UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
for the fiscal year ended December 31, 2010
or
| ¨ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
for the transition period from to
Commission file number 0-24517
ORTHOVITA, INC.
(Exact name of registrant as specified in its charter)
| | |
| Pennsylvania | | 23-2694857 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
77 Great Valley Parkway Malvern, Pennsylvania | | 19355 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (610) 640-1775
Securities registered pursuant to Section 12(b) of the Act:
| | |
(Title of class) | | (Name of Exchange on which Registered) |
| Common Stock, par value $.01 per share | | Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in the definitive proxy statement incorporated by reference in Part III of this annual report on Form 10-K or any amendment to this annual report on Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer | | ¨ | | Accelerated filer | | x |
| | | |
| Non-accelerated filer | | ¨ | | Smaller reporting company | | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The approximate aggregate market value of Common Stock held by non-affiliates of the registrant was $112,844,072 as of June 30, 2010 (For purposes of determining this amount only, the registrant has defined affiliates as including (a) the executive officers of the registrant as of June 30, 2010, (b) all directors of the registrant as of June 30, 2010 and (c) each shareholder that informed the registrant that as of June 30, 2010 it was the beneficial owner of 10% or more of the outstanding common stock of the registrant).
As of March 3, 2011, there were 76,895,151 shares of the registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Proxy Statement relating to the 2010 Annual Meeting of Shareholders (to be filed within 120 days after the end of the year covered by this annual report on Form 10-K) are incorporated into Part III of this annual report on Form 10-K by reference.
TABLE OF CONTENTS
PART I
In addition to historical facts or statements of current conditions, our disclosure and analysis in this Form 10-K contain some forward-looking statements. See the discussion of forward-looking statements in Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operation” included in this Form 10-K.
Unless the context indicates otherwise, the terms Orthovita, Company, we, us or our herein refer to Orthovita, Inc. and, where appropriate, one or more of our subsidiaries.
GENERAL DEVELOPMENT OF OUR BUSINESS
We are a specialty spine and orthopedic company with a portfolio of orthobiologic and biosurgery products. Our products are based on novel and unique proprietary biomaterials that have innovative mechanisms of action in the body.
Our orthobiologic platform includes products for the fusion, regeneration and fixation of human bone. Our orthobiologic products are based on our proprietary Vitoss™ Bone Graft Substitute technology and include the Imbibe™ Bone Marrow Aspiration System used with Vitoss. Vitoss is the market-leading synthetic bone graft in the United States. Several of our Vitoss products incorporate our proprietary bioactive ceramic glass technology which accelerates bone healing. Our orthobiologic products also include our Cortoss™ Bone Augmentation Material and the Aliquot™ Delivery System used with Cortoss, both of which were first available for sale in the U.S. in July 2009. Cortoss is an advanced synthetic biomaterial that hardens to mimic weight-bearing, cortical bone following injection into spinal vertebrae. Cortoss is the first clinically-proven, injectable alternative to polymethylmethacrylate (PMMA) bone cement cleared by the U.S. Food and Drug Administration (FDA) for the treatment of vertebral compression fractures, an often extremely painful condition that occurs in patients with osteoporosis and cancer. In addition to extensive clinical data showing statistically significant improvements in pain relief (at three months following implantation) and function (at twenty-four months following implantation) from the use of Cortoss in comparison to PMMA, we believe that Cortoss has significant ease-of-use advantages over current vertebral augmentation technology.
Our biosurgery products include Vitagel™ Surgical Hemostat, Vitasure™ Absorbable Hemostat, and the CellPaker™ Plasma Collection System used in conjunction with Vitagel, and other accessories and delivery products that complement our Vitagel product. These products incorporate advanced biosurgical materials to help control bleeding during surgeries.
We have historically expanded our product portfolio through internal product development efforts, co-development efforts with strategic partners and the in-licensing of products. We internally developed our Vitoss and Cortoss materials and maintain an ongoing internal research and development program. In addition, we worked with Kensey Nash Corporation (Kensey) to co-develop and commercialize certain Vitoss Foam products, and we have acquired rights from third parties to market Vitagel and Vitasure. We continue to pursue in-licensing, co-development and other opportunities to acquire complementary products and technologies to broaden our product offerings and further leverage our sales force.
Other Sales and Corporate Information
In the U.S., we market and sell our products through a hybrid sales and distribution network of direct sales representatives and independent distributors. As of December 31, 2010, we had 79 direct sales representatives (DSRs), 5 associate sales representatives who support our DSRs, and arrangements with 75 independent distributors and sales agents that we utilize to market and sell our products in the U.S. Of these distributors and agents, 72 were non-stocking and 3 were stocking distributors. We continue to strengthen our hybrid distribution network by optimizing the number of DSRs and adding distributors or sales agents in those territories or markets where we do not have adequate coverage. Outside the U.S., we primarily utilize a network of independent stocking distributors to market our products, although we market these products through a direct sales force in the United Kingdom.
Information regarding our product sales by geographic markets for 2010, 2009, and 2008 is included in note 14 (Product Sales) to the consolidated financial statements included in this report.
We market our products for only the indication(s) or use(s) that have received regulatory approval or clearance.
We incorporated in Pennsylvania in 1992 and maintain a branch operation in Belgium, as well as a wholly-owned subsidiary in the United Kingdom, and a wholly-owned subsidiary incorporated in Delaware to hold certain intangible properties.
Our principal executive offices are located at 77 Great Valley Parkway, Malvern, Pennsylvania 19355, and our telephone number is (610) 640-1775. We maintain a website at www.orthovita.com and make available free of charge on this website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or
1
furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file these materials with, or furnish them to, the U.S. Securities and Exchange Commission. The reference to our website is intended to be an inactive textual reference only.
OUR PRODUCTS
As further discussed below under the caption “GOVERNMENT REGULATION,” our products and product candidates are subject to extensive regulation as medical devices by the FDA, regulatory authorities in Europe and regulatory authorities in other jurisdictions. Product approval or clearance applications for our products must be supported by valid scientific evidence, and may require clinical trial data, to demonstrate the safety and effectiveness of the products.
Our existing orthobiologic and biosurgery products, as well as select product development projects, are described below.
Orthobiologic Products: Vitoss and Cortoss and Related Delivery Systems and Accessories; Select Product Development Projects
A. Vitoss Bone Graft Substitute
| | |
Vitoss Blocks and Morsels | | FDA 510(k) cleared December 2000 Approved in European Union July 2000 |
| |
Vitoss Micro and Macro Morsels | | FDA 510(k) cleared December 2000 Approved in European Union July 2003 |
| |
Vitoss Standard and Micro Canisters | | FDA 510(k) cleared November 2003 Approved in European Union July 2003 |
| |
Vitoss Foam Product Platform, including: | | FDA 510(k) cleared December 2003 Approved in European Union May 2005 |
| |
Vitoss Foam Strips and Cylinders Vitoss Foam Flow Vitoss Foam Shapes Vitoss Foam Pack | | |
| |
Vitoss Bioactive Foam Strips | | FDA 510(k) cleared September 2007 Approved in European Union October 2009 |
| |
Vitoss Bioactive Foam Pack | | FDA 510(k) cleared June 2008 Approved in European Union October 2009 |
| |
Vitoss Bioactive Foam-2X | | FDA 510(k) cleared December 2010 |
| |
Vitoss BA Bimodal | | FDA 510(k) cleared February 2011 |
Vitoss is a high-porous resorbable beta-tricalcium phosphate bone graft substitute used to help the body guide the three-dimensional regeneration of the patient’s own bone. We launched Vitoss in the U.S. and the European Union (EU) in 2001. Vitoss can soak and hold its own volume in blood and bone marrow aspirate due to its high-porosity, and has been shown to perform well in an array of applications in the spine, extremities and pelvis, such as spinal grafting and the treatment of bone defects due to trauma, degenerative disease and tumors, including posterolateral spine fusion procedures. Vitoss integrates well into existing bone and allows for bone in-growth and maturation. Vitoss, the Vitoss Foam products, the Vitoss Bioactive Foam products, Vitoss Bioactive Foam-2X and Vitoss BA Bimodal are covered by certain issued patents and/or patent applications in and outside of the United States.
Since the initial U.S. regulatory clearance and subsequent Vitoss product launch in mid-2001, we have developed and are developing a number of new Vitoss-based products and related devices that are designed to expand the market for our Vitoss products by broadening the range of surgical indications and by addressing additional surgeon preferences and clinical needs. In 2003, we entered into a development, manufacturing and supply agreement with Kensey, leading to the development and launch of the Vitoss Foam product platform. The Vitoss Foam products combine our base Vitoss technology with Kensey’s proprietary resorbable biomaterials to produce a wide array of pliant, flexible, flowable and compression resistant bone graft materials. The Vitoss Foam products have the ability to retain biological fluids such as blood and bone marrow aspirate in pliable and compression resistant forms. These forms can be designed into specific shapes and material characteristics to meet a surgeon’s need for handling and delivery in a variety of surgical approaches and applications. We launched another iteration of the Vitoss Foam product line called Vitoss Bioactive Foam in the United States in 2008 and in the European Union in 2009. In addition to our proprietary Vitoss beta-tricalcium phosphate bone graft substitute and Kensey’s proprietary resorbable biomaterials, Vitoss Bioactive Foam products also contain our proprietary combeite bioactive glass to advance bone healing.
2
In February 2011, we launched in the United States a new iteration of our Vitoss Foam product line called Vitoss Bioactive Foam-2X. As compared to prior Vitoss Bioactive Foam products, Vitoss Bioactive Foam-2X has the same structure and porosity but contains increased levels of bioactive glass. In addition, we expect to launch in the United States in approximately one year another product iteration called Vitoss BA Bimodal.
Bone Defect Grafting. Injury or trauma to the bone, degenerative conditions, disease and aging all affect the health and viability of the human skeleton. These conditions often result in the need for the repair of bone defects through a bone grafting procedure. We estimate that over two million bone grafting procedures on a worldwide basis are performed each year in the spine, extremities and pelvis. Bone grafting material is either (i) autograft material, which is often obtained or harvested from the iliac crest region of the patient’s own hip, (ii) allograft material, which is obtained from a cadaver, or (iii) synthetically derived materials that provide one or more components of either bone-like scaffold (such as Vitoss), cells or signals (such as bone morphogenic proteins). Vitoss has been used in bone grafting procedures as a bone graft substitute to provide a synthetic scaffold in a variety of applications, including those of the extremities, spine and pelvis. When used with the patient’s bone marrow, Vitoss provides all components of scaffold, cells and signals required to aid in the growth of new bone.
As a synthetic beta-tricalcium phosphate material, our Vitoss material has advantages over both autograft and allograft materials. For example, a harvest of autograft material involves an additional surgical procedure that extends surgical time, adding to costs and increasing blood loss and patient risk of infection or adverse reaction from the additional time under anesthesia. In addition, harvesting bone for autograft sometimes causes protracted pain that may necessitate additional medical care after the surgical procedure. Disadvantages of allograft bone include the possibility of disease transmission, antigenic response and quality variability from donor to donor. Using Vitoss instead of autograft or allograft material avoids these potential complications.
Spinal Fusion and Grafting. Many patients affected by severe back pain due to degeneration of one or more discs are often treated with spinal surgical procedures. We estimate that each year approximately 460,000 spinal fusion procedures are performed in the United States. In cases where the patient has advanced disc degeneration or spinal instability, a fusion procedure can involve a surgical incision in the patient’s back or abdomen to access and remove the affected disc material. To provide initial stability and support of the surrounding vertebrae, the resulting defect is filled with a structural implant made of either titanium, shaped bone derived from a human cadaver, or a synthetic material known as polyetheretherketone (PEEK). Adjunctively, these procedures may require the use of bone grafting material to repair defects and facilitate the fusion of two bony elements. We believe the use of Vitoss provides a clinically proven alternative to patient- or cadaver-derived tissues and may be preferable for both patients and their surgeons for its efficacy and safety.
Trauma. Physical trauma such as falls and accidents can result in bone fracture or damage. Fractures of broken bones are often realigned with hardware, such as plates, rods and screws. Once the hardware has been used to recreate the skeletal anatomy and to provide the stability of the bony structure, there are often defects or voids in the bone which remain. Those voids may require the use of bone graft material. The goal of bone grafting in trauma applications is to rapidly heal the damaged bone. Approximately 300,000 trauma-related bone graft repairs are performed annually on a worldwide basis. Autograft, cadaver allograft, as well as synthetic scaffolds like Vitoss, are used for trauma-related bone graft repairs.
Dental, Periodontal, Oral and Cranio-Maxillofacial. In August 2002, we entered into a supply agreement with BioMimetic Therapeutics, Inc. for our proprietary Vitomatrix™ particulate synthetic scaffold biomaterial, which is produced in the same process used to manufacture Vitoss. In January 2008, in connection with the sale of its dental business to Luitpold Pharmaceuticals, Inc., BioMimetic assigned its rights and obligations under the supply agreement to Luitpold with our consent. The supply agreement with Luitpold terminated in March 2010, and we do not expect to sell Vitomatrix as a raw material in the future. In October 2010, we received clearance from the FDA to market Vitomatrix as a standalone product in certain dental procedures. We are seeking to identify potential commercial partners to distribute Vitomatrix or to license the underlying technology.
B. Bone Marrow Aspiration System with Imbibe Needles and Syringes used with Vitoss
| | |
Bone Marrow Aspiration Needle | | FDA 510(k) cleared June 2005 |
| |
Bone Marrow Aspiration Syringe | | FDA 510(k)s cleared September 2001 and March 2003 Approved in European Union September 2004 |
The disposable Imbibe devices provide spine and orthopedic surgeons with a simple method for harvesting a patient’s own bone marrow, mixing it with Vitoss, and delivering the mixture to the bone graft site. We believe Imbibe, when used together with Vitoss, provides greater flexibility and options for surgeons. Imbibe is covered by a U.S.-issued patent.
3
C. Cervical Interbody Fusion Device in Development Phase
We are currently developing a cervical interbody fusion device incorporating our proprietary bioactive glass with polyetheretherketone (PEEK). While we expect to seek regulatory clearance for this device in the United States by filing a 510(k) application for this device by the end of the first quarter of 2011, we are not certain whether the regulatory pathway for this device will entail a 510(k) as opposed to a premarket approval (PMA) application, or whether clinical trials will be required. See “GOVERNMENT REGULATION” below for additional information on U.S. regulatory requirements for the introduction of a new device into the market. There can be no assurance that we can obtain regulatory clearance for this device or the timing of any such clearance.
D. Cortoss Bone Augmentation Material
| | |
Cortoss Bone Augmentation Material | | FDA 510(k) cleared June 2009 for vertebral augmentation Approved in European Union January 2003 for vertebral augmentation Approved in European Union October 2001 for screw augmentation |
Cortoss is a polymer composite which mimics the mechanical characteristics of human bone that has been cleared by the FDA and approved in the EU under the CE mark process. For patients with poor bone healing, as seen in osteoporotic patients, Cortoss may be used in a number of surgical procedures to quickly provide structural stability and reinforcement of the bones after surgery. The surgeon’s goal is to repair the patient’s bone and enhance the patient’s mobility as quickly as possible since prolonged bed rest or inactivity may result in decreased overall health for older or osteoporotic patients. Cortoss’ simple mix-on-demand delivery system design allows for minimum waste and maximum ease of use and flexibility for the surgeon. Cortoss is an injectable material that is delivered through a pre-filled, unit dose, disposable cartridge. Delivery of Cortoss to the surgical site may be started and stopped for a prolonged period of time throughout the surgical procedure as polymerization is initiated only when Cortoss is expressed through its static mix-tip. The polymerization is a self-setting reaction that causes Cortoss to harden within minutes. Cortoss provides two stages of fixation: immediate mechanical interlock into porous bone, followed by intimate bone apposition over time along the contours of its surface. Cortoss is covered by several patents issued in the U.S. and other countries, and other U.S. and non-U.S. patent applications are pending.
Cortoss received regulatory clearance in the U.S. and EU for use in the treatment of vertebral compression fractures (VCFs) of the spine, and may be used in vertebroplasty as well as kyphoplasty procedures. Cortoss also has regulatory approval in the EU for use in screw augmentation. We are in the process of seeking regulatory clearance in the U.S. for the use of Cortoss in cranioplasty, a surgical procedure to repair a defect or deformity of the skull. There can be no assurance that we can obtain regulatory clearance for Cortoss in the use of cranioplasty or the timing of any such clearance.
We have sold Cortoss in Europe since December 2001. We launched Cortoss in the U.S. in the second half of 2009 shortly after its clearance by the FDA for use in vertebral augmentation procedures. We believe that the U.S. launch of Cortoss has been negatively affected by several factors, including two small studies published inThe New England Journal of Medicinein August 2009 that questioned the effectiveness of vertebroplasty, the uncertainty created by the pending reimbursement review of vertebral augmentation procedures by a few of Medicare’s local contractors, and the issuance of clinical practice guidelines in September 2010 by the American Academy of Orthopedic Surgeons (AAOS) that did not support the use of vertebroplasty to treat vertebral compression fractures. While these studies did not evaluate or discuss Cortoss and no reimbursement decisions have yet been announced, we believe that the publication of the studies, the ongoing reimbursement review and the AAOS guidelines have resulted in a notable decrease in the number of vertebroplasty procedures nationwide. We also believe that a recent shift for vertebral augmentation procedures from the hospital in-patient to out-patient settings has adversely affected our launch of Cortoss in the U. S. because the historic call pattern of our sales force is within the in-patient setting. The economic pressure causing this shift was exacerbated during 2009 and 2010 following the imposition of significant fines by the U.S. government on a number of hospitals that treated patients with vertebral compression fractures in the in-patient setting when these patients could have been treated more cost-effectively in the out-patient setting. As a result of these pressures, as well as negative overall economic conditions and health care reform initiatives to reduce costs, we believe the number of vertebral augmentation procedures performed by spine surgeons and neurosurgeons, our primary target audience, has decreased, and a greater number of procedures are being performed in an out-patient setting by interventional radiologists and neuroradiologists, which is a new target physician group for us. We believe that these factors have resulted in U.S. Cortoss sales that are significantly below our initial expectations for the launch of this product. We expect that the vertebral augmentation market will continue to face economic and other pressures at least in the near-term.
Vertebral Augmentation of VCFs. Vertebral augmentation of VCFs is a procedure for repairing fractured vertebrae that can be performed on a hospital in-patient or out-patient basis or in ambulatory surgery centers, clinics and physician offices. We estimate there are approximately 700,000 patients in the U.S. with VCFs caused by osteoporotic bone or bone cancer resulting in severe pain
4
and immobility. Of these, we believe approximately 150,000 fractures are treated annually using vertebroplasty or kyphoplasty techniques. The traditional treatments, e.g., bed rest, bracing, narcotics or anesthetic injections, may not address the underlying fracture. Vertebral augmentation of VCFs has been reported to provide early pain relief in up to 90% of osteoporotic patients. Early relief of pain provided by vertebral augmentation of VCFs enables patients to maintain better functional capacity. Functional capacity, in turn, is believed to be directly related to the ability to live independently and unassisted. We are not aware of any product that has received FDA approval or a CE mark for use in this procedure on the basis of prospective, controlled clinical data. However, since 2004, several versions of PMMA bone cement have been cleared by the FDA under the 510(k) process. Results from our pivotal clinical data comparing Cortoss to PMMA in vertebral augmentation showed statistically significant improvements for Cortoss patients in pain relief (at three months following implantation) and function (at 24 months following implantation). Results from this study further indicated that Cortoss patients treated at one vertebral level with no previous fracture had a lower incidence of subsequent adjacent level fracture. In addition, the material properties of Cortoss described below result in the following advantages over PMMA:
| | • | | Flow characteristics and fill patterns that enhance procedural safety and control; |
| | • | | The ability to be mixed on demand with no time constraints in the pre-injection phase and delivered on a start-stop basis, thereby enhancing ease of use; |
| | • | | Mechanical properties more closely resembling cortical bone; |
| | • | | A lower temperature setting that reduces the risk of tissue necrosis associated with PMMA; and |
| | • | | The absence of volatile, free unreacted methylmethacrylate monomer released into the patient’s body. Although documented complications of monomer released from PMMA may be rare, they are severe adverse events when they occur. |
For the foregoing reasons, we believe that Cortoss has meaningful clinical benefits over PMMA in the treatment of VCFs.
Screw Augmentation. Screw augmentation is a procedure for securing the fixation of bone screws used in patients with weak bone caused by osteoporosis. Cortoss may be used in those procedures where the screws “strip” or fail to hold the integrity of the internal fixation construct due to poor bone quality, as is often the case with osteoporotic bone. Where screws fail to hold, current treatment options include: (i) replacing the screw with a screw of larger diameter, which may further weaken the bone and is not always possible because of the size of the screw holes and/or the bone, or (ii) augmenting the screws with PMMA bone cement, which is cumbersome and time consuming. PMMA must be manually mixed and transferred into a syringe for application. After mixing, there is only a small time window in which the PMMA can be used before it sets, making it difficult to augment more than one screw at a time. Additionally, PMMA bone cement is not approved in either Europe or the U.S. for pedicle, trauma or hip fracture screw augmentation. We believe the use of Cortoss to anchor the screw in a quick and efficient way enables the full function of the screw to be restored. We market Cortoss for use in screw augmentation outside of the U.S. only where we have obtained regulatory approval for this indication.
E. Aliquot Delivery System
Our Aliquot Delivery System is a coaxial system of catheter and syringe dispenser designed specifically to directly deliver our Cortoss material in vertebroplasty procedures. We launched the Aliquot system in the U.S. concurrently with the launch of Cortoss in the second half of 2009. The Aliquot Delivery System is exempt from 510(k) clearance. During the second quarter of 2002, we received CE mark approval from our notified body for certain Aliquot kit configurations.
The U.S. launch of Aliquot was negatively impacted by the same factors mentioned above for Cortoss. Further, prior to and following the U.S. launch of Cortoss and Aliquot and in anticipation of significant product demand, we acquired a significant inventory quantities of Aliquot from third party suppliers with requisite long manufacturing lead times. As a result, during the fourth quarter of 2010, we recorded a $3.5 million inventory charge primarily for Aliquot inventory that either was expected to expire prior to sale or was not expected to be sold based on our current projections of product demand. For additional information, see the risk factor entitled, “If our current products are not commercially successful, our operating results will be impaired and our viability will be jeopardized” under Item 1A. “RISK FACTORS.”
We are currently developing a suite of cavity creation devices that are designed to expand the use of Cortoss in the treatment of vertebral compression fractures for physicians who prefer kyphoplasty over vertebroplasty. We plan to launch the first such device, an Aliquot directional bone tamp, in the second quarter of 2011. This device is intended to create preferential flow channels in existing spinal bone structure prior to a vertebral augmentation procedure. These channels can provide directional filling of bone cement. In addition, we are currently seeking regulatory clearance in the United States for an Aliquot inflatable bone tamp designed for balloon-assisted vertebroplasty with Cortoss bone cement. There can be no assurance that we can obtain regulatory clearance for this device or the timing of any such clearance.
5
Biosugery Products: Vitagel and Vitasure and Related Components and Accessories
A. Vitagel Surgical Hemostat and Accessories
| | |
Vitagel Surgical Hemostat | | PMA approved, distribution rights initially obtained June 2004 Approved in European Union July 2007 (See GOVERNMENT REGULATION below) |
| |
CellPaker Plasma Collection System | | PMA approved, distribution rights initially obtained June 2004 Approved in European Union July 2007 (See GOVERNMENT REGULATION below) |
| |
Malleable Extended Applicator | | PMA approved, distribution rights obtained June 2004 Approved in European Union July 2007 (See GOVERNMENT REGULATION below) |
| |
Laparoscopic Extended Applicator | | PMA approved, U.S. distribution rights obtained June 2004 Approved in European Union July 2007 (See GOVERNMENT REGULATION below) |
| |
Vitagel Spray Set | | FDA 510(k) cleared October 2005 Approved in European Union July 2007 |
Vitagel is an FDA-approved, CE-marked composite liquid hemostat that combines the biomaterials bovine thrombin and bovine collagen with the patient’s autologous plasma. When applied to the surgical site, Vitagel creates a safe adherent matrix and an impermeable barrier to blood flow, and its unique microfibrillar collagen component facilitates healing. Vitagel has been marketed to general surgeons and is applicable in the spine, hip and knee replacement surgery markets, where bleeding is a significant complication for many routine surgeries. After obtaining the right to sell Vitagel and CellPaker from a third party, we commenced sales of the products in the U.S. in 2005 through our existing orthopedics and spine-based sales channel, utilizing the same marketing strategy that we use for all of our Vitoss-related products. This strategy provides our sales network additional functional biomaterial products to offer to customers. During the first half of 2006, we launched the Vitagel Spray Set in the U.S. We obtained the CE mark for Vitagel, CellPaker and related accessories in July 2007 and launched these products in the European Union in the first quarter of 2008. Vitagel and CellPaker are covered by U.S. and non-U.S. issued patents owned by the licensor of the products.
B. Vitasure Absorbable Hemostat
| | |
Vitasure Absorbable Hemostat | | PMA approved, distribution rights initially obtained April 2008 Approved in European Union September 2002 (See GOVERNMENT REGULATION below) |
Vitasure is an FDA-approved, CE-marked, plant-based hemostat product that can be deployed quickly throughout surgery. Through proprietary microporous polysaccharide hemosphere technology, Vitasure acts as a molecular sieve to quickly extract fluids from blood, creating an osmotic action that causes the Vitasure particles to swell and concentrate serum proteins, platelets, and other elements on the surface. The Vitasure particles and their coating of compacted cells then create a matrix for the formation of a tenacious fibrin clot. We originally obtained the right to distribute Vitasure in the U.S. and certain other territories for orthopedic and spine applications in 2008 from Medafor, Inc. We launched the Vitasure product in the U.S. in 2008 and in certain non-U.S. territories in January 2009. Vitasure is covered by U.S. and non-U.S. issued patents owned by Medafor and its licensor.
STRATEGIC PARTNERSHIPS
Kensey - Vitoss Foam
In March 2003, we entered into an agreement with Kensey to jointly develop and commercialize certain orthobiologic products based upon our Vitoss platform. The new products developed under this agreement are based on our internally developed, proprietary Vitoss bone graft substitute material in combination with proprietary resorbable Kensey biomaterials. To date, the products covered by the Kensey agreement for which we have received 510(k) clearance from the FDA are our Vitoss Foam, Vitoss Bioactive Foam, Vitoss Bioactive Foam-2X and Vitoss BA Bimodal products.
Kensey has the exclusive right to manufacture any approved or cleared products that were jointly developed under the agreement, and we market and sell these products worldwide. Following the regulatory approval or clearance of each new product, we have obligations under the agreement to pay Kensey for manufacturing the product and make royalty payments to Kensey based on the net sales of the product. These rights and obligations extend until at least February 2024 for our Vitoss Bioactive Foam, Vitoss
6
Bioactive Foam-2X and Vitoss BA Bimodal products, and until February 2014 for our other products under the Vitoss Foam product platform.
Approximately 67% of our product sales during 2010 were from products based upon our Vitoss Foam platform covered by the Kensey agreement, including Vitoss Bioactive Foam.
Angiotech – Vitagel and CellPaker
We have the right to distribute Vitagel and CellPaker pursuant to a license agreement with Angiotech Pharmaceuticals (U.S.), Inc. (which we refer to collectively with its affiliates and parent company Angiotech Pharmaceuticals, Inc. as Angiotech). We paid Angiotech $9.0 million in 2006 to eliminate any further obligations to pay royalties on our sales of Vitagel and CellPaker under the license agreement.
Under the license agreement, we have exclusive rights to manufacture, market and sell Vitagel products throughout the world for orthopedic indications, and non-exclusive rights to manufacture, market and sell CellPaker products throughout the world for all indications through July 31, 2017. Additionally, under the license agreement, Angiotech has an option for co-exclusive rights outside the orthopedic field which, if exercised, would permit Angiotech to manufacture, market and sell an Angiotech-branded Vitagel product throughout the world. Until Angiotech elects to exercise its option for co-exclusive rights, we have exclusive rights to manufacture, market and sell Vitagel outside of the orthopedic field throughout the world. If Angiotech elects to exercise its option, we would then have co-exclusive rights to manufacture, market and sell Vitagel outside of the orthopedic field throughout the world.
We currently manufacture Vitagel at our facilities in Malvern, Pennsylvania and manufacture CellPaker at our subcontractor’s facility.
On January 28, 2011 Angiotech voluntarily commenced proceedings under theCompanies’ Creditors Arrangement Act (the “CCAA” and such proceedings, the “CCAA Proceedings”), obtaining an Initial Order from the Supreme Court of British Columbia (the “Canadian Court”), inIn re Angiotech Pharmaceuticals, Inc.,et al. In connection with the CCAA Proceedings, Angiotech filed a voluntary petition under chapter 15 of title 11 of the United States Code on January 30, 2011 (the “Chapter 15 Cases” and together with the CCAA Proceedings, the “Proceedings”) in the United States Bankruptcy Court for the District of Delaware (the “U.S. Court”) inIn re Angiotech Pharmaceuticals, Inc. According to Angiotech, the purpose of the Chapter 15 Cases is to obtain recognition and enforcement in the United States of certain relief granted in the CCAA Proceedings, and to obtain U.S. Court assistance to the Canadian Court in effectuating a proposed recapitalization of the Company. We do not believe that the Proceedings will have any effect on the validity of our license from Angiotech.
Medafor - Vitasure
After obtaining certain non-exclusive rights from Medafor, Inc. to distribute Vitasure Absorbable Hemostat pursuant to a distribution agreement, we launched the product in the third quarter of 2008. Our territory under the agreement currently consists of the United States, Australia, Belgium, Germany, Ireland, the Netherlands, South Africa and the United Kingdom. The agreement with Medafor extends through the end of 2013.
SIGNIFICANT CONTRACTUAL OBLIGATIONS
See Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operation” of this report for a discussion of significant contractual arrangements relating to (i) our senior secured note facility, (ii) our leases, (iii) our joint product development and commercialization arrangement with Kensey and (iv) product development milestone and related payments.
RESEARCH AND PRODUCT DEVELOPMENT
We employ the disciplines of composite engineering, polymer science, biologics processing, solution chemistry and nanoparticulate ceramic glass science to create our orthobiologic and biosurgery materials technology platforms. We then utilize technologies developed, co-developed or licensed by us to commercialize orthobiologic and biosurgery products for orthopedic and surgical applications. Patents have been issued and additional patent applications have been filed to protect our key developments. See “PATENTS AND PROPRIETARY INTELLECTUAL PROPERTY” below for additional information. We incurred approximately $5.2 million, $6.8 million, and $6.7 million in research and development expenses in 2010, 2009 and 2008, respectively.
