| UNITED STATES | ||||||||||||||
| SECURITIES AND EXCHANGE COMMISSION | ||||||||||||||
| Washington, D.C. 20549 | ||||||||||||||
FORM 10-K
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the fiscal year ended December 31, 2021
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the transition period from to
Commission file number: 000-33001
NATUS MEDICAL INCORPORATED
(Exact name of registrant as specified in its charter)
| Delaware | 77-0154833 | |||||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |||||||
3150 Pleasant View Road, Middleton, WI 53562
(Address of principal executive offices) (Zip Code)
(608) 829-8500
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
| Common Stock, $0.001 par value per share | NTUS | The Nasdaq Stock Market LLC | ||||||
| (The Nasdaq Global Select Market) | ||||||||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large Accelerated Filer | ☒ | Accelerated filer | ☐ | ||||||||||||||
| Non-accelerated filer | ☐ | Smaller reporting company | ☐ | ||||||||||||||
| Emerging growth company | ☐ | ||||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared and issued its audit report.☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
As of June 30, 2021, the last business day of Registrant’s most recently completed second fiscal quarter, there were 34,132,106 shares of Registrant’s common stock outstanding, and the aggregate market value of such shares held by non-affiliates of Registrant (based upon the closing sale price of such shares on the Nasdaq Global Select Market on June 30, 2021) was $886,752,114. Shares of Registrant’s common stock held by each executive officer and director and by each entity that owns 5% or more of Registrant’s outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
On February 18, 2022, the registrant had 34,408,154 shares of its common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of the Registrant's Definitive Proxy Statement for the 2022 Annual Meeting of Stockholders or an amendment to this Annual Report on Form 10-K, to be filed with the Securities and Exchange Commission, are incorporated by reference into Part III of this Annual Report on Form 10-K.
Auditor Data Elements
Auditor Name: KPMG LLP Auditor Location: San Francisco, CA Auditor Firm ID: 185
NATUS MEDICAL INCORPORATED
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
| ITEM 1. | ||||||||
| ITEM 1A. | ||||||||
| ITEM 1B. | ||||||||
| ITEM 2. | ||||||||
| ITEM 3. | ||||||||
| ITEM 4. | ||||||||
| ITEM 5. | ||||||||
| ITEM 6. | ||||||||
| ITEM 7. | ||||||||
| ITEM 7A. | ||||||||
| ITEM 8. | ||||||||
| ITEM 9. | ||||||||
| ITEM 9A. | ||||||||
| ITEM 10. | ||||||||
| ITEM 11. | ||||||||
| ITEM 12. | ||||||||
| ITEM 13. | ||||||||
| ITEM 14. | ||||||||
| ITEM 15. | ||||||||
| ITEM 16. | ||||||||
PART I
ITEM 1. Business
This Annual Report on Form 10-K (this “Annual Report”) contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 about Natus® Medical Incorporated (“Natus,” “we,” “us,” or “our”). These statements include, among other things, statements concerning our expectations, beliefs, plans, intentions, future operations, financial condition and prospects, and business strategies. The words “may,” “will,” “continue,” “estimate,” “project,” “intend,” “believe,” “expect,” “anticipate,” and other similar expressions generally identify forward-looking statements. Forward-looking statements in this Item 1 include, but are not limited to, statements regarding the effectiveness and advantages of our products, factors relating to demand for and economic advantages of our products, our plan to develop and acquire additional technologies, products or businesses, our marketing, technology enhancement, and product development strategies, our ability to complete all of our backlog orders, and the anticipated timing and effect of the implementation of our new organizational structure.
Forward-looking statements are not guarantees of future performance and are subject to substantial risks and uncertainties that could cause our actual results to differ materially from those that we predicted in the forward-looking statements. Investors should carefully review the information contained under the caption “Risk Factors” contained in Item 1A for a description of risks and uncertainties that could cause actual results to differ from those that we predicted. All forward-looking statements are based on information available to us on the date hereof, and we assume no obligation to update forward-looking statements, except as required by Federal Securities laws.
Natus® and other trademarks of ours appearing in this report are the intellectual property of Natus Medical Incorporated or one of its subsidiaries.
Overview
Natus provides innovative healthcare solutions focused on the diagnosis and treatment of central nervous and sensory system disorders for patients of all ages.
Our broad product portfolio represents a heritage of innovation and leadership. Natus brands have been setting the standard for patient care for over eighty (80) years. Our products are trusted by medical professionals in university medical centers, public and private hospitals, physician offices, clinics, research laboratories, and other sites around the world. Improving quality of human life is at the core of our business.
We use our clinical expertise to support our customers' evolving needs with advanced products, continuing education and outstanding technical service.
Natus provides product solutions for three end markets: Neuro, Newborn Care and Hearing & Balance.
Markets
Neuro — Includes products and services that provide diagnostic, therapeutic and surgical solutions in neurodiagnostics, neurocritical care and neurosurgery. Neuro's comprehensive neurodiagnostic solutions include electroencephalography (“EEG”) and long-term monitoring (“LTM”), Intensive Care Unit (“ICU”) monitoring, electromyography (“EMG”), sleep analysis or polysomnography (“PSG”), and intraoperative monitoring (“IOM”). These solutions enhance the diagnosis of neurological conditions such as epilepsy, sleep disorders and neuromuscular diseases.
Our neurocritical care solutions include management of traumatic brain injury by continuous monitoring of intracranial pressure (“ICP”) and cerebrospinal fluid (“CSF”) drainage, as well as cranial access kits for entry into the cranium. Our neurosurgical solutions such as valves, shunts and related treatment solutions for procedures involving hydrocephalus, in addition to newly launched subdural electrodes for functional brain mapping. Neuro represents approximately 60% of total revenues.
Newborn Care — Includes products and services for newborn care including hearing screening, brain monitoring, eye imaging, jaundice management, live video streaming and various disposable newborn care supplies. These solutions provide important screening, diagnostic and treatment options for newborns in both the neonatal intensive care unit (“NICU”) and well-baby nursery. Newborn Care represents approximately 22% of total revenues.
Hearing & Balance — The Hearing portfolio includes products for hearing assessment and diagnostics, and hearing aid fitting, including computer-based audiological, otoneurologic and vestibular instrumentation for hearing care professionals. Our Balance portfolio provides diagnosis and assessment of vestibular and balance disorders. These solutions have a complete product and brand portfolio known for its sophisticated design technology in the hearing and balance assessment markets. Hearing & Balance represents approximately 18% of total revenues.
Neuro
1
Our Neuro portfolio is comprised of a comprehensive line of neurodiagnostic, neurocritical care, and neurosurgical products that are used by healthcare practitioners in the diagnosis and monitoring of neurological disorders. The environments in which these products are used include outpatient private practice facilities and inpatient hospital environments. Our products can be used throughout the continuum of care through diagnostic procedures and monitoring of patients during admissions, surgery, in post-operative care, and in intensive care units. Our Neuro products and services include:
Neurodiagnostic
•Electroencephalography — Equipment, supplies and services used to monitor and visually display the electrical activity generated by the brain and other key physiological signals for both diagnosis and monitoring of neurological disorders in the hospital, research laboratory, clinician office and patient’s home.
•Electromyography — Equipment and supplies used to measure electrical activity in nerves, muscles, and critical pathways includes EMG, nerve conduction and evoked potential functionality.
•Polysomnography — Equipment and supplies used to measure a variety of respiratory and physiologic functions to assist in the diagnosis and monitoring of sleep disorders, such as insomnia and obstructive sleep apnea.
•Intraoperative monitoring — Equipment and supplies used to monitor the functional integrity of certain neural structures (i.e. nerves, spinal cord and parts of the brain) during surgery. The goal of IOM is to provide real time guidance to the surgeon and anesthesiologist, which will reduce the risk to the patient during surgery.
Neurocritical Care
•Intracranial pressure monitoring and external ventricular drainage — Equipment and catheters used to monitor pressure in the cranium/brain and catheters to drain cerebrospinal fluid from the brain to aid in traumatic brain injury cases and hydrocephalus.
•Cranial access kits — Convenient, pre-packaged sterile sets containing all necessary components for entry into the cranium, to monitor intracranial pressure and provide temporary drainage of CSF.
Neurosurgery
•Shunts — Shunts are used to manage the drainage of cerebrospinal fluid from the brain to maintain appropriate levels of CSF when treating hydrocephalus.
•Subdural electrodes — Subdural electrodes are designed to integrate seamlessly with the Natus EEG equipment, simplifying the set up for functional brain mapping with minimal connections and cables.
Diagnostic EEG and Long-term Monitoring
We design, manufacture, and market a full line of instruments and supplies used to help diagnose the presence of seizure disorders and epilepsy, look for causes of confusion, evaluate head injuries, tumors, infections, degenerative diseases, and metabolic disturbances that affect the brain; and assist in surgical planning. This type of testing is also done to diagnose brain death in comatose patients. These systems and instruments work by detecting, amplifying, and recording the brain’s electrical impulses, as well as other physiological signals needed to support clinical findings. Routine clinical EEG recording is done by placing electrodes on a patient’s scalp over various areas of the brain to record and detect patterns of activity and specific types of electrical events. EEG technologists perform the tests, and neurologists, neurophysiologists and epileptologists review and interpret the results.
Routine outpatient clinical EEG testing is performed in hospital neurology laboratories, private physician offices, and in ambulatory settings such as the patient’s home, providing physicians with a clinical assessment of a patient’s condition. Long-term inpatient monitoring of EEG and video to monitor behavior (LTM) is used to determine complex treatment plans, and for patients with seizures that do not respond to conventional therapeutic approaches, surgical solutions may be appropriate. Patients suffering from severe head trauma and other acute conditions that may affect the brain are monitored in ICUs. In addition, research facilities use EEG equipment to conduct research on humans and laboratory animals.
Diagnostic Electroencephalograph Monitoring Product Lines
Our EEG diagnostic monitoring product lines for neurology consist of signal amplifiers, workstations to capture and store synchronized video and EEG data, and proprietary software. These products are typically used as part of an EEG “system” by the neurology/neurophysiology department of a hospital or clinic to assist in the diagnosis and monitoring of neurological conditions.
•NeuroWorks®; Nicolet®One. Our EEG Systems include a broad range of products, from software licenses and ambulatory monitoring systems to advanced laboratory systems with multiple capabilities for EEG, ICU monitoring,
2
long-term monitoring of up to 256 channels, as well as nursing stations to monitor patients and physician review stations with quantitative EEG analysis capabilities.
•Stellate/Gotman Spike and Seizure; NicoletOne Trends. Our proprietary spike and seizure detection algorithm detects, summarizes, and reports EEG events that save health-care professionals' time by increasing the speed and accuracy of interpretation. NicoletOne Trends provides a comprehensive set of EEG analysis algorithms that are used to generate compressed trends of large amounts of data to assist in the clinical evaluation and data review process.
•Proprietary Signal Amplifiers. Our proprietary signal amplifiers function as the interface between the patient and the computer. The headbox connects electrodes attached to the patient’s head to our EEG monitoring systems.
•Nicolet Cortical Stimulator. This product is our proprietary device that provides cortical stimulation to the brain during functional brain mapping either before or during surgery to help the surgeon protect the eloquent parts of the brain (parts of the brain that control speech, motor and sensory functionality). The device can be used as a standalone unit or with the fully integrated NicoletOne or NeuroWorks software that supports control of the device from the software, automated mapping and comprehensive report generation.
•Supplies. We also manufacture and market a full line of proprietary EEG electrodes and other supplies used in the electroencephalography field.
Electrodiagnostic Monitoring
Our electrodiagnostic systems include electromyography (EMG), nerve conduction (“NCS”), and evoked potential (“EP”) functionality. EMG and NCS involve the measurement of electrical activity of muscles and nerves both at rest and during contraction. Measuring the electrical activity in muscles and nerves can help diagnose diseases of the peripheral, central nervous system or musculature system. An electromyogram is performed to determine if there is any disease present that affects muscle tissue, nerves, or the junctions between nerve and muscle (neuromuscular junctions). An electromyogram can also be used to diagnose the cause of weakness, paralysis, and muscle twitching, and is also used as a primary diagnosis for carpal tunnel syndrome, which is the most frequently encountered peripheral compressive neuropathy. EMG is also used for clinical applications of Botox to relieve muscle spasm and pain. We market both the clinical system and the needles used for these procedures.
Evoked brain potentials are elicited in response to a stimulus. These evoked potentials can come from the sensory pathways (such as hearing and visual) or from the motor pathways. An examination tests the integrity of these pathways including the associated area of the brain. Sophisticated amplifiers are required to recognize and average evoked potential EMG and NCS signals.
Electrodiagnostic Product Lines
•Dantec Keypoint®. The Dantec Keypoint G-4 and Focus EMG and EP family of products features amplifiers, stimulators, and strong signal quality. The Keypoint G4 is used for advanced neurodiagnostic applications such as single fiber EMG, visual and auditory evoked potentials, and in routine nerve conduction studies. The Keypoint Focus system is also available in a portable laptop configuration.
•Dantec Clavis. The Dantec Clavis device is a hand-held EMG stimulation device that provides muscle and nerve localization information to assist with medication and Botox injections. In conjunction with the Bo-ject® or Myoject hypodermic needle and electrodes, physicians can better localize the site of the injection.
•Nicolet EDX® family. A hardware platform of amplifiers, base control units, stimulators and hand-held probes that are sold with Nicolet brand proprietary software. These mid- to high-end systems have full functionality, strong signal quality, and flexibility. They include EMG, NCS, EP’s, IOM and advanced data analysis features.
•Natus UltraPro. With a newly globally launched hardware and software system, this entry level product with add on capabilities offers high quality data collection using the UltraPro S100 amplifier and the proprietary Natus Elite software.
•Supplies. We also manufacture and market a full line of proprietary EMG needles and other supplies used in the electrodiagnostic field.
Diagnostic Polysomnography Monitoring
Polysomnography (“PSG”), which involves the analysis of respiratory patterns, brain electrical activity and other physiological data, has proven critical for the diagnosis and treatment of sleep-related diseases such as apnea, insomnia, and narcolepsy. A full polysomnographic sleep study entails a whole-night recording of brain electrical activity, muscle movement, airflow, respiratory effort, oxygen levels, electrical activity of the heart, and other parameters. In some studies, patients are
3
fitted with treatment devices using Positive Airway Pressure technology (“PAP”) during the sleep study and the proper settings for the treatment devices are determined. In many cases, the sleep study is performed in the patient’s home.
Diagnostic PSG Monitoring Product Lines
We market dedicated diagnostic PSG monitoring products that can be used individually or as part of a networked system for overnight sleep studies to assist in the diagnosis of sleep disorders. Additionally, we offer products that are specifically designed to be used in the patient’s home. Some of our EEG systems described above can also be configured to perform diagnostic PSG monitoring. These products include software licenses, ambulatory monitoring systems, and laboratory systems that combine multiple capabilities, including EEG monitoring, physician review stations, and quantitative PSG analysis capabilities.
•Embla REMlogic, Sandman®; and Xltek SleepWorks®. Our diagnostic PSG systems capture and store all data digitally. The systems enable users to specify rules and personal preferences to be used during analysis, summarizing the results graphically and incorporating them in detailed reports.
•Proprietary Amplifiers. Our data acquisition systems incorporate recent developments in superior amplifiers for sleep analysis. Our amplifiers are used in both hospitals and stand-alone clinics. In addition to exceptional signal quality, headboxes include various tools such as built-in oximeters and controls to allow the user to start and stop a study or perform electrode impedance testing either at the patient’s bedside or from the monitoring room.
•Supplies. We also market a broad line of supplies, disposable products and accessories for the PSG laboratory including the XactTrace® respiratory monitoring belts.
Intraoperative Monitoring
Intraoperative monitoring (“IOM”) is the use of electrophysiological methods such as EEG, EMG, and evoked potentials to monitor the functional integrity of certain neural structures (i.e. nerves, spinal cord and parts of the brain) during surgery. The purpose of IOM is to reduce the risk to the patient’s nervous system, and/or to provide functional guidance to the surgeon and anesthesiologist during surgery.
Diagnostic IOM Product Lines
The Nicolet EDX combo systems are used in IOM applications where a smaller number of channels is sufficient. This approach is primarily followed in international markets that utilize the integrated system approach that allows for the use of the system in EMG clinical applications as well as in IOM applications.
Neurocritical Care Products
Intracranial pressure and temperature provide insight into the health of the brain, especially in patients experiencing a traumatic brain injury, other traumatic, ischemic or hemorrhagic incidents, or a major neurosurgical procedure. A small hole is drilled into the cranium to allow insertion of a catheter that contains a pressure/temperature or pressure only transducer that allows continuous monitoring of brain temperature and/or pressure.
•Camino® ICP Monitor. The Camino ICP Monitor is a compact, portable device that provides tools for continuously determining and monitoring intracranial pressure and intracranial temperature. It has a touch screen interface, physiological alarms, and can output data to either a patient bedside monitor or to remote media types via a USB drive. These systems are used in the intensive care unit (ICU) environment.
•Camino Catheters. Camino catheters use either fiber optic or strain gauge technology to measure either pressure and temperature or just pressure. Camino catheters measure their respective values at the tip of the catheter, which eliminates the need for a fluid-filled system that uses an external transducer to measure pressure. The Camino Flex Ventricular Intracranial Pressure Monitoring Kit has a catheter that allows both the measurement of ICP and CSF drainage.
•Natus EDS 3®. The EDS 3 is a CSF External Drainage System used to manage increased ICP by safely eliminating CSF from the ventricles of the brain or lumbar spine. This drainage can be performed continuously or intermittently. Clinical conditions that may require its use include trauma, stroke or an expansile lesion.
•Cranial Access Kits. Cranial Access Kits are convenient procedural kits that include all the instrumentation and items needed to access the subarachnoid space or the lateral ventricles of the brain. The kit is intended to be used with an external drainage and monitoring system in selected patients to reduce intracranial pressure, to provide temporary drainage of CSF, and to monitor ICP. The kit is a convenient, pre-packaged sterile set containing all necessary components for entry into the cranium and is available with or without drugs and with a variety of drill bits and instrumentation.
Neurosurgical Products
4
Brain surgery is performed to place shunts in the brain to help drain excess CSF either into the body for reabsorption to help treat hydrocephalus or to place subdural electrodes for functional brain mapping.
•Shunts. Shunts are used in the operating room to provide solutions for hydrocephalus or brain trauma. Shunts are used to manage the drainage of cerebrospinal fluid from the brain to maintain appropriate levels of CSF when treating hydrocephalus.
•XactTrode™ Subdural Electrodes. Subdural strip and grid electrodes are used for epilepsy surgical monitoring and brain mapping. The XactTrode portfolio of electrodes are designed to integrate seamlessly with Natus amplifiers and provide increased patient comfort with fewer connections and cables.
Newborn Care
Our newborn care products and services are used by healthcare practitioners in the diagnosis and treatment of common medical ailments in newborn care. Our products are organized in eight modalities and include:
•Newborn Hearing Screening — Products used to screen hearing in newborns.
•Jaundice Management — Products used to treat jaundice, the single largest cause for hospital readmission of newborns in the U.S.
•Newborn Brain Injury — Products used to diagnose the severity of brain injury, monitor the effectiveness of drug therapies, detect seizure activity and monitor general neurological status.
•Eye Imaging — Systems and products used in the advanced science and practice of neonatal and pediatric retinal imaging.
•Essentials — Products used in the everyday operation of NICU and well-baby nursery department within the hospital environment.
•NICVIEW® — Live streaming video for families with babies in the NICU that enables family members and approved friends to see the new baby, 24/7, from anywhere in the world - from any Internet connected device, within a secured environment.
Newborn Hearing Screening
Hearing impairment is the most common treatable chronic disorder in newborns, affecting as many as five babies out of every 1,000 newborns. It is estimated that 20,000 hearing-impaired babies are born in the United States (“U.S.”) every year, and as many as 60,000 more in the rest of the developed world. Until the introduction of newborn hearing screening programs, screening was generally performed only on those newborns that had identifiable risk factors for hearing impairment. However, screening only those newborns with risk factors for hearing impairment overlooks approximately half of newborns with some level of hearing impairment.
Early identification of hearing impairment and early intervention has been shown to improve language development significantly. Undetected hearing impairment often results in the failure to learn, process spoken language, and speak.
Newborn Hearing Screening Techniques
The two traditional technologies used to screen newborns and infants for hearing impairment are auditory brainstem response and otoacoustic emissions.
Auditory brainstem response (“ABR”). ABR technology is the most accurate and comprehensive method for screening and diagnosing hearing impairment. ABR technology is based on detecting the brain’s electrical impulses resulting from a specific auditory stimulus.
Otoacoustic emission (“OAE”). OAEs are sounds created by the active biomechanical processes within the sensory cells of the cochlea. They occur both spontaneously and in response to acoustic stimuli. OAE screening uses a probe placed in the ear canal to deliver auditory stimuli and to measure the response of the sensory cells with a sensitive microphone.
Newborn Hearing Screening Product Lines
Our newborn hearing screening product lines consist of the ALGO®, ABaer®, AuDX®, and Echo-Screen® newborn hearing screeners. These hearing screening products utilize proprietary signal detection technologies to provide accurate and non-invasive hearing screening for newborns and are designed to detect hearing loss at 30 or 35 dB nHL or higher. Each of these devices is designed to generate a PASS or REFER result.
•ALGO® 5 and 3i Newborn Hearing Screeners. These Automated Auditory Brainstem Responses (“AABR”®) devices deliver thousands of soft audible clicks to the newborn’s ears through sound cables and disposable earphones connected to the instrument. Each click elicits an identifiable brain wave, which is detected by disposable
5
electrodes placed on the head of the child and analyzed by the screening device. These devices use our proprietary AABR signal detection algorithm.
•ALGO® 7i. The ALGO® 7i continues the Natus tradition of advancing standards in patient care, combining industry-leading performance with enhanced usability and taking AABR hearing screening to the next level. The ALGO 7i incorporates proven ALGO AABR® technology. The icon-driven interface intuitively guides users through each step of the screening process.
•ABaer Newborn Hearing Screener. The ABaer, which is a PC-based newborn hearing screening device, offers a combination of AABR, OAE, and diagnostic ABR technologies in one system.
•Echo-Screen. Our hand-held Echo-Screen products provide a choice or combination of proprietary ABR and OAE technologies that can also be used for children through adults. The Echo-Screen III device is a compact, multi-modality handheld hearing screener that is tightly integrated with audible Lite Hearing Screening Data Management.
Hearing Screening Supply Products
For infection control, accuracy, and ease of use, the supply products used with our newborn hearing screening devices are designed as single-use, disposable products. Each screening supply product is designed for a specific hearing screening technology.
•ABR Screening Supply Kits. Each ABR screen is carried out with single-use earphones and electrodes, which are alcohol and latex-free. The adhesives used in these supply products are specially formulated for use on the sensitive skin of newborns. To meet the needs of our customers we offer a variety of packaging options. Echo-Screen and ABaer offer the choice of either an earphone or use of ear tips for perform ABR screening.
•OAE Supply Products. Each OAE screen is carried out with single-use ear tips that are supplied in a variety of sizes and packaging options.
Jaundice Management
The American Academy of Pediatrics estimates that each year 60% of the approximately three and a half million newborns in the U.S. become jaundiced. According to the Journal of the American Medical Association, neonatal jaundice is the single largest cause for hospital readmission of newborns in the U.S., and accounts for approximately 50% of readmissions. Because of the serious consequences of hyperbilirubinemia, the American Academy of Pediatrics recommends that all newborns be closely monitored for jaundice and that phototherapy is the standard of care for the treatment of hyperbilirubinemia. The guidelines further recommend that all nurseries have the necessary equipment to provide intensive phototherapy, and specifically recommend the use of the “blue” light as incorporated into our neoBLUE® products.
Jaundice Management Products
•neoBLUE Product Family. This product line consists of our neoBLUE Overhead, neoBLUE Compact and neoBLUE blanket devices, which utilize light emitting diodes (“LEDs”) to generate a high-intensity, narrow spectrum of blue light that is clinically proven to be most effective in the treatment of newborn jaundice. Our neoBLUE phototherapy devices emit significantly less ultraviolet light and heat than conventional phototherapy devices, reducing the risk of skin damage and dehydration for infants undergoing treatment. Because of the high intensity of these lights, the treatment time associated with phototherapy is reduced.
Newborn Brain Injury
For many years, newborn infants admitted to the NICU of a hospital have been routinely monitored for heart activity, temperature, respiration, oxygen saturation, and blood pressure. Recently it has also been considered important to monitor brain activity. A cerebral function monitor, utilizing amplitude-integrated EEGs (“aEEGs”), is a device for monitoring background neurological activity. Our simplified aEEG devices, introduced over ten years ago, are designed to be simple for use by nurses and neonatologists.
Newborn Brain Injury Products
Our newborn brain injury products record and display parameters that the neonatologist uses to assess and monitor neurological status in the newborn. These devices continuously monitor and record brain activity, aiding in the detection and treatment of hypoxic-ischemic encephalopathy (“HIE”), and seizures. The devices also monitor the effects of drugs and other therapies on brain activity and improve the accuracy of newborn neurological assessments. They are used with electrodes attached to the head of the newborn to acquire an EEG signal that is then filtered, compressed, and displayed graphically on the
6
device or as a hardcopy printout. The monitors have touch screens for easy navigation and onscreen keyboards for data entry at the bedside.
•Olympic Brainz Monitor. The Olympic Brainz Monitor is our latest generation Cerebral Function Monitor. The device can be used in single-channel, two-channel or three-channel modes to continuously monitor and record brain activity.
Eye Imaging
Our RetCam® devices incorporate a camera combined with proprietary imaging software that are used to diagnose and monitor a range of ophthalmic maladies in premature infants. RetCam specializes in NICU ophthalmic imaging used in the detection of retinopathy of prematurity (“ROP”) and Retinoblastoma (“RB”) in newborns. ROP and RB are diseases of the retina that must be detected very early after birth and treated immediately, so the RetCam diagnostic camera is a fundamental tool in preventing vision loss and total blindness in infants.
Eye Imaging Products
RetCam images assist physicians in the evaluation of pediatric ocular disease, which have preserved the vision in thousands of infants. Each of the RetCam systems deliver objective and interpretable detail, allow image comparison over time, enable remote consultations, and provide reliable and defensible medico-legal documentation.
•RetCam Envision. The RetCam Envision is a contact type wide-field fundus ophthalmic imaging system used for general ophthalmic, imaging including retinal, corneal, and external imaging. Photo documentation of pediatric ocular diseases, including ROP, are included. The RetCam Envision brings patented Light Shaping Technology that delivers uniform illumination and focus throughout the entire 130 degree field of view.
•RetCam 3. Full-featured imaging systems with a range of interchangeable lenses, Fluorescein Angiography module option.
•RetCam Shuttle. Laptop-based system with a smaller cart and dual wheel casters for improved transportability.
•RetCam Portable. Laptop-based version in a case for maximum portability.
•RetCam DataLink. Cloud-based image review solution that enables anytime, anywhere access to time sensitive image data for rapid review and interpretation. DataLink enables seamless integration with electronic medical records enabling capturing orders, image review and sharing results.
Essentials
The Newborn Care Essentials products include such items as: Biliband® eye protectors, MiniMuffs® noise attenuators, NeatNick® heal lancets, Olympic Circumstraint, Olympic Papoose Boards, Olympic Smart Scales®, OraSwab, Save the Gonads x-ray protection devices and SugarPlum glucose lancets.
Live Video Streaming
Live video streaming offers parents and families secured access to a live video stream of their baby. For hospitals, the system offers a step into family centered care.
Live Video Steaming Products
NICVIEW 2 is a user-friendly, web-based video systems for real-time streaming on any online device via a standard downloadable app. Password-protected access ensures parents can view only their own child, with end-to-end encryption and SSL authentication. The video stream can be turned on/off and repositioned at will, so that NICU staff remain in control of the care process at all times.
Hearing & Balance
Our Hearing & Balance product portfolio provides hearing diagnostic, hearing aid fitting and balance instrumentation and software solutions to hearing and balance care professionals worldwide. For more than 50 years, we have helped hearing and balance care professionals succeed in improving the quality of life for their clients and patients by delivering expert knowledge, reliable solutions and services and trusted partnerships. We will continue this tradition and legacy as we develop, manufacture and market computer-based audiological, otoneurologic and vestibular instrumentation in the future.
Our solutions portfolio covers key application areas within hearing assessment, hearing screening, hearing instrument fitting and balance assessment. Many of our hearing and balance care solutions have set precedent within the hearing care industry and are used by thousands of clinicians around the world.
As an independent provider of hearing care diagnostic solutions, we work closely with leading hearing aid manufacturers to develop new solutions within hearing assessment and hearing aid fitting.
