UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| | | |
| (Mark One) | | |
þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the fiscal year ended December 31, 2009 |
or |
o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the transition period from to |
Commission file number000-23092
NATIONAL DENTEX CORPORATION
(Exact name of registrant as specified in its charter)
| | | |
MASSACHUSETTS
(State or Other Jurisdiction of
Incorporation or Organization) | | 04-2762050
(I.R.S. Employer
Identification No.) |
2 Vision Drive,
Natick, MA
(Address of Principal Executive Offices) | | 01760
(Zip Code) |
(508) 907-7800
(Registrant’s Telephone No., including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
| | | |
Title of Each Class | | Name of Exchange on Which Registered |
| |
| Common Stock, par value $.01 per share | | The NASDAQ Stock Market, LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 ofRegulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” inRule 12b-2 of the Exchange Act. (Check one):
| | | | | | | |
Large accelerated filer o | | Accelerated filer o | | Non-accelerated filer þ | | Smaller reporting company o |
| | | | | (Do not check if a smaller reporting company) | | |
Indicate by check mark whether the registrant is a shell company as defined inRule 12b-2 of the Exchange Act. Yes o No þ
As of June 30, 2009, the aggregate market value of the 5,512,656 outstanding shares of voting stock held by non-affiliates of the registrant was $35,887,391, based upon the closing price of the Common Stock on the NASDAQ Global Market on such date.
As of March 11, 2010, 5,761,363 shares of the registrant’s Common Stock, par value $.01 per share, were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the proxy statement for the 2010 annual stockholders’ meeting, which we plan to file with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2009, are incorporated by reference into Part III.
TABLE OF CONTENTS
PART I
General
We were founded in 1982 as H&M Laboratories Services, Inc., a Massachusetts corporation, which acquired six full-service dental laboratories and related branch laboratories from Healthco, Inc. In 1983, we changed our name to National Dentex Corporation and acquired 20 additional full-service dental laboratories and related branch laboratories from Lifemark Corporation. Our acquisition strategy is to consolidate our position within the dental laboratory industry and use our financial and operational synergies to create a competitive advantage. Over the last five years we have acquired the following stand-alone laboratory facilities: in 2005, Wornson-Polzin Dental Laboratory and Green Dental Laboratories; in 2006, Impact Dental and the Keller Group; and in 2008, Dental Art Laboratories. Impact, located in the Canadian province of Ontario, was our first acquisition outside of the United States.
We currently own and operate 44 dental laboratories, consisting of 40 full-service dental laboratories and 4 branch laboratories located in 30 states throughout the United States and in one province of Canada. Our dental laboratories custom design and fabricate dentures, crowns and fixed bridges, and other dental prosthetic appliances. Each dental laboratory operates under its own business name. Our principal executive offices are located at 2 Vision Drive, Natick, MA 01760, telephone number(508) 907-7800. Our corporate web site is www.nationaldentex.com. We make available free of charge through our website our annual report onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K, and amendments to these reports filed or furnished pursuant to Section 13(a) of the Securities Exchange Act of 1934 as soon as practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission. References to our website address are provided for convenience only and do not constitute, or should be viewed as, an incorporation by reference of the information contained therein. Therefore, such information should not be considered a part of this report.
Information as to Industry and Operating Segments
Our business consists of a single industry segment, which is the design, fabrication, marketing and sale of custom dental prosthetic appliances for and to dentists. We report on three reportable segments within this single industry segment. These three segments are known as Green Dental, representing the operations of Green Dental Laboratories, Inc. of Heber Springs, Arkansas which was acquired in March 2005; Keller Group, representing the operations of Keller Group, Incorporated with laboratories in St. Louis, Missouri and Louisville, Kentucky which was acquired in October, 2006; and NDX Laboratories, which represents our remaining laboratories. You will find information about these segments in Note 10 to the Consolidated Financial Statements “Segment Information”, which you will find in Part II, Item 8 of this annual report.
Description of Business
Our dental laboratories in all three of our reportable segments design and fabricate custom dental prosthetic appliances such as dentures, crowns and bridges. These products are produced by trained technicians working in dental laboratories in accordance with work orders and cases (consisting of impressions, models and occlusal registrations of a patient’s teeth) provided by the dentist. Dentists are the direct purchasers of our products.
Our products are grouped into the following three main categories:
Restorative Products. Restorative products that our dental laboratories sell consist primarily of crowns and bridges. A crown replaces the part of a tooth that is visible, and is usually made of gold, porcelain or zirconia. A bridge is a restoration of one or more missing teeth that is permanently attached to the natural teeth or roots. In addition to the traditional crown, we also make onlays, which are partial crowns which do not cover all of the visible tooth; inlays, which are restorations made to fit a prepared tooth cavity and then cemented into place and precision crowns, which are restorations designed to receive and connect a removable partial denture. We also mill crowns and bridges from zirconia using CAD-CAM (computer-assisted design and computer-assisted manufacturing) technologies.
1
Reconstructive Products. Reconstructive products sold by our dental laboratories consist primarily of partial dentures and full dentures. Partial dentures are removable dental prostheses that replace missing teeth and associated structures. Full dentures are dental prostheses that substitute for the total loss of teeth and associated structures. We also sell precision attachments, which connect a crown and an artificial prosthesis, and implants, which are fixtures anchored securely in the bone of the mouth to which a crown, a partial denture a or full denture is secured by means of screws or clips.
Cosmetic Products. Cosmetic products sold by our dental laboratories consist primarily of porcelain veneers and ceramic crowns. Porcelain veneers are thin coverings of porcelain cemented to the front of a tooth to enhance personal appearance. Ceramic crowns are crowns made from ceramic materials that most closely replicate natural teeth. We also sell composite inlays and onlays, which replace silver fillings for a more natural appearance, and orthodontic appliances, which are products fabricated to move existing teeth to enhance function and appearance.
Additionally, we manufacture and market under an exclusive license in the United States and on a non-exclusive basis in Canada, the laboratory version of the NTI-tss plustm device, an appliance that is an alternative to full-coverage bite guards, which is also approved by the Food & Drug Administration (FDA) for use in the treatment of medically diagnosed migraine pain and jaw disorders. We hold exclusive rights for the production of the laboratory fabricated version of this product through March 2023.
Laboratory and Corporate Operations
Our full-service dental laboratories design and manufacture a full range of custom-made dental prosthetic appliances. These custom products are manufactured from raw materials, such as high noble, noble and predominantly base alloys, zirconia, dental resins, composites and porcelain. There are different production processes for the various types of prosthetic appliances depending upon the product and the materials used in the type of appliance being manufactured, each of which requires different skills, technologies and levels of training. Our dental laboratories perform numerous quality control checks throughout the production cycle to improve the quality of our products and to better ensure that the design and appearance satisfy the needs of the dentist and the patient. Our branch dental laboratories are smaller in size and offer a limited number of products. When a branch receives an order that it cannot fill, the branch refers the order to one of our affiliated full-service dental laboratories.
We operate each of our full-service dental laboratories as stand-alone facilities under the direction of a local manager responsible for operation of the dental laboratory, supervision of its technical and sales staff and delivery of quality products and services. Most of our dental laboratories market and sell their products through their own direct sales force, while the remainder of our laboratories are supported by a team of national client specialists. Corporate level sales management, company-wide marketing programs and group managers are additional resources for our laboratories to draw upon. Employees at each dental laboratory have a direct stake in the growth of the dental laboratory and improvement in earnings through participation in our performance incentive plans.
Our corporate management provides our overall strategy, direction and financial management and negotiates all dental laboratory acquisitions. Corporate personnel also support the operations of our dental laboratories by performing functions that are not directly related to the production and sale of dental laboratory products, such as processing payroll and related benefit programs, obtaining insurance and procuring financing. Our corporate management provides marketing, financial and administrative services, negotiates national purchasing arrangements, and sets quality and performance standards for our dental laboratories. Finally, our corporate management includes industry recognized technical experts who guide and direct our investments in new technology and materials.
Sales and Marketing
The majority of our local dental laboratories market and sell their products through their own direct sales force, while the remainder of our laboratories are supported by a team of national client specialists. The sales force interacts with dentists within its local market area, primarily through visits to dentists’ offices, to introduce the dental laboratory’s services and products offered, and to promote new products and techniques that can assist dentists in expanding their practices. Our dentist-focused marketing and sales programs, are specifically designed to make choosing a dental laboratory an easier decision for dentists and help differentiate our laboratories from their
2
many competitors. We believe that our efforts to assist the dentist and his or her staff to improve chair-time efficiencies while providing exceptional service, superior quality and quick and timely product delivery will enhance our ability to expand our base of business by establishing lasting professional relationships with our customers. Our laboratory sales force is comprised of 31 local and national client specialists and 20 other team members, including inside sales and support personnel. In addition, our dental laboratories, alone or with local dental societies, dental schools or study clubs, sponsor technical training clinics for dentists and their staffs on topics such as advanced clinical techniques. Our dental laboratories also exhibit at state and local dental conventions.
In addition to our local service strategy, since the acquisition of the Keller Group, we now also market more directly to the entire United States dental marketplace using a direct mail and trade advertising approach that focuses upon products that can generate strong revenue growth, such as the NTI-tss plustm device. We believe this additional product offering has helped us to further diversify our business growth strategy.
Competition
The dental laboratory industry is highly competitive and fragmented. A typical dental laboratory’s business originates from dentists located within 50 miles of the dental laboratory. We believe there are currently approximately 12,000 dental laboratories in the United States. We estimate that our sales presently represent less than 3% of the total sales of custom-made dental prosthetic appliances in the United States. We face competition primarily from other dental laboratories in the respective local market areas. The vast majority of dental laboratories consist of single business units, although we recognize that there are several other multiple-location operators, including Dental Services Group, Dental Technologies, Inc. and NovaDent. These groups compete with us in several market areas. We also face competition from various mail order dental laboratories, most notably Glidewell Laboratories.
The domestic industry has experienced growing competition from low-wage countries, particularly from laboratories manufacturing in China. Competition for business in the low-price segment of the marketplace has grown over the last three years. Dental laboratories manufacturing in China, including Dentsply-Prident, Dentalle, Exceldent, DentUSA, Beijing Dental Lab, Sun Dental Lab and Trident operate large, modern facilities. In addition to partnering with laboratories in the United States, some have also reached agreements to provide laboratory services for some of the larger, price focused, economy dental practice chains. In addition, the number of smaller domestic competitors that seek to take advantage of this segment of the market by using price as the main differentiator has continued to grow. While our business has not traditionally focused on this lower cost segment of the market, certain customers are sensitive to price competition. As a result, these competitive pressures have constrained somewhat our ability to increase prices. Beginning in 2008, we partnered with Dentsply-Prident to provide a high quality, economical restoration manufactured in China with FDA registered materials for those price-focused practices who demand it. We believe that this product offering, which has been made available in select marketplaces based solely upon individual customer requests and is coupled with customer level disclosures regarding country of origin, materials and our satisfaction guarantee, provides these dentists with a risk-free, offshore product while allowing us to maintain the relationship to provide other U.S. manufactured products. We continue to evaluate competitive threats as well as opportunities arising from globalization, technology and changing marketplaces to ensure our products and services can continue to be provided at competitive prices.
Our ability to produce quality products locally, to deliver such products on a timely basis, to provide convenience for dentists through the breadth of our product line, to provide technical assistance, and our sponsorship of educational clinics, all provide us with what we consider to be a competitive advantage over other dental laboratories in the local markets in which our dental laboratories operate. Most dentists use a limited number of dental laboratories. We believe they prefer and tend to rely on those laboratories which produce quality products delivered on a timely basis and which carry all of the products which they may need, even if a particular item is a newer specialty product used only sporadically by the dentist. While price is one of the competitive factors in the dental laboratory industry, we believe that most dentists consider product quality and consistency, service, and breadth of product line to also be important factors in selecting dental laboratories. We believe that we compete favorably with respect to all of these factors. Our ability to provide newer specialty products for dental implants, adult orthodontics and cosmetic dentistry, which require highly skilled technicians, more extensive inventories, additional working capital, and investment in both training and capital equipment, also distinguishes us from the
3
many other dental laboratories which do not have comparable resources to provide these products. While such specialty products presently represent less than 20% of our business, we believe that the ability to offer these products will become increasingly essential for dental laboratories to remain competitive. Additionally, our ability to offer CAD-CAM fabricated, metal-free restorations through our own centralized milling centers allows us to participate fully in this fast growing segment.
Employees
As of December 31, 2009, we had 1,745 full time employees, 1,684 of whom worked at individual laboratories. Corporate management and administrative staff totaled 61 people. None of our employees are covered by a collective bargaining agreement. Management considers our employee relations to be good.
Intellectual Property
Our general technological know-how, experience and workforce are important to the conduct of our business. Each of our dental laboratories operates under its own trade name, and we consider these trade names to be important to the conduct of our business. Also important is the development and maintenance of customer relationships. We expect that our continued focus on ensuring that clients get a consistent product that is delivered on time and meets or exceeds their quality expectations, will continue to assist us in generating and maintaining customer relationships and the goodwill of our dental laboratories. We also have licensed long-term, exclusive manufacturing and distribution rights to fabricate and market the laboratory version of the NTI-tss plustm device in the United States. Finally, while we have several other trademarks and licenses to use trademarks, we do not deem these to be material to the overall conduct of our business.
Backlog
Due to the individualized and customized nature of most dental products and a typical turnaround product cycle of less than seven days, there was no significant backlog of orders existing at December 31, 2009 and 2008.
Our business is subject to certain risks that could materially affect our financial condition, results of operations, and the value of our common stock. These risks include, but are not limited to, the ones described below. Additional risks and uncertainties that we are unaware of, or that we may currently deem immaterial, may become important factors that harm our business, financial condition, results of operations, or the value of our common stock.
Our success depends on economic and other external factors that affect consumer decisions about whether and when to have dental procedures performed.
Our business success depends in large measure on consumer decisions to have dental procedures performed. In this respect, demand for our products and our business results are sensitive to external factors that, directly or indirectly, affect consumer confidence, affect levels of disposable consumer income, or otherwise lead consumers to defer or elect not to have dental procedures performed. Examples of such external factors include the timing, duration and effects of adverse changes in overall economic conditions, including rates of job loss or growth, rising food and energy prices, tightening consumer credit and the resulting problems in the housing market, and increases in medical and dental costs, nationally or regionally in the markets we serve. Trends in the dental industry towards managed care may also result in decreased consumer access to dental services and thereby adversely affect demand for our products and our sales and profitability. The precise impact of these external factors is difficult to predict in advance, but one or more of these factors could adversely affect our business to the extent they adversely affect consumer spending on dental procedures.
The US and world economy have experienced a recession that has affected all sectors of the economy, resulting in declines in economic growth and consumer confidence, increases in unemployment rates and uncertainty about economic stability. Recently, there have been some signs that the US economy is recovering; however, the strength and duration of that recovery is unpredictable. Recessions and other economic downturns, especially prolonged downturns, as well as disruptions in the credit and stock markets, can result in lower levels of economic activity,
4
lower employment levels, less consumer disposable income and lower economic confidence. Any of these factors can reduce discretionary consumer spending. Many or our cosmetic dental products may be considered discretionary spending by consumers. If consumers reduce or delay spending on cosmetic dental products, our business and financial performance may be adversely affected.
We operate in a highly competitive and fragmented market that is increasingly global in scope.
The dental laboratory industry is highly competitive and fragmented. We believe there are currently approximately 12,000 dental laboratories in the United States. We estimate that our sales presently represent less than 3% of the total sales of custom-made dental prosthetic appliances in the United States. Competition is primarily from other dental laboratories in the respective local market areas. The vast majority of dental laboratories consist of single business units, although there are several other multiple-location operators, including Dental Services Group, Dental Technologies, Inc., and NovaDent. These groups compete with us in several market areas. We also face competition from various mail order dental laboratories, most notably Glidewell Laboratories. Our success thus depends on our ability to be competitive against many different competitors in each market area we serve. If we fail to anticipate evolving technological innovations and product offerings from our competitors, particularly offerings that seek to leverage lower labor costs available in foreign countries, or fail to offer products that appeal to the changing needs and preferences of our customers in the various markets we serve, demand for our products could decline and our operating results would be adversely affected. While the competitive importance of product quality, price, service and innovation varies from product to product, price is a factor, and we experience pricing pressures from competitors in our markets.
We face increased competitive pressures from foreign-sourced products and technology-based solutions.
The industry in which we operate continues to change and evolve. Increasing competitive pressures from offshore laboratories based in China, India and elsewhere are impacting sales growth and selling prices of certain core products, particularly partial frames and traditional crowns. Technology-based dental laboratory CAD-CAM solutions have required us to make additional investments in capital equipment. While we expect these capital investments to continue to benefit our operations in future periods, there is no assurance that they will be able to do so. Certain of these technology-based solutions also enable dentists to fabricate restorations in their offices rather than purchasing them from an off-site laboratory.
Price pressures from such new sources of competition could erode our margins and cause our financial results of operations to suffer. Our success depends on our ability to evaluate and respond to the threats arising from growing foreign competition, changing marketplaces and new technology and our ability to identify ways in which we can competitively provide the products and services demanded by our customers.
We face increased competitive pressures from multiple location operators and the consolidation in the industry.
The dental laboratory industry has continued to consolidate and has drawn the attention of private equity investors. While the consequences of these changes in the dental laboratory industry are not yet fully known, these competitors may now have greater financial and other resources than previously available to them, which could increase competitive pressures on our operations. These developments could impact the availability of suitable acquisition candidates or otherwise increase the costs of acquiring dental laboratories.
Our failure to generate sufficient cash to meet our liquidity needs may affect our ability to service our indebtedness and grow our business.
Our ability to make payments on and to refinance our indebtedness, principally the amounts borrowed under our credit facility, and to fund planned capital expenditures, expansion efforts and strategic acquisitions we may make in the future, if any, will depend on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive and other factors that are beyond our control.
Based on our current level of operations, we believe our cash flow from operations, together with available cash and available borrowings under our senior credit facility, will be adequate to meet future liquidity needs in the
5
coming year. However, we cannot assure you that our business will generate sufficient cash flow from operations in the future or that future borrowings will be available to us under the senior credit facility in an amount sufficient to enable us to service indebtedness, undertake strategic acquisitions to grow our business, or to fund other liquidity needs. If we need to refinance all or a portion of our indebtedness, we cannot assure you that we will be able to do so on commercially reasonable terms or at all. In the event that our financial performance was to deteriorate, it could result in higher interest rates under our senior credit facility, or a default under the facility, if we are unable to maintain compliance with the financial ratio and other covenants in the facility.
We may be more leveraged than some of our competitors, which could adversely affect our business plans.
We have incurred debt in pursuing our strategic acquisitions and a portion of our cash flow is used to service this debt. This in turn has reduced the funds we have available for working capital, capital expenditures, additional acquisitions, and other purposes and, a significant part of our growth strategy is to acquire additional dental laboratories. Given current credit conditions it may be more difficult for us to make additional borrowings in the future, potentially affecting our growth strategy.
Similarly, our debt levels may increase our vulnerability to, and limit our flexibility in planning for, adverse economic and industry conditions and create other competitive disadvantages compared with other companies with relatively less leverage, especially in times of industry consolidation.
Our Senior Credit Facility contains a number of financial and other covenants that may restrict our ability to engage in some business transactions.
We have entered into a senior credit facility with Bank of America, N.A. which includes a term loan facility and a line of credit of $25 million. As of December 31, 2009, the outstanding balance was $19.6 million on the term loan and $6.5 million on the revolving line of credit. The credit agreement restricts our ability and the ability of our subsidiaries to, among other things, engage in a number of actions including incurring or guaranteeing additional indebtedness, merging or consolidating with, acquiring substantially all of the stock or assets of any other companies, acquiring additional dental laboratories, repurchasing our common stock, making investments, transferring assets, incurring or permitting to exist liens, and making any material changes to the nature of our business. The credit facility also contains other covenants that are typical for credit facilities of this size, type and tenor, such as requirements that we meet specified financial ratios and financial condition tests. Our ability to make additional borrowings under the facility depends upon satisfaction of these covenants. Our ability to meet these covenants and requirements may be affected by events beyond our control.
Our inability to comply with obligations could result in an event of default under the credit facility. A default, if not cured or waived, could permit acceleration of our indebtedness. We cannot be certain that we will be able to remedy any default. If our indebtedness is accelerated, we cannot be certain that we will have funds available to pay the accelerated indebtedness or that we will have the ability to refinance the accelerated indebtedness on terms favorable to us or at all.
An impairment in the carrying value of goodwill or other acquired intangibles could negatively affect our operating results and net worth.
General economic conditions, or other factors resulting in changes in the industry in which we operate, competition, advances in technology, or other factors may lead to reductions in expected sales, profitability or cash flows that could result in impairment of goodwill and other acquired intangible assets. If the value of goodwill or other acquired intangibles is impaired, our earnings, net worth and financial covenants could be adversely affected.
The goodwill impairment analysis is a two-step process. The first step is used to identify potential impairment and involves comparing each reporting unit’s estimated fair value to its carrying value, including goodwill. Fair value is determined by using an income approach, consistent with our valuation of dental laboratories acquired in purchase business combinations. We determine fair value based on the estimated future cash flows of each reporting unit, based on a multiple of annual earnings. Determining the fair value of a reporting unit is judgmental in nature and requires the use of significant estimates and assumptions, including revenue growth rates and profit margin percentages, and future market conditions, among others. Our projections are based on an internal forecast and a
6
business review. If the estimated fair value of a reporting unit exceeds its carrying value, goodwill is not considered to be impaired. However, if the carrying value exceeds estimated fair value, there is an indication of potential impairment and the second step is performed to measure the amount of impairment.
The second step of the goodwill impairment process involves the calculation of an implied fair value of goodwill for the laboratories which step one indicated were impaired. The implied fair value of goodwill is determined similar to how goodwill is calculated in a business combination, by measuring the excess of the estimated fair value of the reporting unit as calculated in step one, over the estimated fair values of the individual assets, liabilities and identifiable intangibles as if the reporting unit was being acquired in a business combination. If the carrying value of goodwill assigned to a reporting unit exceeds the implied fair value of the goodwill, an impairment charge is recorded for the excess. In determining the fair value of assets we utilize valuations of certain intangible assets, including trade names and customer relationships. The analysis we completed for December 31, 2008 determined that the fair value of ten dental laboratories in the NDX Laboratories operating segment was less than their carrying value resulting in goodwill impairment of $6,950,000. During the third quarter of 2009, the Company recorded an impairment charge of $264,000, comprising the entire goodwill balance of one of our smaller laboratories, due to a significant decrease in revenues and operating income during the third quarter of 2009 at this laboratory. As of December 31, 2009, we had $69,616,000 of goodwill remaining on our consolidated balance sheet. If the conditions noted above were to significantly deteriorate, we may need to record additional goodwill impairment charges, which could adversely affect our earnings, net worth and debt covenants.
Risks associated with our strategic acquisitions could adversely affect our business.
We have completed a number of acquisitions over the past five years. Our acquisition strategy depends on our ability to identify laboratories that are suitable acquisition candidates, successfully negotiate and enter into transactions on acceptable terms, and our capacity to integrate and successfully operate newly acquired as well as our previously acquired laboratories. If we fail to locate suitable acquisition candidates, reach mistaken conclusions as to the suitability of laboratories as acquisition candidates, enter into transactions on terms that prove unfavorable to us, or fail to integrate new laboratories following an acquisition, our ability to operate and grow our business in the ways we would like could be materially and adversely affected. While we will continue to consider acquisitions as a means of enhancing shareowner value, acquisitions involve risks and uncertainties, including:
| | |
| | • | difficulties integrating the acquired company, retaining the acquired laboratories’ customers, and achieving the expected benefits of the acquisition, such as revenue increases, cost savings, and increases in geographic or product presence, in the desired time frames, if at all; |
| |
| | • | loss of key employees from the acquired company; |
| |
| | • | implementing and maintaining consistent standards, controls, procedures, policies and information systems; and |
| |
| | • | diversion of management’s attention from other business concerns. |
Our long-term success depends, in part, on our ability to acquire additional dental laboratories in a manner that achieves appropriate returns on our capital invested. If we are unable to generate the required sales or profit levels, as a result of macroeconomic or operational challenges, we will not acquire new dental laboratories and our future financial performance could be materially and adversely affected. Additionally, future acquisitions could cause us to incur additional debt, contingent liabilities, increased interest expense and higher amortization expense related to intangible assets, as well as experience dilution in earnings per share. Impairment losses on goodwill and intangible assets with an indefinite life, or restructuring charges, could also occur as a result of acquisitions, which could adversely affect our results of operations and cause us to violate the covenants under our senior credit facility.
