UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | |
x | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) |
| | OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the fiscal year ended December 31, 2012 |
| | or |
¨ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) |
| | OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission file number 0-19825
SciClone Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
| | |
| Delaware | | 94-3116852 |
(State or other jurisdiction of Incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
950 Tower Lane, Suite 900 Foster City, California | | 94404 |
| (Address of principal executive offices) | | (Zip Code) |
(650) 358-3456
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
| Common Stock, $0.001 par value | | The NASDAQ Global Market of the NASDAQ Stock Market Inc. |
| (Title of Class) | | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨ Smaller Reporting Company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by non-affiliates of SciClone Pharmaceuticals, Inc. was approximately $375,749,000 as of June 30, 2012, based upon the closing price of SciClone Pharmaceuticals Inc.’s Common Stock on The NASDAQ Global Market of the NASDAQ Stock Market Inc. on such date. Shares of Common Stock held by each executive officer and director have been excluded from the calculation because such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 27, 2013, there were 54,008,979 shares of the Registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates by reference from the definitive proxy statement for the Company’s 2013 Annual Meeting of Stockholders to be filed with the Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
EXPLANATORY NOTE
In this Annual Report on Form 10-K for the fiscal year ended December 31, 2012 (“2012 10-K”), we have included revisions to our consolidated financial statements for the year ended December 31, 2011. Because these revisions are treated as corrections to our prior period financial results, the revisions are considered to be a restatement under generally accepted accounting principles. Accordingly, the revised financial information included in this 2012 10-K has been identified as “restated.” Concurrent with the filing of this Form 10-K, we are also filing amended quarterly reports on Form 10-Q/A for each of the first, second and third quarters of 2012 to restate our consolidated financial statements therein, including our financial statements for the second and third quarters and related year to date periods of 2011, and the effects of such restatements are reflected in the items revised herein.
The restatements relate to accounting errors originating with our subsidiary in China, NovaMed, which was acquired on April 18, 2011. The accounting errors relate primarily to the following:
| | • | | The timing of revenue recognition for certain Pfizer products sold by one of the distributors of our subsidiary, NovaMed. Our policy is that all customers’ obligations to pay for product are final once product is delivered. However, we determined that there were various factors, including the override of certain controls, indicating that sales under NovaMed’s distribution arrangement for Pfizer products with such distributor from the date of acquisition of NovaMed through the third quarter of 2012 (the “Relevant Periods”) allowed for contingent payment terms dependent upon when that distributor sold the products. As a result of our review and evaluation of the matter, we believe that instead of recording revenue at the time of sale to that distributor (“sell-in” method), as previously reflected in the financial statements for the Relevant Periods, revenue under the arrangements in effect at our subsidiary NovaMed should have been recognized when we received payment for the products (“sell-through” or “cash receipts” method). Effective as of the fourth quarter of 2012, we entered into a new agreement with that distributor which clarified the “sell-in” method of revenue recognition to provide consistency with our policy regarding revenue recognition on a prospective basis. As of December 31, 2012, we had received payment for all revenues recognized under the “sell-through” method. |
| | • | | The recognition of previously unrecognized product return reserves for sales of Aggrastat® sold by our subsidiary NovaMed prior to the date of the acquisition. We have concluded a liability for expired product existed at the time of the NovaMed acquisition, related to pre-acquisition sales. |
The revisions had the impact of increasing our revenues by $3.0 million for the nine-months ended September 30, 2012, and increasing our net income by $2.0 million for the same period. Basic and diluted earnings per share increased by $0.04 and $0.03, respectively, for the nine-months ended September 30, 2012. As of September 30, 2012, accounts receivable decreased by $1.5 million, inventory increased by $1.3 million and goodwill related to the acquisition of NovaMed increased by $1.9 million. The revisions for the year ended December 31, 2011 had an impact of decreasing our revenues by $1.1 million and decreasing our net income by $0.3 million. Basic and diluted earnings per share decreased by $0.01. As of December 31, 2011, accounts receivable decreased by $3.8 million, inventory increased by $2.3 million and goodwill related to the acquisition of NovaMed increased by $1.9 million.
For more information regarding the impact on our financial results, refer to Part II, Item 6, “Selected Financial Data,” and Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and to our Consolidated Financial Statements included in Part II, Item 8, including Note 2, “Revision of Previously Issued Consolidated Financial Statements”, and Note 20, “Selected Quarterly Financial Data (Unaudited)”.
2
TABLE OF CONTENTS
As used in this Annual Report, the terms “we,” “us,” “our,” the “Company” and “SciClone” mean SciClone Pharmaceuticals, Inc. and its subsidiaries (unless the context indicates a different meaning). SciClone, the SciClone logo and ZADAXIN are registered US trademarks and SCV-07 is a trademark of SciClone Pharmaceuticals, Inc. All other Company names and trademarks included in this Annual Report are trademarks, registered trademarks or trade names of their respective owners.
3
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements are based on our current expectations, estimates and projections about our business, industry, management’s beliefs and certain assumptions made by us. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe” or similar expressions are intended to identify forward-looking statements including those statements we make regarding our future financial results; anticipated product sales of current or anticipated products; the sufficiency of our resources to complete clinical trials and other new product development initiatives; government regulatory actions that may affect product reimbursement, product pricing or otherwise affect the scope of our sales and marketing; the timing and outcome of clinical trials; prospects for ZADAXIN® and our plans for its enhancement and commercialization as well as our expectations regarding other products; future size of the hepatitis B virus (“HBV”) and hepatitis C virus (“HCV”) and other markets, particularly in China; research and development and other expense levels; the ability of our suppliers to continue financially viable production of our products; cash and other asset levels; the allocation of financial resources to certain trials and programs, and expenses related to litigation and regulatory investigations. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict. Therefore, our actual results could differ materially and adversely from those expressed in any forward-looking statements as a result of various factors including, but not limited to, those described under the caption “Risk Factors” in this Annual Report on Form 10-K. We undertake no obligation to revise or update publicly any forward-looking statements for any reason.
PART I
OVERVIEW
SciClone Pharmaceuticals (NASDAQ: SCLN) is a revenue-generating, profitable, United States (“US”)-based, China-focused, specialty pharmaceutical company with a substantial commercial business and a product portfolio of therapies for oncology, infectious diseases, cardiovascular, urological, respiratory and central nervous system disorders. We are focused on continuing to grow our revenue and profitability through our strong sales and marketing efforts. Our business and corporate strategy is focused primarily on the People’s Republic of China (“China”) where we have built a solid reputation and established a strong brand through many years of experience marketing our lead product, ZADAXIN (thymalfasin). In addition, we have an established product promotion business model with large pharmaceutical partners and we are focused on establishing profitability in all of these collaborations. We believe our sales and marketing strengths position us to benefit from the long-term expansion of the pharmaceutical market in China. This pharmaceutical market currently ranks third among the global pharmaceutical markets, and we believe China will rank second among global pharmaceutical markets by 2020. We seek to expand our presence in China and increase revenues by growing sales and profitability of our current product portfolio, launching new products from our development pipeline, adding new, profitable product services agreements and leveraging our strong cash position to in-license additional products.
We operate in two segments which are generally based on the nature and location of our customers: 1) China and 2) the rest of the world which includes our US and Hong Kong operations.
We have two categories of revenues: “product sales revenues” and “promotion services revenues.” Our product sales revenues result from our proprietary and in-licensed products, including our lead product, ZADAXIN, and products from Pfizer Inc. (“Pfizer”) and Iroko Pharmaceuticals LLC. ZADAXIN has the highest margins in our portfolio as it is a premium proprietary product sold exclusively by SciClone. Aggrastat®, an in-licensed product, has higher margins than the products we promote under services agreements and we anticipate revenues from this product will grow significantly as it further penetrates the China market. In addition, we anticipate that new marketed products, when and if introduced, can increase the future revenues and profitability
4
of our pharmaceutical business in China over the coming years. Our “promotion services revenues” result from fees we receive for exclusively promoting products under services agreements with certain pharmaceutical partners, including Sanofi Aventis S.A. (“Sanofi”) and Baxter International, Inc. (“Baxter”) in China. We refer to these agreements as promotion agreements, service agreements and distribution contract rights agreements. We recognize promotion services revenues as a percentage of our collaborators’ product sales revenue for these exclusively promoted products, such as Depakine®, Stilnox® and Tritace®. Over time, as additional proprietary or in-licensed products come to the market, we aim to shift our product mix towards those higher margin products. In January 2013, our promotion agreement with Sanofi was renewed until December 31, 2013 under the same terms as our previous agreement. As we seek renewals of our product services agreements, our strategy is to establish more favorable terms with the goal of achieving profitability under these agreements through improved margins as well as through managing expenses relating to our product services revenue.
ZADAXIN is approved in over 30 countries and may be used for the treatment of HBV, HCV, and certain cancers, and as a vaccine adjuvant according to the local regulatory approvals we have in these countries. In China, thymalfasin is included in the treatment guidelines issued by the Ministry of Health (“MOH”) for liver cancer, as well as guidelines for treatment of chronic HBV (issued by both the Chinese Medical Association and the Asian-Pacific Association for the Study of the Liver) and invasive fungal infections of critically ill patients (issued by the Chinese Medical Association). Our sales force is focused on increasing sales to the country’s largest hospitals (class 3 with over 500 beds) as well as mid-size hospitals (class 2). These hospitals serve Tier 1 and Tier 2 cities located mostly in the eastern part of China which are the largest and generally have the most affluent populations. We are widening our market strategies by targeting numerous smaller hospitals as well as hospitals that are in more rural areas. We are also seeking to expand the indications for which ZADAXIN could be used, including sepsis.
Our market portfolio also includes the anti-epileptic drug Depakine, the hypnotic Stilnox (marketed as Ambien® in the US), the ACE inhibitor Tritace, and Aggrastat, an intervention cardiology product launched in China in 2009.
We are also pursuing the registration of several other therapeutic products in China. These include: DC Bead®, an embolic acting bead with drug loading capabilities that can be used for targeted delivery of cancer chemotherapy drugs directly to the tumor; Loramyc®, a mucoadhesive tablet formulation of miconazole lauriad to treat oropharyngeal candidiasis; Rapinyl®, a sublingual tablet formulation of fentanyl to treat breakthrough cancer pain; and RapidFilm®, an oral film formulation of ondansetron to treat nausea induced by chemotherapy.
We continue to seek to establish profitable in-licensing arrangements for approved or late-stage branded, well-differentiated products which, if not yet approved, have a clear regulatory approval pathway in China based on existing regulatory approval outside of China. Our objective is to in-license products with higher margins that can augment our product sales revenue, and we continue to explore opportunities to optimize our promotion services revenues. We are also working on the final stage of the regulatory approval in China for our in-licensed candidate DC Bead, and on the approval process for our other product candidates, all of which are in clinical trials or in other stages of the regulatory approval process in China.
We acquired NovaMed Pharmaceuticals, Inc. (“NovaMed”) on April 18, 2011 and our results of operations include the operations of NovaMed as of that date forward. Since the acquisition of NovaMed, we have put strategies in place designed to strengthen our sales and marketing infrastructure in China, with the goal of meeting the growing demand for pharmaceuticals in the China market. However, we believe that several developments in 2012 had, or may yet have, material impacts on the growth rate of our business in China and affected the Company’s financial results in the second half of 2012. We are responding to these developments by taking actions to strengthen our business and improve our financial performance in subsequent quarters. These developments include but are not limited to: a reduction in the retail price for ZADAXIN and other products that occurred later in the year than expected; an increase in the channel inventory levels of ZADAXIN during the third quarter and particularly in September 2012; matters relating to our NovaMed acquisition, including a non-
5
cash impairment loss to fully write down the value of the licensing and product services agreement intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; issues in internal control over financial reporting primarily within the NovaMed subsidiary; and changes in management.
Price Reduction for ZADAXIN and Other Products
ZADAXIN’s national reimbursement retail list price in China was recently reviewed by regulatory authorities consistent with the China government’s review of pharmaceutical prices once a product has been included into the Reimbursement Drug List (“RDL”). As a result of this review, the retail list price (or the price at the hospital pharmacy level) of ZADAXIN was reduced by approximately 18%. The reduction was announced in September 2012 and became effective October 8, 2012. Based on an agreement with our primary importer of ZADAXIN into China, this importer will take a larger share of the price reduction impact in exchange for certain exclusive importation rights into China. As a result, the actual impact on SciClone’s revenue and margins is expected to be less than a 5% decrease in our sales price of ZADAXIN to this importer. In addition, the National Development and Reform Commission’s (“NDRC”) retail list prices (or the price at the hospital pharmacy level), for Aggrastat, as well as for several of our oncology products exclusively promoted in China for Pfizer and Baxter, were reduced ranging from 10 to 20%.
ZADAXIN Inventory and Sales
China uses a tiered system to import and distribute products. The distributors make the sales in the country, but the product is imported for them by our exclusive ZADAXIN importer. Our product sales revenues result from the sale of our products to our customers, the importing agent. It takes approximately seven weeks for our customers to clear a shipment of ZADAXIN through the importation process for sale in China. Our China importation customer tends to purchase infrequent large orders of ZADAXIN inventory to facilitate the distribution and sale to Chinese hospital pharmacies. The timing of infrequent large orders may significantly affect the ZADAXIN channel inventory levels and may cause fluctuations to our reported sales and profitability for each quarterly period.
During the third quarter and particularly in September 2012, we estimate that there was an increase of approximately $14 million in ZADAXIN channel inventory levels and we believe that our sales to our customers exceeded the pace at which our customers were able to sell ZADAXIN through to other parties, primarily hospital pharmacies. ZADAXIN channel inventory levels decreased by approximately $5 million in the fourth quarter of 2012, and we expect them to continue to decrease over the next two quarters. As a result, we expect that ZADAXIN revenues will be lower in the first half of 2013, compared to the first half of 2012. We expect our 2013 sales revenue to be lower than or comparable to our 2012 revenues of $156 million. We continue to believe that we will grow demand for ZADAXIN through increased penetration in the market.
In addition, we believe that our sales organization’s efforts relative to ZADAXIN may have been affected by changes to and turnover in management in China, particularly the departures of senior executives. Turnover among sales personnel in the China market generally, including among our own sales staff, is expected to continue, and we may experience additional departures in our China operations. During this transition period, if we are unable to achieve objectives for increased demand, we may experience declines in our quarterly sales revenue in the near term and our sales and profitability for the next few quarters may significantly decrease.
We have taken definitive steps to address the issues affecting product sales. We have revised and we are implementing changes in our strategy designed to increase market penetration and growth, particularly for ZADAXIN. We are widening our market strategies by targeting numerous smaller hospitals as well as hospitals that are in more rural areas with the intent to positively affect sales growth over the next several quarters. In addition, we have already been successful in making significant changes in our China organization with the recruitment of additional senior-level management and the recent appointment of a Chief Executive Officer for
6
our China operations, and we are aggressively recruiting additional management for our China operations. Our recent and continuing executive recruiting in China is intended to bring in additional pharmaceutical company experience in the China market to grow ZADAXIN sales, to increase profitability, to enhance our financial capabilities and internal controls, and to improve and implement our sales strategies.
NovaMed: Intangible Asset Impairment; Contingent Consideration Remeasurement
We acquired NovaMed on April 18, 2011, and our results of operations include the operations of NovaMed as of that date forward. The acquisition increased our portfolio of commercial and development-stage products through exclusive licensing and product service agreements with a number of leading pharmaceutical companies. Although revenues from the NovaMed business have grown since the acquisition, overall revenue growth and profitability have not met the expectations we forecast at the time of the acquisition. This condition was considered to be an impairment indicator with respect to the intangible assets related to our promotion and distribution contract rights. During the third quarter ended September 30, 2012, we determined that the undiscounted cash flows estimated to be generated by the intangible assets were less than the carrying amounts. We further performed a discounted cash flow analysis related to the intangible assets and determined that a full impairment should be recorded. As a result, we recognized a non-cash impairment loss of approximately $42.7 million for the third quarter and full year ended December 31, 2012 on our condensed consolidated statements of operations.
In January 2013, our promotion agreement with Sanofi was renewed until December 31, 2013 under the same terms as the prior agreement. Certain of our other product services agreements with third parties will be expiring soon unless renewed, extended or renegotiated. We are actively negotiating renewals or extensions of these agreements. We are also assessing the terms and conditions of our agreements with the intent to secure more favorable terms to us relative to profitability. If any of these agreements are determined to no longer be beneficial to us and are allowed to expire, or if third parties will not renegotiate, renew or extend the agreements on terms acceptable to us, our revenues and profitability would be affected.
The terms of our acquisition of NovaMed provided for the payment of an additional $43.0 million in earn-out payments upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years (the “earn-out” or “contingent consideration”). We initially recorded $18.9 million as the estimated fair value of the contingent consideration. The fair value of the contingent consideration was remeasured each quarter and changes to the fair value were recorded to contingent consideration expense or gain. As of December 31, 2012, we determined the fair value of the contingent consideration was $0, resulting in a non-cash gain of $15.4 million for the year ended December 31, 2012. The significant reduction in the valuation of the contingent consideration expense during the year was related to revenue and EBITDA (earnings before interest, depreciation and taxes) targets not being achieved, and to the reduced probability of renewal relating to NovaMed’s product distribution agreements, including, in particular, the target of renewing the Depakine services agreement with Sanofi for a five-year term. The Depakine services agreement was extended through December 31, 2013, a term less than five years.
Internal Control Issues — Material Weakness
During the quarter ended September 30, 2012, we identified deficiencies in the design and operating effectiveness of controls primarily associated with product return reserves related to our Aggrastat product line, and the override of certain controls in the financial statement close process related to our NovaMed subsidiary. Certain out of period adjustments and these deficiencies were identified through the performance of controls and processes recently implemented at our NovaMed subsidiary as part of our assessment of internal control over financial reporting in 2012. We concluded that the aggregation of these deficiencies is a material weakness. Furthermore, during the fourth quarter of 2012, we identified deficiencies related to the timing of revenue recognition for our Pfizer products, the override of related controls at our NovaMed subsidiary, and the corporate monitoring thereof. We concluded that these deficiencies identified in the fourth quarter of 2012 are an additional indicator of the material weakness that was identified in the third quarter of 2012.
7
With the oversight of management and our Audit Committee, we have initiated steps to address the material weakness. In the fourth quarter of 2012, we implemented additional controls related to our revenue recognition and product return reserve processes to strengthen our controls and provide that all available information is properly considered. We also re-evaluated the operation of our controls at our NovaMed subsidiary and made adjustments to strengthen these controls, including the recent hiring of our Chief Financial Officer, China Operations who joined the Company in the third quarter of 2012. We terminated personnel who were involved in the override of certain controls in the financial statement close process at our NovaMed subsidiary that resulted in the material weakness. While we have completed certain remediation efforts, our remediation activities are not complete as of December 31, 2012.
China Management Turnover
We announced departures of key personnel from our China organization. These include the individual who was our Chief Executive Officer of SciClone’s China operations and former Chief Executive Officer of NovaMed who resigned in December 2012, and our Chief Operating Officer in China who left the Company in October 2012. There may be additional departures within our China operations. We have and are continuing to recruit executives to address the departures and to expand and strengthen our China operations, including the hiring of both a new Chief Executive Officer, China Operations, who will begin April 1, 2013, and a Chief Financial Officer, China Operations, who began August 2012.
Other Matters
We were developing SCV-07 in a phase 2b clinical trial for the prevention of oral mucositis (“OM”). On March 2, 2012, we announced the discontinuation of this trial based on the pre-planned interim analysis results that indicated the trial would not meet the pre-specified efficacy endpoints, and our intention to further curtail our US-based development efforts. In March 2012, we implemented a reduction in our workforce of 11 full-time employees, primarily in research and development, and recorded severance-related charges of approximately $1.1 million, of which approximately $0.1 million and $1.0 million were recognized to general and administrative and research and development expense, respectively, for the year ended December 31, 2012. We have substantially completed the restructuring.
The US Securities and Exchange Commission (“SEC”) and the US Department of Justice (“DOJ”) are each conducting formal investigations of SciClone regarding a range of matters including the possibility of violations of the Foreign Corrupt Practices Act (“FCPA”). We will continue to cooperate fully with the SEC and DOJ in the conduct of their investigations. In response to these matters, our Board appointed a Special Committee of independent directors (the “Special Committee”) to oversee our response to the government inquiry. The Special Committee substantially concluded its original investigation and on May 4 and 5, 2011 reported its findings and recommendations to the Board of Directors. The Special Committee has also reported findings to the SEC and DOJ. The SEC’s and DOJ’s formal investigations are continuing and the Company is continuing to cooperate with those investigations, including undertaking the review or investigation of additional matters, primarily related to our NovaMed operations.
In our Form 10-Q for the period ended September 30, 2012, filed with the SEC on November 9, 2012, we disclosed, among other things, a non-cash impairment loss to fully write down the value of intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; a significant increase in ZADAXIN channel inventory levels; and internal control issues primarily within the NovaMed organization that resulted in material weakness. Following our disclosure of these items, we received a subpoena from the SEC requesting documents related to these and various other matters regarding the NovaMed acquisition and our operations in China. After review of the subpoena, and in order to respond to inquiries from the DOJ and SEC and to determine if any wrong-doing occurred, our Audit Committee determined to undertake an additional independent investigation as to additional matters including, but not limited to, matters related to our acquisition of NovaMed and FCPA matters.
8
On February 22, 2013, we announced that our reported financial results for each of the second and third quarters of 2011, the year ended December 31, 2011, and the first three quarters of fiscal 2012 could not be relied upon and that we would restate them. Subsequently, we received a purported derivative litigation naming certain of our officers and directors as defendants. We believe the claims lack merit and will vigorously defend against them.
Refer to Part II, Item 8 “Notes to Consolidated Financial Statements” Note 18 “Other Corporate Matters” and Part I, Item 3 “Legal Proceedings” in this Form 10-K for further information regarding the investigation and remedial measures, and related litigation.
BUILDING A LEADING INTERNATIONAL PHARMACEUTICAL BUSINESS
Our Established Business in China
In China, we have established product revenue and positive cash flow. We are committed to building on this base and introducing additional pharmaceutical products to meet the country’s evolving healthcare needs. China state leaders are continuing to implement a health care reform plan which, among other things, is seeking to expand patient access to pharmaceuticals. We believe the China pharmaceutical market may grow approximately15% annually over the next several years.
We launched ZADAXIN in China in 1996 and by 2012 our annual worldwide sales of this product reached more than $112 million, 96% of which were sales to China. Today, ZADAXIN is one of the largest imported pharmaceutical products in China, measured by revenue. We estimate our market share of thymalfasin by units is approximately 13%. Over the last decade, we have established a sales and marketing organization and strong importation relationships with distribution channels which have facilitated strong growth in sales, and profitability, and substantial cash flow. Through our extensive China sales organization of approximately 840 professionals including approximately 770 sales personnel, we believe we have developed a good reputation and relationships with physicians and administrators in over 500 hospitals in the major cities in China. We have built a strong commercial presence in liver disease, cancer and the intensive care setting. We are expanding geographically in China to position the Company for further growth. ZADAXIN has strong brand recognition and is positioned as a high-quality, imported product. ZADAXIN is approved in China for the treatment of HBV and for use as a vaccine adjuvant. It is also included in the treatment guidelines issued by the MOH for liver cancer. In China, orders for ZADAXIN are filled largely by distributors and sub-distributors which purchase ZADAXIN from our selected, established, government-licensed importing agents.
China accounted for approximately 97%, 97%, and 96%, respectively, of our net revenues for the years ended December 31, 2012, 2011 and 2010. In 2012, SinoPharm Lingyun Biopharmaceutical Company Ltd. (“SinoPharm”) (formerly known as Shanghai Lingyun Pharmaceutical Company Ltd.), Sanofi and Jian Wei Trading Company accounted for 53%, 20% and 12% of our net revenues, respectively. In 2011, SinoPharm, Sanofi and Guangdong South Pharmaceutical Foreign Trade Company Ltd. accounted for 62%, 15% and 15% of our net revenues, respectively. In 2010, Shanghai Lingyun Pharmaceutical Company Ltd. and Guangdong South Pharmaceutical Foreign Trade Company Ltd. accounted for 74% and 14% of our net revenues, respectively. We anticipate that SinoPharm will account for a larger percentage of our net revenue in 2013, compared to 2012, as a result of our recent agreement granting certain exclusive importation rights to SinoPharm for ZADAXIN. No other customers accounted for more than 10% of our net revenues in those periods. As of December 31, 2012, approximately $33.7 million or 86% of our accounts receivable were attributable to four customers in China, including $27.6 million or 70% attributable to our largest customer.
The Chinese government is continuing its efforts to reduce overall health care costs, including pricing controls on pharmaceutical products. Individual provinces in China and, in some cases, individual hospitals can and have established pricing requirements for a product to be included on formulary lists. In some cases, these prices have been significantly lower than our distributors have been selling ZADAXIN, in which case we have been removed from formulary lists, which consequently has reduced sales to certain hospitals and could adversely affect our future sales. The price for pharmaceutical products is regulated in China both at the national and at the provincial level. The process
9
and timing for price restrictions is unpredictable. In addition, we are aware that ZADAXIN may be used on an off-label basis, and the Chinese government’s pricing, reimbursement or other actions might reduce such uses.
International Sales and Marketing
ZADAXIN is approved in over 30 countries, primarily in China, the Pacific Rim, Latin America, Eastern Europe, and the Middle East. ZADAXIN’s approvals are principally for the treatment of HBV and as a vaccine adjuvant, with additional approvals in certain countries for the treatment of HCV, or as a chemotherapy adjuvant for cancer patients with weakened immune systems. We sell ZADAXIN in various international markets through our wholly owned subsidiary, SciClone Pharmaceuticals International Ltd. (“SPIL”).
SPIL is registered in the Cayman Islands and its principal office is in Hong Kong. SPIL orders ZADAXIN from our European manufacturer and contracts with a third party for the storage of our finished goods inventory at warehousing facilities in Hong Kong. SPIL then distributes our product worldwide from these warehousing facilities based on purchase orders from our customers. Under our established distribution arrangements, local importers and distributors are responsible for the importation, inventory, distribution and invoicing of ZADAXIN after importation.
Product sales of $112.2 million, $104.8 million, and $85.1 million for the years ended December 31, 2012, 2011 and 2010, respectively, were from sales of ZADAXIN.
SciClone’s Lead Product ZADAXIN (Thymalfasin)
ZADAXIN is SciClone’s synthetic preparation of thymalfasin, scientifically referred to as thymosin alpha 1, a thymic peptide which circulates in the blood naturally and is instrumental in the immune response to certain cancers and infections. Published scientific and clinical studies have shown that thymalfasin helps to stimulate and direct the body’s immune response to eradicate infectious diseases, such as HBV, HCV, bacterial, and fungal infections; to fight certain cancers such as melanoma and liver cancer; and to enhance response to vaccines. Thymalfasin appears to be well tolerated, with few reports of significant side effects or toxicities associated with its use.
Thymalfasin elicits a variety of immune system responses. Acting on intracellular signaling pathways, thymalfasin increases the Th1 subset of T-helper cells that assist with fighting invading viruses and cancers and leads to a boost in production of antibodies in response to vaccines. Thymalfasin also results in decreased CD-4 cell differentiation into the Th2 subset of CD-4 helper cells that produce cytokines, such as IL-4, which are associated with persistence of viral infection, and stimulates several other components of the immune response that help the body attack and kill virally-infected or tumor cells.
Thymalfasin for Treatment of Sepsis
Clinical trials have shown that thymalfasin improves survival in patients in intensive care units being treated for sepsis from severe bacterial infections. A recent publication describes the results from a large, multicenter, single-blind, randomized, and controlled trial in 361 subjects in China. This study showed that the 28-day mortality from any cause was 26% in the ZADAXIN group, versus 35% in the control group, an effect that is clinically important (p = 0.062 non-stratified analysis,p = 0.049 log-rank). Greater improvement in the biomarker HLA-DR was also seen in subjects treated with ZADAXIN (p = 0.037). No serious drug-related adverse events were recorded. These data support the usefulness of ZADAXIN in treating severe sepsis.
Thymalfasin for Enhancement of Response to Vaccines
Clinical trials have demonstrated that thymalfasin increases response to influenza and hepatitis B vaccines in the elderly and in hemodialysis patients. In elderly subjects, thymalfasin was also shown to decrease the incidence of influenza from 19% in subjects given an influenza vaccine alone, to 6% in subjects receiving
10
thymalfasin treatment in addition to the influenza vaccine. For these clinical trials, the treatment regimen involved 8 to 10 injections of 1.6 mg doses of thymalfasin. A clinical study conducted in 2009/2010 by Sigma-Tau in Italy, however, showed that a higher dose of thymalfasin (3.2 or 6.4 mg) given only twice (seven days prior to vaccination and on the day of vaccination) was also effective. ZADAXIN in treatment led to a statistically significant increase in the percent of subjects who seroconverted to the H1N1 vaccine (MF59 adjuvanted monovalent vaccine, Focetria™ from Novartis), and an increase in total titers, when measured at 21 or 42 days after vaccination. While this effect was no longer seen at time points 84 and 168 days after vaccination, the enhancement effect of ZADAXIN provided a significant enhancing effect in the critical first six weeks following vaccination. These promising data further support the utility of thymalfasin for use in vaccine enhancement.
INTELLECTUAL PROPERTY AND PROPRIETARY RIGHTS
Patents
We seek regulatory approval for our products in disease areas with high unmet medical need, significant market potential and where we have a proprietary position through patents covering use, manufacturing process, or composition of matter for our products. For our lead product ZADAXIN, we are the licensee or owner of patents and patent applications relating to thymalfasin and its use for a number of diseases. In particular, we are the licensee or owner of patents and applications in the US or China that are directed to thymalfasin therapy for the treatment of hepatitis B and hepatitis C as a monotherapy or in combination with other therapeutics, including drugs with or without regulatory approval for marketing. In addition, we are the licensee or owner of several patents and applications in the US and internationally that are directed to thymalfasin therapy for vaccine enhancement. The expiring patent terms for issued or to be issued patents are from 2025 to 2030. We are also the licensee or owner of several patents and applications in the US and internationally that are directed to thymalfasin therapy for the treatment of melanoma. The expiring patent terms for issued or to be issued patents are from 2026 to 2028. We are also the licensee or owner of patents in the US and China that are directed to thymalfasin therapy for reducing side effects of chemotherapy. The expiring patent term for issued patents is 2020. We have also applied for patents in the US and internationally that are directed to thymalfasin therapy for the treatment of other infectious diseases, such as acute infection, Aspergillus infection and severe acute respiratory syndrome (“SARS”), as well as other indications, such as treatment of septic shock. In addition, we have patents or patent applications directed to thymalfasin conjugates and the use of thymalfasin to stimulate the immune system in general.
With respect to our issued patents in the US and Europe, we are also entitled to obtain a patent term extension to extend the patent expiration date. For example, in the US, we can apply for a patent term extension of up to five years for one of the patents covering ZADAXIN if ZADAXIN is approved by the FDA. The exact duration of the extension depends on the time we spend in clinical trials as well as getting a new drug application approval from the FDA.
ZADAXIN in China
| | | | | | | | |
Granted Patents Relevant to Approved Indication (not yet expired or abandoned) | | Approved Indication | | | Year of
Expiration | |
ZL 93120725 [China] | | | Chronic Hepatitis B | | | | 2013 | |
ZL 99811382.4 [China] | | | Chronic Hepatitis B | | | | 2019 | |
Proprietary Rights
In addition to patent protection, we intend to use other means to protect our proprietary rights. We may pursue marketing exclusivity periods that are available under regulatory provisions in certain countries, including the US, Europe, Japan, and China. For example, if we are the first to obtain market approval of a product, e.g., thymalfasin in the US, we would expect to receive at least five years of market exclusivity.
11
Furthermore, orphan drug exclusivity has been or may be sought where available. Such exclusivity has a term of seven years in the US and 10 years in Europe. We have obtained orphan drug designation for thymalfasin for the treatment of malignant melanoma and chronic hepatitis B in the US and for the treatment of hepatocellular carcinoma in the US and in Europe. We have filed trademark applications worldwide for ZADAXIN and other trademarks that appear on our commercial packaging and promotional literature. Copyrights for the commercial packaging may prevent counterfeit products or genuine but unauthorized products from entering a particular country by parallel importation. Brand and trademark protection are particularly important to us in China. We are implementing anti-counterfeiting measures on commercial packaging and we are registering the packaging with customs departments in countries where such procedures exist. We rely upon trade secrets, which we seek to protect in part by entering into confidentiality agreements with our employees, consultants, corporate partners, suppliers, and licensees.
MANUFACTURING
ZADAXIN is manufactured for us in Europe by third parties under exclusive contract manufacturing and supply agreements. We closely monitor production runs of ZADAXIN and conduct our own quality assurance audit programs. We believe the manufacturing facilities of our contract suppliers are in compliance with the FDA’s current Good Manufacturing Practices (“GMP”), and European equivalents of such standards. In order to sell ZADAXIN to the licensed importers in China, our manufacturers must 1) be approved by the Italian Ministry of Health (“AIFA”) and 2) be accepted by the SFDA, the Chinese regulatory agency, and we must obtain an Imported Drug License from the SFDA permitting the importation of ZADAXIN into China. The license must be renewed every five years, and our next renewal will be required in December 2017. If we change manufacturers, these changes must 1) be approved by AIFA in Italy and 2) be accepted by the SFDA, and we must obtain a new Imported Drug License from the SFDA.
In the event of the termination of an agreement with any single supplier, we believe that we would be able to enter into arrangements with other suppliers with similar terms. We do not intend at this time to acquire or establish our own dedicated manufacturing facilities for any of our products. We believe that our current manufacturing partners for ZADAXIN have enough manufacturing capacity to meet potential market demand. We also believe that our current manufacturing partners in Europe for our other drugs and drug candidates will be able to meet our clinical trial needs and market demand.
COMPETITION
Our competition for sales of ZADAXIN in China is primarily from generic drug manufacturers located in China that sell their product at lower prices. We compete with them based upon our reputation as a provider of high quality products, including the fact that our products are produced at US and western European GMP facilities.
Our competitors for existing and future products include pharmaceutical companies, biotechnology firms, universities and other research institutions, in the US, China and other territories, that are actively engaged in research and development or marketing of products in the therapeutic areas we are pursuing. We believe that the principal competitive factors in this industry for a marketed drug include the efficacy, safety, price, therapeutic regimen, manufacturing, quality assurance and associated patents and the capabilities of its marketer.
Most of our competitors, particularly large pharmaceutical companies, have substantially greater financial, technical, regulatory, manufacturing, marketing and human resource capabilities than ours. Most of them also have extensive experience in undertaking the preclinical and clinical testing and in obtaining the regulatory approvals necessary to market drugs. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated with our competitors.
For the treatment of HBV, current therapies being marketed by competitors include interferon alpha, in standard and pegylated forms, nucleoside analogues, such as lamivudine and entecavir, and nucleotide analogue
12
adefovir. In addition to these products, in our largest market, China, ZADAXIN faces competition from other synthetic and generic biological extracts that are locally manufactured and significantly lower priced.
Future clinical trials may or may not show ZADAXIN or our other products in the market or in development to have advantages or value over such existing or future competitive products.
Depakine, an anticonvulsant that is used for the treatment of seizures in patients with bipolar disorder and epilepsy attacks, faces competition from both imported western drugs and local drugs, including oxcarbazepine, lamotrigine, levetiracetam, topiramate, olanzapine, quetiapine, diazepam, sodium phenobarbital and others. Depakine is available in a wide range of formulations, including oral solutions, injectables and prolonged release tablets. There are about 10 generic versions of the product available in China.
Aggrastat, an interventional cardiology product, has proven to be effective in improving the results of primary coronary angioplasty in patients with myocardial infarction. There are about five generic versions of the product available in China.
Tritace is an angiotensin converting enzyme (“ACE”) inhibitor for the treatment of hypertension and congestive heart failure post myocardial infarction. As Tritace is the first and only ACE inhibitor available in China indicated for protection against cardiovascular events, the main competition is from locally manufactured generic versions of the product.
RESEARCH AND DEVELOPMENT
Research and development (“R&D”) expenses consist of independent R&D costs and costs associated with in-licensing arrangements. A substantial portion of our development expense is third party cost relating to the conduct of our clinical trials. R&D expenses were $6.1 million, $12.3 million, and $12.4 million, for the years ended December 31, 2012, 2011 and 2010, respectively. On March 2, 2012, we announced the discontinuation of our SCV-07 phase 2b clinical trial in patients with OM based on the pre-planned interim analysis results that indicated that the trial would not meet the pre-specified efficacy endpoints. We expect R&D costs to decrease in the future due to the discontinuation of our SCV-07 phase 2b clinical trial and from further curtailment of our US-based development expenses.
EMPLOYEES
As of December 31, 2012, we had approximately 870 employees: approximately 840 in China, approximately 20 in the US, and approximately 10 in other countries. From time to time, we engage the services of consultants worldwide with pharmaceutical and business backgrounds to assist in our product development and commercialization activities.
GOVERNMENT REGULATION
Regulation by governmental authorities in the US, China and other foreign countries is a significant factor in the manufacturing and marketing of our products, as well as in ongoing research and development activities and in pre-clinical and clinical trials and testing related to our products. Our products in clinical development in the US, China and other foreign countries are subject to approval by the FDA, the SFDA and similar regulatory authorities. Manufacturing establishments are subject to inspections by regulatory authorities at the federal, state and local level and must comply with current GMP as established in various jurisdictions. In complying with GMP standards, manufacturers must continue to expend time, money and effort in the area of production and quality assurance to ensure ongoing full technical compliance. As we do not manufacture our products, we depend on third parties to meet requisite GMP standards.
China
In China, the pharmaceutical industry is subject to extensive government regulation and supervision. The regulatory framework addresses all aspects of operating in the pharmaceutical industry, including approval,
13
pricing, re-imbursement, production, licensing and certification requirements and procedures, periodic renewal and reassessment processes, registration of new drugs and environmental protection.
The SFDA is the authority that monitors and supervises the administration of pharmaceutical products and medical appliances and equipment as well as food, health food and cosmetics in China. The primary responsibilities of the SFDA include:
| | • | | formulating administrative rules and policies concerning the supervision and administration of food, health food, cosmetics and the pharmaceutical industry; |
| | • | | evaluating, registering and approving of new drugs, generic drugs, imported drugs and traditional Chinese medicine; |
| | • | | approving and issuing permits for the manufacture and export/import of pharmaceutical products, medical appliances and equipment and approving the establishment of enterprises to be engaged in the manufacture and distribution of pharmaceutical products; and |
| | • | | examining and evaluating the safety of food, health food and cosmetics and handling significant accidents involving these products. |
The MOH is an authority at the ministerial level under the State Council and is primarily responsible for national public health and has administrative responsibility for the SFDA. The MOH performs a variety of tasks in relation to the health industry such as establishing social medical institutes, promulgating national regulations, and producing professional codes of ethics for public medical personnel. The MOH is also responsible for international issues, such as those pertinent to foreign companies and governments.
Drug Administration Laws and Regulations
The China Drug Administration Law and related regulations provide the legal framework for the establishment of pharmaceutical manufacturing enterprises, and pharmaceutical trading enterprises, and for the administration of pharmaceutical products, including the development and manufacturing of new drugs, the import of pharmaceuticals and the regulation of packaging, trademarking and advertising of pharmaceutical products in China.
Permits and Licenses for Importation, Manufacturing and Registration of Drugs
Imported Drug License. Our strategy to date has been to seek approval for the import into China of drugs approved in other markets. We must obtain an Imported Drug License from the SFDA to import a pharmaceutical product into China.
To qualify to receive an Imported Drug License from the SFDA, each manufacturing establishment must be registered with the FDA or European (“EMA”) regulatory authorities where the product is registered for sale and listed on the Certificate of Pharmaceutical Product (“CPP” or Country of Origin Approval). In general, the SFDA also requires that an imported drug must also have country of origin approval for the same indication for which an Imported Drug License is applied.
As a result, in order to obtain and maintain an Imported Drug License in China, we or our partners must also meet the regulatory requirements for the country of origin of the pharmaceutical products we import, or are seeking to import, into China.
The process for applying for and obtaining an Imported Drug License can be protracted and uncertain. In addition to the submission of clinical data from trials outside China, the SFDA may require additional clinical data, including from studies in China, and it may conduct its own inspection and testing of manufacturing facilities and of finished product. An Imported Drug License needs to be renewed every five years. Further, if the
14
manufacturer of the pharmaceutical product changes, an additional approval is required from the SFDA, and approval will also have to be obtained in the country from which the product is imported.
For ZADAXIN, the CPP is in Italy, and was issued by the AIFA, and the named manufacturer is Patheon Italia S.p.A. Aggrastat received European approval in 1998 under the Mutual Recognition Process (“MRP”) countries in Europe (including Germany, Italy, Spain, Netherlands, Finland, Sweden, Greece, Portugal, United Kingdom, Ireland, Austria, Belgium, Luxembourg and France) and the product is manufactured in Finland by Orion Corporation. We and our partners need to maintain these approvals.
