Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended March 27, 2009
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number0-23832
PSS WORLD MEDICAL, INC.
(Exact name of Registrant as specified in its charter)
| FLORIDA | 59-2280364 | |
| (State of incorporation) | (I.R.S. Employer Identification No.) | |
4345 Southpoint Boulevard Jacksonville, Florida | 32216 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code(904) 332-3000
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of Exchange on which registered | |
| Common Stock, $0.01 par value per share | NASDAQ GS Stock Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yesþ No¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes¨ Noþ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesþ No¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T
(§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes¨ No¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.:
Large accelerated filerþ | Accelerated filer¨ | Non-accelerated filer¨ | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes¨ Noþ
The aggregate market value of common stock held by non-affiliates, computed by reference to the closing price as reported on the NASDAQ GS, as of September 26, 2008 was approximately $1,141,144,259.
The number of shares of Common Stock, $0.01 par value, of the Registrant outstanding at May 15, 2009, was 59,372,323.
Table of Contents
Document Incorporated by Reference
Portions of the Registrant’s Definitive Proxy Statement for the 2009 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission not later than 120 days after March 27, 2009, are incorporated by reference into Part III of this Annual Report on Form 10-K.
Table of Contents
Table of Contents
Forward-Looking Statements
Management may from time-to-time make written or oral forward-looking statements with respect to the Company’s annual or long-term goals, including statements contained in this Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, reports to shareholders, press releases, and other communications. These statements are subject to risks and uncertainties that could cause actual results to differ materially from historical earnings and those currently anticipated or projected. Management cautions readers not to place undue reliance on any of the Company’s forward-looking statements, which speak only as of the date made.
Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “may,” “could,” and similar expressions identify forward-looking statements. Forward-looking statements contained in this Annual Report on Form 10-K that involve risks and uncertainties include, without limitation:
| • | Management’s belief that the medical products distribution industry is expected to experience continued growth due to the aging U.S. population, increased healthcare awareness, the proliferation of medical technology and testing, new pharmacology treatments, and expanded third-party insurance coverage; |
| • | Management’s belief that the elder care market is expected to continue benefiting from the increasing growth rate of the U.S. elderly population and the expansion of provider care into the patient’s home; |
| • | Management’s belief that the physician market is expected to continue benefiting from the shift of procedures and diagnostic testing in hospitals to alternate sites, particularly physician offices, but that near term sales growth rates will be lower than recent historic sales growth, due to general economic conditions, including a reduction in expected elective procedures; |
| • | Management’s belief that the healthcare services industry will continue to be subject to extensive regulation at the federal, state, and local levels and the Company has had, and will continue to have, adequate compliance programs and controls to ensure compliance with the laws and regulations; |
| • | Management’s belief that any new legislation or regulations, or new interpretations of existing statutes and regulations, governing the manner in which the Company conducts its business could have a material adverse impact on the Company and could adversely affect its profitability; |
| • | Management’s belief that a failure of a manufacturer to comply with the requirements of the Food and Drug Administration, the Drug Enforcement Administration and other Federal, state and local authorities, or changes in such requirements, could result in recalls, seizures, manufacturing suspensions or other interruptions in the production, supply, and sale of its products that may result in a material adverse impact on the Company’s business; |
| • | Management’s belief that the Company’s costs associated with complying with the various applicable federal and state statutes and regulations, as they now exist and as they may be modified, could be material; |
| • | Management’s belief that the outcome of legal proceedings or claims which are pending or known to be threatened will not have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations; |
| • | Management’s intention to retain earnings for the growth and development of the Company’s business and not declare cash dividends in the foreseeable future; |
1
Table of Contents
| • | Management’s belief that the global sourcing initiative will continue to improve its cost competitiveness and increase its gross margins on certain products and will continue to increase income from operations during future years; |
| • | Management’s expectation that the remaining federal and state net operating loss carryforwards will be utilized prior to their expiration date; that the Company’s deferred tax assets as of March 27, 2009 will be realizable to offset the projected future taxable income, and that changes in the Company’s current uncertain tax positions will not have a material impact on the results of operations or consolidated balance sheets; |
| • | Management’s expectation to make and integrate strategic business acquisitions in order to increase revenues and market share; |
| • | Management’s expectation that the overall growth in the business will be funded through a combination of cash flows from operating activities, borrowings under the revolving line of credit, capital markets, and/or other financing arrangements; |
| • | Management’s belief that the Company may seek to further retire a portion of its outstanding equity through cash purchases and/or reduce its debt and may also seek to issue additional debt or equity to meet its future liquidity requirements; |
| • | Management’s intent to either renegotiate existing leases or execute new leases upon the expiration date of such agreements; |
| • | Management’s belief that the Company may in future periods negotiate settlement of payments to manufacturers in the local currency of the country providing a product which would then subject the Company to foreign currency risk. |
In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, management is identifying important factors that could affect the Company’s financial performance and could cause actual results for future periods to differ materially from any opinions or statements expressed with respect to future periods in any current statements about the Company’s goals or expectations. The Company’s future results could be adversely affected by a variety of factors, including those discussed in Item 1A.Risk Factors. In addition, all forward-looking statements are qualified by and should be read in conjunction with the risks described or referred to in Item 1A.Risk Factors.
Third-Party Statistical Data
This report contains estimates and other information concerning the Company’s industry, including market size and growth rates, which are based on industry publications, surveys and forecasts. These estimates involve a number of assumptions and limitations, and you are cautioned not to give undue weight to these estimates. Although the Company believes the information in these industry publications, surveys and forecasts is reliable, it has not independently verified and cannot guarantee the accuracy or completeness of the information.
2
Table of Contents
| ITEM 1. | BUSINESS |
THE COMPANY
PSS World Medical, Inc. (the “Company” or “PSSI”), a Florida corporation, began operations in 1983. The Company is a national distributor of medical products and equipment, pharmaceutical products, healthcare information technology and billing services to alternate-site healthcare providers including physician offices, long-term care and assisted living facilities, home health care and hospice providers through 39 full-service distribution centers, which serve all 50 states throughout the United States (“U.S.”). The Company currently conducts business through two operating segments, the Physician Business and the Elder Care Business, which serve a diverse customer base. For information on comparative segment revenue, segment profit and related financial information, refer to Footnote 16,Segment Information, of the consolidated financial statements.
PSSI is a market leader in the alternate-site customer segments it serves as a result of value-added, solutions-based marketing programs; a differentiated customer distribution and service model; a consultative sales force with extensive product, disease state, reimbursement, and supply chain knowledge; unique arrangements with manufacturers; a full line of the Company’s own brand, Select Medical ProductsTM, and specialty brand products (“Select”); innovative information systems and technologies that serve its core markets; and a culture of performance.
THE INDUSTRY
According to industry estimates, the market size of the medical supply and equipment, home health care and office-administered pharmaceutical segments of the U.S. healthcare industry exceeds $53 billion. These market segments consist of medical products, medical equipment, and pharmaceutical products, which are distributed to alternate-site healthcare providers, including physician offices, long-term care and assisted living facilities, home health care providers and agencies, dental offices, and other alternate-site providers, such as outpatient surgery centers, and veterinarians. The Company’s business strategy focuses on the estimated $34 billion sub-segment of this market that includes the distribution of medical products, medical equipment, healthcare information technology and office-administered pharmaceutical products to physician offices, long-term care and assisted living facilities, home health care and hospice providers, and equipment dealers.
The medical products distribution industry is expected to experience continued growth due to the aging U.S. population, increased healthcare awareness, the introduction of new medical technology, new pharmacology treatments, and expanded third-party insurance coverage. The elder care market is expected to benefit from the increasing growth rate of the U.S. elderly population and the expansion of provider care into the patient’s home. The most recent U.S. Bureau of the Census report estimated the U.S. elderly population will more than double within the next 40 years, with Americans age 85 years and older, the population in the greatest need of long-term and elder care services, projected to more than triple during this period. The physician market is expected to grow, due, in part, to the shift of procedures and diagnostic testing from hospitals to alternate sites, particularly physician offices and homecare, and to expected changes in medical reimbursement rates, offset by slower growth due to general economic conditions.
The healthcare industry is subject to extensive governmental regulation, licensure, and operating compliance procedures. National healthcare reform has been the subject of a number of legislative initiatives by Congress and has been identified by President Barack Obama as a key priority of his administration. Government and private insurance programs fund a significant portion of medical costs in the United States. In recent years, state budget deficits and federal and state-imposed limits on reimbursement to hospitals, long-term care and assisted living facilities, physicians, home health care and other healthcare providers have affected spending budgets in certain markets within the medical products industry. In addition to these changes, the nursing home and home health care industries have been impacted by shifts in operations and business strategies, facility divestitures by elder care providers, migration of patient care to private homes as well as overall general economic conditions.
3
Table of Contents
THE PHYSICIAN BUSINESS
Physician Sales & Service, Inc., or the Physician Business, is a leading distributor of medical supplies, diagnostic equipment, and pharmaceutical related products to primary care office-based physicians in the United States based on revenues, number of physician-office customers, number and quality of sales representatives, diagnostic equipment revenues, and number of products distributed under exclusive arrangements. The Physician Business has approximately 700 sales professionals trained in solution-focused selling, disease state management, and diagnostic and therapeutic products used by physicians.
Customers
The Physician Business distributes products to office-based physicians who specialize in internal medicine, family practice, primary care, pediatrics, OB/GYN, general practice, and other specialties. Southern Anesthesia & Surgical, Inc. (“SAS”), the Physician Business’ wholly-owned pharmaceutical supply company, distributes pharmaceutical products, including controlled and non-controlled drugs, to physician offices and other alternate site healthcare providers. The Physician Business’ target market consists of approximately 603,500 physicians practicing at over 236,500 offices in the United States.
Customer pricing for each product is either negotiated directly with the physician or contracted through group purchasing organizations (“GPOs”). GPOs negotiate directly with medical product manufacturers and distributors on behalf of their members, establishing exclusive or multi-supplier relationships.
Distribution Infrastructure
At March 27, 2009, the Physician Business operated a distribution network consisting of 27 full-service distribution centers, 33 break-freight locations, and 2 redistribution facilities to serve customers throughout the United States. The operations of a full-service distribution center include sales support and certain administrative functions, such as customer billing, collections, cash application, and customer service, as well as general warehousing functions, inventory management, and product delivery. Inventory purchasing is centralized at the Company’s headquarters in Jacksonville, Florida. Full-service distribution centers receive inventory directly from manufacturers and redistribution centers. The distribution centers deliver product to customers and break-freight locations on a daily basis via the Company’s fleet of leased vehicles or third party transportation providers. Break-freight locations are interim warehouse facilities that receive packaged customer orders from full-service distribution centers and distribute them directly to customers on a daily basis. The distribution network is complemented by myPSS.com, a customer Internet ordering portal, Instant Customer Order Network (“ICON”), a laptop-based sales force automation tool which enables the Physician Business to extend customer-specific services with local market product and pricing flexibility, and “SmartScan,” a handheld inventory management device that allows customers to order product electronically and provides basic inventory management functions.
Products
The Physician Business distributes approximately 155,000 different products consisting of disposable supplies, pharmaceuticals, diagnostic equipment, non-diagnostic equipment, and healthcare information technology solutions.
Branded Medical-Surgical Disposable Supplies. This product category includes a broad range of medical supplies, including paper goods, needles and syringes, gauze and wound dressings, surgical instruments, sutures, examination gloves, orthopedic soft goods, tongue blades and applicators, sterilization and intravenous solutions, specimen containers, reagents for diagnostic equipment, and diagnostic rapid test kits. The Physician Business offers a broad array of branded products sourced from various medical product manufacturers.
4
Table of Contents
Select Medical-Surgical Disposable Supplies. The Company offers its own brand, Select Medical Products, in connection with its strategy of sourcing through global channels to drive enhanced customer satisfaction and profitability. The Select product category includes a broad range of medical supplies, including paper goods, needles and syringes, gauze and wound dressings, surgical instruments, sutures, examination gloves, orthopedic soft goods, tongue blades and applicators, sterilization products, specimen containers, diagnostic equipment reagents, and diagnostic rapid test kits.
Pharmaceutical Products. This product category includes various vaccines, injectables, inhalants, topicals, opthalmic ointments and solutions, otic solutions and oral analgesics, antacids and antibiotics, and controlled pharmaceutical products, which are used or administered in the physician’s office. Controlled pharmaceutical products include injectable anesthesia agents, narcotics, and pain management drugs.
Diagnostic Equipment. This product category includes various equipment lines such as blood chemistry analyzers, automated cell and differential counters, immunoassay analyzers, bone densitometers, electrocardiograph monitors and defibrillators, cardiac stress systems, cardiac and OB/GYN ultrasound, holter monitors, flexible sigmoidoscopy scopes, and microscopes. Sales of certain diagnostic equipment generate recurring orders of disposable diagnostic reagents consumed in the operation of the equipment.
Non-Diagnostic Equipment. This product category includes all other equipment used in a medical practice such as aesthetic lasers, autoclaves, examination tables, medical scales, and furniture.
Healthcare Information Technology. This product category includes various third party information technology products and services designed to improve the accuracy, efficiency, and effectiveness of physician business practices.
Competition
The Physician Business operates in a highly competitive industry where products and services are readily available to customers from a number of manufacturers, distributors, and suppliers. Competitors of the Physician Business include large, national, full-line distributors, many smaller regional and local distributors, and manufacturers who sell directly to customers. Competitive factors within the medical/surgical supply distribution industry include pricing, product availability, sales force capabilities, delivery time, electronic commerce capabilities, and the ability to meet customer-specific requirements.
THE ELDER CARE BUSINESS
Gulf South Medical Supply, Inc., or the Elder Care Business, is a national distributor of medical supplies and related products to the long-term and elder care industry in the United States. The Elder Care Business serves the nursing home and home care industries as well as the assisted living market segment. The home care industry refers to providers (companies, agencies, and care givers) of medical services, medical supplies, and equipment to patients in a home or residential setting. The Elder Care Business has approximately 130 sales professionals. The Elder Care Business also provides Medicare and Part B billing services, either on a fee-for-service or a full-assignment basis and Medicaid services to the assisted living market.
Customers
The Elder Care Business distributes and provides billing services to independent, regional, and national nursing home facilities, home health agencies, assisted living centers, hospices, and home medical equipment dealers. Approximately 14%, 15%, and 16%, of the Elder Care Business’ net sales for fiscal years 2009, 2008, and 2007, respectively, represent sales to its largest five customers.
5
Table of Contents
Distribution Infrastructure
At March 27, 2009, the Elder Care Business operated a distribution network consisting of 12 full-service distribution centers, 7 break-freight locations, 6 ancillary service centers, and 2 redistribution centers, which serve customers throughout the United States. The operations of a full-service distribution center include general warehousing functions such as inventory management, warehouse management, and product delivery directly to customers on a daily basis. Full-service distribution centers receive inventory directly from manufacturers and redistribution centers and distribute product to customers and break-freight locations. Break-freight locations receive packaged customer orders from full-service distribution centers and distribute them directly to customers on a daily basis. Product is delivered using either the Company’s fleet of leased delivery vehicles or third party transportation providers. Accounts receivable collections, cash application, customer billing, and inventory purchasing are centralized in the Company’s corporate headquarters in Jacksonville, Florida, while customer order processing, customer service, and sales support are centralized in Jackson, Mississippi. Coupled with a team of sales professionals, myGSOnline, an automated customer Internet platform, and SmartScan, the Elder Care Business provides service to customers ranging from independent nursing homes to providers of home health agencies and hospice, sub-acute, rehabilitation, and transitional care.
Products
The Elder Care Business distributes approximately 45,000 different medical and related products consisting of medical supplies, incontinent supplies and personal care items, enteral feeding supplies, point of care testing devices, advanced wound care, home medical equipment, and other supplies required by the long-term care patient. The Elder Care Business offers a broad array of branded products from various manufacturers. In addition, the Company offers its own brand, Select, in connection with its strategy of sourcing through global channels to drive enhanced customer satisfaction and profitability.
Services
The Elder Care Business, through its wholly-owned subsidiary, Proclaim, Inc., provides Medicare Part B billing services on a fee-for-service or a full-assignment basis and Medicaid billing services to the assisted living market.
Competition
The Elder Care Business operates in a highly competitive industry where products and services are readily available to customers from a number of manufacturers, distributors, suppliers, and service providers. Competitors of the Elder Care Business include large, multinational, full-line distributors, many smaller regional and local distributors, manufacturers who sell directly to customers, and Part B billing service providers. Competitive factors within the long-term and elder care industry include pricing, product availability, delivery time, electronic commerce capabilities, and the ability to meet customer-specific requirements.
CORPORATE SHARED SERVICES
Corporate Shared Services is a concentration of Company resources performing administrative functions across the operating segments by providing standardized service levels at an efficient operating cost. The services include accounting and finance; information technology development and support; operations management; regulatory compliance; legal; human resources, training and development; payroll administration; supplier management; and procurement of inventory and non-inventory products and services. Corporate Shared Services allocates a portion of its costs and interest expense to the operating segments. The allocation of shared operating costs is generally proportionate to the revenues of each operating segment. Interest expense is allocated based on (i) an internal carrying value of historical capital used to acquire or develop the operating segments’ operations and (ii) budgeted operating cash flow.
6
Table of Contents
SUPPLIER RELATIONSHIPS
Supplier relationships are an integral part of the Company’s businesses. Sales support, performance incentives, product literature, samples, demonstration units, training, marketing intelligence, distributor discounts and rebates, and new products are important elements of developing successful supplier relationships. The Company seeks to increase profitability by purchasing certain medical supplies, pharmaceutical products, and equipment at the lowest available price through volume discounts, rebates and product line consolidation under contracts with terms negotiated by the supplier management group.
The Company pursues opportunities to market and sell medical equipment and supplies through unique or exclusive marketing arrangements. Manufacturers of medical diagnostic equipment and supplies often seek to optimize the number of distributors selling their products to end users in order to reduce the cost associated with marketing and field sales support. The Company has been successful in obtaining unique or exclusive arrangements to sell certain products based on the size of its sales force and effectiveness of its marketing program.
GLOBAL PRODUCT SOURCING
The Company’s global sourcing strategy involves engaging foreign manufacturers to produce products according to the Company’s specifications. At March 27, 2009, the Company had approximately $39.1 million of globally-sourced product inventory, which represented approximately 1,280 SKUs and 170 product categories. These products were sourced from over 80 manufacturers located in China, Thailand, Malaysia, India, Poland, Cambodia, South Korea, Taiwan, the United Kingdom, and the Philippines. Products sourced from these manufacturers are based on price and availability of raw material.
The Company’s global product sourcing capabilities include identification of manufacturers in foreign locations, selection and specification of products to be manufactured, quality assurance programs and controls, and aligning product availability with customer needs. The Company’s global sourcing team consists of fully dedicated functional experts in areas such as global sourcing, logistics, supply chain design and management, supplier relations, product management, quality assurance and quality controls.
INFORMATION SYSTEMS
The Physician and Elder Care Businesses operate the JD Edwards XE platform (“JDE”) at all distribution centers. The Company’s controlled pharmaceutical operations uses a separate version of JD Edwards XE leveraged from the acquisition of SAS and customized to provide compliance with DEA and FDA regulations.
I2 Supply Chain Management (“SCM”) enables the Company to strategize, plan, and execute its inventory management, and service business processes across multiple locations to increase customer satisfaction and profitability. I2 SCM enables the Company to reduce operating inventory stocking levels, improve fill rates, increase operating inventory turnover, and increase cash flow from operations.
The Physician Business’ laptop-based sales-force automation application, known as “ICON”, carries customer order history and accounts receivable detail, reflects on-hand inventory quantities for the local distribution center, and transmits orders over a secure wireless network. The Physician Business’ internet portal, myPSS.com, provides its customers with sales history, accounts receivable detail, available inventory and supports a number of ordering methods. Approximately 67% of customer orders in the Physician Business are received via ICON or myPSS.com.
The Elder Care Business’ internet portal, myGSOnline.com, provides its customers with sales history, accounts receivable detail, available inventory and supports a number of ordering methods. As a result, the Elder Care Business is an industry leader in eCommerce transactions. Approximately 83% of customer orders in the Elder Care Business are electronic orders.
7
Table of Contents
Online order processing is supplemented in the Physician Business and Elder Care Business with “SmartScan,” a handheld inventory management device that allows customers to order product electronically and provides basic inventory management functions. This device accounts for approximately 7% and 1% of customer orders for the Physician Business and Elder Care Business, respectively.
During fiscal year 2010, the Company will be begin the implementation of an advanced warehouse management system (“WMS”) that will streamline warehouse management, tighten inventory controls and improve customer service. The Company will also implement a new Contracts and Rebates administration system. This new system will provide enhanced visibility into various customer contract scenarios and is intended to lead to a reduction in denied rebates and reduced operating costs.
REGULATORY MATTERS
General
Federal, state, local and foreign government agencies extensively regulate the distribution and sale of medical devices, medical supplies and pharmaceutical products and the billing of government sponsored healthcare programs. Applicable federal, state and foreign statutes and regulations require the Company to meet various standards relating to, among other things, licensure, personnel, physical security, maintenance of proper records, privacy of health information, maintenance and repair of equipment, and quality assurance programs.
The Company’s costs associated with complying with the various applicable federal and state statutes and regulations, as they now exist and as they may be modified, could be material. Although the Company intends to comply with all applicable laws and regulations, many of them have been recently enacted, are broadly worded, and have not been interpreted by regulators and the courts. Consequently, they have been and may continue to be interpreted or applied by governmental authorities in a manner that differs from the Company’s interpretation, which has required and could continue to require the Company to make changes in its operating procedures and increase operating costs. Future allegations by a state or the federal government that the Company has not complied with these laws could have a material adverse impact on the Company. If it is determined that the Company has not complied with these laws, or if the Company enters into settlement agreements to resolve allegations of non-compliance, the Company could be required to make settlement payments, quarantine or destroy inventory, or be subject to civil and criminal penalties, including fines and the loss of licenses or its ability to participate in Medicare, Medicaid and other federal and state healthcare programs. Any of the foregoing could have a material adverse impact on the Company. The Company believes that the healthcare services industry will continue to be subject to extensive regulation at the federal, state, local and foreign levels.
The Food, Drug and Cosmetic Act, Prescription Drug Marketing Act of 1987, Safe Medical Devices Act of 1990, Controlled Substances Act and Various State Regulations
The Company’s business is subject to regulation under the Federal Food, Drug and Cosmetic Act, the Prescription Drug Marketing Act of 1987, the Safe Medical Devices Act of 1990, and state laws applicable to the manufacture, importation and distribution of medical devices and over-the-counter pharmaceutical products, as well as the distribution of prescription pharmaceutical products. In addition, the Company is subject to regulations issued by the United States Food and Drug Administration (“FDA”), the Drug Enforcement Administration, and comparable state agencies.
The Federal Food, Drug, and Cosmetic Act generally regulates the manufacture and importation of drugs and medical devices shipped via interstate commerce, including such matters as labeling, packaging, storage, and handling of such products. The Prescription Drug Marketing Act of 1987, which amended the Federal Food, Drug and Cosmetic Act, establishes certain requirements applicable to the wholesale distribution of prescription drugs, including the requirement that wholesale drug distributors be registered with the Secretary of Health and Human Services or be licensed in each state in which business is conducted in accordance with federally
8
Table of Contents
established guidelines on storage, handling, and records maintenance. The Safe Medical Devices Act of 1990 imposes certain reporting requirements on distributors in the event of an incident involving serious illness, injury, or death caused by a medical device. The Company is also required to maintain licenses and permits for the distribution of pharmaceutical products and medical devices under the laws of the states in which it operates.
Healthcare Fraud and Abuse Laws
The Company is subject to extensive local, state and federal laws and regulations relating to healthcare fraud and abuse. Federal and state governments continue to scrutinize potentially fraudulent practices in the healthcare industry in an attempt to minimize the cost that such practices have on Medicare, Medicaid and other government healthcare programs. Under Medicare, Medicaid, and other government-funded healthcare programs, federal and state governments enforce a Federal law called the Anti-Kickback Statute. The Anti-Kickback Statute, and the related regulations prohibits any person from offering, paying, soliciting or receiving anything of value to or from another person to induce the referral of business, including the sale or purchase of items or services covered by Medicare, Medicaid, or other federally subsidized programs. Many states also have similar anti-kickback statutes.
State and Federal Drug Pedigree Laws
Several states have enacted or proposed laws and regulations designed to protect the integrity of the supply channel. For example, Florida and other states have implemented drug pedigree requirements that require prescription drugs to be distributed with records or information documenting the prior distribution of the drug back to the manufacturers. In addition, California has proposed legislation that will require the implementation of an electronic drug pedigree system that provides “track and trace” chain of custody technologies, such as radio frequency identification, or RFID, technologies by January 1, 2016 for wholesale distributors. There have been increasing efforts by various levels of government to regulate the pharmaceutical distribution system in order to prevent the introduction of counterfeit, adulterated or misbranded pharmaceuticals into the distribution system. At the federal level, the FDA issued final regulations pursuant to the Prescription Drug Marketing Act that became effective in December 2006. The regulations impose drug pedigree and other chain of custody requirements that increase the costs and/or burden of selling our products and handling product returns. In early December 2006, the federal District Court for the Eastern District of New York issued a preliminary injunction, enjoining the implementation of some of the federal drug pedigree requirements, in response to a case initiated by secondary distributors. The case has currently been stayed pending legislative action that would render the issues involved in the case moot. Moreover, the United States Food and Drug Administration Amendments Act of 2007, which went into effect on September 27, 2007, requires the FDA to establish standards and identify and validate effective technologies for the purpose of securing the pharmaceutical supply chain against counterfeit drugs. These standards include track and trace or authentication technologies, such as RFID and other technologies. The FDA must develop a standardized numerical identifier by April 1, 2010. Finally, legislation has been introduced in Congress that would revise and expand the federal pedigree requirements and create a timeline for the adoption of unique identifiers and use of electronic track and trace technologies. If enacted, these pedigree requirements could preempt existing state pedigree requirements and the Company may have to adopt or modify its operations to initiate and transmit electronically-coded pedigree information concerning the purchase and transmittal of prescription drugs in all 50 states.
The Health Insurance Portability and Accountability Act of 1996
The Health Insurance Portability and Accountability Act of 1996 and its implementing regulations (collectively, “HIPAA”) establishes (i) national standards for some types of electronic health information transactions and the data elements used in those transactions, (ii) standards to protect the privacy of individually identifiable health information, and (iii) security standards to ensure the integrity and confidentiality of health information. Health plans, health care clearinghouses and most health care providers, including the Company, are “Covered Entities” subject to HIPAA.
9
Table of Contents
Other Laws
The Company is subject to various additional federal, state and local laws, and regulations in the United States, relating to the safe working conditions and the sales, use and disposal of hazardous or potentially hazardous substances. In addition, laws that affect our foreign operations include U.S. and international import and export laws and regulations that require that the Company abide by certain standards relating to the importation and exportation of finished goods, raw materials, and supplies, and the Foreign Corrupt Practices Act. Furthermore, the Department of Transportation regulates the conveyance of regulated materials, both in Company-leased delivery vehicles and via common carrier.
Impact of Changes in Healthcare Legislation
Federal, state and foreign laws and regulations affecting the Company’s business are subject to change. The Company cannot predict what impact, if any, such changes might have on its business. Any new legislation or regulations, or new interpretations of existing statutes and regulations, governing the manner in which the Company conducts its business could have a material adverse impact on the Company and its results of operations.
The extensive federal and state laws and regulations described above apply not only to the Company, but also to the manufacturers which supply the products distributed by the Company and the Company’s physician and other healthcare customers. For instance, medical product and device manufacturers are subject to design, manufacturing, labeling, promotion and advertising standards imposed on, as well as registration and reporting requirements regarding, their facilities and products. Likewise, pharmaceutical manufacturers are subject to development, manufacturing and distribution regulation by the FDA, the Drug Enforcement Administration and other federal, state and local authorities. Failure of a manufacturer to comply with these requirements, or changes in such requirements, could result in recalls, seizures, manufacturing suspensions or other interruptions in the production, supply, and sale of its products. Such interruptions may result in a material adverse impact on the Company’s business. Similarly, changes in the extensive regulations or in their interpretation or enforcement applicable to the Company’s customers could adversely impact the Company’s business in ways which are difficult for the Company to predict.
See Item 1A “Risk Factors” for a discussion of additional regulatory developments that may affect the Company’s results of operations and financial condition.
PROPRIETARY RIGHTS
The Company has registered with the United States Patent and Trademark Office the marks PSS WORLD MEDICAL (and Design), PSS, PSS ADVANTAGE CLUB THE ADVANTAGE IS SAVING YOU MONEY (and Design), ANSWERS (and Design), SMARTSCAN, SRX (and Design), PHYSICIAN SELECT, NIGHTINGALE, SOUTHERN ANESTHESIA & SURGICAL, INC. (and Design), ADVANCE PLUS + SOUTHERN ANESTHESIA & SURGICAL, Inc. (and Design), GULF SOUTH MEDICAL SUPPLY (and Design), SELECT MEDICAL PRODUCTS (and Design) and SELECT MEDICAL PRODUCTS PSS GULF SOUTH MEDICAL SUPPLY (and Design), among others. The Company’s trademarks generally have a term of ten years. The Company believes that the PSS World Medical, Physician Sales & Service, and Gulf South Medical Supply names are well recognized in the medical supply industry and by healthcare providers and, therefore, are valuable assets of the Company.
EMPLOYEES
As of March 27, 2009, the Company employed approximately 3,680 full-time and part-time employees. The Company believes ongoing employee training is critical to its success and, accordingly, invests significant resources in training, continuing professional education and leadership development. In support of the Company’s mission statement and commitment to being the employer of choice, the Company offers both online
10
Table of Contents
and instructor-led training. The Company’s Center for Career Development (“CCD”) utilizes an Internet-based, enterprise-wide learning management system, “CCD Online,” which is designed to provide a comprehensive learning environment and in-depth training for every employee. At March 27, 2009, there were approximately 110 online classes available to employees and since its inception in 2001, employees have completed over 136,000 classes online. The Company conducted 44 classes and trained over 945 employees in instructor-led classroom sessions during fiscal year 2009. Management believes that relations with employees are strong and the Company’s long-term success depends upon its employees, including its sales professionals.
AVAILABLE INFORMATION
The Company files annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission (“SEC”). Any documents that have been filed with the SEC may be read or copied, at prescribed rates contingent upon a written request, at its Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. These documents are also filed with the SEC electronically and are accessible on the SEC’s Internet website found atwww.sec.gov. Copies of materials filed with the SEC may also be obtained free of charge from the Company’s Internet website found atwww.pssworldmedical.comas soon as reasonably practicable after such material is electronically file with or furnished to the SEC.
The Company also provides public access to its Code of Ethics, Audit Committee Charter and Corporate Governance Committee Charter, which are made available to the public free of charge in the Investor Relations section of the Company’s websitewww.pssworldmedical.com or may be obtained by writing to: PSS World Medical, Inc., Investor Relations, 4345 Southpoint Blvd., Jacksonville, Florida 32216. The Company intends to post amendments to or waivers from its Code of Ethics (to the extent applicable to the Company’s principal executive officer, principal financial officer, or principal accounting officer) on its website.
11
Table of Contents
| ITEM 1A. | RISK FACTORS |
The Company’s continued success depends on management’s ability to identify, prioritize and appropriately manage a wide range of enterprise risk exposures. Readers should carefully consider each of the following risks and additional information set forth in this Annual Report on Form 10-K. These risks and other factors may affect forward-looking statements, including those made by the Company in this document or elsewhere, such as in earnings release web casts, investor conference presentations, or press releases. The risks and uncertainties described herein may not be the only ones facing the Company and are not organized in order of priority. Additional risks and uncertainties not presently known to management or that management currently believes to be immaterial may also adversely affect the Company’s business. If any of the following risks and uncertainties develop into actual events, it could materially affect the Company’s business, financial condition or results of operations, cause the trading price of the Company’s common stock to decline materially, or cause actual results to differ materially from those expected.
General economic conditions, including the current global economic slowdown may materially adversely impact the Company’s operating results.
Current and future economic conditions and other factors including consumer confidence, employment levels, interest rates, tax rates, consumer debt levels, the threat or outbreak of terrorism, fuel and energy costs, the availability of consumer credit, and the impact of state budget deficits on Medicaid reimbursement can reduce consumer spending or change consumer purchasing habits. The current slowdown in the global economy has and may continue to materially adversely affect consumer spending habits and our operating results.
President Barack Obama has identified health care reform as a key priority of his administration. Publicly disclosed initiatives include increased availability of insurance, funding for the use of health care information technology, promotion of patient safety, and increased efficiencies in Medicare and Medicaid. It is unclear at this time what laws and regulations may ultimately be enacted and what impact such laws and regulations will have on the purchasing patterns of the Company’s customers, and as a result, the Company’s financial condition, results of operations and cash flows.
Numerous factors, many of which cannot be controlled by the Company, may cause the Company’s net sales and results of operations to fluctuate, which may adversely affect the market price of the Company’s common stock.
The Company’s net sales and operating results may fluctuate as a result of many factors, including:
| • | general economic conditions; |
| • | demand for the products and services offered by the Company; |
| • | introduction of new products and services offered by the Company and its competitors; |
| • | seasonal vaccine sales; |
| • | retention of sales representatives and other key employees; |
| • | acquisitions, dispositions, or other investments by the Company; |
| • | changes in manufacturers’ pricing policies, contract terms and distribution strategies; |
| • | changes in the level of operating expenses; |
| • | changes in estimates used by management; |
| • | changes in the Company’s business strategies, or those of its competitors; |
| • | product supply shortages; |
| • | product recalls by manufacturers; |
| • | inventory valuation adjustments; |
| • | changes in product mix; |
| • | fuel costs and third party shipping rates; |
| • | inclement weather; |
12
Table of Contents
| • | changes in accounting principles; |
| • | disruptions resulting from implementing strategic business plans; |
| • | disruptions resulting from implementing enterprise resource planning (“ERP”) systems; |
| • | the number of selling days in a period; and |
| • | changes by the government, including healthcare reform, changes in reimbursement rates to providers and regulatory requirements related to the distribution of medical and pharmaceutical products. |
Accordingly, management believes that period-to-period comparisons of the results of operations should not be relied upon as an indication of future performance because these factors may cause the Company’s results of operations to be below analysts’ and investors’ expectations in certain future periods. This could materially and adversely affect the trading price of the Company’s common stock.
The viability of the Company’s customers may be threatened by various factors.
The Company could be negatively impacted if customers experience disruptions resulting from tighter capital and credit markets, or a loss of patient revenue due to a slowdown in the general economy. As a result, customers may modify, delay or cancel plans to purchase the Company’s products or services. Additionally, if customers’ operating and financial performance deteriorates, or if they are unable to make scheduled payments or obtain credit, customers may not be able to pay, or may delay payment of, accounts receivable owed to the Company. Any inability of customers to pay for products and services, may adversely affect the Company’s earnings and cash flow.
The Company’s customers are also impacted by increasing costs of malpractice claims and liability insurance. Insurance rates for customers of the Elder Care and Physician Businesses have greatly increased. If meaningful reform legislation is not adopted or adopted legislation is not effective in reducing these rates, many of the Company’s customers may be adversely affected which, in turn, could affect their profitability. As a result, customer financial viability may adversely impact the Company’s financial condition, net sales, results of operations, and cash flows from operations.
The Company may not be able to continue to successfully compete with other medical supply companies and direct manufacturers.
The medical supply distribution market is highly competitive. The Company’s results of operations could be materially adversely affected if competitors lower prices of products similar to those distributed by the Company. Principal competitors of the Company include full-line and full-service, multi-market medical distributors, internet distributors, and direct selling manufacturers, many of which have a national presence. The Company also faces significant competition from regional and local distributors, telemarketing firms, internet companies, and mail order firms. The Company’s competitors may have the following strengths:
| • | sales representatives competing directly with the Company; |
| • | capability to market products directly to the Company’s customers; |
| • | access to products distributed by the Company from various suppliers; |
| • | substantially greater financial resources than the Company; and |
| • | lower product and operating costs. |
Consolidation within the healthcare industry has resulted in increased competition by direct manufacturers, large national distributors, and drug wholesalers, and may result in lower customer pricing and/or higher operating costs. Continued consolidation of medical supply distributors could result in the following:
| • | competitors obtaining exclusive rights to market products to the Company’s customers; |
| • | potential new entrants to the markets the Company serves; |
| • | national hospital distributors, drug wholesale distributors, and healthcare manufacturers may focus their efforts more directly on the Company’s markets; |
13
Table of Contents
| • | hospitals forming alliances with long-term care facilities or physician practices to create integrated healthcare networks may look to hospital distributors and manufacturers to supply their affiliates; and |
| • | provider networks created through consolidation among physician provider groups, long-term care facilities, and other alternate site providers may shift purchasing decisions to entities with whom the Company has no current relationship. |
There can be no assurance the Company will maintain operating margins and customer relationships and avoid increased competition and significant pricing pressure in the future if medical supply distributor consolidations occur. If the Company is unable to compete successfully with other medical supply distributors and direct manufacturers, the Company’s business, financial condition, and results of operations may be materially adversely affected.