If we fail to develop new or expand existing customer relationships, our ability to grow our business will be impaired.
Our growth depends on our ability to develop new customer relationships with dentists, maintain existing relationships, and to expand existing relationships with our current customers. We cannot guarantee that new customers will be found, that any such new relationships will be successful when they are in place, or that business
7
with current customers will increase. Failure to develop and expand such relationships could have a material adverse effect on our business, results of operations and financial condition.
If we cannot continue to respond to technical innovations we may not be able to compete effectively.
We believe that our future success will depend, in part, upon our ability to continue to respond to technological innovations by the dental industry and introduce innovative design extensions for our existing products and to manufacture and market new products. We cannot assure you that we will be successful in the introduction, manufacturing and marketing of any new products or product innovations, or develop and introduce, in a timely manner, innovations to our existing products that satisfy our dentist customers’ needs or achieve market acceptance. Our failure to introduce new products successfully and in a timely manner, and at favorable margins, could harm our ability to successfully grow our business and could have a material adverse effect on our business, results of operations and financial condition.
Our failure to attract and retain qualified personnel would adversely affect our business.
Our success depends in part on the efforts and abilities of our senior management team and key employees, a number of which are approaching retirement age. Their skills, experience and industry contacts significantly benefit our operations and administration. The failure to attract, retain, and properly motivate the members of our senior management team and key employees, or to find suitable replacements for them in the event of death, ill health, resignation, or retirement, could have a negative effect on our operating results. In addition, our success is also dependent upon our ability to retain qualified employees at our dental laboratories, including dental laboratory technicians who are able to manufacture our dental prosthetics. In the future, if competition for these services increases, the Company may not be able to continue to attract and retain these individuals, which could have a material adverse effect on our revenues.
Our business results are adversely affected by increases in labor, benefits and related costs.
The costs of medical and other benefits have increased in recent years. The increased usage of medical benefits has intensified medical inflation in the United States. If such trends continue, then our business could be negatively affected. Changes in law that may increase the funding of, and the expense reflected for, employee benefits, could also adversely affect our financial results of operations, financial position, and competitiveness.
Our operating results can be adversely affected by changes in the cost or availability of raw materials, particularly precious metals like gold, platinum and palladium.
Pricing and availability of raw materials for use in our businesses — most especially precious metals, like gold, platinum and palladium which are components of many dental alloys — can be volatile due to numerous factors beyond our control, including domestic and international economic and geopolitical conditions, production levels, competition, consumer demand, and investor speculation. This volatility can significantly affect the availability and cost of raw materials for us, and may, therefore, have a material adverse effect on our business, results of operations and financial condition. During periods of rising prices of raw materials, there can be no assurance that we will be able to pass any portion of such increases on to customers. Prolonged higher metal costs may thus have a negative impact on gross profit percentages. Conversely, when raw material prices decline, customer demands for lower prices could result in lower sale prices and, to the extent we have existing inventory, lower margins. As a result, fluctuations in raw material prices could have a material adverse effect on our business, results of operations and financial condition. The combination of higher precious metal prices and increasing offshore competition has made it more difficult for us to pass on these additional costs without impacting our customer base.
Compliance with changing regulation of corporate governance, public disclosure, and accounting standards may result in additional expenses and risks.
Changing laws, regulations and standards relating to corporate governance, public disclosure and accounting practices, new Securities and Exchange Commission regulations and evolving rules applicable to publicly-traded
8
companies on the NASDAQ Global Market (Nasdaq), are creating uncertainty, and hence risks, for companies such as ours. These new or changed laws, regulations and standards are subject to varying interpretations due to the fact that they are new and there has not yet emerged a well-developed body of interpretation. As a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This development could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure, governance and accounting practices.
Our efforts to comply with evolving laws, regulations and standards have resulted in, and are likely to continue to result in, increased general and administrative expenses and an investment of management time and attention from revenue-generating activities to compliance activities. In particular, our efforts to comply with Section 404 of the Sarbanes-Oxley Act of 2002 and the related regulations regarding our required assessment of our internal controls over financial reporting and our external auditors’ audit of that assessment has required the commitment of significant financial and managerial resources. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies, we could face many material and adverse consequences, including, a possible delisting of our common stock.
We are subject to a number of continuing listing standard requirements of Nasdaq Global Market. If we fail to comply with these listing standards we may be subject to delisting.
Our common stock is currently listed on the NASDAQ Global Market (Nasdaq). Nasdaq marketplace rules require the maintenance of a number of continued listing standards. If we fail to comply with any of the continued listing standards and are unable to cure such defect within the allotted time following the receipt of any notice from Nasdaq regarding our failure to maintain such continued listing standard, Nasdaq might delist our common stock. Delisting would have an adverse effect on the liquidity of our common stock and, as a result, the market price for our common stock might become more volatile. Delisting could also make it more difficult for us to raise additional capital.
We may face increased regulatory action by, among other governmental entities, the United States Food and Drug Administration (the “FDA”).
Since 2007, the industry has faced increased scrutiny from the FDA and other governmental entities concerning the safety and efficacy of dental products distributed in the United States from both foreign and domestic laboratories. As a result of this scrutiny, the FDA may propose possible registration, certification, material content and point of origin disclosure. Several states have passed legislation for a combination of material content and point of origin disclosures. Potential changes in laws, regulations and standards involving these issues, while new and evolving, are creating uncertainty in compliance requirements and could result in higher costs in order to comply with any revisions or additions to them and may create new legal liabilities if we fail to do so.
Our business may be adversely affected by the actions of and risks associated with our third-party suppliers.
If we experience declining operating performance, or if we experience liquidity challenges, our suppliers may demand accelerated payment of amounts due to them or require advance payments or letters of credit before goods are shipped to us. These demands could have a significant adverse impact on our operating cash flow and result in a drain on our liquidity. In addition, many of our suppliers may be significantly impacted by current macroeconomic conditions. We may have no warning before a supplier fails, which may have an adverse effect on our business and results of operations. Further, we cannot control the cost of our raw products, and cost increases must either be passed along to our customers or will result in erosion of our earnings.
Forward Looking Statements
Certain statements in this Annual Report, particularly statements contained in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The words “anticipate”, “believe”, “estimate”, “expect”, “plan”, “intend” and other similar expressions are intended to identify these forward-looking statements, but are not the
9
exclusive means of identifying them. Forward-looking statements included in this Annual Report or hereafter included in other publicly available documents filed with the Securities and Exchange Commission (“SEC”), reports to our stockholders and other publicly available statements issued or released by us involve known and unknown risks, uncertainties, and other factors which could cause our actual results, performance (financial or operating) or achievements to differ from the future results, performance (financial or operating) or achievements expressed or implied by such forward-looking statements. Such future results are based upon our best estimates based upon current conditions and the most recent results of operations. We assume no obligation to update these forward-looking statements contained in this report, whether as a result of new information, future events, or otherwise.
Various risks, uncertainties and contingencies could cause our actual results, performance or achievements to differ materially from those expressed in, or implied by, the forward-looking statements contained in this Annual Report. These include, but are not limited to, those listed above in this Item 1A, “Risk Factors.”
| |
| Item 1B. | Unresolved Staff Comments |
Not applicable.
We currently lease a total of approximately 361,000 square feet of space. As of December 31, 2009, the future aggregate minimum rent payable for all of our leased real properties was approximately $16,092,000. We believe that suitable substitute or replacement space is readily available at reasonable rental rates. Our principal executive and administrative offices occupy approximately 15,000 square feet of space in Natick, Massachusetts. Our 38 leased dental laboratories range in size from 1,000 to 40,000 square feet and average approximately$95,000 in annual base rent.
As of December 31, 2009, we owned six of our dental laboratory facilities at locations in Heber Springs, Arkansas; Metairie, Louisiana; Shreveport, Louisiana; Addison, Texas; Houston, Texas; and Waukesha, Wisconsin. These locations total approximately 152,000 square feet and range in building size from 10,000 to 41,000 square feet. In September 2009, we sold our former Denver, Colorado facility comprising approximately 6,000 square feet and recognized a gain on the sale of $365,000.
All of our owned real property is used in connection with our NDX Laboratories operating segment, except for our Heber Springs, Arkansas facility, which is used by our Green Dental operating segment. Our third operating segment, Keller, uses leased property located in St. Louis, Missouri and Louisville, Kentucky. We consider our leased and owned properties to be modern, well maintained and suitable for our purposes and believe that our current facilities are adequate to meet our needs for the foreseeable future.
| |
| Item 3. | Legal Proceedings |
We are involved from time to time in litigation incidental to our business. Our management believes that the outcome of current litigation will not have a material adverse effect upon our operations or financial condition and will not disrupt our normal operations.
| |
| Item 4. | Removed and Reserved |
| |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
10
PART II
Trading Market
The NASDAQ Global Market (“Nasdaq”) is the principal market for our common stock, where our shares are traded under the symbol “NADX”. Our common stock has been publicly traded since December 21, 1993.
The following table sets forth the range of high and low sale prices for our common stock for each of the fiscal quarters of 2008 and 2009. The sale prices set forth below are based on information provided by NASDAQ.
| | | | | | | | | |
| | | Price |
Quarter Ending | | Low | | High |
| |
| 03/31/08 | | $ | 11.50 | | | $ | 16.84 | |
| 06/30/08 | | $ | 10.05 | | | $ | 13.25 | |
| 09/30/08 | | $ | 6.01 | | | $ | 12.60 | |
| 12/31/08 | | $ | 4.19 | | | $ | 6.90 | |
| 03/31/09 | | $ | 1.25 | | | $ | 5.35 | |
| 06/30/09 | | $ | 3.78 | | | $ | 8.00 | |
| 09/30/09 | | $ | 6.25 | | | $ | 8.93 | |
| 12/31/09 | | $ | 6.00 | | | $ | 12.73 | |
Holders
The number of record holders of our common stock as of March 1, 2010 was 612. The number of record owners was determined from our stockholder records, and does not include beneficial owners of our common stock whose shares are held in the names of various security holders, dealers and clearing agencies. We believe that the number of beneficial owners of our common stock held by others in nominee names is approximately 1,200 beneficial holders.
Dividends
We have never paid a cash dividend on our shares of common stock and have no expectation of doing so for the foreseeable future.
Recent Sales of Unregistered Securities
None.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
In November 2002, we announced that our Board of Directors approved the repurchase by us of up to 300,000 shares of our common stock pursuant to a stock repurchase program. During the year ended December 31, 2009, we did not repurchase any shares of our common stock. We continue to consider repurchases on the open market or in privately-negotiated transactions, at management’s discretion, in each case subject to applicable securities law. In addition, before making any repurchases, we are required to obtain approval from our lender under the terms of the credit facility. The following table provides information about our repurchase activity during fiscal 2009 and the number of shares that may yet be purchased under our stock repurchase program.
Issuer Purchases of Equity Securities
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Maximum Number
|
| | | | | | | Total Number of
| | of Shares that
|
| | | | | | | Shares Purchased
| | May Yet Be
|
| | | Total Number
| | Average
| | as Part of Publicly
| | Purchased Under
|
| | | of Shares
| | Price Paid
| | Announced Plans
| | the Plans or
|
Fiscal Period | | Purchased | | per Share | | or Programs | | Programs |
| |
| January 1, 2009 - December 31, 2009 | | | — | | | $ | — | | | | — | | | | 206,700 | |
11
Stock Performance Graph
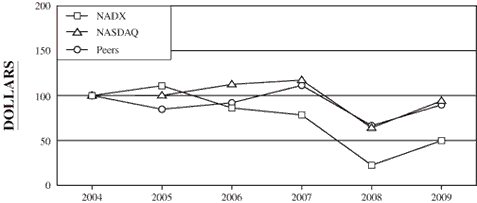
The following graph compares the cumulative total stockholder return of our common stock during the five fiscal years ended December 31, 2009 with the cumulative total return of the NASDAQ Industrial Index and a peer group index described more fully below.
This graph is not deemed to be “filed” with the SEC or subject to the liabilities of Section 18 of the Securities Exchange Act of 1934, and the graph shall not be deemed to be incorporated by reference into any prior or subsequent filings by us under the Securities Act of 1933 or the Securities Exchange Act of 1934.
COMPARISON OF CUMULATIVE TOTAL RETURN (1) AMONG NATIONAL DENTEX (“NADX”),
NASDAQ INDUSTRIAL INDEX AND PEER GROUP INDEX (2)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | 12-31-04 | | | | 12-31-05 | | | | 12-31-06 | | | | 12-31-07 | | | | 12-31-08 | | | | 12-31-09 | |
| NADX | | | | 100.00 | | | | | 111.03 | | | | | 86.20 | | | | | 78.42 | | | | | 22.41 | | | | | 49.75 | |
| NASDAQ Industrial Index | | | | 100.00 | | | | | 100.12 | | | | | 112.52 | | | | | 117.27 | | | | | 64.11 | | | | | 94.07 | |
| Peers | | | | 100.00 | | | | | 84.82 | | | | | 92.01 | | | | | 111.40 | | | | | 66.55 | | | | | 89.65 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Assumes $100 invested on December 31, 2004 in our common stock, the NASDAQ Industrial Index and the Peer Group Index, including reinvestment of any dividends paid on the investment. |
| |
| (2) | | The Peer Group Index consists of Dentsply International, Inc. and Patterson Companies, Inc. We believe that these companies represent the other publicly traded companies within the dental service community. |
12
| |
| Item 6. | Selected Financial Data |
The following selected financial data for the five years ended December 31, 2009 are derived from our audited consolidated financial statements. The data should be read in conjunction with the consolidated financial statements and the related notes included in this Report and in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| | | | | | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2006 | | | 2007 | | | 2008 | | | 2009 | |
| | | (Dollars in thousands, except per share data) | |
| |
Consolidated Statements of Income: | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 135,843 | | | $ | 150,107 | | | $ | 170,361 | | | $ | 171,674 | | | $ | 161,195 | |
| Cost of goods sold | | | 78,381 | | | | 88,269 | | | | 97,739 | | | | 102,184 | | | | 93,119 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 57,462 | | | | 61,838 | | | | 72,622 | | | | 69,490 | | | | 68,076 | |
| Selling, general & administrative expenses | | | 44,728 | | | | 50,097 | | | | 58,562 | | | | 58,588 | | | | 55,755 | |
| Goodwill impairment | | | — | | | | — | | | | — | | | | 6,950 | | | | 264 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating income | | | 12,734 | | | | 11,741 | | | | 14,060 | | | | 3,952 | | | | 12,057 | |
| Other expense | | | 646 | | | | 786 | | | | 771 | | | | 747 | | | | 745 | |
| Interest expense | | | 665 | | | | 1,523 | | | | 2,803 | | | | 2,110 | | | | 1,257 | |
| | | | | | | | | | | | | | | | | | | | | |
| Income before provision for income taxes | | | 11,423 | | | | 9,432 | | | | 10,486 | | | | 1,095 | | | | 10,055 | |
| Provision for income taxes | | | 4,334 | | | | 3,669 | | | | 3,860 | | | | 1,972 | | | | 4,178 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | 7,089 | | | $ | 5,763 | | | $ | 6,626 | | | $ | (877 | ) | | $ | 5,877 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) per share — basic | | $ | 1.33 | | | $ | 1.05 | | | $ | 1.20 | | | $ | (0.16 | ) | | $ | 1.03 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) per share — diluted | | $ | 1.27 | | | $ | 1.01 | | | $ | 1.17 | | | $ | (0.16 | ) | | $ | 1.02 | |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding — basic | | | 5,334 | | | | 5,485 | | | | 5,540 | | | | 5,631 | | | | 5,727 | |
| Weighted average shares outstanding — diluted | | | 5,601 | | | | 5,732 | | | | 5,665 | | | | 5,631 | | | | 5,761 | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Working capital | | $ | 11,126 | | | $ | 6,232 | | | $ | 6,000 | | | $ | 9,527 | | | $ | 9,296 | |
| Total assets | | | 117,119 | | | | 148,490 | | | | 155,639 | | | | 161,515 | | | | 155,295 | |
| Long-term debt, including current portion | | | 18,701 | | | | 35,458 | | | | 29,695 | | | | 39,258 | | | | 26,848 | |
| Stockholders’ equity | | $ | 76,074 | | | $ | 82,794 | | | $ | 91,192 | | | $ | 90,492 | | | $ | 97,790 | |
| |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion should be read in conjunction with the Consolidated Financial Statements
and the related notes that appear elsewhere in this document.
Certain statements in this Annual Report, particularly statements contained in this Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The words “anticipate”, “believe”, “estimate”, “expect”, “plan”, “intend” and other similar expressions are intended to identify these forward-looking statements, but are not the exclusive means of identifying them. Forward-looking statements included in this Annual Report or hereafter included in other publicly available documents filed with the Securities and Exchange Commission (“SEC”), reports to our stockholders and other publicly available statements issued or released by us involve known and unknown risks, uncertainties, and other factors which could cause our actual results, performance (financial or operating) or achievements to differ from the future results, performance (financial or operating) or achievements expressed or implied by such forward-looking statements. Such future results are based upon our best estimates based upon current conditions and the most recent results of operations. Various risks, uncertainties and contingencies could cause our actual results, performance or achievements to differ materially from those
13
expressed in, or implied by, the forward-looking statements contained in this Annual Report. These include, but are not limited to, those described above under Item 1A, “Risk Factors.” We assume no obligation to update these forward-looking statements contained in this report, whether as a result of new information, future events, or otherwise.
Overview
We own and operate 44 dental laboratories located in 30 states and one Canadian province, serving an active customer base of over 24,000 dentists. Our business consists of the design, fabrication, marketing and sale of custom dental prosthetic appliances for dentists located primarily in the domestic marketplace.
Our products are grouped into the following three main categories:
Restorative Products. Restorative products that our dental laboratories sell consist primarily of crowns and bridges. A crown replaces the part of a tooth that is visible, and is usually made of gold, porcelain or zirconia. A bridge is a restoration of one or more missing teeth that are permanently attached to the natural teeth or roots. In addition to the traditional crown, we also make onlays, which are partial crowns which do not cover all of the visible tooth; inlays, which are restorations made to fit a prepared tooth cavity and then cemented into place and precision crowns, which are restorations designed to receive and connect a removable partial denture. We also mill crowns and bridges from zirconia using CAD-CAM (computer-assisted design and computer-assisted manufacturing) technologies.
Reconstructive Products. Reconstructive products sold by our dental laboratories consist primarily of partial dentures and full dentures. Partial dentures are removable dental prostheses that replace missing teeth and associated structures. Full dentures are dental prostheses that substitute for the total loss of teeth and associated structures. We also sell precision attachments, which connect a crown and an artificial prosthesis, and implants, which are fixtures anchored securely in the bone of the mouth to which a crown, partial denture or full denture is secured by means of screws or clips.
Cosmetic Products. Cosmetic products sold by our dental laboratories consist primarily of porcelain veneers and ceramic crowns. Porcelain veneers are thin coverings of porcelain cemented to the front of a tooth to enhance personal appearance. Ceramic crowns are crowns made from ceramic materials that most closely replicate natural teeth. We also sell composite inlays and onlays, which replace silver fillings for a more natural appearance, and orthodontic appliances, which are products fabricated to move existing teeth to enhance function and appearance.
Additionally, we manufacture and market under an exclusive license in the United States and on anon-exclusive basis in Canada, the laboratory version of the NTI-tss plustm device, an appliance that is an alternative to full-coverage bite guards, which is also approved by the FDA for use in the treatment of medically diagnosed migraine pain and jaw disorders.
Recent Trends
We believe that the recent economic recession in the United States has negatively impacted the entire dental laboratory industry, as price-sensitive consumers have postponed elective dental work. Recent economic conditions, coupled with high unemployment, tight credit and continued difficulties in the housing market, dampened consumer confidence and purchasing activities in 2009. Additionally, we believe that the low cost segment for United States manufactured dental prosthetics declined in 2009 as competition from offshore laboratories, primarily those located in China, has become more intensive. While our business has not traditionally focused on this low cost segment of the market, certain customers are sensitive to price competition. As a result, these increasing competitive pressures have somewhat constrained our ability to increase prices. Since 2007, these increasing competitive pressures in the form of low price competition have been partially responsible for decreasing revenues or revenue growth in several marketplaces. While the growth in offshore restorations moderated somewhat during 2009, the deployment of technology-based solutions that allow dentists to fabricate their own restorations without the use of a dental laboratory has increased. These trends appear to be restraining industry growth, and have impacted our results of operations.
14
The main components of our costs are labor and related employee benefits as well as raw materials, including precious metals such as gold and palladium. Beginning in the fourth quarter of 2008 and throughout 2009, we proactively reduced staffing levels to improve profitability and eliminate excess capacity in response to the economic recession and the decline in consumer discretionary spending. As a result of reductions in staffing levels, our costs for labor and related benefits for the year ended December 31, 2009 were significantly lower than incurred during the same period in 2008. We also focused on reducing discretionary operating expenses and as a result our operating expenses were reduced in the year ended December 31, 2009 compared to the prior year.
In addition to our cost reduction efforts, we have made additional investments in capital equipment for technology-based dental laboratory CAD-CAM manufacturing solutions. Our ability to afford and utilize these CAD-CAM systems provides us the opportunity to centrally produce product for many of our laboratories at more efficient and profitable levels. We believe we have begun to recognize these efficiencies and will continue to focus on more completely leveraging these and future technology investments to reduce labor costs. Therefore, we believe that these investments are critical to our long-term business strategy.
As described in more detail below, our focus on controlling costs has resulted in improved profitability, despite the industry wide slowdown in revenues in 2009 primarily due to the economic recession. As the economy recovers, we anticipate that our new cost structure and improvements in productivity will allow us to capitalize on the expected increase in demand for dental services, as patients who deferred these types of expenditures no longer do so.
Acquisitions
We continue to seek strategic acquisitions, which have played an important role in helping us increase sales from $135,843,000 in 2005 to $161,195,000 in 2009. In March 2005, we completed the acquisition of Green Dental Laboratories, Inc. (“Green”). Green is treated as a separate reportable segment for financial reporting purposes. In October 2006, we completed our largest acquisition to date, that of Keller Group, Incorporated (“Keller”) of St. Louis, Missouri. Keller is also treated as a separate reportable segment for financial reporting purposes. The acquisition of Keller has broadened our marketing strategies and product offerings. In recent years Keller has broadened its focus from local markets in the Midwest to the national marketplace. In order to sustain this strategy, Keller invests significantly in product advertising, primarily in dental print publications and direct mail, on products that can generate strong revenue growth. Most recently, in September 2008, we completed the acquisition of Dental Art Laboratories, Inc. (“Dental Art”) of Lansing, Michigan.
We have generally used long-term debt to finance the purchase of our dental laboratories, including Green, Keller and Dental Art. Future acquisitions may also be funded using available debt financing. As a result of these acquisitions, we became more highly leveraged than we were previously. Our interest expense had therefore become a more significant component of our pre-tax earnings, yet has declined in recent years. Interest expense of $2,803,000 in 2007 declined to $2,110,000 in 2008 primarily as a result of lower interest rates. Interest expense in 2009 further declined to $1,257,000 as a result of further decreases in interest rates coupled with a reduction in outstanding debt of $15,350,000 from $42,198,000 at December 31, 2008 to $26,848,000 at December 31, 2009, as we did not acquire any dental laboratories in 2009 increasing the pay down of ourlong-term debt.
Overview of Results of Operations
Since the fourth quarter of 2008, the decline in discretionary consumer spending resulting from the recessionary environment in the United States negatively impacted our revenues and the revenues of the entire dental laboratory industry, as price-sensitive consumers postponed elective dental work. In addition, competitive pressures from offshore laboratories were partially responsible for declines in revenues or revenue growth in several marketplaces.