China requires that products with an Imported Drug License be imported through approved importing agents. At each port of entry, prior to moving the product forward to the distributors, government-licensed importing agents must process and evaluate each shipment to determine whether such shipment satisfies China’s quality control requirements.
GMP Certificates. Our current products and our clinical candidates in China are all manufactured outside China and are subject to GMP standards in the country in which they are manufactured. Our manufacturers are subject to site inspections by the regulatory authorities in the jurisdictions in which they are located. The issuance and renewal of an Imported Drug License is dependent, among other things, upon maintaining manufacturing standards that comply with the GMP standards of a widely recognized regulatory authority, such as the FDA or EMEA.
If we were to manufacture product in China, or obtain product from Chinese contract manufacturers, such manufacture would be subject to similar GMP standards established in China and administered by local authorities.
Distribution of Pharmaceutical Products
According to the China Drug Administration Law and related regulations, a manufacturer of pharmaceutical products in China can only engage in the trading of the pharmaceutical products that the manufacturer has produced itself. In addition, such manufacturer can only sell its products to:
| | • | | wholesalers and retailers holding pharmaceutical trading permits; |
| | • | | other holders of pharmaceutical manufacturing permits; or |
| | • | | medical practitioners holding medical practice permits. |
A pharmaceutical manufacturer in China is prohibited from selling its products to end-users, or individuals or entities other than holders of Pharmaceutical Trading Permits, the pharmaceutical manufacturing permits or the medical practice permits.
A pharmaceutical distributor (including wholesalers and retailers) must satisfy requirements as to: personnel with pharmaceutical expertise, appropriate warehousing and sanitary environments compatible with the distributed pharmaceutical products; quality management and compliance with regulations to ensure the quality of the distributed pharmaceutical products. Operations of pharmaceutical distributors must be conducted in accordance with the Pharmaceutical Operation Quality Management Rules and require a certificate from the SFDA. Pharmaceutical distributors must comply with record-keeping requirements regarding the products sold.
Price Control and Competitive Bidding
The control of prices of pharmaceutical products is vested in the national and provincial price administration authorities. Depending on the categories of pharmaceutical products in question, the prices of pharmaceutical products listed in the State Basic Medical Insurance and Work Injury Insurance Drug Catalogue, drugs with patents and other drugs whose production or trading may constitute monopolies are subject to the control of the
15
NDRC of China and the relevant provincial or local price administration authorities. For pharmaceutical products manufactured or imported into China, the national price administration authority from time to time publishes price control lists specifying pricing ceilings for specific pharmaceuticals. The Ministry of Labor (“MOL”) and Social Security, together with other government authorities, determine which medicines are to be included in or removed from the Catalogue for the national medical insurance program and under which category a medicine should fall, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are based on a number of factors, including price and efficacy. A national medical insurance program participant can be reimbursed for the full cost of a Category A medicine and 60 to 90% of the cost of a Category B medicine. In November 2009, thymalfasin, the generic chemical name for our pharmaceutical product ZADAXIN, was included as a Category B product in the National Reimbursed Drug List (“NRDL”). The main purpose of the NDRC and the NRDL price control policy is to establish new maximum allowable prices for listed pharmaceutical products which, in many cases, will be below previously established prices, thus lowering the prices for many approved pharmaceutical products. The provincial price administration authorities also publish price control lists for pharmaceutical products. Pursuant to the NDRC and Measures for Medicine Pricing by the Government, the price ceiling is determined by whether drugs are deemed essential drugs and are included on the National Essential Drug List (“NEDL”) or non-essential drugs, which could be included in the NRDL and are subject to price control. Price ceiling determinations include references to the quality of the product, whether the products are newly developed products and whether the products have patent protection in China, and the status of implementing the GMP Guidelines by the manufacturer of the relevant product.
The prices of pharmaceutical products included in the price control lists are subject to adjustment upon approval by the price administration authorities from time to time. Pharmaceutical enterprises in China are required to submit cost-related information, such as raw material prices, regularly to the relevant authorities so that the authorities may take into account the prevailing market conditions when setting the prices. The price administration authorities may approve adjustments to the price of pharmaceutical products upon the pharmaceutical manufacturer’s request if material changes in the cost structure of producing the pharmaceutical products or significant changes in demand for these pharmaceutical products are recognized.
In each province where we market our products, distributors participate in a government-sponsored competitive bidding process every year or every few years for procurement by state-owned hospitals of a medicine included in the provincial medicine catalogs. A government-appointed committee reviews bids submitted by pharmaceutical companies and selects one or more medicines for treatment of a particular medical condition. The selection is based on a number of factors, including whether the product is on the NEDL or the NRDL, bid price, quality and manufacturer’s reputation and service. The bid price of the selected medicine will become the price required for purchases of that medicine by all state-owned hospitals in the relevant province or local district.
Health Insurance System
The MOL and Social Security are responsible for the reform of the medical insurance system. As part of the reform of the state basic medical insurance system for employees in the urban areas, the MOL and Social Security, the MOH, the SFDA and various other governmental departments jointly issue the State Basic Medical Insurance and Work Injury Insurance Drug Catalogue, as amended, or Catalogue, with a view to enhancing the management of the use of drugs under the medical insurance system. The drugs listed in the Catalogue are covered by the national medical insurance program.
Third-Party Reimbursement
Although in China the National Medical Insurance Program is designated as a national program, the implementation of the national medical insurance program is delegated to various provincial governments, each of which has established its own medicine catalog. A provincial government must include all Category A
16
medicines listed in the Catalogue in its provincial medicine catalog, but may use its discretion based on its own selection criteria to add other medicines to, or exclude Category B medicines listed in the Catalogue from, its provincial medicine catalog, so long as the combined numbers of the medicines added and excluded do not exceed 15% of the number of the Category B medicines listed in the Catalogue. In addition, provincial governments may not downgrade a nationally classified Category A medicine to Category B. The total amount of reimbursement for the cost of prescription and over-the-counter medicines, in addition to other medical expenses, for an individual program participant in a calendar year is capped at the amount in that participant’s individual account. The amount in a participant’s account varies, depending upon the amount of contributions from the participant and his or her employer. Generally, program participants who are from relatively wealthier eastern parts of China and relatively wealthier metropolitan centers have greater amounts in their individual accounts than those from less developed provinces.
Our ability to successfully commercialize our products may depend in part on the extent to which coverage and reimbursement to patients will be available from government health care programs, private health insurers and other third-party payors or organizations. Significant uncertainty exists as to the reimbursement status of therapeutic products, such as ZADAXIN and Depakine or other drugs we may develop. In most of the markets in which we are currently approved to sell ZADAXIN, reimbursement for ZADAXIN under government or private health insurance programs is not yet widely available, and in many of these countries government resources and per capita income may be so low that our products would be prohibitively expensive. We believe that certain sales of ZADAXIN in China are made with some third-party reimbursement. ZADAXIN’s national reimbursement retail list price in China was recently reviewed by regulatory authorities consistent with the China government’s review of pharmaceutical prices once a product has been included into the NRDL.
Prescription Regulations
As announced by MOH, the Prescription Administrative Measures, regulating prescription of drugs, took effect on May 1, 2007 and stipulates that doctors may only use the generic names of drugs in their prescriptions instead of brand names and that medical institutions offer patients the same type of drug from no more than two separate pharmaceutical companies. The purpose of this legislation is to minimize the practice of doctors receiving kickbacks from pharmaceutical companies for prescribing higher priced or unneeded drugs to patients.
US, Europe and Other Countries
The regulatory regime for the approval for drug distribution and marketing in the US and Europe is similar in many respects to the regulatory system in China. The steps required before a new drug may be distributed commercially generally include:
| | • | | conducting appropriate pre-clinical laboratory evaluations, including animal studies, in compliance with the FDA’s Good Laboratory Practice (“GLP”) requirements, to assess the potential safety and efficacy of the product; |
| | • | | submitting the results of these evaluations and tests to the FDA in an Investigational New Drug Application (“IND”), and receiving approval from the FDA that the clinical studies proposed under the IND are allowed to proceed; |
| | • | | conducting adequate and well-controlled clinical trials in compliance with the FDA’s Good Clinical Practice (“GCP”) requirements that establish the safety and efficacy of the product candidate for the intended use, typically in the same Phase 1, Phase 2 and Phase 3 steps described above for China; |
| | • | | development of manufacturing processes which conform to FDA current Good Manufacturing Practices, or cGMPs, as confirmed by FDA inspection; |
| | • | | submitting to the FDA the results of pre-clinical studies, clinical studies, and adequate data on chemistry, manufacturing and control information to ensure reproducible product quality batch after batch, in a New Drug Application (“NDA”) or Biologics License Application (“BLA”); and |
17
| | • | | obtaining FDA approval of the NDA, including inspection and approval of the product manufacturing facility as compliant with cGMP requirements, prior to any commercial sale or shipment of the pharmaceutical agent. |
After FDA approval has been obtained, the FDA requires post-marketing reporting to monitor the side effects of the drug. This may include phase 4 studies in which the drug is studied in an expanded patient population in a post-approval setting for continued monitoring of safety and sometimes continued efficacy.
We must comply with the regulations of each country in which we seek approval of and intend to market and sell any product.
AGREEMENTS WITH THIRD PARTIES
We hold license, promotion, distribution or marketing agreements with a number of parties for products under development, including agreements with Biocompatibles UK Ltd (“Biocompatibles”) for the distribution of DC Bead in China, with Orexo AB (“Orexo”) for Abstral in China, including Hong Kong and Macau, with BioAlliance Pharma SA (“BioAlliance”) for Loramyc in China, including Hong Kong and Macau, and with Applied Pharma Research s.a. (“APR”) for ondansetron RapidFilm in China including Hong Kong and Macau, and Vietnam. We own the rights, titles and interest in and to the worldwide (except as to Russia) technology and patent rights for SCV-07. We have agreements with each of the following companies for the distribution of certain products in China:
MEDA Pharma GmbH & Co. KG (“MEDA”) Agreement. We are party to an agreement with MEDA for various products under development including Tramadol. See Part I, Item 3 “Legal Proceedings” regarding the status of our agreement with MEDA from whom we license Tramadol.
Sanofi Agreements.In February 2006 and February 2008, one of our wholly-owned subsidiaries, NovaMed Pharmaceuticals (Shanghai) Co. Ltd. (“NovaMed Shanghai”) entered into a series of licensing and distribution agreements with Hangzhou Sanofi-Aventis Minsheng Pharmaceuticals Co. Ltd., Sanofi-Aventis Pharma Beijing Co. Ltd., Sanofi Winthrop Industrie, and Sanofi-Aventis Pharma Beijing Co. Ltd. (collectively, “Sanofi-Aventis”) for the distribution of XatralTM, PerenamTM, Stilnox, Tritace, Depakine and RulideTM in China. Under these agreements, NovaMed Shanghai must purchase product from Sanofi-Aventis for sale in China at prices specified in the agreements. The purchase prices are subject to adjustment in certain circumstances. To maintain NovaMed Shanghai’s exclusive rights, NovaMed Shanghai must meet certain unit volume requirements. In January 2013, the parties extended the term of certain of these agreements for the distribution of Xatral, Stilnox, Tritace, and Depakine in China. Each of these agreements will expire in December 2013, unless renewed.
Baxter Agreement.In September 2007, NovaMed Shanghai entered into a licensing and distribution agreement with Baxter Medical Products Trading (Shanghai) Ltd. (“Baxter”) for the distribution of HoloxanTM, MesnaTM, and EndoxanTM in China. Under the agreement, NovaMed Shanghai must purchase product from Baxter for sale in China, with a promotion fee specified in the agreement. To maintain NovaMed Shanghai’s exclusive rights, NovaMed Shanghai must meet certain unit volume requirements. The agreement will expire April 30, 2013, unless renewed.
Pfizer Agreement.In March 2008, NovaMed Shanghai entered into a licensing and distribution agreement with Pfizer International Trading (Shanghai) Ltd. (“Pfizer”) for the distribution of six products (AdriamycinTM, DaunoblastinaTM, LeucovorinTM, MethotrexateTM, EstracytTM, and FarlutalTM) in China. Under the agreement, NovaMed Shanghai must purchase product from Pfizer for sale in China at prices specified in the agreement. The purchase prices are subject to adjustment in certain circumstances. To maintain NovaMed Shanghai’s exclusive rights, NovaMed Shanghai must meet certain unit volume requirements. The agreement will expire June 30, 2013, unless renewed.
18
Iroko Agreement.In December 2008, NovaMed entered into a licensing and distribution agreement with Iroko Cardio LLC (“Iroko”) for the distribution of Aggrastat in China. Under the agreement, NovaMed must purchase product from Iroko for sale in China at prices specified in the agreement. The purchase prices are subject to adjustment in certain circumstances. To maintain NovaMed’s exclusive rights, NovaMed must meet certain unit volume requirements. The agreement will expire 10 years after the Initial Drug License is granted.
Our continued distribution of approved products depends upon the continuation of these agreements and the renewal of the agreements upon expiration.
AVAILABLE INFORMATION
We file electronically with the SEC our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov.
You may obtain a free copy of our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, on the day of filing with the SEC on our website on the World Wide Web at http://www.sciclone.com, by contacting the Investor Relations Department at our corporate offices by calling 800-724-2566 or by sending an e-mail message to investorrelations@sciclone.com.
You should carefully consider the risks described below, in addition to the other information in this report on Form 10-K, before making an investment decision. Each of these risk factors could adversely affect our business, financial condition, and operating results as well as adversely affect the value of an investment in our common stock.
Our stock price may be volatile, and an investment in our stock could suffer a decline in value.
Although we reported net income of $9.6 million, $28.1 million and $21.1 million for the years ended December 31, 2012, 2011 and 2010, respectively, we have experienced significant operating losses in the past, and as of December 31, 2012, we had an accumulated deficit of approximately $134.4 million. If our operating expenses were to increase or if we were not able to increase or sustain revenue, we may not achieve profitability over the next 12 months.
The market price of our common stock has experienced, and may continue to experience, substantial volatility due to many factors, some of which we have no control over, including:
| | • | | developments related to the pending SEC and DOJ investigations, our efforts to cooperate with the investigations and events related to pending litigations; |
| | • | | government regulatory action affecting our Company or our drug products or our competitors’ drug products in China, the US and other foreign countries, including the effect of government initiatives in China to reduce health care costs, including the change in the governmentally permitted maximum listed price for ZADAXIN (“thymosin alpha 1” or “thymalfasin”) and our other products on the market in China; |
| | • | | actual or anticipated fluctuations in our quarterly operating results some of which may result from acquisition-related expenses including the variation in the valuation of the earn-out, and periodic impairment charges that have and may result from the goodwill and intangible assets recorded in the acquisition; |
19
| | • | | progress and results of clinical trials and the regulatory approval process in Europe and in China; |
| | • | | our ability to manage the risks associated with our acquisition of NovaMed Pharmaceuticals, Inc. (“NovaMed”); |
| | • | | timing and achievement of our corporate milestones; |
| | • | | changes in our agreements or relationships with collaborative partners; |
| | • | | announcements of technological innovations or new products by us or our competitors; |
| | • | | announcement and completion of corporate acquisition, merger, licensing or marketing arrangements, or sales of assets; |
| | • | | developments or disputes concerning patent or proprietary rights; |
| | • | | changes in the composition of our management team or board of directors; |
| | • | | changes in company assessments or financial estimates by securities analysts; |
| | • | | changes in assessments of our internal control over financial reporting; |
| | • | | general stock market conditions and fluctuations for the emerging growth and pharmaceutical market sectors; |
| | • | | unanticipated increases in our G&A expense due to legal and accounting expenses, including expenses relating to the governmental investigations, our disputes with MEDA and with the former stockholders of NovaMed, and arising out of matters relating to any additional or uncorrected control deficiency or related matters; |
| | • | | economic and political conditions in the US or abroad particularly in China; and |
| | • | | broad financial market fluctuations in the US, Europe or Asia. |
Our acquisition of NovaMed involves a number of risks and although we believe we have substantially completed the integration there are some challenges that still could affect us and we may not realize the anticipated benefits of the acquisition; and we may acquire other companies or products that present similar risks.
We believe we have substantially completed the integration of our NovaMed acquisition and the two companies’ operations and personnel and have begun to utilize common business, information and communication systems, operating procedures, financial controls and human resources practices. We have experienced a number of challenges in the process of integration which have had, and could have further adverse affects on our business. These challenges include:
| | • | | diversion of management’s attention from normal daily operations of the business which could adversely affect ongoing operations, including matters relating to the restatement of our financial results and material weakness in our internal control over financial reporting identified in the third and fourth quarters of 2012; |
| | • | | retaining key employees of both organizations, including recently announced executive departures and potential additional turnover; |
| | • | | managing the acquisition and continuing operations in both organizations to successfully achieve the anticipated benefits of the acquisition; |
| | • | | preserving important relationships of both SciClone and NovaMed, including NovaMed’s contractual relationships with pharmaceutical partners; |
| | • | | costs and delays in implementing common systems and procedures; |
| | • | | consolidating and rationalizing information technology and administrative infrastructures; |
20
| | • | | the potential for disputes or litigation related to the escrow and indemnification provisions, including the claims we have made under those provisions, and the uncertainty as to the results of such dispute or any further dispute; |
| | • | | variability in our financial results which may result from acquisition-related expenses including the variation in the valuation of the earn-out, and periodic impairment charges that have and may result from the recording of goodwill in the acquisition; |
| | • | | the effectiveness of our efforts to improve the performance of NovaMed, including our effort to negotiate renewals of agreements with third parties and to improve the financial terms for agreements relating to promotional service revenues; |
| | • | | implementing procedures, policies and processes related to FCPA compliance; and |
| | • | | integrating and documenting processes and controls in conformance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 which were not applicable to NovaMed prior to the acquisition. |
We may enter into other acquisition transactions in the future which could present similar risks and may also cause us to:
| | • | | issue common stock that would dilute our current shareholders’ percentage ownership; |
| | • | | assume liabilities, some of which may be unknown at the time of such acquisitions; |
| | • | | record goodwill and intangible assets that have been and will be subject to impairment testing and potential periodic impairment charges; |
| | • | | incur amortization expenses related to certain intangible assets; and |
| | • | | incur large and immediate write-offs of in-process research and development costs; or become subject to litigation. |
Any one or all of these factors, many of which are outside of our control, may increase operating costs or lower anticipated financial performance following the NovaMed acquisition, or following any future acquisition. In addition, the combined company may lose customers, distributors, suppliers, manufacturers, partners and employees. Any diversion of management’s attention to address these factors and any difficulties associated with the acquisition of NovaMed, or of companies or products we may acquire in the future could have a material adverse effect on the operating results of the company and on the value of our common stock, and could result in our not achieving the anticipated synergies and benefits of the acquisition underlying the two companies’ reasons for the merger. Failure to achieve our objectives could have a material adverse effect on the business and operating results of the company.
During the year ended December 31, 2012, we determined that the carrying value of our intangible assets related to the promotion and distribution contract rights we acquired as part of the acquisition of NovaMed were no longer recoverable. As a result, we recognized a non-cash impairment loss of approximately $42.7 million, which adversely affected our financial results and could adversely affect the market value of our common stock. If the value of our goodwill asset becomes impaired, we may be required to incur further material impairment charges.
Our revenue will continue to be substantially dependent on our sale of ZADAXIN in China. The China government recently imposed price restrictions on ZADAXIN, Aggrastat and several of our oncology products. If we experience difficulties in our sales efforts as a result, our operating results and financial condition will be harmed.
Our product revenue is highly dependent on the sale of ZADAXIN in China. As a result of additional product sales resulting from the NovaMed acquisition in 2011, we expect that the percentage of our revenues that
21
come from the sale of ZADAXIN in China will decline significantly. However, we anticipate that sales of ZADAXIN will continue to be a majority of our revenue for at least the next two years. For the years ended December 31, 2012, 2011 and 2010, approximately 96%, 97% and 96%, respectively, of our ZADAXIN sales were to customers in China. Sales of ZADAXIN in China may be limited due to the low average personal income, lack of patient cost reimbursement, poorly developed infrastructure and competition from other products, including generics. ZADAXIN sales growth in recent years has benefited from the rapidly growing Chinese economy and growing personal disposable income. Sales of ZADAXIN in China could be adversely affected by a slowing or downturn of the Chinese economy and from the recent and future decisions of the National Development and Reform Commission (“NDRC”) and provincial agencies pricing reform.
In China, ZADAXIN is approved for the treatment of hepatitis B virus (“HBV”) and as a vaccine adjuvant. We face competition from pharmaceutical companies who are aggressively marketing competing products for the treatment of HBV and for other indications where we believe ZADAXIN may be used on an off-label basis. In addition, several local companies are selling lower-priced, locally manufactured generic thymalfasin, which is a competitive product and is selling in substantial and increasing quantities. While generic products outsell ZADAXIN in unit volumes, we have been able to maintain a pricing advantage through the reputation of our imported, branded product. We believe such competition will continue with added new local manufacturers of generic thymalfasin and there could be a negative impact on the price and the volume of ZADAXIN sold in China, which would harm our business. Our efforts to in-license or acquire other pharmaceutical products for marketing in China and other markets may be unsuccessful or even if successful may not have a meaningful effect on our dependence on ZADAXIN sales in those markets.
The Chinese government is increasing its efforts to reduce overall health care costs, including pricing controls on pharmaceutical products. Individual provinces in China and, in some cases, individual hospitals can and have established pricing requirements for a product to be included on formulary lists. In some cases, these prices have been significantly lower than our distributors have been selling ZADAXIN, in which case we have been removed from formulary lists, which consequently has reduced sales to certain hospitals and could adversely affect our future sales. The price for pharmaceutical products is regulated in China both at the national and at the provincial level. The process and timing for price restrictions is unpredictable. In addition, we are aware that ZADAXIN may be used on an off-label basis, and the Chinese government’s pricing, reimbursement or other actions might reduce such uses.
In November 2009, thymalfasin, the generic chemical name for our pharmaceutical product ZADAXIN, was included as a Category B product in the NRDL and pricing for ZADAXIN on the NRDL was recently reviewed by the authorities. As a result of this review, the retail list price (or the price at the hospital pharmacy level) of ZADAXIN was reduced by approximately 18% in China. The reduction was announced September 2012 and became effective October 8, 2012. Based on an agreement with our primary importer of ZADAXIN into China, our importer will take a larger share of the price reduction impact, and the actual impact on SciClone’s revenue and margins is expected to be less than a 5% decrease in our sales price of ZADAXIN to the importer in China. In exchange for this favorable arrangement, we expanded exclusivity for the importer. In addition, the NDRC price of Aggrastat, as well as several of our oncology products exclusively promoted in China for Pfizer and Baxter were reduced ranging from 10 to 20%. We have not negotiated any particular arrangement regarding the sharing of these price reductions for products other than ZADAXIN with our partners, but generally, under our agreements, the effect on our per unit revenues would be approximately half of the reduction in retail price at the hospital level.
These regulations, as well as regulation of the importation of pharmaceutical products have reduced and may further reduce prices for ZADAXIN or our other products to levels significantly below those that would prevail in an unregulated market, limit the volume of product which may be imported and sold or place high import duties on the product, any of which may limit the growth of our revenues or cause them to decline.
We expected that a price reduction for ZADAXIN and Aggrastat would be announced during 2012 and assumed a price reduction in our operating plan, and we believe we will be able to successfully manage our
22
business in China through this process; however, further price reductions could be set at some time in the future which could adversely affect our results or require substantial changes in our business model which may be difficult to implement.
Sales of ZADAXIN may also fluctuate significantly from quarter to quarter due to financing limitations on importers, changes in inventory levels at our customers, and surges in sales and inventories due to epidemics. Importers and distributors of ZADAXIN borrow funds in China from banks to purchase, hold and distribute ZADAXIN. Substantial increases in restrictions on fund availability and/or increases in borrowing costs could limit the ability of our importers and distributors to finance their import and distribution process. Further, our customers tend to purchase large orders, and if channels or distribution change, inventory levels may fluctuate significantly, potentially affecting quarterly periodic results.
During the third quarter, and particularly in September 2012, we estimate that there was an increase of approximately $14 million in ZADAXIN channel inventory levels and we believe that our sales to our customers exceeded the pace at which our customers were able to sell ZADAXIN through to other parties, primarily hospital pharmacies. ZADAXIN channel inventory levels decreased by approximately $5 million in the fourth quarter of 2012, and we expect them to continue to decrease over the next two quarters. As a result, we expect that ZADAXIN revenues will be lower in the first half of 2013, compared to the first half of 2012. We expect our 2013 sales revenue to be lower than or comparable to our 2012 revenues of $156 million. We continue to believe that we will grow demand for ZADAXIN through increased penetration in the market.
We have revised and we are implementing changes in our strategy for ZADAXIN market penetration. In addition, we believe that our sales organization’s efforts may have been affected by turnover, particularly at senior management levels. We are recruiting experienced new senior-level sales and other management, particularly following the announced departures of two of our senior executives in China. We are also taking other measures which may address the channel build-up, including working with one importer on an exclusive basis and implementing various programs to expand market demand. There may be additional departures in our China operations. During this transition period, and if we are unable to achieve our objectives for increased demand, we may experience declines in our quarterly sales revenue in the near term and our sales and profitability for the next few quarters may significantly decrease as compared to the preceding quarter.
In addition, during the second quarter of 2009, we experienced a strong upsurge in ZADAXIN sales which we believe was attributable both to the increasing penetration of ZADAXIN within the Chinese market, as well as concerns in China from the H1N1 influenza virus. If distributors and hospitals that purchase ZADAXIN stockpile more ZADAXIN than needed for current use, our sales of ZADAXIN may suffer as distributors and hospitals use ZADAXIN already in their inventory before purchasing additional product from us. This could lead to uneven future revenue results for ZADAXIN and in turn materially impact our cash flow and business condition.
Our revenue will continue to be substantially dependent on our maintaining regulatory licenses and compliance with other regulations.
We have received regulatory approvals to import and market ZADAXIN in China and to manufacture ZADAXIN and export the product from Italy. In order to continue our sales to China, we need to maintain these approvals. Our license to import ZADAXIN into China needs to be renewed every five years and the next renewal is required in 2017. Although we were successful in obtaining a renewal in 2003, 2008 and 2012, there is no assurance that we will receive renewals in the future when applied for or that the renewals will not be conditioned or limited in ways that limit our ability to sell ZADAXIN to China.
Our licenses to manufacture and export ZADAXIN from Italy are dependent upon our continuing compliance with regulations in Italy. Our business would be adversely affected if we are not able to maintain these approvals. In order to sell ZADAXIN to the licensed importers in China, our manufacturers must 1) be
23
approved by the Italian Ministry of Health (“AIFA”) and 2) be accepted by the State Food and Drug Administration of China (“SFDA”). Some manufacturing changes may require: 1) approval by AIFA in Italy and/or 2) be accepted by the SFDA, the Chinese equivalent of the FDA. In addition, we must obtain an Imported Drug License (“IDL”) from the SFDA permitting the importation of ZADAXIN into China in order to sell ZADAXIN to the licensed importers in China. ZADAXIN registration in Italy has been essential to the renewal of our IDL from the SFDA permitting the importation of ZADAXIN into China. Our ability to continue to renew our IDL from the SFDA permitting the importation of ZADAXIN into China could be adversely affected, if we were to fail to maintain ZADAXIN registration in Italy. The SFDA, AIFA and other regulatory agencies may, and have, changed their internal administrative rules in ways that may delay or complicate the regulatory approval process. Those changes are not always disclosed or known to us and we may experience unexpected delays or additional costs as a result of such changes. Our product has been distributed in Italy through BioFutura Pharma Srl (“BioFutura”), a subsidiary of Sigma-Tau Finanziaria, S.p.A. (“Sigma-Tau”). In August 2012, we entered into an agreement with BioFutura to continue to distribute ZADAXIN for SciClone in Italy. However, if we are not able to continue this arrangement, we will need to establish alternative distribution operations in Italy to ensure continuing compliance with regulations in Italy and maintain our Italian licenses.
Our ZADAXIN sales and operations in other parts of China and the world are subject to a number of risks and increasing regulations, including difficulties and delays in obtaining registrations, renewals of registrations, permits, pricing approvals and reimbursement, increasing regulation of product promotion and selling practices, unexpected changes in regulatory requirements and political instability.
We face risks related to the potential outcomes of the SEC investigation regarding FCPA compliance and other matters and DOJ investigation regarding the FCPA including potential penalties, substantial expenses and the use of significant management time and attention, and changes in our marketing and sales practices that could affect our ability to generate revenue, any of which could adversely affect our business.
In August 2010, we received notices of investigations by US government agencies that relate to our operations in China including compliance with the FCPA and we subsequently initiated an internal investigation regarding these matters. In connection with the formal, non public SEC investigation, the SEC issued a subpoena to us requesting documents regarding a range of matters including but not limited to documents relating to potential payments or transfer of anything of value to regulators and government-owned entities in China; documents relating to bids or contracts with state or government-owned entities in China; documents relating to intermediary or local agent of the Company in China; documents regarding the Company’s ethics and anti-corruption policies, training, and audits; and documents relating to certain Company financial and other disclosures made by the Company. The DOJ is currently conducting an investigation of us in connection with compliance with the FCPA, as to which they have advised us that the DOJ has information about the Company’s practices suggesting possible violations. We have been cooperating with, and will continue to cooperate with, the investigations by and inquiries from the SEC and DOJ. In response to these matters, our Board of Directors appointed the Special Committee of independent directors to oversee our response to the government inquiry. The Special Committee conducted an independent investigation as to matters reflected in and arising from the SEC and DOJ investigations including, but not limited to, certain sales and marketing matters in China, in order to evaluate whether any violation of the FCPA or other laws occurred.
The Special Committee substantially concluded its investigation of those matters and on May 4 and 5, 2011 reported its findings and recommendations to the Board of Directors. The Special Committee reached a number of findings, including that we lacked appropriate internal controls to assure compliance with laws, including the FCPA, with respect to sales and marketing practices including payments for, or reimbursement of, third party gifts, travel and entertainment expenses, and sponsorships of certain conferences and symposia. The Special Committee identified evidence of sales and marketing activities that might constitute potential violations of the FCPA. We are undertaking certain remedial measures recommended by the Special Committee and adopted by our Board of Directors.
24
In the Company’s Form 10-Q for the period ending September 30, 2012, filed with the SEC on November 9, 2012, the Company disclosed, among other things, a non-cash impairment loss to fully write down the value of intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; a significant increase in ZADAXIN channel inventory levels; and internal control issues primarily within the NovaMed organization that were concluded to represent a material weakness in internal control over financial reporting. Following our disclosure of these items, the Company received a subpoena from the SEC requesting documents related to these and various other matters regarding the NovaMed acquisition and the Company’s operations in China. After review of the subpoena, and in order to respond to inquiries from the DOJ and SEC and to determine if any wrong-doing occurred, the Audit Committee determined to undertake an additional independent investigation as to additional matters, including, but not limited to, matters related to our acquisition of NovaMed and FCPA matters.
We are unable to predict what consequences any investigation by any regulatory agency or by our Audit or Special Committees may have on us. Our cooperation with these investigations has resulted in substantial legal and accounting expenses, has diverted management’s attention from other business concerns and could harm our business. The ongoing investigations and any other regulatory investigations that might be initiated in the future will result in similar substantial expenses, management diversion and harm to our business. If we fail to comply with regulations or to carry out controls on our Chinese or other foreign operations in a manner that satisfies all applicable laws, our business would be harmed. Any civil or criminal action commenced against us by a regulatory agency could result in administrative orders against us, the imposition of significant penalties and/or fines against us, and/or the imposition of civil or criminal sanctions against certain of our officers, directors and/or employees. The investigations, results of the investigations, or remedial actions we have taken or may take as a result of such investigations may adversely affect our business in China. If we are subject to adverse findings resulting from the SEC and DOJ investigations, or from our own independent investigation, we could be required to pay damages or penalties or have other remedies imposed upon us. In addition, we will incur additional expenses related to remedial measures we are undertaking, and could incur fines or other penalties. The period of time necessary to resolve the investigations by the DOJ and the SEC is uncertain, and these matters could require significant management and financial resources which could otherwise be devoted to the operation of our business.
If we fail to achieve or maintain an effective system of internal controls, we may not be able to accurately report our financial results. As a result, current and potential stockholders could lose confidence in our financial reporting, which would harm our business and the trading price of our stock.
Effective internal controls are necessary for us to provide reliable financial reports and to protect from fraudulent, illegal or unauthorized transactions. If we cannot establish effective controls and provide reliable financial reports, our business and operating results could be harmed. Moreover, as a US-based corporation doing business in China, these controls often need to satisfy the requirements of Chinese law as well as the requirements of US law which frequently differ in certain aspects. We have in the past discovered, and may in the future discover, areas of our internal controls that need improvement. For example, during the third quarter of 2012, our management determined that we had a material weakness in internal control over financial reporting related to the design and operation of our controls primarily associated with product returns reserves and the override of certain controls in the financial statement close process related to our NovaMed subsidiary. Furthermore, during the fourth quarter of 2012, our management determined that we had an additional indicator of the same material weakness related to the timing of revenue recognition for our Pfizer products and the override of related controls at our NovaMed subsidiary, and the corporate monitoring thereof. As of December 31, 2010, we also had two material weaknesses in internal control over financial reporting related to our controls over (i) our implementation of our policy on compliance with laws and (ii) our accounting for income taxes. We continue to work on improvements to our internal controls and there can be no assurance that these or other material weaknesses will not occur in the future, or otherwise cause us to inaccurately report our financial statements. For example, our recently announced restatement of our financial statements for each of our
25
first, second, and third quarters of 2012, and our financial statements for each of the second and third quarters of 2011 and the year ended December 31, 2011, were in part caused by our material weakness related to the design and operation of our controls discussed above. Any failure to implement and maintain controls over our financial reporting or difficulties encountered in the implementation of improvements in our controls, could cause us to fail to meet our reporting obligations. Any failure to improve our internal controls or to address identified weaknesses in the future, if they were to occur, could also cause investors to lose confidence in our reported financial information, which could have a negative impact on the trading price of our stock.
Compliance with changing regulations concerning corporate governance and public disclosure has resulted in and may continue to result in additional expenses. Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, new SEC regulations and The NASDAQ Stock Market rules, are creating uncertainty for companies such as ours and costs are increasing as a result of this uncertainty and other factors. We are committed to maintaining high standards of corporate governance and public disclosure. As a result, we intend to invest all reasonably necessary resources to comply with evolving standards, and this investment has and may continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities.
We may not be able to effectively manage our employees and distribution network, and our reputation, business, prospects and brand may be materially and adversely affected by actions taken by our distributors and third party marketing firms.
We do not control, and therefore have limited ability to manage, the activities of third-parties who assist us in marketing and distributing our products. Our distributors or other third parties could violate the anti-corruption laws of China, the United States or other countries.
Our company policies prohibit our employees from making improper payments to hospitals or otherwise engaging in improper activities to influence the procurement decisions of hospitals, and we take remedial actions, including termination, when employees do not adhere to our policies. However, we may not be able to effectively ensure that every employee complies at all times with our policies. The compensation of our sales and marketing personnel is partially linked to their sales performance. Although we have made numerous changes to ensure compliance with our policies and to attempt to avoid any violation of law, we cannot assure you that employees will not violate the anticorruption laws of China, the United States and other countries. Such violations could have a material adverse effect on our reputation, business, prospects and brand.
Furthermore, our employees in China have access to our facilities and internal systems and we have identified from time to time certain minor instances of improperly submitted expense reporting. Although these instances have involved insignificant sums, our employees may seek to create additional opportunities to engage in misappropriation or other employee malfeasance. If our controls and procedures to prevent such activities fail or are circumvented, our business would be negatively affected by, among other things, the related financial losses, diminished reputation and threat of litigation and regulatory inquiry and investigation.
We do not control, and therefore have limited ability to manage, the activities of third-parties who assist us in marketing and distributing our products. Our distributors or other third parties with whom we do business could take actions which violate the anti-corruption laws of China, the United States or other countries. Failure to adequately manage our employees, and third parties and, or their non-compliance with employment, distribution or marketing agreements, could harm our corporate image among hospitals and end users of our products and disrupt our sales, resulting in a failure to meet our sales goals. Furthermore, we could be liable for actions taken by our employees, distributors or third-party marketing or third party firms, including any violations of applicable law in connection with the marketing or sale of our products, including China’s anticorruption laws and the Foreign Corrupt Practices Act of the United States, or the FCPA. In particular, if our employees, distributors or third-party marketing firms make any payments that are forbidden under the FCPA, we could be subject to civil and criminal penalties imposed by the US government.
26
Recently, the Chinese government has increased its anti-corruption measures. In the pharmaceutical industry, corrupt practices include, among others, acceptance of kickbacks, bribes or other illegal gains or benefits by the hospitals and medical practitioners from pharmaceutical manufacturers and distributors in connection with the prescription of certain pharmaceuticals. Our employees, affiliates, distributors or third-party marketing firms may violate these laws or otherwise engage in illegal practices with respect to their sales or marking of our products or other activities involving our products. If our employees, affiliates, distributors or third-party marketing firms violate these laws, we could be required to pay damages or fines, which could materially and adversely affect our financial condition and results of operations. In addition, Chinese laws regarding what types of payments to promote or sell our products are impermissible are not always clear, and local regulatory authorities enforcing these laws are not always consistent. As a result, we, our employees, affiliates, our distributors or third-party marketing firms could make certain payments in connection with the promotion or sale of our products or other activities involving our products which at the time are considered by us or them to be legal but are later deemed impermissible by the Chinese government, or we may be asked to make payments by local government authorities that may not be permissible under China’s anticorruption laws or the FCPA. Furthermore, our brand and reputation, our sales activities or the price of our common stock could be adversely affected if we become the target of any negative publicity as a result of actions taken by our employees, affiliates, distributors or third-party marketing firms.
Our compliance with the Foreign Corrupt Practices Act may put us at a competitive disadvantage, while our failure to comply with the Foreign Corrupt Practices Act may result in substantial penalties.
As a US reporting company, we are required to comply with the FCPA. If our employees or other agents are found to have engaged in practices in violation of the FCPA, we could suffer severe penalties. Non-US companies, including some of our competitors, are not subject to the provisions of the FCPA. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time to time in mainland China. If our competitors engage in these practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business or from government officials who might give them priority in their business dealings, which would put us at a disadvantage.
Retaliation from terminated employees may damage our reputation or make claims that could subject us to further regulatory action.
From time to time we have terminated the employment of certain employees for performance related reasons, including, in particular, our policies intended to prevent corruption. Employees who are terminated may seek more favorable terms of separation by threatening to damage our reputation in the marketplace. Further, they may seek to retaliate against us by making so-called “whistleblower” claims under the provisions enacted by the Dodd-Frank Act that may entitle persons who report alleged wrong-doing to the SEC to cash rewards. We anticipate that these provisions will result in a significant increase in whistleblower claims across our industry, and dealing with such claims could generate significant expenses and take up significant management time, even for frivolous and non-meritorious claims. Any investigations of whistleblower claims may impose additional expense on us, may require the attention of senior management and members of the Board of Directors and may result in fines and/or reputational damage whether or not we are deemed to have violated any regulations. Furthermore, terminated employees may also seek to retaliate against us by making claims against us to other regulatory agencies, including local regulatory authorities. Inquiries by local regulatory agencies about such claims, even if frivolous and non-meritorious, could also generate significant expenses and take up significant management and Board time.
We are at risk of additional securities class action and derivative lawsuits.
Securities class action and derivative lawsuits are often filed against public companies following a decline in the market price of their securities. After our announcement regarding SEC and DOJ investigations in 2010, we and certain of our officers and directors were named as parties in purported stockholder class actions and
27
derivative lawsuits. Those class action lawsuits were dismissed and we have settled those derivative lawsuits. Our stock price declined following the announcement of a restatement of our financial statements for fiscal 2011 and the first three quarters of fiscal 2012, and that our independent auditing firm had elected not to stand for reappointment for the 2013 fiscal year. Soon after that announcement, we and certain of our officers and directors were named as parties in a purported derivative lawsuit relating to the restatement. We may experience stock price volatility in the future, either related to announcements regarding the SEC and DOJ investigation, our own investigations related thereto or other matters. This risk is especially relevant for us because biotechnology companies have experienced greater than average stock price volatility in recent years. We may be named in additional litigation, which will require significant management time and attention and result in significant legal expenses and may result in an unfavorable outcome which could have a material adverse effect on our business, financial condition, results of operations and cash flows. Such litigation could result in additional substantial costs and a diversion of management’s and the Board’s attention and resources, which could harm our business.
We may not be able to successfully develop or commercialize our products.
Following the acquisition of NovaMed, we have numerous products under development in China and during 2011 and the first quarter of 2012, we were developing SCV-07, a small molecule synthetic peptide with immunomodulating properties, in a phase 2b clinical trial in the US for the delayed onset of oral mucositis (“OM”).
Clinical trials are inherently risky and may reveal that our product candidates are ineffective or have unanticipated side effects and/or drug interactions that may significantly decrease the likelihood of regulatory approval. For example, in March 2012 we announced the discontinuation of our phase 2b clinical trial evaluating SCV-07 for the delayed onset of OM. This decision was based on the results of a pre-planned interim analysis that indicated that the trial would not meet the pre-specified efficacy endpoints, and we have no plans to proceed with further development of SCV-07 at this time.
