costs related to a manufacturing issue during the third quarter of 2005 and by lower average unit sales prices. These decreases in the gross profit percentage were partly offset by the favorable impact of foreign currency rates on sales denominated in those currencies, net of foreign exchange hedge contract losses.
Production Animal Segment. Gross profit for PAS increased $6.6 million, or 31%, to $27.8 million from $21.2 million for the prior year due to increased sales volume, partly offset by a decrease in the gross profit percentage to 62% from 67%. During the same period of the prior year, a reduction of approximately $1.8 million in an estimated liability for a third party claim was accounted for as a reduction in cost of revenue and increased the 2004 gross profit percentage by four percentage points. For 2005, an unfavorable impact on the gross margin percentage of two percentage points was attributable to incremental acquisition integration costs. The gross profit percentage was favorably impacted by higher relative sales of higher margin livestock products and by the favorable impact of foreign currency rates on sales denominated in those currencies, net of foreign exchange hedge contract losses, partially offset by higher net product costs.
Operating Expenses and Operating Income
Total Company. Total operating expenses increased $36.6 million to $207.6 million from $171.0 million for the prior year. As a percentage of revenue, operating expenses increased to 33% from 31% for the prior year.
Operating income increased $7.3 million to $115.3 million from $108.0 million for the prior year. As a percentage of revenue, operating income decreased to 18% from 20%. During 2005, operating income was reduced by acquisition integration costs associated with businesses acquired in the fourth quarter of 2004 and in 2005, including costs incurred in connection with the centralization of our European production animal diagnostics operations in Bern, Switzerland. During 2004, operating income benefited from the settlement of a third party claim, described in the above discussion of Production Animal Segment gross profit, and a payment received in settlement of litigation, partly offset by acquisition integration costs. These discrete items in both years resulted in a reported decrease in operating income as a percentage of total company revenue of one percentage point. The remaining difference in the operating income percentage for 2005, compared to the prior year, was attributable, in part, to the expansion of the CAG sales, customer service and marketing organization during 2004 and the first half of 2005; amortization expense for intangible assets purchased in connection with businesses acquired in the fourth quarter of 2004 and in 2005; and other changes in gross profit and operating expenses described in this discussion.
The following tables present operating expenses and operating income by reportable segment:
For the Twelve Months Ended December 31,
| |
|---|
Operating Expenses
(dollars in thousands)
| 2005
| | Percent of
Revenue
| | 2004
| | Percent of
Revenue
| | Dollar
Change
| | Percentage
Change
| |
|---|
| CAG | | | $ | 167,439 | | | 32.1% | | $ | 137,804 | | | 30.7% | | $ | 29,635 | | | 21.5% | |
| Water | | | | 12,303 | | | 21.7% | | | 11,626 | | | 21.9% | | | 677 | | | 5.8% | |
| PAS | | | | 20,471 | | | 45.5% | | | 14,172 | | | 44.8% | | | 6,299 | | | 44.4% | |
| Other | | | | 3,849 | | | 24.7% | | | 4,202 | | | 26.8% | | | (353 | ) | | (8.4% | ) |
| Unallocated amounts | | | | 3,507 | | | N/A | | | 3,178 | | | N/A | | | 329 | | | 10.3% | |
|
| | |
| | |
| | |
| Total Company | | | $ | 207,569 | | | 32.5% | | $ | 170,982 | | | 31.1% | | $ | 36,587 | | | 21.4% | |
|
| | |
| | |
| | |
37
Operating Income
(dollares in thousands)
| 2005
| | Percent of
Revenue
| | 2004
| | Percent of
Revenue
| | Dollar
Change
| | Percentage
Change
| |
|---|
| CAG | | | $ | 82,970 | | | 15.9% | | $ | 77,123 | | | 17.2% | | $ | 5,847 | | | 7.6% | |
| Water | | | | 25,974 | | | 45.8% | | | 24,259 | | | 45.7% | | | 1,715 | | | 7.1% | |
| PAS | | | | 7,317 | | | 16.3% | | | 7,049 | | | 22.3% | | | 268 | | | 3.8% | |
| Other | | | | 2,577 | | | 16.6% | | | 2,782 | | | 17.7% | | | (205 | ) | | (7.4% | ) |
| Unallocated amounts | | | | (3,507 | ) | | N/A | | | (3,178 | ) | | N/A | | | (329 | ) | | (10.3% | ) |
|
| | |
| | |
| | |
| Total Company | | | $ | 115,331 | | | 18.1% | | $ | 108,035 | | | 19.7% | | $ | 7,296 | | | 6.8% | |
|
| | |
| | |
| | |
Companion Animal Group. Operating expenses for CAG increased $29.6 million, or 22%, to $167.4 million from $137.8 million for the prior year and, as a percentage of revenue, increased to 32% from 31%. The increase was attributable to a 21% ($15.1 million) increase in sales and marketing expense, a 25% ($10.1 million) increase in general and administrative expense, and a 17% ($4.4 million) increase in research and development expense. The increase in sales and marketing expense resulted primarily from the expansion of the worldwide sales, customer service and marketing organization; ongoing expenses attributable to the Vet Med Lab business acquired in the fourth quarter of 2004 and, to a lesser extent, the digital radiography business acquired in the third quarter of 2005 and higher sales commissions as a result of revenue performance. The increase in general and administrative expense resulted primarily from expenses attributable to businesses acquired in the fourth quarter of 2004 and in 2005, comprised of general and administrative expenses of a recurring nature, amortization expense for intangible assets acquired, and integration costs. To a lesser extent, the increase in general and administrative expense was also attributable to higher spending on information technology and other general support functions; the unfavorable impact of exchange rates on foreign currency denominated expenses; and the positive impact in 2004 of a payment received in the second quarter of 2004 to settle certain litigation. The increase in research and development expense resulted primarily from increased spending related to instrument development and, to a lesser extent, rapid assay and pharmaceutical product development.
Water. Operating expenses for Water increased $0.7 million, or 6%, to $12.3 million from $11.6 million for the prior year and, as a percentage of revenue, were approximately constant at 22%. The dollar increase was attributable to a 13% ($0.6 million) increase in general and administrative expense and a 12% ($0.2 million) increase in research and development expense, partly offset by a 2% ($0.1 million) decrease in sales and marketing expense. The increase in general and administrative expense resulted primarily from higher spending on information technology and other corporate functions, and, to a lesser extent, from the unfavorable impact of exchange rates on foreign currency denominated expenses. The increase in research and development expense resulted primarily from increased spending onCryptosporidiumtesting product development. There were no significant individual events or fluctuations in the nature and amounts of sales and marketing expense.
Production Animal Segment. Operating expenses for PAS increased $6.3 million, or 44%, to $20.5 million from $14.2 million for the prior year and, as a percentage of revenue, increased to 46% from 45%. The increase resulted from a 103% ($4.1 million) increase in general and administrative expense, a 26% ($1.5 million) increase in sales and marketing expense, and a 15% ($0.7 million) increase in research and development expense. The increase in general and administrative expense resulted primarily from expenses associated with the acquisition of Bommeli in the fourth quarter of 2004 and the subsequent centralization of our European production animal diagnostics operations in Bern, Switzerland. These costs are composed mainly of general and administrative expenses of a recurring nature to support the Bommeli business, costs related to the cessation of production in our Sweden-based facility, and amortization expense for intangible assets acquired. To a lesser extent, higher spending on information technology and other corporate functions and the unfavorable impact of exchange rates on foreign currency denominated expenses also contributed to the increase in general and administrative expense. The increase in sales and marketing expense resulted primarily from the addition of Bommeli sales and marketing activities and from sales and marketing costs to support the launch of our HerdChek® BSE Antigen Test Kit. The increase in research and development expense was due primarily to the addition of Bommeli research and development activities and to higher compensation costs, partly offset by reduced development activity following the launch of our HerdChek® BSE Antigen Test Kit.
Unallocated Amounts. Operating expenses that are not allocated to our operating segments, consisting primarily of corporate research and development, increased $0.3 million, or 10%, to $3.5 million from $3.2 million for the prior year due mainly to increased long-term development activities.
38
Interest Income and Interest Expense
Interest income was $3.2 million for 2005 and 2004. The impact of higher interest rates was substantially offset by the impact of lower average invested cash balances.
Interest expense was $0.1 million for 2005 compared to $0.2 million in 2004. The decrease was primarily due to interest incurred on a tax payment in December 2004 and the final payment of a note payable in February 2005.
Provision for Income Taxes
Our effective income tax rate was 34.2% for the year ended December 31, 2005 compared with 29.7% for the year ended December 31, 2004. The majority of this rate differential resulted from the favorable impact of the resolution in 2004 of an IRS income tax audit through the year ended December 31, 2001. As a result of completing this audit, we reduced previously accrued taxes. Other rate reductions resulted from the release in 2004 of a valuation allowance on international deferred tax assets as a result of a foreign subsidiary demonstrating consistent sustained profitability and changes in certain state and international tax estimates. In addition, 2005 tax expense increased by $1.0 million and the 2005 effective income tax rate increased by 0.8 percentage points due to incremental taxes on the repatriation of $30.0 million pursuant to theAmerican Jobs Creation Act of 2004.
RECENT ACCOUNTING PRONOUNCEMENTS
A discussion of recent accounting pronouncements is included in Note 2(q) to the consolidated financial statements for the year ended December 31, 2006 included in this Form 10-K.
LIQUIDITY AND CAPITAL RESOURCES
Liquidity
We fund the capital needs of our business through cash generated from operations. At December 31, 2006 and 2005, we had $96.7 million and $132.7 million of cash and cash equivalents and short-term investments, respectively, and working capital of $177.5 million and $192.7 million, respectively.
We consider the operating earnings of non-United States subsidiaries to be indefinitely invested outside the U.S. Changes to this policy could have adverse tax consequences. Subject to this policy, we manage our worldwide cash requirements considering available funds among all of our subsidiaries. Foreign cash balances are generally available without legal restrictions to fund ordinary business operations outside the U.S.
In January 2007, we entered into an unsecured short-term revolving credit facility with a bank in the principal amount of $125.0 million that matures on June 30, 2007. The credit facility may be used for general corporate purposes, including repurchases of our common stock and business acquisitions. The applicable interest rates generally range from 0.375% to 0.875% above the London interbank rate or the Canadian Dollar-denominated bankers’ acceptance rate, dependent on our leverage ratio. Under the credit facility, we pay quarterly commitment fees of 0.08% to 0.20%, dependent on our leverage ratio, on any unused commitment. The credit facility contains financial and other affirmative and negative covenants, as well as customary events of default, that would allow any amounts outstanding under the credit facility to be accelerated, or restrict our ability to borrow thereunder, in the event of noncompliance. The financial covenant requires our ratio of debt to earnings before interest and taxes, as defined by the agreement, not to exceed 3-to-1. We expect to enter into a long-term credit facility with similar terms prior to June 30, 2007.
We believe that current cash and cash equivalents, short-term investments, funds generated from operations, and amounts available under credit facilities will be sufficient to fund our operations, capital purchase requirements, and strategic growth needs. We further believe that we could obtain additional borrowings at comparable interest rates to fund our growth objectives. The extent and timing of acquisitions-related spending and repurchases of our common stock could cause variations on our liquidity and leverage levels.
39
Sources and Uses of Cash
Cash provided by operating activities was $109.8 million for the year ended December 31, 2006, compared to $116.6 million for 2005. The total of net income and net non-cash charges was $120.0 million for the year ended December 31, 2006, compared to $105.8 million for 2005. Reported cash flows from operations were negatively impacted by a change in accounting for the tax benefits from exercises of stock options and disqualifying dispositions of shares acquired in connection with our adoption of SFAS No. 123(R) on January 1, 2006, which reduced reported cash flows from operations by $9.4 million for the year ended December 31, 2006. For the year ended December 31, 2006, the tax benefit on exercises of stock options and disqualifying dispositions of shares was classified as a cash flow from financing activities, whereas the benefit was classified as a cash flow from operating activities in prior years.
In 2006, cash decreased $10.2 million due to changes in operating assets and liabilities, whereas in 2005 cash increased by $10.7 million due to changes in operating assets and liabilities, resulting in a year-to-year change of $21.0 million. The increase in cash used by changes in operating assets and liabilities, compared to 2005, was primarily attributable to $33.1 million of incremental cash used to purchase inventory, partly offset by $7.7 million of additional cash provided by an increase in accrued liabilities during the year ended December 31, 2006. The greater incremental increase in inventories was due, in part, to increased inventories in support of higher sales volumes, to our preparation for a supplier’s production facility transition, and to ensure adequate supply of certain instrument components and accessories that are being discontinued by the manufacturers. The reported increase in cash provided by changes in accrued liabilities, compared to 2005, was primarily attributable to the change in the presentation of tax benefits, noted above, of $9.4 million from exercises of stock options and disqualifying dispositions of shares acquired.
Cash used by investing activities was $40.7 million for the year ended December 31, 2006, compared to cash provided by investing activities of $12.6 million for 2005. The increase in cash used by investing activities for 2006, compared to 2005, was primarily due to the increase in cash used for acquisitions. An incremental increase of $17.6 million in 2006 compared to 2005 was largely due to the $15.2 million paid to acquire a veterinary reference laboratory in Canada. Additionally, in 2006 we had lower incremental net proceeds from net sales and maturities of short- and long-term investments of $13.6 million, we paid $12.1 million for the purchase of land and buildings, and we had incremental purchases of property and equipment of $8.1 million.
We paid cash of $12.1 million for land and buildings, primarily for the purchase of our Westbrook, Maine facility; $32.3 million to purchase other fixed assets; and $1.7 million to acquire rental instruments sold under recourse during the year ended December 31, 2006, principally related to the CAG segment. Our total capital plan for 2007 is approximately $65 million, which includes approximately $23 million towards the renovation and expansion of our headquarters facility in Westbrook, Maine. We preliminarily project additional capital spending of approximately $50 million during 2008 through 2009 to complete this expansion and renovation.
In connection with the purchase of our Westbrook, Maine facility, we assumed a mortgage that is payable in equal monthly installments of approximately $0.1 million through May 1, 2015. The assumed mortgage had a face value of $6.5 million and a stated interest rate of 9.875%. We recorded the mortgage at a fair market value of $7.5 million, based on an effective market interest rate of 6.05%.
The board of directors has authorized the repurchase of up to 18,000,000 shares of our common stock in the open market or in negotiated transactions. From the inception of the program in August 1999 to December 31, 2006, we repurchased 15,285,000 shares. We believe that the repurchase of our common stock is a favorable investment and we also repurchase to offset the dilutive effect of our employee share-based compensation programs. Repurchases of our common stock may vary depending upon the level of other investing activities and the share price. See Note 14 to the consolidated financial statements included in this Form 10-K for additional information about our share repurchases.
40
Other Commitments, Contingencies and Guarantees
Under our workers’ compensation insurance policies for U.S. employees for the years ended December 31, 2006, 2005, 2004 and 2003, we retain the first $250,000 in claim liability per incident and $3.1 million, $2.8 million, $3.0 million and $1.4 million, respectively, in aggregate claim liability. We entered into a similar workers’ compensation insurance policy effective January 1, 2007. The insurance company provides insurance for claims above the individual occurrence and aggregate limits. We estimate claim liability based on claims incurred and the estimated ultimate cost to settle the claims. Based on this analysis, we have recognized cumulative expenses of $1.3 million, $0.6 million, $0.7 million and $0.8 million for claims incurred during the years ended December 31, 2006, 2005, 2004 and 2003, respectively. In connection with these policies, we have outstanding letters of credit totaling $2.1 million to the insurance companies as security for these claims.
Under our employee health care insurance policy, we retain claims liability risk up to $125,000 per incident and an aggregate claim limit based on the number of employees enrolled in the plan per month. We estimate our liability for the uninsured portion of employee health care obligations based on individual and aggregate coverage, our claims experience, the number of employees enrolled in the program, and the average time from when a claim is incurred to the time it is reported. Should actual employee health care claims liability exceed estimates, we are liable for up to an additional $1.5 million for potential uninsured obligations at December 31, 2006. We have insurance coverage of $1.0 million for claims above the aggregate limit. Should employee health insurance claims exceed this coverage, we would have further obligations for the amount in excess of such coverage.
In connection with acquisitions of businesses and intangible assets, we have commitments outstanding at December 31, 2006 for additional purchase price payments of up to $3.9 million, of which $1.8 million is contingent on the achievement by certain acquired businesses of specified milestones.
We previously had a 40% equity interest in a joint venture to market production animal diagnostic products in China. In April 2006, we paid $0.6 million to acquire an additional 55% equity interest in the joint venture from our partner. We also committed to pay an additional $0.2 million in two years in consideration for the additional equity. In addition, the joint venture entered into a contract with the joint venture partner where the partner will provide promotional and agency services and will receive sales commissions at rates escalating from 2.5% to 8.5% annually based on sales volume.
In January 2007, we acquired the Critical Care Division of Osmetech plc. The acquired business is based in the United States and develops, manufactures, and sells point-of-care electrolyte and blood gas analyzers and related consumable products for the human and veterinary diagnostics markets. We paid cash of approximately $45 million.
On January 26, 2007 we announced that we had entered into an agreement to acquire all of the shares of Institut Pourquier. Based in Montpellier, France, Institut Pourquier develops, manufactures and sells production animal diagnostic products. The closing of this transaction is subject to certain conditions and is expected to occur in March 2007. Institut Pourquier had sales of approximately $7.5 million in 2006.
In October 2005, our former supplier of VetAutoread™ Hematology Analyzers and consumables sold this business (including the human hematology testing products division) and we simultaneously entered into a new supply agreement for these products with the acquirer of the business. Under this new supply agreement, we received fixed pricing on certain products through December 31, 2020, among other benefits. In partial consideration for this new supply agreement, we paid cash of $2.5 million to the acquirer and guaranteed the acquirer’s note (the “Note”) in the principal amount of $3.5 million given to our former supplier in partial consideration for the business. The acquirer is obligated to pay the Note through quarterly principal and interest payments through 2008 and to pay the remaining balance in 2008. The principal balance of the note that we have guaranteed is $2.6 million at December 31, 2006. We are obligated to make a second payment of $1.25 million upon the achievement of certain milestones by the acquirer, which we expect to occur in approximately 2007, and a third payment of $1.25 million twelve months later. Our obligations to make the second and third payments are subject to the acquirer’s payment of all amounts under the Note and the release of our guaranty.