For the year ended December 31, 2009, net sales decreased $10,479,000 or 6.1% over year ended December 31, 2008. In September 2008, we acquired Dental Art. Therefore, sales for the year ending December 31, 2008 include only four months of Dental Art sales. Net sales in 2009 included approximately $5,067,000 as a result of eight additional months of sales provided by the Dental Art acquisition, without which net sales would have decreased approximately $15,546,000 or 9.1% for the full year ended December 31, 2009 as compared to 2008. Sales declined
15
$3,089,000, or 7.5%, to $38,175,000 for the fourth quarter of 2009 from $41,264,000 for the fourth quarter of 2008. By operating segment, in the fourth quarter of 2009 compared to the fourth quarter of 2008, sales for Keller declined 4.2%, compared to 7.5% for the first nine months of 2009 as compared to the same period in 2008, sales for Green declined 3.8% compared to 8.4% for the first nine months of 2009 as compared to the same period in 2008, and sales for NDX declined 8.7% compared to 10.2% for the first nine months of 2009 as compared to the same period in 2008, excluding acquired sales from Dental Art.
Despite the decline in revenues of $10,479,000, gross profit for the year ended December 31, 2009 decreased by only $1,414,000 from 2008, and our gross margins increased to 42.2% for the year ended December 31, 2009 from 40.5% in 2008. Within our cost of sales, production labor costs decreased by $4,084,000 and production employee benefits costs decreased by $1,110,000, as a result of reduced staffing levels and reduced overtime. Materials costs declined by $3,302,000 in fiscal 2009 as compared to fiscal 2008, as a result of lower volume and lower costs for precious metals. Laboratory overhead expenses decreased $569,000 in fiscal 2009 as compared to fiscal 2008, primarily as a result of cost reduction efforts.
Operating expenses decreased $2,833,000 or 4.8% for the year ended December 31, 2009, including decreases in labor and benefits of $2,418,000 at our laboratories, as a result of reduced staffing levels, and lower levels of various other expense items including delivery service, travel, and advertising expenses, from cost reduction efforts and lower fuel prices. As a result of improved profitability at many of our laboratories, laboratory incentive compensation increased $1,288,000 to $1,879,000 for the year ended December 31, 2009 from $591,000 for the year ended December 31, 2008. Accruals for corporate incentive compensation increased $1,040,000 to $1,090,000 for the year ended December 31, 2009 from $50,000 in 2008 as a result of the achievement of defined earnings targets. Increases in the cash surrender value of life insurance policies relative to large prior year declines, net of increases in the related deferred compensation accruals for our supplemental executive retirement plans decreased operating expenses by $460,000. Professional fees and consulting decreased by $655,000 to $1,513,000 for the year ended December 31, 2009 from $2,168,000 for 2008. Also in the third quarter of 2009, we recognized a gain of $365,000 resulting from the sale of real estate in Denver, Colorado that had been held for sale since December 2008. Offsetting these improvements in operating expenses, we recognized impairments of $361,000 related to trade names in 2009 compared to $44,000 in 2008. As a result of these factors, our operating expenses decreased $2,833,000 to $55,755,000 for the year ended December 31, 2009 from $58,588,000 in 2008.
In the fourth quarter of 2008, we identified an impairment of $6,950,000 related to ten laboratories in our NDX laboratory segment as a result of the economic recession. We assessed goodwill impairment on June 30, 2009, the date of our annual impairment test, and determined that no impairment charge was required. Although no laboratories were impaired at June 30, 2009, we reassessed goodwill impairment at the end of the third quarter of 2009. As a result of this analysis, we recorded an impairment charge of $264,000, comprising the entire goodwill of one of our smaller laboratories, due to a significant decrease in revenues and operating income in the third quarter at this laboratory compared to the previous forecast at June 30, 2009. During the fourth quarter of 2009, there were no events that triggered a goodwill impairment test or charge to goodwill impairment expense. We will continue to closely monitor the financial results of certain other locations in the NDX laboratories operating segment during subsequent periods in comparison to forecasted financial results and assumptions.
Our operating income increased to $12,057,000 for the year ended December 31, 2009 compared to $3,952,000 for the year ended December 31, 2008 primarily due to our goodwill impairment of $6,950,000 in 2008 and our cost reductions in labor and other expenses mentioned above.
Due to lower interest rates and debt levels, decreases in interest expense contributed $853,000 to pre-tax earnings. As a result of the factors discussed above, particularly goodwill impairment, income before provision for income taxes increased by $8,960,000 to $10,055,000 for the year ended December 31, 2009 from $1,095,000 for the year ended December 31, 2008.
Liquidity and Capital Resources
On August 9, 2005, we entered into an amended and restated financing agreement (the “Amended Agreement”) with Bank of America, N.A. (the “Bank”). The Amended Agreement included a revolving line of credit of $5,000,000, a revolving acquisition line of credit of $20,000,000 and a term loan facility of $20,000,000. The
16
interest rate on both revolving lines of credit and the term loan was the prime rate or, at our option, LIBOR, a cost of funds rate, or the Bank’s fixed rate plus a range of 1.25% to 2.25% per annum, depending on the ratio of consolidated funded debt to consolidated “EBITDA,” as defined in the Amended Agreement. The Amended Agreement required monthly payments of principal on the term loan, based on a seven-year amortization schedule, with a final payment due on the fifth anniversary of the Amended Agreement. The Amended Agreement required compliance with certain covenants, including the maintenance of specified net worth, income and other financial ratios.
In October 2006 we borrowed against our acquisition line of credit to finance our acquisition of Keller. In order to refinance the borrowings incurred for the Keller acquisition, we and the Bank executed a Second Amended and Restated Loan Agreement as of November 7, 2006 (the “Second Agreement”) comprising uncollateralized senior credit facilities that at that time totaled $60,000,000. The Second Agreement amended and restated the Amended Agreement (a) to increase the term loan facility to an aggregate principal amount of $35,000,000 and used the proceeds of the increase in the term loan to repay the portion of the outstanding principal balance under the acquisition line of credit and (b) to adjust the allocation of availability under the lines of credit by increasing the revolving line of credit to $10,000,000 ($5,000,000 of which may be used for future acquisitions) and decreasing the acquisition line of credit from $20,000,000 to $15,000,000. The interest rate on both lines of credit and the term loan was the prime rate or, at our option, LIBOR, a cost of funds rate or the Bank’s fixed rate, plus, in each case, a range of 1.25% to 3.00% per annum, depending on the ratio of consolidated total funded debt to consolidated “EBITDA,” as each is defined in the Second Agreement. The term loan facility portion of the Second Agreement requires monthly interest payments and monthly payments of principal, based on a seven year amortization schedule, with a final payment due on the fifth anniversary of the Second Agreement. The Second Agreement requires compliance with certain covenants, including the maintenance of specified net worth, minimum consolidated total “EBITDA,” debt to income ratio and other financial ratios.
The Second Agreement was amended on May 9, 2008, effective March 31, 2008, to revise certain financial targets within these covenants. Additionally, we and the Bank agreed to consolidate the revolving line of credit with the acquisition line of credit into a single line of credit of $25,000,000 to be used by us for general corporate purposes, including potential acquisitions. The Second Agreement was also amended on September 2, 2008 in connection with the acquisition of Dental Art, which increased our outstanding debt and therefore required an adjustment to an affected financial covenant. We further amended the agreement on December 16, 2008 to extend the maturity of the line of credit to November 7, 2011. The amendment changed the interest rate on both the line of credit and the term loan to prime rate or, at our option, LIBOR, a cost of funds rate, or the Bank’s fixed rate, plus, in each case, a range of 2.50% to 3.50% per annum, depending on the ratio of consolidated total funded debt to consolidated “EBITDA,” as each is defined in the Second Agreement and also increased the commitment fee on the unused portion of the line of credit from 0.125% to 0.50% per annum. In addition, the amendment revised certain financial targets within the covenants. Finally, on March 13, 2009, we amended the Second Agreement to exclude the $6,950,000 goodwill impairment in the fourth quarter of 2008 discussed previously from the calculation of “EBITDA,” used in determining our compliance with certain financial covenants. These amendments did not change the total availability under the Second Agreement. While we were in compliance with our debt covenants as of December 31, 2009, we continue to closely monitor our compliance with these covenants in future periods, particularly minimum consolidated total “EBITDA,” which may be negatively impacted by, among other things, potential declines in future earnings, including declines attributable to additional goodwill impairment.
17
As of December 31, 2009, $18,471,000 was available under the consolidated revolving line of credit.
Long-Term Obligations:
| | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
| Term note | | $ | 24,583,000 | | | $ | 19,583,000 | |
| Borrowings classified as long term under the revolving line of credit | | | 13,800,000 | | | | 6,529,000 | |
| Borrowings classified as short term under the revolving line of credit | | | 2,940,000 | | | | — | |
| Other long-term debt | | | 875,000 | | | | 736,000 | |
| | | | | | | | | |
| Total long-term debt | | | 42,198,000 | | | | 26,848,000 | |
| Less: current maturities | | | 8,055,000 | | | | 5,072,000 | |
| | | | | | | | | |
| Long-term debt, less current portion | | $ | 34,143,000 | | | $ | 21,776,000 | |
| | | | | | | | | |
The table below reflects the expected repayment terms associated with the Company’s long-term debt at December 31, 2009. The weighted average annual interest rate on all of our borrowings was 3.2% as of December 31, 2009.
| | | | | |
| | | December 31, 2009
| |
| | | Principal Due | |
| |
| Fiscal 2010 | | | 5,072,000 | |
| Fiscal 2011 | | | 21,187,000 | |
| Fiscal 2012 | | | 80,000 | |
| Fiscal 2013 | | | 85,000 | |
| Fiscal 2014 | | | 90,000 | |
| Thereafter | | | 334,000 | |
| | | | | |
| Total | | $ | 26,848,000 | |
| | | | | |
Liquidity:
Our working capital decreased by $231,000 to $9,296,000 at December 31, 2009 from $9,527,000 at December 31, 2008. Operating activities provided $15,813,000 in cash flow for the year ended December 31, 2009 compared to $13,327,000 during the year ended December 31, 2008, an increase of $2,486,000. The increase in cash flow provided by operating activities during 2009, as compared to the prior year period, was due primarily to the following factors:
| | |
| | • | increases in accrued expenses and accounts payable of $2,117,000 as a result of increases in payroll related accruals and the timing of vendor payments; |
| |
| | • | decreases of other receivables of $841,000 primarily due to collections of stop loss insurance for medical claims; |
| |
| | • | decreases in benefit for deferred income taxes of $957,000 due to timing differences in the deductibility of depreciation and goodwill impairment; |
partially offset by:
| | |
| | • | increases in inventory of $349,000 primarily due to increased precious metal values; |
| |
| | • | increases in prepaid assets of $681,000 primarily due to the timing of insurance payments, income taxes, and director fees; and; |
| |
| | • | increases in other assets of $722,000 due to improved performance of the cash surrender value of assets held for life insurance policies related to our supplemental employee retirement plans. |
18
Investing activities consumed $1,415,000 in cash flow for the year ended December 31, 2009 compared to $20,516,000 during the year ended December 31, 2008, a decrease of $19,101,000. Capital expenditures decreased $5,466,000 to $1,558,000 for the year ended December 31, 2009 from $7,024,000 for the year ended December 31, 2008, primarily due to lower spending on new facilities as a multi-year phase of laboratory construction had substantially concluded in 2008. In addition, for the year ended December 31, 2008, acquisition related cash outflows totaled $11,578,000, and cash outflows related to notes receivable totaled $2,000,000, while acquisition related cash outflows, consisting of a deferred purchase price payment, totaled only $300,000 in 2009.
For the year ended December 31, 2009, financing activities consumed $15,001,000 compared to providing $7,644,000 for the year ended December 31, 2008. The increased use of cash in financing activities of $22,645,000 is attributable to repayments on our revolving line of credit primarily as a result of greater availability of cash, due in large part to lower investing activities and increases in cash provided by operating activities, as discussed above.
We believe that cash flow from operations and available financing will be sufficient to meet contemplated operating and capital requirements such as those discussed below, for the foreseeable future.
Commitments and Contingencies
The following table represents a list of our contractual obligations and commitments as of December 31, 2009:
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due By Period | |
| | | | | | Less Than
| | | | | | | | | Greater Than
| |
| | | Total | | | 1 Year | | | 1 - 3 Years | | | 4 - 5 Years | | | 5 Years | |
| |
| Term Loan Facility | | $ | 19,583,000 | | | $ | 5,000,000 | | | $ | 14,583,000 | | | $ | — | | | $ | — | |
| Line of Credit | | | 6,529,000 | | | | — | | | | 6,529,000 | | | | — | | | | — | |
| Capital Leases | | | 736,000 | | | | 71,000 | | | | 156,000 | | | | 175,000 | | | | 334,000 | |
| Operating Leases: | | | | | | | | | | | | | | | | | | | | |
| Real Estate | | | 18,386,000 | | | | 3,829,000 | | | | 6,629,000 | | | | 4,165,000 | | | | 3,763,000 | |
| Vehicles | | | 444,000 | | | | 444,000 | | | | — | | | | — | | | | — | |
| Equipment | | | 236,000 | | | | 111,000 | | | | 98,000 | | | | 26,000 | | | | 1,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| TOTAL | | $ | 45,914,000 | | | $ | 9,455,000 | | | $ | 27,995,000 | | | $ | 4,366,000 | | | $ | 4,098,000 | |
| | | | | | | | | | | | | | | | | | | | | |
Bank borrowings on the term loan facility, with repayment terms greater than one year, are classified as long-term debt on the balance sheet.
We are committed under various non-cancelable operating lease agreements covering office space and dental laboratory facilities, vehicles and certain equipment. Certain of these leases also require us to pay maintenance, repairs, insurance and related taxes.
19
Results of Operations
Our results are reported within three operating segments, NDX Laboratories, Green Dental and Keller. The following table sets forth for the periods indicated the percentage of net sales represented by certain items in our Consolidated Financial Statements:
| | | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2007 | | 2008 | | 2009 |
| |
| Net sales | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
| Cost of goods sold | | | 57.4 | | | | 59.5 | | | | 57.8 | |
| | | | | | | | | | | | | |
| Gross profit | | | 42.6 | | | | 40.5 | | | | 42.2 | |
| Selling, general and administrative expenses | | | 34.3 | | | | 34.2 | | | | 34.5 | |
| Goodwill impairment | | | — | | | | 4.0 | | | | 0.2 | |
| | | | | | | | | | | | | |
| Operating income | | | 8.3 | | | | 2.3 | | | | 7.5 | |
| Other expense | | | 0.5 | | | | 0.4 | | | | 0.5 | |
| Interest expense | | | 1.6 | | | | 1.3 | | | | 0.8 | |
| | | | | | | | | | | | | |
| Income before provision for income taxes | | | 6.2 | | | | 0.6 | | | | 6.2 | |
| Provision for income taxes | | | 2.3 | | | | 1.1 | | | | 2.5 | |
| | | | | | | | | | | | | |
| Net income (loss) | | | 3.9 | % | | | (0.5 | )% | | | 3.7 | % |
| | | | | | | | | | | | | |
Year Ended December 31, 2009 Compared with Year Ended December 31, 2008
Net Sales
Since the fourth quarter of 2008, the decline in discretionary consumer spending resulting from the recessionary environment in the United States has negatively impacted our revenues and the revenues of the entire dental laboratory industry, as price-sensitive consumers postponed elective dental work. In addition, competitive pressures from offshore laboratories were partially responsible for declines in revenues or revenue growth in several marketplaces.
For the year ended December 31, 2009, net sales decreased $10,479,000 or 6.1% over the year ended December 31, 2008. We acquired Dental Art in September 2008. Therefore, sales for the year ending December 31, 2008 include only four months of Dental Art sales. Net sales in 2009 included approximately $5,067,000 as a result of eight additional months of sales provided by the Dental Art acquisition, without which net sales would have decreased approximately $15,546,000 or 9.1% for the full year ended December 31, 2009 as compared to 2008. Sales declined $3,089,000 or 7.5% in the fourth quarter of 2009 compared to the fourth quarter of 2008. By operating segment, in the fourth quarter of 2009 compared to the fourth quarter of 2008, sales for Keller declined 4.2%, compared to 7.5% for the first nine months of 2009 as compared to the same period in 2008, sales for Green declined 3.8% compared to 8.4% for the first nine months of 2009 as compared to the same period in 2008 , and sales for NDX declined 8.7% compared to 10.2% for the first nine months of 2009 as compared to the same period in 2008, excluding acquired sales from Dental Art.
Cost of Goods Sold
Our cost of goods sold decreased by $9,066,000 or 8.9% in the year ended December 31, 2009 over the year ended December 31, 2008. As a percentage of sales, cost of goods sold decreased to 57.8% for the year ended December 31, 2009 from 59.5% in 2008, primarily resulting from decreases in labor and related benefits and decreasing materials costs. Production labor decreased by approximately $4,084,000 and related benefits decreased by approximately $1,110,000 for the year ended December 31, 2009 compared to the year ended December 31, 2008. Excluding the acquisition of Dental Art, production labor and related benefits decreased by approximately $6,977,000 for the year ended December 31, 2009 compared to the year ended December 31, 2008. Green’s production labor and benefit costs of 28.6% of sales, as compared to 28.2% in 2008, and Keller’s production labor and benefits costs of 22.7% of sales, as compared to 22.6% in 2008, were fairly consistent on a year to year basis.
20
Within the NDX Laboratories operating segment, production labor and benefits decreased to 35.9% of sales for the year ended December 31, 2009 from 37.3% for the year ended December 31, 2008 primarily as a result of workforce reductions.
The cost of raw materials as a percentage of sales decreased to 15.1% for the year ended December 31, 2009 from 16.1% for the year ended December 31, 2008 as a result of lower commodity costs. We benefited from the downward trend of the average cost of palladium, a precious metal used as a component of many dental alloys, in 2009, particularly in the first three quarters when the average price was down 42.1% compared to the first three quarters of 2008. However, due to increased global demand for palladium, the average price of palladium increased by 75.4% in the fourth quarter of 2009 compared to the fourth quarter of 2008. Similarly, the average price of gold increased 40.6% in the fourth quarter of 2009 compared to the fourth quarter of 2008. Although we are generally able to pass a majority of precious metal cost increases on to our customers, prolonged higher metal costs likely will have a negative impact on gross profit percentages.
Selling, General and Administrative Expenses
Operating expenses, consisting of selling, delivery and administrative expenses both at the laboratory and corporate level, decreased by $2,833,000 for the year ended December 31, 2009 compared to 2008. Operating expenses increased as a percentage of net sales to 34.6% in 2009 from 34.1% in 2008. As a percentage of net sales, delivery expenses decreased to 9.2% for the year ended December 31, 2009 from 9.6% in 2008. Selling expenses remained consistent at 6.6% of sales for the years ended December 31, 2009 and 2008. Selling expenses in 2009 for the Keller segment were 14.5% of sales, or $3,455,000, compared to 15.3% of sales, or $3,914,000 for 2008. Laboratory incentive compensation increased to 1.2% of sales in 2009 from 0.3% of sales in 2008, as the amount increased by $1,288,000 to $1,879,000 for the year ended December 31, 2009 from $591,000 in 2008.
The net decrease of $2,833,000 in our operating expenses in 2009 was primarily attributable to the following decreases:
| | |
| | • | Decreases in delivery costs, resulting from decreases of $1,108,000 in fuel and delivery services and decreases of $857,000 in compensation — $2,006,000; |
| |
| | • | Decreases in administrative expenses at the laboratory level, including $1,095,000 in decreased compensation primarily resulting from workforce reductions, decreases of $276,000 in travel costs, decreases of $168,000 in recruiting and training expenses, and $684,000 of other broad based expense reductions — $2,223,000; |
| |
| | • | Decreases in selling expenses, including $261,000 in decreased sales compensation, $241,000 in decreased marketing expense at Keller, $228,000 in decreased travel related expenses, and $134,000 in decreased promotional related expenses — $886,000; |
| |
| | • | Decreases in consulting and professional fees — $655,000; |
| |
| | • | Decrease in insurance expense resulting from increases in the cash surrender value of insurance, net of increases in the related deferred compensation accruals for our supplemental executive retirement plans — $460,000; |
| |
| | • | The decrease in training and travel expenses at the corporate level — $295,000; and additionally, |
| |
| | • | The gain from the sale of real estate in Denver, Colorado — $365,000; |
partially offset by the following increases:
| | |
| | • | Increases in laboratory incentive compensation as a result of additional operating profit — $1,239,000; |
| |
| | • | Additional operating and amortization expense associated with the Dental Art acquisition — $1,083,000; |
| |
| | • | Increases in corporate incentive compensation accruals due to our improved financial results — $1,040,000; and |
| |
| | • | Increase in amortization due primarily to $317,000 of trade name impairment charges — $380,000. |
21
Goodwill Impairment
Our annual goodwill impairment assessment has historically been completed at the end of the second quarter. In the fourth quarter of 2008, management determined that circumstances had changed enough to perform an additional goodwill impairment test and we recorded goodwill impairment of $6,950,000 related to ten laboratories in the NDX laboratory segment. We performed an annual goodwill impairment test at the end of the second quarter of 2009 and noted no impairment. We later recorded an impairment charge of $264,000 in the third quarter of 2009, comprising the entire goodwill of one of our smaller laboratories in our NDX laboratory segment, due to a significant decrease in revenues and operating income from those forecasted as of June 30, 2009. During the fourth quarter of 2009, there were no events that would trigger an additional goodwill impairment test or result in a charge to goodwill impairment expense. We continue to closely monitor the financial results of certain other locations in the NDX laboratory segment in subsequent periods in comparison to forecast financial results and assumptions made at June 30, 2009.
Operating Income
As a result of the above factors, our operating income increased by $8,105,000 to $12,057,000 for the year ended December 31, 2009 from $3,952,000 in 2008. As a percentage of net sales, operating income increased to 7.5% in 2009 from 2.3% in 2008.
Interest Expense
Due to lower interest rates and lower debt levels, interest expense declined to $1,257,000 for 2009 from $2,110,000 for 2008.
Provision for Income Taxes
For the year ended December 31, 2009, the provision for income taxes increased by $2,206,000 to $4,178,000 from $1,972,000 for the year ended December 31, 2008. For the year ended December 21, 2009, the effective tax rate was 41.6% compared to 180.1% for 2008. In 2008, goodwill impairment recorded in the amount of $6,950,000 had an associated tax benefit of only $833,000, as approximately 70% of the goodwill impairment charge was not deductible for tax purposes. Without the impact of goodwill impairment, our tax provision in 2008 would have resulted in an effective tax rate of 34.9%. The increase in the effective tax rate before adjustment for goodwill impairment was due in part to increased state taxes and additional stock based compensation offset by the absence of recognition of research and experimentation credits, which equaled $604,000 in 2008.
Net Income
As a result of the factors discussed above, particularly the goodwill impairment charge recorded in the fourth quarter of 2008, net income increased $6,754,000 to $5,877,000 or $1.02 per share on a diluted basis for the year ended December 31, 2009 from ($877,000) or ($0.16) per share on a diluted basis in 2008.
Operating Segment Results
Our business consists of a single industry segment, which is the design, fabrication, marketing and sale of custom dental prosthetic appliances for and to dentists in North America. We report on three operating segments within this single industry segment. These three segments are known as Green Dental, representing the operations of Green Dental Laboratories, Inc. of Heber Springs, Arkansas, which we acquired in March 2005; Keller, representing the operations of Keller Group, Incorporated with laboratories in St. Louis, Missouri and Louisville, Kentucky, which we acquired in October, 2006; and NDX Laboratories, which represents our remaining laboratories. We have identified both Green and Keller as separate reportable segments based on the quantitative criteria for financial reporting purposes.