7
Hearing Assessment
From otoacoustic emissions (OAE) and immittance screening to advanced audiological testing and 3D digital ear scanning, we offer a wide range of flexible devices and PC-based solutions that are designed to screen, test and assess patients of all ages. Our hearing assessment solutions offer a range of functionality to support basic audiometric testing to advanced tinnitus and pediatric hearing assessment. Our hearing care solutions help streamline the hearing screening and assessment process making it easier and convenient for the professional and the patient. We also manufacture and market a broad line of supplies and disposable products and accessories for hearing assessment.
Hearing Instrument Fitting and Verification
Hearing fitting solutions help professionals manage the entire hearing aid fitting process - from fitting and verifying the hearing aid to patient counseling and follow up. Used by hearing aid dispensers, audiologists and clinicians around the world, our fitting solutions support otoscopy, audiometry, hearing aid testing and programming, fitting and verification with wireless design and binaural fitting capability. Our fitting solutions are PC-based and supported by integrated audiometric software that helps to streamline the fitting process for greater efficiency and patient satisfaction. We also manufacturer and market a broad line of supplies and disposable products and accessories for hearing instrument fitting and verification.
3D Digital Ear Scanning
Hearing assessment solutions include the breakthrough 3D digital ear scanning solutions Otoscan® that gives hearing care professionals innovative ways to attract and convert more clients while delivering customized hearing care in an efficient way. Otoscan enables hearing care professionals to make digital impressions for custom in-the-ear pieces such as earmolds and hearing aids. The scanner solution applies breakthrough technology to transform images of the ear into 3D digital files that are uploaded to the cloud service, Otocloud®, for immediate use in production of custom products, delivering significant efficiency and quality gains in the production of hearing aids. Otocloud is a web-based portal supported by a dedicated Microsoft Azure server domain.
Balance Assessment
Professionals who evaluate patients with balance disorders use our vestibular diagnostic and ENG/VNG (electronystagmography/videonystagmography) systems and services. These solutions are used by audiologists, otolaryngologists, otologists and neurologists for identifying auditory and vestibular abnormalities. Our balance care solutions are compact and include the world's first portable, gold standard video head impulse test (“vHIT”) and offer modular functionality to support vHIT, video frenzel, positional, oculomotor and SHIMP (suppression head impulse) testing. We also manufacture and market a broad line of supplies, disposable face cushions, and accessories for balance assessment.
Segment and Geographic Information
We determine our reportable segments by first identifying our operating segments, and then by assessing whether any components of these segments constitute a business for which discrete financial information is available and where segment management regularly reviews the operating results of that component. Prior to 2019, our operating segments were based on three strategic business units. Since then, we transitioned from an operating structure of three strategic business units to a single, unified company with globally led operational teams in Sales and Marketing, Manufacturing, Research and Development, Quality, and General and Administrative functions.
We operate as one operating segment and one reportable segment, which provides healthcare products, and services focused on the diagnosis and treatment of central nervous and sensory system disorders for patients of all ages. Financial information is reviewed on a consolidated basis for purposes of making operating decisions and assessing financial performance. Consolidated financial information is accompanied by disaggregated information about revenues by end market and geographic region. We do not assess the performance of our end markets or geographic regions on measures of profit or loss, or asset-based metrics. We have disclosed the revenues for each of our end markets and geographic regions to provide the reader of the financial statements transparency into our operations.
Information regarding our revenues and long-lived assets in the U.S. and in countries outside the U.S. is contained in Note 19—Segment, Customer and Geographic Information of our Consolidated Financial Statements included in this report and is incorporated in this section by this reference.
Revenue by Product Market and Product Category
8
For the years ended December 31, 2021, 2020 and 2019, revenue from our product markets as a percent of total revenue was approximately as follows:
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Neuro | 60 | % | 57 | % | 58 | % | |||||||||||
| Newborn Care | 22 | % | 25 | % | 22 | % | |||||||||||
| Hearing & Balance | 18 | % | 18 | % | 20 | % | |||||||||||
| Total | 100 | % | 100 | % | 100 | % | |||||||||||
We also look at revenue as either being generated from sales of Devices and Systems, which are generally non-recurring, or related Supplies and Services, which are generally recurring. The products that are attributable to these categories are described above. Revenue from Devices and Systems, Supplies and Services as a percent of total revenue for the years ending December 31, 2021, 2020 and 2019 is as follows:
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Devices and Systems | 75 | % | 73 | % | 74 | % | |||||||||||
| Supplies | 22 | % | 23 | % | 22 | % | |||||||||||
| Services | 3 | % | 4 | % | 4 | % | |||||||||||
| Total | 100 | % | 100 | % | 100 | % | |||||||||||
In 2021, 2020 and 2019, no single end-user customer comprised more than 10% of our revenue.
Backlog
In general, we do not manufacture our products against a backlog of orders and do not consider backlog to be a significant indicator of the level of annual future sales activity. Production and inventory levels are based on the level of incoming orders as well as projections of future demand. Therefore, we believe that backlog information is not meaningful to understanding our overall business and should not be considered a reliable indicator of our ability to achieve any particular level of revenue or financial performance.
Marketing and Sales
Marketing
Our marketing strategies differ by product market, incorporating market dynamics, trends and competition in the positioning, promotion and pricing of each product. The value proposition that we communicate is focused on the quality, clinical performance, and customer benefit. We invest in educating our customers worldwide about our products through trade conferences and direct presentations to healthcare professionals.
Domestic Direct and Distributor Sales
We sell our products in North America primarily through a direct sales organization. We believe this direct sales organization allows us to maintain a higher level of customer service and satisfaction than would otherwise be possible by other distribution methods. We also sell certain products under private label and distribution arrangements.
For the years ended December 31, 2021, 2020 and 2019, domestic revenue as a percent of total revenue was approximately as follows:
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Domestic revenue | 60.4 | % | 60.7 | % | 59.0 | % | |||||||||||
9
International Direct and Distributor Sales
We sell some of our products outside the U.S. through direct sales channels in Australia, Canada, China, Denmark, France, Germany, Italy, the Netherlands, New Zealand, the Nordics (Finland, Sweden, Norway), Spain, and the United Kingdom; we sell other products in those regions and into more than 100 other countries through a distributor sales channel.
For the years ended December 31, 2021, 2020 and 2019, international revenue as a percent of total revenue was approximately as follows:
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| International revenue | 39.6 | % | 39.3 | % | 41.0 | % | |||||||||||
We sell products to our distributors under substantially the same terms as sales through our direct sales channels. Terms of sales to international distributors are generally “ex works,” where title and risk of loss are assumed by the distributor at the shipping point. Distributors are generally given exclusive rights in their territories to purchase products from Natus and to resell to end users or sub-distributors in their respective markets. Our distributors typically perform marketing, sales, and technical support functions in their respective markets. Each distributor may sell Natus products to their customer directly, via other distributors or resellers, or both. We actively train our distributors in product marketing, selling, and technical service techniques.
Seasonality in Revenue
We experience seasonality in our revenue. Demand for our products is historically higher in the second half of the year compared to the first. Our seasonality results from the purchasing habits of our hospital-based customers, whose purchases are often governed by calendar year budgets.
Group Purchasing Organizations
More than 90% of the hospitals in the U.S. are members of group purchasing organizations (“GPO”s), which negotiate volume purchase agreements for member hospitals, group practices, and other clinics.
For the years ended December 31, 2021, 2020 and 2019, revenue from direct purchases under a GPO contract as a percent of total revenue was approximately as follows:
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Direct purchases by GPO members | 23.6 | % | 22.5 | % | 18.7 | % | |||||||||||
Third-Party Reimbursement
In the U.S., healthcare providers generally rely on third-party payors, including private health insurance plans, federal Medicare, state Medicaid, and managed care organizations, to reimburse all or part of the cost of the procedures they perform. Third-party payors can affect the pricing or the relative attractiveness of our products by regulating the maximum amount of reimbursement these payors provide for services utilizing our products.
Customer Service and Support
We generally provide a one-year warranty on our medical device and system products. We also sell extended service agreements on our medical device and system products. Service, repair, and calibration services for our domestic customers are provided by Company-owned service centers and our field service specialists. Service for international customers is provided by a combination of Company-owned facilities and vendors on a contract basis.
Manufacturing
We procure a significant portion of the components used in our products from other manufacturers; however, we perform final assembly, testing, and packaging of many of the devices ourselves to control quality and manufacturing efficiency and we are the manufacturer of record. We also use contract manufacturers to manufacture some of our disposable supply and medical device products. We perform regular quality assessments of these contract manufacturers, which include on-site quality audits.
We purchase materials and components from qualified suppliers that are subject to our quality specifications and inspections. We conduct quality audits of our key suppliers, several of which are experienced in the supply of components to manufacturers of finished medical devices or supplies for use with medical devices. Most of our purchased components are available from more than one supplier.
10
Our manufacturing, service, and repair facilities are subject to periodic inspection by local and foreign regulatory authorities. Our quality assurance system is subject to regulation by the U.S. Food and Drug Administration (“FDA”) and other government agencies. We are required to conduct our product design, testing, manufacturing, and control activities in conformance with the FDA’s quality system regulations and to maintain our documentation of these activities in a prescribed manner. In addition, our production facilities have received International Organization for Standardization (“ISO”) 13485 certification. ISO 13485 certification standards for quality operations have been developed to ensure that medical device companies meet the standards of quality on a worldwide basis. We have also received the EC Certificate pursuant to the European Union Medical Device Directive 93/42/EEC, which allows us to place a CE mark on our products.
Research and Development
We are committed to introducing new products and supporting current product offerings in our markets through a combination of internal as well as external efforts that are consistent with our corporate strategy.
Internal product development capabilities. We believe the ability to develop innovative products is essential to providing our customers with new product offerings. We plan to leverage our core technologies by introducing product line extensions as well as new product offerings.
Partnerships that complement our expertise. We continue to seek strategic partners in order to develop products that may not otherwise be available to us. By taking advantage of our core competencies, we believe that we can bring acquired or distributed products to market in an efficient manner and leverage our distribution channels.
New opportunities through technology acquisition. We continue to evaluate new, emerging, and complementary technologies in order to identify new product opportunities. With our knowledge of our current markets, we believe that we can effectively develop acquired technologies into successful new products.
Proprietary Rights
We protect our intellectual property through a combination of patent, copyright, trade secret, and trademark laws. We attempt to protect our intellectual property rights by filing patent applications for new features and products we develop. We enter into confidentiality or license agreements with our employees, consultants, and corporate partners, and seek to control access to our intellectual property, distribution channels, documentation, and other proprietary information. However, we believe that these measures afford only limited protection.
The intellectual rights to some of the original patents for technology incorporated into our products are now in the public domain. However, we do not consider these patents, or any currently viable patent or related group of patents, to be of such importance that their expiration or termination would materially affect our business.
We capitalize the cost of purchased technology and intellectual property, as well as certain costs incurred in obtaining patent rights, and amortize these costs over the estimated economic lives of the related assets.
We have numerous registered trademarks and service marks. Our marks are pending or registered trademarks in the United States and several foreign countries. We intend to file for additional trademarks to strengthen our trademark rights, but we cannot be certain that our trademark applications will result in registration or that our trademarks will be enforceable.
Competition
We sell our products in competitive and rapidly evolving markets. We face competition from other companies in all of our product lines. Our competitors range from small privately-held companies to multinational corporations and their product offerings vary in scope and breadth. We do not believe that any single competitor is dominant in any of our product lines.
We derive a significant portion of our revenue from the sale of disposable supplies that are used with our medical devices. In the U.S., we sell our supply products in a mature market and we expect that our products could face increasing competition, including competitors offering lower prices, which could have an adverse effect on our revenue and profit margins.
We believe the principal factors that will draw clinicians and other buyers to our products, include:
•The clinical performance of our products including the level of specificity, sensitivity, and reliability of the product;
•Time required to obtain results with the product, such as to test for or treat a clinical condition;
•Relative ease of use of the product;
•Our level of expertise in these fields which produces the depth and breadth of the products features;
•Quality of customer support for the product;
•Internet connectivity and cyber-security of the product;
11
•Frequency of product updates;
•Extent of third-party reimbursement of the cost of the product or procedure;
•Extent to which the products conform to standard of care guidelines; and
•Price of the product.
We believe that our primary competitive strength relates to the clinical functionality and reliability of our products.
Government Regulation
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, the medical devices we sell in the United States, must first receive one of the following types of FDA premarket review authorizations under the Food, Drug, and Cosmetics Act, as amended:
•Clearance via Section 510(k); or
•Premarket approval via Section 515 if the FDA has determined that the medical device in question poses a greater risk of injury.
FDA review-time goal for 510(k) applications are 90 days, but clearance may take longer. The process of obtaining premarket approval via Section 515 is much more costly, lengthy, and uncertain. FDA review-time goal for premarket approval applications are 320 days, but approval may take longer. We cannot be sure that the FDA will ever grant either 510(k) clearance or premarket approval for any product we propose to market in the United States.
The FDA decides whether a device must undergo either the 510(k) clearance or premarket approval process based upon statutory criteria. These criteria include the level of risk that the FDA perceives to be associated with the device and a determination of whether the product is a type of device that is substantially equivalent to devices that are already legally marketed. The FDA places devices deemed to pose relatively less risk in either Class I or Class II, which requires the manufacturer to submit a premarket notification requesting 510(k) clearance, unless an exemption applies. The premarket notification under Section 510(k) must demonstrate that the proposed device is substantially equivalent in intended use and in safety and effectiveness to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications.
The FDA places devices deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed to be not substantially equivalent to a predicate device, in its Class III classification. The FDA requires these devices to undergo the premarket approval process via Section 515 in which the manufacturer must prove the safety and effectiveness of the device. A premarket approval application must provide extensive pre-clinical and clinical trial data.
The FDA may require results of clinical trials in support of a 510(k) submission and generally requires clinical trial results for a premarket approval application. In order to conduct a clinical trial on a significant-risk device, the FDA requires manufacturers to apply for and obtain, in advance, an investigational-device exemption. The investigational-device exemption application must be supported by appropriate data, such as animal and laboratory testing results. If the FDA and the Institutional Review Boards at the clinical trial sites approve the investigational-device exemption application for a significant-risk device, the manufacturer may begin the clinical trial. An investigational-device exemption approval provides for a specified clinical protocol, including the number of patients and study sites. If the manufacturer deems the product a non-significant risk device, the product will be eligible for more abbreviated investigational-device exemption requirements. If the Institutional Review Boards at the clinical trial sites concur with the non-significant risk determination, the manufacturer may begin the clinical trial.
Most of our products have been cleared by the FDA as Class II devices.
FDA Regulation
Numerous FDA regulatory requirements apply to our products. These requirements include:
•FDA quality system regulations which require manufacturers to create, implement, and follow design, testing, control, documentation, and other quality assurance procedures;
•Medical device reporting regulations, which require that manufacturers report to the FDA certain types of adverse and other events involving their products; and
•FDA general prohibitions against promoting products for unapproved uses.
Class II and III devices may also be subject to special controls applied to them, such as performance standards, post-market surveillance, patient registries, and FDA guidelines that may not apply to Class I devices. We believe we are in
12
compliance with applicable FDA guidelines, but we could be required to change our compliance activities or be subject to other special controls if the FDA changes existing regulations or adopts new requirements.
We are subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to adequately comply, the FDA can institute a wide variety of enforcement actions, including:
•Issuance of a Form 483 citation;
•Fines, injunctions, and civil penalties;
•Recall or seizure of our products;
•Issuance of public notices or warnings;
•Imposition of operating restrictions, partial suspension, or total shutdown of production;
•Refusal of our requests for 510(k) clearance or pre-market approval of new products;
•Withdrawal of 510(k) clearance or pre-market approval already granted; or
•Criminal prosecution.
The FDA also has the authority to require us to repair or replace any misbranded or adulterated medical device manufactured or distributed by us.
Other Regulations
We also must comply with numerous additional federal, state, and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection, biohazards, fire hazard control, and hazardous substance disposal. We believe we are currently in compliance with such regulations.
Countries outside of the U.S. regulate medical devices in a manner similar to that of the FDA. Our manufacturing facilities are subject to audit and have been certified to be ISO 13485:2016, Medical Device Directive 93/42/EEC, and MDSAP compliant, which allows us to sell our products in Europe, Canada, and other territories around the world. All of our manufacturing facilities are subject to inspection by our notified bodies or other competent authorities, and in some cases without advance notice. We plan to seek approval to sell our products in additional countries, while maintaining our current approvals. The time and cost of obtaining new, and maintaining existing, market authorizations from countries outside of North America, and the requirements for licensing products in these countries may differ significantly from FDA requirements.
In 2017, the European Union (“EU”) adopted the EU Medical Device Regulation (Council Regulations 2017/745) which imposes stricter requirements for the marketing and sale of medical devices, including new quality system and post-market surveillance requirements. The regulation has a three-year implementation period that has now been extended to May 2021 and will replace the existing directives on medical devices in the EU. After May 2021, medical devices marketed in the EU require certification according to these new requirements, except that devices with valid CE certificates, issued pursuant to the Medical Device Directive before May 2021, may be placed on the market until 2024. Complying with this new regulation requires us to incur significant costs on product design history file transition. Failure to meet the requirements of the regulation could adversely impact our business in the European Union and other countries that utilize or rely on European Union requirements for medical device registrations.
Human Capital
Overview
Our Natus Pledge to our Teammates is to create a safe and secure (physical, psychological, financial, and social) environment where each Teammate feels they belong and have the resources and training to respond to the needs of our customers, the quality of life to grow as a person and professional, and time for family, health, and their community.
Workforce Demographics
As of December 31, 2021, we are a workforce of approximately 1,400 full-time Teammates, with about 50% of our Teammates located outside the United States.
We are organized by function, which breaks down as follows: Commercial with 692 Teammates (including Sales, Service & Marketing), Manufacturing Operations with 252 Teammates, Engineering with 173 Teammates, General and Administrative with 177 Teammates and Quality and Regulatory Affairs with 104 Teammates.
Our gender distribution of our Teammates is 34% women and 66% men. Our Teammates' average length of service is 8 years.
13
We experienced an increase in voluntary turnover in 2021 due to the effects of the global pandemic and other related factors, including more competitive demand for talent, low unemployment in core locations, increasing compensation expectations and more retirements than in previous years.
We formalized a flexible and remote work policy in 2021 to support our Teammates safety and well-being. Managers and Teammates were given tools, training and support to make a smooth transition to a more flexible work environment. Today our workforce is 28% flexible, 42% fully remote and 30% on-site as required by their role and/or preference. We believe this transition also opens additional and more diverse talent recruitment opportunities for Natus.
Teammate Engagement
We conduct a semi-annual engagement survey to collect feedback from our Teammates. In 2021, our engagement actions were focused on increasing communications, collaboration across our diverse and global organization, recognition and employee development.
Teammate feedback is also gathered through a variety of focus groups, onboarding and exit surveys and a diverse culture council to help support, retain and attract Teammates. We recognize that a culture of inclusion makes us stronger. Providing appropriate forums where Teammates globally can share ideas and feedback is an important component of our diversity and inclusion efforts.
We believe building a culture of appreciation starts with a simple thank you and acknowledgments, so we started a Peer Appreciation channel which has had hundreds of our Teammates recognizing one another from across the company, across the globe, across teams and functions.
Diversity, Inclusion and Belonging
Our commitment to a diverse and inclusive environment seeks to ensure our global teammates feel a sense of belonging, helping us to produce innovative products that serve our diverse customers and their patients. We know a diverse workforce also strengthens our ability to attract and retain talent who want to grow a career where their perspective is valued and encouraged.
We have an outreach program to recruit and promote our job opportunities to a broad range of organizations and hundreds of job boards with the intent of developing a diverse pool of qualified candidates.
We are committed to providing a work environment free of discrimination or harassment of any kind, supported by policies, training and communication. We have an open and robust support system for Teammates to address any issues, concerns or complaints. We provide management escalation paths, Human Resources support systems and a confidential ethics hotline that is monitored by the Corporate Compliance Officer and the Board of Directors. As part of our onboarding process, all Teammates must go through training on Natus Policies & Procedures and attest to our company Code of Conduct which outlines the standards for appropriate conduct and behavior.
Continuous learning and Development
We provide internal training to Teammates globally through our online learning platforms. In addition to our extensive quality and business process training, we provide comprehensive training resources to Teammates including an on-demand online learning system with over 7,000 courses to support skill building in a wide variety of areas from business, technical, leadership/management to personal productivity and wellness. Our Teammates each took an average of 20 hours of training in 2021.
Total rewards
Our global Total Rewards Philosophy is to establish and maintain total compensation programs that include competitive cash compensation for Teammates in all geographical regions where we operate, short and long-term incentives and comprehensive global health and welfare benefits that meet the varying needs of our Teammates. We added new mental, physical, family and financial wellness resources for Teammates in 2021 to cover a wide variety of personal needs and situations.
All Teammates (except those on commission) are eligible to participate in the corporate bonus program. Payment of the bonus is dependent upon financial and operating goals that our Teammates drive throughout the year.
In 2021, 95% of our Teammates received a merit salary increase based on performance. Our average salary increase was 3% with 6% of our population receiving a merit increase greater than 5%.
Health and safety
Protecting the safety, health, and well-being of our Teammates around the world is a key priority. Throughout the COVID-19 pandemic, we have remained focused on the health and safety of our Teammates by implementing new safety
14
protocols, providing personal protective equipment, increasing cleaning procedures and requiring the wearing of masks globally. In addition, we trained Teammates and increased communications, reconfigured our facilities to add space for physical distancing, implemented remote work where possible and enhanced our IT systems to facilitate working from home and improved cybersecurity.
We mandated the COVID-19 vaccine where permissible. We also anticipated the need for virus testing kits early on and secured a supply for our manufacturing sites and provided Teammates and their families with an onsite COVID-19 vaccination clinic at our largest manufacturing site.
Technology
Our HR Technology Roadmap was accelerated with more Teammates working remotely. In 2021, we implemented various technology enhancements like automation, standardization and increased security features to improve access, enhance the online experience and reduce risk in a globally distributed and often remote workforce.
Executives
The following table lists our executive officers and their ages as of February 25, 2022:
| Name | Age | Position(s) | ||||||||||||
| Thomas J. Sullivan | 58 | President and Chief Executive Officer | ||||||||||||
| B. Drew Davies | 56 | Executive Vice President and Chief Financial Officer | ||||||||||||
| D. Christopher Chung, M.D. | 58 | Vice President of Quality, Regulatory Affairs and Chief Medical Officer | ||||||||||||
| Austin F. Noll, III | 55 | Executive Vice President and Chief Commercial Officer | ||||||||||||
Thomas J. Sullivan began serving as Chief Executive Officer on December 27, 2021, and has served as a member of the Board of Directors since February 2019. Prior to joining Natus, Mr. Sullivan was the President & Chief Executive Officer of Spectrum Plastics Group, a medical device global contract manufacturer. In earlier roles, he led multiple public and privately owned medical device companies including A&E Medical, Symmetry Surgical, and Symmetry Medical. In addition, Mr. Sullivan held numerous executive roles at Johnson & Johnson (“J&J”) from 1990 to 2011 including President of J&J Medical Products Canada and the U.S. Orthopedics division. Mr. Sullivan graduated as a Palmer Scholar from The Wharton School at the University of Pennsylvania in 1991 where he earned an MBA in Strategic Management and Information Technology. He also holds a Bachelor of Science magna cum laude in Applied Mathematics and Computer Science from the University of Pittsburgh. Mr. Sullivan has earned the National Association of Corporate Directors (NACD) Directorship Certification.
B. Drew Davies joined Natus as Executive Vice President and Chief Financial Officer in October 2018. Mr. Davies most recently served as Executive Vice President and Chief Financial Officer of Extreme Networks since June 2016. Before joining Natus, Mr. Davies served as Vice President and Corporate Controller at Marvell Semiconductor Inc. from December 2015 until May 2016. Prior to that, Mr. Davies was the Senior Vice President, Corporate Controller at Spansion, Inc. from August 2012 to December 2015. Prior to Spansion, Mr. Davies was Corporate Controller at Intersil Corporation from April 2009 to August 2012, and served as Operations Controller from March 2008 to April 2009. Mr. Davies also served as Chief Financial Officer of Nanoconduction, Inc. from March 2007 to March 2008, Director of Finance and Administration for STATSChipPac from September 1999 to March 2007, held various finance roles at Micron Custom Manufacturing Services from November 1992 to September 1999. Mr. Davies holds a Master of Business Administration degree from Santa Clara University and a Bachelor of Science, Business Accounting degree from the University of Idaho.
D. Christopher Chung, joined Natus in 2000 as the Medical Director. He has also served as Vice President of R&D and most recently since 2011 as Vice President Medical Affairs, Quality and Regulatory. From 2000 to 2007, Dr. Chung also served as a Pediatric Hospitalist at the California Pacific Medical Center in San Francisco providing patient care in the Neonatal Intensive Care Unit and Newborn Nursery. From 1997 to 2000, Dr. Chung trained as a pediatric resident at Boston Children’s Hospital and Harvard Medical School. From 1986 to 1993, Dr. Chung worked as an R&D engineer at Nellcor Incorporated, a medical device company that pioneered the development of pulse oximetry. Dr. Chung holds a Bachelor of Arts degree in Computer Mathematics from the University of Pennsylvania and a Doctor of Medicine degree from the Medical College of Pennsylvania-Hahnemann University School of Medicine. He is board certified in Pediatrics and is a Fellow of the American Academy of Pediatrics. Dr. Chung has also been awarded nine U.S. Patents in the medical device field.
Austin F. Noll, III currently Chief Commercial Officer, joined Natus in August 2012 as the Vice President and General Manager, Neuro. Prior to joining Natus, Mr. Noll served as the President and CEO of Simpirica Spine, a California-based start-up company that developed and commercialized a novel device for spinal stabilization. Prior to joining Simpirica Spine, Mr. Noll served as the President and CEO of NeoGuide Systems, a medical robotics company acquired by Intuitive Surgical. Prior to joining NeoGuide Systems, Mr. Noll held numerous management positions at Medtronic over a 13-year period, where he served as the Vice President and General Manager of the Powered Surgical Solutions and the Neurosurgery businesses.
15
Before Medtronic, he held sales positions at C.R. Bard and Baxter Healthcare. He received a Bachelor of Science degree in Business Administration from Miami University and a Master of Business Administration degree from the University of Michigan.
Other Information
Natus was incorporated in California in May 1987 and reincorporated in Delaware in August 2000.
We maintain corporate offices at 3150 Pleasant View Road, Middleton, WI 53562. Our telephone number is (608) 829-8500. We maintain a corporate website at www.natus.com. References to our website address do not constitute incorporation by reference of the information contained on the website, and the information contained on the website is not part of this document.
We make available, free of charge on our corporate website, copies of our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, Proxy Statements, and all amendments to these reports, as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission pursuant to Section 13(a) or 15(d) of the Securities Exchange Act. We also show detail about stock trading by corporate insiders by providing access to SEC Forms 3, 4 and 5. This information may also be obtained from the SEC’s on-line database, which is located at www.sec.gov. Our common stock is traded on the Nasdaq Stock Market under the symbol “NTUS”.
Item 1A. Risk Factors
Risks Related to the COVID-19 Pandemic
Our business has been and may continue to be negatively affected by the ongoing COVID-19 pandemic and any future outbreaks of disease.
Our operations and financial performance have and continue to be significantly affected by the ongoing global COVID-19 pandemic and the resulting volatility and uncertainty it has caused in the U.S. and international markets. Over the course of fiscal year 2021, national, state and local authorities have continued to recommend social distancing, vaccinations, wearing masks, and quarantining when individuals are feeling ill or recovering from the effects of COVID-19 in an effort to mitigate the spread of COVID-19. However, this virus has had adverse impacts on the U.S. and foreign economies of uncertain severity and duration and has and may continue to negatively impact our ongoing operations, including our revenue, manufacturing and supply chain. Spikes in the transmission of COVID-19 and other variant strains of the virus may lead to renewed or expanded shutdowns, curfews or other isolation measures and may result in further restriction on, and decreased demand for, elective medical procedures, which may adversely affect our business operations, financial position or consolidated cash flows.
In addition, we have experienced and may continue to see disruption and delays in parts of our direct and indirect supply chain. We have made investments in inventory to help mitigate against further potential supply chain interruptions. These investments include increased inventory and firm purchase orders beyond our typical timeframe in order to secure capacity at our key suppliers. We have experienced, and may continue to experience, increased costs as a result of excess inventory, which in turn has resulted, and may continue to result, in lower gross margins. In addition, our inventory management systems and related supply chain visibility tools were not designed to forecast manage supply of our products and product components under these unprecedented pandemic conditions, and as a result our forecasts could be inaccurate or our supply decisions could be incorrect. We may experience restricted stock availability or delays or difficulty sourcing certain products in the future, which could negatively impact us.