The Company’s future operating results can be affected by its relationships with its customers, sales representatives, and senior management team.
The Company’s ability to retain existing customers and attract new customers is dependent upon hiring and retaining sales representatives. Customer relationships are at risk if a sales representative ceases employment with the Company, particularly where the representative seeks employment with a competitor. The Company has increased the use of employment agreements containing restrictive covenants, which protect the Company’s legitimate business interests. However, these agreements have not been obtained for all sales representatives. In addition, the terms of these agreements, in certain states, may not be enforceable. The inability to adequately hire or retain sales representatives could limit the Company’s ability to expand its business and increase sales.
The Company’s success depends largely on the efforts and abilities of senior management, particularly the executive management team. In addition, the Company is also dependent upon the operations and sales managers at each distribution center because of the decentralized operating structure. Although the Company maintains key man life insurance for its chief executive officer, chief financial officer, and the chief operating officer, the loss of services of one or more of its members of senior management may adversely affect the Company’s business. In addition, the Company may not be successful in attracting and retaining other key personnel.
The Company relies extensively on its relationships, significant distribution agreements and other purchasing arrangements with suppliers.
The Company has distribution agreements and other purchasing arrangements with approximately 2,000 suppliers. The Company relies on these suppliers to manufacture and/or supply products for and to the Company for resale to the Company’s customers. If any distribution agreement or other purchasing arrangement between the Company and a supplier expires or is terminated, if the Company fails to meet the minimum requirements under the agreement, or if the Company and any supplier otherwise cease conducting business with each other, then the Company’s net sales and results of operations may be materially adversely affected.
Since the Company does not manufacture the products it sells, it is dependent on vendors and manufacturers for the supply of products. The Company relies on suppliers to provide, among other things:
| • | field sales representatives’ technical and selling support; |
| • | acceptable purchasing, pricing, and delivery terms; |
| • | sales performance incentives; |
| • | vendor rebates for inventory purchases or sales volume; |
| • | vendor incentives; |
| • | financial support of sales and marketing programs; |
| • | promotional materials; and |
| • | product availability. |
14
Table of Contents
There can be no assurance that the Company will be successful in maintaining good relations with its suppliers. The Company’s global sourcing strategy may threaten or endanger relations with certain suppliers. Additionally, there can be no assurance that the Company will meet forecasted inventory purchases, sales volume, or other criteria required to obtain the benefits outlined in the agreement.
Cost increases for the Company’s products may impact the Company’s results of operations.
Some of the Company’s suppliers have and may continue to accelerate cost increases for products distributed by the Company. While the Company is taking steps to mitigate the effect of these cost increases, there can be no assurance that these cost increases will not materially adversely impact the Company’s gross margins, financial conditions, and results of operations.
Trends in healthcare spending and competitive bidding may impact the Company’s results of operations.
A significant portion of medical costs in the United States are funded by government and private insurance programs, such as Medicare, Medicaid, and corporate health insurance plans. In recent years, government-imposed limits on reimbursement to hospitals, physicians, nursing homes, home health providers, and other healthcare providers have significantly impacted spending in certain markets within the medical-products industry. Future changes in Medicare and state administered Medicaid programs may limit payments to providers and customers served by the Company. Significant reductions in reimbursement levels and adjustments, in combination with rising costs, may negatively impact customers’ financial health and liquidity and may negatively affect the Company’s results of operations. Additionally, the Company’s Medicare Part B billing services are subject to a competitive bidding process. The inability of the Company to successfully compete could impact the Company’s net sales and results of operations.
The Company may face increasing, competitive pricing pressures on its sales to national and regional customers and consolidated provider groups.
Sales to large, national and regional accounts and consolidated provider groups, especially in the long-term care market, represent a significant portion of the Company’s revenue base. Competitive pricing pressures may increase due to:
| • | change in ownership; |
| • | additional negotiating leverage of large, regional and national chains; |
| • | supplier agreements containing volume discounts; |
| • | service specifications; |
| • | financial health of customers; |
| • | activity of competitors; and |
| • | activity of group purchasing organizations (“GPO”). |
The Company’s business strategy may not mitigate the effect of pricing pressures, which could adversely impact the Company’s net sales, gross margins, and results of operations.
Expansion of GPO or hospital purchasing power and the multi-tiered costing structure may place the Company at a competitive disadvantage.
The medical-products industry is subject to a multi-tiered costing structure, which can vary by manufacturer and/or product. Under this structure, certain institutions can obtain more favorable prices for medical products than the Company. The multi-tiered costing structure continues to expand as many large integrated healthcare providers and others with significant purchasing power, such as GPOs, demand more favorable pricing terms. This may threaten the Company’s ability to compete effectively, which would in turn negatively impact the Company’s results of operations. Although the Company is seeking to obtain similar terms from manufacturers and obtain access to lower prices demanded by GPO contracts or other contracts, management cannot assure such terms will be obtained or contracts will be executed.
15
Table of Contents
The Company depends on the availability of multi-tiered priced products from other distributors at prices lower than the manufacturers’ list prices.
The Company depends on the availability of multi-tiered priced products from other distributors at costs that are lower than the manufacturers’ list prices. The reduction of availability of lower-priced product costs could negatively impact the Company’s gross margins. If the Company cannot obtain prices from other distributors that are lower than the manufacturers’ list prices, the Company’s gross margin and results of operations may be materially adversely affected.
The operating costs of the Company’s delivery fleet could increase due to fuel price fluctuations and/or service interruptions by third parties.
The Company’s operating costs are affected by fluctuations in the cost of gasoline and diesel fuel. Products are delivered to customers either by the Company-leased delivery fleet or third party transportation providers. The Physician and Elder Care Businesses currently operate a delivery fleet of over 600 vehicles and management considers utilization of this fleet to be one of the Company’s competitive advantages. Significant fluctuations in the cost of fuel have impacted the Company’s cost to deliver product to customers and the operating costs of transportation providers. Some common carriers have passed increases through to the Company in the form of a fuel surcharge, which had an adverse effect on the Company’s results of operations. In addition, significant increases in the cost of fuel may increase the cost of product obtained from suppliers due to increased inbound freight costs as well as increased shipping costs for globally sourced product. Similarly, strikes or other service interruptions by third party transportation providers may cause an increase in the Company’s operating expenses and adversely affect the Company’s ability to deliver products on a timely basis.
Management anticipates fluctuations in fuel costs may negatively impact gross margin, the cost to deliver, or expected improvements in cost to deliver in future periods. While a customer fuel surcharge may offset a portion of this negative impact, the Company’s operating costs may be negatively affected.
The Company’s strategy for growth may not result in additional net sales or operating income and may have an adverse effect on working capital, operating cash flow, and results of operations.
The Company seeks to increase revenues and operating income by:
| • | expanding product offerings; |
| • | expanding sales support services; |
| • | expanding sales force for home health care customers; |
| • | increasing sales force productivity; |
| • | developing innovative marketing and distribution programs; |
| • | maintaining and expanding vendor incentive programs; |
| • | expanding e-commerce initiatives and development; |
| • | leveraging its infrastructure and information systems; |
| • | improving distribution capability and efficiency through systems development and implementation; |
| • | improving supply chain efficiency through centralization and systems enhancements; and |
| • | improving operating margins through product sourcing initiatives. |
These business strategies for growth may result in increased costs and expenses. There can be no assurance that the Company’s business strategy for growth will result in additional net sales or operating income. In addition, the implementation of the Company’s business strategy for growth may have an adverse effect on working capital, operating cash flow, and results of operations.
16
Table of Contents
Circumstances associated with the Company’s acquisition strategy could adversely affect the Company’s results of operations and financial condition.
An element of the Company’s strategy is to identify, pursue and consummate acquisitions that either expand or compliment the Company’s business. Future acquisitions or investments may be financed by the issuance of equity securities that would increase the number of outstanding common shares and may decrease earnings per share, and through incurring additional debt. Additionally, changes in generally accepted accounting principles and general economic and market conditions may affect the profitability of acquisitions. As a result of these financing and environmental factors, the market price of the Company’s common stock may decline.
Integration of acquisitions involves a number of risks. The Company may be unable to successfully integrate the operations of acquired companies and realize anticipated economic, operational, and other benefits in a timely manner. Integration of an acquired company may be difficult when the acquired business is in a market in which the Company has limited or no expertise, or has a different corporate culture. If the operations of acquired companies are not successfully integrated, the Company may:
| • | incur substantial unanticipated costs and delays; |
| • | experience operational, technical, or financial problems; and |
| • | damage relationships with key customers and employees. |
If the operations of acquired companies are not successfully integrated with the operations of the Company in an efficient manner, the Company’s business, financial condition and results of operations may be adversely affected.
The Company’s ability to carry out its global sourcing strategy, which includes sourcing products from foreign markets subject to political, economic and legal uncertainties, may affect the Company’s overall profitability.
The Company continues to expand its globally sourced product offering, which is marketed under the brand name Select Medical Products. The Company’s global sourcing strategy revolves around sustaining sourcing channels to drive enhanced customer satisfaction and profitability. To attain its strategic objectives, the Company has focused on:
| • | expanding the Select product offering; |
| • | strengthening the global sourcing infrastructure; |
| • | improving product sourcing processes and sourcing partner coordination; |
| • | ensuring the quality of Select brand products; |
| • | supporting increases in volume of globally sourced products; |
| • | effective marketing and promotion of Select brand products; and |
| • | providing appropriate incentives to its sales force in the form of commission and promotions |
The Company’s sourcing strategy involves purchasing certain products directly from foreign manufacturers located in China, Thailand, Malaysia, India, Poland, Cambodia, South Korea, Taiwan, the United Kingdom, and the Philippines. The Company’s business, financial condition and results of operations may be adversely affected by changes in the political, social or economic environment of certain foreign countries. Changes in laws and regulations, or their interpretation, the imposition of surcharges or any material increase in tax rates, restrictions on currency conversion, imports and sources of supply, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on the Company’s ability to conduct business and its results of operations. Additional risks related to the Company’s global sourcing strategy include:
| • | political unrest in certain regions; |
| • | intermittent energy supply interruptions with global manufacturers; |
| • | changes in foreign currency exchange rates; |
17
Table of Contents
| • | shipping disruptions due to transportation delays; |
| • | fluctuations in the cost of commodities; |
| • | availability of raw materials; |
| • | increase in regulation of exports due to increasing trade deficit; |
| • | natural disasters in certain regions; |
| • | regional tensions that adversely affect the development of ongoing agreements; and |
| • | intellectual property violation claims. |
The Company’s failure or inability to execute any of its strategic global sourcing initiatives could adversely impact its future profitability.
The Company’s business is dependent on data processing systems critical to the business operations.
The Company is reliant on its information systems for centralized customer support, operating, and administrative processes. Management relies on the capability, accuracy, timeliness, and stability of its data processing systems to:
| • | receive and ship customer orders, including those received electronically; |
| • | manage customer billings and collections; |
| • | provide accurate point-of-sale product cost information; |
| • | track and report third-party ancillary billing services; |
| • | provide product reporting, such as product purchases and sales by vendor and vendor incentives earned; |
| • | manage inventory procurement and processes; |
| • | track and report regulatory compliance related to certain pharmaceutical products and devices; |
| • | provide manufacturer rebate tracking, compliance, verification, and collection; |
| • | ensure payments to suppliers are made in accordance with negotiated terms; |
| • | ensure certain critical internal controls are operating properly; |
| • | prepare and present accurate financial statements and related information; |
| • | integrate acquisitions; and |
The Company’s business, financial condition and results of operations may be materially adversely affected if, among other things:
| • | data errors are created by the information systems and remain undetected; |
| • | data processing capabilities are interrupted or fail to operate for an extended period of time; |
| • | the data processing system becomes unable to support growth of the business; |
| • | data is lost or unable to be restored; |
| • | data security is breached inadvertently or through malicious intent causing destruction or theft of the Company’s information; |
| • | problems occur with system upgrades and implementations, or such upgrades and implementations do not occur as planned; |
| • | product maintenance and upgrades to the ERP system are no longer provided by suppliers; or |
| • | a revision is made to the estimated useful lives for certain computer software. |
The Company’s future results of operations could be adversely affected by operational disruptions due to natural disasters, particularly in regions susceptible to hurricanes.
A natural disaster such as a hurricane, tornado, earthquake or flood could cause severe damage and disruption to the Company’s operations, property, inventory and the operations of its customers.
18
Table of Contents
The Company has developed disaster recovery plans, which include the use of third party back-up facilities for information system infrastructure. In addition, the Company maintains business interruption insurance for instances of catastrophic loss. There is a risk the Company may fail to execute its disaster recovery plans and incur losses that exceed insurance policy limits or are excluded from policy provisions. Furthermore, the Company may have difficulty obtaining business interruption insurance in the future or similar types of coverage may not be available in the markets in which it operates. The Company’s failure to execute or inability to execute any of its disaster recovery plans and obtain adequate insurance coverage could materially adversely impact the Company’s business and results of operations.
The Company’s significant investment in inventory may be exposed to risk of product obsolescence or decline in market valuation.
In order to provide prompt and complete service to customers, the Company maintains a significant investment in inventory at its full-service distribution centers and redistribution centers. Inventory control procedures and policies are in place to monitor the risk of product obsolescence or declining market prices. Nevertheless, management cannot ensure:
| • | such procedures and policies will continue to be effective; |
| • | the demand for certain product lines will continue; |
| • | unforeseen product development or price changes will not occur; or |
| • | the write-off of any unsold inventory in the future will not be significant. |
Any inventory write-offs could have a material adverse effect on the Company’s business, financial condition, and results of operations.
The Company’s indebtedness may limit its ability to obtain additional financing in the future and may limit its flexibility to react to industry or economic conditions.
The Company maintains an asset-based revolving line of credit (“LOC”), which permits maximum borrowings of up to $200.0 million and may be increased to $250.0 million at the Company’s discretion. Availability of borrowings under the LOC depends upon a borrowing base calculation consisting of accounts receivable and inventory, subject to satisfaction of certain eligibility requirements less any outstanding letters of credit. Any deterioration in the valuation of these assets could reduce the availability of borrowings under the LOC. Additionally, the Company has $230.0 million of 3.125% senior convertible notes outstanding which include provisions that allow note holders to present the notes for redemption should the Company’s debt rating fall below a certain level. Increases in the level of the Company’s indebtedness or changes in the Company’s debt rating could adversely affect the Company’s liquidity and reduce the Company’s ability to:
| • | obtain additional financing for working capital requirements; |
| • | fund the convertible notes if presented for redemption; |
| • | make capital expenditures; |
| • | acquire businesses; and |
| • | react to changes in the industry and economic conditions in general. |
Operating cash requirements are normally funded by cash flows from operating activities and borrowings under the LOC, which expires in 2012. The Company expects that sources of capital to fund future growth in the business will be provided by a combination of cash flows from operating activities, borrowings under the LOC, capital markets, and/or other financing arrangements. However, changes in capital markets or adverse changes to the Company’s operations may disrupt the Company’s ability to maintain adequate levels of liquidity, including its ability to renew its LOC in 2012 on terms acceptable to the Company.
19
Table of Contents
If the Company is unable to generate sufficient cash flow from operating activities, the Company may be forced to adopt strategies that may include the following:
| • | reduce or delay acquisitions and capital expenditures; |
| • | sell assets; |
| • | restructure or refinance existing indebtedness; and |
| • | seek additional equity capital. |
The price of the Company’s common stock and the trading value of the senior convertible notes may be volatile.
The Company’s common stock experiences significant price and volume fluctuations. Trading prices of the Company’s common stock may be influenced by operating results, projections, and economic, financial, regulatory and other factors. In addition, general market conditions, including the level of, and fluctuations in, the trading prices of stocks generally, could affect the price of the Company’s common stock.
The market price of the senior convertible notes (“Notes”) is expected to be significantly affected by the market price of the Company’s common stock as well as the general level of interest rates and the Company’s credit quality. This may result in a significantly greater volatility in the trading value of the Notes than would be expected for nonconvertible debt securities the Company may issue.
The price of the Company’s common stock also could be affected by possible sales of the Company’s common stock by investors who view the Notes as a more attractive means of equity participation in the Company and by hedging or arbitrage activity management expects to develop involving the Company’s common stock as a result of the issuance of the Notes. The hedging or arbitrage activity, in turn, could affect the trading prices of the Notes and common stock.
The Company faces potential litigation and liability exposure for product liability and other claims against the Company.
The Company is primarily a distributor of medical products, equipment, and pharmaceutical products. As a result, there is a risk that injury or other liability arising from the use or transportation of the products may occur and result in litigation against the Company. Accordingly, the Company maintains various insurance policies, including product liability insurance, to cover such exposure at amounts that management considers adequate. In many cases, the manufacturer of the product for any product liability claims may indemnify the Company. However, there can be no assurance the coverage maintained by the Company under various insurance policies is sufficient to cover future claims or will be available in adequate amounts at a reasonable cost. In addition, there can be no assurance that the indemnification agreements provided by manufacturers will adequately protect the Company; particularly the enforceability of indemnification provisions provided by overseas suppliers for globally-sourced products. This risk increases as more of the Company’s sales relate to sourced products. A successful claim brought against the Company in excess of available insurance or indemnification agreements, or any claim that results in significant adverse publicity against the Company, could harm the Company’s business, reputation, and results of operations.
In addition to product liability claims, the Company is subject to various legal and administrative proceedings and claims arising in the normal course of business, which are described in Footnote 17,Commitments and Contingencies, of the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. The outcomes of such proceedings or claims that are unasserted, pending, or known to be threatened could have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations.
20
Table of Contents
The Company faces risk that its proprietary rights may infringe on the rights of third parties and that the protection offered by its proprietary rights may not be adequate.
The Company relies on a combination of patent, copyright and trademark laws, nondisclosure and other contractual provisions to protect a number of its products, services, and intangible assets. These proprietary rights are important to the Company’s ongoing operations. There can be no assurance these protections will provide meaningful protection against competitive products or services or otherwise be commercially valuable or the Company will be successful in obtaining additional intellectual property or enforcing its intellectual property rights against unauthorized users.
From time-to-time, third parties may assert infringement claims against the Company. If the Company was found to be infringing on other’s rights, the Company may be required to pay substantial damage awards, obtain a license, or cease selling the products that contain the infringing property. Such actions may be significant and result in material losses to the Company.
If environmental claims arise, the Company could incur substantial liabilities and costs.
The Company’s operations and properties are subject to various federal, state and local laws and regulations relating to environmental matters. As such, the Company may be responsible for the investigation and remediation of property contaminated by hazardous, toxic, or other chemical substances, regardless of whether the Company is responsible for such contamination. The costs of such investigation and remediation requirements may be substantial. In addition, the Company could be held liable to governmental entities or third parties for any property damage, personal injury, and investigation and cleanup costs incurred by such parties in connection with the contamination. These costs and liabilities could have a material adverse effect on the Company’s business, financial condition, and results of operations. Furthermore, the costs of complying with more stringent standards, as well as any liabilities associated with noncompliance with such standards imposed on the Company, may result in a material adverse effect on the Company’s business, financial condition, and results of operations.
Failure to comply with existing and future regulatory and legal requirements could adversely affect the Company’s results of operations and financial condition.
General
The health care industry is highly regulated and the Company is subject to various federal, state, local and foreign laws and regulations, which include the Drug Enforcement Agency (“DEA”), the Food and Drug Administration (“FDA”), various state boards of pharmacy, state health departments, the United States Department of Health and Human Services (“HHS”), the Occupational Safety and Health Administration (“OSHA”), the Centers for Medicare and Medicaid Services (“CMS”), various State Attorneys General, State Medicaid fraud units, and other comparable agencies. The Company’s global product sourcing initiatives have increased the Company’s exposure to these regulatory agencies. Certain of the Company’s distribution service centers may be required to register for permits and/or licenses with, and comply with operating and security standards of, the DEA, the FDA, HHS, and various state boards of pharmacy, state health departments and/or comparable state agencies as well as certain accrediting bodies depending upon the type of operations and location of product distribution, manufacturing, and sale. Although the Company believes it is in compliance, in all material respects, with applicable laws and regulations, there can be no assurance that a regulatory agency or tribunal would not reach a different conclusion concerning the compliance of the Company’s operations with applicable laws and regulations.
The noncompliance by the Company with applicable laws and regulations or the failure to maintain, renew or obtain necessary permits and licenses could have an adverse effect on the Company’s results of operations and financial condition. In addition, if changes were to occur to the laws and regulations applicable to the Company’s
21
Table of Contents
businesses, such changes could adversely affect many of the Company’s regulated operations or could otherwise restrict the Company’s existing operations, limit the expansion of the Company’s businesses, apply regulations to previously unregulated businesses or otherwise affect the Company adversely. The costs associated with complying with federal and state regulations may be significant and failure to comply with any such laws and regulations could have a material adverse effect on the Company, including criminal and civil penalties, administrative sanctions, quarantine and destruction of inventory, fines, and other adverse actions.
The manufacture, distribution and marketing of certain of the Company’s products are subject to extensive ongoing regulation by the FDA. Failure to comply with the requirements of the FDA could result in warning letters, products recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution of products, civil or criminal sanctions, refusal of the government to grant approvals, restrictions on operations, or withdrawal of existing approvals. Any of these actions could cause a loss of customer confidence in the Company and its products which could adversely affect the Company’s sales. In addition, third parties may file claims against the Company in connection with these issues.
Laws relating to healthcare fraud
We are subject to extensive and frequently changing federal and state laws and regulations relating to healthcare fraud. The federal government continues to strengthen its position on and scrutiny of practices involving healthcare fraud affecting government healthcare programs. Our relationships with manufacturers and healthcare providers subject our business to laws and regulations on fraud and abuse which, among other things, (i) prohibit persons from soliciting, offering, receiving or paying any remuneration in order to induce the referral of a patient for treatment or to induce the ordering, purchasing, leasing or arranging for or recommending ordering, purchasing or leasing of items or services that are in any way paid for by government-sponsored healthcare programs and (ii) impose a number of restrictions upon referring physicians and providers of designated health services under government healthcare programs. While we believe that we are substantially compliant with all applicable laws, many of the regulations applicable to us are vague or indefinite and have not been interpreted by regulators or the courts. They may be interpreted or applied in a manner that could require us to make changes in our operations. In addition, the Federal False Claims Act creates a financial incentive for private individuals, called whistleblowers, to bring suit on behalf of the government to recover funds paid pursuant to a false claim, which may include failure to comply with technical requirements for claim submission, coding, and billing. If we fail to comply with applicable laws and regulations, we could suffer civil and criminal penalties, including substantial fines or penalties, and other sanctions, including exclusion from participation in any federal health care program.
Laws affecting our foreign operations
The Company is subject to the United States Foreign Corrupt Practices Act (“FCPA”), which generally prohibits United States companies from engaging in bribery or prohibited payments to foreign officials for the purpose of obtaining or retaining business. FCPA enforcement has increased significantly in recent years. Certain foreign companies, including some that may compete with the Company, may not be subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in the non-U.S. countries in which the Company conducts business. The Company has implemented safeguards to prevent and discourage such practices by employees and agents. The Company can make no assurance, however, that employees or other agents will not engage in such conduct for which the Company might be held responsible. If employees or other agents are found to have engaged in such practices, the Company could suffer severe penalties and other consequences that may have a material adverse effect on the Company’s business, financial condition and results of operations. The Company is also subject to additional laws relating to its foreign operations including the Patriot Act and anti-boycott and reporting laws. Failure to comply with these laws could subject the Company, its employees or other agents to penalties and other adverse consequences that may have a material adverse effect on the Company’s business, financial condition and results of operations.
22
Table of Contents
Tax legislation initiatives and audits by tax authorities could adversely affect the Company’s net earnings and tax liabilities
The Company is subject to the tax laws and regulations of the United States Federal, state and local governments and certain foreign governments. From time to time, various legislative initiatives may be proposed that could adversely affect the Company’s tax positions. There can be no assurance that the Company’s effective tax rate will not be adversely affected by these initiatives. In addition, United States Federal, state and local tax laws and regulations are extremely complex and subject to varying interpretations. Although the Company believes that its historical tax positions are sound and consistent with applicable laws, regulations and existing precedent, there can be no assurance that the Company’s tax positions will not be challenged by relevant tax authorities or that the Company would be successful in any such challenge.
President Barack Obama recently announced an outline of his administration’s proposal to modify certain aspects of the rules governing the U.S. taxation of certain non-U.S. subsidiaries. Many details of the proposal remain unknown and any legislation enacting such modifications would require Congressional approval; however, changes to these rules could impact the Company’s effective tax rate.
From time to time we are audited by U.S. federal, state and local tax authorities. If these audits result in assessments different from our reserves, our future results may include unfavorable adjustments to our tax liabilities.
See Item 1 “Business—Regulatory Matters” for additional information.
If management fails to maintain an effective system of internal controls, the Company may not be able to accurately report its financial results.
Effective internal controls are necessary to provide reliable financial reports. If the Company cannot provide reliable financial reports, its business and results of operations could be adversely affected. Any failure to maintain effective internal controls over financial reporting may cause the Company to fail to meet its reporting obligations, which could have a negative impact on the trading price of its common stock.
The Articles of Incorporation, Bylaws, and Florida law may inhibit a takeover of the Company or could deprive the Company’s shareholders of the opportunity to obtain a takeover premium for their shares.
The Company’s Amended and Restated Articles of Incorporation and Amended and Restated Bylaws and Florida law contain provisions that may delay, deter, or inhibit a future acquisition that might otherwise result in the Company’s shareholders receiving a takeover premium for their shares. This could occur even if shareholders are offered an attractive value for their shares of common stock or if a substantial number or even a majority of the Company’s shareholders believe the takeover is in their best interest. These provisions are intended to encourage any person interested in acquiring the Company to negotiate with and obtain the approval of the Board of Directors in connection with any transaction. A staggered Board of Directors, the State of Florida’s Affiliated Transaction Statute, or the State of Florida’s Control-Share Acquisition Statute could delay, deter, or inhibit potential offers to acquire the Company.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
The Company has received no written comments regarding its periodic or current reports from the staff of the Securities and Exchange Commission that were issued 180 days or more preceding the end of fiscal year 2009 and that remain unresolved.
23
Table of Contents
| ITEM 2. | PROPERTIES |
The Company leases warehouse and office space for its 39 full-service distribution centers, 40 break-freight locations, 6 ancillary service centers, and 2 redistribution facilities, in various locations across the United States. In the normal course of business, management regularly assesses its business needs and makes changes to the capacity and location of these leased facilities. As of March 27, 2009, the Company believes its facilities are adequate to carry on its business as currently conducted and that, if necessary, it could find additional and/or replacement facilities to lease without suffering a material adverse effect on its business.
The following tables identify the full-service distribution centers, break-freight locations, ancillary service centers, and redistribution facilities for each operating segment:
Physician Business
Full-Service Distribution Center Locations | ||||||
Aiea, HI | Grand Prairie, TX | Memphis, TN | Rochester, NY | |||
Charlotte, NC | Houston, TX | New Orleans, LA | Salt Lake City, UT | |||
Columbia, SC | Jacksonville, FL | Olathe, KS | Schertz, TX | |||
Denver, CO | Kennesaw, GA | Orlando, FL | Seattle, WA | |||
Elgin, IL | Leetsdale, PA | Phoenix, AZ | Wareham, MA | |||
Fairfield, NJ | Louisville, KY | Richmond, VA | West Sacramento, CA | |||
Fullerton, CA | Lubbock, TX | Rogers, MN | ||||
Break Freight Locations | ||||||
Albany, NY | Cincinnati, OH | Little Rock, AR | San Diego, CA | |||
Baltimore, MD | Cleveland, OH | Nashville, TN | St. Louis, MO | |||
Baton Rouge, LA | Columbia, SC | Newark, CA | St. Petersburg, FL | |||
Bethlehem, PA | Fredericksburg, VA | Omaha, NE | Tallahassee, FL | |||
Big Bend, WI | Indianapolis, IN | Pompano Beach, FL | Troy, MI | |||
Birmingham, AL | Knoxville, TN | Portland, OR | Tulsa, OK | |||
Chatsworth, CA | Lafayette, LA | Raleigh, NC | Tyler, TX | |||
Chattanooga, TN | Las Vegas, NV | Roanoke, VA | West Babylon, NY | |||
Chesapeake, VA | ||||||
Redistribution Facilities | ||||||
Fullerton, CA * | Jacksonville, FL * | |||||
Elder Care Business
Full-Service Distribution Center Locations | ||||||
Austell, GA | Mesquite, TX | Omaha, NE | Ridgeland, MS | |||
Gahanna, OH | Middletown, PA | Ontario, CA | Sacramento, CA | |||
Londonderry, NH | Morrisville, NC | Orlando, FL | Windsor, WI | |||
Break Freight Locations | ||||||
Eau Claire, WI | Houston, TX | Olathe, KS | San Antonio, TX | |||
Fairfield, NJ | Indianapolis, IN | Little Rock, AR | ||||
Redistribution Facilities | ||||||
Fullerton, CA * | Jacksonville, FL * | |||||
Ancillary Service Centers | ||||||
Birmingham, AL | Indianapolis, IN | Spokane, WA | Tualatin, OR | |||
Fresno, CA | Redmond, WA | |||||
| * | Redistribution facilities shared by Physician Business and Elder Care Business. |
24
Table of Contents
In the aggregate, the Company’s service center locations consist of approximately 2.6 million square feet of leased space. The lease agreements have expiration dates ranging from April 2009 to August 2016 and facilities ranging in size from approximately 1,000 square feet to 168,500 square feet.
The Company’s corporate office complex consists of approximately 150,000 square feet of leased office space located at 4345 Southpoint Boulevard and 4190 Belfort Road, Jacksonville, Florida 32216. This lease expires in March 2014.
| ITEM 3. | LEGAL PROCEEDINGS |
From time to time the Company is a party to various legal and administrative proceedings and claims arising in the normal course of business. While any litigation contains an element of uncertainty, the Company, after consultation with legal counsel, believes that the outcome of such other proceedings or claims which are pending or known to be threatened will not have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations.
The Company has various insurance policies, including product liability insurance, covering risks in amounts it considers adequate. In many cases in which the Company has been sued in connection with products manufactured by others, the Company is provided indemnification by the manufacturer. There can be no assurance that the insurance coverage maintained by the Company is sufficient or will be available in adequate amounts or at a reasonable cost, or that indemnification agreements will provide adequate protection for the Company.
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
No matters were submitted to a vote of shareholders during the three months ended March 27, 2009.
25
Table of Contents
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Shares of the Company’s common stock are quoted on Nasdaq Stock Market, Inc.’s Global Select Market (“NASDAQ GS”) under the ticker symbol “PSSI.” The following table presents, for the periods indicated, the range of high and low sale prices per share of the Company’s common stock as reported on NASDAQ GS.
Quarter Ended | High | Low | ||
Fiscal year ended March 28, 2008: | ||||
June 29, 2007 | $21.83 | $17.97 | ||
September 28, 2007 | $19.98 | $16.11 | ||
December 28, 2007 | $21.31 | $20.44 | ||
March 28, 2008 | $20.52 | $15.41 | ||
Fiscal year ended March 27, 2009: | ||||
June 27, 2008 | $18.56 | $15.90 | ||
September 26, 2008 | $21.72 | $15.93 | ||
January 2, 2009 | $19.83 | $14.97 | ||
March 27, 2009 | $19.02 | $13.19 | ||
Cash Dividends
Since inception, the Company has neither declared nor paid cash dividends. Management intends to continue to retain earnings for the growth and development of the Company’s business; therefore, the Company does not anticipate that any cash dividends will be declared in the foreseeable future. The Company’s revolving line of credit agreement contains certain covenants that limit the amount of cash dividends that can be declared by the Company.
Holders of Common Stock
As of May 15, 2009, there were approximately 1,550 holders of record of the Company’s common stock.
26
Table of Contents
Performance Graph
The graph below compares the cumulative total stockholder return on $100 invested, assuming reinvestment of dividends, if any, on April 2, 2004, the last trading day before the beginning of the Company’s fiscal year 2005 through the end of fiscal year 2009, with the cumulative return on $100 invested for the same period in the Nasdaq Stock Market (U.S. Companies) Composite Index.
The graph also compares the cumulative stockholder return to an index of companies management believes comprise the Company’s peer group, which includes the following companies: Amerisourcebergen Corporation, Baxter International, Inc., Cardinal Health, Inc., McKesson Corporation, Owens & Minor, Inc., Patterson Companies, Inc., and Henry Schein, Inc.
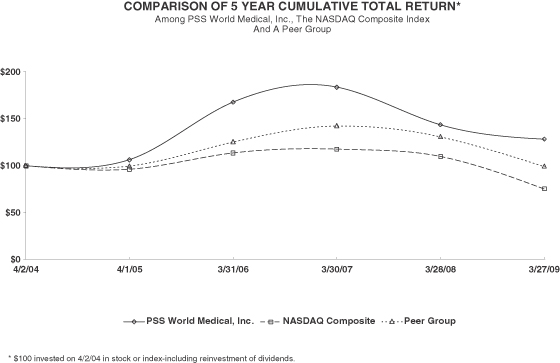
27
Table of Contents
FISCAL YEAR ENDING MARCH 27, 2009
| April 2, 2004 | April 1, 2005 | March 31, 2006 | March 30, 2007 | March 28, 2008 | March 27, 2009 | |||||||
PSS World Medical, Inc. | 100.00 | 106.70 | 167.88 | 183.88 | 143.86 | 128.37 | ||||||
NASDAQ Composite | 100.00 | 96.48 | 113.74 | 117.72 | 109.92 | 75.11 | ||||||
Peer Group | 100.00 | 99.79 | 125.30 | 142.36 | 130.91 | 99.08 |
Information presented above assumes $100 invested on April 2, 2004 and that dividends were reinvested.
Issuer Purchases of Equity Securities
As of March 28, 2008, there were 0.4 million shares available for repurchase under existing stock repurchase programs. During fiscal year 2009, the Company’s Board of Directors approved a stock repurchase program authorizing the Company, depending upon market conditions and other factors, to repurchase up to a maximum of 5% of its common stock, or approximately 3.1 million common shares, in the open market, in privately negotiated transactions, or otherwise. During fiscal year 2009, the Company repurchased approximately 3.4 million shares of common stock under this program at an average price of $15.62 per common share for approximately $52.8 million. At March 27, 2009, approximately 0.1 million shares were available to repurchase under this program.
Subsequent to March 27, 2009, the Board of Directors approved a stock repurchase program authorizing the Company, depending upon market conditions and other factors, to repurchase up to a maximum of 5% of its common stock, or approximately 3.0 million common shares, in the open market, privately negotiated transactions.
The following table summarizes the Company’s repurchase activity during the three months ended March 27, 2009.
Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Number (or Approximate Dollar Value) of Shares that May Yet Be Purchased Under the Plans or Programs | |||||
January 3-January 27 | — | — | — | 1,353,771 | |||||
January 28-February 27 | 670 | $ | 15.81 | 670 | 1,353,101 | ||||
February 28-March 27 | 1,234,700 | 13.80 | 1,234,700 | 118,401 | |||||
Total fourth quarter | 1,235,370 | $ | 13.80 | 1,235,370 | 118,401 | ||||
28
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
The selected financial data for fiscal years 2005 through 2009 have been derived from the Company’s consolidated financial statements, which give retroactive effect to the restatement of Diagnostic Imaging, Inc. as discontinued operations. The selected financial data below should be read in conjunction with the Company’s financial statements and the notes thereto and Item 7,Management’s Discussion and Analysis of Financial Condition and Results of Operations.
| Fiscal Year Ended | ||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||
| (Dollars in thousands, except per share data) | ||||||||||||||||
Statement of Operations Data: | ||||||||||||||||
Net sales | $ | 1,952,691 | $ | 1,855,791 | $ | 1,741,639 | $ | 1,619,417 | $ | 1,473,769 | ||||||
Income from continuing operations | $ | 58,017 | $ | 56,775 | $ | 50,481 | $ | 44,257 | $ | 39,384 | ||||||
Total loss from discontinued operations | — | — | — | — | (412 | ) | ||||||||||
Net income | $ | 58,017 | $ | 56,775 | $ | 50,481 | $ | 44,257 | $ | 38,972 | ||||||
Earnings (loss) per share—Basic: | ||||||||||||||||
Income from continuing operations | $ | 0.97 | $ | 0.88 | $ | 0.75 | $ | 0.67 | $ | 0.61 | ||||||
Total loss from discontinued operations | — | — | — | — | (0.01 | ) | ||||||||||
Net income | $ | 0.97 | $ | 0.88 | $ | 0.75 | $ | 0.67 | $ | 0.60 | ||||||
Earnings (loss) per share—Diluted | ||||||||||||||||
Income from continuing operations | $ | 0.96 | $ | 0.86 | $ | 0.73 | $ | 0.66 | $ | 0.60 | ||||||
Total loss from discontinued operations | — | — | — | — | (0.01 | ) | ||||||||||
Net income | $ | 0.96 | $ | 0.86 | $ | 0.73 | $ | 0.66 | $ | 0.59 | ||||||
Weighted average shares outstanding | ||||||||||||||||
Basic | 59,937 | 64,703 | 67,219 | 65,643 | 64,547 | |||||||||||
Diluted | 60,696 | 66,184 | 69,325 | 66,887 | 65,607 | |||||||||||
Cash dividends declared per common share | — | — | — | — | — | |||||||||||
Ratio of earnings to fixed charges(a) | 4.7 | 5.8 | 5.1 | 4.6 | 3.7 | |||||||||||
Balance Sheet Data: | ||||||||||||||||
Working capital(b) | $ | 323,545 | $ | 93,506 | $ | 304,543 | $ | 265,201 | $ | 228,583 | ||||||
Total assets | $ | 871,830 | $ | 814,825 | $ | 774,975 | $ | 736,976 | $ | 646,358 | ||||||
Long-term liabilities(b) | $ | 286,410 | $ | 64,855 | $ | 211,882 | $ | 200,287 | $ | 180,310 | ||||||
| (a) | For the purpose of calculating the ratio of earnings to fixed charges, earnings consist of income from continuing operations before provision for income taxes, plus fixed charges, less capitalized interest. Fixed charges consist of interest, whether expensed or capitalized, amortization of debt issuance costs, and the portion of rental expense estimated by management to be attributable to interest. |
| (b) | Fiscal year 2008 working capital and long-term liabilities reflect a reclassification of $150 million of 2.25% senior convertible notes from long-term to current liabilities made during fiscal year 2009. |
29
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
THE COMPANY
PSS World Medical, Inc. (the “Company” or “PSSI”), a Florida corporation, began operations in 1983. The Company is a national distributor of medical products and equipment, pharmaceutical products, healthcare information technology and billing services to alternate-site healthcare providers including physician offices, long-term care and assisted living facilities, home health care and hospice providers through 39 full-service distribution centers, which serve all 50 states throughout the United States (“U.S.”). The Company currently conducts business through two operating segments, the Physician Business and the Elder Care Business, which serve a diverse customer base. For information on comparative segment revenue, segment profit and related financial information, refer to Footnote 16,Segment Information, of the consolidated financial statements.
PSSI is a market leader in the alternate-site customer segments it serves as a result of value-added, solutions-based marketing programs; a differentiated customer distribution and service model; a consultative sales force with extensive product, disease state, reimbursement, and supply chain knowledge; unique arrangements with manufacturers; a full line of the Company’s own brand, Select; innovative information systems and technologies that serve its core markets; and a culture of performance.
THE COMPANY’S STRATEGY
The Company’s objective is to be the leading distributor and marketer of medical products and services to select medical market segments in the United States of America, with a goal to grow revenues at twice the market growth rate in the markets it serves. The key components of the Company’s strategy include:
Grow sales through innovative, differentiated marketing programs, customer interface improvements, relationships, products, and services. The Company believes its sales professionals, which consists of approximately 830 employees, and their customer relationships and knowledge are strategic competitive advantages. The Company trains its sales professionals to build unique relationships with customers and provide solutions though innovative marketing programs, exclusive products, and new product or technology offerings. The Company develops and provides unique customer-based solutions and marketing programs. The Company utilizes improved customer interface processes that focus on increasing sales representative bandwidth and improving the customer experience.
Optimize the Company’s product offering. The Company continues to develop its sourcing capabilities, including developing global product sourcing capabilities to optimize its product offering by integrating sourcing and branding initiatives with customer, product and Company strategies to increase profitability. The Company intends to broaden its reach and breadth of products by expanding its Select and specialty brand product offerings, increase product quality and category management and effectively leverage its sourcing capabilities, both foreign and domestic.
Make investments to simplify business activities and leverage existing distribution and shared service capabilities to reduce operating expenses as a ratio to net sales. The Company is making significant investments in its distribution infrastructure, information systems, process reengineering, and training in order to simplify its distribution and administrative infrastructure, develop easy to use scalable processes and systems that enable growth, reduce costs to serve, and achieve the Company’s commitments to superior customer service. Simplification initiatives focus on process redesign, investments in automation and organizational commitments to customer service. During fiscal year 2009, the Company continued to leverage its existing infrastructure investments and process improvements resulting in operating efficiencies, improved customer service levels and operating margins.
30
Table of Contents
Aggressively identify, develop and retain leaders capable of managing a growing corporation. The Company is committed to the effective recruitment, hiring and promotion of employees with outstanding performance, culture and leadership abilities. The Company provides leadership development strategies, individual leadership assessment and development plans, education and coaching programs to its employees. The Company’s goal is to develop a diverse group of individuals capable of leading a fast growing corporation.
Conduct business in a legal and ethical manner. The Company believes each employee is responsible for personal integrity and the consequences of actions, and is expected to follow the highest standards of ethics, honesty, fairness and compliance with the law. The Company provides health, safety, and regulatory education training programs and a safe and positive work environment for its employees.
Be the employer of choice within the industry. The Company believes its management, sales force and employees are its most valuable assets. The Company seeks to foster a culture of performance and execution by designing employee incentive programs aligned with the Company’s business strategies and objectives. The Company strives to be the employer of choice in the markets it serves, in terms of benefits offered to employees, availability of health and wellness programs, professional competency and personal development training.
Make strategic acquisitions. The Company expects to continue to make strategic acquisitions to complement its core business strategies and seek target acquisitions which leverage infrastructure and distribution capabilities, expand its product sourcing and product development expertise, and increase market share and profitability.
EXECUTIVE OVERVIEW
During the fiscal year 2009, the Company continued profitable growth despite the volatile economic environment. Consolidated sales grew by 5.2% during the fiscal year, compared to 6.6% during the prior fiscal year. This slower sales growth was due to general economic conditions and resulting downturn in the overall economy. The Company expects near term sales growth rates to be lower than recent historic sales growth, particularly in the Physician Business, due to general economic conditions.
The Company recognized sales growth throughout most product lines within the Physician Business and across each customer segment within the Elder Care Business during fiscal year 2009 when compared to fiscal year 2008. Net sales for the Physician Business increased by 3.7%, while net sales for the Elder Care Business increased by 8.7%. The Physician and Elder Care Businesses continued sales promotions to increase sales of its Select product line, which positively impacted the Company’s growth and margins. During fiscal year 2009, Select brand product sales grew 14.9% and 28.1% over prior year in the Physician and Elder Care businesses, respectively.
Consolidated income from operations increased approximately $8.7 million, or 9.5% during the fiscal year ended March 27, 2009, while operating margins increased 20 basis points. During fiscal year 2008, operating margins were negatively impacted by costs associated with the Florida Department of Health’s review discussed under the caption “Compliance with Florida Pedigree Laws” below. The remaining fiscal year 2009 increase was primarily a result of the net sales growth leverage discussed above as well as management’s focus on reducing operating costs as a percentage of net sales through its business simplification strategies.
Cash flow from operations during fiscal year 2009 grew to $89.7 million, primarily attributable to growth in profitability and improved collections.
31
Table of Contents
With tightening of business and consumer credit markets, the Company remains focused on credit management and collection of its receivable balances, with additional investments in credit and collections training and continued focus on aged balances. During fiscal year 2009, days sales outstanding decreased from 42.0 days to 40.3 days in the Physician Business and from 49.6 days to 49.3 days in the Elder Care business, when compared to prior year. The Company’s increased operating cash flow and available cash balances funded a portion of the Company’s stock repurchase program and investments in capital projects during the year.
The following significantly impacted the Company’s financial and operating results during fiscal years 2009, 2008, and 2007:
Convertible Debt Transactions
During the second quarter of fiscal year 2009, the Company issued $230.0 million principal amount of 3.125% senior convertible notes. The Company also entered into convertible note hedge transactions with the initial purchaser and/or its affiliates and, separately, sold warrants to the initial purchaser and/or its affiliates. The Company used a portion of the net proceeds to (i) repurchase approximately $35.0 million of its common stock; (ii) pay the costs of its hedge transactions; and (iii) redeem $150.0 million of the Company’s 2.25% senior convertible. Remaining proceeds were used for general corporate purposes. Refer to Footnote 11,Debt, for additional information.
Global Sourcing Initiative
The Company’s global sourcing strategy involves purchasing products directly from contracted manufacturers and is a key initiative for the Company. Milestones reached during fiscal year 2009 and 2008 included (i) expanding the Company’s global sourcing resources in Asia and Europe, (ii) increasing the capacity of the redistribution infrastructure in the United States; (iii) expanding the Select product offering, and (iv) designing and implementing a security assessment program for global manufacturers in compliance with the U.S. Customs Trade Partners Against Terrorism Act. At March 27, 2009 and March 28, 2008, the Company had approximately $39.1 million and $29.5 million of globally-sourced product inventory, respectively, representing an increase of 152 product SKUs and 84 product categories. Management believes this initiative will continue to positively impact the Company’s results of operations in future years.
Investment in athenahealth, Inc.
On June 29, 2007, the Company made a $24.1 million equity investment, including transaction costs, in athenahealth, Inc. (“athena”), a privately held leading provider of internet-based healthcare information technology and business services to physician practices. This equity investment represented approximately 5% of athena’s outstanding shares. On September 20, 2007, an initial public offering of shares of athena’s common stock was made available for sale on the NASDAQ Global Market under the symbol “ATHN,” which impacted the valuation of shares under SFAS 115,Accounting for Certain Investments in Debt and Equity Securities. During fiscal year 2009 and 2008, the Company sold a portion of its investment in athena, resulting in a gain of approximately $0.4 million ($0.3 million, net of tax) and $4.6 million ($2.9 million, net of tax), respectively.
As of March 27, 2009, the aggregate fair value of the Company’s remaining investment was $10.6 million, recorded on the Consolidated Balance sheets as Investment in available for sale securities. During the fiscal year ended March 27, 2009, the Company recorded unrealized holding losses of $0.1 million. In April of 2009, the Company sold its remaining holdings in athena. Refer to Footnote 6,Equity Investment,for additional information.
32
Table of Contents
Compliance with Florida Pedigree Laws
During the fiscal year ended March 28, 2008, the Company’s Florida operations were the subject of an inspection by the Florida Department of Health for compliance with guidelines for the receipt, storage, and distribution of products covered by recently enacted drug pedigree legislation in the state of Florida. Products covered by this legislation included prescription pharmaceutical products used by physicians in their practices, such as vaccines, ointments and creams, anesthetics, and topicals used in office procedures. On January 23, 2008, the Company signed an agreement with the State of Florida resolving issues regarding the interpretation of procedures for documenting the ordering, receipt, storage, and shipping of certain products covered by Florida drug pedigree legislation. Pursuant to the terms of the agreement, the Company reimbursed the State $1.0 million in costs and fees, and donated pharmaceutical inventory valued at $0.5 million. In turn, the State agreed to issue and/or renew permits to the Company’s Florida facilities and release the Company’s quarantined inventory subject to third party validation of pedigree compliance.
Costs associated with the review and inspection were approximately $6.3 million and include costs and fees, inventory exposure, additional operating and legal costs, as well as costs incurred to modify the Company’s compliance systems and processes. These costs were recorded within Corporate Shared Services and the Physician Business.
Influenza Vaccine Product Sales
Influenza vaccine product sales were approximately $48.6 million during the fiscal year ended March 30, 2007 but fell short of expectations due to an oversupply in the market and the cancellation of customer orders due to a mild influenza season. In connection with these environmental issues and as part of the Company’s periodic review of the carrying amount of inventory during fiscal year 2007, management determined that a write-down of $7.0 million, net of recoveries, was required. The Company did not participate in the influenza vaccine market during its fiscal year 2008. The Company reentered the influenza vaccine market on an agency basis during fiscal year 2009. As such, the Company is relieved from inventory and accounts receivable risk and recognizes fees received from its supplier on a net revenue recognition basis.
Acquisition of Activus Healthcare Solutions, Inc.
On May 31, 2007, the Company acquired the stock of Activus Healthcare Solutions, Inc. (“Activus”), a California based distributor of medical supplies and pharmaceuticals to office-based physicians and ambulatory surgery centers. The aggregate purchase price, subject to certain adjustments as set forth in the purchase agreement, was $13.2 million, net of cash acquired. As of March 28, 2008, Activus’ operations have been integrated into the Company’s existing operations. The Company incurred operating losses totaling $2.0 million related to the integration and transition of Activus operations during fiscal year 2008.
33
Table of Contents
RESULTS OF OPERATIONS
FISCAL YEAR ENDED MARCH 27, 2009 VERSUS FISCAL YEAR ENDED MARCH 28, 2008
NET SALES
| For the Fiscal Year Ended | Percent Change | ||||||||||||||
| March 27, 2009 | March 28, 2008 | ||||||||||||||
| (dollars in millions) | Amount | Average Daily Net Sales | Amount | Average Daily Net Sales | |||||||||||
Physician Business | $ | 1,357.4 | $ | 5.4 | $ | 1,308.7 | $ | 5.2 | 3.7 | % | |||||
Elder Care Business | 594.5 | 2.3 | 547.1 | 2.2 | 8.7 | % | |||||||||
Corporate Shared Services | 0.8 | — | — | — | N/A | ||||||||||
Total Company | $ | 1,952.7 | $ | 7.7 | $ | 1,855.8 | $ | 7.4 | 5.2 | % | |||||
Physician Business
Management evaluates the Physician Business by product category. The following table summarizes the growth rate by product category period over period.
| For the Fiscal Year Ended | Percent Change | ||||||||
| (dollars in millions) | March 27, 2009 | March 28, 2008 | |||||||
Branded(a) | $ | 709.3 | $ | 691.3 | 2.6 | % | |||
Select(b) | 168.3 | 146.4 | 14.9 | % | |||||
Pharmaceutical products | 305.2 | 289.2 | 5.5 | % | |||||
Influenza vaccine products | 2.4 | — | N/A | ||||||
Equipment | 137.4 | 146.0 | (5.9 | )% | |||||
Immunoassay product sales | 27.3 | 26.8 | 1.7 | % | |||||
Other | 7.5 | 9.0 | (17.5 | )% | |||||
Total | $ | 1,357.4 | $ | 1,308.7 | 3.7 | % | |||
| (a) | Branded products are comprised of disposables and lab diagnostics from branded manufacturers. |
| (b) | Select products are comprised of the Company’s brand of disposables and lab diagnostics. |
Net sales growth during the year ended March 27, 2009 was driven by continued momentum in the consumable and pharmaceutical growth programs. Select product sales increased due to the Company’s focus on promoting its globally sourced, Select products, which resulted in new customer sales as well as customer conversions from other manufacturer branded products to Select brand products. Equipment sales decreased due to the current economic conditions and tightening credit policies which negatively impacted customers’ ability to obtain equipment financing.
Pharmaceutical sales increased primarily as a result of growth in the Company’s non-controlled drugs product line and, in part, to the introduction of additional products, including additional generic products, to the Company’s offering. The Company reentered the influenza vaccine market on an agency basis during fiscal year 2009. Under the agreement, the Company is relieved from inventory and accounts receivable risk and recognizes fees received from its supplier on a net revenue recognition basis. The agreement requires the sale of minimum amount of influenza vaccine doses annually, the failure of which could result in the termination of the agreement.
Other revenue was impacted by a decrease in leasing incentives due to lower equipment sales, partially offset by an increase in customer freight and handling charges.
The Company expects fuel surcharges to customers to decline in future periods due to lower fuel costs.
34
Table of Contents
Elder Care Business
Management evaluates the Elder Care business by customer category. Net sales were impacted by the utilization of innovative Elder Care customer-specific solution programs, includingMomentum Rewards, a customer incentive programs introduced during fiscal year 2009.
The following table summarizes the change in net sales by customer segment period over period.
| For the Fiscal Year Ended | Percent Change | ||||||||
| (dollars in millions) | March 27, 2009 | March 28, 2008 | |||||||
Nursing home and assisted living facilities | $ | 363.7 | $ | 339.7 | 7.1 | % | |||
Hospice and home health care agencies | 166.1 | 151.6 | 9.5 | % | |||||
Billing services | 13.7 | 13.0 | 5.0 | % | |||||
Other | 51.0 | 42.8 | 19.3 | % | |||||
Total | $ | 594.5 | $ | 547.1 | 8.7 | % | |||
Net sales during the year ended March 27, 2009 compared to the prior year increased approximately $47.4 million, as sales grew in each customer segment. The Company’s growth in the hospice and home health care customer segments during fiscal year 2009 reflects the successful execution of strategies to diversify its customer base through expansion in the home health care market and other non-facility based care. Net sales were also impacted by the continued utilization of innovative customer-specific solution programs and a focus on independent customer segments within the nursing homes market.
Across its Elder Care customer segments, Select product sales increased 28.1% during fiscal year 2009, when compared to fiscal year 2008, due to the Company’s focus on promoting its globally sourced products which resulted in additional sales to new and existing customers.
GROSS PROFIT
Physician Business
Gross profit dollars for the Physician Business increased $23.0 million and gross margins increased 62 basis points during fiscal year 2009. The increase in gross profit was primarily due to the growth in net sales discussed above, in conjunction with the Company’s continued focus on its sourcing strategies and addition of influenza vaccine sales. The Company reentered the influenza vaccine market on an agency basis during fiscal year 2009. As such, the Company is relieved from inventory and accounts receivable risk and recognizes fees received from its supplier on a net revenue recognition basis. The Company’s sourcing strategies are designed to improve the cost competitiveness and increase gross margins on certain products. The increase in gross profit was also attributed to a decrease in vendor rebates denied and inventory sourcing efficiencies.
Elder Care Business
Gross profit dollars in the Elder Care Business increased $17.1 million year over year and were impacted by the increase in sales across the Business’ customer segments. Gross margin percentages increased 60 basis points due to effective inventory cost initiatives, an increase in patient-specific delivery, and increased gross profit from the Company’s Medicare Part B and Medicaid billing service provider, ProClaim, which generates higher gross margin percentages.
35
Table of Contents
GENERAL AND ADMINISTRATIVE EXPENSES
| For the Fiscal Year Ended | ||||||||||||
| March 27, 2009 | March 28, 2008 | |||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||
Physician Business(a) | $ | 195.3 | 14.4 | % | $ | 191.3 | 14.6 | % | ||||
Elder Care Business(a) | 118.1 | 19.9 | % | 106.9 | 19.5 | % | ||||||
Corporate Shared Services(b) | 38.9 | 2.0 | % | 25.8 | 1.4 | % | ||||||
Total Company(b) | $ | 352.3 | 18.0 | % | $ | 324.0 | 17.5 | % | ||||
| (a) | General and administrative expenses as a percentage of net sales are calculated based on reportable segment net sales. |
| (b) | General and administrative expenses as a percentage of net sales are calculated based on consolidated net sales. |
General and administrative expenses are primarily impacted by (i) compensation and employee benefit costs, (ii) cost to deliver, which represents all costs associated with the warehousing, transportation and delivery of products to customers, and (iii) shared services overhead costs.
Physician Business
General and administrative expenses increased $4.0 million and decreased 20 basis points as a percentage of net sales over the prior year. This increase in expenses were primarily attributable to (i) increased compensation expense of $4.7 million due to additional employees and general wage increases; (ii) an increase of $1.7 million related to incentive compensation expense for the year related to a combination of new incentive compensation plans introduced during fiscal year 2009 and stronger operating performance; (iii) an increase of $1.4 million in the corporate overhead allocation from Corporate Shared Services which is primarily related to increased payroll and medical related costs; and (iv) increased cost to deliver of approximately $1.6 million related to increased volume. Partially offsetting these increases are costs incurred in fiscal year 2008 and not repeated in fiscal year 2009; (i) $2.9 million in integration costs for Activus; and (ii) approximately $2.2 million related to additional operating expenses from the Company’s inspection by the Florida Department of Health for compliance with State Pedigree laws.
Elder Care Business
General and administrative expenses increased $11.2 million and 40 basis points as a percentage of net sales over the prior year. This increase was primarily attributable to (i) increased cost to deliver of approximately $3.2 million related to volume; (ii) increased compensation expense of $3.2 million due to additional employees and general wage increases; (iii) an increase of $0.7 million in incentive compensation expense for the year related to a combination of new incentive compensation plans introduced during fiscal year 2009 and stronger operating performance; and (iv) an increase in bad debt expense of $0.6 million due to the recoveries that occurred in fiscal year 2008 that did not occur in fiscal year 2009.
Corporate Shared Services
General and administrative expenses increased $13.1 million during the fiscal year ended 2009 due primarily to (i) an increase of $5.7 million in incentive compensation expense related to a combination of new incentive compensation plans introduced during fiscal year 2009 and an increase in payout estimates based on performance; (ii) a $2.4 million increase in payroll related costs due to an increase in full-time employees and merit increases; (iii) $2.5 million related to an increase in medical insurance costs; (iv) a $2.4 million increase in stock compensation expense related to new long-term incentive plans introduced during fiscal years 2009 and 2008; (v) and an increase in depreciation expense of $1.3 million due to increased investments in IT infrastructure during the fiscal year. Partially offsetting these increases is a $4.1 million decrease in settlement and legal costs related to the Florida Department of Health’s inspection of the Company’s operation for compliance with State Pedigree laws in fiscal year 2008.
36
Table of Contents
SELLING EXPENSES
| For the Fiscal Year Ended | ||||||||||||
| March 27, 2009 | March 28, 2008 | |||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||
Physician Business(a) | $ | 107.7 | 7.9 | % | $ | 105.6 | 8.1 | % | ||||
Elder Care Business(a) | 20.8 | 3.5 | % | 19.7 | 3.6 | % | ||||||
Total Company(b) | $ | 128.5 | 6.6 | % | $ | 125.3 | 6.8 | % | ||||
| (a) | Selling expenses as a percentage of net sales are calculated based on divisional net sales. |
| (b) | Selling expenses as a percentage of net sales are calculated based on consolidated net sales. |
Selling expenses are principally driven by commission expenses, which are generally paid to sales representatives based on gross profit dollars and gross profit as a percentage of net sales. The increase in selling expenses for the Physician Business and Elder Care Business was consistent with the increase in net sales year over year. Selling expense as a percentage of net sales decreased year over year, which is primarily related to leveraging net sales growth across certain fixed selling expenses.
INCOME FROM OPERATIONS
| For the Fiscal Year Ended | ||||||||||||||
| March 27, 2009 | March 28, 2008 | |||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||
Physician Business | $ | 109.8 | 8.1 | % | $ | 93.0 | 7.1 | % | ||||||
Elder Care Business | 30.1 | 5.1 | % | 25.2 | 4.6 | % | ||||||||
Corporate Shared Services | (38.8 | ) | — | (25.8 | ) | — | ||||||||
Total Company | $ | 101.1 | 5.2 | % | $ | 92.4 | 5.0 | % | ||||||
Income from operations for each business segment changed due to the factors discussed above. The Company expects net sales growth to slow within the Physician Business compared to prior years, however, business simplification and lean strategies are expected to offset the effects of the slower sales growth, resulting in continued growth in income from operations.
INTEREST EXPENSE
The Company’s debt structure during fiscal year 2009 consisted of variable rate borrowings under its revolving line of credit (“LOC”) agreement, $150.0 million of 2.25% senior convertible notes, and $230.0 million of 3.125% senior convertible notes issued in August 2008.
The following table summarizes the various components of total interest expense and interest rates applicable to the borrowings outstanding under the LOC.
| For the Fiscal Year Ended | Increase | |||||||||||
| (dollars in millions) | March 27, 2009 | March 28, 2008 | ||||||||||
Components of interest expense: | ||||||||||||
Interest on borrowings | $ | 10.4 | $ | 5.6 | $ | 4.8 | ||||||
Debt issuance costs | 1.8 | 1.4 | 0.4 | |||||||||
Less: Capitalized interest | (0.4 | ) | (0.2 | ) | (0.2 | ) | ||||||
Total interest expense | $ | 11.8 | $ | 6.8 | $ | 5.0 | ||||||
Weighted average interest rate-LOC(a) | 4.00 | % | 5.10 | % | — | |||||||
Average daily borrowings under the LOC | $ | 51.1 | $ | 31.9 | $ | 19.2 | ||||||
| (a) | Weighted average interest rate excludes debt issuance costs and unused line fees. |
37
Table of Contents
On February 14, 2008, the Company entered into a two-year $50.0 million variable-to-fixed interest rate swap, effective February 19, 2008 and expiring February 19, 2010 (“Swap Agreement”). The Swap Agreement effectively sets the interest rate on all or a portion of the borrowings under the LOC to 3.95% (consisting of a fixed interest rate of 2.70% and a credit spread of 1.25%) for a notional amount of $50.0 million. As a result, the Company’s weighted average interest rate decreased 113 bps year over year.
In support of the Swap Agreement, the Company maintains a minimum balance of $50.0 million drawn on the LOC. This is evidenced by the average daily balance of $51.1 million for the year ended March 27, 2009.
OTHER INCOME
| For the Fiscal Year Ended | Percent Change | ||||||||||||
| (dollars in millions) | March 27, 2009 | March 28, 2008 | Decrease | ||||||||||
Total Company | $ | 2.6 | $ | 7.3 | $ | (4.7 | ) | (63.7 | )% | ||||
The Company sold a portion of its investment in Athena during fiscal year 2009 and 2008, recognizing a gain of $0.4 million and $4.6 million, respectively. Excluding the fiscal year 2008 gain on the sale of Athena stock, Other income during fiscal year 2009 remained consistent with prior year and is mainly attributable to customer finance charges. See Footnote 6,Equity Investment, for further information relating the Company’s investment in Athena.
PROVISION FOR INCOME TAXES
| For the Fiscal Year Ended | ||||||||||||
| March 27, 2009 | March 28, 2008 | |||||||||||
| (dollars in millions) | Amount | Effective Rate | Amount | Effective Rate | ||||||||
Total Company | $ | 36.2 | 38.4 | % | $ | 36.8 | 39.3 | % | ||||
The decrease in the provision for income taxes and effective rate year over year was primarily due to certain short term investments that generated tax-exempt interest income and the Company’s growth in non-U.S. earnings which are generally subject to tax at rates lower than the U.S.
FISCAL YEAR ENDED MARCH 28, 2008 VERSUS FISCAL YEAR ENDED MARCH 30, 2007
NET SALES
| For the Fiscal Year Ended | Percent Change | ||||||||||||||
| March 28, 2008 | March 30, 2007 | ||||||||||||||
| (dollars in millions) | Amount | Average Daily Net Sales | Amount | Average Daily Net Sales | |||||||||||
Physician Business | $ | 1,308.7 | $ | 5.2 | $ | 1,227.5 | $ | 4.9 | 6.6 | % | |||||
Elder Care Business | 547.1 | 2.2 | 514.1 | 2.0 | 6.4 | % | |||||||||
Total Company | $ | 1,855.8 | $ | 7.4 | $ | 1,741.6 | $ | 6.9 | 6.6 | % | |||||
38
Table of Contents
Physician Business
Net sales continued to be positively impacted by marketing programs launched to increase the sale of consumable products, pharmaceutical products, and equipment, respectively. Management evaluates the Physician Business by product category. The following table summarizes the growth rate by product category period over period.
| For the Fiscal Year Ended | Percent Change | ||||||||
| (dollars in millions) | March 28, 2008 | March 30, 2007 | |||||||
Branded(a) | $ | 691.3 | $ | 641.6 | 7.7 | % | |||
Select(b) | 146.4 | 114.9 | 27.4 | % | |||||
Pharmaceutical products | 289.2 | 237.7 | 21.7 | % | |||||
Influenza vaccine products | — | 48.6 | N/A | ||||||
Equipment | 146.0 | 147.0 | (0.7 | )% | |||||
Immunoassay product sales | 26.8 | 30.2 | (11.4 | )% | |||||
Other | 9.0 | 7.5 | 18.0 | % | |||||
Total | $ | 1,308.7 | $ | 1,227.5 | 6.6 | % | |||
| (a) | Branded products are comprised of disposables and lab diagnostics from branded manufacturers. |
| (b) | Select products are comprised of the Company’s brand of disposables and lab diagnostics. |
Net sales growth during the year ended March 28, 2008 was driven by continued momentum in the consumable and pharmaceutical growth programs, offset by a decline in influenza vaccine product sales. Influenza vaccine product sales were impacted by the Company’s strategic decision not to participate in the influenza vaccine market during fiscal year 2008 due to oversupply of influenza vaccine in the market in the prior year. Select product sales increased due to the Company’s focus on promoting its globally sourced Select products, which resulted in new customer sales as well as customer conversions from manufacturer branded products to Select brand products.
Elder Care Business
Net sales were impacted by the continued utilization of the following innovative Elder Care customer-specific solution programs. Management evaluates the Elder Care business by customer segment.
The following table summarizes the change in net sales by customer segment period over period.
| For the Fiscal Year Ended | Percent Change | ||||||||
| (dollars in millions) | March 28, 2008 | March 30, 2007 | |||||||
Nursing home and assisted living facilities | $ | 339.7 | $ | 322.1 | 5.5 | % | |||
Hospice and home health care agencies | 151.6 | 141.6 | 7.0 | % | |||||
Billing services | 13.0 | 12.2 | 6.8 | % | |||||
Other | 42.8 | 38.2 | 12.1 | % | |||||
Total | $ | 547.1 | $ | 514.1 | 6.4 | % | |||
Net sales during the year ended March 28, 2008 compared to the prior year increased approximately $33.0 million, as sales continued to grow in each customer segment. During fiscal year 2007, the Elder Care Business implemented strategies to diversify its customer base through expansion in the home health care market and other non-facilities based care and equipment providers. The Company’s growth in the hospice and home health care customer segments during fiscal year 2008 reflects the successful execution of these business growth strategies. Net sales were also impacted by the continued utilization of innovative customer-specific solution programs and a focus on the regional and independent customer segments within the nursing homes market.
39
Table of Contents
Across its Elder Care customer segments, Select product sales increased 24.9% during fiscal year 2008, when compared to fiscal year 2007.
GROSS PROFIT
Physician Business
Gross profit dollars for the Physician Business increased $33.9 million, while gross margins increased 79 basis points during fiscal year 2008. These increases were primarily due to the growth in net sales discussed above, in conjunction with the Company’s continued focus on its global sourcing strategy. The Company’s global sourcing strategy is designed to improve the Physician Business’ cost competitiveness and increase its gross margins on certain products. The increase in gross profit was also affected by a write-down for excess influenza vaccine inventory of $7.1 million during fiscal year 2007. This inventory write-down was the result of a lower-of-cost-or-market valuation on the Physician Business’ remaining influenza inventory due to an oversupply of the product in the market, and a mild influenza season. As a result of the write-down, cost of goods sold was increased by $7.1 million during the year ended March 30, 2007. As a result of the oversupply in the market, the Company determined that it would not participate in the influenza vaccine market during fiscal year 2008. This decision had a positive impact on the Physician Business’ gross margin percentages year over year, as pharmaceutical sales generally have lower margins.
Elder Care Business
Gross profit dollars in the Elder Care Business increased $7.4 million year over year and were impacted by the increase in sales across the Business’ customer segments. While gross margin dollars increased during the year, gross margin percentages decreased 35 basis points and were negatively impacted by the segment’s product mix, as sales to large nursing home customers, which generally have lower margins, offset increases in sales to hospice and home health care agencies and sales of the Company’s Select product line, which generate higher gross margins.
GENERAL AND ADMINISTRATIVE EXPENSES
| For the Fiscal Year Ended | ||||||||||||
| March 28, 2008 | March 30, 2007 | |||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||
Physician Business(a) | $ | 191.3 | 14.6 | % | $ | 174.5 | 14.2 | % | ||||
Elder Care Business(a) | 106.9 | 19.5 | % | 103.4 | 20.1 | % | ||||||
Corporate Shared Services(b) | 25.8 | 1.4 | % | 22.8 | 1.3 | % | ||||||
Total Company(b) | $ | 324.0 | 17.5 | % | $ | 300.7 | 17.3 | % | ||||
| (a) | General and administrative expenses as a percentage of net sales are calculated based on reportable segment net sales. |
| (b) | General and administrative expenses as a percentage of net sales are calculated based on consolidated net sales. |
General and administrative expenses are primarily impacted by (i) compensation and employee benefit costs, (ii) cost to deliver, which represents all costs associated with the transportation and delivery of products to customers, and (iii) shared services overhead costs.
40
Table of Contents
Physician Business
General and administrative expenses increased $16.8 million and 40 basis points as a percentage of net sales over the prior year. This increase was attributable to (i) increased compensation expense of $3.8 million due to additional employees and general wage increases; (ii) additional operating costs of $2.9 million related to the integration of Activus; (iii) approximately $2.2 million related to additional operating expenses from the Company’s inspection by the Florida Department of Health for compliance with State Pedigree laws; (iv) additional costs of $1.3 million related to the Company’s national launch of its new Select and healthcare information technology marketing programs; (v) increased bank service charges of approximately $0.8 million related to fees paid to process payments made via credit cards by the Company’s customers; (vi) an increase of $2.5 million in the corporate overhead allocation from Corporate Shared Services which is primarily related to increased costs associated with the Company’s global sourcing initiative and increased payroll costs and (vii) increased cost to deliver of approximately $2.6 million related to increased fuel costs, shipping and handling charges, fleet repair and maintenance, and delivery professional salaries.
Corporate Shared Services
General and administrative expenses increased during the fiscal year ended 2008 due to (i) approximately $4.1 million related to the Company’s inspection by the Florida Department of Health for compliance with State Pedigree laws, (ii) increased employee compensation and employee benefit expenses of approximately $5.8 million due to additional employees, general wage increases, and increases in healthcare costs, (iii) an increase of $1.8 million related to performance based restricted stock awards granted during fiscal year 2008, (iv) an increase in lease expense of approximately $1.1 million due to a new regional distribution facility for globally-sourced product, general rent increases and the expansion of the Company’s shared service’s leased office space during fiscal year 2008, partially offset by (v) a reduction of $5.9 million in compensation expense related to the Company’s management and employee incentive plans, based on actual of performance achievement and payment estimates, and (vi) a reduction of $1.7 million related to the Company’s deferred compensation plan.
SELLING EXPENSES
| For the Fiscal Year Ended | ||||||||||||
| March 28, 2008 | March 30, 2007 | |||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||
Physician Business(a) | $ | 105.6 | 8.1 | % | $ | 97.3 | 7.9 | % | ||||
Elder Care Business(a) | 19.7 | 3.6 | % | 19.5 | 3.8 | % | ||||||
Total Company(b) | $ | 125.3 | 6.8 | % | $ | 116.8 | 6.7 | % | ||||
| (a) | Selling expenses as a percentage of net sales are calculated based on divisional net sales. |
| (b) | Selling expenses as a percentage of net sales are calculated based on consolidated net sales. |
Selling expenses are driven by commission expense, which are generally paid to sales representatives based on gross profit dollars and gross profit as a percentage of net sales. The increase in selling expenses for the Physician Business was a result of the acquisition of Activus and the related addition of the sales representatives. The growth in the Elder Care Business’ selling expenses was consistent with the increase in net sales year over year. Overall, selling expenses were relatively consistent as a percentage of net sales with the prior year.
41
Table of Contents
INCOME FROM OPERATIONS
| For the Fiscal Year Ended | ||||||||||||||
| March 28, 2008 | March 30, 2007 | |||||||||||||
| (dollars in millions) | Amount | % of Net Sales | Amount | % of Net Sales | ||||||||||
Physician Business | $ | 93.0 | 7.1 | % | $ | 84.1 | 6.9 | % | ||||||
Elder Care Business | 25.2 | 4.6 | % | 21.6 | 4.2 | % | ||||||||
Corporate Shared Services | (25.8 | ) | — | (23.2 | ) | — | ||||||||
Total Company | $ | 92.4 | 5.0 | % | $ | 82.5 | 4.7 | % | ||||||
Income from operations for each business segment changed due to the factors discussed above.