22
| | | | | | | | | | | | | | | | | |
| | | Year Ended December 31 | | | | | | | |
| | | 2008 | | | 2009 | | | $ Change | | | % Change | |
| |
Revenue: | | | | | | | | | | | | | | | | |
| NDX Laboratories | | $ | 126,240,949 | | | $ | 118,801,942 | | | $ | (7,439,007 | ) | | | (5.9 | )% |
| Green Dental Laboratory | | | 20,724,865 | | | | 19,169,497 | | | | (1,555,368 | ) | | | (7.5 | )% |
| Keller Group | | | 25,987,992 | | | | 24,248,404 | | | | (1,739,588 | ) | | | (6.7 | )% |
| | | | | | | | | | | | | | | | | |
| Subtotal | | | 172,953,806 | | | | 162,219,843 | | | | (10,733,963 | ) | | | (6.2 | )% |
| Less: Inter-segment Revenues: | | | 1,279,371 | | | | 1,024,639 | | | | (254,732 | ) | | | (19.9 | )% |
| | | | | | | | | | | | | | | | | |
| Net Sales | | $ | 171,674,435 | | | $ | 161,195,204 | | | $ | (10,479,231 | ) | | | (6.1 | )% |
| | | | | | | | | | | | | | | | | |
Laboratory Operating Income: | | | | | | | | | | | | | | | | |
| NDX Laboratories | | $ | 15,059,508 | | | $ | 16,318,096 | | | $ | 1,258,588 | | | | 8.4 | % |
| Green Dental Laboratory | | | 4,795,136 | | | | 4,418,446 | | | | (376,690 | ) | | | (7.9 | )% |
| Keller Group | | | 3,660,913 | | | | 4,237,948 | | | | 577,035 | | | | 15.8 | % |
| | | | | | | | | | | | | | | | | |
| Laboratory Operating Income | | $ | 23,515,557 | | | $ | 24,974,490 | | | $ | 1,458,933 | | | | 6.2 | % |
| | | | | | | | | | | | | | | | | |
NDX Laboratories
For the year ended December 31, 2009, before elimination of inter-segment revenues, sales in this segment decreased by $7,439,000 or 5.9%. Net of acquired sales of $5,067,000 from Dental Art, sales decreased $12,506,000, or 9.9% for 2009 when compared with 2008. In the fourth quarter of 2009 compared to the fourth quarter of 2008, sales for NDX declined 8.7%. In comparison, sales for the first nine months of 2009 declined by 10.2% compared to the same period in 2008, excluding acquired sales from Dental Art. Gross profit as a percentage of sales increased to 38.9% for the year ended December 31, 2009 from 36.7% for the year ended December 31, 2008. Cost of goods sold decreased by $7,344,000. The decrease in cost of sales is attributable to decreases in manufacturing labor and benefits of approximately $6,205,000 resulting from labor force reductions, decreases in materials costs of $3,288,000 due to lower sales and lower materials costs, and decreased laboratory overhead of $947,000 resulting from less expenditures on supplies and less depreciation related to facilities, partially offset by additional costs of $3,097,000 related to the acquisition of Dental Art.
Laboratory operating income as a percentage of sales for NDX Laboratories increased to 13.8% for the year ended December 31, 2009 from 11.9% for the year ended December 31, 2008 and increased by $1,259,000 or 8.4% as a result of the factors discussed above.
Green Dental Laboratory
For the year ended December 31, 2009, before elimination of inter-segment revenues, sales in this segment decreased by $1,555,000 or 7.5% as compared to fiscal 2008. In the fourth quarter of 2009 compared to the fourth quarter of 2008, sales for Green declined 3.8%. In comparison, sales for the first nine months of 2009 declined by 8.4% compared to the same period in 2008. As a percentage of sales, gross profit remained flat at 48.4% for the year ended December 31, 2009 compared to 48.5% for 2008. Cost of goods sold decreased by $747,000, resulting primarily from decreases in material costs of $475,000 and decreased overtime of $130,000.
As a result of the factors discussed above, laboratory operating income as a percentage of sales for Green decreased slightly to 23.0% for the year ended December 31, 2009 compared to 23.1% for the year ended December 31, 2008 and decreased by $377,000.
Keller Group
For the year ended December 31, 2009, the sales decline before elimination of inter-segment revenues in this segment was $1,740,000 or 6.7%. In the fourth quarter of 2009 compared to the fourth quarter of 2008, sales for Keller declined 4.2%. In comparison, sales for the first nine months of 2009 declined by 7.5% compared to the same period in 2008. As a percentage of sales, gross profit increased to 53.8% for the year ended December 31, 2009 from
23
53.0% for the year ended December 31, 2008, primarily as a result of decreased material costs, including precious metals. Administrative costs decreased by $492,000 primarily due to decreased labor and benefits of $176,000 and various other expense reductions. Selling expense decreased by $459,000 primarily due to decreased labor and benefits of $227,000 and decreased advertising expense of $241,000 for the year ended December 31, 2009 compared to 2008. Delivery costs decreased by $443,000 primarily due to decreased delivery service charges due to lower volume and decreased fuel costs. As a result, delivery costs as a percentage of sales decreased to 11.0% during the year ended December 31, 2009 from 12.0% during the year ended December 31, 2008.
As a result of the factors discussed above, laboratory operating income as a percentage of sales for Keller increased to 17.5% for the year ended December 31, 2009 as compared to 14.1% for the year ended December 31, 2008, an increase of $577,000.
Year Ended December 31, 2008 Compared with Year Ended December 31, 2007
Net Sales
In 2008, competitive pressures from offshore laboratories that can produce crowns at fees lower than crowns manufactured in the United States limited our ability to raise our prices, particularly during a time when we experienced relatively higher costs for precious metals used in manufacturing. In addition, these competitive pressures are partially responsible for declines in revenues or revenue growth in several marketplaces. We also believe that in 2008, the recessionary environment in the United States impacted our revenues and the revenues of the entire dental laboratory industry, as price-sensitive consumers postponed elective dental work. The impact of the recession on our business was particularly noticeable in the fourth quarter of 2008.
For the year ended December 31, 2008, net sales increased $1,314,000 or 0.8% over year ended December 31, 2007. Net sales increased by approximately $2,665,000 as a result of the Dental Art acquisition in September 2008. Excluding the Dental Art acquisition, net sales decreased approximately $1,351,000 for the full year ended December 31, 2008 as compared to 2007. Furthermore, approximately $550,000 of sales growth was attributable to the effect of increased prices due to underlying increases in the prices of precious metals passed through to customers. The decline in sales for laboratories held more than one year was primarily attributable to decreased patient demand, particularly in the fourth quarter of 2008 as consumers started to delay certain dental work due to economic uncertainties. Excluding acquired sales from Dental Art, sales declined $2,112,000 in the fourth quarter of 2008. Sales growth in the fourth quarter of 2008 for Keller, relative to their performance in the first nine months of 2008, was flat at $84,000 or 1.3%. In the fourth quarter, sales declined $89,000 at Green and $2,107,000 at the NDX Laboratories.
Cost of Goods Sold
Our cost of goods sold increased by $4,446,000 or 4.5% in the year ended December 31, 2008 over the year ended December 31, 2007. As a percentage of sales, cost of goods sold increased from 57.4% to 59.5%, primarily resulting from increases in labor and related benefits, increases in laboratory overhead and rising materials costs. Green’s labor costs of 28.2% of sales, as compared to 28.8% in 2007, and Keller’s labor costs of 22.6% of sales, as compared to 22.9% in 2007, lowered the overall percentage while the portion attributable to NDX Laboratories increased to 37.6% of sales for the year ended December 31, 2008 from 35.7% for the year ended December 31, 2007. Included in the increase in the NDX Laboratories segment is a reclassification of approximately $995,000 in base pay increases related to modifications of the Laboratory Plan, which is discussed in Note 7 “Employee Benefit Plans” of our Consolidated Financial Statements included in Item 8 of this Annual Report. Laboratory overhead increased $1,250,000, primarily as a result of increased depreciation and rent for new facilities and increases in technical training. Excluding the acquisition of Dental Art, production labor and related benefits increased by approximately $1,453,000 for the year ended December 31, 2008 compared to the year ended December 31, 2007.
The cost of raw materials as a percentage of sales increased from 15.7% for the year ended December 31, 2007 to 16.1% for the year ended December 31, 2008. For most of 2008, the average cost of gold and palladium, precious metals components of many dental alloys, was on the rise. However, by the end of 2008, due to decreased global demand for palladium, the average price of palladium was essentially unchanged, while gold increased by approximately 25% over average costs in the prior year. Although we were able to pass a majority of precious
24
metal cost increases on to our customers, prolonged higher metal costs have had and likely will continue to have a negative impact on gross profit percentages.
Selling, General and Administrative Expenses
Operating expenses, net of goodwill impairment and consisting of selling, delivery and administrative expenses both at the laboratory and corporate level, increased by $27,000 for the year ended December 31, 2008 compared to 2007. Operating expenses decreased as a percentage of net sales from 34.4% in 2007 to 34.1% in 2008. As a percentage of net sales, delivery expenses increased from 9.2% in the year ended December 31, 2007 to 9.6% in 2008. Selling expenses increased from 6.2% of sales for the year ended December 31, 2007 to 6.6% in 2008. Selling expenses in 2008 for the Keller segment were 15.1% of sales, or $3,914,000. Laboratory incentive compensation decreased from 2.3% of sales in 2007 to 0.3% in 2008, as the amount decreased by $3,281,000 from $3,872,000 for the year ended December 31, 2007 to $591,000 in 2008.
The net increase of $27,000 in our operating expenses in 2008 was primarily attributable to the following increases:
| | |
| | • | Additional operating and amortization expense associated with the Dental Art acquisition — $501,000; |
| |
| | • | Increases in delivery costs, resulting primarily from cost increases in fuel and delivery services — $694,000; |
| |
| | • | Increases in selling expenses, including $271,000 in increased marketing expense at Keller and $454,000 in increased sales compensation, offset by $273,000 of decreases in customer rewards program expenses — $507,000; |
| |
| | • | Increases in administrative expenses at the laboratory level, including $852,000 in increased compensation primarily resulting from increases to base pay of $485,000 related to the change in the Laboratory Plan, offset by $189,000 of decreases to depreciation expense and losses on asset disposals — $758,000; |
| |
| | • | Increases in salaries and benefits at the corporate level— $649,000; and; |
| |
| | • | Decrease of the cash surrender value of life insurance policies as a result of market value declines, net of decreases in the related deferred compensation accruals for our supplemental executive retirement plans — $730,000; |
partially offset by:
| | |
| | • | Decreases in laboratory incentive compensation as a result of the Laboratory Plan restructuring — $3,281,000. |
| |
| | • | Decreases in executive incentive compensation accruals due to our financial results — $400,000; and |
| |
| | • | Decrease in amortization due to fully amortized non-compete agreements — $193,000. |
Goodwill Impairment
Our annual goodwill impairment assessment has historically been completed at the end of the second quarter. Based on our initial assessment for 2008, the fair value of our business units exceeded their carrying value and therefore our goodwill was not impaired. As economic conditions worsened in the fourth quarter and our business performance and outlook was not as strong as anticipated at the end of the second quarter, management determined that circumstances had changed enough to perform an additional goodwill impairment test as of December 31, 2008. Based on our evaluation of goodwill, we determined that the fair value of ten dental laboratories in the NDX Laboratories operating segment was less than their carrying value, resulting in goodwill impairment of $6,950,000.
Operating Income
As a result of the above factors, our operating income decreased by $10,108,000 to $3,952,000 for the year ended December 31, 2008 from $14,060,000 in 2007. As a percentage of net sales, operating income decreased from 8.3% in 2007 to 2.3% in 2008.
25
Interest Expense
Due primarily to lower interest rates, interest expense declined from $2,803,000 for 2007 to $2,110,000 for 2008. Approximately $180,000 of interest expense was related to the borrowings that funded the acquisition of Dental Art.
Provision for Income Taxes
For the year ended December 31, 2008, the provision for income taxes decreased by $1,888,000 from $3,860,000 for the year ended December 31, 2007 to $1,972,000 for the year ended December 31, 2008. However, for the year ended December 31, 2008, income before provision for income taxes was $1,095,000, and the effective tax rate was therefore 180.1%. The reason the tax provision exceeds income before provision for income taxes is due to the tax impact of the impairment of goodwill. Goodwill impairment recorded in the amount of $6,950,000 has an associated tax benefit of only $833,000, as approximately 70% of the goodwill impairment charge is not deductible for tax purposes. Without the impact of goodwill impairment, our tax provision would have resulted in an effective tax rate of 34.9%, which would have represented a decrease from the 36.8% effective tax rate for the year ended December 31, 2007. The decrease in the effective tax rate before adjustment for goodwill impairment was due in part to recognition of research and experimentation credits of $604,000, offset by increases in the amount of certain non-deductible expenses.
Net Income
As a result of all the factors discussed above, particularly the goodwill impairment charge recorded in the fourth quarter, net income decreased $7,503,000 to ($877,000) or ($0.16) per share on a diluted basis for the year ended December 31, 2008 from $6,626,000 or $1.17 per share on a diluted basis in 2007.
Operating Segment Results
The following is a summary of segment reporting for 2007 and 2008:
| | | | | | | | | | | | | | | | | |
| | | Year Ended December 31 | | | | | | | |
| | | 2007 | | | 2008 | | | $ Change | | | % Change | |
| |
Revenue: | | | | | | | | | | | | | | | | |
| NDX Laboratories | | $ | 127,388,588 | | | $ | 126,240,949 | | | $ | (1,147,639 | ) | | | (0.9 | )% |
| Green Dental | | | 19,859,770 | | | | 20,724,865 | | | | 865,095 | | | | 4.4 | % |
| Keller Group | | | 23,843,093 | | | | 25,987,992 | | | | 2,144,899 | | | | 9.0 | % |
| | | | | | | | | | | | | | | | | |
| Subtotal | | | 171,091,451 | | | | 172,953,806 | | | | 1,862,355 | | | | 1.1 | % |
| Less: Inter-segment Revenues: | | | | | | | | | | | | | | | | |
| NDX Laboratories | | | 370,913 | | | | 438,200 | | | | 67,287 | | | | 18.1 | % |
| Green Dental | | | 199,525 | | | | 369,526 | | | | 170,001 | | | | 85.2 | % |
| Keller Group | | | 160,384 | | | | 471,645 | | | | 311,261 | | | | 194.1 | % |
| | | | | | | | | | | | | | | | | |
| Net Sales | | $ | 170,360,629 | | | $ | 171,674,435 | | | $ | 1,313,806 | | | | 0.8 | % |
| | | | | | | | | | | | | | | | | |
Laboratory Operating Income: | | | | | | | | | | | | | | | | |
| NDX Laboratories | | $ | 17,572,491 | | | $ | 15,059,508 | | | $ | (2,512,983 | ) | | | (14.3 | )% |
| Green Dental | | | 4,665,630 | | | | 4,795,136 | | | | 129,506 | | | | 2.8 | % |
| Keller Group | | | 3,228,640 | | | | 3,660,913 | | | | 432,273 | | | | 13.4 | % |
| | | | | | | | | | | | | | | | | |
| Laboratory Operating Income | | $ | 25,466,761 | | | $ | 23,515,557 | | | $ | (1,951,204 | ) | | | (7.7 | )% |
| | | | | | | | | | | | | | | | | |
NDX Laboratories
For the year ended December 31, 2008, before elimination of inter-segment revenues, sales in this segment decreased by $1,148,000 or 0.9%. Net of acquired sales of $2,665,000 from Dental Art, sales decreased $3,813,000,
26
or 3.0% for 2008 when compared with 2007. The decrease in sales in the fourth quarter accounted for $2,107,000 of this amount. Gross profit as a percentage of sales decreased from 40.3% for the year ended December 31, 2007 to 36.9% for the year ended December 31, 2008. Cost of goods sold increased by $3,244,000. The increase was primarily attributable to additional costs of $1,549,000 related to the acquisition of Dental Art, increases in manufacturing labor and benefits of approximately $1,790,000, resulting from increases to base pay of $995,000 related to changes in the Laboratory Plan and increases in health insurance and other benefit costs of $380,000, which were partially offset by lower labor costs resulting from labor force reductions. Materials costs increased approximately $242,000 and laboratory overhead increased by approximately $1,053,000 resulting from depreciation and increased rent for new facilities.
Laboratory operating income as a percentage of sales for NDX Laboratories decreased from 13.9% for the year ended December 31, 2007 to 12.0% for the year ended December 31, 2008 and declined by $2,513,000 or 14.3% as a result of the factors discussed above. Goodwill impairment of $6,950,000 was recorded in the NDX Laboratories operating segment but is not a component of laboratory operating income.
Green Dental Laboratory
Sales growth before elimination of inter-segment revenues for the year ended December 31, 2008 in this segment was $865,000 or 4.4%. Sales increased during the first nine months of 2008, however, in the fourth quarter, sales declined $89,000. As a percentage of sales, gross profit increased from 47.4% for the year ended December 31, 2007 to 47.6% for the year ended December 31, 2008. Cost of goods sold increased by $420,000. In addition to temporary increases in overtime of $72,000 resulting primarily from training and implementation of new production methods, benefit costs increased $107,000, primarily resulting from increases in the cost of providing health insurance. Materials costs increased $106,000 for the year ended December 31, 2008 as compared to the year ended December 31, 2007. Precious metals used declined as precious metal-based unit volume was down in favor of zirconia-based, CAD-CAM produced units. Increased expenses for implant parts, porcelain and zirconia materials resulted from this changing product mix. Laboratory overhead increased by approximately $147,000.
As a result of the factors discussed above, laboratory operating income as a percentage of sales for Green was essentially flat at 23.5% for the year ended December 31, 2007 versus 23.1% for the year ended December 31, 2008 and increased by $130,000.
Keller Group
For the year ended December 31, 2008, sales growth before elimination of inter-segment revenues in this segment was $2,145,000 or 9.0%. Sales growth in the fourth quarter of 2008 for Keller, relative to their performance in the first nine months of 2008, was flat at $84,000 or 1.3%. As a percentage of sales, gross profit increased from 51.0% for the year ended December 31, 2007 to 52.0% for the year ended December 31, 2008, primarily as a result of improved labor efficiency, partially offset by increased materials cost, including precious metals. Delivery costs increased by $483,000 which primarily is the result of increased delivery service charges due to higher volume and increased fuel costs. As a result, delivery costs as a percentage of sales rose to 11.8% during the year ended December 31, 2008 from 10.8% during the year ended December 31, 2007. Advertising expense increased by $271,000 for the year ended December 31, 2008 compared to 2007 due to increased marketing activities.
As a result of the factors discussed above, laboratory operating income as a percentage of sales for Keller increased to 14.1% for the year ended December 31, 2008 as compared to 13.5% for the year ended December 31, 2007, while increasing by $432,000 due to higher sales volumes.
Critical Accounting Policies
Financial Reporting Release No. 60 as released by the SEC requires all companies to include a discussion of critical accounting policies or methods used in the preparation of financial statements. The preparation of our consolidated financial statements requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities and the reported amounts of expenses during the reporting period. A summary of certain of our significant accounting policies is presented below.
27
Summary of Significant Accounting Policies
Revenue Recognition
Revenue is recognized upon transfer of title and risk of loss, generally as the dentists’ orders are shipped. We record shipping and handling fees charged to customers as revenues in accordance with U.S. Generally Accepted Accounting Principles (GAAP). Shipping and handling costs totaled approximately $15,617,000 in fiscal 2007, $16,463,000 in fiscal 2008 and $14,800,000 in fiscal 2009, and are included in selling, general and administrative expense.
Goodwill and Other Indefinite-Lived Intangible Assets Not Subject to Amortization
We continually evaluate whether events and circumstances have occurred that indicate that the value of goodwill has been impaired. In addition, goodwill is evaluated for possible impairment on an annual basis, based on a two-step process. We have selected June 30th as the date for the annual assessment of goodwill impairment.
The first step is used to identify potential impairment and involves comparing each reporting unit’s estimated fair value to its carrying value, including goodwill. A reporting unit is an operating segment or one level below an operating segment (referred to as a component). We have determined that each individual laboratory is a reporting unit. The second step of the goodwill impairment process involves the calculation of an implied fair value of goodwill for the laboratories which step one indicated were impaired. Fair value is determined by using an income approach, consistent with our valuation of dental laboratories acquired in purchase business combinations. We assessed goodwill impairment as of June 30, 2009 and determined that no impairment charge was required. However, our impairment analysis incorporates forecasted financial results and assumptions for several laboratories within the NDX Laboratories operating segment. Although these laboratories were not impaired at June 30, 2009, in the third quarter of 2009, we recorded an impairment charge of $264,000, comprising the entire goodwill balance of one of our smaller laboratories in the NDX laboratories operating segment, due to a significant decrease in revenues and operating income from those forecasted as of June 30, 2009. We continue to closely monitor the financial results of certain other laboratories in the NDX laboratories operating segment in subsequent periods in comparison to forecasted financial results and assumptions made at June 30, 2009 to determine whether any other impairment charges are warranted.
Additionally, we also recognize the existence of value in trade names acquired in business combinations and believe the useful life of this intangible to be indefinite based on a long history of utilizing the laboratory trade name. Accordingly, trade names are also evaluated for impairment on an annual basis using a single step method. Impairment charges related to trade names are recognized when the fair value is less than the carrying value of the asset. Impairment charges related to trade names were recorded in the amount of $94,000, $44,000 and $361,000 for the years ended December 31, 2007, 2008 and 2009, respectively. Trade name impairment charges generally result from a decline in forecasted revenue at specific laboratories in comparison to revenue forecasts used in previous valuation calculations.
Intangible Assets Subject to Amortization
All acquisitions reflected in the accompanying consolidated financial statements have been accounted for as purchase business combinations. Non-competition agreements and customer relationship intangibles arising from dental laboratory acquisitions are amortized over their useful lives. The acquisition date fair value of non-competition agreements are deferred and amortized over their economic useful lives, in accordance with the terms of the agreements, ranging from 2 to 15 years. The acquisition date fair value associated with acquired customer relationships are amortized over their estimated economic useful life, ranging from 9 to 12 years.
Inventories
Inventories, consisting principally of raw materials, are stated at the lower of cost(first-in, first-out) or market. We use estimates based on specific identification to maintain proper reserves for excess and obsolete inventory. Additionally, we estimate work in process inventories by applying current labor, materials and selected overhead
28
expense rates to standard production schedules. We estimate the value of unrefined precious metal scrap based on the application of various return and refining statistics. Finished goods inventory consists of completed orders that were shipped to customers immediately subsequent to period end.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated using the straight-line method over the following estimated depreciable lives:
| | | | | |
| Buildings | | | 25 years | |
| Furniture and fixtures | | | 5 - 10 years | |
| Laboratory equipment | | | 5 - 20 years | |
| Computer equipment | | | 3 - 5 years | |
Leasehold improvements and capital leases are amortized over the lesser of the assets’ estimated useful lives or the remaining lease terms.
Gains and losses are recognized upon the disposal of property and equipment, and the related accumulated depreciation and amortization are removed from the accounts. Maintenance, repairs and betterments that do not enhance the value of or increase the life of the assets are charged to operations as incurred.
Depreciation expense totaled approximately $4,055,000 in fiscal 2007, $4,872,000 in fiscal 2008 and $4,569,000 in fiscal 2009.
Impairment of Long-Lived Assets
At each balance sheet date, management evaluates the recoverability of long-lived assets, including property and equipment and intangible assets, using certain financial indicators, such as historical and future ability to generate income from operations. Our policy is to assess whether an impairment exists in the period when it is determined that the carrying amount of the asset may not be recoverable. The determination is based on an evaluation of such factors as the occurrence of a significant event, a significant change in the environment in which the business operates or if the expected future cash flows become less than the carrying amount of the asset.
Cash Surrender of Life Insurance
The cash surrender value of life insurance policies which are owned by the Company are recorded at net realizable value.
Income Taxes
Deferred tax assets and liabilities are recognized for the expected future tax consequences of events that have been included in the financial statements or tax returns. The amount of deferred tax asset or liability is based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. We have considered our current financial characteristics as well as current tax law and do not believe that the recoverability of various tax assets and liabilities is impaired, and therefore have recorded them at their full value.
Accounting for income taxes prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The minimum threshold is defined as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement.
29
Fair Value Measurements
Effective January 1, 2008, we adopted accounting standards that defined fair value, established a framework for measuring fair value and enhanced disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. These standards establish a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value; describe three levels of inputs that may be used to measure fair value which are provided in the table below; and allow an entity the irrevocable option to elect fair value for the initial and subsequent measurement for certain financial assets and liabilities on acontract-by-contract basis. The adoption of these standards had no material impact on our financial statements.