As a result of the ongoing COVID-19 pandemic, much of our workforce continues to operate under a temporary remote working model, which may result in us experiencing lower work efficiency and productivity, which in turn may adversely affect our business. As our employees work from home and access our systems remotely, we may be subject to heightened security and privacy risks, including the risks of cyberattacks and privacy incidents. Additionally, we have a number of employees who continue to work in our facilities or perform services at our customers' facilities who may be subject to heightened risks for COVID-19 exposure thus potentially impacting their health and future worker compensation claims against us. We may also be subject to lawsuits from employees and others exposed to COVID-19 at our facilities, which could involve large demands and substantial defense costs. Our cyber liability, professional liability, and commercial general liability insurance may not cover all claims against us. Furthermore, if any of our employees are unable to perform his or her duties for a period of time, including as the result of illness, our results of operations or financial condition could be adversely affected. Finally, the widespread pandemic has caused and is expected to continue to cause significant disruption of global financial markets, which may reduce or impair our ability to access capital, or access capital on terms that would be consistent with our expectations.
16
We cannot estimate the length or severity of the COVID-19 pandemic, including the potential for further mutation and the long-term efficacy of vaccines, or the related response, including the length of time it may take for normal economic and operating conditions to resume or the extent to which the disruption may materially impact our business, consolidated financial position, consolidated results of operations or consolidated cash flows. To the extent the COVID-19 pandemic continues to adversely affect our business, it may also have the effect of heightening many of the other risks described here within.
As we allow our employees more flexibility, our organizational culture may be subject to more complexity due to managing employees working at remote locations, working at our office locations, and working in a hybrid workforce model.
Our culture is a key contributor to our success as an organization. While we have begun voluntary phased re-openings in our offices in accordance with guidance provided by government agencies, a significant portion of our workforce have been working remotely and have continued to work in more remote and hybrid work environments. Although many of our employees can effectively perform their responsibilities while working remotely, some work is not well-suited for remote work, and that work may not be completed as efficiently as if it were performed on site. A long-term continuation of these restrictions could, among other things, negatively impact employee morale and productivity. Any failure to preserve our culture could harm our future success, including our ability to retain and recruit personnel, innovate and operate effectively and execute on our business strategy.
Risks Related to Our Manufacturing and Supply Chain
The ability of suppliers to deliver parts, components and manufacturing equipment to our manufacturing facilities, and our ability to manufacture without disruption, could affect our global business performance.
We source components and materials to manufacture our products from a limited number of suppliers, resulting in our product supply being subject to such suppliers' lead times, volume constraints and increasing costs. We have experienced, and may continue to experience, extended lead times and product unavailability due to factory disruptions or closures as well as delays and unanticipated costs associated with the supply of our products, including expedite fees and air freight charges to mitigate delays in product supply, particularly in light of the COVID-19 pandemic. We also expect continued shortages and/or delay of critical components and related services as a result of growing demand in the industry or other sectors. For example, the global semiconductor supply shortages have resulted, and may continue to result, in lower availability, longer lead times, increased prices for such components and increased competition for logistics services. Our supply chain operations span several geographies globally, and we are heavily dependent upon third party logistics and transportation services to deliver our products to customers. We have experienced, and expect to continue to experience, increased competition for and disruptions in logistics and transportation services due to transportation backlogs and labor shortages, which have resulted in longer lead times, increased prices, and surcharges and increased investments in critical components and higher overall costs to manage our supply chain logistics. Extended lead times and shortages could impair our ability to meet our customers' requirements, require us to pay higher prices or incur expedite fees, which would harm our business and negatively impact our gross margin and results of operations.
Adverse economic conditions in markets in which we operate may harm our business.
Unfavorable changes in U.S. and international economic environments may adversely affect our business and financial results. Concerns over the economic stability, the level of U.S. national debt, currency fluctuations and volatility, the growth of economies, unemployment, the availability and cost of credit, inflation levels, trade relations, energy costs and geopolitical uncertainty have contributed to increased volatility in the global economy and markets. During challenging economic times, and in tight credit markets, our customers may delay or reduce capital expenditures. This could result in reductions in sales of our products, longer sales cycles, difficulties in collection of accounts receivable, slower adoption of new technologies, and increased price competition, all of which could impact our results of operations and financial condition. In addition, if these factors are present they will cause us to be more cautious in evaluating potential acquisition opportunities, which could hinder our ability to grow through acquisition while conditions continue.
In addition, we are susceptible to risks related to natural disasters and the COVID-19 pandemic. In particular, the spread of COVID-19 globally could adversely affect our operations, including our manufacturing and supply chain, sales and marketing, and product development efforts depending on the availability of parts and potentially longer lead times. Our direct and indirect supply chain relies on manufacturing at, and transportation of products and materials to and from, facilities located throughout the world, and is accordingly subject to disruption or product contamination. Additionally, our results of operations could be adversely affected to the extent that COVID-19 or any other epidemic harms our business or the economy in general either globally or in any region in which we do business. The extent to which COVID-19 will continue to affect our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, the emergence of new variants, the efficacy of vaccines and other treatments, new information that may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others, and could have an adverse effect on our business and financial condition.
17
Further, the U.S. federal government has called for, or enacted, substantial changes to healthcare, trade, fiscal, and tax policies, which may include changes to existing trade agreements and may have a significant impact on our operations. We cannot predict the impact, if any, that these changes could have on our business. If economic conditions worsen or new legislation is passed related to the healthcare system, trade, fiscal or tax policies, customer demand may not materialize to levels we require to achieve our anticipated financial results, which could have a material adverse effect on our business, financial condition and results of operations.
Uncertain credit markets and concerns regarding the availability of credit could impact consumer and customer demand for our products, as well as our ability to manage normal commercial relationships with our customers, suppliers and creditors, including financial institutions. If the current situation deteriorates, our business could be negatively affected by factors such as reduced demand for our products resulting from a slow‑down or volatility in the general economy, supplier or customer disruptions and/or temporary interruptions in our ability to conduct day‑to‑day transactions through our financial intermediaries involving the payment to or collection of funds from our customers, vendors and suppliers.
If we lose our relationship with any supplier of key product components or our relationship with a supplier deteriorates or key components are not available in sufficient quantities, our manufacturing could be delayed and our business could suffer.
We contract with third parties for the supply of some of the components used in our products and the production of our disposable products. Some of our suppliers are not obligated to continue to supply us. We have relatively few sources of supply for some of the components used in our products and in some cases we rely entirely on sole-source suppliers. In addition, the lead-time involved in the manufacturing of some of these components can be lengthy and unpredictable. If our suppliers become unwilling or unable to supply us with components meeting our requirements, it might be difficult to establish additional or replacement suppliers in a timely manner, or at all. This would cause our product sales to be disrupted and our revenue and operating results to suffer.
Replacement or alternative sources might not be readily obtainable due to regulatory requirements and other factors applicable to our manufacturing operations. Incorporation of components from a new supplier into our products may require a new or supplemental filing with applicable regulatory authorities and clearance or approval of the filing before we could resume product sales. This process may take a substantial period of time, and we may not be able to obtain the necessary regulatory clearance or approval. This could create supply disruptions that would harm our product sales and operating results.
Because we rely on distributors or sub-distributors to sell our products in most of our markets outside of the United States, our revenue could decline if our existing distributors reduce the volume of purchases from us, or if our relationship with any of these distributors is terminated.
We currently rely on our distributors or sub-distributors for a majority of our sales outside the United States. Some distributors also assist us with regulatory approvals and education of clinicians and government agencies. Our contracts with our distributors or sub-distributors do not assure us significant minimum purchase volume. If a contract with a distributor or sub-distributor is terminated for cause or by us for convenience, the distributor or sub-distributor will have no obligation to purchase products from us. We intend to continue our efforts to increase our sales in Europe, Asia, and other developed markets throughout the world. If we fail to sell our products through our international distributors, we would experience a decline in revenue unless we begin to sell our products directly in those markets. We may not be able to attract new international distributors to market our products effectively or provide timely and cost-effective customer support and service. Even if we are successful in selling our products through new distributors, the rate of growth of our revenue could be harmed if our existing distributors do not continue to sell a large dollar volume of our products. None of our existing distributors are obligated to continue selling our products.
We may be subject to foreign laws governing our relationships with our international distributors. These laws may require us to make payments to our distributors if we terminate our relationship for any reason, including for cause. Some countries require termination payments under local law or legislation that may require us to pay to terminate our contractual relationship with the distributor. Any required payments could adversely affect our operating results.
We have substantial international operations which are subject to numerous risks; if our international operations are not successful, our business will be adversely affected.
In 2021, approximately 39.6% of our sales were made outside the United States. We plan to expand our international sales and marketing efforts to increase sales of our products in foreign countries. We may not realize corresponding growth in revenue from growth in international unit sales, due to the lower average selling prices we receive on sales outside of the United States. Even if we are able to successfully expand our international selling efforts, we cannot be certain that we will be able to create or increase demand for our products outside of the United States. Our international operations are subject to other risks, which include:
18
•The ongoing COVID-19 pandemic, which has caused disruptions in our and our distributors', vendors' and customers' respective operations, and any future outbreak of a contagious disease;
•Impact of possible recessions in economies outside the United States;
•Political and economic instability, including instability related to war and terrorist attacks and to political and diplomatic matters;
•Adverse changes in tariffs and trade protection measures;
•Difficulty in obtaining and maintaining foreign regulatory approval and complying with foreign regulations, including the EU Medical Device Regulations;
•Contractual provisions governed by foreign law, such as local law rights to sales commissions by terminated distributors;
•Decreased healthcare spending by foreign governments that would reduce international demand for our products;
•Strengthening of the U.S. dollar relative to foreign currencies that could make our products less competitive because slightly less than half of our international sales are denominated in U.S. dollars;
•Changes in capital and exchange controls affecting international trade;
•Greater difficulty in accounts receivable collection and longer collection periods;
•Difficulties of staffing and managing foreign operations;
•Reduced protection for intellectual property rights in some countries and potentially conflicting intellectual property rights of third parties under the laws of various foreign jurisdictions;
•Attitudes by clinicians, and cost reimbursement policies, towards use of disposable supplies that are potentially unfavorable to our business;
•Complying with U.S. regulations that apply to international operations, including trade laws, the U.S. Foreign Corrupt Practices Act, and anti-boycott laws, as well as international laws such as the U.K. Bribery Act;
•Loss of business through government tenders that are held annually in many cases; and
•Potentially negative consequences from changes in tax laws, including legislative changes concerning taxation of income earned outside of the United States.
Risks Related to Our Business and Operations
We depend upon key employees in a competitive market for skilled personnel, and, without retaining our existing employees and recruiting additional employees, we may not be able to grow or maintain profitability.
Our products and technologies are complex, and we depend substantially on the continued service of our senior management team. The loss of any of our key employees could adversely affect our business and slow our product development process. Our future success will depend, in part, on the continued service of our key management personnel, software engineers, and other research and development employees, and our ability to identify, hire, and retain additional personnel, including customer service, marketing, and sales staff. Demand for these skilled employees in our industry is very competitive due to the limited number of people available with the necessary technical skills and understanding of our product technologies. We may be unable to attract and retain personnel necessary for the development of our business.
We previously identified a material weakness in our internal control over financial reporting in a prior period, and if we fail to maintain proper and effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act in the future, the accuracy and timeliness of our financial reporting may be adversely affected.
The Sarbanes-Oxley Act requires, among other things, that we assess the effectiveness of our internal control over financial reporting annually and disclosure controls and procedures quarterly. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on, and our independent registered public accounting firm to attest to, the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act.
During the fourth quarter of 2019, we identified errors as part of closing our books and concluded that certain deficiencies existed in the Company’s internal control over financial reporting. Specifically, we did not have controls designed to identify and properly account for certain research and development activities related to an arrangement with a third party. Additionally, insufficient training provided to a new control operator and the design of one of our controls over payroll accounts contributed to an error in the period end accrual. The Company has concluded that these deficiencies could have resulted in a material misstatement of the consolidated financial statements that would not have been prevented or detected on a timely basis, and as such, these control deficiencies resulted in a material weakness in our internal control over financial reporting. We have made substantive changes to enhance our design of controls to identify and assess contracts that include research and development and to confirm the accuracy of the payroll accruals. Process updates focused on material weakness remediation were implemented early in 2020 and operated throughout the year. After completing the necessary year-end testing of the enhanced procedures, we concluded that the previously identified material weakness is remediated as of December 31, 2020. However, our current efforts to maintain an effective control environment may not be sufficient to prevent future material
19
weaknesses or significant deficiencies from occurring or to promptly remediate any such future material weaknesses or significant deficiencies.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis. Controls and Procedures-Management’s Report on Internal Control Over Financial Reporting. The existence of this material weakness and of any other ineffective controls over our financial reporting could result in one or more of the following:
•Revision of previously filed financial statements;
•Failure to meet our reporting obligations;
•Loss of investor confidence; and
•Negative impact on the trading price of our common stock.
If other material weaknesses are identified in the future or we are not able to comply with the requirements of Section 404 in a timely manner, we may be unable to accurately report our consolidated financial results, or report them within the timeframes required by the SEC, we could receive an adverse opinion regarding our controls from our independent registered accounting firm and we could be subject to investigations or sanctions by regulatory authorities, which would require additional financial and management resources, and the market price of our stock could decline.
The United Kingdom's withdrawal from the European Union may have a negative effect on global economic conditions, financial markets and our business and operations.
The U.K. exited from the European Union on January 31, 2020 (often referred to as “Brexit”). On December 24, 2020, the U.K. and the European Union entered into a trade and cooperation agreement (the “Trade and Cooperation Agreement”), which was provisionally applied as of January 1, 2021 and entered into force on May 1, 2021 following ratification by the EU. While the economic integration does not reach the level that existed during the time the U.K. was a member state of the European Union, the Trade and Cooperation Agreement sets out preferential arrangements in areas such as trade in goods and in services, digital trade and intellectual property. Negotiations between the U.K. and the European Union are expected to continue in relation to the relationship between the U.K. and the European Union in certain other areas which are not covered by the Trade and Cooperation Agreement. The long-term effects of Brexit will depend on the effects of the implementation and application of the Trade and Cooperation Agreement and any other relevant agreements between the U.K. and the European Union. It is still unclear what terms, if any, may be agreed within the U.K. and between the U.K. and other countries on many aspects of fiscal policy, cross-border trade and international relations, both in the final outcome and for any transitional period. The withdrawal of the U.K. from the European Union could potentially disrupt the free movement of goods, services and people between the U.K. and the European Union, including in Ireland where we have significant manufacturing operations, undermine bilateral cooperation in key geographic areas and significantly disrupt trade between the U.K. and the European Union or other nations as the U.K. pursues independent trade relations. In addition, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the U.K. determines which European Union laws to replace or replicate. Because this is an unprecedented event, it is unclear what long-term economic, financial, trade and legal implications Brexit would have and how it would affect the regulation applicable to our business globally and in the region. Any of these developments, along with any political, economic and regulatory changes that may occur, could cause political and economic uncertainty in Europe and internationally and harm our business and financial results. The impact on Natus from Brexit will depend, in part, on the application of the Trade and Cooperation Agreement and the outcome of other tariff, trade, regulatory and other negotiations. Although we have not observed a material financial impact or identified any trends or potential changes to critical accounting estimates as a result of Brexit at this time we will continue to assess the impact of Brexit on our business and operations, and on accounting and reporting considerations. The effects of Brexit and the application of the Trade and Cooperation Agreement could adversely affect our business, financial condition or future results.
Our operating results may suffer because of our exposure to foreign currency exchange rate fluctuations.
Substantially all of our sales contracts with our U.S. based customers provide for payment in U.S. dollars. With the exception of our Canadian operations, substantially all of the revenue and expenses of our foreign subsidiaries are denominated in the applicable foreign currency for the countries in which they operate. In the past, we have executed only limited foreign currency contracts to hedge these currency risks. Our future revenue and expenses may be subject to volatility due to exchange rate fluctuations that could result in foreign exchange gains and losses associated with foreign currency transactions and the translation of assets and liabilities denominated in foreign currencies.
Substantially all our sales from our U.S. operations to our international distributors provide for payment in U.S. dollars. A strengthening of the U.S. dollar relative to other foreign currencies could increase the effective cost of our products to our
20
international distributors as their functional currency is typically not the U.S. dollar. This could have a potential adverse effect on our ability to increase or maintain average selling prices of our products to our foreign-based customers.
The interest rates on our revolving credit facility are priced using a spread over LIBOR.
LIBOR, the London interbank offered rate, is the basic rate of interest used in lending between banks on the London interbank market and is widely used as a reference for setting the interest rate on loans globally. We typically use LIBOR as a reference rate in our term loans such that the interest due to our creditors pursuant to a term loan extended to us is calculated using LIBOR. Most of our term loan agreements contain a stated minimum value for LIBOR.
Our credit facility permits interest on the outstanding principal balance to be calculated based on LIBOR. On July 27, 2017, the U.K. Financial Conduct Authority (the "FCA") announced that it will no longer require banks to submit rates for the calculation of LIBOR after June 2023, and the U.S. Federal Reserve and the Bank of England have begun publishing a Secured Overnight Funding Rate ("SOFR"), which is currently intended to serve as alternative reference rate to LIBOR. SOFR significantly differs from LIBOR, both in the actual rate and how it is calculated, and therefore is it unclear whether and when markets will adopt this rate as a widely accepted replacement for LIBOR. During the third quarter of 2020, we amended the terms of our credit facility to permit a transition of the reference rate from LIBOR upon the expiration of LIBOR or an earlier election by the parties to the credit facility. At this time, it is not possible to predict the effect of any such changes, any establishment of alternative reference rates or any other reforms to LIBOR that may be implemented in the United Kingdom or elsewhere. Actions in the meantime, by the FCA, other regulators, or law enforcement agencies are expected to influence the method by which LIBOR is calculated. At this time, it is not possible to predict the effect of any such changes or any other reforms to LIBOR that may be enacted in the U.K. or elsewhere. Uncertainty as to the nature of such potential changes, alternative reference rates or other reforms may adversely affect our indebtedness that bear interest at a floating rate determined by reference to LIBOR.
Future changes in technology or market conditions could result in adjustments to our recorded asset balance for intangible assets, including goodwill, resulting in additional charges that could significantly impact our operating results.
Our balance sheet includes significant intangible assets, including goodwill and other acquired intangible assets. The determination of related estimated useful lives and whether these assets are impaired involves significant judgment. Our ability to accurately predict future cash flows related to these intangible assets might be hindered by events over which we have no control. Due to the highly competitive nature of the medical device industry, new technologies could impair the value of our intangible assets if they create market conditions that adversely affect the competitiveness of our products. Further, declines in our market capitalization may be an indicator that our intangible assets or goodwill carrying values exceed their fair values which could lead to potential impairment charges that could impact our operating results.
We have experienced seasonality in the sale of our products.
We experience seasonality in our revenue. For example, our sales typically decline from the second half of our fiscal year to the first half of the fiscal year, due to patterns in the capital budgeting and purchasing cycles of our customers, many of which are government agencies, and the compensation arrangements of our direct sales employees, as those arrangements are tied to calendar-year sales plans. We anticipate that we will continue to experience seasonal fluctuations, which may lead to fluctuations in our quarterly operating results. We believe that you should not rely on our results of operations for interim periods as an indication of our expected results in any future period.
We make regular changes to our information systems that could disrupt our business and our financial results.
We continuously improve and plan to continue to improve our information systems to support the form, functionality, and scale of our business. These types of transitions frequently prove disruptive to the underlying business of an enterprise and may cause us to incur higher costs than we anticipate. Failure to manage a smooth transition to the new systems and the ongoing operations and support of the new systems could materially harm our business operations.
Risks Related to Growth and Competition
Our operating results may decline if we do not succeed in developing, acquiring, and marketing additional products or improving our existing products.
We intend to develop additional products and technologies, including enhancements of existing products, for the screening, detection, treatment, monitoring and tracking of common medical ailments. Developing new products and improving our existing products to meet the needs of current and future customers requires significant investments in research and development. Healthcare products typically receive regulatory approval based on data obtained in controlled clinical trials, which can be time consuming and expensive. Unfavorable or inconsistent clinical data from existing or future clinical trials may adversely affect our ability to secure marketing authorization for certain products. Furthermore, all results, even positive ones, are subject to the interpretation of FDA and other regulatory agencies. If we fail to sell new products, update existing products,
21
or timely react to changes in technology, our operating results may decline as our existing products reach the end of their commercial life cycles.
Demand for some of our products depends on the capital spending policies of our customers, and changes in these policies could harm our business.
A majority of customers for our products are hospitals, physician offices, and clinics. Many factors, including public policy spending provisions, available resources, and economic cycles have a significant effect on the capital spending policies of these entities and the amount that they can spend on our equipment products. If budget resources limit the capital spending of our customers, they will be unlikely to either purchase any new equipment from us or upgrade to any of our newer equipment products. Lack of liquidity in credit markets and uncertainty about future economic conditions can have an adverse effect on the spending patterns of our customers. These factors can have a significant adverse effect on the demand for our products.
Our business results depend on our ability to successfully manage ongoing strategic initiatives and business transformation to drive growth and achieve cost savings and operating efficiency.
Our financial goals assume a level of growth and increased productivity. If we are unable to deliver these expected improvements, or continue to invest in business growth, or if the volume and nature of change require additional resources, our business operations and financial results could be materially and adversely impacted. Our ability to successfully manage and execute these initiatives and realize the related profitability improvement in the amounts and at the times anticipated is important to our business success. Any failure to do so, which could result from our inability to successfully execute organizational change and business transformation plans, changes in global or regional economic conditions, competition, changes in the industries in which we compete, unanticipated costs or charges, loss of key personnel and other factors described herein, could have a material adverse effect on our businesses, financial condition and results of operations.
At previous points in our past, our growth has depended substantially on the completion of acquisitions and we may not be able to complete or successfully capitalize on acquisitions of the same nature or relative size in the future to support a similar level of growth.
The acquisitions that we have completed have contributed to our growth in prior years. We have expended considerable effort in seeking to identify attractive acquisition candidates, and ultimately, to negotiate mutually agreeable acquisition terms. The market for attractive acquisitions is competitive and others with different strategic objectives or greater financial resources than we have may be better positioned than we are to acquire desirable targets. Further, we may not be able to negotiate acquisition terms with target companies that will allow us to achieve acceptable financial returns from the transaction.
If completed, the success of any acquisitions will depend, in part, on our ability to successfully combine and integrate the acquired business into our businesses and realize the anticipated benefits, including synergies, from the transaction. The integration of any acquired businesses may result in material challenges, including: the diversion of management's attention from ongoing business concerns and performance shortfalls at one or both of the companies; maintaining employee morale and retaining key management, sales and other employees; retaining existing business and operational relationships; the possibility of faulty assumptions underlying expectations regarding the integration process; consolidating corporate and administrative infrastructures and eliminating duplicative operations; unanticipated issues in integrating information technology, communications and other systems; and unforeseen costs, expenses and liabilities (including litigation related liabilities) associated with such acquisition.
If we fail in our efforts to educate clinicians, government agency personnel, and third-party payors about the effectiveness of our products, we may not achieve future sales growth.
It is critical to the success of our sales efforts that we educate a sufficient number of clinicians, hospital administrators, and government agencies about our products and the costs and benefits of their use. The commercial success of our products depends upon clinician, government agency, and other third-party payor confidence in the economic and clinical benefits of our products as well as their comfort with the efficacy, reliability, sensitivity, and specificity of our products. We believe that clinicians will not use our products unless they determine, based on published peer-reviewed journal articles and experience, that our products provide an accurate and cost-effective alternative to other means of testing or treatment. Our customers may choose to use competitive products, which may be less expensive or may provide faster results than our devices. Clinicians are traditionally slow to adopt new products, testing practices and clinical treatments, partly because of perceived liability risks and the uncertainty of third-party reimbursement. If clinicians, government agencies and hospital administrators do not adopt our products, we may not maintain profitability. Factors that may adversely affect the medical community’s acceptance of our products include:
•Publication of clinical study results that demonstrate a lack of efficacy or cost-effectiveness of our products;
•Changing governmental and physician group guidelines;
•Actual or perceived performance, quality, price, and total cost of ownership deficiencies of our products relative to other competitive products;
22
•Our ability to maintain and enhance our existing relationships and to form new relationships with leading physicians, physician organizations, hospitals, state laboratory personnel, and third-party payors;
•Changes in federal, state and third-party payor reimbursement policies for our products; and
•Repeal of laws requiring universal newborn hearing screening and metabolic screening.
Sales to members under group purchasing agreements and sales to high volume purchasers may reduce our average selling prices, which could reduce our operating margins.
We have entered and expect in the future to enter into agreements with customers who purchase a high volume of our products. Our agreements with these customers may contain discounts from our normal selling prices and other special pricing considerations, which could cause our operating margins to decline. In addition, we have entered into agreements to sell our products to members of GPOs, which negotiate volume purchase prices for medical devices and supplies for member hospitals, group practices and other clinics. While we make sales directly to GPO members, the GPO members receive volume discounts from our normal selling price and may receive other special pricing considerations from us. Sales to members of all GPOs accounted for approximately 23.6%, 22.5% and 18.7% of our total revenue during 2021, 2020 and 2019, respectively. Certain other existing customers may be members of GPOs with which we do not have agreements. If we enter into agreements with new GPOs and some of our existing customers begin purchasing our products through those GPOs, our operating margins could decline.
Consolidation in the healthcare industry could have an adverse effect on our revenues and results of operations.
Many healthcare industry companies, including our customers and competitors, are consolidating to create new companies with greater market power. As the healthcare industry consolidates, competition to provide goods and services to our customers could become more intense. Our customers may try to use their market power to negotiate price concessions and our competitors may utilize their size and broad product lines to offer cheaper alternatives to our products. If we are forced to reduce our prices because of consolidation in the healthcare industry, our revenues would decrease and our consolidated earnings, financial condition, or cash flow would suffer.
Our markets are very competitive and in the United States we sell certain of our products in a mature market.
We face competition from other companies in all our product lines. Our competitors range from small privately held companies to multinational corporations and their product offerings vary in scope and breadth. We do not believe that any single competitor is dominant in any of our product lines.
The markets for certain of our products in the United States, including the newborn hearing screening, EMG, and EEG monitoring markets, are mature and we are unlikely to see significant growth for such products in the United States. The market for newborn care products is affected by birthrates, and a declining U.S. birthrate has adversely affected our operating results in recent periods. In the United States we derive a significant portion of our revenue from the sale of disposable supplies. Our disposable supply products could face increasing competition, including competitors offering lower prices, which could have an adverse effect on our revenue and margins.
Our competitors may have certain competitive advantages, which include the ability to devote greater resources to the development, promotion, and sale of their products. Consequently, we may need to increase our efforts, and related expenses for research and development, marketing, and selling to maintain or improve our position.
We expect recurring sales to our existing customers to generate much of our revenue in the future, and if our existing customers do not continue to purchase products from us, our revenue may decline.
If healthcare providers are not adequately reimbursed for procedures conducted with our devices or supplies, or if reimbursement policies change adversely, we may not be successful marketing and selling our products or technologies.
Clinicians, hospitals, and government agencies are unlikely to purchase our products if they are not adequately reimbursed for the procedures conducted with our devices or supplies. Unless a sufficient amount of conclusive, peer-reviewed clinical data about our products has been published, third-party payors, including insurance companies and government agencies, may refuse to provide reimbursement. Furthermore, even if reimbursement is provided, it may not be adequate to fully compensate the clinicians or hospitals. Some third-party payors may impose restrictions on the procedures for which they will provide reimbursement. If healthcare providers cannot obtain sufficient reimbursement from third-party payors for our products or the screenings conducted with our products, we may not achieve significant market acceptance of our products. Acceptance of our products in international markets will depend upon the availability of adequate reimbursement or funding within prevailing healthcare payment systems. Reimbursement, funding, and healthcare payment systems vary significantly by country. We may not obtain approvals for reimbursement in a timely manner or at all.
Adverse changes in reimbursement policies in general could harm our business. We are unable to predict changes in the reimbursement methods used by third-party healthcare payors, particularly those in countries and regions outside the United States. For example, some payors are moving toward a managed care system in which providers contract to provide
23
comprehensive healthcare for a fixed cost per person. In a managed care system, the cost of our products may not be incorporated into the overall payment for patient care or there may not be adequate reimbursement for our products separate from reimbursement for other procedures.
Risks Related to Our Compliance and Regulatory Environment
An interruption in or breach of security of our information or manufacturing systems, including the occurrence of a cyber-incident or a vulnerability in our cybersecurity, or disclosure of private patient health information, may result in a loss of business or damage to our reputation.