The regulatory approval processes in the US, Europe and China are demanding, lengthy and expensive. We have committed significant resources, including capital and time, to develop and seek approval for products under development, and if we do not obtain approvals we are seeking, we may be unable to achieve any revenue from these products. All new drugs, including our product candidates, are subject to extensive and rigorous regulation by the FDA, SFDA and similar regulatory agencies. These regulations govern, among other things, the development, testing, manufacturing, labeling, storage, pre-market approval, importation, advertising, promotion, sale and distribution of our products. These regulations may change from time to time and new regulations may be adopted.
Satisfaction of government regulations may take several years and the time needed to satisfy them varies substantially based on the type, complexity and novelty of the pharmaceutical product. As a result, government regulation may cause us to delay the introduction of, or prevent us from marketing, our existing or potential products for a considerable period of time and impose costly procedures upon our activities. We have experienced delays in the regulatory process and continue to experience delays, and there exists risk that we may not receive approval, including with the approval process for DC Bead or other in-licensed products. In addition, the Chinese government is increasing its efforts to reduce overall health care costs, including pricing controls on pharmaceutical products. We cannot determine what the potential government pricing constraints are likely to be for products in development in advance. Therefore, we may be required to abandon the development or commercialization of a product after significant effort and expense if we determine at any time that trends in government pricing constraints will make the commercialization of a product unprofitable.
To fully develop these products and other products we may acquire, substantial resources are required for extensive research, development, pre-clinical testing, clinical trials, and manufacturing scale-up and regulatory approval prior to the potential products being ready for sale. We cannot assure that our efforts will produce commercially viable products. We face significant technological risks inherent in developing these products. We
28
may also abandon some or all of our proposed products before they become commercially viable. We are obligated to make a milestone payment upon regulatory approval of certain products under development. If any of our products, even if developed and approved, cannot be successfully commercialized in a timely manner, our business will be harmed and the price of our stock may decline.
Market acceptance of any product that is successfully developed and approved will depend on many factors, including our ability to convince prospective customers to use our products as an alternative to other treatments and therapies. In addition, doctors must opt to use treatments involving our products. If doctors elect to use a different course of treatment, demand for our drug products would be reduced. In addition, for certain products we may need to convince partners to manufacture or market our products. Failure to do any of the above will lead to an unfavorable outcome on the results of our operations.
Our success is dependent upon the success of our sales and marketing efforts in China, and we may experience difficulties in complying with regulations, slow collections or other matters that could adversely affect our revenue in China.
Following the acquisition of NovaMed, we have numerous products on the market in China in addition to ZADAXIN. Our future revenue growth depends to a great extent on increased sales of ZADAXIN to China and increased sales of the products promoted or marketed by NovaMed. If we fail to continue to successfully market ZADAXIN or NovaMed’s product portfolio, our revenue and operating results will be limited. If unexpected and serious adverse events are reported, or if expected efficacy results are not achieved, it would have a material adverse effect on our business.
Our sales are concentrated in China and we face risks relating to operating in a China, including pricing and other regulations, slow payment cycles and exposure to fluctuations in the Chinese economy.
The Chinese government is increasing its efforts to reduce overall health care costs, including pricing controls on pharmaceutical products. Individual provinces in China and, in some cases, individual hospitals can and have established pricing requirements for a product to be included on formulary lists. The Chinese government has recently imposed price restrictions on ZADAXIN, Aggrastat and various oncology products we promote. The national reimbursement retail list price, or hospital pharmacy level price, of ZADAXIN has been reduced by approximately 18% in China. Based on an agreement with our primary importer of ZADAXIN into China, our importer will take a larger share of the price reduction impact, and the actual impact on SciClone’s revenue and margins is expected to be less than a 5% decrease in our sales price of ZADAXIN to the importer in China. In exchange for this favorable arrangement, we expanded exclusivity for the importer. Over the long term, we believe that the price reductions may positively affect our sales volumes and result in broader penetration into Tier 3 and Tier 2 cities in target geographies, potentially increasing our total sales revenues from these products. However, the process and timing for any price restrictions is unpredictable and further price reduction could be imposed that could adversely affect our business. Further, the successful sales and marketing of all of NovaMed’s products requires continuing compliance with other regulations in China relating to the import, manufacture, approval and distribution of products and if we or our partners are not able to obtain or maintain necessary licenses or other approvals, our operations would be adversely affected.
We experience other issues with managing sales operations in China including long payment cycles, potential difficulties in timely accounts receivable collection and, especially from significant customers, fluctuations in the timing and amount of orders and the adverse effect of any of these issues on our business could be increased due to the concentration of our business with a small number of distributors. Problems with collections from, or sales to, any one of those distributors could materially adversely affect our results. Operations in foreign countries including China also expose us to risks relating to difficulties in enforcing our proprietary rights, currency fluctuations and adverse or deteriorating economic conditions. If we experience problems with these matters, or if significant political, economic or regulatory changes occur, our results could be adversely affected.
29
Our operations throughout the world including China are potentially subject to the laws and regulations of the US including the FCPA, in addition to the laws and regulations of the other countries. Regulation in China of the activities in the pharmaceutical industry has increased and may continue to undergo significant and unanticipated changes. A number of companies have faced significant expenses or fines as a result of the increasing regulation of, and enforcement activity regarding, the pharmaceutical industry.
Currently all of our revenue is generated from customers located outside the US, and a substantial portion of our assets, including employees, are located outside the US. US income taxes and foreign withholding taxes have not been provided on undistributed earnings of non-US subsidiaries, because such earnings are intended to be indefinitely reinvested in the operations of those subsidiaries. The US government may propose initiatives that would substantially reduce our ability to defer US taxes including: repealing deferral of US taxation of foreign earnings, eliminating utilization or substantially reducing our ability to claim foreign tax credits, and eliminating various tax deductions until foreign earnings are repatriated to the US. If any of these proposals are constituted into legislation, they could increase our US income tax liability and as a result have a negative impact on our financial position and results of operations.
Our business strategy is dependent in part upon our agreements with third parties for the rights to develop and commercialize products, or promote products, particularly in China. If we fail to maintain such agreements, or if we fail to enter into additional agreements, our business will suffer.
Our sales and marketing strategy in China depends significantly upon agreements with third parties, and potentially upon entering into additional agreements with third parties, or renegotiating agreements with third parties. Except for ZADAXIN, our rights to develop, market and sell our products in China, including the products currently promoted or sold by our subsidiary, NovaMed, are held by us under license, promotion, distribution or marketing agreements with third parties. These agreements for products on the market including Depakine, Stilnox, Tritace and Aggrastat, and products in the regulatory review process, including DC Bead and several of NovaMed’s products in clinical trials, are held under license, distribution or marketing agreements. In addition, our success in the future may be dependent upon entering into similar agreements with other parties and the renewal of any such agreements. The third parties to these agreements are generally not under an obligation to renew the agreements. If any of these agreements are terminated, or if they are not renewed, our ability to distribute, or develop, the products or product candidates could be terminated and our business could be affected. In addition, if any of such agreements acquired in our NovaMed acquisition are not renewed, we could incur a decline in sales revenues. Renegotiation of agreements can also occur prior to discussions of contract renewals. We have had disputes with collaborators in the past, and are in negotiations regarding disputes with current collaborators regarding product candidates in the regulatory approval process. We are currently in a dispute with MEDA regarding NovaMed’s agreement with MEDA which, if not resolved favorably to us, would prevent us from distributing Tramadol in China which would eliminate Tramadol revenues in the future. See Part I, Item 3 “Legal Proceedings”. Such disputes have arisen, and may arise in the future over the performance of each party’s obligations under the agreements, ownership rights to intellectual property, know-how or technologies developed with our collaborators. Disputes with collaborators or licensors or others could cause us to incur legal costs and could result in the loss of rights to products, loss of potential revenue, or other disruptions in our business.
All of our products were originally obtained by us under licenses, promotion, distribution or similar third-party agreements. We do not conduct product discovery and our ability to bring new products to market is dependent upon our entering into additional acquisition, in-licensing, promotion or distribution agreements, particularly in China. The competition for attractive products is intense, and we cannot assure you that we will be able to negotiate in-license, promotion or distribution agreements for additional products in the future on acceptable terms, if at all.
We may lose market share or otherwise fail to compete effectively in the intensely competitive pharmaceutical industry.
Competition in the pharmaceutical industry in China is intense, and we believe that competition will increase. Our success depends on our ability to compete in this industry, but we cannot assure you that we will be
30
able to successfully compete with our competitors. Increased competitive pressure could lead to intensified price-based competition resulting in lower prices and margins, which would hurt our operating results. We cannot assure you that we will compete successfully against our competitors or that our competitors, or potential competitors, will not develop drugs or other treatments for our targeted indications that will be superior to ours.
We depend on sales to China, and global conditions could negatively affect our operating results or limit our ability to expand our operations in and outside of China. Changes in China’s political, social, regulatory and economic environment may affect our financial performance.
Our business is concentrated in China. Heightened tensions resulting from the current geopolitical conditions in the Middle East, North Korea and elsewhere could worsen, causing disruptions in foreign trade, which would harm our sales. In particular, our commercial product is manufactured in Europe and distributed by us from our operations in Hong Kong. Any disruption of our supply and distribution activities due to geopolitical conditions could decrease our sales and harm our operating results.
With respect to China, our financial performance may be affected by changes in China’s political, social, regulatory and economic environment. The role of the Chinese central and local governments in the Chinese economy is significant. Chinese policies toward economic liberalization, and laws and policies affecting foreign companies, currency exchange rates and other matters could change, resulting in greater restrictions on our ability to do business in China. Any imposition of surcharges or any increase in Chinese tax rates could hurt our operating results. The Chinese government could revoke, terminate or suspend our license for national security and similar reasons without compensation to us. If the government of China were to take any of these actions, we would be prevented from conducting all or part of our business. Any failure on our part to comply with governmental regulations could result in the loss of our ability to market our products in China.
Because of China’s tiered method of importing and distributing finished pharmaceutical products, our quarterly results may vary substantially from one period to the next; we are dependent upon Sinopharm as the exclusive importer of ZADAXIN.
Imported products in China, including ZADAXIN and NovaMed’s imported products, are distributed through a tiered method to import and distribute finished pharmaceutical products. Promoted products are typically sold from our partner companies within China to the primary distributor with the following distribution being the same for imported as well as promoted products. At each port of entry, and prior to moving the product forward to the distributors, government-licensed importing agents must process and evaluate each imported product shipment to determine whether it satisfies China’s quality assurance requirements. In order to efficiently manage this process, the importing agents typically place large, and therefore relatively few, orders within an annual period. Therefore, sales to an importing agent can vary substantially from quarter to quarter depending on the size and timing of the orders, which has in the past and may in the future cause our quarterly results to fluctuate. We rely on a limited number of importers, in any given quarter, to supply our products and most of our ZADAXIN sales have been through three importers. Our receivables from those importers are material, and if we were unable to collect receivables from those importers or any other importer, our business and cash-flow would be adversely affected.
Generally, our importers are not obligated to place purchase orders for our product, and if they determined for any reason not to place purchase orders, we would need to seek alternative licensed importers, which could cause fluctuations in our revenue. As a result of our recent agreement granting certain exclusive importation rights to SinoPharm for ZADAXIN, we are dependent upon SinoPharm’s performance of its obligations under that agreement. We have a long-standing and we believe excellent relationship with SinoPharm, however, if SinoPharm was unable to adequately perform its obligations under, or breached, the agreement our business would be adversely affected.
31
The existence of counterfeit pharmaceutical products in China’s pharmaceutical retail market may damage our brand and reputation and have a material adverse effect on our business, financial condition, results of operations and prospects.
Certain medicine products distributed or sold in China’s pharmaceutical retail market, including those appearing to be our products, may be counterfeit. Counterfeit products are products sold under the same or very similar brand names and/or having a similar appearance to genuine products. Counterfeit products, including counterfeit pharmaceutical products, are a significant problem in China. Such products divert sales from genuine products, often are of lower cost, often are of lower quality (having different ingredients or formulations, for example), and have the potential to damage the reputation for quality and effectiveness of the genuine product. The counterfeit pharmaceutical product regulation control and enforcement system in China is not able to completely eliminate production and sale of counterfeit pharmaceutical products. Any sale of counterfeit products resulting in adverse side effects to consumers may subject us to negative publicity and expenses. It could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be subject to currency exchange rate fluctuations, which could adversely affect our financial performance.
A majority of our product sales are denominated in US dollars and a significant portion of our sales and expenses are denominated in renminbi. Fluctuation in the US dollar exchange rate with local currency directly affects the customer’s cost for our product. In particular, a stronger US dollar vis-à-vis the local currency would tend to have an adverse effect on sales and potentially on collection of accounts receivable. China currently maintains the value of the renminbi in a narrow currency trading band that may or may not fluctuate based upon government policy. Depending on market conditions and the state of the Chinese economy, China has intervened in the foreign exchange market in the past to prevent significant short-term fluctuations in the renminbi exchange rate, and it could make future adjustments, including moving to a managed float system, with opportunistic interventions. This reserve diversification may negatively impact the US dollar and US interest rates. A trend to a stronger US dollar would erode margins earned by our Chinese importers and prompt them to ask us to lower our prices. A weaker US dollar would increase our in-country China operating expenses, and with the addition of NovaMed, our China operating expenses have increased. We are subject to currency exchange rate fluctuations as a result of expenses incurred by our foreign operations. In particular, one of our supply arrangements under which we purchase finished products is denominated in euros and costs of our operations in China are paid in local currency. Consequently, changes in exchange rates could unpredictably and adversely affect our operating results and could result in exchange losses. To date, we have not hedged against the risks associated with fluctuations in exchange rates and, therefore, exchange rate fluctuations could have a material adverse impact on our future operating results and stock price.
We cannot predict the safety profile of the use of ZADAXIN, Depakine, or other drugs we may develop or market when used in combination with other drugs.
Many of our prior trials involved the use of ZADAXIN in combination with other drugs. We cannot predict how ZADAXIN, Depakine, or other drugs we may develop or market will work with other drugs, including causing possible adverse side effects not directly attributable to the other drugs that could compromise the safety profile of ZADAXIN, Depakine, or other drugs we may develop or market when used in certain combination therapies.
If third-party reimbursement is not available or patients cannot otherwise pay for ZADAXIN, Depakine, or other drugs we may develop we may not be able to successfully market them.
Significant uncertainty exists as to the reimbursement status of therapeutic products, such as ZADAXIN and Depakine or other drugs we may develop. We cannot assure you that third-party insurance coverage and reimbursement will be available for therapeutic products we might develop. Although ZADAXIN receives some
32
limited reimbursement in certain provinces in China, we cannot assure you that we will be able to maintain existing reimbursements or increase third-party payments for ZADAXIN or obtain third-party payments for other products which we sell or develop in China. The failure to obtain or maintain third-party reimbursement for our products would harm our business. Further, we cannot assure you that additional limitations will not be imposed in the future in the US or elsewhere on drug coverage and reimbursement due to proposed health care reforms. In many emerging markets where we have marketing rights to ZADAXIN, but where government resources and per capita income may be so low that our products will be prohibitively expensive, we may not be able to market our products on economically favorable terms, if at all.
Recent efforts by governmental and third-party payers to contain or reduce health care costs and the announcement of legislative proposals and reforms to implement government controls has caused us to reduce the prices at which we market our drugs in China, and additional reforms, if they were to occur, could cause us to further reduce our prices which could reduce our gross margins and may harm our business.
We rely on third parties who are our sole source suppliers for our clinical trial and commercial products and their inability to deliver products that meet our quality-control standards could delay or harm one or more important areas of our business including our sales, clinical trials or the regulatory approval process.
We rely on third parties, who are subject to regulatory oversight, to supply our commercial products. Any deficiencies or shortages in supply of our commercial products would adversely affect our ability to realize our sales plans. For example, the manufacturing of the raw material and the processing to finished product of ZADAXIN is done in few batches in any given three-month period and any manufacturing errors have the potential to require a product recall. We currently have only one approved finished vial manufacturer and two approved active pharmaceutical ingredient (“API”) suppliers. If we experience a problem with the manufacturer or our suppliers our sales may suffer. We and NovaMed have each experienced difficulties with obtaining product from manufacturers in the past. During 2012, we have experienced limitations on supply of Depakine IV, Perenan, Methotrexate, and Rulide and the growth in the sales of those products has been affected. During 2011, we experienced manufacturing delays related to repairs for general, non-production-related facilities equipment at one of our API suppliers. During 2010, we experienced difficulties validating upgrades to equipment with one of our API manufacturers. Although we are taking steps to ensure that such problems do not continue, there is no assurance that we will either be successful in doing so with our current supplier or be able to timely and cost-effectively qualify new suppliers for this component. Manufacturing interruptions or failure or delay of product to meet quality assurance specifications could adversely affect shipments and recognition of sales of our products in any period and impair our relationships with customers and our competitive position and may increase the cost of material produced. In addition, each of the products that are marketed through our NovaMed subsidiary is manufactured by, or obtained from, a single source.
We also rely on third parties, who are subject to regulatory oversight, to supply drug product. For example, Bioalliance is the sole supplier of Loramyc. Any unanticipated deficiencies in this supplier, or the suppliers of our raw materials, and/or recall of the manufacturing lots could also impede commercialization of our products and impair our competitive position. In addition, any unanticipated deficiencies in suppliers used in our clinical trials could delay the trials or detract from the integrity of the trial data and its potential use in future regulatory filings. In addition, manufacturing interruptions or failure to comply with regulatory requirements by any of these suppliers could significantly delay clinical development of potential products and reduce third-party or clinical researcher interest and support of proposed trials.
If our thymalfasin API or ZADAXIN products are not shipped and stored at precision temperatures, the products could become damaged, which could negatively affect our sales and operating results.
Thymalfasin API and ZADAXIN are temperature sensitive products. SciClone relies on third party organizations to provide controlled temperature shipping logistics services from the point of ownership transfer from the API contract manufacturer to the point where thymalfasin API is converted to ZADAXIN drug product,
33
and from the ZADAXIN drug product manufacturing site to our storage locations in Hong Kong and then to China. Although some temperature excursions are allowable and thymalfasin and ZADAXIN are relatively stable when exposed to temperatures higher than recommended, if any third party logistics or equipment provider fails to perform their required oversight duties with respect to temperature control or a shipment is delayed in transit for a prolonged period of time, the thymalfasin API or ZADAXIN drug product could become unsuitable for subsequent processing or commercial use. Although we have not experienced cold chain interruptions in the past and our distributor in China may maintain several months supply of our product, were our cold chain distribution or warehouse capability to be interrupted, our ability to timely deliver finished product to China could be adversely affected which in turn could materially adversely affect our sales and operating results.
We rely on third parties for development of our products and the inability of any of these parties to reliably, timely or cost-effectively provide us with their obligated services could materially harm the timing of bringing our products to market and accordingly adversely affect our business.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators, contract laboratories, and collaborative partners in the conduct of clinical trials for our product candidates. If these parties, whom we do not control, do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines or choose not to continue their relationship with us, if the third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical or clinical activities may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize our product candidates.
Commercialization of some of our products depends on collaborations with others. If our collaborators are not successful, or if we are unable to find future collaborators, we may not be able to properly develop and commercialize our products.
We depend in part on our distributors and business partners to develop or promote our drugs, and if they are not successful in their efforts or fail to do so, our business will suffer. For example, Biocompatibles is providing SciClone with product samples, clinical and product data, and the necessary supporting documents to obtain regulatory approval in China for DC Bead. We generally do not have control over the amount and timing of resources that our business partners devote to our collaborative efforts, and some have not always performed as or when expected. If they do not perform their obligations as we expect, particularly obligations regarding clinical trials, our development expenses would increase and the development or sale of our products could be limited or delayed, which could hurt our business and cause our stock price to decline. In addition, our relationships with these companies may not be successful. Disputes may arise with our collaborators, including disputes over ownership rights to intellectual property, know-how or technologies developed with our collaborators. We may not be able to negotiate similar additional arrangements in the future to develop and commercialize ZADAXIN or other products.
If we are unable to retain our key personnel, or are unable to attract and retain additional, highly skilled and experienced personnel, including the ability to expand our sales staff, our business will suffer.
We are highly dependent upon our ability to attract and retain qualified personnel because of the specialized, scientific and worldwide nature of our business. We are also dependent on our ability to appropriately staff these personnel in appropriate positions as our business fluctuates. Further, our efforts to in-license or acquire, develop and commercialize product candidates for China may require the addition of clinical and regulatory personnel and the capabilities to expand our sales and marketing operation. In addition, we assign numerous key responsibilities to a limited number of individuals, and we would experience difficulty in finding immediate replacements for any of them were any one of them to choose to leave employment with us. There is intense competition for qualified management, scientific, clinical, regulatory, and sales and marketing personnel in the pharmaceutical industry.
34
In January 2013, our Senior Vice President and Chief Financial Officer announced his intention to resign from the Company on or around May 31, 2013 to pursue other opportunities, and we are currently recruiting for his replacement.
There is significant turnover in the industry, in China in particular, and we have also experienced turnover in our sales personnel and key employees. We may not be able to attract and retain the qualified personnel we need to grow and develop our business globally. In particular, if we are unable to retain key personnel from the acquisition of NovaMed, particularly sales and marketing personnel with expertise in the products they promote and regulatory personnel, our business may suffer and could result in our not achieving the anticipated benefits of the acquisition.
We recently announced departures of key personnel from our NovaMed subsidiary, including the individual who was our Chief Executive Officer of SciClone’s China operations and former CEO of NovaMed who resigned in December 2012, and our Chief Operating Officer in China who left in October 2012. We also had recent departures in our senior sales personnel in China and may experience additional departures in our China operations. Our future success will depend in part on our recruiting senior sales personnel in China. We are currently recruiting executives to address departures and to expand and strengthen our China operations. In addition, we terminated personnel who were involved in the override of certain controls in the financial statement close process at our NovaMed subsidiary that was concluded to represent a material weakness.
Conversely, if we need to reduce the size of a particular aspect of our business including if we have contracts that are not renewed or renegotiated for products we market or promote, we are also dependent on our ability to make such adjustments while retaining suitably skilled personnel. We have taken corrective measures based upon the findings of our Special Committee relating to its investigation of matters relating to the FCPA and have, and expect to continue to take corrective measures relating to managements’ evaluation of internal control over financial reporting which could have adverse effects on our business, including the loss of personnel, and changes in marketing, sales and educational practices or programs.
If we were unable to attract and retain qualified personnel as needed or promptly replace those employees who are critical to our sales, development and other operations, and in particular senior executives, our financial results and operations would be adversely affected. At this time, we do not maintain “key person” life insurance for any of our personnel.
We may need to obtain additional funding to support our long-term product development and commercialization programs.
We believe our existing cash and investments and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. In November and May 2012, we announced that our Board of Directors has approved increases in our share repurchase program that authorizes the Company to repurchase up to a total of $40.5 million of its outstanding common stock. As of December 31, 2012, $12.4 million of the $40.5 million remained available under the repurchase program for future share repurchases. Further, we may use cash to acquire additional product rights or for future acquisitions. Our ability to achieve and sustain operating profitability is dependent on numerous factors including our ability to achieve our goal of increasing sales of ZADAXIN, securing regulatory approval for DC Bead in China, and for our other products and products we acquired as a result of the NovaMed acquisition, the execution and successful completion of clinical trials in China, securing partnerships for those programs that lead to regulatory approvals in major pharmaceutical markets, and successfully continuing NovaMed’s sales and integrating NovaMed into our business. We cannot assure you that such funds from operating activities will be sufficient, or that we will attain profitable operations in future periods. In addition, we intend to develop other products and we may need additional funds in the future to support such development and to support future growth and achieve profitability. If we need to raise additional funds in the future and such funds are not available on reasonable terms, if at all, our commercialization efforts may be impeded, our revenues may be limited and our operating results may suffer.
35
We are subject to the risk of increased income taxes which could reduce our future operating income.
We have structured our operations in a manner designed to maximize income in countries where:
| | • | | tax incentives have been extended to encourage foreign investment; or |
| | • | | income tax rates are low. |
Our taxes could increase if certain tax holidays or incentives are not renewed upon expiration, or if tax rates applicable to us in such jurisdictions are otherwise increased. For example, on March 16, 2007, the Chinese government passed a unified enterprise income tax law which became effective on January 1, 2008. Among other things, the law cancels many income tax incentives previously applicable to one of our subsidiaries in China. The law provides a transition rule which increased the tax rate of one of our subsidiaries in China over a 5 year period to 25% by 2012. The law also increased the standard withholding rate on earnings distributions to between 5% and 10% depending on the residence of the shareholder. The ultimate effect of these and other changes in Chinese tax laws on our overall tax rate will be affected by, among other things, our China income, the manner in which China interprets, implements and applies the new tax provisions, and by our ability to qualify for any exceptions or new incentives.
In addition, the Company and its subsidiaries are regularly subject to tax return audits and examinations by various taxing jurisdictions, particularly in the US and China. In determining the adequacy of our provision for income taxes, we regularly assess the likelihood of adverse outcomes resulting from tax examinations. While it is often difficult to predict the final outcome or the timing of the resolution of a tax examination, we believe that our reserves for uncertain tax positions reflect the outcome of tax positions that are more likely than not to occur. However, we cannot be certain that the final determination of any tax examinations will not be materially different than that which is reflected in our income tax provisions and accruals. Should additional taxes be assessed as a result of a current or future examination, there could be a material adverse effect on our tax provision, operating results, financial position and cash flows in the period or periods for which that determination is made.
If we fail to protect our products, technologies and trade secrets, we may not be able to successfully use, manufacture, market or sell our products, or we may fail to advance or maintain our competitive position, and we have limited intellectual property protection in China.
Our success depends significantly on our ability to obtain and maintain meaningful patent protection for our products and technologies and to preserve our trade secrets. Our pending patent applications may not result in the issuance of patents in the future. Our patents or patent applications may not have priority over others’ applications. Our existing patents and additional patents that may be issued, if any, may not provide a competitive advantage to us or may be invalidated or circumvented by our competitors. Others may independently develop similar products or design around patents issued or licensed to us. Patents issued to, or patent applications filed by, other companies could harm our ability to use, manufacture, market or sell our products or maintain our competitive position with respect to our products. Although many of our patents relating to thymalfasin have expired, including composition of matter patents, we have rights to other patents and patent applications relating to thymalfasin and thymalfasin analogues, including method of use patents with respect to the use of thymalfasin for certain indications. Additionally, thymosin alpha 1 (“thymalfasin”), the chemical composition of thymalfasin, has received Orphan Drug designation in the US for the treatment of stage 2b through stage 4 melanoma. If other parties develop generic forms of thymalfasin for other indications, including conducting clinical trials for such indications, our patents and other rights might not be sufficient to prohibit them from marketing and selling such generic forms of thymalfasin or their brands of thymalfasin. If other parties develop analogues or derivatives of thymalfasin, our patents and other rights might not be sufficient to prohibit them from marketing these analogues or derivatives.
Pharmaceutical products are either not patentable or have only recently become patentable in some of the countries in which we market or may market thymalfasin. We do not have composition patent claims directed to
36
the same form of thymalfasin currently marketed in China, our largest market, although we do have other type of patent claims, pending or issued, directed to other aspects of thymalfasin therapy. Other companies market generic thymalfasin in China, sometimes in violation of our patent, trademark or other rights which, to date, we have defended by informing physicians and hospitals of the practice. Past enforcement of intellectual property rights in many of these countries, including China in particular, has been limited or non-existent. Future enforcement of patents and proprietary rights in many other countries will likely be problematic or unpredictable. Moreover, the issuance of a patent in one country does not assure the issuance of a similar patent in another country. Claim interpretation and infringement laws vary by nation, so the extent of any patent protection is uncertain and may vary in different jurisdictions.
If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights.
Our commercial success depends in part on our not infringing valid, enforceable patents or proprietary rights of third parties, and not breaching any licenses that may relate to our technologies and products. In addition, we may not be aware of all patents or patent applications that may impact our ability to make, use or sell any of our potential products. For example, US patent applications may be kept confidential for 12 or more months while pending in the Patent and Trademark Office, and patent applications filed in foreign countries are often first published nine months or more after filing. It is possible that we may unintentionally infringe these patents or other patents or proprietary rights of third parties. We may in the future receive notices claiming infringement from third parties as well as invitations to take licenses under third-party patents. Any legal action against us or our collaborative partners claiming damages and seeking to enjoin commercial activities relating to our products and processes affected by third-party rights may require us or our collaborative partners to obtain licenses in order to continue to manufacture or market the affected products and processes. Our efforts to defend against any of these claims, regardless of merit, would require us to devote resources and attention that could have been directed to our operations and growth plans. In addition, these actions may subject us to potential liability for damages. We or our collaborative partners may not prevail in a patent action and any license required under a patent may not be made available on commercially acceptable terms, or at all. Any conflicts resulting from the patent rights of others could significantly reduce the coverage of our patents and limit our ability to obtain meaningful patent protection.
If other companies obtain patents with conflicting claims, we may be required to obtain licenses to those patents or develop or obtain alternative technology to manufacture or market the affected products and processes. We may not be able to obtain any such licenses on acceptable terms or at all. Any failure to obtain such licenses could delay or prevent us from pursuing the development or commercialization of our potential products. Our efforts to defend against any of these claims would require us to devote resources and attention that could have been directed to our operations and growth plans.
We may need to initiate litigation, which could be time-consuming and expensive, to enforce our proprietary rights or to determine the scope and validity of others’ rights. If litigation results, a court may find our patents or those of our licensors invalid or may find that we have infringed on a competitor’s rights. If any of our competitors have filed patent applications in the US which claim technology we also have invented, the Patent and Trademark Office may require us to participate in expensive interference proceedings to determine who has the right to a patent for the technology. These actions may subject us to potential liability for damages. We or our collaborative partners may not prevail in a patent action and any license required under a patent may not be made available on commercially acceptable terms, or at all.
Substantial sales of our stock or the exercise or conversion of options may impact the market price of our common stock.
In March 2012, we filed a Form S-3 Shelf registration with the SEC under which we may offer and sell up to $100.0 million of our securities, assuming we continue to meet the SEC’s eligibility requirements for primary offerings on Form S-3. Subsequently, affiliates of Sigma-Tau sold approximately 6.3 million shares for an aggregate price of
37
approximately $33.1 million under this registration statement, and we have approximately $66.9 million available for future use. In addition, we issued 8,298,110 shares of the Company’s common stock to NovaMed under the terms of the acquisition in April 2011, and former NovaMed stockholders own approximately 15% of our outstanding common stock after the transaction. We have granted registration rights for those shares and the shares are freely tradable. Sales of the shares could lead to a decrease in the market price of our common stock.
Future issuances of substantial amounts of our common stock could adversely affect the market price of our common stock. Similarly, if we raise additional funds through the issuance of common stock or securities convertible into or exercisable for common stock or sell equity in a subsidiary, the percentage ownership of our present stockholders of the respective entities will be reduced and the price of our common stock may fall.
Our cash and investments are subject to certain risks which could materially adversely affect our overall financial position.
We invest our cash in accordance with an established internal policy and customarily in instruments which historically have been highly liquid and carried relatively low risk. However, with turmoil in the credit markets, similar types of investments have experienced losses in value or liquidity issues which differ from their historical pattern. For example, we routinely have invested in money market funds with large financial institutions. One or more of these funds could experience losses or liquidity problems and, although to date some of the largest financial institutions who sponsor such funds have offset similar losses, there is no assurance that our financial institutions would either not incur losses or would offset any losses were they to occur.
Any adjustment to decrease the ratings of our investments by an Interest Rate Rating Agency may have a negative impact on the value of our investments.
Should any of our cash investments permanently lose value or have their liquidity impaired, it would have a material and adverse effect on our overall financial position by imperiling our ability to fund our operations and forcing us to seek additional financing sooner than we would otherwise and such financing may not be available on commercially attractive terms.
In addition, financial instruments may subject us to a concentration of credit risk. Most of our cash, and cash equivalents are held by a limited number of financial institutions. To date, we have not experienced any losses on our deposits of cash and cash equivalents. However, if any of these instruments permanently lost value or had their liquidity impaired, it would also have a material and adverse effect on our overall financial position by imperiling our ability to fund our operations and forcing us to seek additional financing sooner than we would otherwise and such financing may not be available on commercially attractive terms.
We expect that we may need to transfer capital to NovaMed from time to time to fund its operations. We need to obtain regulatory approval from China’s State Administration of Foreign Exchange (“SAFE”) in order to make such transfers and there can be no assurance that we will be able to obtain such approval in a timely manner. We have been able to fund the operations of NovaMed to date through a commercial loan, but we could face difficulties in the future if our efforts to improve profitability and cash flow in NovaMed are not successful, or if we are unable to obtain SAFE approval or obtain further loans.
Furthermore, a majority of our cash is held by our foreign subsidiaries. While such cash is used to fund the operating activities of our foreign subsidiaries and for further investment in foreign operations, and we do not anticipate the need to repatriate cash held by foreign subsidiaries under our current operating plan, if we were to repatriate cash to the US, these amounts may be subject to US income tax upon repatriation.
Our ability to utilize our tax attributes may be limited by an “ownership change.”
Our ability to use our tax attributes, such as our US federal income tax net operating loss carryforwards and our tax credit carryforwards, may be substantially restricted if we have had in the past, or have in the future, an
38
“ownership change” as defined in Section 382 of the US Internal Revenue Code. An ownership change occurs if increases in the percentage of our stock held by “5-percent shareholders” (within the meaning of Section 382, which provides that certain public groups can be treated as 5-percent shareholders) collectively exceed more than fifty percent, comparing the lowest percentage of stock owned by each 5-percent shareholder at any time during the testing period (which is generally a three-year rolling period) to the percentage of stock owned by the 5-percent shareholder immediately after the close of any owner shift testing date. Our issuance of Common Stock in the acquisition of NovaMed, our repurchases of our Common Stock and trading in our stock by stockholders, may have increased the possibility that in the future we could experience an ownership change. Trading by our stockholders, our stock repurchases or other transactions could, in the future, cause an ownership change, resulting in an annual limitation on utilization of our tax attributes. If our tax attribute usage is subject to limitation and if we are profitable, our future cash flows could be adversely affected due to an increased tax liability.
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us, which may be beneficial to our stockholders, more difficult.
Certain anti-takeover provisions of Delaware law and our charter documents as currently in effect may make a change in control of our company more difficult, even if a change in control would be beneficial to our stockholders. Our charter documents contain certain anti-takeover provisions, including provisions in our certificate of incorporation providing that stockholders may not cumulate votes, stockholders’ meetings may be called by stockholders only if they hold 25% or more of our common stock and provisions in our bylaws providing that the stockholders may not take action by written consent. Additionally, our Board of Directors has the authority to issue 10 million shares of preferred stock and to determine the terms of those shares of stock without any further action by the stockholders. The rights of holders of our common stock are subject to the rights of the holders of any preferred stock that may be issued. The issuance of preferred stock could make it more difficult for a third-party to acquire a majority of our outstanding voting stock. Delaware law also prohibits corporations from engaging in a business combination with any holders of 15% or more of their capital stock until the holder has held the stock for three years unless, among other possibilities, the board of directors approves the transaction. Our Board of Directors may use these provisions to prevent changes in the management and control of our company. Also, on December 18, 2006, our Board of Directors declared a dividend distribution of one Preferred Stock Purchase Right (collectively, the “Rights”) for each outstanding share of our Common Stock, each Right which entitles the registered holder to purchase from the Company one one-thousandth of a share of the Company’s Series D Preferred Stock, $0.001 par value, at a price of $25.00 pursuant to a Rights Agreement dated as of December 19, 2006, between the Company and Mellon Investor Services LLC. The Rights have certain anti-takeover effects. Under certain circumstances the Rights could cause substantial dilution to a person or group who attempts to acquire the Company on terms not approved by our Board of Directors. Although the Rights should not interfere with an acquisition of the Company approved by the board, the Rights may have the effect of delaying and perhaps improving the terms of an acquisition for our stockholders, or deterring an acquisition of the Company. Also, under applicable Delaware law, our Board of Directors may adopt additional anti-takeover measures in the future.
We may be subject to product liability lawsuits, and our insurance may be inadequate to cover damages.
Clinical trials of any of our current and potential products or the actual commercial sales of our product may expose us to liability claims from the use of these products. We currently carry product liability insurance. However, we cannot be certain that we will be able to maintain insurance on acceptable terms, if at all, for clinical and commercial activities, that any insurance we have will cover any particular claim that is asserted, or that the insurance would be sufficient to cover any potential product liability claim or recall. If we fail to have sufficient coverage, our business, results of operations and cash flows could be adversely affected.
39
If we are unable to comply with environmental and other laws and regulations, our business may be harmed.
We are subject to various federal, state and local laws, regulations and recommendations relating to the use, manufacture, storage, handling and disposal of hazardous materials and waste products (including radioactive compounds and infectious disease agents), as well as safe working conditions, laboratory and manufacturing practices and the experimental use of animals. The extent of government regulation that might result from future legislation or administrative action in these areas cannot be accurately predicted.
We do not currently maintain hazardous materials at our facilities. While we outsource our research and development programs involving the controlled use of biohazardous materials, if in the future we conduct these programs ourselves, we might be required to incur significant cost to comply with environmental laws and regulations. Further, in the event of an accident, we would be liable for any damages that result, and the liability could exceed our resources.
Our business and operations are subject to the risks of being based in particular locations known for earthquakes, other natural catastrophic disasters and service interruptions.
Our corporate headquarters are located in the Silicon Valley area of Northern California, a region known for seismic activity. Although we maintain a disaster recovery policy that includes storage of important corporate data in a different geographic region of the US, all of our significant corporate data is stored in our headquarters facility and accordingly, a significant natural disaster, such as an earthquake, could have a material adverse impact on our business, operating results, and financial condition. Most of our sales are into China for which we maintain our warehouses for finished goods in Hong Kong, which can experience severe typhoon storms, earthquakes or other natural catastrophic disasters. Although our distributors in China may maintain several months supply of our product, were our warehouse capability to be interrupted, either through a natural disaster such as flooding or through a service interruption, such as a lack of electricity to power required air conditioning, our ability to timely deliver finished product to China could be adversely affected which in turn would materially adversely affect our sales and ensuing operating results.
We May Be Affected by Climate Change and Market or Regulatory Responses to Climate Change.
Climate change, including the impact of global warming, could have a material adverse effect on our results of operations, financial condition, and liquidity if it were to disrupt the demand, supply or delivery of product, management of our business, or result in cost increases as a result of government regulation.
| Item 1B. | Unresolved Staff Comments |
None.
We currently lease approximately 22,000 square feet of office space for our corporate headquarters in Foster City, California, approximately 55,000 square feet of office space in China, primarily in Beijing and Shanghai, and lease approximately 2,000 square feet of combined office space in Hong Kong and Vietnam. Our office space in Foster City exceeds our current needs primarily as a result of the discontinuation of our US-based research and development efforts, including the SCV-07 clinical trial and related restructuring. This lease expires June 30, 2014. We believe that our other existing facilities will be adequate for our current needs and that additional space will be available as needed.
40
The SEC and the DOJ are each conducting formal investigations of us regarding a range of matters including the possibility of violations of the FCPA. We will continue to cooperate fully with the SEC and DOJ in the conduct of their investigations.
In response to these matters, our Board appointed a Special Committee of independent directors (the “Special Committee”) to oversee our response to the government inquiry. Based on an initial review, the Special Committee decided to undertake an independent investigation as to matters reflected in and arising from the SEC and DOJ investigations including, but not limited to, certain sales and marketing matters in China, in order to evaluate whether any violation of the FCPA or other laws occurred.
During the investigation, the Special Committee instructed management to (i) evaluate and to expand the Company’s training of employees regarding understanding and compliance with laws including the FCPA and other anti-bribery laws and regulations, (ii) evaluate existing compliance and anti-bribery policies and guidelines and to prepare new, more detailed policies and guidelines for implementation after review by our Board and/or committees of the Board, (iii) implement a pre-approval policy for certain expenses including payments for, or reimbursement of, travel and entertainment expenses, and sponsorships of certain third party events, and (iv) hire a Vice President of Compliance and Internal Audit to monitor and enforce compliance with our policies.
The Special Committee substantially concluded its investigation of those matters and on May 4 and 5, 2011 reported its findings and recommendations to the Board of Directors. The Special Committee has also reported those findings to the SEC and DOJ, and the Special Committee and the Company have continued to cooperate with the on-going SEC and DOJ investigations.
In the Company’s Form 10-Q for the period ended September 30, 2012, filed with the SEC on November 9, 2012, the Company disclosed, among other things, a non-cash impairment loss to fully write down the value of intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; a significant increase in ZADAXIN channel inventory levels; and internal control issues primarily within the NovaMed organization that was concluded to represent a material weakness in internal control over financial reporting. Following our disclosure of these items, the Company received a subpoena from the SEC requesting documents related to these and various other matters regarding the NovaMed acquisition and the Company’s operations in China. After review of the subpoena, and in order to respond to inquiries from the DOJ and SEC and to determine if any wrong-doing occurred, the Audit Committee determined to undertake an independent investigation as to additional matters, including but not limited to our acquisition of NovaMed and FCPA matters.