7
We continue to develop new products under certain of our approved product platforms. In the near-term, we may seek to bring Vitoss, Cortoss, Vitagel and/or Imbibe product line extensions to market in the U.S. through the 510(k) or PMA regulatory process (see “GOVERNMENT REGULATION” below). As discussed above under OUR PRODUCTS, we are developing a suite of cavity creation devices for the vertebral compression fracture market, as well as a cervical interbody fusion device. We also intend to seek in the near term regulatory clearance in the United States for new indications of use for our existing Cortoss product, including cranioplasty.
In the long-term, we will continue to exploit our biomaterials expertise and manufacturing capacity to develop additional biomaterials for alternative indications related or unrelated to spine and orthopedic uses. Additionally we may utilize our knowledge of the spine and orthopedic markets to identify and develop products that may be unrelated to our biomaterials expertise.
PATENTS AND PROPRIETARY INTELLECTUAL PROPERTY
Our strategy is to seek protection for our product technologies and manufacturing methods through the use of U.S. and non-U.S. patents. We have filed or intend to file applications as appropriate for patents covering our technologies, products and processes. As of March 1, 2011, certain of the products that we have developed or market are covered by one or more of our 24 issued U.S. patents, 20 pending U.S. patent applications and numerous counterparts of certain of these patents and pending patent applications outside the U.S., including Canada, Europe, Mexico, Israel and Japan. Our issued patents are set to expire between 2015 and 2024. Key patents covering Vitoss, Vitoss Foam, Cortoss, Vitagel and Vitasure are set to expire in 2017, 2024, 2022, 2017 and 2019, respectively.
MANUFACTURING AND PRODUCT SUPPLY
The manufacture of our products is subject to regulation and periodic inspection by various regulatory bodies for compliance with current FDA Quality System Regulations, the EU Medical Devices Directive, International Organization for Standardization (ISO) 9000 Series standards, ISO 13485/European Norm (EN) 13485 and equivalent requirements.
In-House Manufacturing. Our 25,800-square foot Vitoss, Vitagel and Cortoss manufacturing facilities that produce our commercial products are leased through July 2017 and are certified as meeting the requirements of ISO 9001 and ISO/EN 13485. These facilities are subject to inspection by the FDA for compliance with FDA device manufacture requirements. We believe our existing manufacturing facilities have the capacity to meet our commercial needs through at least 2013.
In October 2009, we completed renovations at our leased space to establish a 4,000-square foot facility to process collagen used as a raw material in the manufacture of Vitagel. This facility is leased through 2017. During 2010, we received FDA approval for our new collagen processing facility and fully transitioned the processing of collagen for Vitagel in-house. As part of the establishment of our collagen processing facility, in September 2008 we acquired collagen production equipment and a non-exclusive, perpetual, royalty-free, irrevocable license to certain collagen production process technology and know-how from Allergan, Inc. The license rights are limited to certain uses, including those related to the processing and production of collagen for surgical hemostats such as Vitagel.
Third Party Manufacturers. We are manufacturing CellPaker and Aliquot through outside third party contract manufacturers. Our Vitoss is converted to Vitoss Foam and Vitoss Bioactive Foam by our development partner Kensey under a long-term supply agreement. We purchase Vitasure from Medafor, which uses third party manufacturers to make this product. Our and Medafor’s third party manufacturers are regulated by government agencies and are required to be EN 13485 certified or otherwise meet our quality system requirements (See “GOVERNMENT REGULATION” below).
Raw Materials Supply. Our ability to manufacture our products is dependent on a limited number of specialty suppliers of certain raw materials. We have various supply agreements with third parties for raw materials used in products we manufacture.
We have a supply agreement with Kensey for bovine collagen used in our Vitoss Foam, Vitoss Bioactive Foam and Vitoss Bioactive Foam-2X product lines.
A key raw material for manufacturing Vitagel is processed collagen that is derived from bovine hides. During 2010, we entered into a supply agreement to obtain bovine hides, which we process at our new collagen processing facility described above. This supplier of bovine hides is a sole source supplier. See the risk factor entitled, “We are dependent on a limited number of specialty suppliers of certain raw materials, and our business and financial results could be harmed if a specialty supplier no longer provides materials to us,” under Item 1A. “RISK FACTORS.”
We do not have supply agreements for all raw materials. The failure of any supplier to continue to provide us with materials at a price or quality acceptable to us, or at all, or our inability to find an alternative supplier with acceptable prices and quality, would have a material adverse effect on our ability to manufacture our products.
8
SALES AND MARKETING
In the U.S, we have a field sales network consisting of direct sales representatives and independent stocking and non-stocking distributors and agents who market our products. We continually seek to strengthen our hybrid sales and distribution network by optimizing the number of direct sales representatives and adding distributors or sales agents in those territories or markets where we do not have adequate coverage. Outside the U.S., we primarily utilize a network of independent stocking distributors to market our products, although we sell these products through a direct sales force in the United Kingdom.
COMPETITION
Extensive research efforts and rapid technological change characterize the market for products in the orthopedic, spine and orthobiologic and biosurgery markets. We face intense competition from medical device and biomedical technology companies. Our products could be rendered noncompetitive or obsolete by competitors’ technological advances. We may be unable to respond to technological advances through the development and introduction of new products. Moreover, many of our existing and potential competitors have substantially greater financial, marketing, sales, distribution, manufacturing and technological resources than we do. These competitors may also be in the process of seeking FDA or other regulatory approvals, or patent protection, for new products. Our competitors could, therefore, commercialize new competing products in advance of our products. We cannot guarantee that we will be able to compete successfully against current or future competitors or that competition will not have a material adverse effect on our business, financial condition and results of operations.
We believe Vitoss faces competition from numerous synthetic products, bone morphogenic proteins (BMPs), stem cell-based products and cadaver-based products, such as demineralized bone matrix (DBM) products, currently on the market that may be used for the same indications as our products, as well as from other products and technologies that may enter the market in the future. Moreover, we believe that pressure generated by current economic conditions and health care reform has resulted in hospitals becoming more proactive at controlling costs for medical devices. These efforts increase competition on a pricing level among bone graft products.
Cortoss received regulatory clearance in the U.S. in June 2009 for use in the vertebral augmentation of VCFs and received the CE mark several years ago for use in vertebral augmentation of VCFs and in screw augmentation. Cortoss faces competition from the several PMMA bone cement products that have been 510(k) cleared by the FDA since 2004 for this indication. Outside the U.S., Cortoss is facing competition from several other PMMA bone cement products that have received the CE mark since 2004 for this indication. We believe that Cortoss offers advantages over PMMA bone cement in terms of its simple mix-on-demand delivery system design, which allows for minimum waste and maximum ease of use and flexibility for the surgeon.
Vitagel is approved by the FDA as a surgical hemostat where bleeding may be a significant complication for many routine surgeries. As such, Vitagel can be marketed broadly to general surgeons and is applicable in the spine, hip and knee replacement surgery markets. Vitagel faces competition from several established surgical hemostat products marketed by other companies with substantially greater resources than us. However, we believe Vitagel offers unique advantages in comparison to these other products due to Vitagel’s ability to facilitate healing through its collagen component, as well as its use of the patient’s autologous plasma instead of pooled donated human plasma as is the case with the leading products in the market. In 2008, recombinant human thrombin was approved for sale in the U.S. We believe that recombinant human thrombin will be positioned as an improvement to bovine-derived thrombin, a raw material in our Vitagel product, which could adversely affect our sales of Vitagel. It is too early to estimate the effect of this new technology on our Vitagel product. If management believes this effect to be material and adverse to our Vitagel sales, we will consider alternative formulations of Vitagel and the regulatory approvals that may be required for any such alternative formulations.
Vitasure competes with several thrombin products and surgical hemostats on the basis of its safety profile and pricing.
We may also face pricing and competitive pressure as a result of the growing number of physician-owned distributors operating in the spine and orthopedic markets, as discussed below in Item 1A. RISK FACTORS under the risk factor entitled, “If we cannot keep up with technological changes and marketing initiatives of competitors, or if we are unable to effectively compete with the growing number of physician-owned distributors, sales of our products may be harmed.”
GOVERNMENT REGULATION
In order to market our products, we must apply for, be granted and maintain all necessary regulatory approvals, clearances and certifications. In addition, we must comply with all regulatory requirements in each applicable jurisdiction. The section entitled “OUR PRODUCTS” above details the regulatory approvals, clearances and certifications currently in place for our products.
The following discussion summarizes the material regulatory requirements applicable to our products.
9
United States
The medical devices that we manufacture and market, or intend to market, are subject to extensive regulation by the FDA. Pursuant to the Federal Food, Drug and Cosmetic Act (FFD&C Act) and the regulations promulgated thereunder, the FDA regulates the clinical testing, manufacture, labeling, distribution and promotion of medical devices. Noncompliance with applicable requirements can result in, among other things, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure of the government to grant premarket clearance or premarket approval for devices, withdrawal of marketing approvals/clearances and criminal prosecution.
In the U.S., medical devices are classified into one of three classes (Class I, II or III) on the basis of the controls deemed necessary by the FDA to reasonably assure their safety and effectiveness. Under FDA regulations, Class I devices, the least regulated category, are subject to general controls and Class II devices are subject to general and special controls. Generally, Class III devices are those that must receive premarket approval by the FDA to ensure their safety and effectiveness.
Before we can introduce a new device into the market, we must generally obtain market clearance through a 510(k) notification or premarket approval through a premarket approval (PMA) application. A 510(k) clearance will be granted if the submitted information establishes that the proposed device is “substantially equivalent” to a legally marketed Class I or II medical device, or to a Class III medical device for which the FDA has not required a PMA. The FDA may determine that a proposed device is not substantially equivalent to a legally marketed device or that additional information or data are needed before a substantial equivalence determination can be made. A request for additional data may require that clinical studies be performed to establish the device’s “substantial equivalence.”
Commercial distribution of a device for which a 510(k) notification is required can begin only after the FDA issues a letter finding the device to be “substantially equivalent” to a predicate device. Pursuant to the FFD&C Act, the FDA must make a determination with respect to a 510(k) submission within 90 days of its receipt. The FDA may, and often does, extend this time frame by requesting additional data or information.
A “not substantially equivalent” determination, or a request for additional information, could delay or prevent the market introduction of new products for which we file such notifications. For any of our products that are cleared through the 510(k) process, modifications or enhancements that could significantly affect the safety or efficacy of the device or that constitute a major change to the intended use of the device will require new 510(k) submissions. The FDA has implemented a policy under which certain device modifications may be submitted as a “Special 510(k),” which will require only a 30-day review. Special 510(k)s are limited to those device modifications that do not affect the intended use or alter the fundamental scientific technology of the device and for which substantial equivalence can be demonstrated through design controls.
We must file a PMA if our proposed device is not substantially equivalent to a legally marketed Class I or Class II device, or if it is a Class III pre-amendment device (on the market since prior to May 28, 1976) for which FDA has called for PMAs. A PMA must be supported by valid scientific evidence that typically includes extensive data, including pre-clinical and clinical trial data, to demonstrate the safety and effectiveness of the device, as well as extensive manufacturing information.
FDA review of a PMA generally takes one to two years from the date the PMA is accepted for filing, but may take significantly longer. The review time is often significantly extended should the FDA ask for more information or clarification of information already provided in the submission.
During the PMA review period, an advisory committee, typically a panel of clinicians, will likely be convened to review and evaluate the application and provide recommendations to the FDA as to whether the device should be approved. The FDA is not bound by the recommendations of the advisory panel. Toward the end of the PMA review process, the FDA generally will conduct an inspection of the manufacturer’s facilities to ensure that they comply with Quality System Regulation (QSR) requirements.
If the FDA’s evaluations of both the PMA and the manufacturing facilities are favorable, the FDA will either issue an approval letter or an “approvable letter,” which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When, and if, those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue an approval letter, authorizing commercial marketing of the device for certain indications. If the FDA’s evaluation of the PMA or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a “not approvable letter.” The FDA may also determine that additional clinical trials are necessary, in which case PMA approval may be delayed up to several years while additional clinical trials are conducted and submitted as an amendment to the PMA. The PMA process can be expensive, uncertain and lengthy, and a number of devices for which other companies have sought FDA approval have never been approved for marketing.
Modifications to a device that is the subject of an approved PMA (including modifications to its labeling, raw material components or manufacturing process) may require approval by the FDA in the form of a PMA supplement or new PMA. Supplements to a PMA often require the submission of the same type of information required for an initial PMA, except that the
10
supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA.
If clinical trials of a device are required in connection with either a 510(k) notification or a PMA and the device presents a “significant risk,” we will be required to file an investigational device exemption (IDE) application prior to commencing clinical trials. The IDE application must be supported by data, typically including the results of animal and laboratory testing. If the IDE application is reviewed and approved by the FDA and one or more appropriate Institutional Review Boards (IRBs), clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a “non-significant risk” to the patient, we may begin the clinical trials after obtaining approval for the study by one or more appropriate IRBs. For “significant risk” devices, we must submit an IDE supplement to the FDA and receive approval from the FDA before we, or our investigator, may make a change to the investigational plan that may affect its scientific soundness or the rights, safety or welfare of human subjects. IRB approval may be required for changes in the investigational plan for both non-significant risk and significant risk devices.
Any products manufactured or distributed by us pursuant to FDA clearances or approvals are subject to extensive regulation by the FDA, including reporting and record keeping requirements. Device manufacturers are required to register their establishments and list their devices with the FDA and certain state agencies, and are subject to periodic inspections by the FDA and certain state agencies. The FFD&C Act requires devices to be manufactured in accordance with QSR requirements that impose certain procedural and documentation requirements upon us with respect to manufacturing and quality assurance activities. Medical devices are also subject to post-market reporting requirements for deaths or serious injuries when the device may have caused or contributed to the death or serious injury, and for certain device malfunctions that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur. If safety or efficacy problems occur after the product reaches the market, the FDA may impose severe limitations on the use of any approved or cleared product.
Our labeling and promotion activities are subject to scrutiny by the FDA and, in certain instances, by the Federal Trade Commission. The FDA actively enforces regulations prohibiting marketing of products for unapproved or uncleared uses. We, as well as our products, are also subject to a variety of state laws and regulations in those states or localities where our products are or will be marketed. Any applicable state or local regulations may hinder our ability to market our products in those states or localities. We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. There can be no assurance that we will not be required to incur significant costs to comply with such laws and regulations now or in the future or that such laws or regulations will not have a material adverse effect upon our ability to do business. Noncompliance with applicable requirements can result in, among other things, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure of the government to grant the pre-market clearance or premarket approval for devices, withdrawal of marketing approvals, clearances and criminal prosecution.
In August 2010, the FDA issued two comprehensive evaluations containing recommendation to change its regulatory review of medical devices. One report focused on ways to strengthen and clarify the premarket review or 510(k) process for medical devices that do not need to undergo a full premarket approval review. The other report evaluated FDA’s use of science in decision-making. These reports were prepared and issued in response to concerns raised both inside and outside of the FDA about whether the current 510(k) program achieves its goals of making safe and effective devices available to the public while fostering innovation. Concerns about the program have centered on whether it allows devices to enter the market without sufficient safety and effectiveness evidence and whether a lack of predictability, consistency, and transparency is hindering device development.
Following issue of these reports, FDA solicited public feedback on the content and subsequently developed a plan containing 25 actions it intends to implement during 2011 to improve the 510(k) process. Key actions include: 1) Streamlining the “de novo” review process for certain innovative, lower-risk medical devices; 2) Clarifying when clinical data should be submitted in a premarket submission, guidance that will increase the efficiency and transparency of the review process; and 3) Establishing a new Center Science Council of senior FDA experts to assure timely and consistent science-based decision making. FDA seeks to implement more changes after obtaining additional feedback from the public and the Institute of Medicine. These changes to the 510(k) program may significantly alter the review process for our products, and may increase the amount of pre-clinical and clinical data required to obtain product clearances and approvals.
European Union
In order to sell our products within the EU, we are required to achieve compliance with the requirements of the EU Medical Devices Directive, or MDD, and affix a CE mark on our products to attest such compliance. To achieve this, our products must meet the “essential requirements” defined under the MDD relating to safety and performance. Some of our products, such as Vitoss Foam,
11
Vitoss Bioactive Foam and Vitagel, contain materials of animal origin (i.e., bovine collagen and/or bovine thrombin). Demonstrated compliance with the EU Animal Tissue Directive is also required in order to sell these products in the EU.
In addition to demonstrating compliance with these European Directives, we must successfully undergo a verification of our regulatory compliance by an independent notified body referred to as a conformity assessment. The nature of the conformity assessment will depend on the regulatory class of our products. Under European law, our products, other than Imbibe and Aliquot (which are Class I-sterile and Class II-A, respectively), are likely to be in Class III. In the case of Class III products, we must (as a result of the regulatory structure which we have elected to follow) establish and maintain a complete quality system for design and manufacture as described in Annex II of the MDD and demonstrated by compliance with EN 13485:2003. We are certified as meeting the requirements of ISO 9001 and ISO/EN 13485. Our notified body has audited our quality system and determined that it meets the requirements of the MDD. In addition, the notified body must certify the specific design of each device in Class III, IIa and IIb. As part of the design certification process, the notified body must also verify that the products comply with the essential requirements of the MDD. In order to comply with these requirements, we must, among other things, complete a risk analysis and evaluation of the clinical data to demonstrate the product’s conformity with the essential requirements. This clinical evaluation may include a literature review for the subject device, or another comparable device. In the absence of adequate clinical information about the product, we may be required to conduct a clinical investigation for the product.
The clinical data presented by us must provide evidence that the products meet the performance specifications claimed by us, provide sufficient evidence of adequate assessment of unwanted side effects and demonstrate that the benefits to the patient outweigh the risks associated with the device. We are subject to continued surveillance by the notified body and are required to report any serious adverse incidents to the appropriate authorities of the European Union member states. The certification of the notified body is valid for a maximum of five years but may be extended by application for additional five year periods. We also are required to comply with additional national requirements that are outside of the scope of the MDD. There can be no assurance that we will not be required to incur significant costs to comply with such laws and regulations now or in the future or that such laws or regulations will not have a material adverse effect upon our ability to do business.
THIRD PARTY REIMBURSEMENT; HEALTH CARE REFORM
Successful sales of our products in the U.S. and other markets will depend on the availability of adequate coverage and reimbursement from third party payers relative to our products. In the U.S., health care providers, such as hospitals and physicians that purchase or utilize our products for treatment of their patients, generally rely on independent third party payers, such as private insurance companies or HMOs, and governmental payers, such as Medicare and Medicaid, to reimburse all or part of the costs and fees associated with the provision of items and services using our products. Physicians, hospitals, and other health care providers may not purchase our products if they do not receive satisfactory reimbursement from these payers for the cost of procedures using our products.
Each payer has its own process and standards for determining whether it will cover and reimburse a procedure or particular product. Private payers often follow the lead of the governmental payers in rendering coverage and reimbursement determinations. Therefore, achieving favorable Medicare coverage and reimbursement is usually a significant issue for successful introduction of a new product. Medicare is a federally funded program managed by the Centers for Medicare and Medicaid Services (CMS), through local contractors that administer coverage and reimbursement for certain health care items and services furnished to the elderly and disabled. Medicaid is an insurance program for the poor that is both federally and state funded and managed by each state. The federal government sets general guidelines for Medicaid and each state creates specific regulations that govern its individual program.
Medicare provides, among other things, health care benefits that cover, within prescribed limits, the major costs of most medically necessary care for such individuals, subject to certain deductibles and co-payments. The Medicare program has established, and continues to establish, standards and guidelines for the coverage and reimbursement of certain procedures, equipment, supplies and other items and services. Generally, in order to be covered and reimbursed by Medicare, a health care item or service furnished to a Medicare beneficiary must be included in a statutory benefit category, must not be excluded from coverage by statute, and must be reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body part. Medicare’s coverage decisions can be made on a nationwide basis or at the local contractor level, and coverage of an item or service can be reconsidered at any time. Several of Medicare’s contractors have policies governing coverage of our products or procedures using our products, including vertebroplasty and kyphoplasty. In July 2008, CMS identified vertebroplasty and kyphoplasty, procedures for which our Cortoss product is cleared, as one of twenty potential national coverage determination topics. Although CMS has not taken further action nationally since that time, the use of vertebroplasty and kyphoplasty is currently under a coverage review by a few of Medicare’s local contractors. We expect these local contractors will issue their coverage determinations in 2011.
The methodology for determining (1) the coverage status of our products; and (2) the amount of Medicare reimbursement for our products varies based upon, among other factors, the setting in which a Medicare beneficiary received health care items and services. Acute care hospitals are generally reimbursed by Medicare for in-patient operating costs based upon prospectively determined rates. Under the in-patient Prospective Payment System (PPS), acute care hospitals receive a predetermined payment rate
12
based upon the Medicare Severity Diagnosis-Related Group, or MS-DRG, into which each Medicare beneficiary stay is assigned, regardless of the actual cost of the services provided. These payment rates are adjusted for geographic variations in costs, medical education payments, and adjustments for hospitals that serve a disproportionate share of low-income patients. In some cases, hospitals also may receive adjustments for use of certain new technologies. Certain additional or “outlier” payments may be made to a hospital for cases involving unusually high costs. Accordingly, acute care hospitals generally do not receive direct Medicare reimbursement under the in-patient PPS for the distinct costs incurred in purchasing our products. Rather, reimbursement for these costs is deemed to be included within the MS-DRG-based payments made to hospitals for the services furnished to Medicare-eligible in-patients in which our products are utilized. Because in-patient PPS payments are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, acute care hospitals have incentives to lower their in-patient operating costs by utilizing equipment, devices and supplies, including those sold by us, that will reduce the length of in-patient stays, decrease labor or otherwise lower their costs. Our product revenue could be affected negatively if acute care hospitals discontinue product use due to insufficient reimbursement, or if other treatment options are perceived to be more cost-effective. Similarly, some states reimburse certain health care providers for in-patient services under their Medicaid programs by using prospective rates for diagnosis-related groups of illnesses.
Medicare also reimburses most hospital out-patient services on a prospective payment basis, subject to certain adjustments, exceptions, and limitations. Under the Medicare hospital out-patient PPS, services are grouped based on clinical and cost similarity into ambulatory payment classifications (APCs). All services in an APC have the same rate, intended to cover the equipment, supplies, and labor used to perform the service. Unlike the in-patient PPS, if a patient receives multiple services, the hospital will receive reimbursement through multiple APCs. Similar to the in-patient PPS, this system provides hospitals with an incentive to select equipment and supplies that reduce the costs of treating a patient. Therefore, health care providers may refuse to use our products if coverage or reimbursement is inadequate.
Any changes in federal legislation, regulations and policy affecting Medicare coverage and reimbursement relative to our products could have a material effect on our results of operations. Changes in the health care industry in the United States and elsewhere also could adversely affect the demand for our products as well as the way in which we conduct our business. Significantly, the Patient Protection and Affordable Care Act (PPACA) enacted into federal law in 2010 requires most individuals to have health insurance, establishes new regulations on health plans, creates insurance pooling mechanisms, and requires other expanded public health care measures. PPACA will also reduce Medicare spending on services provided by hospitals and other providers and establishes a new 2.3 percent tax on the sale after December 31, 2012 of a taxable medical device by the manufacturer, producer, or importer.
Inadequate coverage or reimbursement by private insurance companies and government programs could significantly reduce usage of our products. In addition, an increasing emphasis on managed care in the U.S. has placed, and we believe will continue to place, greater pressure on medical device pricing. Such pressures could have a material adverse effect on our ability to sell our products profitably. Failure by hospitals and other users of our products to obtain favorable coverage or reimbursement from third party payers or changes in governmental and private third party payers’ policies toward coverage or reimbursement for procedures employing our products could reduce demand for our products.
The European Union has not established a uniform process to govern the pricing and reimbursement of medical devices. Consequently, the individual EU member states operate various combinations of centrally financed health care systems and private health insurance systems. The relative importance of government and private systems varies from country to country. The choice of devices is subject to constraints imposed by the availability of funds within the purchasing institution and by hospital and authority priorities and procedures. Medical devices are most commonly sold to hospitals or health care facilities at a price set by negotiation between the buyer and the seller. A contract to purchase products may result from an individual initiative or as a result of a competitive bidding process. In either case, the purchaser pays the supplier, and payment terms vary widely throughout the EU. Failure to obtain favorable negotiated prices with hospitals or health care facilities could adversely affect sales of our products.
HEALTH CARE FRAUD AND ABUSE LAWS
We are subject to various federal and state laws pertaining to health care fraud and abuse, including anti-kickback laws and false claims laws. Similar regulations have been adopted by EU member states. Violations of these laws are punishable by criminal, civil and/or administrative sanctions, including, in some instances, fines, imprisonment, and exclusion from participation in federal, state and national health care programs, including Medicare, Medicaid and veterans’ health programs. Because of the far-reaching nature of these laws, there can be no assurance that the occurrence of one or more violations of these laws would not result in a material adverse effect on our business, financial condition and results of operations.
Anti-Kickback Laws. Our operations are subject to federal and state anti-kickback laws. The federal anti-kickback law prohibits entities such as us from knowingly and willfully offering, paying, soliciting or receiving any form of remuneration (including any kickback, bribe or rebate) in return for the referral of items or services for which payment may be made under a federal health care
13
program, or in return for the recommendation, arrangement, purchase, lease or order of items or services for which payment may be made under a federal health care program. The definition of remuneration has been broadly interpreted to include anything of value, including for example gifts, discounts, the furnishing of supplies or equipment, payments of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals or otherwise generate business involving goods or services reimbursed in whole or in part under federal health care programs, the statute has been violated. Violation of the federal anti-kickback law is a felony, punishable by criminal fines and imprisonment for up to five years or both. In addition, the Department of Health and Human Services may impose civil penalties and exclude violators from participation in federal health care programs such as Medicare and Medicaid. Exclusion of a manufacturer would preclude any federal health care program from paying for its products. In addition, enforcement officials have argued (and some courts have agreed) that kickback arrangements can provide the basis for an action under the Federal False Claims Act, which is discussed below.
Government officials have focused recent enforcement efforts on, among other things, the sales and marketing activities of health care companies, and recently have brought cases against individuals or entities with personnel who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business. Settlements of these cases by health care companies have involved significant fines and/or penalties and in some instances criminal pleas.
Many states have adopted similar prohibitions against payments intended to induce referrals of products or services paid by Medicaid or other third party payers.
False Claims. The federal False Claims Act imposes civil and criminal liability on individuals or entities who submit (or cause the submission of) false or fraudulent claims for payment to the government. Violations of the federal False Claims Act may result in penalties equal to three times the damages which the government sustained, an assessment of between $5,500 and $11,000 per claim, civil monetary penalties and exclusion from participation in the Medicare and Medicaid programs.
The federal False Claims Act also allows a private individual to bring aqui tam suit on behalf of the government against an individual or entity for violations of the False Claims Act. In aqui tam suit, the private plaintiff is responsible for initiating a lawsuit that may eventually lead to the government recovering money of which it was defrauded. After the private plaintiff has initiated the lawsuit, the government must decide whether to intervene in the lawsuit and become the primary litigant. In the event the government declines to join the lawsuit, the private plaintiff may choose to pursue the case alone, in which case the private plaintiff’s counsel will have primary control over the case (although the government must be kept apprised of the progress of the lawsuit). In return for bringing the suit on the government’s behalf, the statute provides that the private plaintiff is entitled to receive up to 30% of the recovered amount from the litigation proceeds if the litigation is successful plus reasonable expenses and attorneys fees. Recently, the number ofqui tam suits brought against entities in the health care industry has increased dramatically. In addition, a number of states have enacted laws modeled after the False Claims Act that allow those states to recover money which was fraudulently obtained from the state.
Other Fraud and Abuse Laws. The Health Insurance Portability and Accountability Act of 1996 created, in part, two new federal crimes: Health Care Fraud and False Statements Relating to Health Care Matters. The Health Care Fraud statute prohibits the knowing and willful execution of a scheme or artifice to defraud any health care benefit program. A violation of the statute is a felony and may result in fines and/or imprisonment. The False Statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact by any trick, scheme or device or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
The countries of the EU have individual and varying legislative provisions governing health care fraud and abuse, including provisions governing inducement to purchase. Violation of these provisions can lead to action both against the provider of the device and against the health care provider.
PRODUCT LIABILITY AND INSURANCE
We manufacture medical devices used on patients in surgery, and we may be subject to product liability lawsuits. While we have not experienced material product liability claims to date, there can be no assurance that any such product liability claims will not be asserted against us in the future. Under certain of our agreements with our independent distributors and hospital customers, we provide indemnification from product liability claims. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or the inability to secure coverage in the future. In addition, we would have to pay any amount awarded by a court in excess of policy limits. We maintain product liability insurance in the annual aggregate amount of up to $20 million, although our insurance policies have various exclusions. Thus, we may be subject to a product liability claim for which we have no insurance coverage, in which case we may have to pay the entire amount of any award.
14
EMPLOYEES
As of December 31, 2010, we had 256 full-time employees, with 131 employees at our Malvern, Pennsylvania headquarters, 111 employees in other parts of the U.S. and 14 employees in Europe. We consider our relations with our employees to be good.
We have organized the risk factors set forth below that may affect us or our securities into the following four categories: (i) risks related to our business and industry; (ii) risks related to our financial results and financing; (iii) risks related to our intellectual property and litigation; and (iv) risks related to investing in our stock and equity markets.
RISKS RELATED TO OUR BUSINESS AND INDUSTRY
If our current products are not commercially successful, our operating results will be impaired and our viability will be jeopardized.
We are highly dependent on successfully selling our products that have received regulatory approval or clearance. To date, Vitoss, Imbibe, Cortoss, Vitagel, CellPaker and Vitasure are approved or cleared for specified uses in the United States. Vitoss, Cortoss, Aliquot, Vitagel, CellPaker and Vitasure are cleared or approved for specified uses in the European Union and countries adhering to the regulatory standards of the European Union. Vitoss, Cortoss and Aliquot are also cleared for specified uses in Australia. Revenues generated from sales of our approved or cleared products in their approved territories have not been and in the future may not be sufficient to fund operations.
In addition, Cortoss represents a new technology which will generally need to be approved by technology review boards of target hospitals in order for the hospitals to carry the product. The amount of time to obtain approvals from the technology review boards can be difficult to predict and we cannot guarantee that we will be able to secure widespread hospital approvals for Cortoss. We expect our U.S. Cortoss sales will increase as the product launch progresses, we obtain further hospital approvals for the product and our sales force trains more physicians on the product. There is no assurance that Cortoss or our other products will generate revenues sufficient to enable us to fund operations.