We rely on communications, information and manufacturing systems to conduct our business. Any failure, interruption or cyber incident affecting these systems could result in failures or disruptions in our customer relationship management or product manufacturing. Similarly, there can be no assurance that our third-party collaborators, distributors and other contractors and consultants will be successful in protecting our data that is stored on their systems. A cyber incident is an intentional attack or an unintentional event that can include an unauthorized actor gaining access to our systems to disrupt operations, corrupt data, or steal confidential information. In October 2021, we identified a cyber incident that resulted in the unauthorized access to certain personal data, including the personal data of certain of our current and former employees. We are not aware of any evidence that any patient data was accessed, and the breach has not materially impacted our operations. However, the occurrence of any future failures, interruptions or cyber incidents could result in a loss of customer business or reputation and have a material effect on our business, financial condition, results of operations and cash flows. In addition, our products are used in customer networks transmitting a range of sensitive information and any actual or perceived exposure of our products used in customer networks to malicious software or cyber-attacks could adversely affect our business and results of operations.
In the course of performing our business we obtain, from time to time, confidential patient health information. For example, we may learn patient names and be exposed to confidential patient health information when we provide training on our products to our customers’ staff. Complying with federal and state privacy and security requirements imposes compliance related costs, subjects us to potential regulatory audits, and may restrict our business operations. These various laws may be subject to varying interpretations by courts and government agencies creating potentially complex compliance issues for our business. If we were to violate any of our legal obligations to safeguard any confidential patient health information or protected health information against improper use and disclosure, we could lose customers and be exposed to liability, and our reputation and business could be harmed. Concerns or allegations about our practices with regard to the privacy or security of personal health information or other privacy-related matters, even if unfounded, could damage our reputation and harm our business.
We are also subject to new U.S. laws as well as laws and regulations in foreign countries covering data privacy and other protection of health and employee information that may be more onerous than previous corresponding U.S. laws. These laws and regulations impose technical and organizational measures to ensure the security of personal data and may require that we notify regulatory agencies, individuals or the public about any data security breaches. In particular, in the United States, the California Consumer Privacy Act of 2018 (“CCPA”) imposes a private right of action with damages of up to $750 per person in the event of certain data breaches. Other states are considering similar laws. As these laws and regulations develop in the United States and we expand our international operations, we may be required to expend significant time and resources to put in place additional mechanisms to ensure compliance with multiple cybersecurity laws. Failure to comply with these laws may result in significant fines and other administrative penalties and harm our business, and could expose us to significant civil damages.
The FDA has issued guidance advising manufacturers to take cybersecurity risks into account in product design for connected medical devices and systems, to assure that appropriate safeguards are in place to reduce the risk of unauthorized access or modification to medical devices that contain software and reduce the risk of introducing threats into hospital systems that are connected to such devices. The FDA also issued guidance on post market management of cyber security in medical devices. Compliance with these requirements may require changes in business practices, complicate our operations, and add complexity and additional management and oversight needs. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data. Failure to comply with these laws could result in vulnerabilities that would make us susceptible to hackers and other cyber attacks, as well as fines or administrative penalties that, if imposed, would harm our business.
Our ability to market and sell products depends upon receipt of domestic and foreign regulatory clearances of our products and successful inspections of our manufacturing operations. Our failure to obtain or maintain regulatory compliance could negatively affect our business.
Our products and manufacturing operations are subject to extensive regulation in the United States by the FDA and by similar regulatory agencies in other countries. Our products are classified as medical devices. Medical devices are subject to extensive regulation by the FDA pursuant to regulations that are wide ranging and govern, among other things: design and development; manufacturing and testing; labeling; storage and record keeping; advertising, promotion, marketing, sales distribution and export; and surveillance and reporting of deaths or serious injuries.
24
Unless an exemption applies, each medical device that we propose to market in the United States must first receive one of the following types of FDA premarket review authorizations:
•Clearance via Section 510(k) of the Food, Drug, and Cosmetics Act of 1938, as amended; or
•Premarket approval via Section 515 of the Food, Drug, and Cosmetics Act if the FDA has determined that the medical device in question poses a greater risk of injury.
The FDA will clear marketing of a medical device through the 510(k) process if it is demonstrated that the new product is substantially equivalent to other 510(k)-cleared products. The premarket approval application process is much more costly, lengthy and uncertain than the 510(k) process, and must be supported by extensive data from preclinical studies and human clinical trials. The FDA may not grant either 510(k) clearance or premarket approval for any product we propose to market. Further, any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or, possibly, approval of a premarket approval application. The FDA requires every manufacturer to make this determination in terms of the classification of the modification, but the FDA may review any manufacturer’s decision at any time. If the FDA requires us to seek 510(k) clearance or premarket approval for modification of a previously cleared product for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Further, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective.
Delays in receipt of, or failure to receive, clearances or approvals, the loss of previously received clearances or approvals, or the failure to comply with existing or future regulatory requirements could adversely impact our operating results. If the FDA finds that we have failed to comply with these requirements, the FDA can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
•Fines, injunctions and civil penalties;
•Recall or seizure of our products;
•Issuance of public notices or warnings;
•Imposition of operating restrictions, partial suspension, or total shutdown of production;
•Refusal of our requests for Section 510(k) clearance or premarket approval of new products;
•Withdrawal of Section 510(k) clearance or premarket approvals already granted;
•Criminal prosecution;
•Domestic regulation of our products and manufacturing operations, other than that which is administered by the FDA, includes the Environmental Protection Act, the Occupational Safety and Health Act, and state and local counterparts to these Acts; or
Foreign governments and regulatory authorities have, and may continue to, propose and implement regulations that apply to our products and operations. Penalties for regulatory non-compliance could be severe, including fines and revocation or suspension of a company's business license, mandatory price reductions, and criminal sanctions. Future laws and regulations may have a material adverse effect on our business.
Our business would be harmed if regulatory authorities determine that we have failed to comply with applicable regulations governing the manufacture of our products and/or if we are subject to enforcement actions such as seizure, injunction, fines or criminal prosecution.
We and our suppliers are required to demonstrate and maintain compliance with the FDA’s Quality System Regulation. The Quality System Regulation sets forth the FDA’s requirements for good manufacturing practices of medical devices and includes requirements for, among other things, the design, testing, production processes, controls, quality assurance, labeling, packaging, storage and shipping of such products. In addition, we and our suppliers must engage in extensive recordkeeping and reporting and must make available our manufacturing facility and records for periodic unscheduled inspections by federal, state and foreign agencies, including the FDA. We cannot assure you that we and our suppliers are or will continue to be in full compliance with the Quality System Regulation, nor that we will not encounter any manufacturing difficulties.
A failure by us or our third party suppliers and manufacturers to comply with applicable regulations or to adequately respond to any future compliance issues raised by the FDA or regulatory agencies in other countries that we are subject to could result in sanctions being imposed on us, including, among other things, fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of our products, delays, suspension or withdrawal of approvals, seizures or recalls of products and manufacturing restrictions, any of which could harm our business.
If we deliver products with defects, we may incur costs to repair and, possibly, recall that product and market acceptance of our products may decrease.
The manufacturing and marketing of our products involve an inherent risk of our delivering a defective product or products that do not otherwise perform as we expect. We may incur substantial expense to repair any such products and may
25
determine to recall such a product, even if not required to do so under applicable regulations. Any such recall would be time consuming and expensive, diverting management's attention and resources. Product defects or recalls may adversely affect our customers’ acceptance of the recalled and other of our products.
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
We could be subject to healthcare fraud regulation and enforcement by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include: (i) the federal healthcare programs Anti-Kickback Law, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as Medicare or Medicaid, (ii) federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us which provide coding and billing advice to customers, and/or (iii) state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, many of which differ from their federal counterparts in significant ways, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that their provisions are open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Our business may suffer if we are required to revise our labeling or promotional materials, or if regulatory authorities take an enforcement action against us for promoting off-label use of our products.
We are prohibited by the FDA from promoting or advertising our medical device products for uses not within the scope of our clearances or approvals, or from making unsupported promotional claims about the benefits of our products. If the FDA determines that our claims are outside the scope of our clearances, or are unsupported, it could require us to revise our promotional claims or take enforcement action against us. If we were subject to such an action by the FDA, our sales could be delayed, our revenue could decline, and our reputation among clinicians could be harmed. Likewise, if we acquire new products, either through the purchase of products, technology assets, or businesses, that are subsequently deemed to have inadequate supporting data, we may be required to (i) obtain adequate data, which could be costly and impede our ability to market these products, or (ii) modify the labeling on these products, which could impair their marketability, as described above.
We are subject to data privacy laws and our failure to comply with them may require us to make significant changes to our products or incur penalties or other liabilities.
Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personally identifiable information (“PII”), personal health information, financial information, intellectual property and other sensitive information related to our customers and workforce. The collection, maintenance, protection, use, transmission, disclosure and disposal of certain personal information and the security of medical devices are regulated at the U.S. federal and state, international and industry levels. U.S. federal and state laws, such as the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information. We sign business associate agreements with certain customers for which we perform services as business associates under HIPAA, and therefore we are directly subject to certain provisions of HIPAA that are applicable to business associates. Noncompliance with laws and regulations relating to privacy and security of personal information, including HIPAA, or with contractual obligations under any business associate agreement may lead to significant fines, civil and criminal penalties, or liabilities. We also may be required to report breaches of protected health information that we experience. The U.S. Department of Health and Human Services (“HHS”), audits the compliance of business associates and enforces HIPAA privacy and security standards. HHS enforcement activity has become more significant over the last few years and HHS has signaled its intent to continue this trend.
In addition to the regulation of personal health information, a number of states have also adopted laws and regulations that may affect our privacy and data security practices for other kinds of PII, such as state laws that govern the use, disclosure and protection of personal information, such as social security numbers, or that are designed to protect credit card account data. State consumer protection laws, including the CCPA, which became effective on January 1, 2020, may also establish privacy and security standards for use and management of PII, including information related to customers, suppliers and care providers. The CCPA provides individuals certain rights regarding the collection or processing of personal data related to California
26
residents, which may restrict our ability to use personal data, of California residents. Failure to comply with the CCPA could result in penalties of up to $7,500 per violation. Moreover, a new privacy law, the California Privacy Rights Act (“CPRA”) was approved by ballot initiative during the November 3, 2020 general election. Effective starting on January 1, 2023, the CPRA imposes additional obligations on companies covered by the legislation and will significantly modify the CCPA, including by expanding consumers' rights with respect to certain sensitive personal information. The CPRA also creates a new state agency that will be vested with authority to implement and enforce the CCPA and the CPRA. The effects of the CCPA and the CPRA are potentially significant and may require us to modify our data collection or processing practices and policies and to incur substantial costs and expenses to comply and increase our potential exposure to regulatory enforcement and/or litigation. Additionally, the Federal Trade Commission, and many state attorneys general are interpreting federal and state consumer protection laws to impose standards for the online collection, use, dissemination, and security of data. The burdens imposed by the CCPA and other similar laws that have been or may be enacted at the federal and state level may require us to modify our data processing practices and policies and to incur substantial expenditures in order to comply.
Outside the United States, we are impacted by the privacy and data security requirements at the international, national, and regional level, and on an industry specific basis. We serve customers across the globe. Legal requirements in these countries relating to the collection, storage, handling and transfer of personal data and potentially intellectual property continue to evolve with increasingly strict enforcement regimes. More privacy and security laws and regulations are being adopted, and more are being enforced, with potential for significant financial penalties. In the European Union, increasingly stringent data protection and privacy rules that will have substantial impact on the use of patient data across the healthcare industry became effective in May 2018. The European Union General Data Protection Regulation (“GDPR”) applies uniformly across the European Union and to businesses in other countries that target European Union residents and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR also requires companies processing personal data of individuals, including employees, residing in the European Union to comply with European Union privacy and data protection rules. Other international jurisdictions have issued privacy laws that mirror many of the requirements of GDPR. As we expand our international operations, we may be required to expend significant time and resources to put in place additional mechanisms to ensure compliance with multiple data privacy laws. Failure to comply with these laws may result in significant fines and other administrative penalties and harm our business.
Compliance with these laws and regulations may require significant additional costs or changes in our business, which could adversely affect our results of operations or financial condition. Noncompliance with these laws and regulations could result in the imposition of fines and penalties or could result in significant civil and other liabilities. Additionally, the restrictions imposed by these laws and regulations may limit the use and adoption of our products, reduce overall demand for our products, require us to modify our data handling practices and impose additional costs and burdens.
Healthcare reforms, changes in healthcare policies, and changes to third-party reimbursements for our products may affect demand for our products.
Uncertainty in the U.S. healthcare system may influence the way our customers spend on medical devices, supplies, and services in the future. Future rulemakings could affect rebates, prices, or the rate of price increase for healthcare products and services. Cost-containing measures implemented by healthcare providers worldwide could harm our profitability. If we fail to effectively react to healthcare reforms, our business may be adversely affected.
Regulations related to “conflict minerals” may force us to incur additional expenses, may result in damage to our business reputation and may adversely impact our ability to conduct our business.
The Dodd‑Frank Wall Street Reform and Consumer Protection Act and the rules promulgated by the SEC thereunder require companies, including Natus, to disclose the existence in their products of certain metals, known as “conflict minerals,” which are metals mined from the Democratic Republic of the Congo and adjoining countries. These rules require investigative efforts, which has and will continue to cause us to incur associated costs, could adversely affect the sourcing, availability and pricing of minerals used in our products and may cause reputational harm if we determine that certain of our components contain such conflict minerals or if we are unable to alter our processes or sources of supply to avoid using such materials, all of which could adversely impact sales of our products and results of operations.
If guidelines mandating universal newborn hearing screening do not continue to develop in foreign countries and governments do not mandate testing of all newborns as we anticipate, or if those guidelines have a long phase-in period, our sales of newborn hearing screening products may not achieve the revenue growth we have achieved in the past.
We estimate that approximately 95% of the children born in the United States are currently being tested for hearing impairment prior to discharge from the hospital. To date, there has been only limited adoption of newborn hearing screening prior to hospital discharge by foreign governments, and when newborn hearing screening programs are enacted by foreign governments there can be a phase-in period spanning several years. The widespread adoption of guidelines depends, in part, on our ability to educate foreign government agencies, neonatologists, pediatricians, third-party payors, and hospital administrators
27
about the benefits of universal newborn hearing screening as well as the use of our products to perform the screening and monitoring. Our revenue from our newborn hearing screening product lines may not grow if foreign governments do not require universal newborn hearing screening prior to hospital discharge, if physicians or hospitals are slow to comply with those guidelines, or if governments provide for a lengthy phase-in period for compliance.
As a result of increased scrutiny and reporting requirements from customers, shareholders, investors, and government agencies related to environmental, social, and governance (“ESG”) compliance objectives, our organization may be subject to reputational harm and/or monetary impact for any ESG non-compliance or slow progression to ESG compliance.
Organizations are being challenged with a heightened focus related to current and rapidly evolving corporate ESG-related initiatives by its customers, vendors, shareholders, investors, and governmental agencies. Compliance with ESG objectives and expectations may require us to incur additional and ongoing costs and resources, which may have an adverse effect on our business, financial condition and results of operations. Certain influential institutional investors are increasing their focus on ESG practices and are placing importance on the implications and social cost of their investments. If our ESG practices do not meet the standards set by these stockholders, they may choose not to invest in our common stock or if our peer companies outperform us in their ESG initiatives, potential or current investors may elect to invest with our competitors instead. If our organization's ESG practices do not meet or are slow to comply with ESG-imposed standards by interested parties, then our reputation, stock price, growth, employee retention, and customers' and vendors' willingness to do business with us may be negatively impacted for non-compliance or delay in compliance.
If we don’t have a diverse and inclusive environment where our global teammates feel a sense of belonging it may impede our ability to produce innovative products through diversity of perspectives, serve our diverse customers/patients and attract, develop and retain diverse and talented teammates.
Our organization is committed to supporting an environment where we create a sense of safety, respect and belonging where diverse perspectives are valued and encouraged regardless of race, gender association, sexual orientation, age, religion, ethnic origin, national origin and any unique characteristics. There can be no assurances that our initiatives to foster and create a sense of belonging and fair treatment with a diverse workforce will be successful. A failure to adopt or maintain competitive policies and practices that enhance our workplace culture, such as those related to diversity and inclusion, could adversely impact our ability to attract, develop and retain teammates, cause us reputation harm and negatively affect our operations and financial results.
Risks Related to our Intellectual Property and Potential Litigation
We may not be able to preserve the value of our intellectual property because we may not be able to protect access to it or we may lose our intellectual property rights due to expiration of our licenses or patents.
If we fail to protect our intellectual property rights or if our intellectual property rights do not adequately cover the technology we employ, other medical device companies could sell products with features similar to ours, and this could reduce demand for or perceived value of our products. We protect our intellectual property through a combination of patent, copyright, trade secret and trademark laws. Despite our efforts to protect our proprietary rights, others may attempt to copy or otherwise improperly obtain and use our products or technology. Policing unauthorized use of our technology is difficult and expensive, and we cannot be certain that the steps we have taken will prevent misappropriation. Our means of protecting our proprietary rights may be inadequate. Enforcing our intellectual property rights could be costly and time consuming and may divert our management’s attention and resources. Failing to enforce our intellectual property rights could also result in the loss of those rights.
Our financial results may suffer if we were subject to a protracted infringement or products liability litigation.
The medical technology industry is characterized by a substantial amount of litigation and related administrative proceedings regarding patents and intellectual property rights. We expect that medical screening and diagnostic products may become increasingly subject to third-party infringement claims as the number of competitors in our industry grows and the functionality of products overlap. Third parties such as individuals, educational institutions, or other medical device companies may claim that we infringe their intellectual property rights. Any claims, with or without merit, could have any of the following negative consequences:
•Result in costly litigation and damage awards;
•Divert our management’s attention and resources;
•Cause product shipment delays or suspensions; or
•Require us to seek to enter into royalty or licensing agreements.
From time to time, we may be subject to cases based on third-party patent infringement claims. A successful claim of infringement against us from any current or future claim could result in a substantial damage award and materially harm our
28
financial condition. Our failure or inability to license the infringed or similar technology, or design and build non-infringing products, could prevent us from selling our products and adversely affect our business and financial results.
We may also find it necessary to bring infringement actions against third parties to seek to protect our intellectual property rights. Litigation of this nature, even if successful, is often expensive and disruptive of our management’s attention, and in any event may not lead to a successful result relative to the resources dedicated to any such litigation.
In addition, the sale and use of our products could lead to the filing of a product liability claim by someone claiming to have been injured using one of our products or claiming that one of our products failed to perform properly. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business reputation or financial condition. Our product liability insurance may not protect our assets from financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing any coverage in the future.
We license intellectual property rights from third parties and would be adversely affected if our licensors do not appropriately defend their proprietary rights or if we breach any of the agreements under which we license commercialization rights to products or technology from others.
We license rights from third parties for products and technology that are important to our business. If our licensors are unsuccessful in asserting and defending their proprietary rights, including patent rights and trade secrets, we may lose the competitive advantages we have through selling products that we license from third parties. Additionally, if it is found that our licensors infringe on the proprietary rights of others, we may be prohibited from marketing our existing products that incorporate those proprietary rights. Under our licenses, we are subject to commercialization and development, sublicensing, royalty, insurance, and other obligations. If we fail to comply with any of these requirements, or otherwise breach a license agreement, the licensor may have the right to terminate the license in whole or to terminate the exclusive nature of the license.
ITEM 1B. Unresolved Staff Comments.
None.
ITEM 2. Properties
Our corporate headquarters is located in Middleton, Wisconsin, in a facility covering 121,000 square feet pursuant to a lease that expires in April 2024.
We also utilize the following properties:
Company-owned Facilities:
•62,400 square feet in Gort, Ireland, utilized substantially for manufacturing; and
•40,000 square feet in Oakville, Ontario, Canada, primarily utilized for research and development and technical support.
Leased Facilities:
Following is a listing of our most significant leased properties; we have a number of smaller facilities under lease in various countries where we operate.
•55,400 square feet in Taastrup, Denmark, pursuant to a lease with the option to terminate with six months-notice beginning January 2022, that is utilized for manufacturing, research and development, marketing and sales, and general and administrative;
•38,300 square feet in San Diego, California, pursuant to a lease that expires in June 2022, that is utilized substantially for manufacturing; and
•25,100 square feet in Schaumburg, Illinois, pursuant to a lease that expires in November 2026, that is utilized substantially for marketing and sales.
•8,200 square feet in Pleasanton, California, our previous headquarters, pursuant to a lease that expires in January 2025, that is utilized substantially for administrative services.
ITEM 3. Legal Proceedings
We may from time to time become a party to various legal proceedings or claims that arise in the ordinary course of business. We are not currently involved in any legal or administrative proceedings that we believe are likely to have a material
29
effect on our business, financial condition, or results of operations, although we cannot be assured of the outcome of such matters.
ITEM 4. Mine Safety Disclosures
The disclosure required by this item is not applicable.
PART II
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock trades on the Nasdaq Global Select Market under the symbol “NTUS”. The following table sets forth, for the periods indicated, the high and low sale price per share of our common stock, as reported on the Nasdaq Global Select Market.
| High | Low | ||||||||||
| Fiscal Year Ended December 31, 2021: | |||||||||||
| Fourth Quarter | $ | 28.65 | $ | 21.87 | |||||||
| Third Quarter | 27.54 | 22.54 | |||||||||
| Second Quarter | 29.70 | 24.97 | |||||||||
| First Quarter | 27.97 | 19.93 | |||||||||
| Fiscal Year Ended December 31, 2020: | |||||||||||
| Fourth Quarter | $ | 21.98 | $ | 16.60 | |||||||
| Third Quarter | 22.99 | 16.38 | |||||||||
| Second Quarter | 26.97 | 20.25 | |||||||||
| First Quarter | 34.67 | 18.91 | |||||||||
As of February 18, 2022, there were 34,408,154 shares of our common stock issued and outstanding and held by approximately 118 stockholders of record. We estimate that there are approximately 13,770 beneficial owners of our common stock.
Dividends
We have never declared or paid cash dividends on our capital stock and do not anticipate paying any cash dividends in the foreseeable future.
Stock Performance Graph
The following information of Part II Item 5 is being furnished and shall not be deemed to be “soliciting material” or to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section, nor will it be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference thereto.
The following graph shows a comparison, from January 1, 2016 through December 31, 2021, of cumulative total return for our common stock, the Nasdaq Composite Index and the Standard & Poor’s 500 Health Care Equipment Index. Such returns are based on historical results and are not intended to suggest future performance. Data for the Nasdaq Composite Index and the Standard & Poor’s 500 Health Care Equipment Index assumes reinvestment of dividends.
30
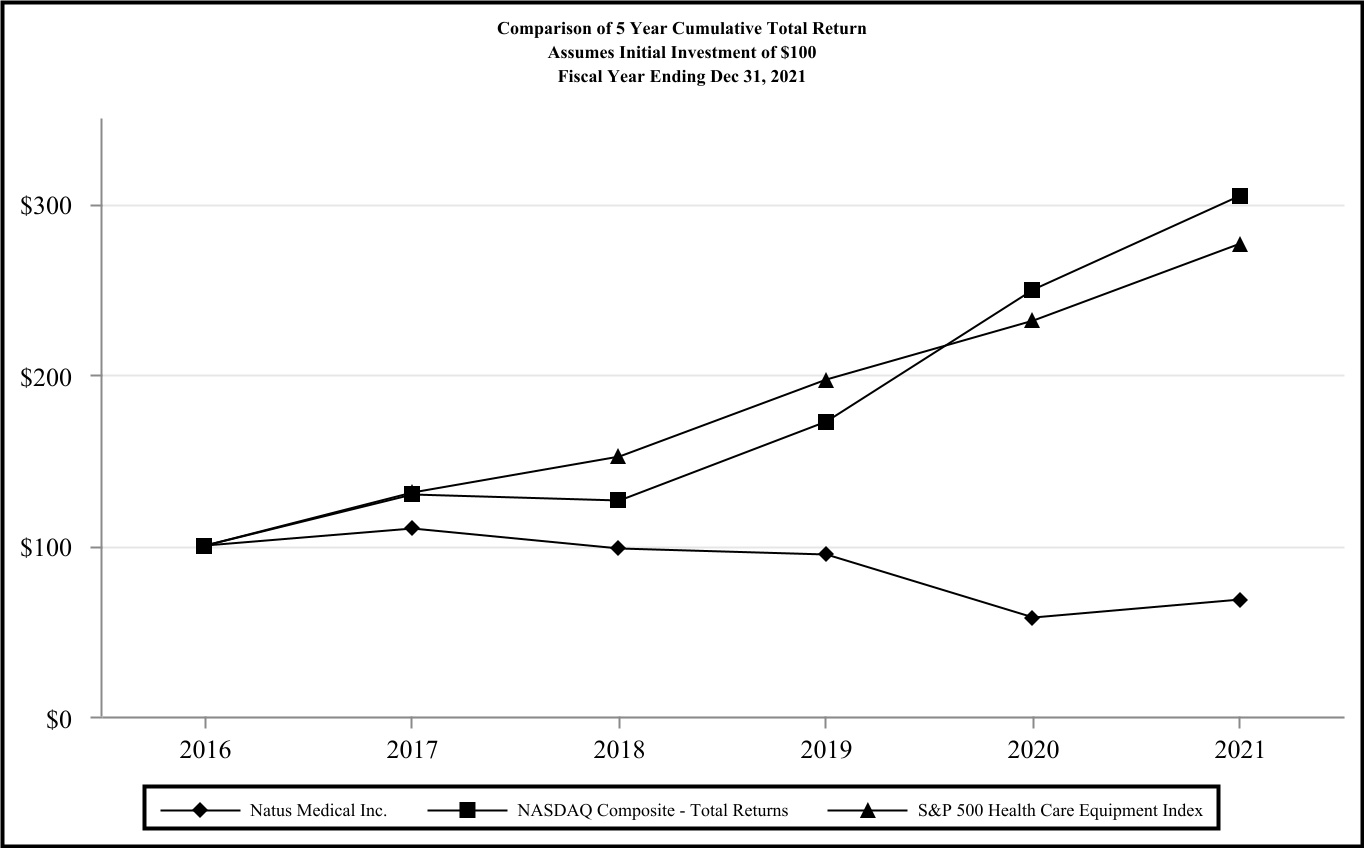
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |||||||||||||||||||||||||||||||||||||||
| Natus Medical Incorporated | Return % | — | 9.77 | (10.92) | (3.06) | (39.25) | 18.41 | |||||||||||||||||||||||||||||||||||||
| Cum $ | 100.00 | 109.77 | 97.78 | 94.79 | 57.58 | 68.19 | ||||||||||||||||||||||||||||||||||||||
| NASDAQ Composite-Total Returns | Return % | — | 29.64 | (2.84) | 36.69 | 44.92 | 22.18 | |||||||||||||||||||||||||||||||||||||
| Cum $ | 100.00 | 129.64 | 125.96 | 172.18 | 249.51 | 304.85 | ||||||||||||||||||||||||||||||||||||||
| S&P 500 Health Care Equipment Index | Return % | — | 30.90 | 16.24 | 29.32 | 17.63 | 19.35 | |||||||||||||||||||||||||||||||||||||
| Cum $ | 100.00 | 130.90 | 152.15 | 196.77 | 231.46 | 276.26 | ||||||||||||||||||||||||||||||||||||||
Purchases of Equity Securities by the Issuer
In December 2019, the Board of Directors approved a share repurchase program, which authorized the repurchase from time to time of up to a maximum of $50.0 million of our outstanding common stock. The program expired on December 12, 2021.
ITEM 6. [Reserved]
Not applicable
31
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) should be read in conjunction with the Consolidated Financial Statements and the accompanying footnotes. MD&A includes the following sections:
Business
We are a leading provider of medical device solutions focused on the diagnosis and treatment of central nervous and sensory system disorders for patients of all ages.
Year 2021 Overview
Our consolidated revenue increased by $57.8 million for the year ended December 31, 2021 compared to the year ended December 31, 2020. This increase was driven by an increase in demand for our Neuro and Hearing and Balance products as a result of the recovery from the impact of the COVID-19 global pandemic.
Net income was $13.2 million, or $0.39 per share in the year ended December 31, 2021, compared with net loss of $16.6 million, or $0.49 per share in the prior year. This increase in income was primarily driven by higher revenue resulting from the recovery of the impact of the COVID-19 pandemic on global demand for our products, a more profitable mix of revenue and impairment of intangibles related to end of sale products in 2020, not repeating to the same level in 2021. This was partly offset by an increase in operating expenses as we returned closer to more normal operating levels of the business related to the impact of the COVID-19 global pandemic. We generated cash flow from operations of $64.0 million.
COVID-19 Update
Healthcare providers and patients continue to depend on our products and services every day. Our team members and partners are continuing to maintain our supply chain, manufacturing and delivery of our products and services. The health and welfare of our employees, our customers and our partners remain our top priority as we continue our business operations.