We purchase the slides sold for use in our VetTest® Chemistry Analyzers under an agreement with Ortho that, as of December 31, 2006, required us to purchase a minimum of $59.0 million of slides through 2010. We purchase our VetAutoread™ Hematology Analyzers, components and consumables under an agreement under which we are required to make aggregate minimum purchases of $15.7 million through 2020, in addition to the payment obligations described above. We also have commitments under certain other agreements that commit us to aggregate future payments of $19.7 million. In addition, we have various minimum royalty payments due through 2024 of $15.1 million.
41
We are contractually obligated to make the following payments in the years below:
(in thousands)
| Total
| | 2007
| | 2008-2009
| | 2010-2011
| | After 2011
| |
|---|
| Long-term debt obligations (1) | | | $ | 7,125 | | $ | 678 | | $ | 1,485 | | $ | 1,676 | | $ | 3,286 | |
| Operating leases | | | | 32,522 | | | 7,369 | | | 11,936 | | | 7,767 | | | 5,450 | |
| Purchase obligations (2) | | | | 168,878 | | | 107,395 | | | 46,908 | | | 8,525 | | | 6,050 | |
| Minimum royalty payments | | | | 15,077 | | | 1,721 | | | 3,135 | | | 3,245 | | | 6,976 | |
| Other long-term liabilities (3) | | | | 5,828 | | | 2,831 | | | 2,797 | | | 200 | | | -- | |
|
| |
| |
| |
| |
| |
| Total contractual cash obligations | | | $ | 229,430 | | $ | 119,994 | | $ | 66,261 | | $ | 21,413 | | $ | 21,762 | |
|
| |
| |
| |
| |
| |
| | |
| (1) | Long-term debt amounts reflect principal payments only, excluding associated interest payments. |
| (2) | Purchase obligations include agreements to purchase goods or services that are enforceable and legally binding that specify all significant terms, including fixed or minimum quantities, pricing, and approximate timing of purchase transactions.Of this amount, $72.0 million represents amounts committed under purchase orders, $59.0 million represents our minimum purchase obligation under our VetTest® supply agreement with Ortho and $18.2 million represents our minimum purchase obligation and related payments under our VetAutoread™ agreements. |
| (3) | Other long-term liabilities are liabilities that are reflected on our consolidated balance sheet in this Annual Report on Form 10-K and include warranty obligations and commitments for additional acquisition purchase price payments. |
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
Our financial market risk consists primarily of foreign currency exchange rate risk. We operate subsidiaries in 16 foreign countries and transact business in local currencies.
The primary purpose of our foreign currency hedging activities is to protect against the volatility associated with foreign currency transactions. We also utilize some natural hedges to mitigate our transaction and commitment exposures. Corporate policy prescribes the range of allowable hedging activity. We enter into exchange contracts with large multinational financial institutions and we do not hold or engage in transactions involving derivative instruments for purposes other than risk management. Our accounting policies for these contracts are based on our designation of such instruments as hedging transactions. Market gains and losses are deferred in prepaid expenses or accruals, as appropriate, until the contract matures, which is the period when the related obligation is settled. We primarily utilize forward exchange contracts with durations of less than 18 months.
Our subsidiaries enter into foreign currency exchange contracts to minimize the impact of foreign currency fluctuations associated with their anticipated intercompany inventory purchases for the next twelve months. From time to time, we may also enter into foreign currency exchange contracts to minimize the impact of foreign currency fluctuations associated with specific, significant transactions. Our hedging strategy is consistent with prior periods. We enter into currency exchange contracts for amounts that are less than the full value of forecasted intercompany sales and for amounts that are equivalent to, or less than, other specific, significant transactions, thus no significant ineffectiveness has resulted or been recorded through the statements of income.
Our hedging strategy related to intercompany inventory purchases provides that we employ the full amount of our hedges for the succeeding year at the conclusion of our budgeting process for that year, which is complete by the end of the preceding year. Quarterly, we enter into contracts to hedge incremental portions of anticipated foreign currency transactions for the following year. Accordingly, our risk with respect to foreign currency exchange rate fluctuations may vary throughout each annual cycle. At December 31, 2006, we had $1.3 million in net unrealized losses on foreign exchange contracts designated as hedges recorded in other comprehensive income, which is net of $0.7 million in taxes.
Our currency rate exposure at December 31, 2006 consisted of local currency revenues and expenses, the impact of hedge contracts and balances denominated in a currency other than the Company’s or its subsidiaries’ functional currency. Based on our overall currency rate exposure, excluding unrealized losses of $2.0 million at December 31, 2006 and unrealized gains of $0.8 million at December 31, 2005 on foreign exchange contracts designated as hedges, a 10% weakening or strengthening of the U.S. dollar relative to foreign currencies at December 31, 2006 would increase or decrease operating income, respectively, by approximately $3.1 million in 2007 and a 10% weakening or strengthening of the U.S. dollar from December 31, 2005 would have increased or decreased operating income, respectively, by approximately $2.6 million in 2006. As of December 31, 2006, a 10% weakening or strengthening of the U.S. dollar relative to foreign currencies, excluding the impact of hedge contracts currently in place, would increase or reduce operating income, respectively, by approximately $13.9 million in 2007, compared to $9.7 million in 2006.
42
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The response to this item is submitted as a separate section of this report commencing on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining disclosure controls and procedures, as defined by the SEC in its Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 as amended (the “Exchange Act”). The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by the company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2006, our chief executive officer and chief financial officer have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures are effective to achieve their stated purpose.
Report of Management on Internal Control Over Financial Reporting
We are responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America and includes those policies and procedures that:
| | • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| | • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the Company; and |
| | • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to risk that controls may become inadequate because of changes in conditions and that the degree of compliance with the policies and procedures may deteriorate.
43
We conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway commission. Based on this evaluation, we conclude that, as of December 31, 2006, our internal control over financial reporting was effective.
Our assessment of the effectiveness of our internal control over financial reporting as of December 31, 2006 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as indicated in their report that is included herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended December 31, 2006 that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Certifications
The certifications with respect to disclosure controls and procedures and internal control over financial reporting of the Company’s chief executive officer and chief financial officer are attached as Exhibits 31.1 and 31.2 to this Annual Report on Form 10-K.
ITEM 9B. OTHER INFORMATION
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item with respect to Directors is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Corporate Governance” and “Election of Directors” in the Company’s definitive proxy statement with respect to its 2007 Annual Meeting of Stockholders, which proxy statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this report.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange, is incorporated herein by reference from the sections entitled “Compensation Discussion and Analysis”, “Executive Compensation and Related Information”, “Corporate Governance – Director Compensation”, “Corporate Governance – Compensation Committee Interlocks and Insider Participation”, and “Compensation Committee Report” in the Company’s definitive proxy statement with respect to its 2007 Annual Meeting of Stockholders, which proxy statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this report.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Executive Compensation and Related Information – Equity Compensation Plan Information” and “Ownership of Common Stock by Directors and Officers” in the Company’s definitive proxy statement with respect to its 2007 Annual Meeting of Stockholders, which proxy statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this report.
44
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Corporate Governance – Related Party Transactions”, “Executive Compensation and Related Information—Employment Agreements” and “Corporate Governance – Director Independence” in the Company’s definitive proxy statement with respect to its 2007 Annual Meeting of Stockholders, which proxy statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this report.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the section entitled “Ratification of Appointment of Independent Auditors—Independent Auditors’ Fees” in the Company’s definitive proxy statement with respect to its 2007 Annual Meeting of Stockholders, which proxy statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this report.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)
| | (1) and (2) | The financial statements set forth in the Index to Consolidated Financial Statements and the Consolidated Financial Statement Schedule are filed as a part of this Annual Report on Form 10-K commencing on page F-1. |
| | (a)(3) and (c) | The exhibits listed in the accompanying Exhibit Index are filed as part of this Annual Report on Form 10-K and incorporated by reference herein. |
45
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
|---|
| | |
| | IDEXX LABORATORIES, INC. |
| | |
| By:/s/ Jonathan W. Ayers
|
| | Jonathan W. Ayers
President and Chief Executive Officer
March 1, 2007 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| SIGNATURE | TITLE | DATE |
|---|
| | | |
| /s/ Jonathan W. Ayers | President, Chief Executive Officer and | March 1, 2007 |
| Jonathan W. Ayers | Chairman of the Board of Directors | |
| | | |
| /s/ Merilee Raines | Corporate Vice President, Chief Financial Officer | March 1, 2007 |
| Merilee Raines | and Treasurer (Principal Financial and Accounting Officer) | |
| | | |
| /s/ Thomas Craig | Director | March 1, 2007 |
| Thomas Craig | | |
| | | |
| /s/ Errol B. De Souza, PhD | Director | March 1, 2007 |
| Errol B. De Souza, PhD | | |
| | | |
| /s/ William T. End | Director | March 1, 2007 |
| William T. End | | |
| | | |
| /s/ Rebecca M. Henderson, PhD | Director | March 1, 2007 |
| Rebecca M. Henderson, PhD | | |
| | | |
| /s/ Barry C. Johnson, PhD | Director | March 1, 2007 |
| Barry C. Johnson, PhD | | |
| | | |
| /s/ Brian P. McKeon | Director | March 1, 2007 |
| Brian P. McKeon | | |
| | | |
| /s/ Robert J. Murray | Director | March 1, 2007 |
| Robert J. Murray | | |
46
EXHIBIT INDEX
| 3.1 | Restated Certificate of Incorporation of the Company, as amended (filed as Exhibit No. 3(i) to Quarterly Report on Form 10-Q for the quarter ended June 30, 2006, File No. 0-19271, and incorporated herein by reference). |
| 3.2 | Amended and Restated By-Laws of the Company (filed as Exhibit No. 3.2 to Quarterly Report on Form 10-Q for the quarter ended September 30, 2000, File No. 0-19271, and incorporated herein by reference). |
| 4.3 | Instruments with respect to other long-term debt of the Company and its consolidated subsidiaries are omitted pursuant to Item 601(b)(4)(iii) of Regulation S-K since the total amount authorized under each such omitted instrument does not exceed 10 percent of the total assets of the Company and its subsidiaries on a consolidated basis. The Company hereby agrees to furnish a copy of any such instrument to the Securities and Exchange Commission upon request. |
| 10.1† | 1991 Stock Option Plan of the Company, as amended (filed herewith). |
| 10.2* | U.S. Supply Agreement, effective as of October 16, 2003, between the Company and Ortho-Clinical Diagnostics, Inc. (“Ortho”) (filed as Exhibit No. 10.7 to Annual Report on Form 10-K for the year ended December 31, 2003, File No. 0-19271 (“2003 Form 10-K”), and incorporated herein by reference). |
| 10.3* | Amendment No. 1 to U.S. Supply Agreement effective as of January 1, 2005, between the Company and Ortho (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended June 30, 2005, File No. 0-19271 (“June 2005 10-Q”), and incorporated herein by reference). |
| 10.4* | European Supply Agreement, effective as of October 17, 2003, between the Company and Ortho (filed as Exhibit No. 10.8 to 2003 Form 10-K, and incorporated herein by reference). |
| 10.5* | Amendment No. 1 to European Supply Agreement effective as of January 1, 2005, between the Company and Ortho (filed as Exhibit No. 10.2 to June 2005 10-Q, and incorporated herein by reference). |
| 10.6† | 1998 Stock Incentive Plan of the Company, as amended (filed herewith). |
| 10.7† | 2000 Director Option Plan of the Company, as amended (filed herewith). |
| 10.8† | Employment Agreement dated January 22, 2002, between the Company and Jonathan W. Ayers (filed as Exhibit No. 10.13 to Annual Report on Form 10-K for the year ended December 31, 2001, File No. 0-19271, and incorporated herein by reference). |
| 10.9† | Executive Employment Agreement dated January 1, 2007, between the Company and Jonathan W. Ayers (filed as Exhibit No. 10.1 to January 5, 2007 Form 8-K, File No. 0-19271 (“January 5, 2007 Form 8-K”), and incorporated herein by reference). |
| 10.10† | Letter Agreement dated August 12, 2003, between the Company and William C. Wallen (filed as Exhibit No. 10.14 to 2003 Form 10-K, and incorporated herein by reference). |
| 10.11† | Executive Employment Agreement dated January 1, 2007, between the Company and William C. Wallen (filed as Exhibit No. 10.2 to January 5, 2007 Form 8-K, and incorporated herein by reference). |
| 10.12† | Executive Employment Agreement dated January 1, 2007, between the Company and Merilee Raines (filed as Exhibit No. 10.3 to January 5, 2007 Form 8-K, and incorporated herein by reference). |
| 10.13† | Executive Employment Agreement dated January 1, 2007, between the Company and Conan R. Deady (filed as Exhibit No. 10.4 to January 5, 2007 Form 8-K, and incorporated herein by reference). |
| 10.14† | Form of Executive Employment Agreement dated January 1, 2007, between the Company and each of Thomas J. Dupree, S. Sam Fratoni, PhD, Robert S. Hulsy, Irene C. Kerr, Ali Naqui, PhD, James F. Polewaczyk, Quentin J. Tonelli, PhD and Michael J. Williams (filed as Exhibit No. 10.5 to January 5, 2007 Form 8-K, and incorporated herein by reference). |
| 10.15 | Amendment, Release and Settlement Agreement dated as of September 12, 2002, among the Company, IDEXX Europe B.V., and Ortho (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended September 30, 2002, File No. 0-19271, and incorporated herein by reference). |
| 10.16† | Restated Director Deferred Compensation Plan (filed as Exhibit No. 10.1 to Current Report on Form 8-K filed on February 28, 2006, File No. 0-19271 (“February 28, 2006 Form 8-K”), and incorporated herein by reference). |
| 10.17† | Amendment No. 1 to Director Deferred Compensation Plan, as amended (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended September 30, 2006, File No. 0-19271, and incorporated herein by reference). |
| 10.18† | 2003 Stock Incentive Plan, as amended (filed herewith). |
| 10.19 | †Form of Stock Option Agreement, as amended pursuant to the 2003 Stock Incentive Plan (filed as Exhibit No. 10.3 to Quarterly Report on Form 10-Q for the quarter ended March 31, 2006, File No. 0-19271 (“March 2006 Form 10-Q”), and incorporated herein by reference). |
| 10.20† | 1997 Employee Stock Purchase Plan, as amended (filed as Exhibit No. 10.19 to Annual Report on Form 10-K for the year ended December 31, 2004, File No. 0-19271, and incorporated herein by reference). |
| 10.21† | Restated Executive Deferred Compensation Plan, as amended (filed as Exhibit No. 10.2 to February 28, 2006 Form 8-K, and incorporated herein by reference). |
| 10.22† | Form of Restricted Stock Unit Agreement (filed as Exhibit 10.22 to Annual Report on Form 10-K for the year ended 2005, File No. 0-19271 (“2005 Form 10-K”), and incorporated herein by reference). |
| 10.23 | Purchase and Sale Agreement dated as of January 17, 2006, between the Company and CW Westbrook Limited Partnership (filed as Exhibit 10.23 to 2005 Form 10-K, and incorporated herein by reference). |
| 10.24† | 2006 Executive Bonus Plan (filed as Exhibit No. 10.2 to March 2006 Form 10-Q, and incorporated herein by reference). |
| 10.25 | Purchase and Sale Agreement among Osmetech plc, Osmetech Inc., Osmetech Technology Inc. and Osmetech GmbH and IDEXX Sciences, Inc. and IDEXX Laboratories, Inc. dated as of December 15, 2006 (filed as Exhibit No. 2.1 to Current Report on Form 8-K filed December 21, 2006, File No. 0-19271, and incorporated herein by reference). |
| 10.26 | Credit Agreement among the Company, as borrower, certain material subsidiaries of the Company, as guarantors, JPMorgan Chase Bank, National Association, as administrative agent, and JPMorgan Chase Bank, National Association, Toronto Branch, as Toronto agent (filed as Exhibit No. 10.1 to Current Report on Form 8-K filed January 31, 2007, File No. 0-19271, and incorporated herein by reference). |
| 21 | Subsidiaries of the Company (filed herewith). |
| 23 | Consent of PricewaterhouseCoopers LLP (filed herewith). |
| 31.1 | Certification by Chief Executive Officer (filed herewith). |
| 31.2 | Certification by Corporate Vice President, Chief Financial Officer and Treasurer (filed herewith). |
| 32.1 | Certification by Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith). |
| 32.2 | Certification by Corporate Vice President, Chief Financial Officer and Treasurer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith). |
| * | Confidential treatment requested as to certain portions. |
| † | Management contract or compensatory arrangement required to be filed as an exhibit pursuant to Item 15(a)(3) of Form 10-K. |
FINANCIAL STATEMENTS AND SUPPLEMENTAL DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
AND
CONSOLIDATED FINANCIAL STATEMENT SCHEDULE
| |
|---|
| | PAGE |
| | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| | |
| Consolidated Balance Sheets as of December 31, 2006 and 2005 | F-4 |
| | |
| Consolidated Statements of Income for the Years Ended December 31, 2006, 2005 and 2004 | F-5 |
| | |
| Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2006, 2005 and 2004 | F-6 |
| | |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2006, 2005 and 2004 | F-7 |
| | |
| Notes to Consolidated Financial Statements | F-8 |
| | |
| Schedule II | |
| Valuation and Qualifying Accounts | F-34 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of IDEXX Laboratories, Inc.:
We have completed integrated audits of IDEXX Laboratories, Inc.‘s consolidated financial statements and of its internal control over financial reporting as of December 31, 2006, in accordance with the standards of the Public Company Accounting Oversight Board (United States). Our opinions, based on our audits, are presented below.
Consolidated financial statements and financial statement schedule
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of IDEXX Laboratories, Inc. and its subsidiaries at December 31, 2006 and 2005, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2006 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit of financial statements includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2(l) to the consolidated financial statements, the Company changed the manner in which it accounts for share-based compensation in 2006.