We use the market approach technique to value our financial instruments and there were no changes in valuation techniques during the year ended December 31, 2009. Our financial assets and liabilities are primarily comprised of investments in insurance contracts held as assets to satisfy outstanding retirement liabilities.
Recent Accounting Pronouncements
In April 2009, the FASB issued additional guidance for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased and for identifying circumstances that indicate a transaction is not orderly. This guidance emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date under current market conditions. This guidance is effective for interim and annual reporting periods ending after June 15, 2009 and its adoption did not have an impact on our unaudited consolidated financial statements.
In May 2009, the FASB issued a pronouncement on subsequent event accounting. The guidance identifies the following: the period after the balance sheet date during which management shall evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements; the circumstances under which an entity shall recognize events or transactions occurring after the balance sheet date in its financial statements; and the disclosures that an entity shall make about events or transactions that occurred after the balance sheet date. This statement was effective for our second quarter 2009, and there was no effect from adoption.
In June 2009, the FASB issued guidance onTheFASB Accounting Standards Codificationtmand the Hierarchy of Generally Accepted Accounting Principles,which is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The adoption of this pronouncement did not have an impact on our consolidated financial statements.
| |
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
Our market risk exposure includes potential price volatility of commodities we use in our manufacturing processes. We purchase dental alloys that contain gold, palladium and other precious metals. We have not participated in hedging transactions. We have relied on pricing practices that attempt to pass some portion, if not all, of our increased costs on to our customers, in conjunction with materials substitution strategies. Our market risk exposure also includes investments in insurance contracts held as assets to satisfy outstanding retirement liabilities, a portion of which are subject to market value fluctuations of the underlying investment. Cash surrender values totaled approximately $5,479,000 for fiscal 2008 and $5,546,000 for fiscal 2009.
At December 31, 2009, we had variable rate debt of $26,112,000 associated with our credit facility. Based on this amount, the earnings and cash flows impact for the next year resulting from a one percentage point increase in interest rates would be approximately $153,000, net of tax, holding other variables constant.
30
We have investments in a Canadian subsidiary. The net assets of this subsidiary are exposed to volatility in current exchange rates. Translation gains and losses related to the net assets of this subsidiary are included in accumulated other comprehensive income.
| |
| Item 8. | Financial Statements and Supplementary Data |
Quarterly Results
The following table sets forth certain selected financial information for the eight fiscal quarters in our two most recently completed fiscal years. In our opinion, this unaudited information has been prepared on the same basis as the audited financial information and includes all adjustments (consisting of only normal, recurring adjustments) necessary to present this information fairly when reviewed in conjunction with our Consolidated Financial Statements and notes thereto contained herein.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended |
| | | March 31,
| | June 30,
| | Sept. 30,
| | Dec. 31,
| | March 31,
| | June 30,
| | Sept. 30,
| | Dec. 31,
|
| | | 2008 | | 2008 | | 2008 | | 2008 | | 2009 | | 2009 | | 2009 | | 2009 |
| | | (Dollars in thousands except per share data) |
| |
| Net sales | | $ | 43,529 | | | $ | 44,580 | | | $ | 42,302 | | | $ | 41,264 | | | $ | 41,260 | | | $ | 42,755 | | | $ | 39,006 | | | $ | 38,175 | |
| Gross profit | | $ | 18,210 | | | $ | 18,665 | | | $ | 16,552 | | | $ | 16,062 | | | $ | 17,622 | | | $ | 18,828 | | | $ | 15,836 | | | $ | 15,790 | |
| Gross margin | | | 41.8 | % | | | 41.9 | % | | | 39.1 | % | | | 38.9 | % | | | 42.7 | % | | | 44.0 | % | | | 40.6 | % | | | 41.4 | % |
| Operating income (loss) | | $ | 3,389 | | | $ | 3,861 | | | $ | 2,183 | | | $ | (5,482 | ) | | $ | 3,898 | | | $ | 4,377 | | | $ | 2,393 | | | $ | 1,388 | |
| Operating margin | | | 7.8 | % | | | 8.7 | % | | | 5.2 | % | | | (13.3 | )% | | | 9.4 | % | | | 10.2 | % | | | 6.1 | % | | | 3.6 | % |
| Net income (loss) | | $ | 1,681 | | | $ | 1,937 | | | $ | 791 | | | $ | (5,286 | ) | | $ | 2,059 | | | $ | 2,330 | | | $ | 1,059 | | | $ | 429 | |
| Net income (loss) per diluted share | | $ | 0.30 | | | $ | 0.34 | | | $ | 0.14 | | | $ | (0.93 | ) | | $ | 0.36 | | | $ | 0.40 | | | $ | 0.18 | | | $ | 0.07 | |
Our results of operations have historically fluctuated on a quarterly basis and are expected to be subject to quarterly fluctuations in the future. As a result, we believe that the results of operations for the interim periods are not necessarily indicative of the results to be expected for any future period or for a full year. Quarterly results are subject to fluctuations resulting from a number of factors, including the number of working days in the quarter for both dentists and our employees, the number of paid vacation days and holidays in the period, general economic conditions and consumer spending patterns. Historically, the second quarter has generated the highest quarterly net sales for the year and has been the most profitable for us due to the greater number of working days in the quarter and more patients scheduling visits with their dentists before departing for summer vacation.
Location of Financial Statements
The consolidated financial statements furnished in connection with this Report are attached immediately following Part IV.
| |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| |
| Item 9A. | Controls and Procedures |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
We carried out an evaluation, with the participation of our principal executive officer and principal financial officer, of the effectiveness of our disclosure controls and procedures as of December 31, 2009. In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and our management necessarily applied its judgment in evaluation of the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our principal executive officer and principal financial officer concluded that, as of December 31, 2009, our disclosure controls and procedures, as defined in the Securities Exchange Act of 1934 (the “Exchange Act”)Rules 13a-15(e) and15d-15(e), were effective to ensure that information required to be
31
disclosed by us in the reports that we file or submit under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and such information is accumulated and communicated to management, including the CEO and CFO, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange ActRules 13a-15(f) and15d-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we carried out an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2009 based on theInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based upon this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2009.
PricewaterhouseCoopers LLP, the independent registered public accounting firm that audited our financial statements included in this Annual Report onForm 10-K, has also audited the effectiveness of internal control over financial reporting as of December 31, 2009, as stated in their report which is included herein.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting (as defined inRules 13a-15(f) and15d-15(f) under the Exchange Act) occurred during the fiscal quarter ended December 31, 2009 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| |
| Item 9B. | Other Information |
None.
PART III
| |
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this Item will be contained in our proxy statement for our 2010 annual meeting of stockholders, which we plan to file with the SEC no later than 120 days after the end of our fiscal year ended December 31, 2009 (the “2010 Proxy Statement”). Such information is hereby incorporated by reference.
We have adopted a written code of business conduct and ethics that applies to all our directors, officers and employees, a copy of which is located on the Investor Relations page of our website which is located atwww.nationaldentex.com. We intend to disclose any amendments to, or waivers from, our code of business conduct and ethics on that same page of our website.
| |
| Item 11. | Executive Compensation |
The information required by this item will be included in our 2010 Proxy Statement and is incorporated herein by reference.
| |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
The information required by this item will be included in our 2010 Proxy Statement and is incorporated herein by reference.
| |
| Item 13. | Certain Relationships and Related Transactions |
The information required by this item will be included in our 2010 Proxy Statement and is incorporated herein by reference.
32
| |
| Item 14. | Principal Accountant Fees and Services |
The information required by this item will be included in our 2010 Proxy Statement and is incorporated herein by reference.
PART IV
| |
| Item 15. | Exhibits and Financial Statement Schedules |
(a) 1. Financial statements:
For a listing of consolidated financial statements which are included in this Report, seepage F-1.
2. Financial Statement Schedules:
All schedules for which provision is made under Item 15(a) (2) are inapplicable and, therefore, have been omitted.
3. Exhibits:
The exhibits listed in the Exhibit Index immediately preceding the exhibits are filed as part of this Annual Report onForm 10-K and are incorporated herein by reference.
(b) Exhibits:
The exhibits listed in the Exhibit Index immediately preceding the exhibits are filed as part of this Annual Report onForm 10-K and are incorporated herein by reference.
(c) Not Applicable
33
NATIONAL DENTEX CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS AND SCHEDULE
| | | | | |
| | | Page |
| |
| Financial Statements: | | | | |
| The consolidated financial statements of National Dentex Corporation included herein are as listed below: | | | | |
| Report of Independent Registered Public Accounting Firm | | | F-2 | |
| Consolidated Balance Sheets as of December 31, 2008 and 2009 | | | F-3 | |
| Consolidated Statements of Income for each of the three years in the period ended December 31, 2009 | | | F-4 | |
| Consolidated Statements of Stockholders’ Equity for each of the three years in the period ended December 31, 2009 | | | F-5 | |
| Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2009 | | | F-6 | |
| Notes to Consolidated Financial Statements | | | F-7 | |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of National Dentex Corporation:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, of shareholders’ equity and of cash flows present fairly, in all material respects, the financial position of National Dentex Corporation and its subsidiaries at December 31, 2009 and December 31, 2008, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 5 to the consolidated financial statements, during the year ended December 31, 2007, the Company changed the manner in which it accounts for uncertain tax positions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Boston, MA
March 11, 2010
F-2
NATIONAL DENTEX CORPORATION
CONSOLIDATED BALANCE SHEETS
| | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
ASSETS |
| CURRENT ASSETS: | | | | | | | | |
| Cash and cash equivalents | | $ | 2,109,943 | | | $ | 1,510,046 | |
| Accounts receivable: | | | | | | | | |
| Trade, less allowance of $492,000 in 2008 and $583,000 in 2009 | | | 16,701,139 | | | | 16,132,939 | |
| Other | | | 2,527,168 | | | | 1,728,591 | |
| Inventories | | | 6,991,385 | | | | 6,890,017 | |
| Prepaid expenses | | | 3,688,057 | | | | 3,784,470 | |
| Deferred tax assets | | | 931,919 | | | | 973,237 | |
| Property held for sale | | | 69,822 | | | | — | |
| | | | | | | | | |
| Total current assets | | | 33,019,433 | | | | 31,019,300 | |
| | | | | | | | | |
| PROPERTY, PLANT AND EQUIPMENT: | | | | | | | | |
| Land and buildings | | | 7,535,015 | | | | 7,535,015 | |
| Leasehold and building improvements | | | 18,890,911 | | | | 19,334,362 | |
| Laboratory equipment | | | 22,503,086 | | | | 20,459,600 | |
| Furniture and fixtures | | | 8,721,724 | | | | 8,337,527 | |
| | | | | | | | | |
| | | | 57,650,736 | | | | 55,666,504 | |
| Less — Accumulated depreciation and amortization | | | 24,213,721 | | | | 24,946,070 | |
| | | | | | | | | |
| Net property, plant and equipment | | | 33,437,015 | | | | 30,720,434 | |
| | | | | | | | | |
| OTHER ASSETS, net: | | | | | | | | |
| Goodwill | | | 69,384,320 | | | | 69,616,034 | |
| Trade names | | | 9,977,917 | | | | 9,490,645 | |
| Customer relationships | | | 6,210,176 | | | | 5,352,962 | |
| Non-competition agreements | | | 1,583,895 | | | | 1,291,824 | |
| Other assets | | | 7,902,147 | | | | 7,803,877 | |
| | | | | | | | | |
| Total other assets | | | 95,058,455 | | | | 93,555,342 | |
| | | | | | | | | |
| Total assets | | $ | 161,514,903 | | | $ | 155,295,076 | |
| | | | | | | | | |
| |
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
| CURRENT LIABILITIES: | | | | | | | | |
| Revolving line of credit | | $ | 2,939,978 | | | $ | — | |
| Current portion of long-term debt | | | 5,115,032 | | | | 5,071,050 | |
| Accounts payable | | | 3,541,996 | | | | 3,495,044 | |
| Accrued liabilities: | | | | | | | | |
| Payroll and employee benefits | | | 7,574,971 | | | | 9,315,821 | |
| Current portion of deferred acquisition costs | | | 300,000 | | | | — | |
| Other accrued expenses | | | 4,019,992 | | | | 3,841,752 | |
| | | | | | | | | |
| Total current liabilities | | | 23,491,969 | | | | 21,723,667 | |
| | | | | | | | | |
| LONG-TERM LIABILITIES: | | | | | | | | |
| Long-term obligations | | | 34,142,891 | | | | 21,776,455 | |
| Deferred compensation | | | 6,114,609 | | | | 6,739,265 | |
| Other accrued expenses | | | 1,419,561 | | | | 1,475,247 | |
| Deferred tax liabilities | | | 5,853,821 | | | | 5,789,956 | |
| | | | | | | | | |
| Total long-term liabilities | | | 47,530,882 | | | | 35,780,923 | |
| | | | | | | | | |
| COMMITMENTS AND CONTINGENCIES (Note 8) | | | | | | | | |
| STOCKHOLDERS’ EQUITY: | | | | | | | | |
| Preferred stock, $.01 par value | | | | | | | | |
| Authorized — 500,000 shares | | | | | | | | |
| None issued and outstanding | | | — | | | | — | |
| Common stock, $.01 par value | | | | | | | | |
| Authorized — 8,000,000 shares | | | | | | | | |
| Issued and Outstanding — 5,663,749 shares at December 31, 2008 and 5,761,363 shares at December 31, 2009 | | | 56,637 | | | | 57,613 | |
| Paid-in capital | | | 19,522,536 | | | | 20,374,197 | |
| Retained earnings | | | 71,312,895 | | | | 77,189,758 | |
| Accumulated other comprehensive (loss) income | | | (400,016 | ) | | | 168,918 | |
| | | | | | | | | |
| Total stockholders’ equity | | | 90,492,052 | | | | 97,790,486 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 161,514,903 | | | $ | 155,295,076 | |
| | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
NATIONAL DENTEX CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
| | | | | | | | | | | | | |
| | | Years Ended | |
| | | December 31,
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2008 | | | 2009 | |
| |
| Net sales | | $ | 170,360,629 | | | $ | 171,674,435 | | | $ | 161,195,204 | |
| Cost of goods sold | | | 97,738,785 | | | | 102,184,879 | | | | 93,119,314 | |
| | | | | | | | | | | | | |
| Gross profit | | | 72,621,844 | | | | 69,489,556 | | | | 68,075,890 | |
| Selling, general and administrative expenses | | | 58,561,368 | | | | 58,587,890 | | | | 55,755,023 | |
| Goodwill impairment | | | — | | | | 6,950,000 | | | | 263,537 | |
| | | | | | | | | | | | | |
| Operating income | | | 14,060,476 | | | | 3,951,666 | | | | 12,057,330 | |
| Other expense | | | 771,660 | | | | 746,633 | | | | 744,867 | |
| Interest expense | | | 2,802,944 | | | | 2,110,113 | | | | 1,257,184 | |
| | | | | | | | | | | | | |
| Income before provision for income taxes | | | 10,485,872 | | | | 1,094,920 | | | | 10,055,279 | |
| Provision for income taxes | | | 3,860,213 | | | | 1,971,963 | | | | 4,178,416 | |
| | | | | | | | | | | | | |
| Net income (loss) | | $ | 6,625,659 | | | $ | (877,043 | ) | | $ | 5,876,863 | |
| | | | | | | | | | | | | |
| Net income (loss) per share — basic | | $ | 1.20 | | | $ | (0.16 | ) | | $ | 1.03 | |
| | | | | | | | | | | | | |
| Net income (loss) per share — diluted | | $ | 1.17 | | | $ | (0.16 | ) | | $ | 1.02 | |
| | | | | | | | | | | | | |
| Weighted average shares outstanding — basic | | | 5,540,496 | | | | 5,631,450 | | | | 5,727,071 | |
| | | | | | | | | | | | | |
| Weighted average shares outstanding — diluted | | | 5,665,042 | | | | 5,631,450 | | | | 5,760,785 | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
NATIONAL DENTEX CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | | | | | | | Cumulative
| | | | |
| | | Number of
| | | $.01 Par
| | | Paid-in
| | | Retained
| | | Translation
| | | | |
| | | Shares | | | Value | | | Capital | | | Earnings | | | Adjustment | | | Total | |
| |
| BALANCE, December 31, 2006 | | | 5,509,412 | | | $ | 55,094 | | | $ | 17,296,170 | | | $ | 65,564,279 | | | $ | (121,911 | ) | | | 82,793,632 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of 42,450 shares of common stock under the stock option plans | | | 42,450 | | | | 425 | | | | 567,407 | | | | | | | | | | | | 567,832 | |
| Issuance of 24,030 shares of common stock under the employee stock purchase program | | | 24,030 | | | | 240 | | | | 295,325 | | | | | | | | | | | | 295,565 | |
| Tax benefit associated with exercise of stock options | | | | | | | | | | | 15,859 | | | | | | | | | | | | 15,859 | |
| Net income | | | | | | | | | | | | | | | 6,625,659 | | | | | | | | 6,625,659 | |
| Issuance of 6,227 shares of common stock as directors’ fees | | | 6,227 | | | | 62 | | | | 165,302 | | | | | | | | | | | | 165,364 | |
| Stock compensation expense | | | | | | | | | | | 161,112 | | | | | | | | | | | | 161,112 | |
| Cumulative translation adjustment | | | | | | | | | | | | | | | | | | | 567,240 | | | | 567,240 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE, December 31, 2007 | | | 5,582,119 | | | | 55,821 | | | | 18,501,175 | | | | 72,189,938 | | | | 445,329 | | | | 91,192,263 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of 12,000 shares of common stock under the stock option plans | | | 20,250 | | | | 202 | | | | 188,799 | | | | | | | | | | | | 189,001 | |
| Issuance of 33,375 shares of common stock under the employee stock purchase program | | | 33,375 | | | | 334 | | | | 371,308 | | | | | | | | | | | | 371,642 | |
| Tax benefit associated with exercise of stock options | | | | | | | | | | | 12,037 | | | | | | | | | | | | 12,037 | |
| Net loss | | | | | | | | | | | | | | | (877,043 | ) | | | | | | | (877,043 | ) |
| Issuance of 28,005 shares of common stock as directors’ fees | | | 28,005 | | | | 280 | | | | 269,720 | | | | | | | | | | | | 270,000 | |
| Stock compensation expense | | | | | | | | | | | 179,497 | | | | | | | | | | | | 179,497 | |
| Cumulative translation adjustment | | | | | | | | | | | | | | | | | | | (845,345 | ) | | | (845,345 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE, December 31, 2008 | | | 5,663,749 | | | | 56,637 | | | | 19,522,536 | | | | 71,312,895 | | | | (400,016 | ) | | | 90,492,052 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of 93,102 shares of common stock under the employee stock purchase program | | | 93,102 | | | | 934 | | | | 315,567 | | | | | | | | | | | | 316,501 | |
| Net income | | | | | | | | | | | | | | | 5,876,863 | | | | | | | | 5,876,863 | |
| Issuance of 4,155 shares of common stock as directors’ fees | | | 4,155 | | | | 42 | | | | 162,708 | | | | | | | | | | | | 162,750 | |
| Stock compensation expense | | | | | | | | | | | 373,386 | | | | | | | | | | | | 373,386 | |
| Cumulative translation adjustment | | | | | | | | | | | | | | | | | | | 568,934 | | | | 568,934 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE, December 31, 2009 | | | 5,761,006 | | | $ | 57,613 | | | $ | 20,374,197 | | | $ | 77,189,758 | | | $ | 168,918 | | | $ | 97,790,486 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
NATIONAL DENTEX CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | |
| | | Years Ended | |
| | | December 31,
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2008 | | | 2009 | |
| |
| Cash flows from operating activities: | | | | | | | | | | | | |
| Net income (loss) | | $ | 6,625,659 | | | $ | (877,043 | ) | | $ | 5,876,863 | |
| Adjustments to reconcile net income to net cash provided by operating activities, net of effects of acquisitions: | | | | | | | | | | | | |
| Depreciation and amortization | | | 5,338,432 | | | | 6,011,564 | | | | 5,825,730 | |
| Goodwill impairment | | | — | | | | 6,950,000 | | | | 263,536 | |
| Loss (gain) on disposal of property, plant and equipment | | | 150,109 | | | | (2,792 | ) | | | (311,472 | ) |
| Benefit for deferred income taxes | | | (265,149 | ) | | | (1,099,572 | ) | | | (142,638 | ) |
| Impairment of long-lived assets | | | 94,000 | | | | 44,000 | | | | 361,443 | |
| Tax benefit associated with exercise of stock options | | | 15,859 | | | | 12,037 | | | | — | |
| Provision for bad debts | | | 151,330 | | | | 148,068 | | | | 274,337 | |
| Losses on write-down of inventories | | | 107,791 | | | | 219,255 | | | | 124,583 | |
| Stock-based compensation expense | | | 326,477 | | | | 449,498 | | | | 536,136 | |
| Other non-cash items | | | 489,375 | | | | — | | | | — | |
| Changes in operating assets and liabilities, net of effects of acquisitions: | | | | | | | | | | | | |
| (Increase) decrease in accounts receivable | | | (701,142 | ) | | | (5,886 | ) | | | 1,159,268 | |
| (Increase) decrease in inventories | | | (226,117 | ) | | | 355,532 | | | | 6,632 | |
| (Increase) decrease in prepaid expenses | | | (2,363,317 | ) | | | 586,798 | | | | (93,747 | ) |
| (Increase) decrease in other assets | | | (19,635 | ) | | | 616,081 | | | | (106,294 | ) |
| Increase (decrease) in accounts payable and accrued liabilities | | | 2,828,757 | | | | (80,844 | ) | | | 2,038,549 | |
| | | | | | | | | | | | | |
| Net cash provided by operating activities | | | 12,552,429 | | | | 13,326,696 | | | | 15,812,926 | |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Payment for acquisitions, net of cash acquired | | | — | | | | (10,000,000 | ) | | | — | |
| Payment of deferred purchase price | | | (2,158,880 | ) | | | (1,577,720 | ) | | | (300,000 | ) |
| Increase in notes receivable | | | — | | | | (2,000,000 | ) | | | — | |
| Premiums paid for life insurance policies | | | (386,621 | ) | | | (324,505 | ) | | | (171,042 | ) |
| Proceeds received from life insurance policies | | | 46,700 | | | | 87,734 | | | | 163,950 | |
| Additions to property, plant and equipment | | | (7,535,578 | ) | | | (7,024,076 | ) | | | (1,557,588 | ) |
| Cash proceeds from the disposition of property, plant, and equipment | | | 183,373 | | | | 322,589 | | | | 450,097 | |
| | | | | | | | | | | | | |
| Net cash used in investing activities | | | (9,851,006 | ) | | | (20,515,978 | ) | | | (1,414,583 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Borrowings of revolving line of credit | | | 55,370,014 | | | | 55,555,496 | | | | 52,894,765 | |
| Repayments of revolving line of credit | | | (52,166,141 | ) | | | (57,162,619 | ) | | | (63,106,023 | ) |
| Borrowings of long-term debt | | | — | | | | 13,800,000 | | | | — | |
| Repayments of long-term debt | | | (5,775,105 | ) | | | (5,109,985 | ) | | | (5,106,371 | ) |
| Net proceeds from issuance of common stock | | | 863,396 | | | | 560,644 | | | | 316,501 | |
| | | | | | | | | | | | | |
| Net cash (used in) provided by financing activities | | | (1,707,836 | ) | | | 7,643,536 | | | | (15,001,128 | ) |
| | | | | | | | | | | | | |
| Effect of Exchange rate changes on cash | | | 47,539 | | | | (33,702 | ) | | | 2,888 | |
| | | | | | | | | | | | | |
| Net increase in cash and cash equivalents | | | 1,041,126 | | | | 420,552 | | | | (599,897 | ) |
| Cash and cash equivalents at beginning of period | | | 648,265 | | | | 1,689,391 | | | | 2,109,943 | |
| | | | | | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 1,689,391 | | | $ | 2,109,943 | | | $ | 1,510,046 | |
| | | | | | | | | | | | | |
| Supplemental disclosures of cash flow information: | | | | | | | | | | | | |
| Interest paid (net of capitalized interest of $37,000 in 2007, $26,000 in 2008 and $3,000 in 2009) | | $ | 2,853,706 | | | $ | 2,211,714 | | | $ | 1,306,242 | |
| | | | | | | | | | | | | |
| Income taxes paid | | $ | 5,792,166 | | | $ | 3,156,077 | | | $ | 4,499,336 | |
| | | | | | | | | | | | | |
| Supplemental schedule of non-cash investing and financing activities: | | | | | | | | | | | | |
| Capital lease obligations | | $ | — | | | $ | (881,000 | ) | | $ | — | |
| The Company purchased the operations of certain dental laboratories in 2008. In connection with these acquisitions, liabilities were assumed as follows: | | | | | | | | | | | | |
| Fair value of assets acquired including acquired cash | | $ | — | | | $ | 11,959,000 | | | $ | — | |
| Cash purchase price | | | — | | | | (10,112,000 | ) | | | — | |
| Deferred purchase price at date of acquisition | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Liabilities assumed | | $ | — | | | $ | 1,847,000 | | | $ | — | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2009
National Dentex Corporation (the “Company”) owns and operates 40 full-service dental laboratories and four branch laboratories in 30 states throughout the United States and one Canadian province as of December 31, 2009. Working from dentists’ work orders, the Company’s dental laboratories custom design and fabricate dentures, crowns and fixed bridges, and other dental prosthetic appliances.
| |
| (2) | Summary of Significant Accounting Policies |
Principles of Consolidation
The Company follows the guidance established by U.S. Generally Accepted Accounting Principles (GAAP) in presenting the consolidated financial statements. Acquisitions are reflected from the date acquired by the Company (see Note 3) to December 31, 2009. The accompanying financial statements include the accounts of the Company and its wholly and majority-owned subsidiaries. All material inter-company balances and transactions have been eliminated in consolidation.