We have implemented safeguards in our facilities to protect team members, including social distancing practices, work from home and other measures consistent with specific regulatory requirements and guidance from health authorities. As an essential supplier of healthcare products and services, all of our manufacturing, engineering, sales and customer support functions remain fully operational and will continue to support customers with vital supplies, service and equipment. Supply chain delays and constraints have impacted our ability to ship products in the last few quarters. We are working with our suppliers to reduce constraints by providing forecasts further into the future and closely monitoring and communicating changes in the forecast, however we remain uncertain of when the supply chain will stabilize.
Impact to our supply chain
Many of our materials are single source and require lengthy qualification periods. Disruptions in our supply chain could negatively impact our ability to produce and supply our finished products. We continue to experience some extended lead times and delays in receiving supplies and finished goods which impacted revenue this period. We are working closely with our suppliers to manage orders and proactively resolve delays in the materials we require to meet our demand.
Liquidity
In the first quarter of 2020 we drew $60.0 million on our credit line as a precaution to ensure we have the necessary capital to continue to reliably serve our customers during an extended period of uncertainty. During the third quarter of 2020 we amended our Credit Agreement which extended the maturity date of the original agreement from September 23, 2021 to September 25, 2023, reduced the aggregate revolving credit facility from $225.0 million to $150.0 million, and amended certain covenants. During 2021, we repaid the full outstanding debt balance under our Credit Agreement and continued to maintain a strong cash position ending the period with $75.6 million in cash.
While we believe that we have sufficient liquidity to operate the Company for the foreseeable future, we are evaluating additional measures we could take to further enhance our liquidity position should negative economic conditions deteriorate for an extended period of time.
Impact to fair-value of intangible assets
We have reviewed the assets on our balance sheet, particularly goodwill and significant intangible assets for indications of impairment related to COVID-19 and determined that there are no indicators of impairment at this time. The values of these assets are particularly sensitive to our market cap and the long term value of their cash flows. If these conditions change
32
significantly, we may need to record an impairment to their value. However, any impairment charges would not require the use of cash and are excluded from the calculation of our debt covenants and therefore would not affect our ability to borrow under our existing credit line.
Government Grants
Various government programs have been established to provide financial relief for businesses affected by COVID-19 including the Canada Emergency Wage Subsidy (“CEWS”) and Canada Emergency Rent Subsidy (“CERS”) under the COVID-19 Economic Response Plan in Canada. We received $4.1 million for payroll subsidies under CEWS and CERS for the year ended December 31, 2021. Our policy is to account for these subsidies in the same manner as an offset to the expense they relate to in the period in which we are reasonably assured to receive payment. For the year ended December 31, 2021 we recognized reductions of $0.5 million in cost of sales, $1.7 million in marketing and selling expense, $1.7 million in research and development expense, and $0.2 million in general and administrative expense in the condensed consolidated statement of operations for these subsidies.
Travel restrictions and use of online technology
The global Natus team is geographically diverse with multiple small locations and hundreds of employees that typically work from home in normal circumstances. We use the latest collaboration technology and have been able to transition to a company-wide work from home model without major interruption. Our manufacturing, distribution and field service operations require physical presence of certain employees as their work requires them to handle our products. In these cases, we have made adjustments to shift size and schedule and limited access to these groups by non-related employees. Our field service technicians are following our customers' requirements for distancing practices but continue to provide service where needed.
Travel restrictions have forced most customer and external partner collaboration to online technology. Using this technology has enabled us to continue operations without incident. However, in-person customer engagement as well as physical presence in laboratory settings is returning and becoming more frequent.
Critical Accounting Estimates
We prepare our financial statements in accordance with accounting principles generally accepted in the United States of America (“GAAP”). In so doing, we must often make estimates and use assumptions that can be subjective and, consequently, our actual results could differ from those estimates. For any given individual estimate or assumption we make, there may also be other estimates or assumptions that are reasonable. Our estimates are based on our historical experience and a variety of other assumptions that we believe to be reasonable under the circumstances. We evaluate our estimates, assumptions, and judgements on a regular basis.
We believe that the following critical accounting estimates require the use of significant assumptions and judgments to have a full understanding of our financial statements. By their nature, these estimates and judgements are subject to an inherent degree of uncertainty. The use of different estimates, assumptions, and judgments could have a material effect on the reported amounts of assets, liabilities, revenue, expenses, and related disclosures as of the date of the financial statements and during the reporting period.
Inventory Valuation
Inventories are carried at the lower of cost or net realizable value, with cost being determined using the first-in, first-out method. The carrying value of our inventory is reduced for any difference between cost and estimated net realizable value of the inventory. We determine net realizable value by evaluating ending inventories for excess quantities, obsolescence, and other factors that could impact our ability to consume inventory for its intended use. The estimate for inventory valuation includes significant assumptions that have a high degree of subjectivity. For example, our evaluation includes an analysis of historical sales by product including judgements with respect to projections of future demand by product and qualitative analysis of obsolescence by product based on expectations from operations. Adjustments to the value of inventory establish a new cost basis and are considered permanent even if circumstances later suggest that increased carrying amounts are recoverable. If demand is higher than expected, we may sell inventory that had previously been written down.
33
Results of Operations
The following table sets forth for the periods indicated selected consolidated statement of income data as a percentage of total revenue. Our historical operating results are not necessarily indicative of the results for any future period.
| Percent of Revenue Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Revenue | 100.0 | % | 100.0 | % | 100.0 | % | |||||||||||
| Cost of revenue | 40.8 | % | 44.7 | % | 39.7 | % | |||||||||||
| Intangibles amortization | 1.4 | % | 3.2 | % | 1.4 | % | |||||||||||
| Gross profit | 57.8 | % | 52.1 | % | 58.9 | % | |||||||||||
| Operating expenses: | |||||||||||||||||
| Marketing and selling | 24.5 | % | 25.8 | % | 26.1 | % | |||||||||||
| Research and development | 11.9 | % | 14.7 | % | 11.9 | % | |||||||||||
| General and administrative | 11.1 | % | 11.8 | % | 12.0 | % | |||||||||||
| Intangibles amortization | 3.6 | % | 3.7 | % | 3.1 | % | |||||||||||
| Restructuring | 1.6 | % | 0.9 | % | 9.0 | % | |||||||||||
| Total operating expenses | 52.8 | % | 56.9 | % | 62.1 | % | |||||||||||
| Income (loss) from operations | 5.0 | % | (4.9) | % | (3.2) | % | |||||||||||
| Other expense, net | (0.9) | % | (0.5) | % | (1.1) | % | |||||||||||
| Income (loss) before provision (benefit) for income tax | 4.1 | % | (5.3) | % | (4.3) | % | |||||||||||
| Provision (benefit) for income tax expense | 1.3 | % | (1.3) | % | (1.1) | % | |||||||||||
| Net income (loss) | 2.8 | % | (4.0) | % | (3.2) | % | |||||||||||
Comparison of 2021 and 2020
Revenue
| Years ended December 31, | |||||||||||||||||
| 2021 | 2020 | Change | |||||||||||||||
| Neuro | |||||||||||||||||
| Devices and Systems | $ | 220,929 | $ | 179,464 | 23 | % | |||||||||||
| Supplies | 63,838 | 56,275 | 13 | % | |||||||||||||
| Total Neuro Revenue | 284,767 | 235,739 | 21 | % | |||||||||||||
| Newborn Care | |||||||||||||||||
| Devices and Systems | 55,174 | 52,284 | 6 | % | |||||||||||||
| Supplies | 33,710 | 36,174 | (7) | % | |||||||||||||
| Services | 16,139 | 16,566 | (3) | % | |||||||||||||
| Total Newborn Care Revenue | 105,023 | 105,024 | — | % | |||||||||||||
| Hearing & Balance | |||||||||||||||||
| Devices and Systems | $ | 78,652 | $ | 70,738 | 11 | % | |||||||||||
| Supplies | 4,996 | 4,183 | 19 | % | |||||||||||||
| Total Hearing & Balance Revenue | 83,648 | 74,921 | 12 | % | |||||||||||||
| Total Revenue | $ | 473,438 | $ | 415,684 | 14 | % | |||||||||||
For the year ended December 31, 2021, Neuro revenue increased by 21% compared to the prior year. Devices and Systems revenue increased by 23% and Supplies revenue increased by 13% compared to the prior year primarily due to the recovery from the impact of the global COVID-19 pandemic, as non-emergent procedures were halted in 2020.
For the year ended December 31, 2021, Newborn Care revenue remained flat compared to the prior year. Devices and Systems revenue increased by 6% due primarily to the recovery from the impact of the global COVID-19 pandemic, particularly in our Hearing Screening business. Supplies revenue decreased 7% compared to the prior year primarily due to
34
lower Hearing Screening supplies shipments, mostly due to production and supply chain constraints. Services revenue decreased by 3% compared to the prior year due to lower revenue from our Newborn Care business.
For the year ended December 31, 2021, Hearing & Balance revenue increased 12% compared to the prior year. Devices and Systems revenue increased by 11% compared to the prior year, particularly in our Hearing Assessment and Fitting businesses. Supplies revenue increased by 19% compared to the prior year, particularly in our Fitting business. The increases are primarily due to the recovery from the impact of the global COVID-19 pandemic, as non-emergent procedures were halted in 2020.
Cost of Revenue and Gross Profit
| Years ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Revenue | $ | 473,438 | $ | 415,684 | |||||||
| Cost of revenue | 193,015 | 185,912 | |||||||||
| Intangibles amortization | 6,743 | 13,241 | |||||||||
| Gross profit | 273,680 | 216,531 | |||||||||
| Gross profit percentage | 57.8 | % | 52.1 | % | |||||||
For the year ended December 31, 2021, our gross profit as a percentage of sales increased by 5.7% compared to the prior year. This increase was primarily attributable to higher revenue resulting from a partial recovery from the impact of the COVID-19 pandemic, a more profitable mix of product shipments, lower inventory scrap and lower amortization relating to an impairment of a product line during 2020.
Operating Costs
| Years ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Marketing and selling | $ | 116,014 | $ | 107,282 | |||||||
| Percentage of revenue | 24.5 | % | 25.8 | % | |||||||
| Research and development | $ | 56,306 | $ | 61,296 | |||||||
| Percentage of revenue | 11.9 | % | 14.7 | % | |||||||
| General and administrative | $ | 52,753 | $ | 49,113 | |||||||
| Percentage of revenue | 11.1 | % | 11.8 | % | |||||||
| Intangibles amortization | $ | 17,129 | $ | 15,224 | |||||||
| Percentage of revenue | 3.6 | % | 3.7 | % | |||||||
| Restructuring | $ | 7,699 | $ | 3,809 | |||||||
| Percentage of revenue | 1.6 | % | 0.9 | % | |||||||
Marketing and Selling
Marketing and selling expenses increased during the year ended December 31, 2021 as compared to the prior year. The increase in expenses is mainly attributable to higher expenses as we moved towards more normal operating levels of the business, as compared to the impact of COVID-19, in the prior year which caused increases in travel expense, tradeshow and other events, payroll, commissions, and service expenses.
Research and Development
Research and development expenses decreased during the year ended December 31, 2021 compared to the prior year. The decrease relates to lower project spend for Medical Device Regulation compliance, consulting and testing, and remediation partly offset by increased spending on new product development.
General and Administrative
General and administrative expenses increased during the year ended December 31, 2021 compared to the prior year. This increase was due to more normal operating levels of the business as we recover from the impact of the global COVID-19 pandemic, resulting in increases in payroll expenses and outside services.
35
Intangibles Amortization
Intangibles amortization increased for the year ended December 31, 2021 compared to the prior year due to an intangible impairment of $2.0 million relating to the Babybe acquisition.
Restructuring
Restructuring costs increased during the year ended December 31, 2021 compared to the prior year. This increase was driven by costs of approximately $6.9 million associated with the Company’s executive management transition, which were primarily comprised of accelerated vesting of stock compensation, severance expense, and sign-on bonus.
Other Income (Expense), net
Other income (expense), net consists of interest income, interest expense, net currency exchange gains and losses, and other miscellaneous income and expense. We reported other expense, net of $4.4 million in the year ended December 31, 2021, compared to $1.9 million in the prior year. We reported $2.0 million of foreign currency exchange losses in the year ended December 31, 2021 versus $2.0 million of foreign currency gains in the prior year. Interest expense was $1.9 million in the year ended December 31, 2021 compared to $3.7 million in the prior year. The reduction in interest expense was driven by accelerated payments on and full repayment of our outstanding third-party bank debt. Other miscellaneous income and expense consisted primarily of a loss on our equity method investment of $0.6 million in the year December 31, 2021.
Provision for Income Tax
The effective tax rate (“ETR”) for the year ended December 31, 2021 was 31.9% as compared to 24.7% for the prior year. Significant items that impact the effective tax rate are the change in geographic mix of income and adjustments and reversals of uncertain tax positions.
For discussion of the year ended December 31, 2020 compared to the year ended December 31, 2019, please refer to Part II, Item 7 “Management's Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2020.
Liquidity and Capital Resources
Liquidity is our ability to generate sufficient cash flows from operating activities to meet our obligations and commitments. In addition, liquidity includes the ability to obtain appropriate financing and to raise capital. Therefore, liquidity cannot be considered separately from capital resources that consist of our current funds and the potential to increase those funds in the future. We plan to use these resources in meeting our commitments and in achieving our business objectives.
We believe that our current cash and cash equivalents and any cash generated from operations will be sufficient to meet our ongoing operating requirements for the foreseeable future.
As of December 31, 2021, we had cash and cash equivalents outside the U.S. in certain of our foreign operations of $30.3 million. We intend to permanently reinvest this cash held by our foreign subsidiaries except for Excel-Tech and Natus Ireland subsidiaries, which we intend to repatriate. If, however, a portion of these permanently reinvested funds were needed and distributed to our operations in the United States, we may be subject to additional U.S. income taxes and foreign withholding taxes depending on facts and circumstances at the time of distribution. The amount of taxes due would depend on the amount and manner of repatriation, as well as the location from where the funds were repatriated.
We have a Credit Agreement with JP Morgan Chase Bank (“JP Morgan”), Citibank, NA (“Citibank”) and Wells Fargo Bank, National Association (“Wells Fargo”). During the third quarter of 2020 we amended the terms of the Credit Agreement to extend the maturity of the original agreement and to reduce the aggregate value of revolving credit facility, and amend certain covenants. The amended Credit Agreement provides for an aggregate $150.0 million of secured revolving credit facility.
The Credit Agreement contains covenants, including covenants relating to maintenance of books and records, financial reporting and notification, compliance with laws, maintenance of properties and insurance, and limitations on guaranties, investments, issuance of debt, lease obligations and capital expenditures. The Credit Agreement provides for events of default, including failure to pay any principal or interest when due, failure to perform or observe covenants, bankruptcy or insolvency events and the occurrence of a material adverse effect. The Credit Agreement matures on September 25, 2023, at which time all principal amounts outstanding under the Credit Agreement will be due and payable. We have no other significant credit
36
facilities. As of December 31, 2021 we had no outstanding balance under the Credit Facility.
| December 31, 2021 | December 31, 2020 | December 31, 2019 | |||||||||||||||
| Cash, cash equivalents, and investments | $ | 75,595 | $ | 82,082 | $ | 63,297 | |||||||||||
| Debt | — | 55,840 | 54,665 | ||||||||||||||
| Working capital | 162,690 | 125,950 | 126,928 | ||||||||||||||
| Year Ended | |||||||||||||||||
| December 31, 2021 | December 31, 2020 | December 31, 2019 | |||||||||||||||
| Net cash provided by operating activities | $ | 63,995 | $ | 34,426 | $ | 60,060 | |||||||||||
| Net cash used in investing activities | (1,946) | (12,606) | (5,339) | ||||||||||||||
| Net cash used in financing activities | (60,662) | (10,877) | (48,532) | ||||||||||||||
Comparison of 2021, 2020, and 2019
During 2021 cash generated from operating activities of $64.0 million was the result of $13.2 million of net income, non-cash adjustments to net income of $43.5 million, and net cash outflows of $7.3 million from changes in operating assets and liabilities. The non-cash adjustments were $28.1 million of depreciation and amortization expense, $14.2 million from share-based compensation, $4.0 million of warranty reserves, and $0.2 million of accounts receivable reserves, offset by deferred taxes of $4.4 million. Cash used in investing activities during the period was $1.9 million and consisted of cash used of $3.6 million to acquire other property and equipment, and $1.0 million of equity method investments offset by $2.7 million of proceeds from disposal of assets held for sale. Cash used in financing activities during the year ended December 31, 2021 was $60.7 million and consisted of repayments of $57.0 million of our outstanding debt under the Credit Facility, $4.1 million for taxes paid related to net share settlement of equity awards, $0.4 million of principal payments of financing lease liability, offset by proceeds from stock option exercises and Employee Stock Purchase Program (“ESPP”) purchases of $0.9 million.
During 2020 cash generated from operating activities of $34.4 million was the result of $16.6 million of net loss, non-cash adjustments to net loss of $49.6 million, and net cash outflows of $1.4 million from changes in operating assets and liabilities. The non-cash adjustments were $28.1 million of depreciation and amortization expense, $9.6 million from share-based compensation, $6.7 million of an impairment of intangible assets, $2.0 million of warranty reserves, $1.9 million of loss on commencement of sales-type leases, $1.4 million of non-cash lease expense, and $1.2 million of accounts receivable reserves, offset by deferred taxes of $1.6 million. Cash used in investing activities during the period was $12.6 million and consisted of cash used of $8.6 million to acquire other property and equipment, $2.0 million of an acquisition, and $2.0 million of equity method investments. Cash used in financing activities during the year ended December 31, 2020 was $10.9 million and consisted of repayments of $58.0 million of our outstanding debt under the Credit Facility, $10.5 million for repurchases of common stock under our share repurchase program, $2.0 million for taxes paid related to net share settlement of equity awards, $1.2 million of deferred debt issuance costs, $0.5 million of principal payments of financing lease liability, offset by proceeds from borrowing of $60.0 million and proceeds from stock option exercises and Employee Stock Purchase Program (“ESPP”) purchases of $1.3 million.
During 2019 cash generated from operating activities of $60.1 million was the result of $15.7 million of net loss, non-cash adjustments to net loss of $65.9 million, and net cash outflows of $9.9 million from changes in operating assets and liabilities. The non-cash adjustments were $30.7 million of depreciation and amortization expense, $24.6 million of impairment for the sale of Medix, $8.4 million from share-based compensation, $4.3 million of accounts receivable reserves, and $2.9 million of warranty reserves, offset by deferred taxes of $5.4 million. Cash used in investing activities during the period was $5.3 million and consisted of cash used to acquire other property and equipment. Cash used in financing activities during the year ended December 31, 2019 was $48.5 million and consisted of repayments of $50.0 million of our outstanding debt under the Credit Facility, $1.7 million for taxes paid related to net share settlement of equity awards, $0.5 million of principal payments of financing lease liability, offset by proceeds from stock option exercises and Employee Stock Purchase Program (“ESPP”) purchases of $3.6 million.
37
Future Liquidity
Our future liquidity and capital requirements will depend on numerous factors, including the:
•Extent to which we make acquisitions;
•Amount and timing of revenue;
•Extent to which our existing and new products gain market acceptance;
•Cost and timing of product development efforts and the success of these development efforts;
•Cost and timing of marketing and selling activities; and
•Availability to borrow under line of credit arrangements and the availability of other means of financing.
Contractual Obligations
In the normal course of business, we enter into obligations and commitments that require future contractual payments. The commitments result primarily from purchase orders placed with contract vendors that manufacture some of the components used in our medical devices and related disposable supply products, purchase orders placed for employee benefits and outside services, as well as commitments for leased office space, leased equipment, and bank debt.
As of December 31, 2021, we have unconditional purchase obligations of $136.5 million that payments are due within the next year and $41.9 million due within one to three years. Purchase obligations are defined as agreements to purchase goods or services that are enforceable and legally binding. Included in the purchase obligations are obligations related to purchase orders for inventory purchases under our standard terms and conditions and under negotiated agreements with vendors. We expect to receive consideration (products or services) for these purchase obligations. The purchase obligation amount does not represent all anticipated purchases in the future, but represent only those items for which we are contractually obligated. This does not include obligations under employment agreements for services rendered in the ordinary course of business.
As of December 31, 2021, we have estimated interest payments of $1.2 million due less than one year and $1.0 million due within one to three years. These interest payments are an estimate of expected interest payments but could vary materially based on the timing of future loan draws and payments. See Note 11 of our Consolidated Financial Statements for further discussion on debt and credit arrangements.
As of December 31, 2021, we have $5.60 million of tax obligation relating to repatriation tax. The repatriation tax is a one-time transition tax on the mandatory deemed repatriation of cumulative foreign earnings as of December 31, 2017 due to the enactment in December 2017 of the Tax Act.
We are not able to reasonably estimate the timing of any potential payments for uncertain tax positions under Accounting Standards Codification (“ASC”) 740, Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement 109, therefore contractual obligations exclude any potential tax payments related to our ASC 740 liability for uncertain positions. See Note 17 of our Consolidated Financial Statements for further discussion on income taxes.
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to various market risks, including changes in foreign currency exchange rates and interest rates that could adversely affect our results of operations and financial condition.
Foreign Exchange Rate Risk
We develop products in the U.S, Canada, and Europe, and sell those products into more than 100 countries throughout the world. As a result, our financial results could be affected by factors such as changes in foreign currency exchange rates or weak economic conditions in foreign markets. Most of our sales in Europe and Asia are denominated in the U.S. Dollar and Euro, with a portion of our sales denominated in the Canadian dollar and British pound. As our sales in currencies other than the U.S. dollar increase, our exposure to foreign currency fluctuations may increase. We do not currently hold derivatives to hedge our exposure to foreign currency exchange rate fluctuations; however, we may choose to hedge our exposure in the future.
In addition, changes in exchange rates also may affect the end-user prices of our products compared to those of our foreign competitors, who may be selling their products based on local currency pricing. These factors may make our products less competitive in some countries.
All of the potential changes noted above are based on sensitivity analyses performed on our financial position as of December 31, 2021. Actual results may differ as our analysis of the effects of changes in interest rates does not account for, changes in the relationship between short-term and long-term interest rates.
38
Interest Rate Risk
In 2018, we entered into an interest rate swap agreement with a notional amount of $40.0 million, designated as a cash flow hedge, to hedge the variability of cash flows in interest payments associated with our floating-rate debt. This interest rate swap agreement matured in September 2021 and converted a portion of our LIBOR floating-Rate debt to fixed-rate debt. We do not have plans to enter into another interest rate swap agreement at this time.
Cautionary Information Regarding Forward Looking Statements
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 about Natus Medical Incorporated. Forward-looking statements can be identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans”, “will”, “outlook” and similar expressions. Forward-looking statements are based on management's current plans, estimates, assumptions and projections, and speak only as of the date they are made. These forward-looking statements within Item 7 include, without limitation, statements regarding the impact of the COVID-19 pandemic on our business, the sufficiency of our current cash, cash equivalents and short-term investment balances, any cash generated from operations to meet our ongoing operating and capital requirements for the foreseeable future, outcomes of new product development, improved operations performance and profitability as the result of restructuring activities, and our intent to acquire additional technologies, products or businesses.
Forward-looking statements are not guarantees of future performance and are subject to substantial risks and uncertainties that could cause the actual results predicted in the forward-looking statements as well as our future financial condition and results of operations to differ materially from our historical results or currently anticipated results. Investors should carefully review the information contained under the caption “Risk Factors” contained in Item 1A of this report for a description of risks and uncertainties. All forward-looking statements are based on information available to us on the date hereof, and we assume no obligation to update forward-looking statements.
ITEM 7A. Quantitative and Qualitative Disclosures About Market Risk
The information required by this Item is set forth in the section entitled Management’s Discussion and Analysis of Financial Condition and Results of Operations—Quantitative and Qualitative Disclosures About Market Risk, and is incorporated by reference in this section.
ITEM 8. Financial Statements and Supplementary Data
The Consolidated Financial Statements and Supplementary Data required by this Item are set forth where indicated in Item 15 of this report.
ITEM 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
ITEM 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the rules of the Securities and Exchange Commission, “disclosure controls and procedures” are controls and other procedures that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in our reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Our management, including our chief executive officer and chief financial officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud due to inherent limitations of internal controls. Because of such limitations, there is a risk that material misstatements will not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Our management, with the participation of our chief executive officer and our chief financial officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Based on that evaluation, our management, including our chief executive officer and chief financial officer, has concluded that our disclosure controls and procedures were effective as of December 31, 2021.
39
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of the inherent limitations of any system of internal control. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses of judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper overriding of controls. As a result of such limitations, there is risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Based on our evaluation under the criteria set forth in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, our management concluded that as of December 31, 2021 our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting.
Our independent registered public accounting firm, KPMG LLP, has audited the consolidated financial statements included in this Annual Report on Form 10-K and also audited the effectiveness of our internal control over financial reporting as of December 31, 2021. KPMG LLP’s report over effectiveness of our internal control over financial reporting is included herein in Part II, Item 9A of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There has been no change in the Company's internal control over financial reporting during the Company's fourth quarter of 2021, that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting.
40
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors
Natus Medical Incorporated:
Opinion on Internal Control Over Financial Reporting
We have audited Natus Medical Incorporated and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2021 and 2020, the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2021, and the related notes and financial statement schedule II: Valuation and Qualifying Accounts (collectively, the consolidated financial statements), and our report dated February 25, 2022 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
San Francisco, California
February 25, 2022
41
ITEM 9B. Other Information
None.
ITEM 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
We will provide information that is responsive to this Part III in our Definitive Proxy Statement for our 2022 Annual Meeting of Stockholders (our “2022 Proxy Statement”) or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report. That information is incorporated into this Part III by reference.
ITEM 10. Directors, Executive Officers, and Corporate Governance
We will provide certain other information that is responsive to this Item 10 in our 2022 Proxy Statement or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report. That information is incorporated into this Item 10 by reference.
Audit Committee and Audit Committee Financial Expert
The members of the Audit Committee of our Board of Directors are Ilan Daskal, Eric J. Guerin, and Alice D. Schroeder. Our Board of Directors has determined that Ilan Daskal is an audit committee financial expert as defined in Item 407(d) of Regulation S-K. All of the members of our audit committee are considered “independent” as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Exchange Act.
Code of Conduct and Ethics
We have a code of conduct and ethics that applies to all of our employees, including our principal executive officer, principal financial officer, and principal accounting officer or controller. This code of conduct and ethics is posted on our internet website. The internet address for our website is www.natus.com, and the code of conduct and ethics may be found in the “Governance” section of our “Investor” webpage.
We intend to satisfy the disclosure requirement under Item 10 of Form 8-K regarding certain amendments to, or waivers from, provisions of this code of conduct and ethics by posting such information on our website, at the address and location specified above, or as otherwise required by The Nasdaq Stock Market.
ITEM 11. Executive Compensation
We will provide information that is responsive to this Item 11 in our 2022 Proxy Statement or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report. That information is incorporated into this Item 11 by reference.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
The following table sets forth information about the number of shares of common stock that can be issued under our 2021 Stock Awards Plan as of December 31, 2021.
| Plan Category | Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants, Awards and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants, Awards and Rights | Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans (excluding securities reflected in the first column) | |||||||||||||||||
| Equity compensation plans approved by security holders | 860,966 | $ | 22.84 | 2,917,938 | ||||||||||||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||||||||||
| Total | 860,966 | 22.84 | 2,917,938 | |||||||||||||||||
42
We will provide certain other information that is responsive to this Item 12 in our 2022 Proxy Statement or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report. That information is incorporated into this Item 12 by reference.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence
We will provide information that is responsive to this Item 13 in our 2022 Proxy Statement or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report. That information is incorporated into this Item 13 by reference.
ITEM 14. Principal Accounting Fees and Services
We will provide information that is responsive to this Item 14 in our 2022 Proxy Statement or in an amendment to this Annual Report not later than 120 days after the end of the fiscal year covered by this Annual Report. That information is incorporated into this Item 14 by reference.
