UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | |
x | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) |
| | OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the fiscal year ended December 31, 2009 |
or
| | |
¨ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) |
| | OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission file number 0-19825
SciClone Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
| | |
| Delaware | | 94-3116852 |
(State or other jurisdiction of Incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
950 Tower Lane, Suite 900 Foster City, California | | 94404 |
| (Address of principal executive offices) | | (Zip Code) |
(650) 358-3456
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
| Common Stock, $0.001 par value | | The NASDAQ Global Market of the NASDAQ Stock Market Inc. |
| (Title of Class) | | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨ Smaller Reporting Company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by non-affiliates of SciClone Pharmaceuticals, Inc. was approximately $117,932,000 as of June 30, 2009, based upon the closing price of SciClone Pharmaceuticals Inc.’s Common Stock on The NASDAQ Global Market of the NASDAQ Stock Market Inc. on such date. Shares of Common Stock held by each executive officer and director have been excluded from the calculation because such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 12, 2010, there were 47,324,232 shares of the Registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates by reference from the definitive proxy statement for the Company’s 2010 Annual Meeting of Stockholders to be filed with the Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K (the “Proxy Statement”).
TABLE OF CONTENTS
As used in this Annual Report, the terms “we,” “us,” “our,” the “Company” and “SciClone” mean SciClone Pharmaceuticals, Inc. and its subsidiaries (unless the context indicates a different meaning). SciClone, the SciClone logo and ZADAXIN are registered U.S. trademarks and SCV-07 is a trademark of SciClone Pharmaceuticals, Inc. All other Company names and trademarks included in this Annual Report are trademarks, registered trademarks or trade names of their respective owners.
2
NOTE REGARDING FORWARD-LOOKING STATEMENTS:
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements are based on our current expectations, estimates and projections about our business, industry, management’s beliefs and certain assumptions made by us. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe” or similar expressions are intended to identify forward-looking statements including those statements we make regarding our future financial results; anticipated product sales; the sufficiency of our resources to complete clinical trials and other new product development initiatives; the timing and outcome of clinical trials; the timing of completion of therapy and observation of our clinical trials; ZADAXIN®’s ability to complement existing therapies; prospects for ZADAXIN and our plans for its enhancement and commercialization; future size of the worldwide hepatitis B virus (“HBV”) and hepatitis C virus (“HCV”) and other markets; research and development and other expense levels, the ability of our suppliers to continue financially viable production of our products; cash and other asset levels; and the allocation of financial resources to certain trials and programs. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict. Therefore, our actual results could differ materially and adversely from those expressed in any forward-looking statements as a result of various factors including, but not limited to, those described under the caption “Risk Factors” in this Annual Report on Form 10-K. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
PART I
OVERVIEW
SciClone Pharmaceuticals (NASDAQ: SCLN) is a profit-focused, global specialty pharmaceutical company with a substantial international business, based primarily in the People’s Republic of China (“China”), and with a product portfolio of novel therapies for cancer and infectious diseases. Our strategy is to concentrate on international sales growth, a cost-containing clinical development strategy, and overall expense management. ZADAXIN®, our brand of thymalfasin or thymosin alpha 1 and our primary product, is sold in over 30 countries for the treatment of the hepatitis B virus and the hepatitis C virus, certain cancers and as a vaccine adjuvant. We believe our current cash and investment position and the gross profit from our product sales provide the financial resources to execute on our strategy for continued growth in our international business and for development of our pipeline of late-stage product candidates.
We plan to expand our commercial operations, currently located primarily in China, with the goal of becoming a significant pharmaceutical company in China’s rapidly growing pharmaceutical market. A key part of our strategy is to leverage our decade of experience in China and to grow our international business by adding commercial stage or near term commercial stage products to our portfolio. We believe we are well-positioned to in-license additional therapeutics for our international business with a focus on China, in part because of our opportunity to commercialize these products utilizing our well established sales and marketing organization in China. Furthermore, our international growth strategy may include additional partnerships and merger and acquisition transactions for products.
We have successfully in-licensed two products that are part of this international commercial growth strategy, DC BeadTM and ondansetron RapidFilmTM. Our DC Bead product candidate is a novel treatment for advanced liver cancer which is currently approved in 40 countries worldwide, including Europe and the U.S. We have commercialization rights for this product in China. We commenced a small local registration trial in the first quarter of 2010, which is expected to enroll approximately 40 advanced liver cancer patients at three State Food and Drug Administration of China (“SFDA”)-certified liver cancer treatment centers. The primary endpoint is safety and the secondary endpoint is efficacy, as measured by tumor response. If the trial results are positive, we believe we may receive regulatory approval for DC Bead by the end of 2010.
3
Our second in-licensed product candidate as part of this international commercial growth strategy is ondansetron RapidFilm. We have commercialization rights for this product candidate in China and Vietnam. Ondansetron RapidFilm, currently being evaluated for regulatory approval in Europe, is an oral thin film formulation of ondansetron, commonly used to treat and prevent nausea and vomiting caused by chemotherapy, radiotherapy and surgery. We plan to file for product registration with the SFDA in 2010. We believe we may receive regulatory approval in China in 2011.
Our clinical development strategy is focused on our portfolio of novel product candidates for cancer and infectious diseases and includes SCV-07 and thymalfasin. SCV-07 is being developed for the delay of onset of severe oral mucositis in patients receiving chemoradiation therapy for the treatment of cancers of the head and neck and for the treatment of HCV. Thymalfasin is being developed in an ongoing clinical trial for enhancement of influenza H1N1 vaccination in immuno-compromised patient populations, and for stage IV melanoma.
As part of this clinical development strategy, we are moving forward our clinical development program for SCV-07, a small molecule synthetic peptide with immunomodulating properties, which is being studied in two indications: oral mucositis and HCV. We are currently conducting a phase 2 multicenter, randomized, double-blind, placebo-controlled, dose ranging study designed to assess the safety and efficacy of SCV-07 for the delay of onset of severe oral mucositis in subjects receiving chemoradiation therapy for the treatment of cancers of the head and neck. The primary efficacy endpoint is delay of onset and severity of severe oral mucositis. We completed enrollment of this trial in the second half of 2009 and expect to report results in the first quarter of 2010.
The second component of the SCV-07 clinical development project is for the treatment of HCV. This multicenter, multidose, open-label study is designed to evaluate the safety and immunomodulatory effects of SCV-07 as a monotherapy or in combination with ribavirin in non–cirrhotic patients with genotype 1 chronic HCV who have relapsed after at least 44 weeks of treatment with pegylated interferon and ribavirin. We expect to report results by the end of 2010. During our previous phase 2a clinical trial of SCV-07 designed to evaluate the effect of SCV-07 on HCV viral load, as well as on other measures of immune response, SCV-07 demonstrated activity in some treated patients in the higher dosage groups, and the decrease in viral load in these patients was accompanied by an increase in a biomarker which is usually correlated with an immunological response against HCV. Additionally, SCV-07 was shown to be generally safe and well-tolerated with no dose limiting toxicities or serious adverse events reported.
We continue to seek development opportunities with ZADAXIN (“thymalfasin”) focused on vaccine enhancement and melanoma. In the fourth quarter of 2009, our partner Sigma-Tau Finanziaria, S.p.A. (“Sigma-Tau”) initiated a study in Italy to evaluate thymalfasin’s ability to enhance immune response to the MF59 adjuvanted H1N1 influenza monovalent vaccine, Focetria™ from Novartis. The study, being conducted in hemodialysis patients reached full enrollment after two days, and in early 2010, we reported promising preliminary results from this study regarding ZADAXIN’s ability to enhance response to H1N1 vaccine. ZADAXIN is currently approved in Italy and more than 10 other countries as an enhancer for the influenza vaccine in immune-compromised patients. In addition, we continue to seek a partner for a phase 3 trial to evaluate thymalfasin for use as a therapy for stage IV melanoma, but we do not intend to proceed with the trial unless we reach agreement with a partner.
Our clinical development strategy is to focus on driving cost-efficient phase 1 and 2 development of promising compounds while seeking development partners for costly phase 3 trials, allowing us to achieve the potential upside of our portfolio of drug candidates.
We believe our cash and investments as of December 31, 2009 and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. Our results may fluctuate from quarter to quarter and we may report quarterly losses in the future.
4
SciClone Pharmaceuticals, Inc. was organized in 1990 as a California corporation and reincorporated in Delaware in 2003. Our corporate headquarters are located in Foster City, California. For information about our revenues from external customers, measures of our profit and loss, our total assets and other financial matters, you should read our Consolidated Financial Statements provided in Part II, Item 8 of this Form 10-K.
BUILDING A LEADING INTERNATIONAL PHARMACEUTICAL BUSINESS
International Sales and Marketing
ZADAXIN is approved in over 30 countries, primarily in Asia, Eastern Europe, the Middle East and Latin America where our distributors are largely responsible for the marketing and sales of the product. ZADAXIN’s approvals are principally for the treatment of HBV, with additional approvals in certain countries for the treatment of HCV, as a vaccine adjuvant, or as a chemotherapy adjuvant for cancer patients with weakened immune systems. We sell ZADAXIN in various international markets through our wholly owned subsidiary, SciClone Pharmaceuticals International Ltd. (“SPIL”).
SPIL is registered in the Cayman Islands and has its principal office in Hong Kong. SPIL orders ZADAXIN from our European manufacturer and contracts with a third party for the storage of our finished goods inventory at warehousing facilities in Hong Kong. SPIL then distributes our product worldwide from these warehousing facilities based on purchase orders from our customers. Under our established distribution arrangements, local importers and distributors are responsible for the importation, inventory, distribution and invoicing of ZADAXIN.
Product sales were $72.4 million, $54.1 million, and $37.0 million for the years ended December 31, 2009, 2008 and 2007, respectively, and all were from sales of ZADAXIN.
Our Established Business in China
In China, we have an established business with growing product revenue and positive cash flow. We are committed to building on this base and introducing new pharmaceutical products to meet the country’s evolving healthcare needs. China state leaders have agreed about a new health care reform plan which, among other things, is seeking to expand patient access to pharmaceuticals. Most experts agree that this market will experience strong growth of approximately 24% over the next five years, and we expect we will see a continued increase in ZADAXIN sales as this trend unfolds.
We launched ZADAXIN in China in 1996 and by 2009 our worldwide sales of this product reached more than $72 million, 96% of which was sales to China. Today, ZADAXIN is one of the top imported pharmaceutical products in China, measured by revenue, and our market share in that country is currently about 5%.
Over the last decade, SciClone China has established a sales and marketing organization and extensive importation and distribution channels, which have resulted in strong growth in sales and substantial cash flow. Through our sales organization of more than 175 medical representatives, we believe we have developed a good reputation and relationships with physicians and administrators in over 500 hospitals in the major cities in China. We are expanding geographically in China to position the Company for further growth. As a result of these efforts, ZADAXIN is one of the top-selling imported pharmaceutical products in China, measured in revenue, and we have a strong commercial presence in liver disease, cancer and the intensive care setting.
ZADAXIN has strong brand recognition and is positioned as a high-quality, imported, premium-priced product. ZADAXIN is approved in China for the treatment of HBV and for use as a vaccine adjuvant. In China, orders for ZADAXIN are fulfilled largely by distributors and sub distributors who purchase ZADAXIN from our selected, established, government-licensed importing agents.
5
China accounted for approximately 96%, 94%, and 92%, respectively, of our product sales for the years ended December 31, 2009, 2008 and 2007. In 2009, Shanghai Lingyun and China National Pharmaceutical Foreign Trade Corporation accounted for 66% and 27% of our product sales, respectively, and two other importers accounted for 3%. In 2008, Shanghai Lingyun and China National Pharmaceutical Foreign Trade Corporation accounted for 68% and 22% of our product sales, respectively, and one other importer accounted for 4%. In 2007, Shanghai Lingyun and China National Pharmaceutical Foreign Trade Corporation accounted for 63% and 21% of our product sales, respectively, and one other importer accounted for 8%. No other customers accounted for more than 10% of product sales in those periods. As of December 31, 2009, approximately $20.8 million or 97% of our accounts receivable were attributable to two customers in China.
Broadening SciClone’s International Product Portfolio
Currently, we are evaluating opportunities to acquire or in-license the marketing rights to other proprietary products in China that can be effectively and efficiently marketed to physicians by our team of medical representatives. To maintain maximum efficiency in our existing operations, we plan to continue to focus on product opportunities in the areas of oncology, including supportive care, infectious disease and intensive care medicine.
We acquired the exclusive China marketing rights to DC Bead, an embolizing and chemotherapy releasing device, for liver cancer for which we filed a regulatory application with the SFDA. We expect to obtain regulatory approval for this product by the end of 2010. DC Bead is currently approved in Europe for use in the treatment of malignant hypervascularized tumors such as hepatocellular carcinoma (“HCC”), or primary liver cancer. China accounts for one-half of the world’s liver cancer cases and more than 300,000 people die annually from this disease in China, making this an important unmet medical need.
In addition, in July 2009, we entered into a licensing agreement with APR Applied Pharma Research S.A. (“APR”) for the commercialization rights of APR’s anti-nausea drug ondansetron RapidFilm in China and Vietnam. Ondansetron RapidFilm is an oral thin film formulation of ondansetron, a serotonin 5-HT3 receptor antagonist commonly used to treat and prevent nausea and vomiting caused by chemotherapy, radiotherapy and surgery. We plan to file for product registration with the SFDA in 2010. We believe we may obtain regulatory approval in China in 2011. We have ten years of marketing and sales exclusivity from APR for China and Vietnam following regulatory approval.
DEVELOPING PRODUCTS FOR THE U.S. AND EUROPEAN MARKETS
SCV-07 for Infectious Diseases and Oncology
Our proprietary drug candidate SCV-07 (gamma-D-glutamyl-L-tryptophan) is a small molecule which stimulates the immune system, possibly through inhibition of STAT3 signaling, and the resulting effects on T-helper 1 cells. SCV-07 has been shown to be efficacious in animal models of immune-sensitive diseases, including viral infections and cancers, and in the enhancement of response to vaccines. SCV-07 has shown safety at varied single and multi-dose levels and is orally bioavailable, differentiating it from other immunomodulators.
Phase 2 Oral Mucositis Trial Protocol
We are currently conducting a phase 2 clinical trial to assess the safety and efficacy of SCV-07 for the delay to onset and severity of severe oral mucositis in subjects receiving chemoradiation therapy for the treatment of cancers of the head and neck. In this multi-center, randomized, double-blind, placebo-controlled dose-ranging study, we have completed the enrollment of 60 patients and expect to report results in the first quarter of 2010.
This study is being conducted at 21 centers in the United States and includes approximately 20 patients in each of the three treatment cohorts. Each cohort receives placebo, SCV-07 at a dose of 0.02 mg/kg, or SCV-07 at a dose of 0.10 mg/kg. The treatment period is approximately seven weeks depending on the patient’s prescribed
6
radiation plan, with a follow-up visit approximately 30 days following the last day of radiation therapy. All the patients have been treated with a standard cisplatin regimen in addition to radiation therapy. The primary efficacy endpoint is delay of onset and severity of severe oral mucositis.
Phase 2 HCV Trial Protocol
We are also conducting a multicenter, multidose, open-label study designed to evaluate the safety and immunomodulatory effects of SCV-07 as a monotherapy or in combination with ribavirin in non–cirrhotic patients with genotype 1 chronic HCV who have relapsed after at least 44 weeks of treatment with pegylated interferon and ribavirin. We expect to report results in the second half of 2010. Should the results of this trial be positive, SCV-07 could be developed as a safe and potentially orally bioavailable HCV therapeutic which could replace or lower the dose of pegylated interferon alpha therapy.
The study, which is monitoring biomarkers of immune activation and HCV viral load dynamics, includes two treatment cohorts of 20 patients each, who will receive SCV-07 at a dose of either 0.1 mg/kg or 1.0 mg/kg. The treatment period is approximately eight weeks long, including four weeks of SCV-07 monotherapy followed by four weeks of SCV-07 in combination with ribavirin. In addition, there will be three follow-up visits within seven weeks after the completion of treatment.
Preclinical Data
Recent publications have highlighted efficacy of SCV-07 treatment in animal models for melanoma, a cancer known to be sensitive to immune modulation (a decrease in tumor growth and increase in survival was seen), as well as several infectious diseases such as herpes, papilloma virus, and Pichinde virus. Efficacy in prevention of severe mucositis in response to radiation therapy has been shown in a hamster model; a publication of these data is in press.
SciClone’s Lead Product ZADAXIN (Thymalfasin)
ZADAXIN is SciClone’s synthetic preparation of thymalfasin, scientifically referred to as thymosin alpha 1, a thymic peptide which circulates in the blood naturally and is instrumental in the immune response to certain cancers and viral infections. Published scientific and clinical studies have shown that thymalfasin helps enhance response to vaccines, and to stimulate and direct the body’s immune response to eradicate infectious diseases like HCV and HBV, as well as certain cancers. Thymalfasin appears to be well tolerated with few reports of significant side effects or toxicities associated with its use.
Thymalfasin elicits a variety of immune system responses. Acting on intracellular signaling pathways, thymalfasin increases production of certain subsets of white blood cells and their differentiation into CD-4 helper cells, specifically towards differentiation into the Th1 subset of CD-4 helper cells (Th1 cells secrete cytokines such as interleukin-2 (IL-2) and gamma interferon that can help the immune response). These effects lead to a boost in production of antibodies in response to vaccines, and assist with fighting invading viruses and cancers. Thymalfasin also results in decreased CD-4 cell differentiation into the Th2 subset of CD-4 helper cells that produce cytokines, such as IL-4, which are associated with persistence of viral infection, and stimulates several other components of the immune response that help the body attack and kill virally-infected or tumor cells.
Thymalfasin for Enhancement of Response to Vaccines
Clinical trials have demonstrated that thymalfasin increases response to influenza and hepatitis B vaccines in the elderly and in hemodialysis patients. In elderly subjects, thymalfasin was also shown to decrease the incidence of influenza from 19% in subjects given an influenza vaccine alone, to 6% in subjects receiving thymalfasin treatment in addition to the influenza vaccine. For these clinical trials, the treatment regimen involved 8 to 10 injections of 1.6 mg doses of thymalfasin. A pilot clinical study conducted in 2009/2010 by our partner Sigma-Tau in Italy showed that higher doses of thymalfasin (3.2 or 6.4 mg) given only twice (seven days
7
prior to vaccination and on the day of vaccination) led to a statistically significant increase in percent of subjects who seroconverted to the H1N1 vaccine (MF59 adjuvanted monovalent vaccine, Focetria™ from Novartis), and an increase in total titers, when measured at 21 or 42 days after vaccination. These promising data support the utility of thymalfasin for use in vaccine enhancement.
Thymalfasin for Advanced-Stage Malignant Melanoma
A 488-patient phase 2 clinical trial with thymalfasin was conducted by Sigma-Tau in Europe, in which thymalfasin achieved its primary endpoint of tumor response and showed promise in extending survival for patients diagnosed with stage IV melanoma, the most advanced form of skin cancer. Results showed that thymalfasin in combination with DTIC chemotherapy tripled the overall response rate and extended overall survival in some arms of the trial by nearly 3 months compared to patients treated with DTIC, the current standard of care, and low dose interferon alpha. These data were reported at the 2007 Annual Meeting of the American Society of Clinical Oncology (“ASCO”), and in November 2008 we reported that we and the U.S. Food and Drug Administration (“FDA”) reached a Special Protocol Assessment (“SPA”) agreement on the design of a phase 3 registration trial for thymalfasin as a potential treatment for stage IV melanoma that adequately addresses the objectives necessary to support a regulatory submission. In addition, thymalfasin has been granted Orphan Drug Designation by the U.S. FDA for the treatment of stage IIb through stage IV melanoma. The Orphan Drug designation will allow us a seven-year period of market exclusivity if and when thymalfasin is approved for this indication in the United States. We have been seeking a partner for this phase 3 trial in melanoma. We estimate the worldwide market for malignant melanoma to be over $300 million.
INTELLECTUAL PROPERTY AND PROPRIETARY RIGHTS
Patents
We seek regulatory approval for our products in disease areas with high unmet medical need, significant market potential and where we have a proprietary position through patents covering use, manufacturing process, or composition of matter for our products. For our lead product ZADAXIN, we are the licensee or owner of patents and patent applications relating to thymosin and its use for a number of diseases. In particular, we are the licensee or owner of a substantial number of patents and applications in the United States and internationally including China that are directed to thymosin therapy for the treatment of hepatitis B and hepatitis C as a monotherapy or in combination with other therapeutics including drugs with or without regulatory approval for marketing. In addition, we are the licensee or owner of several pending patent applications in the United States and internationally that are directed to thymosin therapy for vaccine enhancement. If issued, these patents should have a patent term from 2025 to 2030. We are also the licensee or owner of several pending patent applications in the United States and internationally that are directed to thymosin therapy for the treatment of melanoma. If issued, these patents should have a patent term from 2027 to 2028. We have also applied for patents in the United States and internationally that are directed to thymosin therapy for the treatment of other infectious diseases such as Aspergillus infection, Severe Acute Respiratory Syndrome (“SARS”), as well as other indications. In addition, we have patents or patent applications directed to thymosin conjugates and the use of thymosin to stimulate the immune system in general.
Sigma-Tau holds the European rights to thymalfasin, while we have retained rights in all other regions of the world, including the United States and China.
We are the exclusive licensee of issued patents directed to the composition of matter of SCV-07 and related products as well as their use as an immunomodulator in general in the United States, China and a number of major international markets. These patents have a patent term from 2016 in the United States to 2018 outside of the United States. In addition, we are the exclusive licensee of various applications pending in the United States and internationally that are directed to SCV-07 therapy for the treatment of a number of diseases, including the treatment of oral mucositis as well as the treatment of hepatitis C. If issued, these patents should have a patent term from 2028 to 2030. We also have exclusive rights to patent applications directed to SCV-07 therapy for
8
vaccine enhancement, treatment of asthma as well as various cancers, e.g., lung cancer, melanoma, etc. SCV-07 is approved already in Russia for improvement of depressed immune systems. We acquired exclusive worldwide rights outside of Russia from Verta, Ltd.
Proprietary Rights
In addition to our patent protection, we intend to use other means to protect our proprietary rights. We may pursue marketing exclusivity periods that are available under regulatory provisions in certain countries including the United States, Europe, Japan, and China. For example, we expect to receive at least 5 year market exclusivity in the United States by being the first to obtain market approval of a product, e.g., thymosin or SCV-07 in the United States.
Furthermore, orphan drug exclusivity has been or may be sought where available. Such exclusivity has a term of 7 years in the United States and 10 years in Europe. We have obtained orphan drug designation for thymosin alpha 1 for the treatment of malignant melanoma and chronic hepatitis B in the United States and for the treatment of hepatocellular carcinoma in the United States and in Europe. Orphan drug protection has been or may be sought where available if such protection also grants seven years of market exclusivity. We have filed trademark applications worldwide for ZADAXIN and other trademarks that appear on our commercial packaging and promotional literature. Copyrights for the commercial packaging may prevent counterfeit products or genuine but unauthorized products from entering a particular country by parallel importation. Brand and trademark protection are particularly important to us in China. We have implemented anti-counterfeiting measures on commercial packaging and we are registering the packaging with customs departments in countries where such procedures exist. We rely upon trade secrets, which we seek to protect in part by entering into confidentiality agreements with our employees, consultants, corporate partners, suppliers, and licensees.
MANUFACTURING
ZADAXIN is manufactured for us by third parties under exclusive contract manufacturing and supply agreements. We closely monitor production runs of ZADAXIN and conduct our own quality assurance audit programs. We believe the manufacturing facilities of our contract suppliers are in compliance with the FDA’s current Good Manufacturing Practices, and European equivalents of such standards. In order to sell ZADAXIN to the licensed importers in China, our manufacturers must 1) be approved by the Italian Ministry of Health (AIFA) and 2) be accepted by the SFDA, the Chinese equivalent to the US FDA, and we must obtain an Imported Drug License from the SFDA permitting the importation of ZADAXIN into China. The license must be renewed every five years, and if we change manufacturers, these changes must 1) be approved by AIFA in Italy and 2) be accepted by the SFDA.
In the event of the termination of an agreement with any single supplier, we believe that we would be able to enter into arrangements with other suppliers with similar terms. We do not intend at this time to acquire or establish our own dedicated manufacturing facilities for any of our products. We believe that our current manufacturing partners for ZADAXIN have enough manufacturing capacity to meet potential market demand. We also believe that our current manufacturing partners for our other drug candidates will be able to meet our clinical trial needs.
COMPETITION
Our competition for sales of ZADAXIN in China is primarily from generic drug manufacturers located in China who sell their product at lower prices. We compete with them based upon our reputation as a high quality provider, including the fact that our product is produced outside China.
Our competitors for future products include pharmaceutical companies, biotechnology firms, universities and other research institutions, both in the United States and abroad, that are actively engaged in research and development or marketing of products in the therapeutic areas we are pursuing, particularly certain infectious
9
diseases such as HCV and HBV. Currently, competitors are marketing drugs for HCV, HBV and cancer, or have products in clinical trials. We believe that the principal competitive factors in this industry for a marketed drug include the efficacy, safety, price, therapeutic regimen, manufacturing, quality assurance and associated patents and the capabilities of its marketer.
In addition, most of our competitors, particularly large pharmaceutical companies, have substantially greater financial, technical, regulatory, manufacturing, marketing and human resource capabilities than SciClone. Most of them also have extensive experience in undertaking the preclinical and clinical testing and in obtaining the regulatory approvals necessary to market drugs. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated with our competitors.
We expect continuing advancements in and increasing awareness of the use of therapeutics which boost the immune system to fight cancer and infectious diseases. These developments may create new competitors.
For the treatment of HBV, current therapies being marketed by competitors include interferon alpha, in standard and pegylated forms, nucleoside analogues such as lamivudine, nucleotide analogue adefovir, and nucleoside analogue, entecavir. In addition to these products, in our largest market China, ZADAXIN faces competition from other synthetic and generic biological extracts, which are locally manufactured and significantly lower priced.
Future clinical trials may or may not show ZADAXIN, SCV-07, DC Bead, or ondansetron RapidFilm, to have advantages or clinically significant synergistic value over such existing or future competitive products.
RESEARCH AND DEVELOPMENT
A major portion of our operating expenses to date are related to research and development (“R&D”). R&D expenses consist of independent R&D costs and costs associated with collaborative R&D and in-licensing arrangements. A substantial portion of our development expense is third party expense relating to the conduct of clinical trials. R&D expenses were $16.5 million, $22.9 million and $16.6 million, for the years ended December 31, 2009, 2008, and 2007, respectively. We intend to maintain our strong commitment to R&D as an essential component of our product development effort. Licensed technology as well as products developed by outside parties are an additional source of potential products.
EMPLOYEES
As of December 31, 2009, we had 223 employees, 34 in the United States, 176 in China, and 13 in other countries. From time to time, we engage the services of consultants worldwide with pharmaceutical and business backgrounds to assist in our product development and ZADAXIN commercialization activities. We plan to leverage our key personnel by continuing to make extensive use of clinical research organizations, contract laboratories, development consultants and collaborations with pharmaceutical companies to develop and market our products.
GOVERNMENT REGULATION
Regulation by governmental authorities in the United States, China and other foreign countries is a significant factor in the manufacturing and marketing of our products, as well as in ongoing research and development activities and in pre-clinical and clinical trials and testing related to our products. In China, we are required to renew drug registration certificates and the Imported Drug License every five years. Our products in clinical development in the United States, China and other foreign countries are subject to approval by the FDA, the SFDA and similar regulatory authorities. In addition to obtaining regulatory approval for each product, each manufacturing establishment must be registered with the FDA or European (EMEA) regulatory authorities where the product is registered for sale and listed on the Certificate of Pharmaceutical Product (“CPP” or Country of
10
Origin Approval). (For ZADAXIN the CPP is in Italy and the named manufacturer is Patheon Italia S.p.A, in Italy.) A CPP is necessary in order to obtain an Imported Drug License from the SFDA for sales in China. Manufacturing establishments are subject to inspections by regulatory authorities at the federal, state and local level and must comply with current Good Manufacturing Practices (“GMP”) as established in various jurisdictions. In complying with GMP standards, manufacturers must continue to expend time, money and effort in the area of production and quality assurance to ensure ongoing full technical compliance. As we do not manufacture our products, we depend on third parties to meet requisite GMP standards.