Internal control over financial reporting
Also, in our opinion, management’s assessment, included in the Report of Management on Internal Control Over Financial Reporting appearing under Item 9A, that the Company maintained effective internal control over financial reporting as of December 31, 2006 based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), is fairly stated, in all material respects, based on those criteria. Furthermore, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2006, based on criteria established in Internal Control — Integrated Framework issued by the COSO. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express opinions on management’s assessment and on the effectiveness of the Company’s internal control over financial reporting based on our audit. We conducted our audit of internal control over financial reporting in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. An audit of internal control over financial reporting includes obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we consider necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
F-2
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PRICEWATERHOUSECOOPERS LLP
Boston, Massachusetts
March 1, 2007
F-3
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except per share amounts)
| December 31,
| |
|---|
| 2006
| | 2005
| |
|---|
| ASSETS | | | | | | | | |
| Current Assets: | | | | | | | | |
| Cash and cash equivalents | | | $ | 61,666 | | $ | 67,151 | |
| Short-term investments | | | | 35,000 | | | 65,580 | |
| Accounts receivable, less reserves of $1,783 and $1,221 in 2006 and | | | | | | | | |
| 2005, respectively | | | | 81,389 | | | 71,688 | |
| Inventories | | | | 95,996 | | | 69,369 | |
| Deferred income tax assets | | | | 16,884 | | | 13,778 | |
| Other current assets | | | | 11,328 | | | 11,679 | |
|
| |
| |
| Total current assets | | | | 302,263 | | | 299,245 | |
|
| |
| |
| Property and Equipment, at Cost: | | | | | | | | |
| Land and improvements | | | | 6,062 | | | 1,570 | |
| Buildings and improvements | | | | 50,105 | | | 7,457 | |
| Leasehold improvements | | | | 11,454 | | | 34,645 | |
| Machinery and equipment | | | | 72,146 | | | 58,126 | |
| Office furniture and equipment | | | | 43,632 | | | 35,978 | |
| Construction in progress | | | | 8,139 | | | 5,001 | |
|
| |
| |
| | | | | 191,538 | | | 142,777 | |
| Less accumulated depreciation and amortization | | | | 91,910 | | | 77,080 | |
|
| |
| |
| | | | | 99,628 | | | 65,697 | |
|
| |
| |
| Other Noncurrent Assets: | | | | | | | | |
| Goodwill | | | | 104,826 | | | 88,127 | |
| Other intangible assets, net of accumulated amortization of $15,357 | | | | | | | | |
| and $9,874 for 2006 and 2005, respectively | | | | 43,353 | | | 30,619 | |
| Other noncurrent assets, net | | | | 9,490 | | | 6,988 | |
|
| |
| |
| | | | | 157,669 | | | 125,734 | |
|
| |
| |
| TOTAL ASSETS | | | $ | 559,560 | | $ | 490,676 | |
|
| |
| |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable | | | $ | 24,374 | | $ | 19,842 | |
| Accrued expenses | | | | 21,922 | | | 17,756 | |
| Accrued employee compensation and related expenses | | | | 33,368 | | | 27,550 | |
| Accrued taxes | | | | 18,465 | | | 19,960 | |
| Accrued marketing and customer programs | | | | 15,176 | | | 10,751 | |
| Warranty reserves | | | | 1,784 | | | 2,191 | |
| Current portion of long-term debt | | | | 678 | | | 551 | |
| Deferred revenue | | | | 8,976 | | | 7,965 | |
|
| |
| |
| Total current liabilities | | | | 124,743 | | | 106,566 | |
|
| |
| |
| Long-term Liabilities: | | | | | | | | |
| Deferred income tax liabilities | | | | 7,154 | | | 6,026 | |
| Long-term debt, net of current portion | | | | 6,447 | | | -- | |
| Warranty reserves | | | | 194 | | | 968 | |
| Deferred revenue | | | | 6,834 | | | 7,806 | |
| Other long-term liabilities | | | | 4,327 | | | -- | |
|
| |
| |
| Total long-term liabilities | | | | 24,956 | | | 14,800 | |
|
| |
| |
| Commitments and Contingencies (Note 11) | | | | | | | | |
| | | | | | | | |
| Partner's Interest in Consolidated Subsidiary | | | | -- | | | 300 | |
|
| |
| |
| Stockholders' Equity: | | | | | | | | |
| Common stock, $0.10 par value: Authorized 60,000; Issued: | | | | | | | | |
| 46,621 and 45,938 in 2006 and 2005, respectively | | | | 4,662 | | | 4,594 | |
| Additional paid-in capital | | | | 479,993 | | | 437,394 | |
| Deferred stock units: Outstanding: 31 and 25 units in 2006 and 2005, respectively | | | | 1,852 | | | 1,316 | |
| Retained earnings | | | | 490,614 | | | 396,936 | |
| Accumulated other comprehensive income | | | | 10,566 | | | 866 | |
| Treasury stock, at cost: 15,456 and 14,118 in 2006 and 2005, respectively | | | | (577,826 | ) | | (472,096 | ) |
|
| |
| |
| Total stockholders' equity | | | | 409,861 | | | 369,010 | |
|
| |
| |
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | | | $ | 559,560 | | $ | 490,676 | |
|
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share amounts)
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Revenue: | | | | | | | | | | | |
| Product revenue | | | $ | 525,352 | | $ | 460,495 | | $ | 411,748 | |
| Service revenue | | | | 213,765 | | | 177,600 | | | 137,433 | |
|
| |
| |
| |
| | | | | 739,117 | | | 638,095 | | | 549,181 | |
| Cost of revenue: | | | | | | | | | | | |
| Cost of product revenue | | | | 215,314 | | | 194,252 | | | 174,618 | |
| Cost of service revenue | | | | 144,274 | | | 120,943 | | | 95,546 | |
|
| |
| |
| |
| | | | | 359,588 | | | 315,195 | | | 270,164 | |
|
| |
| |
| |
| Gross profit | | | | 379,529 | | | 322,900 | | | 279,017 | |
|
| |
| |
| |
| Expenses: | | | | | | | | | | | |
| Sales and marketing | | | | 115,882 | | | 101,990 | | | 85,710 | |
| General and administrative | | | | 82,097 | | | 64,631 | | | 49,870 | |
| Research and development | | | | 53,617 | | | 40,948 | | | 35,402 | |
|
| |
| |
| |
| Income from operations | | | | 127,933 | | | 115,331 | | | 108,035 | |
| Interest expense | | | | (462 | ) | | (96 | ) | | (155 | ) |
| Interest income | | | | 3,279 | | | 3,237 | | | 3,223 | |
|
| |
| |
| |
| Income before provisions for income taxes and | | | | | | | | | | | |
| partner's interest | | | | 130,750 | | | 118,472 | | | 111,103 | |
| Provision for income taxes | | | | 37,224 | | | 40,670 | | | 33,165 | |
| Partner's interest in loss of subsidiary | | | | (152 | ) | | (452 | ) | | (394 | ) |
|
| |
| |
| |
| Net income | | | $ | 93,678 | | $ | 78,254 | | $ | 78,332 | |
|
| |
| |
| |
| Earnings per share: | | | | | | | | | | | |
| Basic | | | $ | 2.98 | | $ | 2.41 | | $ | 2.29 | |
|
| |
| |
| |
| Diluted | | | $ | 2.84 | | $ | 2.30 | | $ | 2.19 | |
|
| |
| |
| |
| Weighted average shares outstanding: | | | | | | | | | | | |
| Basic | | | | 31,433 | | | 32,521 | | | 34,214 | |
|
| |
| |
| |
| Diluted | | | | 32,954 | | | 34,055 | | | 35,800 | |
|
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except per share amounts)
| Common Stock
| | | | | | | |
|---|
| Number
of Shares
| | $0.10
Par Value
| | Additional
Paid-in
Capital
| | Deferred
Stock Units
| | Retained
Earnings
| | Accumulated
Other
Comprehensive
Income
(Loss)
| | Treasury
Stock
| | Total
Stockholders'
Equity
| |
|---|
| Balance January 1, 2004 | | | | 44,390 | | $ | 4,439 | | $ | 383,249 | | $ | 138 | | $ | 240,350 | | $ | 4,565 | | $ | (219,449 | ) | $ | 413,292 | |
| Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | | -- | | | -- | | | -- | | | -- | | | 78,332 | | | -- | | | -- | | | 78,332 | |
| Unrealized loss on | | | | | | | | | | | | | | | | | | | | | | | | | | |
| investments, net of | | | | | | | | | | | | | | | | | | | | | | | | | | |
| tax of $57 | | | | -- | | | -- | | | -- | | | -- | | | -- | | | (89 | ) | | -- | | | (89 | ) |
| Unrealized gain on | | | | | | | | | | | | | | | | | | | | | | | | | | |
| foreign currency forward | | | | | | | | | | | | | | | | | | | | | | | | | | |
| contracts, net | | | | | | | | | | | | | | | | | | | | | | | | | | |
| of tax of $24 | | | | -- | | | -- | | | -- | | | -- | | | -- | | | 178 | | | -- | | | 178 | |
| Translation adjustment | | | | -- | | | -- | | | -- | | | -- | | | -- | | | 6,647 | | | -- | | | 6,647 | |
| | | | | | | |
| |
| Total comprehensive income | | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | 85,068 | |
| Purchase of treasury stock | | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | (128,814 | ) | | (128,814 | ) |
| Common Stock issued under | | | | | | | | | | | | | | | | | | | | | | | | | | |
| employee stock option and | | | | | | | | | | | | | | | | | | | | | | | | | | |
| purchase plans, including | | | | | | | | | | | | | | | | | | | | | | | | | | |
| excess tax benefit | | | | 827 | | | 83 | | | 27,568 | | | -- | | | -- | | | -- | | | (64 | ) | | 27,587 | |
| Issuance of deferred stock | | | | | | | | | | | | | | | | | | | | | | | | | | |
| units | | | | -- | | | -- | | | -- | | | 527 | | | -- | | | -- | | | -- | | | 527 | |
|
| |
| |
| |
| |
| |
| |
| |
| |
| Balance December 31, 2004 | | | | 45,217 | | | 4,522 | | | 410,817 | | | 665 | | | 318,682 | | | 11,301 | | | (348,327 | ) | | 397,660 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | | -- | | | -- | | | -- | | | -- | | | 78,254 | | | -- | | | -- | | | 78,254 | |
| Unrealized gain on | | | | | | | | | | | | | | | | | | | | | | | | | | |
| investments, net of | | | | | | | | | | | | | | | | | | | | | | | | | | |
| tax of $15 | | | | -- | | | -- | | | -- | | | -- | | | -- | | | 23 | | | -- | | | 23 | |
| Unrealized gain on | | | | | | | | | | | | | | | | | | | | | | | | | | |
| foreign currency forward | | | | | | | | | | | | | | | | | | | | | | | | | | |
| contracts, net | | | | | | | | | | | | | | | | | | | | | | | | | | |
| of tax of $1,703 | | | | -- | | | -- | | | -- | | | -- | | | -- | | | 3,403 | | | -- | | | 3,403 | |
| Translation adjustment | | | | -- | | | -- | | | -- | | | -- | | | -- | | | (13,861 | ) | | -- | | | (13,861 | ) |
| | | | | | | |
| |
| Total comprehensive income | | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | 67,819 | |
| Purchase of treasury stock | | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | (123,769 | ) | | (123,769 | ) |
| Common Stock issued under | | | | | | | | | | | | | | | | | | | | | | | | | | |
| employee stock option and | | | | | | | | | | | | | | | | | | | | | | | | | | |
| purchase plans, including | | | | | | | | | | | | | | | | | | | | | | | | | | |
| excess tax benefit | | | | 721 | | | 72 | | | 26,577 | | | -- | | | -- | | | -- | | | -- | | | 26,649 | |
| Issuance of deferred stock | | | | | | | | | | | | | | | | | | | | | | | | | | |
| units | | | | -- | | | -- | | | -- | | | 651 | | | -- | | | -- | | | -- | | | 651 | |
|
| |
| |
| |
| |
| |
| |
| |
| |
| Balance December 31, 2005 | | | | 45,938 | | $ | 4,594 | | $ | 437,394 | | $ | 1,316 | | $ | 396,936 | | $ | 866 | | $ | (472,096 | ) | $ | 369,010 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income (loss): | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | | -- | | | -- | | | -- | | | -- | | | 93,678 | | | -- | | | -- | | | 93,678 | |
| Unrealized gain on | | | | | | | | | | | | | | | | | | | | | | | | | | |
| investments, net of | | | | | | | | | | | | | | | | | | | | | | | | | | |
| tax of $29 | | | | -- | | | -- | | | -- | | | -- | | | -- | | | 46 | | | -- | | | 46 | |
| Unrealized loss on | | | | | | | | | | | | | | | | | | | | | | | | | | |
| foreign currency | | | | | | | | | | | | | | | | | | | | | | | | | | |
| forward contracts, net | | | | | | | | | | | | | | | | | | | | | | | | | | |
| of tax of $942 | | | | -- | | | -- | | | -- | | | -- | | | -- | | | (1,873 | ) | | -- | | | (1,873 | ) |
| Translation adjustment | | | | -- | | | -- | | | -- | | | -- | | | -- | | | 11,527 | | | -- | | | 11,527 | |
| | | | | | | |
| |
| Total comprehensive income | | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | 103,378 | |
| Purchase of treasury stock | | | | -- | | | -- | | | -- | | | -- | | | -- | | | -- | | | (105,730 | ) | | (105,730 | ) |
| Common Stock issued under | | | | | | | | | | | | | | | | | | | | | | | | | | |
| employee stock option and | | | | | | | | | | | | | | | | | | | | | | | | | | |
| purchase plans, including | | | | | | | | | | | | | | | | | | | | | | | | | | |
| excess tax benefit | | | | 681 | | | 68 | | | 31,962 | | | -- | | | -- | | | -- | | | -- | | | 32,030 | |
| Common Stock issued under | | | | | | | | | | | | | | | | | | | | | | | | | | |
| employee restricted and | | | | | | | | | | | | | | | | | | | | | | | | | | |
| deferred stock plans | | | | 2 | | | -- | | | 123 | | | (123 | ) | | -- | | | -- | | | -- | | | -- | |
| Issuance of deferred stock | | | | | | | | | | | | | | | | | | | | | | | | | | |
| units | | | | -- | | | -- | | | -- | | | 659 | | | -- | | | -- | | | -- | | | 659 | |
| Share-based compensation | | | | | | | | | | | | | | | | | | | | | | | | | | |
| cost recognized | | | | -- | | | -- | | | 10,514 | | | -- | | | -- | | | -- | | | -- | | | 10,514 | |
|
| |
| |
| |
| |
| |
| |
| |
| |
| Balance December 31, 2006 | | | | 46,621 | | $ | 4,662 | | $ | 479,993 | | $ | 1,852 | | $ | 490,614 | | $ | 10,566 | | $ | (577,826 | ) | $ | 409,861 | |
|
| |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Cash Flows from Operating Activities: | | | | | | | | | | | |
| Net income | | | $ | 93,678 | | $ | 78,254 | | $ | 78,332 | |
| Adjustments to reconcile net income to net cash provided by | | | | | | | | | | | |
| operating activities: | | | | | | | | | | | |
| Depreciation and amortization | | | | 29,816 | | | 24,369 | | | 18,427 | |
| Write-down of non-current assets | | | | 350 | | | -- | | | -- | |
| Partner's interest in loss of subsidiary | | | | (152 | ) | | (452 | ) | | (394 | ) |
| Provision for (recovery of) uncollectible accounts | | | | 1,070 | | | 121 | | | (294 | ) |
| Provision for (benefit of) deferred income taxes | | | | (6,135 | ) | | (4,477 | ) | | 4,599 | |
| Share-based compensation expense | | | | 10,842 | | | 184 | | | 135 | |
| Tax benefit from exercises of stock options | | | | (9,407 | ) | | 7,808 | | | 8,211 | |
| Changes in assets and liabilities, net of acquisitions and disposals | | | | | | | | | | | |
| Accounts receivable | | | | (6,583 | ) | | (9,300 | ) | | (5,162 | ) |
| Inventories | | | | (25,679 | ) | | 7,433 | | | 758 | |
| Other assets | | | | 158 | | | (2,244 | ) | | (26 | ) |
| Accounts payable | | | | 4,352 | | | 4,901 | | | (5,791 | ) |
| Accrued liabilities | | | | 17,882 | | | 10,184 | | | (5,442 | ) |
| Deferred revenue | | | | (366 | ) | | (229 | ) | | 2,026 | |
|
| |
| |
| |
| Net cash provided by operating activities | | | | 109,826 | | | 116,552 | | | 95,379 | |
|
| |
| |
| |
| Cash Flows from Investing Activities: | | | | | | | | | | | |
| Purchase of short- and long-term investments | | | | (79,810 | ) | | (63,619 | ) | | (37,114 | ) |
| Sales and maturities of short- and long-term investments | | | | 110,465 | | | 107,880 | | | 86,010 | |
| Purchase of property and equipment | | | | (32,331 | ) | | (24,199 | ) | | (29,065 | ) |
| Purchase of land and buildings | | | | (12,084 | ) | | -- | | | -- | |
| Net proceeds from sale of land and buildings | | | | -- | | | 2,751 | | | -- | |
| Acquisition of equipment leased to customers | | | | (1,720 | ) | | (2,615 | ) | | (2,640 | ) |
| Acquisition of intangible assets and businesses, net of cash | | | | | | | | | | | |
| acquired | | | | (25,220 | ) | | (7,604 | ) | | (53,942 | ) |
|
| |
| |
| |
| Net cash provided by (used in) investing activities | | | | (40,700 | ) | | 12,594 | | | (36,751 | ) |
|
| |
| |
| |
| Cash Flows from Financing Activities: | | | | | | | | | | | |
| Payment of long-term debt | | | | (877 | ) | | (2,057 | ) | | (356 | ) |
| Purchase of treasury stock | | | | (105,711 | ) | | (123,769 | ) | | (129,191 | ) |
| Proceeds from the exercises of stock options | | | | 20,922 | | | 18,841 | | | 19,376 | |
| Tax benefit from exercises of stock options | | | | 9,407 | | | -- | | | -- | |
|
| |
| |
| |
| Net cash used in financing activities | | | | (76,259 | ) | | (106,985 | ) | | (110,171 | ) |
| Net effect of exchange rates on cash | | | | 1,648 | | | (2,166 | ) | | 1,757 | |
|
| |
| |
| |
| Net increase (decrease) in cash and cash equivalents | | | | (5,485 | ) | | 19,995 | | | (49,786 | ) |
| Cash and cash equivalents at beginning of year | | | | 67,151 | | | 47,156 | | | 96,942 | |
|
| |
| |
| |
| Cash and cash equivalents at end of year | | | $ | 61,666 | | $ | 67,151 | | $ | 47,156 | |
|
| |
| |
| |
| Supplemental Disclosure of Cash Flow Information: | | | | | | | | | | | |
| Interest paid | | | $ | 498 | | $ | 40 | | $ | 33 | |
|
| |
| |
| |
| Income taxes paid | | | $ | 36,100 | | $ | 34,346 | | $ | 25,862 | |
|
| |
| |
| |
| Supplemental Disclosure of Non-Cash Information: | | | | | | | | | | | |
| Market value of common shares received from employees in | | | | | | | | | | | |
| connection with share-based compensation- see Note 13 | | | $ | 18 | | $ | -- | | $ | 64 | |
|
| |
| |
| |
| Receivable for purchase price adjustment of business acquisitions | | | $ | -- | | $ | 22 | | $ | 500 | |
|
| |
| |
| |
| Consideration payable for acquisitions | | | $ | 3,850 | | $ | -- | | $ | 1,000 | |
|
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 NATURE OF BUSINESS
We develop, manufacture and distribute products and provide services for the veterinary and the food and water testing markets. During the year ended December 31, 2006, we operated primarily through three reportable segments: products and services for the veterinary market, which is referred to as the Companion Animal Group (“CAG”), water quality products (“Water”) and products for production animal health, which is referred to as the Production Animal Segment (“PAS”). Our products and services are sold worldwide. See Note 16 for additional information regarding our reportable operating segments, products and services, and geographical areas.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Consolidation
The accompanying consolidated financial statements include the accounts of IDEXX Laboratories, Inc. and our wholly-owned and majority-owned subsidiaries, and all other entities in which we have a variable interest and are determined to be the primary beneficiary. All material intercompany transactions and balances have been eliminated in consolidation.