INTEREST EXPENSE
The Company’s debt structure during fiscal year 2008 and 2007 consisted of the $150.0 million of 2.25% senior convertible notes and variable rate borrowings under its revolving line of credit (“LOC”) agreement. The following table summarizes the various components of total interest expense and interest rates applicable to the borrowings outstanding under the LOC.
| For the Fiscal Year Ended | Increase (Decrease) | |||||||||||
| (dollars in millions) | March 28, 2008 | March 30, 2007 | ||||||||||
Components of interest expense: | ||||||||||||
Interest on borrowings | $ | 5.6 | $ | 4.2 | $ | 1.4 | ||||||
Debt issuance costs | 1.4 | 1.4 | — | |||||||||
Less: Capitalized interest | 0.2 | 0.3 | (0.1 | ) | ||||||||
Total interest expense | $ | 6.8 | $ | 5.3 | $ | 1.5 | ||||||
Weighted average interest rate-LOC(a) | 5.10 | % | 7.60 | % | — | |||||||
Average daily borrowings under the LOC | $ | 31.9 | $ | 1.2 | $ | 30.7 | ||||||
| (a) | Weighted average interest rate excludes debt issuance costs and unused line fees. |
During fiscal year 2008, borrowings under the Company’s LOC were affected by market interest rate decreases by the Federal Open Market Committee (“FOMC”) of the Federal Reserve Board, which consistently reduced the Federal Funds Rate (“FFR”). From September 18, 2007 to March 18, 2008, the FOMC, in a series of rate cuts, reduced the FFR by a cumulative 300 bps. Under the LOC, the Company borrows funds at bank prime plus an applicable margin and/or at LIBOR plus an applicable margin. The interest rate cuts, as well as the effective use of borrowings at LIBOR helped the Company to lower borrowing costs during fiscal year 2008. Borrowings at LIBOR were at various terms with varying notional amounts. As a result, the Company’s weighted average interest rate decreased 250 bps year over year.
On February 14, 2008, the Company entered into a two year $50.0 million variable-to-fixed interest rate swap, effective February 19, 2008 and expiring February 19, 2010 (“Swap Agreement”). The Swap Agreement effectively sets the interest rate on all or a portion of the borrowings under the LOC to 3.95% (consisting of a fixed interest rate of 2.70% and a credit spread of 1.25%) for a notional amount of $50.0 million.
42
Table of Contents
OTHER INCOME
| For the Fiscal Year Ended | Percent Change | |||||||||||
| (dollars in millions) | March 28, 2008 | March 30, 2007 | Increase | |||||||||
Total Company | $ | 7.3 | $ | 2.0 | $ | 5.3 | 263.1 | % | ||||
The Company sold a portion of its investment in Athena during the fourth quarter of fiscal year 2008 for $21.0 million, recognizing a gain of $4.6 million during fiscal year 2008. The Company’s remaining investment in Athena is recorded as Investment in available for sale securities on the Consolidated balance sheets. Excluding the effect of this gain, other income remained consistent year over year. Refer to Footnote 6,Equity Investment, for further information relating the Company’s investment in Athena.
PROVISION FOR INCOME TAXES
| For the Fiscal Year Ended | ||||||||||||
| March 28, 2008 | March 30, 2007 | |||||||||||
| (dollars in millions) | Amount | Effective Rate | Amount | Effective Rate | ||||||||
Total Company | $ | 36.8 | 39.3 | % | $ | 29.9 | 37.2 | % | ||||
The increase in the provision for income taxes and effective rate year over year was attributable to an increase in income from continuing operations and a decrease in favorable permanent adjustments. The decrease in permanent adjustments relates to the change in the cash surrender value of company-owned life insurance policies.
LIQUIDITY AND CAPITAL RESOURCES
Liquidity and Capital Resources Highlights
Cash flows from operations are primarily impacted by segment profitability and operating working capital. Management monitors operating working capital cash flows through the following metrics:
| Fiscal Year Ended | ||||||
| 2009 | 2008 | 2007 | ||||
Days Sales Outstanding:(a) | ||||||
Physician Business | 40.3 | 42.0 | 41.8 | |||
Elder Care Business | 49.3 | 49.6 | 52.0 | |||
Days On Hand:(b) | ||||||
Physician Business | 55.1 | 51.8 | 48.4 | |||
Elder Care Business | 51.9 | 53.4 | 56.6 | |||
Days in Accounts Payable:(c) | ||||||
Physician Business | 39.9 | 42.2 | 44.0 | |||
Elder Care Business | 24.1 | 27.7 | 28.2 | |||
Cash Conversion Days:(d) | ||||||
Physician Business | 55.5 | 51.6 | 46.2 | |||
Elder Care Business | 77.1 | 75.3 | 80.4 | |||
Inventory Turnover:(e) | ||||||
Physician Business | 6.5 | 6.9 | 7.4 | |||
Elder Care Business | 6.9 | 6.7 | 6.4 | |||
| (a) | Days sales outstanding (“DSO”) is average accounts receivable divided by average daily net sales. Average accounts receivable is the sum of accounts receivable, net of the allowance for doubtful accounts, at the beginning and end of the most recent four quarters divided by five. Average daily net sales are net sales for the most recent four quarters divided by 360. |
43
Table of Contents
| (b) | Days on hand (“DOH”) is average inventory divided by average daily cost of goods sold (“COGS”). Average inventory is the sum of inventory at the beginning and end of the most recent four quarters divided by five. Average daily COGS is COGS for the most recent four quarters divided by 360. |
| (c) | Days in accounts payable (“DIP”) is average accounts payable divided by average daily COGS. Average accounts payable is the sum of accounts payable at the beginning and end of the most recent five quarters divided by five. |
| (d) | Cash conversion days is the sum of DSO and DOH, less DIP. |
| (e) | Inventory turnover is 360 divided by DOH. |
In addition to the cash flow metrics described above, the Company monitors and manages other components of liquidity, including the following:
| As of | ||||||||
| (dollars in thousands) | March 27, 2009 | March 28, 2008 | ||||||
Capital Structure: | ||||||||
Senior convertible notes | $ | 230,020 | $ | 150,000 | ||||
Revolving line of credit | 50,000 | 70,000 | ||||||
Other debt | 2,729 | 1,712 | ||||||
Cash and cash equivalents | (82,031 | ) | (21,122 | ) | ||||
Net debt | 200,718 | 200,590 | ||||||
Shareholders’ equity | 346,510 | 335,028 | ||||||
Total capital | $ | 547,228 | $ | 535,618 | ||||
Operating Working Capital: | ||||||||
Accounts receivable, net | $ | 230,361 | $ | 237,248 | ||||
Inventories | 207,593 | 190,846 | ||||||
Accounts payable | (127,300 | ) | (135,930 | ) | ||||
| $ | 310,654 | $ | 292,164 | |||||
Cash Flows from Operating Activities
The primary components of net cash provided by operating activities consist of net income adjusted to reflect the effect of non-cash expenses and changes in operating working capital. Net cash provided by operating activities during fiscal years 2009, 2008, and 2007 was impacted by increases in overall operating profit partially offset by operational working capital needs of approximately $21.7 million, $29.4 million, and $28.6 million, respectively, to support sales growth and long-term strategies. The Company’s net operating working capital levels were impacted in fiscal years 2009 and 2008 by its sourcing initiatives, including global sourcing, which generally require longer supply chain lead times and different payment terms. Management expects this impact to continue as global sourcing activities increase in future years. The Company continues to focus on efforts to increase cash collections from customers, improve inventory turns without impacting customer service levels, and manage the cash disbursements process.
Cash flows from operating activities during fiscal years 2009, 2008, and 2007 reflect the Company’s utilization of $1.2 million (tax effected), $0.8 million (tax effected), and $3.6 million (tax-effected), respectively, of net operating loss (“NOL”) carryforwards to offset cash payments due for Federal and state tax liabilities based on estimated taxable income. Cash flows from operating activities were also impacted by cash payments made to, and refunds received from, Federal and state taxing authorities. During fiscal years 2009, 2008, and 2007, the Company paid cash taxes, net of refunds, of approximately $27.0 million, $31.4 million and $13.0 million, respectively, which primarily related to Federal and state estimated tax payments.
44
Table of Contents
As of March 27, 2009, the Company has a deferred income tax liability of $18.8 million (tax effected) related to interest deductions taken for tax purposes on its 2.25% $150.0 million senior convertible notes. The liability will be fully deferred for 5 years and paid ratably from fiscal year 2014 to fiscal year 2018 in accordance with the American Recovery and Reinvestment Act of 2009.
During the fiscal year ended March 30, 2007, the Company and the Appeals Office of the IRS reached a settlement relating to the audit of the Federal income tax returns for the fiscal years ended March 29, 2002 and March 28, 2003. The resolution of these audits resulted in the IRS issuing the Company a net refund of $1.9 million of overpayment credits which was used to reduce the amount of cash required to satisfy fiscal year 2007 estimated tax payment liabilities.
During fiscal year 2009, the IRS began an examination of the Company’s Federal income tax return for the fiscal year ended March 31, 2006. While the Company is not presently aware of any proposed adjustments, it remains ongoing and therefore the Company cannot determine at this time the impact this examination will have on the Company’s financial condition or results of operations, if any.
Cash Flows from Investing Activities
On June 29, 2007, the Company acquired an equity investment in athenahealth, Inc. for $24.1 million, including $1.6 million of legal and other professional fees, all of which was paid during the fiscal year ended March 28, 2008. During fiscal year 2009, the Company sold a portion of its investment in athena, resulting in a gain of approximately $0.4 million, $0.3 million net of tax. During the fourth quarter of fiscal year 2008, the Company sold a portion of its investment in athena, resulting in a gain of approximately $4.6 million, or $2.9 million net of taxes. Cash proceeds of $22.1 million were received in fiscal year 2009 related to the sale of athena stock during fiscal year 2009 and the fourth quarter of fiscal year 2008.
Subsequent to fiscal year 2009, the Company sold its remaining investment in athena . Refer to Footnote 6,Equity Investment, for additional discussion.
Payments under business combination agreements, net of cash acquired, were $3.7 million, $15.2 million, and $2.9 million during fiscal years 2009, 2008, and 2007, respectively. Refer to Footnote 3,Purchase Business Combinations, for further discussion. The Company expects to continue to make strategic business acquisitions in future periods to grow market share and leverage its existing distribution capabilities, which will impact its cash flows from investing activities.
Capital expenditures totaled $26.9 million, $19.3 million, and $17.3 million, during fiscal years 2009, 2008, and 2007, respectively, of which approximately $ 17.5 million, $12.6 million, and $9.2 million, respectively, related to development and enhancement of the Company’s ERP system, electronic commerce platforms, and supply chain integration. Capital expenditures related to distribution center expansions and enhancements were approximately $5.5 million, $2.6 million, and $4.1 million, during fiscal years 2009, 2008, and 2007, respectively. Capital expenditures are estimated to be approximately $27 million during fiscal year 2010. Such expenditures are expected to be funded by cash flow from operations as well as borrowings under the Company’s revolving line of credit facility.
During fiscal year 2008, the Company received a payment of $2.9 million for the settlement of an obligation for a note receivable from its former chairman and chief executive officer, of which $2.7 million related to the principal amount of this loan. There were no principal payments relating to this note during fiscal year 2007. There were no remaining amounts due from the former chairman and chief executive officer as of March 27, 2009.
45
Table of Contents
Cash Flows from Financing Activities
During fiscal years 2009, 2008 and 2007, the Company repurchased approximately $52.8 million, $112.5 million and $42.9 million of the Company’s common stock, respectively. The share repurchases represented approximately 3.4 million, 6.4 million and 2.1 million shares, respectively.
Subsequent to year end, the Board of Directors for the Company authorized the purchase up to 5% of its outstanding common shares, or approximately 3.0 million common shares. This authorization is in addition to approximately 0.1 million shares remaining available at March 27, 2009 under existing authorized share repurchase programs. Refer to Footnote 13,Shareholders’ Equity, for additional discussion.
The Company recognized excess tax benefits from share-based compensation arrangements of $1.7 million, $1.4 million and $5.6 million during fiscal years 2009, 2008 and 2007, respectively.
The Company issued $230.0 million of 3.125% senior convertible notes during fiscal year 2009. In conjunction with the offering the Company received $25.4 million from the issuance of warrants, paid $54.1 million for the purchase of a convertible note hedge, and paid debt issuance costs of approximately $5.1 million.
During fiscal year 2009, the holders of $149.98 million in principal face value 2.25% senior convertible notes exercised their contractual rights to require the Company to repurchase their notes for a cash repurchase price equal to 100% of the principal face value of the notes to be repurchased plus accrued and unpaid interest. The Company used approximately $101.7 million in available cash on hand from the issuance of $230.0 million of 3.125% senior convertible notes and $50 million from the revolving line of credit to fund the repurchase of such notes and accrued interest on March 16, 2009, for approximately $151.7 million.
During fiscal years 2009 and 2008, the Company repaid approximately $20.0 million and borrowed approximately $70.0 million, respectively on its revolving line of credit. These borrowings were used to fund a portion of the Company’s share repurchases, debt repurchase, acquisition activities, investment strategies, and operating activities during the periods. There were no borrowings on the Company’s line of credit during fiscal year 2007.
Capital Resources
The capital and credit markets have recently experienced adverse conditions. The resulting restricted access to capital along with significant volatility within capital markets have increased the costs associated with issuing or refinancing debt because of increased risk spreads over relevant interest rate benchmarks. The Company continues to be well positioned, however, there can be no guarantee the recent disruptions in the overall economy and the financial markets will not adversely impact the business and results of operations.
The Company finances its business primarily through cash generated from operations, proceeds from the 3.125% $230.0 million senior convertible notes offering and the $200.0 million revolving line of credit. The ability to generate sufficient cash flows from operations is dependent on the continued demand for the Company’s products and services, and access to products and services from suppliers. Given current operating, economic and industry conditions, management believes demand for products and services will grow at slower rates. The Company’s capital structure provides the financial resources to support the business strategies of customer service and revenue growth. The revolving line of credit, which is an asset-based agreement, is primarily collateralized by the Company’s accounts receivable and inventory. The Company’s long-term priorities for use of capital are internal growth, acquisitions, and repurchase of the Company’s common stock.
As the Company’s business grows, its cash and working capital requirements are expected to increase. The Company expects the overall growth in the business will be funded through a combination of cash flows from operating activities, borrowings under the revolving line of credit, capital markets, and/or other financing
46
Table of Contents
arrangements. As of March 27, 2009, the Company has not entered into any material working capital commitments that require funding, other than the items discussed below and the obligations included in the future minimum obligation table below.
Based on prevailing market conditions, liquidity requirements, contractual restrictions, and other factors, the Company may seek to retire a portion of its outstanding equity through cash purchases and/or reduce its debt. The Company may also seek to issue additional debt or equity to meet its future liquidity requirements. Such transactions may occur in the open market, privately negotiated transactions, or otherwise. The amounts involved could be material.
3.125% $230 Million Senior Convertible Notes
In August 2008, the Company issued $230.0 million principal amount of 3.125% senior convertible notes, which mature on August 1, 2014. Interest on the notes is payable semiannually in arrears on February 1 and August 1 of each year. The notes will be convertible into cash up to the principal amount of the notes and shares of the Company’s common stock for any conversion value in excess of the principal amount under the following circumstances: (i) if the Company has called the notes for redemption; (ii) in the event of a Fundamental Change, such as a merger, acquisition, or liquidation; (iii) on or after May 1, 2014 and prior to the close of business on the second scheduled trading day immediately preceding August 1, 2014; (iv) prior to May 1, 2014, during the five consecutive business day period following any five consecutive trading day period in which the trading price for a note for each day of that trading period is less than 98% of the closing sale price of the Company’s common stock on such corresponding trading day multiplied by the applicable conversion rate; (v) prior to May 1, 2014, during any calendar quarter after September 30, 2008 in which the closing sale price of the Company’s common stock for at least 20 of the 30 consecutive trading days ending the day prior to such quarter is greater than 130% of the applicable conversion price of $21.22 per share (“Contingent Conversion Trigger”); or (vi) upon specified corporate events as discussed in the indenture governing the notes.
A note holder may not exercise his/her conversion right with respect to all or any portion of a note, if such conversion would cause the note holder to become a beneficial owner of more than 9.9% of the Company’s outstanding voting stock. The initial conversion rate is 47.1342 shares of common stock per each $1 (in thousands) principal amount of notes and is equivalent to an initial conversion price of $21.22 per share. The conversion rate is subject to adjustment if certain events occur, such as stock dividends or other distributions of cash, securities, indebtedness or assets; stock splits and combinations; issuances of rights or warrants; tender offers; or repurchases.
As of March 27, 2009, the fair value of the senior convertible notes was approximately $203.5 million. (Refer to Footnote 11, Debt, for a detailed discussion regarding the senior convertible notes.)
The ability of note holders to convert is assessed on a quarterly basis and is dependent on the trading price of the Company’s stock during the last 30 trading days of each quarter. The Contingent Conversion Trigger was not met during the three months ended March 27, 2009; therefore, the notes may not be converted during the Company’s first quarter of fiscal year 2010.
The Company used a portion of the net proceeds of the offering to repurchase approximately $35.0 million of its common stock in privately negotiated transactions with institutional investors concurrently with this offering. Refer to Footnote 13,Shareholders’ Equity, for additional information. The Company also used $101.7 million of the net proceeds during the current fiscal year, when holders of 2.25% senior convertible notes required the Company to redeem $149.98 million of the outstanding notes. Remaining proceeds have been used for general corporate purposes.
47
Table of Contents
Convertible Note Hedge Transactions
In connection with the offering of the notes, the Company also entered into convertible note hedge transactions with respect to its common stock (the “purchased options”) with a major financial institution, (the “counterparty”). The Company paid an aggregate amount of $54.1 million to the counterparty for the purchased options. The purchased options cover, subject to anti-dilution adjustments substantially identical to those in the notes, approximately 10.8 million shares of common stock at a strike price that corresponds to the initial conversion price of the notes, also subject to adjustment, and are exercisable at each conversion date of the notes. The purchased options will expire upon the earlier of (i) the last day the notes remain outstanding or (ii) the second scheduled trading day immediately preceding the maturity date of the notes.
The purchased options are intended to reduce the potential dilution upon conversion of the notes in the event that the market value per share of the common stock, as measured under the notes, at the time of exercise is greater than the conversion price of the notes.
The purchased options are separate transactions, entered into by the Company with the counterparty, and are not part of the terms of the notes. Holders of the notes will not have any rights with respect to the purchased options.
Warrant Transactions
The Company also entered into warrant transactions (the “warrants”), whereby the Company sold to the counterparty warrants in an aggregate amount of $25.4 million to acquire, subject to anti-dilution adjustments, up to 10.8 million shares of common stock at a strike price of $28.29 per share of common stock, also subject to adjustment. The warrants will expire after the purchased options in approximately ratable portions on a series of expiration dates commencing on November 3, 2015.
If the market value per share of the common stock, as measured under the warrants, exceeds the strike price of the warrants, the warrants will have a dilutive effect on the Company’s earnings per share. The warrants have been accounted for as an adjustment to the Company’s stockholders’ equity.
The warrants are separate transactions, entered into by the Company with the counterparties, and are not part of the terms of the notes. Holders of the notes do not have any rights with respect to the warrants.
Revolving Line of Credit
The Company had $50.0 million in outstanding borrowings under the revolving line of credit at March 27, 2009. The Credit Agreement permits maximum borrowings of up to $200.0 million, which may be increased to $250.0 million. After reducing availability for outstanding borrowings and letter of credit commitments, the Company has sufficient assets based on eligible accounts receivable and inventory to borrow an additional $149.5 million and $130.0 million (excluding the additional increase of $50 million) under the revolving line of credit at March 27, 2009 and March 28, 2008, respectively. (Refer to Footnote 11,Debt, for a detailed discussion regarding the revolving line of credit.) The average daily interest rate, excluding debt issuance costs and unused line fees, for the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007 was 4.00%, 5.13%, and 7.60%, respectively.
Debt Rating
The Company’s debt is rated by a nationally recognized rating agency, Standard and Poor’s Ratings Services (“S&P”). Companies that have assigned ratings at the top end of the range have, in the opinion of the rating agency, the strongest capacity for repayment of debt or payment of claims, while companies at the bottom end of the range have the weakest capability. The Company has a credit rating of “BB” and during fiscal year 2009, S&P upgraded the ratings outlook from stable to positive. During fiscal year 2009, S&P upgraded the Company’s
48
Table of Contents
$150.0 million 2.25% senior convertible notes rating from “B+” to “BB-” and rated the issuance of the Company’s $230.0 million 3.125% senior convertible notes in August 2008 at “BB-.”
Agency ratings are subject to change, and there can be no assurance that a ratings agency will continue to rate the Company or its debt, and/or maintain its current ratings. Management cannot predict the effect that a change in debt ratings will have on the Company’s liquidity or whether the Company will maintain adequate ratings as required by the 2.25% convertible notes indenture.
Off-Balance Sheet Arrangements
The Company’s most significant off-balance sheet financing arrangements as of March 27, 2009 are non-cancelable operating lease agreements, primarily for warehouse space and equipment rentals, and outstanding letters of credit. As of March 27, 2009, future minimum obligations under operating lease agreements are $77.1 million. The Company has approximately $0.5 million of open letters of credit outstanding at March 27, 2009. The Company does not participate in any off-balance sheet arrangements involving unconsolidated subsidiaries that provide financing or potentially expose the Company to unrecorded financial obligations.
Future Contractual Obligations
In the normal course of business, the Company enters into obligations and commitments that require future contractual payments. The following table presents, in aggregate, scheduled payments under contractual obligations for the Physician Business, the Elder Care Business, and Corporate Shared Services:
| Fiscal Years | |||||||||||||||||||||
| (in thousands) | 2010 | 2011 | 2012 | 2013 | 2014 | Thereafter | Total | ||||||||||||||
Revolving line of credit(a),(b) | $ | 2,295 | $ | 1,875 | $ | 1,875 | $ | 50,938 | $ | — | $ | — | $ | 56,983 | |||||||
Senior convertible notes(b),(c) | 7,188 | 7,188 | 7,188 | 7,188 | 7,188 | 233,617 | 269,557 | ||||||||||||||
Operating lease obligations(d) | 23,089 | 18,816 | 15,218 | 9,685 | 7,066 | 3,270 | 77,144 | ||||||||||||||
Capital lease obligations(b) | 936 | 952 | 832 | 9 | — | — | 2,729 | ||||||||||||||
Purchase commitments(e),(f) | 10,223 | 184 | 193 | — | — | — | 10,600 | ||||||||||||||
Outstanding purchase price(g) | 750 | — | — | — | — | — | 750 | ||||||||||||||
Total(h) | $ | 44,481 | $ | 29,015 | $ | 25,306 | $ | 67,820 | $ | 14,254 | $ | 236,887 | $ | 417,763 | |||||||
| (a) | The revolving line of credit is classified as a current liability in accordance with Emerging Issues Task Force No. 95-22,Balance Sheet Classification of Borrowings Outstanding under Revolving Credit Agreements That Include both a Subjective Acceleration Clause and a Lock-Box Arrangement; however, the credit facility does not expire until September 30, 2012. Interest expense has been estimated using current level borrowings outstanding at current effective interest rates. Actual interest expense may differ due to changes in interest rates or levels of borrowings. |
| (b) | Amounts include interest expense. |
| (c) | The 3.125% $230.0 million senior convertible debt, due fiscal year 2015 is discussed further in Footnote 11,Debt. |
| (d) | Amounts represent contractual obligations for operating leases of the Company as of March 27, 2009. Currently, it is management’s intent to either renegotiate existing leases or execute new leases upon the expiration date of such agreements. |
| (e) | If a supply agreement for Select products between a vendor and the Physician Business or the Elder Care Business were to be terminated, then the Company may be required to purchase from the vendor all remaining finished and unfinished products and product-materials held by the vendor. As of March 27, 2009, the Company had no material obligation to purchase remaining products or materials due to a termination of a supply agreement with a vendor who supplies Select products to the Company. |
| (f) | During the fourth quarter of fiscal year 2008, the Company entered into an agreement to purchase a minimum number of latex and vinyl gloves through February 2010. The pricing of the latex gloves may be periodically adjusted and is based on the price of raw latex as traded on the Malaysian Rubber Exchange. The pricing of the vinyl gloves may also be periodically adjusted and is based on the weighted price of two raw materials, Poly vinyl chloride (PVC) and Dioctylphthalate (DOP), as published on www.icis.com. These purchase commitments are valued at approximately $9.7 million at March 27, 2009 and are based on management’s estimate of current pricing. |
| (g) | Amounts represent estimated additional consideration, including interest, to be paid to the sellers of previously acquired businesses, net of any amounts payable to the Company for indemnity claims that arise under the purchase agreement. |
| (h) | As of March 27, 2009, the Company had gross unrecognized tax benefits of $1.7 million. This amount is excluded from the table above as the Company cannot reasonably estimate the period of cash settlement with the respective taxing authorities. |
49
Table of Contents
CRITICAL ACCOUNTING ESTIMATES
In preparing the consolidated financial statements in conformity with U.S. generally accepted accounting principles, management is required to make certain estimates, judgments, and assumptions that affect the reported amounts of assets and liabilities, including the disclosure of contingent assets and liabilities, at the date of the financial statements and the reported amounts of revenues and expenses during the periods presented. The Company periodically evaluates the accounting policies and estimates it uses to prepare its financial statements, which are then reviewed by the Company’s audit committee. Management’s estimates are based on historical experience and other assumptions considered reasonable with the relevant facts and circumstances. Based on the uncertainty inherent in such estimates, actual results may differ.
The critical accounting estimates are those estimates that require the Company’s management to make assumptions about matters that are highly uncertain at the time the estimate is made and could have a material impact on the Company’s results due to changes in the estimates or the use of different estimates that could reasonably have been used. Additionally, the Company includes those accounting estimates whose initial application had a material impact on the Company’s financial presentation, unless the application resulted solely from the issuance of new accounting literature. The discussion below applies to each of the Company’s reportable segments (Physician Business, Elder Care Business, and Corporate Shared Services), unless otherwise noted.
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses on trade receivables resulting from the inability to collect outstanding amounts due from its customers. The allowances include estimates of specific amounts for those accounts that are likely to be uncollectible, such as bankruptcies, and general allowances for those accounts that management currently believes to be collectible but may later become uncollectible. Management believes the estimates used in determining the allowance for doubtful accounts are critical accounting estimates because changes in credit worthiness and economic conditions, including bankruptcies, have had a material impact on operations in previous fiscal years and could have an impact on the Company’s results from operations in the near future.
The estimates used to determine the allowances for doubtful accounts are based on historical collection experience, current economic trends, credit-worthiness of customers, and changes in customer payment terms. The percentage of each aging category that is reserved is determined by analyzing historical write-offs and current trends in the credit quality of the customer base. Adjustments to credit limits and allowances for bad debts are made based upon payment history and the customer’s current credit worthiness. If the financial condition of the Company’s customers were to deteriorate or improve, allowances may be adjusted, impacting general and administrative expenses and the accounts receivable balance.
During fiscal years 2006 through 2008, the Physician Business’ allowance for doubtful accounts was reduced by $0.8 million, $1.5million, and $1.6 million, respectively, for customer deductions and write-offs and was increased by additional provisions of $1.5 million, $1.4 million and $2.3 million, respectively. During the fiscal years 2008 through 2006, the Company’s allowance for doubtful accounts has represented between 1.7% and 2.0% of the Physician Business’ trade receivable balance. At March 27, 2009 and March 28, 2008, the allowance for doubtful accounts for this business segment was $2.9 million and $3.3 million, respectively. During fiscal year 2009, the Physician Business’ allowance for doubtful accounts was reduced by $2.0 million for customer deductions and write-offs and was increased by additional provisions of $1.5 million. If management were to assume its reserve estimates at March 27, 2009 were based on the historic ranges noted above, the allowance for doubtful accounts as of March 27, 2009 would range from $2.6 million to $3.0 million for the Physician Business.
50
Table of Contents
During fiscal years 2006 through 2008, the Elder Business’ allowance for doubtful accounts was reduced by $3.9 million, $4.9 million, and $3.8 million, respectively, for customer deductions and write-offs and was increased by additional provisions of $4.11 million, $3.2 million and $1.5 million. During fiscal years 2006 through 2008, the Company’s allowance for doubtful accounts has represented between 4.7% and 9.2% of the Elder Care Business’ trade receivable balance. At March 27, 2009 and March 28, 2008, the allowance for doubtful accounts for this business segment was $4.2 million and $3.7 million, respectively. During fiscal year 2009, the Elder Business’ allowance for doubtful accounts was reduced by $1.7 million for customer deductions and write-offs and was increased by additional provisions of $2.1 million. If management were to assume its reserve estimates at March 27, 2009 were based on the historic ranges noted above, the allowance for doubtful accounts as of March 27, 2009 would range from $4.1 million to $8.1 million for the Elder Care Business.
Although the Company believes its judgments, estimates and/or assumptions related to allowances for doubtful accounts are reasonable, making material changes to such judgments, estimates and/or assumptions, and changes in customer’s credit worthiness could materially affect the Company’s financial results.
Inventories
In order to state inventories (medical products, medical equipment, and other related products) at the lower of cost (determined using the first-in, first-out (“FIFO”) method) or market (net realizable value), the Company maintains an allowance for excess or slow moving inventory based on the expectation that certain inventory will become obsolete, sold for less than cost, or become unsaleable altogether. The allowance is estimated based on factors such as historical trends, current market conditions and management’s assessment of when the inventory would likely be sold and the quantities and prices at which the inventory would likely be sold in the normal course of business. Changes in product specifications, customer product preferences or the loss of a customer may result in an unanticipated impairment in net realizable value that may have a material impact on cost of goods sold, gross margin and net income. Obsolete or damaged inventory is disposed of or written down to net realizable value on a quarterly basis. Additional adjustments, if necessary, are made based on management’s specific review of inventory on-hand. Management believes the estimates used in determining the allowance for excess and slow moving inventory are critical accounting estimates as changes in the estimates for both segments could have a material impact on net income and the estimates involve a high degree of judgment.
As part of the Company’s periodic review of the carrying amount of inventory during fiscal year 2007, management determined that a write-down of approximately $7.0 million, net of recoveries, was required for excess influenza vaccine inventory resulting from the cancellation of customer orders due to an oversupply of the flu vaccine in the market, and a mild influenza season. As a result of the write-down, cost of goods sold were increased by $7.0 million during the fiscal year ended March 30, 2007, or $4.4 million net of tax, in the Physician Business. During fiscal year 2008, the Company did not participate in the influenza vaccine market. During fiscal year 2009, the Company participated in this market on an agency basis and therefore did not have inventory risk.
Inventory allowances ranged from 1.7% to 6.2% of inventory for the Physician Business and 1.3% to 2.2% of inventory for the Elder Care Business during fiscal years 2006 through 2008. At March 27, 2009, management estimated the required allowance for excess or slow moving inventory to be approximately $2.1 million and $1.4 million for the Physician Business and Elder Care Business, respectively. If management were to assume inventory write-offs were based on the historic ranges noted above, the allowance for excess and slow moving inventory as of March 27, 2009 would range from $2.7 million and $9.6 million for the Physician Business and $0.9 million and $1.6 million for the Elder Care Business, impacting the Company’s inventory and gross profit balances.
Although the Company believes its judgments, estimates and/or assumptions related to inventory reserves are reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect the Company’s financial results.
51
Table of Contents
Vendor Rebates
The Company receives transaction-based rebates from third party suppliers. Such rebates are classified as a reduction to cost of goods sold in the accompanying statements of operations.
Transaction-based rebates are generally associated with a specific customer contract and are recognized as a reduction to cost of goods sold at the time the transaction occurs. Management establishes a reserve for uncollectible transaction-based vendor rebates based on management’s judgment after considering the status of current outstanding rebate claims, historical denial experience with suppliers, and any other pertinent available information. Management believes the estimates used in determining the reserve for uncollectible transaction-based vendor rebates are critical accounting estimates because changes in the estimates could have a material impact on net income and the estimates involve a high degree of judgment.
Reserves for transaction-based rebates for the year ended March 27, 2009 and March 28, 2008 were $2.7 million and $4.6 million, respectively. Reserves ranged from 12.7% to 48.1% of rebates receivable during fiscal years 2006 through 2008. If management were to assume outstanding future rebate receivable write-offs based on the upper and lower end of the historical range developed in the course of formulating the estimate, the transaction-based rebate reserve as of March 27, 2009 would range from $0.5 million to $1.7 million, impacting the Company’s prepaid and other current assets balance and gross profit.
Although the Company believes its judgments, estimates and/or assumptions related to vendor rebates are reasonable, making material changes to such judgments, estimates and/or assumptions could materially affect the Company’s financial results
Income Taxes
The Company uses the asset and liability method for determining its provision for income taxes and deferred tax assets and liabilities. Under this method, the amount of deferred tax assets and liabilities at the end of each period are determined using the tax rate expected to be in effect when taxes are actually paid or recovered. Valuation allowances are established to reduce deferred tax assets when it is more likely than not that some portion or all of the deferred tax assets will not be realized. In determining the need for valuation allowances, significant judgment and estimates are used as management considers short and long term forecasts of future taxable income as well as prudent and feasible tax planning strategies. These judgments and estimates include some degree of uncertainty and changes could require management to adjust the valuation allowances for deferred tax assets.
The Company had gross deferred income tax assets of $66.5 million and $45.6 million at March 27, 2009 and March 28, 2008, respectively. There were no valuation allowances as of March 27, 2009 and March 28, 2008, as management believes it will fully utilize the Company’s deferred tax assets before their expiration.
The Company’s tax filings are periodically subject to review by the Internal Revenue Service (“IRS”) and other taxing authorities, which may result in assessments of additional tax. Resolution of these situations, either with the taxing authority or the courts, inevitably includes some degree of uncertainty; accordingly, the Company provides taxes only for the amounts management believes will ultimately result from these proceedings. Management’s experience has been that the estimates and assumptions used to provide for future tax assessments have proven to be appropriate. However, past experience is only a guide, and the potential exists, however limited, that adjustments resulting from the resolution of current and potential future tax controversies may differ materially from the amount accrued.
During fiscal year 2008, the Company adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an Interpretation of FAS No. 109” (“FIN 48”) which clarifies the accounting and disclosure for uncertainty in tax positions, as defined. This standard provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes. FIN 48 requires management to make significant
52
Table of Contents
judgments while assessing the probability of possible outcomes of future tax examinations. Upon adoption of FIN 48, effective April 1, 2007, the Company recorded a liability for uncertain tax positions of $2.1 million and a decrease to stockholders equity of approximately $0.2 million. At March 27, 2009, the liability for uncertain tax positions was $1.7 million. Management does not expect the amount of unrecognized tax benefits to change significantly over the next twelve months.
If our estimates or judgments described above were to change, a hypothetical 1% change in our effective tax rate would impact consolidated income from continuing operations by approximately $0.9 million in fiscal year 2009.
Valuation of Intangible Assets, Other Long-lived Assets, and Goodwill
Acquisitions
The Company allocates the purchase price of acquired companies to the tangible and intangible assets acquired and liabilities assumed based on estimated fair values. Such valuations require management to make significant estimates and assumptions. Critical estimates in the valuation of acquired assets include, but are not limited to: (i) expected future cash flows from existing customer contracts and relationships; (ii) assumptions relating to the impact of noncompete agreements on business operations; (iii) assumptions related to the impact on the timing of expected future cash flows; (iv) retention of customers and key business leaders; and (v) the risk inherent in investing in intangible assets. These estimates are inherently uncertain and unpredictable. Assumptions may be incomplete or inaccurate, and unanticipated events and circumstances may occur which may affect the accuracy or validity of such assumptions, estimates, or other actual results. For these reasons, management believes the estimates used in determining the fair value of assets acquired through an acquisition are critical accounting estimates.
During fiscal years 2006 through 2008, the Company made acquisitions for an initial purchase price of $51.2 million. Adjustments to the valuation of acquired assets and liabilities subsequent to the date of purchase based on changes in management’s original estimates were immaterial to the current and previous three fiscal years.
Impairment
Under SFAS No. 142,Goodwill and Other Intangible Assets(“SFAS 142”), goodwill and indefinite-lived intangible assets are not amortized, but instead tested for impairment annually or whenever events or changes in circumstances indicate the carrying amount may be impaired. Goodwill and indefinite-lived intangible assets are reviewed for impairment at each reporting unit annually on the last day of each fiscal year.
The impairment and disposal of long-lived assets is accounted for in accordance with SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets, (“SFAS 144”). SFAS 144 requires that long-lived assets, such as property and equipment and intangible assets subject to amortization, be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. In the event that the carrying value of assets are determined to be unrecoverable, the Company would estimate the fair value of the assets or reporting unit and record an impairment charge for the excess of the carrying value over the fair value. In conducting the impairment analysis, the Company determines the fair value of its reporting units using valuation techniques which may include discounted cash flow analyses requiring management to make certain assumptions regarding estimated future cash flows, revenues, earnings, and other factors, including discount rates, to determine the fair value of these respective assets. The application of different assumptions about such matters as estimated future cash flows or discount rates, or the testing for impairment at a different level of the organization or on a different organization structure, may produce materially different results. For these reasons, management believes the estimates used in evaluating the Company’s goodwill, indefinite-lived intangible assets, and long-lived assets are critical accounting estimates. Based on management’s review, goodwill, intangible assets, and other long-lived assets were not impaired during fiscal years 2008, 2007, and 2006. As of March 27, 2009 and March 28, 2008, the Company’s intangible asset,
53
Table of Contents
other long-lived asset, and goodwill balances totaled $235.8 million, $227.7 million. Based on management’s review, goodwill, intangible assets, and other long-lived assets were not impaired during fiscal year 2009 and management does not believe there were any circumstances which indicated the carrying value of an asset might not be recoverable in the future. Additionally, a hypothetical 5% change in the discount rate utilized in the Company’s discounted cash flow analysis would not have indicated impairment for any of the Company’s reporting units.