China
In China, the pharmaceutical industry is subject to extensive government regulation and supervision. The regulatory framework addresses all aspects of operating in the pharmaceutical industry, including approval, production, licensing and certification requirements and procedures, periodic renewal and reassessment processes, registration of new drugs and environmental protection. In order to sell ZADAXIN in China, our manufacturer must be regulated by and approved by a regulatory body such as the FDA or EMEA, and we must obtain an Imported Drug License from the SFDA for the importation of the product. The Imported Drug License must be renewed every five years, and if we change manufacturers, we must register the new manufacturer and obtain a new Imported Drug License listing the new manufacturer. If we are unable to obtain or renew such permits or certificates or any other regulatory approvals required for our operation we would not be permitted to sell ZADAXIN to China.
Currently, all of our products sold in, or under development in, are imported into China from other countries. In general in China, the SFDA requires that an imported drug must also have country of origin approval for the same indication.
The SFDA is the authority that monitors and supervises the administration of pharmaceutical products and medical appliances and equipment as well as food, health food and cosmetics in China. The primary responsibilities of the SFDA include:
| | • | | monitoring and supervising the administration of pharmaceutical products, medical appliances and equipment as well as food, health food and cosmetics in China; |
| | • | | formulating administrative rules and policies concerning the supervision and administration of food, health food, cosmetics and the pharmaceutical industry; |
| | • | | evaluating, registering and approving of new drugs, generic drugs, imported drugs and traditional Chinese medicine; |
| | • | | approving and issuing permits for the manufacture and export/import of pharmaceutical products and medical appliances and equipment and approving the establishment of enterprises to be engaged in the manufacture and distribution of pharmaceutical products; and |
| | • | | examining and evaluating the safety of food, health food and cosmetics and handling significant accidents involving these products. |
The Ministry of Health (“MOH”) is an authority at the ministerial level under the State Council and is primarily responsible for national public health in China. Following the establishment of the SFDA in 2003, the MOH was put in charge of the overall administration of the national health in China excluding the pharmaceutical industry. In March 2008, the MOH was reorganized and assumed administrative responsibility for the SFDA. The MOH performs a variety of tasks in relation to the health industry such as establishing social medical institutes, promulgating national regulations, and producing professional codes of ethics for public medical personnel. The MOH is also responsible for international issues, such as that pertinent to foreign companies and governments.
11
Drug Administration Laws and Regulations
The China Drug Administration Law and the Implementing Measures of the China Drug Administration Law provide the legal framework for the establishment of pharmaceutical manufacturing enterprises, pharmaceutical trading enterprises and for the administration of pharmaceutical products including the development and manufacturing of new drugs and medicinal preparations by medical institutions. The China Drug Administration Law also regulates the packaging, trademarks and the advertisements of pharmaceutical products in China.
The China Drug Administration Law applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical manufacturers, pharmaceutical trading companies, medicinal preparations of medical institutions and the development, research, manufacturing, distribution, packaging, pricing and advertisements of pharmaceutical products.
Examination and Approval of New Medicines
Under the current applicable regulations in China, new medicines generally refer to those medicines that have not yet been marketed in China. In addition, certain marketed medicines may also be treated as new medicines if the type or application method of such medicines has been changed or new therapeutic functions have been added to such medicines. Generally, the approval of new medicines requires the following steps:
| | • | | Upon completion of the pre-clinical research of the new medicine, application for registration of the new medicine shall be submitted to the drug regulatory authorities at the provincial level for review. After completion of its review, the drug regulatory authorities at the provincial level shall submit their opinion and report to the SFDA for review; |
| | • | | if all the requirements are complied with, the SFDA will issue a notice of acceptance of application and proceed with its assessment on whether or not to grant the approval for conducting the clinical research on the new medicine; |
| | • | | after obtaining the SFDA’s approval for conducting the clinical research, the applicant may proceed with the relevant clinical research (which is generally conducted in three phases for a new medicine under the Medicine Registration Measures) at institutions with appropriate qualification: |
| | • | | Phase 1 refers to the preliminary clinical trial for clinical pharmacology and body safety. It is conducted to observe the human body tolerance for new medicine and pharmacokinetics, so as to provide a basis for determining the prescription plan. |
| | • | | Phase 2 refers to the stage of preliminary evaluation of clinical effectiveness. The purpose is to preliminarily evaluate the clinical effectiveness and safety of the medicine used on patients with targeted indication, as well as to provide a basis for determining the Phase 3 clinical trial research plan and the volume under the prescription plan. |
| | • | | Phase 3 is a clinical trial stage to verify the clinical effectiveness. The purpose is to test and determine the clinical effectiveness and safety of the medicine used on patients with targeted indication, to evaluate the benefits and risks thereof, and, eventually, to provide sufficient basis for review of the medicine registration application. |
| | • | | after completion of the relevant clinical research, the applicant submits a clinical research report and supporting documents to the drug regulatory authorities at the provincial level and provides raw materials of the standard products to the China National Institute for the Control of Pharmaceutical and Biological Products; |
| | • | | the drug regulatory authorities at the provincial level review the relevant documents, conduct site inspections and sample examinations and thereafter submit their opinion, inspection report and other application materials to the SFDA for review; |
12
| | • | | the China National Institute for the Control of Pharmaceutical and Biological Products will arrange for the examination of the sample new drug supplied by the relevant medicine examination institutes and will then issue the examination result report to the SFDA; and |
| | • | | if all the regulatory requirements are satisfied, the SFDA will grant a new drug certificate and a pharmaceutical approval number (assuming the applicant has a valid pharmaceutical manufacturing permit and the requisite production conditions for the new medicine have been met). |
Permits and Licenses for Importation, Manufacturing and Registration of Drugs
Production License. To import a product manufactured outside of China, a permit must be obtained and renewed every five years. If the manufacturing process, or the manufacturer changes, a new permit must be obtained. Currently our product is imported into China by licensed importers. We do not have any plans to manufacture in China at the present time.
To manufacture pharmaceutical products in China, a pharmaceutical manufacturing enterprise must obtain a pharmaceutical manufacturing permit issued by the relevant pharmaceutical administrative authorities at the provincial level where the enterprise is located.
Each pharmaceutical manufacturing permit issued to a pharmaceutical manufacturing enterprise is effective for a period of five years. Any enterprise holding a pharmaceutical manufacturing permit is subject to review by the relevant regulatory authorities on an annual basis.
GMP Certificates. Our current products and our clinical candidates in China are all manufactured outside China and are subject to GMP standards in the country in which they are manufactured. Our manufacturers are subject to site inspections by the regulatory authorities in the jurisdictions in which they are located. The importation of pharmaceutical products into China requires an importation permit which must be renewed every five years. The issuance and renewal of such permit is dependent, among other things, upon maintaining manufacturing standards that comply with the GMP standards of a widely recognized regulatory authority, such as the FDA or EMEA.
If we were to manufacture product in China, or obtain product from Chinese contract manufacturers, such manufacture would be subject to similar GMP standards established in China and administered by local authorities.
Distribution of Pharmaceutical Products
According to the China Drug Administration Law and its implementing regulations and the Administrative Measures on Oversight of Distribution of Pharmaceutical Products, a manufacturer of pharmaceutical products in China can only engage in the trading of the pharmaceutical products that the manufacturer has produced itself. In addition, such manufacturer can only sell its products to:
| | • | | wholesalers and retailers holding pharmaceutical trading permits; |
| | • | | other holders of pharmaceutical manufacturing permits; or |
| | • | | medical practitioners holding medical practice permits. |
A pharmaceutical manufacturer in China is prohibited from selling its products to end-users, or individuals or entities other than holders of Pharmaceutical trading permits, the pharmaceutical manufacturing permits or the medical practice permits.
The granting of a Pharmaceutical Trading Permit to wholesalers requires the approval at the provincial level.
13
A pharmaceutical distributor (including wholesalers and retailers) must satisfy requirements as to personnel with pharmaceutical expertise, appropriate warehousing and sanitary environment compatible to the distributed pharmaceutical products; quality management and compliance with regulations to ensure the quality of the distributed pharmaceutical products.
Operations of pharmaceutical distributors must be conducted in accordance with the Pharmaceutical Operation Quality Management Rules and require a certificate from the SFDA. Pharmaceutical distributors must comply with record keeping requirements regarding the products sold.
Pharmaceutical distributors can only distribute pharmaceutical products obtained from those with a pharmaceutical manufacturing permit or importation license and a Pharmaceutical Trading Permit.
Price Control and Competitive Bidding
The administration of price control of pharmaceutical products is vested in the national and provincial price administration authorities. Depending on the categories of pharmaceutical products in question, the prices of pharmaceutical products listed in the State Basic Medical Insurance and Work Injury Insurance Drug Catalogue, drugs with patents and other drugs whose production or trading may constitute monopolies are subject to the control of the National Development and Reform Commission of China and the relevant provincial or local price administration authorities. For pharmaceutical products manufactured or imported into China, the national price administration authority from time to time publishes price control lists specifying pricing ceilings for specific pharmaceuticals. In November 2009, thymosin alpha 1, the generic chemical name for our pharmaceutical product ZADAXIN, was included as a Category B product in the National Reimbursed Drug List. We will have to negotiate a new price at the time that our product is reviewed for price adjustment by the authorities. The provincial price administration authorities also publish price control lists for pharmaceutical products. The main purpose of the price control policy is to set an upper limit to the prices of pharmaceutical products to prevent excessive increases in the prices of such products. Pursuant to the Measures for Medicine Pricing by the Government, the price ceiling is determined mainly by reference to the quality of the product, whether the products are newly developed products, whether the products have patent protection in China, and the status of implementing the GMP Guidelines by the manufacturer of the relevant product.
The prices of pharmaceutical products included in the price control lists are subject to adjustment upon approval by the price administration authorities from time to time. Pharmaceutical enterprises in China are required to submit cost related information such as raw material prices regularly to the relevant authorities, so that the authorities may take into account the prevailing market conditions when setting the prices. The price administration authorities may approve adjustments to the price of pharmaceutical products upon the pharmaceutical manufacturer’s request if material changes in the cost structure in producing the pharmaceutical products or significant changes in demand for these pharmaceutical products are recognized.
In each province where we market our products, we participate in a government-sponsored competitive bidding process every year or every few years for procurement by state-owned hospitals of a medicine included in the provincial medicine catalogs. A government-appointed committee reviews bids submitted by pharmaceutical companies and selects one or more medicines for treatment of a particular medical condition. The selection is based on a number of factors, including bid price, quality and manufacturer’s reputation and service. The bid price of the selected medicine will become the price required for purchases of that medicine by all state-owned hospitals in the relevant province or local district.
Health Insurance System
The Ministry of Labor and Social Security are responsible for the reform of the medical insurance system. As part of the reform of the state basic medical insurance system for employees in the urban areas, the Ministry of Labor and Social Security, the MOH, the SDFA and various other governmental departments jointly issued the
14
State Basic Medical Insurance and Work Injury Insurance Drug Catalogue, as amended, or Catalogue, with a view to enhancing the management of the use of drugs under the medical insurance system. The drugs listed in the Catalogue are covered by the national medical insurance program.
Reimbursement under the National Medical Insurance Program
The Ministry of Labor and Social Security, together with other government authorities, determines which medicines are to be included in or removed from the Catalogue for the national medical insurance program, and under which category a medicine should fall, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are based on a number of factors, including price and efficacy. A national medical insurance program participant can be reimbursed for the full cost of a Category A medicine and 60 to 90% of the cost of a Category B medicine. Although it is designated as a national program, the implementation of the national medical insurance program is delegated to various provincial governments, each of which has established its own medicine catalog. A provincial government must include all Category A medicines listed in the Catalogue in its provincial medicine catalog, but may use its discretion based on its own selection criteria to add other medicines to, or exclude Category B medicines listed in the Catalogue from, its provincial medicine catalog, so long as the combined numbers of the medicines added and excluded do not exceed 15% of the number of the Category B medicines listed in the Catalogue. In addition, provincial governments may not downgrade a nationally classified Category A medicine to Category B. The total amount of reimbursement for the cost of prescription and over-the-counter medicines, in addition to other medical expenses, for an individual program participant in a calendar year is capped at the amount in that participant’s individual account. The amount in a participant’s account varies, depending upon the amount of contributions from the participant and his or her employer. Generally, program participants who are from relatively wealthier eastern parts of China and relatively wealthier metropolitan centers have greater amounts in their individual accounts than those from less developed provinces.
Prescription Regulations
As announced by Ministry of Health, the Prescription Administrative Measures, regulating prescription of drugs, took effect on May 1, 2007, which stipulates that doctors may only use the generic names of drugs in their prescriptions instead of brand names and that medical institutions offer patients the same type of drug from no more than two separate pharmaceutical companies. The purpose of this legislation is to minimize the practice of doctors receiving kickbacks from pharmaceutical companies for prescribing higher priced, or even unneeded, drugs to patients.
United States, Europe and Other Countries
We have several products in development for markets in the United States, Europe and elsewhere. The regulatory regime in the United States and Europe is similar in many respects to the regulatory system in China, although the regulatory system has been developed over many decades and, we believe, is subject to greater and more certain regulatory oversight than the Chinese regulatory system. The steps required before a new drug may be distributed commercially generally include:
| | • | | conducting appropriate pre-clinical laboratory evaluations, including animal studies, in compliance with the FDA’s Good Laboratory Practice (“GLP”) requirements, to assess the potential safety and efficacy of the product, and to characterize and document the product’s chemistry, manufacturing controls, formulation and stability; |
| | • | | submitting the results of these evaluations and tests to the FDA, along with manufacturing information, analytical data, and protocols for clinical studies, in an Investigational New Drug Application (“IND”), and receiving approval from the FDA that the clinical studies proposed under the IND are allowed to proceed; |
| | • | | obtaining approval of Institutional Review Boards (“IRBs”) to administer the product to humans in clinical studies; |
15
| | • | | conducting adequate and well-controlled human clinical trials in compliance with the FDA’s Good Clinical Practice (“GCP”) requirements that establish the safety and efficacy of the product candidate for the intended use, typically in the same Phase 1, Phase 2 and Phase 3 steps described above for China; |
| | • | | development of manufacturing processes which conform to FDA current Good Manufacturing Practices, or cGMPs, as confirmed by FDA inspection; |
| | • | | submitting to the FDA the results of pre-clinical studies, clinical studies, and adequate data on chemistry, manufacturing and control information to ensure reproducible product quality batch after batch, in a New Drug Application (“NDA”) or Biologics License Application (“BLA”); |
| | • | | obtaining FDA approval of the NDA, including inspection and approval of the product manufacturing facility as compliant with cGMP requirements, prior to any commercial sale or shipment of the pharmaceutical agent. |
When used in connection with trials and filings in other countries, terms such as “phase 1,” “phase 2,” “phase 3,” “phase 4,” “new drug application” and “marketing application” refer to what we believe are comparable trials and filings in these other countries.
The process in the United States of obtaining regulatory approval is lengthy, uncertain, and requires the expenditure of substantial resources. Each NDA must be accompanied by a user fee, pursuant to the requirements of the Prescription Drug User Fee Act, or PDUFA, and its amendments.
After FDA approval has been obtained, the FDA requires post-marketing reporting to monitor the side effects of the drug. This may include phase 4 studies in which the drug is studied in an expanded patient population in a post-approval setting for continued monitoring of safety and sometimes continued efficacy. Further studies may be required to provide additional data on the product’s risks, benefits, and optimal use, and will be required to gain approval for the use of the product as a treatment for clinical indications other than those for which the product was initially tested. Results of post-marketing programs may limit or expand the further marketing of the product. Further, if there are any modifications to the drug, including changes in indication, labeling, or a change in the manufacturing process or manufacturing facility, an NDA or BLA supplement may be required to be submitted to the FDA. We market or intend to market products in other countries and each country has regulations for the regulation of approval, marketing and sale of pharmaceutical products. We must comply with the regulations of each country in which we seek approval of and intend to market and sell any product.
THIRD-PARTY REIMBURSEMENT
Our ability to successfully commercialize our products may depend in part on the extent to which coverage and reimbursement to patients for our products will be available from government health care programs, private health insurers and other third-party payors or organizations. Significant uncertainty exists as to the reimbursement status of new therapeutic products, such as ZADAXIN. In most of the markets in which we are currently approved to sell ZADAXIN, reimbursement for ZADAXIN under government or private health insurance programs is not yet widely available, and in many of these countries government resources and per capita income may be so low that our products will be prohibitively expensive. We believe that most of the sales of ZADAXIN in China are made without third party reimbursement. In the United States, Europe and Japan, proposed health care reforms could limit the amount of governmental or third-party reimbursement available for our products should they be approved for sale in these markets. Various governments and third-party payors are trying to contain or reduce the costs of health care through various means. We expect that there will continue to be legislative efforts and proposals to implement such government controls.
16
AVAILABLE INFORMATION
We file electronically with the Securities and Exchange Commission (“the Commission” or the “SEC”) our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov.
You may obtain a free copy of our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, on the day of filing with the SEC on our website on the World Wide Web at http://www.sciclone.com, by contacting the Investor Relations Department at our corporate offices by calling 800-724-2566 or by sending an e-mail message to investorrelations@sciclone.com.
You should carefully consider the risks described below, in addition to the other information in this report on Form 10-K, before making an investment decision. Each of these risk factors could adversely affect our business, financial condition, and operating results as well as adversely affect the value of an investment in our common stock.
Our stock price may be volatile, and an investment in our stock could suffer a decline in value.
We have a history of operating losses and an accumulated deficit. Although we reported net income of $11.9 million for the year ended December 31, 2009, we have experienced significant operating losses in the past, and as of December 31, 2009, we had an accumulated deficit of approximately $165 million. If our operating expenses were to increase or if we were not able to increase or sustain revenue, we may not achieve profitability over the next 12 months.
The market price of our common stock has experienced, and may continue to experience, substantial volatility due to many factors, some of which we have no control over, including:
| | • | | actual or anticipated fluctuations in our quarterly operating results; |
| | • | | progress and results of clinical trials; |
| | • | | progress of thymalfasin and SCV-07 through the regulatory process, especially regulatory actions and the adequacy of clinical data and documentation for regulatory purposes in the United States, Europe, China and Japan; |
| | • | | finding a partner for the phase 3 trial of thymalfasin for malignant melanoma; |
| | • | | progress of DC BeadTM and ondansetron RapidFilmTM through required clinical studies and the regulatory process, especially regulatory actions and the adequacy of clinical data and documentation for regulatory purposes in China and Vietnam; |
| | • | | timing and achievement of milestones; |
| | • | | changes in our agreements or relationships with collaborative partners; |
| | • | | announcements of technological innovations or new products by us or our competitors; |
| | • | | announcement and completion of corporate acquisition, merger, licensing or marketing arrangements, or sales of assets; |
17
| | • | | government regulatory action affecting our drug products or our competitors’ drug products in China, the United States and other foreign countries; |
| | • | | developments or disputes concerning patent or proprietary rights; |
| | • | | changes in the composition of our management team or board of directors; |
| | • | | changes in company assessments or financial estimates by securities analysts; |
| | • | | changes in assessments of our internal controls over financial reporting; |
| | • | | general stock market conditions and fluctuations for the emerging growth and pharmaceutical market sectors; |
| | • | | economic conditions in the United States or abroad; and |
| | • | | broad financial market fluctuations in the United States, Europe or Asia. |
Our revenue is dependent on our sale of ZADAXIN in China, and if we experience difficulties in our foreign sales efforts, our operating results and financial condition will be harmed.
Our product revenue is highly dependent on the sale of ZADAXIN in China. If we experience difficulties in our foreign sales efforts in China, our business will suffer and our operating results and financial condition will be harmed. For the years ended December 31, 2009 and 2008, approximately 96% and 94%, respectively, of our ZADAXIN sales were to customers in China. Sales of ZADAXIN in China may be limited due to the low average personal income, lack of patient cost reimbursement, poorly developed infrastructure and competition from other products, including generics. ZADAXIN sales growth in recent years has benefited from the rapidly growing Chinese economy and growing personal disposable income. Sales of ZADAXIN in China could be adversely affected by a slowing or downturn of the Chinese economy.
In China, ZADAXIN is approved only for the treatment of hepatitis B virus (“HBV”) and as a vaccine adjuvant. We face competition from pharmaceutical companies who are aggressively marketing competing products for the treatment of HBV and other indications where we believe ZADAXIN may be used on an off-label basis. In addition, several local companies are selling lower-priced, locally manufactured generic thymosin, which is a competitive product and is selling in substantial and increasing quantities. While generic products outsell ZADAXIN in unit volumes, we have been able to maintain an advantage through the reputation of our imported, branded product. We expect such competition to continue and there could be a negative impact on the price and the volume of ZADAXIN sold in China, which would harm our business. Our efforts to in-license or acquire other pharmaceutical products for marketing in China and other markets may be unsuccessful or even if successful may not have a meaningful effect on our dependence on ZADAXIN sales in those markets.
In November 2009, thymosin alpha 1, the generic chemical name for our pharmaceutical product ZADAXIN, was included as a Category B product in the National Reimbursed Drug List. We will have to negotiate a new price at the time that our product is reviewed for price adjustment by the authorities. China regulates pharmaceutical prices and pharmaceutical importation. These regulations may reduce prices for ZADAXIN to levels significantly below those that would prevail in an unregulated market, limit the volume of product which may be imported and sold or place high import duties on the product, any of which may limit the growth of our revenues or cause them to decline. We believe that the Chinese government is increasing its efforts to reduce overall health care costs, including through pricing controls. Individual provinces in China and, in some cases, individual hospitals can and have established pricing requirements for a product to be included on formulary lists. In some cases, these prices have been lower than our distributors have been selling ZADAXIN in which case we have been removed from formulary lists, which consequently has reduced sales to certain hospitals and could adversely affect our future sales. The Chinese national and provincial governments regulate product pricing and the maximum prices for ZADAXIN at the provincial level over the next several years, and
18
potentially at the national level. The process and timing for this is unpredictable. We are working on these regulatory processes as well as on potential changes in our business model depending on potential outcomes. We believe we will be able to successfully manage our business in China through this process, however maximum prices could be set at some time in the future which could adversely affect our results or require substantial changes in our business model which may be difficult to implement.
We have received regulatory approvals to import and market ZADAXIN in China and to manufacture ZADAXIN and export the product from Italy. In order to continue our sales to China, we need to maintain these approvals. Our license to import ZADAXIN into China needs to be renewed every five years and the next renewal is required in 2013. Although we were successful in obtaining a renewal in 2008, there is no assurance that we will receive renewals in the future when applied for or that the renewals will not be conditioned or limited in ways that limit our ability to sell ZADAXIN in China. Further, our licenses to manufacture and export ZADAXIN from Italy are dependent upon our continuing compliance with regulations in Italy. Our business would be adversely affected if we are not able to maintain these approvals. In order to sell ZADAXIN to the licensed importers in China, our manufacturers must 1) be approved by the Italian Ministry of Health (“AIFA”) and 2) be accepted by the State Food and Drug Administration of China (“SFDA”), the Chinese equivalent to the United States Food and Drug Administration (“FDA”), and we must obtain an Imported Drug License from the SFDA permitting the importation of ZADAXIN into China. The license must be renewed every five years, and if we change manufacturers, these changes must 1) be approved by the AIFA in Italy and 2) be accepted by the SFDA. When we change manufacturers we must obtain a new approval. We are currently in the process of changing manufacturer and if we are not successful in obtaining the necessary approval in a timely manner, our business would be adversely affected.
Our ZADAXIN sales and operations in other parts of China and the world are subject to a number of risks and increasing regulations, including difficulties and delays in obtaining registrations, renewals of registrations, permits, pricing approvals and reimbursement, increasing regulation of product promotion and selling practices, unexpected changes in regulatory requirements and political instability. In addition, we recently experienced a strong upsurge in ZADAXIN sales which we believe is attributable both to the increasing penetration of ZADAXIN within the Chinese market, as well as concerns in China from the H1N1 flu virus. Although we believe that ZADAXIN sales will return to levels more consistent with our established business, distributors and hospitals in China could ultimately be found to have been stockpiling more ZADAXIN than needed for current use in anticipation of potential shortages. If distributors and hospitals that purchase ZADAXIN have indeed been stockpiling more ZADAXIN than needed for current use, our sales of ZADAXIN may suffer as distributors and hospitals use ZADAXIN already in their inventory before purchasing additional product from us. This could lead to uneven future revenue results for ZADAXIN and in turn materially impact our cash flow and business condition.
We experience other issues with managing foreign sales operations including long payment cycles, potential difficulties in accounts receivable collection and, especially from significant customers, fluctuations in the timing and amount of orders and the adverse effect of any of these issues on our business could be increased due to the concentration of our business with a small number of distributors. Problems with collections from, or sales to, any one of those distributors could materially adversely affect our results. Operations in foreign countries including China also expose us to risks relating to difficulties in enforcing our proprietary rights, currency fluctuations and adverse or deteriorating economic conditions. If we experience problems with obtaining registrations, complying with reimbursement rules or compliance with foreign country or applicable U.S. laws, adverse or mixed outcome of clinical studies anywhere in the world, or if we experience difficulties in payments or intellectual property matters in foreign jurisdictions, or if significant political, economic or regulatory changes occur, our results could be adversely affected. Moreover, our operations throughout the world including China are potentially subject to the laws and regulations of the United States including the Foreign Corrupt Practices Act, in addition to the laws and regulations of the local countries. Regulation in China of the activities in the pharmaceutical industry has increased and may continue to undergo significant and unanticipated changes. A number of companies have faced significant expenses or fines as a result of the increasing regulation of, and
19
enforcement activity regarding, the pharmaceutical industry. If we fail to comply with regulations or to carry out controls on our Chinese or other foreign operations in a manner that satisfies all applicable laws, our business would be harmed.
The Chinese government has been engaged in an extensive stimulus program in China over the past year. We believe that the Chinese government is reducing these stimulus programs. We believe these programs have had broad effects across the Chinese economy, and the reduction or withdrawal of these programs could adversely affect spending in numerous ways, including reduction of spending on pharmaceutical products including ZADAXIN.
Our sales of ZADAXIN may fluctuate due to seasonality of product orders and sales in any quarter may not be indicative of future sales.
Our sales for the quarter ended June 30, 2009 were greatly affected by the demand in China for ZADAXIN which we believe was in connection with Influenza A (H1N1). Similarly, our sales for the quarter ended June 30, 2003 were greatly affected by the demand in China for ZADAXIN in connection with the treatment of Severe Acute Respiratory Syndrome (“SARS”). To date, SARS has not re-emerged, like influenza, as a seasonal public health problem. However, it is not possible to predict what effect, if any, H1N1, SARS, or similar epidemics would have on future sales of ZADAXIN, if they were to emerge. Although we do not market ZADAXIN for use in treating epidemic diseases such as Influenza A (H1N1) or SARS, if ZADAXIN is purchased in connection with outbreaks of seasonal viral contagions, product sales could become more concentrated in certain quarters of the calendar year, quarterly sales levels could fluctuate and sales in any quarter may not be indicative of sales in future periods.
We may lose market share or otherwise fail to compete effectively in the intensely competitive pharmaceutical industry.
Competition in the pharmaceutical industry in China is intense, and we expect that competition will increase. Our success depends on our ability to compete in this industry, but we cannot assure you that we will be able to successfully compete with our competitors. Increased competitive pressure could lead to intensified price-based competition resulting in lower prices and margins, which would hurt our operating results. We cannot assure you that we will compete successfully against our competitors or that our competitors, or potential competitors, will not develop drugs or other treatments for our targeted indications that will be superior to ours.
We depend on sales in China, and global conditions could negatively affect our operating results or limit our ability to expand our operations outside of China. Changes in China���s political, social and economic environment may affect our financial performance.
A large majority of our sales are in China. Heightened tensions resulting from the current geopolitical conditions in the Middle East, North Korea and elsewhere could worsen, causing disruptions in foreign trade, which would harm our sales. In particular, our commercial product is manufactured in Europe and distributed by us from our operations in Hong Kong. Any disruption of our supply and distribution activities due to geopolitical conditions could decrease our sales and harm our operating results.