Revenue Recognition
Revenue is recognized upon transfer of title and risk of loss, generally as the dentists’ orders are shipped. The Company records shipping and handling fees charged to customers as revenue. Shipping and handling costs totaling approximately $15,617,000, $16,463,000 and $14,800,000 for the years ended December 31, 2007, 2008 and 2009, respectively, are included in selling, general and administrative expense.
Goodwill and Other Indefinite-Lived Intangible Assets Not Subject to Amortization
The Company evaluates goodwill for impairment whenever events and circumstances have occurred that indicate that the value of goodwill may be impaired. In addition, goodwill is evaluated for possible impairment on an annual basis, based on a two-step process. The Company has selected June 30th as the date for the annual assessment of goodwill impairment.
The first step is used to identify potential impairment and involves comparing each reporting unit’s estimated fair value to its carrying value, including goodwill. A reporting unit is an operating segment or one level below an operating segment (referred to as a component). The Company has determined that each individual laboratory is a reporting unit. The second step of the goodwill impairment process involves the calculation of an implied fair value of goodwill for the laboratories which step one indicated were impaired. Fair value is determined by using an income approach, consistent with our valuation of dental laboratories acquired in purchase business combinations. The Company assessed goodwill impairment as of June 30, 2009 and determined that no impairment charge was required. The Company’s impairment analysis incorporates forecasted financial results and assumptions for all laboratories within the NDX Laboratories operating segment. Although these laboratories were not impaired at June 30, 2009, in the third quarter of 2009, the Company recorded an impairment charge of $264,000, comprising the entire goodwill balance of one of its smaller laboratories, due to a significant decrease in revenues and operating income during the third quarter of 2009 at this laboratory. During the fourth quarter of 2009, there were no events that triggered a goodwill impairment test or charge to goodwill impairment expense. The Company will continue to closely monitor the financial results of certain other laboratories in subsequent periods in comparison to forecasted financial results and assumptions made at June 30, 2009 to determine whether any other impairment charges are warranted.
Additionally, the Company also recognizes the existence of value in trade names acquired in business combinations and believes the useful life of this intangible to be indefinite based on a long history of utilizing the laboratory trade names. Accordingly, trade names are also evaluated for impairment on an annual basis using a
F-7
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
single step method. Impairment charges related to trade names are recognized when the fair value is less than the carrying value of the asset. Impairment charges related to trade names were recorded in the amount of $94,000, $44,000 and $361,000 for the years ended December 31, 2007, 2008 and 2009, respectively. Trade name impairment charges generally result from a decline in forecasted revenue at specific laboratories in comparison to revenue forecasts used in previous valuation calculations.
Intangible Assets Subject to Amortization
All acquisitions reflected in the accompanying consolidated financial statements have been accounted for as purchase business combinations. Non-competition agreements and customer relationship intangibles arising from dental laboratory acquisitions are amortized over their useful lives. The acquisition date fair value of non-competition agreements are deferred and amortized over their economic useful lives, in accordance with the terms of the agreements, over 2 to 15 years. The acquisition date fair value associated with acquired customer relationships are amortized over their estimated useful life, generally ranging over 9 to 12 years.
Advertising and Promotional Costs
Advertising, promotional and marketing costs are charged to earnings in the period in which they are incurred. These costs were approximately $2,625,000, $2,773,000 and $2,468,000 for the years ended December 31, 2007, 2008 and 2009, respectively.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with maturities of 90 days or less to be cash equivalents. At certain times the Company may have cash investments including overnight repurchase agreements with financial institutions in excess of the $250,000 insured limit of the Federal Deposit Insurance Corporation.
Accounts Receivable and Allowance for Doubtful Accounts
Trade accounts receivable are recorded at the invoiced amount. Service charges are assessed on balances 60 days past due. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in its existing accounts receivable. The Company determines the allowance based on historical write-off experience. Past due balances over 90 days and over a specified amount are also reviewed individually for collectability. Account balances are charged off against the allowance when the Company determines it is probable the receivable will not be recovered.
Receivables consist of the following at December 31, 2008 and 2009:
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| |
| Trade | | $ | 17,193,121 | | | $ | 16,715,927 | |
| Allowance for doubtful accounts | | | (491,982 | ) | | | (582,988 | ) |
| Employee | | | 298,893 | | | | 50,088 | |
| Income Taxes | | | 1,400,548 | | | | 1,393,000 | |
| Other | | | 827,727 | | | | 285,503 | |
| | | | | | | | | |
| Total Receivables | | $ | 19,228,307 | | | $ | 17,861,530 | |
| | | | | | | | | |
F-8
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Following are the changes in the allowance for doubtful accounts during the years ended December 31, 2007, 2008 and 2009:
| | | | | | | | | | | | | | | | | | | | | |
| | | Balance at
| | Charged to
| | | | Acquired in
| | Balance at
|
| | | Beginning
| | Costs and
| | | | Purchase Business
| | End of
|
| | | of Period | | Expenses | | Write-offs | | Combinations | | Period |
| |
| Allowance for Doubtful Accounts: | | | | | | | | | | | | | | | | | | | | |
| December 31, 2007 | | | 289,992 | | | | 151,330 | | | | 82,770 | | | | — | | | | 358,552 | |
| December 31, 2008 | | | 358,552 | | | | 148,069 | | | | 84,639 | | | | 70,000 | | | | 491,982 | |
| December 31, 2009 | | | 491,982 | | | | 274,337 | | | | 183,331 | | | | — | | | | 582,988 | |
Inventories
Inventories consist of the following:
| | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
| Raw Materials | | $ | 5,783,468 | | | $ | 5,726,274 | |
| Work in Process | | | 985,278 | | | | 945,196 | |
| Finished Goods | | | 222,639 | | | | 218,547 | |
| | | | | | | | | |
| | | $ | 6,991,385 | | | $ | 6,890,017 | |
| | | | | | | | | |
Inventories are stated at the lower of cost(first-in, first-out) or market. Work in process represents an estimate of the value of specific orders in production yet incomplete at period end. Finished goods consist of completed orders that were shipped to customers immediately subsequent to period end.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, less accumulated depreciation. Depreciation is calculated using the straight-line method over the following estimated depreciable lives:
| | | |
| Buildings | | 25 years |
| Furniture and fixtures | | 5 - 10 years |
| Laboratory equipment | | 5 - 20 years |
| Computer equipment | | 3 - 5 years |
Leasehold improvements and capital leases are amortized over the lesser of the assets’ estimated useful lives or the remaining lease term.
Gains and losses are recognized upon the disposal of property and equipment, and the related accumulated depreciation and amortization are removed from the accounts. Maintenance, repairs and betterments that do not enhance the value of or increase the life of the assets are charged to operations as incurred. Interest costs, if incurred, should be capitalized as part of the cost of acquiring or constructing qualifying assets. The Company had two qualifying assets which required a period of time to make ready for their intended use. Capitalized interest which is classified as Leasehold and Building Improvements totaled approximately $37,000, $26,000 and $3,000 for the years ended December 31, 2007, 2008 and 2009, respectively.
Depreciation expense totaled approximately $4,055,000, $4,872,000 and $4,569,000 for the years ended December 31, 2007, 2008 and 2009, respectively.
F-9
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Impairment of Long-Lived Assets
At each balance sheet date, management evaluates the recoverability of the long-lived assets, including property and equipment and intangible assets, using certain financial indicators, such as historical and future ability to generate income from operations. The Company’s policy is to assess long-lived asset impairment in the period when it is determined that the carrying amount of the asset may not be recoverable. The determination is based on an evaluation of such factors as the occurrence of a significant event, a significant change in the environment in which the business operates or if the expected future undiscounted cash flows become less than the carrying amount of the asset.
Cash Surrender of Life Insurance
Life insurance policies, which are owned by the Company and are presented as other assets, are recorded at their net realizable value, which approximates the surrender value of the policy.
Income Taxes
The Company has deferred tax assets and liabilities that are recognized for the expected future tax consequences of events that have been included in the financial statements or tax returns. The amount of deferred tax asset or liability is based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.
Accounting for income taxes prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The minimum threshold is defined as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement.
Earnings per Share
In accordance with disclosure requirements, basic earnings per share is computed by dividing net income by the weighted average number of shares outstanding and diluted earnings per share reflects the dilutive effect of potential common shares. The weighted average number of shares outstanding, the dilutive effects of outstanding stock options and the shares under option plans that were anti-dilutive for the years ended December 31, 2007, 2008 and 2009 are as follows:
| | | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2007 | | 2008 | | 2009 |
| |
| Weighted average number of shares used in basic earnings per share calculation | | | 5,540,496 | | | | 5,631,450 | | | | 5,727,071 | |
| Incremental shares under option and employee stock purchase plans | | | 124,546 | | | | — | | | | 33,714 | |
| | | | | | | | | | | | | |
| Weighted average number of shares used in diluted earnings per share calculation | | | 5,665,042 | | | | 5,631,450 | | | | 5,760,785 | |
| | | | | | | | | | | | | |
| Shares under option plans excluded in computation of diluted earnings per share due to anti-dilutive effects | | | 1,215 | | | | 591,486 | | | | 621,511 | |
| | | | | | | | | | | | | |
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts
F-10
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Disclosures about the Fair Value of Financial Instruments
The Company’s financial instruments mainly consist of cash and cash equivalents, accounts receivable, accounts payable, and current and long-term liabilities. The carrying amounts of the Company’s cash and cash equivalents, accounts receivable and accounts payable approximate their fair value due to the short-term nature of these instruments. The carrying amount of the long-term liabilities also approximates their fair value, based on rates available to the Company for debt with similar terms and remaining maturities.
Comprehensive Income
Comprehensive income is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. The Company’s total comprehensive income was as follows for the periods presented:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
| |
| Net income (loss) | | $ | 6,625,659 | | | $ | (877,043 | ) | | $ | 5,876,863 | |
| Foreign currency translation adjustments | | | 567,240 | | | | (845,345 | ) | | | 568,934 | |
| | | | | | | | | | | | | |
| Total comprehensive income (loss) | | $ | 7,192,899 | | | $ | (1,722,388 | ) | | $ | 6,445,797 | |
| | | | | | | | | | | | | |
Accumulated other comprehensive income (loss) at December 31, 2007, 2008 and 2009 of $445,329, ($400,016) and $168,918, respectively, as presented in the equity section of the consolidated balance sheet is entirely attributable to accumulated foreign currency translation adjustments.
Disclosures about Segments of an Enterprise
Accounting standards for reporting information regarding operating segments in annual financial statements require selected information for those segments to be presented in interim financial reports issued to stockholders and also establish standards for related disclosures about products and services and geographic areas. Operating segments are identified as components of an enterprise about which separate financial information is available for the evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance.
In March 2005, the Company acquired Green Dental Laboratories, Inc. of Heber Springs, Arkansas. In October 2006, the Company acquired Keller Group, Incorporated, a privately-held dental laboratory business with production facilities in both St. Louis, Missouri and Louisville, Kentucky. The Company identified Green and Keller as separate operating segments based on the quantitative criterion of the accounting standard. As a result, the Company has three reportable segments. The accounting policies of these segments are consistent with those described for the consolidated financial statements in the summary of significant accounting policies.
Fair Value Measurements
Effective January 1, 2008, the Company adopted accounting standards that define fair value, establish a framework for measuring fair value and enhances disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. These standards establish a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value; describe three levels of
F-11
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
inputs that may be used to measure fair value which are provided in the table below; and allow an entity the irrevocable option to elect fair value for the initial and subsequent measurement for certain financial assets and liabilities on acontract-by-contract basis. The adoption of these standards had no material impact on the Company’s financial statements.
The Company uses the market approach technique to value its financial instruments and there were no changes in valuation techniques during the year ended December 31, 2009. The Company’s financial assets and liabilities are primarily comprised of investments in insurance contracts held as assets to satisfy outstanding retirement liabilities.
Assets and liabilities carried at fair value are classified and disclosed in one of the following three categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities that the Company has the ability to access.
Level 2: Observable market based inputs or unobservable inputs that are corroborated by market data such as quoted prices, interest rates, and yield curves.
Level 3: Inputs are unobservable data points that are not corroborated by market data.
The following table presents information about the Company’s financial assets measured at fair value on a recurring basis as of December 31, 2009 and 2008. There were no liabilities that required disclosure:
| | | | | | | | | | | | | | | | | |
| | | | | | Quoted Prices in
| | | Significant Other
| | | Significant
| |
| | | As of
| | | Active Markets
| | | Observable Inputs
| | | Unobservable
| |
Description | | December 31, 2009 | | | (Level 1) | | | (Level 2) | | | Inputs (Level 3) | |
| |
Financial Assets | | | | | | | | | | | | | | | | |
| Cash Surrender Value of Life Insurance | | $ | 5,546,000 | | | | — | | | $ | 5,546,000 | | | | — | |
| | | | | | | | | | | | | | | | | |
| Total Financial Assets | | $ | 5,546,000 | | | | — | | | $ | 5,546,000 | | | | — | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | Quoted Prices in
| | | Significant Other
| | | Significant
| |
| | | As of
| | | Active Markets
| | | Observable Inputs
| | | Unobservable
| |
Description | | December 31, 2008 | | | (Level 1) | | | (Level 2) | | | Inputs (Level 3) | |
| |
Financial Assets | | | | | | | | | | | | | | | | |
| Cash Surrender Value of Life Insurance | | $ | 5,479,000 | | | | — | | | $ | 5,479,000 | | | | — | |
| | | | | | | | | | | | | | | | | |
| Total Financial Assets | | $ | 5,479,000 | | | | — | | | $ | 5,479,000 | | | | — | |
| | | | | | | | | | | | | | | | | |
Recent Accounting Pronouncements
In April 2009, the FASB issued additional guidance for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased and for identifying circumstances that indicate a transaction is not orderly. This guidance emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date under current market conditions. This guidance is effective for interim and annual reporting periods ending after June 15, 2009 and its adoption did not have an impact on the Company’s unaudited consolidated financial statements.
In May 2009, the FASB issued a pronouncement on subsequent event accounting. The guidance identifies the following: the period after the balance sheet date during which management shall evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements; the circumstances under which an
F-12
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
entity shall recognize events or transactions occurring after the balance sheet date in its financial statements; and the disclosures that an entity shall make about events or transactions that occurred after the balance sheet date. This statement was effective for the Company’s second quarter 2009, and there was no effect from adoption.
In June 2009, the FASB issued guidance onTheFASB Accounting Standards Codificationtmand the Hierarchy of Generally Accepted Accounting Principles,which is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The adoption of this pronouncement did not have an impact on the Company’s consolidated financial statements.
The Company’s acquisition strategy is to consolidate within the dental laboratory industry and use its financial and operational synergies to create a competitive advantage. Certain factors, such as the laboratory’s assembled workforce and technical skills, result in the recognition of goodwill.
In certain transactions, the Company executes non-compete agreements with the former owners and other key employees. The fair value of these agreements is recognized in purchase accounting as an identifiable intangible asset and is amortized over the estimated economic life of the agreement. All acquisitions have been reflected in the accompanying consolidated financial statements from the date of acquisition and have been accounted for as purchase business combinations. Purchase price is allocated to acquired assets and liabilities based on estimates of their related fair values.
There were no acquisitions of dental laboratories during the year ended December 31, 2009. During 2008, the Company acquired the following dental laboratory operations:
| | | | | | | |
Acquisition | | Form of Acquisition | | Location | | Period Acquired |
| |
| Dental Art Laboratories, Inc. | | All Outstanding Capital Stock | | Lansing, MI | | September, 2008 |
The cost of the acquisition of Dental Art, net of cash acquired, was $10,000,000. The total purchase price has been allocated to the acquired assets and liabilities based on estimates of their related fair values. The total purchase price was allocated as follows as of December 31, 2008:
| | | | | |
| | | Total
| |
Dental Art Laboratories, Inc. | | Acquired | |
| |
| Total Purchase Price | | $ | 10,112,000 | |
| Less Fair Market Values Assigned to Tangible Assets and Liabilities: | | | | |
| Cash | | | 112,000 | |
| Accounts receivable | | | 883,000 | |
| Inventories | | | 252,000 | |
| Property, plant and equipment | | | 332,000 | |
| Other assets | | | 140,000 | |
| Accounts payable | | | (192,000 | ) |
| Accrued liabilities and other | | | (1,655,000 | ) |
| Less Fair Market Values Assigned to Intangible Assets: | | | | |
| Customer relationships | | | 1,500,000 | |
| Trade names | | | 920,000 | |
| Non-compete agreements | | | 150,000 | |
| | | | | |
| Goodwill | | $ | 7,670,000 | |
| | | | | |
Acquired goodwill in certain situations may be tax deductible over a fifteen-year period, as allowed under Internal Revenue Code Section 197. However, acquired goodwill for Dental Art is not tax deductible.
F-13
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following unaudited pro forma operating results of the Company assume the Dental Art acquisition had been made as of January 1, 2007. Such information includes adjustments to reflect additional depreciation, non-compete and customer relationship amortization and interest expense, and is not necessarily indicative of what the results of operations would actually have been or of the results of operations in future periods.
| | | | | | | | | |
| | | Years Ended |
| | | December 31, 2007 | | December 31, 2008 |
| |
| Net sales | | $ | 178,140,000 | | | $ | 176,858,000 | |
| Net income (loss) | | | 7,324,000 | | | | (413,000 | ) |
| Net income (loss) per share: | | | | | | | | |
| Basic | | $ | 1.32 | | | $ | (0.07 | ) |
| Diluted | | $ | 1.29 | | | $ | (0.07 | ) |
(4) Goodwill and Other Intangible Assets
The changes in the carrying amount of goodwill for the years ended December 31, 2008 and 2009 are as follows:
| | | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
| |
NDX Laboratories Segment | | | | | | | | |
| Goodwill, Balance as of January 1 | | $ | 37,636,000 | | | $ | 44,983,000 | |
| Accumulated impairment losses | | | — | | | | (6,950,000 | ) |
| | | | | | | | | |
| | | | 37,636,000 | | | | 38,033,000 | |
| Goodwill acquired during the year | | | 7,501,000 | | | | — | |
| Adjustments related to contingent consideration | | | 300,000 | | | | — | |
| Effects of exchange rate changes | | | (454,000 | ) | | | 316,000 | |
| Impairment losses and other adjustments | | | (6,950,000 | ) | | | (84,000 | ) |
| Balance as of December 31 | | | | | | | | |
| Goodwill | | | 44,983,000 | | | | 45,299,000 | |
| Accumulated impairment losses | | | (6,950,000 | ) | | | (7,034,000 | ) |
| | | | | | | | | |
| | | $ | 38,033,000 | | | $ | 38,265,000 | |
| | | | | | | | | |
| | | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
| |
Green Dental Laboratory Segment | | | | | | | | |
| Goodwill balance | | $ | 15,208,000 | | | $ | 15,208,000 | |
| | | | | | | | | |
| | | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
| |
Keller Group Segment | | | | | | | | |
| Goodwill balance | | $ | 16,143,000 | | | $ | 16,143,000 | |
| | | | | | | | | |
In connection with dental laboratory acquisitions, the Company has identified certain intangible assets including trade names, customer relationships and non-competition agreements.
F-14
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company continually evaluates whether events and circumstances have occurred that indicate that the value of goodwill has been impaired. In addition, goodwill is evaluated for possible impairment on an annual basis, based on a two-step process. The Company has selected June 30th as the date for the annual assessment of goodwill impairment.
The first step is used to identify potential impairment and involves comparing each reporting unit’s estimated fair value to its carrying value, including goodwill. A reporting unit is an operating segment or one level below an operating segment (referred to as a component). The Company has determined that each individual laboratory is a reporting unit. The second step of the goodwill impairment process involves the calculation of an implied fair value of goodwill for the laboratories which step one indicated were impaired. Fair value is determined by using an income approach, consistent with our valuation of dental laboratories acquired in purchase business combinations. The Company assessed goodwill impairment as of June 30, 2009 and determined that no impairment charge was required. The Company’s impairment analysis incorporates forecasted financial results and assumptions for its dental laboratories. Although none of its laboratories were impaired at June 30, 2009, in the third quarter of 2009, the Company recorded an impairment charge of $264,000, comprising the entire goodwill balance of one of its smaller laboratories in the NDX laboratories operating segment, due to a significant decrease in revenues and operating income from those forecasted as of June 30, 2009. During the fourth quarter of 2009, there were no events that triggered an additional goodwill impairment test or result in a charge to goodwill impairment expense. The Company continues to closely monitor the financial results of certain other laboratories in the NDX laboratories operating segment in subsequent periods in comparison to forecasted financial results and assumptions made at June 30, 2009 to determine whether any other impairment charges are warranted for these other laboratories. As of December 31, 2009, the Company had $69,616,000 of goodwill remaining on its consolidated balance sheet.
Trade Names
Trade names, as acquired, are valued using the relief-from-royalty valuation approach. Company practice is to use existing and acquired trade names in perpetuity, and consequently they have been treated as indefinite-lived intangibles. While these assets are not subject to amortization, they are tested for impairment on an annual basis, or as circumstances may require. The Company uses the relief-from-royalty valuation approach at each fiscal year end to determine whether the trade names are impaired. Trade name impairment charges resulted from a decline in forecasted revenue at specific laboratories in comparison to revenue forecasts used in previous valuation calculations. The Company recorded impairment charges of $94,000, $44,000 and $361,000 in 2007, 2008 and 2009, respectively. Impairment charges are a component of selling, general and administrative expense.