Certain factors that may also limit our ability to increase sales include:
| | • | | the ability of our direct sales representatives and independent distributors to develop and retain a customer base and compete effectively with larger sales forces offering more extensive product portfolios; |
| | • | | the introduction of new products based upon competing technologies into the market by competing orthopedic, biomedical and other companies; |
| | • | | increased efforts by hospitals to reduce costs of medical devices; |
| | • | | our dependence in certain geographical areas on the efforts of independent agents and distributors over which we have limited control to promote the use of our products; |
| | • | | our dependence on the continued publication of independent pre-clinical and clinical data to support the use of our products; |
| | • | | our need to educate and train a sufficient number of surgeons to create demand for our products; and |
| | • | | the need for payers to authorize insurance reimbursement for procedures using our products. |
Market acceptance of our products will largely depend on our ability to demonstrate their relative safety, efficacy, cost-effectiveness and ease of use. Surgeons will not use our products unless they determine, based on experience, clinical data and recommendations from prominent surgeons and mentors that our products are safe and effective. Our products are based on newer technologies and compete with more established treatments currently accepted as the standards of care. The attributes of some of our products may require some changes in surgical techniques that have become standard within the medical community, and there may be resistance to change. Therefore, for these products, we must be able to convince surgeons who currently favor existing techniques to switch to new procedures that would use our products. Many surgeons will not purchase our products until there is sufficient, long-term clinical evidence to convince them to alter their existing treatment methods. In addition, surgeons may be slow to change their medical treatment practices because of perceived liability risks arising from the use of new products and the uncertainty of third party reimbursement for our products. Any failure to gain market acceptance of our products could result in lower sales and our inability to become profitable.
An insufficient level of product sales may also result in excess product inventory charges, which would negatively impact operating results. We may manufacture product inventory in anticipation of product launches or order significant inventory from third party suppliers in advance due to long manufacturing lead times. For example, at the time of our U.S. launch of Cortoss and Aliquot (our system used to deliver Cortoss in vertebroplasty) in the second half of 2009, we expected significant revenue growth from these
15
products. We believe that the U.S. launch of Cortoss and Aliquot for vertebral augmentation procedures has been negatively affected by several factors, including the publication of two small studies inThe New England Journal of Medicine in August 2009 that questioned the effectiveness of vertebroplasty; uncertainty created by the pending reimbursement review of vertebral augmentation procedures by a few of Medicare’s local contractors; the issuance of clinical practice guidelines in September 2010 by the American Academy of Orthopedic Surgeons that did not support the use of vertebroplasty to treat vertebral compression fractures; the recent shift of vertebral augmentation procedures from the in-patient to the out-patient setting, which is outside of the historic call pattern of our sales force; overall negative economic conditions; and health care initiatives to reduce costs. Prior to and following the U.S. launch of Cortoss and Aliquot and in anticipation of significant product demand, we acquired significant inventory quantities of Aliquot from a third party supplier with requisite long manufacturing lead times. During the fourth quarter of 2010, we recorded a $3.5 million inventory charge primarily for Aliquot inventory that either was expected to expire prior to sale or was not expected to be sold based on our current projections of product demand.
If we are unable to operate an effective sales and distribution network, our ability to generate sales and become profitable will be impaired.
We have assembled a field sales network of direct sales representatives and independent non-stocking and stocking distributors and agents in the U.S. to market Vitoss, Imbibe, Cortoss, Aliquot, Vitagel, CellPaker and Vitasure. Outside of the U.S., we utilize a network of independent stocking distributors to market Vitoss, Cortoss, Aliquot, Vitagel, CellPaker and Vitasure, although we have a direct sales force in the United Kingdom. We are dependent upon our direct sales representatives, distributors and agents for the sale of our products. Any failure to maintain and effectively manage our distribution network will impair our ability to generate additional sales and become profitable.
In an effort to optimize our sales in a challenging environment, in 2010 we began to increase the number of independent distributors selling our products. For example, to more effectively address the shift in vertebroplasty procedures to the out-patient setting and make an effort to increase Cortoss and Aliquot sales, we began to augment our direct sales efforts in the U.S. in 2010 with distributors that specialize in the out-patient market. We continue to identify and retain distributors and agents to sell our Vitoss and biosurgery products as well. During 2010, we also streamlined our direct sales force, which will continue to focus primarily on the in-patient spine and orthopedic market to leverage our customer relationships by marketing our biosurgery and orthobiologic products to all of our customers. Through an intense focus on our target physician audiences using a combination of our direct sales representatives, sales agents and distributors in a hybrid sales model, our goal is to regain sales momentum and growth in our Vitoss product platform while continuing to increase penetration of Cortoss products and growth of our biosurgery product sales. There can be no assurance that our hybrid sales model will be effective in growing sales or increasing gross profit.
In an effort to further grow our sales, we plan to continue to strengthen our hybrid sales and distribution network by optimizing the number of direct sales representatives or adding distributors and sales agents in those territories or markets where we do not have adequate coverage. At the end of 2008, we added a direct sales force in the United Kingdom, which includes 6 direct sales representatives as of March 1, 2011. The addition of direct sales representatives will increase our operating expenses. Furthermore, there is no assurance that adding direct sales representatives or other selling entities will improve sales or result in the generation of sales sufficient to cover the cost of supporting their sales activities. There is no assurance that we will be able to attract and retain qualified personnel to market or sell our products or that we will successfully manage this type of sales and distribution method.
There can be no assurance that our distributors will perform effectively their obligations in their respective territories or accounts as expected, or that we will continue to derive any revenue from these arrangements. We cannot assure that our interests will continue to coincide with those of our distributors. In addition, we cannot assure that our distributors will not develop independently, or with other companies, competitive products.
Most of our product sales generated by independent U.S. distributors are from Vitoss. These distributors generally sell products for other orthopedic companies. A single distributor may sell Vitoss, as well as hardware manufactured by other orthopedic companies consisting of metal plates, screws and spinal cages, to end user hospitals. Our sales could be adversely affected if, for any reason, one or more of our successful distributors lost their hardware product line provided by other orthopedic companies since Vitoss is typically sold with such hardware products. Additionally, our independent distributors may be unwilling to carry or unable to effectively sell Vitoss as a result of the introduction of new products based upon other technologies that could compete with Vitoss. Our sales could be adversely affected if one or more of our successful distributors eliminated Vitoss from its product line for any other reason or terminated its arrangement with us. Our sales could also be adversely affected if our independent distributors become concerned that their arrangements with us could be eliminated as a result of another company’s offer or threat to acquire us. The complete product line represented by the distributors, including our products, is an important factor in the distributors’ ability to penetrate the markets for our products. Accordingly, our ability to penetrate the markets that we serve or intend to serve is highly dependent upon the quality and breadth of the other product lines carried by our distribution network, the components of which may change from time to time, and over which we have little or no control.
16
Pricing pressure may negatively impact our average sales prices for our products, which could reduce our revenue and gross margins.
The market for our products is very competitive and increasingly subject to pricing pressures due to health care reform and efforts by health care providers to contain costs. Several of our competitors offering multiple product lines provide discounts on certain products such as bone graft substitutes in order to maintain pricing on other products in their portfolio such as hardware. We may also face pricing pressure as a result of the growing number of physician-owned distributors operating in the spine and orthopedic markets, as discussed below under the risk factor entitled, “If we cannot keep up with technological changes and marketing initiatives of competitors, including the growth of physician-owned distributors, sales of our products may be harmed.” As a result of these factors, we believe our products face growing pricing pressure in the near future. If competitive forces drive down the price we are able to charge for our products and we cannot reduce our expenses, our operating results will be adversely affected.
If we do not manage commercial scale manufacturing capability and capacity for our products in compliance with regulatory requirements and in a cost-effective manner, our product sales and operating results may suffer.
The manufacture of our products is subject to regulation and periodic inspection by various regulatory bodies for compliance with the FDA Quality System Regulation (the QSR), and EU Medical Devices Directive requirements, ISO 9001 standards, ISO/European Norm (EN) 13485 and equivalent requirements.
We manufacture our commercial Vitoss, Vitagel and Cortoss products at our 25,800-square foot FDA-registered manufacturing facilities located in Malvern, Pennsylvania. These facilities are leased through July 2017 and are certified as meeting the requirements of ISO9001 and ISO/EN 13485. We also process collagen for Vitagel at our 4,000-square foot FDA-approved processing facility in Malvern, Pennsylvania. We are in the process of seeking regulatory approval of this collagen processing facility under the CE Mark, which would allow us to market in the European Union Vitagel utilizing collagen processed at our facility. We are currently required to demonstrate and maintain compliance with the QSR, which is a complex regulatory scheme that covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. The FDA enforces the QSR through periodic inspections. We have been, and anticipate in the future being, subject to such inspections. We assumed full responsibility for manufacturing Vitagel in 2006 and CellPaker in 2007 after receiving the requisite approvals from the FDA. There can be no assurance that we will be able to comply with FDA requirements to continue to manufacture Vitoss, Cortoss, Vitagel or CellPaker, which could result in delay or inability to manufacture and sell the products. We believe our existing manufacturing facilities have the capacity to meet our commercial needs through at least 2013.
Our third party manufacturers are also regulated by governmental agencies and are required to be EN 13485-certified or otherwise meet our quality system requirements. We manufacture CellPaker and Aliquot through outside third party contract manufacturers. Our Vitoss is converted to Vitoss Foam and Vitoss Bioactive Foam by Kensey, and we purchase Vitasure finished product from Medafor, which uses third party manufacturers to make the product.
Our product sales depend upon, among other things, our ability to manufacture our products in commercial quantities, in compliance with regulatory requirements and in a cost-effective manner. There can be no assurance that regulatory authorities will not, during the course of an inspection of existing or new facilities, identify what they consider to be deficiencies in meeting the applicable quality standards and request or seek remedial action.
Failure to comply with such regulations or a delay in attaining compliance may result in:
| | • | | injunctions suspending the manufacture of products; |
| | • | | civil and criminal penalties; |
| | • | | refusal to grant premarket approvals or clearances, CE Certifications, CE Certification renewals, or clearances to products that are subject to future or pending submissions; |
| | • | | product recalls or seizures of products; |
| | • | | total or partial suspensions of production; and |
| | • | | refusal to permit the import or export of our products. |
The imposition of any of these sanctions could cause our business and operating results to suffer. Furthermore, if we or any of our third-party manufacturers cannot produce our products, our product sales would suffer.
In addition, our gross profit margins may be adversely affected if our manufacturing facilities are under-utilized and result in excess manufacturing capacity charges which are recorded as a component of the cost of sales.
17
We are dependent on a limited number of specialty suppliers of certain raw materials, and our business and financial results could be harmed if a specialty supplier no longer provides materials to us.
Our ability to manufacture Vitoss, Cortoss and Vitagel and to have Vitoss Foam and Vitoss Bioactive Foam manufactured by and for us is dependent on a limited number of specialty suppliers of certain raw materials. We have several single-source suppliers for raw materials used in our products. The failure of a supplier to continue to provide us with these materials at a price or quality acceptable to us, or at all, would have a material adverse effect on our ability to manufacture these products. Although we believe that we maintain good relationships with our suppliers, we cannot assure that such supplies and services will continue to be available with respect to our current and future commercialized products.
In addition, pursuant to our license agreement with Angiotech Pharmaceuticals, we may be obligated in the future, under certain circumstances, to provide CoStasis (an Angiotech-branded Vitagel product) and certain CoStasis materials to Angiotech. We also cannot assure that supply or manufacturing capability will be sufficient to meet our own needs and Angiotech’s needs if Angiotech elects to be supplied by us under our license agreement with them. Our collagen supply or manufacturing capabilities may be inadequate, and we may need to seek additional suppliers for CoStasis materials.
We are dependent on a limited number of specialized manufacturing suppliers for certain products, and our business and financial results could be harmed if a third-party manufacturer fails to supply us with these products.
We are dependent upon Kensey and other third-party manufacturers for the supply of certain products. Approximately 67% of our product sales during 2010 were from products based upon our Vitoss Foam platform co-developed with Kensey, including Vitoss Bioactive Foam. Our Vitoss is converted to Vitoss Foam and Vitoss Bioactive Foam by Kensey. We also manufacture certain delivery accessory products through third party specialty suppliers. There is no assurance that these manufacturers will continue to supply our requirements. If they fail to do so, we cannot guarantee that we will be able to engage another supplier for these products on a timely basis and on acceptable terms. A delay in our ability to obtain adequate supplies of these products may negatively impact our product sales.
If we fail to obtain and maintain the regulatory approvals or clearances necessary to make or sell our products, sales could be delayed or never realized.
The jurisdictions in which we seek to market our products regulate our products as medical devices. In most circumstances, we and our distributors and our agents must obtain regulatory clearances, approvals and certifications and otherwise comply with extensive regulations regarding safety, quality and efficacy standards. These regulations vary from country to country, and the regulatory review can be lengthy, expensive and uncertain. We may not obtain or maintain the regulatory clearances, approvals and certifications necessary to make or market our products in our targeted markets. Moreover, regulatory clearances, approvals and certifications that are obtained may involve significant restrictions on the anatomic sites and types of procedures for which our products can be used. In addition, we may be required to incur significant costs in obtaining or maintaining our regulatory clearances, approvals and certifications. If we do not obtain or maintain regulatory clearances, approvals and certifications to enable us to make or market our products in the U.S. or elsewhere, or if the clearances, approvals and certifications are subject to significant restrictions, we may never generate significant revenues. The regulatory requirements in some of the jurisdictions where we currently market or intend to market our products are summarized below.
United States
Regulation by FDA.The FDA regulates the clinical testing, manufacturing, labeling, distribution and promotion of medical devices. To date, we have received 510(k) clearance from the FDA to market our Vitoss, Vitoss Foam, Vitoss Bioactive Foam, Vitoss Bioactive Foam-2X, Vitoss BA Bimodal, Imbibe and Cortoss products. Aliquot is exempt from 510(k) clearance.
We market Vitoss, Imbibe, Cortoss, Aliquot, Vitagel, CellPaker and Vitasure in the U.S., and we market Vitoss, Cortoss, Aliquot, Vitagel, CellPaker and Vitasure outside the U.S. We directly manufacture Vitoss, Cortoss, Vitagel and CellPaker in the U.S., and we manufacture Imbibe and Aliquot in the U.S. through outside third party contract manufacturers. In addition, under our agreement with Kensey, Kensey has the exclusive right to manufacture any approved or cleared jointly developed products under the agreement. This right extends until February 2014 for the Vitoss Foam product platform and at least until 2024 for the Vitoss Bioactive Foam product platform, which includes Vitoss Bioactive Foam-2X and Vitoss BA Bimodal. We purchase Vitasure from Medafor, which utilizes third party manufacturers to produce this product. Under our distribution agreement with Medafor, Medafor is responsible for maintaining regulatory approval and ensuring compliance with manufacturing requirements for Vitasure.
Vitoss, Cortoss, Vitagel and Vitasure, as well as any other products that we manufacture or distribute following their approval or clearance by the FDA, will be subject to extensive regulation by the FDA. In August 2010, the FDA issued two comprehensive evaluations containing recommendation to change its regulatory review of medical devices. One report focused on ways to strengthen and clarify the premarket review or 510(k) process for medical devices that do not need to undergo a full premarket approval review.
18
The other report evaluated FDA’s use of science in decision-making. These reports were prepared and issued in response to concerns raised both inside and outside of the FDA about whether the current 510(k) program achieves its goals of making safe and effective devices available to the public while fostering innovation. Concerns about the program have centered on whether it allows devices to enter the market without sufficient safety and effectiveness evidence and whether a lack of predictability, consistency, and transparency is hindering device development.
Following issue of these reports, FDA solicited public feedback on the content and subsequently developed a plan containing 25 actions it intends to implement during 2011 to improve the 510(k) process. Key actions include: 1) Streamlining the “de novo” review process for certain innovative, lower-risk medical devices; 2) Clarifying when clinical data should be submitted in a premarket submission, guidance that will increase the efficiency and transparency of the review process; and 3) Establishing a new Center Science Council of senior FDA experts to assure timely and consistent science-based decision making. FDA seeks to implement more changes after obtaining additional feedback from the public and the Institute of Medicine. These changes to the 510(k) program may significantly alter the review process for our products, and may increase the amount of pre-clinical and clinical data required to obtain product clearances and approvals.
European Union and Other International Markets
General.International sales of medical devices are subject to the regulatory requirements of each country in which the products are sold. Accordingly, the introduction of our products in markets outside the United States subject our products to regulatory approvals or clearances in those jurisdictions. The regulatory review process varies from country to country. Many countries impose product standards, packaging, labeling and language requirements and import restrictions on medical devices. In addition, the EU and each country outside of the EU have its own tariff regulations, duties and tax requirements. The approval or clearance of our products by foreign government authorities is uncertain and can be expensive. Our ability to market our products could be substantially limited due to delays in receipt of, or failure to receive, the necessary approvals or clearances.
Requirement of CE Certification in the European Union.To market a product in the European Union, we must be entitled to affix to that product a CE mark, an international symbol of adherence to quality assurance standards and compliance with applicable European medical device directives. A CE mark, preceded by the relevant certification process, enables us to market a product in all of the countries of the European Union, as well as in other countries, such as Switzerland and Turkey, that have mutual recognition agreements with the EU or have adopted the European Union’s regulatory standards. To date, we have received CE mark approval for the use of Vitoss as a bone graft substitute, for the use of Cortoss in screw augmentation and vertebral augmentation procedures, for Vitagel as a surgical hemostat and for certain delivery accessories for Vitoss, Cortoss and Vitagel. Vitasure has CE mark approval for use as a surgical hemostat. There can be no assurance that we will maintain CE mark approval for our products or that we will receive CE mark approval for other devices.
After clearance or approval of our products, we are subject to continuing regulation by the FDA. If we fail to comply with FDA regulations, our business could suffer.
Even after clearance or approval of a product, we are subject to continuing regulation by the FDA, including the requirements that our facility be registered and our devices listed with the agency. We are subject to Medical Device Reporting regulations, which require us to report to the FDA if our products may have caused or contributed to a death or serious injury or malfunction in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. We must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act caused by the device that may present a risk to health, and maintain records of other corrections or removals. The FDA closely regulates promotion and advertising.
The FDA and state authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
| | • | | untitled letters, warning letters, fines, injunctions, consent decrees or civil penalties; |
| | • | | repair, replacement, refunds, recall or seizure of our products; |
| | • | | operating restrictions or partial suspension or total shutdown of production; |
| | • | | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or new intended uses of our existing products; |
| | • | | withdrawing 510(k) clearance or premarket approvals that have already been granted; and |
If any of these events were to occur, they could harm our business.
19
We have modified some of our products without FDA clearance. The FDA could retroactively determine that the modifications were improper and require us to stop marketing and recall the modified products until such clearance is obtained.
Any modification to an FDA-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance; any change that affects the safety or effectiveness of a PMA approved device requires a premarket approval supplement. We have made modifications to some of our products in the past, and may make additional modifications in the future that we believe, based upon FDA’s regulations and guidance, do not or will not require additional clearances or approvals. If the FDA disagrees, we could be required to obtain new clearances or approvals for the modifications. We also may be required to recall and to stop marketing modified products until such clearance or approval is obtained, which could harm our operating results. We may be required to submit extensive pre-clinical and clinical data to support these supplementary submissions, depending on the nature of the changes. We may not be able to obtain additional 510(k) clearances or premarket approvals for modifications to, or additional indications for, our existing products in a timely fashion, or at all. Delays in obtaining future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our operating results.
If we cannot keep up with technological changes and marketing initiatives of competitors, or if we are unable to effectively compete with the growing number of physician-owned distributors, sales of our products may be harmed.
Extensive research efforts and rapid technological change characterize the market for products in the orthopedic and biomaterials markets. We anticipate that we will face intense competition from medical device, medical products and pharmaceutical companies. There are many products that are competitive to our Vitoss platform products. For instance, the use of bone morphogenic proteins, or BMPs, in orthopedic surgery has increased significantly in the past five years and competitive stem cell products have recently entered the market, a trend we expect to continue. With respect to Cortoss, several PMMA bone cements have received 510(k) clearance since 2004 for vertebral augmentation of VCFs. Further, recombinant human thrombin was approved by the FDA in 2008 and may be positioned as a better alternative to bovine-derived thrombin, a raw material in our Vitagel product. Our products could be rendered uncompetitive or obsolete by these and other competitors’ new technologies. We may be unable to respond to technological advances by others through the development and introduction of new products or the modification of existing products. Moreover, many of our existing and potential competitors have substantially greater financial, marketing, sales, distribution, manufacturing and technological resources than us. These competitors may be in the process of seeking FDA or other regulatory approvals or clearances, or patent protection, for competitive products. Our competitors could, therefore, commercialize competing products in advance of our products. They may also enjoy substantial advantages over us in terms of:
| | • | | research and development expertise; |
| | • | | experience in conducting clinical trials; |
| | • | | experience in regulatory matters; |
| | • | | manufacturing efficiency; |
| | • | | sales and marketing expertise; |
| | • | | established distribution channels; and |
| | • | | established relationships with health care providers and payers. |
These advantages may adversely affect our plans for market acceptance of our products.
Further, we may face increasing competition from physician-owned distributors, or PODs, the number of which has grown in the past several years. PODs are entities in the medical device supply chain that derive a proportion of their revenue from selling or arranging for the sale of medical devices ordered by physician-investors for use in procedures they perform on their own patients at hospitals that agreed to purchase from or through the POD, or that otherwise furnish ordering physicians with income that is based directly or indirectly on those orders of medical devices. We believe that the number of PODs in the spine and orthopedic markets will continue to grow as economic pressures increase through the industry, hospitals, insurers and physicians search for ways to reduce costs, and medical device manufacturers seek ways to maintain or grow market share.
If health care providers cannot obtain third party reimbursement for procedures using our products, or if such reimbursement is inadequate, our product sales may be adversely affected and we may never become profitable.
Successful sales of our products in the United States and other markets depend on the availability of adequate reimbursement from third party payers. Inadequate reimbursement by private insurance companies and government programs could significantly reduce usage of our products. In the U.S., health care providers such as hospitals, physicians and ambulatory surgical centers generally
20
rely on third party payers, including private insurance companies, Health Maintenance Organizations, Medicare and Medicaid, to reimburse all or part of the costs of procedures using our products. Health care providers may not perform certain procedures and, accordingly, may not purchase our products, if they do not receive satisfactory reimbursement from these payers. Each payer has its own process and standards for determining whether it will cover and reimburse a procedure or particular product. Private payers often follow the lead of the governmental payers in rendering coverage and reimbursement determinations. Therefore, achieving favorable Medicare coverage and reimbursement is a significant issue for successful introduction of a new product as well as for maintaining or growing sales of existing products.
The Medicare program has established, and continues to establish, standards and guidelines for the coverage and reimbursement of certain procedures, equipment, supplies and other items and services. Medicare’s coverage decisions can be made on a nationwide basis or at the local contractor level, and coverage of an item or service can be reconsidered at any time. Decisions to limit coverage of our products could significantly reduce use of our products, particularly if other payers adopt similar policies. Several of Medicare’s contractors have policies governing coverage of our products or procedures using our products, including vertebroplasty and kyphoplasty. In July 2008, the Centers for Medicare and Medicaid Services (CMS) identified vertebroplasty and kyphoplasty, procedures for which our Cortoss product is cleared for use, as one of twenty potential national coverage determination topics. Although CMS has taken no further action nationally since that time, the use of vertebroplasty and kyphoplasty is currently under a coverage review by a few of Medicare’s local contractors. We expect that these local contractors will issue their coverage determination in 2011.
Any changes in legislation, regulations and policy affecting Medicare coverage and reimbursement relative to our products could have a material effect on our results of operations. Changes in the health care industry in the United States and elsewhere also could adversely affect the demand for our products as well as the way in which we conduct our business. Significantly, the Patient Protection and Affordable Care Act (PPACA) enacted into federal law in 2010 requires most individuals to have health insurance, establishes new regulations on health plans, creates insurance pooling mechanisms, and requires other expanded public health care measures. This legislation will also reduce Medicare spending on services provided by hospitals and other providers. Various health care reform proposals have also emerged at the state level. We cannot predict what health care initiatives, if any, will be further implemented at the federal or state level, or the effect that recently enacted legislation or any future legislation or regulation will have on us.
In addition, an increasing emphasis on managed care in the United States has placed, and we believe will continue to place, greater pressure on medical device pricing. Such pressures could have a material adverse effect on our ability to sell our products or to operate profitably.
If we do not successfully train a sufficient number of surgeons to use our products, demand for our products could be adversely affected.
It is critical to the commercial success of our products that our independent distributors and direct sales representatives succeed in training a sufficient number of surgeons and in providing them adequate instruction in the use of our products. This training requires a commitment of time and money by surgeons which they may be unwilling to give. Even if surgeons are willing, if they are not properly trained, they may misuse or ineffectively use our products. This may result in unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us, any of which could damage our business, reduce product sales and subject us to claims and other liabilities.
We may incur significant liability if it is determined that we are promoting the “off-label” use of our medical devices. The unapproved or “off-label” use of our products could also adversely affect the reputation of our products and our company.
Under the Federal Food, Drug, and Cosmetic Act and other laws, we are prohibited from labeling, training, or promoting our products for off-label uses. This prohibition means that we cannot proactively discuss or provide information or training on the use of our products outside of their cleared or approved uses, except in certain limited scientific and other settings. In the practice of medicine, physicians may use devices for off-label uses. Because the interpretation of these requirements is subjective, the FDA could deem some of our activities as promotional even when we believe in good faith that such activities were not promotional. A company that is found to have improperly promoted off-label uses may be subject to significant liability, including civil and administrative remedies as well as criminal sanctions. Enforcement measures taken against us could harm our business and our reputation.
The medical devices that we manufacture and market, or intend to market, are subject to extensive regulation by the FDA, the European Union Medical Devices Directive and other worldwide regulatory agencies. In order to market our products, we must apply for, receive and maintain all necessary regulatory approvals or clearances in each applicable jurisdiction for specified uses of the products. Under FDA regulations and laws of other countries, we are only permitted to commercially distribute our products for approved indication(s) or use(s); failure to comply with these regulations may subject us to FDA and other enforcement action.
21
Cortoss received CE Certification in January 2003 for use in repairing vertebral compression fractures, and in October 2001 for use in screw augmentation procedures. However, surgeons may have attempted to use Cortoss “off-label” in procedures to repair vertebral compression fractures performed prior to the European Union’s approval of Cortoss for this type of procedure. Furthermore, not all surgeons have been trained in the proper use of Cortoss to repair vertebral compression fractures. A surgeon who has not been properly trained to use Cortoss in a procedure to repair vertebral compression fractures could pose a risk to the reputation of our Cortoss product. In addition, the occurrence of an adverse event while using our product “off-label” in procedures other than the repair of vertebral compression fractures or screw augmentation could adversely affect the reputation of Cortoss or any of our other products as well as us and could subject us to liability.
We could be negatively impacted by future interpretation or implementation of federal and state fraud and abuse laws, including anti-kickback laws and false claims laws.
We are subject to various federal and state laws pertaining to health care fraud and abuse, including anti-kickback laws and false claims laws. Violations of these laws are punishable by criminal, civil, and/or administrative sanctions, including, in some instances, imprisonment and exclusion from participation in federal and state health care programs, including Medicare, Medicaid, and veterans’ health programs. Although we seek to structure our business practices to be in compliance with applicable requirements, these laws are broadly written and broadly interpreted, and it is often difficult to determine precisely how these laws will be applied in specific circumstances.
Health care fraud and abuse regulations are complex, and even minor, inadvertent irregularities can potentially give rise to claims that the law has been violated. Any violations of these laws could result in a material adverse effect on our business, financial condition and results of operations. If there is a change in law, regulation or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which could have a material adverse effect on our business, financial condition and results of operations. Any state or federal review of us, regardless of the outcome, could be costly and time consuming.
We could become subject to false claims litigation under federal or state statutes, which can lead to civil money penalties, criminal fines and imprisonment, and/or exclusion from participation in federal health care programs. These false claims statutes include the federal False Claims Act, which allows any person to bring suit alleging the false or fraudulent submission of claims for payment under federal programs or other violations of the statute and to share in any amounts paid by the entity to the government in fines or settlement. Such suits, known asqui tam actions, have increased significantly in recent years and have increased the risk that companies like us may have to defend a false claim action. We could also become subject to similar false claims litigation under state statutes. If we are unsuccessful in defending any such action, such action may have a material adverse effect on our business, financial condition and results of operations.
The difficulties of operating in international markets may harm sales of our products.
Our sales outside the U.S. totaled $5.2 million in 2010. The international nature of our business subjects us and our representatives, agents and distributors to the laws and regulations of the jurisdictions in which they operate, and in which our products are sold and used. The types of risks that we face in international operations include:
| | • | | the imposition of governmental controls; |
| | • | | logistical difficulties in managing international operations; and |
| | • | | fluctuations in foreign currency exchange rates. |
Our international sales and operations may be limited or disrupted if we cannot successfully meet the challenges of operating internationally.
We may acquire technologies, products or businesses in the future, and these acquisitions could result in disruption of our business, increased costs and dilution to our shareholders.
An acquisition of assets, products or another business entails risks, uncertainties and potential disruptions to our business, and could divert management attention. We also could fail to assimilate the acquired business or company, which could lead to higher operating expenses. In addition, certain acquired technology could require significant additional development work and efforts to obtain regulatory clearance or approval, which could cause us to incur significant expense or to expend significant amounts of cash. Further, any acquired product may not provide the intended complementary fit with our existing product portfolio. Our shareholders could also be diluted if we issue shares of our stock to acquire another company, business or technology.
Our business could suffer if we cannot attract and retain the services of key employees.
We depend substantially upon the continued service and performance of our existing executive officers. We rely on key personnel in formulating and implementing our product research, development and commercialization strategies. Our success will
22
depend in large part on our ability to attract and retain highly skilled employees. We compete for such personnel with other companies, academic institutions, government entities and other organizations. We may not be successful in hiring or retaining qualified personnel. If one or more of our key employees resigns, the loss of that employee could harm our business. If we lose any key personnel, we may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by those former employees, or successfully prevent them from competing against us despite our use of confidentiality and non-compete agreements.
Negative economic conditions may adversely affect our sales, business or cash flows.