With respect to China, our financial performance may be affected by changes in China’s political, social and economic environment. The role of the Chinese central and local governments in the Chinese economy is significant. Chinese policies toward economic liberalization, and laws and policies affecting foreign companies, currency exchange rates and other matters could change, resulting in greater restrictions on our ability to do business in China. Any imposition of surcharges or any increase in Chinese tax rates could hurt our operating results. The Chinese government could revoke, terminate or suspend our license for national security and similar reasons without compensation to us. If the government of China were to take any of these actions, we would be prevented from conducting all or part of our business. Any failure on our part to comply with governmental regulations could result in the loss of our ability to market our products in China.
20
Because of China’s tiered method of importing and distributing finished pharmaceutical products, our quarterly results may vary substantially from one period to the next.
China uses a tiered method to import and distribute finished pharmaceutical products. At each port of entry, and prior to moving the product forward to the distributors, government-licensed importing agents must process and evaluate each shipment to determine whether such shipment satisfies China’s quality assurance requirements. In order to efficiently manage this process, the importing agents typically place large, and therefore relatively few, orders within any nine month period. Therefore, our sales to an importing agent can vary substantially from quarter to quarter depending on the size and timing of the orders, which has in the past and may in the future cause our quarterly results to fluctuate. We rely on a limited number of importers, in any given quarter, to supply substantially all of our product in China. Because we use a small number of importing agents in China, our receivables from any one importing agent are material, and if we were unable to collect receivables from any importer, our business and cash-flow would be adversely affected.
We may be subject to currency exchange rate fluctuations, which could adversely affect our financial performance.
Substantially all of our product sales are denominated in U.S. dollars. Fluctuation in the U.S. dollar exchange rate with local currency directly affects the customer’s cost for our product. In particular, a stronger U.S. dollar vis-à-vis the local currency would tend to have an adverse effect on sales and potentially on collection of accounts receivable. China currently maintains the value of the renminbi in a narrow currency trading band that may or may not fluctuate based upon government policy. Depending on market conditions and the state of the Chinese economy, it is possible that China could make future adjustments, including moving to a managed float system, with opportunistic interventions. This reserve diversification may negatively impact the United States dollar and U.S. interest rates. A trend to a stronger U.S. dollar would erode margins earned by our Chinese importers and prompt them to ask us to lower our prices. We are subject to currency exchange rate fluctuations as a result of expenses incurred by our foreign operations. In particular, one of our supply arrangements under which we purchase finished products is denominated in euros and costs of our operations in China are paid in local currency. Consequently, changes in exchange rates could unpredictably and adversely affect our operating results and could result in exchange losses. To date, we have not hedged against the risks associated with fluctuations in exchange rates and, therefore, exchange rate fluctuations could have a material adverse impact on our future operating results and stock price.
Our business strategy is dependent on our ability to in-license or otherwise acquire the rights to develop and commercialize products, particularly in China. If we fail to acquire such rights or are unsuccessful in our efforts to develop such products and obtain regulatory approval to market and successfully commercialize them, our business will suffer.
All of our products, including ZADAXIN, the only one for which we have sales revenue, have been in-licensed by us. We do not conduct product discovery and typically have in-licensed our products from third parties who have discovered the products and conducted at least some pre-clinical research on them. We are particularly focused on in-licensing products for the China market and the competition for attractive products to in-license is intense, and we cannot assure you that we will be able to in-license products in the future on acceptable terms, if at all. In addition, we face the risks of developing the in-licensed products and the risks and uncertainties of conducting clinical trials and seeking regulatory approval to market the in-licensed products, all of which require years of effort and the commitment of significant financial resources. Our ability to grow our business requires the development and commercialization of additional products. If we are unable to in-license or acquire products on acceptable terms and successfully develop and commercialize them, our business could be harmed.
21
We may engage in acquisitions and must successfully integrate any acquired products or companies in order to avoid a material disruption to our business and material adverse effects to our financial results.
We may engage in one or more acquisitions of products or companies. Acquisitions involve numerous risks, including the following:
| | • | | difficulties and costs in integrating the operations, technologies, products and personnel of the acquired businesses; |
| | • | | inadequate internal control procedures and disclosure controls to comply with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or poor integration of a target company’s or businesses’ procedures and controls; |
| | • | | diversion of management’s attention from normal daily operations of the business; |
| | • | | potential difficulties in completing projects associated with in-process research and development; |
| | • | | difficulties in entering markets in which we have no or limited direct prior experience and where competitors in such markets have stronger market positions; |
| | • | | insufficient net revenue to offset increased expenses associated with acquisitions; |
| | • | | potential failure to achieve commercial expectations; |
| | • | | potential loss of key employees of the acquired companies; and |
| | • | | difficulty in forecasting revenues and margins. |
Acquisitions may also cause us to:
| | • | | issue common stock that would dilute our current shareholders’ percentage ownership; |
| | • | | assume liabilities, some of which may be unknown at the time of such acquisitions; |
| | • | | record goodwill and intangible assets that will be subject to impairment testing and potential periodic impairment charges; |
| | • | | incur amortization expenses related to certain intangible assets; |
| | • | | incur large and immediate write-offs of in-process research and development costs; or |
| | • | | become subject to litigation. |
Mergers and acquisitions of pharmaceutical companies inherently entail risk, and no assurance can be given that any future acquisitions will be successful or will not adversely affect our business, operating results, or financial condition. Failure to manage and successfully integrate acquisitions could harm our business and operating results in a material way. Even when an acquired company has already developed and marketed products, there can be no assurance of the continued prospects or success of such products or that pre-acquisition due diligence will have identified all possible issues that might arise with respect to such products.
We may not be able to successfully develop or commercialize our products.
While we have sales of ZADAXIN (“thymalfasin or thymalfasin alpha 1”) in certain markets, regulatory approval does not exist at this time for ZADAXIN for the key markets of the United States, Europe (outside of Italy) and Japan and, in this respect ZADAXIN is still being developed. In 2006, we acquired the rights to distribute DC Bead in China, but we must receive regulatory approval before we can commercialize this product. We previously believed that regulatory approval for DC Bead in China would be forthcoming in 2009. However, the SFDA required us to conduct a local trial in China to supplement data already obtained from a previous DC Bead study. This study is underway and if the trial results are positive, we expect to receive regulatory approval for DC Bead by the end of 2010. Our other potential products presently are SCV-07 and ondansetron RapidFilm and each of them is in an earlier stage of development than ZADAXIN. Clinical trials outcomes are uncertain.
22
For example, in October 2009, we halted our phase 2 trial of RP101 and we have no plans to proceed with further development of RP101 at this time. We may consider and undertake various strategies to expand our portfolio of potential products, including acquiring product candidate rights through licenses or other relationships, or through other strategic relationships including acquisitions of other companies that may have proprietary rights to other development candidates or the capability to discover new drug candidates. Such transactions could require a substantial amount of our financial resources, or, if equity is involved, may result in substantial dilution to current stockholders. Strategic transactions also require substantial management time and effort and are subject to various risks that could adversely affect us or our financial results.
To fully develop our products, substantial resources are required for extensive research, development, pre-clinical testing, clinical trials, manufacturing scale-up and regulatory approval prior to the potential products being ready for sale. We cannot assure that our efforts will produce commercially viable products. We face significant technological risks inherent in developing these products. We may also abandon some or all of our proposed products before they become commercially viable. For DC Bead, we are obligated to pay half of the costs in our efforts to obtain regulatory approval in China and, due to recent events, the total amount of costs for DC Bead will increase. For ondansetron RapidFilm, we are obligated to make a milestone payment upon regulatory approval. If any of our products, even if developed and approved, cannot be successfully commercialized in a timely manner, our business will be harmed and the price of our stock may decline.
We have not yet sold any product other than ZADAXIN and our sales have been primarily to a single country, China. Our future revenue growth depends to a great extent on increased market acceptance and commercialization of ZADAXIN in additional countries, as well as our ability to increase sales in China. If we fail to successfully market ZADAXIN or if we cannot commercialize this drug in the United States and other additional markets, our revenue and operating results will be limited. If unexpected and serious adverse events are reported, or if expected efficacy results are not achieved, it would have a material adverse effect on our business. Our future revenue will also depend in part on our ability to develop other commercially viable and accepted products, such as DC Bead, ondansetron RapidFilm, and SCV-07. Market acceptance of our products will depend on many factors, including our ability to convince prospective customers to use our products as an alternative to other treatments and therapies and to convince prospective strategic partners to market our products effectively and to manufacture our products in sufficient quantities with acceptable quality and at an acceptable cost. In addition, doctors must opt to use treatments involving our products. If doctors elect to use a different course of treatment, demand for our drug products would be reduced. Failure to do any of the above will lead to an unfavorable outcome on the results of our operations.
Higher than anticipated patient drop out rates in our clinical trials could adversely affect trial results and make it more difficult to obtain regulatory approval.
In December 2005 and June 2006, we announced results from our two U.S. phase 3 HCV clinical trials. These clinical trials did not demonstrate that the combination of ZADAXIN and pegylated interferon alpha provides a statistically significant clinical benefit when compared to the use of pegylated interferon alpha alone in non-responder patients. These results made Sigma-Tau Finanziaria, S.p.A.’s (“Sigma-Tau’s”) efforts to fully recruit patients for the ZADAXIN phase 3 HCV triple therapy combination trial, ongoing at that time, more difficult and enrollment took longer than planned. A patient who drops out at any point during a trial is considered a “failure to respond” in results of the clinical trial. In general, the fewer patients who complete each trial, the higher the positive response rate for the group of remaining treated patients in such trial needs to be in order to demonstrate statistical significance. Therefore, a higher than anticipated dropout rate lowers the chances of proving statistical significance which could adversely affect clinical trial results. Dropouts may affect the oral mucositis phase 2 clinical trial for SCV-07 or our HCV phase 2 trial for SCV-07 and other trials we conduct.
23
We cannot predict the safety profile of the use of thymalfasin, DC Bead, ondansetron RapidFilm or SCV-07 when used in combination with other drugs.
Many of our trials involve the use of thymalfasin in combination with other drugs and our pancreatic cancer clinical trial involved RP101 in combination with gemcitabine. SCV-07 may be developed to be used in combination with other drugs. Some of these drugs, particularly pegylated interferon alpha, ribavirin, non-pegylated interferon alpha, dacarbazine and gemcitabine are known to cause adverse patient reactions. We cannot predict how thymalfasin, DC Bead, ondansetron RapidFilm or SCV-07 will work with other drugs, including causing possible adverse side effects not directly attributable to the other drugs that could compromise the safety profile of thymalfasin, DC Bead, ondansetron RapidFilm or SCV-07 when used in certain combination therapies.
Final results from our proposed or ongoing clinical trials for thymalfasin, SCV-07, ondansetron RapidFilm, and DC Bead may differ materially from interim or pre-clinical trial results. These clinical trials could be affected by the future actions of our partners, unexpected delays, unanticipated patient drop out rates or adverse side effects, future actions by the SFDA or the FDA or equivalent regulatory authorities in Europe or other countries or additional expenses.
Our ability to achieve and sustain operating profitability depends in large part on our ability to commence, execute and complete clinical programs and obtain additional regulatory approvals for ZADAXIN and other drug candidates.
Clinical trials are inherently risky and may reveal that our product candidates are ineffective or have unanticipated side effects and/or drug interactions that may significantly decrease the likelihood of regulatory approval. In October 2009, we announced the discontinuation of our phase 2 clinical trial evaluating RP101, a nucleoside analog known as BVdU, for the treatment of late-stage pancreatic cancer. This decision followed the recommendation of the trial’s Data Safety Monitoring Committee (“DSMC”) based upon the data reviewed at the most recent DSMC meeting and we have no plans to proceed with further development of RP101 at this time. In addition, on November 5, 2008, we announced the top-line results from a large, randomized, phase 3 clinical trial evaluating thymalfasin in combination with pegylated interferon alpha-2a (“peg-IFN-2a”) and ribavirin (“RBV”) as a treatment for patients with hepatitis C virus (“HCV”) who have not responded to prior therapy consisting of peg-IFN and RBV alone (current standard of care). The thymalfasin treated group did not achieve statistical significance for the primary end point of sustained virological response (“SVR”) as assessed in the primary analysis population, i.e. the intent-to-treat population. In the prospectively defined secondary population of patients who completed the full course of 48 weeks of treatment with thymalfasin in addition to peg-IFN-2a and RBV (“Completer Population”), the primary endpoint was achieved with statistical significance. These results did not meet our expectations based upon prior clinical trials. Similarly, the results of our thymalfasin phase 2 melanoma clinical trial do not necessarily predict future clinical or commercial success. Finally, the results of studies in DC Bead, ondansetron RapidFilm, SCV-07, including a phase 2a for HCV, pre-clinical and phase 1 trial results also do not predict clinical or commercial success.
We may face numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent commercialization of our product candidates including: our product candidate clinical trials may not prove statistical significance; negative or inconclusive clinical trial results may require us to conduct further testing or we may choose to abandon projects that we had been expecting to complete; clinical trials may be halted due to safety reasons; patient drop out rates in our clinical trial may be higher than anticipated; the FDA, its European equivalent EMEA or the SFDA may not approve our products for commercialization or may require additional clinical trial data prior to approving our products; and/or our future expenses and ability to proceed with a trial may be dependent in part on finding a partner. Moreover, if the outcome of any of these trials is unsuccessful, or even if successful, does not achieve commercially meaningful results, our business could be harmed.
24
If third-party reimbursement is not available or patients cannot otherwise pay for ZADAXIN, DC Bead, ondansetron RapidFilm, or SCV-07, we may not be able to successfully market them.
Significant uncertainty exists as to the reimbursement status of new therapeutic products, such as ZADAXIN, and once commercialized, DC Bead, ondansetron RapidFilm and SCV-07. We cannot assure you that third-party insurance coverage and reimbursement will be available for therapeutic products we might develop. Reimbursement is generally not available for ZADAXIN in China and we cannot assure you that we will be able to maintain existing reimbursements or increase third-party payments for ZADAXIN or obtain third-party payments for DC Bead in China or ondansetron RapidFilm in China or Vietnam. The failure to obtain or maintain third-party reimbursement for our products would harm our business. Further, we cannot assure you that additional limitations will not be imposed in the future in the United States or elsewhere on drug coverage and reimbursement due to proposed health care reforms. In many emerging markets where we have marketing rights to ZADAXIN, but where government resources and per capita income may be so low that our products will be prohibitively expensive, we may not be able to market our products on economically favorable terms, if at all.
Efforts by governmental and third-party payers to contain or reduce health care costs or the announcement of legislative proposals or reforms to implement government controls could cause us to reduce the prices at which we market our drugs, which would reduce our gross margins and may harm our business.
If we do not obtain regulatory approval for thymalfasin, SCV-07, ondansetron RapidFilm or DC Bead for the intended indications that we are evaluating, our revenues will be limited and our operating results may be materially affected.
Our ability to execute on our business strategy is largely dependent on our ability to obtain regulatory approval for the use of thymalfasin in major markets, for the use of SCV-07 in major markets, excluding Russia, for the use of ondansetron RapidFilm in China and Vietnam, and for the use of DC Bead in China. The regulatory approval processes in the United States, Europe and China are demanding and typically require 12 months or more in the United States and China and 18 months or more in Europe from the date of submission of an NDA. We have committed significant resources, including capital and time, to develop these products, and intend to continue to do so, including the initiation and execution of additional clinical trials, with the goal of obtaining such approvals. If we do not obtain these approvals, we will be unable to achieve any revenue from these products in these major markets and our thymalfasin sales in other jurisdictions could decline.
We believed that regulatory approval for DC Bead would be forthcoming in 2009. However, the SFDA required us to conduct a local trial in China to supplement data already obtained from a previous DC Bead study. The study is underway and if the trial results are positive, we expect to receive regulatory approval for DC Bead by the end of 2010. However, we cannot give assurance that such submission will be approved by the regulatory authorities. If additional clinical trials in China are required as part of the regulatory process, the regulatory submission for marketing approval could be delayed for a considerable period of time, and there can be no assurance that the results of clinical trials would support a regulatory submission or that the regulatory authorities would approve the product to be commercialized and sold in China. To the extent that additional information or clinical trials are required by the regulatory authorities, or we do not receive regulatory approval in China, our future sales potential in China could be harmed.
All new drugs, including our products, which have been developed or are under development, are subject to extensive and rigorous regulation by the FDA, SFDA and similar regulatory agencies. These regulations govern, among other things, the development, testing, manufacturing, labeling, storage, pre-market approval, importation, advertising, promotion, sale and distribution of our products. These regulations may change from time to time and new regulations may be adopted.
Satisfaction of government regulations may take several years and the time needed to satisfy them varies substantially based on the type, complexity and novelty of the pharmaceutical product. As a result, government regulation may cause us to delay the introduction of, or prevent us from marketing, our existing or potential
25
products for a considerable period of time and impose costly procedures upon our activities. Even if we obtain regulatory approval for our products, such approval may impose limitations on the indicated uses for which our products may be marketed. Further unsatisfactory data resulting from clinical trials may also adversely affect our ability to market and sell thymalfasin in markets where it is approved for sale.
We rely on third parties who are our sole source suppliers for our clinical trial and commercial products and their inability to deliver products that meet our quality-control standards could delay or harm one or more important areas of our business including our sales, clinical trials or the regulatory approval process.
We rely on third parties, who are subject to regulatory oversight, to supply our clinical and commercial products. For example, Biocompatibles is the sole supplier of DC Bead, Applied Pharma Research S.A. (“APR”) is the sole supplier of ondansetron RapidFilm, and Solvay Peptides S.A. is our sole supplier of SCV-07. Any unanticipated deficiencies in these suppliers, or the suppliers or our raw materials, and/or recall of the manufacturing lots or similar action regarding the pegylated interferon alpha, ribavirin or gemcitabine used in our clinical trials could delay the trials or detract from the integrity of the trial data and its potential use in future regulatory filings. In addition, manufacturing interruptions or failure to comply with regulatory requirements by any of these suppliers could significantly delay clinical development of potential products and reduce third-party or clinical researcher interest and support of proposed trials. These interruptions or failures could also impede commercialization of our products and impair our competitive position.
Further, any deficiencies or shortages in supply of our commercial products would adversely affect our ability to realize our sales plans. For example, the manufacturing of the raw material and the processing to finished product of ZADAXIN is done in few batches in any given three-month period and any manufacturing errors have the potential to require a product recall. During 2006, we experienced failures and lower yields on production runs from our sole supplier of bulk active pharmaceutical ingredient (“API”) product for the manufacture of ZADAXIN for our current markets, including China. During 2009, we have experienced quality-control problems with a component of our ZADAXIN kit. Although we are taking steps to ensure that such problems do not continue, there is no assurance that we will either be successful in doing so with our current supplier or be able to timely and cost-effectively qualify a new supplier for this component. Manufacturing interruptions or failure or delay of product to meet quality assurance specifications could adversely affect shipments and recognition of sales of ZADAXIN in any period and impair our relationships with customers and our competitive position and may increase the cost of material produced.
We are in the process of registering new suppliers for ZADAXIN with regulatory agencies in markets where thymalfasin is approved for sale, including China. This process, quality assurance and other steps could cause delays or interruptions of supply in certain other markets. In some countries, a manufacturing change may require additional regulatory approvals that may delay thymalfasin marketing approvals in new markets. In addition, if sales of ZADAXIN were to increase dramatically, our third-party suppliers may not be able to supply ZADAXIN either quickly enough or at a commercially reasonable cost, which could limit our ability to satisfy increased demand or could adversely affect the ability of these suppliers to provide products for our clinical trials. If any of our suppliers are unable to match our need for supply, either because of product defects, inability to increase supply in the face of increased demand, or maintain financially viable businesses, our ability to effect our sales and protect our brand reputation would be materially impaired, thereby materially and adversely affecting our sales and results of operations.
We rely on third parties for development of our products and the inability of any of these parties to reliably, timely or cost-effectively provide us with their obligated services could materially harm the timing of bringing our products to market and accordingly adversely affect our business.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators, contract laboratories, and collaborative partners in the conduct of clinical trials for our product candidates. If these parties, whom we do not control, do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines or choose not to continue their relationship with us, if the third parties
26
need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical or clinical activities may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize our product candidates.
Commercialization of some of our products depends on collaborations with others. If our collaborators are not successful, or if we are unable to find future collaborators, we may not be able to properly develop and commercialize our products.
We depend in part on our distributors and business partners to develop or promote our drugs, and if they are not successful in their efforts or fail to do so, our business will suffer. For example, Sigma-Tau is responsible for the development and marketing of ZADAXIN in most of Europe. Biocompatibles is providing SciClone with the necessary supporting documents to obtain regulatory approval in China for DC Bead, APR is providing SciClone with the necessary supporting documents to obtain regulatory approval in China and Vietnam for ondansetron RapidFilm. We generally do not have control over the amount and timing of resources that our business partners devote to our collaborative efforts, and some have not always performed as or when expected. If they do not perform their obligations as we expect, particularly obligations regarding clinical trials, our development expenses would increase and the development or sale of our products could be limited or delayed, which could hurt our business and cause our stock price to decline. In addition, our relationships with these companies may not be successful. Disputes may arise with our collaborators, including disputes over ownership rights to intellectual property, know-how or technologies developed with our collaborators. We may not be able to negotiate similar additional arrangements in the future to develop and commercialize ZADAXIN or other products.
If we are unable to manage our key personnel, or are unable to attract and retain additional, highly skilled and experienced personnel, our business will suffer.
We are highly dependent upon our ability to attract and retain qualified personnel because of the specialized, scientific and worldwide nature of our business. Further, we are also dependent on our ability to appropriately staff these personnel in appropriate positions as our business fluctuates. There is intense competition for qualified management, scientific, clinical, regulatory, and sales and marketing personnel in the pharmaceutical industry. There is significant turnover in the industry in China in particular, and we have recently experienced significant turnover in our sales personnel. We may not be able to attract and retain the qualified personnel we need to grow and develop our business globally. Conversely, in the event that we need to reduce the size of a particular aspect of our business, we are also dependent on our ability to make such adjustments while retaining suitably skilled personnel. Further, our efforts to in-license or acquire, develop and commercialize product candidates for China require the addition of clinical and regulatory personnel and the capabilities to expand our sales and marketing operation. In addition, we assign numerous key responsibilities to a limited number of individuals, and we would experience difficulty in finding immediate replacements for any of them were any one of them to choose to leave employment with us. If we were unable to attract and retain qualified personnel as needed or promptly replace those employees who are critical to our product development and commercialization, the development and commercialization of our products would be adversely affected. At this time, we do not maintain “key person” life insurance for any of our personnel.
We may need to obtain additional funding to support our long-term product development and commercialization programs.
We believe our existing cash and investments and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. However, our ability to achieve and sustain operating profitability is dependent on numerous factors including our ability to achieve our goal of increasing sales of ZADAXIN, securing regulatory approval for DC Bead in China, and for ondansetron RapidFilm in China and Vietnam, the execution and successful completion of thymalfasin, SCV-07, and DC Bead clinical trials and securing partnerships for those programs that lead to regulatory approvals in major
27
pharmaceutical markets. We cannot assure you that such funds from operating activities will be sufficient, or that we will attain profitable operations in future periods. In addition, we intend to develop other products and we may need additional funds in the future to support such development and to support future growth and achieve profitability. We may also need additional cash to fund a portion of the proposed late-stage trial evaluating thymalfasin for stage IV melanoma if we are able to partner that program, and depending on the terms of any partnership. If we need to raise additional funds in the future and such funds are not available on reasonable terms, if at all, our commercialization efforts may be impeded, our revenues may be limited and our operating results may suffer.
If we fail to protect our products, technologies and trade secrets, we may not be able to successfully use, manufacture, market or sell our products, or we may fail to advance or maintain our competitive position, and we have limited intellectual property protection in China.
Our success depends significantly on our ability to obtain and maintain meaningful patent protection for our products and technologies and to preserve our trade secrets. Our pending patent applications may not result in the issuance of patents in the future. Our patents or patent applications may not have priority over others’ applications. Our existing patents and additional patents that may be issued, if any, may not provide a competitive advantage to us or may be invalidated or circumvented by our competitors. Others may independently develop similar products or design around patents issued or licensed to us. Patents issued to, or patent applications filed by, other companies could harm our ability to use, manufacture, market or sell our products or maintain our competitive position with respect to our products. Although many of our patents relating to thymalfasin have expired, including composition of matter patents, we have rights to other patents and patent applications relating to thymalfasin and thymalfasin analogues, including method of use patents with respect to the use of thymalfasin for certain indications. Additionally, thymosin alpha 1 (“thymalfasin”), the chemical composition of thymalfasin, has received Orphan Drug designation in the United States for the treatment of stage IIb through stage IV melanoma. If other parties develop generic forms of thymalfasin for other indications, including conducting clinical trials for such indications, our patents and other rights might not be sufficient to prohibit them from marketing and selling such generic forms of thymalfasin. If other parties develop analogues or derivatives of thymalfasin, our patents and other rights might not be sufficient to prohibit them from marketing these analogues or derivatives.
Pharmaceutical products are either not patentable or have only recently become patentable in some of the countries in which we market or may market thymalfasin. We do not have composition patent claims directed to the same form of thymalfasin currently marketed in China, our largest market, although we do have other type of patent claims, pending or issued, directed to other aspects of thymalfasin therapy. Other companies market generic thymosin alpha 1 in China, sometimes in violation of our patent, trademark or other rights which, to date, we have defended by informing physicians and hospitals of the practice. Past enforcement of intellectual property rights in many of these countries, including China in particular, has been limited or non-existent. Future enforcement of patents and proprietary rights in many other countries will likely be problematic or unpredictable. Moreover, the issuance of a patent in one country does not assure the issuance of a similar patent in another country. Claim interpretation and infringement laws vary by nation, so the extent of any patent protection is uncertain and may vary in different jurisdictions.
If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights.
Our commercial success depends in part on our not infringing valid, enforceable patents or proprietary rights of third parties, and not breaching any licenses that may relate to our technologies and products. In addition, we may not be aware of all patents or patent applications that may impact our ability to make, use or sell any of our potential products. For example, U.S. patent applications may be kept confidential for 12 or more months while pending in the Patent and Trademark Office, and patent applications filed in foreign countries are
28
often first published nine months or more after filing. It is possible that we may unintentionally infringe these patents or other patents or proprietary rights of third parties. We may in the future receive notices claiming infringement from third parties as well as invitations to take licenses under third-party patents. Any legal action against us or our collaborative partners claiming damages and seeking to enjoin commercial activities relating to our products and processes affected by third-party rights may require us or our collaborative partners to obtain licenses in order to continue to manufacture or market the affected products and processes. Our efforts to defend against any of these claims, regardless of merit, would require us to devote resources and attention that could have been directed to our operations and growth plans. In addition, these actions may subject us to potential liability for damages. We or our collaborative partners may not prevail in a patent action and any license required under a patent may not be made available on commercially acceptable terms, or at all. Any conflicts resulting from the patent rights of others could significantly reduce the coverage of our patents and limit our ability to obtain meaningful patent protection.
If other companies obtain patents with conflicting claims, we may be required to obtain licenses to those patents or develop or obtain alternative technology to manufacture or market the affected products and processes. We may not be able to obtain any such licenses on acceptable terms or at all. Any failure to obtain such licenses could delay or prevent us from pursuing the development or commercialization of our potential products. Our efforts to defend against any of these claims would require us to devote resources and attention that could have been directed to our operations and growth plans.
We may need to initiate litigation, which could be time-consuming and expensive, to enforce our proprietary rights or to determine the scope and validity of others’ rights. If litigation results, a court may find our patents or those of our licensors invalid or may find that we have infringed on a competitor’s rights. If any of our competitors have filed patent applications in the United States which claim technology we also have invented, the Patent and Trademark Office may require us to participate in expensive interference proceedings to determine who has the right to a patent for the technology. These actions may subject us to potential liability for damages. We or our collaborative partners may not prevail in a patent action and any license required under a patent may not be made available on commercially acceptable terms, or at all.
Substantial sales of our stock or equity in our subsidiaries or the exercise or conversion of options or convertible securities may impact the market price of our common stock.
Our collaborative partner Sigma-Tau and affiliates hold a substantial amount of our stock. The stock is freely tradable and Sigma-Tau is not under any obligation to SciClone which would prevent it from selling some or all of the stock it holds except for applicable U.S. insider trading regulations with respect to possession of material non-public information by Sigma-Tau or its officers and directors.