The changes in the carrying amount of trade names for the years ended December 31, 2008 and 2009 are as follows:
| | | | | | | | | |
| | | Years Ended | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
| Beginning of year | | $ | 8,998,000 | | | $ | 9,978,000 | |
| Trade names acquired during the year | | | 1,100,000 | | | | — | |
| Effects of exchange rate changes | | | (76,000 | ) | | | 54,000 | |
| | | | | | | | | |
| Trade names | | | 10,022,000 | | | | 10,032,000 | |
| Impairment charges and other adjustments | | | (44,000 | ) | | | (541,000 | ) |
| | | | | | | | | |
| Trade names — end of year, net | | $ | 9,978,000 | | | $ | 9,491,000 | |
| | | | | | | | | |
F-15
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Customer Relationships
Acquired dental laboratories have customer relationships in place with dentists within their market areas. The Company recognizes customer relationship assets when established relationships exist with customers through contract or other contractual relationships such as purchase orders or sales orders. Customer relationships are valued based on an analysis of revenue and customer attrition data and amortized over their useful life. The weighted-average amortization period for the Dental Art acquisition completed in 2008 was 12 years. The amounts assigned to customer relationships are amortized on a straight-line basis over their useful lives. The Company has determined that the straight-line method is appropriate based on an analysis of customer attrition statistics.
| | | | | | | | | |
| | | Years Ended | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
| Beginning of year, gross | | $ | 7,993,000 | | | $ | 9,439,000 | |
| Customer relationships acquired during the year | | | 1,500,000 | | | | — | |
| Effects of exchange rate changes | | | (54,000 | ) | | | 35,000 | |
| | | | | | | | | |
| Customer relationships, gross | | | 9,439,000 | | | | 9,474,000 | |
| Less: Accumulated amortization | | | (3,229,000 | ) | | | (4,121,000 | ) |
| | | | | | | | | |
| Customer relationships, net — end of year | | $ | 6,210,000 | | | $ | 5,353,000 | |
| | | | | | | | | |
Amortization expense associated with customer relationships totaled approximately $771,000, $811,000 and $892,000 for the years ended December 31, 2007, 2008 and 2009, respectively, and is recorded as operating expenses. Future amortization expense of the current customer relationship balance will be approximately:
| | | | | |
| 2010 | | $ | 894,000 | |
| 2011 | | | 894,000 | |
| 2012 | | | 825,000 | |
| 2013 | | | 614,000 | |
| 2014 | | | 483,000 | |
| Thereafter | | | 1,643,000 | |
| | | | | |
| | | $ | 5,353,000 | |
| | | | | |
Non-competition Agreements
In connection with acquisitions, the Company has executed non-compete agreements with certain individuals, ranging over periods of 2 to 15 years. The weighted-average amortization period, which is based on the estimated useful life of the agreement, for the acquisition completed in 2008 was 7.5 years. The amounts assigned to non-competition agreements are amortized on a straight-line basis over the economic useful life of the agreement, and are recorded as operating expenses.
F-16
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| | | | | | | | | |
| | | Years Ended | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
| Beginning of year, gross | | $ | 10,553,000 | | | $ | 10,696,000 | |
| Non-competition agreements acquired during the year | | | 150,000 | | | | — | |
| Effects of exchange rate changes | | | (7,000 | ) | | | 3,000 | |
| | | | | | | | | |
| Non-competition Agreements, gross | | | 10,696,000 | | | | 10,699,000 | |
| Less: Accumulated amortization | | | (9,112,000 | ) | | | (9,407,000 | ) |
| | | | | | | | | |
| Non-competition agreements, net | | $ | 1,584,000 | | | $ | 1,292,000 | |
| | | | | | | | | |
Amortization expense associated with non-competition agreements totaled approximately $489,000, $303,000 and $296,000 for the years ended December 31, 2007, 2008 and 2009, respectively.
Future amortization expense of non-competition agreements will be approximately:
| | | | | |
| 2010 | | $ | 287,000 | |
| 2011 | | | 247,000 | |
| 2012 | | | 192,000 | |
| 2013 | | | 170,000 | |
| 2014 | | | 115,000 | |
| Thereafter | | | 281,000 | |
| | | | | |
| | | $ | 1,292,000 | |
| | | | | |
The following is a summary of the provision (benefit) for income taxes:
| | | | | | | | | | | | | |
| | | Years Ended | |
| | | December 31,
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2008 | | | 2009 | |
| |
| Federal — | | | | | | | | | | | | |
| Current | | $ | 2,985,255 | | | $ | 2,134,884 | | | $ | 3,418,520 | |
| Deferred | | | (189,842 | ) | | | (851,093 | ) | | | (533,076 | ) |
| | | | | | | | | | | | | |
| | | | 2,795,413 | | | | 1,283,791 | | | | 2,885,444 | |
| | | | | | | | | | | | | |
| State — | | | | | | | | | | | | |
| Current | | | 860,500 | | | | 646,717 | | | | 1,168,660 | |
| Deferred | | | (25,700 | ) | | | (188,545 | ) | | | (105,688 | ) |
| | | | | | | | | | | | | |
| | | | 834,800 | | | | 458,172 | | | | 1,062,972 | |
| | | | | | | | | | | | | |
| Foreign — | | | | | | | | | | | | |
| Current | | | 237,848 | | | | 240,108 | | | | 251,460 | |
| Deferred | | | (7,848 | ) | | | (10,108 | ) | | | (21,460 | ) |
| | | | | | | | | | | | | |
| | | | 230,000 | | | | 230,000 | | | | 230,000 | |
| | | | | | | | | | | | | |
| | | $ | 3,860,213 | | | $ | 1,971,963 | | | $ | 4,178,416 | |
| | | | | | | | | | | | | |
Through December 31, 2009, the Company has not provided deferred income taxes on the undistributed earnings of its foreign subsidiary because such earnings are intended to be permanently reinvested outside the
F-17
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
U.S. Determination of the potential deferred income tax liability on these undistributed earnings is not practicable. At December 31, 2009, the Company had 1,511,000 of undistributed earnings in its foreign subsidiary.
Deferred income taxes are comprised of the following at December 31, 2008 and 2009:
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| |
Deferred Tax Assets: | | | | | | | | |
| Non-compete agreements | | $ | 900,252 | | | $ | 811,491 | |
| Other liabilities | | | 2,917,310 | | | | 3,321,180 | |
| Vacation benefits | | | 745,677 | | | | 750,548 | |
| Inventory basis differences | | | 251,687 | | | | 234,018 | |
| Receivables basis differences | | | 44,405 | | | | 83,058 | |
| | | | | | | | | |
| Total deferred tax assets | | | 4,859,331 | | | | 5,200,295 | |
| | | | | | | | | |
Deferred Tax Liabilities: | | | | | | | | |
| Depreciation differences | | | (2,146,150 | ) | | | (2,373,141 | ) |
| Intangible amortization differences | | | (7,635,082 | ) | | | (7,643,873 | ) |
| | | | | | | | | |
| Total deferred tax liabilities | | | (9,781,232 | ) | | | (10,017,014 | ) |
| | | | | | | | | |
| Net deferred tax asset/liability | | $ | (4,921,901 | ) | | $ | (4,816,719 | ) |
| | | | | | | | | |
A reconciliation between the provision for income taxes computed at statutory rates and the amount reflected in the accompanying statements of income is as follows:
| | | | | | | | | | | | | |
| | | December 31,
| | December 31,
| | December 31,
|
| | | 2007 | | 2008 | | 2009 |
| |
| Statutory federal income tax rate | | | 35.0 | % | | | 34.0 | % | | | 34.0 | % |
| State income tax, net of federal income tax benefit | | | 4.9 | | | | 27.6 | | | | 6.9 | |
| Research and experimentation credit | | | — | | | | (55.1 | ) | | | — | |
| Non-deductible goodwill | | | — | | | | 151.3 | | | | 0.9 | |
| Cash surrender value of life insurance | | | (.7 | ) | | | 21.9 | | | | (.2 | ) |
| Domestic production deduction | | | (2.2 | ) | | | (13.2 | ) | | | (2.3 | ) |
| Other | | | (.2 | ) | | | 13.6 | | | | 2.3 | |
| | | | | | | | | | | | | |
| Effective income tax rate | | | 36.8 | % | | | 180.1 | % | | | 41.6 | % |
| | | | | | | | | | | | | |
At December 31, 2007 the Company had recorded $2,033,000 of unrecognized tax benefits and related interest and penalties of $102,000. This liability related to ongoing tax filing positions taken by the Company in its previously filed US Federal and State tax returns. The Company determined this $2,135,000 did not meet the recognition provisions of accounting for tax uncertainties and no material adjustments were required upon adoption. Interest and penalties, as appropriate, are recorded as a component of the Company’s tax liability and tax provision. Interest and penalties recorded for the year ended December 31, 2009 were approximately $21,000.
F-18
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A reconciliation of the beginning and ending amount of unrecognized tax benefits, exclusive of accrued interest and penalties of $154,000 at January 1, 2009 and $175,000 at December 31, 2009, is as follows:
| | | | | |
| Unrecognized tax benefits at January 1, 2008 | | $ | 2,033,000 | |
| Additions based on tax positions related to the current year | | | — | |
| Subtractions for tax positions of prior years | | | (536,000 | ) |
| | | | | |
| Unrecognized tax benefits at December 31, 2008 | | | 1,497,000 | |
| Additions based on tax positions related to the current year | | | — | |
| Subtractions for tax positions of prior years | | | — | |
| | | | | |
| Unrecognized tax benefits at December 31, 2009 | | $ | 1,497,000 | |
| | | | | |
In 2008, tax benefits of $515,000 and the reversal of accrued interest and penalties of $89,000 were recognized as a result of the lapse of the statute of limitations for tax year 2002. The remaining balances represent unrecognized tax benefits that would decrease the Company’s effective tax rate upon recognition. The Company believes that it is reasonably possible unrecognized tax benefits of $1,497,000 and accruals for interest and penalties of $86,000 will reverse in 2010 as a result of the Internal Revenue Service examination of the Company’s U.S. income tax returns for 2004 through 2006. Additionally, there is an amount included in prepaid expenses related to advanced fees which will reverse when the IRS audit is settled. As of December 31, 2009, the tax years that remain subject to examination by the IRS and other jurisdictions are 2004 to 2008.
| |
| (6) | Lines of Credit and Term Loan Facility |
On August 9, 2005, the Company entered into an amended and restated financing agreement (the “Amended Agreement”) with Bank of America, N.A. (the “Bank”). The Amended Agreement included a revolving line of credit of $5,000,000, a revolving acquisition line of credit of $20,000,000 and a term loan facility of $20,000,000. The interest rate on both revolving lines of credit and the term loan was the prime rate or, at the Company’s option, LIBOR, a cost of funds rate or the Bank’s fixed rate plus a range of 1.25% to 2.25% per annum, depending on the ratio of consolidated funded debt to consolidated “EBITDA,” as defined in the Amended Agreement. The Amended Agreement required monthly payments of principal, based on a seven-year amortization schedule, with a final payment due on the fifth anniversary of the Amended Agreement. The Amended Agreement required compliance with certain covenants, including the maintenance of specified net worth, income and other financial ratios.
In October 2006, the Company borrowed against its acquisition line of credit to finance the acquisition of Keller Group, Incorporated (“Keller”). In order to refinance the borrowings incurred for the Keller acquisition, the Company and the Bank executed a Second Amended and Restated Loan Agreement as of November 7, 2006 (the “Second Agreement”) comprised of uncollateralized senior credit facilities that at that time totaled $60,000,000. The Second Agreement amended and restated the Amended Agreement (a) to increase the term loan facility to an aggregate principal amount of $35,000,000 and used the proceeds of the increase in the term loan to repay the outstanding principal balance under the acquisition line of credit and (b) to adjust the allocation of availability under the lines of credit by increasing the revolving line of credit to $10,000,000 ($5,000,000 of which may be used for future acquisitions) and decreasing the acquisition line of credit from $20,000,000 to $15,000,000. The interest rate on both lines of credit and the term loan was the prime rate or, at the Company’s option, LIBOR, a cost of funds rate or the Bank’s fixed rate, plus, in each case, a range of 1.25% to 3.00% per annum, depending on the ratio of consolidated total funded debt to consolidated “EBITDA,” as each is defined in the Second Agreement. The term loan facility portion of the Second Agreement requires monthly interest payments and monthly payments of principal, based on a seven year amortization schedule, with a final payment due on the fifth anniversary of the Second Agreement. The Second Agreement requires compliance with certain covenants, including the maintenance of specified net worth, minimum consolidated total “EBITDA,” debt to income ratio and other financial ratios.
The Second Agreement was amended on May 9, 2008, effective March 31, 2008, to revise certain financial targets within these covenants. Additionally, the Bank and the Company agreed to consolidate the revolving line of
F-19
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
credit with the acquisition line of credit into a single line of credit of $25,000,000 to be used by the Company for general corporate purposes, including potential acquisitions. The Second Agreement was also amended on September 2, 2008 in connection with the acquisition of Dental Art, which increased the Company’s outstanding debt and therefore required an adjustment to an affected financial covenant. The Company further amended the agreement on December 16, 2008 to extend the maturity of the line of credit to November 7, 2011. The amendment changed the interest rate on both the line of credit and the term loan to prime rate or, at the Company’s option, LIBOR, a cost of funds rate, or the Bank’s fixed rate, plus, in each case, a range of 2.50% to 3.50% per annum, depending on the ratio of consolidated total funded debt to consolidated “EBITDA,” as each is defined in the Second Agreement and also increased the commitment fee on the unused portion of the line of credit from 0.125% to 0.50% per annum. In addition, the amendment revised certain financial targets within the covenants. Finally, on March 13, 2009, the Second Agreement was further amended to exclude $6,950,000 of goodwill impairment in the fourth quarter of 2008 from the calculation of “EBITDA,” used in determining compliance with certain financial covenants. These amendments did not change the total availability under the Second Agreement. While the Company was in compliance with its debt covenants as of December 31, 2009, it continues to closely monitor compliance with these covenants in future periods, particularly minimum consolidated total “EBITDA,” which may be negatively impacted by, among other things, potential declines in future earnings, or declines attributable to additional goodwill impairment.
As of December 31, 2009, $18,471,000 was available under the consolidated revolving line of credit.
Long-Term Obligations:
| | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2008 | | | 2009 | |
| |
| Term note | | $ | 24,583,000 | | | $ | 19,583,000 | |
| Borrowings classified as long term under the revolving line of credit | | | 13,800,000 | | | | 6,529,000 | |
| Borrowings classified as short term under the revolving line of credit | | | 2,940,000 | | | | — | |
| Other long-term debt | | | 875,000 | | | | 736,000 | |
| | | | | | | | | |
| Total long-term debt | | | 42,198,000 | | | | 26,848,000 | |
| Less: current maturities | | | 8,055,000 | | | | 5,072,000 | |
| | | | | | | | | |
| Long-term debt, less current portion | | $ | 34,143,000 | | | $ | 21,776,000 | |
| | | | | | | | | |
The table below reflects the expected repayment terms associated with the long-term debt at December 31, 2009. The interest rate associated with the Company’s borrowings as of December 31, 2009 was 3.2%.
| | | | | |
| | | December 31, 2009
| |
| | | Principal Due | |
| |
| Fiscal 2010 | | | 5,072,000 | |
| Fiscal 2011 | | | 21,187,000 | |
| Fiscal 2012 | | | 80,000 | |
| Fiscal 2013 | | | 85,000 | |
| Fiscal 2014 | | | 90,000 | |
| Thereafter | | | 334,000 | |
| | | | | |
| Total | | $ | 26,848,000 | |
| | | | | |
The Company has a qualified retirement plan under Internal Revenue Code Sections 401(a) and 401(k) (the “401(k) Plan”). The 401(k) Plan allows contributions of up to 10% of a participant’s salary, a portion of which is
F-20
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
matched in cash by the Company. The Company contributes cash once a year, within 120 days after December 31, the 401(k) Plan’s year-end. All employees are eligible to participate in the 401(k) Plan after completing one year of service with the Company and the attainment of age 21. Participants are fully vested immediately in employee contributions and become fully vested in the Company’s matching contributions after six years of service or upon attaining age 65. The Company has incurred charges to operations of approximately $826,000, $849,000 and $847,000 to match contributions for the years ended December 31, 2007, 2008 and 2009, respectively.
The Company had a cash incentive plan (the “Laboratory Plan”) for dental laboratory management and other designated key employees who directly influenced the financial performance of an individual dental laboratory. Participant eligibility was determined annually for each laboratory and each participant was eligible to receive an amount based on the achievement of certain earnings levels and other performance metrics by the participant’s laboratory. The Company incurred charges to operations of approximately $3,872,000 for the year ended December 31, 2007 under the Laboratory Plan.
Beginning in 2008, a new incentive program was implemented to provide incentives for growth in profits with participant eligibility to be determined on an ongoing and discretionary basis. The Company has incurred charges to operations of approximately $591,000 and $1,879,000 for the years ended December 31, 2008 and 2009, respectively, under this program.
The Company has an executive bonus plan (the “Executive Plan”) for key executives and management of the Company. Eligibility to participate in this plan is determined annually. Participants are eligible to receive a cash bonus, based on a percentage of salary, dependent upon the achievement of earnings targets, as defined. The bonus is distributed within 75 days after year-end. The Company has incurred aggregate charges to operations of approximately $448,000, $50,000 and $860,000, for the years ended December 31, 2007, 2008 and 2009, respectively, with respect to this plan.
The Company has established Supplemental Executive Retirement Plans (“SERP”) for certain key employees. Benefits are funded by life insurance contracts purchased and owned by the Company. The retirement benefit is based on the value in the individual life insurance policy grossed up for taxes and is payable at the age of 65 or upon retirement, if later, in either a lump sum or equal monthly installments over a period of 10 years. These benefits vest to the participating employees over periods of up to ten years. In one particular plan effective 2006, the retirement benefit is fixed at $125,000 per year for ten years, payable in equal monthly installments upon the individual attaining the age of 75. This benefit vests over five years. The charges to expense for the years ended December 31, 2007, 2008 and 2009, were approximately $727,000, $688,000 and $714,000, respectively and are recorded as accrued liabilities.
| |
| (8) | Commitments and Contingencies |
Operating Leases
The Company is committed under various non-cancelable operating lease agreements covering its office space and dental laboratory facilities and certain equipment. Certain of these leases also require the Company to pay maintenance, repairs, insurance and related taxes. The total rental expense for the years ended December 31, 2007, 2008 and 2009 was approximately $5,061,000, $4,888,000 and $4,778,000 respectively. The approximate aggregate minimum lease commitments under these operating leases as of December 31, 2009 are as follows:
| | | | | |
Year | | Amount | |
| |
| 2010 | | $ | 4,383,000 | |
| 2011 | | | 3,691,000 | |
| 2012 | | | 3,231,000 | |
| 2013 | | | 2,459,000 | |
| 2014 | | | 1,739,000 | |
| Thereafter | | | 3,763,000 | |
| | | | | |
| | | $ | 19,266,000 | |
| | | | | |
F-21
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Employment Contracts andChange-in-Control Arrangements
In April 1995, January 2001, May 2004, October 2006 and August 2008 the Company entered into employment contracts andchange-in-control arrangements with certain key executives. The initial term of these employment contracts is three years and the contracts by their terms renew automatically thereafter until termination by the Company or the executive. Thechange-in-control arrangements provide certain severance benefits in the event that the executive is terminated by the Company without cause or the executive terminates his employment contract for certain specified reasons.
The Company measures and recognizes in its consolidated statement of income the expense associated with all share-based payment awards made to employees and directors. Prior to January 1, 2006, compensation cost was not recognized for options granted because the exercise price of options granted was equal to the market value of the Company’s common stock on the measurement date and the Employees’ Stock Purchase Plan (ESPP) was deemed non-compensatory. Our share-based compensation programs include shares issued under our ESPP, time-vested restricted stock and restricted stock units, and incentive stock options issued under our 1992 Long Term Incentive Plan (LTIP) and our Amended and Restated 2001 Stock Plan (2001 Stock Plan). Compensation cost is measured on the grant date of the option, which is the date the Company’s Board of Directors approves the granting of the option. Compensation cost on discounts associated with ESPP purchases is estimated on the date that share rights are granted. To measure the fair value of stock option grants, the Company utilizes the Black-Scholes option valuation method. The requisite service period for substantially all of the Company’s stock options is the explicit vesting period included in the terms of the stock option award. Accordingly, the Company estimates compensation expense based on the number of options it believes will ultimately vest, which includes an estimate of the number of options expected to be forfeited. The estimated fair value of stock options grants will be recognized on a straight line basis over the requisite service period of the award. The Company periodically reviews its estimate of forfeitures and revises the estimate as facts and circumstances warrant. The following table summarizes share-based compensation expense associated with each of these programs:
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
| |
| Employee stock purchase plan | | $ | 161,000 | | | $ | 179,000 | | | $ | 230,000 | |
| Restricted stock and restricted stock units | | | 165,000 | | | | 270,000 | | | | 163,000 | |
| Performance-based stock options | | | — | | | | — | | | | — | |
| Incentive stock options | | | — | | | | — | | | | 143,000 | |
| | | | | | | | | | | | | |
| Total share-based compensation expense | | $ | 326,000 | | | $ | 449,000 | | | $ | 536,000 | |
| | | | | | | | | | | | | |
No share-based compensation has been capitalized. Windfall tax benefits from the exercises of stock options and ESPP options and issuance of restricted stock and restricted stock units was approximately $20,000, $35,000, and $183,000 for the years ended December 31, 2007, 2008, and 2009 respectively. These amounts have been calculated under the shortcut method.
Share-based Compensation Plans
We have three share-based compensation plans pursuant to which awards have been or are currently being made: (1) the 1992 LTIP, as amended from which no additional options may be granted; (2) the Amended and Restated 2001 Stock Plan from which incentive stock options and restricted shares and share units are granted; and (3) the Employees’ Stock Purchase Plan (ESPP), as amended.
F-22
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Stock Option Plans, Restricted Stock, and Restricted Stock Units
In May 1992, the Company’s Board of Directors (the “Board”) adopted the LTIP. Under the LTIP, the Board may grant stock options, stock appreciation rights, restricted stock, deferred stock, stock purchase rights and other share-based payments to key employees, officers and directors of the Company. As of May 2002, no additional options may be granted under this plan. These options vested over three years from date of grant and have a maximum term of ten years from the date of grant.
In April 2001, the Company’s shareholders approved the 2001 Stock Plan, under which awards may be granted to key employees, officers and directors in the form of stock options. The Board reserved 450,000 shares of common stock for issuance under the 2001 Stock Plan. In April 2004, the Board amended the 2001 Stock Plan to increase the number of shares of common stock reserved for issuance under the plan from 450,000 to 825,000. The Company’s shareholders approved an amendment to the 2001 Stock Plan on May 16, 2006 to allow for the issuance of restricted stock and restricted stock units, subject to a limit of 82,500. At December 31, 2009, options to purchase a total of 590,586 shares were outstanding and 8,450 unvested restricted shares were outstanding. There were 89,051 shares available for grant under the 2001 Stock Plan. Stock option awards granted under the 2001 Stock Plan generally vest ratably over three years on the anniversary date of the grants and are exercisable generally over a period of ten years. Restricted stock and restricted stock units are granted to directors and may vest anywhere from one to two years.
During 2008, the Company granted 275,000 performance-based stock options, which would vest upon the achievement of specific earnings per share targets during 2009, 2010 and 2011. The Company has assumed that none of these performance-based awards will vest and accordingly has not provided for compensation expense associated with the awards. The Company periodically evaluates the likelihood of reaching the performance requirements and would be required to recognize aggregate compensation expense of approximately $820,000 if the targets for 2010 and 2011 are fully met. The fair value of the stock option grants awarded was estimated as of the date of grant using a Black-Scholes option valuation model that uses the following weighted-average assumptions:
| | | | | |
| Weighted-average grant date fair value | | $ | 4.47 | |
| Risk-free interest rate | | | 3.84 | % |
| Expected volatility | | | 30.19 | % |
| Expected holding period in years | | | 6.0 | |
| Expected dividends | | | None | |
The weighted average grant date fair value was calculated under the Black-Scholes option-pricing model. The risk-free interest rate is based on the yield of U.S. Treasury securities that correspond to the expected holding period of the options. After assessing all available information on either historical and implied volatility, or both, the Company has concluded that a combination of both historical and implied volatility provides the best estimate of expected volatility. The expected holding period of options granted is derived using assumed exercise rates based on historical exercise patterns and represents the period of time that options are expected to be outstanding. The dividend yield was based on the Company’s expected dividend rate.