(b) Estimates
The preparation of these financial statements in accordance with accounting principles generally accepted in the United States of America requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate these estimates, including those related to bad debts; goodwill and other intangible assets; income taxes; inventory; investments; revenue recognition, including customer programs and incentives, product returns, and multiple element arrangements; share-based compensation; warranty reserves; and contingencies. We accrue contingent liabilities when it is probable that future expenditures will be made and such expenditures can reasonably be estimated. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates.
(c) Inventories
Inventories include material, labor and overhead, and are stated at the lower of cost (first-in, first-out) or market. We write down inventory for estimated obsolescence when warranted by estimates of future demand and market conditions. If actual market conditions are less favorable than those we estimated, additional inventory write-downs may be required, which would have a negative effect on results of operations.
The components of inventories are as follows(in thousands):
| December 31,
| |
|---|
| 2006
| | 2005
| |
|---|
| Raw materials | | | $ | 33,199 | | $ | 22,517 | |
| Work-in-process | | | | 13,804 | | | 10,583 | |
| Finished goods | | | | 48,993 | | | 36,269 | |
|
| |
| |
| | | | $ | 95,996 | | $ | 69,369 | |
|
| |
| |
| | |
(d) Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. When an item is sold or retired, the cost and related accumulated depreciation is relieved, and the resulting gain or loss, if any, is recognized in the statement of income. We provide for depreciation and amortization primarily using the straight-line method by charges to income in amounts that allocate the cost of property and equipment over their estimated useful lives as follows:
F-8
Asset Classification
| Estimated Useful Life
|
|---|
| Land improvements | | | 15 years | | |
| Buildings and improvements | | | 32-40 years | | |
| Leasehold improvements | | | Shorter of life of lease or | | |
| | | | useful life | | |
| Machinery and equipment | | | 3-5 years | | |
| Office furniture and equipment | | | 3-7 years | | |
We recorded depreciation expense of $21.6 million, $17.8 million and $14.7 million for the years ended December 31, 2006, 2005 and 2004, respectively.
(e) Goodwill and Other Intangible Assets
A significant portion of the purchase prices of our business acquisitions is assigned to intangible assets. Intangible assets other than goodwill are initially valued at the lesser of fair value or, if applicable, fair value proportionately reduced by the excess of the fair value of acquired net assets over cost, (collectively, “fair value”) when acquired. If a market value is not readily available, the fair value of the intangible asset is estimated based on discounted cash flows using market participant assumptions. Goodwill is initially valued based on the excess of the purchase price of a business combination over the fair values of acquired net assets.
We provide for amortization using the straight-line and accelerated methods by charges to income in amounts that allocate the intangible assets over their estimated useful lives as follows:
Asset Classification
| Estimated
Useful Life
|
|---|
| Patents | | | 15 years | | |
| Other product rights | | | 5-15 years | | |
| Customer-related intangible assets | | | 5-15 years | | |
| Other, primarily noncompete agreements | | | 2-10 years | | |
We assess the impairment of identifiable intangible assets and other long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important that could trigger an impairment review include, but are not limited to, the following:
| | • | Significant under-performance relative to historical or projected future operating results; |
| | • | Failures to obtain regulatory approval of certain products; |
| | • | Significant changes in regulations; |
| | • | Significant changes in the manner of our use of the acquired assets or the strategy for our overall business; |
| | • | Significant increase in the discount rate assumed to calculate the present value of future cash flow; |
| | • | Significant negative industry or economic trends; |
| | • | Significant advancements or changes in technology; and |
| | • | Cancellation or significant changes in contractual relationships. |
We continually assess the realizability of intangible assets other than goodwill in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS No. 144”). If an impairment review is triggered, we evaluate the carrying value of long-lived assets by determining if impairment exists based on estimated undiscounted future cash flows over the remaining useful life of the assets and comparing that value to the carrying value of the assets. If the carrying value of the asset is greater than the estimated future cash flows, the asset is written down to its estimated fair value. In determining expected future cash flows, assets are grouped at the lowest level for which cash flows are identifiable and independent of cash flows from other asset groups. The cash flow estimates that are used contain our best estimates, using appropriate and customary assumptions and projections at the time.
F-9
We assess goodwill for impairment annually and whenever events or circumstances indicate an impairment may exist, in accordance with SFAS No. 142, “Goodwill and Other Intangible Assets” (“SFAS No. 142”). For impairment testing, we identify our reporting units, allocate assets and liabilities (including goodwill) to the reporting units and compare the reporting units’ net book value to their estimated fair value. The fair value of the reporting units is estimated using a discounted cash flow approach. The cash flow estimates used contain our best estimates, using appropriate and customary assumptions and projections at the time. If a reporting unit’s net book value exceeds its fair value, then the implied fair value of goodwill is determined. If the net book value of goodwill exceeds the implied fair value of goodwill, a goodwill impairment loss is recognized in an amount equal to that excess. No impairment has been identified as a result of the annual or event-driven reviews during the years ended December 31, 2006, 2005 or 2004.
(f) Warranty Reserves
We provide for the estimated cost of product warranties in cost of product revenue at the time revenue is recognized based on the estimated cost to repair the instrument over its warranty period. Cost of revenue reflects not only estimated warranty expense for the systems sold in the current period, but also any changes in estimated warranty expense for the installed base that results from our quarterly evaluation of service experience. Our actual warranty obligation is affected by product failure rates and service costs incurred in correcting product failures. Should actual product failure rates or service costs differ from our estimates, which are based on historical data, revisions to the estimated warranty liability would be required. Following is a summary of changes in accrued warranty reserve for products sold to customers for the years ended December 31, 2006 and 2005, respectively(in thousands):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| |
|---|
| Balance, beginning of year | | | $ | 3,159 | | $ | 3,679 | |
| Provision for warranty expense | | | | 1,625 | | | 2,479 | |
| Provision for change in estimate of prior warranty expense | | | | (474 | ) | | (276 | ) |
| Settlement of warranty liability | | | | (2,332 | ) | | (2,723 | ) |
|
| |
| |
| Balance, end of year | | | | 1,978 | | | 3,159 | |
| Long-term portion | | | | 194 | | | 968 | |
|
| |
| |
| Current portion of warranty reserves | | | $ | 1,784 | | $ | 2,191 | |
|
| |
| |
(g) Income Taxes
We account for income taxes under SFAS No. 109, “Accounting for Income Taxes.” This statement requires that we recognize a current tax liability or asset for current taxes payable or refundable, respectively, and a deferred tax liability or asset, as the case may be, for the estimated future tax effects of temporary differences between book and tax treatment of assets and liabilities and carryforwards to the extent they are realizable. We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we consider future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for a valuation allowance, in the event we were to determine that we would be able to realize our deferred tax assets in the future in excess of the net recorded amount, an adjustment to the deferred tax asset would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination was made. Significant judgment is required in determining our worldwide provision for income taxes and our income tax filings are regularly under audit by tax authorities. We periodically assess our exposures related to our worldwide provision for income taxes and believe that we have appropriately accrued taxes for contingencies. Any reduction of these contingent liabilities or additional assessment would increase or decrease income, respectively, in the period such determination was made. Interest expense and penalties associated with the underpayment of income taxes are included in income tax expense. See Note 9 for additional information regarding income taxes.
F-10
(h) Sales and Value Added Taxes
We calculate, collect from our customers, and remit to governmental authorities sales, value added and excise taxes assessed by governmental authorities in connection with revenue-producing transactions with our customers. We report these taxes on a net basis and do not include these tax amounts in revenue or costs of sales.
(i) Revenue Recognition
We recognize revenue when four criteria are met: (i) persuasive evidence that an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the sales price is fixed or determinable, and (iv) collectibility is reasonably assured.
| | • | We recognize revenue at the time of shipment to distributors for substantially all products sold through distributors as title and risk of loss pass to these customers on delivery to the common carrier. Our distributors do not have the right to return products. We recognize revenue for the remainder of our customers when the product is delivered to the customer except as noted below. |
| | • | We recognize revenue from the sales of instruments, noncancelable software licenses and hardware systems upon installation (and completion of training if applicable) and the customer’s acceptance of the instrument or system because at this time we have no significant further obligations. |
| | • | We recognize service revenue at the time the service is performed. |
| | • | We recognize revenue associated with extended maintenance agreements over the life of the contracts. Amounts collected in advance of revenue recognition are recorded as a current or long-term liability based on the time from the balance sheet date to the future date of revenue recognition. |
| | • | We recognize revenue on certain instrument systems under rental programs over the life of the rental agreement. Amounts collected in advance of revenue recognition are recorded as a current or long-term liability based on the time from the balance sheet date to the future date of revenue recognition. |
Certain diagnostic instruments and practice information management systems offered for sale may include software that is considered more than incidental to the utility and value of the product. Sales arrangements may provide for software update rights or postcontract customer support. Judgment is required to determine whether sales arrangements include multiple elements.
When multiple products and/or services are sold together, we generally allocate the total consideration received amongst the elements based on their relative fair values, which is determined by amounts charged separately for the delivered and undelivered elements to other customers. When there is objective and reliable evidence of the fair value of the undelivered elements but no such evidence for the delivered elements, the fair value of the undelivered elements is deferred and the residual revenue is allocated to the delivered elements. The delivered elements are recognized as revenue when appropriate under the policies described above. If there is not sufficient evidence of the fair value of the undelivered elements, no revenue is allocated to the delivered elements and the total consideration received is deferred until delivery of those elements for which objective and reliable evidence of the fair value is not available. Shipping costs reimbursed by the customer are included in revenue.
We record estimated reductions to revenue in connection with customer programs and incentive offerings, which may give customers credits, award points, or trade-in rights. Awards points may be applied to trade receivables owed to us and/or toward future purchase of our products and services. We estimate these reductions based on our experience with similar customer programs in prior years. Revenue reductions are recorded on a quarterly basis based on issuance of credits, points actually awarded, and estimates of points to be awarded in the future based on current revenue. For the SNAP Up the Savings™ program, estimates of future points are revised quarterly and finalized annually in the third quarter of each year upon the issuance of points to customers. For our Practice Developer™ volume discount program, we have reduced revenue assuming all points granted will result in future credits because the historical forfeitures have been de minimus. On November 30 of each year, unused points awarded before January 1 of the prior year expire.
F-11
We may offer customers the right to trade in instruments for credit against the purchase price of other instruments acquired in the future. For trade-in rights, we have reduced revenue using estimates regarding the percentage of qualifying instruments that will be traded in and the average trade-in value.
We recognize revenue only in those situations where collection from the customer is reasonably assured. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We base our estimates on detailed analysis of specific customer situations and a percentage of our accounts receivable by aging category. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payment, additional allowances might be required.
(j) Research and Development and Software Development Costs
Research and Development costs are expensed as incurred. In accordance with SFAS No. 86, “Accounting for the Costs of Computer Software to be Sold, Leased or Otherwise Marketed” (“SFAS No. 86”), we evaluate our software research and development costs for capitalization after the technological feasibility of software and products containing software has been established. No software development costs have been capitalized by us because costs eligible for capitalization under SFAS No. 86 have been insignificant. Research and development expenses consist of salaries, employee benefits, materials and consulting costs.
(k) Advertising Costs
Advertising costs, which are recognized as sales and marketing expense in the period in which they are incurred, were $1.5 million, $1.5 million and $1.0 million for the years ended December 31, 2006, 2005 and 2004, respectively.
(l) Share-Based Compensation
In December 2004, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 123(R), “Share-Based Payment” (“SFAS No. 123(R)”), which is a revision of SFAS No. 123, “Accounting for Stock-Based Compensation” and SFAS No. 148, “Accounting for Stock-Based Compensation—Transition and Disclosure—An Amendment of FASB No. 123” (collectively, “SFAS No. 123, as Amended”) and supersedes Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB No. 25”). During 2005 and 2006, the FASB also issued Staff Positions No. FAS 123(R)-1, -2, -3, -4, -5 and -6 to provide application guidance related to SFAS No. 123(R).
SFAS No. 123(R) requires all share-based compensation to employees, including grants of stock options, to be valued at fair value on the date of grant, and to be expensed over the requisite service period (generally the vesting period). Prior to January 1, 2006, we measured costs related to employee share-based compensation plans in accordance with APB No. 25. Accordingly, no employee compensation cost was recognized for these plans prior to January 1, 2006.
We adopted the provisions of SFAS No. 123(R) on January 1, 2006 and elected the modified prospective method of transition to the fair-value-based method of accounting for stock-based employee compensation prescribed by SFAS No. 123(R). Effective January 1, 2006, under the modified prospective method, share-based compensation expense includes expense for unvested awards at December 31, 2005 and all awards granted subsequent to December 31, 2005. Share-based compensation expense for the unvested awards outstanding at December 31, 2005 is based on the grant-date fair value previously calculated in developing the pro forma disclosures in accordance with the provisions of SFAS No. 123, as Amended.
In connection with the adoption of SFAS 123(R), we adopted the straight-line method to prospectively expense share-based awards granted subsequent to December 31, 2005. The graded-vesting, or accelerated, method has been used to record the expense for stock options granted prior to January 1, 2006.
Beginning in 2006, we modified our share-based employee compensation programs to shift from the grant of stock options and employee stock purchase rights only to the grant of a mix of restricted stock units and stock options, along with employee stock purchase rights. There were no modifications to the terms of outstanding options during 2006 or 2005.
F-12
We issue new shares of common stock to satisfy option and employee stock purchase right exercises and to settle restricted stock units and deferred stock units. At December 31, 2006, a remaining total of 797,000 shares of common stock was authorized by our shareholders and was available for future grants of share-based compensation.
(m) Foreign Currency Translation
Assets and liabilities of our foreign subsidiaries are translated using the exchange rate in effect at the balance sheet date. Revenue and expense accounts are translated using a weighted average of exchange rates in effect during the period. Cumulative translation gains and losses are shown in the accompanying consolidated balance sheets as a separate component of accumulated other comprehensive income (loss). Exchange gains and losses arising from transactions denominated in foreign currencies other than our subsidiaries’ respective functional currencies are included in operating expenses. Included in general and administrative expenses are aggregate foreign exchange currency transaction gains of $0.8 million, losses of $0.8 million, and gains of $0.4 million for the years ended December 31, 2006, 2005 and 2004, respectively. Additionally, for the year ended December 31, 2005, a cumulative translation loss of $0.5 million was transferred from accumulated other comprehensive income and included in general and administrative expenses as a result of the closure of our Sweden-based operation and the associated centralization of our European production animal diagnostics operations products manufacturing in Switzerland.
(n) Derivative Instruments and Hedging
We follow SFAS No. 133, “Accounting for Derivative Instruments and Hedging Activities” as amended by SFAS No. 137, “Accounting for Derivative Instruments and Hedging Activities—Deferral of the Effective Date of SFAS No. 133” and SFAS No. 138, “Accounting for Certain Derivative Instruments and Hedging Activities—An Amendment of SFAS No. 133” (“SFAS No. 133, as Amended”). SFAS No. 133, as Amended requires that all derivatives, including forward currency exchange contracts, be recognized on the balance sheet at fair value. Derivatives that are not hedges must be recorded at fair value through earnings. If a derivative is a hedge, depending on the nature of the hedge, changes in the fair value of the derivative are either offset against the change in fair value of the hedged item through earnings or recognized in other comprehensive income until the hedged item is recognized in earnings. We immediately record in earnings the extent to which a hedge is not effective in achieving offsetting changes in fair value.
The primary purpose of our foreign currency hedging activities is to protect against the volatility associated with foreign currency transactions. We also utilize some natural hedges to mitigate our transaction and commitment exposures. We enter into exchange contracts with large multinational financial institutions and we do not hold or engage in transactions involving derivative instruments for purposes other than risk management. Our accounting policies for these contracts are based on our designation of such instruments as hedging transactions. Market gains and losses are deferred in prepaid expenses or accruals, as appropriate, until the contract matures, which is the period when the related obligation is settled. Our subsidiaries enter into foreign currency exchange contracts to minimize the impact of foreign currency fluctuations associated with their anticipated intercompany inventory purchases for the next twelve months. From time to time, we may also enter into foreign currency exchange contracts to minimize the impact of foreign currency fluctuations associated with specific, significant transactions. We enter into currency exchange contracts for amounts that are less than the full value of forecasted intercompany sales and for amounts that are equivalent to, or less than, other specific, significant transactions, thus no significant ineffectiveness has resulted or been recorded through the statements of income.