PART IV
ITEM 15. Exhibits, Financial Statement Schedules
(a)(2) Financial Statement Schedule
SCHEDULE II: VALUATION AND QUALIFYING ACCOUNTS
For the years ended December 31, 2021, 2020 and 2019
(In thousands)
| Balance at Beginning of Period | Additions Charged to Expense | Deductions | Balance at End of Period | ||||||||||||||||||||
| Year ended December 31, 2021 | |||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 6,213 | $ | 181 | $ | (1,123) | $ | 5,271 | |||||||||||||||
| Valuation allowance | 969 | — | (35) | 934 | |||||||||||||||||||
| Warranty reserve | 5,195 | 3,989 | (3,142) | 6,042 | |||||||||||||||||||
| Year ended December 31, 2020 | |||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 7,384 | $ | 1,190 | $ | (2,361) | $ | 6,213 | |||||||||||||||
| Valuation allowance | 606 | 363 | — | 969 | |||||||||||||||||||
| Warranty reserve | 6,404 | 2,009 | (3,218) | 5,195 | |||||||||||||||||||
| Year ended December 31, 2019 | |||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 6,960 | $ | 4,262 | $ | (3,838) | $ | 7,384 | |||||||||||||||
| Valuation allowance | 637 | — | (31) | 606 | |||||||||||||||||||
| Warranty reserve | 9,391 | 3,949 | (6,936) | 6,404 | |||||||||||||||||||
(a)(3) Exhibits
The Exhibits listed in the Index to Exhibits, which appears immediately preceding the signature page and is incorporated herein by reference, are filed as part of this 10-K.
43
NATUS MEDICAL INCORPORATED
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors
Natus Medical Incorporated:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Natus Medical Incorporated and subsidiaries (the Company) as of December 31, 2021 and 2020, the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2021, and the related notes and financial statement schedule II: Valuation and Qualifying Accounts (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 25, 2022 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Evaluation of net realizable value adjustments for excess or obsolete inventory
As discussed in Note 4 to the consolidated financial statements, the Company had inventory with a carrying value of $81.4 million at December 31, 2021, of which $67.7 million is classified as current and $13.7 million is classified as non-current. As discussed in Note 1 to the consolidated financial statements, the Company reduces the carrying value of inventory for any differences between its cost and estimated net realizable value. Net realizable value is estimated by evaluating ending inventories for excess quantities, obsolescence, and other factors that potentially impact the Company’s ability to consume its inventory. In making its estimate of net realizable value, the Company’s evaluation includes an analysis of historical sales by product, projections of future demand by product, and obsolescence by product.
We identified the evaluation of net realizable value adjustments for excess or obsolete inventory as a critical audit matter. A high degree of auditor judgment was required in assessing the identification of the subset of inventory requiring net realizable value adjustments given the inherent uncertainty in projecting future demand and complexity involved in assessing the Company’s analysis of historical sales by product, projections of future demand, and assessment of obsolescence.
F-2
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of an internal control related to the Company's identification of the subset of inventory requiring net realizable value adjustments. In evaluating potential excess inventory, we compared on-hand inventory to the Company's estimate of future inventory consumption. For certain products, we evaluated the estimate of future demand through an analysis of historical inventory sales by product and information obtained from the Company's manufacturing planning department. We obtained internal and external product inspection reports to identify inventory subject to remediation and evaluated the Company's assessment of obsolescence. We also compared the Company's estimate of net realizable value adjustments for identified excess or obsolete inventory to the prior period estimate and to actual inventory scrapping history.
/s/ KPMG LLP
We have served as the Company's auditor since 2014.
San Francisco, California
February 25, 2022
F-3
NATUS MEDICAL INCORPORATED
CONSOLIDATED BALANCE SHEETS
(In thousands, except share amounts)
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| ASSETS | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | 75,595 | $ | 82,082 | |||||||
| Accounts receivable, net of allowance for doubtful accounts of $5,271 and $6,213 | 111,760 | 93,133 | |||||||||
| Inventories | 67,745 | 75,650 | |||||||||
| Prepaid expenses and other current assets | 22,191 | 20,837 | |||||||||
| Total current assets | 277,291 | 271,702 | |||||||||
| Property and equipment, net | 21,783 | 24,516 | |||||||||
| Operating lease right-of-use assets | 9,288 | 11,669 | |||||||||
| Intangible assets, net | 65,513 | 92,741 | |||||||||
| Goodwill | 148,657 | 151,299 | |||||||||
| Deferred income tax | 23,161 | 27,563 | |||||||||
| Other assets | 18,595 | 20,904 | |||||||||
| Total assets | $ | 564,288 | $ | 600,394 | |||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | 36,405 | $ | 23,429 | |||||||
| Current portion of long-term debt | — | 50,000 | |||||||||
| Current portion of operating lease liabilities | 4,964 | 6,779 | |||||||||
| Accrued liabilities | 48,135 | 44,236 | |||||||||
| Deferred revenue | 25,097 | 21,308 | |||||||||
| Total current liabilities | 114,601 | 145,752 | |||||||||
| Long-term liabilities: | |||||||||||
| Other liabilities | 17,237 | 18,451 | |||||||||
| Long-term debt | — | 5,840 | |||||||||
| Operating lease liabilities | 6,567 | 8,959 | |||||||||
| Deferred income tax | 1,133 | 10,298 | |||||||||
| Total liabilities | 139,538 | 189,300 | |||||||||
| Commitments and contingencies (Note 20) | 0 | 0 | |||||||||
| Stockholders’ equity: | |||||||||||
| Common stock, $0.001 par value; 120,000,000 shares authorized; shares issued and outstanding 34,225,682 in 2021 and 33,911,703 in 2020 | 353,737 | 342,828 | |||||||||
| Preferred stock, $0.001 par value; 10,000,000 shares authorized; no shares issued and outstanding in 2021 and in 2020 | — | — | |||||||||
| Retained earnings | 84,486 | 71,309 | |||||||||
| Accumulated other comprehensive loss | (13,473) | (3,043) | |||||||||
| Total stockholders’ equity | 424,750 | 411,094 | |||||||||
| Total liabilities and stockholders’ equity | $ | 564,288 | $ | 600,394 | |||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-4
NATUS MEDICAL INCORPORATED
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Revenue | $ | 473,438 | $ | 415,684 | $ | 495,175 | |||||||||||
| Cost of revenue | 193,015 | 185,912 | 196,551 | ||||||||||||||
| Intangibles amortization | 6,743 | 13,241 | 6,916 | ||||||||||||||
| Gross profit | 273,680 | 216,531 | 291,708 | ||||||||||||||
| Operating expenses: | |||||||||||||||||
| Marketing and selling | 116,014 | 107,282 | 129,109 | ||||||||||||||
| Research and development | 56,306 | 61,296 | 58,733 | ||||||||||||||
| General and administrative | 52,753 | 49,113 | 59,649 | ||||||||||||||
| Intangibles amortization | 17,129 | 15,224 | 15,144 | ||||||||||||||
| Restructuring | 7,699 | 3,809 | 44,739 | ||||||||||||||
| Total operating expenses | 249,901 | 236,724 | 307,374 | ||||||||||||||
| Income (loss) from operations | 23,779 | (20,193) | (15,666) | ||||||||||||||
| Other expense, net | (4,424) | (1,872) | (5,591) | ||||||||||||||
| Income (loss) before provision (benefit) for income tax | 19,355 | (22,065) | (21,257) | ||||||||||||||
| Provision (benefit) for income tax | 6,178 | (5,452) | (5,586) | ||||||||||||||
| Net income (loss) | $ | 13,177 | $ | (16,613) | $ | (15,671) | |||||||||||
| Net income (loss) per share: | |||||||||||||||||
| Basic | $ | 0.39 | $ | (0.49) | $ | (0.47) | |||||||||||
| Diluted | $ | 0.39 | $ | (0.49) | $ | (0.47) | |||||||||||
| Weighted average shares used in the calculation of net income (loss) per share: | |||||||||||||||||
| Basic | 33,670 | 33,562 | 33,696 | ||||||||||||||
| Diluted | 33,974 | 33,562 | 33,696 | ||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-5
NATUS MEDICAL INCORPORATED
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In thousands, except per share amounts)
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net income (loss) | 13,177 | (16,613) | (15,671) | ||||||||||||||
| Other comprehensive income (loss): | |||||||||||||||||
| Foreign currency translation adjustment | (10,640) | 13,205 | (1,576) | ||||||||||||||
| Interest rate swap designated as a cash flow hedge | 119 | 27 | (180) | ||||||||||||||
| Reclassification of losses on interest rate swap designated as a cash flow hedge | 91 | — | — | ||||||||||||||
| Reclassification of stranded tax effects upon adoption of ASU 2018-02 | — | — | (1,332) | ||||||||||||||
Reclassification of deferred foreign currency related adjustments related to the sale of Medix (See FN 22) | — | — | 24,845 | ||||||||||||||
| Total other comprehensive income (loss) | (10,430) | 13,232 | 21,757 | ||||||||||||||
| Comprehensive income (loss) | $ | 2,747 | $ | (3,381) | $ | 6,086 | |||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-6
NATUS MEDICAL INCORPORATED
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| Common Stock | Retained Earnings | Accumulated Other Comprehensive Loss | Stockholders’ Equity | ||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||
| Balances, December 31, 2018 | 33,804,379 | $ | 334,215 | $ | 102,261 | $ | (38,032) | $ | 398,444 | ||||||||||||||||||||
| Reclassification of stranded tax effects for ASU 2018-02 | — | — | 1,332 | (1,332) | — | ||||||||||||||||||||||||
| Vesting of restricted stock units | 42,130 | — | — | — | — | ||||||||||||||||||||||||
| Net issuance of restricted stock awards | 175,833 | — | — | — | — | ||||||||||||||||||||||||
| Employee stock purchase plan | 53,839 | 1,354 | — | — | 1,354 | ||||||||||||||||||||||||
| Stock-based compensation expense | — | 8,315 | — | — | 8,315 | ||||||||||||||||||||||||
| Taxes paid related to net share settlement of equity awards | (51,784) | (1,689) | — | — | (1,689) | ||||||||||||||||||||||||
| Exercise of stock options | 124,303 | 2,281 | — | — | 2,281 | ||||||||||||||||||||||||
| Other comprehensive income | — | — | — | 23,089 | 23,089 | ||||||||||||||||||||||||
| Net loss | — | — | (15,671) | — | (15,671) | ||||||||||||||||||||||||
| Balances, December 31, 2019 | 34,148,700 | $ | 344,476 | $ | 87,922 | $ | (16,275) | $ | 416,123 | ||||||||||||||||||||
| Vesting of restricted stock units | 14,033 | — | — | — | — | ||||||||||||||||||||||||
| Net issuance of restricted stock awards | 204,057 | — | — | — | — | ||||||||||||||||||||||||
| Employee stock purchase plan | 73,347 | 1,314 | — | — | 1,314 | ||||||||||||||||||||||||
| Stock-based compensation expense | — | 9,527 | — | — | 9,527 | ||||||||||||||||||||||||
| Repurchase of company stock | (465,117) | (10,495) | — | — | (10,495) | ||||||||||||||||||||||||
| Taxes paid related to net share settlement of equity awards | (63,317) | (1,994) | — | — | (1,994) | ||||||||||||||||||||||||
| Other comprehensive income | — | — | — | 13,232 | 13,232 | ||||||||||||||||||||||||
| Net loss | — | — | (16,613) | — | (16,613) | ||||||||||||||||||||||||
| Balances, December 31, 2020 | 33,911,703 | $ | 342,828 | $ | 71,309 | $ | (3,043) | $ | 411,094 | ||||||||||||||||||||
| Vesting of restricted stock units | 205,082 | — | — | — | — | ||||||||||||||||||||||||
| Net issuance of restricted stock awards | 252,064 | — | — | — | — | ||||||||||||||||||||||||
| Employee stock purchase plan | 28,158 | 611 | — | — | 611 | ||||||||||||||||||||||||
| Stock-based compensation expense | — | 14,161 | — | — | 14,161 | ||||||||||||||||||||||||
| Taxes paid related to net share settlement of equity awards | (183,112) | (4,107) | — | — | (4,107) | ||||||||||||||||||||||||
| Exercise of stock options | 11,787 | 244 | — | — | 244 | ||||||||||||||||||||||||
| Other comprehensive income | — | — | — | (10,430) | (10,430) | ||||||||||||||||||||||||
| Net loss | — | — | 13,177 | 13,177 | |||||||||||||||||||||||||
| Balances, December 31, 2021 | 34,225,682 | $ | 353,737 | $ | 84,486 | $ | (13,473) | $ | 424,750 | ||||||||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-7
NATUS MEDICAL INCORPORATED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Operating activities: | |||||||||||||||||
| Net income (loss) | $ | 13,177 | $ | (16,613) | $ | (15,671) | |||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||||||||
| Provision for losses on accounts receivable | 181 | 1,190 | 4,262 | ||||||||||||||
| Depreciation and amortization | 28,091 | 28,115 | 30,722 | ||||||||||||||
| Loss on commencement of sales-type leases | 3 | 1,881 | — | ||||||||||||||
| Loss on equity method investment adjustment | 602 | 133 | — | ||||||||||||||
| (Gain) Loss on disposal of property and equipment | (1,426) | 268 | 449 | ||||||||||||||
| Impairment of intangible assets | 2,004 | 6,678 | — | ||||||||||||||
| Impairment of property and equipment | 279 | — | — | ||||||||||||||
| Impairment charge for sale of entity | — | — | 24,571 | ||||||||||||||
| Warranty reserve | 3,989 | 2,009 | 2,886 | ||||||||||||||
| Stock-based compensation | 14,161 | 9,566 | 8,352 | ||||||||||||||
| Non cash lease expense | — | 1,353 | — | ||||||||||||||
| Deferred taxes | (4,386) | (1,577) | (5,364) | ||||||||||||||
| Changes in operating assets and liabilities, net of assets and liabilities acquired in acquisitions: | |||||||||||||||||
| Accounts receivable | (13,111) | 17,651 | 7,139 | ||||||||||||||
| Inventories | 4,830 | 1,939 | 7,185 | ||||||||||||||
| Other assets | (1,880) | (1,055) | (2,486) | ||||||||||||||
| Accounts payable | 13,530 | (4,523) | (1,367) | ||||||||||||||
| Accrued liabilities | (507) | (13,427) | (4,010) | ||||||||||||||
| Deferred revenue | 4,458 | 838 | 3,392 | ||||||||||||||
| Net cash provided by operating activities | 63,995 | 34,426 | 60,060 | ||||||||||||||
| Investing activities: | |||||||||||||||||
| Acquisition of businesses, net of cash acquired | — | (1,997) | — | ||||||||||||||
| Acquisition of property and equipment | (3,620) | (8,609) | (5,326) | ||||||||||||||
| Acquisition of intangible assets | — | — | (13) | ||||||||||||||
| Proceeds from sale of property and equipment | 2,674 | — | — | ||||||||||||||
| Purchases of equity investments | (1,000) | (2,000) | — | ||||||||||||||
| Net cash used in investing activities | (1,946) | (12,606) | (5,339) | ||||||||||||||
| Financing activities: | |||||||||||||||||
| Proceeds from stock option exercises and ESPP | 855 | 1,314 | 3,635 | ||||||||||||||
| Principal payments of financing lease liability | (410) | (527) | (478) | ||||||||||||||
| Repurchase of company stock | — | (10,495) | — | ||||||||||||||
| Taxes paid related to net share settlement of equity awards | (4,107) | (1,994) | (1,689) | ||||||||||||||
| Proceeds from long-term borrowings | — | 60,000 | — | ||||||||||||||
| Deferred debt issuance costs | — | (1,175) | — | ||||||||||||||
| Payments on borrowings | (57,000) | (58,000) | (50,000) | ||||||||||||||
| Net cash used in financing activities | (60,662) | (10,877) | (48,532) | ||||||||||||||
| Exchange rate effect on cash and cash equivalents | (7,874) | 7,842 | 735 | ||||||||||||||
| Net increase (decrease) in cash and cash equivalents | (6,487) | 18,785 | 6,924 | ||||||||||||||
| Cash and cash equivalents, beginning of year | 82,082 | 63,297 | 56,373 | ||||||||||||||
| Cash and cash equivalents, end of year | $ | 75,595 | $ | 82,082 | $ | 63,297 | |||||||||||
| Supplemental disclosure of cash flow information: | |||||||||||||||||
| Cash paid for interest | $ | 1,180 | $ | 3,110 | $ | 4,580 | |||||||||||
| Cash paid for income taxes | $ | 3,782 | $ | 6,825 | $ | 6,445 | |||||||||||
| Non-cash investing activities: | |||||||||||||||||
| Property and equipment included in accounts payable | $ | 56 | $ | (51) | $ | 69 | |||||||||||
| Inventory transferred to property and equipment | $ | 1,167 | $ | 1,303 | $ | 300 | |||||||||||
| Transfer of leased assets to sales-type leases | $ | 13 | $ | 4,107 | $ | — | |||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-8
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2021, 2020 and 2019
1—ORGANIZATION AND SIGNIFICANT ACCOUNTING POLICIES
Organization
Natus Medical Incorporated (“we”, “our”, “us”) was incorporated in California in May 1987 and reincorporated in Delaware in August 2000. We are a leading provider of medical device solutions focused on the diagnosis and treatment of central nervous and sensory system disorders for patients of all ages. Product offerings include computerized neurodiagnostic systems for audiology, neurology, polysomnography, and neonatology, as well as newborn care products such as hearing screening systems, phototherapy devices for the treatment of newborn jaundice, software systems for managing and tracking disorders and diseases for public health laboratories, computer-based audiological, otoneurologic and vestibular instrumentation for hearing and balance care professionals.
Basis of Presentation and Principles of Consolidation
The accompanying Consolidated Financial Statements includes our accounts and accounts of our wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation. Certain reclassifications to the prior periods have been made to conform to the current period presentation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities in the Consolidated Financial Statements and the reported amount of revenue and expenses during the reporting period. Such estimates include allowances for potentially uncollectible accounts receivable, valuation of inventory, intangible assets, goodwill, share-based compensation, valuation of deferred income taxes, reserves for warranty obligations, and the provision for income taxes including uncertain positions. Actual results could differ from those estimates.
Revenue recognition
Revenue is recognized when obligations under the terms of a contract with a customer are satisfied; generally this occurs with the transfer of control of devices, supplies, or services. Revenue is measured as the amount of consideration we expect to receive in exchange for transferring goods or providing services.
For the majority of devices and supplies, we transfer control and recognize revenue when products ship from the warehouse to the customer. We generally do not provide rights of return on devices and supplies. Freight charges billed to customers are included in revenue and freight-related expenses are charged to cost of revenue.
Depending on the terms of the arrangement, we may also defer the recognition of a portion of the consideration received because we have to satisfy a future obligation (e.g. installation). Judgment is required to determine the standalone selling price for each distinct performance obligation. Our estimate of SSP is a point estimate. The estimate is calculated annually for each performance obligation that is not sold separately. In instances where SSP is not directly observable, such as when we do not sell the product or service separately, the SSP is determined using information that may include market conditions and other observable inputs.
We sell separately-priced service contracts that extend maintenance coverages for both medical devices and data management systems beyond the base agreements to customers. The separately priced service contracts range from 12 months to 60 months. We receive payment at the inception of the contract and recognize revenue ratably over the service period.
For products containing embedded software, we have determined that the hardware and software components function together to deliver the products' essential functionality and are considered a combined performance obligation. Revenue recognition policies for sales of these products are substantially the same as for other tangible products.
Inventory Valuation
Inventories are carried at the lower of cost or net realizable value, with cost being determined using the first-in, first-out method. The carrying value of our inventory is reduced for any difference between cost and estimated net realizable value of the inventory. We determine net realizable value by evaluating ending inventories for excess quantities, obsolescence, and other factors that could impact our ability to consume inventory for its intended use. Our evaluation includes an analysis of historical sales by product, projections of future demand by product, and an analysis of obsolescence by product. Adjustments to the
F-9
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
value of inventory establish a new cost basis and are considered permanent even if circumstances later suggest that increased carrying amounts are recoverable. If demand is higher than expected, we may sell inventory that had previously been written down.
Intangible assets
We amortize intangible assets with finite lives over the estimate of their useful lives. Any future changes that would limit their useful lives or any determination that these assets are carried at amounts greater than their estimated fair value could result in acceleration of amortization over a revised useful life.
We review intangible assets with indefinite lives for impairment on an annual basis and finite lives for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of the finite-lived intangible assets is assessed based on the estimated undiscounted future cash flows expected to result from the use and eventual disposition of the asset. If the undiscounted future cash flows are less than the carrying amount, the finite-lived intangible assets are considered to be impaired. The amount of the impairment loss, if any, is measured as the difference between the carrying amount of the asset and its fair value. We estimate the fair value of finite-lived intangible assets by using an income approach or, when available and appropriate, using a market approach.
Goodwill
Goodwill is not amortized but is subject to an annual impairment analysis, which is performed as of October 1st; this assessment is also performed whenever there is a change in circumstances that indicates the carrying value of goodwill may be impaired.
Goodwill is tested for impairment at the reporting unit level. Our 3 strategic business units, Neuro, Newborn Care and Hearing & Balance are consolidated into one unit. We have concluded that we have 1 operating segment and 1 reporting unit for purposes of goodwill impairment testing in 2021, 2020, and 2019.
In accordance with accounting standards we perform a qualitative assessment to test goodwill for impairment prior to the performing the first step of the goodwill impairment process. Qualitative factors considered in this assessment include industry and market considerations, overall financial performance and other relevant events and factors affecting the reporting unit.
Based on the qualitative assessment in 2021, 2020, and 2019, we determined the fair value of goodwill was more likely than not greater than its carrying amount, and no further analysis was needed.
Lessee Leases
We determine if an arrangement is a lease at inception of the lease. Right-of-use (“ROU”) assets represent the right to use an underlying asset for the lease term, and lease liabilities represent an obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit borrowing rate, generally we use an incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at the lease commencement date. We use the implicit rate when readily determinable. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. Our lease terms may include options to exclude or terminate the lease when it is reasonably certain that they will exercise that option. Lease expense for lease payments are recognized on a straight-line basis over the lease term.
Operating leases are included in operating lease ROU assets, accrued liabilities, and operating lease liabilities in our consolidated balance sheet. Finance leases are included in property and equipment, accrued liabilities, and other liabilities in the consolidated balance sheet.
We have lease agreements with lease and non-lease components, which are generally accounted for based on the type of asset. For real estate and telecom leases, we account for these components separately. For equipment leases, such as office equipment and vehicles, we account for the lease and non-lease components as a single lease component.
Lessor Leases
We determine if an arrangement is a lease at inception of the lease. We entered into a five-year non-cancelable exclusive supply agreement with a customer to provide hearing screening products which commenced on January 1, 2020. The agreement includes automatic renewal options for successive three-year periods. Notice of non-renewal must be provided to Natus at least twelve months prior to the end of the current term. The agreement does not contain any purchase options or residual value guarantees.
F-10
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
Pricing of the exclusive supply agreement is structured on a fee per baby screened basis. The per baby fee is inclusive of all products and services to be provided under the terms of the agreement which include hearing screening hardware, consumables required to complete each screen, and related maintenance. The pricing structure also includes a guaranteed minimum payment per unit per contract year for each unit of equipment supplied.
Sales-Type Leases
The guaranteed minimum payment for each unit of equipment supplied represents the fixed transaction price and is allocated to separate performance obligations, consisting of hardware products, consumables, and maintenance, proportionally based on the standalone selling price of each performance obligation. Standalone selling price is best evidenced by the price we charge for the good or service when selling it separately in similar circumstances to similar customers. Other than for the renewal of annual support services contracts, our products and services are not generally sold separately. We use an amount discounted from the list price as a best estimated selling price.
For sales-type leases, we recognize revenues for hearing screening hardware products at the net present value of their guaranteed minimum payment standalone selling price allocation upon delivery of the hardware. We recognize consumables and maintenance revenues associated with the sales-type leases over the term of the agreement on a weighted average as actual screens are performed.
Operating Leases
Under the terms of the exclusive supply agreement, hospitals where we were previously providing hearing screening services were provided the option to transition to the exclusive supply agreement. Hospitals which chose to transition continued to use their existing Natus hardware. Based on the remaining useful life of the existing hardware and the date new hardware is supplied after the commencement of the lease, some of these leases were classified as operating leases.
For operating leases, rental income is recognized on a straight-line basis over the term of the associated lease. Leased assets classified as operating leases are carried at amortized cost net of accumulated depreciation in property and equipment, net on the condensed consolidated balance sheet. The depreciation expense of the leased assets is recognized on a straight-line basis over the useful life of the asset, and recorded in cost of revenue in the condensed consolidated statements of operations.
Long lived assets
We continually monitor events and changes in circumstances that could indicate that carrying amounts of our long-lived assets, including property and equipment, intangible assets and leases, may not be recoverable. When such events or changes in circumstances occur, we will assess the recoverability by determining whether the carrying value of an asset group will be recovered through undiscounted expected future cash flows. If the future undiscounted cash flows are less than the carrying amount of the asset group, we will recognize an impairment loss based on the excess of the carrying amount over the fair value of the assets.
Liability for product warranties
We provide a warranty for products that is generally one year in length. In some cases, regulations may require us to provide repair or remediation beyond the typical warranty period. If any products contain defects, we may be required to incur additional repair and remediation costs. Service, repair and calibration services are provided by a combination of our owned facilities and vendors on a contract basis.
We accrue estimated product warranty costs at the time of sale based on historical experience. A warranty reserve is included in accrued liabilities for the expected future costs of servicing products. Additions to the reserve are based on management’s best estimate of probable liability. We consider a combination of factors including material and labor costs, regulatory requirements, and other judgments in determining the amount of the reserve. The reserve is reduced as costs are incurred to honor existing warranty and regulatory obligations.
Share-based compensation
We recognize share-based compensation expense associated with employee stock options under the single-option straight line method over the requisite service period, which is generally a four-year vesting period and ten-year contractual term pursuant to ASC Topic 718, Compensation-Stock Compensation. See Note 15 of the Consolidated Financial Statements.
For employee stock options, the value of each option is estimated on the date of grant using the Black-Scholes option pricing model, which was developed for use in estimating the value of freely traded options. Similar to other option pricing models, the Black-Scholes method requires the input of highly subjective assumptions, including stock price volatility. Changes in the subjective input assumptions can materially affect the estimated fair value of the employee stock options.
F-11
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
We recognize share-based compensation associated with Restricted Stock Awards (“RSA”) and Restricted Stock Units (“RSU”). RSAs and RSUs vest ratably over a three-year period for employees. RSAs and RSUs for executives vest over a four-year period; 25% on each of the annual anniversaries. RSAs and RSUs for non-employees (Board of Directors) vest over a one-year period; 100% on the first anniversary. The value is estimated based on the market value of Natus common stock on the date of issuance pursuant to ASC Topic 718, Compensation-Stock Compensation.
We grant market stock unit (“MSU”) awards to certain employees. We estimate the fair value of MSUs at the date of grant using a Monte Carlo simulation model and amortize those fair values over the requisite service period, which is generally three years. The Monte Carlo simulation model that we use to estimate the fair value of market-based MSUs at the date of grant incorporates into the valuation the possibility that the market condition may not be satisfied. Provided that the requisite service is rendered, the total fair value of the market-based MSUs, which is determined at the date of grant, must be recognized as compensation expense even if the market condition is not achieved. However, the number of shares that ultimately vest can vary significantly with the achievement of the specified market criteria.
We also grant performance stock unit (“PSU”) awards to our CEO and CFO. These PSUs fully vest after a performance period and have separate performance goals than MSUs. We estimate fair value of performance stock unit awards based on the share price and other pertinent factors on the grant date. Compensation expense for performance stock unit awards are recognized on a straight-line basis over the requisite service period of the award when performance condition is probable to occur. We assess the achievement probability of the performance conditions each period and adjust compensation expense if necessary. Provided that the requisite service is rendered, the shares will become vested and payout will occur based on the outcome of the performance condition. Any unrecognized compensation cost shall be recognized when the award becomes vested.
We issue new shares of common stock upon the exercise of stock options and the vesting of RSAs, RSUs, MSUs, and PSUs.
Forfeitures of employee stock options and awards are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from initial estimates. Share-based compensation expense is recorded net of estimated forfeitures, such that expense is recorded only for those share-based awards that are expected to vest.
Cash Equivalents
All highly liquid investments purchased with an original maturity or remaining maturity upon purchase of three months or less are classified as cash equivalents.
Allowance for Doubtful Accounts
We estimate the allowance for potentially uncollectible accounts receivable based on historical collection experience within the markets in which we operate and other customer-specific information, such as bankruptcy filings or customer liquidity problems. When all internal efforts have been exhausted to collect the receivable, it is written off and relieved from the reserve.
Fair Value of Financial Instruments
Financial instruments include cash and cash equivalents, accounts receivable, and accounts payable. Cash is reported at its fair value on the balance sheet dates. The recorded carrying amounts of accounts receivable and accounts payable approximate their fair values due to the short-term maturities.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation expense is computed using the straight-line method over estimated useful lives of the respective assets, which are three to five years for office furniture and equipment, computer software and hardware, demonstration and loaned equipment, and 30 to 40 years for buildings. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the improvement. Land is not depreciated. Costs associated with acquiring and installing software to be used for internal purposes are capitalized and amortized on a straight-line basis over three years.
Research & Development Costs
Costs incurred in research and development are charged to operations as incurred.