We are unable to predict what consequences that any investigation by any regulatory agency or by our Special Committee may have on us. Our cooperation with these investigations has resulted in substantial legal and accounting expenses, has diverted management’s attention from other business concerns and could harm our business. The ongoing investigations and any other regulatory investigations that might be initiated in the future, will result in similar substantial expenses, management diversion and harm to our business. If we fail to comply with regulations or to carry out controls in our Chinese or other foreign operations in a manner that satisfies all applicable laws, our business would be harmed. Any civil or criminal action commenced against us by a regulatory agency could result in administrative orders against us, the imposition of significant penalties and/or fines against us, and/or the imposition of civil or criminal sanctions against certain of our officers, directors and/or employees. The investigations, results of the investigations or remedial actions we have taken or may take, if any, as a result of such investigations, may adversely affect our business in China. If we are subject to adverse findings resulting from the SEC and DOJ investigations, or from our own independent investigation, we could be required to pay damages or penalties or have other remedies imposed upon us. In addition, we will incur additional expenses related to remedial measures we are undertaking, and could incur fines or other penalties. The period of time necessary to resolve the investigations by the DOJ and the SEC is uncertain, and these matters could require significant management, Board and financial resources which could otherwise be devoted to the operation of our business.
41
NovaMed is a party to a Distribution and Supply Agreement with MEDA. Following our acquisition of NovaMed, NovaMed continued to perform this agreement; however, MEDA claimed it had a right to terminate the agreement under a change of control provision. NovaMed does not believe that MEDA had a right of termination under the agreement. NovaMed and MEDA were in negotiations since the acquisition regarding potential amendments to the agreement that would resolve the disagreement. However no resolution was reached. MEDA notified NovaMed that the termination was effective as of May 2011, however as provided in the agreement, disputes, including disputes regarding termination must be resolved in binding arbitration. We do not expect any significant revenues from this agreement until 2014. NovaMed filed an application for binding arbitration with the China International Economic and Trade Arbitration Commission (“CIETAC”) on July 26, 2012. On October 10, 2012, MEDA filed a response and counterclaim with CIETAC claiming MEDA’s termination was valid and demanding the transfer of certain information and rights, as well as demanding the award of damages, including attorneys’ fees in an unspecified amount. NovaMed continues to perform its obligations under the agreement pending resolution of the dispute, and has notified MEDA that the dispute has been submitted to arbitration. The arbitration process is proceeding and we cannot predict the outcome of this matter at this time.
On October 16, 2012, we made a claim against the former stockholders of NovaMed pursuant to the acquisition agreement relating to our acquisition of NovaMed. As a result of our claim, approximately $1.4 million in cash held in escrow and 622,363 shares of our common stock held in escrow were not released to the former NovaMed stockholders pending the outcome of our claim. The claim relates to damages we incurred as a result of various matters, including in particular the understatement of product return reserves for Aggrastat product in the balance sheet of NovaMed at the date of the acquisition, and related expenses and damages. On October 29, 2012, the shareholder representative of the former NovaMed stockholders sent us a response notice disputing the validity of our claims. If the matter is not resolved in negotiation between the parties it will be submitted to binding arbitration. We cannot predict the outcome of this matter at this time.
On March 11, 2013, Adam Crum filed a derivative lawsuit, purportedly in the name of SciClone, against Friedhelm Blobel, Gary Titus, Jon Saxe, Peter Barrett, Richard Hawkins, Gregg Lapointe and Ira Lawrence in California Superior Court, San Mateo County, captionedCrum v. Blobel, et al, Case No. CIV520331. The lawsuit alleges, based on the restatement resulting from the timing of revenue recognition for certain products sold by the Company’s subsidiary NovaMed, that the board and management breached their fiduciary duties to the Company by not exercising oversight in such a way that they allowed the Company to file financial statements that were materially inaccurate. Plaintiff asserts claims for breach of fiduciary duty, abuse of control and mismanagement. Plaintiff seeks, among other things, injunctive relief, disgorgement, undisclosed damages and attorneys’ fees and costs. We believe the claims lack merit and will vigorously defend against them.
| Item 4. | Mine Safety Disclosures |
Not applicable.
42
PART II
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock trades on The NASDAQ Global Market of the NASDAQ Stock Market under the symbol “SCLN.”
The following table sets forth the high and low sales prices per share for the quarterly periods indicated, as reported by The NASDAQ Stock Market. The quotations shown represent inter-dealer prices without adjustment for retail markups, markdowns, or commissions, and may not necessarily reflect actual transactions.
| | | | | | | | |
| | | Price Range
Common Stock | |
| | | High | | | Low | |
2012 | | | | | | | | |
4th quarter | | $ | 6.29 | | | $ | 3.68 | |
3rd quarter | | | 7.58 | | | | 4.38 | |
2nd quarter | | | 7.45 | | | | 5.35 | |
1st quarter | | | 6.97 | | | | 4.15 | |
2011 | | | | | | | | |
4th quarter | | $ | 4.86 | | | $ | 3.41 | |
3rd quarter | | | 6.88 | | | | 3.70 | |
2nd quarter | | | 6.40 | | | | 3.93 | |
1st quarter | | | 4.49 | | | | 3.30 | |
Stockholders
As of March 26, 2013, there were approximately 280 holders of record of our common stock and 54,008,979 shares of common stock issued and outstanding.
Dividends
We have not paid any dividends on our common stock during the fiscal years ended December 31, 2012, 2011, and 2010 and do not currently have plans to pay any cash dividends.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by Item 201(d) of Regulation S-K is incorporated by reference from the section entitled “Securities Authorized for Issuance under Equity Compensation Plans” in Part III, Item 12 of this Form 10-K.
43
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Common stock repurchases in the fourth quarter of fiscal 2012 were as follows(in thousands, except average price paid per share):
| | | | | | | | | | | | | | | | |
| | | Total
Number
of Shares
Purchased | | | Average
Price
Paid
per Share | | | Total Number
of Shares
Purchased as Part
of Publicly
Announced
Plans
or Programs | | | Approximate
Dollar
Value of Shares
that May Yet Be
Purchased
Under the
Plans or Programs(1) | |
October 1, 2012 through October 31, 2012 | | | 422 | | | $ | 5.89 | | | | 422 | | | $ | 16,191 | |
November 1, 2012 through November 30, 2012 | | | 568 | | | | 4.43 | | | | 568 | | | | 13,671 | |
December 1, 2012 through December 31, 2012 | | | 300 | | | | 4.37 | | | | 300 | | | | 12,358 | |
| | | | | | | | | | | | | | | | |
Total Fiscal 2012 Fourth Quarter | | | 1,290 | | | | 4.90 | | | | 1,290 | | | | | |
| | | | | | | | | | | | | | | | |
| (1) | “Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs” reflects increases of $10.5 million and $10.0 million announced May 2012 and November 2012, respectively, bringing the total authorized since the program’s inception in October 2011 to $40.5 million, less the $28.1 million we repurchased through December 31, 2012. |
Performance Graph
The following line graph compares the annual percentage change in (i) the cumulative total stockholder return on the Company’s Common Stock since December 31, 2007, with (ii) the cumulative total return on (a) The NASDAQ Composite Index and (b) the NASDAQ Biotechnology Index. The comparison assumes (i) an investment of $100 on December 31, 2007 in each of the foregoing indices and (ii) reinvestment of dividends, if any. The stock price performance shown on the graph below is not necessarily indicative of future stock price performance.
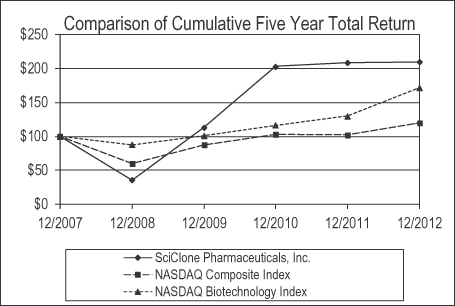
44
| Item 6. | Selected Financial Data |
This section presents selected historical financial data for each of the last five fiscal years and is qualified by reference to and should be read in conjunction with the consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K. The selected balance sheet data at December 31, 2012 and 2011 and the selected statement of operations data for each year ended December 31, 2012, 2011, and 2010 have been derived from our audited financial statements that are included elsewhere in this report. The selected balance sheet data at December 31, 2010, 2009, and 2008 and the selected statement of operations data for each year ended December 31, 2009 and 2008 have been derived from our audited financial statements not included in this report. Historical results are not necessarily indicative of the results to be expected in the future.
The 2011 information presented in the following table has been restated as a result of the accounting errors originating with our subsidiary in China, NovaMed, as is more fully described in the “Explanatory Note” immediately preceding Part I, Item 1 and in Note 2, “Revision of Previously Issued Consolidated Financial Statements,” to our Consolidated and Combined Financial Statements in Part II, Item 8.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | (in thousands, except per share data) | |
| | | 2012(1) | | | 2011(2)
Restated | | | 2010 | | | 2009 | | | 2008 | |
Statement of Operations data: | | | | | | | | | | | | | | | | | | | | |
Total net revenues | | $ | 156,269 | | | $ | 132,565 | | | $ | 85,112 | | | $ | 72,411 | | | $ | 54,113 | |
Net income (loss) | | $ | 9,620 | | | $ | 28,122 | | | $ | 21,081 | | | $ | 11,945 | | | $ | (8,348 | ) |
Basic net income (loss) per share | | $ | 0.17 | | | $ | 0.51 | | | $ | 0.44 | | | $ | 0.26 | | | $ | (0.18 | ) |
Diluted net income (loss) per share | | $ | 0.16 | | | $ | 0.49 | | | $ | 0.43 | | | $ | 0.25 | | | $ | (0.18 | ) |
Shares used in computing: | | | | | | | | | | | | | | | | | | | | |
Basic net income (loss) per share | | | 56,637 | | | | 55,110 | | | | 47,624 | | | | 46,574 | | | | 46,212 | |
Diluted net income (loss) per share | | | 58,483 | | | | 57,387 | | | | 49,414 | | | | 47,135 | | | | 46,212 | |
| | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2012(1) | | | 2011(2)
Restated | | | 2010 | | | 2009 | | | 2008 | |
| | | (in thousands) | |
Balance Sheet data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents, and short-term investments | | $ | 84,228 | | | $ | 66,654 | | | $ | 56,142 | | | $ | 31,333 | | | $ | 27,773 | |
Restricted cash and investments | | | 2,759 | | | | 364 | | | | — | | | | — | | | | — | |
Accounts receivable, net of allowance | | | 38,109 | | | | 38,465 | | | | 30,671 | | | | 21,394 | | | | 11,927 | |
Inventories | | | 10,424 | | | | 11,141 | | | | 7,078 | | | | 10,149 | | | | 6,056 | |
Deferred tax assets | | | 369 | | | | 1,788 | | | | — | | | | — | | | | — | |
Total current assets | | | 137,329 | | | | 119,694 | | | | 95,948 | | | | 64,465 | | | | 48,016 | |
Intangible assets, net | | | — | | | | 45,185 | | | | — | | | | — | | | | — | |
Goodwill | | | 34,313 | | | | 33,868 | | | | — | | | | — | | | | — | |
Total assets | | | 174,071 | | | | 200,844 | | | | 97,807 | | | | 66,900 | | | | 51,905 | |
Borrowings | | | 1,445 | | | | 2,500 | | | | 2,500 | | | | — | | | | — | |
Contingent consideration | | | — | | | | 15,400 | | | | — | | | | — | | | | — | |
Deferred tax liabilities | | | 153 | | | | 8,407 | | | | — | | | | — | | | | — | |
Other long-term liabilities | | | 237 | | | | 469 | | | | 990 | | | | 979 | | | | 779 | |
Total stockholders’ equity | | | 143,022 | | | | 150,119 | | | | 82,188 | | | | 57,393 | | | | 40,903 | |
| (1) | During fiscal 2012, we identified an impairment indicator with respect to our intangible assets related to our promotion and distribution contract rights and recorded losses of approximately $42.7 million to recognize the full impairment. We recorded a benefit for income tax of approximately $6.8 million for the year ended December 31, 2012 due to the impairment of intangible assets, which resulted in a reversal of deferred tax liabilities. This was partially offset by the impact of recognizing a full valuation allowance on any |
45
| | remaining NovaMed deferred tax assets. In addition, we recorded a non-cash gain of $15.4 million related to the contingent consideration because revenue and EBITDA targets of NovaMed were not achieved. |
| (2) | On April 18, 2011, we acquired NovaMed Pharmaceuticals, Inc. (“NovaMed”) for approximately $24.6 million in cash and 8,298,110 shares of SciClone common stock and a contingent right to receive additional cash consideration (“the contingent consideration”) of up to $43.0 million based upon achievement of revenue and earnings targets for the 2011 and 2012 fiscal years. Commencing April 18, 2011, the Company’s financial statements include the assets, liabilities, and operating results of NovaMed. |
46
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the “Selected Financial Data” and our consolidated financial statements and related notes thereto included elsewhere in this Annual Report on Form 10-K. This Management’s Discussion and Analysis of Financial Condition and Results of Operations and other parts of this Annual Report on Form 10-K contain forward-looking statements which involve risks and uncertainties. See “Note Regarding Forward-Looking Statements” and “Risk Factors” contained in this Annual Report on Form 10-K.
Overview
SciClone Pharmaceuticals (NASDAQ: SCLN) is a revenue-generating, profitable, United States (“US”)-based, China-focused, specialty pharmaceutical company with a substantial commercial business and a product portfolio of therapies for oncology, infectious diseases, cardiovascular, urological, respiratory and central nervous system disorders. We are focused on continuing to grow our revenue and profitability through our strong sales and marketing efforts. Our business and corporate strategy is focused primarily on the People’s Republic of China (“China”) where we have built a solid reputation and established a strong brand through many years of experience marketing our lead product, ZADAXIN® (thymalfasin). In addition, we have an established product promotion business model with large pharmaceutical partners and we are focused on establishing profitability in all of these collaborations. We believe our sales and marketing strengths position us to benefit from the long-term expansion of the pharmaceutical market in China. This pharmaceutical market currently ranks third among the global pharmaceutical markets, and we believe China will rank second among global pharmaceutical markets by 2020. We seek to expand our presence in China and increase revenues by growing sales and profitability of our current product portfolio, launching new products from our development pipeline, adding new, profitable product services agreements and leveraging our strong cash position to in-license additional products.
We operate in two segments which are generally based on the nature and location of our customers: 1) China and 2) the rest of the world which includes our US and Hong Kong operations.
We have two categories of revenues: “product sales revenues” and “promotion services revenues.” Our product sales revenues result from our proprietary and in-licensed products, including our lead product, ZADAXIN, and products from Pfizer Inc. (“Pfizer”) and Iroko Pharmaceuticals LLC. ZADAXIN has the highest margins in our portfolio as it is a premium proprietary product sold exclusively by SciClone. Aggrastat®, an in-licensed product, has higher margins than the products we promote under services agreements and we anticipate revenues from this product will grow significantly as it further penetrates the China market. In addition, we anticipate that new marketed products, when and if introduced, can increase the future revenues and profitability of our pharmaceutical business in China over the coming years. Our “promotion services revenues” result from fees we receive for exclusively promoting products under services agreements with certain pharmaceutical partners, including Sanofi Aventis S.A. (“Sanofi”) and Baxter International, Inc. (“Baxter”) in China. We refer to these agreements as promotion agreements, service agreements and distribution contract rights agreements. We recognize promotion services revenues as a percentage of our collaborators’ product sales revenue for these exclusively promoted products, such as Depakine®, Stilnox® and Tritace®. Over time, as additional proprietary or in-licensed products come to the market, we aim to shift our product mix towards those higher margin products. In January 2013, our promotion agreement with Sanofi was renewed until December 31, 2013 under the same terms as our previous agreement. As we seek renewals of our product services agreements, our strategy is to establish more favorable terms with the goal of achieving profitability under these agreements through improved margins as well as through managing expenses relating to our product services revenue.
ZADAXIN is approved in over 30 countries and may be used for the treatment of HBV, HCV, and certain cancers, and as a vaccine adjuvant according to the local regulatory approvals we have in these countries. In China, thymalfasin is included in the treatment guidelines issued by the Ministry of Health (“MOH”) for liver cancer, as well as guidelines for treatment of chronic HBV (issued by both the Chinese Medical Association and the Asian-Pacific Association for the Study of the Liver) and invasive fungal infections of critically ill patients
47
(issued by the Chinese Medical Association). Our sales force is focused on increasing sales to the country’s largest hospitals (class 3 with over 500 beds) as well as mid-size hospitals (class 2). These hospitals serve Tier 1 and Tier 2 cities located mostly in the eastern part of China which are the largest and generally have the most affluent populations. We are widening our market strategies by targeting numerous smaller hospitals as well as hospitals that are in more rural areas. We are also seeking to expand the indications for which ZADAXIN could be used, including sepsis.
Our market portfolio also includes the anti-epileptic drug Depakine, the hypnotic Stilnox (marketed as Ambien® in the US), the ACE inhibitor Tritace, and Aggrastat, an intervention cardiology product launched in China in 2009.
We are also pursuing the registration of several other therapeutic products in China. These include: DC Bead®, an embolic acting bead with drug loading capabilities that can be used for targeted delivery of cancer chemotherapy drugs directly to the tumor; Loramyc®, a mucoadhesive tablet formulation of miconazole lauriad to treat oropharyngeal candidiasis; Rapinyl®, a sublingual tablet formulation of fentanyl to treat breakthrough cancer pain; and RapidFilm®, an oral film formulation of ondansetron to treat nausea induced by chemotherapy.
We continue to seek to establish profitable in-licensing arrangements for approved or late-stage branded, well-differentiated products which if not yet approved, have a clear regulatory approval pathway in China based on existing regulatory approval outside of China. Our objective is to in-license products with higher margins that can augment our product sales revenue, and we continue to explore opportunities to optimize our promotion services revenues. We are also working on the final stage of the regulatory approval in China for our in-licensed candidate DC Bead, and on the approval process for our other product candidates, all of which are in clinical trials or in other stages of the regulatory approval process in China.
We acquired NovaMed Pharmaceuticals, Inc. (“NovaMed”) on April 18, 2011 and our results of operations include the operations of NovaMed as of that date forward. Since the acquisition of NovaMed, we have put strategies in place designed to strengthen our sales and marketing infrastructure in China, with the goal of meeting the growing demand for pharmaceuticals in the China market. However, we believe that several developments in 2012 had, or may yet have, material impacts on the growth rate of our business in China and affected the Company’s financial results in the second half of 2012. We are responding to these developments by taking actions to strengthen our business and improve our financial performance in subsequent quarters. These developments include, but are not limited to: a reduction in the retail price for ZADAXIN and other products that occurred later in the year than expected; an increase in the channel inventory levels of ZADAXIN during the third quarter and particularly in September of 2012; matters relating to our NovaMed acquisition, including a non-cash impairment loss to fully write down the value of the licensing and product services agreement intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; issues in internal control over financial reporting primarily within the NovaMed subsidiary; and changes in management.
Price Reduction for ZADAXIN and Other Products
ZADAXIN’s national reimbursement retail list price in China was recently reviewed by regulatory authorities consistent with the China government’s review of pharmaceutical prices once a product has been included into the Reimbursement Drug List (“RDL”). As a result of this review, the retail list price (or the price at the hospital pharmacy level) of ZADAXIN was reduced by approximately 18%. The reduction was announced in September 2012 and became effective October 8, 2012. Based on an agreement with our primary importer of ZADAXIN into China, this importer will take a larger share of the price reduction impact in exchange for certain exclusive importation rights into China. As a result, the actual impact on SciClone’s revenue and margins is expected to be less than a 5% decrease in our sales price of ZADAXIN to this importer. In addition, the National Development and Reform Commission’s (“NDRC”) retail list prices (or the price at the hospital pharmacy level), for Aggrastat, as well as for several of our oncology products exclusively promoted in China for Pfizer and Baxter, were reduced ranging from 10 to 20%.
48
ZADAXIN Inventory and Sales
China uses a tiered system to import and distribute products. The distributors make the sales in the country, but the product is imported for them by our exclusive ZADAXIN importer. Our product sales revenues result from the sale of our products to our customers, the importing agent. It takes approximately seven weeks for our customers to clear a shipment of ZADAXIN through the importation process for sale in China. Our China importation customer tends to purchase infrequent large orders of ZADAXIN inventory to facilitate the distribution and sale to Chinese hospital pharmacies. The timing of infrequent large orders may significantly affect the ZADAXIN channel inventory levels and may cause fluctuations to our reported sales and profitability for each quarterly period.
During the third quarter and particularly in September 2012, we estimate that there was an increase of approximately $14 million in ZADAXIN channel inventory levels and we believe that our sales to our customers exceeded the pace at which our customers were able to sell ZADAXIN through to other parties, primarily hospital pharmacies. ZADAXIN channel inventory levels decreased by approximately $5 million in the fourth quarter of 2012, and we expect them to continue to decrease over the next two quarters. As a result, we expect that ZADAXIN revenues will be lower in the first half of 2013, compared to the first half of 2012. We expect our 2013 sales revenue to be lower than or comparable to our 2012 revenues of $156 million. We continue to believe that we will grow demand for ZADAXIN through increased penetration in the market.
In addition, we believe that our sales organization’s efforts relative to ZADAXIN may have been affected by changes to and turnover in management in China, particularly the departures of senior executives. Turnover among sales personnel in the China market generally, including among our own sales staff, is expected to continue, and we may experience additional departures in our China operations. During this transition period, if we are unable to achieve objectives for increased demand, we may experience declines in our quarterly sales revenue in the near term and our sales and profitability for the next few quarters may significantly decrease.
We have taken definitive steps to address the issues affecting product sales. We have revised and we are implementing changes in our strategy designed to increase market penetration and growth, particularly for ZADAXIN. We are widening our market strategies by targeting numerous smaller hospitals as well as hospitals that are in more rural areas with the intent to positively affect sales growth over the next several quarters. In addition, we have already been successful in making significant changes in our China organization with the recruitment of additional senior level management and the recent appointment of a Chief Executive Officer for our China operations, and we are aggressively recruiting additional management for our China operations. Our recent and continuing executive recruiting in China is intended to bring in additional pharmaceutical company experience in the China market to grow ZADAXIN sales, to increase profitability, to enhance our financial capabilities and internal controls, and to improve and implement our sales strategies.
NovaMed: Intangible Asset Impairment; Contingent Consideration Remeasurement
We acquired NovaMed on April 18, 2011, and our results of operations include the operations of NovaMed as of that date forward. The acquisition increased our portfolio of commercial and development-stage products through exclusive licensing and product service agreements with a number of leading pharmaceutical companies. Although revenues from the NovaMed business have grown since the acquisition, overall revenue growth and profitability have not met the expectations we forecast at the time of the acquisition. This condition was considered to be an impairment indicator with respect to the intangible assets related to our promotion and distribution contract rights. During the third quarter ended September 30, 2012, we determined that the undiscounted cash flows estimated to be generated by the intangible assets were less than the carrying amounts. We further performed a discounted cash flow analysis related to the intangible assets and determined that a full impairment should be recorded. As a result, we recognized a non-cash impairment loss of approximately $42.7 million for the third quarter and full year ended December 31, 2012 on our consolidated statements of operations.
In January 2013, our promotion agreement with Sanofi was renewed until December 31, 2013 under the same terms as the prior agreement. Certain of our other product services agreements with third parties will be
49
expiring soon unless renewed, extended or renegotiated. We are actively negotiating renewals or extensions of these agreements. We are also assessing the terms and conditions of our agreements with the intent to secure more favorable terms to us relative to profitability. If any of these agreements are determined to no longer be beneficial to us and are allowed to expire, or if third parties will not renegotiate, renew or extend the agreements on terms acceptable to us, our revenues and profitability would be affected.
The terms of our acquisition of NovaMed provided for the payment of an additional $43.0 million in earn-out payments upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years (the “earn-out” or “contingent consideration”). We initially recorded $18.9 million as the estimated fair value of the contingent consideration. The fair value of the contingent consideration was remeasured each quarter and changes to the fair value were recorded to contingent consideration expense or gain. As of December 31, 2012, we determined the fair value of the contingent consideration was $0, resulting in a non-cash gain of $15.4 million for the year ended December 31, 2012. The significant reduction in the valuation of the contingent consideration expense during the year was related to revenue and EBITDA (earnings before interest, depreciation and taxes) targets not being achieved and to the reduced probability of renewal relating to NovaMed’s product distribution agreements, including, in particular, the target of renewing the Depakine services agreement with Sanofi for a five-year term. The Depakine services agreement was extended through December 31, 2013, a term less than five years.
Restatement of Financial Results
We have revised our consolidated financial statements for the year ended December 31, 2011 and certain quarterly periods in 2011 and 2012. Accordingly, the revised financial information included in this 2012 Form 10-K has been identified as “restated.” Concurrent with the filing of this Form 10-K, we are also filing amended quarterly reports on Form 10-Q/A for each of the first, second and third quarters of 2012 to restate our consolidated financial statements therein, and the effects of such restatements are reflected in the items revised herein.
The restatements relate to accounting errors originating with our subsidiary in China, NovaMed, which was acquired on April 18, 2011. The accounting errors relate primarily to the following:
| | • | | The timing of revenue recognition for certain Pfizer products sold by one of our distributors of our subsidiary NovaMed. Our policy is that all customers’ obligations to pay for product are final once product is delivered. However, we have determined that there were various factors, including the override of certain controls, indicating that sales under NovaMed’s distribution arrangement for Pfizer products with such distributor from the date of acquisition of NovaMed through the third quarter of 2012 (the “Relevant Periods”) allowed for contingent payment terms dependent upon when that distributor sold the products. As a result of our review and evaluation of the matter, we believe that instead of recording revenue at the time of sale to that distributor (“sell-in” method), as previously reflected in the financial statements for the Relevant Periods, revenue under the arrangements in effect at our subsidiary NovaMed should have been recognized when we received payment for the products (“sell-through” or “cash receipts” method). Effective as of the fourth quarter of 2012, we entered into a new agreement with that distributor which clarified the “sell-in” method of revenue recognition to provide consistency with our policy regarding revenue recognition on a prospective basis. As of December 31, 2012, we had received payment for all revenues recognized under the “sell-through” method. |
| | • | | The recognition of previously unrecognized product return reserves for sales of Aggrastat sold by our subsidiary NovaMed prior to the date of acquisition. We have concluded a liability for expired product existed at the time of the NovaMed acquisition, related to pre-acquisition sales. |
The revisions had the impact of increasing our revenues by $3.0 million for the nine-months ended September 30, 2012, and increasing our net income by $2.0 million for the same period. Basic and diluted
50
earnings per share increased by $0.04 and $0.03, respectively, for the nine-months ended September 30, 2012. As of September 30, 2012, accounts receivable decreased by $1.5 million, inventory increased by $1.3 million and goodwill related to the acquisition of NovaMed increased by $1.9 million. The revisions for the year ended December 31, 2011 had an impact of decreasing our revenues by $1.1 million and decreasing our net income by $0.3 million. Basic and diluted earnings per share decreased by $0.01. As of December 31, 2011, accounts receivable decreased by $3.8 million, inventory increased by $2.3 million and goodwill related to the acquisition of NovaMed increased by $1.9 million.
Internal Control Issues — Material Weakness
During the quarter ended September 30, 2012, we identified deficiencies in the design and operating effectiveness of controls primarily associated with product return reserves related to our Aggrastat product line, and the override of certain controls in the financial statement close process related to our NovaMed subsidiary. Certain out of period adjustments and these deficiencies were identified through the performance of controls and processes recently implemented at our NovaMed subsidiary as part of our assessment of internal control over financial reporting in 2012. We concluded that the aggregation of these deficiencies is a material weakness. Furthermore, during the fourth quarter of 2012, we identified deficiencies related to the timing of revenue recognition for our Pfizer products, the override of related controls at our NovaMed subsidiary, and the corporate monitoring thereof. We concluded that these deficiencies identified in the fourth quarter of 2012 are an additional indicator of the material weakness that was identified in the third quarter of 2012.
With the oversight of management and our Audit Committee, we have initiated steps to address the material weakness. In the fourth quarter of 2012, we implemented additional controls related to our revenue recognition and product return reserve processes to strengthen our controls and provide that all available information is properly considered. We also re-evaluated the operation of our controls at our NovaMed subsidiary and made adjustments to strengthen these controls, including the recent hiring of our Chief Financial Officer, China Operations who joined the Company in the third quarter of 2012. We terminated personnel who were involved in the override of certain controls in the financial statement close process at our NovaMed subsidiary that resulted in the material weakness. While we have completed certain remediation efforts, our remediation activities are not complete as of December 31, 2012.
China Management Turnover
We announced departures of key personnel from our China organization. These include the individual who was our Chief Executive Officer of SciClone’s China operations and former Chief Executive Officer of NovaMed who resigned in December 2012, and our Chief Operating Officer in China who left the Company in October 2012. There may be additional departures within our China operations. We have and are continuing to recruit executives to address the departures and to expand and strengthen our China operations, including the hiring of both a new Chief Executive Officer, China Operations who will begin April 1, 2013, and a Chief Financial Officer, China Operations who began August 2012.
Other Matters
We were developing SCV-07 in a phase 2b clinical trial for the prevention of oral mucositis (“OM”). On March 2, 2012, we announced the discontinuation of this trial based on the pre-planned interim analysis results that indicated the trial would not meet the pre-specified efficacy endpoints, and our intention to further curtail our US-based development efforts. In March 2012, we implemented a reduction in our workforce of 11 full-time employees, primarily in research and development, and recorded severance-related charges of approximately $1.1 million, of which approximately $0.1 million and $1.0 million were recognized to general and administrative and research and development expense, respectively, for the year ended December 31, 2012. We have substantially completed the restructuring.
51
The US Securities and Exchange Commission (“SEC”) and the US Department of Justice (“DOJ”) are each conducting formal investigations of SciClone regarding a range of matters, including the possibility of violations of the Foreign Corrupt Practices Act (“FCPA”). We will continue to cooperate fully with the SEC and DOJ in the conduct of their investigations. In response to these matters, our Board appointed a Special Committee of independent directors (the “Special Committee”) to oversee our response to the government inquiry. The Special Committee substantially concluded its original investigation, and on May 4 and 5, 2011 reported its findings and recommendations to the Board of Directors. The Special Committee has also reported findings to the SEC and DOJ.
In our Form 10-Q for the period ended September 30, 2012, filed with the SEC on November 9, 2012, we disclosed, among other things, a non-cash impairment loss to fully write down the value of intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; a significant increase in ZADAXIN channel inventory levels; and internal control issues primarily within the NovaMed organization that was concluded to represent a material weakness in internal control over financial reporting. Following our disclosure of these items, we received a subpoena from the SEC requesting documents related to these and various other matters regarding the NovaMed acquisition and our operations in China. After review of the subpoena, and in order to respond to inquiries from the DOJ and SEC and to determine if any wrong-doing occurred, our Audit Committee determined to undertake an additional independent investigation as to additional matters including, but not limited to, matters related to our acquisition of NovaMed and FCPA matters.
We are unable to predict what consequences any investigation by any regulatory agency or by our Special Committee may have on us. Our cooperation with these investigations has resulted in substantial legal and accounting expenses, has diverted management’s attention from other business concerns and could harm our business. The ongoing investigations and any other regulatory investigations that might be initiated in the future will result in similar substantial expenses, management diversion and harm to our business. If we fail to comply with regulations or to carry out controls on our Chinese or other foreign operations in a manner that satisfies all applicable laws, our business would be harmed. Any civil or criminal action commenced against us by a regulatory agency could result in administrative orders against us, the imposition of significant penalties and/or fines against us and/or the imposition of civil or criminal sanctions against certain of our officers, directors and/or employees. The investigations, results of the investigations, or remedial actions we have taken or may take as a result of such investigations may adversely affect our business in China. If we are subject to adverse findings resulting from the SEC and DOJ investigations, or from our own independent investigation, we could be required to pay damages or penalties or have other remedies imposed upon us. In addition, we will incur additional expenses related to remedial measures we are undertaking, and could incur fines or other penalties. The period of time necessary to resolve the investigations by the DOJ and the SEC is uncertain, and these matters could require significant management and financial resources which could otherwise be devoted to the operation of our business. We cannot predict what the outcome of those investigations will be, or the timing of any resolution.
On February 22, 2013, we announced that our reported financial results for each of the second and third quarters of 2011, the year ended December 31, 2011, and the first three quarters of fiscal 2012 could not be relied upon and that we would restate them. Subsequently, we received a purported derivative litigation naming certain of our officers and directors as defendants. We believe the claims lack merit and will vigorously defend against them.
Refer to Part II, Item 8 “Notes to Consolidated Financial Statements” Note 18 “Other Corporate Matters” and Part I, Item 3 “Legal Proceedings” in this Form 10-K for further information regarding the investigation and remedial measures, and related litigation.
We believe our cash and investments as of December 31, 2012 and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. Our results may fluctuate from quarter to quarter and we may report losses in the future.
52
Results of Operations
Revenues:
The following table summarizes the year over year changes in our product sales and promotion services revenues(in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2012 | | | Change | | | 2011
Restated | | | Change | | | 2010 | |
Product Sales, net | | $ | 123,171 | | | | 10 | % | | $ | 111,951 | | | | 32 | % | | $ | 85,112 | |
Promotion Services | | | 33,098 | | | | 61 | % | | | 20,614 | | | | 100 | % | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total Net Revenues | | $ | 156,269 | | | | 18 | % | | $ | 132,565 | | | | 56 | % | | $ | 85,112 | |
| | | | | | | | | | | | | | | | | | | | |
Product sales were $123.2 million, $112.0 million and $85.1 million for the years ended December 31, 2012, 2011, and 2010, respectively. The increases of $11.2 million, or 10%, for the year ended December 31, 2012 compared to 2011, and $26.8 million, or 32%, for the year ended December 31, 2011, compared to 2010 were primarily attributable to increased sales of ZADAXIN to our customers and to a lesser extent related to oncology and Aggrastat products. The increase in the 2011 period was also related to the addition of NovaMed product sales as a result of the acquisition of NovaMed in April 2011. ZADAXIN sales were $112.2 million for the year ended December 31, 2012, compared to $104.8 million and $85.1 million for the years ended December 2011 and 2010, respectively.
Promotion services revenue relates to the distribution of products under promotion contracts as a result of the acquisition of NovaMed in 2011. Promotion services revenue was $33.1 million, for the year ended December 31, 2012, compared to $20.6 million for the corresponding period in 2011. The increase of $12.5 million or 61% reflects the addition of promotion services revenue as a result of the acquisition in April 2011 and also reflects an increase in Depakine product sales in the year ended December 31, 2012.
Total revenues attributable to China were $152.2 million, $128.9 million and $82.0 million, or 97%, 97%, and 96% of sales for the years ended December 31, 2012, 2011, and 2010, respectively.
For the year ended December 31, 2012, sales to three customers in China accounted for approximately 53%, 20% and 12% of our revenues. For the year ended December 31, 2011, sales to three customers in China accounted for approximately 62%, 15% and 15% of our revenues. For the year ended December 31, 2010, sales to two customers in China accounted for approximately 74% and 14% of our revenues. Our experience with our customers has been good and we anticipate that we will continue to sell a majority of our product to them.
China uses a tiered method to import and distribute products. The distributors make the sales in the country, but the product is imported for them by our exclusive importer. Our product sales revenues result from the sale of the Company’s proprietary or in-licensed products to importing agents. Our promotion services revenues result from fees received for exclusively promoting products for certain partners. These importing agents, distributors and partners are the Company’s customers. It takes approximately seven weeks for our China importation customer to clear a shipment of ZADAXIN through the importation process for sale in China. Our customers tend to purchase infrequent large orders of ZADAXIN inventory to facilitate the distribution and sale to Chinese hospital pharmacies. The timing of infrequent large orders may significantly affect the ZADAXIN channel inventory levels and may cause fluctuations to our reported sales and profitability for each quarterly period.
During the third quarter and particularly in September 2012, we estimate that there was an increase of approximately $14 million in ZADAXIN channel inventory levels and we believe that our sales to our customers exceeded the pace at which our customers were able to sell ZADAXIN through to other parties, primarily hospital pharmacies. ZADAXIN channel inventory levels decreased by approximately $5 million in the fourth quarter of 2012, and we expect them to continue to decrease over the next two quarters. As a result, we expect
53
that ZADAXIN revenues will be lower in the first half of 2013, compared to the first half of 2012. We expect our 2013 sales revenue to be lower than or comparable to our 2012 revenues of $156 million. We continue to believe that we will grow demand for ZADAXIN through increased penetration in the market.
In January 2013, our promotion agreement with Sanofi was renewed until December 31, 2013 under the same terms. Certain of our other product services agreements with third parties will be expiring over the next several months unless renewed, extended or renegotiated. We are actively negotiating renewals or extensions of these agreements. We are also in the process of assessing the financial performance of the products we promote under these agreements and their overall value within our entire portfolio of products. We are also assessing the terms and conditions of our agreements with the intent to secure more favorable terms to us relative to profitability. If any of these agreements are determined to no longer be beneficial to us and are allowed to expire, or if third parties will not renegotiate, renew or extend the agreements on terms acceptable to us, our revenues and profitability would be adversely affected.
Cost of Product Sales:
The following table summarizes the year over year changes in our cost of product sales(in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2012 | | | Change | | | 2011
Restated | | | Change | | | 2010 | |
Cost of Product Sales | | $ | 21,996 | | | | 13 | % | | $ | 19,409 | | | | 53 | % | | $ | 12,691 | |
Cost of product sales was $22.0 million for the year ended December 31, 2012, compared to $19.4 million and $12.7 million for years ended December 31, 2011 and 2010, respectively. The increase of $2.6 million, or 13%, for the year ended December 31, 2012, compared to the year ended December 31, 2011, was attributable to an increase of $1.2 million in ZADAXIN cost of sales related to an increase in ZADAXIN sales and an increase of $1.4 million in oncology and Aggrastat cost of sales related to an increase in those product sales and deliveries. The increase of $6.7 million, or 53%, for the year ended December 31, 2011, compared to the year ended December 31, 2010, was attributable to higher ZADAXIN sales and the addition of NovaMed cost of product sales in 2011 as a result of the acquisition of NovaMed. ZADAXIN cost of sales were $16.2 million for the year ended December 31, 2012, compared to $15.0 million and $12.7 million for the years ended December 31, 2011 and 2010, respectively. Gross margin for ZADAXIN was 85.7% for the year ended December 31, 2012, compared to 85.7% and 85.1% for the years ended December 31, 2011 and 2010, respectively. The increase in gross margin for ZADAXIN for the year ended December 31, 2011, compared to the years ended December 31, 2010 was due primarily to lower per vial production costs mainly as a result of volume efficiencies.
We expect our ZADAXIN cost of product sales and gross margins to fluctuate from period to period depending upon the level of sales and price of our products, the absorption of product-related fixed costs, currency exchange fluctuations, any charges associated with excess or expiring finished product inventory, and the timing of other inventory period costs such as manufacturing process improvements for the goal of future cost reductions
Overall, we expect our gross margin percentages in 2013 to remain comparable to 2012, although they may fluctuate from quarter to quarter.
Sales and Marketing:
The following tables summarize the year over year changes in our sales and marketing expenses(in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2012 | | | Change | | | 2011
Restated | | | Change | | | 2010 | |
Sales and Marketing | | $ | 70,327 | | | | 44 | % | | $ | 48,853 | | | | 122 | % | | $ | 22,006 | |
54
Sales and marketing expenses were $70.3 million, $48.9 million, and $22.0 million for the years ended December 31, 2012, 2011, and 2010, respectively. Sales and marketing expenses increased $21.5 million or 44% for the year ended December 31, 2012, compared to the year ended December 31, 2011. In 2012, we recorded 12 months of sales and marketing expense for NovaMed, compared to the same period of 2011 that included sales and marketing expenses for NovaMed from the date of acquisition on April 18, 2011 through December 31, 2012. Additional increases in sales and marketing expenses for the year ended December 31, 2012 related to increased growth in support of our commercial efforts to expand more deeply and widely throughout the China market.
Sales and marketing expenses increased $26.8 million for the year ended December 31, 2011, compared to the year ended December 31, 2010. An increase in sales and marketing expenses of $22.6 million for the year ended December 31, 2011, compared to the year ended December 31, 2010, was primarily related to NovaMed operations as a result of the acquisition of NovaMed and its sales professionals of over 440 individuals. The remaining increases of $4.2 million for the year ended December 31, 2011, related to increased growth in the ZADAXIN sales force in China of approximately 100 additional sales individuals, and further market penetration in China resulting in increased sales and marketing costs for compensation and benefits, sales incentives, travel, medical training, and business taxes. We expect sales and marketing expenses for the year ending December 31, 2013 to be comparable to those incurred for the year ended December 31, 2012.
Amortization and Impairment of Acquired Intangible Assets:
For the year ended December 31, 2012 and 2011, we recognized $2.6 million and $2.5 million, respectively, in amortization of acquired intangible assets expense. Amortization of acquired intangible assets reflects the amortization of services and distribution contract intangible assets acquired as part of the NovaMed acquisition on April 18, 2011.