Long-Term Incentive Compensation
Equity Incentive Plans
As of March 27, 2009, the Company has grants of incentive stock options, nonqualified stock options, time-based restricted stock and performance-based restricted stock outstanding.
Estimates are required to determine the number of share-based awards which will ultimately vest, and, in the case of performance-based restricted stock, estimates of the Company’s future performance. Changes in the estimated forfeiture rate, differences between actual and estimated forfeitures when an award vests or changes in estimates regarding the Company’s performance can have material effects on share-based compensation expense. For this reason, management has determined that the estimates used to determine equity-based compensation expense are critical accounting estimates.
When estimating forfeitures, the Company considers termination behaviors as well as trends of actual equity-based awards forfeited. Management re-assesses the estimated forfeiture rate established upon grant periodically throughout the required service period. Such estimates are revised if they differ materially from actual forfeitures. As required, forfeiture estimates are adjusted to reflect actual forfeitures when an award vests. Actual forfeitures in the future reporting periods could be materially higher or lower than management’s current estimates, which could have a material impact on equity-based compensation expense recognized in future years.
When estimating the Company’s earnings per share goals for performance-based restricted stock, the Company reviews historical performance, internal plans and goals, economic conditions, and other performance metrics. These future performance estimates are re-assessed throughout the service period. Such estimates are revised, if necessary, if they differ materially from the original assessment and may have an impact on the vesting of an award. If actual performance differs significantly from management’s current estimates, it could have a material impact on equity-based compensation expense recognized in fiscal year 2010 and in future years. Specifically, a change in the Company’s future earnings per share performance estimate, which would meet certain milestones, would have a material impact on equity based compensation expense.
Total compensation expense for restricted stock grants during the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, was $5.6 million, $3.4 million, and $1.7 million, respectively. Current forfeiture rates average 1.0% per quarter, with actual rates ranging from 0.1% to 2.7% per quarter for fiscal years 2006-2008. If management had used the low and high end of these actual ranges during fiscal year 2009, equity-based compensation expense, included in general and administrative expenses, would have been $5.9 million and $3.5 million, respectively. Holding forfeiture rates static, if management had estimated the Company’s future performance at the minimum and maximum earnings per share ranges, equity-based compensation expense would have been $5.1 million and $7.7 million, respectively, during the fiscal year ended March 27, 2009. Refer to Footnote 13,Shareholders’ Equity, for additional information.
Cash-based incentive plans
The Company maintains a cash-based long-term incentive plan, the Shareholder Value Plan (“SVP”) for certain employees. The SVP provides incentive to enhance shareholder value through the achievement of cumulative earnings per share goals. The performance period under the current SVP is the 3-year period from fiscal year 2009 through fiscal year 2011.
54
Table of Contents
Estimates are required to determine the Company’s expected future performance and cumulative earnings per share at the end of the three- year performance period. Changes in estimates regarding the Company’s performance can have a material effect on cash-based incentive compensation expense. For this reason, management has determined that the estimates used to determine the amounts accrued for cash-based compensation expense are critical accounting estimates.
When estimating the Company’s earnings per share goals for the SVP, the Company reviews historical performance, internal plans and goals, economic conditions, and other performance metrics. These performance goals are re-assessed throughout the service period. Such estimates are revised, if necessary, if they differ materially from the original assessment. If actual performance differs significantly from management’s estimates, it could have a material impact on cash-based compensation expense recognized in future years.
The Company’s three-year performance period for the 2006 SVP ended on March 28, 2008, with a payment of $4.2 million made during the first quarter of fiscal year 2009. The Company accrued $4.5 million and $5.0 million within Accrued expenses on the Consolidated balance sheets as of March 28, 2008 and March 30, 2007, respectively.
During fiscal year 2009, the Compensation Committee approved the 2008 Shareholder Value Plan (“2008 SVP”). The performance period under the 2008 SVP is the 36-month period from March 31, 2008 to March 25, 2011. The Company accrued approximately $1.9 million of compensation cost based on actual results as of March 27, 2009. The Company expects the target payout to be approximately $6.5 million with minimum and maximum target payouts of $3.4 million and $10.2 million, respectively. If management had estimated the Company’s future performance at the minimum and maximum target payouts, cash-based compensation expense would have been $0.9 million and $2.8 million, respectively, during the fiscal year ended March 27, 2009.
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS 157”). SFAS 157 defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements by establishing a fair value hierarchy based on the quality of inputs used to measure fair value. SFAS 157 does not require any new fair value measurements, but applies to other accounting pronouncements that require or permit fair value measurements. SFAS 157 is effective for financial statements for fiscal years beginning after November 15, 2007. The Company adopted SFAS 157 on March 29, 2008 and adoption did not have a material impact on its financial position, results of operations or cash flows. FSP SFAS No. 157-2, “Effective Date of FASB Statement No. 157” (“FSP SFAS 157-2”), delays the effective date of SFAS 157 with respect to nonfinancial assets and nonfinancial liabilities not remeasured at fair value on a recurring basis until fiscal years beginning after November 15, 2008, or the Company’s fiscal year 2010. Accordingly, the Company has not yet applied the requirements of SFAS 157 to certain such nonfinancial assets for which fair value measurements are determined only when there is an indication of potential impairment.
During February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB SFAS 115 (“SFAS 159”). SFAS 159 permits entities to choose to measure financial instruments and certain other items at fair value that are not currently required to be measured at fair value. SFAS 159 mandates certain financial statement presentation and disclosure requirements when a company elects to report assets and liabilities at fair value under SFAS 159. SFAS 159 is effective for fiscal years beginning after November 15, 2007, or the Company’s fiscal year 2009. The Company adopted SFAS 159 on March 29, 2008 and adoption did not have an impact on its financial position, results of operations or cash flows, as the Company did not elect fair value measurements for any financial instruments or other items upon adoption.
55
Table of Contents
In April 2008, the FASB issued FSP No. 142-3,Determination of the Useful Life of Intangible Assets (“FSP 142-3”). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset and the disclosure requirements under FASB Statement No. 142,Goodwill and Other Intangibles. FSP 142-3 requires that an entity consider its historical experience in renewing or extending similar arrangements in determining the useful life of a recognized intangible asset. Determining the useful life of a recognized intangible asset under FSP 142-3 applies prospectively to intangible assets acquired after the effective date. The disclosure requirements of FSP 142-3 will be applied prospectively to all intangible assets recognized as of, and subsequent to, the effective date. FSP 142-3 is effective for financial statements issued for fiscal years beginning after December 15, 2008, or the Company’s fiscal year 2010, and interim periods within those fiscal years. Early adoption of FSP 142-3 is prohibited. The Company is currently assessing the effect, if any, the adoption of FSP 142-3 will have on its financial position, results of operations or cash flows.
In May 2008, the FASB issued FASB Staff Position No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) (“FSP APB 14-1”). FSP APB 14-1 requires entities with convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) to separately account for the liability and equity components in a manner that reflects an estimate of the entity’s nonconvertible debt borrowing rate when interest expense is recognized in subsequent periods. FSP APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, or the Company’s fiscal year 2010. Entities are required to apply FSP APB 14-1 retrospectively for all periods presented. Upon adoption, the Company estimates FSP APB 14-1 will increase non-cash interest expense by $5.9 million (or approximately $3.6 million after-tax), $10.9 million (or approximately $6.7 million after-tax), and $7.7 million (or approximately $4.7 million after-tax) for fiscal years 2008, 2009, and 2010, respectively.
In June 2008, the FASB ratified EITF Issue No. 07-5,Determining Whether an Instrument (or an Embedded Feature) Is Indexed to an Entity’s Own Stock (“EITF 07-5”). EITF 07-5 provides that an entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. It also clarifies the impact of foreign currency denominated strike prices and market-based employee stock option valuation instruments on the evaluation. EITF 07-5 is effective for fiscal years beginning after December 15, 2008, or the Company’s fiscal year 2010. This pronouncement will not effect future reporting periods, as the Company currently accounts for their financial instruments under the guidance set forth by EITF 07-5.
During December 2007, the FASB issued SFAS No. 141 (revised 2007),Business Combinations (“SFAS 141(R)”) and Statement No. 160,Accounting and Reporting of Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB No. 51 (“Statement No. 160”). These new standards significantly change the accounting for business combinations and noncontrolling (or minority) interests in a number of areas including the treatment of contingent consideration, preacquisition contingencies, allocation of acquisition costs, in-process research and development, and restructuring costs. In addition, under SFAS No. 141(R), changes in an acquired entity’s deferred tax assets and uncertain tax positions after the measurement period may impact income tax expense. Statement No. 141(R) is required to be adopted concurrently with Statement No. 160 and is effective for business combination transactions for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. Early adoption is prohibited. Application of Statement No. 141(R) and Statement No. 160 is required to be adopted prospectively, except for certain provisions of Statement No. 160, which are required to be adopted retrospectively. Business combination transactions accounted for before adoption of Statement No. 141(R) should be accounted for in accordance with Statement No. 141 and that accounting previously completed under Statement No. 141 should not be modified as of or after the date of adoption of Statement No. 141(R). The Company is currently assessing the effect that adopting SFAS 141(R) and Statement No. 160 will have on its financial position, results of operations and cash flows.
56
Table of Contents
In March 2008, the FASB issued SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities—an amendment of FASB Statement No. 133 (“SFAS 161”). This Statement requires enhanced disclosures about an entity’s derivative and hedging activities, including (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities (“SFAS 133”), and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. SFAS 161 is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008. The Company adopted SFAS during the current fiscal year. The adoption did not have an impact on the Company’s financial position, results of operations or cash flows.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Market Risk. The Company’s objective in managing market risk exposures is to identify and limit the potential impact of changes in interest rates, commodity availability, and access of capital on earnings and cash flow. The following assessment of the Company’s market risk does not include uncertainties that are either nonfinancial or nonquantifiable, such as political uncertainty, economic uncertainty, impact of future tax legislation, and credit risks.
Interest Rate Risk. The Company’s primary interest rate exposure relates to cash and cash equivalents and fixed and variable rate debt. During fiscal year 2009, the Company’s debt obligations consisted of (i) $150.0 million senior convertible notes with a fixed rate of 2.25%, (ii) $230.0 million senior convertible notes with a fixed rate of 3.125%, and (iii) variable rate borrowings under the revolving line of credit, which bear interest at the bank’s prime rate plus an applicable margin based on a fixed charge coverage ratio, or at LIBOR plus an applicable margin based on a fixed charge coverage ratio. Fixed charge coverage is determined by the ratio of Earnings before Interest, Tax, Depreciation and Amortization to the sum of fixed charges, including but not limited to, Interest Expense, Capital Expenditures, payment of debt principal, and cash paid for taxes.
Changes in interest rates affect interest payments under the Company’s variable rate revolving line of credit agreement. The Company periodically enters into interest rate swap agreements to hedge the variable interest rate of its revolving line of credit. During fiscal year 2008, the Company entered into an interest rate swap agreement with a notional value of $50.0 million (“2008 Swap”). Under the terms of the 2008 Swap, the Company makes payments based on the fixed rate and will receive interest payments based on 1-month LIBOR which effectively fixes the notional amount covered by the swap at a borrowing rate of 2.70% plus a credit spread.
The changes in market value of these financial instruments are highly correlated with changes in market value of the hedged item both at inception and over the life of the agreement. Amounts received or paid under the interest rate swap agreement are recorded as reductions or additions to interest expense. In accordance with Statement of Financial Accounting Standards (“SFAS”) No. 133,Accounting for Derivative Instruments and Hedging Activities,SFAS No. 138,Accounting for Certain Derivative Instruments and Certain Hedging Activities, an amendment of FASB Statement No. 133, SFAS No. 149, Amendment of Statement 133 on Derivative Instruments and Hedging Activities,and SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities, and amendment of FASB Statement No. 133, the Company’s interest rate swap agreement has been designated as a cash flow hedge with changes in fair value recognized in accumulated other comprehensive income (loss) in the accompanying consolidated balance sheets.
As of March 27, 2009, the 2008 Swap carries a notional principal amount of $50.0 million and effectively fixes the interest rate on a portion of the revolving line of credit to 2.70%, prior to applying the applicable margin discussed above. The 2008 Swap agreement expires on February 19, 2010 and settles monthly until expiration. During the fiscal years ended March 27, 2009 and March 28, 2008, the Company recorded unrealized losses, net of tax, of approximately $0.4 million and $0.2 million, respectively, for the estimated fair value of the 2008 Swap agreement in accumulated other comprehensive income in the accompanying consolidated balance sheet.
57
Table of Contents
Changes in interest rates also affect rates of return on the Company’s cash equivalents and short-term investments, which generally consist of money market accounts.
During fiscal year 2009, the Company had average daily variable rate borrowings under its line of credit of $51.1 million. Excluding the impact of the interest rate swap, a hypothetical 1% increase/decrease in prevailing interest rates at March 27, 2009, would result in a corresponding increase/decrease in interest expense of approximately $0.5 million.
During fiscal year 2008, the Company had average daily variable rate borrowings under its line of credit of $31.9 million. Excluding the impact of the interest rate swap, a hypothetical 1% increase/decrease in prevailing interest rates at March 28, 2008, would result in a corresponding increase/decrease in interest expense of approximately $0.3 million.
Currency Risk. The Company’s currency rate exposures relate to products that are globally sourced from manufacturers primarily in Southeast Asia. Currently, the Company has negotiated settlement of payments to manufacturers in U.S. dollars. However, over time, local country currency fluctuations may increase or decrease the negotiated cost that the Company must pay for these products. In addition, the Company may in future periods negotiate settlement of payments to manufacturers in the local currency of the country providing a product which would then subject the Company to foreign currency risk.
Commodity Risk. The Company’s primary commodity exposures relate to fluctuations in the price of gasoline and diesel fuel and the procurement of certain medical supplies in which the product cost is dependent upon the price of raw materials.
The Company’s direct fuel exposure relates to fluctuations in fuel costs that affect the Company-leased delivery fleet or third-party delivery charges. Significant increases in the cost of gasoline and diesel fuel have impacted, and may continue to impact, the Company’s gross margin, cost to deliver, and the operating costs of third part transportation providers. Some of these common carriers have passed these increases through to the Company in the form of a fuel surcharge, which has had an adverse effect on the Company’s results of operations. The Company implemented a fuel surcharge to its customers during fiscal year 2006 to pass on a portion of the increased cost of gasoline and diesel fuel. There can be no assurance that the Company will be able to fully pass along further significant increases in fuel costs to its customers due to the competitive nature of the medical supply distribution industry.
As of March 2007, March 2008, and March 2009, the U.S. National average for unleaded gasoline ranged from $2.54, $3.25 and $2.03/gallon, respectively, and the U.S. National average for diesel fuel ranged from $2.79, $3.96, and $2.22/gallon, respectively. With respect to the Company’s direct fuel purchases, a hypothetical 10% increase/decrease in diesel and unleaded fuel costs during fiscal years 2009 and 2008 would have resulted in a corresponding decrease/increase in fuel expense of approximately $0.7 million and $0.6 million, respectively.
The Company purchases latex and vinyl gloves through agreements in which the pricing of the gloves is based on the price of latex as traded on the Malaysian Rubber Exchange and the weighted price of two raw materials, Poly vinyl chloride (PVC) and Dioctylphthalate (DOP), as published onwww.icis.com. Latex, PVC, and DOP in their raw form are only one of many components used on the manufacture of gloves. However, based on estimates of component mix, a hypothetical 10% increase/decrease in latex costs during fiscal years 2009 and 2008 would have resulted in a corresponding decrease/increase in the cost of latex gloves of approximately $1.0 million and $0.1 million, respectively. In addition, a hypothetical 10% increase/decrease in PVC and DOP costs during fiscal years 2009 and 2008 would have resulted in a corresponding decrease/increase in the cost of vinyl gloves of approximately $1.5 million and $0.1 million respectively.
58
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||
| F-2 | ||
Consolidated Balance Sheets—March 27, 2009 and March 28, 2008 | F-3 | |
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-7 | ||
| F-42 | ||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
PSS World Medical, Inc.:
We have audited the accompanying consolidated balance sheets of PSS World Medical, Inc. and subsidiaries (the Company) as of March 27, 2009 and March 28, 2008, and the related consolidated statements of operations, shareholders’ equity and cash flows for each of the years in the three-year period ended March 27, 2009. In connection with our audits of the consolidated financial statements, we also audited the financial statement schedule for each of the years in the three-year period ended March 27, 2009. These consolidated financial statements and the financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of PSS World Medical, Inc. and subsidiaries as of March 27, 2009 and March 28, 2008, and the results of their operations and their cash flows for each of the years in the three-year period ended March 27, 2009, in conformity with U.S. generally accepted accounting principles. Also, in our opinion the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 2 to the consolidated financial statements, the Company adopted the provisions of Financial Accounting Standards Board Interpretation No. 48,Accounting for Uncertainty in Income Taxes, effective March 31, 2007.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of the Company’s internal control over financial reporting as of March 27, 2009, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated May 20, 2009 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
May 20, 2009
Jacksonville, Florida
Certified Public Accountants
F-2
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
MARCH 27, 2009 AND MARCH 28, 2008
(Dollars in Thousands, Except Share Data)
| ASSETS | ||||||
| 2009 | 2008 | |||||
Current Assets: | ||||||
Cash and cash equivalents | $ | 82,031 | $ | 21,122 | ||
Investment in available for sale securities | 10,592 | — | ||||
Accounts receivable, net | 230,361 | 237,248 | ||||
Inventories | 207,593 | 190,846 | ||||
Deferred tax assets, net | 8,059 | 10,488 | ||||
Prepaid expenses and other | 23,819 | 48,744 | ||||
Total current assets | 562,455 | 508,448 | ||||
Property and equipment, net | 100,115 | 90,680 | ||||
Other Assets: | ||||||
Goodwill | 112,768 | 110,679 | ||||
Intangibles, net | 22,958 | 26,305 | ||||
Investment in available for sale securities | — | 11,318 | ||||
Other | 73,534 | 67,395 | ||||
Total assets | $ | 871,830 | $ | 814,825 | ||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||
Current Liabilities: | ||||||
Accounts payable | $ | 127,300 | $ | 135,930 | ||
Accrued expenses | 52,718 | 46,056 | ||||
Revolving line of credit and current portion of long-term debt | 50,937 | 220,987 | ||||
Other | 7,955 | 11,969 | ||||
Total current liabilities | 238,910 | 414,942 | ||||
Long-term debt, excluding current portion | 231,812 | 725 | ||||
Other noncurrent liabilities | 54,598 | 64,130 | ||||
Total liabilities | 525,320 | 479,797 | ||||
Commitments and contingencies (Notes 2, 4, 10, 11, 12, 13, 14, 15 and 17) | ||||||
Shareholders’ Equity: | ||||||
Preferred stock, $0.01 par value; 1,000,000 shares authorized, no shares issued and outstanding | — | — | ||||
Common stock, $0.01 par value; 150,000,000 shares authorized, 59,316,697 and 61,847,679 shares issued and outstanding at March 27, 2009 and March 28, 2008, respectively | 583 | 609 | ||||
Additional paid in capital | 149,336 | 195,657 | ||||
Retained earnings | 194,939 | 136,718 | ||||
Accumulated other comprehensive income | 1,652 | 2,044 | ||||
Total shareholders’ equity | 346,510 | 335,028 | ||||
Total liabilities and shareholders’ equity | $ | 871,830 | $ | 814,825 | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED MARCH 27, 2009, MARCH 28, 2008, AND MARCH 30, 2007
(In Thousands, Except Per Share Data)
| 2009 | 2008 | 2007 | ||||||||||
Net sales | $ | 1,952,691 | $ | 1,855,791 | $ | 1,741,639 | ||||||
Cost of goods sold | 1,370,781 | 1,314,119 | 1,241,629 | |||||||||
Gross profit | 581,910 | 541,672 | 500,010 | |||||||||
General and administrative expenses | 352,260 | 323,973 | 300,700 | |||||||||
Selling expenses | 128,505 | 125,296 | 116,823 | |||||||||
Income from operations | 101,145 | 92,403 | 82,487 | |||||||||
Other (expense) income: | ||||||||||||
Interest expense | (11,843 | ) | (6,773 | ) | (5,346 | ) | ||||||
Interest and investment income | 2,304 | 691 | 1,194 | |||||||||
Other income, net | 2,643 | 7,284 | 2,006 | |||||||||
Other income (expense) | (6,896 | ) | 1,202 | (2,146 | ) | |||||||
Income before provision for income taxes | 94,249 | 93,605 | 80,341 | |||||||||
Provision for income taxes | 36,232 | 36,830 | 29,860 | |||||||||
Net income | $ | 58,017 | $ | 56,775 | $ | 50,481 | ||||||
Basic earnings per common share | $ | 0.97 | $ | 0.88 | $ | 0.75 | ||||||
Diluted earnings per common share | $ | 0.96 | $ | 0.86 | $ | 0.73 | ||||||
Weighted average common shares outstanding, Basic | 59,937 | 64,703 | 67,219 | |||||||||
Weighted average common shares outstanding, Diluted | 60,696 | 66,184 | 69,325 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
FOR THE YEARS ENDED MARCH 27, 2009, MARCH 28, 2008 AND MARCH 30, 2007
(In Thousands, Except Share Data)
| Shares | Amount | Additional Paid-In Capital | Retained Earnings | Accumulated Other Comprehensive (Loss) Income | Totals | ||||||||||||||||||
Balance at March 31, 2006 | 67,476,682 | 674 | 318,441 | 29,698 | — | 348,813 | |||||||||||||||||
Net income | — | — | — | 50,481 | — | 50,481 | |||||||||||||||||
Repurchases and retirement of common stock | (2,126,728 | ) | (21 | ) | (42,878 | ) | — | — | (42,899 | ) | |||||||||||||
Exercise of stock options and related income tax benefit | 1,597,668 | 16 | 22,214 | — | — | 22,230 | |||||||||||||||||
Issuance of restricted stock, net of forfeitures | (277,991 | ) | (2 | ) | 2 | — | — | — | |||||||||||||||
Vesting of restricted stock and related income tax benefit | 95,563 | 1 | 2,001 | — | — | 2,002 | |||||||||||||||||
Employee benefits and other | 9,487 | — | 234 | — | — | 234 | |||||||||||||||||
Balance at March 30, 2007, as reported | 66,774,681 | 668 | 300,014 | 80,179 | — | 380,861 | |||||||||||||||||
FIN 48 Adjustment | — | — | — | (236 | ) | — | (236 | ) | |||||||||||||||
Balance at March 30, 2007, as adjusted | 66,774,681 | 668 | 300,014 | 79,943 | — | 380,625 | |||||||||||||||||
Net income | — | — | — | 56,775 | — | 56,775 | |||||||||||||||||
Unrealized holding gains on available-for-sale investments, net of taxes of $3,128 | — | — | — | — | 5,125 | 5,125 | |||||||||||||||||
Reclassification adjustment for gains on available-for-sale investments included in net income, net of taxes of $1,757 | — | — | — | — | (2,879 | ) | (2,879 | ) | |||||||||||||||
Unrealized loss on interest rate swap, net of income tax benefit of $123 | — | — | — | — | (202 | ) | (202 | ) | |||||||||||||||
Total comprehensive income | 58,819 | ||||||||||||||||||||||
Repurchases and retirement of common stock | (6,363,828 | ) | (63 | ) | (112,464 | ) | — | — | (112,527 | ) | |||||||||||||
Exercise of stock options and related income tax benefit | 320,860 | 3 | 3,975 | — | — | 3,978 | |||||||||||||||||
Vesting of restricted stock and related income tax benefit | 141,208 | 1 | 3,681 | — | — | 3,682 | |||||||||||||||||
Employee benefits and other | 21,705 | — | 451 | — | — | 451 | |||||||||||||||||
Balance at March 28, 2008 | 60,894,626 | $ | 609 | $ | 195,657 | $ | 136,718 | $ | 2,044 | $ | 335,028 | ||||||||||||
Net income | — | — | — | 58,017 | — | 58,017 | |||||||||||||||||
Unrealized holding gain on available-for-sale investments, net of taxes of $140 | — | — | — | — | 234 | 234 | |||||||||||||||||
Reclassification adjustment for gains on available-for-sale investments included in net income, net of taxes of $169 | — | — | — | — | (275 | ) | (275 | ) | |||||||||||||||
Unrealized loss on interest rate swap, net of income tax benefit of $216 | — | — | — | — | (351 | ) | (351 | ) | |||||||||||||||
Total comprehensive income | 57,625 | ||||||||||||||||||||||
Repurchases and retirement of common stock | (3,377,385 | ) | (34 | ) | (52,734 | ) | — | — | (52,768 | ) | |||||||||||||
Exercise of stock options and related income tax benefit | 604,974 | 6 | 7,870 | — | — | 7,876 | |||||||||||||||||
Stock-based compensation | — | — | 247 | — | — | 247 | |||||||||||||||||
Vesting of restricted stock and related income tax benefit | 156,012 | 2 | 5,626 | — | — | 5,628 | |||||||||||||||||
Convertible note hedge transaction net of income tax benefit | — | — | (33,103 | ) | — | — | (33,103 | ) | |||||||||||||||
Warrant transaction | — | — | 25,368 | — | — | 25,368 | |||||||||||||||||
Employee benefits and other | 23,026 | — | 405 | 204 | — | 609 | |||||||||||||||||
Balance at March 27, 2009 | 58,301,253 | $ | 583 | $ | 149,336 | $ | 194,939 | $ | 1,652 | $ | 346,510 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED MARCH 27, 2009, MARCH 28, 2008, AND MARCH 30, 2007
(Dollars in Thousands)
| 2009 | 2008 | 2007 | ||||||||||
Cash Flows From Operating Activities: | ||||||||||||
Net income | $ | 58,017 | $ | 56,775 | $ | 50,481 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation | 19,784 | 18,706 | 16,750 | |||||||||
Provision (benefit) for deferred income taxes | 9,935 | 6,602 | (1,661 | ) | ||||||||
Noncash compensation expense | 6,747 | 3,893 | 5,086 | |||||||||
Amortization of intangible assets | 5,489 | 5,586 | 5,908 | |||||||||
Provision for doubtful accounts | 3,680 | 3,787 | 4,533 | |||||||||
Provision for deferred compensation | 1,210 | 9 | 2,021 | |||||||||
Amortization of debt issuance costs | 1,760 | 1,417 | 1,434 | |||||||||
(Gain) loss on sales of property and equipment | (9 | ) | 113 | 191 | ||||||||
Gain on sale of available for sale securities | (444 | ) | (4,637 | ) | — | |||||||
Changes in operating assets and liabilities, net of effects from business combinations: | ||||||||||||
Accounts receivable, net | 3,912 | (15,164 | ) | (18,142 | ) | |||||||
Inventories | (16,358 | ) | (14,548 | ) | (672 | ) | ||||||
Prepaid expenses and other current assets | 2,486 | 3,188 | 1,664 | |||||||||
Other assets | (9,213 | ) | (11,056 | ) | (3,490 | ) | ||||||
Accounts payable | (9,209 | ) | 286 | (9,822 | ) | |||||||
Accrued expenses and other liabilities | 11,930 | 14,888 | 9,260 | |||||||||
Net cash provided by operating activities | 89,717 | 69,845 | 63,541 | |||||||||
Cash Flows From Investing Activities: | ||||||||||||
Capital expenditures | (26,936 | ) | (19,263 | ) | (17,266 | ) | ||||||
Payments for business combinations, net of cash acquired of $ -, $285, and $ -, respectively | (3,744 | ) | (15,245 | ) | (2,869 | ) | ||||||
Payments for investment in available for sale securities | — | (24,064 | ) | — | ||||||||
Proceeds from sale of available for sale securities | 22,098 | — | — | |||||||||
Proceeds from note receivable | — | 2,737 | — | |||||||||
Proceeds from sales of property and equipment | 19 | 51 | 34 | |||||||||
Other | (240 | ) | (151 | ) | (1,187 | ) | ||||||
Net cash used in investing activities | (8,803 | ) | (55,935 | ) | (21,288 | ) | ||||||
Cash Flows From Financing Activities: | ||||||||||||
Proceeds from issuance of convertible debt | 230,000 | — | — | |||||||||
Proceeds from issuance of warrants | 25,368 | — | — | |||||||||
Proceeds from exercise of stock options | 6,208 | 2,796 | 16,963 | |||||||||
Excess tax benefits from share-based compensation arrangements | 1,700 | 1,404 | 5,599 | |||||||||
Proceeds from borrowings | — | — | 1,500 | |||||||||
Payments for extinguishment of convertible notes | (149,980 | ) | — | — | ||||||||
Payment for purchase of hedge on convertible notes | (54,096 | ) | — | — | ||||||||
Purchase of common stock | (52,768 | ) | (112,527 | ) | (42,899 | ) | ||||||
Net (payments) proceeds on the revolving line of credit | (20,000 | ) | 70,000 | — | ||||||||
Payment for debt issue costs | (5,147 | ) | (206 | ) | — | |||||||
Payment under capital lease obligations | (1,290 | ) | (913 | ) | (625 | ) | ||||||
Net cash used in financing activities | (20,005 | ) | (39,446 | ) | (19,462 | ) | ||||||
Net increase (decrease) in cash and cash equivalents | 60,909 | (25,536 | ) | 22,791 | ||||||||
Cash and cash equivalents, beginning of period | 21,122 | 46,658 | 23,867 | |||||||||
Cash and cash equivalents, end of period | $ | 82,031 | $ | 21,122 | $ | 46,658 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
MARCH 27, 2009, MARCH 28, 2008, AND MARCH 30, 2007
(Dollars and Shares in Thousands, Except Per Share Data, Unless Otherwise Noted)
| 1. | NATURE OF OPERATIONS |
PSS World Medical, Inc. (the “Company” or “PSSI”), a Florida corporation which began operations in 1983, is a national distributor of medical products, equipment, and pharmaceutical related products to alternate-site healthcare providers including physician offices, long-term care and assisted living facilities, and home health care providers through 39 full-service distribution centers, which serve all 50 states throughout the United States (“U.S.”). The Company currently conducts business through two operating segments, the Physician Business and the Elder Care Business, which serve a diverse customer base. A third reporting segment, Corporate Shared Services, includes allocated and unallocated costs of corporate departments that provide services to the operating segments.
The Physician Business, or the Physician Sales & Service division, is a leading distributor of medical supplies, diagnostic equipment, pharmaceutical related products and healthcare information technology to primary care office-based physicians in the U.S. The Physician Business currently operates 27 full-service distribution centers, 33 break-freight locations, and 2 redistribution facilities serving physician offices in all 50 states.
The Elder Care Business, or the Gulf South Medical Supply, Inc. division, is a national distributor of medical supplies and related products to the long-term and elder care industry in the U.S. In addition, the Elder Care Business provides Medicare Part B reimbursable products and billing services, either on a fee-for-service or a full-assignment basis and Medicaid billing services to the assisted living market. The Elder Care Business currently operates 12 full-service distribution centers, 7 break-freight locations, 6 ancillary service centers, and 2 redistribution facilities serving independent, regional, and national skilled nursing facilities, assisted living centers, and home health care providers in all 50 states.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and include the accounts of PSS World Medical, Inc. and its wholly-owned subsidiaries. The Company held an interest in a variable interest entity (“VIE”) that was consolidated by the Company as it determined that it should, as the primary beneficiary, absorb the majority of the VIE’s expected losses and/or expected residual returns. During fiscal year 2009, the Company terminated its agreement with and deconsolidated the VIE. Refer to Footnote 4,Variable Interest Entity,for additional information. All significant intercompany balances and transactions have been eliminated in consolidation.
The Company reports its year-end financial position, results of operations, and cash flows on the Friday closest to March 31. Fiscal years 2009, 2008, and 2007 each consisted of 52 weeks.
Use of Estimates
The preparation of financial statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant items subject to such estimates and assumptions include the carrying amount of inventories, property and equipment, goodwill, and intangibles; allowances for doubtful accounts receivables, and vendor rebate receivables; valuation allowances for deferred income taxes; liabilities for loss contingencies; cash and stock-based compensation expense, and valuations associated with business combinations. Actual results could differ from the estimates and assumptions used in preparing the consolidated financial statements.
F-7
Table of Contents
Reclassification
During the first quarter of fiscal year 2009, the Company corrected the classification of its 2.25% $150.0 million senior convertible notes at March 28, 2008 from long-term debt to short-term debt, reflecting the one-day put option afforded to the holders of the notes on March 15, 2009 in accordance with the terms thereof. Refer to Footnote 11,Debt, for additional information regarding the note holders’ put option. Under Staff Accounting Bulletin (“SAB”) 99,Materiality, and SAB 108,Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements,the Company evaluated the quantitative and qualitative aspects of the adjustment and determined the correction was not material. There was no impact on the Company’s results of operations or cash flow statements for the fiscal year ended March 28, 2008.
Additionally, certain items previously reported in combined financial statement captions have been reclassified to conform to the current financial statement presentation.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, including cash and cash equivalents, short-term trade receivables, and accounts payable, approximate their fair values due to the short-term nature of these assets and liabilities. The carrying value of the Company’s 3.125% senior convertible notes at March 27, 2009 was $230,000 and the fair value, which is estimated using quoted market prices, was approximately $203,500. The carrying value of the Company’s 2.25% senior convertible notes at March 28, 2008 was $150,000 and the fair value, which is estimated using quoted market prices, was approximately $164,700.
Cash and Cash Equivalents
Cash and cash equivalents generally consist of demand deposits with financial institutions and highly liquid investment grade instruments having maturities of three months or less at the date of purchase. Cash and cash equivalents are stated at cost, which approximates market value.
Accounts Receivable
Trade accounts receivable consist of amounts owed to the Company and are stated at cost, which approximates fair value due to the short-term nature of the asset. The Company’s outstanding accounts receivable are exposed to credit risk and valuation allowances are established for estimated losses resulting from non-collection of outstanding amounts due from customers. The valuation allowances include specific amounts for those accounts that are deemed likely to be uncollectible, such as customer bankruptcies and disputed amounts, and general allowances for accounts that management currently believes to be collectible but that may later become uncollectible. Estimates are used to determine the valuation allowances and are generally based on historical collection results, current economic trends, credit-worthiness of customers, and changes in customer payment terms. Cash flows related to changes in accounts receivable balances are classified as operating activities within the Consolidated Statements of Cash Flows.
The Physician Business’ trade accounts receivable consist of many individual accounts; none of which are individually significant to the Company. The Physician Business had allowances for doubtful accounts of approximately $2,889 and $3,323 at March 27, 2009 and March 28, 2008, respectively. During fiscal years 2009, 2008, and 2007, bad debt expense was less than 1% of net sales.
F-8
Table of Contents
The Elder Care Business’ trade accounts receivable have a number of large, national chain customer accounts that are significant to its business. Approximately 14%, 15%, and 16%, of the Elder Care Business’ net sales for the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, respectively, represent sales to its largest five customers. As of March 27, 2009 and March 28, 2008, the outstanding accounts receivable balances of these customers represented approximately 10% and 9% of accounts receivable, net of allowance for doubtful accounts, respectively. The Elder Care Business had allowances for doubtful accounts of approximately $4,152 and $3,688 at March 27, 2009 and March 28, 2008, respectively. During fiscal years 2009, 2008, and 2007, bad debt expense was less than 1% of net sales.
Over the past three years, the Company’s allowance for doubtful accounts has represented 2% of the Physician Business’ gross accounts receivable balance, and 5% to 8% of the Elder Care business’ gross accounts receivable balance.
Inventories
Inventories consist of medical products, medical equipment, and other related products and are stated at the lower of cost or market. Cost is determined using the first-in, first-out (“FIFO”) method. Market is defined as net realizable value. The net realizable value of excess and slow moving inventory is determined using judgment as to when inventory will be sold and the quantities and prices at which inventory will be sold in the normal course of business. Obsolete or damaged inventory is disposed of or written down to net realizable value on a quarterly basis. Additional adjustments, if necessary, are made based on management’s specific review of each full-service distribution center’s inventory on-hand. Cash flows related to changes in inventory are classified as operating activities within the Consolidated Statements of Cash Flows.
As part of the Company’s periodic review of the carrying amount of inventory during fiscal year 2007, management determined that a write-down of $7,027, net of recoveries, was required for excess influenza vaccine inventory resulting from the cancellation of customer orders due to an oversupply of the product in the market, and a mild influenza season. In addition to these factors, management based its decision on the likelihood of locating alternate markets for the product and the viability of the product based on its limited life span. As a result of the write-down, cost of goods sold increased by $7,027, net of recoveries, during the fiscal year ended March 30, 2007, or $4,364 net of tax.