In addition to hedges for anticipated 2007 intercompany inventory purchases, we had a foreign currency exchange contract outstanding at December 31, 2006 to hedge the repayment by our Canadian subsidiary of an intercompany loan denominated in U.S. Dollars that the subsidiary used to fund the acquisition of a veterinary reference laboratory business, which had a U.S dollar equivalent of $11.2 million at December 31, 2006. This contract expires on February 28, 2007, at which time the intercompany loan will be repaid using funds borrowed under our credit facility. At December 31, 2006, we recorded $2.0 million in unrealized losses through accumulated other comprehensive loss from foreign exchange contracts with 2007 expiration dates. At December 31, 2005, we recorded $0.8 million in unrealized gains through accumulated other comprehensive income from foreign exchange contracts with 2006 expiration dates. The foreign currency contracts, which extend through December 31, 2007 and 2006, respectively, consisted of the following(in thousands):
F-13
| U.S. Dollar Equivalent
| |
|---|
Currency Sold
| 2006
| | 2005
| |
|---|
| Euro | | | $ | 47,918 | | $ | 44,511 | |
| British Pound | | | | 20,650 | | | 18,046 | |
| Canadian Dollar | | | | 24,215 | | | 11,825 | |
| Swiss Franc | | | | 6,689 | | | 7,664 | |
| Australian Dollar | | | | 4,451 | | | 2,756 | |
| Japanese Yen | | | | 4,495 | | | 2,644 | |
|
| |
| |
| | | | $ | 108,418 | | $ | 87,446 | |
|
| |
| |
Gains and losses on foreign exchange contracts intended as hedges for intercompany sales of goods are recorded in cost of product revenue. Included in cost of product revenue are foreign exchange losses of $2.8 million, $0.1 million and $5.2 million for the years ended December 31, 2006, 2005 and 2004, respectively.
(o) Disclosure of Fair Value of Financial Instruments and Concentration of Risk
Financial instruments consist mainly of cash and cash equivalents, investments, accounts receivable, accounts payable and notes payable. Financial instruments that potentially subject us to concentrations of credit risk are principally cash, cash equivalents, investments and accounts receivable. We place our investments in highly rated financial institutions and investment grade money market funds and municipal bonds. Concentration of credit risk with respect to accounts receivable is limited to certain customers to whom we make substantial sales. To reduce risk, we routinely assess the financial strength of our customers and, as a consequence, believe that our accounts receivable credit risk exposure is limited. We maintain an allowance for potential credit losses, but historically have not experienced any significant credit losses related to an individual customer or group of customers in any particular industry or geographic area. The carrying amounts of our financial instruments approximate fair market value. See Note 16 for further discussion of concentration of credit risk of accounts receivable.
We currently purchase many products and materials from single sources or a limited number of sources. Some of the products that we purchase from these sources are proprietary, and, therefore, cannot be readily or easily replaced by alternative sources. If we are unable to obtain adequate quantities of these products in the future, we could face cost increases or reductions, delays or discontinuations in product shipments, which could have a material adverse effect on our results of operations.
(p) Comprehensive Income
SFAS No. 130, “Reporting Comprehensive Income,” requires us to report all changes in equity during a period, resulting from net income and transactions or other events and circumstances from non-owner sources, in a financial statement for the period in which they are recognized. We have chosen to disclose comprehensive income, which encompasses net income, foreign currency translation adjustments and the difference between the cost and the fair market value of investments in debt securities and foreign exchange contracts, in the Consolidated Statement of Stockholders’ Equity. We consider the foreign currency cumulative translation adjustment to be permanently invested and, therefore, have not provided income taxes on those amounts.
Accumulated other comprehensive income consists of the following at December 31, 2006 and 2005, respectively, (in thousands):
| December 31,
| |
|---|
| 2006
| | 2005
| |
|---|
| Unrealized loss on investments, net of tax | | | $ | -- | | $ | (46 | ) |
| Unrealized gain (loss) on forward exchange contracts, | | | | | | | | |
| net of tax | | | | (1,320 | ) | | 553 | |
| Cumulative translation adjustment | | | | 11,886 | | | 359 | |
|
| |
| |
| | | | $ | 10,566 | | $ | 866 | |
|
| |
| |
| | |
F-14
(q) Recent Accounting Pronouncements
In June 2006, the FASB issued FASB Interpretation (“FIN”) No. 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”). FIN 48 clarifies the accounting for uncertainty in income taxes recognized in financial statements and prescribes a comprehensive model for the recognition, measurement, and financial statement disclosure of uncertain tax positions taken or expected to be taken in tax returns. The provisions of FIN 48 are effective for fiscal years beginning after December 15, 2006. We are adopting the provisions of FIN 48 for the quarter ending March 31, 2007. We are studying this pronouncement and awaiting further interpretive guidance and, as such, we have not yet determined the expected impact of the implementation of FIN 48. We will record the change in net assets that results from the adoption of FIN 48 as of January 1, 2007 as an adjustment to retained earnings. While the ultimate realization of income tax benefits and expenses is not impacted by the provisions of FIN 48, the timing of recognition of the effects of uncertain tax positions in the results of operations for specific periods, and the financial position at specific points in time, prior to the ultimate settlement of such uncertain tax positions could be materially different under the provisions of FIN 48 compared to the timing of recognition under historical methods used prior to the adoption of FIN 48. The adoption of FIN 48 will not have an effect on our cash flows.
In June 2006, the FASB ratified the Emerging Issues Task Force (“EITF”) consensus on Issue 06-2, “Accounting for Sabbatical Leave and Other Similar Benefits Pursuant to FASB Statement No. 43, Accounting for Compensated Absences” (“EITF 06-2”). EITF 06-2 requires that the costs associated with unrestricted sabbaticals and other similar benefit arrangements be recognized over the service period during which the employee earns the benefit. We provide an additional four weeks of compensated personal growth leave to all U.S. salaried employees in their tenth anniversary year of employment and again at each fifth year thereafter. The provisions of EITF 06-2 are effective for fiscal years beginning after December 15, 2006. Accordingly, we will record a cumulative effect adjustment of approximately $1.8 million to recognize an estimated liability, net of a tax benefit of $1.2 million, as of January 1, 2007 for eligible employees’ rights to receive future paid leave. Beginning in 2007, we will recognize estimated costs for such future compensated leave benefits earned. We estimate that approximately $0.3 million of compensation expense, before tax, will be recognized during the year ending December 31, 2007 in accordance with EITF 06-2. The adoption of EITF 06-2 will not have an effect on our cash flows.
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS No. 157”). SFAS No. 157 establishes a framework for measuring fair value and expands financial statement disclosures about fair value measurements. The provisions of SFAS No. 157 are required as of the beginning of the first fiscal year beginning after November 15, 2007 and shall generally be applied prospectively. We are studying SFAS No. 157 and have not yet determined the expected impact of the implementation of this pronouncement on our financial position and results of operations, if any. The adoption of SFAS No. 157 will not have an effect on our cash flows.
In September 2006, the Securities and Exchange Commission issued Staff Accounting Bulletin (“SAB”) No. 108 (“SAB No. 108”) which provides guidance on the consideration of the effects of prior year misstatements in quantifying current year misstatements for the purpose of a materiality assessment. The provisions of SAB No. 108 are effective for the first annual financial statements for years ending after November 15, 2006. We adopted the provisions of SAB No. 108 as of December 31, 2006. The adoption of SAB No. 108 did not have a material effect on our financial position, results of operations or cash flows.
In September 2006, the FASB ratified the Emerging Issues Task Force consensus on Issue 06-1, “Accounting for Consideration Given by a Service Provider to Manufacturers or Resellers of Equipment Necessary for an End-Customer to Receive Service from the Service Provider” (“EITF 06-1”). EITF 06-1 requires that if the consideration given by a service provider to a manufacturer or reseller (that is not a customer of the service provider) can be linked contractually to the benefit received by the service provider’s customer, a service provider should account for the characterization of the consideration in accordance with EITF Issue 01-9. The provisions of EITF 06-1 are effective for the first annual reporting period beginning after June 15, 2007. We do not expect the adoption of EITF 06-1 to have a material effect on our financial position and results of operations. The adoption of EITF 06-1 will not have an effect on our cash flows.
F-15
NOTE 3 BUSINESS ACQUISITIONS
We paid cash of $23.9 million, $7.5 million and $53.6 million to acquire businesses during the years ended December 31, 2006, 2005 and 2004, respectively, and recognized liabilities, including contingent liabilities and deferred tax liabilities associated with purchase accounting, of $4.6 million, $2.0 million and $14.3 million, respectively. We also agreed to make subsequent purchase price payments to sellers for various acquisitions and issued a note payable of $1.0 million associated with a 2004 acquisition.
In connection with acquisitions of businesses and intangible assets, we have commitments outstanding at December 31, 2006 for additional purchase price payments of up to $3.9 million, of which $1.8 million is contingent on the achievement by certain acquired businesses of specified milestones. In addition to these purchase price payments of $3.9 million, we also have agreed to make payments of up to $0.8 million to sellers of certain acquired businesses that are conditional upon those sellers providing future services to IDEXX for specified periods of time. These contingent payments will be recognized as compensation and consulting expense over the remaining service periods when management deems payment to be probable.
The 2004 acquisitions included veterinary reference laboratories in the United States and Europe and production animal diagnostics businesses in the United States and Europe. Goodwill of $28.0 million and $7.4 million were assigned to the Companion Animal Group segment and the Production Animal Segment, respectively. Amortizable intangible assets of $15.1 million and $11.0 million were assigned to the Companion Animal Group segment and the Production Animal Segment, respectively.
The 2005 acquisitions included veterinary reference laboratories in the United States and Europe and a digital radiography business based in the United States. As of December 31, 2005, goodwill and amortizable intangible assets of $2.1 million and $5.3 million, respectively, were assigned to the Companion Animal Group segment. During the year ended December 31, 2006, we recognized incremental amortizable intangible assets of $1.9 million in connection with the finalization of purchase price allocations for certain 2005 acquisitions.
The 2006 acquisitions included veterinary reference laboratories in the United States, Canada and South Africa; a veterinary practice information management software business based in the United States; and certain intellectual property and distribution rights from a diagnostics company based in Australia. Goodwill and amortizable intangible assets of $11.0 million and $13.3 million, respectively, were assigned to the Companion Animal Group segment.
The results of operations of the acquired businesses have been included since their respective acquisition dates. Pro forma information has not been presented because such information is not material to the financial statements taken as a whole. The final purchase price allocations for certain 2006 acquisitions are subject to finalization of the valuation of certain assets and liabilities and, as a result, preliminary amounts assigned to assets and liabilities are subject to revision in future periods.
In January 2007, we acquired the Critical Care Division of Osmetech plc. The acquired business is based in the United States and develops, manufactures, and sells point-of-care electrolyte and blood gas analyzers and related consumable products for the human and veterinary diagnostics markets. We paid cash of approximately $45 million.
F-16
NOTE 4 SHARE-BASED COMPENSATION
Selected financial impacts of share-based compensation, excluding the impact of deferred stock units issued under our Director Deferred Compensation Plan or our Executive Deferred Compensation Plan that do not have vesting conditions (which are described below), are presented in the table below (in thousands, except per share amounts):
| For the
Year Ended
December 31,
2006
| |
|---|
| Share-based compensation expense included in cost of revenue | | | $ | 1,671 | |
| Share-based compensation expense included in operating expense | | | | 8,986 | |
|
| |
| Total share-based compensation expense | | | | 10,657 | |
|
| |
| Income tax benefit in net income for share-based compensation expense | | | | (1,845 | ) |
| Income tax benefit in net income for employees' disqualifying dispositions of shares | | |
| acquired through the exercise of stock options and employee stock purchase rights | | | | (57 | ) |
|
| |
| Total income tax benefit | | | | (1,902 | ) |
|
| |
| Net impact of share-based compensation on net income | | | $ | 8,755 | |
|
| |
| Net impact of share-based compensation on: | | | | | |
| Earnings per share, basic | | | $ | 0.28 | |
| Earnings per share, diluted | | | | 0.27 | |
Share-based compensation costs are classified in costs of sales and operating expenses consistently with the classification of cash compensation paid to the employees receiving such share-based compensation. Capitalized share-based employee compensation cost at December 31, 2006 was $0.2 million, which was included in inventory on the consolidated balance sheet.
Our financial statements for periods ending prior to January 1, 2006 have not been restated or revised. Had compensation cost for the Company’s share-based compensation for the years ended December 31, 2005 and 2004 been determined consistent with the provisions of SFAS No. 123, as Amended, the Company’s net income and net income per common share would have been reduced to the following pro forma amounts (in thousands, except per share amounts):
| For the Years Ended December 31,
| |
|---|
| 2005
| | 2004
| |
|---|
| Net income: | | | | | | | | |
| As reported | | | $ | 78,254 | | $ | 78,332 | |
| Pro forma share-based employee compensation, net of tax | | | | (8,701 | ) | | (7,975 | ) |
|
| |
| |
| Pro forma net income | | | $ | 69,553 | | $ | 70,357 | |
|
| |
| |
| Earnings per share: | | | | | | | | |
| Basic: as reported | | | $ | 2.41 | | $ | 2.29 | |
| Basic: pro forma | | | | 2.14 | | | 2.06 | |
| Diluted: as reported | | | | 2.30 | | | 2.19 | |
| Diluted: pro forma | | | | 2.05 | | | 1.97 | |
The following table represents cash proceeds from employees’ exercise of stock options and employee stock purchase rights and the reduction of income taxes payable due to employees’ share-based compensation tax events(in thousands):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Cash proceeds from employee stock purchases and option | | | | | | | | | | | |
| exercised under all share-based payment arrangements | | | $ | 20,922 | | $ | 18,841 | | $ | 19,376 | |
| | | | | | | | | | | | |
| Reduction of income taxes payable due to employee's | | | | | | | | | | | |
| share-based compensation tax | | | $ | 10,692 | | $ | 7,808 | | $ | 8,211 | |
F-17
Prior to the adoption of SFAS 123(R), we reported all income tax benefits resulting from the exercise of stock options as operating cash inflows in our consolidated statements of cash flows. SFAS 123(R) requires the benefits of tax deductions from the exercise of options in excess of the compensation cost for those options to be reported as financing cash inflows. FASB Staff Position (“FSP”) No. FAS 123(R)-3, “Transition Election Related to Accounting for the Tax Effects of Share-Based Payment Awards” provides an alternative transitional method of calculating the excess tax benefits available to absorb tax deficiencies recognized subsequent to the adoption of SFAS 123(R). In accordance with FSP No. FAS 123(R)-3, which we elected, the full amount of tax benefits related to exercises after December 31, 2005 of employee share-based compensation awards that were fully vested as of December 31, 2005 are reported as financing cash inflows. For the year ended December 31, 2006, $9.4 million of tax benefits were reported as financing cash inflows rather than operating cash inflows.
The fair value of options, restricted stock units, deferred stock units with vesting conditions, and employee stock purchase rights awarded during the years ended December 31, 2006, 2005 and 2004 totaled $11.9 million, $15.7 million and $13.4 million, respectively. The total unrecognized compensation cost for unvested share-based compensation awards outstanding at December 31, 2006, net of estimated forfeitures, was $14.6 million. Approximately $6.3 million is expected to be recognized in the year ending December 31, 2007 for outstanding awards and decreasing amounts of the total expense are expected to be recognized over the subsequent five years, resulting in a weighted average remaining expense recognition period of approximately 1.5 years.
Stock Incentive Plan
During 2003, our Board of Directors approved the 2003 Stock Incentive Plan, as amended (the “2003 Stock Plan”) pursuant to which our employees and Directors may receive various types of share-based incentives, including stock options, restricted stock units, stock appreciation rights and deferred stock units. A total of 1,850,000 shares of common stock are authorized for issuance under the 2003 Stock Plan, provided that no more than 1,500,000 shares will be available for the grant of incentive stock options, and no more than 600,000 shares will be available for awards other than stock options and stock appreciation rights (such as restricted stock). In addition, if any options granted under our prior plans, including the 1991 Stock Option Plan, the 1998 Stock Incentive Plan or the 2000 Director Option Plan, terminate, expire or are forfeited without having been exercised in full, the shares subject to such unexercised options are available for issuance under the 2003 Stock Plan. Options granted under the 2003 Stock Plan and prior plans may not be granted at an exercise price less than the fair market value of the common stock on the date granted (or less than 110% of the fair market value in the case of incentive stock options granted to holders of more than 10% of our Common Stock). Options may not be granted for a term of more than ten years. The vesting schedule of all options granted under the 2003 Stock Plan is determined by the Compensation Committee of the Board of Directors at the time of grant.
Options
Option awards are granted to employees with an exercise price equal to the closing market price of our common stock at the date of grant and generally vest ratably over five years on each anniversary of the date of grant, conditional on continuous service. Options granted to non-employee directors in 2005 vested fully on the first anniversary of the date of grant. Upon any change in control of the company, 25% of the unvested stock options then outstanding will vest and become exercisable. However, if the acquiring entity does not assume outstanding options, then all options will vest immediately prior to the change in control.
We use the Black-Scholes-Merton option-pricing model to determine the fair value of options granted. Option-pricing models require the input of highly subjective assumptions, particularly for the expected stock price volatility. Changes in the subjective input assumptions can materially affect the fair value estimate. Our expected stock price volatility assumptions are based on the historical volatility of our stock for the expected term and other relevant factors. The risk-free interest rate is based on the U.S. Treasury yields for the expected term in effect at the approximate date of grant. We have never paid any cash dividends on our common stock and we have no present intention to pay a dividend; therefore, we assumed that no dividends will be paid over the expected terms of option awards.