In May 2009, we filed a Form S-3 Shelf registration with the Securities and Exchange Commission (“SEC”) which was later declared effective by the SEC and will allow us to sell securities in one or more offerings. Future issuances of substantial amounts of our common stock could adversely affect the market price of our common stock. Similarly, if we raise additional funds through the issuance of common stock or securities convertible into or exercisable for common stock or sell equity in a subsidiary, the percentage ownership of our present stockholders of the respective entities will be reduced and the price of our common stock may fall.
Our cash and investments are subject to certain risks which could materially adversely affect our overall financial position.
We invest our cash in accordance with an established internal policy and customarily in instruments which historically have been highly liquid and carried relatively low risk. However, with turmoil in the credit markets, similar types of investments have experienced losses in value or liquidity issues which differ from their historical pattern. For example, we routinely have invested in money market funds with large financial institutions. One or more of these funds could experience losses or liquidity problems and, although to date some of the largest
29
financial institutions who sponsor such funds have offset similar losses, there is no assurance that our financial institutions would either not incur losses or would offset any losses were they to occur. Further, we hold auction rate securities (“ARS”) which are collateralized by student loans with most of such collateral in the aggregate being guaranteed by the U.S. government. These ARS are intended to provide liquidity via an auction process that resets the applicable interest rate at predetermined calendar intervals, usually every month. As of December 31, 2009, we held $1.6 million of auction rate security investments and all of these had experienced failed auctions since February 2008. An auction failure means that the parties wishing to sell securities could not. When an auction fails, the interest rate on such securities resets to a default rate which is usually higher and would require payment by the issuer, affecting its creditworthiness and therefore potentially its ability to redeem such obligations and therefore avoid the higher interest payments.
In November 2008, we accepted an Auction Rate Securities Rights Offer (“Rights Offer”) from UBS AG under which, in return for a general release of claims and the grant of a right to UBS AG to purchase our ARS at any time for full par value, we received the right to require UBS AG to purchase at par value our ARS beginning in 2010 (“the Rights”). The Rights are non-transferable. Upon acceptance, we granted UBS AG the sole discretion and right to sell or dispose of, and/or enter orders in the auction process with respect to the eligible ARS on our behalf-without prior notification to us from UBS AG, as long as we receive a payment at par upon any sale or disposition.
As a result, our ability to liquidate these investments and fully recover the carrying value of these investments in the near term is limited or may not exist. We have written down our ARS investments by $0.2 million as of December 31, 2009. Should our ARS investments continue to lose value, or should any of our other cash investments permanently lose value or have their liquidity impaired, it would have a material and adverse effect on our overall financial position by imperiling our ability to fund our operations and forcing us to seek additional financing sooner than we would otherwise and such financing may not be available on commercially attractive terms.
In addition, financial instruments may subject us to a concentration of credit risk. Substantially all of our cash, and cash equivalents are held by a limited number of financial institutions including all of our term deposits held by financial institutions in Hong Kong and offshore. Such term deposits do not exceed federally insured limits and to date, we have not experienced any losses on our deposits of cash and cash equivalents. However, if any of these instruments permanently lost value or had their liquidity impaired, it would also have a material and adverse effect on our overall financial position by imperiling our ability to fund our operations and forcing us to seek additional financing sooner than we would otherwise and such financing may not be available on commercially attractive terms.
New accounting pronouncements may impact our financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected fluctuations in the timing of the recognition of revenues or expenses and may affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with frequency and may occur in the future and this may lead to changes in our accounting policies in the future.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results. As a result, current and potential stockholders could lose confidence in our financial reporting, which would harm our business and the trading price of our stock.
Effective internal controls are necessary for us to provide reliable financial reports and to protect from fraudulent, illegal or unauthorized transactions. If we cannot provide effective controls and reliable financial reports, our business and operating results could be harmed. Moreover, as a United States-based corporation doing business in China, these controls often need to satisfy the requirements of Chinese law as well as the requirements of United States law which frequently differ in certain aspects. We have in the past discovered, and
30
may in the future discover, areas of our internal controls that need improvement. For example, our management determined that as of December 31, 2008 we had a material weakness in our internal controls related to our failure to expense approximately $540,000 in research and development costs related to changes in the scope of activities performed by our clinical research organization during 2008. Although we have since taken steps to rectify this material weakness, there can be no assurance that we will be successful in this regard. Any failure to implement and maintain controls over our financial reporting, or difficulties encountered in the implementation of improvements in our controls, could cause us to fail to meet our reporting obligations. Any failure to improve our internal controls or to address identified weaknesses in the future, including with respect to our clinical research expenses, if they were to occur, could also cause investors to lose confidence in our reported financial information, which could have a negative impact on the trading price of our stock.
New legislation may impact our financial position or results of operations.
Compliance with changing regulations concerning corporate governance and public disclosure has resulted in and may continue to result in additional expenses. Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, new SEC regulations and The NASDAQ Stock Market rules, are creating uncertainty for companies such as ours and costs are increasing as a result of this uncertainty and other factors. We are committed to maintaining high standards of corporate governance and public disclosure. As a result, we intend to invest all reasonably necessary resources to comply with evolving standards, and this investment has and may continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities.
Currently a majority of our revenue is generated form customers located outside the United States, and a substantial portion of our assets, including employees, are located outside the United States. United States income taxes and foreign withholding taxes have not been provided on undistributed earnings of non-United States subsidiaries, because such earnings are intended to be indefinitely reinvested in the operations of those subsidiaries. President Obama’s administration has recently announced initiatives that would substantially reduce our ability to defer U.S. taxes including: repeal deferral of U.S. taxation of foreign earnings, eliminate utilization or substantially reduce our ability to claim foreign tax credits, and eliminates various tax deductions until foreign earnings are repatriated to the United States. If any of these proposals are constituted into legislation, they could have a negative impact on our financial position and results of operations.
We may be subject to product liability lawsuits, and our insurance may be inadequate to cover damages.
Clinical trials of any of our current and potential products or the actual commercial sales of our product may expose us to liability claims from the use of these products. We currently carry product liability insurance. However, we cannot be certain that we will be able to maintain insurance on acceptable terms, if at all, for clinical and commercial activities or that the insurance would be sufficient to cover any potential product liability claim or recall. If we fail to have sufficient coverage, our business, results of operations and cash flows could be adversely affected.
If we are unable to comply with environmental and other laws and regulations, our business may be harmed.
We are subject to various federal, state and local laws, regulations and recommendations relating to the use, manufacture, storage, handling and disposal of hazardous materials and waste products (including radioactive compounds and infectious disease agents), as well as safe working conditions, laboratory and manufacturing practices and the experimental use of animals. The extent of government regulation that might result from future legislation or administrative action in these areas cannot be accurately predicted.
31
We do not currently maintain hazardous materials at our facilities. While we outsource our research and development programs involving the controlled use of biohazardous materials, if in the future we conduct these programs ourselves, we might be required to incur significant cost to comply with environmental laws and regulations. Further, in the event of an accident, we would be liable for any damages that result, and the liability could exceed our resources.
We are at risk of securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced greater than average stock price volatility in recent years. If we faced such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us, which may be beneficial to our stockholders, more difficult.
Certain anti-takeover provisions of Delaware law and our charter documents as currently in effect may make a change in control of our company more difficult, even if a change in control would be beneficial to our stockholders. Our charter documents contain certain anti-takeover provisions, including provisions in our certificate of incorporation providing that stockholders may not cumulate votes, stockholders’ meetings may be called by stockholders only if they hold 25% or more of our common stock and provisions in our bylaws providing that the stockholders may not take action by written consent. Additionally, our board of directors has the authority to issue 10 million shares of preferred stock and to determine the terms of those shares of stock without any further action by the stockholders. The rights of holders of our common stock are subject to the rights of the holders of any preferred stock that may be issued. The issuance of preferred stock could make it more difficult for a third-party to acquire a majority of our outstanding voting stock. Delaware law also prohibits corporations from engaging in a business combination with any holders of 15% or more of their capital stock until the holder has held the stock for three years unless, among other possibilities, the board of directors approves the transaction. Our board of directors may use these provisions to prevent changes in the management and control of our company. Also, on December 18, 2006, our Board of Directors declared a dividend distribution of one Preferred Stock Purchase Right (collectively, the “Rights”) for each outstanding share of our Common Stock, each Right which entitles the registered holder to purchase from the Company one one-thousandth of a share of the Company’s Series D Preferred Stock, $0.001 par value, at a price of $25.00 pursuant to a Rights Agreement dated as of December 19, 2006, between the Company and Mellon Investor Services LLC. The Rights have certain anti-takeover effects. Under certain circumstances the Rights could cause substantial dilution to a person or group who attempts to acquire the Company on terms not approved by our Board of Directors. Although the Rights should not interfere with an acquisition of the Company approved by the Board, the Rights may have the effect of delaying and perhaps improving the terms of an acquisition for our stockholders, or deterring an acquisition of the Company. Also, under applicable Delaware law, our board of directors may adopt additional anti-takeover measures in the future.
Our business and operations are subject to the risks of being based in particular locations known for earthquakes, other natural catastrophic disasters and service interruptions.
Our corporate headquarters are located in the Silicon Valley area of Northern California, a region known for seismic activity. Although we maintain a disaster recovery policy that includes storage of important corporate data in a different geographic region of the United States, all of our significant corporate data is stored in our headquarters facility and accordingly, a significant natural disaster, such as an earthquake, could have a material adverse impact on our business, operating results, and financial condition. Most of our sales are into China for which we maintain a sole warehouse for finished goods in Hong Kong, which can experience severe typhoon storms. Although our distributors in China maintain several months supply of our product, were our warehouse
32
capability to be interrupted, either through a natural disaster such as flooding or through a service interruption, such as a lack of electricity to power required air conditioning, our ability to timely deliver finished product to China could be adversely affected which in turn would materially adversely affect our sales and ensuing operating results.
We May Be Affected by Climate Change and Market or Regulatory Responses to Climate Change
Climate change, including the impact of global warming, could have a material adverse effect on our results of operations, financial condition, and liquidity if it were to disrupt the demand, supply or delivery of product, management of our business, or result in cost increases as a result of government regulation.
| Item 1B. | Unresolved Staff Comments |
None.
We currently lease approximately 22,000 square feet of office space for our corporate headquarters in Foster City, California and lease approximately 12,000 square feet of office space in Beijing, Hong Kong, Shanghai, Singapore, Guangzhou, Vietnam and Sao Paulo. We believe that our existing facilities will be adequate for our current needs and that additional space will be available as needed.
None.
| Item 4. | Removed and Reserved |
33
PART II
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock trades on The NASDAQ Global Market of the NASDAQ Stock Market under the symbol “SCLN.”
The following table sets forth the high and low sales prices per share for the quarterly periods indicated, as reported by The NASDAQ Stock Market. The quotations shown represent inter-dealer prices without adjustment for retail markups, markdowns, or commissions, and may not necessarily reflect actual transactions.
| | | | | | |
| | | Price Range
Common Stock |
| | | High | | Low |
2009 | | | | | | |
4th quarter | | $ | 4.41 | | $ | 2.09 |
3rd quarter | | | 5.33 | | | 2.50 |
2nd quarter | | | 2.77 | | | 1.21 |
1st quarter | | | 1.41 | | | 0.75 |
2008 | | | | | | |
4th quarter | | $ | 1.34 | | $ | 0.63 |
3rd quarter | | | 1.79 | | | 1.11 |
2nd quarter | | | 2.05 | | | 1.53 |
1st quarter | | | 2.19 | | | 1.72 |
Stockholders
As of March 12, 2010, there were approximately 356 holders of record of our common stock and 47,324,232 shares of common stock issued and outstanding.
Dividends
We have not paid any dividends on our common stock during the fiscal years ended December 31, 2009, 2008, and 2007 and currently intend to retain any future earnings for use in our business.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by Item 201(d) of Regulation S-K is incorporated by reference from the section entitled “Securities Authorized for Issuance under Equity Compensation Plans” in Part III, Item 12 of this Form 10-K.
34
Performance Graph
The following line graph compares the annual percentage change in (i) the cumulative total stockholder return on the Company’s Common Stock since December 31, 2004, with (ii) the cumulative total return on (a) The NASDAQ Composite Index and (b) the NASDAQ Biotechnology Index. The comparison assumes (i) an investment of $100 on December 31, 2004 in each of the foregoing indices and (ii) reinvestment of dividends, if any. The stock price performance shown on the graph below is not necessarily indicative of future stock price performance.
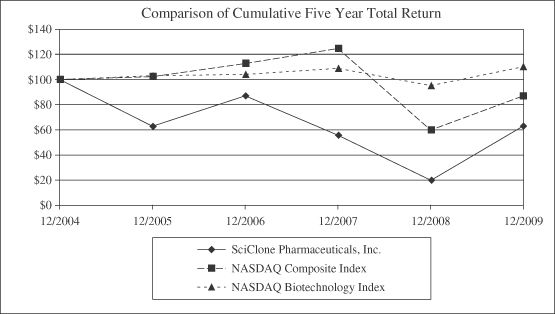
35
| Item 6. | Selected Financial Data |
This section presents selected historical financial data for each of the last five fiscal years and is qualified by reference to and should be read in conjunction with the consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K. The selected balance sheets data at December 31, 2009 and 2008 and the selected statement of operations data for each year ended December 31, 2009, 2008, and 2007 have been derived from our audited financial statements that are included elsewhere in this report. The selected balance sheets data at December 31, 2007, 2006, and 2005 and the statements of operations for each year ended December 31, 2006 and 2005 have been derived from our audited financial statements not included in this report. Historical results are not necessarily indicative of the results to be expected in the future.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | (in thousands, except per share data) | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
Statement of Operations data: | | | | | | | | | | | | | | | | | | | | |
Product sales | | $ | 72,411 | | | $ | 54,108 | | | $ | 37,038 | | | $ | 32,433 | | | $ | 27,842 | |
Contract revenue | | | — | | | | 5 | | | | 20 | | | | 229 | | | | 492 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenues | | | 72,411 | | | | 54,113 | | | | 37,058 | | | | 32,662 | | | | 28,334 | |
Cost of product sales | | | 11,960 | | | | 10,299 | | | | 7,088 | | | | 7,235 | | | | 5,179 | |
| | | | | | | | | | | | | | | | | | | | |
Gross margin | | | 60,451 | | | | 43,814 | | | | 29,970 | | | | 25,427 | | | | 23,155 | |
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 16,531 | | | | 22,857 | | | | 16,552 | | | | 13,399 | | | | 13,786 | |
Sales and marketing | | | 18,805 | | | | 16,853 | | | | 13,575 | | | | 11,223 | | | | 9,933 | |
General and administrative | | | 12,521 | | | | 12,538 | | | | 11,139 | | | | 9,729 | | | | 8,077 | |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 47,857 | | | | 52,248 | | | | 41,266 | | | | 34,351 | | | | 31,796 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) from operations | | | 12,594 | | | | (8,434 | ) | | | (11,296 | ) | | | (8,924 | ) | | | (8,641 | ) |
Interest and investment income | | | 153 | | | | 608 | | | | 1,629 | | | | 1,764 | | | | 1,273 | |
Interest expense | | | (179 | ) | | | (31 | ) | | | (20 | ) | | | (94 | ) | | | (345 | ) |
Other income (expense), net | | | 18 | | | | (16 | ) | | | 40 | | | | 7,981 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before provision for income tax | | | 12,586 | | | | (7,873 | ) | | | (9,647 | ) | | | 727 | | | | (7,713 | ) |
Provision for income tax | | | 641 | | | | 475 | | | | 301 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | 11,945 | | | $ | (8,348 | ) | | $ | (9,948 | ) | | $ | 727 | | | $ | (7,713 | ) |
| | | | | | | | | | | | | | | | | | | | |
Earnings (loss) per share: | | | | | | | | | | | | | | | | | | | | |
Basic net income (loss) per share | | $ | 0.26 | | | $ | (0.18 | ) | | $ | (0.22 | ) | | $ | 0.02 | | | $ | (0.17 | ) |
Diluted net income (loss) per share | | $ | 0.25 | | | $ | (0.18 | ) | | $ | (0.22 | ) | | $ | 0.02 | | | $ | (0.17 | ) |
| | | | | |
Weighted average shares used in computing: | | | | | | | | | | | | | | | | | | | | |
Basic net income (loss) per share | | | 46,574 | | | | 46,212 | | | | 46,100 | | | | 45,901 | | | | 45,329 | |
Diluted net income (loss) per share | | | 47,404 | | | | 46,212 | | | | 46,100 | | | | 46,072 | | | | 45,329 | |
| | | | | | | | | | | | | | | |
| | | As of December 31, |
| | 2009 | | 2008 | | 2007 | | 2006 | | 2005 |
| | (in thousands) |
Balance Sheet data: | | | | | | | | | | | | | | | |
Cash, cash equivalents, and short-term investments | | $ | 31,333 | | $ | 27,773 | | $ | 35,209 | | $ | 41,894 | | $ | 41,564 |
Accounts receivable | | | 21,394 | | | 11,927 | | | 12,650 | | | 13,277 | | | 9,701 |
Inventories | | | 10,149 | | | 6,056 | | | 5,579 | | | 3,232 | | | 3,272 |
Long-term investments | | | — | | | 1,485 | | | — | | | — | | | — |
Restricted investments | | | 486 | | | 457 | | | 72 | | | 698 | | | 692 |
Working capital | | | 55,937 | | | 37,793 | | | 45,400 | | | 53,079 | | | 48,735 |
Total assets | | | 66,900 | | | 51,905 | | | 58,659 | | | 62,584 | | | 59,515 |
Long-term liabilities | | | 979 | | | 779 | | | 341 | | | 68 | | | 68 |
Total stockholders’ equity | | | 57,393 | | | 40,903 | | | 47,259 | | | 54,634 | | | 51,063 |
Convertible notes payable | | | — | | | — | | | — | | | — | �� | | 1,600 |
36
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the “Selected Financial Data” and our consolidated financial statements and related notes thereto included elsewhere in this Annual Report on Form 10-K. This Management’s Discussion and Analysis of Financial Condition and Results of Operations and other parts of this Annual Report on Form 10-K contain forward-looking statements which involve risks and uncertainties. See “Note Regarding Forward-Looking Statements” and “Risk Factors” contained in this Annual Report on Form 10-K.
Overview
SciClone Pharmaceuticals (NASDAQ: SCLN) is a profit-focused, global specialty pharmaceutical company with a substantial international business, based primarily in the People’s Republic of China (“China”), and with a product portfolio of novel therapies for cancer and infectious diseases. Our strategy is focused on international sales growth, a cost-containing clinical development strategy, and overall expense management. ZADAXIN®, our brand of thymalfasin or thymosin alpha 1 and our primary product, is sold in over 30 countries for the treatment of the hepatitis B virus (“HBV”) and the hepatitis C virus (“HCV”), certain cancers and as a vaccine adjuvant. We believe our current cash and investment position and the gross profit from our product sales provide the financial resources to execute on our strategy for continued growth in our international business and for development of our pipeline of late-stage product candidates.
We plan to expand our commercial operations, currently located primarily in China, with the goal of becoming a significant pharmaceutical company in China’s rapidly growing pharmaceutical market. A key part of our strategy is to leverage our decade of experience in China and to grow our international business by adding commercial stage or near term commercial stage products to our portfolio. We believe we are well-positioned to in-license additional therapeutics for our international business with a focus on China, in part because of our opportunity to commercialize these products utilizing our well established sales and marketing organization in China. Furthermore, our international growth strategy may include additional partnerships and merger and acquisition transactions for products.
We have successfully in-licensed two products that are part of this international commercial growth strategy, DC BeadTM and ondansetron RapidFilmTM. Our DC Bead product candidate is a novel treatment for advanced liver cancer which is currently approved in 40 countries worldwide, including Europe and the U.S. We have commercialization rights for this product in China. We commenced a small local registration trial in the first quarter of 2010, which is expected to enroll approximately 40 advanced liver cancer patients at three SFDA-certified liver cancer treatment centers. The primary endpoint is safety and the secondary endpoint is efficacy, as measured by tumor response. If the trial results are positive, we believe we may receive regulatory approval for DC Bead by the end of 2010.
Our second in-licensed product candidate as part of this international commercial growth strategy, is ondansetron RapidFilm. We have commercialization rights for this product candidate in China and Vietnam. Ondansetron RapidFilm, currently being evaluated for regulatory approval in Europe, is an oral thin film formulation of ondansetron, a serotonin 5-HT3 receptor antagonist commonly used to treat and prevent nausea and vomiting caused by chemotherapy, radiotherapy and surgery. We plan to file for product registration with the State Food and Drug Administration of China (“SFDA”) in 2010. We believe we may receive regulatory approval in China in 2011.
Our clinical development strategy is focused on our portfolio of novel product candidates for cancer and infectious diseases and includes SCV-07 and thymalfasin. SCV-07 is being developed for the delay of onset of severe oral mucositis in patients receiving chemoradiation therapy for the treatment of cancers of the head and neck and for the treatment of HCV. Thymalfasin is being developed in an ongoing clinical trial for enhancement of influenza H1N1 vaccination in immuno-compromised patient populations, and for stage IV melanoma.
37
As part of this clinical development strategy, we are moving forward our clinical development program for SCV-07, a small molecule synthetic peptide with immunomodulating properties, which is being studied in two indications: oral mucositis and HCV. We are currently conducting a phase 2 multicenter, randomized, double-blind, placebo-controlled, dose ranging study designed to assess the safety and efficacy of SCV-07 for the delay of onset of severe oral mucositis in subjects receiving chemoradiation therapy for the treatment of cancers of the head and neck. The primary efficacy endpoint is delay of onset and severity of severe oral mucositis. We completed enrollment of this trial in the second half of 2009 and expect to report results in the first quarter of 2010.
The second component of the SCV-07 clinical development project is for the treatment of HCV. This multicenter, multidose, open-label study is designed to evaluate the safety and immunomodulatory effects of SCV-07 as a monotherapy or in combination with ribavirin in non–cirrhotic patients with genotype 1 chronic HCV who have relapsed after at least 44 weeks of treatment with pegylated interferon and ribavirin. We expect to report results by the end of 2010. During our previous phase 2a clinical trial of SCV-07 designed to evaluate the effect of SCV-07 on HCV viral load, as well as on other measures of immune response, SCV-07 demonstrated activity in some treated patients in the higher dosage groups, and the decrease in viral load in these patients was accompanied by an increase in a biomarker which is usually correlated with an immunological response against HCV. Additionally, SCV-07 was shown to be generally safe and well-tolerated with no dose limiting toxicities or serious adverse events reported.
We continue to seek development opportunities with ZADAXIN (“thymalfasin”) focused on vaccine enhancement and melanoma. In the fourth quarter of 2009, our partner Sigma-Tau Finanziaria, S.p.A. (“Sigma-Tau”) initiated a study in Italy to evaluate ZADAXIN’s (thymalfasin) ability to enhance immune response to the MF59 adjuvanted H1N1 influenza monovalent vaccine, Focetria™ from Novartis. The study, being conducted in hemodialysis patients reached full enrollment after two days, and in early 2010, we reported promising preliminary results from this study regarding ZADAXIN’s ability to enhance response to H1N1 vaccine. ZADAXIN is currently approved in Italy and more than 10 other countries as an enhancer for the influenza vaccine in immune-compromised patients. In addition, we continue to seek a partner for a phase 3 trial to evaluate thymalfasin for use as a therapy for stage IV melanoma, but we do not intend to proceed with the trial unless we reach agreement with a partner.
SciClone’s clinical development strategy is to focus on driving cost-efficient phase 1 and 2 development of promising compounds while seeking development partners for costly phase 3 trials, allowing us to achieve the potential upside of our portfolio of drug candidates.
We believe our cash and investments as of December 31, 2009 and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. Our results may fluctuate from quarter to quarter and we may report quarterly losses in the future.
Critical Accounting Policies and Significant Judgments and Estimates
General
We have identified the policies below as critical to our business operations and the understanding of our results of operations. The impact and any associated risks related to these policies on our business operations is discussed throughout “Management’s Discussion and Analysis of Financial Condition and Results of Operations” where such policies affect our reported and expected financial results. For a detailed discussion on the application of these and other accounting policies, see Note 1 in the “Notes to our Consolidated Financial Statements” in Part II, Item 8 of this Annual Report on Form 10-K. Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United Sates, which requires us to make estimates and assumptions that affect the reported amount of assets and liabilities, disclosure of contingent assets and liabilities at the date of our financial statements, and the reported amounts of revenue and
38
expenses during the reporting period. On an on-going basis, we evaluate the relevance of our estimates and judgments. We base our estimates on historical experience and on various other market specific assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. There can be no assurance that actual results will not differ from those estimates.
Revenue Recognition
We recognize revenue from product sales at the time of delivery. There are no significant customer acceptance requirements or post-shipment obligations on our part, except for sales to a new market where acceptance requirements have to be met. Sales to importing agents or distributors are recognized at time of shipment when title to the product is transferred to them. Importing agents or distributors do not have contractual rights of return except under limited terms regarding product quality. However, we are expected to replace products that have expired or are deemed to be damaged or defective when delivered. We estimate expected returns primarily on historical patterns. Historically, we have had no product returns of damaged, defective or expired product. As such, no amount was accrued for product returns as of December 31, 2009 and 2008 in the respective consolidated balance sheets. Payments by the importing agents and distributors are not contingent upon sale to the end user by the importing agents or distributors. Amounts invoiced relating to arrangements where collectibility is uncertain and revenue cannot be recognized are reflected on our balance sheet as deferred revenue and recognized as the applicable revenue recognition criteria are satisfied.
Cash Equivalents and Investments
We record investments at fair value, as determined by available information on the consolidated balance sheet date. An investment is considered impaired if the fair value of the investment is less than its cost. If, after consideration of all available evidence to evaluate the realizable value of its investment, impairment is determined to be other-than-temporary, then an impairment loss is recognized in the Consolidated Statement of Operations.
Fair Value of Financial Instruments
We record our financial assets including cash equivalents and accrued liabilities at cost, which approximates fair value due to their short-term nature. Investments in marketable securities and our put option are recorded at their estimated fair value. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of input are:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Where quoted prices are available in an active market, we determine fair value based upon quoted market prices, and classify these values in level 1 of the valuation hierarchy. If quoted market prices are not available, fair values are based upon observable inputs such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities and are classified in level 2 of the valuation hierarchy.
39
When quoted prices and observable inputs are unavailable, fair values are based on internally developed cash flow models and are classified in level 3 of the valuation hierarchy. The internally developed cash flow models primarily use, as inputs, estimates for interest rates and discount rates including yields of comparable traded instruments adjusted for illiquidity and other risk factors, amount of cash flows and expected holding periods of the assets. These inputs reflect our assumptions about the assumptions market participants would use in pricing the assets including assumptions about risk developed based on the best information available in the circumstances. Our assessment of the significance of a particular input to the fair value measurements requires judgment, and may affect the valuation of the assets being measured and their placement within the fair value hierarchy.
Accounts Receivable
Accounts receivable are recorded net of the allowance for doubtful accounts. We maintain an allowance for doubtful accounts for estimated losses resulting from the inability of customers to make required payments, when appropriate. We record our allowance for doubtful accounts based on our assessment of various factors. When estimating the need for an allowance for doubtful accounts, we consider historical payment patterns of our customers, the circumstances of each individual customer and their geographic region including a review of the local economic environment, the age of the accounts receivable balances, credit quality of our customers, and other factors that may affect customers’ ability to pay. At December 31, 2009, no allowance for doubtful accounts was considered necessary.
Inventories
Our inventories are stated at the lower of cost or market, with cost determined on a first-in, first-out basis and include amounts related to materials, labor and overhead. In assessing the ultimate realization of inventories, we are required to make judgments as to future demand requirements and compare that with the current inventory levels. If our current assumptions about future production or inventory levels and demand were to change or if actual market conditions are less favorable than those projected by management, inventory write-downs may be required which could negatively impact our gross margins and results of operations. If obsolete items are observed and there are no alternate uses for the inventory, we will record a write-down to net realizable value in the period that the impairment is first recognized.