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line method over the following estimated useful lives of the respective classes of assets.
| Useful Life | ||
Equipment | 2 to 10 years | |
Computer hardware and software | 3 to 15 years | |
Capitalized internal-use software costs | 5 to 15 years |
Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life. Management is required to use judgment in determining the estimated useful lives of such assets. Changes in circumstances such as technological advances, changes to the Company’s business model, changes in the Company’s business strategy, or changes in the planned use of property and equipment could result in the actual useful lives differing from the Company’s current estimates. In those cases where the Company determines the useful life of property and equipment should be shortened or extended, the Company depreciates the net book value in excess of the estimated salvage value over its revised remaining useful life.
F-9
Table of Contents
The Company capitalizes the following costs associated with developing internal-use computer software: (i) external direct costs of materials and services consumed in developing or obtaining internal-use computer software; (ii) certain payroll and payroll-related costs for Company employees who are directly associated with the development of internal-use software, to the extent of time spent directly on the project; and (iii) interest costs incurred while developing internal-use computer software. Internal-use software costs capitalized subsequent to March 23, 2005 are amortized over the estimated useful lives of the software, ranging from 5 to 15 years. Statement of Financial Accounting Standards (“SFAS”) No. 34,Capitalization of Interest Cost, requires the capitalization of interest cost as a part of the historical cost of acquiring certain assets, such as assets that are constructed or produced for a company’s own use. The amount of capitalized interest during fiscal years 2009, 2008, and 2007 was $450, $222, and $262, respectively.
Gains or losses upon retirement or disposal of property and equipment are recorded in other income in the accompanying consolidated statements of operations. Normal repair and maintenance costs that do not substantially extend the life of the property and equipment are expensed as incurred.
Goodwill
Goodwill represents the excess of the cost of an acquired company over the fair value of identifiable assets and liabilities acquired. In accordance with the provisions of SFAS No. 142,Goodwill and Other Intangible Assets(“SFAS 142”), goodwill is reviewed for impairment annually as of the last day of the fiscal year. An interim review is performed between annual tests whenever events or changes in circumstances indicate the carrying amount of the goodwill may be impaired. Because the estimated fair value of the reporting units exceeded the carrying amount of the goodwill, there was no impairment at March 27, 2009 and March 28, 2008.
Intangibles
SFAS 142 requires intangible assets with finite useful lives be amortized over their respective estimated useful lives. Amortization is computed using the straight-line method.
Nonsolicitation Agreements
Certain sales representatives employed by the Physician and Elder Care Businesses have executed employment agreements in exchange for a cash payment (“Nonsolicitation Agreements”). These employment agreements include nonsolicitation covenants, which state that the sales representative can neither solicit nor accept business from certain of the Company’s customers for a stated period of time subsequent to the date the sales representative ceases employment with the Company. The costs associated with these Nonsolicitation Agreements are capitalized and amortized on a straight-line basis over their estimated useful lives, plus the stated nonsolicitation period. If a sales representative terminates employment prior to the end of the estimated useful life of the agreement, the remaining net book value of the asset is amortized over the stated nonsolicitation period.
During the period the sales representatives remain employed with the Company, the nonsolicitation intangible asset is evaluated for impairment in accordance with the provisions of SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets (“SFAS 144”). SFAS 144 requires the Company to test for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Certain factors which may indicate an impairment exists include, but are not limited to: (i) a change in a state’s legal system that would impact any legal opinion relied upon when assessing enforceability of the nonsolicitation covenants, (ii) a decline in gross profit generated by a sales representative below the amount that the nonsolicitation was based upon, (iii) death, or (iv) full retirement by the sales representative. In the event the carrying value of the assets were to be determined unrecoverable, the Company would estimate the fair value of the assets and record an impairment charge for the excess of the carrying value over the fair value. There were no impairments at March 27, 2009 or March 28, 2008.
F-10
Table of Contents
Impairment of Long-Lived Assets
Long-lived assets, other than goodwill and indefinite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable in accordance with SFAS 144. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted future cash flows expected to result from the use and eventual disposition of the asset. The impairment loss is measured as the amount by which the carrying amount of the long-lived asset exceeds fair value.
The Company evaluates the recoverability of indefinite-lived intangible assets annually in accordance with SFAS 142. An interim review may be performed more frequently, if events or changes in circumstances, such as a decline in sales, earnings, or cash flows, or material adverse changes in the business climate, indicate that the carrying value of an asset might be impaired.
There were no impairments at March 27, 2009 or March 28, 2008.
Accounts Payable
Outstanding checks in excess of cash balances available for a legal right of offset are reclassified to accounts payable. Amounts reclassified to accounts payable were $21,361 and $22,197 at March 27, 2009 and March 28, 2008, respectively.
Insurance Coverage
The Company has a self-funded program for employee and dependent health insurance. This program includes an administrator, a large provider network, and stop loss reinsurance to cover individual claims in excess of $225 per person, with an additional aggregate specific deductible of $190 annually, and up to $2,000 catastrophic loss maximum per lifetime benefit per person. Claims incurred but not reported are recorded based on estimates of claims provided by the third party administrator and are included in accrued expenses in the accompanying consolidated balance sheets.
The Company maintains a primary casualty insurance program for its automobile liability, employer’s liability, and general liability risks, which in general provides limits of up to $2,000, $1,000, and $2,000, respectively. In addition, the Company maintains workers compensation policies which have statutory limits that are based on state regulations. The primary program contains a deductible of $250 per occurrence for each line of coverage, subject to a primary aggregate stop loss of approximately $7,473 for the current plan year. In addition, the Company maintains an umbrella/excess liability program to cover occurrences in excess of the underlying primary limits.
Contingent Loss Accruals
In determining the accrual necessary for probable loss contingencies as defined by SFAS No. 5,Accounting for Contingencies(“SFAS 5”), the Company includes estimates for professional fees, such as legal, accounting, and consulting, and other related costs to be incurred, unless such fees and related costs are not probable of being incurred or are not reasonably estimable.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the tax consequences attributable to temporary differences between the financial statement carrying amounts and the respective tax bases in existing assets and liabilities. Deferred tax assets and liabilities are measured using the tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the consolidated statements of operations in the period that includes the enactment date.
F-11
Table of Contents
In the first quarter of fiscal year 2008, the Company adopted FIN 48, which provided guidance on recognition, classification and interest and penalties. In accordance with FIN 48, the Company only recognizes income tax benefits for tax positions that are more likely than not of being sustained upon examination based on the technical merits of the position. The amount of income tax benefit recognized is determined as the largest amount of benefit that has more than a 50 percent likelihood of being realized upon ultimate settlement. Refer to Footnote 12,Income Taxes, for further discussion.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the selling price is fixed or determinable, and collectibility of the resulting accounts receivable is reasonably assured. The Company assesses collectibility based upon a thorough evaluation of current and prospective customers’ credit history and ability to pay. The Company establishes and adjusts credit terms and limits to reflect customer credit worthiness based upon this evaluation. Customer credit evaluations are updated periodically and for specific events or circumstances such as deterioration in the aging of account balances, bankruptcy filings, or notice of financial difficulties.
Consolidated sales allowances are immaterial and generally represent less than 1.0% of gross sales.
Physician Business. The Physician Business has two primary sources of revenue: the sale of consumable products and the sale of equipment. Revenue from the sale of consumable products is recognized when products are shipped or delivered since at that time there is persuasive evidence that an arrangement exists, the price is fixed and determinable, and the collection of the resulting accounts receivable is reasonably assured. Revenue from the sale of single deliverable equipment is generally recognized when the equipment is shipped, unless there are multiple deliverables, in which case revenue is recognized when all obligations to the customer are fulfilled. Obligations to the customer are typically satisfied when installation and training are complete.
Customers have the right to return consumable products and equipment. Sales allowances are recorded as a reduction of revenue for potential product returns and estimated billing errors. Management analyzes sales allowances quarterly using historical data adjusted for significant changes in volume and business conditions, as well as specific identification of significant returns or billing errors.
Elder Care Business. The Elder Care Business has four primary sources of revenue: the sale of consumable products to (i) skilled nursing home and assisted living facilities and (ii) hospice and home health care providers; (iii) the sale of equipment, and (iv) service fees earned for providing Medicare Part B and Medicaid billing services.
Revenue from the sale of consumable products to skilled nursing home facilities, assisted living facilities, and home health care providers is recognized when products are shipped or delivered. Revenue for these products is recorded upon shipment since at that time there is persuasive evidence that an arrangement exists, the price is fixed and determinable, and the collection of the resulting accounts receivable is reasonably assured.
Revenue from the sale of single deliverable equipment is generally recognized when the equipment is shipped, unless there are multiple deliverables, in which case revenue is recognized when all obligations to the customer are fulfilled.
F-12
Table of Contents
Revenue from providing Medicare Part B and Medicaid billing services on a full-assignment basis is recognized during the period the supplies being provided to eligible patients are consumed. Revenue is recorded at the amounts expected to be collected from Medicare, Medicaid, other third-party payers, and directly from customers. Reimbursement from Medicare is subject to review by appropriate government regulators. Revenue from providing Medicare Part B and Medicaid billing services on a fee-for-service basis is recognized when billing services are rendered to the customer.
Customers have the right to return consumable products and equipment. Sales allowances are recorded as a reduction of revenue for (i) potential product and equipment returns, (ii) revenue adjustments related to actual usage of products by eligible Medicare Part B and Medicaid patients, and (iii) Medicare Part B and Medicaid reimbursement denials, and billing errors. Management analyzes product returns and billing errors quarterly using historical data adjusted for significant changes in volume and business conditions, as well as specific identification of significant returns and billing errors. Management analyzes revenue adjustments related to estimated usage of products by eligible Medicare Part B patients and Medicare Part B reimbursement denials using historical actual cash collection and actual adjustments to gross revenue for a certain period of time. Additional allowances are recorded for any significant specific adjustments known to management.
Vendor Rebates
The Company receives transaction-based and performance-based rebates from third party suppliers. Such rebates are classified as either (i) a reduction to cost of goods sold, (ii) a reduction of cost, or (iii) an increase to net sales in the accompanying Consolidated Statements of Operations.
Transaction-based rebates are generally associated with a specific customer contract and are recognized as a reduction to cost of goods sold at the time the transaction occurs. Management establishes a reserve for uncollectible transaction-based vendor rebates based on management’s judgment after considering the status of current outstanding rebate claims, historical denial experience with suppliers, and any other pertinent available information.
In accordance with Emerging Issues Task Force (“EITF”) No. 02-16,Accounting by a Customer (Including a Reseller) for Certain Consideration Received from a Vendor (“EITF 02-16”), performance-based rebates are recognized based on a systematic estimation of the consideration to be received relative to the transaction that marks the progress of the Company toward earning vendor rebates, provided the collection of the amounts is, in the judgment of management, reasonably assured. The factors the Company considers in estimating performance-based rebates include forecasted inventory purchases or sales volumes, in conjunction with vendor rebate contract terms, which generally provide for increasing rebates based on either increased purchases or sales volume. Performance-based rebates are recognized in income only if the related inventory has been sold.
In accordance with EITF No. 03-10:Application of Issue No. 02-16 by Resellers to Sales Incentives Offered to Consumers by Manufacturers, sales incentive arrangements that meet certain criteria are not subject to guidance in EITF 02-16. Accordingly, reimbursements from manufacturers under these arrangements are recognized by the Company as revenue rather than a reduction of cost of sales.
Transaction-based and performance-based rebate contracts are negotiated periodically with vendors.
F-13
Table of Contents
The following table summarizes the financial statement impact of transaction-based and performance-based vendor rebates recognized by the Company and each of its segments during fiscal years 2009, 2008, and 2007.
| Physician Business | |||||||||
| (in thousands) | 2009 | 2008 | 2007 | ||||||
Net sales | $ | 4,339 | $ | 5,664 | $ | 4,618 | |||
Cost of goods sold | 104,452 | 91,916 | 92,512 | ||||||
General and administrative expenses | 8,019 | 7,864 | 6,930 | ||||||
Total | $ | 116,810 | $ | 105,444 | $ | 104,060 | |||
| Elder Care Business | |||||||||
| 2009 | 2008 | 2007 | |||||||
Cost of goods sold | $ | 100,934 | $ | 89,273 | $ | 85,126 | |||
General and administrative expenses | 1,373 | 1,033 | 991 | ||||||
Total | $ | 102,307 | $ | 90,306 | $ | 86,117 | |||
| Total Company | |||||||||
| 2009 | 2008 | 2007 | |||||||
Net sales | $ | 4,339 | $ | 5,664 | $ | 4,618 | |||
Cost of goods sold | 205,386 | 181,189 | 177,638 | ||||||
General and administrative expenses | 9,392 | 8,897 | 7,921 | ||||||
Total | $ | 219,117 | $ | 195,750 | $ | 190,177 | |||
Shipping and Handling Costs
Shipping and handling costs billed to customers are included in net sales and totaled approximately $13,668, $10,195, and $8,716, for fiscal years 2009, 2008, and 2007, respectively. Shipping and handling costs incurred by the Company, which are included in general and administrative expenses, totaled approximately $102,836, $98,916, and $89,968, for fiscal years 2009, 2008, and 2007, respectively.
Derivative Financial Instruments
Derivative financial instruments are accounted for under SFAS 133, “Accounting for Derivative Instruments and Hedging Activities”, as amended (“SFAS 133”) and disclosed in accordance with SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities—an amendment of FASB Statement No. 133 (“SFAS 161”).
Under SFAS 133, all derivatives are recorded on the balance sheet as assets or liabilities and measured at fair value. For derivatives designated as hedges of the fair value of assets or liabilities, the changes in fair values of both the derivatives and the hedged items are recorded in current earnings. For derivatives designated as cash flow hedges, the effective portion of the changes in fair value of the derivatives are recorded in Other Comprehensive Income and subsequently recognized in earnings when the hedged items impact earnings, typically upon settlement. Changes in the fair value of derivatives not designated as hedges and the ineffective portion of cash flow hedges are recorded in current earnings.
SFAS 161 requires enhanced disclosures about an entity’s derivative and hedging activities, including (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities (“SFAS 133”), and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows.
F-14
Table of Contents
Derivative financial instruments are used principally in the management of the Company’s interest rate exposure. During the fiscal year ended March 28, 2008, the Company entered into an interest rate swap agreement to hedge the variable interest rate of its revolving line of credit. The interest rate swap was designated as a cash flow hedge in accordance with SFAS 133. Amounts received or paid upon settlement of the interest rate swap agreement will be recorded as reductions or additions to interest expense. Refer to Footnote 11,Debt, for additional information regarding the Company’s interest rate swap agreement.
Earnings Per Share
Basic and diluted earnings per share are presented in accordance with SFAS No. 128,Earnings Per Share(“SFAS 128”). Basic earnings per share is computed by dividing net income by the weighted average number of common shares outstanding during the period. Diluted earnings per share is computed by dividing net income by the weighted average number of common and common equivalent shares outstanding during the period adjusted for the potential dilutive effect of stock options using the treasury stock method and the conversion of the senior convertible notes. Common equivalent shares are excluded from the computation in periods in which they have an anti-dilutive effect.
The following table sets forth computational data for the denominator in the basic and diluted earnings per share calculation for fiscal years ended March 27, 2009, March 28, 2008 and March 30, 2007:
| (in thousands) | 2009 | 2008 | 2007 | |||
Denominator-Weighted average shares outstanding used in computing basic earnings per share | 59,937 | 64,703 | 67,219 | |||
Assumed exercise price of stock options(a) | 425 | 616 | 934 | |||
Assumed vesting of restricted stock | 152 | 109 | 102 | |||
Assumed conversion of 2.25% senior convertible notes | 182 | 756 | 1,070 | |||
Denominator-Weighted average shares outstanding used in computing diluted earnings per share | 60,696 | 66,184 | 69,325 | |||
| (a) | Options to purchase approximately 200, 55, and 188, shares of common stock that were outstanding during fiscal years 2009, 2008, and 2007, respectively, were not included in the computation of diluted earnings per share for each of the respective periods because the options’ exercise prices exceeded the average fair market value of the Company’s common stock for each fiscal year. |
In accordance with EITF Issue No. 04-8,The Effect of Contingently Convertible Debt on Diluted Earnings Per Share(“EITF 04-8”) and the Company’s stated policy to settle the principal amount of the $150.0 million 2.25% convertible notes in cash, the Company was required to include any shares underlying the $150.0 million 2.25% convertible notes in its diluted weighted average shares outstanding when the average stock price per share for the period exceeded $17.10 (the conversion price for the senior convertible notes). The average price of the Company’s common stock during fiscal years 2009, 2008 and 2007 exceeded the 2.25% convertible notes’ conversion price of $17.10. As such, 182, 756, and 1,070 potential common shares, respectively, were included in the calculation of diluted weighted average shares outstanding.
The Company is not required to include any shares underlying the $230 million 3.125% convertible notes in its diluted weighted average shares outstanding until the average stock price per share for the period exceeds $21.22 (the conversion price for the senior convertible notes). At such time, only the number of shares that would be issuable (under the treasury stock method of accounting for share dilution) would be included, which is based upon the amount by which the average stock price exceeds the conversion price. The average price of the Company’s common stock during fiscal year 2009 did not exceed the 3.125% convertible notes’ conversion price of $21.22. As such, no potential common shares were included in the calculation of diluted weighted average shares outstanding.
F-15
Table of Contents
Stock-Based Compensation
Effective April 1, 2006, the Company adopted the provisions of SFAS No. 123 (Revised 2004),Share-Based Payment, (“SFAS 123(R)”) using the modified prospective transition method, and therefore, has not restated results for prior periods. Prior to the adoption of SFAS 123(R), the Company elected to account for stock-based awards using the intrinsic-value recognition and measurement principles of APB Opinion No. 25,Accounting for Stock Issued to Employees, and related interpretations. Refer to Footnote 13,Shareholders’ Equity,for additional information
Comprehensive Income
Comprehensive income represents all changes in equity of an enterprise that result from recognized transactions and other economic events during the period. Other comprehensive income refers to revenues, expenses, gains, and losses that under GAAP are included in comprehensive income but excluded from net income, such as the unrealized gain or loss on the interest rate swap and unrealized holding gain or loss on available-for-sale investments.
Marketable Securities
Equity securities are considered to be available for sale and carried at fair value as of the balance sheet dates. Fair values are based on quoted market prices.
Realized gains and losses on the sale of investments are determined on the basis of the cost of the specific investments sold and are credited or charged to income on a trade date basis. Unrealized gains or losses on equity securities which are classified as available for sale, net of applicable deferred income taxes (benefits), are excluded from earnings and credited or charged directly to a separate component of stockholders’ equity. If any unrealized losses on equity securities are deemed other-than-temporary, such unrealized losses are recognized as realized losses. Unrealized losses are deemed other-than-temporary if factors exist that cause management to believe that the value will not increase to a level sufficient to recover the Company’s cost basis. As of March 27, 2009, there were no other-than-temporary impairments related to the Company’s available for sale securities.
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements” (“SFAS 157”). SFAS 157 defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements by establishing a fair value hierarchy based on the quality of inputs used to measure fair value. SFAS 157 does not require any new fair value measurements, but applies to other accounting pronouncements that require or permit fair value measurements. SFAS 157 is effective for financial statements for fiscal years beginning after November 15, 2007. The Company adopted SFAS 157 on March 29, 2008 and adoption did not have a material impact on its financial position, results of operations or cash flows. FSP SFAS No. 157-2, “Effective Date of FASB Statement No. 157” (“FSP SFAS 157-2”), delays the effective date of SFAS 157 with respect to nonfinancial assets and nonfinancial liabilities not remeasured at fair value on a recurring basis until fiscal years beginning after November 15, 2008, or the Company’s fiscal year 2010. Accordingly, the Company has not yet applied the requirements of SFAS 157 to certain such nonfinancial assets for which fair value measurements are determined only when there is an indication of potential impairment.
During February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB SFAS 115 (“SFAS 159”). SFAS 159 permits entities to choose to measure financial instruments and certain other items at fair value that are not currently required to be measured at fair value. SFAS 159 mandates certain financial statement presentation and disclosure requirements when a company elects to report assets and liabilities at fair value under SFAS 159. SFAS 159 is effective for fiscal years beginning after November 15, 2007, or the Company’s fiscal year 2009. The Company adopted SFAS 159 on March 29, 2008 and adoption did not have an impact on its financial position, results of operations or cash flows, as the Company did not elect fair value measurements for any financial instruments or other items upon adoption.
F-16
Table of Contents
In April 2008, the FASB issued FSP No. 142-3,Determination of the Useful Life of Intangible Assets (“FSP 142-3”). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset and the disclosure requirements under FASB Statement No. 142,Goodwill and Other Intangibles. FSP 142-3 requires that an entity consider its historical experience in renewing or extending similar arrangements in determining the useful life of a recognized intangible asset. Determining the useful life of a recognized intangible asset under FSP 142-3 applies prospectively to intangible assets acquired after the effective date. The disclosure requirements of FSP 142-3 will be applied prospectively to all intangible assets recognized as of, and subsequent to, the effective date. FSP 142-3 is effective for financial statements issued for fiscal years beginning after December 15, 2008, or the Company’s fiscal year 2010, and interim periods within those fiscal years. Early adoption of FSP 142-3 is prohibited. The Company is currently assessing the effect, if any, the adoption of FSP 142-3 will have on its financial position, results of operations or cash flows.
In May 2008, the FASB issued FASB Staff Position No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement) (“FSP APB 14-1”). FSP APB 14-1 requires entities with convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) to separately account for the liability and equity components in a manner that reflects an estimate of the entity’s nonconvertible debt borrowing rate when interest expense is recognized in subsequent periods. FSP APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, or the Company’s fiscal year 2010. Entities are required to apply FSP APB 14-1 retrospectively for all periods presented. Upon adoption, the Company estimates FSP APB 14-1 will increase non-cash interest expense by $5.9 million (or approximately $3.6 million after-tax), $10.9 million (or approximately $6.7 million after-tax), and $7.7 million (or approximately $4.7 million after-tax) for fiscal years 2008, 2009, and 2010, respectively.
In June 2008, the FASB ratified EITF Issue No. 07-5,Determining Whether an Instrument (or an Embedded Feature) Is Indexed to an Entity’s Own Stock (“EITF 07-5”). EITF 07-5 provides that an entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. It also clarifies the impact of foreign currency denominated strike prices and market-based employee stock option valuation instruments on the evaluation. EITF 07-5 is effective for fiscal years beginning after December 15, 2008, or the Company’s fiscal year 2010. This pronouncement will not effect future reporting periods, as the Company currently accounts for their financial instruments under the guidance set forth by EITF 07-5.
During December 2007, the FASB issued SFAS No. 141 (revised 2007),Business Combinations (“SFAS 141(R)”) and Statement No. 160,Accounting and Reporting of Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB No. 51 (“Statement No. 160”). These new standards significantly change the accounting for business combinations and noncontrolling (or minority) interests in a number of areas including the treatment of contingent consideration, preacquisition contingencies, allocation of acquisition costs, in-process research and development, and restructuring costs. In addition, under SFAS No. 141(R), changes in an acquired entity’s deferred tax assets and uncertain tax positions after the measurement period may impact income tax expense. Statement No. 141(R) is required to be adopted concurrently with Statement No. 160 and is effective for business combination transactions for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. Early adoption is prohibited. Application of Statement No. 141(R) and Statement No. 160 is required to be adopted prospectively, except for certain provisions of Statement No. 160, which are required to be adopted retrospectively. Business combination transactions accounted for before adoption of Statement No. 141(R) should be accounted for in accordance with Statement No. 141 and that accounting previously completed under Statement No. 141 should not be modified as of or after the date of adoption of Statement No. 141(R). The Company is currently assessing the effect that adopting SFAS 141(R) and Statement No. 160 will have on its financial position, results of operations and cash flows.
F-17
Table of Contents
In March 2008, the FASB issued SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities—an amendment of FASB Statement No. 133 (“SFAS 161”). This Statement requires enhanced disclosures about an entity’s derivative and hedging activities, including (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities (“SFAS 133”), and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. SFAS 161 is effective for financial statements issued for fiscal years and interim periods beginning after November 15, 2008. The Company adopted SFAS during the current fiscal year. The adoption did not have an impact on the Company’s financial position, results of operations or cash flows.
| 3. | PURCHASE BUSINESS COMBINATIONS |
The following acquisitions were accounted for under the purchase method of accounting in accordance with SFAS No. 141,Business Combinations; accordingly, the operations of the acquired companies have been included in the Company’s results of operations subsequent to the date of acquisition. The assets acquired and liabilities assumed were recorded at their estimated fair values at the date of the acquisition as determined by management based on information currently available and independent valuations. Supplemental unaudited pro forma information, assuming these acquisitions were made at the beginning of the immediate preceding period, is not presented as the results would not differ materially from the amounts reported in the accompanying Consolidated Statements of Operations.
Fiscal Year 2008
On May 31, 2007, the Physician Business acquired 100% of the outstanding stock of Activus Healthcare Solutions, Inc. (“Activus”), a California based distributor of medical supplies and pharmaceuticals to office-based physicians and ambulatory surgery centers. The aggregate purchase price, subject to certain adjustments as set forth in the purchase agreement, was approximately $13,278 (net of cash acquired of $285). Payments totaling $13,278 were made during fiscal year 2008, of which $1,000 was held in escrow and was released upon the satisfaction of certain sales representative retention milestones, net of any amounts payable to the Company for potential indemnity claims. These funds were classified as restricted cash within other current assets and accrued liabilities on the Consolidated Balance Sheet at March, 28, 2008. The premium paid in excess of the fair value of the net assets acquired was primarily for Activus’ tenured sales force.
Goodwill of $3,235, which includes purchase price adjustments of $296, is not deductible for tax purposes. Based on the final purchase price allocation, acquired intangible assets totaled approximately $1,912 and were assigned to customer relationships with a useful life of ten years.
F-18
Table of Contents
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition.
Cash | $ | 285 | |
Accounts receivable | 2,836 | ||
Inventory | 2,134 | ||
Other current assets | 520 | ||
Property and equipment | 427 | ||
Goodwill | 3,235 | ||
Intangibles | 1,912 | ||
Deferred tax asset | 6,582 | ||
Other noncurrent assets | 29 | ||
Total assets acquired | 17,960 | ||
Less: Current liabilities | 4,397 | ||
Net assets acquired | $ | 13,563 | |
There were no material acquisitions during fiscal year 2009 and 2007.
| 4. | VARIABLE INTEREST ENTITY |
PSS World Medical, Inc., and its wholly owned subsidiary, World Med Shared Services, Inc. (collectively the “Company”), entered into a Sourcing Services Agreement, dated as of January 19, 2005 (the “Agreement”), with Tiger Specialty Sourcing Limited, Tiger Shanghai Specialty Sourcing Co. Ltd., and its principals (collectively “Tiger Medical”). Subject to the terms and conditions of the Agreement, the Company agreed to purchase certain medical and other products from Chinese suppliers and manufacturers using the exclusive sourcing services of Tiger Medical.
Pursuant to the terms of the Agreement, the Company acquired a minority interest in Tiger Medical on January 25, 2005 for $1,000. Under the agreement, the Company ultimately had the right to increase its ownership interest in Tiger Medical to 100% during fiscal years 2006 through 2009 if certain performance targets were achieved. The Company did not make any cash payments during fiscal years 2009, 2008 and 2007 to increase its ownership interest in Tiger Medical.
For the fiscal year ended March 28, 2008, the Company’s interest in Tiger Medical was considered to be a VIE as defined by Financial Accounting Standards Board (“FASB”) Interpretation No. 46 (Revised 2003),Consolidation of Variable Interest Entities-an Interpretation of Accounting Research Bulletin No. 51(“FIN 46R”).The Company was considered the primary beneficiary as Tiger Medical provided sourcing services exclusively to the Company. Therefore, the financial statements of Tiger Medical were consolidated with the Company’s financial statements for fiscal year ended March 28, 2008. The Company is not obligated on any of the indebtedness of Tiger Medical nor is the Company obligated to fund any operating losses of Tiger Medical. The impact of consolidating the statement of operations of Tiger Medical with the Company’s statement of operations for the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007 was immaterial.
During the fiscal year ended March 27, 2009, the Company terminated its agreement with and exchanged its minority interest in Tiger Medical as partial consideration for a non-competition agreement. As of March 27, 2009, Tiger Medical did not meet the definition of a VIE under FIN 46R.Therefore, the financial statements of Tiger Medical are not consolidated with the Company’s financial statements as of March 27, 2009. The impact of deconsolidating the financial statements of Tiger Medical was immaterial.
| 5. | FAIR VALUE MEASUREMENTS |
As discussed in the Recent Accounting Pronouncements section of Footnote 2,Summary of Significant Accounting Policies, the Company adopted SFAS 157 (as impacted by FSP 157-2) effective March 29, 2008, with respect to fair value measurements of (i) nonfinancial assets and liabilities that are recognized or disclosed at fair value in the Company’s financial statements on a recurring basis (at least annually) and (ii) all financial assets and liabilities.
F-19
Table of Contents
SFAS 157 provides a framework for measuring fair value, expands disclosures about fair value measurements, and establishes a fair value hierarchy which prioritizes the inputs used in measuring fair value summarized as follows:
Level 1: Inputs using unadjusted quoted prices for identical assets or liabilities in an active market that the Company has the ability to access.
Level 2: Inputs other than quoted prices in markets that are observable for the asset or liability, either directly or indirectly.
Level 3: Inputs that are both significant to the fair value measurement and unobservable.
As of March 27, 2009, the fair value of the Company’s financial assets and/or liabilities are measured using Level 1 or Level 2 inputs. The following table presents the Company’s assets and liabilities which are measured at fair value on a recurring basis as of March 27, 2009, by level within the fair value hierarchy:
| Level 1 | Level 2 | Total | ||||||||
| (in thousands) | ||||||||||
Assets: | ||||||||||
Available-for-sale equity securities(a) | $ | 10,592 | $ | — | $ | 10,592 | ||||
Total assets | $ | 10,592 | $ | — | $ | 10,592 | ||||
Liabilities: | ||||||||||
Interest rate swap(b) | $ | — | $ | 891 | $ | 891 | ||||
Deferred compensation(c) | 45,932 | — | 45,932 | |||||||
Total liabilities | $ | 45,932 | $ | 891 | $ | 46,823 | ||||
| (a) | Relates to the Company’s investment in athenahealth, Inc., which is included in “Investment in available for sale securities” on the Company’s Consolidated balance sheets. This investment is measured using quoted market prices. |
| (b) | Relates to the Company’s interest rate swap on its revolving line of credit. See Footnote 11,Debt, for further information. The interest rate swap is valued using an internal model that utilizes as its basis readily observable market inputs such as credit spreads, current market interest rates, and forward interest rates. |
| (c) | Relates to the Company’s obligation to pay benefits under its non-qualified deferred compensation plans, which is included in “Other noncurrent liabilities” on the Company’s Consolidated balance sheets. The obligation to pay benefits is based off of participants’ allocation percentages to plan investments. The investments are measured using quoted market prices. |
As indicated in Footnote 2,Summary of Significant Accounting Policies,FSP 157-2 delays the effective date of SFAS 157 with respect to nonfinancial assets and nonfinancial liabilities that are not remeasured at fair value on a recurring basis until fiscal years beginning after November 15, 2008. Accordingly, the Company has not yet applied the disclosure requirements of SFAS 157 to certain such nonfinancial assets for which fair value measurements are determined only when there is an indication of potential impairment.
| 6. | EQUITY INVESTMENT |
On June 29, 2007, the Company made a $24,064 investment (including $1,564 of legal and other professional fees) in athenahealth, Inc. (“athena”), a leading provider of internet-based healthcare information technology and business services to physician practices. At the investment date, athena was a privately held entity and the Company’s investment represented an ownership interest of approximately 5% of outstanding shares. On September 20, 2007, an initial public offering of shares of athena’s common stock was made available for sale on the NASDAQ Global Market under the symbol “ATHN.”
This investment was initially recorded at cost in accordance with Accounting Principles Board Opinion No. 18,The Equity Method of Accounting for Investments in Common Stock, which governs the accounting for privately held investments. As a result of the initial public offering, the Company recorded investment as “available-for-sale” in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities. This investment was marked to market based on quoted market prices as of March 27, 2009 and March 28, 2008. During fiscal year 2009 and 2008, the Company sold a portion of its investment in athena, resulting in a gain of approximately $444, or $275 net of tax and $4,637, or $2,879 net of tax. This gain was determined on a specific identification method and recognized in Other income on the Consolidated statement of operations. Proceeds of $22,098 were received during fiscal year 2009 related to the sales of athena stock in fiscal years 2009 and 2008.
F-20
Table of Contents
As of March 27, 2009 and March 28, 2008, the aggregate fair value of the remaining investment of $10,592 and $11,318, was recorded on the Consolidated Balance Sheet in Investment in available for sale securities. Cumulative gross unrealized holding gains were $3,547 and $3,617, March 27, 2009 and March 28, 2008, respectively. During the years ended March 27, 2009 and March 28, 2008, the Company recorded a net unrealized holding loss of $70, of which $41 was recorded in other comprehensive income net of related income taxes of $29 and a net unrealized holding gain of $3,617, of which $2,246 was recorded in other comprehensive income net of related income taxes of $1,371, respectively.
Subsequent to year end the Company sold its remaining investment in athena for $10,681. The sale resulted in a gain of $3,635, or $2,254 net of tax.
| 7. | PROPERTY AND EQUIPMENT |
Property and equipment are summarized as follows:
| 2009 | 2008 | |||||||
Land | $ | — | $ | 9 | ||||
Computer hardware under capital leases | 5,343 | 2,777 | ||||||
Office equipment under capital leases | 963 | 807 | ||||||
Leasehold improvements | 15,785 | 13,595 | ||||||
Equipment | 28,703 | 28,333 | ||||||
Computer hardware and software | 148,589 | 133,656 | ||||||
| 199,383 | 179,177 | |||||||
Accumulated depreciation | (99,268 | ) | (88,497 | ) | ||||
Property and equipment, net | $ | 100,115 | $ | 90,680 | ||||
Depreciation expense, which includes amortization of capital leases, is included in general and administrative expenses in the accompanying consolidated statements of operations, and approximated $19,784, $18,706, and $16,750, for fiscal years 2009, 2008, and 2007, respectively.
| 8. | GOODWILL |
The change in the carrying value of goodwill for the fiscal years ended March 27, 2009 and March 28, 2008 is as follows:
| Physician Business | Elder Care Business | Corporate Shared Services | Total | ||||||||||||
Balance as of March 30, 2007 | $ | 27,279 | $ | 79,338 | $ | 749 | $ | 107,366 | |||||||
Purchase business combinations | 3,550 | — | — | 3,550 | |||||||||||
Purchase price allocation adjustments | (237 | ) | — | — | (237 | ) | |||||||||
Balance as of March 28, 2008 | 30,592 | 79,338 | 749 | 110,679 | |||||||||||
Purchase business combinations | — | 1,696 | — | 1,696 | |||||||||||
Purchase price allocation adjustments | 9 | 1,133 | — | 1,142 | |||||||||||
Termination of variable interest entity | — | — | (749 | ) | (749 | ) | |||||||||
Balance as of March 27, 2009 | $ | 30,601 | $ | 82,167 | $ | — | $ | 112,768 | |||||||
F-21
Table of Contents
| 9. | INTANGIBLES, NET |
The following table summarizes the gross carrying amount and accumulated amortization for existing intangible assets by business segment and major asset class.
| As of | ||||||||||||||||||||
| March 27, 2009 | March 28, 2008 | |||||||||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||||||||
INTANGIBLES SUBJECT TO AMORTIZATION | ||||||||||||||||||||
Customer Relationships: | ||||||||||||||||||||
Physician Business | $ | 9,355 | $ | (3,438 | ) | $ | 5,917 | $ | 10,147 | $ | (3,168 | ) | $ | 6,979 | ||||||
Elder Care Business | 14,362 | (9,798 | ) | 4,564 | 13,762 | (7,790 | ) | 5,972 | ||||||||||||
| 23,717 | (13,236 | ) | 10,481 | 23,909 | (10,958 | ) | 12,951 | |||||||||||||
Nonsolicitation Agreements: | ||||||||||||||||||||
Physician Business | 10,783 | (5,454 | ) | 5,329 | 11,573 | (4,638 | ) | 6,935 | ||||||||||||
Elder Care Business | 248 | (70 | ) | 178 | 264 | (139 | ) | 125 | ||||||||||||
| 11,031 | (5,524 | ) | 5,507 | 11,837 | (4,777 | ) | 7,060 | |||||||||||||
Noncompetition Agreements: | ||||||||||||||||||||
Physician Business | 417 | (367 | ) | 50 | 717 | (535 | ) | 182 | ||||||||||||
Elder Care Business | 640 | (448 | ) | 192 | 640 | (309 | ) | 331 | ||||||||||||
Corporate Shared Services | 1,027 | (146 | ) | 881 | 52 | (28 | ) | 24 | ||||||||||||
| 2,084 | (961 | ) | 1,123 | 1,409 | (872 | ) | 537 | |||||||||||||
Signing Bonuses: | ||||||||||||||||||||
Physician Business | 694 | (264 | ) | 430 | 858 | (653 | ) | 205 | ||||||||||||
Elder Care Business | — | — | — | 500 | (403 | ) | 97 | |||||||||||||
Corporate Shared Services | 22 | (5 | ) | 17 | — | — | — | |||||||||||||
| 716 | (269 | ) | 447 | 1,358 | (1,056 | ) | 302 | |||||||||||||
Other Intangibles: | ||||||||||||||||||||
Corporate Shared Services | — | — | — | 226 | (171 | ) | 55 | |||||||||||||
| — | — | — | 226 | (171 | ) | 55 | ||||||||||||||
Total intangible assets subject to amortization | $ | 37,548 | $ | (19,990 | ) | $ | 17,558 | $ | 38,739 | $ | (17,834 | ) | $ | 20,905 | ||||||
INTANGIBLES NOT SUBJECT TO AMORTIZATION | ||||||||||||||||||||
Tradename: | ||||||||||||||||||||
Physician Business | $ | 5,400 | $ | — | $ | 5,400 | $ | 5,400 | $ | — | $ | 5,400 | ||||||||
Total unamortized intangibleassets | 5,400 | — | 5,400 | 5,400 | — | 5,400 | ||||||||||||||
Total intangible assets | $ | 42,948 | $ | (19,990 | ) | $ | 22,958 | $ | 44,139 | $ | (17,834 | ) | $ | 26,305 | ||||||
Total amortization expense for intangible assets for the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, was $5,489, $5,586, and $5,908, respectively.