F-12
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
Income Taxes
We account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statements carrying value of assets and liabilities and the tax basis of those assets and liabilities, using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
We record net deferred tax assets to the extent it is more likely than not that the assets will be realized. In making such determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial operations. To the extent that previously reserved deferred tax assets are estimated to be realizable, we adjust the valuation allowance which reduces the provision for income taxes.
We recognize the tax benefit of uncertain tax positions in the financial statements as defined in ASC Topic 740, Income Tax. When the tax position is deemed more likely than not of being sustained, we recognize the largest amount of tax benefit that is greater than 50 percent likely of being ultimately realized upon settlement, as defined in ASC 740-10-05.
Foreign Currency
The functional currency of our subsidiaries outside of North America is generally the local currency of the country where the subsidiary is located. Accordingly, foreign currency translation adjustments relating to the translation of foreign subsidiary financial statements are included as a component of accumulated other comprehensive loss. We have recorded $(10.6) million, $13.2 million, and $(1.6) million of foreign currency translation gains (losses) for the years ended December 31, 2021, 2020 and 2019, respectively.
Gains and losses from transactions denominated in currencies other than the functional currencies are included in other income and expense. In 2021, 2020, and 2019, net foreign currency transaction gains (losses) were $(2.0) million, $2.0 million, and $(0.8) million, respectively. Foreign currency gains and losses result primarily from fluctuations in the exchange rate between the U.S. dollar, Canadian dollar, Euro, British pound, and Danish kroner.
We divested our wholly owned subsidiary, Medix, on April 2, 2019 via a stock sale. Included in the year ended December 31, 2019 is the impact of the sale of Medix, which was completed as of June 30, 2019, and the deferred foreign currency related translation adjustments previously in accumulated other comprehensive income have been released from the balance sheet along with the held for sale accrual (See Note 22 - Sale of a Certain Subsidiary).
Comprehensive Income
We break out the major components and report as a single total the change in net assets during the period as defined in ASC Topic 220, Comprehensive Income. The consolidated statement of comprehensive income (loss) has been separately stated from the consolidated statements of operations. Accumulated other comprehensive loss consists of translation gains and losses on foreign subsidiary financial statements, interest rate swap designated as a cash flow hedge, reclassifications from the adoption of ASU 2018-02, and reclassification of previously recorded deferred foreign currency related translation adjustment losses upon the divestiture of Medix.
Basic and Diluted Net Income per Share
We compute net income per share as defined in ASC Topic 260, Earnings per Share. Basic net income per share is based upon the weighted average number of common shares outstanding during the period. Diluted net income per share is based upon the weighted average number of common shares outstanding and dilutive common stock equivalents outstanding during the period. Common stock equivalents are options granted, shares of restricted stock, and shares of market stock issued under the stock awards plans and are calculated under the treasury stock method. Common equivalent shares from unexercised stock options and restricted stock are excluded from the computation when there is a loss as the effect is anti-dilutive, or if the exercise price of such options is greater than the average market price of the stock for the period.
Recently Adopted Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740), Simplifying the Accounting for Income Tax. This update includes removal of certain exceptions to the general principles of ASC 740, Income taxes, and simplification in several other areas such as accounting for franchise tax (or similar tax) that is partially based on income. We early adopted the ASU in the second quarter of 2020. Upon adoption, we recorded an additional income tax benefit of $0.9 million due to the elimination of the year-to-date loss limitation rules that limited the interim tax period tax benefit.
F-13
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
In October 2020, the FASB issued ASU 2020-10, Codification Improvements. This update improves consistency by amending the Codification to include all disclosure guidance in appropriate disclosure sections. Also, the update clarifies application of various provisions in the Codification by amending and adding new headings, cross-referencing to other guidance, and refining or correcting terminology. ASU 2020-10 was effective for annual period starting December 15, 2020. The adoption of this ASU did not have an impact on our consolidated financial statements.
In July 2021, the FASB issued ASU 2021-05, Leases (Topic 842): Lessors-Certain Leases with Variable Lease Payments. This update addresses stakeholders' concerns by amending the lease classification requirements for lessors to align them with practice under Topic 840. Lessors should classify and account for a lease with variable lease payments that do not depend on a reference index or a rate as an operating lease if the lease would have been classified as a sales-type lease or a direct financing lease in accordance with 842-10-25-2 through 25-3 and the lessors would have otherwise recognized a day-one loss. ASU 2021-05 is effective for fiscal years beginning after December 15, 2021. We have early adopted the ASU on a prospective basis in the third quarter of the current fiscal year. The adoption of ASU 2021-05 did not have an impact on our consolidated financial statements.
2—REVENUE
Contract assets for the periods presented primarily represent the difference between revenue recognized based on the relative selling price of the related performance obligations and the contractual billing terms in the arrangements. Deferred revenue for the periods presented was primarily related to extended service contracts, installation, and training, for which the service fees are billed up-front. The associated deferred revenue is generally recognized ratably over the extended service period or when installation and training are complete.
The following table summarized the changes in the contract assets and contract liability balances for the year ended December 31, 2021 (in thousands):
| Unbilled AR, December 31, 2020 | $ | 1,925 | |||
| Additions | 58 | ||||
| Transferred to Trade Receivable | (1,731) | ||||
| Unbilled AR, December 31, 2021 | $ | 252 | |||
| Deferred Revenue, December 31, 2020 | $ | 25,723 | |||
| Additions | 24,053 | ||||
| Revenue Recognized | (19,679) | ||||
| Deferred Revenue, December 31, 2021 | $ | 30,097 | |||
At December 31, 2021, the contract assets of $0.3 million were included in accounts receivable in the consolidated balance sheet. At December 31, 2021, the short-term portion of the contract liability of $25.1 million and the long-term portion of $5.0 million were included in deferred revenue and other long-term liabilities respectively, in the consolidated balance sheet. As of December 31, 2021, we expect to recognize revenue associated with deferred revenue of approximately $25.0 million in 2022, $2.7 million in 2023, $1.5 million in 2024, $0.5 million in 2025, and $0.4 million thereafter.
F-14
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
3—ALLOWANCE FOR DOUBTFUL ACCOUNTS
We estimate the lifetime allowance for doubtful, potentially uncollectible, accounts receivable upon their inception based on historical collection experience within the markets in which we operate, customer-specific information such as bankruptcy filings or customer liquidity problems, current conditions, and reasonable and supportable forecasts about the future.
Our allowance for doubtful accounts is presented as a reduction to accounts receivable on our consolidated balance sheet. When all internal efforts have been exhausted to collect the receivable, it is written off and relieved from the reserve.
The details of activity in allowance for doubtful accounts are as follows (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Balance, beginning of period | $ | 6,213 | $ | 7,384 | |||||||
| Additions charged to expense | 181 | 1,190 | |||||||||
| Write-offs charged against allowance | (1,123) | (2,361) | |||||||||
| Balance, end of period | $ | 5,271 | $ | 6,213 | |||||||
4—INVENTORIES
Inventories consist of (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Raw materials and subassemblies | $ | 20,750 | $ | 22,583 | |||||||
| Work in process | 2,825 | 2,294 | |||||||||
| Finished goods | 57,836 | 65,695 | |||||||||
| Total Inventories | 81,411 | 90,572 | |||||||||
| Less: Non-current Inventories | (13,666) | (14,922) | |||||||||
| Inventories | $ | 67,745 | $ | 75,650 | |||||||
Non-current inventory consists of service components used to repair products held by customers pursuant to warranty obligations and extended service contracts, including service components for products that we no longer sell, inventory purchased for lifetime buys, inventory built as a last-time build, and inventory that is turning at a slow rate. We believe that these inventories will be utilized for their intended purpose.
5—PROPERTY AND EQUIPMENT
Property and equipment consist of (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Land | $ | 1,782 | $ | 1,792 | |||||||
| Buildings | 7,238 | 7,365 | |||||||||
| Leasehold improvements | 7,722 | 8,050 | |||||||||
| Finance lease right-of-use assets | 2,356 | 2,555 | |||||||||
| Office furniture and equipment | 20,637 | 22,148 | |||||||||
| Computer software and hardware | 11,308 | 10,246 | |||||||||
| Demonstration and loaned equipment | 4,050 | 3,086 | |||||||||
| 55,093 | 55,242 | ||||||||||
| Accumulated depreciation | (33,310) | (30,726) | |||||||||
| Total | $ | 21,783 | $ | 24,516 | |||||||
Depreciation expense of property and equipment was $6.3 million, $6.2 million, and $6.6 million in the years ending December 31, 2021, 2020 and 2019, respectively.
F-15
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
6—INTANGIBLE ASSETS
The following table summarizes the components of gross and net intangible asset balances (in thousands):
| December 31, 2021 | December 31, 2020 | ||||||||||||||||||||||||||||||||||||||||||||||
| Gross Carrying Amount | Accumulated Impairment | Accumulated Amortization | Net Book Value | Gross Carrying Amount | Accumulated Impairment | Accumulated Amortization | Net Book Value | ||||||||||||||||||||||||||||||||||||||||
| Intangible assets with definite lives: | |||||||||||||||||||||||||||||||||||||||||||||||
| Technology | $ | 99,920 | (6,039) | $ | (66,679) | $ | 27,202 | $ | 112,138 | (12,480) | $ | (64,203) | $ | 35,455 | |||||||||||||||||||||||||||||||||
| Customer related | 89,794 | (50) | (57,133) | 32,611 | 94,526 | (50) | (51,247) | 43,229 | |||||||||||||||||||||||||||||||||||||||
| Trade names | 45,593 | (3,260) | (36,960) | 5,373 | 47,058 | (3,677) | (31,890) | 11,491 | |||||||||||||||||||||||||||||||||||||||
| Internally developed software | 13,281 | — | (12,985) | 296 | 13,281 | — | (12,845) | 436 | |||||||||||||||||||||||||||||||||||||||
| Patents | 2,705 | (133) | (2,572) | — | 2,810 | (133) | (2,677) | — | |||||||||||||||||||||||||||||||||||||||
| Service Agreements | 800 | — | (769) | 31 | 1,190 | — | (1,119) | 71 | |||||||||||||||||||||||||||||||||||||||
| Total definite-lived intangible assets | 252,093 | (9,482) | (177,098) | 65,513 | 271,003 | (16,340) | (163,981) | 90,682 | |||||||||||||||||||||||||||||||||||||||
| Intangible assets with indefinite lives: | |||||||||||||||||||||||||||||||||||||||||||||||
| Intellectual Property | 2,004 | (2,004) | — | — | 2,059 | — | — | 2,059 | |||||||||||||||||||||||||||||||||||||||
| Total intangible assets | 254,097 | (11,486) | (177,098) | 65,513 | 273,062 | (16,340) | (163,981) | 92,741 | |||||||||||||||||||||||||||||||||||||||
Finite lived intangible assets are amortized over their weighted average lives, which are 14 years for technology, 13 years for patents, 10 years for customer-related intangibles, 7 years for trade names, 6 years for internally developed software, 2 years for service agreements, and 11 years weighted average in total.
Internally developed software consists of $11.1 million relating to costs incurred for development of internal use computer software and $2.2 million for development of software to be sold.
During the third quarter of 2020 we recorded an impairment charge related to intangible assets of $6.7 million of which $6.4 million was recorded within intangibles amortization within cost of revenue and $0.3 million was recorded within intangibles amortization within operating expense on our income statement. The impairment related to an end of life decision for an acquired tradename and technology at which time we made the decision to discontinue sales of the related product rather than continuing to invest in the product.
During the fourth quarter of 2021 we recorded an impairment charge related to intangible assets of $2.0 million which was recorded within intangibles amortization within operating expense on our income statement. The impairment was a management decision to abandon further development of the product using intellectual property.
Amortization expense related to intangible assets with finite and indefinite lives, including impairment charges described above, was as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Technology | $ | 6,907 | $ | 13,417 | $ | 6,906 | |||||||||||
| Customer related | 9,040 | 8,825 | 8,662 | ||||||||||||||
| Trade names | 5,881 | 6,182 | 6,111 | ||||||||||||||
| Intellectual Property | 2,004 | — | — | ||||||||||||||
| Internally developed software | 141 | 239 | 1,438 | ||||||||||||||
| Patents | — | — | 60 | ||||||||||||||
| Service Agreements | 40 | 40 | 322 | ||||||||||||||
| Total amortization | $ | 24,013 | $ | 28,703 | $ | 23,499 | |||||||||||
The amortization expense amounts shown above include internally developed software not held for sale of $0.1 million, $0.2 million, and $1.3 million for the years ended 2021, 2020, and 2019, respectively. The amortization expense for internally developed software not held for sale is recorded within our income statement as a general and administrative operating expense. The amount in the table shown above for intellectual property relates to the impairment charge recorded during the fourth quarter of 2021.
F-16
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
Expected annual amortization expense related to amortizable intangible assets is as follows (in thousands):
| 2022 | $ | 17,634 | |||
| 2023 | 14,865 | ||||
| 2024 | 12,970 | ||||
| 2025 | 12,362 | ||||
| 2026 | 2,408 | ||||
| Thereafter | 5,274 | ||||
| Total expected amortization expense | $ | 65,513 | |||
7—GOODWILL
The carrying amount of goodwill and the changes in those balances are as follows (in thousands):
| As of December 31, 2019 | $ | 146,367 | |||
| Purchase Accounting Adjustments | 1,207 | ||||
| Foreign currency translation | $ | 3,725 | |||
| As of December 31, 2020 | 151,299 | ||||
| Foreign currency translation | (2,642) | ||||
| As of December 31, 2021 | $ | 148,657 | |||
8—LEASES
Lessee Leases
We have operating and finance leases for offices, warehouses, and certain equipment. The leases have remaining lease terms of one to eight years, some of which include options to extend the leases for up to ten years. Our leases do not have any residual value guarantees or any restrictions or covenants imposed by leases.
Components of lease cost were as follows (in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Operating lease cost | $ | 6,046 | $ | 6,696 | |||||||
| Finance lease cost: | |||||||||||
| Amortization of right-of-use assets (principal payments) | 384 | 395 | |||||||||
| Interest on lease liabilities | 34 | 49 | |||||||||
| Short-term lease cost | 22 | 7 | |||||||||
| Variable lease cost | 1,958 | 2,541 | |||||||||
| Sublease income | (81) | (61) | |||||||||
| Total lease cost | $ | 8,363 | $ | 9,627 | |||||||
Supplemental cash flow information related to leases was as follows (in thousands):
F-17
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Cash paid for amounts included in the measurement of lease liabilities: | |||||||||||
| Operating cash flows from operating leases | $ | 12,636 | $ | 13,979 | |||||||
| Operating cash flows from finance leases | 19 | 32 | |||||||||
| Financing cash flows from finance leases | 410 | 527 | |||||||||
| Right-of-use assets obtained in exchange for lease obligations: | |||||||||||
| Operating leases | 3,055 | 3,290 | |||||||||
| Finance leases | 98 | 337 | |||||||||
Supplemental balance sheet information related to leases was as follows (in thousands):
| December 31, 2021 | December 31, 2020 | ||||||||||
| Operating Leases | |||||||||||
| Operating lease right-of-use assets | $ | 9,288 | $ | 11,669 | |||||||
| Current portion of operating lease liabilities | $ | 4,964 | $ | 6,779 | |||||||
| Operating lease liabilities | 6,567 | 8,959 | |||||||||
| Total operating lease liabilities | $ | 11,531 | $ | 15,738 | |||||||
| Finance Leases | |||||||||||
| Property and equipment, gross | $ | 2,356 | $ | 2,555 | |||||||
| Accumulated amortization | (1,906) | (1,705) | |||||||||
| Property and equipment, net | $ | 450 | $ | 850 | |||||||
| Accrued liabilities | $ | 231 | $ | 414 | |||||||
| Other liabilities | 230 | 446 | |||||||||
| Total finance lease liabilities | $ | 461 | $ | 860 | |||||||
| Weighted Average Remaining Lease Term | |||||||||||
| Operating leases | 2.97 years | 3.33 years | |||||||||
| Finance leases | 2.40 years | 2.68 years | |||||||||
| Weighted Average Discount Rate | |||||||||||
| Operating leases | 5.4 | % | 5.4 | % | |||||||
| Finance leases | 5.1 | % | 5.1 | % | |||||||
As of December 31, 2021, future minimum lease payments included in the measurement of lease liabilities on the consolidated balance sheet, for the following five fiscal years and thereafter, were as follows (in thousands):
F-18
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
| Year ending December 31, | Operating Leases | Finance Leases | |||||||||
| 2022 | $ | 5,440 | $ | 240 | |||||||
| 2023 | 3,404 | 159 | |||||||||
| 2024 | 1,959 | 63 | |||||||||
| 2025 | 957 | 17 | |||||||||
| 2026 | 630 | — | |||||||||
| Thereafter | 105 | — | |||||||||
| Total lease payments | 12,495 | 479 | |||||||||
| Less imputed interest | (964) | (18) | |||||||||
| Total | $ | 11,531 | $ | 461 | |||||||
Lessor Leases
Pricing of our exclusive hearing screening supply agreement is structured on a fee per baby screened basis and includes a guaranteed minimum payment per unit per contract year for each unit of equipment supplied. We recognize revenues for the hardware products upon commencement of each lease at the net present value of their guaranteed minimum payment standalone selling price allocation. The remaining variable fees are recognized as revenue over the remaining lease term. The following table provides the profit recognized for sales-type leases at their commencement (net sales includes the initial allocation of fixed costs plus allocation of incremental variable fees and excludes the revenue associated with non-lease components associated with hearing screening supplies and maintenance that are recognized as revenue over the remaining lease term) (in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Net Sales | $ | 10 | $ | 2,226 | |||||||
| Cost of Sales | 13 | 4,107 | |||||||||
| Gross Loss | $ | (3) | $ | (1,881) | |||||||
The following table provides lease income related to variable lease payments on our sales-type leases not included in the measurement of the lease receivable (in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Variable lease income | $ | 877 | $ | 420 | |||||||
The following table provides lease income on our operating leases (in thousands):
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Lease income on operating leases | $ | 197 | $ | 291 | |||||||
The receivables as a result of our sales-type leases are collateralized by the underlying equipment leased and consist of the following components (in thousands):
F-19
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
| Year Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Net minimum lease payments to be received | $ | 1,209 | $ | 1,777 | |||||||
| Less: Unearned interest income portion | — | — | |||||||||
| Net investment in sales-type leases | $ | 1,209 | $ | 1,777 | |||||||
| Less: Current portion | (447) | (444) | |||||||||
| Long-term net investment in sales-type leases | $ | 762 | $ | 1,333 | |||||||
As of December 31, 2021, future minimum lease payments and the reconciliation to net investment in sales-type leases reported on the consolidated balance sheet, for the following five fiscal years and thereafter, were as follows (in thousands):
| Year Ended December 31, | Sales-Type Leases | Operating Leases | |||||||||
| 2022 | 447 | — | |||||||||
| 2023 | 399 | — | |||||||||
| 2024 | 363 | — | |||||||||
| 2025 | — | — | |||||||||
| 2026 | — | — | |||||||||
| Thereafter | — | — | |||||||||
| Total future minimum lease payments | 1,209 | — | |||||||||
| Less: imputed interest | — | — | |||||||||
| Total | $ | 1,209 | $ | — | |||||||
9—ACCRUED LIABILITIES
Accrued liabilities consist of (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Compensation and related benefits | $ | 22,734 | $ | 20,409 | |||||||
| Accrued federal, state, and local taxes | 9,855 | 9,118 | |||||||||
| Warranty reserve | 6,042 | 5,195 | |||||||||
| Accrued professional fees | 3,573 | 3,699 | |||||||||
| Accrued amounts due to customers | 2,597 | 2,743 | |||||||||
| Self-funded insurance expense | 835 | 911 | |||||||||
| Accrued travel | 794 | 123 | |||||||||
| Accrued selling expenses | 494 | 311 | |||||||||
| Other | 1,211 | 1,727 | |||||||||
| Total | $ | 48,135 | $ | 44,236 | |||||||
10—LONG-TERM OTHER LIABILITIES
F-20
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
Long-term other liabilities consist of (in thousands):
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Long-term taxes payable | $ | 10,678 | $ | 10,949 | |||||||
| Non-current deferred revenue | 5,001 | 4,416 | |||||||||
| Long-term earnout payable | 1,184 | 1,286 | |||||||||
| Finance lease liabilities | 230 | 446 | |||||||||
| CARES Act payroll tax payable | — | 1,354 | |||||||||
| Other | 144 | — | |||||||||
| Total | $ | 17,237 | $ | 18,451 | |||||||
11—DEBT AND CREDIT ARRANGEMENTS
We have a Credit Agreement with JP Morgan Chase Bank ("JP Morgan"), Citibank, NA (“Citibank”), and Wells Fargo Bank, National Association (“Wells Fargo”). During the third quarter of 2020 we amended the terms of the Credit Agreement to extend the maturity date of the original agreement, reduce the aggregate value of the revolving facility, and amend certain covenants. The amended Credit Agreement provides for an aggregate $150 million of secured revolving credit facility. The Credit Agreement contains covenants, including covenants relating to maintenance of books and records, financial reporting and notification, compliance with laws, maintenance of properties and insurance, and limitations on guaranties, investments, issuance of debt, lease obligations and capital expenditures, and is secured by virtually all of our assets. The Credit Agreement provides for events of default, including failure to pay any principal or interest when due, failure to perform or observe covenants, bankruptcy or insolvency events and the occurrence of the event has a material adverse effect. We have no other significant credit facilities. As of December 31, 2021, no amounts were outstanding under the Credit Agreement.
In addition to the customary restrictive covenants listed above, the Credit Agreement also contains financial covenants that require us to maintain a certain leverage ratio and fixed charge coverage ratio, each as defined in the Credit Agreement:
•Leverage Ratio, as defined, to be no higher than 3.25 to 1.00.
•Interest Coverage Ratio, as defined, to be at least 1.75 to 1.00 at all times.
Pursuant to the terms of the Credit Agreement, the outstanding principal balance will bear interest at either (a) a fluctuating rate per annum equal to the Applicable Rate, as defined in the Credit Agreement, depending on our leverage ratio plus the higher of (i) the federal funds rate plus one-half of one percent per annum; (ii) the prime rate in effect on such a day; and (iii) the LIBOR rate plus one percent, or (b) a fluctuating rate per annum of LIBOR Rate plus the Applicable Rate, which ranges between 2.25% to 3.50%. The Credit Agreement matures on September 25, 2023, at which time all principal amounts outstanding under the Credit Agreement will be due and payable.
As of June 30, 2021, we have discontinued our interest rate swap derivative. There are no amounts outstanding under our Credit Agreement and the termination date of the hedge was September 23, 2021. Upon the discontinuation of the hedge relationship, we have reclassified the unrealized gains or losses recorded in accumulated other comprehensive income (“AOCI”) to earnings.
12—RESERVE FOR PRODUCT WARRANTIES
We provide a warranty for products that is generally one year in length and in some cases, regulations may require us to provide repair or remediation beyond the typical warranty period. If any of the products contain defects, we may be required to incur additional repair and remediation costs. Service, repair and calibration services are provided by a combination of our owned facilities and vendors on a contract basis.
A warranty reserve is included in accrued liabilities for the expected future costs of servicing products. Additions to the reserve are based on management's best estimate of probable liability. We consider a combination of factors including material and labor costs, regulatory requirements, and other judgments in determining the amount of reserve. The reserve is reduced as costs are incurred to honor existing warranty and regulatory obligations.
As of December 31, 2021, we have accrued $6.0 million for product related warranties. The estimates we use in projecting future product warranty costs may prove to be incorrect. Any future determination that product warranty reserves are understated could result in increases to cost of sales and reductions in operating profits and results of operations.
F-21
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
13—STOCKHOLDERS’ EQUITY
Common Stock—We have 120,000,000 shares of common stock authorized at a par value or $0.001 per share.
Preferred Stock—We have 10,000,000 shares of preferred stock authorized at a par value of $0.001 per share. In accordance with the terms of the amended and restated certificate of incorporation, the Board of Directors is authorized to provide for the issuance of one or more series of preferred stock, including increases or decreases to the series. The Board of Directors has the authority to set the rights, preferences, and terms of such shares. As of December 31, 2021, no shares of preferred stock were issued and outstanding.
14—EARNINGS PER SHARE
The components of basic and diluted EPS are as follows (in thousands, except per share amounts):
| December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Net income (loss) | $ | 13,177 | $ | (16,613) | $ | (15,671) | |||||||||||
| Weighted average common shares | 33,670 | 33,562 | 33,696 | ||||||||||||||
| Dilutive effect of stock based awards | 304 | — | — | ||||||||||||||
| Diluted Shares | 33,974 | 33,562 | 33,696 | ||||||||||||||
| Basic income (loss) per share | $ | 0.39 | $ | (0.49) | $ | (0.47) | |||||||||||
| Diluted income (loss) per share | $ | 0.39 | $ | (0.49) | $ | (0.47) | |||||||||||
| Shares excluded from calculation of diluted EPS | — | 66 | 104 | ||||||||||||||
15—SHARE-BASED COMPENSATION
Share-Based Compensation Expense—We account for share-based compensation in accordance with ASC Topic 718, Compensation—Stock Compensation. Share-based compensation was recognized as follows in the consolidated statement of income (in thousands):
| December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Cost of revenue | $ | 394 | $ | 319 | $ | 264 | |||||||||||
| Marketing and selling | 2,201 | 1,938 | 1,024 | ||||||||||||||
| Research and development | 1,406 | 1,099 | 800 | ||||||||||||||
| General and administrative | 10,160 | 6,171 | 6,227 | ||||||||||||||
| Total expense | $ | 14,161 | $ | 9,527 | $ | 8,315 | |||||||||||
A significant modification impacted stock compensation expense related to the CEO transition in late December 2021. As part of the separation agreement, the former CEO received the benefit of immediate accelerated vesting for all forms of unvested stock (stock options, restricted stock awards, restricted stock units, performance-based stock and market-based stock). The accelerated vesting was not subject to any future service conditions. Under ASC 718, the stock options, restricted stock awards and restricted stock units service periods were modified to end on the separation date. The acceleration was contemplated in the CEO's original employment agreement, which resulted in compensation cost recognized at the grant date fair value of the original grants. The performance-based and market-based units were treated as a Type III modification (from improbable to probable) as the stock would be forfeited if the full vesting period was not achieved due to separation. As such, the terms and conditions of the performance-based and market-based units were modified to change the vesting conditions where two measurements were computed, and the net impact of truing up the cumulative compensation expense to the fair value of the modified award was recorded in the income statement, resulting in a $1.1 million net benefit in compensation cost, classified as restructuring expense in the consolidated statements of operations, for the year ended December 31, 2021.
Stock Awards Plans—Natus' 2021 Stock Awards Plan (the “Plan”) provides for the granting of the following:
•Incentive stock options to employees;
F-22
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
•Non-statutory stock options to employees, directors and consultants;
•Restricted stock awards and restricted stock units;
•Market stock units;
•Performance stock units;
•Stock bonuses; and
•Stock appreciation rights.
As of December 31, 2021, there were 2,917,938 shares available for future awards under the plan.
Under the Plan, stock options may be issued at not less than the fair market value of the common stock on the date of grant, as determined by the Board of Directors. Options issued under the Plan become exercisable as determined by the Board of Directors and expire no more than six years after the date of grant. Most options vest ratably over four years.
Stock Option Activity—Stock option activity under the stock awards plans for the year ended December 31, 2021 is summarized as follows:
| Number of Shares | Weighted Average Exercise Price | ||||||||||
| Outstanding, December 31, 2020 (37,062 shares exercisable at a weighted average exercise price of $35.25 per share) | 331,585 | $ | 23.94 | ||||||||
| Granted | 200,146 | $ | 20.61 | ||||||||
| Exercised | (11,787) | $ | 20.68 | ||||||||
| Forfeited | (25,387) | $ | 20.68 | ||||||||
| Outstanding, December 31, 2021 (320,512 shares exercisable at a weighted average exercise price of $24.01 per share) | 494,557 | $ | — | ||||||||
As of December 31, 2021, unrecognized compensation related to the unvested portion of stock options was approximately $1.0 million, which is expected to be recognized over a weighted average period of 3.1 years. The intrinsic value of options exercised, representing the difference between the closing stock price of common stock on the date of the exercise and the exercise price, in the years ended December 31, 2021, 2020 and 2019 was $0.0 million, $0.0 million, and $1.4 million, respectively.
As of December 31, 2021, there were: (i) 484,507 options vested and expected to vest with a weighted average exercise price of $22.88, an intrinsic value of $1.3 million, and a weighted average remaining contractual term of 4.0 years; and (ii) 320,512 options exercisable with a weighted average exercise price of $24.01, an intrinsic value of $0.8 million, and a weighted average remaining contractual term of 1.5 years.