During the year ended December 31, 2012, we identified an impairment indicator with respect to the intangible assets related to our promotion and distribution contract rights. We determined that the undiscounted cash flows estimated to be generated by the intangible assets were less than the carrying amounts. We further performed a discounted cash flow analysis related to the intangible assets and determined that a full impairment should be recorded. As a result, we recognized a non-cash impairment loss of approximately $42.7 million for the year ended December 31, 2012. No further amortization expense will be recorded in future periods related to our acquired intangible assets.
Research and Development (“R&D”):
The following tables summarize the year over year changes in our R&D expenses(in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2012 | | | Change | | | 2011 | | | Change | | | 2010 | |
Research and Development | | $ | 6,143 | | | | -50 | % | | $ | 12,346 | | | | -1 | % | | $ | 12,415 | |
Research and development (“R&D”) expenses were $6.1 million, $12.3 million and $12.4 million for the years ended December 31, 2012, 2011, and 2010, respectively. On March 2, 2012, we announced the discontinuation of our SCV-07 phase 2b clinical trial for the delay to onset of severe OM based on the results of the pre-planned interim analysis that indicated that the trial would not meet the pre-specified efficacy endpoints. R&D expenses decreased $6.2 million or 50% for the year ended December 31, 2012, compared to the year ended December 31, 2011, primarily related to the discontinuation of this trial. These decreases were offset partially by employee severance costs of $1.0 million recognized to R&D expenses for the year ended December 31, 2012.
During the year ended December 31, 2011, R&D expenses primarily related to our SCV-07 phase 2b clinical trial which began enrollment in January 2011 for the treatment of OM and to a lesser extent to the addition of NovaMed R&D expenses due to our acquisition of NovaMed. For the year ended December 31, 2010, R&D expenses primarily
55
related to our phase 2a clinical trial of SCV-07 for the delay to onset of severe OM, the initiation of our SCV-07 phase 2b clinical trial for the treatment of OM, and our SCV-07 phase 2b clinical trial for the treatment of HCV.
The major components of R&D expenses include salaries and other personnel-related expenses, including associated stock-based compensation, facility-related expenses, depreciation of facilities and equipment, license-related fees, services performed by clinical research organizations and research institutions and other outside service providers.
We expect our research and development expenses to decrease significantly in 2013, compared to 2012, as a result of the discontinuation of the SCV-07 phase 2b clinical trial. We continue to evaluate opportunities to in-license the marketing rights to proprietary products primarily in China, which may result in increased research and development expenses due to license fee payments, local registration clinical trials, or other expenses related to in-licensing and development of new products in the future.
General and Administrative:
The following table summarizes the year over year changes in our general and administrative expenses(in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2012 | | | Change | | | 2011 | | | Change | | | 2010 | |
General and Administrative | | $ | 21,442 | | | | -11 | % | | $ | 24,032 | | | | 54 | % | | $ | 15,606 | |
General and administrative expenses were $21.4 million, $24.0 million, and $15.6 million for the years ended December 31, 2012, 2011, and 2010, respectively. General and administrative expenses for the year ended December 31, 2012 decreased by $2.6 million, or 11%, compared to the year ended December 31, 2011. The decrease was attributable to $3.8 million lower NovaMed acquisition-related costs, offset partially by increased general and administrative expenses related to NovaMed as a result of our acquisition of NovaMed on April 18, 2011, and increased professional fees related to NovaMed internal control issues identified in the third and fourth quarters of 2012 and the SEC and DOJ’s ongoing investigation.
During the year ended December 31, 2011, general and administrative expenses increased $8.4 million or 54% compared to the year ended December 31, 2010, primarily related to the acquisition of NovaMed in April 2011, including the general and administrative expenses attributable to NovaMed operations, acquisition related transaction costs (including legal, banker fees, accounting and advisory services) of $3.8 million, and higher professional services primarily related to our FCPA compliance efforts and various tax planning and compliance matters.
We expect our general and administrative expenses in 2013 to increase compared to 2012 as we anticipate higher legal and accounting costs in 2013. Our ongoing investigations with the SEC and DOJ are unpredictable and may result in higher legal costs or fines or penalties and could affect our expenses or the timing thereof. We do not expect to incur any significant acquisition-related costs in 2013, though we continue to evaluate opportunities in China, which may result in increased general and administrative expenses in the future. See Part I, Item 3 “Legal Proceedings”.
Contingent Consideration:
As part of the acquisition of NovaMed, we would have been required to pay up to an additional $43.0 million in earn-out payments upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years (the “earn-out” or “contingent consideration”). We initially recorded $18.9 million as the estimated fair value of the contingent consideration. The fair value of the contingent consideration was remeasured each quarter, and changes to the fair value were recorded to contingent consideration expense or gain. As of December 31, 2012, the fair value of the earn-out was determined to be $0, resulting in a gain of $15.4 million for the year ended December 31, 2012. Through September 30, 2012, the Company used the assistance of a third-party valuation expert to estimate the fair value of the contingent consideration using a
56
Monte Carlo simulation model. The estimated fair value of the contingent consideration was subject to fluctuations as a result of adjustments to certain performance metric projections used to estimate the fair value that resulted in a gain of $3.5 million for the year ended December 31, 2011. Our fair value estimates were based on a variety of factors that significantly fluctuated from period to period, including the likelihood that earn-out targets would be achieved and present value factors associated with the timing of the earn-out targets. The significant reduction in the valuation of the contingent consideration expense during 2012 and 2011 was primarily due to revenue and EBITDA (earnings before interest, depreciation and taxes) targets not being achieved and to the reduced probability of renewal relating to NovaMed’s product distribution agreements including, in particular, the target of renewing the Depakine services agreement with Sanofi for a five-year term. The Depakine services agreement was extended through December 31, 2013, a term less than five years.
Other Income:
In November 2010, we were awarded approximately $1.0 million in non-taxable grants as part of the US Department of Treasury’s Therapeutic Discovery Project Program related to our research and development activities in SCV-07 and ZADAXIN. There were no similar grants awarded to us during the years ended December 31, 2012 or 2011, and we do not expect any income related to non-taxable grants for the year ending December 31, 2013.
(Benefit) Provision for Income Tax:
The (benefit) provision for income tax relates to our foreign operations in China. The benefit for income tax was $3.3 million for the year ended December 31, 2012, compared to tax expense of $0.7 million and $2.2 million for the years ended December 31, 2011 and 2010, respectively. The tax benefit increased $4.0 million for the year ended December 31, 2012, compared to 2011, primarily related to the reversal of deferred tax liabilities due to the impairment loss of approximately $42.7 million recorded related to our intangible assets, partially offset by the impact of recording a full valuation allowance on NovaMed deferred tax assets, and by increased tax expense related to growth in our China operations.
Tax expense decreased $1.5 million for the year ended December 31, 2011, compared to the year ended December 31, 2010. The decreases related to tax benefits recognized of $1.0 million for the year ended December 31, 2011 on deferred tax assets and liabilities mainly as a result of our acquisition of NovaMed in April 2011 and lower tax expense recorded for the year ended December 31, 2011 related to an uncertain tax position in China, offset partially by an increase in tax expense due to growth in our China operations and an increase in the statutory tax rate of certain of our operations in China from 22% in 2010 to 24%-25% in 2011. The statutory tax rate in China was 25% in 2012, 24-25% in 2011, and was 22% in 2010. Our statutory tax rate in China is 25% in 2013. We expect the provision for income tax to increase for the year ending December 31, 2013, compared to the year ended December 31, 2012, as we do not expect the tax benefits we recorded during 2012 related to the reversal of deferred tax liabilities, due to the impairment loss, to recur in 2013.
We have not recorded any US federal or state income tax expense for the years ended December 31, 2012, 2011, and 2010. Undistributed earnings of our foreign subsidiaries amounted to approximately $45.0 million at December 31, 2012. These earnings are considered to be permanently reinvested and accordingly, no deferred US income taxes have been provided thereon.
At December 31, 2012, we had net operating loss carryforwards for federal income tax purposes of approximately $87.4 million that expire in the years 2020 through 2030. At December 31, 2012, we had federal research and development, orphan drug and investment tax credit carryforwards of approximately $11.7 million that expire in the years 2018 through 2031.
Because of the “change in ownership” provisions of the Internal Revenue Code, a portion of our net operating loss carryforwards and tax credit carryforwards may be subject to an annual limitation regarding their
57
utilization against taxable income in future periods. As a result of the annual limitation, a portion of these carryforwards may expire before ultimately becoming available to reduce future income tax liabilities.
Liquidity and Capital Resources
We continue to closely manage our liquidity and capital resources. We rely on our operating cash flows, cash and cash equivalents, short-term investments and our short-term loan arrangement to provide for our liquidity requirements. We continue to believe that we have the ability to meet our liquidity needs for at least the next 12 months to fund our working capital requirements of our operations, including investments in our business, share repurchases, to pay down our short-term borrowing arrangements, and to fund our business development activities.
The following tables summarize our cash and investments and our cash flow activities as of the end of, and for each of, the years presented(in thousands):
| | | | | | | | |
| | | As of December 31, | |
| | | 2012 | | | 2011 | |
Cash and investments | | $ | 84,228 | | | $ | 66,654 | |
As of December 31, 2012, we had $84.2 million in cash and investments of which $56.4 million was located in subsidiaries of the Company outside the US. Cash held by subsidiaries outside the US is held primarily in US dollars. Such cash is used to fund the operating activities of our foreign subsidiaries and for further investment in foreign operations which may include in-licensing new products, particularly for China, and for potential acquisitions. Generally, we consider such cash to be permanently reinvested in our foreign operations. Based on our current operating plan for the next 12 months, we do not anticipate the repatriation of cash held by foreign subsidiaries, if any, would result in payment of US Federal taxes.
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
Cash provided by (used in): | | | | | | | | | | | | |
Operating activities | | $ | 43,521 | | | $ | 29,455 | | | $ | 20,660 | |
Investing activities | | $ | (1,203 | ) | | $ | (18,840 | ) | | $ | (1,316 | ) |
Financing activities | | $ | (24,699 | ) | | $ | 2,827 | | | $ | 3,901 | |
Net cash provided by operating activities was $43.5 million for year ended December 31, 2012 and primarily reflected the net income for the period, adjusted for non-cash items such as intangible asset impairment, the change in the fair value of the contingent consideration, stock-based compensation expense, depreciation and amortization expense, and changes in operating assets and liabilities. Such changes included a $2.2 million increase in accounts payable mainly related to professional and manufacturing services, and a $3.0 million increase in accrued liabilities mainly related to increases in sales and marketing and tax accruals.
Net cash provided by operating activities was $29.5 million for year ended December 31, 2011 and primarily reflected the net income for the period, adjusted for non-cash items such as stock-based compensation expense, depreciation and amortization expense and changes in operating assets and liabilities. Such changes included a decrease in cash received from customers for accounts receivable of $2.7 million. The majority of our sales are to importers and distributors in China where our accounts receivable collections have standard credit terms generally ranging from 60 to 180 days. Changes also included an increase in inventory levels of $2.3 million related to higher inventory stock levels to support increased demand for ZADAXIN product and related to oncology products, and an increase in accounts payable and accrued expenses of $4.6 million primarily as a result of our acquisition of NovaMed.
Net cash used in investing activities was $1.2 million, $18.8 million, and $1.3 million for the years ended December 31, 2012, 2011, and 2010, respectively. For the year ended December 31, 2012, net cash used in
58
investing activities was primarily related to the purchases of property and equipment. Cash used in investing activities for the year ended December 31, 2011 was primarily related to the acquisition of NovaMed which used cash of approximately $21.3 million, and to a lesser extent related to the sale of available-for-sale investments, net of purchases of available-for-sale investments, and purchases of property and equipment. Cash used in investing activities for the year ended December 31, 2010 was primarily related to purchases of investments, net of proceeds from sale or maturities of investments, and purchases of property and equipment.
Net cash (used in) provided by financing activities was ($24.7) million, $2.8 million, and $3.9 million for the years ended December 31, 2012, 2011, and 2010. During the years ended December 31, 2012 and 2011, we used $24.8 million and $3.5 million, respectively, to repurchase and retire approximately 4.7 million and 781,000 shares, respectively, of our common stock under our stock repurchase program. During 2012, we also used $2.5 million for the repayment of borrowings on our loan and security agreement with Silicon Valley Bank (“SVB”) (“the Credit Facility”), $2.3 million for restricted cash to secure a letter of credit related to our loan agreement with Shanghai Pudong Development Bank Co. Ltd., and we borrowed $1.4 million under our loan agreement with Shanghai Pudong Development Bank Co. Ltd. For the years ended December 31, 2012, 2011, and 2010, we also received $3.5 million, $6.3 million, and $1.4 million, respectively, of proceeds from the issuances of common stock made under our stock award plans.
The following summarizes our future contractual obligations as of December 31, 2012 (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
Contractual Obligations | | Total | | | Less than 1 Year | | | 1-3 Years | | | 3-5 Years | | | More Than 5 Years | |
Short-term debt obligation(1) | | $ | 1,517 | | | $ | 1,517 | | | $ | — | | | $ | — | | | $ | — | |
Operating leases(2) | | | 5,035 | | | | 3,204 | | | | 1,582 | | | | 242 | | | | 7 | |
Purchase obligations(3) | | | 12,402 | | | | 12,402 | | | | — | | | | — | | | | — | |
Uncertain tax position(4) | | | 3,343 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 22,297 | | | $ | 17,123 | | | $ | 1,582 | | | $ | 242 | | | $ | 7 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Our short-term debt obligations include our expected principal and interest obligations related to our loan agreement with Shanghai Pudong Development Bank Co. Ltd. Our calculations of expected principal and interest payments incorporate only current period assumptions for interest rates and borrowings. |
| (2) | These are future minimum rental commitments for office space and copiers leased under non-cancelable operating lease arrangements. |
| (3) | These consist of purchase obligations with manufacturers and distributors. |
| (4) | As we are not able to reasonably estimate the timing of the payments or the amount by which our obligations for unrecognized tax benefits will increase or decrease over time, the related balances have not been reflected in the “Payments Due by Period” section of the table. |
In August 2012, our subsidiary, NovaMed Pharmaceuticals (Shanghai) Co. Ltd. entered into a loan agreement with Shanghai Pudong Development Bank Co. Ltd to borrow up to 12.5 million renminbi (approximately $2.0 million USD as of December 31, 2012). As of December 31, 2012, there were 9.0 million renminbi ($1.4 million USD) in borrowings under the loan agreement. The loan agreement is secured by a $2.3 million letter of credit. We are required to maintain $2.3 million of cash in a restricted bank account to secure the letter of credit. The loan agreement bears interest on borrowed funds at 7.5% and expires August 29, 2013. Any amounts borrowed must be repaid by the expiration date.
On March 15, 2012, we filed a shelf registration with the SEC under which we may offer and sell up to $100.0 million of our securities, assuming we continue to meet the SEC’s eligibility requirements for primary offerings on Form S-3. Subsequently, affiliates of Sigma-Tau sold approximately 6.3 million shares for an aggregate price of approximately $33.1 million under this registration statement, and we have approximately
59
$66.9 million available for future use. On April 18, 2011, we issued 8,298,110 shares of common stock to former stockholders of NovaMed as part of our acquisition of NovaMed.
In May and November 2012, we announced that our Board of Directors approved increases of $10.5 million and $10.0 million, respectively, to the Company’s stock repurchase program bringing the total authorized since the program’s inception in October 2011 to $40.5 million. Under this program, we repurchased and retired approximately 5.5 million shares at a cost of $28.1 million through December 31, 2012. The current repurchase program will expire October 2013, unless extended or renewed. We consider several factors in determining when to make share repurchases including, among other things, our cash needs, the availability of funding and the market price of our stock. We expect that cash provided by future operating activities, as well as available cash and cash equivalents and short-term investments, will be the sources of funding for our share repurchase program.
We believe that our existing cash and investments and ongoing revenue generating business operations will be sufficient to support our current operating plan for at least the next 12 months. We have no current commitments to offer and sell any securities that may be offered or sold pursuant to our registration statement. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience dilution. Debt financing, if available, may subject us to restrictive covenants and significant interest costs. To the extent that we raise additional funds through collaboration and licensing arrangements, we would be required to relinquish some rights to our technologies, product candidates or marketing territories. Additional financing or collaboration and licensing arrangements may not be available when needed either at all or, on favorable terms.
We intend to continue to explore alternatives for financing to provide additional flexibility in managing our operations, in-licensing new products, particularly for China, and potential acquisitions, as may be required. The unavailability or the inopportune timing of any financing could prevent or delay our long-term product development and commercialization programs, either of which could hurt our business. We cannot assure you that funds from financings, if any, will be sufficient to in-license additional products. The need, timing and amount of any such financing would depend upon numerous factors, including the status of the pending regulatory investigations and pending litigations, the level and price of our products, the timing and amount of manufacturing costs related to our products, the availability of complementary products, technologies and businesses, the initiation and continuation of preclinical and clinical trials and testing, the timing of regulatory approvals, developments in relationships with existing or future collaborative parties, the status of competitive products, and various alternatives for financing. We have not determined the timing or structure of any transaction.
Off-Balance Sheet Arrangements
We do not have any off-sheet balance sheet arrangements.
Critical Accounting Policies and Significant Judgments and Estimates
General
We have identified the policies below as critical to our business operations and the understanding of our results of operations. The impact and any associated risks related to these policies on our business operations is discussed throughout “Management’s Discussion and Analysis of Financial Condition and Results of Operations” where such policies affect our reported and expected financial results. For a detailed discussion on the application of these and other accounting policies, see Note 1 in the “Notes to our Consolidated Financial Statements” in Part II, Item 8 of this Annual Report on Form 10-K. Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United Sates, which require us to make estimates and assumptions that affect the reported amount of assets and liabilities, disclosure of contingent assets and liabilities at the date of our financial statements, and the reported amounts of revenue and expenses during the reporting period. On an on-going basis, we evaluate the relevance of our estimates and
60
judgments. We base our estimates on historical experience and on various other market specific assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. There can be no assurance that actual results will not differ from those estimates.
Revenue Recognition
We recognize revenue when persuasive evidence of an arrangement exists, services have been rendered or delivery has occurred, the price to the buyer is fixed or determinable and collectability is reasonably assured.
Product Revenue. We earn product revenue from selling manufactured ZADAXIN product at the time of delivery. Sales of ZADAXIN to importing agents or distributors are recognized at time of shipment when title to the product is transferred to them. We also earn product revenue from purchasing medical products from pharmaceutical companies and selling them directly to importers or distributors. Sales of Pfizer products, from the date of the acquisition of NovaMed into October 2012, were based on the “sell-through” method as our distribution arrangement for these products allowed for payment terms dependent on when the distributor sold the product. We did not maintain information on the timing of “sell-through” of the Pfizer products by the distributor through this period, therefore we applied the cash receipts approach for the application of the “sell-through” method as it was the most reliable information available. Accordingly, during this period of time, revenue for sales of the Pfizer products was recognized upon receipt of cash from the distributor. Effective as of the fourth quarter of 2012, we entered into a new agreement with the distributor which clarified and confirmed the “sell-in” method of revenue recognition to ensure consistency with the Company’s policy regarding revenue recognition on a prospective basis. All other product sales are recognized on the “sell-in” method, or when the medical products have been delivered to the importers or distributors.
Promotion Services Revenue. We recognize promotion services revenue after designated medical products are delivered to the distributors as specified in the promotional contract, which marks the period when marketing and promotional services have been rendered, and when all of the above revenue recognition criteria are met.
Accounts Receivable
Receivable Reserve.We record a receivable reserve based upon a specific review of our overdue invoices. Our estimate for a reserve is determined after considering our existing contractual payment terms, payment patterns of our customers and individual customer circumstances, the age of any outstanding receivables and our current customer relationships. At December 31, 2012, we recorded a receivable reserve of approximately $1.0 million, related to total accounts receivable from one customer of $3.5 million, where actual collections could range from $0 to $3.5 million. The receivable reserve represents our best estimate of these results, though actual results may vary and we continue to pursue the full amount of the receivables.
Reserve for Product Returns. We maintain a reserve for product returns based on estimates of the amount of product to be returned by our customers which may result from expired product or for price reductions on the related sales and is based on historical patterns, analysis of market demand and/or a percentage of sales based on industry trends, and management’s evaluation of specific factors that may increase the risk of product returns. Importing agents or distributors do not have contractual rights of return except under limited terms regarding product quality. However, we are expected to replace products that have expired or are deemed to be damaged or defective when delivered. The calculation of the product returns reserve requires estimates and involves a high degree of subjectivity and judgment. As a result of the uncertainties involved in estimating the product returns reserve, there is a possibility that materially different amounts could be reported under different conditions or using different assumptions. As of December 31, 2012 and 2011, we estimated a product returns reserve of $0.1 million on our consolidated balance sheet, related to oncology products and Aggrastat product sales. As part of our restatement of our financial statements, we recorded adjustments to correct errors in our product returns reserve for sales of Aggrastat sold by our subsidiary, NovaMed, prior to the date of acquisition. We have concluded a liability
61
for expired product existed at the time of the NovaMed acquisition, related to pre-acquisition sales. At December 31, 2012 and 2011, our liability for Aggrastat expired product was $0.3 million and $0.8 million, respectively. We evaluate our returns reserve quarterly and adjust it when events indicate that a change in estimate is appropriate. Changes in estimates could materially affect our results of operations or financial position. It is possible that we may need to adjust our estimates in future periods.
Inventories
Our inventories are valued at the lower of cost or market (net realizable value), with cost determined on a first-in, first-out basis and include amounts related to materials, labor and overhead. In assessing the ultimate realization of inventories, we are required to make judgments as to future demand requirements and compare that with the current inventory levels. If our current assumptions about future production or inventory levels and demand were to change or if actual market conditions are less favorable than those projected by management, inventory write-downs may be required which could negatively impact our gross margins and results of operations. If obsolete items or excess are observed and there are no alternate uses for the inventory, we will record a write-down to net realizable value in the period that the impairment is first recognized.
Business Combinations
We accounted for the acquisition of NovaMed in April 2011 in accordance with ASC Topic 805,Business Combinations. ASC Topic 805 establishes principles and requirements for recognizing and measuring the total consideration transferred to and the assets acquired and liabilities assumed in the acquired target in a business combination. The consideration paid to acquire NovaMed is required to be measured at fair value and included contingent consideration, which are earn-out payments that will be paid upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years. After the total consideration transferred was calculated by determining the fair value of the contingent consideration and SciClone common stock, plus the cash consideration, we assigned the purchase price of NovaMed to the fair value assets acquired and liabilities assumed. This resulted in recognition of intangible assets related to promotion and distribution contract rights and goodwill. The determination and recognition of the consideration transferred requires management to make significant estimates and assumptions, especially at the acquisition date with respect to the fair value of the contingent consideration and intangible assets acquired.
As part of the acquisition of NovaMed, we would have been required to pay up to $43.0 million in contingent consideration upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years. As of December 31, 2012, fair value of the earn-out was determined to be $0. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. When quoted prices and observable inputs are unavailable, fair values are based on internally developed cash flow models and are classified in level 3 of the valuation hierarchy. Through September 30, 2012, we estimated the fair value of the contingent consideration of the contingent consideration using a Monte Carlo simulation model and classified it in level 3 of the valuation hierarchy. The inputs used in the Monte Carlo simulation model reflected assumptions and estimates including for the risk-adjusted discount rate and volatility. Our assessment of the inputs used in the fair value measurement required judgment, and affected the valuation of the contingent consideration being measured.
The earn-out was based upon certain financial performance metrics, including a revenue-based formula and an adjusted EBITDA based formula. The earn-out provisions were subject to a number of adjustments and acceleration provisions. The total earn-out payments described above could have been increased by $10.0 million (but not exceeding a total maximum contingent cash consideration of $43.0 million) or reduced by $10.0 million, depending upon whether we were able to achieve targets relating to product distribution agreements. We were required to remeasure the contingent consideration each reporting period until the amount of the payment was finalized, which
62
occurred December 31, 2012. The change in the estimated fair value of the contingent consideration was recognized as an adjustment to operating expenses. From the acquisition date of April 18, 2011 to December 31, 2011 and for the year ended December 31, 2012, the estimated fair value of the contingent consideration decreased by $3.5 million and $15.4 million, respectively, primarily related to the decrease in the estimated probability of achieving revenue and EBITDA targets and to the reduced probability of renewal relating to NovaMed’s product distribution agreements, including, in particular, the target of renewing the Depakine services agreement with Sanofi for a five-year term. The Depakine services agreement was extended through December 31, 2013, a term less than five years.
Goodwill and Other Intangible Assets
We account for goodwill and other intangible assets in accordance with ASC Topic 805, and ASC Topic 350,Intangibles — Goodwill and Other. ASC Topic 805 requires that the purchase method of accounting be used for all business combinations and specifies the criteria that must be met in order for intangible assets acquired in a business combination to be recognized and reported apart from goodwill. Intangible assets and goodwill are tested for impairment at least annually or whenever events or circumstances occur that indicate impairment might have occurred or circumstances suggest that the carrying value of these assets may not be recoverable and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amounts of those assets in accordance with ASC Topic 350.
Judgment regarding the existence of impairment indicators will be based on operating results, changes in the manner of our use of the acquired assets or our overall business strategy, and market and economic trends.
As part of the acquisition of NovaMed, we recorded intangible assets related to promotion and distribution contract rights. The promotion and distribution contracts expire in one to ten years, with the majority expiring in one to two years, but are subject to renewal or extension. The estimated useful life was approximately 13.5 years. Although revenues from the NovaMed business have grown since the acquisition, overall revenue growth and profitability have not met our expectations at the time of the acquisition. This condition was considered to be an impairment indicator with respect to the intangible assets related to our promotion and distribution contract rights. We determined that the undiscounted cash flows estimated to be generated by the intangible assets were less than the carrying amounts. We further performed a discounted cash flow analysis related to the intangible assets and determined that a full impairment should be recorded. As a result, we recognized a non-cash impairment loss of approximately $42.7 million for year ended December 31, 2012 related to our intangible assets.
Contingent Liabilities
We record as liabilities estimated amounts for litigation, claims or other legal actions that are probable and can be reasonably estimated. The likelihood of a material change in these estimated reserves is dependent on the possible outcome of settlement negotiations, regulatory or judicial review and the development of facts and circumstances in extended litigation which could change claims or assessments when both the amount and range of loss on some outstanding litigation is uncertain. We disclose in the footnotes of the financial statements when we are unable to make a reasonable estimate of a material liability that could result from unfavorable outcomes. As events occur, we will assess the potential liability related to any pending litigation, claims or other legal actions and adjust our estimates accordingly. Such adjustments could materially impact our financial statements.
Stock-Based Compensation
We record stock-based compensation costs relating to share-based payment transactions, including stock options, restricted stock units (“RSUs”) and employee stock purchase plans. Stock-based compensation expense for stock options and the employee stock purchase plan is estimated at the date of grant based on the fair value of the award using the Black-Scholes option-pricing model. Stock-based compensation expense for RSUs is estimated at the date of grant based on the number of shares granted and the quoted price of the Company’s common stock on the grant date. Stock-based compensation expense values are recognized as expense on a
63
straight-line basis over the requisite service period, net of estimated forfeitures. The stock-based compensation costs that are ultimately expected to vest are recognized as expense ratably (as the awards vest) over the requisite service period, which is generally one or four years for stock options and RSUs and three months for the employee stock purchase plan. The Company estimates pre-vesting forfeitures at the time of grant by analyzing historical data and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. The total expense recognized over the vesting period will only be for those awards that ultimately vest.
Certain target-stock-price-based options were valued using the Monte Carlo simulation options pricing model and recognized to expense over the service periods for each of the vesting portions of these awards which were six or eight years. The option-pricing models require the use of certain subjective assumptions, including the expected volatility of the market price of the underlying stock and the expected term of the award. Expected volatility is based on the historical volatility of our stock. Expected term is derived from historical data on employee exercises and terminations, or the contractual life of the award for target-stock-price-based options. We review our valuation assumptions at each grant date, and, as a result, valuation assumptions used to value stock-based compensation of awards granted in future periods may change.
Income Taxes
Income taxes are accounted for under the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates that will be in effect when these differences reverse. We provide a valuation allowance against net deferred tax assets if, based upon the available evidence, it is more-likely-than-not that the deferred tax assets will not be realized. Realization of our deferred tax assets is dependent upon the generation of future taxable income, the timing and amount of which are uncertain. The tax years 1995-2012 remain open to examination by the major taxing jurisdictions to which we are subject, although the Internal Revenue Service completed their examination of our 2009 and 2008 US Federal tax returns with no additional tax assessments or proposed adjustments relating to taxable income for any years.
We record liabilities related to uncertain tax positions in accordance with the guidance that clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements by prescribing a minimum recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. We do not believe any such uncertain tax positions currently pending will have a material adverse effect on our consolidated financial statements, although an adverse resolution of one or more of these uncertain tax positions in any period could have a material impact on the results of operations for that period.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Foreign Currency Exchange Rate Risk
We do not hold any derivative financial instruments for speculation or trading purposes. The majority of our sales have been in US dollars, although a significant amount of our sales are denominated in renminbi. Our purchases with contract manufacturers are denominated in US dollars and euros and costs of our marketing efforts in China are paid in local currency. In addition, we have certain cash balances and other assets and liabilities denominated in euros, renminbi and Hong Kong dollars. As a result, we are exposed to foreign currency rate fluctuations, and we do not hedge against the risk associated with such fluctuations. Consequently, changes in exchange rates could result in material exchange losses and could unpredictably, materially and adversely affect our operating results and stock price. A hypothetical 1% increase or decrease in foreign exchange rates would result in an approximate $0.1 million and $0.2 million increase or decrease, respectively, in our financial assets and liabilities denominated in euros, renminbi and Hong Kong dollars, at December 21, 2012 and 2011, respectively. These potential changes are based on a sensitivity analyses performed on our financial position at December 31, 2012 and
64
2011. Actual results may differ materially. We do not hold any derivative financial instruments to manage our foreign currency exchange rate risks. Such losses have not been significant to date.
Interest Rate Risk
The primary objective of our investment activities is to preserve principal while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in money market funds, term deposits, commercial paper, corporate bonds, US treasury, or government agency notes. All of our investments mature within one year from date of purchase except for our Italian state bonds which mature in 2013. Our investment securities may be subject to interest rate risk and could decrease in value if market interest rates rise. To minimize this risk, we primarily hold securities that are short-term in duration and maintain an average maturity of less than one year. We believe that our exposure to interest rate risk is not significant and a 1% movement in market interest rates would not have a significant impact to the total value of our investment portfolio at December 31, 2012.
65
| Item 8. | Financial Statements and Supplementary Data |
SCICLONE PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
66
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of SciClone Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of SciClone Pharmaceuticals, Inc. as of December 31, 2012 and 2011, and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2012. Our audits also included the financial statement schedule at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of SciClone Pharmaceuticals, Inc. at December 31, 2012 and 2011, and the consolidated results of its operations, and its cash flows for each of the three years in the period ended December 31, 2012, in conformity with US generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
The financial statements for the year ended December 31, 2011 have been restated as a result of the accounting errors primarily related to revenue recognition originating with SciClone Pharmaceuticals, Inc.’s subsidiary in China, NovaMed, as is more fully described in Note 2, “Revision of Previously Issued Consolidated Financial Statements,” of the financial statements.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), SciClone Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated April 1, 2013 expressed an adverse opinion thereon.
/s/ Ernst & Young LLP
Redwood City, California
April 1, 2013
67
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| | | | | | | | |
| | | December 31,
2012 | | | December 31,
2011
Restated
(Note 2) | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 84,228 | | | $ | 66,654 | |
Accounts receivable, net of allowance of $1,169 and $116 at December 31, 2012 and 2011, respectively | | | 38,109 | | | | 38,465 | |
Inventories | | | 10,424 | | | | 11,141 | |
Restricted cash and investments | | | 2,759 | | | | — | |
Prepaid expenses and other current assets | | | 1,809 | | | | 1,646 | |
Deferred tax assets | | | — | | | | 1,788 | |
| | | | | | | | |
Total current assets | | | 137,329 | | | | 119,694 | |
Property and equipment, net | | | 1,377 | | | | 984 | |
Deferred tax assets | | | 369 | | | | — | |
Restricted investments | | | — | | | | 364 | |
Intangible assets, net | | | — | | | | 45,185 | |
Goodwill | | | 34,313 | | | | 33,868 | |
Other assets | | | 683 | | | | 749 | |
| | | | | | | | |
Total assets | | $ | 174,071 | | | $ | 200,844 | |
| | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 7,787 | | | $ | 5,592 | |
Accrued and other current liabilities | | | 21,427 | | | | 18,357 | |
Deferred tax liabilities | | | 153 | | | | — | |
Short-term borrowings on credit facilities | | | 1,445 | | | | 2,500 | |
| | | | | | | | |
Total current liabilities | | | 30,812 | | | | 26,449 | |
Contingent consideration | | | — | | | | 15,400 | |
Deferred tax liabilities | | | — | | | | 8,407 | |
Other long-term liabilities | | | 237 | | | | 469 | |
Commitments and contingencies | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Preferred stock; $0.001 par value; 10,000,000 shares authorized; no shares issued and outstanding | | | — | | | | — | |
Common stock; $0.001 par value; 100,000,000 shares authorized; 54,484,053 and 57,847,367 shares issued and outstanding at December 31, 2012 and 2011, respectively | | | 54 | | | | 58 | |
Additional paid-in capital | | | 274,387 | | | | 266,913 | |
Accumulated other comprehensive income | | | 2,988 | | | | 2,344 | |
Accumulated deficit | | | (134,407 | ) | | | (119,196 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 143,022 | | | | 150,119 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 174,071 | | | $ | 200,844 | |
| | | | | | | | |
See notes to consolidated financial statements.
68
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF INCOME
(In thousands, except per share amounts)
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Net revenues: | | | | | | | | | | | | |
Product sales, net | | $ | 123,171 | | | $ | 111,951 | | | $ | 85,112 | |
Promotion services | | | 33,098 | | | | 20,614 | | | | — | |
| | | | | | | | | | | | |
Total net revenues | | | 156,269 | | | | 132,565 | | | | 85,112 | |
Operating expenses: | | | | | | | | | | | | |
Cost of product sales | | | 21,996 | | | | 19,409 | | | | 12,691 | |
Sales and marketing | | | 70,327 | | | | 48,853 | | | | 22,006 | |
Amortization of acquired intangible assets,related to sales and marketing | | | 2,645 | | | | 2,465 | | | | — | |
Research and development | | | 6,143 | | | | 12,346 | | | | 12,415 | |
General and administrative | | | 21,442 | | | | 24,032 | | | | 15,606 | |
Intangible asset impairment (Note 7) | | | 42,728 | | | | — | | | | — | |
Contingent consideration (Notes 4 & 9) | | | (15,422 | ) | | | (3,495 | ) | | | — | |
| | | | | | | | | | | | |
Total operating expenses | | | 149,859 | | | | 103,610 | | | | 62,718 | |
Income from operations | | | 6,410 | | | | 28,955 | | | | 22,394 | |
Non-operating income (expense): | | | | | | | | | | | | |
Interest and investment income | | | 89 | | | | 71 | | | | 105 | |
Interest and investment expense | | | (185 | ) | | | (213 | ) | | | (195 | ) |
Other (expense) income, net | | | (42 | ) | | | (21 | ) | | | 953 | |
| | | | | | | | | | | | |
Income before provision for income tax | | | 6,272 | | | | 28,792 | | | | 23,257 | |
(Benefit) provision for income tax | | | (3,348 | ) | | | 670 | | | | 2,176 | |
| | | | | | | | | | | | |
Net income | | $ | 9,620 | | | $ | 28,122 | | | $ | 21,081 | |
| | | | | | | | | | | | |
Basic net income per share | | $ | 0.17 | | | $ | 0.51 | | | $ | 0.44 | |
Diluted net income per share | | $ | 0.16 | | | $ | 0.49 | | | $ | 0.43 | |
See notes to consolidated financial statements.
69
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In thousands)
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Net income | | $ | 9,620 | | | $ | 28,122 | | | $ | 21,081 | |
Other comprehensive income: | | | | | | | | | | | | |
Net change in unrealized gain (loss) and foreign currency translation on foreign currency denominated available-for-sale securities | | | 19 | | | | (16 | ) | | | (36 | ) |
Net change in unrealized losses on available-for-sale securities | | | — | | | | — | | | | (2 | ) |
Foreign currency translation adjustment | | | 625 | | | | 2,293 | | | | 83 | |
| | | | | | | | | | | | |
Total other comprehensive income | | | 644 | | | | 2,277 | | | | 45 | |
| | | | | | | | | | | | |
Total comprehensive income | | $ | 10,264 | | | $ | 30,399 | | | $ | 21,126 | |
| | | | | | | | | | | | |
See notes to consolidated financial statements.
70
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Accumulated
Other
Comprehensive
Income | | | | | | | |
| | | | | | | | | Additional
Paid-in Capital | | | | | | | Total
Stockholders’
Equity | |
| | | Common stock | | | | | Accumulated
Deficit | | |
| | | Shares | | | Amount | | | | | |
Balance at December 31, 2009 | | | 47,218 | | | $ | 47 | | | $ | 222,229 | | | $ | 22 | | | $ | (164,905 | ) | | $ | 57,393 | |
Net income | | | — | | | | — | | | | — | | | | — | | | | 21,081 | | | | 21,081 | |
Other comprehensive income | | | — | | | | — | | | | — | | | | 45 | | | | — | | | | 45 | |
Issuance of common stock from exercise of stock options and employee stock purchase plan | | | 746 | | | | 1 | | | | 1,401 | | | | — | | | | — | | | | 1,402 | |
Issuance of common stock from exercise of warrants, net of repurchases | | | 47 | | | | — | | | | — | | | | — | | | | — | | | | — | |
Compensation related to stock option awards | | | — | | | | — | | | | 2,267 | | | | — | | | | — | | | | 2,267 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | 48,011 | | | | 48 | | | | 225,897 | | | | 67 | | | | (143,824 | ) | | | 82,188 | |
Net income (restated) | | | — | | | | — | | | | — | | | | — | | | | 28,122 | | | | 28,122 | |
Other comprehensive income | | | — | | | | — | | | | — | | | | 2,277 | | | | — | | | | 2,277 | |
Issuance of common stock for acquisition of NovaMed | | | 8,298 | | | | 8 | | | | 31,522 | | | | — | | | | — | | | | 31,530 | |
Issuance of common stock from exercise of stock options and employee stock purchase plan | | | 2,319 | | | | 3 | | | | 6,319 | | | | — | | | | — | | | | 6,322 | |
Compensation related to stock option awards | | | — | | | | — | | | | 3,175 | | | | — | | | | — | | | | 3,175 | |
Repurchase of common stock | | | (781 | ) | | | (1 | ) | | | — | | | | — | | | | (3,494 | ) | | | (3,495 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 (restated) | | | 57,847 | | | | 58 | | | | 266,913 | | | | 2,344 | | | | (119,196 | ) | | | 150,119 | |
Net income | | | — | | | | — | | | | — | | | | — | | | | 9,620 | | | | 9,620 | |
Other comprehensive income | | | — | | | | — | | | | — | | | | 644 | | | | — | | | | 644 | |
Issuance of common stock from exercise of stock options, restricted stock units, and employee stock purchase plan | | | 1,346 | | | | 1 | | | | 3,491 | | | | — | | | | — | | | | 3,492 | |
Compensation related to stock option awards | | | — | | | | — | | | | 3,983 | | | | — | | | | — | | | | 3,983 | |
Repurchase of common stock | | | (4,709 | ) | | | (5 | ) | | | — | | | | — | | | | (24,831 | ) | | | (24,836 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2012 | | | 54,484 | | | $ | 54 | | | $ | 274,387 | | | $ | 2,988 | | | $ | (134,407 | ) | | $ | 143,022 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
See notes to consolidated financial statements.