F-22
Table of Contents
The estimated amortization expense for the next five fiscal years is as follows:
Fiscal Year: | |||
2010 | $ | 4,663 | |
2011 | 3,647 | ||
2012 | 2,367 | ||
2013 | 1,535 | ||
2014 | 1,253 | ||
Thereafter | 4,093 | ||
Total | $ | 17,558 | |
The remaining weighted-average amortization period, in total and by major asset class, is as follows:
| (in years) | March 27, 2009 | March 28, 2008 | ||
Customer relationships | 6.2 | 6.4 | ||
Nonsolicitation agreements | 7.2 | 7.5 | ||
Noncompetition agreements | 3.7 | 2.2 | ||
Signing bonuses | 3.0 | 1.3 | ||
Other intangibles | — | 1.0 | ||
Total weighted-average period | 6.3 | 6.6 | ||
| 10. | ACCRUED EXPENSES |
Accrued expenses at March 27, 2009 and March 28, 2008 were as follows:
| 2009 | 2008 | |||||
Accrued payroll | $ | 24,662 | $ | 20,883 | ||
Accrued incentive compensation | 12,699 | 9,801 | ||||
Other | 15,357 | 15,372 | ||||
Accrued expenses | $ | 52,718 | $ | 46,056 | ||
| 11. | DEBT |
Outstanding debt consists of the following, in order of priority:
| As of | ||||||
| March 27, 2009 | March 28, 2008 | |||||
Revolving line of credit | $ | 50,000 | $ | 70,000 | ||
2.25% senior convertible notes | 20 | 150,000 | ||||
3.125% senior convertible notes | 230,000 | — | ||||
Capital lease obligations | 2,729 | 1,712 | ||||
Total debt | 282,749 | 221,712 | ||||
Less: Current portion of debt | 50,937 | 220,987 | ||||
Long-term debt | $ | 231,812 | $ | 725 | ||
2.25% Senior Convertible Notes
During fiscal year 2004, the Company issued $150.0 million principal amount of 2.25% senior convertible notes, which mature on March 15, 2024. Interest on the notes was payable semiannually in arrears on March 15 and September 15 of each year. Contingent interest was also payable during any six-month interest period, beginning with the six-month interest period commencing on March 15, 2009, if the average trading price of the notes for the five trading days ending on the second trading day immediately preceding such six-month interest period equaled or exceeded 120% of the principal amount of the notes. The amount of contingent interest payable per note in respect of any six-month interest period was equal to 0.25% of the average trading price of a note for the trading period referenced above.
F-23
Table of Contents
On each of March 15, 2009, March 15, 2014, and March 15, 2019, holders of the 2.25% $150.0 million senior convertible notes have the option to require the Company to purchase the notes at 100% of the principal amount of the notes plus accrued and unpaid interest, and other considerations, including contingent interest, if applicable (“put option”). On March 15, 2009, the holders of $149.98 million in principal face value of 2.25% senior convertible notes exercised the put option. The Company used approximately $101.7 million in available cash on hand from the issuance of $230.0 million of 3.125% senior convertible notes and $50 million from its asset-based revolving line of credit to fund the repurchase of such notes and accrued interest on March 16, 2009, for approximately $151.7 million.
The fair value of the remaining senior convertible notes at March 27, 2009 was immaterial, while the fair value at March 28, 2008 was approximately $164.7 million.
As of March 27, 2009, the Company has a deferred income tax liability of $18.8 million (tax effected) related to interest deductions taken for tax purposes on its 2.25% $150.0 million senior convertible notes. The liability will be deferred and paid ratably from fiscal year 2014 to fiscal year 2018 in accordance with the American Recovery and Reinvestment Act of 2009.
During the first quarter of fiscal year 2009, the Company corrected the classification of its 2.25% $150.0 million senior convertible notes at March 28, 2008 from long-term debt to short-term debt. Refer to Footnote 2,Summary of Significant Accounting Policies, for additional information.
3.125% Senior Convertible Notes
In August 2008, the Company issued $230.0 million principal amount of 3.125% senior convertible notes, which mature on August 1, 2014. Interest on the notes is payable semiannually in arrears on February 1 and August 1 of each year. The notes will be convertible into cash up to the principal amount of the notes and shares of the Company’s common stock for any conversion value in excess of the principal amount under the following circumstances: (i) if the Company has called the notes for redemption; (ii) in the event of a fundamental change, as defined in the indenture, such as a merger, acquisition, or liquidation; (iii) on or after May 1, 2014 and prior to the close of business on the second scheduled trading day immediately preceding August 1, 2014; (iv) prior to May 1, 2014, during the five consecutive business day period following any five consecutive trading day period in which the trading price for a note for each day of that trading period is less than 98% of the closing sale price of the Company’s common stock on such corresponding trading day multiplied by the applicable conversion rate; (v) prior to May 1, 2014, during any calendar quarter after September 30, 2008 in which the closing sale price of the Company’s common stock for at least 20 of the 30 consecutive trading days ending the day prior to such quarter is greater than 130% of the applicable conversion price of $21.22 per share (“Contingent Conversion Trigger”); or (vi) upon certain specified corporate events as discussed in the indenture governing the notes.
A note holder may not exercise its conversion right with respect to all or any portion of a note, if such conversion would cause the note holder to become a beneficial owner of more than 9.9% of the Company’s outstanding voting stock. The initial conversion rate is 47.1342 shares of common stock per each $1 (in thousands) principal amount of notes and is equivalent to an initial conversion price of $21.22 per share. The conversion rate is subject to adjustment upon the occurrence of certain events.
As of March 27, 2009, the fair value of the 3.125% $230.0 million senior convertible notes was approximately $203.5 million.
F-24
Table of Contents
The ability of note holders to convert is assessed on a quarterly basis and is dependent on the trading price of the Company’s stock during the last 30 trading days of each quarter. The Contingent Conversion Trigger was not met during the three months ended March 27, 2009; therefore, the notes may not be converted during the Company’s first quarter of fiscal year 2010.
The Company used a portion of the net proceeds of the offering to repurchase approximately $35.0 million of its common stock in privately negotiated transactions with institutional investors concurrently with this offering. See Stock Repurchase Program in Footnote 13,Shareholders’ Equity, for additional information. The Company used $101.7 million of the net proceeds during the year, when holders of the 2.25% senior convertible notes required the Company to redeem approximately all of their outstanding notes plus accrued interest. Remaining proceeds will be used for general corporate purposes.
Convertible Note Hedge Transactions
In connection with the offering of the notes, the Company also entered into convertible note hedge transactions with respect to its common stock (the “purchased options”) with a major financial institution (the “counterparty”). The Company paid an aggregate amount of $54.1 million to the counterparty for the purchased options. The purchased options cover, subject to anti-dilution adjustments substantially identical to those in the notes, approximately 10.8 million shares of common stock at a strike price that corresponds to the initial conversion price of the notes, also subject to adjustment, and are exercisable at each conversion date of the notes. The purchased options will expire upon the earlier of (i) the last day the notes remain outstanding or (ii) the second scheduled trading day immediately preceding the maturity date of the notes.
The purchased options are intended to reduce the potential dilution upon conversion of the notes in the event that the market value per share of the common stock, as measured under the notes, at the time of exercise is greater than the conversion price of the notes. The options have been accounted for as an adjustment to the Company’s stockholders’ equity, net of deferred tax assets of $21.0 million.
The purchased options are separate transactions, entered into by the Company with the counterparty, and are not part of the terms of the notes. Holders of the notes will not have any rights with respect to the purchased options.
Warrant Transactions
The Company also entered into warrant transactions (the “warrants”), whereby the Company sold to the counterparty warrants in an aggregate amount of $25.4 million to acquire, subject to anti-dilution adjustments, up to 10.8 million shares of common stock at a strike price of $28.29 per share of common stock, also subject to adjustment. The warrants will expire after the purchased options in approximately ratable portions on a series of expiration dates commencing on November 3, 2015.
If the market value per share of the common stock, as measured under the warrants, exceeds the strike price of the warrants, the warrants will have a dilutive effect on the Company’s earnings per share. The warrants have been accounted for as an adjustment to the Company’s stockholders’ equity.
The warrants are separate transactions, entered into by the Company with the counterparties, and are not part of the terms of the notes. Holders of the notes do not have any rights with respect to the warrants.
Revolving Line of Credit
The Company maintains an asset-based revolving line of credit (the “Credit Agreement”), which matures on September 30, 2012 and is classified as a current liability in accordance with EITF No. 95-22,Balance Sheet Classification of Borrowings Outstanding under Revolving Credit Agreements That Include both a Subjective Acceleration Clause and a Lock-Box Arrangement.The Credit Agreement permits maximum borrowings of up to $200.0 million, which may be increased to $250.0 million. Availability of borrowings (“Availability”) depends upon a borrowing base calculation consisting of accounts receivable and inventory, subject to satisfaction of certain eligibility requirements less any outstanding letters of credit. Borrowings under the revolving line of credit bear interest at the bank’s prime rate plus an applicable margin based on a fixed charge coverage ratio, or at LIBOR plus an applicable margin based on a fixed charge coverage ratio. Additionally, the Credit Agreement bears interest at a fixed rate of 0.25% for any unused portion of the facility.
F-25
Table of Contents
Under the Credit Agreement, the Company and its subsidiaries are subject to certain covenants, including but not limited to, limitations on (i) paying dividends and repurchasing the Company’s common stock, (ii) selling or transferring assets, (iii) making certain investments including acquisitions, (iv) incurring additional indebtedness and liens, and (v) annual capital expenditures. However, these covenants may not apply if the Company maintains sufficient Availability under the credit facility.
Borrowings under the revolving line of credit are anticipated to fund future requirements for working capital, capital expenditures, acquisitions, and the issuance of letters of credit. As of March 27, 2009, the Company had outstanding letters of credit in the amount of $0.5 million.
Outstanding borrowings under the revolving line of credit were $50 million as of March 27, 2009 and $70 million as of March 28, 2008. After reducing Availability for outstanding borrowings and letter of credit commitments, the Company has sufficient assets based on eligible accounts receivable and inventory to borrow an additional $149.5 million (excluding the additional increase of $50 million) under the revolving line of credit. Outstanding borrowings during the fiscal year ended March 27, 2009 bore interest in two forms, either at the bank’s prime rate plus an applicable margin or at LIBOR plus an applicable margin. Average daily borrowings during fiscal year 2009 were $51.1 million and during fiscal year 2008 were $31.9 million. Excluding the impact of the interest rate swap, a hypothetical 1% increase/decrease in prevailing interest rates at March 27, 2009, would result in a corresponding increase/decrease in interest expense of approximately $0.5 million. The average daily interest rate, excluding debt issuance costs and unused line fees, for the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, was 4.00%, 5.13%, and 7.60%, respectively.
From time-to-time, the Company has amended the Credit Agreement to meet specific business objectives and requirements. The Credit Agreement, originally dated May 20, 2003, was last amended on January 23, 2008.
This amendment (i) extended the term of the agreement to September 30, 2012, and (ii) set the determination of applicable margin level for Base Rate loans and LIBOR loans from Availability to a Fixed Charge Coverage Ratio and reduced the maximum applicable margin to 1.75%.
Interest Rate Swap Agreement
Changes in interest rates affect interest payments under the Company’s variable rate Credit Agreement. During fiscal year 2008, the Company entered into an interest rate swap agreement which matures on February 19, 2010. The purpose of this swap agreement is to hedge the variable interest rate of its Credit Agreement. The notional amount of the swap is $50.0 million. The interest rate swap effectively fixed the interest rate on a portion of the revolving line of credit to 2.70%, plus an applicable margin as determined by the Credit Agreement.
These interest rate swaps have been designated as cash flow hedges in accordance with the provisions of SFAS No. 133,Accounting for Derivative Instruments and Hedging Activities,SFAS No. 138,Accounting for Certain Derivative Instruments and Certain Hedging Activities, an amendment of FASB Statement No. 133,SFAS No. 149, Amendment of Statement 133 on Derivative Instruments and Hedging Activities,and SFAS No 161,Disclosures about Derivative Instruments and Hedging Activities, and amendment of FASB Statement No. 133.Therefore, changes in fair value were recognized in Accumulated other comprehensive income in the accompanying Consolidated balance sheets. Under the terms of the interest rate swap agreements, the Company makes monthly payments based on the fixed rate and receives interest payments based on 1-month LIBOR. The changes in market value of these financial instruments were highly correlated with changes in market value of the hedged item both at inception and over the lives of the agreements. Amounts received or paid under these interest rate swap agreements are recorded as reductions or additions to interest expense.
F-26
Table of Contents
The interest rate swap is disclosed on the Consolidated balance sheets and Consolidated statements of shareholders’ equity as follows:
| March 27, 2009 | March 28, 2008 | |||||||||
| (in thousands) | Balance Sheet Location | Fair Value | Balance Sheet Location | Fair Value | ||||||
Derivatives designated as hedging instrument Interest rate swap | Other current liabilities | $ | 891 | Other current liabilities | $ | 325 | ||||
Total derivatives designated as hedging instrument | $ | 891 | $ | 325 | ||||||
Total derivatives | $ | 891 | $ | 325 | ||||||
Derivatives in Cash Flow Hedging Relationships | Amount of Gain (Loss) Recognized in OCI on Derivative (Effective Portion) | ||||||||||
| (in thousands) | March 27, 2009 | March 28, 2008 | March 30, 2007 | ||||||||
Interest rate swap, net of tax | $ | (351 | ) | $ | (202 | ) | $ | — | |||
Total | $ | (351 | ) | $ | (202 | ) | $ | — | |||
Capital Lease Obligations
The Company leases certain computer hardware and office equipment at an aggregate annual rental of approximately $1,093. The equipment is capitalized at its fair market value, which approximates the present value of the future minimum lease payments, and is amortized over the useful life of the asset. The following table is a schedule by year of future minimum lease payments under capital leases, together with the present value of the net minimum lease payments as of March 27, 2009.
Fiscal Year: | |||
2010 | $ | 1,093 | |
2011 | 1,042 | ||
2012 | 859 | ||
2013 | 9 | ||
Total minimum lease payments | 3,003 | ||
Less: amount representing interest | 274 | ||
Present value of net minimum lease payments | $ | 2,729 | |
| 12. | INCOME TAXES |
The provision for income taxes from continuing operations is detailed below:
| 2009 | 2008 | 2007 | ||||||||
Current tax provision: | ||||||||||
Federal | $ | 23,233 | $ | 26,784 | $ | 28,054 | ||||
State | 3,064 | 3,444 | 3,467 | |||||||
Total current provision | 26,297 | 30,228 | 31,521 | |||||||
Deferred tax (benefit) provision: | ||||||||||
Federal | 8,778 | 5,850 | (1,478 | ) | ||||||
State | 1,157 | 752 | (183 | ) | ||||||
Total deferred provision (benefit) | 9,935 | 6,602 | (1,661 | ) | ||||||
Total income tax provision | $ | 36,232 | $ | 36,830 | $ | 29,860 | ||||
F-27
Table of Contents
Total income tax expense for the years ended March 27, 2009, March 28, 2008 and March 30, 2007 was allocated as follows:
| 2009 | 2008 | 2007 | ||||||||||
Tax expense per Consolidated statements of operations | $ | 36,232 | $ | 36,830 | $ | 29,860 | ||||||
Other comprehensive income: | ||||||||||||
Unrealized holding gains on equity securities recognized for financial reporting purposes | (29 | ) | 1,371 | — | ||||||||
Unrealized losses on interest rate swap recognized for financial reporting purposes | (216 | ) | (123 | ) | — | |||||||
Total income tax expense (benefit) allocated to other comprehensive income | (245 | ) | 1,248 | — | ||||||||
Benefit for compensation expense for tax purposes in excess of amounts recognized for financial reporting purposes | (1,700 | ) | (1,452 | ) | (5,599 | ) | ||||||
Benefit for income taxes recorded as a reduction | ||||||||||||
Total income tax expense | $ | 34,287 | $ | 36,626 | $ | 24,261 | ||||||
The difference between income tax computed at the Federal statutory rate and the actual tax provision is shown below:
| 2009 | 2008 | 2007 | ||||||||||
Income from continuing operations before provision for income taxes | $ | 94,249 | $ | 93,605 | $ | 80,341 | ||||||
Tax provision at the 35% statutory rate | 32,987 | 32,762 | 28,119 | |||||||||
Increase (decrease) in taxes: | ||||||||||||
State income tax, net of Federal benefit | 2,744 | 2,728 | 2,135 | |||||||||
Other, net | 501 | 1,340 | (394 | ) | ||||||||
Total increase in taxes | 3,245 | 4,068 | 1,741 | |||||||||
Total income tax provision | $ | 36,232 | $ | 36,830 | $ | 29,860 | ||||||
Effective tax rate | 38.4 | % | 39.3 | % | 37.2 | % | ||||||
At March 27, 2009 and March 28, 2008, the Company recorded an income tax receivable of $3,712 and $1,742, respectively, related to current income tax filings and Internal Revenue Service (“IRS”) audit settlements.
F-28
Table of Contents
Deferred income taxes for fiscal years 2009 and 2008, reflect the impact of temporary differences between the financial statement and tax basis of assets and liabilities. The tax effect of temporary differences, which create deferred tax assets and liabilities, at March 27, 2009 and March 28, 2008 are detailed below:
| 2009 | 2008 | |||||||
Deferred tax assets: | ||||||||
Deferred compensation | $ | 25,843 | $ | 22,470 | ||||
Original issue discount on 3.125% convertible debt | 19,134 | — | ||||||
Net operating loss and tax credit carryforwards | 6,792 | 7,738 | ||||||
Allowance for doubtful accounts and sales returns | 4,482 | 6,591 | ||||||
Accrued incentive compensation | 4,477 | 2,912 | ||||||
Inventory uniform cost capitalization | 2,643 | 2,573 | ||||||
Inventory obsolescence | 1,707 | 2,043 | ||||||
Other | 1,467 | 1,265 | ||||||
Gross deferred tax assets | 66,545 | 45,592 | ||||||
Deferred tax liabilities: | ||||||||
Excess of tax depreciation over book depreciation | (13,346 | ) | (12,400 | ) | ||||
Capitalized software development costs | (2,522 | ) | (714 | ) | ||||
Interest on 2.25% convertible debt | (18,807 | ) | (13,050 | ) | ||||
Excess of tax amortization over book amortization | (5,881 | ) | (4,929 | ) | ||||
Unrealized gain on equity investment | (1,003 | ) | (1,371 | ) | ||||
Other | (3,729 | ) | (2,859 | ) | ||||
Gross deferred tax liabilities | (45,288 | ) | (35,323 | ) | ||||
Deferred tax assets, net | $ | 21,257 | $ | 10,269 | ||||
The deferred tax accounts at March 27, 2009 and March 28, 2008 include current deferred income tax assets of $8,059 and $10,488, respectively, included in current assets; non-current deferred income tax assets of $13,198 and $0, respectively, included in other assets; and non-current deferred income tax liabilities of $0 and $219, respectively, included in other noncurrent liabilities.
At March 27, 2009 and March 28, 2008, the Company had federal and state net operating loss (“NOL”) carryforwards resulting in deferred tax assets of $6,504 and $7,489, respectively. The federal NOL carryforwards result in deferred tax assets at March 27, 2009 and March 28, 2008 of $4,200 and $4,656, respectively, which expire in 2025 to 2028. The state NOL carryforwards result in deferred tax assets at March 27, 2009 and March 28, 2008 of $2,304 and $2,833, respectively, which expire in 2010 to 2028. Management expects to utilize these NOL carryforwards prior to their expiration.
Management believes it is more likely than not the deferred tax assets will be realized through the reversal of existing deferred tax liabilities and future taxable income and, therefore, no valuation allowance has been recorded as of March 27, 2009.
The Company has not provided for U.S. income taxes on accumulated and undistributed earnings attributable to foreign operations as the Company intends to permanently reinvest these undistributed earnings. These earnings relate to ongoing operations and were $1,648 at March 27, 2009.
During fiscal year 2008, the Company adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—and Interpretation of FAS No. 109” (“FIN 48”) which clarifies the accounting and disclosure for uncertainty in tax positions. The adoption of FIN 48, effective April 1, 2007, resulted in a one-time decrease to stockholders equity of approximately $236.
F-29
Table of Contents
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
Unrecognized Tax Benefits at March 31, 2008 | $ | 2,155 | ||
Gross Increases for tax positions of prior years | 143 | |||
Gross Increases for tax positions of current year | 401 | |||
Lapse of Statute of Limitations | (1,029 | ) | ||
Unrecognized Tax Benefits at March 27, 2009 | $ | 1,670 | ||
The Company classifies interest and penalties related to income tax matters as a component of income tax expense. As of March 27, 2009 and March 28, 2008 the Company had $475 and $93 of accrued interest related to uncertain tax positions, respectively.
The total amount of unrecognized tax benefits that would affect the effective income tax rate if recognized is $1,121 as of March 27, 2009. It is reasonably possible that the amount of the unrecognized tax benefit with respect to certain of our unrecognized tax positions will increase or decease over the next twelve months; however, management does not expect the change, if any, to have a material effect on the Company’s financial condition or results of operations within the next twelve months.
During the three months ended December 31, 2004, the IRS completed fieldwork on the audit of the Federal income tax returns for the fiscal years ended March 29, 2002 and March 28, 2003. The Company appealed certain findings, which primarily related to timing of tax deductions, with the Appeals Office of the IRS. During the three months ended March 30, 2007, the Company and the Appeals Office of the IRS reached a settlement. The resolution of these audits resulted in the IRS issuing the Company a net refund of $1,863 of overpayment credits which was used to reduce the amount of cash required to satisfy fiscal year 2007 estimated tax payment liabilities.
The Company files a United States federal income tax return and income tax returns in various states and foreign jurisdictions. With limited exceptions, the Company is no longer subject to income tax examinations for years prior to the fiscal year ended April 1, 2005.
During fiscal year 2009, the IRS began an examination of the Company’s federal income tax return for the fiscal year ended March 31, 2006. While the Company is not presently aware of any proposed adjustments, it remains ongoing and therefore the Company cannot determine at this time the impact this examination will have on the Company’s financial condition or results of operations.
| 13. | SHAREHOLDERS’ EQUITY |
Stock Repurchase Programs
The Company repurchases its common stock under stock repurchase programs authorized by the Company’s Board of Directors. As of March 27, 2009, there were 0.1 million shares available for repurchase under existing stock repurchase programs.
Subsequent to year end, on March 31, 2009, the Company’s Board of Directors authorized the purchase of its outstanding common shares. Depending on current market conditions and other factors, the Company is authorized to repurchase up to a maximum of 5% of its total common stock, or approximately 3.0 million common shares. Repurchases can be made in the open market, privately negotiated transactions, and other transactions that will be disclosed publicly through filings with the Securities and Exchange Commission (SEC). This authorization is in addition to any shares remaining available under existing repurchase programs.
F-30
Table of Contents
The following table summarizes the common stock repurchases and Board of Directors authorizations during fiscal years 2009, 2008, 2007, as well as the shares available for repurchase under the stock repurchase program at March 27, 2009, March 28, 2008, March 30, 2007, and March 31, 2006:
| (in thousands) | Number of Shares | ||
Shares available for repurchase at March 31, 2006 | 2,233 | ||
Shares authorized for repurchase | 3,393 | ||
Shares repurchased | (2,127 | ) | |
Shares available for repurchase at March 30, 2007 | 3,499 | ||
Shares authorized for repurchase | 3,267 | ||
Shares repurchased | (6,363 | ) | |
Shares available for repurchase at March 28, 2008 | 403 | ||
Shares authorized for repurchase | 3,092 | ||
Shares repurchased | (3,377 | ) | |
Shares available for repurchase at March 27, 2009 | 118 | ||
During fiscal year 2009, the Company repurchased approximately 3.4 million shares of common stock at an average price of $15.62 common share for approximately $52,768. During fiscal year 2008, the Company repurchased approximately 6.4 million shares of common stock at an average price of $17.68 per common share for approximately $112,527.
Equity Incentive Plans
The Company has equity incentive plans for the benefit of certain officers, directors, and employees. The Compensation Committee of the Board of Directors has discretion to make grants under these plans in the form of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, performance units, dividend equivalents, other stock-based awards, or other rights or interests relating to common stock or cash.
On June 7, 2006, the Board of Directors approved the PSS World Medical, Inc. 2006 Incentive Plan (the “2006 Plan”), a stock incentive plan under which equity may be granted to the Company’s officers, directors, and employees. The 2006 Plan became effective as of August 24, 2006, the date on which shareholders approved the plan. The 2006 Plan replaced the 1999 Long-Term Incentive Plan and the 1999 Broad-Based Employee Stock Plan (collectively, the “Prior Plans”). Grants under the 2006 Plan may be made in the form of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, restricted or deferred stock units, performance awards, dividend equivalents, performance-based cash awards, and other stock-based awards. Subject to adjustment as provided in the plan, the aggregate number of shares of common stock reserved and available for issuance pursuant to awards granted under the 2006 Plan is approximately 867 as of March 27, 2009.
In addition to the 2006 Plan, the Company maintains the 2004 Non-Employee Directors Compensation Plan (the “2004 Directors Plan”), which permits the grant of restricted stock to the Company’s non-employee directors. Subject to adjustment as provided in the plan, the aggregate number of shares of common stock reserved and available for issuance pursuant to awards granted under the 2004 Directors Plan is approximately 303 as of March 27, 2009. It is the Company’s policy to issue shares of common stock upon exercise of stock options or the grant of restricted stock from those shares reserved for issuance under the stock incentive plans.
F-31
Table of Contents
Outstanding stock-based awards granted under equity incentive plans are as follows:
| (in thousands) | March 27, 2009 | March 28, 2008 | March 30, 2007 | |||
Stock options(a) | 1,306 | 1,774 | 2,366 | |||
Restricted stock(b) | 1,015 | 953 | 405 | |||
Restricted stock units(a) | 221 | 99 | — | |||
Deferred stock units(a) | 9 | 9 | 9 | |||
Total outstanding stock based awards | 2,551 | 2,835 | 2,780 | |||
| (a) | Amounts are excluded from shares of common stock issued and outstanding. |
| (b) | Amounts as of March 27, 2009 and March 28, 2008 are included in shares of common stock issued and outstanding on the face of the balance sheet and in calculating weighted average shares outstanding, but are not considered outstanding for accounting purposes under SFAS 123(R) until restrictions lapse. |
Effective April 1, 2006, the Company adopted the provisions of SFAS 123(R) using the modified prospective transition method. SFAS 123(R) requires companies to recognize the cost of employee services received in exchange for awards of equity instruments in the financial statements based on the grant date fair value of those awards, net of estimated forfeitures over the awards’ vesting period. SFAS 123(R) requires forfeitures to be estimated at the time of grant and adjusted, if necessary in subsequent periods if actual forfeitures differ from those estimates. When estimating forfeitures, the Company considers voluntary termination behaviors as well as trends of actual equity based awards forfeited. The Company recorded a reduction of compensation expense of approximately $197 during the fiscal year ended March 30, 2007 to comply with the forfeiture provisions of SFAS 123(R).
There was no impact to the Company’s statements of operations for outstanding stock options during fiscal year 2007 as a result of adopting SFAS 123(R) because on June 7, 2004, the Compensation Committee of the Board of Directors approved an amendment to accelerate the vesting of all unvested stock options outstanding as of April 1, 2005.
Stock Option Awards
On June 6, 2008, the Compensation Committee of the Company’s Board of Directors approved a retention award of 200 stock options under the Company’s 2006 Incentive Stock Plan to the Company’s Chairman and Chief Executive Officer. The stock options awarded will cliff-vest on the five-year anniversary of the grant date.
The fair value of stock options granted was estimated as of the date of grant using a Black-Scholes option-pricing model with the following weighted-average assumptions:
| 2009 | |||
Expected dividend yield | — | ||
Expected stock price volatility | 29 | % | |
Risk-free interest rate | 3.94 | % | |
Expected term of options (years) | 10 |
The risk free interest rate used in the calculation is the rate that corresponds to the weighted average expected life on an option. The expected term of the options granted is derived from the expiration date of the options. Expected volatilities are based upon historical volatilities of the Company’s shares. Based on these assumptions, the estimated fair value of the options granted during the first quarter of fiscal year 2009 was approximately $1,481.
As of March 27, 2009, there was $1,234 of unrecognized compensation cost related to these options which is expected to be recognized over a weighted average period of 4.3 years.
F-32
Table of Contents
The following table summarizes the number of common shares to be issued upon exercise of outstanding options and the number of common shares remaining available for future issuance under the existing stock incentive plans at March 27, 2009:
| (in thousands) | Number of securities to be issued upon exercise of outstanding options | Number of securities remaining available for future issuance | ||
Equity compensation plans approved by shareholders: | ||||
1999 Long Term Incentive Plan(a) | 723 | — | ||
Amended and Restated Directors’ Stock Plan(a) | 183 | — | ||
PSS World Medical, Inc. 2006 Incentive Plan(b) | 200 | 867 | ||
2004 Non-Employee Directors Compensation Plan(c) | — | 303 | ||
| 1,106 | 1,170 | |||
Equity compensation plan not approved by shareholders: | ||||
1999 Broad Based Employee Stock Plan(a) | 200 | — | ||
Total | 1,306 | 1,170 | ||
| (a) | These plans are terminated; however, options remain outstanding at March 27, 2009 which are exercisable. |
| (b) | This plan superseded the 1999 Long Term Incentive Plan and the 1999 Broad Based Employee Stock Plan and was approved by shareholders on August 24, 2006. |
| (c) | This plan superseded the Amended and Restated Directors’ Stock Plan and was approved by shareholders during fiscal year 2005. |
The following table summarizes the stock option activity during the period from March 31, 2006 to March 27, 2009:
| Shares | Weighted Average Exercise Price | Weighted Average Contractual Term | Aggregate Intrinsic Value | ||||||||
Outstanding at, March 31, 2006 | 3,985 | $ | 10.42 | ||||||||
Exercised | (1,598 | ) | $ | 10.62 | |||||||
Expired | (21 | ) | $ | 8.90 | |||||||
Outstanding at, March 30, 2007 | 2,366 | $ | 10.30 | 2.8 | $ | 25,962 | |||||
Exercised | (321 | ) | $ | 8.72 | |||||||
Expired | (271 | ) | $ | 20.69 | |||||||
Outstanding at, March 28, 2008 | 1,774 | $ | 9.00 | 2.3 | $ | 13,729 | |||||
Granted | 200 | $ | 17.98 | ||||||||
Exercised | (605 | ) | $ | 10.26 | |||||||
Expired | (63 | ) | $ | 21.37 | |||||||
Outstanding at, March 27, 2009 | 1,306 | $ | 9.20 | 3.4 | $ | 7,909 | |||||
Exercisable at, March 27, 2009 | 1,106 | $ | 7.60 | 2.3 | $ | 7,909 | |||||
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (the difference between the Company’s closing stock price of $14.75 on the last trading day of the Company’s fiscal year end and the exercise price, multiplied by the number of outstanding stock options) that would have been received by the option holders had all option holders exercised their options on March 27, 2009. This amount changes over time based on changes in the fair market value of the Company’s stock.
The total intrinsic value of stock options exercised during fiscal years March 27, 2009 and March 28, 2008 was $4,391 and $3,118, respectively. Cash received from stock option exercises during the fiscal year ended March 27, 2009 and March 28, 2008 was approximately $6,208 and $2,796, respectively. The actual tax benefit realized for the tax deductions from stock option exercises totaled approximately $1,669 and $1,182 during the fiscal years ended March 27, 2009 and March 28, 2008, respectively.
F-33
Table of Contents
Restricted Stock Awards
The Company issues (i) restricted stock which vests based on the recipient’s continued service over time (“Time-Based Awards”) and (ii) restricted stock or restricted stock units which vest based on the Company achieving specified performance measurements (“Performance-Based Awards”).
Time-Based Awards
The Company measures the fair value of Time-Based Awards on the date of grant based on the closing stock price. The related compensation expense is recognized on a straight-line basis over the vesting period, net of estimated forfeitures.
Performance-Based Awards
The Company issues performance-based restricted stock units (“Performance Shares”) and performance-accelerated restricted stock (“PARS”) under the Company’s 2006 Incentive Plan.
The Performance Shares cliff vest three years from the date of grant and convert to shares of common stock based on the Company’s achievement of certain cumulative earnings per share growth targets. These awards, which are denominated in terms of a target number of shares, will be forfeited if performance falls below a designated threshold level and may increase up to 250% of the target number of shares for exceptional performance. The ultimate number of shares delivered to recipients and the related compensation cost recognized as expense will be based on actual performance. The Company recognizes compensation expense on a straight-line basis (net of estimated forfeitures) over the awards vesting period based on the Company’s estimate of what will ultimately vest. This estimate may be adjusted in future periods based on actual experience and changes in management assumptions.
The PARS awards vest on the five-year anniversary of the grant date, subject to accelerated vesting after three years if the Company achieves a cumulative earnings per share growth target. The Company measures stock-based compensation at the grant date, based on the estimated fair value of the award, and recognizes the cost as compensation expense on a straight-line basis (net of estimated forfeitures) over the awards’ vesting period of five years based on the Company’s estimate of its cumulative earnings per share growth rate. This estimate may be adjusted in future periods based on actual experience and changes in management assumptions.
The following table summarizes the activity of restricted stock and restricted stock units during the period from March 31, 2006 to March 27, 2009:
| Performance-Based Awards | Time-Based Awards | ||||||||||||||||
| Performance Shares | PARS | Shares | Weighted Average Grant Date Fair Value | ||||||||||||||
| Shares | Weighted Average Grant Date Fair Value | Shares | Weighted Average Grant Date Fair Value | ||||||||||||||
Balance, March 31, 2006 | — | — | — | — | 286 | $ | 12.62 | ||||||||||
Granted | — | — | — | — | 237 | 19.73 | |||||||||||
Vested | — | — | — | — | (103 | ) | 11.73 | ||||||||||
Forfeited | — | — | — | — | (15 | ) | 14.01 | ||||||||||
Balance, March 30, 2007 | — | — | — | — | 405 | $ | 16.96 | ||||||||||
Granted | 99 | $ | 18.52 | 610 | $ | 18.49 | 96 | 18.24 | |||||||||
Vested | — | — | — | — | (141 | ) | 15.11 | ||||||||||
Forfeited | — | — | — | — | (16 | ) | 17.68 | ||||||||||
Balance, March 28, 2008 | 99 | $ | 18.52 | 610 | $ | 18.49 | 344 | $ | 16.64 | ||||||||
Granted | 122 | 18.47 | 191 | 18.04 | 78 | 19.26 | |||||||||||
Vested | — | — | — | — | (156 | ) | 16.90 | ||||||||||
Forfeited | — | — | (34 | ) | 18.50 | (18 | ) | 18.82 | |||||||||
Balance, March 27, 2009 | 221 | $ | 18.49 | 767 | $ | 18.38 | 248 | $ | 19.23 | ||||||||
F-34
Table of Contents
Total compensation expense for restricted stock grants during the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, was $5,570, $3,412, and $1,744, respectively, with related income tax benefits of $2,119, $1,271, and $661, respectively. The total fair value of shares vested during the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, was $2,824, $2,589, and $2,023, respectively.