During 2009, the Company granted 120,000 incentive stock options. The estimated fair value of the options is recognized over the options’ vesting periods. Of those 120,000 options, 90,000 options vest up to one third in 2010, 2011, and 2012 and 30,000 options vest up to one half in 2010 and 2011. These options expire in April 2019. As of December 31, 2009, there was approximately $167,000 of unrecognized compensation cost related to incentive stock options. That cost will be recognized over a weighted average period of 10 months. The fair value of the stock option grants awarded was estimated as of the date of grant using the Black-Scholes option valuation model using the following weighted-average assumptions:
| | | | | |
| Weighted-average grant date fair value | | $ | 2.58 | |
| Risk-free interest rate | | | 2.91 | % |
| Expected volatility | | | 58.02 | % |
| Expected holding period in years | | | 8.0 | |
| Expected dividends | | | None | |
F-23
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A summary of stock option activity and related information for the year ended December 31, 2009 is as follows:
| | | | | | | | | | | | | | | | | |
| | | LTIP | | | 2001 Stock Plan | |
| | | | | | Weighted-
| | | | | | Weighted-
| |
| | | Shares
| | | Average
| | | Shares
| | | Average
| |
| | | Underlying
| | | Exercise
| | | Underlying
| | | Exercise
| |
| | | Options | | | Price | | | Options | | | Price | |
| |
| Outstanding December 31, 2008 | | | 154,330 | | | $ | 12.18 | | | | 563,000 | | | $ | 13.25 | |
| Granted | | | — | | | | — | | | | 120,000 | | | | 4.07 | |
| Exercised | | | — | | | | — | | | | — | | | | — | |
| Canceled | | | (3,405 | ) | | | 11.52 | | | | (92,414 | ) | | | 12.02 | |
| | | | | | | | | | | | | | | | | |
| Outstanding December 31, 2009 | | | 150,925 | | | $ | 12.20 | | | | 590,586 | | | $ | 11.58 | |
| Exercisable at end of period: | | | 150,925 | | | $ | 12.20 | | | | 287,250 | | | $ | 14.45 | |
The weighted-average remaining contractual term for options exercisable as of December 31, 2009 was 1.6 years. While, the intrinsic value of options exercised was $107,973 for the year ended December 31, 2007, there was no intrinsic value for options exercised or exercisable for the years ended December 31, 2008 and 2009.
Time-vested restricted stock and restricted stock units under the 2001 Stock Plan
For the year ended December 31, 2009, there were no grants of restricted stock or restricted stock units to our Board of Directors. For the year ended December 31, 2008, the Company granted 20,280 shares of restricted stock and 10,140 restricted stock units as directors’ fees under the 2001 Stock Plan, as amended in May 2006. For the year ended December 31, 2007, the Company granted 9,002 shares of restricted stock and 6,003 restricted stock units as directors’ fees under the 2001 Stock Plan.
The following table summarizes restricted stock and restricted stock unit awards for the year ended December 31, 2009:
| | | | | | | | | | | | | | | | | |
| | | Restricted Stock | | | Restricted Stock Units | |
| | | | | | Weighted-Average
| | | | | | Weighted-Average
| |
| | | | | | Grant Date Fair
| | | | | | Grant Date Fair
| |
| | | Number of Shares | | | Value | | | Number of Shares | | | Value | |
| |
| Nonvested December 31, 2006 | | | 1,596 | | | $ | 22.55 | | | | 3,192 | | | $ | 22.55 | |
| Granted | | | 9,002 | | | | 17.99 | | | | 6,003 | | | | 17.99 | |
| Vested | | | 1,596 | | | | 22.55 | | | | 3,192 | | | | 22.55 | |
| Cancelled | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Nonvested December 31, 2007 | | | 9,002 | | | | 17.99 | | | | 6,003 | | | | 17.99 | |
| Granted | | | 20,280 | | | | 10.65 | | | | 10,140 | | | | 10.65 | |
| Vested | | | 6,502 | | | | 17.99 | | | | 6,003 | | | | 17.99 | |
| Cancelled | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Nonvested December 31, 2008 | | | 22,780 | | | | 11.46 | | | | 10,140 | | | | 10.65 | |
| Granted | | | — | | | | — | | | | — | | | | — | |
| Vested | | | 14,330 | | | | 11.93 | | | | 10,140 | | | | 10.65 | |
| Cancelled | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Nonvested December 31, 2009 | | | 8,450 | | | $ | 10.65 | | | | — | | | $ | — | |
As of December 31, 2009, there was $33,750 of total unrecognized compensation cost for restricted stock. That cost will be recognized over a weighted average period of 4.5 months. The total fair value of shares vested during 2007, 2008, and 2009 was $65,404, $134,405, and $172,447 respectively.
F-24
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Employees’ Stock Purchase Plan (ESPP)
Also, the Company has the ESPP, under which an aggregate of 450,000 shares of the Company’s common stock have been reserved. Upon enrollment, employees purchase shares of the Company’s common stock at the end of each plan year, through payroll deductions, at a discount of 15% of the lower of the market price on the date of grant or the date of exercise, as quoted on NASDAQ. These shares may be purchased in the current plan year, through a payroll deduction program, at a price equal to 85% of the fair market value of the common stock on either April 1, 2008 or March 31, 2009, whichever is lower. Approximately 273,195 shares are available for future purchases as of December 31, 2009. The number of shares of common stock purchased through the Stock Purchase Plan for 2007, 2008 and 2009 were 24,030, 33,375, and 93,102 respectively.
The following tables summarize the significant assumptions used to estimate stock compensation costs for the ESPP for the periods indicated:
| | | | | | | | | |
| | | January 1-
| | April 1-
|
| | | March 31,
| | December 31,
|
| | | 2009 | | 2009 |
| |
| Weighted-Average Grant Date Fair Value | | $ | 3.91 | | | $ | 1.82 | |
| Risk-free Interest Rate | | | 2.25 | % | | | 0.56 | % |
| Expected Volatility | | | 40.54 | % | | | 78.76 | % |
| Expected Holding Period in Years | | | 1.0 | | | | 1.0 | |
| Expected Dividends | | | None | | | | None | |
| | | | | | | | | |
| | | January 1-
| | April 1-
|
| | | March 31,
| | December 31,
|
| | | 2008 | | 2008 |
| |
| Weighted-Average Grant Date Fair Value | | $ | 4.18 | | | $ | 3.91 | |
| Risk-free Interest Rate | | | 4.92 | % | | | 2.25 | % |
| Expected Volatility | | | 30.29 | % | | | 40.54 | % |
| Expected Holding Period in Years | | | 1.0 | | | | 1.0 | |
| Expected Dividends | | | None | | | | None | |
| | | | | | | | | |
| | | January 1-
| | April 1-
|
| | | March 31,
| | December 31,
|
| | | 2007 | | 2007 |
| |
| Weighted-Average Grant Date Fair Value | | $ | 6.69 | | | $ | 4.18 | |
| Risk-free Interest Rate | | | 5.27 | % | | | 4.92 | % |
| Expected Volatility | | | 33.19 | % | | | 30.29 | % |
| Expected Holding Period | | | 1.0 Year | | | | 1.0 Year | |
| Expected Dividends | | | None | | | | None | |
F-25
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The risk-free interest rate is based on the yield of U.S. Treasury securities that correspond to the expected holding period of the options. The1-year historical volatility period was selected since that period corresponds with the expected holding period. The expected forfeiture rate was determined based on the historical ESPP forfeiture data. The dividend yield was based on the Company’s expected dividend rate.
The Company follows accounting standards for disclosing information about reportable segments in financial statements. Laboratory operating income includes the direct profits generated by laboratories owned by the Company and excludes general and administrative expenses of the Company’s corporate location including amortization expenses associated with the Company’s intangible assets as well as interest expense.
In March 2005, the Company acquired Green Dental Laboratories, Inc. of Heber Springs, Arkansas. In October 2006, the Company acquired Keller Group, Incorporated, a privately-held dental laboratory business with production facilities in both St. Louis, Missouri and Louisville, Kentucky. The Company has identified both Green and Keller as separate reportable segments based on the quantitative criteria for financial reporting purposes. All of the Company’s remaining laboratories are included under the NDX Laboratories segment. As a result, the Company has three reportable segments. The accounting policies of this segment are consistent with those described for the consolidated financial statements in the summary of significant accounting policies.
F-26
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table sets forth information about the Company’s reportable segments for the years ended December 31, 2007, 2008 and 2009. Prior to the fourth quarter of 2006 the Company had two reportable segments and prior to 2005 the Company had only one reportable segment.
| | | | | | | | | | | | | |
| | | Year Ended
| | | Year Ended
| | | Year Ended
| |
| | | December 31,
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2008 | | | 2009 | |
| |
Revenue: | | | | | | | | | | | | |
| NDX Laboratories | | $ | 127,388,588 | | | $ | 126,240,949 | | | $ | 118,801,942 | |
| Green Dental Laboratory | | | 19,859,770 | | | | 20,724,865 | | | | 19,169,497 | |
| Keller Group | | | 23,843,093 | | | | 25,987,992 | | | | 24,248,404 | |
| | | | | | | | | | | | | |
| Subtotal | | | 171,091,451 | | | | 172,953,806 | | | | 162,219,843 | |
Inter-segment Revenues: | | | | | | | | | | | | |
| NDX Laboratories | | | 370,913 | | | | 438,200 | | | | 328,783 | |
| Green Dental Laboratory | | | 199,525 | | | | 369,526 | | | | 281,469 | |
| Keller Group | | | 160,384 | | | | 471,645 | | | | 414,387 | |
| | | | | | | | | | | | | |
| Net Sales | | $ | 170,360,629 | | | $ | 171,674,435 | | | $ | 161,195,204 | |
| | | | | | | | | | | | | |
Laboratory Operating Income: | | | | | | | | | | | | |
| NDX Laboratories | | $ | 17,572,491 | | | $ | 15,059,508 | | | $ | 16,318,096 | |
| Green Dental Laboratory | | | 4,665,630 | | | | 4,795,136 | | | | 4,418,446 | |
| Keller Group | | | 3,228,640 | | | | 3,660,913 | | | | 4,237,948 | |
| | | | | | | | | | | | | |
| | | $ | 25,466,761 | | | $ | 23,515,557 | | | $ | 24,974,490 | |
| | | | | | | | | | | | | |
Total Assets: | | | | | | | | | | | | |
| NDX Laboratories | | $ | 87,811,020 | | | $ | 93,661,985 | | | $ | 89,146,022 | |
| Green Dental Laboratory | | | 26,756,893 | | | | 26,140,524 | | | | 25,758,671 | |
| Keller Group | | | 25,769,930 | | | | 25,634,168 | | | | 25,132,647 | |
| Corporate | | | 15,301,300 | | | | 16,078,226 | | | | 15,257,735 | |
| | | | | | | | | | | | | |
| | | $ | 155,639,143 | | | $ | 161,514,903 | | | $ | 155,295,075 | |
| | | | | | | | | | | | | |
Capital Expenditures: | | | | | | | | | | | | |
| NDX Laboratories | | $ | 4,604,908 | | | $ | 5,606,100 | | | $ | 1,582,078 | |
| Green Dental Laboratory | | | 463,763 | | | | 161,415 | | | | 34,073 | |
| Keller Group | | | 756,581 | | | | 420,628 | | | | 86,039 | |
| Corporate | | | 1,871,058 | | | | 899,243 | | | | 288,694 | |
| | | | | | | | | | | | | |
| | | $ | 7,696,310 | | | $ | 7,087,386 | | | $ | 1,990,884 | |
| | | | | | | | | | | | | |
Depreciation & Amortization on Property, Plant & Equipment: | | | | | | | | | | | | |
| NDX Laboratories | | $ | 2,579,090 | | | $ | 3,114,183 | | | $ | 2,853,540 | |
| Green Dental Laboratory | | | 330,820 | | | | 330,698 | | | | 342,671 | |
| Keller Group | | | 452,935 | | | | 532,410 | | | | 524,395 | |
| Corporate | | | 691,769 | | | | 894,591 | | | | 848,332 | |
| | | | | | | | | | | | | |
| | | $ | 4,054,614 | | | $ | 4,871,882 | | | $ | 4,568,938 | |
| | | | | | | | | | | | | |
F-27
NATIONAL DENTEX CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Reconciliation of Laboratory Operating Income with reported Consolidated Operating Income:
| | | | | | | | | | | | | |
| | | Year Ended
| | | Year Ended
| | | Year Ended
| |
| | | December 31,
| | | December 31,
| | | December 31,
| |
| | | 2007 | | | 2008 | | | 2009 | |
| |
| Laboratory Operating Income | | $ | 25,466,761 | | | $ | 23,515,557 | | | $ | 24,974,490 | |
| Less: | | | | | | | | | | | | |
| Corporate Selling, General and Administrative Expenses | | | 10,894,225 | | | | 12,220,728 | | | | 11,780,255 | |
| Amortization Expense — Intangible Assets | | | 1,283,720 | | | | 1,139,796 | | | | 1,618,236 | |
| Goodwill Impairment | | | — | | | | 6,950,000 | | | | 263,536 | |
| Add: | | | | | | | | | | | | |
| Other Expense | | | 771,660 | | | | 746,633 | | | | 744,867 | |
| | | | | | | | | | | | | |
| Consolidated Operating Income | | $ | 14,060,476 | | | $ | 3,951,666 | | | $ | 12,057,330 | |
| | | | | | | | | | | | | |
(11) Subsequent Events
The Company has assessed the impact of subsequent events and has concluded that there were no such events that require adjustment to the audited consolidated financial statements or disclosure in the notes to the audited consolidated financial statements.
F-28
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
NATIONAL DENTEX CORPORATION
David L. Brown, Chief Executive Officer
March 12, 2010
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | |
Signature | | Title | | Date |
| |
| | | | | |
/s/ DAVID V. HARKINS
David V. Harkins | | Director | | March 12, 2010 |
| | | | | |
/s/ JACK R. CROSBY
Jack R. Crosby | | Director | | March 12, 2010 |
| | | | | |
/s/ THOMAS E. CALLAHAN
Thomas E. Callahan | | Director | | March 12, 2010 |
| | | | | |
/s/ NORMAN F. STRATE
Norman F. Strate | | Director | | March 12, 2010 |
| | | | | |
/s/ JAMES E. MULVIHILL, D.M.D.
James E. Mulvihill, D.M.D. | | Director | | March 12, 2010 |
| | | | | |
/s/ DAVID L. BROWN
David L. Brown | | Chairman and CEO
(Principal Executive Officer) | | March 12, 2010 |
| | | | | |
/s/ WAYNE M. COLL
Wayne M. Coll | | Vice President & Chief Financial Officer (Principal Financial Officer) | | March 12, 2010 |
EXHIBIT INDEX
| | | | | |
Exhibit No. | | Description of Exhibit |
| |
| | 3 | .1(2) | | Restated Articles of Organization of the Company, filed with the Massachusetts Secretary of State on October 14, 1993. |
| | 3 | .2(2) | | Articles of Amendment, filed with the Massachusetts Secretary of State on September 26, 1995. |
| | 3 | .3(17) | | Amended and Restated By-Laws of the Company, as amended on March 25, 2008. |
| | 10 | .1(5)* | | Amended & Restated 2001 Stock Plan, as amended on May 16, 2006. |
| | 10 | .1a(6)* | | Form of Annual Director Fee Deferral and Restricted Stock/RSU Subscription Agreement. |
| | 10 | .1b(6)* | | Form of Restricted Stock Unit Agreement for Employees and Directors Under the Company’s Amended and Restated 2001 Stock Plan. |
| | 10 | .1c(6)* | | Form of Restricted Stock Agreement (Non-Employee Director). |
| | 10 | .1d(18)* | | Amended and Restated 2001 Stock Plan Form of Incentive Stock Option Agreement for Employees. |
| | 10 | .1e(18)* | | Amended and Restated 2001 Stock Plan Form of Non-Qualified Stock Option Agreement for Employees. |
| | 10 | .1f(18)* | | Amended and Restated 2001 Stock Plan Form of Restricted Stock Agreement for Employees. |
| | 10 | .1g(18)* | | Amended and Restated 2001 Stock Plan Form of Restricted Stock Unit Agreement for Employees. |
| | 10 | .1h(25)* | | Amended and Restated 2001 Stock Plan Form of Incentive Stock Option Agreement for Employees with Extended Exercisability upon Retirement. |
| | 10 | .1i(25)* | | Amended and Restated 2001 Stock Plan Form of Non-Qualified Stock Option Agreement for Employees with Extended Exercisability upon Retirement. |
| | 10 | .2(20)* | | Form of Amended and Restated Change of Control Severance Agreement with David L. Brown, Richard F. Becker, Jr., and Arthur B. Champagne. |
| | 10 | .3(20)* | | Form of Change of Control Severance Agreement with Wayne Coll And John F. Green. |
| | 10 | .4(1)* | | 1992 Long-Term Incentive Plan, as amended. |
| | 10 | .5(1)* | | Employment Agreement between the Company and Richard F. Becker, Jr., dated April 1, 1995. |
| | 10 | .5a(20)* | | First Amendment to Employment Agreement between the Company and Richard F. Becker, Jr., dated July 28, 2008. |
| | 10 | .6(1)* | | Employment Agreement between the Company and David L. Brown, dated April 1, 1995. |
| | 10 | .6b (20)* | | First Amendment to Employment Agreement between the Company and David L. Brown dated July 28, 2008. |
| | 10 | .7(4)* | | National Dentex Corporation Key Employee and Corporate Support Group Incentive Compensation Plan. |
| | 10 | .7a(26)* | | Amended National Dentex Corporation Key Employee and Corporate Support Group Incentive Compensation Plan. |
| | 10 | .8(4)* | | National Dentex Corporation Employees’ Stock Purchase Plan, as amended effective April 4, 2000. |
| | 10 | .8a(15)* | | Second Amendment to National Dentex Corporation Employees’ Stock Purchase Plan. |
| | 10 | .8b(27)* | | Third Amendment to National Dentex Corporation Employees’ Stock Purchase Plan. |
| | 10 | .9(9) | | Second Amended and Restated Loan Agreement by and between Bank of America, N.A., National Dentex Corporation and Green Dental Laboratories, Inc. dated November 7, 2006. |
| | 10 | .9a(14) | | Amendment dated October 24, 2007 to Second Amended and Restated Loan Agreement by and between Bank of America, N.A., National Dentex Corporation, and its subsidiaries listed therein. |
| | 10 | .9b(13) | | Loan Modification Agreement dated as of March 29, 2007 by and between Bank of America, N.A., National Dentex Corporation, Green Dental Laboratories, Inc., Keller Group, Inc., Keller Laboratories, Incorporation — Midwest, and Keller Laboratories, Incorporation — Southwest. |
| | 10 | .9c(18) | | Amendment No. 2 to Second Amended and Restated Loan Agreement by and among Bank of America, N.A., National Dentex Corporation and the subsidiaries of National Dentex Corporation therein named. |
| | 10 | .9d(21) | | Amendment No. 3 to Second Amended and Restated Loan Agreement by and between Bank of America, N.A., National Dentex Corporation and the subsidiaries therein named dated September 2, 2008. |
| | | | | |
Exhibit No. | | Description of Exhibit |
| |
| | 10 | .9e(22) | | Amendment No. 4 to Second Amended and Restated Loan Agreement by and between Bank of America, N.A., National Dentex Corporation and the subsidiaries therein named dated December 11, 2008. |
| | 10 | .9f(26) | | Amendment No. 5 to Second Amended and Restated Loan Agreement by and between Bank of America, N.A., National Dentex Corporation and the subsidiaries therein named dated March 13, 2009. |
| | 10 | .10(4)* | | National Dentex Supplemental Executive Retirement Plan. |
| | 10 | .10a(10)* | | Amendment No. 1 to Supplemental Executive Retirement Plan dated as of January 17, 2006. |
| | 10 | .10b(10)* | | Amendment No. 2 to Supplemental Executive Retirement Plan dated as of January 17, 2006. |
| | 10 | .10c(23)* | | Amendment No. 3 to National Dentex Supplemental Executive Retirement Plan, dated December 31, 2008. |
| | 10 | .11(4)* | | National Dentex Supplemental Laboratory Executive Retirement Plan. |
| | 10 | .11a(23)* | | Amendment No. 2 to National Dentex Supplemental Laboratory Executive Retirement Plan., dated December 31, 2008. |
| | 10 | .12(3) | | Stock Purchase Agreement by and among John W. Green IV, Richard M. Nordskog and the Company dated as of March 1, 2005. |
| | 10 | .13(7)* | | Supplemental Executive Retirement Plan VI effective as of August 11, 2006. |
| | 10 | .13a(23)* | | Amendment No. 1 to Supplemental Executive Retirement Plan VI effective as of December 31, 2008. |
| | 10 | .14(8) | | Stock Purchase Agreement by and among William G. Keller, Thomas A. Keller and the Company dated October 5, 2006. |
| | 10 | .15* | | Written Summary of Compensation Arrangements with Named Executive Officers. |
| | 10 | .16(24)* | | Written Summary of Non-Employee Director Compensation Arrangements. |
| | 10 | .17(16)* | | Retirement Agreement by and among National Dentex Corporation and Donald Merz dated January 2, 2008. |
| | 21 | (12) | | Subsidiaries of the Company. |
| | 23 | | | Consent of PricewaterhouseCoopers LLP. |
| | 31 | .1 | | Certification pursuant to Section 302 of the Sarbanes-Oxley Act (Chief Executive Officer). |
| | 31 | .2 | | Certification pursuant to Section 302 of the Sarbanes-Oxley Act (Chief Financial Officer). |
| | 32 | .1 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act (Chief Executive Officer). |
| | 32 | .2 | | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act (Chief Financial Officer). |
Unless otherwise noted all exhibits are filed herewith. The file number for our Exchange Act reports is0-23092. |
| * These exhibits relate to a management contract or to a compensatory plan or arrangement. |
| | |
| (1) | | Incorporated by reference from theForm 10-K for the fiscal year ended December 31, 2003 as filed with the Commission on March 12, 2004. |
| |
| (2) | | Incorporated by reference from the Annual Report onForm 10-K for the fiscal year ended December 31, 2004 as filed with the Commission on May 24, 2005. |
| |
| (3) | | Incorporated by reference from the Current Report onForm 8-K/A as filed with the Commission on January 26, 2006. |
| |
| (4) | | Incorporated by reference from the Annual Report onForm 10-K for the fiscal year ended December 31, 2005 as filed with the Commission on March 16, 2006. |
| |
| (5) | | Incorporated by reference from the Proxy Statement filed on Schedule 14A with the Commission on March 29, 2006. |
| |
| (6) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on May 22, 2006. |
| | |
| (7) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on August 14, 2006. |
| |
| (8) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on October 6, 2006. |
| |
| (9) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on November 8, 2006. |
| |
| (10) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on December 12, 2006. |
| |
| (11) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on January 29, 2007. |
| |
| (12) | | Incorporated by reference from theForm 10-K for the fiscal year ended December 31, 2006 filed with the Commission on March 13, 2007. |
| |
| (13) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on April 2, 2007. |
| |
| (14) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on October 30, 2007. |
| |
| (15) | | Incorporated by reference from the Registrant’s Registration Statement onForm S-8 as filed on November 16, 2007. |
| |
| (16) | | Incorporated by reference from the Current Report onForm 8-K as filed with the Commission on January 1, 2008. |
| |
| (17) | | Incorporated by reference from the Current Report onForm 8-K filed with the Commission on March 27, 2008. |
| |
| (18) | | Incorporated by reference from the Quarterly Report onForm 10-Q for the quarter ended March 31, 2008 as filed with the Commission on May 12, 2008. |
| |
| (19) | | Incorporated by reference from the Quarterly Report onForm 10-Q for the quarter ended June 30, 2008 as filed with the Commission on August 8, 2008. |
| |
| (20) | | Incorporated by reference from the Current Report onForm 8-K filed with the Commission on August 1, 2008. |
| |
| (21) | | Incorporated by reference from the Current Report onForm 8-K filed with the Commission on September 8, 2008. |
| |
| (22) | | Incorporated by reference from the Current Report onForm 8-K filed with the Commission on December 17, 2008. |
| |
| (23) | | Incorporated by reference from the Current Report onForm 8-K filed with the Commission on January 6, 2009. |
| |
| (24) | | Incorporated by reference from theForm 10-K for the fiscal year ended December 31, 2007 filed with the Commission on March 12, 2008. |
| |
| (25) | | Incorporated by reference from the Current Report onForm 8-K filed with the Commission on April 23, 2009. |
| |
| (26) | | Incorporated by reference from the Annual Report onForm 10-K filed with the Commission on March 16, 2009. |
| |
| (27) | | Incorporated by reference from the Proxy Statement filed on Schedule 14A with the Commission on April 9, 2009. |