71
SCICLONE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | |
| | | Year ended December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Operating activities: | | | | | | | | | | | | |
Net income | | $ | 9,620 | | | $ | 28,122 | | | $ | 21,081 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
Non-cash expense related to stock-based compensation | | | 3,897 | | | | 3,108 | | | | 2,224 | |
Depreciation and amortization | | | 3,449 | | | | 2,942 | | | | 556 | |
Realized gain on investments | | | — | | | | — | | | | (226 | ) |
Other non-cash expense | | | — | | | | — | | | | 249 | |
Intangible asset impairment | | | 42,728 | | | | — | | | | — | |
Change in fair value of contingent consideration | | | (15,422 | ) | | | (3,495 | ) | | | — | |
Deferred taxes, net | | | (6,785 | ) | | | (1,602 | ) | | | — | |
Other long-term liabilities | | | (232 | ) | | | (222 | ) | | | 11 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Accounts receivable, net | | | 356 | | | | (2,730 | ) | | | (9,277 | ) |
Inventories | | | 801 | | | | (2,269 | ) | | | 3,115 | |
Prepaid expenses and other assets | | | (156 | ) | | | 976 | | | | (674 | ) |
Accounts payable | | | 2,195 | | | | 945 | | | | 1,543 | |
Accrued and other current liabilities | | | 3,070 | | | | 3,680 | | | | 2,199 | |
Deferred revenue | | | — | | | | — | | | | (141 | ) |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 43,521 | | | | 29,455 | | | | 20,660 | |
| | | | | | | | | | | | |
Investing activities: | | | | | | | | | | | | |
Acquisition of NovaMed, net of cash acquired | | | — | | | | (21,256 | ) | | | — | |
Purchases of available-for-sale investments | | | (75 | ) | | | (1,580 | ) | | | (10,266 | ) |
Proceeds from the sale or maturities of available-for-sale investments | | | — | | | | 4,682 | | | | 7,283 | |
Proceeds from the sale or maturity of trading security investments | | | — | | | | — | | | | 1,800 | |
Purchases of property and equipment | | | (1,128 | ) | | | (686 | ) | | | (133 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (1,203 | ) | | | (18,840 | ) | | | (1,316 | ) |
| | | | | | | | | | | | |
Financing activities: | | | | | | | | | | | | |
Repurchase of common stock | | | (24,836 | ) | | | (3,495 | ) | | | — | |
Repayment of line of credit | | | (2,500 | ) | | | — | | | | — | |
Proceeds from borrowing on short-term loan | | | 1,445 | | | | — | | | | 2,500 | |
Increase in restricted cash related to short-term loan | | | (2,300 | ) | | | — | | | | — | |
Proceeds from issuances of common stock | | | 3,492 | | | | 6,322 | | | | 1,401 | |
| | | | | | | | | | | | |
Net cash (used in) provided by financing activities | | | (24,699 | ) | | | 2,827 | | | | 3,901 | |
Effect of exchange rate changes on cash and cash equivalents | | | (45 | ) | | | 195 | | | | 85 | |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | 17,574 | | | | 13,637 | | | | 23,330 | |
Cash and cash equivalents, beginning of period | | | 66,654 | | | | 53,017 | | | | 29,687 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 84,228 | | | $ | 66,654 | | | $ | 53,017 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Income taxes paid related to foreign operations | | $ | 1,893 | | | $ | 1,102 | | | $ | 829 | |
| | | | | | | | | | | | |
Interest and unused line fees paid related to borrowings | | $ | 136 | | | $ | 169 | | | $ | 37 | |
| | | | | | | | | | | | |
See notes to consolidated financial statements.
72
SCICLONE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 — The Company and Summary of Significant Accounting Policies
Description of Business
SciClone Pharmaceuticals, Inc. (“SciClone” or the “Company”), incorporated in 1990, is a revenue-generating, specialty pharmaceutical company with a substantial commercial business in China and a product portfolio of therapies for oncology, infectious diseases and cardiovascular, urological, respiratory, and central nervous system disorders. The Company’s lead product, ZADAXIN® (thymalfasin) is approved in over 30 countries and may be used for the treatment of hepatitis B (HBV), hepatitis C (HCV), as a vaccine adjuvant, and certain cancers according to the local regulatory approvals. In addition to ZADAXIN, SciClone markets approximately 14 mostly partnered products in China, including Depakine®, a widely prescribed broad-spectrum anti-convulsant in China; Tritace®, an ACE inhibitor for the treatment of hypertension; Stilnox®, a fast-acting hypnotic for the short-term treatment of insomnia (marketed as Ambien® in the US); and Aggrastat®, an interventional cardiology product. SciClone is also pursuing the registration of several other therapeutic products in China.
Presentation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated from the consolidated financial statements.
On April 18, 2011, SciClone acquired NovaMed Pharmaceuticals, Inc. (“NovaMed”). See Note 9. Commencing April 18, 2011, the Company’s financial statements include the assets, liabilities, operating results and cash flows of NovaMed.
Use of Estimates
The preparation of financial statements in conformity with US generally accepted accounting principles requires management to make informed estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ materially from those estimates.
Cash Equivalents and Investments
Cash equivalents consist of highly liquid investments with original maturities of three months or less on the date of purchase. The Company records its investments at fair value, as determined by available information on the consolidated balance sheet date. The Company’s available-for-sale portfolio at December 31, 2012 consisted of money market funds, a certificate of deposit, and restricted Italian state bonds.
Unrealized gains or losses on available-for-sale securities are included in accumulated other comprehensive income on the consolidated balance sheet. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are included in earnings. Gains or losses and declines in value judged to be other-than-temporary on trading securities are included in earnings. The amortized cost of securities is adjusted for amortization of premiums and accretion of discounts to maturity and is included in earnings. The cost of securities sold is based on the specific identification method.
Available-for-sale investments are evaluated for impairment each reporting period. An investment is considered impaired if the fair value of the investment is less than its cost. If, after consideration of all available evidence to evaluate the realizable value of its investment, impairment is determined to be other-than-temporary, then an impairment loss is recognized in the consolidated statement of income.
73
For the year ended December 31, 2010, the Company sold its auction rate securities that had been classified as trading securities and realized gains related to the investments of $0.2 million.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Fair value measurements are based on a three-tier hierarchy that prioritizes the inputs used to measure fair value. The three levels of input are:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Where quoted prices are available in an active market, the Company determines fair value based upon quoted market prices, and classifies these values in level 1 of the valuation hierarchy. If quoted market prices are not available, fair values are based upon observable inputs such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities and are classified in level 2 of the valuation hierarchy. When quoted prices and observable inputs are unavailable, fair values are based on cash flow models and are classified in level 3 of the valuation hierarchy. The cash flow models use inputs specific to the asset or liability including estimates for interest rates and discount rates including yields of comparable traded instruments adjusted for illiquidity and other risk factors, amount of cash flows and expected holding periods of the assets and liabilities. These inputs reflect the Company’s assumptions about the assumptions market participants would use in pricing the assets and liability including assumptions about risk developed based on the best information available in the circumstances. The Company’s assessment of the significance of a particular input to the fair value measurements requires judgment, and may materially affect the valuation of the assets and liability being measured and their placement within the fair value hierarchy.
Other financial instruments, including accrued short-term liabilities, are carried at cost, which the Company believes approximates fair value because of the short-term maturity of these instruments.
Concentration of Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash, cash equivalents, investments and accounts receivable. The Company is exposed to credit risk in the event of default by the institutions holding the cash, cash equivalents and investments to the extent of the amounts recorded on the consolidated balance sheet. Most of the Company’s cash and cash equivalents are held by financial institutions that the Company believes are of high credit quality. At times, deposits may exceed government insured limits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
The People’s Republic of China (“China”) uses a tiered method to import and distribute products. The distributors make the sales in the country, but the product is imported for them by licensed importers. Product sales revenues result from the sale of the Company’s proprietary or in-licensed products to importing agents and distributors. Promotion services revenues result from fees received for exclusively promoting products for certain partners. These importing agents, distributors and partners are the Company’s customers.
74
For the year ended December 31, 2012, three customers in China accounted for 53%, 20% and 12% of the Company’s revenues. For the year ended December 31, 2011, three customers in China accounted for 62%, 15% and 15% of the Company’s revenues. In 2010, the two largest customers accounted for 74% and 14% of revenues. No other customer accounted for more than 10% of sales in 2012, 2011, or 2010. The Company’s largest customer was the same for all periods. A third party holds a majority interest in the Company’s largest customer. As of December 31, 2012, approximately $33.7 million, or 86%, of the Company’s accounts receivable were attributable to four customers in China including $27.6 million or 70% attributable to its largest customer. The Company generally does not require collateral from its customers. The Company maintains reserves for potential credit losses and such actual losses may vary significantly from its estimates.
The Company currently relies on two suppliers to provide key components to its ZADAXIN manufacturing supply. Although there are a limited number of manufacturers who would be able to meet the requirements to manufacture these components, the Company believes that other suppliers could provide similar components on comparable terms. A change in suppliers, however, could cause a delay in manufacturing and a possible loss of sales, which would affect operating results adversely.
The majority of the Company’s product sales are in US dollars. However, a significant portion of the Company’s revenues and expenses are denominated in renminbi (“RMB”) and a significant portion of the Company’s assets and liabilities are denominated in RMB and are exposed to foreign exchange risk. RMB is not freely convertible into foreign currencies. In China, foreign exchange transactions are required by law to be transacted only by authorized financial institutions at the exchange rates quoted by People’s Bank of China. Remittances in currencies other than RMB by the Company in China require certain supporting documentation in order to affect the remittance.
Accounts Receivable
Receivable Reserve:The Company records a receivable reserve based upon a specific review of its overdue invoices. The Company’s estimate for a reserve is determined after considering its existing contractual payment terms, payment patterns of its customers and individual customer circumstances, the age of any outstanding receivables and its current customer relationships. At December 31, 2012, the Company recorded a receivable reserve of approximately $1.0 million related to total accounts receivable from one customer of $3.5 million, where actual collections could range from $0 to $3.5 million. The receivable reserve represents the Company’s best estimate of these results, though actual results may vary and the Company continues to pursue the full amount of the receivables.
Reserve for Product Returns: The Company maintains a reserve for product returns based on estimates of the amount of product to be returned by its customers which may result from expired product or for price reductions on the related sales and is based on historical patterns, analysis of market demand and/or a percentage of sales based on industry trends, and management’s evaluation of specific factors that may increase the risk of product returns. Importing agents or distributors do not have contractual rights of return except under limited terms regarding product quality. However, the Company is expected to replace products that have expired or are deemed to be damaged or defective when delivered. The calculation of the product returns reserve requires estimates and involves a high degree of subjectivity and judgment. As a result of the uncertainties involved in estimating the product returns reserve, there is a possibility that materially different amounts could be reported under different conditions or using different assumptions. As of December 31, 2012 and 2011, the Company had estimated a product returns reserve of $0.1 million on its consolidated balance sheet related to oncology products and Aggrastat product sales. No reserve was considered necessary as of December 31, 2010, and there was no expense recorded during the year ended December 31, 2010 for product returns. As part of the Company’s restatement of its financial statements, the Company recorded adjustments to correct errors in its product returns reserve for sales of Aggrastat sold by its subsidiary, NovaMed, prior to the date of acquisition. The Company has concluded a liability for expired product existed at the time of the NovaMed acquisition, related to pre-acquisition sales. At December 31, 2012 and 2011, the liability for Aggrastat expired product was $0.3 million
75
and $0.8 million, respectively. The Company evaluates its returns reserve quarterly and adjusts it when events indicate that a change in estimate is appropriate. Changes in estimates could materially affect the Company’s results of operations or financial position. It is possible that the Company may need to adjust its estimates in future periods.
Inventories
Inventories consist of raw materials, work in progress and finished goods products. Inventories are valued at the lower of cost or market (net realizable value), with cost determined on a first-in, first-out basis, and include amounts related to materials, labor and overhead. The Company periodically reviews the inventory in order to identify excess and obsolete items. If obsolete or excess items are observed and there are no alternate uses for the inventory, the Company will record a write-down to net realizable value in the period that the impairment is first recognized.
Property and Equipment
Property and equipment is stated at cost, less accumulated depreciation. Depreciation is recorded over the estimated useful lives of the respective assets (generally three to five years) on the straight-line basis. Leasehold improvements are amortized over the shorter of the estimated useful life or lease term on the straight-line basis. The Company’s policy is to identify and record impairment losses, if necessary, on property and equipment when events and circumstances indicate that the assets might be impaired and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amounts of those assets.
Intangible Assets
Intangible assets are reviewed for impairment when changes in facts or circumstances suggest that the carrying value of these assets may not be recoverable. The Company’s policy is to identify and record impairment losses, if necessary, on intangible product rights when events and circumstances indicate that the assets might be impaired and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amounts of those assets. It is the Company’s policy to expense costs as incurred in connection with the renewal or extension of its intangible assets.
As part of the acquisition of NovaMed, the Company recorded intangible assets related to promotion and distribution contract rights. During the year ended December 31, 2012, the Company identified impairment indicators related to the intangible assets and determined that a full impairment should be recorded. Refer toNet Intangible Assets in Note 7 for further information.
Goodwill
The Company accounted for the acquisition of NovaMed under the purchase method of accounting in accordance with the Financial Accounting Standards Board Accounting Standards Codification (“ASC”) Topic 805,Business Combinations. Under the purchase method of accounting, the total acquisition-date fair value of the assets and liabilities are recognized as of the closing date. The total consideration paid by SciClone to NovaMed consisted of cash, SciClone common stock, and contingent consideration. The excess of the fair value of the total consideration transferred over the acquisition-date fair value of net tangible and intangible assets and liabilities assumed was allocated to goodwill. Goodwill is tested for impairment at least annually, or whenever events or circumstances occur that indicate impairment might have occurred in accordance with ASC Topic 350,Intangibles — Goodwill and Other.
Contingent Consideration
As part of the acquisition of NovaMed, the Company would have been required to pay up to an additional $43.0 million in earn-out upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years (the “earn-out” or “contingent consideration”). The fair value of the earn-out was re-measured each
76
period, and changes in the fair value were recorded to “contingent consideration” in operating expenses. As of December 31, 2012, the earn-out was determined to be zero. Through September 30, 2012, the Company used the assistance of a third-party valuation expert to estimate the fair value of the contingent consideration using a Monte Carlo simulation model. Refer toContingent Consideration in Note 4 andAcquisition Note 9 for further information.
Accrued Expenses
The Company makes estimates of its accrued expenses as of each balance sheet date in its consolidated financial statements based on facts and circumstances known to them. Examples of estimated accrued expenses include fees paid to contract research organizations and investigative sites in connection with clinical trials, fees paid to contract manufacturers in connection with the production of clinical trial materials, and professional services. The Company periodically confirms the accuracy of its estimates with selected service providers and makes adjustments, if necessary, in the periods identified.
Expenses related to clinical trials generally are accrued based on estimates of work performed or the level of patient enrollment and activities according to the protocols and agreements. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under certain contracts depend on factors such as the achievement of certain events, the successful enrollment of patients, and the completion of portions of the clinical trial or similar conditions. The objective of the Company’s accrual policy is to match the recording of expenses to the actual services received and efforts expended. The Company monitors planned protocols, work performed, patient enrollment levels and related activities to the extent possible and adjusts estimates accordingly.
The Company records as liabilities estimated amounts for litigation, claims or other legal actions that are probable and can be reasonably estimated. The likelihood of a material change in these estimated reserves is dependent on the possible outcome of settlement negotiations, regulatory or judicial review and the development of facts and circumstances in extended litigation which could change claims or assessments when both the amount and range of loss on some outstanding litigation is uncertain. The Company discloses in the footnotes of the financial statements when it is unable to make a reasonable estimate of a material liability that could result from unfavorable outcomes. As events occur, the Company assesses the potential liability related to any pending litigation, claims or other legal actions and adjusts its estimates accordingly. Such adjustments could materially impact its financial statements.
Foreign Currency Translation
The Company translates the assets and liabilities of its foreign subsidiaries stated in local functional currencies to US dollars at the rates of exchange in effect at the end of the period. Revenues and expenses are translated using average rates of exchange in effect during the period. Intangible assets and goodwill are generally recorded in the local currency which is the functional currency of our subsidiaries located in China. As a result, the carrying values of intangible assets and goodwill may fluctuate with the value of the renminbi as compared to the US dollar. Gains and losses from the translation of financial statements denominated in foreign currencies are included as a separate component of accumulated other comprehensive income in the statement of stockholders’ equity.
The Company records foreign currency transactions at the exchange rate prevailing at the date of the transaction with resultant gains and losses being included in results of operations. Foreign currency transaction gains and losses have not been significant for any period presented.
Revenue Recognition
The Company recognizes revenue when persuasive evidence of an arrangement exists, services have been rendered or delivery has occurred, the price to the buyer is fixed or determinable and collectability is reasonably assured.
77
Product Revenue. The Company earns product revenue from selling manufactured ZADAXIN product at the time of delivery. Sales of ZADAXIN to importing agents or distributors are recognized at time of shipment when title to the product is transferred to them. The Company also earns product revenue from purchasing medical products from pharmaceutical companies and selling them directly to importers or distributors. Sales of Pfizer products, from the date of the acquisition of the NovaMed subsidiary in April of 2011 into October 2012, were based on the “sell-through” method as our distribution arrangement for these products allowed for payment terms dependent on when the distributor sold the product. The Company did not maintain information on the timing of “sell-through” of the Pfizer products by the distributor through this period, therefore, the Company applied the cash receipts approach for the application of the “sell-through” method as it was the most reliable information available. Accordingly, during this period of time, revenue for sales of the Pfizer products was recognized upon receipt of cash from the distributor. Effective as of the fourth quarter of 2012, the Company entered into a new agreement with the distributor which clarified the “sell-in” method of revenue recognition to provide consistency with the Company’s policy regarding revenue recognition on a prospective basis. All other product sales are recognized on the “sell-in” method, or when the medical products have been delivered to the importers or distributors.
Promotion Services Revenue. The Company recognizes promotion services revenue after designated medical products are delivered to the distributors as specified in the promotional contract, which marks the period when marketing and promotional services have been rendered, and when all of the above revenue recognition criteria are met.
Sales Tax and Surcharge Expense
Sales taxes and surcharge costs are expensed as incurred and are included in sales and marketing expense. The Company is generally subject to a 5% business tax and surcharge for its promotion services of medical products under the relevant taxation laws in China. Sales tax and surcharge costs amounted to approximately $3.8 million, $2.6 million and $1.0 million, for the years ended December 31, 2012, 2011, and 2010, respectively.
Research and Development Expenses
Research and development costs are expensed as incurred. These costs consist primarily of salaries and other personnel-related expenses, including associated stock-based compensation, facility-related expenses, depreciation of facilities and equipment, license-related fees, and services performed by clinical research organizations and research institutions and other outside service providers.
Expenses related to clinical trials generally are accrued based on estimates of work performed or the level of patient enrollment and activities according to the protocols and agreements. The Company monitors planned protocols, work performed, patient enrollment levels and related activities to the extent possible and adjusts estimates accordingly. Nonrefundable advance payments for research and development goods or services are recognized as expense as the related goods are delivered or the related services are provided.
Shipping and Handling Costs
Shipping and handling costs incurred for inventory purchases and product shipments are included in cost of product sales for all periods presented.
Advertising Expenses
Advertising costs are expensed as incurred and are included in sales and marketing expenses for all periods presented. Advertising expenses for each of the years ended December 31, 2012, 2011, and 2010 were $0.2 million.
78
Stock-Based Compensation
The Company records stock-based compensation costs relating to share-based payment transactions, including stock options, restricted stock units (“RSUs”) and employee stock purchase plans. Stock-based compensation expense for stock options and the employee stock purchase plan is estimated at the date of grant based on the fair value of the award using the Black-Scholes option-pricing model. Stock-based compensation expense for RSUs is estimated at the date of grant based on the number of shares granted and the quoted price of the Company’s common stock on the grant date. Stock-based compensation expense values are recognized as expense on a straight-line basis over the requisite service period, net of estimated forfeitures. The stock-based compensation costs that are ultimately expected to vest are recognized as expense ratably (as the awards vest) over the requisite service period, which is generally one or four years for stock options and RSUs and three months for the employee stock purchase plan. The Company estimates pre-vesting forfeitures at the time of grant by analyzing historical data and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. The total expense recognized over the vesting period will only be for those awards that ultimately vest.
Certain target-stock-price-based options were valued using the Monte Carlo simulation options pricing model and recognized to expense over the service periods for each of the vesting portions of these awards which were six or eight years. Refer also to Note 14, “Stockholders’ Equity,” in the notes to consolidated financial statements for further information regarding stock-based compensation.
Income Taxes
Income taxes are accounted for under the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates that will be in effect when these differences reverse. The Company provides a valuation allowance against net deferred tax assets if, based upon the available evidence, it is more-likely-than-not that the deferred tax assets will not be realized. When the Company establishes or reduces the valuation allowance against its deferred tax assets, its provision for income taxes will increase or decrease, respectively, in the period such determination is made. The Company’s policy is to recognize interest and penalties related to the estimated obligations for tax positions as a component of income tax expense. The amount of accrued interest related to tax positions taken on our tax returns and included in accrued and other current liabilities was $0.8 million and $0.4 million at December 31, 2012 and 2011, respectively.
The Company records liabilities related to uncertain tax positions in accordance with the guidance that clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements by prescribing a minimum recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The Company does not believe any such uncertain tax positions currently pending will have a material adverse effect on its consolidated financial statements, although an adverse resolution of one or more of these uncertain tax positions in any period could have a material impact on the results of operations for that period.
Net Income Per Share
Basic net income per share has been computed by dividing net income by the weighted-average number of shares of common stock outstanding for the period. Diluted net income per share is computed by dividing net income by the weighted-average number of common equivalent shares outstanding for the period. Diluted net income per share includes any dilutive impact from outstanding stock options and the employee stock purchase plan using the treasury stock method.
79
The following is a reconciliation of the numerator and denominators of the basic and diluted net income per share computations for the year ended December 31(in thousands, except per share amounts):
| | | | | | | | | | | | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Numerator: | | | | | | | | | | | | |
Net income | | $ | 9,620 | | | $ | 28,122 | | | $ | 21,081 | |
Denominator: | | | | | | | | | | | | |
Weighted-average shares outstanding used to compute basic net income per share | | | 56,637 | | | | 55,110 | | | | 47,624 | |
Effect of dilutive securities | | | 1,846 | | | | 2,277 | | | | 1,790 | |
| | | | | | | | | | | | |
Weighted-average shares outstanding used to compute diluted net income per share | | | 58,483 | | | | 57,387 | | | | 49,414 | |
| | | | | | | | | | | | |
Basic net income per share | | $ | 0.17 | | | $ | 0.51 | | | $ | 0.44 | |
Diluted net income per share | | $ | 0.16 | | | $ | 0.49 | | | $ | 0.43 | |
For the years ended December 31, 2012 and 2011, shares of common stock outstanding decreased by approximately 4,709,000 and 781,000 shares, respectively, as a result of stock repurchases. For the years ended December 31, 2012, 2011, and 2010, shares of common stock outstanding increased by approximately 1,270,000, 2,288,000, and 730,000 shares, respectively, as a result of stock exercises. In addition, for the year ended December 31, 2011, shares of common stock outstanding increased by 8,298,110 shares due to the acquisition of NovaMed (refer to Note 9).
For the years ended December 31, 2012, 2011, and 2010, approximately 3,280,492, 3,030,664, and 2,872,513 shares, respectively, related to outstanding stock options were excluded from the calculation of diluted net income per share because their inclusion would have been anti-dilutive. In addition, for the years ended December 31, 2012, 2011, and 2010, 118,046, 35,068 and 155,000 shares, respectively, subject to market or performance conditions were excluded from the calculation of diluted net income per share because the performance or market criteria had not been met.
Segment Information
The Company operates in two segments (refer to Note 19).
Note 2 — Revision to Previously Issued Consolidated Financial Statements
The Company revised its consolidated financial statements for the year ended December 31, 2011. The revised financial information included in this 2012 Form 10-K has been identified as “restated.” Concurrent with the filing of this Form 10-K, the Company is also filing amended quarterly reports on Form 10-Q/A for each of the first, second and third quarters of 2012 to restate its consolidated financial statements therein and its consolidated financial statements for the second and third quarters, and related year to date periods of 2011, and the effects of such restatements are reflected in the items revised herein.
The restatements relate to accounting errors originating with the Company’s subsidiary in China, NovaMed, which was acquired on April 18, 2011. The accounting errors relate primarily to the following:
| | • | | The timing of revenue recognition for certain Pfizer products sold by one of the distributors of the Company’s subsidiary NovaMed. The Company’s policy is that all customers’ obligations to pay for product are final once product is delivered. However, the Company determined that there were various factors, including the override of certain controls, indicating that sales under NovaMed’s distribution arrangement for Pfizer products with such distributor from the date of acquisition of NovaMed through the third quarter of 2012 (the “Relevant Periods”) allowed for contingent payment terms dependent |
80
| | upon when that distributor sold the products. As a result of its review and evaluation of the matter, the Company believes that instead of recording revenue at the time of sale to that distributor (“sell-in” method), as previously reflected in the financial statements for the Relevant Periods, revenue under the arrangements in effect at the Company’s subsidiary NovaMed should have been recognized when the Company received payment for the products (“sell-through” or “cash receipts” method). Effective as of the fourth quarter of 2012, the Company entered into a new agreement with that distributor which clarified the “sell-in” method of revenue recognition to provide consistency with the Company’s policy regarding revenue recognition on a prospective basis. As of December 31, 2012, the Company had received payment for all revenues recognized under the “sell-through” method. |
| | • | | The recognition of previously unrecognized product return reserves for sales of Aggrastat sold by the Company’s subsidiary NovaMed prior to the date of acquisition. The Company has concluded a liability for expired product existed at the time of the NovaMed acquisition, related to pre-acquisition sales. |
The revisions had the impact of increasing the Company’s revenues by $3.0 million for the nine-months ended September 30, 2012, and increasing the Company’s net income by $2.0 million for the same period. Basic and diluted earnings per share increased by $0.04 and $0.03, respectively, for the nine-months ended September 30, 2012. As of September 30, 2012, accounts receivable decreased by $1.5 million, inventory increased by $1.3 million and goodwill related to the acquisition of NovaMed increased by $1.9 million. The revisions for the year ended December 31, 2011 had an impact of decreasing the Company’s revenues by $1.1 million and decreasing the Company’s net income by $0.3 million. Basic and diluted earnings per share decreased by $0.01. As of December 31, 2011, accounts receivable decreased by $3.8 million, inventory increased by $2.3 million and goodwill related to the acquisition of NovaMed increased by $1.9 million.
The revisions to the consolidated statements of cash flows did not have a material impact on any amounts previously reported for net cash from operating activities, investing activities, or financing activities and as a result, there was no net impact to net change in cash and equivalents for any previously reported periods.
The following table presents the impact of the revisions on the Company’s previously issued consolidated balance sheet as of December 31, 2011(in thousands):
| | | | | | | | |
| | | December 31, 2011 | |
| | | As Reported | | | As Restated | |
ASSETS | | | | | | | | |
Accounts receivable, net | | $ | 42,226 | | | $ | 38,465 | (1) |
Inventories | | | 8,813 | | | | 11,141 | (1) |
Deferred tax assets | | | 1,732 | | | | 1,788 | |
Total current assets | | | 121,071 | | | | 119,694 | |
Goodwill | | | 31,973 | | | | 33,868 | (2) |
Total assets | | | 200,326 | | | | 200,844 | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Accrued and other current liabilities | | $ | 17,192 | | | $ | 18,357 | (2) |
Total current liabilities | | | 25,284 | | | | 26,449 | |
Deferred tax liabilities | | | 8,715 | | | | 8,407 | |
Accumulated other comprehensive income | | | 2,341 | | | | 2,344 | |
Accumulated deficit | | | (118,854 | ) | | | (119,196 | ) |
Total stockholders’ equity | | | 150,458 | | | | 150,119 | |
Total liabilities and stockholders’ equity | | | 200,326 | | | | 200,844 | |
| (1) | Accounts receivable decreased $3.8 million and inventory increased $2.3 million related to the Company changing from the “sell-in” method to the “sell-through” method of revenue recognition for certain Pfizer products sold by the Company. |
81
| (2) | Goodwill increased $1.0 million and accrued and other current liabilities increased $1.0 million related to the Company recording a liability for expired product that existed at the time of the NovaMed acquisition, related to pre-acquisition Aggrastat product sales. Goodwill also increased $0.9 million related to the Company changing from the “sell-in” method to the “sell-through” method of revenue recognition for certain Pfizer products sold by the Company. |
The following table presents the impact of the revisions on the Company’s previously issued consolidated statement of income for the year ended December 31, 2011 (in thousands):
| | | | | | | | |
| | | For the Year Ended
December 31, 2011 | |
| | | As Reported | | | As Restated | |
Net Revenues: | | | | | | | | |
Product sales, net | | $ | 113,027 | | | $ | 111,951 | (3) |
Promotion services | | | 20,614 | | | | 20,614 | |
| | | | | | | | |
Total net revenues | | | 133,641 | | | | 132,565 | |
Operating expenses: | | | | | | | | |
Cost of product sales | | | 20,013 | | | | 19,409 | (3) |
Sales and marketing | | | 48,855 | | | | 48,853 | (4) |
Amortization of acquired intangible assets,related to sales and marketing | | | 2,465 | | | | 2,465 | |
Research and development | | | 12,346 | | | | 12,346 | |
General and administrative | | | 24,032 | | | | 24,032 | |
Contingent consideration | | | (3,495 | ) | | | (3,495 | ) |
| | | | | | | | |
Total operating expenses | | | 104,216 | | | | 103,610 | |
Income from operations | | | 29,425 | | | | 28,955 | |
Non-operating income (expense): | | | | | | | | |
Interest and investment income | | | 71 | | | | 71 | |
Interest and investment expense | | | (213 | ) | | | (213 | ) |
Other (expense) income, net | | | (21 | ) | | | (21 | ) |
| | | | | | | | |
Income before provision for income tax | | | 29,262 | | | | 28,792 | |
Provision for income tax | | | 798 | | | | 670 | |
| | | | | | | | |
Net income | | $ | 28,464 | | | $ | 28,122 | |
| | | | | | | | |
Comprehensive income | | $ | 30,738 | | | $ | 30,399 | |
Basic net income per share | | $ | 0.52 | | | $ | 0.51 | |
Diluted net income per share | | $ | 0.50 | | | $ | 0.49 | |
Basic shares outstanding | | | 55,110 | | | | 55,110 | |
Diluted shares outstanding | | | 57,387 | | | | 57,387 | |
| (3) | Revenue decreased $1.1 million and cost of product sales decreased $0.6 million mainly related to the Company changing from the “sell-in” method to the “sell-through” method of revenue recognition for certain Pfizer products sold by the Company’s NovaMed subsidiary. |
| (4) | Sales and marketing expense decreased $0.3 million related to the payment of the liability for expired Aggrastat product, offset by a liability for sales commissions. |
The following table presents the impact of the revisions on the Company’s previously issued consolidated statement of cash flows for the year ended December 31, 2011 (in thousands). The restatement had no effect on
82
net cash provided by operating activities, net cash used in investing activities, net cash (used in) provided by financing activities or on the net increase in cash and cash equivalents.
| | | | | | | | |
| | | For the Year Ended
December 31, 2011 | |
| | | As Reported | | | As Restated | |
Cash Flows From Operating activities: | | | | | | | | |
Net income | | $ | 28,464 | | | $ | 28,122 | |
Deferred taxes, net | | | (1,241 | ) | | | (1,602 | ) |
Accounts receivable, net | | | (3,907 | ) | | | (2,730 | )(5) |
Inventories | | | (1,665 | ) | | | (2,269 | )(5) |
Accrued and other current liabilities | | | 3,550 | | | | 3,680 | |
| (5) | Changes to net cash used in accounts receivable and inventory mainly related to the Company changing from the “sell-in” method to the “sell-through” method of revenue recognition for certain Pfizer products sold by the Company. |
Note 3 — Available for Sale Investments
The following is a summary of available-for-sale investments(in thousands):
| | | | | | | | | | | | | | | | |
| | | December 31, 2012 | |
| | | Amortized
Cost | | | Unrealized
Gains | | | Unrealized
Losses for
More Than
12 Months | | | Estimated
Fair
Value | |
Certificate of deposit | | $ | 75 | | | $ | — | | | $ | — | | | $ | 75 | |
Money market funds | | | 43,505 | | | | — | | | | — | | | | 43,505 | |
Restricted Italian state bonds maturing in 2013 | | | 451 | | | | — | | | | (67 | ) | | | 384 | |
| | | | | | | | | | | | | | | | |
Total available-for-sale investments | | $ | 44,031 | | | $ | — | | | $ | (67 | ) | | $ | 43,964 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | December 31, 2011 | |
| | | Amortized
Cost | | | Unrealized
Gains | | | Unrealized
Losses for
More Than
12 Months | | | Estimated
Fair
Value | |
Money market funds | | $ | 31,849 | | | $ | — | | | $ | — | | | $ | 31,849 | |
Restricted Italian state bonds maturing in 2013 | | | 450 | | | | — | | | | (86 | ) | | | 364 | |
| | | | | | | | | | | | | | | | |
Total available-for-sale investments | | $ | 32,299 | | | $ | — | | | $ | (86 | ) | | $ | 32,213 | |
| | | | | | | | | | | | | | | | |
The cost of securities sold is based on the specific identification method.
The Company’s restricted Italian state bonds secure its Italian value added tax filing arrangements. The unrealized losses on the bonds mainly relate to loss on foreign currency translation. The Company has concluded that it has the ability and it is more likely than not that it will hold its restricted Italian state bond investments until maturity or recovery of its cost basis.
The Company’s certificate of deposit for $0.1 million at December 31, 2012 secures the Company’s letter of credit required under its European value added tax filing arrangements.
The Company’s money market funds at December 31, 2012 include $2.3 million of cash in a restricted account to secure a letter of credit associated with its loan agreement with Shanghai Pudong Development Bank Co. Ltd. Refer also to Note 11 for further information regarding the Company’s loan agreement.
83
Note 4 — Fair Value Measurements
The following table represents the Company’s fair value hierarchy for its financial assets (available-for-sale investments) and liability measured at fair value on a recurring basis(in thousands):
| | | | | | | | | | | | | | | | |
| | | Fair Value Measurements at December 31, 2012 Using | |
Description | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | | Significant
Other
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | | | Balance as of
December 31, 2012 | |
Assets: | | | | | | | | | | | | | | | | |
Certificate of deposit | | $ | — | | | $ | 75 | | | $ | — | | | $ | 75 | |
Money market funds | | | 43,505 | | | | — | | | | — | | | | 43,505 | |
Restricted Italian state bonds | | | 384 | | | | — | | | | — | | | | 384 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 43,889 | | | $ | 75 | | | $ | — | | | $ | 43,964 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | Fair Value Measurements at December 31, 2011 Using | |
Description | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | | Significant
Other
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | | | Balance as of
December 31, 2011 | |
Assets: | | | | | | | | | | | | | | | | |
Money market funds | | $ | 31,849 | | | $ | — | | | $ | — | | | $ | 31,849 | |
Restricted Italian state bonds | | | 364 | | | | — | | | | — | | | | 364 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 32,213 | | | $ | — | | | $ | — | | | $ | 32,213 | |
| | | | | | | | | | | | | | | | |
Liability: | | | | | | | | | | | | | | | | |
Contingent consideration | | $ | — | | | $ | — | | | $ | 15,400 | | | $ | 15,400 | |
| | | | | | | | | | | | | | | | |
Total | | $ | — | | | $ | — | | | $ | 15,400 | | | $ | 15,400 | |
| | | | | | | | | | | | | | | | |
The following table provides a summary of changes in fair value of the Company’s level 3 financial assets and liability during fiscal 2010, 2011, and 2012(in thousands):
| | | | | | | | | | | | |
| | | Auction Rate
Securities | | | Put
Option | | | Contingent
Consideration | |
Balance at December 31, 2009 | | $ | 1,588 | | | $ | 202 | | | $ | — | |
Proceeds from sales | | | (1,800 | ) | | | — | | | | — | |
Total realized gain (loss) included in other income (expense) | | | 212 | | | | (202 | ) | | | — | |
| | | | | | | | | | | | |
Balance at December 31, 2010 | | | — | | | | — | | | | — | |
Fair value at acquisition date | | | — | | | | — | | | | 18,870 | |
Change in the estimated fair value of the contingent consideration liability | | | — | | | | — | | | | (3,495 | ) |
Translation adjustments | | | — | | | | — | | | | 25 | |
| | | | | | | | | | | | |
Balance at December 31, 2011 | | | — | | | | — | | | | 15,400 | |
Change in the estimated fair value of the contingent consideration liability | | | — | | | | — | | | | (15,422 | ) |
Translation adjustments | | | — | | | | — | | | | 22 | |
| | | | | | | | | | | | |
Balance at December 31, 2012 | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
84
Contingent Consideration
As part of the acquisition of NovaMed, the Company would have been required to pay up to an additional $43.0 million in earn-out payments upon the successful achievement of revenue and earnings targets for the 2011 and 2012 fiscal years (the “earn-out” or “contingent consideration”). The measurement period for the contingent consideration ended on December 31, 2012 and the payout of the contingent consideration was determined to be zero. The terms governing the earn-out and information regarding the estimated fair value of the earn-out are disclosed in Note 9,Acquistion.
Auction Rate Securities and Put Option
In November 2008, the Company accepted an Auction Rate Securities Rights Offer (the “Settlement Agreement”) from UBS AG under which, in return for a general release of claims and the grant of a right to UBS AG to purchase the Company’s ARS at any time for full par value, the Company received the right to require UBS AG to purchase the Company’s ARS beginning in June 2010 (the “Rights”). By entering into the Settlement Agreement, the Company (1) received the right (the “Put Option”) to sell these auction rate securities back to the investment firm at par, at its sole discretion, anytime during the period from June 30, 2010 through July 2, 2012, and (2) gave the investment firm the right to purchase these auction rate securities or sell them on the Company’s behalf at par anytime after the execution of the Settlement Agreement through July 2, 2012. On June 30, 2010, the Company exercised the Put Option and sold its ARS back to the investment firm.
Note 5 — Inventories
Inventories consisted of the following(in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | |
Raw materials | | $ | 3,184 | | | $ | 1,797 | |
Work in progress | | | 904 | | | | 84 | |
Finished goods | | | 6,336 | | | | 9,260 | |
| | | | | | | | |
| | $ | 10,424 | | | $ | 11,141 | |
| | | | | | | | |
As of December 31, 2012 and 2011, the Company had $2.3 million in inventory held at distributors related to products sold by its NovaMed subsidiary.
Note 6 — Property and Equipment
Property and equipment consisted of the following(in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2012 | | | 2011 | |
Office equipment | | $ | 1,379 | | | $ | 1,098 | |
Leasehold improvements | | | 1,647 | | | | 922 | |
Office furniture and fixtures | | | 606 | | | | 617 | |
Software | | | 204 | | | | 198 | |
Vehicle | | | 68 | | | | 68 | |
| | | | | | | | |
| | | 3,904 | | | | 2,903 | |
Less accumulated depreciation | | | (2,527 | ) | | | (1,919 | ) |
| | | | | | | | |
Net property and equipment | | $ | 1,377 | | | $ | 984 | |
| | | | | | | | |
85
Depreciation expense was $0.6 million for the year ended December 31, 2012, and was $0.3 million for each of the years ended December 31, 2011 and 2010.
Note 7 — Net Intangible Assets
Net intangible assets consisted of the following(in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2012 | | | 2011 | |
Promotion and distribution contract rights | | $ | — | | | $ | 47,687 | |
Less accumulated amortization | | | — | | | | (2,502 | ) |
| | | | | | | | |
| | $ | — | | | $ | 45,185 | |
| | | | | | | | |
As part of the acquisition of NovaMed, the Company recorded intangible assets related to promotion and distribution contract rights that were included in the Company’s China segment. During the third quarter of 2012, the Company identified impairment indicators related to the intangible assets. The Company determined that the undiscounted cash flows estimated to be generated by the intangible assets were less than the carrying amounts. The Company further performed a discounted cash flow analysis related to the intangible assets and determined that a full impairment should be recorded as the estimated fair value was determined to be zero. As a result, the Company recognized a non-cash impairment loss of approximately $42.7 million on its consolidated statement of income for the year ended December 31, 2012. Fair value measurements are based on a three-tier hierarchy that prioritizes the inputs used to measure fair value (see Note 1). The significant Level 3 unobservable inputs used in the fair value measurement of the intangible assets were estimates of projected revenues and earnings, a discount rate of approximately 20%, and assumptions regarding the probability of renewal of the customer contracts. Significant changes in the estimated revenues and earnings would have resulted in adjustments in the fair value measurement. A change in the renewal probability rates of the customer contracts would have also resulted in a significant change in the fair value measurement.
Acquired promotion and distribution contract intangible assets were being amortized on a straight-line basis over 13.5 years, based on their estimated useful life. Amortization expense was $2.6 million, $2.5 million, and $0 for the years ended December 31, 2012, 2011, and 2010, respectively. No further amortization expense will be recorded in future periods related to the acquired promotion and distribution contract intangible assets.
Note 8 — Accrued Liabilities
The following is a summary of accrued liabilities(in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | |
Accrued sales and marketing expenses | | $ | 9,051 | | | $ | 7,124 | |
Accrued taxes, tax reserves and interest | | | 4,410 | | | | 3,500 | |
Accrued compensation and benefits | | | 3,232 | | | | 4,409 | |
Accrued professional fees | | | 1,570 | | | | 666 | |
Other | | | 3,164 | | | | 2,658 | |
| | | | | | | | |
| | $ | 21,427 | | | $ | 18,357 | |
| | | | | | | | |
Note 9 — Acquisition
On April 18, 2011, SciClone acquired all the outstanding shares of NovaMed pursuant to the terms of a Share Purchase Agreement (the “Agreement”) dated April 18, 2011 between SciClone, NovaMed, the shareholders of NovaMed and SciClone Pharmaceuticals Hong Kong Limited, a wholly-owned subsidiary of
86
SciClone. The Company acquired NovaMed to bring additional broad sales and marketing, as well as regulatory and extensive business capabilities and pharmaceutical assets, on the market as well as in the regulatory approval stage, to its growing and profitable China focused specialty pharmaceutical business. Under the terms of the Agreement, the purchase price is comprised of up-front payments of approximately $24.6 million in cash, 8,298,110 shares of SciClone common stock and a contingent right to receive additional cash consideration of up to $43.0 million (the “earn out” or “contingent consideration”), based upon achievement of revenue and earnings targets for the 2011 and 2012 fiscal years.