F-18
The use of the Black-Scholes-Merton option-pricing model, the general methods employed to develop the above-described option valuation assumptions, and the vesting conditions of option awards are consistent with prior periods. Beginning in 2006, the contractual terms of employee option grants were reduced from ten years to seven years and we elected to use the simplified method described in the Securities and Exchange Commission Staff Accounting Bulletin No. 107, which is based on vesting and contractual terms, to develop the expected term assumption for 2006 option awards. Additionally, beginning in 2006, share-based compensation expense is reduced for an estimate of the number of awards that are expected to be forfeited. The estimate is based on historical data and other factors, and compensation expense is adjusted for actual results.
The weighted average valuation assumptions used to determine the fair value of each option grant on the date of grant and the weighted average estimated fair values were as follows:
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Expected stock price volatility | | | | 30 | % | | 40 | % | | 40 | % |
| Expected term, in years | | | | 5.0 | | | 5.8 | | | 5.8 | |
| Risk-free interest rate | | | | 4.6 | % | | 4.2 | % | | 3.1 | % |
| Weighted average fair value of options granted | | | $ | 26.78 | | $ | 25.17 | | $ | 21.59 | |
A summary of the status of options granted under our share-based compensation plans at December 31, 2006, and changes during the year then ended, are presented in the table below:
| Number of
Options (000)
| | Weighted
Average
Exercise Price
| | Average
Remaining
Contractual
Term
| | Aggregate
Intrinsic Value
($000)
| |
|---|
| Outstanding at December 31, 2005 | | | | 3,747 | | $ | 35.17 | | | | | | | |
| Granted | | | | 165 | | | 76.67 | | | | | | | |
| Exercised | | | | (648 | ) | | 28.88 | | | | | | | |
| Forfeited | | | | (124 | ) | | 51.63 | | | | | | | |
| Expired | | | | (2 | ) | | 26.99 | | | | | | | |
|
| |
| | | |
| Outstanding at December 31, 2006 | | | | 3,138 | | $ | 38.02 | | | 5.7 | | $ | 129,537 | |
| | | | | | | | | | | | | | | |
| Fully vested at December 31, 2006 | | | | 1,787 | | $ | 29.99 | | | 4.9 | | $ | 88,093 | |
| Fully vested and expected to vest, at December 31, 2006 | | | | 3,079 | | $ | 37.58 | | | 5.7 | | $ | 128,482 | |
Intrinsic value represents the amount by which the market price of the common stock exceeded the exercise price of the options, before applicable income taxes. The closing sale price of the common stock was $79.30 on the last business day of the year ended December 31, 2006. During the years ended December 31, 2006, 2005 and 2004, the total intrinsic value of stock options exercised was $34.4 million, $25.4 million and $25.0 million, respectively.
The total fair value of options vested during the years ended December 31, 2006, 2005 and 2004 was $12.8 million, $10.3 million and $9.1 million, respectively.
Employee Stock Purchase Plan
During 1997, the Board of Directors approved the 1997 Employee Stock Purchase Plan, under which we reserved and may issue up to an aggregate of 620,000 shares of Common Stock in periodic offerings. Also during 1997, the Board of Directors approved the 1997 International Employee Stock Purchase Plan, under which we reserved and could issue up to an aggregate of 30,000 shares of Common Stock in semiannual offerings. The 1997 International Employee Stock Purchase Plan was terminated in February 2005, and there were no shares remaining thereunder at the time of termination.
F-19
Prior to July 1, 2005, stock was sold under each of these plans at 85% of its fair market value, as defined in the plans as the lower of the closing price of our common stock at the beginning of the period and the closing price of our common stock at the end of the period. For periods ended prior to July 1, 2005, in order to determine the pro forma impact under SFAS No. 123, as Amended, the fair value of the purchase rights issued under the employee stock purchase plan was estimated on the date of grant using the Black-Scholes-Merton option-pricing model. The following weighted average assumptions were used to determine the fair value of employee purchase rights for the six months ended June 30, 2005 and the year ended December 31, 2004:
| For the Periods Ended,
| |
|---|
| June 30, 2005
| | December 31, 2004
| |
|---|
| Expected stock price volatility | | | | 33.0 | % | | 33.0 | % |
| Expected term, in years | | | | 0.5 | | | 0.5 | |
| Risk-free interest rate | | | | 3.4 | % | | 2.0 | % |
| Dividend yield | | | | None | | | None | |
Effective July 1, 2005, we amended our employee stock purchase plan to provide that stock is sold at 85% of the closing price of the stock on the last day of the period and to change the subscription period from six months to three months. The fair value of purchase rights under the revised program equals the 15% discount from the market price at the exercise date, which is the last day of the subscription period.
The following summarizes information about purchase rights issued under the employee stock purchase plan(in thousands, except per share amounts):
| For the Year Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Number of purchase rights issued | | | | 31 | | | 39 | | | 44 | |
| Fair value per purchase right issued | | | $ | 12.48 | | $ | 12.33 | | $ | 12.38 | |
Restricted and Other Deferred Stock Units With Vesting Conditions
Restricted stock unit awards to employees either vest ratably over five years on each anniversary of the date of grant, or vest on the third anniversary of the date of grant. Vesting is conditional on continuous service. Restricted stock units are converted to an equivalent number of shares of common stock upon vesting. Upon any change in control of the company, 25% of the unvested restricted stock units then outstanding under the 2003 Stock Incentive Plan will vest, provided, however, that if the acquiring entity does not assume the restricted stock units, then all such units will vest immediately prior to the change in control. Deferred stock units with vesting conditions awarded to non-employee directors under the Director Deferred Compensation Plan vest fully on the first anniversary of the date of grant. Except upon a change in control, as defined in the Director Deferred Compensation Plan, or certain limited circumstances, all deferred stock units will be exchanged for an equivalent number of shares of common stock one year following a director’s resignation or retirement. Upon a change in control, unvested deferred stock units vest immediately.
The fair values of restricted and deferred stock units with vesting conditions are based on the closing sale price of the common stock on the date of grant. We use historical data and other factors to estimate employee termination behavior and to evaluate whether particular groups of employees have significantly different forfeiture behaviors. Share-based compensation expense is reduced for an estimate of the number of awards that are expected to be forfeited. The estimate is based on historical data and other factors, and compensation expense is adjusted for actual results.
The weighted average fair value per unit of restricted stock units granted during the year ended December 31, 2006 was $78.01. The weighted average fair value per unit of deferred stock units with vesting conditions granted during the year ended December 31, 2006 was $77.47. There were no restricted stock units or deferred stock units with vesting conditions granted in 2005 or 2004.
A summary of the status of restricted and other deferred stock units with vesting conditions granted under our share-based compensation plans at December 31, 2006, and changes during the period then ended, are presented in the table below:
F-20
| Number
of Units
(000)
| | Weighted
Average
Remaining
Contractual
Term
| | Aggregate
Intrinsic Value
($000)
| |
|---|
| Outstanding at December 31, 2005 | | | | -- | | | | | | | |
| Granted | | | | 91 | | | | | | | |
| Settled | | | | -- | | | | | | | |
| Forfeited | | | | (5 | ) | | | | | | |
|
| | | |
| Outstanding at December 31, 2006 | | | | 86 | | | 2.0 | | $ | 6,817 | |
| | | | | | | | | | | | |
| Fully vested at December 31, 2006 | | | | -- | | | -- | | | -- | |
| | | | | | | | | | | | |
| Fully vested and expected to vest, at December 31, 2006 | | | | 78 | | | 1.9 | | | 6,166 | |
Deferred Stock Units With No Vesting Conditions
Under our Director Deferred Compensation Plan, non-employee directors also may defer a portion of their cash fees in the form of vested deferred stock units, each of which represents the right to receive one unissued share of our common stock. Directors receive a number of deferred stock units equal to the amount of cash fees deferred divided by the closing sale price of the common stock on the date of deferral. Under our Executive Deferred Compensation Plan (the “Executive Plan”), certain members of our management may elect to defer a portion of their cash compensation in deferred stock units. These deferred stock units will be exchanged for a fixed number of shares of common stock on dates determined by the employee, subject to the limitations of the Executive Plan and applicable law. Except upon a change in control, as defined in the Director Deferred Compensation Plan and the Executive Plan, or certain other limited circumstances, directors and officers may not receive shares of common stock in settlement of deferred stock units earlier than one year following their resignation from the board or termination of their employment, respectively.
During the years ended December 31, 2006, 2005 and 2004, approximately 8,000, 11,000 and 10,000 deferred stock units valued at $0.7 million, $0.7 million and $0.5 million were issued, respectively.
During the year ended December 31, 2006, approximately 2,000 shares of common stock were issued to settle deferred stock units.
NOTE 5 CASH EQUIVALENTS AND SHORT-TERM INVESTMENTS
Cash equivalents are highly liquid investments purchased with original maturities of less than three months.
We account for investments under SFAS No. 115, “Accounting for Certain Investments in Debt and Equity Securities” as available-for-sale. Investments are recorded at amortized cost and adjusted to fair market value through other comprehensive income. Short-term investments, which had cost bases of $35.0 million and $65.7 million at December 31, 2006 and 2005, respectively, are investment securities with original maturities of greater than three months, but less than one year, from the balance sheet date and consist of the following(in thousands):
| December 31,
| |
|---|
| 2006
| | 2005
| |
|---|
| Municipal bonds | | | $ | -- | | $ | 19,263 | |
| Municipal auction rate securities | | | | 35,000 | | | 44,600 | |
| Canadian certificates of deposit | | | | -- | | | 1,717 | |
|
| |
| |
| | | | $ | 35,000 | | $ | 65,580 | |
|
| |
| |
At December 31, 2006 and 2005, we held $35.0 million and $65.6 million, respectively, of short-term investments, which included $35.0 million and $44.6 million, respectively, of auction rate municipal securities classified as available-for-sale securities. Our investments in these securities are recorded at cost, which approximates fair market value due to their variable interest rates, which typically reset every 28 to 35 days, and, despite the long-term nature of their stated contractual maturities, we have the ability to quickly liquidate these securities. As a result, we had no cumulative gross unrealized holding gains (losses) or gross realized gains (losses) from these short-term investments. All income generated from these short-term investments was recorded as interest income in the consolidated statements of income.
F-21
NOTE 6 OTHER NONCURRENT ASSETS, INTANGIBLE ASSETS AND GOODWILL
Other noncurrent assets are as follows(in thousands):
| December 31,
| |
|---|
Description
| 2006
| | 2005
| |
|---|
| Deferred tax asset | | | $ | 3,253 | | $ | 420 | |
| Cost of rental instruments sold under recourse, net | | | | 3,121 | | | 4,115 | |
| Other assets | | | | 3,116 | | | 2,453 | |
|
| |
| |
| | | | $ | 9,490 | | $ | 6,988 | |
|
| |
| |
Rental instruments sold under recourse are amortized over their estimated useful life of three years. Amortization expense of rental instruments sold under recourse was $2.7 million, $2.5 million and $2.0 million for the years ended December 31, 2006, 2005 and 2004, respectively.
Intangible assets other than goodwill consist of the following(in thousands):
| December 31, 2006
| | December 31, 2005
| |
|---|
| Cost
| | Accumulated
Amortization
| | Cost
| | Accumulated
Amortization
| |
|---|
| Patents | | | $ | 10,491 | | $ | 2,932 | | $ | 5,810 | | $ | 1,934 | |
| Other product rights | | | | 18,743 | | | 7,660 | | | 15,662 | | | 5,059 | |
| Customer-related intangible assets | | | | 25,955 | | | 3,496 | | | 15,814 | | | 1,878 | |
| Other, primarily noncompete agreements | | | | 3,521 | | | 1,269 | | | 3,207 | | | 1,003 | |
|
| |
| |
| |
| |
| | | | $ | 58,710 | | $ | 15,357 | | $ | 40,493 | | $ | 9,874 | |
|
| |
| |
| |
| |
Amortization expense of intangible assets was $5.4 million, $3.9 million and $1.6 million for the years ended December 31, 2006, 2005 and 2004, respectively.
During the year ended December 31, 2006, we acquired $13.3 million of amortizable intangible assets related to business acquisitions and $0.7 million related to the acquisition of the additional ownership interest in a joint venture in China. During the year ended December 31, 2006, we also recognized incremental amortizable intangible assets of $1.9 million in connection with the finalization of purchase price allocations for certain 2005 business acquisitions and we acquired licenses for $0.4 million. The weighted average amortization periods for patents, other product rights, customer-related intangible assets, and other intangible assets acquired during 2006 in connection with business acquisitions were 8 years, 3 years, 12 years and 7 years, respectively. During the year ended December 31, 2005, we acquired $5.3 million of amortizable intangible assets related to business acquisitions. The weighted average amortization periods for product rights other than patents, customer-related intangible assets, and other intangible assets acquired during 2005 in connection with business acquisitions were 5 years, 15 years and 4 years, respectively. See Notes 3 and 11 for additional information. The remaining change in the cost of intangible assets other than goodwill during the years ended December 31, 2006 and 2005 resulted primarily from changes in foreign currency exchange rates.
The aggregate amortization expense associated with intangible assets owned at December 31, 2006 is expected to be as follows for each of the next five years (in thousands):
| Amortization
Expense
| |
|---|
| 2007 | | | $ | 5,911 | |
| 2008 | | | | 5,499 | |
| 2009 | | | | 5,086 | |
| 2010 | | | | 4,709 | |
| 2011 | | | | 4,281 | |
F-22
Goodwill consists of the following (in thousands):
| December 31,
2006
| | December 31,
2005
| |
|---|
| CAG segment: | | | | | | | | |
| Rapid assay products | | | $ | 1,952 | | $ | -- | |
| Reference laboratory and consulting services | | | | 63,485 | | | 51,311 | |
| Practice information management systems and digital radiography | | | | 1,453 | | | 1,453 | |
| Pharmaceutical products | | | | 13,745 | | | 13,745 | |
| Other | | | | 117 | | | 113 | |
| Water segment | | | | 17,282 | | | 15,184 | |
| Production animal segment | | | | 6,792 | | | 6,321 | |
|
| |
| |
| | | | $ | 104,826 | | $ | 88,127 | |
|
| |
| |
During the year ended December 31, 2006, we acquired $11.0 million of goodwill (of which $2.0 million is expected to be tax deductible) related to business acquisitions. During the year ended December 31, 2005, we acquired $2.1 million of goodwill (of which $1.3 million is expected to be tax deductible) related to business acquisitions. During the year ended December 31, 2006, we also recognized purchase accounting adjustments related to goodwill. See Note 3 for additional information. The remaining changes in the cost of goodwill during the year ended December 31, 2006 resulted primarily from changes in foreign currency exchange rates.
NOTE 7 NOTES PAYABLE
In connection with the February 2004 acquisition of a veterinary reference laboratory, we issued a note payable to the sellers for $1.0 million. The note bears interest at the prime rate. The balance outstanding at December 31, 2005 was $0.6 million. We paid $0.4 million in February 2005 and the remaining $0.5 million, plus accrued interest, in March 2006.
In May 2006, we acquired our Westbrook, Maine facility. We paid cash of $11.5 million and assumed a mortgage that had a face value of $6.5 million and a stated interest rate of 9.875%. We recorded the mortgage at a fair market value of $7.5 million, based on an effective market interest rate of 6.05%. The mortgage is payable in equal monthly installments of approximately $0.1 million through May 1, 2015. Annual mortgage principal payments as of December 31, 2006, based on the fair market value of the mortgage at the assumption date, are as follows(in thousands):
Years Ending December 31,
| Amount
| |
|---|
| 2007 | | | $ | 678 | |
| 2008 | | | | 720 | |
| 2009 | | | | 765 | |
| 2010 | | | | 813 | |
| 2011 | | | | 863 | |
| Thereafter | | | | 3,286 | |
|
| |
| | | | $ | 7,125 | |
|
| |
In January 2007, we entered into an unsecured short-term revolving credit facility with a bank in the principal amount of $125.0 million that matures on June 30, 2007. The credit facility may be used for general corporate purposes, including repurchases of our common stock and business acquisitions. The applicable interest rates generally range from 0.375% to 0.875% above the London interbank rate or the Canadian Dollar-denominated bankers’ acceptance rate, dependent on our leverage ratio. Under the credit facility, we pay quarterly commitment fees of 0.08% to 0.20%, dependent on our leverage ratio, on any unused commitment. The credit facility contains financial and other affirmative and negative covenants, as well as customary events of default, that would allow any amounts outstanding under the credit facility to be accelerated, or restrict our ability to borrow thereunder, in the event of noncompliance. The financial covenant requires our ratio of debt to earnings before interest and taxes, as defined by the agreement, not to exceed 3-to-1.
F-23
NOTE 8 EXIT ACTIVITY
During the year ended December 31, 2005, we centralized our European production animal diagnostics manufacturing operations in Bern, Switzerland, the location of the production animal diagnostics company acquired in December 2004. In connection with this centralization, we ceased operations in Sweden. We recognized expenses of $1.0 million associated with this exit activity during the year ended December 31, 2005. The total costs included a cumulative translation adjustment write-off of $0.5 million, one-time employee termination benefits of $0.2 million, and building lease termination costs of $0.1 million, which are included in general and administrative expenses in the consolidated statement of income. The total costs also included one-time employee termination benefits of $0.2 million that are included in costs of product revenue. At December 31, 2005, accrued expenses included building lease termination costs of $0.1 million which were subsequently paid in January 2006. We did not incur significant expenses associated with this activity during the year ended December 31, 2006.