The current economic conditions and recent financial crisis affecting the banking system and financial markets have resulted in a tightening in the credit markets, a low level of liquidity in many financial markets, and extreme volatility in fixed income, credit and equity markets. The impact of these conditions could result in a number of follow-on adverse effects on our business, liquidity and results of operations, including but not limited to:
| | • | | the inability of one or more of our key suppliers to obtain credit to finance their operations or the insolvency of one or more of our key suppliers, which could adversely affect our product supply and reduce our sales; |
| | • | | the inability of customers to obtain credit to finance purchases of our products and/or customer insolvencies, which could reduce our sales and collectability of our receivables; |
| | • | | an unwillingness or inability of our current or potential business partners to continue existing collaborations or enter into transactions with us; |
| | • | | a negative impact on our current investment portfolio; and |
| | • | | our inability to raise funds through equity or debt financings that may be necessary in the future for various purposes, including acquisitions of products, technologies or other companies. |
There was a slowdown in the market during 2010 for spine procedures, which we believe contributed to lower sales growth for our products in 2010. Further, U.S. unemployment remains at relatively high levels. If patients become unemployed and lose health insurance previously obtained through their employers’ plans, or elect to defer undergoing surgery in a soft employment market, we could continue to see a decrease in elective surgeries that use our products, which would adversely affect our sales and could result in write offs of excess inventory and additional excess manufacturing capacity charges.
Health care reform legislation may adversely affect our business and financial position.
The United States enacted the Patient Protection and Affordable Care Act (PPACA) into law in 2010 in order to expand access to health insurance and reduce the cost of health care. The PPACA will require most individuals to have health insurance, establish new regulations on health plans, create insurance pooling mechanisms, and other expanded public health care measures, in addition to reducing Medicare spending on services provided by hospitals and other healthcare providers. An expansion in government’s role in the U.S. health care industry may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business. In addition, there have been and continue to be proposals by the federal government, state governments, regulators and third-party payors to control health care costs. Moreover, as discussed below, the PPACA would impose significant new taxes on medical device makers such as us. The implementation of the PPACA and other legislation may have a material adverse effect on our financial position and results of operations.
With respect to our industry in particular, the PPACA imposes a new 2.3 percent tax on the sale after December 31, 2012 of a taxable medical device by the manufacturer, producer or importer. This tax could negatively impact our cash flow from operations and delay our ability to become profitable or, if we become profitable, this tax could reduce our profitability.
RISKS RELATED TO OUR FINANCIAL RESULTS AND FINANCING
If our losses continue in the long term, they could limit our growth and jeopardize our viability.
We have experienced negative operating cash flows from inception through 2009 and experienced nominal positive operating cash flow in 2010. We have funded our operations primarily from proceeds received from sales of our securities and borrowings under a debt facility. In November and December 2006, we raised approximately $26.6 million in net proceeds from the sale of approximately 8.8 million shares of our common stock to institutional investors. In July 2007, we sold approximately 12.3 million shares of our common stock for net proceeds of approximately $32.2 million. In addition, in July 2007, we entered into a senior secured note purchase facility under which we have issued $35.0 million of our 10% senior secured notes due July 30, 2012. To date, we have not been profitable. We have incurred substantial operating losses since our inception and at December 31, 2010, had an accumulated deficit of $186.6 million. These losses have resulted principally from:
| | • | | the development and patenting of our technologies; |
23
| | • | | pre-clinical and clinical studies; |
| | • | | preparation of submissions to the FDA and foreign regulatory bodies; |
| | • | | the development of manufacturing, sales and marketing capabilities; and |
| | • | | the repurchase of a revenue interest from Royalty Securitization Trust I in July 2007. |
We may incur operating losses in the future unless or until we can consistently leverage our product portfolio, our direct sales force, our manufacturing capacity and our product development capability to generate product sales in amounts that exceed our cost of sales and operating expenses. We may never be able to achieve or maintain profitability in the future, and our products may never be commercially accepted or generate sufficient product sales to fund operations.
While we believe that our existing cash, cash equivalents, and short-term investments of $22.4 million as of December 31, 2010 together with expected positive cash flow from operations will be sufficient to meet our currently estimated operating and investing requirements for the foreseeable future, we will need to repay or refinance our outstanding senior secured notes in the aggregate principal amount of $35.0 million by July 30, 2012, as discussed above. We are currently seeking to refinance this obligation; however, there is no assurance that we will be able to execute this refinancing and, if we are able to refinance this indebtedness, that the terms of such a refinancing would be equal to or better than the terms of the existing senior secured note facility. In addition, we are required to pay a prepayment premium in the event that we refinance this obligation prior to July 30, 2012. The amount of the prepayment premium depends on the timing of the prepayment and decreases over time. Assuming we repaid this obligation in full on March 31, 2011, the prepayment premium would be approximately $1.9 million. Additionally, our cash flows and cash resources would be negatively impacted if we elected to repay the existing outstanding principal indebtedness with a combination of our existing cash and cash equivalents and new borrowings.
If we default under our senior secured note purchase facility, we may be required to repay amounts due under the facility, and we may incur additional interest and prepayment penalties. If we are unable to repay amounts due under the note facility, the lender could foreclose on certain assets that are essential to our operations.
We have $35.0 million in outstanding principal indebtedness under a senior secured note purchase facility. Notes issued under the facility are due July 30, 2012. We initially issued $25.0 million principal amount of our 10% senior secured notes under the facility and used most of the proceeds to repurchase a revenue interest obligation. On July 31, 2008 we issued an additional $10.0 million principal amount of notes under the facility primarily to fund our acquisition of the collagen raw material, equipment and technology license from Allergan. In connection with borrowings made under the facility, the note purchaser received five-year warrants, expiring in 2012, to purchase approximately 1.1 million shares of our common stock at an exercise price of $3.41 per share, which are currently exercisable and outstanding.
Borrowings under the facility are guaranteed by us and one of our wholly-owned subsidiaries. The facility is secured by a first priority lien on substantially all of our assets (including intellectual property) other than those exclusively related to Cortoss and Aliquot. We are required to make quarterly interest only payments to the note holder. Outstanding principal amounts under the notes bear annual interest at 10%, provided that interest would accrue at 12% per year during the continuance of any event of default and would be payable on demand. Based on the amount outstanding under the facility as of December 31, 2010, we will incur quarterly interest expense of $875,000 during 2011. The unpaid principal amount under the notes and accrued interest and all other obligations would become due and payable immediately if we are insolvent, are in bankruptcy proceedings or have a custodian or receiver appointed for any substantial part of our property. If an event of default not described in the preceding sentence occurs and is continuing (including a change of control of the Company), then the holders of at least two-thirds in principal amount of notes then outstanding may declare the unpaid principal amount of the notes, accrued interest and all other obligations due and payable immediately. In addition, if the notes become due and payable, whether automatically or by declaration, by reason of any of the following events of default, then we must pay a prepayment premium of up to approximately $1.9 million, depending on the timing of the prepayment:
| | • | | we fail to pay any principal on any note or prepayment premiums, if any, when due and payable; |
| | • | | we fail to pay any interest on any note or other amount (other than principal or prepayment premiums) for more than three business days after becoming due and payable; |
| | • | | we are insolvent, in bankruptcy proceedings or have a custodian or receiver appointed for any substantial part of our property; or |
| | • | | a change of control of our company occurs. |
Under the facility, we must comply with various financial and non-financial covenants. For example, we are required to maintain a minimum cash balance equal to at least 25% of the then outstanding principal amount under the notes in a separate account that is pledged as collateral for the loan. As of December 31, 2010, we must maintain a minimum cash balance of $8.75 million. In addition, if the balance in the separate account falls below an amount equal to 40% of the principal amount outstanding under the
24
notes, we must obtain and maintain for the benefit of favor of the note holder a letter of credit in the amount of 25% of the principal amount outstanding under the notes. As of December 31, 2010, we must maintain a minimum balance of $14 million in cash, cash equivalents, and short-term investments in order to avoid obtaining a letter of credit. Currently we are not required to obtain a letter of credit. The primary non-financial covenants limit our ability to incur indebtedness or liens, sell assets, conduct mergers or acquisitions, make investments and pay dividends.
As of December 31, 2010, we had $35.0 million outstanding under the facility, had accrued $875,000 in interest thereon, and were in compliance with all covenants under the facility. Based on our current operating plans, we expect to be in compliance with the covenants under the facility within the next twelve months from the date of filing of this report. However, if we default under the facility and cannot repay amounts then due, the lender could foreclose on the pledged assets, we could be forced into bankruptcy and we could be otherwise harmed.
We are currently seeking to refinance this obligation; however, there is no assurance that we will be able to execute this refinancing or, if we are able to refinance this indebtedness, that the terms of such a refinancing would be equal to or better than the terms of the current note purchase facility. In addition, we are required to pay a prepayment premium and we expect to recognize additional costs in the event that we refinance this obligation prior to July 30, 2012. The amount of the prepayment premium depends on the timing of the prepayment and decreases over time. At December 31, 2010, the prepayment premium would have been approximately $2.2 million. Assuming we repaid this indebtedness in full on March 31, 2011, the prepayment premium would be approximately $1.9 million. This prepayment premium would negatively impact our cash flows and cash resources. Additionally, our cash flows and cash resources would be negatively impacted if we elected to repay the existing outstanding principal indebtedness with a combination of our existing cash and cash equivalents and new borrowings. In addition to the prepayment premium, if we repay this indebtedness in full or in part prior to July 30, 2012, we would be required to write-off the unamortized debt discount and debt issuance costs remaining on our balance sheet at the date of such refinancing and recognize a non-cash loss. Assuming this debt was fully repaid on March 31, 2011, we would expect to recognize a charge of approximately $2.5 million in connection with the early extinguishment of debt.
We may seek to obtain additional funds through equity and or debt financings, or strategic alliances with third parties either alone or in combination. This could adversely affect our financial condition and cause dilution to our existing shareholders.
There are factors that may cause our future capital requirements to be greater than anticipated or could accelerate our need for funds including, without limitation:
| | • | | inability to increase sales of our cleared or approved products; |
| | • | | unforeseen difficulties in operating an effective direct sales and distribution network; |
| | • | | delayed, insufficient or lack of market acceptance of our products; |
| | • | | unanticipated expenditures in research and product development or manufacturing activities; |
| | • | | unforeseen changes in health care reimbursement for procedures using our products; |
| | • | | unforeseen developments during our pre-clinical and clinical trials; |
| | • | | unanticipated expenditures in the acquisition, enforcement and defense of intellectual property rights; |
| | • | | inability to train a sufficient number of surgeons to create demand for our products; |
| | • | | lack of financial resources to adequately support our operations; |
| | • | | difficulties in maintaining commercial scale manufacturing capacity and capability; |
| | • | | unforeseen problems with our third party manufacturers and service providers or with our specialty suppliers of certain raw materials; |
| | • | | delays in the timing of receipt of required regulatory approvals or clearances to make or sell our products; |
| | • | | unanticipated difficulties in operating in international markets; |
| | • | | the need to respond to technological changes and increased competition; |
| | • | | unforeseen problems in attracting and retaining qualified personnel to market our products; |
| | • | | enactment of new legislation or administrative regulation or changes in the regulatory climate affecting our products or the markets in which we compete; |
| | • | | the application to our business of new court decisions and regulatory interpretations; |
25
| | • | | claims that might be brought in excess of our insurance coverage; |
| | • | | any imposition of penalties for failure to comply with regulatory guidelines; and |
| | • | | management’s perception that uncertainties relating to these factors may be increasing. |
In addition, we may seek to expand our operations and product portfolio through acquisitions or joint ventures. Any such acquisitions or joint ventures may increase our capital requirements.
We cannot be certain that funding will be available on terms acceptable to us, if at all, particularly in light of current economic conditions. Any public or private financings may involve issuances of debt or common stock or other classes of our equity, which could further dilute existing shareholders’ percentage ownership. If we are unable to obtain funding to support operations, we may be forced to curtail our operations or be unable to develop products.
We are required annually, or on an interim basis as needed, to review the carrying value of our long-lived assets for impairment. Our long-lived assets consist of property and equipment and intangible assets. If sales of our products decline because of, for example, competition or an inability to manufacture sufficient supply of product, the value of our long-lived assets could become impaired.
As of December 31, 2010, we had approximately $16.8 million of property and equipment, net of accumulated depreciation, and $9.9 million of license and technology intangible assets, net of accumulated amortization. Our property and equipment consist primarily of assets used to manufacture Vitoss, Vitagel and Cortoss. Our intangible assets, net of accumulated amortization, consist of (i) $5.6 million for our prepayment in December 2006 of the profit-sharing license royalties on Vitagel and CellPaker, (ii) $3.9 million recorded for the value of the collagen-processing technology license acquired from Allergan in the third quarter of 2008 and (iii) $0.4 million recorded for the value of a license acquired from a business partner related to the development of a new product. If a change in circumstances causes us to lower our future sales forecast for our products, we may be required to write off a portion or all of the net book value of the affected property and equipment or intangible assets associated with that product. Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by comparing the carrying amount of the asset against the estimated undiscounted future cash flows expected to be generated by this asset. If the sum of the expected future net cash flows is less than the carrying value of the asset being evaluated, an impairment charge would be recognized. The impairment charge would be calculated as the amount by which the carrying value of the asset exceeds its fair value. An impairment charge to our net intangible assets, depending on the size, could result in a material adverse effect on our business, financial condition and results of operations.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY AND LITIGATION
Our business will be damaged if we are unable to protect our proprietary rights to our products, and we may be subject to intellectual property infringement claims by others.
We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, nondisclosure and confidentiality agreements and other contractual restrictions to protect our proprietary technology. However, these measures afford only limited protection and may not adequately protect our rights. For example, our patents may be challenged, invalidated or circumvented by third parties. As of March 1, 2011, we own or control 24 issued U.S. patents and 20 pending patent applications in the U.S., and counterparts of certain of these patents and pending patent applications worldwide, including Canada, Europe, Mexico, Israel and Japan. There can be no assurance that patents will issue from any of the pending patent applications. Moreover, we cannot be certain that we were the first creator of inventions covered by pending patent applications or we were the first to file patent applications for the relevant inventions for the following reasons:
| | • | | patent applications filed prior to December 2000 in the U.S. are maintained in secrecy until issued; |
| | • | | patent applications filed after November 2000 in the U.S. are maintained in secrecy until 18 months after the date of filing; and |
| | • | | the publication of discoveries in the scientific or patent literature tends to lag behind actual discoveries. |
If we do receive a patent, it may not be broad enough to protect our proprietary position in the technology or to be commercially useful to us. In addition, if we lose certain key personnel, we may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by those former employees. Furthermore, the laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. Even if our intellectual property rights are adequately protected, litigation or other proceedings may be necessary to enforce our intellectual property rights, which could result in substantial costs to us and divert management attention. If our intellectual property is not adequately protected, our competitors could use the intellectual property that we have developed to enhance their products and compete more directly with us, which could damage our business.
26
In addition to the risk of failing to adequately protect our proprietary rights, there is a risk that we may become subject to a claim that we infringe upon the proprietary rights of others. Although we do not believe that we are infringing the rights of others, third parties may claim that we are doing so. There is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry generally, and in the spinal market segments particularly. If the holder of a proprietary right brought an infringement action against us, the cost of litigating the claim could be substantial and divert management attention. In addition, if a court determined that one of our products infringed a proprietary right, we could be prevented from selling that product unless we could obtain a license from the owner of the right. Such a license may not be available on terms acceptable to us, if at all. Modification of our products or development of new products to avoid infringement may require us to conduct additional clinical trials for these modified or new products and to revise our filings with the FDA, which is time consuming and expensive. If we are not successful in obtaining a license or redesigning our product, our business could suffer.
We have obtained certain of our intellectual property rights and our rights to develop and commercialize certain products, and made certain commitments regarding our intellectual property and these rights, through license and development agreements with third parties. These agreements generally may be terminated by our counterparty if we materially breach any of our obligations under the agreement. We may disagree with these third parties as to our and their obligations under the agreements or the interpretation of provisions in the agreements. Any such disagreements could cause these third parties to seek to terminate our rights under the particular agreement. If any of our agreements were terminated, we could lose the rights to the product, product candidate or intellectual property covered by the agreement, reducing our revenue, potential revenues and significantly harming our business. Whether or not such a termination were proper, if a dispute arises under any of our agreements, the need to resolve the dispute could consume significant resources and divert management’s time and attention.
We distribute Vitasure, which we purchase from Medafor and is manufactured by third parties on behalf of Medafor. Medafor and these third parties have patents, licenses, and proprietary technologies related to Vitasure. We cannot be certain that no one will challenge the validity or enforceability of any patent that they own. We cannot be certain that if anyone does make such a challenge, that these third parties will be able to successfully defend that challenge, with or without our assistance. In addition, others may infringe these third parties’ patents or use their proprietary rights inappropriately. If others infringe patents owned or licensed by these third parties and related to Vitasure, we may choose to assist in bringing an infringement action and may incur substantial costs in any efforts to uphold the validity and prevent infringement of a patent or to protect proprietary technologies and methods. Furthermore, we cannot be certain that competitors will not independently develop similar technologies, duplicate technologies, design around the patented aspects of such technologies, or attempt to duplicate their proprietary technologies that have no patent protection. In addition, we cannot be sure that these third parties’ technologies will not infringe patents or other rights owned by others.
If we are sued in a product liability action, we could be forced to incur substantial defense costs, pay substantial damages and the attention of our management team may be diverted from operating our business.
We manufacture medical devices that are used on patients in surgery, and we may be subject to product liability lawsuits. In particular, the market for spine products has a history of product liability litigation. Under certain of our agreements with our distributors and hospital customers, we indemnify the distributor or hospital from product liability claims. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or the inability to secure coverage in the future. In addition, we could incur substantial costs to defend these claims and would have to pay any amount awarded by a court in excess of policy limits. We maintain product liability insurance in the annual aggregate amount of up to $20 million, although our insurance policies have various exclusions. Thus, we may be subject to a product liability claim for which we have no insurance coverage, in which case we may have to pay the entire amount of any defense costs, award or settlement. Even in the absence of a claim, our insurance rates may rise in the future to a point where we may decide to carry a lesser amount of this insurance or to not carry this type of insurance. A meritless or unsuccessful product liability claim would be time-consuming and expensive to defend and could divert management’s attention from our core business. A successful product liability claim or series of claims brought against us in excess of our coverage could have a material adverse effect on our business, financial condition and results of operations. Any product liability claim – even if unsuccessful – could adversely affect our reputation and otherwise adversely affect our business and the market for our common stock.
RISKS RELATED TO INVESTING IN OUR STOCK AND EQUITY MARKETS
Our stock price may be volatile for many reasons, including fluctuations in our results of operations due to factors out of our control.
The market price of our common stock may fluctuate substantially, which could adversely affect the value of an investment in our common stock or shareholders’ ability to sell our shares. Our stock price may fluctuate due to fluctuations in our results of operations or for other reasons. Future levels of our product sales and the timing of regulatory clearances and product introductions are difficult to predict, and product sales to date may not be indicative of future sales levels. We currently sell Vitagel and CellPaker under a license agreement, the termination of which would have a material adverse effect on our sales revenues. Our results of
27
operations may fluctuate significantly in the future as a result of a number of additional factors, many of which are outside of our control. These factors include, but are not limited to:
| | • | | the medical community’s acceptance of our products; |
| | • | | pricing pressures faced by our products and industry; |
| | • | | the public perception of our company and the reputation of our business practices and products; |
| | • | | maintaining adequate commercial-scale manufacturing capabilities; |
| | • | | disruptions with the manufacturing of our products, including with respect to our third party manufacturers and suppliers of raw materials; |
| | • | | difficulties in establishing and expanding our sales and distribution channels; |
| | • | | the mix of our products as profit margins differ between our products; |
| | • | | the timing of governmental approvals or clearances for our products and our competitors’ products; |
| | • | | the timing of product introductions by us or our competitors; |
| | • | | third party reimbursement of our products; |
| | • | | unanticipated events associated with clinical and pre-clinical trials of our products; |
| | • | | the success of competitive products; |
| | • | | our ability to enter into strategic alliances with other companies; |
| | • | | expenses associated with development and protection of intellectual property matters; |
| | • | | events affecting logistics and elective surgery trends; |
| | • | | the timing of expenses related to commercialization of new products; |
| | • | | competitive disruptions to our sales and distribution channels; |
| | • | | fluctuations in sales levels, which may be caused by seasonality of elective surgeries, the timing of orders from hospitals or distributor stocking orders, and other factors; |
| | • | | news concerning economic or market conditions in the general economy or within our industry, or the movement of capital into or out of our industry; |
| | • | | changes in U.S. or foreign government regulations impacting our industry; |
| | • | | the adequate training of a sufficient number of surgeons in the use of our products; and |
| | • | | the timing of governmental approvals or clearances for us to manufacture the products we sell. |
The results of our operations may fluctuate significantly from quarter to quarter and may not meet expectations of securities analysts and investors. This may also cause our stock price to be volatile.
In general, equity markets, including NASDAQ, have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These broad market fluctuations, including the following factors, may adversely affect the market price of our common stock:
| | • | | changes in the financial guidance we provide to the investment community; |
| | • | | changes in stock market analyst recommendations regarding our stock; |
| | • | | sales by existing holders of significant numbers of shares of our stock into the market, including sales by the three institutional investors that we believe held approximately one-third of our outstanding shares at or about December 31, 2010; |
| | • | | FDA and international regulatory actions regarding us or our competitors; |
| | • | | determinations by governments and insurance companies regarding reimbursement for medical procedures using our or our competitors’ products; |
| | • | | product sales growth rates; |
| | • | | public perception of our company and the reputation of our business practices and products; |
| | • | | developments with respect to patents or proprietary rights; |
| | • | | public concern as to the safety of products developed by us or by others; |
28
| | • | | changes in health care policy in the United States and internationally; |
| | • | | acquisitions or strategic alliances by us or our competitors; |
| | • | | business conditions affecting other medical device companies or the medical device industry generally; |
| | • | | general market conditions, particularly for companies with small market capitalizations; and |
| | • | | pending or threatened offers to acquire us. |
29
Provisions of Pennsylvania law or our Articles of Incorporation may deter a third party from seeking to obtain control of us or may affect your rights as a shareholder.
Certain provisions of Pennsylvania law could make it more difficult for a third party to acquire us, or could discourage a third party from attempting to acquire us. These provisions could limit the price that certain investors might be willing to pay in the future for shares of our common stock. In addition, our Articles of Incorporation enable our board of directors to issue up to 19,998,100 shares of preferred stock having rights, privileges and preferences as are determined by the board of directors. Accordingly, our board is empowered, without shareholder approval, to issue preferred stock with dividend, liquidation, conversion, voting or other rights superior to those of holders of our common stock. For example, an issuance of preferred stock could:
| | • | | adversely affect the voting power of the common shareholders; |
| | • | | make it more difficult for a third party to gain control of us; |
| | • | | discourage bids for our common stock at a premium; or |
| | • | | otherwise adversely affect the market price of our common stock. |
Future sales of our common stock in the public market could adversely affect our stock price.
We cannot predict the effect, if any, that future sales of our common stock or the availability for future sales of shares of our common stock or securities convertible into or exercisable for our common stock will have on the market price of our common stock prevailing from time to time.
As of December 31, 2010, we had outstanding options to purchase approximately 8.1 million shares of our common stock at a weighted average exercise price of $3.54 per share; restricted stock and restricted stock units granted to employees and/or directors under our equity compensation plan for an aggregate 0.2 million shares of our common stock; and warrants to purchase approximately 1.1 million shares of our common stock at an exercise price of $3.41 per share, which expire in July 2012. In order to attract and retain key personnel, we may issue additional securities, including stock options, restricted stock grants and shares of common stock, in connection with our equity compensation plan. The sale, or the availability for sale, of substantial amounts of common stock by our existing shareholders pursuant to an effective registration statement or under Rule 144, through the exercise of registration rights or the issuance of shares of common stock upon the exercise of stock options or warrants, or the perception that such sales or issuances could occur, could adversely affect the prevailing market prices for our common stock.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None
Our headquarters are located at the Great Valley Corporate Center in Malvern, Pennsylvania, which is a suburb of Philadelphia. We lease several buildings within the Great Valley Corporate Center as follows:
| | | | | | | | |
Purpose | | Approximate
Square Feet | | | Lease Expiration | |
Executive offices | | | 13,700 | | | | July 2017 | |
Manufacturing | | | 25,800 | | | | July 2017 | |
Processing facility for Vitagel collagen | | | 4,000 | | | | July 2017 | |
Warehouse, logistics and quality assurance | | | 36,500 | | | | July 2017 | |
Offices and research and development laboratories | | | 7,900 | | | | July 2017 | |
We believe our existing manufacturing facilities have the capacity to meet our commercial needs through at least 2013.
We conduct the majority of our international sales and marketing activities through administrative office space of approximately 1,733 square feet in Leuven, Belgium, which we have leased through May 2018.
None.
30
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is quoted on the NASDAQ Global Market (NASDAQ) under the symbol “VITA.” The following table reflects the ranges of high and low sale prices for our common stock as reported on NASDAQ for the stated periods.
| | | | | | | | |
| | | High | | | Low | |
Fiscal year 2010: | | | | | | | | |
First quarter | | $ | 4.33 | | | $ | 3.35 | |
Second quarter | | | 4.66 | | | | 2.03 | |
Third quarter | | | 2.30 | | | | 1.64 | |
Fourth quarter | | | 2.25 | | | | 1.86 | |
| | |
Fiscal year 2009: | | | | | | | | |
First quarter | | $ | 3.78 | | | $ | 2.47 | |
Second quarter | | | 6.10 | | | | 2.56 | |
Third quarter | | | 6.91 | | | | 4.09 | |
Fourth quarter | | | 4.46 | | | | 3.33 | |
As of March 3, 2011, there were 204 holders of record for shares of our common stock. Since a portion of our common stock is held in “street” or nominee name, we are unable to determine the exact number of beneficial holders. On March 3, 2011, the last sale price of our common stock as reported by NASDAQ was $2.40 per share. We have never declared or paid cash dividends on shares of our common stock and do not intend to pay any cash dividends in the foreseeable future on shares of our common stock. See Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder matters” included in this report for information regarding our equity compensation plans approved by security holders, which information is incorporated herein by reference.
No repurchases of equity securities were made by the Company in the quarter or year ended December 31, 2010.
| ITEM 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
The following table presents selected historical consolidated financial data derived from the consolidated financial statements of Orthovita, Inc. and subsidiaries as of and for each of the five years in the period ended December 31, 2010. This data should be read in conjunction with our consolidated financial statements, including notes thereto, and “MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION,” included in this report.
31
| | | | | | | | | | | | | | | | | | | | |
| | | | | | Year ended December 31 | | | | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | (In thousands except per share amounts) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Product sales | | $ | 94,663 | | | $ | 92,853 | | | $ | 76,915 | | | $ | 58,046 | | | $ | 46,828 | |
Cost of sales | | | 34,945 | | | | 29,914 | | | | 25,929 | | | | 20,544 | | | | 17,783 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 59,718 | | | | 62,939 | | | | 50,986 | | | | 37,502 | | | | 29,045 | |
Operating expenses | | | 61,757 | | | | 64,028 | | | | 60,170 | | | | 51,080 | | | | 46,006 | |
| | | | | | | | | | | | | | | | | | | | |
Operating loss | | | (2,039 | ) | | | (1,089 | ) | | | (9,184 | ) | | | (13,578 | ) | | | (16,960 | ) |
Interest expense, net of interest income | | | (3,591 | ) | | | (2,830 | ) | | | (1,506 | ) | | | (339 | ) | | | (503 | ) |
(Loss) gain on sale of product line and related assets | | | — | | | | — | | | | (72 | ) | | | 372 | | | | — | |
Charge for repurchase of revenue interest obligation | | | — | | | | — | | | | — | | | | (16,605 | ) | | | — | |
Income tax (expense) benefit | | | (76 | ) | | | 13 | | | | 10 | | | | 268 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Net loss | | $ | (5,706 | ) | | $ | (3,906 | ) | | $ | (10,752 | ) | | $ | (29,882 | ) | | $ | (17,464 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Net loss per share, basic and diluted | | $ | (0.07 | ) | | $ | (0.05 | ) | | $ | (0.14 | ) | | $ | (0.44 | ) | | $ | (0.33 | ) |
| | | | | | | | | | | | | | | | | | | | |
Shares used in computing basic and diluted net loss per share | | | 76,675 | | | | 76,176 | | | | 75,804 | | | | 67,181 | | | | 53,354 | |
| | | | | | | | | | | | | | | | | | | | |
| | | |
| | | | | | As of December 31 | | | | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | | | | | | | (In thousands) | | | | | | | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 22,364 | �� | | $ | 23,106 | | | $ | 32,291 | | | $ | 48,408 | | | $ | 28,339 | |
Accounts receivable, net | | | 11,877 | | | | 12,324 | | | | 10,881 | | | | 8,438 | | | | 8,755 | |
Inventories | | | 22,035 | | | | 26,058 | | | | 19,757 | | | | 15,612 | | | | 9,444 | |
Property and equipment, net | | | 16,809 | | | | 17,940 | | | | 14,438 | | | | 8,252 | | | | 5,295 | |
License and technology intangible assets, net | | | 9,884 | | | | 11,376 | | | | 12,354 | | | | 8,150 | | | | 9,000 | |
Total assets | | | 84,075 | | | | 92,629 | | | | 91,171 | | | | 90,217 | | | | 62,215 | |
Current liabilities | | | 7,700 | | | | 13,367 | | | | 11,410 | | | | 11,282 | | | | 8,164 | |
Notes payable, net of debt discount | | | 34,415 | | | | 34,095 | | | | 33,809 | | | | 23,895 | | | | 1,338 | |
Long-term liabilities | | | 387 | | | | 408 | | | | 310 | | | | 511 | | | | 9,451 | |
Total shareholders’ equity | | | 41,573 | | | | 44,759 | | | | 45,642 | | | | 54,529 | | | | 43,262 | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION |
UNDERSTANDING OUR FINANCIAL INFORMATION
The following discussion and analysis provides information management believes to be relevant in understanding our financial condition and results of operation. In addition to reading management’s discussion and analysis, you should review our consolidated financial statements and accompanying notes thereto as of December 31, 2010 and 2009 and for each of the years in the three-year period ended December 31, 2010, which begin on page F-1 of this report.