Accrued Expenses
We make estimates of our accrued expenses as of each balance sheet date in our consolidated financial statements based on facts and circumstances known to us. Examples of estimated accrued expenses include fees paid to contract research organizations and investigative sites in connection with clinical trials, fees paid to contract manufacturers in connection with the production of clinical trial materials, and professional services. We periodically confirm the accuracy of our estimates with selected service providers and make adjustments, if necessary. Expenses related to clinical trials generally are accrued based on estimates of work performed or the level of patient enrollment and activities according to the protocols and agreements. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under certain contracts depend on factors such as the achievement of certain events, the successful enrollment of patients, and the completion of portions of the clinical trial or similar conditions. The objective of our accrual policy is to match the recording of expenses to the actual services received and efforts expended. We monitor planned protocols, work performed, patient enrollment levels and related activities to the extent possible and adjust estimates accordingly.
Stock-Based Compensation
Stock-based compensation is estimated at the date of grant based on the fair value of the award using the Black-Scholes option-pricing model and is recognized as expense ratably (as the awards vest) over the requisite service period, which is generally one or four years. We value certain target-stock-price-based options using the
40
Monte Carlo simulation options pricing model and recognize expense over the service periods for each of the vesting portions of these awards which are six or eight years. The option-pricing models require the use of certain subjective assumptions, including the expected volatility of the market price of the underlying stock and the expected term of the award. Expected volatility is based on the historical volatility of our stock. Expected term is derived from historical data on employee exercises and terminations, or the contractual life of the award for target-stock-price-based options. We review our valuation assumptions at each grant date, and, as a result, valuation assumptions used to value stock-based compensation of awards granted in future periods may change.
Income Taxes
Income taxes are accounted for under the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates that will be in effect when these differences reverse. We provide a valuation allowance against net deferred tax assets if, based upon the available evidence, it is more-likely-than-not that the deferred tax assets will not be realized. Realization of our deferred tax assets is dependent upon the generation of future taxable income, the timing and amount of which are uncertain. Accordingly, our deferred tax assets have been fully offset by a valuation allowance. The tax years 1996-2009 remain open to examination by the major taxing jurisdictions to which we are subject.
Results of Operations
Product sales were $72.4 million, $54.1 million, and $37.0 million for the years ended December 31, 2009, 2008, and 2007. Our revenue growth was attributable to a higher volume of ZADAXIN due to further market penetration in China and modest price increases from the 2007 to 2009 periods. For the year ended December 31, 2009, we believe there was also increased demand for ZADAXIN as a result of the H1N1 flu virus. Sales to customers in China are denominated in U.S. dollars and accounted for approximately 96%, 94%, and 92% of product sales for the years ended December 31, 2009, 2008 and 2007, respectively. In 2009, Shanghai Lingyun and China National Pharmaceutical Foreign Trade Corporation accounted for 66% and 27% of our sales, respectively, and two others importer accounted for 3% of our sales. In 2008, Shanghai Lingyun and China National Pharmaceutical Foreign Trade Corporation accounted for 68% and 22% of our sales, respectively, and one other importer accounted for 4% of our sales. In 2007, Shanghai Lingyun and China National Pharmaceutical Foreign Trade Corporation accounted for 63% and 21% of our sales, respectively, and one other importer accounted for 8% of our sales. No other customers accounted for more than 10% of sales in those periods. As of December 31, 2009, approximately $20.8 million or 97% of our accounts receivable were attributable to four customers in China. We perform on-going credit evaluations of our customers’ financial condition, and generally do not require collateral from our customers.
Gross margin was 83%, 81%, and 81% for the years ended December 31, 2009, 2008, and 2007, respectively. The increase in gross margin for the year ended December 31, 2009, compared to the years ended December 31, 2008 and 2007, was attributable to higher sales of ZADAXIN resulting in volume discounts and lower per vial production costs, and was also attributable to lower royalty expense in the 2009 period. In addition, we recorded a charge of $630,000 associated with our finished goods inventory related to vials made obsolete due to labeling requirements arising from our renewed import license in 2008. No significant charges were recorded by us associated with our product inventory during the year ended December 31, 2009.
We estimate that our future cost of product sales will increase for the year ending December 31, 2010 in relation to an expected increase in the level of ZADAXIN sales. We expect cost of product sales and hence gross margin to vary from year to year, depending upon the level of ZADAXIN sales, the absorption of product-related fixed costs, and any charges associated with excess or expiring finished product inventory.
Research and development (“R&D”) expenses were $16.5 million, $22.9 million, and $16.6 million for the years ended December 31, 2009, 2008, and 2007, respectively. The decrease of $6.4 million, or 28%, in R&D expenses for the year ended December 31, 2009, compared to the corresponding period of 2008, was primarily
41
related to the discontinuation of our RP101 clinical trial that occurred in the year ended December 31, 2009, offset partially by increased expense related to our SCV-07 phase 2 clinical trials for the treatment of oral mucositis and HCV. Further, we made a $1.3 million milestone payment upon first patient dosing in the RP101 phase 2 clinical trial that occurred during the year ended December 31, 2008, and there was no corresponding payment in 2009. These decreases were partially offset by a $1.0 million license fee payment related to our licensing agreement with APR Applied Pharma Research S.A. (“APR”) that occurred during the year ended December 31, 2009.
The increase of $6.3 million, or 38%, in R&D expenses for the year ended December 31, 2008, compared to the year ended December 31, 2007, related to our phase 2 clinical trials for RP101 and SCV-07, and to the development planning for a late-stage clinical trial for melanoma. The increase included approximately $2.0 million for clinical trial management, statistical services and investigator expenses; an increase of approximately $2.7 million for drug development and manufacturing costs related to the scale up for RP101 and purchase of gemcitabine for the RP101 clinical trial, and manufacturing of SCV-07 and ribavirin for the SCV-07 clinical trial; a $1.3 million milestone payment upon first patient dosing in the RP101 phase 2 clinical trial; an increase in compensation and benefits expense and recruiting fees due to an increase in the number of research and development personnel; and an increase in consulting and travel expenses. These increases were partially offset by a decrease of approximately $2.2 million of acquisition and legal costs related to the acquisition of exclusive rights for the United States and Canada to develop and commercialize RP101 recorded during the year ended December 31, 2007.
The major components of R&D expenses include salaries and other personnel-related expenses, including associated stock-based compensation, facility-related expenses, depreciation of facilities and equipment, license-related fees, services performed by clinical research organizations and research institutions and other outside service providers, and the sharing of certain costs for the development of ZADAXIN by our partner, Sigma-Tau.
The initiation and continuation of our current clinical development programs has had and is expected to continue to have a significant effect on our research and development expenses. Although it is not possible to determine the total cost expected to be incurred in any particular year for each clinical trial due to the uncertain nature of the clinical trial process, we expect that our future costs for the year ending December 31, 2010 relating to research and development of our programs will decrease primarily due to the discontinuation of our RP101 phase 2 clinical trial which we announced in October 2009, and the conclusion of our SCV-07 phase 2 trial for the treatment of oral mucositis for which we expect to report results in the first quarter of 2010, partially offset by our ongoing phase 2 clinical trial for SCV-07 for the treatment of HCV. The actual costs incurred in future periods will vary depending in particular upon timeline and design for further clinical trials and final decisions regarding the timing and expense sharing arrangements for these trials. An expansion or significant extension of our clinical development programs may require us to seek additional capital resources. We are evaluating opportunities to acquire or in-license the marketing rights to proprietary products in China, which may result in increased research and development expenses due to license fee payments or other expenses related to in-licensing a new product in the future.
Sales and marketing expenses were $18.8 million, $16.9 million, and $13.6 million for the years ended December 31, 2009, 2008, and 2007, respectively. The increase in sales and marketing expenses of $1.9 million or 12% for the year ended December 31, 2009, compared to the year ended December 31, 2008, was primarily due to an increase of $1.2 million in conference and seminar expenses, an increase of $0.5 million in compensation and benefits expense as a result of increased bonus and sales incentives and an increase in the number of sales personnel, and an increase of $0.3 million in rent and business tax expense, all related to our expanding sales efforts. The increase in sales and marketing expenses of $3.3 million or 24% for the year ended December 31, 2008 compared to the year ended December 31, 2007, was primarily due to an increase of $1.4 million in conferences and medical education seminar expenses, an increase of $0.8 million in salesforce incentives, an increase of $0.6 million in compensation and benefits expense due to an increase in the number of
42
sales personnel, and an increase of $0.4 million in travel and entertainment expenses, all related to our expanding sales and marketing efforts. We expect sales and marketing expenses for the year ending December 31, 2010 to be higher than those incurred for the year ended December 31, 2009 due to increased sales efforts, primarily in China.
General and administrative expenses were $12.5 million, $12.5 million, and $11.1 million for the years ended December 31, 2009, 2008, and 2007, respectively. During the year ended December 31, 2009, general and administrative expenses decreased $17,000 or 0% compared to the year ended December 31, 2008. Although we incurred increased expenses related to business development efforts in China, the increases were offset by decreased accounting and legal fees. General and administrative expenses increased $1.4 million or 13% for the year December 31, 2008, compared to the year ended December 31, 2007, primarily due to an increase in severance costs of approximately $0.4 million related to the departure of a former officer, an increase in accounting and professional fees of approximately $0.4 million, an increase in legal fees of approximately $0.5 million, an increase in employment recruiting costs of approximately $0.2 million, and a net increase in compensation and benefits related expenses due to an increase in headcount for the year ended December 31, 2008, offset partially by a decrease in stock based compensation expense. We expect general and administrative expenses for the year ending December 31, 2010 will be comparable to general and administrative expenses for the year ended December 31, 2009.
Interest and investment income was approximately $0.2 million, $0.6 million, and $1.6 million for the years ended December 31, 2009, 2008, and 2007, respectively. The decrease for the year ended December 31, 2009 compared to the years ended December 31, 2008 and 2007, resulted from lower cash and investments balances and lower returns due to a declining interest rate environment in the 2009 and 2008 periods.
Interest and investment expense was $0.2 million, $31,000, and $20,000 for the years ended December 31, 2009, 2008, and 2007, respectively. The increase in interest expense for the year ended December 31, 2009, compared to the years ended December 31, 2008 and 2007, resulted from the amortization of loan origination fees related to our Silicon Valley Bank line of credit that was established in November 2008.
Provision for income tax of $0.6 million, $0.5 million and $0.3 million for the years ended December 31, 2009, 2008 and 2007, respectively, relates to our foreign operations in China. The increases resulted from an increase in operating activities in China and an increase in the statutory tax rate in China from 18% for the year ended December 31, 2008 to 20% for the year ended December 31, 2009. We expect the provision for income tax to increase for the year ending December 31, 2010, compared to the year ended December 31, 2009, due to increased operating activities in China and an increased statutory tax rate in China of 22% in 2010.
Income Taxes
We have not recorded any U.S. federal or state income tax expense for the years ended December 31, 2009, 2008, and 2007. Undistributed earnings of our foreign subsidiaries amounted to approximately $2.2 million at December 31, 2009. These earnings are considered to be permanently reinvested and accordingly, no deferred U.S. income taxes have been provided thereon.
At December 31, 2009, we had net operating loss carryforwards for federal income tax purposes of approximately $130.6 million that expire in the years 2010 through 2029. The difference between the cumulative losses for financial reporting purposes and federal income tax purposes is primarily attributable to losses incurred by our foreign subsidiaries. At December 31, 2009, we had federal tax credit carryforwards of approximately $9.5 million that expire in the years 2010 through 2029.
Because of the “change in ownership” provisions of the Internal Revenue Code, a portion of our net operating loss carryforwards and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods. As a result of the annual limitation, a portion of these carryforwards may expire before ultimately becoming available to reduce future income tax liabilities.
43
Liquidity and Capital Resources
Days’ sales outstanding in accounts receivable, using the average receivables method, were 101, 103, and 137 days in 2009, 2008, and 2007, respectively. The majority of our sales are to customers in China where our accounts receivable collections have standard credit terms ranging from 90 to 180 days. During the fourth quarter of 2008, our largest customer in China agreed to payment terms of 90 days instead of 180 days in order to procure from us increased volume of ZADAXIN and a volume discount. This change resulted in a reduction in our days’ sales outstanding in 2009 and 2008 compared to 2007. We believe that the 90 day credit terms with our largest customer in China will continue into the foreseeable future.
At December 31, 2009 and 2008, we had $31.8 million and $29.7 million, respectively, in cash and investments, including $1.6 million and $1.5 million, respectively, of ARS recorded at fair value as trading securities. The ARS are investments with contractual maturities generally between 20-32 years whose underlying assets are student loans that are substantially backed by the federal government with interest rates resetting approximately every 30 days through an auction process. At the end of each reset period, investors may sell or continue to hold the securities at par. Disruptions in the credit markets have adversely affected the market for ARS. However, we do not believe that the underlying securities or collateral have been permanently affected and we continue to earn interest on our ARS.
In November 2008, we accepted an Auction Rate Securities Rights Offer (the “Rights Offer”) from UBS AG under which, in return for a general release of claims and the grant of a right to UBS AG to purchase our ARS at any time for full par value, we received the right to require UBS AG to purchase at par value our ARS beginning in June 2010 (the “Rights”). The Rights are non-transferable. Upon acceptance, we granted UBS AG the sole discretion and right to sell or dispose of, and/or enter orders in the auction process with respect to the eligible ARS on our behalf without prior notification to us from UBS AG, as long as we receive a payment at par upon any sale or disposition. We expect to sell our ARS under the Rights. However, if the Rights are not exercised before July 2, 2012, they will expire and UBS will have no further rights or obligation to buy our ARS.
Net cash used in operating activities was $0.5 million, $4.9 million, and $7.0 million, for the years ended December 31, 2009, 2008, and 2007, respectively. Net cash used in operating activities for the years ended December 31, 2009, 2008 and 2007 primarily reflected the net income (loss) for the period, adjusted for non-cash items such as stock-based compensation expense, depreciation and amortization expense and changes in operating assets and liabilities. For the year ended December 31, 2009, such changes included a $9.5 million increase in accounts receivable, compared to the year ended December 31, 2008 related to increased sales and a reduction in payment terms that occurred at the end of fiscal 2008 with our largest customer in China from 180 days to 90 days. In addition, inventory levels increased by $4.1 million for the year ended December 31, 2009 compared to the year ended December 31, 2008 to meet expected sales demands.
Net cash (used in) provided by investing activities was ($0.1) million, $0.6 million and $13.0 million for the years ended December 31, 2009, 2008 and 2007, respectively. Cash (used in) provided by investing activities was primarily related to purchases of investments, net of proceeds from the maturities of investments, and purchases of property and equipment.
Net cash provided by financing activities was $2.6 million, $0.1 million and $0.2 million for the years ended December 31, 2009, 2008 and 2007, respectively, and consisted of proceeds from stock option exercises made under our stock award plans.
44
The following table summarizes our future contractual obligations as of December 31, 2009 (in thousands).
| | | | | | | | | | | | |
| | | Payments Due by Period |
Contractual Obligations | | Total | | Less than 1 | | 1-3 years | | 3-5 years |
Operating leases(1) | | $ | 7,133 | | $ | 1,682 | | $ | 3,274 | | $ | 2,177 |
Purchase obligations(2) | | | 4,357 | | | 4,357 | | | — | | | — |
Other liabilities(3) | | | 150 | | | — | | | 150 | | | — |
| | | | | | | | | | | | |
Total | | $ | 11,640 | | $ | 6,039 | | $ | 3,424 | | $ | 2,177 |
| | | | | | | | | | | | |
| (1) | These are future minimum rental commitments for office space and copiers leased under non-cancelable operating lease arrangements. |
| (2) | These consist of purchase obligations with manufacturers. |
| (3) | This amount represents a discretionary accrued bonus payable to our chief executive officer as of December 31, 2009 based on the achievement of performance targets over the years 2009-2011. |
In November 2008, SciClone and its subsidiaries, SPIL and SPIL China as borrowers, entered into a loan and security agreement and an unconditional guaranty and security agreement with Silicon Valley Bank (the “Credit Facility”). The Credit Facility is a line-of-credit that permits borrowing up to $6 million for a term of 36 months, subject to certain sublimits, including $2.5 million for letters of credit. The Credit Facility is secured by a first priority secured interest in all of our assets, other than intellectual property. The Credit Facility also requires us to obtain a credit insurance policy insuring certain foreign account receivable assets. SciClone Pharmaceuticals, Inc., as the parent of SPIL and SPIL China, is the guarantor of the Credit Facility. The Credit Facility expires in November 2011, and upon termination all amounts borrowed must be repaid in full. As of December 31, 2009, there were no outstanding borrowings under the Credit Facility.
The Credit Facility bears interest at the bank’s prime rate plus 1.75% on outstanding balances. We are required to meet certain financial covenants, including minimum consolidated revenue, and minimum consolidated earnings before interest and income tax, as defined, and since November 14, 2009, have been subject to certain minimum fees and interest payments. We are also required to meet certain operating covenants that limit our ability to incur liabilities, create liens, make capital expenditures, pay dividends or distributions, make investments, and dispose of assets and to carry credit insurance.
In November 2009, we received a limited waiver to the Credit Facility to waive certain minimum fees and interest payments, and to waive the requirement to maintain a credit insurance policy. We are currently in compliance with the Credit Facility after giving effect to the limited waiver.
In May 2009, we filed a shelf registration statement on Form S-3 with the SEC under which we may offer and sell up to $50.0 million of our securities, (plus an additional $10.0 million that we could sell under an immediately effective related registration statement), assuming we continue to meet the SEC’s eligibility requirements for primary offerings on Form S-3.
We believe that our existing cash and investments and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. We have no current commitments to offer and sell any securities that may be offered or sold pursuant to our registration statement. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience dilution. Debt financing, if available, may subject us to restrictive covenants and significant interest costs. To the extent that we raise additional funds through collaboration and licensing arrangements, we would be required to relinquish some rights to our technologies, product candidates or marketing territories. Additional financing or collaboration and licensing arrangements may not be available when needed either at all or, on favorable terms.
We intend to continue to explore alternatives for financing to provide additional flexibility in managing our operations, in-licensing or acquiring new products, particularly in China, and funding our clinical trials. The
45
unavailability or the inopportune timing of any financing could prevent or delay our long-term product development and commercialization programs, either of which could hurt our business. We cannot assure you that funds from financings will be sufficient to conduct and complete further clinical trials or to acquire or in-license additional products. The need, timing and amount of any such financing would depend upon numerous factors, including the level of ZADAXIN sales, the timing and amount of manufacturing costs related to ZADAXIN, the availability of complementary products, technologies and businesses, the initiation and continuation of preclinical and clinical trials and testing, the timing of regulatory approvals, developments in relationships with existing or future collaborative parties, the status of competitive products, and various alternatives for financing. We have not determined the timing or structure of any transaction.
Related Party Transactions
None of our officers or directors, other than Dr. Camerini, Dr. Jones and Mr. Lapointe, as set forth below, was involved in related party transactions in 2009.
We have licensed to Sigma-Tau exclusive ZADAXIN development and marketing rights that cover all countries in the European Union as defined on January 1, 1995, in addition to Iceland, Norway and Switzerland. Sigma-Tau and affiliated persons were our stockholders at the time this transaction was entered into, and are currently our largest shareholder. Under our agreement with Sigma-Tau, Sigma-Tau conducted a multi-center phase 3 hepatitis C triple therapy clinical trial in Europe with 553 patients. The objective of the European trial was to provide data on thymalfasin’s use as part of a triple therapy in treating HCV patients. We paid Sigma-Tau approximately $4.0 million of funding support during the course of patient enrollment and trial including completion of the final report in October 2009. Our accounting policy for recording funding amounts due to Sigma-Tau was to record the amounts to research and development expense over the trial period including the period of completion of the final report. Based on the level of activity in this trial, we have recorded approximately $0.1 million, $0.3 million and $0.5 million of research and development expense related to this trial for the years ended December 31, 2009, 2008, and 2007, respectively. As of December 31, 2009, no amounts were owed by us to Sigma-Tau. As of December 31, 2008, we had accrued clinical trial expenses of $1.4 million due to Sigma-Tau.
On March 30, 2009, we entered into a settlement agreement (the “Settlement Agreement”) with the affiliates of Sigma-Tau (the “Sigma-Tau Group”). We issued a press release on March 31, 2009 (the “Press Release”), announcing the execution of the Settlement Agreement and a commercial agreement referred to herein as the “ZADAXIN Agreement,” as well as certain actions that we have taken to implement the provisions of the Settlement Agreement. The Press Release, Settlement Agreement and ZADAXIN Agreement are exhibits to our Current Report on Form 8-K which we filed with the SEC on April 1, 2009. Under the Settlement Agreement, Dr. Camerini, Dr. Jones and Mr. Lapointe were appointed to our Board of Directors and pursuant to the unanimous recommendation of our Board, were elected by our stockholders at the 2009 annual meeting of stockholders. Certain obligations under the Settlement Agreement have expired and it expires in its entirety on or around June 15, 2010. The Company is no longer under any obligation to nominate Dr. Camerini, Dr. Jones and Mr. Lapointe. However, our Board has independently decided to re-nominate them at the annual stockholder’s meeting in 2010.
Except as a result of the relationships of Dr. Camerini, Dr. Jones and Mr. Lapointe with Sigma Tau, none of our officers or directors, other than, as set forth above, was involved in related party transactions in 2009.
Off-Balance Sheet Arrangements
There were no off-balance sheet arrangements in 2009, 2008, or 2007.
Recent Accounting Guidance
In December 2007, the Financial Accounting Standards Board (“FASB”) issued guidance concerning: determining whether an arrangement constitutes a collaborative arrangement; how costs incurred and revenue
46
generated on sales to third parties and how payments between parties to the collaborative arrangement should be reported in the income statement; how an entity should characterize payments on the income statement; and what participants should disclose in the notes to the financial statements about a collaborative arrangement. We adopted this guidance on January 1, 2009. The adoption of this guidance did not have a material impact on our consolidated financial statements.
In December 2007, the FASB issued authoritative guidance for business combinations which establishes principles and requirements for how the acquirer in a business combination (i) recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree, (ii) recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase and (iii) determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. The guidance was effective on a prospective basis and will impact business combination transactions for which the acquisition date occurs after December 15, 2008. We adopted this guidance on January 1, 2009. The adoption of this guidance did not have a material impact on our consolidated financial statements.
In October 2008 and April 2009, the FASB issued authoritative guidance that clarifies determining fair value measurements and annual and interim disclosures in a market that is not active and identifying transactions that are not orderly. This guidance is applicable to the valuation of auction-rate securities that we held for which there was an inactive market as of December 31, 2009. The adoption of this guidance did not have a material impact on our consolidated results of operations or consolidated financial position.
In April 2009, the FASB also issued guidance regarding Recognition and Presentation of Other-Than-Temporary Impairments which amends the other-than-temporary impairment guidance in U.S. GAAP for debt securities to make the guidance more operational and to improve the presentation and disclosure of other-than-temporary impairments for debt and equity securities. We adopted this guidance on January 1, 2009. The adoption of this guidance did not have a material impact on our consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
The primary objective of our investment activities is to preserve principal while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in money market funds, certificates of deposit, term deposits, U.S. Treasury, or government agency notes. In the past, we also invested in highly-rated, auction rate securities. The auction rate securities are classified as trading securities as of December 31, 2009, and consequently, are recorded on the balance sheet at fair value with realized gains or losses reported as income (loss). All of our investments mature within one year from date of purchase except for our auction rate securities, which we have the right to sell at par value, at our sole discretion, anytime during the period from June 30, 2010 through July 2, 2012, and our Italian state bonds which mature in 2013. Our investment securities may be subject to interest rate risk and could decrease in value if market interest rates rise. To minimize this risk, we primarily hold securities that are short-term in duration and maintain an average maturity of less than one year. We believe that our exposure to interest rate risk is not significant and a 1% movement in market interest rates would not have a significant impact to the total value of our investment portfolio at December 31, 2009. We do not hold any derivative financial instruments for speculation or trading purposes.
We determined the fair market values of our financial instruments based on the fair value hierarchy that requires an entity to maximize the use of observable inputs (level 1 and level 2 inputs) and minimize the use of unobservable inputs (level 3 inputs) when measuring fair value. We categorized our $1.8 million par value ARS as level 3 in short-term investments due to the failures in the markets to provide quoted market prices for the ARS securities as well as the lack of any correlation to these instruments to other observable market data. We valued these securities using a pricing model. Significant inputs that went into the model were the credit quality of the issuer, the percentage and types of guarantees (such as Federal Family Education Loan Program-FFELP),
47
the probability of the auction succeeding or the security being called, an illiquidity discount factor, and tax status. At December 31, 2009, the ARS were recorded at their determined fair value of $1.6 million and the loss of $0.2 million had been realized in our financial statements. Changes in the assumptions of the model based on dynamic market conditions could have a significant impact on the valuation of these securities, which may lead us in the future to take further losses or gains for these securities.
We have recorded our Put Option at its fair value of $0.2 million in prepaid expenses and other current assets on our accompanying consolidated balance sheet as of December 31, 2009. We estimate the fair value of the Put Option based on a discounted cash flow model. The assumptions used in preparing the discounted cash flow model include estimates for interest rates, estimates for discount rates using yields of comparable traded instruments adjusted for illiquidity and other risk factors, and amount of cash flows for the expected holding period. These inputs reflect our assumptions about risk, developed based on the best information available in the circumstances. Changes in the assumptions of the model based on dynamic market conditions could have a significant impact on the valuation of this asset, which may lead us in the future to record gains or losses for this asset.
Substantially all our sales and most of our manufacturing costs to date have been in U.S. dollars. However, some of our purchases with contract manufacturers are denominated in euros and costs of our marketing efforts in China are paid in local currency. In addition, we have certain cash balances denominated in euros. As a result, we are exposed to foreign currency rate fluctuations, and we do not hedge against the risk associated with such fluctuations. Consequently, changes in exchange rates could result in material exchange losses and could unpredictably, materially and adversely affect our operating results and stock price. Such losses have been insignificant to date.
48
| Item 8. | Financial Statements and Supplementary Data |
SCICLONE PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
49
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of SciClone Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of SciClone Pharmaceuticals, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2009. Our audits also included the financial statement schedule listed at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of SciClone Pharmaceuticals, Inc. at December 31, 2009 and 2008, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2009, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), SciClone Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 16, 2010 expressed an unqualified opinion on the effectiveness of internal control over financial reporting.
/s/ Ernst & Young LLP
Palo Alto, California
March 16, 2010
50
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| | | | | | | | |
| | | December 31,
2009 | | | December 31,
2008 | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 29,687 | | | $ | 27,673 | |
Short-term investments | | | 1,646 | | | | 100 | |
Accounts receivable | | | 21,394 | | | | 11,927 | |
Inventories | | | 10,149 | | | | 6,056 | |
Prepaid expenses and other current assets | | | 1,518 | | | | 2,191 | |
Restricted short-term investments | | | 71 | | | | 69 | |
| | | | | | | | |
Total current assets | | | 64,465 | | | | 48,016 | |
Property and equipment, net | | | 771 | | | | 861 | |
Long-term investments | | | — | | | | 1,485 | |
Restricted investments | | | 415 | | | | 388 | |
Other assets | | | 1,249 | | | | 1,155 | |
| | | | | | | | |
Total assets | | $ | 66,900 | | | $ | 51,905 | |
| | | | | | | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 2,339 | | | $ | 2,195 | |
Accrued liabilities (including $0 and $1,442 due to related party at December 31, 2008 and 2009, respectively) | | | 6,048 | | | | 8,028 | |
Deferred revenue | | | 141 | | | | — | |
| | | | | | | | |
Total current liabilities | | | 8,528 | | | | 10,223 | |
Long-term liabilities | | | 979 | | | | 779 | |
Commitments and contingencies (see Note 8) | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Preferred stock; $0.001 par value; 10,000,000 shares authorized; no shares outstanding | | | — | | | | — | |
Common stock; $0.001 par value; 75,000,000 shares authorized; 47,217,944 and 46,219,562 shares issued and outstanding at December 31, 2009 and 2008, respectively | | | 47 | | | | 46 | |
Additional paid-in capital | | | 222,229 | | | | 217,704 | |
Accumulated other comprehensive income | | | 22 | | | | 3 | |
Accumulated deficit | | | (164,905 | ) | | | (176,850 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 57,393 | | | | 40,903 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 66,900 | | | $ | 51,905 | |
| | | | | | | | |
See notes to consolidated financial statements.