Scheduled vesting for outstanding restricted stock and restricted stock units is as follows:
| Number of Shares | ||
Fiscal Year: | ||
2010 | 195 | |
2011 | 85 | |
2012 | 158 | |
2013 | 668 | |
2014 and thereafter | 130 | |
Total | 1,236 | |
As of March 27, 2009, there was $15,215 of unrecognized compensation cost related to non-vested restricted stock and restricted stock units granted under the stock incentive plans. The estimated compensation expense for the next five fiscal years is expected to be recognized over a weighted average period of 4.1 years and is as follows:
Fiscal Year: | |||
2010 | $ | 5,557 | |
2011 | 4,453 | ||
2012 | 3,209 | ||
2013 | 1,837 | ||
2014 and thereafter | 159 | ||
Total | $ | 15,215 | |
| 14. | EMPLOYEE BENEFIT PLANS |
PSS World Medical, Inc. Savings Plan
The PSS World Medical, Inc. Savings Plan (the “Plan”) provides an opportunity for tax-deferred savings, enabling eligible employees to invest in a variety of investments, including an interest in the common stock of the Company. Employees become eligible to participate in the Plan upon the completion of 30 days of service. Employees may elect to defer up to 85% but not less than 1% of their compensation to the Plan, subject to certain limitations imposed by the Internal Revenue Code. The Company matches an amount equal to the lesser of (i) 50% of the employee deferrals up to 6% of their compensation or (ii) $1,200. This match can be invested in various mutual funds or the common stock of the Company at the discretion of the participant and vests over a six-year period. During the fiscal years ended March 27, 2009, March 28, 2008, and March 30, 2007, the Company contributed approximately $1,840, $1,756, and $1,626, respectively, to the Plan under this matching arrangement. The Plan owned approximately 1.7 million, 1.7 million, and 1.8 million shares of the Company’s common stock at March 27, 2009, March 28, 2008, and March 30, 2007, respectively.
Employee Stock Purchase Plan
The Company also has an employee stock purchase plan available to all employees with at least six months of service. The plan allows eligible employees to purchase Company stock acquired in the open market through after-tax payroll deductions.
F-35
Table of Contents
Deferred Compensation Program
The Company offers a deferred compensation program (the “Program”) to qualified executives, management, and sales representatives. The Program consists of a deferred compensation plan and also previously consisted of a stock option program. Under the deferred compensation plan, participants can elect to defer up to 100% of their total compensation; however, the Company matching contribution program only applies to deferrals of up to 10% or 15% of the participant’s compensation. The Company’s matching contribution ranges from 10% to 125% of the participant’s deferral. Participant contributions are always 100% vested. The Company’s matching contribution vests in 20% increments beginning after participating in the plan for 4 years and becomes fully vested after participating in the plan for 8 years.
Upon (i) retirement or termination from the Company and (ii) at age 60, or at age 55 with ten years of participation in the Program, the retirement benefit is distributed to participants in five equal annual installments, or in a lump sum payment if the vested account balance is less than $25. The retirement benefit is distributed in a lump sum upon death and over five years upon disability. In the event of termination of employment, 100% of the participant’s vested balance will be distributed in five equal installments or in a lump-sum payment if the vested account balance is less than $25. In the event of a change in control, if the successor terminates the plan, all participants become 100% vested in their accounts, including the Company’s matching contributions, discretionary Company contributions, and allocated return thereon. The Company has purchased corporate-owned, life insurance policies for certain participants in the Program to fund the future payments related to the deferred compensation liability.
During fiscal years 2009, 2008, and 2007, the Company matched approximately $1,930, $1,967, and $1,728, respectively, of employee deferrals. The cash surrender value of the corporate-owned, life insurance policies, which is recorded in Other assets in the accompanying Consolidated Balance Sheets, was approximately $51,549 and $61,246, at March 27, 2009 and March 28, 2008, respectively. In addition, the deferred compensation liability, which is recorded in Other noncurrent liabilities in the accompanying Consolidated Balance Sheets, was approximately $45,932 and $57,863, at March 27, 2009 and March 28, 2008, respectively.
Directors’ Deferred Compensation Plan
Effective January 1, 2004, the Company offers a deferred compensation plan to non-employee members of the Board of Directors. Participants may elect to defer up to 100% receipt of their annual retainer, meeting fees, other director’s fees, and other cash compensation and invest their deferrals in a variety of investment options. A participant’s deferred compensation account balance will be distributed, at the election of the participant, in a single lump sum payment following the participant’s termination of service on the board of directors, or in up to ten annual installments. The deferred compensation account balance will be distributed in a lump sum payment upon the death of the participant, or in the event of a change in control of the Company.
Certain Officer Long-Term Executive Cash-Based Incentive Plans
During fiscal year 2006, the Compensation Committee of the Board of Directors approved a cash-based performance award program under the 1999 Long-Term Incentive Plan, known as the 2006 Shareholder Value Plan (“2006 SVP”). The performance period under the 2006 SVP is the 36-month period from April 2, 2005 to March 28, 2008. Target awards under the 2006 SVP are calculated as three times the participant’s base salary times an award factor ranging from 15% to 55% and performance goals are based on planned cumulative earnings per share. Participants have the opportunity to earn from 50% to 150% of their target award. The Company accrued approximately $4,572 and $5,000 of compensation cost as of March 28, 2008 and March 30, 2007, respectively. The Company paid the 2006 SVP awards during the first quarter of fiscal year 2009.
During fiscal year 2009, the Compensation Committee approved the 2008 Shareholder Value Plan (“2008 SVP”). The performance period under the 2008 SVP is the 36-month period from March 31, 2008 to March 25, 2011. The Company accrued approximately $1.9 million of compensation cost as of March 27, 2009. The Company expects the target payout to be approximately $6.5 million.
F-36
Table of Contents
Long-Term Equity-Based Incentive Plans
The Company has equity incentive plans for the benefit of certain officers, directors, and employees. The Compensation Committee of the Board of Directors has discretion to make grants under these plans in the form of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, performance units, dividend equivalents, other stock-based awards, or other rights or interests relating to common stock or cash. Refer to Footnote 13,Shareholders’ Equity, for further discussion related to the Company’s equity-based incentive programs.
| 15. | OPERATING LEASE COMMITMENTS |
The Company leases various facilities and equipment under operating leases. Certain lease commitments provide that the Company pays taxes, insurance, and maintenance expenses related to the leased assets. Many of the Company’s leases contain predetermined fixed escalations of the minimum rentals during the initial term. For these leases, the Company has recognized the related rental expense on a straight-line basis and has recorded the difference between the expense charged to income and amounts payable under the leases as other non-current liabilities in the accompanying balance sheets.
Rent expense, including common area maintenance, for operating leases approximated $28,018, $26,166, and $24,499, for fiscal years 2009, 2008, and 2007, respectively. As of March 27, 2009, future minimum payments by fiscal year and in the aggregate, required under non-cancelable operating leases are as follows:
Fiscal Year: | |||
2010 | $ | 23,089 | |
2011 | 18,816 | ||
2012 | 15,218 | ||
2013 | 9,685 | ||
2014 | 7,066 | ||
Thereafter | 3,270 | ||
Total | $ | 77,144 | |
| 16. | SEGMENT INFORMATION |
The Company’s reportable segments are strategic businesses that offer different products to different segments of the healthcare industry, and are the basis on which management regularly evaluates the Company. These segments are managed separately because of different customers and products. Refer to Footnote 1, Nature of Operations, for descriptive information about the Company’s operating segments. The Company primarily evaluates the operating performance of its segments based on net sales and income from operations. Corporate Shared Services allocates a portion of its costs and interest expense to the operating segments. The allocation of shared operating costs is generally proportionate to the revenues of each operating segment. Interest expense is allocated based on (i) an internal carrying value of historical capital used to acquire or develop the operating segments’ operations and (ii) budgeted operating cash flow. The following tables present financial information about the Company’s business segments:
| 2009 | 2008 | 2007 | |||||||
NET SALES: | |||||||||
Physician Business | $ | 1,357,382 | $ | 1,308,685 | $ | 1,227,520 | |||
Elder Care Business | 594,509 | 547,106 | 514,119 | ||||||
Corporate Shared Services | 800 | — | — | ||||||
Total net sales | $ | 1,952,691 | $ | 1,855,791 | $ | 1,741,639 | |||
F-37
Table of Contents
| 2009 | 2008 | 2007 | ||||||||||
NET SALES BY PRODUCT TYPE: | ||||||||||||
Consumable products | $ | 1,439,611 | $ | 1,352,797 | $ | 1,247,541 | ||||||
Pharmaceutical products | 327,502 | 307,824 | 302,892 | |||||||||
Equipment | 154,092 | 166,316 | 165,659 | |||||||||
Billing services | 15,080 | 13,041 | 12,215 | |||||||||
Customer freight charges | 13,482 | 10,149 | 8,714 | |||||||||
Vendor incentive income | 2,924 | 5,664 | 4,618 | |||||||||
Total net sales | $ | 1,952,691 | $ | 1,855,791 | $ | 1,741,639 | ||||||
INCOME FROM OPERATIONS: | ||||||||||||
Physician Business | $ | 109,800 | $ | 92,965 | $ | 84,112 | ||||||
Elder Care Business | 30,140 | 25,216 | 21,640 | |||||||||
Corporate Shared Services | (38,795 | ) | (25,778 | ) | (23,265 | ) | ||||||
Total income from operations | $ | 101,145 | $ | 92,403 | $ | 82,487 | ||||||
DEPRECIATION: | ||||||||||||
Physician Business | $ | 7,494 | $ | 8,131 | $ | 7,847 | ||||||
Elder Care Business | 4,948 | 4,557 | 4,036 | |||||||||
Corporate Shared Services | 7,342 | 6,018 | 4,867 | |||||||||
Total depreciation | $ | 19,784 | $ | 18,706 | $ | 16,750 | ||||||
AMORTIZATION OF INTANGIBLE ASSETS: | ||||||||||||
Physician Business | $ | 3,008 | $ | 3,074 | $ | 3,079 | ||||||
Elder Care Business | 2,290 | 2,443 | 2,712 | |||||||||
Corporate Shared Services | 191 | 69 | 117 | |||||||||
Total amortization of intangible assets | $ | 5,489 | $ | 5,586 | $ | 5,908 | ||||||
PROVISIONS FOR DOUBTFUL ACCOUNTS AND NOTES RECEIVABLE: | ||||||||||||
Physician Business | $ | 1,547 | $ | 2,303 | $ | 1,382 | ||||||
Elder Care Business | 2,133 | 1,484 | 3,151 | |||||||||
Total provision for doubtful accounts and notes receivable | $ | 3,680 | $ | 3,787 | $ | 4,533 | ||||||
INTEREST EXPENSE: | ||||||||||||
Physician Business | $ | 4,051 | $ | 4,769 | $ | 4,101 | ||||||
Elder Care Business | 7,982 | 7,837 | 7,840 | |||||||||
Corporate Shared Services | (190 | ) | (5,833 | ) | (6,595 | ) | ||||||
Total interest expense | $ | 11,843 | $ | 6,773 | $ | 5,346 | ||||||
PROVISION FOR INCOME TAXES: | ||||||||||||
Physician Business | $ | 41,115 | $ | 35,578 | $ | 30,885 | ||||||
Elder Care Business | 8,496 | 6,924 | 5,274 | |||||||||
Corporate Shared Services | (13,379 | ) | (5,672 | ) | (6,299 | ) | ||||||
Total provision for income taxes | $ | 36,232 | $ | 36,830 | $ | 29,860 | ||||||
CAPITAL EXPENDITURES: | ||||||||||||
Physician Business | $ | 5,201 | $ | 3,039 | $ | 4,531 | ||||||
Elder Care Business | 568 | 436 | 447 | |||||||||
Corporate Shared Services | 21,167 | 15,788 | 12,288 | |||||||||
Total capital expenditures | $ | 26,936 | $ | 19,263 | $ | 17,266 | ||||||
ASSETS: | ||||||||||||
Physician Business | $ | 426,117 | $ | 447,711 | $ | 413,646 | ||||||
Elder Care Business | 272,311 | 264,977 | 266,472 | |||||||||
Corporate Shared Services | 173,402 | 102,137 | 94,855 | |||||||||
Total assets | $ | 871,830 | $ | 814,825 | $ | 774,973 | ||||||
F-38
Table of Contents
| 17. | COMMITMENTS AND CONTINGENCIES |
State of Florida Pedigree Compliance
During the first quarter of fiscal 2008, the Company’s Florida distribution operations were inspected by the Florida Department of Health for compliance with state drug pedigree legislation, which covers the receipt, storage, and distribution of pharmaceutical products within the State of Florida. Based on these inspections, on January 23, 2008, the Company signed an agreement with the State of Florida relating to its compliance with the State’s drug pedigree legislation. This agreement resolved issues regarding the interpretation of procedures for documenting the ordering, receipt, storage, and shipping of prescription pharmaceutical products covered by the legislation. Pursuant to the terms of the agreement, the Company reimbursed the State $1.0 million in costs and fees, and donated pharmaceutical inventory valued at $0.5 million, which were charged against previously established reserves within Accrued expenses on the Consolidated Balance Sheet. The Company paid the $1.0 million in the fourth quarter of fiscal year 2008 and donated the pharmaceutical inventory in the first quarter of fiscal year 2009. In the fourth quarter of fiscal year 2008, the State also issued and/or renewed permits to the Company’s existing Florida facilities, and released the quarantined inventory.
As a result of the foregoing, the Company incurred and recorded approximately $6.3 million relating to additional operating, legal and other costs to modify and transition the Company’s compliance system and processes during fiscal 2008. These costs were recorded as general and administrative expenses within the Company’s Corporate Shared Services and Physician Business.
Other Litigation Matters
The Company is party to various legal and administrative proceedings and claims arising in the normal course of business. While any litigation contains an element of uncertainty, the Company, after consultation with outside legal counsel, believes that the outcome of such other proceedings or claims which are pending or known to be threatened will not have a material adverse effect on the Company’s consolidated financial position, liquidity, or results of operations.
The Company has various insurance policies, including product liability insurance, covering risks and in amounts it considers adequate. In many cases in which the Company has been sued in connection with products manufactured by others, the Company is provided indemnification by the manufacturer. There can be no assurance that the insurance coverage maintained by the Company is sufficient or will be available in adequate amounts or at a reasonable cost, or that indemnification agreements will provide adequate protection for the Company, including agreements with foreign vendors.
Purchase Commitments
During the fourth quarter of fiscal year 2008, the Company entered into an agreement to purchase a minimum number of latex and vinyl gloves through February 2010. The pricing of the latex gloves may be periodically adjusted and is based on the price of raw latex as traded on the Malaysian Rubber Exchange. The pricing of the vinyl gloves may also be periodically adjusted and is based on the weighted price of two raw materials, Poly vinyl chloride (PVC) and Dioctylphthalate (DOP), as published on www.icis.com. These purchase commitments are valued at approximately $9,735 at March 27, 2009 and are based on management’s estimate of current pricing.
Commitments and Other Contingencies
The Company has employment agreements with certain executive officers which provide that in the event of their termination or resignation, under certain conditions, the Company may be required to pay severance to the executive officers in amounts ranging from one-fourth to two times their base salary and target annual bonus. In the event that a termination or resignation follows or is in connection with a change in control, the Company may be required to pay severance to the executive officers in amounts ranging from three-fourths to three times their base salary and target annual bonus. The Company may also be required to continue welfare benefit plan coverage for the executive officers following a termination or resignation for a period ranging from three months to three years.
F-39
Table of Contents
If a supply agreement for Select products between a vendor and the Physician Business or the Elder Care Business were to be terminated, then the Company may be required to purchase from the vendor all remaining finished and unfinished products and product-materials held by the vendor. As of March 27, 2009, the Company had no material obligation to purchase remaining products or materials due to a termination of a supply agreement with a vendor who supplies Select products to the Company.
| 18. | SUPPLEMENTAL CASH FLOW INFORMATION |
The Company’s supplemental disclosures for the years ended March 27, 2009, March 28, 2008 and March 30, 2007 are as follows:
| 2009 | 2008 | 2007 | |||||||
Cash paid for: | |||||||||
Interest | $ | 9,608 | $ | 5,450 | $ | 4,172 | |||
Income taxes, net | $ | 26,986 | $ | 31,351 | $ | 13,027 | |||
During fiscal year 2009, the Company had $2,306 relating to new capital lease obligations.
During fiscal year 2008, the Company had $296 in non-cash adjustments relating to the Company’s purchase accounting for Activus, $1,500 relating to investments in capital assets, and $1,238 relating to new capital lease obligations.
During fiscal year 2007, the Company had $1,173 in non-cash adjustments relating to the Company’s purchase accounting for business combinations, and $674 relating to new capital lease obligations.
F-40
Table of Contents
| 19. | QUARTERLY RESULTS OF OPERATIONS (UNAUDITED) |
The following tables present summarized unaudited quarterly results of operations for fiscal years 2009 and 2008. The Company believes all necessary adjustments have been included in the amounts stated below to present fairly the following selected information when read in conjunction with the consolidated financial statements of the Company. Future quarterly operating results may fluctuate depending on a number of factors, including the number of selling days in a quarter, the timing of business combinations, and changes in customer’s buying patterns of supplies, equipment, and pharmaceutical products. Results of operations for any particular quarter are not necessarily indicative of results of operations for a full year or any other quarter.
| Fiscal Year 2009 | |||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Total | |||||||||||
Net sales | $ | 472,215 | $ | 491,603 | $ | 519,145 | $ | 469,728 | $ | 1,952,691 | |||||
Gross profit | 138,931 | 147,255 | 154,863 | 140,860 | 581,909 | ||||||||||
Net income | 10,317 | 13,898 | 18,158 | 15,644 | 58,017 | ||||||||||
Earnings per share—Basic(a) | $ | 0.17 | $ | 0.23 | $ | 0.31 | $ | 0.26 | $ | 0.97 | |||||
Earnings per share—Diluted(a) | $ | 0.17 | $ | 0.23 | $ | 0.30 | $ | 0.26 | $ | 0.96 | |||||
| Fiscal Year 2008 | |||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Total | |||||||||||
Net sales | $ | 438,910 | $ | 457,930 | $ | 465,208 | $ | 493,743 | $ | 1,855,791 | |||||
Gross profit | 127,683 | 132,540 | 134,993 | 146,456 | 541,672 | ||||||||||
Net income | 8,687 | 14,483 | 14,162 | 19,443 | 56,775 | ||||||||||
Earnings per share—Basic(a) | $ | 0.13 | $ | 0.22 | $ | 0.22 | $ | 0.31 | $ | 0.88 | |||||
Earnings per share—Diluted(a) | $ | 0.13 | $ | 0.22 | $ | 0.22 | $ | 0.31 | $ | 0.86 | |||||
| (a) | Due to the changes in the number of average common shares and common stock equivalents outstanding during the year, the sum of earnings per share amounts for each quarter do not necessarily sum in the aggregate to the earnings per share amounts for the full year. |
The Company reports its year-end and quarter-end financial position, results of operations, and cash flows as of the Friday closest to calendar month end, determined using the number of business days. Fiscal years 2009 and 2008 each consist of 52 weeks or 253 selling days, respectively. The three months ended March 27, 2009 consisted of 60 selling days, while the three months ended March 28, 2008 consisted of 64 selling days. The three months ended January 2, 2009 consisted of 66 selling days, while the three months ended December 28, 2007 consisted of 62 selling days. The remaining quarterly selling days during fiscal year 2009 and 2008 were consistent with each other.
F-41
Table of Contents
PSS WORLD MEDICAL, INC. AND SUBSIDIARIES
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED MARCH 27, 2009, MARCH 28, 2008, AND MARCH 30, 2007
(Dollars in Thousands)
Valuation Allowance For Accounts Receivable | Balance at Beginning of Period | Provision Charged to Expense | Write-Offs(1) | Balance at End of Period | ||||||||
Fiscal Year Ended March 30, 2007 | $ | 10,498 | $ | 4,533 | $ | 6,345 | $ | 8,686 | ||||
Fiscal Year Ended March 28, 2008 | $ | 8,686 | $ | 3,787 | $ | 5,462 | $ | 7,011 | ||||
Fiscal Year Ended March 27, 2009 | $ | 7,011 | $ | 3,680 | $ | 3,650 | $ | 7,041 | ||||
| (1) | Uncollectible Accounts Written Off, Net of Recoveries. |
See Report of Independent Registered Public Accounting Firm
F-42
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s Principal Executive Officer and Principal Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this report (the “Evaluation Date”). Based on the evaluation, the Principal Executive Officer and the Principal Financial Officer concluded that, as of the Evaluation Date, the Company’s disclosure controls and procedures are effective in recording, processing, summarizing and reporting, on a timely basis, information required to be disclosed by the Company in the reports that it files or submits under the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) and are effective in ensuring that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act, is accumulated and communicated to the Company’s management, including the Company’s Principal Executive Officer and Principal Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Internal Control Over Financial Reporting
(a) Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining effective internal controls over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f).
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the Company’s financial statements.
Management, with the participation of the Company’s principal executive and principal financial officers, assessed the effectiveness of the Company’s internal control over financial reporting as of March 27, 2009. This assessment was performed using the criteria established under the Internal Control-Integrated Framework established by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations, including the possibility of human error or circumvention or overriding of internal control. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance with respect to financial statement preparation and reporting and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Based on the assessment performed using the criteria established by COSO, management has concluded that the Company maintained effective internal control over financial reporting as of March 27, 2009.
59
Table of Contents
KPMG LLP, the independent registered public accounting firm that audited the financial statements included in this Annual Report on Form 10-K for the fiscal year ended March 27, 2009, has issued an audit report on the effectiveness of the Company’s internal control over financial reporting. Such report appears immediately below.
(b) Attestation Report of the Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
PSS World Medical, Inc.:
We have audited PSS World Medical, Inc. and subsidiaries (the Company) internal control over financial reporting as of March 27, 2009, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). PSS World Medical, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying management’s annual report on internal control over financial reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, PSS World Medical, Inc. maintained, in all material respects, effective internal control over financial reporting as of March 27, 2009, based on criteria established inInternal Control—Integrated Framework issued by COSO.
60
Table of Contents
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of PSS World Medical, Inc. and subsidiaries as of March 27, 2009 and March 28, 2008, and the related consolidated statements of operations, shareholders’ equity, and cash flows for each of the years in the three-year period ended March 27, 2009 and the financial statement schedule and our report dated May 20, 2009, expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
May 20, 2009
Jacksonville, Florida
Certified Public Accountants
(c) Changes in Internal Control Over Financial Reporting
There were no changes in the Company’s internal control over financial reporting that occurred during the three months ended March 27, 2009 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
61
Table of Contents
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information called for by this Item 10 regarding the Company’s directors, executive officers and corporate governance is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC under the Exchange Act, relating to the Company’s fiscal year 2009 Annual Meeting of Shareholders under the sections“Corporate Governance”and“Management.”
The Company has adopted a Code of Ethics that applies to the Chief Executive Officer, Chief Financial Officer and Corporate Controller. The Code of Ethics may be viewed free of charge on the Company’s web sitewww.pssworldmedical.com. The Company intends to post amendments to or waivers from its Code of Ethics (to the extent applicable to the Company’s Chief Executive Officer, Chief Financial Officer, or Corporate Controller) on its web site.
Information required by this item concerning compliance with Section 16(a) of the Securities Exchange Act of 1934 is hereby incorporated by reference to the Section entitled“Section 16(a) Beneficial Ownership Reporting Compliance.”
There have been no changes to the procedures by which stockholders may recommend nominees to the Company’s Board of Directors since the Company’s last disclosure of such procedures, which appeared in the Company’s definitive 2008 Proxy Statement filed pursuant to Regulation 14A on July 25, 2008.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information called for by this Item 11 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2009 Annual Meeting of Shareholders under the caption “Executive Compensation.”
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information called for by this Item 12 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2009 Annual Meeting of Shareholders under the caption “Security Ownership of Certain Beneficial Owners and Management”and“Executive Compensation.”
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information called for by this Item 13 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2009 Annual Meeting of Shareholders under the caption“Certain Relationships and Related Transactions”and“Director Independence.”
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information called for by this Item 14 is incorporated herein by reference to the Company’s definitive proxy statement, to be filed with the SEC pursuant to Regulation 14A of the Exchange Act, relating to the Company’s fiscal year 2009 Annual Meeting of Shareholders under the caption“Independent Registered Public Accounting Firm.”
62
Table of Contents
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a)(1) The following financial statements are included in Item 8 of this report:
| Page | ||
| F-2 | ||
Consolidated Balance Sheets—March 27, 2009 and March 28, 2008 | F-3 | |
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-7 |
(a)(2) The following supplemental schedule is included in this report:
| Page | ||
| F-42 |
(a)(3) Exhibits required by Item 601 of Regulation S-K:
Exhibit Number | Description | |
| 3.1 | Restatement of Amended and Restated Articles of Incorporation, dated December 12, 2008 (24) | |
| 3.2 | Amended and Restated Bylaws, dated December 12, 2008 (24) | |
| 4.1 | Registration Rights Agreement, dated as of March 8, 2004, by and among the Company, Goldman, Sachs & Co., Banc of America Securities LLC and Lehman Brothers Inc. (9) | |
| 4.2 | Indenture, dated as of March 8, 2004, by and between the Company and Wachovia Bank, N.A., as Trustee. (9) | |
| 4.3 | Form of 2.25% Convertible Senior Note due 2024. (9) | |
| 4.4 | Indenture related to the 3.125% Convertible Senior Notes due 2014, dated as of August 4, 2008, among PSS World Medical, Inc., as issuer, U.S. Bank National Association, as trustee. (23) | |
| 4.5 | Form of 3.125% Convertible Senior Note due 2014. (23) | |
| 10.1* | Amended and Restated Directors Stock Plan. (6) | |
| 10.2* | 1994 Employee Stock Purchase Plan. (1) | |
| 10.3* | 1999 Long-term Incentive Plan (Amended and Restated as of July 25, 2001). (6) | |
| 10.4 | 1999 Broad-Based Employee Stock Plan (6) | |
| 10.5* | PSS World Medical, Inc. 2006 Incentive Plan (18) | |
| 10.6 | Shareholder Value Plan (Portions omitted pursuant to a request for confidentiality treatment, separately filed with the SEC). | |
| 10.7* | Description of Executive Officer Annual Incentive Bonus Program. (17) | |
63
Table of Contents
Exhibit Number | Description | |
| 10.8 | Distributorship Agreement between Abbott Laboratories and the Company (Portions omitted pursuant to a request for confidential treatment – Separately filed with the SEC). (7) | |
| 10.9* | Amended and Restated Savings Plan. (3) | |
| 10.9a* | First Amendment to the Amended and Restated Savings Plan. (4) | |
| 10.9b* | Second Amendment to the Amended and Restated Savings Plan. (5) | |
| 10.9c* | Third Amendment to the Amended and Restated Savings Plan. (6) | |
| 10.9d* | Fourth Amendment to the Amended and Restated Savings Plan. (7) | |
| 10.9e* | Fifth Amendment to the Amended and Restated Savings Plan. (8) | |
| 10.9f* | Sixth Amendment to the Amended and Restated Savings Plan. (13) | |
| 10.9g* | Seventh Amendment to the Amended and Restated Savings Plan. (20) | |
| 10.9h* | Eighth Amendment to the Amended and Restated Savings Plan (25) | |
| 10.10 | Amended and Restated Credit Agreement, dated as of May 20, 2003, by and among the Company, each of the Company’s subsidiaries therein named, the Lenders from time to time party thereto, Bank of America, N.A., as Agent, and Banc of America Securities LLC, as Arranger. (6) | |
| 10.10a | Second Amendment to Credit Agreement, dated as of December 16, 2003, by and among the Company, each of the Company’s subsidiaries therein named, the Lenders from time to time party thereto, Bank of America, N.A., as Agent, and Banc of America Securities LLC, as Arranger. (8) | |
| 10.10b | Third Amendment to Credit Agreement, dated as of March 1, 2004, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (9) | |
| 10.10c | Fourth Amendment to Credit Agreement, dated as of June 1, 2004, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (11) | |
| 10.10d | Fifth Amendment to Credit Agreement, dated as of October 1, 2004, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (12) | |
| 10.10e | Sixth Amendment to Credit Agreement, dated as of June 30, 2005, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (16) | |
| 10.10f | Seventh Amendment to the Credit Agreement, dated as of January 23, 2008, among the Company, each of the Company’s subsidiaries therein named, the Lenders party to the amendment, and Bank of America, N.A., as agent for the Lenders. (22) | |
| 10.11* | Employment Agreement, dated as of April 1, 2003, by and between the Company and David M. Bronson. (5) | |
| 10.12* | Employment Agreement, dated as of August 16, 2005, by and between the Company and Gary A. Corless. (14) | |
| 10.13* | Employment Agreement, dated as of April 1, 2004, by and between the Company and Kevin P. English. (10) | |
64
Table of Contents
Exhibit Number | Description | |
| 10.14* | Employment Agreement, dated as of January 1, 2002, by and between the Company and Bradley J. Hilton. (13) | |
| 10.15* | Employment Agreement, dated as of April 1, 1998, by and between the Company and John F. Sasen, Sr. (2) | |
| 10.15a* | Amendment to Employment Agreement, dated as of April 17, 2000, by and between the Company and John F. Sasen, Sr. (2) | |
| 10.16* | Employment Agreement, dated as of July 10, 2003, by and between the Company and David A. Smith. (6) | |
| 10.17* | PSS World Medical, Inc. Amended and Restated Officer Deferred Compensation Plan, as amended through July 1, 2004. (12) | |
| 10.17a* | Amendment No. 1 to the PSS World Medical, Inc. Amended and Restated Officer Deferred Compensation Plan, as amended through July 1, 2004. (15) | |
| 10.17b* | PSS World Medical, Inc. Amended and Restated Officer Stock Option Grant Program, as amended through July 1, 2004. (12) | |
| 10.17c* | PSS World Medical Inc. Amended and Restated Director’s Deferred Compensation Plan, as amended and restated effective January 1, 2009 (25) | |
| 10.17d* | PSS World Medical Inc. Amended and Restated Officer Deferred Compensation Plan, as amended and restated effective January 1, 2009 (25) | |
| 10.18* | PSS World Medical, Inc. Amended and Restated ELITe Deferred Compensation Plan, as amended through July 1, 2004. (12) | |
| 10.18a* | Amendment No. 1 to the PSS World Medical, Inc. Amended and Restated ELITe Deferred Compensation Plan, as amended through July 1, 2004. (15) | |
| 10.18b* | PSS World Medical, Inc. Amended and Restated ELITe Stock Option Grant Program, as amended through July 1, 2004. (12) | |
| 10.18c* | PSS World Medical Inc. Amended and Restated ELITe Deferred Compensation Plan, as amended and restated effective January 1, 2009 (25) | |
| 10.19* | PSS World Medical, Inc. Amended and Restated Leader’s Deferral Plan, as amended through July 1, 2004. (12) | |
| 10.19a* | Amendment No. 1 to the PSS World Medical, Inc. Amended and Restated Leader’s Deferral Plan, as amended through July 1, 2004. (15) | |
| 10.19b* | PSS World Medical, Inc. Leader’s Stock Option Grant Program, as amended through July 1, 2004. (12) | |
| 10.19c* | PSS World Medical Inc. Amended and Restated Leader’s Deferral Plan, as amended and restated effective January 1, 2009 (25) | |
| 10.20* | PSS World Medical, Inc. Directors’ Deferred Compensation Plan. (10) | |
| 10.21* | PSS World Medical, Inc. 2004 Non-Employee Directors’ Deferred Compensation Plan. (12) | |
| 10.21a* | Amendment No. 1 to the PSS World Medical, Inc. Amended and Restated 2004 Non-Employee Directors’ Deferred Compensation Plan, as amended through August 24, 2006. (19) | |
| 10.22* | Form of Restricted Stock Award Agreement. (14) | |
65
Table of Contents
Exhibit Number | Description | |
| 10.23* | Form of Performance-Accelerated Restricted Stock Award Agreement. (21) | |
| 10.24* | Form of Performance-Based Restricted Stock Unit Agreement. (21) | |
| 10.25 | Form of Non-statutory Stock Option Award. (23) | |
| 10.26 | Purchase Agreement dated as of July 29, 2008 among PSS World Medical and Goldman, Sachs & Co. (23) | |
| 10.27 | Convertible Bond Hedge Transaction Confirmation, dated July 29, 2008 between Goldman, Sachs & Co. and PSS World Medical, Inc. (23) | |
| 10.28 | Issuer Warrant Transaction Confirmation dated July 29, 2008 between Goldman, Sachs & Co. and PSS World Medical, Inc. (23) | |
| 10.29* | Amended and Restated Employment Agreement related to 409A, dated as of December 29, 2008, by and between the Company and David A. Smith (25) | |
| 10.30* | Amended and Restated Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and David M. Bronson (25) | |
| 10.31* | Amended and Restated Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and John F. Sasen Sr. (25) | |
| 10.32* | Amended and Restated Employment Agreement related to 409A, dated as of December 29, 2008, by and between the Company and Gary A. Corless (25) | |
| 10.33* | Amendment to Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and Kevin P. English (25) | |
| 10.34* | Amendment to Employment Agreement related to 409A, dated as of December 30, 2008, by and between the Company and Bradley J. Hilton (25) | |
| 12 | Computation of Consolidated Ratios of Earnings to Fixed Charges. | |
| 21 | List of Subsidiaries of PSS World Medical, Inc. | |
| 23 | Consent of Independent Registered Public Accounting Firm. | |
| 31.1 | Rule 13a-14(a) Certification of the Chief Executive Officer. | |
| 31.2 | Rule 13a-14(a) Certification of the Chief Financial Officer. | |
| 32.1 | Section 1350 Certification of the Chief Executive Officer. | |
| 32.2 | Section 1350 Certification of the Chief Financial Officer. | |
| * Represents a management contract or compensatory plan or arrangement. | ||
66
Table of Contents
Footnote References | ||||
(1) | Incorporated by Reference to the Company’s Registration Statement on Form S-8, Registration No. 33-80657. | |||
(2) | Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 30, 2001. | |||
(3) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 28, 2002. | |||
(4) | Incorporated by Reference to the Company’s Current Report on Form 10-Q, for the quarter ended September 27, 2002. | |||
(5) | Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 28, 2003. | |||
(6) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. | |||
(7) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended October 3, 2003. | |||
(8) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2003. | |||
(9) | Incorporated by Reference to the Company’s Current Report on Form 8-K, filed March 9, 2004. | |||
(10) | Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended April 2, 2004. | |||
(11) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004. | |||
(12) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended October 1, 2004. | |||
(13) | Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended April 1, 2005. | |||
(14) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. | |||
(15) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended December 30, 2005. | |||
(16) | Incorporated by Reference to the Company’s Current Report on Form 8-K, filed July 7, 2005. | |||
(17) | Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 31, 2006. | |||
(18) | Incorporated by Reference to the Company’s Current Report on Form 8-K, filed August 29, 2006. | |||
(19) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 29, 2006. | |||
(20) | Incorporated by Reference to the Company’s Annual Report on Form 10-K for the year ended March 30, 2007. | |||
(21) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended July 29, 2007. | |||
(22) | Incorporated by Reference to the Company’s Current Report on Form 8-K, filed January 29, 2008. | |||
(23) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 27, 2008. | |||
(24) | Incorporated by Reference to the Company’s Current Report on Form 8-K, filed December 17, 2008 | |||
(25) | Incorporated by Reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended January 2, 2009. |
67
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Jacksonville, State of Florida, on May 20, 2009.
| PSS WORLD MEDICAL, INC. | ||
| By: | /s/ David M. Bronson | |
| David M. Bronson | ||
| Executive Vice President and Chief Financial Officer (Principal Financial Officer/Principal Accounting Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signatures | Title | Date | ||
/s/ David A. Smith David A. Smith | Chairman of the Board of Directors and Chief Executive Officer | May 20, 2009 | ||
/s/ Charles E. Adair Charles E. Adair | Director | May 20, 2009 | ||
/s/ Alvin R. Carpenter Alvin R. Carpenter | Director | May 20, 2009 | ||
/s/ Steven T. Halverson Steven T. Halverson | Director | May 20, 2009 | ||
/s/ Melvin L. Hecktman Melvin L. Hecktman | Director | May 20, 2009 | ||
/s/ Delores P. Kesler Delores P. Kesler | Director | May 20, 2009 | ||
/s/ Stephen H. Rogers Stephen H. Rogers | Director | May 20, 2009 | ||
/s/ Jeffrey C. Crowe Jeffrey C. Crowe | Director | May 20, 2009 | ||
/s/ David M. Bronson David M. Bronson | Executive Vice President and Chief Financial Officer (Principal Financial Officer/Principal Accounting Officer) | May 20, 2009 | ||
68