This discussion and analysis contains forward-looking statements that provide our current expectations, forecasts of future events or goals. You can identify these statements as those that do not relate strictly to historical or current facts. These statements could include words such as “may,” “will,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “seek” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. Any or all of our forward-looking statements in this Form 10-K may turn out to be incorrect. They can be affected by inaccurate assumptions we might
32
make, or by known or unknown risks and uncertainties. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially. There are many important factors that could cause actual events or results to differ materially from those expressed or implied by forward-looking statements including, without limitation, the development, demand, market acceptance and hospital approval of our products; third party reimbursement rates and health care reform; our ability to compete in increasingly price-sensitive markets; our ability to grow revenues and optimize our hybrid sales model to reach and maintain profitability; our ability to identify and obtain successful third-party distributors and sales agents and our ability to better leverage our existing sales and administrative infrastructures; our ability to manage our manufacturing facilities and increase utilization; our ability to forecast sales during difficult market conditions; the ability of our competitors to develop more technically advanced products; our ability to obtain and maintain regulatory approval of our products; the development of our sales network, sales product mix and related margins; capital expenditures; our ability to repay or refinance our existing debt; future liquidity; uses of cash; the achievement of product development goals and the amount and timing of related milestone and other payments; cost and availability of raw materials; inventory levels; development costs for existing and new products; equity compensation expense; foreign currency exchange rates; fluctuations in our stock price; and the other risk factors addressed in ITEM 1A. “RISK FACTORS” in this report.
We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised, however, to consult any further disclosures we make on related subjects in our filings with the U.S. Securities and Exchange Commission (the SEC). This discussion is provided as permitted by the Private Securities Litigation Reform Act of 1995.
EXECUTIVE LEVEL OVERVIEW
We are a specialty spine and orthopedic company with a portfolio of orthobiologic and biosurgery products based on novel and unique proprietary biomaterials that have innovative mechanisms of action in the body.
Our orthobiologic platform includes products for the fusion, regeneration and fixation of human bone. Our orthobiologic products are based on our proprietary Vitoss™ Bone Graft Substitute technology and include the Imbibe™ Bone Marrow Aspiration System. Vitoss is the market-leading synthetic bone graft in the United States and has been used in more than 325,000 implantations worldwide. Several of our Vitoss products incorporate our proprietary ceramic glass technology which accelerates bone healing. We continue to develop next generation Vitoss products which we believe will result in improved patient outcomes and maintain Vitoss average selling prices in an increasingly competitive market. In December 2010, the FDA granted regulatory clearance for our Vitoss Bioactive Foam-2X Bone Graft Substitute product, which has the same structure and porosity as Vitoss Bioactive products, but contains increased levels of bioactive glass. In February 2011, the FDA granted regulatory clearance for Vitoss BA Bimodal, another Vitoss product that differs from previous versions by incorporating varying size bioactive glass particles that accelerates the resorption of the bioactive glass. We launched Vitoss Bioactive Foam-2X in February 2011 and expect to launch Vitoss BA Bimodal by the first quarter of 2012.
Our orthobiologic products also include our Cortoss™ Bone Augmentation Material and the Aliquot™ Delivery System. These products were first available for sale in the U.S. in the second half of 2009. Cortoss is an advanced synthetic biomaterial which has regulatory clearance in the U.S. for both vertebroplasty and kyphoplasty. Although the Aliquot system is exempt from regulatory clearance in the U.S., it is currently designed to deliver Cortoss in vertebroplasty procedures only. Following injection into spinal vertebrae, Cortoss hardens to mimic weight-bearing, cortical bone. Cortoss is the first clinically-proven, injectable FDA-cleared alternative to polymethylmethacrylate (PMMA) bone cement for the treatment of vertebral compression fractures, an often extremely painful condition that occurs in patients with osteoporosis and cancer. In addition to extensive clinical data showing statistically significant improvements in pain relief (at three months following implantation) and function (at twenty-four months following implantation) from the use of Cortoss in comparison to PMMA, we believe that Cortoss has significant ease-of-use advantages over current vertebral augmentation technology. We believe that the U.S. launch of Cortoss and Aliquot has been negatively affected by several factors, including two small studies published inThe New England Journal of Medicinein August 2009 that questioned the effectiveness of vertebroplasty, a procedure in which Cortoss is used, by uncertainty created by the pending reimbursement review of vertebroplasty and kyphoplasty by a few of Medicare’s local contractors, and by the issuance of clinical practice guidelines in September 2010 by the American Academy of Orthopedic Surgeons (AAOS) that did not support the use of vertebroplasty to treat vertebral compression fractures. While these studies did not evaluate or discuss Cortoss and no reimbursement decisions have yet been announced, we believe that the publication of the studies, the ongoing reimbursement review and the AAOS guidelines have resulted in a notable decrease in the number of vertebroplasty procedures nationwide. We also believe that a recent shift for vertebral augmentation procedures from the hospital in-patient to out-patient settings has adversely affected our launch of Cortoss and Aliquot in the U. S. because the historic call pattern of our sales force is within the in-patient setting. The economic pressure causing this shift was exacerbated during 2009 and 2010 following the imposition of significant fines by the U.S. government on a number of hospitals that treated patients with vertebral compression fractures in the in-patient setting when these patients could have been treated more cost-effectively in the out-patient setting. As a result of these pressures, as well as negative overall economic conditions and health care reform initiatives to reduce costs, we believe the number of vertebral augmentation procedures performed by spine surgeons and
33
neurosurgeons, our primary target audience, has decreased, and a greater number of procedures are being performed in an out-patient setting by interventional radiologists and neuroradiologists, which is a new target physician group for us We also expect that the vertebral augmentation market will continue to face economic and other pressures at least in the near-term. We believe that these factors have resulted in U.S. Cortoss and Aliquot sales that are significantly below our initial expectations for the launch of these products. Prior to and following the launch of Cortoss and Aliquot and in anticipation of significant product demand, we acquired significant inventory quantities of Aliquot from a third party supplier with requisite long manufacturing lead times. Based upon the increasingly negative trends and developments related to vertebroplasty and current sales forecasts, during the fourth quarter of 2010, we recorded a $3.5 million inventory charge primarily for Aliquot inventory that either was expected to expire prior to sale or was not expected to be sold based on our current projections of product demand.
We are currently developing a suite of cavity creation devices that are designed to expand the use of Cortoss in the treatment of vertebral compression fractures for physicians who prefer kyphoplasty over vertebroplasty.
Over the longer term, we plan to seek U.S. regulatory clearance of Cortoss for additional indications that could expand its usage within the hospital in-patient surgery setting. These development efforts are currently ongoing and to date, we have submitted a
510(k) application to the FDA for the use of Cortoss in cranial defect repair.
Our biosurgery products include Vitagel™ Surgical Hemostat, Vitasure™ Absorbable Hemostat, and the CellPaker™ Plasma Collection System used in conjunction with Vitagel, and other accessories and delivery products that complement our Vitagel product. These products incorporate advanced biosurgical materials and product engineering to help control bleeding during surgeries. In an effort to increase our biosurgery sales, in 2011 we launched new marketing programs to promote the use of our Vitagel products in orthopedic joint reconstruction, obstetrics/gynecology and vascular surgery.
We sell our products worldwide and focus on the United States orthopedic and spine market. In the United States our products are sold through our direct sales force, third-party distributors and sales agents. Our “hybrid” distribution organization focuses its marketing efforts on orthopedic surgeons, interventional radiologists, neuroradiologists and hospital administrators. Our products can be used in hospital in-patient procedures, hospital out-patient procedures and ancillary surgical center procedures. Outside the United States we use a direct sales force to sell our products in the United Kingdom and third-party distributors to sell our products in other parts of the world.
We manufacture the majority of our products at our headquarters in Malvern, Pennsylvania. We are highly dependent on Kensey Nash Corporation (Kensey) as a sole source provider of certain raw materials used in the manufacture of Vitoss Foam products. We co-developed the Vitoss Foam platform with Kensey and our cost of goods sold includes royalty expense to Kensey. We obtain from a limited number of other third party manufacturers additional raw materials and a majority of the disposable components that accompany and are used with our products. We expect to continue to use third party manufacturers for these elements in order to reduce costs and increase product gross margins.
We manage our orthobiologic and biosurgery platforms as a single business unit. These platforms are marketed and sold by a single sales force to the same customer base. Accordingly, we report our financial results under a single operating segment – the development, manufacture and sale of specialty spine and orthopedic products.
In addition to the impact of theNew England Journal of Medicine articles and reimbursement review discussed above, our business has been negatively affected by macroeconomic and other key factors that could continue to affect us in 2011 and beyond:
| | • | | Continued high unemployment and the slow economic recovery have resulted in a drop in spine procedures nationwide, which has contributed to a decline in Vitoss revenues. |
| | • | | Increased focus on the general cost of healthcare has triggered notable changes in payer and hospital behavior. Payers and hospitals are becoming increasingly price sensitive and are aggressively seeking price concessions and allowances and, in some cases, seek lower cost alternatives to our products. This price pressure has and will continue to place pressure on our average selling prices. |
| | • | | There has been a significant shift of vertebral augmentation procedures from the hospital in-patient to out-patient settings, which adversely affected our launch of Cortoss and Aliquot in the U. S. The economic pressure causing this shift was exacerbated during 2009 and 2010 following the imposition of significant fines by the U.S. government on a number of hospitals that treated patients with vertebral compression fractures in the in-patient setting when these patients could have been treated more cost-effectively in the out-patient setting. As a result of these pressures, we believe the number of vertebral augmentation procedures performed by spine surgeons and neurosurgeons, our primary target audience, has decreased, and a greater number of procedures are being performed in an out-patient setting by interventional radiologists and neuroradiologists, which is a new target physician group for us. We believe |
34
| | that our initial efforts to reach this new target group with our existing sales force requires significantly more effort on the part of our sales force and, during 2009 and 2010, diluted our efforts to sell Vitoss in the in-patient market. |
| | • | | We may face increasing competition from physician-owned distributors, or PODs. PODs are entities in the medical device supply chain that derive a proportion of their revenue from selling or arranging for the sale of medical devices ordered by physician-investors for use in procedures they perform on their own patients at hospitals that agreed to purchase from or through the POD, or that otherwise furnish ordering physicians with income that is based directly or indirectly on those orders of medical devices. We believe that the number of PODs in the spine and orthopedic markets will continue to grow as economic pressures increase through the industry, hospitals, insurers and physicians search for ways to reduce costs, and medical device manufacturers seek ways to maintain or grow market share. |
We continue to optimize our hybrid sales force in order to proactively respond to these factors by (i) augmenting our direct sales efforts with distributors and/or sales agents that specialize in the out-patient market or who are particularly strong in a particular region of the country or with particular customers; (ii) streamlining our direct sales force to achieve profitability of our direct sales force, and (iii) developing next generation products to improve patient outcomes and broaden the market opportunities for our products. Our goal is to regain sales momentum, grow our Vitoss platform, increase penetration of Cortoss and grow our biosurgery product sales. There are many factors that will affect our ability to achieve these objectives and there is no guarantee that we will be able to achieve any of these goals. Some of these risks are contained in the forward-looking statements overview at the introduction of this discussion. Other risk factors are addressed in ITEM 1A. “RISK FACTORS” in this report.
We remain committed to our mission to develop and market quality products backed by clinical research that will improve patient outcomes and meet the needs of our surgical customers with maximum effectiveness and safety. Our strategy to accomplish this is threefold: (i) maximize the growth of existing products by continuing to optimize our direct sales force, develop additional clinical data and manage the lifecycles of product portfolios; (ii) continue product pipeline development of our own internally developed products; and (iii) pursue in-licensing, joint venture and acquisition opportunities to augment our product portfolio.
You should also read the additional detailed commentary of product sales, gross profit, operating expenses and other analyses of the year ended December 31, 2010 compared to 2009, as well as similar comparisons for the year ended December 31, 2009 to 2008 contained later in this discussion.
Summary Full Year 2010 Financial Results
Product sales for 2010 increased to $94.7 million from $92.9 million in 2009, an increase of 2%. This increase was primarily due to higher sales of Vitoss Bioactive Foam and our Biosurgery products, and a full year of U.S. Cortoss and Aliquot sales (which we launched in July 2009); these increases were offset partially by lower sales of other Vitoss products.
Gross profit for 2010 was $59.7 million, or 63% of product sales, compared to $62.9 million, or 68% of product sales, for 2009. This decrease was primarily attributable to a $3.5 million inventory reserve charge recorded in the fourth quarter of 2010 principally related to Aliquot inventory which either was expected to expire prior to its anticipated sale or was not expected to be sold based upon current forecasts of product demand. Also contributing to the lower gross profit in 2010 as compared to 2009 were higher excess manufacturing capacity charges. Excess manufacturing capacity charges result when actual inventory production is below normal productive capacity.
In June 2010, we implemented a headcount reduction and selectively reduced certain discretionary expenses, which resulted in lower operating expenses in 2010 compared to 2009. Operating expenses for 2010 were $61.8 million, or 65% of product sales, as compared to $64.0 million, or 69% of product sales for 2009. As a percentage of sales, each of the categories of operating expenses shown in the table below has been declining or remaining constant.
35
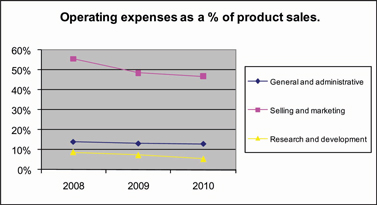
We expect operating expenses as a percentage of sales to decline in future periods as we succeed in leveraging our existing sales and administrative infrastructure to generate increased product sales, optimizing our hybrid sales model in order to improve productivity and to reach and maintain profitability, and continuing to manage all other expenditures.
Please read the additional detailed commentary contained later in this discussion of product sales, gross profit, operating expenses and other analyses of 2010 compared to 2009, as well as similar comparisons for 2009 versus 2008.
CRITICAL ACCOUNTING POLICIES
The preparation of the consolidated financial statements requires us to make assumptions, estimates and judgments that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates, including, but not limited to, those related to accounts receivable, inventories and realizability of long-lived assets. We use historical experience and other assumptions as the basis for making estimates. Illiquid credit markets, volatile equity, foreign currency and energy markets, and declines in consumer spending have combined to increase the uncertainty inherent in such estimates and assumptions. As future events and their effects cannot be determined with precision, actual results could differ significantly from these estimates. Changes in those estimates will be reflected in the financial statements in those future periods. The critical accounting policies addressed below have been reviewed with the Audit Committee of our Board of Directors and reflect our most significant judgments and estimates used in the preparation of our consolidated financial statements.
The list below is not intended to be a comprehensive list of all our accounting policies. See our consolidated financial statements and notes thereto included in this report which contains accounting policies and other disclosures required by GAAP.
Revenue Recognition
Revenue is not recognized until persuasive evidence of an arrangement exists, performance or shipment has occurred, the sales price is fixed or determinable and collection is probable. In the U.S. and the U.K., we recognize product sales revenue upon either shipment of purchased product to the customer, or receipt of documentation from the customer indicating its consumption of product from consigned inventory. Outside the U.S. and the U.K., revenue from product sales is recognized upon shipment of the product to the independent stocking distributors.
We have no obligations to our customers once title has been transferred but allow the return or exchange of ambient temperature products within five days after their original delivery. Subsequent to the end of every quarter we adjust sales, cost of sales, commission expense, inventory, accounts receivable and accrued commission expense for the effect of such returns. We generally require each of our U.S. and U.K. customers to pay on a net 30-day basis. Outside of the U.S. and the U.K., independent stocking distributors are generally required to pay on a net 60-day basis.
Allowance for Doubtful Accounts
We maintain an accounts receivable allowance for an estimated amount of losses that may result from a customer’s failure to pay for product purchased. The allowance for doubtful accounts is reviewed quarterly and is estimated based on historical bad debts, customer concentrations, known trends with current customers, and changes in customer payment patterns. If the financial condition
36
of our customers or the overall condition of the health care industry were to deteriorate and result in an impairment of our customers’ ability to make payments, additional allowances could be required.
Inventories
Inventories are stated at the lower of cost or market value using the first-in first-out basis, or FIFO and consist of raw materials, Kensey raw material conversion costs, labor and overhead. The majority of our inventories are subject to expiration dating. We continually evaluate the carrying value of our inventories, and when, in the opinion of management, factors indicate that impairment has occurred, either a reserve is established against the inventories’ carrying value to reduce inventory costs to their net realizable values or the inventories are completely written off. We base these decisions on the level of inventories on hand in relation to our estimated forecast of product demand, production requirements over the next twelve months and the expiration dates of raw materials and finished goods. The cost of producing inventory in the reporting periods prior to the receipt of regulatory approval or clearance are recorded as research and development expense.
Income Taxes
We account for income taxes in accordance with an asset and liability approach requiring the recognition of deferred tax assets and liabilities for the expected tax consequences of events that have been recognized in the financial statements or tax returns. Deferred tax assets and liabilities are recorded without consideration as to their ability to be realized. The deferred tax asset includes net operating loss and credit carryforwards, and the cumulative temporary differences related to certain research and patent costs, which have been charged to expense in our consolidated statements of operations but have been recorded as assets for tax return purposes. The portion of any deferred tax asset, for which it is more likely than not that a tax benefit will not be realized, must then be offset by recording a valuation allowance against the asset.
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. With the exception of the Texas margin tax credit described in note 15 to our consolidated financial statements included in this report, management believes it is more likely than not that we will not realize the deferred tax assets in excess of deferred tax liabilities, and as such, a full valuation allowance is maintained against the net deferred tax assets in excess of the Texas Margin tax credit.
While we believe that our tax positions are fully supportable, there is a risk that certain positions could be challenged successfully. In these instances, we look to establish reserves. If we determine that a tax position is more likely than not of being sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that has likelihood greater than 50% of being realized upon settlement. We presume that all tax positions will be examined by a taxing authority with full knowledge of all relevant information. We regularly monitor our tax positions, tax assets and tax liabilities. We reevaluate the technical merits of our tax positions and recognize an uncertain tax benefit or derecognize a previously recorded tax benefit when (i) there is a completion of a tax audit, (ii) there is a change in applicable tax law including a tax case or legislative guidance, or (iii) there is an expiration of the statute of limitations. Significant judgment is required in accounting for tax reserves.
Stock-based Compensation
We measure all employee stock-based compensation awards using a fair value method and record such expense in our consolidated financial statements. Such expense is amortized on a straight line basis over the requisite service period of the award.
We estimate the grant date fair value of stock options using the Black-Scholes option-pricing model which requires the input of highly subjective assumptions. These assumptions include estimating the expected term of the award and the estimated volatility of our stock price over the expected term. Changes in these assumptions and in the estimated forfeitures of stock option awards may materially affect the amount of stock-based compensation recognized in our consolidated statements of operations.
Long-Lived Assets and Intangible Assets
We test all long-lived assets and intangible assets for recoverability whenever events or changes in circumstances indicate that we may not be able to recover an asset’s carrying amount. We evaluate the recoverability of an asset by comparing its carrying amount to the undiscounted cash flows expected to result from the use and eventual disposition of that asset. If the undiscounted cash flows are not sufficient to recover the carrying amount, we measure any impairment loss as the excess of the carrying amount of the asset over its fair value. Events which could trigger asset impairment include significant underperformance relative to historical or projected future operating results, significant changes in the manner or use of an asset or in our overall business strategy, significant negative industry or economic trends, shortening of product life-cycles, negative changes in third party reimbursement, or changes in technology. We currently believe the future cash flows to be received from all long-lived assets will exceed their carrying value, and
37
accordingly we have not recognized any impairment losses for the years ended December 31, 2010, 2009 or 2008. Any unanticipated significant impairment in the future, however, could have a material adverse impact to our balance sheet and future operating results.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
In January 2010, the Financial Accounting Standards Board (FASB) issued amended guidance requiring additional fair value disclosures related to inputs and valuation techniques used to measure fair value as well as disclosures about significant transfers between levels in the hierarchy of fair value measurement. This guidance became effective for us beginning on January 1, 2010 and did not have a significant impact on our consolidated financial statements.
38
RESULTS OF OPERATIONS
We have incurred annual operating losses since inception. We may continue to incur operating losses in the future unless or until we can consistently leverage our product portfolio, our sales and administrative infrastructure, our manufacturing capacity and our product development capability to generate product sales in amounts that exceed our cost of sales and operating expenses. Our ability to increase revenue and to attain profitability are affected by the risk factors addressed in ITEM 1A. “RISK FACTORS” in this report. The following information summarizes our results of operations for the years ended December 31, 2010, 2009 and 2008:
Comparison of the Year Ended December 31, 2010 to the Year Ended December 31, 2009
Product Sales
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
Orthobiologics | | $ | 71,773 | | | $ | 71,297 | | | | 1 | % |
Biosurgery | | | 22,890 | | | | 21,556 | | | | 6 | % |
| | | | | | | | | | | | |
| | | |
| | $ | 94,663 | | | $ | 92,853 | | | | 2 | % |
| | | | | | | | | | | | |
Product sales increased $1.8 million, or 2%, to $94.7 million in 2010, from $92.9 million in 2009. For the year ended December 31, 2010, 95% of product sales were in the U.S. and 5% were outside the U.S. as compared with 94 % and 6 %, respectively, in 2009.
Orthobiologic product sales represented 76% and 77 % of total product sales in 2010 and 2009, respectively. Orthobiologic product sales increased $0.5 million, or 1%, to $71.8 million in 2010, from $71.3 million in 2009. Orthobiologic product sales for 2010 included $4.0 million of U.S. product sales of Cortoss and Aliquot as compared to $1.1 million in 2009. Cortoss was launched in the United States in the second half of 2009. Orthobiologic product sales for 2010 and 2009 included $1.0 million and $0.6 million, respectively, from our sale under a supply agreement of Vitomatrix material used in the manufacture of dental implants. This supply agreement was terminated in March 2010, and no further sales relating to this agreement are expected. Excluding the Vitomatrix sale, Orthobiologic sales were $70.7 million in both 2010 and 2009, with a 6% increase in average selling price offset by a 6% decrease in unit volume in 2010 versus 2009.
Vitoss product sales in 2010 were 4% lower than in 2009. Within the Vitoss product family, sales of Vitoss Bioactive Foam, our Vitoss product launched in 2008 that accounted for 63% of total Vitoss sales, increased 8% in 2010 compared to 2009, but this increase was more than offset by decreases in sales of Vitoss Foam products and Vitoss morsels. Sales of Vitoss products have been, and may continue to be, negatively affected by the current economic recession and high unemployment, the associated reduction of surgical procedures traditionally using Vitoss products and increasing efforts by hospitals to contain and reduce costs. We continue to manage the Vitoss product life cycle by developing next generation Vitoss products. Vitoss Bioactive Foam-2X and Vitoss Bimodal received FDA clearance in December 2010 and February 2011, respectively. We launched Vitoss Bioactive Foam-2X in the United States in February 2011 and expect to launch Vitoss Bimodal in the United States by the first quarter of 2012.
Biosurgery product sales represented 24% and 23% of total product sales for 2010 and 2009, respectively. Biosurgery product sales increased $1.3 million, or 6%, to $22.9 million in 2010, from $21.6 million, primarily due to higher Vitagel sales with 5% attributable to higher average selling prices and 1% due to increased unit volume.
Gross Profit
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
Gross profit | | $ | 59,718 | | | $ | 62,939 | | | | (5 | )% |
Gross profit as a percentage of product sales | | | 63 | % | | | 68 | % | | | | |
Gross profit was $59.7 million, or 63% of product sales, for 2010 and $62.9 million, or 68% of product sales, for 2009. The decrease in gross profit and gross profit as a percentage of product sales in 2010 from 2009 is primarily due to increased inventory reserve and excess manufacturing capacity charges. Inventory reserve charges were $4.1 million in 2010 compared to $0.4 million in 2009; excess manufacturing capacity charges were $1.6 million in 2010 compared to $0.3 million in 2009. The inventory reserve
39
charge of $4.1 million included a fourth quarter charge of $3.5 million primarily for Aliquot inventory that was expected either to expire prior to sale or to remain unsold based upon current forecasts of product demand. Aliquot is used to deliver Cortoss in vertebroplasty procedures. As mentioned above under EXECUTIVE LEVEL OVERVIEW, in connection with the U.S. launch of Cortoss in the second half of 2009, we purchased significant quantities of Aliquot raw materials from a third party supplier, which had long manufacturing lead times, in anticipation of significant demand for Cortoss. Sales of Cortoss have been significantly lower than anticipated and have resulted in the inventory reserve charge described above.
Our gross profit may fluctuate from quarter to quarter based on excess manufacturing capacity charges and inventory reserve charges, as well as the mix of products sold from period to period.
Operating Expenses
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
Operating expenses | | $ | 61,757 | | | $ | 64,028 | | | | (4 | )% |
Operating expenses as a % of product sales | | | 65 | % | | | 69 | % | | | | |
Operating expenses decreased $2.2 million, or 4%, to $61.8 million in 2010, from $64.0 million in 2009. Operating expenses as a percentage of product sales were 65% and 69% for 2010 and 2009, respectively. The decrease is primarily due to lower selling and marketing expenses (including lower commission expense) and lower research and development expenses, partially offset by higher general and administrative expenses.
General and administrative expense
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
General and administrative expense | | $ | 12,240 | | | $ | 12,172 | | | | 1 | % |
General and administrative expense as a % of product sales | | | 13 | % | | | 13 | % | | | | |
General and administrative expenses increased less than $0.1 million, or 1%, in 2010 as compared to 2009. The slight increase was primarily due to increased expenditures to provide a sustainable information technology infrastructure to support current and expected future sales growth. As a percentage of product sales, general and administrative expenses were 13% for each of 2010 and 2009.
Selling and marketing expense
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
Selling and marketing expense | | $ | 44,359 | | | $ | 45,070 | | | | (2 | )% |
Selling and marketing expense as a % of product sales | | | 47 | % | | | 49 | % | | | | |
Selling and marketing expenses decreased $0.7 million, or 2%, to $44.4 million in 2010, from $45.1 million in 2009. As a percentage of product sales, selling and marketing expenses were 47% and 49% in 2010 and 2009, respectively. At December 31, 2010, our direct sales force was composed of 84 sales representatives, while at December 31, 2009, our direct sales force was composed of 103 sales representatives. This decrease in the number of direct sales representatives reflects our effort to optimize our hybrid sale model and to use distributors and sales agents where appropriate, and resulted in lower personnel–related expenditures in 2010 compared to 2009. Also contributing to lower selling and marketing expense in 2010 compared to 2009 was a lower overall commission rate. These lower expenditures for personnel and commissions were partially offset by increased expenditures for post-approval marketing studies, which primarily related to our Vitoss products. Selling and marketing expenses for 2009 also included costs associated with the launch of Cortoss in the U. S. in the second half of 2009. We expect that selling and marketing expenses as a percentage of product sales will decrease over time as we increase product sales and further optimize our hybrid sales model.
40
Research and development expense
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
Research and development expense | | $ | 5,158 | | | $ | 6,786 | | | | (24 | )% |
Research and development expense as a % of product sales | | | 5 | % | | | 7 | % | | | | |
Research and development decreased $1.6 million, or 24%, to $5.2 million in 2010, from $6.8 million in 2009, primarily attributable to lower Cortoss and certain other product development costs partially offset by a $0.5 million write-off relating to a prepayment in connection with a development project where the supplier was unable to satisfy its obligations under our agreement. Nevertheless, we anticipate continuing the development of this product with another party. Research and development expenses were 5% and 7% of product sales for 2010 and 2009, respectively.
Other income (expense), net
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2010 | | | 2009 | | | % Change | |
Other income (expense), net | | $ | (3,591 | ) | | $ | (2,830 | ) | | | 27 | % |
Other income (expense), net includes interest expense and interest income. We recorded ($3.6) million and ($2.8) million of other income (expense), net for 2010 and 2009, respectively. Other income (expense), net for 2010 was higher than in 2009 primarily due to higher interest expense in 2010 than in 2009 as we capitalized more interest in 2009 as compared to 2010. Interest was capitalized as part of the construction of our collagen production facility which became operational in the third quarter of 2010. Also contributing to the higher other income (expense), net in 2010 compared to 2009 was lower interest income primarily resulting from lower interest rates combined with lower cash, cash equivalents and short-term investment balances in 2010.
Comparison of the Year Ended December 31, 2009 to the Year Ended December 31, 2008
Product Sales
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
Orthobiologics | | $ | 71,297 | | | $ | 58,170 | | | | 23 | % |
Biosurgery | | | 21,556 | | | | 18,745 | | | | 15 | % |
| | | | | | | | | | | | |
| | | |
| | $ | 92,853 | | | $ | 76,915 | | | | 21 | % |
| | | | | | | | | | | | |
Product sales increased $15.9 million, or 21%, to $92.9 million in 2009, from $76.9 million in 2008. Orthobiologic product sales and biosurgery product sales increased 23% and 15%, respectively. For both 2009 and 2008, 94% of product sales were in the United States and 6% were outside the U.S. Approximately 67% of our product sales during 2009 were from products based upon our Vitoss Foam platform co-developed with Kensey, including Vitoss Bioactive Foam.
Orthobiologic product sales represented 77% of total product sales in 2009 compared to 76% in 2008. Orthobiologic product sales increased $13.1 million, or 23%, to $71.3 million in 2009, from $58.2 million in 2008, primarily due to higher sales of Vitoss Bioactive Foam products, which we launched in 2008. Sales of Cortoss in the U.S. also contributed to the higher orthobiologic product sales in 2009. We began to sell Cortoss in the U.S. in the second half of 2009. Sales of Cortoss and Aliquot in the United States were $1.1 million in 2009.
Biosurgery product sales represented 23% of total product sales in 2009 compared to 24% in 2008. Biosurgery product sales
41
increased $2.8 million, or 15%, to $21.6 million in 2009 from $18.7 million in 2008, with the increase primarily due to an increase in Vitagel sales.
Gross Profit
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
Gross profit | | $ | 62,939 | | | $ | 50,986 | | | | 23 | % |
Gross profit as a percentage of product sales | | | 68 | % | | | 66 | % | | | | |
Gross profit for 2009 and 2008 was $62.9 million and $51.0 million, respectively. As a percentage of product sales, gross profit was 68% for 2009, compared to 66% for 2008. Gross profit increases were seen in both the orthobiologic and biosurgery product families and primarily reflect more favorable product mix and successful efforts to reduce the cost of disposable and plastic components that are included in our product kits. Our gross profit may fluctuate from quarter to quarter based on the mix of products sold from period to period.