51
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | 2009 | | | 2008 | | | 2007 | |
Revenues: | | | | | | | | | | | | |
Product sales | | $ | 72,411 | | | $ | 54,108 | | | $ | 37,038 | |
Contract revenue | | | — | | | | 5 | | | | 20 | |
| | | | | | | | | | | | |
Total revenues | | | 72,411 | | | | 54,113 | | | | 37,058 | |
Cost of product sales | | | 11,960 | | | | 10,299 | | | | 7,088 | |
| | | | | | | | | | | | |
Gross margin | | | 60,451 | | | | 43,814 | | | | 29,970 | |
| | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development (including $58, $322 and $507 of related party research and development in 2009, 2008 and 2007, respectively) | | | 16,531 | | | | 22,857 | | | | 16,552 | |
Sales and marketing | | | 18,805 | | | | 16,853 | | | | 13,575 | |
General and administrative | | | 12,521 | | | | 12,538 | | | | 11,139 | |
| | | | | | | | | | | | |
Total operating expenses | | | 47,857 | | | | 52,248 | | | | 41,266 | |
| | | | | | | | | | | | |
Income (loss) from operations | | | 12,594 | | | | (8,434 | ) | | | (11,296 | ) |
Interest and investment income | | | 153 | | | | 608 | | | | 1,629 | |
Interest and investment expense | | | (179 | ) | | | (31 | ) | | | (20 | ) |
Other income (expense), net | | | 18 | | | | (16 | ) | | | 40 | |
| | | | | | | | | | | | |
Income (loss) before provision for income tax | | | 12,586 | | | | (7,873 | ) | | | (9,647 | ) |
Provision for income tax | | | 641 | | | | 475 | | | | 301 | |
| | | | | | | | | | | | |
Net income (loss) | | $ | 11,945 | | | $ | (8,348 | ) | | $ | (9,948 | ) |
| | | | | | | | | | | | |
Basic net income (loss) per share | | $ | 0.26 | | | $ | (0.18 | ) | | $ | (0.22 | ) |
Diluted net income (loss) per share | | $ | 0.25 | | | $ | (0.18 | ) | | $ | (0.22 | ) |
Weighted average shares used in computing: | | | | | | | | | | | | |
Basic net income (loss) per share | | | 46,574 | | | | 46,212 | | | | 46,100 | |
Diluted net income (loss) per share | | | 47,404 | | | | 46,212 | | | | 46,100 | |
See notes to consolidated financial statements.
52
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Common stock | | Additional
Paid-in
Capital | | Accumulated
Other
Comprehensive
Income | | | Accumulated
Deficit | | | Total
Stockholders’
Equity | |
| | Shares | | Amount | | | | |
Balance at December 31, 2006 | | 46,001 | | $ | 46 | | $ | 213,064 | | $ | 78 | | | $ | (158,554 | ) | | $ | 54,634 | |
Issuance of common stock from exercise of stock options and employee stock purchase plan | | 120 | | | — | | | 241 | | | — | | | | — | | | | 241 | |
Compensation related to stock option awards | | — | | | — | | | 2,328 | | | — | | | | — | | | | 2,328 | |
Net loss | | — | | | — | | | — | | | — | | | | (9,948 | ) | | | (9,948 | ) |
Net change in unrealized gain on available-for-sale securities | | — | | | — | | | — | | | 22 | | | | — | | | | 22 | |
Foreign currency translation | | — | | | — | | | — | | | (18 | ) | | | — | | | | (18 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | (9,944 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | 46,121 | | | 46 | | | 215,633 | | | 82 | | | | (168,502 | ) | | | 47,259 | |
Issuance of common stock from exercise of stock options | | 98 | | | — | | | 146 | | | — | | | | — | | | | 146 | |
Stock short-swing profit | | — | | | — | | | 3 | | | — | | | | — | | | | 3 | |
Issuance of warrants related to line of credit | | — | | | — | | | 30 | | | — | | | | — | | | | 30 | |
Compensation related to stock option awards | | — | | | — | | | 1,892 | | | — | | | | — | | | | 1,892 | |
Net loss | | — | | | — | | | — | | | — | | | | (8,348 | ) | | | (8,348 | ) |
Net change in unrealized losses and foreign currency translation on foreign-denominated available-for- sale securities | | — | | | — | | | — | | | (60 | ) | | | — | | | | (60 | ) |
Net change in unrealized gains/losses on available-for-sale securities | | — | | | — | | | — | | | (102 | ) | | | — | | | | (102 | ) |
Foreign currency translation | | — | | | — | | | — | | | 83 | | | | — | | | | 83 | |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | (8,427 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | 46,219 | | | 46 | | | 217,704 | | | 3 | | | | (176,850 | ) | | | 40,903 | |
Issuance of common stock from exercise of stock options | | 999 | | | 1 | | | 2,624 | | | — | | | | — | | | | 2,625 | |
Compensation related to stock option awards | | — | | | — | | | 1,901 | | | — | | | | — | | | | 1,901 | |
Net income | | — | | | — | | | — | | | — | | | | 11,945 | | | | 11,945 | |
Net change in unrealized losses and foreign currency translation on foreign-denominated available-for- sale securities | | — | | | — | | | — | | | 26 | | | | — | | | | 26 | |
Net change in unrealized gains/losses on available-for-sale securities | | — | | | — | | | — | | | 4 | | | | — | | | | 4 | |
Foreign currency translation | | — | | | — | | | — | | | (11 | ) | | | — | | | | (11 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | 11,964 | |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | 47,218 | | $ | 47 | | $ | 222,229 | | $ | 22 | | | $ | (164,905 | ) | | $ | 57,393 | |
| | | | | | | | | | | | | | | | | | | | |
See notes to consolidated financial statements.
53
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | 2009 | | | 2008 | | | 2007 | |
Operating activities: | | | | | | | | | | | | |
Net income (loss) | | $ | 11,945 | | | $ | (8,348 | ) | | $ | (9,948 | ) |
Adjustments to reconcile net income (loss) to net cash used in operating activities: | | | | | | | | | | | | |
Non-cash expense related to stock options | | | 1,867 | | | | 1,843 | | | | 2,243 | |
Amortization of interest on investments held-to-maturity | | | — | | | | — | | | | 95 | |
Depreciation and amortization | | | 530 | | | | 353 | | | | 248 | |
Realized (gain) loss on auction rate securities | | | (103 | ) | | | 315 | | | | — | |
Loss on disposal of property and equipment | | | — | | | | 37 | | | | — | |
Other non-cash expense (income) | | | 83 | | | | (285 | ) | | | 1 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Accounts receivable | | | (9,467 | ) | | | 723 | | | | 627 | |
Inventories | | | (4,059 | ) | | | (428 | ) | | | (2,262 | ) |
Prepaid expenses and other assets | | | 247 | | | | 1,243 | | | | (1,259 | ) |
Accounts payable | | | 144 | | | | 258 | | | | 974 | |
Accrued liabilities (including ($1.4) million, ($0.2) million and $21,000 due to related party in 2009, 2008 and 2007, respectively) | | | (1,980 | ) | | | (1,057 | ) | | | 2,199 | |
Deferred revenue | | | 141 | | | | (37 | ) | | | (25 | ) |
Long-term liabilities | | | 200 | | | | 438 | | | | 96 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (452 | ) | | | (4,945 | ) | | | (7,011 | ) |
| | | | | | | | | | | | |
Investing activities: | | | | | | | | | | | | |
Purchases of property and equipment | | | (192 | ) | | | (373 | ) | | | (446 | ) |
Proceeds from maturities of investments | | | 44 | | | | 2,593 | | | | 23,082 | |
Purchases of investments | | | — | | | | (1,648 | ) | | | (9,642 | ) |
| | | | | | | | | | | | |
Net cash (used in) provided by investing activities | | | (148 | ) | | | 572 | | | | 12,994 | |
| | | | | | | | | | | | |
Financing activities: | | | | | | | | | | | | |
Proceeds from issuances of common stock | | | 2,625 | | | | 149 | | | | 241 | |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 2,625 | | | | 149 | | | | 241 | |
| | | | | | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents | | | (11 | ) | | | 80 | | | | (22 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 2,014 | | | | (4,144 | ) | | | 6,202 | |
Cash and cash equivalents, beginning of year | | | 27,673 | | | | 31,817 | | | | 25,615 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of year | | $ | 29,687 | | | $ | 27,673 | | | $ | 31,817 | |
| | | | | | | | | | | | |
Supplemental disclosures of cash flow information: | | | | | | | | | | | | |
Income taxes paid related to foreign operations | | $ | 608 | | | $ | 559 | | | $ | 299 | |
| | | | | | | | | | | | |
Non-cash investing activities: | | | | | | | | | | | | |
Leasehold improvements reimbursed by tenant allowance | | $ | — | | | $ | — | | | $ | 206 | |
| | | | | | | | | | | | |
See notes to consolidated financial statements.
54
SCICLONE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 — The Company and Summary of Significant Accounting Policies
The Company
SciClone Pharmaceuticals, Inc. (“SciClone” or the “Company”) is a profit-focused, global specialty pharmaceutical company with a substantial international business, based primarily in the People’s Republic of China (“China”), and with a product portfolio of novel therapies for cancer and infectious diseases. ZADAXIN®, the Company’s brand of thymalfasin or thymosin alpha 1 and its primary product, is sold in over 30 countries, primarily to China, through the Company’s wholly-owned subsidiary SciClone Pharmaceuticals International Ltd. The Company continues to seek development opportunities with ZADAXIN focused on vaccine enhancement and melanoma. The Company’s proprietary in-licensed compound SCV-07 is in phase 2 clinical development for the treatment of oral mucositis and hepatitis C virus (“HCV”). The Company has in-licensed the commercialization rights of DC BeadTM in China, a novel treatment for advanced liver cancer which is currently approved in 40 countries worldwide, including Europe and the U.S., and commenced a small local registration trial in China in the first quarter of 2010 as required by the Chinese State Food and Drug Administration for regulatory approval. The Company has also in-licensed the commercialization rights to an anti-nausea drug ondansetron RapidFilmTM in China and Vietnam, for which the Company intends to seek regulatory approval.
Presentation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries, SciClone Pharmaceuticals International Ltd. (“SPIL”), SciClone Pharmaceuticals International China Holding Ltd. (“SPIL China”), SciClone Pharmaceuticals (China) Ltd., SciClone Italy S.R.L., and SciClone do Brasil–Produtos Farmaceuticos Ltda. SPIL is registered in the Cayman Islands with its principal office located in Hong Kong. SPIL China is registered in Cayman Islands with its principal office located in Hong Kong. SciClone Pharmaceuticals (China) Ltd. is registered in China with its principal office located in Shanghai. SciClone Italy S.R.L. is registered in Italy with its principal office located in Rome. SciClone do Brasil is registered in Brazil with its principal office located in Sao Paulo. All significant intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash Equivalents and Investments
Cash equivalents consist of highly liquid investments with maturities of three months or less on the date of purchase. The Company classifies its investments as available-for-sale or trading securities. The Company records the investments at fair value, as determined by available information on the consolidated balance sheet date. The Company’s available-for-sale portfolio primarily consists of a certificate of deposit, corporate equity securities and restricted long-term Italian state bonds. The Company’s trading portfolio consists of auction-rate securities (“ARS”).
Unrealized gains or losses on available-for-sale securities are included in accumulated other comprehensive income on the consolidated balance sheet. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are included in earnings. Gains or losses and declines in value judged to be other-than-temporary on trading securities are included in earnings. The amortized cost of securities is adjusted for amortization of premiums and accretion of discounts to maturity and is included in earnings. The cost of securities sold is based on the specific identification method.
55
Available-for-sale investments are evaluated for impairment each reporting period. An investment is considered impaired if the fair value of the investment is less than its cost. If, after consideration of all available evidence to evaluate the realizable value of its investment, impairment is determined to be other-than-temporary, then an impairment loss is recognized in the Consolidated Statement of Operations.
For the years ended December 31, 2009, 2008 and 2007, net change in unrealized (loss) gains, including foreign currency translation on foreign-denominated securities, of approximately $30,000, ($0.2) million, and $22,000, on available-for-sale securities, respectively, were included in accumulated other comprehensive income.
For the years ended December 31, 2009 and 2008, the Company realized gains (losses) related to its ARS trading securities of $0.1 million and ($0.3) million, respectively. There were no other realized gains or losses for the years ended December 31, 2009, 2008 or 2007 on the Company’s investments.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of input are:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Where quoted prices are available in an active market, the Company determines fair value based upon quoted market prices, and classifies these values in level 1 of the valuation hierarchy. If quoted market prices are not available, fair values are based upon observable inputs such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities and are classified in level 2 of the valuation hierarchy. When quoted prices and observable inputs are unavailable, fair values are based on internally developed cash flow models and are classified in level 3 of the valuation hierarchy. The internally developed cash flow models primarily use, as inputs, estimates for interest rates and discount rates including yields of comparable traded instruments adjusted for illiquidity and other risk factors, amount of cash flows and expected holding periods of the assets. These inputs reflect the Company’s assumptions about the assumptions market participants would use in pricing the assets including assumptions about risk developed based on the best information available in the circumstances. The Company’s assessment of the significance of a particular input to the fair value measurements requires judgment, and may affect the valuation of the assets being measured and their placement within the fair value hierarchy.
Other financial instruments, including accrued liabilities, are carried at cost, which the Company believes approximates fair value because of the short-term maturity of these instruments.
Concentration of Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash, cash equivalents and investments. The Company is exposed to credit risk in the event of default by the institutions holding the cash, cash equivalents and investments to the extent of the amounts recorded on the
56
consolidated balance sheet. Substantially all the Company’s cash and cash equivalents are held by financial institutions that the Company believes are of high credit quality. At times, deposits may exceed government insured limits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
China uses a tiered method to import and distribute products. The distributors make the sales in the country, but the product is imported for them by licensed importers. For the years ended December 31, 2009, 2008 and 2007, sales to four importing agents in China accounted for 96%, 94% and 92%, respectively, of the Company’s product sales. In 2009, the two largest customers accounted for 66% and 27% of sales, respectively. In 2008, the two largest customers accounted for 68% and 22% of sales, respectively. In 2007, the two largest customers accounted for 63% and 21% of sales, respectively. No other customer accounted for more than 10% of sales in 2009, 2008 or 2007. As of December 31, 2009, approximately $20.8 million, or 97%, of the Company’s accounts receivable were attributable to four importing agents in China. The Company performs on-going credit evaluations of its customers’ financial condition, and generally does not require collateral from its customers.
The Company currently relies on two suppliers to provide key components to its ZADAXIN manufacturing supply. Although there are a limited number of manufacturers who would be able to meet the requirements to manufacture these components, management believes that other suppliers could provide similar components on comparable terms. A change in suppliers, however, could cause a delay in manufacturing and a possible loss of sales, which would affect operating results adversely.
Accounts Receivable
Accounts receivable are recorded net of the allowance for doubtful accounts. The Company maintains allowances for doubtful accounts for estimated losses resulting from the inability of customers to make required payments, when appropriate. The Company records its allowance for doubtful accounts based on its assessment of various factors. When estimating the need for an allowance for doubtful accounts, the Company considers historical payment patterns of its customers, the circumstances of each individual customer and their geographic region including a review of the local economic environment, the age of the accounts receivable balances, credit quality of its customers, and other factors that may affect customers’ ability to pay. At December 31, 2009, no allowance for doubtful accounts was considered necessary.
Inventories
Inventories consist of raw materials, work in progress and finished goods products. Inventories are stated at the lower of cost or market, with cost determined on a first-in, first-out basis, and include amounts related to materials, labor and overhead. The Company periodically reviews the inventory in order to identify obsolete items. If obsolete items are observed and there are no alternate uses for the inventory, the Company will record a write-down to net realizable value in the period that the impairment is first recognized.
Property and Equipment
Property and equipment is stated at cost, less accumulated depreciation. Depreciation is recorded over the estimated useful lives of the respective assets (three to five years) on the straight-line basis. Leasehold improvements are amortized over the shorter of the estimated useful life or lease term on the straight-line basis.
Intangible Assets
Intangible assets with definite lives are amortized over their estimated useful lives and are reviewed for impairment when facts or circumstances suggest that the carrying value of these assets may not be recoverable. The Company’s policy is to identify and record impairment losses, if necessary, on intangible product rights when events and circumstances indicate that the assets might be impaired and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amounts of those assets. The Company to date has not identified any impairment losses on its intangible assets.
57
Foreign Currency Translation
The Company translates the assets and liabilities of its foreign subsidiaries stated in local functional currencies to U.S. dollars at the rates of exchange in effect at the end of the period. Revenues and expenses are translated using rates of exchange in effect during the period. Gains and losses from the translation of financial statements denominated in foreign currencies are included as a separate component of accumulated other comprehensive income in the statement of stockholders’ equity.
The Company records foreign currency transactions at the exchange rate prevailing at the date of the transaction with resultant gains and losses being included in results of operations. Foreign currency transaction gains and losses have not been significant for any period presented.
Revenue Recognition
The Company recognizes revenue from product sales at the time of delivery. There are no significant customer acceptance requirements or post-shipment obligations on the part of the Company, except for sales to a new market where acceptance requirements have to be met. Sales to importing agents or distributors are recognized at time of shipment when title to the product is transferred to them. Importing agents or distributors do not have contractual rights of return except under limited terms regarding product quality. However, the Company is expected to replace products that have expired or are deemed to be damaged or defective when delivered. The Company estimates expected returns primarily on historical patterns. Historically, the Company has had no product returns of damaged, defective or expired product. As such, no amount was accrued for product returns as of December 31, 2009 and 2008 in the respective consolidated balance sheets. Payments by the importing agents and distributors are not contingent upon sale to the end user by the importing agents or distributors. Amounts invoiced relating to arrangements where collectibility is uncertain and revenue cannot be recognized are reflected on the Company’s balance sheet as deferred revenue and recognized as the applicable revenue recognition criteria are satisfied.
Contract revenue for research and development is recorded as earned based on performance requirements of the contract. Nonrefundable contract fees for which no further performance obligations exist, and for which there is no continuing involvement by the Company, are recognized as contract revenue on the earlier of when the payments are received or when collection is reasonably assured.
Revenue associated with substantive performance milestones is recognized based on the achievement of the milestones, as defined in the respective agreements and provided that (i) the milestone event is substantive and its achievement is not reasonably assured at the inception of the agreement and (ii) there are no future performance obligations associated with the milestone payment.
Research and Development Expenses
Research and development costs are expensed as incurred. These costs consist primarily of salaries and other personnel-related expenses, including associated stock-based compensation, facility-related expenses, depreciation of facilities and equipment, license-related fees, services performed by clinical research organizations and research institutions and other outside service providers, and the sharing of certain costs for the development of ZADAXIN by the Company’s partner, Sigma-Tau Finanziaria S.p.A (“Sigma-Tau”).
Expenses related to clinical trials generally are accrued based on estimates of work performed or the level of patient enrollment and activities according to the protocols and agreements. The Company monitors planned protocols, work performed, patient enrollment levels and related activities to the extent possible and adjusts estimates accordingly. Nonrefundable advance payments for research and development goods or services are recognized as expense as the related goods are delivered or the related services are provided.
58
Shipping and Handling Costs
Shipping and handling costs incurred for inventory purchases and product shipments are included in cost of product sales for all periods presented.
Advertising Expenses
Advertising costs are expensed as incurred and are included in sales and marketing expenses for all periods presented. Advertising expenses for the years ended December 31, 2009, 2008 and 2007 were $0.3 million, $0.2 million, and $0.4 million, respectively.
Stock-Based Compensation
The Company records stock-based compensation costs relating to share-based payment transactions, including stock options and employee stock purchase plans. Stock-based compensation expense is estimated at the date of grant based on the fair value of the award using the Black-Scholes option-pricing model. These stock-based compensation costs are recognized as expense ratably (as the awards vest) over the requisite service period, which is generally one or four years. Refer also to note 13, “Stockholders’ Equity,” in the Notes to Consolidated Financial Statements for further information regarding stock-based compensation.
Income Taxes
Income taxes are accounted for under the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates that will be in effect when these differences reverse. The Company provides a valuation allowance against net deferred tax assets if, based upon the available evidence, it is more-likely-than-not that the deferred tax assets will not be realized. The Company’s policy is to recognize interest and penalties related to the underpayment of income taxes as a component of income tax expense. To date, there have been no interest or penalties charged to the Company in relation to the underpayment of income taxes.
Comprehensive Income
Comprehensive income is comprised of net income (loss) and other comprehensive income (loss). The following table summarizes the components of accumulated other comprehensive income(in thousands):
| | | | | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
Net unrealized gain (loss) on available-for-sale securities | | $ | 2 | | | $ | (2 | ) | | $ | 100 | |
Net unrealized loss and foreign currency translation adjustment on foreign-denominated available-for-sale securities | | | (34 | ) | | | (60 | ) | | | — | |
Cumulative foreign currency translation adjustment | | | 54 | | | | 65 | | | | (18 | ) |
| | | | | | | | | | | | |
Accumulated other comprehensive income | | $ | 22 | | | $ | 3 | | | $ | 82 | |
| | | | | | | | | | | | |
Net Income (Loss) Per Share
Basic net income (loss) per share has been computed by dividing net income (loss) by the weighted-average number of shares of common stock outstanding for the period. Diluted net income (loss) per share is computed by dividing net income (loss) by the weighted-average number of common equivalent shares outstanding for the period. Diluted net income (loss) per share includes any dilutive impact from stock options and warrants outstanding using the treasury stock method.
59
The following is a reconciliation of the numerator and denominators of the basic and diluted net income (loss) per share computations for the year ended December 31(in thousands, except per share amounts):
| | | | | | | | | | | |
| | | 2009 | | 2008 | | | 2007 | |
Numerator: | | | | | | | | | | | |
Net income (loss) | | $ | 11,945 | | $ | (8,348 | ) | | $ | (9,948 | ) |
Denominator: | | | | | | | | | | | |
Weighted-average shares outstanding used to compute basic net income (loss) per share | | | 46,574 | | | 46,212 | | | | 46,100 | |
Effect of dilutive securities | | | 830 | | | — | | | | — | |
| | | | | | | | | | | |
Weighted-average shares outstanding used to compute diluted net income (loss) per share | | | 47,404 | | | 46,212 | | | | 46,100 | |
| | | | | | | | | | | |
Basic net income (loss) per share | | $ | 0.26 | | $ | (0.18 | ) | | $ | (0.22 | ) |
Diluted net income (loss) per share | | $ | 0.25 | | $ | (0.18 | ) | | $ | (0.22 | ) |
For the years ended December 31, 2009, 2008 and 2007, approximately 6,107,498, 7,968,269 and 7,324,688 shares, respectively, related to outstanding stock options and warrants were excluded from the calculation of diluted net income (loss) per share because their inclusion would have been anti-dilutive.
Segment Information
The Company operates in one segment (refer to note 15).
Recent Accounting Guidance
In December 2007, the Financial Accounting Standards Board (“FASB”) issued guidance concerning: determining whether an arrangement constitutes a collaborative arrangement; how costs incurred and revenue generated on sales to third parties and how payments between parties to the collaborative arrangement should be reported in the income statement; how an entity should characterize payments on the income statement; and what participants should disclose in the notes to the financial statements about a collaborative arrangement. The Company adopted this guidance on January 1, 2009. The adoption did not have a material impact on the Company’s consolidated financial statements.
In December 2007, the FASB issued authoritative guidance for business combinations which establishes principles and requirements for how the acquirer in a business combination (i) recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed and any noncontrolling interest in the acquiree, (ii) recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase and (iii) determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. The Company adopted this guidance on January 1, 2009. The adoption did not have a material impact on the Company’s consolidated financial statements.
In October 2008 and April 2009, the FASB issued authoritative guidance that clarifies determining fair value measurements and annual and interim disclosures in a market that is not active and identifying transactions that are not orderly. This guidance is applicable to the valuation of auction-rate securities held by the Company for which there was an inactive market as of December 31, 2009. The adoption of this guidance did not have a material impact on the Company’s consolidated results of operations or consolidated financial position.
In April 2009, the FASB also issued guidance regarding Recognition and Presentation of Other-Than-Temporary Impairments which amends the other-than-temporary impairment guidance in U.S. GAAP for debt securities to make the guidance more operational and to improve the presentation and disclosure of other-than-temporary impairments for debt and equity securities. The Company adopted this guidance on January 1, 2009. The adoption did not have a material impact on the Company’s consolidated financial statements.
60
Reclassifications
The Company reclassified shipping and handling costs related to product shipments of $0.5 million and $0.4 million from sales and marketing expense to cost of product sales, and reclassified $0.7 million and $0.9 million of legal expense from research and development expense to general and administrative expense for the years ended December 31, 2008 and 2007, respectively, to conform to the current year presentation. These reclassifications had no effect on prior years’ net loss or stockholders’ equity.
Note 2 — Cash, Cash Equivalents and Investments
The following is a summary of cash, cash equivalents, and investments(in thousands):
| | | | | | | | | | | | | |
| | | December 31, 2009 |
| | | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses for
More Than
12 Months | | | Estimated
Fair Value |
Cash and cash equivalents: | | | | | | | | | | | | | |
Cash | | $ | 17,541 | | $ | — | | $ | — | | | $ | 17,541 |
Money market funds | | | 16 | | | — | | | — | | | | 16 |
Foreign U.S. dollar term deposits | | | 12,130 | | | — | | | — | | | | 12,130 |
| | | | | | | | | | | | | |
Total cash and cash equivalents | | $ | 29,687 | | $ | — | | $ | — | | | $ | 29,687 |
| | | | | | | | | | | | | |
Available-for-sale investments: | | | | | | | | | | | | | |
Certificates of deposit maturing within 1 year | | $ | 76 | | $ | — | | $ | — | | | $ | 76 |
Restricted long-term Italian state bonds maturing in 2013 | | | 449 | | | — | | | (34 | ) | | | 415 |
Corporate equity securities | | | 51 | | | 2 | | | — | | | | 53 |
| | | | | | | | | | | | | |
Total available-for-sale investments | | $ | 576 | | $ | 2 | | $ | (34 | ) | | $ | 544 |
| | | | | | | | | | | | | |
Trading security investments: | | | | | | | | | | | | | |
Auction rate securities maturing after 20 years | | $ | 1,588 | | $ | — | | $ | — | | | $ | 1,588 |
| | | | | | | | | | | | | |
Total trading security investments | | $ | 1,588 | | $ | — | | $ | — | | | $ | 1,588 |
| | | | | | | | | | | | | |
| |
| | | December 31, 2008 |
| | | Amortized
Cost | | Unrealized
Gains | | Unrealized
Losses | | | Estimated
Fair Value |
Cash and cash equivalents: | | | | | | | | | | | | | |
Cash | | $ | 9,277 | | $ | — | | $ | — | | | $ | 9,277 |
Money market funds | | | 1,969 | | | — | | | — | | | | 1,969 |
Foreign U.S. dollar term deposits | | | 16,427 | | | — | | | — | | | | 16,427 |
| | | | | | | | | | | | | |
Total cash and cash equivalents | | $ | 27,673 | | $ | — | | $ | — | | | $ | 27,673 |
| | | | | | | | | | | | | |
Available-for-sale investments: | | | | | | | | | | | | | |
Certificates of deposit maturing within 1 year | | $ | 120 | | $ | — | | $ | — | | | $ | 120 |
Restricted long-term Italian state bonds maturing in 2013 | | | 448 | | | — | | | (60 | ) | | | 388 |
Corporate equity securities | | | 51 | | | — | | | (2 | ) | | | 49 |
| | | | | | | | | | | | | |
Total available-for-sale investments | | $ | 619 | | $ | — | | $ | (62 | ) | | $ | 557 |
| | | | | | | | | | | | | |
Long-term trading security investments: | | | | | | | | | | | | | |
Long-term auction rate securities maturing after 20 years | | $ | 1,485 | | $ | — | | $ | — | | | $ | 1,485 |
| | | | | | | | | | | | | |
Total long-term trading security investments | | $ | 1,485 | | $ | — | | $ | — | | | $ | 1,485 |
| | | | | | | | | | | | | |
As of December 31, 2009 and 2008, available-for-sale securities included $0.1 million in short-term investments and $0.5 million in restricted investments. The Company intends to hold its restricted Italian state bond investments until recovery.
61
The Company’s certificate of deposit for $0.1 million secures the Company’s letter of credit required under its European value added tax filing arrangements.