Under the Agreement, the earn-out is based upon certain financial performance metrics, including a revenue-based formula and an adjusted EBITDA (earnings before interest, depreciation and taxes) based formula. The earn-out provisions provide that: (i) if cumulative revenue in China for legacy NovaMed products for the two fiscal years ending December 31, 2012 exceed $94.2 million, a cash payment ranging from $9.2 million to $11.5 million will be paid, with the full amount payable if such revenue is $117.8 million or more; and (ii) if adjusted EBITDA for the two year period ending December 31, 2012 exceeds $91.8 million, a cash payment from $17.2 million to $21.5 million will be paid with the full amount payable if such adjusted EBITDA is $137.8 million or more. Adjusted EBITDA is defined in the Agreement to exclude certain expenses which are not generally related to operating results in China, including SciClone’s US research and development expense, certain share-based compensation, license fees paid by SciClone for new products, certain legal and advisory fees related to the Agreement or to change-in-control transactions, and certain fees and expenses, including legal fees and governmental fines or settlements paid with respect to the pending formal, non-public investigation being conducted by the US Securities and Exchange Commission (“SEC”).
The earn-out provisions were subject to a number of adjustments and acceleration provisions. The total earn-out payments described above could have been increased by $10.0 million (a total maximum contingent cash consideration of $43.0 million) or reduced by $10.0 million, depending upon whether the Company was able to achieve targets relating to the renewal of the Depakine services agreement with Sanofi. The earn-out payments would be due 20 business days after completion of the Company’s audit for the fiscal year ending December 31, 2012. The earn-out provisions are subject to various limitations and conditions specified in the Agreement.
The fair value of the earn-out was re-measured each period, and changes in the fair value were recorded to “contingent consideration” in operating expenses. As of December 31, 2012, the earn-out was determined to be zero. Through September 30, 2012, the Company used the assistance of a third-party valuation expert to estimate the fair value of the contingent consideration using a Monte Carlo simulation model. The estimated fair value of the contingent consideration was subject to fluctuations as a result of adjustments to certain performance metric projections used to estimate the fair value. As of December 31, 2011, the significant unobservable inputs used in the fair value measurement of the contingent consideration were revenue volatility of 40%; revenue discount rate of 20%; risk free rate of 0.15%; earn-out discount rate, including counterparty risk of 5%; estimates of projected revenues and earnings before taxes; and other assumptions regarding customer conditions, and employment termination considerations. Significant increases (decreases) in the estimated revenues, earnings before taxes, customer conditions, and revenue volatility would have resulted in a significantly higher (lower) fair value measurement. Generally, an increase (decrease) in the assumption used for revenue discount rate was accompanied by a decrease (increase) in the fair value of the contingent consideration.
The Company initially recorded $18.9 million as the estimated fair value of the contingent consideration. The fair value of the contingent consideration was remeasured each quarter, and changes to the fair value were recorded to contingent consideration expense or gain. As of December 31, 2012 and 2011, the Company estimated the fair value of the contingent consideration to be $0 and $15.4 million, resulting in a non-cash gain of $15.4 million and $3.5 million for the years ended December 31, 2012 and 2011, respectively. The significant reduction in the valuation of the contingent consideration expense for 2012 was primarily due to revenue and EBITDA targets not being achieved and to the reduced probability of renewal relating to NovaMed’s product distribution agreements, including, in particular, the target of renewing the Depakine services agreement with Sanofi for a five-year term. The Depakine services agreement was extended through December 31, 2013, a term less than five years.
87
The total purchase price of NovaMed was approximately $75.0 million, comprised as follows(in thousands):
| | | | |
Cash consideration | | $ | 24,578 | |
SciClone shares of common stock at estimated fair value on closing date | | | 31,530 | |
Contingent consideration (earn-out) at estimated fair value | | | 18,870 | |
| | | | |
Total purchase price | | $ | 74,978 | |
| | | | |
Under the purchase method of accounting, the total acquisition-date fair value of the assets and liabilities are recognized as of the closing date, and the excess of the consideration transferred over the acquisition date fair value of net assets acquired is recorded as goodwill. The total purchase price of the net assets acquired and included in the Company’s consolidated balance sheet is as follows (in thousands and restated):
| | | | |
Cash | | $ | 3,322 | |
Accounts receivable | | | 4,827 | |
Inventory | | | 1,724 | |
Prepaid and other assets | | | 486 | |
Property and equipment | | | 80 | |
Deferred tax assets | | | 1,389 | |
Intangible assets — promotion and distribution contract rights | | | 46,310 | |
Goodwill | | | 32,843 | |
Deferred tax liabilities | | | (9,352 | ) |
Liabilities assumed | | | (6,651 | ) |
| | | | |
Total net assets acquired (Restated) | | $ | 74,978 | |
| | | | |
The fair value of the acquired promotion and distribution contract intangible assets was estimated using the income approach. The income approach uses valuation techniques to convert future amounts to a single present amount (discounted). The Company’s measurement is based on the value indicated by current market expectations about those future amounts. The fair value considered the Company’s estimates at the time of the acquisition of future incremental earnings that could be achieved by the promotion and distribution contract intangible assets, and included estimated acquired useful lives of approximately 13.5 years and a discount rate of 29%.
Goodwill is calculated as the excess of the consideration transferred over the net assets recognized and represents the future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Specifically, the goodwill recorded as part of the acquisition of NovaMed includes benefits that the Company believes will result from combining the operations of NovaMed with the operations of SciClone and any intangible assets that do not qualify for separate recognition, as well as future, yet unidentified products. Goodwill is not amortized and is not deductible for tax purposes.
The following table represents the changes in goodwill for the years ended December 31, 2012 and 2011 (in thousands and restated):
| | | | |
Balance at December 31, 2010 | | $ | — | |
Goodwill for the acquisition of NovaMed | | | 32,843 | |
Translation adjustments | | | 1,025 | |
| | | | |
Balance at December 31, 2011 (restated) | | $ | 33,868 | |
Translation adjustments | | | 445 | |
| | | | |
Balance at December 31, 2012 | | $ | 34,313 | |
| | | | |
88
Deferred tax liabilities as of December 31, 2011, reflected non-deductible amortization expenses associated with the promotion and distribution contract intangible assets recognized as part of the acquisition.
The results of operations of NovaMed are included in the Company’s financial statements from April 18, 2011, the date of acquisition. The following summary, prepared on an unaudited pro-forma basis, reflects consolidated results of operations for the year ended December 31, 2011, and 2010, assuming NovaMed had been acquired on January 1, 2010. The pro forma results of continuing operations are prepared for comparative purposes only and do not necessarily reflect the results that would have occurred had the acquisition occurred at the beginning of the years presented or the results which may occur in the future. (in thousands, except per share data):
| | | | | | | | |
| | | For the Year Ended
December 31, | |
| | | 2011
Restated
(Note 2) | | | 2010 | |
Total revenues | | $ | 140,604 | | | $ | 116,520 | |
Net income | | $ | 24,849 | | | $ | 20,021 | |
Basic net income per share | | $ | 0.45 | | | $ | 0.42 | |
Diluted net income per share | | $ | 0.43 | | | $ | 0.41 | |
The following table presents information for NovaMed that is included in SciClone’s consolidated statement of income from April 18, 2011 through December 31, 2011 and 2012(in thousands):
| | | | | | | | |
| | | For the Year Ended
December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | |
Total revenues | | $ | 44,100 | | | $ | 27,852 | |
Net loss | | $ | (40,258 | ) | | $ | (3,835 | ) |
For the year ended December 31, 2011, the Company recorded acquisition-related transaction costs of $3.8 million which were included in general and administrative expense in the consolidated statements of income.
Note 10 — Commitments
Leases
In May 2007, the Company entered into a non-cancelable operating lease agreement for its corporate headquarters (“the Lease”) effective from July 1, 2007 through June 30, 2014, with an option to renew for an additional five year period. In September 2008, the Company entered into an amendment to the Lease for additional office space (“the Expansion Agreement”) that expires on June 30, 2014, with an option to renew for an additional five year period. Both the Lease and Expansion Agreements contain rent escalations of approximately 4% and 6% per year, respectively. The Company is recognizing the rental expense on a straight-line basis over the lease terms. Under the terms of the Lease and the Expansion Agreements, the Company was provided allowances in the amounts of approximately $0.2 million and $0.5 million, respectively, towards the cost of its leasehold improvements and as an incentive to rent, respectively. The Company has recorded these allowances as deferred rent which is being amortized over the lease terms as a reduction of rent expense. The leases require the Company to pay insurance and taxes and its pro-rata share of operating expenses.
In October 2011, the Company entered into two non-cancelable operating lease agreements for its primary office space in China (“the China Lease”) for fixed lease terms from October 15, 2011 through October 14, 2014, with options to renew. The Company will recognize the rental expense on a straight-line basis over the lease term. The leases require the Company to pay insurance and its pro-rata share of operating expenses.
89
The Company also leases other office facilities and equipment outside the US under non-cancelable operating lease agreements and subleases certain office facilities to a third party. Rent expense for the years ended December 31, 2012, 2011, and 2010 was $2.9 million, $2.1 million, and $1.6 million, respectively. Future minimum lease payments and sublease rental income under non-cancelable facility and equipment operating lease agreements as of December 31, 2012, were as follows(in thousands):
| | | | | | | | | | | | |
Year ended: | | Minimum Lease
Payments | | | Sublease Rental
Income | | | Net Minimum
Lease Payments | |
2013 | | $ | 3,291 | | | $ | 87 | | | $ | 3,204 | |
2014 | | | 1,619 | | | | 37 | | | | 1,582 | |
2015 | | | 242 | | | | — | | | | 242 | |
2016 | | | 7 | | | | — | | | | 7 | |
| | | | | | | | | | | | |
| | $ | 5,159 | | | $ | 124 | | | $ | 5,035 | |
| | | | | | | | | | | | |
Note 11 — Credit Facilities
Line of Credit
Our loan and security agreement with Silicon Valley Bank (“SVB”) (“the Credit Facility”) for a financing facility up to $15 million expired on October 1, 2012. The Credit Facility bore interest on borrowed funds at the bank’s prime rate plus 1.25% (5.25% in 2012) on outstanding balances.
Loan Agreement
In August 2012, the Company’s subsidiary, NovaMed Pharmaceuticals (Shanghai) Co. Ltd., entered into a loan agreement with Shanghai Pudong Development Bank Co. Ltd. for 12.5 million renminbi (approximately $2.0 million USD). As of December 31, 2012, 9.0 million renminbi (approximately $1.4 million USD) in borrowings were outstanding on the loan. The loan is secured by a $2.3 million letter of credit. The Company is required to maintain $2.3 million of cash in a restricted bank account to secure the letter of credit. The loan bears interest on borrowed funds at 7.5% and expires August 29, 2013. Any amounts borrowed must be repaid by the expiration date.
Note 12 — Reduction in Workforce
In March 2012, the Company implemented a reduction in its workforce of 11 full-time employees, primarily in research and development that were included in the Company’s Rest of the World (including the US) segment. The restructuring follows the discontinuation of the Company’s SCV-07 phase 2b clinical trial. The reduction in workforce resulted in severance-related charges of approximately $1.1 million, of which $0.1 million and $1.0 million was recognized in general and administrative and research and development expense, respectively, in the consolidated statement of income for year ended December 31, 2012. As of December 31, 2012, the Company had paid $0.9 million and had accrued $0.2 million of the severance-related charges. The Company had substantially completed its restructuring activities as of December 31, 2012.
Note 13 — Income Taxes
The Company recorded income tax (benefit) expense of ($3.3) million, $0.7 million, and $2.2 million for the years ended December 31, 2012, 2011, and 2010, respectively, related to its operations in China. The Company’s statutory tax rate in China was 25% in 2012, 24-25% in 2011, and 22% for the year ended 2010. The Company has not recorded any US federal or state income tax expense for the years ended December 31, 2012, 2011, and 2010. Undistributed earnings of the Company’s foreign subsidiaries that are considered to be permanently invested outside the US and for which no US taxes have been provided amounted to approximately $45.0 million at December 31, 2012. Upon distribution of those earnings, the Company may be subject to US federal and state income taxes, although determining the amount is not practical as it is dependent on the amount of US tax losses at the time of the repatriation.
90
The domestic and foreign components of income (loss) before (benefit) provision for tax for the years ended December 31 are as follows(in thousands):
| | | | | | | | | | | | | | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Domestic | | $ | (11,541 | ) | | $ | (17,939 | ) | | | | $ | (19,315 | ) |
Foreign | | | 17,813 | | | | 46,731 | | | | | | 42,572 | |
| | | | | | | | | | | | | | |
Pre-tax income | | $ | 6,272 | | | $ | 28,792 | | | | | $ | 23,257 | |
| | | | | | | | | | | | | | |
A reconciliation of the statutory federal income tax rate of 34% to the actual tax rate for the years ended December 31 is as follows(in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Tax at federal statutory rate | | $ | 2,132 | | | $ | 9,789 | | | $ | 7,907 | |
Foreign income tax at different rates | | | (10,405 | ) | | | (16,137 | ) | | | (13,085 | ) |
Taxable dividend from foreign subsidiary | | | 11,900 | | | | 10,506 | | | | 6,724 | |
Uncertain tax positions accrual | | | 999 | | | | 925 | | | | 795 | |
Change in valuation allowance | | | (7,945 | ) | | | (4,674 | ) | | | (318 | ) |
Stock-based compensation | | | (41 | ) | | | 30 | | | | 53 | |
Non deductible expenses | | | 11 | | | | 237 | | | | 350 | |
Other | | | 1 | | | | (6 | ) | | | (250 | ) |
| | | | | | | | | | | | |
Income tax (benefit) expense | | $ | (3,348 | ) | | $ | 670 | | | $ | 2,176 | |
| | | | | | | | | | | | |
The (benefit) provision for income taxes for the years ended December 31 consisted of the following (in thousands):
| | | | | | | | | | | | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
Federal | | $ | — | | | $ | (5 | ) | | $ | (9 | ) |
State | | | 1 | | | | 1 | | | | 1 | |
Foreign | | | 3,445 | | | | 2,259 | | | | 2,184 | |
| | | | | | | | | | | | |
Total current | | | 3,446 | | | | 2,255 | | | | 2,176 | |
Federal | | | — | | | | — | | | | — | |
State | | | — | | | | — | | | | — | |
Foreign | | | (6,794 | ) | | | (1,585 | ) | | | — | |
| | | | | | | | | | | | |
Total deferred | | | (6,794 | ) | | | (1,585 | ) | | | — | |
| | | | | | | | | | | | |
Income tax (benefit) expense | | $ | (3,348 | ) | | $ | 670 | | | $ | 2,176 | |
| | | | | | | | | | | | |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
91
Significant components of the Company’s deferred tax assets and liabilities at December 31 are as follows (in thousands):
| | | | | | | | |
| | | 2012 | | | 2011
Restated
(Note 2) | |
Deferred tax assets: | | | | | | | | |
Net operating loss carryforwards | | $ | 28,625 | | | $ | 37,501 | |
Research and development credit carryforwards | | | 10,143 | | | | 10,600 | |
Intangibles | | | 388 | | | | — | |
Other | | | 4,627 | | | | 5,455 | |
| | | | | | | | |
Gross deferred tax assets | | | 43,783 | | | | 53,556 | |
Valuation allowance | | | (42,932 | ) | | | (50,606 | ) |
| | | | | | | | |
Total deferred tax assets | | | 851 | | | | 2,950 | |
| | | | | | | | |
Deferred tax liabilities: | | | | | | | | |
Intangibles | | | — | | | | (9,569 | ) |
Other | | | (635 | ) | | | — | |
| | | | | | | | |
Total deferred tax liabilities | | | (635 | ) | | | (9,569 | ) |
| | | | | | | | |
Net deferred tax liabilities | | $ | 216 | | | $ | (6,619 | ) |
| | | | | | | | |
Realization of deferred tax assets is dependent upon the Company generating future taxable income, the timing and amount of which are uncertain. Accordingly, the deferred tax assets have been largely offset by a valuation allowance. The valuation allowance decreased by approximately $7.7 million, $4.9 million, and $0.7 million, in the years ended December 31, 2012, 2011, and 2010, respectively.
At December 31, 2012, the Company had federal net operating loss carryforwards of approximately $87.4 million that expire in the years 2020 through 2030, and federal research and development, orphan drug and investment tax credit carryforwards of approximately $11.7 million that expire in the years 2018 through 2031. At December 31, 2012, the Company has state net operating loss carryforwards of approximately $18.9 million that expire in the years 2028 through 2030, if not utilized, and state research and development tax credit carryforwards of approximately $2.1 million that do not expire. Approximately $4.2 million of the operating loss carryforwards relate to benefits associated with stock option deductions that, when recognized, will be credited directly to stockholders’ equity.
Utilization of the Company’s net operating loss and credit carryforwards may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such annual limitation could result in the expiration of the net operating loss and credit carryforwards before utilization.
As of December 31, 2012, the unrecognized tax benefit was $6.1 million, of which $2.5 million, if recognized would affect the effective tax rate, and $3.6 million would be offset by a valuation allowance. A reconciliation of the beginning and ending amount of unrecognized tax benefit is as follows(in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2012 | | | 2011 | |
Balance beginning of period | | $ | 5,715 | | | $ | 4,225 | |
Tax positions related to current year: | | | | | | | | |
Additions for current year items | | | 1,050 | | | | 955 | |
Additions for prior year items | | | — | | | | 510 | |
Reductions for prior year items | | | (747 | ) | | | (33 | ) |
Changes for foreign currency translation | | | 35 | | | | 58 | |
| | | | | | | | |
Balance end of period | | $ | 6,053 | | | $ | 5,715 | |
| | | | | | | | |
92
Tax years 1995-2012 remain open to examination by the major taxing jurisdictions to which the Company is subject, although during 2012 the Internal Revenue Service concluded its examination of the Company’s 2008 and 2009 US federal tax returns with no additional tax assessments or proposed adjustments relating to taxable income for any years. Although the timing of further income tax examinations is uncertain, and the amounts ultimately paid, if any, upon resolution of the issues raised by the taxing authorities may differ materially from the amounts accrued for each year, we do not anticipate any material change to the amount of our unrecognized tax benefits over the next 12 months.
In January 2013, the United States enacted an extension of the federal research tax credit through December 31, 2013. As a result, the Company expects that its income tax provision for the first quarter of 2013 will include an increase in the gross deferred tax assets of $0.3 million reflecting the full year 2012 federal research tax credit. However, due to the Company’s full valuation allowance against its deferred tax assets it will not impact the Company’s provision as the increase in the gross deferred tax asset will be offset by the increase in the valuation allowance.
Note 14 — Stockholders’ Equity
Stock Award Plans
The Company’s 1995 Equity Incentive Plan has reserved 6,100,000 shares of common stock for issuance and permits the grant of incentive stock options, nonstatutory stock options and other forms of equity compensation. Although the 1995 Plan has expired, the outstanding stock options relating to it are fully valid.
The Company’s 2005 Equity Incentive Plan (the “2005 Plan”) has reserved 13,600,000 shares of common stock for issuance. The 2005 Plan permits the grant of incentive stock options, nonstatutory stock options, restricted stock units, performance shares and other forms of equity compensation. As of December 31, 2012, approximately 4,167,000 shares of common stock were available for future issuance under the 2005 Plan.
Under the 1995 and 2005 Plans, options are exercisable upon conditions determined by the board of directors and expire ten years from the date of grant. Options are generally granted at fair market value on the date of grant and vest over time, generally four years, or upon achievement of certain market and service conditions. SeeStock-Based Compensation.
The Company’s 1995 Nonemployee Director Stock Option Plan (the “1995 Director Plan”) had reserved 750,000 shares of common stock for issuance. The 1995 Director Plan permits the grant of nonqualified stock options to nonemployee directors. Although the 1995 Directors Plan has expired, the outstanding stock options relating to it are fully valid.
The Company’s 2004 Outside Directors Stock Option Plan (the “2004 Director Plan”) has reserved 1,765,000 shares of common stock for issuance. The 2004 Director Plan automatically grants nonqualified stock options to nonemployee directors upon their appointment or first election to the Company’s board of directors (“Initial Grant”) and annually upon their reelection to the board of directors at the Company’s Annual Meeting of Stockholders (“Annual Grant”). As of December 31, 2012, approximately 184,000 shares of common stock were available for future issuance under the 2004 Director Plan.
Under the 1995 and 2004 Director Plans, options are granted at fair market value on the date of grant and expire ten years from the date of grant. Initial Grants become exercisable in three equal annual installments beginning on the first anniversary of the date of grant, and Annual Grants become exercisable in twelve equal monthly installments from the date of grant, subject in each case to the director’s continuous service on the Company’s board of directors.
Certain stock option awards are subject to accelerated vesting if there is a change in control.
93
Stock-Based Compensation
The following table summarizes the stock-based compensation expenses included in our consolidated statements of income (in thousands):
| | | | | | | | | | | | |
| | | For the Year Ended
December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
Sales and marketing | | $ | 1,169 | | | $ | 779 | | | $ | 873 | |
Research and development | | | 212 | | | | 424 | | | | 257 | |
General and administrative | | | 2,516 | | | | 1,905 | | | | 1,094 | |
| | | | | | | | | | | | |
| | $ | 3,897 | | | $ | 3,108 | | | $ | 2,224 | |
| | | | | | | | | | | | |
Compensation cost capitalized in inventory was $0.1 million, $0.1 million, and $44,000, respectively, for the years ended December 31, 2012, 2011, and 2010. There has been no income tax benefit recognized in the income statement for share-based compensation arrangements.
Valuation Assumptions
The fair value granted under the Company’s stock option and ESPP plans is estimated on the date of grant using the Black-Scholes option valuation model and the single option approach with the following weighted-average assumptions for the years ended December 31:
| | | | | | | | | | | | |
| | �� | 2012 | | | 2011 | | | 2010 | |
Risk-free interest rate: | | | | | | | | | | | | |
Time-based stock options | | | 0.92 | % | | | 2.34 | % | | | 2.39 | % |
Performance-based stock options | | | 0.96 | | | | 1.96 | | | | 2.43 | |
ESPP | | | 0.09 | | | | 0.06 | | | | 0.15 | |
Volatility factor of the market price of our common stock: | | | | | | | | | | | | |
Time-based stock options | | | 63.79 | % | | | 65.16 | % | | | 72.50 | % |
Performance-based stock options | | | 63.66 | | | | 65.14 | | | | 72.66 | |
ESPP | | | 56.79 | | | | 50.67 | | | | 68.92 | |
Weighted-average expected life (years): | | | | | | | | | | | | |
Time-based stock options | | | 5.21 | | | | 5.28 | | | | 5.03 | |
Performance-based stock options | | | 5.22 | | | | 5.28 | | | | 5.02 | |
ESPP | | | 0.25 | | | | 0.25 | | | | 0.25 | |
Dividend yield | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % |
The risk-free interest rate is based on the US Treasury yield curve in effect at the time of grant. The expected dividend yield is based on the Company’s history and expectations of no dividend payouts. Expected volatility is based on the historical volatility of the Company’s stock. The expected term of options granted is derived from historical data on employee exercises and terminations.
94
Stock Options
The following table summarizes stock option activity as of December 31, 2012, and changes during the year then ended is presented below(in thousands, except per share and term amounts):
| | | | | | | | | | | | | | | | |
| | | Options Outstanding | |
| | | Number
of
Shares | | | Weighted-
Average
Exercise
Price Per
Share | | | Weighted-
Average
Remaining
Contractual
Term (Years) | | | Aggregate
Intrinsic
Value | |
Balance at December 31, 2009 | | | 7,939 | | | $ | 2.93 | | | | 6.34 | | | $ | 3,181 | |
Options forfeited | | | (1,223 | ) | | | 5.35 | | | | | | | | | |
Options granted | | | 1,475 | | | | 3.46 | | | | | | | | | |
Options exercised | | | (730 | ) | | | 1.87 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | 7,461 | | | $ | 2.74 | | | | 6.22 | | | $ | 12,034 | |
Options forfeited | | | (762 | ) | | | 3.84 | | | | | | | | | |
Options granted | | | 2,504 | | | | 5.06 | | | | | | | | | |
Options exercised | | | (2,288 | ) | | | 2.71 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | 6,915 | | | $ | 3.46 | | | | 6.98 | | | $ | 8,130 | |
Options forfeited | | | (1,536 | ) | | | 5.13 | | | | | | | | | |
Options granted | | | 1,889 | | | | 6.14 | | | | | | | | | |
Options exercised | | | (1,270 | ) | | | 2.66 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2012 | | | 5,998 | | | $ | 4.05 | | | | 6.06 | | | $ | 5,575 | |
| | | | | | | | | | | | | | | | |
Vested and expected to vest after | | | | | | | | | | | | | | | | |
December 31, 2012 | | | 5,673 | | | $ | 3.96 | | | | 5.92 | | | $ | 5,552 | |
Exercisable at December 31, 2012 | | | 3,648 | | | $ | 3.25 | | | | 4.66 | | | $ | 5,055 | |
In 2008, the Company granted to its chief executive officer target-stock-price-based options to purchase 600,000 and 300,000 shares of the Company’s common stock at an exercise price per share of $2.49 and $1.81, respectively. The options have a term of 10 years and shares of such option were to vest upon the Company’s common stock trading for at least 30 consecutive calendar days at or greater than a target closing stock price as reported on The NASDAQ Stock Market, of (a) $4.50 on or before June 2, 2009 for 150,000 shares, (b) $6.00 on or before June 2, 2010 for 150,000 shares, (c) $8.00 on or before June 2, 2011 for 150,000 shares, (d) $10.00 on or before June 2, 2012 for 150,000 shares, (e) $12.00 on or before June 2, 2013 for 150,000 shares, and (f) $14.00 on or before June 2, 2014 for 150,000 shares, each price as adjusted for stock dividends, stock splits or similar changes in the Company’s capital structure. These grants were considered awards with market and service conditions and compensation expense was recognized for these options as long as the service requirements were met, even if the market conditions were not reached. Because of the market conditions of the grants, the Monte Carlo simulation option pricing model was used to calculate the grant date fair value per share of $1.70 and $0.88, respectively, related to each of the six vesting portions of these awards with the assumptions of a risk-free interest rate of 5.10% and 3.88%, respectively, a volatility factor of 94% and 89.14%, respectively, dividend yield of 0%, and an expected life of 10 years. The related compensation costs were being expensed over the service periods for each of the six vesting portions of the awards.
In May 2010, the Company amended the target-stock-price-based options to purchase an aggregate of 750,000 shares of the Company’s common stock that were still subject to vesting that had been granted to its chief executive officer. The amendment modified the vesting provisions of the options such that 1/36th of the unvested stock options vest monthly over a three-year period, with initial vesting occurring on June 1, 2010. The fair value of the modified award was estimated using the Black-Scholes option valuation model with the assumptions of a risk-free rate of 2.28%, a volatility factor of 71.83%, a dividend yield of 0%, and an expected life of 5.07 years. The incremental compensation cost resulting from the modification and the remaining
95
unrecognized compensation expense from the original award are being recognized ratably over the three-year vesting period. The Company recorded expense of $0.4 million, $0.4 million, and $0.3 million for the years ended December 31, 2012, 2011, and 2010, respectively, related to these options.
The Company has granted certain performance-based options to purchase shares of the Company’s common stock at an exercise price equal to the closing price of a share of the Company’s common stock as of the grant date. The options will fully vest upon meeting a performance goal within an established time frame. If the performance goal is met for the option within the established time frame, the option generally has a ten-year term measured from the date of grant. If the performance goal is not met within the established time frame, the option expires in its entirety. The Company recognizes expense related to a performance-based option over the period of time the Company determines that it is probable that the performance goal will be achieved. If it is subsequently determined that the performance goal is not probable of achievement, the expense related to the performance-based option is reversed. For years ended December 31, 2012, 2011, and 2010, the Company recognized approximately $36,000, $0.2 million and $0.4 million, respectively, of expense related to performance-based options.
The weighted-average fair value of stock options granted for the years ended December 31, 2012, 2011, and 2010 was $3.36, $2.79, and $2.10, respectively. The intrinsic value of options at time of exercise was $4.5 million, $5.8 million, and $1.3 million, for the years ended December 31, 2012, 2011, and 2010, respectively. The estimated fair value of shares vested for the years ended December 31, 2012, 2011, and 2010 was $4.1 million, $3.3 million, and $1.9 million, respectively. As of December 31, 2012, unamortized compensation expense related to unvested options was approximately $5.0 million, net of forfeitures. The weighted average period over which compensation expense related to these options will be recognized is approximately 2.47 years. Cash received from stock option exercises was $3.4 million, $6.2 million, and $1.4 million for the years ended December 31, 2012, 2011, and 2010, respectively.
RSUs
The following table summarizes RSU activity as of December 31, 2012, and changes during the year then ended is presented below(in thousands):
| | | | | | | | |
| | | Restricted Stock
Units Outstanding | |
| | | Number
of
Shares | | | Aggregate
Intrinsic
Value | |
Balance at December 31, 2010 | | | — | | | | | |
Awarded | | | 192 | | | | | |
Vested/Released | | | — | | | | | |
Forfeited | | | (15 | ) | | | | |
| | | | | | | | |
Balance at December 31, 2011 | | | 177 | | | $ | 758 | |
Awarded | | | 190 | | | | | |
Vested/Released | | | (47 | ) | | | | |
Forfeited | | | (60 | ) | | | | |
| | | | | | | | |
Balance at December 31, 2012 | | | 260 | | | $ | 1,121 | |
| | | | | | | | |
Vested and expected to vest after December 31, 2012 | | | 223 | | | $ | 961 | |
Ending Exercisable at December 31, 2012 (Vested and deferred) | | | — | | | | — | |
The RSUs generally vest 25% approximately one year after grant date with the remaining shares vesting either approximately annually or quarterly in equal installments over a three year period, depending on the terms of the grant. The weighted average fair value at grant date of the RSUs was $6.49 and $5.81 for the years ended December 31, 2012 and 2011, respectively. There were no RSUs granted by the Company in any period prior to 2011. As of December 31, 2012, there was approximately $0.9 million of unrecognized compensation cost, net of
96
forfeitures, related to non-vested RSUs, which is expected to be recognized over a weighted average remaining period of approximately 1.42 years.
Employee Stock Purchase Plan
As of December 31, 2012, 1,300,000 shares of our common stock are reserved for issuance under the Company’s Employee Stock Purchase Plan (“ESPP”). Under the terms of the ESPP, eligible employees may choose to have up to 15% of their salary withheld to purchase the Company’s common stock and may purchase up to 1,000 shares per offering period. Each offering under the ESPP is for a three-month period. Commencing with the offering period beginning June 1, 2010, the purchase price of the stock issued under the ESPP will be equal to 85% of the lower of the fair market value of a share of common stock on the first day of the offering or on the final day of the offering period. As of December 31, 2012, approximately 505,821 shares of common stock were available for issuance under the ESPP.
Repurchase of Common Stock
In May and November 2012, the Company’s Board of Directors approved an increase of approximately $10.5 million and $10.0 million, respectively, to the existing $20 million share repurchase program initiated in October 2011, bringing the total authorized under the program since inception to approximately $40.5 million. The Company repurchased and retired approximately 781,000 and 4,709,000 shares at a cost of $3.5 million and $24.8 million, respectively, during the years ended December 31, 2011 and 2012. As of December 31, 2012, $12.4 million of the $40.5 million share repurchase program authorized by the Board was available for future share repurchases. Repurchased shares have been retired and constitute authorized but unissued shares.
Stockholder Rights Agreement
On December 18, 2006, the Company’s Board of Directors declared a dividend distribution of one Preferred Stock Purchase Right (collectively, the “Rights”) for each outstanding share of the Company’s Common Stock, each Right which entitles the registered holder to purchase from the Company one one-thousandth of a share of the Company’s Series D Preferred Stock, $0.001 par value, at a price of $25.00 pursuant to a Rights Agreement dated as of December 19, 2006, between the Company and Mellon Investor Services LLC (the “Rights Agreement”). The Rights, which will initially trade with the Common Stock, become exercisable when a person or group acquires 15% or more of the Company’s Common Stock without prior board approval. In that event, the Rights permit the Company’s stockholders, other than the acquirer, to purchase the Company’s Common Stock having a market value of twice the exercise price of the Rights, in lieu of the Preferred Stock. Alternatively, when the Rights become exercisable, the Company’s Board of Directors may authorize the issuance of one share of the Company’s Common Stock in exchange for each Right that is then exercisable. In addition, in the event of certain business combinations, the Rights permit the purchase of the Common Stock of an acquirer at a 50% discount. Rights held by the acquirer will become null and void in each case. Prior to a person or group acquiring 15%, the Rights can be redeemed for $0.001 each by action of the Board. The Rights Agreement contains an exception to the 15% ownership threshold for shares currently beneficially owned by Sigma-Tau Finanziaria S.p.A. The Rights expire on December 19, 2016. The Rights Agreement includes a requirement that a committee of independent directors evaluate the Rights Agreement at least every three years.
97
Note 15 — Accumulated Other Comprehensive Income (Loss)
Changes in the composition of accumulated other comprehensive income (loss) for the years ended December 31, 2012, 2011 and 2012 are as follows(in thousands):
| | | | | | | | | | | | | | | | |
| | | Foreign
Currency
Translation
Adjustments | | | Net Change in
Gains (Losses)
and Foreign
Currency
Translation
on Foreign-
Denominated
Available-
for-Sale
Securities | | | Net
Change in
Unrealized
Losses on
Available-
Sale
Securities | | | Total | |
Balances as of January 1, 2010 | | $ | 54 | | | $ | (34 | ) | | $ | 2 | | | $ | 22 | |
Other comprehensive income (loss) | | | 83 | | | | (36 | ) | | | (2 | ) | | | 45 | |
| | | | | | | | | | | | | | | | |
Balances as of December 31, 2010 | | | 137 | | | | (70 | ) | | | — | | | | 67 | |
Other comprehensive income (loss) | | | 2,293 | | | | (16 | ) | | | — | | | | 2,277 | |
| | | | | | | | | | | | | | | | |
Balances as of December 31, 2011 (Restated) | | | 2,430 | | | | (86 | ) | | | — | | | | 2,344 | |
Other comprehensive income | | | 625 | | | | 19 | | | | — | | | | 644 | |
| | | | | | | | | | | | | | | | |
Balances as of December 31, 2012 | | $ | 3,055 | | | $ | (67 | ) | | $ | — | | | $ | 2,988 | |
| | | | | | | | | | | | | | | | |
Note 16 — 401k Plan
The Company has a pre-tax savings plan covering most US employees, which qualifies under Section 401(k) of the Internal Revenue Code. Under the plan, eligible employees may contribute a portion of their pre-tax salary, subject to certain limitations. The Company contributes and matches 50% of the employee contributions. Company contributions, which can be terminated at the Company’s discretion, were approximately $0.2 million, $0.3 million and $0.2 million for the years ended December 31, 2012, 2011, and 2010, respectively.
Note 17 — Other Income
Other income for the year ended December 31, 2010 included approximately $1.0 million in non-taxable grants awarded to the Company in November 2010 related to its research and development activities in SCV-07 and ZADAXIN as part of the US Department of Treasury’s Therapeutic Discovery Project Program.
Note 18 — Other Corporate Matters
On August 5, 2010, SciClone was contacted by the SEC and advised that the SEC has initiated a formal, non-public investigation of SciClone, and the SEC issued a subpoena to SciClone requesting a variety of documents and other information. The subpoena requests documents relating to a range of matters including, but not limited to, potential payments or transfers of anything of value to regulators and government-owned entities in China, bids or contracts with state or government-owned entities in China, any joint venture partner, intermediary or local agent of the Company in China, the Company’s ethics and anti-corruption policies, training, and audits, and certain company financial and other disclosures. On August 6, 2010, the Company received a letter from the US Department of Justice (“DOJ”) indicating that the DOJ was investigating Foreign Corrupt Practices Act (“FCPA”) issues in the pharmaceutical industry generally, and that the DOJ had information about the Company’s practices suggesting possible violations. The Company will continue to cooperate fully with the SEC and DOJ in the conduct of their investigations.
In response to these matters, the Company’s Board appointed a Special Committee of independent directors (the “Special Committee”) to oversee the Company’s response to the government inquiry. Based on an initial review, the Special Committee decided to undertake an independent investigation as to matters reflected in and
98
arising from the SEC and DOJ investigations including, but not limited to, certain sales and marketing matters in China, in order to evaluate whether any violation of the FCPA or other laws occurred.
During the investigation, the Special Committee instructed management to (i) evaluate and to expand the Company’s training of employees regarding understanding and compliance with laws including the FCPA and other anti-bribery laws and regulations, (ii) evaluate existing compliance and anti-bribery policies and guidelines and to prepare new, more detailed policies and guidelines for implementation after review by SciClone’s Board and/or committees of the Board, (iii) implement a pre-approval policy for certain expenses including payments for, or reimbursement of, travel and entertainment expenses, and sponsorships of certain third party events, and (iv) hire a Vice President of Compliance and Internal Audit to monitor and enforce compliance with the Company’s policies.
The Special Committee substantially concluded its investigation of those matters and on May 4 and 5, 2011 reported its findings and recommendations to the Board of Directors. The Special Committee has also reported those findings to the SEC and DOJ, and the Special Committee and the Company have continued to cooperate with the on-going SEC and DOJ investigations.
In the Company’s Form 10-Q for the period ended September 30, 2012, filed with the SEC on November 9, 2012, the Company disclosed, among other things, a non-cash impairment loss to fully write down the value of intangible assets recorded as part of the NovaMed acquisition; a remeasurement of the valuation of the contingent consideration expense recorded as part of the NovaMed acquisition; a significant increase in ZADAXIN channel inventory levels; and internal control issues primarily within the NovaMed organization that was concluded to be a material weakness in internal control over financial reporting. Following our disclosure of these items, the Company received a subpoena from the SEC requesting documents related to these and various other matters regarding the NovaMed acquisition and the Company’s operations in China. After review of the subpoena, and in order to respond to inquiries from the DOJ and SEC and to determine if any wrong-doing occurred, the Audit Committee determined to undertake an additional independent investigation as to additional matters including but not limited to matters related to our acquisition of NovaMed and FCPA matters.
The Company is unable to predict what consequences that any investigation by any regulatory agency or by our Audit Committee or Special Committee may have on it. The Company’s cooperation with these investigations has resulted in substantial legal and accounting expenses, has diverted management’s attention from other business concerns and could harm its business. The ongoing investigations and any other regulatory investigations that might be initiated in the future, will result in similar substantial expenses, management diversion and harm to its business. If the Company fails to comply with regulations or to carry out controls on its Chinese or other foreign operations in a manner that satisfies all applicable laws, its business would be harmed. Any civil or criminal action commenced against us by a regulatory agency could result in administrative orders against the Company, the imposition of significant penalties and/or fines against it, and/or the imposition of civil or criminal sanctions against certain of its officers, directors and/or employees. The investigations, results of the investigations, or remedial actions the Company has taken or may take, if any, as a result of such investigations, may adversely affect its business in China. If the Company is subject to adverse findings resulting from the SEC and DOJ investigations, or from its own independent investigation, it could be required to pay damages or penalties or have other remedies imposed upon it. In addition, the Company will incur additional expenses related to remedial measures it is undertaking, and could incur fines or other penalties. The period of time necessary to resolve the investigations by the DOJ and the SEC is uncertain, and these matters could require significant management and financial resources which could otherwise be devoted to the operation of our business. The SEC’s and DOJ’s formal investigations are continuing.
The Company cannot predict what the outcome of those investigations will be, or the timing of any resolution. However, due to the significant uncertainty as to the outcome of the investigation, the Company is unable to reasonably estimate the loss or range of loss that may be incurred. As of December 31, 2012, the Company has therefore not accrued a liability for the contingent loss related to the FCPA investigation. Any fines or penalties that may be imposed, or other losses that may be realized related to the investigations, could
99
materially impact the Company’s financial statements. The Company will continue to reassess the potential liability related to the investigations and adjust its estimates accordingly in future periods.
Following the Company’s announcement of these investigations, purported class actions naming SciClone and certain of its officers as defendants were filed and derivate lawsuits purportedly on behalf of the Company were filed naming certain of its officers and directors as defendants. On January 13, 2011, the derivative lawsuits were consolidated into a single action (“the Consolidated Derivative Action”). On October 3, 2011, the parties to the Consolidated Action reached an agreement to settle the lawsuit. On December 13, 2011, the Court granted final approval of the settlement, including the payment of approximately $2.5 million in attorney’s fees, and entered a final judgment dismissing all claims. The settlement provides for the actions against the defendants to be dismissed with prejudice and for the release of certain known or unknown claims that have been or could have been brought later in court arising out of the same allegations. The Company agreed to adopt certain corporate governance measures, to be in effect for at least three years, and agreed to the payment of approximately $2.5 million in attorney’s fees to counsel for the plaintiffs, with $2.5 million paid by SciClone’s insurers under its director and officer liability policy.