Operating expenses
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
Operating expenses | | $ | 64,028 | | | $ | 60,170 | | | | 6 | % |
Operating expenses as a % of product sales | | | 69 | % | | | 78 | % | | | | |
Operating expenses increased $3.8 million or 6%, to $64.0 million in 2009 from $60.2 million in 2008. Operating expenses as a percentage of product sales declined to 69% in 2009 from 78% in 2008. All components of operating expenses as a percentage of product sales have been decreasing as we leverage our infrastructure to generate incremental sales. The increase in operating expenses for 2009 over 2008 was due to higher commissions associated with increased product sales, infrastructure expansion expenses, and increased marketing costs related to the launch of Cortoss in the U. S., partially offset by more effective cost management of certain sales force discretionary spending.
General and administrative expense
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
General and administrative expense | | $ | 12,172 | | | $ | 10,753 | | | | 13 | % |
General and administrative expense as a % of product sales | | | 13 | % | | | 14 | % | | | | |
General and administrative expenses increased $1.4 million, or 13%, to $12.2 million in 2009, from $10.8 million in 2008. The increase is primarily due to additional finance, human resources and information technology staff required to provide sustainable infrastructure for future sales growth. Expenses for 2009 also included severance costs of $0.4 million associated with the departure of a senior executive officer. As a percentage of product sales, general and administrative expenses were 13% of product sales in 2009 compared to 14% of product sales in 2008
42
Selling and marketing expense
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
Selling and marketing expense | | $ | 45,070 | | | $ | 42,706 | | | | 6 | % |
Selling and marketing expense as a % of product sales | | | 49 | % | | | 56 | % | | | | |
Selling and marketing expenses increased $2.4 million, or 6%, to $45.1 million in 2009, from $42.7 million in 2008. Higher product sales commissions combined with costs associated with the launch of Cortoss in the U. S. were partially offset by reduced sales force discretionary spending and lower recruiting costs. Our field sales team increased to 111 employees at December 31, 2009, of which 90 were direct sales representatives, 13 were associate sales representatives and 8 were managers, from 96 employees at December 31, 2008, of which 76 were direct sales representatives, 11 were associate sales representatives and 9 were managers. As a percentage of product sales, selling and marketing expenses were 49% of product sales in 2009 compared to 56% of product sales in 2008.
Research and development expense
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
Research and development expense | | $ | 6,786 | | | $ | 6,711 | | | | 1 | % |
Research and development expense as a % of product sales | | | 7 | % | | | 9 | % | | | | |
Research and development expenses increased $0.1 million, or 1%, to $6.8 million in 2009, from $6.7 million in 2008. The increase for 2009 was due to increased next generation and new product development costs offset by lower Cortoss development and clinical trial costs. As a percentage of product sales, research and development expenses were 7% of product sales in 2009 compared to 9% of product sales in 2008.
Other income (expense), net
| | | | | | | | | | | | |
| | | December 31, | | | | |
| (dollars in thousands) | | 2009 | | | 2008 | | | % Change | |
Other income (expense), net | | $ | (2,830 | ) | | $ | (1,578 | ) | | | 79 | % |
Other income (expense), net, includes interest income, interest expense and gain or loss on asset disposals. We recorded ($2.8) million and ($1.6) million of other income (expense), net for 2009 and 2008, respectively. Other income (expense), net for 2009 was higher than in 2008 primarily due to lower interest income resulting from lower interest rates and lower cash, cash equivalents and short-term investment balances in 2009, combined with higher interest expense in 2009 than in 2008. The higher 2009 interest expense was due to a higher level of borrowings outstanding for a longer period of time during 2009 as compared to 2008.
LIQUIDITY AND CAPITAL RESOURCES
We experienced negative annual operating cash flows from our inception through 2009 and we have funded our operations primarily from the proceeds we received from sales of our common stock and other debt and equity securities. For 2010, we had positive cash flow from operations of $0.2 million. At December 31, 2010 and 2009, key liquidity indicators were as follows:
| | | | | | | | |
| | | December 31, | |
| (dollars in thousands) | | 2010 | | | 2009 | |
Cash and cash equivalents | | $ | 11,059 | | | $ | 7,757 | |
Short-term investments | | | 11,305 | | | | 15,349 | |
| | | | | | | | |
Total cash, cash equivalents and short-term investments | | $ | 22,364 | | | $ | 23,106 | |
| | | | | | | | |
Working capital | | $ | 49,200 | | | $ | 48,905 | |
| | | | | | | | |
Notes payable | | $ | 34,415 | | | $ | 34,095 | |
| | | | | | | | |
43
The Company’s cash flow activity was as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (Dollars in thousands) | |
Net cash provided by (used in) operating activities | | $ | 186 | | | $ | (5,064 | ) | | $ | (14,210 | ) |
Net cash provided by investing activities | | | 2,314 | | | | 2,813 | | | | 2,720 | |
Net cash provided by financing activities | | | 920 | | | | 1,446 | | | | 10,196 | |
Effect of exchange rate changes on cash and cash equivalents | | | (118 | ) | | | 44 | | | | (374 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | $ | 3,302 | | | $ | (761 | ) | | $ | (1,668 | ) |
| | | | | | | | | | | | |
Although we expect to generate positive cash flows from operations in 2011, our ability to consistently generate positive cash flows is heavily dependent upon: (i) maintaining and growing product sales; (ii) our product sales mix as relative increases in sales of our lower margin products result in lower gross profit; (iii) the amount of inventory, including raw materials and work-in-process, that we maintain to support product sales, anticipated product sales and anticipated product launches; (iv) the overall level of our research and development activities, which will depend on the development status and costs of products in our pipeline and any new products that we could pursue in the future, and (v) costs associated with the refinancing of our existing $35 million debt. Accordingly, for the foreseeable future, our operating cash requirements may continue to be subject to quarterly volatility. If we are unable to improve cash flows from operations, we may need to consider and evaluate alternatives including reduction of capital expenditures and other cost reductions, and will need to refinance or renegotiate the terms of our outstanding debt. Additionally, cash resources could be affected by in-licensing, joint ventures, acquisitions and other investment opportunities that we may pursue.
We have $35 million in outstanding principal indebtedness under a senior secured note facility which is due July 30, 2012. We are currently seeking to refinance this obligation; however, there is no assurance that we will be able to execute this refinancing and, if we are able to refinance this indebtedness, that the terms of such a refinancing would equal to or better than the terms of the existing senior secured note facility. In addition, we are required to pay a prepayment premium in the event that we refinance this obligation prior to July 30, 2012. The amount of the prepayment premium depends on the timing of the prepayment and decreases over time. Assuming we repaid this obligation in full on March 31, 2011, the prepayment premium would be approximately $1.9 million. This prepayment premium would negatively impact our cash flows and cash resources. In addition to the prepayment premium, if we repay this indebtedness in full or in part prior to July 30, 2012, we would be required to write-off the unamortized debt discount and debt issuance costs remaining on our balance sheet at the date of such refinancing and recognize a non-cash loss on the early extinguishment of debt. Assuming this debt was fully repaid on March 31, 2011, we would expect to recognize a charge would be approximately $2.5 million in connection with the early extinguishment of debt. Additionally, our cash flows and cash resources could be negatively impacted if we elected to repay the existing outstanding principal indebtedness with a combination of our existing cash and cash equivalents and new borrowings.
Discussion of Cash Flows
Cash Flows Provided by (Used in) Operating Activities
Net cash provided by operating activities was $0.2 million for 2010, compared to net cash used in operating activities of $5.1 million for 2009. This $5.3 million improvement is attributable to reduced operating expenses, increased collection efforts over accounts receivable and lower purchases of inventory, which were partially offset by reduced accounts payable and other current liabilities.
During 2010, we used cash of $3.7 million and $0.3 million to fund operations in the quarters ended March 31, 2010 and June 30, 2010, respectively; however, we generated positive cash flows from operations of $2.4 million and $1.8 million in the quarters ended September 30, 2010 and December 31, 2010, respectively. Our cash usage may fluctuate on a quarterly basis.
Cash Flows Provided by Investing Activities
Net cash provided by investing activities was $2.3 million in 2010 as compared to $2.8 million for 2009. Net proceeds from the sale and maturity of investments decreased by $4.4 million in 2010 compared to 2009. Purchase of property and equipment totaled $1.5 million and $4.9 million in 2010 and 2009, respectively. For 2011, we expect that expenditures for property and equipment will approximate 2010 spending.
We invest our excess cash in highly liquid investment-grade marketable securities, including corporate debt securities and government-sponsored enterprise debt securities. Marketable securities having maturities greater than three months and less than one year are classified as short-term investments. Until we achieve sales at levels that consistently enable us to fund operations and
44
investing activities, we expect to continue to use cash, cash equivalents and proceeds from sales of short-term investments to fund operating and investing activities.
Cash Flows Provided by Financing Activities
Net cash provided by financing activities was $0.9 million in 2010 and $1.4 million in 2009, all of which was derived from the exercise of employee stock options and the sale of our common stock under our employee stock purchase plan. The extent and timing of proceeds from future stock option and warrant exercises, if any, are primarily dependent upon future trading prices for our common stock and the expiration dates of these instruments.
CONTRACTUAL OBLIGATIONS AND COMMITMENTS
Below is a table that presents our contractual obligations and commitments as of December 31, 2010:
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | More | |
| | | | | | Less | | | | | | | | | Than | |
| | | | | | Than | | | | | | | | | Five | |
Contractual Obligations (3) | | Total | | | One Year | | | 1-3 Years | | | 3-5 Years | | | Years | |
| | | | | |
Notes Payable (1) | | $ | 35,000 | | | $ | — | | | $ | 35,000 | | | $ | — | | | $ | — | |
Lease Obligations (2) | | | 6,442 | | | | 885 | | | | 1,883 | | | | 2,001 | | | | 1,673 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Total | | $ | 41,442 | | | $ | 885 | | | $ | 36,883 | | | $ | 2,001 | | | $ | 1,673 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Senior Secured Note Purchase Facility.We have $35 million in outstanding principal indebtedness under a senior secured note purchase facility. Notes issued under the facility are due July 30, 2012. |
Borrowings under the facility are guaranteed by us and one of our wholly-owned subsidiaries. The facility is secured by a first priority lien on substantially all of our assets (including intellectual property) other than those exclusively related to Cortoss and Aliquot. We are required to make quarterly interest-only payments to the note holder. Outstanding principal amounts under the notes bear annual interest at 10%, except that during the continuance of any event of defaults, interest would accrue at the rate of 12% per year and the notes would be payable on demand. As of December 31, 2010, we were in compliance with all covenants under the facility. Based on our current operating plans, we expect to be in compliance with the covenants under the facility for at least the next twelve months from the date of filing of this report. We expect to incur quarterly interest payments of $875,000 under our debt facility during 2011 and thereafter until maturity on July 30, 2012. Prepayment of the obligation under our facility is subject to a prepayment premium, the amount of which depends on the timing of the prepayment. As discussed above, we are seeking to refinance this obligation. Any prepayment premium and the write off of the remaining debt discount and unamortized debt issuance costs would be recognized as an operating expense in the period of the prepayment and recorded as a loss on the early extinguishment of debt.
| (2) | Leases. We lease facilities under non-cancelable operating leases that extend through July 31, 2017. As we continue to expand, we expect to lease additional facilities. |
| (3) | In addition to the contractual obligations and commitments set forth above, we have the following contractual obligations: |
Agreement with Kensey Nash Corporation. Approximately 67% of our product sales during 2010 were from products based upon our Vitoss Foam platform co-developed with Kensey, including Vitoss Bioactive Foam. As of December 31, 2010, we owed Kensey $0.7 million for manufactured product inventory and royalties, which amount is included in accounts payable and other accrued expenses on our consolidated balance sheet as of December 31, 2010 included in this report.
Excluded from the above table are certain product development milestones and related payments. In connection with the development of new products with business partners, we may contractually agree to make milestone payments upon achievement of specified developmental goals. The timing and actual amount of these payments can be difficult to
45
| | determine as they depend upon satisfactory achievement of product development and other milestones, which will be determined based on events that may occur in the future. During 2009 payments of $1.9 million were made for license fees and product development milestones pursuant to the contractual obligations that were incurred in 2009. If these products under development are brought to market, we may be obligated to make certain launch-related milestone payments of up to $1 million, as well as additional payments which may be made depending upon the potential achievements of clinical and sales milestones in the future. There were no such product development payments made in 2010. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Foreign Currency Risk
The functional currency for our European branch operation is the Euro. The functional currency for our U.K. subsidiary operation is the British pound sterling. All assets and liabilities related to these operations are translated at the current exchange rates at the end of each period. Revenues and expenses are translated at average exchange rates in effect during the period. The resulting translation adjustments are accumulated in a separate component of shareholders’ equity (accumulated other comprehensive income (loss)). Foreign currency transaction gains and losses, if any, are included in our results of operations.
As of December 31, 2010, our total exposure to foreign currency risk in U.S. dollar terms was approximately $3.2 million, or 4% of our total assets. The potential impact of a hypothetical 10% decline in the foreign exchange rates would result in a total decline in the fair value of our assets of approximately $0.3 million at December 31, 2010.
Market Risk
We may be exposed to market risk through changes in market interest rates that could affect the value of our short-term investments; however, we do not believe the fair value of our investment portfolio or related income would be significantly affected by changes in interest rates due mainly to the relatively short-term nature of the majority of our investment portfolio.
As of December 31, 2010, our short-term investments consisted of highly liquid investment-grade marketable securities. The impact on our future interest income and future changes in investment yields will depend on the gross amount of our investments and various external economic factors.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The consolidated financial statements of the Company and its subsidiaries and supplementary data required by this item are attached to this annual report on Form 10-K beginning on page F-1.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
CONCLUSION REGARDING EFFECTIVENESS OF DISCLOSURE CONTROLS AND PROCEDURES
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to costs. Because of the inherent limitation in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected. However, our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2010. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2010, our disclosure controls and procedures are functioning effectively to provide reasonable assurance that the information required to be disclosed by us in reports filed under the Securities Exchange Act of
46
1934 is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding disclosure.
MANAGEMENT’S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of Orthovita, Inc. (the Company) is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, provide reasonable assurance that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management evaluated the Company’s internal control over financial reporting as of December 31, 2010. In making this assessment, management used the framework established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Management’s assessment included an evaluation of the design of the Company’s internal control over financial reporting and testing of the operational effectiveness of the Company’s internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of the Company’s Board of Directors. Based on that assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2010, the Company’s internal control over financial reporting was effective.
The Company’s independent registered public accounting firm, KPMG LLP, has audited the effectiveness of the Company’s internal control over financial reporting as of December 31, 2010. KPMG LLP’s report on the effectiveness of the Company’s internal control over financial reporting appears below.
| | |
| Antony Koblish | | Nancy C. Broadbent |
| President and Chief Executive Officer | | Senior Vice President and Chief Financial Officer |
March 16, 2011
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
There have been no changes in our internal control over financial reporting during the quarter ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
47
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Orthovita, Inc.:
We have audited Orthovita, Inc. and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).Orthovita, Inc. and subsidiaries’ management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Orthovita, Inc. and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Orthovita, Inc. and subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of operations, shareholders’ equity and comprehensive loss, and cash flows for each of the years in the three-year period ended December 31, 2010, and our report dated March 16, 2011 expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 16, 2011
| ITEM 9B. | OTHER INFORMATION |
Not applicable.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
(a) Identification of Directors
Information with respect to the members of the Board of Directors of the Company is set forth under the caption “Election of Directors” in the Company’s definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, which information is incorporated herein by reference.
48
(b) Identification of Executive Officers
Information with respect to the executive officers of the Company is set forth under the caption “Executive Officers of Orthovita” in the Company’s definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, which information is incorporated herein by reference.
(c) Section 16(a) Beneficial Ownership Reporting Compliance
Information with respect to the Section 16(a) compliance of the directors and executive officers of the Company is set forth under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” in the Company’s definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, which information is incorporated herein by reference.
(d) Code of Conduct and Ethics
Information with respect to the Company’s Code of Conduct is set forth under the caption “Governance of the Company-Code of Conduct” in the Company’s definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, which information is incorporated herein by reference.
(e) Audit Committee Financial Expert
Information with respect to the Company’s Audit Committee Financial Expert is set forth under the caption “Governance of the Company-Audit Committee” in the Company’s definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, which information is incorporated herein by reference.
| ITEM 11. | EXECUTIVE COMPENSATION |
Information required by this item is set forth under the caption “Compensation of Executive Officers and Directors” in the Company’s definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, which information is incorporated herein by reference.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Equity Compensation Plan Information as of December 31, 2010
| | | | | | | | | | | | |
Plan Category | | Number of securities to be
issued upon exercise of
outstanding options,
warrants and rights
(a) | | | Weighted-average
exercise price of
outstanding options,
warrants and rights
(b) | | | Number of securities remaining
available for future issuance under
equity compensation plans (excluding
securities reflected in column (a)
(c) | |
Equity compensation plans approved by security holders | | | 8,136,000 | | | $ | 3.54 | | | | 4,609,000 | |
The information called for by this item not contained in this annual report on Form 10-K will be set forth under the captions “Stock Ownership-Securities Ownership of Certain Beneficial Owners and Management” in our definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, and is incorporated herein by reference.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information called for by this item not contained in this annual report on Form 10-K will be set forth under the caption “Certain Relationships and Related Transactions, and Director Independence” in our definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, and is incorporated herein by reference.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information called for by this item not contained in this annual report on Form 10-K will be set forth under the caption “Ratification of Appointment of Independent Registered Public Accounting Firm” in our definitive proxy statement, to be filed within 120 days after the end of the year covered by this annual report on Form 10-K, and is incorporated herein by reference.
49
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) Documents filed as part of this Report
1. Financial Statements. The following consolidated financial statements and notes thereto which are attached hereto beginning on page F-1 have been included by reference into Item 8 of this part of the annual report on Form 10-K:
2. Financial Statement Schedules. All schedules are omitted because they are inapplicable, or not required, or the information is shown in the Financial Statements or notes thereto.
3. Exhibits. The information required by this item is set forth in the Exhibit Index hereto which is incorporated herein by reference.
50
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
Date: March 16, 2011 | | ORTHOVITA, INC. |
| | |
| | By: | | /s/ ANTONY KOBLISH |
| | | | President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Capacity | | Date |
| | |
/S/ ANTONY KOBLISH | | President, Chief Executive Officer and Director | | March 16, 2011 |
| | (principal executive officer) | | |
| | |
/S/ WILLIAM E. TIDMORE, JR. | | Chairman | | March 16, 2011 |
| | | | |
| | |
/S/ NANCY C. BROADBENT | | Senior Vice President and Chief Financial Officer | | March 16, 2011 |
| | (principal financial and accounting officer) | | |
| | |
/S/ R. SCOTT BARRY | | Director | | March 16, 2011 |
| | | | |
| | |
/S/ MORRIS CHESTON, JR. | | Director | | March 16, 2011 |
| | | | |
| | |
/S/ MARY E. PAETZOLD | | Director | | March 16, 2011 |
| | | | |
| | |
/S/ PAUL G. THOMAS | | Director | | March 16, 2011 |
| | | | |
| | |
/S/ PAUL T. TOUHEY, JR. | | Director | | March 16, 2011 |
| | | | |
51
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Orthovita, Inc.:
We have audited the accompanying consolidated balance sheets of Orthovita, Inc. and subsidiaries (the Company) as of December 31, 2010 and 2009, and the related consolidated statements of operations, shareholders’ equity and comprehensive loss, and cash flows for each of the years in the three-year period ended December 31, 2010. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Orthovita, Inc. and subsidiaries as of December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Orthovita, Inc. and subsidiaries’ internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 16, 2011 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 16, 2011
F-1
ORTHOVITA, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In thousands except per share data)
| | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2009 | |
| ASSETS | | | | | | | | |
CURRENT ASSETS: | | | | | | | | |
Cash and cash equivalents | | $ | 11,059 | | | $ | 7,757 | |
Short-term investments | | | 11,305 | | | | 15,349 | |
Accounts receivable, net of allowance for doubtful accounts of $282 and $217, respectively | | | 11,877 | | | | 12,324 | |
Inventories | | | 22,035 | | | | 26,058 | |
Other current assets | | | 631 | | | | 784 | |
| | | | | | | | |
Total current assets | | | 56,907 | | | | 62,272 | |
| | |
Property and equipment, net | | | 16,809 | | | | 17,940 | |
License and technology intangible assets, net | | | 9,884 | | | | 11,376 | |
Other assets | | | 475 | | | | 1,041 | |
| | | | | | | | |
Total assets | | $ | 84,075 | | | $ | 92,629 | |
| | | | | | | | |
| | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
CURRENT LIABILITIES: | | | | | | | | |
Accounts payable | | $ | 1,191 | | | $ | 5,028 | |
Accrued compensation and related expenses | | | 1,106 | | | | 2,805 | |
Other accrued expenses | | | 5,403 | | | | 5,534 | |
| | | | | | | | |
Total current liabilities | | | 7,700 | | | | 13,367 | |
| | | | | | | | |
| | |
LONG-TERM LIABILITIES: | | | | | | | | |
Notes payable, net of debt discount of $585 and $905, respectively | | | 34,415 | | | | 34,095 | |
Other long-term liabilities | | | 387 | | | | 408 | |
| | | | | | | | |
Total long-term liabilities | | | 34,802 | | | | 34,503 | |
| | | | | | | | |
| | |
Total liabilities | | | 42,502 | | | | 47,870 | |
| | | | | | | | |
| | |
COMMITMENTS (Note 16) | | | | | | | | |
| | |
SHAREHOLDERS’ EQUITY: | | | | | | | | |
Common stock, $.01 par value, 100,000 shares authorized, 76,881 and
76,542 shares issued and outstanding | | | 769 | | | | 765 | |
Additional paid-in capital | | | 227,569 | | | | 224,734 | |
Accumulated deficit | | | (186,284 | ) | | | (180,578 | ) |
Accumulated other comprehensive loss | | | (481 | ) | | | (162 | ) |
| | | | | | | | |
Total shareholders’ equity | | | 41,573 | | | | 44,759 | |
| | | | | | | | |
| | |
Total liabilities and shareholders’ equity | | $ | 84,075 | | | $ | 92,629 | |
| | | | | | | | |
The accompanying notes are an integral part of these statements.
F-2
ORTHOVITA, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands except per share data)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
PRODUCT SALES | | $ | 94,663 | | | $ | 92,853 | | | $ | 76,915 | |
COST OF SALES | | | 34,945 | | | | 29,914 | | | | 25,929 | |
| | | | | | | | | | | | |
GROSS PROFIT | | | 59,718 | | | | 62,939 | | | | 50,986 | |
| | | | | | | | | | | | |
| | | |
OPERATING EXPENSES: | | | | | | | | | | | | |
General and administrative | | | 12,240 | | | | 12,172 | | | | 10,753 | |
Selling and marketing | | | 44,359 | | | | 45,070 | | | | 42,706 | |
Research and development | | | 5,158 | | | | 6,786 | | | | 6,711 | |
| | | | | | | | | | | | |
Total operating expenses | | | 61,757 | | | | 64,028 | | | | 60,170 | |
| | | | | | | | | | | | |
| | | |
OPERATING LOSS | | | (2,039 | ) | | | (1,089 | ) | | | (9,184 | ) |
| | | | | | | | | | | | |
| | | |
OTHER INCOME (EXPENSE), NET: | | | | | | | | | | | | |
Interest expense | | | (3,641 | ) | | | (3,112 | ) | | | (2,777 | ) |
Interest income | | | 50 | | | | 282 | | | | 1,271 | |
Loss on sale of product line and related assets | | | — | | | | — | | | | (72 | ) |
| | | | | | | | | | | | |
Total other expense, net | | | (3,591 | ) | | | (2,830 | ) | | | (1,578 | ) |
| | | | | | | | | | | | |
LOSS BEFORE INCOME TAXES | | | (5,630 | ) | | | (3,919 | ) | | | (10,762 | ) |
INCOME TAX (EXPENSE) BENEFIT | | | (76 | ) | | | 13 | | | | 10 | |
| | | | | | | | | | | | |
NET LOSS | | $ | (5,706 | ) | | $ | (3,906 | ) | | $ | (10,752 | ) |
| | | | | | | | | | | | |
| | | |
NET LOSS PER SHARE, BASIC AND DILUTED | | $ | (0.07 | ) | | $ | (0.05 | ) | | $ | (0.14 | ) |
| | | | | | | | | | | | |
| | | |
SHARES USED IN COMPUTING BASIC AND DILUTED NET | | | | | | | | | | | | |
LOSS PER SHARE | | | 76,675 | | | | 76,176 | | | | 75,804 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these statements.
F-3
ORTHOVITA, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY AND COMPREHENSIVE LOSS
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Accumulated | | | | | | | |
| | | | | | | | | Additional | | | | | | Other | | | | | | | |
| | | Common Stock | | | Paid-in | | | Accumulated | | | Comprehensive | | | Comprehensive | | | | |
| | | Number of shares | | | Amount | | | Capital | | | Deficit | | | Income (Loss) | | | Loss | | | Total | |
| | | | | | | |
BALANCE, JANUARY 1, 2008 | | | 75,684 | | | $ | 756 | | | $ | 219,444 | | | $ | (165,920 | ) | | $ | 248 | | | $ | | | | $ | 54,528 | |
Purchase of shares by employees, non-employees and exercise of warrants | | | 80 | | | | 1 | | | | 195 | | | | — | | | | — | | | | — | | | | 196 | |
Issuance of shares under restricted stock award | | | 28 | | | | 1 | | | | 40 | | | | — | | | | — | | | | — | | | | 41 | |
Issuance of shares for services | | | 7 | | | | — | | | | 21 | | | | — | | | | — | | | | — | | | | 21 | |
| | | | | | | |
Issuance of warrants in connection with debt facility | | | — | | | | — | | | | 286 | | | | — | | | | — | | | | — | | | | 286 | |
Issuance of shares in exchange for non-employee consultant stock options | | | 131 | | | | 1 | | | | 415 | | | | — | | | | — | | | | — | | | | 416 | |
Stock-based compensation expense related to stock options and restricted stock units | | | — | | | | — | | | | 1,232 | | | | — | | | | — | | | | — | | | | 1,232 | |
Comprehensive loss: | | | | | | | — | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | — | | | | — | | | | — | | | | (10,752 | ) | | | — | | | | (10,752 | ) | | | (10,752 | ) |
Other comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized gain on investments | | | — | | | | — | | | | — | | | | — | | | | 101 | | | | 101 | | | | 101 | |
Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | (427 | ) | | | (427 | ) | | | (427 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | $ | (11,078 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
BALANCE DECEMBER 31, 2008 | | | 75,930 | | | | 759 | | | | 221,633 | | | | (176,672 | ) | | | (78 | ) | | | | | | | 45,642 | |
Purchase of shares by employees, non-employees and exercise of warrants | | | 428 | | | | 4 | | | | 1,442 | | | | — | | | | — | | | | — | | | | 1,446 | |
Issuance of shares under restricted stock award | | | 28 | | | | — | | | | 95 | | | | — | | | | — | | | | — | | | | 95 | |
| | | | | | | |
Stock-based compensation expense related to stock options and restricted stock units | | | 156 | | | | 2 | | | | 1,564 | | | | — | | | | — | | | | — | | | | 1,566 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | — | | | | — | | | | — | | | | (3,906 | ) | | | — | | | | (3,906 | ) | | | (3,906 | ) |
Other comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized loss on investments | | | — | | | | — | | | | — | | | | — | | | | (150 | ) | | | (150 | ) | | | (150 | ) |
Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 66 | | | | 66 | | | | 66 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | $ | (3,990 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
BALANCE DECEMBER 31, 2009 | | | 76,542 | | | | 765 | | | | 224,734 | | | | (180,578 | ) | | | (162 | ) | | | | | | | 44,759 | |
Purchase of shares by employees | | | 311 | | | | 3 | | | | 917 | | | | — | | | | — | | | | — | | | | 920 | |
Issuance of shares under restricted stock award | | | 28 | | | | 1 | | | | 89 | | | | — | | | | — | | | | — | | | | 90 | |
Stock-based compensation expense related to stock options and restricted stock units | | | — | | | | — | | | | 1,829 | | | | — | | | | — | | | | — | | | | 1,829 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | — | |
Net loss | | | — | | | | — | | | | — | | | | (5,706 | ) | | | — | | | | (5,706 | ) | | | (5,706 | ) |
Other comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | — | |
Currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | (319 | ) | | | (319 | ) | | | (319 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | $ | (6,025 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
BALANCE DECEMBER 31, 2010 | | | 76,881 | | | $ | 769 | | | $ | 227,569 | | | $ | (186,284 | ) | | $ | (481 | ) | | | | | | $ | 41,573 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these statements.
F-4
ORTHOVITA, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
OPERATING ACTIVITIES: | | | | | | | | | | | | |
Net loss | | $ | (5,706 | ) | | $ | (3,906 | ) | | $ | (10,752 | ) |
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
Amortization of discounts and premiums related to investments | | | 244 | | | | 36 | | | | (389 | ) |
Depreciation and amortization of property and equipment | | | 2,772 | | | | 1,831 | | | | 1,728 | |
Amortization of license and technology intangible assets | | | 1,492 | | | | 1,493 | | | | 995 | |
Amortization of debt discount | | | 320 | | | | 286 | | | | 199 | |
Common stock issued for services rendered | | | — | | | | — | | | | 21 | |
Compensation related to restricted stock awards, restricted stock units and stock option accounting for employees and non-employees | | | 1,838 | | | | 1,669 | | | | 1,260 | |
Charge related to exchange of non-employee stock options for common stock | | | — | | | | — | | | | 170 | |
Deferred tax expense | | | — | | | | 7 | | | | 7 | |
Provision for doubtful accounts | | | 71 | | | | 18 | | | | 97 | |
Loss on disposal of property and equipment | | | — | | | | — | | | | 72 | |
Increase in inventory reserves | | | 4,141 | | | | 433 | | | | 465 | |
Noncash asset impairment charge | | | 500 | | | | — | | | | — | |
Decrease (increase) in (net of business acquisition in 2008)— | | | | | | | | | | | | |
Accounts receivable | | | 184 | | | | (1,444 | ) | | | (2,578 | ) |
Inventories | | | (157 | ) | | | (6,723 | ) | | | (3,652 | ) |
Other current assets | | | 116 | | | | (48 | ) | | | (71 | ) |
Other assets | | | (37 | ) | | | (425 | ) | | | (212 | ) |
(Decrease) increase in — | | | — | | | | — | | | | | |
Accounts payable | | | (3,858 | ) | | | 901 | | | | (773 | ) |
Accrued compensation and related expenses | | | (1,505 | ) | | | 357 | | | | 202 | |
Other accrued expenses | | | (305 | ) | | | 375 | | | | (1,056 | ) |
Other long-term liabilities | | | 76 | | | | 76 | | | | 57 | |
| | | | | | | | | | | | |
Net cash provided by (used in) operating activities | | | 186 | | | | (5,064 | ) | | | (14,210 | ) |
| | | | | | | | | | | | |
| | | |
INVESTING ACTIVITIES: | | | | | | | | | | | | |
Purchase of investments | | | (18,521 | ) | | | (29,936 | ) | | | (40,750 | ) |
Proceeds from sale and maturity of investments | | | 22,321 | | | | 38,174 | | | | 55,674 | |
Purchases of property and equipment | | | (1,486 | ) | | | (4,910 | ) | | | (5,852 | ) |
Purchase of intangible asset | | | — | | | | (515 | ) | | | — | |
Proceeds from sale of property and equipment | | | — | | | | — | | | | 200 | |
Acquisition of business | | | — | | | | — | | | | (6,552 | ) |
| | | | | | | | | | | | |
Net cash provided by investing activities | | | 2,314 | | | | 2,813 | | | | 2,720 | |
| | | | | | | | | | | | |
| | | |
FINANCING ACTIVITIES: | | | | | | | | | | | | |
Proceeds from notes payable | | | — | | | | — | | | | 10,000 | |
Proceeds from issuance of common stock | | | 920 | | | | 1,446 | | | | 196 | |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 920 | | | | 1,446 | | | | 10,196 | |
| | | | | | | | | | | | |
| | | |
EFFECT OF EXCHANGE RATE CHANGES ON CASH AND CASH EQUIVALENTS | | | (118 | ) | | | 44 | | | | (374 | ) |
| | | | | | | | | | | | |
| | | |
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | | | 3,302 | | | | (761 | ) | | | (1,668 | ) |
| | | |
CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR | | | 7,757 | | | | 8,518 | | | | 10,186 | |
| | | | | | | | | | | | |
| | | |
CASH AND CASH EQUIVALENTS, END OF YEAR | | $ | 11,059 | | | $ | 7,757 | | | $ | 8,518 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these statements.