As of December 31, 2009, the Company held $1.6 million in fair value of ARS as trading securities in short-term investments. The ARS are highly rated investments by one or more of the major independent rating agencies and have contractual maturities generally between 20 and 32 years whose underlying assets are student loans that are substantially backed by the federal government with interest rates resetting approximately every 30 days through an auction process. At the end of each reset period, investors may sell or continue to hold the securities at par. Disruptions in the credit markets have adversely affected the market for ARS. However, the Company does not believe that the underlying securities or collateral have been permanently affected.
In November 2008, the Company accepted an Auction Rate Securities Rights Offer (the “Settlement Agreement”) from UBS AG under which, in return for a general release of claims and the grant of a right to UBS AG to purchase the Company’s ARS at any time for full par value, the Company received the right to require UBS AG to purchase the Company’s ARS beginning in June 2010 (the “Rights”). The Company held approximately $1.8 million, at par value, of eligible ARS with UBS as of December 31, 2009. By entering into the Settlement Agreement, the Company (1) received the right (the “Put Option”) to sell these auction rate securities back to the investment firm at par, at its sole discretion, anytime during the period from June 30, 2010 through July 2, 2012, and (2) gave the investment firm the right to purchase these auction rate securities or sell them on the Company’s behalf at par anytime after the execution of the Settlement Agreement through July 2, 2012. The Company has recorded the fair value of the Put Option in prepaid expenses and other current assets in its consolidated balance sheet as of December 31, 2009. The Company’s fair value election was intended to create accounting symmetry with its fair value accounting of the ARS.
The recording of the fair value changes of the Put Option resulted in net realized (losses) gains of ($0.1) million and $0.3 million for the years ended December 31, 2009 and 2008, respectively, recorded to other expense in the Company’s consolidated statements of operations. The recording of the fair value changes to the ARS trading securities resulted in net realized gains (losses) of $0.1 million and ($0.3) million for the years ended December 31, 2009 and 2008, respectively, recorded to other income in the Company’s consolidated statements of operations. As of December 31, 2009 the combined fair value of the Put Option and ARS was $1.8 million and represented 99% of the par value of the ARS. The Company anticipates that future changes in the fair value of the Put Option will approximate fair value movements in the related ARS. Further, the Company expects to receive the ARS par value of $1.8 million subsequent to June 29, 2010 related to its Settlement Agreement.
Note 3 — Fair Value Measurements
The following table represents the Company’s fair value hierarchy for its financial assets (cash equivalents, investments and other current assets) measured at fair value on a recurring basis(in thousands):
| | | | | | | | | | | | |
| | | Fair Value Measurements at December 31, 2009 Using |
Description | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | | Balance as of
December 31, 2009 |
Money market funds | | $ | 16 | | $ | — | | $ | — | | $ | 16 |
Foreign U.S. dollar term deposits | | | — | | | 12,130 | | | — | | | 12,130 |
Certificates of deposit | | | — | | | 76 | | | — | | | 76 |
Corporate equity securities | | | 53 | | | — | | | — | | | 53 |
Restricted long-term Italian state bonds | | | 415 | | | — | | | — | | | 415 |
Auction rate securities | | | — | | | — | | | 1,588 | | | 1,588 |
Put Option | | | — | | | — | | | 202 | | | 202 |
| | | | | | | | | | | | |
Total | | $ | 484 | | $ | 12,206 | | $ | 1,790 | | $ | 14,480 |
| | | | | | | | | | | | |
62
The following table provides a summary of changes in fair value of the Company’s level 3 financial assets during fiscal 2009(in thousands):
| | | | | | | |
| | | Auction Rate
Securities | | Put
Option | |
Balance at December 31, 2008 | | $ | 1,485 | | $ | 285 | |
Total realized gain (loss) included in net income | | | 103 | | | (83 | ) |
| | | | | | | |
Balance at December 31, 2009 | | $ | 1,588 | | $ | 202 | |
| | | | | | | |
Note 4 — Inventories
Inventories consisted of the following(in thousands):
| | | | | | |
| | | December 31, |
| | | 2009 | | 2008 |
Raw materials | | $ | 2,446 | | $ | 1,605 |
Work in progress | | | 1,048 | | | 1,525 |
Finished goods | | | 6,655 | | | 2,926 |
| | | | | | |
| | $ | 10,149 | | $ | 6,056 |
| | | | | | |
Note 5 — Property and Equipment
Property and equipment consisted of the following(in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
Office furniture and fixtures | | $ | 616 | | | $ | 528 | |
Office equipment | | | 861 | | | | 860 | |
Leasehold improvements | | | 732 | | | | 732 | |
Vehicle | | | 57 | | | | — | |
| | | | | | | | |
| | | 2,266 | | | | 2,120 | |
Less accumulated depreciation | | | (1,495 | ) | | | (1,259 | ) |
| | | | | | | | |
Net property and equipment | | $ | 771 | | | $ | 861 | |
| | | | | | | | |
Depreciation expense was $0.3 million for each of the years ended December 31, 2009 and 2008, and was $0.2 million for the year ended December 31, 2007.
Note 6 — Accrued Liabilities
The following is a summary of accrued liabilities(in thousands):
| | | | | | |
| | | December 31, |
| | | 2009 | | 2008 |
Accrued compensation | | $ | 1,913 | | $ | 2,578 |
Accrued sales and marketing expenses | | | 1,394 | | | 1,228 |
Accrued manufacturing costs | | | 808 | | | 657 |
Accrued clinical trial expense (including $0 and $1,442 due to related party at December 31, 2009 and 2008, respectively) | | | 452 | | | 1,757 |
Accrued professional fees | | | 394 | | | 727 |
Other | | | 1,087 | | | 1,081 |
| | | | | | |
| | $ | 6,048 | | $ | 8,028 |
| | | | | | |
63
Note 7 — Long-term Liabilities
The following is a summary of long-term liabilities(in thousands):
| | | | | | |
| | | December 31, |
| | | 2009 | | 2008 |
Long-term deferred rent | | $ | 829 | | $ | 768 |
Accrued compensation | | | 150 | | | — |
Long-term lease deposit | | | — | | | 11 |
| | | | | | |
| | $ | 979 | | $ | 779 |
| | | | | | |
Note 8 — Commitments and Contingencies
Leases
In May 2007, the Company entered into a non-cancelable operating lease agreement for its corporate headquarters (“the Lease”) effective from July 1, 2007 through June 30, 2014, with an option to renew for an additional five year period. In September 2008, the Company entered into an amendment to the Lease for additional office space (“the Expansion Agreement”) that expires on June 30, 2014, with an option to renew for an additional five year period. Both the Lease and Expansion Agreements contain rent escalations of approximately 4% and 6% per year, respectively. The Company is recognizing the rental expense on a straight-line basis over the lease terms. Under the terms of the Lease and the Expansion Agreements, the Company was provided allowances in the amounts of approximately $0.2 million and $0.5 million, respectively, towards the cost of its leasehold improvements and as an incentive to rent, respectively. The Company has recorded these allowances as deferred rent which are being amortized over the lease terms as a reduction of rent expense. The leases require the Company to pay insurance and taxes and its pro-rata share of operating expenses.
The Company also leases office facilities and equipment outside the U.S. under non-cancelable operating lease agreements and subleases certain office facilities to a third party. Rent expense for the years ended December 31, 2009, 2008, and 2007 was $1.6 million, $1.1 million and $1.4 million, respectively. Future minimum lease payments and sublease rental income under non-cancelable facility and equipment operating lease agreements as of December 31, 2009, were as follows(in thousands):
| | | | | | | | | |
Year ended: | | Minimum Lease
Payments | | Sublease Rental
Income | | Net Minimum
Lease Payments |
2010 | | $ | 1,803 | | $ | 121 | | $ | 1,682 |
2011 | | | 1,784 | | | — | | | 1,784 |
2012 | | | 1,490 | | | — | | | 1,490 |
2013 | | | 1,432 | | | — | | | 1,432 |
2014 | | | 745 | | | — | | | 745 |
| | | | | | | | | |
| | $ | 7,254 | | $ | 121 | | $ | 7,133 |
| | | | | | | | | |
Note 9 — Lines-of-Credit
Silicon Valley Bank
In November 2008, the Company and its subsidiaries, SPIL and SPIL China as borrowers, entered into a loan and security agreement and an unconditional guaranty and security agreement with Silicon Valley Bank (the “Credit Facility”). The Credit Facility permits borrowing up to $6 million for a term of 36 months, subject to certain sublimits, including $2.5 million for letters of credit. The Credit Facility is secured by a first priority secured interest in all of the Company’s assets, other than intellectual property. The Credit facility expires in November 2011, and upon termination all amounts borrowed must be repaid in full. As of December 31, 2009, there were no outstanding borrowings under the Credit Facility. The Credit Facility bears interest at the bank’s
64
prime rate plus 1.75% on outstanding balances. The Company is required to meet certain financial covenants, including minimum consolidated revenue, and minimum consolidated earnings before interest and income tax, as defined, and since November 14, 2009, has been subject to certain minimum fees and interest payments. The Company is also required to meet certain operating covenants that limit its ability to incur liabilities, create liens, make capital expenditures, pay dividends or distributions, make investments, and dispose of assets and to carry credit insurance.
In November 2009, the Company received a limited waiver to the Credit Facility to waive certain minimum fees and interest payments, and to waive the requirement to maintain a credit insurance policy. The Company capitalized $0.2 million in costs associated with the origination of the facility, which are being amortized to interest and investment expense over the term of the Credit Facility.
In connection with the Credit Facility, the Company issued Silicon Valley Bank a five-year warrant to purchase 60,000 shares of the Company’s common stock at an exercise price of $0.86 per share. Accordingly, the Company recorded a cost for the fair value of the warrants issued using the Black-Scholes option pricing model with the following assumptions, risk-free interest rate of 2.29%; dividend yield of 0%; volatility factor of 68.40% and expected term of 5 years. The resulting warrant value of $30,000 is being amortized over the term of the Credit Facility.
UBS AG
On April 30, 2009, the Company was approved for a Credit Line (the “UBS Credit Line”) with UBS AG that will provide up to an aggregate amount of $1.1 million in the form of an uncommitted, demand, revolving line of credit in connection with its previous acceptance of an offer from UBS AG and its affiliates (“UBS”) of certain rights to require UBS to repurchase $1.8 million (par value) of auction rate securities that UBS had previously sold to the Company. The UBS Credit Line will be secured only by such auction rate securities and proceeds from sales of the auction rate securities will be applied to repayment of the UBS Credit Line.
Advances under the UBS Credit Line will be made on a “no net cost” basis, meaning that the interest paid by the Company on such advances will not exceed the interest or dividends paid to the Company by the issuer of the auction rate securities.
The UBS Credit Line also provides, among other things, that UBS AG may demand full or partial repayment of the UBS Credit Line or terminate and cancel the UBS Credit Line at its sole discretion and without cause at any time; provided that in such case UBS AG would be required to provide the Company alternative financing on substantially similar terms or repurchase the auction rate securities at par, unless the demand right was exercised as a result of certain specified events or the customer relationship between UBS AG and the Company is terminated for cause by UBS AG. As of December 31, 2009, the Company had no outstanding borrowings under the UBS Credit Line.
Note 10 — Related Party Transactions
The Company has licensed to Sigma-Tau exclusive ZADAXIN (thymalfasin or thymosin alpha 1) development and marketing rights that cover all countries in the European Union as defined on January 1, 1995, in addition to Iceland, Norway and Switzerland. Sigma-Tau and affiliated persons were stockholders of the Company at the time this transaction was entered into, and are currently the Company’s largest shareholder. Under the agreement with Sigma-Tau, Sigma-Tau conducted a multi-center phase 3 hepatitis C triple therapy clinical trial in Europe with 553 patients. The objective of the European trial was to provide data on ZADAXIN’s use as part of a triple therapy in treating HCV patients. The Company paid Sigma-Tau approximately $4.0 million through December 31, 2009 of funding support during the course of patient enrollment and trial period including the period of completion of the final report. The Company’s accounting policy for recording funding amounts due to Sigma-Tau was to record the amounts to research and development expense over the trial period including the period of completion of the final report. Based on the level of activity in this trial, the Company
65
recorded $0.1 million, $0.3 million, and $0.5 million of research and development expense related to this trial in the years ended December 31, 2009, 2008 and 2007, respectively. The Company had no amounts payable to Sigma-Tau as of December 31, 2009. As of December 31, 2008, the Company had accrued clinical trial expenses of $1.4 million due to Sigma-Tau.
On March 30, 2009, the Company entered into a settlement agreement (the “Settlement Agreement”) with the affiliates of Sigma-Tau (the “Sigma-Tau Group”). The Company issued a press release on March 31, 2009 (the “Press Release”), announcing the execution of the Settlement Agreement and a commercial agreement referred to herein as the “ZADAXIN Agreement,” as well as certain actions that the Company has taken to implement the provisions of the Settlement Agreement. The Press Release, Settlement Agreement and ZADAXIN Agreement are exhibits to the Company’s Current Report on Form 8-K which the Company filed with the Securities and Exchange Commission on April 1, 2009. Under the Settlement Agreement, Dr. Camerini, Dr. Jones and Mr. Lapointe were appointed to our Board of Directors and pursuant to the unanimous recommendation of our Board, were elected by our stockholders at the 2009 annual meeting of stockholders. Certain obligations under the Settlement Agreement have expired and it expires in its entirety on or around June 15, 2010. The Company is no longer under any obligation to nominate Dr. Camerini, Dr. Jones and Mr. Lapointe. However, the Board has independently decided to re-nominate them at the annual stockholder’s meeting in 2010.
Except as a result of the relationships of Dr. Camerini, Dr. Jones and Mr. Lapointe with Sigma Tau, none of the Company’s officers or directors, other than, as set forth above, was involved in related party transactions in 2009.
Note 11 — Income Taxes
The Company recorded income tax expense of $0.6 million, $0.5 million, and $0.3 million for the years ended December 31, 2009, 2008 and 2007, respectively, related to its operations in China. The Company’s statutory tax rate in China is 20% for 2009. The Company has not recorded any federal or state income tax expense for the years ended December 31, 2009, 2008 and 2007. Undistributed earnings of the Company’s foreign subsidiaries that are considered to be permanently invested outside the U.S. and for which no U.S. taxes have been provided amounted to approximately $2.2 million at December 31, 2009. The tax years 1996-2009 remain open to examination by the major taxing jurisdictions to which the Company is subject.
The domestic and foreign components of pre-tax income (loss) for the years ended December 31 are as follows(in thousands):
| | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
Domestic | | $ | (19,554 | ) | | $ | (25,922 | ) | | $ | (18,975 | ) |
Foreign | | | 32,140 | | | | 18,049 | | | | 9,328 | |
| | | | | | | | | | | | |
Pre-tax income (loss) | | $ | 12,586 | | | $ | (7,873 | ) | | $ | (9,647 | ) |
| | | | | | | | | | | | |
A reconciliation of the statutory federal income tax rate of 34% to the actual tax rate for the years ended December 31 is as follows(in thousands):
| | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
Tax at federal statutory rate | | $ | 4,279 | | | $ | (2,677 | ) | | $ | (3,280 | ) |
Foreign income tax at different rates | | | (10,279 | ) | | | (5,661 | ) | | | (2,870 | ) |
Taxable dividend from foreign subsidiary | | | 5,440 | | | | 4,177 | | | | 3,041 | |
Net operating losses not benefited | | | 916 | | | | 4,239 | | | | 3,162 | |
Stock based compensation | | | 122 | | | | 91 | | | | 94 | |
Non deductible expenses | | | 281 | | | | 308 | | | | 192 | |
Other | | | (118 | ) | | | (2 | ) | | | (38 | ) |
| | | | | | | | | | | | |
Income tax expense | | $ | 641 | | | $ | 475 | | | $ | 301 | |
| | | | | | | | | | | | |
66
Significant components of the Company’s deferred tax assets at December 31 are as follows(in thousands):
| | | | | | | | |
| | | 2009 | | | 2008 | |
Net operating loss carryforwards | | $ | 47,008 | | | $ | 50,480 | |
Research and development credit carryforwards | | | 8,010 | | | | 9,631 | |
Other | | | 4,731 | | | | 5,815 | |
| | | | | | | | |
Gross deferred tax assets | | | 59,749 | | | | 65,926 | |
Valuation allowance | | | (59,749 | ) | | | (65,926 | ) |
| | | | | | | | |
Total deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
Realization of deferred tax assets is dependent upon the Company generating future taxable income, the timing and amount of which are uncertain. Accordingly, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance (decreased) increased by approximately $(6.2) million, $13.1 million, and $0.8 million, in the years ended December 31, 2009, 2008 and 2007, respectively.
At December 31, 2009, the Company had federal net operating loss carryforwards of approximately $130.6 million that expire in the years 2010 through 2029, and federal research and development, orphan drug and investment tax credit carryforwards of approximately $9.5 million that expire in the years 2010 through 2029. At December 31, 2009, the Company has state net operating loss carryforwards of approximately $40.0 million that begin to expire in the year 2012, if not utilized, and state research and development tax credit carryforwards of approximately $1.7 million that do not expire.
Approximately $3.6 million of the valuation allowance relates to benefits associated with stock option deductions that, when recognized, will be credited directly to stockholders’ equity.
Utilization of the Company’s net operating loss and credit carryforwards may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such annual limitation could result in the expiration of the net operating loss and credit carryforwards before utilization.
As of December 31, 2009, the unrecognized tax benefit was $2.8 million, all of which is offset by a full valuation allowance. A reconciliation of the beginning and ending amount of unrecognized tax benefit is as follows(in thousands):
| | | |
Balance as of January 1, 2009 | | $ | — |
Tax positions related to current year: | | | |
Additions for current year items | | | 261 |
Additions for prior year items | | | 2,557 |
| | | |
Balance as of December 31, 2009 | | $ | 2,818 |
| | | |
Note 12 — Restructuring Charge
In October 2009, the Company implemented a reduction in its workforce of seven full-time employees, primarily in research and development. The restructuring follows the discontinuation of the Company’s RP101 phase 2 clinical trial and reflects the Company’s focus on a cost-containing clinical development strategy and expense management. The Company completed the restructuring in the fourth quarter of 2009. The reduction in workforce resulted in a one-time severance-related charge of approximately $0.3 million, which was primarily recognized in research and development expenses in the fourth quarter of 2009. As of December 31, 2009, substantially all severance-related charges have been paid.
67
Note 13 — Stockholders’ Equity
Stock Award Plans
The Company’s 1991 Stock Plan, 1992 Stock Plan and 1995 Equity Incentive Plan (collectively the “1991, 1992 and 1995 Plans”) have reserved 3,450,000, 240,000 and 6,100,000 shares of common stock for issuance, respectively. The 1991, 1992 and 1995 Plans permit the grant of incentive stock options, nonstatutory stock options and other forms of equity compensation. Although the 1991, 1992 and 1995 Plans have expired, the outstanding stock options relating to them are fully valid.
The Company’s 2005 Equity Incentive Plan (the “2005 Plan”) has reserved 7,800,000 shares of common stock for issuance. The 2005 Plan permits the grant of incentive stock options, nonstatutory stock options, restricted stock units, performance shares and other forms of equity compensation. As of December 31, 2009, approximately 1,648,000 shares of common stock were available for future issuance under the 2005 Plan.
Under the 1991, 1992, 1995 and 2005 Plans, options are exercisable upon conditions determined by the Board of Directors and expire ten years from the date of grant. Options are generally granted at fair market value on the date of grant and vest over time, generally four years, or upon achievement of certain market and service conditions. See Stock-Based Compensation.
The Company’s 1995 Nonemployee Director Stock Option Plan (the “1995 Director Plan”) had reserved 750,000 shares of common stock for issuance. The 1995 Director Plan permits the grant of nonqualified stock options to nonemployee directors. Although the 1995 Directors Plan has expired, the outstanding stock options relating to it are fully valid.
The Company’s 2004 Outside Directors Stock Option Plan (the “2004 Director Plan”) has reserved 1,765,000 shares of common stock for issuance. The 2004 Director Plan automatically grants nonqualified stock options to nonemployee directors upon their appointment or first election to the Company’s Board of Directors (“Initial Grant”) and annually upon their reelection to the Board of Directors at the Company’s Annual Meeting of Stockholders (“Annual Grant”). As of December 31, 2009, approximately 635,000 shares of common stock were available for future issuance under the 2004 Director Plan.
Under the 1995 and 2004 Director Plans, options are granted at fair market value on the date of grant and expire ten years from the date of grant. Initial Grants become exercisable in three equal annual installments beginning on the first anniversary of the date of grant, and Annual Grants become exercisable in twelve equal monthly installments from the date of grant, subject in each case to the director’s continuous service on the Company’s Board of Directors.
Certain stock option awards are subject to accelerated vesting if there is a change in control.
Stock-Based Compensation
The compensation cost that has been charged against income for the stock award plans was $1.9 million, $1.8 million and $2.2 million, respectively, for the years ended December 31, 2009, 2008, and 2007. Compensation cost capitalized in inventory was $35,000, $49,000 and $0.1 million, respectively, for the years ended December 31, 2009, 2008, and 2007. There has been no income tax benefit recognized in the income statement for share-based compensation arrangements.
68
The fair value of each non performance-based and non target stock-price-based option granted under the Company’s stock award plans is estimated on the date of grant using the Black-Scholes option valuation model and the single option approach with the following weighted-average assumptions for the years ended December 31:
| | | | | | | | | |
Stock Option Plan | | 2009 | | | 2008 | | | 2007 | |
Risk-free interest rate | | 1.79 | % | | 2.76 | % | | 4.64 | % |
Dividend yield | | 0.00 | % | | 0.00 | % | | 0.00 | % |
Volatility factor of the expected market price of our common stock | | 70.38 | % | | 66.57 | % | | 70.01 | % |
Weighted-average expected life of option (years) | | 4.83 | | | 4.83 | | | 4.64 | |
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The expected dividend yield is based on the Company’s history and expectations of no dividend payouts. Expected volatility is based on the historical volatility of the Company’s stock. The expected term of options granted is derived from historical data on employee exercises and terminations.
The following table summarizes stock option activity as of December 31, 2009, and changes during the year then ended is presented below(in thousands, except per share and year amounts):
| | | | | | | | | | | | | | |
| | | | | | Options Outstanding |
| | | Shares
Available For
Grant | | | Number
of
Shares | | | Weighted-
Average
Exercise
Price Per
Share | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value |
Balance at December 31, 2008 | | 3,603 | | | 8,343 | | | $ | 3.34 | | | | | |
Options cancelled | | 1,674 | | | (1,674 | ) | | $ | 2.94 | | | | | |
Options granted | | (2,269 | ) | | 2,269 | | | $ | 1.28 | | | | | |
Options exercised | | — | | | (999 | ) | | $ | 2.63 | | | | | |
Plan shares expired | | (725 | ) | | — | | | | | | | | | |
| | | | | | | | | | | | | | |
Balance at December 31, 2009 | | 2,283 | | | 7,939 | | | $ | 2.93 | | 6.34 | | $ | 3,181 |
| | | | | | | | | | | | | | |
Vested and expected to vest afterDecember 31, 2009 | | | | | 7,038 | | | $ | 3.07 | | 6.12 | | $ | 2,623 |
Exercisable at December 31, 2009 | | | | | 4,184 | | | $ | 4.08 | | 4.52 | | $ | 306 |
The Company has granted to its chief executive officer target-stock-price-based options to purchase 600,000 and 300,000 shares of the Company’s common stock at an exercise price per share of $2.49 and $1.81, respectively. The options have a term of 10 years and shares of such option will vest upon the Company’s common stock trading for at least 30 consecutive calendar days at or greater than a target closing stock price as reported on The NASDAQ Stock Market, of (a) $4.50 on or before June 2, 2009 for 150,000 shares, (b) $6.00 on or before June 2, 2010 for 150,000 shares, (c) $8.00 on or before June 2, 2011 for 150,000 shares, (d) $10.00 on or before June 2, 2012 for 150,000 shares, (e) $12.00 on or before June 2, 2013 for 150,000 shares, and (f) $14.00 on or before June 2, 2014 for 150,000 shares, each price as adjusted for stock dividends, stock splits or similar changes in the Company’s capital structure. These grants are considered awards with market and service conditions and compensation expense is recognized for an option with market conditions as long as service requirements are met, even if the market conditions are not reached. Because the probability of satisfying the market conditions had to be considered in the estimate of grant-date fair value for these awards, the Monte Carlo simulation option pricing model was used to calculate the grant date fair value per share of $1.70 and $0.88, respectively, related to each of the six vesting portions of these awards with the assumptions of a risk-free interest rate of 5.10% and 3.88% respectively, a volatility factor of 94% and 89.14%, respectively, dividend yield of 0%, and an expected life of 10 years. The related compensation costs are being expensed over the service periods for each of the six vesting portions of the awards.
69
The Company has granted to certain employees performance-based options to purchase shares of the Company’s common stock at an exercise price equal to the closing price of a share of the Company’s common stock as of the grant date. The options will fully vest upon the employee meeting a performance goal within an established time frame. If the performance goal is met for the option within the established time frame, the option has a ten year term measured from the date of grant. If the performance goal is not met within the established time frame, the option expires in its entirety. The grant date fair value per share of the awards has been calculated using the Black-Scholes option pricing model using the following assumptions: expected term of 4.83 years, volatility factor ranging from 69.06 to 70.19%, and risk free interest rates ranging from 1.72-3.07%. The Company recognizes expense related to the performance-based options over the period of time the Company determines that it is probable that the performance goal will be achieved.
During 2009, the Company extended the period in which two of the Company’s departed board members may exercise their outstanding vested stock options following the cessation of their service to the Company from ninety days to the second anniversary of the date of cessation of service which ceased on June 9, 2009. The Company recorded expense of $0.1 million for the year ended December 31, 2009 related to these modifications.
The weighted-average fair value of stock options granted for the years ended December 31, 2009, 2008 and 2007 was $0.76, $0.98, and $1.58, respectively. The intrinsic value of options at time of exercise was $1.7 million, $0.1 million, and $0.1 million for the years ended December 31, 2009, 2008, and 2007, respectively. The estimated fair value of shares vested for the years ended December 31, 2009, 2008, and 2007 was $1.5 million, $1.6 million, and $1.9 million, respectively. As of December 31, 2009, unamortized compensation expense related to unvested options was approximately $2.3 million. The weighted average period over which compensation expense related to these options will be recognized is approximately 3.1 years. Cash received from stock option exercises was $2.6 million, $0.1 million, and $0.2 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Employee Stock Purchase Plan
The Company’s 1996 Employee Stock Purchase Plan (the “ESPP”) has reserved 1,000,000 shares for issuance thereunder. Under the terms of the ESPP, eligible employees may choose to have up to 15% of their salary withheld to purchase the Company’s common stock. The purchase price of the stock shall be equal to 95% of the fair market value of a share of the Company’s common stock on the purchase date (or such other amount as may be established by the Company’s Board of Directors). No ESPP shares were issued by the Company in 2009, 2008 or 2007. As of December 31, 2009, approximately 283,000 shares of common stock were available for issuance under the ESPP.
Stockholder Rights Agreement
On December 18, 2006, the Company’s Board of Directors declared a dividend distribution of one Preferred Stock Purchase Right (collectively, the “Rights”) for each outstanding share of the Company’s Common Stock, each Right which entitles the registered holder to purchase from the Company one one-thousandth of a share of the Company’s Series D Preferred Stock, $0.001 par value, at a price of $25.00 pursuant to a Rights Agreement dated as of December 19, 2006, between the Company and Mellon Investor Services LLC (the “Rights Agreement”). The Rights, which will initially trade with the Common Stock, become exercisable when a person or group acquires 15% or more of the Company’s Common Stock without prior Board approval. In that event, the Rights permit the Company’s stockholders, other than the acquiror, to purchase the Company’s Common Stock having a market value of twice the exercise price of the Rights, in lieu of the Preferred Stock. Alternatively, when the Rights become exercisable, the Company’s Board of Directors may authorize the issuance of one share of the Company’s Common Stock in exchange for each Right that is then exercisable. In addition, in the event of certain business combinations, the Rights permit the purchase of the Common Stock of an acquiror at a 50% discount. Rights held by the acquiror will become null and void in each case. Prior to a person or group acquiring 15%, the Rights can be redeemed for $0.001 each by action of the Board. The Rights
70
Agreement contains an exception to the 15% ownership threshold for shares currently beneficially owned by Sigma-Tau Finanziaria S.p.A. The Rights expire on December 19, 2016. The Rights Agreement includes a requirement that a committee of independent directors evaluate the Rights Agreement at least every three years.