On March 11, 2013, Adam Crum filed a derivative lawsuit, purportedly in the name of SciClone, against Friedhelm Blobel, Gary Titus, Jon Saxe, Peter Barrett, Richard Hawkins, Gregg Lapointe and Ira Lawrence in California Superior Court, San Mateo County, captionedCrum v. Blobel, et al, Case No. CIV520331. The lawsuit alleges, based on the restatement, that the board and management breached their fiduciary duties to the company by not exercising oversight in such a way that they allowed the company to file financial statements that were materially inaccurate. Plaintiff asserts claims for breach of fiduciary duty, abuse of control and mismanagement. Plaintiff seeks, among other things, injunctive relief, disgorgement, undisclosed damages and attorneys’ fees and costs. The Company believes the claims lack merit and will vigorously defend against them.
Note 19 — Segment Information and Geographic Data
The Company reports segment information based on the internal reporting used by management for evaluating segment performance based on management’s estimates of the appropriate allocation of resources to segments.
The Company operates and manages its business primarily on a geographic basis. Accordingly, the Company determined its operating segments and reporting units, which are generally based on the nature and location of its customers, to be 1) China, and 2) Rest of the World, including the US.
The Company evaluates the performance of its operating segments based on revenues and operating income (loss). Revenues for geographic segments are generally based on the location of customers. Operating income for each segment includes revenues, related cost of sales and operating expenses directly attributable to the segment. Operating income (loss) for each segment excludes non-operating income and expense.
100
Summary information by operating segments is as follows (in thousands):
| | | | | | | | | | | | |
| | | For the Year Ended
December 31, | |
| | | 2012 | | | 2011
Restated
(Note 2) | | | 2010 | |
China: | | | | | | | | | | | | |
Net Revenues: | | | | | | | | | | | | |
ZADAXIN product sales | | $ | 108,127 | | | $ | 101,193 | | | $ | 82,012 | |
Other product sales | | | 11,002 | | | | 7,121 | | | | — | |
| | | | | | | | | | | | |
Total net product sales | | | 119,129 | | | | 108,314 | | | | 82,012 | |
Promotion services revenue | | | 33,098 | | | | 20,614 | | | | — | |
| | | | | | | | | | | | |
Total net revenues | | | 152,227 | | | | 128,928 | | | | 82,012 | |
Operating income | | | 4,216 | (1) | | | 47,154 | | | | 43,754 | |
Long-lived assets | | | 35,541 | | | | 79,694 | | | | 202 | |
| | | |
Rest of the World (including the US): | | | | | | | | | | | | |
Net Revenues: | | | | | | | | | | | | |
ZADAXIN product sales | | $ | 4,042 | | | $ | 3,637 | | | $ | 3,100 | |
| | | | | | | | | | | | |
Total net revenues | | | 4,042 | | | | 3,637 | | | | 3,100 | |
Operating income (loss)(2) | | | 2,194 | | | | (18,199 | ) | | | (21,360 | ) |
Long-lived assets | | | 832 | | | | 1,092 | | | | 1,277 | |
| (1) | The operating income for the China segment for the year ended December 31, 2012, includes the $42.7 million impairment charge on the intangible assets. |
| (2) | Operating income (loss) for the Rest of the World segment includes the change in the fair value of the contingent consideration and all research and development expense for the Company’s SCV-07 trial, which was discontinued in the first quarter of 2012. |
Note 20 — Selected Quarterly Financial Data (unaudited)
Information presented below for the three months ended March 31, June 30 and September 30, 2012 and for the three months ended June 30, September 30, and December 31, 2011 has been restated. Refer to the Company’s March 31, June 30, and September 30, 2012 Forms 10-Q/A filed with the Securities and Exchange Commission and Note 2 above for further information regarding the restatement.
| | | | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31,(1)
2012
Restated
(Note 2) | | | June 30,(2)
2012
Restated
(Note 2) | | | September 30,(3)
2012 Restated
(Note 2) | | | December 31,
2012 | |
| | | (in thousands, except per share amounts) | |
Net product sales | | $ | 33,163 | | | $ | 33,284 | | | $ | 32,137 | | | $ | 24,587 | |
Promotion services revenue | | | 7,905 | | | | 8,085 | | | | 8,556 | | | | 8,552 | |
Cost of product sales | | | 5,532 | | | | 5,664 | | | | 5,474 | | | | 5,326 | |
Net income (loss) | | | 9,665 | | | | 11,228 | | | | (13,161 | )(4) | | | 1,888 | |
Basic net income (loss) per share | | $ | 0.17 | | | $ | 0.20 | | | $ | (0.23 | ) | | $ | 0.03 | |
Diluted net income (loss) per share | | $ | 0.16 | | | $ | 0.19 | | | $ | (0.23 | ) | | $ | 0.03 | |
101
| | | | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31,
2011 | | | June 30,(5)(6)
2011
Restated
(Note 2) | | | September 30,(7)
2011 Restated
(Note 2) | | | December 31,(8)
2011 Restated
(Note 2) | |
| | | (in thousands, except per share amounts) | |
Net product sales | | $ | 21,662 | | | $ | 26,207 | | | $ | 32,310 | | | $ | 31,772 | |
Promotion services revenue | | | — | | | | 5,719 | | | | 6,992 | | | | 7,903 | |
Cost of product sales | | | 3,103 | | | | 4,205 | | | | 6,262 | | | | 5,839 | |
Net income | | | 3,849 | | | | 1,891 | | | | 10,832 | | | | 11,550 | |
Basic net income per share | | $ | 0.08 | | | $ | 0.03 | | | $ | 0.19 | | | $ | 0.20 | |
Diluted net income per share | | $ | 0.08 | | | $ | 0.03 | | | $ | 0.18 | | | $ | 0.19 | |
| (1) | For the three months ended March, 2012, the restatement revisions had an impact of increasing revenues, cost of product sales, and net income by $1.9 million, $0.6 million and $1.0 million, respectively, and increasing basic and diluted earnings per share by $0.02 and $0.01, respectively. |
| (2) | For the three months ended June 30, 2012, the restatement revisions had an impact of increasing revenues, cost of product sales, and net income by $1.1 million, $0.4 million and $0.6 million, respectively, and increasing basic and diluted earnings per share by $0.01. |
| (3) | For the three months ended September 30, 2012, the restatement revisions had an impact of increasing revenues, decreasing cost of product sales, and increasing net income by $7,000, $1,000 and $0.4 million, respectively, and increasing basic and diluted earnings per share by $0.01. |
| (4) | During the three months ended September 30, 2012, the Company identified an impairment indicator with respect to its intangible assets related to its promotion and distribution contract rights and recorded losses of approximately $42.7 million to recognize the full impairment and recorded a benefit for income tax of approximately $6.8 million due to the impairment of intangible assets, which resulted in a reversal of deferred tax liabilities. This was partially offset by the impact of recognizing a full valuation allowance on any remaining NovaMed deferred tax assets. In addition, for the same period, the Company recorded a non-cash gain of $12.8 million, primarily related to the decrease in estimated probability of achieving targets relating to NovaMed’s product distributor agreements, including the renewal of the agreement with Sanofi for a five-year term. |
| (5) | For the three months ended June 30, 2011, the restatement revisions had an impact of decreasing revenues, cost of product sales, and net income by $1.2 million, $1.0 million and $0.1 million, respectively, and decreasing diluted earnings per share by $0.01. |
| (6) | On April 18, 2011, SciClone acquired NovaMed. Commencing April 18, 2011, the Company’s financial statements include the assets, liabilities, operating results and cash flows of NovaMed. |
| (7) | For the three months ended September 30, 2011, the restatement revisions had an impact of increasing revenues, cost of product sales, and net income by $1.9 million, $1.2 million and $0.6 million, respectively, and increasing basic and diluted earnings per share by $0.01. |
| (8) | For the three months ended December 31, 2011, the restatement revisions had an impact of decreasing revenues, cost of product sales, and net income by $1.8 million, $0.9 million, and $0.9 million, respectively, and decreasing basic and diluted earnings per share by $0.01 and $0.02, respectively. |
102
The following table presents the impact of the revisions on the Company’s previously issued selected quarterly financial data for the three months ended December 31, 2011 (in thousands):
| | | | | | | | |
| | | For the Three Months Ended
December 31, 2011 | |
| | | As
Reported | | | As
Restated(9) | |
Net product sales | | $ | 33,543 | | | $ | 31,772 | |
Cost of product sales | | | 6,712 | | | | 5,839 | |
Net income | | | 12,402 | | | | 11,550 | |
Basic net income per share | | $ | 0.21 | | | $ | 0.20 | |
Diluted net income per share | | $ | 0.21 | | | $ | 0.19 | |
| (9) | Changes to the selected quarterly financial data for the three months ended December 31, 2011 mainly related to the Company changing from the “sell-in” method to the “sell-through” method of revenue recognition for certain Pfizer products sold by the Company. |
Note 21 — Subsequent Event
The Company repurchased and retired 502,400 shares at a cost of $2.5 million from January 1, 2013 through March 27, 2013 under its share repurchase program. As of March 27, 2013, $9.9 million of the $40.5 million share repurchase program authorized by our Board of Directors was available for future share repurchase.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
Not applicable.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our principal executive officer and our principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. As of the end of the period covered by this report, the Company carried out an evaluation under the supervision and with the participation of its management, including the Company’s President and Chief Executive Officer (“CEO”) and its Senior Vice President and Chief Financial Officer (“CFO”), of the effectiveness of the design and operation of the Company’s disclosure controls and procedures in ensuring that material information required to be disclosed in the Company’s reports filed or submitted under the Exchange Act, has been made known to them in a timely fashion. Based on this evaluation, the CEO and CFO concluded that the Company’s disclosure controls and procedures were not effective as of December 31, 2012 due to a material weaknesses in our internal control over financial reporting, which is disclosed below.
Material Weakness and Remediation Activities
During the quarter ended September 30, 2012, we identified deficiencies in the design and operating effectiveness of controls primarily associated with product return reserves related to our Aggrastat product line, and the override of certain controls in the financial statement close process related to our NovaMed subsidiary. Certain out of period adjustments and these deficiencies were identified through the performance of controls and processes recently implemented at our NovaMed subsidiary as part of our assessment of internal control over financial reporting in 2012. We concluded that the aggregation of these deficiencies is a material weakness. Furthermore, during the fourth quarter of 2012, we identified deficiencies related to the timing of revenue recognition for our Pfizer products, the override of related controls at our
103
NovaMed subsidiary, and the corporate monitoring thereof. We concluded that these deficiencies identified in the fourth quarter of 2012 are an additional indicator of the material weakness that was identified in the third quarter of 2012.
With the oversight of management and our Audit Committee, we have initiated steps to address the material weakness. In the fourth quarter of 2012, we implemented additional controls related to our revenue recognition and product return reserve processes to strengthen our controls and provide that all available information is properly considered. We also re-evaluated the operation of our controls at our NovaMed subsidiary and made adjustments to strengthen these controls, including the recent hiring of our Chief Financial Officer, China Operations who joined the Company in the third quarter of 2012. We terminated personnel who were involved in the override of certain controls in the financial statement close process at our NovaMed subsidiary that resulted in the material weakness. While we have completed certain remediation efforts, our remediation activities are not complete as of December 31, 2012.
Management’s Annual Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. All control systems have inherent limitations so that no evaluation of controls can provide absolute assurance that all control issues are detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, we assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2012, based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework. Based on the results of our assessment, we concluded that there was a material weakness in the design and operating effectiveness of our internal control over financial reporting as of December 31, 2012. A material weakness is defined as a deficiency or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
During the year ended December 31, 2012, we identified deficiencies in the design and operating effectiveness of controls primarily associated with the timing of revenue recognition for our Pfizer products and product returns reserves related to our Aggrastat product line, the override of certain controls in the financial statement close process related to our NovaMed subsidiary, and the corporate monitoring thereof. We have concluded the aggregation of these deficiencies is a material weakness. Because of the effect of the material weaknesses described above on the achievement of the objectives of the control criteria, we have concluded that the Company has not maintained effective internal control over financial reporting as of December 31, 2012, based on the COSO criteria.
Ernst & Young LLP, an independent registered public accounting firm, has audited and issued an attestation report on the Company’s internal control over financial reporting as of December 31, 2012, as stated in their report which is included below.
104
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of SciClone Pharmaceuticals, Inc.
We have audited SciClone Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2012, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). SciClone Pharmaceuticals, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness has been identified and included in management’s assessment. In its assessment, management has identified deficiencies in the design and operating effectiveness of controls primarily associated with the timing of revenue recognition for its Pfizer products and product returns reserves related to the Aggrastat product line, the override of certain controls in the financial statement close process related to the NovaMed subsidiary, and the corporate monitoring thereof. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of SciClone Pharmaceuticals, Inc. as of December 31, 2012 and 2011, and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2012. This material weakness was considered in determining the nature, timing, and extent of audit tests applied in our audit of those financial statements, and this report does not affect our report dated April 1, 2013, which expressed an unqualified opinion on those financial statements.
In our opinion, because of the effect of the material weaknesses described above on the achievement of the objectives of the control criteria, SciClone Pharmaceuticals, Inc. has not maintained effective internal control over financial reporting as of December 31, 2012, based on the COSO criteria.
/s/ Ernst & Young LLP
Redwood City, California
April 1, 2013
105
Changes in Internal Controls
There were no other changes, beyond that described above, in our internal control over financial reporting during the fourth quarter of fiscal 2012 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations of the Effectiveness of Internal Controls
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the internal control system are met. Because of inherent limitations in any control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. We are continuously seeking to improve the efficiency and effectiveness of our operations and of our internal controls. This results in refinements to processes throughout our organization.
| Item 9B. | Other Information |
None.
PART III
Certain information required by Part III is incorporated by reference from our definitive Proxy in connection with the solicitation of proxies for our 2013 Annual Meeting of Stockholders (the “Proxy Statement”) or in an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission, in each case, not later than 120 days after the end of the fiscal year covered by this report, and certain information therein is incorporated in this report by reference.
| Item 10. | Directors, Executive Officers, and Corporate Governance |
The information required by Item 401 of Regulation S-K is incorporated by reference from the Proxy Statement under the caption “Proposal No. 1 Election of Directors – Nominees,” and “Proposal No. 1 Corporate Governance — Board Meetings and Committees.” Information relating to the executive officers of the Company is incorporated by reference from the Proxy Statement under the caption “Executive Compensation and Other Matters — Executive Officers”.
The information required by Item 405 of Regulation S-K is incorporated by reference from the Proxy Statement under the caption “Executive Compensation and Other Matters — Section 16(a) Beneficial Ownership Reporting Compliance”.
Code of Ethics
We have adopted a code of business conduct and ethics, and a policy providing for the reporting of potential violations of the code, for directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller) and employees, known as the Corporate Code of Conduct and Ethics (including “Whistle blowing” in the case of Violations of the Company Policies) (the “Code of Conduct”). The Code of Conduct is available on our website atwww.sciclone.com under the section “Investor Relations — Corporate Governance”. Additionally, stockholders may request a free copy of the Code of Conduct by contacting the Investor Relations Department at our corporate offices by calling 800-724-2566 or by sending an e-mail message to investorrelations@sciclone.com.
| Item 11. | Executive Compensation |
The information required by this Item 11 is incorporated by reference from the Proxy Statement under the captions “Executive Compensation and Other Matters” and “Corporate Governance.”
106
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides certain information regarding our compensation plans in effect as of December 31, 2012(in thousands, except per share amounts):
| | | | | | | | | | | | |
| | | Number of
securities
to be issued upon
exercise of
outstanding
options, warrants
and rights
(a) | | | Weighted-
average exercise
price of
outstanding
options,
warrants and
rights
(b) | | | Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities
reflected in column (a))
(c) | |
Equity compensation plans approved by security holders: | | | | | | | | | | | | |
1995 Equity Incentive Plan(1) | | | 95 | | | $ | 5.61 | | | | — | |
1995 Nonemployee Director Stock Option Plan(1) | | | 20 | | | $ | 8.90 | | | | — | |
SciClone Pharmaceuticals, Inc. Employee Stock Purchase Plan | | | — | | | | — | | | | 506 | |
2004 Outside Directors Stock Option Plan | | | 925 | | | $ | 3.95 | | | | 184 | |
2005 Equity Incentive Plan | | | 5,218 | | | $ | 4.02 | | | | 4,167 | |
Equity compensation plans not approve by security holders: | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total | | | 6,258 | | | $ | 4.05 | | | | 4,857 | |
| | | | | | | | | | | | |
| (1) | Although the 1995 Equity Incentive Plan and 1995 Nonemployee Director Stock Option Plan have expired, the outstanding stock options relating to them are fully valid. |
The information required by Item 403 of Regulation S-K is incorporated by reference from the Proxy Statement under the caption “Executive Compensation and Other Matters — Security Ownership of Certain Beneficial Owners and Management.”
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item is incorporated by reference from the Proxy Statement under the captions “Executive Compensation and Other Matters — Transactions with Related Persons” and “Corporate Governance — Director Independence.”
| Item 14. | Principal Accountant Fees and Services |
The information required by this Item 14 is incorporated by reference from the Proxy Statement under the caption “Proposal No. 3 Ratification of Appointment of Independent Registered Public Accounting Firm — Principal Accountant Fees.”
107
PART IV
| Item 15. | Exhibits, Financial Statement Schedules |
Item 15 (a). The following documents are filed as part of this Annual Report on Form 10-K:
(1)Financial Statements.The following financial statements of the Company are contained in Item 8, Part II on pages 68-103 of this Annual Report on Form 10-K:
Report of Independent Registered Public Accounting Firm.
Consolidated Balance Sheets at December 31, 2012 and 2011.
Consolidated Statements of Income for each of the three years in the period ended December 31, 2012.
Consolidated Statements of Comprehensive Income for each of the three years in the period ended December 31, 2012.
Consolidated Statements of Stockholders’ Equity for each of the three years ended December 31, 2012.
Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2012.
Notes to Consolidated Financial Statements.
(2)Financial Statement Schedules
The following schedule required to be filed by Item 8 of this form and Item 15(d) is contained on page 114 of this Report:
Schedule II — Valuation and Qualifying Accounts for each of the three years in the period ended December 31, 2012.
All other schedules have been omitted because they are either inapplicable or the required information has been given in the consolidated financial statements or the notes thereto.
(3)Exhibits.
Refer to Item 15(b) below.
Item 15 (b). Exhibits.
Exhibits (numbered in accordance with Item 601 of Regulation S-K):
| | |
Exhibit Number | | Description |
| |
| 3(i).1(1) | | Amended and Restated Certificate of Incorporation. |
| |
| 3(i).2(19) | | Certificate of Amendment to the Amended and Restated Certificate of Incorporation. |
| |
| 3(i).3(10) | | Certificate of Designation, Preferences and Rights of the Terms of the Series D Preferred Stock, as filed by SciClone Pharmaceuticals, Inc. with the Secretary of State of the State of Delaware as of December 22, 2006. |
| |
| 3(ii).1(11) | | Amended and Restated Bylaws. |
| |
| 4.9(9) | | Rights Agreement, effective as of December 19, 2006, between the Company and Mellon Investor Services LLC, as rights agent (including as Exhibit A the form of Certificate of Designation, Preferences and Rights of the Series D Preferred Stock, as Exhibit B the form of Right Certificate, and as Exhibit C the Summary of Terms of Rights Agreement). |
| |
| 10.1(2)** | | Registrant’s 1995 Equity Incentive Plan, together with forms of agreement thereunder. |
| |
| 10.2(2)** | | Registrant’s 1995 Nonemployee Director Stock Option Plan, together with forms of agreement thereunder. |
108
| | |
Exhibit Number | | Description |
| |
| 10.3(24)** | | Registrant’s 2005 Equity Incentive Plan, effective as of June 7, 2005, as amended on June 25, 2012. |
| |
| 10.4(8)** | | Registrant’s 2004 Outside Directors Stock Option Plan, as amended on June 7, 2005 and as further amended on February 22, 2007. |
| |
| 10.5(5) ** | | Form of Indemnity Agreement by and between the Registrant and each director and executive officer of SciClone Pharmaceuticals, Inc. |
| |
| 10.6(3) | | Alpha Rights Acquisition Agreement by and between the Registrant and Alpha 1 Biomedicals, Inc., dated December 17, 1997. |
| |
| 10.7(4) | | Acquisition Agreement between the Company and Sclavo S.p.A. dated April 20, 1998. |
| |
| 10.8(4) | | First Amendment to Acquisition Agreement between the Company and Sclavo S.p.A., dated April 20, 1998. |
| |
| 10.9(6)* | | Manufacturing and Supply Agreement between SciClone Pharmaceuticals International Ltd. and Patheon Italia S.p.A. dated as of November 1, 2002. |
| |
| 10.10(7)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.11(7)** | | Change in Control Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.12(12)* | | Manufacturing Supply Agreement Between SciClone Pharmaceuticals International Ltd. and Lonza Sales Ltd., executed as of May 23, 2008. |
| |
| 10.13(13)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Gary Titus, dated as of November 21, 2008 and effective as of December 8, 2008. |
| |
| 10.14(14)** | | Amendment No. 1 to Change of Control Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.15(14)** | | Amendment No. 1 to Employment Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.16(15)** | | Change in Control Agreement between Mr. Gary Titus and SciClone Pharmaceuticals, Inc. dated December 8, 2008. |
| |
| 10.17(16)** | | The SciClone Pharmaceuticals, Inc. Employee Stock Purchase Plan, as amended. |
| |
| 10.18(25)** | | Description of Executive Incentive Plan. |
| |
| 10.19(17) | | Assignment and Purchase of Intellectual Property Rights Agreement by and among Edward T. Wei, Cragmont Pharmaceuticals, LLC, and certain Russian Inventors and Russian Companies and SciClone Pharmaceuticals, Inc., dated as of December 28, 2010. |
| |
| 10.20(17)* | | Amendment No. 1 to Manufacturing and Supply Agreement between SciClone Pharmaceuticals International Ltd. and Lonza Sales Ltd., effective December 31, 2010. |
| |
| 10.21(17)** | | Executive Severance Agreement between Gary Titus and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.22(17)** | | Executive Severance Agreement between Israel Rios, M.D. and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.23(17)** | | Amendment No. 2 to Change of Control Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
109
| | |
Exhibit Number | | Description |
| |
| 10.24(17)** | | Amendment No. 2 to Employment Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.25(17)** | | Amendment No. 1 to Change of Control Agreement between Gary S. Titus and SciClone Pharmaceuticals, Inc. effective July 9, 2010. |
| |
| 10.26(18) | | Share Purchase Agreement dated April 18, 2011 by and among SciClone Pharmaceuticals, Inc., SciClone Pharmaceuticals Hong Kong Limited, NovaMed Pharmaceuticals, Inc. and the listed sellers represented by Mark Lotter. |
| |
| 10.27(18)** | | Change in Control Agreement effective April 18, 2011 by and between Mr. Mark Lotter and SciClone Pharmaceuticals Hong Kong Limited. |
| |
| 10.28(18)** | | Employment Agreement by and between SciClone Pharmaceuticals Hong Kong Limited and Mark Lotter, dated April 18, 2011. |
| |
| 10.29(18)** | | Secondment Contract between SciClone Pharmaceuticals Hong Kong Limited and NovaMed Pharmaceuticals (Shanghai) Co., Ltd. and Mark Lotter, dated April 18, 2011. |
| |
| 10.30(18)** | | Director Services Agreement between SciClone Pharmaceuticals International Ltd. and Mr. Mark Lotter, dated April 18, 2011. |
| |
| 10.31(18)** | | Director Services Agreement between NovaMed Pharmaceuticals, Inc. and Mr. Mark Lotter, dated April 18, 2011. |
| |
| 10.32(21)** | | Separation Agreement between SciClone Pharmaceuticals, Inc. and Israel Rios, dated April 6, 2012. |
| |
| 10.33(22)** | | Change in Control Agreement effective June 11, 2012 by and between Min Yin and SciClone Pharmaceuticals, Inc. |
| |
| 10.34(22)** | | Change in Control Agreement effective June 11, 2012 by and between Jackie Guan and SciClone Pharmaceuticals, Inc. |
| |
| 10.35(22)** | | Executive Severance Agreement effective June 11, 2012 by and between Min Yin and SciClone Pharmaceuticals, Inc. |
| |
| 10.36(22)** | | Executive Severance Agreement effective June 11, 2012 by and between Jackie Guan and SciClone Pharmaceuticals, Inc. |
| |
| 10.37(23)** | | Change in Control Agreement effective August 3, 2012 by and between Stephanie Wong and SciClone Pharmaceuticals, Inc. |
| |
| 10.38(23)** | | Change in Control Agreement effective August 3, 2012 by and between Lan Xie and SciClone Pharmaceuticals, Inc. |
| |
| 10.39(23)** | | Executive Severance Agreement effective August 3, 2012 by and between Stephanie Wong and SciClone Pharmaceuticals, Inc. |
| |
| 10.40(23)** | | Executive Severance Agreement effective August 3, 2012 by and between Lan Xie and SciClone Pharmaceuticals, Inc. |
| |
| 10.41(20)** | | Form of Amendment to Change in Control Agreements between SciClone Pharmaceuticals, Inc. and Executive Officers effective August 4, 2011. |
| |
| 10.42(25)* | | Re-Exportation Agreement between SciClone Pharmaceuticals International China Holding Ltd., Sinopharm Holding Hong Kong Co., Limited and Sinopharm Holding Lingyun Biopharmaceutical (Shanghai) Co. Limited effective January 1, 2013. |
110
| | |
Exhibit Number | | Description |
| |
| 10.43(25)** | | Stay Bonus Agreement by and between Gary Titus and SciClone Pharmaceuticals, Inc. effective January 16, 2013. |
| |
| 14 | | See “Code of Ethics” in Item 10: Executive Officers and Directors, of this Annual Report on Form 10-K. |
| |
| 21.1(25) | | Subsidiaries of Registrant. |
| |
| 23.1(25) | | Consent of Independent Registered Public Accounting Firm. |
| |
| 24.1(25) | | Power of Attorney. See page 113. |
| |
| 31.1(25) | | Rule 13a-14(a) Certification of Principal Executive Officer. |
| |
| 31.2(25) | | Rule 13a-14(a) Certification of Principal Financial Officer. |
| |
| 32.1(25) | | Section 1350 Certification of Principal Executive Officer. |
| |
| 32.2(25) | | Section 1350 Certification of Principal Financial Officer. |
| |
| 101*** | | The following materials from Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets at December 31, 2012 and 2011, (ii) Consolidated Statements of Income for the years ended December 31, 2012, 2011, and 2010, (iii) Consolidated Statements of Comprehensive Income (iv) Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2012, 2011 and 2010, (v) Consolidated Statements of Cash Flows for the years ended December 2012, 2011, and 2010 and (vi) Notes to the Consolidated Financial Statements. |
| * | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4), 200.83 and 230.46. |
| ** | Management compensatory plan or arrangement. |
| *** | XBRL information is furnished and not filed or a part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Exchange Act of 1933, as amended, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections. |
| (1) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on July 28, 2003. |
| (2) | Incorporated by reference from the Company’s Registration Statement on Form S-8 (No. 33-80911) filed with the Commission on December 28, 1995. |
| (3) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on January 26, 1998. |
| (4) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 1998. |
| (5) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2003. |
| (6) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. |
| (7) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 25, 2006. |
| (8) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2007 filed on August 8, 2007. |
111
| (9) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 22, 2006. |
| (10) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 28, 2006. |
| (11) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 21, 2007. |
| (12) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2008 filed on August 1, 2008. |
| (13) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 4, 2008. |
| (14) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 8, 2009. |
| (15) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 11, 2009. |
| (16) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on August 9, 2010. |
| (17) | Incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 31, 2011. |
| (18) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 10, 2011. |
| (19) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on July 6, 2011. |
| (20) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on November 9, 2011. |
| (21) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 10, 2012. |
| (22) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on August 8, 2012. |
| (23) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on November 9, 2012. |
| (24) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on June 7, 2012. |
Item 15 (c). See Item 15(a) above.
112
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| SCICLONE PHARMACEUTICALS, INC. |
| |
| By: | | /S/ FRIEDHELM BLOBEL, PH.D. |
| | Friedhelm Blobel, Ph.D. President and Chief Executive Officer (Principal Executive Officer) |
Date: April 1, 2013
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Friedhelm Blobel, Ph.D. and Gary Titus, and each of them, his attorneys-in-fact and agents, each with the power of substitution and resubstitution, for him in any and all capacities, to sign this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting to said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary, to be done in connection therewith, as fully as to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/S/ FRIEDHELM BLOBEL, PH.D. (Friedhelm Blobel, Ph.D.) | | President and Chief Executive Officer,
Director (Principal Executive Officer) | | March 29, 2013 |
| | |
/S/ GARY S. TITUS (Gary S. Titus) | | Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | | March 29, 2013 |
| | |
/S/ PETER BARRETT (Peter Barrett) | | Director | | March 29, 2013 |
| | |
/S/ RICHARD J. HAWKINS (Richard J. Hawkins) | | Director | | March 29, 2013 |
| | |
/S/ GREGG A. LAPOINTE (Gregg A. Lapointe) | | Director | | March 29, 2013 |
| | |
/S/ IRA D. LAWRENCE, M.D. (Ira D. Lawrence, M.D.) | | Director | | March 29, 2013 |
| | |
/S/ SIMON LI (Simon Li) | | Director | | March 29, 2013 |
| | |
/S/ JON S. SAXE (Jon S. Saxe) | | Chairman of the Board of Directors | | March 29, 2013 |
113
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
SCICLONE PHARMACEUTICALS, INC.
Receivable and Product Returns Reserves (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Balance at
Beginning of
Period | | | Charges for
Amounts
Reserved | | | Deductions for
Amounts
Recovered | | | Deductions for
Amounts
Written Off | | | Balance at
End of
Period | |
Receivables Reserve: | | | | | | | | | | | | | | | | | | | | |
Year Ended December 31, 2012 | | $ | — | | | $ | (2,235 | ) | | $ | — | | | $ | 1,189 | | | $ | (1,046 | ) |
Year Ended December 31, 2011 | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Year Ended December 31, 2010 | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | |
Reserve for Product Returns: | | | | | | | | | | | | | | | | | | | | |
Year Ended December 31, 2012 | | $ | (116 | ) | | $ | (58 | ) | | $ | 51 | | | $ | — | | | $ | (123 | ) |
Year Ended December 31, 2011 | | $ | — | | | $ | (1,214 | )(1) | | $ | — | | | $ | 1,098 | | | $ | (116 | ) |
Year Ended December 31, 2010 | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| (1) | Includes $1.1 million of reserve for Aggrastat product related to pre-acquisition sales acquired from NovaMed at the date of acquisition, April 18, 2011. |
114
INDEX TO EXHIBITS
| | |
Exhibit Number | | Description |
| |
| 3(i).1(1) | | Amended and Restated Certificate of Incorporation. |
| |
| 3(i).2(19) | | Certificate of Amendment to the Amended and Restated Certificate of Incorporation. |
| |
| 3(i).3(10) | | Certificate of Designation, Preferences and Rights of the Terms of the Series D Preferred Stock, as filed by SciClone Pharmaceuticals, Inc. with the Secretary of State of the State of Delaware as of December 22, 2006. |
| |
| 3(ii).1(11) | | Amended and Restated Bylaws. |
| |
| 4.9(9) | | Rights Agreement, effective as of December 19, 2006, between the Company and Mellon Investor Services LLC, as rights agent (including as Exhibit A the form of Certificate of Designation, Preferences and Rights of the Series D Preferred Stock, as Exhibit B the form of Right Certificate, and as Exhibit C the Summary of Terms of Rights Agreement). |
| |
| 10.1(2)** | | Registrant’s 1995 Equity Incentive Plan, together with forms of agreement thereunder. |
| |
| 10.2(2)** | | Registrant’s 1995 Nonemployee Director Stock Option Plan, together with forms of agreement thereunder. |
| |
| 10.3(24)** | | Registrant’s 2005 Equity Incentive Plan, effective as of June 7, 2005, as amended on June 7, 2012. |
| |
| 10.4(8)** | | Registrant’s 2004 Outside Directors Stock Option Plan, as amended on June 7, 2005 and as further amended on February 22, 2007. |
| |
| 10.5(5)** | | Form of Indemnity Agreement by and between the Registrant and each director and executive officer of SciClone Pharmaceuticals, Inc. |
| |
| 10.6(3) | | Alpha Rights Acquisition Agreement by and between the Registrant and Alpha 1 Biomedicals, Inc., dated December 17, 1997. |
| |
| 10.7(4) | | Acquisition Agreement between the Company and Sclavo S.p.A. dated April 20, 1998. |
| |
| 10.8(4) | | First Amendment to Acquisition Agreement between the Company and Sclavo S.p.A., dated April 20, 1998. |
| |
| 10.9(6)* | | Manufacturing and Supply Agreement between SciClone Pharmaceuticals International Ltd. and Patheon Italia S.p.A. dated as of November 1, 2002. |
| |
| 10.10(7)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.11(7)** | | Change in Control Agreement between SciClone Pharmaceuticals, Inc. and Friedhelm Blobel, Ph.D. dated as of April 23, 2006 and effective as of June 2, 2006. |
| |
| 10.12(12)* | | Manufacturing Supply Agreement Between SciClone Pharmaceuticals International Ltd. and Lonza Sales Ltd., executed as of May 23, 2008. |
| |
| 10.13(13)** | | Employment Agreement between SciClone Pharmaceuticals, Inc. and Gary Titus, dated as of November 21, 2008 and effective as of December 8, 2008. |
| |
| 10.14(14)** | | Amendment No. 1 to Change of Control Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
| |
| 10.15(14)** | | Amendment No. 1 to Employment Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. dated April 7, 2009. |
115
| | |
Exhibit Number | | Description |
| |
| 10.16(15)** | | Change in Control Agreement between Mr. Gary Titus and SciClone Pharmaceuticals, Inc. dated December 8, 2008. |
| |
| 10.17(16)** | | The SciClone Pharmaceuticals, Inc. Employee Stock Purchase Plan, as amended. |
| |
| 10.18(25)** | | Description of Executive Incentive Plan. |
| |
| 10.19(17) | | Assignment and Purchase of Intellectual Property Rights Agreement by and among Edward T. Wei, Cragmont Pharmaceuticals, LLC, and certain Russian Inventors and Russian Companies and SciClone Pharmaceuticals, Inc., dated as of December 28, 2010. |
| |
| 10.20(17)* | | Amendment No. 1 to Manufacturing and Supply Agreement between SciClone Pharmaceuticals International Ltd. and Lonza Sales Ltd., effective December 31, 2010. |
| |
| 10.21(17)** | | Executive Severance Agreement between Gary Titus and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.22(17)** | | Executive Severance Agreement between Israel Rios, M.D. and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.23(17)** | | Amendment No. 2 to Change of Control Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.24(17)** | | Amendment No. 2 to Employment Agreement between Dr. Friedhelm Blobel and SciClone Pharmaceuticals, Inc. effective May 4, 2010. |
| |
| 10.25(17)** | | Amendment No. 1 to Change of Control Agreement between Gary S. Titus and SciClone Pharmaceuticals, Inc. effective July 9, 2010. |
| |
| 10.26(18) | | Share Purchase Agreement dated April 18, 2011 by and among SciClone Pharmaceuticals, Inc., SciClone Pharmaceuticals Hong Kong Limited, NovaMed Pharmaceuticals, Inc. and the listed sellers represented by Mark Lotter. |
| |
| 10.27(18)** | | Change in Control Agreement effective April 18, 2011 by and between Mr. Mark Lotter and SciClone Pharmaceuticals Hong Kong Limited. |
| |
| 10.28(18)** | | Employment Agreement by and between SciClone Pharmaceuticals Hong Kong Limited and Mark Lotter, dated April 18, 2011. |
| |
| 10.29(18)** | | Secondment Contract between SciClone Pharmaceuticals Hong Kong Limited and NovaMed Pharmaceuticals (Shanghai) Co., Ltd. and Mark Lotter, dated April 18, 2011. |
| |
| 10.30(18)** | | Director Services Agreement between SciClone Pharmaceuticals International Ltd. and Mr. Mark Lotter, dated April 18, 2011. |
| |
| 10.31(18)** | | Director Services Agreement between NovaMed Pharmaceuticals, Inc. and Mr. Mark Lotter, dated April 18, 2011. |
| |
| 10.32(21)** | | Separation Agreement between SciClone Pharmaceuticals, Inc. and Israel Rios, dated April 6, 2012. |
| |
| 10.33(22)** | | Change in Control Agreement effective June 11, 2012 by and between Min Yin and SciClone Pharmaceuticals, Inc. |
| |
| 10.34(22)** | | Change in Control Agreement effective June 11, 2012 by and between Jackie Guan and SciClone Pharmaceuticals, Inc. |
| |
| 10.35(22)** | | Executive Severance Agreement effective June 11, 2012 by and between Min Yin and SciClone Pharmaceuticals, Inc. |
116
| | |
Exhibit Number | | Description |
| |
| 10.36(22)** | | Executive Severance Agreement effective June 11, 2012 by and between Jackie Guan and SciClone Pharmaceuticals, Inc. |
| |
| 10.37(23)** | | Change in Control Agreement effective August 3, 2012 by and between Stephanie Wong and SciClone Pharmaceuticals, Inc. |
| |
| 10.38(23)** | | Change in Control Agreement effective August 3, 2012 by and between Lan Xie and SciClone Pharmaceuticals, Inc. |
| |
| 10.39(23)** | | Executive Severance Agreement effective August 3, 2012 by and between Stephanie Wong and SciClone Pharmaceuticals, Inc. |
| |
| 10.40(23)** | | Executive Severance Agreement effective August 3, 2012 by and between Lan Xie and SciClone Pharmaceuticals, Inc. |
| |
| 10.41(20)** | | Form of Amendment to Change in Control Agreements between SciClone Pharmaceuticals, Inc. and Executive Officers effective August 4, 2011. |
| |
| 10.42(25)* | | Re-Exportation Agreement between SciClone Pharmaceuticals International China Holding Ltd., Sinopharm Holding Hong Kong Co., Limited and Sinopharm Holding Lingyun Biopharmaceutical (Shanghai) Co. Limited effective January 1, 2013. |
| |
| 10.43(25)** | | Stay Bonus Agreement by and between Gary Titus and SciClone Pharmaceuticals, Inc. effective January 16, 2013. |
| |
| 14 | | See “Code of Ethics” in Item 10: Executive Officers and Directors, of this Annual Report on Form 10-K. |
| |
| 21.1(25) | | Subsidiaries of Registrant. |
| |
| 23.1(25) | | Consent of Independent Registered Public Accounting Firm. |
| |
| 24.1(25) | | Power of Attorney. See page 113. |
| |
| 31.1(25) | | Rule 13a-14(a) Certification of Principal Executive Officer. |
| |
| 31.2(25) | | Rule 13a-14(a) Certification of Principal Financial Officer. |
| |
| 32.1(25) | | Section 1350 Certification of Principal Executive Officer. |
| |
| 32.2(25) | | Section 1350 Certification of Principal Financial Officer. |
| |
| 101*** | | The following materials from Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets at December 31, 2012 and 2011, (ii) Consolidated Statements of Income for the years ended December 31, 2012, 2011, and 2010, (iii) Consolidated Statements of Comprehensive Income, (iv) Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2012, 2011 and 2010, (v) Consolidated Statements of Cash Flows for the years ended December 2012, 2011, and 2010 and (vi) Notes to the Consolidated Financial Statements. |
| * | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4), 200.83 and 230.46. |
| ** | Management compensatory plan or arrangement. |
| *** | XBRL information is furnished and not filed or a part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Exchange Act of 1933, as amended, is deemed not filed for purposes |
117
| | of section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections. |
| (1) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on July 28, 2003. |
| (2) | Incorporated by reference from the Company’s Registration Statement on Form S-8 (No. 33-80911) filed with the Commission on December 28, 1995. |
| (3) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on January 26, 1998. |
| (4) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 1998. |
| (5) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2003. |
| (6) | Incorporated by reference from the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. |
| (7) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 25, 2006. |
| (8) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2007 filed on August 8, 2007. |
| (9) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 22, 2006. |
| (10) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 28, 2006. |
| (11) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 21, 2007. |
| (12) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2008 filed on August 1, 2008. |
| (13) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on December 4, 2008. |
| (14) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on April 8, 2009. |
| (15) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 11, 2009. |
| (16) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on August 9, 2010. |
| (17) | Incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 31, 2011. |
| (18) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 10, 2011. |
| (19) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on July 6, 2011. |
| (20) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on November 9, 2011. |
| (21) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on May 10, 2012. |
| (22) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on August 8, 2012. |
| (23) | Incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on November 9, 2012. |
| (24) | Incorporated by reference from the Company’s Current Report on Form 8-K filed on June 7, 2012. |
Item 15 (c). See Item 15(a) above.
118