NOTE 9 INCOME TAXES
Earnings before income taxes for each year were as follows (in thousands):
| 2006
| | 2005
| | 2004
| |
|---|
| Domestic | | | $ | 100,446 | | $ | 85,401 | | $ | 78,605 | |
| International | | | | 30,457 | | | 33,523 | | | 32,892 | |
|
| |
| |
| |
| | | | $ | 130,903 | | $ | 118,924 | | $ | 111,497 | |
|
| |
| |
| |
The provisions for income taxes for the years ended December 31, 2006, 2005 and 2004 are comprised of the following (in thousands):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Current | | | | | | | | | | | |
| Federal | | | $ | 35,409 | | $ | 30,070 | | $ | 19,438 | |
| State | | | | 5,512 | | | 4,680 | | | 2,628 | |
| International | | | | 2,438 | | | 10,397 | | | 6,500 | |
|
| |
| |
| |
| | | | | 43,359 | | | 45,147 | | | 28,566 | |
|
| |
| |
| |
| Deferred | | | | | | | | | | | |
| Federal | | | | (4,064 | ) | | (3,020 | ) | | 5,328 | |
| State | | | | (555 | ) | | (277 | ) | | 556 | |
| International | | | | (1,516 | ) | | (1,180 | ) | | (1,285 | ) |
|
| |
| |
| |
| | | | | (6,135 | ) | | (4,477 | ) | | 4,599 | |
|
| |
| |
| |
| | | | $ | 37,224 | | $ | 40,670 | | $ | 33,165 | |
|
| |
| |
| |
The provisions for income taxes differ from the amounts computed by applying the statutory federal income tax rate as follows:
| December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| U.S. federal statutory rate | | | | 35.0 | % | | 35.0 | % | | 35.0 | % |
| State income tax, net of federal tax benefit | | | | 2.3 | | | 2.4 | | | 1.9 | |
| International income taxes | | | | (4.5 | ) | | (1.9 | ) | | (3.5 | ) |
| Extraterritorial income exclusions | | | | (1.0 | ) | | (0.6 | ) | | (0.7 | ) |
| Nontaxable interest income | | | | (0.5 | ) | | (0.6 | ) | | (0.6 | ) |
| Domestic manufacturing exclusions | | | | (0.4 | ) | | (0.5 | ) | | -- | |
| Tax on dividend repatriations | | | | -- | | | 0.5 | | | -- | |
| Other, net | | | | (2.5 | ) | | (0.1 | ) | | (2.4 | ) |
|
| |
| |
| |
| Effective tax rate | | | | 28.4 | % | | 34.2 | % | | 29.7 | % |
|
| |
| |
| |
F-24
Our effective income tax rate was 28.4% for the year ended December 31, 2006 compared with 34.2% for the year ended December 31, 2005. The majority of this rate differential resulted from the favorable impact of the resolution in 2006 of an IRS income tax audit for the years ended December 31, 2003 and 2004. As a result of completing this audit, we reduced previously accrued taxes and recognized a tax benefit of 3.7% of income before tax. Other items that decreased our effective tax rate for the year ended December 31, 2006 included a reduction of previously recorded international deferred tax liabilities as a result of obtaining certain multi-year tax incentives and the release of a valuation allowance on international deferred tax assets as a result of a subsidiary demonstrating consistent sustained profitability. In addition, the effective rate for the year ended December 31, 2006 was less than the effective rate for the year ended December 31, 2005 due to the incremental tax expense in 2005 on the repatriation of $30.0 million pursuant to theAmerican Jobs Creation Act of 2004. These rate reductions were partly offset by the nonrecognition, in 2006, of tax benefits on compensation expense for incentive stock options and employee stock purchase rights that were recorded in accordance with SFAS No. 123(R) effective January 1, 2006.
Our effective tax rate was 34.2% for the year ended December 31, 2005, compared with 29.7% for the year ended December 31, 2004. The majority of this rate differential resulted from the favorable impact of several rate reducing items occurring in 2004. The 2004 rate was favorably impacted by the resolution in 2004 of an IRS income tax audit through the year ended December 31, 2001. As a result of completing this audit, we reduced previously accrued taxes. Other rate reductions resulted from the release in 2004 of a valuation allowance on international deferred tax assets as a result of a foreign subsidiary demonstrating consistent sustained profitability and changes in certain state and international tax estimates. In addition, 2005 tax expense increased by $1.0 million and the 2005 effective income tax rate increased by 0.8 percentage points due to incremental taxes on repatriations of $30.0 million pursuant to theAmerican Jobs Creation Act of 2004.
The components of the net deferred tax asset (liability) included in the accompanying consolidated balance sheets are as follows (in thousands):
| 2006
| | 2005
| |
|---|
| Current
| | Long-Term
| | Current
| | Long-Term
| |
|---|
| Assets: | | | | | | | | | | | | | | |
| Accrued expenses | | | $ | 9,188 | | $ | -- | | $ | 8,380 | | $ | -- | |
| Accounts receivable reserves | | | | 401 | | | -- | | | 363 | | | -- | |
| Deferred revenue | | | | 2,482 | | | 1,989 | | | 2,198 | | | 2,453 | |
| Inventory basis differences | | | | 3,409 | | | -- | | | 2,612 | | | -- | |
| Property-based differences | | | | -- | | | 1,475 | | | -- | | | 366 | |
| Share-based compensation | | | | 784 | | | 1,750 | | | 507 | | | -- | |
| Other | | | | 20 | | | 5 | | | -- | | | 5 | |
| Net operating loss carryforwards | | | | -- | | | 4,019 | | | 58 | | | 4,424 | |
| Unrealized losses on foreign exchange | | | | | | | | | | | | | | |
| contracts and investments | | | | 659 | | | -- | | | 29 | | | -- | |
|
| |
| |
| |
| |
| Total assets | | | | 16,943 | | | 9,238 | | | 14,147 | | | 7,248 | |
|
| |
| |
| |
| |
| Valuation allowance | | | | (59 | ) | | (4,015 | ) | | (369 | ) | | (4,527 | ) |
|
| |
| |
| |
| |
| Total assets, net of valuation | | | | | | | | | | | | | | |
| allowance | | | | 16,884 | | | 5,223 | | | 13,778 | | | 2,721 | |
|
| |
| |
| |
| |
| Liabilities: | | | | | | | | | | | | | | |
| Cost of rental instruments sold under recourse | | | | -- | | | (774 | ) | | -- | | | (1,158 | ) |
| Property-based differences | | | | -- | | | (297 | ) | | -- | | | (410 | ) |
| Intangible basis differences | | | | -- | | | (7,920 | ) | | -- | | | (6,758 | ) |
| Unrealized gains on foreign exchange contracts | | | | -- | | | -- | | | (307 | ) | | -- | |
| Other | | | | (107 | ) | | (147 | ) | | -- | | | -- | |
|
| |
| |
| |
| |
| Total liabilities | | | | (107 | ) | | (9,138 | ) | | (307 | ) | | (8,326 | ) |
|
| |
| |
| |
| |
| Net deferred tax assets (liabilities) | | | $ | 16,777 | | | (3,915 | ) | $ | 13,471 | | $ | (5,605 | ) |
|
| |
| |
| |
| |
| | |
At December 31, 2006, we had United States federal domestic net operating loss carryforwards of approximately $0.1 million available to offset future taxable income. Net operating loss carryforwards expire at various dates through 2014. The Tax Reform Act of 1986 contains provisions that limit annual availability of the net operating loss carryforwards due to a more than 50% change in ownership that occurred upon the acquisition of some companies.
At December 31, 2006, we had net operating loss carryforwards in foreign and state jurisdictions of approximately $56.8 million available to offset future taxable income. Most of these net operating loss carryforwards expire at various dates through 2022 and the remainder have indefinite lives. We have recorded a valuation allowance for these assets because realizability is uncertain.
We consider the operating earnings of non-United States subsidiaries to be indefinitely invested outside the United States. No provision has been made for United States federal and state, or international taxes that may result from future remittances of undistributed earnings of non-United States subsidiaries, the cumulative amount of which is $111.4 million at December 31, 2006.
F-25
NOTE 10 EARNINGS PER SHARE
Basic earnings per share is computed by dividing net income by the weighted average number of shares of common stock and deferred stock units outstanding during the year. The computation of diluted earnings per share is similar to the computation of basic earnings per share, except that the denominator is increased for the assumed exercise of dilutive options and other potentially dilutive securities using the treasury stock method unless the effect is anti-dilutive.
The following is a reconciliation of shares outstanding for basic and diluted earnings per share (in thousands):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Shares Outstanding for Basic Earnings per Share: | | | | | | | | | | | |
| Weighted average shares outstanding | | | | 31,403 | | | 32,498 | | | 34,203 | |
| Weighted average deferred stock units outstanding | | | | 30 | | | 23 | | | 11 | |
|
| |
| |
| |
| | | | | 31,433 | | | 32,521 | | | 34,214 | |
|
| |
| |
| |
| Shares Outstanding for Diluted Earnings per Share: | | | | | | | | | | | |
| Shares outstanding for basic earnings per share | | | | 31,433 | | | 32,521 | | | 34,214 | |
| Dilutive effect of options issued to employees and directors | | | | 1,507 | | | 1,534 | | | 1,586 | |
| Dilutive effect of restricted stock units issued to employees | | | | 8 | | | -- | | | -- | |
| Dilutive effect of nonvested deferred stock units issued to | | | | | | | | | | | |
| directors | | | | 6 | | | -- | | | -- | |
|
| |
| |
| |
| | | | | 32,954 | | | 34,055 | | | 35,800 | |
|
| |
| |
| |
Certain deferred stock units outstanding are included in shares outstanding for basic and diluted earnings per share because the associated shares of our common stock are issuable for no cash consideration, the number of shares of our common stock to be issued is fixed and issuance is not contingent. See Note 4 for additional information regarding deferred compensation plans.
Certain options to acquire shares and restricted stock units have been excluded from the calculation of shares outstanding for dilutive earnings per share because they were anti-dilutive. The following table presents information concerning those anti-dilutive options(in thousands, except per share amounts):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Weighted average number of shares underlying anti-dilutive options | | | | 138 | | | -- | | | 24 | |
| Weighted average exercise price per underlying share of anti-dilutive | | | | | | | | | | | |
| options | | | $ | 76.67 | | $ | -- | | $ | 60.70 | |
| Weighted average number of shares underlying anti-dilutive restricted | | | | | | | | | | | |
| stock units | | | | 2 | | | -- | | | -- | |
The following table presents additional information concerning the exercise prices of vested and unvested options outstanding at the end of the period (in thousands, except per share amounts):
| December 31,
|
|---|
| 2006
| | 2005
| |
|---|
| Closing price per share of our common stock | | | $ | 79.30 | | $ | 71.98 | |
|
| |
| |
| Number of shares underlying options outstanding with exercise prices below the | | | | | | | | |
| closing price | | | | 3,138 | | | 3,747 | |
| Number of shares underlying options outstanding with exercise prices equal to | | | | | | | | |
| or above the closing price | | | | -- | | | -- | |
|
| |
| |
| Total number of shares underlying outstanding options | | | | 3,138 | | | 3,747 | |
|
| |
| |
F-26
NOTE 11 COMMITMENTS, CONTINGENCIES AND GUARANTEES
We lease our facilities under operating leases that expire through 2021. In addition, we are responsible for the real estate taxes and operating expenses related to these facilities. We also have lease commitments for automobiles and office equipment.
Minimum annual rental payments under these agreements are as follows (in thousands):
Years Ending December 31,
| Amount
| |
|---|
| 2007 | | | $ | 7,369 | |
| 2008 | | | | 6,593 | |
| 2009 | | | | 5,343 | |
| 2010 | | | | 4,162 | |
| 2011 | | | | 3,605 | |
| Thereafter | | | | 5,450 | |
|
| |
| | | | $ | 32,522 | |
|
| |
Rent expense charged to operations under operating leases was approximately $8.8 million, $7.7 million and $6.6 million for the years ended December 31, 2006, 2005 and 2004, respectively.
We purchase the slides sold for use in our VetTest® Chemistry Analyzers under an agreement with Ortho that, as of December 31, 2006, required us to purchase a minimum of $59.0 million of slides through 2010. We purchase our VetAutoread™ Hematology Analyzers, components and consumables under an agreement under which we are required to make aggregate minimum purchases of $15.7 million through 2020, in addition to the payment obligations described in the section below titled “Guarantees.” We also have commitments under certain other agreements that commit us to aggregate future payments of $19.7 million. In addition, we have various minimum royalty payments due through 2024 of $15.1 million.
In connection with the acquisitions of businesses and intangible assets, we have commitments outstanding at December 31, 2006 for additional purchase price payments of up to $3.9 million, of which $1.8 million is contingent on the achievement by certain acquired businesses of specified milestones.
We previously had a 40% equity interest in a joint venture to market production animal diagnostic products in China. In April 2006, we paid $0.6 million to acquire an additional 55% equity interest in the joint venture from our partner and we also committed to pay an additional $0.2 million over two years in consideration for the additional equity. In addition, the joint venture entered into a contract with the joint venture partner where the partner will provide promotional and agency services and will receive sales commissions at rates escalating from 2.5% to 8.5% annually based on sales volume. In connection with this step acquisition, we recognized $0.7 million of intangible assets in the Production Animal Segment.
Contingencies
We are subject to claims that arise in the ordinary course of business, including with respect to actual and threatened litigation and other matters. We accrue contingent liabilities when it is probable that future expenditures will be made and such expenditures can reasonably be estimated. However, our actual losses with respect to these contingencies could exceed our accruals.
In October 2004, we resolved a contingent liability for a third-party claim related to alleged patent infringement. As a result, we recognized reductions of previously accrued expenses during 2004 of $1.8 million in cost of product revenue.
Under our workers’ compensation insurance policy for U.S. employees for the years ended December 31, 2006, 2005, 2004 and 2003, we retain the first $250,000 in claim liability per incident and $3.1 million, $2.8 million, $3.0 million and $1.4 million, respectively, in aggregate claim liability. We entered into a similar workers’ compensation insurance policy effective January 1, 2007. The insurance company administers and pays these claims, and we reimburse the insurance company for our portion of these claims. The insurance company provides insurance for claims above the individual occurrence and aggregate limits. We estimate claim liability based on claims incurred and the estimated ultimate cost to settle the claims. Based on this analysis, we have recognized cumulative expenses of $1.3 million, $0.6 million, $0.7 million and $0.8 million for claims incurred during the years ended December 31, 2006, 2005, 2004 and 2003, respectively. In connection with these policies, we have outstanding letters of credit totaling $2.1 million to the insurance companies as security for these claims.
F-27
Under our employee health care insurance policy, we retain claims liability risk up to $125,000 per incident and an aggregate claim limit based on the number of employees enrolled in the plan per month. We estimate our liability for the uninsured portion of employee health care obligations based on individual and aggregate coverage, our claims experience, the number of employees enrolled in the program, and the average time from when a claim is incurred to the time it is reported. Should actual employee health care claims liability exceed estimates, we are liable for up to an additional $1.5 million for potential uninsured obligations at December 31, 2006. We have insurance coverage of $1.0 million for claims above the aggregate limit. Should employee health insurance claims exceed this coverage, we would have further obligations for the amount in excess of such coverage.
We have entered into employment agreements with two of our officers whereby payments may be required if we terminate their employment without cause other than following a change in control. The amounts payable are based upon the executives’ salaries at the time of termination and the cost to us of continuing to provide certain benefits. Had both of such officers been terminated as of December 31, 2006, we would have had aggregate obligations for salaries and benefits of approximately $2.0 million under such agreements. We have entered into employment agreements with each of our officers that require us to make certain payments in the event the officer’s employment is terminated under certain circumstances within a certain period following a change in control of our stock. The amounts payable by us under these agreements is based on the officer’s salary and bonus history at the time of termination and the cost to us of continuing to provide certain benefits. Had all of our officers been terminated following a change in control as of December 31, 2006, we would have had aggregate obligations of approximately $11.5 million under these agreements. These agreements also provide for the acceleration of the vesting of all stock options and restricted stock units upon any qualifying termination following a change in control.
From time to time, we have received notices alleging that our products infringe third-party proprietary rights, although we are not aware of any pending litigation with respect to such claims. Patent litigation frequently is complex and expensive, and the outcome of patent litigation can be difficult to predict. There can be no assurance that we will prevail in any infringement proceedings that may be commenced against us. If we lose any such litigation, we may be stopped from selling certain products and/or we may be required to pay damages as a result of the litigation.
Guarantees
The following is a summary of our agreements and obligations that we have determined to be within the scope of FIN 45, “Guarantor’s Accounting and Disclosure Requirements for Guarantees Including Indirect Guarantees of Indebtedness of Others, an Interpretation of FASB No. 5, 57 and 107 and a Rescission of FASB Interpretation No. 34” (“FIN 45”).
In October 2005, our former supplier of VetAutoread™ Hematology Analyzers and consumables sold this business (including the human hematology testing products division) and we simultaneously entered into a new supply agreement for these products with the acquirer of the business. Under this new supply agreement, we received fixed pricing on certain products through December 31, 2020, among other benefits. In partial consideration for this new supply agreement, we paid cash of $2.5 million to the acquirer and guaranteed the acquirer’s note (the “Note”) in the principal amount of $3.5 million given to our former supplier in partial consideration for the business. The acquirer is obligated to pay the Note through quarterly principal and interest payments through 2008 and to pay the remaining balance in 2008. The principal balance of the note that we have guaranteed is $2.6 million at December 31, 2006.We are obligated to make a second payment of $1.25 million upon the achievement of certain milestones by the acquirer, which we expect to occur in 2007, and a third payment of $1.25 million twelve months later. Our obligations to make the second and third payments are subject to the acquirer’s payment of all amounts under the Note and the release of our guaranty. We recorded the fair value of the guaranty of $0.5 million and recognized the associated assets as of the effective date of the agreements.
We enter into agreements with third parties in the ordinary course of business under which we are obligated to indemnify such third parties for and against various risks and losses. The precise terms of such indemnities vary with the nature of the agreement. In many cases, we limit the maximum amount of our indemnification obligations, but in some cases, those obligations may be theoretically unlimited. We have not incurred material expenses in discharging any of these indemnification obligations, and based on our analysis of the nature of the risks involved, we believe that the fair value of these agreements is minimal. Accordingly, we have recorded no liabilities for these obligations as of December 31, 2006 and 2005.
F-28
When acquiring a business, we sometimes assume liability for certain events or occurrences that took place prior to the date of acquisition. However, we do not believe that we have any probable pre-acquisition liabilities or guarantees that should be recognized as of December 31, 2006 and 2005.
NOTE 12 PREFERRED STOCK
Our Board of Directors is authorized, subject to any limitations prescribed by law, without further stockholder approval, to issue from time to time up to 500,000 shares of Preferred Stock, $1.00 par value per share (“Preferred Stock”), in one or more series. Each such series of Preferred Stock shall have such number of shares, designations, preferences, voting powers, qualifications and special or relative rights or privileges as shall be determined by the Board of Directors, which may include, among others, dividend rights, voting rights, redemption and sinking fund provisions, liquidation preferences, conversion rights and preemptive rights.