F-5
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
1. The Company:
We are a specialty spine and orthopedic company with a portfolio of orthobiologic and biosurgery products. Our products are based on novel and unique proprietary biomaterials that have innovative mechanisms of action in the body. Our orthobiologic platform offers products for the fusion, regeneration and fixation of human bone. Our biosurgery platform offers products for controlling intra-operative bleeding, also known as hemostasis. In the United States, we primarily market our products through a direct sales force but also utilize non-stocking distributors and independent sales agents in certain geographical areas. Outside the United States, we market our products through a direct sales force in the United Kingdom and through independent stocking distributors in other countries.
Orthobiologics
Vitoss™ Bone Graft Substitute is our primary fusion and regeneration product, and Cortoss™ Bone Augmentation Material is our primary fixation product. Our Vitoss product line, which includes Vitoss Foam and Vitoss Bioactive Foam products, provides the non-structural bone graft market with synthetic, bioactive alternatives to patient- and cadaver-derived bone tissue. An injectable polymer composite which mimics the structural characteristics of human bone, Cortoss provides the vertebral compression fracture market with a synthetic, bioactive alternative to polymethylmethacrylate bone cement.
Biosurgery
The primary products in our hemostasis portfolio are Vitagel™ Surgical Hemostat, a proprietary, collagen-based matrix that controls bleeding and facilitates healing, and Vitasure™ Absorbable Hemostat, a proprietary, plant-based product that can be deployed quickly throughout surgery.
We also market accessories and delivery products which complement the orthobiologic and biosurgery platforms. In addition, we seek to expand our product portfolio through internal product development efforts, co-development efforts with strategic partners, and acquisition and in-licensing opportunities.
2. Summary of Significant Accounting Policies:
Basis of Presentation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
The financial statements of our international operations are translated into U.S. dollars using the exchange rate at each balance sheet date for assets and liabilities, and an average exchange rate for each period of revenues, expenses, and gains and losses. The functional currency of our non-U.S. operations is the local currency. Adjustments resulting from the translation of financial statements are reflected in accumulated other comprehensive income (loss). Transaction gains and losses are charged to operations.
Preparation of Financial Statements and Use of Estimates
The preparation of the consolidated financial statements requires us to make assumptions, estimates and judgments that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates, including, but not limited to, those related to accounts receivable, inventories and recoverability of long-lived assets. We use historical experience and other assumptions as the basis for making estimates. Illiquid credit markets, volatile equity, foreign currency and energy markets, and declines in consumer spending have combined to increase the uncertainty inherent in such estimates and assumptions. As future events and their effects cannot be determined with precision, actual results could differ significantly from these estimates. Changes in those estimates will be reflected in the financial statements in those future periods.
We evaluated all subsequent events through the date and time our financial statements were issued. No subsequent event occurred during this period that requires recognition or disclosure in these consolidated financial statements.
F-6
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
Cash and Cash Equivalents and Short-Term Investments
We consider all highly liquid instruments with an original maturity of 90 days or less at the time of purchase to be cash equivalents. Cash equivalents consist of deposits that are readily convertible into cash (see note 4).
For financial reporting purposes, our entire short-term investment portfolio is classified as available-for-sale and is stated at fair value as determined by quoted market values. Accordingly, any unrealized holding gains and losses are included in accumulated other comprehensive income (loss), as a separate component of shareholders’ equity. Discounts and premiums are amortized over the term of the security and reported in interest income. The investments are reviewed on a periodic basis for other-than-temporary impairments (see notes 4 and 5).
Fair Value Measurements
The Financial Accounting Standards Board’s (FASB) Accounting Standards Codification (ASC) Topic 820,Fair Value Measurements and Disclosures, defines fair value, establishes a framework for measuring fair value and expands the related disclosure requirements. The guidance applies under other accounting pronouncements that require or permit fair value measurements and indicates, among other things, that a fair value measurement assumes that the transaction to sell an asset or transfer a liability occurs in the principal market for the asset or liability or, in the absence of a principal market, the most advantageous market for the asset or liability. ASC 820 defines fair value based upon an exit price model.
Our financial instruments are categorized into a three-level fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). If the inputs used to measure fair value fall within different levels of the hierarchy, the category level is based on the lowest priority level input that is significant to the fair value measurement of the instrument. Financial assets recorded at fair value on our consolidated balance sheets are categorized as follows:
| | • | | Level 1: Observable inputs such as quoted prices in active markets; |
| | • | | Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
| | • | | Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
Concentrations of Credit Risk
Our policy is to limit the amount of credit exposure to any one financial institution, and place investments with financial institutions evaluated as being creditworthy, or in short-term money market funds which are exposed to minimal interest rate and credit risk. We maintain our cash primarily in investment accounts within large financial institutions. Currently, the Federal Deposit Insurance Corporation insures these balances up to $250 per bank. We have not experienced any losses on our bank deposits and we believe these deposits do not expose us to any significant credit risk.
We grant credit, generally without collateral, to our customers, which are primarily in the health care market. Consequently, we are subject to potential credit risk related to changes in economic conditions within that market. However, we believe that our billing and collection policies are adequate to minimize the potential credit risk.
Concentrations of Supply Risk
We are highly dependent on Kensey Nash Corporation (Kensey Nash) to convert our Vitoss to Vitoss Foam and Vitoss Bioactive Foam Products (see note 16). We also source other raw materials and a majority of the disposable components that accompany and are used with our products with a limited number of third party manufacturers and are highly dependent upon these manufacturers.
Inventories
Inventories are stated at the lower of cost or market value using the first-in first-out basis, or FIFO. A provision is recorded to reduce the cost of inventories to the estimated net realizable values, if required. The costs of producing inventory in the reporting periods prior to the receipt of regulatory approval or clearance are recorded as research and development expense (see note 6).
F-7
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
Property and Equipment
Property and equipment are recorded at cost. Depreciation is calculated on a straight-line basis over the estimated useful life of each asset, generally three to five years. The useful life for leasehold improvements is generally the term of the facility lease. Expenditures for major renewals and improvements are capitalized, and expenditures for maintenance and repairs are charged to operations as incurred. Property and equipment includes assets designated as construction in-progress and not placed in service. We begin to depreciate these assets when they are placed into service or, if the property and equipment requires approval from the U.S. Food and Drug Administration (FDA), when such approval is obtained and the asset is placed in service (see note 7).
Interest cost that is incurred on borrowed funds used to expand the manufacturing facilities is capitalized and is recorded as part of the asset to which it relates, and is amortized over the asset’s estimated useful life.
Impairment of Long-Lived Assets and Intangible Assets
We evaluate the recoverability of long-lived assets and intangible assets whenever events or changes in circumstances indicate that an asset’s carrying amount may not be recoverable. Such circumstances could include, but are not limited to (1) a significant decrease in the market value of an asset, (2) a significant adverse change in the extent or manner in which an asset is used, or (3) an accumulation of costs significantly in excess of the amount originally expected for the acquisition of an asset. We measure the carrying amount of the asset against the estimated undiscounted future cash flows associated with it. If the sum of the expected future net cash flows is less than the carrying value of the asset being evaluated, an impairment charge would be recognized. The impairment charge would be calculated as the amount by which the carrying value of the asset exceeds its fair value. The determination of fair value is based on quoted market prices, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including the discounted value of estimated future cash flows. At December 31, 2010 and 2009, the carrying amounts of our long-lived assets and intangible assets with finite lives were not impaired.
Amortization of Intangible Assets
The carrying value of intangible assets, which consist of acquired technology and license rights, are generally amortized on a straight line basis or the unit of sales method.
Revenue Recognition
Revenue is not recognized until persuasive evidence of an arrangement exists, performance or shipment has occurred, the sales price is fixed or determinable and collection is probable. In the U.S. and the U.K., we recognize product sales revenue upon either shipment of purchased product to the customer, or receipt of documentation from the customer indicating its consumption of product from consigned inventory. Outside the U.S. and the U.K., revenue from product sales is recognized upon shipment of the product to the independent stocking distributors.
We have no obligations to our customers once title has been transferred but allow the return or exchange of ambient temperature products within five days after their original delivery. Subsequent to the end of every quarter we adjust sales, cost of sales, inventory and accounts receivable for the effect of such returns. We generally require each of our U.S. and U.K. customers to pay on a net 30-day basis. Outside of the U.S. and the U.K., independent stocking distributors are generally required to pay on a net 60-day basis.
Net Loss per Common Share
Basic net loss per share excludes securities exercisable for or convertible into shares of common stock, and is computed by dividing net loss applicable to common shareholders by the weighted average number of shares of common stock outstanding for the period. Diluted net loss per common share data is computed assuming the conversion or exercise of all dilutive securities such as common stock options and warrants.
Equity awards and warrants exercisable to purchase 9,459, 9,282 and 8,904 shares were excluded from the computation of diluted net loss per common share for 2010, 2009 and 2008, respectively, because the inclusion of the shares in the calculation would have been anti-dilutive due to the losses.
F-8
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The asset and liability method requires that deferred tax assets and liabilities be recorded without consideration as to their realizability. The deferred tax asset primarily includes net operating loss and tax credit carryforwards, accrued expenses not currently deductible and the cumulative temporary differences related to certain research and patent costs, which have been charged to expense in the accompanying statements of operations but have been recorded as assets for income tax purposes. The portion of any deferred tax asset for which it is more likely than not that a tax benefit will not be realized must then be offset by recording a valuation allowance. A valuation allowance has been established against all of the deferred tax assets, with the exception of the Texas margin tax credit (see note 15), as it is more likely than not that these assets will not be realized given the history of operating losses.
While we believe that our tax positions are fully supportable, there is a risk that certain positions could be challenged successfully. In these instances, we look to establish reserves. If we determine that a tax position is more likely than not of being sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that has likelihood greater than 50% of being realized upon settlement. We presume that all tax positions will be examined by a taxing authority with full knowledge of all relevant information. We regularly monitor our tax positions, tax assets and tax liabilities. We reevaluate the technical merits of our tax positions and recognize an uncertain tax benefit or derecognize a previously recorded tax benefit when (i) there is a completion of a tax audit, (ii) there is a change in applicable tax law including a tax case or legislative guidance, or (iii) there is an expiration of the statute of limitations. Significant judgment is required in accounting for tax reserves.
Stock-based Compensation
Stock-based compensation is accounted for at grant-date fair value and amortized on a straight-line basis over the vesting terms of the awards in the consolidated statement of operations based upon the portion of the awards that are expected to vest. We estimate pre-vesting forfeitures by analyzing historical data and revise those estimates in subsequent periods if actual forfeitures differ from those estimates (see note 13).
The following table sets forth the total stock-based compensation expense included in the consolidated statements of operations for the years ended December 31, 2010, 2009, and 2008:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Cost of sales | | $ | 53 | | | $ | 74 | | | $ | 42 | |
General and administrative | | | 936 | | | | 829 | | | | 584 | |
Selling and marketing | | | 676 | | | | 540 | | | | 464 | |
Research and development | | | 254 | | | | 218 | | | | 183 | |
| | | | | | | | | | | | |
| | | |
Total | | $ | 1,919 | | | $ | 1,661 | | | $ | 1,273 | |
| | | | | | | | | | | | |
We estimated the fair value of each option grant using the Black-Scholes option-pricing model using the following weighted average assumptions:
F-9
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Risk-free interest rate | | | 2.91 | % | | | 2.85 | % | | | 3.78 | % |
Expected volatility | | | 55 | % | | | 55 | % | | | 58 | % |
Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
Expected life | | | 6 years | | | | 6 years | | | | 6 years | |
The weighted average fair value per share of options granted in 2010, 2009, and 2008 was $2.02, $2.01, and $1.70, respectively.
Expected volatility was calculated based upon the historical daily closing prices of our common stock as quoted on the NASDAQ Global Market (NASDAQ) over a prior period having a term equal to the expected life of the stock options.
Non-employee Stock Based Compensation
The cost of stock-based compensation awards issued to non-employees for services are recorded at the fair value of instruments issued in exchange for such services. Historically, the common stock options granted to non-employee consultants as compensation were fully vested on the date of the grant. In certain circumstances, these awards may have to be settled in cash through the expiration of the option or until it is exercised, whichever is earlier. Accordingly, the fair value of the fully-vested non-employee consultant stock options are classified as liabilities with changes in fair value between reporting periods recognized in our consolidated statement of operations.
The fair value of the derivative liability associated with non-employee consultant stock options was estimated using the Black-Scholes option pricing model. For the years ended December 31, 2010, 2009, and 2008, the income (expense) associated with the change in fair value of the derivative liability was $81, $(8) and $13, respectively, and was recorded in selling and marketing expenses in our consolidated statement of operations.
Research & Development Costs
Research and development costs are expensed as incurred.
Segment Information
We are managed and operated as one business. The entire business is managed by a single management team that reports to the chief executive officer. We do not operate separate lines of business and do not prepare discrete financial information with respect to separate product areas or by location and therefore do not have separate reportable segments.
Comprehensive Loss
Comprehensive loss is the change in equity of a business enterprise during a period from non-owner sources. Excluding net loss, our sources of other comprehensive loss are from net unrealized appreciation (depreciation) on available-for-sale securities and foreign currency translation adjustments. Reclassification adjustments result from the recognition in net loss of unrealized gains or losses that were included in comprehensive loss in prior periods.
Supplemental Cash Flow Information
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | |
Supplemental disclosure of non-cash transactions: | | | | | | | | | | | | |
Warrants issued in connection with Senior Secured credit facility (note 9) | | $ | — | | | $ | — | | | $ | 286 | |
Exchange of stock options for common stock (note 13) | | | — | | | | — | | | | 170 | |
Purchase of property and equipment included in accounts payable and accrued expenses | | | 76 | | | | — | | | | — | |
Change in the fair market value of non-employee stock options | | | (81 | ) | | | 8 | | | | (13 | ) |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | |
Cash paid for interest | | | 3,319 | | | | 2,826 | | | | 2,325 | |
Cash paid for taxes | | | 54 | | | | 85 | | | | 82 | |
F-10
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
Recent Accounting Pronouncements
In January 2010, the FASB issued amended guidance requiring additional fair value disclosures related to inputs and valuation techniques used to measure fair value, as well as disclosures about significant transfers between levels in the hierarchy of fair value measurement. This guidance became effective for us beginning on January 1, 2010 and did not have a significant impact on our consolidated financial statements.
3. Business Acquisition:
In September 2008, we purchased collagen raw materials, equipment and a technology license from Allergan, Inc. and its affiliate Allergan Sales, LLC under a supply and license agreement dated November 2007 for an aggregate purchase price of $6,552 in cash, which was funded through a senior secured note purchase facility (see note 9). We accounted for the purchase of the assets and the related business at fair value with the purchase price being allocated among the assets purchased. The raw materials, equipment and license acquired from Allergan relate primarily to the production of the Vitagel Surgical Hemostat product. The purchase price was allocated to the fair value of the assets purchased as follows: $980 of raw material inventory, $373 of equipment, and $5,199 of acquired technology. The purchase price allocation was based upon a third party independent valuation. The acquired technology is being amortized over approximately 9 years, which is the estimated remaining life of the relevant Vitagel patent. There were no liabilities assumed in the transaction.
4. Cash, Cash Equivalents and Short-Term Investments:
At December 31, 2010 and 2009 cash, cash equivalents and short-term investments consisted of the following:
| | | | | | | | | | | | | | | | |
| | | Gross Unrealized | |
| | | Amortized Cost | | | Gains | | | Losses | | | Fair Market Value | |
December 31, 2010: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 11,059 | | | $ | — | | | $ | — | | | $ | 11,059 | |
| | | | | | | | | | | | | | | | |
Short-Term Investments: | | | | | | | | | | | | | | | | |
Corporate debt securities | | | 2,006 | | | | — | | | | — | | | | 2,006 | |
U.S. Treasury securities | | | 2,022 | | | | — | | | | — | | | | 2,022 | |
Government-sponsored enterprise debt securities | | | 7,277 | | | | — | | | | — | | | | 7,277 | |
| | | | | | | | | | | | | | | | |
| | | 11,305 | | | | — | | | | — | | | | 11,305 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 22,364 | | | $ | — | | | $ | — | | | $ | 22,364 | |
| | | | | | | | | | | | | | | | |
| |
| | | Gross Unrealized | |
| | | Amortized Cost | | | Gains | | | Losses | | | Fair Market Value | |
December 31, 2009: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 7,757 | | | $ | — | | | $ | — | | | $ | 7,757 | |
| | | | | | | | | | | | | | | | |
Short-Term Investments: | | | | | | | | | | | | | | | | |
U.S. Treasury securities | | | 1,770 | | | | — | | | | (1 | ) | | | 1,769 | |
Government-sponsored enterprise debt securities | | | 13,579 | | | | 1 | | | | — | | | | 13,580 | |
| | | | | | | | | | | | | | | | |
| | | 15,349 | | | | 1 | | | | (1 | ) | | | 15,349 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 23,106 | | | $ | 1 | | | $ | (1 | ) | | $ | 23,106 | |
| | | | | | | | | | | | | | | | |
Amortization of premiums and discounts related to investments resulted in expense of $244 and $36 and income of $389 for the years ended December 31, 2010, 2009 and 2008, respectively.
As of December 31, 2010 and 2009, all short-term investments mature within one year of the balance sheet date.
F-11
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
5. Fair Value Measurements:
The following tables provide the assets carried at fair value measured on a recurring basis as of December 31, 2010 and 2009:
| | | | | | | | | | | | | | | | |
| | | Carrying | | | Fair Value Measurement at December 31, 2010 | |
| | | Value | | | Level 1 | | | Level 2 | | | Level 3 | |
Cash and Cash Equivalents: | | | | | | | | | | | | | | | | |
Bank deposits | | $ | 3,703 | | | $ | 3,703 | | | $ | — | | | $ | — | |
Corporate debt securities | | | 500 | | | | 500 | | | | — | | | | — | |
U.S. Treasury security money market fund | | | 5,152 | | | | 5,152 | | | | — | | | | — | |
Government-sponsored enterprise debt securities | | | 1,704 | | | | 1,704 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| | $ | 11,059 | | | $ | 11,059 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Short-Term Investments: | | | | | | | | | | | | | | | | |
Corporate debt securities | | $ | 2,006 | | | $ | 2,006 | | | $ | — | | | $ | — | |
U.S. Treasury Securities | | | 2,022 | | | | 2,022 | | | | — | | | | — | |
Government-sponsored enterprise debt securities | | | 7,277 | | | | 7,277 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| | $ | 11,305 | | | $ | 11,305 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
| | |
| | | Carrying | | | Fair Value Measurement at December 31, 2009 | |
| | | Value | | | Level 1 | | | Level 2 | | | Level 3 | |
Cash and Cash Equivalents: | | | | | | | | | | | | | | | | |
Bank deposits | | $ | 1,763 | | | $ | 1,763 | | | | — | | | | — | |
U.S. Treasury security money market fund | | | 5,994 | | | | 5,994 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| | $ | 7,757 | | | $ | 7,757 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Short-Term Investments: | | | | | | | | | | | | | | | | |
U.S. Treasury securities | | $ | 1,769 | | | $ | 1,769 | | | | — | | | | — | |
Government-sponsored enterprise debt securities | | | 13,580 | | | | 13,580 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| | $ | 15,349 | | | $ | 15,349 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
6. Inventories:
As of December 31, 2010 and 2009, inventories consisted of the following:
| | | | | | | | |
| | | 2010 | | | 2009 | |
Raw materials | | $ | 3,618 | | | $ | 5,538 | |
Work-in-process | | | 8,781 | | | | 8,816 | |
Finished goods | | | 9,636 | | | | 11,704 | |
| | | | | | | | |
| | $ | 22,035 | | | $ | 26,058 | |
| | | | | | | | |
Included in cost of sales for each of the years ended December 31, 2010, 2009 and 2008 are inventory reserve charges of $4,141, $433 and $465, respectively. The 2010 inventory provision included a fourth quarter charge of $3,500 primarily related to Aliquot inventory that was expected either to expire prior to sale or to remain unsold based upon current forecasts of product demand.
F-12
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
7. Property and Equipment:
As of December 31, 2010 and 2009, property and equipment consisted of the following:
| | | | | | | | |
| | | 2010 | | | 2009 | |
Construction in-progress | | $ | 329 | | | $ | 9,898 | |
Machinery and equipment | | | 10,778 | | | | 7,797 | |
Furniture and computers, sales, marketing and office equipment | | | 6,782 | | | | 5,790 | |
Leasehold improvements | | | 16,305 | | | | 9,083 | |
| | | | | | | | |
| | | 34,194 | | | | 32,568 | |
Less—Accumulated depreciation and amortization | | | (17,385 | ) | | | (14,628 | ) |
| | | | | | | | |
| | $ | 16,809 | | | $ | 17,940 | |
| | | | | | | | |
As of December 31, 2010, and 2009, property and equipment included assets designated as construction in-progress and not placed in service. Construction in-progress at December 31, 2009 consisted primarily of construction costs for our collagen manufacturing facility. This facility was approved by the FDA and the assets relating to this facility that were previously included in construction in-progress were placed in service in July 2010.
Total interest cost capitalized was $180, $674 and $265 for 2010, 2009 and 2008, respectively. Included in construction in-progress at December 31, 2009 is capitalized interest of $779.
Depreciation and amortization expense relating to property and equipment for the years ended December 31, 2010, 2009 and 2008 was $2,772, $1,831, and $1,728, respectively.
8. License and Technology Intangible Assets:
As of December 31, 2010 and 2009, license and technology intangible assets consisted of the following:
| | | | | | | | | | | | |
| | | Life | | | 2010 | | | 2009 | |
| | | |
Acquired technology-Vitagel and CellPaker products | | | 11 years | | | $ | 9,000 | | | $ | 9,000 | |
Collagen processing technology (Note 3) | | | 9 years | | | | 5,199 | | | | 5,199 | |
Other licensed technology (Note 16) | | | 8 years | | | | 515 | | | | 515 | |
| | | | | | | | | | | | |
| | | | | | | 14,714 | | | | 14,714 | |
Less — Accumulated amortization | | | | | | | (4,830 | ) | | | (3,338 | ) |
| | | | | | | | | | | | |
| | | |
| | | | | | $ | 9,884 | | | $ | 11,376 | |
| | | | | | | | | | | | |
Acquired technology – Vitagel and Cellpaker products represents an intangible asset relating to our cash payment of $9,000 in 2006 to Angiotech Pharmaceuticals, Inc. (Angiotech) in order to eliminate any further obligations to pay royalties on future sales of Vitagel and CellPaker products under our license agreement with Angiotech. This intangible asset is being amortized based upon the greater of (a) straight-line amortization through July 31, 2017, or (b) actual units sold in a given period in relation to the total estimated units to be sold over the expected life of the applicable patent, which is through July 31, 2017.
Under the license agreement with Angiotech, we have exclusive rights to manufacture, market and sell Vitagel products throughout the world for orthopedic indications, and non-exclusive rights to manufacture, market and sell CellPaker products throughout the world for all indications through July 31, 2017. Additionally, under the license agreement, Angiotech has an option for co-exclusive rights outside the orthopedic field which, if exercised, would permit Angiotech to manufacture, market and sell an Angiotech-branded Vitagel product throughout the world. Until Angiotech elects to exercise its option for co-exclusive rights, we have exclusive rights to manufacture, market and sell Vitagel outside of the orthopedic field throughout the world. If Angiotech elects to exercise its option, we would then have co-exclusive rights to manufacture, market and sell Vitagel outside of the orthopedic field throughout the world.
F-13
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
Amortization expense was $1,492, $1,493 and $995 for 2010, 2009 and 2008, respectively, and has been recorded as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Cost of sales | | $ | 1,428 | | | $ | 1,429 | | | $ | 995 | |
Research and development | | | 64 | | | | 64 | | | | — | |
| | | | | | | | | | | | |
| | | |
Total | | $ | 1,492 | | | $ | 1,493 | | | $ | 995 | |
| | | | | | | | | | | | |
Amortization expense for the five years subsequent to the year ended December 31, 2010 is expected to be $1,492 per year.
9. Senior Secured Note Purchase Facility:
We have $35,000 in outstanding principal indebtedness under a senior secured note purchase facility. Notes issued under the facility are due July 30, 2012. Outstanding principal amounts under the notes bear annual interest at 10%, except that during the continuance of any event of defaults, interest would accrue at the rate of 12% per year and the notes would be payable on demand. In connection with entering into the facility, we issued to the note purchaser five-year warrants to purchase 1,466 shares of our common stock at an exercise price of $3.41 per share. Of these warrants, warrants to purchase 1,100 shares of our common stock have vested and, as of January 30, 2010, the remaining 366 warrants were no longer eligible to vest and are no longer considered outstanding. All warrants expire in July 2012.
We issued $25,000 principal amount of notes under the facility on July 30, 2007 and used most of the proceeds to repurchase a revenue interest obligation. In conjunction with the issuance of these notes, warrants to purchase 733 shares of common stock became exercisable. The fair value of these warrants of $1,206 was recorded in our consolidated balance sheet as a discount to the initial loan amount of $25,000 and is being amortized into interest expense over the remaining term of the facility.
On July 31, 2008, we issued an additional $10,000 principal amount of notes under the facility. We applied the proceeds of this note toward payment of (i) the $6,552 purchase price for the collagen raw material, equipment and technology license acquired under our supply and license agreement with Allergan (see note 3); and (ii) costs to expand our manufacturing capacity for Vitagel and ancillary products such as Aliquot, Imbibe and CellPaker. In conjunction with the issuance of the note, warrants to purchase 367 shares of common stock became exercisable. The fair value of these warrants of $286 has been recorded as a discount to the initial loan amount of $10,000 and is being amortized into interest expense over the remaining term of the facility.
Borrowings under the facility are guaranteed by us and one of our wholly-owned subsidiaries. The facility is secured by a first priority lien on substantially all of our assets (including intellectual property) other than those exclusively related to Cortoss and Aliquot. We are required to make quarterly interest-only payments to the note holder. Upon the occurrence of various events, including our receipt of (i) proceeds in excess of 5% of our total assets for certain asset dispositions; (ii) more than $1,000 in aggregate cash insurance proceeds for damaged or destroyed property that is not applied to the repair or replacement of the property within one year; or (iii) more than $7,500 in gross cash proceeds from judgment awards or settlements, the note holders are entitled to prepayment of the outstanding principal amount of the notes to the extent of the net cash proceeds that we receive. We may prepay at any time any part of the outstanding balance under the notes, in a minimum amount of $2,000 and in increments of at least $1,000 in excess of such minimum, together with interest accrued thereon. Both mandatory and optional prepayments are subject to a prepayment premium of up to 16% of the principal balance of the notes then outstanding, depending on the timing of the prepayment. The unpaid principal amount under the notes and accrued interest and all other obligations shall become due and payable immediately if we are insolvent, are in bankruptcy proceedings or have a custodian or receiver appointed for any substantial part of our property. If an event of default not described in the preceding sentence occurs and is continuing (including a change of control of the Company), then the holders of at least two-thirds in principal amount of notes then outstanding may declare the unpaid principal amount of the notes, accrued interest and all other obligations due and payable immediately. In addition, if the notes become due and payable, whether automatically or by declaration, by reason of any of the following events of default, then we must pay a prepayment premium depending on the timing of the prepayment:
| | • | | failure to pay any principal on any note or prepayment premiums, if any, when due and payable; |
| | • | | failure to pay any interest on any note or other amount (other than principal or prepayment premiums) for more than three business days after becoming due and payable; |
F-14
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
| | • | | we become insolvent, are in bankruptcy proceedings or have a custodian or receiver appointed for any substantial part of our property; or |
| | • | | the occurrence of a change of control of the Company as defined in the facility. |
The carrying value of the non-variable rate notes under the facility approximates fair value at December 31, 2010 based on rates that would be available to the Company for debt with similar terms.
Under the facility, we must comply with various financial and non-financial covenants. Under the financial covenant, we are required to maintain a minimum cash balance equal to at least 25% of the then-outstanding principal amount under the notes in a separate interest-bearing deposit or other similar demand investment account that is pledged as collateral for the loan. As of December 31, 2010, we must maintain a minimum cash, cash equivalent, and short-term investment balance of $8,750. If the balance in the separate account falls below an amount equal to 40% of the principal amount outstanding under the notes, we must obtain and maintain for the benefit of favor of the note holder a letter of credit in the amount of 25% of the principal amount outstanding under the notes. As of December 31, 2010, we must maintain a minimum balance of $14,000 in cash, cash equivalents, and short-term investments in order to avoid obtaining a letter of credit. Currently we are not required to obtain a letter of credit. The primary non-financial covenants limit our ability to incur indebtedness or liens, sell assets, conduct mergers or acquisitions as defined in the facility terms, make investments and pay dividends.