Note 14 — 401k Plan
The Company has a pre-tax savings plan covering substantially all U.S. employees, which qualifies under Section 401(k) of the Internal Revenue Code. Under the plan, eligible employees may contribute a portion of their pre-tax salary, subject to certain limitations. The Company contributes and matches 50% of the employee contributions, up to 15% of an employee’s salary. Company contributions, which can be terminated at the Company’s discretion, were approximately $0.2 million for each of the years ended December 31, 2009, 2008, and 2007.
Note 15 — Significant Geographic Information
The Company operates in one business segment: the development and commercialization of novel therapeutics to treat chronic and life threatening diseases. Through December 31, 2009, all of the Company’s product revenues were generated from the sale of ZADAXIN.
The President and CEO has been identified as the Chief Operating Decision Maker (“CODM”) because he has final authority over resource allocation decisions and performance assessment. The CODM does not receive discrete financial information at a level below that of the business segment.
The Company’s domestic operations primarily consist of research and development. The Company’s wholly owned international subsidiary, SciClone Pharmaceuticals International Ltd., is engaged in sales and marketing and product distribution worldwide.
Information regarding geographic areas is as follows(in thousands):
| | | | | | | | | |
| | | Product Sales
Revenue for
the Year
Ended
December 31, | | Contract
Revenue for
the Year
Ended
December 31, | | Long Lived
Assets
December 31, |
2009 | | | | | | | | | |
U.S. | | $ | — | | $ | — | | $ | 877 |
China | | | 69,696 | | | — | | | 217 |
Rest of world | | | 2,715 | | | — | | | 926 |
| | | | | | | | | |
Total | | $ | 72,411 | | $ | — | | $ | 2,020 |
| | | | | | | | | |
2008 | | | | | | | | | |
U.S. | | $ | — | | $ | — | | $ | 1,549 |
China | | | 50,724 | | | — | | | 287 |
Rest of world | | | 3,384 | | | 5 | | | 180 |
| | | | | | | | | |
Total | | $ | 54,108 | | $ | 5 | | $ | 2,016 |
| | | | | | | | | |
2007 | | | | | | | | | |
U.S. | | $ | — | | $ | — | | $ | 1,798 |
China | | | 34,240 | | | — | | | 137 |
Rest of world | | | 2,798 | | | 20 | | | 265 |
| | | | | | | | | |
Total | | $ | 37,038 | | $ | 20 | | $ | 2,200 |
| | | | | | | | | |
71
Note 16 — Selected Quarterly Financial Data (unaudited)
| | | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31 | | | June 30 | | | September 30 | | December 31 | |
| | | (in thousands, except per share amounts) | |
2009 | | | | | | | | | | | | | | | |
Product sales | | $ | 15,069 | | | $ | 21,971 | | | $ | 17,240 | | $ | 18,131 | |
Contract revenue | | | — | | | | — | | | | — | | | — | |
Cost of product sales | | | 2,665 | | | | 3,422 | | | | 3,098 | | | 2,775 | |
Gross margin | | | 12,404 | | | | 18,549 | | | | 14,142 | | | 15,356 | |
Net income | | | 96 | | | | 7,339 | (1) | | | 2,064 | | | 2,446 | |
Basic and diluted net income per share | | $ | 0.00 | | | $ | 0.16 | | | $ | 0.04 | | $ | 0.05 | |
2008 | | | | | | | | | | | | | | | |
Product sales | | $ | 10,634 | | | $ | 13,834 | | | $ | 14,328 | | $ | 15,312 | |
Contract revenue | | | — | | | | — | | | | — | | | 5 | |
Cost of product sales | | | 2,105 | | | | 2,584 | | | | 2,419 | | | 3,191 | |
Gross margin | | | 8,529 | | | | 11,250 | | | | 11,909 | | | 12,126 | |
Net (loss) income | | | (5,659 | )(2) | | | (319 | ) | | | 773 | | | (3,143 | )(3) |
Basic and diluted net (loss) income per share | | $ | (0.12 | ) | | $ | (0.01 | ) | | $ | 0.02 | | $ | (0.07 | ) |
| (1) | During the three months ended June 30, 2009, product sales increased attributable to higher volume of ZADAXIN which the Company believes was primarily due to increased demand as a result of the H1N1 flu virus and further market penetration in China. |
| (2) | During the three months ended March 31, 2008, research and development expenses increased as a result of the Company’s phase 2 trial for RP101. |
| (3) | During the three months ended December 31, 2008, operating expenses increased primarily related to the Company’s phase 2 trial for RP101, the purchase and manufacture of drugs for use in the Company’s clinical trials, and due to an increase in legal and professional fees. |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, the Company carried out an evaluation, under the supervision and with the participation of its management, including the Company’s President and Chief Executive Officer and its Senior Vice President and Chief Financial Officer, of the effectiveness of the design and operation of the Company’s disclosure controls and procedures in ensuring that material information required to be disclosed in the Company’s reports filed or submitted under the Securities Exchange Act of 1934, as amended, has been made known to them in a timely fashion. Based on this evaluation, the Company’s President and Chief Executive Officer and its Senior Vice President and Chief Financial Officer concluded that the Company’s disclosure controls and procedures are effective in reaching a reasonable level of assurance that information required to be disclosed by the Company in the reports that it files or submits under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
72
Management’s Annual Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. All control systems have inherent limitations so that no evaluation of controls can provide absolute assurance that all control issues are detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, we assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2009, based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on this assessment and those criteria, management believes that the Company maintained effective internal control over financial reporting as of December 31, 2009.
Ernst & Young LLP, an independent registered public accounting firm, has audited and issued an attestation report on the Company’s internal control over financial reporting as of December 31, 2009, as stated in their report which is included below.
73
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of SciClone Pharmaceuticals, Inc.
We have audited SciClone Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). SciClone Pharmaceuticals, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, SciClone Pharmaceuticals, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the 2009 consolidated financial statements of SciClone Pharmaceuticals, Inc. and our report dated March 16, 2010 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 16, 2010
74
Changes in Internal Controls
There has been no change in the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Act of 1934, as amended) that was identified in connection with the evaluation thereof that occurred during the fourth quarter of 2009 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
Limitations of the Effectiveness of Internal Controls
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the internal control system are met. Because of inherent limitations in any control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. We are continuously seeking to improve the efficiency and effectiveness of our operations and of our internal controls. This results in refinements to processes throughout our organization.
| Item 9B. | Other Information |
None.
PART III
Certain information required by Part III is incorporated by reference from our definitive Proxy Statement to be filed with the Securities and Exchange Commission in connection with the solicitation of proxies for our 2010 Annual Meeting of Stockholders (the “Proxy Statement”) not later than 120 days after the end of the fiscal year covered by this report, and certain information therein is incorporated in this report by reference.
| Item 10. | Directors, Executive Officers, and Corporate Governance |
The information required by Item 401 of Regulation S-K is incorporated by reference from the Proxy Statement under the caption “Proposal No. 1 Election of Directors — Nominees,” and “Proposal No. 1 Election of Directors — Board Meetings and Committees — Audit Committee.” Information relating to the executive officers of the Company is incorporated by reference from the Proxy Statement under the caption “Executive Compensation and Other Matters — Executive Officers”.
The information required by Item 405 of Regulation S-K is incorporated by reference from the Proxy Statement under the caption “Compensation Committee Report — Section 16(a) Beneficial Ownership Reporting Compliance”.
Code of Ethics
We have adopted a code of business conduct and ethics, and a policy providing for the reporting of potential violations of the code, for directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller) and employees, known as the Corporate Code of Conduct and Ethics (including “Whistle blowing” in the case of Violations of the Company Policies) (the “Code of Conduct”). The Code of Conduct is available on our website atwww.sciclone.com under the section “Investor Relations — Corporate Governance”. Additionally, stockholders may request a free copy of the Code of Conduct by contacting the Investor Relations Department at our corporate offices by calling 800-724-2566 or by sending an e-mail message to investorrelations@sciclone.com.
| Item 11. | Executive Compensation |
The information required by this Item 11 is incorporated by reference from the Proxy Statement under the captions “Executive Compensation and Other Matters” and “Corporate Governance — Compensation of Directors.”
75
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides certain information regarding our compensation plans in effect as of December 31, 2009(in thousands, except per share amounts):
| | | | | | | |
Plan Category | | Number of securities
to be issued upon
exercise of
outstanding
options,
warrants and
rights
(a) | | Weighted-
average exercise
price of
outstanding
options,
warrants and
rights
(b) | | Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding securities
reflected in column (a))
(c) |
Equity compensation plans approved by security holders: | | | | | | | |
1991 Stock Plan(1) | | 68 | | $ | 9.08 | | — |
1992 Stock Plan(1) | | 32 | | | 10.75 | | — |
1995 Equity Incentive Plan(1) | | 1,183 | | | 5.91 | | — |
1995 Nonemployee Director Stock Option Plan(1) | | 270 | | | 7.17 | | — |
1996 Employee Stock Purchase Plan | | — | | | — | | 283 |
2004 Outside Directors Stock Option Plan | | 1,130 | | | 2.66 | | 635 |
2005 Equity Incentive Plan | | 5,256 | | | 1.97 | | 1,648 |
Equity compensation plans not approved by security holders: | | — | | | — | | — |
| | | | | | | |
Total | | 7,939 | | $ | 2.93 | | 2,566 |
| | | | | | | |
| (1) | Although the 1991 Stock Plan, 1992 Stock Plan, 1995 Equity Incentive Plan and 1995 Nonemployee Director Stock Option Plan have expired, the outstanding stock options relating to them are fully valid. |
The information required by Item 403 of Regulation S-K is incorporated by reference from the Proxy Statement under the caption “Security Ownership of Certain Beneficial Owners and Management.”
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item is incorporated by reference from the Proxy Statement under the captions “Compensation Committee Report — Transactions with Related Persons” and “Corporate Governance — Director Independence.”
| Item 14. | Principal Accounting Fees and Services |
The information required by this Item 14 is incorporated by reference from the Proxy Statement under the caption “Proposal No. 2 Ratification of Appointment of Independent Registered Public Accounting Firm — Principal Accountant Fees.”
PART IV
| Item 15. | Exhibits, Financial Statement Schedules |
Item 15 (a). The following documents are filed as part of this Annual Report on Form 10-K:
(1)Financial Statements.The following financial statements of the Company are contained in Item 8, Part II on pages 50-72 of this Annual Report on Form 10-K:
Report of Independent Registered Public Accounting Firm.
76
Consolidated Balance Sheets at December 31, 2009 and 2008.
Consolidated Statements of Operations for each of the three years in the period ended December 31, 2009.
Consolidated Statements of Stockholders’ Equity for the three years ended December 31, 2009.
Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2009.
Notes to Consolidated Financial Statements.
(2)Financial Statement Schedules
The following schedule required to be filed by Item 8 of this form and Item 15(d) is contained on page 82 of this Report:
Schedule II — Valuation and Qualifying Accounts for each of the three years in the period ended December 31, 2009.
All other schedules have been omitted because they are either inapplicable or the required information has been given in the consolidated financial statements or the notes thereto.
(3)Exhibits.
Refer to Item 15(b) below.
Item 15 (b). Exhibits.
Exhibits (numbered in accordance with Item 601 of Regulation S-K):
| | |
Exhibit Number | | Description |
| |
| 3(i).1(1) | | Amended and Restated Certificate of Incorporation. |
| |
| 3(i).2(17) | | Certificate of Designation, Preferences and Rights of the Terms of the Series D Preferred Stock, as filed by SciClone Pharmaceuticals, Inc. with the Secretary of State of the State of Delaware as of December 22, 2006. |
| |
| 3(ii).1(1) | | Bylaws. |
| |
| 4.9(16) | | Rights Agreement, effective as of December 19, 2006, between the Company and Mellon Investor Services LLC, as rights agent (including as Exhibit A the form of Certificate of Designation, Preferences and Rights of the Series D Preferred Stock, as Exhibit B the form of Right Certificate, and as Exhibit C the Summary of Terms of Rights Agreement). |
| |
| 10.1(3)** | | Registrant’s 1991 Stock Plan, together with forms of agreements thereunder. |
| |
| 10.2(2)** | | Registrant’s 1992 Stock Plan, together with forms of agreements thereunder. |
| |
| 10.3(4)** | | Registrant’s 1995 Equity Incentive Plan, together with forms of agreement thereunder. |
| |
| 10.4(4)** | | Registrant’s 1995 Nonemployee Director Stock Option Plan, together with forms of agreement thereunder. |
| |
| 10.5(9)** | | Registrant’s 1996 Employee Stock Purchase Plan, as amended. |
| |
| 10.6(15)** | | Registrant’s 2005 Equity Incentive Plan, effective as of June 7, 2005, as amended on February 22, 2007. |
77
| | |
Exhibit Number | | Description |
| |
| 10.7(15)** | | Registrant’s 2004 Outside Directors Stock Option Plan, as amended on June 7, 2005 and as further amended on February 22, 2007. |
| |
| 10.18(9)** | | Change in Control Agreement between the Company and Hans P. Schmid dated as of April 22, 2003. |
| |
| 10.19(10)** | | Form of Indemnity Agreement by and between the Registrant and each director and executive officer of SciClone Pharmaceuticals, Inc. |
| |
| 10.22(5) | | Alpha Rights Acquisition Agreement by and between the Registrant and Alpha 1 Biomedicals, Inc., dated December 17, 1997. |
| |
| 10.23(6)* | | Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement by and between the Company and Sigma-Tau Industrie Farmadeutiche Riunite S.p.A. dated as of March 3, 2000. |
| |
| 10.24(8)* | | Amendment No. 1 to the Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement by and between the Company and Sigma-Tau Farmadeutiche Riunite S.p.A. dated as of December 19, 2001. |
| |
| 10.25(12)* | | Amendment No. 2 to the Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement between the Registrant and Sigma-Tau Industrie Farmacetiche Riunite S.p.A., dated May 20, 2004. |
| |
| 10.26(12)* | | Amendment No. 3 to the Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement between the Registrant and Sigma-Tau Industrie Farmacetiche Riunite S.p.A., dated May 21, 2004. |
| |
| 10.27(7) | | Acquisition Agreement between the Company and Sclavo S.p.A. dated April 20, 1998. |
| |
| 10.28(7) | | First Amendment to Acquisition Agreement between the Company and Sclavo S.p.A., dated April 20, 1998. |
| |
| 10.31(11)* | | Manufacturing and Supply Agreement between SciClone Pharmaceuticals International Ltd. and Patheon Italia S.p.A. dated as of November 1, 2002. |
| |
| 10.39(13)** | | Amendment to Offer Letter dated as of February 24, 2006 by and between SciClone Pharmaceuticals International, Ltd. and Hans P. Schmid including offer letter dated May 21, 2001 between SciClone Pharmaceuticals International, Ltd. and Hans P. Schmid as Exhibit A thereto. |
| |
| 10.41(14)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.42(14)** | | Change in Control Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.52(18)* | | Manufacturing Supply Agreement Between SciClone Pharmaceuticals International Ltd. and Lonza Sales Ltd., executed as of May 23, 2008. |
| |
| 10.53(20)* | | Loan and Security Agreement by and among SciClone Phamaceuticals International Ltd., SciClone Pharmaceuticals International China Holding Ltd., and Silicon Valley Bank, dated as of November 14, 2008. |
| |
| 10.54(19)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Gary Titus, dated as of November 21, 2008 and effective as of December 8, 2008. |
| |
| 10.55(21) | | Settlement Agreement between Sigma-Tau Finanziaria, S.p.A. and SciClone Pharmaceuticals, Inc. dated March 30, 2009. |
78
| | |
Exhibit Number | | Description |
| |
| 10.56(21) | | Letter Agreement related to Thymosin Alpha 1 between SciClone Pharmaceuticals, Inc. and Sigma-Tau Industrie Farmaceutiche Riunite S.p.A., dated March 30, 2009. |
| |
| 10.57(22)** | | Amendment No. 1 to Change of Control Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.58(22)** | | Amendment No. 1 to Employment Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.59(23)** | | Change in Control Agreement between Mr. Gary Titus and SciClone Pharmaceuticals, Inc. dated December 8, 2008. |
| |
| 10.60(23) | | Amendment No. 1 to Loan and Security Agreement between Silicon Valley Bank, SciClone Pharmaceuticals International Ltd., and SciClone Pharmaceuticals International China Holding Ltd. dated March 30, 2009. |
| |
| 10.61(24) | | Description of Executive Incentive Plan. |
| |
| 14 | | See “Code of Ethics” in Item 10: Executive Officers and Directors, of this Annual Report on Form 10-K. |
| |
| 21.1(24) | | Subsidiaries of Registrant. |
| |
| 23.1(24) | | Consent of Independent Registered Public Accounting Firm. |
| |
| 24.1(24) | | Powers of Attorney. See page 81. |
| |
| 31.1(24) | | Rule 13a-14(a) Certification of Principal Executive Officer. |
| |
| 31.2(24) | | Rule 13a-14(a) Certification of Principal Financial Officer. |
| |
| 32.1(24) | | Section 1350 Certification of Principal Executive Officer. |
| |
| 32.2(24) | | Section 1350 Certification of Principal Financial Officer. |
| * | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4), 200.83 and 230.46. |
| ** | Management compensatory plan or arrangement. |
| (1) | Incorporated by reference from the Company’s Current Report on 8-K filed on July 28, 2003. |
| (2) | Incorporated by reference from the Company’s Registration Statement on Form S-l (No. 33-45446), declared effective by the Commission on March 17, 1992. |
| (3) | Incorporated by reference from the Company’s Registration Statement on Form S-8 (No. 33-66832) filed with the Commission on August 3, 1993. |
| (4) | Incorporated by reference from the Company’s Registration Statement on Form S-8 (No. 33-80911) filed with the Commission on December 28, 1995. |
| (5) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on January 26, 1998. |
| (6) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 20, 2000. |
| (7) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 1998. |
| (8) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2002. |
79
| (9) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2003. |
| (10) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2003. |
| (11) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. |
| (12) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2004. |
| (13) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on February 28, 2006. |
| (14) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 25, 2006. |
| (15) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2007 filed on August 8, 2007. |
| (16) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 22, 2006. |
| (17) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 28, 2006. |
| (18) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2008 filed on August 1, 2008. |
| (19) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 4, 2008. |
| (20) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2008. |
| (21) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 1, 2009. |
| (22) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 8, 2009. |
| (23) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 11, 2009. |
Item 15 (c). See Item 15(a) above.
80
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| SCICLONE PHARMACEUTICALS, INC. |
| |
| By: | | /S/ FRIEDHELM BLOBEL, PH.D. |
| | Friedhelm Blobel, Ph.D. President and Chief Executive Officer (Principal Executive Officer) |
Date: March 16, 2010
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Friedhelm Blobel, Ph.D. and Gary Titus, and each of them, his attorneys-in-fact and agents, each with the power of substitution and resubstitution, for him in any and all capacities, to sign this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting to said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary, to be done in connection therewith, as fully as to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/S/ FRIEDHELM BLOBEL, PH.D. (Friedhelm Blobel, Ph.D.) | | President and Chief Executive Officer, Director (Principal Executive Officer) | | March 16, 2010 |
| | |
/S/ GARY S. TITUS (Gary S. Titus) | | Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | | March 16, 2010 |
| | |
/S/ ROBERTO CAMERINI, M.D. (Roberto Camerini, M.D.) | | Director | | March 16, 2010 |
| | |
/S/ RICHARD J. HAWKINS (Richard J. Hawkins) | | Director | | March 16, 2010 |
| | |
/S/ TREVOR M. JONES, PH.D. (Trevor M. Jones, Ph.D.) | | Director | | March 16, 2010 |
| | |
/S/ IRA D. LAWRENCE, M.D. (Ira D. Lawrence. M.D.) | | Director | | March 16, 2010 |
| | |
/S/ GREGG A. LAPOINTE (Gregg A. Lapointe) | | Director | | March 16, 2010 |
| | |
/S/ JON S. SAXE (Jon S. Saxe) | | Chairman of the Board of Directors | | March 16, 2010 |
| | |
/S/ DEAN S. WOODMAN (Dean S. Woodman) | | Director | | March 16, 2010 |
81
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
SCICLONE PHARMACEUTICALS, INC.
Accounts Receivable Allowances for Uncollectible Accounts (in thousands):
| | | | | | | | | | | | | | | | |
| | | Balance at
Beginning
of Period | | Charged to
Costs and
Expenses | | Deductions for
Amounts
Recovered | | | Deductions for
Amounts
Written Off | | Balance at
End of
Period |
Year Ended December 31, 2009 | | $ | — | | $ | — | | $ | — | | | $ | — | | $ | — |
Year Ended December 31, 2008 | | $ | 15 | | $ | — | | $ | (15 | ) | | $ | — | | $ | — |
Year Ended December 31, 2007 | | $ | 50 | | $ | — | | $ | (35 | ) | | $ | — | | $ | 15 |
82
INDEX TO EXHIBITS
| | |
Exhibit Number | | Description |
| 3(i).1(1) | | Amended and Restated Certificate of Incorporation. |
| |
| 3(i).2(17) | | Certificate of Designation, Preferences and Rights of the Terms of the Series D Preferred Stock, as filed by SciClone Pharmaceuticals, Inc. with the Secretary of State of the State of Delaware as of December 22, 2006. |
| |
| 3(ii).1(1) | | Bylaws. |
| |
| 4.9(16) | | Rights Agreement, effective as of December 19, 2006, between the Company and Mellon Investor Services LLC, as rights agent (including as Exhibit A the form of Certificate of Designation, Preferences and Rights of the Series D Preferred Stock, as Exhibit B the form of Right Certificate, and as Exhibit C the Summary of Terms of Rights Agreement). |
| |
| 10.1(3)** | | Registrant’s 1991 Stock Plan, together with forms of agreements thereunder. |
| |
| 10.2(2)** | | Registrant’s 1992 Stock Plan, together with forms of agreements thereunder. |
| |
| 10.3(4)** | | Registrant’s 1995 Equity Incentive Plan, together with forms of agreement thereunder. |
| |
| 10.4(4)** | | Registrant’s 1995 Nonemployee Director Stock Option Plan, together with forms of agreement thereunder. |
| |
| 10.5(9)** | | Registrant’s 1996 Employee Stock Purchase Plan, as amended. |
| |
| 10.6(15)** | | Registrant’s 2005 Equity Incentive Plan, effective as of June 7, 2005, as amended on February 22, 2007. |
| |
| 10.7(15)** | | Registrant’s 2004 Outside Directors Stock Option Plan, as amended on June 7, 2005 and as further amended on February 22, 2007. |
| |
| 10.18(9)** | | Change in Control Agreement between the Company and Hans P. Schmid dated as of April 22, 2003. |
| |
| 10.19(10)** | | Form of Indemnity Agreement by and between the Registrant and each director and executive officer of SciClone Pharmaceuticals, Inc. |
| |
| 10.22(5) | | Alpha Rights Acquisition Agreement by and between the Registrant and Alpha 1 Biomedicals, Inc., dated December 17, 1997. |
| |
| 10.23(6)* | | Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement by and between the Company and Sigma-Tau Industrie Farmadeutiche Riunite S.p.A. dated as of March 3, 2000. |
| |
| 10.24(8)* | | Amendment No. 1 to the Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement by and between the Company and Sigma-Tau Farmadeutiche Riunite S.p.A. dated as of December 19, 2001. |
| |
| 10.25(12)* | | Amendment No. 2 to the Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement between the Registrant and Sigma-Tau Industrie Farmacetiche Riunite S.p.A., dated May 20, 2004. |
| |
| 10.26(12)* | | Amendment No. 3 to the Expanded and Amended Thymosin Alpha 1 License, Distributorship and Supply Agreement between the Registrant and Sigma-Tau Industrie Farmacetiche Riunite S.p.A., dated May 21, 2004. |
| |
| 10.27(7) | | Acquisition Agreement between the Company and Sclavo S.p.A. dated April 20, 1998. |
83
| | |
Exhibit Number | | Description |
| 10.28(7) | | First Amendment to Acquisition Agreement between the Company and Sclavo S.p.A., dated April 20, 1998. |
| |
| 10.31(11)* | | Manufacturing and Supply Agreement between SciClone Pharmaceuticals International Ltd. and Patheon Italia S.p.A. dated as of November 1, 2002. |
| |
| 10.39(13)** | | Amendment to Offer Letter dated as of February 24, 2006 by and between SciClone Pharmaceuticals International, Ltd. and Hans P. Schmid including offer letter dated May 21, 2001 between SciClone Pharmaceuticals International, Ltd. and Hans P. Schmid as Exhibit A thereto. |
| |
| 10.41(14)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.42(14)** | | Change in Control Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.52(18)* | | Manufacturing Supply Agreement Between SciClone Pharmaceuticals International Ltd. and Lonza Sales Ltd., executed as of May 23, 2008. |
| |
| 10.53(20)* | | Loan and Security Agreement by and among SciClone Phamaceuticals International Ltd., SciClone Pharmaceuticals International China Holding Ltd., and Silicon Valley Bank, dated as of November 14, 2008. |
| |
| 10.54(19)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Gary Titus, dated as of November 21, 2008 and effective as of December 8, 2008. |
| |
| 10.55(21) | | Settlement Agreement between Sigma-Tau Finanziaria, S.p.A. and SciClone Pharmaceuticals, Inc. dated March 30, 2009. |
| |
| 10.56(21) | | Letter Agreement related to Thymosin Alpha 1 between SciClone Pharmaceuticals, Inc. and Sigma-Tau Industrie Farmaceutiche Riunite S.p.A., dated March 30, 2009. |
| |
| 10.57(22)** | | Amendment No. 1 to Change of Control Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.58(22)** | | Amendment No. 1 to Employment Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.59(23)** | | Change in Control Agreement between Mr. Gary Titus and SciClone Pharmaceuticals, Inc. dated December 8, 2008. |
| |
| 10.60(23) | | Amendment No. 1 to Loan and Security Agreement between Silicon Valley Bank, SciClone Pharmaceuticals International Ltd., and SciClone Pharmaceuticals International China Holding Ltd. dated March 30, 2009. |
| |
| 10.61(24) | | Description of Executive Incentive Plan. |
| |
| 14 | | See “Code of Ethics” in Item 10: Executive Officers and Directors, of this Annual Report on Form 10-K. |
| |
| 21.1(24) | | Subsidiaries of Registrant. |
| |
| 23.1(24) | | Consent of Independent Registered Public Accounting Firm. |
| |
| 24.1(24) | | Powers of Attorney. See page 81. |
| |
| 31.1(24) | | Rule 13a-14(a) Certification of Principal Executive Officer. |
| |
| 31.2(24) | | Rule 13a-14(a) Certification of Principal Financial Officer. |
84
| | |
Exhibit Number | | Description |
| 32.1(24) | | Section 1350 Certification of Principal Executive Officer. |
| |
| 32.2(24) | | Section 1350 Certification of Principal Financial Officer. |
| * | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4), 200.83 and 230.46. |
| ** | Management compensatory plan or arrangement. |
| (1) | Incorporated by reference from the Company’s Current Report on 8-K filed on July 28, 2003. |
| (2) | Incorporated by reference from the Company’s Registration Statement on Form S-l (No. 33-45446), declared effective by the Commission on March 17, 1992. |
| (3) | Incorporated by reference from the Company’s Registration Statement on Form S-8 (No. 33-66832) filed with the Commission on August 3, 1993. |
| (4) | Incorporated by reference from the Company’s Registration Statement on Form S-8 (No. 33-80911) filed with the Commission on December 28, 1995. |
| (5) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on January 26, 1998. |
| (6) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 20, 2000. |
| (7) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 1998. |
| (8) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2002. |
| (9) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2003. |
| (10) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2003. |
| (11) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. |
| (12) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2004. |
| (13) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on February 28, 2006. |
| (14) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 25, 2006. |
| (15) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2007 filed on August 8, 2007. |
| (16) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 22, 2006. |
| (17) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 28, 2006. |
| (18) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2008 filed on August 1, 2008. |
| (19) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 4, 2008. |
| (20) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2008. |
| (21) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 1, 2009. |
85
| (22) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 8, 2009. |
| (23) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 11, 2009. |
86