Black-Scholes Inputs—The fair value of option grants was estimated using the Black-Scholes option pricing model with the following weighted average assumptions:
| December 31, | December 31, | ||||||||||
| 2021 | 2020 | ||||||||||
| Weighted-average fair value of options granted | $7.50 | $6.54 | |||||||||
| Expected life in years | 5.9 | 4.5 | |||||||||
| Risk-free interest rate | 0.3% | 0.2% | |||||||||
| Expected volatility | 38% | 38% | |||||||||
| Dividend yield | None | None | |||||||||
We did not grant any stock options during December 31, 2019.
The expected life of options is based primarily on historical share option exercise experience of the employees for options granted. All options are treated as a single group in the determination of expected life, as we do not currently expect substantially different exercise or post-vesting termination behavior among the employee population. The risk-free interest rate is based on the U.S. Treasury yield for a term consistent with the expected life of the awards in effect at the time of grant.
F-23
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
Expected volatility is based primarily on historical volatility data of our common stock. We have no history or expectation of paying dividends on common stock.
Share-based compensation expense associated with options is based on awards ultimately expected to vest. At the time of an option grant, we estimate the expected future rate of forfeitures based on historical experience. These estimates are revised, if necessary, in subsequent periods if actual forfeiture rates differ from those estimates. If the actual forfeiture rate is lower than estimated we will record additional expense and if the actual forfeiture is higher than estimated we will record a recovery of prior expense.
Restricted Stock Awards Activity—The following table summarizes the activity for restricted stock awards during the year ended December 31, 2021:
| Shares | Weighted Average Grant Date Fair Value | ||||||||||
| Unvested at December 31, 2020 | 380,089 | $ | 31.84 | ||||||||
| Granted | 320,814 | $ | 22.04 | ||||||||
| Vested | (320,541) | $ | 29.54 | ||||||||
| Forfeited | (40,592) | $ | 25.54 | ||||||||
| Unvested at December 31, 2021 | 339,770 | $ | 26.15 | ||||||||
As of December 31, 2021, unrecognized compensation related to the unvested portion of stock awards was $5.1 million, which is expected to be recognized over a weighted average period of 2.1 years. The fair market value of outstanding restricted stock awards at December 31, 2021 was $8.1 million. For the restricted stock awards granted during the years ended December 31, 2021, 2020, 2019, the weighted average grant date fair values were $22.04, $29.74, and $31.53, respectively. The total grant date fair value of restricted stock awards vested during fiscal year 2021, 2020, and 2019 was $8.6 million, $5.5 million, and $4.7 million, respectively. For the restricted stock awards that vested during the years ended December 31, 2021, 2020, and 2019, the total intrinsic value was $6.7 million, $4.8 million, and $4.0 million, respectively.
Restricted Stock Units Activity—The following table summarizes restricted stock units activity, including performance and market stock units granted at the valuation date (which may be subject to additional awards or forfeitures depending on the outcome of the performance measures at the end of the performance period) for the year ended December 31, 2021:
| Shares | Weighted Average Grant Date Fair Value | ||||||||||
| Outstanding at December 31, 2020 | 212,601 | $ | 37.27 | ||||||||
| Awarded | 400,005 | $ | 26.43 | ||||||||
| Released | (205,082) | $ | 31.34 | ||||||||
| Forfeited | (40,959) | $ | 36.20 | ||||||||
| Outstanding at December 31, 2021 | 366,565 | $ | 28.87 | ||||||||
As of December 31, 2021, unrecognized compensation related to the unvested portion of stock units was $8.0 million, which is expected to be recognized over a weighted average period of 2.9 years. The aggregate intrinsic value of outstanding restricted stock units at December 31, 2021 was $8.7 million. For the restricted stock units granted during the years December 31, 2021, 2020, 2019, the weighted average grant date fair values were $26.43, $35.42, and $38.62, respectively. The total grant date fair value of restricted stock units vested during fiscal year 2021, 2020, and 2019 was $5.7 million, $0.5 million, and $1.4 million, respectively. For the restricted stock units that vested during the years ended December 31, 2021, 2020, and 2019, the total intrinsic value was $4.7 million, $0.5 million, and $1.3 million, respectively.
Employee Stock Purchase Plan—Under Natus' 2011 Employee Stock Purchase Plan (the “ESPP”), U.S. employees can elect to have salary withholdings of up to 15% of eligible compensation to a maximum of $10,625 per offering period, to purchase shares of common stock on April 30 and October 31 of each year. The purchase price for shares acquired under the
F-24
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
ESPP is 85% of the fair market value on the last day of the offering period. As of December 31, 2021, there are no shares reserved for future issuance under the ESPP.
Because the ESPP does not have a “look back” feature, the compensation expense associated with the Plan is not measured by the use of the Black-Scholes pricing model, but rather by measuring the difference between the fair market value of common stock on the last day of the offering period and the purchase price for the offering period, which is 85% of the fair market value. Compensation expense associated with the ESPP for the years ended December 31, 2021, 2020 and 2019, respectively, was $0.1 million, $0.2 million, and $0.2 million.
16—OTHER INCOME (EXPENSE), NET
Other income (expense), net consists of (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Interest income | $ | 87 | $ | 11 | $ | 250 | |||||||||||
| Interest expense | (1,872) | (3,656) | (4,941) | ||||||||||||||
| Foreign currency gain (loss) | (1,957) | 1,988 | (765) | ||||||||||||||
| Other | (682) | (215) | (135) | ||||||||||||||
| Total other expense, net | $ | (4,424) | $ | (1,872) | $ | (5,591) | |||||||||||
17—INCOME TAXES
Income (loss) before provision for income tax is as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| U.S. | $ | 18,629 | $ | 1,908 | $ | (22,851) | |||||||||||
| Foreign | 726 | (23,973) | 1,594 | ||||||||||||||
| Income (loss) before provision for income tax | $ | 19,355 | $ | (22,065) | $ | (21,257) | |||||||||||
The components of income tax expense (benefit) for the years ended December 31, 2021, 2020 and 2019 (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Current | |||||||||||||||||
| U.S. Federal | $ | 946 | $ | (3,976) | $ | (948) | |||||||||||
| U.S. State and local | 368 | 145 | 561 | ||||||||||||||
| Non-U.S. | 7,523 | 1,241 | 8,386 | ||||||||||||||
| Total current tax expense (benefit) | 8,837 | (2,590) | 7,999 | ||||||||||||||
| Deferred | |||||||||||||||||
| U.S. Federal | 4,583 | 2,049 | (7,491) | ||||||||||||||
| U.S. State and local | 184 | 533 | (816) | ||||||||||||||
| Non-U.S. | (7,426) | (5,444) | (5,278) | ||||||||||||||
| Total deferred tax benefit | (2,659) | (2,862) | (13,585) | ||||||||||||||
| Total income expense (benefit) | $ | 6,178 | $ | (5,452) | $ | (5,586) | |||||||||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of deferred tax assets and liabilities as of December 31, 2021 and 2020 are as follows (in thousands):
F-25
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
| December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Deferred tax assets: | |||||||||||
| Net operating loss carryforwards | $ | 12,439 | $ | 9,099 | |||||||
| Credit carryforwards | 1,213 | 5,768 | |||||||||
| Accruals deductible in different periods | 15,650 | 15,368 | |||||||||
| Employee benefits | 925 | 1,016 | |||||||||
| Operating leases | 2,766 | 3,967 | |||||||||
| Total deferred tax assets | 32,993 | 35,218 | |||||||||
| Valuation allowance | (934) | (969) | |||||||||
| Total net deferred tax assets | $ | 32,059 | $ | 34,249 | |||||||
| Deferred tax liabilities: | |||||||||||
| Basis difference in fixed and intangible assets | (7,007) | (13,214) | |||||||||
| Operating leases | (2,224) | (2,970) | |||||||||
| Foreign earnings to be repatriated | (800) | (800) | |||||||||
| Total deferred tax liabilities | (10,031) | (16,984) | |||||||||
| Total net deferred tax assets | $ | 22,028 | $ | 17,265 | |||||||
The income tax expense (benefit) in the accompanying statements of income differs from the provision calculated by applying the U.S. federal statutory income tax rate of 21% in 2021, 2020, and 2019 to income before taxes due to the following:
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Federal statutory tax expense | $ | 4,065 | $ | (4,634) | $ | (4,464) | |||||||||||
| State tax expense | 605 | 661 | (300) | ||||||||||||||
| Foreign taxes at rates less than U.S. rates | (609) | 1,050 | (2,205) | ||||||||||||||
| Equity compensation | 2,581 | 1,161 | 824 | ||||||||||||||
| Tax credits | (1,393) | (1,641) | (1,428) | ||||||||||||||
| Uncertain tax position | 1,553 | 3,002 | 2,910 | ||||||||||||||
| Lapse of statute | (365) | (2,718) | (3,961) | ||||||||||||||
| Repatriation tax net of foreign tax credits | — | — | 172 | ||||||||||||||
| Federal NOL Carryback | (454) | (1,621) | — | ||||||||||||||
| Tax audits | 97 | (224) | — | ||||||||||||||
| Withholding taxes | 254 | — | 1,107 | ||||||||||||||
| Global intangible low-taxed income net of foreign tax credits | — | — | 1,601 | ||||||||||||||
| Return to provision | (704) | (946) | 560 | ||||||||||||||
| Other | 548 | 458 | (402) | ||||||||||||||
| Total expense (benefit) | $ | 6,178 | $ | (5,452) | $ | (5,586) | |||||||||||
At December 31, 2021, we had U.S. state net operating loss carryforwards of $16.9 million, of which immaterial portions will begin to expire in 2022 and the majority have long carryforward periods ranging primarily from 10 years to indefinite. At December 31, 2021, we had U.S. state R&D credit carryforwards of $1.3 million, which will begin to expire in 2022. At December 31, 2021, we had $0.1 million of U.S. foreign tax credit carryforwards that can be used to offset future U.S. tax liabilities related to foreign source taxable income. The foreign tax credits will start to expire in 2022.
At December 31, 2021, certain foreign subsidiaries had deferred tax assets attributable to net operating loss carryforwards as follows: $0.8 million in France, $8.7 million in Denmark, $0.3 million in Canada, $0.2 million in China, and $0.3 million in Germany. These foreign net operating loss carryforwards, ranging from 6 years utilization period to indefinite utilization period, if not utilized to offset taxable income in future periods, will begin to expire in various amounts beginning in 2026.
F-26
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Accordingly, valuation allowances of $0.9 million and $1.0 million were recorded at December 31, 2021 and 2020, respectively. The decrease of $0.1 million in valuation allowance was primarily due to a valuation allowance recorded against certain U.S. state credits, which are expected to expire before fully utilized, and release of prior Canadian valuation allowances.
The realizability of the deferred tax assets is primarily dependent on our ability to generate sufficient taxable income in future periods. We record net deferred tax assets to the extent it is more likely than not that the assets will be realized. In making such determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial operations. To the extent that previously reserved deferred tax assets are estimated to be realizable, we adjust the valuation allowance which reduces the provision for income taxes. Weighing all of the positive and negative evidence, we have recorded a valuation allowance related primarily to net operating losses in certain foreign jurisdictions where it is more likely than not that the tax benefit of the net operating losses will not be realized.
There are no changes to the position on our permanent reinvestment of earnings from foreign operations. As of December 31, 2021, we intend to distribute all of the earnings from Excel-Tech and Natus Ireland in excess of their operational needs. We intend to permanently reinvest the earnings of remaining foreign subsidiaries. The other remaining foreign subsidiaries have both the intent and ability to indefinitely reinvest its undistributed earnings.
Uncertain Tax Positions
A reconciliation of the beginning and ending amount of unrecognized tax benefits (excluding interest and penalties) is as follows (in thousands):
| Balance at December 31, 2018 | $ | 7,227 | |||
| Decreases for tax positions related to prior years | (48) | ||||
| Increases for tax positions related to the current year | 495 | ||||
| Decrease due to lapse of statutes of limitations | (3,763) | ||||
| Foreign exchange difference | 6 | ||||
| Balance at December 31, 2019 | $ | 3,917 | |||
| Increases for tax positions related to prior years | 2,454 | ||||
| Increases for tax positions related to the current year | 104 | ||||
| Decrease due to lapse of statutes of limitations | (2,416) | ||||
| Foreign exchange difference | 83 | ||||
| Balance at December 31, 2020 | $ | 4,142 | |||
| Increases for tax positions related to the current year | 1,067 | ||||
| Decrease due to lapse of statutes of limitations | (279) | ||||
| Foreign exchange difference | (82) | ||||
| Balance at December 31, 2021 | $ | 4,848 | |||
For the year ended December 31, 2021, our net unrecognized tax benefits increased by $0.7 million resulting in a corresponding $0.7 million increase in income tax expense. The increase was primarily attributable to the increase in uncertain tax positions related to the current year in certain jurisdictions.
The unrecognized tax benefits for the tax years ended December 31, 2021, 2020 and 2019 were $4.8 million, $4.1 million and $3.9 million, respectively which include $4.6 million, $3.8 million and $3.6 million, respectively, that would impact the effective tax rate if recognized.
We expect a range from zero to $0.8 million of unrecognized tax benefit that will impact the effective tax rate in the next 12 months due to the lapse of statute of limitations provided that no taxing authority conducts a new examination.
At December 31, 2021, 2020 and 2019, we had cumulatively accrued $0.9 million, $0.5 million, and $0.4 million for estimated interest and penalties related to uncertain tax positions. We record interest and penalties related to unrecognized tax positions as a component of income tax expense (benefit), which totaled approximately $0.3 million, $0.1 million, and $(0.1) million for the years ended December 31, 2021, 2020, and 2019, respectively.
F-27
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
We are currently unaware of any uncertain tax positions that could result in significant additional payments, accruals, or other material deviation in this estimate over the next 12 months.
As of December 31, 2021, we are currently under U.S. federal IRS examination for tax years 2018 and 2019.
Our tax returns remain open to examination as follows: U.S. federal, 2017 through 2020; U.S. states, generally 2016 through 2020; and significant foreign jurisdictions, generally 2016 through 2020.
18—EMPLOYEE BENEFIT PLAN
We offer pre-tax and after-tax 401(k) savings plan options under which eligible U.S. employees may elect to have a portion of their salary deferred and contributed to the plan. Employer matching contributions are determined by management and are discretionary. Employer matching contributions were $3.7 million, $3.2 million, and $3.6 million respectively, in the years ended December 31, 2021, 2020, and 2019. For new hires, employer contributions vest ratably over the first two years of employment.
19—SEGMENT, CUSTOMER, AND GEOGRAPHIC INFORMATION
We determine our reportable segments by first identifying our operating segments, and then by assessing whether any components of these segments constitute a business for which discrete financial information is available and where segment management regularly reviews the operating results of that component. Historically, our operating segments were based on its 3 strategic business units. In January 2019, we announced the transition of our operating structure from three strategic business units to a single, unified company with globally led operational teams in Sales and Marketing, Manufacturing, Research and Development, Quality, and General and Administrative functions.
Following the reorganization, we operate as 1 operating segment and 1 reportable segment, which provides medical device solutions focused on the diagnosis and treatment of central nervous and sensory system disorders for patients of all ages. Financial information is reviewed on a consolidated basis for purposes of making operating decisions and assessing financial performance. Consolidated financial information is accompanied by disaggregated information about revenues by end market and geographic region. We do not assess the performance of our end markets or geographic regions on measures of profit or loss, or asset-based metrics. We have disclosed the revenues for each end market and geographic region to provide the reader of the financial statements transparency into our operations.
The following tables present revenue and long-lived asset information by end market and geographic region. Revenue is based on the destination of the shipments and long-lived assets are based on the physical location of the assets (in thousands):
F-28
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2021, 2020 and 2019
| Years Ended December 31, | |||||||||||||||||
| 2021 | 2020 | 2019 | |||||||||||||||
| Consolidated Revenue: | |||||||||||||||||
| United States | $ | 285,979 | $ | 252,496 | $ | 292,400 | |||||||||||
| Foreign countries | 187,459 | 163,188 | 202,775 | ||||||||||||||
| $ | 473,438 | $ | 415,684 | $ | 495,175 | ||||||||||||
| Revenue by End Market: | |||||||||||||||||
| Neuro | |||||||||||||||||
| Devices and Systems | $ | 220,929 | $ | 179,464 | $ | 220,306 | |||||||||||
| Supplies | 63,838 | 56,275 | 66,059 | ||||||||||||||
| Services | — | — | 871 | ||||||||||||||
| Total Neuro Revenue | $ | 284,767 | $ | 235,739 | $ | 287,236 | |||||||||||
| Newborn Care | |||||||||||||||||
| Devices and Systems | $ | 55,174 | $ | 52,284 | $ | 53,465 | |||||||||||
| Supplies | 33,710 | 36,174 | 38,264 | ||||||||||||||
| Services | 16,139 | 16,566 | 19,183 | ||||||||||||||
| Total Newborn Care Revenue | $ | 105,023 | $ | 105,024 | $ | 110,912 | |||||||||||
| Hearing & Balance | |||||||||||||||||
| Devices and Systems | $ | 78,652 | $ | 70,738 | $ | 92,050 | |||||||||||
| Supplies | 4,996 | 4,183 | 4,977 | ||||||||||||||
| Total Hearing & Balance Revenue | $ | 83,648 | $ | 74,921 | $ | 97,027 | |||||||||||
| Total Revenue | $ | 473,438 | $ | 415,684 | $ | 495,175 | |||||||||||
Long-lived asset information by geographic region is as follows (in thousands):
| Years Ended December 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Property and equipment, net: | |||||||||||
| United States | $ | 9,642 | $ | 10,998 | |||||||
| Ireland | 6,223 | 6,716 | |||||||||
| Canada | 3,594 | 3,775 | |||||||||
| Denmark | 1,262 | 1,787 | |||||||||
| Other foreign countries | 1,062 | 1,240 | |||||||||
| $ | 21,783 | $ | 24,516 | ||||||||
During the years ended December 31, 2021, 2020 and 2019, no single customer or foreign country contributed to more than 10% of revenue.
20—COMMITMENTS AND CONTINGENCIES
Purchase commitments—We have various purchase obligations for goods or services totaling $178.4 million at December 31, 2021, which are expected to be paid $136.5 million in 2022 and $41.9 million thereafter.
Legal matters—We currently are, and may from time to time become, a party to various legal proceedings or claims that arise in the ordinary course of business. Our managements reviews these matters if and when they arise and believes that the resolution of any such matters currently known will not have a material effect on our results of operations or financial position.
F-29
NATUS MEDICAL INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS-(Continued)
Years Ended December 31, 2019, 2018 and 2017
21—FAIR VALUE MEASUREMENTS
ASC 820 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. Fair value is defined under ASC 820 as the exit price associated with the sale of an asset or transfer of a liability in an orderly transaction between market participants at the measurement date. ASC 820 establishes the following three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value:
Level 1—Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
During the third quarter of 2021, we sold vacant land located in Canada for $1.6 million resulting in a gain of $0.7 million. Also during this period, we sold a building located in the United Kingdom for $1.1 million resulting in a gain of $0.8 million. The gain on disposal of these assets is reported in general and administrative expense in the condensed consolidated statements of operations. The book value of these assets was in other current assets on the accompanying condensed consolidated balance sheet.
The following financial instruments are not measured at fair value on the consolidated balance sheet as of December 31, 2021 and 2020, but require disclosure of fair values: cash and cash equivalents, accounts receivable, and accounts payable. The carrying value of these financial instruments approximates fair values because of the relatively short maturity.
The carrying amount of our short-term and long-term debt approximates fair value based on Level 2 inputs since the debt carries a variable interest rate that is tied to the current LIBOR rate plus a spread.
22—SALE OF CERTAIN SUBSIDIARY
We divested our wholly owned subsidiary, Medix, on April 2, 2019, via a stock sale to the local managing director, a related party. In exchange for the stock, we received $2.5 thousand in cash and provided Medix with a $2.2 million limited-recourse loan secured by real estate assets of Medix. The fair market value of the loan was adjusted as necessary to reflect any changes in market environment or the value of the building. During the second quarter of 2021, we received a $0.9 million payment of which the loan receivable of $0.7 million was fully repaid and a $0.2 million gain was recognized.
The held for sale criteria under GAAP was met in the first quarter of 2019. As such, we completed an asset impairment analysis which resulted in the full impairment of all assets held for sale. We recognized an impairment loss of $24.6 million which included an accrual for the anticipated realization of deferred foreign currency related translation adjustments in accumulated other comprehensive income of $24.8 million, net of tax, and an adjustment of $4.6 million for assets with a book value in excess of their fair market value. Included in the year ended December 31, 2019, is the impact of the sale of Medix, which was completed as of June 30, 2019, and the deferred foreign currency related translation adjustments previously in accumulated other comprehensive income have been released from the balance sheet along with the held for sale accrual.
ITEM 16. Form 10-K Summary
Not Applicable.
F-30
EXHIBIT INDEX
| Incorporated By Reference | ||||||||||||||||||||||||||||||||
| Exhibit No. | Exhibit | Filing | Exhibit No. | File No. | File Date | |||||||||||||||||||||||||||
| S-1 | 3.1.1 | 333-44138 | 8/18/2000 | |||||||||||||||||||||||||||||
| 8-K | 3.1 | 000-33001 | 9/13/2012 | |||||||||||||||||||||||||||||
| 8-K | 3.1 | 000-33001 | 6/7/2019 | |||||||||||||||||||||||||||||
| 8-K | 3.1 | 000-33001 | 12/16/2019 | |||||||||||||||||||||||||||||
| S-1/A | 4.1 | 333-44138 | 2/9/2001 | |||||||||||||||||||||||||||||
| 8-A | 3.1.2 | 000-33001 | 9/6/2002 | |||||||||||||||||||||||||||||
| 10-K | 4.3 | 000-33001 | 12/31/2019 | |||||||||||||||||||||||||||||
| S-1 | 10.1 | 333-44138 | 8/18/2000 | |||||||||||||||||||||||||||||
| 8-K | 10.1 | 000-33001 | 12/18/2018 | |||||||||||||||||||||||||||||
| 8-K | 10.1.1 | 000-33001 | 12/18/2018 | |||||||||||||||||||||||||||||
| 8-K | 10.1.2 | 000-33001 | 12/18/2018 | |||||||||||||||||||||||||||||
| 8-K | 10.1.3 | 000-33001 | 12/18/2018 | |||||||||||||||||||||||||||||
| 8-K | 10.1.4 | 000-33001 | 12/18/2018 | |||||||||||||||||||||||||||||
10.2* | 8-K | 10.1 | 000-33001 | 1/4/2006 | ||||||||||||||||||||||||||||
| S-1 | 10.3.1 | 333-44138 | 8/18/2000 | |||||||||||||||||||||||||||||
| 10-Q | 10.2 | 000-33001 | 8/9/2006 | |||||||||||||||||||||||||||||
| 10-K | 10.2.3 | 000-33001 | 3/14/2008 | |||||||||||||||||||||||||||||
10.3* | 10-Q | 10.02 | 000-33001 | 5/9/2008 | ||||||||||||||||||||||||||||
| S-1 | 10.4.1 | 333-44138 | 8/18/2000 | |||||||||||||||||||||||||||||
10.4* | S-1 | 10.15 | 333-44138 | 2/9/2001 | ||||||||||||||||||||||||||||
| S-1 | 10.15.1 | 333-44138 | 2/9/2001 | |||||||||||||||||||||||||||||
| Incorporated By Reference | ||||||||||||||||||||||||||||||||
| Exhibit No. | Exhibit | Filing | Exhibit No. | File No. | File Date | |||||||||||||||||||||||||||
10.5* | 8-K | 10.2 | 000-33001 | 1/4/2006 | ||||||||||||||||||||||||||||
10.6* | 14-A | — | 000-33001 | 4/20/2011 | ||||||||||||||||||||||||||||
| 10-Q | 10.1 | 000-33001 | 11/7/2011 | |||||||||||||||||||||||||||||
| 10-Q | 10.2 | 000-33001 | 11/7/2011 | |||||||||||||||||||||||||||||
| 10-Q | 10.3 | 000-33001 | 11/7/2011 | |||||||||||||||||||||||||||||
10.7* | 14-A | — | 000-33001 | 4/20/2011 | ||||||||||||||||||||||||||||
| 14-A | — | 000-33001 | 4/20/2011 | |||||||||||||||||||||||||||||
10.8* | 10-K | 10.10 | 000-33001 | 3/10/2009 | ||||||||||||||||||||||||||||
| 10-K | 000-33001 | 3/16/2015 | ||||||||||||||||||||||||||||||
10.9* | 8-K | 99.1 | 000-33001 | 4/22/2013 | ||||||||||||||||||||||||||||
| 10-Q | 10.16 | 000-33001 | 8/8/2018 | |||||||||||||||||||||||||||||
| 8-K | 10.1 | 000-33001 | 10/9/2015 | |||||||||||||||||||||||||||||
| 10-Q | 000-33001 | 2/29/2016 | ||||||||||||||||||||||||||||||
| 10-Q | 10.2 | 000-33001 | 11/3/2016 | |||||||||||||||||||||||||||||
| 10-Q | 10.1 | 000-33001 | 11/3/2016 | |||||||||||||||||||||||||||||
| 10-Q | 10.3 | 000-33001 | 11/3/2016 | |||||||||||||||||||||||||||||
| 8-K | 99.1 | 000-33001 | 8/29/2018 | |||||||||||||||||||||||||||||
| 10-Q | 10.18 | 000-33001 | 11/8/2018 | |||||||||||||||||||||||||||||
| 10-Q | 10.1 | 000-33001 | 9/30/2020 | |||||||||||||||||||||||||||||
| Incorporated By Reference | ||||||||||||||||||||||||||||||||
| Exhibit No. | Exhibit | Filing | Exhibit No. | File No. | File Date | |||||||||||||||||||||||||||
| 14-A | — | 000-33001 | 4/28/2021 | |||||||||||||||||||||||||||||
| 8-K | 10.1 | 000-33001 | 12/13/2021 | |||||||||||||||||||||||||||||
| 8-K | 10.2 | 000-33001 | 12/13/2021 | |||||||||||||||||||||||||||||
| 101 | The following financial information from Natus Medical Incorporated Annual Report on Form 10-K for the fiscal year ended December 31, 2021, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2021 and December 31, 2020, (ii) Consolidated Statements of Operations for the years ended December 31, 2021, 2020 and 2019, (iii) Consolidated Statements of Comprehensive Income for the years ended December 31, 2021, 2020 and 2019 (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2021, 2020and 2019, (v) Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2021, 2020 and 2019, and (vi) the Notes to Consolidated Financial Statements. | |||||||||||||||||||||||||||||||
| 104 | The cover page of the Annual Report on Form 10-K formatted in Inline XBRL (included in Exhibit 101). | |||||||||||||||||||||||||||||||
* Indicates a management contract or compensatory plan or arrangement
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned thereunto duly authorized.
NATUS MEDICAL INCORPORATED | ||||||||
| By | /s/ THOMAS J. SULLIVAN | |||||||
| Thomas J. Sullivan President, Chief Executive Officer and Director | ||||||||
| By | /s/ B. DREW DAVIES | |||||||
| B. Drew Davies Executive Vice President and Chief Financial Officer | ||||||||
Dated: February 25, 2022
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Thomas J. Sullivan and B. Drew Davies and each of them acting individually, as his or her attorney-in-fact, each with full power of substitution, for him or her in any and all capacities, to sign any and all amendments to this Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed by the following persons on behalf of the registrant and in the capacity and dates indicated:
| Signature | Title | Date | ||||||||||||
/S/ THOMAS J. SULLIVAN | President, Chief Executive Officer and Director (Principal Executive Officer) | February 25, 2022 | ||||||||||||
| (Thomas J. Sullivan) | ||||||||||||||
/S/ B. DREW DAVIES | Executive Vice President & Chief Financial Officer (Principal Financial and Accounting Officer) | February 25, 2022 | ||||||||||||
| (B. Drew Davies) | ||||||||||||||
/S/ JOSHUA H. LEVINE | Chairperson of the Board of Directors | February 25, 2022 | ||||||||||||
| (Joshua H. Levine) | ||||||||||||||
/S/ ILAN DASKAL | Director | February 25, 2022 | ||||||||||||
| (Ilan Daskal) | ||||||||||||||
/S/ ERIC J. GUERIN | Director | February 25, 2022 | ||||||||||||
| (Eric J. Guerin) | ||||||||||||||
/S/ LISA W. HEINE | Director | February 25, 2022 | ||||||||||||
| (Lisa W. Heine) | ||||||||||||||
/S/ BRYANT M. MOORE | Director | February 25, 2022 | ||||||||||||
| (Bryant M. Moore) | ||||||||||||||
/S/ ALICE D. SCHROEDER | Director | February 25, 2022 | ||||||||||||
| (Alice D. Schroeder) | ||||||||||||||