Interest expense on notes payable was $3,641, $3,112, and $2,777 for the years ended December 31, 2010, 2009 and 2008, respectively.
We are currently seeking to refinance this obligation; however, there is no assurance that we will be able to execute this refinancing or, if we are able to refinance this indebtedness, that the terms of such a refinancing would be equal to or better than the terms of the existing senior secured note purchase facility. Additionally, our cash flows and cash resources would be negatively impacted if we elected to repay the existing outstanding principal indebtedness with a combination of our existing cash and cash equivalents and new borrowings. If this obligation would have been paid in full at December 31, 2010, the prepayment premium would have been approximately $2,200. The amount of the prepayment premium decreases quarterly by $400. In addition to the prepayment premium, if we repay this indebtedness in full or in part prior to July 30, 2012, we would be required to write-off the unamortized debt discount and debt issuance costs remaining on our balance sheet at the date of such refinancing and recognize a non-cash loss. Assuming this debt was fully repaid on March 31, 2011, we would expect to recognize a charge of approximately $2,500 in connection with the early extinguishment of debt.
11. Other Accrued Expenses:
As of December 31, 2010 and 2009, other accrued expenses consisted of the following:
| | | | | | | | |
| | | 2010 | | | 2009 | |
Commissions payable | | $ | 2,238 | | | $ | 2,423 | |
Interest payable | | | 875 | | | | 875 | |
Royalties | | | 488 | | | | 567 | |
Professional fees | | | 428 | | | | 527 | |
Other | | | 1,374 | | | | 1,142 | |
| | | | | | | | |
| | $ | 5,403 | | | $ | 5,534 | |
| | | | | | | | |
12. Profit Sharing Plan:
We have a Section 401(k) plan for all qualified employees, as defined. Contributions are discretionary and determined annually and were $634, $692 and $667 for the years ended December 31, 2010, 2009 and 2008, respectively.
13. Shareholders’ Equity:
Equity Compensation Plan
Our equity compensation plan (the Plan) provides for incentive and nonqualified stock options, restricted stock awards, restricted stock units and other equity incentives to be granted to directors, employees, and consultants. Our shareholders have approved the Plan.
F-15
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
At December 31, 2010, there were 17,850 shares authorized for issuance under the Plan. The following table shows the number of shares underlying outstanding awards under the Plan at December 31, 2010 and the number of shares remaining available for grant under the Plan:
| | | | |
Shares authorized for issuance under the Plan | | | 17,850 | |
Common stock options currently issued and outstanding | | | (8,136 | ) |
Unvested restricted common stock units | | | (113 | ) |
Restricted common stock currently issued and outstanding | | | (110 | ) |
Shares issued upon exercise of common stock options and vesting of restricted stock units | | | (4,882 | ) |
| | | | |
Shares available for grant under the Plan at December 31, 2010 | | | 4,609 | |
| | | | |
Performance-based Equity Awards
In 2008, the Company granted performance-based equity awards under the Plan to certain executive officers whereby up to an aggregate of 421 shares of the Company’s common stock, valued at $2.68 per share, could be issued. The number of shares of common stock issuable under each award was dependent upon the Company’s achievement of pre-determined levels of Cortoss and Aliquot product sales in the U.S. during a twelve-month period that ended on June 30, 2010. Since none of the performance objectives were achieved, the awards have been cancelled. No compensation expense has been recorded for these performance-based equity awards during the years ended December 31, 2010, 2009 and 2008.
Common Stock
We have never declared or paid cash dividends on shares of our common stock and do not intend to pay any cash dividends in the foreseeable future on shares of our common stock.
During 2008, we exchanged options to purchase 131 shares of common stock for 131 common shares with certain non-employee consultants. In connection with this exchange, we incurred a non-cash charge of $170 in 2008. In 2008, we issued 7 shares of common stock valued at $21 for consulting services rendered pursuant to consulting agreements.
Restricted Common Stock and Restricted Common Stock Units
In 2009, we issued 156 shares of our common stock upon the vesting of certain of the restricted common stock units granted in 2007. As of December 31, 2010, 113 restricted common stock units are scheduled to vest in March 2011, subject to the continued employment of the holders of such units.
During 2010, 2009 and 2008, each year we issued an aggregate of 28 restricted common shares to non-employee directors in consideration of their services. These shares were valued at $54, $138 and $66, respectively. The shares granted vest in equal one-third installments on each anniversary of the date of grant, or earlier upon either (i) a change of control of the Company, (ii) the recipient’s death or (iii) the recipient’s departure in good standing from the board of directors provided he or she served on the board for at least five years or the sum of his or her age and length of services is at least 65. As of December 31, 2010, 62 restricted common shares are scheduled to vest in the future in accordance with the terms of the awards.
We have $156 of unrecognized cost related to unvested restricted common stock and restricted common stock units as of December 31, 2010, which is expected to be recognized over a weighted average period of approximately 0.6 years. The compensation expense recorded for these awards during 2010, 2009 and 2008 was $183, $266 and $368, respectively, and is included in stock-based compensation expense (see note 2).
Common Stock Options
Options are granted with exercise prices equal to or greater than the fair market value of the common stock on the date of grant. Generally, incentive stock options become exercisable in equal installments over a four-year period and nonqualified stock options to non-employee consultants are fully vested at the date of grant. The options generally remain exercisable for a maximum period of ten years.
During 2010, 2009 and 2008, we granted options to purchase 1,758, 1,310 and 1,787 shares of common stock, respectively,
F-16
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
under the Plan to our employees and non-employee directors. The respective aggregate fair values of these grants was $3,473, $2,638 and $3,046, and are being amortized over the four-year vesting period. Compensation expense related to the common stock options was $1,736, $1,395 and $903 for 2010, 2009 and 2008, respectively.
In July 2008, we issued to non-employee directors options to purchase 84 shares of common stock in the aggregate. These options have an exercise price of $2.35 per share and vest as to 25% of the underlying shares on each of the four successive anniversaries of the date of grant.
In July 2009, we issued to non-employee directors options to purchase 84 shares of common stock in the aggregate. These options have an exercise price of $4.89 per share and vest as to 25% of the underlying shares on each of the four successive anniversaries of the date of grant.
In July 2010, we issued to non-employee directors options to purchase 84 shares of common stock in the aggregate. These options have an exercise price of $1.92 per share and vest as to 25% of the underlying shares on each of the four successive anniversaries of the date of grant.
As of December 31, 2010, we had $4,720 of unrecognized compensation cost related to unvested common stock options which we expect to recognize over a weighted average period of approximately 2.4 years.
For all options outstanding as of December 31, 2010, the weighted average exercise price per share was $3.54, with a weighted average remaining contractual life of approximately 6 years and an aggregate intrinsic value of $31.
At December 31, 2010, there were 4,916 options exercisable. These options have a weighted average exercise price per share of $3.59, with a weighted average remaining contractual life of approximately 6 years and an aggregate intrinsic value of $1.
Summary stock option information is as follows:
| | | | | | | | | | | | | | |
| | | Aggregate
Number | | | Aggregate
Exercise Price | | | Exercise Price
Range | | Weighted
Average
Exercise Price | |
Outstanding, December 31, 2007 | | | 5,412 | | | $ | 20,977 | | | $1.65 – 11.25 | | $ | 3.74 | |
Granted | | | 1,787 | | | | 4,861 | | | 2.01 – 3.40 | | | 2.72 | |
Exercised | | | (5 | ) | | | (12 | ) | | 2.32 – 3.50 | | | 2.32 | |
Cancelled | | | (464 | ) | | | (1,494 | ) | | 1.69 – 6.60 | | | 3.22 | |
Expired | | | (54 | ) | | | (273 | ) | | 4.25 – 11.25 | | | 5.05 | |
| | | | | | | | | | | | | | |
Outstanding, December 31, 2008 | | | 6,676 | | | | 24,059 | | | 1.69 – 11.25 | | | 3.55 | |
Granted | | | 1,310 | | | | 4,757 | | | 2.60 – 6.73 | | | 3.63 | |
Exercised | | | (360 | ) | | | (1,206 | ) | | 2.48 – 4.48 | | | 3.35 | |
Cancelled | | | (290 | ) | | | (1,014 | ) | | 2.01 – 7.80 | | | 3.49 | |
Expired | | | (137 | ) | | | (703 | ) | | 4.75 – 6.60 | | | 5.17 | |
| | | | | | | | | | | | | | |
Outstanding, December 31, 2009 | | | 7,199 | | | | 25,893 | | | 1.65 – 6.75 | | | 3.55 | |
Granted | | | 1,758 | | | | 6,084 | | | 1.67 – 4.28 | | | 3.46 | |
Exercised | | | (212 | ) | | | (689 | ) | | 2.64 – 3.69 | | | 3.25 | |
Cancelled | | | (392 | ) | | | (1,223 | ) | | 1.94 – 6.73 | | | 3.12 | |
Expired | | | (217 | ) | | | (950 | ) | | 2.01 – 6.75 | | | 4.38 | |
| | | | | | | | | | | | | | |
Outstanding, December 31, 2010 | | | 8,136 | | | $ | 29,115 | | | $1.67 – 6.73 | | | 3.54 | |
| | | | | | | | | | | | | | |
As of December 31, 2010, non-employee consultants held fully-vested stock options to purchase 62 shares of common stock, at an average exercise price of $3.20 per share and an average remaining term of approximately three years. These options are classified as a non-current liability of $23 and $104 at December 31, 2010 and 2009, respectively, and are included in other long-term liabilities on our consolidated balance sheets as management does not believe that any financial liability underlying the options will need to be satisfied using current assets within the next twelve months.
F-17
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
Employee Stock Purchase Plan
We have an Employee Stock Purchase Plan (ESPP) under which eligible employees may purchase shares of our common stock at a per share price equal to 95% of the closing price of our common stock as reported on NASDAQ on the last trading day of each calendar quarter. Under the terms of the ESPP, eligible employees may have up to 10% of eligible compensation deducted from their pay to purchase common stock. The term of the ESPP expires in 2018.
Employees purchased 99, 68 and 75 shares under the ESPP during 2010, 2009 and 2008, respectively, for cash proceeds of $231, $240 and $184, respectively. In June 2009, our shareholders approved an amendment to the ESPP to increase by 200 the number of shares available for issuance under the ESPP. As of December 31, 2010, there were 500 shares of common stock authorized for issuance under the ESPP, of which 51 shares remained available for purchase and issue.
Common Stock Purchase Warrants
As of December 31, 2010, we had outstanding warrants to purchase 1,466 shares of common stock at an exercise price of $3.41 per share. All of these warrants were granted pursuant to the Senior Secured Note Purchase Facility (see note 9). Of the outstanding warrants, warrants to purchase 1,100 shares were exercisable as of December 31, 2010 and will expire in July 2012 if not exercised before that date. As of January 30, 2010, the remaining 366 warrants were no longer eligible to vest.
14. Product Sales:
For 2010, 2009 and 2008, product sales by geographic market were as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
United States | | $ | 89,459 | | | $ | 87,719 | | | $ | 71,997 | |
Outside the United States | | | 5,204 | | | | 5,134 | | | | 4,918 | |
| | | | | | | | | | | | |
Total product sales | | $ | 94,663 | | | $ | 92,853 | | | $ | 76,915 | |
| | | | | | | | | | | | |
Approximately 67%, 67%, and 63% of product sales during 2010, 2009 and 2008, respectively, were from products based upon the Vitoss Foam platform co-developed with Kensey (see note 16).
15. Income Taxes:
The components of income taxes are as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Current | | $ | (69 | ) | | $ | 20 | | | $ | 17 | |
Deferred | | | (417 | ) | | | 1,263 | | | | 3,317 | |
| | | | | | | | | | | | |
| | | (486 | ) | | | 1,283 | | | | 3,334 | |
Valuation allowance | | | 410 | | | | (1,270 | ) | | | (3,324 | ) |
| | | | | | | | | | | | |
Income tax (expense) benefit | | $ | (76 | ) | | $ | 13 | | | $ | 10 | |
| | | | | | | | | | | | |
Reconciliation between the (provision) benefit for income taxes, computed by applying the statutory federal income tax rate of 34% to income before income taxes, and the actual (provision) benefit for income taxes follows:
F-18
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
| | | | | | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Federal income tax provision at statutory rate | | | (34 | )% | | | (34 | )% | | | (34 | )% |
State tax (benefit) - Texas Gross Margin Tax | | | 1 | | | | 1 | | | | — | |
State income taxes, net of federal income tax | | | 8 | | | | (2 | ) | | | (4 | ) |
Change in state rate | | | 8 | | | | (2 | ) | | | — | |
Change in valuation allowance | | | (8 | ) | | | 32 | | | | 31 | |
Foreign net operating losses | | | 11 | | | | (11 | ) | | | — | |
Non-deductible expenses | | | 15 | | | | 17 | | | | 6 | |
Other | | | — | | | | (1 | ) | | | 1 | |
| | | | | | | | | | | | |
Actual income tax provision (benefit) effective tax rate | | | 1 | % | | | — | % | | | — | % |
| | | | | | | | | | | | |
Components of deferred tax asset as of December 31, 2010 and 2009 were as follows:
| | | | | | | | |
| | | December 31 | |
| | | 2010 | | | 2009 | |
Deferred tax assets: | | | | | | | | |
Net operating loss carryforwards | | $ | 53,985 | | | $ | 50,458 | |
Accrued expenses not currently deductible and other | | | 5,489 | | | | 4,164 | |
Research and patent costs capitalized for tax purposes | | | 7,286 | | | | 12,395 | |
Research and development credits | | | 1,889 | | | | 2,042 | |
Texas margin tax temporary credit | | | 282 | | | | 289 | |
| | | | | | | | |
Total deferred tax asset | | | 68,931 | | | | 69,348 | |
Valuation allowance | | | (68,649 | ) | | | (69,059 | ) |
| | | | | | | | |
Net deferred tax asset | | $ | 282 | | | $ | 289 | |
| | | | | | | | |
In October 2008, the United States Congress enacted the Housing Assistance Act of 2008 (Public Law 100-289), which enabled us to make a federal tax election to claim accelerated research tax credits in lieu of bonus depreciation. As a result, we recognized a current income tax benefit of $64 and $55 in the years ended December 31, 2009 and 2008, respectively. The ability to make this election was not extended for research tax credits for the year ended December 31, 2010.
In 2006, Texas enacted a margin tax which restructured the state business tax by replacing the taxable capital components of the current franchise tax with a new “taxable margin” component. The Texas margin tax was effective for taxable years ended on or after January 1, 2007. Under the Texas margin tax, we have the right to claim a temporary credit against our Texas margin tax liability over a 20 year period. Accordingly, during 2010, 2009 and 2008, we recognized current and deferred state income tax expense of $76, $51 and $45, respectively, and a deferred tax asset of $282 and $289 as of December 31, 2010 and 2009, respectively, specifically related to the Texas temporary credit on taxable margin. This deferred tax asset has been included in Other Assets on our consolidated balance sheets. We have recognized a deferred tax asset related to the Texas credit as we believe it is more likely than not to be realized based upon projections of future Texas margin tax liabilities.
As of December 31, 2010 we had approximately $131,212 of federal net operating loss carryforwards, $161,473 of state net operating loss carryforwards and $11,239 of foreign net operating loss carryforwards. Federal net operating loss carryforwards begin to expire in 2011 and will fully expire in 2028, while the state net operating carryforwards began to expire prior to 2010 and will fully expire in 2028. The federal net operating losses have a carryforward period of 20 years. The state net operating losses have carryforward periods ranging from five to 20 years, with the Pennsylvania net operating loss having a carryforward period of 20 years. The foreign net losses have an unlimited carryforward period. Federal tax years for the years ended December 31, 2007 and forward remain open to audit as well as prior years to the extent that available net operating losses are utilized. The amount of U.S. federal net operating loss and credit carryforwards which can be utilized in any one period may be limited by federal and state income tax
F-19
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
regulations, since a change in ownership as defined in Section 382 and 383 of the Internal Revenue Code may have occurred in prior years. The amount of net operating loss carryforwards which can be utilized in any one period on the Pennsylvania corporate income tax return is limited to the greater of $3,000 or 20% of apportioned net taxable income for tax years beginning after December 31, 2009. Federal and Pennsylvania research and development tax credits of $1,889 and $226 begin to expire in 2012 and 2013, respectively.
As of December 31, 2010, we determined that we had no liability for uncertain income taxes. Our policy is to recognize potential accrued interest as a component of interest expense and penalties as a component of tax expense related to the liability for uncertain income taxes benefits, if applicable.
16. Commitments:
Operating Leases
For the years ended December 31, 2010, 2009 and 2008, lease expense was $918, $897 and $783, respectively. At December 31, 2010, future minimum rental payments under operating leases are as follows:
| | | | |
2011 | | $ | 885 | |
2012 | | | 923 | |
2013 | | | 960 | |
2014 | | | 987 | |
2015 and thereafter | | | 2,687 | |
| | | | |
| | $ | 6,442 | |
| | | | |
Agreement with Kensey Nash Corporation
We have an agreement with Kensey Nash Corporation (Kensey) to jointly develop and commercialize certain biomaterials-based products based upon the Vitoss platform. The products developed under this agreement are based on our internally developed proprietary Vitoss bone void filler material in combination with proprietary resorbable Kensey biomaterials. Kensey has the exclusive right to manufacture any approved or cleared jointly developed product under the agreement, and we will market and sell the product worldwide. Under the agreement, we are obligated to pay Kensey both a transfer price for manufacturing products and royalties based on the net sales of such products. These rights and obligations extend until February 2014 for the Vitoss Foam product platform and until February 2024 for the Vitoss Bioactive Foam product platform, which includes Vitoss Bioactive Foam-2X and Vitoss BA Bimodal.
During 2010, 2009 and 2008, we purchased $6,483, $8,497 and $7,887, respectively, of product inventory manufactured by Kensey. As of December 31, 2010 and 2009, we owed Kensey $946 and $2,420, respectively, for manufactured product inventory and royalties, which are included in accounts payable and other accrued expenses in our consolidated balance sheets. All product royalty expense payable to Kensey is included in cost of sales on our consolidated statements of operations as we recognize product sales revenue from our customers.
Separate from the royalty payment obligation described above, we pay additional royalties to Kensey pursuant to a contractual royalty obligation that Kensey purchased from a co-inventor of Vitoss technology. Under this arrangement, we are obligated to pay no more than $5,000 in aggregate royalties on Vitoss product sales. For the years ended December 31, 2010, 2009 and 2008, we recognized $606, $645 and $593, respectively, as royalty expense under this contractual arrangement. From inception of the royalty arrangement through December 31, 2010, we have made aggregate royalty payments of $3,865.
Product Development Milestones
In 2009, we contractually agreed to make milestone payments upon achievement of specified goals in connection with the development of new products with one or more business partners. The timing and actual amount of these payments can be difficult to determine as these payments depend upon satisfactory achievement of product development and other milestones, which will be determined based on events that may occur in the future. During 2009, we made payments of $1,915 related to this product development program: $500 was recorded as a prepaid asset; $515 was recorded as an intangible asset for the value of a license acquired which is being amortized over the life of the licensed patents and the remaining amount of $900 was recorded as research and development expense. During the fourth quarter of 2010, we wrote off the $500 prepaid asset as the supplier was unable to satisfy its obligations under the agreement.
F-20
ORTHOVITA, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(All amounts in thousands except per share data)
If these products under development are brought to market, we may be obligated to make certain launch-related milestone payments of up to $1,000, as well as additional payments which may be made depending upon the potential achievements of clinical and sales milestones in the future.
Employment Agreements
Under the terms of employment agreements with two executive officers, extending through April 2012 and May 2012, subject to renewal, we are required to pay each individual a base salary for continuing employment with us. The agreements currently require aggregate payment of $692 in 2011. The agreements also provide for other benefits, including certain obligations that may be triggered by a change in control, termination with cause or resignation for good reason.
17. Quarterly Financial Data (Unaudited):
| | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31 | | | June 30 | | | September 30 | | | December 31 | | | Total | |
2010 | | | | | | | | | | | | | | | | | | | | |
Product sales | | $ | 24,077 | | | $ | 23,852 | | | $ | 23,327 | | | $ | 23,407 | | | $ | 94,663 | |
Gross profit | | | 16,678 | | | | 15,744 | | | | 15,089 | | | | 12,207 | | | | 59,718 | |
Total operating expenses | | | 17,088 | | | | 15,340 | | | | 14,410 | | | | 14,919 | | | | 61,757 | |
Net interest expense | | | (761 | ) | | | (946 | ) | | | (946 | ) | | | (938 | ) | | | (3,591 | ) |
Net loss | | | (1,186 | ) | | | (557 | ) | | | (283 | ) | | | (3,680 | ) | | | (5,706 | ) |
Net loss per share, basic and diluted | | $ | (0.02 | ) | | $ | (0.01 | ) | | $ | (0.00 | ) | | $ | (0.05 | ) | | $ | (0.07 | ) |
| |
| | | Three Months Ended | |
| | | March 31 | | | June 30 | | | September 30 | | | December 31 | | | Total | |
2009 | | | | | | | | | | | | | | | | | | | | |
Product sales | | $ | 21,690 | | | $ | 24,491 | | | $ | 22,295 | | | $ | 24,377 | | | $ | 92,853 | |
Gross profit | | | 14,680 | | | | 16,825 | | | | 15,145 | | | | 16,289 | | | | 62,939 | |
Total operating expenses | | | 15,053 | | | | 16,922 | | | | 16,149 | | | | 15,904 | | | | 64,028 | |
Net interest expense | | | (789 | ) | | | (587 | ) | | | (722 | ) | | | (732 | ) | | | (2,830 | ) |
Net loss | | | (1,176 | ) | | | (698 | ) | | | (1,741 | ) | | | (291 | ) | | | (3,906 | ) |
Net loss per share, basic and diluted | | $ | (0.02 | ) | | $ | (0.01 | ) | | $ | (0.02 | ) | | $ | (0.00 | ) | | $ | (0.05 | ) |
18. Valuation and Qualifying Accounts:
| | | | | | | | | | | | | | | | |
Description | | Balance,
Beginning
of Period | | | Cost and
Expenses | | | Other
adjustments(1) | | | Balance,
End of
Period | |
For the year ended December 31, 2010: | | | | | | | | | | | | | | | | |
Reserve for doubtful accounts | | $ | 217 | | | $ | 71 | | | $ | (6 | ) | | $ | 282 | |
| | | | |
For the year ended December 31, 2009: | | | | | | | | | | | | | | | | |
Reserve for doubtful accounts | | $ | 252 | | | $ | 18 | | | $ | (53 | ) | | $ | 217 | |
| | | | |
For the year ended December 31, 2008: | | | | | | | | | | | | | | | | |
Reserve for doubtful accounts | | $ | 218 | | | $ | 97 | | | $ | (63 | ) | | $ | 252 | |
| (1) | Represents write-offs of specific accounts receivable and other adjustments. |
F-21
EXHIBIT INDEX
| | |
Exhibit | | Description |
| |
| 3.1 | | Composite Amended and Restated Articles of Incorporation of the Company, as amended(16) |
| |
| 3.2 | | Amended and Restated Bylaws of the Company, as amended(16) |
| |
| 4.1 | | Specimen of Common Stock Certificate of the Company(2) |
| |
| *10.1 | | Amended and Restated Employment Agreement, as amended as of March 9, 2010, by and between the Company and Antony Koblish(7) |
| |
| *10.2 | | Amended and Restated Employment Agreement, dated as of March 9, 2010, by and between the Company and Nancy C. Broadbent(7) |
| |
| *10.3 | | Employment Letter, dated as of March 12, 1999, by and between the Company and Dr. Maarten Persenaire(3) |
| |
| *10.4 | | Confidentiality and Non-Disclosure Agreement, dated as of July 3, 2007, by and between the Company and Dr. Maarten Persenaire(5) |
| |
| *10.5 | | Severance and Change of Control Agreement, dated as of August 4, 2010, between the Company and Christopher H. Smith(11) |
| |
| *10.6 | | Severance and Change of Control Agreement, dated as of August 4, 2010, between the Company and Maarten Persenaire(11) |
| |
| *10.7 | | Form of Severance and Change of Control Agreement between Orthovita and Vice Presidents(1) |
| |
| *10.8 | | Form of Indemnification Agreement between the Company and directors(8) |
| |
| *10.9 | | 2007 Omnibus Equity Compensation Plan, as amended on June 23, 2010(17) |
| |
| *10.10 | | Form of Incentive Stock Option Grant(6) |
| |
| *10.11 | | Form of Nonqualified Stock Option Grant(6) |
| |
| *10.12 | | Form of Performance Stock Option Grant(18) |
| |
| *10.13 | | Form of Restricted Stock Unit Agreement(14) |
| |
| *10.14 | | Amended and Restated Employee Stock Purchase Plan(10) |
| | |
Exhibit | | Description |
| |
| 10.15 | | Senior Secured Note and Warrant Purchase Agreement, dated as of July 30, 2007, among the Purchasers named therein, the Company, and LB I Group Inc., as Collateral Agent, including form of 10% Senior Secured Promissory Note due July 30, 2012(15) |
| |
| 10.16 | | Form of Warrant to Purchase Common Stock of the Company(15) |
| |
| 10.17 | | Registration Rights Agreement, dated as of July 30, 2007, between the Company and the Purchasers named therein(15) |
| |
| 10.18 | | Security Agreement, dated as of July 30, 2007, among the Company, the other entities listed on Schedule A thereto, as Pledgors, and LB I Group Inc., as Collateral Agent(15) |
| |
| 10.19 | | Company Stock Pledge Agreement, dated as of July 30, 2007, by the Company in favor of LB I Group Inc. for the benefit of holders of those certain 10% Senior Secured Promissory Notes described in the Note Purchase Agreement(15) |
| |
| 10.20 | | Company Intellectual Property Security Agreement, dated as of July 30, 2007, by the Company in favor of LB I Group Inc. for the benefit of holders of those certain 10% Senior Secured Promissory Notes described in the Note Purchase Agreement(15) |
| |
| 10.21 | | Subsidiary Guaranty Agreement, dated as of July 30, 2007, among Vita Licensing, Inc., Orthovita International Services, Inc., Partisyn Corp. and Vita Special Purpose Corp., as Guarantors, and LB I Group Inc., as Collateral Agent(15) |
| |
| 10.22 | | Subsidiary Stock Pledge Agreement, dated as of July 30, 2007, by Vita Licensing, Inc. in favor of LB I Group Inc. for the benefit of holders of those certain 10% Senior Secured Promissory Notes described in the Note Purchase Agreement(15) |
| |
| 10.23 | | Subsidiary Intellectual Property Security Agreement, dated as of July 30, 2007, by each of Vita Licensing, Inc., Orthovita International Services, Inc., Partisyn Corp. and Vita Special Purpose Corp. in favor of LB I Group Inc. for the benefit of holders of those certain 10% Senior Secured Promissory Notes described in the Note Purchase Agreement(15) |
| |
| 10.24 | | Development, Manufacturing and Supply Agreement, dated as of March 25, 2003, between the Company and Kensey Nash Corporation (portions of this document have been omitted pursuant to the Company’s confidential treatment request under Exchange Act Rule 24b-2)(12) |
| |
| 10.25 | | Amendment to Development, Manufacturing and Supply Agreement, dated as of February 25, 2005, between the Company and Kensey Nash Corporation (portions of this document have been omitted and were filed separately with the SEC pursuant to a confidential treatment request under Exchange Act Rule 24b-2)(9) |
| |
| 10.26 | | Fifth Amendment to the Development, Manufacturing and Supply Agreement, dated as of February 21, 2007, between the Company and Kensey Nash Corporation (portions of this document have been omitted and were filed separately with the SEC pursuant to a confidential treatment request under Exchange Act Rule 24b-2)(5) |
| |
| 10.27 | | Amended and Restated License Agreement, dated as of December 29, 2006, between the Company and Angiotech Pharmaceuticals (U.S.), Inc. (portions of this document have been omitted and were filed separately with the SEC pursuant to a confidential treatment request under Exchange Act Rule 24b-2)(4) |
| |
| 10.28 | | Royalty Sale Agreement, dated as of December 29, 2006, between the Company and Angiotech Pharmaceuticals (U.S.), Inc.(13) |
| |
| 10.29 | | Supply and License Agreement, dated as of November 5, 2007, by and between Allergan, Inc. and Allergan Sales, LLC and Orthovita, Inc. (portions of this document have been omitted and were filed separately with the SEC pursuant to a confidential treatment request under Exchange Act Rule 24b-2)(5) |
| |
| 21.1 | | Subsidiaries(1) |
| |
| 23.1 | | Consent of KPMG LLP(1) |
| | |
Exhibit | | Description |
| |
| 31.1 | | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended(1) |
| |
| 31.2 | | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended(1) |
| |
| 32.1 | | Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002(1) |
| |
| 32.2 | | Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002(1) |
| * | Constitutes management contract or compensatory plan or arrangement required to be filed as an exhibit to this form. |
| (2) | Filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2000 and incorporated herein by reference. |
| (3) | Filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002 and incorporated herein by reference. |
| (4) | Filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006 and incorporated herein by reference. |
| (5) | Filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007 and incorporated herein by reference. |
| (6) | Filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008 and incorporated herein by reference. |
| (7) | Filed as an Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2009 and incorporated herein by reference. |
| (8) | Filed as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004 and incorporated herein by reference. |
| (9) | Filed as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2005 and incorporated herein by reference. |
| (10) | Filed as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008 and incorporated herein by reference. |
| (11) | Filed as an Exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 and incorporated herein by reference. |
| (12) | Filed as an Exhibit to the Company’s Current Report on Form 8-K filed on June 30, 2004 and incorporated herein by reference. |
| (13) | Filed as an Exhibit to the Company’s Current Report on Form 8-K filed on December 7, 2006 and incorporated herein by reference. |
| (14) | Filed as an Exhibit to the Company’s Current Report on Form 8-K filed on March 12, 2007 and incorporated herein by reference. |
| (15) | Filed as an Exhibit to the Company’s Current Report on Form 8-K filed on July 31, 2007 and incorporated herein by reference. |
| (16) | Filed as an Exhibit to the Company’s Current Report on Form 8-K filed on December 27, 2007 and incorporated herein by reference. |
| (17) | Filed as an Exhibit to the Company’s Current Report on Form 8-K/A filed on June 25, 2009 and incorporated herein by reference. |
| (18) | Filed as an Exhibit to the Company’s Current Report on Form 8-K filed on March 14, 2011 and incorporated herein by reference. |