Series A Junior Participating Preferred Stock
On December 17, 1996, we designated 100,000 shares of Preferred Stock as Series A Junior Participating Preferred Stock (“Series A Stock”) in connection with the adoption of our Shareholder Rights Plan. In general, each share of Series A Stock will: (i) be entitled to a minimum preferential quarterly dividend of $10 per share and to an aggregate dividend of 1,000 times the dividend declared per share of Common Stock, (ii) in the event of liquidation, be entitled to a minimum preferential liquidation payment of $1,000 per share (plus accrued and unpaid dividends) and to an aggregate payment of 1,000 times the payment made per share of Common Stock, (iii) have 1,000 votes, voting together with the Common Stock, (iv) in the event of any merger, consolidation or other transaction in which Common Stock is exchanged, be entitled to receive 1,000 times the amount received per share of Common Stock and (v) not be redeemable. These rights are protected by customary anti-dilution provisions. There are no shares of Series A Stock outstanding.
NOTE 13 PREFERRED STOCK PURCHASE RIGHTS
On December 17, 1996, we adopted a Shareholder Rights Plan and declared a dividend of one preferred stock purchase right for each outstanding share of Common Stock to stockholders of record at the close of business on December 30, 1996. Those rights expired December 31, 2006. There are no preferred stock purchase rights outstanding.
NOTE 14 TREASURY STOCK
The board of directors has authorized the repurchase of up to 18,000,000 shares of our common stock in the open market or in negotiated transactions. We believe that the repurchase of our common stock is a favorable investment and we also repurchase to offset the dilutive effect of our employee share-based compensation programs. Repurchases of our common stock may vary depending upon the level of other investing activities and the share price.
From the inception of the program in August 1999 to December 31, 2006, we repurchased 15,285,000 shares for $571.8 million. From the inception of the program to December 31, 2006, we also received approximately 171,000 shares of stock with a market value of $6.0 million that were surrendered by employees in payment for the minimum required withholding taxes due on the exercise of stock options, vesting of restricted stock units and settlement of deferred stock units, and in payment for the exercise price of stock options.
F-29
Information about our treasury stock purchases and other receipts is presented in the table below (in thousands, except per share amounts):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Increase in the number of treasury shares | | | | 1,338 | | | 1,993 | | | 2,415 | |
| Total cost of treasury shares | | | $ | 105,729 | | $ | 123,770 | | $ | 128,878 | |
| Average cost per share | | | $ | 79.02 | | $ | 62.11 | | $ | 53.37 | |
NOTE 15 IDEXX RETIREMENT AND INCENTIVE SAVINGS PLAN
We have established the IDEXX Retirement and Incentive Savings Plan (the “401(k) Plan”). Employees eligible to participate in the 401(k) Plan may contribute specified percentages of their salaries, a portion of which will be matched by us. We matched $2.7 million, $2.4 million, and $2.0 million for the years ended December 31, 2006, 2005 and 2004, respectively. In addition, we may make contributions to the 401(k) Plan at the discretion of the Board of Directors. There were no discretionary contributions in 2006, 2005 and 2004.
NOTE 16 SEGMENT REPORTING
We disclose information regarding our segments in accordance with the provisions of SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information” (“SFAS No. 131”). SFAS No. 131 requires disclosures about operating segments in annual financial statements and requires selected information about operating segments in interim financial statements. It also requires related disclosures about products and services and geographic areas. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision-maker, or decision-making group, in deciding how to allocate resources and in assessing performance. Our chief operating decision-maker is the Chief Executive Officer.
We are organized into business units by market and customer group. Our reportable segments include: products and services for the veterinary market, which we refer to as our Companion Animal Group (“CAG”), water quality products (“Water”), and products for production animal health, which we refer to as the Production Animal Segment (“PAS”). CAG develops, designs, manufactures, and distributes products and performs services for veterinarians. Water develops, designs, manufactures and distributes products to detect contaminants in water. PAS develops, designs, manufactures and distributes products to detect disease in production animals. Our operating segment that comprises products for dairy quality does not meet the quantitative thresholds described in SFAS 131 and is presented in an “Other” category. Unallocated items that are not allocated to our operating segments are comprised primarily of share-based compensation costs (effective January 1, 2006), corporate research and development expenses, interest income and expense, and income taxes. The segment information for the years ended December 31, 2005 and 2004 has been restated to conform to our presentation of reportable segments for the year ended December 31, 2006. Previously, PAS and Dairy were aggregated into a single reportable segment, which we referred to as the Food Diagnostics Group.
F-30
The accounting policies of the operating segments are the same as those described in the summary of significant accounting policies except that most interest income and expenses and income taxes are not allocated to individual operating segments. Below is our segment information (in thousands):
| For the Years Ended December 31,
| |
|---|
| CAG
| | Water
| | PAS
| | Other
| | Unallocated
Amounts
| | Consolidated
Total
| |
|---|
| 2006 | | | | | | | | | | | | | | | | | | | | |
| Revenues | | | $ | 606,319 | | $ | 58,466 | | $ | 58,940 | | $ | 15,392 | | $ | -- | | $ | 739,117 | |
|
| |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | $ | 100,760 | | $ | 25,762 | | $ | 16,172 | | | 1,852 | | $ | (16,613 | ) | $ | 127,933 | |
|
| |
| |
| |
| |
| | |
| Interest income, net | | | | | | | | | | | | | | | | | | | 2,817 | |
| | | | | |
| |
| Income before provisions for income | | | | | | | | | | | | | | | | | | | | |
| taxes and partner's interest | | | | | | | | | | | | | | | | | | | 130,750 | |
| Provision for income taxes | | | | | | | | | | | | | | | | | | | 37,224 | |
| Partner's interest in loss of subsidiary | | | | | | | | | | | | | | | | | | | (152 | ) |
| | | | | |
| |
| Net income | | | | | | | | | | | | | | | | | | $ | 93,678 | |
| | | | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | $ | 25,643 | | $ | 647 | | $ | 3,456 | | $ | 70 | | $ | -- | | $ | 29,816 | |
| Segment assets | | | | 353,585 | | | 35,042 | | | 38,516 | | | 4,184 | | | 128,233 | | | 559,560 | |
| Expenditures for long-lived assets (1) | | | | 49,448 | | | 2,345 | | | 2,352 | | | 13 | | | -- | | | 54,158 | |
| For the Years Ended December 31,
| |
|---|
| CAG
| | Water
| | PAS
| | Other
| | Unallocated
Amounts
| | Consolidated
Total
| |
|---|
| 2005 | | | | | | | | | | | | | | | | | | | | |
| Revenues | | | $ | 520,830 | | $ | 56,760 | | $ | 44,945 | | $ | 15,560 | | $ | -- | | $ | 638,095 | |
|
| |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | $ | 82,970 | | $ | 25,974 | | $ | 7,317 | | | 2,577 | | $ | (3,507 | ) | $ | 115,331 | |
|
| |
| |
| |
| |
| | |
| Interest income, net | | | | | | | | | | | | | | | | | | | 3,141 | |
| | | | | |
| |
| Income before provisions for income | | | | | | | | | | | | | | | | | | | | |
| taxes and partner's interest | | | | | | | | | | | | | | | | | | | 118,472 | |
| Provision for income taxes | | | | | | | | | | | | | | | | | | | 40,670 | |
| Partner's interest in loss of subsidiary | | | | | | | | | | | | | | | | | | | (452 | ) |
| | | | | |
| |
| Net income | | | | | | | | | | | | | | | | | | $ | 78,254 | |
| | | | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | $ | 21,236 | | $ | 447 | | $ | 2,667 | | $ | 19 | | $ | -- | | $ | 24,369 | |
| Segment assets | | | | 266,207 | | | 29,685 | | | 34,107 | | | 3,981 | | | 156,696 | | | 490,676 | |
| Expenditures for long-lived assets (1) | | | | 23,402 | | | 119 | | | 1,172 | | | 139 | | | -- | | | 24,832 | |
| For the Years Ended December 31,
| |
|---|
| CAG
| | Water
| | PAS
| | Other
| | Unallocated
Amounts
| | Consolidated
Total
| |
|---|
| 2004 | | | | | | | | | | | | | | | | | | | | |
| Revenues | | | $ | 448,687 | | $ | 53,098 | | $ | 31,690 | | $ | 15,706 | | $ | -- | | $ | 549,181 | |
|
| |
| |
| |
| |
| |
| |
| Income (loss) from operations | | | $ | 77,123 | | $ | 24,259 | | $ | 7,049 | | | 2,782 | | $ | (3,178 | ) | $ | 108,035 | |
|
| |
| |
| |
| |
| | |
| Interest income, net | | | | | | | | | | | | | | | | | | | 3,068 | |
| | | | | |
| |
| Income before provisions for income | | | | | | | | | | | | | | | | | | | | |
| taxes and partner's interest | | | | | | | | | | | | | | | | | | | 111,103 | |
| Provision for income taxes | | | | | | | | | | | | | | | | | | | 33,165 | |
| Partner's interest in loss of subsidiary | | | | | | | | | | | | | | | | | | | (394 | ) |
| | | | | |
| |
| Net income | | | | | | | | | | | | | | | | | | $ | 78,332 | |
| | | | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | $ | 16,794 | | $ | 507 | | $ | 1,109 | | $ | 17 | | $ | -- | | $ | 18,427 | |
| Segment assets | | | | 263,858 | | | 30,832 | | | 34,780 | | | 5,040 | | | 179,727 | | | 514,237 | |
| Expenditures for long-lived assets (1) | | | | 27,541 | | | 694 | | | 3,613 | | | 17 | | | -- | | | 31,865 | |
| (1) | Expenditures for long-lived assets exclude expenditures for intangible assets. See Note 3 for information regarding acquisitions of goodwill and other intangible assets in connection with business acquisitions. Expenditures for long-lived assets for the year ended December 31, 2006 include $2.5 million for property acquired in connection with CAG business acquisitions and $19.0 million related to the purchase of our Westbrook, Maine headquarters facility, of which $7.5 million was financed through the assumption of a mortgage. Expenditures for long-lived assets for the year ended December 31, 2005 include $0.6 million for property acquired in connection with CAG business acquisitions. Expenditures for long-lived assets for the year ended December 31, 2004 include $2.1 million and $0.7 million for property acquired in connection with CAG and PAS business acquisitions, respectively. |
F-31
Revenues by product and service categories were as follows (in thousands):
| December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| CAG segment revenue: | | | | | | | | | | | |
| Instruments and consumables | | | $ | 242,312 | | $ | 217,537 | | $ | 197,939 | |
| Rapid assay products | | | | 114,536 | | | 100,255 | | | 93,506 | |
| Reference laboratory and consulting services | | | | 187,114 | | | 156,425 | | | 118,596 | |
| Practice information management systems and digital radiography | | | | 44,427 | | | 32,589 | | | 28,163 | |
| Pharmaceutical products | | | | 17,930 | | | 14,024 | | | 10,483 | |
|
| |
| |
| |
| Net CAG segment revenue | | | | 606,319 | | | 520,830 | | | 448,687 | |
|
| |
| |
| |
| Net water segment revenue | | | | 58,466 | | | 56,760 | | | 53,098 | |
| Net production animal segment revenue | | | | 58,940 | | | 44,945 | | | 31,690 | |
| Other segment revenue | | | | 15,392 | | | 15,560 | | | 15,706 | |
|
| |
| |
| |
| Net revenue | | | $ | 739,117 | | $ | 638,095 | | $ | 549,181 | |
|
| |
| |
| |
Revenue by principal geographic area, based on customers’ domiciles, was as follows (in thousands):
| For the Years Ended December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Americas | | | | | | | | | | | |
| United States | | | $ | 478,172 | | $ | 418,565 | | $ | 373,615 | |
| Canada | | | | 22,070 | | | 18,428 | | | 16,486 | |
| Other Americas | | | | 6,076 | | | 6,235 | | | 4,766 | |
|
| |
| |
| |
| | | | | 506,318 | | | 443,228 | | | 394,867 | |
|
| |
| |
| |
| Europe | | | | | | | | | | | |
| United Kingdom | | | | 53,296 | | | 46,419 | | | 43,365 | |
| Germany | | | | 45,391 | | | 38,994 | | | 20,595 | |
| France | | | | 26,884 | | | 19,300 | | | 15,148 | |
| Other Europe | | | | 62,251 | | | 49,468 | | | 35,045 | |
|
| |
| |
| |
| | | | | 187,822 | | | 154,181 | | | 114,153 | |
|
| |
| |
| |
| | | |
| Asia Pacific Region | | | | | | | | | | | |
| Japan | | | | 19,271 | | | 17,531 | | | 16,533 | |
| Australia | | | | 17,378 | | | 15,618 | | | 16,308 | |
| Other Asia Pacific | | | | 8,328 | | | 7,537 | | | 7,320 | |
|
| |
| |
| |
| | | | | 44,977 | | | 40,686 | | | 40,161 | |
|
| |
| |
| |
| Total | | | $ | 739,117 | | $ | 638,095 | | $ | 549,181 | |
|
| |
| |
| |
Our largest customers are our U.S. distributors of our products in the CAG segment. In 2005, two of our CAG distributors merged and, as a result, the combined company, Butler Animal Health Supply, LLC, accounted for 9% and 10% of our 2006 and 2005 revenue, respectively, and 5% and 4% of our net accounts receivable at December 31, 2006 and 2005, respectively.
F-32
Net long-lived assets by principal geographic areas include net property and equipment, goodwill and other intangible assets. These long-lived assets are subject to geographic risks because they are generally difficult to move and effectively utilize in another geographic area in a reasonable time period and because they are relatively illiquid. Net long-lived assets by principal geographic areas were as follows (in thousands):
| December 31,
| |
|---|
| 2006
| | 2005
| | 2004
| |
|---|
| Americas | | | | | | | | | | | |
| United States | | | $ | 133,989 | | $ | 97,268 | | $ | 94,573 | |
| Canada | | | | 16,392 | | | 174 | | | 170 | |
|
| |
| |
| |
| | | | | 150,381 | | | 97,442 | | | 94,743 | |
|
| |
| |
| |
| Europe | | | | | | | | | | | |
| United Kingdom | | | | 30,926 | | | 26,878 | | | 25,336 | |
| Germany | | | | 34,797 | | | 32,282 | | | 35,817 | |
| Switzerland | | | | 18,166 | | | 17,009 | | | 20,351 | |
| France | | | | 2,091 | | | 1,297 | | | 58 | |
| Netherlands | | | | 2,895 | | | 2,632 | | | 2,505 | |
| Other Europe | | | | 1,410 | | | 80 | | | 659 | |
|
| |
| |
| |
| | | | | 90,285 | | | 80,178 | | | 84,726 | |
|
| |
| |
| |
| Asia Pacific Region | | | | | | | | | | | |
| Japan | | | | 468 | | | 386 | | | 518 | |
| Australia | | | | 6,375 | | | 5,846 | | | 6,454 | |
| Other Asia Pacific | | | | 298 | | | 592 | | | 683 | |
|
| |
| |
| |
| | | | | 7,141 | | | 6,824 | | | 7,655 | |
|
| |
| |
| |
| Total | | | $ | 247,807 | | $ | 184,444 | | $ | 187,124 | |
|
| |
| |
| |
NOTE 17 SUMMARY OF QUARTERLY DATA (UNAUDITED)
A summary of quarterly data follows (in thousands, except per share data):
| For the Quarters Ended
| |
|---|
| March 31,
| | June 30,
| | September 30,
| | December 31,
| |
|---|
| 2006 | | | | | | | | | | | | | | |
| Revenue | | | $ | 168,164 | | $ | 191,364 | | $ | 187,380 | | $ | 192,209 | |
| Gross profit | | | | 86,025 | | | 99,036 | | | 98,199 | | | 96,269 | |
| Operating income | | | | 26,975 | | | 37,026 | | | 34,462 | | | 29,470 | |
| Net income | | | | 18,273 | | | 25,780 | | | 24,953 | | | 24,672 | |
| Earnings per share: | | | | | | | | | | | | | | |
| Basic | | | $ | 0.57 | | $ | 0.82 | | $ | 0.80 | | $ | 0.79 | |
| Diluted | | | $ | 0.55 | | $ | 0.78 | | $ | 0.76 | | $ | 0.75 | |
| | | | | | | | | | | | | | | |
| 2005 | | | | | | | | | | | | | | |
| Revenue | | | $ | 152,426 | | $ | 160,630 | | $ | 158,069 | | $ | 166,970 | |
| Gross profit | | | | 76,080 | | | 80,575 | | | 81,329 | | | 84,916 | |
| Operating income | | | | 26,138 | | | 28,886 | | | 30,123 | | | 30,184 | |
| Net income | | | | 17,690 | | | 19,933 | | | 20,604 | | | 20,027 | |
| Earnings per share: | | | | | | | | | | | | | | |
| Basic | | | $ | 0.54 | | $ | 0.61 | | $ | 0.63 | | $ | 0.63 | |
| Diluted | | | $ | 0.51 | | $ | 0.59 | | $ | 0.61 | | $ | 0.60 | |
F-33
SCHEDULE II
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
VALUATION AND QUALIFYING ACCOUNTS
(in thousands)
| Balance at
Beginning
of Year
| | Charges to
Costs and
Expenses
| | Write-Offs/
Cash
Payments
| | Balance
at End
of Year
| |
|---|
| Reserves for doubtful accounts receivable: | | | | | | | | | | | | | | |
| December 31, 2004 | | | $ | 1,950 | | $ | (294 | ) | $ | 162 | | $ | 1,494 | |
| December 31, 2005 | | | | 1,494 | | | 121 | | | 394 | | | 1,221 | |
| December 31, 2006 | | | | 1,221 | | | 1,070 | | | 508 | | | 1,783 | |
| | | | | | | | | | | | | | | |
| Valuation allowance for deferred tax assets: | | | | | | | | | | | | | | |
| December 31, 2004 | | | $ | 5,627 | | $ | (615 | ) | $ | 69 | | $ | 4,943 | |
| December 31, 2005 | | | | 4,943 | | | 541 | | | 588 | | | 4,896 | |
| December 31, 2006 | | | | 4,896 | | | (88 | ) | | 734 | | | 4,074 | |
F-34
